User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Researchers discover brain abnormalities in babies who had SIDS
For decades, researchers have been trying to understand why some otherwise healthy babies under 1 year old mysteriously die during their sleep. SIDS is the leading cause of infant death in the U.S., affecting 103 out of every 100,000 babies.
The new study found that babies who died of SIDS had abnormalities in certain brain receptors responsible for waking and restoring breathing. The scientists decided to look at the babies’ brains at the molecular level because previous research showed that the same kind of brain receptors in rodents are responsible for protective breathing functions during sleep.
The study was published in the Journal of Neuropathology & Experimental Neurology. The researchers compared brain stems from 70 babies, some of whom died of SIDS and some who died of other causes.
Despite discovering the differences in the babies’ brains, the lead author of the paper said more study is needed.
Robin Haynes, PhD, who studies SIDS at Boston Children’s Hospital, said in a statement that “the relationship between the abnormalities and cause of death remains unknown.”
She said there is no way to identify babies with the brain abnormalities, and “thus, adherence to safe-sleep practices remains critical.”
The American Academy of Pediatrics recommends numerous steps for creating a safe sleeping environment for babies, including placing babies on their backs on a firm surface. Education campaigns targeting parents and caregivers in the 1990s are largely considered successful, but SIDS rates have remained steady since the practices became widely used.
A version of this article first appeared on WebMD.com.
For decades, researchers have been trying to understand why some otherwise healthy babies under 1 year old mysteriously die during their sleep. SIDS is the leading cause of infant death in the U.S., affecting 103 out of every 100,000 babies.
The new study found that babies who died of SIDS had abnormalities in certain brain receptors responsible for waking and restoring breathing. The scientists decided to look at the babies’ brains at the molecular level because previous research showed that the same kind of brain receptors in rodents are responsible for protective breathing functions during sleep.
The study was published in the Journal of Neuropathology & Experimental Neurology. The researchers compared brain stems from 70 babies, some of whom died of SIDS and some who died of other causes.
Despite discovering the differences in the babies’ brains, the lead author of the paper said more study is needed.
Robin Haynes, PhD, who studies SIDS at Boston Children’s Hospital, said in a statement that “the relationship between the abnormalities and cause of death remains unknown.”
She said there is no way to identify babies with the brain abnormalities, and “thus, adherence to safe-sleep practices remains critical.”
The American Academy of Pediatrics recommends numerous steps for creating a safe sleeping environment for babies, including placing babies on their backs on a firm surface. Education campaigns targeting parents and caregivers in the 1990s are largely considered successful, but SIDS rates have remained steady since the practices became widely used.
A version of this article first appeared on WebMD.com.
For decades, researchers have been trying to understand why some otherwise healthy babies under 1 year old mysteriously die during their sleep. SIDS is the leading cause of infant death in the U.S., affecting 103 out of every 100,000 babies.
The new study found that babies who died of SIDS had abnormalities in certain brain receptors responsible for waking and restoring breathing. The scientists decided to look at the babies’ brains at the molecular level because previous research showed that the same kind of brain receptors in rodents are responsible for protective breathing functions during sleep.
The study was published in the Journal of Neuropathology & Experimental Neurology. The researchers compared brain stems from 70 babies, some of whom died of SIDS and some who died of other causes.
Despite discovering the differences in the babies’ brains, the lead author of the paper said more study is needed.
Robin Haynes, PhD, who studies SIDS at Boston Children’s Hospital, said in a statement that “the relationship between the abnormalities and cause of death remains unknown.”
She said there is no way to identify babies with the brain abnormalities, and “thus, adherence to safe-sleep practices remains critical.”
The American Academy of Pediatrics recommends numerous steps for creating a safe sleeping environment for babies, including placing babies on their backs on a firm surface. Education campaigns targeting parents and caregivers in the 1990s are largely considered successful, but SIDS rates have remained steady since the practices became widely used.
A version of this article first appeared on WebMD.com.
FROM THE JOURNAL OF NEUROPATHY & EXPERIMENTAL NEUROLOGY
Standard measure may underestimate OSA in Black patients
Measurement error may be the culprit in underdiagnosing obstructive sleep apnea in Black patients, compared with White patients, based on data from nearly 2,000 individuals.
, according to Ali Azarbarzin, PhD, of Harvard Medical School, Boston.
“We wanted to examine the implications for obstructive sleep apnea,” which is often caused by a reduction in air flow, Dr. Azarbarzin said in an interview.
In a study presented at the American Thoracic Society’s international conference, Dr. Azarbarzin and colleagues examined data from 1,955 adults who were enrolled in the Multi-Ethnic Study of Atherosclerosis (MESA) Exam 5. The study participants underwent unattended 15-channel polysomnography that included a finger pulse oximeter. The mean age of the participants was 68.3 years, and 53.7% were women. A total of 12.1%, 23.7%, 27.7%, and 36.5% of the participants were Asian, Hispanic, Black, and White, respectively.
Apnea hypopnea index (AHI3P) was similar between Black and White patients, at approximately 19 events per hour. Black participants had higher wake SpO2, higher current smoking rates, and higher body mass index, compared with White participants, but these differences were not significant.
Severity of obstructive sleep apnea (OSA) was based on the hypoxic burden, which was defined as the total area under the respiratory curve. The total ventilatory burden was defined as the event-specific area under the ventilation signal and identified by amplitude changes in the nasal pressure signal. The researchers then calculated desaturation sensitivity (the primary outcome) as hypoxic burden divided by ventilatory burden.
In an unadjusted analysis, desaturation sensitivity was significantly lower in Black patients and Asian patients, compared with White patients (P < .001 and P < .02, respectively). After adjusting for age, sex, body mass index, and time spent in a supine position, desaturation sensitivity was lower only in Black patients, compared with White patients, and this difference persisted in both men and women.
The difference in desaturation sensitivity by race could be caused by differences in physiology or in measurement error, Dr. Azarbarzin told this news organization. If measurement error is the culprit, “we may be underestimating OSA severity in [Black people],” especially in Black women, he said.
However, more research is needed to understand the potential impact of both physiology and device accuracy on differences in oxygen saturation across ethnicities and to effectively identify and treat OSA in all patients, Dr. Azarbarzin said.
The MESA Study was supported by the National Institutes of Health and the National Institute on Aging. Data from MESA were obtained through support from the National Heart, Lung, and Blood Institute and the National Center for Advancing Translational Sciences. Dr. Azarbarzin disclosed funding from the National Institutes of Health, the American Health Association, and the American Academy of Sleep Medicine.
A version of this article first appeared on Medscape.com.
Measurement error may be the culprit in underdiagnosing obstructive sleep apnea in Black patients, compared with White patients, based on data from nearly 2,000 individuals.
, according to Ali Azarbarzin, PhD, of Harvard Medical School, Boston.
“We wanted to examine the implications for obstructive sleep apnea,” which is often caused by a reduction in air flow, Dr. Azarbarzin said in an interview.
In a study presented at the American Thoracic Society’s international conference, Dr. Azarbarzin and colleagues examined data from 1,955 adults who were enrolled in the Multi-Ethnic Study of Atherosclerosis (MESA) Exam 5. The study participants underwent unattended 15-channel polysomnography that included a finger pulse oximeter. The mean age of the participants was 68.3 years, and 53.7% were women. A total of 12.1%, 23.7%, 27.7%, and 36.5% of the participants were Asian, Hispanic, Black, and White, respectively.
Apnea hypopnea index (AHI3P) was similar between Black and White patients, at approximately 19 events per hour. Black participants had higher wake SpO2, higher current smoking rates, and higher body mass index, compared with White participants, but these differences were not significant.
Severity of obstructive sleep apnea (OSA) was based on the hypoxic burden, which was defined as the total area under the respiratory curve. The total ventilatory burden was defined as the event-specific area under the ventilation signal and identified by amplitude changes in the nasal pressure signal. The researchers then calculated desaturation sensitivity (the primary outcome) as hypoxic burden divided by ventilatory burden.
In an unadjusted analysis, desaturation sensitivity was significantly lower in Black patients and Asian patients, compared with White patients (P < .001 and P < .02, respectively). After adjusting for age, sex, body mass index, and time spent in a supine position, desaturation sensitivity was lower only in Black patients, compared with White patients, and this difference persisted in both men and women.
The difference in desaturation sensitivity by race could be caused by differences in physiology or in measurement error, Dr. Azarbarzin told this news organization. If measurement error is the culprit, “we may be underestimating OSA severity in [Black people],” especially in Black women, he said.
However, more research is needed to understand the potential impact of both physiology and device accuracy on differences in oxygen saturation across ethnicities and to effectively identify and treat OSA in all patients, Dr. Azarbarzin said.
The MESA Study was supported by the National Institutes of Health and the National Institute on Aging. Data from MESA were obtained through support from the National Heart, Lung, and Blood Institute and the National Center for Advancing Translational Sciences. Dr. Azarbarzin disclosed funding from the National Institutes of Health, the American Health Association, and the American Academy of Sleep Medicine.
A version of this article first appeared on Medscape.com.
Measurement error may be the culprit in underdiagnosing obstructive sleep apnea in Black patients, compared with White patients, based on data from nearly 2,000 individuals.
, according to Ali Azarbarzin, PhD, of Harvard Medical School, Boston.
“We wanted to examine the implications for obstructive sleep apnea,” which is often caused by a reduction in air flow, Dr. Azarbarzin said in an interview.
In a study presented at the American Thoracic Society’s international conference, Dr. Azarbarzin and colleagues examined data from 1,955 adults who were enrolled in the Multi-Ethnic Study of Atherosclerosis (MESA) Exam 5. The study participants underwent unattended 15-channel polysomnography that included a finger pulse oximeter. The mean age of the participants was 68.3 years, and 53.7% were women. A total of 12.1%, 23.7%, 27.7%, and 36.5% of the participants were Asian, Hispanic, Black, and White, respectively.
Apnea hypopnea index (AHI3P) was similar between Black and White patients, at approximately 19 events per hour. Black participants had higher wake SpO2, higher current smoking rates, and higher body mass index, compared with White participants, but these differences were not significant.
Severity of obstructive sleep apnea (OSA) was based on the hypoxic burden, which was defined as the total area under the respiratory curve. The total ventilatory burden was defined as the event-specific area under the ventilation signal and identified by amplitude changes in the nasal pressure signal. The researchers then calculated desaturation sensitivity (the primary outcome) as hypoxic burden divided by ventilatory burden.
In an unadjusted analysis, desaturation sensitivity was significantly lower in Black patients and Asian patients, compared with White patients (P < .001 and P < .02, respectively). After adjusting for age, sex, body mass index, and time spent in a supine position, desaturation sensitivity was lower only in Black patients, compared with White patients, and this difference persisted in both men and women.
The difference in desaturation sensitivity by race could be caused by differences in physiology or in measurement error, Dr. Azarbarzin told this news organization. If measurement error is the culprit, “we may be underestimating OSA severity in [Black people],” especially in Black women, he said.
However, more research is needed to understand the potential impact of both physiology and device accuracy on differences in oxygen saturation across ethnicities and to effectively identify and treat OSA in all patients, Dr. Azarbarzin said.
The MESA Study was supported by the National Institutes of Health and the National Institute on Aging. Data from MESA were obtained through support from the National Heart, Lung, and Blood Institute and the National Center for Advancing Translational Sciences. Dr. Azarbarzin disclosed funding from the National Institutes of Health, the American Health Association, and the American Academy of Sleep Medicine.
A version of this article first appeared on Medscape.com.
FROM ATS 2023
States move to curb insurers’ prior authorization requirements as federal reforms lag
Amid growing criticism of health insurers’ onerous prior authorization practices, lawmakers in 30 states have introduced bills this year that aim to rein in insurer gatekeeping and improve patient care.
“This is something that goes on in every doctor’s office every day; the frustrations, the delays, and the use of office staff time are just unbelievable,” said Steven Orland, MD, a board-certified urologist and president of the Medical Society of New Jersey.
The bills, which cover private health plans and insurers that states regulate, may provide some relief for physicians as federal efforts to streamline prior authorization for some Medicare patients have lagged.
Last year, Congress failed to pass the Improving Seniors’ Timely Access to Care Act of 2021, despite 326 co-sponsors. The bill would have compelled insurers covering Medicare Advantage enrollees to speed up prior authorizations, make the process more transparent, and remove obstacles such as requiring fax machine submissions.
Last month, however, the Centers for Medicare & Medicaid Services issued a final rule that will improve some aspects of prior authorizations in Medicare Advantage insurance plans and ensure that enrollees have the same access to necessary care as traditional Medicare enrollees.
The insurance industry has long defended prior authorization requirements and opposed legislation that would limit them.
America’s Health Insurance Plans (AHIP) and the Blue Cross Blue Shield Association said in a 2019 letter to a congressional committee when the federal legislation was first introduced, “Prior authorizations enforce best practices and guidelines for care management and help physicians identify and avoid care techniques that would harm patient outcomes, such as designating prescriptions that could feed into an opioid addiction.” AHIP didn’t respond to repeated requests for comment.
But some major insurers now appear willing to compromise and voluntarily reduce the volume of prior authorizations they require. Days before the federal final rule was released, three major insurers – United HealthCare, Cigna, and Aetna CVS Health – announced they plan to drop some prior authorization requirements and automate processes.
United HealthCare said it will eliminate almost 20% of its prior authorizations for some nonurgent surgeries and procedures starting this summer. It also will create a national Gold Card program in 2024 for physicians who meet its eligibility requirements, which would eliminate prior authorization requirements for most procedures. Both initiatives will apply to commercial, Medicare Advantage, and Medicaid businesses, said the insurer in a statement.
However, United HealthCare also announced that in June it will start requiring prior authorization for diagnostic (not screening) gastrointestinal endoscopies for its nearly 27 million privately insured patients, citing data it says shows potentially harmful overuse of scopes. Physician groups have publicly criticized the move, saying it could delay lifesaving treatment, and have asked the insurer to reconsider.
Cigna and Aetna also have moved to pare back prior authorization processes. Scott Josephs, national medical officer for Cigna, told Healthcare Dive that Cigna has removed prior authorization reviews from nearly 500 services since 2020.
An Aetna spokesperson told Healthcare Dive that the CVS-owned payer has implemented a gold card program and rolled back prior authorization requirements on cataract surgeries, video EEGs, and home infusion for some drugs, according to Healthcare Dive.
Cigna has faced increased scrutiny from some state regulators since a ProPublica/The Capitol Forum article revealed in March that its doctors were denying claims without opening patients’ files, contrary to what insurance laws and regulations require in many states.
Over a period of 2 months last year, Cigna doctors denied over 300,000 requests for payments using this method, spending an average of 1.2 seconds on each case, the investigation found. In a written response, Cigna said the reporting by ProPublica and The Capitol Forum was “biased and incomplete.”
States aim to reduce prior authorization volume
The American Medical Association said it has been tracking nearly 90 prior authorization reform bills in 30 states. More than a dozen bills are still being considered in this legislative session, including in Arkansas, California, New Jersey, North Carolina, Maryland, and Washington, D.C.
“The groundswell of activity in the states reflects how big a problem this is,” said an AMA legislative expert. “The issue used to be ‘how can we automate and streamline processes’; now the issue is focused on reducing the volume of prior authorizations and the harm that can cause patients.”
The state bills use different strategies to reduce excessive prior authorization requirements. Maryland’s proposed bill, for example, would require just one prior authorization to stay on a prescription drug, if the insurer has previously approved the drug and the patient continues to successfully be treated by the drug.
Washington, D.C. and New Jersey have introduced comprehensive reform bills that include a “grace period” of 60 days, to ensure continuity of care when a patient switches health plans. They also would eliminate repeat authorizations for chronic and long-term conditions, set explicit timelines for insurers to respond to prior authorization requests and appeals, and require that practicing physicians review denials that are appealed.
Many state bills also would require insurers to be more transparent by posting information on their websites about which services and drugs require prior authorization and what their approval rates are for them, said AMA’s legislative expert.
“There’s a black hole of information that insurers have access to. We would really like to know how many prior authorization requests are denied, the time it takes to deny them, and the reasons for denial,” said Josh Bengal, JD, the director of government relations for the Medical Society of New Jersey.
The legislation in New Jersey and other states faces stiff opposition from the insurance lobby, especially state associations of health plans affiliated with AHIP. The California Association of Health Plans, for example, opposes a “gold card” bill (SB 598), introduced in February, that would allow a select group of high-performing doctors to skip prior authorizations for 1 year.
The CAHP states, “Californians deserve safe, high quality, high-value health care. Yet SB 598 will derail the progress we have made in our health care system by lowering the value and safety that Californians should expect from their health care providers,” according to a fact sheet.
The fact-sheet defines “low-value care” as medical services for which there is little to no benefit and poses potential physical or financial harm to patients, such as unnecessary CT scans or MRIs for uncomplicated conditions.
California is one of about a dozen states that have introduced gold card legislation this year. If enacted, they would join five states with gold card laws: West Virginia, Texas, Vermont, Michigan, and Louisiana.
How do gold cards work?
Physicians who achieve a high approval rate of prior authorizations from insurers for 1 year are eligible to be exempted from obtaining prior authorizations the following year.
The approval rate is at least 90% for a certain number of eligible health services, but the number of prior authorizations required to qualify can range from 5 to 30, depending on the state law.
Gold card legislation typically also gives the treating physician the right to have an appeal of a prior authorization denial by a physician peer of the same or similar specialty.
California’s bill would also apply to all covered health services, which is broader than what United HealthCare has proposed for its gold card exemption. The bill would also require a plan or insurer to annually monitor rates of prior authorization approval, modification, appeal, and denial, and to discontinue services, items, and supplies that are approved 95% of the time.
“These are important reforms that will help ensure that patients can receive the care they need, when they need it,” said CMA president Donaldo Hernandez, MD.
However, it’s not clear how many physicians will meet “gold card” status based on Texas’ recent experience with its own “gold card” law.
The Texas Department of Insurance estimated that only 3.3% of licensed physicians in the state have met “gold card” status since the bill became law in 2021, said Zeke Silva, MD, an interventional radiologist who serves on the Council of Legislation for the Texas Medical Association.
He noted that the legislation has had a limited effect for several reasons. Commercial health plans only make up only about 20% of all health plans in Texas. Also, the final regulations didn’t go into effect until last May and physicians are evaluated by health plans for “gold card” status every 6 months, said Dr. Silva.
In addition, physicians must have at least five prior authorizations approved for the same health service, which the law left up to the health plans to define, said Dr. Silva.
Now, the Texas Medical Association is lobbying for legislative improvements. “We want to reduce the number of eligible services that health plans require for prior authorizations and have more oversight of prior authorization denials by the Texas Department of Insurance and the Texas Medical Board,” said Dr. Silva.
He’s optimistic that if the bill becomes law, the number of physicians eligible for gold cards may increase.
Meanwhile, the AMA’s legislative expert, who declined to be identified because of organization policy, acknowledged the possibility that some prior authorization bills will die in state legislatures this year.
“We remain hopeful, but it’s an uphill battle. The state medical associations face a lot of opposition from health plans who don’t want to see these reforms become law.”
A version of this article originally appeared on Medscape.com.
Amid growing criticism of health insurers’ onerous prior authorization practices, lawmakers in 30 states have introduced bills this year that aim to rein in insurer gatekeeping and improve patient care.
“This is something that goes on in every doctor’s office every day; the frustrations, the delays, and the use of office staff time are just unbelievable,” said Steven Orland, MD, a board-certified urologist and president of the Medical Society of New Jersey.
The bills, which cover private health plans and insurers that states regulate, may provide some relief for physicians as federal efforts to streamline prior authorization for some Medicare patients have lagged.
Last year, Congress failed to pass the Improving Seniors’ Timely Access to Care Act of 2021, despite 326 co-sponsors. The bill would have compelled insurers covering Medicare Advantage enrollees to speed up prior authorizations, make the process more transparent, and remove obstacles such as requiring fax machine submissions.
Last month, however, the Centers for Medicare & Medicaid Services issued a final rule that will improve some aspects of prior authorizations in Medicare Advantage insurance plans and ensure that enrollees have the same access to necessary care as traditional Medicare enrollees.
The insurance industry has long defended prior authorization requirements and opposed legislation that would limit them.
America’s Health Insurance Plans (AHIP) and the Blue Cross Blue Shield Association said in a 2019 letter to a congressional committee when the federal legislation was first introduced, “Prior authorizations enforce best practices and guidelines for care management and help physicians identify and avoid care techniques that would harm patient outcomes, such as designating prescriptions that could feed into an opioid addiction.” AHIP didn’t respond to repeated requests for comment.
But some major insurers now appear willing to compromise and voluntarily reduce the volume of prior authorizations they require. Days before the federal final rule was released, three major insurers – United HealthCare, Cigna, and Aetna CVS Health – announced they plan to drop some prior authorization requirements and automate processes.
United HealthCare said it will eliminate almost 20% of its prior authorizations for some nonurgent surgeries and procedures starting this summer. It also will create a national Gold Card program in 2024 for physicians who meet its eligibility requirements, which would eliminate prior authorization requirements for most procedures. Both initiatives will apply to commercial, Medicare Advantage, and Medicaid businesses, said the insurer in a statement.
However, United HealthCare also announced that in June it will start requiring prior authorization for diagnostic (not screening) gastrointestinal endoscopies for its nearly 27 million privately insured patients, citing data it says shows potentially harmful overuse of scopes. Physician groups have publicly criticized the move, saying it could delay lifesaving treatment, and have asked the insurer to reconsider.
Cigna and Aetna also have moved to pare back prior authorization processes. Scott Josephs, national medical officer for Cigna, told Healthcare Dive that Cigna has removed prior authorization reviews from nearly 500 services since 2020.
An Aetna spokesperson told Healthcare Dive that the CVS-owned payer has implemented a gold card program and rolled back prior authorization requirements on cataract surgeries, video EEGs, and home infusion for some drugs, according to Healthcare Dive.
Cigna has faced increased scrutiny from some state regulators since a ProPublica/The Capitol Forum article revealed in March that its doctors were denying claims without opening patients’ files, contrary to what insurance laws and regulations require in many states.
Over a period of 2 months last year, Cigna doctors denied over 300,000 requests for payments using this method, spending an average of 1.2 seconds on each case, the investigation found. In a written response, Cigna said the reporting by ProPublica and The Capitol Forum was “biased and incomplete.”
States aim to reduce prior authorization volume
The American Medical Association said it has been tracking nearly 90 prior authorization reform bills in 30 states. More than a dozen bills are still being considered in this legislative session, including in Arkansas, California, New Jersey, North Carolina, Maryland, and Washington, D.C.
“The groundswell of activity in the states reflects how big a problem this is,” said an AMA legislative expert. “The issue used to be ‘how can we automate and streamline processes’; now the issue is focused on reducing the volume of prior authorizations and the harm that can cause patients.”
The state bills use different strategies to reduce excessive prior authorization requirements. Maryland’s proposed bill, for example, would require just one prior authorization to stay on a prescription drug, if the insurer has previously approved the drug and the patient continues to successfully be treated by the drug.
Washington, D.C. and New Jersey have introduced comprehensive reform bills that include a “grace period” of 60 days, to ensure continuity of care when a patient switches health plans. They also would eliminate repeat authorizations for chronic and long-term conditions, set explicit timelines for insurers to respond to prior authorization requests and appeals, and require that practicing physicians review denials that are appealed.
Many state bills also would require insurers to be more transparent by posting information on their websites about which services and drugs require prior authorization and what their approval rates are for them, said AMA’s legislative expert.
“There’s a black hole of information that insurers have access to. We would really like to know how many prior authorization requests are denied, the time it takes to deny them, and the reasons for denial,” said Josh Bengal, JD, the director of government relations for the Medical Society of New Jersey.
The legislation in New Jersey and other states faces stiff opposition from the insurance lobby, especially state associations of health plans affiliated with AHIP. The California Association of Health Plans, for example, opposes a “gold card” bill (SB 598), introduced in February, that would allow a select group of high-performing doctors to skip prior authorizations for 1 year.
The CAHP states, “Californians deserve safe, high quality, high-value health care. Yet SB 598 will derail the progress we have made in our health care system by lowering the value and safety that Californians should expect from their health care providers,” according to a fact sheet.
The fact-sheet defines “low-value care” as medical services for which there is little to no benefit and poses potential physical or financial harm to patients, such as unnecessary CT scans or MRIs for uncomplicated conditions.
California is one of about a dozen states that have introduced gold card legislation this year. If enacted, they would join five states with gold card laws: West Virginia, Texas, Vermont, Michigan, and Louisiana.
How do gold cards work?
Physicians who achieve a high approval rate of prior authorizations from insurers for 1 year are eligible to be exempted from obtaining prior authorizations the following year.
The approval rate is at least 90% for a certain number of eligible health services, but the number of prior authorizations required to qualify can range from 5 to 30, depending on the state law.
Gold card legislation typically also gives the treating physician the right to have an appeal of a prior authorization denial by a physician peer of the same or similar specialty.
California’s bill would also apply to all covered health services, which is broader than what United HealthCare has proposed for its gold card exemption. The bill would also require a plan or insurer to annually monitor rates of prior authorization approval, modification, appeal, and denial, and to discontinue services, items, and supplies that are approved 95% of the time.
“These are important reforms that will help ensure that patients can receive the care they need, when they need it,” said CMA president Donaldo Hernandez, MD.
However, it’s not clear how many physicians will meet “gold card” status based on Texas’ recent experience with its own “gold card” law.
The Texas Department of Insurance estimated that only 3.3% of licensed physicians in the state have met “gold card” status since the bill became law in 2021, said Zeke Silva, MD, an interventional radiologist who serves on the Council of Legislation for the Texas Medical Association.
He noted that the legislation has had a limited effect for several reasons. Commercial health plans only make up only about 20% of all health plans in Texas. Also, the final regulations didn’t go into effect until last May and physicians are evaluated by health plans for “gold card” status every 6 months, said Dr. Silva.
In addition, physicians must have at least five prior authorizations approved for the same health service, which the law left up to the health plans to define, said Dr. Silva.
Now, the Texas Medical Association is lobbying for legislative improvements. “We want to reduce the number of eligible services that health plans require for prior authorizations and have more oversight of prior authorization denials by the Texas Department of Insurance and the Texas Medical Board,” said Dr. Silva.
He’s optimistic that if the bill becomes law, the number of physicians eligible for gold cards may increase.
Meanwhile, the AMA’s legislative expert, who declined to be identified because of organization policy, acknowledged the possibility that some prior authorization bills will die in state legislatures this year.
“We remain hopeful, but it’s an uphill battle. The state medical associations face a lot of opposition from health plans who don’t want to see these reforms become law.”
A version of this article originally appeared on Medscape.com.
Amid growing criticism of health insurers’ onerous prior authorization practices, lawmakers in 30 states have introduced bills this year that aim to rein in insurer gatekeeping and improve patient care.
“This is something that goes on in every doctor’s office every day; the frustrations, the delays, and the use of office staff time are just unbelievable,” said Steven Orland, MD, a board-certified urologist and president of the Medical Society of New Jersey.
The bills, which cover private health plans and insurers that states regulate, may provide some relief for physicians as federal efforts to streamline prior authorization for some Medicare patients have lagged.
Last year, Congress failed to pass the Improving Seniors’ Timely Access to Care Act of 2021, despite 326 co-sponsors. The bill would have compelled insurers covering Medicare Advantage enrollees to speed up prior authorizations, make the process more transparent, and remove obstacles such as requiring fax machine submissions.
Last month, however, the Centers for Medicare & Medicaid Services issued a final rule that will improve some aspects of prior authorizations in Medicare Advantage insurance plans and ensure that enrollees have the same access to necessary care as traditional Medicare enrollees.
The insurance industry has long defended prior authorization requirements and opposed legislation that would limit them.
America’s Health Insurance Plans (AHIP) and the Blue Cross Blue Shield Association said in a 2019 letter to a congressional committee when the federal legislation was first introduced, “Prior authorizations enforce best practices and guidelines for care management and help physicians identify and avoid care techniques that would harm patient outcomes, such as designating prescriptions that could feed into an opioid addiction.” AHIP didn’t respond to repeated requests for comment.
But some major insurers now appear willing to compromise and voluntarily reduce the volume of prior authorizations they require. Days before the federal final rule was released, three major insurers – United HealthCare, Cigna, and Aetna CVS Health – announced they plan to drop some prior authorization requirements and automate processes.
United HealthCare said it will eliminate almost 20% of its prior authorizations for some nonurgent surgeries and procedures starting this summer. It also will create a national Gold Card program in 2024 for physicians who meet its eligibility requirements, which would eliminate prior authorization requirements for most procedures. Both initiatives will apply to commercial, Medicare Advantage, and Medicaid businesses, said the insurer in a statement.
However, United HealthCare also announced that in June it will start requiring prior authorization for diagnostic (not screening) gastrointestinal endoscopies for its nearly 27 million privately insured patients, citing data it says shows potentially harmful overuse of scopes. Physician groups have publicly criticized the move, saying it could delay lifesaving treatment, and have asked the insurer to reconsider.
Cigna and Aetna also have moved to pare back prior authorization processes. Scott Josephs, national medical officer for Cigna, told Healthcare Dive that Cigna has removed prior authorization reviews from nearly 500 services since 2020.
An Aetna spokesperson told Healthcare Dive that the CVS-owned payer has implemented a gold card program and rolled back prior authorization requirements on cataract surgeries, video EEGs, and home infusion for some drugs, according to Healthcare Dive.
Cigna has faced increased scrutiny from some state regulators since a ProPublica/The Capitol Forum article revealed in March that its doctors were denying claims without opening patients’ files, contrary to what insurance laws and regulations require in many states.
Over a period of 2 months last year, Cigna doctors denied over 300,000 requests for payments using this method, spending an average of 1.2 seconds on each case, the investigation found. In a written response, Cigna said the reporting by ProPublica and The Capitol Forum was “biased and incomplete.”
States aim to reduce prior authorization volume
The American Medical Association said it has been tracking nearly 90 prior authorization reform bills in 30 states. More than a dozen bills are still being considered in this legislative session, including in Arkansas, California, New Jersey, North Carolina, Maryland, and Washington, D.C.
“The groundswell of activity in the states reflects how big a problem this is,” said an AMA legislative expert. “The issue used to be ‘how can we automate and streamline processes’; now the issue is focused on reducing the volume of prior authorizations and the harm that can cause patients.”
The state bills use different strategies to reduce excessive prior authorization requirements. Maryland’s proposed bill, for example, would require just one prior authorization to stay on a prescription drug, if the insurer has previously approved the drug and the patient continues to successfully be treated by the drug.
Washington, D.C. and New Jersey have introduced comprehensive reform bills that include a “grace period” of 60 days, to ensure continuity of care when a patient switches health plans. They also would eliminate repeat authorizations for chronic and long-term conditions, set explicit timelines for insurers to respond to prior authorization requests and appeals, and require that practicing physicians review denials that are appealed.
Many state bills also would require insurers to be more transparent by posting information on their websites about which services and drugs require prior authorization and what their approval rates are for them, said AMA’s legislative expert.
“There’s a black hole of information that insurers have access to. We would really like to know how many prior authorization requests are denied, the time it takes to deny them, and the reasons for denial,” said Josh Bengal, JD, the director of government relations for the Medical Society of New Jersey.
The legislation in New Jersey and other states faces stiff opposition from the insurance lobby, especially state associations of health plans affiliated with AHIP. The California Association of Health Plans, for example, opposes a “gold card” bill (SB 598), introduced in February, that would allow a select group of high-performing doctors to skip prior authorizations for 1 year.
The CAHP states, “Californians deserve safe, high quality, high-value health care. Yet SB 598 will derail the progress we have made in our health care system by lowering the value and safety that Californians should expect from their health care providers,” according to a fact sheet.
The fact-sheet defines “low-value care” as medical services for which there is little to no benefit and poses potential physical or financial harm to patients, such as unnecessary CT scans or MRIs for uncomplicated conditions.
California is one of about a dozen states that have introduced gold card legislation this year. If enacted, they would join five states with gold card laws: West Virginia, Texas, Vermont, Michigan, and Louisiana.
How do gold cards work?
Physicians who achieve a high approval rate of prior authorizations from insurers for 1 year are eligible to be exempted from obtaining prior authorizations the following year.
The approval rate is at least 90% for a certain number of eligible health services, but the number of prior authorizations required to qualify can range from 5 to 30, depending on the state law.
Gold card legislation typically also gives the treating physician the right to have an appeal of a prior authorization denial by a physician peer of the same or similar specialty.
California’s bill would also apply to all covered health services, which is broader than what United HealthCare has proposed for its gold card exemption. The bill would also require a plan or insurer to annually monitor rates of prior authorization approval, modification, appeal, and denial, and to discontinue services, items, and supplies that are approved 95% of the time.
“These are important reforms that will help ensure that patients can receive the care they need, when they need it,” said CMA president Donaldo Hernandez, MD.
However, it’s not clear how many physicians will meet “gold card” status based on Texas’ recent experience with its own “gold card” law.
The Texas Department of Insurance estimated that only 3.3% of licensed physicians in the state have met “gold card” status since the bill became law in 2021, said Zeke Silva, MD, an interventional radiologist who serves on the Council of Legislation for the Texas Medical Association.
He noted that the legislation has had a limited effect for several reasons. Commercial health plans only make up only about 20% of all health plans in Texas. Also, the final regulations didn’t go into effect until last May and physicians are evaluated by health plans for “gold card” status every 6 months, said Dr. Silva.
In addition, physicians must have at least five prior authorizations approved for the same health service, which the law left up to the health plans to define, said Dr. Silva.
Now, the Texas Medical Association is lobbying for legislative improvements. “We want to reduce the number of eligible services that health plans require for prior authorizations and have more oversight of prior authorization denials by the Texas Department of Insurance and the Texas Medical Board,” said Dr. Silva.
He’s optimistic that if the bill becomes law, the number of physicians eligible for gold cards may increase.
Meanwhile, the AMA’s legislative expert, who declined to be identified because of organization policy, acknowledged the possibility that some prior authorization bills will die in state legislatures this year.
“We remain hopeful, but it’s an uphill battle. The state medical associations face a lot of opposition from health plans who don’t want to see these reforms become law.”
A version of this article originally appeared on Medscape.com.
Researchers locate signals in brain related to chronic pain
a new study in Nature Neuroscience concluded.
The researchers used the devices on four patients who had felt endless nerve pain for more than a year. The devices recorded several times a day, which could pave “the way for implanted devices to one day predict pain signals or even short-circuit them,” The New York Times reported.
The study says the pain “was associated with electrical fluctuations in the orbitofrontal cortex, an area involved in emotion regulation, self-evaluation, and decision-making,” The Times reported. “The research suggests that such patterns of brain activity could serve as biomarkers to guide diagnosis and treatment for millions of people with shooting or burning chronic pain linked to a damaged nervous system.”
Ajay Wasan, MD, and a pain specialist at the University of Pittsburgh who was not involved in the study praised it to the Times.
“The study really advances a whole generation of research that has shown that the functioning of the brain is really important to processing and perceiving pain,” he said.
Chronic pain is defined as persistent or recurring and lasting more than three months. The Centers for Disease Control and Prevention says about 20% of Americans experience it. It has been linked with depression, Alzheimer’s disease and other dementias, suicide, and substance use.
Yet, the study’s authors noted, “pain severity is often measured through subjective report, while objective biomarkers that may guide diagnosis and treatment are lacking.”
Medtronic provided devices for the study. The study authors reported no conflicts of interest.
A version of this article first appeared on WebMD.com.
a new study in Nature Neuroscience concluded.
The researchers used the devices on four patients who had felt endless nerve pain for more than a year. The devices recorded several times a day, which could pave “the way for implanted devices to one day predict pain signals or even short-circuit them,” The New York Times reported.
The study says the pain “was associated with electrical fluctuations in the orbitofrontal cortex, an area involved in emotion regulation, self-evaluation, and decision-making,” The Times reported. “The research suggests that such patterns of brain activity could serve as biomarkers to guide diagnosis and treatment for millions of people with shooting or burning chronic pain linked to a damaged nervous system.”
Ajay Wasan, MD, and a pain specialist at the University of Pittsburgh who was not involved in the study praised it to the Times.
“The study really advances a whole generation of research that has shown that the functioning of the brain is really important to processing and perceiving pain,” he said.
Chronic pain is defined as persistent or recurring and lasting more than three months. The Centers for Disease Control and Prevention says about 20% of Americans experience it. It has been linked with depression, Alzheimer’s disease and other dementias, suicide, and substance use.
Yet, the study’s authors noted, “pain severity is often measured through subjective report, while objective biomarkers that may guide diagnosis and treatment are lacking.”
Medtronic provided devices for the study. The study authors reported no conflicts of interest.
A version of this article first appeared on WebMD.com.
a new study in Nature Neuroscience concluded.
The researchers used the devices on four patients who had felt endless nerve pain for more than a year. The devices recorded several times a day, which could pave “the way for implanted devices to one day predict pain signals or even short-circuit them,” The New York Times reported.
The study says the pain “was associated with electrical fluctuations in the orbitofrontal cortex, an area involved in emotion regulation, self-evaluation, and decision-making,” The Times reported. “The research suggests that such patterns of brain activity could serve as biomarkers to guide diagnosis and treatment for millions of people with shooting or burning chronic pain linked to a damaged nervous system.”
Ajay Wasan, MD, and a pain specialist at the University of Pittsburgh who was not involved in the study praised it to the Times.
“The study really advances a whole generation of research that has shown that the functioning of the brain is really important to processing and perceiving pain,” he said.
Chronic pain is defined as persistent or recurring and lasting more than three months. The Centers for Disease Control and Prevention says about 20% of Americans experience it. It has been linked with depression, Alzheimer’s disease and other dementias, suicide, and substance use.
Yet, the study’s authors noted, “pain severity is often measured through subjective report, while objective biomarkers that may guide diagnosis and treatment are lacking.”
Medtronic provided devices for the study. The study authors reported no conflicts of interest.
A version of this article first appeared on WebMD.com.
FROM NATURE NEUROSCIENCE
The weird world of hydrogels: How they’ll change health care
Imagine a day when a simple injection prompts a broken bone to heal. When tiny, ingestible devices linger in the body, unnoticed, tracking our health or delivering life-saving medications. When brain and heart implants mesh with flesh so seamlessly that the body thinks they’ve been there all along.
These are the dreams of materials scientists who have toiled for decades to mimic the complex architecture of the human body in hopes of replacing broken parts or treating disease.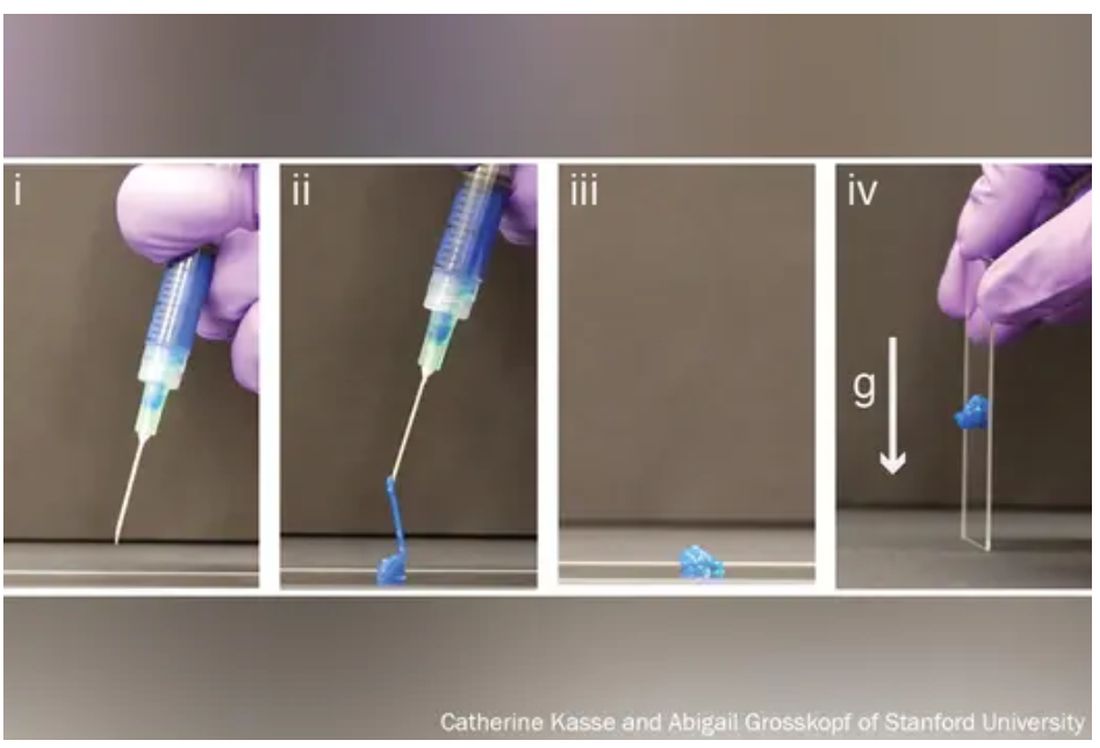
The problem, say bioengineers, is that most replacement and corrective parts – from prosthetics to pacemakers – are made of hard, dry, lifeless materials, like metal or plastic, while biological tissue is soft, wet, and living.
The body knows the difference and tends to reject imitations.
Enter hydrogels, three-dimensional networks of molecules swollen with – by definition – water.
First described in 1960 by creators of soft contact lenses, these weird, shape-shifting substances are able to morph from liquid to solid to a squishy in-between. (Early, simple uses include hair gel or Jell-O.). Slow to gain attention, growing to just 1,000 studies published by 1982, they’ve become the subject of intense study recently, with 100,000 papers published by 2020, and 3,800 already this year alone.
As chemists, biologists, and engineers begin to work more with one another and with medical doctors,
“We are, essentially, hydrogels,” said Benjamin Wiley, PhD, a chemistry professor at Duke University in Durham, N.C. “As people develop new hydrogels that more closely match the tissues in our body, we’ll be able to treat a whole host of ailments we couldn’t treat before.”
From contact lenses to brain implants
Put simply, a hydrogel is like a mesh bag of water.
The mesh is made of polymers, or spaghetti-like strands of molecules, stitched together in a repeating pattern and swollen with H2O, much like the way 3D matrixes in our body surround, support, and give structure to our cells and tissues.
“Imagine a soccer net, with all of these long fibers woven together to create the net,” said Eric Appel, PhD, associate professor of materials science and engineering at Stanford (Calif.) University.
While the broader category of “gels” could be filled with anything, including chemical solvents, water is the key ingredient that sets hydrogels apart, making them ideal for, as some scientists put it, “merging humans and machines.”
Human bones are about 25% water, while muscles hover around 70% and the brain is 85%. The precious liquid plays a host of critical roles, from shuttling nutrients in and waste out to helping cells talk to each other.
Lab-made hydrogels can be loaded with cargo (like a ball in the net), including cells or drugs that help mimic some of those functions.
Hydrogels are soft and pliable like flesh. So, if used in implants, they may be less likely to damage surrounding tissue.
“Think about a metal spoon in your bowl of pudding. As you’re shaking the bowl, the spoon doesn’t stay in place, and you get scarring around the spoon,” said Christina Tringides, PhD, a materials scientist who studies neural engineering. That, she says, is exactly what happens to brain implants when patients breathe or move. “It’s a mechanical mismatch. But with hydrogels, you could get perfect mechanical matching.”
Hydrogels also tend to be nontoxic, so the immune system may be less likely to attack them as foreign bodies.
All this has made hydrogels the new darling of the bioengineering world.
“There has been an absolute explosion of interest in these materials,” Dr. Appel said.
Smarter drug delivery and ingestible electronics
Early versions of hydrogels were thick and gooey, making it hard to get them inside the body.
“Think of a block of Jell-O. You couldn’t inject something like that,” Dr. Appel said.
But Dr. Appel, whose lab develops new drug delivery systems, has been tinkering with gel formulas for years in hopes that these high-tech globs could someday ferry timed-release drugs to just the right spot in the body.
His new hydrogels start as fully formed gels (which help preserve the drug contents) inside a syringe. But once the plunger is pushed, they magically shape-shift to a liquid thin enough to flow easily through a standard needle. Upon exit, they immediately reform into gels, protecting the inherent cargo from degrading.
This could be a game changer at a time when many cutting-edge drugs – think Humira for arthritis or Ozempic for type 2 diabetes – are made of quickly degrading proteins too large and complex to simply jam into a pill. Instead, they must be injected, often frequently.
“Because the gel takes months to dissolve, it slowly delivers the drug over time,” Dr. Appel said. “You could conceivably go from a shot once a week to once every 4 months.”
Such slow-release hydrogels could make vaccines last longer, in turn teaching the body to better resist emerging virus variants, and deliver tumor-busting therapies more precisely, said Dr. Appel, who has formed a startup and hopes to fast-track the first hydrogel drug delivery system to clinical trials within a few years.
Meanwhile, another team at the Massachusetts Institute of Technology has taken a different approach, developing a standard-sized ingestible hydrogel pill that swells up like a puffer fish in the stomach, lasting a month and slowly releasing drugs all the while. To remove the pill, a patient simply drinks a salt-based solution that shrivels the ping-pong ball–sized device so it can be passed out of the body.
In a paper in Nature Communications, the scientists showed the puffer fish pill could also be loaded with tiny cameras or monitors to track conditions like ulcers or cancer.
“The dream is to have a Jell-O-like smart pill that, once swallowed, stays in the stomach and monitors the patient’s health,” said Xuanhe Zhao, PhD, a researcher on the project and associate professor of mechanical engineering at MIT.
Building joints and regrowing bones
Since the 1970s, researchers have mulled using hydrogels to replace human cartilage, a remarkably strong and flexible tissue made of about 90% water but able to withstand the weight of a car on an area about the size of a coin.
Until recently, those efforts have largely failed. Meaning when knee cartilage wears down, things like cartilage transplants, drilling holes to stimulate new growth, or total joint replacements – all of which require lengthy rehab – are the only options.
But that may be about to change.
Dr. Wiley and his colleagues at Duke recently reported that they’d developed the first gel-based cartilage substitute even stronger and more durable than the real thing.
By attaching their hydrogel to a titanium backing to help stick it in place, they hope to repair damaged cartilage “much like a dentist fills a cavity” long before surgery is necessary.
They too have partnered with industry to bring their hydrogel to market – starting with knees.
“Ultimately, the goal is to do any joint – hips, ankles, fingers, and toes,” Dr. Wiley said.
At the University of Toronto, chemist Karina Carneiro, PhD, and dentist Christopher McCulloch, DDS, are also thinking big.
In a recent paper in Proceedings of the National Academy of Sciences, they describe a hydrogel, designed by Dr. Carneiro and made of DNA, that can be injected, migrate to a defect in bone – an irreparable break, hole from surgery, or jawbone withered by age – and fill in the gap like putty. But not only does it patch the hole, it prompts the bone to regenerate.
In rats with holes in their skulls due to surgery, they found that the treatment did not work as well as the existing gold standard for repairing holes in bone – grafting bone from elsewhere in the body. But it did work.
“These are very early days for DNA hydrogels,” cautioned Dr. McCulloch, a study coauthor and professor in the Faculty of Dentistry, noting that it will likely be a decade or more before such technology could be available to patients. “But there is the potential that DNA hydrogel could someday grow bone without having to have highly invasive surgical procedures. That’s a significant advancement.”
A sci-fi future
Perhaps the wildest, and weirdest, potential applications of hydrogels come in the realm of human-machine interaction.
Numerous companies are already dabbling in neural prosthetic or brain computer interfaces that might someday, for instance, let someone who is paralyzed and can’t speak write on a laptop using their thoughts.
The spoon-in-the-Jell-O problem has been a major stumbling block.
But Dr. Tringides, who recently earned her PhD in biophysics from Harvard, is working on it.
She and her team have developed a seaweed-based hydrogel loaded with tiny flecks of nanomaterials that can not only meld nicely into squishy brain tissue but also conduct electricity.
Within a decade, she says, this could replace the clunky platinum metal discs used for electrocorticography – recording electrical activity in the brain to identify where seizures start or doing precise brain surgery.
In 30 to 50 years? Let your imagination run wild.
“I’m a skeptic. I like to take research step by step,” she said. “But things are definitely progressing in an interesting direction.”
A version of this article first appeared on WebMD.com.
Imagine a day when a simple injection prompts a broken bone to heal. When tiny, ingestible devices linger in the body, unnoticed, tracking our health or delivering life-saving medications. When brain and heart implants mesh with flesh so seamlessly that the body thinks they’ve been there all along.
These are the dreams of materials scientists who have toiled for decades to mimic the complex architecture of the human body in hopes of replacing broken parts or treating disease.
The problem, say bioengineers, is that most replacement and corrective parts – from prosthetics to pacemakers – are made of hard, dry, lifeless materials, like metal or plastic, while biological tissue is soft, wet, and living.
The body knows the difference and tends to reject imitations.
Enter hydrogels, three-dimensional networks of molecules swollen with – by definition – water.
First described in 1960 by creators of soft contact lenses, these weird, shape-shifting substances are able to morph from liquid to solid to a squishy in-between. (Early, simple uses include hair gel or Jell-O.). Slow to gain attention, growing to just 1,000 studies published by 1982, they’ve become the subject of intense study recently, with 100,000 papers published by 2020, and 3,800 already this year alone.
As chemists, biologists, and engineers begin to work more with one another and with medical doctors,
“We are, essentially, hydrogels,” said Benjamin Wiley, PhD, a chemistry professor at Duke University in Durham, N.C. “As people develop new hydrogels that more closely match the tissues in our body, we’ll be able to treat a whole host of ailments we couldn’t treat before.”
From contact lenses to brain implants
Put simply, a hydrogel is like a mesh bag of water.
The mesh is made of polymers, or spaghetti-like strands of molecules, stitched together in a repeating pattern and swollen with H2O, much like the way 3D matrixes in our body surround, support, and give structure to our cells and tissues.
“Imagine a soccer net, with all of these long fibers woven together to create the net,” said Eric Appel, PhD, associate professor of materials science and engineering at Stanford (Calif.) University.
While the broader category of “gels” could be filled with anything, including chemical solvents, water is the key ingredient that sets hydrogels apart, making them ideal for, as some scientists put it, “merging humans and machines.”
Human bones are about 25% water, while muscles hover around 70% and the brain is 85%. The precious liquid plays a host of critical roles, from shuttling nutrients in and waste out to helping cells talk to each other.
Lab-made hydrogels can be loaded with cargo (like a ball in the net), including cells or drugs that help mimic some of those functions.
Hydrogels are soft and pliable like flesh. So, if used in implants, they may be less likely to damage surrounding tissue.
“Think about a metal spoon in your bowl of pudding. As you’re shaking the bowl, the spoon doesn’t stay in place, and you get scarring around the spoon,” said Christina Tringides, PhD, a materials scientist who studies neural engineering. That, she says, is exactly what happens to brain implants when patients breathe or move. “It’s a mechanical mismatch. But with hydrogels, you could get perfect mechanical matching.”
Hydrogels also tend to be nontoxic, so the immune system may be less likely to attack them as foreign bodies.
All this has made hydrogels the new darling of the bioengineering world.
“There has been an absolute explosion of interest in these materials,” Dr. Appel said.
Smarter drug delivery and ingestible electronics
Early versions of hydrogels were thick and gooey, making it hard to get them inside the body.
“Think of a block of Jell-O. You couldn’t inject something like that,” Dr. Appel said.
But Dr. Appel, whose lab develops new drug delivery systems, has been tinkering with gel formulas for years in hopes that these high-tech globs could someday ferry timed-release drugs to just the right spot in the body.
His new hydrogels start as fully formed gels (which help preserve the drug contents) inside a syringe. But once the plunger is pushed, they magically shape-shift to a liquid thin enough to flow easily through a standard needle. Upon exit, they immediately reform into gels, protecting the inherent cargo from degrading.
This could be a game changer at a time when many cutting-edge drugs – think Humira for arthritis or Ozempic for type 2 diabetes – are made of quickly degrading proteins too large and complex to simply jam into a pill. Instead, they must be injected, often frequently.
“Because the gel takes months to dissolve, it slowly delivers the drug over time,” Dr. Appel said. “You could conceivably go from a shot once a week to once every 4 months.”
Such slow-release hydrogels could make vaccines last longer, in turn teaching the body to better resist emerging virus variants, and deliver tumor-busting therapies more precisely, said Dr. Appel, who has formed a startup and hopes to fast-track the first hydrogel drug delivery system to clinical trials within a few years.
Meanwhile, another team at the Massachusetts Institute of Technology has taken a different approach, developing a standard-sized ingestible hydrogel pill that swells up like a puffer fish in the stomach, lasting a month and slowly releasing drugs all the while. To remove the pill, a patient simply drinks a salt-based solution that shrivels the ping-pong ball–sized device so it can be passed out of the body.
In a paper in Nature Communications, the scientists showed the puffer fish pill could also be loaded with tiny cameras or monitors to track conditions like ulcers or cancer.
“The dream is to have a Jell-O-like smart pill that, once swallowed, stays in the stomach and monitors the patient’s health,” said Xuanhe Zhao, PhD, a researcher on the project and associate professor of mechanical engineering at MIT.
Building joints and regrowing bones
Since the 1970s, researchers have mulled using hydrogels to replace human cartilage, a remarkably strong and flexible tissue made of about 90% water but able to withstand the weight of a car on an area about the size of a coin.
Until recently, those efforts have largely failed. Meaning when knee cartilage wears down, things like cartilage transplants, drilling holes to stimulate new growth, or total joint replacements – all of which require lengthy rehab – are the only options.
But that may be about to change.
Dr. Wiley and his colleagues at Duke recently reported that they’d developed the first gel-based cartilage substitute even stronger and more durable than the real thing.
By attaching their hydrogel to a titanium backing to help stick it in place, they hope to repair damaged cartilage “much like a dentist fills a cavity” long before surgery is necessary.
They too have partnered with industry to bring their hydrogel to market – starting with knees.
“Ultimately, the goal is to do any joint – hips, ankles, fingers, and toes,” Dr. Wiley said.
At the University of Toronto, chemist Karina Carneiro, PhD, and dentist Christopher McCulloch, DDS, are also thinking big.
In a recent paper in Proceedings of the National Academy of Sciences, they describe a hydrogel, designed by Dr. Carneiro and made of DNA, that can be injected, migrate to a defect in bone – an irreparable break, hole from surgery, or jawbone withered by age – and fill in the gap like putty. But not only does it patch the hole, it prompts the bone to regenerate.
In rats with holes in their skulls due to surgery, they found that the treatment did not work as well as the existing gold standard for repairing holes in bone – grafting bone from elsewhere in the body. But it did work.
“These are very early days for DNA hydrogels,” cautioned Dr. McCulloch, a study coauthor and professor in the Faculty of Dentistry, noting that it will likely be a decade or more before such technology could be available to patients. “But there is the potential that DNA hydrogel could someday grow bone without having to have highly invasive surgical procedures. That’s a significant advancement.”
A sci-fi future
Perhaps the wildest, and weirdest, potential applications of hydrogels come in the realm of human-machine interaction.
Numerous companies are already dabbling in neural prosthetic or brain computer interfaces that might someday, for instance, let someone who is paralyzed and can’t speak write on a laptop using their thoughts.
The spoon-in-the-Jell-O problem has been a major stumbling block.
But Dr. Tringides, who recently earned her PhD in biophysics from Harvard, is working on it.
She and her team have developed a seaweed-based hydrogel loaded with tiny flecks of nanomaterials that can not only meld nicely into squishy brain tissue but also conduct electricity.
Within a decade, she says, this could replace the clunky platinum metal discs used for electrocorticography – recording electrical activity in the brain to identify where seizures start or doing precise brain surgery.
In 30 to 50 years? Let your imagination run wild.
“I’m a skeptic. I like to take research step by step,” she said. “But things are definitely progressing in an interesting direction.”
A version of this article first appeared on WebMD.com.
Imagine a day when a simple injection prompts a broken bone to heal. When tiny, ingestible devices linger in the body, unnoticed, tracking our health or delivering life-saving medications. When brain and heart implants mesh with flesh so seamlessly that the body thinks they’ve been there all along.
These are the dreams of materials scientists who have toiled for decades to mimic the complex architecture of the human body in hopes of replacing broken parts or treating disease.
The problem, say bioengineers, is that most replacement and corrective parts – from prosthetics to pacemakers – are made of hard, dry, lifeless materials, like metal or plastic, while biological tissue is soft, wet, and living.
The body knows the difference and tends to reject imitations.
Enter hydrogels, three-dimensional networks of molecules swollen with – by definition – water.
First described in 1960 by creators of soft contact lenses, these weird, shape-shifting substances are able to morph from liquid to solid to a squishy in-between. (Early, simple uses include hair gel or Jell-O.). Slow to gain attention, growing to just 1,000 studies published by 1982, they’ve become the subject of intense study recently, with 100,000 papers published by 2020, and 3,800 already this year alone.
As chemists, biologists, and engineers begin to work more with one another and with medical doctors,
“We are, essentially, hydrogels,” said Benjamin Wiley, PhD, a chemistry professor at Duke University in Durham, N.C. “As people develop new hydrogels that more closely match the tissues in our body, we’ll be able to treat a whole host of ailments we couldn’t treat before.”
From contact lenses to brain implants
Put simply, a hydrogel is like a mesh bag of water.
The mesh is made of polymers, or spaghetti-like strands of molecules, stitched together in a repeating pattern and swollen with H2O, much like the way 3D matrixes in our body surround, support, and give structure to our cells and tissues.
“Imagine a soccer net, with all of these long fibers woven together to create the net,” said Eric Appel, PhD, associate professor of materials science and engineering at Stanford (Calif.) University.
While the broader category of “gels” could be filled with anything, including chemical solvents, water is the key ingredient that sets hydrogels apart, making them ideal for, as some scientists put it, “merging humans and machines.”
Human bones are about 25% water, while muscles hover around 70% and the brain is 85%. The precious liquid plays a host of critical roles, from shuttling nutrients in and waste out to helping cells talk to each other.
Lab-made hydrogels can be loaded with cargo (like a ball in the net), including cells or drugs that help mimic some of those functions.
Hydrogels are soft and pliable like flesh. So, if used in implants, they may be less likely to damage surrounding tissue.
“Think about a metal spoon in your bowl of pudding. As you’re shaking the bowl, the spoon doesn’t stay in place, and you get scarring around the spoon,” said Christina Tringides, PhD, a materials scientist who studies neural engineering. That, she says, is exactly what happens to brain implants when patients breathe or move. “It’s a mechanical mismatch. But with hydrogels, you could get perfect mechanical matching.”
Hydrogels also tend to be nontoxic, so the immune system may be less likely to attack them as foreign bodies.
All this has made hydrogels the new darling of the bioengineering world.
“There has been an absolute explosion of interest in these materials,” Dr. Appel said.
Smarter drug delivery and ingestible electronics
Early versions of hydrogels were thick and gooey, making it hard to get them inside the body.
“Think of a block of Jell-O. You couldn’t inject something like that,” Dr. Appel said.
But Dr. Appel, whose lab develops new drug delivery systems, has been tinkering with gel formulas for years in hopes that these high-tech globs could someday ferry timed-release drugs to just the right spot in the body.
His new hydrogels start as fully formed gels (which help preserve the drug contents) inside a syringe. But once the plunger is pushed, they magically shape-shift to a liquid thin enough to flow easily through a standard needle. Upon exit, they immediately reform into gels, protecting the inherent cargo from degrading.
This could be a game changer at a time when many cutting-edge drugs – think Humira for arthritis or Ozempic for type 2 diabetes – are made of quickly degrading proteins too large and complex to simply jam into a pill. Instead, they must be injected, often frequently.
“Because the gel takes months to dissolve, it slowly delivers the drug over time,” Dr. Appel said. “You could conceivably go from a shot once a week to once every 4 months.”
Such slow-release hydrogels could make vaccines last longer, in turn teaching the body to better resist emerging virus variants, and deliver tumor-busting therapies more precisely, said Dr. Appel, who has formed a startup and hopes to fast-track the first hydrogel drug delivery system to clinical trials within a few years.
Meanwhile, another team at the Massachusetts Institute of Technology has taken a different approach, developing a standard-sized ingestible hydrogel pill that swells up like a puffer fish in the stomach, lasting a month and slowly releasing drugs all the while. To remove the pill, a patient simply drinks a salt-based solution that shrivels the ping-pong ball–sized device so it can be passed out of the body.
In a paper in Nature Communications, the scientists showed the puffer fish pill could also be loaded with tiny cameras or monitors to track conditions like ulcers or cancer.
“The dream is to have a Jell-O-like smart pill that, once swallowed, stays in the stomach and monitors the patient’s health,” said Xuanhe Zhao, PhD, a researcher on the project and associate professor of mechanical engineering at MIT.
Building joints and regrowing bones
Since the 1970s, researchers have mulled using hydrogels to replace human cartilage, a remarkably strong and flexible tissue made of about 90% water but able to withstand the weight of a car on an area about the size of a coin.
Until recently, those efforts have largely failed. Meaning when knee cartilage wears down, things like cartilage transplants, drilling holes to stimulate new growth, or total joint replacements – all of which require lengthy rehab – are the only options.
But that may be about to change.
Dr. Wiley and his colleagues at Duke recently reported that they’d developed the first gel-based cartilage substitute even stronger and more durable than the real thing.
By attaching their hydrogel to a titanium backing to help stick it in place, they hope to repair damaged cartilage “much like a dentist fills a cavity” long before surgery is necessary.
They too have partnered with industry to bring their hydrogel to market – starting with knees.
“Ultimately, the goal is to do any joint – hips, ankles, fingers, and toes,” Dr. Wiley said.
At the University of Toronto, chemist Karina Carneiro, PhD, and dentist Christopher McCulloch, DDS, are also thinking big.
In a recent paper in Proceedings of the National Academy of Sciences, they describe a hydrogel, designed by Dr. Carneiro and made of DNA, that can be injected, migrate to a defect in bone – an irreparable break, hole from surgery, or jawbone withered by age – and fill in the gap like putty. But not only does it patch the hole, it prompts the bone to regenerate.
In rats with holes in their skulls due to surgery, they found that the treatment did not work as well as the existing gold standard for repairing holes in bone – grafting bone from elsewhere in the body. But it did work.
“These are very early days for DNA hydrogels,” cautioned Dr. McCulloch, a study coauthor and professor in the Faculty of Dentistry, noting that it will likely be a decade or more before such technology could be available to patients. “But there is the potential that DNA hydrogel could someday grow bone without having to have highly invasive surgical procedures. That’s a significant advancement.”
A sci-fi future
Perhaps the wildest, and weirdest, potential applications of hydrogels come in the realm of human-machine interaction.
Numerous companies are already dabbling in neural prosthetic or brain computer interfaces that might someday, for instance, let someone who is paralyzed and can’t speak write on a laptop using their thoughts.
The spoon-in-the-Jell-O problem has been a major stumbling block.
But Dr. Tringides, who recently earned her PhD in biophysics from Harvard, is working on it.
She and her team have developed a seaweed-based hydrogel loaded with tiny flecks of nanomaterials that can not only meld nicely into squishy brain tissue but also conduct electricity.
Within a decade, she says, this could replace the clunky platinum metal discs used for electrocorticography – recording electrical activity in the brain to identify where seizures start or doing precise brain surgery.
In 30 to 50 years? Let your imagination run wild.
“I’m a skeptic. I like to take research step by step,” she said. “But things are definitely progressing in an interesting direction.”
A version of this article first appeared on WebMD.com.
Care bundle improves outcome in ICH: INTERACT3
The INTERACT3 study showed that timely administration of a care bundle that included early intensive lowering of systolic blood pressure, strict glucose control, treatment of fever, and rapid reversal of abnormal anticoagulation led to less disability, lower rates of death, and better overall quality of life.
“This is a groundbreaking result. It is the first-ever published trial in ICH patients to show a clear benefit on functional outcomes and on mortality,” lead investigator Craig Anderson, MD, director of global brain health at the George Institute for Global Health, Sydney, said in an interview.
“These results show that, if we can organize care and focus on optimal management of these four aspects of the health of the patient, they do better,” Dr. Anderson said.
‘Game changer’
“This is a game changer because now we have level A evidence showing something is definitely beneficial for these patients,” Dr. Anderson added. “That means hospitals have the imperative to organize their systems to do these things and maximize care. We have never had that before.”
Dr. Anderson noted that, while some previous studies have suggested benefit from various interventions, such as early lowering of blood pressure, the results have not been conclusive.
“This means the intervention has not always been implemented, leading to large variations in clinical practice. But now we have a package that is proven to work; this should become a guideline-recommended practice,” he commented.
The INTERACT-3 results were presented at the European Stroke Organisation conference in Munich. They were also simultaneously published online in The Lancet.
Dr. Anderson explained that, until now, there haven’t been any proven treatments for ICH. “There has been a lot of energy and research put into the field, but this has resulted in several interventions that are ‘probably useful’ or which have a level B recommendation,” he said. “No therapy has been shown to be beneficial in a totally conclusive way, so we are still not entirely sure exactly whether the treatments we use actually make a difference.”
The INTERACT3 researchers therefore decided to evaluate a care package consisting of a bundle of several treatments in the hope that they may have additive or synergistic effects.
The study involved 7,036 patients with imaging-confirmed spontaneous ICH who presented within 6 hours of symptom onset to one of 121 hospitals in 10 mainly low- and middle-income countries: Brazil, China, India, Mexico, Nigeria, Pakistan, Peru, Sri Lanka, Vietnam, and Chile.
Using a cluster design, all hospitals started with usual care as a control and then at some point during the study started using the care bundle intervention.
The care-bundle protocol included the early intensive lowering of systolic blood pressure (target, < 140 mm Hg), strict glucose control (target, 6.1-7.8 mmol/L in those without diabetes and 7.8-10.0 mmol/L in those with diabetes), antipyrexia treatment (target body temperature, ≤ 37.5° C), and rapid reversal of warfarin-related anticoagulation (target international normalized ratio, 1.5) in patients for whom these variables were abnormal.
Overall, the modified intention-to-treat population included 3,221 patients who were assigned to the care-bundle group and 3,815 who were assigned to the usual-care group. Primary outcome data were available for 2,892 patients in the care-bundle group and 3,363 patients in the usual-care group.
The primary outcome was functional recovery, measured with the Modified Rankin Scale at 6 months. Results show that the likelihood of a poor functional outcome was lower in the care-bundle group (common odds ratio, 0.86; P = .015).
Patients who received the interventional care bundle also had a significantly lower rate of serious adverse events (16.0% vs. 20.1%) and mortality (14.1% vs. 17.0%).
NNT of 35 to save one life free of disability
“The number needed to treat (NNT) is just 35 to save a life free of disability,” Dr. Anderson commented. “That’s pretty good. We estimate that this care bundle would save tens of thousands of lives a year if universally adopted.”
The intervention group also spent less time in hospital and had improved health-related quality of life.
Dr. Anderson pointed out that the interventions included in the care bundle were all relatively easy to perform.
“They just require a bit more nursing time and the use of a few inexpensive medicines and maybe infusion pumps, but we’re not talking about the need for skilled surgery or a new therapy costing hundreds of thousands of dollars, so this care bundle should be very straightforward to implement. While we haven’t done a formal cost-effectiveness analysis, I would say it will definitely be good value for money.”
Dr. Anderson believes the rapid lowering of blood pressure is a very important part of the care bundle. He noted that target levels were achieved, on average, in 2.3 hours, compared with 4.0 hours in the control group. But he stressed that this was not just a trial of blood pressure reduction and that the whole package is important.
He gave a couple of possible reasons why this trial was successful whereas previous trials did not show a clear benefit of blood pressure lowering in ICH.
“Firstly, it was a very large trial with more than 7,000 patients – that is more than three times larger than any other trial in ICH. And secondly, the package of care means there are several different interventions that together show a real benefit,” he said. “It’s like the polypill, or a rehabilitation program – if you put several different things together, the whole package can show really positive results.”
Dr. Anderson also pointed out that the study included a wide spectrum of ICH patients, and the benefit of the care bundle was seen across all groups and all stroke severities.
“There were a lot of patients with a large ICH, and if anything, they showed an even larger benefit with the bundle of care,” he said.
The researchers note that the burden of ICH is greatest in low- and middle-income countries. In 2019, 30% of all stroke cases in these countries were ICH, almost double the proportion seen in high-income countries (16%). This is in part attributable to high rates of hypertension and limited resources for primary prevention, including identification and management of stroke risk factors by health care services.
‘Outstanding example’ of less therapeutic negativity
Lili Song, MD, PhD, joint lead author and head of the Stroke Program at the George Institute China, Beijing, said, “A lack of proven treatments for ICH has led to a pessimistic view that not much can be done for these patients.
“However, with INTERACT3, we demonstrate on a large scale how readily available treatments can be used to improve outcomes in resource-limited settings,” she said. “We hope this evidence will inform clinical practice guidelines across the globe and help save many lives.”
In a comment that accompanied the article, Wendy Ziai, MD, Matthew Bower, MD, and Daniel Hanley, MD, Johns Hopkins University, Baltimore, say the INTERACT3 study shows that “an intracerebral hemorrhage care bundle focused on physiological control interventions, whether synergistic or not, might promote better outcomes in hospitals where care has not previously optimized sustained interventions.”
Pointing out that the care bundle has minimal risks of cost and coordination and a high public health effect, they conclude: “This effort is an outstanding example of why less therapeutic negativity, and more intervention might benefit survivors of intracerebral hemorrhage.”
The INTERACT3 study was funded by the Department of Health and Social Care, the Foreign, Commonwealth and Development Office, the Medical Research Council, and the Wellcome Trust (all in the United Kingdom), the West China Hospital Outstanding Discipline Development 1–3-5 Programme, the National Health and Medical Research Council of Australia, Sichuan Credit Pharmaceutical, and Takeda (China).
A version of this article first appeared on Medscape.com.
The INTERACT3 study showed that timely administration of a care bundle that included early intensive lowering of systolic blood pressure, strict glucose control, treatment of fever, and rapid reversal of abnormal anticoagulation led to less disability, lower rates of death, and better overall quality of life.
“This is a groundbreaking result. It is the first-ever published trial in ICH patients to show a clear benefit on functional outcomes and on mortality,” lead investigator Craig Anderson, MD, director of global brain health at the George Institute for Global Health, Sydney, said in an interview.
“These results show that, if we can organize care and focus on optimal management of these four aspects of the health of the patient, they do better,” Dr. Anderson said.
‘Game changer’
“This is a game changer because now we have level A evidence showing something is definitely beneficial for these patients,” Dr. Anderson added. “That means hospitals have the imperative to organize their systems to do these things and maximize care. We have never had that before.”
Dr. Anderson noted that, while some previous studies have suggested benefit from various interventions, such as early lowering of blood pressure, the results have not been conclusive.
“This means the intervention has not always been implemented, leading to large variations in clinical practice. But now we have a package that is proven to work; this should become a guideline-recommended practice,” he commented.
The INTERACT-3 results were presented at the European Stroke Organisation conference in Munich. They were also simultaneously published online in The Lancet.
Dr. Anderson explained that, until now, there haven’t been any proven treatments for ICH. “There has been a lot of energy and research put into the field, but this has resulted in several interventions that are ‘probably useful’ or which have a level B recommendation,” he said. “No therapy has been shown to be beneficial in a totally conclusive way, so we are still not entirely sure exactly whether the treatments we use actually make a difference.”
The INTERACT3 researchers therefore decided to evaluate a care package consisting of a bundle of several treatments in the hope that they may have additive or synergistic effects.
The study involved 7,036 patients with imaging-confirmed spontaneous ICH who presented within 6 hours of symptom onset to one of 121 hospitals in 10 mainly low- and middle-income countries: Brazil, China, India, Mexico, Nigeria, Pakistan, Peru, Sri Lanka, Vietnam, and Chile.
Using a cluster design, all hospitals started with usual care as a control and then at some point during the study started using the care bundle intervention.
The care-bundle protocol included the early intensive lowering of systolic blood pressure (target, < 140 mm Hg), strict glucose control (target, 6.1-7.8 mmol/L in those without diabetes and 7.8-10.0 mmol/L in those with diabetes), antipyrexia treatment (target body temperature, ≤ 37.5° C), and rapid reversal of warfarin-related anticoagulation (target international normalized ratio, 1.5) in patients for whom these variables were abnormal.
Overall, the modified intention-to-treat population included 3,221 patients who were assigned to the care-bundle group and 3,815 who were assigned to the usual-care group. Primary outcome data were available for 2,892 patients in the care-bundle group and 3,363 patients in the usual-care group.
The primary outcome was functional recovery, measured with the Modified Rankin Scale at 6 months. Results show that the likelihood of a poor functional outcome was lower in the care-bundle group (common odds ratio, 0.86; P = .015).
Patients who received the interventional care bundle also had a significantly lower rate of serious adverse events (16.0% vs. 20.1%) and mortality (14.1% vs. 17.0%).
NNT of 35 to save one life free of disability
“The number needed to treat (NNT) is just 35 to save a life free of disability,” Dr. Anderson commented. “That’s pretty good. We estimate that this care bundle would save tens of thousands of lives a year if universally adopted.”
The intervention group also spent less time in hospital and had improved health-related quality of life.
Dr. Anderson pointed out that the interventions included in the care bundle were all relatively easy to perform.
“They just require a bit more nursing time and the use of a few inexpensive medicines and maybe infusion pumps, but we’re not talking about the need for skilled surgery or a new therapy costing hundreds of thousands of dollars, so this care bundle should be very straightforward to implement. While we haven’t done a formal cost-effectiveness analysis, I would say it will definitely be good value for money.”
Dr. Anderson believes the rapid lowering of blood pressure is a very important part of the care bundle. He noted that target levels were achieved, on average, in 2.3 hours, compared with 4.0 hours in the control group. But he stressed that this was not just a trial of blood pressure reduction and that the whole package is important.
He gave a couple of possible reasons why this trial was successful whereas previous trials did not show a clear benefit of blood pressure lowering in ICH.
“Firstly, it was a very large trial with more than 7,000 patients – that is more than three times larger than any other trial in ICH. And secondly, the package of care means there are several different interventions that together show a real benefit,” he said. “It’s like the polypill, or a rehabilitation program – if you put several different things together, the whole package can show really positive results.”
Dr. Anderson also pointed out that the study included a wide spectrum of ICH patients, and the benefit of the care bundle was seen across all groups and all stroke severities.
“There were a lot of patients with a large ICH, and if anything, they showed an even larger benefit with the bundle of care,” he said.
The researchers note that the burden of ICH is greatest in low- and middle-income countries. In 2019, 30% of all stroke cases in these countries were ICH, almost double the proportion seen in high-income countries (16%). This is in part attributable to high rates of hypertension and limited resources for primary prevention, including identification and management of stroke risk factors by health care services.
‘Outstanding example’ of less therapeutic negativity
Lili Song, MD, PhD, joint lead author and head of the Stroke Program at the George Institute China, Beijing, said, “A lack of proven treatments for ICH has led to a pessimistic view that not much can be done for these patients.
“However, with INTERACT3, we demonstrate on a large scale how readily available treatments can be used to improve outcomes in resource-limited settings,” she said. “We hope this evidence will inform clinical practice guidelines across the globe and help save many lives.”
In a comment that accompanied the article, Wendy Ziai, MD, Matthew Bower, MD, and Daniel Hanley, MD, Johns Hopkins University, Baltimore, say the INTERACT3 study shows that “an intracerebral hemorrhage care bundle focused on physiological control interventions, whether synergistic or not, might promote better outcomes in hospitals where care has not previously optimized sustained interventions.”
Pointing out that the care bundle has minimal risks of cost and coordination and a high public health effect, they conclude: “This effort is an outstanding example of why less therapeutic negativity, and more intervention might benefit survivors of intracerebral hemorrhage.”
The INTERACT3 study was funded by the Department of Health and Social Care, the Foreign, Commonwealth and Development Office, the Medical Research Council, and the Wellcome Trust (all in the United Kingdom), the West China Hospital Outstanding Discipline Development 1–3-5 Programme, the National Health and Medical Research Council of Australia, Sichuan Credit Pharmaceutical, and Takeda (China).
A version of this article first appeared on Medscape.com.
The INTERACT3 study showed that timely administration of a care bundle that included early intensive lowering of systolic blood pressure, strict glucose control, treatment of fever, and rapid reversal of abnormal anticoagulation led to less disability, lower rates of death, and better overall quality of life.
“This is a groundbreaking result. It is the first-ever published trial in ICH patients to show a clear benefit on functional outcomes and on mortality,” lead investigator Craig Anderson, MD, director of global brain health at the George Institute for Global Health, Sydney, said in an interview.
“These results show that, if we can organize care and focus on optimal management of these four aspects of the health of the patient, they do better,” Dr. Anderson said.
‘Game changer’
“This is a game changer because now we have level A evidence showing something is definitely beneficial for these patients,” Dr. Anderson added. “That means hospitals have the imperative to organize their systems to do these things and maximize care. We have never had that before.”
Dr. Anderson noted that, while some previous studies have suggested benefit from various interventions, such as early lowering of blood pressure, the results have not been conclusive.
“This means the intervention has not always been implemented, leading to large variations in clinical practice. But now we have a package that is proven to work; this should become a guideline-recommended practice,” he commented.
The INTERACT-3 results were presented at the European Stroke Organisation conference in Munich. They were also simultaneously published online in The Lancet.
Dr. Anderson explained that, until now, there haven’t been any proven treatments for ICH. “There has been a lot of energy and research put into the field, but this has resulted in several interventions that are ‘probably useful’ or which have a level B recommendation,” he said. “No therapy has been shown to be beneficial in a totally conclusive way, so we are still not entirely sure exactly whether the treatments we use actually make a difference.”
The INTERACT3 researchers therefore decided to evaluate a care package consisting of a bundle of several treatments in the hope that they may have additive or synergistic effects.
The study involved 7,036 patients with imaging-confirmed spontaneous ICH who presented within 6 hours of symptom onset to one of 121 hospitals in 10 mainly low- and middle-income countries: Brazil, China, India, Mexico, Nigeria, Pakistan, Peru, Sri Lanka, Vietnam, and Chile.
Using a cluster design, all hospitals started with usual care as a control and then at some point during the study started using the care bundle intervention.
The care-bundle protocol included the early intensive lowering of systolic blood pressure (target, < 140 mm Hg), strict glucose control (target, 6.1-7.8 mmol/L in those without diabetes and 7.8-10.0 mmol/L in those with diabetes), antipyrexia treatment (target body temperature, ≤ 37.5° C), and rapid reversal of warfarin-related anticoagulation (target international normalized ratio, 1.5) in patients for whom these variables were abnormal.
Overall, the modified intention-to-treat population included 3,221 patients who were assigned to the care-bundle group and 3,815 who were assigned to the usual-care group. Primary outcome data were available for 2,892 patients in the care-bundle group and 3,363 patients in the usual-care group.
The primary outcome was functional recovery, measured with the Modified Rankin Scale at 6 months. Results show that the likelihood of a poor functional outcome was lower in the care-bundle group (common odds ratio, 0.86; P = .015).
Patients who received the interventional care bundle also had a significantly lower rate of serious adverse events (16.0% vs. 20.1%) and mortality (14.1% vs. 17.0%).
NNT of 35 to save one life free of disability
“The number needed to treat (NNT) is just 35 to save a life free of disability,” Dr. Anderson commented. “That’s pretty good. We estimate that this care bundle would save tens of thousands of lives a year if universally adopted.”
The intervention group also spent less time in hospital and had improved health-related quality of life.
Dr. Anderson pointed out that the interventions included in the care bundle were all relatively easy to perform.
“They just require a bit more nursing time and the use of a few inexpensive medicines and maybe infusion pumps, but we’re not talking about the need for skilled surgery or a new therapy costing hundreds of thousands of dollars, so this care bundle should be very straightforward to implement. While we haven’t done a formal cost-effectiveness analysis, I would say it will definitely be good value for money.”
Dr. Anderson believes the rapid lowering of blood pressure is a very important part of the care bundle. He noted that target levels were achieved, on average, in 2.3 hours, compared with 4.0 hours in the control group. But he stressed that this was not just a trial of blood pressure reduction and that the whole package is important.
He gave a couple of possible reasons why this trial was successful whereas previous trials did not show a clear benefit of blood pressure lowering in ICH.
“Firstly, it was a very large trial with more than 7,000 patients – that is more than three times larger than any other trial in ICH. And secondly, the package of care means there are several different interventions that together show a real benefit,” he said. “It’s like the polypill, or a rehabilitation program – if you put several different things together, the whole package can show really positive results.”
Dr. Anderson also pointed out that the study included a wide spectrum of ICH patients, and the benefit of the care bundle was seen across all groups and all stroke severities.
“There were a lot of patients with a large ICH, and if anything, they showed an even larger benefit with the bundle of care,” he said.
The researchers note that the burden of ICH is greatest in low- and middle-income countries. In 2019, 30% of all stroke cases in these countries were ICH, almost double the proportion seen in high-income countries (16%). This is in part attributable to high rates of hypertension and limited resources for primary prevention, including identification and management of stroke risk factors by health care services.
‘Outstanding example’ of less therapeutic negativity
Lili Song, MD, PhD, joint lead author and head of the Stroke Program at the George Institute China, Beijing, said, “A lack of proven treatments for ICH has led to a pessimistic view that not much can be done for these patients.
“However, with INTERACT3, we demonstrate on a large scale how readily available treatments can be used to improve outcomes in resource-limited settings,” she said. “We hope this evidence will inform clinical practice guidelines across the globe and help save many lives.”
In a comment that accompanied the article, Wendy Ziai, MD, Matthew Bower, MD, and Daniel Hanley, MD, Johns Hopkins University, Baltimore, say the INTERACT3 study shows that “an intracerebral hemorrhage care bundle focused on physiological control interventions, whether synergistic or not, might promote better outcomes in hospitals where care has not previously optimized sustained interventions.”
Pointing out that the care bundle has minimal risks of cost and coordination and a high public health effect, they conclude: “This effort is an outstanding example of why less therapeutic negativity, and more intervention might benefit survivors of intracerebral hemorrhage.”
The INTERACT3 study was funded by the Department of Health and Social Care, the Foreign, Commonwealth and Development Office, the Medical Research Council, and the Wellcome Trust (all in the United Kingdom), the West China Hospital Outstanding Discipline Development 1–3-5 Programme, the National Health and Medical Research Council of Australia, Sichuan Credit Pharmaceutical, and Takeda (China).
A version of this article first appeared on Medscape.com.
FROM ESO 2023
Younger age of type 2 diabetes onset linked to dementia risk
, new findings suggest.
Moreover, the new data from the prospective Atherosclerosis Risk in Communities (ARIC) cohort also suggest that the previously identified increased risk for dementia among people with prediabetes appears to be entirely explained by the subset who go on to develop type 2 diabetes.
“Our findings suggest that preventing prediabetes progression, especially in younger individuals, may be an important way to reduce the dementia burden,” wrote PhD student Jiaqi Hu of Johns Hopkins University, Baltimore, and colleagues. Their article was published online in Diabetologia.
The result builds on previous findings linking dysglycemia and cognitive decline, the study’s lead author, Elizabeth Selvin, PhD, of the Bloomberg School of Public Health at Johns Hopkins, said in an interview.
“Our prior work in the ARIC study suggests that improving glucose control could help prevent dementia in later life,” she said.
Other studies have also linked higher A1c levels and diabetes in midlife to increased rates of cognitive decline. In addition, Dr. Selvin noted, “There is growing evidence that focusing on vascular health, especially focusing on diabetes and blood pressure, in midlife can stave off dementia in later life.”
This new study is the first to examine the effect of diabetes in the relationship between prediabetes and dementia, as well as the age of diabetes onset on subsequent dementia.
Prediabetes linked to dementia via diabetes development
Of the 11,656 ARIC participants without diabetes at baseline during 1990-1992 (age 46-70 years), 20.0% had prediabetes (defined as A1c 5.7%-6.4% or 39-46 mmol/mol). During a median follow-up of 15.9 years, 3,143 participants developed diabetes. The proportions of patients who developed diabetes were 44.6% among those with prediabetes at baseline versus 22.5% of those without.
Dementia developed in 2,247 participants over a median follow-up of 24.7 years. The cumulative incidence of dementia was 23.9% among those who developed diabetes versus 20.5% among those who did not.
After adjustment for demographics and for the Alzheimer’s disease–linked apolipoprotein E (APOE) gene, prediabetes was significantly associated with incident dementia (hazard ratio [HR], 1.19). However, significance disappeared after adjustment for incident diabetes (HR, 1.09), the researchers reported.
Younger age at diabetes diagnosis raises dementia risk
Age at diabetes diagnosis made a difference in dementia risk. With adjustments for lifestyle, demographic, and clinical factors, those diagnosed with diabetes before age 60 years had a nearly threefold increased risk for dementia compared with those who never developed diabetes (HR, 2.92; P < .001).
The dementia risk was also significantly increased, although to a lesser degree, among those aged 60-69 years at diabetes diagnosis (HR, 1.73; P < .001) and age 70-79 years at diabetes diagnosis (HR, 1.23; P < .001). The relationship was not significant for those aged 80 years and older (HR, 1.13).
“Prevention efforts in people with diabetes diagnosed younger than 65 years should be a high priority,” the authors urged.
Taken together, the data suggest that prolonged exposure to hyperglycemia plays a major role in dementia development.
“Putative mechanisms include acute and chronic hyperglycemia, glucose toxicity, insulin resistance, and microvascular dysfunction of the central nervous system. ... Glucose toxicity and microvascular dysfunction are associated with increased inflammatory and oxidative stress, leading to increased blood–brain permeability,” the researchers wrote.
Dr. Selvin said that her group is pursuing further work in this area using continuous glucose monitoring. “We plan to look at ... how glycemic control and different patterns of glucose in older adults may be linked to cognitive decline and other neurocognitive outcomes.”
The researchers reported no relevant financial relationships. Dr. Selvin has reported being on the advisory board for Diabetologia; she had no role in peer review of the manuscript.
A version of this article first appeared on Medscape.com.
, new findings suggest.
Moreover, the new data from the prospective Atherosclerosis Risk in Communities (ARIC) cohort also suggest that the previously identified increased risk for dementia among people with prediabetes appears to be entirely explained by the subset who go on to develop type 2 diabetes.
“Our findings suggest that preventing prediabetes progression, especially in younger individuals, may be an important way to reduce the dementia burden,” wrote PhD student Jiaqi Hu of Johns Hopkins University, Baltimore, and colleagues. Their article was published online in Diabetologia.
The result builds on previous findings linking dysglycemia and cognitive decline, the study’s lead author, Elizabeth Selvin, PhD, of the Bloomberg School of Public Health at Johns Hopkins, said in an interview.
“Our prior work in the ARIC study suggests that improving glucose control could help prevent dementia in later life,” she said.
Other studies have also linked higher A1c levels and diabetes in midlife to increased rates of cognitive decline. In addition, Dr. Selvin noted, “There is growing evidence that focusing on vascular health, especially focusing on diabetes and blood pressure, in midlife can stave off dementia in later life.”
This new study is the first to examine the effect of diabetes in the relationship between prediabetes and dementia, as well as the age of diabetes onset on subsequent dementia.
Prediabetes linked to dementia via diabetes development
Of the 11,656 ARIC participants without diabetes at baseline during 1990-1992 (age 46-70 years), 20.0% had prediabetes (defined as A1c 5.7%-6.4% or 39-46 mmol/mol). During a median follow-up of 15.9 years, 3,143 participants developed diabetes. The proportions of patients who developed diabetes were 44.6% among those with prediabetes at baseline versus 22.5% of those without.
Dementia developed in 2,247 participants over a median follow-up of 24.7 years. The cumulative incidence of dementia was 23.9% among those who developed diabetes versus 20.5% among those who did not.
After adjustment for demographics and for the Alzheimer’s disease–linked apolipoprotein E (APOE) gene, prediabetes was significantly associated with incident dementia (hazard ratio [HR], 1.19). However, significance disappeared after adjustment for incident diabetes (HR, 1.09), the researchers reported.
Younger age at diabetes diagnosis raises dementia risk
Age at diabetes diagnosis made a difference in dementia risk. With adjustments for lifestyle, demographic, and clinical factors, those diagnosed with diabetes before age 60 years had a nearly threefold increased risk for dementia compared with those who never developed diabetes (HR, 2.92; P < .001).
The dementia risk was also significantly increased, although to a lesser degree, among those aged 60-69 years at diabetes diagnosis (HR, 1.73; P < .001) and age 70-79 years at diabetes diagnosis (HR, 1.23; P < .001). The relationship was not significant for those aged 80 years and older (HR, 1.13).
“Prevention efforts in people with diabetes diagnosed younger than 65 years should be a high priority,” the authors urged.
Taken together, the data suggest that prolonged exposure to hyperglycemia plays a major role in dementia development.
“Putative mechanisms include acute and chronic hyperglycemia, glucose toxicity, insulin resistance, and microvascular dysfunction of the central nervous system. ... Glucose toxicity and microvascular dysfunction are associated with increased inflammatory and oxidative stress, leading to increased blood–brain permeability,” the researchers wrote.
Dr. Selvin said that her group is pursuing further work in this area using continuous glucose monitoring. “We plan to look at ... how glycemic control and different patterns of glucose in older adults may be linked to cognitive decline and other neurocognitive outcomes.”
The researchers reported no relevant financial relationships. Dr. Selvin has reported being on the advisory board for Diabetologia; she had no role in peer review of the manuscript.
A version of this article first appeared on Medscape.com.
, new findings suggest.
Moreover, the new data from the prospective Atherosclerosis Risk in Communities (ARIC) cohort also suggest that the previously identified increased risk for dementia among people with prediabetes appears to be entirely explained by the subset who go on to develop type 2 diabetes.
“Our findings suggest that preventing prediabetes progression, especially in younger individuals, may be an important way to reduce the dementia burden,” wrote PhD student Jiaqi Hu of Johns Hopkins University, Baltimore, and colleagues. Their article was published online in Diabetologia.
The result builds on previous findings linking dysglycemia and cognitive decline, the study’s lead author, Elizabeth Selvin, PhD, of the Bloomberg School of Public Health at Johns Hopkins, said in an interview.
“Our prior work in the ARIC study suggests that improving glucose control could help prevent dementia in later life,” she said.
Other studies have also linked higher A1c levels and diabetes in midlife to increased rates of cognitive decline. In addition, Dr. Selvin noted, “There is growing evidence that focusing on vascular health, especially focusing on diabetes and blood pressure, in midlife can stave off dementia in later life.”
This new study is the first to examine the effect of diabetes in the relationship between prediabetes and dementia, as well as the age of diabetes onset on subsequent dementia.
Prediabetes linked to dementia via diabetes development
Of the 11,656 ARIC participants without diabetes at baseline during 1990-1992 (age 46-70 years), 20.0% had prediabetes (defined as A1c 5.7%-6.4% or 39-46 mmol/mol). During a median follow-up of 15.9 years, 3,143 participants developed diabetes. The proportions of patients who developed diabetes were 44.6% among those with prediabetes at baseline versus 22.5% of those without.
Dementia developed in 2,247 participants over a median follow-up of 24.7 years. The cumulative incidence of dementia was 23.9% among those who developed diabetes versus 20.5% among those who did not.
After adjustment for demographics and for the Alzheimer’s disease–linked apolipoprotein E (APOE) gene, prediabetes was significantly associated with incident dementia (hazard ratio [HR], 1.19). However, significance disappeared after adjustment for incident diabetes (HR, 1.09), the researchers reported.
Younger age at diabetes diagnosis raises dementia risk
Age at diabetes diagnosis made a difference in dementia risk. With adjustments for lifestyle, demographic, and clinical factors, those diagnosed with diabetes before age 60 years had a nearly threefold increased risk for dementia compared with those who never developed diabetes (HR, 2.92; P < .001).
The dementia risk was also significantly increased, although to a lesser degree, among those aged 60-69 years at diabetes diagnosis (HR, 1.73; P < .001) and age 70-79 years at diabetes diagnosis (HR, 1.23; P < .001). The relationship was not significant for those aged 80 years and older (HR, 1.13).
“Prevention efforts in people with diabetes diagnosed younger than 65 years should be a high priority,” the authors urged.
Taken together, the data suggest that prolonged exposure to hyperglycemia plays a major role in dementia development.
“Putative mechanisms include acute and chronic hyperglycemia, glucose toxicity, insulin resistance, and microvascular dysfunction of the central nervous system. ... Glucose toxicity and microvascular dysfunction are associated with increased inflammatory and oxidative stress, leading to increased blood–brain permeability,” the researchers wrote.
Dr. Selvin said that her group is pursuing further work in this area using continuous glucose monitoring. “We plan to look at ... how glycemic control and different patterns of glucose in older adults may be linked to cognitive decline and other neurocognitive outcomes.”
The researchers reported no relevant financial relationships. Dr. Selvin has reported being on the advisory board for Diabetologia; she had no role in peer review of the manuscript.
A version of this article first appeared on Medscape.com.
FROM DIABETOLOGIA
Earlier anticoagulation safe in stroke with AFib: ELAN
, a new study suggests.
The ELAN trial found that starting DOAC treatment earlier was not associated with an increased risk for intracranial hemorrhage (ICH) but rather was linked to a lower rate of ischemic events.
“We conclude that there is no reason to delay DOAC treatment in these patients. Our results suggest that early DOAC treatment is reasonable; it is unlikely to cause harm, and it is probably better at reducing ischemic events,” lead investigator of the study, Urs Fischer, MD, professor of neurology at University Hospital Basel (Switzerland), commented in an interview.
“This trial will change clinical practice in that we can feel much more reassured that starting DOAC treatment early in these patients will not cause harm,” he said.
Senior investigator Jesse Dawson, MD, professor of stroke medicine at Queen Elizabeth University Hospital, Glasgow, added: “This issue of timing of DOAC treatment causes a lot of anxiety in our daily workload. Clinicians are scared of causing an ICH, so they tend to wait. These results will ease a lot of that anxiety.”
Dr. Fischer presented the results of the ELAN trial at the European Stroke Organisation Conference (ESOC) in Munich. The trial was also simultaneously published online in The New England Journal of Medicine.
He explained that patients presenting with acute ischemic stroke who are found to have atrial fibrillation need to be started on anticoagulation to reduce the risk for a recurrent stroke. But there are no clear guidelines on when to start anticoagulation in these patients at present, with concerns that starting very early may increase the risk for hemorrhagic transformation and ICH.
Based on observations that patients with larger strokes have a higher risk for ICH in the early post-stroke period, some guidelines advise different times for starting anticoagulation for different stroke severities: 1 day for a transient ischemic attack, 3 days for a minor stroke, 6 days for a moderate stroke, and 12 days for a severe stroke – known as the 1-, 3-, 6-, 12-day rule.
“But this is not based on evidence – just on expert opinion,” Dr. Fischer noted. “The ELAN trial was conducted to obtain more solid information on optimal timing for starting anticoagulation and whether we can safely start a DOAC earlier than these guidelines currently advise.”
For the trial, which was conducted in 15 countries, 2,013 patients with an acute ischemic stroke and found to have AFib were randomly selected to start DOAC treatment earlier or later.
The later-treatment strategy followed the current approach of starting treatment at day 3 or 4 after a minor stroke, day 6 or 7 after a moderate stroke, or day 12, 13, or 14 after a major stroke, whereas the earlier-treatment group started DOAC treatment within 48 hours after a minor or moderate stroke or on day 6 or 7 after a major stroke.
In terms of stroke severity, which was defined on imaging-based criteria, 37% of patients had a minor stroke, 40% had a moderate stroke, and 23% had a major stroke.
The primary outcome was a composite of recurrent ischemic stroke, systemic embolism, major extracranial bleeding, symptomatic intracranial hemorrhage, or vascular death within 30 days after randomization.
Results showed that this occurred in 2.9% in the early-treatment group and 4.1% in the later-treatment group (risk difference, –1.18 percentage points; 95% confidence interval, –2.84-0.47) by 30 days.
Recurrent ischemic stroke occurred in 1.4% in the early-treatment group and 2.5% in the later-treatment group (odds ratio, 0.57; 95% CI, 0.29-1.07). Symptomatic intracranial hemorrhage occurred in two participants (0.2%) in both groups by 30 days.
The rates of the outcomes increased only slightly more at 90 days than at 30 days, “findings that suggest there was not an excessive risk associated with early anticoagulation through that period,” the researchers report in the NEJM paper.
“Early treatment initiation can therefore be supported if indicated or if desired,” they conclude.
“The most important finding was that among 2,000 patients randomized, there was a very low rate of bleeding complications and no increase in any bleeding complication in the early DOAC group. This has been a major worry about starting anticoagulation early,” Dr. Fischer commented.
“These are very practical findings in that we can keep things simple,” Dr. Dawson added. “If the patient has a big stroke, anticoagulation with a DOAC can now be started at 6 days. For everyone else, we can start DOAC treatment as soon as possible without fear of causing harm. So, we can now confidently give patients with a minor or moderate stroke, as defined by imaging, a beneficial treatment as soon as we establish they are having an ischemic stroke and have AFib.”
Dr. Dawson pointed out that about 25% of patients with ischemic stroke are found to have AFib on admission ECG, and in another 4%-5%, AFib is found in the first 48 hours. “These are the patients we are targeting in this study.”
The researchers note that the trial did not have a statistical superiority or noninferiority design but rather aimed to estimate the treatment effects of early initiation versus later initiation of DOACs.
“This trial was slightly different in that we weren’t testing a strict statistical hypothesis because we didn’t have any data with which to formulate what sort of effect size to aim for, so we performed a qualitative trial to look at what the event rates were with the two approaches,” Dr. Fischer explained. “Our main findings are that ICH rates were not increased with early DOAC treatment and that ischemic event rates were numerically reduced, but because we didn’t have strict statistical limits, we can only say this is a high probability but not a certainty.”
Dr. Dawson added: “We can say from these results that there is a high level of probability that early DOAC treatment does not cause harm and a reasonable probability that it reduces risks of a recurrent stroke or other ischemic event.”
The researchers give an estimate of the effect size for the primary composite endpoint, which combines the major ischemic and bleeding events, ranging from a 2.8% lower risk to a 0.5% higher risk with early DOAC treatment.
“So, it is very likely that the composite endpoint would be lower,” Dr. Dawson said.
Dr. Fischer noted that a previous study (TIMING) tried to address the issue of earlier versus later anticoagulation in these patients but was stopped early after 880 patients had been enrolled because of slow recruitment.
“Results from this study failed to show superiority of early versus late DOAC treatment but they did suggest noninferiority, and they also found no increase in major bleeding complications, which is an added reassurance,” he commented.
Another trial looking at early versus late anticoagulation in these patients, OPTIMAS, is ongoing in the United Kingdom and is aiming to randomize 3,500 patients.
Imaging-based assessment of stroke severity
In the ELAN trial, the definition of stroke severity was based on imaging rather than on the National Institutes of Health Stroke Scale (NIHSS).
“We took a cautious approach by using imaging to define stroke severity. So, when using these results in clinical practice, it is important that patients are selected for the timing of DOAC treatment based on the imaging results,” Dr. Dawson explained. “This is very straightforward, as the size of the stroke can be seen clearly on the routine CT imaging that all patients receive up front. This is a very pragmatic and simple protocol. And advanced imaging is not required.”
He noted that though clinicians tend to use the NIHSS clinical symptom score to define mild, moderate, and severe stroke, the imaging approach is actually more accurate when determining the risk for bleeding and ICH. And though imaging results often correlate with NIHSS scores, there can be some exceptions.
Commenting on the ELAN trial results at the ESOC meeting, Georgios Tsivgoulis, MD, professor of neurology, University of Athens, said that the trial showed that early administration of DOACs in these patients was safe and did not increase the rate of ICH.
“There was a very low ICH rate with only two events in each group. And then there was above a 1% reduction in the composite outcome including ischemic vascular events and bleeding,” he noted.
“This is important because there are many thousands of patients with acute ischemic stroke and AFib, and now we have a large study showing we can treat them with a DOAC early, and this appears to be safe and it appears also be more effective in terms of outcome events,” Dr. Tsivgoulis said.
But he highlighted one important caveat: The majority of patients had mild or moderate stroke.
A version of this article first appeared on Medscape.com.
, a new study suggests.
The ELAN trial found that starting DOAC treatment earlier was not associated with an increased risk for intracranial hemorrhage (ICH) but rather was linked to a lower rate of ischemic events.
“We conclude that there is no reason to delay DOAC treatment in these patients. Our results suggest that early DOAC treatment is reasonable; it is unlikely to cause harm, and it is probably better at reducing ischemic events,” lead investigator of the study, Urs Fischer, MD, professor of neurology at University Hospital Basel (Switzerland), commented in an interview.
“This trial will change clinical practice in that we can feel much more reassured that starting DOAC treatment early in these patients will not cause harm,” he said.
Senior investigator Jesse Dawson, MD, professor of stroke medicine at Queen Elizabeth University Hospital, Glasgow, added: “This issue of timing of DOAC treatment causes a lot of anxiety in our daily workload. Clinicians are scared of causing an ICH, so they tend to wait. These results will ease a lot of that anxiety.”
Dr. Fischer presented the results of the ELAN trial at the European Stroke Organisation Conference (ESOC) in Munich. The trial was also simultaneously published online in The New England Journal of Medicine.
He explained that patients presenting with acute ischemic stroke who are found to have atrial fibrillation need to be started on anticoagulation to reduce the risk for a recurrent stroke. But there are no clear guidelines on when to start anticoagulation in these patients at present, with concerns that starting very early may increase the risk for hemorrhagic transformation and ICH.
Based on observations that patients with larger strokes have a higher risk for ICH in the early post-stroke period, some guidelines advise different times for starting anticoagulation for different stroke severities: 1 day for a transient ischemic attack, 3 days for a minor stroke, 6 days for a moderate stroke, and 12 days for a severe stroke – known as the 1-, 3-, 6-, 12-day rule.
“But this is not based on evidence – just on expert opinion,” Dr. Fischer noted. “The ELAN trial was conducted to obtain more solid information on optimal timing for starting anticoagulation and whether we can safely start a DOAC earlier than these guidelines currently advise.”
For the trial, which was conducted in 15 countries, 2,013 patients with an acute ischemic stroke and found to have AFib were randomly selected to start DOAC treatment earlier or later.
The later-treatment strategy followed the current approach of starting treatment at day 3 or 4 after a minor stroke, day 6 or 7 after a moderate stroke, or day 12, 13, or 14 after a major stroke, whereas the earlier-treatment group started DOAC treatment within 48 hours after a minor or moderate stroke or on day 6 or 7 after a major stroke.
In terms of stroke severity, which was defined on imaging-based criteria, 37% of patients had a minor stroke, 40% had a moderate stroke, and 23% had a major stroke.
The primary outcome was a composite of recurrent ischemic stroke, systemic embolism, major extracranial bleeding, symptomatic intracranial hemorrhage, or vascular death within 30 days after randomization.
Results showed that this occurred in 2.9% in the early-treatment group and 4.1% in the later-treatment group (risk difference, –1.18 percentage points; 95% confidence interval, –2.84-0.47) by 30 days.
Recurrent ischemic stroke occurred in 1.4% in the early-treatment group and 2.5% in the later-treatment group (odds ratio, 0.57; 95% CI, 0.29-1.07). Symptomatic intracranial hemorrhage occurred in two participants (0.2%) in both groups by 30 days.
The rates of the outcomes increased only slightly more at 90 days than at 30 days, “findings that suggest there was not an excessive risk associated with early anticoagulation through that period,” the researchers report in the NEJM paper.
“Early treatment initiation can therefore be supported if indicated or if desired,” they conclude.
“The most important finding was that among 2,000 patients randomized, there was a very low rate of bleeding complications and no increase in any bleeding complication in the early DOAC group. This has been a major worry about starting anticoagulation early,” Dr. Fischer commented.
“These are very practical findings in that we can keep things simple,” Dr. Dawson added. “If the patient has a big stroke, anticoagulation with a DOAC can now be started at 6 days. For everyone else, we can start DOAC treatment as soon as possible without fear of causing harm. So, we can now confidently give patients with a minor or moderate stroke, as defined by imaging, a beneficial treatment as soon as we establish they are having an ischemic stroke and have AFib.”
Dr. Dawson pointed out that about 25% of patients with ischemic stroke are found to have AFib on admission ECG, and in another 4%-5%, AFib is found in the first 48 hours. “These are the patients we are targeting in this study.”
The researchers note that the trial did not have a statistical superiority or noninferiority design but rather aimed to estimate the treatment effects of early initiation versus later initiation of DOACs.
“This trial was slightly different in that we weren’t testing a strict statistical hypothesis because we didn’t have any data with which to formulate what sort of effect size to aim for, so we performed a qualitative trial to look at what the event rates were with the two approaches,” Dr. Fischer explained. “Our main findings are that ICH rates were not increased with early DOAC treatment and that ischemic event rates were numerically reduced, but because we didn’t have strict statistical limits, we can only say this is a high probability but not a certainty.”
Dr. Dawson added: “We can say from these results that there is a high level of probability that early DOAC treatment does not cause harm and a reasonable probability that it reduces risks of a recurrent stroke or other ischemic event.”
The researchers give an estimate of the effect size for the primary composite endpoint, which combines the major ischemic and bleeding events, ranging from a 2.8% lower risk to a 0.5% higher risk with early DOAC treatment.
“So, it is very likely that the composite endpoint would be lower,” Dr. Dawson said.
Dr. Fischer noted that a previous study (TIMING) tried to address the issue of earlier versus later anticoagulation in these patients but was stopped early after 880 patients had been enrolled because of slow recruitment.
“Results from this study failed to show superiority of early versus late DOAC treatment but they did suggest noninferiority, and they also found no increase in major bleeding complications, which is an added reassurance,” he commented.
Another trial looking at early versus late anticoagulation in these patients, OPTIMAS, is ongoing in the United Kingdom and is aiming to randomize 3,500 patients.
Imaging-based assessment of stroke severity
In the ELAN trial, the definition of stroke severity was based on imaging rather than on the National Institutes of Health Stroke Scale (NIHSS).
“We took a cautious approach by using imaging to define stroke severity. So, when using these results in clinical practice, it is important that patients are selected for the timing of DOAC treatment based on the imaging results,” Dr. Dawson explained. “This is very straightforward, as the size of the stroke can be seen clearly on the routine CT imaging that all patients receive up front. This is a very pragmatic and simple protocol. And advanced imaging is not required.”
He noted that though clinicians tend to use the NIHSS clinical symptom score to define mild, moderate, and severe stroke, the imaging approach is actually more accurate when determining the risk for bleeding and ICH. And though imaging results often correlate with NIHSS scores, there can be some exceptions.
Commenting on the ELAN trial results at the ESOC meeting, Georgios Tsivgoulis, MD, professor of neurology, University of Athens, said that the trial showed that early administration of DOACs in these patients was safe and did not increase the rate of ICH.
“There was a very low ICH rate with only two events in each group. And then there was above a 1% reduction in the composite outcome including ischemic vascular events and bleeding,” he noted.
“This is important because there are many thousands of patients with acute ischemic stroke and AFib, and now we have a large study showing we can treat them with a DOAC early, and this appears to be safe and it appears also be more effective in terms of outcome events,” Dr. Tsivgoulis said.
But he highlighted one important caveat: The majority of patients had mild or moderate stroke.
A version of this article first appeared on Medscape.com.
, a new study suggests.
The ELAN trial found that starting DOAC treatment earlier was not associated with an increased risk for intracranial hemorrhage (ICH) but rather was linked to a lower rate of ischemic events.
“We conclude that there is no reason to delay DOAC treatment in these patients. Our results suggest that early DOAC treatment is reasonable; it is unlikely to cause harm, and it is probably better at reducing ischemic events,” lead investigator of the study, Urs Fischer, MD, professor of neurology at University Hospital Basel (Switzerland), commented in an interview.
“This trial will change clinical practice in that we can feel much more reassured that starting DOAC treatment early in these patients will not cause harm,” he said.
Senior investigator Jesse Dawson, MD, professor of stroke medicine at Queen Elizabeth University Hospital, Glasgow, added: “This issue of timing of DOAC treatment causes a lot of anxiety in our daily workload. Clinicians are scared of causing an ICH, so they tend to wait. These results will ease a lot of that anxiety.”
Dr. Fischer presented the results of the ELAN trial at the European Stroke Organisation Conference (ESOC) in Munich. The trial was also simultaneously published online in The New England Journal of Medicine.
He explained that patients presenting with acute ischemic stroke who are found to have atrial fibrillation need to be started on anticoagulation to reduce the risk for a recurrent stroke. But there are no clear guidelines on when to start anticoagulation in these patients at present, with concerns that starting very early may increase the risk for hemorrhagic transformation and ICH.
Based on observations that patients with larger strokes have a higher risk for ICH in the early post-stroke period, some guidelines advise different times for starting anticoagulation for different stroke severities: 1 day for a transient ischemic attack, 3 days for a minor stroke, 6 days for a moderate stroke, and 12 days for a severe stroke – known as the 1-, 3-, 6-, 12-day rule.
“But this is not based on evidence – just on expert opinion,” Dr. Fischer noted. “The ELAN trial was conducted to obtain more solid information on optimal timing for starting anticoagulation and whether we can safely start a DOAC earlier than these guidelines currently advise.”
For the trial, which was conducted in 15 countries, 2,013 patients with an acute ischemic stroke and found to have AFib were randomly selected to start DOAC treatment earlier or later.
The later-treatment strategy followed the current approach of starting treatment at day 3 or 4 after a minor stroke, day 6 or 7 after a moderate stroke, or day 12, 13, or 14 after a major stroke, whereas the earlier-treatment group started DOAC treatment within 48 hours after a minor or moderate stroke or on day 6 or 7 after a major stroke.
In terms of stroke severity, which was defined on imaging-based criteria, 37% of patients had a minor stroke, 40% had a moderate stroke, and 23% had a major stroke.
The primary outcome was a composite of recurrent ischemic stroke, systemic embolism, major extracranial bleeding, symptomatic intracranial hemorrhage, or vascular death within 30 days after randomization.
Results showed that this occurred in 2.9% in the early-treatment group and 4.1% in the later-treatment group (risk difference, –1.18 percentage points; 95% confidence interval, –2.84-0.47) by 30 days.
Recurrent ischemic stroke occurred in 1.4% in the early-treatment group and 2.5% in the later-treatment group (odds ratio, 0.57; 95% CI, 0.29-1.07). Symptomatic intracranial hemorrhage occurred in two participants (0.2%) in both groups by 30 days.
The rates of the outcomes increased only slightly more at 90 days than at 30 days, “findings that suggest there was not an excessive risk associated with early anticoagulation through that period,” the researchers report in the NEJM paper.
“Early treatment initiation can therefore be supported if indicated or if desired,” they conclude.
“The most important finding was that among 2,000 patients randomized, there was a very low rate of bleeding complications and no increase in any bleeding complication in the early DOAC group. This has been a major worry about starting anticoagulation early,” Dr. Fischer commented.
“These are very practical findings in that we can keep things simple,” Dr. Dawson added. “If the patient has a big stroke, anticoagulation with a DOAC can now be started at 6 days. For everyone else, we can start DOAC treatment as soon as possible without fear of causing harm. So, we can now confidently give patients with a minor or moderate stroke, as defined by imaging, a beneficial treatment as soon as we establish they are having an ischemic stroke and have AFib.”
Dr. Dawson pointed out that about 25% of patients with ischemic stroke are found to have AFib on admission ECG, and in another 4%-5%, AFib is found in the first 48 hours. “These are the patients we are targeting in this study.”
The researchers note that the trial did not have a statistical superiority or noninferiority design but rather aimed to estimate the treatment effects of early initiation versus later initiation of DOACs.
“This trial was slightly different in that we weren’t testing a strict statistical hypothesis because we didn’t have any data with which to formulate what sort of effect size to aim for, so we performed a qualitative trial to look at what the event rates were with the two approaches,” Dr. Fischer explained. “Our main findings are that ICH rates were not increased with early DOAC treatment and that ischemic event rates were numerically reduced, but because we didn’t have strict statistical limits, we can only say this is a high probability but not a certainty.”
Dr. Dawson added: “We can say from these results that there is a high level of probability that early DOAC treatment does not cause harm and a reasonable probability that it reduces risks of a recurrent stroke or other ischemic event.”
The researchers give an estimate of the effect size for the primary composite endpoint, which combines the major ischemic and bleeding events, ranging from a 2.8% lower risk to a 0.5% higher risk with early DOAC treatment.
“So, it is very likely that the composite endpoint would be lower,” Dr. Dawson said.
Dr. Fischer noted that a previous study (TIMING) tried to address the issue of earlier versus later anticoagulation in these patients but was stopped early after 880 patients had been enrolled because of slow recruitment.
“Results from this study failed to show superiority of early versus late DOAC treatment but they did suggest noninferiority, and they also found no increase in major bleeding complications, which is an added reassurance,” he commented.
Another trial looking at early versus late anticoagulation in these patients, OPTIMAS, is ongoing in the United Kingdom and is aiming to randomize 3,500 patients.
Imaging-based assessment of stroke severity
In the ELAN trial, the definition of stroke severity was based on imaging rather than on the National Institutes of Health Stroke Scale (NIHSS).
“We took a cautious approach by using imaging to define stroke severity. So, when using these results in clinical practice, it is important that patients are selected for the timing of DOAC treatment based on the imaging results,” Dr. Dawson explained. “This is very straightforward, as the size of the stroke can be seen clearly on the routine CT imaging that all patients receive up front. This is a very pragmatic and simple protocol. And advanced imaging is not required.”
He noted that though clinicians tend to use the NIHSS clinical symptom score to define mild, moderate, and severe stroke, the imaging approach is actually more accurate when determining the risk for bleeding and ICH. And though imaging results often correlate with NIHSS scores, there can be some exceptions.
Commenting on the ELAN trial results at the ESOC meeting, Georgios Tsivgoulis, MD, professor of neurology, University of Athens, said that the trial showed that early administration of DOACs in these patients was safe and did not increase the rate of ICH.
“There was a very low ICH rate with only two events in each group. And then there was above a 1% reduction in the composite outcome including ischemic vascular events and bleeding,” he noted.
“This is important because there are many thousands of patients with acute ischemic stroke and AFib, and now we have a large study showing we can treat them with a DOAC early, and this appears to be safe and it appears also be more effective in terms of outcome events,” Dr. Tsivgoulis said.
But he highlighted one important caveat: The majority of patients had mild or moderate stroke.
A version of this article first appeared on Medscape.com.
FROM ESOC 2023
People still want their medical intelligence in human form
Doctors or AI? Lukewarm vote of confidence goes to …
Well, we’ve got some good news for the physicians out there, and we’ve got some bad news. Which do you want first? Okay, we’re mostly hearing good news, so here goes: Most people would choose a human doctor over artificial intelligence for the diagnosis and treatment of their medical conditions.
And the bad news? In the survey we’re talking about, “most” was 53%, so not exactly a huge victory for the carbon-based life forms. Yup, about 47% of the 2,472 respondents said they would prefer an AI-based clinic over a human specialist, and that number went up if individuals were told that their primary care physicians were on board with AI, “or otherwise nudged to consider AI as good,” the research team said in a written statement released by the University of Arizona, Tucson.
They went on to add that “this signaled the significance of the human physician in guiding a patient’s decision.” So patients will still need their doctors in the future to … um … this is a bit awkward … tell them how good the AI is?
And yes, we know that ChatGPT is already doing the same thing to journalists, but could it write a medical-humor column? Not a chance. Probably can’t even tell a joke.
How do ghosts get rid of wrinkles? Boo-tox. There, let’s see ChatGPT do that.
Explaining the joke makes it funnier, right?
Here at LOTME headquarters, we live by one simple rule, passed down directly from the Buddha himself: “Never let a good presurgical assessment of refractory epilepsy go to waste. Also, don’t believe everything you read on the Internet.”
This human-created joke has been brought to you by the leading theory of humor, which states that comedy stems from our brain reacting to an incongruous part of reality in a positive way. These positive emotions light up our neurons in a specific fashion, and boom, comedy is achieved.
Previous studies into the science of comedy have typically used functional MRI to analyze the brain while it was gripped in the throes of a comedic reaction. Unfortunately, fMRI cannot detect the entirety of the electromagnetic spectrum generated by the brain during these moments, so observing scientists have been, quite literally, missing out on some of the joke. And that’s where a new study from France comes in.
In the study, the researchers showed a group of patients with epilepsy who were hooked up to deep brain electrodes and a high-tech neuroimaging machine – part of the aforementioned presurgical assessment – a 3-minute excerpt from a Charlie Chaplin movie and analyzed their brain activity. Why Charlie Chaplin? Simple. Slapstick is perhaps the most accessible form of comedy across cultures. We can all appreciate a man getting hit in the head with a coconut. The world’s oldest bar joke or whatever this is? Not so much.
During the funniest scenes, all study participants showed increased high-frequency gamma waves (indicating high cognitive engagement) and a decrease in low-frequency waves (indicating reduced inattention and introspection). During unfunny scenes, such as transition moments, the opposite occurred. Importantly, this inverse relationship occurred in the temporal lobe but not in other regions, supporting previous research that indicated humor was mainly processed in the temporal lobe.
The investigators suggested future research should focus on longer videos with more complex forms of comedy, such as jokes, irony, sarcasm, or reference humor. So, uh, a guy getting hit in the head with two coconuts? That’s high-brow stuff right there.
Hot take: Humans aren’t that special
We humans have always prided ourselves on being different from “the animals” in an exceptional way. News flash! We aren’t. We may be the apex predator, but new research shows that humans, as part of the animal kingdom, just aren’t special.
Not special? How can they say that? Are gorillas doing open-heart surgery? Do wolverines tell jokes? At a more basic level, though, the way we operate as mammals in societies is not unique or even new. Elephants are known to mourn their deceased and to have funeral-like practices, ants invented agriculture, and we’re certainly not the only species that has figured out how to use tools.
This new research just demonstrates another way we aren’t exceptional, and that’s in our mating practices and outcomes.
“Humans appear to resemble mammals that live in monogamous partnerships and to some extent, those classified as cooperative breeders, where breeding individuals have to rely on the help of others to raise their offspring,” Monique Borgerhoff Mulder, PhD, professor emerita of anthropology at the University of California, Davis, said in a written statement.
The research team, which consisted of over 100 investigators, looked at 90 human populations based on data from over 80,000 people globally and compared the human data with 49 different nonhuman mammal species. In polygynous societies in which men take several wives, they found, women have more access to resources like food, shelter, and parenting help. Monogamy, on the other hand, “can drive significant inequalities among women,” Dr. Borgerhoff Mulder said, by promoting large differences in the number of children couples produce.
Human day-to-day behavior and child-rearing habits – one parent taking a daughter to ballet class and fixing dinner so the other parent can get to exercise class before picking up the son from soccer practice – may have us thinking that we are part of an evolved society, but really we are not much different than other mammals that hunt, forage for food, and rear and teach their children, the researchers suggested.
So, yes, humans can travel to the moon, create a vaccine for smallpox, and hit other humans with coconuts, but when it comes to simply having offspring or raising them, we’re not all that special. Get over it.
Doctors or AI? Lukewarm vote of confidence goes to …
Well, we’ve got some good news for the physicians out there, and we’ve got some bad news. Which do you want first? Okay, we’re mostly hearing good news, so here goes: Most people would choose a human doctor over artificial intelligence for the diagnosis and treatment of their medical conditions.
And the bad news? In the survey we’re talking about, “most” was 53%, so not exactly a huge victory for the carbon-based life forms. Yup, about 47% of the 2,472 respondents said they would prefer an AI-based clinic over a human specialist, and that number went up if individuals were told that their primary care physicians were on board with AI, “or otherwise nudged to consider AI as good,” the research team said in a written statement released by the University of Arizona, Tucson.
They went on to add that “this signaled the significance of the human physician in guiding a patient’s decision.” So patients will still need their doctors in the future to … um … this is a bit awkward … tell them how good the AI is?
And yes, we know that ChatGPT is already doing the same thing to journalists, but could it write a medical-humor column? Not a chance. Probably can’t even tell a joke.
How do ghosts get rid of wrinkles? Boo-tox. There, let’s see ChatGPT do that.
Explaining the joke makes it funnier, right?
Here at LOTME headquarters, we live by one simple rule, passed down directly from the Buddha himself: “Never let a good presurgical assessment of refractory epilepsy go to waste. Also, don’t believe everything you read on the Internet.”
This human-created joke has been brought to you by the leading theory of humor, which states that comedy stems from our brain reacting to an incongruous part of reality in a positive way. These positive emotions light up our neurons in a specific fashion, and boom, comedy is achieved.
Previous studies into the science of comedy have typically used functional MRI to analyze the brain while it was gripped in the throes of a comedic reaction. Unfortunately, fMRI cannot detect the entirety of the electromagnetic spectrum generated by the brain during these moments, so observing scientists have been, quite literally, missing out on some of the joke. And that’s where a new study from France comes in.
In the study, the researchers showed a group of patients with epilepsy who were hooked up to deep brain electrodes and a high-tech neuroimaging machine – part of the aforementioned presurgical assessment – a 3-minute excerpt from a Charlie Chaplin movie and analyzed their brain activity. Why Charlie Chaplin? Simple. Slapstick is perhaps the most accessible form of comedy across cultures. We can all appreciate a man getting hit in the head with a coconut. The world’s oldest bar joke or whatever this is? Not so much.
During the funniest scenes, all study participants showed increased high-frequency gamma waves (indicating high cognitive engagement) and a decrease in low-frequency waves (indicating reduced inattention and introspection). During unfunny scenes, such as transition moments, the opposite occurred. Importantly, this inverse relationship occurred in the temporal lobe but not in other regions, supporting previous research that indicated humor was mainly processed in the temporal lobe.
The investigators suggested future research should focus on longer videos with more complex forms of comedy, such as jokes, irony, sarcasm, or reference humor. So, uh, a guy getting hit in the head with two coconuts? That’s high-brow stuff right there.
Hot take: Humans aren’t that special
We humans have always prided ourselves on being different from “the animals” in an exceptional way. News flash! We aren’t. We may be the apex predator, but new research shows that humans, as part of the animal kingdom, just aren’t special.
Not special? How can they say that? Are gorillas doing open-heart surgery? Do wolverines tell jokes? At a more basic level, though, the way we operate as mammals in societies is not unique or even new. Elephants are known to mourn their deceased and to have funeral-like practices, ants invented agriculture, and we’re certainly not the only species that has figured out how to use tools.
This new research just demonstrates another way we aren’t exceptional, and that’s in our mating practices and outcomes.
“Humans appear to resemble mammals that live in monogamous partnerships and to some extent, those classified as cooperative breeders, where breeding individuals have to rely on the help of others to raise their offspring,” Monique Borgerhoff Mulder, PhD, professor emerita of anthropology at the University of California, Davis, said in a written statement.
The research team, which consisted of over 100 investigators, looked at 90 human populations based on data from over 80,000 people globally and compared the human data with 49 different nonhuman mammal species. In polygynous societies in which men take several wives, they found, women have more access to resources like food, shelter, and parenting help. Monogamy, on the other hand, “can drive significant inequalities among women,” Dr. Borgerhoff Mulder said, by promoting large differences in the number of children couples produce.
Human day-to-day behavior and child-rearing habits – one parent taking a daughter to ballet class and fixing dinner so the other parent can get to exercise class before picking up the son from soccer practice – may have us thinking that we are part of an evolved society, but really we are not much different than other mammals that hunt, forage for food, and rear and teach their children, the researchers suggested.
So, yes, humans can travel to the moon, create a vaccine for smallpox, and hit other humans with coconuts, but when it comes to simply having offspring or raising them, we’re not all that special. Get over it.
Doctors or AI? Lukewarm vote of confidence goes to …
Well, we’ve got some good news for the physicians out there, and we’ve got some bad news. Which do you want first? Okay, we’re mostly hearing good news, so here goes: Most people would choose a human doctor over artificial intelligence for the diagnosis and treatment of their medical conditions.
And the bad news? In the survey we’re talking about, “most” was 53%, so not exactly a huge victory for the carbon-based life forms. Yup, about 47% of the 2,472 respondents said they would prefer an AI-based clinic over a human specialist, and that number went up if individuals were told that their primary care physicians were on board with AI, “or otherwise nudged to consider AI as good,” the research team said in a written statement released by the University of Arizona, Tucson.
They went on to add that “this signaled the significance of the human physician in guiding a patient’s decision.” So patients will still need their doctors in the future to … um … this is a bit awkward … tell them how good the AI is?
And yes, we know that ChatGPT is already doing the same thing to journalists, but could it write a medical-humor column? Not a chance. Probably can’t even tell a joke.
How do ghosts get rid of wrinkles? Boo-tox. There, let’s see ChatGPT do that.
Explaining the joke makes it funnier, right?
Here at LOTME headquarters, we live by one simple rule, passed down directly from the Buddha himself: “Never let a good presurgical assessment of refractory epilepsy go to waste. Also, don’t believe everything you read on the Internet.”
This human-created joke has been brought to you by the leading theory of humor, which states that comedy stems from our brain reacting to an incongruous part of reality in a positive way. These positive emotions light up our neurons in a specific fashion, and boom, comedy is achieved.
Previous studies into the science of comedy have typically used functional MRI to analyze the brain while it was gripped in the throes of a comedic reaction. Unfortunately, fMRI cannot detect the entirety of the electromagnetic spectrum generated by the brain during these moments, so observing scientists have been, quite literally, missing out on some of the joke. And that’s where a new study from France comes in.
In the study, the researchers showed a group of patients with epilepsy who were hooked up to deep brain electrodes and a high-tech neuroimaging machine – part of the aforementioned presurgical assessment – a 3-minute excerpt from a Charlie Chaplin movie and analyzed their brain activity. Why Charlie Chaplin? Simple. Slapstick is perhaps the most accessible form of comedy across cultures. We can all appreciate a man getting hit in the head with a coconut. The world’s oldest bar joke or whatever this is? Not so much.
During the funniest scenes, all study participants showed increased high-frequency gamma waves (indicating high cognitive engagement) and a decrease in low-frequency waves (indicating reduced inattention and introspection). During unfunny scenes, such as transition moments, the opposite occurred. Importantly, this inverse relationship occurred in the temporal lobe but not in other regions, supporting previous research that indicated humor was mainly processed in the temporal lobe.
The investigators suggested future research should focus on longer videos with more complex forms of comedy, such as jokes, irony, sarcasm, or reference humor. So, uh, a guy getting hit in the head with two coconuts? That’s high-brow stuff right there.
Hot take: Humans aren’t that special
We humans have always prided ourselves on being different from “the animals” in an exceptional way. News flash! We aren’t. We may be the apex predator, but new research shows that humans, as part of the animal kingdom, just aren’t special.
Not special? How can they say that? Are gorillas doing open-heart surgery? Do wolverines tell jokes? At a more basic level, though, the way we operate as mammals in societies is not unique or even new. Elephants are known to mourn their deceased and to have funeral-like practices, ants invented agriculture, and we’re certainly not the only species that has figured out how to use tools.
This new research just demonstrates another way we aren’t exceptional, and that’s in our mating practices and outcomes.
“Humans appear to resemble mammals that live in monogamous partnerships and to some extent, those classified as cooperative breeders, where breeding individuals have to rely on the help of others to raise their offspring,” Monique Borgerhoff Mulder, PhD, professor emerita of anthropology at the University of California, Davis, said in a written statement.
The research team, which consisted of over 100 investigators, looked at 90 human populations based on data from over 80,000 people globally and compared the human data with 49 different nonhuman mammal species. In polygynous societies in which men take several wives, they found, women have more access to resources like food, shelter, and parenting help. Monogamy, on the other hand, “can drive significant inequalities among women,” Dr. Borgerhoff Mulder said, by promoting large differences in the number of children couples produce.
Human day-to-day behavior and child-rearing habits – one parent taking a daughter to ballet class and fixing dinner so the other parent can get to exercise class before picking up the son from soccer practice – may have us thinking that we are part of an evolved society, but really we are not much different than other mammals that hunt, forage for food, and rear and teach their children, the researchers suggested.
So, yes, humans can travel to the moon, create a vaccine for smallpox, and hit other humans with coconuts, but when it comes to simply having offspring or raising them, we’re not all that special. Get over it.
Which interventions could lessen the burden of dementia?
Using a microsimulation algorithm that accounts for the effect on mortality, a team from Marseille, France, has shown that interventions targeting the three main vascular risk factors for dementia – hypertension, diabetes, and physical inactivity – could significantly reduce the burden of dementia by 2040.
Although these modeling results could appear too optimistic, since total disappearance of the risk factors was assumed, the authors say the results do show that targeted interventions for these factors could be effective in reducing the future burden of dementia.
Increasing prevalence
According to the World Alzheimer Report 2018, 50 million people around the world were living with dementia; a population roughly around the size of South Korea or Spain. That community is likely to rise to about 152 million people by 2050, which is similar to the size of Russia or Bangladesh, the result of an aging population.
Among modifiable risk factors, many studies support a deleterious effect of hypertension, diabetes, and physical inactivity on the risk of dementia. However, since the distribution of these risk factors could have a direct impact on mortality, reducing it should increase life expectancy and the number of cases of dementia.
The team, headed by Hélène Jacqmin-Gadda, PhD, research director at the University of Bordeaux (France), has developed a microsimulation model capable of predicting the burden of dementia while accounting for the impact on mortality. The team used this approach to assess the impact of interventions targeting these three main risk factors on the burden of dementia in France by 2040.
Removing risk factors
The researchers estimated the incidence of dementia for men and women using data from the 2020 PAQUID cohort, and these data were combined with the projections forecast by the French National Institute of Statistics and Economic Studies to account for mortality with and without dementia.
Without intervention, the prevalence rate of dementia in 2040 would be 9.6% among men and 14% among women older than 65 years.
These figures would decrease to 6.4% (−33%) and 10.4% (−26%), respectively, under the intervention scenario whereby the three modifiable vascular risk factors (hypertension, diabetes, and physical inactivity) would be removed simultaneously beginning in 2020. The prevalence rates are significantly reduced for men and women from age 75 years. In this scenario, life expectancy without dementia would increase by 3.4 years in men and 2.6 years in women, the result of men being more exposed to these three risk factors.
Other scenarios have estimated dementia prevalence with the disappearance of just one of these risk factors. For example, the disappearance of hypertension alone from 2020 could decrease dementia prevalence by 21% in men and 16% in women (because this risk factor is less common in women than in men) by 2040. This reduction would be associated with a decrease in the lifelong probability of dementia among men and women and a gain in life expectancy without dementia of 2 years in men and 1.4 years in women.
Among the three factors, hypertension has the largest impact on dementia burden in the French population, since this is, by far, the most prevalent (69% in men and 49% in women), while intervention targeting only diabetes or physical inactivity would lead to a reduction in dementia prevalence of only 4%-7%.
The authors reported no conflicts of interest.
This article was translated from Univadis France. A version appeared on Medscape.com.
Using a microsimulation algorithm that accounts for the effect on mortality, a team from Marseille, France, has shown that interventions targeting the three main vascular risk factors for dementia – hypertension, diabetes, and physical inactivity – could significantly reduce the burden of dementia by 2040.
Although these modeling results could appear too optimistic, since total disappearance of the risk factors was assumed, the authors say the results do show that targeted interventions for these factors could be effective in reducing the future burden of dementia.
Increasing prevalence
According to the World Alzheimer Report 2018, 50 million people around the world were living with dementia; a population roughly around the size of South Korea or Spain. That community is likely to rise to about 152 million people by 2050, which is similar to the size of Russia or Bangladesh, the result of an aging population.
Among modifiable risk factors, many studies support a deleterious effect of hypertension, diabetes, and physical inactivity on the risk of dementia. However, since the distribution of these risk factors could have a direct impact on mortality, reducing it should increase life expectancy and the number of cases of dementia.
The team, headed by Hélène Jacqmin-Gadda, PhD, research director at the University of Bordeaux (France), has developed a microsimulation model capable of predicting the burden of dementia while accounting for the impact on mortality. The team used this approach to assess the impact of interventions targeting these three main risk factors on the burden of dementia in France by 2040.
Removing risk factors
The researchers estimated the incidence of dementia for men and women using data from the 2020 PAQUID cohort, and these data were combined with the projections forecast by the French National Institute of Statistics and Economic Studies to account for mortality with and without dementia.
Without intervention, the prevalence rate of dementia in 2040 would be 9.6% among men and 14% among women older than 65 years.
These figures would decrease to 6.4% (−33%) and 10.4% (−26%), respectively, under the intervention scenario whereby the three modifiable vascular risk factors (hypertension, diabetes, and physical inactivity) would be removed simultaneously beginning in 2020. The prevalence rates are significantly reduced for men and women from age 75 years. In this scenario, life expectancy without dementia would increase by 3.4 years in men and 2.6 years in women, the result of men being more exposed to these three risk factors.
Other scenarios have estimated dementia prevalence with the disappearance of just one of these risk factors. For example, the disappearance of hypertension alone from 2020 could decrease dementia prevalence by 21% in men and 16% in women (because this risk factor is less common in women than in men) by 2040. This reduction would be associated with a decrease in the lifelong probability of dementia among men and women and a gain in life expectancy without dementia of 2 years in men and 1.4 years in women.
Among the three factors, hypertension has the largest impact on dementia burden in the French population, since this is, by far, the most prevalent (69% in men and 49% in women), while intervention targeting only diabetes or physical inactivity would lead to a reduction in dementia prevalence of only 4%-7%.
The authors reported no conflicts of interest.
This article was translated from Univadis France. A version appeared on Medscape.com.
Using a microsimulation algorithm that accounts for the effect on mortality, a team from Marseille, France, has shown that interventions targeting the three main vascular risk factors for dementia – hypertension, diabetes, and physical inactivity – could significantly reduce the burden of dementia by 2040.
Although these modeling results could appear too optimistic, since total disappearance of the risk factors was assumed, the authors say the results do show that targeted interventions for these factors could be effective in reducing the future burden of dementia.
Increasing prevalence
According to the World Alzheimer Report 2018, 50 million people around the world were living with dementia; a population roughly around the size of South Korea or Spain. That community is likely to rise to about 152 million people by 2050, which is similar to the size of Russia or Bangladesh, the result of an aging population.
Among modifiable risk factors, many studies support a deleterious effect of hypertension, diabetes, and physical inactivity on the risk of dementia. However, since the distribution of these risk factors could have a direct impact on mortality, reducing it should increase life expectancy and the number of cases of dementia.
The team, headed by Hélène Jacqmin-Gadda, PhD, research director at the University of Bordeaux (France), has developed a microsimulation model capable of predicting the burden of dementia while accounting for the impact on mortality. The team used this approach to assess the impact of interventions targeting these three main risk factors on the burden of dementia in France by 2040.
Removing risk factors
The researchers estimated the incidence of dementia for men and women using data from the 2020 PAQUID cohort, and these data were combined with the projections forecast by the French National Institute of Statistics and Economic Studies to account for mortality with and without dementia.
Without intervention, the prevalence rate of dementia in 2040 would be 9.6% among men and 14% among women older than 65 years.
These figures would decrease to 6.4% (−33%) and 10.4% (−26%), respectively, under the intervention scenario whereby the three modifiable vascular risk factors (hypertension, diabetes, and physical inactivity) would be removed simultaneously beginning in 2020. The prevalence rates are significantly reduced for men and women from age 75 years. In this scenario, life expectancy without dementia would increase by 3.4 years in men and 2.6 years in women, the result of men being more exposed to these three risk factors.
Other scenarios have estimated dementia prevalence with the disappearance of just one of these risk factors. For example, the disappearance of hypertension alone from 2020 could decrease dementia prevalence by 21% in men and 16% in women (because this risk factor is less common in women than in men) by 2040. This reduction would be associated with a decrease in the lifelong probability of dementia among men and women and a gain in life expectancy without dementia of 2 years in men and 1.4 years in women.
Among the three factors, hypertension has the largest impact on dementia burden in the French population, since this is, by far, the most prevalent (69% in men and 49% in women), while intervention targeting only diabetes or physical inactivity would lead to a reduction in dementia prevalence of only 4%-7%.
The authors reported no conflicts of interest.
This article was translated from Univadis France. A version appeared on Medscape.com.
FROM THE EUROPEAN JOURNAL OF EPIDEMIOLOGY