User login
Large Language Models Cut Time, Cost of Guideline Development
, according to a pilot study from the American Gastroenterological Association (AGA).
Faster, cheaper study screening could allow societies to update clinical recommendations more frequently, improving alignment with the latest evidence, lead author Sunny Chung, MD, of Yale School of Medicine, New Haven, Connecticut, and colleagues, reported.
“Each guideline typically requires 5 to 15 systematic reviews, making the process time-consuming (averaging more than 60 weeks) and costly (more than $140,000),” the investigators wrote in Gastroenterology . “One of the most critical yet time-consuming steps in systematic reviews is title and abstract screening. LLMs have the potential to make this step more efficient.”
To test this approach, the investigators developed, validated, and applied a dual-model LLM screening pipeline with human-in-the-loop oversight, focusing on randomized controlled trials in AGA guidelines.
The system was built using the 2021 guideline on moderate-to-severe Crohn’s disease, targeting biologic therapies for induction and maintenance of remission.
Using chain-of-thought prompting and structured inclusion criteria based on the PICO framework, the investigators deployed GPT-4o (OpenAI) and Gemini-1.5-Pro (Google DeepMind) as independent screeners, each assessing titles and abstracts according to standardized logic encoded in JavaScript Object Notation. This approach mimicked a traditional double-reviewer system.
After initial testing, the pipeline was validated in a 2025 update of the same guideline, this time spanning 6 focused clinical questions on advanced therapies and immunomodulators. Results were compared against manual screening by 2 experienced human reviewers, with total screening time documented.
The system was then tested across 4 additional guideline topics: fecal microbiota transplantation (FMT) for irritable bowel syndrome and Clostridioides difficile, gastroparesis, and hepatocellular carcinoma. A final test applied the system to a forthcoming guideline on complications of acute pancreatitis.
Across all topics, the dual-LLM system achieved 100% sensitivity in identifying randomized controlled trials (RCTs). For the 2025 update of the AGA guideline on Crohn’s disease, the models flagged 418 of 4,377 abstracts for inclusion, captur-ing all 25 relevant RCTs in just 48 minutes. Manual screening of the same dataset previously took almost 13 hours.
Comparable accuracy and time savings were observed for the other topics.
The pipeline correctly flagged all 13 RCTs in 4,820 studies on FMT for irritable bowel syndrome, and all 16 RCTs in 5,587 studies on FMT for Clostridioides difficile, requiring 27 and 66 minutes, respectively. Similarly, the system captured all 11 RCTs in 3,919 hepatocellular carcinoma abstracts and all 18 RCTs in 1,578 studies on gastroparesis, completing each task in under 65 minutes. Early testing on the upcoming guideline for pancreatitis yielded similar results.
Cost analysis underscored the efficiency of this approach. At an estimated $175–200 per hour for expert screeners, traditional abstract screening would cost around $2,500 per review, versus approximately $100 for the LLM approach—a 96% reduction.
The investigators cautioned that human oversight remains necessary to verify the relevance of studies flagged by the models. While the system’s sensitivity was consistent, it also selected articles that were ultimately excluded by expert reviewers. Broader validation will be required to assess performance across non-RCT study designs, such as observational or case-control studies, they added.
“As medical literature continues to expand, the integration of artificial intelligence into evidence synthesis processes will become increasingly vital,” Dr. Chung and colleagues wrote. “With further refinement and broader validation, this LLM-based pipeline has the potential to revolutionize evidence synthesis and set a new standard for guideline development.”
This study was funded by National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. The investigators reported no conflicts of interest.
Ethan Goh, MD, executive director of the Stanford AI Research and Science Evaluation (ARISE) Network, described the AGA pilot as both timely and promising.
“I’m certainly bullish about the use case,” he said in an interview. “Their study design and application is also robust, so I would congratulate them.”
Goh, a general editor for BMJ Digital Health & AI, predicted “huge potential” in the strategy for both clinicians and the general population, who benefit from the most up-to-date guidelines possible.
“I believe that using AI can represent a much faster, more cost effective, efficient way of gathering all these information sources,” he said.
Still, humans will need to be involved in the process.
“[This AI-driven approach] will always need some degree of expert oversight and judgement,” Goh said.
Speaking more broadly about automating study aggregation, Goh said AI may still struggle to determine which studies are most clinically relevant.
“When we use [AI models] to pull out medical references, anecdotally, I don’t think they’re always getting the best ones all the time, or even necessarily the right ones,” he said.
And as AI models grow more impressive, these shortcomings become less apparent, potentially lulling humans into overconfidence.
“Humans are humans,” Goh said. “We get lazy over time. That will be one of the challenges. As the systems get increasingly good, humans start to defer more and more of their judgment to them and say, ‘All right, AI, you’re doing good. Just do 100% automation.’ And then [people] start fact checking or reviewing even less.”
AI could also undermine automated reviews in another way: AI-generated publications that appear genuine, but aren’t, may creep into the dataset.
Despite these concerns, Goh concluded on an optimistic note.
“I think that there are huge ways to use AI, tools, not to replace, but to augment and support human judgment,” he said.
Ethan Goh, MD, is senior research engineer and executive director of the Stanford AI Research and Science Evaluation (ARISE) Network, at Stanford (Calif.) University. He declared no conflicts of interest.
Ethan Goh, MD, executive director of the Stanford AI Research and Science Evaluation (ARISE) Network, described the AGA pilot as both timely and promising.
“I’m certainly bullish about the use case,” he said in an interview. “Their study design and application is also robust, so I would congratulate them.”
Goh, a general editor for BMJ Digital Health & AI, predicted “huge potential” in the strategy for both clinicians and the general population, who benefit from the most up-to-date guidelines possible.
“I believe that using AI can represent a much faster, more cost effective, efficient way of gathering all these information sources,” he said.
Still, humans will need to be involved in the process.
“[This AI-driven approach] will always need some degree of expert oversight and judgement,” Goh said.
Speaking more broadly about automating study aggregation, Goh said AI may still struggle to determine which studies are most clinically relevant.
“When we use [AI models] to pull out medical references, anecdotally, I don’t think they’re always getting the best ones all the time, or even necessarily the right ones,” he said.
And as AI models grow more impressive, these shortcomings become less apparent, potentially lulling humans into overconfidence.
“Humans are humans,” Goh said. “We get lazy over time. That will be one of the challenges. As the systems get increasingly good, humans start to defer more and more of their judgment to them and say, ‘All right, AI, you’re doing good. Just do 100% automation.’ And then [people] start fact checking or reviewing even less.”
AI could also undermine automated reviews in another way: AI-generated publications that appear genuine, but aren’t, may creep into the dataset.
Despite these concerns, Goh concluded on an optimistic note.
“I think that there are huge ways to use AI, tools, not to replace, but to augment and support human judgment,” he said.
Ethan Goh, MD, is senior research engineer and executive director of the Stanford AI Research and Science Evaluation (ARISE) Network, at Stanford (Calif.) University. He declared no conflicts of interest.
Ethan Goh, MD, executive director of the Stanford AI Research and Science Evaluation (ARISE) Network, described the AGA pilot as both timely and promising.
“I’m certainly bullish about the use case,” he said in an interview. “Their study design and application is also robust, so I would congratulate them.”
Goh, a general editor for BMJ Digital Health & AI, predicted “huge potential” in the strategy for both clinicians and the general population, who benefit from the most up-to-date guidelines possible.
“I believe that using AI can represent a much faster, more cost effective, efficient way of gathering all these information sources,” he said.
Still, humans will need to be involved in the process.
“[This AI-driven approach] will always need some degree of expert oversight and judgement,” Goh said.
Speaking more broadly about automating study aggregation, Goh said AI may still struggle to determine which studies are most clinically relevant.
“When we use [AI models] to pull out medical references, anecdotally, I don’t think they’re always getting the best ones all the time, or even necessarily the right ones,” he said.
And as AI models grow more impressive, these shortcomings become less apparent, potentially lulling humans into overconfidence.
“Humans are humans,” Goh said. “We get lazy over time. That will be one of the challenges. As the systems get increasingly good, humans start to defer more and more of their judgment to them and say, ‘All right, AI, you’re doing good. Just do 100% automation.’ And then [people] start fact checking or reviewing even less.”
AI could also undermine automated reviews in another way: AI-generated publications that appear genuine, but aren’t, may creep into the dataset.
Despite these concerns, Goh concluded on an optimistic note.
“I think that there are huge ways to use AI, tools, not to replace, but to augment and support human judgment,” he said.
Ethan Goh, MD, is senior research engineer and executive director of the Stanford AI Research and Science Evaluation (ARISE) Network, at Stanford (Calif.) University. He declared no conflicts of interest.
, according to a pilot study from the American Gastroenterological Association (AGA).
Faster, cheaper study screening could allow societies to update clinical recommendations more frequently, improving alignment with the latest evidence, lead author Sunny Chung, MD, of Yale School of Medicine, New Haven, Connecticut, and colleagues, reported.
“Each guideline typically requires 5 to 15 systematic reviews, making the process time-consuming (averaging more than 60 weeks) and costly (more than $140,000),” the investigators wrote in Gastroenterology . “One of the most critical yet time-consuming steps in systematic reviews is title and abstract screening. LLMs have the potential to make this step more efficient.”
To test this approach, the investigators developed, validated, and applied a dual-model LLM screening pipeline with human-in-the-loop oversight, focusing on randomized controlled trials in AGA guidelines.
The system was built using the 2021 guideline on moderate-to-severe Crohn’s disease, targeting biologic therapies for induction and maintenance of remission.
Using chain-of-thought prompting and structured inclusion criteria based on the PICO framework, the investigators deployed GPT-4o (OpenAI) and Gemini-1.5-Pro (Google DeepMind) as independent screeners, each assessing titles and abstracts according to standardized logic encoded in JavaScript Object Notation. This approach mimicked a traditional double-reviewer system.
After initial testing, the pipeline was validated in a 2025 update of the same guideline, this time spanning 6 focused clinical questions on advanced therapies and immunomodulators. Results were compared against manual screening by 2 experienced human reviewers, with total screening time documented.
The system was then tested across 4 additional guideline topics: fecal microbiota transplantation (FMT) for irritable bowel syndrome and Clostridioides difficile, gastroparesis, and hepatocellular carcinoma. A final test applied the system to a forthcoming guideline on complications of acute pancreatitis.
Across all topics, the dual-LLM system achieved 100% sensitivity in identifying randomized controlled trials (RCTs). For the 2025 update of the AGA guideline on Crohn’s disease, the models flagged 418 of 4,377 abstracts for inclusion, captur-ing all 25 relevant RCTs in just 48 minutes. Manual screening of the same dataset previously took almost 13 hours.
Comparable accuracy and time savings were observed for the other topics.
The pipeline correctly flagged all 13 RCTs in 4,820 studies on FMT for irritable bowel syndrome, and all 16 RCTs in 5,587 studies on FMT for Clostridioides difficile, requiring 27 and 66 minutes, respectively. Similarly, the system captured all 11 RCTs in 3,919 hepatocellular carcinoma abstracts and all 18 RCTs in 1,578 studies on gastroparesis, completing each task in under 65 minutes. Early testing on the upcoming guideline for pancreatitis yielded similar results.
Cost analysis underscored the efficiency of this approach. At an estimated $175–200 per hour for expert screeners, traditional abstract screening would cost around $2,500 per review, versus approximately $100 for the LLM approach—a 96% reduction.
The investigators cautioned that human oversight remains necessary to verify the relevance of studies flagged by the models. While the system’s sensitivity was consistent, it also selected articles that were ultimately excluded by expert reviewers. Broader validation will be required to assess performance across non-RCT study designs, such as observational or case-control studies, they added.
“As medical literature continues to expand, the integration of artificial intelligence into evidence synthesis processes will become increasingly vital,” Dr. Chung and colleagues wrote. “With further refinement and broader validation, this LLM-based pipeline has the potential to revolutionize evidence synthesis and set a new standard for guideline development.”
This study was funded by National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. The investigators reported no conflicts of interest.
, according to a pilot study from the American Gastroenterological Association (AGA).
Faster, cheaper study screening could allow societies to update clinical recommendations more frequently, improving alignment with the latest evidence, lead author Sunny Chung, MD, of Yale School of Medicine, New Haven, Connecticut, and colleagues, reported.
“Each guideline typically requires 5 to 15 systematic reviews, making the process time-consuming (averaging more than 60 weeks) and costly (more than $140,000),” the investigators wrote in Gastroenterology . “One of the most critical yet time-consuming steps in systematic reviews is title and abstract screening. LLMs have the potential to make this step more efficient.”
To test this approach, the investigators developed, validated, and applied a dual-model LLM screening pipeline with human-in-the-loop oversight, focusing on randomized controlled trials in AGA guidelines.
The system was built using the 2021 guideline on moderate-to-severe Crohn’s disease, targeting biologic therapies for induction and maintenance of remission.
Using chain-of-thought prompting and structured inclusion criteria based on the PICO framework, the investigators deployed GPT-4o (OpenAI) and Gemini-1.5-Pro (Google DeepMind) as independent screeners, each assessing titles and abstracts according to standardized logic encoded in JavaScript Object Notation. This approach mimicked a traditional double-reviewer system.
After initial testing, the pipeline was validated in a 2025 update of the same guideline, this time spanning 6 focused clinical questions on advanced therapies and immunomodulators. Results were compared against manual screening by 2 experienced human reviewers, with total screening time documented.
The system was then tested across 4 additional guideline topics: fecal microbiota transplantation (FMT) for irritable bowel syndrome and Clostridioides difficile, gastroparesis, and hepatocellular carcinoma. A final test applied the system to a forthcoming guideline on complications of acute pancreatitis.
Across all topics, the dual-LLM system achieved 100% sensitivity in identifying randomized controlled trials (RCTs). For the 2025 update of the AGA guideline on Crohn’s disease, the models flagged 418 of 4,377 abstracts for inclusion, captur-ing all 25 relevant RCTs in just 48 minutes. Manual screening of the same dataset previously took almost 13 hours.
Comparable accuracy and time savings were observed for the other topics.
The pipeline correctly flagged all 13 RCTs in 4,820 studies on FMT for irritable bowel syndrome, and all 16 RCTs in 5,587 studies on FMT for Clostridioides difficile, requiring 27 and 66 minutes, respectively. Similarly, the system captured all 11 RCTs in 3,919 hepatocellular carcinoma abstracts and all 18 RCTs in 1,578 studies on gastroparesis, completing each task in under 65 minutes. Early testing on the upcoming guideline for pancreatitis yielded similar results.
Cost analysis underscored the efficiency of this approach. At an estimated $175–200 per hour for expert screeners, traditional abstract screening would cost around $2,500 per review, versus approximately $100 for the LLM approach—a 96% reduction.
The investigators cautioned that human oversight remains necessary to verify the relevance of studies flagged by the models. While the system’s sensitivity was consistent, it also selected articles that were ultimately excluded by expert reviewers. Broader validation will be required to assess performance across non-RCT study designs, such as observational or case-control studies, they added.
“As medical literature continues to expand, the integration of artificial intelligence into evidence synthesis processes will become increasingly vital,” Dr. Chung and colleagues wrote. “With further refinement and broader validation, this LLM-based pipeline has the potential to revolutionize evidence synthesis and set a new standard for guideline development.”
This study was funded by National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. The investigators reported no conflicts of interest.
FROM GASTROENTEROLOGY
New Guidelines for Pregnancy and IBD Aim to Quell Fears
, suggesting this approach will not harm the fetus.
The guidelines also recommend that all women with IBD receive preconception counseling and be followed as high-risk pregnancies.
“Management of chronic illness in pregnant women has always been defined by fear of harming the fetus,” said Uma Mahadevan, MD, AGAF, director of the Colitis and Crohn’s Disease Center at the University of California San Francisco and chair of the Global Consensus Consortium that developed the guidelines.
As a result, pregnant women are excluded from clinical trials of experimental therapies for IBD. And when a new therapy achieves regulatory approval, there are no human pregnancy safety data, only animal data. To fill this gap, the PIANO study, of which Mahadevan is principal investigator, looked at the safety of IBD medications in pregnancy and short- and long-term outcomes of the children.
“With our ongoing work in pregnancy in the patient with IBD, we realized that inflammation in the mother is the leading cause of poor outcome for the infant,” she told GI & Hepatology News.
“We also have a better understanding of placental transfer of biologic agents” and the lack of exposure to the fetus during the first trimester, “a key period of organogenesis,” she added.
Final recommendations were published simultaneously in six international journals, namely, Clinical Gastroenterology and Hepatology, American Journal of Gastroenterology, GUT, Inflammatory Bowel Diseases, Journal of Crohn’s and Colitis, and Alimentary Pharmacology and Therapeutics.
Surprising, Novel Findings
Limited provider knowledge led to varied practices in caring for women with IBD who become pregnant, according to the consensus authors. Practices are affected by local dogma, available resources, individual interpretation of the literature, and fear of harming the fetus.
“The variations in guidelines by different societies and countries reflect this and lead to confusion for physicians and patients alike,” the authors of the guidelines wrote.
Therefore, the Global Consensus Consortium — a group of 39 IBD experts, including teratologists and maternal fetal medicine specialists and seven patient advocates from six continents — convened to review and assess current data and come to an agreement on best practices. The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) process was used when sufficient published data were available, and the Research and Development process when expert opinion was needed to guide consistent practice.
“Some of the findings were expected, but others were novel,” said Mahadevan.
Recommendations that might surprise clinicians include GRADE statement 9, which suggests that pregnant women with IBD take low-dose aspirin by 12 to 16 weeks’ gestation to prevent preterm preeclampsia. “This is based on the ASPRE study, showing that women at risk of preeclampsia can lower their risk by taking low-dose aspirin,” with no risk for flare, Mahadevan said.
In addition, GRADE statements 17-20 recommend/suggest that women continue their biologic throughout pregnancy without stopping. “North America has always recommended continuing during the third trimester, while Europe only recently has come to this,” Mahadevan said. “However, there was always some looseness about stopping at week X, Y, or Z. Now, we do recommend continuing the dose on schedule with no holding.”
Continuing medications considered low risk for use during pregnancy, such as 5-amino salicylic acids, sulfasalazine, thiopurines, and all monoclonal antibodies during preconception, pregnancy, and lactation, was also recommended.
However, small-molecule drugs such as S1P receptor molecules and JAK inhibitors should be avoided for at least 1 month, and in some cases for 3 months prior to attempting conception, unless there is no alternative for the health of the mother. They should also be avoided during lactation.
Grade statement 33, which suggests that live rotavirus vaccine may be provided in children with in utero exposure to biologics, is also new, Mahadevan noted. “All prior recommendations were that no live vaccine should be given in the first 6 months or longer if infants were exposed to biologics in utero, but based on a prospective Canadian study, there is no harm when given to these infants.”
Another novel recommendation is that women with IBD on any monoclonal antibodies, including newer interleukin-23s, may breastfeed even though there are not clinical trial data at this point. The recommendation to continue them through pregnancy and lactation is based on placental physiology, as well as on the physiology of monoclonal antibody transfer in breast milk, according to the consortium.
Furthermore, the authors noted, there was no increase in infant infections at 4 months or 12 months if they were exposed to a biologic or thiopurine (or both) during pregnancy.
Overall, the consortium recommended that all pregnancies for women with IBD be considered as “high risk” for complications. This is due to the fact that many parts of the world, including the US, are “resource-limited,” Mahadevan explained. Since maternal fetal medicine specialists are not widely available, the consortium suggested all these patients be followed with increased monitoring and surveillance based on available resources.
In addition to the guidelines, patient videos in seven languages, a professional slide deck in English and Spanish, and a video on the global consensus are all available at https://pianostudy.org/.
This study was funded by The Leona B. and Harry H. Helmsley Charitable Trust.
Mahadevan reported being a consultant for AbbVie, Bristol Myers Squibb, Boehringer Ingelheim, Celltrion, Enveda, Gilead, Janssen, Lilly, Merck, Pfizer, Protagonist, Roivant, and Takeda.
A version of this article appeared on Medscape.com.
, suggesting this approach will not harm the fetus.
The guidelines also recommend that all women with IBD receive preconception counseling and be followed as high-risk pregnancies.
“Management of chronic illness in pregnant women has always been defined by fear of harming the fetus,” said Uma Mahadevan, MD, AGAF, director of the Colitis and Crohn’s Disease Center at the University of California San Francisco and chair of the Global Consensus Consortium that developed the guidelines.
As a result, pregnant women are excluded from clinical trials of experimental therapies for IBD. And when a new therapy achieves regulatory approval, there are no human pregnancy safety data, only animal data. To fill this gap, the PIANO study, of which Mahadevan is principal investigator, looked at the safety of IBD medications in pregnancy and short- and long-term outcomes of the children.
“With our ongoing work in pregnancy in the patient with IBD, we realized that inflammation in the mother is the leading cause of poor outcome for the infant,” she told GI & Hepatology News.
“We also have a better understanding of placental transfer of biologic agents” and the lack of exposure to the fetus during the first trimester, “a key period of organogenesis,” she added.
Final recommendations were published simultaneously in six international journals, namely, Clinical Gastroenterology and Hepatology, American Journal of Gastroenterology, GUT, Inflammatory Bowel Diseases, Journal of Crohn’s and Colitis, and Alimentary Pharmacology and Therapeutics.
Surprising, Novel Findings
Limited provider knowledge led to varied practices in caring for women with IBD who become pregnant, according to the consensus authors. Practices are affected by local dogma, available resources, individual interpretation of the literature, and fear of harming the fetus.
“The variations in guidelines by different societies and countries reflect this and lead to confusion for physicians and patients alike,” the authors of the guidelines wrote.
Therefore, the Global Consensus Consortium — a group of 39 IBD experts, including teratologists and maternal fetal medicine specialists and seven patient advocates from six continents — convened to review and assess current data and come to an agreement on best practices. The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) process was used when sufficient published data were available, and the Research and Development process when expert opinion was needed to guide consistent practice.
“Some of the findings were expected, but others were novel,” said Mahadevan.
Recommendations that might surprise clinicians include GRADE statement 9, which suggests that pregnant women with IBD take low-dose aspirin by 12 to 16 weeks’ gestation to prevent preterm preeclampsia. “This is based on the ASPRE study, showing that women at risk of preeclampsia can lower their risk by taking low-dose aspirin,” with no risk for flare, Mahadevan said.
In addition, GRADE statements 17-20 recommend/suggest that women continue their biologic throughout pregnancy without stopping. “North America has always recommended continuing during the third trimester, while Europe only recently has come to this,” Mahadevan said. “However, there was always some looseness about stopping at week X, Y, or Z. Now, we do recommend continuing the dose on schedule with no holding.”
Continuing medications considered low risk for use during pregnancy, such as 5-amino salicylic acids, sulfasalazine, thiopurines, and all monoclonal antibodies during preconception, pregnancy, and lactation, was also recommended.
However, small-molecule drugs such as S1P receptor molecules and JAK inhibitors should be avoided for at least 1 month, and in some cases for 3 months prior to attempting conception, unless there is no alternative for the health of the mother. They should also be avoided during lactation.
Grade statement 33, which suggests that live rotavirus vaccine may be provided in children with in utero exposure to biologics, is also new, Mahadevan noted. “All prior recommendations were that no live vaccine should be given in the first 6 months or longer if infants were exposed to biologics in utero, but based on a prospective Canadian study, there is no harm when given to these infants.”
Another novel recommendation is that women with IBD on any monoclonal antibodies, including newer interleukin-23s, may breastfeed even though there are not clinical trial data at this point. The recommendation to continue them through pregnancy and lactation is based on placental physiology, as well as on the physiology of monoclonal antibody transfer in breast milk, according to the consortium.
Furthermore, the authors noted, there was no increase in infant infections at 4 months or 12 months if they were exposed to a biologic or thiopurine (or both) during pregnancy.
Overall, the consortium recommended that all pregnancies for women with IBD be considered as “high risk” for complications. This is due to the fact that many parts of the world, including the US, are “resource-limited,” Mahadevan explained. Since maternal fetal medicine specialists are not widely available, the consortium suggested all these patients be followed with increased monitoring and surveillance based on available resources.
In addition to the guidelines, patient videos in seven languages, a professional slide deck in English and Spanish, and a video on the global consensus are all available at https://pianostudy.org/.
This study was funded by The Leona B. and Harry H. Helmsley Charitable Trust.
Mahadevan reported being a consultant for AbbVie, Bristol Myers Squibb, Boehringer Ingelheim, Celltrion, Enveda, Gilead, Janssen, Lilly, Merck, Pfizer, Protagonist, Roivant, and Takeda.
A version of this article appeared on Medscape.com.
, suggesting this approach will not harm the fetus.
The guidelines also recommend that all women with IBD receive preconception counseling and be followed as high-risk pregnancies.
“Management of chronic illness in pregnant women has always been defined by fear of harming the fetus,” said Uma Mahadevan, MD, AGAF, director of the Colitis and Crohn’s Disease Center at the University of California San Francisco and chair of the Global Consensus Consortium that developed the guidelines.
As a result, pregnant women are excluded from clinical trials of experimental therapies for IBD. And when a new therapy achieves regulatory approval, there are no human pregnancy safety data, only animal data. To fill this gap, the PIANO study, of which Mahadevan is principal investigator, looked at the safety of IBD medications in pregnancy and short- and long-term outcomes of the children.
“With our ongoing work in pregnancy in the patient with IBD, we realized that inflammation in the mother is the leading cause of poor outcome for the infant,” she told GI & Hepatology News.
“We also have a better understanding of placental transfer of biologic agents” and the lack of exposure to the fetus during the first trimester, “a key period of organogenesis,” she added.
Final recommendations were published simultaneously in six international journals, namely, Clinical Gastroenterology and Hepatology, American Journal of Gastroenterology, GUT, Inflammatory Bowel Diseases, Journal of Crohn’s and Colitis, and Alimentary Pharmacology and Therapeutics.
Surprising, Novel Findings
Limited provider knowledge led to varied practices in caring for women with IBD who become pregnant, according to the consensus authors. Practices are affected by local dogma, available resources, individual interpretation of the literature, and fear of harming the fetus.
“The variations in guidelines by different societies and countries reflect this and lead to confusion for physicians and patients alike,” the authors of the guidelines wrote.
Therefore, the Global Consensus Consortium — a group of 39 IBD experts, including teratologists and maternal fetal medicine specialists and seven patient advocates from six continents — convened to review and assess current data and come to an agreement on best practices. The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) process was used when sufficient published data were available, and the Research and Development process when expert opinion was needed to guide consistent practice.
“Some of the findings were expected, but others were novel,” said Mahadevan.
Recommendations that might surprise clinicians include GRADE statement 9, which suggests that pregnant women with IBD take low-dose aspirin by 12 to 16 weeks’ gestation to prevent preterm preeclampsia. “This is based on the ASPRE study, showing that women at risk of preeclampsia can lower their risk by taking low-dose aspirin,” with no risk for flare, Mahadevan said.
In addition, GRADE statements 17-20 recommend/suggest that women continue their biologic throughout pregnancy without stopping. “North America has always recommended continuing during the third trimester, while Europe only recently has come to this,” Mahadevan said. “However, there was always some looseness about stopping at week X, Y, or Z. Now, we do recommend continuing the dose on schedule with no holding.”
Continuing medications considered low risk for use during pregnancy, such as 5-amino salicylic acids, sulfasalazine, thiopurines, and all monoclonal antibodies during preconception, pregnancy, and lactation, was also recommended.
However, small-molecule drugs such as S1P receptor molecules and JAK inhibitors should be avoided for at least 1 month, and in some cases for 3 months prior to attempting conception, unless there is no alternative for the health of the mother. They should also be avoided during lactation.
Grade statement 33, which suggests that live rotavirus vaccine may be provided in children with in utero exposure to biologics, is also new, Mahadevan noted. “All prior recommendations were that no live vaccine should be given in the first 6 months or longer if infants were exposed to biologics in utero, but based on a prospective Canadian study, there is no harm when given to these infants.”
Another novel recommendation is that women with IBD on any monoclonal antibodies, including newer interleukin-23s, may breastfeed even though there are not clinical trial data at this point. The recommendation to continue them through pregnancy and lactation is based on placental physiology, as well as on the physiology of monoclonal antibody transfer in breast milk, according to the consortium.
Furthermore, the authors noted, there was no increase in infant infections at 4 months or 12 months if they were exposed to a biologic or thiopurine (or both) during pregnancy.
Overall, the consortium recommended that all pregnancies for women with IBD be considered as “high risk” for complications. This is due to the fact that many parts of the world, including the US, are “resource-limited,” Mahadevan explained. Since maternal fetal medicine specialists are not widely available, the consortium suggested all these patients be followed with increased monitoring and surveillance based on available resources.
In addition to the guidelines, patient videos in seven languages, a professional slide deck in English and Spanish, and a video on the global consensus are all available at https://pianostudy.org/.
This study was funded by The Leona B. and Harry H. Helmsley Charitable Trust.
Mahadevan reported being a consultant for AbbVie, Bristol Myers Squibb, Boehringer Ingelheim, Celltrion, Enveda, Gilead, Janssen, Lilly, Merck, Pfizer, Protagonist, Roivant, and Takeda.
A version of this article appeared on Medscape.com.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
SGLT2 Inhibition Promising for Patients With Cirrhosis and on Diuretics
, a large cohort study of more than 10,000 patients found.
Reporting in JAMA Network Open, Dian J. Chiang, MD, MPH, a section head of Hepatology at the Cleveland Clinic in Cleveland looked at the impact of these antihyperglycemic agents, also known as gliflozins, used in diabetes and kidney disease to block the reabsorption of glucose in the kidneys and causing more glucose to be excreted in the urine.
“Patients with cirrhosis were previously not included in SGLT2 inhibition clinical trials, and there is no large real-world evidence on the safety and effectiveness of this class of medication in patients with cirrhosis. Therefore, we decided to conduct the study to assess its safety and effectiveness,” Chiang told GI & Hepatology News.
The study’s primary endpoint was a composite of serious hepatic events, defined as ascites, varices, hyponatremia, and all-cause mortality. Secondary outcomes included variceal bleeding, paracentesis, spontaneous bacterial peritonitis, hepatic encephalopathy, hepatorenal syndrome, liver carcinoma, hypoglycemia, and all-cause hospitalizations.
The cohort consisted of 10,660 propensity-matched adult patients with cirrhosis from more than 120 healthcare organizations in the TriNetX database who were receiving furosemide and spironolactone from January 2013 to July 2021. Those also receiving SGLT2 inhibitors (n = 5330) were compared with a matched control group receiving diuretics only (n = 5330). The mean age of participants was 63.8 years, 57.8% were men, 66.3% were White individuals, and all were followed for 3 years.
The SGLT2 inhibitor group had a 32% lower incidence of serious liver events than the control group, for a hazard ratio (HR) of 0.68 (95% CI, 0.66-0.71; P < .001).
Secondary risk reductions in the intervention group were as follows:
- Hepatorenal syndrome: HR, 0.47 (95% CI, 0.40-0.56)
- Spontaneous bacterial peritonitis: HR, 0.55 (95% CI, 0.46-0.65)
- Paracentesis: HR, 0.54 (95% CI, 0.50-0.60)
- Variceal bleeding: HR, 0.79 (95% CI, 0.73-0.84)
- Hypoglycemia: HR, 0.75 (95% CI, 0.62-0.91)
- All-cause hospitalizations: HR, 0.67 (95% CI, 0.63-0.71)
The authors conjectured that SGLT2 inhibition might also benefit patients with other stages of liver disease. They pointed to a 2020 study in patients with diabetes, metabolic dysfunction-associated steatotic liver disease (MASLD), and high baseline fibrosis that revealed a significant reduction in fibrosis after 12 months’ SGLT2 inhibition.
The study findings also align with those of another large propensity-matched cohort in which patients with type 2 diabetes and cirrhosis receiving metformin plus SGLT2 inhibition showed significantly lower 5-year mortality, decreased incidence of decompensated cirrhosis, and reduced hepatocellular carcinoma incidence compared with those taking metformin alone.
Prospective trials are needed to further evaluate safety and efficacy, however, the authors stressed. Future studies should specifically examine changes in sodium levels following SGLT2 inhibitor initiation, as well as the incidence of recurrent urinary tract infections and euglycemic diabetic ketoacidosis, given that these are known adverse effects of this drug class. Additionally, research comparing different types and dosing regimens would provide valuable insights into optimizing treatment.
Commenting on the analysis but not participating in it, Karn Wijarnpreecha, MD, MPH, a hepatologist at College of Medicine, The University of Arizona, Phoenix, said the study was interesting but did not show the adjusted HR for all-cause mortality separately from other serious liver events, “so we do not know if SGLT2 inhibitor group was associated with lower mortality.”
It would be premature to conclude that using SGLT2 inhibitors in patients with cirrhosis and ascites and on diuretics will decrease the need for liver transplant or significantly improve liver-related outcomes based on this study, Wijarnpreecha told GI & Hepatology News. “Moreover, we do not know the dose of diuretics or specific drugs and doses for SGLT2 inhibitors that were used in the study. Indications for using SGLT2 inhibitors are mainly from diabetes and heart failure, so this may not apply to those with cirrhosis without these two conditions as well.”
In addition, the etiology of cirrhosis in this database study is unknown. “Is it mainly from MASLD or alcohol or other conditions such as viral hepatitis or autoimmmunity? We need more thorough study to answer this question.” He also pointed out that the authors urged caution in using SGLT2 inhibitors in the context of hepatic encephalopathy (HE), which could be worsened with these agents. “This should be taken into consideration before starting medication in decompensated cirrhosis with HE,” Wijarnpreecha said.
In an accompanying commentary, Mohamed I. Elsaid, PhD, MPH, a biomedical informatics researcher and assistant professor at The Ohio State University in Columbus, Ohio, said that if confirmed, the findings could substantially improve cirrhosis care. “The signal is exciting but needs strong confirmation from large observational studies and prospective trials,” he wrote. “To turn promise into practice, the next wave of observational studies must embrace the target-trial emulation framework for bolstering firm causal conclusions and doubly robust learners that tease apart who benefits, who does not, and why.”
He added that head-to-head comparisons with the type 2 diabetes drugs known as incretin mimetics will clarify the best antihyperglycemic agents for different patient phenotypes. “With these advanced causal-inference approaches, repurposed type 2 diabetes therapies could shift cirrhosis management from reactive to proactive, improving quality of life and bending the mortality curve,” Elsaid wrote.
For Wijarnpreecha, important pending questions include the benefits of SGLT2 inhibition in cirrhosis without diabetes or heart failure “Can it be used to prevent cirrhosis in MASLD if we start at the early fibrosis stage in F0-F3?”
Chiang conceded that the study had limitations as it relied on 10th revision of the International Classification of Diseases codes to define outcomes, which may not have captured the complexity of cirrhotic complications. “And the retrospective design may have introduced confounding, selection, and information bias, which could have impacted the results,” he said. “Future prospective studies may help confirm our findings.”
No specific funding was reported for this study. The study authors and Wijarnpreecha had no relevant conflicts of interest to declare. Elsaid reported receiving research funding from Genentech and AstraZeneca outside of the submitted work.
A version of this article first appeared on Medscape.com.
, a large cohort study of more than 10,000 patients found.
Reporting in JAMA Network Open, Dian J. Chiang, MD, MPH, a section head of Hepatology at the Cleveland Clinic in Cleveland looked at the impact of these antihyperglycemic agents, also known as gliflozins, used in diabetes and kidney disease to block the reabsorption of glucose in the kidneys and causing more glucose to be excreted in the urine.
“Patients with cirrhosis were previously not included in SGLT2 inhibition clinical trials, and there is no large real-world evidence on the safety and effectiveness of this class of medication in patients with cirrhosis. Therefore, we decided to conduct the study to assess its safety and effectiveness,” Chiang told GI & Hepatology News.
The study’s primary endpoint was a composite of serious hepatic events, defined as ascites, varices, hyponatremia, and all-cause mortality. Secondary outcomes included variceal bleeding, paracentesis, spontaneous bacterial peritonitis, hepatic encephalopathy, hepatorenal syndrome, liver carcinoma, hypoglycemia, and all-cause hospitalizations.
The cohort consisted of 10,660 propensity-matched adult patients with cirrhosis from more than 120 healthcare organizations in the TriNetX database who were receiving furosemide and spironolactone from January 2013 to July 2021. Those also receiving SGLT2 inhibitors (n = 5330) were compared with a matched control group receiving diuretics only (n = 5330). The mean age of participants was 63.8 years, 57.8% were men, 66.3% were White individuals, and all were followed for 3 years.
The SGLT2 inhibitor group had a 32% lower incidence of serious liver events than the control group, for a hazard ratio (HR) of 0.68 (95% CI, 0.66-0.71; P < .001).
Secondary risk reductions in the intervention group were as follows:
- Hepatorenal syndrome: HR, 0.47 (95% CI, 0.40-0.56)
- Spontaneous bacterial peritonitis: HR, 0.55 (95% CI, 0.46-0.65)
- Paracentesis: HR, 0.54 (95% CI, 0.50-0.60)
- Variceal bleeding: HR, 0.79 (95% CI, 0.73-0.84)
- Hypoglycemia: HR, 0.75 (95% CI, 0.62-0.91)
- All-cause hospitalizations: HR, 0.67 (95% CI, 0.63-0.71)
The authors conjectured that SGLT2 inhibition might also benefit patients with other stages of liver disease. They pointed to a 2020 study in patients with diabetes, metabolic dysfunction-associated steatotic liver disease (MASLD), and high baseline fibrosis that revealed a significant reduction in fibrosis after 12 months’ SGLT2 inhibition.
The study findings also align with those of another large propensity-matched cohort in which patients with type 2 diabetes and cirrhosis receiving metformin plus SGLT2 inhibition showed significantly lower 5-year mortality, decreased incidence of decompensated cirrhosis, and reduced hepatocellular carcinoma incidence compared with those taking metformin alone.
Prospective trials are needed to further evaluate safety and efficacy, however, the authors stressed. Future studies should specifically examine changes in sodium levels following SGLT2 inhibitor initiation, as well as the incidence of recurrent urinary tract infections and euglycemic diabetic ketoacidosis, given that these are known adverse effects of this drug class. Additionally, research comparing different types and dosing regimens would provide valuable insights into optimizing treatment.
Commenting on the analysis but not participating in it, Karn Wijarnpreecha, MD, MPH, a hepatologist at College of Medicine, The University of Arizona, Phoenix, said the study was interesting but did not show the adjusted HR for all-cause mortality separately from other serious liver events, “so we do not know if SGLT2 inhibitor group was associated with lower mortality.”
It would be premature to conclude that using SGLT2 inhibitors in patients with cirrhosis and ascites and on diuretics will decrease the need for liver transplant or significantly improve liver-related outcomes based on this study, Wijarnpreecha told GI & Hepatology News. “Moreover, we do not know the dose of diuretics or specific drugs and doses for SGLT2 inhibitors that were used in the study. Indications for using SGLT2 inhibitors are mainly from diabetes and heart failure, so this may not apply to those with cirrhosis without these two conditions as well.”
In addition, the etiology of cirrhosis in this database study is unknown. “Is it mainly from MASLD or alcohol or other conditions such as viral hepatitis or autoimmmunity? We need more thorough study to answer this question.” He also pointed out that the authors urged caution in using SGLT2 inhibitors in the context of hepatic encephalopathy (HE), which could be worsened with these agents. “This should be taken into consideration before starting medication in decompensated cirrhosis with HE,” Wijarnpreecha said.
In an accompanying commentary, Mohamed I. Elsaid, PhD, MPH, a biomedical informatics researcher and assistant professor at The Ohio State University in Columbus, Ohio, said that if confirmed, the findings could substantially improve cirrhosis care. “The signal is exciting but needs strong confirmation from large observational studies and prospective trials,” he wrote. “To turn promise into practice, the next wave of observational studies must embrace the target-trial emulation framework for bolstering firm causal conclusions and doubly robust learners that tease apart who benefits, who does not, and why.”
He added that head-to-head comparisons with the type 2 diabetes drugs known as incretin mimetics will clarify the best antihyperglycemic agents for different patient phenotypes. “With these advanced causal-inference approaches, repurposed type 2 diabetes therapies could shift cirrhosis management from reactive to proactive, improving quality of life and bending the mortality curve,” Elsaid wrote.
For Wijarnpreecha, important pending questions include the benefits of SGLT2 inhibition in cirrhosis without diabetes or heart failure “Can it be used to prevent cirrhosis in MASLD if we start at the early fibrosis stage in F0-F3?”
Chiang conceded that the study had limitations as it relied on 10th revision of the International Classification of Diseases codes to define outcomes, which may not have captured the complexity of cirrhotic complications. “And the retrospective design may have introduced confounding, selection, and information bias, which could have impacted the results,” he said. “Future prospective studies may help confirm our findings.”
No specific funding was reported for this study. The study authors and Wijarnpreecha had no relevant conflicts of interest to declare. Elsaid reported receiving research funding from Genentech and AstraZeneca outside of the submitted work.
A version of this article first appeared on Medscape.com.
, a large cohort study of more than 10,000 patients found.
Reporting in JAMA Network Open, Dian J. Chiang, MD, MPH, a section head of Hepatology at the Cleveland Clinic in Cleveland looked at the impact of these antihyperglycemic agents, also known as gliflozins, used in diabetes and kidney disease to block the reabsorption of glucose in the kidneys and causing more glucose to be excreted in the urine.
“Patients with cirrhosis were previously not included in SGLT2 inhibition clinical trials, and there is no large real-world evidence on the safety and effectiveness of this class of medication in patients with cirrhosis. Therefore, we decided to conduct the study to assess its safety and effectiveness,” Chiang told GI & Hepatology News.
The study’s primary endpoint was a composite of serious hepatic events, defined as ascites, varices, hyponatremia, and all-cause mortality. Secondary outcomes included variceal bleeding, paracentesis, spontaneous bacterial peritonitis, hepatic encephalopathy, hepatorenal syndrome, liver carcinoma, hypoglycemia, and all-cause hospitalizations.
The cohort consisted of 10,660 propensity-matched adult patients with cirrhosis from more than 120 healthcare organizations in the TriNetX database who were receiving furosemide and spironolactone from January 2013 to July 2021. Those also receiving SGLT2 inhibitors (n = 5330) were compared with a matched control group receiving diuretics only (n = 5330). The mean age of participants was 63.8 years, 57.8% were men, 66.3% were White individuals, and all were followed for 3 years.
The SGLT2 inhibitor group had a 32% lower incidence of serious liver events than the control group, for a hazard ratio (HR) of 0.68 (95% CI, 0.66-0.71; P < .001).
Secondary risk reductions in the intervention group were as follows:
- Hepatorenal syndrome: HR, 0.47 (95% CI, 0.40-0.56)
- Spontaneous bacterial peritonitis: HR, 0.55 (95% CI, 0.46-0.65)
- Paracentesis: HR, 0.54 (95% CI, 0.50-0.60)
- Variceal bleeding: HR, 0.79 (95% CI, 0.73-0.84)
- Hypoglycemia: HR, 0.75 (95% CI, 0.62-0.91)
- All-cause hospitalizations: HR, 0.67 (95% CI, 0.63-0.71)
The authors conjectured that SGLT2 inhibition might also benefit patients with other stages of liver disease. They pointed to a 2020 study in patients with diabetes, metabolic dysfunction-associated steatotic liver disease (MASLD), and high baseline fibrosis that revealed a significant reduction in fibrosis after 12 months’ SGLT2 inhibition.
The study findings also align with those of another large propensity-matched cohort in which patients with type 2 diabetes and cirrhosis receiving metformin plus SGLT2 inhibition showed significantly lower 5-year mortality, decreased incidence of decompensated cirrhosis, and reduced hepatocellular carcinoma incidence compared with those taking metformin alone.
Prospective trials are needed to further evaluate safety and efficacy, however, the authors stressed. Future studies should specifically examine changes in sodium levels following SGLT2 inhibitor initiation, as well as the incidence of recurrent urinary tract infections and euglycemic diabetic ketoacidosis, given that these are known adverse effects of this drug class. Additionally, research comparing different types and dosing regimens would provide valuable insights into optimizing treatment.
Commenting on the analysis but not participating in it, Karn Wijarnpreecha, MD, MPH, a hepatologist at College of Medicine, The University of Arizona, Phoenix, said the study was interesting but did not show the adjusted HR for all-cause mortality separately from other serious liver events, “so we do not know if SGLT2 inhibitor group was associated with lower mortality.”
It would be premature to conclude that using SGLT2 inhibitors in patients with cirrhosis and ascites and on diuretics will decrease the need for liver transplant or significantly improve liver-related outcomes based on this study, Wijarnpreecha told GI & Hepatology News. “Moreover, we do not know the dose of diuretics or specific drugs and doses for SGLT2 inhibitors that were used in the study. Indications for using SGLT2 inhibitors are mainly from diabetes and heart failure, so this may not apply to those with cirrhosis without these two conditions as well.”
In addition, the etiology of cirrhosis in this database study is unknown. “Is it mainly from MASLD or alcohol or other conditions such as viral hepatitis or autoimmmunity? We need more thorough study to answer this question.” He also pointed out that the authors urged caution in using SGLT2 inhibitors in the context of hepatic encephalopathy (HE), which could be worsened with these agents. “This should be taken into consideration before starting medication in decompensated cirrhosis with HE,” Wijarnpreecha said.
In an accompanying commentary, Mohamed I. Elsaid, PhD, MPH, a biomedical informatics researcher and assistant professor at The Ohio State University in Columbus, Ohio, said that if confirmed, the findings could substantially improve cirrhosis care. “The signal is exciting but needs strong confirmation from large observational studies and prospective trials,” he wrote. “To turn promise into practice, the next wave of observational studies must embrace the target-trial emulation framework for bolstering firm causal conclusions and doubly robust learners that tease apart who benefits, who does not, and why.”
He added that head-to-head comparisons with the type 2 diabetes drugs known as incretin mimetics will clarify the best antihyperglycemic agents for different patient phenotypes. “With these advanced causal-inference approaches, repurposed type 2 diabetes therapies could shift cirrhosis management from reactive to proactive, improving quality of life and bending the mortality curve,” Elsaid wrote.
For Wijarnpreecha, important pending questions include the benefits of SGLT2 inhibition in cirrhosis without diabetes or heart failure “Can it be used to prevent cirrhosis in MASLD if we start at the early fibrosis stage in F0-F3?”
Chiang conceded that the study had limitations as it relied on 10th revision of the International Classification of Diseases codes to define outcomes, which may not have captured the complexity of cirrhotic complications. “And the retrospective design may have introduced confounding, selection, and information bias, which could have impacted the results,” he said. “Future prospective studies may help confirm our findings.”
No specific funding was reported for this study. The study authors and Wijarnpreecha had no relevant conflicts of interest to declare. Elsaid reported receiving research funding from Genentech and AstraZeneca outside of the submitted work.
A version of this article first appeared on Medscape.com.
Clinical Characteristics and Outcomes of Tall Cell Carcinoma with Reversed Polarity
Background
Tall cell carcinoma with reversed polarity (TCCRP) is a rare and distinct subtype of invasive breast carcinoma, defined by tall columnar cells with eosinophilic cytoplasm and reversed nuclear polarity. TCCRP remains poorly characterized in the literature, with limited population-level evidence to guide management and prognostication. This study uses the National Cancer Database (NCDB) to examine the epidemiology, clinical features, and outcomes of this neoplasm.
Methods
A retrospective cohort analysis included 951 patients diagnosed with TCCRP (ICD-O-3 code 8509) from 2018–2020 using the NCDB. Demographic and treatment variables were analyzed using descriptive statistics. Incidence trends were assessed using linear regression, and overall survival was evaluated using Kaplan-Meier methods.
Results
Most patients were female (98.1%) with a mean age of 69.1 years. The majority were White (82.0%), followed by Black (9.0%) and Hispanic (8.7%). Primary tumor sites included overlapping breast lesions (28.5%) and the upper-inner quadrant (27.0%). Incidence remained stable (R2 = 0.0). Most patients were diagnosed at Stage I (58.4%) and had a Charlson-Deyo score of 0 (76.2%). Socioeconomically, 41.8% lived in the highest income quartile (≥$74,063), and most had Medicare (64.7%). The most common treatment settings were comprehensive community cancer programs (40.3%). Surgery was performed in 95.6% of cases, with negative margins in 91.1%. Radiation therapy (46.6%) and hormone therapy (44.3%) were frequently used. Mortality was 1.1% at 30 days and 1.7% at 90 days. Survival was 98.9% at 2 years, 97.3% at 5 years, and 94.5% at 10 years, with a mean survival of 46.4 months.
Conclusions
This is the first NCDB-based study of TCCRP, highlighting favorable outcomes and distinct clinicodemographic features. Patients were predominantly older, White, and Medicare-insured, often receiving care at community cancer programs. These findings suggest that socioeconomic factors may influence access and treatment. Results may inform strategies to promote equitable care delivery across health systems and guide further research on clinical management and survivorship in TCCRP, particularly for rare cancers within community-based settings such as the VHA.
Background
Tall cell carcinoma with reversed polarity (TCCRP) is a rare and distinct subtype of invasive breast carcinoma, defined by tall columnar cells with eosinophilic cytoplasm and reversed nuclear polarity. TCCRP remains poorly characterized in the literature, with limited population-level evidence to guide management and prognostication. This study uses the National Cancer Database (NCDB) to examine the epidemiology, clinical features, and outcomes of this neoplasm.
Methods
A retrospective cohort analysis included 951 patients diagnosed with TCCRP (ICD-O-3 code 8509) from 2018–2020 using the NCDB. Demographic and treatment variables were analyzed using descriptive statistics. Incidence trends were assessed using linear regression, and overall survival was evaluated using Kaplan-Meier methods.
Results
Most patients were female (98.1%) with a mean age of 69.1 years. The majority were White (82.0%), followed by Black (9.0%) and Hispanic (8.7%). Primary tumor sites included overlapping breast lesions (28.5%) and the upper-inner quadrant (27.0%). Incidence remained stable (R2 = 0.0). Most patients were diagnosed at Stage I (58.4%) and had a Charlson-Deyo score of 0 (76.2%). Socioeconomically, 41.8% lived in the highest income quartile (≥$74,063), and most had Medicare (64.7%). The most common treatment settings were comprehensive community cancer programs (40.3%). Surgery was performed in 95.6% of cases, with negative margins in 91.1%. Radiation therapy (46.6%) and hormone therapy (44.3%) were frequently used. Mortality was 1.1% at 30 days and 1.7% at 90 days. Survival was 98.9% at 2 years, 97.3% at 5 years, and 94.5% at 10 years, with a mean survival of 46.4 months.
Conclusions
This is the first NCDB-based study of TCCRP, highlighting favorable outcomes and distinct clinicodemographic features. Patients were predominantly older, White, and Medicare-insured, often receiving care at community cancer programs. These findings suggest that socioeconomic factors may influence access and treatment. Results may inform strategies to promote equitable care delivery across health systems and guide further research on clinical management and survivorship in TCCRP, particularly for rare cancers within community-based settings such as the VHA.
Background
Tall cell carcinoma with reversed polarity (TCCRP) is a rare and distinct subtype of invasive breast carcinoma, defined by tall columnar cells with eosinophilic cytoplasm and reversed nuclear polarity. TCCRP remains poorly characterized in the literature, with limited population-level evidence to guide management and prognostication. This study uses the National Cancer Database (NCDB) to examine the epidemiology, clinical features, and outcomes of this neoplasm.
Methods
A retrospective cohort analysis included 951 patients diagnosed with TCCRP (ICD-O-3 code 8509) from 2018–2020 using the NCDB. Demographic and treatment variables were analyzed using descriptive statistics. Incidence trends were assessed using linear regression, and overall survival was evaluated using Kaplan-Meier methods.
Results
Most patients were female (98.1%) with a mean age of 69.1 years. The majority were White (82.0%), followed by Black (9.0%) and Hispanic (8.7%). Primary tumor sites included overlapping breast lesions (28.5%) and the upper-inner quadrant (27.0%). Incidence remained stable (R2 = 0.0). Most patients were diagnosed at Stage I (58.4%) and had a Charlson-Deyo score of 0 (76.2%). Socioeconomically, 41.8% lived in the highest income quartile (≥$74,063), and most had Medicare (64.7%). The most common treatment settings were comprehensive community cancer programs (40.3%). Surgery was performed in 95.6% of cases, with negative margins in 91.1%. Radiation therapy (46.6%) and hormone therapy (44.3%) were frequently used. Mortality was 1.1% at 30 days and 1.7% at 90 days. Survival was 98.9% at 2 years, 97.3% at 5 years, and 94.5% at 10 years, with a mean survival of 46.4 months.
Conclusions
This is the first NCDB-based study of TCCRP, highlighting favorable outcomes and distinct clinicodemographic features. Patients were predominantly older, White, and Medicare-insured, often receiving care at community cancer programs. These findings suggest that socioeconomic factors may influence access and treatment. Results may inform strategies to promote equitable care delivery across health systems and guide further research on clinical management and survivorship in TCCRP, particularly for rare cancers within community-based settings such as the VHA.
Forceps Assistance Improves Outcomes in Difficult ERCP Cannulations
The results emerged from the small, single-center SOCCER trial of 152 patients recruited from March 2022 to October 2024 and are published in The American Journal of Gastroenterology.
Both groups had a slightly higher number of female participants, and the mean ages of the participants were 61.9 years in the forceps group and 68.3 years in the no forceps group.
First author Steven M. Hadley Jr, an MD candidate at Northwestern Feinberg School of Medicine in Chicago, and colleagues reported that forceps assistance in difficult cannulations yielded significantly higher success rates than no forceps assistance (100% vs 83.9%; P < .001).
The investigators noted that difficult cannulations during ERCP have a frequency of 42%. Cannulation failure is associated with increased morbidity — including longer hospitalization, increased ICU admissions, readmissions, and increased financial cost — as well as mortality rates of up to 10%.
SOCCER defined difficult cannulation as a papilla in or on the rim of a diverticulum, five or more attempts, attempts lasting 5 or more minutes, or two or more unintended pancreatic duct wire passages. Other features were redundant tissue overlaying the papilla or a type 2, 3, or 4 papilla.
The study found forceps assistance also had a nonstatistically significant lower rate of difficult cannulations than no forceps (57.1% vs 69.1%; P = .132). The rate of post-ERCP pancreatitis (PEP) was similarly low in both groups: 5.7% with forceps vs 3.7% without forceps (P = .705). The no forceps group had significantly more cannulation attempts after randomization than the forceps group (14 vs 8.3; P = .026).
Patients who crossed over to forceps assistance all had successful cannulations.
The technique has long been used to overcome cannulation difficulties, said Timothy B. Gardner, MD, MS, a gastroenterologist at the Dartmouth Hitchcock Medical Center in Lebanon, New Hampshire, and a coauthor of the study. “It was particularly effective for cannulations with redundant tissue limiting access to the papilla,” Gardner told GI & Hepatology News. “We decided to design a randomized trial to determine the extent to which this technique worked. We believed our study would answer an important question that would hopefully lead to an improvement in endoscopy practice.”
While a few case reports and video demos had described the technique, no trials had assessed its effectiveness, Hadley added. “We found the technique to be effective based on our experience, but it was exciting to see that a rigorously designed randomized trial proved that it is indeed a very effective technique to facilitate cannulation.”
Hadley noted the technique does not increase PEP incidence, unlike the commonly used precut sphincterotomy and the double-wire method for difficult cannulations. “As a result, the forceps-assisted technique may be an effective first-line option and may reduce the need for additional, more invasive procedures including surgery and repeat ERCP to obtain the therapeutic intent of the original ERCP.”
The paper outlines the technique’s methodology, he added, “so we believe endoscopists who read the manuscript will be able to start implementing the technique into their practice.”
Commenting on the paper but not involved in it, Christopher J. DiMaio, MD, regional director of Endoscopy for Northwell Health Physician Partners Gastroenterology and a gastroenterologist in Greenlawn, New York, called it potentially helpful but aimed at a niche group of expert practitioners. “The technique appears safe and very effective, which is the number one concern, and I would definitely keep it in my back pocket,” he said. “I expect it will be used more commonly now because of this study.”
He added that although expert endoscopists are familiar with the approach, they use more time-tested and sometimes more aggressive maneuvers to cope with difficult cannulations. “But this is a simple technique using a device that should be available to most high-volume endoscopists.”
DiMaio also noted that he would have liked to see an actual decrease in PEP incidence in the intervention group.
Looking ahead, Hadley said it would be interesting to compare the effectiveness of the double-wire technique against forceps-assisted cannulation in a randomized context. “A study we’re already looking into is seeing whether physician experience with the technique impacts outcomes.”
This study was supported by the American College of Gastroenterology. The authors and DiMaio reported having no relevant competing interests.
A version of this article first appeared on Medscape.com.
The results emerged from the small, single-center SOCCER trial of 152 patients recruited from March 2022 to October 2024 and are published in The American Journal of Gastroenterology.
Both groups had a slightly higher number of female participants, and the mean ages of the participants were 61.9 years in the forceps group and 68.3 years in the no forceps group.
First author Steven M. Hadley Jr, an MD candidate at Northwestern Feinberg School of Medicine in Chicago, and colleagues reported that forceps assistance in difficult cannulations yielded significantly higher success rates than no forceps assistance (100% vs 83.9%; P < .001).
The investigators noted that difficult cannulations during ERCP have a frequency of 42%. Cannulation failure is associated with increased morbidity — including longer hospitalization, increased ICU admissions, readmissions, and increased financial cost — as well as mortality rates of up to 10%.
SOCCER defined difficult cannulation as a papilla in or on the rim of a diverticulum, five or more attempts, attempts lasting 5 or more minutes, or two or more unintended pancreatic duct wire passages. Other features were redundant tissue overlaying the papilla or a type 2, 3, or 4 papilla.
The study found forceps assistance also had a nonstatistically significant lower rate of difficult cannulations than no forceps (57.1% vs 69.1%; P = .132). The rate of post-ERCP pancreatitis (PEP) was similarly low in both groups: 5.7% with forceps vs 3.7% without forceps (P = .705). The no forceps group had significantly more cannulation attempts after randomization than the forceps group (14 vs 8.3; P = .026).
Patients who crossed over to forceps assistance all had successful cannulations.
The technique has long been used to overcome cannulation difficulties, said Timothy B. Gardner, MD, MS, a gastroenterologist at the Dartmouth Hitchcock Medical Center in Lebanon, New Hampshire, and a coauthor of the study. “It was particularly effective for cannulations with redundant tissue limiting access to the papilla,” Gardner told GI & Hepatology News. “We decided to design a randomized trial to determine the extent to which this technique worked. We believed our study would answer an important question that would hopefully lead to an improvement in endoscopy practice.”
While a few case reports and video demos had described the technique, no trials had assessed its effectiveness, Hadley added. “We found the technique to be effective based on our experience, but it was exciting to see that a rigorously designed randomized trial proved that it is indeed a very effective technique to facilitate cannulation.”
Hadley noted the technique does not increase PEP incidence, unlike the commonly used precut sphincterotomy and the double-wire method for difficult cannulations. “As a result, the forceps-assisted technique may be an effective first-line option and may reduce the need for additional, more invasive procedures including surgery and repeat ERCP to obtain the therapeutic intent of the original ERCP.”
The paper outlines the technique’s methodology, he added, “so we believe endoscopists who read the manuscript will be able to start implementing the technique into their practice.”
Commenting on the paper but not involved in it, Christopher J. DiMaio, MD, regional director of Endoscopy for Northwell Health Physician Partners Gastroenterology and a gastroenterologist in Greenlawn, New York, called it potentially helpful but aimed at a niche group of expert practitioners. “The technique appears safe and very effective, which is the number one concern, and I would definitely keep it in my back pocket,” he said. “I expect it will be used more commonly now because of this study.”
He added that although expert endoscopists are familiar with the approach, they use more time-tested and sometimes more aggressive maneuvers to cope with difficult cannulations. “But this is a simple technique using a device that should be available to most high-volume endoscopists.”
DiMaio also noted that he would have liked to see an actual decrease in PEP incidence in the intervention group.
Looking ahead, Hadley said it would be interesting to compare the effectiveness of the double-wire technique against forceps-assisted cannulation in a randomized context. “A study we’re already looking into is seeing whether physician experience with the technique impacts outcomes.”
This study was supported by the American College of Gastroenterology. The authors and DiMaio reported having no relevant competing interests.
A version of this article first appeared on Medscape.com.
The results emerged from the small, single-center SOCCER trial of 152 patients recruited from March 2022 to October 2024 and are published in The American Journal of Gastroenterology.
Both groups had a slightly higher number of female participants, and the mean ages of the participants were 61.9 years in the forceps group and 68.3 years in the no forceps group.
First author Steven M. Hadley Jr, an MD candidate at Northwestern Feinberg School of Medicine in Chicago, and colleagues reported that forceps assistance in difficult cannulations yielded significantly higher success rates than no forceps assistance (100% vs 83.9%; P < .001).
The investigators noted that difficult cannulations during ERCP have a frequency of 42%. Cannulation failure is associated with increased morbidity — including longer hospitalization, increased ICU admissions, readmissions, and increased financial cost — as well as mortality rates of up to 10%.
SOCCER defined difficult cannulation as a papilla in or on the rim of a diverticulum, five or more attempts, attempts lasting 5 or more minutes, or two or more unintended pancreatic duct wire passages. Other features were redundant tissue overlaying the papilla or a type 2, 3, or 4 papilla.
The study found forceps assistance also had a nonstatistically significant lower rate of difficult cannulations than no forceps (57.1% vs 69.1%; P = .132). The rate of post-ERCP pancreatitis (PEP) was similarly low in both groups: 5.7% with forceps vs 3.7% without forceps (P = .705). The no forceps group had significantly more cannulation attempts after randomization than the forceps group (14 vs 8.3; P = .026).
Patients who crossed over to forceps assistance all had successful cannulations.
The technique has long been used to overcome cannulation difficulties, said Timothy B. Gardner, MD, MS, a gastroenterologist at the Dartmouth Hitchcock Medical Center in Lebanon, New Hampshire, and a coauthor of the study. “It was particularly effective for cannulations with redundant tissue limiting access to the papilla,” Gardner told GI & Hepatology News. “We decided to design a randomized trial to determine the extent to which this technique worked. We believed our study would answer an important question that would hopefully lead to an improvement in endoscopy practice.”
While a few case reports and video demos had described the technique, no trials had assessed its effectiveness, Hadley added. “We found the technique to be effective based on our experience, but it was exciting to see that a rigorously designed randomized trial proved that it is indeed a very effective technique to facilitate cannulation.”
Hadley noted the technique does not increase PEP incidence, unlike the commonly used precut sphincterotomy and the double-wire method for difficult cannulations. “As a result, the forceps-assisted technique may be an effective first-line option and may reduce the need for additional, more invasive procedures including surgery and repeat ERCP to obtain the therapeutic intent of the original ERCP.”
The paper outlines the technique’s methodology, he added, “so we believe endoscopists who read the manuscript will be able to start implementing the technique into their practice.”
Commenting on the paper but not involved in it, Christopher J. DiMaio, MD, regional director of Endoscopy for Northwell Health Physician Partners Gastroenterology and a gastroenterologist in Greenlawn, New York, called it potentially helpful but aimed at a niche group of expert practitioners. “The technique appears safe and very effective, which is the number one concern, and I would definitely keep it in my back pocket,” he said. “I expect it will be used more commonly now because of this study.”
He added that although expert endoscopists are familiar with the approach, they use more time-tested and sometimes more aggressive maneuvers to cope with difficult cannulations. “But this is a simple technique using a device that should be available to most high-volume endoscopists.”
DiMaio also noted that he would have liked to see an actual decrease in PEP incidence in the intervention group.
Looking ahead, Hadley said it would be interesting to compare the effectiveness of the double-wire technique against forceps-assisted cannulation in a randomized context. “A study we’re already looking into is seeing whether physician experience with the technique impacts outcomes.”
This study was supported by the American College of Gastroenterology. The authors and DiMaio reported having no relevant competing interests.
A version of this article first appeared on Medscape.com.
Unique Presentation of Postpartum Hypereosinophilic Syndrome With Atypical Features and Therapeutic Challenges
Unique Presentation of Postpartum Hypereosinophilic Syndrome With Atypical Features and Therapeutic Challenges
Hypereosinophilic syndrome (HES) is defined by marked, persistent absolute eosinophil count (AEC) > 1500 cells/μL on ≥ 2 peripheral smears separated by ≥ 1 month with evidence of accompanied end-organ damage, in the absence of other causes of eosinophilia such as malignancy, atopy, or parasitic infections.1-5 Hypereosinophilic infiltration can impact almost every organ system; however, the most profound complications in patients with HES are related to leukemias and cardiac manifestations of the disease.3,4 Although rare, the associated morbidity and mortality of HES are considerable, making prompt recognition and treatment essential. Management involves targeted therapy based on pathologic classification of HES and on decreasing associated inflammation, fibrosis, and end-organ damage.3,5-7
The patient in this case report met the diagnostic criteria for HES. However, this patient had several clinical and laboratory features that made it difficult to characterize a specific HES variant. Moreover, she had additional immunomodulating factors in the setting of pregnancy. This is the first documented case of HES of undetermined etiology diagnosed postpartum and managed in the setting of a new pregnancy.2,8
CASE PRESENTATION
A 32-year-old female active-duty military service member with allergic rhinitis and a history of childhood eczema was referred to allergy/immunology for evaluation of a new, progressive pruritic rash. Symptoms started 3 months after the birth of her first child, with a new diffuse erythematous skin rash sparing her palms, soles, and mucosal surfaces. Given her history of atopy, the rash was initially treated as severe atopic dermatitis with appropriate topical medications. The rash gradually worsened, with the development of intermittent facial swelling, night sweats, dyspnea, recurrent epigastric abdominal pain, and nausea with vomiting, resulting in decreased oral intake and weight loss.
The patient was hospitalized and received an expedited multidisciplinary evaluation by dermatology, hematology/oncology, and gastroenterology. Her AEC of 4787 cells/μL peaked on admission and was markedly elevated from the 1070 cells/μL reported in the third trimester of her pregnancy. She was found to have mature eosinophilia on skin biopsy (Figure 1), endoscopic duodenal biopsy (Figure 2), peripheral blood smear (Figure 3), and bone marrow biopsy (Figure 4).
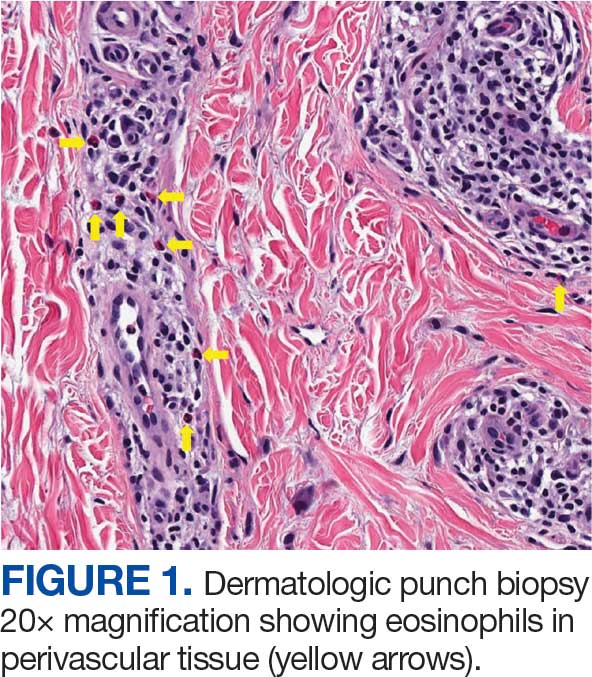
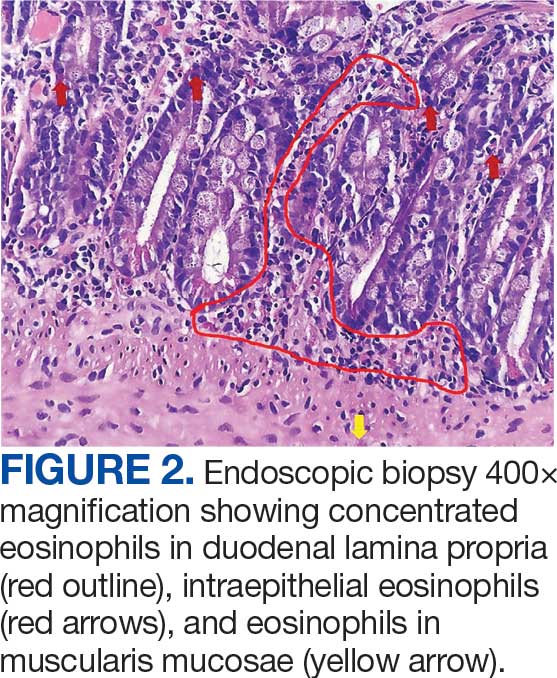
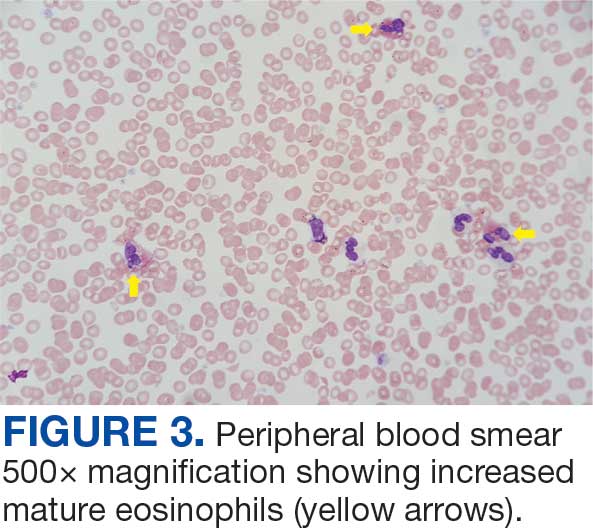
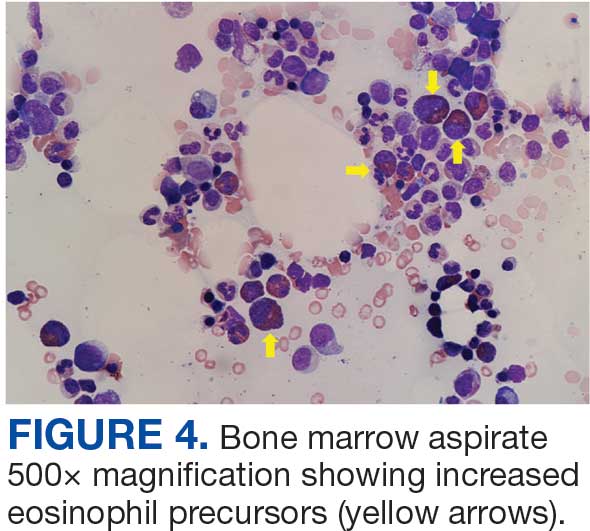
Radiographic imaging of the chest, abdomen, and pelvis revealed hepatomegaly without detectable neoplasm. There was no clinical evidence of cardiac involvement, and evaluation with electrocardiography and echocardiography did not indicate myocarditis. Extensive laboratory testing revealed no genetic mutations indicative of familial, myeloproliferative, or lymphocytic variants of HES.
The patient received topical emollients, omeprazole 40 mg daily, and ondansetron 8 mg 3 times daily as needed for symptom management, and was started on oral prednisone 40 mg daily with improvement in dyspnea, night sweats, and gastrointestinal complaints. During the patient's 6-day hospitalization and treatment, her AECs gradually decreased to 2110 cells/μL, and decreased to 1600 cells/μL over the course of a month, remaining in the hypereosinophilic range. The patient was discovered to be pregnant while symptoms were improving, resulting in stepwise discontinuation of oral steroids, but she reported continued improvement in symptoms.
DISCUSSION
Peripheral eosinophilia has a broad differential diagnoses, including HES, parasitic infections, atopic hypersensitivity diseases, eosinophilic lung diseases, eosinophilic gastrointestinal diseases, vasculitides such as eosinophilic granulomatosis with polyangiitis, genetic syndromes predisposing to eosinophilia, episodic angioedema with eosinophilia, and chronic metabolic disease with adrenal insufficiency.1-5 HES, although rare, is a disease process with potentially devastating associated morbidity and mortality if not promptly recognized and treated. HES is further delineated by hypereosinophilia with associated eosinophil-mediated organ damage or dysfunction.3-5
Clinical manifestations of HES can differ greatly depending on the HES variant and degree of organ involvement at the time of diagnosis and throughout the disease course. Patients with HES, as well as those with asymptomatic eosinophilia or hypereosinophilia, should be closely monitored for disease progression. In addition to trending peripheral AECs, clinicians should screen for symptoms of organ involvement and perform targeted evaluation of the suspected organs to promptly identify early signs of organ involvement and initiate treatment.1-4 Recommendations regarding screening intervals vary widely from monthly to annually, depending on a patient’s specific clinical picture.
HES has been subdivided into clinically relevant variants, including myeloproliferative (M-HES), T lymphocytic (L-HES), organ-restricted (or overlap) HES, familial HES, idiopathic HES, and specific syndromes with associated hypereosinophilia.3-5,9 Patients with M-HES have elevated circulating leukocyte precursors and clinical manifestations, including but not limited to hepatosplenomegaly, anemia, and thrombocytopenia. The most commonly associated genetic mutations include the FIP1L1-PDGFR-α fusion, BCR-ABL1, PDGFRA/B, JAK2, KIT, and FGFR1.3-6 L-HES usually has predominant skin and soft tissue involvement secondary to immunoglobulin E-mediated actions with clonal expansion of T cells (most commonly CD3-4+ or CD3+CD4-CD8-).3,5,6 Familial HES, a rare variant, follows an autosomal dominant inheritance pattern and is usually present at birth. It involves chromosome 5, which contains genes coding for cytokines that drive eosinophilic proliferation, including interleukin (IL)-3, IL-5, and granulocyte-macrophage colony-stimulating factor.5,9 Hypereosinophilia in the setting of end-organ damage restricted to a single organ is considered organ-restricted HES. There can be significant hepatic and gastrointestinal dysfunction, with or without malabsorption.
HES can also manifest with hematologic malignancy, restrictive obliterative cardiomyopathies, renal injury manifested by hematuria and electrolyte derangements, and neurologic complications including hemiparesis, dysarthria, and even coma.6 Endothelial damage due to eosinophil-driven inflammation can result in thrombus formation and increased risk of thromboembolic events in various organs.3 Idiopathic HES, otherwise known as HES of unknown etiology or significance, is a diagnosis of exclusion and constitutes a cohort of patients who do not fit into the other delineated categories.3-5 These patients often have multisystem involvement, making classification and treatment a challenge.5
The patient described in this case met the diagnostic criteria for HES, but her complicated clinical and laboratory features were challenging to characterize into a specific variant of HES. Organ-restricted HES was ruled out due to skin, marrow, and duodenal infiltration. She also had the potential for lung involvement based on her clinical symptoms, however no biopsy was obtained. Laboratory testing revealed no deletions or mutations indicative of familial, myeloproliferative, or lymphocytic variants. Her multisystem involvement without an underlying associated syndrome suggests idiopathic HES or HES of undetermined significance.1-5
Most patients with HES are diagnosed between the ages of 20 and 50 years.10 While HES has its peak incidence in the fourth decade of life, acute onset of new symptoms 3 months postpartum makes this an unusual presentation. In this unique case, it is important to highlight the role of the physiologic changes of pregnancy in inflammatory mediation. The physiologic changes that occur in pregnancy to ensure fetal tolerance can have profound implications for leukocyte count, AEC, and subsequent inflammatory responses. The phenomenon of inflammatory amelioration during pregnancy is well-documented, but there has only been 1 known published case report discussing decreasing HES symptoms during pregnancy with prepregnancy and postpartum hypereosinophilia.8 It is suggested that this amelioration is secondary to cortisol and progesterone shifts that occur in pregnancy. Physiologic increases in adrenocorticotropic hormone in pregnancy leads to subsequent secretion of endogenous steroids by the adrenal cortex. In turn, pregnancy can lead to leukocytosis and eosinopenia.8 Overall, pregnancy can have beneficial immunomodulating properties in the spectrum of hypereosinophilic syndromes. Even so, this patient with HES diagnosed postpartum remains at risk for the sequelae of hypereosinophilia, regardless of potential for AEC reduction during pregnancy. Therefore, treatment considerations need to be made with the safety of the maternal-fetal dyad as a priority.
Treatment
The treatment of symptomatic HES without acute life-threatening features or associated malignancy is generally determined by clinical variant.2-4 There is insufficient data to support initiation of treatment solely based on persistently elevated AEC. Patients with peripheral eosinophilia and hypereosinophilia should be monitored periodically with appropriate subspecialist evaluation for occult end-organ involvement, and targeted therapies should be deferred until an HES diagnosis.1-4 First-line therapy in most HES variants is systemic glucocorticoids.2,3,7 Since the disease course for this patient did not precisely match an HES variant, it was challenging to ascertain the optimal personalized treatment regimen. The approach to therapy was further complicated by newly identified pregnancy necessitating cessation of systemic glucocorticoids. In addition to glucocorticoids, hydroxyurea and interferon-α are among treatments historically used for HES, with tyrosine kinase inhibitors and monoclonal antibodies targeting IL-5 becoming more common.1-4 Although this patient may ultimately benefit from an IL-5 targeting biologic medication such as mepolizumab, safety in pregnancy is not well-studied and may require close clinical monitoring with treatment deferred until after delivery if possible.3,7,8,11
Military service members with frequent geographic relocation have additional barriers to timely diagnosis with often-limited access to subspecialty care depending on the duty station. While the patient was able to receive care at a large military medical center with many subspecialists, prompt recognition and timely referral to specialists would be even more critical at a smaller treatment facility. Depending on the severity and variant of HES, patients may warrant evaluation and treatment by hematology/oncology, cardiology, pulmonology, and immunology. Although HES can present in young children and older adults, this condition is most often diagnosed during the third and fourth decades of life, putting clinicians on the front line of hypereosinophilia identification and evaluation.10 Military physicians have the additional duty to not only think ahead in their diverse clinical settings to ensure proper care for patients, but also maintain a broad differential inclusive of more rare disease processes such as HES.
CONCLUSIONS
This case emphasizes how uncontrolled or untreated HES can lead to significant end-organ damage involving multiple systems and high morbidity. Prompt recognition of hypereosinophilia with potential HES can help expedite coordination of multidisciplinary care across multiple specialties to minimize delays in diagnosis and treatment. Doing so may minimize associated morbidity and mortality, especially in individuals located at more remote duty stations or deployed to austere environments.
- Cogan E, Roufosse F. Clinical management of the hypereosinophilic syndromes. Expert Rev Hematol. 2012;5:275-290. doi: 10.1586/ehm.12.14
- Klion A. Hypereosinophilic syndrome: approach to treatment in the era of precision medicine. Hematology Am Soc Hematol Educ Program. 2018;2018:326-331. doi:10.1182/asheducation-2018.1.326
- Shomali W, Gotlib J. World health organization-defined eosinophilic disorders: 2022 update on diagnosis, risk stratification, and management. Am J Hematol. 2022;97:129-148. doi:10.1002/ajh.26352
- Helbig G, Klion AD. Hypereosinophilic syndromes - an enigmatic group of disorders with an intriguing clinical spectrum and challenging treatment. Blood Rev. 2021;49:100809. doi:10.1016/j.blre.2021.100809
- Valent P, Klion AD, Horny HP, et al. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. J Allergy Clin Immunol. 2012;130:607-612.e9. doi:10.1016/j.jaci.2012.02.019
- Roufosse FE, Goldman M, Cogan E. Hypereosinophilic syndromes. Orphanet J Rare Dis. 2007;2:37. doi:10.1186/1750-1172-2-37
- Pitlick MM, Li JT, Pongdee T. Current and emerging biologic therapies targeting eosinophilic disorders. World Allergy Organ J. 2022;15:100676. doi:10.1016/j.waojou.2022.10067
- Ault P, Cortes J, Lynn A, Keating M, Verstovsek S. Pregnancy in a patient with hypereosinophilic syndrome. Leuk Res. 2009;33:186-187. doi:10.1016/j.leukres.2008.05.013
- Rioux JD, Stone VA, Daly MJ, et al. Familial eosinophilia maps to the cytokine gene cluster on human chromosomal region 5q31-q33. Am J Hum Genet. 1998;63:1086-1094. doi:10.1086/302053
- Williams KW, Ware J, Abiodun A, et al. Hypereosinophilia in children and adults: a retrospective comparison. J Allergy Clin Immunol Pract. 2016;4:941-947.e1. doi:10.1016/j.jaip.2016.03.020
- Pane F, Lefevre G, Kwon N, et al. Characterization of disease flares and impact of mepolizumab in patients with hypereosinophilic syndrome. Front Immunol. 2022;13:935996. doi:10.3389/fimmu.2022.935996
Hypereosinophilic syndrome (HES) is defined by marked, persistent absolute eosinophil count (AEC) > 1500 cells/μL on ≥ 2 peripheral smears separated by ≥ 1 month with evidence of accompanied end-organ damage, in the absence of other causes of eosinophilia such as malignancy, atopy, or parasitic infections.1-5 Hypereosinophilic infiltration can impact almost every organ system; however, the most profound complications in patients with HES are related to leukemias and cardiac manifestations of the disease.3,4 Although rare, the associated morbidity and mortality of HES are considerable, making prompt recognition and treatment essential. Management involves targeted therapy based on pathologic classification of HES and on decreasing associated inflammation, fibrosis, and end-organ damage.3,5-7
The patient in this case report met the diagnostic criteria for HES. However, this patient had several clinical and laboratory features that made it difficult to characterize a specific HES variant. Moreover, she had additional immunomodulating factors in the setting of pregnancy. This is the first documented case of HES of undetermined etiology diagnosed postpartum and managed in the setting of a new pregnancy.2,8
CASE PRESENTATION
A 32-year-old female active-duty military service member with allergic rhinitis and a history of childhood eczema was referred to allergy/immunology for evaluation of a new, progressive pruritic rash. Symptoms started 3 months after the birth of her first child, with a new diffuse erythematous skin rash sparing her palms, soles, and mucosal surfaces. Given her history of atopy, the rash was initially treated as severe atopic dermatitis with appropriate topical medications. The rash gradually worsened, with the development of intermittent facial swelling, night sweats, dyspnea, recurrent epigastric abdominal pain, and nausea with vomiting, resulting in decreased oral intake and weight loss.
The patient was hospitalized and received an expedited multidisciplinary evaluation by dermatology, hematology/oncology, and gastroenterology. Her AEC of 4787 cells/μL peaked on admission and was markedly elevated from the 1070 cells/μL reported in the third trimester of her pregnancy. She was found to have mature eosinophilia on skin biopsy (Figure 1), endoscopic duodenal biopsy (Figure 2), peripheral blood smear (Figure 3), and bone marrow biopsy (Figure 4).




Radiographic imaging of the chest, abdomen, and pelvis revealed hepatomegaly without detectable neoplasm. There was no clinical evidence of cardiac involvement, and evaluation with electrocardiography and echocardiography did not indicate myocarditis. Extensive laboratory testing revealed no genetic mutations indicative of familial, myeloproliferative, or lymphocytic variants of HES.
The patient received topical emollients, omeprazole 40 mg daily, and ondansetron 8 mg 3 times daily as needed for symptom management, and was started on oral prednisone 40 mg daily with improvement in dyspnea, night sweats, and gastrointestinal complaints. During the patient's 6-day hospitalization and treatment, her AECs gradually decreased to 2110 cells/μL, and decreased to 1600 cells/μL over the course of a month, remaining in the hypereosinophilic range. The patient was discovered to be pregnant while symptoms were improving, resulting in stepwise discontinuation of oral steroids, but she reported continued improvement in symptoms.
DISCUSSION
Peripheral eosinophilia has a broad differential diagnoses, including HES, parasitic infections, atopic hypersensitivity diseases, eosinophilic lung diseases, eosinophilic gastrointestinal diseases, vasculitides such as eosinophilic granulomatosis with polyangiitis, genetic syndromes predisposing to eosinophilia, episodic angioedema with eosinophilia, and chronic metabolic disease with adrenal insufficiency.1-5 HES, although rare, is a disease process with potentially devastating associated morbidity and mortality if not promptly recognized and treated. HES is further delineated by hypereosinophilia with associated eosinophil-mediated organ damage or dysfunction.3-5
Clinical manifestations of HES can differ greatly depending on the HES variant and degree of organ involvement at the time of diagnosis and throughout the disease course. Patients with HES, as well as those with asymptomatic eosinophilia or hypereosinophilia, should be closely monitored for disease progression. In addition to trending peripheral AECs, clinicians should screen for symptoms of organ involvement and perform targeted evaluation of the suspected organs to promptly identify early signs of organ involvement and initiate treatment.1-4 Recommendations regarding screening intervals vary widely from monthly to annually, depending on a patient’s specific clinical picture.
HES has been subdivided into clinically relevant variants, including myeloproliferative (M-HES), T lymphocytic (L-HES), organ-restricted (or overlap) HES, familial HES, idiopathic HES, and specific syndromes with associated hypereosinophilia.3-5,9 Patients with M-HES have elevated circulating leukocyte precursors and clinical manifestations, including but not limited to hepatosplenomegaly, anemia, and thrombocytopenia. The most commonly associated genetic mutations include the FIP1L1-PDGFR-α fusion, BCR-ABL1, PDGFRA/B, JAK2, KIT, and FGFR1.3-6 L-HES usually has predominant skin and soft tissue involvement secondary to immunoglobulin E-mediated actions with clonal expansion of T cells (most commonly CD3-4+ or CD3+CD4-CD8-).3,5,6 Familial HES, a rare variant, follows an autosomal dominant inheritance pattern and is usually present at birth. It involves chromosome 5, which contains genes coding for cytokines that drive eosinophilic proliferation, including interleukin (IL)-3, IL-5, and granulocyte-macrophage colony-stimulating factor.5,9 Hypereosinophilia in the setting of end-organ damage restricted to a single organ is considered organ-restricted HES. There can be significant hepatic and gastrointestinal dysfunction, with or without malabsorption.
HES can also manifest with hematologic malignancy, restrictive obliterative cardiomyopathies, renal injury manifested by hematuria and electrolyte derangements, and neurologic complications including hemiparesis, dysarthria, and even coma.6 Endothelial damage due to eosinophil-driven inflammation can result in thrombus formation and increased risk of thromboembolic events in various organs.3 Idiopathic HES, otherwise known as HES of unknown etiology or significance, is a diagnosis of exclusion and constitutes a cohort of patients who do not fit into the other delineated categories.3-5 These patients often have multisystem involvement, making classification and treatment a challenge.5
The patient described in this case met the diagnostic criteria for HES, but her complicated clinical and laboratory features were challenging to characterize into a specific variant of HES. Organ-restricted HES was ruled out due to skin, marrow, and duodenal infiltration. She also had the potential for lung involvement based on her clinical symptoms, however no biopsy was obtained. Laboratory testing revealed no deletions or mutations indicative of familial, myeloproliferative, or lymphocytic variants. Her multisystem involvement without an underlying associated syndrome suggests idiopathic HES or HES of undetermined significance.1-5
Most patients with HES are diagnosed between the ages of 20 and 50 years.10 While HES has its peak incidence in the fourth decade of life, acute onset of new symptoms 3 months postpartum makes this an unusual presentation. In this unique case, it is important to highlight the role of the physiologic changes of pregnancy in inflammatory mediation. The physiologic changes that occur in pregnancy to ensure fetal tolerance can have profound implications for leukocyte count, AEC, and subsequent inflammatory responses. The phenomenon of inflammatory amelioration during pregnancy is well-documented, but there has only been 1 known published case report discussing decreasing HES symptoms during pregnancy with prepregnancy and postpartum hypereosinophilia.8 It is suggested that this amelioration is secondary to cortisol and progesterone shifts that occur in pregnancy. Physiologic increases in adrenocorticotropic hormone in pregnancy leads to subsequent secretion of endogenous steroids by the adrenal cortex. In turn, pregnancy can lead to leukocytosis and eosinopenia.8 Overall, pregnancy can have beneficial immunomodulating properties in the spectrum of hypereosinophilic syndromes. Even so, this patient with HES diagnosed postpartum remains at risk for the sequelae of hypereosinophilia, regardless of potential for AEC reduction during pregnancy. Therefore, treatment considerations need to be made with the safety of the maternal-fetal dyad as a priority.
Treatment
The treatment of symptomatic HES without acute life-threatening features or associated malignancy is generally determined by clinical variant.2-4 There is insufficient data to support initiation of treatment solely based on persistently elevated AEC. Patients with peripheral eosinophilia and hypereosinophilia should be monitored periodically with appropriate subspecialist evaluation for occult end-organ involvement, and targeted therapies should be deferred until an HES diagnosis.1-4 First-line therapy in most HES variants is systemic glucocorticoids.2,3,7 Since the disease course for this patient did not precisely match an HES variant, it was challenging to ascertain the optimal personalized treatment regimen. The approach to therapy was further complicated by newly identified pregnancy necessitating cessation of systemic glucocorticoids. In addition to glucocorticoids, hydroxyurea and interferon-α are among treatments historically used for HES, with tyrosine kinase inhibitors and monoclonal antibodies targeting IL-5 becoming more common.1-4 Although this patient may ultimately benefit from an IL-5 targeting biologic medication such as mepolizumab, safety in pregnancy is not well-studied and may require close clinical monitoring with treatment deferred until after delivery if possible.3,7,8,11
Military service members with frequent geographic relocation have additional barriers to timely diagnosis with often-limited access to subspecialty care depending on the duty station. While the patient was able to receive care at a large military medical center with many subspecialists, prompt recognition and timely referral to specialists would be even more critical at a smaller treatment facility. Depending on the severity and variant of HES, patients may warrant evaluation and treatment by hematology/oncology, cardiology, pulmonology, and immunology. Although HES can present in young children and older adults, this condition is most often diagnosed during the third and fourth decades of life, putting clinicians on the front line of hypereosinophilia identification and evaluation.10 Military physicians have the additional duty to not only think ahead in their diverse clinical settings to ensure proper care for patients, but also maintain a broad differential inclusive of more rare disease processes such as HES.
CONCLUSIONS
This case emphasizes how uncontrolled or untreated HES can lead to significant end-organ damage involving multiple systems and high morbidity. Prompt recognition of hypereosinophilia with potential HES can help expedite coordination of multidisciplinary care across multiple specialties to minimize delays in diagnosis and treatment. Doing so may minimize associated morbidity and mortality, especially in individuals located at more remote duty stations or deployed to austere environments.
Hypereosinophilic syndrome (HES) is defined by marked, persistent absolute eosinophil count (AEC) > 1500 cells/μL on ≥ 2 peripheral smears separated by ≥ 1 month with evidence of accompanied end-organ damage, in the absence of other causes of eosinophilia such as malignancy, atopy, or parasitic infections.1-5 Hypereosinophilic infiltration can impact almost every organ system; however, the most profound complications in patients with HES are related to leukemias and cardiac manifestations of the disease.3,4 Although rare, the associated morbidity and mortality of HES are considerable, making prompt recognition and treatment essential. Management involves targeted therapy based on pathologic classification of HES and on decreasing associated inflammation, fibrosis, and end-organ damage.3,5-7
The patient in this case report met the diagnostic criteria for HES. However, this patient had several clinical and laboratory features that made it difficult to characterize a specific HES variant. Moreover, she had additional immunomodulating factors in the setting of pregnancy. This is the first documented case of HES of undetermined etiology diagnosed postpartum and managed in the setting of a new pregnancy.2,8
CASE PRESENTATION
A 32-year-old female active-duty military service member with allergic rhinitis and a history of childhood eczema was referred to allergy/immunology for evaluation of a new, progressive pruritic rash. Symptoms started 3 months after the birth of her first child, with a new diffuse erythematous skin rash sparing her palms, soles, and mucosal surfaces. Given her history of atopy, the rash was initially treated as severe atopic dermatitis with appropriate topical medications. The rash gradually worsened, with the development of intermittent facial swelling, night sweats, dyspnea, recurrent epigastric abdominal pain, and nausea with vomiting, resulting in decreased oral intake and weight loss.
The patient was hospitalized and received an expedited multidisciplinary evaluation by dermatology, hematology/oncology, and gastroenterology. Her AEC of 4787 cells/μL peaked on admission and was markedly elevated from the 1070 cells/μL reported in the third trimester of her pregnancy. She was found to have mature eosinophilia on skin biopsy (Figure 1), endoscopic duodenal biopsy (Figure 2), peripheral blood smear (Figure 3), and bone marrow biopsy (Figure 4).




Radiographic imaging of the chest, abdomen, and pelvis revealed hepatomegaly without detectable neoplasm. There was no clinical evidence of cardiac involvement, and evaluation with electrocardiography and echocardiography did not indicate myocarditis. Extensive laboratory testing revealed no genetic mutations indicative of familial, myeloproliferative, or lymphocytic variants of HES.
The patient received topical emollients, omeprazole 40 mg daily, and ondansetron 8 mg 3 times daily as needed for symptom management, and was started on oral prednisone 40 mg daily with improvement in dyspnea, night sweats, and gastrointestinal complaints. During the patient's 6-day hospitalization and treatment, her AECs gradually decreased to 2110 cells/μL, and decreased to 1600 cells/μL over the course of a month, remaining in the hypereosinophilic range. The patient was discovered to be pregnant while symptoms were improving, resulting in stepwise discontinuation of oral steroids, but she reported continued improvement in symptoms.
DISCUSSION
Peripheral eosinophilia has a broad differential diagnoses, including HES, parasitic infections, atopic hypersensitivity diseases, eosinophilic lung diseases, eosinophilic gastrointestinal diseases, vasculitides such as eosinophilic granulomatosis with polyangiitis, genetic syndromes predisposing to eosinophilia, episodic angioedema with eosinophilia, and chronic metabolic disease with adrenal insufficiency.1-5 HES, although rare, is a disease process with potentially devastating associated morbidity and mortality if not promptly recognized and treated. HES is further delineated by hypereosinophilia with associated eosinophil-mediated organ damage or dysfunction.3-5
Clinical manifestations of HES can differ greatly depending on the HES variant and degree of organ involvement at the time of diagnosis and throughout the disease course. Patients with HES, as well as those with asymptomatic eosinophilia or hypereosinophilia, should be closely monitored for disease progression. In addition to trending peripheral AECs, clinicians should screen for symptoms of organ involvement and perform targeted evaluation of the suspected organs to promptly identify early signs of organ involvement and initiate treatment.1-4 Recommendations regarding screening intervals vary widely from monthly to annually, depending on a patient’s specific clinical picture.
HES has been subdivided into clinically relevant variants, including myeloproliferative (M-HES), T lymphocytic (L-HES), organ-restricted (or overlap) HES, familial HES, idiopathic HES, and specific syndromes with associated hypereosinophilia.3-5,9 Patients with M-HES have elevated circulating leukocyte precursors and clinical manifestations, including but not limited to hepatosplenomegaly, anemia, and thrombocytopenia. The most commonly associated genetic mutations include the FIP1L1-PDGFR-α fusion, BCR-ABL1, PDGFRA/B, JAK2, KIT, and FGFR1.3-6 L-HES usually has predominant skin and soft tissue involvement secondary to immunoglobulin E-mediated actions with clonal expansion of T cells (most commonly CD3-4+ or CD3+CD4-CD8-).3,5,6 Familial HES, a rare variant, follows an autosomal dominant inheritance pattern and is usually present at birth. It involves chromosome 5, which contains genes coding for cytokines that drive eosinophilic proliferation, including interleukin (IL)-3, IL-5, and granulocyte-macrophage colony-stimulating factor.5,9 Hypereosinophilia in the setting of end-organ damage restricted to a single organ is considered organ-restricted HES. There can be significant hepatic and gastrointestinal dysfunction, with or without malabsorption.
HES can also manifest with hematologic malignancy, restrictive obliterative cardiomyopathies, renal injury manifested by hematuria and electrolyte derangements, and neurologic complications including hemiparesis, dysarthria, and even coma.6 Endothelial damage due to eosinophil-driven inflammation can result in thrombus formation and increased risk of thromboembolic events in various organs.3 Idiopathic HES, otherwise known as HES of unknown etiology or significance, is a diagnosis of exclusion and constitutes a cohort of patients who do not fit into the other delineated categories.3-5 These patients often have multisystem involvement, making classification and treatment a challenge.5
The patient described in this case met the diagnostic criteria for HES, but her complicated clinical and laboratory features were challenging to characterize into a specific variant of HES. Organ-restricted HES was ruled out due to skin, marrow, and duodenal infiltration. She also had the potential for lung involvement based on her clinical symptoms, however no biopsy was obtained. Laboratory testing revealed no deletions or mutations indicative of familial, myeloproliferative, or lymphocytic variants. Her multisystem involvement without an underlying associated syndrome suggests idiopathic HES or HES of undetermined significance.1-5
Most patients with HES are diagnosed between the ages of 20 and 50 years.10 While HES has its peak incidence in the fourth decade of life, acute onset of new symptoms 3 months postpartum makes this an unusual presentation. In this unique case, it is important to highlight the role of the physiologic changes of pregnancy in inflammatory mediation. The physiologic changes that occur in pregnancy to ensure fetal tolerance can have profound implications for leukocyte count, AEC, and subsequent inflammatory responses. The phenomenon of inflammatory amelioration during pregnancy is well-documented, but there has only been 1 known published case report discussing decreasing HES symptoms during pregnancy with prepregnancy and postpartum hypereosinophilia.8 It is suggested that this amelioration is secondary to cortisol and progesterone shifts that occur in pregnancy. Physiologic increases in adrenocorticotropic hormone in pregnancy leads to subsequent secretion of endogenous steroids by the adrenal cortex. In turn, pregnancy can lead to leukocytosis and eosinopenia.8 Overall, pregnancy can have beneficial immunomodulating properties in the spectrum of hypereosinophilic syndromes. Even so, this patient with HES diagnosed postpartum remains at risk for the sequelae of hypereosinophilia, regardless of potential for AEC reduction during pregnancy. Therefore, treatment considerations need to be made with the safety of the maternal-fetal dyad as a priority.
Treatment
The treatment of symptomatic HES without acute life-threatening features or associated malignancy is generally determined by clinical variant.2-4 There is insufficient data to support initiation of treatment solely based on persistently elevated AEC. Patients with peripheral eosinophilia and hypereosinophilia should be monitored periodically with appropriate subspecialist evaluation for occult end-organ involvement, and targeted therapies should be deferred until an HES diagnosis.1-4 First-line therapy in most HES variants is systemic glucocorticoids.2,3,7 Since the disease course for this patient did not precisely match an HES variant, it was challenging to ascertain the optimal personalized treatment regimen. The approach to therapy was further complicated by newly identified pregnancy necessitating cessation of systemic glucocorticoids. In addition to glucocorticoids, hydroxyurea and interferon-α are among treatments historically used for HES, with tyrosine kinase inhibitors and monoclonal antibodies targeting IL-5 becoming more common.1-4 Although this patient may ultimately benefit from an IL-5 targeting biologic medication such as mepolizumab, safety in pregnancy is not well-studied and may require close clinical monitoring with treatment deferred until after delivery if possible.3,7,8,11
Military service members with frequent geographic relocation have additional barriers to timely diagnosis with often-limited access to subspecialty care depending on the duty station. While the patient was able to receive care at a large military medical center with many subspecialists, prompt recognition and timely referral to specialists would be even more critical at a smaller treatment facility. Depending on the severity and variant of HES, patients may warrant evaluation and treatment by hematology/oncology, cardiology, pulmonology, and immunology. Although HES can present in young children and older adults, this condition is most often diagnosed during the third and fourth decades of life, putting clinicians on the front line of hypereosinophilia identification and evaluation.10 Military physicians have the additional duty to not only think ahead in their diverse clinical settings to ensure proper care for patients, but also maintain a broad differential inclusive of more rare disease processes such as HES.
CONCLUSIONS
This case emphasizes how uncontrolled or untreated HES can lead to significant end-organ damage involving multiple systems and high morbidity. Prompt recognition of hypereosinophilia with potential HES can help expedite coordination of multidisciplinary care across multiple specialties to minimize delays in diagnosis and treatment. Doing so may minimize associated morbidity and mortality, especially in individuals located at more remote duty stations or deployed to austere environments.
- Cogan E, Roufosse F. Clinical management of the hypereosinophilic syndromes. Expert Rev Hematol. 2012;5:275-290. doi: 10.1586/ehm.12.14
- Klion A. Hypereosinophilic syndrome: approach to treatment in the era of precision medicine. Hematology Am Soc Hematol Educ Program. 2018;2018:326-331. doi:10.1182/asheducation-2018.1.326
- Shomali W, Gotlib J. World health organization-defined eosinophilic disorders: 2022 update on diagnosis, risk stratification, and management. Am J Hematol. 2022;97:129-148. doi:10.1002/ajh.26352
- Helbig G, Klion AD. Hypereosinophilic syndromes - an enigmatic group of disorders with an intriguing clinical spectrum and challenging treatment. Blood Rev. 2021;49:100809. doi:10.1016/j.blre.2021.100809
- Valent P, Klion AD, Horny HP, et al. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. J Allergy Clin Immunol. 2012;130:607-612.e9. doi:10.1016/j.jaci.2012.02.019
- Roufosse FE, Goldman M, Cogan E. Hypereosinophilic syndromes. Orphanet J Rare Dis. 2007;2:37. doi:10.1186/1750-1172-2-37
- Pitlick MM, Li JT, Pongdee T. Current and emerging biologic therapies targeting eosinophilic disorders. World Allergy Organ J. 2022;15:100676. doi:10.1016/j.waojou.2022.10067
- Ault P, Cortes J, Lynn A, Keating M, Verstovsek S. Pregnancy in a patient with hypereosinophilic syndrome. Leuk Res. 2009;33:186-187. doi:10.1016/j.leukres.2008.05.013
- Rioux JD, Stone VA, Daly MJ, et al. Familial eosinophilia maps to the cytokine gene cluster on human chromosomal region 5q31-q33. Am J Hum Genet. 1998;63:1086-1094. doi:10.1086/302053
- Williams KW, Ware J, Abiodun A, et al. Hypereosinophilia in children and adults: a retrospective comparison. J Allergy Clin Immunol Pract. 2016;4:941-947.e1. doi:10.1016/j.jaip.2016.03.020
- Pane F, Lefevre G, Kwon N, et al. Characterization of disease flares and impact of mepolizumab in patients with hypereosinophilic syndrome. Front Immunol. 2022;13:935996. doi:10.3389/fimmu.2022.935996
- Cogan E, Roufosse F. Clinical management of the hypereosinophilic syndromes. Expert Rev Hematol. 2012;5:275-290. doi: 10.1586/ehm.12.14
- Klion A. Hypereosinophilic syndrome: approach to treatment in the era of precision medicine. Hematology Am Soc Hematol Educ Program. 2018;2018:326-331. doi:10.1182/asheducation-2018.1.326
- Shomali W, Gotlib J. World health organization-defined eosinophilic disorders: 2022 update on diagnosis, risk stratification, and management. Am J Hematol. 2022;97:129-148. doi:10.1002/ajh.26352
- Helbig G, Klion AD. Hypereosinophilic syndromes - an enigmatic group of disorders with an intriguing clinical spectrum and challenging treatment. Blood Rev. 2021;49:100809. doi:10.1016/j.blre.2021.100809
- Valent P, Klion AD, Horny HP, et al. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. J Allergy Clin Immunol. 2012;130:607-612.e9. doi:10.1016/j.jaci.2012.02.019
- Roufosse FE, Goldman M, Cogan E. Hypereosinophilic syndromes. Orphanet J Rare Dis. 2007;2:37. doi:10.1186/1750-1172-2-37
- Pitlick MM, Li JT, Pongdee T. Current and emerging biologic therapies targeting eosinophilic disorders. World Allergy Organ J. 2022;15:100676. doi:10.1016/j.waojou.2022.10067
- Ault P, Cortes J, Lynn A, Keating M, Verstovsek S. Pregnancy in a patient with hypereosinophilic syndrome. Leuk Res. 2009;33:186-187. doi:10.1016/j.leukres.2008.05.013
- Rioux JD, Stone VA, Daly MJ, et al. Familial eosinophilia maps to the cytokine gene cluster on human chromosomal region 5q31-q33. Am J Hum Genet. 1998;63:1086-1094. doi:10.1086/302053
- Williams KW, Ware J, Abiodun A, et al. Hypereosinophilia in children and adults: a retrospective comparison. J Allergy Clin Immunol Pract. 2016;4:941-947.e1. doi:10.1016/j.jaip.2016.03.020
- Pane F, Lefevre G, Kwon N, et al. Characterization of disease flares and impact of mepolizumab in patients with hypereosinophilic syndrome. Front Immunol. 2022;13:935996. doi:10.3389/fimmu.2022.935996
Unique Presentation of Postpartum Hypereosinophilic Syndrome With Atypical Features and Therapeutic Challenges
Unique Presentation of Postpartum Hypereosinophilic Syndrome With Atypical Features and Therapeutic Challenges
GI Disorders Linked With Sleep Problems
“Emerging evidence suggests a bidirectional relationship between GI diseases and sleep disorders, whereby dysfunction in one domain may exacerbate the other,” wrote Shicheng Ye, PhD, of The Third Clinical Medical College of Guangzhou University of Chinese Medicine, and colleagues. However, previous studies on the association between GI and sleep problems have been small, and the role of depression as a mediator has not been well explored.
In the study, which was published online in BMC Gastroenterology, the researchers reviewed data from the US National Health and Nutrition Examination Survey between 2005 and 2014. The study population included 10,626 adults aged 20 years or older, with a mean age of 45.6 years, 50.8% of whom were women. Of these, 6444 were identified as having GI disease on the basis of a “yes” response to the question of whether they had a stomach or intestinal illness with vomiting or diarrhea within the past 30 days.
Researchers also examined responses to survey questions related to sleep duration, trouble sleeping, and diagnosis of a sleep disorder. Individuals with vs without GI diseases had a significantly higher prevalence of sleep trouble (37.99% vs 24.21%; P < .001) and a greater frequency of diagnosed sleep disorders (14.99% vs 8.08%; P < .001).
An analysis adjusted for demographic, lifestyle, and clinical factors found that individuals with vs without GI diseases were 70% more likely to have sleep trouble. Individuals with vs without GI diseases were also significantly more likely to have a diagnosed sleep disorder and a reduction in sleep duration (adjusted odds ratio, 1.8; adjusted beta, -0.15).
The association between GI diseases and sleep problems remained consistent across individuals of multiple subgroups, including those without hypertension, diabetes, or a history of smoking. It also remained significant among individuals with coronary heart disease and higher scores on the dietary index for gut microbiota. No significant interaction effects related to age, sex, or chronic disease appeared in any subgroup (P > .05).
An additional mediation analysis found that depression partly mediated the associations between GI diseases and sleep issues. Depression accounted for 21.29% of the total effect on sleep problems, 19.23% of the effect on sleep disorders, and 26.68% of the effect on sleep duration.
The mediating role of depression on the association between GI disease and sleep problems may not be exclusive, the researchers wrote. Other potential mechanisms may include systemic inflammation, visceral hypersensitivity, and metabolic dysfunction.
The findings were limited by several factors, including the possibly underpowered sample size for machine-learning models and the reliance on self-reports of GI diseases, sleep outcomes, and coronary heart disease, the researchers noted. Other limitations included the inability to adjust for confounding factors, including obstructive sleep apnea, chronic pain, and hypertension.
However, the results illustrate the need to address both psychological and GI factors in clinical practice to improve sleep health, the researchers wrote. More research is needed to identify causal pathways and develop targeted, multidimensional interventions for this interconnected trio of health problems.
Increasing Evidence for Gut-Brain Interaction
Both sleep disorders and disorders of GBI (DGBI) are highly prevalent worldwide, Jatin Roper, MD, gastroenterologist and associate professor of medicine at Duke University, Durham, North Carolina, told GI & Hepatology News.
“A growing body of evidence suggests that DGBI, including irritable bowel syndrome, are caused by imbalances in signaling between the brain and the intestine, which include the vagus nerve, hormonal signals, the gut microbiota, and immune system,” said Roper, who was not involved in the current study.
“Since many sleep disturbances are centrally mediated, it is plausible that sleep and gastrointestinal disorders could be mechanistically linked,” he said. Rigorous analysis of patient databases for a possible association between sleep and GI disorders, as was done in the current study, is an important step.
The current study findings were not unexpected, “particularly the finding that depression may mediate a link between sleep and GI disorders, because depression is well known to be associated to sleep disturbances and DGBI,” Roper said.
However, GI doctors often do not ask patients about problems with sleep, and pulmonary doctors or sleep specialists may not ask patients about GI symptoms, Roper noted. Similarly, patients may not bring up all their symptoms when seeing these specialists.
“The current study underscores the need for comprehensive, multisystem evaluations in specialty clinics for sleep and GI conditions and appropriate referrals to specialists, when necessary,” he said.
The research raised an important question of whether sleep and GI disorders are associated with each other because of other underlying medical conditions, which may be difficult to control for in cross-sectional studies, or whether sleep problems cause GI problems or vice versa, Roper said. Other uncertainties include whether the conditions are biologically linked, possibly through shared changes in the brain-gut axis.
Long-term observational studies would be useful to identify whether sleep disturbances precede DGBI or vice versa, Roper added.
The study received no outside funding. The researchers and Roper had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
“Emerging evidence suggests a bidirectional relationship between GI diseases and sleep disorders, whereby dysfunction in one domain may exacerbate the other,” wrote Shicheng Ye, PhD, of The Third Clinical Medical College of Guangzhou University of Chinese Medicine, and colleagues. However, previous studies on the association between GI and sleep problems have been small, and the role of depression as a mediator has not been well explored.
In the study, which was published online in BMC Gastroenterology, the researchers reviewed data from the US National Health and Nutrition Examination Survey between 2005 and 2014. The study population included 10,626 adults aged 20 years or older, with a mean age of 45.6 years, 50.8% of whom were women. Of these, 6444 were identified as having GI disease on the basis of a “yes” response to the question of whether they had a stomach or intestinal illness with vomiting or diarrhea within the past 30 days.
Researchers also examined responses to survey questions related to sleep duration, trouble sleeping, and diagnosis of a sleep disorder. Individuals with vs without GI diseases had a significantly higher prevalence of sleep trouble (37.99% vs 24.21%; P < .001) and a greater frequency of diagnosed sleep disorders (14.99% vs 8.08%; P < .001).
An analysis adjusted for demographic, lifestyle, and clinical factors found that individuals with vs without GI diseases were 70% more likely to have sleep trouble. Individuals with vs without GI diseases were also significantly more likely to have a diagnosed sleep disorder and a reduction in sleep duration (adjusted odds ratio, 1.8; adjusted beta, -0.15).
The association between GI diseases and sleep problems remained consistent across individuals of multiple subgroups, including those without hypertension, diabetes, or a history of smoking. It also remained significant among individuals with coronary heart disease and higher scores on the dietary index for gut microbiota. No significant interaction effects related to age, sex, or chronic disease appeared in any subgroup (P > .05).
An additional mediation analysis found that depression partly mediated the associations between GI diseases and sleep issues. Depression accounted for 21.29% of the total effect on sleep problems, 19.23% of the effect on sleep disorders, and 26.68% of the effect on sleep duration.
The mediating role of depression on the association between GI disease and sleep problems may not be exclusive, the researchers wrote. Other potential mechanisms may include systemic inflammation, visceral hypersensitivity, and metabolic dysfunction.
The findings were limited by several factors, including the possibly underpowered sample size for machine-learning models and the reliance on self-reports of GI diseases, sleep outcomes, and coronary heart disease, the researchers noted. Other limitations included the inability to adjust for confounding factors, including obstructive sleep apnea, chronic pain, and hypertension.
However, the results illustrate the need to address both psychological and GI factors in clinical practice to improve sleep health, the researchers wrote. More research is needed to identify causal pathways and develop targeted, multidimensional interventions for this interconnected trio of health problems.
Increasing Evidence for Gut-Brain Interaction
Both sleep disorders and disorders of GBI (DGBI) are highly prevalent worldwide, Jatin Roper, MD, gastroenterologist and associate professor of medicine at Duke University, Durham, North Carolina, told GI & Hepatology News.
“A growing body of evidence suggests that DGBI, including irritable bowel syndrome, are caused by imbalances in signaling between the brain and the intestine, which include the vagus nerve, hormonal signals, the gut microbiota, and immune system,” said Roper, who was not involved in the current study.
“Since many sleep disturbances are centrally mediated, it is plausible that sleep and gastrointestinal disorders could be mechanistically linked,” he said. Rigorous analysis of patient databases for a possible association between sleep and GI disorders, as was done in the current study, is an important step.
The current study findings were not unexpected, “particularly the finding that depression may mediate a link between sleep and GI disorders, because depression is well known to be associated to sleep disturbances and DGBI,” Roper said.
However, GI doctors often do not ask patients about problems with sleep, and pulmonary doctors or sleep specialists may not ask patients about GI symptoms, Roper noted. Similarly, patients may not bring up all their symptoms when seeing these specialists.
“The current study underscores the need for comprehensive, multisystem evaluations in specialty clinics for sleep and GI conditions and appropriate referrals to specialists, when necessary,” he said.
The research raised an important question of whether sleep and GI disorders are associated with each other because of other underlying medical conditions, which may be difficult to control for in cross-sectional studies, or whether sleep problems cause GI problems or vice versa, Roper said. Other uncertainties include whether the conditions are biologically linked, possibly through shared changes in the brain-gut axis.
Long-term observational studies would be useful to identify whether sleep disturbances precede DGBI or vice versa, Roper added.
The study received no outside funding. The researchers and Roper had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
“Emerging evidence suggests a bidirectional relationship between GI diseases and sleep disorders, whereby dysfunction in one domain may exacerbate the other,” wrote Shicheng Ye, PhD, of The Third Clinical Medical College of Guangzhou University of Chinese Medicine, and colleagues. However, previous studies on the association between GI and sleep problems have been small, and the role of depression as a mediator has not been well explored.
In the study, which was published online in BMC Gastroenterology, the researchers reviewed data from the US National Health and Nutrition Examination Survey between 2005 and 2014. The study population included 10,626 adults aged 20 years or older, with a mean age of 45.6 years, 50.8% of whom were women. Of these, 6444 were identified as having GI disease on the basis of a “yes” response to the question of whether they had a stomach or intestinal illness with vomiting or diarrhea within the past 30 days.
Researchers also examined responses to survey questions related to sleep duration, trouble sleeping, and diagnosis of a sleep disorder. Individuals with vs without GI diseases had a significantly higher prevalence of sleep trouble (37.99% vs 24.21%; P < .001) and a greater frequency of diagnosed sleep disorders (14.99% vs 8.08%; P < .001).
An analysis adjusted for demographic, lifestyle, and clinical factors found that individuals with vs without GI diseases were 70% more likely to have sleep trouble. Individuals with vs without GI diseases were also significantly more likely to have a diagnosed sleep disorder and a reduction in sleep duration (adjusted odds ratio, 1.8; adjusted beta, -0.15).
The association between GI diseases and sleep problems remained consistent across individuals of multiple subgroups, including those without hypertension, diabetes, or a history of smoking. It also remained significant among individuals with coronary heart disease and higher scores on the dietary index for gut microbiota. No significant interaction effects related to age, sex, or chronic disease appeared in any subgroup (P > .05).
An additional mediation analysis found that depression partly mediated the associations between GI diseases and sleep issues. Depression accounted for 21.29% of the total effect on sleep problems, 19.23% of the effect on sleep disorders, and 26.68% of the effect on sleep duration.
The mediating role of depression on the association between GI disease and sleep problems may not be exclusive, the researchers wrote. Other potential mechanisms may include systemic inflammation, visceral hypersensitivity, and metabolic dysfunction.
The findings were limited by several factors, including the possibly underpowered sample size for machine-learning models and the reliance on self-reports of GI diseases, sleep outcomes, and coronary heart disease, the researchers noted. Other limitations included the inability to adjust for confounding factors, including obstructive sleep apnea, chronic pain, and hypertension.
However, the results illustrate the need to address both psychological and GI factors in clinical practice to improve sleep health, the researchers wrote. More research is needed to identify causal pathways and develop targeted, multidimensional interventions for this interconnected trio of health problems.
Increasing Evidence for Gut-Brain Interaction
Both sleep disorders and disorders of GBI (DGBI) are highly prevalent worldwide, Jatin Roper, MD, gastroenterologist and associate professor of medicine at Duke University, Durham, North Carolina, told GI & Hepatology News.
“A growing body of evidence suggests that DGBI, including irritable bowel syndrome, are caused by imbalances in signaling between the brain and the intestine, which include the vagus nerve, hormonal signals, the gut microbiota, and immune system,” said Roper, who was not involved in the current study.
“Since many sleep disturbances are centrally mediated, it is plausible that sleep and gastrointestinal disorders could be mechanistically linked,” he said. Rigorous analysis of patient databases for a possible association between sleep and GI disorders, as was done in the current study, is an important step.
The current study findings were not unexpected, “particularly the finding that depression may mediate a link between sleep and GI disorders, because depression is well known to be associated to sleep disturbances and DGBI,” Roper said.
However, GI doctors often do not ask patients about problems with sleep, and pulmonary doctors or sleep specialists may not ask patients about GI symptoms, Roper noted. Similarly, patients may not bring up all their symptoms when seeing these specialists.
“The current study underscores the need for comprehensive, multisystem evaluations in specialty clinics for sleep and GI conditions and appropriate referrals to specialists, when necessary,” he said.
The research raised an important question of whether sleep and GI disorders are associated with each other because of other underlying medical conditions, which may be difficult to control for in cross-sectional studies, or whether sleep problems cause GI problems or vice versa, Roper said. Other uncertainties include whether the conditions are biologically linked, possibly through shared changes in the brain-gut axis.
Long-term observational studies would be useful to identify whether sleep disturbances precede DGBI or vice versa, Roper added.
The study received no outside funding. The researchers and Roper had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
Advanced Imaging Techniques Use in Giant Cell Arteritis Diagnosis: The Experience at Walter Reed National Military Medical Center
Advanced Imaging Techniques Use in Giant Cell Arteritis Diagnosis: The Experience at Walter Reed National Military Medical Center
Giant cell arteritis (GCA), the most commonly diagnosed systemic vasculitis, is a large- and medium-vessel vasculitis that can lead to significant morbidity due to aneurysm formation or vascular occlusion if not diagnosed in a timely manner.1,2 Diagnosis is typically based on clinical history and inflammatory markers. Laboratory inflammatory markers may be normal in the early stages of GCA but can be abnormal due to other unrelated reasons leading to a false positive diagnosis.3 Delayed treatment may lead to visual loss, jaw or limb claudication, or ischemic stroke.2 Initial treatment typically includes high-dose steroids that can lead to significant adverse reactions such as hypothalamic-pituitary-adrenal axis dysfunction, metabolic syndrome, premature atherosclerosis, and increased risk of infection.4-6
The 1990 American College of Rheumatology (ACR) criteria for GCA are widely recognized (Table 1).7 The criteria focuses on clinical manifestations, including new onset headache, temporal artery tenderness, age ≥ 50 years, erythrocyte sedimentation rate (ESR) ≥ 50 mm/hr, and temporal artery biopsy with positive anatomical findings.8 When 3 of the 5 1990 ACR criteria are present, the sensitivity and specificity is estimated to be > 90% for GCA vs alternative vasculitides.7

Although the 1990 ACR criteria do not include imaging, modalities such as ultrasound, computed tomography angiography (CTA), 18F-FDG positron emission tomography (PET), and magnetic resonance imaging (MRI)/magnetic resonance angiography (MRA) may be used in GCA diagnosis.8-10 These imaging modalities have been added to the proposed ACR classification criteria for GCA.11 For this updated point system standard, age ≥ 50 years is a requirement and includes a positive temporal artery biopsy or temporal artery halo sign on ultrasound (+5 points), an ESR ≥ 50 mm/h or C-reactive protein (CRP) ≥ 10 mg/L (+3 points), or sudden visual loss (+3 points). Scalp tenderness, jaw or tongue claudication, new temporal headache, morning stiffness in shoulders or neck, temporal artery abnormality on vascular examination, bilateral axillary vessel involvement on imaging, and 18F-FDG PET activity throughout the aorta are scored +2 points each. With these new criteria, a cumulative score ≥ 6 is classified as GCA. Diagnostic accuracy is further improved with imaging: ultrasonography (sensitivity 55% and specificity 95%) and 18F-FDG PET (sensitivity 69% and specificity 92%), CTA (sensitivity 71% and specificity 86%), and MRI/MRA (sensitivity 73% and specificity 88%).12-15
In recent years, clinicians have reported increased glucose uptake in arteries observed on PET imaging that suggests GCA.9,10,16-20 18F-FDG accumulates in cells with high metabolic activity rates, such as areas of inflammation. In assessing temporal arteries or other involved vasculature (eg, axillary or great vessels) for GCA, this modality indicates increased glucose uptake in the lining of vessel walls. The inflammation of vessel walls can then be visualized with PET. 18F-FDG PET presents a noninvasive imaging technique for evaluating GCA but its use has been limited in the United States due to its high cost.
Methods
Approval for a retrospective chart review of patients evaluated for suspected GCA was obtained from the Walter Reed National Military Medical Center (WRNMMC) Institutional Review Board. The review included patients who underwent diagnostic procedures such as ultrasound, MRI, CT angiogram, and PET studies from 2016 through 2022. International Classification of Diseases codes used for case identification included: M31.6, M31.5, I77.6, I77.8, I77.89, I67.7, and I68.2. The Current Procedural Terminology code used for temporal artery biopsy is 37609.
Results
Seventy-eight charts were reviewed and 42 patients (54%) were diagnosed with GCA (Table 2). This study sample had a much higher proportion of African American subjects (31%) when compared with the civilian population, likely reflecting the higher representation of African Americans in the armed forces. Twenty-eight females (67%) were GCA positive. The most common presenting symptoms included 27 patients (64%) with headache, 17 (40%) with scalp tenderness, and 14 (33%) with jaw pain. The mean 1990 ACR score was 3.8 (range, 2-5). With respect to the score criteria: 41 patients (98%) were aged ≥ 50 years, 31 (74%) had new onset headache, and 31 (74%) had elevated ESR (Table 3). Acute ischemic optic neuropathy was documented in 4 patients (10%) with confirmed GCA. The mean ESR and CRP values at diagnosis were 66.2 mm/h (range, 7-122 mm/h) and 8.711 μg/mL (range, 0.054 – 92.690 μg/mL), respectively. Twenty-seven patients (64%) underwent biopsy: 24 (89%) were unilateral and 3 (11%) were bilateral (Table 4). Four patients with GCA (10%) were missing biopsy data. Nineteen patients with GCA (70%) had biopsies with pathologic findings consistent with GCA.
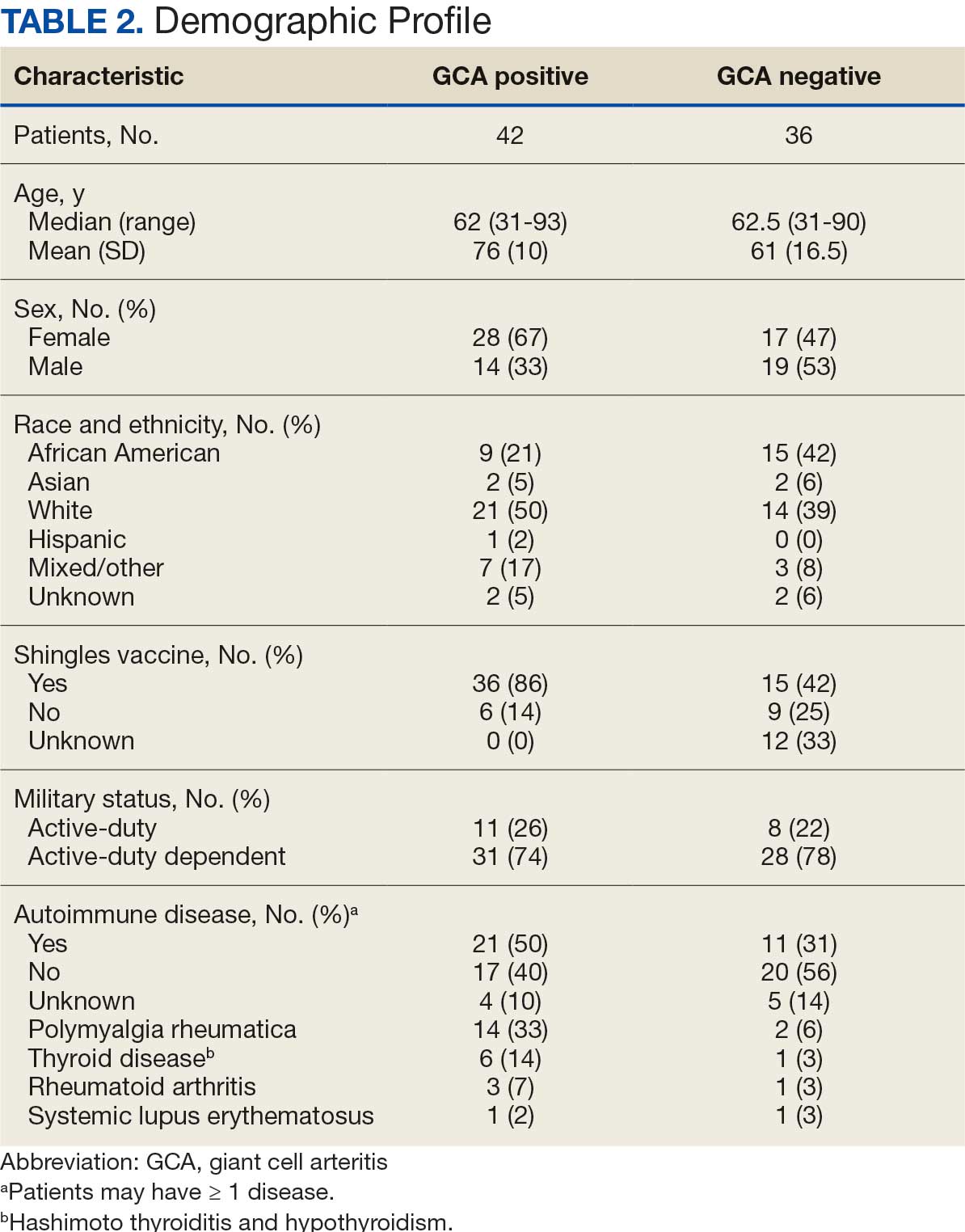
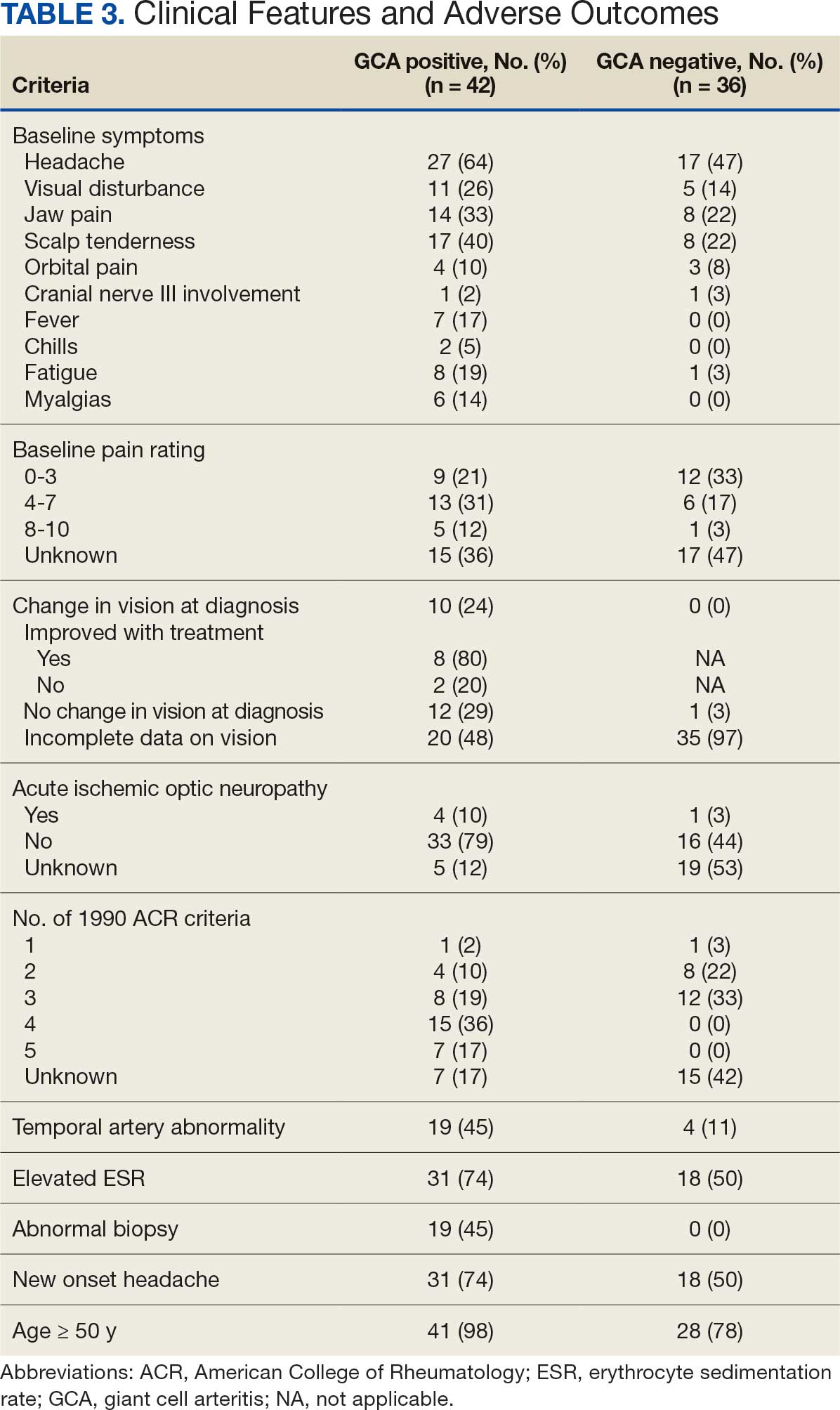
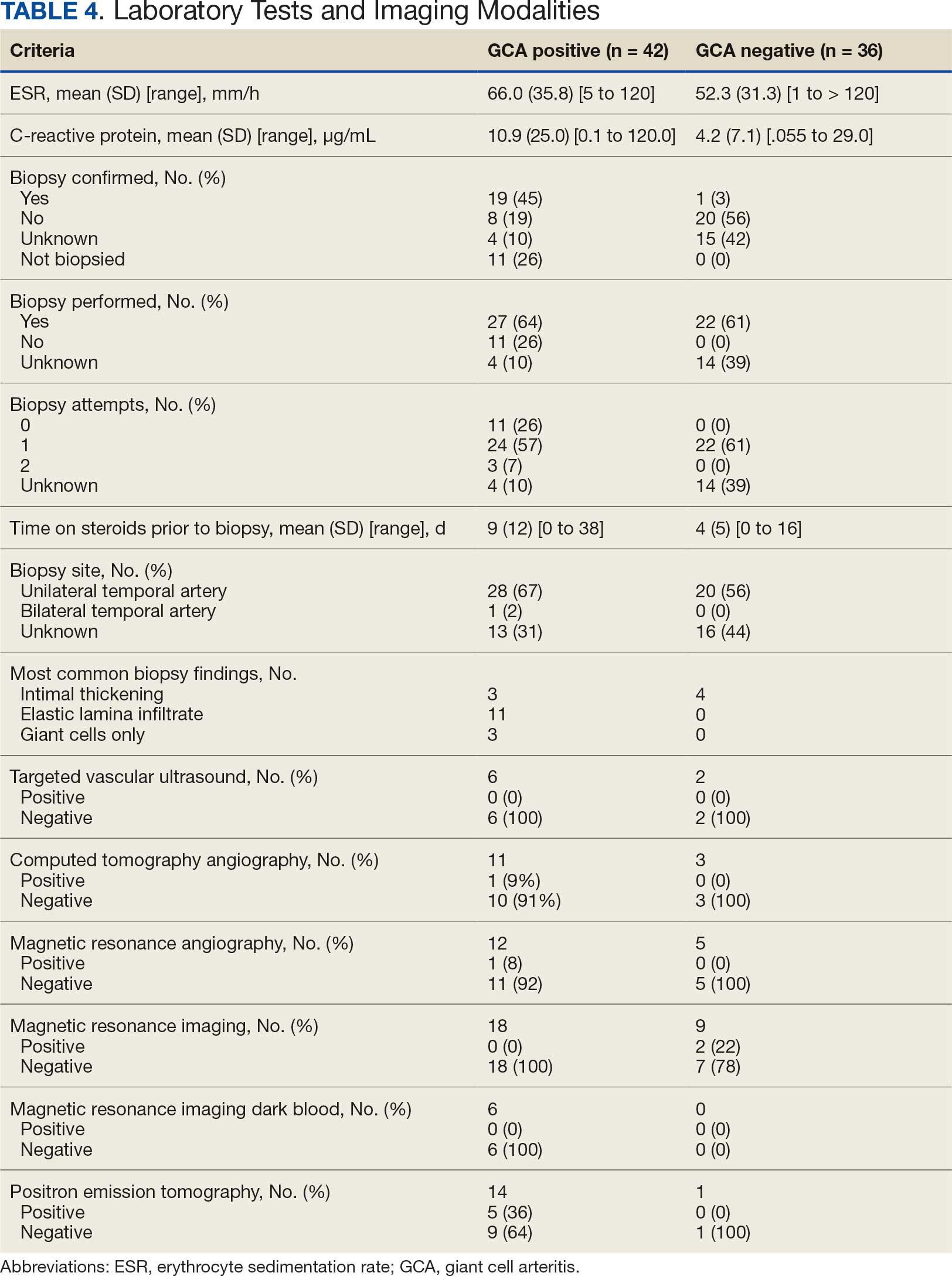
Twenty-five patients with GCA (60%) received ≥ 1 imaging modality. The most common imaging modality was MRI, which was used for 18 (43%) patients. Fourteen patients (33%) had 18F-FDG PET, 12 patients (29%) had MRA, and 11 patients (26%) had CTA. The small number of patients who underwent point-of-care ultrasound (POCUS), brain MRI, or dark blood MRI were negative for disease. Five patients who underwent 18F-FDG PET had findings consistent with GCA. One patient with GCA had CTA of the head and neck with radiographic findings supportive of GCA.
Discussion
The available evidence supports the use of additional screening tests to increase the temporal artery biopsy yield for GCA. Inflammatory laboratory markers demonstrate some sensitivity but are nonspecific for GCA. In this study, only 60% of patients with GCA underwent diagnostic imaging as part of the workup. There are multiple factors that may contribute to the underutilization of advanced imaging in the diagnosis of GCA, including outdated standardized diagnostic criteria, limited resources (direct access to modalities), and lack of clinician awareness of diagnostic testing options. In this retrospective review, 30 patients (71%) were diagnosed with GCA with a 1990 ACR GCA score ≤ 3. Of these 30 patients, 19 underwent confirmatory biopsy followed by prolonged courses of steroid therapy. In addition, only 25 patients underwent advanced imaging to increase diagnostic accuracy of the suspected syndrome.
A large meta-analysis demonstrated a sensitivity of 77.3% (95% CI, 71.8-81.9%) for temporal artery biopsy.21 The overall yield was 40% in the meta-analysis. Advanced noninvasive imaging represents an appropriate method of evaluating GCA.8-20 In our study, 18F-FDG PET demonstrated the highest sensitivity (36%) for the diagnosis of GCA. Ultrasonography is recommended as an initial screening tool to identify the noncompressible halo sign (a hypoechoic circumferential wall thickening due to edema) as a cost-effective and widely available technology.22 Other research has corroborated the beneficial use of ultrasonography in improving diagnostic accuracy by detecting the noncompressible halo sign in temporal arteries.22,23 GCA diagnostic performance has been significantly improved with the use of B-mode probes ≥ 15 MHz as well as proposals to incorporate a compression sign or interrogating the axillary vessels, showing a sensitivity of 54% to 77%.23,24
POCUS may reduce the risk of a false-negative biopsy and improve yield with more frequent utilization. However, ultrasonography may be limited by operator skills and visualization of the great vessels. The accuracy of ultrasonography is dependent on the experience and adeptness of the operator. Additional studies are needed to establish a systematic standard for POCUS training to ensure accurate interpretation and uniform interrogation procedure.24 Artificial intelligence (AI) may aid in interpreting results of POCUS and bridging the operator skill gap among operators.25,26 AI and machine learning techniques can assist in detecting the noncompressible halo sign and compression sign in temporal arteries and other affected vessels.
In comparing the WRNMMC patient population with other US civilian GCA cohorts, there are some differences and similarities. There was a high representation of African American patients in the study, which may reflect a greater severity of autoimmune disease expression in this population.27 We also observed a higher number of females and an association with polymyalgia rheumatica in the data, consistent with previous reports.28,29 The females in this study were primarily civilians and therefore more similar to the general population of individuals with GCA. In contrast, male patients were more likely to be active-duty service members or have prior service experience with increased exposure to novel environmental factors linked to increased risk of autoimmune disease. This includes an increased risk of Guillain-Barré syndrome and Graves disease among Vietnam veterans exposed to Agent Orange.30,31 Other studies have found that veterans with posttraumatic stress disorder are at increased risk for severe autoimmune diseases.32,33 As more women join the active-duty military, the impact of autoimmune disease in the military service population is expected to grow, requiring further research.
Conclusions
Early diagnosis and treatment of GCA are critical to preventing serious outcomes, such as visual loss, jaw or limb claudication, or ischemic stroke. The incidence of autoimmune disease is expected to rise in the armed forces and veteran populations due to exposure to novel environmental factors and the increasing representation of women in the military. The use of additional screening tools can aid in earlier diagnosis of GCA. The 2022 ACR classification criteria for GCA represent significant updates to the 1990 criteria, incorporating ancillary tests such as the temporal artery halo sign on ultrasound, bilateral axillary vessel screening on imaging, and 18F-FDG PET activity throughout the aorta. The updated criteria require further validation and supports the adoption of a multidisciplinary approach that includes ultrasonography, vascular MRI/CT, and 18F-FDG PET. Furthermore, AI may play a future key role in ultrasound interpretation and study interrogation procedure. Ultimately, ultrasonography is a noninvasive and promising technique for the early diagnosis of GCA. A target goal is to increase the yield of positive temporal artery biopsies to ≥ 70%.
- Jennette JC. Overview of the 2012 revised International Chapel Hill Consensus Conference nomenclature of vasculitides. Clin Exp Nephrol. 2013;17:603-606. doi:10.1007/s10157-013-0869-6
- Kale N, Eggenberger E. Diagnosis and management of giant cell arteritis: a review. Curr Opin Ophthalmol. 2010;21:417-422. doi:10.1097/ICU.0b013e32833eae8b
- Smetana GW, Shmerling RH. Does this patient have temporal arteritis? JAMA. 2002;287:92-101.
- Schäcke H, Döcke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96:23-43. doi:10.1016/s0163-7258(02)00297-8
- Curtis JR, Patkar N, Xie A, et al. Risk of serious bacterial infections among rheumatoid arthritis patients exposed to tumor necrosis factor alpha antagonists. Arthritis Rheum. 2007;56:1125-1133. doi:10.1002/art.22504
- Hoes JN, van der Goes MC, van Raalte DH, et al. Glucose tolerance, insulin sensitivity and ß-cell function in patients with rheumatoid arthritis treated with or without low-to-medium dose glucocorticoids. Ann Rheum Dis. 2011;70:1887-1894. doi:10.1136/ard.2011.151464
- Hunder GG, Bloch DA, Michel BA, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum. 1990;33:1122-1128. doi:10.1002/art.1780330810
- Dejaco C, Duftner C, Buttgereit F, Matteson EL, Dasgupta B. The spectrum of giant cell arteritis and polymyalgia rheumatica: revisiting the concept of the disease. Rheumatology (Oxford). 2017;56:506-515. doi:10.1093/rheumatology/kew273
- Slart RHJ, Nienhuis PH, Glaudemans AWJM, et al. Role of 18F-FDG PET/CT in large vessel vasculitis and polymyalgia rheumatica. J Nucl Med. 2023;64:515-521. doi:10.2967/jnumed.122.265016
- Shimol JB, Amital H, Lidar M, Domachevsky L, Shoenfeld Y, Davidson T. The utility of PET/CT in large vessel vasculitis. Sci Rep. 2020;10:17709. doi:10.1038/s41598-020-73818-2
- Ponte C, Grayson PC, Robson JC, et al. 2022 American College of Rheumatology/EULAR Classification Criteria for Giant Cell Arteritis. Arthritis Rheumatol. 2022;74:1881-1889. doi:10.1002/art.42325
- He J, Williamson L, Ng B, et al. The diagnostic accuracy of temporal artery ultrasound and temporal artery biopsy in giant cell arteritis: a single center Australian experience over 10 years. Int J Rheum Dis. 2022;25:447-453. doi:10.1111/1756-185X.14288
- Stellingwerff MD, Brouwer E, Lensen KDF, et al. Different scoring methods of FDG PET/CT in giant cell arteritis: need for standardization. Medicine (Baltimore). 2015;94:e1542. doi:10.1097/MD.0000000000001542
- Conway R, Smyth AE, Kavanagh RG, et al. Diagnostic utility of computed tomographic angiography in giant-cell arteritis. Stroke. 2018;49:2233-2236. doi:10.1161/STROKEAHA.118.021995
- Duftner C, Dejaco C, Sepriano A, et al. Imaging in diagnosis, outcome prediction and monitoring of large vessel vasculitis: a systematic literature review and meta-analysis informing the EULAR recommendations. RMD Open. 2018;4:e000612. doi:10.1136/rmdopen-2017-000612
- Rehak Z, Vasina J, Ptacek J, et al. PET/CT in giant cell arteritis: high 18F-FDG uptake in the temporal, occipital and vertebral arteries. Rev Esp Med Nucl Imagen Mol. 2016;35:398-401. doi:10.1016/j.remn.2016.03.007
- Salvarani C, Soriano A, Muratore F, et al. Is PET/CT essential in the diagnosis and follow-up of temporal arteritis? Autoimmun Rev. 2017;16:1125-1130. doi:10.1016/j.autrev.2017.09.007
- Brodmann M, Lipp RW, Passath A, et al. The role of 2-18F-fluoro-2-deoxy-D-glucose positron emission tomography in the diagnosis of giant cell arteritis of the temporal arteries. Rheumatology (Oxford). 2004;43:241-242. doi:10.1093/rheumatology/keh025
- Flaus A, Granjon D, Habouzit V, Gaultier JB, Prevot-Bitot N. Unusual and diffuse hypermetabolism in routine 18F-FDG PET/CT of the supra-aortic vessels in biopsy-positive giant cell arteritis. Clin Nucl Med. 2018;43:e336-e337. doi:10.1097/RLU.0000000000002198
- Berger CT, Sommer G, Aschwanden M, et al. The clinical benefit of imaging in the diagnosis and treatment of giant cell arteritis. Swiss Med Wkly. 2018;148:w14661. doi:10.4414/smw.2018.14661
- Rubenstein E, Maldini C, Gonzalez-Chiappe S, et al. Sensitivity of temporal artery biopsy in the diagnosis of giant cell arteritis: a systematic literature review and meta-analysis. Rheumatology (Oxford). 2020;59:1011-1020. doi:10.1093/rheumatology/kez385
- Tsivgoulis G, Heliopoulos I, Vadikolias K, et al. Teaching neuroimages: ultrasound findings in giant-cell arteritis. Neurology. 2010;75:e67-e68. doi:10.1212/WNL.0b013e3181f881e9
- Nakajima E, Moon FH, Canvas Jr N, et al. Accuracy of Doppler ultrasound in the diagnosis of giant cell arteritis: a systematic review and meta-analysis. Adv Rheumatol. 2023;63:5. doi:10.1186/s42358-023-00286-3
- Naumegni SR, Hoffmann C, Cornec D, et al. Temporal artery ultrasound to diagnose giant cell arteritis: a practical guide. Ultrasound Med Biol. 2021;47:201-213. doi:10.1016/j.ultrasmedbio.2020.10.004
- Kim YH. Artificial intelligence in medical ultrasonography: driving on an unpaved road. Ultrasonography. 2021;40:313-317. doi:10.14366/usg.21031
- Sultan LR, Mohamed MH, Andronikou S. ChatGPT-4: a breakthrough in ultrasound image analysis. Radiol Adv. 2024;1:umae006. doi:10.1093/radadv/umae006
- Cipriani VP, Klein S. Clinical characteristics of multiple sclerosis in African-Americans. Curr Neurol Neurosci Rep. 2019;19:87. doi:10.1007/s11910-019-1000-5
- Sturm A, Dechant C, Proft F, et al. Gender differences in giant cell arteritis: a case-control study. Clin Exp Rheumatol. 2016;34:S70-72.
- Li KJ, Semenov D, Turk M, et al. A meta-analysis of the epidemiology of giant cell arteritis across time and space. Arthritis Res Ther. 2021;23:82. doi:10.1186/s13075-021-02450-w
- Nelson L, Gormley R, Riddle MS, Tribble DR, Porter CK. The epidemiology of Guillain-Barré syndrome in U.S. military personnel: a case-control study. BMC Res Notes. 2009;2:171. doi:10.1186/1756-0500-2-171
- Spaulding SW. The possible roles of environmental factors and the aryl hydrocarbon receptor in the prevalence of thyroid diseases in Vietnam era veterans. Curr Opin Endocrinol Diabetes Obes. 2011;18:315-320.
- O’Donovan A, Cohen BE, Seal KH, et al. Elevated risk for autoimmune disorders in Iraq and Afghanistan veterans with posttraumatic stress disorder. Biol Psychiatry. 2015;77:365-374. doi:10.1016/j.biopsych.2014.06.015
- Bookwalter DB, Roenfeldt KA, LeardMann CA, Kong SY, Riddle MS, Rull RP. Posttraumatic stress disorder and risk of selected autoimmune diseases among US military personnel. BMC Psychiatry. 2020;20:23. doi:10.1186/s12888-020-2432-9
Giant cell arteritis (GCA), the most commonly diagnosed systemic vasculitis, is a large- and medium-vessel vasculitis that can lead to significant morbidity due to aneurysm formation or vascular occlusion if not diagnosed in a timely manner.1,2 Diagnosis is typically based on clinical history and inflammatory markers. Laboratory inflammatory markers may be normal in the early stages of GCA but can be abnormal due to other unrelated reasons leading to a false positive diagnosis.3 Delayed treatment may lead to visual loss, jaw or limb claudication, or ischemic stroke.2 Initial treatment typically includes high-dose steroids that can lead to significant adverse reactions such as hypothalamic-pituitary-adrenal axis dysfunction, metabolic syndrome, premature atherosclerosis, and increased risk of infection.4-6
The 1990 American College of Rheumatology (ACR) criteria for GCA are widely recognized (Table 1).7 The criteria focuses on clinical manifestations, including new onset headache, temporal artery tenderness, age ≥ 50 years, erythrocyte sedimentation rate (ESR) ≥ 50 mm/hr, and temporal artery biopsy with positive anatomical findings.8 When 3 of the 5 1990 ACR criteria are present, the sensitivity and specificity is estimated to be > 90% for GCA vs alternative vasculitides.7

Although the 1990 ACR criteria do not include imaging, modalities such as ultrasound, computed tomography angiography (CTA), 18F-FDG positron emission tomography (PET), and magnetic resonance imaging (MRI)/magnetic resonance angiography (MRA) may be used in GCA diagnosis.8-10 These imaging modalities have been added to the proposed ACR classification criteria for GCA.11 For this updated point system standard, age ≥ 50 years is a requirement and includes a positive temporal artery biopsy or temporal artery halo sign on ultrasound (+5 points), an ESR ≥ 50 mm/h or C-reactive protein (CRP) ≥ 10 mg/L (+3 points), or sudden visual loss (+3 points). Scalp tenderness, jaw or tongue claudication, new temporal headache, morning stiffness in shoulders or neck, temporal artery abnormality on vascular examination, bilateral axillary vessel involvement on imaging, and 18F-FDG PET activity throughout the aorta are scored +2 points each. With these new criteria, a cumulative score ≥ 6 is classified as GCA. Diagnostic accuracy is further improved with imaging: ultrasonography (sensitivity 55% and specificity 95%) and 18F-FDG PET (sensitivity 69% and specificity 92%), CTA (sensitivity 71% and specificity 86%), and MRI/MRA (sensitivity 73% and specificity 88%).12-15
In recent years, clinicians have reported increased glucose uptake in arteries observed on PET imaging that suggests GCA.9,10,16-20 18F-FDG accumulates in cells with high metabolic activity rates, such as areas of inflammation. In assessing temporal arteries or other involved vasculature (eg, axillary or great vessels) for GCA, this modality indicates increased glucose uptake in the lining of vessel walls. The inflammation of vessel walls can then be visualized with PET. 18F-FDG PET presents a noninvasive imaging technique for evaluating GCA but its use has been limited in the United States due to its high cost.
Methods
Approval for a retrospective chart review of patients evaluated for suspected GCA was obtained from the Walter Reed National Military Medical Center (WRNMMC) Institutional Review Board. The review included patients who underwent diagnostic procedures such as ultrasound, MRI, CT angiogram, and PET studies from 2016 through 2022. International Classification of Diseases codes used for case identification included: M31.6, M31.5, I77.6, I77.8, I77.89, I67.7, and I68.2. The Current Procedural Terminology code used for temporal artery biopsy is 37609.
Results
Seventy-eight charts were reviewed and 42 patients (54%) were diagnosed with GCA (Table 2). This study sample had a much higher proportion of African American subjects (31%) when compared with the civilian population, likely reflecting the higher representation of African Americans in the armed forces. Twenty-eight females (67%) were GCA positive. The most common presenting symptoms included 27 patients (64%) with headache, 17 (40%) with scalp tenderness, and 14 (33%) with jaw pain. The mean 1990 ACR score was 3.8 (range, 2-5). With respect to the score criteria: 41 patients (98%) were aged ≥ 50 years, 31 (74%) had new onset headache, and 31 (74%) had elevated ESR (Table 3). Acute ischemic optic neuropathy was documented in 4 patients (10%) with confirmed GCA. The mean ESR and CRP values at diagnosis were 66.2 mm/h (range, 7-122 mm/h) and 8.711 μg/mL (range, 0.054 – 92.690 μg/mL), respectively. Twenty-seven patients (64%) underwent biopsy: 24 (89%) were unilateral and 3 (11%) were bilateral (Table 4). Four patients with GCA (10%) were missing biopsy data. Nineteen patients with GCA (70%) had biopsies with pathologic findings consistent with GCA.



Twenty-five patients with GCA (60%) received ≥ 1 imaging modality. The most common imaging modality was MRI, which was used for 18 (43%) patients. Fourteen patients (33%) had 18F-FDG PET, 12 patients (29%) had MRA, and 11 patients (26%) had CTA. The small number of patients who underwent point-of-care ultrasound (POCUS), brain MRI, or dark blood MRI were negative for disease. Five patients who underwent 18F-FDG PET had findings consistent with GCA. One patient with GCA had CTA of the head and neck with radiographic findings supportive of GCA.
Discussion
The available evidence supports the use of additional screening tests to increase the temporal artery biopsy yield for GCA. Inflammatory laboratory markers demonstrate some sensitivity but are nonspecific for GCA. In this study, only 60% of patients with GCA underwent diagnostic imaging as part of the workup. There are multiple factors that may contribute to the underutilization of advanced imaging in the diagnosis of GCA, including outdated standardized diagnostic criteria, limited resources (direct access to modalities), and lack of clinician awareness of diagnostic testing options. In this retrospective review, 30 patients (71%) were diagnosed with GCA with a 1990 ACR GCA score ≤ 3. Of these 30 patients, 19 underwent confirmatory biopsy followed by prolonged courses of steroid therapy. In addition, only 25 patients underwent advanced imaging to increase diagnostic accuracy of the suspected syndrome.
A large meta-analysis demonstrated a sensitivity of 77.3% (95% CI, 71.8-81.9%) for temporal artery biopsy.21 The overall yield was 40% in the meta-analysis. Advanced noninvasive imaging represents an appropriate method of evaluating GCA.8-20 In our study, 18F-FDG PET demonstrated the highest sensitivity (36%) for the diagnosis of GCA. Ultrasonography is recommended as an initial screening tool to identify the noncompressible halo sign (a hypoechoic circumferential wall thickening due to edema) as a cost-effective and widely available technology.22 Other research has corroborated the beneficial use of ultrasonography in improving diagnostic accuracy by detecting the noncompressible halo sign in temporal arteries.22,23 GCA diagnostic performance has been significantly improved with the use of B-mode probes ≥ 15 MHz as well as proposals to incorporate a compression sign or interrogating the axillary vessels, showing a sensitivity of 54% to 77%.23,24
POCUS may reduce the risk of a false-negative biopsy and improve yield with more frequent utilization. However, ultrasonography may be limited by operator skills and visualization of the great vessels. The accuracy of ultrasonography is dependent on the experience and adeptness of the operator. Additional studies are needed to establish a systematic standard for POCUS training to ensure accurate interpretation and uniform interrogation procedure.24 Artificial intelligence (AI) may aid in interpreting results of POCUS and bridging the operator skill gap among operators.25,26 AI and machine learning techniques can assist in detecting the noncompressible halo sign and compression sign in temporal arteries and other affected vessels.
In comparing the WRNMMC patient population with other US civilian GCA cohorts, there are some differences and similarities. There was a high representation of African American patients in the study, which may reflect a greater severity of autoimmune disease expression in this population.27 We also observed a higher number of females and an association with polymyalgia rheumatica in the data, consistent with previous reports.28,29 The females in this study were primarily civilians and therefore more similar to the general population of individuals with GCA. In contrast, male patients were more likely to be active-duty service members or have prior service experience with increased exposure to novel environmental factors linked to increased risk of autoimmune disease. This includes an increased risk of Guillain-Barré syndrome and Graves disease among Vietnam veterans exposed to Agent Orange.30,31 Other studies have found that veterans with posttraumatic stress disorder are at increased risk for severe autoimmune diseases.32,33 As more women join the active-duty military, the impact of autoimmune disease in the military service population is expected to grow, requiring further research.
Conclusions
Early diagnosis and treatment of GCA are critical to preventing serious outcomes, such as visual loss, jaw or limb claudication, or ischemic stroke. The incidence of autoimmune disease is expected to rise in the armed forces and veteran populations due to exposure to novel environmental factors and the increasing representation of women in the military. The use of additional screening tools can aid in earlier diagnosis of GCA. The 2022 ACR classification criteria for GCA represent significant updates to the 1990 criteria, incorporating ancillary tests such as the temporal artery halo sign on ultrasound, bilateral axillary vessel screening on imaging, and 18F-FDG PET activity throughout the aorta. The updated criteria require further validation and supports the adoption of a multidisciplinary approach that includes ultrasonography, vascular MRI/CT, and 18F-FDG PET. Furthermore, AI may play a future key role in ultrasound interpretation and study interrogation procedure. Ultimately, ultrasonography is a noninvasive and promising technique for the early diagnosis of GCA. A target goal is to increase the yield of positive temporal artery biopsies to ≥ 70%.
Giant cell arteritis (GCA), the most commonly diagnosed systemic vasculitis, is a large- and medium-vessel vasculitis that can lead to significant morbidity due to aneurysm formation or vascular occlusion if not diagnosed in a timely manner.1,2 Diagnosis is typically based on clinical history and inflammatory markers. Laboratory inflammatory markers may be normal in the early stages of GCA but can be abnormal due to other unrelated reasons leading to a false positive diagnosis.3 Delayed treatment may lead to visual loss, jaw or limb claudication, or ischemic stroke.2 Initial treatment typically includes high-dose steroids that can lead to significant adverse reactions such as hypothalamic-pituitary-adrenal axis dysfunction, metabolic syndrome, premature atherosclerosis, and increased risk of infection.4-6
The 1990 American College of Rheumatology (ACR) criteria for GCA are widely recognized (Table 1).7 The criteria focuses on clinical manifestations, including new onset headache, temporal artery tenderness, age ≥ 50 years, erythrocyte sedimentation rate (ESR) ≥ 50 mm/hr, and temporal artery biopsy with positive anatomical findings.8 When 3 of the 5 1990 ACR criteria are present, the sensitivity and specificity is estimated to be > 90% for GCA vs alternative vasculitides.7

Although the 1990 ACR criteria do not include imaging, modalities such as ultrasound, computed tomography angiography (CTA), 18F-FDG positron emission tomography (PET), and magnetic resonance imaging (MRI)/magnetic resonance angiography (MRA) may be used in GCA diagnosis.8-10 These imaging modalities have been added to the proposed ACR classification criteria for GCA.11 For this updated point system standard, age ≥ 50 years is a requirement and includes a positive temporal artery biopsy or temporal artery halo sign on ultrasound (+5 points), an ESR ≥ 50 mm/h or C-reactive protein (CRP) ≥ 10 mg/L (+3 points), or sudden visual loss (+3 points). Scalp tenderness, jaw or tongue claudication, new temporal headache, morning stiffness in shoulders or neck, temporal artery abnormality on vascular examination, bilateral axillary vessel involvement on imaging, and 18F-FDG PET activity throughout the aorta are scored +2 points each. With these new criteria, a cumulative score ≥ 6 is classified as GCA. Diagnostic accuracy is further improved with imaging: ultrasonography (sensitivity 55% and specificity 95%) and 18F-FDG PET (sensitivity 69% and specificity 92%), CTA (sensitivity 71% and specificity 86%), and MRI/MRA (sensitivity 73% and specificity 88%).12-15
In recent years, clinicians have reported increased glucose uptake in arteries observed on PET imaging that suggests GCA.9,10,16-20 18F-FDG accumulates in cells with high metabolic activity rates, such as areas of inflammation. In assessing temporal arteries or other involved vasculature (eg, axillary or great vessels) for GCA, this modality indicates increased glucose uptake in the lining of vessel walls. The inflammation of vessel walls can then be visualized with PET. 18F-FDG PET presents a noninvasive imaging technique for evaluating GCA but its use has been limited in the United States due to its high cost.
Methods
Approval for a retrospective chart review of patients evaluated for suspected GCA was obtained from the Walter Reed National Military Medical Center (WRNMMC) Institutional Review Board. The review included patients who underwent diagnostic procedures such as ultrasound, MRI, CT angiogram, and PET studies from 2016 through 2022. International Classification of Diseases codes used for case identification included: M31.6, M31.5, I77.6, I77.8, I77.89, I67.7, and I68.2. The Current Procedural Terminology code used for temporal artery biopsy is 37609.
Results
Seventy-eight charts were reviewed and 42 patients (54%) were diagnosed with GCA (Table 2). This study sample had a much higher proportion of African American subjects (31%) when compared with the civilian population, likely reflecting the higher representation of African Americans in the armed forces. Twenty-eight females (67%) were GCA positive. The most common presenting symptoms included 27 patients (64%) with headache, 17 (40%) with scalp tenderness, and 14 (33%) with jaw pain. The mean 1990 ACR score was 3.8 (range, 2-5). With respect to the score criteria: 41 patients (98%) were aged ≥ 50 years, 31 (74%) had new onset headache, and 31 (74%) had elevated ESR (Table 3). Acute ischemic optic neuropathy was documented in 4 patients (10%) with confirmed GCA. The mean ESR and CRP values at diagnosis were 66.2 mm/h (range, 7-122 mm/h) and 8.711 μg/mL (range, 0.054 – 92.690 μg/mL), respectively. Twenty-seven patients (64%) underwent biopsy: 24 (89%) were unilateral and 3 (11%) were bilateral (Table 4). Four patients with GCA (10%) were missing biopsy data. Nineteen patients with GCA (70%) had biopsies with pathologic findings consistent with GCA.



Twenty-five patients with GCA (60%) received ≥ 1 imaging modality. The most common imaging modality was MRI, which was used for 18 (43%) patients. Fourteen patients (33%) had 18F-FDG PET, 12 patients (29%) had MRA, and 11 patients (26%) had CTA. The small number of patients who underwent point-of-care ultrasound (POCUS), brain MRI, or dark blood MRI were negative for disease. Five patients who underwent 18F-FDG PET had findings consistent with GCA. One patient with GCA had CTA of the head and neck with radiographic findings supportive of GCA.
Discussion
The available evidence supports the use of additional screening tests to increase the temporal artery biopsy yield for GCA. Inflammatory laboratory markers demonstrate some sensitivity but are nonspecific for GCA. In this study, only 60% of patients with GCA underwent diagnostic imaging as part of the workup. There are multiple factors that may contribute to the underutilization of advanced imaging in the diagnosis of GCA, including outdated standardized diagnostic criteria, limited resources (direct access to modalities), and lack of clinician awareness of diagnostic testing options. In this retrospective review, 30 patients (71%) were diagnosed with GCA with a 1990 ACR GCA score ≤ 3. Of these 30 patients, 19 underwent confirmatory biopsy followed by prolonged courses of steroid therapy. In addition, only 25 patients underwent advanced imaging to increase diagnostic accuracy of the suspected syndrome.
A large meta-analysis demonstrated a sensitivity of 77.3% (95% CI, 71.8-81.9%) for temporal artery biopsy.21 The overall yield was 40% in the meta-analysis. Advanced noninvasive imaging represents an appropriate method of evaluating GCA.8-20 In our study, 18F-FDG PET demonstrated the highest sensitivity (36%) for the diagnosis of GCA. Ultrasonography is recommended as an initial screening tool to identify the noncompressible halo sign (a hypoechoic circumferential wall thickening due to edema) as a cost-effective and widely available technology.22 Other research has corroborated the beneficial use of ultrasonography in improving diagnostic accuracy by detecting the noncompressible halo sign in temporal arteries.22,23 GCA diagnostic performance has been significantly improved with the use of B-mode probes ≥ 15 MHz as well as proposals to incorporate a compression sign or interrogating the axillary vessels, showing a sensitivity of 54% to 77%.23,24
POCUS may reduce the risk of a false-negative biopsy and improve yield with more frequent utilization. However, ultrasonography may be limited by operator skills and visualization of the great vessels. The accuracy of ultrasonography is dependent on the experience and adeptness of the operator. Additional studies are needed to establish a systematic standard for POCUS training to ensure accurate interpretation and uniform interrogation procedure.24 Artificial intelligence (AI) may aid in interpreting results of POCUS and bridging the operator skill gap among operators.25,26 AI and machine learning techniques can assist in detecting the noncompressible halo sign and compression sign in temporal arteries and other affected vessels.
In comparing the WRNMMC patient population with other US civilian GCA cohorts, there are some differences and similarities. There was a high representation of African American patients in the study, which may reflect a greater severity of autoimmune disease expression in this population.27 We also observed a higher number of females and an association with polymyalgia rheumatica in the data, consistent with previous reports.28,29 The females in this study were primarily civilians and therefore more similar to the general population of individuals with GCA. In contrast, male patients were more likely to be active-duty service members or have prior service experience with increased exposure to novel environmental factors linked to increased risk of autoimmune disease. This includes an increased risk of Guillain-Barré syndrome and Graves disease among Vietnam veterans exposed to Agent Orange.30,31 Other studies have found that veterans with posttraumatic stress disorder are at increased risk for severe autoimmune diseases.32,33 As more women join the active-duty military, the impact of autoimmune disease in the military service population is expected to grow, requiring further research.
Conclusions
Early diagnosis and treatment of GCA are critical to preventing serious outcomes, such as visual loss, jaw or limb claudication, or ischemic stroke. The incidence of autoimmune disease is expected to rise in the armed forces and veteran populations due to exposure to novel environmental factors and the increasing representation of women in the military. The use of additional screening tools can aid in earlier diagnosis of GCA. The 2022 ACR classification criteria for GCA represent significant updates to the 1990 criteria, incorporating ancillary tests such as the temporal artery halo sign on ultrasound, bilateral axillary vessel screening on imaging, and 18F-FDG PET activity throughout the aorta. The updated criteria require further validation and supports the adoption of a multidisciplinary approach that includes ultrasonography, vascular MRI/CT, and 18F-FDG PET. Furthermore, AI may play a future key role in ultrasound interpretation and study interrogation procedure. Ultimately, ultrasonography is a noninvasive and promising technique for the early diagnosis of GCA. A target goal is to increase the yield of positive temporal artery biopsies to ≥ 70%.
- Jennette JC. Overview of the 2012 revised International Chapel Hill Consensus Conference nomenclature of vasculitides. Clin Exp Nephrol. 2013;17:603-606. doi:10.1007/s10157-013-0869-6
- Kale N, Eggenberger E. Diagnosis and management of giant cell arteritis: a review. Curr Opin Ophthalmol. 2010;21:417-422. doi:10.1097/ICU.0b013e32833eae8b
- Smetana GW, Shmerling RH. Does this patient have temporal arteritis? JAMA. 2002;287:92-101.
- Schäcke H, Döcke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96:23-43. doi:10.1016/s0163-7258(02)00297-8
- Curtis JR, Patkar N, Xie A, et al. Risk of serious bacterial infections among rheumatoid arthritis patients exposed to tumor necrosis factor alpha antagonists. Arthritis Rheum. 2007;56:1125-1133. doi:10.1002/art.22504
- Hoes JN, van der Goes MC, van Raalte DH, et al. Glucose tolerance, insulin sensitivity and ß-cell function in patients with rheumatoid arthritis treated with or without low-to-medium dose glucocorticoids. Ann Rheum Dis. 2011;70:1887-1894. doi:10.1136/ard.2011.151464
- Hunder GG, Bloch DA, Michel BA, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum. 1990;33:1122-1128. doi:10.1002/art.1780330810
- Dejaco C, Duftner C, Buttgereit F, Matteson EL, Dasgupta B. The spectrum of giant cell arteritis and polymyalgia rheumatica: revisiting the concept of the disease. Rheumatology (Oxford). 2017;56:506-515. doi:10.1093/rheumatology/kew273
- Slart RHJ, Nienhuis PH, Glaudemans AWJM, et al. Role of 18F-FDG PET/CT in large vessel vasculitis and polymyalgia rheumatica. J Nucl Med. 2023;64:515-521. doi:10.2967/jnumed.122.265016
- Shimol JB, Amital H, Lidar M, Domachevsky L, Shoenfeld Y, Davidson T. The utility of PET/CT in large vessel vasculitis. Sci Rep. 2020;10:17709. doi:10.1038/s41598-020-73818-2
- Ponte C, Grayson PC, Robson JC, et al. 2022 American College of Rheumatology/EULAR Classification Criteria for Giant Cell Arteritis. Arthritis Rheumatol. 2022;74:1881-1889. doi:10.1002/art.42325
- He J, Williamson L, Ng B, et al. The diagnostic accuracy of temporal artery ultrasound and temporal artery biopsy in giant cell arteritis: a single center Australian experience over 10 years. Int J Rheum Dis. 2022;25:447-453. doi:10.1111/1756-185X.14288
- Stellingwerff MD, Brouwer E, Lensen KDF, et al. Different scoring methods of FDG PET/CT in giant cell arteritis: need for standardization. Medicine (Baltimore). 2015;94:e1542. doi:10.1097/MD.0000000000001542
- Conway R, Smyth AE, Kavanagh RG, et al. Diagnostic utility of computed tomographic angiography in giant-cell arteritis. Stroke. 2018;49:2233-2236. doi:10.1161/STROKEAHA.118.021995
- Duftner C, Dejaco C, Sepriano A, et al. Imaging in diagnosis, outcome prediction and monitoring of large vessel vasculitis: a systematic literature review and meta-analysis informing the EULAR recommendations. RMD Open. 2018;4:e000612. doi:10.1136/rmdopen-2017-000612
- Rehak Z, Vasina J, Ptacek J, et al. PET/CT in giant cell arteritis: high 18F-FDG uptake in the temporal, occipital and vertebral arteries. Rev Esp Med Nucl Imagen Mol. 2016;35:398-401. doi:10.1016/j.remn.2016.03.007
- Salvarani C, Soriano A, Muratore F, et al. Is PET/CT essential in the diagnosis and follow-up of temporal arteritis? Autoimmun Rev. 2017;16:1125-1130. doi:10.1016/j.autrev.2017.09.007
- Brodmann M, Lipp RW, Passath A, et al. The role of 2-18F-fluoro-2-deoxy-D-glucose positron emission tomography in the diagnosis of giant cell arteritis of the temporal arteries. Rheumatology (Oxford). 2004;43:241-242. doi:10.1093/rheumatology/keh025
- Flaus A, Granjon D, Habouzit V, Gaultier JB, Prevot-Bitot N. Unusual and diffuse hypermetabolism in routine 18F-FDG PET/CT of the supra-aortic vessels in biopsy-positive giant cell arteritis. Clin Nucl Med. 2018;43:e336-e337. doi:10.1097/RLU.0000000000002198
- Berger CT, Sommer G, Aschwanden M, et al. The clinical benefit of imaging in the diagnosis and treatment of giant cell arteritis. Swiss Med Wkly. 2018;148:w14661. doi:10.4414/smw.2018.14661
- Rubenstein E, Maldini C, Gonzalez-Chiappe S, et al. Sensitivity of temporal artery biopsy in the diagnosis of giant cell arteritis: a systematic literature review and meta-analysis. Rheumatology (Oxford). 2020;59:1011-1020. doi:10.1093/rheumatology/kez385
- Tsivgoulis G, Heliopoulos I, Vadikolias K, et al. Teaching neuroimages: ultrasound findings in giant-cell arteritis. Neurology. 2010;75:e67-e68. doi:10.1212/WNL.0b013e3181f881e9
- Nakajima E, Moon FH, Canvas Jr N, et al. Accuracy of Doppler ultrasound in the diagnosis of giant cell arteritis: a systematic review and meta-analysis. Adv Rheumatol. 2023;63:5. doi:10.1186/s42358-023-00286-3
- Naumegni SR, Hoffmann C, Cornec D, et al. Temporal artery ultrasound to diagnose giant cell arteritis: a practical guide. Ultrasound Med Biol. 2021;47:201-213. doi:10.1016/j.ultrasmedbio.2020.10.004
- Kim YH. Artificial intelligence in medical ultrasonography: driving on an unpaved road. Ultrasonography. 2021;40:313-317. doi:10.14366/usg.21031
- Sultan LR, Mohamed MH, Andronikou S. ChatGPT-4: a breakthrough in ultrasound image analysis. Radiol Adv. 2024;1:umae006. doi:10.1093/radadv/umae006
- Cipriani VP, Klein S. Clinical characteristics of multiple sclerosis in African-Americans. Curr Neurol Neurosci Rep. 2019;19:87. doi:10.1007/s11910-019-1000-5
- Sturm A, Dechant C, Proft F, et al. Gender differences in giant cell arteritis: a case-control study. Clin Exp Rheumatol. 2016;34:S70-72.
- Li KJ, Semenov D, Turk M, et al. A meta-analysis of the epidemiology of giant cell arteritis across time and space. Arthritis Res Ther. 2021;23:82. doi:10.1186/s13075-021-02450-w
- Nelson L, Gormley R, Riddle MS, Tribble DR, Porter CK. The epidemiology of Guillain-Barré syndrome in U.S. military personnel: a case-control study. BMC Res Notes. 2009;2:171. doi:10.1186/1756-0500-2-171
- Spaulding SW. The possible roles of environmental factors and the aryl hydrocarbon receptor in the prevalence of thyroid diseases in Vietnam era veterans. Curr Opin Endocrinol Diabetes Obes. 2011;18:315-320.
- O’Donovan A, Cohen BE, Seal KH, et al. Elevated risk for autoimmune disorders in Iraq and Afghanistan veterans with posttraumatic stress disorder. Biol Psychiatry. 2015;77:365-374. doi:10.1016/j.biopsych.2014.06.015
- Bookwalter DB, Roenfeldt KA, LeardMann CA, Kong SY, Riddle MS, Rull RP. Posttraumatic stress disorder and risk of selected autoimmune diseases among US military personnel. BMC Psychiatry. 2020;20:23. doi:10.1186/s12888-020-2432-9
- Jennette JC. Overview of the 2012 revised International Chapel Hill Consensus Conference nomenclature of vasculitides. Clin Exp Nephrol. 2013;17:603-606. doi:10.1007/s10157-013-0869-6
- Kale N, Eggenberger E. Diagnosis and management of giant cell arteritis: a review. Curr Opin Ophthalmol. 2010;21:417-422. doi:10.1097/ICU.0b013e32833eae8b
- Smetana GW, Shmerling RH. Does this patient have temporal arteritis? JAMA. 2002;287:92-101.
- Schäcke H, Döcke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96:23-43. doi:10.1016/s0163-7258(02)00297-8
- Curtis JR, Patkar N, Xie A, et al. Risk of serious bacterial infections among rheumatoid arthritis patients exposed to tumor necrosis factor alpha antagonists. Arthritis Rheum. 2007;56:1125-1133. doi:10.1002/art.22504
- Hoes JN, van der Goes MC, van Raalte DH, et al. Glucose tolerance, insulin sensitivity and ß-cell function in patients with rheumatoid arthritis treated with or without low-to-medium dose glucocorticoids. Ann Rheum Dis. 2011;70:1887-1894. doi:10.1136/ard.2011.151464
- Hunder GG, Bloch DA, Michel BA, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum. 1990;33:1122-1128. doi:10.1002/art.1780330810
- Dejaco C, Duftner C, Buttgereit F, Matteson EL, Dasgupta B. The spectrum of giant cell arteritis and polymyalgia rheumatica: revisiting the concept of the disease. Rheumatology (Oxford). 2017;56:506-515. doi:10.1093/rheumatology/kew273
- Slart RHJ, Nienhuis PH, Glaudemans AWJM, et al. Role of 18F-FDG PET/CT in large vessel vasculitis and polymyalgia rheumatica. J Nucl Med. 2023;64:515-521. doi:10.2967/jnumed.122.265016
- Shimol JB, Amital H, Lidar M, Domachevsky L, Shoenfeld Y, Davidson T. The utility of PET/CT in large vessel vasculitis. Sci Rep. 2020;10:17709. doi:10.1038/s41598-020-73818-2
- Ponte C, Grayson PC, Robson JC, et al. 2022 American College of Rheumatology/EULAR Classification Criteria for Giant Cell Arteritis. Arthritis Rheumatol. 2022;74:1881-1889. doi:10.1002/art.42325
- He J, Williamson L, Ng B, et al. The diagnostic accuracy of temporal artery ultrasound and temporal artery biopsy in giant cell arteritis: a single center Australian experience over 10 years. Int J Rheum Dis. 2022;25:447-453. doi:10.1111/1756-185X.14288
- Stellingwerff MD, Brouwer E, Lensen KDF, et al. Different scoring methods of FDG PET/CT in giant cell arteritis: need for standardization. Medicine (Baltimore). 2015;94:e1542. doi:10.1097/MD.0000000000001542
- Conway R, Smyth AE, Kavanagh RG, et al. Diagnostic utility of computed tomographic angiography in giant-cell arteritis. Stroke. 2018;49:2233-2236. doi:10.1161/STROKEAHA.118.021995
- Duftner C, Dejaco C, Sepriano A, et al. Imaging in diagnosis, outcome prediction and monitoring of large vessel vasculitis: a systematic literature review and meta-analysis informing the EULAR recommendations. RMD Open. 2018;4:e000612. doi:10.1136/rmdopen-2017-000612
- Rehak Z, Vasina J, Ptacek J, et al. PET/CT in giant cell arteritis: high 18F-FDG uptake in the temporal, occipital and vertebral arteries. Rev Esp Med Nucl Imagen Mol. 2016;35:398-401. doi:10.1016/j.remn.2016.03.007
- Salvarani C, Soriano A, Muratore F, et al. Is PET/CT essential in the diagnosis and follow-up of temporal arteritis? Autoimmun Rev. 2017;16:1125-1130. doi:10.1016/j.autrev.2017.09.007
- Brodmann M, Lipp RW, Passath A, et al. The role of 2-18F-fluoro-2-deoxy-D-glucose positron emission tomography in the diagnosis of giant cell arteritis of the temporal arteries. Rheumatology (Oxford). 2004;43:241-242. doi:10.1093/rheumatology/keh025
- Flaus A, Granjon D, Habouzit V, Gaultier JB, Prevot-Bitot N. Unusual and diffuse hypermetabolism in routine 18F-FDG PET/CT of the supra-aortic vessels in biopsy-positive giant cell arteritis. Clin Nucl Med. 2018;43:e336-e337. doi:10.1097/RLU.0000000000002198
- Berger CT, Sommer G, Aschwanden M, et al. The clinical benefit of imaging in the diagnosis and treatment of giant cell arteritis. Swiss Med Wkly. 2018;148:w14661. doi:10.4414/smw.2018.14661
- Rubenstein E, Maldini C, Gonzalez-Chiappe S, et al. Sensitivity of temporal artery biopsy in the diagnosis of giant cell arteritis: a systematic literature review and meta-analysis. Rheumatology (Oxford). 2020;59:1011-1020. doi:10.1093/rheumatology/kez385
- Tsivgoulis G, Heliopoulos I, Vadikolias K, et al. Teaching neuroimages: ultrasound findings in giant-cell arteritis. Neurology. 2010;75:e67-e68. doi:10.1212/WNL.0b013e3181f881e9
- Nakajima E, Moon FH, Canvas Jr N, et al. Accuracy of Doppler ultrasound in the diagnosis of giant cell arteritis: a systematic review and meta-analysis. Adv Rheumatol. 2023;63:5. doi:10.1186/s42358-023-00286-3
- Naumegni SR, Hoffmann C, Cornec D, et al. Temporal artery ultrasound to diagnose giant cell arteritis: a practical guide. Ultrasound Med Biol. 2021;47:201-213. doi:10.1016/j.ultrasmedbio.2020.10.004
- Kim YH. Artificial intelligence in medical ultrasonography: driving on an unpaved road. Ultrasonography. 2021;40:313-317. doi:10.14366/usg.21031
- Sultan LR, Mohamed MH, Andronikou S. ChatGPT-4: a breakthrough in ultrasound image analysis. Radiol Adv. 2024;1:umae006. doi:10.1093/radadv/umae006
- Cipriani VP, Klein S. Clinical characteristics of multiple sclerosis in African-Americans. Curr Neurol Neurosci Rep. 2019;19:87. doi:10.1007/s11910-019-1000-5
- Sturm A, Dechant C, Proft F, et al. Gender differences in giant cell arteritis: a case-control study. Clin Exp Rheumatol. 2016;34:S70-72.
- Li KJ, Semenov D, Turk M, et al. A meta-analysis of the epidemiology of giant cell arteritis across time and space. Arthritis Res Ther. 2021;23:82. doi:10.1186/s13075-021-02450-w
- Nelson L, Gormley R, Riddle MS, Tribble DR, Porter CK. The epidemiology of Guillain-Barré syndrome in U.S. military personnel: a case-control study. BMC Res Notes. 2009;2:171. doi:10.1186/1756-0500-2-171
- Spaulding SW. The possible roles of environmental factors and the aryl hydrocarbon receptor in the prevalence of thyroid diseases in Vietnam era veterans. Curr Opin Endocrinol Diabetes Obes. 2011;18:315-320.
- O’Donovan A, Cohen BE, Seal KH, et al. Elevated risk for autoimmune disorders in Iraq and Afghanistan veterans with posttraumatic stress disorder. Biol Psychiatry. 2015;77:365-374. doi:10.1016/j.biopsych.2014.06.015
- Bookwalter DB, Roenfeldt KA, LeardMann CA, Kong SY, Riddle MS, Rull RP. Posttraumatic stress disorder and risk of selected autoimmune diseases among US military personnel. BMC Psychiatry. 2020;20:23. doi:10.1186/s12888-020-2432-9
Advanced Imaging Techniques Use in Giant Cell Arteritis Diagnosis: The Experience at Walter Reed National Military Medical Center
Advanced Imaging Techniques Use in Giant Cell Arteritis Diagnosis: The Experience at Walter Reed National Military Medical Center
Demographic and Clinical Factors Associated With PD-L1 Testing of Veterans With Advanced Non-Small Cell Lung Cancer
Background
Programmed death-ligand 1 (PD-L1) checkpoint inhibitors revolutionized the treatment of advanced non-small cell lung cancer (aNSCLC) by improving overall survival compared to chemotherapy. PD-L1 biomarker testing is paramount for informing treatment decisions in aNSCLC. Real-world data describing patterns of PD-L1 testing within the Veteran Health Administration (VHA) are limited. This retrospective study seeks to evaluate demographic and clinical factors associated with PD-L1 testing in VHA.
Methods
Veterans diagnosed with aNSCLC from 2019-2022 were identified using VHA’s Corporate Data Warehouse. Wilcoxon Rank Sum and Chi- Square tests measured association between receipt of PD-L1 testing and patient demographic and clinical characteristics at aNSCLC diagnosis. Logistic regression assessed predictors of PD-L1 testing, and subgroup analyses were performed for significant interactions.
Results
Our study included 4575 patients with aNSCLC; 57.0% received PD-L1 testing. The likelihood of PD-L1 testing increased among patients diagnosed with aNSCLC after 2019 vs during 2019 (OR≥1.118, p≤0.035) and in Black vs White patients (OR=1.227, p=0.011). However, the following had decreased likelihood of PD-L1 testing: patients with stage IIIB vs IV cancer (OR=0.683, p=0.004); non vs current/former smokers (OR=0.733, p=0.039); squamous (OR=0.863, p=0.030) or NOS (OR=0.695,p=0.013) vs. adenocarcinoma histology. Interactions were observed between patient residential region and residential rurality (p=0.003), and region and receipt of oncology community care consults (OCCC) (p=0.030). Patients in rural Midwest (OR=0.445,p=0.004) and rural South (OR=0.566, p=0.032) were less likely to receive PD-L1 testing than Metropolitan patients. Across patients with OCCC, Western US patients were more likely to receive PD-L1 testing (OR=1.554, p=0.001) than patients in other regions. However, within Midwestern patients, those without a OCCC were more likely to receive PD-L1 testing (OR=1.724, p< 0.001) than those with a OCCC. High comorbidity index (CCI≥3) is associated with an increased likelihood of PD-L1 testing in a univariable model (OR=1.286 vs. CCI=0,p=0.009), but not in the multivariable model (p=0.278).
Conclusions
We identified demographic and clinical factors, including regional differences in rurality and OCCC patterns, associated with PD-L1 testing. These factors can focus ongoing efforts to improve PD-L1 testing and efforts to be more in line with recommended care.
Background
Programmed death-ligand 1 (PD-L1) checkpoint inhibitors revolutionized the treatment of advanced non-small cell lung cancer (aNSCLC) by improving overall survival compared to chemotherapy. PD-L1 biomarker testing is paramount for informing treatment decisions in aNSCLC. Real-world data describing patterns of PD-L1 testing within the Veteran Health Administration (VHA) are limited. This retrospective study seeks to evaluate demographic and clinical factors associated with PD-L1 testing in VHA.
Methods
Veterans diagnosed with aNSCLC from 2019-2022 were identified using VHA’s Corporate Data Warehouse. Wilcoxon Rank Sum and Chi- Square tests measured association between receipt of PD-L1 testing and patient demographic and clinical characteristics at aNSCLC diagnosis. Logistic regression assessed predictors of PD-L1 testing, and subgroup analyses were performed for significant interactions.
Results
Our study included 4575 patients with aNSCLC; 57.0% received PD-L1 testing. The likelihood of PD-L1 testing increased among patients diagnosed with aNSCLC after 2019 vs during 2019 (OR≥1.118, p≤0.035) and in Black vs White patients (OR=1.227, p=0.011). However, the following had decreased likelihood of PD-L1 testing: patients with stage IIIB vs IV cancer (OR=0.683, p=0.004); non vs current/former smokers (OR=0.733, p=0.039); squamous (OR=0.863, p=0.030) or NOS (OR=0.695,p=0.013) vs. adenocarcinoma histology. Interactions were observed between patient residential region and residential rurality (p=0.003), and region and receipt of oncology community care consults (OCCC) (p=0.030). Patients in rural Midwest (OR=0.445,p=0.004) and rural South (OR=0.566, p=0.032) were less likely to receive PD-L1 testing than Metropolitan patients. Across patients with OCCC, Western US patients were more likely to receive PD-L1 testing (OR=1.554, p=0.001) than patients in other regions. However, within Midwestern patients, those without a OCCC were more likely to receive PD-L1 testing (OR=1.724, p< 0.001) than those with a OCCC. High comorbidity index (CCI≥3) is associated with an increased likelihood of PD-L1 testing in a univariable model (OR=1.286 vs. CCI=0,p=0.009), but not in the multivariable model (p=0.278).
Conclusions
We identified demographic and clinical factors, including regional differences in rurality and OCCC patterns, associated with PD-L1 testing. These factors can focus ongoing efforts to improve PD-L1 testing and efforts to be more in line with recommended care.
Background
Programmed death-ligand 1 (PD-L1) checkpoint inhibitors revolutionized the treatment of advanced non-small cell lung cancer (aNSCLC) by improving overall survival compared to chemotherapy. PD-L1 biomarker testing is paramount for informing treatment decisions in aNSCLC. Real-world data describing patterns of PD-L1 testing within the Veteran Health Administration (VHA) are limited. This retrospective study seeks to evaluate demographic and clinical factors associated with PD-L1 testing in VHA.
Methods
Veterans diagnosed with aNSCLC from 2019-2022 were identified using VHA’s Corporate Data Warehouse. Wilcoxon Rank Sum and Chi- Square tests measured association between receipt of PD-L1 testing and patient demographic and clinical characteristics at aNSCLC diagnosis. Logistic regression assessed predictors of PD-L1 testing, and subgroup analyses were performed for significant interactions.
Results
Our study included 4575 patients with aNSCLC; 57.0% received PD-L1 testing. The likelihood of PD-L1 testing increased among patients diagnosed with aNSCLC after 2019 vs during 2019 (OR≥1.118, p≤0.035) and in Black vs White patients (OR=1.227, p=0.011). However, the following had decreased likelihood of PD-L1 testing: patients with stage IIIB vs IV cancer (OR=0.683, p=0.004); non vs current/former smokers (OR=0.733, p=0.039); squamous (OR=0.863, p=0.030) or NOS (OR=0.695,p=0.013) vs. adenocarcinoma histology. Interactions were observed between patient residential region and residential rurality (p=0.003), and region and receipt of oncology community care consults (OCCC) (p=0.030). Patients in rural Midwest (OR=0.445,p=0.004) and rural South (OR=0.566, p=0.032) were less likely to receive PD-L1 testing than Metropolitan patients. Across patients with OCCC, Western US patients were more likely to receive PD-L1 testing (OR=1.554, p=0.001) than patients in other regions. However, within Midwestern patients, those without a OCCC were more likely to receive PD-L1 testing (OR=1.724, p< 0.001) than those with a OCCC. High comorbidity index (CCI≥3) is associated with an increased likelihood of PD-L1 testing in a univariable model (OR=1.286 vs. CCI=0,p=0.009), but not in the multivariable model (p=0.278).
Conclusions
We identified demographic and clinical factors, including regional differences in rurality and OCCC patterns, associated with PD-L1 testing. These factors can focus ongoing efforts to improve PD-L1 testing and efforts to be more in line with recommended care.