User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Pushing the Boundaries in Multiple Sclerosis (MS): Reimagining Treatment and Care
It’s undeniable that great strides have been made in advancing the science of multiple sclerosis (MS) over the past 20 years. Continued pursuit of research and discovery has revolutionized the understanding, diagnosis, and treatment of this debilitating condition that causes symptoms that may impact nearly every part of the body and mind. Despite these advancements, there is still more work to be done.
As the search for a cure continues, it’s imperative to ask the big questions: How does MS develop and progress? How can we reduce health disparities in care and treatment? How can we improve existing treatments and expedite the discovery of new ones? To continue making progress, we must stretch the boundaries of scientific understanding until we have answers and solutions to these and other important questions.
We know that MS is a leading cause of non-traumatic disability for young people.1,2 Yet, one of the biggest challenges for neurologists is that every person’s experience with MS is unique, making it difficult to predict what symptoms will manifest, how disruptive the symptoms and relapses will be and how a person will respond to treatment.
Twenty years ago, it was widely thought that MS was mainly driven by T cells, but in the late 1990’s, a team of researchers made a groundbreaking discovery: B cells also played a key role in MS. This discovery redefined how the scientific community thought about the underlying biology of MS and the central role B cells can play.
It was this bold thinking that led researchers at the University of California, San Francisco (UCSF) and scientists at Genentech, a member of the Roche Group, to explore whether a medication that depleted B cells would have an impact on MS. This collaboration, in turn, inspired the clinical research behind Ocrevus® (ocrelizumab), the first and only treatment approved by the FDA for both relapsing MS (RMS) and primary progressive MS (PPMS). MS is a progressive disease from the start. An important goal of treating MS is to reduce disease progression as soon as possible to slow how quickly a person’s disability changes and prevent the long-term consequences of disease progression.3 Ocrevus is administered via infusion twice yearly, with the initial dose split into two separate infusions and given two weeks apart. As with any therapy, this treatment has side effects, which can be found in the full Prescribing Information and Medication Guide. Ocrevus is contraindicated in patients with active hepatitis B virus infection and in patients with a history of life-threatening infusion reactions to Ocrevus. The warnings and precautions for Ocrevus are infusion reactions, and infections, which include respiratory tract infections, herpes, progressive multifocal leukoencephalopathy (PML), and hepatitis B virus (HBV) reactivation. Additional warnings are possible increased risk of immunosuppressant effects with other immunosuppressants, reduction in immunoglobulins, and malignancies.
Ensuring a Continuum of Care
Another significant challenge facing the MS community is ensuring ongoing support and care for people living with the condition, especially throughout the COVID-19 pandemic. “We know that seeking early and appropriate care for MS is critical to slowing disability progression and achieving successful patient outcomes,” said Dr. Ashish Pradhan, Executive Director of Neuroimmunology at Genentech. “The pandemic has created additional barriers to early diagnosis and treatment, but we are committed to continuing to do everything we can to support patients and physicians at this stressful time.” Dr. Pradhan also observed that FDA's approval of a shorter infusion time for Ocrevus in December 2020 could potentially contribute to reducing some of the burden on the healthcare system in the long-term.
Being able to connect with others in the MS community is a vital part of navigating the uncertainties of living with the condition. This past April, to shine a light on the diverse MS community, Genentech hosted an #MSVisibility Virtual Concert that gathered people from across the world with different backgrounds and MS experiences and encouraged people living with MS to continue seeking appropriate support and care during the pandemic.
Continued support of the MS community through advocacy partnerships and grants over the years has played a sustained role in Genentech’s efforts to ensure people with MS receive necessary medical care and treatment. For example, Genentech partnered with an MS advocacy organization to fund a program that helps people get to and from their medical appointments. In addition, for more than 30 years, Genentech has helped more than two million people get the medicine they need through patient assistance programs like Genentech Access Solutions and the Genentech Patient Foundation.
of Neuroimmunology at Genentech
Advancing Inclusive Research in MS
MS has been shown to impact individuals and specific populations in markedly different ways. Those who identify as Black or of African descent and Hispanic/Latinos for example can experience more severe symptoms and faster disease progression than their Caucasian counterparts.4,5 Unfortunately, it’s not clear exactly why, largely because of their vast underrepresentation in clinical trials (less than 5% and 1%, respectively).6 While there are multiple explanations for these disparities, around one-in-three minority patients don’t participate in clinical trials due to lack of trust and 52% feel that the healthcare system is not only flawed, but is actually working against them.7
“Increased diversity in research is paramount to ensuring that the healthcare system is serving every person living with MS and ultimately reducing health inequities while providing more tailored treatment options,” commented Dr. Pradhan. “To advance the care of undertreated and underserved people with MS, we are creating and supporting programs, grants and other initiatives focused on people of color and inclusive and minority research to better understand how MS presents itself and develops in different patient populations. We want to make sure that all generations of people living with MS are equally represented in clinical research and have access to the treatments that will work best for them.”
Refining Existing Approaches and Leading Treatment Innovation
For decades, Genentech and Roche have been conducting neuroscience research and clinical trials to make forward progress in understanding and treating a variety of neurological conditions, including MS. While the treatment landscape for MS has evolved significantly over the past two decades, continued investment in research and development is critical. Continuing to review and refine existing treatment approaches may improve patient outcomes and discovering new treatment approaches may play an important role in advancing the treatment paradigm.
Dr. Pradhan added, “as the scientific community continues to think boldly, and re-imagine treatment and care for MS, we hope to continue to improve our understanding of the condition for all people with the disease, and advance treatment options. Pushing boundaries means refusing to stop until everyone living with MS can be treated effectively and a cure is found. This sentiment reminds me of a Ralph Waldo Emerson quote, ‘Don’t follow where the path may lead. Go instead where there is no path and leave a trail.’ That’s what we are trying to do at Genentech.”
To learn more, please visit Ocrevus.com.
Indications and Important Safety Information
OCREVUS is indicated for the treatment of:
- Relapsing forms of multiple sclerosis (MS), to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults.
- Primary progressive MS, in adults.
Contraindications
OCREVUS is contraindicated in patients with active hepatitis B virus infection and in patients with a history of life-threatening infusion reaction to OCREVUS.
Warnings and Precautions
Infusion Reactions
OCREVUS can cause infusion reactions, which can include pruritus, rash, urticaria, erythema, bronchospasm, throat irritation, oropharyngeal pain, dyspnea, pharyngeal or laryngeal edema, flushing, hypotension, pyrexia, fatigue, headache, dizziness, nausea, tachycardia, and anaphylaxis. In multiple sclerosis (MS) clinical trials, the incidence of infusion reactions in OCREVUS-treated patients [who received methylprednisolone (or an equivalent steroid) and possibly other pre-medication to reduce the risk of infusion reactions prior to each infusion] was 34-40%, with the highest incidence with the first infusion. There were no fatal infusion reactions, but 0.3% of OCREVUS-treated MS patients experienced infusion reactions that were serious, some requiring hospitalization.
Observe patients treated with OCREVUS for infusion reactions during the infusion and for at least one hour after completion of the infusion. Inform patients that infusion reactions can occur up to 24 hours after the infusion. Administer pre-medication (e.g., methylprednisolone or an equivalent corticosteroid, and an antihistamine) to reduce the frequency and severity of infusion reactions. The addition of an antipyretic (e.g., acetaminophen) may also be considered. For life-threatening infusion reactions, immediately and permanently stop OCREVUS and administer appropriate supportive treatment. For less severe infusion reactions, management may involve temporarily stopping the infusion, reducing the infusion rate, and/or administering symptomatic treatment.
Infections
A higher proportion of OCREVUS-treated patients experienced infections compared to patients taking REBIF or placebo. In RMS trials, 58% of OCREVUS-treated patients experienced one or more infections compared to 52% of REBIF-treated patients. In the PPMS trial, 70% of OCREVUS-treated patients experienced one or more infections compared to 68% of patients on placebo. OCREVUS increased the risk for upper respiratory tract infections, lower respiratory tract infections, skin infections, and herpes-related infections. OCREVUS was not associated with an increased risk of serious infections in MS patients. Delay OCREVUS administration in patients with an active infection until the infection is resolved.
Respiratory Tract Infections
A higher proportion of OCREVUS-treated patients experienced respiratory tract infections compared to patients taking REBIF or placebo. In RMS trials, 40% of OCREVUS-treated patients experienced upper respiratory tract infections compared to 33% of REBIF-treated patients, and 8% of OCREVUS-treated patients experienced lower respiratory tract infections compared to 5% of REBIF-treated patients. In the PPMS trial, 49% of OCREVUS-treated patients experienced upper respiratory tract infections compared to 43% of patients on placebo and 10% of OCREVUS-treated patients experienced lower respiratory tract infections compared to 9% of patients on placebo. The infections were predominantly mild to moderate and consisted mostly of upper respiratory tract infections and bronchitis.
Herpes
In active-controlled (RMS) clinical trials, herpes infections were reported more frequently in OCREVUS-treated patients than in REBIF-treated patients, including herpes zoster (2.1% vs. 1.0%), herpes simplex (0.7% vs. 0.1%), oral herpes (3.0% vs. 2.2%), genital herpes (0.1% vs. 0%), and herpes virus infection (0.1% vs. 0%). Infections were predominantly mild to moderate in severity. In the placebo-controlled (PPMS) clinical trial, oral herpes was reported more frequently in the OCREVUS-treated patients than in the patients on placebo (2.7% vs 0.8%).
Serious cases of infections caused by herpes simplex virus and varicella zoster virus, including central nervous system infections (encephalitis and meningitis), intraocular infections, and disseminated skin and soft tissue infections, have been reported in the postmarketing setting in multiple sclerosis patients receiving OCREVUS. Serious herpes virus infections may occur at any time during treatment with OCREVUS. Some cases were life-threatening.
If serious herpes infections occur, OCREVUS should be discontinued or withheld until the infection has resolved, and appropriate treatment should be administered.
Progressive Multifocal Leukoencephalopathy (PML)
PML is an opportunistic viral infection of the brain caused by the John Cunningham (JC) virus that typically only occurs in patients who are immunocompromised, and that usually leads to death or severe disability. Although no cases of PML were identified in OCREVUS clinical trials, JC virus infection resulting in PML has been observed in patients treated with other anti-CD20 antibodies and other MS therapies and has been associated with some risk factors (e.g., immunocompromised patients, polytherapy with immunosuppressants). At the first sign or symptom suggestive of PML, withhold OCREVUS and perform an appropriate diagnostic evaluation. MRI findings may be apparent before clinical signs or symptoms. Typical symptoms associated with PML are diverse, progress over days to weeks, and include progressive weakness on one side of the body or clumsiness of limbs, disturbance of vision, and changes in thinking, memory, and orientation leading to confusion and personality changes (per USPI).
Hepatitis B Virus (HBV) Reactivation
Hepatitis B reactivation has been reported in MS patients treated with OCREVUS in the postmarketing setting. Fulminant hepatitis, hepatic failure, and death caused by HBV reactivation have occurred in patients treated with anti-CD20 antibodies. Perform HBV screening in all patients before initiation of treatment with OCREVUS. Do not administer OCREVUS to patients with active HBV confirmed by positive results for HBsAg and anti-HB tests. For patients who are negative for surface antigen [HBsAg] and positive for HB core antibody [HBcAb+] or are carriers of HBV [HBsAg+], consult liver disease experts before starting and during treatment.
Possible Increased Risk of Immunosuppressant Effects with Other Immunosuppressants
When initiating OCREVUS after an immunosuppressive therapy or initiating an immunosuppressive therapy after OCREVUS, consider the potential for increased immunosuppressive effect. OCREVUS has not been studied in combination with other MS therapies.
Vaccinations
Administer all immunizations according to immunization guidelines at least 4 weeks prior to initiation of OCREVUS for live or live-attenuated vaccines and, whenever possible, at least 2 weeks prior to initiation of OCREVUS for non-live vaccines. OCREVUS may interfere with the effectiveness of non-live vaccines. The safety of immunization with live or live-attenuated vaccines following OCREVUS therapy has not been studied, and vaccination with live-attenuated or live vaccines is not recommended during treatment and until B-cell repletion.
Vaccination of Infants Born to Mothers Treated with OCREVUS During Pregnancy
In infants of mothers exposed to OCREVUS during pregnancy, do not administer live or live-attenuated vaccines before confirming the recovery of B-cell counts as measured by CD19+ B-cells. Depletion of B-cells in these infants may increase the risks from live or live-attenuated vaccines.
You may administer non-live vaccines, as indicated, prior to recovery from B-cell depletion, but should consider assessing vaccine immune responses, including consultation with a qualified specialist, to assess whether a protective immune response was mounted.
Reduction in Immunoglobulins
As expected with any B-cell depleting therapy, decreased immunoglobulin levels are observed with OCREVUS treatment. The pooled data of OCREVUS clinical studies (RMS and PPMS) and their open-label extensions (up to approximately 7 years of exposure) have shown an association between decreased levels of immunoglobulin G (IgG<LLN) and increased rates of serious infections. Monitor the levels of quantitative serum immunoglobulins during OCREVUS treatment and after discontinuation of treatment, until B-cell repletion, and especially in the setting of recurrent serious infections. Consider discontinuing OCREVUS therapy in patients with serious opportunistic or recurrent serious infections, and if prolonged hypogammaglobulinemia requires treatment with intravenous immunoglobulins.
Malignancies
An increased risk of malignancy with OCREVUS may exist. In controlled trials, malignancies, including breast cancer, occurred more frequently in OCREVUS-treated patients. Breast cancer occurred in 6 of 781 females treated with OCREVUS and none of 668 females treated with REBIF or placebo. Patients should follow standard breast cancer screening guidelines.
Use in Specific Populations
Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy and fetal/neonatal/infant outcomes in women exposed to OCREVUS during pregnancy. Physicians are encouraged to register patients and pregnant women are encouraged to register themselves by calling 1-833-872-4370 or visiting www.ocrevuspregnancyregistry.com.
There are no adequate data on the developmental risk associated with use of OCREVUS in pregnant women. There are no data on B-cell levels in human neonates following maternal exposure to OCREVUS. However, transient peripheral B-cell depletion and lymphocytopenia have been reported in infants born to mothers exposed to other anti-CD20 antibodies during pregnancy. OCREVUS is a humanized monoclonal antibody of an immunoglobulin G1 subtype and immunoglobulins are known to cross the placental barrier.
Lactation
There are no data on the presence of ocrelizumab in human milk, the effects on the breastfed infant, or the effects of the drug on milk production. Ocrelizumab was excreted in the milk of ocrelizumab-treated monkeys. Human IgG is excreted in human milk, and the potential for absorption of ocrelizumab to lead to B-cell depletion in the infant is unknown. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for OCREVUS and any potential adverse effects on the breastfed infant from OCREVUS or from the underlying maternal condition.
Females and Males of Reproductive Potential
Women of childbearing potential should use effective contraception while receiving OCREVUS and for 6 months after the last infusion of OCREVUS.
Most Common Adverse Reactions
RMS: The most common adverse reactions in RMS trials (incidence ≥10% and >REBIF) were upper respiratory tract infections (40%) and infusion reactions (34%).
PPMS: The most common adverse reactions in PPMS trials (incidence ≥10% and >placebo) were upper respiratory tract infections (49%), infusion reactions (40%), skin infections (14%), and lower respiratory tract infections (10%).
For additional safety information, please see the full Prescribing Information and Medication Guide.
1 Murray TJ. (2006). Diagnosis and Treatment of Multiple Sclerosis. BMJ, 322 (7540):525-527.
2 Tullman M. (2013). Overview of the Epidemiology, Diagnosis, and Disease Progression Associated With Multiple Sclerosis. The American Journal of Managed Care. 19 (2): S15-S20.
3 National Institutes of Health-National Institute of Neurological Disorders and Stroke. (2015). Multiple Sclerosis: Hope Through Research. Available at: https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Hope-Through-Research/Multiple-Sclerosis-Hope-Through-Research.
4 National MS Society. "Who Gets MS? - African Americans." Retrieved November 2020. nationalmssociety.org/What-is-MS/Who-Gets-MS/African-American-Resources.
5 Langer-Gould A, et al. Neurology. 2013;80:1734-1739; 2. Wallin MT, et al. Brain. 2012;135:1778-1785.
6 U.S. Food and Drug Administration. “Clinical Trials Shed Light on Minority Health.” U.S. Food and Drug Administration Website. https://wayback.archive-it.org/7993/20180908114418/https://www.fda.gov/ForConsumers/ConsumerUpdates/ucm349063.htm. Published 2018.
7 Genentech. “A New Perspective on Health Inequity.” https://www.gene.com/stories/a-new-perspective-on-health-inequity.

It’s undeniable that great strides have been made in advancing the science of multiple sclerosis (MS) over the past 20 years. Continued pursuit of research and discovery has revolutionized the understanding, diagnosis, and treatment of this debilitating condition that causes symptoms that may impact nearly every part of the body and mind. Despite these advancements, there is still more work to be done.
As the search for a cure continues, it’s imperative to ask the big questions: How does MS develop and progress? How can we reduce health disparities in care and treatment? How can we improve existing treatments and expedite the discovery of new ones? To continue making progress, we must stretch the boundaries of scientific understanding until we have answers and solutions to these and other important questions.
We know that MS is a leading cause of non-traumatic disability for young people.1,2 Yet, one of the biggest challenges for neurologists is that every person’s experience with MS is unique, making it difficult to predict what symptoms will manifest, how disruptive the symptoms and relapses will be and how a person will respond to treatment.
Twenty years ago, it was widely thought that MS was mainly driven by T cells, but in the late 1990’s, a team of researchers made a groundbreaking discovery: B cells also played a key role in MS. This discovery redefined how the scientific community thought about the underlying biology of MS and the central role B cells can play.
It was this bold thinking that led researchers at the University of California, San Francisco (UCSF) and scientists at Genentech, a member of the Roche Group, to explore whether a medication that depleted B cells would have an impact on MS. This collaboration, in turn, inspired the clinical research behind Ocrevus® (ocrelizumab), the first and only treatment approved by the FDA for both relapsing MS (RMS) and primary progressive MS (PPMS). MS is a progressive disease from the start. An important goal of treating MS is to reduce disease progression as soon as possible to slow how quickly a person’s disability changes and prevent the long-term consequences of disease progression.3 Ocrevus is administered via infusion twice yearly, with the initial dose split into two separate infusions and given two weeks apart. As with any therapy, this treatment has side effects, which can be found in the full Prescribing Information and Medication Guide. Ocrevus is contraindicated in patients with active hepatitis B virus infection and in patients with a history of life-threatening infusion reactions to Ocrevus. The warnings and precautions for Ocrevus are infusion reactions, and infections, which include respiratory tract infections, herpes, progressive multifocal leukoencephalopathy (PML), and hepatitis B virus (HBV) reactivation. Additional warnings are possible increased risk of immunosuppressant effects with other immunosuppressants, reduction in immunoglobulins, and malignancies.
Ensuring a Continuum of Care
Another significant challenge facing the MS community is ensuring ongoing support and care for people living with the condition, especially throughout the COVID-19 pandemic. “We know that seeking early and appropriate care for MS is critical to slowing disability progression and achieving successful patient outcomes,” said Dr. Ashish Pradhan, Executive Director of Neuroimmunology at Genentech. “The pandemic has created additional barriers to early diagnosis and treatment, but we are committed to continuing to do everything we can to support patients and physicians at this stressful time.” Dr. Pradhan also observed that FDA's approval of a shorter infusion time for Ocrevus in December 2020 could potentially contribute to reducing some of the burden on the healthcare system in the long-term.
Being able to connect with others in the MS community is a vital part of navigating the uncertainties of living with the condition. This past April, to shine a light on the diverse MS community, Genentech hosted an #MSVisibility Virtual Concert that gathered people from across the world with different backgrounds and MS experiences and encouraged people living with MS to continue seeking appropriate support and care during the pandemic.
Continued support of the MS community through advocacy partnerships and grants over the years has played a sustained role in Genentech’s efforts to ensure people with MS receive necessary medical care and treatment. For example, Genentech partnered with an MS advocacy organization to fund a program that helps people get to and from their medical appointments. In addition, for more than 30 years, Genentech has helped more than two million people get the medicine they need through patient assistance programs like Genentech Access Solutions and the Genentech Patient Foundation.
of Neuroimmunology at Genentech
Advancing Inclusive Research in MS
MS has been shown to impact individuals and specific populations in markedly different ways. Those who identify as Black or of African descent and Hispanic/Latinos for example can experience more severe symptoms and faster disease progression than their Caucasian counterparts.4,5 Unfortunately, it’s not clear exactly why, largely because of their vast underrepresentation in clinical trials (less than 5% and 1%, respectively).6 While there are multiple explanations for these disparities, around one-in-three minority patients don’t participate in clinical trials due to lack of trust and 52% feel that the healthcare system is not only flawed, but is actually working against them.7
“Increased diversity in research is paramount to ensuring that the healthcare system is serving every person living with MS and ultimately reducing health inequities while providing more tailored treatment options,” commented Dr. Pradhan. “To advance the care of undertreated and underserved people with MS, we are creating and supporting programs, grants and other initiatives focused on people of color and inclusive and minority research to better understand how MS presents itself and develops in different patient populations. We want to make sure that all generations of people living with MS are equally represented in clinical research and have access to the treatments that will work best for them.”
Refining Existing Approaches and Leading Treatment Innovation
For decades, Genentech and Roche have been conducting neuroscience research and clinical trials to make forward progress in understanding and treating a variety of neurological conditions, including MS. While the treatment landscape for MS has evolved significantly over the past two decades, continued investment in research and development is critical. Continuing to review and refine existing treatment approaches may improve patient outcomes and discovering new treatment approaches may play an important role in advancing the treatment paradigm.
Dr. Pradhan added, “as the scientific community continues to think boldly, and re-imagine treatment and care for MS, we hope to continue to improve our understanding of the condition for all people with the disease, and advance treatment options. Pushing boundaries means refusing to stop until everyone living with MS can be treated effectively and a cure is found. This sentiment reminds me of a Ralph Waldo Emerson quote, ‘Don’t follow where the path may lead. Go instead where there is no path and leave a trail.’ That’s what we are trying to do at Genentech.”
To learn more, please visit Ocrevus.com.
Indications and Important Safety Information
OCREVUS is indicated for the treatment of:
- Relapsing forms of multiple sclerosis (MS), to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults.
- Primary progressive MS, in adults.
Contraindications
OCREVUS is contraindicated in patients with active hepatitis B virus infection and in patients with a history of life-threatening infusion reaction to OCREVUS.
Warnings and Precautions
Infusion Reactions
OCREVUS can cause infusion reactions, which can include pruritus, rash, urticaria, erythema, bronchospasm, throat irritation, oropharyngeal pain, dyspnea, pharyngeal or laryngeal edema, flushing, hypotension, pyrexia, fatigue, headache, dizziness, nausea, tachycardia, and anaphylaxis. In multiple sclerosis (MS) clinical trials, the incidence of infusion reactions in OCREVUS-treated patients [who received methylprednisolone (or an equivalent steroid) and possibly other pre-medication to reduce the risk of infusion reactions prior to each infusion] was 34-40%, with the highest incidence with the first infusion. There were no fatal infusion reactions, but 0.3% of OCREVUS-treated MS patients experienced infusion reactions that were serious, some requiring hospitalization.
Observe patients treated with OCREVUS for infusion reactions during the infusion and for at least one hour after completion of the infusion. Inform patients that infusion reactions can occur up to 24 hours after the infusion. Administer pre-medication (e.g., methylprednisolone or an equivalent corticosteroid, and an antihistamine) to reduce the frequency and severity of infusion reactions. The addition of an antipyretic (e.g., acetaminophen) may also be considered. For life-threatening infusion reactions, immediately and permanently stop OCREVUS and administer appropriate supportive treatment. For less severe infusion reactions, management may involve temporarily stopping the infusion, reducing the infusion rate, and/or administering symptomatic treatment.
Infections
A higher proportion of OCREVUS-treated patients experienced infections compared to patients taking REBIF or placebo. In RMS trials, 58% of OCREVUS-treated patients experienced one or more infections compared to 52% of REBIF-treated patients. In the PPMS trial, 70% of OCREVUS-treated patients experienced one or more infections compared to 68% of patients on placebo. OCREVUS increased the risk for upper respiratory tract infections, lower respiratory tract infections, skin infections, and herpes-related infections. OCREVUS was not associated with an increased risk of serious infections in MS patients. Delay OCREVUS administration in patients with an active infection until the infection is resolved.
Respiratory Tract Infections
A higher proportion of OCREVUS-treated patients experienced respiratory tract infections compared to patients taking REBIF or placebo. In RMS trials, 40% of OCREVUS-treated patients experienced upper respiratory tract infections compared to 33% of REBIF-treated patients, and 8% of OCREVUS-treated patients experienced lower respiratory tract infections compared to 5% of REBIF-treated patients. In the PPMS trial, 49% of OCREVUS-treated patients experienced upper respiratory tract infections compared to 43% of patients on placebo and 10% of OCREVUS-treated patients experienced lower respiratory tract infections compared to 9% of patients on placebo. The infections were predominantly mild to moderate and consisted mostly of upper respiratory tract infections and bronchitis.
Herpes
In active-controlled (RMS) clinical trials, herpes infections were reported more frequently in OCREVUS-treated patients than in REBIF-treated patients, including herpes zoster (2.1% vs. 1.0%), herpes simplex (0.7% vs. 0.1%), oral herpes (3.0% vs. 2.2%), genital herpes (0.1% vs. 0%), and herpes virus infection (0.1% vs. 0%). Infections were predominantly mild to moderate in severity. In the placebo-controlled (PPMS) clinical trial, oral herpes was reported more frequently in the OCREVUS-treated patients than in the patients on placebo (2.7% vs 0.8%).
Serious cases of infections caused by herpes simplex virus and varicella zoster virus, including central nervous system infections (encephalitis and meningitis), intraocular infections, and disseminated skin and soft tissue infections, have been reported in the postmarketing setting in multiple sclerosis patients receiving OCREVUS. Serious herpes virus infections may occur at any time during treatment with OCREVUS. Some cases were life-threatening.
If serious herpes infections occur, OCREVUS should be discontinued or withheld until the infection has resolved, and appropriate treatment should be administered.
Progressive Multifocal Leukoencephalopathy (PML)
PML is an opportunistic viral infection of the brain caused by the John Cunningham (JC) virus that typically only occurs in patients who are immunocompromised, and that usually leads to death or severe disability. Although no cases of PML were identified in OCREVUS clinical trials, JC virus infection resulting in PML has been observed in patients treated with other anti-CD20 antibodies and other MS therapies and has been associated with some risk factors (e.g., immunocompromised patients, polytherapy with immunosuppressants). At the first sign or symptom suggestive of PML, withhold OCREVUS and perform an appropriate diagnostic evaluation. MRI findings may be apparent before clinical signs or symptoms. Typical symptoms associated with PML are diverse, progress over days to weeks, and include progressive weakness on one side of the body or clumsiness of limbs, disturbance of vision, and changes in thinking, memory, and orientation leading to confusion and personality changes (per USPI).
Hepatitis B Virus (HBV) Reactivation
Hepatitis B reactivation has been reported in MS patients treated with OCREVUS in the postmarketing setting. Fulminant hepatitis, hepatic failure, and death caused by HBV reactivation have occurred in patients treated with anti-CD20 antibodies. Perform HBV screening in all patients before initiation of treatment with OCREVUS. Do not administer OCREVUS to patients with active HBV confirmed by positive results for HBsAg and anti-HB tests. For patients who are negative for surface antigen [HBsAg] and positive for HB core antibody [HBcAb+] or are carriers of HBV [HBsAg+], consult liver disease experts before starting and during treatment.
Possible Increased Risk of Immunosuppressant Effects with Other Immunosuppressants
When initiating OCREVUS after an immunosuppressive therapy or initiating an immunosuppressive therapy after OCREVUS, consider the potential for increased immunosuppressive effect. OCREVUS has not been studied in combination with other MS therapies.
Vaccinations
Administer all immunizations according to immunization guidelines at least 4 weeks prior to initiation of OCREVUS for live or live-attenuated vaccines and, whenever possible, at least 2 weeks prior to initiation of OCREVUS for non-live vaccines. OCREVUS may interfere with the effectiveness of non-live vaccines. The safety of immunization with live or live-attenuated vaccines following OCREVUS therapy has not been studied, and vaccination with live-attenuated or live vaccines is not recommended during treatment and until B-cell repletion.
Vaccination of Infants Born to Mothers Treated with OCREVUS During Pregnancy
In infants of mothers exposed to OCREVUS during pregnancy, do not administer live or live-attenuated vaccines before confirming the recovery of B-cell counts as measured by CD19+ B-cells. Depletion of B-cells in these infants may increase the risks from live or live-attenuated vaccines.
You may administer non-live vaccines, as indicated, prior to recovery from B-cell depletion, but should consider assessing vaccine immune responses, including consultation with a qualified specialist, to assess whether a protective immune response was mounted.
Reduction in Immunoglobulins
As expected with any B-cell depleting therapy, decreased immunoglobulin levels are observed with OCREVUS treatment. The pooled data of OCREVUS clinical studies (RMS and PPMS) and their open-label extensions (up to approximately 7 years of exposure) have shown an association between decreased levels of immunoglobulin G (IgG<LLN) and increased rates of serious infections. Monitor the levels of quantitative serum immunoglobulins during OCREVUS treatment and after discontinuation of treatment, until B-cell repletion, and especially in the setting of recurrent serious infections. Consider discontinuing OCREVUS therapy in patients with serious opportunistic or recurrent serious infections, and if prolonged hypogammaglobulinemia requires treatment with intravenous immunoglobulins.
Malignancies
An increased risk of malignancy with OCREVUS may exist. In controlled trials, malignancies, including breast cancer, occurred more frequently in OCREVUS-treated patients. Breast cancer occurred in 6 of 781 females treated with OCREVUS and none of 668 females treated with REBIF or placebo. Patients should follow standard breast cancer screening guidelines.
Use in Specific Populations
Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy and fetal/neonatal/infant outcomes in women exposed to OCREVUS during pregnancy. Physicians are encouraged to register patients and pregnant women are encouraged to register themselves by calling 1-833-872-4370 or visiting www.ocrevuspregnancyregistry.com.
There are no adequate data on the developmental risk associated with use of OCREVUS in pregnant women. There are no data on B-cell levels in human neonates following maternal exposure to OCREVUS. However, transient peripheral B-cell depletion and lymphocytopenia have been reported in infants born to mothers exposed to other anti-CD20 antibodies during pregnancy. OCREVUS is a humanized monoclonal antibody of an immunoglobulin G1 subtype and immunoglobulins are known to cross the placental barrier.
Lactation
There are no data on the presence of ocrelizumab in human milk, the effects on the breastfed infant, or the effects of the drug on milk production. Ocrelizumab was excreted in the milk of ocrelizumab-treated monkeys. Human IgG is excreted in human milk, and the potential for absorption of ocrelizumab to lead to B-cell depletion in the infant is unknown. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for OCREVUS and any potential adverse effects on the breastfed infant from OCREVUS or from the underlying maternal condition.
Females and Males of Reproductive Potential
Women of childbearing potential should use effective contraception while receiving OCREVUS and for 6 months after the last infusion of OCREVUS.
Most Common Adverse Reactions
RMS: The most common adverse reactions in RMS trials (incidence ≥10% and >REBIF) were upper respiratory tract infections (40%) and infusion reactions (34%).
PPMS: The most common adverse reactions in PPMS trials (incidence ≥10% and >placebo) were upper respiratory tract infections (49%), infusion reactions (40%), skin infections (14%), and lower respiratory tract infections (10%).
For additional safety information, please see the full Prescribing Information and Medication Guide.
1 Murray TJ. (2006). Diagnosis and Treatment of Multiple Sclerosis. BMJ, 322 (7540):525-527.
2 Tullman M. (2013). Overview of the Epidemiology, Diagnosis, and Disease Progression Associated With Multiple Sclerosis. The American Journal of Managed Care. 19 (2): S15-S20.
3 National Institutes of Health-National Institute of Neurological Disorders and Stroke. (2015). Multiple Sclerosis: Hope Through Research. Available at: https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Hope-Through-Research/Multiple-Sclerosis-Hope-Through-Research.
4 National MS Society. "Who Gets MS? - African Americans." Retrieved November 2020. nationalmssociety.org/What-is-MS/Who-Gets-MS/African-American-Resources.
5 Langer-Gould A, et al. Neurology. 2013;80:1734-1739; 2. Wallin MT, et al. Brain. 2012;135:1778-1785.
6 U.S. Food and Drug Administration. “Clinical Trials Shed Light on Minority Health.” U.S. Food and Drug Administration Website. https://wayback.archive-it.org/7993/20180908114418/https://www.fda.gov/ForConsumers/ConsumerUpdates/ucm349063.htm. Published 2018.
7 Genentech. “A New Perspective on Health Inequity.” https://www.gene.com/stories/a-new-perspective-on-health-inequity.

It’s undeniable that great strides have been made in advancing the science of multiple sclerosis (MS) over the past 20 years. Continued pursuit of research and discovery has revolutionized the understanding, diagnosis, and treatment of this debilitating condition that causes symptoms that may impact nearly every part of the body and mind. Despite these advancements, there is still more work to be done.
As the search for a cure continues, it’s imperative to ask the big questions: How does MS develop and progress? How can we reduce health disparities in care and treatment? How can we improve existing treatments and expedite the discovery of new ones? To continue making progress, we must stretch the boundaries of scientific understanding until we have answers and solutions to these and other important questions.
We know that MS is a leading cause of non-traumatic disability for young people.1,2 Yet, one of the biggest challenges for neurologists is that every person’s experience with MS is unique, making it difficult to predict what symptoms will manifest, how disruptive the symptoms and relapses will be and how a person will respond to treatment.
Twenty years ago, it was widely thought that MS was mainly driven by T cells, but in the late 1990’s, a team of researchers made a groundbreaking discovery: B cells also played a key role in MS. This discovery redefined how the scientific community thought about the underlying biology of MS and the central role B cells can play.
It was this bold thinking that led researchers at the University of California, San Francisco (UCSF) and scientists at Genentech, a member of the Roche Group, to explore whether a medication that depleted B cells would have an impact on MS. This collaboration, in turn, inspired the clinical research behind Ocrevus® (ocrelizumab), the first and only treatment approved by the FDA for both relapsing MS (RMS) and primary progressive MS (PPMS). MS is a progressive disease from the start. An important goal of treating MS is to reduce disease progression as soon as possible to slow how quickly a person’s disability changes and prevent the long-term consequences of disease progression.3 Ocrevus is administered via infusion twice yearly, with the initial dose split into two separate infusions and given two weeks apart. As with any therapy, this treatment has side effects, which can be found in the full Prescribing Information and Medication Guide. Ocrevus is contraindicated in patients with active hepatitis B virus infection and in patients with a history of life-threatening infusion reactions to Ocrevus. The warnings and precautions for Ocrevus are infusion reactions, and infections, which include respiratory tract infections, herpes, progressive multifocal leukoencephalopathy (PML), and hepatitis B virus (HBV) reactivation. Additional warnings are possible increased risk of immunosuppressant effects with other immunosuppressants, reduction in immunoglobulins, and malignancies.
Ensuring a Continuum of Care
Another significant challenge facing the MS community is ensuring ongoing support and care for people living with the condition, especially throughout the COVID-19 pandemic. “We know that seeking early and appropriate care for MS is critical to slowing disability progression and achieving successful patient outcomes,” said Dr. Ashish Pradhan, Executive Director of Neuroimmunology at Genentech. “The pandemic has created additional barriers to early diagnosis and treatment, but we are committed to continuing to do everything we can to support patients and physicians at this stressful time.” Dr. Pradhan also observed that FDA's approval of a shorter infusion time for Ocrevus in December 2020 could potentially contribute to reducing some of the burden on the healthcare system in the long-term.
Being able to connect with others in the MS community is a vital part of navigating the uncertainties of living with the condition. This past April, to shine a light on the diverse MS community, Genentech hosted an #MSVisibility Virtual Concert that gathered people from across the world with different backgrounds and MS experiences and encouraged people living with MS to continue seeking appropriate support and care during the pandemic.
Continued support of the MS community through advocacy partnerships and grants over the years has played a sustained role in Genentech’s efforts to ensure people with MS receive necessary medical care and treatment. For example, Genentech partnered with an MS advocacy organization to fund a program that helps people get to and from their medical appointments. In addition, for more than 30 years, Genentech has helped more than two million people get the medicine they need through patient assistance programs like Genentech Access Solutions and the Genentech Patient Foundation.
of Neuroimmunology at Genentech
Advancing Inclusive Research in MS
MS has been shown to impact individuals and specific populations in markedly different ways. Those who identify as Black or of African descent and Hispanic/Latinos for example can experience more severe symptoms and faster disease progression than their Caucasian counterparts.4,5 Unfortunately, it’s not clear exactly why, largely because of their vast underrepresentation in clinical trials (less than 5% and 1%, respectively).6 While there are multiple explanations for these disparities, around one-in-three minority patients don’t participate in clinical trials due to lack of trust and 52% feel that the healthcare system is not only flawed, but is actually working against them.7
“Increased diversity in research is paramount to ensuring that the healthcare system is serving every person living with MS and ultimately reducing health inequities while providing more tailored treatment options,” commented Dr. Pradhan. “To advance the care of undertreated and underserved people with MS, we are creating and supporting programs, grants and other initiatives focused on people of color and inclusive and minority research to better understand how MS presents itself and develops in different patient populations. We want to make sure that all generations of people living with MS are equally represented in clinical research and have access to the treatments that will work best for them.”
Refining Existing Approaches and Leading Treatment Innovation
For decades, Genentech and Roche have been conducting neuroscience research and clinical trials to make forward progress in understanding and treating a variety of neurological conditions, including MS. While the treatment landscape for MS has evolved significantly over the past two decades, continued investment in research and development is critical. Continuing to review and refine existing treatment approaches may improve patient outcomes and discovering new treatment approaches may play an important role in advancing the treatment paradigm.
Dr. Pradhan added, “as the scientific community continues to think boldly, and re-imagine treatment and care for MS, we hope to continue to improve our understanding of the condition for all people with the disease, and advance treatment options. Pushing boundaries means refusing to stop until everyone living with MS can be treated effectively and a cure is found. This sentiment reminds me of a Ralph Waldo Emerson quote, ‘Don’t follow where the path may lead. Go instead where there is no path and leave a trail.’ That’s what we are trying to do at Genentech.”
To learn more, please visit Ocrevus.com.
Indications and Important Safety Information
OCREVUS is indicated for the treatment of:
- Relapsing forms of multiple sclerosis (MS), to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults.
- Primary progressive MS, in adults.
Contraindications
OCREVUS is contraindicated in patients with active hepatitis B virus infection and in patients with a history of life-threatening infusion reaction to OCREVUS.
Warnings and Precautions
Infusion Reactions
OCREVUS can cause infusion reactions, which can include pruritus, rash, urticaria, erythema, bronchospasm, throat irritation, oropharyngeal pain, dyspnea, pharyngeal or laryngeal edema, flushing, hypotension, pyrexia, fatigue, headache, dizziness, nausea, tachycardia, and anaphylaxis. In multiple sclerosis (MS) clinical trials, the incidence of infusion reactions in OCREVUS-treated patients [who received methylprednisolone (or an equivalent steroid) and possibly other pre-medication to reduce the risk of infusion reactions prior to each infusion] was 34-40%, with the highest incidence with the first infusion. There were no fatal infusion reactions, but 0.3% of OCREVUS-treated MS patients experienced infusion reactions that were serious, some requiring hospitalization.
Observe patients treated with OCREVUS for infusion reactions during the infusion and for at least one hour after completion of the infusion. Inform patients that infusion reactions can occur up to 24 hours after the infusion. Administer pre-medication (e.g., methylprednisolone or an equivalent corticosteroid, and an antihistamine) to reduce the frequency and severity of infusion reactions. The addition of an antipyretic (e.g., acetaminophen) may also be considered. For life-threatening infusion reactions, immediately and permanently stop OCREVUS and administer appropriate supportive treatment. For less severe infusion reactions, management may involve temporarily stopping the infusion, reducing the infusion rate, and/or administering symptomatic treatment.
Infections
A higher proportion of OCREVUS-treated patients experienced infections compared to patients taking REBIF or placebo. In RMS trials, 58% of OCREVUS-treated patients experienced one or more infections compared to 52% of REBIF-treated patients. In the PPMS trial, 70% of OCREVUS-treated patients experienced one or more infections compared to 68% of patients on placebo. OCREVUS increased the risk for upper respiratory tract infections, lower respiratory tract infections, skin infections, and herpes-related infections. OCREVUS was not associated with an increased risk of serious infections in MS patients. Delay OCREVUS administration in patients with an active infection until the infection is resolved.
Respiratory Tract Infections
A higher proportion of OCREVUS-treated patients experienced respiratory tract infections compared to patients taking REBIF or placebo. In RMS trials, 40% of OCREVUS-treated patients experienced upper respiratory tract infections compared to 33% of REBIF-treated patients, and 8% of OCREVUS-treated patients experienced lower respiratory tract infections compared to 5% of REBIF-treated patients. In the PPMS trial, 49% of OCREVUS-treated patients experienced upper respiratory tract infections compared to 43% of patients on placebo and 10% of OCREVUS-treated patients experienced lower respiratory tract infections compared to 9% of patients on placebo. The infections were predominantly mild to moderate and consisted mostly of upper respiratory tract infections and bronchitis.
Herpes
In active-controlled (RMS) clinical trials, herpes infections were reported more frequently in OCREVUS-treated patients than in REBIF-treated patients, including herpes zoster (2.1% vs. 1.0%), herpes simplex (0.7% vs. 0.1%), oral herpes (3.0% vs. 2.2%), genital herpes (0.1% vs. 0%), and herpes virus infection (0.1% vs. 0%). Infections were predominantly mild to moderate in severity. In the placebo-controlled (PPMS) clinical trial, oral herpes was reported more frequently in the OCREVUS-treated patients than in the patients on placebo (2.7% vs 0.8%).
Serious cases of infections caused by herpes simplex virus and varicella zoster virus, including central nervous system infections (encephalitis and meningitis), intraocular infections, and disseminated skin and soft tissue infections, have been reported in the postmarketing setting in multiple sclerosis patients receiving OCREVUS. Serious herpes virus infections may occur at any time during treatment with OCREVUS. Some cases were life-threatening.
If serious herpes infections occur, OCREVUS should be discontinued or withheld until the infection has resolved, and appropriate treatment should be administered.
Progressive Multifocal Leukoencephalopathy (PML)
PML is an opportunistic viral infection of the brain caused by the John Cunningham (JC) virus that typically only occurs in patients who are immunocompromised, and that usually leads to death or severe disability. Although no cases of PML were identified in OCREVUS clinical trials, JC virus infection resulting in PML has been observed in patients treated with other anti-CD20 antibodies and other MS therapies and has been associated with some risk factors (e.g., immunocompromised patients, polytherapy with immunosuppressants). At the first sign or symptom suggestive of PML, withhold OCREVUS and perform an appropriate diagnostic evaluation. MRI findings may be apparent before clinical signs or symptoms. Typical symptoms associated with PML are diverse, progress over days to weeks, and include progressive weakness on one side of the body or clumsiness of limbs, disturbance of vision, and changes in thinking, memory, and orientation leading to confusion and personality changes (per USPI).
Hepatitis B Virus (HBV) Reactivation
Hepatitis B reactivation has been reported in MS patients treated with OCREVUS in the postmarketing setting. Fulminant hepatitis, hepatic failure, and death caused by HBV reactivation have occurred in patients treated with anti-CD20 antibodies. Perform HBV screening in all patients before initiation of treatment with OCREVUS. Do not administer OCREVUS to patients with active HBV confirmed by positive results for HBsAg and anti-HB tests. For patients who are negative for surface antigen [HBsAg] and positive for HB core antibody [HBcAb+] or are carriers of HBV [HBsAg+], consult liver disease experts before starting and during treatment.
Possible Increased Risk of Immunosuppressant Effects with Other Immunosuppressants
When initiating OCREVUS after an immunosuppressive therapy or initiating an immunosuppressive therapy after OCREVUS, consider the potential for increased immunosuppressive effect. OCREVUS has not been studied in combination with other MS therapies.
Vaccinations
Administer all immunizations according to immunization guidelines at least 4 weeks prior to initiation of OCREVUS for live or live-attenuated vaccines and, whenever possible, at least 2 weeks prior to initiation of OCREVUS for non-live vaccines. OCREVUS may interfere with the effectiveness of non-live vaccines. The safety of immunization with live or live-attenuated vaccines following OCREVUS therapy has not been studied, and vaccination with live-attenuated or live vaccines is not recommended during treatment and until B-cell repletion.
Vaccination of Infants Born to Mothers Treated with OCREVUS During Pregnancy
In infants of mothers exposed to OCREVUS during pregnancy, do not administer live or live-attenuated vaccines before confirming the recovery of B-cell counts as measured by CD19+ B-cells. Depletion of B-cells in these infants may increase the risks from live or live-attenuated vaccines.
You may administer non-live vaccines, as indicated, prior to recovery from B-cell depletion, but should consider assessing vaccine immune responses, including consultation with a qualified specialist, to assess whether a protective immune response was mounted.
Reduction in Immunoglobulins
As expected with any B-cell depleting therapy, decreased immunoglobulin levels are observed with OCREVUS treatment. The pooled data of OCREVUS clinical studies (RMS and PPMS) and their open-label extensions (up to approximately 7 years of exposure) have shown an association between decreased levels of immunoglobulin G (IgG<LLN) and increased rates of serious infections. Monitor the levels of quantitative serum immunoglobulins during OCREVUS treatment and after discontinuation of treatment, until B-cell repletion, and especially in the setting of recurrent serious infections. Consider discontinuing OCREVUS therapy in patients with serious opportunistic or recurrent serious infections, and if prolonged hypogammaglobulinemia requires treatment with intravenous immunoglobulins.
Malignancies
An increased risk of malignancy with OCREVUS may exist. In controlled trials, malignancies, including breast cancer, occurred more frequently in OCREVUS-treated patients. Breast cancer occurred in 6 of 781 females treated with OCREVUS and none of 668 females treated with REBIF or placebo. Patients should follow standard breast cancer screening guidelines.
Use in Specific Populations
Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy and fetal/neonatal/infant outcomes in women exposed to OCREVUS during pregnancy. Physicians are encouraged to register patients and pregnant women are encouraged to register themselves by calling 1-833-872-4370 or visiting www.ocrevuspregnancyregistry.com.
There are no adequate data on the developmental risk associated with use of OCREVUS in pregnant women. There are no data on B-cell levels in human neonates following maternal exposure to OCREVUS. However, transient peripheral B-cell depletion and lymphocytopenia have been reported in infants born to mothers exposed to other anti-CD20 antibodies during pregnancy. OCREVUS is a humanized monoclonal antibody of an immunoglobulin G1 subtype and immunoglobulins are known to cross the placental barrier.
Lactation
There are no data on the presence of ocrelizumab in human milk, the effects on the breastfed infant, or the effects of the drug on milk production. Ocrelizumab was excreted in the milk of ocrelizumab-treated monkeys. Human IgG is excreted in human milk, and the potential for absorption of ocrelizumab to lead to B-cell depletion in the infant is unknown. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for OCREVUS and any potential adverse effects on the breastfed infant from OCREVUS or from the underlying maternal condition.
Females and Males of Reproductive Potential
Women of childbearing potential should use effective contraception while receiving OCREVUS and for 6 months after the last infusion of OCREVUS.
Most Common Adverse Reactions
RMS: The most common adverse reactions in RMS trials (incidence ≥10% and >REBIF) were upper respiratory tract infections (40%) and infusion reactions (34%).
PPMS: The most common adverse reactions in PPMS trials (incidence ≥10% and >placebo) were upper respiratory tract infections (49%), infusion reactions (40%), skin infections (14%), and lower respiratory tract infections (10%).
For additional safety information, please see the full Prescribing Information and Medication Guide.
1 Murray TJ. (2006). Diagnosis and Treatment of Multiple Sclerosis. BMJ, 322 (7540):525-527.
2 Tullman M. (2013). Overview of the Epidemiology, Diagnosis, and Disease Progression Associated With Multiple Sclerosis. The American Journal of Managed Care. 19 (2): S15-S20.
3 National Institutes of Health-National Institute of Neurological Disorders and Stroke. (2015). Multiple Sclerosis: Hope Through Research. Available at: https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Hope-Through-Research/Multiple-Sclerosis-Hope-Through-Research.
4 National MS Society. "Who Gets MS? - African Americans." Retrieved November 2020. nationalmssociety.org/What-is-MS/Who-Gets-MS/African-American-Resources.
5 Langer-Gould A, et al. Neurology. 2013;80:1734-1739; 2. Wallin MT, et al. Brain. 2012;135:1778-1785.
6 U.S. Food and Drug Administration. “Clinical Trials Shed Light on Minority Health.” U.S. Food and Drug Administration Website. https://wayback.archive-it.org/7993/20180908114418/https://www.fda.gov/ForConsumers/ConsumerUpdates/ucm349063.htm. Published 2018.
7 Genentech. “A New Perspective on Health Inequity.” https://www.gene.com/stories/a-new-perspective-on-health-inequity.
Certain DMTs in MS linked to more psoriasis
, a new study finds. However, overall rates of reported disease are very low, and there’s no confirmation of a connection.
“People with MS and comorbid psoriasis – or those at a known high-risk for developing psoriasis – may benefit from a careful consideration of disease-modifying therapy (DMT), specifically when B cell-depleting therapies are considered,” study coauthor and Medical College of Wisconsin neurologist Ahmed Obeidat, MD, PhD, said in an interview. The findings were presented at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
Dr. Obeidat and colleagues launched the study after noticing cases of psoriasis that developed months to years after patients started taking ocrelizumab, a B cell-depleting therapy. “We referred to the published literature and only found very scant reports of MS, psoriasis, and B cell-depleting therapy use,” he said. “Thus we decided to pursue an investigation of a large [Food and Drug Administration] database to examine for possible out-of-proportion reports for psoriasis in patients with MS who were receiving B cell-depleting therapy.”
The researchers tracked case reports of psoriasis in patients with MS on DMTs from 2009 to 2020 via the FDA Adverse Event Reporting System. They found 517 psoriasis reports among 45,547 reports of skin/cutaneous conditions. The reports were linked to interferon beta 1a (136 reports, 26% of total), natalizumab (107, 21%), fingolimod (75, 15%), dimethyl fumarate (64, 12%), ocrelizumab (49, 10%), teriflunomide (28, 5%), interferon beta 1b (22, 4%), glatiramer acetate (12, 2%), rituximab (10, 2%), and alemtuzumab (9, 2%).
The total numbers of cases is low, but this may reflect underreporting due to the assumption that “autoimmunity begets autoimmunity” and therefore cases of psoriasis in MS are not alarming, medical student Mokshal H. Porwal, the study lead author, said in an interview.
The average age of patients – 48-51 – was similar for all of the drugs except alemtuzumab (mean age 41), which had a very small number of cases. The percentage of cases in females was 71%-77% for most of the drugs, with a few exceptions: rituximab (60%), ocrelizumab (63%), and alemtuzumab (33%).
Other drugs – cladribine, siponimod, and ozanimod – had 1, 1, and 0 reports, respectively, and were not included in the age and gender analyses.
The researchers also found that psoriasis made up about 65% of all skin/cutaneous adverse reports for rituximab, the highest number among DMTs. By comparison, that number was about 30% for ocrelizumab and under 1% for dimethyl fumarate and alemtuzumab.
Links between psoriasis and MS are murky, Dr. Obeidat said. “Some studies consider the presence of psoriasis as a possible indicator of increased future risk for MS, but there’s no clear association between the two conditions,” he said.
As for DMTs, “a few case reports of psoriasis in association with interferon-beta and rare case reports in association with ocrelizumab therapy have been published. However, the possible association between certain DMTs and psoriasis remains unclear,” he said.
Going forward, “we advise that patients with psoriasis on B cell-depleting agents are monitored more closely,” Dr. Obeidat said. “If the psoriasis worsens, it may be beneficial to think about potential alternative therapies.”
No study funding is reported. Dr. Obeidat reports various disclosures; the other authors report no disclosures.
, a new study finds. However, overall rates of reported disease are very low, and there’s no confirmation of a connection.
“People with MS and comorbid psoriasis – or those at a known high-risk for developing psoriasis – may benefit from a careful consideration of disease-modifying therapy (DMT), specifically when B cell-depleting therapies are considered,” study coauthor and Medical College of Wisconsin neurologist Ahmed Obeidat, MD, PhD, said in an interview. The findings were presented at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
Dr. Obeidat and colleagues launched the study after noticing cases of psoriasis that developed months to years after patients started taking ocrelizumab, a B cell-depleting therapy. “We referred to the published literature and only found very scant reports of MS, psoriasis, and B cell-depleting therapy use,” he said. “Thus we decided to pursue an investigation of a large [Food and Drug Administration] database to examine for possible out-of-proportion reports for psoriasis in patients with MS who were receiving B cell-depleting therapy.”
The researchers tracked case reports of psoriasis in patients with MS on DMTs from 2009 to 2020 via the FDA Adverse Event Reporting System. They found 517 psoriasis reports among 45,547 reports of skin/cutaneous conditions. The reports were linked to interferon beta 1a (136 reports, 26% of total), natalizumab (107, 21%), fingolimod (75, 15%), dimethyl fumarate (64, 12%), ocrelizumab (49, 10%), teriflunomide (28, 5%), interferon beta 1b (22, 4%), glatiramer acetate (12, 2%), rituximab (10, 2%), and alemtuzumab (9, 2%).
The total numbers of cases is low, but this may reflect underreporting due to the assumption that “autoimmunity begets autoimmunity” and therefore cases of psoriasis in MS are not alarming, medical student Mokshal H. Porwal, the study lead author, said in an interview.
The average age of patients – 48-51 – was similar for all of the drugs except alemtuzumab (mean age 41), which had a very small number of cases. The percentage of cases in females was 71%-77% for most of the drugs, with a few exceptions: rituximab (60%), ocrelizumab (63%), and alemtuzumab (33%).
Other drugs – cladribine, siponimod, and ozanimod – had 1, 1, and 0 reports, respectively, and were not included in the age and gender analyses.
The researchers also found that psoriasis made up about 65% of all skin/cutaneous adverse reports for rituximab, the highest number among DMTs. By comparison, that number was about 30% for ocrelizumab and under 1% for dimethyl fumarate and alemtuzumab.
Links between psoriasis and MS are murky, Dr. Obeidat said. “Some studies consider the presence of psoriasis as a possible indicator of increased future risk for MS, but there’s no clear association between the two conditions,” he said.
As for DMTs, “a few case reports of psoriasis in association with interferon-beta and rare case reports in association with ocrelizumab therapy have been published. However, the possible association between certain DMTs and psoriasis remains unclear,” he said.
Going forward, “we advise that patients with psoriasis on B cell-depleting agents are monitored more closely,” Dr. Obeidat said. “If the psoriasis worsens, it may be beneficial to think about potential alternative therapies.”
No study funding is reported. Dr. Obeidat reports various disclosures; the other authors report no disclosures.
, a new study finds. However, overall rates of reported disease are very low, and there’s no confirmation of a connection.
“People with MS and comorbid psoriasis – or those at a known high-risk for developing psoriasis – may benefit from a careful consideration of disease-modifying therapy (DMT), specifically when B cell-depleting therapies are considered,” study coauthor and Medical College of Wisconsin neurologist Ahmed Obeidat, MD, PhD, said in an interview. The findings were presented at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
Dr. Obeidat and colleagues launched the study after noticing cases of psoriasis that developed months to years after patients started taking ocrelizumab, a B cell-depleting therapy. “We referred to the published literature and only found very scant reports of MS, psoriasis, and B cell-depleting therapy use,” he said. “Thus we decided to pursue an investigation of a large [Food and Drug Administration] database to examine for possible out-of-proportion reports for psoriasis in patients with MS who were receiving B cell-depleting therapy.”
The researchers tracked case reports of psoriasis in patients with MS on DMTs from 2009 to 2020 via the FDA Adverse Event Reporting System. They found 517 psoriasis reports among 45,547 reports of skin/cutaneous conditions. The reports were linked to interferon beta 1a (136 reports, 26% of total), natalizumab (107, 21%), fingolimod (75, 15%), dimethyl fumarate (64, 12%), ocrelizumab (49, 10%), teriflunomide (28, 5%), interferon beta 1b (22, 4%), glatiramer acetate (12, 2%), rituximab (10, 2%), and alemtuzumab (9, 2%).
The total numbers of cases is low, but this may reflect underreporting due to the assumption that “autoimmunity begets autoimmunity” and therefore cases of psoriasis in MS are not alarming, medical student Mokshal H. Porwal, the study lead author, said in an interview.
The average age of patients – 48-51 – was similar for all of the drugs except alemtuzumab (mean age 41), which had a very small number of cases. The percentage of cases in females was 71%-77% for most of the drugs, with a few exceptions: rituximab (60%), ocrelizumab (63%), and alemtuzumab (33%).
Other drugs – cladribine, siponimod, and ozanimod – had 1, 1, and 0 reports, respectively, and were not included in the age and gender analyses.
The researchers also found that psoriasis made up about 65% of all skin/cutaneous adverse reports for rituximab, the highest number among DMTs. By comparison, that number was about 30% for ocrelizumab and under 1% for dimethyl fumarate and alemtuzumab.
Links between psoriasis and MS are murky, Dr. Obeidat said. “Some studies consider the presence of psoriasis as a possible indicator of increased future risk for MS, but there’s no clear association between the two conditions,” he said.
As for DMTs, “a few case reports of psoriasis in association with interferon-beta and rare case reports in association with ocrelizumab therapy have been published. However, the possible association between certain DMTs and psoriasis remains unclear,” he said.
Going forward, “we advise that patients with psoriasis on B cell-depleting agents are monitored more closely,” Dr. Obeidat said. “If the psoriasis worsens, it may be beneficial to think about potential alternative therapies.”
No study funding is reported. Dr. Obeidat reports various disclosures; the other authors report no disclosures.
FROM CMSC 2021
Boxed warnings: Legal risks that many physicians never see coming
Almost all physicians write prescriptions, and each prescription requires a physician to assess the risks and benefits of the drug. If an adverse drug reaction occurs, physicians may be called on to defend their risk-benefit assessment in court.
The assessment of risk is complicated when there is a boxed warning that describes potentially serious and life-threatening adverse reactions associated with a drug. Some of our most commonly prescribed drugs have boxed warnings, and drugs that were initially approved by the Food and Drug Administration without boxed warnings may have them added years later.
One serious problem with boxed warnings is that there are no reliable mechanisms for making sure that physicians are aware of them. The warnings are typically not seen by physicians as printed product labels, just as physicians often don’t see the pills and capsules that they prescribe. Pharmacists who receive packaged drugs from manufacturers may be the only ones to see an actual printed boxed warning, but even those pharmacists have little reason to read each label and note changes when handling many bulk packages.
This problem is aggravated by misperceptions that many physicians have about boxed warnings and the increasingly intense scrutiny given to them by mass media and the courts. Lawyers can use boxed warnings to make a drug look dangerous, even when it’s not, and to make physicians look reckless when prescribing it. Therefore, it is important for physicians to understand what boxed warnings are, what they are not, the problems they cause, and how to minimize these problems.
What is a ‘boxed warning’?
The marketing and sale of drugs in the United States requires approval by the FDA. Approval requires manufacturers to prepare a document containing “Full Prescribing Information” for the drug and to include a printed copy in every package of the drug that is sold. This document is commonly called a “package insert,” but the FDA designates this document as the manufacturer’s product “label.”
In 1979, the FDA began requiring some labels to appear within thick, black rectangular borders; these have come to be known as boxed warnings. Boxed warnings are usually placed at the beginning of a label. They may be added to the label of a previously approved drug already on the market or included in the product label when first approved and marketed.
The requirement for a boxed warning most often arises when a signal appears during review of postmarketing surveillance data suggesting a possible and plausible association between a drug and an adverse reaction. Warnings may also be initiated in response to petitions from public interest groups, or upon the discovery of serious toxicity in animals. Regardless of their origin, the intent of a boxed warning is to highlight information that may have important therapeutic consequences and warrants heightened awareness among physicians.
What a boxed warning is not
A boxed warning is not “issued” by the FDA; it is merely required by the FDA. Specific wording or a template may be suggested by the FDA, but product labels and boxed warnings are written and issued by the manufacturer. This distinction may seem minor, but extensive litigation has occurred over whether manufacturers have met their duty to warn consumers about possible risks when using their products, and this duty cannot be shifted to the FDA.
A boxed warning may not be added to a product label at the option of a manufacturer. The FDA allows a boxed warning only if it requires the warning, to preserve its impact. It should be noted that some medical information sources (e.g., PDR.net) may include a “BOXED WARNING” in their drug monographs, but monographs not written by a manufacturer are not regulated by the FDA, and the text of their boxed warnings do not always correspond to the boxed warning that was approved by the FDA.
A boxed warning is not an indication that revocation of FDA approval is being considered or that it is likely to be revoked. FDA approval is subject to ongoing review and may be revoked at any time, without a prior boxed warning.
A boxed warning is not the highest level of warning. The FDA may require a manufacturer to send out a “Dear Health Care Provider” (DHCP) letter when an even higher or more urgent level of warning is deemed necessary. DHCP letters are usually accompanied by revisions of the product label, but most label revisions – and even most boxed warnings – are not accompanied by DHCP letters.
A boxed warning is not a statement about causation. Most warnings describe an “association” between a drug and an adverse effect, or “increased risk,” or instances of a particular adverse effect that “have been reported” in persons taking a drug. The words in a boxed warning are carefully chosen and require careful reading; in most cases they refrain from stating that a drug actually causes an adverse effect. The postmarketing surveillance data on which most warnings are based generally cannot provide the kind of evidence required to establish causation, and an association may be nothing more than an uncommon manifestation of the disorder for which the drug has been prescribed.
A boxed warning is not a statement about the probability of an adverse reaction occurring. The requirement for a boxed warning correlates better to the new recognition of a possible association than to the probability of an association. For example, penicillin has long been known to cause fatal anaphylaxis in 1/100,000 first-time administrations, but it does not have a boxed warning. The adverse consequences described in boxed warnings are often far less frequent – so much so that most physicians will never see them.
A boxed warning does not define the standard of care. The warning is a requirement imposed on the manufacturer, not on the practice of medicine. For legal purposes, the “standard of care” for the practice of medicine is defined state by state and is typically cast in terms such as “what most physicians would do in similar circumstances.” Physicians often prescribe drugs in spite of boxed warnings, just as they often prescribe drugs for “off label” indications, always balancing risk versus benefit.
A boxed warning does not constitute a contraindication to the use of a medication. Some warnings state that a drug is contraindicated in some situations, but product labels have another mandated section for listing contraindications, and most boxed warnings have no corresponding entry in that section.
A boxed warning does not necessarily constitute current information, nor is it always updated when new or contrary information becomes available. Revisions to boxed warnings, and to product labels in general, are made only after detailed review at the FDA, and the process of deciding whether an existing boxed warning continues to be appropriate may divert limited regulatory resources from more urgent priorities. Consequently, revisions to a boxed warning may lag behind the data that justify a revision by months or years. Revisions may never occur if softening or eliminating a boxed warning is deemed to be not worth the cost by a manufacturer.
Boxed warning problems for physicians
There is no reliable mechanism for manufacturers or the FDA to communicate boxed warnings directly to physicians, so it’s not clear how physicians are expected to stay informed about the issuance or revision of boxed warnings. They may first learn about new or revised warnings in the mass media, which is paying ever-increasing attention to press releases from the FDA. However, it can be difficult for the media to accurately convey the subtle and complex nature of a boxed warning in nontechnical terms.
Many physicians subscribe to various medical news alerts and attend continuing medical education (CME) programs, which often do an excellent job of highlighting new warnings, while hospitals, clinics, and pharmacies may broadcast news about boxed warnings in newsletters or other notices. But these notifications are ephemeral and may be missed by physicians who are overwhelmed by email, notices, newsletters, and CME programs.
The warnings that pop up in electronic medical records systems are often so numerous that physicians become trained to ignore them. Printed advertisements in professional journals must include mandated boxed warnings, but their visibility is waning as physicians increasingly read journals online.
Another conundrum is how to inform the public about boxed warnings.
Manufacturers are prohibited from direct-to-consumer advertising of drugs with boxed warnings, although the warnings are easily found on the Internet. Some patients expect and welcome detailed information from their physicians, so it’s a good policy to always and repeatedly review this information with them, especially if they are members of an identified risk group. However, that policy may be counterproductive if it dissuades anxious patients from needed therapy despite risk-benefit considerations that strongly favor it. Boxed warnings are well known to have “spillover effects” in which the aspersions cast by a boxed warning for a relatively small subgroup of patients causes use of a drug to decline among all patients.
Compounding this conundrum is that physicians rarely have sufficient information to gauge the magnitude of a risk, given that boxed warnings are often based on information from surveillance systems that cannot accurately quantify the risk or even establish a causal relationship. The text of a boxed warning generally does not provide the information needed for evidence-based clinical practice such as a quantitative estimate of effect, information about source and trustworthiness of the evidence, and guidance on implementation. For these and other reasons, FDA policies about various boxed warnings have been the target of significant criticism.
Medication guides are one mechanism to address the challenge of informing patients about the risks of drugs they are taking. FDA-approved medication guides are available for most drugs dispensed as outpatient prescriptions, they’re written in plain language for the consumer, and they include paraphrased versions of any boxed warning. Ideally, patients review these guides with their physicians or pharmacists, but the guides may be lengthy and raise questions that may not be answerable (e.g., about incidence rates). Patients may decline to review this information when a drug is prescribed or dispensed, and they may discard printed copies given to them without reading.
What can physicians do to minimize boxed warning problems?
Physicians should periodically review the product labels for drugs they commonly prescribe, including drugs they’ve prescribed for a long time. Prescription renewal requests can be used as a prompt to check for changes in a patient’s condition or other medications that might place a patient in the target population of a boxed warning. Physicians can subscribe to newsletters that announce and discuss significant product label changes, including alerts directly from the FDA. Physicians may also enlist their office staff to find and review boxed warnings for drugs being prescribed, noting which ones should require a conversation with any patient who has been or will be receiving this drug. They may want to make explicit mention in their encounter record that a boxed warning, medication guide, or overall risk-benefit assessment has been discussed.
Summary
The nature of boxed warnings, the means by which they are disseminated, and their role in clinical practice are all in great need of improvement. Until that occurs, boxed warnings offer some, but only very limited, help to patients and physicians who struggle to understand the risks of medications.
Dr. Axelsen is professor in the departments of pharmacology, biochemistry, and biophysics, and of medicine, infectious diseases section, University of Pennsylvania, Philadelphia. He disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
Almost all physicians write prescriptions, and each prescription requires a physician to assess the risks and benefits of the drug. If an adverse drug reaction occurs, physicians may be called on to defend their risk-benefit assessment in court.
The assessment of risk is complicated when there is a boxed warning that describes potentially serious and life-threatening adverse reactions associated with a drug. Some of our most commonly prescribed drugs have boxed warnings, and drugs that were initially approved by the Food and Drug Administration without boxed warnings may have them added years later.
One serious problem with boxed warnings is that there are no reliable mechanisms for making sure that physicians are aware of them. The warnings are typically not seen by physicians as printed product labels, just as physicians often don’t see the pills and capsules that they prescribe. Pharmacists who receive packaged drugs from manufacturers may be the only ones to see an actual printed boxed warning, but even those pharmacists have little reason to read each label and note changes when handling many bulk packages.
This problem is aggravated by misperceptions that many physicians have about boxed warnings and the increasingly intense scrutiny given to them by mass media and the courts. Lawyers can use boxed warnings to make a drug look dangerous, even when it’s not, and to make physicians look reckless when prescribing it. Therefore, it is important for physicians to understand what boxed warnings are, what they are not, the problems they cause, and how to minimize these problems.
What is a ‘boxed warning’?
The marketing and sale of drugs in the United States requires approval by the FDA. Approval requires manufacturers to prepare a document containing “Full Prescribing Information” for the drug and to include a printed copy in every package of the drug that is sold. This document is commonly called a “package insert,” but the FDA designates this document as the manufacturer’s product “label.”
In 1979, the FDA began requiring some labels to appear within thick, black rectangular borders; these have come to be known as boxed warnings. Boxed warnings are usually placed at the beginning of a label. They may be added to the label of a previously approved drug already on the market or included in the product label when first approved and marketed.
The requirement for a boxed warning most often arises when a signal appears during review of postmarketing surveillance data suggesting a possible and plausible association between a drug and an adverse reaction. Warnings may also be initiated in response to petitions from public interest groups, or upon the discovery of serious toxicity in animals. Regardless of their origin, the intent of a boxed warning is to highlight information that may have important therapeutic consequences and warrants heightened awareness among physicians.
What a boxed warning is not
A boxed warning is not “issued” by the FDA; it is merely required by the FDA. Specific wording or a template may be suggested by the FDA, but product labels and boxed warnings are written and issued by the manufacturer. This distinction may seem minor, but extensive litigation has occurred over whether manufacturers have met their duty to warn consumers about possible risks when using their products, and this duty cannot be shifted to the FDA.
A boxed warning may not be added to a product label at the option of a manufacturer. The FDA allows a boxed warning only if it requires the warning, to preserve its impact. It should be noted that some medical information sources (e.g., PDR.net) may include a “BOXED WARNING” in their drug monographs, but monographs not written by a manufacturer are not regulated by the FDA, and the text of their boxed warnings do not always correspond to the boxed warning that was approved by the FDA.
A boxed warning is not an indication that revocation of FDA approval is being considered or that it is likely to be revoked. FDA approval is subject to ongoing review and may be revoked at any time, without a prior boxed warning.
A boxed warning is not the highest level of warning. The FDA may require a manufacturer to send out a “Dear Health Care Provider” (DHCP) letter when an even higher or more urgent level of warning is deemed necessary. DHCP letters are usually accompanied by revisions of the product label, but most label revisions – and even most boxed warnings – are not accompanied by DHCP letters.
A boxed warning is not a statement about causation. Most warnings describe an “association” between a drug and an adverse effect, or “increased risk,” or instances of a particular adverse effect that “have been reported” in persons taking a drug. The words in a boxed warning are carefully chosen and require careful reading; in most cases they refrain from stating that a drug actually causes an adverse effect. The postmarketing surveillance data on which most warnings are based generally cannot provide the kind of evidence required to establish causation, and an association may be nothing more than an uncommon manifestation of the disorder for which the drug has been prescribed.
A boxed warning is not a statement about the probability of an adverse reaction occurring. The requirement for a boxed warning correlates better to the new recognition of a possible association than to the probability of an association. For example, penicillin has long been known to cause fatal anaphylaxis in 1/100,000 first-time administrations, but it does not have a boxed warning. The adverse consequences described in boxed warnings are often far less frequent – so much so that most physicians will never see them.
A boxed warning does not define the standard of care. The warning is a requirement imposed on the manufacturer, not on the practice of medicine. For legal purposes, the “standard of care” for the practice of medicine is defined state by state and is typically cast in terms such as “what most physicians would do in similar circumstances.” Physicians often prescribe drugs in spite of boxed warnings, just as they often prescribe drugs for “off label” indications, always balancing risk versus benefit.
A boxed warning does not constitute a contraindication to the use of a medication. Some warnings state that a drug is contraindicated in some situations, but product labels have another mandated section for listing contraindications, and most boxed warnings have no corresponding entry in that section.
A boxed warning does not necessarily constitute current information, nor is it always updated when new or contrary information becomes available. Revisions to boxed warnings, and to product labels in general, are made only after detailed review at the FDA, and the process of deciding whether an existing boxed warning continues to be appropriate may divert limited regulatory resources from more urgent priorities. Consequently, revisions to a boxed warning may lag behind the data that justify a revision by months or years. Revisions may never occur if softening or eliminating a boxed warning is deemed to be not worth the cost by a manufacturer.
Boxed warning problems for physicians
There is no reliable mechanism for manufacturers or the FDA to communicate boxed warnings directly to physicians, so it’s not clear how physicians are expected to stay informed about the issuance or revision of boxed warnings. They may first learn about new or revised warnings in the mass media, which is paying ever-increasing attention to press releases from the FDA. However, it can be difficult for the media to accurately convey the subtle and complex nature of a boxed warning in nontechnical terms.
Many physicians subscribe to various medical news alerts and attend continuing medical education (CME) programs, which often do an excellent job of highlighting new warnings, while hospitals, clinics, and pharmacies may broadcast news about boxed warnings in newsletters or other notices. But these notifications are ephemeral and may be missed by physicians who are overwhelmed by email, notices, newsletters, and CME programs.
The warnings that pop up in electronic medical records systems are often so numerous that physicians become trained to ignore them. Printed advertisements in professional journals must include mandated boxed warnings, but their visibility is waning as physicians increasingly read journals online.
Another conundrum is how to inform the public about boxed warnings.
Manufacturers are prohibited from direct-to-consumer advertising of drugs with boxed warnings, although the warnings are easily found on the Internet. Some patients expect and welcome detailed information from their physicians, so it’s a good policy to always and repeatedly review this information with them, especially if they are members of an identified risk group. However, that policy may be counterproductive if it dissuades anxious patients from needed therapy despite risk-benefit considerations that strongly favor it. Boxed warnings are well known to have “spillover effects” in which the aspersions cast by a boxed warning for a relatively small subgroup of patients causes use of a drug to decline among all patients.
Compounding this conundrum is that physicians rarely have sufficient information to gauge the magnitude of a risk, given that boxed warnings are often based on information from surveillance systems that cannot accurately quantify the risk or even establish a causal relationship. The text of a boxed warning generally does not provide the information needed for evidence-based clinical practice such as a quantitative estimate of effect, information about source and trustworthiness of the evidence, and guidance on implementation. For these and other reasons, FDA policies about various boxed warnings have been the target of significant criticism.
Medication guides are one mechanism to address the challenge of informing patients about the risks of drugs they are taking. FDA-approved medication guides are available for most drugs dispensed as outpatient prescriptions, they’re written in plain language for the consumer, and they include paraphrased versions of any boxed warning. Ideally, patients review these guides with their physicians or pharmacists, but the guides may be lengthy and raise questions that may not be answerable (e.g., about incidence rates). Patients may decline to review this information when a drug is prescribed or dispensed, and they may discard printed copies given to them without reading.
What can physicians do to minimize boxed warning problems?
Physicians should periodically review the product labels for drugs they commonly prescribe, including drugs they’ve prescribed for a long time. Prescription renewal requests can be used as a prompt to check for changes in a patient’s condition or other medications that might place a patient in the target population of a boxed warning. Physicians can subscribe to newsletters that announce and discuss significant product label changes, including alerts directly from the FDA. Physicians may also enlist their office staff to find and review boxed warnings for drugs being prescribed, noting which ones should require a conversation with any patient who has been or will be receiving this drug. They may want to make explicit mention in their encounter record that a boxed warning, medication guide, or overall risk-benefit assessment has been discussed.
Summary
The nature of boxed warnings, the means by which they are disseminated, and their role in clinical practice are all in great need of improvement. Until that occurs, boxed warnings offer some, but only very limited, help to patients and physicians who struggle to understand the risks of medications.
Dr. Axelsen is professor in the departments of pharmacology, biochemistry, and biophysics, and of medicine, infectious diseases section, University of Pennsylvania, Philadelphia. He disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
Almost all physicians write prescriptions, and each prescription requires a physician to assess the risks and benefits of the drug. If an adverse drug reaction occurs, physicians may be called on to defend their risk-benefit assessment in court.
The assessment of risk is complicated when there is a boxed warning that describes potentially serious and life-threatening adverse reactions associated with a drug. Some of our most commonly prescribed drugs have boxed warnings, and drugs that were initially approved by the Food and Drug Administration without boxed warnings may have them added years later.
One serious problem with boxed warnings is that there are no reliable mechanisms for making sure that physicians are aware of them. The warnings are typically not seen by physicians as printed product labels, just as physicians often don’t see the pills and capsules that they prescribe. Pharmacists who receive packaged drugs from manufacturers may be the only ones to see an actual printed boxed warning, but even those pharmacists have little reason to read each label and note changes when handling many bulk packages.
This problem is aggravated by misperceptions that many physicians have about boxed warnings and the increasingly intense scrutiny given to them by mass media and the courts. Lawyers can use boxed warnings to make a drug look dangerous, even when it’s not, and to make physicians look reckless when prescribing it. Therefore, it is important for physicians to understand what boxed warnings are, what they are not, the problems they cause, and how to minimize these problems.
What is a ‘boxed warning’?
The marketing and sale of drugs in the United States requires approval by the FDA. Approval requires manufacturers to prepare a document containing “Full Prescribing Information” for the drug and to include a printed copy in every package of the drug that is sold. This document is commonly called a “package insert,” but the FDA designates this document as the manufacturer’s product “label.”
In 1979, the FDA began requiring some labels to appear within thick, black rectangular borders; these have come to be known as boxed warnings. Boxed warnings are usually placed at the beginning of a label. They may be added to the label of a previously approved drug already on the market or included in the product label when first approved and marketed.
The requirement for a boxed warning most often arises when a signal appears during review of postmarketing surveillance data suggesting a possible and plausible association between a drug and an adverse reaction. Warnings may also be initiated in response to petitions from public interest groups, or upon the discovery of serious toxicity in animals. Regardless of their origin, the intent of a boxed warning is to highlight information that may have important therapeutic consequences and warrants heightened awareness among physicians.
What a boxed warning is not
A boxed warning is not “issued” by the FDA; it is merely required by the FDA. Specific wording or a template may be suggested by the FDA, but product labels and boxed warnings are written and issued by the manufacturer. This distinction may seem minor, but extensive litigation has occurred over whether manufacturers have met their duty to warn consumers about possible risks when using their products, and this duty cannot be shifted to the FDA.
A boxed warning may not be added to a product label at the option of a manufacturer. The FDA allows a boxed warning only if it requires the warning, to preserve its impact. It should be noted that some medical information sources (e.g., PDR.net) may include a “BOXED WARNING” in their drug monographs, but monographs not written by a manufacturer are not regulated by the FDA, and the text of their boxed warnings do not always correspond to the boxed warning that was approved by the FDA.
A boxed warning is not an indication that revocation of FDA approval is being considered or that it is likely to be revoked. FDA approval is subject to ongoing review and may be revoked at any time, without a prior boxed warning.
A boxed warning is not the highest level of warning. The FDA may require a manufacturer to send out a “Dear Health Care Provider” (DHCP) letter when an even higher or more urgent level of warning is deemed necessary. DHCP letters are usually accompanied by revisions of the product label, but most label revisions – and even most boxed warnings – are not accompanied by DHCP letters.
A boxed warning is not a statement about causation. Most warnings describe an “association” between a drug and an adverse effect, or “increased risk,” or instances of a particular adverse effect that “have been reported” in persons taking a drug. The words in a boxed warning are carefully chosen and require careful reading; in most cases they refrain from stating that a drug actually causes an adverse effect. The postmarketing surveillance data on which most warnings are based generally cannot provide the kind of evidence required to establish causation, and an association may be nothing more than an uncommon manifestation of the disorder for which the drug has been prescribed.
A boxed warning is not a statement about the probability of an adverse reaction occurring. The requirement for a boxed warning correlates better to the new recognition of a possible association than to the probability of an association. For example, penicillin has long been known to cause fatal anaphylaxis in 1/100,000 first-time administrations, but it does not have a boxed warning. The adverse consequences described in boxed warnings are often far less frequent – so much so that most physicians will never see them.
A boxed warning does not define the standard of care. The warning is a requirement imposed on the manufacturer, not on the practice of medicine. For legal purposes, the “standard of care” for the practice of medicine is defined state by state and is typically cast in terms such as “what most physicians would do in similar circumstances.” Physicians often prescribe drugs in spite of boxed warnings, just as they often prescribe drugs for “off label” indications, always balancing risk versus benefit.
A boxed warning does not constitute a contraindication to the use of a medication. Some warnings state that a drug is contraindicated in some situations, but product labels have another mandated section for listing contraindications, and most boxed warnings have no corresponding entry in that section.
A boxed warning does not necessarily constitute current information, nor is it always updated when new or contrary information becomes available. Revisions to boxed warnings, and to product labels in general, are made only after detailed review at the FDA, and the process of deciding whether an existing boxed warning continues to be appropriate may divert limited regulatory resources from more urgent priorities. Consequently, revisions to a boxed warning may lag behind the data that justify a revision by months or years. Revisions may never occur if softening or eliminating a boxed warning is deemed to be not worth the cost by a manufacturer.
Boxed warning problems for physicians
There is no reliable mechanism for manufacturers or the FDA to communicate boxed warnings directly to physicians, so it’s not clear how physicians are expected to stay informed about the issuance or revision of boxed warnings. They may first learn about new or revised warnings in the mass media, which is paying ever-increasing attention to press releases from the FDA. However, it can be difficult for the media to accurately convey the subtle and complex nature of a boxed warning in nontechnical terms.
Many physicians subscribe to various medical news alerts and attend continuing medical education (CME) programs, which often do an excellent job of highlighting new warnings, while hospitals, clinics, and pharmacies may broadcast news about boxed warnings in newsletters or other notices. But these notifications are ephemeral and may be missed by physicians who are overwhelmed by email, notices, newsletters, and CME programs.
The warnings that pop up in electronic medical records systems are often so numerous that physicians become trained to ignore them. Printed advertisements in professional journals must include mandated boxed warnings, but their visibility is waning as physicians increasingly read journals online.
Another conundrum is how to inform the public about boxed warnings.
Manufacturers are prohibited from direct-to-consumer advertising of drugs with boxed warnings, although the warnings are easily found on the Internet. Some patients expect and welcome detailed information from their physicians, so it’s a good policy to always and repeatedly review this information with them, especially if they are members of an identified risk group. However, that policy may be counterproductive if it dissuades anxious patients from needed therapy despite risk-benefit considerations that strongly favor it. Boxed warnings are well known to have “spillover effects” in which the aspersions cast by a boxed warning for a relatively small subgroup of patients causes use of a drug to decline among all patients.
Compounding this conundrum is that physicians rarely have sufficient information to gauge the magnitude of a risk, given that boxed warnings are often based on information from surveillance systems that cannot accurately quantify the risk or even establish a causal relationship. The text of a boxed warning generally does not provide the information needed for evidence-based clinical practice such as a quantitative estimate of effect, information about source and trustworthiness of the evidence, and guidance on implementation. For these and other reasons, FDA policies about various boxed warnings have been the target of significant criticism.
Medication guides are one mechanism to address the challenge of informing patients about the risks of drugs they are taking. FDA-approved medication guides are available for most drugs dispensed as outpatient prescriptions, they’re written in plain language for the consumer, and they include paraphrased versions of any boxed warning. Ideally, patients review these guides with their physicians or pharmacists, but the guides may be lengthy and raise questions that may not be answerable (e.g., about incidence rates). Patients may decline to review this information when a drug is prescribed or dispensed, and they may discard printed copies given to them without reading.
What can physicians do to minimize boxed warning problems?
Physicians should periodically review the product labels for drugs they commonly prescribe, including drugs they’ve prescribed for a long time. Prescription renewal requests can be used as a prompt to check for changes in a patient’s condition or other medications that might place a patient in the target population of a boxed warning. Physicians can subscribe to newsletters that announce and discuss significant product label changes, including alerts directly from the FDA. Physicians may also enlist their office staff to find and review boxed warnings for drugs being prescribed, noting which ones should require a conversation with any patient who has been or will be receiving this drug. They may want to make explicit mention in their encounter record that a boxed warning, medication guide, or overall risk-benefit assessment has been discussed.
Summary
The nature of boxed warnings, the means by which they are disseminated, and their role in clinical practice are all in great need of improvement. Until that occurs, boxed warnings offer some, but only very limited, help to patients and physicians who struggle to understand the risks of medications.
Dr. Axelsen is professor in the departments of pharmacology, biochemistry, and biophysics, and of medicine, infectious diseases section, University of Pennsylvania, Philadelphia. He disclosed no relevant financial relationships. A version of this article first appeared on Medscape.com.
A Clinical Review of Eslicarbazepine Acetate
When managing seizures, physicians have multiple treatment choices developed over the past half century. Partial-onset seizures, or focal seizures, represent the majority of cases. Neurologists and primary care providers are tasked with choosing the first-, second-, or third-line option for monotherapy, and determining when treatment-refractory cases require adjunct treatment.
This supplement reviews Eslicarbazepine Acetate and its effectiveness as a first-line or later adjunctive therapy in patients with partial-onset seizures.
When managing seizures, physicians have multiple treatment choices developed over the past half century. Partial-onset seizures, or focal seizures, represent the majority of cases. Neurologists and primary care providers are tasked with choosing the first-, second-, or third-line option for monotherapy, and determining when treatment-refractory cases require adjunct treatment.
This supplement reviews Eslicarbazepine Acetate and its effectiveness as a first-line or later adjunctive therapy in patients with partial-onset seizures.
When managing seizures, physicians have multiple treatment choices developed over the past half century. Partial-onset seizures, or focal seizures, represent the majority of cases. Neurologists and primary care providers are tasked with choosing the first-, second-, or third-line option for monotherapy, and determining when treatment-refractory cases require adjunct treatment.
This supplement reviews Eslicarbazepine Acetate and its effectiveness as a first-line or later adjunctive therapy in patients with partial-onset seizures.
80% of Americans research recommendations post-visit
Confusion over health information and doctor advice is even higher among people who care for patients than among those who don’t provide care to their loved ones, the nationally representative survey from the AHIMA Foundation found.
The survey also shows that 80% of Americans – and an even higher portion of caregivers – are likely to research medical recommendations online after a doctor’s visit. But 1 in 4 people don’t know how to access their own medical records or find it difficult to do so.
The findings reflect the same low level of health literacy in the U.S. population that earlier surveys did. The results also indicate that little has changed since the Department of Health and Human Services released a National Action Plan to Improve Health Literacy in 2010.
That plan emphasized the need to develop and share accurate health information that helps people make decisions; to promote changes in the health care system that improve health information, communication, informed decision-making, and access to health services; and to increase the sharing and use of evidence-based health literacy practices.
According to the AHIMA Foundation report, 62% of Americans are not sure they understand their doctor’s advice and the health information discussed during a visit. Twenty-four percent say they don’t comprehend any of it, and 31% can’t remember what was said during the visit. Fifteen percent of those surveyed said they were more confused about their health than they were before the encounter with their doctor.
Caregivers have special issues
Forty-three percent of Americans are caregivers, the report notes, and 91% of those play an active role in managing someone else’s health. Millennials (65%) and Gen Xers (50%) are significantly more likely than Gen Zers (39%) and Boomers (20%) to be a caregiver.
Most caregivers have concerns about their loved ones’ ability to manage their own health. Most of them believe that doctors provide enough information, but 38% don’t believe a doctor can communicate effectively with the patient if the caregiver is not present.
Forty-three percent of caretakers don’t think their loved ones can understand medical information on their own. On the other hand, caregivers are more likely than people who don’t provide care to say the doctor confused them and to research the doctor’s advice after an appointment.
For many patients and caregivers, communications break down when they are with their health care provider. Twenty-two percent of Americans say they do not feel comfortable asking their doctor certain health questions. This inability to have a satisfactory dialogue with their doctor means that many patients leave their appointments without getting clear answers to their questions (24%) or without having an opportunity to ask any questions at all (17%).
This is not surprising, considering that a 2018 study found that doctors spend only 11 seconds, on average, listening to patients before interrupting them.
Depending on the internet
Overall, the AHIMA survey found, 42% of Americans research their doctor’s recommendations after an appointment. A higher percentage of caregivers than noncaregiver peers do so (47% vs. 38%). Eighty percent of respondents say they are “likely” to research their doctor’s advice online after a visit.
When they have a medical problem or a question about their condition, just as many Americans (59%) turn to the internet for an answer as contact their doctor directly, the survey found. Twenty-nine percent of the respondents consult friends, family, or colleagues; 23% look up medical records if they’re easily accessible; 19% ask pharmacists for advice; and 6% call an unspecified 800 number.
Americans feel secure in the health information they find on the internet. Among those who go online to look up information, 86% are confident that it is credible. And 42% report feeling relieved that they can find a lot of information about their health concerns. Respondents also say that the information they gather allows them to feel more confident in their doctor’s recommendations (35%) and that they feel better after having learned more on the internet than their doctor had told them (39%). Men are more likely than women to say that their confidence in their doctor’s recommendations increased after doing online research (40% vs. 30%).
Access to health records
Access to medical records would help people better understand their condition or diagnosis. But nearly half of Americans (48%) admit they don’t usually review their medical records until long after an appointment, and 52% say they rarely access their records at all.
One in four Americans say that they don’t know where to go to access their health information or that they didn’t find the process easy. More than half of those who have never had to find their records think the process would be difficult if they had to try.
Eighty-one percent of Americans use an online platform or portal to access their medical records or health information. Two-thirds of Americans who use an online portal trust that their medical information is kept safe and not shared with other people or organizations.
Four in five respondents agree that if they had access to all of their health information, including medical records, recommendations, conditions, and test results, they’d see an improvement in their health management. Fifty-nine percent of them believe they’d also be more confident about understanding their health, and 47% say they’d have greater trust in their doctor’s recommendations. Higher percentages of caregivers than noncaregivers say the same.
Younger people, those with a high school degree or less, and those who earn less than $50,000 are less likely than older, better educated, and more affluent people to understand their doctor’s health information and to ask questions of their providers.
People of color struggle with their relationships with doctors, are less satisfied than white people with the information they receive during visits, and are more likely than white peers to feel that if they had access to all their health information, they’d manage their health better and be more confident in their doctors’ recommendations, the survey found.
A version of this article first appeared on WebMD.com.
Confusion over health information and doctor advice is even higher among people who care for patients than among those who don’t provide care to their loved ones, the nationally representative survey from the AHIMA Foundation found.
The survey also shows that 80% of Americans – and an even higher portion of caregivers – are likely to research medical recommendations online after a doctor’s visit. But 1 in 4 people don’t know how to access their own medical records or find it difficult to do so.
The findings reflect the same low level of health literacy in the U.S. population that earlier surveys did. The results also indicate that little has changed since the Department of Health and Human Services released a National Action Plan to Improve Health Literacy in 2010.
That plan emphasized the need to develop and share accurate health information that helps people make decisions; to promote changes in the health care system that improve health information, communication, informed decision-making, and access to health services; and to increase the sharing and use of evidence-based health literacy practices.
According to the AHIMA Foundation report, 62% of Americans are not sure they understand their doctor’s advice and the health information discussed during a visit. Twenty-four percent say they don’t comprehend any of it, and 31% can’t remember what was said during the visit. Fifteen percent of those surveyed said they were more confused about their health than they were before the encounter with their doctor.
Caregivers have special issues
Forty-three percent of Americans are caregivers, the report notes, and 91% of those play an active role in managing someone else’s health. Millennials (65%) and Gen Xers (50%) are significantly more likely than Gen Zers (39%) and Boomers (20%) to be a caregiver.
Most caregivers have concerns about their loved ones’ ability to manage their own health. Most of them believe that doctors provide enough information, but 38% don’t believe a doctor can communicate effectively with the patient if the caregiver is not present.
Forty-three percent of caretakers don’t think their loved ones can understand medical information on their own. On the other hand, caregivers are more likely than people who don’t provide care to say the doctor confused them and to research the doctor’s advice after an appointment.
For many patients and caregivers, communications break down when they are with their health care provider. Twenty-two percent of Americans say they do not feel comfortable asking their doctor certain health questions. This inability to have a satisfactory dialogue with their doctor means that many patients leave their appointments without getting clear answers to their questions (24%) or without having an opportunity to ask any questions at all (17%).
This is not surprising, considering that a 2018 study found that doctors spend only 11 seconds, on average, listening to patients before interrupting them.
Depending on the internet
Overall, the AHIMA survey found, 42% of Americans research their doctor’s recommendations after an appointment. A higher percentage of caregivers than noncaregiver peers do so (47% vs. 38%). Eighty percent of respondents say they are “likely” to research their doctor’s advice online after a visit.
When they have a medical problem or a question about their condition, just as many Americans (59%) turn to the internet for an answer as contact their doctor directly, the survey found. Twenty-nine percent of the respondents consult friends, family, or colleagues; 23% look up medical records if they’re easily accessible; 19% ask pharmacists for advice; and 6% call an unspecified 800 number.
Americans feel secure in the health information they find on the internet. Among those who go online to look up information, 86% are confident that it is credible. And 42% report feeling relieved that they can find a lot of information about their health concerns. Respondents also say that the information they gather allows them to feel more confident in their doctor’s recommendations (35%) and that they feel better after having learned more on the internet than their doctor had told them (39%). Men are more likely than women to say that their confidence in their doctor’s recommendations increased after doing online research (40% vs. 30%).
Access to health records
Access to medical records would help people better understand their condition or diagnosis. But nearly half of Americans (48%) admit they don’t usually review their medical records until long after an appointment, and 52% say they rarely access their records at all.
One in four Americans say that they don’t know where to go to access their health information or that they didn’t find the process easy. More than half of those who have never had to find their records think the process would be difficult if they had to try.
Eighty-one percent of Americans use an online platform or portal to access their medical records or health information. Two-thirds of Americans who use an online portal trust that their medical information is kept safe and not shared with other people or organizations.
Four in five respondents agree that if they had access to all of their health information, including medical records, recommendations, conditions, and test results, they’d see an improvement in their health management. Fifty-nine percent of them believe they’d also be more confident about understanding their health, and 47% say they’d have greater trust in their doctor’s recommendations. Higher percentages of caregivers than noncaregivers say the same.
Younger people, those with a high school degree or less, and those who earn less than $50,000 are less likely than older, better educated, and more affluent people to understand their doctor’s health information and to ask questions of their providers.
People of color struggle with their relationships with doctors, are less satisfied than white people with the information they receive during visits, and are more likely than white peers to feel that if they had access to all their health information, they’d manage their health better and be more confident in their doctors’ recommendations, the survey found.
A version of this article first appeared on WebMD.com.
Confusion over health information and doctor advice is even higher among people who care for patients than among those who don’t provide care to their loved ones, the nationally representative survey from the AHIMA Foundation found.
The survey also shows that 80% of Americans – and an even higher portion of caregivers – are likely to research medical recommendations online after a doctor’s visit. But 1 in 4 people don’t know how to access their own medical records or find it difficult to do so.
The findings reflect the same low level of health literacy in the U.S. population that earlier surveys did. The results also indicate that little has changed since the Department of Health and Human Services released a National Action Plan to Improve Health Literacy in 2010.
That plan emphasized the need to develop and share accurate health information that helps people make decisions; to promote changes in the health care system that improve health information, communication, informed decision-making, and access to health services; and to increase the sharing and use of evidence-based health literacy practices.
According to the AHIMA Foundation report, 62% of Americans are not sure they understand their doctor’s advice and the health information discussed during a visit. Twenty-four percent say they don’t comprehend any of it, and 31% can’t remember what was said during the visit. Fifteen percent of those surveyed said they were more confused about their health than they were before the encounter with their doctor.
Caregivers have special issues
Forty-three percent of Americans are caregivers, the report notes, and 91% of those play an active role in managing someone else’s health. Millennials (65%) and Gen Xers (50%) are significantly more likely than Gen Zers (39%) and Boomers (20%) to be a caregiver.
Most caregivers have concerns about their loved ones’ ability to manage their own health. Most of them believe that doctors provide enough information, but 38% don’t believe a doctor can communicate effectively with the patient if the caregiver is not present.
Forty-three percent of caretakers don’t think their loved ones can understand medical information on their own. On the other hand, caregivers are more likely than people who don’t provide care to say the doctor confused them and to research the doctor’s advice after an appointment.
For many patients and caregivers, communications break down when they are with their health care provider. Twenty-two percent of Americans say they do not feel comfortable asking their doctor certain health questions. This inability to have a satisfactory dialogue with their doctor means that many patients leave their appointments without getting clear answers to their questions (24%) or without having an opportunity to ask any questions at all (17%).
This is not surprising, considering that a 2018 study found that doctors spend only 11 seconds, on average, listening to patients before interrupting them.
Depending on the internet
Overall, the AHIMA survey found, 42% of Americans research their doctor’s recommendations after an appointment. A higher percentage of caregivers than noncaregiver peers do so (47% vs. 38%). Eighty percent of respondents say they are “likely” to research their doctor’s advice online after a visit.
When they have a medical problem or a question about their condition, just as many Americans (59%) turn to the internet for an answer as contact their doctor directly, the survey found. Twenty-nine percent of the respondents consult friends, family, or colleagues; 23% look up medical records if they’re easily accessible; 19% ask pharmacists for advice; and 6% call an unspecified 800 number.
Americans feel secure in the health information they find on the internet. Among those who go online to look up information, 86% are confident that it is credible. And 42% report feeling relieved that they can find a lot of information about their health concerns. Respondents also say that the information they gather allows them to feel more confident in their doctor’s recommendations (35%) and that they feel better after having learned more on the internet than their doctor had told them (39%). Men are more likely than women to say that their confidence in their doctor’s recommendations increased after doing online research (40% vs. 30%).
Access to health records
Access to medical records would help people better understand their condition or diagnosis. But nearly half of Americans (48%) admit they don’t usually review their medical records until long after an appointment, and 52% say they rarely access their records at all.
One in four Americans say that they don’t know where to go to access their health information or that they didn’t find the process easy. More than half of those who have never had to find their records think the process would be difficult if they had to try.
Eighty-one percent of Americans use an online platform or portal to access their medical records or health information. Two-thirds of Americans who use an online portal trust that their medical information is kept safe and not shared with other people or organizations.
Four in five respondents agree that if they had access to all of their health information, including medical records, recommendations, conditions, and test results, they’d see an improvement in their health management. Fifty-nine percent of them believe they’d also be more confident about understanding their health, and 47% say they’d have greater trust in their doctor’s recommendations. Higher percentages of caregivers than noncaregivers say the same.
Younger people, those with a high school degree or less, and those who earn less than $50,000 are less likely than older, better educated, and more affluent people to understand their doctor’s health information and to ask questions of their providers.
People of color struggle with their relationships with doctors, are less satisfied than white people with the information they receive during visits, and are more likely than white peers to feel that if they had access to all their health information, they’d manage their health better and be more confident in their doctors’ recommendations, the survey found.
A version of this article first appeared on WebMD.com.
FDA authorizes Pfizer’s COVID-19 vaccine for kids
The move brings families with young children a step closer to resuming their normal activities, and it should help further slow transmission of the coronavirus virus in the United States.
States have already placed their orders for initial doses of the vaccines. The Oct. 29 FDA authorization triggers the shipment of millions of doses to pediatricians, family practice doctors, children’s hospitals, community health centers, and pharmacies.
Next, a panel of experts known as the Advisory Committee on Immunization Practices, or ACIP, will meet Nov. 2 to vote on recommendations for use of the vaccine.
As soon as the Centers for Disease Control and Prevention’s director signs off on those recommendations, children can get the shots, perhaps as early as Nov. 3.
Pfizer’s vaccine for children is 10 micrograms, or one-third of the dose given to teens and adults. Kids get two doses of the vaccine 3 weeks apart. In clinical trials, the most common side effects were pain at the injection site, fatigue, and headache. These side effects were mild and disappeared quickly. There were no serious adverse events detected in the studies, which included about 3,100 children. In one study, the vaccine was 90% effective at preventing COVID-19 infections with symptoms in younger children.
There are about 28 million children in the United States between the ages of 5 and 12.
“As a mother and a physician, I know that parents, caregivers, school staff, and children have been waiting for today’s authorization. Vaccinating younger children against COVID-19 will bring us closer to returning to a sense of normalcy,” Acting FDA Commissioner Janet Woodcock, MD, said in an FDA news release.
“Our comprehensive and rigorous evaluation of the data pertaining to the vaccine’s safety and effectiveness should help assure parents and guardians that this vaccine meets our high standards,” she said.
A version of this article first appeared on WebMD.com.
The move brings families with young children a step closer to resuming their normal activities, and it should help further slow transmission of the coronavirus virus in the United States.
States have already placed their orders for initial doses of the vaccines. The Oct. 29 FDA authorization triggers the shipment of millions of doses to pediatricians, family practice doctors, children’s hospitals, community health centers, and pharmacies.
Next, a panel of experts known as the Advisory Committee on Immunization Practices, or ACIP, will meet Nov. 2 to vote on recommendations for use of the vaccine.
As soon as the Centers for Disease Control and Prevention’s director signs off on those recommendations, children can get the shots, perhaps as early as Nov. 3.
Pfizer’s vaccine for children is 10 micrograms, or one-third of the dose given to teens and adults. Kids get two doses of the vaccine 3 weeks apart. In clinical trials, the most common side effects were pain at the injection site, fatigue, and headache. These side effects were mild and disappeared quickly. There were no serious adverse events detected in the studies, which included about 3,100 children. In one study, the vaccine was 90% effective at preventing COVID-19 infections with symptoms in younger children.
There are about 28 million children in the United States between the ages of 5 and 12.
“As a mother and a physician, I know that parents, caregivers, school staff, and children have been waiting for today’s authorization. Vaccinating younger children against COVID-19 will bring us closer to returning to a sense of normalcy,” Acting FDA Commissioner Janet Woodcock, MD, said in an FDA news release.
“Our comprehensive and rigorous evaluation of the data pertaining to the vaccine’s safety and effectiveness should help assure parents and guardians that this vaccine meets our high standards,” she said.
A version of this article first appeared on WebMD.com.
The move brings families with young children a step closer to resuming their normal activities, and it should help further slow transmission of the coronavirus virus in the United States.
States have already placed their orders for initial doses of the vaccines. The Oct. 29 FDA authorization triggers the shipment of millions of doses to pediatricians, family practice doctors, children’s hospitals, community health centers, and pharmacies.
Next, a panel of experts known as the Advisory Committee on Immunization Practices, or ACIP, will meet Nov. 2 to vote on recommendations for use of the vaccine.
As soon as the Centers for Disease Control and Prevention’s director signs off on those recommendations, children can get the shots, perhaps as early as Nov. 3.
Pfizer’s vaccine for children is 10 micrograms, or one-third of the dose given to teens and adults. Kids get two doses of the vaccine 3 weeks apart. In clinical trials, the most common side effects were pain at the injection site, fatigue, and headache. These side effects were mild and disappeared quickly. There were no serious adverse events detected in the studies, which included about 3,100 children. In one study, the vaccine was 90% effective at preventing COVID-19 infections with symptoms in younger children.
There are about 28 million children in the United States between the ages of 5 and 12.
“As a mother and a physician, I know that parents, caregivers, school staff, and children have been waiting for today’s authorization. Vaccinating younger children against COVID-19 will bring us closer to returning to a sense of normalcy,” Acting FDA Commissioner Janet Woodcock, MD, said in an FDA news release.
“Our comprehensive and rigorous evaluation of the data pertaining to the vaccine’s safety and effectiveness should help assure parents and guardians that this vaccine meets our high standards,” she said.
A version of this article first appeared on WebMD.com.
Which agent is best for neuromyelitis optica?
The Alexion-sponsored study was presented at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) by Dean Wingerchuk, MD, of the Mayo Clinic in Scottsdale, Ariz.
Other experts in the field have highlighted limitations to the analysis and pointed out that all three agents are very effective in treating AQP4+ NMOSD, and many other considerations need to be taken into account as well as time to first relapse when selecting a therapy, leaving the door open for all three agents.
Dr. Wingerchuk explained that NMOSD is a rare severely disabling complement-mediated autoimmune neuroinflammatory disease of the central nervous system, characterized by devastating and unpredictable attacks (relapses) that can cause immediate and irreversible damage.
There are three recently approved monoclonal antibody treatment options in the United States for adults with AQP4+ NMOSD: eculizumab (Soliris, Alexion), inebilizumab (Uplizna, Horizon), and satralizumab (Enspryng, Genentech). A comparison of the relative treatment effects of these drugs would facilitate the treatment selection process, Dr. Wingerchuk said.
The objective of this study was to perform an indirect treatment comparison on the efficacy of these three FDA-approved treatment options for adults with AQP4+ NMOSD, in the absence of any head-to-head studies.
Using published data from randomized controlled trials, which were identified by a systematic literature review in September 2020, the researchers performed a Bayesian network meta-analysis to estimate the relative effects between eculizumab, inebilizumab, and satralizumab.
Network meta-analyses were performed for clinically relevant subpopulations based on three treatment networks: (1) patients who received monotherapy with one of the monoclonal antibodies or in combination with an immunosuppressant therapy; (2) patients who received monotherapy with the monoclonal antibody alone; and (3) patients who received a combination of both the monoclonal antibody and immunosuppressant therapy.
Time to first relapse was the primary efficacy outcome assessed. Relative treatment effects were expressed as hazard ratios and the probability that a treatment was the best at delaying time to first relapse was also evaluated.
In the systematic literature review, 29 publications from four unique clinical trials were identified and include in the network meta-analysis. These included publications from congress proceedings and peer-reviewed journals.
The four clinical trials were the N-MOmentum trial of inebilizumab versus placebo; the PREVENT trial of eculizumab with or without immunosuppressant therapy versus placebo with or without immunosuppressant therapy; the SAkuraSky trial of satralizumab plus immunosuppressant therapy versus placebo plus immunosuppressant therapy; and the SAkuraStar trial of satralizumab versus placebo.
Results showed that for the first analysis of mono or combination therapy, patients treated with eculizumab with or without immunosuppressant therapy were 76% less likely to experience a first relapse when compared with patients treated with satralizumab with or without immunosuppressant therapy.
In the monotherapy network, patients on eculizumab were 90% less likely to experience a first relapse when compared with patients treated with satralizumab, and patients on eculizumab were 89% less likely to experience a first relapse when compared with patients treated with inebilizumab.
In the third network analysis – a comparison of eculizumab plus immunosuppressant therapy with inebilizumab plus immunosuppressant therapy (Table 1) – the point estimate appeared to favor eculizumab but the confidence intervals were wide and not significant.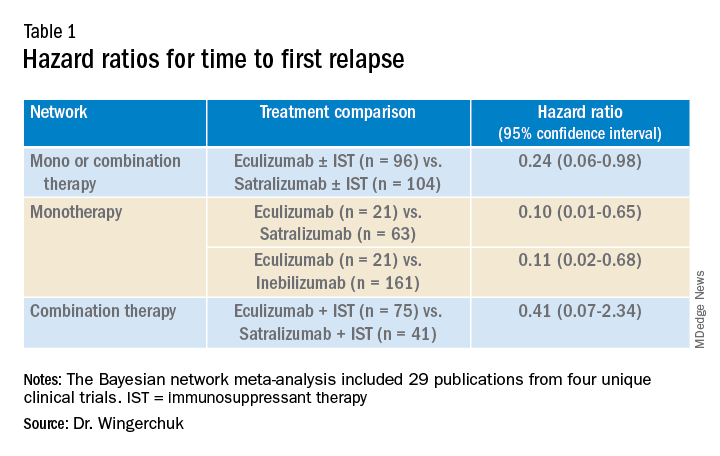
A subsequent analysis looked at the rank order of the best treatment option, with eculizumab coming out first in all three networks (Table 2).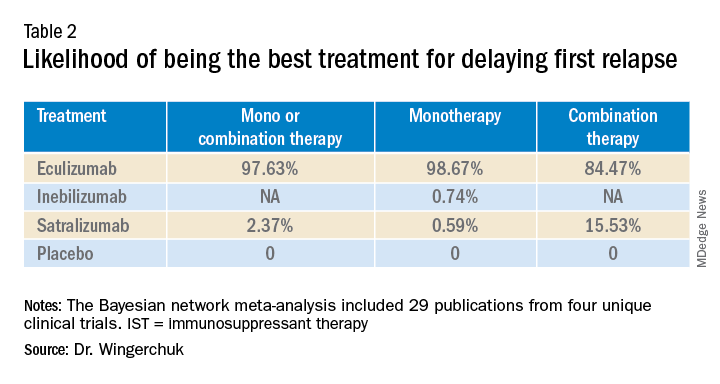
Dr. Wingerchuk acknowledged that there were many limitations to this study, including that analyses for annualized relapse rate, disability, and quality of life were not included because of a lack of consistent outcome reporting by AQP4+ status in the randomized trials.
Safety outcomes were excluded because of a lack of standardized baseline risks and inconsistent reporting by AQP4+ status across trials.
Because this study focused on drugs approved in the United States in a rare disease area, there were a limited number of studies with intervention effects.
There were differences in follow-up durations across the different trials, with N-MOmentum having a follow-up of 197 days compared with 144 weeks for other trials.
“In the absence of head-to-head trials, this network meta-analysis provides important evidence on the relative efficacy of eculizumab versus satralizumab or inebilizumab for the treatment of patients with AQP4+ NMOSD, with significant differences in two out of the three treatment comparison scenarios observed,” Dr. Wingerchuk concluded.
“Based on current evidence, monotherapy and mono-combination therapy with eculizumab appear to more efficacious at preventing relapses than satralizumab or inebilizumab for the treatment of adults with AQP4+ NMOSD. These findings appear to suggest that C5 complement inhibition with treatments such as eculizumab appear to prevent relapses more effectively than other mechanisms involving IL-6 receptor or CD19 inhibition among adults with AQP4+ NMOSD,” he added.
Experts respond
Commenting on the study, several experts in the field provided some balancing views.
Bruce Cree, MD, University of California San Francisco, who was the chief investigator of the N-MOmentum study with inebilizumab, said he was skeptical about this new indirect comparison. “The results of this study seem too good to be true; a 90% difference between agents has to be an overestimate,” he said.
“We know from independent studies that all three drugs are very effective. If we take each trial separately, eculizumab reduced attack risk by 90% versus placebo; and the other two drugs by 77% to 78% versus placebo. For eculizumab to be 90% better than inebilizumab or satralizumab its basically like saying these drugs perform like placebo, but we know that is not the case,” Dr. Cree argued.
He pointed out that when comparing results across studies there are many factors that have to be considered, including the different patient populations included in the different studies, with the characteristic of each population in each trial being unique to that dataset.
In addition, Dr. Cree suggested that all the studies included in the comparison were relatively small for this type of analysis. “Normally this type of analysis is done with much larger studies, so the resulting database is closer to a representation of the disease state itself,” he said.
Dr. Cree also questioned the role of the sponsor in this meta-analysis. “The analysis was sponsored by Alexion and several coauthors were employees of Alexion. There was not much description available of how the statistics were done. I am concerned that the company was involved in the analysis, which could introduce bias. I look forward to seeing details of the statistical methodology,” he said.
“This is definitely a provocative study. They have thrown down the gauntlet. If they are so confident in the results they should now do a head-to-head study to back this result up. If they don’t do that, then I think physicians should ignore it as there are just too many problems with this analysis,” Dr. Cree stated.
Dr. Cree acknowledged that when looking at the four trials separately, eculizumab does look a little better than the other two agents in delaying time to first relapse. “But there are some caveats. Despite a larger reduction in relapse rate there was no reduction in disability in the eculizumab trial. Whereas the inebilizumab trial did show a reduction in disability. And while the PREVENT trial with eculizumab was a good study, during the course of the trial the definition of clinical relapse was changed, and as a consequence that increased the product’s performance – that’s a little bit curious,” he added.
How to choose?
On how to choose between the three agents, Dr. Cree said they are all “extraordinarily effective” at reducing relapse activity. “They are all ‘home run’ products, but they have differences in safety,” he said.
“Inebilizumab is linked to hypogammaglobulinemia over time – we haven’t seen an increase in infection risk linked to this, but with enough time, I would expect that there probably will be. But inebilizumab is a B-cell-depleting agent like the agents used in MS, and we now have a lot of experiences with this type of product, which gives us more confidence on the safety profile,” Dr. Cree noted.
“Eculizumab was linked to a risk of meningococcal meningitis and other bacterial infections, and satralizumab seems to [be] overall well tolerated with no obvious safety concerns to date, but the studies have been quite small,” he added.
On routes of administration and frequency of dosing, Dr. Cree pointed out that while all three drugs have an intensive loading schedule, for maintenance, eculizumab needs to be given as an IV infusion every 2 weeks. Inebilizumab needs just two infusions per year for maintenance, while satralizumab is given by subcutaneous injection once per month.
“It may be that eculizumab could be used at the time of an acute attack but then treatment could be switched to one of the other two for long-term maintenance,” he suggested.
But Dr. Cree pointed out that the biggest challenge for all three agents is access. “The costs are astronomically high ($200,000-$770,000). They are prohibitively expensive and very few insurance companies are covering them.”
Also commenting, Brian Weinshenker, MD, from the Mayo Clinic in Rochester, Minn., who was a member of the attack adjudication committee for both PREVENT and N-MOmentum studies, pointed out that as well as differences in the populations enrolled, and study designs, the studies with the three different drugs also had differences in attack adjudication criteria.
“These factors make it very difficult to compare across studies, which is what was done in this analysis, so I would be reluctant to reach many conclusions about differences.”
Dr. Weinshenker added: “All three treatments provided strong benefit. We are still learning about long-term benefits, but emerging data have suggested that all three seem to provide persistent benefits for the length of the open-label extension study. We don’t have much evidence about the severity of the attacks that did occur, although some limited data suggest that both eculizumab and inebilizumab reduce attack severity.”
Dennis Bourdette, MD, professor emeritus, department of neurology, Oregon Health & Science University, Portland, who was not involved in any of the studies, said he thought the new analysis was “a worthwhile effort to determine the relative effectiveness of the three different drugs in treating AQP4+ NMOSD.
“Given the rarity of APQ4+ NMOSD, it will be difficult to perform randomized head-to-head clinical trials of the agents, so this type of comparison is the best we can do at this time,” he said.
While Dr. Bourdette feels this study supports the notion that eculizumab is more effective at delaying time to first relapse than inebilizumab and satralizumab, he does not believe the results should have a major impact on decisions about which agent to use in clinical practice.
“A difference in delaying time to first relapse tells us little about the relative effectiveness of the long-term benefit of these [agents], particularly with regards to permanent disability or frequency of relapses. However, it is possible that the difference reflects the efficacy kinetics of the agents with eculizumab working faster than the other two agents, which would be useful in making a decision about a patient with very active NMOSD where one wants to get the disease under control as quickly as possible,” Dr. Bourdette noted.
But he added that neurologists should also consider safety profile, convenience, and contraindications. “Eculizumab is clearly less convenient in terms of dosing schedule than the other two agents, and patient convenience is important for long-term compliance.”
Dr. Bourdette pointed out that another consideration is prior treatment. “Many patients with NMOSD will receive the anti-CD20 monoclonal antibody, rituximab – which depletes B cells – off label. Inebilizumab also depletes B cells, so a patient who has had continued NMOSD disease activity on rituximab probably should not be treated with inebilizumab, making eculizumab or satralizumab preferable,” he suggested.
Finally, Dr. Bourdette highlighted the sponsorship of the current study by the manufacturer of eculizumab, Alexion, and that all of the authors have some financial relationship with Alexion as described in their disclosures. “Whether this resulted in any biases about the design, conduct, or interpretation of the study is uncertain but is always a concern,” he said.
Company statements
The companies selling inebilizumab and satralizumab sent statements on the new analysis and repeated many of the above points.
Genentech noted that new longer-term data presented at ECTRIMS show that satralizumab is effective in significantly reducing relapses over 4 years of treatment in people with AQP4+ NMOSD, with a favorable safety profile both as a monotherapy and in conjunction with immunosuppressive therapy. More than 70% of people treated with satralizumab remained relapse free after 4 years in the SAkuraStar (73%) and SAkuraSky (71%) open-label extension studies, and 90% and 91%, respectively, were free from severe relapse, the company reported.
Horizon said: “We are confident in the efficacy and safety of Uplizna (inebilizumab) – a convenient, twice-annual monotherapy – that was studied in the largest randomized, placebo-controlled, global trial of a monotherapy in NMOSD. The endpoints in this trial were prospectively defined and assessed by an adjudication committee as published in The Lancet, with long-term follow-up data now published in the Multiple Sclerosis Journal that further support the efficacy and safety.”
The current study was funded by Alexion–AstraZeneca Rare Disease. Dr. Wingerchuk has participated on data safety monitoring or advisory boards for Roche, Viela Bio, Genentech, Biogen, Reistone, TG Therapeutics, Celgene, Novartis, and Alexion–AstraZeneca Rare Disease. He has received grants for clinical trials through Alexion–AstraZeneca Rare Disease and Terumo BCT, and has been paid consulting fees by Mitsubishi Tanabe. Several coauthors of this study are employees of Alexion Pharmaceutics. Dr. Cree was principal investigator on the N-MOmentum study with inebilizumab. He has a grant from Genentech for MS research, and has consulted for Alexion in the past. Dr. Weinshenker has served as a member of the attack adjudication committee for both PREVENT and N-MOmentum studies and has financial relationships with the manufacturers of all three drugs. Dr. Bourdette has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Alexion-sponsored study was presented at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) by Dean Wingerchuk, MD, of the Mayo Clinic in Scottsdale, Ariz.
Other experts in the field have highlighted limitations to the analysis and pointed out that all three agents are very effective in treating AQP4+ NMOSD, and many other considerations need to be taken into account as well as time to first relapse when selecting a therapy, leaving the door open for all three agents.
Dr. Wingerchuk explained that NMOSD is a rare severely disabling complement-mediated autoimmune neuroinflammatory disease of the central nervous system, characterized by devastating and unpredictable attacks (relapses) that can cause immediate and irreversible damage.
There are three recently approved monoclonal antibody treatment options in the United States for adults with AQP4+ NMOSD: eculizumab (Soliris, Alexion), inebilizumab (Uplizna, Horizon), and satralizumab (Enspryng, Genentech). A comparison of the relative treatment effects of these drugs would facilitate the treatment selection process, Dr. Wingerchuk said.
The objective of this study was to perform an indirect treatment comparison on the efficacy of these three FDA-approved treatment options for adults with AQP4+ NMOSD, in the absence of any head-to-head studies.
Using published data from randomized controlled trials, which were identified by a systematic literature review in September 2020, the researchers performed a Bayesian network meta-analysis to estimate the relative effects between eculizumab, inebilizumab, and satralizumab.
Network meta-analyses were performed for clinically relevant subpopulations based on three treatment networks: (1) patients who received monotherapy with one of the monoclonal antibodies or in combination with an immunosuppressant therapy; (2) patients who received monotherapy with the monoclonal antibody alone; and (3) patients who received a combination of both the monoclonal antibody and immunosuppressant therapy.
Time to first relapse was the primary efficacy outcome assessed. Relative treatment effects were expressed as hazard ratios and the probability that a treatment was the best at delaying time to first relapse was also evaluated.
In the systematic literature review, 29 publications from four unique clinical trials were identified and include in the network meta-analysis. These included publications from congress proceedings and peer-reviewed journals.
The four clinical trials were the N-MOmentum trial of inebilizumab versus placebo; the PREVENT trial of eculizumab with or without immunosuppressant therapy versus placebo with or without immunosuppressant therapy; the SAkuraSky trial of satralizumab plus immunosuppressant therapy versus placebo plus immunosuppressant therapy; and the SAkuraStar trial of satralizumab versus placebo.
Results showed that for the first analysis of mono or combination therapy, patients treated with eculizumab with or without immunosuppressant therapy were 76% less likely to experience a first relapse when compared with patients treated with satralizumab with or without immunosuppressant therapy.
In the monotherapy network, patients on eculizumab were 90% less likely to experience a first relapse when compared with patients treated with satralizumab, and patients on eculizumab were 89% less likely to experience a first relapse when compared with patients treated with inebilizumab.
In the third network analysis – a comparison of eculizumab plus immunosuppressant therapy with inebilizumab plus immunosuppressant therapy (Table 1) – the point estimate appeared to favor eculizumab but the confidence intervals were wide and not significant.
A subsequent analysis looked at the rank order of the best treatment option, with eculizumab coming out first in all three networks (Table 2).
Dr. Wingerchuk acknowledged that there were many limitations to this study, including that analyses for annualized relapse rate, disability, and quality of life were not included because of a lack of consistent outcome reporting by AQP4+ status in the randomized trials.
Safety outcomes were excluded because of a lack of standardized baseline risks and inconsistent reporting by AQP4+ status across trials.
Because this study focused on drugs approved in the United States in a rare disease area, there were a limited number of studies with intervention effects.
There were differences in follow-up durations across the different trials, with N-MOmentum having a follow-up of 197 days compared with 144 weeks for other trials.
“In the absence of head-to-head trials, this network meta-analysis provides important evidence on the relative efficacy of eculizumab versus satralizumab or inebilizumab for the treatment of patients with AQP4+ NMOSD, with significant differences in two out of the three treatment comparison scenarios observed,” Dr. Wingerchuk concluded.
“Based on current evidence, monotherapy and mono-combination therapy with eculizumab appear to more efficacious at preventing relapses than satralizumab or inebilizumab for the treatment of adults with AQP4+ NMOSD. These findings appear to suggest that C5 complement inhibition with treatments such as eculizumab appear to prevent relapses more effectively than other mechanisms involving IL-6 receptor or CD19 inhibition among adults with AQP4+ NMOSD,” he added.
Experts respond
Commenting on the study, several experts in the field provided some balancing views.
Bruce Cree, MD, University of California San Francisco, who was the chief investigator of the N-MOmentum study with inebilizumab, said he was skeptical about this new indirect comparison. “The results of this study seem too good to be true; a 90% difference between agents has to be an overestimate,” he said.
“We know from independent studies that all three drugs are very effective. If we take each trial separately, eculizumab reduced attack risk by 90% versus placebo; and the other two drugs by 77% to 78% versus placebo. For eculizumab to be 90% better than inebilizumab or satralizumab its basically like saying these drugs perform like placebo, but we know that is not the case,” Dr. Cree argued.
He pointed out that when comparing results across studies there are many factors that have to be considered, including the different patient populations included in the different studies, with the characteristic of each population in each trial being unique to that dataset.
In addition, Dr. Cree suggested that all the studies included in the comparison were relatively small for this type of analysis. “Normally this type of analysis is done with much larger studies, so the resulting database is closer to a representation of the disease state itself,” he said.
Dr. Cree also questioned the role of the sponsor in this meta-analysis. “The analysis was sponsored by Alexion and several coauthors were employees of Alexion. There was not much description available of how the statistics were done. I am concerned that the company was involved in the analysis, which could introduce bias. I look forward to seeing details of the statistical methodology,” he said.
“This is definitely a provocative study. They have thrown down the gauntlet. If they are so confident in the results they should now do a head-to-head study to back this result up. If they don’t do that, then I think physicians should ignore it as there are just too many problems with this analysis,” Dr. Cree stated.
Dr. Cree acknowledged that when looking at the four trials separately, eculizumab does look a little better than the other two agents in delaying time to first relapse. “But there are some caveats. Despite a larger reduction in relapse rate there was no reduction in disability in the eculizumab trial. Whereas the inebilizumab trial did show a reduction in disability. And while the PREVENT trial with eculizumab was a good study, during the course of the trial the definition of clinical relapse was changed, and as a consequence that increased the product’s performance – that’s a little bit curious,” he added.
How to choose?
On how to choose between the three agents, Dr. Cree said they are all “extraordinarily effective” at reducing relapse activity. “They are all ‘home run’ products, but they have differences in safety,” he said.
“Inebilizumab is linked to hypogammaglobulinemia over time – we haven’t seen an increase in infection risk linked to this, but with enough time, I would expect that there probably will be. But inebilizumab is a B-cell-depleting agent like the agents used in MS, and we now have a lot of experiences with this type of product, which gives us more confidence on the safety profile,” Dr. Cree noted.
“Eculizumab was linked to a risk of meningococcal meningitis and other bacterial infections, and satralizumab seems to [be] overall well tolerated with no obvious safety concerns to date, but the studies have been quite small,” he added.
On routes of administration and frequency of dosing, Dr. Cree pointed out that while all three drugs have an intensive loading schedule, for maintenance, eculizumab needs to be given as an IV infusion every 2 weeks. Inebilizumab needs just two infusions per year for maintenance, while satralizumab is given by subcutaneous injection once per month.
“It may be that eculizumab could be used at the time of an acute attack but then treatment could be switched to one of the other two for long-term maintenance,” he suggested.
But Dr. Cree pointed out that the biggest challenge for all three agents is access. “The costs are astronomically high ($200,000-$770,000). They are prohibitively expensive and very few insurance companies are covering them.”
Also commenting, Brian Weinshenker, MD, from the Mayo Clinic in Rochester, Minn., who was a member of the attack adjudication committee for both PREVENT and N-MOmentum studies, pointed out that as well as differences in the populations enrolled, and study designs, the studies with the three different drugs also had differences in attack adjudication criteria.
“These factors make it very difficult to compare across studies, which is what was done in this analysis, so I would be reluctant to reach many conclusions about differences.”
Dr. Weinshenker added: “All three treatments provided strong benefit. We are still learning about long-term benefits, but emerging data have suggested that all three seem to provide persistent benefits for the length of the open-label extension study. We don’t have much evidence about the severity of the attacks that did occur, although some limited data suggest that both eculizumab and inebilizumab reduce attack severity.”
Dennis Bourdette, MD, professor emeritus, department of neurology, Oregon Health & Science University, Portland, who was not involved in any of the studies, said he thought the new analysis was “a worthwhile effort to determine the relative effectiveness of the three different drugs in treating AQP4+ NMOSD.
“Given the rarity of APQ4+ NMOSD, it will be difficult to perform randomized head-to-head clinical trials of the agents, so this type of comparison is the best we can do at this time,” he said.
While Dr. Bourdette feels this study supports the notion that eculizumab is more effective at delaying time to first relapse than inebilizumab and satralizumab, he does not believe the results should have a major impact on decisions about which agent to use in clinical practice.
“A difference in delaying time to first relapse tells us little about the relative effectiveness of the long-term benefit of these [agents], particularly with regards to permanent disability or frequency of relapses. However, it is possible that the difference reflects the efficacy kinetics of the agents with eculizumab working faster than the other two agents, which would be useful in making a decision about a patient with very active NMOSD where one wants to get the disease under control as quickly as possible,” Dr. Bourdette noted.
But he added that neurologists should also consider safety profile, convenience, and contraindications. “Eculizumab is clearly less convenient in terms of dosing schedule than the other two agents, and patient convenience is important for long-term compliance.”
Dr. Bourdette pointed out that another consideration is prior treatment. “Many patients with NMOSD will receive the anti-CD20 monoclonal antibody, rituximab – which depletes B cells – off label. Inebilizumab also depletes B cells, so a patient who has had continued NMOSD disease activity on rituximab probably should not be treated with inebilizumab, making eculizumab or satralizumab preferable,” he suggested.
Finally, Dr. Bourdette highlighted the sponsorship of the current study by the manufacturer of eculizumab, Alexion, and that all of the authors have some financial relationship with Alexion as described in their disclosures. “Whether this resulted in any biases about the design, conduct, or interpretation of the study is uncertain but is always a concern,” he said.
Company statements
The companies selling inebilizumab and satralizumab sent statements on the new analysis and repeated many of the above points.
Genentech noted that new longer-term data presented at ECTRIMS show that satralizumab is effective in significantly reducing relapses over 4 years of treatment in people with AQP4+ NMOSD, with a favorable safety profile both as a monotherapy and in conjunction with immunosuppressive therapy. More than 70% of people treated with satralizumab remained relapse free after 4 years in the SAkuraStar (73%) and SAkuraSky (71%) open-label extension studies, and 90% and 91%, respectively, were free from severe relapse, the company reported.
Horizon said: “We are confident in the efficacy and safety of Uplizna (inebilizumab) – a convenient, twice-annual monotherapy – that was studied in the largest randomized, placebo-controlled, global trial of a monotherapy in NMOSD. The endpoints in this trial were prospectively defined and assessed by an adjudication committee as published in The Lancet, with long-term follow-up data now published in the Multiple Sclerosis Journal that further support the efficacy and safety.”
The current study was funded by Alexion–AstraZeneca Rare Disease. Dr. Wingerchuk has participated on data safety monitoring or advisory boards for Roche, Viela Bio, Genentech, Biogen, Reistone, TG Therapeutics, Celgene, Novartis, and Alexion–AstraZeneca Rare Disease. He has received grants for clinical trials through Alexion–AstraZeneca Rare Disease and Terumo BCT, and has been paid consulting fees by Mitsubishi Tanabe. Several coauthors of this study are employees of Alexion Pharmaceutics. Dr. Cree was principal investigator on the N-MOmentum study with inebilizumab. He has a grant from Genentech for MS research, and has consulted for Alexion in the past. Dr. Weinshenker has served as a member of the attack adjudication committee for both PREVENT and N-MOmentum studies and has financial relationships with the manufacturers of all three drugs. Dr. Bourdette has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Alexion-sponsored study was presented at the annual meeting of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) by Dean Wingerchuk, MD, of the Mayo Clinic in Scottsdale, Ariz.
Other experts in the field have highlighted limitations to the analysis and pointed out that all three agents are very effective in treating AQP4+ NMOSD, and many other considerations need to be taken into account as well as time to first relapse when selecting a therapy, leaving the door open for all three agents.
Dr. Wingerchuk explained that NMOSD is a rare severely disabling complement-mediated autoimmune neuroinflammatory disease of the central nervous system, characterized by devastating and unpredictable attacks (relapses) that can cause immediate and irreversible damage.
There are three recently approved monoclonal antibody treatment options in the United States for adults with AQP4+ NMOSD: eculizumab (Soliris, Alexion), inebilizumab (Uplizna, Horizon), and satralizumab (Enspryng, Genentech). A comparison of the relative treatment effects of these drugs would facilitate the treatment selection process, Dr. Wingerchuk said.
The objective of this study was to perform an indirect treatment comparison on the efficacy of these three FDA-approved treatment options for adults with AQP4+ NMOSD, in the absence of any head-to-head studies.
Using published data from randomized controlled trials, which were identified by a systematic literature review in September 2020, the researchers performed a Bayesian network meta-analysis to estimate the relative effects between eculizumab, inebilizumab, and satralizumab.
Network meta-analyses were performed for clinically relevant subpopulations based on three treatment networks: (1) patients who received monotherapy with one of the monoclonal antibodies or in combination with an immunosuppressant therapy; (2) patients who received monotherapy with the monoclonal antibody alone; and (3) patients who received a combination of both the monoclonal antibody and immunosuppressant therapy.
Time to first relapse was the primary efficacy outcome assessed. Relative treatment effects were expressed as hazard ratios and the probability that a treatment was the best at delaying time to first relapse was also evaluated.
In the systematic literature review, 29 publications from four unique clinical trials were identified and include in the network meta-analysis. These included publications from congress proceedings and peer-reviewed journals.
The four clinical trials were the N-MOmentum trial of inebilizumab versus placebo; the PREVENT trial of eculizumab with or without immunosuppressant therapy versus placebo with or without immunosuppressant therapy; the SAkuraSky trial of satralizumab plus immunosuppressant therapy versus placebo plus immunosuppressant therapy; and the SAkuraStar trial of satralizumab versus placebo.
Results showed that for the first analysis of mono or combination therapy, patients treated with eculizumab with or without immunosuppressant therapy were 76% less likely to experience a first relapse when compared with patients treated with satralizumab with or without immunosuppressant therapy.
In the monotherapy network, patients on eculizumab were 90% less likely to experience a first relapse when compared with patients treated with satralizumab, and patients on eculizumab were 89% less likely to experience a first relapse when compared with patients treated with inebilizumab.
In the third network analysis – a comparison of eculizumab plus immunosuppressant therapy with inebilizumab plus immunosuppressant therapy (Table 1) – the point estimate appeared to favor eculizumab but the confidence intervals were wide and not significant.
A subsequent analysis looked at the rank order of the best treatment option, with eculizumab coming out first in all three networks (Table 2).
Dr. Wingerchuk acknowledged that there were many limitations to this study, including that analyses for annualized relapse rate, disability, and quality of life were not included because of a lack of consistent outcome reporting by AQP4+ status in the randomized trials.
Safety outcomes were excluded because of a lack of standardized baseline risks and inconsistent reporting by AQP4+ status across trials.
Because this study focused on drugs approved in the United States in a rare disease area, there were a limited number of studies with intervention effects.
There were differences in follow-up durations across the different trials, with N-MOmentum having a follow-up of 197 days compared with 144 weeks for other trials.
“In the absence of head-to-head trials, this network meta-analysis provides important evidence on the relative efficacy of eculizumab versus satralizumab or inebilizumab for the treatment of patients with AQP4+ NMOSD, with significant differences in two out of the three treatment comparison scenarios observed,” Dr. Wingerchuk concluded.
“Based on current evidence, monotherapy and mono-combination therapy with eculizumab appear to more efficacious at preventing relapses than satralizumab or inebilizumab for the treatment of adults with AQP4+ NMOSD. These findings appear to suggest that C5 complement inhibition with treatments such as eculizumab appear to prevent relapses more effectively than other mechanisms involving IL-6 receptor or CD19 inhibition among adults with AQP4+ NMOSD,” he added.
Experts respond
Commenting on the study, several experts in the field provided some balancing views.
Bruce Cree, MD, University of California San Francisco, who was the chief investigator of the N-MOmentum study with inebilizumab, said he was skeptical about this new indirect comparison. “The results of this study seem too good to be true; a 90% difference between agents has to be an overestimate,” he said.
“We know from independent studies that all three drugs are very effective. If we take each trial separately, eculizumab reduced attack risk by 90% versus placebo; and the other two drugs by 77% to 78% versus placebo. For eculizumab to be 90% better than inebilizumab or satralizumab its basically like saying these drugs perform like placebo, but we know that is not the case,” Dr. Cree argued.
He pointed out that when comparing results across studies there are many factors that have to be considered, including the different patient populations included in the different studies, with the characteristic of each population in each trial being unique to that dataset.
In addition, Dr. Cree suggested that all the studies included in the comparison were relatively small for this type of analysis. “Normally this type of analysis is done with much larger studies, so the resulting database is closer to a representation of the disease state itself,” he said.
Dr. Cree also questioned the role of the sponsor in this meta-analysis. “The analysis was sponsored by Alexion and several coauthors were employees of Alexion. There was not much description available of how the statistics were done. I am concerned that the company was involved in the analysis, which could introduce bias. I look forward to seeing details of the statistical methodology,” he said.
“This is definitely a provocative study. They have thrown down the gauntlet. If they are so confident in the results they should now do a head-to-head study to back this result up. If they don’t do that, then I think physicians should ignore it as there are just too many problems with this analysis,” Dr. Cree stated.
Dr. Cree acknowledged that when looking at the four trials separately, eculizumab does look a little better than the other two agents in delaying time to first relapse. “But there are some caveats. Despite a larger reduction in relapse rate there was no reduction in disability in the eculizumab trial. Whereas the inebilizumab trial did show a reduction in disability. And while the PREVENT trial with eculizumab was a good study, during the course of the trial the definition of clinical relapse was changed, and as a consequence that increased the product’s performance – that’s a little bit curious,” he added.
How to choose?
On how to choose between the three agents, Dr. Cree said they are all “extraordinarily effective” at reducing relapse activity. “They are all ‘home run’ products, but they have differences in safety,” he said.
“Inebilizumab is linked to hypogammaglobulinemia over time – we haven’t seen an increase in infection risk linked to this, but with enough time, I would expect that there probably will be. But inebilizumab is a B-cell-depleting agent like the agents used in MS, and we now have a lot of experiences with this type of product, which gives us more confidence on the safety profile,” Dr. Cree noted.
“Eculizumab was linked to a risk of meningococcal meningitis and other bacterial infections, and satralizumab seems to [be] overall well tolerated with no obvious safety concerns to date, but the studies have been quite small,” he added.
On routes of administration and frequency of dosing, Dr. Cree pointed out that while all three drugs have an intensive loading schedule, for maintenance, eculizumab needs to be given as an IV infusion every 2 weeks. Inebilizumab needs just two infusions per year for maintenance, while satralizumab is given by subcutaneous injection once per month.
“It may be that eculizumab could be used at the time of an acute attack but then treatment could be switched to one of the other two for long-term maintenance,” he suggested.
But Dr. Cree pointed out that the biggest challenge for all three agents is access. “The costs are astronomically high ($200,000-$770,000). They are prohibitively expensive and very few insurance companies are covering them.”
Also commenting, Brian Weinshenker, MD, from the Mayo Clinic in Rochester, Minn., who was a member of the attack adjudication committee for both PREVENT and N-MOmentum studies, pointed out that as well as differences in the populations enrolled, and study designs, the studies with the three different drugs also had differences in attack adjudication criteria.
“These factors make it very difficult to compare across studies, which is what was done in this analysis, so I would be reluctant to reach many conclusions about differences.”
Dr. Weinshenker added: “All three treatments provided strong benefit. We are still learning about long-term benefits, but emerging data have suggested that all three seem to provide persistent benefits for the length of the open-label extension study. We don’t have much evidence about the severity of the attacks that did occur, although some limited data suggest that both eculizumab and inebilizumab reduce attack severity.”
Dennis Bourdette, MD, professor emeritus, department of neurology, Oregon Health & Science University, Portland, who was not involved in any of the studies, said he thought the new analysis was “a worthwhile effort to determine the relative effectiveness of the three different drugs in treating AQP4+ NMOSD.
“Given the rarity of APQ4+ NMOSD, it will be difficult to perform randomized head-to-head clinical trials of the agents, so this type of comparison is the best we can do at this time,” he said.
While Dr. Bourdette feels this study supports the notion that eculizumab is more effective at delaying time to first relapse than inebilizumab and satralizumab, he does not believe the results should have a major impact on decisions about which agent to use in clinical practice.
“A difference in delaying time to first relapse tells us little about the relative effectiveness of the long-term benefit of these [agents], particularly with regards to permanent disability or frequency of relapses. However, it is possible that the difference reflects the efficacy kinetics of the agents with eculizumab working faster than the other two agents, which would be useful in making a decision about a patient with very active NMOSD where one wants to get the disease under control as quickly as possible,” Dr. Bourdette noted.
But he added that neurologists should also consider safety profile, convenience, and contraindications. “Eculizumab is clearly less convenient in terms of dosing schedule than the other two agents, and patient convenience is important for long-term compliance.”
Dr. Bourdette pointed out that another consideration is prior treatment. “Many patients with NMOSD will receive the anti-CD20 monoclonal antibody, rituximab – which depletes B cells – off label. Inebilizumab also depletes B cells, so a patient who has had continued NMOSD disease activity on rituximab probably should not be treated with inebilizumab, making eculizumab or satralizumab preferable,” he suggested.
Finally, Dr. Bourdette highlighted the sponsorship of the current study by the manufacturer of eculizumab, Alexion, and that all of the authors have some financial relationship with Alexion as described in their disclosures. “Whether this resulted in any biases about the design, conduct, or interpretation of the study is uncertain but is always a concern,” he said.
Company statements
The companies selling inebilizumab and satralizumab sent statements on the new analysis and repeated many of the above points.
Genentech noted that new longer-term data presented at ECTRIMS show that satralizumab is effective in significantly reducing relapses over 4 years of treatment in people with AQP4+ NMOSD, with a favorable safety profile both as a monotherapy and in conjunction with immunosuppressive therapy. More than 70% of people treated with satralizumab remained relapse free after 4 years in the SAkuraStar (73%) and SAkuraSky (71%) open-label extension studies, and 90% and 91%, respectively, were free from severe relapse, the company reported.
Horizon said: “We are confident in the efficacy and safety of Uplizna (inebilizumab) – a convenient, twice-annual monotherapy – that was studied in the largest randomized, placebo-controlled, global trial of a monotherapy in NMOSD. The endpoints in this trial were prospectively defined and assessed by an adjudication committee as published in The Lancet, with long-term follow-up data now published in the Multiple Sclerosis Journal that further support the efficacy and safety.”
The current study was funded by Alexion–AstraZeneca Rare Disease. Dr. Wingerchuk has participated on data safety monitoring or advisory boards for Roche, Viela Bio, Genentech, Biogen, Reistone, TG Therapeutics, Celgene, Novartis, and Alexion–AstraZeneca Rare Disease. He has received grants for clinical trials through Alexion–AstraZeneca Rare Disease and Terumo BCT, and has been paid consulting fees by Mitsubishi Tanabe. Several coauthors of this study are employees of Alexion Pharmaceutics. Dr. Cree was principal investigator on the N-MOmentum study with inebilizumab. He has a grant from Genentech for MS research, and has consulted for Alexion in the past. Dr. Weinshenker has served as a member of the attack adjudication committee for both PREVENT and N-MOmentum studies and has financial relationships with the manufacturers of all three drugs. Dr. Bourdette has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ECTRIMS 2021
Managing simple febrile seizures without lumbar puncture safe: 15-year study
Most children with simple febrile seizures (SFSs) can be safely managed without lumbar puncture or other diagnostic tests without risking delayed diagnosis of bacterial meningitis, new data gathered from a 15-year span suggest.
Vidya R. Raghavan, MD, with the division of emergency medicine at Boston Children’s Hospital and Harvard Medical School, also in Boston, published their findings in Pediatrics.
In 2011, researchers published the American Academy of Pediatrics simple febrile seizure guideline, which recommends limiting lumbar puncture to non–low-risk patients. The guidelines also specified that neuroimaging and hematologic testing are not routinely recommended.
Dr. Raghavan and coauthors studied evaluation and management trends of the patients before and after the guidelines. They identified 142,121 children diagnosed with SFS who presented to 1 of 49 pediatric tertiary EDs and met other study criteria. Changes in management of SFS had started years before the guideline and positive effects continued after the guideline publication.
Researchers found a significant 95% decline in rates of lumbar puncture between 2005 and 2019 from 11.6% (95% confidence interval, 10.8%-12.4%) of children in 2005 to 0.6% (95% CI, 0.5%-0.8%; P < .001) in 2019. The most significant declines were among infants 6 months to 1 year.
“We found similar declines in rates of diagnostic laboratory and radiologic testing, intravenous antibiotic administration, hospitalization, and costs,” the authors wrote.
“Importantly,” they wrote, “the decrease in testing was not associated with a concurrent increase in delayed diagnoses of bacterial meningitis.”
The number of hospital admissions and total costs also dropped significantly over the 15-year span of the study. After adjusting for inflation, the authors wrote, costs dropped from an average $1,523 in 2005 to $605 (P < .001) in 2019.
Among first-time presentations for SFSs, 19.2% (95% CI, 18.3%-20.2%) resulted in admission in 2005. That rate dropped to 5.2% (95% CI, 4.8%-5.6%) in 2019 (P < .001), although the authors noted that trend largely plateaued after the guideline was published.
“Our findings are consistent with smaller studies published before 2011 in which researchers found declining rates of LP [lumbar puncture] in children presenting to the ED with their first SFS,” the authors wrote.
Mercedes Blackstone, MD, an emergency physician at the Children’s Hospital of Philadelphia, said in an interview that the paper offers reassurance for changed practice over the last decade.
She said there was substantial relief in pediatrics when the 2011 guidelines recognized formally that protocols were outdated, especially as bacterial meningitis had become increasingly rare with widespread use of pneumococcal and Haemophilus influenzae vaccines. Practitioners had already started to limit the spinal taps on their own.
“We were not really complying with the prior recommendation to do a spinal tap in all those children because it often felt like doing a pretty invasive procedure with a very low yield in what was often a very well child in front of you,” she said.
In 2007, the authors noted, a few years before the guidelines, rates of bacterial meningitis had decreased to 7 per 100,000 in children aged between 2 and 23 months and 0.56 per 100,000 in children aged between 2 and 10 years.
However, Dr. Blackstone said, there was still a worry among some practitioners that there could be missed cases of bacterial meningitis.
“It’s very helpful to see that in all those years, the guidelines have been very validated and there were really no missed cases,” said Dr. Blackstone, author of CHOP’s febrile seizures clinical pathway.
It was good to see the number of CT scans drop as well, she said. Dr. Raghavan’s team found they decreased from 10.6% to 1.6%; P < .001, over the study period.
“Earlier work had shown that there was still a fair amount of head CTs happening and that’s radiation to the young brain,” Dr. Blackstone noted. “This is great news.”
Dr. Blackstone said it was great to see so many children from so many children’s hospitals included in the study.
The paper confirmed that “we’ve reduced a lot of unnecessary testing, saved a lot of cost, and had no increased risk to the patients,” she said.
Dr. Blackstone pointed out that the authors include a limitation that many children are seen in nonpediatric centers in community adult ED and she said those settings tend to have more testing.
“Hopefully, these guidelines have penetrated into the whole community,” she said. “With this paper they should feel reassured that they can spare children some of these tests and procedures.”
Dr. Raghavan and Dr. Blackstone declared no relevant financial relationships.
Most children with simple febrile seizures (SFSs) can be safely managed without lumbar puncture or other diagnostic tests without risking delayed diagnosis of bacterial meningitis, new data gathered from a 15-year span suggest.
Vidya R. Raghavan, MD, with the division of emergency medicine at Boston Children’s Hospital and Harvard Medical School, also in Boston, published their findings in Pediatrics.
In 2011, researchers published the American Academy of Pediatrics simple febrile seizure guideline, which recommends limiting lumbar puncture to non–low-risk patients. The guidelines also specified that neuroimaging and hematologic testing are not routinely recommended.
Dr. Raghavan and coauthors studied evaluation and management trends of the patients before and after the guidelines. They identified 142,121 children diagnosed with SFS who presented to 1 of 49 pediatric tertiary EDs and met other study criteria. Changes in management of SFS had started years before the guideline and positive effects continued after the guideline publication.
Researchers found a significant 95% decline in rates of lumbar puncture between 2005 and 2019 from 11.6% (95% confidence interval, 10.8%-12.4%) of children in 2005 to 0.6% (95% CI, 0.5%-0.8%; P < .001) in 2019. The most significant declines were among infants 6 months to 1 year.
“We found similar declines in rates of diagnostic laboratory and radiologic testing, intravenous antibiotic administration, hospitalization, and costs,” the authors wrote.
“Importantly,” they wrote, “the decrease in testing was not associated with a concurrent increase in delayed diagnoses of bacterial meningitis.”
The number of hospital admissions and total costs also dropped significantly over the 15-year span of the study. After adjusting for inflation, the authors wrote, costs dropped from an average $1,523 in 2005 to $605 (P < .001) in 2019.
Among first-time presentations for SFSs, 19.2% (95% CI, 18.3%-20.2%) resulted in admission in 2005. That rate dropped to 5.2% (95% CI, 4.8%-5.6%) in 2019 (P < .001), although the authors noted that trend largely plateaued after the guideline was published.
“Our findings are consistent with smaller studies published before 2011 in which researchers found declining rates of LP [lumbar puncture] in children presenting to the ED with their first SFS,” the authors wrote.
Mercedes Blackstone, MD, an emergency physician at the Children’s Hospital of Philadelphia, said in an interview that the paper offers reassurance for changed practice over the last decade.
She said there was substantial relief in pediatrics when the 2011 guidelines recognized formally that protocols were outdated, especially as bacterial meningitis had become increasingly rare with widespread use of pneumococcal and Haemophilus influenzae vaccines. Practitioners had already started to limit the spinal taps on their own.
“We were not really complying with the prior recommendation to do a spinal tap in all those children because it often felt like doing a pretty invasive procedure with a very low yield in what was often a very well child in front of you,” she said.
In 2007, the authors noted, a few years before the guidelines, rates of bacterial meningitis had decreased to 7 per 100,000 in children aged between 2 and 23 months and 0.56 per 100,000 in children aged between 2 and 10 years.
However, Dr. Blackstone said, there was still a worry among some practitioners that there could be missed cases of bacterial meningitis.
“It’s very helpful to see that in all those years, the guidelines have been very validated and there were really no missed cases,” said Dr. Blackstone, author of CHOP’s febrile seizures clinical pathway.
It was good to see the number of CT scans drop as well, she said. Dr. Raghavan’s team found they decreased from 10.6% to 1.6%; P < .001, over the study period.
“Earlier work had shown that there was still a fair amount of head CTs happening and that’s radiation to the young brain,” Dr. Blackstone noted. “This is great news.”
Dr. Blackstone said it was great to see so many children from so many children’s hospitals included in the study.
The paper confirmed that “we’ve reduced a lot of unnecessary testing, saved a lot of cost, and had no increased risk to the patients,” she said.
Dr. Blackstone pointed out that the authors include a limitation that many children are seen in nonpediatric centers in community adult ED and she said those settings tend to have more testing.
“Hopefully, these guidelines have penetrated into the whole community,” she said. “With this paper they should feel reassured that they can spare children some of these tests and procedures.”
Dr. Raghavan and Dr. Blackstone declared no relevant financial relationships.
Most children with simple febrile seizures (SFSs) can be safely managed without lumbar puncture or other diagnostic tests without risking delayed diagnosis of bacterial meningitis, new data gathered from a 15-year span suggest.
Vidya R. Raghavan, MD, with the division of emergency medicine at Boston Children’s Hospital and Harvard Medical School, also in Boston, published their findings in Pediatrics.
In 2011, researchers published the American Academy of Pediatrics simple febrile seizure guideline, which recommends limiting lumbar puncture to non–low-risk patients. The guidelines also specified that neuroimaging and hematologic testing are not routinely recommended.
Dr. Raghavan and coauthors studied evaluation and management trends of the patients before and after the guidelines. They identified 142,121 children diagnosed with SFS who presented to 1 of 49 pediatric tertiary EDs and met other study criteria. Changes in management of SFS had started years before the guideline and positive effects continued after the guideline publication.
Researchers found a significant 95% decline in rates of lumbar puncture between 2005 and 2019 from 11.6% (95% confidence interval, 10.8%-12.4%) of children in 2005 to 0.6% (95% CI, 0.5%-0.8%; P < .001) in 2019. The most significant declines were among infants 6 months to 1 year.
“We found similar declines in rates of diagnostic laboratory and radiologic testing, intravenous antibiotic administration, hospitalization, and costs,” the authors wrote.
“Importantly,” they wrote, “the decrease in testing was not associated with a concurrent increase in delayed diagnoses of bacterial meningitis.”
The number of hospital admissions and total costs also dropped significantly over the 15-year span of the study. After adjusting for inflation, the authors wrote, costs dropped from an average $1,523 in 2005 to $605 (P < .001) in 2019.
Among first-time presentations for SFSs, 19.2% (95% CI, 18.3%-20.2%) resulted in admission in 2005. That rate dropped to 5.2% (95% CI, 4.8%-5.6%) in 2019 (P < .001), although the authors noted that trend largely plateaued after the guideline was published.
“Our findings are consistent with smaller studies published before 2011 in which researchers found declining rates of LP [lumbar puncture] in children presenting to the ED with their first SFS,” the authors wrote.
Mercedes Blackstone, MD, an emergency physician at the Children’s Hospital of Philadelphia, said in an interview that the paper offers reassurance for changed practice over the last decade.
She said there was substantial relief in pediatrics when the 2011 guidelines recognized formally that protocols were outdated, especially as bacterial meningitis had become increasingly rare with widespread use of pneumococcal and Haemophilus influenzae vaccines. Practitioners had already started to limit the spinal taps on their own.
“We were not really complying with the prior recommendation to do a spinal tap in all those children because it often felt like doing a pretty invasive procedure with a very low yield in what was often a very well child in front of you,” she said.
In 2007, the authors noted, a few years before the guidelines, rates of bacterial meningitis had decreased to 7 per 100,000 in children aged between 2 and 23 months and 0.56 per 100,000 in children aged between 2 and 10 years.
However, Dr. Blackstone said, there was still a worry among some practitioners that there could be missed cases of bacterial meningitis.
“It’s very helpful to see that in all those years, the guidelines have been very validated and there were really no missed cases,” said Dr. Blackstone, author of CHOP’s febrile seizures clinical pathway.
It was good to see the number of CT scans drop as well, she said. Dr. Raghavan’s team found they decreased from 10.6% to 1.6%; P < .001, over the study period.
“Earlier work had shown that there was still a fair amount of head CTs happening and that’s radiation to the young brain,” Dr. Blackstone noted. “This is great news.”
Dr. Blackstone said it was great to see so many children from so many children’s hospitals included in the study.
The paper confirmed that “we’ve reduced a lot of unnecessary testing, saved a lot of cost, and had no increased risk to the patients,” she said.
Dr. Blackstone pointed out that the authors include a limitation that many children are seen in nonpediatric centers in community adult ED and she said those settings tend to have more testing.
“Hopefully, these guidelines have penetrated into the whole community,” she said. “With this paper they should feel reassured that they can spare children some of these tests and procedures.”
Dr. Raghavan and Dr. Blackstone declared no relevant financial relationships.
FROM PEDIATRICS
Two diets linked to improved cognition, fatigue in MS
A Paleolithic elimination diet (Wahls diet) or a low-saturated fat diet (Swank diet) are associated with improved cognition, among other clinical outcomes, in relapsing-remitting multiple sclerosis (RRMS), new research suggests.
In a randomized study of patients with RRMS, the group that followed a Wahls diet and the group that followed a Swank diet both showed significant, unique improvement in measures of cognitive dysfunction, fatigue, and quality of life.
“Several dietary intervention studies have demonstrated favorable results on MS-related fatigue and quality of life. However, these results are among the first to show favorable reductions in cognitive dysfunction,” said co-investigator Tyler Titcomb, PhD, department of internal medicine, University of Iowa, Iowa City.
The results were presented at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
Similar diets
The CMSC findings came from a secondary analysis of a randomized trial published online in July in the Multiple Sclerosis Journal Experimental, Translational, and Clinical (MSJ-ETC).
The primary analysis of the single-blind, parallel group, randomized trial showed the Wahls and Swank diets were linked to significant improvement in outcomes on the Fatigue Severity Scale (FSS), the Modified Fatigue Impact Scale (MFIS), and other measures among participants with RRMS. There were no significant differences between the two dietary regimens.
The Swank diet restricts saturated fat to a maximum of 15 g per day while providing 20 g to 50 g (4 to 10 teaspoons) of unsaturated fat per day, with four servings each of whole grains, fruits, and vegetables.
The Wahls diet recommends six to nine servings of fruits and vegetables per day, in addition to 6 to 12 ounces of meat per day, according to gender. Grains, legumes, eggs, and dairy, with the exception of clarified butter or ghee, are not permitted on this diet. Both diets eschew processed foods.
To further evaluate the diets’ effects on perceived fatigue and cognitive dysfunction, the researchers returned to the trial, which enrolled 95 adults with stable RRMS at the University of Iowa Prevention Intervention Center between August 2016 and May 2019.
After a 12-week run-in period with support and education from registered dietitians, participants were randomly assigned to either the Swank or Wahls diets in a 24-week intervention that did not include dietitian support.
Inclusion criteria included having moderate to severe fatigue, as shown by an FSS score of at least 4.0, while not having severe mental impairment, an eating disorder, or liver or kidney disease. There were no significant differences in baseline demographic or clinical characteristics between the groups.
Of the patients, 77 completed the 12-week run-in (38 in the Swank diet group and 39 in the Wahls group). A total of 72 participants completed the 24-week follow-up (37 and 35, respectively).
Reduction in fatigue, cognitive dysfunction
After the researchers controlled for smoking, alcohol consumption, age, sex, baseline distance 6-minute walk test, body mass index, serum vitamin D, and years with MS, results at 12 and 24 weeks showed significant improvements from baseline in the key outcomes of fatigue and cognitive function, as measured by the Fatigue Scale for Motor and Cognitive Function (FSMC).
Scores were −5.7 and −9.0, respectively, for the Swank diet group and −9.3 and −14.9 for the Wahls group (P ≤ .001 for all comparisons).
In addition, there was a significant reduction in both groups on the total Perceived Deficits Questionnaire (PDQ) at 12 and 24 weeks (Swank, −7.4 and −6.3, respectively; Wahls, −6.8 and −10.8; P ≤ .001 for all).
There were similar improvements for both diets in an analysis of the mental and physical scores on FSMC and on the subscales on PDQ of attention, retrospective memory, prospective memory, and planning.
As observed in the primary analysis, there were no significant differences between the two groups in absolute mean scores on FSMC, PDQ, or their subscales at any timepoint.
“Both diets led to significant reductions in fatigue and cognitive dysfunction,” Dr. Titcomb said.
Of note, the primary analysis further showed statistically and clinically significant increases in the 6-minute walk test at 24-weeks of 6% in the Wahls group (P = .007). After removal of nonadherent participants, the improvement was still significant at 24 weeks in the Wahls group (P = .02), as well as in the Swank group (P = .001).
Dr. Titcomb noted that the majority of study participants were taking disease-modifying therapies (DMTs). However, there were no interactions between any specific DMTs and dietary benefits.
Potential mechanisms
Although the similar outcomes between the diets point to a common mechanism, there are also various other possibilities, said Dr. Titcomb. These include modulation of the microbiome, inflammation, immune system, or micronutrient optimization, he said.
Previous research has shown reduced mass and diversity in the gut microbiota among patients with MS compared with those without MS, potentially promoting inflammation. Other research has shown improvements in those factors with dietary modification.
While there is no evidence of gut microbiota changes with the Wahls and Swank diets, each is rich in fiber and plant-derived phytochemicals, which are known to be associated with improvements in gut microbiota and neuroinflammation, the investigators noted.
Dr. Titcomb reported that research into the diets is continuing as they evaluate longer-term and other effects. “This trial was a short-term parallel arm trial that did not include MRI or a control group,” he said, adding that the investigators will soon start recruiting for a follow-up study that will include a control group, long-term follow-up, and MRIs.
That upcoming study “has the potential to answer several of the unknown questions regarding the effect of diet on MS,” Dr. Titcomb said.
Notable research with limitations
Commenting on the study, Rebecca Spain, MD, MSPH, associate professor of neurology at the Oregon Health & Science University, and associate director of clinical care at VA MS Center of Excellence West in Portland, said there were several notable findings.
This includes that most people with MS “were able to adhere to the protocols for significant lengths of time, even without the support of dietitians for the final 12 weeks of the study,” said Spain, who was not involved with the research.
A significant limitation was the lack of a control group. Without that, “it’s hard to know for sure if the improvements in fatigue and cognition were from the diets or were simply from the social support of participating in a research study,” she said
Nevertheless, trials reporting on dietary effects in MS such as the current study are important, Dr. Spain noted. They demonstrate “that it is feasible and safe to conduct dietary studies and suggest which key MS symptoms may benefit and should be evaluated in future studies.”
“Critically, diet studies address one of the most frequent concerns of people with MS, promoting self-management and empowerment,” Dr. Spain concluded.
General guidelines for common dietary elements with evidence of improving fatigue, cognition, and mood are available on the National MS Society’s website.
The study received no outside funding. Dr. Titcomb and Dr. Spain have disclosed no relevant financial relationships. Terry L. Wahls, MD, who developed the Wahls diet, was a senior author of the study.
A version of this article first appeared on Medscape.com.
A Paleolithic elimination diet (Wahls diet) or a low-saturated fat diet (Swank diet) are associated with improved cognition, among other clinical outcomes, in relapsing-remitting multiple sclerosis (RRMS), new research suggests.
In a randomized study of patients with RRMS, the group that followed a Wahls diet and the group that followed a Swank diet both showed significant, unique improvement in measures of cognitive dysfunction, fatigue, and quality of life.
“Several dietary intervention studies have demonstrated favorable results on MS-related fatigue and quality of life. However, these results are among the first to show favorable reductions in cognitive dysfunction,” said co-investigator Tyler Titcomb, PhD, department of internal medicine, University of Iowa, Iowa City.
The results were presented at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
Similar diets
The CMSC findings came from a secondary analysis of a randomized trial published online in July in the Multiple Sclerosis Journal Experimental, Translational, and Clinical (MSJ-ETC).
The primary analysis of the single-blind, parallel group, randomized trial showed the Wahls and Swank diets were linked to significant improvement in outcomes on the Fatigue Severity Scale (FSS), the Modified Fatigue Impact Scale (MFIS), and other measures among participants with RRMS. There were no significant differences between the two dietary regimens.
The Swank diet restricts saturated fat to a maximum of 15 g per day while providing 20 g to 50 g (4 to 10 teaspoons) of unsaturated fat per day, with four servings each of whole grains, fruits, and vegetables.
The Wahls diet recommends six to nine servings of fruits and vegetables per day, in addition to 6 to 12 ounces of meat per day, according to gender. Grains, legumes, eggs, and dairy, with the exception of clarified butter or ghee, are not permitted on this diet. Both diets eschew processed foods.
To further evaluate the diets’ effects on perceived fatigue and cognitive dysfunction, the researchers returned to the trial, which enrolled 95 adults with stable RRMS at the University of Iowa Prevention Intervention Center between August 2016 and May 2019.
After a 12-week run-in period with support and education from registered dietitians, participants were randomly assigned to either the Swank or Wahls diets in a 24-week intervention that did not include dietitian support.
Inclusion criteria included having moderate to severe fatigue, as shown by an FSS score of at least 4.0, while not having severe mental impairment, an eating disorder, or liver or kidney disease. There were no significant differences in baseline demographic or clinical characteristics between the groups.
Of the patients, 77 completed the 12-week run-in (38 in the Swank diet group and 39 in the Wahls group). A total of 72 participants completed the 24-week follow-up (37 and 35, respectively).
Reduction in fatigue, cognitive dysfunction
After the researchers controlled for smoking, alcohol consumption, age, sex, baseline distance 6-minute walk test, body mass index, serum vitamin D, and years with MS, results at 12 and 24 weeks showed significant improvements from baseline in the key outcomes of fatigue and cognitive function, as measured by the Fatigue Scale for Motor and Cognitive Function (FSMC).
Scores were −5.7 and −9.0, respectively, for the Swank diet group and −9.3 and −14.9 for the Wahls group (P ≤ .001 for all comparisons).
In addition, there was a significant reduction in both groups on the total Perceived Deficits Questionnaire (PDQ) at 12 and 24 weeks (Swank, −7.4 and −6.3, respectively; Wahls, −6.8 and −10.8; P ≤ .001 for all).
There were similar improvements for both diets in an analysis of the mental and physical scores on FSMC and on the subscales on PDQ of attention, retrospective memory, prospective memory, and planning.
As observed in the primary analysis, there were no significant differences between the two groups in absolute mean scores on FSMC, PDQ, or their subscales at any timepoint.
“Both diets led to significant reductions in fatigue and cognitive dysfunction,” Dr. Titcomb said.
Of note, the primary analysis further showed statistically and clinically significant increases in the 6-minute walk test at 24-weeks of 6% in the Wahls group (P = .007). After removal of nonadherent participants, the improvement was still significant at 24 weeks in the Wahls group (P = .02), as well as in the Swank group (P = .001).
Dr. Titcomb noted that the majority of study participants were taking disease-modifying therapies (DMTs). However, there were no interactions between any specific DMTs and dietary benefits.
Potential mechanisms
Although the similar outcomes between the diets point to a common mechanism, there are also various other possibilities, said Dr. Titcomb. These include modulation of the microbiome, inflammation, immune system, or micronutrient optimization, he said.
Previous research has shown reduced mass and diversity in the gut microbiota among patients with MS compared with those without MS, potentially promoting inflammation. Other research has shown improvements in those factors with dietary modification.
While there is no evidence of gut microbiota changes with the Wahls and Swank diets, each is rich in fiber and plant-derived phytochemicals, which are known to be associated with improvements in gut microbiota and neuroinflammation, the investigators noted.
Dr. Titcomb reported that research into the diets is continuing as they evaluate longer-term and other effects. “This trial was a short-term parallel arm trial that did not include MRI or a control group,” he said, adding that the investigators will soon start recruiting for a follow-up study that will include a control group, long-term follow-up, and MRIs.
That upcoming study “has the potential to answer several of the unknown questions regarding the effect of diet on MS,” Dr. Titcomb said.
Notable research with limitations
Commenting on the study, Rebecca Spain, MD, MSPH, associate professor of neurology at the Oregon Health & Science University, and associate director of clinical care at VA MS Center of Excellence West in Portland, said there were several notable findings.
This includes that most people with MS “were able to adhere to the protocols for significant lengths of time, even without the support of dietitians for the final 12 weeks of the study,” said Spain, who was not involved with the research.
A significant limitation was the lack of a control group. Without that, “it’s hard to know for sure if the improvements in fatigue and cognition were from the diets or were simply from the social support of participating in a research study,” she said
Nevertheless, trials reporting on dietary effects in MS such as the current study are important, Dr. Spain noted. They demonstrate “that it is feasible and safe to conduct dietary studies and suggest which key MS symptoms may benefit and should be evaluated in future studies.”
“Critically, diet studies address one of the most frequent concerns of people with MS, promoting self-management and empowerment,” Dr. Spain concluded.
General guidelines for common dietary elements with evidence of improving fatigue, cognition, and mood are available on the National MS Society’s website.
The study received no outside funding. Dr. Titcomb and Dr. Spain have disclosed no relevant financial relationships. Terry L. Wahls, MD, who developed the Wahls diet, was a senior author of the study.
A version of this article first appeared on Medscape.com.
A Paleolithic elimination diet (Wahls diet) or a low-saturated fat diet (Swank diet) are associated with improved cognition, among other clinical outcomes, in relapsing-remitting multiple sclerosis (RRMS), new research suggests.
In a randomized study of patients with RRMS, the group that followed a Wahls diet and the group that followed a Swank diet both showed significant, unique improvement in measures of cognitive dysfunction, fatigue, and quality of life.
“Several dietary intervention studies have demonstrated favorable results on MS-related fatigue and quality of life. However, these results are among the first to show favorable reductions in cognitive dysfunction,” said co-investigator Tyler Titcomb, PhD, department of internal medicine, University of Iowa, Iowa City.
The results were presented at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
Similar diets
The CMSC findings came from a secondary analysis of a randomized trial published online in July in the Multiple Sclerosis Journal Experimental, Translational, and Clinical (MSJ-ETC).
The primary analysis of the single-blind, parallel group, randomized trial showed the Wahls and Swank diets were linked to significant improvement in outcomes on the Fatigue Severity Scale (FSS), the Modified Fatigue Impact Scale (MFIS), and other measures among participants with RRMS. There were no significant differences between the two dietary regimens.
The Swank diet restricts saturated fat to a maximum of 15 g per day while providing 20 g to 50 g (4 to 10 teaspoons) of unsaturated fat per day, with four servings each of whole grains, fruits, and vegetables.
The Wahls diet recommends six to nine servings of fruits and vegetables per day, in addition to 6 to 12 ounces of meat per day, according to gender. Grains, legumes, eggs, and dairy, with the exception of clarified butter or ghee, are not permitted on this diet. Both diets eschew processed foods.
To further evaluate the diets’ effects on perceived fatigue and cognitive dysfunction, the researchers returned to the trial, which enrolled 95 adults with stable RRMS at the University of Iowa Prevention Intervention Center between August 2016 and May 2019.
After a 12-week run-in period with support and education from registered dietitians, participants were randomly assigned to either the Swank or Wahls diets in a 24-week intervention that did not include dietitian support.
Inclusion criteria included having moderate to severe fatigue, as shown by an FSS score of at least 4.0, while not having severe mental impairment, an eating disorder, or liver or kidney disease. There were no significant differences in baseline demographic or clinical characteristics between the groups.
Of the patients, 77 completed the 12-week run-in (38 in the Swank diet group and 39 in the Wahls group). A total of 72 participants completed the 24-week follow-up (37 and 35, respectively).
Reduction in fatigue, cognitive dysfunction
After the researchers controlled for smoking, alcohol consumption, age, sex, baseline distance 6-minute walk test, body mass index, serum vitamin D, and years with MS, results at 12 and 24 weeks showed significant improvements from baseline in the key outcomes of fatigue and cognitive function, as measured by the Fatigue Scale for Motor and Cognitive Function (FSMC).
Scores were −5.7 and −9.0, respectively, for the Swank diet group and −9.3 and −14.9 for the Wahls group (P ≤ .001 for all comparisons).
In addition, there was a significant reduction in both groups on the total Perceived Deficits Questionnaire (PDQ) at 12 and 24 weeks (Swank, −7.4 and −6.3, respectively; Wahls, −6.8 and −10.8; P ≤ .001 for all).
There were similar improvements for both diets in an analysis of the mental and physical scores on FSMC and on the subscales on PDQ of attention, retrospective memory, prospective memory, and planning.
As observed in the primary analysis, there were no significant differences between the two groups in absolute mean scores on FSMC, PDQ, or their subscales at any timepoint.
“Both diets led to significant reductions in fatigue and cognitive dysfunction,” Dr. Titcomb said.
Of note, the primary analysis further showed statistically and clinically significant increases in the 6-minute walk test at 24-weeks of 6% in the Wahls group (P = .007). After removal of nonadherent participants, the improvement was still significant at 24 weeks in the Wahls group (P = .02), as well as in the Swank group (P = .001).
Dr. Titcomb noted that the majority of study participants were taking disease-modifying therapies (DMTs). However, there were no interactions between any specific DMTs and dietary benefits.
Potential mechanisms
Although the similar outcomes between the diets point to a common mechanism, there are also various other possibilities, said Dr. Titcomb. These include modulation of the microbiome, inflammation, immune system, or micronutrient optimization, he said.
Previous research has shown reduced mass and diversity in the gut microbiota among patients with MS compared with those without MS, potentially promoting inflammation. Other research has shown improvements in those factors with dietary modification.
While there is no evidence of gut microbiota changes with the Wahls and Swank diets, each is rich in fiber and plant-derived phytochemicals, which are known to be associated with improvements in gut microbiota and neuroinflammation, the investigators noted.
Dr. Titcomb reported that research into the diets is continuing as they evaluate longer-term and other effects. “This trial was a short-term parallel arm trial that did not include MRI or a control group,” he said, adding that the investigators will soon start recruiting for a follow-up study that will include a control group, long-term follow-up, and MRIs.
That upcoming study “has the potential to answer several of the unknown questions regarding the effect of diet on MS,” Dr. Titcomb said.
Notable research with limitations
Commenting on the study, Rebecca Spain, MD, MSPH, associate professor of neurology at the Oregon Health & Science University, and associate director of clinical care at VA MS Center of Excellence West in Portland, said there were several notable findings.
This includes that most people with MS “were able to adhere to the protocols for significant lengths of time, even without the support of dietitians for the final 12 weeks of the study,” said Spain, who was not involved with the research.
A significant limitation was the lack of a control group. Without that, “it’s hard to know for sure if the improvements in fatigue and cognition were from the diets or were simply from the social support of participating in a research study,” she said
Nevertheless, trials reporting on dietary effects in MS such as the current study are important, Dr. Spain noted. They demonstrate “that it is feasible and safe to conduct dietary studies and suggest which key MS symptoms may benefit and should be evaluated in future studies.”
“Critically, diet studies address one of the most frequent concerns of people with MS, promoting self-management and empowerment,” Dr. Spain concluded.
General guidelines for common dietary elements with evidence of improving fatigue, cognition, and mood are available on the National MS Society’s website.
The study received no outside funding. Dr. Titcomb and Dr. Spain have disclosed no relevant financial relationships. Terry L. Wahls, MD, who developed the Wahls diet, was a senior author of the study.
A version of this article first appeared on Medscape.com.
FROM CMSC 2021
MS and COVID: Docs switched DMTs but maybe didn’t need to
These are the messages of a pair of new studies that examine the impact of the pandemic on the treatment of MS.
One report finds that 80% of specialists surveyed in the summer of 2020 said the pandemic may have changed how they prescribe disease-modifying therapies (DMTs). However, the other report finds no evidence that choice of DMT affects risk of COVID-19 infection. Both studies were presented at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
For the survey, researchers led by neurologist Elizabeth H. Morrison-Banks, MD, of the University of California, Riverside, sent questions to 188 clinicians who serve on regional National Multiple Sclerosis Society Healthcare Provider Councils. A total of 86 people responded: 45 physicians, 18 rehabilitation therapists, 7 psychologists, 8 advanced practice clinicians, 4 social workers, 2 nurses, a pharmacist, and a researcher.
The results, which were published earlier in 2021 in Multiple Sclerosis and Related Disorders, revealed that the survey participants were prescribing certain DMTs more often: beta-interferons (prescribed more by 28.6% of prescribers), natalizumab (23.8%), and glatiramer acetate (21.4%). Those prescribed less included alemtuzumab (64.2% prescribed it less), cladribine (52.4%), and B cell–depleting agents including ocrelizumab and rituximab (50%). Some specialists suspended drugs entirely (21.4% for alemtuzumab, 16.7% for B cell–depleting agents) or extending dosing intervals (38.1% for natalizumab, 11.9% for fingolimod and siponimod).
“We suspect that some of the lower-efficacy therapies were prescribed more often because these therapies were much less immunosuppressive, and because they did not require in-person visits that would increase risk of viral exposure from infusion center staff, or from other infusion patients,” Dr. Morrison-Banks said in an interview. “We also suspect that some of our survey respondents may have increased the dosing intervals for higher-efficacy therapies such as B cell–modulating agents – or even avoided these therapies altogether – because they were concerned that immunosuppressive agents might trigger severe complications from COVID-19.”
As she noted, “in retrospect, at least some of the concerns expressed in our survey may not have been entirely warranted, but then again, we all knew even less then about COVID-19.”
Indeed, researchers led by neurologist Tyler E. Smith, MD, of New York University Langone Multiple Sclerosis Care Center are reporting that they couldn’t find any link between the following DMTs and higher rates of COVID-19 at the New York City center: rituximab, ocrelizumab, fumerate (dimethyl fumarate, monomethyl fumarate, diroximel fumarate), sphingosine-1-phosphate modulators (fingolimod, siponimod), and natalizumab.
The researchers tracked 1,439 patients with MS who were taking the DMTs from March 2020 to March 2021. Of those, 16.0% were infected with COVID-19 (75% lab confirmed), 6.5% were hospitalized, and 0.9% died.
“We did not find an association between the choice of disease-modifying therapy and developing COVID-19 infection, nor having increased disease severity,” Dr. Smith said in an interview. “We are still analyzing data and hope to publish an updated analysis, but at this point, we don’t have conclusive evidence that DMTs, including anti-CD20 agents, need to be changed to lower the risk of COVID-19.”
Instead, he said, “at this point, we feel our energies should be spent on educating our patients on importance of vaccines and boosters. I don’t think it is necessary to switch DMTs because of COVID-19 concerns. However, this should be reviewed on a case-by-case basis.”
No funding is reported for the survey study, and the authors reported various disclosures. The DMT study was funded by an investigator-initiated grant from the Consortium of Multiple Sclerosis Centers, and the authors reported various disclosures.
These are the messages of a pair of new studies that examine the impact of the pandemic on the treatment of MS.
One report finds that 80% of specialists surveyed in the summer of 2020 said the pandemic may have changed how they prescribe disease-modifying therapies (DMTs). However, the other report finds no evidence that choice of DMT affects risk of COVID-19 infection. Both studies were presented at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
For the survey, researchers led by neurologist Elizabeth H. Morrison-Banks, MD, of the University of California, Riverside, sent questions to 188 clinicians who serve on regional National Multiple Sclerosis Society Healthcare Provider Councils. A total of 86 people responded: 45 physicians, 18 rehabilitation therapists, 7 psychologists, 8 advanced practice clinicians, 4 social workers, 2 nurses, a pharmacist, and a researcher.
The results, which were published earlier in 2021 in Multiple Sclerosis and Related Disorders, revealed that the survey participants were prescribing certain DMTs more often: beta-interferons (prescribed more by 28.6% of prescribers), natalizumab (23.8%), and glatiramer acetate (21.4%). Those prescribed less included alemtuzumab (64.2% prescribed it less), cladribine (52.4%), and B cell–depleting agents including ocrelizumab and rituximab (50%). Some specialists suspended drugs entirely (21.4% for alemtuzumab, 16.7% for B cell–depleting agents) or extending dosing intervals (38.1% for natalizumab, 11.9% for fingolimod and siponimod).
“We suspect that some of the lower-efficacy therapies were prescribed more often because these therapies were much less immunosuppressive, and because they did not require in-person visits that would increase risk of viral exposure from infusion center staff, or from other infusion patients,” Dr. Morrison-Banks said in an interview. “We also suspect that some of our survey respondents may have increased the dosing intervals for higher-efficacy therapies such as B cell–modulating agents – or even avoided these therapies altogether – because they were concerned that immunosuppressive agents might trigger severe complications from COVID-19.”
As she noted, “in retrospect, at least some of the concerns expressed in our survey may not have been entirely warranted, but then again, we all knew even less then about COVID-19.”
Indeed, researchers led by neurologist Tyler E. Smith, MD, of New York University Langone Multiple Sclerosis Care Center are reporting that they couldn’t find any link between the following DMTs and higher rates of COVID-19 at the New York City center: rituximab, ocrelizumab, fumerate (dimethyl fumarate, monomethyl fumarate, diroximel fumarate), sphingosine-1-phosphate modulators (fingolimod, siponimod), and natalizumab.
The researchers tracked 1,439 patients with MS who were taking the DMTs from March 2020 to March 2021. Of those, 16.0% were infected with COVID-19 (75% lab confirmed), 6.5% were hospitalized, and 0.9% died.
“We did not find an association between the choice of disease-modifying therapy and developing COVID-19 infection, nor having increased disease severity,” Dr. Smith said in an interview. “We are still analyzing data and hope to publish an updated analysis, but at this point, we don’t have conclusive evidence that DMTs, including anti-CD20 agents, need to be changed to lower the risk of COVID-19.”
Instead, he said, “at this point, we feel our energies should be spent on educating our patients on importance of vaccines and boosters. I don’t think it is necessary to switch DMTs because of COVID-19 concerns. However, this should be reviewed on a case-by-case basis.”
No funding is reported for the survey study, and the authors reported various disclosures. The DMT study was funded by an investigator-initiated grant from the Consortium of Multiple Sclerosis Centers, and the authors reported various disclosures.
These are the messages of a pair of new studies that examine the impact of the pandemic on the treatment of MS.
One report finds that 80% of specialists surveyed in the summer of 2020 said the pandemic may have changed how they prescribe disease-modifying therapies (DMTs). However, the other report finds no evidence that choice of DMT affects risk of COVID-19 infection. Both studies were presented at the 2021 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
For the survey, researchers led by neurologist Elizabeth H. Morrison-Banks, MD, of the University of California, Riverside, sent questions to 188 clinicians who serve on regional National Multiple Sclerosis Society Healthcare Provider Councils. A total of 86 people responded: 45 physicians, 18 rehabilitation therapists, 7 psychologists, 8 advanced practice clinicians, 4 social workers, 2 nurses, a pharmacist, and a researcher.
The results, which were published earlier in 2021 in Multiple Sclerosis and Related Disorders, revealed that the survey participants were prescribing certain DMTs more often: beta-interferons (prescribed more by 28.6% of prescribers), natalizumab (23.8%), and glatiramer acetate (21.4%). Those prescribed less included alemtuzumab (64.2% prescribed it less), cladribine (52.4%), and B cell–depleting agents including ocrelizumab and rituximab (50%). Some specialists suspended drugs entirely (21.4% for alemtuzumab, 16.7% for B cell–depleting agents) or extending dosing intervals (38.1% for natalizumab, 11.9% for fingolimod and siponimod).
“We suspect that some of the lower-efficacy therapies were prescribed more often because these therapies were much less immunosuppressive, and because they did not require in-person visits that would increase risk of viral exposure from infusion center staff, or from other infusion patients,” Dr. Morrison-Banks said in an interview. “We also suspect that some of our survey respondents may have increased the dosing intervals for higher-efficacy therapies such as B cell–modulating agents – or even avoided these therapies altogether – because they were concerned that immunosuppressive agents might trigger severe complications from COVID-19.”
As she noted, “in retrospect, at least some of the concerns expressed in our survey may not have been entirely warranted, but then again, we all knew even less then about COVID-19.”
Indeed, researchers led by neurologist Tyler E. Smith, MD, of New York University Langone Multiple Sclerosis Care Center are reporting that they couldn’t find any link between the following DMTs and higher rates of COVID-19 at the New York City center: rituximab, ocrelizumab, fumerate (dimethyl fumarate, monomethyl fumarate, diroximel fumarate), sphingosine-1-phosphate modulators (fingolimod, siponimod), and natalizumab.
The researchers tracked 1,439 patients with MS who were taking the DMTs from March 2020 to March 2021. Of those, 16.0% were infected with COVID-19 (75% lab confirmed), 6.5% were hospitalized, and 0.9% died.
“We did not find an association between the choice of disease-modifying therapy and developing COVID-19 infection, nor having increased disease severity,” Dr. Smith said in an interview. “We are still analyzing data and hope to publish an updated analysis, but at this point, we don’t have conclusive evidence that DMTs, including anti-CD20 agents, need to be changed to lower the risk of COVID-19.”
Instead, he said, “at this point, we feel our energies should be spent on educating our patients on importance of vaccines and boosters. I don’t think it is necessary to switch DMTs because of COVID-19 concerns. However, this should be reviewed on a case-by-case basis.”
No funding is reported for the survey study, and the authors reported various disclosures. The DMT study was funded by an investigator-initiated grant from the Consortium of Multiple Sclerosis Centers, and the authors reported various disclosures.
FROM CMSC 2021





