User login
European Academy of Dermatology & Venereology (EADV): Annual Congress
EADV: DLX105 is a novel treatment strategy for Behçet’s flares
COPENHAGEN – An ultrasmall yet highly potent single-chain antibody fragment directed against tumor necrosis factor–alpha showed promise for the treatment of Behçet’s disease flares in a pilot phase II study presented at the annual congress of the European Academy of Dermatology and Venereology.
“We believe that we have something in our hands that may make a difference to these patients. Further development with follow-up studies is planned,” said Dr. Thomas Jung, chief medical officer at Delenex Therapeutics.
The agent, known for now as DLX105, utilizes the company’s proprietary PentraBody platform. DLX105 inhibits soluble as well as membrane-bound TNF-alpha. Because the protein antibody is so small, it has the capacity to penetrate into inflamed tissue, be it skin or cartilage. DLX105 is also being explored as a potential therapy for other flaring inflammatory skin and autoimmune disorders, he added.
“The antibody also leaves the tissue very rapidly. It doesn’t stick around as long as an IgG antibody. The typical half-life of this molecule is about 1 day. We believe this is actually an advantage when we talk about treating a flaring disease such as Behçet’s, where we want exposure for a certain time frame, but we don’t want to overexpose the patient over weeks and months when it is not really necessary,” Dr. Jung explained.
Behçet’s disease is a chronic autoimmune vasculitic disease which presents most often as oral ulcers, papulopustular skin lesions, genital ulcers, uveitis, and/or arthritis. Cardiac, gastrointestinal, and CNS involvement occurs less frequently. The pathogenesis of the disease is unknown; no specific cause or triggers have been identified. Behçet’s disease affects an estimated 20,000 people in the United States, but is far more common in Turkey, the Middle East, and Asia. All treatment is off-label; there is no approved therapy for Behçet’s disease. The most widely used agents are corticosteroids, colchicine, and cyclosporine, with the biologic TNF inhibitors often being utilized in an effort to prevent blindness when uveitis occurs.
Dr. Jung presented results of the small phase II open-label study, which involved six patients with Behçet’s disease for a mean of 10 years. All presented with a disease flare. All six had oral aphthous ulcers, four had skin lesions, three had joint pain, two had erythema nodosum, and one had genital ulcers. All participants received a single intravenous infusion of DLX105 at 10 mg/kg.
All of the oral ulcers healed within 1 week following the single dose of DLX105. Patients with joint pain reported it was substantially improved within 1-2 days after treatment. Genital lesions healed completely within 2 weeks. The skin lesions were also markedly improved. The clinical improvement was maintained up to 4 weeks post treatment.
The improvement in joint symptoms was not unexpected. DLX105 has been shown to inhibit human TNF-alpha–induced joint swelling in rats with an efficacy comparable to infliximab (Remicade), according to Dr. Jung.
Perhaps most impressively, Dr. Jung observed, erythema nodosum healed completely in both affected patients. Erythema nodosum can be notoriously difficult to treat. Indeed, one of the patients had erythema nodosum for 5 years during which multiple systemic therapies were employed without benefit.
COPENHAGEN – An ultrasmall yet highly potent single-chain antibody fragment directed against tumor necrosis factor–alpha showed promise for the treatment of Behçet’s disease flares in a pilot phase II study presented at the annual congress of the European Academy of Dermatology and Venereology.
“We believe that we have something in our hands that may make a difference to these patients. Further development with follow-up studies is planned,” said Dr. Thomas Jung, chief medical officer at Delenex Therapeutics.
The agent, known for now as DLX105, utilizes the company’s proprietary PentraBody platform. DLX105 inhibits soluble as well as membrane-bound TNF-alpha. Because the protein antibody is so small, it has the capacity to penetrate into inflamed tissue, be it skin or cartilage. DLX105 is also being explored as a potential therapy for other flaring inflammatory skin and autoimmune disorders, he added.
“The antibody also leaves the tissue very rapidly. It doesn’t stick around as long as an IgG antibody. The typical half-life of this molecule is about 1 day. We believe this is actually an advantage when we talk about treating a flaring disease such as Behçet’s, where we want exposure for a certain time frame, but we don’t want to overexpose the patient over weeks and months when it is not really necessary,” Dr. Jung explained.
Behçet’s disease is a chronic autoimmune vasculitic disease which presents most often as oral ulcers, papulopustular skin lesions, genital ulcers, uveitis, and/or arthritis. Cardiac, gastrointestinal, and CNS involvement occurs less frequently. The pathogenesis of the disease is unknown; no specific cause or triggers have been identified. Behçet’s disease affects an estimated 20,000 people in the United States, but is far more common in Turkey, the Middle East, and Asia. All treatment is off-label; there is no approved therapy for Behçet’s disease. The most widely used agents are corticosteroids, colchicine, and cyclosporine, with the biologic TNF inhibitors often being utilized in an effort to prevent blindness when uveitis occurs.
Dr. Jung presented results of the small phase II open-label study, which involved six patients with Behçet’s disease for a mean of 10 years. All presented with a disease flare. All six had oral aphthous ulcers, four had skin lesions, three had joint pain, two had erythema nodosum, and one had genital ulcers. All participants received a single intravenous infusion of DLX105 at 10 mg/kg.
All of the oral ulcers healed within 1 week following the single dose of DLX105. Patients with joint pain reported it was substantially improved within 1-2 days after treatment. Genital lesions healed completely within 2 weeks. The skin lesions were also markedly improved. The clinical improvement was maintained up to 4 weeks post treatment.
The improvement in joint symptoms was not unexpected. DLX105 has been shown to inhibit human TNF-alpha–induced joint swelling in rats with an efficacy comparable to infliximab (Remicade), according to Dr. Jung.
Perhaps most impressively, Dr. Jung observed, erythema nodosum healed completely in both affected patients. Erythema nodosum can be notoriously difficult to treat. Indeed, one of the patients had erythema nodosum for 5 years during which multiple systemic therapies were employed without benefit.
COPENHAGEN – An ultrasmall yet highly potent single-chain antibody fragment directed against tumor necrosis factor–alpha showed promise for the treatment of Behçet’s disease flares in a pilot phase II study presented at the annual congress of the European Academy of Dermatology and Venereology.
“We believe that we have something in our hands that may make a difference to these patients. Further development with follow-up studies is planned,” said Dr. Thomas Jung, chief medical officer at Delenex Therapeutics.
The agent, known for now as DLX105, utilizes the company’s proprietary PentraBody platform. DLX105 inhibits soluble as well as membrane-bound TNF-alpha. Because the protein antibody is so small, it has the capacity to penetrate into inflamed tissue, be it skin or cartilage. DLX105 is also being explored as a potential therapy for other flaring inflammatory skin and autoimmune disorders, he added.
“The antibody also leaves the tissue very rapidly. It doesn’t stick around as long as an IgG antibody. The typical half-life of this molecule is about 1 day. We believe this is actually an advantage when we talk about treating a flaring disease such as Behçet’s, where we want exposure for a certain time frame, but we don’t want to overexpose the patient over weeks and months when it is not really necessary,” Dr. Jung explained.
Behçet’s disease is a chronic autoimmune vasculitic disease which presents most often as oral ulcers, papulopustular skin lesions, genital ulcers, uveitis, and/or arthritis. Cardiac, gastrointestinal, and CNS involvement occurs less frequently. The pathogenesis of the disease is unknown; no specific cause or triggers have been identified. Behçet’s disease affects an estimated 20,000 people in the United States, but is far more common in Turkey, the Middle East, and Asia. All treatment is off-label; there is no approved therapy for Behçet’s disease. The most widely used agents are corticosteroids, colchicine, and cyclosporine, with the biologic TNF inhibitors often being utilized in an effort to prevent blindness when uveitis occurs.
Dr. Jung presented results of the small phase II open-label study, which involved six patients with Behçet’s disease for a mean of 10 years. All presented with a disease flare. All six had oral aphthous ulcers, four had skin lesions, three had joint pain, two had erythema nodosum, and one had genital ulcers. All participants received a single intravenous infusion of DLX105 at 10 mg/kg.
All of the oral ulcers healed within 1 week following the single dose of DLX105. Patients with joint pain reported it was substantially improved within 1-2 days after treatment. Genital lesions healed completely within 2 weeks. The skin lesions were also markedly improved. The clinical improvement was maintained up to 4 weeks post treatment.
The improvement in joint symptoms was not unexpected. DLX105 has been shown to inhibit human TNF-alpha–induced joint swelling in rats with an efficacy comparable to infliximab (Remicade), according to Dr. Jung.
Perhaps most impressively, Dr. Jung observed, erythema nodosum healed completely in both affected patients. Erythema nodosum can be notoriously difficult to treat. Indeed, one of the patients had erythema nodosum for 5 years during which multiple systemic therapies were employed without benefit.
AT THE EADV CONGRESS
Key clinical point: DLX105, a highly potent single-chain antibody fragment, shows early promise as a fast-acting treatment of flares of Behçet’s disease.
Major finding: Cutaneous, mucosal, and joint flares of Behçet’s disease resolved almost completely within several days following a single intravenous dose of DLX105 at 10 mg/kg.
Data source: This was a phase II open-label study of the effects of a single intravenous dose of DLX105 in 6 patients with flares of Behçet’s disease.
Disclosures: The study was sponsored by Delenex Therapeutics and presented by the Swiss company’s chief medical officer.
EADV: Vismodegib treatment breaks don’t hurt efficacy
COPENHAGEN – Treatment breaks due to adverse events in patients taking vismodegib for advanced basal cell carcinoma don’t appear to compromise the oral hedgehog pathway inhibitor’s efficacy; in fact, they might even enhance it, according to a prespecified interim analysis of the STEVIE trial.
STEVIE is an ongoing phase II, long-term, open-label international study designed primarily to assess the safety of vismodegib (Erivedge) in a situation similar to routine clinical practice. Efficacy and impact on quality of life are secondary endpoints. Although STEVIE has enrolled 1,227 patients, a prespecified interim analysis was conducted in the first 499 followed for at least 12 months, of whom 468 had locally advanced basal cell carcinoma (BCC) and 31 had metastatic BCC, explained Dr. Johan Hansson, an oncologist at the Karolinska Institute in Stockholm.
The drug was dosed at 150 mg once daily continuously in 28-day cycles until disease progression, intolerable toxicity, or study withdrawal. Safety follow-up was conducted at 1, 3, 5, 9, and 12 months. In an earlier report, the complete and partial response rates were 34% and 33%, respectively, in patients with locally advanced BCC, and 7% and 31% in those with metastatic disease (Lancet Oncol. 2015 Jun;16[6]:729-36).
Dr. Hansson presented new data on efficacy outcomes broken down according to treatment breaks, as well as quality of life results, at the annual congress of the European Academy of Dermatology and Venereology.
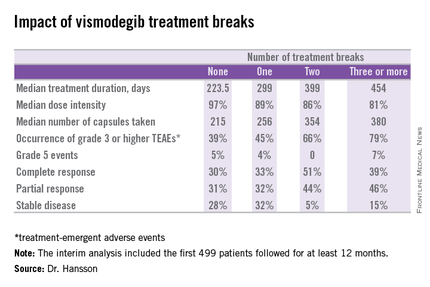
Twenty-six percent of patients had one or more treatment breaks. Seventy-six patients had one, 41 had two, and 14 had three or more. The median duration of the breaks was 22 days. The two most frequent reasons for treatment breaks were intolerable adverse events in 53% of cases, and lesser adverse events in 23%.
Close to 100% of STEVIE participants had treatment-emergent adverse events. The most common were muscle spasms, alopecia, altered sense of smell, and weight loss.
Although the number of patients with treatment breaks was relatively small, the response rates were higher in patients with more treatment breaks. So was median treatment duration as well as the median number of capsules taken.
Median progression-free survival was 19.8 months in patients with no treatment breaks, was 19.0 months in those with one, and hasn’t yet been reached in patients with two or more breaks.
In interpreting these findings, Dr. Hansson said, “We have to remember that although intriguing, these are tentative results from an exploratory analysis of subgroups in an ongoing study and should be interpreted with caution.”
The oncologist added, however, based upon these promising results he and his coinvestigators plan to look further into the concept of deliberate intermittent dosing of vismodegib.
Quality of life was assessed using the Skindex-16 questionnaire at baseline, again after two and seven 28-day cycles of vismodegib, and at 12 months. Three domains were examined: emotion, function, and symptoms.
A clinically meaningful improvement – defined as a 10-point or greater reduction from baseline – was seen in the emotion domain at all time points in patients with locally advanced BCC, with median improvements of 14.3 points after two cycles and 23.8 points after seven cycles and at the 12-month mark. Clinically meaningful improvement in symptom scores on the Skindex-16 were noted in patients aged 65 and older, in women, and in those with BCCs in locations other than the head or neck. However, no clinically meaningful improvement in the domain of function was seen at any time in patients with locally advanced BCC.
Patients with metastatic BCC didn’t show significant improvement in any of the three quality of life domains at any time point, added Dr. Hansson.
The STEVIE trial is sponsored by F. Hoffmann–La Roche/Genentech. Dr. Hansson reported receiving research grants from and serving as a consultant to Bristol-Myers Squibb, GlaxoSmithKline, Merck, Novartis, and Roche.
COPENHAGEN – Treatment breaks due to adverse events in patients taking vismodegib for advanced basal cell carcinoma don’t appear to compromise the oral hedgehog pathway inhibitor’s efficacy; in fact, they might even enhance it, according to a prespecified interim analysis of the STEVIE trial.
STEVIE is an ongoing phase II, long-term, open-label international study designed primarily to assess the safety of vismodegib (Erivedge) in a situation similar to routine clinical practice. Efficacy and impact on quality of life are secondary endpoints. Although STEVIE has enrolled 1,227 patients, a prespecified interim analysis was conducted in the first 499 followed for at least 12 months, of whom 468 had locally advanced basal cell carcinoma (BCC) and 31 had metastatic BCC, explained Dr. Johan Hansson, an oncologist at the Karolinska Institute in Stockholm.
The drug was dosed at 150 mg once daily continuously in 28-day cycles until disease progression, intolerable toxicity, or study withdrawal. Safety follow-up was conducted at 1, 3, 5, 9, and 12 months. In an earlier report, the complete and partial response rates were 34% and 33%, respectively, in patients with locally advanced BCC, and 7% and 31% in those with metastatic disease (Lancet Oncol. 2015 Jun;16[6]:729-36).
Dr. Hansson presented new data on efficacy outcomes broken down according to treatment breaks, as well as quality of life results, at the annual congress of the European Academy of Dermatology and Venereology.
Twenty-six percent of patients had one or more treatment breaks. Seventy-six patients had one, 41 had two, and 14 had three or more. The median duration of the breaks was 22 days. The two most frequent reasons for treatment breaks were intolerable adverse events in 53% of cases, and lesser adverse events in 23%.
Close to 100% of STEVIE participants had treatment-emergent adverse events. The most common were muscle spasms, alopecia, altered sense of smell, and weight loss.
Although the number of patients with treatment breaks was relatively small, the response rates were higher in patients with more treatment breaks. So was median treatment duration as well as the median number of capsules taken.
Median progression-free survival was 19.8 months in patients with no treatment breaks, was 19.0 months in those with one, and hasn’t yet been reached in patients with two or more breaks.
In interpreting these findings, Dr. Hansson said, “We have to remember that although intriguing, these are tentative results from an exploratory analysis of subgroups in an ongoing study and should be interpreted with caution.”
The oncologist added, however, based upon these promising results he and his coinvestigators plan to look further into the concept of deliberate intermittent dosing of vismodegib.
Quality of life was assessed using the Skindex-16 questionnaire at baseline, again after two and seven 28-day cycles of vismodegib, and at 12 months. Three domains were examined: emotion, function, and symptoms.
A clinically meaningful improvement – defined as a 10-point or greater reduction from baseline – was seen in the emotion domain at all time points in patients with locally advanced BCC, with median improvements of 14.3 points after two cycles and 23.8 points after seven cycles and at the 12-month mark. Clinically meaningful improvement in symptom scores on the Skindex-16 were noted in patients aged 65 and older, in women, and in those with BCCs in locations other than the head or neck. However, no clinically meaningful improvement in the domain of function was seen at any time in patients with locally advanced BCC.
Patients with metastatic BCC didn’t show significant improvement in any of the three quality of life domains at any time point, added Dr. Hansson.
The STEVIE trial is sponsored by F. Hoffmann–La Roche/Genentech. Dr. Hansson reported receiving research grants from and serving as a consultant to Bristol-Myers Squibb, GlaxoSmithKline, Merck, Novartis, and Roche.

COPENHAGEN – Treatment breaks due to adverse events in patients taking vismodegib for advanced basal cell carcinoma don’t appear to compromise the oral hedgehog pathway inhibitor’s efficacy; in fact, they might even enhance it, according to a prespecified interim analysis of the STEVIE trial.
STEVIE is an ongoing phase II, long-term, open-label international study designed primarily to assess the safety of vismodegib (Erivedge) in a situation similar to routine clinical practice. Efficacy and impact on quality of life are secondary endpoints. Although STEVIE has enrolled 1,227 patients, a prespecified interim analysis was conducted in the first 499 followed for at least 12 months, of whom 468 had locally advanced basal cell carcinoma (BCC) and 31 had metastatic BCC, explained Dr. Johan Hansson, an oncologist at the Karolinska Institute in Stockholm.
The drug was dosed at 150 mg once daily continuously in 28-day cycles until disease progression, intolerable toxicity, or study withdrawal. Safety follow-up was conducted at 1, 3, 5, 9, and 12 months. In an earlier report, the complete and partial response rates were 34% and 33%, respectively, in patients with locally advanced BCC, and 7% and 31% in those with metastatic disease (Lancet Oncol. 2015 Jun;16[6]:729-36).
Dr. Hansson presented new data on efficacy outcomes broken down according to treatment breaks, as well as quality of life results, at the annual congress of the European Academy of Dermatology and Venereology.
Twenty-six percent of patients had one or more treatment breaks. Seventy-six patients had one, 41 had two, and 14 had three or more. The median duration of the breaks was 22 days. The two most frequent reasons for treatment breaks were intolerable adverse events in 53% of cases, and lesser adverse events in 23%.
Close to 100% of STEVIE participants had treatment-emergent adverse events. The most common were muscle spasms, alopecia, altered sense of smell, and weight loss.
Although the number of patients with treatment breaks was relatively small, the response rates were higher in patients with more treatment breaks. So was median treatment duration as well as the median number of capsules taken.
Median progression-free survival was 19.8 months in patients with no treatment breaks, was 19.0 months in those with one, and hasn’t yet been reached in patients with two or more breaks.
In interpreting these findings, Dr. Hansson said, “We have to remember that although intriguing, these are tentative results from an exploratory analysis of subgroups in an ongoing study and should be interpreted with caution.”
The oncologist added, however, based upon these promising results he and his coinvestigators plan to look further into the concept of deliberate intermittent dosing of vismodegib.
Quality of life was assessed using the Skindex-16 questionnaire at baseline, again after two and seven 28-day cycles of vismodegib, and at 12 months. Three domains were examined: emotion, function, and symptoms.
A clinically meaningful improvement – defined as a 10-point or greater reduction from baseline – was seen in the emotion domain at all time points in patients with locally advanced BCC, with median improvements of 14.3 points after two cycles and 23.8 points after seven cycles and at the 12-month mark. Clinically meaningful improvement in symptom scores on the Skindex-16 were noted in patients aged 65 and older, in women, and in those with BCCs in locations other than the head or neck. However, no clinically meaningful improvement in the domain of function was seen at any time in patients with locally advanced BCC.
Patients with metastatic BCC didn’t show significant improvement in any of the three quality of life domains at any time point, added Dr. Hansson.
The STEVIE trial is sponsored by F. Hoffmann–La Roche/Genentech. Dr. Hansson reported receiving research grants from and serving as a consultant to Bristol-Myers Squibb, GlaxoSmithKline, Merck, Novartis, and Roche.

AT THE EADV CONGRESS
Key clinical point: Treatment breaks due to adverse events in patients taking vismodegib for advanced basal cell carcinoma don’t compromise efficacy.
Major finding: The complete response rate to vismodegib in patients with advanced BCC was intriguingly higher in those with more treatment breaks due to adverse events.
Data source: A prespecified interim analysis of the first 499 patients with advanced BCC enrolled in STEVIE, a large ongoing phase II, long-term, open-label international safety study of vismodegib.
Disclosures: The STEVIE trial is sponsored by F. Hoffmann–La Roche/Genentech. The presenter reported receiving research grants from and serving as a consultant to Bristol-Myers Squibb, GlaxoSmithKline, Merck, Novartis, and Roche.
Family History of Cardiovascular Disease Is Key in Psoriasis Patients
COPENHAGEN – The increased risk of MI and stroke in patients who develop psoriasis as young adults is essentially confined to those having a positive family history of cardiovascular disease, according to a Danish national study presented at the annual congress of the European Academy of Dermatology and Venereology.
“We found a significantly increased risk of MACE [major adverse cardiovascular events] in patients with psoriasis only when a family history of cardiovascular disease was present. This just highlights why it’s important that future studies of cardiovascular risk in psoriasis should include family history. Also, an increased focus on cardiovascular disease in relatives may be appropriate in the cardiovascular risk assessment of patients with psoriasis,” said Dr. Alexander Egeberg of the University of Copenhagen.
He presented a population-based study involving 15 years of follow-up of 30,278 Danes diagnosed with psoriasis in their 20s and a control group consisting of nearly 2.7 million of their Danish contemporaries who were not. None had personal history of acute MI or stroke at baseline. Family medical history, including whether cardiovascular disease occurred in first-degree relatives, was available for all subjects.
Dr. Egeberg and coinvestigators mapped the incidence of acute MI, ischemic stroke, or cardiovascular death in psoriasis patients and the general population controls during follow-up.
“When you look at the patients who developed psoriasis and didn’t have a positive family history of cardiovascular disease, there are almost no cardiovascular events for the entire country,” Dr. Egeberg observed.
In contrast, in a multivariate analysis adjusted for age, gender, socioeconomic status, comorbid cardiovascular disease, smoking, and the use of cardiovascular medications, patients with mild psoriasis and a positive family history for cardiovascular disease had a 28% greater risk of a premature cardiovascular event than the general population during follow-up out to roughly age 40. Those with a positive family history and severe psoriasis as defined by the use of systemic therapies had a 62% increase in risk. Both of these elevated risks were statistically significant.
Among young adult Danes with a positive family history for cardiovascular disease, there were 222 MACE events during 62,225 person-years of follow-up in the mild psoriasis group and 31 events during 6,848 person-years in the 4,504 subjects with severe psoriasis. The resultant incidence rates in both groups were significantly higher than in the control group, who experienced 28,846 MACE events during 16.1 million person-years of follow-up.
In contrast, fewer than 10 MACE events occurred in Danish psoriasis patients without a family history of cardiovascular disease.
A positive family history was also associated with increased MACE in the nonpsoriatic general population, although it didn’t confer as great a risk as in the Danes with psoriasis.
A point worthy of consideration, Dr. Egeberg noted, is that the epidemiology of psoriasis in Denmark apparently differs in several important ways from psoriasis in the United States and some other countries. For one, the prevalence is higher in Scandinavian countries – 7.1% in a Danish national cross-sectional study (Int J Dermatol. 2013 Jun;52[6]:681-3) and 8% in neighboring Norway – as compared with 2%-3% in much of the rest of the world.
Moreover, according to the same cross-sectional study, the prevalence of traditional cardiovascular risk factors, such as smoking and the components of the metabolic syndrome, isn’t higher in Danish psoriasis patients than in the country’s general population. That’s in contrast to the situation in the United Kingdom, where Dr. Joel M. Gelfand of the University of Pennsylvania and associates reported a decade ago in a landmark study that the prevalence of hypertension, obesity, hyperlipidemia, diabetes, and smoking were all higher in persons with psoriasis than in the general population (J Am Acad Dermatol. 2006 Nov;55[5]:829-35). Similar findings were subsequently reported in U.S. psoriasis patients.
Despite their absence of elevated levels of the standard cardiovascular risk factors, Danish psoriasis patients as a group do face a clinically significant increase in cardiovascular risk, compared with the general population, as shown in yet another Danish national cohort study in which the rate ratios for cardiovascular death for mild and severe psoriasis were 1.14 and 1.57, respectively, compared with controls (J Intern Med. 2011 Aug;270[2]:147-57).
In an even more recent Danish nationwide study, the overall death rate was found to be 25.4 per 1,000 person-years in patients with severe psoriasis, 17.0 in those with mild psoriasis, and 13.8 per 1,000 person-years in the general population (J Eur Acad Dermatol Venereol. 2015 May;29[5]:1002-5).
Dr. Egeberg said his new Danish findings suggest that even in psoriasis patients with a greater burden of systemic inflammation as expressed in severe disease, that burden alone doesn’t translate into increased cardiovascular risk. Rather, elevated cardiovascular risk appears to be a consequence of heritable factors, Dr. Egeberg said.
An important caveat regarding this study, he continued, is that the mean age at which participants were diagnosed with psoriasis was 26.6 years. It’s unclear whether the study findings extend to individuals who develop the dermatologic disease later in life.
Dr. Egeberg reported having no financial conflicts regarding this study, supported by Danish national research funding.
COPENHAGEN – The increased risk of MI and stroke in patients who develop psoriasis as young adults is essentially confined to those having a positive family history of cardiovascular disease, according to a Danish national study presented at the annual congress of the European Academy of Dermatology and Venereology.
“We found a significantly increased risk of MACE [major adverse cardiovascular events] in patients with psoriasis only when a family history of cardiovascular disease was present. This just highlights why it’s important that future studies of cardiovascular risk in psoriasis should include family history. Also, an increased focus on cardiovascular disease in relatives may be appropriate in the cardiovascular risk assessment of patients with psoriasis,” said Dr. Alexander Egeberg of the University of Copenhagen.
He presented a population-based study involving 15 years of follow-up of 30,278 Danes diagnosed with psoriasis in their 20s and a control group consisting of nearly 2.7 million of their Danish contemporaries who were not. None had personal history of acute MI or stroke at baseline. Family medical history, including whether cardiovascular disease occurred in first-degree relatives, was available for all subjects.
Dr. Egeberg and coinvestigators mapped the incidence of acute MI, ischemic stroke, or cardiovascular death in psoriasis patients and the general population controls during follow-up.
“When you look at the patients who developed psoriasis and didn’t have a positive family history of cardiovascular disease, there are almost no cardiovascular events for the entire country,” Dr. Egeberg observed.
In contrast, in a multivariate analysis adjusted for age, gender, socioeconomic status, comorbid cardiovascular disease, smoking, and the use of cardiovascular medications, patients with mild psoriasis and a positive family history for cardiovascular disease had a 28% greater risk of a premature cardiovascular event than the general population during follow-up out to roughly age 40. Those with a positive family history and severe psoriasis as defined by the use of systemic therapies had a 62% increase in risk. Both of these elevated risks were statistically significant.
Among young adult Danes with a positive family history for cardiovascular disease, there were 222 MACE events during 62,225 person-years of follow-up in the mild psoriasis group and 31 events during 6,848 person-years in the 4,504 subjects with severe psoriasis. The resultant incidence rates in both groups were significantly higher than in the control group, who experienced 28,846 MACE events during 16.1 million person-years of follow-up.
In contrast, fewer than 10 MACE events occurred in Danish psoriasis patients without a family history of cardiovascular disease.
A positive family history was also associated with increased MACE in the nonpsoriatic general population, although it didn’t confer as great a risk as in the Danes with psoriasis.
A point worthy of consideration, Dr. Egeberg noted, is that the epidemiology of psoriasis in Denmark apparently differs in several important ways from psoriasis in the United States and some other countries. For one, the prevalence is higher in Scandinavian countries – 7.1% in a Danish national cross-sectional study (Int J Dermatol. 2013 Jun;52[6]:681-3) and 8% in neighboring Norway – as compared with 2%-3% in much of the rest of the world.
Moreover, according to the same cross-sectional study, the prevalence of traditional cardiovascular risk factors, such as smoking and the components of the metabolic syndrome, isn’t higher in Danish psoriasis patients than in the country’s general population. That’s in contrast to the situation in the United Kingdom, where Dr. Joel M. Gelfand of the University of Pennsylvania and associates reported a decade ago in a landmark study that the prevalence of hypertension, obesity, hyperlipidemia, diabetes, and smoking were all higher in persons with psoriasis than in the general population (J Am Acad Dermatol. 2006 Nov;55[5]:829-35). Similar findings were subsequently reported in U.S. psoriasis patients.
Despite their absence of elevated levels of the standard cardiovascular risk factors, Danish psoriasis patients as a group do face a clinically significant increase in cardiovascular risk, compared with the general population, as shown in yet another Danish national cohort study in which the rate ratios for cardiovascular death for mild and severe psoriasis were 1.14 and 1.57, respectively, compared with controls (J Intern Med. 2011 Aug;270[2]:147-57).
In an even more recent Danish nationwide study, the overall death rate was found to be 25.4 per 1,000 person-years in patients with severe psoriasis, 17.0 in those with mild psoriasis, and 13.8 per 1,000 person-years in the general population (J Eur Acad Dermatol Venereol. 2015 May;29[5]:1002-5).
Dr. Egeberg said his new Danish findings suggest that even in psoriasis patients with a greater burden of systemic inflammation as expressed in severe disease, that burden alone doesn’t translate into increased cardiovascular risk. Rather, elevated cardiovascular risk appears to be a consequence of heritable factors, Dr. Egeberg said.
An important caveat regarding this study, he continued, is that the mean age at which participants were diagnosed with psoriasis was 26.6 years. It’s unclear whether the study findings extend to individuals who develop the dermatologic disease later in life.
Dr. Egeberg reported having no financial conflicts regarding this study, supported by Danish national research funding.
COPENHAGEN – The increased risk of MI and stroke in patients who develop psoriasis as young adults is essentially confined to those having a positive family history of cardiovascular disease, according to a Danish national study presented at the annual congress of the European Academy of Dermatology and Venereology.
“We found a significantly increased risk of MACE [major adverse cardiovascular events] in patients with psoriasis only when a family history of cardiovascular disease was present. This just highlights why it’s important that future studies of cardiovascular risk in psoriasis should include family history. Also, an increased focus on cardiovascular disease in relatives may be appropriate in the cardiovascular risk assessment of patients with psoriasis,” said Dr. Alexander Egeberg of the University of Copenhagen.
He presented a population-based study involving 15 years of follow-up of 30,278 Danes diagnosed with psoriasis in their 20s and a control group consisting of nearly 2.7 million of their Danish contemporaries who were not. None had personal history of acute MI or stroke at baseline. Family medical history, including whether cardiovascular disease occurred in first-degree relatives, was available for all subjects.
Dr. Egeberg and coinvestigators mapped the incidence of acute MI, ischemic stroke, or cardiovascular death in psoriasis patients and the general population controls during follow-up.
“When you look at the patients who developed psoriasis and didn’t have a positive family history of cardiovascular disease, there are almost no cardiovascular events for the entire country,” Dr. Egeberg observed.
In contrast, in a multivariate analysis adjusted for age, gender, socioeconomic status, comorbid cardiovascular disease, smoking, and the use of cardiovascular medications, patients with mild psoriasis and a positive family history for cardiovascular disease had a 28% greater risk of a premature cardiovascular event than the general population during follow-up out to roughly age 40. Those with a positive family history and severe psoriasis as defined by the use of systemic therapies had a 62% increase in risk. Both of these elevated risks were statistically significant.
Among young adult Danes with a positive family history for cardiovascular disease, there were 222 MACE events during 62,225 person-years of follow-up in the mild psoriasis group and 31 events during 6,848 person-years in the 4,504 subjects with severe psoriasis. The resultant incidence rates in both groups were significantly higher than in the control group, who experienced 28,846 MACE events during 16.1 million person-years of follow-up.
In contrast, fewer than 10 MACE events occurred in Danish psoriasis patients without a family history of cardiovascular disease.
A positive family history was also associated with increased MACE in the nonpsoriatic general population, although it didn’t confer as great a risk as in the Danes with psoriasis.
A point worthy of consideration, Dr. Egeberg noted, is that the epidemiology of psoriasis in Denmark apparently differs in several important ways from psoriasis in the United States and some other countries. For one, the prevalence is higher in Scandinavian countries – 7.1% in a Danish national cross-sectional study (Int J Dermatol. 2013 Jun;52[6]:681-3) and 8% in neighboring Norway – as compared with 2%-3% in much of the rest of the world.
Moreover, according to the same cross-sectional study, the prevalence of traditional cardiovascular risk factors, such as smoking and the components of the metabolic syndrome, isn’t higher in Danish psoriasis patients than in the country’s general population. That’s in contrast to the situation in the United Kingdom, where Dr. Joel M. Gelfand of the University of Pennsylvania and associates reported a decade ago in a landmark study that the prevalence of hypertension, obesity, hyperlipidemia, diabetes, and smoking were all higher in persons with psoriasis than in the general population (J Am Acad Dermatol. 2006 Nov;55[5]:829-35). Similar findings were subsequently reported in U.S. psoriasis patients.
Despite their absence of elevated levels of the standard cardiovascular risk factors, Danish psoriasis patients as a group do face a clinically significant increase in cardiovascular risk, compared with the general population, as shown in yet another Danish national cohort study in which the rate ratios for cardiovascular death for mild and severe psoriasis were 1.14 and 1.57, respectively, compared with controls (J Intern Med. 2011 Aug;270[2]:147-57).
In an even more recent Danish nationwide study, the overall death rate was found to be 25.4 per 1,000 person-years in patients with severe psoriasis, 17.0 in those with mild psoriasis, and 13.8 per 1,000 person-years in the general population (J Eur Acad Dermatol Venereol. 2015 May;29[5]:1002-5).
Dr. Egeberg said his new Danish findings suggest that even in psoriasis patients with a greater burden of systemic inflammation as expressed in severe disease, that burden alone doesn’t translate into increased cardiovascular risk. Rather, elevated cardiovascular risk appears to be a consequence of heritable factors, Dr. Egeberg said.
An important caveat regarding this study, he continued, is that the mean age at which participants were diagnosed with psoriasis was 26.6 years. It’s unclear whether the study findings extend to individuals who develop the dermatologic disease later in life.
Dr. Egeberg reported having no financial conflicts regarding this study, supported by Danish national research funding.
AT THE EADV CONGRESS
EADV: Family history of cardiovascular disease is key in psoriasis patients
COPENHAGEN – The increased risk of MI and stroke in patients who develop psoriasis as young adults is essentially confined to those having a positive family history of cardiovascular disease, according to a Danish national study presented at the annual congress of the European Academy of Dermatology and Venereology.
“We found a significantly increased risk of MACE [major adverse cardiovascular events] in patients with psoriasis only when a family history of cardiovascular disease was present. This just highlights why it’s important that future studies of cardiovascular risk in psoriasis should include family history. Also, an increased focus on cardiovascular disease in relatives may be appropriate in the cardiovascular risk assessment of patients with psoriasis,” said Dr. Alexander Egeberg of the University of Copenhagen.
He presented a population-based study involving 15 years of follow-up of 30,278 Danes diagnosed with psoriasis in their 20s and a control group consisting of nearly 2.7 million of their Danish contemporaries who were not. None had personal history of acute MI or stroke at baseline. Family medical history, including whether cardiovascular disease occurred in first-degree relatives, was available for all subjects.
Dr. Egeberg and coinvestigators mapped the incidence of acute MI, ischemic stroke, or cardiovascular death in psoriasis patients and the general population controls during follow-up.
“When you look at the patients who developed psoriasis and didn’t have a positive family history of cardiovascular disease, there are almost no cardiovascular events for the entire country,” Dr. Egeberg observed.
In contrast, in a multivariate analysis adjusted for age, gender, socioeconomic status, comorbid cardiovascular disease, smoking, and the use of cardiovascular medications, patients with mild psoriasis and a positive family history for cardiovascular disease had a 28% greater risk of a premature cardiovascular event than the general population during follow-up out to roughly age 40. Those with a positive family history and severe psoriasis as defined by the use of systemic therapies had a 62% increase in risk. Both of these elevated risks were statistically significant.
Among young adult Danes with a positive family history for cardiovascular disease, there were 222 MACE events during 62,225 person-years of follow-up in the mild psoriasis group and 31 events during 6,848 person-years in the 4,504 subjects with severe psoriasis. The resultant incidence rates in both groups were significantly higher than in the control group, who experienced 28,846 MACE events during 16.1 million person-years of follow-up.
In contrast, fewer than 10 MACE events occurred in Danish psoriasis patients without a family history of cardiovascular disease.
A positive family history was also associated with increased MACE in the nonpsoriatic general population, although it didn’t confer as great a risk as in the Danes with psoriasis.
A point worthy of consideration, Dr. Egeberg noted, is that the epidemiology of psoriasis in Denmark apparently differs in several important ways from psoriasis in the United States and some other countries. For one, the prevalence is higher in Scandinavian countries – 7.1% in a Danish national cross-sectional study (Int J Dermatol. 2013 Jun;52[6]:681-3) and 8% in neighboring Norway – as compared with 2%-3% in much of the rest of the world.
Moreover, according to the same cross-sectional study, the prevalence of traditional cardiovascular risk factors, such as smoking and the components of the metabolic syndrome, isn’t higher in Danish psoriasis patients than in the country’s general population. That’s in contrast to the situation in the United Kingdom, where Dr. Joel M. Gelfand of the University of Pennsylvania and associates reported a decade ago in a landmark study that the prevalence of hypertension, obesity, hyperlipidemia, diabetes, and smoking were all higher in persons with psoriasis than in the general population (J Am Acad Dermatol. 2006 Nov;55[5]:829-35). Similar findings were subsequently reported in U.S. psoriasis patients.
Despite their absence of elevated levels of the standard cardiovascular risk factors, Danish psoriasis patients as a group do face a clinically significant increase in cardiovascular risk, compared with the general population, as shown in yet another Danish national cohort study in which the rate ratios for cardiovascular death for mild and severe psoriasis were 1.14 and 1.57, respectively, compared with controls (J Intern Med. 2011 Aug;270[2]:147-57).
In an even more recent Danish nationwide study, the overall death rate was found to be 25.4 per 1,000 person-years in patients with severe psoriasis, 17.0 in those with mild psoriasis, and 13.8 per 1,000 person-years in the general population (J Eur Acad Dermatol Venereol. 2015 May;29[5]:1002-5).
Dr. Egeberg said his new Danish findings suggest that even in psoriasis patients with a greater burden of systemic inflammation as expressed in severe disease, that burden alone doesn’t translate into increased cardiovascular risk. Rather, elevated cardiovascular risk appears to be a consequence of heritable factors, Dr. Egeberg said.
An important caveat regarding this study, he continued, is that the mean age at which participants were diagnosed with psoriasis was 26.6 years. It’s unclear whether the study findings extend to individuals who develop the dermatologic disease later in life.
Dr. Egeberg reported having no financial conflicts regarding this study, supported by Danish national research funding.
COPENHAGEN – The increased risk of MI and stroke in patients who develop psoriasis as young adults is essentially confined to those having a positive family history of cardiovascular disease, according to a Danish national study presented at the annual congress of the European Academy of Dermatology and Venereology.
“We found a significantly increased risk of MACE [major adverse cardiovascular events] in patients with psoriasis only when a family history of cardiovascular disease was present. This just highlights why it’s important that future studies of cardiovascular risk in psoriasis should include family history. Also, an increased focus on cardiovascular disease in relatives may be appropriate in the cardiovascular risk assessment of patients with psoriasis,” said Dr. Alexander Egeberg of the University of Copenhagen.
He presented a population-based study involving 15 years of follow-up of 30,278 Danes diagnosed with psoriasis in their 20s and a control group consisting of nearly 2.7 million of their Danish contemporaries who were not. None had personal history of acute MI or stroke at baseline. Family medical history, including whether cardiovascular disease occurred in first-degree relatives, was available for all subjects.
Dr. Egeberg and coinvestigators mapped the incidence of acute MI, ischemic stroke, or cardiovascular death in psoriasis patients and the general population controls during follow-up.
“When you look at the patients who developed psoriasis and didn’t have a positive family history of cardiovascular disease, there are almost no cardiovascular events for the entire country,” Dr. Egeberg observed.
In contrast, in a multivariate analysis adjusted for age, gender, socioeconomic status, comorbid cardiovascular disease, smoking, and the use of cardiovascular medications, patients with mild psoriasis and a positive family history for cardiovascular disease had a 28% greater risk of a premature cardiovascular event than the general population during follow-up out to roughly age 40. Those with a positive family history and severe psoriasis as defined by the use of systemic therapies had a 62% increase in risk. Both of these elevated risks were statistically significant.
Among young adult Danes with a positive family history for cardiovascular disease, there were 222 MACE events during 62,225 person-years of follow-up in the mild psoriasis group and 31 events during 6,848 person-years in the 4,504 subjects with severe psoriasis. The resultant incidence rates in both groups were significantly higher than in the control group, who experienced 28,846 MACE events during 16.1 million person-years of follow-up.
In contrast, fewer than 10 MACE events occurred in Danish psoriasis patients without a family history of cardiovascular disease.
A positive family history was also associated with increased MACE in the nonpsoriatic general population, although it didn’t confer as great a risk as in the Danes with psoriasis.
A point worthy of consideration, Dr. Egeberg noted, is that the epidemiology of psoriasis in Denmark apparently differs in several important ways from psoriasis in the United States and some other countries. For one, the prevalence is higher in Scandinavian countries – 7.1% in a Danish national cross-sectional study (Int J Dermatol. 2013 Jun;52[6]:681-3) and 8% in neighboring Norway – as compared with 2%-3% in much of the rest of the world.
Moreover, according to the same cross-sectional study, the prevalence of traditional cardiovascular risk factors, such as smoking and the components of the metabolic syndrome, isn’t higher in Danish psoriasis patients than in the country’s general population. That’s in contrast to the situation in the United Kingdom, where Dr. Joel M. Gelfand of the University of Pennsylvania and associates reported a decade ago in a landmark study that the prevalence of hypertension, obesity, hyperlipidemia, diabetes, and smoking were all higher in persons with psoriasis than in the general population (J Am Acad Dermatol. 2006 Nov;55[5]:829-35). Similar findings were subsequently reported in U.S. psoriasis patients.
Despite their absence of elevated levels of the standard cardiovascular risk factors, Danish psoriasis patients as a group do face a clinically significant increase in cardiovascular risk, compared with the general population, as shown in yet another Danish national cohort study in which the rate ratios for cardiovascular death for mild and severe psoriasis were 1.14 and 1.57, respectively, compared with controls (J Intern Med. 2011 Aug;270[2]:147-57).
In an even more recent Danish nationwide study, the overall death rate was found to be 25.4 per 1,000 person-years in patients with severe psoriasis, 17.0 in those with mild psoriasis, and 13.8 per 1,000 person-years in the general population (J Eur Acad Dermatol Venereol. 2015 May;29[5]:1002-5).
Dr. Egeberg said his new Danish findings suggest that even in psoriasis patients with a greater burden of systemic inflammation as expressed in severe disease, that burden alone doesn’t translate into increased cardiovascular risk. Rather, elevated cardiovascular risk appears to be a consequence of heritable factors, Dr. Egeberg said.
An important caveat regarding this study, he continued, is that the mean age at which participants were diagnosed with psoriasis was 26.6 years. It’s unclear whether the study findings extend to individuals who develop the dermatologic disease later in life.
Dr. Egeberg reported having no financial conflicts regarding this study, supported by Danish national research funding.
COPENHAGEN – The increased risk of MI and stroke in patients who develop psoriasis as young adults is essentially confined to those having a positive family history of cardiovascular disease, according to a Danish national study presented at the annual congress of the European Academy of Dermatology and Venereology.
“We found a significantly increased risk of MACE [major adverse cardiovascular events] in patients with psoriasis only when a family history of cardiovascular disease was present. This just highlights why it’s important that future studies of cardiovascular risk in psoriasis should include family history. Also, an increased focus on cardiovascular disease in relatives may be appropriate in the cardiovascular risk assessment of patients with psoriasis,” said Dr. Alexander Egeberg of the University of Copenhagen.
He presented a population-based study involving 15 years of follow-up of 30,278 Danes diagnosed with psoriasis in their 20s and a control group consisting of nearly 2.7 million of their Danish contemporaries who were not. None had personal history of acute MI or stroke at baseline. Family medical history, including whether cardiovascular disease occurred in first-degree relatives, was available for all subjects.
Dr. Egeberg and coinvestigators mapped the incidence of acute MI, ischemic stroke, or cardiovascular death in psoriasis patients and the general population controls during follow-up.
“When you look at the patients who developed psoriasis and didn’t have a positive family history of cardiovascular disease, there are almost no cardiovascular events for the entire country,” Dr. Egeberg observed.
In contrast, in a multivariate analysis adjusted for age, gender, socioeconomic status, comorbid cardiovascular disease, smoking, and the use of cardiovascular medications, patients with mild psoriasis and a positive family history for cardiovascular disease had a 28% greater risk of a premature cardiovascular event than the general population during follow-up out to roughly age 40. Those with a positive family history and severe psoriasis as defined by the use of systemic therapies had a 62% increase in risk. Both of these elevated risks were statistically significant.
Among young adult Danes with a positive family history for cardiovascular disease, there were 222 MACE events during 62,225 person-years of follow-up in the mild psoriasis group and 31 events during 6,848 person-years in the 4,504 subjects with severe psoriasis. The resultant incidence rates in both groups were significantly higher than in the control group, who experienced 28,846 MACE events during 16.1 million person-years of follow-up.
In contrast, fewer than 10 MACE events occurred in Danish psoriasis patients without a family history of cardiovascular disease.
A positive family history was also associated with increased MACE in the nonpsoriatic general population, although it didn’t confer as great a risk as in the Danes with psoriasis.
A point worthy of consideration, Dr. Egeberg noted, is that the epidemiology of psoriasis in Denmark apparently differs in several important ways from psoriasis in the United States and some other countries. For one, the prevalence is higher in Scandinavian countries – 7.1% in a Danish national cross-sectional study (Int J Dermatol. 2013 Jun;52[6]:681-3) and 8% in neighboring Norway – as compared with 2%-3% in much of the rest of the world.
Moreover, according to the same cross-sectional study, the prevalence of traditional cardiovascular risk factors, such as smoking and the components of the metabolic syndrome, isn’t higher in Danish psoriasis patients than in the country’s general population. That’s in contrast to the situation in the United Kingdom, where Dr. Joel M. Gelfand of the University of Pennsylvania and associates reported a decade ago in a landmark study that the prevalence of hypertension, obesity, hyperlipidemia, diabetes, and smoking were all higher in persons with psoriasis than in the general population (J Am Acad Dermatol. 2006 Nov;55[5]:829-35). Similar findings were subsequently reported in U.S. psoriasis patients.
Despite their absence of elevated levels of the standard cardiovascular risk factors, Danish psoriasis patients as a group do face a clinically significant increase in cardiovascular risk, compared with the general population, as shown in yet another Danish national cohort study in which the rate ratios for cardiovascular death for mild and severe psoriasis were 1.14 and 1.57, respectively, compared with controls (J Intern Med. 2011 Aug;270[2]:147-57).
In an even more recent Danish nationwide study, the overall death rate was found to be 25.4 per 1,000 person-years in patients with severe psoriasis, 17.0 in those with mild psoriasis, and 13.8 per 1,000 person-years in the general population (J Eur Acad Dermatol Venereol. 2015 May;29[5]:1002-5).
Dr. Egeberg said his new Danish findings suggest that even in psoriasis patients with a greater burden of systemic inflammation as expressed in severe disease, that burden alone doesn’t translate into increased cardiovascular risk. Rather, elevated cardiovascular risk appears to be a consequence of heritable factors, Dr. Egeberg said.
An important caveat regarding this study, he continued, is that the mean age at which participants were diagnosed with psoriasis was 26.6 years. It’s unclear whether the study findings extend to individuals who develop the dermatologic disease later in life.
Dr. Egeberg reported having no financial conflicts regarding this study, supported by Danish national research funding.
AT THE EADV CONGRESS
Key clinical point: A family history of cardiovascular disease takes on extra importance in assessing cardiovascular risk in young adult psoriasis patients.
Major finding: Danes with mild or severe psoriasis plus a family history of cardiovascular disease were respectively 28% and 62% more likely to have an early cardiovascular event than the general population. In contrast, Danish psoriasis patients without a positive family history were not at increased risk of a cardiovascular event.
Data source: A population-based study of 2.7 million Danish young adults, including more than 30,000 diagnosed with psoriasis in their 20s, who were followed for 15 years.
Disclosures: The presenter reported having no financial conflicts regarding this study, which was supported by Danish national research funding.
EADV: New oral psoriasis drug shows excellent safety
COPENHAGEN – An oral small molecule with a novel mechanism of action for treatment of moderate to severe plaque psoriasis is being developed as a potential first-line systemic treatment in view of its highly favorable safety profile.
The investigational drug, known for now as CF101, is a first-in-class agonist of the A3 adenosine receptor. This cell surface receptor is upregulated in the pathologic cells of certain inflammatory diseases, but has little or no expression in normal cells. This high degree of specificity accounts for its safety, which in a recent phase II/III trial was essentially indistinguishable from placebo, making it an attractive potential alternative to methotrexate or biologics as a starting point in systemic therapy, Pnina Fishman, Ph.D., said at the annual congress of the European Academy of Dermatology and Venereology.
Planning is underway for a pivotal phase III trial of CF101 in psoriasis, which is also being organized for CF101 in rheumatoid arthritis on the strength of favorable phase II findings, according to Dr. Fishman, CEO of Can-Fite BioPharma, an Israeli biotech company that is developing the drug.
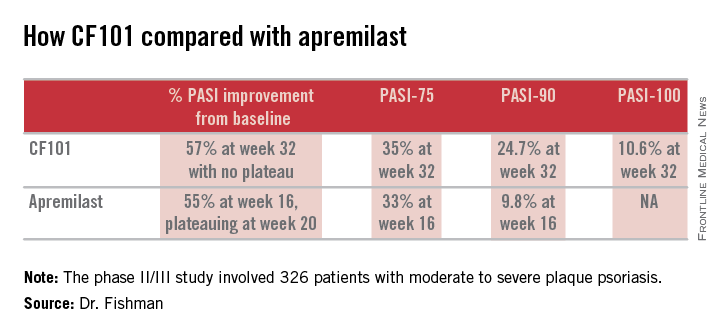
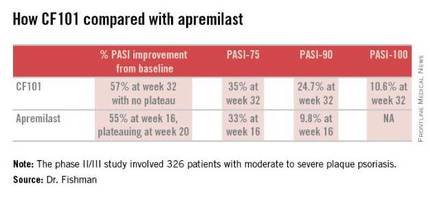
The phase II/III psoriasis trial was a 326-patient, double-blind, placebo-controlled study. It showed efficacy comparable to and in some respects better than that reported in the phase III ESTEEM-1 study of the oral phosphodiesterase 4 inhibitor apremilast (J Am Acad Dermatol. 2015 Jul;73:37-49).
Moreover, it appears that the twice-daily 2-mg dosing of CF101 studied in the phase II/III trial was suboptimal in light of the observed linear increase in Psoriasis Area and Severity Index (PASI)-75, -90, and -100 response rates over time. Those response rates rose steadily until the study’s end at week 32 with no evidence of a plateau. In contrast, in ESTEEM-1, the improvement with apremilast (Otezla) leveled off starting at about week 20, she observed.
In the recent phase II/III trial, the PASI-75 rate for CF101 at week 12 – the prespecified primary endpoint – was not significantly better than placebo was. However, the PASI-75 response rate continued to climb such that by week 32, it was 35.3%, similar to the 33.1% PASI-75 response seen at 16 weeks in ESTEEM-1. PASI improved by an average of 57% from baseline to week 32 with CF101 with no plateau in sight, and by 55% with apremilast at week 16, with a leveling off at week 20. This is why the upcoming phase III trial will employ a higher dose of CF101 than twice-daily 2-mg dose used in the phase II/III study and will run longer. The goal is to achieve higher PASI response rates faster than obtainable with 2 mg BID, Dr. Fishman explained.
She added that the PASI-90 response data in the phase II/III trial bode particularly well for the future of CF101 as a first-line systemic agent. At week 32, this stringent outcome measure was achieved by 26.9% of participants who hadn’t previously been on methotrexate or a biologic and by 13.7% of those who had. And as was the case for the PASI-75 results, the PASI-90 response increased in linear fashion out to 32 weeks with no plateau. In ESTEEM-1, the PASI-90 rate was 9.8% at week 16.
No treatment-related adverse events were seen in the phase II/III CF101 study, Dr. Fishman reported.
The study was sponsored by Can-Fite BioPharma and presented by Dr. Fishman, who is the company’s CEO.
COPENHAGEN – An oral small molecule with a novel mechanism of action for treatment of moderate to severe plaque psoriasis is being developed as a potential first-line systemic treatment in view of its highly favorable safety profile.
The investigational drug, known for now as CF101, is a first-in-class agonist of the A3 adenosine receptor. This cell surface receptor is upregulated in the pathologic cells of certain inflammatory diseases, but has little or no expression in normal cells. This high degree of specificity accounts for its safety, which in a recent phase II/III trial was essentially indistinguishable from placebo, making it an attractive potential alternative to methotrexate or biologics as a starting point in systemic therapy, Pnina Fishman, Ph.D., said at the annual congress of the European Academy of Dermatology and Venereology.

Planning is underway for a pivotal phase III trial of CF101 in psoriasis, which is also being organized for CF101 in rheumatoid arthritis on the strength of favorable phase II findings, according to Dr. Fishman, CEO of Can-Fite BioPharma, an Israeli biotech company that is developing the drug.
The phase II/III psoriasis trial was a 326-patient, double-blind, placebo-controlled study. It showed efficacy comparable to and in some respects better than that reported in the phase III ESTEEM-1 study of the oral phosphodiesterase 4 inhibitor apremilast (J Am Acad Dermatol. 2015 Jul;73:37-49).
Moreover, it appears that the twice-daily 2-mg dosing of CF101 studied in the phase II/III trial was suboptimal in light of the observed linear increase in Psoriasis Area and Severity Index (PASI)-75, -90, and -100 response rates over time. Those response rates rose steadily until the study’s end at week 32 with no evidence of a plateau. In contrast, in ESTEEM-1, the improvement with apremilast (Otezla) leveled off starting at about week 20, she observed.
In the recent phase II/III trial, the PASI-75 rate for CF101 at week 12 – the prespecified primary endpoint – was not significantly better than placebo was. However, the PASI-75 response rate continued to climb such that by week 32, it was 35.3%, similar to the 33.1% PASI-75 response seen at 16 weeks in ESTEEM-1. PASI improved by an average of 57% from baseline to week 32 with CF101 with no plateau in sight, and by 55% with apremilast at week 16, with a leveling off at week 20. This is why the upcoming phase III trial will employ a higher dose of CF101 than twice-daily 2-mg dose used in the phase II/III study and will run longer. The goal is to achieve higher PASI response rates faster than obtainable with 2 mg BID, Dr. Fishman explained.
She added that the PASI-90 response data in the phase II/III trial bode particularly well for the future of CF101 as a first-line systemic agent. At week 32, this stringent outcome measure was achieved by 26.9% of participants who hadn’t previously been on methotrexate or a biologic and by 13.7% of those who had. And as was the case for the PASI-75 results, the PASI-90 response increased in linear fashion out to 32 weeks with no plateau. In ESTEEM-1, the PASI-90 rate was 9.8% at week 16.
No treatment-related adverse events were seen in the phase II/III CF101 study, Dr. Fishman reported.
The study was sponsored by Can-Fite BioPharma and presented by Dr. Fishman, who is the company’s CEO.
COPENHAGEN – An oral small molecule with a novel mechanism of action for treatment of moderate to severe plaque psoriasis is being developed as a potential first-line systemic treatment in view of its highly favorable safety profile.
The investigational drug, known for now as CF101, is a first-in-class agonist of the A3 adenosine receptor. This cell surface receptor is upregulated in the pathologic cells of certain inflammatory diseases, but has little or no expression in normal cells. This high degree of specificity accounts for its safety, which in a recent phase II/III trial was essentially indistinguishable from placebo, making it an attractive potential alternative to methotrexate or biologics as a starting point in systemic therapy, Pnina Fishman, Ph.D., said at the annual congress of the European Academy of Dermatology and Venereology.

Planning is underway for a pivotal phase III trial of CF101 in psoriasis, which is also being organized for CF101 in rheumatoid arthritis on the strength of favorable phase II findings, according to Dr. Fishman, CEO of Can-Fite BioPharma, an Israeli biotech company that is developing the drug.
The phase II/III psoriasis trial was a 326-patient, double-blind, placebo-controlled study. It showed efficacy comparable to and in some respects better than that reported in the phase III ESTEEM-1 study of the oral phosphodiesterase 4 inhibitor apremilast (J Am Acad Dermatol. 2015 Jul;73:37-49).
Moreover, it appears that the twice-daily 2-mg dosing of CF101 studied in the phase II/III trial was suboptimal in light of the observed linear increase in Psoriasis Area and Severity Index (PASI)-75, -90, and -100 response rates over time. Those response rates rose steadily until the study’s end at week 32 with no evidence of a plateau. In contrast, in ESTEEM-1, the improvement with apremilast (Otezla) leveled off starting at about week 20, she observed.
In the recent phase II/III trial, the PASI-75 rate for CF101 at week 12 – the prespecified primary endpoint – was not significantly better than placebo was. However, the PASI-75 response rate continued to climb such that by week 32, it was 35.3%, similar to the 33.1% PASI-75 response seen at 16 weeks in ESTEEM-1. PASI improved by an average of 57% from baseline to week 32 with CF101 with no plateau in sight, and by 55% with apremilast at week 16, with a leveling off at week 20. This is why the upcoming phase III trial will employ a higher dose of CF101 than twice-daily 2-mg dose used in the phase II/III study and will run longer. The goal is to achieve higher PASI response rates faster than obtainable with 2 mg BID, Dr. Fishman explained.
She added that the PASI-90 response data in the phase II/III trial bode particularly well for the future of CF101 as a first-line systemic agent. At week 32, this stringent outcome measure was achieved by 26.9% of participants who hadn’t previously been on methotrexate or a biologic and by 13.7% of those who had. And as was the case for the PASI-75 results, the PASI-90 response increased in linear fashion out to 32 weeks with no plateau. In ESTEEM-1, the PASI-90 rate was 9.8% at week 16.
No treatment-related adverse events were seen in the phase II/III CF101 study, Dr. Fishman reported.
The study was sponsored by Can-Fite BioPharma and presented by Dr. Fishman, who is the company’s CEO.
AT THE EADV CONGRESS
Key clinical point: The oral A3 adenosine receptor agonist CF1-1 shows promise as a potential first-line systemic therapy for moderate to severe plaque psoriasis.
Major finding: The PASI-75 response rate with the twice-daily 2-mg dose of oral CF101 was 35.3% at 32 weeks in a phase II/III study.
Data source: This was a randomized, double-blind, placebo-controlled, 32-week phase II/III study including 326 patients with moderate-to-severe plaque psoriasis.
Disclosures: The study was sponsored by Can-Fite BioPharma of Israel and presented by the Dr. Fishman, the company’s CEO.
EADV: Oral nicotinamide reduces transepidermal water loss
COPENHAGEN – Oral nicotinamide markedly reduces transepidermal water loss (TEWL), raising the prospect that it could be effective in the treatment of atopic dermatitis, Dr. Andrew C. Chen reported at the annual congress of the European Academy of Dermatology and Venereology.
“Nicotinamide is safe, accessible over the counter as a vitamin supplement, and inexpensive. It may be a new systemic therapy for conditions with impaired skin barrier function,” according to Dr. Chen of Royal Alfred Hospital in Sydney.
While nicotinic acid, also known as niacin, famously causes vasodilatory side effects including pronounced flushing and headache, nicotinamide is something else altogether: an amide form of vitamin B3 with a well-established safety profile and no vasodilatory side effects, he explained.
Dr. Chen presented a substudy of the phase III double-blind, multicenter, placebo-controlled Oral Nicotinamide to Reduce Actinic Cancer trial (ONTRAC), in which 386 patients with at least two nonmelanoma skin cancers in the previous 5 years were randomized to oral nicotinamide at 500 mg twice daily or placebo for 12 months. This landmark study showed that nicotinamide did indeed reduce the incidence of nonmelanoma skin cancer (N Engl J Med 2015;373:1618-26).
A secondary endpoint in ONTRAC was oral nicotinamide’s effect on skin barrier function as measured by TEWL. Among the 292 ONTRAC participants with standardized TEWL measurements obtained at the forehead, left forearm, and left leg at baseline and every 3 months thereafter, nicotinamide resulted in significantly less TEWL than placebo at every time point after baseline. At the midforehead, for example, TEWL was 5% less than with placebo at 3 months and 6 months, 6% less at 9 months, and 6% less at the 12-month mark. At the forearm and midleg, the difference was 7% at 12 months. Side effects were similar to placebo.
During the course of the 12-month study, TEWL was 15% greater in controls during the Australian winter as compared with summer. Oral nicotinamide essentially reduced TEWL by a magnitude equivalent to roughly half the variation between the extremes of summer and winter, Dr. Chen noted.
The ONTRAC trial was supported by the Australian National Health and Medical Research Council. Dr. Chen reported having no financial conflicts regarding the study.
COPENHAGEN – Oral nicotinamide markedly reduces transepidermal water loss (TEWL), raising the prospect that it could be effective in the treatment of atopic dermatitis, Dr. Andrew C. Chen reported at the annual congress of the European Academy of Dermatology and Venereology.
“Nicotinamide is safe, accessible over the counter as a vitamin supplement, and inexpensive. It may be a new systemic therapy for conditions with impaired skin barrier function,” according to Dr. Chen of Royal Alfred Hospital in Sydney.
While nicotinic acid, also known as niacin, famously causes vasodilatory side effects including pronounced flushing and headache, nicotinamide is something else altogether: an amide form of vitamin B3 with a well-established safety profile and no vasodilatory side effects, he explained.
Dr. Chen presented a substudy of the phase III double-blind, multicenter, placebo-controlled Oral Nicotinamide to Reduce Actinic Cancer trial (ONTRAC), in which 386 patients with at least two nonmelanoma skin cancers in the previous 5 years were randomized to oral nicotinamide at 500 mg twice daily or placebo for 12 months. This landmark study showed that nicotinamide did indeed reduce the incidence of nonmelanoma skin cancer (N Engl J Med 2015;373:1618-26).
A secondary endpoint in ONTRAC was oral nicotinamide’s effect on skin barrier function as measured by TEWL. Among the 292 ONTRAC participants with standardized TEWL measurements obtained at the forehead, left forearm, and left leg at baseline and every 3 months thereafter, nicotinamide resulted in significantly less TEWL than placebo at every time point after baseline. At the midforehead, for example, TEWL was 5% less than with placebo at 3 months and 6 months, 6% less at 9 months, and 6% less at the 12-month mark. At the forearm and midleg, the difference was 7% at 12 months. Side effects were similar to placebo.
During the course of the 12-month study, TEWL was 15% greater in controls during the Australian winter as compared with summer. Oral nicotinamide essentially reduced TEWL by a magnitude equivalent to roughly half the variation between the extremes of summer and winter, Dr. Chen noted.
The ONTRAC trial was supported by the Australian National Health and Medical Research Council. Dr. Chen reported having no financial conflicts regarding the study.
COPENHAGEN – Oral nicotinamide markedly reduces transepidermal water loss (TEWL), raising the prospect that it could be effective in the treatment of atopic dermatitis, Dr. Andrew C. Chen reported at the annual congress of the European Academy of Dermatology and Venereology.
“Nicotinamide is safe, accessible over the counter as a vitamin supplement, and inexpensive. It may be a new systemic therapy for conditions with impaired skin barrier function,” according to Dr. Chen of Royal Alfred Hospital in Sydney.
While nicotinic acid, also known as niacin, famously causes vasodilatory side effects including pronounced flushing and headache, nicotinamide is something else altogether: an amide form of vitamin B3 with a well-established safety profile and no vasodilatory side effects, he explained.
Dr. Chen presented a substudy of the phase III double-blind, multicenter, placebo-controlled Oral Nicotinamide to Reduce Actinic Cancer trial (ONTRAC), in which 386 patients with at least two nonmelanoma skin cancers in the previous 5 years were randomized to oral nicotinamide at 500 mg twice daily or placebo for 12 months. This landmark study showed that nicotinamide did indeed reduce the incidence of nonmelanoma skin cancer (N Engl J Med 2015;373:1618-26).
A secondary endpoint in ONTRAC was oral nicotinamide’s effect on skin barrier function as measured by TEWL. Among the 292 ONTRAC participants with standardized TEWL measurements obtained at the forehead, left forearm, and left leg at baseline and every 3 months thereafter, nicotinamide resulted in significantly less TEWL than placebo at every time point after baseline. At the midforehead, for example, TEWL was 5% less than with placebo at 3 months and 6 months, 6% less at 9 months, and 6% less at the 12-month mark. At the forearm and midleg, the difference was 7% at 12 months. Side effects were similar to placebo.
During the course of the 12-month study, TEWL was 15% greater in controls during the Australian winter as compared with summer. Oral nicotinamide essentially reduced TEWL by a magnitude equivalent to roughly half the variation between the extremes of summer and winter, Dr. Chen noted.
The ONTRAC trial was supported by the Australian National Health and Medical Research Council. Dr. Chen reported having no financial conflicts regarding the study.
AT THE EADV CONGRESS
Key clinical point: Oral nicotinamide is a safe, inexpensive vitamin, which may be of value in patients with disorders of skin barrier function, such as atopic dermatitis.
Major finding: Transepidermal water loss was essentially cut in half with oral nicotinamide at 500 mg twice daily, compared with placebo.
Data source: This double-blind, randomized trial of oral nicotinamide versus placebo included 292 subjects with transepidermal water loss measurements obtained at multiple body sites every 3 months for 1 year.
Disclosures: The ONTRAC trial was supported by the Australian National Health and Medical Research Council. The presenter reported having no financial conflicts regarding the study.
EADV: Fresolimumab shows early promise in scleroderma
COPENHAGEN – The recent success of fresolimumab in treating early diffuse cutaneous systemic sclerosis in a proof-of-concept study signals better days ahead in the treatment of scleroderma, Dr. Thomas Krieg predicted at the annual congress of the European Academy of Dermatology and Venereology.
“There will be new and better treatments based upon our improved understanding of the pathophysiology of this complex multifaceted disease. New targeted therapies are likely,” according to Dr. Krieg, chairman of the department of dermatology and venereology and dean of the medical faculty at the University of Cologne (Germany).
One such therapy is fresolimumab. This high-affinity neutralizing antibody targets all three isoforms of transforming growth factor–beta (TGF-beta), long believed to be a key player in the development of fibrosis. Fibrosis in patients with systemic sclerosis can affect the skin, lungs, heart, kidneys, and GI tract.
The recent U.S. open-label fresolimumab proof-of-concept study led by investigators at Boston University included 15 patients with early diffuse cutaneous systemic sclerosis. Eight patients received a single 5-mg/kg dose, while the other seven got two 1-mg/kg doses. All were then followed for 24 weeks.
The results make a strong case for TGF-beta as playing a pivotal role in the pathogenesis of systemic sclerosis, according to Dr. Krieg, who wasn’t involved in the study. The investigators characterized the rate of decline in clinical skin disease in response to fresolimumab as assessed using the modified Rodnan skin score (mRSS) as “unprecedented.” The subjects averaged mRSS reductions of 5.1 points at week 3 or 4, increasing to 6.3 points at week 7 and 8 points at week 11 (J Clin Invest. 2015;125[7]:2795-807). To put that into context, the median improvement in mRSS scores in seven previous clinical trials of other agents was an anemic 2.9 points at 6 months and 3.4 at 1 year.
Skin biopsies showed that the fresolimumab-induced rapid decline in mRSS was accompanied by a sharp drop in skin biomarkers of TGF-beta activity, including expression of the genes thrombospondin-1, cartilage oligomeric protein, and connective tissue growth factor. In addition, dermal myofibroblast infiltration dropped off.
The logical next steps in the research effort are to go larger and longer in terms of clinical trials of fresolimumab for scleroderma skin disease, and also to assess whether fresolimumab is effective in treating the fibrotic disease involving the internal organs of scleroderma patients.
Fresolimumab was well tolerated in this study. That’s also been true thus far in studies using higher doses and multiple doses for other diseases where fibrosis figures prominently. As the TGF-beta inhibitor’s side-effect profile becomes better characterized, it will be important to compare the adverse events associated with fresolimumab to the known substantial morbidity associated with current therapies.
The current treatments for the fibrotic disease in systemic sclerosis are unsatisfactory: cyclophosphamide is not terribly effective and carries the risk of malignancy, while “the jury is still out” on stem cell transplantation for systemic sclerosis, as ongoing studies attempt to establish the risk-benefit ratio of this intervention, the dermatologist said.
As for corticosteroids, Dr. Krieg believes they’re overused in systemic sclerosis.
“I have to tell you, many patients come to our center on relatively high doses of corticosteroids. Yet if you go through the literature there’s more or less no evidence for a beneficial role for corticosteroids in systemic sclerosis. And there is an increased risk of developing severe renal crisis, especially with higher doses in patients with diffuse systemic sclerosis,” the dermatologist cautioned.
More than a dozen drugs are now in clinical trials for systemic sclerosis. In addition to fresolimumab, tyrosine kinase inhibitors including imatinib (Gleevec) and nilotinib (Tasigna) are being evaluated for their antifibrotic effects. Other investigational agents target the inflammatory and vascular components of the disease.
Fresolimumab is owned by Sanofi. The fresolimumab study was supported by the National Institutes of Health. Dr. Krieg reported having no financial conflicts regarding his presentation.
COPENHAGEN – The recent success of fresolimumab in treating early diffuse cutaneous systemic sclerosis in a proof-of-concept study signals better days ahead in the treatment of scleroderma, Dr. Thomas Krieg predicted at the annual congress of the European Academy of Dermatology and Venereology.
“There will be new and better treatments based upon our improved understanding of the pathophysiology of this complex multifaceted disease. New targeted therapies are likely,” according to Dr. Krieg, chairman of the department of dermatology and venereology and dean of the medical faculty at the University of Cologne (Germany).
One such therapy is fresolimumab. This high-affinity neutralizing antibody targets all three isoforms of transforming growth factor–beta (TGF-beta), long believed to be a key player in the development of fibrosis. Fibrosis in patients with systemic sclerosis can affect the skin, lungs, heart, kidneys, and GI tract.
The recent U.S. open-label fresolimumab proof-of-concept study led by investigators at Boston University included 15 patients with early diffuse cutaneous systemic sclerosis. Eight patients received a single 5-mg/kg dose, while the other seven got two 1-mg/kg doses. All were then followed for 24 weeks.
The results make a strong case for TGF-beta as playing a pivotal role in the pathogenesis of systemic sclerosis, according to Dr. Krieg, who wasn’t involved in the study. The investigators characterized the rate of decline in clinical skin disease in response to fresolimumab as assessed using the modified Rodnan skin score (mRSS) as “unprecedented.” The subjects averaged mRSS reductions of 5.1 points at week 3 or 4, increasing to 6.3 points at week 7 and 8 points at week 11 (J Clin Invest. 2015;125[7]:2795-807). To put that into context, the median improvement in mRSS scores in seven previous clinical trials of other agents was an anemic 2.9 points at 6 months and 3.4 at 1 year.
Skin biopsies showed that the fresolimumab-induced rapid decline in mRSS was accompanied by a sharp drop in skin biomarkers of TGF-beta activity, including expression of the genes thrombospondin-1, cartilage oligomeric protein, and connective tissue growth factor. In addition, dermal myofibroblast infiltration dropped off.
The logical next steps in the research effort are to go larger and longer in terms of clinical trials of fresolimumab for scleroderma skin disease, and also to assess whether fresolimumab is effective in treating the fibrotic disease involving the internal organs of scleroderma patients.
Fresolimumab was well tolerated in this study. That’s also been true thus far in studies using higher doses and multiple doses for other diseases where fibrosis figures prominently. As the TGF-beta inhibitor’s side-effect profile becomes better characterized, it will be important to compare the adverse events associated with fresolimumab to the known substantial morbidity associated with current therapies.
The current treatments for the fibrotic disease in systemic sclerosis are unsatisfactory: cyclophosphamide is not terribly effective and carries the risk of malignancy, while “the jury is still out” on stem cell transplantation for systemic sclerosis, as ongoing studies attempt to establish the risk-benefit ratio of this intervention, the dermatologist said.
As for corticosteroids, Dr. Krieg believes they’re overused in systemic sclerosis.
“I have to tell you, many patients come to our center on relatively high doses of corticosteroids. Yet if you go through the literature there’s more or less no evidence for a beneficial role for corticosteroids in systemic sclerosis. And there is an increased risk of developing severe renal crisis, especially with higher doses in patients with diffuse systemic sclerosis,” the dermatologist cautioned.
More than a dozen drugs are now in clinical trials for systemic sclerosis. In addition to fresolimumab, tyrosine kinase inhibitors including imatinib (Gleevec) and nilotinib (Tasigna) are being evaluated for their antifibrotic effects. Other investigational agents target the inflammatory and vascular components of the disease.
Fresolimumab is owned by Sanofi. The fresolimumab study was supported by the National Institutes of Health. Dr. Krieg reported having no financial conflicts regarding his presentation.
COPENHAGEN – The recent success of fresolimumab in treating early diffuse cutaneous systemic sclerosis in a proof-of-concept study signals better days ahead in the treatment of scleroderma, Dr. Thomas Krieg predicted at the annual congress of the European Academy of Dermatology and Venereology.
“There will be new and better treatments based upon our improved understanding of the pathophysiology of this complex multifaceted disease. New targeted therapies are likely,” according to Dr. Krieg, chairman of the department of dermatology and venereology and dean of the medical faculty at the University of Cologne (Germany).
One such therapy is fresolimumab. This high-affinity neutralizing antibody targets all three isoforms of transforming growth factor–beta (TGF-beta), long believed to be a key player in the development of fibrosis. Fibrosis in patients with systemic sclerosis can affect the skin, lungs, heart, kidneys, and GI tract.
The recent U.S. open-label fresolimumab proof-of-concept study led by investigators at Boston University included 15 patients with early diffuse cutaneous systemic sclerosis. Eight patients received a single 5-mg/kg dose, while the other seven got two 1-mg/kg doses. All were then followed for 24 weeks.
The results make a strong case for TGF-beta as playing a pivotal role in the pathogenesis of systemic sclerosis, according to Dr. Krieg, who wasn’t involved in the study. The investigators characterized the rate of decline in clinical skin disease in response to fresolimumab as assessed using the modified Rodnan skin score (mRSS) as “unprecedented.” The subjects averaged mRSS reductions of 5.1 points at week 3 or 4, increasing to 6.3 points at week 7 and 8 points at week 11 (J Clin Invest. 2015;125[7]:2795-807). To put that into context, the median improvement in mRSS scores in seven previous clinical trials of other agents was an anemic 2.9 points at 6 months and 3.4 at 1 year.
Skin biopsies showed that the fresolimumab-induced rapid decline in mRSS was accompanied by a sharp drop in skin biomarkers of TGF-beta activity, including expression of the genes thrombospondin-1, cartilage oligomeric protein, and connective tissue growth factor. In addition, dermal myofibroblast infiltration dropped off.
The logical next steps in the research effort are to go larger and longer in terms of clinical trials of fresolimumab for scleroderma skin disease, and also to assess whether fresolimumab is effective in treating the fibrotic disease involving the internal organs of scleroderma patients.
Fresolimumab was well tolerated in this study. That’s also been true thus far in studies using higher doses and multiple doses for other diseases where fibrosis figures prominently. As the TGF-beta inhibitor’s side-effect profile becomes better characterized, it will be important to compare the adverse events associated with fresolimumab to the known substantial morbidity associated with current therapies.
The current treatments for the fibrotic disease in systemic sclerosis are unsatisfactory: cyclophosphamide is not terribly effective and carries the risk of malignancy, while “the jury is still out” on stem cell transplantation for systemic sclerosis, as ongoing studies attempt to establish the risk-benefit ratio of this intervention, the dermatologist said.
As for corticosteroids, Dr. Krieg believes they’re overused in systemic sclerosis.
“I have to tell you, many patients come to our center on relatively high doses of corticosteroids. Yet if you go through the literature there’s more or less no evidence for a beneficial role for corticosteroids in systemic sclerosis. And there is an increased risk of developing severe renal crisis, especially with higher doses in patients with diffuse systemic sclerosis,” the dermatologist cautioned.
More than a dozen drugs are now in clinical trials for systemic sclerosis. In addition to fresolimumab, tyrosine kinase inhibitors including imatinib (Gleevec) and nilotinib (Tasigna) are being evaluated for their antifibrotic effects. Other investigational agents target the inflammatory and vascular components of the disease.
Fresolimumab is owned by Sanofi. The fresolimumab study was supported by the National Institutes of Health. Dr. Krieg reported having no financial conflicts regarding his presentation.
EXPERT ANALYSIS FROM THE EADV CONGRESS
EADV: Pursuing ‘clear’ in psoriasis worthwhile, expert says
COPENHAGEN – It is worthwhile to push harder for complete skin clearance in a psoriasis patient whose treatment regimen provides a Physician Global Assessment score of 1, or “almost clear,” according to Dr. Kristian Reich.
He presented an analysis of pooled data from the active treatment arms of three 12-week phase III clinical trials of the investigational interleukin-17 inhibitor brodalumab. The 1,260 patients with a static Physician Global Assessment (PGA) score of 1, or almost clear, at week 12 had significantly greater residual disease in terms of involved body surface area, fewer symptom-free days, and greater health-related quality of life impairment than did the 1,097 participants who achieved a static PGA of 0.
“These results suggest that clear versus almost clear represents meaningfully different levels of disease,” Dr. Reich of Dermatologikum Hamburg (Germany) said at the annual congress of the European Academy of Dermatology and Venereology.
The newer-generation biologics that target IL-17 or IL-23 provide a significantly greater rate of complete skin clearance than do the older anti–tumor necrosis factor biologic agents. But the newer biologics are also typically costlier and have a shorter safety record. So the question arises: Does the residual disease that defines a static PGA of 1 matter to patients in terms of quality of life and days free of symptoms? Or do they want even better results, if achievable? This is what Dr. Reich and his coworkers set out to answer.
Thirty percent of patients with a static PGA of 1 had clinically significant residual disease at week 12 as defined by a body surface area involvement of 6% or greater, as did 0.3% of those with a static PGA of 0. Fourteen percent of patients with a static PGA of 1 had a Dermatology Life Quality Index score of 6 or more, indicative of at least moderate impairment in quality of life, compared with 6.4% of psoriasis patients with a static PGA of 0.
Moreover, 42% of patients with a static PGA of 0 were 100% symptom free every day from week 10 to week 12 of the study as defined by a score of 0 on the eight-item Psoriasis Symptom Inventory, compared with 12% of patients with a static PGA of 1.
Dr. Reich reported receiving research support from Amgen, which funded the study.
COPENHAGEN – It is worthwhile to push harder for complete skin clearance in a psoriasis patient whose treatment regimen provides a Physician Global Assessment score of 1, or “almost clear,” according to Dr. Kristian Reich.
He presented an analysis of pooled data from the active treatment arms of three 12-week phase III clinical trials of the investigational interleukin-17 inhibitor brodalumab. The 1,260 patients with a static Physician Global Assessment (PGA) score of 1, or almost clear, at week 12 had significantly greater residual disease in terms of involved body surface area, fewer symptom-free days, and greater health-related quality of life impairment than did the 1,097 participants who achieved a static PGA of 0.
“These results suggest that clear versus almost clear represents meaningfully different levels of disease,” Dr. Reich of Dermatologikum Hamburg (Germany) said at the annual congress of the European Academy of Dermatology and Venereology.
The newer-generation biologics that target IL-17 or IL-23 provide a significantly greater rate of complete skin clearance than do the older anti–tumor necrosis factor biologic agents. But the newer biologics are also typically costlier and have a shorter safety record. So the question arises: Does the residual disease that defines a static PGA of 1 matter to patients in terms of quality of life and days free of symptoms? Or do they want even better results, if achievable? This is what Dr. Reich and his coworkers set out to answer.
Thirty percent of patients with a static PGA of 1 had clinically significant residual disease at week 12 as defined by a body surface area involvement of 6% or greater, as did 0.3% of those with a static PGA of 0. Fourteen percent of patients with a static PGA of 1 had a Dermatology Life Quality Index score of 6 or more, indicative of at least moderate impairment in quality of life, compared with 6.4% of psoriasis patients with a static PGA of 0.
Moreover, 42% of patients with a static PGA of 0 were 100% symptom free every day from week 10 to week 12 of the study as defined by a score of 0 on the eight-item Psoriasis Symptom Inventory, compared with 12% of patients with a static PGA of 1.
Dr. Reich reported receiving research support from Amgen, which funded the study.
COPENHAGEN – It is worthwhile to push harder for complete skin clearance in a psoriasis patient whose treatment regimen provides a Physician Global Assessment score of 1, or “almost clear,” according to Dr. Kristian Reich.
He presented an analysis of pooled data from the active treatment arms of three 12-week phase III clinical trials of the investigational interleukin-17 inhibitor brodalumab. The 1,260 patients with a static Physician Global Assessment (PGA) score of 1, or almost clear, at week 12 had significantly greater residual disease in terms of involved body surface area, fewer symptom-free days, and greater health-related quality of life impairment than did the 1,097 participants who achieved a static PGA of 0.
“These results suggest that clear versus almost clear represents meaningfully different levels of disease,” Dr. Reich of Dermatologikum Hamburg (Germany) said at the annual congress of the European Academy of Dermatology and Venereology.
The newer-generation biologics that target IL-17 or IL-23 provide a significantly greater rate of complete skin clearance than do the older anti–tumor necrosis factor biologic agents. But the newer biologics are also typically costlier and have a shorter safety record. So the question arises: Does the residual disease that defines a static PGA of 1 matter to patients in terms of quality of life and days free of symptoms? Or do they want even better results, if achievable? This is what Dr. Reich and his coworkers set out to answer.
Thirty percent of patients with a static PGA of 1 had clinically significant residual disease at week 12 as defined by a body surface area involvement of 6% or greater, as did 0.3% of those with a static PGA of 0. Fourteen percent of patients with a static PGA of 1 had a Dermatology Life Quality Index score of 6 or more, indicative of at least moderate impairment in quality of life, compared with 6.4% of psoriasis patients with a static PGA of 0.
Moreover, 42% of patients with a static PGA of 0 were 100% symptom free every day from week 10 to week 12 of the study as defined by a score of 0 on the eight-item Psoriasis Symptom Inventory, compared with 12% of patients with a static PGA of 1.
Dr. Reich reported receiving research support from Amgen, which funded the study.
AT THE EADV CONGRESS
Key clinical point: A Physician Global Assessment of “clear” is worth striving for in psoriasis patients because it represents a clinically important lesser level of disease than “almost clear.”
Major finding: Fourteen percent of psoriasis patients with an on-treatment physician assessment of “almost clear” had at least moderately impaired health care quality of life, compared with 6.4% of those rated “clear.”
Data source: A secondary pooled analysis of quality of life–related outcomes among psoriasis patients rated “clear” as opposed to “almost clear” at week 12 of three phase III double-blind randomized trials of the interleukin-17 inhibitor brodalumab.
Disclosures: The presenter reported receiving research support from Amgen, which sponsored the study.
EADV: Focus on non-UV triggers of melanoma
COPENHAGEN – Sunlight is recognized as the 800-pound gorilla of melanoma risk, with roughly 65% of all melanomas worldwide attributable to exposure to UV radiation. But what about the other 35%?
About 10% of melanomas are due to an inherited germline mutation, and new germline mutations are being discovered all the time. However, the incidence of melanoma has been rising steadily in industrialized countries since the 1950s, and genetic predisposition can’t explain that phenomenon. Environmental and lifestyle factors are likely to be involved. Indeed, melanomas arising from epigenetic modifications by extrinsic environmental and lifestyle factors other than UV exposure are drawing increasing research attention because of the potential for preventing the malignancy through avoidance of these risk factors, Dr. Veronique del Marmol said at the annual congress of the European Academy of Dermatology and Venereology.
One promising avenue of investigation focuses on microRNAs (miRs) as environmental drivers of melanoma risk. The miRs are important posttranscriptional regulators of gene expression. In particular, overexpression of miR-21 has been shown to accompany the transition of benign melanocytes into melanoma. miR-21 affects numerous genes critical to oncogenesis, including genes promoting sustained proliferation, angiogenesis, evasion from apoptosis, genetic instability, oxidative stress, and invasion and metastasis, as detailed recently [J Transl Med. 2015 Jun 27;13:202] by Dr. Bodo C. Melnik of the department of dermatology, environmental medicine, and health theory, University of Osnabrück (Germany).
Translational work by Dr. Melnik and others has shown that miR-21 expression is upregulated by several elements that investigators have long suspected are associated with an increased risk of melanoma, including smoking, air pollution, polychlorinated biphenyls and other noxious chemicals, chronic inflammation, a high-fat/high-sugar diet, and a sedentary lifestyle. Even cow’s milk has generated suspicion, since bovine miR-21 is identical to human miR-21, noted Dr. del Marmol, head of the department of dermatology at Erasmus Hospital in Brussels and chair of the pan-European Euromelanoma project.
Two noteworthy factors that have generated interest recently are coffee consumption, shown in a large observational study to protect against melanoma, and sildenafil (Viagra), which was shown in an analysis of 25,848 physicians enrolled in the Health Professionals’ Follow-Up Study to be associated with a 2.24-fold increased risk of subsequently developing melanoma during prospective follow-up (JAMA Intern Med. 2014 Jun;174[6]:964-70).
An association between increased risk of melanoma and the use of sildenafil or other phosphodiesterase-5 (PDE-5) inhibitors commonly used by men with erectile dysfunction is biologically plausible, according to Dr. del Marmol. Activation of oncogenic BRAF is a hallmark of 40%-60% of melanomas, and BRAF activation, like sildenafil use, downregulates phosphodiesterase-5A, which increases the invasiveness of melanoma cells.
The coffee connection was identified through an analysis of food frequency questionnaires completed by 447,357 non-Hispanic whites enrolled in the NIH-AARP Diet and Health Study, a prospective study developed at the National Cancer Institute.
During 4.3 million person-years of follow-up, 2,904 individuals were diagnosed with malignant melanoma. In a multivariate analysis, quaffing 4 or more cups of caffeinated coffee daily was associated with a 25% reduction in the risk of developing melanoma. Lesser consumption had no significant impact on melanoma risk (J Natl Cancer Inst. 2015 Jan 20;107[2]: pii: dju421. doi: 10.1093/jnci/dju421].
COPENHAGEN – Sunlight is recognized as the 800-pound gorilla of melanoma risk, with roughly 65% of all melanomas worldwide attributable to exposure to UV radiation. But what about the other 35%?
About 10% of melanomas are due to an inherited germline mutation, and new germline mutations are being discovered all the time. However, the incidence of melanoma has been rising steadily in industrialized countries since the 1950s, and genetic predisposition can’t explain that phenomenon. Environmental and lifestyle factors are likely to be involved. Indeed, melanomas arising from epigenetic modifications by extrinsic environmental and lifestyle factors other than UV exposure are drawing increasing research attention because of the potential for preventing the malignancy through avoidance of these risk factors, Dr. Veronique del Marmol said at the annual congress of the European Academy of Dermatology and Venereology.
One promising avenue of investigation focuses on microRNAs (miRs) as environmental drivers of melanoma risk. The miRs are important posttranscriptional regulators of gene expression. In particular, overexpression of miR-21 has been shown to accompany the transition of benign melanocytes into melanoma. miR-21 affects numerous genes critical to oncogenesis, including genes promoting sustained proliferation, angiogenesis, evasion from apoptosis, genetic instability, oxidative stress, and invasion and metastasis, as detailed recently [J Transl Med. 2015 Jun 27;13:202] by Dr. Bodo C. Melnik of the department of dermatology, environmental medicine, and health theory, University of Osnabrück (Germany).
Translational work by Dr. Melnik and others has shown that miR-21 expression is upregulated by several elements that investigators have long suspected are associated with an increased risk of melanoma, including smoking, air pollution, polychlorinated biphenyls and other noxious chemicals, chronic inflammation, a high-fat/high-sugar diet, and a sedentary lifestyle. Even cow’s milk has generated suspicion, since bovine miR-21 is identical to human miR-21, noted Dr. del Marmol, head of the department of dermatology at Erasmus Hospital in Brussels and chair of the pan-European Euromelanoma project.
Two noteworthy factors that have generated interest recently are coffee consumption, shown in a large observational study to protect against melanoma, and sildenafil (Viagra), which was shown in an analysis of 25,848 physicians enrolled in the Health Professionals’ Follow-Up Study to be associated with a 2.24-fold increased risk of subsequently developing melanoma during prospective follow-up (JAMA Intern Med. 2014 Jun;174[6]:964-70).
An association between increased risk of melanoma and the use of sildenafil or other phosphodiesterase-5 (PDE-5) inhibitors commonly used by men with erectile dysfunction is biologically plausible, according to Dr. del Marmol. Activation of oncogenic BRAF is a hallmark of 40%-60% of melanomas, and BRAF activation, like sildenafil use, downregulates phosphodiesterase-5A, which increases the invasiveness of melanoma cells.
The coffee connection was identified through an analysis of food frequency questionnaires completed by 447,357 non-Hispanic whites enrolled in the NIH-AARP Diet and Health Study, a prospective study developed at the National Cancer Institute.
During 4.3 million person-years of follow-up, 2,904 individuals were diagnosed with malignant melanoma. In a multivariate analysis, quaffing 4 or more cups of caffeinated coffee daily was associated with a 25% reduction in the risk of developing melanoma. Lesser consumption had no significant impact on melanoma risk (J Natl Cancer Inst. 2015 Jan 20;107[2]: pii: dju421. doi: 10.1093/jnci/dju421].
COPENHAGEN – Sunlight is recognized as the 800-pound gorilla of melanoma risk, with roughly 65% of all melanomas worldwide attributable to exposure to UV radiation. But what about the other 35%?
About 10% of melanomas are due to an inherited germline mutation, and new germline mutations are being discovered all the time. However, the incidence of melanoma has been rising steadily in industrialized countries since the 1950s, and genetic predisposition can’t explain that phenomenon. Environmental and lifestyle factors are likely to be involved. Indeed, melanomas arising from epigenetic modifications by extrinsic environmental and lifestyle factors other than UV exposure are drawing increasing research attention because of the potential for preventing the malignancy through avoidance of these risk factors, Dr. Veronique del Marmol said at the annual congress of the European Academy of Dermatology and Venereology.
One promising avenue of investigation focuses on microRNAs (miRs) as environmental drivers of melanoma risk. The miRs are important posttranscriptional regulators of gene expression. In particular, overexpression of miR-21 has been shown to accompany the transition of benign melanocytes into melanoma. miR-21 affects numerous genes critical to oncogenesis, including genes promoting sustained proliferation, angiogenesis, evasion from apoptosis, genetic instability, oxidative stress, and invasion and metastasis, as detailed recently [J Transl Med. 2015 Jun 27;13:202] by Dr. Bodo C. Melnik of the department of dermatology, environmental medicine, and health theory, University of Osnabrück (Germany).
Translational work by Dr. Melnik and others has shown that miR-21 expression is upregulated by several elements that investigators have long suspected are associated with an increased risk of melanoma, including smoking, air pollution, polychlorinated biphenyls and other noxious chemicals, chronic inflammation, a high-fat/high-sugar diet, and a sedentary lifestyle. Even cow’s milk has generated suspicion, since bovine miR-21 is identical to human miR-21, noted Dr. del Marmol, head of the department of dermatology at Erasmus Hospital in Brussels and chair of the pan-European Euromelanoma project.
Two noteworthy factors that have generated interest recently are coffee consumption, shown in a large observational study to protect against melanoma, and sildenafil (Viagra), which was shown in an analysis of 25,848 physicians enrolled in the Health Professionals’ Follow-Up Study to be associated with a 2.24-fold increased risk of subsequently developing melanoma during prospective follow-up (JAMA Intern Med. 2014 Jun;174[6]:964-70).
An association between increased risk of melanoma and the use of sildenafil or other phosphodiesterase-5 (PDE-5) inhibitors commonly used by men with erectile dysfunction is biologically plausible, according to Dr. del Marmol. Activation of oncogenic BRAF is a hallmark of 40%-60% of melanomas, and BRAF activation, like sildenafil use, downregulates phosphodiesterase-5A, which increases the invasiveness of melanoma cells.
The coffee connection was identified through an analysis of food frequency questionnaires completed by 447,357 non-Hispanic whites enrolled in the NIH-AARP Diet and Health Study, a prospective study developed at the National Cancer Institute.
During 4.3 million person-years of follow-up, 2,904 individuals were diagnosed with malignant melanoma. In a multivariate analysis, quaffing 4 or more cups of caffeinated coffee daily was associated with a 25% reduction in the risk of developing melanoma. Lesser consumption had no significant impact on melanoma risk (J Natl Cancer Inst. 2015 Jan 20;107[2]: pii: dju421. doi: 10.1093/jnci/dju421].
EXPERT ANALYSIS FROM THE EADV CONGRESS
EADV: Fresh insights into Gorlin syndrome
COPENHAGEN – A glimpse into the massive burden imposed upon patients with basal cell nevus syndrome (BCNS) is provided by the initial report from the first U.S. registry of patients affected with the disorder to include individuals who’ve tried the Hedgehog signaling pathway inhibitor vismodegib.
The first 94 patients (average age, 56 years) with BCNS to enroll in the prospective registry had a reported lifetime mean of 312 basal cell carcinomas (BCCs), Dr. Marieke Peters reported at the annual congress of the European Academy of Dermatology and Venereology.
The patients had an average of 36 new BCCs or other tumors in the previous 2 years, and 70% were categorized as having moderate to severe BCNS (also known as Gorlin syndrome), as defined by more than 10 new BCCs during that time period.
Most (94%) of tumors were on sun-exposed areas. Patients reported a lifetime mean of 202 surgical excisions, according to Dr. Peters of Catharina Hospital Eindhoven, the Netherlands.
The median age at diagnosis of BCNS was 15 years, and most patients were diagnosed clinically. Only 26% had undergone genetic testing for PTCH1. (The syndrome is caused by mutations in the PTCH1 gene). Sixty-two percent of subjects reported a family history of BCNS.
Other abnormalities associated with BCNS were common: Eighty percent of patients had jaw cysts, 82% had palmer pitting, 50% had various bone abnormalities, 21% reported ovarian fibromas, 4% reported medulloblastomas, and 4% had other tumors.
Fifty-seven percent of patients had tried vismodegib (Erivedge). However, only 15% were currently on the drug at enrollment; the rest had discontinued it, mainly because of side effects; less frequently because of loss of efficacy due to the development of new mutations. The chief side effects were nausea, vomiting, and diarrhea; less frequent side effects were weight loss, fatigue, muscle cramps, and alopecia, according to the dermatologist.
Gorlin syndrome is a rare autosomal dominant disorder caused mainly by mutations in the PTCH1 gene. The U.S. registry is directed by investigators at Children’s Hospital Oakland Research Institute and Stanford (Calif.) University.
The patient questionnaire used in the BCNS registry is available at https://redcap.stanford.edu/surveys/?s=7MWW9E37ND. For more information about the registry, contact Dr. Ervin Epstein, at [email protected].
Dr. Peters reported having no financial conflicts regarding her report. At the time of the EADV meeting, she was a dermatology resident at the Academic Medical Center, Amsterdam.
*This story was updated 12/15/2015.
COPENHAGEN – A glimpse into the massive burden imposed upon patients with basal cell nevus syndrome (BCNS) is provided by the initial report from the first U.S. registry of patients affected with the disorder to include individuals who’ve tried the Hedgehog signaling pathway inhibitor vismodegib.
The first 94 patients (average age, 56 years) with BCNS to enroll in the prospective registry had a reported lifetime mean of 312 basal cell carcinomas (BCCs), Dr. Marieke Peters reported at the annual congress of the European Academy of Dermatology and Venereology.
The patients had an average of 36 new BCCs or other tumors in the previous 2 years, and 70% were categorized as having moderate to severe BCNS (also known as Gorlin syndrome), as defined by more than 10 new BCCs during that time period.
Most (94%) of tumors were on sun-exposed areas. Patients reported a lifetime mean of 202 surgical excisions, according to Dr. Peters of Catharina Hospital Eindhoven, the Netherlands.
The median age at diagnosis of BCNS was 15 years, and most patients were diagnosed clinically. Only 26% had undergone genetic testing for PTCH1. (The syndrome is caused by mutations in the PTCH1 gene). Sixty-two percent of subjects reported a family history of BCNS.
Other abnormalities associated with BCNS were common: Eighty percent of patients had jaw cysts, 82% had palmer pitting, 50% had various bone abnormalities, 21% reported ovarian fibromas, 4% reported medulloblastomas, and 4% had other tumors.
Fifty-seven percent of patients had tried vismodegib (Erivedge). However, only 15% were currently on the drug at enrollment; the rest had discontinued it, mainly because of side effects; less frequently because of loss of efficacy due to the development of new mutations. The chief side effects were nausea, vomiting, and diarrhea; less frequent side effects were weight loss, fatigue, muscle cramps, and alopecia, according to the dermatologist.
Gorlin syndrome is a rare autosomal dominant disorder caused mainly by mutations in the PTCH1 gene. The U.S. registry is directed by investigators at Children’s Hospital Oakland Research Institute and Stanford (Calif.) University.
The patient questionnaire used in the BCNS registry is available at https://redcap.stanford.edu/surveys/?s=7MWW9E37ND. For more information about the registry, contact Dr. Ervin Epstein, at [email protected].
Dr. Peters reported having no financial conflicts regarding her report. At the time of the EADV meeting, she was a dermatology resident at the Academic Medical Center, Amsterdam.
*This story was updated 12/15/2015.
COPENHAGEN – A glimpse into the massive burden imposed upon patients with basal cell nevus syndrome (BCNS) is provided by the initial report from the first U.S. registry of patients affected with the disorder to include individuals who’ve tried the Hedgehog signaling pathway inhibitor vismodegib.
The first 94 patients (average age, 56 years) with BCNS to enroll in the prospective registry had a reported lifetime mean of 312 basal cell carcinomas (BCCs), Dr. Marieke Peters reported at the annual congress of the European Academy of Dermatology and Venereology.
The patients had an average of 36 new BCCs or other tumors in the previous 2 years, and 70% were categorized as having moderate to severe BCNS (also known as Gorlin syndrome), as defined by more than 10 new BCCs during that time period.
Most (94%) of tumors were on sun-exposed areas. Patients reported a lifetime mean of 202 surgical excisions, according to Dr. Peters of Catharina Hospital Eindhoven, the Netherlands.
The median age at diagnosis of BCNS was 15 years, and most patients were diagnosed clinically. Only 26% had undergone genetic testing for PTCH1. (The syndrome is caused by mutations in the PTCH1 gene). Sixty-two percent of subjects reported a family history of BCNS.
Other abnormalities associated with BCNS were common: Eighty percent of patients had jaw cysts, 82% had palmer pitting, 50% had various bone abnormalities, 21% reported ovarian fibromas, 4% reported medulloblastomas, and 4% had other tumors.
Fifty-seven percent of patients had tried vismodegib (Erivedge). However, only 15% were currently on the drug at enrollment; the rest had discontinued it, mainly because of side effects; less frequently because of loss of efficacy due to the development of new mutations. The chief side effects were nausea, vomiting, and diarrhea; less frequent side effects were weight loss, fatigue, muscle cramps, and alopecia, according to the dermatologist.
Gorlin syndrome is a rare autosomal dominant disorder caused mainly by mutations in the PTCH1 gene. The U.S. registry is directed by investigators at Children’s Hospital Oakland Research Institute and Stanford (Calif.) University.
The patient questionnaire used in the BCNS registry is available at https://redcap.stanford.edu/surveys/?s=7MWW9E37ND. For more information about the registry, contact Dr. Ervin Epstein, at [email protected].
Dr. Peters reported having no financial conflicts regarding her report. At the time of the EADV meeting, she was a dermatology resident at the Academic Medical Center, Amsterdam.
*This story was updated 12/15/2015.
AT THE EADV CONGRESS
Key clinical point: Gorlin syndrome imposes an impressively heavy burden on affected patients.
Major finding: Patients with basal cell nevus syndrome reported a lifetime mean of 312 basal cell carcinomas and 202 surgical excisions.
Data source: The initial report from a prospective U.S. registry of patients with basal cell nevus syndrome, which is still enrolling participants.
Disclosures: The presenter reported having no financial conflicts regarding her study.