User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Worsening motor function tied to post COVID syndrome in Parkinson’s disease
, new research suggests.
Results from a small, international retrospective case study show that about half of participants with Parkinson’s disease who developed post–COVID-19 syndrome experienced a worsening of motor symptoms and that their need for anti-Parkinson’s medication increased.
“In our series of 27 patients with Parkinson’s disease, 85% developed post–COVID-19 symptoms,” said lead investigator Valentina Leta, MD, Parkinson’s Foundation Center of Excellence, Kings College Hospital, London.
The most common long-term effects were worsening of motor function and an increase in the need for daily levodopa. Other adverse effects included fatigue; cognitive disturbances, including brain fog, loss of concentration, and memory deficits; and sleep disturbances, such as insomnia, Dr. Leta said.
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
Long-term sequelae
Previous studies have documented worsening of motor and nonmotor symptoms among patients with Parkinson’s disease in the acute phase of COVID-19. Results of these studies suggest that mortality may be higher among patients with more advanced Parkinson’s disease, comorbidities, and frailty.
Dr. Leta noted that long-term sequelae with so-called long COVID have not been adequately explored, prompting the current study.
The case series included 27 patients with Parkinson’s disease in the United Kingdom, Italy, Romania, and Mexico who were also affected by COVID-19. The investigators defined post–COVID-19 syndrome as “signs and symptoms that develop during or after an infection consistent with COVID-19, continue for more than 12 weeks, and are not explained by an alternative diagnosis.”
Because some of the symptoms are also associated with Parkinson’s disease, symptoms were attributed to post–COVID-19 only if they occurred after a confirmed severe acute respiratory infection with SARS-CoV-2 or if patients experienced an acute or subacute worsening of a pre-existing symptom that had previously been stable.
Among the participants, 59.3% were men. The mean age at the time of Parkinson’s disease diagnosis was 59.0 ± 12.7 years, and the mean Parkinson’s disease duration was 9.2 ± 7.8 years. The patients were in Hoehn and Yahr stage 2.0 ± 1.0 at the time of their COVID-19 diagnosis.
Charlson Comorbidity Index score at COVID-19 diagnosis was 2.0 ± 1.5, and the levodopa equivalent daily dose (LEDD) was 1053.5 ± 842.4 mg.
Symptom worsening
“Cognitive disturbances” were defined as brain fog, concentration difficulty, or memory problems. “Peripheral neuropathy symptoms” were defined as having feelings of pins and needles or numbness.
By far, the most prevalent sequelae were worsening motor symptoms and increased need for anti-Parkinson’s medications. Each affected about half of the study cohort, the investigators noted.
Dr. Leta added the non-Parkinson’s disease-specific findings are in line with the existing literature on long COVID in the general population. The severity of COVID-19, as indicated by a history of hospitalization, did not seem to correlate with development of post–COVID-19 syndrome in patients with Parkinson’s disease.
In this series, few patients had respiratory, cardiovascular, gastrointestinal, musculoskeletal, or dermatologic symptoms. Interestingly, only four patients reported a loss of taste or smell.
The investigators noted that in addition to viral illness, the stress of prolonged lockdown during the pandemic and reduced access to health care and rehabilitation programs may contribute to the burden of post–COVID-19 syndrome in patients with Parkinson’s disease.
Study limitations cited include the relatively small sample size and the lack of a control group. The researchers noted the need for larger studies to elucidate the natural history of COVID-19 among patients with Parkinson’s disease in order to raise awareness of their needs and to help develop personalized management strategies.
Meaningful addition
Commenting on the findings, Kyle Mitchell, MD, movement disorders neurologist, Duke University, Durham, N.C., said he found the study to be a meaningful addition in light of the fact that data on the challenges that patients with Parkinson’s disease may face after having COVID-19 are limited.
“What I liked about this study was there’s data from multiple countries, what looks like a diverse population of study participants, and really just addressing a question that we get asked a lot in clinic and we see a fair amount, but we don’t really know a lot about: how people with Parkinson’s disease will do during and post COVID-19 infection,” said Dr. Mitchell, who was not involved with the research.
He said the worsening of motor symptoms and the need for increased dopaminergic medication brought some questions to mind.
“Is this increase in medications permanent, or is it temporary until post-COVID resolves? Or is it truly something where they stay on a higher dose?” he asked.
Dr. Mitchell said he does not believe the worsening of symptoms is specific to COVID-19 and that he sees individuals with Parkinson’s disease who experience setbacks “from any number of infections.” These include urinary tract infections and influenza, which are associated with worsening mobility, rigidity, tremor, fatigue, and cognition.
“People with Parkinson’s disease seem to get hit harder by infections in general,” he said.
The study had no outside funding. Dr. Leta and Dr. Mitchell have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
Results from a small, international retrospective case study show that about half of participants with Parkinson’s disease who developed post–COVID-19 syndrome experienced a worsening of motor symptoms and that their need for anti-Parkinson’s medication increased.
“In our series of 27 patients with Parkinson’s disease, 85% developed post–COVID-19 symptoms,” said lead investigator Valentina Leta, MD, Parkinson’s Foundation Center of Excellence, Kings College Hospital, London.
The most common long-term effects were worsening of motor function and an increase in the need for daily levodopa. Other adverse effects included fatigue; cognitive disturbances, including brain fog, loss of concentration, and memory deficits; and sleep disturbances, such as insomnia, Dr. Leta said.
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
Long-term sequelae
Previous studies have documented worsening of motor and nonmotor symptoms among patients with Parkinson’s disease in the acute phase of COVID-19. Results of these studies suggest that mortality may be higher among patients with more advanced Parkinson’s disease, comorbidities, and frailty.
Dr. Leta noted that long-term sequelae with so-called long COVID have not been adequately explored, prompting the current study.
The case series included 27 patients with Parkinson’s disease in the United Kingdom, Italy, Romania, and Mexico who were also affected by COVID-19. The investigators defined post–COVID-19 syndrome as “signs and symptoms that develop during or after an infection consistent with COVID-19, continue for more than 12 weeks, and are not explained by an alternative diagnosis.”
Because some of the symptoms are also associated with Parkinson’s disease, symptoms were attributed to post–COVID-19 only if they occurred after a confirmed severe acute respiratory infection with SARS-CoV-2 or if patients experienced an acute or subacute worsening of a pre-existing symptom that had previously been stable.
Among the participants, 59.3% were men. The mean age at the time of Parkinson’s disease diagnosis was 59.0 ± 12.7 years, and the mean Parkinson’s disease duration was 9.2 ± 7.8 years. The patients were in Hoehn and Yahr stage 2.0 ± 1.0 at the time of their COVID-19 diagnosis.
Charlson Comorbidity Index score at COVID-19 diagnosis was 2.0 ± 1.5, and the levodopa equivalent daily dose (LEDD) was 1053.5 ± 842.4 mg.
Symptom worsening
“Cognitive disturbances” were defined as brain fog, concentration difficulty, or memory problems. “Peripheral neuropathy symptoms” were defined as having feelings of pins and needles or numbness.
By far, the most prevalent sequelae were worsening motor symptoms and increased need for anti-Parkinson’s medications. Each affected about half of the study cohort, the investigators noted.
Dr. Leta added the non-Parkinson’s disease-specific findings are in line with the existing literature on long COVID in the general population. The severity of COVID-19, as indicated by a history of hospitalization, did not seem to correlate with development of post–COVID-19 syndrome in patients with Parkinson’s disease.
In this series, few patients had respiratory, cardiovascular, gastrointestinal, musculoskeletal, or dermatologic symptoms. Interestingly, only four patients reported a loss of taste or smell.
The investigators noted that in addition to viral illness, the stress of prolonged lockdown during the pandemic and reduced access to health care and rehabilitation programs may contribute to the burden of post–COVID-19 syndrome in patients with Parkinson’s disease.
Study limitations cited include the relatively small sample size and the lack of a control group. The researchers noted the need for larger studies to elucidate the natural history of COVID-19 among patients with Parkinson’s disease in order to raise awareness of their needs and to help develop personalized management strategies.
Meaningful addition
Commenting on the findings, Kyle Mitchell, MD, movement disorders neurologist, Duke University, Durham, N.C., said he found the study to be a meaningful addition in light of the fact that data on the challenges that patients with Parkinson’s disease may face after having COVID-19 are limited.
“What I liked about this study was there’s data from multiple countries, what looks like a diverse population of study participants, and really just addressing a question that we get asked a lot in clinic and we see a fair amount, but we don’t really know a lot about: how people with Parkinson’s disease will do during and post COVID-19 infection,” said Dr. Mitchell, who was not involved with the research.
He said the worsening of motor symptoms and the need for increased dopaminergic medication brought some questions to mind.
“Is this increase in medications permanent, or is it temporary until post-COVID resolves? Or is it truly something where they stay on a higher dose?” he asked.
Dr. Mitchell said he does not believe the worsening of symptoms is specific to COVID-19 and that he sees individuals with Parkinson’s disease who experience setbacks “from any number of infections.” These include urinary tract infections and influenza, which are associated with worsening mobility, rigidity, tremor, fatigue, and cognition.
“People with Parkinson’s disease seem to get hit harder by infections in general,” he said.
The study had no outside funding. Dr. Leta and Dr. Mitchell have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
Results from a small, international retrospective case study show that about half of participants with Parkinson’s disease who developed post–COVID-19 syndrome experienced a worsening of motor symptoms and that their need for anti-Parkinson’s medication increased.
“In our series of 27 patients with Parkinson’s disease, 85% developed post–COVID-19 symptoms,” said lead investigator Valentina Leta, MD, Parkinson’s Foundation Center of Excellence, Kings College Hospital, London.
The most common long-term effects were worsening of motor function and an increase in the need for daily levodopa. Other adverse effects included fatigue; cognitive disturbances, including brain fog, loss of concentration, and memory deficits; and sleep disturbances, such as insomnia, Dr. Leta said.
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
Long-term sequelae
Previous studies have documented worsening of motor and nonmotor symptoms among patients with Parkinson’s disease in the acute phase of COVID-19. Results of these studies suggest that mortality may be higher among patients with more advanced Parkinson’s disease, comorbidities, and frailty.
Dr. Leta noted that long-term sequelae with so-called long COVID have not been adequately explored, prompting the current study.
The case series included 27 patients with Parkinson’s disease in the United Kingdom, Italy, Romania, and Mexico who were also affected by COVID-19. The investigators defined post–COVID-19 syndrome as “signs and symptoms that develop during or after an infection consistent with COVID-19, continue for more than 12 weeks, and are not explained by an alternative diagnosis.”
Because some of the symptoms are also associated with Parkinson’s disease, symptoms were attributed to post–COVID-19 only if they occurred after a confirmed severe acute respiratory infection with SARS-CoV-2 or if patients experienced an acute or subacute worsening of a pre-existing symptom that had previously been stable.
Among the participants, 59.3% were men. The mean age at the time of Parkinson’s disease diagnosis was 59.0 ± 12.7 years, and the mean Parkinson’s disease duration was 9.2 ± 7.8 years. The patients were in Hoehn and Yahr stage 2.0 ± 1.0 at the time of their COVID-19 diagnosis.
Charlson Comorbidity Index score at COVID-19 diagnosis was 2.0 ± 1.5, and the levodopa equivalent daily dose (LEDD) was 1053.5 ± 842.4 mg.
Symptom worsening
“Cognitive disturbances” were defined as brain fog, concentration difficulty, or memory problems. “Peripheral neuropathy symptoms” were defined as having feelings of pins and needles or numbness.
By far, the most prevalent sequelae were worsening motor symptoms and increased need for anti-Parkinson’s medications. Each affected about half of the study cohort, the investigators noted.
Dr. Leta added the non-Parkinson’s disease-specific findings are in line with the existing literature on long COVID in the general population. The severity of COVID-19, as indicated by a history of hospitalization, did not seem to correlate with development of post–COVID-19 syndrome in patients with Parkinson’s disease.
In this series, few patients had respiratory, cardiovascular, gastrointestinal, musculoskeletal, or dermatologic symptoms. Interestingly, only four patients reported a loss of taste or smell.
The investigators noted that in addition to viral illness, the stress of prolonged lockdown during the pandemic and reduced access to health care and rehabilitation programs may contribute to the burden of post–COVID-19 syndrome in patients with Parkinson’s disease.
Study limitations cited include the relatively small sample size and the lack of a control group. The researchers noted the need for larger studies to elucidate the natural history of COVID-19 among patients with Parkinson’s disease in order to raise awareness of their needs and to help develop personalized management strategies.
Meaningful addition
Commenting on the findings, Kyle Mitchell, MD, movement disorders neurologist, Duke University, Durham, N.C., said he found the study to be a meaningful addition in light of the fact that data on the challenges that patients with Parkinson’s disease may face after having COVID-19 are limited.
“What I liked about this study was there’s data from multiple countries, what looks like a diverse population of study participants, and really just addressing a question that we get asked a lot in clinic and we see a fair amount, but we don’t really know a lot about: how people with Parkinson’s disease will do during and post COVID-19 infection,” said Dr. Mitchell, who was not involved with the research.
He said the worsening of motor symptoms and the need for increased dopaminergic medication brought some questions to mind.
“Is this increase in medications permanent, or is it temporary until post-COVID resolves? Or is it truly something where they stay on a higher dose?” he asked.
Dr. Mitchell said he does not believe the worsening of symptoms is specific to COVID-19 and that he sees individuals with Parkinson’s disease who experience setbacks “from any number of infections.” These include urinary tract infections and influenza, which are associated with worsening mobility, rigidity, tremor, fatigue, and cognition.
“People with Parkinson’s disease seem to get hit harder by infections in general,” he said.
The study had no outside funding. Dr. Leta and Dr. Mitchell have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM MDS VIRTUAL CONGRESS 2021
Cook your amphibians before you eat them
Novel food for thought
When you were growing up, your parents probably told you to brush your teeth before you went to bed, warned you not to run with the scissors or play with matches, and punished you whenever you used the neighbor children to play Schrödinger’s cat.
They did those things for your own good, of course, and now the nation’s mother – the Centers for Disease Control and Prevention – is doing the same by warning us about novel outbreak–associated foods. As in, “Put down that novel outbreak–associated food! You don’t know where it’s been!”
Seriously, you don’t know where it’s been. CDC investigators identified 28 novel foods that were linked to 36 foodborne-disease outbreaks that occurred during 2007-2016, including moringa leaf (herb/spice), tempeh (grain), frog, sprouted nut butter, and skate.
The novel foods implicated in these outbreaks were more likely to be imported, compared with 14,216 outbreaks that occurred from 1973 to 2016, and about half didn’t require refrigeration. Two-thirds did not need to be cooked after purchase. Another thing your parents wouldn’t like: Some can’t be washed, like sheep milk, sugar cane, or the aforementioned nut butter.
We wanted to get a food expert to comment on these novel foods, but our editor said that the assistant manager of our local Burger King wasn’t expert enough, so we’ve commandeered someone else’s expert. Cynthia Sears, MD, of Johns Hopkins University in Baltimore, told Today.com all about the dangers of frogs: “Essentially all amphibians are contaminated, often with salmonella. Eating any amphibian that is not thoroughly cooked is a risk.”
Be sure to cook your amphibians before you eat them. Advice that your parents would be proud to share.
Dieters should stay away from diet drinks
When a drink is labeled “diet” many assume that the calorie-free beverage is the best choice. However, one of the largest studies to date on artificial sweeteners is out to set the record straight.
Artificial sweeteners, or nonnutritive sweeteners (NNS), are used in most if not all diet products to give the illusion of sweetness without the caloric guilt. Some studies say they help with weight loss for that very reason, but others say they can contribute to weight gain. So which is it?
Researchers at the University of Southern California sought to add some clarity to the research already out there.
They looked at an even-gendered split of 74 participants who drank 300 mL of drinks sweetened with NNS, table sugar, or water. The researchers then used functional MRI to see how parts of the brain responsible for appetite and cravings responded to images of high-calorie foods. They also looked at glucose, insulin, and other metabolic hormone levels, as well as how much food the participants ate at their free buffet. (In the participants’ defense, who can say no to a free buffet?)
The researchers made some interesting observations:
- Women who drank the NNS drink ate more than did the table-sugar group, but all men ate the same.
- Images of those calorie-packed goodies increased cravings and appetite for obese men and women in the NNS group, compared with the table-sugar group.
- For all participants who drank the NNS drink, there was a decrease in the hormone that tells the body it’s full.
“By studying different groups we were able to show that females and people with obesity may be more sensitive to artificial sweeteners. For these groups, drinking artificially sweetened drinks may trick the brain into feeling hungry, which may in turn result in more calories being consumed,” Kathleen Page, MD, the study’s corresponding author, said in a separate statement.
Today’s lesson? Don’t believe every label you read.
Instagram vegetables and the triumph of peer pressure
You and your family are sitting down for dinner. You’ve taken the time to prepare a healthy, nutritious meal. Vegetables, rice, seafood – all the right things. But the children around you refuse to partake. What can you do? Why, show them a highly liked photo of broccoli on Instagram!
In reality, kids will probably never like to eat their vegetables, but according to a study published in Appetite, viewing highly liked images on social media can compel adults to eat theirs.
The investigators recruited a group of 169 adults aged 18-28 (average age, 21) and showed them a series of mock Instagram posts of all sorts of food, everything from Brussels sprouts to chocolate cake, as well as nonfood images to act as a baseline. The images had a varying amount of likes. After viewing the images, study participants were offered a snack buffet consisting of grapes and cookies.
The results were a triumph of peer pressure. Those who viewed highly liked images of nutritious foods ate a significantly larger proportion of grapes, compared with those who saw highly liked images of unhealthy food or nonfood.
The authors cautioned that more research is needed, but they said that they’re onto something in the eternal struggle of getting people to eat better. If Mikey liked it, maybe you should, too. Just as long as you don’t try to encourage the eating of peas. That is a dark road none should take, and no one should ever be subjected to that cursed food.
It’s nice to share … hypertension?
You may have heard that, over time, you begin to resemble your spouse. You may have also heard that, as time goes by, your pet might start to resemble you, but that is a story for another time.
A lot of the time, it’s human nature that people partner with someone who is similar to them in physical and environmental status. If you like to go jogging at 5 a.m., you might want a spouse who does the same. A study done using data from couples in Japan and the Netherlands found that couples who had the same lifestyle had similar levels of blood pressure, cholesterol, and triglycerides. They also had similar illnesses such as hypertension and diabetes.
It’s important to note that many of the couples were not very genetically similar but had similar lifestyles. Encourage your partner to have a healthier lifestyle, so you can live on for many years to come!
Novel food for thought
When you were growing up, your parents probably told you to brush your teeth before you went to bed, warned you not to run with the scissors or play with matches, and punished you whenever you used the neighbor children to play Schrödinger’s cat.
They did those things for your own good, of course, and now the nation’s mother – the Centers for Disease Control and Prevention – is doing the same by warning us about novel outbreak–associated foods. As in, “Put down that novel outbreak–associated food! You don’t know where it’s been!”
Seriously, you don’t know where it’s been. CDC investigators identified 28 novel foods that were linked to 36 foodborne-disease outbreaks that occurred during 2007-2016, including moringa leaf (herb/spice), tempeh (grain), frog, sprouted nut butter, and skate.
The novel foods implicated in these outbreaks were more likely to be imported, compared with 14,216 outbreaks that occurred from 1973 to 2016, and about half didn’t require refrigeration. Two-thirds did not need to be cooked after purchase. Another thing your parents wouldn’t like: Some can’t be washed, like sheep milk, sugar cane, or the aforementioned nut butter.
We wanted to get a food expert to comment on these novel foods, but our editor said that the assistant manager of our local Burger King wasn’t expert enough, so we’ve commandeered someone else’s expert. Cynthia Sears, MD, of Johns Hopkins University in Baltimore, told Today.com all about the dangers of frogs: “Essentially all amphibians are contaminated, often with salmonella. Eating any amphibian that is not thoroughly cooked is a risk.”
Be sure to cook your amphibians before you eat them. Advice that your parents would be proud to share.
Dieters should stay away from diet drinks
When a drink is labeled “diet” many assume that the calorie-free beverage is the best choice. However, one of the largest studies to date on artificial sweeteners is out to set the record straight.
Artificial sweeteners, or nonnutritive sweeteners (NNS), are used in most if not all diet products to give the illusion of sweetness without the caloric guilt. Some studies say they help with weight loss for that very reason, but others say they can contribute to weight gain. So which is it?
Researchers at the University of Southern California sought to add some clarity to the research already out there.
They looked at an even-gendered split of 74 participants who drank 300 mL of drinks sweetened with NNS, table sugar, or water. The researchers then used functional MRI to see how parts of the brain responsible for appetite and cravings responded to images of high-calorie foods. They also looked at glucose, insulin, and other metabolic hormone levels, as well as how much food the participants ate at their free buffet. (In the participants’ defense, who can say no to a free buffet?)
The researchers made some interesting observations:
- Women who drank the NNS drink ate more than did the table-sugar group, but all men ate the same.
- Images of those calorie-packed goodies increased cravings and appetite for obese men and women in the NNS group, compared with the table-sugar group.
- For all participants who drank the NNS drink, there was a decrease in the hormone that tells the body it’s full.
“By studying different groups we were able to show that females and people with obesity may be more sensitive to artificial sweeteners. For these groups, drinking artificially sweetened drinks may trick the brain into feeling hungry, which may in turn result in more calories being consumed,” Kathleen Page, MD, the study’s corresponding author, said in a separate statement.
Today’s lesson? Don’t believe every label you read.
Instagram vegetables and the triumph of peer pressure
You and your family are sitting down for dinner. You’ve taken the time to prepare a healthy, nutritious meal. Vegetables, rice, seafood – all the right things. But the children around you refuse to partake. What can you do? Why, show them a highly liked photo of broccoli on Instagram!
In reality, kids will probably never like to eat their vegetables, but according to a study published in Appetite, viewing highly liked images on social media can compel adults to eat theirs.
The investigators recruited a group of 169 adults aged 18-28 (average age, 21) and showed them a series of mock Instagram posts of all sorts of food, everything from Brussels sprouts to chocolate cake, as well as nonfood images to act as a baseline. The images had a varying amount of likes. After viewing the images, study participants were offered a snack buffet consisting of grapes and cookies.
The results were a triumph of peer pressure. Those who viewed highly liked images of nutritious foods ate a significantly larger proportion of grapes, compared with those who saw highly liked images of unhealthy food or nonfood.
The authors cautioned that more research is needed, but they said that they’re onto something in the eternal struggle of getting people to eat better. If Mikey liked it, maybe you should, too. Just as long as you don’t try to encourage the eating of peas. That is a dark road none should take, and no one should ever be subjected to that cursed food.
It’s nice to share … hypertension?
You may have heard that, over time, you begin to resemble your spouse. You may have also heard that, as time goes by, your pet might start to resemble you, but that is a story for another time.
A lot of the time, it’s human nature that people partner with someone who is similar to them in physical and environmental status. If you like to go jogging at 5 a.m., you might want a spouse who does the same. A study done using data from couples in Japan and the Netherlands found that couples who had the same lifestyle had similar levels of blood pressure, cholesterol, and triglycerides. They also had similar illnesses such as hypertension and diabetes.
It’s important to note that many of the couples were not very genetically similar but had similar lifestyles. Encourage your partner to have a healthier lifestyle, so you can live on for many years to come!
Novel food for thought
When you were growing up, your parents probably told you to brush your teeth before you went to bed, warned you not to run with the scissors or play with matches, and punished you whenever you used the neighbor children to play Schrödinger’s cat.
They did those things for your own good, of course, and now the nation’s mother – the Centers for Disease Control and Prevention – is doing the same by warning us about novel outbreak–associated foods. As in, “Put down that novel outbreak–associated food! You don’t know where it’s been!”
Seriously, you don’t know where it’s been. CDC investigators identified 28 novel foods that were linked to 36 foodborne-disease outbreaks that occurred during 2007-2016, including moringa leaf (herb/spice), tempeh (grain), frog, sprouted nut butter, and skate.
The novel foods implicated in these outbreaks were more likely to be imported, compared with 14,216 outbreaks that occurred from 1973 to 2016, and about half didn’t require refrigeration. Two-thirds did not need to be cooked after purchase. Another thing your parents wouldn’t like: Some can’t be washed, like sheep milk, sugar cane, or the aforementioned nut butter.
We wanted to get a food expert to comment on these novel foods, but our editor said that the assistant manager of our local Burger King wasn’t expert enough, so we’ve commandeered someone else’s expert. Cynthia Sears, MD, of Johns Hopkins University in Baltimore, told Today.com all about the dangers of frogs: “Essentially all amphibians are contaminated, often with salmonella. Eating any amphibian that is not thoroughly cooked is a risk.”
Be sure to cook your amphibians before you eat them. Advice that your parents would be proud to share.
Dieters should stay away from diet drinks
When a drink is labeled “diet” many assume that the calorie-free beverage is the best choice. However, one of the largest studies to date on artificial sweeteners is out to set the record straight.
Artificial sweeteners, or nonnutritive sweeteners (NNS), are used in most if not all diet products to give the illusion of sweetness without the caloric guilt. Some studies say they help with weight loss for that very reason, but others say they can contribute to weight gain. So which is it?
Researchers at the University of Southern California sought to add some clarity to the research already out there.
They looked at an even-gendered split of 74 participants who drank 300 mL of drinks sweetened with NNS, table sugar, or water. The researchers then used functional MRI to see how parts of the brain responsible for appetite and cravings responded to images of high-calorie foods. They also looked at glucose, insulin, and other metabolic hormone levels, as well as how much food the participants ate at their free buffet. (In the participants’ defense, who can say no to a free buffet?)
The researchers made some interesting observations:
- Women who drank the NNS drink ate more than did the table-sugar group, but all men ate the same.
- Images of those calorie-packed goodies increased cravings and appetite for obese men and women in the NNS group, compared with the table-sugar group.
- For all participants who drank the NNS drink, there was a decrease in the hormone that tells the body it’s full.
“By studying different groups we were able to show that females and people with obesity may be more sensitive to artificial sweeteners. For these groups, drinking artificially sweetened drinks may trick the brain into feeling hungry, which may in turn result in more calories being consumed,” Kathleen Page, MD, the study’s corresponding author, said in a separate statement.
Today’s lesson? Don’t believe every label you read.
Instagram vegetables and the triumph of peer pressure
You and your family are sitting down for dinner. You’ve taken the time to prepare a healthy, nutritious meal. Vegetables, rice, seafood – all the right things. But the children around you refuse to partake. What can you do? Why, show them a highly liked photo of broccoli on Instagram!
In reality, kids will probably never like to eat their vegetables, but according to a study published in Appetite, viewing highly liked images on social media can compel adults to eat theirs.
The investigators recruited a group of 169 adults aged 18-28 (average age, 21) and showed them a series of mock Instagram posts of all sorts of food, everything from Brussels sprouts to chocolate cake, as well as nonfood images to act as a baseline. The images had a varying amount of likes. After viewing the images, study participants were offered a snack buffet consisting of grapes and cookies.
The results were a triumph of peer pressure. Those who viewed highly liked images of nutritious foods ate a significantly larger proportion of grapes, compared with those who saw highly liked images of unhealthy food or nonfood.
The authors cautioned that more research is needed, but they said that they’re onto something in the eternal struggle of getting people to eat better. If Mikey liked it, maybe you should, too. Just as long as you don’t try to encourage the eating of peas. That is a dark road none should take, and no one should ever be subjected to that cursed food.
It’s nice to share … hypertension?
You may have heard that, over time, you begin to resemble your spouse. You may have also heard that, as time goes by, your pet might start to resemble you, but that is a story for another time.
A lot of the time, it’s human nature that people partner with someone who is similar to them in physical and environmental status. If you like to go jogging at 5 a.m., you might want a spouse who does the same. A study done using data from couples in Japan and the Netherlands found that couples who had the same lifestyle had similar levels of blood pressure, cholesterol, and triglycerides. They also had similar illnesses such as hypertension and diabetes.
It’s important to note that many of the couples were not very genetically similar but had similar lifestyles. Encourage your partner to have a healthier lifestyle, so you can live on for many years to come!
Migraine history linked to more severe hot flashes in postmenopausal women
Women with a history of migraine are more likely to experience severe or very severe hot flashes than women without migraines, according to research presented Sept. 24 at the hybrid annual meeting of the North American Menopause Society. An estimated one in five women experience migraine, and women tend to have greater migraine symptoms and disability, the authors note in their background information. Since migraines are also linked to a higher risk of cardiovascular disease, the authors sought to learn whether migraines were associated with vasomotor symptoms, another cardiovascular risk factor.
“The question in my mind is, can we do better at predicting cardiovascular risk in women because the risk prediction models that we have really don’t work all that well in women because they were designed for use in men,” Stephanie S. Faubion, MD, MBA, Penny and Bill George Director for Mayo Clinic’s Center for Women’s Health said in an interview. “My ultimate goal is to see if we can somehow use big data, artificial intelligence to figure out how to weight some of these female-specific or female-predominant factors to come up with a better model for cardiovascular risk prediction.”
The researchers analyzed cross-sectional data from 3,308 women who participated in the Data Registry on the Experiences of Aging, Menopause and Sexuality (DREAMS) study through Mayo Clinic sites in Rochester, Minn.; Scottsdale, Ariz.; and Jacksonville, Fla.. The women ranged in age from 45 to 60 years old, with an average age of 53, and the vast majority of them were white (95%) and had at least some college (93%). Most were also in a long-term relationship (85%), and a majority were employed (69%) and postmenopausal (67%).
The data, collected between May 2015 and December 2019, included a self-reported history of migraine and questionnaires that included the Menopause Rating Scale of menopause-related symptoms.
The researchers adjusted their findings to account for body mass index (BMI), menopause status, smoking status, depression, anxiety, current use of hormone therapy, and presence of low back pain within the past year. ”The diagnosis of low back pain, another pain disorder, was used to test the specificity of the association of migraine and vasomotor symptoms,” the authors write.
Just over a quarter of the women (27%) reported a history of migraine, and these women’s Menopause Rating Scale scores were an average 1.36 points greater than women without a history of migraines (P < .001). Women with self-reported migraine were also 40% more likely than women without migraines to report severe or very severe flashes versus reporting no hot flashes at all (odds ratio, 1.4; P = .02).
“The odds of reporting more severe hot flashes increased monotonically in women with a history of migraine,” the authors report. “In addition, women with low back pain had higher Menopause Rating Scale scores, but were no more likely to have severe/very severe hot flashes than those without back pain, confirming the specificity of the link between vasomotor symptoms and migraine.”
It’s not clear if migraine or hot flashes are risk factors that add to a woman’s existing cardiovascular risk profile or whether they are simply biomarkers of a shared pathway, Dr Faubion said in an interview. She speculates that the common link between migraine and vasomotor symptoms could be neurovascular dysregulation.
Rachael B. Smith, DO, of the department of ob.gyn. at the University of Arizona, Phoenix, was not involved in the research but found that hypothesis plausible as well.
“Our neurologic and vascular systems are coordinated physiologic processes working together for basic brain and body function,” Dr. Smith said in an interview. Some of the symptoms of migraines and menopause are similar and both are often explained by the dysfunction of these systems. The association between history of migraines and severity of vasomotor symptoms is very likely to be explained by this dysregulation between the neurologic and vascular systems.”
Dr. Smith also pointed out, however, that the largely homogeneous study population, all from the same national clinic system, makes it difficult to know how generalizable these findings are.
The primary clinical implications of these findings are that women’s providers need to be sure they’re asking their patients about migraine history and symptoms.
“The counseling we provide on menopausal symptoms should be better tailored to our patients’ medical history, specifically inquiring about history of migraines and how this may impact their symptoms,” Dr. Smith said.
The research was funded by the National Institutes of Health. Dr. Faubion and Dr. Smith had no disclosures.
Women with a history of migraine are more likely to experience severe or very severe hot flashes than women without migraines, according to research presented Sept. 24 at the hybrid annual meeting of the North American Menopause Society. An estimated one in five women experience migraine, and women tend to have greater migraine symptoms and disability, the authors note in their background information. Since migraines are also linked to a higher risk of cardiovascular disease, the authors sought to learn whether migraines were associated with vasomotor symptoms, another cardiovascular risk factor.
“The question in my mind is, can we do better at predicting cardiovascular risk in women because the risk prediction models that we have really don’t work all that well in women because they were designed for use in men,” Stephanie S. Faubion, MD, MBA, Penny and Bill George Director for Mayo Clinic’s Center for Women’s Health said in an interview. “My ultimate goal is to see if we can somehow use big data, artificial intelligence to figure out how to weight some of these female-specific or female-predominant factors to come up with a better model for cardiovascular risk prediction.”
The researchers analyzed cross-sectional data from 3,308 women who participated in the Data Registry on the Experiences of Aging, Menopause and Sexuality (DREAMS) study through Mayo Clinic sites in Rochester, Minn.; Scottsdale, Ariz.; and Jacksonville, Fla.. The women ranged in age from 45 to 60 years old, with an average age of 53, and the vast majority of them were white (95%) and had at least some college (93%). Most were also in a long-term relationship (85%), and a majority were employed (69%) and postmenopausal (67%).
The data, collected between May 2015 and December 2019, included a self-reported history of migraine and questionnaires that included the Menopause Rating Scale of menopause-related symptoms.
The researchers adjusted their findings to account for body mass index (BMI), menopause status, smoking status, depression, anxiety, current use of hormone therapy, and presence of low back pain within the past year. ”The diagnosis of low back pain, another pain disorder, was used to test the specificity of the association of migraine and vasomotor symptoms,” the authors write.
Just over a quarter of the women (27%) reported a history of migraine, and these women’s Menopause Rating Scale scores were an average 1.36 points greater than women without a history of migraines (P < .001). Women with self-reported migraine were also 40% more likely than women without migraines to report severe or very severe flashes versus reporting no hot flashes at all (odds ratio, 1.4; P = .02).
“The odds of reporting more severe hot flashes increased monotonically in women with a history of migraine,” the authors report. “In addition, women with low back pain had higher Menopause Rating Scale scores, but were no more likely to have severe/very severe hot flashes than those without back pain, confirming the specificity of the link between vasomotor symptoms and migraine.”
It’s not clear if migraine or hot flashes are risk factors that add to a woman’s existing cardiovascular risk profile or whether they are simply biomarkers of a shared pathway, Dr Faubion said in an interview. She speculates that the common link between migraine and vasomotor symptoms could be neurovascular dysregulation.
Rachael B. Smith, DO, of the department of ob.gyn. at the University of Arizona, Phoenix, was not involved in the research but found that hypothesis plausible as well.
“Our neurologic and vascular systems are coordinated physiologic processes working together for basic brain and body function,” Dr. Smith said in an interview. Some of the symptoms of migraines and menopause are similar and both are often explained by the dysfunction of these systems. The association between history of migraines and severity of vasomotor symptoms is very likely to be explained by this dysregulation between the neurologic and vascular systems.”
Dr. Smith also pointed out, however, that the largely homogeneous study population, all from the same national clinic system, makes it difficult to know how generalizable these findings are.
The primary clinical implications of these findings are that women’s providers need to be sure they’re asking their patients about migraine history and symptoms.
“The counseling we provide on menopausal symptoms should be better tailored to our patients’ medical history, specifically inquiring about history of migraines and how this may impact their symptoms,” Dr. Smith said.
The research was funded by the National Institutes of Health. Dr. Faubion and Dr. Smith had no disclosures.
Women with a history of migraine are more likely to experience severe or very severe hot flashes than women without migraines, according to research presented Sept. 24 at the hybrid annual meeting of the North American Menopause Society. An estimated one in five women experience migraine, and women tend to have greater migraine symptoms and disability, the authors note in their background information. Since migraines are also linked to a higher risk of cardiovascular disease, the authors sought to learn whether migraines were associated with vasomotor symptoms, another cardiovascular risk factor.
“The question in my mind is, can we do better at predicting cardiovascular risk in women because the risk prediction models that we have really don’t work all that well in women because they were designed for use in men,” Stephanie S. Faubion, MD, MBA, Penny and Bill George Director for Mayo Clinic’s Center for Women’s Health said in an interview. “My ultimate goal is to see if we can somehow use big data, artificial intelligence to figure out how to weight some of these female-specific or female-predominant factors to come up with a better model for cardiovascular risk prediction.”
The researchers analyzed cross-sectional data from 3,308 women who participated in the Data Registry on the Experiences of Aging, Menopause and Sexuality (DREAMS) study through Mayo Clinic sites in Rochester, Minn.; Scottsdale, Ariz.; and Jacksonville, Fla.. The women ranged in age from 45 to 60 years old, with an average age of 53, and the vast majority of them were white (95%) and had at least some college (93%). Most were also in a long-term relationship (85%), and a majority were employed (69%) and postmenopausal (67%).
The data, collected between May 2015 and December 2019, included a self-reported history of migraine and questionnaires that included the Menopause Rating Scale of menopause-related symptoms.
The researchers adjusted their findings to account for body mass index (BMI), menopause status, smoking status, depression, anxiety, current use of hormone therapy, and presence of low back pain within the past year. ”The diagnosis of low back pain, another pain disorder, was used to test the specificity of the association of migraine and vasomotor symptoms,” the authors write.
Just over a quarter of the women (27%) reported a history of migraine, and these women’s Menopause Rating Scale scores were an average 1.36 points greater than women without a history of migraines (P < .001). Women with self-reported migraine were also 40% more likely than women without migraines to report severe or very severe flashes versus reporting no hot flashes at all (odds ratio, 1.4; P = .02).
“The odds of reporting more severe hot flashes increased monotonically in women with a history of migraine,” the authors report. “In addition, women with low back pain had higher Menopause Rating Scale scores, but were no more likely to have severe/very severe hot flashes than those without back pain, confirming the specificity of the link between vasomotor symptoms and migraine.”
It’s not clear if migraine or hot flashes are risk factors that add to a woman’s existing cardiovascular risk profile or whether they are simply biomarkers of a shared pathway, Dr Faubion said in an interview. She speculates that the common link between migraine and vasomotor symptoms could be neurovascular dysregulation.
Rachael B. Smith, DO, of the department of ob.gyn. at the University of Arizona, Phoenix, was not involved in the research but found that hypothesis plausible as well.
“Our neurologic and vascular systems are coordinated physiologic processes working together for basic brain and body function,” Dr. Smith said in an interview. Some of the symptoms of migraines and menopause are similar and both are often explained by the dysfunction of these systems. The association between history of migraines and severity of vasomotor symptoms is very likely to be explained by this dysregulation between the neurologic and vascular systems.”
Dr. Smith also pointed out, however, that the largely homogeneous study population, all from the same national clinic system, makes it difficult to know how generalizable these findings are.
The primary clinical implications of these findings are that women’s providers need to be sure they’re asking their patients about migraine history and symptoms.
“The counseling we provide on menopausal symptoms should be better tailored to our patients’ medical history, specifically inquiring about history of migraines and how this may impact their symptoms,” Dr. Smith said.
The research was funded by the National Institutes of Health. Dr. Faubion and Dr. Smith had no disclosures.
FROM NAMS 2021
Greater portal use gives patients access, doctors headaches
The use of patient portals that provide access to electronic health records has dramatically increased in the past several years, and patients whose health care practitioner encouraged them to use their online portal accessed them at a higher rate than those who were not encouraged to do so.
These were among the top-line results of a national survey of U.S. adults conducted by the National Institutes of Health from January 2020 to April 2020. Although the COVID-19 pandemic hit the United States in the middle of that period, a report on the survey by the Office of the National Coordinator for Health IT stated, “These findings largely reflect prepandemic rates of individuals being offered and subsequently using their online medical record, also known as a patient portal.”
But with more patient access can come additional work for physicians and other health care practitioners, ranging from an onslaught of patient communications to managing data sent to them by patients.
According to the report, 59% of individuals were offered access to their patient portal, and 38% accessed their record at least once in 2020. By comparison, in 2014, just 42% were offered access to their portal, and 25% used it. But these percentages hardly changed from 2019 to 2020.
The increase in the percentage of people who accessed portals reflects the fact that more people were offered access. In addition, there were signs of rising activity among portal users.
Among patients offered access to their patient portal, 64% accessed it at least once in 2020 – 11 percentage points more than in 2017. Twenty-seven percent of those who had access to a portal used it once or twice; 20% accessed it three to five times; and 18% used it six or more times. The latter two percentages were significantly higher than in 2017.
Of the respondents who were offered access to portals but didn’t use them, 69% said they didn’t access the portal because they preferred to speak with their health care practitioner directly. Sixty-three percent said they didn’t see a need to use their online medical record. This was similar to the percentage 3 years earlier. Other reasons included respondents’ concerns about the privacy/security of online medical records (24%), their lack of comfort with computers (20%), and their lack of Internet access (13%).
The pros and cons of patient portals, greater access
Among portal users who accessed their records through a mobile health app, 51% used the app to facilitate discussions with their health care practitioner in 2020, an 8–percentage point increase from 2017. Fifty-percent of the mobile health app users utilized it to make a decision about how to treat an illness or condition, up from 45% in 2017. And 71% of these individuals used their app to track progress on a health-related goal, just a bit more than in 2017.
Individuals who were encouraged by their health care practitioner to use their patient portal viewed clinical notes and exchanged secure messages with their practitioner at higher rates than those who had not been encouraged. This is not surprising, but it reflects an unintended result of patient portals that many physicians have found burdensome, especially during the pandemic: overflowing electronic in-boxes.
Robert Wachter, MD, chairman of the department of medicine at the University of California, San Francisco, recently tweeted, “We’re seeing huge uptick in in-box messages for MDs during COVID – now seems like biggest driver of MD burnout. The fundamental problem: We turned on 24/7/365 access for patients (who of course like it) with no operational or business model to handle it. Crucial that we fix this.”
Steven Waldren, MD, vice president and chief medical informatics officer at the American Academy of Family Physicians, told this news organization that he agrees that this is a major challenge. “In-box management is a burden on physicians and practices,” he said. “However, it can be done better, either through a team in-box or through better use of technology.”
The team in-box he refers to is a mechanism for triaging patient messages. For example, a triage nurse can look at the messages and decide which ones can be handled by staff and which ones the doctor needs to see. Or physicians and front office staff can see the messages at the same time; a nurse can triage some messages according to protocols, and the physician can respond to any message, depending on what he or she knows about the patient.
Technology can also be enlisted in the effort, he suggested, perhaps by automating the triaging of messages such as prescription refill requests or using artificial intelligence to sort messages by content.
Making patient records portable
Nearly 40% of portal users accessed it using a smartphone app (17%) or with both their smartphone app and their computer (22%). Sixty-one percent of users relied exclusively on computers to access their portals.
About a third of patient portal users downloaded their online medical records in 2020. This proportion has nearly doubled from 17% since 2017, the ONC report noted.
Although the survey didn’t ask about multiple downloads, it appears that most people had to download their records separately from the patient portal of each practitioner who cared for them. Although the Apple Health app allows people to download records to their iPhones from multiple portals using a standard application programming interface, the ONC report says that only 5% of respondents transmitted their records to a service or app, up slightly from 3% in 2017.
Dr. Waldren hopes most patients will have the ability to download and integrate records from multiple practitioners in a few years, but he wouldn’t bet on it.
“A fair amount of work needs to be done on the business side and on figuring out how the data get connected together,” he said. “And there are still privacy concerns with apps.”
Overall, 21% of portal users transmitted their data to at least one outside party in 2020, compared with 14% in 2017. Seventeen percent of them sent their records to another health care practitioner, up from 10% in 2017. Five percent of the users transmitted their records to a caregiver, slightly more than in 2017.
Managing data is a challenge
Asked how physicians feel about portal users adding information to their record or correcting inaccurate information, Dr. Waldren says, “Doctors are already comfortable with patient-generated data. The challenge is managing it. If the patient provides data that’s not easy to put in the EHR, that’s going to add work, and they don’t want to see 100 blood pressure readings.
“You’d be hard-pressed to find a doctor who doesn’t welcome additional information about the patient’s health, but it can be onerous and can take time to enter the data,” Dr. Waldren said.
Overall, he said, “Giving patients the ability to take more ownership of their health and participate in their own care is good and can help us move forward. How this will be integrated into patient care is another question.”
A version of this article first appeared on Medscape.com.
The use of patient portals that provide access to electronic health records has dramatically increased in the past several years, and patients whose health care practitioner encouraged them to use their online portal accessed them at a higher rate than those who were not encouraged to do so.
These were among the top-line results of a national survey of U.S. adults conducted by the National Institutes of Health from January 2020 to April 2020. Although the COVID-19 pandemic hit the United States in the middle of that period, a report on the survey by the Office of the National Coordinator for Health IT stated, “These findings largely reflect prepandemic rates of individuals being offered and subsequently using their online medical record, also known as a patient portal.”
But with more patient access can come additional work for physicians and other health care practitioners, ranging from an onslaught of patient communications to managing data sent to them by patients.
According to the report, 59% of individuals were offered access to their patient portal, and 38% accessed their record at least once in 2020. By comparison, in 2014, just 42% were offered access to their portal, and 25% used it. But these percentages hardly changed from 2019 to 2020.
The increase in the percentage of people who accessed portals reflects the fact that more people were offered access. In addition, there were signs of rising activity among portal users.
Among patients offered access to their patient portal, 64% accessed it at least once in 2020 – 11 percentage points more than in 2017. Twenty-seven percent of those who had access to a portal used it once or twice; 20% accessed it three to five times; and 18% used it six or more times. The latter two percentages were significantly higher than in 2017.
Of the respondents who were offered access to portals but didn’t use them, 69% said they didn’t access the portal because they preferred to speak with their health care practitioner directly. Sixty-three percent said they didn’t see a need to use their online medical record. This was similar to the percentage 3 years earlier. Other reasons included respondents’ concerns about the privacy/security of online medical records (24%), their lack of comfort with computers (20%), and their lack of Internet access (13%).
The pros and cons of patient portals, greater access
Among portal users who accessed their records through a mobile health app, 51% used the app to facilitate discussions with their health care practitioner in 2020, an 8–percentage point increase from 2017. Fifty-percent of the mobile health app users utilized it to make a decision about how to treat an illness or condition, up from 45% in 2017. And 71% of these individuals used their app to track progress on a health-related goal, just a bit more than in 2017.
Individuals who were encouraged by their health care practitioner to use their patient portal viewed clinical notes and exchanged secure messages with their practitioner at higher rates than those who had not been encouraged. This is not surprising, but it reflects an unintended result of patient portals that many physicians have found burdensome, especially during the pandemic: overflowing electronic in-boxes.
Robert Wachter, MD, chairman of the department of medicine at the University of California, San Francisco, recently tweeted, “We’re seeing huge uptick in in-box messages for MDs during COVID – now seems like biggest driver of MD burnout. The fundamental problem: We turned on 24/7/365 access for patients (who of course like it) with no operational or business model to handle it. Crucial that we fix this.”
Steven Waldren, MD, vice president and chief medical informatics officer at the American Academy of Family Physicians, told this news organization that he agrees that this is a major challenge. “In-box management is a burden on physicians and practices,” he said. “However, it can be done better, either through a team in-box or through better use of technology.”
The team in-box he refers to is a mechanism for triaging patient messages. For example, a triage nurse can look at the messages and decide which ones can be handled by staff and which ones the doctor needs to see. Or physicians and front office staff can see the messages at the same time; a nurse can triage some messages according to protocols, and the physician can respond to any message, depending on what he or she knows about the patient.
Technology can also be enlisted in the effort, he suggested, perhaps by automating the triaging of messages such as prescription refill requests or using artificial intelligence to sort messages by content.
Making patient records portable
Nearly 40% of portal users accessed it using a smartphone app (17%) or with both their smartphone app and their computer (22%). Sixty-one percent of users relied exclusively on computers to access their portals.
About a third of patient portal users downloaded their online medical records in 2020. This proportion has nearly doubled from 17% since 2017, the ONC report noted.
Although the survey didn’t ask about multiple downloads, it appears that most people had to download their records separately from the patient portal of each practitioner who cared for them. Although the Apple Health app allows people to download records to their iPhones from multiple portals using a standard application programming interface, the ONC report says that only 5% of respondents transmitted their records to a service or app, up slightly from 3% in 2017.
Dr. Waldren hopes most patients will have the ability to download and integrate records from multiple practitioners in a few years, but he wouldn’t bet on it.
“A fair amount of work needs to be done on the business side and on figuring out how the data get connected together,” he said. “And there are still privacy concerns with apps.”
Overall, 21% of portal users transmitted their data to at least one outside party in 2020, compared with 14% in 2017. Seventeen percent of them sent their records to another health care practitioner, up from 10% in 2017. Five percent of the users transmitted their records to a caregiver, slightly more than in 2017.
Managing data is a challenge
Asked how physicians feel about portal users adding information to their record or correcting inaccurate information, Dr. Waldren says, “Doctors are already comfortable with patient-generated data. The challenge is managing it. If the patient provides data that’s not easy to put in the EHR, that’s going to add work, and they don’t want to see 100 blood pressure readings.
“You’d be hard-pressed to find a doctor who doesn’t welcome additional information about the patient’s health, but it can be onerous and can take time to enter the data,” Dr. Waldren said.
Overall, he said, “Giving patients the ability to take more ownership of their health and participate in their own care is good and can help us move forward. How this will be integrated into patient care is another question.”
A version of this article first appeared on Medscape.com.
The use of patient portals that provide access to electronic health records has dramatically increased in the past several years, and patients whose health care practitioner encouraged them to use their online portal accessed them at a higher rate than those who were not encouraged to do so.
These were among the top-line results of a national survey of U.S. adults conducted by the National Institutes of Health from January 2020 to April 2020. Although the COVID-19 pandemic hit the United States in the middle of that period, a report on the survey by the Office of the National Coordinator for Health IT stated, “These findings largely reflect prepandemic rates of individuals being offered and subsequently using their online medical record, also known as a patient portal.”
But with more patient access can come additional work for physicians and other health care practitioners, ranging from an onslaught of patient communications to managing data sent to them by patients.
According to the report, 59% of individuals were offered access to their patient portal, and 38% accessed their record at least once in 2020. By comparison, in 2014, just 42% were offered access to their portal, and 25% used it. But these percentages hardly changed from 2019 to 2020.
The increase in the percentage of people who accessed portals reflects the fact that more people were offered access. In addition, there were signs of rising activity among portal users.
Among patients offered access to their patient portal, 64% accessed it at least once in 2020 – 11 percentage points more than in 2017. Twenty-seven percent of those who had access to a portal used it once or twice; 20% accessed it three to five times; and 18% used it six or more times. The latter two percentages were significantly higher than in 2017.
Of the respondents who were offered access to portals but didn’t use them, 69% said they didn’t access the portal because they preferred to speak with their health care practitioner directly. Sixty-three percent said they didn’t see a need to use their online medical record. This was similar to the percentage 3 years earlier. Other reasons included respondents’ concerns about the privacy/security of online medical records (24%), their lack of comfort with computers (20%), and their lack of Internet access (13%).
The pros and cons of patient portals, greater access
Among portal users who accessed their records through a mobile health app, 51% used the app to facilitate discussions with their health care practitioner in 2020, an 8–percentage point increase from 2017. Fifty-percent of the mobile health app users utilized it to make a decision about how to treat an illness or condition, up from 45% in 2017. And 71% of these individuals used their app to track progress on a health-related goal, just a bit more than in 2017.
Individuals who were encouraged by their health care practitioner to use their patient portal viewed clinical notes and exchanged secure messages with their practitioner at higher rates than those who had not been encouraged. This is not surprising, but it reflects an unintended result of patient portals that many physicians have found burdensome, especially during the pandemic: overflowing electronic in-boxes.
Robert Wachter, MD, chairman of the department of medicine at the University of California, San Francisco, recently tweeted, “We’re seeing huge uptick in in-box messages for MDs during COVID – now seems like biggest driver of MD burnout. The fundamental problem: We turned on 24/7/365 access for patients (who of course like it) with no operational or business model to handle it. Crucial that we fix this.”
Steven Waldren, MD, vice president and chief medical informatics officer at the American Academy of Family Physicians, told this news organization that he agrees that this is a major challenge. “In-box management is a burden on physicians and practices,” he said. “However, it can be done better, either through a team in-box or through better use of technology.”
The team in-box he refers to is a mechanism for triaging patient messages. For example, a triage nurse can look at the messages and decide which ones can be handled by staff and which ones the doctor needs to see. Or physicians and front office staff can see the messages at the same time; a nurse can triage some messages according to protocols, and the physician can respond to any message, depending on what he or she knows about the patient.
Technology can also be enlisted in the effort, he suggested, perhaps by automating the triaging of messages such as prescription refill requests or using artificial intelligence to sort messages by content.
Making patient records portable
Nearly 40% of portal users accessed it using a smartphone app (17%) or with both their smartphone app and their computer (22%). Sixty-one percent of users relied exclusively on computers to access their portals.
About a third of patient portal users downloaded their online medical records in 2020. This proportion has nearly doubled from 17% since 2017, the ONC report noted.
Although the survey didn’t ask about multiple downloads, it appears that most people had to download their records separately from the patient portal of each practitioner who cared for them. Although the Apple Health app allows people to download records to their iPhones from multiple portals using a standard application programming interface, the ONC report says that only 5% of respondents transmitted their records to a service or app, up slightly from 3% in 2017.
Dr. Waldren hopes most patients will have the ability to download and integrate records from multiple practitioners in a few years, but he wouldn’t bet on it.
“A fair amount of work needs to be done on the business side and on figuring out how the data get connected together,” he said. “And there are still privacy concerns with apps.”
Overall, 21% of portal users transmitted their data to at least one outside party in 2020, compared with 14% in 2017. Seventeen percent of them sent their records to another health care practitioner, up from 10% in 2017. Five percent of the users transmitted their records to a caregiver, slightly more than in 2017.
Managing data is a challenge
Asked how physicians feel about portal users adding information to their record or correcting inaccurate information, Dr. Waldren says, “Doctors are already comfortable with patient-generated data. The challenge is managing it. If the patient provides data that’s not easy to put in the EHR, that’s going to add work, and they don’t want to see 100 blood pressure readings.
“You’d be hard-pressed to find a doctor who doesn’t welcome additional information about the patient’s health, but it can be onerous and can take time to enter the data,” Dr. Waldren said.
Overall, he said, “Giving patients the ability to take more ownership of their health and participate in their own care is good and can help us move forward. How this will be integrated into patient care is another question.”
A version of this article first appeared on Medscape.com.
FDA okays new oral CGRP antagonist for migraine prevention
the manufacturer announced in a release.
The once-daily medication will be available in doses of 10 mg, 30 mg, and 60 mg.
“Qulipta provides a simple oral treatment option specifically developed to prevent migraine attacks and target CGRP, which is believed to be crucially involved in migraine in many patients,” coinvestigator Peter J. Goadsby, MD, PhD, DSc, neurologist and professor at the University of California, Los Angeles, and King’s College London, said in the release.
Approval was based partly on the findings from the phase 3 ADVANCE trial, in which patients with episodic migraine were randomly assigned to receive placebo or a 10-mg, 30-mg, or 60-mg daily dose of atogepant for 12 weeks.
As reported by this news organization, all three doses of atogepant reduced the number of mean monthly migraine days.
With this approval, neurologists will be able to choose from four monoclonal antibodies and two gepants for the preventive treatment of migraine.
“Having another gepant that can also be given preventively is a good idea, because one may be better than the other for a patient,” Alan M. Rapoport, MD, past president of the International Headache Society and founder and director emeritus of the New England Center for Headache, Stamford, Conn., told this news organization.
“Once we have a year or so of experience with atogepant, we’ll have a pretty good idea of which one works better preventively,” said Dr. Rapoport, who was not involved with the research.
Practice changing?
In the ADVANCE trial, there was a reduction of 3.69 migraine days with the 10-mg dose, 3.86 days with the 30-mg dose, and 4.2 days with the 60-mg dose. Placebo was associated with a reduction of 2.48 migraine days.
In addition, more than half of patients in each atogepant arm achieved a reduction in mean monthly migraine days of 50% or greater. This outcome occurred in 55.6% of the 10-mg atogepant group, 58.7% of the 30-mg group, and 60.8% of the 60-mg group. Approximately 29% patients who received placebo achieved this outcome.
The data indicated that atogepant has a favorable safety profile. The most common adverse events associated with treatment were constipation, nausea, and upper respiratory tract infection.
Dr. Rapoport, who is also a clinical professor of neurology at UCLA, noted that he was impressed with the efficacy.
“I’m not as impressed with the adverse events, but they’re not serious, and they don’t necessarily last,” he said.
Although being able to prescribe a single drug for acute and preventive treatment may be an advantage, it remains to be seen whether the tolerability and price of atogepant will be barriers for patients, Dr. Rapoport added.
How the approval will affect clinical practice is also unclear, he noted.
“If you’re going to start someone on a preventive, especially if it’s a woman of childbearing potential, you might just consider one of the two gepants. Doctors will decide once they see how they work,” said Dr. Rapoport.
Not a ‘breakthrough’ treatment
Also commenting ahead of the approval, Elizabeth W. Loder, MD, vice chair for academic affairs in the department of neurology at Brigham and Women’s Hospital, Boston, noted that the “safety of these CGRP medications in pregnancy is uncertain, and there are theoretical reasons to be concerned about it.”
Unlike injectable CGRP medications, atogepant is eliminated from the body relatively quickly after the patient stops taking it, said Dr. Loder, who is also professor of neurology at Harvard Medical School, Boston. However, atogepant may not otherwise differ greatly from other medications of its type.
“I don’t see a reason to think that one of these oral CGRP medicines is much more effective than another one,” said Dr. Loder.
“In my mind, as a clinician who will be prescribing these for patients, it will be cost and the ease of getting it covered that makes the difference,” she added.
These questions may raise concerns. “Those of us who treat patients who do not have private insurance find it very difficult to get these medications for them, even in situations where they have exhausted other alternatives,” said Dr. Loder.
Patients insured by Medicare or Medicaid “usually have no avenue to get some of these new, expensive treatments,” she said.
The approval of atogepant for acute and preventive treatment shows that the distinction between these indications may be artificial, Dr. Loder noted. The approval “will, I hope, help people think more flexibly about the way in which we use medications.”
It is a positive that atogepant has emerged as another option for preventive therapy, but the treatment cannot be considered a breakthrough, Dr. Loder added. The efficacy of atogepant, like that of other preventive treatments for migraine, is modest.
“It would be so nice if we could find things that were more effective than the treatments we currently have,” said Dr. Loder.
A version of this article first appeared on Medscape.com.
the manufacturer announced in a release.
The once-daily medication will be available in doses of 10 mg, 30 mg, and 60 mg.
“Qulipta provides a simple oral treatment option specifically developed to prevent migraine attacks and target CGRP, which is believed to be crucially involved in migraine in many patients,” coinvestigator Peter J. Goadsby, MD, PhD, DSc, neurologist and professor at the University of California, Los Angeles, and King’s College London, said in the release.
Approval was based partly on the findings from the phase 3 ADVANCE trial, in which patients with episodic migraine were randomly assigned to receive placebo or a 10-mg, 30-mg, or 60-mg daily dose of atogepant for 12 weeks.
As reported by this news organization, all three doses of atogepant reduced the number of mean monthly migraine days.
With this approval, neurologists will be able to choose from four monoclonal antibodies and two gepants for the preventive treatment of migraine.
“Having another gepant that can also be given preventively is a good idea, because one may be better than the other for a patient,” Alan M. Rapoport, MD, past president of the International Headache Society and founder and director emeritus of the New England Center for Headache, Stamford, Conn., told this news organization.
“Once we have a year or so of experience with atogepant, we’ll have a pretty good idea of which one works better preventively,” said Dr. Rapoport, who was not involved with the research.
Practice changing?
In the ADVANCE trial, there was a reduction of 3.69 migraine days with the 10-mg dose, 3.86 days with the 30-mg dose, and 4.2 days with the 60-mg dose. Placebo was associated with a reduction of 2.48 migraine days.
In addition, more than half of patients in each atogepant arm achieved a reduction in mean monthly migraine days of 50% or greater. This outcome occurred in 55.6% of the 10-mg atogepant group, 58.7% of the 30-mg group, and 60.8% of the 60-mg group. Approximately 29% patients who received placebo achieved this outcome.
The data indicated that atogepant has a favorable safety profile. The most common adverse events associated with treatment were constipation, nausea, and upper respiratory tract infection.
Dr. Rapoport, who is also a clinical professor of neurology at UCLA, noted that he was impressed with the efficacy.
“I’m not as impressed with the adverse events, but they’re not serious, and they don’t necessarily last,” he said.
Although being able to prescribe a single drug for acute and preventive treatment may be an advantage, it remains to be seen whether the tolerability and price of atogepant will be barriers for patients, Dr. Rapoport added.
How the approval will affect clinical practice is also unclear, he noted.
“If you’re going to start someone on a preventive, especially if it’s a woman of childbearing potential, you might just consider one of the two gepants. Doctors will decide once they see how they work,” said Dr. Rapoport.
Not a ‘breakthrough’ treatment
Also commenting ahead of the approval, Elizabeth W. Loder, MD, vice chair for academic affairs in the department of neurology at Brigham and Women’s Hospital, Boston, noted that the “safety of these CGRP medications in pregnancy is uncertain, and there are theoretical reasons to be concerned about it.”
Unlike injectable CGRP medications, atogepant is eliminated from the body relatively quickly after the patient stops taking it, said Dr. Loder, who is also professor of neurology at Harvard Medical School, Boston. However, atogepant may not otherwise differ greatly from other medications of its type.
“I don’t see a reason to think that one of these oral CGRP medicines is much more effective than another one,” said Dr. Loder.
“In my mind, as a clinician who will be prescribing these for patients, it will be cost and the ease of getting it covered that makes the difference,” she added.
These questions may raise concerns. “Those of us who treat patients who do not have private insurance find it very difficult to get these medications for them, even in situations where they have exhausted other alternatives,” said Dr. Loder.
Patients insured by Medicare or Medicaid “usually have no avenue to get some of these new, expensive treatments,” she said.
The approval of atogepant for acute and preventive treatment shows that the distinction between these indications may be artificial, Dr. Loder noted. The approval “will, I hope, help people think more flexibly about the way in which we use medications.”
It is a positive that atogepant has emerged as another option for preventive therapy, but the treatment cannot be considered a breakthrough, Dr. Loder added. The efficacy of atogepant, like that of other preventive treatments for migraine, is modest.
“It would be so nice if we could find things that were more effective than the treatments we currently have,” said Dr. Loder.
A version of this article first appeared on Medscape.com.
the manufacturer announced in a release.
The once-daily medication will be available in doses of 10 mg, 30 mg, and 60 mg.
“Qulipta provides a simple oral treatment option specifically developed to prevent migraine attacks and target CGRP, which is believed to be crucially involved in migraine in many patients,” coinvestigator Peter J. Goadsby, MD, PhD, DSc, neurologist and professor at the University of California, Los Angeles, and King’s College London, said in the release.
Approval was based partly on the findings from the phase 3 ADVANCE trial, in which patients with episodic migraine were randomly assigned to receive placebo or a 10-mg, 30-mg, or 60-mg daily dose of atogepant for 12 weeks.
As reported by this news organization, all three doses of atogepant reduced the number of mean monthly migraine days.
With this approval, neurologists will be able to choose from four monoclonal antibodies and two gepants for the preventive treatment of migraine.
“Having another gepant that can also be given preventively is a good idea, because one may be better than the other for a patient,” Alan M. Rapoport, MD, past president of the International Headache Society and founder and director emeritus of the New England Center for Headache, Stamford, Conn., told this news organization.
“Once we have a year or so of experience with atogepant, we’ll have a pretty good idea of which one works better preventively,” said Dr. Rapoport, who was not involved with the research.
Practice changing?
In the ADVANCE trial, there was a reduction of 3.69 migraine days with the 10-mg dose, 3.86 days with the 30-mg dose, and 4.2 days with the 60-mg dose. Placebo was associated with a reduction of 2.48 migraine days.
In addition, more than half of patients in each atogepant arm achieved a reduction in mean monthly migraine days of 50% or greater. This outcome occurred in 55.6% of the 10-mg atogepant group, 58.7% of the 30-mg group, and 60.8% of the 60-mg group. Approximately 29% patients who received placebo achieved this outcome.
The data indicated that atogepant has a favorable safety profile. The most common adverse events associated with treatment were constipation, nausea, and upper respiratory tract infection.
Dr. Rapoport, who is also a clinical professor of neurology at UCLA, noted that he was impressed with the efficacy.
“I’m not as impressed with the adverse events, but they’re not serious, and they don’t necessarily last,” he said.
Although being able to prescribe a single drug for acute and preventive treatment may be an advantage, it remains to be seen whether the tolerability and price of atogepant will be barriers for patients, Dr. Rapoport added.
How the approval will affect clinical practice is also unclear, he noted.
“If you’re going to start someone on a preventive, especially if it’s a woman of childbearing potential, you might just consider one of the two gepants. Doctors will decide once they see how they work,” said Dr. Rapoport.
Not a ‘breakthrough’ treatment
Also commenting ahead of the approval, Elizabeth W. Loder, MD, vice chair for academic affairs in the department of neurology at Brigham and Women’s Hospital, Boston, noted that the “safety of these CGRP medications in pregnancy is uncertain, and there are theoretical reasons to be concerned about it.”
Unlike injectable CGRP medications, atogepant is eliminated from the body relatively quickly after the patient stops taking it, said Dr. Loder, who is also professor of neurology at Harvard Medical School, Boston. However, atogepant may not otherwise differ greatly from other medications of its type.
“I don’t see a reason to think that one of these oral CGRP medicines is much more effective than another one,” said Dr. Loder.
“In my mind, as a clinician who will be prescribing these for patients, it will be cost and the ease of getting it covered that makes the difference,” she added.
These questions may raise concerns. “Those of us who treat patients who do not have private insurance find it very difficult to get these medications for them, even in situations where they have exhausted other alternatives,” said Dr. Loder.
Patients insured by Medicare or Medicaid “usually have no avenue to get some of these new, expensive treatments,” she said.
The approval of atogepant for acute and preventive treatment shows that the distinction between these indications may be artificial, Dr. Loder noted. The approval “will, I hope, help people think more flexibly about the way in which we use medications.”
It is a positive that atogepant has emerged as another option for preventive therapy, but the treatment cannot be considered a breakthrough, Dr. Loder added. The efficacy of atogepant, like that of other preventive treatments for migraine, is modest.
“It would be so nice if we could find things that were more effective than the treatments we currently have,” said Dr. Loder.
A version of this article first appeared on Medscape.com.
Fraudulent misbranding of PPE nets $22 million settlement
Avanos medical to pay $22 million to resolve criminal charge for fraudulent misbranding of PPE
A U.S.-based multinational medical device corporation will pay more than $22 million to resolve a criminal charge regarding fraudulent misbranding of their surgical gowns.
Avanos Medical Inc, which as its U.S. headquarters in Alpharetta, Georgia, is charged with one count of introducing misbranded surgical gowns into interstate commerce with the intent to defraud and mislead.
According to the Department of Justice, the company knowingly falsely labeled its MicroCool surgical gowns as providing AAMI Level 4 protection (the highest level) against fluid and virus penetration. Under the standards set by the American National Standards Institute (ANSI) and the Association for the Advancement of Medical Instrumentation (AAMI), the highest protection level for surgical gowns is reserved for gowns intended to be used in surgeries and other high-risk medical procedures on patients suspected of having infectious diseases.
Avanos admitted to selling hundreds of thousands of MicroCool gowns that were falsely labeled as AAMI Level 4 between late 2014 and early 2015, as well as directly lying to customers about the gowns’ protective capacities. In total, Avanos sold almost $9 million of misbranded MicroCool gowns.
“The last thing health care workers should have to worry about is whether their personal protective equipment lives up to manufacturers’ claims,” said Acting U.S. Attorney Prerak Shah for the Northern District of Texas. “Misbranded PPE can pose serious risks to medical professionals and patients alike.”
Company pays $38.75 million to settle allegations of knowingly selling defective devices
Medical device manufacturers Alere and Alere San Diego (collectively, Alere) have agreed to pay almost $39 million to resolve allegations that they violated the False Claims Act by billing, and causing others to bill, the Medicare program for defective rapid point-of-care testing devices.
From 2008 to 2016, the Department of Justice alleges, Alere knowingly sold defective INRatio blood coagulation monitors used by Medicare beneficiaries who were taking anticoagulants. The software algorithms in the monitors contained a material defect, which Alere had found in their research, to cause inaccurate readings. Blood coagulation monitoring is essential for the safety of these patients, enabling them to maintain a safe dosage of their medications. Taking too much of an anticoagulant can cause major bleeding, while taking too little can cause blood clots that lead to strokes.
While Alere was aware that these devices were linked to over a dozen deaths and hundreds of injuries, the company continued to conceal the defect and billed Medicare for the devices.
In 2016, the product was taken off the market at the request of the FDA.
Mass. doctor, wife charged in international money laundering, fraud scheme
Massachusetts psychiatrist Rahim Shafa, MD, and his wife and office manager, Nahid Tormosi Shafa, are charged in connection to an international money laundering scheme involving importing illegal and misbranded drugs.
Through Shafa’s company, Novel Psychopharmacology, the two allegedly filed false and fraudulent Medicare reimbursement claims from 2016-2019, then deposited the money in their bank accounts, according to federal officials. From 2008-2018, the couple also engaged in an international money laundering scheme to purchase naltrexone pellet implants, disulfiram pellet implants, and injections from Hong Kong that were not approved by the FDA. According to officials, they falsified shipping documents, disguising the naltrexone pellet implants as “plastic beads in plastic tubes” to receive the drugs. They then offered to sell these drugs to patients of Novel Psychopharmacology.
Rahim Shafa was indicted on conspiracies of international money laundering, health care fraud, and defrauding the United States, as well as illegally importing merchandise and purposely delivering misbranded drugs. His wife was indicted on one count each of health care fraud conspiracy and international money laundering conspiracy.
Jury convicts medical equipment company owners of $27 million fraud
A federal jury in Texas convicted the owners of two durable medical equipment (DME) companies linked to a scheme to defraud Medicare.
Leah Hagen, 49, and Michael Hagen, 54, were convicted of one count of conspiracy to defraud the United States and to pay and receive health care kickbacks and one count of conspiracy to commit money laundering. The defendants owned and operated Metro DME Supply and Ortho Pain Solutions.
Ms. Hagen and Mr. Hagen paid a fixed rate per DME item in exchange for prescriptions and paperwork completed by telemedicine doctors that were used to submit false claims to Medicare, which totaled about $59 million. They were paid $27 million, and wired millions to their personal bank accounts. The defendants paid illegal bribes and kickbacks and wired money to their co-conspirator’s call center in the Philippines that provided signed doctor’s orders for orthotic braces.
At trial, evidence showed emails between Leah and Michael Hagen and their co-conspirators outlining a per-product pricing structure for orthotic braces, but not disclosing their agreement as one for marketing and other services.
At sentencing, the Hagens each face a maximum sentence of 25 years in prison.
A version of this article first appeared on Medscape.com.
Avanos medical to pay $22 million to resolve criminal charge for fraudulent misbranding of PPE
A U.S.-based multinational medical device corporation will pay more than $22 million to resolve a criminal charge regarding fraudulent misbranding of their surgical gowns.
Avanos Medical Inc, which as its U.S. headquarters in Alpharetta, Georgia, is charged with one count of introducing misbranded surgical gowns into interstate commerce with the intent to defraud and mislead.
According to the Department of Justice, the company knowingly falsely labeled its MicroCool surgical gowns as providing AAMI Level 4 protection (the highest level) against fluid and virus penetration. Under the standards set by the American National Standards Institute (ANSI) and the Association for the Advancement of Medical Instrumentation (AAMI), the highest protection level for surgical gowns is reserved for gowns intended to be used in surgeries and other high-risk medical procedures on patients suspected of having infectious diseases.
Avanos admitted to selling hundreds of thousands of MicroCool gowns that were falsely labeled as AAMI Level 4 between late 2014 and early 2015, as well as directly lying to customers about the gowns’ protective capacities. In total, Avanos sold almost $9 million of misbranded MicroCool gowns.
“The last thing health care workers should have to worry about is whether their personal protective equipment lives up to manufacturers’ claims,” said Acting U.S. Attorney Prerak Shah for the Northern District of Texas. “Misbranded PPE can pose serious risks to medical professionals and patients alike.”
Company pays $38.75 million to settle allegations of knowingly selling defective devices
Medical device manufacturers Alere and Alere San Diego (collectively, Alere) have agreed to pay almost $39 million to resolve allegations that they violated the False Claims Act by billing, and causing others to bill, the Medicare program for defective rapid point-of-care testing devices.
From 2008 to 2016, the Department of Justice alleges, Alere knowingly sold defective INRatio blood coagulation monitors used by Medicare beneficiaries who were taking anticoagulants. The software algorithms in the monitors contained a material defect, which Alere had found in their research, to cause inaccurate readings. Blood coagulation monitoring is essential for the safety of these patients, enabling them to maintain a safe dosage of their medications. Taking too much of an anticoagulant can cause major bleeding, while taking too little can cause blood clots that lead to strokes.
While Alere was aware that these devices were linked to over a dozen deaths and hundreds of injuries, the company continued to conceal the defect and billed Medicare for the devices.
In 2016, the product was taken off the market at the request of the FDA.
Mass. doctor, wife charged in international money laundering, fraud scheme
Massachusetts psychiatrist Rahim Shafa, MD, and his wife and office manager, Nahid Tormosi Shafa, are charged in connection to an international money laundering scheme involving importing illegal and misbranded drugs.
Through Shafa’s company, Novel Psychopharmacology, the two allegedly filed false and fraudulent Medicare reimbursement claims from 2016-2019, then deposited the money in their bank accounts, according to federal officials. From 2008-2018, the couple also engaged in an international money laundering scheme to purchase naltrexone pellet implants, disulfiram pellet implants, and injections from Hong Kong that were not approved by the FDA. According to officials, they falsified shipping documents, disguising the naltrexone pellet implants as “plastic beads in plastic tubes” to receive the drugs. They then offered to sell these drugs to patients of Novel Psychopharmacology.
Rahim Shafa was indicted on conspiracies of international money laundering, health care fraud, and defrauding the United States, as well as illegally importing merchandise and purposely delivering misbranded drugs. His wife was indicted on one count each of health care fraud conspiracy and international money laundering conspiracy.
Jury convicts medical equipment company owners of $27 million fraud
A federal jury in Texas convicted the owners of two durable medical equipment (DME) companies linked to a scheme to defraud Medicare.
Leah Hagen, 49, and Michael Hagen, 54, were convicted of one count of conspiracy to defraud the United States and to pay and receive health care kickbacks and one count of conspiracy to commit money laundering. The defendants owned and operated Metro DME Supply and Ortho Pain Solutions.
Ms. Hagen and Mr. Hagen paid a fixed rate per DME item in exchange for prescriptions and paperwork completed by telemedicine doctors that were used to submit false claims to Medicare, which totaled about $59 million. They were paid $27 million, and wired millions to their personal bank accounts. The defendants paid illegal bribes and kickbacks and wired money to their co-conspirator’s call center in the Philippines that provided signed doctor’s orders for orthotic braces.
At trial, evidence showed emails between Leah and Michael Hagen and their co-conspirators outlining a per-product pricing structure for orthotic braces, but not disclosing their agreement as one for marketing and other services.
At sentencing, the Hagens each face a maximum sentence of 25 years in prison.
A version of this article first appeared on Medscape.com.
Avanos medical to pay $22 million to resolve criminal charge for fraudulent misbranding of PPE
A U.S.-based multinational medical device corporation will pay more than $22 million to resolve a criminal charge regarding fraudulent misbranding of their surgical gowns.
Avanos Medical Inc, which as its U.S. headquarters in Alpharetta, Georgia, is charged with one count of introducing misbranded surgical gowns into interstate commerce with the intent to defraud and mislead.
According to the Department of Justice, the company knowingly falsely labeled its MicroCool surgical gowns as providing AAMI Level 4 protection (the highest level) against fluid and virus penetration. Under the standards set by the American National Standards Institute (ANSI) and the Association for the Advancement of Medical Instrumentation (AAMI), the highest protection level for surgical gowns is reserved for gowns intended to be used in surgeries and other high-risk medical procedures on patients suspected of having infectious diseases.
Avanos admitted to selling hundreds of thousands of MicroCool gowns that were falsely labeled as AAMI Level 4 between late 2014 and early 2015, as well as directly lying to customers about the gowns’ protective capacities. In total, Avanos sold almost $9 million of misbranded MicroCool gowns.
“The last thing health care workers should have to worry about is whether their personal protective equipment lives up to manufacturers’ claims,” said Acting U.S. Attorney Prerak Shah for the Northern District of Texas. “Misbranded PPE can pose serious risks to medical professionals and patients alike.”
Company pays $38.75 million to settle allegations of knowingly selling defective devices
Medical device manufacturers Alere and Alere San Diego (collectively, Alere) have agreed to pay almost $39 million to resolve allegations that they violated the False Claims Act by billing, and causing others to bill, the Medicare program for defective rapid point-of-care testing devices.
From 2008 to 2016, the Department of Justice alleges, Alere knowingly sold defective INRatio blood coagulation monitors used by Medicare beneficiaries who were taking anticoagulants. The software algorithms in the monitors contained a material defect, which Alere had found in their research, to cause inaccurate readings. Blood coagulation monitoring is essential for the safety of these patients, enabling them to maintain a safe dosage of their medications. Taking too much of an anticoagulant can cause major bleeding, while taking too little can cause blood clots that lead to strokes.
While Alere was aware that these devices were linked to over a dozen deaths and hundreds of injuries, the company continued to conceal the defect and billed Medicare for the devices.
In 2016, the product was taken off the market at the request of the FDA.
Mass. doctor, wife charged in international money laundering, fraud scheme
Massachusetts psychiatrist Rahim Shafa, MD, and his wife and office manager, Nahid Tormosi Shafa, are charged in connection to an international money laundering scheme involving importing illegal and misbranded drugs.
Through Shafa’s company, Novel Psychopharmacology, the two allegedly filed false and fraudulent Medicare reimbursement claims from 2016-2019, then deposited the money in their bank accounts, according to federal officials. From 2008-2018, the couple also engaged in an international money laundering scheme to purchase naltrexone pellet implants, disulfiram pellet implants, and injections from Hong Kong that were not approved by the FDA. According to officials, they falsified shipping documents, disguising the naltrexone pellet implants as “plastic beads in plastic tubes” to receive the drugs. They then offered to sell these drugs to patients of Novel Psychopharmacology.
Rahim Shafa was indicted on conspiracies of international money laundering, health care fraud, and defrauding the United States, as well as illegally importing merchandise and purposely delivering misbranded drugs. His wife was indicted on one count each of health care fraud conspiracy and international money laundering conspiracy.
Jury convicts medical equipment company owners of $27 million fraud
A federal jury in Texas convicted the owners of two durable medical equipment (DME) companies linked to a scheme to defraud Medicare.
Leah Hagen, 49, and Michael Hagen, 54, were convicted of one count of conspiracy to defraud the United States and to pay and receive health care kickbacks and one count of conspiracy to commit money laundering. The defendants owned and operated Metro DME Supply and Ortho Pain Solutions.
Ms. Hagen and Mr. Hagen paid a fixed rate per DME item in exchange for prescriptions and paperwork completed by telemedicine doctors that were used to submit false claims to Medicare, which totaled about $59 million. They were paid $27 million, and wired millions to their personal bank accounts. The defendants paid illegal bribes and kickbacks and wired money to their co-conspirator’s call center in the Philippines that provided signed doctor’s orders for orthotic braces.
At trial, evidence showed emails between Leah and Michael Hagen and their co-conspirators outlining a per-product pricing structure for orthotic braces, but not disclosing their agreement as one for marketing and other services.
At sentencing, the Hagens each face a maximum sentence of 25 years in prison.
A version of this article first appeared on Medscape.com.
Novel drug effective for essential tremor, but with significant side effects
new research suggests.
The phase 2 KINETIC trial involved patients with essential tremor. Among patients treated with SAGE-324 for 28 days, there was a statistically significant reduction in upper-limb tremors on day 29 – meeting the primary endpoint of the study.
However, moderate to severe treatment-emergent adverse events (TEAEs) led to many treatment and/or study discontinuations, the investigators reported.
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
Mechanism of action
Essential tremor affects an estimated 6.4 million adults in the United States. Available drugs are not helpful for 30%-50% of these patients. No new drug for this condition has been approved by the Food and Drug Administration for the past 50 years. Of the several drugs used to treat essential tremor, propranolol is the only one that has been approved, according to the American Academy of Neurology.
Deficits in inhibitory signaling via the gamma-aminobutyric acid system may have a role in the pathophysiology of essential tremor because the GABAergic system is the major neuroinhibitory system in the brain.
SAGE-324 is a steroid-positive allosteric modulator of the GABAA receptor. It acts on the receptor distant from the neuronal synapse to enhance GABAergic (inhibitory) signaling.
In the phase 2 multicenter KINETIC trial, investigators enrolled 69 patients aged 18-80 years. The patients had moderate to severe essential tremor, as determined on the basis of their having a score of 10 or higher on item 4 of the Essential Tremor Rating Assessment Scale (TETRAS) on screening day and at baseline/day 1 of the trial.
Participants did not take medications for essential tremor during the 28-day washout period. They were randomly assigned in a 1:1 ratio to receive SAGE-324 60 mg (n = 34) or placebo (n = 35) once daily. Dose reductions were allowed.
The groups were reasonably matched for age (mean, 69.4 years for SAGE-324 vs. 64.7 years for placebo) and dominant hand (right, 85.3% for SAGE-324 vs. 88.6% for placebo). Women composed 35.3% of the drug group and 57.1% of the placebo group.
The primary endpoint of the trial was change from baseline for the active drug in comparison with placebo on day 29 (1 day after the final dose) for upper-limb tremor, as measured by item 4 of TETRAS. There was also a 2-week follow-up with assessments on day 42.
Primary endpoint met, high dropout rate
Baseline mean TETRAS Performance Subscale item 4 scores were 12.82 for the SAGE-324 group and 12.28 for the placebo group.
On day 29, the least squares mean difference from baseline was –2.31 with SAGE-324 (n = 21) versus –1.24 with placebo (n = 33; P = .049). There was no difference between the SAGE-324 and placebo groups on day 42.
“Their significant reduction in upper-limb tremor score at day 29 corresponds to a 36% reduction from baseline in tremor amplitude in patients receiving SAGE-324, compared with a 21% reduction in tremor amplitude in patients receiving a placebo,” said lead investigator Kemi Bankole, MBBCh, of Sage Therapeutics.
“A reduction in tremor amplitude of 36% is a clinically significant improvement for most patients with essential tremor. For patients with moderate-severe tremor, a 41% improvement would be clinically noticeable and appreciated,” said Helen Colquhoun, MBChB, vice president at Sage.
“We believe patients with more severe tremor, that is, a TETRAS score of greater than 12, represent the majority of [essential tremor] patients getting diagnosed and seeking treatment today,” Dr. Colquhoun said.
There was an even greater reduction in tremor amplitude for the subgroup of patients with more severe tremor at baseline, meaning those with a median TETRAS score of 12 or greater (–2.75 for SAGE-324 vs. –1.05 for placebo; P = .0066).
These figures represented a 41% reduction from baseline in tremor amplitude for the SAGE-324 group, versus an 18% reduction in the placebo group. Again, the effect had disappeared in comparison with placebo at the 2-week off-drug follow-up on day 42.
Tolerability of SAGE-324 was a major problem, leading to dose reductions, treatment discontinuations, and study discontinuations. Of the 34 patients who received SAGE-324, 13 dropped out of the study, compared with 2 of 35 patients who received placebo.
Most TEAEs were moderate or severe in the SAGE-324 group, whereas most were mild in the placebo group.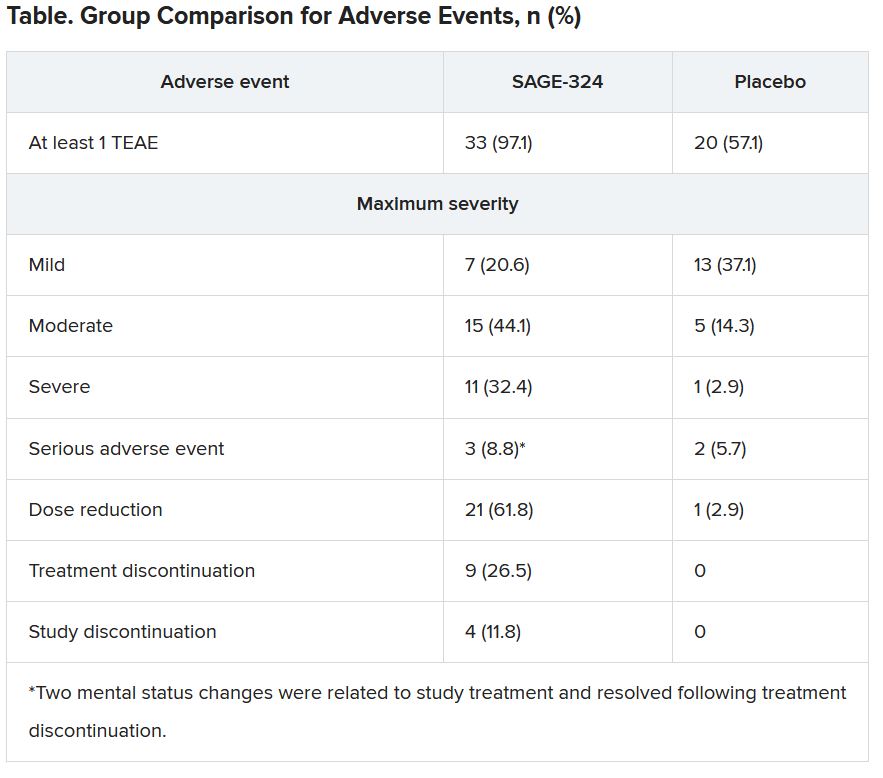
The most common TEAEs for participants who received SAGE-324 were somnolence (67.6%) and dizziness (38.2%), followed by balance problems, diplopia, dysarthria, and gait disturbance. In the placebo group, somnolence affected 5.7%, and dizziness affected 11.4%. There were no deaths in either group.
Dr. Colquhoun said these findings “are in line with our expectations for the 60-mg dose.”
More than one-third of the SAGE-324 group discontinued treatment before the end of the trial, and continuing treatment often required dose reductions. Only 24% completed the trial while taking the 60-mg dose; 15% completed the trial while taking 45 mg; and 24% did so while taking 30 mg.
Dr. Colquhoun noted that the company plans to initiate a phase 2b dose-ranging study later this year to optimize the dosing regimen with regard to tolerability and sustained tremor control.
No advantage over older drugs?
Commenting on the findings, Michele Tagliati, MD, director of the movement disorders program at Cedars-Sinai Medical Center, Los Angeles, said he had been aware of the study and was interested in seeing the results. However, he does not see an advantage with this drug, compared with what is already used for essential tremor.
“The response of people is not that different than when we treat them with the old barbiturates and benzodiazepines,” said Dr. Tagliati, who was not involved with the research.
He also noted the high rate of adverse events, particularly somnolence, and said that in his experience with current treatments, some patients prefer to live with their tremors rather than be sleepy and not thinking well.
Dr. Tagliati said he thinks use of SAGE-324 is going to be limited to patients who can tolerate it, “which was not that many.”
In addition, the trial was limited by its relatively small size, a “huge placebo effect,” and a high dropout rate in the active treatment arm, he concluded.
The study was funded by Sage Therapeutics and Biogen. Dr. Bankole and Dr. Calquhoun are employees of Sage. Dr. Tagliati reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
The phase 2 KINETIC trial involved patients with essential tremor. Among patients treated with SAGE-324 for 28 days, there was a statistically significant reduction in upper-limb tremors on day 29 – meeting the primary endpoint of the study.
However, moderate to severe treatment-emergent adverse events (TEAEs) led to many treatment and/or study discontinuations, the investigators reported.
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
Mechanism of action
Essential tremor affects an estimated 6.4 million adults in the United States. Available drugs are not helpful for 30%-50% of these patients. No new drug for this condition has been approved by the Food and Drug Administration for the past 50 years. Of the several drugs used to treat essential tremor, propranolol is the only one that has been approved, according to the American Academy of Neurology.
Deficits in inhibitory signaling via the gamma-aminobutyric acid system may have a role in the pathophysiology of essential tremor because the GABAergic system is the major neuroinhibitory system in the brain.
SAGE-324 is a steroid-positive allosteric modulator of the GABAA receptor. It acts on the receptor distant from the neuronal synapse to enhance GABAergic (inhibitory) signaling.
In the phase 2 multicenter KINETIC trial, investigators enrolled 69 patients aged 18-80 years. The patients had moderate to severe essential tremor, as determined on the basis of their having a score of 10 or higher on item 4 of the Essential Tremor Rating Assessment Scale (TETRAS) on screening day and at baseline/day 1 of the trial.
Participants did not take medications for essential tremor during the 28-day washout period. They were randomly assigned in a 1:1 ratio to receive SAGE-324 60 mg (n = 34) or placebo (n = 35) once daily. Dose reductions were allowed.
The groups were reasonably matched for age (mean, 69.4 years for SAGE-324 vs. 64.7 years for placebo) and dominant hand (right, 85.3% for SAGE-324 vs. 88.6% for placebo). Women composed 35.3% of the drug group and 57.1% of the placebo group.
The primary endpoint of the trial was change from baseline for the active drug in comparison with placebo on day 29 (1 day after the final dose) for upper-limb tremor, as measured by item 4 of TETRAS. There was also a 2-week follow-up with assessments on day 42.
Primary endpoint met, high dropout rate
Baseline mean TETRAS Performance Subscale item 4 scores were 12.82 for the SAGE-324 group and 12.28 for the placebo group.
On day 29, the least squares mean difference from baseline was –2.31 with SAGE-324 (n = 21) versus –1.24 with placebo (n = 33; P = .049). There was no difference between the SAGE-324 and placebo groups on day 42.
“Their significant reduction in upper-limb tremor score at day 29 corresponds to a 36% reduction from baseline in tremor amplitude in patients receiving SAGE-324, compared with a 21% reduction in tremor amplitude in patients receiving a placebo,” said lead investigator Kemi Bankole, MBBCh, of Sage Therapeutics.
“A reduction in tremor amplitude of 36% is a clinically significant improvement for most patients with essential tremor. For patients with moderate-severe tremor, a 41% improvement would be clinically noticeable and appreciated,” said Helen Colquhoun, MBChB, vice president at Sage.
“We believe patients with more severe tremor, that is, a TETRAS score of greater than 12, represent the majority of [essential tremor] patients getting diagnosed and seeking treatment today,” Dr. Colquhoun said.
There was an even greater reduction in tremor amplitude for the subgroup of patients with more severe tremor at baseline, meaning those with a median TETRAS score of 12 or greater (–2.75 for SAGE-324 vs. –1.05 for placebo; P = .0066).
These figures represented a 41% reduction from baseline in tremor amplitude for the SAGE-324 group, versus an 18% reduction in the placebo group. Again, the effect had disappeared in comparison with placebo at the 2-week off-drug follow-up on day 42.
Tolerability of SAGE-324 was a major problem, leading to dose reductions, treatment discontinuations, and study discontinuations. Of the 34 patients who received SAGE-324, 13 dropped out of the study, compared with 2 of 35 patients who received placebo.
Most TEAEs were moderate or severe in the SAGE-324 group, whereas most were mild in the placebo group.
The most common TEAEs for participants who received SAGE-324 were somnolence (67.6%) and dizziness (38.2%), followed by balance problems, diplopia, dysarthria, and gait disturbance. In the placebo group, somnolence affected 5.7%, and dizziness affected 11.4%. There were no deaths in either group.
Dr. Colquhoun said these findings “are in line with our expectations for the 60-mg dose.”
More than one-third of the SAGE-324 group discontinued treatment before the end of the trial, and continuing treatment often required dose reductions. Only 24% completed the trial while taking the 60-mg dose; 15% completed the trial while taking 45 mg; and 24% did so while taking 30 mg.
Dr. Colquhoun noted that the company plans to initiate a phase 2b dose-ranging study later this year to optimize the dosing regimen with regard to tolerability and sustained tremor control.
No advantage over older drugs?
Commenting on the findings, Michele Tagliati, MD, director of the movement disorders program at Cedars-Sinai Medical Center, Los Angeles, said he had been aware of the study and was interested in seeing the results. However, he does not see an advantage with this drug, compared with what is already used for essential tremor.
“The response of people is not that different than when we treat them with the old barbiturates and benzodiazepines,” said Dr. Tagliati, who was not involved with the research.
He also noted the high rate of adverse events, particularly somnolence, and said that in his experience with current treatments, some patients prefer to live with their tremors rather than be sleepy and not thinking well.
Dr. Tagliati said he thinks use of SAGE-324 is going to be limited to patients who can tolerate it, “which was not that many.”
In addition, the trial was limited by its relatively small size, a “huge placebo effect,” and a high dropout rate in the active treatment arm, he concluded.
The study was funded by Sage Therapeutics and Biogen. Dr. Bankole and Dr. Calquhoun are employees of Sage. Dr. Tagliati reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
The phase 2 KINETIC trial involved patients with essential tremor. Among patients treated with SAGE-324 for 28 days, there was a statistically significant reduction in upper-limb tremors on day 29 – meeting the primary endpoint of the study.
However, moderate to severe treatment-emergent adverse events (TEAEs) led to many treatment and/or study discontinuations, the investigators reported.
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
Mechanism of action
Essential tremor affects an estimated 6.4 million adults in the United States. Available drugs are not helpful for 30%-50% of these patients. No new drug for this condition has been approved by the Food and Drug Administration for the past 50 years. Of the several drugs used to treat essential tremor, propranolol is the only one that has been approved, according to the American Academy of Neurology.
Deficits in inhibitory signaling via the gamma-aminobutyric acid system may have a role in the pathophysiology of essential tremor because the GABAergic system is the major neuroinhibitory system in the brain.
SAGE-324 is a steroid-positive allosteric modulator of the GABAA receptor. It acts on the receptor distant from the neuronal synapse to enhance GABAergic (inhibitory) signaling.
In the phase 2 multicenter KINETIC trial, investigators enrolled 69 patients aged 18-80 years. The patients had moderate to severe essential tremor, as determined on the basis of their having a score of 10 or higher on item 4 of the Essential Tremor Rating Assessment Scale (TETRAS) on screening day and at baseline/day 1 of the trial.
Participants did not take medications for essential tremor during the 28-day washout period. They were randomly assigned in a 1:1 ratio to receive SAGE-324 60 mg (n = 34) or placebo (n = 35) once daily. Dose reductions were allowed.
The groups were reasonably matched for age (mean, 69.4 years for SAGE-324 vs. 64.7 years for placebo) and dominant hand (right, 85.3% for SAGE-324 vs. 88.6% for placebo). Women composed 35.3% of the drug group and 57.1% of the placebo group.
The primary endpoint of the trial was change from baseline for the active drug in comparison with placebo on day 29 (1 day after the final dose) for upper-limb tremor, as measured by item 4 of TETRAS. There was also a 2-week follow-up with assessments on day 42.
Primary endpoint met, high dropout rate
Baseline mean TETRAS Performance Subscale item 4 scores were 12.82 for the SAGE-324 group and 12.28 for the placebo group.
On day 29, the least squares mean difference from baseline was –2.31 with SAGE-324 (n = 21) versus –1.24 with placebo (n = 33; P = .049). There was no difference between the SAGE-324 and placebo groups on day 42.
“Their significant reduction in upper-limb tremor score at day 29 corresponds to a 36% reduction from baseline in tremor amplitude in patients receiving SAGE-324, compared with a 21% reduction in tremor amplitude in patients receiving a placebo,” said lead investigator Kemi Bankole, MBBCh, of Sage Therapeutics.
“A reduction in tremor amplitude of 36% is a clinically significant improvement for most patients with essential tremor. For patients with moderate-severe tremor, a 41% improvement would be clinically noticeable and appreciated,” said Helen Colquhoun, MBChB, vice president at Sage.
“We believe patients with more severe tremor, that is, a TETRAS score of greater than 12, represent the majority of [essential tremor] patients getting diagnosed and seeking treatment today,” Dr. Colquhoun said.
There was an even greater reduction in tremor amplitude for the subgroup of patients with more severe tremor at baseline, meaning those with a median TETRAS score of 12 or greater (–2.75 for SAGE-324 vs. –1.05 for placebo; P = .0066).
These figures represented a 41% reduction from baseline in tremor amplitude for the SAGE-324 group, versus an 18% reduction in the placebo group. Again, the effect had disappeared in comparison with placebo at the 2-week off-drug follow-up on day 42.
Tolerability of SAGE-324 was a major problem, leading to dose reductions, treatment discontinuations, and study discontinuations. Of the 34 patients who received SAGE-324, 13 dropped out of the study, compared with 2 of 35 patients who received placebo.
Most TEAEs were moderate or severe in the SAGE-324 group, whereas most were mild in the placebo group.
The most common TEAEs for participants who received SAGE-324 were somnolence (67.6%) and dizziness (38.2%), followed by balance problems, diplopia, dysarthria, and gait disturbance. In the placebo group, somnolence affected 5.7%, and dizziness affected 11.4%. There were no deaths in either group.
Dr. Colquhoun said these findings “are in line with our expectations for the 60-mg dose.”
More than one-third of the SAGE-324 group discontinued treatment before the end of the trial, and continuing treatment often required dose reductions. Only 24% completed the trial while taking the 60-mg dose; 15% completed the trial while taking 45 mg; and 24% did so while taking 30 mg.
Dr. Colquhoun noted that the company plans to initiate a phase 2b dose-ranging study later this year to optimize the dosing regimen with regard to tolerability and sustained tremor control.
No advantage over older drugs?
Commenting on the findings, Michele Tagliati, MD, director of the movement disorders program at Cedars-Sinai Medical Center, Los Angeles, said he had been aware of the study and was interested in seeing the results. However, he does not see an advantage with this drug, compared with what is already used for essential tremor.
“The response of people is not that different than when we treat them with the old barbiturates and benzodiazepines,” said Dr. Tagliati, who was not involved with the research.
He also noted the high rate of adverse events, particularly somnolence, and said that in his experience with current treatments, some patients prefer to live with their tremors rather than be sleepy and not thinking well.
Dr. Tagliati said he thinks use of SAGE-324 is going to be limited to patients who can tolerate it, “which was not that many.”
In addition, the trial was limited by its relatively small size, a “huge placebo effect,” and a high dropout rate in the active treatment arm, he concluded.
The study was funded by Sage Therapeutics and Biogen. Dr. Bankole and Dr. Calquhoun are employees of Sage. Dr. Tagliati reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM MDS VIRTUAL CONGRESS 2021
Apple devices identify early Parkinson’s disease
, new research shows. Results from the WATCH-PD study show clear differences in a finger-tapping task in the Parkinson’s disease versus control group. The finger-tapping task also correlated with “traditional measures,” such as the Movement Disorder Society–Unified Parkinson’s Disease Rating Scale (MDS-UPDRS), investigators reported.
“And then the smartphone and smartwatch also showed differences in gait between groups,” said lead investigator Jamie Adams, MD, University of Rochester, New York.
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
WATCH-PD
The 12-month WATCH-PD study included 132 individuals at 17 Parkinson’s Study Group sites, 82 with Parkinson’s disease and 50 controls.
Participants with Parkinson’s disease were untreated, were no more than 2 years out from diagnosis (mean disease duration, 10.0 ±7.3 months), and were in Hoehn and Yahr stage 1 or 2.
Apple Watches and iPhones were provided to participants, all of whom underwent in-clinic assessments at baseline and at months 1, 3, 6, 9, and 12. The assessments included motor and cognitive tasks using the devices, which contained motion sensors.
The phone also contained an app that could assess verbal, cognitive, and other abilities. Participants wore a set of inertial sensors (APDM Mobility Lab) while performing the MDS-UPDRS Part III motor examination.
In addition, there were biweekly at-home tasks. Questions and tests on the watch assessed symptoms of mood, fatigue, cognition, and falls as well as cognitive performance involving perceptual, verbal, visual spatial, and fine motor abilities. Both the watch and iPhone were used to gauge gait, balance, and tremor.
Ages of the participants were approximately the same in the Parkinson’s disease and control groups (63.3 years vs. 60.2 years, respectively), but male to female ratios differed between the groups. There were more men in the Parkinson’s disease cohort (56% men vs. 44% women) and more women in the control cohort (36% vs. 64%; P =.03).
Between-group differences
Results showed that MDS-UPDRS total scores and on all individual parts of the rating scale were significantly better for the control group (lower scores are better), as shown in the following table.
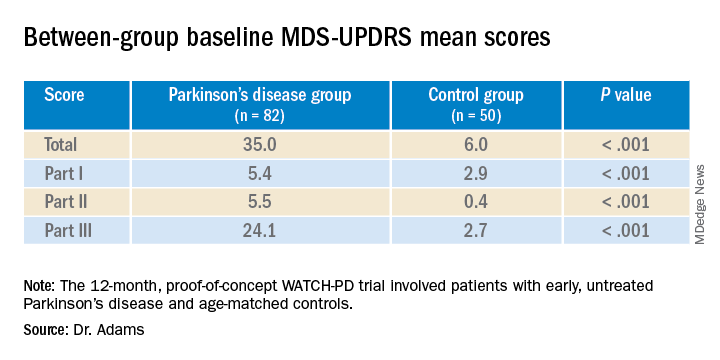
Similarly, the control group performed better than the Parkinson’s disease group on the Montreal Cognitive Assessment (MoCA), with higher scores showing better performance on the 0 to 30 scale (28.1 vs. 27.6, respectively).
Touchscreen assessments on the phone also showed group differences in a finger-tapping task, with more taps by the control group than by the Parkinson’s disease group. The difference was more pronounced when the dominant hand was used.
The median numbers of taps in 20 seconds for the dominant hand were 103.7 for the Parkinson’s disease cohort versus 131.9 for control cohort (P < .005); and for the nondominant hand the numbers of taps were 106.6 versus 122.1 (P < .05), respectively. The control group also scored better on tests of hand fine-motor control (P < .01) and on the mobile digit symbols modalities test (P < .05)
Measures of gait in a 1-minute walk test also showed group differences.
“The five gait measures that differed most were cadence, which is steps per minute, double support, arm swing amplitude, arm swing variation, and turn duration,” Dr. Adams said.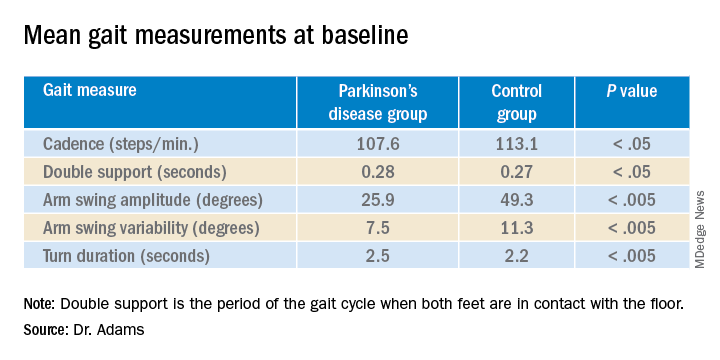
‘Tremendous interest’
Commenting on the findings, Ludy Shih, MD, MMSc, of Boston University, noted that in the future, devices such as the ones used in this study may help clinicians remotely monitor their patients’ Parkinson’s disease conditions and response to therapy.
That would “eliminate some of the transportation barrier for people with Parkinson’s disease,” said Dr. Shih, who was not involved with the research.
The devices can give objective measurements, reducing inter-rater variability in assessment of movements, she noted.
“I think there’s tremendous interest in using digital measures to pick up on subtle disease phenotypes earlier than a clinical diagnosis can be made,” Dr. Shih said.
She also referred to literature “going back a few decades” showing that finger tapping can be used as a pharmacodynamic measure of how well a patient’s dopaminergic medications are working, so the devices may be a way to remotely assess treatment efficacy and decide when it is time to make adjustments.
Dr. Shih said she thinks regulatory agencies are now open “to consider these as part of the totality of evidence that a therapeutic [device] might be working.”
Whether these would need to be professional grade and approved as medical devices or if patients could just buy smartwatches and smartphones to generate useful data is still a question, she said. Already, there are several Parkinson’s apps that the public can download to track symptoms, improve voice, provide exercises, find support groups or research studies, and more.
Dr. Shih predicted that the biweekly at-home tasks, as in the current protocol, could be a burden to some people. If only a segment of the population were willing to comply, it could call into question how generalizable the results were, she added.
“There’s even a prior publication showing that compliance rate really dropped like a rock,” she noted. However, for those people willing to perform the tasks on a regular schedule, the results could be valuable, Dr. Shih said.
Dr. Adams concurred, saying that she had received feedback from some of her study participants that the biweekly tasks were a bit much.
The study was supported by Biogen and Takeda Pharmaceuticals. Dr. Adams receives research support from Biogen. Dr. Shih has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows. Results from the WATCH-PD study show clear differences in a finger-tapping task in the Parkinson’s disease versus control group. The finger-tapping task also correlated with “traditional measures,” such as the Movement Disorder Society–Unified Parkinson’s Disease Rating Scale (MDS-UPDRS), investigators reported.
“And then the smartphone and smartwatch also showed differences in gait between groups,” said lead investigator Jamie Adams, MD, University of Rochester, New York.
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
WATCH-PD
The 12-month WATCH-PD study included 132 individuals at 17 Parkinson’s Study Group sites, 82 with Parkinson’s disease and 50 controls.
Participants with Parkinson’s disease were untreated, were no more than 2 years out from diagnosis (mean disease duration, 10.0 ±7.3 months), and were in Hoehn and Yahr stage 1 or 2.
Apple Watches and iPhones were provided to participants, all of whom underwent in-clinic assessments at baseline and at months 1, 3, 6, 9, and 12. The assessments included motor and cognitive tasks using the devices, which contained motion sensors.
The phone also contained an app that could assess verbal, cognitive, and other abilities. Participants wore a set of inertial sensors (APDM Mobility Lab) while performing the MDS-UPDRS Part III motor examination.
In addition, there were biweekly at-home tasks. Questions and tests on the watch assessed symptoms of mood, fatigue, cognition, and falls as well as cognitive performance involving perceptual, verbal, visual spatial, and fine motor abilities. Both the watch and iPhone were used to gauge gait, balance, and tremor.
Ages of the participants were approximately the same in the Parkinson’s disease and control groups (63.3 years vs. 60.2 years, respectively), but male to female ratios differed between the groups. There were more men in the Parkinson’s disease cohort (56% men vs. 44% women) and more women in the control cohort (36% vs. 64%; P =.03).
Between-group differences
Results showed that MDS-UPDRS total scores and on all individual parts of the rating scale were significantly better for the control group (lower scores are better), as shown in the following table.

Similarly, the control group performed better than the Parkinson’s disease group on the Montreal Cognitive Assessment (MoCA), with higher scores showing better performance on the 0 to 30 scale (28.1 vs. 27.6, respectively).
Touchscreen assessments on the phone also showed group differences in a finger-tapping task, with more taps by the control group than by the Parkinson’s disease group. The difference was more pronounced when the dominant hand was used.
The median numbers of taps in 20 seconds for the dominant hand were 103.7 for the Parkinson’s disease cohort versus 131.9 for control cohort (P < .005); and for the nondominant hand the numbers of taps were 106.6 versus 122.1 (P < .05), respectively. The control group also scored better on tests of hand fine-motor control (P < .01) and on the mobile digit symbols modalities test (P < .05)
Measures of gait in a 1-minute walk test also showed group differences.
“The five gait measures that differed most were cadence, which is steps per minute, double support, arm swing amplitude, arm swing variation, and turn duration,” Dr. Adams said.
‘Tremendous interest’
Commenting on the findings, Ludy Shih, MD, MMSc, of Boston University, noted that in the future, devices such as the ones used in this study may help clinicians remotely monitor their patients’ Parkinson’s disease conditions and response to therapy.
That would “eliminate some of the transportation barrier for people with Parkinson’s disease,” said Dr. Shih, who was not involved with the research.
The devices can give objective measurements, reducing inter-rater variability in assessment of movements, she noted.
“I think there’s tremendous interest in using digital measures to pick up on subtle disease phenotypes earlier than a clinical diagnosis can be made,” Dr. Shih said.
She also referred to literature “going back a few decades” showing that finger tapping can be used as a pharmacodynamic measure of how well a patient’s dopaminergic medications are working, so the devices may be a way to remotely assess treatment efficacy and decide when it is time to make adjustments.
Dr. Shih said she thinks regulatory agencies are now open “to consider these as part of the totality of evidence that a therapeutic [device] might be working.”
Whether these would need to be professional grade and approved as medical devices or if patients could just buy smartwatches and smartphones to generate useful data is still a question, she said. Already, there are several Parkinson’s apps that the public can download to track symptoms, improve voice, provide exercises, find support groups or research studies, and more.
Dr. Shih predicted that the biweekly at-home tasks, as in the current protocol, could be a burden to some people. If only a segment of the population were willing to comply, it could call into question how generalizable the results were, she added.
“There’s even a prior publication showing that compliance rate really dropped like a rock,” she noted. However, for those people willing to perform the tasks on a regular schedule, the results could be valuable, Dr. Shih said.
Dr. Adams concurred, saying that she had received feedback from some of her study participants that the biweekly tasks were a bit much.
The study was supported by Biogen and Takeda Pharmaceuticals. Dr. Adams receives research support from Biogen. Dr. Shih has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows. Results from the WATCH-PD study show clear differences in a finger-tapping task in the Parkinson’s disease versus control group. The finger-tapping task also correlated with “traditional measures,” such as the Movement Disorder Society–Unified Parkinson’s Disease Rating Scale (MDS-UPDRS), investigators reported.
“And then the smartphone and smartwatch also showed differences in gait between groups,” said lead investigator Jamie Adams, MD, University of Rochester, New York.
The findings were presented at the International Congress of Parkinson’s Disease and Movement Disorders.
WATCH-PD
The 12-month WATCH-PD study included 132 individuals at 17 Parkinson’s Study Group sites, 82 with Parkinson’s disease and 50 controls.
Participants with Parkinson’s disease were untreated, were no more than 2 years out from diagnosis (mean disease duration, 10.0 ±7.3 months), and were in Hoehn and Yahr stage 1 or 2.
Apple Watches and iPhones were provided to participants, all of whom underwent in-clinic assessments at baseline and at months 1, 3, 6, 9, and 12. The assessments included motor and cognitive tasks using the devices, which contained motion sensors.
The phone also contained an app that could assess verbal, cognitive, and other abilities. Participants wore a set of inertial sensors (APDM Mobility Lab) while performing the MDS-UPDRS Part III motor examination.
In addition, there were biweekly at-home tasks. Questions and tests on the watch assessed symptoms of mood, fatigue, cognition, and falls as well as cognitive performance involving perceptual, verbal, visual spatial, and fine motor abilities. Both the watch and iPhone were used to gauge gait, balance, and tremor.
Ages of the participants were approximately the same in the Parkinson’s disease and control groups (63.3 years vs. 60.2 years, respectively), but male to female ratios differed between the groups. There were more men in the Parkinson’s disease cohort (56% men vs. 44% women) and more women in the control cohort (36% vs. 64%; P =.03).
Between-group differences
Results showed that MDS-UPDRS total scores and on all individual parts of the rating scale were significantly better for the control group (lower scores are better), as shown in the following table.

Similarly, the control group performed better than the Parkinson’s disease group on the Montreal Cognitive Assessment (MoCA), with higher scores showing better performance on the 0 to 30 scale (28.1 vs. 27.6, respectively).
Touchscreen assessments on the phone also showed group differences in a finger-tapping task, with more taps by the control group than by the Parkinson’s disease group. The difference was more pronounced when the dominant hand was used.
The median numbers of taps in 20 seconds for the dominant hand were 103.7 for the Parkinson’s disease cohort versus 131.9 for control cohort (P < .005); and for the nondominant hand the numbers of taps were 106.6 versus 122.1 (P < .05), respectively. The control group also scored better on tests of hand fine-motor control (P < .01) and on the mobile digit symbols modalities test (P < .05)
Measures of gait in a 1-minute walk test also showed group differences.
“The five gait measures that differed most were cadence, which is steps per minute, double support, arm swing amplitude, arm swing variation, and turn duration,” Dr. Adams said.
‘Tremendous interest’
Commenting on the findings, Ludy Shih, MD, MMSc, of Boston University, noted that in the future, devices such as the ones used in this study may help clinicians remotely monitor their patients’ Parkinson’s disease conditions and response to therapy.
That would “eliminate some of the transportation barrier for people with Parkinson’s disease,” said Dr. Shih, who was not involved with the research.
The devices can give objective measurements, reducing inter-rater variability in assessment of movements, she noted.
“I think there’s tremendous interest in using digital measures to pick up on subtle disease phenotypes earlier than a clinical diagnosis can be made,” Dr. Shih said.
She also referred to literature “going back a few decades” showing that finger tapping can be used as a pharmacodynamic measure of how well a patient’s dopaminergic medications are working, so the devices may be a way to remotely assess treatment efficacy and decide when it is time to make adjustments.
Dr. Shih said she thinks regulatory agencies are now open “to consider these as part of the totality of evidence that a therapeutic [device] might be working.”
Whether these would need to be professional grade and approved as medical devices or if patients could just buy smartwatches and smartphones to generate useful data is still a question, she said. Already, there are several Parkinson’s apps that the public can download to track symptoms, improve voice, provide exercises, find support groups or research studies, and more.
Dr. Shih predicted that the biweekly at-home tasks, as in the current protocol, could be a burden to some people. If only a segment of the population were willing to comply, it could call into question how generalizable the results were, she added.
“There’s even a prior publication showing that compliance rate really dropped like a rock,” she noted. However, for those people willing to perform the tasks on a regular schedule, the results could be valuable, Dr. Shih said.
Dr. Adams concurred, saying that she had received feedback from some of her study participants that the biweekly tasks were a bit much.
The study was supported by Biogen and Takeda Pharmaceuticals. Dr. Adams receives research support from Biogen. Dr. Shih has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM MDS VIRTUAL CONGRESS 2021
The soccer punch
Soccer is the most popular sport on Earth.
A recent JAMA Neurology article noted the incidence of neurodegenerative disease in retired professional soccer players. It found that, not surprisingly, the frequency of such was higher than in the general population, highest amongst defenders and lowest in goalkeepers (presumably because the latter can use their hands).
The point here shouldn’t surprise anyone: Repeatedly hitting your head on solid objects is a bad idea.
Somewhere, a long time ago, early vertebrates developed a bony case to protect their centralized nervous system. Its success is shown by the fact that skulls and spinal cords among vertebrates have more similarities than differences: They work. It’s true that some ungulates use their heads to fight, but their skulls are adapted for such, being thicker and having horns and antlers to lessen the impacts.
But humans? Nope. The skull can support up to nine tons of (slowly-applied) weight (don’t try this at home) but repeated impacts aren’t good for its contents.
There is no degree of external protection that will prevent this, either. We talk about helmets, but the reality is that, while they definitely reduce exterior injuries, they do very little to prevent the effects of rapid acceleration/deceleration on the brain inside. This is what results in concussions, coup & contra-coup injuries, and the shearing effects of diffuse axonal injury.
I’m not saying we should ban soccer, or football, or any of the other activities that clearly have a high risk of repeated head trauma. They’re ingrained into the cultures of our societies.
At this point it’s pretty much impossible for participants and their family members to not be aware of the risks posed by these sports. The popular press has covered it in great detail.
At some point there’s only so much you can warn people about. Like tobacco smoking or riding without a helmet, you accept the risks, fully aware of the serious potential consequences. For those who wish to participate, it’s their decision.
But it’s also time to stop blinding ourselves to the simple facts. Minimizing them, pointing out their delayed onset, and turning a blind eye won’t change that.
If we’re going to continue enjoying contact sports, we have to accept that someone is going to pay the price for it, even if they’ve been forewarned. And no amount of protective gear, at today’s technology, is going to change that.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Soccer is the most popular sport on Earth.
A recent JAMA Neurology article noted the incidence of neurodegenerative disease in retired professional soccer players. It found that, not surprisingly, the frequency of such was higher than in the general population, highest amongst defenders and lowest in goalkeepers (presumably because the latter can use their hands).
The point here shouldn’t surprise anyone: Repeatedly hitting your head on solid objects is a bad idea.
Somewhere, a long time ago, early vertebrates developed a bony case to protect their centralized nervous system. Its success is shown by the fact that skulls and spinal cords among vertebrates have more similarities than differences: They work. It’s true that some ungulates use their heads to fight, but their skulls are adapted for such, being thicker and having horns and antlers to lessen the impacts.
But humans? Nope. The skull can support up to nine tons of (slowly-applied) weight (don’t try this at home) but repeated impacts aren’t good for its contents.
There is no degree of external protection that will prevent this, either. We talk about helmets, but the reality is that, while they definitely reduce exterior injuries, they do very little to prevent the effects of rapid acceleration/deceleration on the brain inside. This is what results in concussions, coup & contra-coup injuries, and the shearing effects of diffuse axonal injury.
I’m not saying we should ban soccer, or football, or any of the other activities that clearly have a high risk of repeated head trauma. They’re ingrained into the cultures of our societies.
At this point it’s pretty much impossible for participants and their family members to not be aware of the risks posed by these sports. The popular press has covered it in great detail.
At some point there’s only so much you can warn people about. Like tobacco smoking or riding without a helmet, you accept the risks, fully aware of the serious potential consequences. For those who wish to participate, it’s their decision.
But it’s also time to stop blinding ourselves to the simple facts. Minimizing them, pointing out their delayed onset, and turning a blind eye won’t change that.
If we’re going to continue enjoying contact sports, we have to accept that someone is going to pay the price for it, even if they’ve been forewarned. And no amount of protective gear, at today’s technology, is going to change that.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Soccer is the most popular sport on Earth.
A recent JAMA Neurology article noted the incidence of neurodegenerative disease in retired professional soccer players. It found that, not surprisingly, the frequency of such was higher than in the general population, highest amongst defenders and lowest in goalkeepers (presumably because the latter can use their hands).
The point here shouldn’t surprise anyone: Repeatedly hitting your head on solid objects is a bad idea.
Somewhere, a long time ago, early vertebrates developed a bony case to protect their centralized nervous system. Its success is shown by the fact that skulls and spinal cords among vertebrates have more similarities than differences: They work. It’s true that some ungulates use their heads to fight, but their skulls are adapted for such, being thicker and having horns and antlers to lessen the impacts.
But humans? Nope. The skull can support up to nine tons of (slowly-applied) weight (don’t try this at home) but repeated impacts aren’t good for its contents.
There is no degree of external protection that will prevent this, either. We talk about helmets, but the reality is that, while they definitely reduce exterior injuries, they do very little to prevent the effects of rapid acceleration/deceleration on the brain inside. This is what results in concussions, coup & contra-coup injuries, and the shearing effects of diffuse axonal injury.
I’m not saying we should ban soccer, or football, or any of the other activities that clearly have a high risk of repeated head trauma. They’re ingrained into the cultures of our societies.
At this point it’s pretty much impossible for participants and their family members to not be aware of the risks posed by these sports. The popular press has covered it in great detail.
At some point there’s only so much you can warn people about. Like tobacco smoking or riding without a helmet, you accept the risks, fully aware of the serious potential consequences. For those who wish to participate, it’s their decision.
But it’s also time to stop blinding ourselves to the simple facts. Minimizing them, pointing out their delayed onset, and turning a blind eye won’t change that.
If we’re going to continue enjoying contact sports, we have to accept that someone is going to pay the price for it, even if they’ve been forewarned. And no amount of protective gear, at today’s technology, is going to change that.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Top questions answered about COVID-19 boosters for your patients
Confusion continues to circulate in the wake of decisions on booster doses of the Pfizer/BioNTech COVID-19 vaccine, all announced within 1 week. Many people – including those now eligible and those who officially have to wait for their shot at a third dose – have questions.
Multiple agencies are involved in the booster decisions, and they have put out multiple – and sometimes conflicting – messages about booster doses, leaving more questions than answers for many people.
On Sept. 22, the Food and Drug Administration granted an emergency use authorization (EUA) for a booster dose of the Pfizer mRNA COVID-19 vaccine for those 65 and older and those at high risk for severe illness from the coronavirus, including essential workers whose jobs increase their risk for infection – such as frontline health care workers.
The Centers for Disease Control and Prevention Director Rochelle Walensky, MD, then overruled advice from the agency’s Advisory Committee on Immunization Practices (ACIP) to recommend boosters for essential workers such as those working on the front lines during the pandemic.
As it stands now, the CDC recommends that the following groups should get a third dose of the Pfizer vaccine:
- People aged 65 years and older.
- People aged 18 years and older in long-term care settings.
- People aged 50-64 years with underlying medical conditions.
The CDC also recommends that the following groups may receive a booster shot of the Pfizer vaccine, based on their individual benefits and risks:
- People aged 18-49 years with underlying medical conditions.
- People aged 18-64 years at increased risk for COVID-19 exposure and transmission because of occupational or institutional setting.
The CDC currently considers the following groups at increased risk for COVID-19:
- First responders (health care workers, firefighters, police, congregate care staff).
- Education staff (teachers, support staff, day care workers).
- Food and agriculture workers.
- Manufacturing workers.
- Corrections workers.
- U.S. Postal Service workers.
- Public transit workers.
- Grocery store workers.
Health care professionals, among the most trusted sources of COVID-19 information, are likely to encounter a number of patients wondering how all this will work.
“It’s fantastic that boosters will be available for those who the data supports need [them],” Rachael Piltch-Loeb, PhD, said during a media briefing on Sept. 23, held between the FDA and CDC decisions.
“But we’re really in a place where we have a lot more questions and answers about what the next phase of the vaccine availability and updates are going to be in the United States,” added Dr. Piltch-Loeb, preparedness fellow in the division of policy translation and leadership development and a research associate in the department of biostatistics at the Harvard T. H. Chan School of Public Health in Boston.
1. What is the biggest concern you are hearing from patients about getting a booster?
“The biggest concerns are that everyone wants it and they don’t know where to get it. In health care’s defense, the CDC just figured out what to do,” said Janet Englund, MD, professor of pediatric infectious diseases and an infectious disease and virology expert at Seattle Children’s Hospital in Washington.
“Everyone thinks they should be eligible for a booster ... people in their 50s who are not yet 65+, people with young grandchildren, etc.,” she added. “I’m at Seattle Children’s Hospital, so people are asking about booster shots and about getting their children vaccinated.”
Boosters for all COVID-19 vaccines are completely free.
“All COVID-19 vaccines, including booster doses, will be provided free of charge to the U.S. population,” the CDC has said.
2. Will patients need to prove they meet eligibility criteria for a booster shot or will it be the honor system?
“No, patients will only need to attest that they fall into one of the high-risk groups for whom a booster vaccine is authorized,” said Robert Atmar, MD, professor of infectious diseases at Baylor College of Medicine in Houston.
Dr. Piltch-Loeb agreed. “It is likely to be an honor system. It is very unlikely that there will be punishments or other ramifications ... if doses are administered, beyond the approved usage.”
3. If a patient who had the Moderna or the Johnson and Johnson vaccination requests a booster, can health care workers give them Pfizer?
The short answer is no. “This only applies to individuals who have received the Pfizer vaccine,” Dr. Piltch-Loeb said.
More data will be needed before other vaccine boosters are authorized, she added.
“My understanding is the Moderna people have just recently submitted their information, all of their data to the FDA and J&J is in line to do that very shortly,” said William Schaffner, MD, professor of preventive medicine and infectious diseases at Vanderbilt University in Nashville, Tenn. “I would hope that within the next month to 6 weeks, we will get information about both of those vaccines,” Dr. Schaffner said.
4. When are the “mix-and-match” vaccine study results expected to come out?
“We expect that data from the study will be available in the coming weeks,” said Dr. Atmar, who is the national co-principal investigator of a mix-and-match booster trial launched in June 2021.
5. Are side effects of a booster vaccine expected to be about the same as what people experienced during their first or second immunization?
“I’m expecting the side effects will be similar to the second dose,” Dr. Englund said.
“The data presented ... at ACIP suggests that the side effects from the third shot are either the same or actually less than the first two shots,” said Carlos del Rio, MD, distinguished professor of medicine, epidemiology, and global health, and executive associate dean of Emory University School of Medicine at Grady Health System in Atlanta.
”Everyone reacts very differently to vaccines, regardless of vaccine type,” said Eric Ascher, MD, a family medicine physician at Lenox Hill Hospital in New York City. “I have had patients (as well as personal experience) where there were none to minimal symptoms, and others who felt they had a mild flu for 24 hours.”
“I expect no side effects greater than what was felt with you prior doses,” he said. “The vaccine is very safe and the benefit of vaccination outweighs the risks of any mild side effects.”
6. Is it unethical to give a booster to someone outside the approved groups if there are doses remaining at the end of the day in an open vial?
“Offering a booster shot to someone outside of approved groups if remaining doses will go to waste at the end of the day seems like a prudent decision, and relatively harmless action,” said Faith Fletcher, PhD, assistant professor at the Center for Medical Ethics and Health Policy at Baylor College of Medicine.
“However, if doses continue to fall in the laps of unapproved groups, we must evaluate the vaccine systems and structures that advantage some groups and disadvantage others,” she added. “We know that the distribution of COVID-19 vaccines has not been equitable – and some groups have been left behind.”
“I am not an ethicist and there are many competing concerns that this question addresses,” Dr. Atmar said. For example, “there is not a limitation of vaccine supply in the U.S., so that using leftover vaccine to prevent waste is no longer a major concern in the U.S.”
It could be more of a legal than ethical question, Dr. Atmar said. For an individual outside the authorized groups, legally, the FDA’s EUA for boosting does not allow the vaccine to be administered to this person, he said.
“The rationale for the restricted use in the EUA is that at this time the safety and risks associated with such administration are not known, and the benefits also have not been determined,” Dr. Atmar said. “Members of the ACIP raised concerns about other individuals who may potentially benefit from a booster but are not eligible and the importance of making boosters available to them, but from a legal standpoint – I am also not a lawyer, so this is my understanding – administration of the vaccine is limited to those identified in the EUA.”
7. What is the likelihood that one shot will combine COVID and flu protection in the near future?
It is not likely, Dr. Englund said. “The reason is that the flu vaccine changes so much, and it already has four different antigens. This is assuming we keep the same method of making the flu vaccine – the answer could be different if the flu vaccine becomes an mRNA vaccine in the future.”
Companies such as Moderna and Novavax are testing single-dose shots for COVID-19 and influenza, but they are still far from having anything ready for this flu season in the United States.
8. Is there any chance a booster shot distributed now will need to be redesigned for a future variant?
“Absolutely,” Dr. Englund said. “And a booster dose is the time we may want to consider re-engineering a vaccine.”
9. Do you think the FDA/CDC limitations on who is eligible for a booster was in any way influenced by the World Health Organization call for prioritizing shots for the unvaccinated in lower-resource countries?
“This is absolutely still a global problem,” Dr. Piltch-Loeb said. “We need to get more vaccine to more countries and more people as soon as possible, because if there’s anything we’ve seen about the variants it is that ... they can come from all different places.”
“That being said, I think that it is unlikely to change the course of action in the U.S.,” she added, when it comes to comparing the global need with the domestic policy priorities of the administration.
Dr. Atmar was more direct. “No,” he said. “The WHO recommends against boosting of anyone. The U.S. decisions about boosting those in this country who are eligible are aimed toward addressing perceived needs domestically at the same time that vaccines are being provided to other countries.
“The philosophy is to address both ‘needs’ at the same time,” Dr. Atmar said.
10. What does the future hold for booster shots?
“Predicting the future is really hard, especially when it involves COVID,” Dr. del Rio said.
“Having said that, COVID is not the flu, so I doubt there will be need for annual boosters. I think the population eligible for boosters will be expanded ... and the major population not addressed at this point is the people that received either Moderna or J&J [vaccines].”
Kelly Davis contributed to this feature. A version of this article first appeared on Medscape.com.
Confusion continues to circulate in the wake of decisions on booster doses of the Pfizer/BioNTech COVID-19 vaccine, all announced within 1 week. Many people – including those now eligible and those who officially have to wait for their shot at a third dose – have questions.
Multiple agencies are involved in the booster decisions, and they have put out multiple – and sometimes conflicting – messages about booster doses, leaving more questions than answers for many people.
On Sept. 22, the Food and Drug Administration granted an emergency use authorization (EUA) for a booster dose of the Pfizer mRNA COVID-19 vaccine for those 65 and older and those at high risk for severe illness from the coronavirus, including essential workers whose jobs increase their risk for infection – such as frontline health care workers.
The Centers for Disease Control and Prevention Director Rochelle Walensky, MD, then overruled advice from the agency’s Advisory Committee on Immunization Practices (ACIP) to recommend boosters for essential workers such as those working on the front lines during the pandemic.
As it stands now, the CDC recommends that the following groups should get a third dose of the Pfizer vaccine:
- People aged 65 years and older.
- People aged 18 years and older in long-term care settings.
- People aged 50-64 years with underlying medical conditions.
The CDC also recommends that the following groups may receive a booster shot of the Pfizer vaccine, based on their individual benefits and risks:
- People aged 18-49 years with underlying medical conditions.
- People aged 18-64 years at increased risk for COVID-19 exposure and transmission because of occupational or institutional setting.
The CDC currently considers the following groups at increased risk for COVID-19:
- First responders (health care workers, firefighters, police, congregate care staff).
- Education staff (teachers, support staff, day care workers).
- Food and agriculture workers.
- Manufacturing workers.
- Corrections workers.
- U.S. Postal Service workers.
- Public transit workers.
- Grocery store workers.
Health care professionals, among the most trusted sources of COVID-19 information, are likely to encounter a number of patients wondering how all this will work.
“It’s fantastic that boosters will be available for those who the data supports need [them],” Rachael Piltch-Loeb, PhD, said during a media briefing on Sept. 23, held between the FDA and CDC decisions.
“But we’re really in a place where we have a lot more questions and answers about what the next phase of the vaccine availability and updates are going to be in the United States,” added Dr. Piltch-Loeb, preparedness fellow in the division of policy translation and leadership development and a research associate in the department of biostatistics at the Harvard T. H. Chan School of Public Health in Boston.
1. What is the biggest concern you are hearing from patients about getting a booster?
“The biggest concerns are that everyone wants it and they don’t know where to get it. In health care’s defense, the CDC just figured out what to do,” said Janet Englund, MD, professor of pediatric infectious diseases and an infectious disease and virology expert at Seattle Children’s Hospital in Washington.
“Everyone thinks they should be eligible for a booster ... people in their 50s who are not yet 65+, people with young grandchildren, etc.,” she added. “I’m at Seattle Children’s Hospital, so people are asking about booster shots and about getting their children vaccinated.”
Boosters for all COVID-19 vaccines are completely free.
“All COVID-19 vaccines, including booster doses, will be provided free of charge to the U.S. population,” the CDC has said.
2. Will patients need to prove they meet eligibility criteria for a booster shot or will it be the honor system?
“No, patients will only need to attest that they fall into one of the high-risk groups for whom a booster vaccine is authorized,” said Robert Atmar, MD, professor of infectious diseases at Baylor College of Medicine in Houston.
Dr. Piltch-Loeb agreed. “It is likely to be an honor system. It is very unlikely that there will be punishments or other ramifications ... if doses are administered, beyond the approved usage.”
3. If a patient who had the Moderna or the Johnson and Johnson vaccination requests a booster, can health care workers give them Pfizer?
The short answer is no. “This only applies to individuals who have received the Pfizer vaccine,” Dr. Piltch-Loeb said.
More data will be needed before other vaccine boosters are authorized, she added.
“My understanding is the Moderna people have just recently submitted their information, all of their data to the FDA and J&J is in line to do that very shortly,” said William Schaffner, MD, professor of preventive medicine and infectious diseases at Vanderbilt University in Nashville, Tenn. “I would hope that within the next month to 6 weeks, we will get information about both of those vaccines,” Dr. Schaffner said.
4. When are the “mix-and-match” vaccine study results expected to come out?
“We expect that data from the study will be available in the coming weeks,” said Dr. Atmar, who is the national co-principal investigator of a mix-and-match booster trial launched in June 2021.
5. Are side effects of a booster vaccine expected to be about the same as what people experienced during their first or second immunization?
“I’m expecting the side effects will be similar to the second dose,” Dr. Englund said.
“The data presented ... at ACIP suggests that the side effects from the third shot are either the same or actually less than the first two shots,” said Carlos del Rio, MD, distinguished professor of medicine, epidemiology, and global health, and executive associate dean of Emory University School of Medicine at Grady Health System in Atlanta.
”Everyone reacts very differently to vaccines, regardless of vaccine type,” said Eric Ascher, MD, a family medicine physician at Lenox Hill Hospital in New York City. “I have had patients (as well as personal experience) where there were none to minimal symptoms, and others who felt they had a mild flu for 24 hours.”
“I expect no side effects greater than what was felt with you prior doses,” he said. “The vaccine is very safe and the benefit of vaccination outweighs the risks of any mild side effects.”
6. Is it unethical to give a booster to someone outside the approved groups if there are doses remaining at the end of the day in an open vial?
“Offering a booster shot to someone outside of approved groups if remaining doses will go to waste at the end of the day seems like a prudent decision, and relatively harmless action,” said Faith Fletcher, PhD, assistant professor at the Center for Medical Ethics and Health Policy at Baylor College of Medicine.
“However, if doses continue to fall in the laps of unapproved groups, we must evaluate the vaccine systems and structures that advantage some groups and disadvantage others,” she added. “We know that the distribution of COVID-19 vaccines has not been equitable – and some groups have been left behind.”
“I am not an ethicist and there are many competing concerns that this question addresses,” Dr. Atmar said. For example, “there is not a limitation of vaccine supply in the U.S., so that using leftover vaccine to prevent waste is no longer a major concern in the U.S.”
It could be more of a legal than ethical question, Dr. Atmar said. For an individual outside the authorized groups, legally, the FDA’s EUA for boosting does not allow the vaccine to be administered to this person, he said.
“The rationale for the restricted use in the EUA is that at this time the safety and risks associated with such administration are not known, and the benefits also have not been determined,” Dr. Atmar said. “Members of the ACIP raised concerns about other individuals who may potentially benefit from a booster but are not eligible and the importance of making boosters available to them, but from a legal standpoint – I am also not a lawyer, so this is my understanding – administration of the vaccine is limited to those identified in the EUA.”
7. What is the likelihood that one shot will combine COVID and flu protection in the near future?
It is not likely, Dr. Englund said. “The reason is that the flu vaccine changes so much, and it already has four different antigens. This is assuming we keep the same method of making the flu vaccine – the answer could be different if the flu vaccine becomes an mRNA vaccine in the future.”
Companies such as Moderna and Novavax are testing single-dose shots for COVID-19 and influenza, but they are still far from having anything ready for this flu season in the United States.
8. Is there any chance a booster shot distributed now will need to be redesigned for a future variant?
“Absolutely,” Dr. Englund said. “And a booster dose is the time we may want to consider re-engineering a vaccine.”
9. Do you think the FDA/CDC limitations on who is eligible for a booster was in any way influenced by the World Health Organization call for prioritizing shots for the unvaccinated in lower-resource countries?
“This is absolutely still a global problem,” Dr. Piltch-Loeb said. “We need to get more vaccine to more countries and more people as soon as possible, because if there’s anything we’ve seen about the variants it is that ... they can come from all different places.”
“That being said, I think that it is unlikely to change the course of action in the U.S.,” she added, when it comes to comparing the global need with the domestic policy priorities of the administration.
Dr. Atmar was more direct. “No,” he said. “The WHO recommends against boosting of anyone. The U.S. decisions about boosting those in this country who are eligible are aimed toward addressing perceived needs domestically at the same time that vaccines are being provided to other countries.
“The philosophy is to address both ‘needs’ at the same time,” Dr. Atmar said.
10. What does the future hold for booster shots?
“Predicting the future is really hard, especially when it involves COVID,” Dr. del Rio said.
“Having said that, COVID is not the flu, so I doubt there will be need for annual boosters. I think the population eligible for boosters will be expanded ... and the major population not addressed at this point is the people that received either Moderna or J&J [vaccines].”
Kelly Davis contributed to this feature. A version of this article first appeared on Medscape.com.
Confusion continues to circulate in the wake of decisions on booster doses of the Pfizer/BioNTech COVID-19 vaccine, all announced within 1 week. Many people – including those now eligible and those who officially have to wait for their shot at a third dose – have questions.
Multiple agencies are involved in the booster decisions, and they have put out multiple – and sometimes conflicting – messages about booster doses, leaving more questions than answers for many people.
On Sept. 22, the Food and Drug Administration granted an emergency use authorization (EUA) for a booster dose of the Pfizer mRNA COVID-19 vaccine for those 65 and older and those at high risk for severe illness from the coronavirus, including essential workers whose jobs increase their risk for infection – such as frontline health care workers.
The Centers for Disease Control and Prevention Director Rochelle Walensky, MD, then overruled advice from the agency’s Advisory Committee on Immunization Practices (ACIP) to recommend boosters for essential workers such as those working on the front lines during the pandemic.
As it stands now, the CDC recommends that the following groups should get a third dose of the Pfizer vaccine:
- People aged 65 years and older.
- People aged 18 years and older in long-term care settings.
- People aged 50-64 years with underlying medical conditions.
The CDC also recommends that the following groups may receive a booster shot of the Pfizer vaccine, based on their individual benefits and risks:
- People aged 18-49 years with underlying medical conditions.
- People aged 18-64 years at increased risk for COVID-19 exposure and transmission because of occupational or institutional setting.
The CDC currently considers the following groups at increased risk for COVID-19:
- First responders (health care workers, firefighters, police, congregate care staff).
- Education staff (teachers, support staff, day care workers).
- Food and agriculture workers.
- Manufacturing workers.
- Corrections workers.
- U.S. Postal Service workers.
- Public transit workers.
- Grocery store workers.
Health care professionals, among the most trusted sources of COVID-19 information, are likely to encounter a number of patients wondering how all this will work.
“It’s fantastic that boosters will be available for those who the data supports need [them],” Rachael Piltch-Loeb, PhD, said during a media briefing on Sept. 23, held between the FDA and CDC decisions.
“But we’re really in a place where we have a lot more questions and answers about what the next phase of the vaccine availability and updates are going to be in the United States,” added Dr. Piltch-Loeb, preparedness fellow in the division of policy translation and leadership development and a research associate in the department of biostatistics at the Harvard T. H. Chan School of Public Health in Boston.
1. What is the biggest concern you are hearing from patients about getting a booster?
“The biggest concerns are that everyone wants it and they don’t know where to get it. In health care’s defense, the CDC just figured out what to do,” said Janet Englund, MD, professor of pediatric infectious diseases and an infectious disease and virology expert at Seattle Children’s Hospital in Washington.
“Everyone thinks they should be eligible for a booster ... people in their 50s who are not yet 65+, people with young grandchildren, etc.,” she added. “I’m at Seattle Children’s Hospital, so people are asking about booster shots and about getting their children vaccinated.”
Boosters for all COVID-19 vaccines are completely free.
“All COVID-19 vaccines, including booster doses, will be provided free of charge to the U.S. population,” the CDC has said.
2. Will patients need to prove they meet eligibility criteria for a booster shot or will it be the honor system?
“No, patients will only need to attest that they fall into one of the high-risk groups for whom a booster vaccine is authorized,” said Robert Atmar, MD, professor of infectious diseases at Baylor College of Medicine in Houston.
Dr. Piltch-Loeb agreed. “It is likely to be an honor system. It is very unlikely that there will be punishments or other ramifications ... if doses are administered, beyond the approved usage.”
3. If a patient who had the Moderna or the Johnson and Johnson vaccination requests a booster, can health care workers give them Pfizer?
The short answer is no. “This only applies to individuals who have received the Pfizer vaccine,” Dr. Piltch-Loeb said.
More data will be needed before other vaccine boosters are authorized, she added.
“My understanding is the Moderna people have just recently submitted their information, all of their data to the FDA and J&J is in line to do that very shortly,” said William Schaffner, MD, professor of preventive medicine and infectious diseases at Vanderbilt University in Nashville, Tenn. “I would hope that within the next month to 6 weeks, we will get information about both of those vaccines,” Dr. Schaffner said.
4. When are the “mix-and-match” vaccine study results expected to come out?
“We expect that data from the study will be available in the coming weeks,” said Dr. Atmar, who is the national co-principal investigator of a mix-and-match booster trial launched in June 2021.
5. Are side effects of a booster vaccine expected to be about the same as what people experienced during their first or second immunization?
“I’m expecting the side effects will be similar to the second dose,” Dr. Englund said.
“The data presented ... at ACIP suggests that the side effects from the third shot are either the same or actually less than the first two shots,” said Carlos del Rio, MD, distinguished professor of medicine, epidemiology, and global health, and executive associate dean of Emory University School of Medicine at Grady Health System in Atlanta.
”Everyone reacts very differently to vaccines, regardless of vaccine type,” said Eric Ascher, MD, a family medicine physician at Lenox Hill Hospital in New York City. “I have had patients (as well as personal experience) where there were none to minimal symptoms, and others who felt they had a mild flu for 24 hours.”
“I expect no side effects greater than what was felt with you prior doses,” he said. “The vaccine is very safe and the benefit of vaccination outweighs the risks of any mild side effects.”
6. Is it unethical to give a booster to someone outside the approved groups if there are doses remaining at the end of the day in an open vial?
“Offering a booster shot to someone outside of approved groups if remaining doses will go to waste at the end of the day seems like a prudent decision, and relatively harmless action,” said Faith Fletcher, PhD, assistant professor at the Center for Medical Ethics and Health Policy at Baylor College of Medicine.
“However, if doses continue to fall in the laps of unapproved groups, we must evaluate the vaccine systems and structures that advantage some groups and disadvantage others,” she added. “We know that the distribution of COVID-19 vaccines has not been equitable – and some groups have been left behind.”
“I am not an ethicist and there are many competing concerns that this question addresses,” Dr. Atmar said. For example, “there is not a limitation of vaccine supply in the U.S., so that using leftover vaccine to prevent waste is no longer a major concern in the U.S.”
It could be more of a legal than ethical question, Dr. Atmar said. For an individual outside the authorized groups, legally, the FDA’s EUA for boosting does not allow the vaccine to be administered to this person, he said.
“The rationale for the restricted use in the EUA is that at this time the safety and risks associated with such administration are not known, and the benefits also have not been determined,” Dr. Atmar said. “Members of the ACIP raised concerns about other individuals who may potentially benefit from a booster but are not eligible and the importance of making boosters available to them, but from a legal standpoint – I am also not a lawyer, so this is my understanding – administration of the vaccine is limited to those identified in the EUA.”
7. What is the likelihood that one shot will combine COVID and flu protection in the near future?
It is not likely, Dr. Englund said. “The reason is that the flu vaccine changes so much, and it already has four different antigens. This is assuming we keep the same method of making the flu vaccine – the answer could be different if the flu vaccine becomes an mRNA vaccine in the future.”
Companies such as Moderna and Novavax are testing single-dose shots for COVID-19 and influenza, but they are still far from having anything ready for this flu season in the United States.
8. Is there any chance a booster shot distributed now will need to be redesigned for a future variant?
“Absolutely,” Dr. Englund said. “And a booster dose is the time we may want to consider re-engineering a vaccine.”
9. Do you think the FDA/CDC limitations on who is eligible for a booster was in any way influenced by the World Health Organization call for prioritizing shots for the unvaccinated in lower-resource countries?
“This is absolutely still a global problem,” Dr. Piltch-Loeb said. “We need to get more vaccine to more countries and more people as soon as possible, because if there’s anything we’ve seen about the variants it is that ... they can come from all different places.”
“That being said, I think that it is unlikely to change the course of action in the U.S.,” she added, when it comes to comparing the global need with the domestic policy priorities of the administration.
Dr. Atmar was more direct. “No,” he said. “The WHO recommends against boosting of anyone. The U.S. decisions about boosting those in this country who are eligible are aimed toward addressing perceived needs domestically at the same time that vaccines are being provided to other countries.
“The philosophy is to address both ‘needs’ at the same time,” Dr. Atmar said.
10. What does the future hold for booster shots?
“Predicting the future is really hard, especially when it involves COVID,” Dr. del Rio said.
“Having said that, COVID is not the flu, so I doubt there will be need for annual boosters. I think the population eligible for boosters will be expanded ... and the major population not addressed at this point is the people that received either Moderna or J&J [vaccines].”
Kelly Davis contributed to this feature. A version of this article first appeared on Medscape.com.








