User login
Transporting stroke patients directly to thrombectomy boosts outcomes
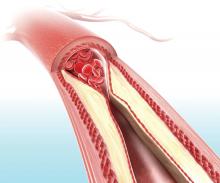
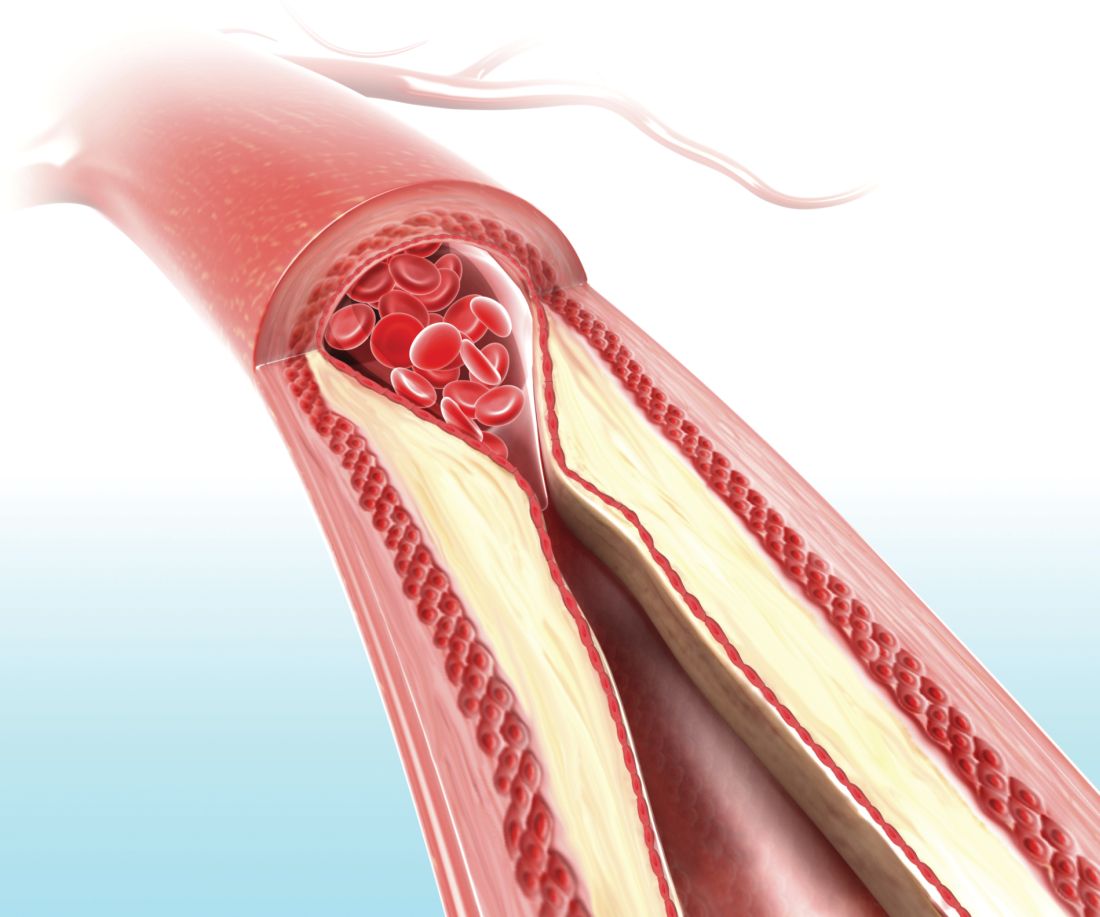
LOS ANGELES – Evidence continues to mount that in the new era of thrombectomy treatment for selected acute ischemic stroke patients outcomes are better when patients go directly to the closest comprehensive stroke center that offers intravascular procedures rather than first being taken to a closer hospital and then needing transfer.
Nils H. Mueller-Kronast, MD, presented a modeled analysis of data collected in a registry on 236 real-world U.S. patients who underwent mechanical thrombectomy for an acute, large-vessel occlusion stroke following transfer from a hospital that could perform thrombolysis but couldn’t offer thrombectomy. The analysis showed that if the patients had instead gone directly to the closest thrombectomy center the result would have been a 16-percentage-point increase in patients with a modified Rankin Scale (mRS) score of 0-1 after 90 days, and a 9-percentage-point increase in mRS 0-2 outcomes, Dr. Mueller-Kronast said at the International Stroke Conference, sponsored by the American Heart Association.
The analysis he presented used data from the Systematic Evaluation of Patients Treated With Stroke Devices for Acute Ischemic Stroke (STRATIS) registry, which included 984 acute ischemic stroke patients who underwent mechanical thrombectomy at any one of 55 participating U.S. sites (Stroke. 2017 Oct;48[10]:2760-8). A previously-reported analysis of the STRATIS data showed that the 55% of patients taken directly to a center that performed thrombectomy had a 60% rate of mRS score 0-2 after 90 days, compared with 52% of patients taken first to a hospital unable to perform thrombectomy and then transferred (Circulation. 2017 Dec 12;136[24]:2311-21).
The current analysis focused on 236 of the transferred patients with complete information on their location at the time of their stroke and subsequent time intervals during their transport and treatment, including 117 patients with ground transfer from their first hospital to the thrombectomy site, 114 with air transfer, and 5 with an unreported means of transport.
Dr. Mueller-Kronast and his associates calculated the time it would have taken each of the 117 ground transported patients to have gone directly to the closest thrombectomy center (adjusted by traffic conditions at the time of the stroke), and modeled the likely outcomes of these patients based on the data collected in the registry. This projected a 47% rate of mRS scores 0-1 (good outcomes) after 90 days, and a 60% rate of mRS 0-2 scores with a direct-to-thrombectomy strategy, compared with actual rates of 31% and 51%, respectively, among the patients who were transferred from their initial hospital.
“Bypass to an endovascular-capable center may be an option to improve rapid access to mechanical thrombectomy,” he concluded.
The STRATIS registry is sponsored by Medtronic. Dr. Mueller-Kronast has been a consultant to Medtronic.
SOURCE: Mueller-Kronast N et al. Abstract LB12.
LOS ANGELES – Evidence continues to mount that in the new era of thrombectomy treatment for selected acute ischemic stroke patients outcomes are better when patients go directly to the closest comprehensive stroke center that offers intravascular procedures rather than first being taken to a closer hospital and then needing transfer.
Nils H. Mueller-Kronast, MD, presented a modeled analysis of data collected in a registry on 236 real-world U.S. patients who underwent mechanical thrombectomy for an acute, large-vessel occlusion stroke following transfer from a hospital that could perform thrombolysis but couldn’t offer thrombectomy. The analysis showed that if the patients had instead gone directly to the closest thrombectomy center the result would have been a 16-percentage-point increase in patients with a modified Rankin Scale (mRS) score of 0-1 after 90 days, and a 9-percentage-point increase in mRS 0-2 outcomes, Dr. Mueller-Kronast said at the International Stroke Conference, sponsored by the American Heart Association.
The analysis he presented used data from the Systematic Evaluation of Patients Treated With Stroke Devices for Acute Ischemic Stroke (STRATIS) registry, which included 984 acute ischemic stroke patients who underwent mechanical thrombectomy at any one of 55 participating U.S. sites (Stroke. 2017 Oct;48[10]:2760-8). A previously-reported analysis of the STRATIS data showed that the 55% of patients taken directly to a center that performed thrombectomy had a 60% rate of mRS score 0-2 after 90 days, compared with 52% of patients taken first to a hospital unable to perform thrombectomy and then transferred (Circulation. 2017 Dec 12;136[24]:2311-21).
The current analysis focused on 236 of the transferred patients with complete information on their location at the time of their stroke and subsequent time intervals during their transport and treatment, including 117 patients with ground transfer from their first hospital to the thrombectomy site, 114 with air transfer, and 5 with an unreported means of transport.
Dr. Mueller-Kronast and his associates calculated the time it would have taken each of the 117 ground transported patients to have gone directly to the closest thrombectomy center (adjusted by traffic conditions at the time of the stroke), and modeled the likely outcomes of these patients based on the data collected in the registry. This projected a 47% rate of mRS scores 0-1 (good outcomes) after 90 days, and a 60% rate of mRS 0-2 scores with a direct-to-thrombectomy strategy, compared with actual rates of 31% and 51%, respectively, among the patients who were transferred from their initial hospital.
“Bypass to an endovascular-capable center may be an option to improve rapid access to mechanical thrombectomy,” he concluded.
The STRATIS registry is sponsored by Medtronic. Dr. Mueller-Kronast has been a consultant to Medtronic.
SOURCE: Mueller-Kronast N et al. Abstract LB12.
LOS ANGELES – Evidence continues to mount that in the new era of thrombectomy treatment for selected acute ischemic stroke patients outcomes are better when patients go directly to the closest comprehensive stroke center that offers intravascular procedures rather than first being taken to a closer hospital and then needing transfer.
Nils H. Mueller-Kronast, MD, presented a modeled analysis of data collected in a registry on 236 real-world U.S. patients who underwent mechanical thrombectomy for an acute, large-vessel occlusion stroke following transfer from a hospital that could perform thrombolysis but couldn’t offer thrombectomy. The analysis showed that if the patients had instead gone directly to the closest thrombectomy center the result would have been a 16-percentage-point increase in patients with a modified Rankin Scale (mRS) score of 0-1 after 90 days, and a 9-percentage-point increase in mRS 0-2 outcomes, Dr. Mueller-Kronast said at the International Stroke Conference, sponsored by the American Heart Association.
The analysis he presented used data from the Systematic Evaluation of Patients Treated With Stroke Devices for Acute Ischemic Stroke (STRATIS) registry, which included 984 acute ischemic stroke patients who underwent mechanical thrombectomy at any one of 55 participating U.S. sites (Stroke. 2017 Oct;48[10]:2760-8). A previously-reported analysis of the STRATIS data showed that the 55% of patients taken directly to a center that performed thrombectomy had a 60% rate of mRS score 0-2 after 90 days, compared with 52% of patients taken first to a hospital unable to perform thrombectomy and then transferred (Circulation. 2017 Dec 12;136[24]:2311-21).
The current analysis focused on 236 of the transferred patients with complete information on their location at the time of their stroke and subsequent time intervals during their transport and treatment, including 117 patients with ground transfer from their first hospital to the thrombectomy site, 114 with air transfer, and 5 with an unreported means of transport.
Dr. Mueller-Kronast and his associates calculated the time it would have taken each of the 117 ground transported patients to have gone directly to the closest thrombectomy center (adjusted by traffic conditions at the time of the stroke), and modeled the likely outcomes of these patients based on the data collected in the registry. This projected a 47% rate of mRS scores 0-1 (good outcomes) after 90 days, and a 60% rate of mRS 0-2 scores with a direct-to-thrombectomy strategy, compared with actual rates of 31% and 51%, respectively, among the patients who were transferred from their initial hospital.
“Bypass to an endovascular-capable center may be an option to improve rapid access to mechanical thrombectomy,” he concluded.
The STRATIS registry is sponsored by Medtronic. Dr. Mueller-Kronast has been a consultant to Medtronic.
SOURCE: Mueller-Kronast N et al. Abstract LB12.
REPORTING FROM ISC 2018
Key clinical point: A direct-to-thrombectomy strategy maximizes good stroke outcomes.
Major finding: Modeling showed a 47% rate of good 90-day outcomes by taking patients to the closest thrombectomy center, compared with an actual 31% rate with transfers.
Study details: A simulation-model analysis of data collected by the STRATIS registry of acute stroke thrombectomies.
Disclosures: The STRATIS registry is sponsored by Medtronic. Dr. Mueller-Kronast has been a consultant to Medtronic.
Source: Mueller-Kronast N et al. Abstract LB12.
Thrombectomy’s success treating strokes prompts rethinking of selection criteria
LOS ANGELES – CT or magnetic resonance brain imaging of acute ischemic stroke patients was the key triage tool in two groundbreaking thrombectomy trials, DAWN and DEFUSE; results from both showed that patients found by imaging to have limited infarcted cores could safely benefit from endovascular thrombectomy, even when they are more than 6 hours out from their stroke onset, breaking the 6-hour barrier created 3 years ago by the first wave of thrombectomy trials.
But some stroke neurologists studying thrombectomy are now convinced that imaging is not needed and may actually harm acute ischemic stroke patients early on by introducing an unnecessary time delay when they present within the first 6 hours after stroke onset.
This new thinking on how to best use brain imaging in acute ischemic stroke patients is part of the rapid evolution of acute stroke management as experts process new data and refine their approach both within 6 hours of stroke onset and during the new treatment window of 6-24 hours post onset. The dramatic success achieved with thrombectomy in highly selected late-window patients prompted researchers to promote pushing the boundaries further to find less-selected late patients who could also potentially benefit from thrombectomy.
The downside of early imaging
“Early on, we have a mix of fast and slow progressors. Slow progressors are about a third to half the patients, so there is a lot of potential for [late] treatment, but the majority of patients during the first 6 hours after onset are fast progressors,” patients who won’t benefit from thrombectomy delivery beyond 6 hours, said Dr. Jovin, an interventional neurologist and director of the Stroke Institute of the University of Pittsburgh Medical Center.
“Time is very precious in the 0- to 6-hour window. When we’re dealing with a lot of fast progressors, we pay a price [in added time to treatment] for any imaging we do. We need to understand that this is a real price we pay, even when CT takes perhaps 24 minutes, and MRI adds about 12 minutes. It’s not the case in all patients that doing CT angiography just adds 5 minutes. It can take 15, 20 minutes,” especially at centers that don’t treat these types of stroke patients day in and day out. “There is no question that imaging slows us down,” Dr. Jovin said.
He highlighted that “the main role of imaging is to exclude patients from treatment, a treatment that has unbelievable effects.” Imaging can rule out patients who have a hemorrhage, lack an occlusion, have a large infarcted core, or have none of the brain at risk or just a small amount, he noted. “Excluding hemorrhage is reasonable, but we can do that in the angiography suite, when the patient is on the table. The main benefit from advanced imaging is to more precisely define the core,” but for most patients the size of their core is not important because the vast majority of acute ischemic stroke patients seen within 6 hours of onset have cores smaller than 70 mL.
“Is this much ado about nothing – especially because we punish all the other patients [with smaller cores] who need to be treated [quickly] when we do additional imaging?” asked Dr. Jovin, who was one of the two lead investigators for the Clinical Mismatch in the Triage of Wake Up and Late Presenting Strokes Undergoing Neurointervention With Trevo (DAWN) trial (N Engl J Med. 2018 Jan 4;378[1]:11-21). Another factor undercutting the value of imaging and determining core size is registry results that show patients who undergo thrombectomy with a large infarcted core are not harmed by treatment.
Current practice often uses imaging “to exclude the 20% of patients who may not have a large vessel occlusion or may not get benefit but whom we are unlikely to harm. But we harm the other 80% by delaying treatment by 30 minutes because of imaging. That’s why we need to rethink imaging and minimize its use.”
Dr. Jovin noted that at his center in Pittsburgh, the stroke institute staff began in 2013 to take patients transported from other stroke facilities and already diagnosed with a large vessel occlusion directly to the angiography suite, bypassing further brain imaging. By doing this, they cut their average door-to-groin puncture time down to 22 minutes from what had been an average of 81 minutes with imaging (Stroke. 2017 July;48[7]:1884-9). Right now the door-to-groin time is even lower, he added.
“Don’t waste time imaging,” said Dr. Nogueira, a stroke neurologist who is director of the neuroendovascular division of the Marcus Stroke and Neuroscience Center and professor of neurology, neurosurgery, and radiology at Emory University, both in Atlanta, as well as the second lead investigator for DAWN. “Time is crucial and trumps patient selection. Most selection criteria are informative rather than truly selective. It is important to understand that in every time window, we do not yet know who doesn’t benefit from treatment. Select faster, select less, and treat more” during the 0- to 6-hour window, he told his colleagues.
Expanding thrombectomy 6-24 hours after stroke onset
While Dr. Jovin and Dr. Nogueira called for more aggressive and less selective use of thrombectomy in patients who present within 6 hours of their stroke onset, they acknowledged that for patients who present during the 6- to 24-hour window, selection for thrombectomy should follow the rules applied in DAWN and in the more inclusive Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke (DEFUSE 3) trial (N Engl J Med. 2018 Feb 22;378:708-18). That means using imaging to confirm that the patient’s infarcted core is within the enrollment ceiling, but both neurologists also downplayed the need for the more sophisticated imaging approaches that were often used in both trials as well as in current routine practice at comprehensive stroke centers. They agreed that noncontrast CT, a widely available method, seems adequate for patient selection, based on the admittedly limited data available right now.
Dr. Nogueira cited data from the Trevo stent retriever registry, collected from nearly 1,000 U.S. acute ischemic stroke patients who underwent thrombectomy with this device. (Researchers at the conference reported updated registry data with nearly 2,000 patients with findings similar to what Dr. Nogueira referenced.) Although these patients underwent treatment before results of DAWN and DEFUSE 3 came out and before release of the new U.S. acute stroke management guidelines (Stroke. 2018 Jan 24; doi: 10.1161/STR.0000000000000158) that endorsed targeted thrombectomy for patients 6-24 hours out from their stroke onset, 278 (28%) of the registry patients underwent thrombectomy treatment during the 6- to 24-hour time window. In this subgroup, 34 patients underwent noncontrast CT to assess their infarcted core prior to thrombectomy, while the other patients underwent perfusion CT, MRI, or both. The noncontrast CT patients had recanalization rates, adverse event rates, and 90-day modified Rankin Scale (mRS) scores comparable with those of patients assessed with more advanced imaging.
Based on this, “just looking at CT only seems reasonable. Noncontrast CT is a pretty valid way to select patients,” Dr. Nogueira said.
“This is the direction we should follow to simplify the paradigm for treating beyond 6 hours,” agreed Dr. Jovin, who also called for further advances in patient selection to expand the pool of patients who qualify for thrombectomy during the 6- to 24-hour postonset period.
“We want the DAWN results to be generalizable, to be simpler. We are exploring some more easily generalizable inclusion criteria that would allow us to treat more patients in more parts of the world,” Dr. Jovin added.
Both clinicians cited the remarkably low number needed to treat found in both DAWN and DEFUSE 3 of roughly three patients to produce one additional patient with a statistically significant and clinically meaningful improvement in their 90-day mRS score, compared with controls, as an unmistakable sign that the treatment in both trials was too targeted.
“When we planned the DAWN and DEFUSE 3 trials we didn’t expect how powerful the treatment effect would be. There are probably other patients who could also benefit, so how low can we go? How liberal can we be in our inclusion criteria and still get benefit?” Dr. Jovin asked.
“When intravenous TPA [tissue plasminogen activator] first came out, we went by the book [for patient selection], but as we got to know the treatment and became more comfortable with it, we began to bend the rules. Now we’re at the point of getting comfortable with endovascular treatment, and we need to figure out where to bend the rules by building the database. There is no doubt that the rules need bending because of the treatment effect that we’ve seen. We need to get our patients to endovascular treatment,” she said in her presentation at the conference.
But these physicians realize that for the time being, standard of care will follow the imaging and data processing primarily used in DAWN and DEFUSE 3, which not only involved perfusion CT or MRI but also a proprietary, automated image processing software, RAPID, that takes imaging data and calculates the amount and ratio of infarcted core and hypoperfused, ischemic brain tissue.
“I asked our imaging experts [at the University of Cincinnati] what should my threshold be [for mismatch between the infarcted core and ischemic tissue], and they said, ‘Use the automated software,’ ” Dr. Khatri said. If centers managing acute ischemic stroke patients don’t already have this software, “they need it. I think there is no way around that. It’s the only way we’ll be able to do this,” she commented. Most U.S. community hospitals that admit stroke patients currently lack this software, largely because of its high cost, she added.
“We’re struggling because it is very difficult to get some community hospitals – primary stroke centers – to invest in the software, but that’s really the only way we’ll be able to do this. There are issues of cost, and of getting technicians trained,” she noted.
That’s because DEFUSE 3 enrolled patients with a baseline National Institutes of Health Stroke Scale (NIHSS) score of at least 6, while patients in DAWN required a score of at least 10, a loosening that allowed inclusion of 31 patients with scores of 6-9 in DEFUSE 3. Another, less restrictive criterion was the patient’s prestroke mRS score, which could have been 0-2 in DEFUSE 3 but could be only 0-1 in DAWN. Thirteen of the DEFUSE 3 patients had prestroke mRS scores of 2. DEFUSE 3 also had somewhat more liberal criteria for the size of a patient’s infarcted core at enrollment, with 41 patients who would have been excluded from DAWN because of an overly large infarcted core, Dr. Albers said in his presentation at the conference.
Currently, for patients presenting more than 16 hours following their stroke onset but within 24 hours, the DAWN enrollment criteria exclusively determine which patients should get thrombectomy.
One area where these rules could be bent is by a more thoughtful approach to the prestroke mRS score rule out, such as patients with orthopedic or rheumatic complications that limit mobility and give them an mRS score of 3. “We don’t have data for patients with back pain who can’t walk. I currently take these patients to endovascular therapy, and I’m sure many others do, too,” Dr. Khatri said.
Another potential way to grow the inclusion criteria is to investigate thrombectomy in patients with larger infarcted cores than were enrolled in DAWN and DEFUSE 3, but assessing this will require a new prospective study, Dr. Albers said.
Running the 6- to 24-hour numbers
Adoption of the 6- to 24-hour time window for endovascular intervention in selected patients means that suddenly the U.S. acute stroke infrastructure needs to accommodate a significantly increased number of patients. Just how many added patients this means is uncertain for the time being, and will vary from region to region and center to center. Dr. Albers roughly guessed that the new late time window might double the number of stroke patients undergoing thrombectomy at his center in Stanford. Dr. Khatri put together a more data-driven but still very speculative estimate that at the University of Cincinnati, it will mean about 40% more stroke patients going to thrombectomy. She shared the numbers behind this estimate in a report she gave at the conference.
To calculate the incremental change produced by the late time window, she used data collected on 2,297 acute ischemic stroke patients from the Greater Cincinnati/Northern Kentucky region who were seen at the University of Cincinnati during 2010. Prior analysis by Dr. Khatri and her associates showed that 159 of these patients presented quickly enough and with an appropriate stroke to qualify for thrombolytic therapy, and that 29 patients would have qualified for thrombectomy performed during the 0- to 6-hour time window.
In the new analysis Dr. Khatri calculated that 791 patients presented at 5-23 hours, and of these 34 had other features that would have made them eligible for enrollment in DAWN. Because no imaging data existed for these 2010 patients, she applied an estimate that 22% of these patients would qualify by the size of their infarcted core and ischemic penumbra, resulting in seven additional thrombectomy-eligible patients. Accounting for patients who would qualify by the more liberal DEFUSE 3 criteria added another 5 patients for a total increment of 12 patients during 2010 who would have been eligible for thrombectomy, about 40% of the number from the 0- to 6-hour window.
She noted that “this is likely an underestimate,” and “too small a sample to project to national estimates,” but concluded that “resources must be adapted to account for this increased volume in endovascular treatment.”
Dr. Khatri acknowledged that the new 6- to 24-hour window for endovascular therapy, and concerns about imaging delays in 0- to 6-hour patients, raise challenging issues regarding the message to give U.S. clinicians about treating acute ischemic stroke patients.
“We have a mandate to figure it out in every region. There is no doubt that stroke patients need access to this care. We need to become a lot more aggressive with endovascular treatment. It’s so gratifying to see the outcomes that we’re seeing,” Dr. Khatri said. “A lot of work is needed to accommodate endovascular therapy–eligible patients in an extended time window. We need more refined prehospital triage tools, we need to adequately implement imaging software, and we need increased capacity to perform endovascular treatment with additional procedure suites, operators, and ICU beds.”
Dr. Jovin has been a consultant to Anaconda Biomed, Blockade Medical, Cerenovus, FreeOx Biotech, and Silk Road Medical. Dr. Nogueira has received travel expense reimbursement from Stryker. Dr. Khatri has been a consultant to Biogen, Medpace/Novartis, and St. Jude; has received travel support from Neuravi and EmstoPA; and has received research support from Genentech, Lumosa, and Neurospring. Dr. Albers has an ownership interest in iSchemaView, the company that markets the RAPID imaging software, and is a consultant to iSchemaView and Medtronic.
LOS ANGELES – CT or magnetic resonance brain imaging of acute ischemic stroke patients was the key triage tool in two groundbreaking thrombectomy trials, DAWN and DEFUSE; results from both showed that patients found by imaging to have limited infarcted cores could safely benefit from endovascular thrombectomy, even when they are more than 6 hours out from their stroke onset, breaking the 6-hour barrier created 3 years ago by the first wave of thrombectomy trials.
But some stroke neurologists studying thrombectomy are now convinced that imaging is not needed and may actually harm acute ischemic stroke patients early on by introducing an unnecessary time delay when they present within the first 6 hours after stroke onset.
This new thinking on how to best use brain imaging in acute ischemic stroke patients is part of the rapid evolution of acute stroke management as experts process new data and refine their approach both within 6 hours of stroke onset and during the new treatment window of 6-24 hours post onset. The dramatic success achieved with thrombectomy in highly selected late-window patients prompted researchers to promote pushing the boundaries further to find less-selected late patients who could also potentially benefit from thrombectomy.
The downside of early imaging
“Early on, we have a mix of fast and slow progressors. Slow progressors are about a third to half the patients, so there is a lot of potential for [late] treatment, but the majority of patients during the first 6 hours after onset are fast progressors,” patients who won’t benefit from thrombectomy delivery beyond 6 hours, said Dr. Jovin, an interventional neurologist and director of the Stroke Institute of the University of Pittsburgh Medical Center.
“Time is very precious in the 0- to 6-hour window. When we’re dealing with a lot of fast progressors, we pay a price [in added time to treatment] for any imaging we do. We need to understand that this is a real price we pay, even when CT takes perhaps 24 minutes, and MRI adds about 12 minutes. It’s not the case in all patients that doing CT angiography just adds 5 minutes. It can take 15, 20 minutes,” especially at centers that don’t treat these types of stroke patients day in and day out. “There is no question that imaging slows us down,” Dr. Jovin said.
He highlighted that “the main role of imaging is to exclude patients from treatment, a treatment that has unbelievable effects.” Imaging can rule out patients who have a hemorrhage, lack an occlusion, have a large infarcted core, or have none of the brain at risk or just a small amount, he noted. “Excluding hemorrhage is reasonable, but we can do that in the angiography suite, when the patient is on the table. The main benefit from advanced imaging is to more precisely define the core,” but for most patients the size of their core is not important because the vast majority of acute ischemic stroke patients seen within 6 hours of onset have cores smaller than 70 mL.
“Is this much ado about nothing – especially because we punish all the other patients [with smaller cores] who need to be treated [quickly] when we do additional imaging?” asked Dr. Jovin, who was one of the two lead investigators for the Clinical Mismatch in the Triage of Wake Up and Late Presenting Strokes Undergoing Neurointervention With Trevo (DAWN) trial (N Engl J Med. 2018 Jan 4;378[1]:11-21). Another factor undercutting the value of imaging and determining core size is registry results that show patients who undergo thrombectomy with a large infarcted core are not harmed by treatment.
Current practice often uses imaging “to exclude the 20% of patients who may not have a large vessel occlusion or may not get benefit but whom we are unlikely to harm. But we harm the other 80% by delaying treatment by 30 minutes because of imaging. That’s why we need to rethink imaging and minimize its use.”
Dr. Jovin noted that at his center in Pittsburgh, the stroke institute staff began in 2013 to take patients transported from other stroke facilities and already diagnosed with a large vessel occlusion directly to the angiography suite, bypassing further brain imaging. By doing this, they cut their average door-to-groin puncture time down to 22 minutes from what had been an average of 81 minutes with imaging (Stroke. 2017 July;48[7]:1884-9). Right now the door-to-groin time is even lower, he added.
“Don’t waste time imaging,” said Dr. Nogueira, a stroke neurologist who is director of the neuroendovascular division of the Marcus Stroke and Neuroscience Center and professor of neurology, neurosurgery, and radiology at Emory University, both in Atlanta, as well as the second lead investigator for DAWN. “Time is crucial and trumps patient selection. Most selection criteria are informative rather than truly selective. It is important to understand that in every time window, we do not yet know who doesn’t benefit from treatment. Select faster, select less, and treat more” during the 0- to 6-hour window, he told his colleagues.
Expanding thrombectomy 6-24 hours after stroke onset
While Dr. Jovin and Dr. Nogueira called for more aggressive and less selective use of thrombectomy in patients who present within 6 hours of their stroke onset, they acknowledged that for patients who present during the 6- to 24-hour window, selection for thrombectomy should follow the rules applied in DAWN and in the more inclusive Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke (DEFUSE 3) trial (N Engl J Med. 2018 Feb 22;378:708-18). That means using imaging to confirm that the patient’s infarcted core is within the enrollment ceiling, but both neurologists also downplayed the need for the more sophisticated imaging approaches that were often used in both trials as well as in current routine practice at comprehensive stroke centers. They agreed that noncontrast CT, a widely available method, seems adequate for patient selection, based on the admittedly limited data available right now.
Dr. Nogueira cited data from the Trevo stent retriever registry, collected from nearly 1,000 U.S. acute ischemic stroke patients who underwent thrombectomy with this device. (Researchers at the conference reported updated registry data with nearly 2,000 patients with findings similar to what Dr. Nogueira referenced.) Although these patients underwent treatment before results of DAWN and DEFUSE 3 came out and before release of the new U.S. acute stroke management guidelines (Stroke. 2018 Jan 24; doi: 10.1161/STR.0000000000000158) that endorsed targeted thrombectomy for patients 6-24 hours out from their stroke onset, 278 (28%) of the registry patients underwent thrombectomy treatment during the 6- to 24-hour time window. In this subgroup, 34 patients underwent noncontrast CT to assess their infarcted core prior to thrombectomy, while the other patients underwent perfusion CT, MRI, or both. The noncontrast CT patients had recanalization rates, adverse event rates, and 90-day modified Rankin Scale (mRS) scores comparable with those of patients assessed with more advanced imaging.
Based on this, “just looking at CT only seems reasonable. Noncontrast CT is a pretty valid way to select patients,” Dr. Nogueira said.
“This is the direction we should follow to simplify the paradigm for treating beyond 6 hours,” agreed Dr. Jovin, who also called for further advances in patient selection to expand the pool of patients who qualify for thrombectomy during the 6- to 24-hour postonset period.
“We want the DAWN results to be generalizable, to be simpler. We are exploring some more easily generalizable inclusion criteria that would allow us to treat more patients in more parts of the world,” Dr. Jovin added.
Both clinicians cited the remarkably low number needed to treat found in both DAWN and DEFUSE 3 of roughly three patients to produce one additional patient with a statistically significant and clinically meaningful improvement in their 90-day mRS score, compared with controls, as an unmistakable sign that the treatment in both trials was too targeted.
“When we planned the DAWN and DEFUSE 3 trials we didn’t expect how powerful the treatment effect would be. There are probably other patients who could also benefit, so how low can we go? How liberal can we be in our inclusion criteria and still get benefit?” Dr. Jovin asked.
“When intravenous TPA [tissue plasminogen activator] first came out, we went by the book [for patient selection], but as we got to know the treatment and became more comfortable with it, we began to bend the rules. Now we’re at the point of getting comfortable with endovascular treatment, and we need to figure out where to bend the rules by building the database. There is no doubt that the rules need bending because of the treatment effect that we’ve seen. We need to get our patients to endovascular treatment,” she said in her presentation at the conference.
But these physicians realize that for the time being, standard of care will follow the imaging and data processing primarily used in DAWN and DEFUSE 3, which not only involved perfusion CT or MRI but also a proprietary, automated image processing software, RAPID, that takes imaging data and calculates the amount and ratio of infarcted core and hypoperfused, ischemic brain tissue.
“I asked our imaging experts [at the University of Cincinnati] what should my threshold be [for mismatch between the infarcted core and ischemic tissue], and they said, ‘Use the automated software,’ ” Dr. Khatri said. If centers managing acute ischemic stroke patients don’t already have this software, “they need it. I think there is no way around that. It’s the only way we’ll be able to do this,” she commented. Most U.S. community hospitals that admit stroke patients currently lack this software, largely because of its high cost, she added.
“We’re struggling because it is very difficult to get some community hospitals – primary stroke centers – to invest in the software, but that’s really the only way we’ll be able to do this. There are issues of cost, and of getting technicians trained,” she noted.
That’s because DEFUSE 3 enrolled patients with a baseline National Institutes of Health Stroke Scale (NIHSS) score of at least 6, while patients in DAWN required a score of at least 10, a loosening that allowed inclusion of 31 patients with scores of 6-9 in DEFUSE 3. Another, less restrictive criterion was the patient’s prestroke mRS score, which could have been 0-2 in DEFUSE 3 but could be only 0-1 in DAWN. Thirteen of the DEFUSE 3 patients had prestroke mRS scores of 2. DEFUSE 3 also had somewhat more liberal criteria for the size of a patient’s infarcted core at enrollment, with 41 patients who would have been excluded from DAWN because of an overly large infarcted core, Dr. Albers said in his presentation at the conference.
Currently, for patients presenting more than 16 hours following their stroke onset but within 24 hours, the DAWN enrollment criteria exclusively determine which patients should get thrombectomy.
One area where these rules could be bent is by a more thoughtful approach to the prestroke mRS score rule out, such as patients with orthopedic or rheumatic complications that limit mobility and give them an mRS score of 3. “We don’t have data for patients with back pain who can’t walk. I currently take these patients to endovascular therapy, and I’m sure many others do, too,” Dr. Khatri said.
Another potential way to grow the inclusion criteria is to investigate thrombectomy in patients with larger infarcted cores than were enrolled in DAWN and DEFUSE 3, but assessing this will require a new prospective study, Dr. Albers said.
Running the 6- to 24-hour numbers
Adoption of the 6- to 24-hour time window for endovascular intervention in selected patients means that suddenly the U.S. acute stroke infrastructure needs to accommodate a significantly increased number of patients. Just how many added patients this means is uncertain for the time being, and will vary from region to region and center to center. Dr. Albers roughly guessed that the new late time window might double the number of stroke patients undergoing thrombectomy at his center in Stanford. Dr. Khatri put together a more data-driven but still very speculative estimate that at the University of Cincinnati, it will mean about 40% more stroke patients going to thrombectomy. She shared the numbers behind this estimate in a report she gave at the conference.
To calculate the incremental change produced by the late time window, she used data collected on 2,297 acute ischemic stroke patients from the Greater Cincinnati/Northern Kentucky region who were seen at the University of Cincinnati during 2010. Prior analysis by Dr. Khatri and her associates showed that 159 of these patients presented quickly enough and with an appropriate stroke to qualify for thrombolytic therapy, and that 29 patients would have qualified for thrombectomy performed during the 0- to 6-hour time window.
In the new analysis Dr. Khatri calculated that 791 patients presented at 5-23 hours, and of these 34 had other features that would have made them eligible for enrollment in DAWN. Because no imaging data existed for these 2010 patients, she applied an estimate that 22% of these patients would qualify by the size of their infarcted core and ischemic penumbra, resulting in seven additional thrombectomy-eligible patients. Accounting for patients who would qualify by the more liberal DEFUSE 3 criteria added another 5 patients for a total increment of 12 patients during 2010 who would have been eligible for thrombectomy, about 40% of the number from the 0- to 6-hour window.
She noted that “this is likely an underestimate,” and “too small a sample to project to national estimates,” but concluded that “resources must be adapted to account for this increased volume in endovascular treatment.”
Dr. Khatri acknowledged that the new 6- to 24-hour window for endovascular therapy, and concerns about imaging delays in 0- to 6-hour patients, raise challenging issues regarding the message to give U.S. clinicians about treating acute ischemic stroke patients.
“We have a mandate to figure it out in every region. There is no doubt that stroke patients need access to this care. We need to become a lot more aggressive with endovascular treatment. It’s so gratifying to see the outcomes that we’re seeing,” Dr. Khatri said. “A lot of work is needed to accommodate endovascular therapy–eligible patients in an extended time window. We need more refined prehospital triage tools, we need to adequately implement imaging software, and we need increased capacity to perform endovascular treatment with additional procedure suites, operators, and ICU beds.”
Dr. Jovin has been a consultant to Anaconda Biomed, Blockade Medical, Cerenovus, FreeOx Biotech, and Silk Road Medical. Dr. Nogueira has received travel expense reimbursement from Stryker. Dr. Khatri has been a consultant to Biogen, Medpace/Novartis, and St. Jude; has received travel support from Neuravi and EmstoPA; and has received research support from Genentech, Lumosa, and Neurospring. Dr. Albers has an ownership interest in iSchemaView, the company that markets the RAPID imaging software, and is a consultant to iSchemaView and Medtronic.
LOS ANGELES – CT or magnetic resonance brain imaging of acute ischemic stroke patients was the key triage tool in two groundbreaking thrombectomy trials, DAWN and DEFUSE; results from both showed that patients found by imaging to have limited infarcted cores could safely benefit from endovascular thrombectomy, even when they are more than 6 hours out from their stroke onset, breaking the 6-hour barrier created 3 years ago by the first wave of thrombectomy trials.
But some stroke neurologists studying thrombectomy are now convinced that imaging is not needed and may actually harm acute ischemic stroke patients early on by introducing an unnecessary time delay when they present within the first 6 hours after stroke onset.
This new thinking on how to best use brain imaging in acute ischemic stroke patients is part of the rapid evolution of acute stroke management as experts process new data and refine their approach both within 6 hours of stroke onset and during the new treatment window of 6-24 hours post onset. The dramatic success achieved with thrombectomy in highly selected late-window patients prompted researchers to promote pushing the boundaries further to find less-selected late patients who could also potentially benefit from thrombectomy.
The downside of early imaging
“Early on, we have a mix of fast and slow progressors. Slow progressors are about a third to half the patients, so there is a lot of potential for [late] treatment, but the majority of patients during the first 6 hours after onset are fast progressors,” patients who won’t benefit from thrombectomy delivery beyond 6 hours, said Dr. Jovin, an interventional neurologist and director of the Stroke Institute of the University of Pittsburgh Medical Center.
“Time is very precious in the 0- to 6-hour window. When we’re dealing with a lot of fast progressors, we pay a price [in added time to treatment] for any imaging we do. We need to understand that this is a real price we pay, even when CT takes perhaps 24 minutes, and MRI adds about 12 minutes. It’s not the case in all patients that doing CT angiography just adds 5 minutes. It can take 15, 20 minutes,” especially at centers that don’t treat these types of stroke patients day in and day out. “There is no question that imaging slows us down,” Dr. Jovin said.
He highlighted that “the main role of imaging is to exclude patients from treatment, a treatment that has unbelievable effects.” Imaging can rule out patients who have a hemorrhage, lack an occlusion, have a large infarcted core, or have none of the brain at risk or just a small amount, he noted. “Excluding hemorrhage is reasonable, but we can do that in the angiography suite, when the patient is on the table. The main benefit from advanced imaging is to more precisely define the core,” but for most patients the size of their core is not important because the vast majority of acute ischemic stroke patients seen within 6 hours of onset have cores smaller than 70 mL.
“Is this much ado about nothing – especially because we punish all the other patients [with smaller cores] who need to be treated [quickly] when we do additional imaging?” asked Dr. Jovin, who was one of the two lead investigators for the Clinical Mismatch in the Triage of Wake Up and Late Presenting Strokes Undergoing Neurointervention With Trevo (DAWN) trial (N Engl J Med. 2018 Jan 4;378[1]:11-21). Another factor undercutting the value of imaging and determining core size is registry results that show patients who undergo thrombectomy with a large infarcted core are not harmed by treatment.
Current practice often uses imaging “to exclude the 20% of patients who may not have a large vessel occlusion or may not get benefit but whom we are unlikely to harm. But we harm the other 80% by delaying treatment by 30 minutes because of imaging. That’s why we need to rethink imaging and minimize its use.”
Dr. Jovin noted that at his center in Pittsburgh, the stroke institute staff began in 2013 to take patients transported from other stroke facilities and already diagnosed with a large vessel occlusion directly to the angiography suite, bypassing further brain imaging. By doing this, they cut their average door-to-groin puncture time down to 22 minutes from what had been an average of 81 minutes with imaging (Stroke. 2017 July;48[7]:1884-9). Right now the door-to-groin time is even lower, he added.
“Don’t waste time imaging,” said Dr. Nogueira, a stroke neurologist who is director of the neuroendovascular division of the Marcus Stroke and Neuroscience Center and professor of neurology, neurosurgery, and radiology at Emory University, both in Atlanta, as well as the second lead investigator for DAWN. “Time is crucial and trumps patient selection. Most selection criteria are informative rather than truly selective. It is important to understand that in every time window, we do not yet know who doesn’t benefit from treatment. Select faster, select less, and treat more” during the 0- to 6-hour window, he told his colleagues.
Expanding thrombectomy 6-24 hours after stroke onset
While Dr. Jovin and Dr. Nogueira called for more aggressive and less selective use of thrombectomy in patients who present within 6 hours of their stroke onset, they acknowledged that for patients who present during the 6- to 24-hour window, selection for thrombectomy should follow the rules applied in DAWN and in the more inclusive Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke (DEFUSE 3) trial (N Engl J Med. 2018 Feb 22;378:708-18). That means using imaging to confirm that the patient’s infarcted core is within the enrollment ceiling, but both neurologists also downplayed the need for the more sophisticated imaging approaches that were often used in both trials as well as in current routine practice at comprehensive stroke centers. They agreed that noncontrast CT, a widely available method, seems adequate for patient selection, based on the admittedly limited data available right now.
Dr. Nogueira cited data from the Trevo stent retriever registry, collected from nearly 1,000 U.S. acute ischemic stroke patients who underwent thrombectomy with this device. (Researchers at the conference reported updated registry data with nearly 2,000 patients with findings similar to what Dr. Nogueira referenced.) Although these patients underwent treatment before results of DAWN and DEFUSE 3 came out and before release of the new U.S. acute stroke management guidelines (Stroke. 2018 Jan 24; doi: 10.1161/STR.0000000000000158) that endorsed targeted thrombectomy for patients 6-24 hours out from their stroke onset, 278 (28%) of the registry patients underwent thrombectomy treatment during the 6- to 24-hour time window. In this subgroup, 34 patients underwent noncontrast CT to assess their infarcted core prior to thrombectomy, while the other patients underwent perfusion CT, MRI, or both. The noncontrast CT patients had recanalization rates, adverse event rates, and 90-day modified Rankin Scale (mRS) scores comparable with those of patients assessed with more advanced imaging.
Based on this, “just looking at CT only seems reasonable. Noncontrast CT is a pretty valid way to select patients,” Dr. Nogueira said.
“This is the direction we should follow to simplify the paradigm for treating beyond 6 hours,” agreed Dr. Jovin, who also called for further advances in patient selection to expand the pool of patients who qualify for thrombectomy during the 6- to 24-hour postonset period.
“We want the DAWN results to be generalizable, to be simpler. We are exploring some more easily generalizable inclusion criteria that would allow us to treat more patients in more parts of the world,” Dr. Jovin added.
Both clinicians cited the remarkably low number needed to treat found in both DAWN and DEFUSE 3 of roughly three patients to produce one additional patient with a statistically significant and clinically meaningful improvement in their 90-day mRS score, compared with controls, as an unmistakable sign that the treatment in both trials was too targeted.
“When we planned the DAWN and DEFUSE 3 trials we didn’t expect how powerful the treatment effect would be. There are probably other patients who could also benefit, so how low can we go? How liberal can we be in our inclusion criteria and still get benefit?” Dr. Jovin asked.
“When intravenous TPA [tissue plasminogen activator] first came out, we went by the book [for patient selection], but as we got to know the treatment and became more comfortable with it, we began to bend the rules. Now we’re at the point of getting comfortable with endovascular treatment, and we need to figure out where to bend the rules by building the database. There is no doubt that the rules need bending because of the treatment effect that we’ve seen. We need to get our patients to endovascular treatment,” she said in her presentation at the conference.
But these physicians realize that for the time being, standard of care will follow the imaging and data processing primarily used in DAWN and DEFUSE 3, which not only involved perfusion CT or MRI but also a proprietary, automated image processing software, RAPID, that takes imaging data and calculates the amount and ratio of infarcted core and hypoperfused, ischemic brain tissue.
“I asked our imaging experts [at the University of Cincinnati] what should my threshold be [for mismatch between the infarcted core and ischemic tissue], and they said, ‘Use the automated software,’ ” Dr. Khatri said. If centers managing acute ischemic stroke patients don’t already have this software, “they need it. I think there is no way around that. It’s the only way we’ll be able to do this,” she commented. Most U.S. community hospitals that admit stroke patients currently lack this software, largely because of its high cost, she added.
“We’re struggling because it is very difficult to get some community hospitals – primary stroke centers – to invest in the software, but that’s really the only way we’ll be able to do this. There are issues of cost, and of getting technicians trained,” she noted.
That’s because DEFUSE 3 enrolled patients with a baseline National Institutes of Health Stroke Scale (NIHSS) score of at least 6, while patients in DAWN required a score of at least 10, a loosening that allowed inclusion of 31 patients with scores of 6-9 in DEFUSE 3. Another, less restrictive criterion was the patient’s prestroke mRS score, which could have been 0-2 in DEFUSE 3 but could be only 0-1 in DAWN. Thirteen of the DEFUSE 3 patients had prestroke mRS scores of 2. DEFUSE 3 also had somewhat more liberal criteria for the size of a patient’s infarcted core at enrollment, with 41 patients who would have been excluded from DAWN because of an overly large infarcted core, Dr. Albers said in his presentation at the conference.
Currently, for patients presenting more than 16 hours following their stroke onset but within 24 hours, the DAWN enrollment criteria exclusively determine which patients should get thrombectomy.
One area where these rules could be bent is by a more thoughtful approach to the prestroke mRS score rule out, such as patients with orthopedic or rheumatic complications that limit mobility and give them an mRS score of 3. “We don’t have data for patients with back pain who can’t walk. I currently take these patients to endovascular therapy, and I’m sure many others do, too,” Dr. Khatri said.
Another potential way to grow the inclusion criteria is to investigate thrombectomy in patients with larger infarcted cores than were enrolled in DAWN and DEFUSE 3, but assessing this will require a new prospective study, Dr. Albers said.
Running the 6- to 24-hour numbers
Adoption of the 6- to 24-hour time window for endovascular intervention in selected patients means that suddenly the U.S. acute stroke infrastructure needs to accommodate a significantly increased number of patients. Just how many added patients this means is uncertain for the time being, and will vary from region to region and center to center. Dr. Albers roughly guessed that the new late time window might double the number of stroke patients undergoing thrombectomy at his center in Stanford. Dr. Khatri put together a more data-driven but still very speculative estimate that at the University of Cincinnati, it will mean about 40% more stroke patients going to thrombectomy. She shared the numbers behind this estimate in a report she gave at the conference.
To calculate the incremental change produced by the late time window, she used data collected on 2,297 acute ischemic stroke patients from the Greater Cincinnati/Northern Kentucky region who were seen at the University of Cincinnati during 2010. Prior analysis by Dr. Khatri and her associates showed that 159 of these patients presented quickly enough and with an appropriate stroke to qualify for thrombolytic therapy, and that 29 patients would have qualified for thrombectomy performed during the 0- to 6-hour time window.
In the new analysis Dr. Khatri calculated that 791 patients presented at 5-23 hours, and of these 34 had other features that would have made them eligible for enrollment in DAWN. Because no imaging data existed for these 2010 patients, she applied an estimate that 22% of these patients would qualify by the size of their infarcted core and ischemic penumbra, resulting in seven additional thrombectomy-eligible patients. Accounting for patients who would qualify by the more liberal DEFUSE 3 criteria added another 5 patients for a total increment of 12 patients during 2010 who would have been eligible for thrombectomy, about 40% of the number from the 0- to 6-hour window.
She noted that “this is likely an underestimate,” and “too small a sample to project to national estimates,” but concluded that “resources must be adapted to account for this increased volume in endovascular treatment.”
Dr. Khatri acknowledged that the new 6- to 24-hour window for endovascular therapy, and concerns about imaging delays in 0- to 6-hour patients, raise challenging issues regarding the message to give U.S. clinicians about treating acute ischemic stroke patients.
“We have a mandate to figure it out in every region. There is no doubt that stroke patients need access to this care. We need to become a lot more aggressive with endovascular treatment. It’s so gratifying to see the outcomes that we’re seeing,” Dr. Khatri said. “A lot of work is needed to accommodate endovascular therapy–eligible patients in an extended time window. We need more refined prehospital triage tools, we need to adequately implement imaging software, and we need increased capacity to perform endovascular treatment with additional procedure suites, operators, and ICU beds.”
Dr. Jovin has been a consultant to Anaconda Biomed, Blockade Medical, Cerenovus, FreeOx Biotech, and Silk Road Medical. Dr. Nogueira has received travel expense reimbursement from Stryker. Dr. Khatri has been a consultant to Biogen, Medpace/Novartis, and St. Jude; has received travel support from Neuravi and EmstoPA; and has received research support from Genentech, Lumosa, and Neurospring. Dr. Albers has an ownership interest in iSchemaView, the company that markets the RAPID imaging software, and is a consultant to iSchemaView and Medtronic.
EXPERT ANALYSIS FROM ISC 2018
VIDEO: Stroke benefits from stem cells maintained for 2 years
LOS ANGELES – , Gary K. Steinberg, MD, said at the International Stroke Conference, sponsored by the American Heart Association.
Seeing sustained benefit out to 2 years was “quite surprising. We thought we’d lose the benefit,” Dr. Steinberg said in a video interview.
The findings “change our notion of what happens after a stroke. The damaged circuits can be resurrected,” said Dr. Steinberg, professor and chair of neurosurgery at Stanford (Calif.) University.
He reported long-term follow-up data for 18 chronic stroke patients who had received transplantation of allogeneic bone marrow–derived stem cells. The study’s primary efficacy endpoint, at 6 months after treatment, showed clinically meaningful improvements in several measures of stroke disability and function in 13 of the 18 patients (72%), including a rise of at least 10 points in the Fugl-Meyer total motor function score.
His new report on 2-year follow-up showed that these 6-month improvements continued. The average increase in Fugl-Meyer score over baseline was about 18 points at 6, 12, and 24 months of follow-up.
Based on the promise shown in this pilot study, Dr. Steinberg and his associates are running a randomized study with 156 patients. Enrollment recently completed, and the results should be available during the second half of 2019, Dr. Steinberg said.
SanBio funded the study. Dr. Steinberg has been a consultant or advisor to Qool Therapeutics, Peter Lazic US, and NeuroSave.
SOURCE: Steinberg K et al. International Stroke Conference 2018, Abstract LB14.
LOS ANGELES – , Gary K. Steinberg, MD, said at the International Stroke Conference, sponsored by the American Heart Association.
Seeing sustained benefit out to 2 years was “quite surprising. We thought we’d lose the benefit,” Dr. Steinberg said in a video interview.
The findings “change our notion of what happens after a stroke. The damaged circuits can be resurrected,” said Dr. Steinberg, professor and chair of neurosurgery at Stanford (Calif.) University.
He reported long-term follow-up data for 18 chronic stroke patients who had received transplantation of allogeneic bone marrow–derived stem cells. The study’s primary efficacy endpoint, at 6 months after treatment, showed clinically meaningful improvements in several measures of stroke disability and function in 13 of the 18 patients (72%), including a rise of at least 10 points in the Fugl-Meyer total motor function score.
His new report on 2-year follow-up showed that these 6-month improvements continued. The average increase in Fugl-Meyer score over baseline was about 18 points at 6, 12, and 24 months of follow-up.
Based on the promise shown in this pilot study, Dr. Steinberg and his associates are running a randomized study with 156 patients. Enrollment recently completed, and the results should be available during the second half of 2019, Dr. Steinberg said.
SanBio funded the study. Dr. Steinberg has been a consultant or advisor to Qool Therapeutics, Peter Lazic US, and NeuroSave.
SOURCE: Steinberg K et al. International Stroke Conference 2018, Abstract LB14.
LOS ANGELES – , Gary K. Steinberg, MD, said at the International Stroke Conference, sponsored by the American Heart Association.
Seeing sustained benefit out to 2 years was “quite surprising. We thought we’d lose the benefit,” Dr. Steinberg said in a video interview.
The findings “change our notion of what happens after a stroke. The damaged circuits can be resurrected,” said Dr. Steinberg, professor and chair of neurosurgery at Stanford (Calif.) University.
He reported long-term follow-up data for 18 chronic stroke patients who had received transplantation of allogeneic bone marrow–derived stem cells. The study’s primary efficacy endpoint, at 6 months after treatment, showed clinically meaningful improvements in several measures of stroke disability and function in 13 of the 18 patients (72%), including a rise of at least 10 points in the Fugl-Meyer total motor function score.
His new report on 2-year follow-up showed that these 6-month improvements continued. The average increase in Fugl-Meyer score over baseline was about 18 points at 6, 12, and 24 months of follow-up.
Based on the promise shown in this pilot study, Dr. Steinberg and his associates are running a randomized study with 156 patients. Enrollment recently completed, and the results should be available during the second half of 2019, Dr. Steinberg said.
SanBio funded the study. Dr. Steinberg has been a consultant or advisor to Qool Therapeutics, Peter Lazic US, and NeuroSave.
SOURCE: Steinberg K et al. International Stroke Conference 2018, Abstract LB14.
REPORTING FROM ISC 2018
Key clinical point: The stroke benefits from cell transplantation continued during 2-year follow-up.
Major finding: Among 18 treated patients, 13 (72%) had a sustained, clinically meaningful rise in their total motor function score.
Study details: Review of 18 patients who received intracranial cell transplantations at two U.S. sites.
Disclosures: SanBio funded the study. Dr. Steinberg has been a consultant or adviser to Qool Therapeutics, Peter Lazic US, and NeuroSave.
Source: Steinberg K et al. International Stroke Conference 2018, Abstract LB14.
EMS stroke field triage improves outcomes
LOS ANGELES – An emergency medical services protocol to identify large vessel occlusions and deliver patients to a comprehensive stroke center if it is within 30 minutes of travel time reduced the time to recanalization when compared against a separate protocol that optimized transfer of such patients from primary to comprehensive stroke centers.
The findings, which come from a sequential study conducted in an urban Rhode Island region, offer evidence to resolve the controversy over whether field triage in emergency medical services (EMS) units will improve outcomes, because field stroke severity scores won’t always be accurate, and longer travel to a comprehensive stroke center (CSC) could delay treatment to a patient who doesn’t need thrombectomy.
The region where the study was carried out has one CSC and eight primary stroke centers (PSCs). The large vessel occlusions transfer protocol instructed PSCs to contact the CSC when a patient scored 4 or 5 on the Los Angeles Motor Scale (LAMS), followed by CT and CT angiography. They then shared the resulting images with the CSC, which could make a decision whether to transfer the patient.
The field-based protocol relied on a LAMS score assessment by EMS personnel. Patients scoring 4 or 5 would then be delivered to the CSC if it was within 30 minutes from their current location. Patients scoring less than 4 would be brought to the nearest facility. In cases when the field LAMS score was 4 or greater and the nearest CSC was more than 30 miles away, EMS personnel were instructed to travel to the closest PSC, but immediately send word of an inbound patient that might need a transfer to a CSC. In those cases, the PSC’s goal was to get images to the CSC for review within 45 minutes. The protocol was executed out to 24 hours after the patient was last known well.
Even in patients who were closer to a PSC than a CSC, process outcomes were better with the field triage protocol. “Despite 8 additional minutes of transport time, IV TPA was given 17 minutes earlier, and recanalization occurred almost an hour earlier,” said Dr. McTaggart. “That would indicate that perhaps even a 30-minute window is too conservative of a protocol, because the number needed to treat for mechanical thrombectomy is 2 or 3, so you have this tremendously powerful treatment effect for these patients. If you can get it to them an hour earlier, it’s a no-brainer to me that they need to go to the right place the first time,” he said.
Instituting the changes was no picnic. Dr. McTaggart spent thousands of hours working with EMS personnel and emergency department physicians at PSCs. “It’s a lot of work, but the downstream gains are huge, not only from a disability standpoint for patients but for the economics of the health care system. We’re potentially saving patients from disability health care costs,” he said.
The study population included consecutive stroke patients in the region whose first contact was with EMS personnel during three time periods: before either change was made: (pre PSC-CSC transfer optimization, pre field triage, July 2015 to January 2016), after PSC optimization but only voluntary field triage (January 2016 to January 2017), and when both PSC optimization and field triage were mandatory (January 2017 to January 2018).
The patients had an anterior large vessel occlusion and mild to moderate early ischemic change. Outcomes included time from hospital arrival (PSC or CSC) to alteplase treatment, arterial puncture, and recanalization. Clinical measures included favorable outcomes (modified Rankin scale score 0-2) at 90 days, or discharge with a National Institutes of Health Stroke Scale score of 4 or less, in cases where 90-day follow-up did not occur.
A total of 38 patients were seen before any change, 100 after PSC optimization, and 94 after both PSC optimization and field triage were implemented. A Google Maps analysis showed that the median additional time required to travel to a CSC instead of a PSC was 8 minutes (interquartile range 4-12).
The time to first use of IV alteplase dropped from 54 minutes before any change to 49 minutes after PSC optimization, and 36 minutes after both PSC optimization and field triage. Similar drops were seen in time to arterial puncture (105 minutes, 101 minutes, 88 minutes) and time to recanalization (156 minutes, 132 minutes, 116 minutes). These differences did not reach statistical significance.
The clinical outcomes also became more favorable: 58% had a favorable outcome at 90 days with both protocols in place, compared with 51% with only PSC optimization and 31% before any changes (P = .049 for 31% to 58% comparison).
The researchers conducted a subanalysis of 150 patients for whom the PSC was closest. Of these, 94 went to a CSC and 56 went to a PSC. The elapsed time between EMS leaving the scene with the patient aboard and IV TPA treatment was an average of 51 minutes in patients taken to the CSC, compared with 68 minutes in patients taken to PSCs (P = .012). The time to arterial puncture was also shorter (98 minutes versus 155 minutes; P less than .001), as was time to recanalization (131 minutes versus 174 minutes; P less than .001).
CSC patients were more likely to have a favorable outcome (65% versus 42%; P = .01).
The study received no external funding. Dr. McTaggart reported having no financial disclosures.
SOURCE: Jayaraman M et al. ISC 2018 Abstract 95 (Stroke. 2018 Jan;49[Suppl 1]:A95)
LOS ANGELES – An emergency medical services protocol to identify large vessel occlusions and deliver patients to a comprehensive stroke center if it is within 30 minutes of travel time reduced the time to recanalization when compared against a separate protocol that optimized transfer of such patients from primary to comprehensive stroke centers.
The findings, which come from a sequential study conducted in an urban Rhode Island region, offer evidence to resolve the controversy over whether field triage in emergency medical services (EMS) units will improve outcomes, because field stroke severity scores won’t always be accurate, and longer travel to a comprehensive stroke center (CSC) could delay treatment to a patient who doesn’t need thrombectomy.
The region where the study was carried out has one CSC and eight primary stroke centers (PSCs). The large vessel occlusions transfer protocol instructed PSCs to contact the CSC when a patient scored 4 or 5 on the Los Angeles Motor Scale (LAMS), followed by CT and CT angiography. They then shared the resulting images with the CSC, which could make a decision whether to transfer the patient.
The field-based protocol relied on a LAMS score assessment by EMS personnel. Patients scoring 4 or 5 would then be delivered to the CSC if it was within 30 minutes from their current location. Patients scoring less than 4 would be brought to the nearest facility. In cases when the field LAMS score was 4 or greater and the nearest CSC was more than 30 miles away, EMS personnel were instructed to travel to the closest PSC, but immediately send word of an inbound patient that might need a transfer to a CSC. In those cases, the PSC’s goal was to get images to the CSC for review within 45 minutes. The protocol was executed out to 24 hours after the patient was last known well.
Even in patients who were closer to a PSC than a CSC, process outcomes were better with the field triage protocol. “Despite 8 additional minutes of transport time, IV TPA was given 17 minutes earlier, and recanalization occurred almost an hour earlier,” said Dr. McTaggart. “That would indicate that perhaps even a 30-minute window is too conservative of a protocol, because the number needed to treat for mechanical thrombectomy is 2 or 3, so you have this tremendously powerful treatment effect for these patients. If you can get it to them an hour earlier, it’s a no-brainer to me that they need to go to the right place the first time,” he said.
Instituting the changes was no picnic. Dr. McTaggart spent thousands of hours working with EMS personnel and emergency department physicians at PSCs. “It’s a lot of work, but the downstream gains are huge, not only from a disability standpoint for patients but for the economics of the health care system. We’re potentially saving patients from disability health care costs,” he said.
The study population included consecutive stroke patients in the region whose first contact was with EMS personnel during three time periods: before either change was made: (pre PSC-CSC transfer optimization, pre field triage, July 2015 to January 2016), after PSC optimization but only voluntary field triage (January 2016 to January 2017), and when both PSC optimization and field triage were mandatory (January 2017 to January 2018).
The patients had an anterior large vessel occlusion and mild to moderate early ischemic change. Outcomes included time from hospital arrival (PSC or CSC) to alteplase treatment, arterial puncture, and recanalization. Clinical measures included favorable outcomes (modified Rankin scale score 0-2) at 90 days, or discharge with a National Institutes of Health Stroke Scale score of 4 or less, in cases where 90-day follow-up did not occur.
A total of 38 patients were seen before any change, 100 after PSC optimization, and 94 after both PSC optimization and field triage were implemented. A Google Maps analysis showed that the median additional time required to travel to a CSC instead of a PSC was 8 minutes (interquartile range 4-12).
The time to first use of IV alteplase dropped from 54 minutes before any change to 49 minutes after PSC optimization, and 36 minutes after both PSC optimization and field triage. Similar drops were seen in time to arterial puncture (105 minutes, 101 minutes, 88 minutes) and time to recanalization (156 minutes, 132 minutes, 116 minutes). These differences did not reach statistical significance.
The clinical outcomes also became more favorable: 58% had a favorable outcome at 90 days with both protocols in place, compared with 51% with only PSC optimization and 31% before any changes (P = .049 for 31% to 58% comparison).
The researchers conducted a subanalysis of 150 patients for whom the PSC was closest. Of these, 94 went to a CSC and 56 went to a PSC. The elapsed time between EMS leaving the scene with the patient aboard and IV TPA treatment was an average of 51 minutes in patients taken to the CSC, compared with 68 minutes in patients taken to PSCs (P = .012). The time to arterial puncture was also shorter (98 minutes versus 155 minutes; P less than .001), as was time to recanalization (131 minutes versus 174 minutes; P less than .001).
CSC patients were more likely to have a favorable outcome (65% versus 42%; P = .01).
The study received no external funding. Dr. McTaggart reported having no financial disclosures.
SOURCE: Jayaraman M et al. ISC 2018 Abstract 95 (Stroke. 2018 Jan;49[Suppl 1]:A95)
LOS ANGELES – An emergency medical services protocol to identify large vessel occlusions and deliver patients to a comprehensive stroke center if it is within 30 minutes of travel time reduced the time to recanalization when compared against a separate protocol that optimized transfer of such patients from primary to comprehensive stroke centers.
The findings, which come from a sequential study conducted in an urban Rhode Island region, offer evidence to resolve the controversy over whether field triage in emergency medical services (EMS) units will improve outcomes, because field stroke severity scores won’t always be accurate, and longer travel to a comprehensive stroke center (CSC) could delay treatment to a patient who doesn’t need thrombectomy.
The region where the study was carried out has one CSC and eight primary stroke centers (PSCs). The large vessel occlusions transfer protocol instructed PSCs to contact the CSC when a patient scored 4 or 5 on the Los Angeles Motor Scale (LAMS), followed by CT and CT angiography. They then shared the resulting images with the CSC, which could make a decision whether to transfer the patient.
The field-based protocol relied on a LAMS score assessment by EMS personnel. Patients scoring 4 or 5 would then be delivered to the CSC if it was within 30 minutes from their current location. Patients scoring less than 4 would be brought to the nearest facility. In cases when the field LAMS score was 4 or greater and the nearest CSC was more than 30 miles away, EMS personnel were instructed to travel to the closest PSC, but immediately send word of an inbound patient that might need a transfer to a CSC. In those cases, the PSC’s goal was to get images to the CSC for review within 45 minutes. The protocol was executed out to 24 hours after the patient was last known well.
Even in patients who were closer to a PSC than a CSC, process outcomes were better with the field triage protocol. “Despite 8 additional minutes of transport time, IV TPA was given 17 minutes earlier, and recanalization occurred almost an hour earlier,” said Dr. McTaggart. “That would indicate that perhaps even a 30-minute window is too conservative of a protocol, because the number needed to treat for mechanical thrombectomy is 2 or 3, so you have this tremendously powerful treatment effect for these patients. If you can get it to them an hour earlier, it’s a no-brainer to me that they need to go to the right place the first time,” he said.
Instituting the changes was no picnic. Dr. McTaggart spent thousands of hours working with EMS personnel and emergency department physicians at PSCs. “It’s a lot of work, but the downstream gains are huge, not only from a disability standpoint for patients but for the economics of the health care system. We’re potentially saving patients from disability health care costs,” he said.
The study population included consecutive stroke patients in the region whose first contact was with EMS personnel during three time periods: before either change was made: (pre PSC-CSC transfer optimization, pre field triage, July 2015 to January 2016), after PSC optimization but only voluntary field triage (January 2016 to January 2017), and when both PSC optimization and field triage were mandatory (January 2017 to January 2018).
The patients had an anterior large vessel occlusion and mild to moderate early ischemic change. Outcomes included time from hospital arrival (PSC or CSC) to alteplase treatment, arterial puncture, and recanalization. Clinical measures included favorable outcomes (modified Rankin scale score 0-2) at 90 days, or discharge with a National Institutes of Health Stroke Scale score of 4 or less, in cases where 90-day follow-up did not occur.
A total of 38 patients were seen before any change, 100 after PSC optimization, and 94 after both PSC optimization and field triage were implemented. A Google Maps analysis showed that the median additional time required to travel to a CSC instead of a PSC was 8 minutes (interquartile range 4-12).
The time to first use of IV alteplase dropped from 54 minutes before any change to 49 minutes after PSC optimization, and 36 minutes after both PSC optimization and field triage. Similar drops were seen in time to arterial puncture (105 minutes, 101 minutes, 88 minutes) and time to recanalization (156 minutes, 132 minutes, 116 minutes). These differences did not reach statistical significance.
The clinical outcomes also became more favorable: 58% had a favorable outcome at 90 days with both protocols in place, compared with 51% with only PSC optimization and 31% before any changes (P = .049 for 31% to 58% comparison).
The researchers conducted a subanalysis of 150 patients for whom the PSC was closest. Of these, 94 went to a CSC and 56 went to a PSC. The elapsed time between EMS leaving the scene with the patient aboard and IV TPA treatment was an average of 51 minutes in patients taken to the CSC, compared with 68 minutes in patients taken to PSCs (P = .012). The time to arterial puncture was also shorter (98 minutes versus 155 minutes; P less than .001), as was time to recanalization (131 minutes versus 174 minutes; P less than .001).
CSC patients were more likely to have a favorable outcome (65% versus 42%; P = .01).
The study received no external funding. Dr. McTaggart reported having no financial disclosures.
SOURCE: Jayaraman M et al. ISC 2018 Abstract 95 (Stroke. 2018 Jan;49[Suppl 1]:A95)
REPORTING FROM ISC 2018
Key clinical point: EMS field triage may improve stroke patient management.
Major finding: Even when a primary stroke center was closer, the time to recanalization was shortened by 43 minutes when patients were taken to a comprehensive stroke center instead.
Data source: Prospective study of 232 consecutive stroke patients.
Disclosures: The study received no external funding. Dr. McTaggart reported having no financial disclosures.
Source: Jayaraman M et al. ISC 2018 Abstract 95 (Stroke. 2018 Jan;49[Suppl 1]:A95)
Survey highlights challenges in Asian American stroke patients
LOS ANGELES – A large survey of Asian Americans suggests that the group experiences more severe ischemic strokes and is less likely to receive intravenous tissue plasminogen activator (tPA) than do white patients, among other discrepancies. The research found that whites had declining stroke severity between 2004 and 2016, but there was no change in Asian Americans.
The research encompasses all self-identified Asian Americans in the Get-with-the-Guidelines stroke database, which is a voluntary stroke quality improvement program begun by the American Heart Association in 2003. The analysis included 64,337 Asian Americans and 1,707,962 white Americans at 2,171 hospitals nationwide that participated in the program during 2004-2016.
“I think the most important finding is that they’re not getting as much tPA and having more tPA complications, such as bleeding more. I think it gives it an urgency that maybe was lacking, an urgency that we really need to address this issue by finding innovative ways to reach Asian Americans, to educate them about stroke. We need to find culturally appropriate ways to reach out to Asian populations,” said Dr. Song, who is a vascular neurologist at Rush Medical College, Chicago.
Dr. Song is working on small-scale interventions that are culturally tailored for Asian populations. “I think the way to approach any insular community is to work from within, so that’s my goal,” Dr. Song said.
One particular finding suggested a need for better education among Asian American communities. Asian Americans were less likely than whites to report a clinical history of having heightened levels of low-density lipoproteins. “They didn’t know that they had high cholesterol, but they had a higher LDL [cholesterol levels] than Caucasians on average,” said Dr. Song. The mean LDL cholesterol value was 101 mg/dL in Asian Americans, compared with 95 mg/dL in whites, which was statistically significant.
White patients had higher rates of atrial fibrillation (21.2% vs. 16.0%), coronary artery disease (27.8% vs. 17.5%), and stenosis (4.7% vs. 2.0%), while Asian Americans were more prone to diabetes (38.0% vs. 29.2%).
Severe strokes (National Institutes of Health stroke score of 16 or greater) were more common among Asian Americans (odds ratio, 1.35; P less than .0001). After adjustment for stroke severity, the researchers found that Asian Americans were less likely to receive tPA (OR, 0.90; P less than .0001) and more likely to experience symptomatic intracerebral hemorrhage within 36 hours of receiving tPA (OR, 1.23; P = .003). “I think that may have something to do with the pathophysiology of Asian stroke that we don’t quite understand yet, but we can see there is a problem,” Dr. Song said.
Although in-hospital mortality was initially higher among Asian Americans, this trend switched after researchers corrected for stroke severity, leading to a better outcome for Asian Americans (OR, 0.95; P = .008). Some quality of care measures also favored Asian Americans, including receipt of stroke education (OR, 1.08; P = .02), receipt of IV tPA within 60 minutes of arrival (OR, 1.14; P = .0006), LDL cholesterol documentation (OR, 1.19; P less than .0001), and receipt of intensive statin therapy (OR, 1.15; P less than .0001). However, Asian Americans were less likely to receive a CT scan within 25 minutes of arrival (OR, 0.92; P less than .0001).
Between 2004 and 2016, both groups benefited from similar improvements, but there were differences. In 2016, a stroke in a white patient was less likely to be severe than in 2004 (OR, 0.97; P less than .0001), while there was no change in Asian Americans (OR, 1.00; P = .95).
The study is limited by the fact that the database is voluntary, which could lead to selection bias, and all Asian Americans are combined into one group. “One can argue that South Asian stroke is not the same as Japanese stroke or stroke in the Philippines,” Dr. Song said. Still, the findings suggest problems that need to be addressed. “I think it highlights the problem that Asian ischemic stroke patients don’t do as well, they bleed more, and they receive less tPA. I think that’s a big deal.”
The study received no specific funding. Dr. Song reported having no financial disclosures.
SOURCE: Song S et al. ISC 2018, Abstract TMP75.
LOS ANGELES – A large survey of Asian Americans suggests that the group experiences more severe ischemic strokes and is less likely to receive intravenous tissue plasminogen activator (tPA) than do white patients, among other discrepancies. The research found that whites had declining stroke severity between 2004 and 2016, but there was no change in Asian Americans.
The research encompasses all self-identified Asian Americans in the Get-with-the-Guidelines stroke database, which is a voluntary stroke quality improvement program begun by the American Heart Association in 2003. The analysis included 64,337 Asian Americans and 1,707,962 white Americans at 2,171 hospitals nationwide that participated in the program during 2004-2016.
“I think the most important finding is that they’re not getting as much tPA and having more tPA complications, such as bleeding more. I think it gives it an urgency that maybe was lacking, an urgency that we really need to address this issue by finding innovative ways to reach Asian Americans, to educate them about stroke. We need to find culturally appropriate ways to reach out to Asian populations,” said Dr. Song, who is a vascular neurologist at Rush Medical College, Chicago.
Dr. Song is working on small-scale interventions that are culturally tailored for Asian populations. “I think the way to approach any insular community is to work from within, so that’s my goal,” Dr. Song said.
One particular finding suggested a need for better education among Asian American communities. Asian Americans were less likely than whites to report a clinical history of having heightened levels of low-density lipoproteins. “They didn’t know that they had high cholesterol, but they had a higher LDL [cholesterol levels] than Caucasians on average,” said Dr. Song. The mean LDL cholesterol value was 101 mg/dL in Asian Americans, compared with 95 mg/dL in whites, which was statistically significant.
White patients had higher rates of atrial fibrillation (21.2% vs. 16.0%), coronary artery disease (27.8% vs. 17.5%), and stenosis (4.7% vs. 2.0%), while Asian Americans were more prone to diabetes (38.0% vs. 29.2%).
Severe strokes (National Institutes of Health stroke score of 16 or greater) were more common among Asian Americans (odds ratio, 1.35; P less than .0001). After adjustment for stroke severity, the researchers found that Asian Americans were less likely to receive tPA (OR, 0.90; P less than .0001) and more likely to experience symptomatic intracerebral hemorrhage within 36 hours of receiving tPA (OR, 1.23; P = .003). “I think that may have something to do with the pathophysiology of Asian stroke that we don’t quite understand yet, but we can see there is a problem,” Dr. Song said.
Although in-hospital mortality was initially higher among Asian Americans, this trend switched after researchers corrected for stroke severity, leading to a better outcome for Asian Americans (OR, 0.95; P = .008). Some quality of care measures also favored Asian Americans, including receipt of stroke education (OR, 1.08; P = .02), receipt of IV tPA within 60 minutes of arrival (OR, 1.14; P = .0006), LDL cholesterol documentation (OR, 1.19; P less than .0001), and receipt of intensive statin therapy (OR, 1.15; P less than .0001). However, Asian Americans were less likely to receive a CT scan within 25 minutes of arrival (OR, 0.92; P less than .0001).
Between 2004 and 2016, both groups benefited from similar improvements, but there were differences. In 2016, a stroke in a white patient was less likely to be severe than in 2004 (OR, 0.97; P less than .0001), while there was no change in Asian Americans (OR, 1.00; P = .95).
The study is limited by the fact that the database is voluntary, which could lead to selection bias, and all Asian Americans are combined into one group. “One can argue that South Asian stroke is not the same as Japanese stroke or stroke in the Philippines,” Dr. Song said. Still, the findings suggest problems that need to be addressed. “I think it highlights the problem that Asian ischemic stroke patients don’t do as well, they bleed more, and they receive less tPA. I think that’s a big deal.”
The study received no specific funding. Dr. Song reported having no financial disclosures.
SOURCE: Song S et al. ISC 2018, Abstract TMP75.
LOS ANGELES – A large survey of Asian Americans suggests that the group experiences more severe ischemic strokes and is less likely to receive intravenous tissue plasminogen activator (tPA) than do white patients, among other discrepancies. The research found that whites had declining stroke severity between 2004 and 2016, but there was no change in Asian Americans.
The research encompasses all self-identified Asian Americans in the Get-with-the-Guidelines stroke database, which is a voluntary stroke quality improvement program begun by the American Heart Association in 2003. The analysis included 64,337 Asian Americans and 1,707,962 white Americans at 2,171 hospitals nationwide that participated in the program during 2004-2016.
“I think the most important finding is that they’re not getting as much tPA and having more tPA complications, such as bleeding more. I think it gives it an urgency that maybe was lacking, an urgency that we really need to address this issue by finding innovative ways to reach Asian Americans, to educate them about stroke. We need to find culturally appropriate ways to reach out to Asian populations,” said Dr. Song, who is a vascular neurologist at Rush Medical College, Chicago.
Dr. Song is working on small-scale interventions that are culturally tailored for Asian populations. “I think the way to approach any insular community is to work from within, so that’s my goal,” Dr. Song said.
One particular finding suggested a need for better education among Asian American communities. Asian Americans were less likely than whites to report a clinical history of having heightened levels of low-density lipoproteins. “They didn’t know that they had high cholesterol, but they had a higher LDL [cholesterol levels] than Caucasians on average,” said Dr. Song. The mean LDL cholesterol value was 101 mg/dL in Asian Americans, compared with 95 mg/dL in whites, which was statistically significant.
White patients had higher rates of atrial fibrillation (21.2% vs. 16.0%), coronary artery disease (27.8% vs. 17.5%), and stenosis (4.7% vs. 2.0%), while Asian Americans were more prone to diabetes (38.0% vs. 29.2%).
Severe strokes (National Institutes of Health stroke score of 16 or greater) were more common among Asian Americans (odds ratio, 1.35; P less than .0001). After adjustment for stroke severity, the researchers found that Asian Americans were less likely to receive tPA (OR, 0.90; P less than .0001) and more likely to experience symptomatic intracerebral hemorrhage within 36 hours of receiving tPA (OR, 1.23; P = .003). “I think that may have something to do with the pathophysiology of Asian stroke that we don’t quite understand yet, but we can see there is a problem,” Dr. Song said.
Although in-hospital mortality was initially higher among Asian Americans, this trend switched after researchers corrected for stroke severity, leading to a better outcome for Asian Americans (OR, 0.95; P = .008). Some quality of care measures also favored Asian Americans, including receipt of stroke education (OR, 1.08; P = .02), receipt of IV tPA within 60 minutes of arrival (OR, 1.14; P = .0006), LDL cholesterol documentation (OR, 1.19; P less than .0001), and receipt of intensive statin therapy (OR, 1.15; P less than .0001). However, Asian Americans were less likely to receive a CT scan within 25 minutes of arrival (OR, 0.92; P less than .0001).
Between 2004 and 2016, both groups benefited from similar improvements, but there were differences. In 2016, a stroke in a white patient was less likely to be severe than in 2004 (OR, 0.97; P less than .0001), while there was no change in Asian Americans (OR, 1.00; P = .95).
The study is limited by the fact that the database is voluntary, which could lead to selection bias, and all Asian Americans are combined into one group. “One can argue that South Asian stroke is not the same as Japanese stroke or stroke in the Philippines,” Dr. Song said. Still, the findings suggest problems that need to be addressed. “I think it highlights the problem that Asian ischemic stroke patients don’t do as well, they bleed more, and they receive less tPA. I think that’s a big deal.”
The study received no specific funding. Dr. Song reported having no financial disclosures.
SOURCE: Song S et al. ISC 2018, Abstract TMP75.
REPORTING FROM ISC 2018
Key clinical point: Asian stroke patients don’t do as well as whites on some outcomes and quality measures.
Major finding: Asian Americans had a 35% higher frequency of severe stroke.
Data source: Retrospective analysis (n = 1,772,299).
Disclosures: The study received no specific funding. Dr. Song reported having no financial disclosures.
Source: Song S et al. ISC 2018, Abstract TMP75.
On-label stent use looks safe in intracranial atherosclerotic disease
LOS ANGELES – A postmarketing study of the Wingspan stent shows that the safety of the device in the treatment of intracranial atherosclerotic disease (ICAD) is good enough to be a reasonable alternative to medical management in these patients, but only if the device is used on label.
The results may reassure some interventionalists who were alarmed by results from the Stenting versus Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) trial (N Engl J Med. 2011;365:993-1003), which showed a 30-day rate of stroke or death of 14.7%. The new study showed a frequency of 2.6%, so long as the device was used on label. Off-label use yielded a frequency of 23.9%.
Up to 10% of strokes in the United States result from ICAD, and in China the rate is an estimated 20%-46%. The condition can also be treated medically. Early trials of the Wingspan device showed initial success with complication rates of 4.5%-6.2%, but the SAMMPRIS trial, which directly compared stenting to aggressive medical management, showed superior outcomes with medical treatment. The 30-day rate of stroke or death of 14.7% was too high to compete with medical therapy, which included aspirin 325 mg per day, clopidogrel 75 mg per day for 90 days after enrollment, and management of primary and secondary risk factors.
Dr. Alexander believes that the SAMMPRIS trial did not employ favorable patient selection. “ICAD is variable. Some patients present with hemodynamic compromise, where stenting is probably beneficial. Some present with embolic stroke, and some present with small-vessel perforator strokes that are unlikely to be responsive to stenting and better treated with medical therapy. All these patients were grouped together” in SAMMPRIS, said Dr. Alexander, who is director of the Neurovascular Center and endovascular surgery at Cedars-Sinai in Los Angeles.
The SAMMPRIS findings put a damper on stenting, and use of the procedure has dropped at many U.S. hospitals. But studies conducted prior to SAMMPRIS had shown much lower periprocedural morbidity, and those studies looked at on-label use of stenting. SAMMPRIS was off label.
Now the WEAVE study, which was an Food and Drug Administration–mandated, postmarketing surveillance study of Stryker’s Wingspan stent, suggests that the off-label use of the system in the SAMMPRIS trial may explain the poor results. SAMMPRIS had attempted to extend the approved boundaries of stenting by treating patients who presented with transient ischemic attacks only, patients who had failed medical therapy, and patients who had experienced a stroke in the past 7 days. In fact, about half of the patients were treated within 7 days of the previous event, sometimes within 24 hours.
Previous studies had shown that risk factors for poor outcomes included stenting within 10 days of the last event, stenting a posterior circulation target lesion, stenting presentations other than stroke, and sites with a low patient volume for stenting. Patient selection is vital to success, according to Dr. Alexander. Patients with hemodynamic compromise are good candidates for stenting, while those with perforator stroke alone are better off with medical therapy. Embolic stroke patients are candidates for either approach.
WEAVE looked at 152 consecutive patients treated on label at 24 institutions. The primary analysis group consisted of patients with a 70%-99% stenotic intracranial atherosclerotic lesion who were refractory to medical treatment, 22-80 years of age, and had a modified Rankin Scale (mRS) score of 3 or less at baseline. The treatment was performed at least 7 days after the last stroke. Finally, patients had to have experienced two or more strokes. This last requirement presented a problem for recruitment, according to Dr. Alexander. “That was never a criterion for any of the [previous] trials, so it’s not clear why FDA added that. That made it very difficult to enroll for this trial – to have patients who had two or more strokes and still had a functional mRS score,” he said.
The study protocol aimed for a frequency of 6.6% for periprocedural morbidity, defined as a stroke or death within 72 hours.
An interim analysis at 100 patients showed that the periprocedural morbidity frequency was below 4%, which met the agency’s requirement and allowed the trial to be halted once the trial enrolled 150 on-label patients. The total number of on-label patients reached 152, and the researchers analyzed the results from another 46 patients who were treated with stenting despite not meeting the study’s inclusion criteria, and these patients were considered to be off-label use. The final analysis showed that the on-label group had a periprocedural morbidity of 2.6%, compared with 23.9% in the off-label group (P = .0001).
The most glaring difference in the patient populations was that half of the off-label group received the stent within 7 days of experiencing a stroke. What might be the reason for worse outcomes when stenting is performed within 7 days? “There’s speculation that the plaques might be hot, and those patients might have a higher thrombotic risk with putting a foreign body in the vessel, or there’s capillary instability, so reperfusing a vessel that has a 99% stenosis has a higher risk for reperfusion hemorrhage,” Dr. Alexander said.
Experience may also be a factor. Interventionalists participating in the WEAVE trial had performed a stent using Wingspan an average of 37 times before the study began, compared with a mean of 10 cases for physicians in the SAMMPRIS trial. Those who had performed over 50 procedures had no periprocedural morbidity outcomes at all.
The study was funded by Stryker Neurovascular. Dr. Alexander has consulted for Stryker.
SOURCE: Alexander M et al. ISC 2018 Abstract LB13.
LOS ANGELES – A postmarketing study of the Wingspan stent shows that the safety of the device in the treatment of intracranial atherosclerotic disease (ICAD) is good enough to be a reasonable alternative to medical management in these patients, but only if the device is used on label.
The results may reassure some interventionalists who were alarmed by results from the Stenting versus Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) trial (N Engl J Med. 2011;365:993-1003), which showed a 30-day rate of stroke or death of 14.7%. The new study showed a frequency of 2.6%, so long as the device was used on label. Off-label use yielded a frequency of 23.9%.
Up to 10% of strokes in the United States result from ICAD, and in China the rate is an estimated 20%-46%. The condition can also be treated medically. Early trials of the Wingspan device showed initial success with complication rates of 4.5%-6.2%, but the SAMMPRIS trial, which directly compared stenting to aggressive medical management, showed superior outcomes with medical treatment. The 30-day rate of stroke or death of 14.7% was too high to compete with medical therapy, which included aspirin 325 mg per day, clopidogrel 75 mg per day for 90 days after enrollment, and management of primary and secondary risk factors.
Dr. Alexander believes that the SAMMPRIS trial did not employ favorable patient selection. “ICAD is variable. Some patients present with hemodynamic compromise, where stenting is probably beneficial. Some present with embolic stroke, and some present with small-vessel perforator strokes that are unlikely to be responsive to stenting and better treated with medical therapy. All these patients were grouped together” in SAMMPRIS, said Dr. Alexander, who is director of the Neurovascular Center and endovascular surgery at Cedars-Sinai in Los Angeles.
The SAMMPRIS findings put a damper on stenting, and use of the procedure has dropped at many U.S. hospitals. But studies conducted prior to SAMMPRIS had shown much lower periprocedural morbidity, and those studies looked at on-label use of stenting. SAMMPRIS was off label.
Now the WEAVE study, which was an Food and Drug Administration–mandated, postmarketing surveillance study of Stryker’s Wingspan stent, suggests that the off-label use of the system in the SAMMPRIS trial may explain the poor results. SAMMPRIS had attempted to extend the approved boundaries of stenting by treating patients who presented with transient ischemic attacks only, patients who had failed medical therapy, and patients who had experienced a stroke in the past 7 days. In fact, about half of the patients were treated within 7 days of the previous event, sometimes within 24 hours.
Previous studies had shown that risk factors for poor outcomes included stenting within 10 days of the last event, stenting a posterior circulation target lesion, stenting presentations other than stroke, and sites with a low patient volume for stenting. Patient selection is vital to success, according to Dr. Alexander. Patients with hemodynamic compromise are good candidates for stenting, while those with perforator stroke alone are better off with medical therapy. Embolic stroke patients are candidates for either approach.
WEAVE looked at 152 consecutive patients treated on label at 24 institutions. The primary analysis group consisted of patients with a 70%-99% stenotic intracranial atherosclerotic lesion who were refractory to medical treatment, 22-80 years of age, and had a modified Rankin Scale (mRS) score of 3 or less at baseline. The treatment was performed at least 7 days after the last stroke. Finally, patients had to have experienced two or more strokes. This last requirement presented a problem for recruitment, according to Dr. Alexander. “That was never a criterion for any of the [previous] trials, so it’s not clear why FDA added that. That made it very difficult to enroll for this trial – to have patients who had two or more strokes and still had a functional mRS score,” he said.
The study protocol aimed for a frequency of 6.6% for periprocedural morbidity, defined as a stroke or death within 72 hours.
An interim analysis at 100 patients showed that the periprocedural morbidity frequency was below 4%, which met the agency’s requirement and allowed the trial to be halted once the trial enrolled 150 on-label patients. The total number of on-label patients reached 152, and the researchers analyzed the results from another 46 patients who were treated with stenting despite not meeting the study’s inclusion criteria, and these patients were considered to be off-label use. The final analysis showed that the on-label group had a periprocedural morbidity of 2.6%, compared with 23.9% in the off-label group (P = .0001).
The most glaring difference in the patient populations was that half of the off-label group received the stent within 7 days of experiencing a stroke. What might be the reason for worse outcomes when stenting is performed within 7 days? “There’s speculation that the plaques might be hot, and those patients might have a higher thrombotic risk with putting a foreign body in the vessel, or there’s capillary instability, so reperfusing a vessel that has a 99% stenosis has a higher risk for reperfusion hemorrhage,” Dr. Alexander said.
Experience may also be a factor. Interventionalists participating in the WEAVE trial had performed a stent using Wingspan an average of 37 times before the study began, compared with a mean of 10 cases for physicians in the SAMMPRIS trial. Those who had performed over 50 procedures had no periprocedural morbidity outcomes at all.
The study was funded by Stryker Neurovascular. Dr. Alexander has consulted for Stryker.
SOURCE: Alexander M et al. ISC 2018 Abstract LB13.
LOS ANGELES – A postmarketing study of the Wingspan stent shows that the safety of the device in the treatment of intracranial atherosclerotic disease (ICAD) is good enough to be a reasonable alternative to medical management in these patients, but only if the device is used on label.
The results may reassure some interventionalists who were alarmed by results from the Stenting versus Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) trial (N Engl J Med. 2011;365:993-1003), which showed a 30-day rate of stroke or death of 14.7%. The new study showed a frequency of 2.6%, so long as the device was used on label. Off-label use yielded a frequency of 23.9%.
Up to 10% of strokes in the United States result from ICAD, and in China the rate is an estimated 20%-46%. The condition can also be treated medically. Early trials of the Wingspan device showed initial success with complication rates of 4.5%-6.2%, but the SAMMPRIS trial, which directly compared stenting to aggressive medical management, showed superior outcomes with medical treatment. The 30-day rate of stroke or death of 14.7% was too high to compete with medical therapy, which included aspirin 325 mg per day, clopidogrel 75 mg per day for 90 days after enrollment, and management of primary and secondary risk factors.
Dr. Alexander believes that the SAMMPRIS trial did not employ favorable patient selection. “ICAD is variable. Some patients present with hemodynamic compromise, where stenting is probably beneficial. Some present with embolic stroke, and some present with small-vessel perforator strokes that are unlikely to be responsive to stenting and better treated with medical therapy. All these patients were grouped together” in SAMMPRIS, said Dr. Alexander, who is director of the Neurovascular Center and endovascular surgery at Cedars-Sinai in Los Angeles.
The SAMMPRIS findings put a damper on stenting, and use of the procedure has dropped at many U.S. hospitals. But studies conducted prior to SAMMPRIS had shown much lower periprocedural morbidity, and those studies looked at on-label use of stenting. SAMMPRIS was off label.
Now the WEAVE study, which was an Food and Drug Administration–mandated, postmarketing surveillance study of Stryker’s Wingspan stent, suggests that the off-label use of the system in the SAMMPRIS trial may explain the poor results. SAMMPRIS had attempted to extend the approved boundaries of stenting by treating patients who presented with transient ischemic attacks only, patients who had failed medical therapy, and patients who had experienced a stroke in the past 7 days. In fact, about half of the patients were treated within 7 days of the previous event, sometimes within 24 hours.
Previous studies had shown that risk factors for poor outcomes included stenting within 10 days of the last event, stenting a posterior circulation target lesion, stenting presentations other than stroke, and sites with a low patient volume for stenting. Patient selection is vital to success, according to Dr. Alexander. Patients with hemodynamic compromise are good candidates for stenting, while those with perforator stroke alone are better off with medical therapy. Embolic stroke patients are candidates for either approach.
WEAVE looked at 152 consecutive patients treated on label at 24 institutions. The primary analysis group consisted of patients with a 70%-99% stenotic intracranial atherosclerotic lesion who were refractory to medical treatment, 22-80 years of age, and had a modified Rankin Scale (mRS) score of 3 or less at baseline. The treatment was performed at least 7 days after the last stroke. Finally, patients had to have experienced two or more strokes. This last requirement presented a problem for recruitment, according to Dr. Alexander. “That was never a criterion for any of the [previous] trials, so it’s not clear why FDA added that. That made it very difficult to enroll for this trial – to have patients who had two or more strokes and still had a functional mRS score,” he said.
The study protocol aimed for a frequency of 6.6% for periprocedural morbidity, defined as a stroke or death within 72 hours.
An interim analysis at 100 patients showed that the periprocedural morbidity frequency was below 4%, which met the agency’s requirement and allowed the trial to be halted once the trial enrolled 150 on-label patients. The total number of on-label patients reached 152, and the researchers analyzed the results from another 46 patients who were treated with stenting despite not meeting the study’s inclusion criteria, and these patients were considered to be off-label use. The final analysis showed that the on-label group had a periprocedural morbidity of 2.6%, compared with 23.9% in the off-label group (P = .0001).
The most glaring difference in the patient populations was that half of the off-label group received the stent within 7 days of experiencing a stroke. What might be the reason for worse outcomes when stenting is performed within 7 days? “There’s speculation that the plaques might be hot, and those patients might have a higher thrombotic risk with putting a foreign body in the vessel, or there’s capillary instability, so reperfusing a vessel that has a 99% stenosis has a higher risk for reperfusion hemorrhage,” Dr. Alexander said.
Experience may also be a factor. Interventionalists participating in the WEAVE trial had performed a stent using Wingspan an average of 37 times before the study began, compared with a mean of 10 cases for physicians in the SAMMPRIS trial. Those who had performed over 50 procedures had no periprocedural morbidity outcomes at all.
The study was funded by Stryker Neurovascular. Dr. Alexander has consulted for Stryker.
SOURCE: Alexander M et al. ISC 2018 Abstract LB13.
REPORTING FROM ISC 2018
Key clinical point:
Major finding: On-label 72-hour death and stroke rate was 2.6%, compared with 23.9% off label.
Data source: Postmarketing analysis of 198 consecutive patients.
Disclosures: The study was funded by Stryker Neurovascular. Dr. Alexander has consulted for Stryker.
Source: Alexander M et al. ISC 2018 Abstract LB13.
Aspirin blunts early stroke risk from preeclampsia
LOS ANGELES – Women with a history of preeclampsia have a significantly increased risk for an early-onset stroke, but that risk is blunted in women taking aspirin, an epidemiologic analysis of data from more than 83,000 women in the California Teachers Study showed.
Among the 4,072 women in the study with a history of preeclampsia, 3,003 were not on aspirin, and during follow-up they had a greater than 1% incidence of stroke – ischemic and hemorrhagic combined – before turning 60 years of age. Their incidence rate was 40% higher than in the roughly 60,000 women without a preeclampsia history who were not taking aspirin, a statistically significant difference after adjustment for demographics, smoking, obesity, diabetes, and hypertension, Eliza C. Miller, MD, said at the International Stroke Conference, sponsored by the American Heart Association.
Her analysis used data drawn from more than 133,000 women enrolled starting in 1995 in the California Teachers Study. She focused on 83,790 women who entered the study when they were younger than 60 years old, who had no history of stroke, and who provided data on their history of preeclampsia. The prevalence of a preeclampsia history was 4.9% overall, and 6.1% among women who had been pregnant at least once, an incidence rate similar to what has been found in other large populations of women, Dr. Miller said.
The average age of the women with preeclampsia was 44, and 46 for those without preeclampsia. The women with a history of preeclampsia also had a higher prevalence rate of obesity, hypertension, diabetes, and chronic kidney disease. Roughly a quarter of all women were regularly taking aspirin.
After adjustment of the data for demographic and clinical differences, women with a history of preeclampsia had a 20% higher overall rate of a subsequent stroke before reaching age 60 years, but this difference was not significant in an analysis that included both women taking aspirin and those not on the drug. When the analysis focused only on the women not on aspirin, the increased stroke rate linked with a preeclampsia history rose to 40% higher than in women without a preeclampsia history, a statistically significant difference. In contrast, among the quarter of women on aspirin, the two subgroups – with a preeclampsia history and without – had similar rates of incident strokes.
SOURCE: Miller E et al. International Stroke Conference 2018, A174 (Stroke. 2018 Jan;49[Suppl1]:A174).
LOS ANGELES – Women with a history of preeclampsia have a significantly increased risk for an early-onset stroke, but that risk is blunted in women taking aspirin, an epidemiologic analysis of data from more than 83,000 women in the California Teachers Study showed.
Among the 4,072 women in the study with a history of preeclampsia, 3,003 were not on aspirin, and during follow-up they had a greater than 1% incidence of stroke – ischemic and hemorrhagic combined – before turning 60 years of age. Their incidence rate was 40% higher than in the roughly 60,000 women without a preeclampsia history who were not taking aspirin, a statistically significant difference after adjustment for demographics, smoking, obesity, diabetes, and hypertension, Eliza C. Miller, MD, said at the International Stroke Conference, sponsored by the American Heart Association.
Her analysis used data drawn from more than 133,000 women enrolled starting in 1995 in the California Teachers Study. She focused on 83,790 women who entered the study when they were younger than 60 years old, who had no history of stroke, and who provided data on their history of preeclampsia. The prevalence of a preeclampsia history was 4.9% overall, and 6.1% among women who had been pregnant at least once, an incidence rate similar to what has been found in other large populations of women, Dr. Miller said.
The average age of the women with preeclampsia was 44, and 46 for those without preeclampsia. The women with a history of preeclampsia also had a higher prevalence rate of obesity, hypertension, diabetes, and chronic kidney disease. Roughly a quarter of all women were regularly taking aspirin.
After adjustment of the data for demographic and clinical differences, women with a history of preeclampsia had a 20% higher overall rate of a subsequent stroke before reaching age 60 years, but this difference was not significant in an analysis that included both women taking aspirin and those not on the drug. When the analysis focused only on the women not on aspirin, the increased stroke rate linked with a preeclampsia history rose to 40% higher than in women without a preeclampsia history, a statistically significant difference. In contrast, among the quarter of women on aspirin, the two subgroups – with a preeclampsia history and without – had similar rates of incident strokes.
SOURCE: Miller E et al. International Stroke Conference 2018, A174 (Stroke. 2018 Jan;49[Suppl1]:A174).
LOS ANGELES – Women with a history of preeclampsia have a significantly increased risk for an early-onset stroke, but that risk is blunted in women taking aspirin, an epidemiologic analysis of data from more than 83,000 women in the California Teachers Study showed.
Among the 4,072 women in the study with a history of preeclampsia, 3,003 were not on aspirin, and during follow-up they had a greater than 1% incidence of stroke – ischemic and hemorrhagic combined – before turning 60 years of age. Their incidence rate was 40% higher than in the roughly 60,000 women without a preeclampsia history who were not taking aspirin, a statistically significant difference after adjustment for demographics, smoking, obesity, diabetes, and hypertension, Eliza C. Miller, MD, said at the International Stroke Conference, sponsored by the American Heart Association.
Her analysis used data drawn from more than 133,000 women enrolled starting in 1995 in the California Teachers Study. She focused on 83,790 women who entered the study when they were younger than 60 years old, who had no history of stroke, and who provided data on their history of preeclampsia. The prevalence of a preeclampsia history was 4.9% overall, and 6.1% among women who had been pregnant at least once, an incidence rate similar to what has been found in other large populations of women, Dr. Miller said.
The average age of the women with preeclampsia was 44, and 46 for those without preeclampsia. The women with a history of preeclampsia also had a higher prevalence rate of obesity, hypertension, diabetes, and chronic kidney disease. Roughly a quarter of all women were regularly taking aspirin.
After adjustment of the data for demographic and clinical differences, women with a history of preeclampsia had a 20% higher overall rate of a subsequent stroke before reaching age 60 years, but this difference was not significant in an analysis that included both women taking aspirin and those not on the drug. When the analysis focused only on the women not on aspirin, the increased stroke rate linked with a preeclampsia history rose to 40% higher than in women without a preeclampsia history, a statistically significant difference. In contrast, among the quarter of women on aspirin, the two subgroups – with a preeclampsia history and without – had similar rates of incident strokes.
SOURCE: Miller E et al. International Stroke Conference 2018, A174 (Stroke. 2018 Jan;49[Suppl1]:A174).
REPORTING FROM ISC 2018
Key clinical point: Aspirin use dampened the rise in early-onset strokes seen after preeclampsia.
Major finding: Study details: A review of data collected from 83,790 women enrolled in the California Teachers Study.
Disclosures: Dr. Miller had no relevant financial disclosures.
Source: Miller E et al. International Stroke Conference 2018, A174 (Stroke. 2018 Jan;49[Suppl 1]:A174).
Imaging methods for stroke thrombectomy eligibility yield similar results
LOS ANGELES – The benefits of mechanical thrombectomy observed in the DAWN trial for patients with acute ischemic stroke and a mismatch between core imaging and clinical presentation out to 24 hours appear to apply regardless of whether their eligibility is determined by CT perfusion or diffusion-weighted magnetic resonance imaging, according to a subanalysis of the trial data.
Diffusion-weighted magnetic resonance imaging (DW-MRI) is considered the gold standard, but it is not as widely available as CT perfusion (CTP) and previous studies have shown that MR is associated with longer times between stroke onset and treatment randomization. “Though MR was originally preferred in DAWN, it was pretty clear that CT perfusion was going to need to be employed in the trial as well,” Cathy Sila, MD, said during her presentation of the results of the subanalysis at the International Stroke Conference 2018, sponsored by the American Heart Association.
The research sought to determine if the two imaging methods perform similarly. CTP is more readily available, but it has some issues. In patients with severe heart failure, a severe proximal stenosis, or a contralateral severe stenosis, the technique may struggle to accurately image the core infarct, which has led some to wonder if the outcomes would be as good using CTP as selection criteria. “In our institution, we’ve had this conversation very frequently,” said Dr. Sila, who is a vascular neurologist and the director of the University Hospitals Systems stroke program in Cleveland.
To be eligible for DAWN, the core infarct had to correspond to at least a 30% decrease in regional blood flow in the CTP map, or an apparent diffusion coefficient of less than 620 on DW-MRI.
The researchers included all 206 patients in the DAWN study (N Engl J Med. 2018;378:11-21), separating them into DW-MRI or CTP groups based on which imaging method was used to randomize them during the trial. There were no statistically significant differences in any of the baseline characteristics between the two imaging groups.
The 26 sites participating in DAWN had clear differences in their preferences for imaging techniques; 19 exclusively used CTP, 4 used only DW-MRI, and 3 sites used a combination of both imaging methods.
There were no statistically significant differences between the two groups in any of the measured clinical outcomes, including neurologic deterioration in hospital (22.8% with CTP vs. 15.7% with DW-MRII, P = .286), symptomatic intracranial hemorrhage (4.1% with CTP vs. 4.8% with DW-MRI, P = 1.000), or death related to stroke (19.5% with CTP vs. 13.3% with DW-MRI, P = .263). Outcomes at 90 days proved to be similar between CTP and DW-MRI for achieving functional independence (29.3% vs. 34.9%, respectively; P = .445) and utility-weighted modified Rankin Scale scores (4.2 vs. 4.9, respectively; P = .172).
Multivariate analyses showed that 90-day functional independence was predicted by thrombectomy treatment, age, blood glucose level, baseline National Institutes of Health Stroke Scale score, and core lab ASPECTS (Alberta Stroke Program Early CT Score), but not the method of imaging.
“The efficacy and safety of mechanical thrombectomy for patients meeting those clinical mismatch criteria at 6-24 hours were comparable whether the small core infarcts were measured by diffusion imaging or cerebral blood flow imaging. I believe that future clinical trials aiming to extend the eligibility outside of this prespecified population should include both imaging modalities to determine whether these results are generalizable,” Dr. Sila said.
The DAWN study was funded by Stryker Neurovascular. Dr. Sila has reported receiving honoraria from Medtronic.
SOURCE: Sila C et al. ISC 2018, abstract LB11.
LOS ANGELES – The benefits of mechanical thrombectomy observed in the DAWN trial for patients with acute ischemic stroke and a mismatch between core imaging and clinical presentation out to 24 hours appear to apply regardless of whether their eligibility is determined by CT perfusion or diffusion-weighted magnetic resonance imaging, according to a subanalysis of the trial data.
Diffusion-weighted magnetic resonance imaging (DW-MRI) is considered the gold standard, but it is not as widely available as CT perfusion (CTP) and previous studies have shown that MR is associated with longer times between stroke onset and treatment randomization. “Though MR was originally preferred in DAWN, it was pretty clear that CT perfusion was going to need to be employed in the trial as well,” Cathy Sila, MD, said during her presentation of the results of the subanalysis at the International Stroke Conference 2018, sponsored by the American Heart Association.
The research sought to determine if the two imaging methods perform similarly. CTP is more readily available, but it has some issues. In patients with severe heart failure, a severe proximal stenosis, or a contralateral severe stenosis, the technique may struggle to accurately image the core infarct, which has led some to wonder if the outcomes would be as good using CTP as selection criteria. “In our institution, we’ve had this conversation very frequently,” said Dr. Sila, who is a vascular neurologist and the director of the University Hospitals Systems stroke program in Cleveland.
To be eligible for DAWN, the core infarct had to correspond to at least a 30% decrease in regional blood flow in the CTP map, or an apparent diffusion coefficient of less than 620 on DW-MRI.
The researchers included all 206 patients in the DAWN study (N Engl J Med. 2018;378:11-21), separating them into DW-MRI or CTP groups based on which imaging method was used to randomize them during the trial. There were no statistically significant differences in any of the baseline characteristics between the two imaging groups.
The 26 sites participating in DAWN had clear differences in their preferences for imaging techniques; 19 exclusively used CTP, 4 used only DW-MRI, and 3 sites used a combination of both imaging methods.
There were no statistically significant differences between the two groups in any of the measured clinical outcomes, including neurologic deterioration in hospital (22.8% with CTP vs. 15.7% with DW-MRII, P = .286), symptomatic intracranial hemorrhage (4.1% with CTP vs. 4.8% with DW-MRI, P = 1.000), or death related to stroke (19.5% with CTP vs. 13.3% with DW-MRI, P = .263). Outcomes at 90 days proved to be similar between CTP and DW-MRI for achieving functional independence (29.3% vs. 34.9%, respectively; P = .445) and utility-weighted modified Rankin Scale scores (4.2 vs. 4.9, respectively; P = .172).
Multivariate analyses showed that 90-day functional independence was predicted by thrombectomy treatment, age, blood glucose level, baseline National Institutes of Health Stroke Scale score, and core lab ASPECTS (Alberta Stroke Program Early CT Score), but not the method of imaging.
“The efficacy and safety of mechanical thrombectomy for patients meeting those clinical mismatch criteria at 6-24 hours were comparable whether the small core infarcts were measured by diffusion imaging or cerebral blood flow imaging. I believe that future clinical trials aiming to extend the eligibility outside of this prespecified population should include both imaging modalities to determine whether these results are generalizable,” Dr. Sila said.
The DAWN study was funded by Stryker Neurovascular. Dr. Sila has reported receiving honoraria from Medtronic.
SOURCE: Sila C et al. ISC 2018, abstract LB11.
LOS ANGELES – The benefits of mechanical thrombectomy observed in the DAWN trial for patients with acute ischemic stroke and a mismatch between core imaging and clinical presentation out to 24 hours appear to apply regardless of whether their eligibility is determined by CT perfusion or diffusion-weighted magnetic resonance imaging, according to a subanalysis of the trial data.
Diffusion-weighted magnetic resonance imaging (DW-MRI) is considered the gold standard, but it is not as widely available as CT perfusion (CTP) and previous studies have shown that MR is associated with longer times between stroke onset and treatment randomization. “Though MR was originally preferred in DAWN, it was pretty clear that CT perfusion was going to need to be employed in the trial as well,” Cathy Sila, MD, said during her presentation of the results of the subanalysis at the International Stroke Conference 2018, sponsored by the American Heart Association.
The research sought to determine if the two imaging methods perform similarly. CTP is more readily available, but it has some issues. In patients with severe heart failure, a severe proximal stenosis, or a contralateral severe stenosis, the technique may struggle to accurately image the core infarct, which has led some to wonder if the outcomes would be as good using CTP as selection criteria. “In our institution, we’ve had this conversation very frequently,” said Dr. Sila, who is a vascular neurologist and the director of the University Hospitals Systems stroke program in Cleveland.
To be eligible for DAWN, the core infarct had to correspond to at least a 30% decrease in regional blood flow in the CTP map, or an apparent diffusion coefficient of less than 620 on DW-MRI.
The researchers included all 206 patients in the DAWN study (N Engl J Med. 2018;378:11-21), separating them into DW-MRI or CTP groups based on which imaging method was used to randomize them during the trial. There were no statistically significant differences in any of the baseline characteristics between the two imaging groups.
The 26 sites participating in DAWN had clear differences in their preferences for imaging techniques; 19 exclusively used CTP, 4 used only DW-MRI, and 3 sites used a combination of both imaging methods.
There were no statistically significant differences between the two groups in any of the measured clinical outcomes, including neurologic deterioration in hospital (22.8% with CTP vs. 15.7% with DW-MRII, P = .286), symptomatic intracranial hemorrhage (4.1% with CTP vs. 4.8% with DW-MRI, P = 1.000), or death related to stroke (19.5% with CTP vs. 13.3% with DW-MRI, P = .263). Outcomes at 90 days proved to be similar between CTP and DW-MRI for achieving functional independence (29.3% vs. 34.9%, respectively; P = .445) and utility-weighted modified Rankin Scale scores (4.2 vs. 4.9, respectively; P = .172).
Multivariate analyses showed that 90-day functional independence was predicted by thrombectomy treatment, age, blood glucose level, baseline National Institutes of Health Stroke Scale score, and core lab ASPECTS (Alberta Stroke Program Early CT Score), but not the method of imaging.
“The efficacy and safety of mechanical thrombectomy for patients meeting those clinical mismatch criteria at 6-24 hours were comparable whether the small core infarcts were measured by diffusion imaging or cerebral blood flow imaging. I believe that future clinical trials aiming to extend the eligibility outside of this prespecified population should include both imaging modalities to determine whether these results are generalizable,” Dr. Sila said.
The DAWN study was funded by Stryker Neurovascular. Dr. Sila has reported receiving honoraria from Medtronic.
SOURCE: Sila C et al. ISC 2018, abstract LB11.
REPORTING FROM ISC 2018
Key clinical point: DW-MRI is the gold standard for imaging, but CTP is more widely available.
Major finding: Rates of neurologic deterioration in hospital, symptomatic intracranial hemorrhage, and death related to stroke were similar regardless of whether CT or MR imaging was used to assess patients’ infarcts.
Data source: A subanalysis of the DAWN randomized, controlled trial (n = 206).
Disclosures: The DAWN study was funded by Stryker Neurovascular. Dr. Sila reported receiving honoraria from Medtronic.
Source: Sila C et al. ISC 2018, abstract LB11.
VIDEO: Rivaroxaban plus aspirin halves ischemic strokes
LOS ANGELES – Combined treatment with a low dosage of the anticoagulant rivaroxaban plus aspirin cut the incidence of ischemic strokes nearly in half, compared with aspirin alone, in a multicenter, randomized trial of more than 27,000 patients with stable atherosclerotic vascular disease.
This dramatic reduction in ischemic strokes as well as in all-cause strokes by adding low-dose rivaroxaban(Xarelto) occurred without any significant increase in hemorrhagic strokes but with a small increase in total major bleeding events, such as gastrointestinal bleeds, Mike Sharma, MD, said at the International Stroke Conference, sponsored by the American Heart Association.
“There was a consistent effect across all strata of stroke risk. For patients who had a prior stroke, it’s pretty clear to use rivaroxaban plus aspirin because it had a big benefit” with no increase in intracranial hemorrhages, Dr. Sharma said in a video interview.
“We think these results will fundamentally change how we approach stroke prevention,” added Dr. Sharma, a stroke neurologist in the Population Health Research Institute of McMaster University in Hamilton, Ont.
The results he reported came from a secondary analysis of data collected in the COMPASS (Rivaroxaban for the Prevention of Major Cardiovascular Events in Coronary or Peripheral Artery Disease) trial, which enrolled 27,395 patients with stable coronary or peripheral artery disease at 602 centers in 33 countries.
The primary outcome of the trial, reported in 2017, was the combined rate of cardiovascular death, MI, or stroke during an average 23 months of follow-up, which occurred in 4.1% of patients treated with 2.5 mg rivaroxaban twice daily plus 100 mg aspirin once daily, 4.9% of patients who received 5.0 mg rivaroxaban twice daily, and 5.4% in patients who received 100 mg aspirin daily, a statistically significant 24% relative risk reduction in the combined treatment group, compared with aspirin only. The rivaroxaban only–treated patients did not significantly differ from the control patients who received only aspirin (N Engl J Med. 2017 Oct 5;377[14]:1319-30). The main results showed a 1.2% increase in the rate of major bleeds in patients treated with rivaroxaban plus aspirin, compared with aspirin only, but the rate of nonfatal symptomatic intracranial hemorrhages was identical in the two treatment groups.
The new results Dr. Sharma reported at the conference focused on various measures of stroke. The rate of all strokes was 42% lower among the patients treated with rivaroxaban plus aspirin, compared with the aspirin alone patients, and ischemic strokes were 49% lower with the dual therapy, compared with aspirin only. Both differences were statistically significant. In contrast, the rivaroxaban alone regimen did not significantly reduce all-cause strokes. It did significantly reduce ischemic strokes, compared with aspirin only, but it also significantly increased hemorrhagic strokes, compared with aspirin only, an adverse effect not caused by the combination of low-dose rivaroxaban plus aspirin.
Rivaroxaban plus aspirin surpassed aspirin alone for preventing both mild and severe strokes and for preventing strokes both in patients with a history of a prior stroke and in those who never had a prior stroke. The stroke reduction produced by rivaroxaban plus aspirin was greatest in the highest risk patients – those with a prior stroke. On the combined regimen, these patients had an average stroke incidence of 0.7% per year, compared with an annual 3.4% rate among the patients on aspirin only, a 2.7% absolute reduction by using rivaroxaban plus aspirin that translated into a number needed to treat of 37 patients with a history of stroke to prevent one new stroke per year.
The 2017 report of the main COMPASS results included a net clinical benefit analysis that factored together the primary endpoint events and major bleeding events. The net rate of all these events was 4.7% with rivaroxaban plus aspirin and 5.9% with aspirin only, a statistically significant 20% relative risk reduction for all adverse outcomes with dual therapy. This net clinical benefit suggests that adding rivaroxaban has a cost-effective benefit. Assessment of rivaroxaban’s cost benefit in COMPASS is in process, Dr. Sharma said.
Rivaroxaban received Food and Drug Administration marketing approval in 2011 for preventing deep vein thrombosis and preventing stroke in patients with atrial fibrillation at dosages higher than what was used in COMPASS. The approved rivaroxaban dosage for preventing deep vein thrombosis is 10 mg/day, and 20 mg/day for preventing stroke in atrial fibrillation patients. The 2.5-mg formulation of rivaroxaban that was given twice daily had the best safety and efficacy in COMPASS, but it is not available now on the U.S. market, although it is available in Europe. Johnson & Johnson, which markets rivaroxaban globally with Bayer, submitted an application to the FDA in December for marketing approval of the 2.5-mg formulation in twice-daily dosing for use as in the COMPASS trial.
COMPASS was sponsored by Bayer, the company that markets rivaroxaban in collaboration with Johnson & Johnson. Dr. Sharma has been a consultant or adviser to Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, and Daiichi-Sankyo.
SOURCE: Sharma M et al. ISC 2018, Abstract LB7.
LOS ANGELES – Combined treatment with a low dosage of the anticoagulant rivaroxaban plus aspirin cut the incidence of ischemic strokes nearly in half, compared with aspirin alone, in a multicenter, randomized trial of more than 27,000 patients with stable atherosclerotic vascular disease.
This dramatic reduction in ischemic strokes as well as in all-cause strokes by adding low-dose rivaroxaban(Xarelto) occurred without any significant increase in hemorrhagic strokes but with a small increase in total major bleeding events, such as gastrointestinal bleeds, Mike Sharma, MD, said at the International Stroke Conference, sponsored by the American Heart Association.
“There was a consistent effect across all strata of stroke risk. For patients who had a prior stroke, it’s pretty clear to use rivaroxaban plus aspirin because it had a big benefit” with no increase in intracranial hemorrhages, Dr. Sharma said in a video interview.
“We think these results will fundamentally change how we approach stroke prevention,” added Dr. Sharma, a stroke neurologist in the Population Health Research Institute of McMaster University in Hamilton, Ont.
The results he reported came from a secondary analysis of data collected in the COMPASS (Rivaroxaban for the Prevention of Major Cardiovascular Events in Coronary or Peripheral Artery Disease) trial, which enrolled 27,395 patients with stable coronary or peripheral artery disease at 602 centers in 33 countries.
The primary outcome of the trial, reported in 2017, was the combined rate of cardiovascular death, MI, or stroke during an average 23 months of follow-up, which occurred in 4.1% of patients treated with 2.5 mg rivaroxaban twice daily plus 100 mg aspirin once daily, 4.9% of patients who received 5.0 mg rivaroxaban twice daily, and 5.4% in patients who received 100 mg aspirin daily, a statistically significant 24% relative risk reduction in the combined treatment group, compared with aspirin only. The rivaroxaban only–treated patients did not significantly differ from the control patients who received only aspirin (N Engl J Med. 2017 Oct 5;377[14]:1319-30). The main results showed a 1.2% increase in the rate of major bleeds in patients treated with rivaroxaban plus aspirin, compared with aspirin only, but the rate of nonfatal symptomatic intracranial hemorrhages was identical in the two treatment groups.
The new results Dr. Sharma reported at the conference focused on various measures of stroke. The rate of all strokes was 42% lower among the patients treated with rivaroxaban plus aspirin, compared with the aspirin alone patients, and ischemic strokes were 49% lower with the dual therapy, compared with aspirin only. Both differences were statistically significant. In contrast, the rivaroxaban alone regimen did not significantly reduce all-cause strokes. It did significantly reduce ischemic strokes, compared with aspirin only, but it also significantly increased hemorrhagic strokes, compared with aspirin only, an adverse effect not caused by the combination of low-dose rivaroxaban plus aspirin.
Rivaroxaban plus aspirin surpassed aspirin alone for preventing both mild and severe strokes and for preventing strokes both in patients with a history of a prior stroke and in those who never had a prior stroke. The stroke reduction produced by rivaroxaban plus aspirin was greatest in the highest risk patients – those with a prior stroke. On the combined regimen, these patients had an average stroke incidence of 0.7% per year, compared with an annual 3.4% rate among the patients on aspirin only, a 2.7% absolute reduction by using rivaroxaban plus aspirin that translated into a number needed to treat of 37 patients with a history of stroke to prevent one new stroke per year.
The 2017 report of the main COMPASS results included a net clinical benefit analysis that factored together the primary endpoint events and major bleeding events. The net rate of all these events was 4.7% with rivaroxaban plus aspirin and 5.9% with aspirin only, a statistically significant 20% relative risk reduction for all adverse outcomes with dual therapy. This net clinical benefit suggests that adding rivaroxaban has a cost-effective benefit. Assessment of rivaroxaban’s cost benefit in COMPASS is in process, Dr. Sharma said.
Rivaroxaban received Food and Drug Administration marketing approval in 2011 for preventing deep vein thrombosis and preventing stroke in patients with atrial fibrillation at dosages higher than what was used in COMPASS. The approved rivaroxaban dosage for preventing deep vein thrombosis is 10 mg/day, and 20 mg/day for preventing stroke in atrial fibrillation patients. The 2.5-mg formulation of rivaroxaban that was given twice daily had the best safety and efficacy in COMPASS, but it is not available now on the U.S. market, although it is available in Europe. Johnson & Johnson, which markets rivaroxaban globally with Bayer, submitted an application to the FDA in December for marketing approval of the 2.5-mg formulation in twice-daily dosing for use as in the COMPASS trial.
COMPASS was sponsored by Bayer, the company that markets rivaroxaban in collaboration with Johnson & Johnson. Dr. Sharma has been a consultant or adviser to Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, and Daiichi-Sankyo.
SOURCE: Sharma M et al. ISC 2018, Abstract LB7.
LOS ANGELES – Combined treatment with a low dosage of the anticoagulant rivaroxaban plus aspirin cut the incidence of ischemic strokes nearly in half, compared with aspirin alone, in a multicenter, randomized trial of more than 27,000 patients with stable atherosclerotic vascular disease.
This dramatic reduction in ischemic strokes as well as in all-cause strokes by adding low-dose rivaroxaban(Xarelto) occurred without any significant increase in hemorrhagic strokes but with a small increase in total major bleeding events, such as gastrointestinal bleeds, Mike Sharma, MD, said at the International Stroke Conference, sponsored by the American Heart Association.
“There was a consistent effect across all strata of stroke risk. For patients who had a prior stroke, it’s pretty clear to use rivaroxaban plus aspirin because it had a big benefit” with no increase in intracranial hemorrhages, Dr. Sharma said in a video interview.
“We think these results will fundamentally change how we approach stroke prevention,” added Dr. Sharma, a stroke neurologist in the Population Health Research Institute of McMaster University in Hamilton, Ont.
The results he reported came from a secondary analysis of data collected in the COMPASS (Rivaroxaban for the Prevention of Major Cardiovascular Events in Coronary or Peripheral Artery Disease) trial, which enrolled 27,395 patients with stable coronary or peripheral artery disease at 602 centers in 33 countries.
The primary outcome of the trial, reported in 2017, was the combined rate of cardiovascular death, MI, or stroke during an average 23 months of follow-up, which occurred in 4.1% of patients treated with 2.5 mg rivaroxaban twice daily plus 100 mg aspirin once daily, 4.9% of patients who received 5.0 mg rivaroxaban twice daily, and 5.4% in patients who received 100 mg aspirin daily, a statistically significant 24% relative risk reduction in the combined treatment group, compared with aspirin only. The rivaroxaban only–treated patients did not significantly differ from the control patients who received only aspirin (N Engl J Med. 2017 Oct 5;377[14]:1319-30). The main results showed a 1.2% increase in the rate of major bleeds in patients treated with rivaroxaban plus aspirin, compared with aspirin only, but the rate of nonfatal symptomatic intracranial hemorrhages was identical in the two treatment groups.
The new results Dr. Sharma reported at the conference focused on various measures of stroke. The rate of all strokes was 42% lower among the patients treated with rivaroxaban plus aspirin, compared with the aspirin alone patients, and ischemic strokes were 49% lower with the dual therapy, compared with aspirin only. Both differences were statistically significant. In contrast, the rivaroxaban alone regimen did not significantly reduce all-cause strokes. It did significantly reduce ischemic strokes, compared with aspirin only, but it also significantly increased hemorrhagic strokes, compared with aspirin only, an adverse effect not caused by the combination of low-dose rivaroxaban plus aspirin.
Rivaroxaban plus aspirin surpassed aspirin alone for preventing both mild and severe strokes and for preventing strokes both in patients with a history of a prior stroke and in those who never had a prior stroke. The stroke reduction produced by rivaroxaban plus aspirin was greatest in the highest risk patients – those with a prior stroke. On the combined regimen, these patients had an average stroke incidence of 0.7% per year, compared with an annual 3.4% rate among the patients on aspirin only, a 2.7% absolute reduction by using rivaroxaban plus aspirin that translated into a number needed to treat of 37 patients with a history of stroke to prevent one new stroke per year.
The 2017 report of the main COMPASS results included a net clinical benefit analysis that factored together the primary endpoint events and major bleeding events. The net rate of all these events was 4.7% with rivaroxaban plus aspirin and 5.9% with aspirin only, a statistically significant 20% relative risk reduction for all adverse outcomes with dual therapy. This net clinical benefit suggests that adding rivaroxaban has a cost-effective benefit. Assessment of rivaroxaban’s cost benefit in COMPASS is in process, Dr. Sharma said.
Rivaroxaban received Food and Drug Administration marketing approval in 2011 for preventing deep vein thrombosis and preventing stroke in patients with atrial fibrillation at dosages higher than what was used in COMPASS. The approved rivaroxaban dosage for preventing deep vein thrombosis is 10 mg/day, and 20 mg/day for preventing stroke in atrial fibrillation patients. The 2.5-mg formulation of rivaroxaban that was given twice daily had the best safety and efficacy in COMPASS, but it is not available now on the U.S. market, although it is available in Europe. Johnson & Johnson, which markets rivaroxaban globally with Bayer, submitted an application to the FDA in December for marketing approval of the 2.5-mg formulation in twice-daily dosing for use as in the COMPASS trial.
COMPASS was sponsored by Bayer, the company that markets rivaroxaban in collaboration with Johnson & Johnson. Dr. Sharma has been a consultant or adviser to Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, and Daiichi-Sankyo.
SOURCE: Sharma M et al. ISC 2018, Abstract LB7.
REPORTING FROM ISC 2018
Key clinical point: Rivaroxaban plus aspirin cuts strokes in patients with stable atherosclerotic vascular disease.
Major finding: Rivaroxaban plus aspirin cut the rate of ischemic strokes by 49%, compared with aspirin only.
Study details: Secondary analysis from the COMPASS trial, a multicenter, randomized trial with 27,395 patients.
Disclosures: COMPASS was sponsored by Bayer, the company that markets rivaroxaban in collaboration with Johnson & Johnson. Dr. Sharma has been a consultant or adviser to Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, and Daiichi-Sankyo.
Source: Sharma M et al. ISC 2018, Abstract LB7.
VIDEO: Retinal infarctions get missed as stroke harbingers
LOS ANGELES – Retinal infarctions are often going missed as important red flags for future ischemic strokes.
Among U.S. Medicare beneficiaries older than 65 years who had a retinal infarction (RI), “only one-third underwent adequate stroke risk factor evaluation,” Alexander E. Merkler, MD, reported in a poster presented at the International Stroke Conference sponsored by the American Heart Association. And fewer than 10% underwent assessment by a neurologist, based on a review of 5,688 of these older Medicare beneficiaries who had a RI sometime during 2009-2015.
The high-risk profile of these patients was affirmed by a 1% ischemic stroke incidence during the 90 days following their RI diagnosis, a rate roughly fourfold higher than in similar patients without a recent RI.
“A lot of people don’t recognize that a retinal infarction is a type of stroke,” Dr. Merkler said in a video interview. To test this hypothesis, Dr. Merkler and his associates examined the follow-up run on elderly Medicare beneficiaries following a RI diagnosis.”The guidelines recommend evaluating why these patients had a stroke [a retinal infarction] and treating risk factors to reduce the risk of a future stroke,” said Dr. Merkler, a neurologist at Weill Cornell Medicine in New York.
The review showed that 34% of the RI patients underwent cervical carotid imaging, 29% had heart rhythm monitoring, 23% underwent echocardiography, and 8% had assessment by a neurologist.
Dr. Merkler had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SOURCE: Merkler A et al. ISC 2018 Abstract TMP76 (Stroke. 2018 Jan;49[Suppl 1]:ATMP76).
LOS ANGELES – Retinal infarctions are often going missed as important red flags for future ischemic strokes.
Among U.S. Medicare beneficiaries older than 65 years who had a retinal infarction (RI), “only one-third underwent adequate stroke risk factor evaluation,” Alexander E. Merkler, MD, reported in a poster presented at the International Stroke Conference sponsored by the American Heart Association. And fewer than 10% underwent assessment by a neurologist, based on a review of 5,688 of these older Medicare beneficiaries who had a RI sometime during 2009-2015.
The high-risk profile of these patients was affirmed by a 1% ischemic stroke incidence during the 90 days following their RI diagnosis, a rate roughly fourfold higher than in similar patients without a recent RI.
“A lot of people don’t recognize that a retinal infarction is a type of stroke,” Dr. Merkler said in a video interview. To test this hypothesis, Dr. Merkler and his associates examined the follow-up run on elderly Medicare beneficiaries following a RI diagnosis.”The guidelines recommend evaluating why these patients had a stroke [a retinal infarction] and treating risk factors to reduce the risk of a future stroke,” said Dr. Merkler, a neurologist at Weill Cornell Medicine in New York.
The review showed that 34% of the RI patients underwent cervical carotid imaging, 29% had heart rhythm monitoring, 23% underwent echocardiography, and 8% had assessment by a neurologist.
Dr. Merkler had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SOURCE: Merkler A et al. ISC 2018 Abstract TMP76 (Stroke. 2018 Jan;49[Suppl 1]:ATMP76).
LOS ANGELES – Retinal infarctions are often going missed as important red flags for future ischemic strokes.
Among U.S. Medicare beneficiaries older than 65 years who had a retinal infarction (RI), “only one-third underwent adequate stroke risk factor evaluation,” Alexander E. Merkler, MD, reported in a poster presented at the International Stroke Conference sponsored by the American Heart Association. And fewer than 10% underwent assessment by a neurologist, based on a review of 5,688 of these older Medicare beneficiaries who had a RI sometime during 2009-2015.
The high-risk profile of these patients was affirmed by a 1% ischemic stroke incidence during the 90 days following their RI diagnosis, a rate roughly fourfold higher than in similar patients without a recent RI.
“A lot of people don’t recognize that a retinal infarction is a type of stroke,” Dr. Merkler said in a video interview. To test this hypothesis, Dr. Merkler and his associates examined the follow-up run on elderly Medicare beneficiaries following a RI diagnosis.”The guidelines recommend evaluating why these patients had a stroke [a retinal infarction] and treating risk factors to reduce the risk of a future stroke,” said Dr. Merkler, a neurologist at Weill Cornell Medicine in New York.
The review showed that 34% of the RI patients underwent cervical carotid imaging, 29% had heart rhythm monitoring, 23% underwent echocardiography, and 8% had assessment by a neurologist.
Dr. Merkler had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SOURCE: Merkler A et al. ISC 2018 Abstract TMP76 (Stroke. 2018 Jan;49[Suppl 1]:ATMP76).
REPORTING FROM ISC 2018
Key clinical point: Retinal infarction patients often fail to undergo stroke assessment.
Major finding: One-third of Medicare beneficiaries with retinal infarction received adequate evaluation for stroke risk factors.
Study details: Review of 5,688 Medicare patients with a retinal infarction during 2009-2015.
Disclosures: Dr. Merkler had no disclosures.
Source: Merkler A et al. ISC 2018 Abstract TMP76 (Stroke. 2018 Jan;49[Suppl 1]:ATMP76).