User login
Assessing the Impact of Antidepressants on Cancer Treatment: A Retrospective Analysis of 14 Antineoplastic Agents
Assessing the Impact of Antidepressants on Cancer Treatment: A Retrospective Analysis of 14 Antineoplastic Agents
Cancer patients experience depression at rates > 5 times that of the general population.1-11 Despite an increase in palliative care use, depression rates continued to rise.2-4 Between 5% to 16% of outpatients, 4% to 14% of inpatients, and up to 49% of patients receiving palliative care experience depression.5 This issue also impacts families and caregivers.1 A 2021 meta-analysis found that 23% of active military personnel and 20% of veterans experience depression.11
Antidepressants approved by the US Food and Drug Administration (FDA) target the serotonin, norepinephrine, or dopamine systems and include boxed warnings about an increased risk of suicidal thoughts in adults aged 18 to 24 years.12,13 These medications are categorized into several classes: monoamine oxidase inhibitors (MAOIs), tricyclic antidepressants (TCAs), tetracyclic antidepressants (TeCAs), norepinephrine-dopamine reuptake inhibitors (NDRIs), selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), serotonin receptor modulators (SRMs), serotonin-melatonin receptor antagonists (SMRAs), and N—methyl-D-aspartate receptor antagonists (NMDARAs).14,15 The first FDA-approved antidepressants, iproniazid (an MAOI) and imipramine (a TCA) laid the foundation for the development of newer classes like SSRIs and SNRIs.15-17
Older antidepressants such as MAOIs and TCAs are used less due to their adverse effects (AEs) and drug interactions. MAOIs, such as iproniazid, selegiline, moclobemide, tranylcypromine, isocarboxazid, and phenelzine, have numerous AEs and drug interactions, making them unsuitable for first- or second-line treatment of depression.14,18-21 TCAs such as doxepin, amitriptyline, nortriptyline, imipramine, desipramine, clomipramine, trimipramine, protriptyline, maprotiline, and amoxapine have a narrow therapeutic index requiring careful monitoring for signs of toxicity such as QRS widening, tremors, or confusion. Despite the issues, TCAs are generally classified as second-line agents for major depressive disorder (MDD). TCAs have off-label uses for migraine prophylaxis, treatment of obsessive-compulsive disorder (OCD), insomnia, and chronic pain management first-line.14,22-29
Newer antidepressants, including TeCAs and NDRIs, are typically more effective, but also come with safety concerns. TeCAs like mirtazapine interact with several medications, including MAOIs, serotonin-increasing drugs, alcohol, cannabidiol, and marijuana. Mirtazapine is FDA-approved for the treatment of moderate to severe depression in adults. It is also used off-label to treat insomnia, panic disorder, posttraumatic stress disorder (PTSD), generalized anxiety disorder (GAD), social anxiety disorder (SAD), headaches, and migraines. Compared to other antidepressants, mirtazapine is effective for all stages of depression and addresses a broad range of related symptoms.14,30-34 NDRIs, such as bupropion, also interact with various medications, including MAOIs, other antidepressants, stimulants, and alcohol. Bupropion is FDA-approved for smoking cessation and to treat depression and SAD. It is also used off-label for depression- related bipolar disorder or sexual dysfunction, attention-deficit/hyperactivity disorder (ADHD), and obesity.14,35-42
SSRIs, SNRIs, and SRMs should be used with caution. SSRIs such as sertraline, citalopram, escitalopram, fluoxetine, paroxetine, and fluvoxamine are first-line treatments for depression and various psychiatric disorders due to their safety and efficacy. Common AEs of SSRIs include sexual dysfunction, sleep disturbances, weight changes, and gastrointestinal (GI) issues. SSRIs can prolong the QT interval, posing a risk of life-threatening arrhythmia, and may interact with other medications, necessitating treatment adjustments. The FDA approved SSRIs for MDD, GAD, bulimia nervosa, bipolar depression, OCD, panic disorder, premenstrual dysphoric disorder, treatment-resistant depression, PTSD, and SAD. Off-label uses include binge eating disorder, body dysmorphic disorder, fibromyalgia, premature ejaculation, paraphilias, autism, Raynaud phenomenon, and vasomotor symptoms associated with menopause. Among SSRIs, sertraline and escitalopram are noted for their effectiveness and tolerability.14,43-53
SNRIs, including duloxetine, venlafaxine, desvenlafaxine, milnacipran, and levomilnacipran, may increase bleeding risk, especially when taken with blood thinners. They can also elevate blood pressure, which may worsen if combined with stimulants. SNRIs may interact with other medications that affect serotonin levels, increasing the risk of serotonin syndrome when taken with triptans, pain medications, or other antidepressants.14 Desvenlafaxine has been approved by the FDA (but not by the European Medicines Agency).54-56 Duloxetine is FDA-approved for the treatment of depression, neuropathic pain, anxiety disorders, fibromyalgia, and musculoskeletal disorders. It is used off-label to treat chemotherapy-induced peripheral neuropathy and stress urinary incontinence.57-61 Venlafaxine is FDA-approved for depression, SAD, and panic disorder, and is prescribed off-label to treat ADHD, neuropathy, fibromyalgia, cataplexy, and PTSD, either alone or in combination with other medications.62,63 Milnacipran is not approved for MDD; levomilnacipran received approval in 2013.64
SRMs such as trazodone, nefazodone, vilazodone, and vortioxetine also function as serotonin reuptake inhibitors.14,15 Trazodone is FDA-approved for MDD. It has been used off-label to treat anxiety, Alzheimer disease, substance misuse, bulimia nervosa, insomnia, fibromyalgia, and PTSD when first-line SSRIs are ineffective. A notable AE of trazodone is orthostatic hypotension, which can lead to dizziness and increase the risk of falls, especially in geriatric patients.65-70 Nefazodone was discontinued in Europe in 2003 due to rare cases of liver toxicity but remains available in the US.71-74 Vilazodone and vortioxetine are FDA-approved.
The latest classes of antidepressants include SMRAs and NMDARAs.14 Agomelatine, an SMRA, was approved in Europe in 2009 but rejected by the FDA in 2011 due to liver toxicity.75 NMDARAs like esketamine and a combination of dextromethorphan and bupropion received FDA approval in 2019 and 2022, respectively.76,77
This retrospective study analyzes noncancer drugs used during systemic chemotherapy based on a dataset of 14 antineoplastic agents. It sought to identify the most dispensed noncancer drug groups, discuss findings, compare patients with and without antidepressant prescriptions, and examine trends in antidepressant use from 2002 to 2023. This analysis expands on prior research.78-81
Methods
The Walter Reed National Military Medical Center Institutional Review Board approved the study protocol and ensured compliance with the Health Insurance Portability and Accountability Act as an exempt protocol. The Joint Pathology Center (JPC) of the US Department of Defense (DoD) Cancer Registry Program and Military Health System (MHS) data experts from the Comprehensive Ambulatory/Professional Encounter Record (CAPER) and Pharmacy Data Transaction Service (PDTS) provided data for the analysis.
Data Sources
The JPC DoD Cancer Registry Program contains data from 1998 to 2024. CAPER and PDTS are part of the MHS Data Repository/Management Analysis and Reporting Tool database. Each observation in CAPER represents an ambulatory encounter at a military treatment facility (MTF). CAPER records are available from 2003 to 2024. PDTS records are available from 2002 to 2004. Each observation in PDTS represents a prescription filled for an MHS beneficiary, excluding those filled at international civilian pharmacies and inpatient pharmacy prescriptions.
This cross-sectional analysis requested data extraction for specific cancer drugs from the DoD Cancer Registry, focusing on treatment details, diagnosis dates, patient demographics, and physicians’ comments on AEs. After identifying patients, CAPER was used to identify additional health conditions. PDTS was used to compile a list of prescription medications filled during systemic cancer treatment or < 2 years postdiagnosis.
The 2016 Surveillance, Epidemiology, and End Results Program Coding and Staging Manual and International Classification of Diseases for Oncology, 3rd edition, 1st revision, were used to decode disease and cancer types.82,83 Data sorting and analysis were performed using Microsoft Excel. The percentage for the total was calculated by using the number of patients or data available within the subgroup divided by the total number of patients or data variables. To compare the mean number of dispensed antidepressants to those without antidepressants, a 2-tailed, 2-sample z test was used to calculate the P value and determine statistical significance (P < .05) using socscistatistics.com.
Data were extracted 3 times between 2021 and 2023. The initial 2021 protocol focused on erlotinib and gefitinib. A modified protocol in 2022 added paclitaxel, cisplatin, docetaxel, pemetrexed, and crizotinib; further modification in 2023 included 8 new antineoplastic agents and 2 anticoagulants. Sotorasib has not been prescribed in the MHS, and JPC lacks records for noncancer drugs. The 2023 dataset comprised 2210 patients with cancer treated with 14 antineoplastic agents; 2104 had documented diagnoses and 2113 had recorded prescriptions. Data for erlotinib, gefitinib, and paclitaxel have been published previously.78,79
Results
Of 2113 patients with recorded prescriptions, 1297 patients (61.4%) received 109 cancer drugs, including 96 antineoplastics, 7 disease-modifying antirheumatic agents, 4 biologic response modifiers, and 2 calcitonin gene-related peptides. Fourteen antineoplastic agents had complete data from JPC, while others were noted for combination therapies or treatment switches from the PDTS (Table 1). Seventy-six cancer drugs were prescribed with antidepressants in 489 patients (eAppendix).
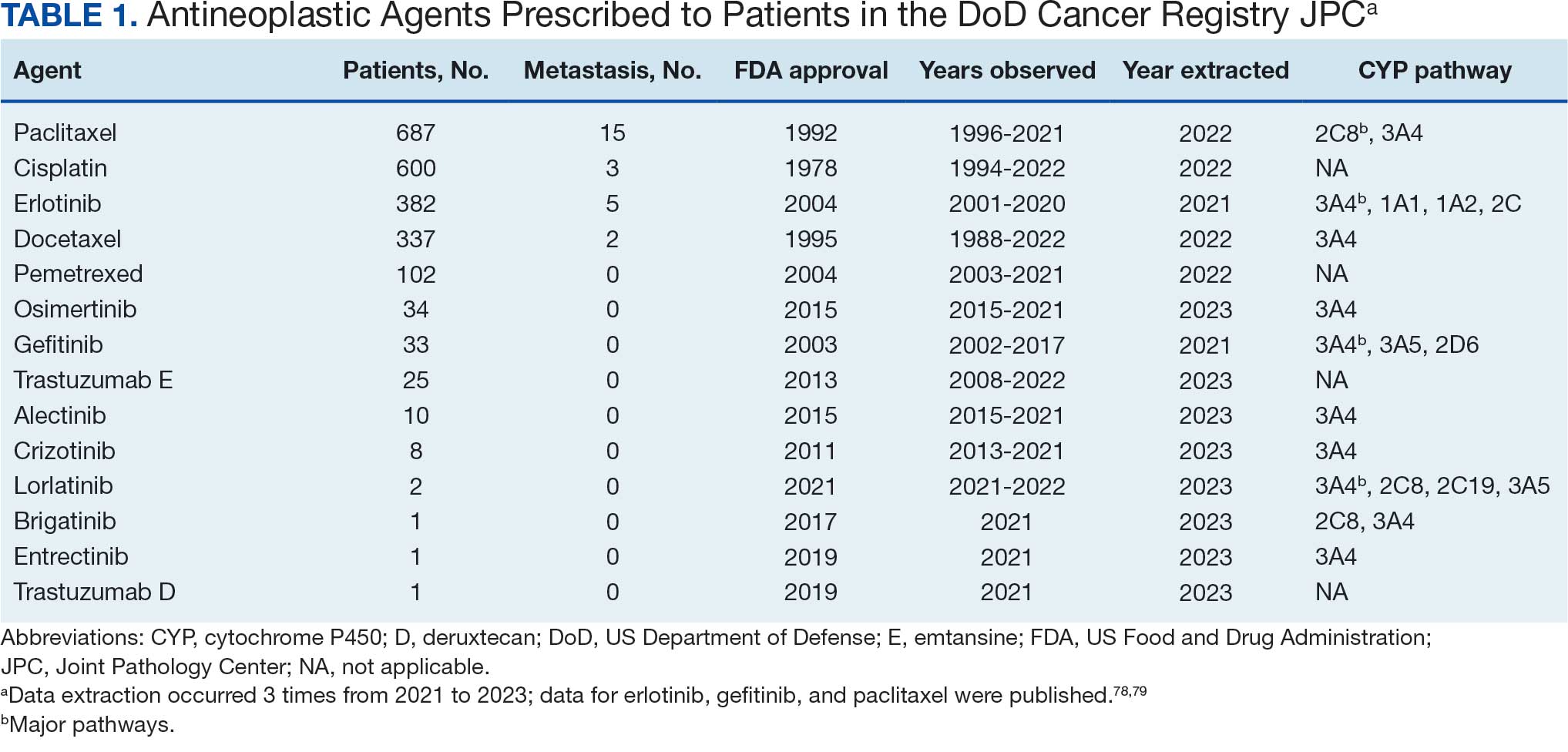
The JPC provided 2242 entries for 2210 patients, ranging in age from 2 months to 88 years (mean, 56 years), documenting treatment from September 1988 to January 2023. Thirty-two patients had duplicate entries due to multiple cancer locations or occurrences. Of the 2242 patients, 1541 (68.7%) were aged > 50 years, 975 patients (43.5%) had cancers that were stage III or IV, and 1267 (56.5%) had cancers that were stage 0, I, II, or not applicable/unknown. There were 51 different types of cancer: breast, lung, testicular, endometrial, and ovarian were most common (n ≥ 100 patients). Forty-two cancer types were documented among 750 patients prescribed antidepressants (Table 2).
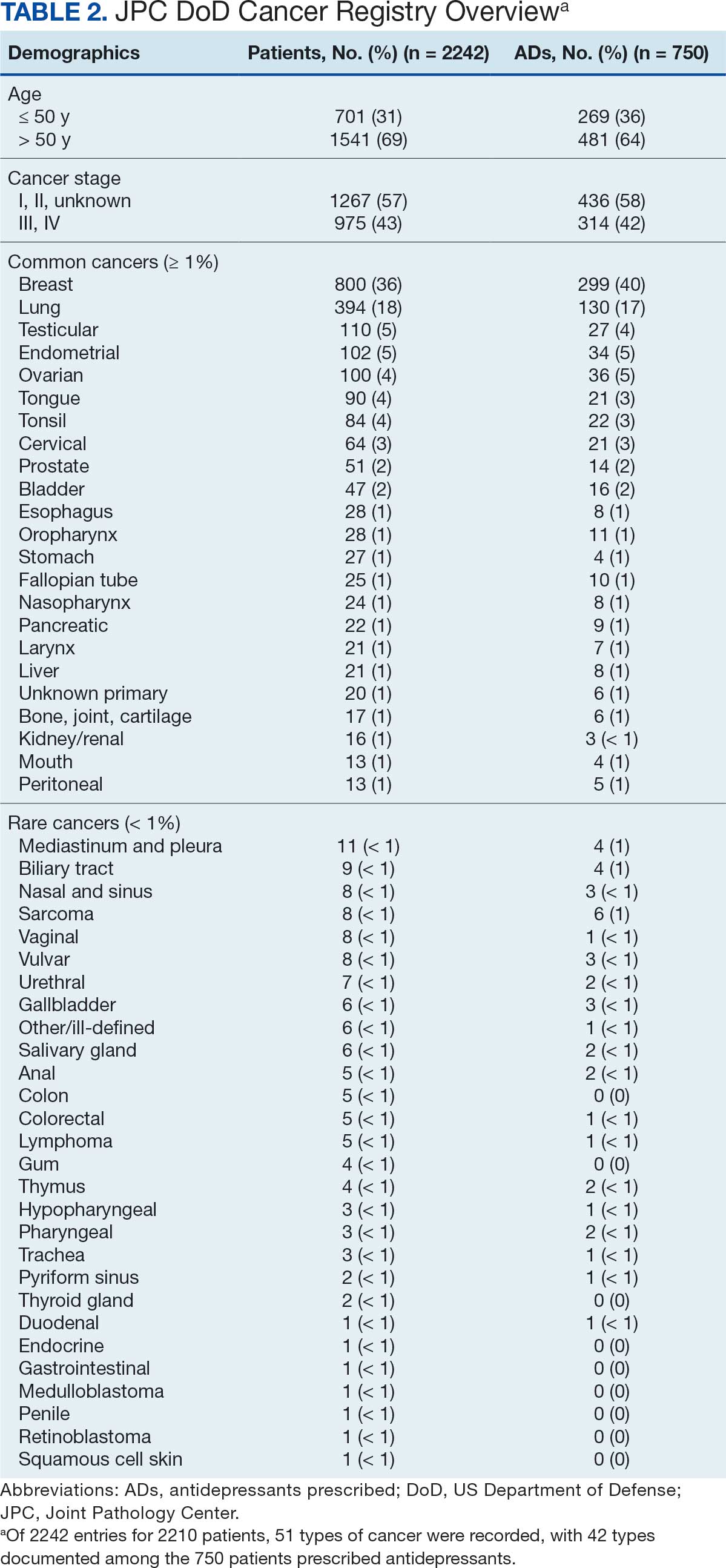
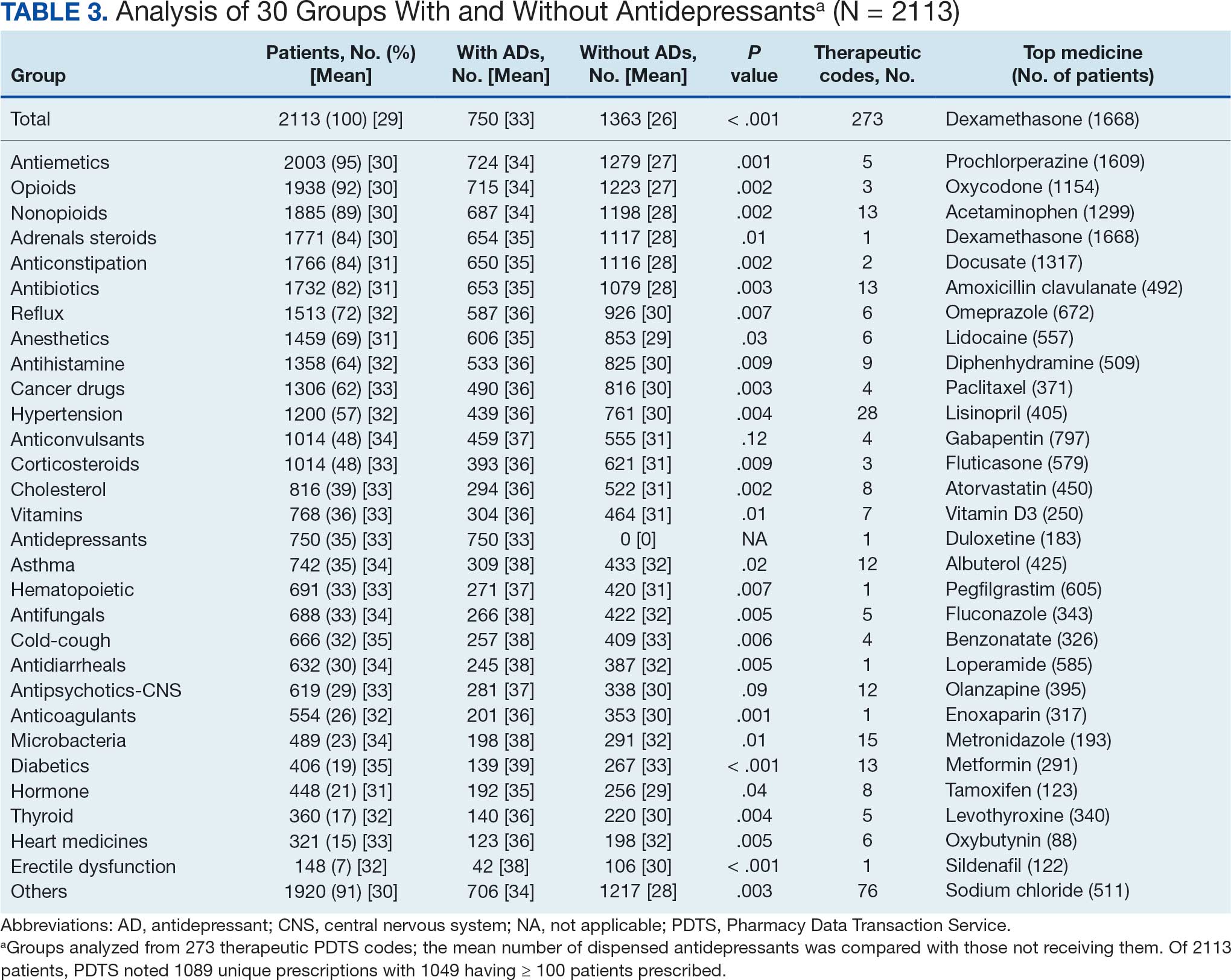
The CAPER database recorded 8882 unique diagnoses for 2104 patients, while PDTS noted 1089 unique prescriptions within 273 therapeutic codes for 2113 patients. Nine therapeutic codes (opiate agonists, adrenals, cathartics-laxatives, nonsteroidal anti-inflammatory agents, antihistamines for GI conditions, 5-HT3 receptor antagonists, analgesics and antipyretic miscellanea, antineoplastic agents, and proton-pump inhibitors) and 8 drugs (dexamethasone, prochlorperazine, ondansetron, docusate, acetaminophen, ibuprofen, oxycodone, and polyethylene glycol 3350) were associated with > 1000 patients (≥ 50%). Patients had between 1 and 275 unique health conditions and filled 1 to 108 prescriptions. The mean (SD) number of diagnoses and prescriptions was 50 (28) and 29 (12), respectively. Of the 273 therapeutic codes, 30 groups were analyzed, with others categorized into miscellaneous groups such as lotions, vaccines, and devices. Significant differences in mean number of prescriptions were found for patients taking antidepressants compared to those not (P < .05), except for anticonvulsants and antipsychotics (P = .12 and .09, respectively) (Table 3).
Antidepressants
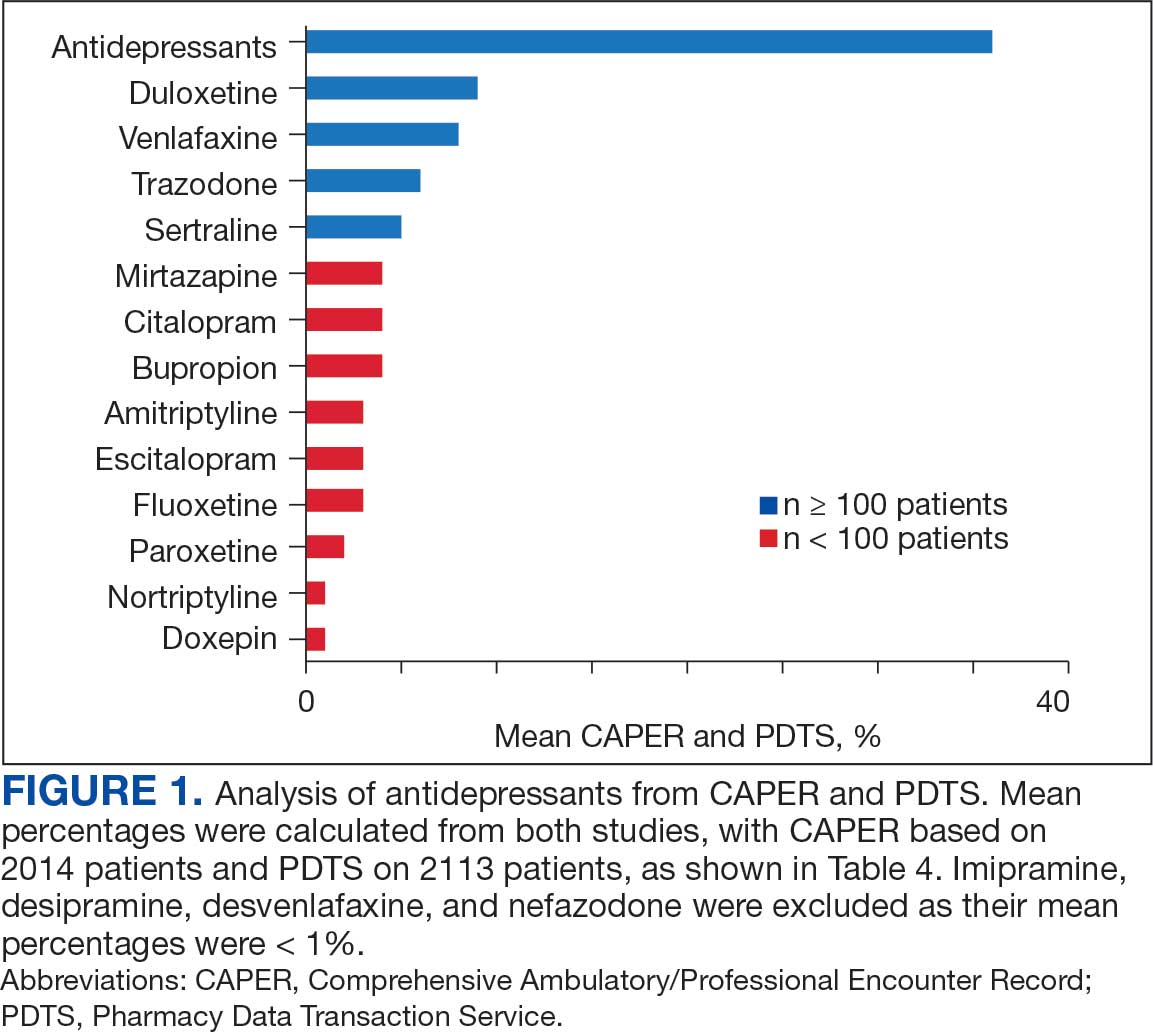
Of the 2113 patients with recorded prescriptions, 750 (35.5%) were dispensed 17 different antidepressants. Among these 17 antidepressants, 183 (8.7%) patients received duloxetine, 158 (7.5%) received venlafaxine, 118 (5.6%) received trazodone, and 107 (5.1%) received sertraline (Figure 1, Table 4). Of the 750 patients, 509 (67.9%) received 1 antidepressant, 168 (22.4%) received 2, 60 (8.0%) received 3, and 13 (1.7%) received > 3. Combinations varied, but only duloxetine and trazodone were prescribed to > 10 patients.
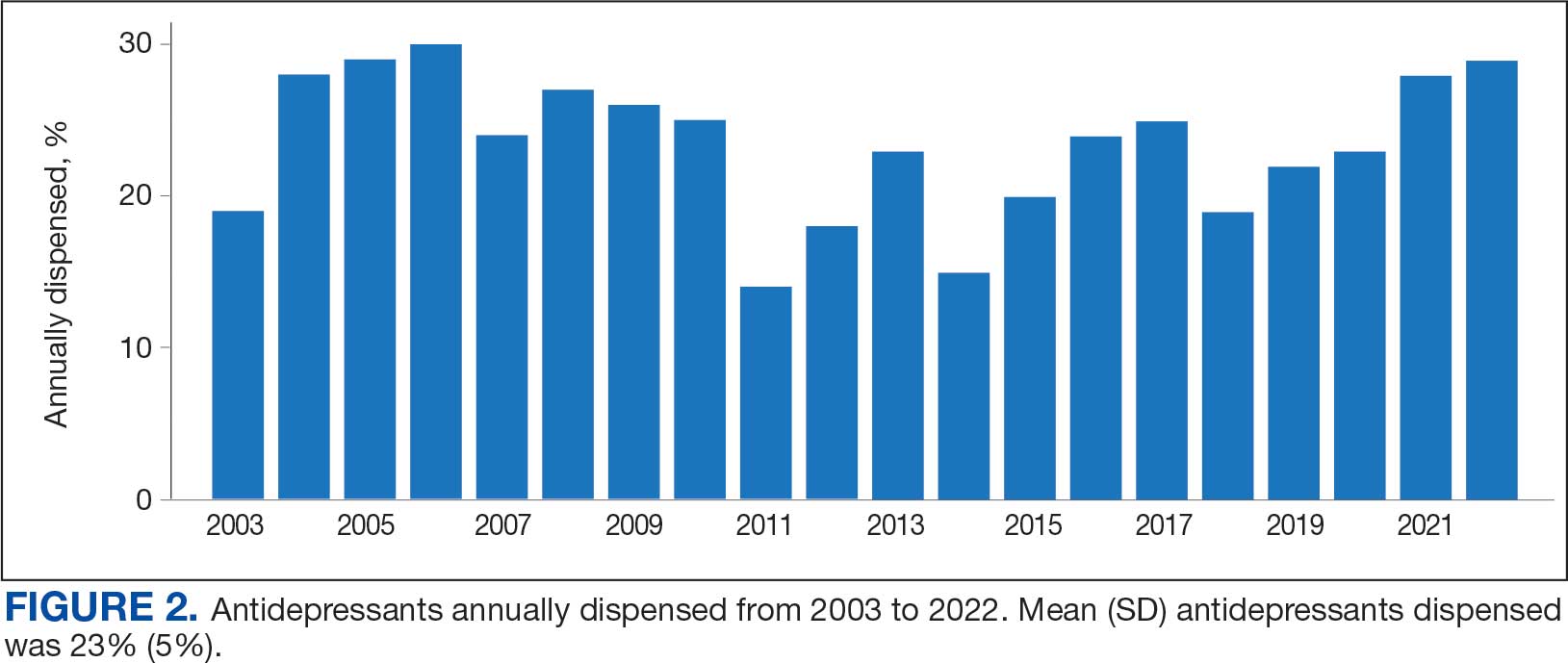
Antidepressants were prescribed annually at an overall mean (SD) rate of 23% (5%) from 2003 to 2022 (Figure 2). Patients on antidepressants during systemic therapy had a greater number of diagnosed medical conditions and received more prescription medications compared to those not taking antidepressants (P < .001) (Figure 3). The 745 patients taking antidepressants in CAPER data had between 1 and 275 diagnosed medical issues, with a mean (SD) of 55 (31) vs a range of 1 to 209 and a mean (SD) of 46 (26) for the 1359 patients not taking antidepressants. The 750 patients on antidepressants in PDTS data had between 8 and 108 prescriptions dispensed, with a mean (SD) of 32 (12), vs a range of 1 to 65 prescriptions and a mean (SD) of 29 (12) for 1363 patients not taking antidepressants.
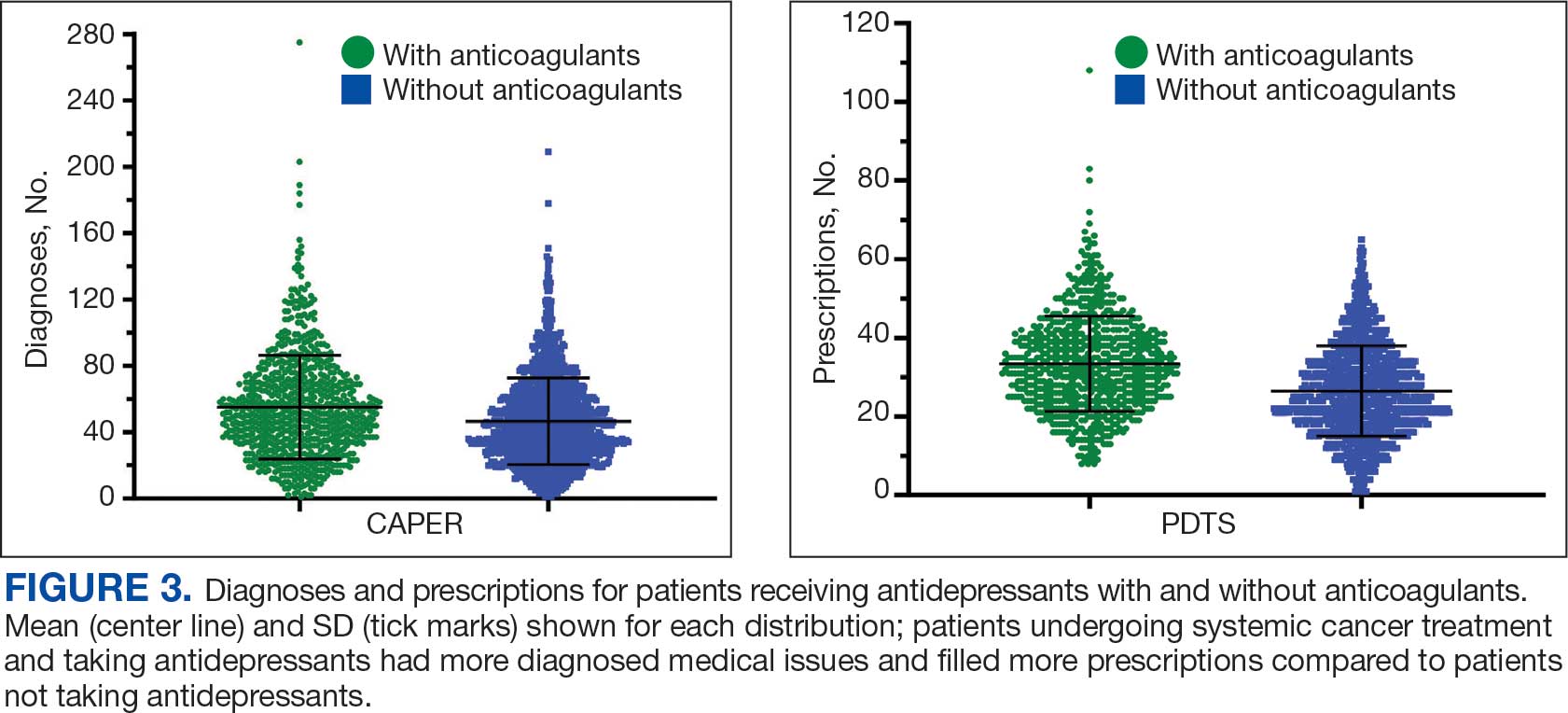
Discussion
The JPC DoD Cancer Registry includes information on cancer types, stages, treatment regimens, and physicians’ notes, while noncancer drugs are sourced from the PDTS database. The pharmacy uses a different documentation system, leading to varied classifications.
Database reliance has its drawbacks. For example, megestrol is coded as a cancer drug, although it’s primarily used for endometrial or gynecologic cancers. Many drugs have multiple therapeutic codes assigned to them, including 10 antineoplastic agents: diclofenac, Bacillus Calmette-Guérin (BCG), megestrol acetate, tamoxifen, anastrozole, letrozole, leuprolide, goserelin, degarelix, and fluorouracil. Diclofenac, BCG, and mitomycin have been repurposed for cancer treatment.84-87 From 2003 to 2023, diclofenac was prescribed to 350 patients for mild-to-moderate pain, with only 2 patients receiving it for cancer in 2018. FDA-approved for bladder cancer in 1990, BCG was prescribed for cancer treatment for 1 patient in 2021 after being used for vaccines between 2003 and 2018. Tamoxifen, used for hormone receptor-positive breast cancer from 2004 to 2017 with 53 patients, switched to estrogen agonist-antagonists from 2017 to 2023 with 123 patients. Only a few of the 168 patients were prescribed tamoxifen using both codes.88-91 Anastrozole and letrozole were coded as antiestrogens for 7 and 18 patients, respectively, while leuprolide and goserelin were coded as gonadotropins for 59 and 18 patients. Degarelix was coded as antigonadotropins, fluorouracil as skin and mucous membrane agents miscellaneous, and megestrol acetate as progestins for 7, 6, and 3 patients, respectively. Duloxetine was given to 186 patients, primarily for depression from 2005 to 2023, with 7 patients treated for fibromyalgia from 2022 to 2023.
Antidepressants Observed
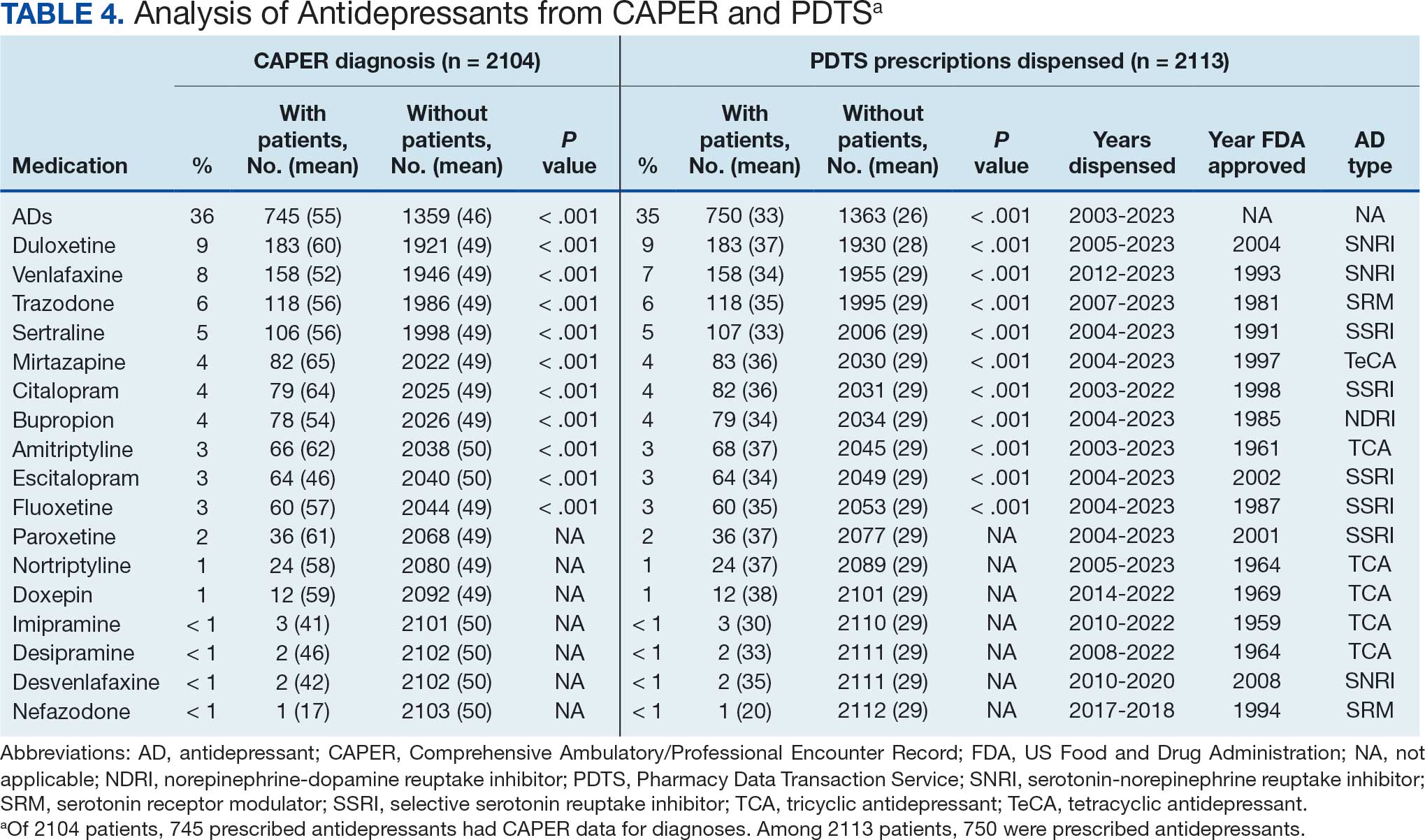
Tables 1 and 5 provide insight into the FDA approval of 14 antineoplastics and antidepressants and their CYP metabolic pathways.92-122 In Table 4, the most prescribed antidepressant classes are SNRIs, SRMs, SSRIs, TeCAs, NDRIs, and TCAs. This trend highlights a preference for newer medications with weak CYP inhibition. A total of 349 patients were prescribed SSRIs, 343 SNRIs, 119 SRMs, 109 TCAs, 83 TeCAs, and 79 NDRIs. MAOIs, SMRAs, and NMDARAs were not observed in this dataset. While there are instances of dextromethorphan-bupropion and sertraline-escitalopram being dispensed together, it remains unclear whether these were NMDARA combinations.
Among the 14 specific antineoplastic agents, 10 are metabolized by CYP isoenzymes, primarily CYP3A4. Duloxetine neither inhibits nor is metabolized by CYP3A4, a reason it is often recommended, following venlafaxine.
Both duloxetine and venlafaxine are used off-label for chemotherapy-induced peripheral neuropathy related to paclitaxel and docetaxel. According to the CYP metabolized pathway, duloxetine tends to have more favorable DDIs than venlafaxine. In PDTS data, 371 patients were treated with paclitaxel and 180 with docetaxel, with respective antidepressant prescriptions of 156 and 70. Of the 156 patients dispensed paclitaxel, 62 (40%) were dispensed with duloxetine compared to 43 (28%) with venlafaxine. Of the 70 patients dispensed docetaxel, 23 (33%) received duloxetine vs 24 (34%) with venlafaxine.
Of 85 patients prescribed duloxetine, 75 received it with either paclitaxel or docetaxel (5 received both). Five patients had documented AEs (1 neuropathy related). Of 67 patients prescribed venlafaxine, 66 received it with either paclitaxel or docetaxel. Two patients had documented AEs (1 was neuropathy related, the same patient who received duloxetine). Of the 687 patients treated with paclitaxel and 337 with docetaxel in all databases, 4 experienced neuropathic AEs from both medications.79
Antidepressants can increase the risk of bleeding, especially when combined with blood thinners, and may elevate blood pressure, particularly alongside stimulants. Of the 554 patients prescribed 9 different anticoagulants, enoxaparin, apixaban, and rivaroxaban were the most common (each > 100 patients). Among these, 201 patients (36%) received both anticoagulants and antidepressants: duloxetine for 64 patients, venlafaxine for 30, trazodone for 35, and sertraline for 26. There were no data available to assess bleeding rates related to the evaluation of DDIs between these medication classes.
Antidepressants can be prescribed for erectile dysfunction. Of the 148 patients prescribed an antidepressant for erectile dysfunction, duloxetine, trazodone, and mirtazapine were the most common. Antidepressant preferences varied by cancer type. Duloxetine was the only antidepressant used for all types of cancer. Venlafaxine, duloxetine, trazodone, sertraline, and escitalopram were the most prescribed antidepressants for breast cancer, while duloxetine, mirtazapine, citalopram, sertraline, and trazodone were the most prescribed for lung cancer. Sertraline, duloxetine, trazodone, amitriptyline, and escitalopram were most common for testicular cancer. Duloxetine, venlafaxine, trazodone, amitriptyline, and sertraline were the most prescribed for endometrial cancer, while duloxetine, venlafaxine, amitriptyline, citalopram, and sertraline were most prescribed for ovarian cancer.
The broadness of International Statistical Classification of Diseases, Tenth Revision codes made it challenging to identify nondepression diagnoses in the analyzed population. However, if all antidepressants were prescribed to treat depression, service members with cancer exhibited a higher depression rate (35%) than the general population (25%). Of 2104 patients, 191 (9.1%) had mood disorders, and 706 (33.6%) had mental disorders: 346 (49.0%) had 1 diagnosis, and 360 (51.0%) had multiple diagnoses. The percentage of diagnoses varied yearly, with notable drops in 2003, 2007, 2011, 2014, and 2018, and peaks in 2006, 2008, 2013, 2017, and 2022. This fluctuation was influenced by events like the establishment of PDTS in 2002, the 2008 economic recession, a hospital relocation in 2011, the 2014 Ebola outbreak, and the COVID-19 pandemic. Although the number of patients receiving antidepressants increased from 2019 to 2022, the overall percentage of patients receiving them did not significantly change from 2003 to 2022, aligning with previous research.5,125
Many medications have potential uses beyond what is detailed in the prescribing information. Antidepressants can relieve pain, while pain medications may help with depression. Opioids were once thought to effectively treat depression, but this perspective has changed with a greater understanding of their risks, including misuse.126-131 Pain is a severe and often unbearable AE of cancer. Of 2113 patients, 92% received opioids; 34% received both opioids and antidepressants; 2% received only antidepressants; and 7% received neither. This study didn’t clarify whether those on opioids alone recognized their depression or if those on both were aware of their dependence. While SSRIs are generally not addictive, they can lead to physical dependence, and any medication can be abused if not managed properly.132-134
Conclusions
This retrospective study analyzes data from antineoplastic agents used in systemic cancer treatment between 1988 and 2023, with a particular focus on the use of antidepressants. Data on antidepressant prescriptions are incomplete and specific to these agents, which means the findings cannot be generalized to all antidepressants. Hence, the results indicate that patients taking antidepressants had more diagnosed health issues and received more medications compared to patients who were not on these drugs.
This study underscores the need for further research into the effects of antidepressants on cancer treatment, utilizing all data from the DoD Cancer Registry. Future research should explore DDIs between antidepressants and other cancer and noncancer medications, as this study did not assess AE documentation, unlike in studies involving erlotinib, gefitinib, and paclitaxel.78,79 Further investigation is needed to evaluate the impact of discontinuing antidepressant use during cancer treatment. This comprehensive overview provides insights for clinicians to help them make informed decisions regarding the prescription of antidepressants in the context of cancer treatment.
- National Cancer Institute. Depression (PDQ)-Health Professional Version. Updated July 25, 2024. Accessed April 4, 2025. https://www.cancer.gov/about-cancer/coping/feelings/depression-hp-pdq
- Krebber AM, Buffart LM, Kleijn G, et al. Prevalence of depression in cancer patients: a meta-analysis of diagnostic interviews and self-report instruments. Psychooncology. 2014;23(2):121-130. doi:10.1002/pon.3409
- Xiang X, An R, Gehlert S. Trends in antidepressant use among U.S. cancer survivors, 1999-2012. Psychiatr Serv. 2015;66(6):564. doi:10.1176/appi.ps.201500007
- Hartung TJ, Brähler E, Faller H, et al. The risk of being depressed is significantly higher in cancer patients than in the general population: Prevalence and severity of depressive symptoms across major cancer types. Eur J Cancer. 2017;72:46-53. doi:10.1016/j.ejca.2016.11.017
- Walker J, Holm Hansen C, Martin P, et al. Prevalence of depression in adults with cancer: a systematic review. Ann Oncol. 2013;24(4):895-900. doi:10.1093/annonc/mds575
- Pitman A, Suleman S, Hyde N, Hodgkiss A. Depression and anxiety in patients with cancer. BMJ. 2018;361:k1415. doi:10.1136/bmj.k1415
- Kisely S, Alotiby MKN, Protani MM, Soole R, Arnautovska U, Siskind D. Breast cancer treatment disparities in patients with severe mental illness: a systematic review and meta-analysis. Psychooncology. 2023;32(5):651-662. doi:10.1002/pon.6120
- Massie MJ. Prevalence of depression in patients with cancer. J Natl Cancer Inst Monogr. 2004;(32):57-71. doi:10.1093/jncimonographs/lgh014
- Rodin G, Katz M, Lloyd N, Green E, Mackay JA, Wong RK. Treatment of depression in cancer patients. Curr Oncol. 2007;14(5):180-188. doi:10.3747/co.2007.146
- Wilson KG, Chochinov HM, Skirko MG, et al. Depression and anxiety disorders in palliative cancer care. J Pain Symptom Manage. 2007;33(2):118-129. doi:10.1016/j.jpainsymman.2006.07.016
- Moradi Y, Dowran B, Sepandi M. The global prevalence of depression, suicide ideation, and attempts in the military forces: a systematic review and meta-analysis of cross sectional studies. BMC Psychiatry. 2021;21(1):510. doi:10.1186/s12888-021-03526-2
- Lu CY, Zhang F, Lakoma MD, et al. Changes in antidepressant use by young people and suicidal behavior after FDA warnings and media coverage: quasi-experimental study. BMJ. 2014;348:g3596. doi:10.1136/bmj.g3596
- Friedman RA. Antidepressants’ black-box warning--10 years later. N Engl J Med. 2014;371(18):1666-1668. doi:10.1056/NEJMp1408480
- Sheffler ZM, Patel P, Abdijadid S. Antidepressants. In: StatPearls. StatPearls Publishing. Updated May 26, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK538182/
- Miller JJ. Antidepressants, Part 1: 100 Years and Counting. Accessed April 4, 2025. Psychiatric Times. https:// www.psychiatrictimes.com/view/antidepressants-part-1-100-years-and-counting
- Hillhouse TM, Porter JH. A brief history of the development of antidepressant drugs: from monoamines to glutamate. Exp Clin Psychopharmacol. 2015;23(1):1-21. doi:10.1037/a0038550
- Mitchell PB, Mitchell MS. The management of depression. Part 2. The place of the new antidepressants. Aust Fam Physician. 1994;23(9):1771-1781.
- Sabri MA, Saber-Ayad MM. MAO Inhibitors. In: Stat- Pearls. StatPearls Publishing. Updated June 5, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK557395/
- Sub Laban T, Saadabadi A. Monoamine Oxidase Inhibitors (MAOI). In: StatPearls. StatPearls Publishing. Updated July 17, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK539848/
- Fiedorowicz JG, Swartz KL. The role of monoamine oxidase inhibitors in current psychiatric practice. J Psychiatr Pract. 2004;10(4):239-248. doi:10.1097/00131746-200407000-00005
- Flockhart DA. Dietary restrictions and drug interactions with monoamine oxidase inhibitors: an update. J Clin Psychiatry. 2012;73 Suppl 1:17-24. doi:10.4088/JCP.11096su1c.03
- Moraczewski J, Awosika AO, Aedma KK. Tricyclic Antidepressants. In: StatPearls. StatPearls Publishing. Updated August 17, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK557791/
- Almasi A, Patel P, Meza CE. Doxepin. In: StatPearls. StatPearls Publishing. Updated February 14, 2024. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK542306/
- Thour A, Marwaha R. Amitriptyline. In: StatPearls. Stat- Pearls Publishing. Updated July 18, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK537225/
- Radley DC, Finkelstein SN, Stafford RS. Off-label prescribing among office-based physicians. Arch Intern Med. 2006;166(9):1021-1026. doi:10.1001/archinte.166.9.1021
- Tesfaye S, Sloan G, Petrie J, et al. Comparison of amitriptyline supplemented with pregabalin, pregabalin supplemented with amitriptyline, and duloxetine supplemented with pregabalin for the treatment of diabetic peripheral neuropathic pain (OPTION-DM): a multicentre, double-blind, randomised crossover trial. Lancet. 2022;400(10353):680- 690. doi:10.1016/S0140-6736(22)01472-6
- Farag HM, Yunusa I, Goswami H, Sultan I, Doucette JA, Eguale T. Comparison of amitriptyline and US Food and Drug Administration-approved treatments for fibromyalgia: a systematic review and network metaanalysis. JAMA Netw Open. 2022;5(5):e2212939. doi:10.1001/jamanetworkopen.2022.12939
- Merwar G, Gibbons JR, Hosseini SA, Saadabadi A. Nortriptyline. In: StatPearls. StatPearls Publishing. Updated June 5, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK482214/
- Fayez R, Gupta V. Imipramine. In: StatPearls. StatPearls Publishing. Updated May 22, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK557656/
- Jilani TN, Gibbons JR, Faizy RM, Saadabadi A. Mirtazapine. In: StatPearls. StatPearls Publishing. Updated August 28, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK519059/
- Nutt DJ. Tolerability and safety aspects of mirtazapine. Hum Psychopharmacol. 2002;17 Suppl 1:S37-S41. doi:10.1002/hup.388
- Gandotra K, Chen P, Jaskiw GE, Konicki PE, Strohl KP. Effective treatment of insomnia with mirtazapine attenuates concomitant suicidal ideation. J Clin Sleep Med. 2018;14(5):901-902. doi:10.5664/jcsm.7142
- Anttila SA, Leinonen EV. A review of the pharmacological and clinical profile of mirtazapine. CNS Drug Rev. 2001;7(3):249-264. doi:10.1111/j.1527-3458.2001.tb00198.x
- Wang SM, Han C, Bahk WM, et al. Addressing the side effects of contemporary antidepressant drugs: a comprehensive review. Chonnam Med J. 2018;54(2):101-112. doi:10.4068/cmj.2018.54.2.101
- Huecker MR, Smiley A, Saadabadi A. Bupropion. In: StatPearls. StatPearls Publishing. Updated April 9, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK470212/
- Hsieh MT, Tseng PT, Wu YC, et al. Effects of different pharmacologic smoking cessation treatments on body weight changes and success rates in patients with nicotine dependence: a network meta-analysis. Obes Rev. 2019;20(6):895- 905. doi:10.1111/obr.12835
- Hankosky ER, Bush HM, Dwoskin LP, et al. Retrospective analysis of health claims to evaluate pharmacotherapies with potential for repurposing: association of bupropion and stimulant use disorder remission. AMIA Annu Symp Proc. 2018;2018:1292-1299.
- Livingstone-Banks J, Norris E, Hartmann-Boyce J, West R, Jarvis M, Hajek P. Relapse prevention interventions for smoking cessation. Cochrane Database Syst Rev. 2019; 2(2):CD003999. doi:10.1002/14651858.CD003999.pub5
- Clayton AH, Kingsberg SA, Goldstein I. Evaluation and management of hypoactive sexual desire disorder. Sex Med. 2018;6(2):59-74. doi:10.1016/j.esxm.2018.01.004
- Verbeeck W, Bekkering GE, Van den Noortgate W, Kramers C. Bupropion for attention deficit hyperactivity disorder (ADHD) in adults. Cochrane Database Syst Rev. 2017;10(10):CD009504. doi:10.1002/14651858.CD009504.pub2
- Ng QX. A systematic review of the use of bupropion for attention-deficit/hyperactivity disorder in children and adolescents. J Child Adolesc Psychopharmacol. 2017;27(2):112-116. doi:10.1089/cap.2016.0124
- Fava M, Rush AJ, Thase ME, et al. 15 years of clinical experience with bupropion HCl: from bupropion to bupropion SR to bupropion XL. Prim Care Companion J Clin Psychiatry. 2005;7(3):106-113. doi:10.4088/pcc.v07n0305
- Chu A, Wadhwa R. Selective Serotonin Reuptake Inhibitors. In: StatPearls. StatPearls Publishing. Updated May 1, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK554406/
- Singh HK, Saadabadi A. Sertraline. In: StatPearls. Stat- Pearls Publishing. Updated February 13, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK547689/
- MacQueen G, Born L, Steiner M. The selective serotonin reuptake inhibitor sertraline: its profile and use in psychiatric disorders. CNS Drug Rev. 2001;7(1):1-24. doi:10.1111/j.1527-3458.2001.tb00188.x
- Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet. 2009;373(9665):746-758. doi:10.1016/S0140-6736(09)60046-5
- Nelson JC. The STAR*D study: a four-course meal that leaves us wanting more. Am J Psychiatry. 2006;163(11):1864-1866. doi:10.1176/ajp.2006.163.11.1864
- Sharbaf Shoar N, Fariba KA, Padhy RK. Citalopram. In: StatPearls. StatPearls Publishing. Updated November 7, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK482222/
- Landy K, Rosani A, Estevez R. Escitalopram. In: Stat- Pearls. StatPearls Publishing. Updated November 10, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK557734/
- Cavanah LR, Ray P, Goldhirsh JL, Huey LY, Piper BJ. Rise of escitalopram and the fall of citalopram. medRxiv. Preprint published online May 8, 2023. doi:10.1101/2023.05.07.23289632
- Sohel AJ, Shutter MC, Patel P, Molla M. Fluoxetine. In: StatPearls. StatPearls Publishing. Updated July 4, 2022. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK459223/
- Wong DT, Perry KW, Bymaster FP. Case history: the discovery of fluoxetine hydrochloride (Prozac). Nat Rev Drug Discov. 2005;4(9):764-774. doi:10.1038/nrd1821
- Shrestha P, Fariba KA, Abdijadid S. Paroxetine. In: Stat- Pearls. StatPearls Publishing. Updated July 17, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK526022/
- Naseeruddin R, Rosani A, Marwaha R. Desvenlafaxine. In: StatPearls. StatPearls Publishing. Updated July 10, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK534829
- Lieberman DZ, Massey SH. Desvenlafaxine in major depressive disorder: an evidence-based review of its place in therapy. Core Evid. 2010;4:67-82. doi:10.2147/ce.s5998
- Withdrawal assessment report for Ellefore (desvenlafaxine). European Medicine Agency. 2009. Accessed April 4, 2025. https://www.ema.europa.eu/en/documents/withdrawal-report/withdrawal-assessment-report-ellefore_en.pdf
- Dhaliwal JS, Spurling BC, Molla M. Duloxetine. In: Stat- Pearls. Statpearls Publishing. Updated May 29, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK549806/
- Hershman DL, Lacchetti C, Dworkin RH, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32(18):1941-1967. doi:10.1200/JCO.2013.54.0914
- Sommer C, Häuser W, Alten R, et al. Medikamentöse Therapie des Fibromyalgiesyndroms. Systematische Übersicht und Metaanalyse [Drug therapy of fibromyalgia syndrome. Systematic review, meta-analysis and guideline]. Schmerz. 2012;26(3):297-310. doi:10.1007/s00482-012-1172-2
- Bril V, England J, Franklin GM, et al. Evidence-based guideline: treatment of painful diabetic neuropathy: report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2011;76(20):1758-1765. doi:10.1212/WNL.0b013e3182166ebe
- Attal N, Cruccu G, Baron R, et al. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol. 2010;17(9):1113-e88. doi:10.1111/j.1468-1331.2010.02999.x
- Singh D, Saadabadi A. Venlafaxine. In: StatPearls. StatePearls Publishing. Updated February 26, 2024. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK535363/
- Sertraline and venlafaxine: new indication. Prevention of recurrent depression: no advance. Prescrire Int. 2005;14(75):19-20.
- Bruno A, Morabito P, S p i n a E , M u s c a t ello MRl. The role of levomilnacipran in the management of major depressive disorder: a comprehensive review. Curr Neuro pharmacol. 2016;14(2):191-199. doi:10.2174/1570159x14666151117122458
- Shin JJ, Saadabadi A. Trazodone. In: StatPearls. StatPearls Publishing. Updated February 29, 2024. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK470560/
- Khouzam HR. A review of trazodone use in psychiatric and medical conditions. Postgrad Med. 2017;129(1):140-148. doi:10.1080/00325481.2017.1249265
- Smales ET, Edwards BA, Deyoung PN, et al. Trazodone effects on obstructive sleep apnea and non-REM arousal threshold. Ann Am Thorac Soc. 2015;12(5):758-764. doi:10.1513/AnnalsATS.201408-399OC
- Eckert DJ, Malhotra A, Wellman A, White DP. Trazodone increases the respiratory arousal threshold in patients with obstructive sleep apnea and a low arousal threshold. Sleep. 2014;37(4):811-819. doi:10.5665/sleep.3596
- Schatzberg AF, Nemeroff CB, eds. The American Psychiat ric Association Publishing Textbook of Psychopharmacology. 4th ed. American Psychiatric Publishing; 2009.
- Ruxton K, Woodman RJ, Mangoni AA. Drugs with anticholinergic effects and cognitive impairment, falls and allcause mortality in older adults: a systematic review and meta-analysis. Br J Clin Pharmacol. 2015;80(2):209-220. doi:10.1111/bcp.12617
- Nefazodone. In: LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. National Institute of Diabetes and Digestive and Kidney Diseases. Updated March 6, 2020. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK548179/
- Drugs of Current Interest: Nefazodone. WHO Pharmaceuticals Newsletter:(1). 2003(1):7. https://web.archive.org/web/20150403165029/http:/apps.who.int/medicinedocs/en/d/Js4944e/3.html
- Choi S. Nefazodone (serzone) withdrawn because of hepatotoxicity. CMAJ. 2003;169(11):1187.
- Teva Nefazodone Statement. News release. Teva USA. December 20, 2021. Accessed April 4, 2025. https:// www.tevausa.com/news-and-media/press-releases/teva-nefazodone-statement/
- Levitan MN, Papelbaum M, Nardi AE. Profile of agomelatine and its potential in the treatment of generalized anxiety disorder. Neuropsychiatr Dis Treat. 2015;11:1149- 1155. doi:10.2147/NDT.S67470
- Fu DJ, Ionescu DF, Li X, et al. Esketamine nasal spray for rapid reduction of major depressive disorder symptoms in patients who have active suicidal ideation with intent: double-blind, randomized study (ASPIRE I). J Clin Psychiatry. 2020;81(3):19m13191. doi:10.4088/JCP.19m13191
- Iosifescu DV, Jones A, O’Gorman C, et al. Efficacy and safety of AXS-05 (dextromethorphan-bupropion) in patients with major depressive disorder: a phase 3 randomized clinical trial (GEMINI). J Clin Psychiatry. 2022;83(4): 21m14345. doi:10.4088/JCP.21m14345
- Luong TT, Powers CN, Reinhardt BJ, et al. Retrospective evaluation of drug-drug interactions with erlotinib and gefitinib use in the Military Health System. Fed Pract. 2023;40(Suppl 3):S24-S34. doi:10.12788/fp.0401
- Luong TT, Shou KJ, Reinhard BJ, Kigelman OF, Greenfield KM. Paclitaxel drug-drug interactions in the Military Health System. Fed Pract. 2024;41(Suppl 3):S70-S82. doi:10.12788/fp.0499
- Luong TT, Powers CN, Reinhardt BJ, Weina PJ. Preclinical drug-drug interactions (DDIs) of gefitinib with/without losartan and selective serotonin reuptake inhibitors (SSRIs): citalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, and venlafaxine. Curr Res Pharmacol Drug Discov. 2022;3:100112. doi:10.1016/j.crphar.2022.100112
- Luong TT, McAnulty MJ, Evers DL, Reinhardt BJ, Weina PJ. Pre-clinical drug-drug interaction (DDI) of gefitinib or erlotinib with cytochrome P450 (CYP) inhibiting drugs, fluoxetine and/or losartan. Curr Res Toxicol. 2021;2:217- 224. doi:10.1016/j.crtox.2021.05.006,
- Adamo M, Dickie L, Ruhl J. SEER program coding and staging manual 2016. National Cancer Institute; 2016. Accessed April 4, 2025. https://seer.cancer.gov/archive/manuals/2016/SPCSM_2016_maindoc.pdf
- World Health Organization. International classification of diseases for oncology (ICD-O). 3rd ed, 1st revision. World Health Organization; 2013. Accessed April 4, 2025. https://apps.who.int/iris/handle/10665/96612
- Simon S. FDA approves Jelmyto (mitomycin gel) for urothelial cancer. American Cancer Society. April 20, 2020. Accessed April 4, 2025. https://www.cancer.org/cancer/latest-news/fda-approves-jelmyto-mitomycin-gel-for-urothelial-cancer.html
- Pantziarka P, Sukhatme V, Bouche G, Meheus L, Sukhatme VP. Repurposing drugs in oncology (ReDO)- diclofenac as an anti-cancer agent. Ecancermedicalscience. 2016;10:610. doi:10.3332/ecancer.2016.610
- Choi S, Kim S, Park J, Lee SE, Kim C, Kang D. Diclofenac: a nonsteroidal anti-inflammatory drug inducing cancer cell death by inhibiting microtubule polymerization and autophagy flux. Antioxidants (Basel). 2022;11(5):1009. doi:10.3390/antiox11051009
- Tontonoz M. The ABCs of BCG: oldest approved immunotherapy gets new explanation. Memorial Sloan Kettering Cancer Center. July 17, 2020. Accessed April 4, 2025. https://www.mskcc.org/news/oldest-approved-immunotherapy-gets-new-explanation
- Jordan VC. Tamoxifen as the first targeted long-term adjuvant therapy for breast cancer. Endocr Relat Cancer. 2014;21(3):R235-R246. doi:10.1530/ERC-14-0092
- Cole MP, Jones CT, Todd ID. A new anti-oestrogenic agent in late breast cancer. An early clinical appraisal of ICI46474. Br J Cancer. 1971;25(2):270-275. doi:10.1038/bjc.1971.33
- Jordan VC. Tamoxifen: a most unlikely pioneering medicine. Nat Rev Drug Discov. 2003;2(3):205-213. doi:10.1038/nrd1031
- Maximov PY, McDaniel RE, Jordan VC. Tamoxifen: Pioneering Medicine in Breast Cancer. Springer Basel; 2013. Accessed April 4, 2025. https://link.springer.com/book/10.1007/978-3-0348-0664-0
- Taxol (paclitaxel). Prescribing information. Bristol-Myers Squibb Company; 2011. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020262s049lbl.pdf
- Abraxane (paclitaxel). Prescribing information. Celgene Corporation; 2020. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/021660s047lbl.pdf
- Tarceva (erlotinib). Prescribing information. OSI Pharmaceuticals, LLC; 2016. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/021743s025lbl.pdf
- Docetaxel. Prescribing information. Sichuan Hyiyu Pharmaceutical Co.; 2022. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215813s000lbl.pdf
- Alimta (pemetrexed). Prescribing information. Teva Pharmaceuticals; 2022. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/208419s004lbl.pdf
- Tagrisso (osimertinib). Prescribing information. Astra- Zeneca Pharmaceuticals; 2020. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208065s021lbl.pdf
- Iressa (gefitinib). Prescribing information. AstraZeneca Pharmaceuticals; 2018. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/206995s003lbl.pdf
- Kadcyla (ado-trastuzumab emtansine). Prescribing information. Genentech, Inc.; 2013. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/125427lbl.pdf
- Alecensa (alectinib). Prescribing information. Genetech, Inc.; 2017. Accessed April 4, 2025. https:// www.accessdata.fda.gov/drugsatfda_docs/label/2017/208434s003lbl.pdf
- Xalkori (crizotinib). Prescribing information. Pfizer Laboratories; 2022. Accessed April 4, 2025. https:// www.accessdata.fda.gov/drugsatfda_docs/label/2022/202570s033lbl.pdf
- Lorbrena (lorlatinib). Prescribing information. Pfizer Laboratories; 2018. Accessed April 14, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210868s000lbl.pdf
- Alunbrig (brigatinib). Prescribing information. Takeda Pharmaceutical Company; 2020. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208772s008lbl.pdf
- Rozlytrek (entrectinib). Prescribing information. Genentech, Inc.; 2019. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212725s000lbl.pdf
- Herceptin (trastuzumab). Prescribing information. Genentech, Inc.; 2010. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/103792s5250lbl.pdf
- Cybalta (duloxetine). Prescribing information. Eli Lilly and Company; 2017. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/021427s049lbl.pdf
- Effexor XR (venlafaxine). Prescribing information. Pfizer Wyeth Pharmaceuticals Inc; 2022. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/020699s112lbl.pdf
- Desyrel (trazodone hydrochloride). Prescribing information. Pragma Pharmaceuticals; 2017. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018207s032lbl.pdf
- Sertraline hydrochloride. Prescribing information. Almatica Pharma LLC; 2021. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/215133s000lbl.pdf
- Remeron (mirtazapine). Prescribing information. Merck & Co. Inc; 2020. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020415s029,%20021208s019lbl.pdf
- Celexa (citalopram). Prescribing information. Allergan USA Inc; 2022. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/020822s041lbl.pdf
- information. GlaxoSmithKline; 2019. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/020358s066lbl.pdf
- Amitriptyline hydrochloride tablet. Prescribing information. Quality Care Products LLC; 2022. Accessed April 4, 2025. https://www.accessdata.fda.gov/spl/data/0f12f50f-7087-46e7-a2e6-356b4c566c9f/0f12f50f-7087-46e7-a2e6-356b4c566c9f.xml
- Lexapro (escitalopram). Prescribing information. AbbVie Inc; 2023. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/021323s055,021365s039lbl.pdf
- Fluoxetine. Prescribing information. Edgemont Pharmaceutical, LLC; 2017. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/202133s004s005lbl.pdf
- Paxil (paroxetine). Prescribing Information. Apotex Inc; 2021. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/020031s077lbl.pdf
- Pamelor (nortriptyline HCl). Prescribing information. Mallinckrodt, Inc; 2012. Accessed April 4, 2025. https:// www.accessdata.fda.gov/drugsatfda_docs/label/2012/018012s029,018013s061lbl.pdf
- Silenor (doxepin). Prescribing information. Currax Pharmaceuticals; 2020. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/022036s006lbl.pdf
- Tofranil-PM (imipramine pamote). Prescribing information. Mallinckrodt, Inc; 2014. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/017090s078lbl.pdf
- Norpramin (desipramine hydrochloride). Prescribing information. Sanofi-aventis U.S. LLC; 2014. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/014399s069lbl.pdf
- Khedezla (desvenlafaxine). Prescribing information. Osmotical Pharmaceutical US LLC; 2019. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/204683s006lbl.pdf
- Nefazodone hydrochloride. Prescribing information. Bryant Ranch Prepack; 2022. Accessed April 4, 2025. https://www.accessdata.fda.gov/spl/data/0bd4c34a-4f43-4c84-8b98-1d074cba97d5/0bd4c34a-4f43-4c84-8b98-1d074cba97d5.xml
- Grassi L, Nanni MG, Rodin G, Li M, Caruso R. The use of antidepressants in oncology: a review and practical tips for oncologists. Ann Oncol. 2018;29(1):101-111. doi:10.1093/annonc/mdx526
- Lee E, Park Y, Li D, Rodriguez-Fuguet A, Wang X, Zhang WC. Antidepressant use and lung cancer risk and survival: a meta-analysis of observational studies. Cancer Res Commun. 2023;3(6):1013-1025. doi:10.1158/2767-9764.CRC-23-0003
- Olfson M, Marcus SC. National patterns in antidepressant medication treatment. Arch Gen Psychiatry. 2009;66(8):848 -856. doi:10.1001/archgenpsychiatry.2009.81
- Grattan A, Sullivan MD, Saunders KW, Campbell CI, Von Korff MR. Depression and prescription opioid misuse among chronic opioid therapy recipients with no history of substance abuse. Ann Fam Med. 2012;10(4):304-311. doi:10.1370/afm.1371
- Cowan DT, Wilson-Barnett J, Griffiths P, Allan LG. A survey of chronic noncancer pain patients prescribed opioid analgesics. Pain Med. 2003;4(4):340-351. doi:10.1111/j.1526-4637.2003.03038.x
- Breckenridge J, Clark JD. Patient characteristics associated with opioid versus nonsteroidal anti-inflammatory drug management of chronic low back pain. J Pain. 2003;4(6):344-350. doi:10.1016/s1526-5900(03)00638-2
- Edlund MJ, Martin BC, Devries A, Fan MY, Braden JB, Sullivan MD. Trends in use of opioids for chronic noncancer pain among individuals with mental health and substance use disorders: the TROUP study. Clin J Pain. 2010;26(1):1-8. doi:10.1097/AJP.0b013e3181b99f35
- Sullivan MD, Edlund MJ, Fan MY, DeVries A, Braden JB, Martin BC. Risks for possible and probable opioid misuse among recipients of chronic opioid therapy in commercial and medicaid insurance plans: the TROUP study. Pain. 2010;150(2):332-339. doi:10.1016/j.pain.2010.05.020
- Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152(2):85-92. doi:10.7326/0003-4819-152-2-201001190-00006
- Haddad P. Do antidepressants have any potential to cause addiction? J Psychopharmacol. 1999;13(3):300- 307. doi:10.1177/026988119901300321
- Lakeview Health Staff. America’s most abused antidepressants. Lakeview Health. January 24, 2004. Accessed April 4, 2025. https://www.lakeviewhealth.com/blog/us-most-abused-antidepressants/
- Greenhouse Treatment Center Editorial Staff. Addiction to antidepressants: is it possible? America Addiction Centers: Greenhouse Treatment Center. Updated April 23, 2024. Accessed April 4, 2025. https://greenhousetreatment.com/prescription-medication/antidepressants/
Cancer patients experience depression at rates > 5 times that of the general population.1-11 Despite an increase in palliative care use, depression rates continued to rise.2-4 Between 5% to 16% of outpatients, 4% to 14% of inpatients, and up to 49% of patients receiving palliative care experience depression.5 This issue also impacts families and caregivers.1 A 2021 meta-analysis found that 23% of active military personnel and 20% of veterans experience depression.11
Antidepressants approved by the US Food and Drug Administration (FDA) target the serotonin, norepinephrine, or dopamine systems and include boxed warnings about an increased risk of suicidal thoughts in adults aged 18 to 24 years.12,13 These medications are categorized into several classes: monoamine oxidase inhibitors (MAOIs), tricyclic antidepressants (TCAs), tetracyclic antidepressants (TeCAs), norepinephrine-dopamine reuptake inhibitors (NDRIs), selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), serotonin receptor modulators (SRMs), serotonin-melatonin receptor antagonists (SMRAs), and N—methyl-D-aspartate receptor antagonists (NMDARAs).14,15 The first FDA-approved antidepressants, iproniazid (an MAOI) and imipramine (a TCA) laid the foundation for the development of newer classes like SSRIs and SNRIs.15-17
Older antidepressants such as MAOIs and TCAs are used less due to their adverse effects (AEs) and drug interactions. MAOIs, such as iproniazid, selegiline, moclobemide, tranylcypromine, isocarboxazid, and phenelzine, have numerous AEs and drug interactions, making them unsuitable for first- or second-line treatment of depression.14,18-21 TCAs such as doxepin, amitriptyline, nortriptyline, imipramine, desipramine, clomipramine, trimipramine, protriptyline, maprotiline, and amoxapine have a narrow therapeutic index requiring careful monitoring for signs of toxicity such as QRS widening, tremors, or confusion. Despite the issues, TCAs are generally classified as second-line agents for major depressive disorder (MDD). TCAs have off-label uses for migraine prophylaxis, treatment of obsessive-compulsive disorder (OCD), insomnia, and chronic pain management first-line.14,22-29
Newer antidepressants, including TeCAs and NDRIs, are typically more effective, but also come with safety concerns. TeCAs like mirtazapine interact with several medications, including MAOIs, serotonin-increasing drugs, alcohol, cannabidiol, and marijuana. Mirtazapine is FDA-approved for the treatment of moderate to severe depression in adults. It is also used off-label to treat insomnia, panic disorder, posttraumatic stress disorder (PTSD), generalized anxiety disorder (GAD), social anxiety disorder (SAD), headaches, and migraines. Compared to other antidepressants, mirtazapine is effective for all stages of depression and addresses a broad range of related symptoms.14,30-34 NDRIs, such as bupropion, also interact with various medications, including MAOIs, other antidepressants, stimulants, and alcohol. Bupropion is FDA-approved for smoking cessation and to treat depression and SAD. It is also used off-label for depression- related bipolar disorder or sexual dysfunction, attention-deficit/hyperactivity disorder (ADHD), and obesity.14,35-42
SSRIs, SNRIs, and SRMs should be used with caution. SSRIs such as sertraline, citalopram, escitalopram, fluoxetine, paroxetine, and fluvoxamine are first-line treatments for depression and various psychiatric disorders due to their safety and efficacy. Common AEs of SSRIs include sexual dysfunction, sleep disturbances, weight changes, and gastrointestinal (GI) issues. SSRIs can prolong the QT interval, posing a risk of life-threatening arrhythmia, and may interact with other medications, necessitating treatment adjustments. The FDA approved SSRIs for MDD, GAD, bulimia nervosa, bipolar depression, OCD, panic disorder, premenstrual dysphoric disorder, treatment-resistant depression, PTSD, and SAD. Off-label uses include binge eating disorder, body dysmorphic disorder, fibromyalgia, premature ejaculation, paraphilias, autism, Raynaud phenomenon, and vasomotor symptoms associated with menopause. Among SSRIs, sertraline and escitalopram are noted for their effectiveness and tolerability.14,43-53
SNRIs, including duloxetine, venlafaxine, desvenlafaxine, milnacipran, and levomilnacipran, may increase bleeding risk, especially when taken with blood thinners. They can also elevate blood pressure, which may worsen if combined with stimulants. SNRIs may interact with other medications that affect serotonin levels, increasing the risk of serotonin syndrome when taken with triptans, pain medications, or other antidepressants.14 Desvenlafaxine has been approved by the FDA (but not by the European Medicines Agency).54-56 Duloxetine is FDA-approved for the treatment of depression, neuropathic pain, anxiety disorders, fibromyalgia, and musculoskeletal disorders. It is used off-label to treat chemotherapy-induced peripheral neuropathy and stress urinary incontinence.57-61 Venlafaxine is FDA-approved for depression, SAD, and panic disorder, and is prescribed off-label to treat ADHD, neuropathy, fibromyalgia, cataplexy, and PTSD, either alone or in combination with other medications.62,63 Milnacipran is not approved for MDD; levomilnacipran received approval in 2013.64
SRMs such as trazodone, nefazodone, vilazodone, and vortioxetine also function as serotonin reuptake inhibitors.14,15 Trazodone is FDA-approved for MDD. It has been used off-label to treat anxiety, Alzheimer disease, substance misuse, bulimia nervosa, insomnia, fibromyalgia, and PTSD when first-line SSRIs are ineffective. A notable AE of trazodone is orthostatic hypotension, which can lead to dizziness and increase the risk of falls, especially in geriatric patients.65-70 Nefazodone was discontinued in Europe in 2003 due to rare cases of liver toxicity but remains available in the US.71-74 Vilazodone and vortioxetine are FDA-approved.
The latest classes of antidepressants include SMRAs and NMDARAs.14 Agomelatine, an SMRA, was approved in Europe in 2009 but rejected by the FDA in 2011 due to liver toxicity.75 NMDARAs like esketamine and a combination of dextromethorphan and bupropion received FDA approval in 2019 and 2022, respectively.76,77
This retrospective study analyzes noncancer drugs used during systemic chemotherapy based on a dataset of 14 antineoplastic agents. It sought to identify the most dispensed noncancer drug groups, discuss findings, compare patients with and without antidepressant prescriptions, and examine trends in antidepressant use from 2002 to 2023. This analysis expands on prior research.78-81
Methods
The Walter Reed National Military Medical Center Institutional Review Board approved the study protocol and ensured compliance with the Health Insurance Portability and Accountability Act as an exempt protocol. The Joint Pathology Center (JPC) of the US Department of Defense (DoD) Cancer Registry Program and Military Health System (MHS) data experts from the Comprehensive Ambulatory/Professional Encounter Record (CAPER) and Pharmacy Data Transaction Service (PDTS) provided data for the analysis.
Data Sources
The JPC DoD Cancer Registry Program contains data from 1998 to 2024. CAPER and PDTS are part of the MHS Data Repository/Management Analysis and Reporting Tool database. Each observation in CAPER represents an ambulatory encounter at a military treatment facility (MTF). CAPER records are available from 2003 to 2024. PDTS records are available from 2002 to 2004. Each observation in PDTS represents a prescription filled for an MHS beneficiary, excluding those filled at international civilian pharmacies and inpatient pharmacy prescriptions.
This cross-sectional analysis requested data extraction for specific cancer drugs from the DoD Cancer Registry, focusing on treatment details, diagnosis dates, patient demographics, and physicians’ comments on AEs. After identifying patients, CAPER was used to identify additional health conditions. PDTS was used to compile a list of prescription medications filled during systemic cancer treatment or < 2 years postdiagnosis.
The 2016 Surveillance, Epidemiology, and End Results Program Coding and Staging Manual and International Classification of Diseases for Oncology, 3rd edition, 1st revision, were used to decode disease and cancer types.82,83 Data sorting and analysis were performed using Microsoft Excel. The percentage for the total was calculated by using the number of patients or data available within the subgroup divided by the total number of patients or data variables. To compare the mean number of dispensed antidepressants to those without antidepressants, a 2-tailed, 2-sample z test was used to calculate the P value and determine statistical significance (P < .05) using socscistatistics.com.
Data were extracted 3 times between 2021 and 2023. The initial 2021 protocol focused on erlotinib and gefitinib. A modified protocol in 2022 added paclitaxel, cisplatin, docetaxel, pemetrexed, and crizotinib; further modification in 2023 included 8 new antineoplastic agents and 2 anticoagulants. Sotorasib has not been prescribed in the MHS, and JPC lacks records for noncancer drugs. The 2023 dataset comprised 2210 patients with cancer treated with 14 antineoplastic agents; 2104 had documented diagnoses and 2113 had recorded prescriptions. Data for erlotinib, gefitinib, and paclitaxel have been published previously.78,79
Results
Of 2113 patients with recorded prescriptions, 1297 patients (61.4%) received 109 cancer drugs, including 96 antineoplastics, 7 disease-modifying antirheumatic agents, 4 biologic response modifiers, and 2 calcitonin gene-related peptides. Fourteen antineoplastic agents had complete data from JPC, while others were noted for combination therapies or treatment switches from the PDTS (Table 1). Seventy-six cancer drugs were prescribed with antidepressants in 489 patients (eAppendix).

The JPC provided 2242 entries for 2210 patients, ranging in age from 2 months to 88 years (mean, 56 years), documenting treatment from September 1988 to January 2023. Thirty-two patients had duplicate entries due to multiple cancer locations or occurrences. Of the 2242 patients, 1541 (68.7%) were aged > 50 years, 975 patients (43.5%) had cancers that were stage III or IV, and 1267 (56.5%) had cancers that were stage 0, I, II, or not applicable/unknown. There were 51 different types of cancer: breast, lung, testicular, endometrial, and ovarian were most common (n ≥ 100 patients). Forty-two cancer types were documented among 750 patients prescribed antidepressants (Table 2).

The CAPER database recorded 8882 unique diagnoses for 2104 patients, while PDTS noted 1089 unique prescriptions within 273 therapeutic codes for 2113 patients. Nine therapeutic codes (opiate agonists, adrenals, cathartics-laxatives, nonsteroidal anti-inflammatory agents, antihistamines for GI conditions, 5-HT3 receptor antagonists, analgesics and antipyretic miscellanea, antineoplastic agents, and proton-pump inhibitors) and 8 drugs (dexamethasone, prochlorperazine, ondansetron, docusate, acetaminophen, ibuprofen, oxycodone, and polyethylene glycol 3350) were associated with > 1000 patients (≥ 50%). Patients had between 1 and 275 unique health conditions and filled 1 to 108 prescriptions. The mean (SD) number of diagnoses and prescriptions was 50 (28) and 29 (12), respectively. Of the 273 therapeutic codes, 30 groups were analyzed, with others categorized into miscellaneous groups such as lotions, vaccines, and devices. Significant differences in mean number of prescriptions were found for patients taking antidepressants compared to those not (P < .05), except for anticonvulsants and antipsychotics (P = .12 and .09, respectively) (Table 3).

Antidepressants
Of the 2113 patients with recorded prescriptions, 750 (35.5%) were dispensed 17 different antidepressants. Among these 17 antidepressants, 183 (8.7%) patients received duloxetine, 158 (7.5%) received venlafaxine, 118 (5.6%) received trazodone, and 107 (5.1%) received sertraline (Figure 1, Table 4). Of the 750 patients, 509 (67.9%) received 1 antidepressant, 168 (22.4%) received 2, 60 (8.0%) received 3, and 13 (1.7%) received > 3. Combinations varied, but only duloxetine and trazodone were prescribed to > 10 patients.



Antidepressants were prescribed annually at an overall mean (SD) rate of 23% (5%) from 2003 to 2022 (Figure 2). Patients on antidepressants during systemic therapy had a greater number of diagnosed medical conditions and received more prescription medications compared to those not taking antidepressants (P < .001) (Figure 3). The 745 patients taking antidepressants in CAPER data had between 1 and 275 diagnosed medical issues, with a mean (SD) of 55 (31) vs a range of 1 to 209 and a mean (SD) of 46 (26) for the 1359 patients not taking antidepressants. The 750 patients on antidepressants in PDTS data had between 8 and 108 prescriptions dispensed, with a mean (SD) of 32 (12), vs a range of 1 to 65 prescriptions and a mean (SD) of 29 (12) for 1363 patients not taking antidepressants.


Discussion
The JPC DoD Cancer Registry includes information on cancer types, stages, treatment regimens, and physicians’ notes, while noncancer drugs are sourced from the PDTS database. The pharmacy uses a different documentation system, leading to varied classifications.
Database reliance has its drawbacks. For example, megestrol is coded as a cancer drug, although it’s primarily used for endometrial or gynecologic cancers. Many drugs have multiple therapeutic codes assigned to them, including 10 antineoplastic agents: diclofenac, Bacillus Calmette-Guérin (BCG), megestrol acetate, tamoxifen, anastrozole, letrozole, leuprolide, goserelin, degarelix, and fluorouracil. Diclofenac, BCG, and mitomycin have been repurposed for cancer treatment.84-87 From 2003 to 2023, diclofenac was prescribed to 350 patients for mild-to-moderate pain, with only 2 patients receiving it for cancer in 2018. FDA-approved for bladder cancer in 1990, BCG was prescribed for cancer treatment for 1 patient in 2021 after being used for vaccines between 2003 and 2018. Tamoxifen, used for hormone receptor-positive breast cancer from 2004 to 2017 with 53 patients, switched to estrogen agonist-antagonists from 2017 to 2023 with 123 patients. Only a few of the 168 patients were prescribed tamoxifen using both codes.88-91 Anastrozole and letrozole were coded as antiestrogens for 7 and 18 patients, respectively, while leuprolide and goserelin were coded as gonadotropins for 59 and 18 patients. Degarelix was coded as antigonadotropins, fluorouracil as skin and mucous membrane agents miscellaneous, and megestrol acetate as progestins for 7, 6, and 3 patients, respectively. Duloxetine was given to 186 patients, primarily for depression from 2005 to 2023, with 7 patients treated for fibromyalgia from 2022 to 2023.
Antidepressants Observed
Tables 1 and 5 provide insight into the FDA approval of 14 antineoplastics and antidepressants and their CYP metabolic pathways.92-122 In Table 4, the most prescribed antidepressant classes are SNRIs, SRMs, SSRIs, TeCAs, NDRIs, and TCAs. This trend highlights a preference for newer medications with weak CYP inhibition. A total of 349 patients were prescribed SSRIs, 343 SNRIs, 119 SRMs, 109 TCAs, 83 TeCAs, and 79 NDRIs. MAOIs, SMRAs, and NMDARAs were not observed in this dataset. While there are instances of dextromethorphan-bupropion and sertraline-escitalopram being dispensed together, it remains unclear whether these were NMDARA combinations.
Among the 14 specific antineoplastic agents, 10 are metabolized by CYP isoenzymes, primarily CYP3A4. Duloxetine neither inhibits nor is metabolized by CYP3A4, a reason it is often recommended, following venlafaxine.
Both duloxetine and venlafaxine are used off-label for chemotherapy-induced peripheral neuropathy related to paclitaxel and docetaxel. According to the CYP metabolized pathway, duloxetine tends to have more favorable DDIs than venlafaxine. In PDTS data, 371 patients were treated with paclitaxel and 180 with docetaxel, with respective antidepressant prescriptions of 156 and 70. Of the 156 patients dispensed paclitaxel, 62 (40%) were dispensed with duloxetine compared to 43 (28%) with venlafaxine. Of the 70 patients dispensed docetaxel, 23 (33%) received duloxetine vs 24 (34%) with venlafaxine.
Of 85 patients prescribed duloxetine, 75 received it with either paclitaxel or docetaxel (5 received both). Five patients had documented AEs (1 neuropathy related). Of 67 patients prescribed venlafaxine, 66 received it with either paclitaxel or docetaxel. Two patients had documented AEs (1 was neuropathy related, the same patient who received duloxetine). Of the 687 patients treated with paclitaxel and 337 with docetaxel in all databases, 4 experienced neuropathic AEs from both medications.79
Antidepressants can increase the risk of bleeding, especially when combined with blood thinners, and may elevate blood pressure, particularly alongside stimulants. Of the 554 patients prescribed 9 different anticoagulants, enoxaparin, apixaban, and rivaroxaban were the most common (each > 100 patients). Among these, 201 patients (36%) received both anticoagulants and antidepressants: duloxetine for 64 patients, venlafaxine for 30, trazodone for 35, and sertraline for 26. There were no data available to assess bleeding rates related to the evaluation of DDIs between these medication classes.
Antidepressants can be prescribed for erectile dysfunction. Of the 148 patients prescribed an antidepressant for erectile dysfunction, duloxetine, trazodone, and mirtazapine were the most common. Antidepressant preferences varied by cancer type. Duloxetine was the only antidepressant used for all types of cancer. Venlafaxine, duloxetine, trazodone, sertraline, and escitalopram were the most prescribed antidepressants for breast cancer, while duloxetine, mirtazapine, citalopram, sertraline, and trazodone were the most prescribed for lung cancer. Sertraline, duloxetine, trazodone, amitriptyline, and escitalopram were most common for testicular cancer. Duloxetine, venlafaxine, trazodone, amitriptyline, and sertraline were the most prescribed for endometrial cancer, while duloxetine, venlafaxine, amitriptyline, citalopram, and sertraline were most prescribed for ovarian cancer.
The broadness of International Statistical Classification of Diseases, Tenth Revision codes made it challenging to identify nondepression diagnoses in the analyzed population. However, if all antidepressants were prescribed to treat depression, service members with cancer exhibited a higher depression rate (35%) than the general population (25%). Of 2104 patients, 191 (9.1%) had mood disorders, and 706 (33.6%) had mental disorders: 346 (49.0%) had 1 diagnosis, and 360 (51.0%) had multiple diagnoses. The percentage of diagnoses varied yearly, with notable drops in 2003, 2007, 2011, 2014, and 2018, and peaks in 2006, 2008, 2013, 2017, and 2022. This fluctuation was influenced by events like the establishment of PDTS in 2002, the 2008 economic recession, a hospital relocation in 2011, the 2014 Ebola outbreak, and the COVID-19 pandemic. Although the number of patients receiving antidepressants increased from 2019 to 2022, the overall percentage of patients receiving them did not significantly change from 2003 to 2022, aligning with previous research.5,125
Many medications have potential uses beyond what is detailed in the prescribing information. Antidepressants can relieve pain, while pain medications may help with depression. Opioids were once thought to effectively treat depression, but this perspective has changed with a greater understanding of their risks, including misuse.126-131 Pain is a severe and often unbearable AE of cancer. Of 2113 patients, 92% received opioids; 34% received both opioids and antidepressants; 2% received only antidepressants; and 7% received neither. This study didn’t clarify whether those on opioids alone recognized their depression or if those on both were aware of their dependence. While SSRIs are generally not addictive, they can lead to physical dependence, and any medication can be abused if not managed properly.132-134
Conclusions
This retrospective study analyzes data from antineoplastic agents used in systemic cancer treatment between 1988 and 2023, with a particular focus on the use of antidepressants. Data on antidepressant prescriptions are incomplete and specific to these agents, which means the findings cannot be generalized to all antidepressants. Hence, the results indicate that patients taking antidepressants had more diagnosed health issues and received more medications compared to patients who were not on these drugs.
This study underscores the need for further research into the effects of antidepressants on cancer treatment, utilizing all data from the DoD Cancer Registry. Future research should explore DDIs between antidepressants and other cancer and noncancer medications, as this study did not assess AE documentation, unlike in studies involving erlotinib, gefitinib, and paclitaxel.78,79 Further investigation is needed to evaluate the impact of discontinuing antidepressant use during cancer treatment. This comprehensive overview provides insights for clinicians to help them make informed decisions regarding the prescription of antidepressants in the context of cancer treatment.
Cancer patients experience depression at rates > 5 times that of the general population.1-11 Despite an increase in palliative care use, depression rates continued to rise.2-4 Between 5% to 16% of outpatients, 4% to 14% of inpatients, and up to 49% of patients receiving palliative care experience depression.5 This issue also impacts families and caregivers.1 A 2021 meta-analysis found that 23% of active military personnel and 20% of veterans experience depression.11
Antidepressants approved by the US Food and Drug Administration (FDA) target the serotonin, norepinephrine, or dopamine systems and include boxed warnings about an increased risk of suicidal thoughts in adults aged 18 to 24 years.12,13 These medications are categorized into several classes: monoamine oxidase inhibitors (MAOIs), tricyclic antidepressants (TCAs), tetracyclic antidepressants (TeCAs), norepinephrine-dopamine reuptake inhibitors (NDRIs), selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), serotonin receptor modulators (SRMs), serotonin-melatonin receptor antagonists (SMRAs), and N—methyl-D-aspartate receptor antagonists (NMDARAs).14,15 The first FDA-approved antidepressants, iproniazid (an MAOI) and imipramine (a TCA) laid the foundation for the development of newer classes like SSRIs and SNRIs.15-17
Older antidepressants such as MAOIs and TCAs are used less due to their adverse effects (AEs) and drug interactions. MAOIs, such as iproniazid, selegiline, moclobemide, tranylcypromine, isocarboxazid, and phenelzine, have numerous AEs and drug interactions, making them unsuitable for first- or second-line treatment of depression.14,18-21 TCAs such as doxepin, amitriptyline, nortriptyline, imipramine, desipramine, clomipramine, trimipramine, protriptyline, maprotiline, and amoxapine have a narrow therapeutic index requiring careful monitoring for signs of toxicity such as QRS widening, tremors, or confusion. Despite the issues, TCAs are generally classified as second-line agents for major depressive disorder (MDD). TCAs have off-label uses for migraine prophylaxis, treatment of obsessive-compulsive disorder (OCD), insomnia, and chronic pain management first-line.14,22-29
Newer antidepressants, including TeCAs and NDRIs, are typically more effective, but also come with safety concerns. TeCAs like mirtazapine interact with several medications, including MAOIs, serotonin-increasing drugs, alcohol, cannabidiol, and marijuana. Mirtazapine is FDA-approved for the treatment of moderate to severe depression in adults. It is also used off-label to treat insomnia, panic disorder, posttraumatic stress disorder (PTSD), generalized anxiety disorder (GAD), social anxiety disorder (SAD), headaches, and migraines. Compared to other antidepressants, mirtazapine is effective for all stages of depression and addresses a broad range of related symptoms.14,30-34 NDRIs, such as bupropion, also interact with various medications, including MAOIs, other antidepressants, stimulants, and alcohol. Bupropion is FDA-approved for smoking cessation and to treat depression and SAD. It is also used off-label for depression- related bipolar disorder or sexual dysfunction, attention-deficit/hyperactivity disorder (ADHD), and obesity.14,35-42
SSRIs, SNRIs, and SRMs should be used with caution. SSRIs such as sertraline, citalopram, escitalopram, fluoxetine, paroxetine, and fluvoxamine are first-line treatments for depression and various psychiatric disorders due to their safety and efficacy. Common AEs of SSRIs include sexual dysfunction, sleep disturbances, weight changes, and gastrointestinal (GI) issues. SSRIs can prolong the QT interval, posing a risk of life-threatening arrhythmia, and may interact with other medications, necessitating treatment adjustments. The FDA approved SSRIs for MDD, GAD, bulimia nervosa, bipolar depression, OCD, panic disorder, premenstrual dysphoric disorder, treatment-resistant depression, PTSD, and SAD. Off-label uses include binge eating disorder, body dysmorphic disorder, fibromyalgia, premature ejaculation, paraphilias, autism, Raynaud phenomenon, and vasomotor symptoms associated with menopause. Among SSRIs, sertraline and escitalopram are noted for their effectiveness and tolerability.14,43-53
SNRIs, including duloxetine, venlafaxine, desvenlafaxine, milnacipran, and levomilnacipran, may increase bleeding risk, especially when taken with blood thinners. They can also elevate blood pressure, which may worsen if combined with stimulants. SNRIs may interact with other medications that affect serotonin levels, increasing the risk of serotonin syndrome when taken with triptans, pain medications, or other antidepressants.14 Desvenlafaxine has been approved by the FDA (but not by the European Medicines Agency).54-56 Duloxetine is FDA-approved for the treatment of depression, neuropathic pain, anxiety disorders, fibromyalgia, and musculoskeletal disorders. It is used off-label to treat chemotherapy-induced peripheral neuropathy and stress urinary incontinence.57-61 Venlafaxine is FDA-approved for depression, SAD, and panic disorder, and is prescribed off-label to treat ADHD, neuropathy, fibromyalgia, cataplexy, and PTSD, either alone or in combination with other medications.62,63 Milnacipran is not approved for MDD; levomilnacipran received approval in 2013.64
SRMs such as trazodone, nefazodone, vilazodone, and vortioxetine also function as serotonin reuptake inhibitors.14,15 Trazodone is FDA-approved for MDD. It has been used off-label to treat anxiety, Alzheimer disease, substance misuse, bulimia nervosa, insomnia, fibromyalgia, and PTSD when first-line SSRIs are ineffective. A notable AE of trazodone is orthostatic hypotension, which can lead to dizziness and increase the risk of falls, especially in geriatric patients.65-70 Nefazodone was discontinued in Europe in 2003 due to rare cases of liver toxicity but remains available in the US.71-74 Vilazodone and vortioxetine are FDA-approved.
The latest classes of antidepressants include SMRAs and NMDARAs.14 Agomelatine, an SMRA, was approved in Europe in 2009 but rejected by the FDA in 2011 due to liver toxicity.75 NMDARAs like esketamine and a combination of dextromethorphan and bupropion received FDA approval in 2019 and 2022, respectively.76,77
This retrospective study analyzes noncancer drugs used during systemic chemotherapy based on a dataset of 14 antineoplastic agents. It sought to identify the most dispensed noncancer drug groups, discuss findings, compare patients with and without antidepressant prescriptions, and examine trends in antidepressant use from 2002 to 2023. This analysis expands on prior research.78-81
Methods
The Walter Reed National Military Medical Center Institutional Review Board approved the study protocol and ensured compliance with the Health Insurance Portability and Accountability Act as an exempt protocol. The Joint Pathology Center (JPC) of the US Department of Defense (DoD) Cancer Registry Program and Military Health System (MHS) data experts from the Comprehensive Ambulatory/Professional Encounter Record (CAPER) and Pharmacy Data Transaction Service (PDTS) provided data for the analysis.
Data Sources
The JPC DoD Cancer Registry Program contains data from 1998 to 2024. CAPER and PDTS are part of the MHS Data Repository/Management Analysis and Reporting Tool database. Each observation in CAPER represents an ambulatory encounter at a military treatment facility (MTF). CAPER records are available from 2003 to 2024. PDTS records are available from 2002 to 2004. Each observation in PDTS represents a prescription filled for an MHS beneficiary, excluding those filled at international civilian pharmacies and inpatient pharmacy prescriptions.
This cross-sectional analysis requested data extraction for specific cancer drugs from the DoD Cancer Registry, focusing on treatment details, diagnosis dates, patient demographics, and physicians’ comments on AEs. After identifying patients, CAPER was used to identify additional health conditions. PDTS was used to compile a list of prescription medications filled during systemic cancer treatment or < 2 years postdiagnosis.
The 2016 Surveillance, Epidemiology, and End Results Program Coding and Staging Manual and International Classification of Diseases for Oncology, 3rd edition, 1st revision, were used to decode disease and cancer types.82,83 Data sorting and analysis were performed using Microsoft Excel. The percentage for the total was calculated by using the number of patients or data available within the subgroup divided by the total number of patients or data variables. To compare the mean number of dispensed antidepressants to those without antidepressants, a 2-tailed, 2-sample z test was used to calculate the P value and determine statistical significance (P < .05) using socscistatistics.com.
Data were extracted 3 times between 2021 and 2023. The initial 2021 protocol focused on erlotinib and gefitinib. A modified protocol in 2022 added paclitaxel, cisplatin, docetaxel, pemetrexed, and crizotinib; further modification in 2023 included 8 new antineoplastic agents and 2 anticoagulants. Sotorasib has not been prescribed in the MHS, and JPC lacks records for noncancer drugs. The 2023 dataset comprised 2210 patients with cancer treated with 14 antineoplastic agents; 2104 had documented diagnoses and 2113 had recorded prescriptions. Data for erlotinib, gefitinib, and paclitaxel have been published previously.78,79
Results
Of 2113 patients with recorded prescriptions, 1297 patients (61.4%) received 109 cancer drugs, including 96 antineoplastics, 7 disease-modifying antirheumatic agents, 4 biologic response modifiers, and 2 calcitonin gene-related peptides. Fourteen antineoplastic agents had complete data from JPC, while others were noted for combination therapies or treatment switches from the PDTS (Table 1). Seventy-six cancer drugs were prescribed with antidepressants in 489 patients (eAppendix).

The JPC provided 2242 entries for 2210 patients, ranging in age from 2 months to 88 years (mean, 56 years), documenting treatment from September 1988 to January 2023. Thirty-two patients had duplicate entries due to multiple cancer locations or occurrences. Of the 2242 patients, 1541 (68.7%) were aged > 50 years, 975 patients (43.5%) had cancers that were stage III or IV, and 1267 (56.5%) had cancers that were stage 0, I, II, or not applicable/unknown. There were 51 different types of cancer: breast, lung, testicular, endometrial, and ovarian were most common (n ≥ 100 patients). Forty-two cancer types were documented among 750 patients prescribed antidepressants (Table 2).

The CAPER database recorded 8882 unique diagnoses for 2104 patients, while PDTS noted 1089 unique prescriptions within 273 therapeutic codes for 2113 patients. Nine therapeutic codes (opiate agonists, adrenals, cathartics-laxatives, nonsteroidal anti-inflammatory agents, antihistamines for GI conditions, 5-HT3 receptor antagonists, analgesics and antipyretic miscellanea, antineoplastic agents, and proton-pump inhibitors) and 8 drugs (dexamethasone, prochlorperazine, ondansetron, docusate, acetaminophen, ibuprofen, oxycodone, and polyethylene glycol 3350) were associated with > 1000 patients (≥ 50%). Patients had between 1 and 275 unique health conditions and filled 1 to 108 prescriptions. The mean (SD) number of diagnoses and prescriptions was 50 (28) and 29 (12), respectively. Of the 273 therapeutic codes, 30 groups were analyzed, with others categorized into miscellaneous groups such as lotions, vaccines, and devices. Significant differences in mean number of prescriptions were found for patients taking antidepressants compared to those not (P < .05), except for anticonvulsants and antipsychotics (P = .12 and .09, respectively) (Table 3).

Antidepressants
Of the 2113 patients with recorded prescriptions, 750 (35.5%) were dispensed 17 different antidepressants. Among these 17 antidepressants, 183 (8.7%) patients received duloxetine, 158 (7.5%) received venlafaxine, 118 (5.6%) received trazodone, and 107 (5.1%) received sertraline (Figure 1, Table 4). Of the 750 patients, 509 (67.9%) received 1 antidepressant, 168 (22.4%) received 2, 60 (8.0%) received 3, and 13 (1.7%) received > 3. Combinations varied, but only duloxetine and trazodone were prescribed to > 10 patients.



Antidepressants were prescribed annually at an overall mean (SD) rate of 23% (5%) from 2003 to 2022 (Figure 2). Patients on antidepressants during systemic therapy had a greater number of diagnosed medical conditions and received more prescription medications compared to those not taking antidepressants (P < .001) (Figure 3). The 745 patients taking antidepressants in CAPER data had between 1 and 275 diagnosed medical issues, with a mean (SD) of 55 (31) vs a range of 1 to 209 and a mean (SD) of 46 (26) for the 1359 patients not taking antidepressants. The 750 patients on antidepressants in PDTS data had between 8 and 108 prescriptions dispensed, with a mean (SD) of 32 (12), vs a range of 1 to 65 prescriptions and a mean (SD) of 29 (12) for 1363 patients not taking antidepressants.


Discussion
The JPC DoD Cancer Registry includes information on cancer types, stages, treatment regimens, and physicians’ notes, while noncancer drugs are sourced from the PDTS database. The pharmacy uses a different documentation system, leading to varied classifications.
Database reliance has its drawbacks. For example, megestrol is coded as a cancer drug, although it’s primarily used for endometrial or gynecologic cancers. Many drugs have multiple therapeutic codes assigned to them, including 10 antineoplastic agents: diclofenac, Bacillus Calmette-Guérin (BCG), megestrol acetate, tamoxifen, anastrozole, letrozole, leuprolide, goserelin, degarelix, and fluorouracil. Diclofenac, BCG, and mitomycin have been repurposed for cancer treatment.84-87 From 2003 to 2023, diclofenac was prescribed to 350 patients for mild-to-moderate pain, with only 2 patients receiving it for cancer in 2018. FDA-approved for bladder cancer in 1990, BCG was prescribed for cancer treatment for 1 patient in 2021 after being used for vaccines between 2003 and 2018. Tamoxifen, used for hormone receptor-positive breast cancer from 2004 to 2017 with 53 patients, switched to estrogen agonist-antagonists from 2017 to 2023 with 123 patients. Only a few of the 168 patients were prescribed tamoxifen using both codes.88-91 Anastrozole and letrozole were coded as antiestrogens for 7 and 18 patients, respectively, while leuprolide and goserelin were coded as gonadotropins for 59 and 18 patients. Degarelix was coded as antigonadotropins, fluorouracil as skin and mucous membrane agents miscellaneous, and megestrol acetate as progestins for 7, 6, and 3 patients, respectively. Duloxetine was given to 186 patients, primarily for depression from 2005 to 2023, with 7 patients treated for fibromyalgia from 2022 to 2023.
Antidepressants Observed
Tables 1 and 5 provide insight into the FDA approval of 14 antineoplastics and antidepressants and their CYP metabolic pathways.92-122 In Table 4, the most prescribed antidepressant classes are SNRIs, SRMs, SSRIs, TeCAs, NDRIs, and TCAs. This trend highlights a preference for newer medications with weak CYP inhibition. A total of 349 patients were prescribed SSRIs, 343 SNRIs, 119 SRMs, 109 TCAs, 83 TeCAs, and 79 NDRIs. MAOIs, SMRAs, and NMDARAs were not observed in this dataset. While there are instances of dextromethorphan-bupropion and sertraline-escitalopram being dispensed together, it remains unclear whether these were NMDARA combinations.
Among the 14 specific antineoplastic agents, 10 are metabolized by CYP isoenzymes, primarily CYP3A4. Duloxetine neither inhibits nor is metabolized by CYP3A4, a reason it is often recommended, following venlafaxine.
Both duloxetine and venlafaxine are used off-label for chemotherapy-induced peripheral neuropathy related to paclitaxel and docetaxel. According to the CYP metabolized pathway, duloxetine tends to have more favorable DDIs than venlafaxine. In PDTS data, 371 patients were treated with paclitaxel and 180 with docetaxel, with respective antidepressant prescriptions of 156 and 70. Of the 156 patients dispensed paclitaxel, 62 (40%) were dispensed with duloxetine compared to 43 (28%) with venlafaxine. Of the 70 patients dispensed docetaxel, 23 (33%) received duloxetine vs 24 (34%) with venlafaxine.
Of 85 patients prescribed duloxetine, 75 received it with either paclitaxel or docetaxel (5 received both). Five patients had documented AEs (1 neuropathy related). Of 67 patients prescribed venlafaxine, 66 received it with either paclitaxel or docetaxel. Two patients had documented AEs (1 was neuropathy related, the same patient who received duloxetine). Of the 687 patients treated with paclitaxel and 337 with docetaxel in all databases, 4 experienced neuropathic AEs from both medications.79
Antidepressants can increase the risk of bleeding, especially when combined with blood thinners, and may elevate blood pressure, particularly alongside stimulants. Of the 554 patients prescribed 9 different anticoagulants, enoxaparin, apixaban, and rivaroxaban were the most common (each > 100 patients). Among these, 201 patients (36%) received both anticoagulants and antidepressants: duloxetine for 64 patients, venlafaxine for 30, trazodone for 35, and sertraline for 26. There were no data available to assess bleeding rates related to the evaluation of DDIs between these medication classes.
Antidepressants can be prescribed for erectile dysfunction. Of the 148 patients prescribed an antidepressant for erectile dysfunction, duloxetine, trazodone, and mirtazapine were the most common. Antidepressant preferences varied by cancer type. Duloxetine was the only antidepressant used for all types of cancer. Venlafaxine, duloxetine, trazodone, sertraline, and escitalopram were the most prescribed antidepressants for breast cancer, while duloxetine, mirtazapine, citalopram, sertraline, and trazodone were the most prescribed for lung cancer. Sertraline, duloxetine, trazodone, amitriptyline, and escitalopram were most common for testicular cancer. Duloxetine, venlafaxine, trazodone, amitriptyline, and sertraline were the most prescribed for endometrial cancer, while duloxetine, venlafaxine, amitriptyline, citalopram, and sertraline were most prescribed for ovarian cancer.
The broadness of International Statistical Classification of Diseases, Tenth Revision codes made it challenging to identify nondepression diagnoses in the analyzed population. However, if all antidepressants were prescribed to treat depression, service members with cancer exhibited a higher depression rate (35%) than the general population (25%). Of 2104 patients, 191 (9.1%) had mood disorders, and 706 (33.6%) had mental disorders: 346 (49.0%) had 1 diagnosis, and 360 (51.0%) had multiple diagnoses. The percentage of diagnoses varied yearly, with notable drops in 2003, 2007, 2011, 2014, and 2018, and peaks in 2006, 2008, 2013, 2017, and 2022. This fluctuation was influenced by events like the establishment of PDTS in 2002, the 2008 economic recession, a hospital relocation in 2011, the 2014 Ebola outbreak, and the COVID-19 pandemic. Although the number of patients receiving antidepressants increased from 2019 to 2022, the overall percentage of patients receiving them did not significantly change from 2003 to 2022, aligning with previous research.5,125
Many medications have potential uses beyond what is detailed in the prescribing information. Antidepressants can relieve pain, while pain medications may help with depression. Opioids were once thought to effectively treat depression, but this perspective has changed with a greater understanding of their risks, including misuse.126-131 Pain is a severe and often unbearable AE of cancer. Of 2113 patients, 92% received opioids; 34% received both opioids and antidepressants; 2% received only antidepressants; and 7% received neither. This study didn’t clarify whether those on opioids alone recognized their depression or if those on both were aware of their dependence. While SSRIs are generally not addictive, they can lead to physical dependence, and any medication can be abused if not managed properly.132-134
Conclusions
This retrospective study analyzes data from antineoplastic agents used in systemic cancer treatment between 1988 and 2023, with a particular focus on the use of antidepressants. Data on antidepressant prescriptions are incomplete and specific to these agents, which means the findings cannot be generalized to all antidepressants. Hence, the results indicate that patients taking antidepressants had more diagnosed health issues and received more medications compared to patients who were not on these drugs.
This study underscores the need for further research into the effects of antidepressants on cancer treatment, utilizing all data from the DoD Cancer Registry. Future research should explore DDIs between antidepressants and other cancer and noncancer medications, as this study did not assess AE documentation, unlike in studies involving erlotinib, gefitinib, and paclitaxel.78,79 Further investigation is needed to evaluate the impact of discontinuing antidepressant use during cancer treatment. This comprehensive overview provides insights for clinicians to help them make informed decisions regarding the prescription of antidepressants in the context of cancer treatment.
- National Cancer Institute. Depression (PDQ)-Health Professional Version. Updated July 25, 2024. Accessed April 4, 2025. https://www.cancer.gov/about-cancer/coping/feelings/depression-hp-pdq
- Krebber AM, Buffart LM, Kleijn G, et al. Prevalence of depression in cancer patients: a meta-analysis of diagnostic interviews and self-report instruments. Psychooncology. 2014;23(2):121-130. doi:10.1002/pon.3409
- Xiang X, An R, Gehlert S. Trends in antidepressant use among U.S. cancer survivors, 1999-2012. Psychiatr Serv. 2015;66(6):564. doi:10.1176/appi.ps.201500007
- Hartung TJ, Brähler E, Faller H, et al. The risk of being depressed is significantly higher in cancer patients than in the general population: Prevalence and severity of depressive symptoms across major cancer types. Eur J Cancer. 2017;72:46-53. doi:10.1016/j.ejca.2016.11.017
- Walker J, Holm Hansen C, Martin P, et al. Prevalence of depression in adults with cancer: a systematic review. Ann Oncol. 2013;24(4):895-900. doi:10.1093/annonc/mds575
- Pitman A, Suleman S, Hyde N, Hodgkiss A. Depression and anxiety in patients with cancer. BMJ. 2018;361:k1415. doi:10.1136/bmj.k1415
- Kisely S, Alotiby MKN, Protani MM, Soole R, Arnautovska U, Siskind D. Breast cancer treatment disparities in patients with severe mental illness: a systematic review and meta-analysis. Psychooncology. 2023;32(5):651-662. doi:10.1002/pon.6120
- Massie MJ. Prevalence of depression in patients with cancer. J Natl Cancer Inst Monogr. 2004;(32):57-71. doi:10.1093/jncimonographs/lgh014
- Rodin G, Katz M, Lloyd N, Green E, Mackay JA, Wong RK. Treatment of depression in cancer patients. Curr Oncol. 2007;14(5):180-188. doi:10.3747/co.2007.146
- Wilson KG, Chochinov HM, Skirko MG, et al. Depression and anxiety disorders in palliative cancer care. J Pain Symptom Manage. 2007;33(2):118-129. doi:10.1016/j.jpainsymman.2006.07.016
- Moradi Y, Dowran B, Sepandi M. The global prevalence of depression, suicide ideation, and attempts in the military forces: a systematic review and meta-analysis of cross sectional studies. BMC Psychiatry. 2021;21(1):510. doi:10.1186/s12888-021-03526-2
- Lu CY, Zhang F, Lakoma MD, et al. Changes in antidepressant use by young people and suicidal behavior after FDA warnings and media coverage: quasi-experimental study. BMJ. 2014;348:g3596. doi:10.1136/bmj.g3596
- Friedman RA. Antidepressants’ black-box warning--10 years later. N Engl J Med. 2014;371(18):1666-1668. doi:10.1056/NEJMp1408480
- Sheffler ZM, Patel P, Abdijadid S. Antidepressants. In: StatPearls. StatPearls Publishing. Updated May 26, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK538182/
- Miller JJ. Antidepressants, Part 1: 100 Years and Counting. Accessed April 4, 2025. Psychiatric Times. https:// www.psychiatrictimes.com/view/antidepressants-part-1-100-years-and-counting
- Hillhouse TM, Porter JH. A brief history of the development of antidepressant drugs: from monoamines to glutamate. Exp Clin Psychopharmacol. 2015;23(1):1-21. doi:10.1037/a0038550
- Mitchell PB, Mitchell MS. The management of depression. Part 2. The place of the new antidepressants. Aust Fam Physician. 1994;23(9):1771-1781.
- Sabri MA, Saber-Ayad MM. MAO Inhibitors. In: Stat- Pearls. StatPearls Publishing. Updated June 5, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK557395/
- Sub Laban T, Saadabadi A. Monoamine Oxidase Inhibitors (MAOI). In: StatPearls. StatPearls Publishing. Updated July 17, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK539848/
- Fiedorowicz JG, Swartz KL. The role of monoamine oxidase inhibitors in current psychiatric practice. J Psychiatr Pract. 2004;10(4):239-248. doi:10.1097/00131746-200407000-00005
- Flockhart DA. Dietary restrictions and drug interactions with monoamine oxidase inhibitors: an update. J Clin Psychiatry. 2012;73 Suppl 1:17-24. doi:10.4088/JCP.11096su1c.03
- Moraczewski J, Awosika AO, Aedma KK. Tricyclic Antidepressants. In: StatPearls. StatPearls Publishing. Updated August 17, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK557791/
- Almasi A, Patel P, Meza CE. Doxepin. In: StatPearls. StatPearls Publishing. Updated February 14, 2024. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK542306/
- Thour A, Marwaha R. Amitriptyline. In: StatPearls. Stat- Pearls Publishing. Updated July 18, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK537225/
- Radley DC, Finkelstein SN, Stafford RS. Off-label prescribing among office-based physicians. Arch Intern Med. 2006;166(9):1021-1026. doi:10.1001/archinte.166.9.1021
- Tesfaye S, Sloan G, Petrie J, et al. Comparison of amitriptyline supplemented with pregabalin, pregabalin supplemented with amitriptyline, and duloxetine supplemented with pregabalin for the treatment of diabetic peripheral neuropathic pain (OPTION-DM): a multicentre, double-blind, randomised crossover trial. Lancet. 2022;400(10353):680- 690. doi:10.1016/S0140-6736(22)01472-6
- Farag HM, Yunusa I, Goswami H, Sultan I, Doucette JA, Eguale T. Comparison of amitriptyline and US Food and Drug Administration-approved treatments for fibromyalgia: a systematic review and network metaanalysis. JAMA Netw Open. 2022;5(5):e2212939. doi:10.1001/jamanetworkopen.2022.12939
- Merwar G, Gibbons JR, Hosseini SA, Saadabadi A. Nortriptyline. In: StatPearls. StatPearls Publishing. Updated June 5, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK482214/
- Fayez R, Gupta V. Imipramine. In: StatPearls. StatPearls Publishing. Updated May 22, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK557656/
- Jilani TN, Gibbons JR, Faizy RM, Saadabadi A. Mirtazapine. In: StatPearls. StatPearls Publishing. Updated August 28, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK519059/
- Nutt DJ. Tolerability and safety aspects of mirtazapine. Hum Psychopharmacol. 2002;17 Suppl 1:S37-S41. doi:10.1002/hup.388
- Gandotra K, Chen P, Jaskiw GE, Konicki PE, Strohl KP. Effective treatment of insomnia with mirtazapine attenuates concomitant suicidal ideation. J Clin Sleep Med. 2018;14(5):901-902. doi:10.5664/jcsm.7142
- Anttila SA, Leinonen EV. A review of the pharmacological and clinical profile of mirtazapine. CNS Drug Rev. 2001;7(3):249-264. doi:10.1111/j.1527-3458.2001.tb00198.x
- Wang SM, Han C, Bahk WM, et al. Addressing the side effects of contemporary antidepressant drugs: a comprehensive review. Chonnam Med J. 2018;54(2):101-112. doi:10.4068/cmj.2018.54.2.101
- Huecker MR, Smiley A, Saadabadi A. Bupropion. In: StatPearls. StatPearls Publishing. Updated April 9, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK470212/
- Hsieh MT, Tseng PT, Wu YC, et al. Effects of different pharmacologic smoking cessation treatments on body weight changes and success rates in patients with nicotine dependence: a network meta-analysis. Obes Rev. 2019;20(6):895- 905. doi:10.1111/obr.12835
- Hankosky ER, Bush HM, Dwoskin LP, et al. Retrospective analysis of health claims to evaluate pharmacotherapies with potential for repurposing: association of bupropion and stimulant use disorder remission. AMIA Annu Symp Proc. 2018;2018:1292-1299.
- Livingstone-Banks J, Norris E, Hartmann-Boyce J, West R, Jarvis M, Hajek P. Relapse prevention interventions for smoking cessation. Cochrane Database Syst Rev. 2019; 2(2):CD003999. doi:10.1002/14651858.CD003999.pub5
- Clayton AH, Kingsberg SA, Goldstein I. Evaluation and management of hypoactive sexual desire disorder. Sex Med. 2018;6(2):59-74. doi:10.1016/j.esxm.2018.01.004
- Verbeeck W, Bekkering GE, Van den Noortgate W, Kramers C. Bupropion for attention deficit hyperactivity disorder (ADHD) in adults. Cochrane Database Syst Rev. 2017;10(10):CD009504. doi:10.1002/14651858.CD009504.pub2
- Ng QX. A systematic review of the use of bupropion for attention-deficit/hyperactivity disorder in children and adolescents. J Child Adolesc Psychopharmacol. 2017;27(2):112-116. doi:10.1089/cap.2016.0124
- Fava M, Rush AJ, Thase ME, et al. 15 years of clinical experience with bupropion HCl: from bupropion to bupropion SR to bupropion XL. Prim Care Companion J Clin Psychiatry. 2005;7(3):106-113. doi:10.4088/pcc.v07n0305
- Chu A, Wadhwa R. Selective Serotonin Reuptake Inhibitors. In: StatPearls. StatPearls Publishing. Updated May 1, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK554406/
- Singh HK, Saadabadi A. Sertraline. In: StatPearls. Stat- Pearls Publishing. Updated February 13, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK547689/
- MacQueen G, Born L, Steiner M. The selective serotonin reuptake inhibitor sertraline: its profile and use in psychiatric disorders. CNS Drug Rev. 2001;7(1):1-24. doi:10.1111/j.1527-3458.2001.tb00188.x
- Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet. 2009;373(9665):746-758. doi:10.1016/S0140-6736(09)60046-5
- Nelson JC. The STAR*D study: a four-course meal that leaves us wanting more. Am J Psychiatry. 2006;163(11):1864-1866. doi:10.1176/ajp.2006.163.11.1864
- Sharbaf Shoar N, Fariba KA, Padhy RK. Citalopram. In: StatPearls. StatPearls Publishing. Updated November 7, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK482222/
- Landy K, Rosani A, Estevez R. Escitalopram. In: Stat- Pearls. StatPearls Publishing. Updated November 10, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK557734/
- Cavanah LR, Ray P, Goldhirsh JL, Huey LY, Piper BJ. Rise of escitalopram and the fall of citalopram. medRxiv. Preprint published online May 8, 2023. doi:10.1101/2023.05.07.23289632
- Sohel AJ, Shutter MC, Patel P, Molla M. Fluoxetine. In: StatPearls. StatPearls Publishing. Updated July 4, 2022. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK459223/
- Wong DT, Perry KW, Bymaster FP. Case history: the discovery of fluoxetine hydrochloride (Prozac). Nat Rev Drug Discov. 2005;4(9):764-774. doi:10.1038/nrd1821
- Shrestha P, Fariba KA, Abdijadid S. Paroxetine. In: Stat- Pearls. StatPearls Publishing. Updated July 17, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK526022/
- Naseeruddin R, Rosani A, Marwaha R. Desvenlafaxine. In: StatPearls. StatPearls Publishing. Updated July 10, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK534829
- Lieberman DZ, Massey SH. Desvenlafaxine in major depressive disorder: an evidence-based review of its place in therapy. Core Evid. 2010;4:67-82. doi:10.2147/ce.s5998
- Withdrawal assessment report for Ellefore (desvenlafaxine). European Medicine Agency. 2009. Accessed April 4, 2025. https://www.ema.europa.eu/en/documents/withdrawal-report/withdrawal-assessment-report-ellefore_en.pdf
- Dhaliwal JS, Spurling BC, Molla M. Duloxetine. In: Stat- Pearls. Statpearls Publishing. Updated May 29, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK549806/
- Hershman DL, Lacchetti C, Dworkin RH, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32(18):1941-1967. doi:10.1200/JCO.2013.54.0914
- Sommer C, Häuser W, Alten R, et al. Medikamentöse Therapie des Fibromyalgiesyndroms. Systematische Übersicht und Metaanalyse [Drug therapy of fibromyalgia syndrome. Systematic review, meta-analysis and guideline]. Schmerz. 2012;26(3):297-310. doi:10.1007/s00482-012-1172-2
- Bril V, England J, Franklin GM, et al. Evidence-based guideline: treatment of painful diabetic neuropathy: report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2011;76(20):1758-1765. doi:10.1212/WNL.0b013e3182166ebe
- Attal N, Cruccu G, Baron R, et al. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol. 2010;17(9):1113-e88. doi:10.1111/j.1468-1331.2010.02999.x
- Singh D, Saadabadi A. Venlafaxine. In: StatPearls. StatePearls Publishing. Updated February 26, 2024. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK535363/
- Sertraline and venlafaxine: new indication. Prevention of recurrent depression: no advance. Prescrire Int. 2005;14(75):19-20.
- Bruno A, Morabito P, S p i n a E , M u s c a t ello MRl. The role of levomilnacipran in the management of major depressive disorder: a comprehensive review. Curr Neuro pharmacol. 2016;14(2):191-199. doi:10.2174/1570159x14666151117122458
- Shin JJ, Saadabadi A. Trazodone. In: StatPearls. StatPearls Publishing. Updated February 29, 2024. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK470560/
- Khouzam HR. A review of trazodone use in psychiatric and medical conditions. Postgrad Med. 2017;129(1):140-148. doi:10.1080/00325481.2017.1249265
- Smales ET, Edwards BA, Deyoung PN, et al. Trazodone effects on obstructive sleep apnea and non-REM arousal threshold. Ann Am Thorac Soc. 2015;12(5):758-764. doi:10.1513/AnnalsATS.201408-399OC
- Eckert DJ, Malhotra A, Wellman A, White DP. Trazodone increases the respiratory arousal threshold in patients with obstructive sleep apnea and a low arousal threshold. Sleep. 2014;37(4):811-819. doi:10.5665/sleep.3596
- Schatzberg AF, Nemeroff CB, eds. The American Psychiat ric Association Publishing Textbook of Psychopharmacology. 4th ed. American Psychiatric Publishing; 2009.
- Ruxton K, Woodman RJ, Mangoni AA. Drugs with anticholinergic effects and cognitive impairment, falls and allcause mortality in older adults: a systematic review and meta-analysis. Br J Clin Pharmacol. 2015;80(2):209-220. doi:10.1111/bcp.12617
- Nefazodone. In: LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. National Institute of Diabetes and Digestive and Kidney Diseases. Updated March 6, 2020. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK548179/
- Drugs of Current Interest: Nefazodone. WHO Pharmaceuticals Newsletter:(1). 2003(1):7. https://web.archive.org/web/20150403165029/http:/apps.who.int/medicinedocs/en/d/Js4944e/3.html
- Choi S. Nefazodone (serzone) withdrawn because of hepatotoxicity. CMAJ. 2003;169(11):1187.
- Teva Nefazodone Statement. News release. Teva USA. December 20, 2021. Accessed April 4, 2025. https:// www.tevausa.com/news-and-media/press-releases/teva-nefazodone-statement/
- Levitan MN, Papelbaum M, Nardi AE. Profile of agomelatine and its potential in the treatment of generalized anxiety disorder. Neuropsychiatr Dis Treat. 2015;11:1149- 1155. doi:10.2147/NDT.S67470
- Fu DJ, Ionescu DF, Li X, et al. Esketamine nasal spray for rapid reduction of major depressive disorder symptoms in patients who have active suicidal ideation with intent: double-blind, randomized study (ASPIRE I). J Clin Psychiatry. 2020;81(3):19m13191. doi:10.4088/JCP.19m13191
- Iosifescu DV, Jones A, O’Gorman C, et al. Efficacy and safety of AXS-05 (dextromethorphan-bupropion) in patients with major depressive disorder: a phase 3 randomized clinical trial (GEMINI). J Clin Psychiatry. 2022;83(4): 21m14345. doi:10.4088/JCP.21m14345
- Luong TT, Powers CN, Reinhardt BJ, et al. Retrospective evaluation of drug-drug interactions with erlotinib and gefitinib use in the Military Health System. Fed Pract. 2023;40(Suppl 3):S24-S34. doi:10.12788/fp.0401
- Luong TT, Shou KJ, Reinhard BJ, Kigelman OF, Greenfield KM. Paclitaxel drug-drug interactions in the Military Health System. Fed Pract. 2024;41(Suppl 3):S70-S82. doi:10.12788/fp.0499
- Luong TT, Powers CN, Reinhardt BJ, Weina PJ. Preclinical drug-drug interactions (DDIs) of gefitinib with/without losartan and selective serotonin reuptake inhibitors (SSRIs): citalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, and venlafaxine. Curr Res Pharmacol Drug Discov. 2022;3:100112. doi:10.1016/j.crphar.2022.100112
- Luong TT, McAnulty MJ, Evers DL, Reinhardt BJ, Weina PJ. Pre-clinical drug-drug interaction (DDI) of gefitinib or erlotinib with cytochrome P450 (CYP) inhibiting drugs, fluoxetine and/or losartan. Curr Res Toxicol. 2021;2:217- 224. doi:10.1016/j.crtox.2021.05.006,
- Adamo M, Dickie L, Ruhl J. SEER program coding and staging manual 2016. National Cancer Institute; 2016. Accessed April 4, 2025. https://seer.cancer.gov/archive/manuals/2016/SPCSM_2016_maindoc.pdf
- World Health Organization. International classification of diseases for oncology (ICD-O). 3rd ed, 1st revision. World Health Organization; 2013. Accessed April 4, 2025. https://apps.who.int/iris/handle/10665/96612
- Simon S. FDA approves Jelmyto (mitomycin gel) for urothelial cancer. American Cancer Society. April 20, 2020. Accessed April 4, 2025. https://www.cancer.org/cancer/latest-news/fda-approves-jelmyto-mitomycin-gel-for-urothelial-cancer.html
- Pantziarka P, Sukhatme V, Bouche G, Meheus L, Sukhatme VP. Repurposing drugs in oncology (ReDO)- diclofenac as an anti-cancer agent. Ecancermedicalscience. 2016;10:610. doi:10.3332/ecancer.2016.610
- Choi S, Kim S, Park J, Lee SE, Kim C, Kang D. Diclofenac: a nonsteroidal anti-inflammatory drug inducing cancer cell death by inhibiting microtubule polymerization and autophagy flux. Antioxidants (Basel). 2022;11(5):1009. doi:10.3390/antiox11051009
- Tontonoz M. The ABCs of BCG: oldest approved immunotherapy gets new explanation. Memorial Sloan Kettering Cancer Center. July 17, 2020. Accessed April 4, 2025. https://www.mskcc.org/news/oldest-approved-immunotherapy-gets-new-explanation
- Jordan VC. Tamoxifen as the first targeted long-term adjuvant therapy for breast cancer. Endocr Relat Cancer. 2014;21(3):R235-R246. doi:10.1530/ERC-14-0092
- Cole MP, Jones CT, Todd ID. A new anti-oestrogenic agent in late breast cancer. An early clinical appraisal of ICI46474. Br J Cancer. 1971;25(2):270-275. doi:10.1038/bjc.1971.33
- Jordan VC. Tamoxifen: a most unlikely pioneering medicine. Nat Rev Drug Discov. 2003;2(3):205-213. doi:10.1038/nrd1031
- Maximov PY, McDaniel RE, Jordan VC. Tamoxifen: Pioneering Medicine in Breast Cancer. Springer Basel; 2013. Accessed April 4, 2025. https://link.springer.com/book/10.1007/978-3-0348-0664-0
- Taxol (paclitaxel). Prescribing information. Bristol-Myers Squibb Company; 2011. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020262s049lbl.pdf
- Abraxane (paclitaxel). Prescribing information. Celgene Corporation; 2020. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/021660s047lbl.pdf
- Tarceva (erlotinib). Prescribing information. OSI Pharmaceuticals, LLC; 2016. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/021743s025lbl.pdf
- Docetaxel. Prescribing information. Sichuan Hyiyu Pharmaceutical Co.; 2022. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215813s000lbl.pdf
- Alimta (pemetrexed). Prescribing information. Teva Pharmaceuticals; 2022. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/208419s004lbl.pdf
- Tagrisso (osimertinib). Prescribing information. Astra- Zeneca Pharmaceuticals; 2020. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208065s021lbl.pdf
- Iressa (gefitinib). Prescribing information. AstraZeneca Pharmaceuticals; 2018. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/206995s003lbl.pdf
- Kadcyla (ado-trastuzumab emtansine). Prescribing information. Genentech, Inc.; 2013. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/125427lbl.pdf
- Alecensa (alectinib). Prescribing information. Genetech, Inc.; 2017. Accessed April 4, 2025. https:// www.accessdata.fda.gov/drugsatfda_docs/label/2017/208434s003lbl.pdf
- Xalkori (crizotinib). Prescribing information. Pfizer Laboratories; 2022. Accessed April 4, 2025. https:// www.accessdata.fda.gov/drugsatfda_docs/label/2022/202570s033lbl.pdf
- Lorbrena (lorlatinib). Prescribing information. Pfizer Laboratories; 2018. Accessed April 14, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210868s000lbl.pdf
- Alunbrig (brigatinib). Prescribing information. Takeda Pharmaceutical Company; 2020. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208772s008lbl.pdf
- Rozlytrek (entrectinib). Prescribing information. Genentech, Inc.; 2019. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212725s000lbl.pdf
- Herceptin (trastuzumab). Prescribing information. Genentech, Inc.; 2010. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/103792s5250lbl.pdf
- Cybalta (duloxetine). Prescribing information. Eli Lilly and Company; 2017. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/021427s049lbl.pdf
- Effexor XR (venlafaxine). Prescribing information. Pfizer Wyeth Pharmaceuticals Inc; 2022. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/020699s112lbl.pdf
- Desyrel (trazodone hydrochloride). Prescribing information. Pragma Pharmaceuticals; 2017. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018207s032lbl.pdf
- Sertraline hydrochloride. Prescribing information. Almatica Pharma LLC; 2021. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/215133s000lbl.pdf
- Remeron (mirtazapine). Prescribing information. Merck & Co. Inc; 2020. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020415s029,%20021208s019lbl.pdf
- Celexa (citalopram). Prescribing information. Allergan USA Inc; 2022. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/020822s041lbl.pdf
- information. GlaxoSmithKline; 2019. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/020358s066lbl.pdf
- Amitriptyline hydrochloride tablet. Prescribing information. Quality Care Products LLC; 2022. Accessed April 4, 2025. https://www.accessdata.fda.gov/spl/data/0f12f50f-7087-46e7-a2e6-356b4c566c9f/0f12f50f-7087-46e7-a2e6-356b4c566c9f.xml
- Lexapro (escitalopram). Prescribing information. AbbVie Inc; 2023. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/021323s055,021365s039lbl.pdf
- Fluoxetine. Prescribing information. Edgemont Pharmaceutical, LLC; 2017. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/202133s004s005lbl.pdf
- Paxil (paroxetine). Prescribing Information. Apotex Inc; 2021. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/020031s077lbl.pdf
- Pamelor (nortriptyline HCl). Prescribing information. Mallinckrodt, Inc; 2012. Accessed April 4, 2025. https:// www.accessdata.fda.gov/drugsatfda_docs/label/2012/018012s029,018013s061lbl.pdf
- Silenor (doxepin). Prescribing information. Currax Pharmaceuticals; 2020. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/022036s006lbl.pdf
- Tofranil-PM (imipramine pamote). Prescribing information. Mallinckrodt, Inc; 2014. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/017090s078lbl.pdf
- Norpramin (desipramine hydrochloride). Prescribing information. Sanofi-aventis U.S. LLC; 2014. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/014399s069lbl.pdf
- Khedezla (desvenlafaxine). Prescribing information. Osmotical Pharmaceutical US LLC; 2019. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/204683s006lbl.pdf
- Nefazodone hydrochloride. Prescribing information. Bryant Ranch Prepack; 2022. Accessed April 4, 2025. https://www.accessdata.fda.gov/spl/data/0bd4c34a-4f43-4c84-8b98-1d074cba97d5/0bd4c34a-4f43-4c84-8b98-1d074cba97d5.xml
- Grassi L, Nanni MG, Rodin G, Li M, Caruso R. The use of antidepressants in oncology: a review and practical tips for oncologists. Ann Oncol. 2018;29(1):101-111. doi:10.1093/annonc/mdx526
- Lee E, Park Y, Li D, Rodriguez-Fuguet A, Wang X, Zhang WC. Antidepressant use and lung cancer risk and survival: a meta-analysis of observational studies. Cancer Res Commun. 2023;3(6):1013-1025. doi:10.1158/2767-9764.CRC-23-0003
- Olfson M, Marcus SC. National patterns in antidepressant medication treatment. Arch Gen Psychiatry. 2009;66(8):848 -856. doi:10.1001/archgenpsychiatry.2009.81
- Grattan A, Sullivan MD, Saunders KW, Campbell CI, Von Korff MR. Depression and prescription opioid misuse among chronic opioid therapy recipients with no history of substance abuse. Ann Fam Med. 2012;10(4):304-311. doi:10.1370/afm.1371
- Cowan DT, Wilson-Barnett J, Griffiths P, Allan LG. A survey of chronic noncancer pain patients prescribed opioid analgesics. Pain Med. 2003;4(4):340-351. doi:10.1111/j.1526-4637.2003.03038.x
- Breckenridge J, Clark JD. Patient characteristics associated with opioid versus nonsteroidal anti-inflammatory drug management of chronic low back pain. J Pain. 2003;4(6):344-350. doi:10.1016/s1526-5900(03)00638-2
- Edlund MJ, Martin BC, Devries A, Fan MY, Braden JB, Sullivan MD. Trends in use of opioids for chronic noncancer pain among individuals with mental health and substance use disorders: the TROUP study. Clin J Pain. 2010;26(1):1-8. doi:10.1097/AJP.0b013e3181b99f35
- Sullivan MD, Edlund MJ, Fan MY, DeVries A, Braden JB, Martin BC. Risks for possible and probable opioid misuse among recipients of chronic opioid therapy in commercial and medicaid insurance plans: the TROUP study. Pain. 2010;150(2):332-339. doi:10.1016/j.pain.2010.05.020
- Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152(2):85-92. doi:10.7326/0003-4819-152-2-201001190-00006
- Haddad P. Do antidepressants have any potential to cause addiction? J Psychopharmacol. 1999;13(3):300- 307. doi:10.1177/026988119901300321
- Lakeview Health Staff. America’s most abused antidepressants. Lakeview Health. January 24, 2004. Accessed April 4, 2025. https://www.lakeviewhealth.com/blog/us-most-abused-antidepressants/
- Greenhouse Treatment Center Editorial Staff. Addiction to antidepressants: is it possible? America Addiction Centers: Greenhouse Treatment Center. Updated April 23, 2024. Accessed April 4, 2025. https://greenhousetreatment.com/prescription-medication/antidepressants/
- National Cancer Institute. Depression (PDQ)-Health Professional Version. Updated July 25, 2024. Accessed April 4, 2025. https://www.cancer.gov/about-cancer/coping/feelings/depression-hp-pdq
- Krebber AM, Buffart LM, Kleijn G, et al. Prevalence of depression in cancer patients: a meta-analysis of diagnostic interviews and self-report instruments. Psychooncology. 2014;23(2):121-130. doi:10.1002/pon.3409
- Xiang X, An R, Gehlert S. Trends in antidepressant use among U.S. cancer survivors, 1999-2012. Psychiatr Serv. 2015;66(6):564. doi:10.1176/appi.ps.201500007
- Hartung TJ, Brähler E, Faller H, et al. The risk of being depressed is significantly higher in cancer patients than in the general population: Prevalence and severity of depressive symptoms across major cancer types. Eur J Cancer. 2017;72:46-53. doi:10.1016/j.ejca.2016.11.017
- Walker J, Holm Hansen C, Martin P, et al. Prevalence of depression in adults with cancer: a systematic review. Ann Oncol. 2013;24(4):895-900. doi:10.1093/annonc/mds575
- Pitman A, Suleman S, Hyde N, Hodgkiss A. Depression and anxiety in patients with cancer. BMJ. 2018;361:k1415. doi:10.1136/bmj.k1415
- Kisely S, Alotiby MKN, Protani MM, Soole R, Arnautovska U, Siskind D. Breast cancer treatment disparities in patients with severe mental illness: a systematic review and meta-analysis. Psychooncology. 2023;32(5):651-662. doi:10.1002/pon.6120
- Massie MJ. Prevalence of depression in patients with cancer. J Natl Cancer Inst Monogr. 2004;(32):57-71. doi:10.1093/jncimonographs/lgh014
- Rodin G, Katz M, Lloyd N, Green E, Mackay JA, Wong RK. Treatment of depression in cancer patients. Curr Oncol. 2007;14(5):180-188. doi:10.3747/co.2007.146
- Wilson KG, Chochinov HM, Skirko MG, et al. Depression and anxiety disorders in palliative cancer care. J Pain Symptom Manage. 2007;33(2):118-129. doi:10.1016/j.jpainsymman.2006.07.016
- Moradi Y, Dowran B, Sepandi M. The global prevalence of depression, suicide ideation, and attempts in the military forces: a systematic review and meta-analysis of cross sectional studies. BMC Psychiatry. 2021;21(1):510. doi:10.1186/s12888-021-03526-2
- Lu CY, Zhang F, Lakoma MD, et al. Changes in antidepressant use by young people and suicidal behavior after FDA warnings and media coverage: quasi-experimental study. BMJ. 2014;348:g3596. doi:10.1136/bmj.g3596
- Friedman RA. Antidepressants’ black-box warning--10 years later. N Engl J Med. 2014;371(18):1666-1668. doi:10.1056/NEJMp1408480
- Sheffler ZM, Patel P, Abdijadid S. Antidepressants. In: StatPearls. StatPearls Publishing. Updated May 26, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK538182/
- Miller JJ. Antidepressants, Part 1: 100 Years and Counting. Accessed April 4, 2025. Psychiatric Times. https:// www.psychiatrictimes.com/view/antidepressants-part-1-100-years-and-counting
- Hillhouse TM, Porter JH. A brief history of the development of antidepressant drugs: from monoamines to glutamate. Exp Clin Psychopharmacol. 2015;23(1):1-21. doi:10.1037/a0038550
- Mitchell PB, Mitchell MS. The management of depression. Part 2. The place of the new antidepressants. Aust Fam Physician. 1994;23(9):1771-1781.
- Sabri MA, Saber-Ayad MM. MAO Inhibitors. In: Stat- Pearls. StatPearls Publishing. Updated June 5, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK557395/
- Sub Laban T, Saadabadi A. Monoamine Oxidase Inhibitors (MAOI). In: StatPearls. StatPearls Publishing. Updated July 17, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK539848/
- Fiedorowicz JG, Swartz KL. The role of monoamine oxidase inhibitors in current psychiatric practice. J Psychiatr Pract. 2004;10(4):239-248. doi:10.1097/00131746-200407000-00005
- Flockhart DA. Dietary restrictions and drug interactions with monoamine oxidase inhibitors: an update. J Clin Psychiatry. 2012;73 Suppl 1:17-24. doi:10.4088/JCP.11096su1c.03
- Moraczewski J, Awosika AO, Aedma KK. Tricyclic Antidepressants. In: StatPearls. StatPearls Publishing. Updated August 17, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK557791/
- Almasi A, Patel P, Meza CE. Doxepin. In: StatPearls. StatPearls Publishing. Updated February 14, 2024. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK542306/
- Thour A, Marwaha R. Amitriptyline. In: StatPearls. Stat- Pearls Publishing. Updated July 18, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK537225/
- Radley DC, Finkelstein SN, Stafford RS. Off-label prescribing among office-based physicians. Arch Intern Med. 2006;166(9):1021-1026. doi:10.1001/archinte.166.9.1021
- Tesfaye S, Sloan G, Petrie J, et al. Comparison of amitriptyline supplemented with pregabalin, pregabalin supplemented with amitriptyline, and duloxetine supplemented with pregabalin for the treatment of diabetic peripheral neuropathic pain (OPTION-DM): a multicentre, double-blind, randomised crossover trial. Lancet. 2022;400(10353):680- 690. doi:10.1016/S0140-6736(22)01472-6
- Farag HM, Yunusa I, Goswami H, Sultan I, Doucette JA, Eguale T. Comparison of amitriptyline and US Food and Drug Administration-approved treatments for fibromyalgia: a systematic review and network metaanalysis. JAMA Netw Open. 2022;5(5):e2212939. doi:10.1001/jamanetworkopen.2022.12939
- Merwar G, Gibbons JR, Hosseini SA, Saadabadi A. Nortriptyline. In: StatPearls. StatPearls Publishing. Updated June 5, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK482214/
- Fayez R, Gupta V. Imipramine. In: StatPearls. StatPearls Publishing. Updated May 22, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK557656/
- Jilani TN, Gibbons JR, Faizy RM, Saadabadi A. Mirtazapine. In: StatPearls. StatPearls Publishing. Updated August 28, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK519059/
- Nutt DJ. Tolerability and safety aspects of mirtazapine. Hum Psychopharmacol. 2002;17 Suppl 1:S37-S41. doi:10.1002/hup.388
- Gandotra K, Chen P, Jaskiw GE, Konicki PE, Strohl KP. Effective treatment of insomnia with mirtazapine attenuates concomitant suicidal ideation. J Clin Sleep Med. 2018;14(5):901-902. doi:10.5664/jcsm.7142
- Anttila SA, Leinonen EV. A review of the pharmacological and clinical profile of mirtazapine. CNS Drug Rev. 2001;7(3):249-264. doi:10.1111/j.1527-3458.2001.tb00198.x
- Wang SM, Han C, Bahk WM, et al. Addressing the side effects of contemporary antidepressant drugs: a comprehensive review. Chonnam Med J. 2018;54(2):101-112. doi:10.4068/cmj.2018.54.2.101
- Huecker MR, Smiley A, Saadabadi A. Bupropion. In: StatPearls. StatPearls Publishing. Updated April 9, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK470212/
- Hsieh MT, Tseng PT, Wu YC, et al. Effects of different pharmacologic smoking cessation treatments on body weight changes and success rates in patients with nicotine dependence: a network meta-analysis. Obes Rev. 2019;20(6):895- 905. doi:10.1111/obr.12835
- Hankosky ER, Bush HM, Dwoskin LP, et al. Retrospective analysis of health claims to evaluate pharmacotherapies with potential for repurposing: association of bupropion and stimulant use disorder remission. AMIA Annu Symp Proc. 2018;2018:1292-1299.
- Livingstone-Banks J, Norris E, Hartmann-Boyce J, West R, Jarvis M, Hajek P. Relapse prevention interventions for smoking cessation. Cochrane Database Syst Rev. 2019; 2(2):CD003999. doi:10.1002/14651858.CD003999.pub5
- Clayton AH, Kingsberg SA, Goldstein I. Evaluation and management of hypoactive sexual desire disorder. Sex Med. 2018;6(2):59-74. doi:10.1016/j.esxm.2018.01.004
- Verbeeck W, Bekkering GE, Van den Noortgate W, Kramers C. Bupropion for attention deficit hyperactivity disorder (ADHD) in adults. Cochrane Database Syst Rev. 2017;10(10):CD009504. doi:10.1002/14651858.CD009504.pub2
- Ng QX. A systematic review of the use of bupropion for attention-deficit/hyperactivity disorder in children and adolescents. J Child Adolesc Psychopharmacol. 2017;27(2):112-116. doi:10.1089/cap.2016.0124
- Fava M, Rush AJ, Thase ME, et al. 15 years of clinical experience with bupropion HCl: from bupropion to bupropion SR to bupropion XL. Prim Care Companion J Clin Psychiatry. 2005;7(3):106-113. doi:10.4088/pcc.v07n0305
- Chu A, Wadhwa R. Selective Serotonin Reuptake Inhibitors. In: StatPearls. StatPearls Publishing. Updated May 1, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK554406/
- Singh HK, Saadabadi A. Sertraline. In: StatPearls. Stat- Pearls Publishing. Updated February 13, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK547689/
- MacQueen G, Born L, Steiner M. The selective serotonin reuptake inhibitor sertraline: its profile and use in psychiatric disorders. CNS Drug Rev. 2001;7(1):1-24. doi:10.1111/j.1527-3458.2001.tb00188.x
- Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet. 2009;373(9665):746-758. doi:10.1016/S0140-6736(09)60046-5
- Nelson JC. The STAR*D study: a four-course meal that leaves us wanting more. Am J Psychiatry. 2006;163(11):1864-1866. doi:10.1176/ajp.2006.163.11.1864
- Sharbaf Shoar N, Fariba KA, Padhy RK. Citalopram. In: StatPearls. StatPearls Publishing. Updated November 7, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK482222/
- Landy K, Rosani A, Estevez R. Escitalopram. In: Stat- Pearls. StatPearls Publishing. Updated November 10, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK557734/
- Cavanah LR, Ray P, Goldhirsh JL, Huey LY, Piper BJ. Rise of escitalopram and the fall of citalopram. medRxiv. Preprint published online May 8, 2023. doi:10.1101/2023.05.07.23289632
- Sohel AJ, Shutter MC, Patel P, Molla M. Fluoxetine. In: StatPearls. StatPearls Publishing. Updated July 4, 2022. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK459223/
- Wong DT, Perry KW, Bymaster FP. Case history: the discovery of fluoxetine hydrochloride (Prozac). Nat Rev Drug Discov. 2005;4(9):764-774. doi:10.1038/nrd1821
- Shrestha P, Fariba KA, Abdijadid S. Paroxetine. In: Stat- Pearls. StatPearls Publishing. Updated July 17, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK526022/
- Naseeruddin R, Rosani A, Marwaha R. Desvenlafaxine. In: StatPearls. StatPearls Publishing. Updated July 10, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK534829
- Lieberman DZ, Massey SH. Desvenlafaxine in major depressive disorder: an evidence-based review of its place in therapy. Core Evid. 2010;4:67-82. doi:10.2147/ce.s5998
- Withdrawal assessment report for Ellefore (desvenlafaxine). European Medicine Agency. 2009. Accessed April 4, 2025. https://www.ema.europa.eu/en/documents/withdrawal-report/withdrawal-assessment-report-ellefore_en.pdf
- Dhaliwal JS, Spurling BC, Molla M. Duloxetine. In: Stat- Pearls. Statpearls Publishing. Updated May 29, 2023. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK549806/
- Hershman DL, Lacchetti C, Dworkin RH, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32(18):1941-1967. doi:10.1200/JCO.2013.54.0914
- Sommer C, Häuser W, Alten R, et al. Medikamentöse Therapie des Fibromyalgiesyndroms. Systematische Übersicht und Metaanalyse [Drug therapy of fibromyalgia syndrome. Systematic review, meta-analysis and guideline]. Schmerz. 2012;26(3):297-310. doi:10.1007/s00482-012-1172-2
- Bril V, England J, Franklin GM, et al. Evidence-based guideline: treatment of painful diabetic neuropathy: report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2011;76(20):1758-1765. doi:10.1212/WNL.0b013e3182166ebe
- Attal N, Cruccu G, Baron R, et al. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol. 2010;17(9):1113-e88. doi:10.1111/j.1468-1331.2010.02999.x
- Singh D, Saadabadi A. Venlafaxine. In: StatPearls. StatePearls Publishing. Updated February 26, 2024. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK535363/
- Sertraline and venlafaxine: new indication. Prevention of recurrent depression: no advance. Prescrire Int. 2005;14(75):19-20.
- Bruno A, Morabito P, S p i n a E , M u s c a t ello MRl. The role of levomilnacipran in the management of major depressive disorder: a comprehensive review. Curr Neuro pharmacol. 2016;14(2):191-199. doi:10.2174/1570159x14666151117122458
- Shin JJ, Saadabadi A. Trazodone. In: StatPearls. StatPearls Publishing. Updated February 29, 2024. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK470560/
- Khouzam HR. A review of trazodone use in psychiatric and medical conditions. Postgrad Med. 2017;129(1):140-148. doi:10.1080/00325481.2017.1249265
- Smales ET, Edwards BA, Deyoung PN, et al. Trazodone effects on obstructive sleep apnea and non-REM arousal threshold. Ann Am Thorac Soc. 2015;12(5):758-764. doi:10.1513/AnnalsATS.201408-399OC
- Eckert DJ, Malhotra A, Wellman A, White DP. Trazodone increases the respiratory arousal threshold in patients with obstructive sleep apnea and a low arousal threshold. Sleep. 2014;37(4):811-819. doi:10.5665/sleep.3596
- Schatzberg AF, Nemeroff CB, eds. The American Psychiat ric Association Publishing Textbook of Psychopharmacology. 4th ed. American Psychiatric Publishing; 2009.
- Ruxton K, Woodman RJ, Mangoni AA. Drugs with anticholinergic effects and cognitive impairment, falls and allcause mortality in older adults: a systematic review and meta-analysis. Br J Clin Pharmacol. 2015;80(2):209-220. doi:10.1111/bcp.12617
- Nefazodone. In: LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. National Institute of Diabetes and Digestive and Kidney Diseases. Updated March 6, 2020. Accessed April 4, 2025. https://www.ncbi.nlm.nih.gov/books/NBK548179/
- Drugs of Current Interest: Nefazodone. WHO Pharmaceuticals Newsletter:(1). 2003(1):7. https://web.archive.org/web/20150403165029/http:/apps.who.int/medicinedocs/en/d/Js4944e/3.html
- Choi S. Nefazodone (serzone) withdrawn because of hepatotoxicity. CMAJ. 2003;169(11):1187.
- Teva Nefazodone Statement. News release. Teva USA. December 20, 2021. Accessed April 4, 2025. https:// www.tevausa.com/news-and-media/press-releases/teva-nefazodone-statement/
- Levitan MN, Papelbaum M, Nardi AE. Profile of agomelatine and its potential in the treatment of generalized anxiety disorder. Neuropsychiatr Dis Treat. 2015;11:1149- 1155. doi:10.2147/NDT.S67470
- Fu DJ, Ionescu DF, Li X, et al. Esketamine nasal spray for rapid reduction of major depressive disorder symptoms in patients who have active suicidal ideation with intent: double-blind, randomized study (ASPIRE I). J Clin Psychiatry. 2020;81(3):19m13191. doi:10.4088/JCP.19m13191
- Iosifescu DV, Jones A, O’Gorman C, et al. Efficacy and safety of AXS-05 (dextromethorphan-bupropion) in patients with major depressive disorder: a phase 3 randomized clinical trial (GEMINI). J Clin Psychiatry. 2022;83(4): 21m14345. doi:10.4088/JCP.21m14345
- Luong TT, Powers CN, Reinhardt BJ, et al. Retrospective evaluation of drug-drug interactions with erlotinib and gefitinib use in the Military Health System. Fed Pract. 2023;40(Suppl 3):S24-S34. doi:10.12788/fp.0401
- Luong TT, Shou KJ, Reinhard BJ, Kigelman OF, Greenfield KM. Paclitaxel drug-drug interactions in the Military Health System. Fed Pract. 2024;41(Suppl 3):S70-S82. doi:10.12788/fp.0499
- Luong TT, Powers CN, Reinhardt BJ, Weina PJ. Preclinical drug-drug interactions (DDIs) of gefitinib with/without losartan and selective serotonin reuptake inhibitors (SSRIs): citalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, and venlafaxine. Curr Res Pharmacol Drug Discov. 2022;3:100112. doi:10.1016/j.crphar.2022.100112
- Luong TT, McAnulty MJ, Evers DL, Reinhardt BJ, Weina PJ. Pre-clinical drug-drug interaction (DDI) of gefitinib or erlotinib with cytochrome P450 (CYP) inhibiting drugs, fluoxetine and/or losartan. Curr Res Toxicol. 2021;2:217- 224. doi:10.1016/j.crtox.2021.05.006,
- Adamo M, Dickie L, Ruhl J. SEER program coding and staging manual 2016. National Cancer Institute; 2016. Accessed April 4, 2025. https://seer.cancer.gov/archive/manuals/2016/SPCSM_2016_maindoc.pdf
- World Health Organization. International classification of diseases for oncology (ICD-O). 3rd ed, 1st revision. World Health Organization; 2013. Accessed April 4, 2025. https://apps.who.int/iris/handle/10665/96612
- Simon S. FDA approves Jelmyto (mitomycin gel) for urothelial cancer. American Cancer Society. April 20, 2020. Accessed April 4, 2025. https://www.cancer.org/cancer/latest-news/fda-approves-jelmyto-mitomycin-gel-for-urothelial-cancer.html
- Pantziarka P, Sukhatme V, Bouche G, Meheus L, Sukhatme VP. Repurposing drugs in oncology (ReDO)- diclofenac as an anti-cancer agent. Ecancermedicalscience. 2016;10:610. doi:10.3332/ecancer.2016.610
- Choi S, Kim S, Park J, Lee SE, Kim C, Kang D. Diclofenac: a nonsteroidal anti-inflammatory drug inducing cancer cell death by inhibiting microtubule polymerization and autophagy flux. Antioxidants (Basel). 2022;11(5):1009. doi:10.3390/antiox11051009
- Tontonoz M. The ABCs of BCG: oldest approved immunotherapy gets new explanation. Memorial Sloan Kettering Cancer Center. July 17, 2020. Accessed April 4, 2025. https://www.mskcc.org/news/oldest-approved-immunotherapy-gets-new-explanation
- Jordan VC. Tamoxifen as the first targeted long-term adjuvant therapy for breast cancer. Endocr Relat Cancer. 2014;21(3):R235-R246. doi:10.1530/ERC-14-0092
- Cole MP, Jones CT, Todd ID. A new anti-oestrogenic agent in late breast cancer. An early clinical appraisal of ICI46474. Br J Cancer. 1971;25(2):270-275. doi:10.1038/bjc.1971.33
- Jordan VC. Tamoxifen: a most unlikely pioneering medicine. Nat Rev Drug Discov. 2003;2(3):205-213. doi:10.1038/nrd1031
- Maximov PY, McDaniel RE, Jordan VC. Tamoxifen: Pioneering Medicine in Breast Cancer. Springer Basel; 2013. Accessed April 4, 2025. https://link.springer.com/book/10.1007/978-3-0348-0664-0
- Taxol (paclitaxel). Prescribing information. Bristol-Myers Squibb Company; 2011. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020262s049lbl.pdf
- Abraxane (paclitaxel). Prescribing information. Celgene Corporation; 2020. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/021660s047lbl.pdf
- Tarceva (erlotinib). Prescribing information. OSI Pharmaceuticals, LLC; 2016. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/021743s025lbl.pdf
- Docetaxel. Prescribing information. Sichuan Hyiyu Pharmaceutical Co.; 2022. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215813s000lbl.pdf
- Alimta (pemetrexed). Prescribing information. Teva Pharmaceuticals; 2022. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/208419s004lbl.pdf
- Tagrisso (osimertinib). Prescribing information. Astra- Zeneca Pharmaceuticals; 2020. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208065s021lbl.pdf
- Iressa (gefitinib). Prescribing information. AstraZeneca Pharmaceuticals; 2018. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/206995s003lbl.pdf
- Kadcyla (ado-trastuzumab emtansine). Prescribing information. Genentech, Inc.; 2013. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/125427lbl.pdf
- Alecensa (alectinib). Prescribing information. Genetech, Inc.; 2017. Accessed April 4, 2025. https:// www.accessdata.fda.gov/drugsatfda_docs/label/2017/208434s003lbl.pdf
- Xalkori (crizotinib). Prescribing information. Pfizer Laboratories; 2022. Accessed April 4, 2025. https:// www.accessdata.fda.gov/drugsatfda_docs/label/2022/202570s033lbl.pdf
- Lorbrena (lorlatinib). Prescribing information. Pfizer Laboratories; 2018. Accessed April 14, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210868s000lbl.pdf
- Alunbrig (brigatinib). Prescribing information. Takeda Pharmaceutical Company; 2020. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208772s008lbl.pdf
- Rozlytrek (entrectinib). Prescribing information. Genentech, Inc.; 2019. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212725s000lbl.pdf
- Herceptin (trastuzumab). Prescribing information. Genentech, Inc.; 2010. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/103792s5250lbl.pdf
- Cybalta (duloxetine). Prescribing information. Eli Lilly and Company; 2017. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/021427s049lbl.pdf
- Effexor XR (venlafaxine). Prescribing information. Pfizer Wyeth Pharmaceuticals Inc; 2022. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/020699s112lbl.pdf
- Desyrel (trazodone hydrochloride). Prescribing information. Pragma Pharmaceuticals; 2017. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018207s032lbl.pdf
- Sertraline hydrochloride. Prescribing information. Almatica Pharma LLC; 2021. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/215133s000lbl.pdf
- Remeron (mirtazapine). Prescribing information. Merck & Co. Inc; 2020. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020415s029,%20021208s019lbl.pdf
- Celexa (citalopram). Prescribing information. Allergan USA Inc; 2022. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/020822s041lbl.pdf
- information. GlaxoSmithKline; 2019. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/020358s066lbl.pdf
- Amitriptyline hydrochloride tablet. Prescribing information. Quality Care Products LLC; 2022. Accessed April 4, 2025. https://www.accessdata.fda.gov/spl/data/0f12f50f-7087-46e7-a2e6-356b4c566c9f/0f12f50f-7087-46e7-a2e6-356b4c566c9f.xml
- Lexapro (escitalopram). Prescribing information. AbbVie Inc; 2023. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/021323s055,021365s039lbl.pdf
- Fluoxetine. Prescribing information. Edgemont Pharmaceutical, LLC; 2017. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/202133s004s005lbl.pdf
- Paxil (paroxetine). Prescribing Information. Apotex Inc; 2021. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/020031s077lbl.pdf
- Pamelor (nortriptyline HCl). Prescribing information. Mallinckrodt, Inc; 2012. Accessed April 4, 2025. https:// www.accessdata.fda.gov/drugsatfda_docs/label/2012/018012s029,018013s061lbl.pdf
- Silenor (doxepin). Prescribing information. Currax Pharmaceuticals; 2020. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/022036s006lbl.pdf
- Tofranil-PM (imipramine pamote). Prescribing information. Mallinckrodt, Inc; 2014. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/017090s078lbl.pdf
- Norpramin (desipramine hydrochloride). Prescribing information. Sanofi-aventis U.S. LLC; 2014. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/014399s069lbl.pdf
- Khedezla (desvenlafaxine). Prescribing information. Osmotical Pharmaceutical US LLC; 2019. Accessed April 4, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/204683s006lbl.pdf
- Nefazodone hydrochloride. Prescribing information. Bryant Ranch Prepack; 2022. Accessed April 4, 2025. https://www.accessdata.fda.gov/spl/data/0bd4c34a-4f43-4c84-8b98-1d074cba97d5/0bd4c34a-4f43-4c84-8b98-1d074cba97d5.xml
- Grassi L, Nanni MG, Rodin G, Li M, Caruso R. The use of antidepressants in oncology: a review and practical tips for oncologists. Ann Oncol. 2018;29(1):101-111. doi:10.1093/annonc/mdx526
- Lee E, Park Y, Li D, Rodriguez-Fuguet A, Wang X, Zhang WC. Antidepressant use and lung cancer risk and survival: a meta-analysis of observational studies. Cancer Res Commun. 2023;3(6):1013-1025. doi:10.1158/2767-9764.CRC-23-0003
- Olfson M, Marcus SC. National patterns in antidepressant medication treatment. Arch Gen Psychiatry. 2009;66(8):848 -856. doi:10.1001/archgenpsychiatry.2009.81
- Grattan A, Sullivan MD, Saunders KW, Campbell CI, Von Korff MR. Depression and prescription opioid misuse among chronic opioid therapy recipients with no history of substance abuse. Ann Fam Med. 2012;10(4):304-311. doi:10.1370/afm.1371
- Cowan DT, Wilson-Barnett J, Griffiths P, Allan LG. A survey of chronic noncancer pain patients prescribed opioid analgesics. Pain Med. 2003;4(4):340-351. doi:10.1111/j.1526-4637.2003.03038.x
- Breckenridge J, Clark JD. Patient characteristics associated with opioid versus nonsteroidal anti-inflammatory drug management of chronic low back pain. J Pain. 2003;4(6):344-350. doi:10.1016/s1526-5900(03)00638-2
- Edlund MJ, Martin BC, Devries A, Fan MY, Braden JB, Sullivan MD. Trends in use of opioids for chronic noncancer pain among individuals with mental health and substance use disorders: the TROUP study. Clin J Pain. 2010;26(1):1-8. doi:10.1097/AJP.0b013e3181b99f35
- Sullivan MD, Edlund MJ, Fan MY, DeVries A, Braden JB, Martin BC. Risks for possible and probable opioid misuse among recipients of chronic opioid therapy in commercial and medicaid insurance plans: the TROUP study. Pain. 2010;150(2):332-339. doi:10.1016/j.pain.2010.05.020
- Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152(2):85-92. doi:10.7326/0003-4819-152-2-201001190-00006
- Haddad P. Do antidepressants have any potential to cause addiction? J Psychopharmacol. 1999;13(3):300- 307. doi:10.1177/026988119901300321
- Lakeview Health Staff. America’s most abused antidepressants. Lakeview Health. January 24, 2004. Accessed April 4, 2025. https://www.lakeviewhealth.com/blog/us-most-abused-antidepressants/
- Greenhouse Treatment Center Editorial Staff. Addiction to antidepressants: is it possible? America Addiction Centers: Greenhouse Treatment Center. Updated April 23, 2024. Accessed April 4, 2025. https://greenhousetreatment.com/prescription-medication/antidepressants/
Assessing the Impact of Antidepressants on Cancer Treatment: A Retrospective Analysis of 14 Antineoplastic Agents
Assessing the Impact of Antidepressants on Cancer Treatment: A Retrospective Analysis of 14 Antineoplastic Agents
Associations Between Prescreening Dietary Patterns and Longitudinal Colonoscopy Outcomes in Veterans
Associations Between Prescreening Dietary Patterns and Longitudinal Colonoscopy Outcomes in Veterans
Screening for colorectal cancer (CRC) with colonoscopy enables the identification and removal of CRC precursors (colonic adenomas) and has been associated with reduced risk of CRC incidence and mortality.1-3 Furthermore, there is consensus that diet and lifestyle may be associated with forestalling CRC pathogenesis at the intermediate adenoma stages.4-7 However, studies have shown that US veterans have poorer diet quality and a higher risk for neoplasia compared with nonveterans, reinforcing the need for tailored clinical approaches.8,9 Combining screening with conversations about modifiable environmental and lifestyle risk factors, such as poor diet, is a highly relevant and possibly easily leveraged prevention for those at high risk. However, there is limited evidence for any particular dietary patterns or dietary features that are most important over time.7
Several dietary components have been shown to be associated with CRC risk,10 either as potentially chemopreventive (fiber, fruits and vegetables,11 dairy,12 supplemental vitamin D,13 calcium,14 and multivitamins15) or carcinogenic (red meat16 and alcohol17). Previous studies of veterans have similarly shown that higher intake of fiber and vitamin D reduced risk, and red meat is associated with an increased risk for finding CRC precursors during colonoscopy.18 However, these dietary categories are often analyzed in isolation. Studying healthy dietary patterns in aggregate may be more clinically relevant and easier to implement for prevention of CRC and its precursors.19-21 Healthy dietary patterns, such as the US Dietary Guidelines for Americans represented by the Healthy Eating Index (HEI), the Mediterranean diet (MD), and the Dietary Approaches to Stop Hypertension (DASH) diet, have been associated with lower risk for chronic disease.22-24 Despite the extant literature, no known studies have compared these dietary patterns for associations with risk of CRC precursor or CRC development among US veterans undergoing long-term screening and follow-up after a baseline colonoscopy.
The objective of this study was to test for associations between baseline scores of healthy dietary patterns and the most severe colonoscopy findings (MSCFs) over ≥ 10 years following a baseline screening colonoscopy in veterans.
Methods
Participants in the Cooperative Studies Program (CSP) #380 cohort study included 3121 asymptomatic veterans aged 50 to 75 years at baseline who had consented to initial screening colonoscopy between 1994 and 1997, with subsequent follow-up and surveillance.25 Prior to their colonoscopy, all participants completed a baseline study survey that included questions about cancer risk factors including family history of CRC, diet, physical activity, and medication use.
Included in this cross-sectional analysis were data from a sample of veteran participants of the CSP #380 cohort with 1 baseline colonoscopy, follow-up surveillance through 2009, a cancer risk factor survey collected at baseline, and complete demographic and clinical indicator data. Excluded from the analysis were 67 participants with insufficient responses to the dietary food frequency questionnaire (FFQ) and 31 participants with missing body mass index (BMI), 3023 veterans.
Measures
MSCF. The outcome of interest in this study was the MSCF recorded across all participant colonoscopies during the study period. MSCF was categorized as either (1) no neoplasia; (2) < 2 nonadvanced adenomas, including small adenomas (diameter < 10 mm) with tubular histology; or (3) advanced neoplasia (AN), which is characterized by adenomas > 10 mm in diameter, with villous histology, with high-grade dysplasia, or CRC.
Dietary patterns. Dietary pattern scores representing dietary quality and calculated based on recommendations of the US Dietary Guidelines for Americans using the HEI, MD, and DASH diets were independent variables.26-28 These 3 dietary patterns were chosen for their hypothesized relationship with CRC risk, but each weighs food categories differently (Appendix 1).22-24,29 Dietary pattern scores were calculated using the CSP #380 self-reported responses to 129 baseline survey questions adapted from a well-established and previously validated semiquantitative FFQ.30 The form was administered by mail twice to a sample of 127 participants at baseline and at 1 year. During this interval, men completed 1-week diet records twice, spaced about 6 months apart. Mean values for intake of most nutrients assessed by the 2 methods were similar. Intraclass correlation coefficients for the baseline and 1-year FFQ-assessed nutrient intakes that ranged from 0.47 for vitamin E (without supplements) to 0.80 for vitamin C (with supplements). Correlation coefficients between the energy-adjusted nutrient intakes were measured by diet records and the 1-year FFQ, which asked about diet during the year encompassing the diet records. Higher raw and percent scores indicated better alignment with recommendations from each respective dietary pattern. Percent scores were calculated as a standardizing method and used in analyses for ease of comparing the dietary patterns. Scoring can be found in Appendix 2.
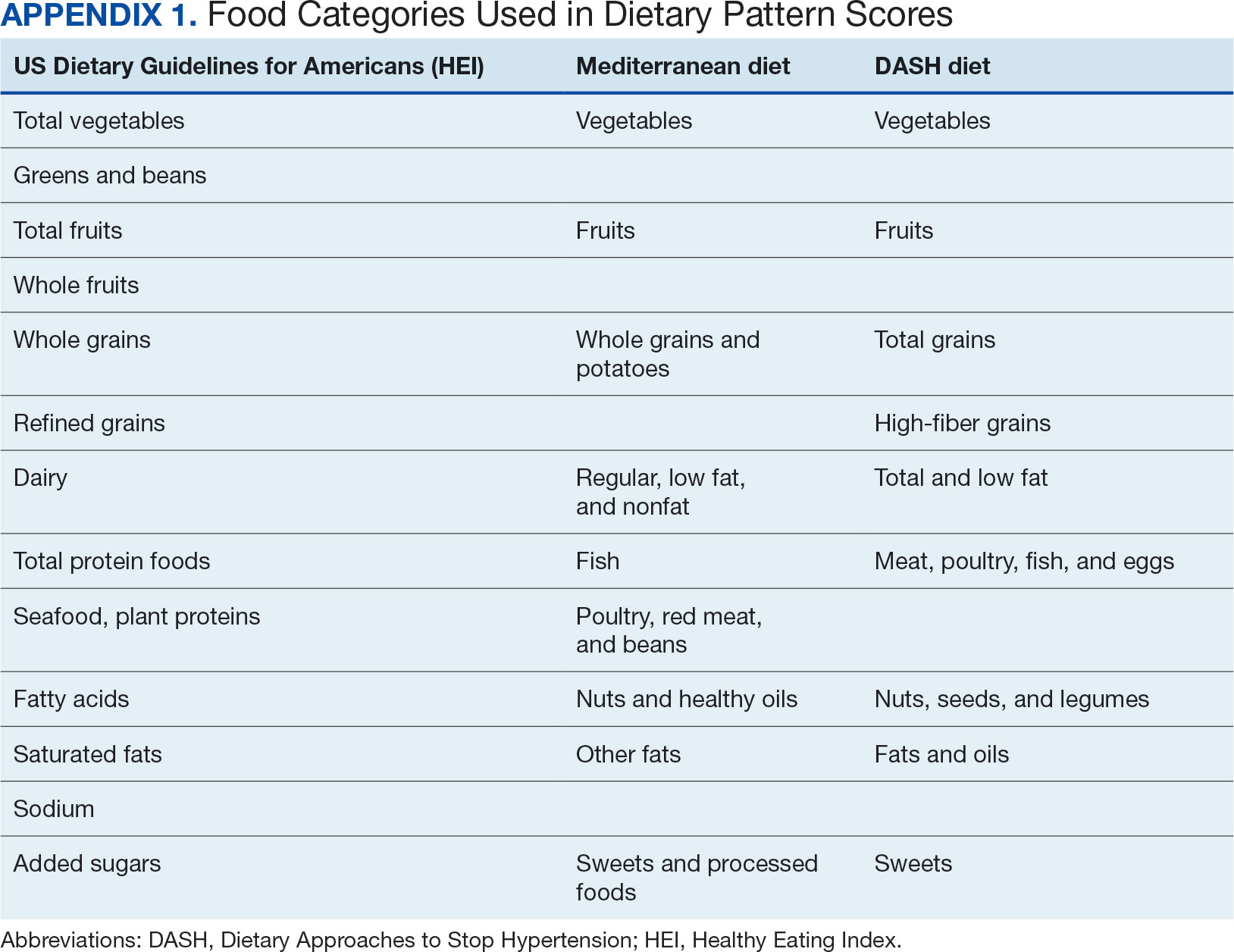
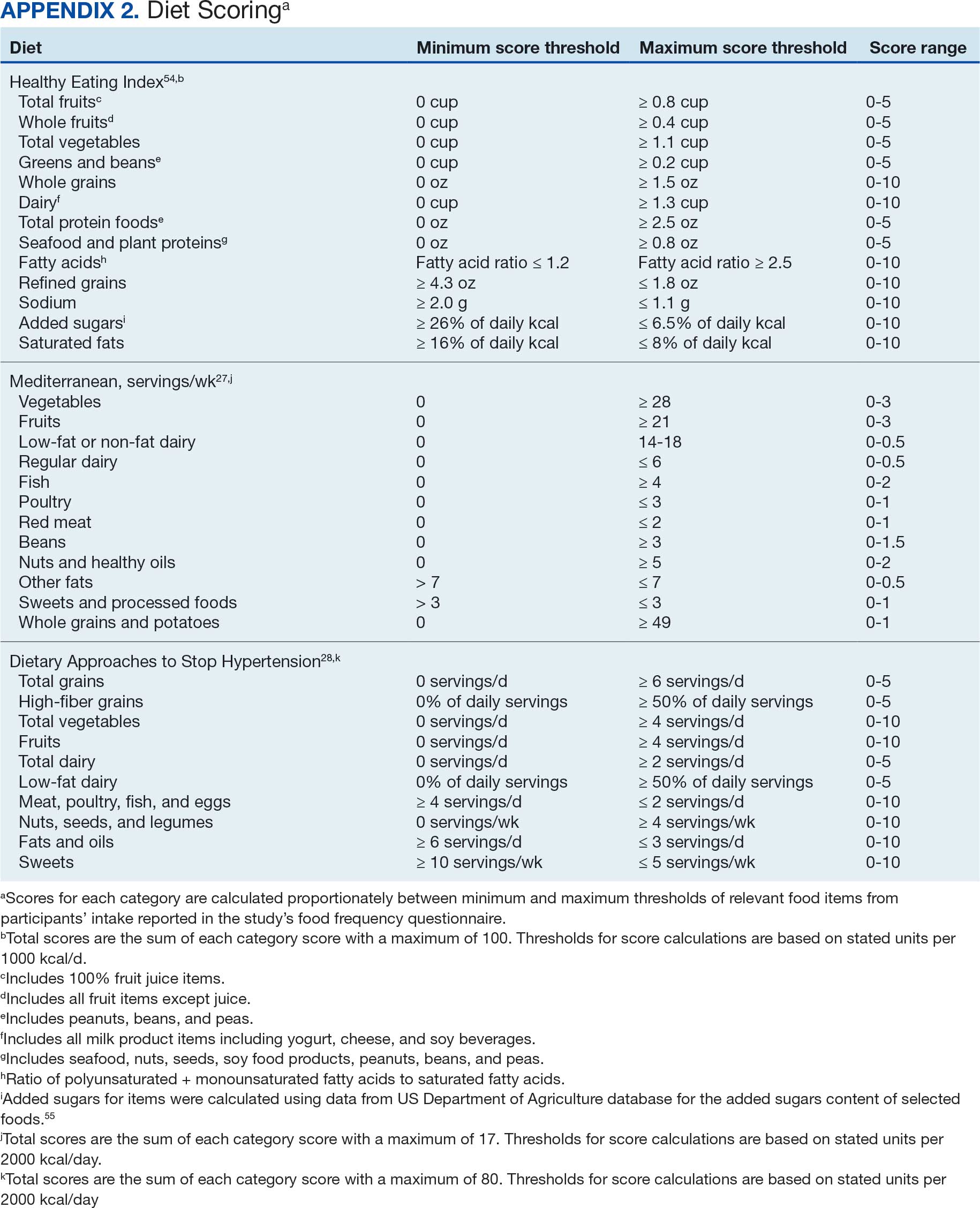
Demographic characteristics and clinical indicators. Demographic characteristics included age categories, sex, and race/ethnicity. Clinical indicators included BMI, the number of comorbid conditions used to calculate the Charlson Comorbidity Index, family history of CRC in first-degree relatives, number of follow-up colonoscopies across the study period, and food-based vitamin D intake.31 These variables were chosen for their applicability found in previous CSP #380 cohort studies.18,32,33 Self-reported race and ethnicity were collapsed due to small numbers in some groups. The authors acknowledge these are distinct concepts and the variable has limited utility other than for controlling for systemic racism in the model.
Statistical Analyses
Descriptive statistics were used to describe distributional assumptions for all variables, including demographics, clinical indicators, colonoscopy results, and dietary patterns. Pairwise correlations between the total dietary pattern scores and food category scores were calculated with Pearson correlation (r).
Multinomial logistic regression models were created using SAS procedure LOGISTIC with the outcome of the categorical MSCF (no neoplasia, nonadvanced adenoma, or AN).34 A model was created for each independent predictor variable of interest (ie, the HEI, MD, or DASH percentage-standardized dietary pattern score and each food category comprising each dietary pattern score). All models were adjusted for age, sex, race/ethnicity, BMI, number of comorbidities, family history of CRC, number of follow-up colonoscopies, and estimated daily food-derived vitamin D intake. The demographic and clinical indicators were included in the models as they are known to be associated with CRC risk.18 The number of colonoscopies was included to control for surveillance intensity presuming risk for AN is reduced as polyps are removed. Because colonoscopy findings from an initial screening have unique clinical implications compared with follow- up and surveillance, MSCF was observed in 2 ways in sensitivity analyses: (1) baseline and (2) aggregate follow-up and surveillance only, excluding baseline findings.
Adjusted odds ratios (aORs) and 95% CIs for each of the MSCF outcomes with a reference finding of no neoplasia for the models are presented. We chose not to adjust for multiple comparisons across the different dietary patterns given the correlation between dietary pattern total and category scores but did adjust for multiple comparisons for dietary categories within each dietary pattern. Tests for statistical significance used α= .05 for the dietary pattern total scores and P values for the dietary category scores for each dietary pattern controlled for false discovery rate using the MULTTEST SAS procedure.35 All data manipulations and analyses were performed using SAS version 9.4.
Results
The study included 3023 patients. All were aged 50 to 75 years, 2923 (96.7%) were male and 2532 (83.8%) were non-Hispanic White (Table 1). Most participants were overweight or obese (n = 2535 [83.8%]), 2024 (67.0%) had ≤ 2 comorbidities, and 2602 (86.1%) had no family history of CRC. The MSCF for 1628 patients (53.9%) was no neoplasia, 966 patients (32.0%) was nonadvanced adenoma, and 429 participants (14.2%) had AN.
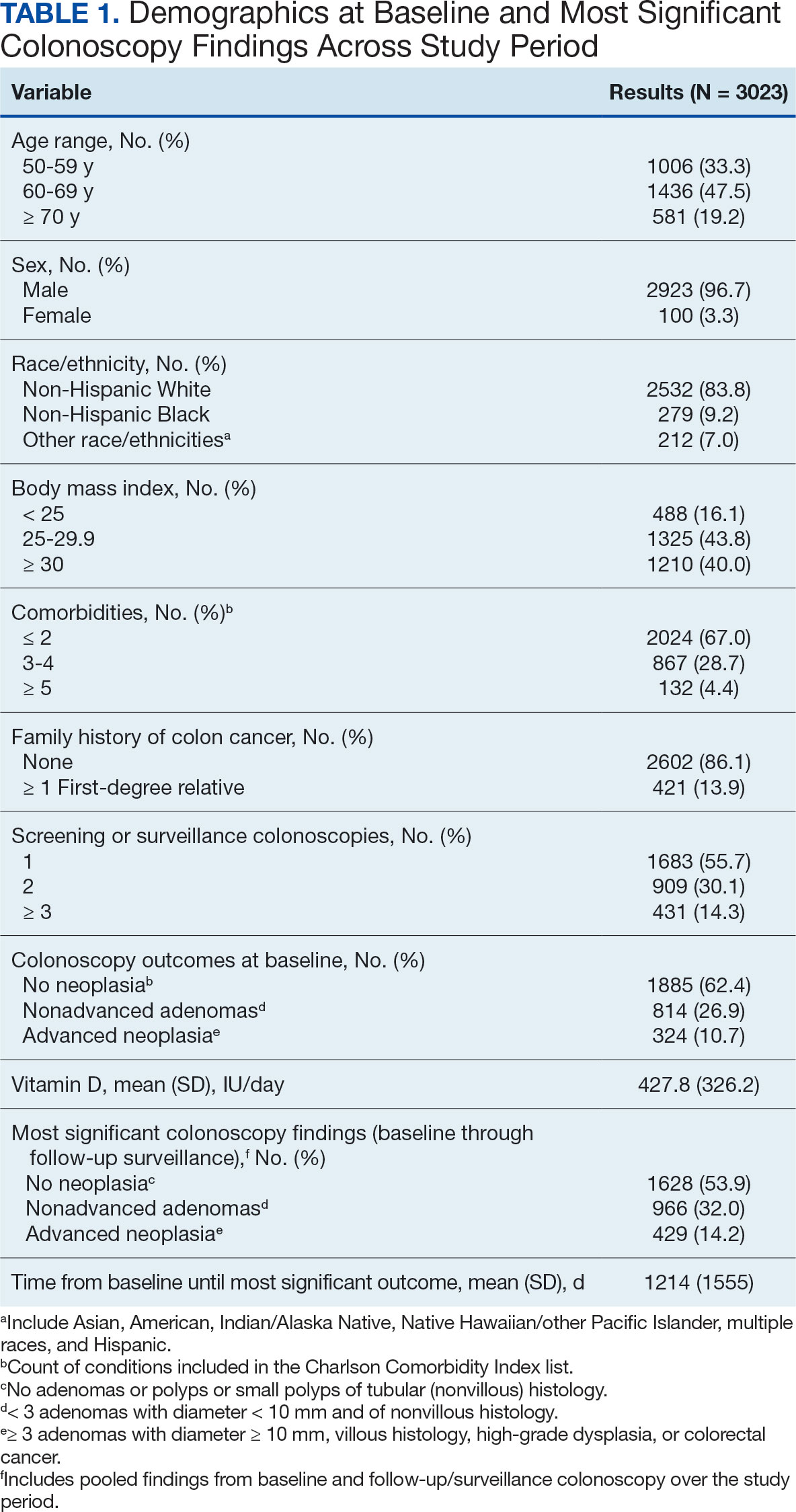
Mean percent scores were 58.5% for HEI, 38.2% for MD, and 63.1% for the DASH diet, with higher percentages indicating greater alignment with the recommendations for each diet (Table 2). All 3 dietary patterns scores standardized to percentages were strongly and significantly correlated in pairwise comparisons: HEI:MD, r = 0.62 (P < .001); HEI:DASH, r = 0.60 (P < .001); and MD:DASH, r = 0.72 (P < .001). Likewise, food category scores were significantly correlated across dietary patterns. For example, whole grain and fiber values from each dietary score were strongly correlated in pairwise comparisons: HEI Whole Grain:MD Grain, r = 0.64 (P < .001); HEI Whole Grain:DASH Fiber, r = 0.71 (P < .001); and MD Grain:DASH Fiber, r = 0.70 (P < .001).
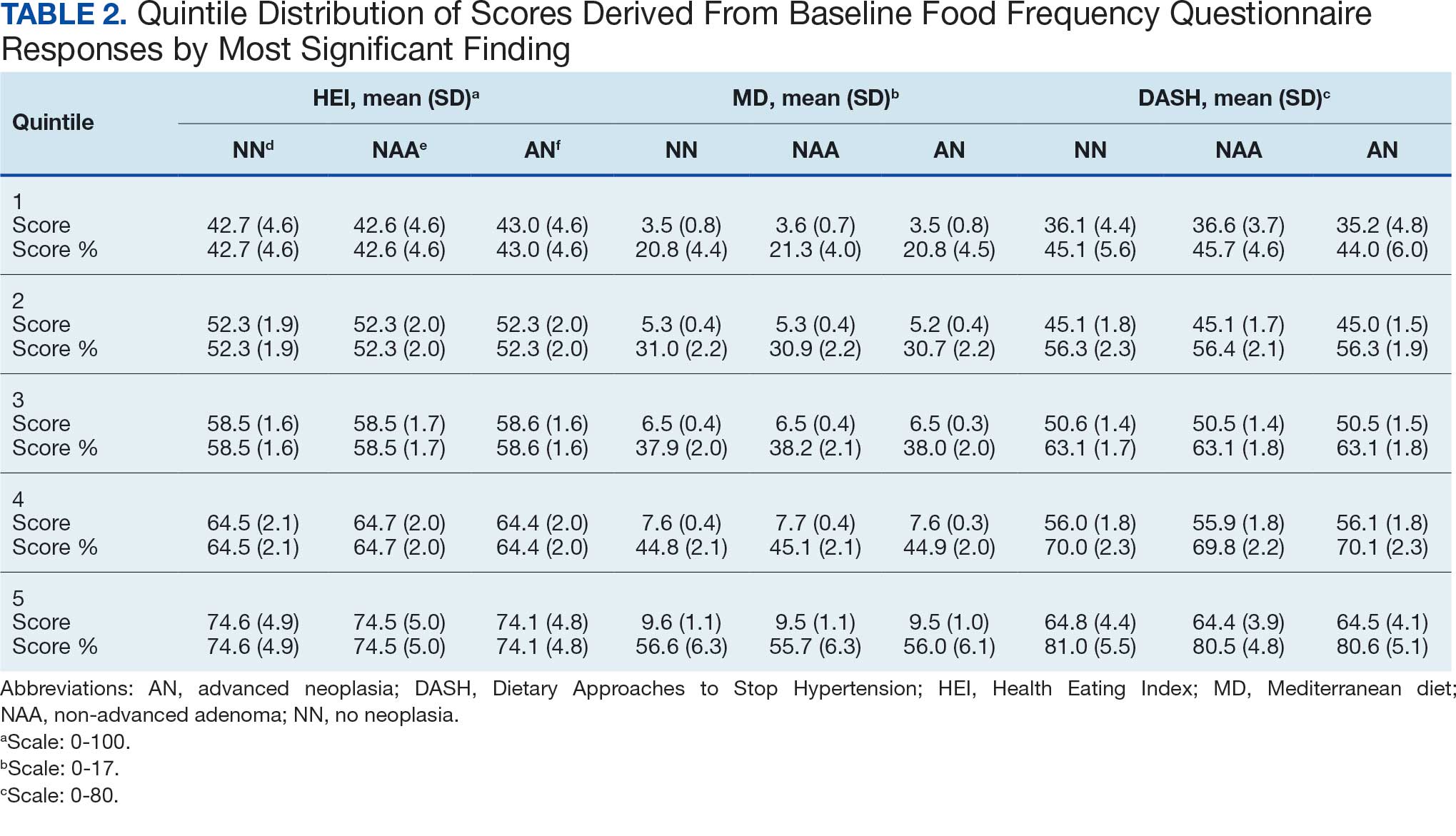
Associations between individual participants' dietary pattern scores and the outcome of their pooled MSCF from baseline screening and ≥ 10 years of surveillance are presented in Table 3. For each single-point increases in dietary pattern scores (reflecting better dietary quality), aORs for nonadvanced adenoma vs no neoplasia were slightly lower but not statistically significantly: HEI, aOR, 1.00 (95% CI, 0.99-1.01); MD, aOR, 0.98 (95% CI, 0.94-1.02); and DASH, aOR, 0.99 (95% CI, 0.99-1.00). aORs for AN vs no neoplasia were slightly lower for each dietary pattern assessed, and only the MD and DASH scores were significantly different from 1.00: HEI, aOR, 1.00 (95% CI, 0.99-1.01); MD, aOR, 0.95 (95% CI, 0.90-1.00); and DASH, aOR, 0.99 (95% CI, 0.98-1.00).
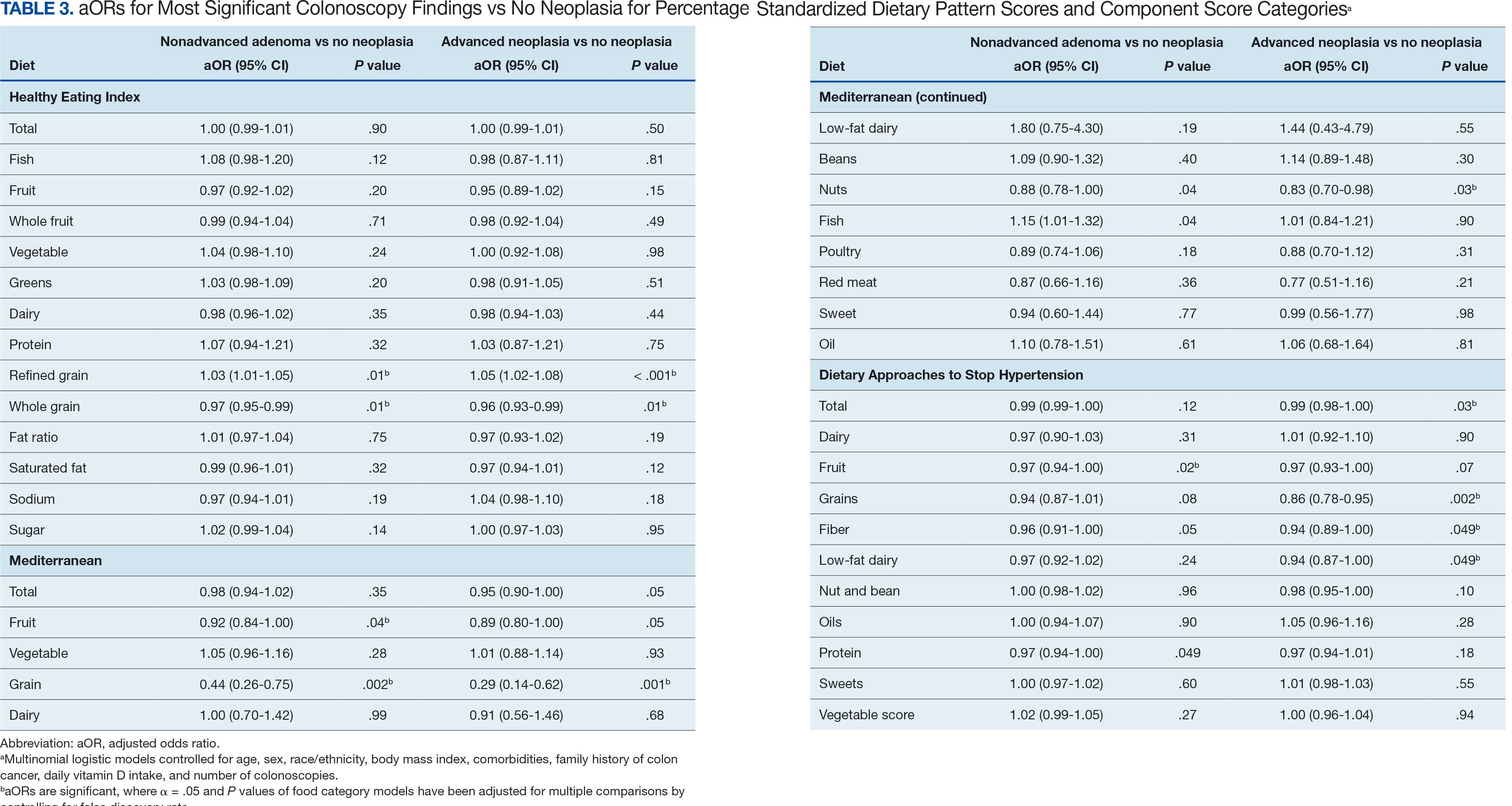
We observed lower odds for nonadvanced adenoma and AN among all these dietary patterns when there was greater alignment with the recommended intake of whole grains and fiber. In separate models conducted using food categories comprising the dietary patterns as independent variables and after correcting for multiple tests, higher scores for the HEI Refined Grain category were associated with higher odds for nonadvanced adenoma (aOR, 1.03 [95% CI, 1.01-1.05]; P = .01) and AN (aOR, 1.05 [95% CI, 1.02-1.08]; P < .001). Higher scores for the HEI Whole Grain category were associated with lower odds for nonadvanced adenoma (aOR, 0.97 [95% CI, 0.95-0.99]; P = .01) and AN (aOR, 0.96 [95% CI, 0.93-0.99]; P = .01). Higher scores for the MD Grain category were significantly associated with lower odds for nonadvanced adenoma (aOR, 0.44 [95% CI, 0.26-0.75]; P = .002) and AN (aOR, 0.29 [95% CI, 0.14-0.62]; P = .001). The DASH Grains category also was significantly associated with lower odds for AN (aOR, 0.86 [95% CI, 0.78-0.95]; P = .002).
Discussion
In this study of 3023 veterans undergoing first-time screening colonoscopy and ≥ 10 years of surveillance, we found that healthy dietary patterns, as assessed by the MD and DASH diet, were significantly associated with lower risk of AN. Additionally, we identified lower odds for AN and nonadvanced adenoma compared with no neoplasia for higher grain scores for all the dietary patterns studied. Other food categories that comprise the dietary pattern scores had mixed associations with the MSCF outcomes. Several other studies have examined associations between dietary patterns and risk for CRC but to our knowledge, no studies have explored these associations among US veterans.
These results also indicate study participants had better than average (based on a 50% threshold) dietary quality according to the HEI and DASH diet scoring methods we used, but poor dietary quality according to the MD scoring method. The mean HEI scores for the present study were higher than a US Department of Agriculture study by Dong et al that compared dietary quality between veterans and nonveterans using the HEI, for which veterans’ expected HEI score was 45.6 of 100.8 This could be explained by the fact that the participants needed to be healthy to be eligible and those with healthier behaviors overall may have self-selected into the study due to motivation for screening during a time when screening was not yet commonplace. 36 Similarly, participants of the present study had higher adherence to the DASH diet (63.1%) than adolescents with diabetes in a study by Günther et al. Conversely, firefighters who were coached to use a Mediterranean-style dietary pattern and dietary had higher adherence to MD than did participants in this study.27
A closer examination of specific food category component scores that comprise the 3 distinct dietary patterns revealed mixed results from the multinomial modeling, which may have to do with the guideline thresholds used to calculate the dietary scores. When analyzed separately in the logistic regression models for their associations with nonadvanced adenomas and AN compared with no neoplasia, higher MD and DASH fruit scores (but not HEI fruit scores) were found to be significant. Other studies have had mixed findings when attempting to test for associations of fruit intake with adenoma recurrence.10,37
This study had some unexpected findings. Vegetable intake was not associated with nonadvanced adenomas or AN risk. Studies of food categories have consistently found vegetable (specifically cruciferous ones) intake to be linked with lower odds for cancers.38 Likewise, the red meat category, which was only a unique food category in the MD score, was not associated with nonadvanced adenomas or AN. Despite consistent literature suggesting higher intake of red meat and processed meats increases CRC risk, in 2019 the Nutritional Recommendations Consortium indicated that the evidence was weak.39,40 This study showed higher DASH diet scores for low-fat dairy, which were maximized when participants reported at least 50% of their dairy servings per day as being low-fat, had lower odds for AN. Yet, the MD scores for low-fat dairy had no association with either outcome; their calculation was based on total number of servings per week. This difference in findings suggests the fat intake ratio may be more relevant to CRC risk than intake quantity.
The literature is mixed regarding fatty acid intake and CRC risk, which may be relevant to both dairy and meat intake. One systematic review and meta-analysis found dietary fat and types of fatty acid intake had no association with CRC risk.41 However, a more recent meta-analysis that assessed both dietary intake and plasma levels of fatty acids did find some statistically significant differences for various types of fatty acids and CRC risk.42
The findings in the present study that grain intake is associated with lower odds for more severe colonoscopy findings among veterans are notable.43 Lieberman et al, using the CSP #380 data, found that cereal fiber intake was associated with a lower odds for AN compared with hyperplastic polyps (OR, 0.98 [95% CI, 0.96- 1.00]).18 Similarly, Hullings et al determined that older adults in the highest quintile of cereal fiber intake had significantly lower odds of CRC than those in lower odds for CRC when compared with lowest quintile (OR, 0.89 [95% CI, 0.83- 0.96]; P < .001).44 These findings support existing guidance that prioritizes whole grains as a key source of dietary fiber for CRC prevention.
A recent literature review on fiber, fat, and CRC risk suggested a consensus regarding one protective mechanism: dietary fiber from grains modulates the gut microbiota by promoting butyrate synthesis.45 Butyrate is a short-chain fatty acid that supports energy production in colonocytes and has tumor-suppressing properties.46 Our findings suggest there could be more to learn about the relationship between butyrate production and reduction of CRC risk through metabolomic studies that use measurements of plasma butyrate. These studies may examine associations between not just a singular food or food category, but rather food patterns that include fruits, vegetables, nuts and seeds, and whole grains known to promote butyrate production and plasma butyrate.47
Improved understanding of mechanisms and risk-modifying lifestyle factors such as dietary patterns may enhance prevention strategies. Identifying the collective chemopreventive characteristics of a specific dietary pattern (eg, MD) will be helpful to clinicians and health care staff to promote healthy eating to reduce cancer risk. More studies are needed to understand whether such promotion is more clinically applicable and effective for patients, as compared with eating more or less of specific foods (eg, more whole grains, less red meat). Furthermore, considering important environmental factors collectively beyond dietary patterns may offer a way to better tailor screening and implement a variety of lifestyle interventions. In the literature, this is often referred to as a teachable moment when patients’ attentions are captured and may position them to be more receptive to guidance.48
Limitations
This study has several important limitations and leaves opportunities for future studies that explore the role of dietary patterns and AN or CRC risk. First, the FFQ data used to calculate dietary pattern scores used in analysis were only captured at baseline, and there are nearly 3 decades across the study period. However, it is widely assumed that the diets of older adults, like those included in this study, remain stable over time which is appropriate given our sample population was aged 50 to 75 years when the baseline FFQ data were collected.49-51 Additionally, while the HEI is a well-documented, standard scoring method for dietary quality, there are multitudes of dietary pattern scoring approaches for MD and DASH.23,52,53 Finally, findings from this study using the sample of veterans may not be generalizable to a broader population. Future longitudinal studies that test for a clinically significant change threshold are warranted.
Conclusion
Results of this study suggest future research should further explore the effects of dietary patterns, particularly intake of specific food groups in combination, as opposed to individual nutrients or food items, on AN and CRC risk. Possible studies might explore these dietary patterns for their mechanistic role in altering the microbiome metabolism, which may influence CRC outcomes or include diet in a more comprehensive, holistic risk score that could be used to predict colonic neoplasia risk or in intervention studies that assess the effects of dietary changes on long-term CRC prevention. We suggest there are differences in people’s dietary intake patterns that might be important to consider when implementing tailored approaches to CRC risk mitigation.
- Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectalcancer deaths. N Engl J Med. 2012;366(8):687-696. doi:10.1056/NEJMoa1100370
- Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;369(12):1095-1105. doi:10.1056/NEJMoa1301969
- Bretthauer M, Løberg M, Wieszczy P, et al. Effect of colonoscopy screening on risks of colorectal cancer and related death. N Engl J Med. 2022;387(17):1547-1556. doi:10.1056/NEJMoa2208375
- Cottet V, Bonithon-Kopp C, Kronborg O, et al. Dietary patterns and the risk of colorectal adenoma recurrence in a European intervention trial. Eur J Cancer Prev. 2005;14(1):21.
- Miller PE, Lesko SM, Muscat JE, Lazarus P, Hartman TJ. Dietary patterns and colorectal adenoma and cancer risk: a review of the epidemiological evidence. Nutr Cancer. 2010;62(4):413-424. doi:10.1080/01635580903407114
- Godos J, Bella F, Torrisi A, Sciacca S, Galvano F, Grosso G. Dietary patterns and risk of colorectal adenoma: a systematic review and meta-analysis of observational studies. J Hum Nutr Diet Off J Br Diet Assoc. 2016;29(6):757-767. doi:10.1111/jhn.12395
- Haggar FA, Boushey RP. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg. 2009;22(4):191-197. doi:10.1055/s-0029-1242458
- Dong D, Stewart H, Carlson AC. An Examination of Veterans’ Diet Quality. U.S. Department of Agriculture, Economic Research Service; 2019:32.
- El-Halabi MM, Rex DK, Saito A, Eckert GJ, Kahi CJ. Defining adenoma detection rate benchmarks in average-risk male veterans. Gastrointest Endosc. 2019;89(1):137-143. doi:10.1016/j.gie.2018.08.021
- Alberts DS, Hess LM, eds. Fundamentals of Cancer Prevention. Springer International Publishing; 2019. doi:10.1007/978-3-030-15935-1
- Dahm CC, Keogh RH, Spencer EA, et al. Dietary fiber and colorectal cancer risk: a nested case-control study using food diaries. J Natl Cancer Inst. 2010;102(9):614-626. doi:10.1093/jnci/djq092
- Aune D, Lau R, Chan DSM, et al. Dairy products and colorectal cancer risk: a systematic review and metaanalysis of cohort studies. Ann Oncol. 2012;23(1):37-45. doi:10.1093/annonc/mdr269
- Lee JE, Li H, Chan AT, et al. Circulating levels of vitamin D and colon and rectal cancer: the Physicians’ Health Study and a meta-analysis of prospective studies. Cancer Prev Res Phila Pa. 2011;4(5):735-743. doi:10.1158/1940-6207.CAPR-10-0289
- Carroll C, Cooper K, Papaioannou D, Hind D, Pilgrim H, Tappenden P. Supplemental calcium in the chemoprevention of colorectal cancer: a systematic review and meta-analysis. Clin Ther. 2010;32(5):789-803. doi:10.1016/j.clinthera.2010.04.024
- Park Y, Spiegelman D, Hunter DJ, et al. Intakes of vitamins A, C, and E and use of multiple vitamin supplements and risk of colon cancer: a pooled analysis of prospective cohort studies. Cancer Causes Control CCC. 2010;21(11):1745- 1757. doi:10.1007/s10552-010-9549-y
- Alexander DD, Weed DL, Miller PE, Mohamed MA. Red meat and colorectal cancer: a quantitative update on the state of the epidemiologic science. J Am Coll Nutr. 2015;34(6):521-543. doi:10.1080/07315724.2014.992553
- Park SY, Wilkens LR, Setiawan VW, Monroe KR, Haiman CA, Le Marchand L. Alcohol intake and colorectal cancer risk in the multiethnic cohort study. Am J Epidemiol. 2019;188(1):67-76. doi:10.1093/aje/kwy208
- Lieberman DA. Risk Factors for advanced colonic neoplasia and hyperplastic polyps in asymptomatic individuals. JAMA. 2003;290(22):2959. doi:10.1001/jama.290.22.2959
- Archambault AN, Jeon J, Lin Y, et al. Risk stratification for early-onset colorectal cancer using a combination of genetic and environmental risk scores: an international multi-center study. J Natl Cancer Inst. 2022;114(4):528-539. doi:10.1093/jnci/djac003
- Carr PR, Weigl K, Edelmann D, et al. Estimation of absolute risk of colorectal cancer based on healthy lifestyle, genetic risk, and colonoscopy status in a populationbased study. Gastroenterology. 2020;159(1):129-138.e9. doi:10.1053/j.gastro.2020.03.016
- Sullivan BA, Qin X, Miller C, et al. Screening colonoscopy findings are associated with noncolorectal cancer mortality. Clin Transl Gastroenterol. 2022;13(4):e00479. doi:10.14309/ctg.0000000000000479
- Erben V, Carr PR, Holleczek B, Stegmaier C, Hoffmeister M, Brenner H. Dietary patterns and risk of advanced colorectal neoplasms: A large population based screening study in Germany. Prev Med. 2018;111:101-109. doi:10.1016/j.ypmed.2018.02.025
- Donovan MG, Selmin OI, Doetschman TC, Romagnolo DF. Mediterranean diet: prevention of colorectal cancer. Front Nutr. 2017;4:59. doi:10.3389/fnut.2017.00059
- Mohseni R, Mohseni F, Alizadeh S, Abbasi S. The Association of Dietary Approaches to Stop Hypertension (DASH) diet with the risk of colorectal cancer: a meta-analysis of observational studies.Nutr Cancer. 2020;72(5):778-790. doi:10.1080/01635581.2019.1651880
- Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. N Engl J Med. 2000;343(3):162-168. doi:10.1056/NEJM200007203430301
- Developing the Healthy Eating Index (HEI) | EGRP/ DCCPS/NCI/NIH. Accessed July 22, 2025. https://epi.grants.cancer.gov/hei/developing.html#2015c
- Reeve E, Piccici F, Feairheller DL. Validation of a Mediterranean diet scoring system for intervention based research. J Nutr Med Diet Care. 2021;7(1):053. doi:10.23937/2572-3278/1510053
- Günther AL, Liese AD, Bell RA, et al. ASSOCIATION BETWEEN THE DIETARY APPROACHES TO HYPERTENSION (DASH) DIET AND HYPERTENSION IN YOUTH WITH DIABETES. Hypertens Dallas Tex 1979. 2009;53(1):6-12. doi:10.1161/HYPERTENSIONAHA.108.116665
- Buckland G, Agudo A, Luján L, et al. Adherence to a Mediterranean diet and risk of gastric adenocarcinoma within the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort study. Am J Clin Nutr. 2010;91(2):381- 390. doi:10.3945/ajcn.2009.28209
- Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135(10):1114-1126. doi:10.1093/oxfordjournals.aje.a116211
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi:10.1016/0021-9681(87)90171-8
- Lieberman DA, Weiss DG, Harford WV, et al. Fiveyear colon surveillance after screening colonoscopy. Gastroenterology. 2007;133(4):1077-1085. doi:10.1053/j.gastro.2007.07.006
- Lieberman D, Sullivan BA, Hauser ER, et al. Baseline colonoscopy findings associated with 10-year outcomes in a screening cohort undergoing colonoscopy surveillance. Gastroenterology. 2020;158(4):862-874.e8. doi:10.1053/j.gastro.2019.07.052
- PROC LOGISTIC: PROC LOGISTIC Statement : SAS/STAT(R) 9.22 User’s Guide. Accessed July 22, 2025. https://support.sas.com/documentation/cdl/en/statug/63347/HTML/default/viewer.htm#statug_logistic_sect004.htm
- PROC MULTTEST: PROC MULTTEST Statement : SAS/ STAT(R) 9.22 User’s Guide. Accessed July 22, 2025. https://support.sas.com/documentation/cdl/en/statug/63347/HTML/default/viewer.htm#statug_multtest_sect005.htm
- Elston DM. Participation bias, self-selection bias, and response bias. J Am Acad Dermatol. Published online June 18, 2021. doi:10.1016/j.jaad.2021.06.025
- Sansbury LB, Wanke K, Albert PS, et al. The effect of strict adherence to a high-fiber, high-fruit and -vegetable, and low-fat eating pattern on adenoma recurrence. Am J Epidemiol. 2009;170(5):576-584. doi:10.1093/aje/kwp169
- Borgas P, Gonzalez G, Veselkov K, Mirnezami R. Phytochemically rich dietary components and the risk of colorectal cancer: A systematic review and meta-analysis of observational studies. World J Clin Oncol. 2021;12(6):482- 499. doi:10.5306/wjco.v12.i6.482
- Papadimitriou N, Markozannes G, Kanellopoulou A, et al. An umbrella review of the evidence associating diet and cancer risk at 11 anatomical sites. Nat Commun. 2021;12(1):4579. doi:10.1038/s41467-021-24861-8
- Johnston BC, Zeraatkar D, Han MA, et al. Unprocessed red meat and processed meat consumption: dietary guideline recommendations from the nutritional recommendations (NutriRECS) Consortium. Ann Intern Med. 2019;171(10):756-764. doi:10.7326/M19-1621
- Kim M, Park K. Dietary fat intake and risk of colorectal cancer: a systematic review and meta-analysis of prospective studies. Nutrients. 2018;10(12):1963. doi:10.3390/nu10121963
- Lu Y, Li D, Wang L, et al. Comprehensive investigation on associations between dietary intake and blood levels of fatty acids and colorectal cancer risk. Nutrients. 2023;15(3):730. doi:10.3390/nu15030730
- Gherasim A, Arhire LI, Ni.a O, Popa AD, Graur M, Mihalache L. The relationship between lifestyle components and dietary patterns. Proc Nutr Soc. 2020;79(3):311-323. doi:10.1017/S0029665120006898
- Hullings AG, Sinha R, Liao LM, Freedman ND, Graubard BI, Loftfield E. Whole grain and dietary fiber intake and risk of colorectal cancer in the NIH-AARP Diet and Health Study cohort. Am J Clin Nutr. 2020;112(3):603- 612. doi:10.1093/ajcn/nqaa161
- Ocvirk S, Wilson AS, Appolonia CN, Thomas TK, O’Keefe SJD. Fiber, fat, and colorectal cancer: new insight into modifiable dietary risk factors. Curr Gastroenterol Rep. 2019;21(11):62. doi:10.1007/s11894-019-0725-2
- O’Keefe SJD. Diet, microorganisms and their metabolites, and colon cancer. Nat Rev Gastroenterol Hepatol. 2016;13(12):691-706. doi:10.1038/nrgastro.2016.165
- The health benefits and side effects of Butyrate Cleveland Clinic. July 11, 2022. Accessed July 22, 2025. https://health.clevelandclinic.org/butyrate-benefits/
- Knudsen MD, Wang L, Wang K, et al. Changes in lifestyle factors after endoscopic screening: a prospective study in the United States. Clin Gastroenterol Hepatol Off ClinPract J Am Gastroenterol Assoc. 2022;20(6):e1240-e1249. doi:10.1016/j.cgh.2021.07.014
- Thorpe MG, Milte CM, Crawford D, McNaughton SA. Education and lifestyle predict change in dietary patterns and diet quality of adults 55 years and over. Nutr J. 2019;18(1):67. doi:10.1186/s12937-019-0495-6
- Chapman K, Ogden J. How do people change their diet?: an exploration into mechanisms of dietary change. J Health Psychol. 2009;14(8):1229-1242. doi:10.1177/1359105309342289
- Djoussé L, Petrone AB, Weir NL, et al. Repeated versus single measurement of plasma omega-3 fatty acids and risk of heart failure. Eur J Nutr. 2014;53(6):1403-1408. doi:10.1007/s00394-013-0642-3
- Bach-Faig A, Berry EM, Lairon D, et al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011;14(12A):2274-2284. doi:10.1017/S1368980011002515
- Miller PE, Cross AJ, Subar AF, et al. Comparison of 4 established DASH diet indexes: examining associations of index scores and colorectal cancer123. Am J Clin Nutr. 2013;98(3):794-803. doi:10.3945/ajcn.113.063602
- Krebs-Smith SM, Pannucci TE, Subar AF, et al. Update of the Healthy Eating Index: HEI-2015. J Acad Nutr Diet. 2018;118(9):1591-1602. doi:10.1016/j.jand.2018.05.021
- P.R. Pehrsson, Cutrufelli RL, Gebhardt SE, et al. USDA Database for the Added Sugars Content of Selected Foods. USDA; 2005. www.ars.usda.gov/nutrientdata
Screening for colorectal cancer (CRC) with colonoscopy enables the identification and removal of CRC precursors (colonic adenomas) and has been associated with reduced risk of CRC incidence and mortality.1-3 Furthermore, there is consensus that diet and lifestyle may be associated with forestalling CRC pathogenesis at the intermediate adenoma stages.4-7 However, studies have shown that US veterans have poorer diet quality and a higher risk for neoplasia compared with nonveterans, reinforcing the need for tailored clinical approaches.8,9 Combining screening with conversations about modifiable environmental and lifestyle risk factors, such as poor diet, is a highly relevant and possibly easily leveraged prevention for those at high risk. However, there is limited evidence for any particular dietary patterns or dietary features that are most important over time.7
Several dietary components have been shown to be associated with CRC risk,10 either as potentially chemopreventive (fiber, fruits and vegetables,11 dairy,12 supplemental vitamin D,13 calcium,14 and multivitamins15) or carcinogenic (red meat16 and alcohol17). Previous studies of veterans have similarly shown that higher intake of fiber and vitamin D reduced risk, and red meat is associated with an increased risk for finding CRC precursors during colonoscopy.18 However, these dietary categories are often analyzed in isolation. Studying healthy dietary patterns in aggregate may be more clinically relevant and easier to implement for prevention of CRC and its precursors.19-21 Healthy dietary patterns, such as the US Dietary Guidelines for Americans represented by the Healthy Eating Index (HEI), the Mediterranean diet (MD), and the Dietary Approaches to Stop Hypertension (DASH) diet, have been associated with lower risk for chronic disease.22-24 Despite the extant literature, no known studies have compared these dietary patterns for associations with risk of CRC precursor or CRC development among US veterans undergoing long-term screening and follow-up after a baseline colonoscopy.
The objective of this study was to test for associations between baseline scores of healthy dietary patterns and the most severe colonoscopy findings (MSCFs) over ≥ 10 years following a baseline screening colonoscopy in veterans.
Methods
Participants in the Cooperative Studies Program (CSP) #380 cohort study included 3121 asymptomatic veterans aged 50 to 75 years at baseline who had consented to initial screening colonoscopy between 1994 and 1997, with subsequent follow-up and surveillance.25 Prior to their colonoscopy, all participants completed a baseline study survey that included questions about cancer risk factors including family history of CRC, diet, physical activity, and medication use.
Included in this cross-sectional analysis were data from a sample of veteran participants of the CSP #380 cohort with 1 baseline colonoscopy, follow-up surveillance through 2009, a cancer risk factor survey collected at baseline, and complete demographic and clinical indicator data. Excluded from the analysis were 67 participants with insufficient responses to the dietary food frequency questionnaire (FFQ) and 31 participants with missing body mass index (BMI), 3023 veterans.
Measures
MSCF. The outcome of interest in this study was the MSCF recorded across all participant colonoscopies during the study period. MSCF was categorized as either (1) no neoplasia; (2) < 2 nonadvanced adenomas, including small adenomas (diameter < 10 mm) with tubular histology; or (3) advanced neoplasia (AN), which is characterized by adenomas > 10 mm in diameter, with villous histology, with high-grade dysplasia, or CRC.
Dietary patterns. Dietary pattern scores representing dietary quality and calculated based on recommendations of the US Dietary Guidelines for Americans using the HEI, MD, and DASH diets were independent variables.26-28 These 3 dietary patterns were chosen for their hypothesized relationship with CRC risk, but each weighs food categories differently (Appendix 1).22-24,29 Dietary pattern scores were calculated using the CSP #380 self-reported responses to 129 baseline survey questions adapted from a well-established and previously validated semiquantitative FFQ.30 The form was administered by mail twice to a sample of 127 participants at baseline and at 1 year. During this interval, men completed 1-week diet records twice, spaced about 6 months apart. Mean values for intake of most nutrients assessed by the 2 methods were similar. Intraclass correlation coefficients for the baseline and 1-year FFQ-assessed nutrient intakes that ranged from 0.47 for vitamin E (without supplements) to 0.80 for vitamin C (with supplements). Correlation coefficients between the energy-adjusted nutrient intakes were measured by diet records and the 1-year FFQ, which asked about diet during the year encompassing the diet records. Higher raw and percent scores indicated better alignment with recommendations from each respective dietary pattern. Percent scores were calculated as a standardizing method and used in analyses for ease of comparing the dietary patterns. Scoring can be found in Appendix 2.


Demographic characteristics and clinical indicators. Demographic characteristics included age categories, sex, and race/ethnicity. Clinical indicators included BMI, the number of comorbid conditions used to calculate the Charlson Comorbidity Index, family history of CRC in first-degree relatives, number of follow-up colonoscopies across the study period, and food-based vitamin D intake.31 These variables were chosen for their applicability found in previous CSP #380 cohort studies.18,32,33 Self-reported race and ethnicity were collapsed due to small numbers in some groups. The authors acknowledge these are distinct concepts and the variable has limited utility other than for controlling for systemic racism in the model.
Statistical Analyses
Descriptive statistics were used to describe distributional assumptions for all variables, including demographics, clinical indicators, colonoscopy results, and dietary patterns. Pairwise correlations between the total dietary pattern scores and food category scores were calculated with Pearson correlation (r).
Multinomial logistic regression models were created using SAS procedure LOGISTIC with the outcome of the categorical MSCF (no neoplasia, nonadvanced adenoma, or AN).34 A model was created for each independent predictor variable of interest (ie, the HEI, MD, or DASH percentage-standardized dietary pattern score and each food category comprising each dietary pattern score). All models were adjusted for age, sex, race/ethnicity, BMI, number of comorbidities, family history of CRC, number of follow-up colonoscopies, and estimated daily food-derived vitamin D intake. The demographic and clinical indicators were included in the models as they are known to be associated with CRC risk.18 The number of colonoscopies was included to control for surveillance intensity presuming risk for AN is reduced as polyps are removed. Because colonoscopy findings from an initial screening have unique clinical implications compared with follow- up and surveillance, MSCF was observed in 2 ways in sensitivity analyses: (1) baseline and (2) aggregate follow-up and surveillance only, excluding baseline findings.
Adjusted odds ratios (aORs) and 95% CIs for each of the MSCF outcomes with a reference finding of no neoplasia for the models are presented. We chose not to adjust for multiple comparisons across the different dietary patterns given the correlation between dietary pattern total and category scores but did adjust for multiple comparisons for dietary categories within each dietary pattern. Tests for statistical significance used α= .05 for the dietary pattern total scores and P values for the dietary category scores for each dietary pattern controlled for false discovery rate using the MULTTEST SAS procedure.35 All data manipulations and analyses were performed using SAS version 9.4.
Results
The study included 3023 patients. All were aged 50 to 75 years, 2923 (96.7%) were male and 2532 (83.8%) were non-Hispanic White (Table 1). Most participants were overweight or obese (n = 2535 [83.8%]), 2024 (67.0%) had ≤ 2 comorbidities, and 2602 (86.1%) had no family history of CRC. The MSCF for 1628 patients (53.9%) was no neoplasia, 966 patients (32.0%) was nonadvanced adenoma, and 429 participants (14.2%) had AN.

Mean percent scores were 58.5% for HEI, 38.2% for MD, and 63.1% for the DASH diet, with higher percentages indicating greater alignment with the recommendations for each diet (Table 2). All 3 dietary patterns scores standardized to percentages were strongly and significantly correlated in pairwise comparisons: HEI:MD, r = 0.62 (P < .001); HEI:DASH, r = 0.60 (P < .001); and MD:DASH, r = 0.72 (P < .001). Likewise, food category scores were significantly correlated across dietary patterns. For example, whole grain and fiber values from each dietary score were strongly correlated in pairwise comparisons: HEI Whole Grain:MD Grain, r = 0.64 (P < .001); HEI Whole Grain:DASH Fiber, r = 0.71 (P < .001); and MD Grain:DASH Fiber, r = 0.70 (P < .001).

Associations between individual participants' dietary pattern scores and the outcome of their pooled MSCF from baseline screening and ≥ 10 years of surveillance are presented in Table 3. For each single-point increases in dietary pattern scores (reflecting better dietary quality), aORs for nonadvanced adenoma vs no neoplasia were slightly lower but not statistically significantly: HEI, aOR, 1.00 (95% CI, 0.99-1.01); MD, aOR, 0.98 (95% CI, 0.94-1.02); and DASH, aOR, 0.99 (95% CI, 0.99-1.00). aORs for AN vs no neoplasia were slightly lower for each dietary pattern assessed, and only the MD and DASH scores were significantly different from 1.00: HEI, aOR, 1.00 (95% CI, 0.99-1.01); MD, aOR, 0.95 (95% CI, 0.90-1.00); and DASH, aOR, 0.99 (95% CI, 0.98-1.00).

We observed lower odds for nonadvanced adenoma and AN among all these dietary patterns when there was greater alignment with the recommended intake of whole grains and fiber. In separate models conducted using food categories comprising the dietary patterns as independent variables and after correcting for multiple tests, higher scores for the HEI Refined Grain category were associated with higher odds for nonadvanced adenoma (aOR, 1.03 [95% CI, 1.01-1.05]; P = .01) and AN (aOR, 1.05 [95% CI, 1.02-1.08]; P < .001). Higher scores for the HEI Whole Grain category were associated with lower odds for nonadvanced adenoma (aOR, 0.97 [95% CI, 0.95-0.99]; P = .01) and AN (aOR, 0.96 [95% CI, 0.93-0.99]; P = .01). Higher scores for the MD Grain category were significantly associated with lower odds for nonadvanced adenoma (aOR, 0.44 [95% CI, 0.26-0.75]; P = .002) and AN (aOR, 0.29 [95% CI, 0.14-0.62]; P = .001). The DASH Grains category also was significantly associated with lower odds for AN (aOR, 0.86 [95% CI, 0.78-0.95]; P = .002).
Discussion
In this study of 3023 veterans undergoing first-time screening colonoscopy and ≥ 10 years of surveillance, we found that healthy dietary patterns, as assessed by the MD and DASH diet, were significantly associated with lower risk of AN. Additionally, we identified lower odds for AN and nonadvanced adenoma compared with no neoplasia for higher grain scores for all the dietary patterns studied. Other food categories that comprise the dietary pattern scores had mixed associations with the MSCF outcomes. Several other studies have examined associations between dietary patterns and risk for CRC but to our knowledge, no studies have explored these associations among US veterans.
These results also indicate study participants had better than average (based on a 50% threshold) dietary quality according to the HEI and DASH diet scoring methods we used, but poor dietary quality according to the MD scoring method. The mean HEI scores for the present study were higher than a US Department of Agriculture study by Dong et al that compared dietary quality between veterans and nonveterans using the HEI, for which veterans’ expected HEI score was 45.6 of 100.8 This could be explained by the fact that the participants needed to be healthy to be eligible and those with healthier behaviors overall may have self-selected into the study due to motivation for screening during a time when screening was not yet commonplace. 36 Similarly, participants of the present study had higher adherence to the DASH diet (63.1%) than adolescents with diabetes in a study by Günther et al. Conversely, firefighters who were coached to use a Mediterranean-style dietary pattern and dietary had higher adherence to MD than did participants in this study.27
A closer examination of specific food category component scores that comprise the 3 distinct dietary patterns revealed mixed results from the multinomial modeling, which may have to do with the guideline thresholds used to calculate the dietary scores. When analyzed separately in the logistic regression models for their associations with nonadvanced adenomas and AN compared with no neoplasia, higher MD and DASH fruit scores (but not HEI fruit scores) were found to be significant. Other studies have had mixed findings when attempting to test for associations of fruit intake with adenoma recurrence.10,37
This study had some unexpected findings. Vegetable intake was not associated with nonadvanced adenomas or AN risk. Studies of food categories have consistently found vegetable (specifically cruciferous ones) intake to be linked with lower odds for cancers.38 Likewise, the red meat category, which was only a unique food category in the MD score, was not associated with nonadvanced adenomas or AN. Despite consistent literature suggesting higher intake of red meat and processed meats increases CRC risk, in 2019 the Nutritional Recommendations Consortium indicated that the evidence was weak.39,40 This study showed higher DASH diet scores for low-fat dairy, which were maximized when participants reported at least 50% of their dairy servings per day as being low-fat, had lower odds for AN. Yet, the MD scores for low-fat dairy had no association with either outcome; their calculation was based on total number of servings per week. This difference in findings suggests the fat intake ratio may be more relevant to CRC risk than intake quantity.
The literature is mixed regarding fatty acid intake and CRC risk, which may be relevant to both dairy and meat intake. One systematic review and meta-analysis found dietary fat and types of fatty acid intake had no association with CRC risk.41 However, a more recent meta-analysis that assessed both dietary intake and plasma levels of fatty acids did find some statistically significant differences for various types of fatty acids and CRC risk.42
The findings in the present study that grain intake is associated with lower odds for more severe colonoscopy findings among veterans are notable.43 Lieberman et al, using the CSP #380 data, found that cereal fiber intake was associated with a lower odds for AN compared with hyperplastic polyps (OR, 0.98 [95% CI, 0.96- 1.00]).18 Similarly, Hullings et al determined that older adults in the highest quintile of cereal fiber intake had significantly lower odds of CRC than those in lower odds for CRC when compared with lowest quintile (OR, 0.89 [95% CI, 0.83- 0.96]; P < .001).44 These findings support existing guidance that prioritizes whole grains as a key source of dietary fiber for CRC prevention.
A recent literature review on fiber, fat, and CRC risk suggested a consensus regarding one protective mechanism: dietary fiber from grains modulates the gut microbiota by promoting butyrate synthesis.45 Butyrate is a short-chain fatty acid that supports energy production in colonocytes and has tumor-suppressing properties.46 Our findings suggest there could be more to learn about the relationship between butyrate production and reduction of CRC risk through metabolomic studies that use measurements of plasma butyrate. These studies may examine associations between not just a singular food or food category, but rather food patterns that include fruits, vegetables, nuts and seeds, and whole grains known to promote butyrate production and plasma butyrate.47
Improved understanding of mechanisms and risk-modifying lifestyle factors such as dietary patterns may enhance prevention strategies. Identifying the collective chemopreventive characteristics of a specific dietary pattern (eg, MD) will be helpful to clinicians and health care staff to promote healthy eating to reduce cancer risk. More studies are needed to understand whether such promotion is more clinically applicable and effective for patients, as compared with eating more or less of specific foods (eg, more whole grains, less red meat). Furthermore, considering important environmental factors collectively beyond dietary patterns may offer a way to better tailor screening and implement a variety of lifestyle interventions. In the literature, this is often referred to as a teachable moment when patients’ attentions are captured and may position them to be more receptive to guidance.48
Limitations
This study has several important limitations and leaves opportunities for future studies that explore the role of dietary patterns and AN or CRC risk. First, the FFQ data used to calculate dietary pattern scores used in analysis were only captured at baseline, and there are nearly 3 decades across the study period. However, it is widely assumed that the diets of older adults, like those included in this study, remain stable over time which is appropriate given our sample population was aged 50 to 75 years when the baseline FFQ data were collected.49-51 Additionally, while the HEI is a well-documented, standard scoring method for dietary quality, there are multitudes of dietary pattern scoring approaches for MD and DASH.23,52,53 Finally, findings from this study using the sample of veterans may not be generalizable to a broader population. Future longitudinal studies that test for a clinically significant change threshold are warranted.
Conclusion
Results of this study suggest future research should further explore the effects of dietary patterns, particularly intake of specific food groups in combination, as opposed to individual nutrients or food items, on AN and CRC risk. Possible studies might explore these dietary patterns for their mechanistic role in altering the microbiome metabolism, which may influence CRC outcomes or include diet in a more comprehensive, holistic risk score that could be used to predict colonic neoplasia risk or in intervention studies that assess the effects of dietary changes on long-term CRC prevention. We suggest there are differences in people’s dietary intake patterns that might be important to consider when implementing tailored approaches to CRC risk mitigation.
Screening for colorectal cancer (CRC) with colonoscopy enables the identification and removal of CRC precursors (colonic adenomas) and has been associated with reduced risk of CRC incidence and mortality.1-3 Furthermore, there is consensus that diet and lifestyle may be associated with forestalling CRC pathogenesis at the intermediate adenoma stages.4-7 However, studies have shown that US veterans have poorer diet quality and a higher risk for neoplasia compared with nonveterans, reinforcing the need for tailored clinical approaches.8,9 Combining screening with conversations about modifiable environmental and lifestyle risk factors, such as poor diet, is a highly relevant and possibly easily leveraged prevention for those at high risk. However, there is limited evidence for any particular dietary patterns or dietary features that are most important over time.7
Several dietary components have been shown to be associated with CRC risk,10 either as potentially chemopreventive (fiber, fruits and vegetables,11 dairy,12 supplemental vitamin D,13 calcium,14 and multivitamins15) or carcinogenic (red meat16 and alcohol17). Previous studies of veterans have similarly shown that higher intake of fiber and vitamin D reduced risk, and red meat is associated with an increased risk for finding CRC precursors during colonoscopy.18 However, these dietary categories are often analyzed in isolation. Studying healthy dietary patterns in aggregate may be more clinically relevant and easier to implement for prevention of CRC and its precursors.19-21 Healthy dietary patterns, such as the US Dietary Guidelines for Americans represented by the Healthy Eating Index (HEI), the Mediterranean diet (MD), and the Dietary Approaches to Stop Hypertension (DASH) diet, have been associated with lower risk for chronic disease.22-24 Despite the extant literature, no known studies have compared these dietary patterns for associations with risk of CRC precursor or CRC development among US veterans undergoing long-term screening and follow-up after a baseline colonoscopy.
The objective of this study was to test for associations between baseline scores of healthy dietary patterns and the most severe colonoscopy findings (MSCFs) over ≥ 10 years following a baseline screening colonoscopy in veterans.
Methods
Participants in the Cooperative Studies Program (CSP) #380 cohort study included 3121 asymptomatic veterans aged 50 to 75 years at baseline who had consented to initial screening colonoscopy between 1994 and 1997, with subsequent follow-up and surveillance.25 Prior to their colonoscopy, all participants completed a baseline study survey that included questions about cancer risk factors including family history of CRC, diet, physical activity, and medication use.
Included in this cross-sectional analysis were data from a sample of veteran participants of the CSP #380 cohort with 1 baseline colonoscopy, follow-up surveillance through 2009, a cancer risk factor survey collected at baseline, and complete demographic and clinical indicator data. Excluded from the analysis were 67 participants with insufficient responses to the dietary food frequency questionnaire (FFQ) and 31 participants with missing body mass index (BMI), 3023 veterans.
Measures
MSCF. The outcome of interest in this study was the MSCF recorded across all participant colonoscopies during the study period. MSCF was categorized as either (1) no neoplasia; (2) < 2 nonadvanced adenomas, including small adenomas (diameter < 10 mm) with tubular histology; or (3) advanced neoplasia (AN), which is characterized by adenomas > 10 mm in diameter, with villous histology, with high-grade dysplasia, or CRC.
Dietary patterns. Dietary pattern scores representing dietary quality and calculated based on recommendations of the US Dietary Guidelines for Americans using the HEI, MD, and DASH diets were independent variables.26-28 These 3 dietary patterns were chosen for their hypothesized relationship with CRC risk, but each weighs food categories differently (Appendix 1).22-24,29 Dietary pattern scores were calculated using the CSP #380 self-reported responses to 129 baseline survey questions adapted from a well-established and previously validated semiquantitative FFQ.30 The form was administered by mail twice to a sample of 127 participants at baseline and at 1 year. During this interval, men completed 1-week diet records twice, spaced about 6 months apart. Mean values for intake of most nutrients assessed by the 2 methods were similar. Intraclass correlation coefficients for the baseline and 1-year FFQ-assessed nutrient intakes that ranged from 0.47 for vitamin E (without supplements) to 0.80 for vitamin C (with supplements). Correlation coefficients between the energy-adjusted nutrient intakes were measured by diet records and the 1-year FFQ, which asked about diet during the year encompassing the diet records. Higher raw and percent scores indicated better alignment with recommendations from each respective dietary pattern. Percent scores were calculated as a standardizing method and used in analyses for ease of comparing the dietary patterns. Scoring can be found in Appendix 2.


Demographic characteristics and clinical indicators. Demographic characteristics included age categories, sex, and race/ethnicity. Clinical indicators included BMI, the number of comorbid conditions used to calculate the Charlson Comorbidity Index, family history of CRC in first-degree relatives, number of follow-up colonoscopies across the study period, and food-based vitamin D intake.31 These variables were chosen for their applicability found in previous CSP #380 cohort studies.18,32,33 Self-reported race and ethnicity were collapsed due to small numbers in some groups. The authors acknowledge these are distinct concepts and the variable has limited utility other than for controlling for systemic racism in the model.
Statistical Analyses
Descriptive statistics were used to describe distributional assumptions for all variables, including demographics, clinical indicators, colonoscopy results, and dietary patterns. Pairwise correlations between the total dietary pattern scores and food category scores were calculated with Pearson correlation (r).
Multinomial logistic regression models were created using SAS procedure LOGISTIC with the outcome of the categorical MSCF (no neoplasia, nonadvanced adenoma, or AN).34 A model was created for each independent predictor variable of interest (ie, the HEI, MD, or DASH percentage-standardized dietary pattern score and each food category comprising each dietary pattern score). All models were adjusted for age, sex, race/ethnicity, BMI, number of comorbidities, family history of CRC, number of follow-up colonoscopies, and estimated daily food-derived vitamin D intake. The demographic and clinical indicators were included in the models as they are known to be associated with CRC risk.18 The number of colonoscopies was included to control for surveillance intensity presuming risk for AN is reduced as polyps are removed. Because colonoscopy findings from an initial screening have unique clinical implications compared with follow- up and surveillance, MSCF was observed in 2 ways in sensitivity analyses: (1) baseline and (2) aggregate follow-up and surveillance only, excluding baseline findings.
Adjusted odds ratios (aORs) and 95% CIs for each of the MSCF outcomes with a reference finding of no neoplasia for the models are presented. We chose not to adjust for multiple comparisons across the different dietary patterns given the correlation between dietary pattern total and category scores but did adjust for multiple comparisons for dietary categories within each dietary pattern. Tests for statistical significance used α= .05 for the dietary pattern total scores and P values for the dietary category scores for each dietary pattern controlled for false discovery rate using the MULTTEST SAS procedure.35 All data manipulations and analyses were performed using SAS version 9.4.
Results
The study included 3023 patients. All were aged 50 to 75 years, 2923 (96.7%) were male and 2532 (83.8%) were non-Hispanic White (Table 1). Most participants were overweight or obese (n = 2535 [83.8%]), 2024 (67.0%) had ≤ 2 comorbidities, and 2602 (86.1%) had no family history of CRC. The MSCF for 1628 patients (53.9%) was no neoplasia, 966 patients (32.0%) was nonadvanced adenoma, and 429 participants (14.2%) had AN.

Mean percent scores were 58.5% for HEI, 38.2% for MD, and 63.1% for the DASH diet, with higher percentages indicating greater alignment with the recommendations for each diet (Table 2). All 3 dietary patterns scores standardized to percentages were strongly and significantly correlated in pairwise comparisons: HEI:MD, r = 0.62 (P < .001); HEI:DASH, r = 0.60 (P < .001); and MD:DASH, r = 0.72 (P < .001). Likewise, food category scores were significantly correlated across dietary patterns. For example, whole grain and fiber values from each dietary score were strongly correlated in pairwise comparisons: HEI Whole Grain:MD Grain, r = 0.64 (P < .001); HEI Whole Grain:DASH Fiber, r = 0.71 (P < .001); and MD Grain:DASH Fiber, r = 0.70 (P < .001).

Associations between individual participants' dietary pattern scores and the outcome of their pooled MSCF from baseline screening and ≥ 10 years of surveillance are presented in Table 3. For each single-point increases in dietary pattern scores (reflecting better dietary quality), aORs for nonadvanced adenoma vs no neoplasia were slightly lower but not statistically significantly: HEI, aOR, 1.00 (95% CI, 0.99-1.01); MD, aOR, 0.98 (95% CI, 0.94-1.02); and DASH, aOR, 0.99 (95% CI, 0.99-1.00). aORs for AN vs no neoplasia were slightly lower for each dietary pattern assessed, and only the MD and DASH scores were significantly different from 1.00: HEI, aOR, 1.00 (95% CI, 0.99-1.01); MD, aOR, 0.95 (95% CI, 0.90-1.00); and DASH, aOR, 0.99 (95% CI, 0.98-1.00).

We observed lower odds for nonadvanced adenoma and AN among all these dietary patterns when there was greater alignment with the recommended intake of whole grains and fiber. In separate models conducted using food categories comprising the dietary patterns as independent variables and after correcting for multiple tests, higher scores for the HEI Refined Grain category were associated with higher odds for nonadvanced adenoma (aOR, 1.03 [95% CI, 1.01-1.05]; P = .01) and AN (aOR, 1.05 [95% CI, 1.02-1.08]; P < .001). Higher scores for the HEI Whole Grain category were associated with lower odds for nonadvanced adenoma (aOR, 0.97 [95% CI, 0.95-0.99]; P = .01) and AN (aOR, 0.96 [95% CI, 0.93-0.99]; P = .01). Higher scores for the MD Grain category were significantly associated with lower odds for nonadvanced adenoma (aOR, 0.44 [95% CI, 0.26-0.75]; P = .002) and AN (aOR, 0.29 [95% CI, 0.14-0.62]; P = .001). The DASH Grains category also was significantly associated with lower odds for AN (aOR, 0.86 [95% CI, 0.78-0.95]; P = .002).
Discussion
In this study of 3023 veterans undergoing first-time screening colonoscopy and ≥ 10 years of surveillance, we found that healthy dietary patterns, as assessed by the MD and DASH diet, were significantly associated with lower risk of AN. Additionally, we identified lower odds for AN and nonadvanced adenoma compared with no neoplasia for higher grain scores for all the dietary patterns studied. Other food categories that comprise the dietary pattern scores had mixed associations with the MSCF outcomes. Several other studies have examined associations between dietary patterns and risk for CRC but to our knowledge, no studies have explored these associations among US veterans.
These results also indicate study participants had better than average (based on a 50% threshold) dietary quality according to the HEI and DASH diet scoring methods we used, but poor dietary quality according to the MD scoring method. The mean HEI scores for the present study were higher than a US Department of Agriculture study by Dong et al that compared dietary quality between veterans and nonveterans using the HEI, for which veterans’ expected HEI score was 45.6 of 100.8 This could be explained by the fact that the participants needed to be healthy to be eligible and those with healthier behaviors overall may have self-selected into the study due to motivation for screening during a time when screening was not yet commonplace. 36 Similarly, participants of the present study had higher adherence to the DASH diet (63.1%) than adolescents with diabetes in a study by Günther et al. Conversely, firefighters who were coached to use a Mediterranean-style dietary pattern and dietary had higher adherence to MD than did participants in this study.27
A closer examination of specific food category component scores that comprise the 3 distinct dietary patterns revealed mixed results from the multinomial modeling, which may have to do with the guideline thresholds used to calculate the dietary scores. When analyzed separately in the logistic regression models for their associations with nonadvanced adenomas and AN compared with no neoplasia, higher MD and DASH fruit scores (but not HEI fruit scores) were found to be significant. Other studies have had mixed findings when attempting to test for associations of fruit intake with adenoma recurrence.10,37
This study had some unexpected findings. Vegetable intake was not associated with nonadvanced adenomas or AN risk. Studies of food categories have consistently found vegetable (specifically cruciferous ones) intake to be linked with lower odds for cancers.38 Likewise, the red meat category, which was only a unique food category in the MD score, was not associated with nonadvanced adenomas or AN. Despite consistent literature suggesting higher intake of red meat and processed meats increases CRC risk, in 2019 the Nutritional Recommendations Consortium indicated that the evidence was weak.39,40 This study showed higher DASH diet scores for low-fat dairy, which were maximized when participants reported at least 50% of their dairy servings per day as being low-fat, had lower odds for AN. Yet, the MD scores for low-fat dairy had no association with either outcome; their calculation was based on total number of servings per week. This difference in findings suggests the fat intake ratio may be more relevant to CRC risk than intake quantity.
The literature is mixed regarding fatty acid intake and CRC risk, which may be relevant to both dairy and meat intake. One systematic review and meta-analysis found dietary fat and types of fatty acid intake had no association with CRC risk.41 However, a more recent meta-analysis that assessed both dietary intake and plasma levels of fatty acids did find some statistically significant differences for various types of fatty acids and CRC risk.42
The findings in the present study that grain intake is associated with lower odds for more severe colonoscopy findings among veterans are notable.43 Lieberman et al, using the CSP #380 data, found that cereal fiber intake was associated with a lower odds for AN compared with hyperplastic polyps (OR, 0.98 [95% CI, 0.96- 1.00]).18 Similarly, Hullings et al determined that older adults in the highest quintile of cereal fiber intake had significantly lower odds of CRC than those in lower odds for CRC when compared with lowest quintile (OR, 0.89 [95% CI, 0.83- 0.96]; P < .001).44 These findings support existing guidance that prioritizes whole grains as a key source of dietary fiber for CRC prevention.
A recent literature review on fiber, fat, and CRC risk suggested a consensus regarding one protective mechanism: dietary fiber from grains modulates the gut microbiota by promoting butyrate synthesis.45 Butyrate is a short-chain fatty acid that supports energy production in colonocytes and has tumor-suppressing properties.46 Our findings suggest there could be more to learn about the relationship between butyrate production and reduction of CRC risk through metabolomic studies that use measurements of plasma butyrate. These studies may examine associations between not just a singular food or food category, but rather food patterns that include fruits, vegetables, nuts and seeds, and whole grains known to promote butyrate production and plasma butyrate.47
Improved understanding of mechanisms and risk-modifying lifestyle factors such as dietary patterns may enhance prevention strategies. Identifying the collective chemopreventive characteristics of a specific dietary pattern (eg, MD) will be helpful to clinicians and health care staff to promote healthy eating to reduce cancer risk. More studies are needed to understand whether such promotion is more clinically applicable and effective for patients, as compared with eating more or less of specific foods (eg, more whole grains, less red meat). Furthermore, considering important environmental factors collectively beyond dietary patterns may offer a way to better tailor screening and implement a variety of lifestyle interventions. In the literature, this is often referred to as a teachable moment when patients’ attentions are captured and may position them to be more receptive to guidance.48
Limitations
This study has several important limitations and leaves opportunities for future studies that explore the role of dietary patterns and AN or CRC risk. First, the FFQ data used to calculate dietary pattern scores used in analysis were only captured at baseline, and there are nearly 3 decades across the study period. However, it is widely assumed that the diets of older adults, like those included in this study, remain stable over time which is appropriate given our sample population was aged 50 to 75 years when the baseline FFQ data were collected.49-51 Additionally, while the HEI is a well-documented, standard scoring method for dietary quality, there are multitudes of dietary pattern scoring approaches for MD and DASH.23,52,53 Finally, findings from this study using the sample of veterans may not be generalizable to a broader population. Future longitudinal studies that test for a clinically significant change threshold are warranted.
Conclusion
Results of this study suggest future research should further explore the effects of dietary patterns, particularly intake of specific food groups in combination, as opposed to individual nutrients or food items, on AN and CRC risk. Possible studies might explore these dietary patterns for their mechanistic role in altering the microbiome metabolism, which may influence CRC outcomes or include diet in a more comprehensive, holistic risk score that could be used to predict colonic neoplasia risk or in intervention studies that assess the effects of dietary changes on long-term CRC prevention. We suggest there are differences in people’s dietary intake patterns that might be important to consider when implementing tailored approaches to CRC risk mitigation.
- Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectalcancer deaths. N Engl J Med. 2012;366(8):687-696. doi:10.1056/NEJMoa1100370
- Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;369(12):1095-1105. doi:10.1056/NEJMoa1301969
- Bretthauer M, Løberg M, Wieszczy P, et al. Effect of colonoscopy screening on risks of colorectal cancer and related death. N Engl J Med. 2022;387(17):1547-1556. doi:10.1056/NEJMoa2208375
- Cottet V, Bonithon-Kopp C, Kronborg O, et al. Dietary patterns and the risk of colorectal adenoma recurrence in a European intervention trial. Eur J Cancer Prev. 2005;14(1):21.
- Miller PE, Lesko SM, Muscat JE, Lazarus P, Hartman TJ. Dietary patterns and colorectal adenoma and cancer risk: a review of the epidemiological evidence. Nutr Cancer. 2010;62(4):413-424. doi:10.1080/01635580903407114
- Godos J, Bella F, Torrisi A, Sciacca S, Galvano F, Grosso G. Dietary patterns and risk of colorectal adenoma: a systematic review and meta-analysis of observational studies. J Hum Nutr Diet Off J Br Diet Assoc. 2016;29(6):757-767. doi:10.1111/jhn.12395
- Haggar FA, Boushey RP. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg. 2009;22(4):191-197. doi:10.1055/s-0029-1242458
- Dong D, Stewart H, Carlson AC. An Examination of Veterans’ Diet Quality. U.S. Department of Agriculture, Economic Research Service; 2019:32.
- El-Halabi MM, Rex DK, Saito A, Eckert GJ, Kahi CJ. Defining adenoma detection rate benchmarks in average-risk male veterans. Gastrointest Endosc. 2019;89(1):137-143. doi:10.1016/j.gie.2018.08.021
- Alberts DS, Hess LM, eds. Fundamentals of Cancer Prevention. Springer International Publishing; 2019. doi:10.1007/978-3-030-15935-1
- Dahm CC, Keogh RH, Spencer EA, et al. Dietary fiber and colorectal cancer risk: a nested case-control study using food diaries. J Natl Cancer Inst. 2010;102(9):614-626. doi:10.1093/jnci/djq092
- Aune D, Lau R, Chan DSM, et al. Dairy products and colorectal cancer risk: a systematic review and metaanalysis of cohort studies. Ann Oncol. 2012;23(1):37-45. doi:10.1093/annonc/mdr269
- Lee JE, Li H, Chan AT, et al. Circulating levels of vitamin D and colon and rectal cancer: the Physicians’ Health Study and a meta-analysis of prospective studies. Cancer Prev Res Phila Pa. 2011;4(5):735-743. doi:10.1158/1940-6207.CAPR-10-0289
- Carroll C, Cooper K, Papaioannou D, Hind D, Pilgrim H, Tappenden P. Supplemental calcium in the chemoprevention of colorectal cancer: a systematic review and meta-analysis. Clin Ther. 2010;32(5):789-803. doi:10.1016/j.clinthera.2010.04.024
- Park Y, Spiegelman D, Hunter DJ, et al. Intakes of vitamins A, C, and E and use of multiple vitamin supplements and risk of colon cancer: a pooled analysis of prospective cohort studies. Cancer Causes Control CCC. 2010;21(11):1745- 1757. doi:10.1007/s10552-010-9549-y
- Alexander DD, Weed DL, Miller PE, Mohamed MA. Red meat and colorectal cancer: a quantitative update on the state of the epidemiologic science. J Am Coll Nutr. 2015;34(6):521-543. doi:10.1080/07315724.2014.992553
- Park SY, Wilkens LR, Setiawan VW, Monroe KR, Haiman CA, Le Marchand L. Alcohol intake and colorectal cancer risk in the multiethnic cohort study. Am J Epidemiol. 2019;188(1):67-76. doi:10.1093/aje/kwy208
- Lieberman DA. Risk Factors for advanced colonic neoplasia and hyperplastic polyps in asymptomatic individuals. JAMA. 2003;290(22):2959. doi:10.1001/jama.290.22.2959
- Archambault AN, Jeon J, Lin Y, et al. Risk stratification for early-onset colorectal cancer using a combination of genetic and environmental risk scores: an international multi-center study. J Natl Cancer Inst. 2022;114(4):528-539. doi:10.1093/jnci/djac003
- Carr PR, Weigl K, Edelmann D, et al. Estimation of absolute risk of colorectal cancer based on healthy lifestyle, genetic risk, and colonoscopy status in a populationbased study. Gastroenterology. 2020;159(1):129-138.e9. doi:10.1053/j.gastro.2020.03.016
- Sullivan BA, Qin X, Miller C, et al. Screening colonoscopy findings are associated with noncolorectal cancer mortality. Clin Transl Gastroenterol. 2022;13(4):e00479. doi:10.14309/ctg.0000000000000479
- Erben V, Carr PR, Holleczek B, Stegmaier C, Hoffmeister M, Brenner H. Dietary patterns and risk of advanced colorectal neoplasms: A large population based screening study in Germany. Prev Med. 2018;111:101-109. doi:10.1016/j.ypmed.2018.02.025
- Donovan MG, Selmin OI, Doetschman TC, Romagnolo DF. Mediterranean diet: prevention of colorectal cancer. Front Nutr. 2017;4:59. doi:10.3389/fnut.2017.00059
- Mohseni R, Mohseni F, Alizadeh S, Abbasi S. The Association of Dietary Approaches to Stop Hypertension (DASH) diet with the risk of colorectal cancer: a meta-analysis of observational studies.Nutr Cancer. 2020;72(5):778-790. doi:10.1080/01635581.2019.1651880
- Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. N Engl J Med. 2000;343(3):162-168. doi:10.1056/NEJM200007203430301
- Developing the Healthy Eating Index (HEI) | EGRP/ DCCPS/NCI/NIH. Accessed July 22, 2025. https://epi.grants.cancer.gov/hei/developing.html#2015c
- Reeve E, Piccici F, Feairheller DL. Validation of a Mediterranean diet scoring system for intervention based research. J Nutr Med Diet Care. 2021;7(1):053. doi:10.23937/2572-3278/1510053
- Günther AL, Liese AD, Bell RA, et al. ASSOCIATION BETWEEN THE DIETARY APPROACHES TO HYPERTENSION (DASH) DIET AND HYPERTENSION IN YOUTH WITH DIABETES. Hypertens Dallas Tex 1979. 2009;53(1):6-12. doi:10.1161/HYPERTENSIONAHA.108.116665
- Buckland G, Agudo A, Luján L, et al. Adherence to a Mediterranean diet and risk of gastric adenocarcinoma within the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort study. Am J Clin Nutr. 2010;91(2):381- 390. doi:10.3945/ajcn.2009.28209
- Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135(10):1114-1126. doi:10.1093/oxfordjournals.aje.a116211
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi:10.1016/0021-9681(87)90171-8
- Lieberman DA, Weiss DG, Harford WV, et al. Fiveyear colon surveillance after screening colonoscopy. Gastroenterology. 2007;133(4):1077-1085. doi:10.1053/j.gastro.2007.07.006
- Lieberman D, Sullivan BA, Hauser ER, et al. Baseline colonoscopy findings associated with 10-year outcomes in a screening cohort undergoing colonoscopy surveillance. Gastroenterology. 2020;158(4):862-874.e8. doi:10.1053/j.gastro.2019.07.052
- PROC LOGISTIC: PROC LOGISTIC Statement : SAS/STAT(R) 9.22 User’s Guide. Accessed July 22, 2025. https://support.sas.com/documentation/cdl/en/statug/63347/HTML/default/viewer.htm#statug_logistic_sect004.htm
- PROC MULTTEST: PROC MULTTEST Statement : SAS/ STAT(R) 9.22 User’s Guide. Accessed July 22, 2025. https://support.sas.com/documentation/cdl/en/statug/63347/HTML/default/viewer.htm#statug_multtest_sect005.htm
- Elston DM. Participation bias, self-selection bias, and response bias. J Am Acad Dermatol. Published online June 18, 2021. doi:10.1016/j.jaad.2021.06.025
- Sansbury LB, Wanke K, Albert PS, et al. The effect of strict adherence to a high-fiber, high-fruit and -vegetable, and low-fat eating pattern on adenoma recurrence. Am J Epidemiol. 2009;170(5):576-584. doi:10.1093/aje/kwp169
- Borgas P, Gonzalez G, Veselkov K, Mirnezami R. Phytochemically rich dietary components and the risk of colorectal cancer: A systematic review and meta-analysis of observational studies. World J Clin Oncol. 2021;12(6):482- 499. doi:10.5306/wjco.v12.i6.482
- Papadimitriou N, Markozannes G, Kanellopoulou A, et al. An umbrella review of the evidence associating diet and cancer risk at 11 anatomical sites. Nat Commun. 2021;12(1):4579. doi:10.1038/s41467-021-24861-8
- Johnston BC, Zeraatkar D, Han MA, et al. Unprocessed red meat and processed meat consumption: dietary guideline recommendations from the nutritional recommendations (NutriRECS) Consortium. Ann Intern Med. 2019;171(10):756-764. doi:10.7326/M19-1621
- Kim M, Park K. Dietary fat intake and risk of colorectal cancer: a systematic review and meta-analysis of prospective studies. Nutrients. 2018;10(12):1963. doi:10.3390/nu10121963
- Lu Y, Li D, Wang L, et al. Comprehensive investigation on associations between dietary intake and blood levels of fatty acids and colorectal cancer risk. Nutrients. 2023;15(3):730. doi:10.3390/nu15030730
- Gherasim A, Arhire LI, Ni.a O, Popa AD, Graur M, Mihalache L. The relationship between lifestyle components and dietary patterns. Proc Nutr Soc. 2020;79(3):311-323. doi:10.1017/S0029665120006898
- Hullings AG, Sinha R, Liao LM, Freedman ND, Graubard BI, Loftfield E. Whole grain and dietary fiber intake and risk of colorectal cancer in the NIH-AARP Diet and Health Study cohort. Am J Clin Nutr. 2020;112(3):603- 612. doi:10.1093/ajcn/nqaa161
- Ocvirk S, Wilson AS, Appolonia CN, Thomas TK, O’Keefe SJD. Fiber, fat, and colorectal cancer: new insight into modifiable dietary risk factors. Curr Gastroenterol Rep. 2019;21(11):62. doi:10.1007/s11894-019-0725-2
- O’Keefe SJD. Diet, microorganisms and their metabolites, and colon cancer. Nat Rev Gastroenterol Hepatol. 2016;13(12):691-706. doi:10.1038/nrgastro.2016.165
- The health benefits and side effects of Butyrate Cleveland Clinic. July 11, 2022. Accessed July 22, 2025. https://health.clevelandclinic.org/butyrate-benefits/
- Knudsen MD, Wang L, Wang K, et al. Changes in lifestyle factors after endoscopic screening: a prospective study in the United States. Clin Gastroenterol Hepatol Off ClinPract J Am Gastroenterol Assoc. 2022;20(6):e1240-e1249. doi:10.1016/j.cgh.2021.07.014
- Thorpe MG, Milte CM, Crawford D, McNaughton SA. Education and lifestyle predict change in dietary patterns and diet quality of adults 55 years and over. Nutr J. 2019;18(1):67. doi:10.1186/s12937-019-0495-6
- Chapman K, Ogden J. How do people change their diet?: an exploration into mechanisms of dietary change. J Health Psychol. 2009;14(8):1229-1242. doi:10.1177/1359105309342289
- Djoussé L, Petrone AB, Weir NL, et al. Repeated versus single measurement of plasma omega-3 fatty acids and risk of heart failure. Eur J Nutr. 2014;53(6):1403-1408. doi:10.1007/s00394-013-0642-3
- Bach-Faig A, Berry EM, Lairon D, et al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011;14(12A):2274-2284. doi:10.1017/S1368980011002515
- Miller PE, Cross AJ, Subar AF, et al. Comparison of 4 established DASH diet indexes: examining associations of index scores and colorectal cancer123. Am J Clin Nutr. 2013;98(3):794-803. doi:10.3945/ajcn.113.063602
- Krebs-Smith SM, Pannucci TE, Subar AF, et al. Update of the Healthy Eating Index: HEI-2015. J Acad Nutr Diet. 2018;118(9):1591-1602. doi:10.1016/j.jand.2018.05.021
- P.R. Pehrsson, Cutrufelli RL, Gebhardt SE, et al. USDA Database for the Added Sugars Content of Selected Foods. USDA; 2005. www.ars.usda.gov/nutrientdata
- Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectalcancer deaths. N Engl J Med. 2012;366(8):687-696. doi:10.1056/NEJMoa1100370
- Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;369(12):1095-1105. doi:10.1056/NEJMoa1301969
- Bretthauer M, Løberg M, Wieszczy P, et al. Effect of colonoscopy screening on risks of colorectal cancer and related death. N Engl J Med. 2022;387(17):1547-1556. doi:10.1056/NEJMoa2208375
- Cottet V, Bonithon-Kopp C, Kronborg O, et al. Dietary patterns and the risk of colorectal adenoma recurrence in a European intervention trial. Eur J Cancer Prev. 2005;14(1):21.
- Miller PE, Lesko SM, Muscat JE, Lazarus P, Hartman TJ. Dietary patterns and colorectal adenoma and cancer risk: a review of the epidemiological evidence. Nutr Cancer. 2010;62(4):413-424. doi:10.1080/01635580903407114
- Godos J, Bella F, Torrisi A, Sciacca S, Galvano F, Grosso G. Dietary patterns and risk of colorectal adenoma: a systematic review and meta-analysis of observational studies. J Hum Nutr Diet Off J Br Diet Assoc. 2016;29(6):757-767. doi:10.1111/jhn.12395
- Haggar FA, Boushey RP. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg. 2009;22(4):191-197. doi:10.1055/s-0029-1242458
- Dong D, Stewart H, Carlson AC. An Examination of Veterans’ Diet Quality. U.S. Department of Agriculture, Economic Research Service; 2019:32.
- El-Halabi MM, Rex DK, Saito A, Eckert GJ, Kahi CJ. Defining adenoma detection rate benchmarks in average-risk male veterans. Gastrointest Endosc. 2019;89(1):137-143. doi:10.1016/j.gie.2018.08.021
- Alberts DS, Hess LM, eds. Fundamentals of Cancer Prevention. Springer International Publishing; 2019. doi:10.1007/978-3-030-15935-1
- Dahm CC, Keogh RH, Spencer EA, et al. Dietary fiber and colorectal cancer risk: a nested case-control study using food diaries. J Natl Cancer Inst. 2010;102(9):614-626. doi:10.1093/jnci/djq092
- Aune D, Lau R, Chan DSM, et al. Dairy products and colorectal cancer risk: a systematic review and metaanalysis of cohort studies. Ann Oncol. 2012;23(1):37-45. doi:10.1093/annonc/mdr269
- Lee JE, Li H, Chan AT, et al. Circulating levels of vitamin D and colon and rectal cancer: the Physicians’ Health Study and a meta-analysis of prospective studies. Cancer Prev Res Phila Pa. 2011;4(5):735-743. doi:10.1158/1940-6207.CAPR-10-0289
- Carroll C, Cooper K, Papaioannou D, Hind D, Pilgrim H, Tappenden P. Supplemental calcium in the chemoprevention of colorectal cancer: a systematic review and meta-analysis. Clin Ther. 2010;32(5):789-803. doi:10.1016/j.clinthera.2010.04.024
- Park Y, Spiegelman D, Hunter DJ, et al. Intakes of vitamins A, C, and E and use of multiple vitamin supplements and risk of colon cancer: a pooled analysis of prospective cohort studies. Cancer Causes Control CCC. 2010;21(11):1745- 1757. doi:10.1007/s10552-010-9549-y
- Alexander DD, Weed DL, Miller PE, Mohamed MA. Red meat and colorectal cancer: a quantitative update on the state of the epidemiologic science. J Am Coll Nutr. 2015;34(6):521-543. doi:10.1080/07315724.2014.992553
- Park SY, Wilkens LR, Setiawan VW, Monroe KR, Haiman CA, Le Marchand L. Alcohol intake and colorectal cancer risk in the multiethnic cohort study. Am J Epidemiol. 2019;188(1):67-76. doi:10.1093/aje/kwy208
- Lieberman DA. Risk Factors for advanced colonic neoplasia and hyperplastic polyps in asymptomatic individuals. JAMA. 2003;290(22):2959. doi:10.1001/jama.290.22.2959
- Archambault AN, Jeon J, Lin Y, et al. Risk stratification for early-onset colorectal cancer using a combination of genetic and environmental risk scores: an international multi-center study. J Natl Cancer Inst. 2022;114(4):528-539. doi:10.1093/jnci/djac003
- Carr PR, Weigl K, Edelmann D, et al. Estimation of absolute risk of colorectal cancer based on healthy lifestyle, genetic risk, and colonoscopy status in a populationbased study. Gastroenterology. 2020;159(1):129-138.e9. doi:10.1053/j.gastro.2020.03.016
- Sullivan BA, Qin X, Miller C, et al. Screening colonoscopy findings are associated with noncolorectal cancer mortality. Clin Transl Gastroenterol. 2022;13(4):e00479. doi:10.14309/ctg.0000000000000479
- Erben V, Carr PR, Holleczek B, Stegmaier C, Hoffmeister M, Brenner H. Dietary patterns and risk of advanced colorectal neoplasms: A large population based screening study in Germany. Prev Med. 2018;111:101-109. doi:10.1016/j.ypmed.2018.02.025
- Donovan MG, Selmin OI, Doetschman TC, Romagnolo DF. Mediterranean diet: prevention of colorectal cancer. Front Nutr. 2017;4:59. doi:10.3389/fnut.2017.00059
- Mohseni R, Mohseni F, Alizadeh S, Abbasi S. The Association of Dietary Approaches to Stop Hypertension (DASH) diet with the risk of colorectal cancer: a meta-analysis of observational studies.Nutr Cancer. 2020;72(5):778-790. doi:10.1080/01635581.2019.1651880
- Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. N Engl J Med. 2000;343(3):162-168. doi:10.1056/NEJM200007203430301
- Developing the Healthy Eating Index (HEI) | EGRP/ DCCPS/NCI/NIH. Accessed July 22, 2025. https://epi.grants.cancer.gov/hei/developing.html#2015c
- Reeve E, Piccici F, Feairheller DL. Validation of a Mediterranean diet scoring system for intervention based research. J Nutr Med Diet Care. 2021;7(1):053. doi:10.23937/2572-3278/1510053
- Günther AL, Liese AD, Bell RA, et al. ASSOCIATION BETWEEN THE DIETARY APPROACHES TO HYPERTENSION (DASH) DIET AND HYPERTENSION IN YOUTH WITH DIABETES. Hypertens Dallas Tex 1979. 2009;53(1):6-12. doi:10.1161/HYPERTENSIONAHA.108.116665
- Buckland G, Agudo A, Luján L, et al. Adherence to a Mediterranean diet and risk of gastric adenocarcinoma within the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort study. Am J Clin Nutr. 2010;91(2):381- 390. doi:10.3945/ajcn.2009.28209
- Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135(10):1114-1126. doi:10.1093/oxfordjournals.aje.a116211
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi:10.1016/0021-9681(87)90171-8
- Lieberman DA, Weiss DG, Harford WV, et al. Fiveyear colon surveillance after screening colonoscopy. Gastroenterology. 2007;133(4):1077-1085. doi:10.1053/j.gastro.2007.07.006
- Lieberman D, Sullivan BA, Hauser ER, et al. Baseline colonoscopy findings associated with 10-year outcomes in a screening cohort undergoing colonoscopy surveillance. Gastroenterology. 2020;158(4):862-874.e8. doi:10.1053/j.gastro.2019.07.052
- PROC LOGISTIC: PROC LOGISTIC Statement : SAS/STAT(R) 9.22 User’s Guide. Accessed July 22, 2025. https://support.sas.com/documentation/cdl/en/statug/63347/HTML/default/viewer.htm#statug_logistic_sect004.htm
- PROC MULTTEST: PROC MULTTEST Statement : SAS/ STAT(R) 9.22 User’s Guide. Accessed July 22, 2025. https://support.sas.com/documentation/cdl/en/statug/63347/HTML/default/viewer.htm#statug_multtest_sect005.htm
- Elston DM. Participation bias, self-selection bias, and response bias. J Am Acad Dermatol. Published online June 18, 2021. doi:10.1016/j.jaad.2021.06.025
- Sansbury LB, Wanke K, Albert PS, et al. The effect of strict adherence to a high-fiber, high-fruit and -vegetable, and low-fat eating pattern on adenoma recurrence. Am J Epidemiol. 2009;170(5):576-584. doi:10.1093/aje/kwp169
- Borgas P, Gonzalez G, Veselkov K, Mirnezami R. Phytochemically rich dietary components and the risk of colorectal cancer: A systematic review and meta-analysis of observational studies. World J Clin Oncol. 2021;12(6):482- 499. doi:10.5306/wjco.v12.i6.482
- Papadimitriou N, Markozannes G, Kanellopoulou A, et al. An umbrella review of the evidence associating diet and cancer risk at 11 anatomical sites. Nat Commun. 2021;12(1):4579. doi:10.1038/s41467-021-24861-8
- Johnston BC, Zeraatkar D, Han MA, et al. Unprocessed red meat and processed meat consumption: dietary guideline recommendations from the nutritional recommendations (NutriRECS) Consortium. Ann Intern Med. 2019;171(10):756-764. doi:10.7326/M19-1621
- Kim M, Park K. Dietary fat intake and risk of colorectal cancer: a systematic review and meta-analysis of prospective studies. Nutrients. 2018;10(12):1963. doi:10.3390/nu10121963
- Lu Y, Li D, Wang L, et al. Comprehensive investigation on associations between dietary intake and blood levels of fatty acids and colorectal cancer risk. Nutrients. 2023;15(3):730. doi:10.3390/nu15030730
- Gherasim A, Arhire LI, Ni.a O, Popa AD, Graur M, Mihalache L. The relationship between lifestyle components and dietary patterns. Proc Nutr Soc. 2020;79(3):311-323. doi:10.1017/S0029665120006898
- Hullings AG, Sinha R, Liao LM, Freedman ND, Graubard BI, Loftfield E. Whole grain and dietary fiber intake and risk of colorectal cancer in the NIH-AARP Diet and Health Study cohort. Am J Clin Nutr. 2020;112(3):603- 612. doi:10.1093/ajcn/nqaa161
- Ocvirk S, Wilson AS, Appolonia CN, Thomas TK, O’Keefe SJD. Fiber, fat, and colorectal cancer: new insight into modifiable dietary risk factors. Curr Gastroenterol Rep. 2019;21(11):62. doi:10.1007/s11894-019-0725-2
- O’Keefe SJD. Diet, microorganisms and their metabolites, and colon cancer. Nat Rev Gastroenterol Hepatol. 2016;13(12):691-706. doi:10.1038/nrgastro.2016.165
- The health benefits and side effects of Butyrate Cleveland Clinic. July 11, 2022. Accessed July 22, 2025. https://health.clevelandclinic.org/butyrate-benefits/
- Knudsen MD, Wang L, Wang K, et al. Changes in lifestyle factors after endoscopic screening: a prospective study in the United States. Clin Gastroenterol Hepatol Off ClinPract J Am Gastroenterol Assoc. 2022;20(6):e1240-e1249. doi:10.1016/j.cgh.2021.07.014
- Thorpe MG, Milte CM, Crawford D, McNaughton SA. Education and lifestyle predict change in dietary patterns and diet quality of adults 55 years and over. Nutr J. 2019;18(1):67. doi:10.1186/s12937-019-0495-6
- Chapman K, Ogden J. How do people change their diet?: an exploration into mechanisms of dietary change. J Health Psychol. 2009;14(8):1229-1242. doi:10.1177/1359105309342289
- Djoussé L, Petrone AB, Weir NL, et al. Repeated versus single measurement of plasma omega-3 fatty acids and risk of heart failure. Eur J Nutr. 2014;53(6):1403-1408. doi:10.1007/s00394-013-0642-3
- Bach-Faig A, Berry EM, Lairon D, et al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011;14(12A):2274-2284. doi:10.1017/S1368980011002515
- Miller PE, Cross AJ, Subar AF, et al. Comparison of 4 established DASH diet indexes: examining associations of index scores and colorectal cancer123. Am J Clin Nutr. 2013;98(3):794-803. doi:10.3945/ajcn.113.063602
- Krebs-Smith SM, Pannucci TE, Subar AF, et al. Update of the Healthy Eating Index: HEI-2015. J Acad Nutr Diet. 2018;118(9):1591-1602. doi:10.1016/j.jand.2018.05.021
- P.R. Pehrsson, Cutrufelli RL, Gebhardt SE, et al. USDA Database for the Added Sugars Content of Selected Foods. USDA; 2005. www.ars.usda.gov/nutrientdata
Associations Between Prescreening Dietary Patterns and Longitudinal Colonoscopy Outcomes in Veterans
Associations Between Prescreening Dietary Patterns and Longitudinal Colonoscopy Outcomes in Veterans
Earlier Vaccinations Helped Limit Marine Adenovirus Outbreak
Earlier Vaccinations Helped Limit Marine Adenovirus Outbreak
During an adenovirus (AdV) outbreak among recruits and staff at the Marine Corps Recruit Depot (MCRD) in San Diego, an investigation revealed that the earlier individuals working at the site received vaccination, the better. The clinical team found that accelerating the vaccination schedule could help prevent further outbreaks, medical separations, and training disruption.
From July 1, 2024, through September 23, 2024, a total of 212 trainees and staff developed AdV and 28 were hospitalized. Nine patients were hospitalized with AdV pneumonia within a 2-week period; 3 were admitted to the intensive care unit. Outpatient acute respiratory disease (ARD) cases also increased, with recruits accounting for nearly 97% of the AdV outbreak cases.
AdV is a frequent cause of illness among military recruits. Research has found that up to 80% of cases of febrile ARD in recruits are due to AdV, and 20% result in hospitalization.
The military developed and implemented a live, oral vaccine against AdV serotypes 4 and 7 (most common in recruits) starting in the 1970s, reducing febrile respiratory illness in recruit training sites by 50% and AdV infection by > 90%. However, the manufacturer halted production of the vaccine in 1995. By 1999, vaccine supply was depleted, and ARD cases rose. A replacement vaccine introduced in 2011 proved 99% effective, leading to a dramatic 100-fold decline in AdV disease among military trainees.
While the vaccine is effective, outbreaks are still possible among closely congregating groups like military trainees. AdV pneumonia cases spiked as the virus spread through the training companies and into new companies when they arrived at the MCRD in early July 2024. Most new infections were in recruits who had missed the AdV vaccination day.
Early symptoms of AdV may be very mild, and some recruits were likely already symptomatic when vaccinated. Aggressive environmental cleaning, separation of sick and well recruits, masking, and other nonpharmaceutical interventions did not slow the spread.
The preventive medicine and public health teams noted that AdV vaccination was being administered 11 days postarrival, to allow for pregnancy testing, and for assessing vaccine titers. US Department of Defense regulations do not dictate precise vaccination schedules. Implementation of the regulation varies among military training sites.
After reviewing other training sites’ vaccine timing schedules (most required vaccination by day 6 postarrival) and determining the time required for immunity, the medical teams at MCRD recommended shifting AdV vaccine administration, along with other standard vaccines, from day 11 to day 1 postarrival. Two weeks after the schedule change, overall incidence began declining rapidly.
Nearly 75% of patients had coinfections with other respiratory pathogens, most notably seasonal coronaviruses, COVID-19, and rhinovirus/enterovirus, suggesting that infection with AdV may increase susceptibility to other viruses, a finding that has not been identified in previous AdV outbreaks. Newly increased testing sensitivity associated with multiplex respiratory pathogen PCR availability may have been a factor in coinfection identification during this outbreak.
AdV is a significant medical threat to military recruits. Early vaccination, the investigators advise, should remain “a central tenet for prevention and control of communicable diseases in these high-risk, congregate settings.”
During an adenovirus (AdV) outbreak among recruits and staff at the Marine Corps Recruit Depot (MCRD) in San Diego, an investigation revealed that the earlier individuals working at the site received vaccination, the better. The clinical team found that accelerating the vaccination schedule could help prevent further outbreaks, medical separations, and training disruption.
From July 1, 2024, through September 23, 2024, a total of 212 trainees and staff developed AdV and 28 were hospitalized. Nine patients were hospitalized with AdV pneumonia within a 2-week period; 3 were admitted to the intensive care unit. Outpatient acute respiratory disease (ARD) cases also increased, with recruits accounting for nearly 97% of the AdV outbreak cases.
AdV is a frequent cause of illness among military recruits. Research has found that up to 80% of cases of febrile ARD in recruits are due to AdV, and 20% result in hospitalization.
The military developed and implemented a live, oral vaccine against AdV serotypes 4 and 7 (most common in recruits) starting in the 1970s, reducing febrile respiratory illness in recruit training sites by 50% and AdV infection by > 90%. However, the manufacturer halted production of the vaccine in 1995. By 1999, vaccine supply was depleted, and ARD cases rose. A replacement vaccine introduced in 2011 proved 99% effective, leading to a dramatic 100-fold decline in AdV disease among military trainees.
While the vaccine is effective, outbreaks are still possible among closely congregating groups like military trainees. AdV pneumonia cases spiked as the virus spread through the training companies and into new companies when they arrived at the MCRD in early July 2024. Most new infections were in recruits who had missed the AdV vaccination day.
Early symptoms of AdV may be very mild, and some recruits were likely already symptomatic when vaccinated. Aggressive environmental cleaning, separation of sick and well recruits, masking, and other nonpharmaceutical interventions did not slow the spread.
The preventive medicine and public health teams noted that AdV vaccination was being administered 11 days postarrival, to allow for pregnancy testing, and for assessing vaccine titers. US Department of Defense regulations do not dictate precise vaccination schedules. Implementation of the regulation varies among military training sites.
After reviewing other training sites’ vaccine timing schedules (most required vaccination by day 6 postarrival) and determining the time required for immunity, the medical teams at MCRD recommended shifting AdV vaccine administration, along with other standard vaccines, from day 11 to day 1 postarrival. Two weeks after the schedule change, overall incidence began declining rapidly.
Nearly 75% of patients had coinfections with other respiratory pathogens, most notably seasonal coronaviruses, COVID-19, and rhinovirus/enterovirus, suggesting that infection with AdV may increase susceptibility to other viruses, a finding that has not been identified in previous AdV outbreaks. Newly increased testing sensitivity associated with multiplex respiratory pathogen PCR availability may have been a factor in coinfection identification during this outbreak.
AdV is a significant medical threat to military recruits. Early vaccination, the investigators advise, should remain “a central tenet for prevention and control of communicable diseases in these high-risk, congregate settings.”
During an adenovirus (AdV) outbreak among recruits and staff at the Marine Corps Recruit Depot (MCRD) in San Diego, an investigation revealed that the earlier individuals working at the site received vaccination, the better. The clinical team found that accelerating the vaccination schedule could help prevent further outbreaks, medical separations, and training disruption.
From July 1, 2024, through September 23, 2024, a total of 212 trainees and staff developed AdV and 28 were hospitalized. Nine patients were hospitalized with AdV pneumonia within a 2-week period; 3 were admitted to the intensive care unit. Outpatient acute respiratory disease (ARD) cases also increased, with recruits accounting for nearly 97% of the AdV outbreak cases.
AdV is a frequent cause of illness among military recruits. Research has found that up to 80% of cases of febrile ARD in recruits are due to AdV, and 20% result in hospitalization.
The military developed and implemented a live, oral vaccine against AdV serotypes 4 and 7 (most common in recruits) starting in the 1970s, reducing febrile respiratory illness in recruit training sites by 50% and AdV infection by > 90%. However, the manufacturer halted production of the vaccine in 1995. By 1999, vaccine supply was depleted, and ARD cases rose. A replacement vaccine introduced in 2011 proved 99% effective, leading to a dramatic 100-fold decline in AdV disease among military trainees.
While the vaccine is effective, outbreaks are still possible among closely congregating groups like military trainees. AdV pneumonia cases spiked as the virus spread through the training companies and into new companies when they arrived at the MCRD in early July 2024. Most new infections were in recruits who had missed the AdV vaccination day.
Early symptoms of AdV may be very mild, and some recruits were likely already symptomatic when vaccinated. Aggressive environmental cleaning, separation of sick and well recruits, masking, and other nonpharmaceutical interventions did not slow the spread.
The preventive medicine and public health teams noted that AdV vaccination was being administered 11 days postarrival, to allow for pregnancy testing, and for assessing vaccine titers. US Department of Defense regulations do not dictate precise vaccination schedules. Implementation of the regulation varies among military training sites.
After reviewing other training sites’ vaccine timing schedules (most required vaccination by day 6 postarrival) and determining the time required for immunity, the medical teams at MCRD recommended shifting AdV vaccine administration, along with other standard vaccines, from day 11 to day 1 postarrival. Two weeks after the schedule change, overall incidence began declining rapidly.
Nearly 75% of patients had coinfections with other respiratory pathogens, most notably seasonal coronaviruses, COVID-19, and rhinovirus/enterovirus, suggesting that infection with AdV may increase susceptibility to other viruses, a finding that has not been identified in previous AdV outbreaks. Newly increased testing sensitivity associated with multiplex respiratory pathogen PCR availability may have been a factor in coinfection identification during this outbreak.
AdV is a significant medical threat to military recruits. Early vaccination, the investigators advise, should remain “a central tenet for prevention and control of communicable diseases in these high-risk, congregate settings.”
Earlier Vaccinations Helped Limit Marine Adenovirus Outbreak
Earlier Vaccinations Helped Limit Marine Adenovirus Outbreak
Alarming Rise in Early-Onset GI Cancers Calls for Early Screening, Lifestyle Change
, said the authors of a JAMA review.
In the US, early-onset GI cancers are increasing faster than any other type of early-onset cancer, including breast cancer. The trend is not limited to colorectal cancer (CRC). Gastric, pancreatic, esophageal, as well as many biliary tract and appendix cancers, are also on the rise in young adults, Kimmie Ng, MD, MPH, and Thejus Jayakrishnan, MD, both with Dana-Farber Cancer Institute, Boston, noted in their article.
The increase in early-onset GI cancers follows a “birth cohort effect,” with generational variation in risk, suggesting a potential association with changes in environmental exposures, Ng explained in an accompanying JAMA podcast.
All these GI cancers link strongly to multiple modifiable risk factors, and it is a “top area of investigation to determine exactly what environmental exposures are at play,” Ng added.
For many of these GI cancers, obesity has been the “leading hypothesis” given that rising rates seem to parallel the increase in incidence of these early-onset GI cancers, Ng explained.
“But we also have evidence, particularly strong for colorectal cancer, that dietary patterns, such as consuming a Western diet, as well as sedentary behavior and lifestyles seem to be associated with a significantly higher risk of developing these cancers at an age under 50,” Ng said.
Rising Incidence
Globally, among early-onset GI cancers reported in 2022, CRC was the most common (54%), followed by gastric cancer (24%), esophageal cancer (13%), and pancreatic cancer (9%).
In the US in 2022, 20,805 individuals were diagnosed with early-onset CRC, 2689 with early-onset gastric cancer, 2657 with early-onset pancreatic cancer, and 875 with early-onset esophageal cancer.
Since the mid-1990s, CRC among adults of all ages in the US declined by 1.3%-4.2% annually but early-onset CRC increased by roughly 2% per year in both men and women, and currently makes up about 14% of all CRC cases.
Early-onset pancreatic cancer and esophageal cancer each currently make up about 5% of all cases of these cancers in the US.
Between 2010 and 2019, the number of newly diagnosed cases of early-onset GI cancers rose by nearly about 15%, with Black, Hispanic, Indigenous ancestry, and women disproportionately affected, Ng and coauthors noted in a related review published in the British Journal of Surgery.
Modifiable and Nonmodifiable Risk Factors
Along with obesity and poor diet, other modifiable risk factors for early-onset GI cancers include sedentary lifestyle, cigarette smoking, and alcohol consumption.
Nonmodifiable risk factors include family history, hereditary cancer syndromes such as Lynch syndrome and inflammatory bowel disease.
Roughly 15%-30% of early-onset GI cancers have pathogenic germline variants in genes such as DNA mismatch repair genes and BRCA1/2.
All individuals with early-onset GI cancers should undergo germline and somatic genetic testing to guide treatment, screen for other cancers (eg, endometrial cancer in Lynch syndrome), and assess familial risk, Ng and Jayakrishnan advised.
Treatment Challenges
Treatment for early-onset GI cancers is generally similar to later-onset GI cancers and prognosis for patients with early-onset GI cancers is “similar to or worse” than that for patients with later-onset GI cancers, highlighting the need for improved methods of prevention and early detection, the authors said.
Ng noted that younger cancer patients often face more challenges after diagnosis than older patients and benefit from multidisciplinary care, including referral for fertility counseling and preservation if appropriate, and psychosocial support.
“It is very difficult and challenging to receive a cancer diagnosis no matter what age you are, but when a person is diagnosed in their 20s, 30s, or 40s, there are unique challenges,” Ng said.
Studies have documented “much higher levels of psychosocial distress, depression and anxiety” in early-onset cancer patients, “and they also often experience more financial toxicity, disruptions in their education as well as their career and there may be fertility concerns,” Ng added.
Diagnostic Delays and Screening
Currently, screening is not recommended for most early-onset GI cancers — with the exception of CRC, with screening recommended for average-risk adults in the US starting at age 45.
Yet, despite this recommendation, fewer than 1 in 5 (19.7%) US adults aged 45-49 years were screened in 2021, indicating a significant gap in early detection efforts.
High-risk individuals, such as those with Lynch syndrome, a first-degree relative with CRC, or advanced colorectal adenoma, should begin CRC screening earlier, at an age determined by the specific risk factor.
“Studies have shown significant delays in diagnosis among younger patients. It’s important that prompt diagnosis happens so that these patients do not end up being diagnosed with advanced or metastatic stages of cancer, as they often are,” Ng said.
“Screening adherence is absolutely critical,” co-author Jayakrishnan added in a news release.
“We have strong evidence that colorectal cancer screening saves lives by reducing both the number of people who develop colorectal cancer and the number of people who die from it. Each missed screening is a lost opportunity to detect cancer early when it is more treatable, or to prevent cancer altogether by identifying and removing precancerous polyps,” Jayakrishnan said.This research had no funding. Ng reported receipt of nonfinancial support from Pharmavite, institutional grants from Janssen, and personal fees from Bayer, Seagen, GlaxoSmithKline, Pfizer, CytomX, Jazz Pharmaceuticals, Revolution Medicines, Redesign Health, AbbVie, Etiome, and CRICO. Ng is an associate editor of JAMA but was not involved in any of the decisions regarding review of the manuscript or its acceptance. Jayakrishnan had no disclosures.
A version of this article appeared on Medscape.com.
, said the authors of a JAMA review.
In the US, early-onset GI cancers are increasing faster than any other type of early-onset cancer, including breast cancer. The trend is not limited to colorectal cancer (CRC). Gastric, pancreatic, esophageal, as well as many biliary tract and appendix cancers, are also on the rise in young adults, Kimmie Ng, MD, MPH, and Thejus Jayakrishnan, MD, both with Dana-Farber Cancer Institute, Boston, noted in their article.
The increase in early-onset GI cancers follows a “birth cohort effect,” with generational variation in risk, suggesting a potential association with changes in environmental exposures, Ng explained in an accompanying JAMA podcast.
All these GI cancers link strongly to multiple modifiable risk factors, and it is a “top area of investigation to determine exactly what environmental exposures are at play,” Ng added.
For many of these GI cancers, obesity has been the “leading hypothesis” given that rising rates seem to parallel the increase in incidence of these early-onset GI cancers, Ng explained.
“But we also have evidence, particularly strong for colorectal cancer, that dietary patterns, such as consuming a Western diet, as well as sedentary behavior and lifestyles seem to be associated with a significantly higher risk of developing these cancers at an age under 50,” Ng said.
Rising Incidence
Globally, among early-onset GI cancers reported in 2022, CRC was the most common (54%), followed by gastric cancer (24%), esophageal cancer (13%), and pancreatic cancer (9%).
In the US in 2022, 20,805 individuals were diagnosed with early-onset CRC, 2689 with early-onset gastric cancer, 2657 with early-onset pancreatic cancer, and 875 with early-onset esophageal cancer.
Since the mid-1990s, CRC among adults of all ages in the US declined by 1.3%-4.2% annually but early-onset CRC increased by roughly 2% per year in both men and women, and currently makes up about 14% of all CRC cases.
Early-onset pancreatic cancer and esophageal cancer each currently make up about 5% of all cases of these cancers in the US.
Between 2010 and 2019, the number of newly diagnosed cases of early-onset GI cancers rose by nearly about 15%, with Black, Hispanic, Indigenous ancestry, and women disproportionately affected, Ng and coauthors noted in a related review published in the British Journal of Surgery.
Modifiable and Nonmodifiable Risk Factors
Along with obesity and poor diet, other modifiable risk factors for early-onset GI cancers include sedentary lifestyle, cigarette smoking, and alcohol consumption.
Nonmodifiable risk factors include family history, hereditary cancer syndromes such as Lynch syndrome and inflammatory bowel disease.
Roughly 15%-30% of early-onset GI cancers have pathogenic germline variants in genes such as DNA mismatch repair genes and BRCA1/2.
All individuals with early-onset GI cancers should undergo germline and somatic genetic testing to guide treatment, screen for other cancers (eg, endometrial cancer in Lynch syndrome), and assess familial risk, Ng and Jayakrishnan advised.
Treatment Challenges
Treatment for early-onset GI cancers is generally similar to later-onset GI cancers and prognosis for patients with early-onset GI cancers is “similar to or worse” than that for patients with later-onset GI cancers, highlighting the need for improved methods of prevention and early detection, the authors said.
Ng noted that younger cancer patients often face more challenges after diagnosis than older patients and benefit from multidisciplinary care, including referral for fertility counseling and preservation if appropriate, and psychosocial support.
“It is very difficult and challenging to receive a cancer diagnosis no matter what age you are, but when a person is diagnosed in their 20s, 30s, or 40s, there are unique challenges,” Ng said.
Studies have documented “much higher levels of psychosocial distress, depression and anxiety” in early-onset cancer patients, “and they also often experience more financial toxicity, disruptions in their education as well as their career and there may be fertility concerns,” Ng added.
Diagnostic Delays and Screening
Currently, screening is not recommended for most early-onset GI cancers — with the exception of CRC, with screening recommended for average-risk adults in the US starting at age 45.
Yet, despite this recommendation, fewer than 1 in 5 (19.7%) US adults aged 45-49 years were screened in 2021, indicating a significant gap in early detection efforts.
High-risk individuals, such as those with Lynch syndrome, a first-degree relative with CRC, or advanced colorectal adenoma, should begin CRC screening earlier, at an age determined by the specific risk factor.
“Studies have shown significant delays in diagnosis among younger patients. It’s important that prompt diagnosis happens so that these patients do not end up being diagnosed with advanced or metastatic stages of cancer, as they often are,” Ng said.
“Screening adherence is absolutely critical,” co-author Jayakrishnan added in a news release.
“We have strong evidence that colorectal cancer screening saves lives by reducing both the number of people who develop colorectal cancer and the number of people who die from it. Each missed screening is a lost opportunity to detect cancer early when it is more treatable, or to prevent cancer altogether by identifying and removing precancerous polyps,” Jayakrishnan said.This research had no funding. Ng reported receipt of nonfinancial support from Pharmavite, institutional grants from Janssen, and personal fees from Bayer, Seagen, GlaxoSmithKline, Pfizer, CytomX, Jazz Pharmaceuticals, Revolution Medicines, Redesign Health, AbbVie, Etiome, and CRICO. Ng is an associate editor of JAMA but was not involved in any of the decisions regarding review of the manuscript or its acceptance. Jayakrishnan had no disclosures.
A version of this article appeared on Medscape.com.
, said the authors of a JAMA review.
In the US, early-onset GI cancers are increasing faster than any other type of early-onset cancer, including breast cancer. The trend is not limited to colorectal cancer (CRC). Gastric, pancreatic, esophageal, as well as many biliary tract and appendix cancers, are also on the rise in young adults, Kimmie Ng, MD, MPH, and Thejus Jayakrishnan, MD, both with Dana-Farber Cancer Institute, Boston, noted in their article.
The increase in early-onset GI cancers follows a “birth cohort effect,” with generational variation in risk, suggesting a potential association with changes in environmental exposures, Ng explained in an accompanying JAMA podcast.
All these GI cancers link strongly to multiple modifiable risk factors, and it is a “top area of investigation to determine exactly what environmental exposures are at play,” Ng added.
For many of these GI cancers, obesity has been the “leading hypothesis” given that rising rates seem to parallel the increase in incidence of these early-onset GI cancers, Ng explained.
“But we also have evidence, particularly strong for colorectal cancer, that dietary patterns, such as consuming a Western diet, as well as sedentary behavior and lifestyles seem to be associated with a significantly higher risk of developing these cancers at an age under 50,” Ng said.
Rising Incidence
Globally, among early-onset GI cancers reported in 2022, CRC was the most common (54%), followed by gastric cancer (24%), esophageal cancer (13%), and pancreatic cancer (9%).
In the US in 2022, 20,805 individuals were diagnosed with early-onset CRC, 2689 with early-onset gastric cancer, 2657 with early-onset pancreatic cancer, and 875 with early-onset esophageal cancer.
Since the mid-1990s, CRC among adults of all ages in the US declined by 1.3%-4.2% annually but early-onset CRC increased by roughly 2% per year in both men and women, and currently makes up about 14% of all CRC cases.
Early-onset pancreatic cancer and esophageal cancer each currently make up about 5% of all cases of these cancers in the US.
Between 2010 and 2019, the number of newly diagnosed cases of early-onset GI cancers rose by nearly about 15%, with Black, Hispanic, Indigenous ancestry, and women disproportionately affected, Ng and coauthors noted in a related review published in the British Journal of Surgery.
Modifiable and Nonmodifiable Risk Factors
Along with obesity and poor diet, other modifiable risk factors for early-onset GI cancers include sedentary lifestyle, cigarette smoking, and alcohol consumption.
Nonmodifiable risk factors include family history, hereditary cancer syndromes such as Lynch syndrome and inflammatory bowel disease.
Roughly 15%-30% of early-onset GI cancers have pathogenic germline variants in genes such as DNA mismatch repair genes and BRCA1/2.
All individuals with early-onset GI cancers should undergo germline and somatic genetic testing to guide treatment, screen for other cancers (eg, endometrial cancer in Lynch syndrome), and assess familial risk, Ng and Jayakrishnan advised.
Treatment Challenges
Treatment for early-onset GI cancers is generally similar to later-onset GI cancers and prognosis for patients with early-onset GI cancers is “similar to or worse” than that for patients with later-onset GI cancers, highlighting the need for improved methods of prevention and early detection, the authors said.
Ng noted that younger cancer patients often face more challenges after diagnosis than older patients and benefit from multidisciplinary care, including referral for fertility counseling and preservation if appropriate, and psychosocial support.
“It is very difficult and challenging to receive a cancer diagnosis no matter what age you are, but when a person is diagnosed in their 20s, 30s, or 40s, there are unique challenges,” Ng said.
Studies have documented “much higher levels of psychosocial distress, depression and anxiety” in early-onset cancer patients, “and they also often experience more financial toxicity, disruptions in their education as well as their career and there may be fertility concerns,” Ng added.
Diagnostic Delays and Screening
Currently, screening is not recommended for most early-onset GI cancers — with the exception of CRC, with screening recommended for average-risk adults in the US starting at age 45.
Yet, despite this recommendation, fewer than 1 in 5 (19.7%) US adults aged 45-49 years were screened in 2021, indicating a significant gap in early detection efforts.
High-risk individuals, such as those with Lynch syndrome, a first-degree relative with CRC, or advanced colorectal adenoma, should begin CRC screening earlier, at an age determined by the specific risk factor.
“Studies have shown significant delays in diagnosis among younger patients. It’s important that prompt diagnosis happens so that these patients do not end up being diagnosed with advanced or metastatic stages of cancer, as they often are,” Ng said.
“Screening adherence is absolutely critical,” co-author Jayakrishnan added in a news release.
“We have strong evidence that colorectal cancer screening saves lives by reducing both the number of people who develop colorectal cancer and the number of people who die from it. Each missed screening is a lost opportunity to detect cancer early when it is more treatable, or to prevent cancer altogether by identifying and removing precancerous polyps,” Jayakrishnan said.This research had no funding. Ng reported receipt of nonfinancial support from Pharmavite, institutional grants from Janssen, and personal fees from Bayer, Seagen, GlaxoSmithKline, Pfizer, CytomX, Jazz Pharmaceuticals, Revolution Medicines, Redesign Health, AbbVie, Etiome, and CRICO. Ng is an associate editor of JAMA but was not involved in any of the decisions regarding review of the manuscript or its acceptance. Jayakrishnan had no disclosures.
A version of this article appeared on Medscape.com.
Sterile Water Bottles Deemed Unnecessary for Endoscopy
Like diners saving on drinks,
“No direct evidence supports the recommendation and widespread use of sterile water during gastrointestinal endosco-py procedures,” lead author Deepak Agrawal, MD, chief of gastroenterology & hepatology at the Dell Medical School, University Texas at Austin, and colleagues, wrote in Gastro Hep Advances. “Guidelines recommending sterile water during endoscopy are based on limited evidence and mostly expert opinions.”
After reviewing the literature back to 1975, Dr. Agrawal and colleagues considered the use of sterile water in endoscopy via three frameworks: medical evidence and guidelines, environmental and broader health effects, and financial costs.
Only 2 studies – both from the 1990s – directly compared sterile and tap water use in endoscopy. Neither showed an increased risk of infection from tap water. In fact, some cultures from allegedly sterile water bottles grew pathogenic bacteria, while no patient complications were reported in either study.
“The recommendations for sterile water contradict observations in other medical care scenarios, for example, for the irrigation of open wounds,” Dr. Agrawal and colleagues noted. “Similarly, there is no benefit in using sterile water for enteral feeds in immunosuppressed patients, and tap water enemas are routinely acceptable for colon cleansing before sigmoidoscopies in all patients, irrespective of immune status.”
Current guidelines, including the 2021 US multisociety guideline on reprocessing flexible GI endoscopes and accessories, recommend sterile water for procedures involving mucosal penetration but acknowledge low-quality supporting evidence. These recommendations are based on outdated studies, some unrelated to GI endoscopy, Dr. Agrawal and colleagues pointed out, and rely heavily on cross-referenced opinion statements rather than clinical data.
They went on to suggest a concerning possibility: all those plastic bottles may actually cause more health problems than prevent them. The review estimates that the production and transportation of sterile water bottles contributes over 6,000 metric tons of emissions per year from US endoscopy units alone. What’s more, as discarded bottles break down, they release greenhouse gases and microplastics, the latter of which have been linked to cardiovascular disease, inflammatory bowel disease, and endocrine disruption.
Dr. Agrawal and colleagues also underscored the financial toxicity of sterile water bottles. Considering a 1-liter bottle of sterile water costs $3-10, an endoscopy unit performing 30 procedures per day spends approximately $1,000-3,000 per month on bottled water alone. Scaled nationally, the routine use of sterile water costs tens of millions of dollars each year, not counting indirect expenses associated with stocking and waste disposal.
Considering the dubious clinical upside against the apparent environmental and financial downsides, Dr. Agrawal and colleagues urged endoscopy units to rethink routine sterile water use.
They proposed a pragmatic model: start the day with a new sterile or reusable bottle, refill with tap water for subsequent cases, and recycle the bottle at day’s end. Institutions should ensure their tap water meets safety standards, they added, such as those outlined in the Joint Commission’s 2022 R3 Report on standards for water management.
Dr. Agrawal and colleagues also called on GI societies to revise existing guidance to reflect today’s clinical and environmental realities. Until strong evidence supports the need for sterile water, they wrote, the smarter, safer, and more sustainable option may be simply turning on the tap.
The investigators disclosed relationships with Guardant, Exact Sciences, Freenome, and others.
In an editorial accompanying the study and comments to GI & Hepatology News, Dr. Seth A. Gross of NYU Langone Health urged gastroenterologists to reconsider the use of sterile water in endoscopy.
While the rationale for bottled water has centered on infection prevention, Gross argued that the evidence does not hold up, noting that this practice contradicts modern values around sustainability and evidence-based care.
The two relevant clinical studies comparing sterile versus tap water in endoscopy are almost 30 years old, he said, and neither detected an increased risk of infection with tap water, leading both to conclude that tap water is “safe and practical” for routine endoscopy.
Gross also pointed out the inconsistency of sterile water use in medical practice, noting that tap water is acceptable in procedures with higher infection risk than endoscopy.
“Lastly,” he added, “most people drink tap water and not sterile water on a daily basis without outbreaks of gastroenteritis from bacterial infections.”
Gross’s comments went beyond the data to emphasize the obvious but overlooked environmental impacts of sterile water bottles. He suggested several challenging suggestions to make medicine more ecofriendly, like reducing travel to conferences, increasing the availability of telehealth, and choosing reusable devices over disposables.
But “what’s hiding in plain sight,” he said, “is our use of sterile water.”
While acknowledging that some patients, like those who are immunocompromised, might still warrant sterile water, Gross supported the review’s recommendation to use tap water instead. He called on GI societies and regulatory bodies to re-examine current policy and pursue updated guidance.
“Sometimes going back to the basics,” he concluded, “could be the most innovative strategy with tremendous impact.”
Seth A. Gross, MD, AGAF, is clinical chief in the Division of Gastroenterology & Hepatology at NYU Langone Health, and professor at the NYU Grossman School of Medicine, both in New York City. He reported no conflicts of interest.
In an editorial accompanying the study and comments to GI & Hepatology News, Dr. Seth A. Gross of NYU Langone Health urged gastroenterologists to reconsider the use of sterile water in endoscopy.
While the rationale for bottled water has centered on infection prevention, Gross argued that the evidence does not hold up, noting that this practice contradicts modern values around sustainability and evidence-based care.
The two relevant clinical studies comparing sterile versus tap water in endoscopy are almost 30 years old, he said, and neither detected an increased risk of infection with tap water, leading both to conclude that tap water is “safe and practical” for routine endoscopy.
Gross also pointed out the inconsistency of sterile water use in medical practice, noting that tap water is acceptable in procedures with higher infection risk than endoscopy.
“Lastly,” he added, “most people drink tap water and not sterile water on a daily basis without outbreaks of gastroenteritis from bacterial infections.”
Gross’s comments went beyond the data to emphasize the obvious but overlooked environmental impacts of sterile water bottles. He suggested several challenging suggestions to make medicine more ecofriendly, like reducing travel to conferences, increasing the availability of telehealth, and choosing reusable devices over disposables.
But “what’s hiding in plain sight,” he said, “is our use of sterile water.”
While acknowledging that some patients, like those who are immunocompromised, might still warrant sterile water, Gross supported the review’s recommendation to use tap water instead. He called on GI societies and regulatory bodies to re-examine current policy and pursue updated guidance.
“Sometimes going back to the basics,” he concluded, “could be the most innovative strategy with tremendous impact.”
Seth A. Gross, MD, AGAF, is clinical chief in the Division of Gastroenterology & Hepatology at NYU Langone Health, and professor at the NYU Grossman School of Medicine, both in New York City. He reported no conflicts of interest.
In an editorial accompanying the study and comments to GI & Hepatology News, Dr. Seth A. Gross of NYU Langone Health urged gastroenterologists to reconsider the use of sterile water in endoscopy.
While the rationale for bottled water has centered on infection prevention, Gross argued that the evidence does not hold up, noting that this practice contradicts modern values around sustainability and evidence-based care.
The two relevant clinical studies comparing sterile versus tap water in endoscopy are almost 30 years old, he said, and neither detected an increased risk of infection with tap water, leading both to conclude that tap water is “safe and practical” for routine endoscopy.
Gross also pointed out the inconsistency of sterile water use in medical practice, noting that tap water is acceptable in procedures with higher infection risk than endoscopy.
“Lastly,” he added, “most people drink tap water and not sterile water on a daily basis without outbreaks of gastroenteritis from bacterial infections.”
Gross’s comments went beyond the data to emphasize the obvious but overlooked environmental impacts of sterile water bottles. He suggested several challenging suggestions to make medicine more ecofriendly, like reducing travel to conferences, increasing the availability of telehealth, and choosing reusable devices over disposables.
But “what’s hiding in plain sight,” he said, “is our use of sterile water.”
While acknowledging that some patients, like those who are immunocompromised, might still warrant sterile water, Gross supported the review’s recommendation to use tap water instead. He called on GI societies and regulatory bodies to re-examine current policy and pursue updated guidance.
“Sometimes going back to the basics,” he concluded, “could be the most innovative strategy with tremendous impact.”
Seth A. Gross, MD, AGAF, is clinical chief in the Division of Gastroenterology & Hepatology at NYU Langone Health, and professor at the NYU Grossman School of Medicine, both in New York City. He reported no conflicts of interest.
Like diners saving on drinks,
“No direct evidence supports the recommendation and widespread use of sterile water during gastrointestinal endosco-py procedures,” lead author Deepak Agrawal, MD, chief of gastroenterology & hepatology at the Dell Medical School, University Texas at Austin, and colleagues, wrote in Gastro Hep Advances. “Guidelines recommending sterile water during endoscopy are based on limited evidence and mostly expert opinions.”
After reviewing the literature back to 1975, Dr. Agrawal and colleagues considered the use of sterile water in endoscopy via three frameworks: medical evidence and guidelines, environmental and broader health effects, and financial costs.
Only 2 studies – both from the 1990s – directly compared sterile and tap water use in endoscopy. Neither showed an increased risk of infection from tap water. In fact, some cultures from allegedly sterile water bottles grew pathogenic bacteria, while no patient complications were reported in either study.
“The recommendations for sterile water contradict observations in other medical care scenarios, for example, for the irrigation of open wounds,” Dr. Agrawal and colleagues noted. “Similarly, there is no benefit in using sterile water for enteral feeds in immunosuppressed patients, and tap water enemas are routinely acceptable for colon cleansing before sigmoidoscopies in all patients, irrespective of immune status.”
Current guidelines, including the 2021 US multisociety guideline on reprocessing flexible GI endoscopes and accessories, recommend sterile water for procedures involving mucosal penetration but acknowledge low-quality supporting evidence. These recommendations are based on outdated studies, some unrelated to GI endoscopy, Dr. Agrawal and colleagues pointed out, and rely heavily on cross-referenced opinion statements rather than clinical data.
They went on to suggest a concerning possibility: all those plastic bottles may actually cause more health problems than prevent them. The review estimates that the production and transportation of sterile water bottles contributes over 6,000 metric tons of emissions per year from US endoscopy units alone. What’s more, as discarded bottles break down, they release greenhouse gases and microplastics, the latter of which have been linked to cardiovascular disease, inflammatory bowel disease, and endocrine disruption.
Dr. Agrawal and colleagues also underscored the financial toxicity of sterile water bottles. Considering a 1-liter bottle of sterile water costs $3-10, an endoscopy unit performing 30 procedures per day spends approximately $1,000-3,000 per month on bottled water alone. Scaled nationally, the routine use of sterile water costs tens of millions of dollars each year, not counting indirect expenses associated with stocking and waste disposal.
Considering the dubious clinical upside against the apparent environmental and financial downsides, Dr. Agrawal and colleagues urged endoscopy units to rethink routine sterile water use.
They proposed a pragmatic model: start the day with a new sterile or reusable bottle, refill with tap water for subsequent cases, and recycle the bottle at day’s end. Institutions should ensure their tap water meets safety standards, they added, such as those outlined in the Joint Commission’s 2022 R3 Report on standards for water management.
Dr. Agrawal and colleagues also called on GI societies to revise existing guidance to reflect today’s clinical and environmental realities. Until strong evidence supports the need for sterile water, they wrote, the smarter, safer, and more sustainable option may be simply turning on the tap.
The investigators disclosed relationships with Guardant, Exact Sciences, Freenome, and others.
Like diners saving on drinks,
“No direct evidence supports the recommendation and widespread use of sterile water during gastrointestinal endosco-py procedures,” lead author Deepak Agrawal, MD, chief of gastroenterology & hepatology at the Dell Medical School, University Texas at Austin, and colleagues, wrote in Gastro Hep Advances. “Guidelines recommending sterile water during endoscopy are based on limited evidence and mostly expert opinions.”
After reviewing the literature back to 1975, Dr. Agrawal and colleagues considered the use of sterile water in endoscopy via three frameworks: medical evidence and guidelines, environmental and broader health effects, and financial costs.
Only 2 studies – both from the 1990s – directly compared sterile and tap water use in endoscopy. Neither showed an increased risk of infection from tap water. In fact, some cultures from allegedly sterile water bottles grew pathogenic bacteria, while no patient complications were reported in either study.
“The recommendations for sterile water contradict observations in other medical care scenarios, for example, for the irrigation of open wounds,” Dr. Agrawal and colleagues noted. “Similarly, there is no benefit in using sterile water for enteral feeds in immunosuppressed patients, and tap water enemas are routinely acceptable for colon cleansing before sigmoidoscopies in all patients, irrespective of immune status.”
Current guidelines, including the 2021 US multisociety guideline on reprocessing flexible GI endoscopes and accessories, recommend sterile water for procedures involving mucosal penetration but acknowledge low-quality supporting evidence. These recommendations are based on outdated studies, some unrelated to GI endoscopy, Dr. Agrawal and colleagues pointed out, and rely heavily on cross-referenced opinion statements rather than clinical data.
They went on to suggest a concerning possibility: all those plastic bottles may actually cause more health problems than prevent them. The review estimates that the production and transportation of sterile water bottles contributes over 6,000 metric tons of emissions per year from US endoscopy units alone. What’s more, as discarded bottles break down, they release greenhouse gases and microplastics, the latter of which have been linked to cardiovascular disease, inflammatory bowel disease, and endocrine disruption.
Dr. Agrawal and colleagues also underscored the financial toxicity of sterile water bottles. Considering a 1-liter bottle of sterile water costs $3-10, an endoscopy unit performing 30 procedures per day spends approximately $1,000-3,000 per month on bottled water alone. Scaled nationally, the routine use of sterile water costs tens of millions of dollars each year, not counting indirect expenses associated with stocking and waste disposal.
Considering the dubious clinical upside against the apparent environmental and financial downsides, Dr. Agrawal and colleagues urged endoscopy units to rethink routine sterile water use.
They proposed a pragmatic model: start the day with a new sterile or reusable bottle, refill with tap water for subsequent cases, and recycle the bottle at day’s end. Institutions should ensure their tap water meets safety standards, they added, such as those outlined in the Joint Commission’s 2022 R3 Report on standards for water management.
Dr. Agrawal and colleagues also called on GI societies to revise existing guidance to reflect today’s clinical and environmental realities. Until strong evidence supports the need for sterile water, they wrote, the smarter, safer, and more sustainable option may be simply turning on the tap.
The investigators disclosed relationships with Guardant, Exact Sciences, Freenome, and others.
FROM GASTRO HEP ADVANCES
Cirrhosis Mortality Prediction Boosted by Machine Learning
“This highly inclusive, representative, and globally derived model has been externally validated,” Jasmohan Bajaj, MD, AGAF, professor of medicine at Virginia Commonwealth University in Richmond, Virginia, told GI & Hepatology News. “This gives us a crystal ball. It helps hospital teams, transplant centers, gastroenterology and intensive care unit services triage and prioritize patients more effectively.”
The study supporting the model, which Bajaj said “could be used at this stage,” was published online in Gastroenterology. The model is available for downloading at https://silveys.shinyapps.io/app_cleared/.
CLEARED Cohort Analyzed
Wide variations across the world regarding available resources, outpatient services, reasons for admission, and etiologies of cirrhosis can influence patient outcomes, according to Bajaj and colleagues. Therefore, they sought to use ML approaches to improve prognostication for all countries.
They analyzed admission-day data from the prospective Chronic Liver Disease Evolution And Registry for Events and Decompensation (CLEARED) consortium, which includes inpatients with cirrhosis enrolled from six continents. The analysis compared ML approaches with logistical regression to predict inpatient mortality.
The researchers performed internal validation (75/25 split) and subdivision using World-Bank income status: low/low-middle (L-LMIC), upper middle (UMIC), and high (HIC). They determined that the ML model with the best area-under-the-curve (AUC) would be externally validated in a US-Veteran cirrhosis inpatient population.
The CLEARED cohort included 7239 cirrhosis inpatients (mean age, 56 years; 64% men; median MELD-Na, 25) from 115 centers globally; 22.5% of centers belonged to LMICs, 41% to UMICs, and 34% to HICs.
A total of 808 patients (11.1%) died in the hospital.
Random-Forest analysis showed the best AUC (0.815) with high calibration. This was significantly better than parametric logistic regression (AUC, 0.774) and LASSO (AUC, 0.787) models.
Random-Forest also was better than logistic regression regardless of country income-level: HIC (AUC,0.806), UMIC (AUC, 0.867), and L-LMICs (AUC, 0.768).
Of the top 15 important variables selected from Random-Forest, admission for acute kidney injury, hepatic encephalopathy, high MELD-Na/white blood count, and not being in high income country were variables most predictive of mortality.
In contrast, higher albumin, hemoglobin, diuretic use on admission, viral etiology, and being in a high-income country were most protective.
The Random-Forest model was validated in 28,670 veterans (mean age, 67 years; 96% men; median MELD-Na,15), with an inpatient mortality of 4% (1158 patients).
The final Random-Forest model, using 48 of the 67 original covariates, attained a strong AUC of 0.859. A refit version using only the top 15 variables achieved a comparable AUC of 0.851.
Clinical Relevance
“Cirrhosis and resultant organ failures remain a dynamic and multidisciplinary problem,” Bajaj noted. “Machine learning techniques are one part of multi-faceted management strategy that is required in this population.”
If patients fall into the high-risk category, he said, “careful consultation with patients, families, and clinical teams is needed before providing information, including where this model was derived from. The results of these discussions could be instructive regarding decisions for transfer, more aggressive monitoring/ICU transfer, palliative care or transplant assessments.”
Meena B. Bansal, MD, system chief, Division of Liver Diseases, Mount Sinai Health System in New York City, called the tool “very promising.” However, she told GI & Hepatology News, “it was validated on a VA [Veterans Affairs] cohort, which is a bit different than the cohort of patients seen at Mount Sinai. Therefore, validation in more academic tertiary care medical centers with high volume liver transplant would be helpful.”
Furthermore, said Bansal, who was not involved in the study, “they excluded those that receiving a liver transplant, and while only a small number, this is an important limitation.”
Nevertheless, she added, “Artificial intelligence has great potential in predictive risk models and will likely be a tool that assists for risk stratification, clinical management, and hopefully improved clinical outcomes.”
This study was partly supported by a VA Merit review to Bajaj and the National Center for Advancing Translational Sciences, National Institutes of Health. No conflicts of interest were reported by any author.
A version of this article appeared on Medscape.com.
“This highly inclusive, representative, and globally derived model has been externally validated,” Jasmohan Bajaj, MD, AGAF, professor of medicine at Virginia Commonwealth University in Richmond, Virginia, told GI & Hepatology News. “This gives us a crystal ball. It helps hospital teams, transplant centers, gastroenterology and intensive care unit services triage and prioritize patients more effectively.”
The study supporting the model, which Bajaj said “could be used at this stage,” was published online in Gastroenterology. The model is available for downloading at https://silveys.shinyapps.io/app_cleared/.
CLEARED Cohort Analyzed
Wide variations across the world regarding available resources, outpatient services, reasons for admission, and etiologies of cirrhosis can influence patient outcomes, according to Bajaj and colleagues. Therefore, they sought to use ML approaches to improve prognostication for all countries.
They analyzed admission-day data from the prospective Chronic Liver Disease Evolution And Registry for Events and Decompensation (CLEARED) consortium, which includes inpatients with cirrhosis enrolled from six continents. The analysis compared ML approaches with logistical regression to predict inpatient mortality.
The researchers performed internal validation (75/25 split) and subdivision using World-Bank income status: low/low-middle (L-LMIC), upper middle (UMIC), and high (HIC). They determined that the ML model with the best area-under-the-curve (AUC) would be externally validated in a US-Veteran cirrhosis inpatient population.
The CLEARED cohort included 7239 cirrhosis inpatients (mean age, 56 years; 64% men; median MELD-Na, 25) from 115 centers globally; 22.5% of centers belonged to LMICs, 41% to UMICs, and 34% to HICs.
A total of 808 patients (11.1%) died in the hospital.
Random-Forest analysis showed the best AUC (0.815) with high calibration. This was significantly better than parametric logistic regression (AUC, 0.774) and LASSO (AUC, 0.787) models.
Random-Forest also was better than logistic regression regardless of country income-level: HIC (AUC,0.806), UMIC (AUC, 0.867), and L-LMICs (AUC, 0.768).
Of the top 15 important variables selected from Random-Forest, admission for acute kidney injury, hepatic encephalopathy, high MELD-Na/white blood count, and not being in high income country were variables most predictive of mortality.
In contrast, higher albumin, hemoglobin, diuretic use on admission, viral etiology, and being in a high-income country were most protective.
The Random-Forest model was validated in 28,670 veterans (mean age, 67 years; 96% men; median MELD-Na,15), with an inpatient mortality of 4% (1158 patients).
The final Random-Forest model, using 48 of the 67 original covariates, attained a strong AUC of 0.859. A refit version using only the top 15 variables achieved a comparable AUC of 0.851.
Clinical Relevance
“Cirrhosis and resultant organ failures remain a dynamic and multidisciplinary problem,” Bajaj noted. “Machine learning techniques are one part of multi-faceted management strategy that is required in this population.”
If patients fall into the high-risk category, he said, “careful consultation with patients, families, and clinical teams is needed before providing information, including where this model was derived from. The results of these discussions could be instructive regarding decisions for transfer, more aggressive monitoring/ICU transfer, palliative care or transplant assessments.”
Meena B. Bansal, MD, system chief, Division of Liver Diseases, Mount Sinai Health System in New York City, called the tool “very promising.” However, she told GI & Hepatology News, “it was validated on a VA [Veterans Affairs] cohort, which is a bit different than the cohort of patients seen at Mount Sinai. Therefore, validation in more academic tertiary care medical centers with high volume liver transplant would be helpful.”
Furthermore, said Bansal, who was not involved in the study, “they excluded those that receiving a liver transplant, and while only a small number, this is an important limitation.”
Nevertheless, she added, “Artificial intelligence has great potential in predictive risk models and will likely be a tool that assists for risk stratification, clinical management, and hopefully improved clinical outcomes.”
This study was partly supported by a VA Merit review to Bajaj and the National Center for Advancing Translational Sciences, National Institutes of Health. No conflicts of interest were reported by any author.
A version of this article appeared on Medscape.com.
“This highly inclusive, representative, and globally derived model has been externally validated,” Jasmohan Bajaj, MD, AGAF, professor of medicine at Virginia Commonwealth University in Richmond, Virginia, told GI & Hepatology News. “This gives us a crystal ball. It helps hospital teams, transplant centers, gastroenterology and intensive care unit services triage and prioritize patients more effectively.”
The study supporting the model, which Bajaj said “could be used at this stage,” was published online in Gastroenterology. The model is available for downloading at https://silveys.shinyapps.io/app_cleared/.
CLEARED Cohort Analyzed
Wide variations across the world regarding available resources, outpatient services, reasons for admission, and etiologies of cirrhosis can influence patient outcomes, according to Bajaj and colleagues. Therefore, they sought to use ML approaches to improve prognostication for all countries.
They analyzed admission-day data from the prospective Chronic Liver Disease Evolution And Registry for Events and Decompensation (CLEARED) consortium, which includes inpatients with cirrhosis enrolled from six continents. The analysis compared ML approaches with logistical regression to predict inpatient mortality.
The researchers performed internal validation (75/25 split) and subdivision using World-Bank income status: low/low-middle (L-LMIC), upper middle (UMIC), and high (HIC). They determined that the ML model with the best area-under-the-curve (AUC) would be externally validated in a US-Veteran cirrhosis inpatient population.
The CLEARED cohort included 7239 cirrhosis inpatients (mean age, 56 years; 64% men; median MELD-Na, 25) from 115 centers globally; 22.5% of centers belonged to LMICs, 41% to UMICs, and 34% to HICs.
A total of 808 patients (11.1%) died in the hospital.
Random-Forest analysis showed the best AUC (0.815) with high calibration. This was significantly better than parametric logistic regression (AUC, 0.774) and LASSO (AUC, 0.787) models.
Random-Forest also was better than logistic regression regardless of country income-level: HIC (AUC,0.806), UMIC (AUC, 0.867), and L-LMICs (AUC, 0.768).
Of the top 15 important variables selected from Random-Forest, admission for acute kidney injury, hepatic encephalopathy, high MELD-Na/white blood count, and not being in high income country were variables most predictive of mortality.
In contrast, higher albumin, hemoglobin, diuretic use on admission, viral etiology, and being in a high-income country were most protective.
The Random-Forest model was validated in 28,670 veterans (mean age, 67 years; 96% men; median MELD-Na,15), with an inpatient mortality of 4% (1158 patients).
The final Random-Forest model, using 48 of the 67 original covariates, attained a strong AUC of 0.859. A refit version using only the top 15 variables achieved a comparable AUC of 0.851.
Clinical Relevance
“Cirrhosis and resultant organ failures remain a dynamic and multidisciplinary problem,” Bajaj noted. “Machine learning techniques are one part of multi-faceted management strategy that is required in this population.”
If patients fall into the high-risk category, he said, “careful consultation with patients, families, and clinical teams is needed before providing information, including where this model was derived from. The results of these discussions could be instructive regarding decisions for transfer, more aggressive monitoring/ICU transfer, palliative care or transplant assessments.”
Meena B. Bansal, MD, system chief, Division of Liver Diseases, Mount Sinai Health System in New York City, called the tool “very promising.” However, she told GI & Hepatology News, “it was validated on a VA [Veterans Affairs] cohort, which is a bit different than the cohort of patients seen at Mount Sinai. Therefore, validation in more academic tertiary care medical centers with high volume liver transplant would be helpful.”
Furthermore, said Bansal, who was not involved in the study, “they excluded those that receiving a liver transplant, and while only a small number, this is an important limitation.”
Nevertheless, she added, “Artificial intelligence has great potential in predictive risk models and will likely be a tool that assists for risk stratification, clinical management, and hopefully improved clinical outcomes.”
This study was partly supported by a VA Merit review to Bajaj and the National Center for Advancing Translational Sciences, National Institutes of Health. No conflicts of interest were reported by any author.
A version of this article appeared on Medscape.com.
FROM GASTROENTEROLOGY
Colonoscopy Costs Rise When Private Equity Acquires GI Practices, but Quality Does Not
Price increases ranged from about 5% to about 7%.
In view of the growing trend to such acquisitions, policy makers should monitor the impact of PE investment in medical practices, according to researchers led by health economist Daniel R. Arnold, PhD, of the Department of Health Services, Policy & Practice in the School of Public Health at Brown University in Providence, Rhode Island. “In a previous study of ours, gastroenterology stood out as a particularly attractive specialty to private equity,” Arnold told GI & Hepatology News.
Published in JAMA Health Forum, the economic evaluation of more than 1.1 million patients and 1.3 million colonoscopies concluded that PE acquisitions of GI sites are difficult to justify.
The Study
This difference-in-differences event study and economic evaluation analyzed data from US GI practices acquired by PE firms from 2015 to 2021. Commercial insurance claims covering more than 50 million enrollees were used to calculate price, spending, utilization, and quality measures from 2012 to 2021, with all data analyzed from April to September 2024.
The main outcomes were price, spending per physician, number of colonoscopies per physician, number of unique patients per physician, and quality, as defined by polyp detection, incomplete colonoscopies, and four adverse event measures: cardiovascular, serious and nonserious GI events, and any other adverse events.
The mean age of patients was 47.1 years, and 47.8% were men. The sample included 718, 851 colonoscopies conducted by 1494 physicians in 590, 900 patients across 1240 PE-acquired practice sites and 637, 990 control colonoscopies conducted by 2550 physicians in 527,380 patients across 2657 independent practice sites.
Among the findings:
- Colonoscopy prices at PE-acquired sites increased by 4.5% (95% CI, 2.5-6.6; P < .001) vs independent practices. That increase was much lower than that reported by Singh and colleagues for .
- The estimated price increase was 6.7% (95% CI, 4.2-9.3; P < .001) when only colonoscopies at PE practices with market shares above the 75th percentile (24.4%) in 2021 were considered. Both increases were in line with other research, Arnold said.
- Colonoscopy spending per physician increased by 16.0% (95% CI, 8.4%-24.0%; P < .001), while the number of colonoscopies and the number of unique patients per physician increased by 12.1% (95% CI, 5.3-19.4; P < .001) and 11.3% (95% CI, 4.4%-18.5%; P < .001), respectively. These measures, however, were already increasing before PE acquisition.
- No statistically significant associations were detected for the six quality measures analyzed.
Could such cost-raising acquisitions potentially be blocked by concerned regulators?
“No. Generally the purchases are at prices below what would require notification to federal authorities,” Arnold said. “The Department of Justice/Federal Trade Commission hinted at being willing to look at serial acquisitions in their 2023 Merger Guidelines, but until that happens, these will likely continue to fly under the radar.”
Still, as evidence of PE-associated poorer quality outcomes as well as clinician burnout continues to emerge, Arnold added, “I would advise physicians who get buyout offers from PE to educate themselves on what could happen to patients and staff if they choose to sell.”
Offering an outsider’s perspective on the study, health economist Atul Gupta, PhD, an assistant professor of healthcare management in the Wharton School at the University of Pennsylvania in Philadelphia, called it an “excellent addition to the developing literature examining the effects of private equity ownership of healthcare providers.” Very few studies have examined the effects on prices and quality for the same set of deals and providers. “This is important because we want to be able to do an apples-to-apples comparison of the effects on both outcomes before judging PE ownership,” he told GI & Hepatology News.
In an accompanying editorial , primary care physician Jane M. Zhu, MD, an associate professor of medicine at Oregon Health & Science University in Portland, Oregon, and not involved in the commercial-insurance-based study, said one interpretation of the findings may be that PE acquisition focuses on reducing inefficiencies, improving access by expanding practice capacity, and increasing throughput. “Another interpretation may be that PE acquisition is focused on the strategic exploitation of market and pricing power. The latter may have less of an impact on clinical measures like quality of care, but potentially, both strategies could be at play.”
Since this analysis focused on the commercial population, understanding how patient demographics may change after PE acquisition is a future avenue for exploration. “For instance, a potential explanation for both the price and utilization shifts might be if payer mix shifted toward more commercially insured patients at the expense of Medicaid or Medicare patients,” she wrote.
Zhu added that the impact of PE on prices and spending, by now replicated across different settings and specialties, is far clearer than the effect of PE on access and quality. “The analysis by Arnold et al is a welcome addition to the literature, generating important questions for future study and transparent monitoring as investor-owners become increasingly influential in healthcare.”
Going forward, said Gupta, an open question is whether the harmful effects of PE ownership of practices are differentially worse than those of other corporate entities such as insurers and hospital systems.
“There are reasons to believe that PE could be worse in theory. For example, their short-term investment horizon may force them to take measures that others will not as well as avoid investing into capital improvements that have a long-run payoff,” he said. “Their uniquely high dependence on debt and unbundling of real estate can severely hurt financial solvency of providers.” But high-quality evidence is lacking to compare the effects from these two distinct forms of corporatization.
The trend away from individual private practice is a reality, Arnold said. “The administrative burden on solo docs is becoming too much and physicians just seem to want to treat patients and not deal with it. So the options at this point really become selling to a hospital system or private equity.”
This study was funded by a grant from the philanthropic foundation Arnold Ventures (no family relation to Daniel Arnold).
Arnold reported receiving grants from Arnold Ventures during the conduct of the study. Gupta had no competing interests to declare. Zhu reported receiving grants from the Agency for Healthcare Research and Quality during the submitted work and from the National Institutes of Health, National Institute for Health Care Management Foundation, and American Psychological Association, as well as personal fees from Cambia outside the submitted work.
A version of this article appeared on Medscape.com.
Price increases ranged from about 5% to about 7%.
In view of the growing trend to such acquisitions, policy makers should monitor the impact of PE investment in medical practices, according to researchers led by health economist Daniel R. Arnold, PhD, of the Department of Health Services, Policy & Practice in the School of Public Health at Brown University in Providence, Rhode Island. “In a previous study of ours, gastroenterology stood out as a particularly attractive specialty to private equity,” Arnold told GI & Hepatology News.
Published in JAMA Health Forum, the economic evaluation of more than 1.1 million patients and 1.3 million colonoscopies concluded that PE acquisitions of GI sites are difficult to justify.
The Study
This difference-in-differences event study and economic evaluation analyzed data from US GI practices acquired by PE firms from 2015 to 2021. Commercial insurance claims covering more than 50 million enrollees were used to calculate price, spending, utilization, and quality measures from 2012 to 2021, with all data analyzed from April to September 2024.
The main outcomes were price, spending per physician, number of colonoscopies per physician, number of unique patients per physician, and quality, as defined by polyp detection, incomplete colonoscopies, and four adverse event measures: cardiovascular, serious and nonserious GI events, and any other adverse events.
The mean age of patients was 47.1 years, and 47.8% were men. The sample included 718, 851 colonoscopies conducted by 1494 physicians in 590, 900 patients across 1240 PE-acquired practice sites and 637, 990 control colonoscopies conducted by 2550 physicians in 527,380 patients across 2657 independent practice sites.
Among the findings:
- Colonoscopy prices at PE-acquired sites increased by 4.5% (95% CI, 2.5-6.6; P < .001) vs independent practices. That increase was much lower than that reported by Singh and colleagues for .
- The estimated price increase was 6.7% (95% CI, 4.2-9.3; P < .001) when only colonoscopies at PE practices with market shares above the 75th percentile (24.4%) in 2021 were considered. Both increases were in line with other research, Arnold said.
- Colonoscopy spending per physician increased by 16.0% (95% CI, 8.4%-24.0%; P < .001), while the number of colonoscopies and the number of unique patients per physician increased by 12.1% (95% CI, 5.3-19.4; P < .001) and 11.3% (95% CI, 4.4%-18.5%; P < .001), respectively. These measures, however, were already increasing before PE acquisition.
- No statistically significant associations were detected for the six quality measures analyzed.
Could such cost-raising acquisitions potentially be blocked by concerned regulators?
“No. Generally the purchases are at prices below what would require notification to federal authorities,” Arnold said. “The Department of Justice/Federal Trade Commission hinted at being willing to look at serial acquisitions in their 2023 Merger Guidelines, but until that happens, these will likely continue to fly under the radar.”
Still, as evidence of PE-associated poorer quality outcomes as well as clinician burnout continues to emerge, Arnold added, “I would advise physicians who get buyout offers from PE to educate themselves on what could happen to patients and staff if they choose to sell.”
Offering an outsider’s perspective on the study, health economist Atul Gupta, PhD, an assistant professor of healthcare management in the Wharton School at the University of Pennsylvania in Philadelphia, called it an “excellent addition to the developing literature examining the effects of private equity ownership of healthcare providers.” Very few studies have examined the effects on prices and quality for the same set of deals and providers. “This is important because we want to be able to do an apples-to-apples comparison of the effects on both outcomes before judging PE ownership,” he told GI & Hepatology News.
In an accompanying editorial , primary care physician Jane M. Zhu, MD, an associate professor of medicine at Oregon Health & Science University in Portland, Oregon, and not involved in the commercial-insurance-based study, said one interpretation of the findings may be that PE acquisition focuses on reducing inefficiencies, improving access by expanding practice capacity, and increasing throughput. “Another interpretation may be that PE acquisition is focused on the strategic exploitation of market and pricing power. The latter may have less of an impact on clinical measures like quality of care, but potentially, both strategies could be at play.”
Since this analysis focused on the commercial population, understanding how patient demographics may change after PE acquisition is a future avenue for exploration. “For instance, a potential explanation for both the price and utilization shifts might be if payer mix shifted toward more commercially insured patients at the expense of Medicaid or Medicare patients,” she wrote.
Zhu added that the impact of PE on prices and spending, by now replicated across different settings and specialties, is far clearer than the effect of PE on access and quality. “The analysis by Arnold et al is a welcome addition to the literature, generating important questions for future study and transparent monitoring as investor-owners become increasingly influential in healthcare.”
Going forward, said Gupta, an open question is whether the harmful effects of PE ownership of practices are differentially worse than those of other corporate entities such as insurers and hospital systems.
“There are reasons to believe that PE could be worse in theory. For example, their short-term investment horizon may force them to take measures that others will not as well as avoid investing into capital improvements that have a long-run payoff,” he said. “Their uniquely high dependence on debt and unbundling of real estate can severely hurt financial solvency of providers.” But high-quality evidence is lacking to compare the effects from these two distinct forms of corporatization.
The trend away from individual private practice is a reality, Arnold said. “The administrative burden on solo docs is becoming too much and physicians just seem to want to treat patients and not deal with it. So the options at this point really become selling to a hospital system or private equity.”
This study was funded by a grant from the philanthropic foundation Arnold Ventures (no family relation to Daniel Arnold).
Arnold reported receiving grants from Arnold Ventures during the conduct of the study. Gupta had no competing interests to declare. Zhu reported receiving grants from the Agency for Healthcare Research and Quality during the submitted work and from the National Institutes of Health, National Institute for Health Care Management Foundation, and American Psychological Association, as well as personal fees from Cambia outside the submitted work.
A version of this article appeared on Medscape.com.
Price increases ranged from about 5% to about 7%.
In view of the growing trend to such acquisitions, policy makers should monitor the impact of PE investment in medical practices, according to researchers led by health economist Daniel R. Arnold, PhD, of the Department of Health Services, Policy & Practice in the School of Public Health at Brown University in Providence, Rhode Island. “In a previous study of ours, gastroenterology stood out as a particularly attractive specialty to private equity,” Arnold told GI & Hepatology News.
Published in JAMA Health Forum, the economic evaluation of more than 1.1 million patients and 1.3 million colonoscopies concluded that PE acquisitions of GI sites are difficult to justify.
The Study
This difference-in-differences event study and economic evaluation analyzed data from US GI practices acquired by PE firms from 2015 to 2021. Commercial insurance claims covering more than 50 million enrollees were used to calculate price, spending, utilization, and quality measures from 2012 to 2021, with all data analyzed from April to September 2024.
The main outcomes were price, spending per physician, number of colonoscopies per physician, number of unique patients per physician, and quality, as defined by polyp detection, incomplete colonoscopies, and four adverse event measures: cardiovascular, serious and nonserious GI events, and any other adverse events.
The mean age of patients was 47.1 years, and 47.8% were men. The sample included 718, 851 colonoscopies conducted by 1494 physicians in 590, 900 patients across 1240 PE-acquired practice sites and 637, 990 control colonoscopies conducted by 2550 physicians in 527,380 patients across 2657 independent practice sites.
Among the findings:
- Colonoscopy prices at PE-acquired sites increased by 4.5% (95% CI, 2.5-6.6; P < .001) vs independent practices. That increase was much lower than that reported by Singh and colleagues for .
- The estimated price increase was 6.7% (95% CI, 4.2-9.3; P < .001) when only colonoscopies at PE practices with market shares above the 75th percentile (24.4%) in 2021 were considered. Both increases were in line with other research, Arnold said.
- Colonoscopy spending per physician increased by 16.0% (95% CI, 8.4%-24.0%; P < .001), while the number of colonoscopies and the number of unique patients per physician increased by 12.1% (95% CI, 5.3-19.4; P < .001) and 11.3% (95% CI, 4.4%-18.5%; P < .001), respectively. These measures, however, were already increasing before PE acquisition.
- No statistically significant associations were detected for the six quality measures analyzed.
Could such cost-raising acquisitions potentially be blocked by concerned regulators?
“No. Generally the purchases are at prices below what would require notification to federal authorities,” Arnold said. “The Department of Justice/Federal Trade Commission hinted at being willing to look at serial acquisitions in their 2023 Merger Guidelines, but until that happens, these will likely continue to fly under the radar.”
Still, as evidence of PE-associated poorer quality outcomes as well as clinician burnout continues to emerge, Arnold added, “I would advise physicians who get buyout offers from PE to educate themselves on what could happen to patients and staff if they choose to sell.”
Offering an outsider’s perspective on the study, health economist Atul Gupta, PhD, an assistant professor of healthcare management in the Wharton School at the University of Pennsylvania in Philadelphia, called it an “excellent addition to the developing literature examining the effects of private equity ownership of healthcare providers.” Very few studies have examined the effects on prices and quality for the same set of deals and providers. “This is important because we want to be able to do an apples-to-apples comparison of the effects on both outcomes before judging PE ownership,” he told GI & Hepatology News.
In an accompanying editorial , primary care physician Jane M. Zhu, MD, an associate professor of medicine at Oregon Health & Science University in Portland, Oregon, and not involved in the commercial-insurance-based study, said one interpretation of the findings may be that PE acquisition focuses on reducing inefficiencies, improving access by expanding practice capacity, and increasing throughput. “Another interpretation may be that PE acquisition is focused on the strategic exploitation of market and pricing power. The latter may have less of an impact on clinical measures like quality of care, but potentially, both strategies could be at play.”
Since this analysis focused on the commercial population, understanding how patient demographics may change after PE acquisition is a future avenue for exploration. “For instance, a potential explanation for both the price and utilization shifts might be if payer mix shifted toward more commercially insured patients at the expense of Medicaid or Medicare patients,” she wrote.
Zhu added that the impact of PE on prices and spending, by now replicated across different settings and specialties, is far clearer than the effect of PE on access and quality. “The analysis by Arnold et al is a welcome addition to the literature, generating important questions for future study and transparent monitoring as investor-owners become increasingly influential in healthcare.”
Going forward, said Gupta, an open question is whether the harmful effects of PE ownership of practices are differentially worse than those of other corporate entities such as insurers and hospital systems.
“There are reasons to believe that PE could be worse in theory. For example, their short-term investment horizon may force them to take measures that others will not as well as avoid investing into capital improvements that have a long-run payoff,” he said. “Their uniquely high dependence on debt and unbundling of real estate can severely hurt financial solvency of providers.” But high-quality evidence is lacking to compare the effects from these two distinct forms of corporatization.
The trend away from individual private practice is a reality, Arnold said. “The administrative burden on solo docs is becoming too much and physicians just seem to want to treat patients and not deal with it. So the options at this point really become selling to a hospital system or private equity.”
This study was funded by a grant from the philanthropic foundation Arnold Ventures (no family relation to Daniel Arnold).
Arnold reported receiving grants from Arnold Ventures during the conduct of the study. Gupta had no competing interests to declare. Zhu reported receiving grants from the Agency for Healthcare Research and Quality during the submitted work and from the National Institutes of Health, National Institute for Health Care Management Foundation, and American Psychological Association, as well as personal fees from Cambia outside the submitted work.
A version of this article appeared on Medscape.com.
Less Invasive Sponge Test Stratifies Risk in Patients With Barrett’s Esophagus
The biomarker risk panel collected by the panesophageal Cytosponge-on-a-string in more than 900 UK patients helped identify those at highest risk for dysplasia or cancer and needing endoscopy. It was found safe for following low-risk patients who did not need endoscopy.
Endoscopic surveillance is the clinical standard for BE, but its effectiveness is inconsistent, wrote Rebecca C. Fitzgerald, MD, AGAF, professor in the Early Cancer Institute at the University of Cambridge in Cambridge, England, and colleagues in The Lancet.
“It is often performed by nonspecialists, and recent trials show that around 10% of cases of dysplasia and cancer are missed, which means some patients re-present within a year of their surveillance procedure with a symptomatic cancer that should have been diagnosed earlier,” Fitzgerald told GI & Hepatology News.
Moreover, repeated endoscopy monitoring is stressful. “A simple nonendoscopic capsule sponge test done nearer to home is less scary and could be less operator-dependent. By reducing the burden of endoscopy in patients at very low risk we can focus more on the patients at higher risk,” she said.
In 2022, her research group had reported that the capsule sponge test, coupled with a centralized lab test for p53 and atypia, can risk-stratify patients into low-, moderate-, and high-risk groups. “In the current study, we wanted to check this risk stratification capsule sponge test in the real world. Our main aim was to see if we could conform the 2022 results with the hypothesis that the low-risk patients — more than 50% of patients in surveillance — would have a risk of high-grade dysplasia or cancer that was sufficiently low — that is, less than from 3% — and could therefore have follow-up with the capsule sponge without requiring endoscopy.”
The investigators hypothesized that the 15% at high risk would have a significant chance of dysplasia warranting endoscopy in a specialist center.
“Our results showed that in the low-risk group the risk of high-grade dysplasia or cancer was 0.4%, suggesting these patients could be offered follow-up with the capsule sponge test,” Fitzgerald said.
The high-risk group with a double biomarker positive (p53 and atypia) had an 85% risk for dysplasia or cancer. “We call this a tier 1 or ultra-high risk, and this suggests these cases merit a specialist endoscopy in a center that could treat the dysplasia/cancer,” she said.
Study Details
Adult participants (n = 910) were recruited from August 2020 to December 2024 in two multicenter, prospective, pragmatic implementation studies from 13 hospitals. Patients with nondysplastic BE on last endoscopy had a capsule sponge test.
Patient risk was assigned as low (clinical and capsule sponge biomarkers negative), moderate (negative for capsule sponge biomarkers, positive clinical biomarkers: age, sex, and segment length), or high risk (p53 abnormality, glandular atypia regardless of clinical biomarkers, or both). The primary outcome was a diagnosis of high-grade dysplasia or cancer necessitating treatment, according to the risk group.
In the cohort, 138 (15%) were classified as having high risk, 283 (31%) had moderate risk, and 489 (54%) had low risk.
The positive predictive value for any dysplasia or worse in the high-risk group was 37.7% (95% CI, 29.7-46.4). Patients with both atypia and aberrant p53 had the highest risk for high-grade dysplasia or cancer with a relative risk of 135.8 (95% CI, 32.7-564.0) vs the low-risk group.
The prevalence of high-grade dysplasia or cancer in the low-risk group was, as mentioned, just 0.4% (95% CI, 0.1-1.6), while the negative predictive value for any dysplasia or cancer was 97.8% (95% CI, 95.9-98.8). Applying a machine learning algorithm reduced the proportion needing p53 pathology review to 32% without missing any positive cases.
Offering a US perspective on the study, Nicholas J. Shaheen, MD, MPH, AGAF, professor of medicine and director of the NC Translational & Clinical Sciences Institute at the University of North Carolina School of Medicine in Chapel Hill, called the findings “very provocative.”
“We have known for some time that nonendoscopic techniques could be used to screen for Barrett’s esophagus and esophageal cancer, allowing us to screen larger groups of patients in a more cost-effective manner compared to traditional upper endoscopy,” he told GI & Hepatology News. “This study suggests that, in addition to case-finding for Barrett’s [esophagus], a nonendoscopic sponge-based technique can also help us stratify risk, finding cases that either already harbor cancer or are at high risk to do so.”
Shaheen said these cases deserve immediate attention since they are most likely to benefit from timely endoscopic intervention. “The study also suggests that a nonendoscopic result could someday be used to decide subsequent follow-up, with low-risk patients undergoing further nonendoscopic surveillance, while higher-risk patients would move on to endoscopy. Such a paradigm could unburden our endoscopy units from low-risk patients unlikely to benefit from endoscopy as well as increase the numbers of patients who are able to be screened.”
Fitzgerald added, “The GI community is realizing that we need a better approach to managing patients with Barrett’s [esophagus]. In the UK this evidence is being considered by our guideline committee, and it would influence the upcoming guidelines in 2025 with a requirement to continue to audit the results. Outside of the UK we hope this will pave the way for nonendoscopic approaches to Barrett’s [esophagus] surveillance.”
One ongoing goal is to optimize the biomarkers, Fitzgerald said. “For patients with longer segments we would like to add additional genomic biomarkers to refine the risk predictions,” she said. “We need a more operator-independent, consistent method for monitoring Barrett’s [esophagus]. This large real-world study is highly encouraging for a more personalized and patient-friendly approach to Barrett’s [esophagus] surveillance.”
This study was funded by Innovate UK, Cancer Research UK, National Health Service England Cancer Alliance. Cytosponge technology is licensed by the Medical Research Council to Medtronic. Fitzgerald declared holding patents related to this test. Fitzgerald reported being a shareholder in Cyted Health.
Shaheen reported receiving research funding from Lucid Diagnostics and Cyted Health, both of which are manufacturers of nonendoscopic screening devices for BE.
A version of this article appeared on Medscape.com.
The biomarker risk panel collected by the panesophageal Cytosponge-on-a-string in more than 900 UK patients helped identify those at highest risk for dysplasia or cancer and needing endoscopy. It was found safe for following low-risk patients who did not need endoscopy.
Endoscopic surveillance is the clinical standard for BE, but its effectiveness is inconsistent, wrote Rebecca C. Fitzgerald, MD, AGAF, professor in the Early Cancer Institute at the University of Cambridge in Cambridge, England, and colleagues in The Lancet.
“It is often performed by nonspecialists, and recent trials show that around 10% of cases of dysplasia and cancer are missed, which means some patients re-present within a year of their surveillance procedure with a symptomatic cancer that should have been diagnosed earlier,” Fitzgerald told GI & Hepatology News.
Moreover, repeated endoscopy monitoring is stressful. “A simple nonendoscopic capsule sponge test done nearer to home is less scary and could be less operator-dependent. By reducing the burden of endoscopy in patients at very low risk we can focus more on the patients at higher risk,” she said.
In 2022, her research group had reported that the capsule sponge test, coupled with a centralized lab test for p53 and atypia, can risk-stratify patients into low-, moderate-, and high-risk groups. “In the current study, we wanted to check this risk stratification capsule sponge test in the real world. Our main aim was to see if we could conform the 2022 results with the hypothesis that the low-risk patients — more than 50% of patients in surveillance — would have a risk of high-grade dysplasia or cancer that was sufficiently low — that is, less than from 3% — and could therefore have follow-up with the capsule sponge without requiring endoscopy.”
The investigators hypothesized that the 15% at high risk would have a significant chance of dysplasia warranting endoscopy in a specialist center.
“Our results showed that in the low-risk group the risk of high-grade dysplasia or cancer was 0.4%, suggesting these patients could be offered follow-up with the capsule sponge test,” Fitzgerald said.
The high-risk group with a double biomarker positive (p53 and atypia) had an 85% risk for dysplasia or cancer. “We call this a tier 1 or ultra-high risk, and this suggests these cases merit a specialist endoscopy in a center that could treat the dysplasia/cancer,” she said.
Study Details
Adult participants (n = 910) were recruited from August 2020 to December 2024 in two multicenter, prospective, pragmatic implementation studies from 13 hospitals. Patients with nondysplastic BE on last endoscopy had a capsule sponge test.
Patient risk was assigned as low (clinical and capsule sponge biomarkers negative), moderate (negative for capsule sponge biomarkers, positive clinical biomarkers: age, sex, and segment length), or high risk (p53 abnormality, glandular atypia regardless of clinical biomarkers, or both). The primary outcome was a diagnosis of high-grade dysplasia or cancer necessitating treatment, according to the risk group.
In the cohort, 138 (15%) were classified as having high risk, 283 (31%) had moderate risk, and 489 (54%) had low risk.
The positive predictive value for any dysplasia or worse in the high-risk group was 37.7% (95% CI, 29.7-46.4). Patients with both atypia and aberrant p53 had the highest risk for high-grade dysplasia or cancer with a relative risk of 135.8 (95% CI, 32.7-564.0) vs the low-risk group.
The prevalence of high-grade dysplasia or cancer in the low-risk group was, as mentioned, just 0.4% (95% CI, 0.1-1.6), while the negative predictive value for any dysplasia or cancer was 97.8% (95% CI, 95.9-98.8). Applying a machine learning algorithm reduced the proportion needing p53 pathology review to 32% without missing any positive cases.
Offering a US perspective on the study, Nicholas J. Shaheen, MD, MPH, AGAF, professor of medicine and director of the NC Translational & Clinical Sciences Institute at the University of North Carolina School of Medicine in Chapel Hill, called the findings “very provocative.”
“We have known for some time that nonendoscopic techniques could be used to screen for Barrett’s esophagus and esophageal cancer, allowing us to screen larger groups of patients in a more cost-effective manner compared to traditional upper endoscopy,” he told GI & Hepatology News. “This study suggests that, in addition to case-finding for Barrett’s [esophagus], a nonendoscopic sponge-based technique can also help us stratify risk, finding cases that either already harbor cancer or are at high risk to do so.”
Shaheen said these cases deserve immediate attention since they are most likely to benefit from timely endoscopic intervention. “The study also suggests that a nonendoscopic result could someday be used to decide subsequent follow-up, with low-risk patients undergoing further nonendoscopic surveillance, while higher-risk patients would move on to endoscopy. Such a paradigm could unburden our endoscopy units from low-risk patients unlikely to benefit from endoscopy as well as increase the numbers of patients who are able to be screened.”
Fitzgerald added, “The GI community is realizing that we need a better approach to managing patients with Barrett’s [esophagus]. In the UK this evidence is being considered by our guideline committee, and it would influence the upcoming guidelines in 2025 with a requirement to continue to audit the results. Outside of the UK we hope this will pave the way for nonendoscopic approaches to Barrett’s [esophagus] surveillance.”
One ongoing goal is to optimize the biomarkers, Fitzgerald said. “For patients with longer segments we would like to add additional genomic biomarkers to refine the risk predictions,” she said. “We need a more operator-independent, consistent method for monitoring Barrett’s [esophagus]. This large real-world study is highly encouraging for a more personalized and patient-friendly approach to Barrett’s [esophagus] surveillance.”
This study was funded by Innovate UK, Cancer Research UK, National Health Service England Cancer Alliance. Cytosponge technology is licensed by the Medical Research Council to Medtronic. Fitzgerald declared holding patents related to this test. Fitzgerald reported being a shareholder in Cyted Health.
Shaheen reported receiving research funding from Lucid Diagnostics and Cyted Health, both of which are manufacturers of nonendoscopic screening devices for BE.
A version of this article appeared on Medscape.com.
The biomarker risk panel collected by the panesophageal Cytosponge-on-a-string in more than 900 UK patients helped identify those at highest risk for dysplasia or cancer and needing endoscopy. It was found safe for following low-risk patients who did not need endoscopy.
Endoscopic surveillance is the clinical standard for BE, but its effectiveness is inconsistent, wrote Rebecca C. Fitzgerald, MD, AGAF, professor in the Early Cancer Institute at the University of Cambridge in Cambridge, England, and colleagues in The Lancet.
“It is often performed by nonspecialists, and recent trials show that around 10% of cases of dysplasia and cancer are missed, which means some patients re-present within a year of their surveillance procedure with a symptomatic cancer that should have been diagnosed earlier,” Fitzgerald told GI & Hepatology News.
Moreover, repeated endoscopy monitoring is stressful. “A simple nonendoscopic capsule sponge test done nearer to home is less scary and could be less operator-dependent. By reducing the burden of endoscopy in patients at very low risk we can focus more on the patients at higher risk,” she said.
In 2022, her research group had reported that the capsule sponge test, coupled with a centralized lab test for p53 and atypia, can risk-stratify patients into low-, moderate-, and high-risk groups. “In the current study, we wanted to check this risk stratification capsule sponge test in the real world. Our main aim was to see if we could conform the 2022 results with the hypothesis that the low-risk patients — more than 50% of patients in surveillance — would have a risk of high-grade dysplasia or cancer that was sufficiently low — that is, less than from 3% — and could therefore have follow-up with the capsule sponge without requiring endoscopy.”
The investigators hypothesized that the 15% at high risk would have a significant chance of dysplasia warranting endoscopy in a specialist center.
“Our results showed that in the low-risk group the risk of high-grade dysplasia or cancer was 0.4%, suggesting these patients could be offered follow-up with the capsule sponge test,” Fitzgerald said.
The high-risk group with a double biomarker positive (p53 and atypia) had an 85% risk for dysplasia or cancer. “We call this a tier 1 or ultra-high risk, and this suggests these cases merit a specialist endoscopy in a center that could treat the dysplasia/cancer,” she said.
Study Details
Adult participants (n = 910) were recruited from August 2020 to December 2024 in two multicenter, prospective, pragmatic implementation studies from 13 hospitals. Patients with nondysplastic BE on last endoscopy had a capsule sponge test.
Patient risk was assigned as low (clinical and capsule sponge biomarkers negative), moderate (negative for capsule sponge biomarkers, positive clinical biomarkers: age, sex, and segment length), or high risk (p53 abnormality, glandular atypia regardless of clinical biomarkers, or both). The primary outcome was a diagnosis of high-grade dysplasia or cancer necessitating treatment, according to the risk group.
In the cohort, 138 (15%) were classified as having high risk, 283 (31%) had moderate risk, and 489 (54%) had low risk.
The positive predictive value for any dysplasia or worse in the high-risk group was 37.7% (95% CI, 29.7-46.4). Patients with both atypia and aberrant p53 had the highest risk for high-grade dysplasia or cancer with a relative risk of 135.8 (95% CI, 32.7-564.0) vs the low-risk group.
The prevalence of high-grade dysplasia or cancer in the low-risk group was, as mentioned, just 0.4% (95% CI, 0.1-1.6), while the negative predictive value for any dysplasia or cancer was 97.8% (95% CI, 95.9-98.8). Applying a machine learning algorithm reduced the proportion needing p53 pathology review to 32% without missing any positive cases.
Offering a US perspective on the study, Nicholas J. Shaheen, MD, MPH, AGAF, professor of medicine and director of the NC Translational & Clinical Sciences Institute at the University of North Carolina School of Medicine in Chapel Hill, called the findings “very provocative.”
“We have known for some time that nonendoscopic techniques could be used to screen for Barrett’s esophagus and esophageal cancer, allowing us to screen larger groups of patients in a more cost-effective manner compared to traditional upper endoscopy,” he told GI & Hepatology News. “This study suggests that, in addition to case-finding for Barrett’s [esophagus], a nonendoscopic sponge-based technique can also help us stratify risk, finding cases that either already harbor cancer or are at high risk to do so.”
Shaheen said these cases deserve immediate attention since they are most likely to benefit from timely endoscopic intervention. “The study also suggests that a nonendoscopic result could someday be used to decide subsequent follow-up, with low-risk patients undergoing further nonendoscopic surveillance, while higher-risk patients would move on to endoscopy. Such a paradigm could unburden our endoscopy units from low-risk patients unlikely to benefit from endoscopy as well as increase the numbers of patients who are able to be screened.”
Fitzgerald added, “The GI community is realizing that we need a better approach to managing patients with Barrett’s [esophagus]. In the UK this evidence is being considered by our guideline committee, and it would influence the upcoming guidelines in 2025 with a requirement to continue to audit the results. Outside of the UK we hope this will pave the way for nonendoscopic approaches to Barrett’s [esophagus] surveillance.”
One ongoing goal is to optimize the biomarkers, Fitzgerald said. “For patients with longer segments we would like to add additional genomic biomarkers to refine the risk predictions,” she said. “We need a more operator-independent, consistent method for monitoring Barrett’s [esophagus]. This large real-world study is highly encouraging for a more personalized and patient-friendly approach to Barrett’s [esophagus] surveillance.”
This study was funded by Innovate UK, Cancer Research UK, National Health Service England Cancer Alliance. Cytosponge technology is licensed by the Medical Research Council to Medtronic. Fitzgerald declared holding patents related to this test. Fitzgerald reported being a shareholder in Cyted Health.
Shaheen reported receiving research funding from Lucid Diagnostics and Cyted Health, both of which are manufacturers of nonendoscopic screening devices for BE.
A version of this article appeared on Medscape.com.