User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
MS fundraising during a pandemic
Fundraising walks for multiple sclerosis (MS) should be familiar to everyone nationwide. They serve to raise money for MS, bolster public awareness of the disease, and build a sense of community. But such in-person events took a big hit during the pandemic.
Recently, this news organization spoke with Kristin Gibbs, vice president of Walk MS for the National MS Society.
How has the National MS Society raised money before the pandemic?
We are a peer-to-peer fundraising event. That means our registered participants ask their family, friends and coworkers to support them by donating. More than 90% of our participants are friends-and-family teams, and nearly everyone who participates in Walk MS has a connection to MS. We do also have corporate and national teams that fundraise, as well as national and local sponsors that provide monetary support of Walk MS.
About how many Walk MS events were held nationally in an average prepandemic year?
Going back to 2019, we held almost 400 Walk MS events. Next year, the Society will host 234 events, with at least one in each state. The reduction in the number of events reflects a prepandemic strategy of focusing our limited resources in areas where we can have the biggest impact.
How has the pandemic impacted fundraising and community building/outreach?
Fewer people registered and participated in our virtual events in 2020 and 2021, and the pandemic made it challenging for participants to fundraise. While normally we might see more than 200,000 participants nationally, in 2021 we attracted 40,000. Our fundraising decreased from nearly $40 million in prepandemic years to around $20 million in 2021. Our experience is similar to that of most nonprofit peer-to-peer events. However, we were encouraged by the individuals who did support Walk MS during the pandemic, as their fundraising averages were higher than prepandemic campaigns.
What kinds of ‘virtual events’ were held during the pandemic lockdowns?
When it comes to building community, during the pandemic we innovatively utilized online gathering technology, especially Teams, to bring our Walk MS participants together. We held numerous meetings for Team Captains and conducted pre-event pep rallies online to help share information and generate excitement. We produced Facebook Live broadcasts and launched a cutting-edge online version of a Walk MS event called Walk MS On Demand. On Demand visitors could create a virtual bib, learn about the Society, watch inspirational videos, and secure information from national and local sponsors.
How is fundraising handled nationally and locally?
In 2022 we will have 234 Walk MS events spread across the country. We are anticipating 100,000 participants will register and our goal is to raise $24 million. Our fundraising will come from individuals, teams, and corporations who contribute at the local and national levels. We are hopeful the excitement surrounding safely being back in person will allow the Walk MS campaign to quickly regain its financial and community-building impact.
Has the pandemic impacted corporate contributions?
We were extremely lucky to maintain support of our national sponsors, and to engage a strong number of local partners. Because we offered the Walk MS On Demand online experience where sponsors could showcase their companies in innovative ways, even though we were virtual we could provide our important partners with a unique way to connect to our constituents. That made a tremendous difference. Also, our partners are strongly committed to the mission and knew their continued support during the pandemic was critical to our organization.
How is the money distributed? Who benefits and how?
Walk MS is the United States’ 7th-largest nonprofit walk series, and the 12th-largest nonprofit event overall. Our Walk MS funds help provide support, programming, and research for individuals diagnosed with MS. Over the history of Walk MS, participants and sponsors have generated more than $1 billion to support those who live with MS.
How can clinicians and health care practitioners get involved?
There are several exciting ways for clinicians and health care practitioners to get involved in Walk MS. Many health care practitioners and clinicians form their own Walk MS teams and fundraise for the event – sometimes inviting patients to join them. Being at Walk MS with your team is an experience like no other when it comes to engaging with the MS community. Several health care organizations also sponsor their local Walk MS event and are able to showcase their brand in front of an important target audience. Still others support Walk MS as volunteers and many clinicians and health care practitioners spread awareness by promoting Walk MS to their patients. You can find ideas for Walk MS engagement and sponsorship details at WalkMS.org.
How do individuals with MS benefit from Walk MS initiatives?
Over its 30-plus-year history, Walk MS has generated more than $1 billion to support the Society’s mission to cure MS while empowering people affected by MS to live their best lives. Funds raised at Walk MS fuel cutting-edge MS research, power advocacy, generate awareness, and provide access to resources that connect those affected by MS to the information and people they need to live their best lives.
Any future plans?
Walk MS historically has been the society’s largest gathering. We are excited in 2022 to return to in-person events after a nearly 2-year hiatus. Society-hosted events will occur at 234 locations across the United States. The Walk MS season spans from February to June and you can register at WalkMS.org. New this year – and a carry-over from our pandemic experience – we’re offering a Your Way option. No matter where you are located or how you want to commemorate Walk MS, you can participate in this virtual option and still receive fundraising support and exciting prizes.
Fundraising walks for multiple sclerosis (MS) should be familiar to everyone nationwide. They serve to raise money for MS, bolster public awareness of the disease, and build a sense of community. But such in-person events took a big hit during the pandemic.
Recently, this news organization spoke with Kristin Gibbs, vice president of Walk MS for the National MS Society.
How has the National MS Society raised money before the pandemic?
We are a peer-to-peer fundraising event. That means our registered participants ask their family, friends and coworkers to support them by donating. More than 90% of our participants are friends-and-family teams, and nearly everyone who participates in Walk MS has a connection to MS. We do also have corporate and national teams that fundraise, as well as national and local sponsors that provide monetary support of Walk MS.
About how many Walk MS events were held nationally in an average prepandemic year?
Going back to 2019, we held almost 400 Walk MS events. Next year, the Society will host 234 events, with at least one in each state. The reduction in the number of events reflects a prepandemic strategy of focusing our limited resources in areas where we can have the biggest impact.
How has the pandemic impacted fundraising and community building/outreach?
Fewer people registered and participated in our virtual events in 2020 and 2021, and the pandemic made it challenging for participants to fundraise. While normally we might see more than 200,000 participants nationally, in 2021 we attracted 40,000. Our fundraising decreased from nearly $40 million in prepandemic years to around $20 million in 2021. Our experience is similar to that of most nonprofit peer-to-peer events. However, we were encouraged by the individuals who did support Walk MS during the pandemic, as their fundraising averages were higher than prepandemic campaigns.
What kinds of ‘virtual events’ were held during the pandemic lockdowns?
When it comes to building community, during the pandemic we innovatively utilized online gathering technology, especially Teams, to bring our Walk MS participants together. We held numerous meetings for Team Captains and conducted pre-event pep rallies online to help share information and generate excitement. We produced Facebook Live broadcasts and launched a cutting-edge online version of a Walk MS event called Walk MS On Demand. On Demand visitors could create a virtual bib, learn about the Society, watch inspirational videos, and secure information from national and local sponsors.
How is fundraising handled nationally and locally?
In 2022 we will have 234 Walk MS events spread across the country. We are anticipating 100,000 participants will register and our goal is to raise $24 million. Our fundraising will come from individuals, teams, and corporations who contribute at the local and national levels. We are hopeful the excitement surrounding safely being back in person will allow the Walk MS campaign to quickly regain its financial and community-building impact.
Has the pandemic impacted corporate contributions?
We were extremely lucky to maintain support of our national sponsors, and to engage a strong number of local partners. Because we offered the Walk MS On Demand online experience where sponsors could showcase their companies in innovative ways, even though we were virtual we could provide our important partners with a unique way to connect to our constituents. That made a tremendous difference. Also, our partners are strongly committed to the mission and knew their continued support during the pandemic was critical to our organization.
How is the money distributed? Who benefits and how?
Walk MS is the United States’ 7th-largest nonprofit walk series, and the 12th-largest nonprofit event overall. Our Walk MS funds help provide support, programming, and research for individuals diagnosed with MS. Over the history of Walk MS, participants and sponsors have generated more than $1 billion to support those who live with MS.
How can clinicians and health care practitioners get involved?
There are several exciting ways for clinicians and health care practitioners to get involved in Walk MS. Many health care practitioners and clinicians form their own Walk MS teams and fundraise for the event – sometimes inviting patients to join them. Being at Walk MS with your team is an experience like no other when it comes to engaging with the MS community. Several health care organizations also sponsor their local Walk MS event and are able to showcase their brand in front of an important target audience. Still others support Walk MS as volunteers and many clinicians and health care practitioners spread awareness by promoting Walk MS to their patients. You can find ideas for Walk MS engagement and sponsorship details at WalkMS.org.
How do individuals with MS benefit from Walk MS initiatives?
Over its 30-plus-year history, Walk MS has generated more than $1 billion to support the Society’s mission to cure MS while empowering people affected by MS to live their best lives. Funds raised at Walk MS fuel cutting-edge MS research, power advocacy, generate awareness, and provide access to resources that connect those affected by MS to the information and people they need to live their best lives.
Any future plans?
Walk MS historically has been the society’s largest gathering. We are excited in 2022 to return to in-person events after a nearly 2-year hiatus. Society-hosted events will occur at 234 locations across the United States. The Walk MS season spans from February to June and you can register at WalkMS.org. New this year – and a carry-over from our pandemic experience – we’re offering a Your Way option. No matter where you are located or how you want to commemorate Walk MS, you can participate in this virtual option and still receive fundraising support and exciting prizes.
Fundraising walks for multiple sclerosis (MS) should be familiar to everyone nationwide. They serve to raise money for MS, bolster public awareness of the disease, and build a sense of community. But such in-person events took a big hit during the pandemic.
Recently, this news organization spoke with Kristin Gibbs, vice president of Walk MS for the National MS Society.
How has the National MS Society raised money before the pandemic?
We are a peer-to-peer fundraising event. That means our registered participants ask their family, friends and coworkers to support them by donating. More than 90% of our participants are friends-and-family teams, and nearly everyone who participates in Walk MS has a connection to MS. We do also have corporate and national teams that fundraise, as well as national and local sponsors that provide monetary support of Walk MS.
About how many Walk MS events were held nationally in an average prepandemic year?
Going back to 2019, we held almost 400 Walk MS events. Next year, the Society will host 234 events, with at least one in each state. The reduction in the number of events reflects a prepandemic strategy of focusing our limited resources in areas where we can have the biggest impact.
How has the pandemic impacted fundraising and community building/outreach?
Fewer people registered and participated in our virtual events in 2020 and 2021, and the pandemic made it challenging for participants to fundraise. While normally we might see more than 200,000 participants nationally, in 2021 we attracted 40,000. Our fundraising decreased from nearly $40 million in prepandemic years to around $20 million in 2021. Our experience is similar to that of most nonprofit peer-to-peer events. However, we were encouraged by the individuals who did support Walk MS during the pandemic, as their fundraising averages were higher than prepandemic campaigns.
What kinds of ‘virtual events’ were held during the pandemic lockdowns?
When it comes to building community, during the pandemic we innovatively utilized online gathering technology, especially Teams, to bring our Walk MS participants together. We held numerous meetings for Team Captains and conducted pre-event pep rallies online to help share information and generate excitement. We produced Facebook Live broadcasts and launched a cutting-edge online version of a Walk MS event called Walk MS On Demand. On Demand visitors could create a virtual bib, learn about the Society, watch inspirational videos, and secure information from national and local sponsors.
How is fundraising handled nationally and locally?
In 2022 we will have 234 Walk MS events spread across the country. We are anticipating 100,000 participants will register and our goal is to raise $24 million. Our fundraising will come from individuals, teams, and corporations who contribute at the local and national levels. We are hopeful the excitement surrounding safely being back in person will allow the Walk MS campaign to quickly regain its financial and community-building impact.
Has the pandemic impacted corporate contributions?
We were extremely lucky to maintain support of our national sponsors, and to engage a strong number of local partners. Because we offered the Walk MS On Demand online experience where sponsors could showcase their companies in innovative ways, even though we were virtual we could provide our important partners with a unique way to connect to our constituents. That made a tremendous difference. Also, our partners are strongly committed to the mission and knew their continued support during the pandemic was critical to our organization.
How is the money distributed? Who benefits and how?
Walk MS is the United States’ 7th-largest nonprofit walk series, and the 12th-largest nonprofit event overall. Our Walk MS funds help provide support, programming, and research for individuals diagnosed with MS. Over the history of Walk MS, participants and sponsors have generated more than $1 billion to support those who live with MS.
How can clinicians and health care practitioners get involved?
There are several exciting ways for clinicians and health care practitioners to get involved in Walk MS. Many health care practitioners and clinicians form their own Walk MS teams and fundraise for the event – sometimes inviting patients to join them. Being at Walk MS with your team is an experience like no other when it comes to engaging with the MS community. Several health care organizations also sponsor their local Walk MS event and are able to showcase their brand in front of an important target audience. Still others support Walk MS as volunteers and many clinicians and health care practitioners spread awareness by promoting Walk MS to their patients. You can find ideas for Walk MS engagement and sponsorship details at WalkMS.org.
How do individuals with MS benefit from Walk MS initiatives?
Over its 30-plus-year history, Walk MS has generated more than $1 billion to support the Society’s mission to cure MS while empowering people affected by MS to live their best lives. Funds raised at Walk MS fuel cutting-edge MS research, power advocacy, generate awareness, and provide access to resources that connect those affected by MS to the information and people they need to live their best lives.
Any future plans?
Walk MS historically has been the society’s largest gathering. We are excited in 2022 to return to in-person events after a nearly 2-year hiatus. Society-hosted events will occur at 234 locations across the United States. The Walk MS season spans from February to June and you can register at WalkMS.org. New this year – and a carry-over from our pandemic experience – we’re offering a Your Way option. No matter where you are located or how you want to commemorate Walk MS, you can participate in this virtual option and still receive fundraising support and exciting prizes.
Good data is lacking on best first-line MS drug strategies
Personalized medicine is just about the biggest buzzword in health. But neurologist Ellen M. Mowry, MD, of Johns Hopkins University, Baltimore, steers patients with multiple sclerosis (MS) away from the concept when she first starts talking to them about initial therapy options and possible ways to forestall disability down the line.
“I try to be quite honest,” she told colleagues at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
Observational and clinical trials offer extremely limited insight, she said in a keynote address, so there aren’t any simple answers about the best strategies. However, she highlighted new research projects that aim to provide more reliable answers.
As Dr. Mowry noted, patients tend to do well early on regardless of the choice of drug, so the question isn’t how to immediately control MS. “I personally find that most people can have control of relapses and the development of new lesions. I don’t find that there are too many individuals these days who don’t achieve control of their inflammatory activity,” she said. “I’m really interested in understanding whether the treatment choices a person with MS and myself make – at the time of their diagnosis – matters with respect to how they’re doing several years down the road. One major question is: Should I be using higher-efficacy therapy right out of the gate to better impact long-term disability?”
Research suggests that disability in MS is declining dramatically, she said, although it’s not quite clear if this is caused by evolving definitions of the disease or better medications. If the latter is the case, it’s useful to know that “there have been several publications suggesting that using stronger therapies right out of the gate may have an even greater impact on the long-term disability trajectory [than lower-efficacy treatments],” she said.
But some studies in this area are observational and come with various weaknesses, she said. Clinical trials offer data of their own, but “the conditions of clinical trials are also not real world or generalizable.” They often have healthier subjects than physicians actually see, and their requirements – such as requiring patients to have failed certain therapies – can muddy the messages of their outcomes. And, she added, people are more complicated in real life than in these trials, with many having a mix of both higher- and lower-risk features.
So how can physicians make the best decisions? Dr. Mowry recommends considering several factors, such patient comorbidities and reproductive status, the way drugs are administered, monitoring requirements, and cost. Safety is crucial too. She noted that newly diagnosed patients with MS are very concerned about safety – “they’re very much afraid of risks of stronger medications” – and many choose escalation therapy instead of immediately embracing higher-efficacy therapy for that reason.
At her clinic, she doesn’t push quick decisions. “I find that the treatment-decision discussion with individuals with MS takes several appointments, which we offer typically in quick succession. If we go with the escalation route, we are very strongly conscientious about escalating if there’s breakthrough disease. For me, that means after the medication should have kicked in, we may indeed escalate that therapy right away if there’s a new relapse or more than one new lesion.”
As for the future, Dr. Mowry highlighted two ongoing clinical trials that are expected to provide guidance about first-line therapy options. One is TREAT-MS, which will track intermediate-term risk of disability based on choices regarding first-line and later therapy. The pragmatic trial aims to enroll 900 subjects for up to 5 years. The other is DELIVER-MS, which aims to track how treatment choices affect brain volume.
“We really do need more definitive data to support the early treatment choices that people need to make,” she said.
Dr. Mowry disclosed grant/research support from Biogen, Teva, and Genentech, as welll as honoraria (editorial royalties) from UpToDate.
Personalized medicine is just about the biggest buzzword in health. But neurologist Ellen M. Mowry, MD, of Johns Hopkins University, Baltimore, steers patients with multiple sclerosis (MS) away from the concept when she first starts talking to them about initial therapy options and possible ways to forestall disability down the line.
“I try to be quite honest,” she told colleagues at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
Observational and clinical trials offer extremely limited insight, she said in a keynote address, so there aren’t any simple answers about the best strategies. However, she highlighted new research projects that aim to provide more reliable answers.
As Dr. Mowry noted, patients tend to do well early on regardless of the choice of drug, so the question isn’t how to immediately control MS. “I personally find that most people can have control of relapses and the development of new lesions. I don’t find that there are too many individuals these days who don’t achieve control of their inflammatory activity,” she said. “I’m really interested in understanding whether the treatment choices a person with MS and myself make – at the time of their diagnosis – matters with respect to how they’re doing several years down the road. One major question is: Should I be using higher-efficacy therapy right out of the gate to better impact long-term disability?”
Research suggests that disability in MS is declining dramatically, she said, although it’s not quite clear if this is caused by evolving definitions of the disease or better medications. If the latter is the case, it’s useful to know that “there have been several publications suggesting that using stronger therapies right out of the gate may have an even greater impact on the long-term disability trajectory [than lower-efficacy treatments],” she said.
But some studies in this area are observational and come with various weaknesses, she said. Clinical trials offer data of their own, but “the conditions of clinical trials are also not real world or generalizable.” They often have healthier subjects than physicians actually see, and their requirements – such as requiring patients to have failed certain therapies – can muddy the messages of their outcomes. And, she added, people are more complicated in real life than in these trials, with many having a mix of both higher- and lower-risk features.
So how can physicians make the best decisions? Dr. Mowry recommends considering several factors, such patient comorbidities and reproductive status, the way drugs are administered, monitoring requirements, and cost. Safety is crucial too. She noted that newly diagnosed patients with MS are very concerned about safety – “they’re very much afraid of risks of stronger medications” – and many choose escalation therapy instead of immediately embracing higher-efficacy therapy for that reason.
At her clinic, she doesn’t push quick decisions. “I find that the treatment-decision discussion with individuals with MS takes several appointments, which we offer typically in quick succession. If we go with the escalation route, we are very strongly conscientious about escalating if there’s breakthrough disease. For me, that means after the medication should have kicked in, we may indeed escalate that therapy right away if there’s a new relapse or more than one new lesion.”
As for the future, Dr. Mowry highlighted two ongoing clinical trials that are expected to provide guidance about first-line therapy options. One is TREAT-MS, which will track intermediate-term risk of disability based on choices regarding first-line and later therapy. The pragmatic trial aims to enroll 900 subjects for up to 5 years. The other is DELIVER-MS, which aims to track how treatment choices affect brain volume.
“We really do need more definitive data to support the early treatment choices that people need to make,” she said.
Dr. Mowry disclosed grant/research support from Biogen, Teva, and Genentech, as welll as honoraria (editorial royalties) from UpToDate.
Personalized medicine is just about the biggest buzzword in health. But neurologist Ellen M. Mowry, MD, of Johns Hopkins University, Baltimore, steers patients with multiple sclerosis (MS) away from the concept when she first starts talking to them about initial therapy options and possible ways to forestall disability down the line.
“I try to be quite honest,” she told colleagues at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
Observational and clinical trials offer extremely limited insight, she said in a keynote address, so there aren’t any simple answers about the best strategies. However, she highlighted new research projects that aim to provide more reliable answers.
As Dr. Mowry noted, patients tend to do well early on regardless of the choice of drug, so the question isn’t how to immediately control MS. “I personally find that most people can have control of relapses and the development of new lesions. I don’t find that there are too many individuals these days who don’t achieve control of their inflammatory activity,” she said. “I’m really interested in understanding whether the treatment choices a person with MS and myself make – at the time of their diagnosis – matters with respect to how they’re doing several years down the road. One major question is: Should I be using higher-efficacy therapy right out of the gate to better impact long-term disability?”
Research suggests that disability in MS is declining dramatically, she said, although it’s not quite clear if this is caused by evolving definitions of the disease or better medications. If the latter is the case, it’s useful to know that “there have been several publications suggesting that using stronger therapies right out of the gate may have an even greater impact on the long-term disability trajectory [than lower-efficacy treatments],” she said.
But some studies in this area are observational and come with various weaknesses, she said. Clinical trials offer data of their own, but “the conditions of clinical trials are also not real world or generalizable.” They often have healthier subjects than physicians actually see, and their requirements – such as requiring patients to have failed certain therapies – can muddy the messages of their outcomes. And, she added, people are more complicated in real life than in these trials, with many having a mix of both higher- and lower-risk features.
So how can physicians make the best decisions? Dr. Mowry recommends considering several factors, such patient comorbidities and reproductive status, the way drugs are administered, monitoring requirements, and cost. Safety is crucial too. She noted that newly diagnosed patients with MS are very concerned about safety – “they’re very much afraid of risks of stronger medications” – and many choose escalation therapy instead of immediately embracing higher-efficacy therapy for that reason.
At her clinic, she doesn’t push quick decisions. “I find that the treatment-decision discussion with individuals with MS takes several appointments, which we offer typically in quick succession. If we go with the escalation route, we are very strongly conscientious about escalating if there’s breakthrough disease. For me, that means after the medication should have kicked in, we may indeed escalate that therapy right away if there’s a new relapse or more than one new lesion.”
As for the future, Dr. Mowry highlighted two ongoing clinical trials that are expected to provide guidance about first-line therapy options. One is TREAT-MS, which will track intermediate-term risk of disability based on choices regarding first-line and later therapy. The pragmatic trial aims to enroll 900 subjects for up to 5 years. The other is DELIVER-MS, which aims to track how treatment choices affect brain volume.
“We really do need more definitive data to support the early treatment choices that people need to make,” she said.
Dr. Mowry disclosed grant/research support from Biogen, Teva, and Genentech, as welll as honoraria (editorial royalties) from UpToDate.
FROM CMSC 2021
The devil in the (masking) details
The Devil’s own face covering?
It’s been over a year and a half since the COVID-19 emergency was declared in the United States, and we’ve been starting to wonder what our good friend SARS-CoV-2 has left to give. The collective cynic/optimist in us figures that the insanity can’t last forever, right?
Maybe not forever, but …
A group of parents is suing the Central Bucks (Pa.) School District over school mask mandates, suggesting that the district has no legal authority to enforce such measures. Most of their arguments, Philadelphia Magazine says, are pretty standard stuff: Masks are causing depression, anxiety, and discomfort in their children; masks are a violation of their constitutional rights; and “masks are being used as a control mechanism over the population.”
There are some unusual claims, though. One of the parents, Shannon Harris, said that “wearing masks interferes with their religious duty to spread the word of God and forces them to participate in a satanic ritual,” according to the Philadelphia Inquirer.
Philadelphia Magazine decided to check on that “satanic ritual” claim by asking an expert, in this case a spokesperson for the Church of Satan. The Reverend Raul Antony said that “simply ‘wearing a mask’ is not a Satanic ritual, and anyone that genuinely thinks otherwise is a blithering idiot,” adding that the group’s rituals were available on its website.
COVID, you never let us down.
You’re the (hurricane) wind beneath my wings
Marriage isn’t easy. From finances to everyday stressors like work and children, maintaining a solid relationship is tough. Then a natural disaster shows up on top of everything else, and marriages actually improve, researchers found.
In a study published by Psychological Science, researchers surveyed 231 newlywed couples about the satisfaction of their marriage before and after Hurricane Harvey in 2017. They found after the hurricane couples had a “significant boost” in the satisfaction of their relationship.
One would think something like this would create what researchers call a “stress spillover,” creating a decrease in relationship satisfaction. Destruction to your home or even displacement after a natural disaster seems pretty stressful. But, “a natural disaster can really put things in perspective. People realize how important their partner is to them when they are jolted out of the day-to-day stress of life,” said Hannah Williamson, PhD, the lead author of the study.
And although everyone saw an increase, the biggest jumps in relationship satisfaction belonged to the people who were most unhappy before the hurricane. Unfortunately, the researchers also found that the effects were only temporary and the dissatisfaction came back within a year.
Dr. Williamson thinks there may be something to these findings that can be beneficial from a therapy standpoint where “couples can shift their perspective in a similar way without having to go through a natural disaster.”
Let’s hope she’s right, because the alternative is to seek out a rampaging hurricane every time your relationship is on the rocks, and that just seems impractical after the second or third year.
Not-so-essential oils
Many people use essential oils as a way to unwind and relax. Stressed? Can’t sleep? There’s probably an essential oil for that. However, it seems like these days a lot of things we love and/or think are good for us have a side that’s not so.
According to the Centers for Disease Control and Prevention, a woman from Georgia died from a rare bacteria called Burkholderia pseudomallei. There have been three previous infections in Kansas, Minnesota, and Texas throughout 2021; two of the four infections were in children. Melioidosis, the disease caused by B. pseudomallei, is usually found in southeast Asia and isn’t obvious or easy to diagnose, especially in places like decidedly untropical Minnesota.
The Georgia case was the real break in this medical mystery, as the infection was traced back to a Walmart product called “Better Homes and Gardens Essential Oil Infused Aromatherapy Room Spray with Gemstones” (a very pithy name). The bacteria were in the lavender and chamomile scent. The CDC is investigating all other product scents, and Walmart has recalled all lots of the product.
If you’ve got that particular essential oil, it’s probably for the best that you stop using it. Don’t worry, we’re sure there’s plenty of other essential oil–infused aromatherapy room sprays with gemstones out there for your scent-based needs.
Welcome to the Ministry of Sleep-Deprived Walks
Walking is simple, right? You put one foot in front of the other, and soon you’re walking out the door. Little kids can do it. Even zombies can walk, and they don’t even have brains.
Research from MIT and the University of São Paulo has shown that walking is a little trickier than we might think. One researcher in particular noticed that student volunteers tended to perform worse toward the end of semesters, as project deadlines and multiple exams crashed over their heads and they were deprived of solid sleep schedules.
In a study published in Scientific Reports, our intrepid walking researchers had a collection of students monitor their sleep patterns for 2 weeks; on average, the students got 6 hours per night, though some were able to compensate on weekends. On the final day of a 14-day period, some students pulled all-nighters while the rest were allowed to sleep as usual. Then all students performed a walking test involving keeping time with a metronome.
To absolutely no one’s surprise, the students who performed all-nighters before being tested walked the worst, but between the other students, the ones who compensated for sleep deprivation on weekends did better than those who got 6 hours every night, despite getting a similar amount of sleep overall. This effect persisted even when the compensating students performed their walking tests late in the week, just before they got their weekend beauty sleep.
The moral of the story? Sleep is good, and you should get more of it. But if you can’t, sleep in on weekends. Science has given you permission. All those suburban dads looking to get their teenagers up at 8 in the morning must be sweating right now.
The Devil’s own face covering?
It’s been over a year and a half since the COVID-19 emergency was declared in the United States, and we’ve been starting to wonder what our good friend SARS-CoV-2 has left to give. The collective cynic/optimist in us figures that the insanity can’t last forever, right?
Maybe not forever, but …
A group of parents is suing the Central Bucks (Pa.) School District over school mask mandates, suggesting that the district has no legal authority to enforce such measures. Most of their arguments, Philadelphia Magazine says, are pretty standard stuff: Masks are causing depression, anxiety, and discomfort in their children; masks are a violation of their constitutional rights; and “masks are being used as a control mechanism over the population.”
There are some unusual claims, though. One of the parents, Shannon Harris, said that “wearing masks interferes with their religious duty to spread the word of God and forces them to participate in a satanic ritual,” according to the Philadelphia Inquirer.
Philadelphia Magazine decided to check on that “satanic ritual” claim by asking an expert, in this case a spokesperson for the Church of Satan. The Reverend Raul Antony said that “simply ‘wearing a mask’ is not a Satanic ritual, and anyone that genuinely thinks otherwise is a blithering idiot,” adding that the group’s rituals were available on its website.
COVID, you never let us down.
You’re the (hurricane) wind beneath my wings
Marriage isn’t easy. From finances to everyday stressors like work and children, maintaining a solid relationship is tough. Then a natural disaster shows up on top of everything else, and marriages actually improve, researchers found.
In a study published by Psychological Science, researchers surveyed 231 newlywed couples about the satisfaction of their marriage before and after Hurricane Harvey in 2017. They found after the hurricane couples had a “significant boost” in the satisfaction of their relationship.
One would think something like this would create what researchers call a “stress spillover,” creating a decrease in relationship satisfaction. Destruction to your home or even displacement after a natural disaster seems pretty stressful. But, “a natural disaster can really put things in perspective. People realize how important their partner is to them when they are jolted out of the day-to-day stress of life,” said Hannah Williamson, PhD, the lead author of the study.
And although everyone saw an increase, the biggest jumps in relationship satisfaction belonged to the people who were most unhappy before the hurricane. Unfortunately, the researchers also found that the effects were only temporary and the dissatisfaction came back within a year.
Dr. Williamson thinks there may be something to these findings that can be beneficial from a therapy standpoint where “couples can shift their perspective in a similar way without having to go through a natural disaster.”
Let’s hope she’s right, because the alternative is to seek out a rampaging hurricane every time your relationship is on the rocks, and that just seems impractical after the second or third year.
Not-so-essential oils
Many people use essential oils as a way to unwind and relax. Stressed? Can’t sleep? There’s probably an essential oil for that. However, it seems like these days a lot of things we love and/or think are good for us have a side that’s not so.
According to the Centers for Disease Control and Prevention, a woman from Georgia died from a rare bacteria called Burkholderia pseudomallei. There have been three previous infections in Kansas, Minnesota, and Texas throughout 2021; two of the four infections were in children. Melioidosis, the disease caused by B. pseudomallei, is usually found in southeast Asia and isn’t obvious or easy to diagnose, especially in places like decidedly untropical Minnesota.
The Georgia case was the real break in this medical mystery, as the infection was traced back to a Walmart product called “Better Homes and Gardens Essential Oil Infused Aromatherapy Room Spray with Gemstones” (a very pithy name). The bacteria were in the lavender and chamomile scent. The CDC is investigating all other product scents, and Walmart has recalled all lots of the product.
If you’ve got that particular essential oil, it’s probably for the best that you stop using it. Don’t worry, we’re sure there’s plenty of other essential oil–infused aromatherapy room sprays with gemstones out there for your scent-based needs.
Welcome to the Ministry of Sleep-Deprived Walks
Walking is simple, right? You put one foot in front of the other, and soon you’re walking out the door. Little kids can do it. Even zombies can walk, and they don’t even have brains.
Research from MIT and the University of São Paulo has shown that walking is a little trickier than we might think. One researcher in particular noticed that student volunteers tended to perform worse toward the end of semesters, as project deadlines and multiple exams crashed over their heads and they were deprived of solid sleep schedules.
In a study published in Scientific Reports, our intrepid walking researchers had a collection of students monitor their sleep patterns for 2 weeks; on average, the students got 6 hours per night, though some were able to compensate on weekends. On the final day of a 14-day period, some students pulled all-nighters while the rest were allowed to sleep as usual. Then all students performed a walking test involving keeping time with a metronome.
To absolutely no one’s surprise, the students who performed all-nighters before being tested walked the worst, but between the other students, the ones who compensated for sleep deprivation on weekends did better than those who got 6 hours every night, despite getting a similar amount of sleep overall. This effect persisted even when the compensating students performed their walking tests late in the week, just before they got their weekend beauty sleep.
The moral of the story? Sleep is good, and you should get more of it. But if you can’t, sleep in on weekends. Science has given you permission. All those suburban dads looking to get their teenagers up at 8 in the morning must be sweating right now.
The Devil’s own face covering?
It’s been over a year and a half since the COVID-19 emergency was declared in the United States, and we’ve been starting to wonder what our good friend SARS-CoV-2 has left to give. The collective cynic/optimist in us figures that the insanity can’t last forever, right?
Maybe not forever, but …
A group of parents is suing the Central Bucks (Pa.) School District over school mask mandates, suggesting that the district has no legal authority to enforce such measures. Most of their arguments, Philadelphia Magazine says, are pretty standard stuff: Masks are causing depression, anxiety, and discomfort in their children; masks are a violation of their constitutional rights; and “masks are being used as a control mechanism over the population.”
There are some unusual claims, though. One of the parents, Shannon Harris, said that “wearing masks interferes with their religious duty to spread the word of God and forces them to participate in a satanic ritual,” according to the Philadelphia Inquirer.
Philadelphia Magazine decided to check on that “satanic ritual” claim by asking an expert, in this case a spokesperson for the Church of Satan. The Reverend Raul Antony said that “simply ‘wearing a mask’ is not a Satanic ritual, and anyone that genuinely thinks otherwise is a blithering idiot,” adding that the group’s rituals were available on its website.
COVID, you never let us down.
You’re the (hurricane) wind beneath my wings
Marriage isn’t easy. From finances to everyday stressors like work and children, maintaining a solid relationship is tough. Then a natural disaster shows up on top of everything else, and marriages actually improve, researchers found.
In a study published by Psychological Science, researchers surveyed 231 newlywed couples about the satisfaction of their marriage before and after Hurricane Harvey in 2017. They found after the hurricane couples had a “significant boost” in the satisfaction of their relationship.
One would think something like this would create what researchers call a “stress spillover,” creating a decrease in relationship satisfaction. Destruction to your home or even displacement after a natural disaster seems pretty stressful. But, “a natural disaster can really put things in perspective. People realize how important their partner is to them when they are jolted out of the day-to-day stress of life,” said Hannah Williamson, PhD, the lead author of the study.
And although everyone saw an increase, the biggest jumps in relationship satisfaction belonged to the people who were most unhappy before the hurricane. Unfortunately, the researchers also found that the effects were only temporary and the dissatisfaction came back within a year.
Dr. Williamson thinks there may be something to these findings that can be beneficial from a therapy standpoint where “couples can shift their perspective in a similar way without having to go through a natural disaster.”
Let’s hope she’s right, because the alternative is to seek out a rampaging hurricane every time your relationship is on the rocks, and that just seems impractical after the second or third year.
Not-so-essential oils
Many people use essential oils as a way to unwind and relax. Stressed? Can’t sleep? There’s probably an essential oil for that. However, it seems like these days a lot of things we love and/or think are good for us have a side that’s not so.
According to the Centers for Disease Control and Prevention, a woman from Georgia died from a rare bacteria called Burkholderia pseudomallei. There have been three previous infections in Kansas, Minnesota, and Texas throughout 2021; two of the four infections were in children. Melioidosis, the disease caused by B. pseudomallei, is usually found in southeast Asia and isn’t obvious or easy to diagnose, especially in places like decidedly untropical Minnesota.
The Georgia case was the real break in this medical mystery, as the infection was traced back to a Walmart product called “Better Homes and Gardens Essential Oil Infused Aromatherapy Room Spray with Gemstones” (a very pithy name). The bacteria were in the lavender and chamomile scent. The CDC is investigating all other product scents, and Walmart has recalled all lots of the product.
If you’ve got that particular essential oil, it’s probably for the best that you stop using it. Don’t worry, we’re sure there’s plenty of other essential oil–infused aromatherapy room sprays with gemstones out there for your scent-based needs.
Welcome to the Ministry of Sleep-Deprived Walks
Walking is simple, right? You put one foot in front of the other, and soon you’re walking out the door. Little kids can do it. Even zombies can walk, and they don’t even have brains.
Research from MIT and the University of São Paulo has shown that walking is a little trickier than we might think. One researcher in particular noticed that student volunteers tended to perform worse toward the end of semesters, as project deadlines and multiple exams crashed over their heads and they were deprived of solid sleep schedules.
In a study published in Scientific Reports, our intrepid walking researchers had a collection of students monitor their sleep patterns for 2 weeks; on average, the students got 6 hours per night, though some were able to compensate on weekends. On the final day of a 14-day period, some students pulled all-nighters while the rest were allowed to sleep as usual. Then all students performed a walking test involving keeping time with a metronome.
To absolutely no one’s surprise, the students who performed all-nighters before being tested walked the worst, but between the other students, the ones who compensated for sleep deprivation on weekends did better than those who got 6 hours every night, despite getting a similar amount of sleep overall. This effect persisted even when the compensating students performed their walking tests late in the week, just before they got their weekend beauty sleep.
The moral of the story? Sleep is good, and you should get more of it. But if you can’t, sleep in on weekends. Science has given you permission. All those suburban dads looking to get their teenagers up at 8 in the morning must be sweating right now.
Which specialties get the biggest markups over Medicare rates?
Anesthesiologists charge private insurers more than 300% above Medicare rates, a markup that is higher than that of 16 other specialties, according to a study released by the Urban Institute.
The Washington-based nonprofit institute found that the lowest markups were in psychiatry, ophthalmology, ob.gyn., family medicine, gastroenterology, and internal medicine, at 110%-120% of Medicare rates. .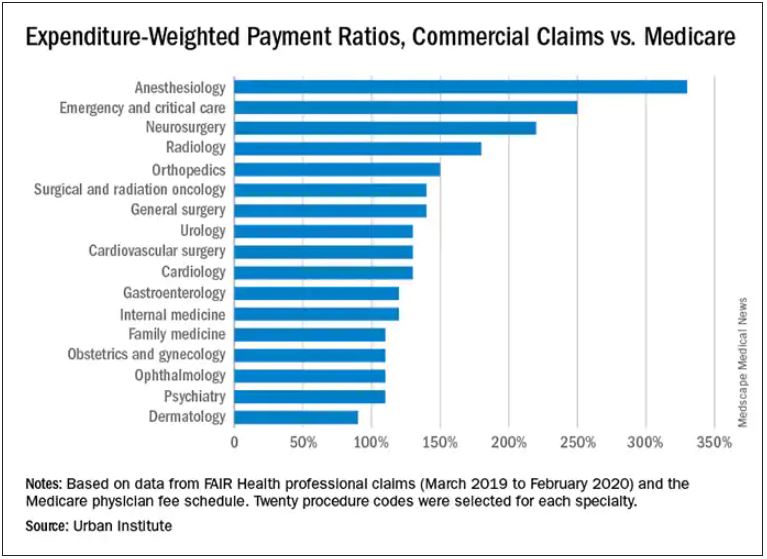
In the middle are cardiology and cardiovascular surgery (130%), urology (130%), general surgery, surgical and radiation oncology (all at 140%), and orthopedics (150%).
At the top end were radiology (180%), neurosurgery (220%), emergency and critical care (250%), and anesthesiology (330%).
The wide variation in payments could be cited in support of the idea of applying Medicare rates across all physician specialties, say the study authors. Although lowering practitioner payments might lead to savings, it “will also create more pushback from providers, especially if these rates are introduced in the employer market,” write researchers Stacey McMorrow, PhD, Robert A. Berenson, MD, and John Holahan, PhD.
It is not known whether lowering commercial payment rates might decrease patient access, they write.
The authors also note that specialties in which the potential for a fee reduction was greatest were also the specialties for which baseline compensation was highest – from $350,000 annually for emergency physicians to $800,000 a year for neurosurgeons. Annual compensation for ob.gyns., dermatologists, and opthalmologists is about $350,000 a year, which suggests that “these specialties are similarly well compensated by both Medicare and commercial insurers,” the authors write.
The investigators assessed the top 20 procedure codes by expenditure in each of 17 physician specialties. They estimated the commercial-to-Medicare payment ratio for each service and constructed weighted averages across services for each specialty at the national level and for 12 states for which data for all the specialties and services were available.
The researchers analyzed claims from the FAIR Health database between March 2019 and March 2020. That database represents 60 insurers covering 150 million people.
Pediatric and geriatric specialties, nonphysician practitioners, out-of-network clinicians, and ambulatory surgery center claims were excluded. Codes with modifiers, J codes, and clinical laboratory services were also not included.
The charges used in the study were not the actual contracted rates. The authors instead used “imputed allowed amounts” for each claim line. That method was used to protect the confidentiality of the negotiated rates.
With regard to all specialties, the lowest compensated services were procedures, evaluation and management, and tests, which received 140%-150% of the Medicare rate. Treatments and imaging were marked up 160%. Anesthesia was reimbursed at a rate 330% higher than the rate Medicare would pay.
The authors also assessed geographic variation for the 12 states for which they had data.
Similar to findings in other studies, the researchers found that the markup was lowest in Pennsylvania (120%) and highest in Wisconsin (260%). The U.S. average was 160%. California and Missouri were at 150%; Michigan was right at the average.
For physicians in Illinois, Louisiana, Colorado, Texas, and New York, markups were 170%-180% over the Medicare rate. Markups for clinicians in New Jersey (190%) and Arizona (200%) were closest to the Wisconsin rate.
The authors note some study limitations, including the fact that they excluded out-of-network practitioners, “and such payments may disproportionately affect certain specialties.”
A version of this article first appeared on Medscape.com.
Anesthesiologists charge private insurers more than 300% above Medicare rates, a markup that is higher than that of 16 other specialties, according to a study released by the Urban Institute.
The Washington-based nonprofit institute found that the lowest markups were in psychiatry, ophthalmology, ob.gyn., family medicine, gastroenterology, and internal medicine, at 110%-120% of Medicare rates. .
In the middle are cardiology and cardiovascular surgery (130%), urology (130%), general surgery, surgical and radiation oncology (all at 140%), and orthopedics (150%).
At the top end were radiology (180%), neurosurgery (220%), emergency and critical care (250%), and anesthesiology (330%).
The wide variation in payments could be cited in support of the idea of applying Medicare rates across all physician specialties, say the study authors. Although lowering practitioner payments might lead to savings, it “will also create more pushback from providers, especially if these rates are introduced in the employer market,” write researchers Stacey McMorrow, PhD, Robert A. Berenson, MD, and John Holahan, PhD.
It is not known whether lowering commercial payment rates might decrease patient access, they write.
The authors also note that specialties in which the potential for a fee reduction was greatest were also the specialties for which baseline compensation was highest – from $350,000 annually for emergency physicians to $800,000 a year for neurosurgeons. Annual compensation for ob.gyns., dermatologists, and opthalmologists is about $350,000 a year, which suggests that “these specialties are similarly well compensated by both Medicare and commercial insurers,” the authors write.
The investigators assessed the top 20 procedure codes by expenditure in each of 17 physician specialties. They estimated the commercial-to-Medicare payment ratio for each service and constructed weighted averages across services for each specialty at the national level and for 12 states for which data for all the specialties and services were available.
The researchers analyzed claims from the FAIR Health database between March 2019 and March 2020. That database represents 60 insurers covering 150 million people.
Pediatric and geriatric specialties, nonphysician practitioners, out-of-network clinicians, and ambulatory surgery center claims were excluded. Codes with modifiers, J codes, and clinical laboratory services were also not included.
The charges used in the study were not the actual contracted rates. The authors instead used “imputed allowed amounts” for each claim line. That method was used to protect the confidentiality of the negotiated rates.
With regard to all specialties, the lowest compensated services were procedures, evaluation and management, and tests, which received 140%-150% of the Medicare rate. Treatments and imaging were marked up 160%. Anesthesia was reimbursed at a rate 330% higher than the rate Medicare would pay.
The authors also assessed geographic variation for the 12 states for which they had data.
Similar to findings in other studies, the researchers found that the markup was lowest in Pennsylvania (120%) and highest in Wisconsin (260%). The U.S. average was 160%. California and Missouri were at 150%; Michigan was right at the average.
For physicians in Illinois, Louisiana, Colorado, Texas, and New York, markups were 170%-180% over the Medicare rate. Markups for clinicians in New Jersey (190%) and Arizona (200%) were closest to the Wisconsin rate.
The authors note some study limitations, including the fact that they excluded out-of-network practitioners, “and such payments may disproportionately affect certain specialties.”
A version of this article first appeared on Medscape.com.
Anesthesiologists charge private insurers more than 300% above Medicare rates, a markup that is higher than that of 16 other specialties, according to a study released by the Urban Institute.
The Washington-based nonprofit institute found that the lowest markups were in psychiatry, ophthalmology, ob.gyn., family medicine, gastroenterology, and internal medicine, at 110%-120% of Medicare rates. .
In the middle are cardiology and cardiovascular surgery (130%), urology (130%), general surgery, surgical and radiation oncology (all at 140%), and orthopedics (150%).
At the top end were radiology (180%), neurosurgery (220%), emergency and critical care (250%), and anesthesiology (330%).
The wide variation in payments could be cited in support of the idea of applying Medicare rates across all physician specialties, say the study authors. Although lowering practitioner payments might lead to savings, it “will also create more pushback from providers, especially if these rates are introduced in the employer market,” write researchers Stacey McMorrow, PhD, Robert A. Berenson, MD, and John Holahan, PhD.
It is not known whether lowering commercial payment rates might decrease patient access, they write.
The authors also note that specialties in which the potential for a fee reduction was greatest were also the specialties for which baseline compensation was highest – from $350,000 annually for emergency physicians to $800,000 a year for neurosurgeons. Annual compensation for ob.gyns., dermatologists, and opthalmologists is about $350,000 a year, which suggests that “these specialties are similarly well compensated by both Medicare and commercial insurers,” the authors write.
The investigators assessed the top 20 procedure codes by expenditure in each of 17 physician specialties. They estimated the commercial-to-Medicare payment ratio for each service and constructed weighted averages across services for each specialty at the national level and for 12 states for which data for all the specialties and services were available.
The researchers analyzed claims from the FAIR Health database between March 2019 and March 2020. That database represents 60 insurers covering 150 million people.
Pediatric and geriatric specialties, nonphysician practitioners, out-of-network clinicians, and ambulatory surgery center claims were excluded. Codes with modifiers, J codes, and clinical laboratory services were also not included.
The charges used in the study were not the actual contracted rates. The authors instead used “imputed allowed amounts” for each claim line. That method was used to protect the confidentiality of the negotiated rates.
With regard to all specialties, the lowest compensated services were procedures, evaluation and management, and tests, which received 140%-150% of the Medicare rate. Treatments and imaging were marked up 160%. Anesthesia was reimbursed at a rate 330% higher than the rate Medicare would pay.
The authors also assessed geographic variation for the 12 states for which they had data.
Similar to findings in other studies, the researchers found that the markup was lowest in Pennsylvania (120%) and highest in Wisconsin (260%). The U.S. average was 160%. California and Missouri were at 150%; Michigan was right at the average.
For physicians in Illinois, Louisiana, Colorado, Texas, and New York, markups were 170%-180% over the Medicare rate. Markups for clinicians in New Jersey (190%) and Arizona (200%) were closest to the Wisconsin rate.
The authors note some study limitations, including the fact that they excluded out-of-network practitioners, “and such payments may disproportionately affect certain specialties.”
A version of this article first appeared on Medscape.com.
Hot temperatures in outdoor lockboxes increase sample errors
, according to results from a recent study published in the American Journal of Clinical Pathology.
“Our findings indicate that samples (centrifuged or not centrifuged) were impacted by extreme summer temperatures when stored for short periods of time inside commonly used steel lockboxes,” Joseph R. Wiencek, PhD, medical director of clinical chemistry, Vanderbilt University School of Medicine Core Laboratory in Nashville, said in an interview.
Dr. Wiencek and colleagues picked two dates during the summer of 2019 in a mid-Atlantic state to place two courier lockboxes (LabLocker-KF300) outside in hot temperatures (32º C) starting at 11 a.m., with one lockbox containing two 24-oz cold packs (Nordic NI24) and the other containing no cold packs. The researchers monitored the temperatures of each lockbox over the course of 4 hours.
Overall, eight participants had seven samples in lithium heparin drawn for two studies evaluating centrifuged or not centrifuged samples. In the first study, four participants had seven samples drawn, with one centrifuged sample serving as a control for each patient. The other six samples were wrapped in paper towels, placed in resealable plastic bags, and distributed evenly in the warm and cold lockboxes. The samples did not directly touch the cold packs in the cold lockbox. At 1 hour, 2 hours, and 4 hours, a participant’s sample was removed from each lockbox and centrifuged.
In the second study, another four participants had seven samples drawn. As in the first study, all samples were centrifuged and placed in the lockboxes. For both studies, when samples were centrifuged, plasma from samples was left on the gel barrier when analyzed for concentrations of C-reactive protein, a comprehensive metabolic panel, lactate dehydrogenase (LDH), a lipid panel, magnesium, and phosphorus (Abbott Architect c16000).
In the study of uncentrifuged samples, Dr. Wiencek and colleagues found that when the temperature outside ranged from 28.2º to 44.0º C (mean 40.4º C), the temperature of the cold lockbox was between 16.5º to 22.3º C (mean 22.3º C). The temperature ranged between 34.4º to 46.9º C (mean 42.6º C) in the warm lockbox. For centrifuged samples, the cold lockbox temperature was between 12.2º to 23.0º C (mean 18.0º C) and the warm lockbox was between 25. to 40.8º C (mean 35.2º C) when the outdoor temperature ranged from 27.2º to 46.3º C (mean 37.9º C).
The researchers also calculated the significant change limit (SCL) for each analyte in each sample, finding that aspartate aminotransferase, glucose, LDH, and potassium significantly exceeded the SCL in both the centrifuged and uncentrifuged samples, with the greatest changes seen at the 4-hour timepoint for samples in the warm lockbox (P < .05 for all).
Lockbox instructions are “consistently inconsistent”
In viewing instructions for lockboxes across institutions, Dr. Wiencek said the “outdoor courier lockbox instructions among private, academic and reference laboratories were consistently inconsistent.” For example, no laboratories cited time restrictions for samples in lockboxes, and their descriptions on the number of cold packs a laboratory should use and where the lockbox should be placed varied. The inconsistencies “highlighted the emergent need for standardization and guidance documents for institutions to implement,” Dr. Wiencek said.
One unanswered question is how widespread the problem is. It is unclear how many outdoor courier lockboxes are currently in use in the United States or globally; however, experts agreed it was a common occurrence, with some of the largest laboratory service providers offering outdoor courier lockboxes to their clients.
“Courier lockboxes are everywhere. All you need to do is walk around your clinics that are at your hospitals or clinics located around your grocery store to find them,” Dr. Wiencek said. “Some hang on doors, while others can be found on the ground in direct sunlight on a hot summer day.”
What’s more, institutions may not realize how leaving samples outdoors for extended periods can affect results. “Care teams are commonly unaware that samples placed in these poorly designed lockboxes can experience extreme summer or winter temperatures that may lead to incorrect results,” Dr. Wiencek said. “Healthcare providers need to understand the hidden dangers courier lockboxes have on the quality of their patient’s test results.”
Amy L. Pyle-Eilola, PhD, clinical chemistry director at Nationwide Children’s Hospital in Columbus, Ohio, said a major strength of the study by Dr. Wiencek and colleagues “is just that it was done at all.”
“I appreciate the real-world nature of this study and that it provides a snapshot of what conditions are really like in a lockbox in the summer,” she said in an interview.
In the clinical lab, receiving samples that had been sitting in a courier lockbox “is not uncommon,” Dr. Pyle-Eilola said.
“When I have encountered these situations, I have struggled to decide if it is still appropriate to run the tests. I always look to the medical literature for assistance with these situations, but there has been a paucity of information available on the impact of lockbox storage,” she explained.
The study by Dr. Wiencek and colleagues “provides some much-needed evidence for what is acceptable for lockbox storage conditions,” she said.
Areas of future research
Rodney E. Rohde, PhD, university distinguished chair and professor of the Clinical Laboratory Science (CLS) Program at Texas State University in San Marcos, said in an interview that the study “does a nice job of looking at multiple analytes and controlling for several variables,” but the sample size is small and the results may be difficult to generalize.
Dr. Pyle-Eilola highlighted another limitation — “a common shortcoming of these kinds of studies” — in the use of healthy donors for patient samples, which narrows the range of assay results.
“It is possible that more significant variation in results may be observed in additional analytes if the samples had higher concentrations of those analytes,” she said. “Moreover, this is clinically relevant as the samples stored in such lockboxes are not always from healthy individuals and have abnormal concentrations of analytes.”
Mario Plebani, MD, professor of clinical biochemistry and clinical molecular biology and chief of the department of laboratory medicine at University Hospital of Padova in Padova, Italy, agreed with that assessment.
“[T]he risks for errors and patient safety are higher for values near to the upper or lower reference value, and in general for samples collected in patients with particular diseases and clinical conditions,” he said in an interview.
“This paper deserves a commenting editorial to better highlight the urgent need for further studies on the same issue and in general on the risk in the pre-pre-analytical phase, including sample storage and transportation,” he noted.
Another area of future research is studying patient samples exposed to hotter or colder temperatures in outdoor courier lockboxes outside the mid-Atlantic area. “Here in Texas, temperatures can reach extreme heat levels,” Dr. Rohde said, who added that use of outdoor lockboxes is “very common in my region.”
Dr. Wiencek disclosed he has been a consultant on this research topic for Roche Diagnostics and received an honorarium for speaking on the subject from the American Association for Clinical Chemistry and American Society of Clinical Pathology. The other authors have no relevant conflict of interest. Dr. Pyle-Eilola, Dr. Rohde, and Dr. Plebani have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to results from a recent study published in the American Journal of Clinical Pathology.
“Our findings indicate that samples (centrifuged or not centrifuged) were impacted by extreme summer temperatures when stored for short periods of time inside commonly used steel lockboxes,” Joseph R. Wiencek, PhD, medical director of clinical chemistry, Vanderbilt University School of Medicine Core Laboratory in Nashville, said in an interview.
Dr. Wiencek and colleagues picked two dates during the summer of 2019 in a mid-Atlantic state to place two courier lockboxes (LabLocker-KF300) outside in hot temperatures (32º C) starting at 11 a.m., with one lockbox containing two 24-oz cold packs (Nordic NI24) and the other containing no cold packs. The researchers monitored the temperatures of each lockbox over the course of 4 hours.
Overall, eight participants had seven samples in lithium heparin drawn for two studies evaluating centrifuged or not centrifuged samples. In the first study, four participants had seven samples drawn, with one centrifuged sample serving as a control for each patient. The other six samples were wrapped in paper towels, placed in resealable plastic bags, and distributed evenly in the warm and cold lockboxes. The samples did not directly touch the cold packs in the cold lockbox. At 1 hour, 2 hours, and 4 hours, a participant’s sample was removed from each lockbox and centrifuged.
In the second study, another four participants had seven samples drawn. As in the first study, all samples were centrifuged and placed in the lockboxes. For both studies, when samples were centrifuged, plasma from samples was left on the gel barrier when analyzed for concentrations of C-reactive protein, a comprehensive metabolic panel, lactate dehydrogenase (LDH), a lipid panel, magnesium, and phosphorus (Abbott Architect c16000).
In the study of uncentrifuged samples, Dr. Wiencek and colleagues found that when the temperature outside ranged from 28.2º to 44.0º C (mean 40.4º C), the temperature of the cold lockbox was between 16.5º to 22.3º C (mean 22.3º C). The temperature ranged between 34.4º to 46.9º C (mean 42.6º C) in the warm lockbox. For centrifuged samples, the cold lockbox temperature was between 12.2º to 23.0º C (mean 18.0º C) and the warm lockbox was between 25. to 40.8º C (mean 35.2º C) when the outdoor temperature ranged from 27.2º to 46.3º C (mean 37.9º C).
The researchers also calculated the significant change limit (SCL) for each analyte in each sample, finding that aspartate aminotransferase, glucose, LDH, and potassium significantly exceeded the SCL in both the centrifuged and uncentrifuged samples, with the greatest changes seen at the 4-hour timepoint for samples in the warm lockbox (P < .05 for all).
Lockbox instructions are “consistently inconsistent”
In viewing instructions for lockboxes across institutions, Dr. Wiencek said the “outdoor courier lockbox instructions among private, academic and reference laboratories were consistently inconsistent.” For example, no laboratories cited time restrictions for samples in lockboxes, and their descriptions on the number of cold packs a laboratory should use and where the lockbox should be placed varied. The inconsistencies “highlighted the emergent need for standardization and guidance documents for institutions to implement,” Dr. Wiencek said.
One unanswered question is how widespread the problem is. It is unclear how many outdoor courier lockboxes are currently in use in the United States or globally; however, experts agreed it was a common occurrence, with some of the largest laboratory service providers offering outdoor courier lockboxes to their clients.
“Courier lockboxes are everywhere. All you need to do is walk around your clinics that are at your hospitals or clinics located around your grocery store to find them,” Dr. Wiencek said. “Some hang on doors, while others can be found on the ground in direct sunlight on a hot summer day.”
What’s more, institutions may not realize how leaving samples outdoors for extended periods can affect results. “Care teams are commonly unaware that samples placed in these poorly designed lockboxes can experience extreme summer or winter temperatures that may lead to incorrect results,” Dr. Wiencek said. “Healthcare providers need to understand the hidden dangers courier lockboxes have on the quality of their patient’s test results.”
Amy L. Pyle-Eilola, PhD, clinical chemistry director at Nationwide Children’s Hospital in Columbus, Ohio, said a major strength of the study by Dr. Wiencek and colleagues “is just that it was done at all.”
“I appreciate the real-world nature of this study and that it provides a snapshot of what conditions are really like in a lockbox in the summer,” she said in an interview.
In the clinical lab, receiving samples that had been sitting in a courier lockbox “is not uncommon,” Dr. Pyle-Eilola said.
“When I have encountered these situations, I have struggled to decide if it is still appropriate to run the tests. I always look to the medical literature for assistance with these situations, but there has been a paucity of information available on the impact of lockbox storage,” she explained.
The study by Dr. Wiencek and colleagues “provides some much-needed evidence for what is acceptable for lockbox storage conditions,” she said.
Areas of future research
Rodney E. Rohde, PhD, university distinguished chair and professor of the Clinical Laboratory Science (CLS) Program at Texas State University in San Marcos, said in an interview that the study “does a nice job of looking at multiple analytes and controlling for several variables,” but the sample size is small and the results may be difficult to generalize.
Dr. Pyle-Eilola highlighted another limitation — “a common shortcoming of these kinds of studies” — in the use of healthy donors for patient samples, which narrows the range of assay results.
“It is possible that more significant variation in results may be observed in additional analytes if the samples had higher concentrations of those analytes,” she said. “Moreover, this is clinically relevant as the samples stored in such lockboxes are not always from healthy individuals and have abnormal concentrations of analytes.”
Mario Plebani, MD, professor of clinical biochemistry and clinical molecular biology and chief of the department of laboratory medicine at University Hospital of Padova in Padova, Italy, agreed with that assessment.
“[T]he risks for errors and patient safety are higher for values near to the upper or lower reference value, and in general for samples collected in patients with particular diseases and clinical conditions,” he said in an interview.
“This paper deserves a commenting editorial to better highlight the urgent need for further studies on the same issue and in general on the risk in the pre-pre-analytical phase, including sample storage and transportation,” he noted.
Another area of future research is studying patient samples exposed to hotter or colder temperatures in outdoor courier lockboxes outside the mid-Atlantic area. “Here in Texas, temperatures can reach extreme heat levels,” Dr. Rohde said, who added that use of outdoor lockboxes is “very common in my region.”
Dr. Wiencek disclosed he has been a consultant on this research topic for Roche Diagnostics and received an honorarium for speaking on the subject from the American Association for Clinical Chemistry and American Society of Clinical Pathology. The other authors have no relevant conflict of interest. Dr. Pyle-Eilola, Dr. Rohde, and Dr. Plebani have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to results from a recent study published in the American Journal of Clinical Pathology.
“Our findings indicate that samples (centrifuged or not centrifuged) were impacted by extreme summer temperatures when stored for short periods of time inside commonly used steel lockboxes,” Joseph R. Wiencek, PhD, medical director of clinical chemistry, Vanderbilt University School of Medicine Core Laboratory in Nashville, said in an interview.
Dr. Wiencek and colleagues picked two dates during the summer of 2019 in a mid-Atlantic state to place two courier lockboxes (LabLocker-KF300) outside in hot temperatures (32º C) starting at 11 a.m., with one lockbox containing two 24-oz cold packs (Nordic NI24) and the other containing no cold packs. The researchers monitored the temperatures of each lockbox over the course of 4 hours.
Overall, eight participants had seven samples in lithium heparin drawn for two studies evaluating centrifuged or not centrifuged samples. In the first study, four participants had seven samples drawn, with one centrifuged sample serving as a control for each patient. The other six samples were wrapped in paper towels, placed in resealable plastic bags, and distributed evenly in the warm and cold lockboxes. The samples did not directly touch the cold packs in the cold lockbox. At 1 hour, 2 hours, and 4 hours, a participant’s sample was removed from each lockbox and centrifuged.
In the second study, another four participants had seven samples drawn. As in the first study, all samples were centrifuged and placed in the lockboxes. For both studies, when samples were centrifuged, plasma from samples was left on the gel barrier when analyzed for concentrations of C-reactive protein, a comprehensive metabolic panel, lactate dehydrogenase (LDH), a lipid panel, magnesium, and phosphorus (Abbott Architect c16000).
In the study of uncentrifuged samples, Dr. Wiencek and colleagues found that when the temperature outside ranged from 28.2º to 44.0º C (mean 40.4º C), the temperature of the cold lockbox was between 16.5º to 22.3º C (mean 22.3º C). The temperature ranged between 34.4º to 46.9º C (mean 42.6º C) in the warm lockbox. For centrifuged samples, the cold lockbox temperature was between 12.2º to 23.0º C (mean 18.0º C) and the warm lockbox was between 25. to 40.8º C (mean 35.2º C) when the outdoor temperature ranged from 27.2º to 46.3º C (mean 37.9º C).
The researchers also calculated the significant change limit (SCL) for each analyte in each sample, finding that aspartate aminotransferase, glucose, LDH, and potassium significantly exceeded the SCL in both the centrifuged and uncentrifuged samples, with the greatest changes seen at the 4-hour timepoint for samples in the warm lockbox (P < .05 for all).
Lockbox instructions are “consistently inconsistent”
In viewing instructions for lockboxes across institutions, Dr. Wiencek said the “outdoor courier lockbox instructions among private, academic and reference laboratories were consistently inconsistent.” For example, no laboratories cited time restrictions for samples in lockboxes, and their descriptions on the number of cold packs a laboratory should use and where the lockbox should be placed varied. The inconsistencies “highlighted the emergent need for standardization and guidance documents for institutions to implement,” Dr. Wiencek said.
One unanswered question is how widespread the problem is. It is unclear how many outdoor courier lockboxes are currently in use in the United States or globally; however, experts agreed it was a common occurrence, with some of the largest laboratory service providers offering outdoor courier lockboxes to their clients.
“Courier lockboxes are everywhere. All you need to do is walk around your clinics that are at your hospitals or clinics located around your grocery store to find them,” Dr. Wiencek said. “Some hang on doors, while others can be found on the ground in direct sunlight on a hot summer day.”
What’s more, institutions may not realize how leaving samples outdoors for extended periods can affect results. “Care teams are commonly unaware that samples placed in these poorly designed lockboxes can experience extreme summer or winter temperatures that may lead to incorrect results,” Dr. Wiencek said. “Healthcare providers need to understand the hidden dangers courier lockboxes have on the quality of their patient’s test results.”
Amy L. Pyle-Eilola, PhD, clinical chemistry director at Nationwide Children’s Hospital in Columbus, Ohio, said a major strength of the study by Dr. Wiencek and colleagues “is just that it was done at all.”
“I appreciate the real-world nature of this study and that it provides a snapshot of what conditions are really like in a lockbox in the summer,” she said in an interview.
In the clinical lab, receiving samples that had been sitting in a courier lockbox “is not uncommon,” Dr. Pyle-Eilola said.
“When I have encountered these situations, I have struggled to decide if it is still appropriate to run the tests. I always look to the medical literature for assistance with these situations, but there has been a paucity of information available on the impact of lockbox storage,” she explained.
The study by Dr. Wiencek and colleagues “provides some much-needed evidence for what is acceptable for lockbox storage conditions,” she said.
Areas of future research
Rodney E. Rohde, PhD, university distinguished chair and professor of the Clinical Laboratory Science (CLS) Program at Texas State University in San Marcos, said in an interview that the study “does a nice job of looking at multiple analytes and controlling for several variables,” but the sample size is small and the results may be difficult to generalize.
Dr. Pyle-Eilola highlighted another limitation — “a common shortcoming of these kinds of studies” — in the use of healthy donors for patient samples, which narrows the range of assay results.
“It is possible that more significant variation in results may be observed in additional analytes if the samples had higher concentrations of those analytes,” she said. “Moreover, this is clinically relevant as the samples stored in such lockboxes are not always from healthy individuals and have abnormal concentrations of analytes.”
Mario Plebani, MD, professor of clinical biochemistry and clinical molecular biology and chief of the department of laboratory medicine at University Hospital of Padova in Padova, Italy, agreed with that assessment.
“[T]he risks for errors and patient safety are higher for values near to the upper or lower reference value, and in general for samples collected in patients with particular diseases and clinical conditions,” he said in an interview.
“This paper deserves a commenting editorial to better highlight the urgent need for further studies on the same issue and in general on the risk in the pre-pre-analytical phase, including sample storage and transportation,” he noted.
Another area of future research is studying patient samples exposed to hotter or colder temperatures in outdoor courier lockboxes outside the mid-Atlantic area. “Here in Texas, temperatures can reach extreme heat levels,” Dr. Rohde said, who added that use of outdoor lockboxes is “very common in my region.”
Dr. Wiencek disclosed he has been a consultant on this research topic for Roche Diagnostics and received an honorarium for speaking on the subject from the American Association for Clinical Chemistry and American Society of Clinical Pathology. The other authors have no relevant conflict of interest. Dr. Pyle-Eilola, Dr. Rohde, and Dr. Plebani have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pain in MS: Focus on flexibility, multiple strategies, and nondrug treatments
Flexibility and multiple strategies are key, especially considering that pain can evolve over time because of changes in MS and related conditions.
“Pain syndromes are incredibly common. They can happen in monophasic, neurological attacks, or relapsing conditions,” neurologist Scott Newsome, DO, of Johns Hopkins University, Baltimore, said in a presentation about pain at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC). “The good news is there are a lot of things that we can do to help our patients, and the buck does not just stop with oral medications.”
Dr. Newsome, president of the CMSC’s foundation, noted that pain syndromes affect most people who have spinal cord attacks. Research has suggested that the severity of initial attacks is a predictor of the severity of pain syndromes to come.
“There’s a number of triggers that can worsen these pain syndromes – not sleeping well the night before, anxiety, or when someone overheats,” he said. “A lot of our patients during the summertime, when they go out, they want to enjoy themselves and hang out with their family. If the ambient temperature is to a degree where they have increased symptoms, it really impacts their quality of life.”
Dr. Newsome urged colleagues to consider the three types of pain – primary, such as those related to spasticity or tonic spasms; secondary, which can be caused by weakness, reaction to weakness, and spasticity; and tertiary, which is the emotional response to pain.
Tertiary and secondary pain are often overlooked. On the latter front, “early on in my career, I was a big offender,” he said. “I would just focus how a person had a direct injury to the nervous system and not realize that their hip isn’t hurting because of it. It’s a compensatory mechanism after the direct injury, affecting the muscle skeletal system adversely, and having this wear-and-tear phenomenon – setting them up for advanced arthritis, or even a vascular necrosis.”
In regard to MS, he said, it’s helpful to understand pain syndromes. One type is neuropathic: pain that’s worse at night, doesn’t respond well to standard painkillers, and needs multiple therapies. Another type is paroxysmal cord phenomena, which include tonic spasms, Lhermitte’s sign (“an uncomfortable, shocking, vibrating, electrical pain that goes right down their spine” when the neck is flexed), and a condition known as MS hug. “Our patients will come in and say: ‘Oh, it feels like someone’s given me a bear hug or is strangling me.’”
What works as therapy for primary pain syndromes? “I personally don’t like opioids for any pain syndrome, for a lot of reasons,” he said, but a combination of other drugs can be helpful at low doses to start. “I’m a big believer in combining treatments that have different mechanism of actions” instead of, say, combining gabapentin with pregabalin, nerve drugs which work in similar ways.
Dr. Newsome recalled seeing a patient recently who said: “Oh, I tried that drug, I tried this drug, they didn’t help, and I couldn’t tolerate them.” Turns out the patient was taking maximum doses. “No wonder you didn’t tolerate it,” Dr. Newsome said.
Nonpharmaceutical interventions can play an important role, he said. “Believe it or not, we’ve had a lot of people get benefit from acupuncture and massage therapy. And we’ve had some people actually undergo spinal cord stimulation and get stimulators placed. It’s rare, but that’s a consideration for individuals who are refractory to everything you do.”
Medical marijuana, Botox, ketamine, and intrathecal baclofen are other options, he said.
Finally, he said, slowly taper a patient off pain medications if they’re pain free for 3 months. “If someone is doing nonpharmacological interventions, and they’re having a good deal of pain relief, then that’s definitely an opportunity to cut back on the pain medications.”
Flexibility and multiple strategies are key, especially considering that pain can evolve over time because of changes in MS and related conditions.
“Pain syndromes are incredibly common. They can happen in monophasic, neurological attacks, or relapsing conditions,” neurologist Scott Newsome, DO, of Johns Hopkins University, Baltimore, said in a presentation about pain at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC). “The good news is there are a lot of things that we can do to help our patients, and the buck does not just stop with oral medications.”
Dr. Newsome, president of the CMSC’s foundation, noted that pain syndromes affect most people who have spinal cord attacks. Research has suggested that the severity of initial attacks is a predictor of the severity of pain syndromes to come.
“There’s a number of triggers that can worsen these pain syndromes – not sleeping well the night before, anxiety, or when someone overheats,” he said. “A lot of our patients during the summertime, when they go out, they want to enjoy themselves and hang out with their family. If the ambient temperature is to a degree where they have increased symptoms, it really impacts their quality of life.”
Dr. Newsome urged colleagues to consider the three types of pain – primary, such as those related to spasticity or tonic spasms; secondary, which can be caused by weakness, reaction to weakness, and spasticity; and tertiary, which is the emotional response to pain.
Tertiary and secondary pain are often overlooked. On the latter front, “early on in my career, I was a big offender,” he said. “I would just focus how a person had a direct injury to the nervous system and not realize that their hip isn’t hurting because of it. It’s a compensatory mechanism after the direct injury, affecting the muscle skeletal system adversely, and having this wear-and-tear phenomenon – setting them up for advanced arthritis, or even a vascular necrosis.”
In regard to MS, he said, it’s helpful to understand pain syndromes. One type is neuropathic: pain that’s worse at night, doesn’t respond well to standard painkillers, and needs multiple therapies. Another type is paroxysmal cord phenomena, which include tonic spasms, Lhermitte’s sign (“an uncomfortable, shocking, vibrating, electrical pain that goes right down their spine” when the neck is flexed), and a condition known as MS hug. “Our patients will come in and say: ‘Oh, it feels like someone’s given me a bear hug or is strangling me.’”
What works as therapy for primary pain syndromes? “I personally don’t like opioids for any pain syndrome, for a lot of reasons,” he said, but a combination of other drugs can be helpful at low doses to start. “I’m a big believer in combining treatments that have different mechanism of actions” instead of, say, combining gabapentin with pregabalin, nerve drugs which work in similar ways.
Dr. Newsome recalled seeing a patient recently who said: “Oh, I tried that drug, I tried this drug, they didn’t help, and I couldn’t tolerate them.” Turns out the patient was taking maximum doses. “No wonder you didn’t tolerate it,” Dr. Newsome said.
Nonpharmaceutical interventions can play an important role, he said. “Believe it or not, we’ve had a lot of people get benefit from acupuncture and massage therapy. And we’ve had some people actually undergo spinal cord stimulation and get stimulators placed. It’s rare, but that’s a consideration for individuals who are refractory to everything you do.”
Medical marijuana, Botox, ketamine, and intrathecal baclofen are other options, he said.
Finally, he said, slowly taper a patient off pain medications if they’re pain free for 3 months. “If someone is doing nonpharmacological interventions, and they’re having a good deal of pain relief, then that’s definitely an opportunity to cut back on the pain medications.”
Flexibility and multiple strategies are key, especially considering that pain can evolve over time because of changes in MS and related conditions.
“Pain syndromes are incredibly common. They can happen in monophasic, neurological attacks, or relapsing conditions,” neurologist Scott Newsome, DO, of Johns Hopkins University, Baltimore, said in a presentation about pain at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC). “The good news is there are a lot of things that we can do to help our patients, and the buck does not just stop with oral medications.”
Dr. Newsome, president of the CMSC’s foundation, noted that pain syndromes affect most people who have spinal cord attacks. Research has suggested that the severity of initial attacks is a predictor of the severity of pain syndromes to come.
“There’s a number of triggers that can worsen these pain syndromes – not sleeping well the night before, anxiety, or when someone overheats,” he said. “A lot of our patients during the summertime, when they go out, they want to enjoy themselves and hang out with their family. If the ambient temperature is to a degree where they have increased symptoms, it really impacts their quality of life.”
Dr. Newsome urged colleagues to consider the three types of pain – primary, such as those related to spasticity or tonic spasms; secondary, which can be caused by weakness, reaction to weakness, and spasticity; and tertiary, which is the emotional response to pain.
Tertiary and secondary pain are often overlooked. On the latter front, “early on in my career, I was a big offender,” he said. “I would just focus how a person had a direct injury to the nervous system and not realize that their hip isn’t hurting because of it. It’s a compensatory mechanism after the direct injury, affecting the muscle skeletal system adversely, and having this wear-and-tear phenomenon – setting them up for advanced arthritis, or even a vascular necrosis.”
In regard to MS, he said, it’s helpful to understand pain syndromes. One type is neuropathic: pain that’s worse at night, doesn’t respond well to standard painkillers, and needs multiple therapies. Another type is paroxysmal cord phenomena, which include tonic spasms, Lhermitte’s sign (“an uncomfortable, shocking, vibrating, electrical pain that goes right down their spine” when the neck is flexed), and a condition known as MS hug. “Our patients will come in and say: ‘Oh, it feels like someone’s given me a bear hug or is strangling me.’”
What works as therapy for primary pain syndromes? “I personally don’t like opioids for any pain syndrome, for a lot of reasons,” he said, but a combination of other drugs can be helpful at low doses to start. “I’m a big believer in combining treatments that have different mechanism of actions” instead of, say, combining gabapentin with pregabalin, nerve drugs which work in similar ways.
Dr. Newsome recalled seeing a patient recently who said: “Oh, I tried that drug, I tried this drug, they didn’t help, and I couldn’t tolerate them.” Turns out the patient was taking maximum doses. “No wonder you didn’t tolerate it,” Dr. Newsome said.
Nonpharmaceutical interventions can play an important role, he said. “Believe it or not, we’ve had a lot of people get benefit from acupuncture and massage therapy. And we’ve had some people actually undergo spinal cord stimulation and get stimulators placed. It’s rare, but that’s a consideration for individuals who are refractory to everything you do.”
Medical marijuana, Botox, ketamine, and intrathecal baclofen are other options, he said.
Finally, he said, slowly taper a patient off pain medications if they’re pain free for 3 months. “If someone is doing nonpharmacological interventions, and they’re having a good deal of pain relief, then that’s definitely an opportunity to cut back on the pain medications.”
FROM CMSC 2021
FDA panel votes to approve Pfizer’s vaccine for children
Seventeen of the 18 members of the Vaccines and Related Biological Products Advisory Committee (VRBPAC) on Oct. 26 voted to recommend the 10-microgram shot for kids, which is one-third the dose given to adults.
One member, Michael Kurilla, MD, director of the division of clinical innovation at the National Institutes of Health, Bethesda, Md., abstained from voting.
If the FDA follows the recommendation, as it typically does, and issues an Emergency Use Authorization for the vaccine, the shots could be available within days.
After the FDA’s final decision, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices will meet to make specific recommendations for its use. The CDC committee must stick closely to the conditions for use spelled out in the EUA, so their recommendations are likely to be similar to those made by the FDA. Their next meeting is scheduled for Nov. 2 and 3.
In the end, some on the panel felt uneasy with their decision.
“I voted yes primarily because I wanted to make sure that children who really need this vaccine, the Black and brown children of our country, get the vaccine,” said James Hildreth, MD, PhD, president and CEO of Meharry Medical College in Nashville.
“But to be honest, the best way to protect the health of some children will be to do nothing because they will be just fine,” he said.
Others said they were surprised by how difficult the decision had been.
“This is a much tougher one than we had expected going into it,” said committee member Eric Rubin, MD, editor and chief of the New England Journal of Medicine, during the FDA advisory committee’s meeting.
Ahead of the vote, the committee heard presentations outlining the expected benefits of vaccinating children along with potential risks.
“Children have been greatly impacted by the pandemic,” said Fiona Havers, MD, a medical officer with the CDC in Atlanta who reviewed the epidemiology of COVID-19 in kids.
In the second year of the pandemic, as more seniors have been vaccinated against the virus, COVID cases have largely shifted from older to younger age groups.
So far, there have been more than 1.9 million COVID-19 cases in children ages 5 through 11 in the United States.. Cases in kids saw a big jump in July and August with summer travel, schools reopening, and the dominance of the Delta variant.
And those are just the cases reported to the CDC. Regular testing of anonymous blood samples collected at sites across the United States indicates that 6 times as many kids have had COVID than what is reflected in official counts.
Last winter, blood sample testing showed about 13% of children had antibodies against the virus, suggesting they’d been infected. By this summer, that number had risen to 42%.
That figure clearly made an impression on many members of the committee who asked the FDA’s vaccine reviewers if they had tried to account for immunity from past infections in their modeling. They had not.
Some felt that even with a highly effective vaccine — new data presented by Pfizer showed the children’s dose was 90% effective at preventing symptomatic infections in kids — caution was warranted as much is still unknown about myocarditis, a rare side effect of the mRNA vaccines.
Myocarditis has been more common in younger age groups. It usually goes away over time but requires hospital care. It’s not known if myocarditis could have lingering effects for those who experience it.
There were no cases of myocarditis seen in Pfizer’s studies of the vaccine in children, and no other serious events were seen. Vaccine side effects reported in the Pfizer studies were mostly mild and included fatigue, headache, and pain at the injection site.
“We think we have optimized the immune response and minimized our reactions,” said William Gruber, MD, senior vice president vaccine research and clinical development at Pfizer.
But the studies didn’t include enough participants to pick up rare, but serious adverse events like myocarditis.
“We’re worried about a side effect that we can’t measure yet, but it’s probably real, and we see a benefit that isn’t the same as it is in older age groups,” said Dr. Rubin.
Benefits vs. risks
FDA modeled the benefits and risks for children under a variety of scenarios. The benefits of the vaccines to children very much depend on the amount of transmission in the community.
When transmission is high, the benefits to children — in terms of infections, hospitalizations, ICU admissions — clearly outweigh its risks.
But when COVID-19 rates are low in the community, as they were in June, FDA analysts predicted the vaccines might send more children to the hospital for myocarditis than the virus would.
The FDA noted that kids who are hospitalized for myocarditis tend not to be as ill as children with COVID-19, however.
“If the trends continue the way they are going, the emergency for children is not what we might think it would be. That was my concern,” Dr. Hildreth said.
But others warned against complacency.
“Thinking that this is going to be the end of the wave permanently may be a little overly optimistic,” said committee chairman Arnold Monto, MD, a professor of public health and epidemiology at the University of Michigan, Ann Arbor.
The majority of COVID-19 cases in children are mild. Only about 1% of kids are hospitalized for their infections, according to CDC data. But the rates of hospitalizations in kids are about 3 times higher for people of color — including Blacks, Hispanics, and Native Americans, as compared to Whites and Asian Americans.
Since the start of the pandemic, 94 children ages 5 to 11 have died, making it the eighth leading cause of death for kids this age last year.
More than 5,200 children have developed a delayed complication from their infections called Multi-System Inflammatory Syndrome (MIS-C).
MIS-C can be severe and require hospital care and can lead to myocarditis. Children ages 5 to 11 are the age group at greatest risk for this complication.
Kids can also get long COVID. There’s not a lot of data on how often this happens, though it appears to be less frequent in children than in adults.
But a survey in the United Kingdom found that 7%-8% of kids have symptoms from their infections that last longer than 12 weeks, Dr. Havers said. Symptoms that can linger for kids include fatigue, cough, muscle and joint pain, headaches, and insomnia.
More than 1 million children have been impacted by school closures so far this year, and quarantines have had lasting impacts on learning, social development, and mental health.
Even though kids aren’t usually COVID superspreaders, they can still pass the infection on to others.
“What is clear is that secondary transmission from children, both to other children and to adults, does occur,” Dr. Havers said.
For that reason, they can continue the spread of the virus and give it opportunities to mutate and become more dangerous.
Safety monitoring to continue
Some committee members referenced thousands of letters they had received within the past few days urging them to vote against the vaccine.
Jay Portnoy, MD, a professor of pediatrics at Children’s Mercy Hospital in Kansas City, Mo., said he had personally received about 4,000 emails.
“But I feel like I need to also represent the consumers, the parents that I see every day in the clinic who are terrified of sending their children to school because they’re not protected against COVID,” he said, explaining his vote to recommend authorization.
“Our kids are going to be dealing with this virus for many years to come. It’s going to come repeatedly. Getting this vaccine is just the first step that they can take to protect themselves from having bad outcomes,” Dr. Portnoy said.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, reminded members of the committee that there were several government surveillance systems in place to catch any potential safety issues in near real time.
“I really appreciate very much the concern here. The safety monitoring of this vaccine will continue,” Dr. Marks said. “I do view this as one of our greatest responsibilities.”
“I really am so grateful that we had this discussion and voted to approve,” said Capt. Amanda Cohn, MD, chief medical officer at the National Center for Immunization and Respiratory Diseases.
“I think the benefits in this age group really are super important even if they are lower than for other age groups.”
This article was updated 10/27/21.
A version of this article first appeared on WebMD.com.
Seventeen of the 18 members of the Vaccines and Related Biological Products Advisory Committee (VRBPAC) on Oct. 26 voted to recommend the 10-microgram shot for kids, which is one-third the dose given to adults.
One member, Michael Kurilla, MD, director of the division of clinical innovation at the National Institutes of Health, Bethesda, Md., abstained from voting.
If the FDA follows the recommendation, as it typically does, and issues an Emergency Use Authorization for the vaccine, the shots could be available within days.
After the FDA’s final decision, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices will meet to make specific recommendations for its use. The CDC committee must stick closely to the conditions for use spelled out in the EUA, so their recommendations are likely to be similar to those made by the FDA. Their next meeting is scheduled for Nov. 2 and 3.
In the end, some on the panel felt uneasy with their decision.
“I voted yes primarily because I wanted to make sure that children who really need this vaccine, the Black and brown children of our country, get the vaccine,” said James Hildreth, MD, PhD, president and CEO of Meharry Medical College in Nashville.
“But to be honest, the best way to protect the health of some children will be to do nothing because they will be just fine,” he said.
Others said they were surprised by how difficult the decision had been.
“This is a much tougher one than we had expected going into it,” said committee member Eric Rubin, MD, editor and chief of the New England Journal of Medicine, during the FDA advisory committee’s meeting.
Ahead of the vote, the committee heard presentations outlining the expected benefits of vaccinating children along with potential risks.
“Children have been greatly impacted by the pandemic,” said Fiona Havers, MD, a medical officer with the CDC in Atlanta who reviewed the epidemiology of COVID-19 in kids.
In the second year of the pandemic, as more seniors have been vaccinated against the virus, COVID cases have largely shifted from older to younger age groups.
So far, there have been more than 1.9 million COVID-19 cases in children ages 5 through 11 in the United States.. Cases in kids saw a big jump in July and August with summer travel, schools reopening, and the dominance of the Delta variant.
And those are just the cases reported to the CDC. Regular testing of anonymous blood samples collected at sites across the United States indicates that 6 times as many kids have had COVID than what is reflected in official counts.
Last winter, blood sample testing showed about 13% of children had antibodies against the virus, suggesting they’d been infected. By this summer, that number had risen to 42%.
That figure clearly made an impression on many members of the committee who asked the FDA’s vaccine reviewers if they had tried to account for immunity from past infections in their modeling. They had not.
Some felt that even with a highly effective vaccine — new data presented by Pfizer showed the children’s dose was 90% effective at preventing symptomatic infections in kids — caution was warranted as much is still unknown about myocarditis, a rare side effect of the mRNA vaccines.
Myocarditis has been more common in younger age groups. It usually goes away over time but requires hospital care. It’s not known if myocarditis could have lingering effects for those who experience it.
There were no cases of myocarditis seen in Pfizer’s studies of the vaccine in children, and no other serious events were seen. Vaccine side effects reported in the Pfizer studies were mostly mild and included fatigue, headache, and pain at the injection site.
“We think we have optimized the immune response and minimized our reactions,” said William Gruber, MD, senior vice president vaccine research and clinical development at Pfizer.
But the studies didn’t include enough participants to pick up rare, but serious adverse events like myocarditis.
“We’re worried about a side effect that we can’t measure yet, but it’s probably real, and we see a benefit that isn’t the same as it is in older age groups,” said Dr. Rubin.
Benefits vs. risks
FDA modeled the benefits and risks for children under a variety of scenarios. The benefits of the vaccines to children very much depend on the amount of transmission in the community.
When transmission is high, the benefits to children — in terms of infections, hospitalizations, ICU admissions — clearly outweigh its risks.
But when COVID-19 rates are low in the community, as they were in June, FDA analysts predicted the vaccines might send more children to the hospital for myocarditis than the virus would.
The FDA noted that kids who are hospitalized for myocarditis tend not to be as ill as children with COVID-19, however.
“If the trends continue the way they are going, the emergency for children is not what we might think it would be. That was my concern,” Dr. Hildreth said.
But others warned against complacency.
“Thinking that this is going to be the end of the wave permanently may be a little overly optimistic,” said committee chairman Arnold Monto, MD, a professor of public health and epidemiology at the University of Michigan, Ann Arbor.
The majority of COVID-19 cases in children are mild. Only about 1% of kids are hospitalized for their infections, according to CDC data. But the rates of hospitalizations in kids are about 3 times higher for people of color — including Blacks, Hispanics, and Native Americans, as compared to Whites and Asian Americans.
Since the start of the pandemic, 94 children ages 5 to 11 have died, making it the eighth leading cause of death for kids this age last year.
More than 5,200 children have developed a delayed complication from their infections called Multi-System Inflammatory Syndrome (MIS-C).
MIS-C can be severe and require hospital care and can lead to myocarditis. Children ages 5 to 11 are the age group at greatest risk for this complication.
Kids can also get long COVID. There’s not a lot of data on how often this happens, though it appears to be less frequent in children than in adults.
But a survey in the United Kingdom found that 7%-8% of kids have symptoms from their infections that last longer than 12 weeks, Dr. Havers said. Symptoms that can linger for kids include fatigue, cough, muscle and joint pain, headaches, and insomnia.
More than 1 million children have been impacted by school closures so far this year, and quarantines have had lasting impacts on learning, social development, and mental health.
Even though kids aren’t usually COVID superspreaders, they can still pass the infection on to others.
“What is clear is that secondary transmission from children, both to other children and to adults, does occur,” Dr. Havers said.
For that reason, they can continue the spread of the virus and give it opportunities to mutate and become more dangerous.
Safety monitoring to continue
Some committee members referenced thousands of letters they had received within the past few days urging them to vote against the vaccine.
Jay Portnoy, MD, a professor of pediatrics at Children’s Mercy Hospital in Kansas City, Mo., said he had personally received about 4,000 emails.
“But I feel like I need to also represent the consumers, the parents that I see every day in the clinic who are terrified of sending their children to school because they’re not protected against COVID,” he said, explaining his vote to recommend authorization.
“Our kids are going to be dealing with this virus for many years to come. It’s going to come repeatedly. Getting this vaccine is just the first step that they can take to protect themselves from having bad outcomes,” Dr. Portnoy said.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, reminded members of the committee that there were several government surveillance systems in place to catch any potential safety issues in near real time.
“I really appreciate very much the concern here. The safety monitoring of this vaccine will continue,” Dr. Marks said. “I do view this as one of our greatest responsibilities.”
“I really am so grateful that we had this discussion and voted to approve,” said Capt. Amanda Cohn, MD, chief medical officer at the National Center for Immunization and Respiratory Diseases.
“I think the benefits in this age group really are super important even if they are lower than for other age groups.”
This article was updated 10/27/21.
A version of this article first appeared on WebMD.com.
Seventeen of the 18 members of the Vaccines and Related Biological Products Advisory Committee (VRBPAC) on Oct. 26 voted to recommend the 10-microgram shot for kids, which is one-third the dose given to adults.
One member, Michael Kurilla, MD, director of the division of clinical innovation at the National Institutes of Health, Bethesda, Md., abstained from voting.
If the FDA follows the recommendation, as it typically does, and issues an Emergency Use Authorization for the vaccine, the shots could be available within days.
After the FDA’s final decision, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices will meet to make specific recommendations for its use. The CDC committee must stick closely to the conditions for use spelled out in the EUA, so their recommendations are likely to be similar to those made by the FDA. Their next meeting is scheduled for Nov. 2 and 3.
In the end, some on the panel felt uneasy with their decision.
“I voted yes primarily because I wanted to make sure that children who really need this vaccine, the Black and brown children of our country, get the vaccine,” said James Hildreth, MD, PhD, president and CEO of Meharry Medical College in Nashville.
“But to be honest, the best way to protect the health of some children will be to do nothing because they will be just fine,” he said.
Others said they were surprised by how difficult the decision had been.
“This is a much tougher one than we had expected going into it,” said committee member Eric Rubin, MD, editor and chief of the New England Journal of Medicine, during the FDA advisory committee’s meeting.
Ahead of the vote, the committee heard presentations outlining the expected benefits of vaccinating children along with potential risks.
“Children have been greatly impacted by the pandemic,” said Fiona Havers, MD, a medical officer with the CDC in Atlanta who reviewed the epidemiology of COVID-19 in kids.
In the second year of the pandemic, as more seniors have been vaccinated against the virus, COVID cases have largely shifted from older to younger age groups.
So far, there have been more than 1.9 million COVID-19 cases in children ages 5 through 11 in the United States.. Cases in kids saw a big jump in July and August with summer travel, schools reopening, and the dominance of the Delta variant.
And those are just the cases reported to the CDC. Regular testing of anonymous blood samples collected at sites across the United States indicates that 6 times as many kids have had COVID than what is reflected in official counts.
Last winter, blood sample testing showed about 13% of children had antibodies against the virus, suggesting they’d been infected. By this summer, that number had risen to 42%.
That figure clearly made an impression on many members of the committee who asked the FDA’s vaccine reviewers if they had tried to account for immunity from past infections in their modeling. They had not.
Some felt that even with a highly effective vaccine — new data presented by Pfizer showed the children’s dose was 90% effective at preventing symptomatic infections in kids — caution was warranted as much is still unknown about myocarditis, a rare side effect of the mRNA vaccines.
Myocarditis has been more common in younger age groups. It usually goes away over time but requires hospital care. It’s not known if myocarditis could have lingering effects for those who experience it.
There were no cases of myocarditis seen in Pfizer’s studies of the vaccine in children, and no other serious events were seen. Vaccine side effects reported in the Pfizer studies were mostly mild and included fatigue, headache, and pain at the injection site.
“We think we have optimized the immune response and minimized our reactions,” said William Gruber, MD, senior vice president vaccine research and clinical development at Pfizer.
But the studies didn’t include enough participants to pick up rare, but serious adverse events like myocarditis.
“We’re worried about a side effect that we can’t measure yet, but it’s probably real, and we see a benefit that isn’t the same as it is in older age groups,” said Dr. Rubin.
Benefits vs. risks
FDA modeled the benefits and risks for children under a variety of scenarios. The benefits of the vaccines to children very much depend on the amount of transmission in the community.
When transmission is high, the benefits to children — in terms of infections, hospitalizations, ICU admissions — clearly outweigh its risks.
But when COVID-19 rates are low in the community, as they were in June, FDA analysts predicted the vaccines might send more children to the hospital for myocarditis than the virus would.
The FDA noted that kids who are hospitalized for myocarditis tend not to be as ill as children with COVID-19, however.
“If the trends continue the way they are going, the emergency for children is not what we might think it would be. That was my concern,” Dr. Hildreth said.
But others warned against complacency.
“Thinking that this is going to be the end of the wave permanently may be a little overly optimistic,” said committee chairman Arnold Monto, MD, a professor of public health and epidemiology at the University of Michigan, Ann Arbor.
The majority of COVID-19 cases in children are mild. Only about 1% of kids are hospitalized for their infections, according to CDC data. But the rates of hospitalizations in kids are about 3 times higher for people of color — including Blacks, Hispanics, and Native Americans, as compared to Whites and Asian Americans.
Since the start of the pandemic, 94 children ages 5 to 11 have died, making it the eighth leading cause of death for kids this age last year.
More than 5,200 children have developed a delayed complication from their infections called Multi-System Inflammatory Syndrome (MIS-C).
MIS-C can be severe and require hospital care and can lead to myocarditis. Children ages 5 to 11 are the age group at greatest risk for this complication.
Kids can also get long COVID. There’s not a lot of data on how often this happens, though it appears to be less frequent in children than in adults.
But a survey in the United Kingdom found that 7%-8% of kids have symptoms from their infections that last longer than 12 weeks, Dr. Havers said. Symptoms that can linger for kids include fatigue, cough, muscle and joint pain, headaches, and insomnia.
More than 1 million children have been impacted by school closures so far this year, and quarantines have had lasting impacts on learning, social development, and mental health.
Even though kids aren’t usually COVID superspreaders, they can still pass the infection on to others.
“What is clear is that secondary transmission from children, both to other children and to adults, does occur,” Dr. Havers said.
For that reason, they can continue the spread of the virus and give it opportunities to mutate and become more dangerous.
Safety monitoring to continue
Some committee members referenced thousands of letters they had received within the past few days urging them to vote against the vaccine.
Jay Portnoy, MD, a professor of pediatrics at Children’s Mercy Hospital in Kansas City, Mo., said he had personally received about 4,000 emails.
“But I feel like I need to also represent the consumers, the parents that I see every day in the clinic who are terrified of sending their children to school because they’re not protected against COVID,” he said, explaining his vote to recommend authorization.
“Our kids are going to be dealing with this virus for many years to come. It’s going to come repeatedly. Getting this vaccine is just the first step that they can take to protect themselves from having bad outcomes,” Dr. Portnoy said.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, reminded members of the committee that there were several government surveillance systems in place to catch any potential safety issues in near real time.
“I really appreciate very much the concern here. The safety monitoring of this vaccine will continue,” Dr. Marks said. “I do view this as one of our greatest responsibilities.”
“I really am so grateful that we had this discussion and voted to approve,” said Capt. Amanda Cohn, MD, chief medical officer at the National Center for Immunization and Respiratory Diseases.
“I think the benefits in this age group really are super important even if they are lower than for other age groups.”
This article was updated 10/27/21.
A version of this article first appeared on WebMD.com.
The missing puzzle piece
Mrs. Stevens died last week. She was 87.
That’s nothing new. The nature of medicine is such that you’ll see patients pass on.
But Mrs. Stevens bothers me, because even to the end I’m not sure I ever had an answer.
Her case began with somewhat nebulous, but clearly neurological, symptoms. An initial workup was normal, as was the secondary one.
The third stage of increasingly esoteric tests turned up some clues as to what was going wrong, even as she continued to dwindle. I could at least start working on a differential, even if none of it was good.
I met with her and her husband, and they wanted an answer, good or bad.
I pulled some strings at a local tertiary subspecialty center and got her in. They agreed with my suspicions, though also couldn’t find something definitive. They even repeated the tests, and came to the same conclusions – narrowed down to a few things, but no smoking gun.
Throughout all of this Mrs. Stevens kept spiraling down. After a few hospital admissions and even a biopsy of an abdominal mass we thought would give us the answer, we still didn’t solve the puzzle.
At some point she and her husband grew tired of looking and accepted that it wouldn’t change anything. Her internist called hospice in. They kept her comfortable for her last few weeks.
They didn’t want an autopsy, so the secret stayed with her.
Looking back, I agree with their decision to stop the workup. When looking further won’t change anything, why bother?
But, as a doctor, it’s frustrating. There’s a degree of intellectual curiosity that drives us. We want answers. We want to solve puzzles.
And sometimes we never get that final piece. Even if it’s the right decision for the patient, at the end of the day it’s still an unsolved crime to us. A reminder that,
We probably never will.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Mrs. Stevens died last week. She was 87.
That’s nothing new. The nature of medicine is such that you’ll see patients pass on.
But Mrs. Stevens bothers me, because even to the end I’m not sure I ever had an answer.
Her case began with somewhat nebulous, but clearly neurological, symptoms. An initial workup was normal, as was the secondary one.
The third stage of increasingly esoteric tests turned up some clues as to what was going wrong, even as she continued to dwindle. I could at least start working on a differential, even if none of it was good.
I met with her and her husband, and they wanted an answer, good or bad.
I pulled some strings at a local tertiary subspecialty center and got her in. They agreed with my suspicions, though also couldn’t find something definitive. They even repeated the tests, and came to the same conclusions – narrowed down to a few things, but no smoking gun.
Throughout all of this Mrs. Stevens kept spiraling down. After a few hospital admissions and even a biopsy of an abdominal mass we thought would give us the answer, we still didn’t solve the puzzle.
At some point she and her husband grew tired of looking and accepted that it wouldn’t change anything. Her internist called hospice in. They kept her comfortable for her last few weeks.
They didn’t want an autopsy, so the secret stayed with her.
Looking back, I agree with their decision to stop the workup. When looking further won’t change anything, why bother?
But, as a doctor, it’s frustrating. There’s a degree of intellectual curiosity that drives us. We want answers. We want to solve puzzles.
And sometimes we never get that final piece. Even if it’s the right decision for the patient, at the end of the day it’s still an unsolved crime to us. A reminder that,
We probably never will.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Mrs. Stevens died last week. She was 87.
That’s nothing new. The nature of medicine is such that you’ll see patients pass on.
But Mrs. Stevens bothers me, because even to the end I’m not sure I ever had an answer.
Her case began with somewhat nebulous, but clearly neurological, symptoms. An initial workup was normal, as was the secondary one.
The third stage of increasingly esoteric tests turned up some clues as to what was going wrong, even as she continued to dwindle. I could at least start working on a differential, even if none of it was good.
I met with her and her husband, and they wanted an answer, good or bad.
I pulled some strings at a local tertiary subspecialty center and got her in. They agreed with my suspicions, though also couldn’t find something definitive. They even repeated the tests, and came to the same conclusions – narrowed down to a few things, but no smoking gun.
Throughout all of this Mrs. Stevens kept spiraling down. After a few hospital admissions and even a biopsy of an abdominal mass we thought would give us the answer, we still didn’t solve the puzzle.
At some point she and her husband grew tired of looking and accepted that it wouldn’t change anything. Her internist called hospice in. They kept her comfortable for her last few weeks.
They didn’t want an autopsy, so the secret stayed with her.
Looking back, I agree with their decision to stop the workup. When looking further won’t change anything, why bother?
But, as a doctor, it’s frustrating. There’s a degree of intellectual curiosity that drives us. We want answers. We want to solve puzzles.
And sometimes we never get that final piece. Even if it’s the right decision for the patient, at the end of the day it’s still an unsolved crime to us. A reminder that,
We probably never will.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Sleep time ‘sweet spot’ to slow cognitive decline identified?
In a longitudinal study, investigators found older adults who slept less than 4.5 hours or more than 6.5 hours a night reported significant cognitive decline over time, but cognitive scores for those with sleep duration in between that range remained stable.
“This really suggests that there’s this middle range, a ‘sweet spot,’ where your sleep is really optimal,” said lead author Brendan Lucey, MD, MSCI, associate professor of neurology and director of the Washington University Sleep Medicine Center, St. Louis.
The study, published online Oct. 20 in Brain, is part of a growing body of research that seeks to determine if sleep can be used as a marker of Alzheimer’s disease progression.
A complex relationship
Studies suggest a strong relationship between sleep patterns and Alzheimer’s disease, which affects nearly 6 million Americans. The challenge, Dr. Lucey said, is unwinding the complex links between sleep, Alzheimer’s disease, and cognitive function.
An earlier study by Dr. Lucey and colleagues found that poor sleep quality is associated with early signs of Alzheimer’s disease, and a report published in September found that elderly people who slept less than 6 hours a night had a greater burden of amyloid beta, a hallmark sign of Alzheimer’s disease.
For this new study, researchers monitored sleep-wake activity over 4-6 nights in 100 participants who underwent annual cognitive assessments and clinical studies, including APOE genotyping, as part of a longitudinal study at the Knight Alzheimer Disease Research Center at Washington University. Participants also provided cerebrospinal fluid (CSF) total tau and amyloid-beta42 and wore a small EEG device on their forehead while they slept.
The majority of participants had a clinical dementia rating (CDR) score of 0, indicating no cognitive impairment. Twelve individuals had a CDR >0, with most reporting mild cognitive impairment.
As expected, CSF analysis showed greater evidence of Alzheimer’s disease pathology in those with a baseline CDR greater than 0.
Changes in cognitive function were measured using a Preclinical Alzheimer Cognitive Composite (PACC) score, a composite of results from a neuropsychological testing battery that included the Free and Cued Selective Reminding Test, the Logical Memory Delayed Recall Test from the Wechsler Memory Scale-Revised, the Digit Symbol Substitution Test from the Wechsler Adult Intelligence Scale-Revised, and the Mini-Mental State Examination.
Researchers found an upside-down U-shaped relationship between PACC scores and sleep duration, with dramatic cognitive decline in those who slept less than 4.5 hours or more than 6.5 hours a night (P < .001 for both). The U-shaped relationship was also found with measures of sleep phases, including time spent in rapid eye movement and in non-REM sleep (P < .001 for both).
The findings persisted even after controlling for confounders that can affect sleep and cognition, such as age, CSF total tau/amyloid-beta-42 ratio, APOE ε4 allele carrier status, years of education, and sex.
Understanding how sleep changes at different stages of Alzheimer’s disease could help researchers determine if sleep can be used as a marker of disease progression, Dr. Lucey said. That could lead to interventions to slow that process.
“We’re not at the point yet where we can say that we need to monitor someone’s sleep time and then do an intervention to see if it would improve their risk for cognitive decline,” said Dr. Lucey, who plans to repeat this sleep study with the same cohort to track changes in sleep patterns and cognitive function over time. “But that’s a question I’m very excited to try to answer.”
A component of cognitive health
Commenting on the findings, Heather Snyder, PhD, vice president of medical and scientific relations for the Alzheimer’s Association, noted that the study adds to a body of evidence linking sleep and cognition, especially how sleep quality can optimize brain function.
“We’ve seen previous research that’s shown poor sleep contributes to dementia risk, as well as research showing sleep duration may play a role in cognition,” she said.
“We also need studies that look at sleep as an intervention for cognitive health,” Dr. Snyder said. “Sleep is an important aspect of our overall health. Clinicians should have conversations with their patients about sleep as part of standard discussions about their health habits and wellness.”
The study was funded by the National Institutes of Health, the American Sleep Medicine Foundation, the Roger and Paula Riney Fund, and the Daniel J. Brennan, MD Fund. Dr. Lucey consults for Merck and Eli Lilly. Dr. Snyder has disclosed no relevant financial relationships. Full disclosures are included in the original article.
A version of this article first appeared on Medscape.com.
In a longitudinal study, investigators found older adults who slept less than 4.5 hours or more than 6.5 hours a night reported significant cognitive decline over time, but cognitive scores for those with sleep duration in between that range remained stable.
“This really suggests that there’s this middle range, a ‘sweet spot,’ where your sleep is really optimal,” said lead author Brendan Lucey, MD, MSCI, associate professor of neurology and director of the Washington University Sleep Medicine Center, St. Louis.
The study, published online Oct. 20 in Brain, is part of a growing body of research that seeks to determine if sleep can be used as a marker of Alzheimer’s disease progression.
A complex relationship
Studies suggest a strong relationship between sleep patterns and Alzheimer’s disease, which affects nearly 6 million Americans. The challenge, Dr. Lucey said, is unwinding the complex links between sleep, Alzheimer’s disease, and cognitive function.
An earlier study by Dr. Lucey and colleagues found that poor sleep quality is associated with early signs of Alzheimer’s disease, and a report published in September found that elderly people who slept less than 6 hours a night had a greater burden of amyloid beta, a hallmark sign of Alzheimer’s disease.
For this new study, researchers monitored sleep-wake activity over 4-6 nights in 100 participants who underwent annual cognitive assessments and clinical studies, including APOE genotyping, as part of a longitudinal study at the Knight Alzheimer Disease Research Center at Washington University. Participants also provided cerebrospinal fluid (CSF) total tau and amyloid-beta42 and wore a small EEG device on their forehead while they slept.
The majority of participants had a clinical dementia rating (CDR) score of 0, indicating no cognitive impairment. Twelve individuals had a CDR >0, with most reporting mild cognitive impairment.
As expected, CSF analysis showed greater evidence of Alzheimer’s disease pathology in those with a baseline CDR greater than 0.
Changes in cognitive function were measured using a Preclinical Alzheimer Cognitive Composite (PACC) score, a composite of results from a neuropsychological testing battery that included the Free and Cued Selective Reminding Test, the Logical Memory Delayed Recall Test from the Wechsler Memory Scale-Revised, the Digit Symbol Substitution Test from the Wechsler Adult Intelligence Scale-Revised, and the Mini-Mental State Examination.
Researchers found an upside-down U-shaped relationship between PACC scores and sleep duration, with dramatic cognitive decline in those who slept less than 4.5 hours or more than 6.5 hours a night (P < .001 for both). The U-shaped relationship was also found with measures of sleep phases, including time spent in rapid eye movement and in non-REM sleep (P < .001 for both).
The findings persisted even after controlling for confounders that can affect sleep and cognition, such as age, CSF total tau/amyloid-beta-42 ratio, APOE ε4 allele carrier status, years of education, and sex.
Understanding how sleep changes at different stages of Alzheimer’s disease could help researchers determine if sleep can be used as a marker of disease progression, Dr. Lucey said. That could lead to interventions to slow that process.
“We’re not at the point yet where we can say that we need to monitor someone’s sleep time and then do an intervention to see if it would improve their risk for cognitive decline,” said Dr. Lucey, who plans to repeat this sleep study with the same cohort to track changes in sleep patterns and cognitive function over time. “But that’s a question I’m very excited to try to answer.”
A component of cognitive health
Commenting on the findings, Heather Snyder, PhD, vice president of medical and scientific relations for the Alzheimer’s Association, noted that the study adds to a body of evidence linking sleep and cognition, especially how sleep quality can optimize brain function.
“We’ve seen previous research that’s shown poor sleep contributes to dementia risk, as well as research showing sleep duration may play a role in cognition,” she said.
“We also need studies that look at sleep as an intervention for cognitive health,” Dr. Snyder said. “Sleep is an important aspect of our overall health. Clinicians should have conversations with their patients about sleep as part of standard discussions about their health habits and wellness.”
The study was funded by the National Institutes of Health, the American Sleep Medicine Foundation, the Roger and Paula Riney Fund, and the Daniel J. Brennan, MD Fund. Dr. Lucey consults for Merck and Eli Lilly. Dr. Snyder has disclosed no relevant financial relationships. Full disclosures are included in the original article.
A version of this article first appeared on Medscape.com.
In a longitudinal study, investigators found older adults who slept less than 4.5 hours or more than 6.5 hours a night reported significant cognitive decline over time, but cognitive scores for those with sleep duration in between that range remained stable.
“This really suggests that there’s this middle range, a ‘sweet spot,’ where your sleep is really optimal,” said lead author Brendan Lucey, MD, MSCI, associate professor of neurology and director of the Washington University Sleep Medicine Center, St. Louis.
The study, published online Oct. 20 in Brain, is part of a growing body of research that seeks to determine if sleep can be used as a marker of Alzheimer’s disease progression.
A complex relationship
Studies suggest a strong relationship between sleep patterns and Alzheimer’s disease, which affects nearly 6 million Americans. The challenge, Dr. Lucey said, is unwinding the complex links between sleep, Alzheimer’s disease, and cognitive function.
An earlier study by Dr. Lucey and colleagues found that poor sleep quality is associated with early signs of Alzheimer’s disease, and a report published in September found that elderly people who slept less than 6 hours a night had a greater burden of amyloid beta, a hallmark sign of Alzheimer’s disease.
For this new study, researchers monitored sleep-wake activity over 4-6 nights in 100 participants who underwent annual cognitive assessments and clinical studies, including APOE genotyping, as part of a longitudinal study at the Knight Alzheimer Disease Research Center at Washington University. Participants also provided cerebrospinal fluid (CSF) total tau and amyloid-beta42 and wore a small EEG device on their forehead while they slept.
The majority of participants had a clinical dementia rating (CDR) score of 0, indicating no cognitive impairment. Twelve individuals had a CDR >0, with most reporting mild cognitive impairment.
As expected, CSF analysis showed greater evidence of Alzheimer’s disease pathology in those with a baseline CDR greater than 0.
Changes in cognitive function were measured using a Preclinical Alzheimer Cognitive Composite (PACC) score, a composite of results from a neuropsychological testing battery that included the Free and Cued Selective Reminding Test, the Logical Memory Delayed Recall Test from the Wechsler Memory Scale-Revised, the Digit Symbol Substitution Test from the Wechsler Adult Intelligence Scale-Revised, and the Mini-Mental State Examination.
Researchers found an upside-down U-shaped relationship between PACC scores and sleep duration, with dramatic cognitive decline in those who slept less than 4.5 hours or more than 6.5 hours a night (P < .001 for both). The U-shaped relationship was also found with measures of sleep phases, including time spent in rapid eye movement and in non-REM sleep (P < .001 for both).
The findings persisted even after controlling for confounders that can affect sleep and cognition, such as age, CSF total tau/amyloid-beta-42 ratio, APOE ε4 allele carrier status, years of education, and sex.
Understanding how sleep changes at different stages of Alzheimer’s disease could help researchers determine if sleep can be used as a marker of disease progression, Dr. Lucey said. That could lead to interventions to slow that process.
“We’re not at the point yet where we can say that we need to monitor someone’s sleep time and then do an intervention to see if it would improve their risk for cognitive decline,” said Dr. Lucey, who plans to repeat this sleep study with the same cohort to track changes in sleep patterns and cognitive function over time. “But that’s a question I’m very excited to try to answer.”
A component of cognitive health
Commenting on the findings, Heather Snyder, PhD, vice president of medical and scientific relations for the Alzheimer’s Association, noted that the study adds to a body of evidence linking sleep and cognition, especially how sleep quality can optimize brain function.
“We’ve seen previous research that’s shown poor sleep contributes to dementia risk, as well as research showing sleep duration may play a role in cognition,” she said.
“We also need studies that look at sleep as an intervention for cognitive health,” Dr. Snyder said. “Sleep is an important aspect of our overall health. Clinicians should have conversations with their patients about sleep as part of standard discussions about their health habits and wellness.”
The study was funded by the National Institutes of Health, the American Sleep Medicine Foundation, the Roger and Paula Riney Fund, and the Daniel J. Brennan, MD Fund. Dr. Lucey consults for Merck and Eli Lilly. Dr. Snyder has disclosed no relevant financial relationships. Full disclosures are included in the original article.
A version of this article first appeared on Medscape.com.
From Brain
Judge dismisses Lyme disease lawsuit against IDSA, doctors, but the ordeal has left its scars
Years ago, when rheumatologist Leonard Sigal, MD, was undertaking research on Lyme disease and treating patients with the condition at the Robert Wood Johnson Medical School, New Brunswick, N.J., a regular stream of abuse and threats became the usual background noise of his work. He didn’t get used to it, but it never stopped.
“I was accused of incredibly heinous crimes,” Dr. Sigal said in an interview. “I was accused of lying, cheating, of doing things to make money that were against the public interest and against the interest of patients in general.”
It’s an experience many doctors who treat Lyme disease have endured, so much so that some infectious disease doctors aren’t comfortable treating patients with Lyme disease, according to Timothy Flanigan, MD, a professor of infectious disease at Brown University, Providence, R.I.
But it wasn’t until Dr. Sigal left academia in 2003 that he realized the toll all that background abuse had been taking on him.
“It was a breath of fresh air,” he said. “I didn’t have to go into clinic and argue with people. I didn’t have to read articles in the newspaper that made no sense whatsoever. I didn’t have to hear through second and third parties how such and such was saying horrible things about me. I didn’t have to fight anymore. When I was in industry and working on stuff that had nothing to do with Lyme disease, I realized what a relief it was not to have that burden.”
So the last thing Dr. Sigal expected after all these years was to find himself named in a lawsuit alleging that he was part of a conspiracy to deny patients of what they claimed was appropriate treatment for Lyme disease. Yet, that’s exactly what happened in November 2017, when a group of 24 patients with Lyme disease, led by Texas resident Lisa Torrey, filed a lawsuit against the Infectious Diseases Society of America, eight insurance companies, and 7 of the doctors involved in producing the IDSA guidelines on Lyme disease diagnosis and management. Dr. Sigal himself had not even participated in writing the guidelines. He simply reviewed them, made a few grammatical suggestions, and said they looked good. Over the next 4 years, however, he and his fellow defendants rode an emotional roller coaster of seemingly endless motions, amendments, and other legal developments, waiting to find out whether they would owe millions of dollars for simply summarizing – or just reviewing – the available medical literature on Lyme disease.
“There were times I was on the verge of real anger. I was frustrated. There were times I was frightened, and, occasionally, I would just think of it as being silly. But when I thought of it as being silly, I had to remember I was being sued in Texas, because who knows what’s going to happen,” Dr. Sigal said. “It’s not as though I was being sued in a jurisdiction where anybody knew about Lyme disease. There are examples of physicians who are convicted of doing things they didn’t do because they were sued in the wrong jurisdiction.”
Several individuals who spoke with this news organization on condition of anonymity said that the district court where the suit was filed is notorious for being especially friendly to plaintiffs. But in legal rulings issued on Sept. 1 and Sept. 20, 2021, a federal judge in Texas dismissed all the patient group’s claims. The plaintiffs filed an appeal on Oct. 19. It’s unclear whether that has any reasonable chance of success.
“One of the things this court case does is validate the fact that our [guidelines] process is a legitimate process and there isn’t outside influence from insurance companies or pharma firms,” Daniel McQuillen, MD, president of IDSA, said in an interview. “We don’t really want anything other than to be vindicated, which we were, 100%.”
But that vindication came with a cost, both emotional and financial. Although IDSA’s insurance covered many of its legal costs, “it’s not a trivial expense,” Dr. McQuillen said. “We’re left with a baseless lawsuit with no facts that went on for 4 years, and our [medical] society basically bore all that expense, which isn’t really particularly fair.”
‘Preposterous’ accusations
The lawsuit alleged that the IDSA, the seven named physicians, and the insurance companies had “engaged in a decades-long conspiracy to deny the existence and prevent treatment of chronic Lyme disease.” The patient group claimed that the doctors knew that many patients with Lyme disease do not respond to short-term antibiotic treatment and instead need “long-term antibiotic treatment until the symptoms are resolved,” an assertion not supported by the scientific evidence.
What many patients call “chronic Lyme disease” is termed posttreatment Lyme disease syndrome (PTDLS), a constellation of symptoms that include pain, fatigue, and cognitive difficulties that some people experience after a 2- to 4-week course of antibiotics for Lyme disease. It took years of patient advocacy before the Centers for Disease Control and Prevention recognized PTLDS as a condition, but awareness of it has been increasing, said Dr. Flanigan, who was not involved in the lawsuit but treats patients with Lyme disease and PTLDS.
“Long haulers and sequelae of COVID have really opened the eyes of many practitioners that these long-term inflammatory conditions are real and very challenging to treat, and we need to work with patients to help them improve their health,” Dr. Flanigan said. “It’s a sad commentary on our society that the difficulty in treating patients with posttreatment Lyme disease syndrome, or what is commonly referred to by patients as chronic Lyme, ends up in a lawsuit in court.” He said he’s glad the lawsuit was dismissed but added that “there’s a crying need for additional high-quality, evidence-based research to help patients who are suffering from posttreatment Lyme disease syndrome.”
Patients fought for broader recognition of their condition, and some of them organized. They came up with their own ideas of what was causing their symptoms to persist. One that especially took hold was that infection from Borrelia burgdorferi, the bacteria that causes Lyme disease, persists after initial antibiotic treatment, causing so-called chronic Lyme disease. The cause of PTLDS is still under investigation, and the evidence does not support the idea of a persistent bacterial infection. Multiple studies from the National Institutes of Health have shown that long-term use of antibiotics does not benefit patients who continue to experience symptoms after initial treatment. Several studies have shown that severe adverse effects can result from extended intravenous antibiotic treatment, including death.
Nevertheless, the plaintiffs in the lawsuit argued that the insurance companies “enlisted the help of doctors who were researching Lyme disease – the IDSA panelists – and paid them large fees to develop arbitrary guidelines for testing Lyme disease,” thereby enabling the insurance companies to deny coverage for long-term antibiotic treatment to patients.
“The assertions were just preposterous,” Dr. McQuillen said.
In addition to the conspiracy charge, the plaintiffs brought additional accusations to the lawsuit over the years, including racketeering and claims that the guidelines contain false representations regarding Lyme disease testing and treatment. The plaintiffs claimed that the guidelines didn’t acknowledge that treatment can fail and included false information about how to test for Lyme disease. In reality, however, the guidelines do acknowledge that not all patients respond to the recommended 2- to 4-week course of antibiotics and that some diagnoses should be made clinically rather than on the basis of testing.
Regardless, guidelines are not stipulations. They’re a summation of the medical and scientific findings on Lyme disease based on careful review of hundreds of studies.
“They make really clear that adherence to the guidelines [is] voluntary. They aren’t a standard of care from which deviation of care is a problem,” Dr. McQuillen said. “You take those guidelines and apply it to the patient in front of you, and you see what fits best for that patient, because not every patient is going to fit into guidelines.”
Further, the authors said that IDSA vets their recommendations for any potential conflicts of interest in accordance with the organization’s guidelines practices.
“The point of the guidelines is to have people on the committee who don’t care what the guidelines are as long as we have good patient care,” Dr. McQuillen said.
Choosing to fight
Malpractice insurance does not cover this kind of lawsuit, because the doctors named in it did not personally treat any of the patients who filed it. Thus, the doctors were at risk of losing thousands, or millions, of dollars in legal fees, even if they ultimately prevail. Several of the physicians’ academic and health care institutions stepped in to cover some fees, and IDSA covered the rest in a joint defense.
“The IDSA provided me a lawyer at no cost to me, and I felt protected by them,” Dr. Sigal said. “They took care of me and made sure I was safe, and I am grateful to them for that.”
Dr. McQuillen said the expenses exceeded what the organization’s umbrella insurance covered. The physicians had invested their time and effort into the guidelines without any financial compensation.
“They’ve basically put a lot of sweat equity into producing guidelines” that follow the organization’s practices and ethics, Dr. McQuillen said. “To leave them out on an island by themselves is just not the right thing to do. We wouldn’t do that for any of our members who did something on behalf of our society.”
IDSA could have chosen to settle the lawsuit, as the insurance companies did.
“None of us on the board felt that was the right thing to do, because we believe in the process, and the science is right, and you shouldn’t be able to try to change that by having a lawsuit that’s baseless,” Dr. McQuillen said.
Several of the doctors named in the suit spoke with this news organization off the record about the exhaustion, frustration, and general suffering the suit has caused them over the past several years, including ongoing harassment that targeted their families and often became quite personal. But none expressed any wish that IDSA had chosen the faster, cheaper, easier route of settling.
“I love the organization for having done this rather than caving and paying,” Dr. Sigal said. “They showed real moral character, real integrity in fighting this suit, because they had done nothing wrong.”
Fighting the suit was about more than standing by the science, though. It’s essential to ensure physicians continue to conduct research and write clinical guidelines, even about ambiguous or controversial topics, said Raymond J. Dattwyler, MD, a professor of microbiology, immunology, and medicine at New York Medical College, Valhalla, who wrote the treatment part of the guidelines and was named in the suit.
“I was really surprised that someone would sue for scientific guidelines, because guidelines are common across medicine, and they’re just a roadmap to help practicing physicians understand how to handle evaluation or treatment of any number of particular problems,” Dr. Dattwyler said in an interview. But he wasn’t surprised that IDSA chose to fight the accusations, “because the principle involved is so compelling. It’s really standing up for all medical societies, and it’s very important to have guidelines. For the health and welfare of the American public, you need to have good information readily available to the practicing physicians.”
If the patient group had won in a settlement, it could potentially have led to less rigorous guidelines from other medical organizations, which would have had an adverse effect on public health, Dr. Dattwyler said. Such a chilling effect could reverberate far beyond the management of Lyme disease.
“One of the problems with our legal system is anybody can sue anybody, but it costs so much to defend yourself,” Dr. Dattwyler said. “This lawsuit costs millions, so that’s chilling. That’s going to inhibit guidelines, and it’s not only guidelines for infectious disease but it’s guidelines for cancer, guidelines for allergic diseases, guidelines for any number of things.”
To an extent, the threats and harassment that patient groups have directed toward different doctors have already had a chilling effect.
“For the people who gave of their time in good faith to generate these guidelines to get harassed everywhere, all the time, sometimes at home, sometimes at their place of work, it’s just unfair,” Dr. McQuillen said. “It also might discourage people from working in research to try to figure out better diagnostics or get a vaccine that actually works. Even if you really find it incredibly interesting, if laying over you is the threat that someone is going to sue you baselessly, and you’re going to have to put the time and effort into defending that, not to mention the money, I can’t see how that would be considered a positive that would encourage you to do it. In some ways, attacking people that are trying to help may drive them away from trying to help.
“At the same time, professional disagreements among practitioners – including a small minority who do treat patients with lengthy courses of antibiotics – can ultimately harm patient care, Dr. Flanigan said.
“There’s a lot of energy being expended fighting among different care providers, and often the individual needs of the patients seem to be not addressed,” Dr. Flanigan said. “The discord between different approaches often seems more important than spending time with the individual patient and trying to find a tailored approach to treatment which can benefit the patient best.”
At the same time, Dr. Sigal said he believes most of the clinicians who use non–evidence-based treatments for their patients do so because they genuinely believe it’s the right thing to do.
“I think they’re motivated by the same concerns that I have, and that is, I need to do what’s best for my patient,” Dr. Sigal said. Ultimately, the evidence should lead the way. “The only arbiter we possibly have in deciding these things is the medical scientific literature,” he added, “and if you can’t subscribe to that, then this way lies madness.”
A version of this article first appeared on Medscape.com.
Years ago, when rheumatologist Leonard Sigal, MD, was undertaking research on Lyme disease and treating patients with the condition at the Robert Wood Johnson Medical School, New Brunswick, N.J., a regular stream of abuse and threats became the usual background noise of his work. He didn’t get used to it, but it never stopped.
“I was accused of incredibly heinous crimes,” Dr. Sigal said in an interview. “I was accused of lying, cheating, of doing things to make money that were against the public interest and against the interest of patients in general.”
It’s an experience many doctors who treat Lyme disease have endured, so much so that some infectious disease doctors aren’t comfortable treating patients with Lyme disease, according to Timothy Flanigan, MD, a professor of infectious disease at Brown University, Providence, R.I.
But it wasn’t until Dr. Sigal left academia in 2003 that he realized the toll all that background abuse had been taking on him.
“It was a breath of fresh air,” he said. “I didn’t have to go into clinic and argue with people. I didn’t have to read articles in the newspaper that made no sense whatsoever. I didn’t have to hear through second and third parties how such and such was saying horrible things about me. I didn’t have to fight anymore. When I was in industry and working on stuff that had nothing to do with Lyme disease, I realized what a relief it was not to have that burden.”
So the last thing Dr. Sigal expected after all these years was to find himself named in a lawsuit alleging that he was part of a conspiracy to deny patients of what they claimed was appropriate treatment for Lyme disease. Yet, that’s exactly what happened in November 2017, when a group of 24 patients with Lyme disease, led by Texas resident Lisa Torrey, filed a lawsuit against the Infectious Diseases Society of America, eight insurance companies, and 7 of the doctors involved in producing the IDSA guidelines on Lyme disease diagnosis and management. Dr. Sigal himself had not even participated in writing the guidelines. He simply reviewed them, made a few grammatical suggestions, and said they looked good. Over the next 4 years, however, he and his fellow defendants rode an emotional roller coaster of seemingly endless motions, amendments, and other legal developments, waiting to find out whether they would owe millions of dollars for simply summarizing – or just reviewing – the available medical literature on Lyme disease.
“There were times I was on the verge of real anger. I was frustrated. There were times I was frightened, and, occasionally, I would just think of it as being silly. But when I thought of it as being silly, I had to remember I was being sued in Texas, because who knows what’s going to happen,” Dr. Sigal said. “It’s not as though I was being sued in a jurisdiction where anybody knew about Lyme disease. There are examples of physicians who are convicted of doing things they didn’t do because they were sued in the wrong jurisdiction.”
Several individuals who spoke with this news organization on condition of anonymity said that the district court where the suit was filed is notorious for being especially friendly to plaintiffs. But in legal rulings issued on Sept. 1 and Sept. 20, 2021, a federal judge in Texas dismissed all the patient group’s claims. The plaintiffs filed an appeal on Oct. 19. It’s unclear whether that has any reasonable chance of success.
“One of the things this court case does is validate the fact that our [guidelines] process is a legitimate process and there isn’t outside influence from insurance companies or pharma firms,” Daniel McQuillen, MD, president of IDSA, said in an interview. “We don’t really want anything other than to be vindicated, which we were, 100%.”
But that vindication came with a cost, both emotional and financial. Although IDSA’s insurance covered many of its legal costs, “it’s not a trivial expense,” Dr. McQuillen said. “We’re left with a baseless lawsuit with no facts that went on for 4 years, and our [medical] society basically bore all that expense, which isn’t really particularly fair.”
‘Preposterous’ accusations
The lawsuit alleged that the IDSA, the seven named physicians, and the insurance companies had “engaged in a decades-long conspiracy to deny the existence and prevent treatment of chronic Lyme disease.” The patient group claimed that the doctors knew that many patients with Lyme disease do not respond to short-term antibiotic treatment and instead need “long-term antibiotic treatment until the symptoms are resolved,” an assertion not supported by the scientific evidence.
What many patients call “chronic Lyme disease” is termed posttreatment Lyme disease syndrome (PTDLS), a constellation of symptoms that include pain, fatigue, and cognitive difficulties that some people experience after a 2- to 4-week course of antibiotics for Lyme disease. It took years of patient advocacy before the Centers for Disease Control and Prevention recognized PTLDS as a condition, but awareness of it has been increasing, said Dr. Flanigan, who was not involved in the lawsuit but treats patients with Lyme disease and PTLDS.
“Long haulers and sequelae of COVID have really opened the eyes of many practitioners that these long-term inflammatory conditions are real and very challenging to treat, and we need to work with patients to help them improve their health,” Dr. Flanigan said. “It’s a sad commentary on our society that the difficulty in treating patients with posttreatment Lyme disease syndrome, or what is commonly referred to by patients as chronic Lyme, ends up in a lawsuit in court.” He said he’s glad the lawsuit was dismissed but added that “there’s a crying need for additional high-quality, evidence-based research to help patients who are suffering from posttreatment Lyme disease syndrome.”
Patients fought for broader recognition of their condition, and some of them organized. They came up with their own ideas of what was causing their symptoms to persist. One that especially took hold was that infection from Borrelia burgdorferi, the bacteria that causes Lyme disease, persists after initial antibiotic treatment, causing so-called chronic Lyme disease. The cause of PTLDS is still under investigation, and the evidence does not support the idea of a persistent bacterial infection. Multiple studies from the National Institutes of Health have shown that long-term use of antibiotics does not benefit patients who continue to experience symptoms after initial treatment. Several studies have shown that severe adverse effects can result from extended intravenous antibiotic treatment, including death.
Nevertheless, the plaintiffs in the lawsuit argued that the insurance companies “enlisted the help of doctors who were researching Lyme disease – the IDSA panelists – and paid them large fees to develop arbitrary guidelines for testing Lyme disease,” thereby enabling the insurance companies to deny coverage for long-term antibiotic treatment to patients.
“The assertions were just preposterous,” Dr. McQuillen said.
In addition to the conspiracy charge, the plaintiffs brought additional accusations to the lawsuit over the years, including racketeering and claims that the guidelines contain false representations regarding Lyme disease testing and treatment. The plaintiffs claimed that the guidelines didn’t acknowledge that treatment can fail and included false information about how to test for Lyme disease. In reality, however, the guidelines do acknowledge that not all patients respond to the recommended 2- to 4-week course of antibiotics and that some diagnoses should be made clinically rather than on the basis of testing.
Regardless, guidelines are not stipulations. They’re a summation of the medical and scientific findings on Lyme disease based on careful review of hundreds of studies.
“They make really clear that adherence to the guidelines [is] voluntary. They aren’t a standard of care from which deviation of care is a problem,” Dr. McQuillen said. “You take those guidelines and apply it to the patient in front of you, and you see what fits best for that patient, because not every patient is going to fit into guidelines.”
Further, the authors said that IDSA vets their recommendations for any potential conflicts of interest in accordance with the organization’s guidelines practices.
“The point of the guidelines is to have people on the committee who don’t care what the guidelines are as long as we have good patient care,” Dr. McQuillen said.
Choosing to fight
Malpractice insurance does not cover this kind of lawsuit, because the doctors named in it did not personally treat any of the patients who filed it. Thus, the doctors were at risk of losing thousands, or millions, of dollars in legal fees, even if they ultimately prevail. Several of the physicians’ academic and health care institutions stepped in to cover some fees, and IDSA covered the rest in a joint defense.
“The IDSA provided me a lawyer at no cost to me, and I felt protected by them,” Dr. Sigal said. “They took care of me and made sure I was safe, and I am grateful to them for that.”
Dr. McQuillen said the expenses exceeded what the organization’s umbrella insurance covered. The physicians had invested their time and effort into the guidelines without any financial compensation.
“They’ve basically put a lot of sweat equity into producing guidelines” that follow the organization’s practices and ethics, Dr. McQuillen said. “To leave them out on an island by themselves is just not the right thing to do. We wouldn’t do that for any of our members who did something on behalf of our society.”
IDSA could have chosen to settle the lawsuit, as the insurance companies did.
“None of us on the board felt that was the right thing to do, because we believe in the process, and the science is right, and you shouldn’t be able to try to change that by having a lawsuit that’s baseless,” Dr. McQuillen said.
Several of the doctors named in the suit spoke with this news organization off the record about the exhaustion, frustration, and general suffering the suit has caused them over the past several years, including ongoing harassment that targeted their families and often became quite personal. But none expressed any wish that IDSA had chosen the faster, cheaper, easier route of settling.
“I love the organization for having done this rather than caving and paying,” Dr. Sigal said. “They showed real moral character, real integrity in fighting this suit, because they had done nothing wrong.”
Fighting the suit was about more than standing by the science, though. It’s essential to ensure physicians continue to conduct research and write clinical guidelines, even about ambiguous or controversial topics, said Raymond J. Dattwyler, MD, a professor of microbiology, immunology, and medicine at New York Medical College, Valhalla, who wrote the treatment part of the guidelines and was named in the suit.
“I was really surprised that someone would sue for scientific guidelines, because guidelines are common across medicine, and they’re just a roadmap to help practicing physicians understand how to handle evaluation or treatment of any number of particular problems,” Dr. Dattwyler said in an interview. But he wasn’t surprised that IDSA chose to fight the accusations, “because the principle involved is so compelling. It’s really standing up for all medical societies, and it’s very important to have guidelines. For the health and welfare of the American public, you need to have good information readily available to the practicing physicians.”
If the patient group had won in a settlement, it could potentially have led to less rigorous guidelines from other medical organizations, which would have had an adverse effect on public health, Dr. Dattwyler said. Such a chilling effect could reverberate far beyond the management of Lyme disease.
“One of the problems with our legal system is anybody can sue anybody, but it costs so much to defend yourself,” Dr. Dattwyler said. “This lawsuit costs millions, so that’s chilling. That’s going to inhibit guidelines, and it’s not only guidelines for infectious disease but it’s guidelines for cancer, guidelines for allergic diseases, guidelines for any number of things.”
To an extent, the threats and harassment that patient groups have directed toward different doctors have already had a chilling effect.
“For the people who gave of their time in good faith to generate these guidelines to get harassed everywhere, all the time, sometimes at home, sometimes at their place of work, it’s just unfair,” Dr. McQuillen said. “It also might discourage people from working in research to try to figure out better diagnostics or get a vaccine that actually works. Even if you really find it incredibly interesting, if laying over you is the threat that someone is going to sue you baselessly, and you’re going to have to put the time and effort into defending that, not to mention the money, I can’t see how that would be considered a positive that would encourage you to do it. In some ways, attacking people that are trying to help may drive them away from trying to help.
“At the same time, professional disagreements among practitioners – including a small minority who do treat patients with lengthy courses of antibiotics – can ultimately harm patient care, Dr. Flanigan said.
“There’s a lot of energy being expended fighting among different care providers, and often the individual needs of the patients seem to be not addressed,” Dr. Flanigan said. “The discord between different approaches often seems more important than spending time with the individual patient and trying to find a tailored approach to treatment which can benefit the patient best.”
At the same time, Dr. Sigal said he believes most of the clinicians who use non–evidence-based treatments for their patients do so because they genuinely believe it’s the right thing to do.
“I think they’re motivated by the same concerns that I have, and that is, I need to do what’s best for my patient,” Dr. Sigal said. Ultimately, the evidence should lead the way. “The only arbiter we possibly have in deciding these things is the medical scientific literature,” he added, “and if you can’t subscribe to that, then this way lies madness.”
A version of this article first appeared on Medscape.com.
Years ago, when rheumatologist Leonard Sigal, MD, was undertaking research on Lyme disease and treating patients with the condition at the Robert Wood Johnson Medical School, New Brunswick, N.J., a regular stream of abuse and threats became the usual background noise of his work. He didn’t get used to it, but it never stopped.
“I was accused of incredibly heinous crimes,” Dr. Sigal said in an interview. “I was accused of lying, cheating, of doing things to make money that were against the public interest and against the interest of patients in general.”
It’s an experience many doctors who treat Lyme disease have endured, so much so that some infectious disease doctors aren’t comfortable treating patients with Lyme disease, according to Timothy Flanigan, MD, a professor of infectious disease at Brown University, Providence, R.I.
But it wasn’t until Dr. Sigal left academia in 2003 that he realized the toll all that background abuse had been taking on him.
“It was a breath of fresh air,” he said. “I didn’t have to go into clinic and argue with people. I didn’t have to read articles in the newspaper that made no sense whatsoever. I didn’t have to hear through second and third parties how such and such was saying horrible things about me. I didn’t have to fight anymore. When I was in industry and working on stuff that had nothing to do with Lyme disease, I realized what a relief it was not to have that burden.”
So the last thing Dr. Sigal expected after all these years was to find himself named in a lawsuit alleging that he was part of a conspiracy to deny patients of what they claimed was appropriate treatment for Lyme disease. Yet, that’s exactly what happened in November 2017, when a group of 24 patients with Lyme disease, led by Texas resident Lisa Torrey, filed a lawsuit against the Infectious Diseases Society of America, eight insurance companies, and 7 of the doctors involved in producing the IDSA guidelines on Lyme disease diagnosis and management. Dr. Sigal himself had not even participated in writing the guidelines. He simply reviewed them, made a few grammatical suggestions, and said they looked good. Over the next 4 years, however, he and his fellow defendants rode an emotional roller coaster of seemingly endless motions, amendments, and other legal developments, waiting to find out whether they would owe millions of dollars for simply summarizing – or just reviewing – the available medical literature on Lyme disease.
“There were times I was on the verge of real anger. I was frustrated. There were times I was frightened, and, occasionally, I would just think of it as being silly. But when I thought of it as being silly, I had to remember I was being sued in Texas, because who knows what’s going to happen,” Dr. Sigal said. “It’s not as though I was being sued in a jurisdiction where anybody knew about Lyme disease. There are examples of physicians who are convicted of doing things they didn’t do because they were sued in the wrong jurisdiction.”
Several individuals who spoke with this news organization on condition of anonymity said that the district court where the suit was filed is notorious for being especially friendly to plaintiffs. But in legal rulings issued on Sept. 1 and Sept. 20, 2021, a federal judge in Texas dismissed all the patient group’s claims. The plaintiffs filed an appeal on Oct. 19. It’s unclear whether that has any reasonable chance of success.
“One of the things this court case does is validate the fact that our [guidelines] process is a legitimate process and there isn’t outside influence from insurance companies or pharma firms,” Daniel McQuillen, MD, president of IDSA, said in an interview. “We don’t really want anything other than to be vindicated, which we were, 100%.”
But that vindication came with a cost, both emotional and financial. Although IDSA’s insurance covered many of its legal costs, “it’s not a trivial expense,” Dr. McQuillen said. “We’re left with a baseless lawsuit with no facts that went on for 4 years, and our [medical] society basically bore all that expense, which isn’t really particularly fair.”
‘Preposterous’ accusations
The lawsuit alleged that the IDSA, the seven named physicians, and the insurance companies had “engaged in a decades-long conspiracy to deny the existence and prevent treatment of chronic Lyme disease.” The patient group claimed that the doctors knew that many patients with Lyme disease do not respond to short-term antibiotic treatment and instead need “long-term antibiotic treatment until the symptoms are resolved,” an assertion not supported by the scientific evidence.
What many patients call “chronic Lyme disease” is termed posttreatment Lyme disease syndrome (PTDLS), a constellation of symptoms that include pain, fatigue, and cognitive difficulties that some people experience after a 2- to 4-week course of antibiotics for Lyme disease. It took years of patient advocacy before the Centers for Disease Control and Prevention recognized PTLDS as a condition, but awareness of it has been increasing, said Dr. Flanigan, who was not involved in the lawsuit but treats patients with Lyme disease and PTLDS.
“Long haulers and sequelae of COVID have really opened the eyes of many practitioners that these long-term inflammatory conditions are real and very challenging to treat, and we need to work with patients to help them improve their health,” Dr. Flanigan said. “It’s a sad commentary on our society that the difficulty in treating patients with posttreatment Lyme disease syndrome, or what is commonly referred to by patients as chronic Lyme, ends up in a lawsuit in court.” He said he’s glad the lawsuit was dismissed but added that “there’s a crying need for additional high-quality, evidence-based research to help patients who are suffering from posttreatment Lyme disease syndrome.”
Patients fought for broader recognition of their condition, and some of them organized. They came up with their own ideas of what was causing their symptoms to persist. One that especially took hold was that infection from Borrelia burgdorferi, the bacteria that causes Lyme disease, persists after initial antibiotic treatment, causing so-called chronic Lyme disease. The cause of PTLDS is still under investigation, and the evidence does not support the idea of a persistent bacterial infection. Multiple studies from the National Institutes of Health have shown that long-term use of antibiotics does not benefit patients who continue to experience symptoms after initial treatment. Several studies have shown that severe adverse effects can result from extended intravenous antibiotic treatment, including death.
Nevertheless, the plaintiffs in the lawsuit argued that the insurance companies “enlisted the help of doctors who were researching Lyme disease – the IDSA panelists – and paid them large fees to develop arbitrary guidelines for testing Lyme disease,” thereby enabling the insurance companies to deny coverage for long-term antibiotic treatment to patients.
“The assertions were just preposterous,” Dr. McQuillen said.
In addition to the conspiracy charge, the plaintiffs brought additional accusations to the lawsuit over the years, including racketeering and claims that the guidelines contain false representations regarding Lyme disease testing and treatment. The plaintiffs claimed that the guidelines didn’t acknowledge that treatment can fail and included false information about how to test for Lyme disease. In reality, however, the guidelines do acknowledge that not all patients respond to the recommended 2- to 4-week course of antibiotics and that some diagnoses should be made clinically rather than on the basis of testing.
Regardless, guidelines are not stipulations. They’re a summation of the medical and scientific findings on Lyme disease based on careful review of hundreds of studies.
“They make really clear that adherence to the guidelines [is] voluntary. They aren’t a standard of care from which deviation of care is a problem,” Dr. McQuillen said. “You take those guidelines and apply it to the patient in front of you, and you see what fits best for that patient, because not every patient is going to fit into guidelines.”
Further, the authors said that IDSA vets their recommendations for any potential conflicts of interest in accordance with the organization’s guidelines practices.
“The point of the guidelines is to have people on the committee who don’t care what the guidelines are as long as we have good patient care,” Dr. McQuillen said.
Choosing to fight
Malpractice insurance does not cover this kind of lawsuit, because the doctors named in it did not personally treat any of the patients who filed it. Thus, the doctors were at risk of losing thousands, or millions, of dollars in legal fees, even if they ultimately prevail. Several of the physicians’ academic and health care institutions stepped in to cover some fees, and IDSA covered the rest in a joint defense.
“The IDSA provided me a lawyer at no cost to me, and I felt protected by them,” Dr. Sigal said. “They took care of me and made sure I was safe, and I am grateful to them for that.”
Dr. McQuillen said the expenses exceeded what the organization’s umbrella insurance covered. The physicians had invested their time and effort into the guidelines without any financial compensation.
“They’ve basically put a lot of sweat equity into producing guidelines” that follow the organization’s practices and ethics, Dr. McQuillen said. “To leave them out on an island by themselves is just not the right thing to do. We wouldn’t do that for any of our members who did something on behalf of our society.”
IDSA could have chosen to settle the lawsuit, as the insurance companies did.
“None of us on the board felt that was the right thing to do, because we believe in the process, and the science is right, and you shouldn’t be able to try to change that by having a lawsuit that’s baseless,” Dr. McQuillen said.
Several of the doctors named in the suit spoke with this news organization off the record about the exhaustion, frustration, and general suffering the suit has caused them over the past several years, including ongoing harassment that targeted their families and often became quite personal. But none expressed any wish that IDSA had chosen the faster, cheaper, easier route of settling.
“I love the organization for having done this rather than caving and paying,” Dr. Sigal said. “They showed real moral character, real integrity in fighting this suit, because they had done nothing wrong.”
Fighting the suit was about more than standing by the science, though. It’s essential to ensure physicians continue to conduct research and write clinical guidelines, even about ambiguous or controversial topics, said Raymond J. Dattwyler, MD, a professor of microbiology, immunology, and medicine at New York Medical College, Valhalla, who wrote the treatment part of the guidelines and was named in the suit.
“I was really surprised that someone would sue for scientific guidelines, because guidelines are common across medicine, and they’re just a roadmap to help practicing physicians understand how to handle evaluation or treatment of any number of particular problems,” Dr. Dattwyler said in an interview. But he wasn’t surprised that IDSA chose to fight the accusations, “because the principle involved is so compelling. It’s really standing up for all medical societies, and it’s very important to have guidelines. For the health and welfare of the American public, you need to have good information readily available to the practicing physicians.”
If the patient group had won in a settlement, it could potentially have led to less rigorous guidelines from other medical organizations, which would have had an adverse effect on public health, Dr. Dattwyler said. Such a chilling effect could reverberate far beyond the management of Lyme disease.
“One of the problems with our legal system is anybody can sue anybody, but it costs so much to defend yourself,” Dr. Dattwyler said. “This lawsuit costs millions, so that’s chilling. That’s going to inhibit guidelines, and it’s not only guidelines for infectious disease but it’s guidelines for cancer, guidelines for allergic diseases, guidelines for any number of things.”
To an extent, the threats and harassment that patient groups have directed toward different doctors have already had a chilling effect.
“For the people who gave of their time in good faith to generate these guidelines to get harassed everywhere, all the time, sometimes at home, sometimes at their place of work, it’s just unfair,” Dr. McQuillen said. “It also might discourage people from working in research to try to figure out better diagnostics or get a vaccine that actually works. Even if you really find it incredibly interesting, if laying over you is the threat that someone is going to sue you baselessly, and you’re going to have to put the time and effort into defending that, not to mention the money, I can’t see how that would be considered a positive that would encourage you to do it. In some ways, attacking people that are trying to help may drive them away from trying to help.
“At the same time, professional disagreements among practitioners – including a small minority who do treat patients with lengthy courses of antibiotics – can ultimately harm patient care, Dr. Flanigan said.
“There’s a lot of energy being expended fighting among different care providers, and often the individual needs of the patients seem to be not addressed,” Dr. Flanigan said. “The discord between different approaches often seems more important than spending time with the individual patient and trying to find a tailored approach to treatment which can benefit the patient best.”
At the same time, Dr. Sigal said he believes most of the clinicians who use non–evidence-based treatments for their patients do so because they genuinely believe it’s the right thing to do.
“I think they’re motivated by the same concerns that I have, and that is, I need to do what’s best for my patient,” Dr. Sigal said. Ultimately, the evidence should lead the way. “The only arbiter we possibly have in deciding these things is the medical scientific literature,” he added, “and if you can’t subscribe to that, then this way lies madness.”
A version of this article first appeared on Medscape.com.
















