User login
Oncology Mergers Are on the Rise. How Can Independent Practices Survive?
When he completed his fellowship at Fox Chase Cancer Center in Philadelphia, Moshe Chasky, MD, joined a small five-person practice that rented space from the city’s Jefferson Hospital in Philadelphia. The arrangement seemed to work well for the hospital and the small practice, which remained independent.
Within 10 years, the hospital sought to buy the practice, Alliance Cancer Specialists.
But the oncologists at Alliance did not want to join Jefferson.
The hospital eventually entered into an exclusive agreement with its own medical group to provide inpatient oncology/hematology services at three Jefferson Health–Northeast hospitals and stripped Dr. Chasky and his colleagues of their privileges at those facilities, Medscape Medical News reported last year.
said Jeff Patton, MD, CEO of OneOncology, a management services organization.
A 2020 report from the Community Oncology Alliance (COA), for instance, tracked mergers, acquisitions, and closures in the community oncology setting and found the number of practices acquired by hospitals, known as vertical integration, nearly tripled from 2010 to 2020.
“Some hospitals are pretty predatory in their approach,” Dr. Patton said. If hospitals have their own oncology program, “they’ll employ the referring doctors and then discourage them or prevent them from referring patients to our independent practices that are not owned by the hospital.”
Still, in the face of growing pressure to join hospitals, some community oncology practices are finding ways to survive and maintain their independence.
A Growing Trend
The latest data continue to show a clear trend: Consolidation in oncology is on the rise.
A 2024 study revealed that the pace of consolidation seems to be increasing.
The analysis found that, between 2015 and 2022, the number of medical oncologists increased by 14% and the number of medical oncologists per practice increased by 40%, while the number of practices decreased by 18%.
While about 44% of practices remain independent, the percentage of medical oncologists working in practices with more than 25 clinicians has increased from 34% in 2015 to 44% in 2022. By 2022, the largest 102 practices in the United States employed more than 40% of all medical oncologists.
“The rate of consolidation seems to be rapid,” study coauthor Parsa Erfani, MD, an internal medicine resident at Brigham & Women’s Hospital, Boston, explained.
Consolidation appears to breed more consolidation. The researchers found, for instance, that markets with greater hospital consolidation and more hospital beds per capita were more likely to undergo consolidation in oncology.
Consolidation may be higher in these markets “because hospitals or health systems are buying up oncology practices or conversely because oncology practices are merging to compete more effectively with larger hospitals in the area,” Dr. Erfani told this news organization.
Mergers among independent practices, known as horizontal integration, have also been on the rise, according to the 2020 COA report. These mergers can help counter pressures from hospitals seeking to acquire community practices as well as prevent practices and their clinics from closing.
Although Dr. Erfani’s research wasn’t designed to determine the factors behind consolidation, he and his colleagues point to the Affordable Care Act (ACA) and the federal 340B Drug Pricing Program as potential drivers of this trend.
The ACA encouraged consolidation as a way to improve efficiency and created the need for ever-larger information systems to collect and report quality data. But these data collection and reporting requirements have become increasingly difficult for smaller practices to take on.
The 340B Program, however, may be a bigger contributing factor to consolidation. Created in 1992, the 340B Program allows qualifying hospitals and clinics that treat low-income and uninsured patients to buy outpatient prescription drugs at a 25%-50% discount.
Hospitals seeking to capitalize on the margins possible under the 340B Program will “buy all the referring physicians in a market so that the medical oncology group is left with little choice but to sell to the hospital,” said Dr. Patton.
“Those 340B dollars are worth a lot to hospitals,” said David A. Eagle, MD, a hematologist/oncologist with New York Cancer & Blood Specialists and past president of COA. The program “creates an appetite for nonprofit hospitals to want to grow their medical oncology programs,” he told this news organization.
Declining Medicare reimbursement has also hit independent practices hard.
Over the past 15 years, compared with inflation, physicians have gotten “a pay rate decrease from Medicare,” said Dr. Patton. Payers have followed that lead and tried to cut pay for clinicians, especially those who do not have market share, he said. Paying them less is “disingenuous knowing that our costs of providing care are going up,” he said.
Less Access, Higher Costs, Worse Care?
Many studies have demonstrated that, when hospitals become behemoths in a given market, healthcare costs go up.
“There are robust data showing that consolidation increases healthcare costs by reducing competition, including in oncology,” wrote Dr. Erfani and colleagues.
Oncology practices that are owned by hospitals bill facility fees for outpatient chemotherapy treatment, adding another layer of cost, the researchers explained, citing a 2019 Health Economics study.
Another analysis, published in 2020, found that hospital prices for the top 37 infused cancer drugs averaged 86% more per unit than the price charged by physician offices. Hospital outpatient departments charged even more, on average, for drugs — 128% more for nivolumab and 428% more for fluorouracil, for instance.
In their 2024 analysis, Dr. Erfani and colleagues also found that increased hospital market concentration was associated with worse quality of care, across all assessed patient satisfaction measures, and may result in worse access to care as well.
Overall, these consolidation “trends have important implications for cancer care cost, quality, and access,” the authors concluded.
Navigating the Consolidation Trend
In the face of mounting pressure to join hospitals, community oncology practices have typically relied on horizontal mergers to maintain their independence. An increasing number of practices, however, are now turning to another strategy: Management services organizations.
According to some oncologists, a core benefit of joining a management services organization is their community practices can maintain autonomy, hold on to referrals, and benefit from access to a wider network of peers and recently approved treatments such as chimeric antigen receptor T-cell therapies.
In these arrangements, the management company also provides business assistance to practices, including help with billing and collection, payer negotiations, supply chain issues, and credentialing, as well as recruiting, hiring, and marketing.
These management organizations, which include American Oncology Network, Integrated Oncology Network, OneOncology, and Verdi Oncology, are, however, backed by private equity. According to a 2022 report, private equity–backed management organizations have ramped up arrangements with community oncology practices over the past few years — a trend that has concerned some experts.
The authors of a recent analysis in JAMA Internal Medicine explained that, although private equity involvement in physician practices may enable operational efficiencies, “critics point to potential conflicts of interest” and highlight concerns that patients “may face additional barriers to both accessibility and affordability of care.”
The difference, according to some oncologists, is their practices are not owned by the management services organization; instead, the practices enter contracts that outline the boundaries of the relationship and stipulate fees to the management organizations.
In 2020, Dr. Chasky’s practice, Alliance Cancer Specialists, joined The US Oncology Network, a management services organization wholly owned by McKesson. The organization provides the practice with capital and other resources, as well as access to the Sarah Cannon Research Institute, so patients can participate in clinical trials.
“We totally function as an independent practice,” said Dr. Chasky. “We make our own management decisions,” he said. For instance, if Alliance wants to hire a new clinician, US Oncology helps with the recruitment. “But at the end of the day, it’s our practice,” he said.
Davey Daniel, MD — whose community practice joined the management services organization OneOncology — has seen the benefits of being part of a larger network. For instance, bispecific therapies for leukemias, lymphomas, and multiple myeloma are typically administered at academic centers because of the risk for cytokine release syndrome.
However, physician leaders in the OneOncology network “came up with a playbook on how to do it safely” in the community setting, said Dr. Daniel. “It meant that we were adopting FDA newly approved therapies in a very short course.”
Being able to draw from a wider pool of expertise has had other advantages. Dr. Daniel can lean on pathologists and research scientists in the network for advice on targeted therapy use. “We’re actually bringing precision medicine expertise to the community,” Dr. Daniel said.
Dr. Chasky and Dr. Eagle, whose practice is also part of OneOncology, said that continuing to work in the community setting has allowed them greater flexibility.
Dr. Eagle explained that New York Cancer & Blood Specialists tries to offer patients an appointment within 2 days of a referral, and it allows walk-in visits.
Dr. Chasky leans into the flexibility by having staff stay late, when needed, to ensure that all patients are seen. “We’re there for our patients at all hours,” Dr. Chasky said, adding that often “you don’t have that flexibility when you work for a big hospital system.”
The bottom line is community oncology can still thrive, said Nick Ferreyros, managing director of COA, “as long as we have a healthy competitive ecosystem where [we] are valued and seen as an important part of our cancer care system.”
A version of this article first appeared on Medscape.com.
When he completed his fellowship at Fox Chase Cancer Center in Philadelphia, Moshe Chasky, MD, joined a small five-person practice that rented space from the city’s Jefferson Hospital in Philadelphia. The arrangement seemed to work well for the hospital and the small practice, which remained independent.
Within 10 years, the hospital sought to buy the practice, Alliance Cancer Specialists.
But the oncologists at Alliance did not want to join Jefferson.
The hospital eventually entered into an exclusive agreement with its own medical group to provide inpatient oncology/hematology services at three Jefferson Health–Northeast hospitals and stripped Dr. Chasky and his colleagues of their privileges at those facilities, Medscape Medical News reported last year.
said Jeff Patton, MD, CEO of OneOncology, a management services organization.
A 2020 report from the Community Oncology Alliance (COA), for instance, tracked mergers, acquisitions, and closures in the community oncology setting and found the number of practices acquired by hospitals, known as vertical integration, nearly tripled from 2010 to 2020.
“Some hospitals are pretty predatory in their approach,” Dr. Patton said. If hospitals have their own oncology program, “they’ll employ the referring doctors and then discourage them or prevent them from referring patients to our independent practices that are not owned by the hospital.”
Still, in the face of growing pressure to join hospitals, some community oncology practices are finding ways to survive and maintain their independence.
A Growing Trend
The latest data continue to show a clear trend: Consolidation in oncology is on the rise.
A 2024 study revealed that the pace of consolidation seems to be increasing.
The analysis found that, between 2015 and 2022, the number of medical oncologists increased by 14% and the number of medical oncologists per practice increased by 40%, while the number of practices decreased by 18%.
While about 44% of practices remain independent, the percentage of medical oncologists working in practices with more than 25 clinicians has increased from 34% in 2015 to 44% in 2022. By 2022, the largest 102 practices in the United States employed more than 40% of all medical oncologists.
“The rate of consolidation seems to be rapid,” study coauthor Parsa Erfani, MD, an internal medicine resident at Brigham & Women’s Hospital, Boston, explained.
Consolidation appears to breed more consolidation. The researchers found, for instance, that markets with greater hospital consolidation and more hospital beds per capita were more likely to undergo consolidation in oncology.
Consolidation may be higher in these markets “because hospitals or health systems are buying up oncology practices or conversely because oncology practices are merging to compete more effectively with larger hospitals in the area,” Dr. Erfani told this news organization.
Mergers among independent practices, known as horizontal integration, have also been on the rise, according to the 2020 COA report. These mergers can help counter pressures from hospitals seeking to acquire community practices as well as prevent practices and their clinics from closing.
Although Dr. Erfani’s research wasn’t designed to determine the factors behind consolidation, he and his colleagues point to the Affordable Care Act (ACA) and the federal 340B Drug Pricing Program as potential drivers of this trend.
The ACA encouraged consolidation as a way to improve efficiency and created the need for ever-larger information systems to collect and report quality data. But these data collection and reporting requirements have become increasingly difficult for smaller practices to take on.
The 340B Program, however, may be a bigger contributing factor to consolidation. Created in 1992, the 340B Program allows qualifying hospitals and clinics that treat low-income and uninsured patients to buy outpatient prescription drugs at a 25%-50% discount.
Hospitals seeking to capitalize on the margins possible under the 340B Program will “buy all the referring physicians in a market so that the medical oncology group is left with little choice but to sell to the hospital,” said Dr. Patton.
“Those 340B dollars are worth a lot to hospitals,” said David A. Eagle, MD, a hematologist/oncologist with New York Cancer & Blood Specialists and past president of COA. The program “creates an appetite for nonprofit hospitals to want to grow their medical oncology programs,” he told this news organization.
Declining Medicare reimbursement has also hit independent practices hard.
Over the past 15 years, compared with inflation, physicians have gotten “a pay rate decrease from Medicare,” said Dr. Patton. Payers have followed that lead and tried to cut pay for clinicians, especially those who do not have market share, he said. Paying them less is “disingenuous knowing that our costs of providing care are going up,” he said.
Less Access, Higher Costs, Worse Care?
Many studies have demonstrated that, when hospitals become behemoths in a given market, healthcare costs go up.
“There are robust data showing that consolidation increases healthcare costs by reducing competition, including in oncology,” wrote Dr. Erfani and colleagues.
Oncology practices that are owned by hospitals bill facility fees for outpatient chemotherapy treatment, adding another layer of cost, the researchers explained, citing a 2019 Health Economics study.
Another analysis, published in 2020, found that hospital prices for the top 37 infused cancer drugs averaged 86% more per unit than the price charged by physician offices. Hospital outpatient departments charged even more, on average, for drugs — 128% more for nivolumab and 428% more for fluorouracil, for instance.
In their 2024 analysis, Dr. Erfani and colleagues also found that increased hospital market concentration was associated with worse quality of care, across all assessed patient satisfaction measures, and may result in worse access to care as well.
Overall, these consolidation “trends have important implications for cancer care cost, quality, and access,” the authors concluded.
Navigating the Consolidation Trend
In the face of mounting pressure to join hospitals, community oncology practices have typically relied on horizontal mergers to maintain their independence. An increasing number of practices, however, are now turning to another strategy: Management services organizations.
According to some oncologists, a core benefit of joining a management services organization is their community practices can maintain autonomy, hold on to referrals, and benefit from access to a wider network of peers and recently approved treatments such as chimeric antigen receptor T-cell therapies.
In these arrangements, the management company also provides business assistance to practices, including help with billing and collection, payer negotiations, supply chain issues, and credentialing, as well as recruiting, hiring, and marketing.
These management organizations, which include American Oncology Network, Integrated Oncology Network, OneOncology, and Verdi Oncology, are, however, backed by private equity. According to a 2022 report, private equity–backed management organizations have ramped up arrangements with community oncology practices over the past few years — a trend that has concerned some experts.
The authors of a recent analysis in JAMA Internal Medicine explained that, although private equity involvement in physician practices may enable operational efficiencies, “critics point to potential conflicts of interest” and highlight concerns that patients “may face additional barriers to both accessibility and affordability of care.”
The difference, according to some oncologists, is their practices are not owned by the management services organization; instead, the practices enter contracts that outline the boundaries of the relationship and stipulate fees to the management organizations.
In 2020, Dr. Chasky’s practice, Alliance Cancer Specialists, joined The US Oncology Network, a management services organization wholly owned by McKesson. The organization provides the practice with capital and other resources, as well as access to the Sarah Cannon Research Institute, so patients can participate in clinical trials.
“We totally function as an independent practice,” said Dr. Chasky. “We make our own management decisions,” he said. For instance, if Alliance wants to hire a new clinician, US Oncology helps with the recruitment. “But at the end of the day, it’s our practice,” he said.
Davey Daniel, MD — whose community practice joined the management services organization OneOncology — has seen the benefits of being part of a larger network. For instance, bispecific therapies for leukemias, lymphomas, and multiple myeloma are typically administered at academic centers because of the risk for cytokine release syndrome.
However, physician leaders in the OneOncology network “came up with a playbook on how to do it safely” in the community setting, said Dr. Daniel. “It meant that we were adopting FDA newly approved therapies in a very short course.”
Being able to draw from a wider pool of expertise has had other advantages. Dr. Daniel can lean on pathologists and research scientists in the network for advice on targeted therapy use. “We’re actually bringing precision medicine expertise to the community,” Dr. Daniel said.
Dr. Chasky and Dr. Eagle, whose practice is also part of OneOncology, said that continuing to work in the community setting has allowed them greater flexibility.
Dr. Eagle explained that New York Cancer & Blood Specialists tries to offer patients an appointment within 2 days of a referral, and it allows walk-in visits.
Dr. Chasky leans into the flexibility by having staff stay late, when needed, to ensure that all patients are seen. “We’re there for our patients at all hours,” Dr. Chasky said, adding that often “you don’t have that flexibility when you work for a big hospital system.”
The bottom line is community oncology can still thrive, said Nick Ferreyros, managing director of COA, “as long as we have a healthy competitive ecosystem where [we] are valued and seen as an important part of our cancer care system.”
A version of this article first appeared on Medscape.com.
When he completed his fellowship at Fox Chase Cancer Center in Philadelphia, Moshe Chasky, MD, joined a small five-person practice that rented space from the city’s Jefferson Hospital in Philadelphia. The arrangement seemed to work well for the hospital and the small practice, which remained independent.
Within 10 years, the hospital sought to buy the practice, Alliance Cancer Specialists.
But the oncologists at Alliance did not want to join Jefferson.
The hospital eventually entered into an exclusive agreement with its own medical group to provide inpatient oncology/hematology services at three Jefferson Health–Northeast hospitals and stripped Dr. Chasky and his colleagues of their privileges at those facilities, Medscape Medical News reported last year.
said Jeff Patton, MD, CEO of OneOncology, a management services organization.
A 2020 report from the Community Oncology Alliance (COA), for instance, tracked mergers, acquisitions, and closures in the community oncology setting and found the number of practices acquired by hospitals, known as vertical integration, nearly tripled from 2010 to 2020.
“Some hospitals are pretty predatory in their approach,” Dr. Patton said. If hospitals have their own oncology program, “they’ll employ the referring doctors and then discourage them or prevent them from referring patients to our independent practices that are not owned by the hospital.”
Still, in the face of growing pressure to join hospitals, some community oncology practices are finding ways to survive and maintain their independence.
A Growing Trend
The latest data continue to show a clear trend: Consolidation in oncology is on the rise.
A 2024 study revealed that the pace of consolidation seems to be increasing.
The analysis found that, between 2015 and 2022, the number of medical oncologists increased by 14% and the number of medical oncologists per practice increased by 40%, while the number of practices decreased by 18%.
While about 44% of practices remain independent, the percentage of medical oncologists working in practices with more than 25 clinicians has increased from 34% in 2015 to 44% in 2022. By 2022, the largest 102 practices in the United States employed more than 40% of all medical oncologists.
“The rate of consolidation seems to be rapid,” study coauthor Parsa Erfani, MD, an internal medicine resident at Brigham & Women’s Hospital, Boston, explained.
Consolidation appears to breed more consolidation. The researchers found, for instance, that markets with greater hospital consolidation and more hospital beds per capita were more likely to undergo consolidation in oncology.
Consolidation may be higher in these markets “because hospitals or health systems are buying up oncology practices or conversely because oncology practices are merging to compete more effectively with larger hospitals in the area,” Dr. Erfani told this news organization.
Mergers among independent practices, known as horizontal integration, have also been on the rise, according to the 2020 COA report. These mergers can help counter pressures from hospitals seeking to acquire community practices as well as prevent practices and their clinics from closing.
Although Dr. Erfani’s research wasn’t designed to determine the factors behind consolidation, he and his colleagues point to the Affordable Care Act (ACA) and the federal 340B Drug Pricing Program as potential drivers of this trend.
The ACA encouraged consolidation as a way to improve efficiency and created the need for ever-larger information systems to collect and report quality data. But these data collection and reporting requirements have become increasingly difficult for smaller practices to take on.
The 340B Program, however, may be a bigger contributing factor to consolidation. Created in 1992, the 340B Program allows qualifying hospitals and clinics that treat low-income and uninsured patients to buy outpatient prescription drugs at a 25%-50% discount.
Hospitals seeking to capitalize on the margins possible under the 340B Program will “buy all the referring physicians in a market so that the medical oncology group is left with little choice but to sell to the hospital,” said Dr. Patton.
“Those 340B dollars are worth a lot to hospitals,” said David A. Eagle, MD, a hematologist/oncologist with New York Cancer & Blood Specialists and past president of COA. The program “creates an appetite for nonprofit hospitals to want to grow their medical oncology programs,” he told this news organization.
Declining Medicare reimbursement has also hit independent practices hard.
Over the past 15 years, compared with inflation, physicians have gotten “a pay rate decrease from Medicare,” said Dr. Patton. Payers have followed that lead and tried to cut pay for clinicians, especially those who do not have market share, he said. Paying them less is “disingenuous knowing that our costs of providing care are going up,” he said.
Less Access, Higher Costs, Worse Care?
Many studies have demonstrated that, when hospitals become behemoths in a given market, healthcare costs go up.
“There are robust data showing that consolidation increases healthcare costs by reducing competition, including in oncology,” wrote Dr. Erfani and colleagues.
Oncology practices that are owned by hospitals bill facility fees for outpatient chemotherapy treatment, adding another layer of cost, the researchers explained, citing a 2019 Health Economics study.
Another analysis, published in 2020, found that hospital prices for the top 37 infused cancer drugs averaged 86% more per unit than the price charged by physician offices. Hospital outpatient departments charged even more, on average, for drugs — 128% more for nivolumab and 428% more for fluorouracil, for instance.
In their 2024 analysis, Dr. Erfani and colleagues also found that increased hospital market concentration was associated with worse quality of care, across all assessed patient satisfaction measures, and may result in worse access to care as well.
Overall, these consolidation “trends have important implications for cancer care cost, quality, and access,” the authors concluded.
Navigating the Consolidation Trend
In the face of mounting pressure to join hospitals, community oncology practices have typically relied on horizontal mergers to maintain their independence. An increasing number of practices, however, are now turning to another strategy: Management services organizations.
According to some oncologists, a core benefit of joining a management services organization is their community practices can maintain autonomy, hold on to referrals, and benefit from access to a wider network of peers and recently approved treatments such as chimeric antigen receptor T-cell therapies.
In these arrangements, the management company also provides business assistance to practices, including help with billing and collection, payer negotiations, supply chain issues, and credentialing, as well as recruiting, hiring, and marketing.
These management organizations, which include American Oncology Network, Integrated Oncology Network, OneOncology, and Verdi Oncology, are, however, backed by private equity. According to a 2022 report, private equity–backed management organizations have ramped up arrangements with community oncology practices over the past few years — a trend that has concerned some experts.
The authors of a recent analysis in JAMA Internal Medicine explained that, although private equity involvement in physician practices may enable operational efficiencies, “critics point to potential conflicts of interest” and highlight concerns that patients “may face additional barriers to both accessibility and affordability of care.”
The difference, according to some oncologists, is their practices are not owned by the management services organization; instead, the practices enter contracts that outline the boundaries of the relationship and stipulate fees to the management organizations.
In 2020, Dr. Chasky’s practice, Alliance Cancer Specialists, joined The US Oncology Network, a management services organization wholly owned by McKesson. The organization provides the practice with capital and other resources, as well as access to the Sarah Cannon Research Institute, so patients can participate in clinical trials.
“We totally function as an independent practice,” said Dr. Chasky. “We make our own management decisions,” he said. For instance, if Alliance wants to hire a new clinician, US Oncology helps with the recruitment. “But at the end of the day, it’s our practice,” he said.
Davey Daniel, MD — whose community practice joined the management services organization OneOncology — has seen the benefits of being part of a larger network. For instance, bispecific therapies for leukemias, lymphomas, and multiple myeloma are typically administered at academic centers because of the risk for cytokine release syndrome.
However, physician leaders in the OneOncology network “came up with a playbook on how to do it safely” in the community setting, said Dr. Daniel. “It meant that we were adopting FDA newly approved therapies in a very short course.”
Being able to draw from a wider pool of expertise has had other advantages. Dr. Daniel can lean on pathologists and research scientists in the network for advice on targeted therapy use. “We’re actually bringing precision medicine expertise to the community,” Dr. Daniel said.
Dr. Chasky and Dr. Eagle, whose practice is also part of OneOncology, said that continuing to work in the community setting has allowed them greater flexibility.
Dr. Eagle explained that New York Cancer & Blood Specialists tries to offer patients an appointment within 2 days of a referral, and it allows walk-in visits.
Dr. Chasky leans into the flexibility by having staff stay late, when needed, to ensure that all patients are seen. “We’re there for our patients at all hours,” Dr. Chasky said, adding that often “you don’t have that flexibility when you work for a big hospital system.”
The bottom line is community oncology can still thrive, said Nick Ferreyros, managing director of COA, “as long as we have a healthy competitive ecosystem where [we] are valued and seen as an important part of our cancer care system.”
A version of this article first appeared on Medscape.com.
Platinum Add-On Improves Survival in Early TNBC
presented at the annual meeting of the American Society of Clinical Oncology (ASCO).
The outcomes of the South Korean study, dubbed PEARLY, provide strong evidence for incorporating carboplatin into both the neoadjuvant and adjuvant settings in patients with early-stage TNBC, said lead investigator and presenter Joohyuk Sohn, MD, PhD, a medical oncologist at Yonsei University, Seoul, South Korea.
In early-stage TNBC, carboplatin is already being incorporated into the neoadjuvant setting on the basis of trial results from KEYNOTE-522 that demonstrated improved pathologic complete response rates and event-free survival with the platinum alongside pembrolizumab.
However, the overall survival benefit of carboplatin in this setting remains unclear, as does the benefit of platinum add-on in the adjuvant setting, Dr. Sohn explained.
Dr. Sohn and colleagues randomized 868 patients evenly to either standard treatment — doxorubicin, anthracycline, and cyclophosphamide followed by a taxane — or an experimental arm that added carboplatin to the taxane phase of treatment.
About 30% of women were treated in the adjuvant setting, the rest in the neoadjuvant setting. The two arms of the study were generally well balanced — about 80% of patients had stage II disease, half were node negative, and 11% had deleterious germline mutations.
The primary endpoint, event-free survival, was broadly defined. Events included disease progression, local or distant recurrence, occurrence of a second primary cancer, inoperable status after neoadjuvant therapy, or death from any cause.
Adding carboplatin increased 5-year event-free survival rates from 75.1% to 82.3% (hazard ratio [HR], 0.67; P = .012) with the benefit holding across various subgroup analyses and particularly strong for adjuvant carboplatin (HR, 0.26).
Five-year overall survival was also better in the carboplatin arm — 90.7% vs 87% in the control arm (HR, 0.65; 95% CI, 0.42-1.02) — but that benefit did not reach statistical significance (P = .057)
Invasive disease-free survival (HR, 0.73) and distant recurrence-free survival (HR, 0.77) favored carboplatin, but the results also weren’t statistically significant.
Overall, 46% of patients had a pathologic complete response with carboplatin vs nearly 40% in the control arm. The pathologic complete response benefit from carboplatin add-on was consistent with past reports.
As expected, adding carboplatin to treatment increased hematologic toxicity and other adverse events, with three-quarters of patients experiencing grade 3 or worse adverse events vs 56.7% of control participants. There was one death in the carboplatin arm from pneumonia and two in the control arm — one from septic shock and the other from suicide.
Dr. Sohn and colleagues, however, did not observe a quality of life difference between the two groups.
“The PEARLY trial provides compelling evidence for including carboplatin in the treatment of early-stage TNBC,” Dr. Sohn concluded, adding that the results underscore the benefit in the neoadjuvant setting and suggest “potential applicability in the adjuvant setting post surgery.”
Study discussant Javier Cortes, MD, PhD, believes that the PEARLY provides a strong signal for adding carboplatin in the adjuvant setting.
“That’s something I would do in my clinical practice,” said Dr. Cortes, head of the International Breast Cancer Center in Barcelona, Spain. “After ASCO this year, I would offer taxanes plus carboplatin following anthracyclines.”
An audience member, William Sikov, MD, a breast cancer specialist at Brown University in Providence, Rhode Island, said he hopes “we’ve reached the end of a road that started many years ago in terms of incorporating carboplatin as part of neoadjuvant and adjuvant therapy for triple-negative breast cancer, where we finally [reach] consensus that this is necessary in our triple-negative patients.”
The work was funded by the government of South Korea and others. Dr. Sohn reported stock in Daiichi Sankyo and research funding from Daiichi and other companies. Dr. Cortes disclosed numerous industry ties, including honoraria, research funding, and/or travel expenses from AstraZeneca, Daiichi, and others.
A version of this article first appeared on Medscape.com.
presented at the annual meeting of the American Society of Clinical Oncology (ASCO).
The outcomes of the South Korean study, dubbed PEARLY, provide strong evidence for incorporating carboplatin into both the neoadjuvant and adjuvant settings in patients with early-stage TNBC, said lead investigator and presenter Joohyuk Sohn, MD, PhD, a medical oncologist at Yonsei University, Seoul, South Korea.
In early-stage TNBC, carboplatin is already being incorporated into the neoadjuvant setting on the basis of trial results from KEYNOTE-522 that demonstrated improved pathologic complete response rates and event-free survival with the platinum alongside pembrolizumab.
However, the overall survival benefit of carboplatin in this setting remains unclear, as does the benefit of platinum add-on in the adjuvant setting, Dr. Sohn explained.
Dr. Sohn and colleagues randomized 868 patients evenly to either standard treatment — doxorubicin, anthracycline, and cyclophosphamide followed by a taxane — or an experimental arm that added carboplatin to the taxane phase of treatment.
About 30% of women were treated in the adjuvant setting, the rest in the neoadjuvant setting. The two arms of the study were generally well balanced — about 80% of patients had stage II disease, half were node negative, and 11% had deleterious germline mutations.
The primary endpoint, event-free survival, was broadly defined. Events included disease progression, local or distant recurrence, occurrence of a second primary cancer, inoperable status after neoadjuvant therapy, or death from any cause.
Adding carboplatin increased 5-year event-free survival rates from 75.1% to 82.3% (hazard ratio [HR], 0.67; P = .012) with the benefit holding across various subgroup analyses and particularly strong for adjuvant carboplatin (HR, 0.26).
Five-year overall survival was also better in the carboplatin arm — 90.7% vs 87% in the control arm (HR, 0.65; 95% CI, 0.42-1.02) — but that benefit did not reach statistical significance (P = .057)
Invasive disease-free survival (HR, 0.73) and distant recurrence-free survival (HR, 0.77) favored carboplatin, but the results also weren’t statistically significant.
Overall, 46% of patients had a pathologic complete response with carboplatin vs nearly 40% in the control arm. The pathologic complete response benefit from carboplatin add-on was consistent with past reports.
As expected, adding carboplatin to treatment increased hematologic toxicity and other adverse events, with three-quarters of patients experiencing grade 3 or worse adverse events vs 56.7% of control participants. There was one death in the carboplatin arm from pneumonia and two in the control arm — one from septic shock and the other from suicide.
Dr. Sohn and colleagues, however, did not observe a quality of life difference between the two groups.
“The PEARLY trial provides compelling evidence for including carboplatin in the treatment of early-stage TNBC,” Dr. Sohn concluded, adding that the results underscore the benefit in the neoadjuvant setting and suggest “potential applicability in the adjuvant setting post surgery.”
Study discussant Javier Cortes, MD, PhD, believes that the PEARLY provides a strong signal for adding carboplatin in the adjuvant setting.
“That’s something I would do in my clinical practice,” said Dr. Cortes, head of the International Breast Cancer Center in Barcelona, Spain. “After ASCO this year, I would offer taxanes plus carboplatin following anthracyclines.”
An audience member, William Sikov, MD, a breast cancer specialist at Brown University in Providence, Rhode Island, said he hopes “we’ve reached the end of a road that started many years ago in terms of incorporating carboplatin as part of neoadjuvant and adjuvant therapy for triple-negative breast cancer, where we finally [reach] consensus that this is necessary in our triple-negative patients.”
The work was funded by the government of South Korea and others. Dr. Sohn reported stock in Daiichi Sankyo and research funding from Daiichi and other companies. Dr. Cortes disclosed numerous industry ties, including honoraria, research funding, and/or travel expenses from AstraZeneca, Daiichi, and others.
A version of this article first appeared on Medscape.com.
presented at the annual meeting of the American Society of Clinical Oncology (ASCO).
The outcomes of the South Korean study, dubbed PEARLY, provide strong evidence for incorporating carboplatin into both the neoadjuvant and adjuvant settings in patients with early-stage TNBC, said lead investigator and presenter Joohyuk Sohn, MD, PhD, a medical oncologist at Yonsei University, Seoul, South Korea.
In early-stage TNBC, carboplatin is already being incorporated into the neoadjuvant setting on the basis of trial results from KEYNOTE-522 that demonstrated improved pathologic complete response rates and event-free survival with the platinum alongside pembrolizumab.
However, the overall survival benefit of carboplatin in this setting remains unclear, as does the benefit of platinum add-on in the adjuvant setting, Dr. Sohn explained.
Dr. Sohn and colleagues randomized 868 patients evenly to either standard treatment — doxorubicin, anthracycline, and cyclophosphamide followed by a taxane — or an experimental arm that added carboplatin to the taxane phase of treatment.
About 30% of women were treated in the adjuvant setting, the rest in the neoadjuvant setting. The two arms of the study were generally well balanced — about 80% of patients had stage II disease, half were node negative, and 11% had deleterious germline mutations.
The primary endpoint, event-free survival, was broadly defined. Events included disease progression, local or distant recurrence, occurrence of a second primary cancer, inoperable status after neoadjuvant therapy, or death from any cause.
Adding carboplatin increased 5-year event-free survival rates from 75.1% to 82.3% (hazard ratio [HR], 0.67; P = .012) with the benefit holding across various subgroup analyses and particularly strong for adjuvant carboplatin (HR, 0.26).
Five-year overall survival was also better in the carboplatin arm — 90.7% vs 87% in the control arm (HR, 0.65; 95% CI, 0.42-1.02) — but that benefit did not reach statistical significance (P = .057)
Invasive disease-free survival (HR, 0.73) and distant recurrence-free survival (HR, 0.77) favored carboplatin, but the results also weren’t statistically significant.
Overall, 46% of patients had a pathologic complete response with carboplatin vs nearly 40% in the control arm. The pathologic complete response benefit from carboplatin add-on was consistent with past reports.
As expected, adding carboplatin to treatment increased hematologic toxicity and other adverse events, with three-quarters of patients experiencing grade 3 or worse adverse events vs 56.7% of control participants. There was one death in the carboplatin arm from pneumonia and two in the control arm — one from septic shock and the other from suicide.
Dr. Sohn and colleagues, however, did not observe a quality of life difference between the two groups.
“The PEARLY trial provides compelling evidence for including carboplatin in the treatment of early-stage TNBC,” Dr. Sohn concluded, adding that the results underscore the benefit in the neoadjuvant setting and suggest “potential applicability in the adjuvant setting post surgery.”
Study discussant Javier Cortes, MD, PhD, believes that the PEARLY provides a strong signal for adding carboplatin in the adjuvant setting.
“That’s something I would do in my clinical practice,” said Dr. Cortes, head of the International Breast Cancer Center in Barcelona, Spain. “After ASCO this year, I would offer taxanes plus carboplatin following anthracyclines.”
An audience member, William Sikov, MD, a breast cancer specialist at Brown University in Providence, Rhode Island, said he hopes “we’ve reached the end of a road that started many years ago in terms of incorporating carboplatin as part of neoadjuvant and adjuvant therapy for triple-negative breast cancer, where we finally [reach] consensus that this is necessary in our triple-negative patients.”
The work was funded by the government of South Korea and others. Dr. Sohn reported stock in Daiichi Sankyo and research funding from Daiichi and other companies. Dr. Cortes disclosed numerous industry ties, including honoraria, research funding, and/or travel expenses from AstraZeneca, Daiichi, and others.
A version of this article first appeared on Medscape.com.
FROM ASCO 2024
Anti-CD20 Therapy for Relapsing Multiple Sclerosis
Data have shown that CD20-expressing B cells are crucial to the pathogenesis of multiple sclerosis (MS). First approved by the US Food and Drug Administration for MS in 2017, anti-CD20 monoclonal antibody therapies including ocrelizumab, ofatumumab, and ublituximab have proven effective at controlling the symptoms of relapsing-remitting MS (RRMS).
In this ReCAP, Dr Fred D. Lublin, of the Mount Sinai School of Medicine, discusses recent data on anti-CD20 agents for RRMS, including results presented at the 2024 meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
He discusses a protocol examining the effect on RRMS of extending dosage intervals or stopping anti-CD20 therapy after 1 or 2 years of treatment based on results suggesting that the B cells that return post depletion are predominantly regulatory rather than pathogenic.
Next, Dr Lublin discusses a paper presented at CMSC on risks for serious infections in individuals taking ocrelizumab or ofatumumab. Major predictors were found to be progressive disease, prior use of a disease-modifying therapy, and longer duration of therapy.
Finally, he considers recent studies comparing rituximab, an anti-CD20 therapy not approved for MS in the United States but commonly used off-label internationally, with more recent therapies such as ocrelizumab. Data currently indicate that an increased risk for infections are associated with rituximab vs ocrelizumab, but further research is under way.
--
Fred D. Lublin, MD, Director, The Corinne Goldsmith Dickinson Center for Multiple Sclerosis, Icahn School of Medicine at Mount Sinai, New York, NY
Fred D. Lublin, MD, has disclosed the following relevant financial relationships:
Sources of Funding for Research: Novartis; Biogen; Sanofi; NMSS; NIH; Brainstorm Cell Therapeutics
Consulting Agreements/Advisory Boards/DSMB: Biogen; EMD Serono; Novartis; Actelion/Janssen; Sanofi/Genzyme; Roche/Genentech; Horizon Therapeutics/Amgen; Bristol Myers Squibb; Mapi Pharma; Brainstorm Cell Therapeutics; Mylan/Viatris; Immunic; Avotres; Neurogene; LabCorp; Entelexo Biotherapeutics; Neuralight; SetPoint Medical; Hexal/Sandoz; Baim Institute; Sudo Biosciences; Lapix Therapeutics; Biohaven Pharmaceuticals; Abata Therapeutics; Cognito Therapeutics; ImmPACT Bio
Speaker: Sanofi
Stock Options: Avotres; Neuralight; Lapix Therapeutics; Entelexo
I may discuss unapproved agents that are in the MS developmental pipeline without any recommendation on their use.
Data have shown that CD20-expressing B cells are crucial to the pathogenesis of multiple sclerosis (MS). First approved by the US Food and Drug Administration for MS in 2017, anti-CD20 monoclonal antibody therapies including ocrelizumab, ofatumumab, and ublituximab have proven effective at controlling the symptoms of relapsing-remitting MS (RRMS).
In this ReCAP, Dr Fred D. Lublin, of the Mount Sinai School of Medicine, discusses recent data on anti-CD20 agents for RRMS, including results presented at the 2024 meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
He discusses a protocol examining the effect on RRMS of extending dosage intervals or stopping anti-CD20 therapy after 1 or 2 years of treatment based on results suggesting that the B cells that return post depletion are predominantly regulatory rather than pathogenic.
Next, Dr Lublin discusses a paper presented at CMSC on risks for serious infections in individuals taking ocrelizumab or ofatumumab. Major predictors were found to be progressive disease, prior use of a disease-modifying therapy, and longer duration of therapy.
Finally, he considers recent studies comparing rituximab, an anti-CD20 therapy not approved for MS in the United States but commonly used off-label internationally, with more recent therapies such as ocrelizumab. Data currently indicate that an increased risk for infections are associated with rituximab vs ocrelizumab, but further research is under way.
--
Fred D. Lublin, MD, Director, The Corinne Goldsmith Dickinson Center for Multiple Sclerosis, Icahn School of Medicine at Mount Sinai, New York, NY
Fred D. Lublin, MD, has disclosed the following relevant financial relationships:
Sources of Funding for Research: Novartis; Biogen; Sanofi; NMSS; NIH; Brainstorm Cell Therapeutics
Consulting Agreements/Advisory Boards/DSMB: Biogen; EMD Serono; Novartis; Actelion/Janssen; Sanofi/Genzyme; Roche/Genentech; Horizon Therapeutics/Amgen; Bristol Myers Squibb; Mapi Pharma; Brainstorm Cell Therapeutics; Mylan/Viatris; Immunic; Avotres; Neurogene; LabCorp; Entelexo Biotherapeutics; Neuralight; SetPoint Medical; Hexal/Sandoz; Baim Institute; Sudo Biosciences; Lapix Therapeutics; Biohaven Pharmaceuticals; Abata Therapeutics; Cognito Therapeutics; ImmPACT Bio
Speaker: Sanofi
Stock Options: Avotres; Neuralight; Lapix Therapeutics; Entelexo
I may discuss unapproved agents that are in the MS developmental pipeline without any recommendation on their use.
Data have shown that CD20-expressing B cells are crucial to the pathogenesis of multiple sclerosis (MS). First approved by the US Food and Drug Administration for MS in 2017, anti-CD20 monoclonal antibody therapies including ocrelizumab, ofatumumab, and ublituximab have proven effective at controlling the symptoms of relapsing-remitting MS (RRMS).
In this ReCAP, Dr Fred D. Lublin, of the Mount Sinai School of Medicine, discusses recent data on anti-CD20 agents for RRMS, including results presented at the 2024 meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
He discusses a protocol examining the effect on RRMS of extending dosage intervals or stopping anti-CD20 therapy after 1 or 2 years of treatment based on results suggesting that the B cells that return post depletion are predominantly regulatory rather than pathogenic.
Next, Dr Lublin discusses a paper presented at CMSC on risks for serious infections in individuals taking ocrelizumab or ofatumumab. Major predictors were found to be progressive disease, prior use of a disease-modifying therapy, and longer duration of therapy.
Finally, he considers recent studies comparing rituximab, an anti-CD20 therapy not approved for MS in the United States but commonly used off-label internationally, with more recent therapies such as ocrelizumab. Data currently indicate that an increased risk for infections are associated with rituximab vs ocrelizumab, but further research is under way.
--
Fred D. Lublin, MD, Director, The Corinne Goldsmith Dickinson Center for Multiple Sclerosis, Icahn School of Medicine at Mount Sinai, New York, NY
Fred D. Lublin, MD, has disclosed the following relevant financial relationships:
Sources of Funding for Research: Novartis; Biogen; Sanofi; NMSS; NIH; Brainstorm Cell Therapeutics
Consulting Agreements/Advisory Boards/DSMB: Biogen; EMD Serono; Novartis; Actelion/Janssen; Sanofi/Genzyme; Roche/Genentech; Horizon Therapeutics/Amgen; Bristol Myers Squibb; Mapi Pharma; Brainstorm Cell Therapeutics; Mylan/Viatris; Immunic; Avotres; Neurogene; LabCorp; Entelexo Biotherapeutics; Neuralight; SetPoint Medical; Hexal/Sandoz; Baim Institute; Sudo Biosciences; Lapix Therapeutics; Biohaven Pharmaceuticals; Abata Therapeutics; Cognito Therapeutics; ImmPACT Bio
Speaker: Sanofi
Stock Options: Avotres; Neuralight; Lapix Therapeutics; Entelexo
I may discuss unapproved agents that are in the MS developmental pipeline without any recommendation on their use.

Treatment of Infantile Hemangiomas in Concomitant Tuberous Sclerosis Complex Should Prompt Evaluation for Cardiac Rhabdomyomas Prior to Initiation of Propranolol
To the Editor:
Cardiac rhabdomyomas are benign hamartomas that are common in patients with tuberous sclerosis complex (TSC).1 We describe a patient who presented with large infantile hemangiomas (IHs) and hypopigmented macules, which prompted further testing that eventually showed concomitant multiple cardiac rhabdomyomas in the context of TSC.
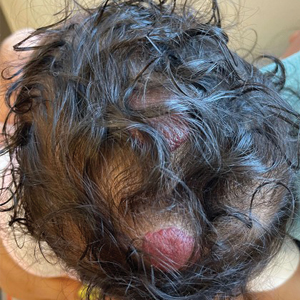
A 5-week-old girl—who was born at 38 weeks and 3 days’ gestation via uncomplicated vaginal delivery—was referred to our pediatric dermatology clinic for evaluation of multiple erythematous lesions on the scalp and left buttock that were first noticed 2 weeks prior to presentation. There was a family history of seizures in the patient’s mother. The patient’s older brother did not have similar symptoms.
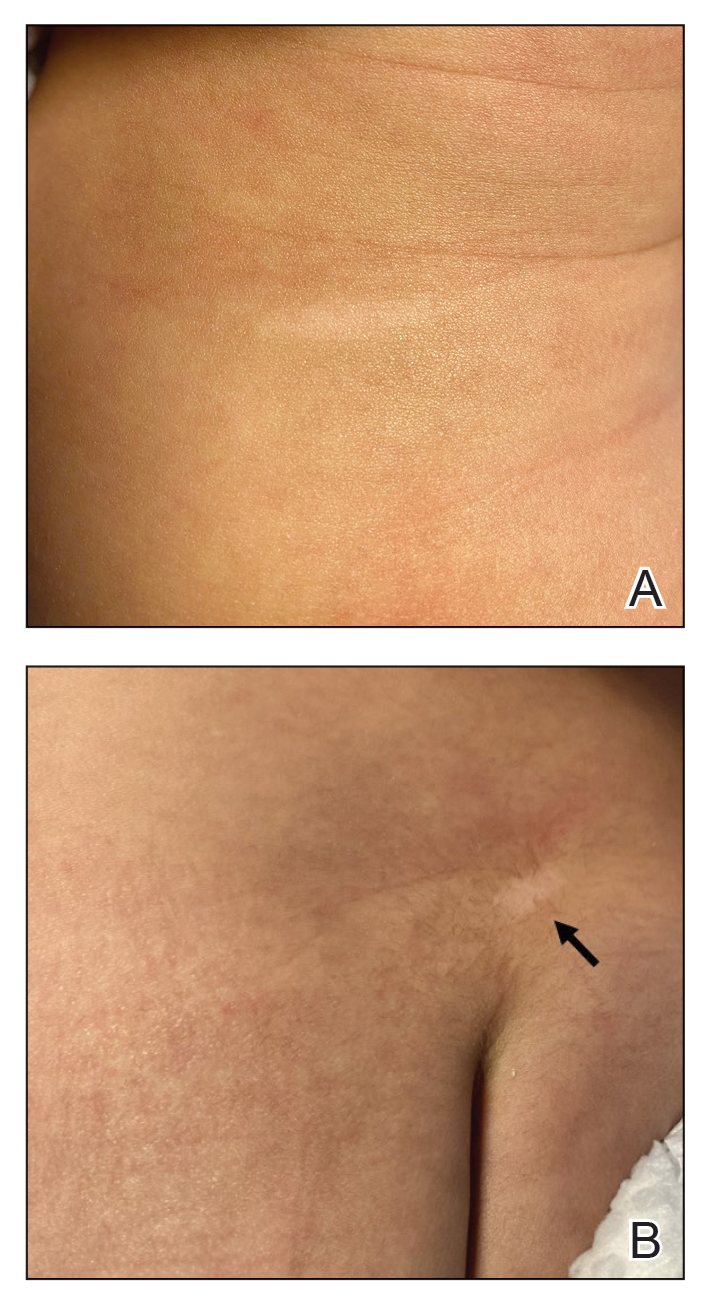
Physical examination revealed 2 nonulcerating erythematous nodules on the middle and posterior left vertex scalp that measured 2.5×2 cm (Figure 1A) as well as 1 bright red plaque on the left buttock (Figure 1B). Five hypopigmented macules, ranging from 5 mm to 1.5 cm in diameter, also were detected on the left thorax (Figure 2A) as well as the middle and lower back (Figure 2B). These findings, along with the history of seizures in the patient’s mother, prompted further evaluation of the family history, which uncovered TSC in the patient’s mother, maternal aunt, and maternal grandmother.
The large IHs on the scalp did not pose concerns for potential functional impairment but were still considered high risk for permanent alopecia based on clinical practice guidelines for the management of IH.2 Treatment with oral propranolol was recommended; however, because of a strong suspicion of TSC due to the presence of 5 hypopigmented macules measuring more than 5 mm in diameter (≥3 hypopigmented macules of ≥5 mm is one of the major criterion for TSC), the patient was referred to cardiology prior to initiation of propranolol.
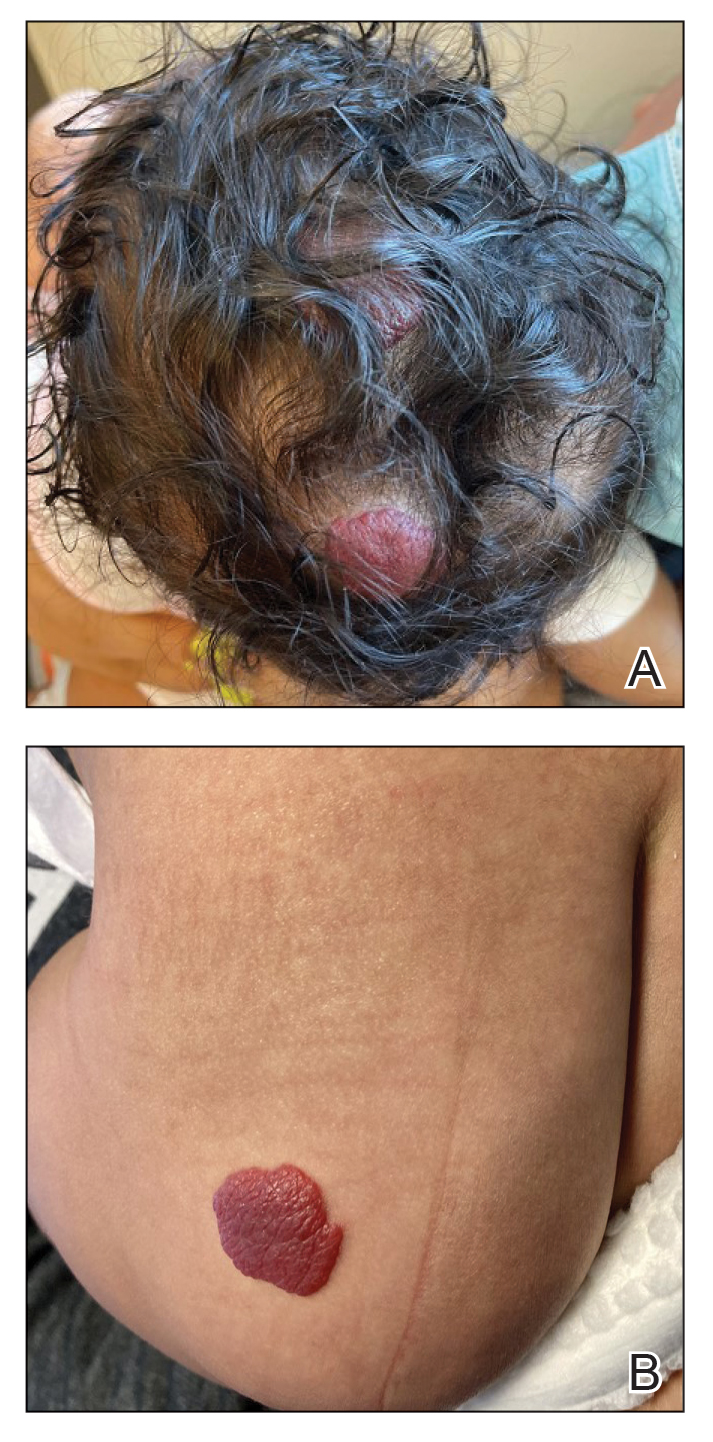
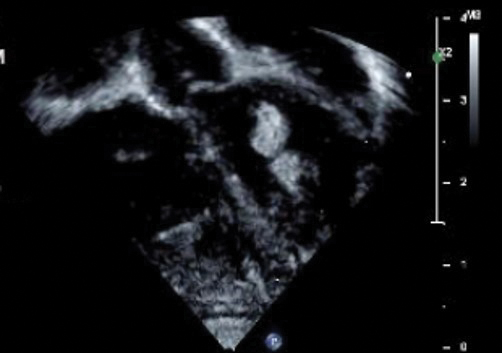
Echocardiography revealed 3 intracardiac masses measuring 4 to 5 mm in diameter in the left ventricle (LV), along the interventricular septum and the LV posterior wall. These masses were consistent with rhabdomyomas (Figure 3)—a major criterion for TSC—which had not been detected by prenatal ultrasonography. No obstruction to LV inflow or outflow was observed. Additionally, no arrhythmias were detected on electrocardiography.
The patient was cleared for propranolol, which was slowly uptitrated to 2 mg/kg/d. She completed the course without adverse effects. The treatment of IH was successful with substantial reduction in size over the following months until clearance. She also was referred to neurology for magnetic resonance imaging of the brain, which showed a 3-mm subependymal nodule in the lateral right ventricle, another major feature of TSC.
Cardiac rhabdomyomas are benign hamartomas that affect as many as 80% of patients with TSC1 and are primarily localized in the ventricles. Although cardiac rhabdomyomas usually regress over time, they can compromise ventricular function or valvular function, or both, and result in outflow obstruction, arrhythmias, and Wolff- Parkinson-White syndrome.3 Surgical resection may be needed in patients whose condition is refractory to medical management for heart failure.
The pathophysiologic mechanism behind the natural involution of cardiac rhabdomyomas has not been fully elucidated. It has been hypothesized that these masses stem from the inability of rhabdomyoma cells to divide after birth due to their embryonic myocyte derivation.4
According to the TSC diagnostic criteria from the Tuberous Sclerosis Complex International Consensus Group, at least 2 major features or 1 major and 2 minor features are required to make a definitive diagnosis of TSC. Cutaneous signs represent more than one-third of major features of TSC; almost all patients with TSC have skin findings.5
Identification of pathogenic mutations in either TSC1 (on chromosome 9q34.3, encoding for hamartin) or TSC2 (on chromosome 16p13.3, encoding for tuberin), resulting in constitutive activation of mammalian target of rapamycin and subsequent increased cell growth, is sufficient for a definitive diagnosis of TSC. However, mutations cannot be identified by conventional genetic testing in as many as one-quarter of patients with TSC; therefore, a negative result does not exclude TSC if the patient meets clinical diagnostic criteria.
Although a cardiology workup is indicated prior to initiating propranolol in the presence of possible cardiac rhabdomyomas, most of those lesions are hemodynamically stable and do not require treatment. There also is no contraindication for β-blocker therapy. In fact, propranolol has been reported as a successful treatment in rhabdomyoma-associated arrhythmias in children.6 Notably, obstructive cardiac rhabdomyomas have been successfully treated with mammalian target of rapamycin inhibitors, such as sirolimus7 and everolimus.8
Baseline cardiology screening with echocardiography prior to initiating propranolol for treatment of IH is not routinely indicated in babies with uncomplicated IH. However, in a patient with TSC, cardiology screening is necessary to rule out rhabdomyomas with associated arrhythmias or obstructed blood flow, or both, prior to initiating treatment.
We presented a case of concomitant IH and TSC in a patient with cardiac rhabdomyomas. The manifestation of large IHs in our patient prompted further testing that revealed multiple cardiac rhabdomyomas in the context of TSC. It is imperative for cardiologists, cardiac surgeons, and dermatologists to be familiar with the TSC diagnostic criteria so that they can reach a prompt diagnosis and make appropriate referrals for further evaluation of cardiac, neurologic, and ophthalmologic signs.
- Frudit P, Vitturi BK, Navarro FC, et al. Multiple cardiac rhabdomyomas in tuberous sclerosis complex: case report and review of the literature. Autops Case Rep. 2019;9:e2019125. doi:10.4322/acr.2019.125
- Krowchuk DP, Frieden IJ, Mancini AJ, et al; Subcommittee on the Management of Infantile Hemangiomas. Clinical practice guideline for the management of infantile hemangiomas. Pediatrics. 2019;143:e20183475. doi:10.1542/peds.2018-3475
- Venugopalan P, Babu JS, Al-Bulushi A. Right atrial rhabdomyoma acting as the substrate for Wolff-Parkinson-White syndrome in a 3-month-old infant. Acta Cardiol. 2005;60:543-545. doi:10.2143/AC.60.5.2004977
- DiMario FJ Jr, Diana D, Leopold H, et al. Evolution of cardiac rhabdomyoma in tuberous sclerosis complex. Clin Pediatr (Phila). 1996;35:615-619. doi:10.1177/000992289603501202
- Northrup H, Krueger DA; International Tuberous Sclerosis Complex Consensus Group. Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol. 2013;49:243-254. doi:10.1016/j.pediatrneurol.2013.08.001
- Kathare PA, Muthuswamy KS, Sadasivan J, et al. Incessant ventricular tachycardia due to multiple cardiac rhabdomyomas in an infant with tuberous sclerosis. Indian Heart J. 2013;65:111-113. doi:10.1016/j.ihj.2012.12.003
- Breathnach C, Pears J, Franklin O, et al. Rapid regression of left ventricular outflow tract rhabdomyoma after sirolimus therapy. Pediatrics. 2014;134:e1199-e1202. doi:10.1542/peds.2013-3293
- Chang J-S, Chiou P-Y, Yao S-H, et al. Regression of neonatal cardiac rhabdomyoma in two months through low-dose everolimus therapy: a report of three cases. Pediatr Cardiol. 2017;38:1478-1484. doi:10.1007/s00246-017-1688-4
To the Editor:
Cardiac rhabdomyomas are benign hamartomas that are common in patients with tuberous sclerosis complex (TSC).1 We describe a patient who presented with large infantile hemangiomas (IHs) and hypopigmented macules, which prompted further testing that eventually showed concomitant multiple cardiac rhabdomyomas in the context of TSC.
A 5-week-old girl—who was born at 38 weeks and 3 days’ gestation via uncomplicated vaginal delivery—was referred to our pediatric dermatology clinic for evaluation of multiple erythematous lesions on the scalp and left buttock that were first noticed 2 weeks prior to presentation. There was a family history of seizures in the patient’s mother. The patient’s older brother did not have similar symptoms.
Physical examination revealed 2 nonulcerating erythematous nodules on the middle and posterior left vertex scalp that measured 2.5×2 cm (Figure 1A) as well as 1 bright red plaque on the left buttock (Figure 1B). Five hypopigmented macules, ranging from 5 mm to 1.5 cm in diameter, also were detected on the left thorax (Figure 2A) as well as the middle and lower back (Figure 2B). These findings, along with the history of seizures in the patient’s mother, prompted further evaluation of the family history, which uncovered TSC in the patient’s mother, maternal aunt, and maternal grandmother.
The large IHs on the scalp did not pose concerns for potential functional impairment but were still considered high risk for permanent alopecia based on clinical practice guidelines for the management of IH.2 Treatment with oral propranolol was recommended; however, because of a strong suspicion of TSC due to the presence of 5 hypopigmented macules measuring more than 5 mm in diameter (≥3 hypopigmented macules of ≥5 mm is one of the major criterion for TSC), the patient was referred to cardiology prior to initiation of propranolol.
Echocardiography revealed 3 intracardiac masses measuring 4 to 5 mm in diameter in the left ventricle (LV), along the interventricular septum and the LV posterior wall. These masses were consistent with rhabdomyomas (Figure 3)—a major criterion for TSC—which had not been detected by prenatal ultrasonography. No obstruction to LV inflow or outflow was observed. Additionally, no arrhythmias were detected on electrocardiography.
The patient was cleared for propranolol, which was slowly uptitrated to 2 mg/kg/d. She completed the course without adverse effects. The treatment of IH was successful with substantial reduction in size over the following months until clearance. She also was referred to neurology for magnetic resonance imaging of the brain, which showed a 3-mm subependymal nodule in the lateral right ventricle, another major feature of TSC.

Cardiac rhabdomyomas are benign hamartomas that affect as many as 80% of patients with TSC1 and are primarily localized in the ventricles. Although cardiac rhabdomyomas usually regress over time, they can compromise ventricular function or valvular function, or both, and result in outflow obstruction, arrhythmias, and Wolff- Parkinson-White syndrome.3 Surgical resection may be needed in patients whose condition is refractory to medical management for heart failure.
The pathophysiologic mechanism behind the natural involution of cardiac rhabdomyomas has not been fully elucidated. It has been hypothesized that these masses stem from the inability of rhabdomyoma cells to divide after birth due to their embryonic myocyte derivation.4
According to the TSC diagnostic criteria from the Tuberous Sclerosis Complex International Consensus Group, at least 2 major features or 1 major and 2 minor features are required to make a definitive diagnosis of TSC. Cutaneous signs represent more than one-third of major features of TSC; almost all patients with TSC have skin findings.5
Identification of pathogenic mutations in either TSC1 (on chromosome 9q34.3, encoding for hamartin) or TSC2 (on chromosome 16p13.3, encoding for tuberin), resulting in constitutive activation of mammalian target of rapamycin and subsequent increased cell growth, is sufficient for a definitive diagnosis of TSC. However, mutations cannot be identified by conventional genetic testing in as many as one-quarter of patients with TSC; therefore, a negative result does not exclude TSC if the patient meets clinical diagnostic criteria.


Although a cardiology workup is indicated prior to initiating propranolol in the presence of possible cardiac rhabdomyomas, most of those lesions are hemodynamically stable and do not require treatment. There also is no contraindication for β-blocker therapy. In fact, propranolol has been reported as a successful treatment in rhabdomyoma-associated arrhythmias in children.6 Notably, obstructive cardiac rhabdomyomas have been successfully treated with mammalian target of rapamycin inhibitors, such as sirolimus7 and everolimus.8
Baseline cardiology screening with echocardiography prior to initiating propranolol for treatment of IH is not routinely indicated in babies with uncomplicated IH. However, in a patient with TSC, cardiology screening is necessary to rule out rhabdomyomas with associated arrhythmias or obstructed blood flow, or both, prior to initiating treatment.
We presented a case of concomitant IH and TSC in a patient with cardiac rhabdomyomas. The manifestation of large IHs in our patient prompted further testing that revealed multiple cardiac rhabdomyomas in the context of TSC. It is imperative for cardiologists, cardiac surgeons, and dermatologists to be familiar with the TSC diagnostic criteria so that they can reach a prompt diagnosis and make appropriate referrals for further evaluation of cardiac, neurologic, and ophthalmologic signs.
To the Editor:
Cardiac rhabdomyomas are benign hamartomas that are common in patients with tuberous sclerosis complex (TSC).1 We describe a patient who presented with large infantile hemangiomas (IHs) and hypopigmented macules, which prompted further testing that eventually showed concomitant multiple cardiac rhabdomyomas in the context of TSC.
A 5-week-old girl—who was born at 38 weeks and 3 days’ gestation via uncomplicated vaginal delivery—was referred to our pediatric dermatology clinic for evaluation of multiple erythematous lesions on the scalp and left buttock that were first noticed 2 weeks prior to presentation. There was a family history of seizures in the patient’s mother. The patient’s older brother did not have similar symptoms.
Physical examination revealed 2 nonulcerating erythematous nodules on the middle and posterior left vertex scalp that measured 2.5×2 cm (Figure 1A) as well as 1 bright red plaque on the left buttock (Figure 1B). Five hypopigmented macules, ranging from 5 mm to 1.5 cm in diameter, also were detected on the left thorax (Figure 2A) as well as the middle and lower back (Figure 2B). These findings, along with the history of seizures in the patient’s mother, prompted further evaluation of the family history, which uncovered TSC in the patient’s mother, maternal aunt, and maternal grandmother.
The large IHs on the scalp did not pose concerns for potential functional impairment but were still considered high risk for permanent alopecia based on clinical practice guidelines for the management of IH.2 Treatment with oral propranolol was recommended; however, because of a strong suspicion of TSC due to the presence of 5 hypopigmented macules measuring more than 5 mm in diameter (≥3 hypopigmented macules of ≥5 mm is one of the major criterion for TSC), the patient was referred to cardiology prior to initiation of propranolol.
Echocardiography revealed 3 intracardiac masses measuring 4 to 5 mm in diameter in the left ventricle (LV), along the interventricular septum and the LV posterior wall. These masses were consistent with rhabdomyomas (Figure 3)—a major criterion for TSC—which had not been detected by prenatal ultrasonography. No obstruction to LV inflow or outflow was observed. Additionally, no arrhythmias were detected on electrocardiography.
The patient was cleared for propranolol, which was slowly uptitrated to 2 mg/kg/d. She completed the course without adverse effects. The treatment of IH was successful with substantial reduction in size over the following months until clearance. She also was referred to neurology for magnetic resonance imaging of the brain, which showed a 3-mm subependymal nodule in the lateral right ventricle, another major feature of TSC.

Cardiac rhabdomyomas are benign hamartomas that affect as many as 80% of patients with TSC1 and are primarily localized in the ventricles. Although cardiac rhabdomyomas usually regress over time, they can compromise ventricular function or valvular function, or both, and result in outflow obstruction, arrhythmias, and Wolff- Parkinson-White syndrome.3 Surgical resection may be needed in patients whose condition is refractory to medical management for heart failure.
The pathophysiologic mechanism behind the natural involution of cardiac rhabdomyomas has not been fully elucidated. It has been hypothesized that these masses stem from the inability of rhabdomyoma cells to divide after birth due to their embryonic myocyte derivation.4
According to the TSC diagnostic criteria from the Tuberous Sclerosis Complex International Consensus Group, at least 2 major features or 1 major and 2 minor features are required to make a definitive diagnosis of TSC. Cutaneous signs represent more than one-third of major features of TSC; almost all patients with TSC have skin findings.5
Identification of pathogenic mutations in either TSC1 (on chromosome 9q34.3, encoding for hamartin) or TSC2 (on chromosome 16p13.3, encoding for tuberin), resulting in constitutive activation of mammalian target of rapamycin and subsequent increased cell growth, is sufficient for a definitive diagnosis of TSC. However, mutations cannot be identified by conventional genetic testing in as many as one-quarter of patients with TSC; therefore, a negative result does not exclude TSC if the patient meets clinical diagnostic criteria.


Although a cardiology workup is indicated prior to initiating propranolol in the presence of possible cardiac rhabdomyomas, most of those lesions are hemodynamically stable and do not require treatment. There also is no contraindication for β-blocker therapy. In fact, propranolol has been reported as a successful treatment in rhabdomyoma-associated arrhythmias in children.6 Notably, obstructive cardiac rhabdomyomas have been successfully treated with mammalian target of rapamycin inhibitors, such as sirolimus7 and everolimus.8
Baseline cardiology screening with echocardiography prior to initiating propranolol for treatment of IH is not routinely indicated in babies with uncomplicated IH. However, in a patient with TSC, cardiology screening is necessary to rule out rhabdomyomas with associated arrhythmias or obstructed blood flow, or both, prior to initiating treatment.
We presented a case of concomitant IH and TSC in a patient with cardiac rhabdomyomas. The manifestation of large IHs in our patient prompted further testing that revealed multiple cardiac rhabdomyomas in the context of TSC. It is imperative for cardiologists, cardiac surgeons, and dermatologists to be familiar with the TSC diagnostic criteria so that they can reach a prompt diagnosis and make appropriate referrals for further evaluation of cardiac, neurologic, and ophthalmologic signs.
- Frudit P, Vitturi BK, Navarro FC, et al. Multiple cardiac rhabdomyomas in tuberous sclerosis complex: case report and review of the literature. Autops Case Rep. 2019;9:e2019125. doi:10.4322/acr.2019.125
- Krowchuk DP, Frieden IJ, Mancini AJ, et al; Subcommittee on the Management of Infantile Hemangiomas. Clinical practice guideline for the management of infantile hemangiomas. Pediatrics. 2019;143:e20183475. doi:10.1542/peds.2018-3475
- Venugopalan P, Babu JS, Al-Bulushi A. Right atrial rhabdomyoma acting as the substrate for Wolff-Parkinson-White syndrome in a 3-month-old infant. Acta Cardiol. 2005;60:543-545. doi:10.2143/AC.60.5.2004977
- DiMario FJ Jr, Diana D, Leopold H, et al. Evolution of cardiac rhabdomyoma in tuberous sclerosis complex. Clin Pediatr (Phila). 1996;35:615-619. doi:10.1177/000992289603501202
- Northrup H, Krueger DA; International Tuberous Sclerosis Complex Consensus Group. Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol. 2013;49:243-254. doi:10.1016/j.pediatrneurol.2013.08.001
- Kathare PA, Muthuswamy KS, Sadasivan J, et al. Incessant ventricular tachycardia due to multiple cardiac rhabdomyomas in an infant with tuberous sclerosis. Indian Heart J. 2013;65:111-113. doi:10.1016/j.ihj.2012.12.003
- Breathnach C, Pears J, Franklin O, et al. Rapid regression of left ventricular outflow tract rhabdomyoma after sirolimus therapy. Pediatrics. 2014;134:e1199-e1202. doi:10.1542/peds.2013-3293
- Chang J-S, Chiou P-Y, Yao S-H, et al. Regression of neonatal cardiac rhabdomyoma in two months through low-dose everolimus therapy: a report of three cases. Pediatr Cardiol. 2017;38:1478-1484. doi:10.1007/s00246-017-1688-4
- Frudit P, Vitturi BK, Navarro FC, et al. Multiple cardiac rhabdomyomas in tuberous sclerosis complex: case report and review of the literature. Autops Case Rep. 2019;9:e2019125. doi:10.4322/acr.2019.125
- Krowchuk DP, Frieden IJ, Mancini AJ, et al; Subcommittee on the Management of Infantile Hemangiomas. Clinical practice guideline for the management of infantile hemangiomas. Pediatrics. 2019;143:e20183475. doi:10.1542/peds.2018-3475
- Venugopalan P, Babu JS, Al-Bulushi A. Right atrial rhabdomyoma acting as the substrate for Wolff-Parkinson-White syndrome in a 3-month-old infant. Acta Cardiol. 2005;60:543-545. doi:10.2143/AC.60.5.2004977
- DiMario FJ Jr, Diana D, Leopold H, et al. Evolution of cardiac rhabdomyoma in tuberous sclerosis complex. Clin Pediatr (Phila). 1996;35:615-619. doi:10.1177/000992289603501202
- Northrup H, Krueger DA; International Tuberous Sclerosis Complex Consensus Group. Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol. 2013;49:243-254. doi:10.1016/j.pediatrneurol.2013.08.001
- Kathare PA, Muthuswamy KS, Sadasivan J, et al. Incessant ventricular tachycardia due to multiple cardiac rhabdomyomas in an infant with tuberous sclerosis. Indian Heart J. 2013;65:111-113. doi:10.1016/j.ihj.2012.12.003
- Breathnach C, Pears J, Franklin O, et al. Rapid regression of left ventricular outflow tract rhabdomyoma after sirolimus therapy. Pediatrics. 2014;134:e1199-e1202. doi:10.1542/peds.2013-3293
- Chang J-S, Chiou P-Y, Yao S-H, et al. Regression of neonatal cardiac rhabdomyoma in two months through low-dose everolimus therapy: a report of three cases. Pediatr Cardiol. 2017;38:1478-1484. doi:10.1007/s00246-017-1688-4
Practice Points
- Dermatologists may see patients with infantile hemangiomas (IHs) and tuberous sclerosis complex (TSC); therefore, they should be familiar with TSC diagnostic criteria to reach a prompt diagnosis and make appropriate referrals.
- Cardiologic evaluation is not routinely required prior to systemic treatment of IH, but knowledge of cardiac findings in TSC should prompt cardiologic clearance prior to β-blocker initiation.
Diabetic Foot Infections: A Peptide’s Potential Promise
At the recent American Diabetes Association (ADA) Scientific Sessions, researchers unveiled promising data on a novel antimicrobial peptide PL-5 spray. This innovative treatment shows significant promise for managing mild to moderate infected diabetic foot ulcers.
Of the 1.6 million people with diabetes in the United States and the tens of millions of similar people worldwide, 50% will require antimicrobials at some time during their life cycle. Diabetic foot infections are difficult to treat because of their resistance to conventional therapies, often leading to severe complications, including amputations.
To address this issue, the antimicrobial peptide PL-5 spray was developed with a novel mechanism of action to potentially improve treatment outcomes. The study aimed to assess the clinical efficacy and safety of the PL-5 spray combined with standard debridement procedures in treating mild to moderate diabetic foot ulcers.
This multicenter, randomized, double-blind, placebo-controlled clinical trial was conducted in four hospitals across China. Participants with mild to moderate diabetic foot ulcers were randomly assigned in a 2:1 ratio to either the PL-5 group or the placebo group, both receiving standard debridement. The primary endpoint was clinical efficacy at day 1 after the end of treatment (EOT1). Secondary endpoints included clinical efficacy at day 7 (EOT7), microbiological efficacy, drug-resistant bacteria clearance rate, wound healing rate, and safety outcomes evaluated at both EOT1 and EOT7.
The study included 47 participants, with 32 in the PL-5 group and 15 in the placebo group. Both groups had statistically comparable demographic and clinical characteristics. The primary endpoint showed a higher clinical efficacy (cure/improvement ratio) in the PL-5 group, compared with the control group (1.33 vs 0.55; P =.0764), suggesting a positive trend but not reaching statistical significance in this population.
Among the secondary endpoints, clinical efficacy at EOT7 was significantly higher in the PL-5 group than in the control group (1.6 vs 0.86). Microbial eradication rates were notably better in the PL-5 group at both EOT1 (57.89% vs 33.33%) and EOT7 (64.71% vs 40.00%). The clearance rates of drug-resistant bacteria were also higher in the PL-5 group at EOT1 (71.43% vs 50%).
Of importance, safety parameters showed no significant differences between the two groups (24.24% vs 33.33%), highlighting the favorable safety profile of PL-5 spray.
The study presented at the ADA Scientific Sessions provides a glint of promising evidence supporting the potential efficacy and safety of PL-5 spray in treating mild to moderate diabetic foot infections. Despite the limited sample size, the results suggest that PL-5 spray may enhance the recovery speed of diabetic foot wounds, particularly in clearing drug-resistant bacterial infections. These findings justify further investigation with larger sample sizes to confirm or refute the efficacy and potentially establish PL-5 spray as a standard treatment option in diabetic foot care.
The novel antimicrobial peptide PL-5 spray shows potential in addressing the challenging issue of diabetic foot infections. This recent ADA presentation sparked significant interest and discussions about the future of diabetic foot ulcer treatments, emphasizing the importance of innovative approaches in managing complex diabetic complications.
Dr. Armstrong is a professor of surgery and director of limb preservation at the University of Southern California, Los Angeles. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
At the recent American Diabetes Association (ADA) Scientific Sessions, researchers unveiled promising data on a novel antimicrobial peptide PL-5 spray. This innovative treatment shows significant promise for managing mild to moderate infected diabetic foot ulcers.
Of the 1.6 million people with diabetes in the United States and the tens of millions of similar people worldwide, 50% will require antimicrobials at some time during their life cycle. Diabetic foot infections are difficult to treat because of their resistance to conventional therapies, often leading to severe complications, including amputations.
To address this issue, the antimicrobial peptide PL-5 spray was developed with a novel mechanism of action to potentially improve treatment outcomes. The study aimed to assess the clinical efficacy and safety of the PL-5 spray combined with standard debridement procedures in treating mild to moderate diabetic foot ulcers.
This multicenter, randomized, double-blind, placebo-controlled clinical trial was conducted in four hospitals across China. Participants with mild to moderate diabetic foot ulcers were randomly assigned in a 2:1 ratio to either the PL-5 group or the placebo group, both receiving standard debridement. The primary endpoint was clinical efficacy at day 1 after the end of treatment (EOT1). Secondary endpoints included clinical efficacy at day 7 (EOT7), microbiological efficacy, drug-resistant bacteria clearance rate, wound healing rate, and safety outcomes evaluated at both EOT1 and EOT7.
The study included 47 participants, with 32 in the PL-5 group and 15 in the placebo group. Both groups had statistically comparable demographic and clinical characteristics. The primary endpoint showed a higher clinical efficacy (cure/improvement ratio) in the PL-5 group, compared with the control group (1.33 vs 0.55; P =.0764), suggesting a positive trend but not reaching statistical significance in this population.
Among the secondary endpoints, clinical efficacy at EOT7 was significantly higher in the PL-5 group than in the control group (1.6 vs 0.86). Microbial eradication rates were notably better in the PL-5 group at both EOT1 (57.89% vs 33.33%) and EOT7 (64.71% vs 40.00%). The clearance rates of drug-resistant bacteria were also higher in the PL-5 group at EOT1 (71.43% vs 50%).
Of importance, safety parameters showed no significant differences between the two groups (24.24% vs 33.33%), highlighting the favorable safety profile of PL-5 spray.
The study presented at the ADA Scientific Sessions provides a glint of promising evidence supporting the potential efficacy and safety of PL-5 spray in treating mild to moderate diabetic foot infections. Despite the limited sample size, the results suggest that PL-5 spray may enhance the recovery speed of diabetic foot wounds, particularly in clearing drug-resistant bacterial infections. These findings justify further investigation with larger sample sizes to confirm or refute the efficacy and potentially establish PL-5 spray as a standard treatment option in diabetic foot care.
The novel antimicrobial peptide PL-5 spray shows potential in addressing the challenging issue of diabetic foot infections. This recent ADA presentation sparked significant interest and discussions about the future of diabetic foot ulcer treatments, emphasizing the importance of innovative approaches in managing complex diabetic complications.
Dr. Armstrong is a professor of surgery and director of limb preservation at the University of Southern California, Los Angeles. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
At the recent American Diabetes Association (ADA) Scientific Sessions, researchers unveiled promising data on a novel antimicrobial peptide PL-5 spray. This innovative treatment shows significant promise for managing mild to moderate infected diabetic foot ulcers.
Of the 1.6 million people with diabetes in the United States and the tens of millions of similar people worldwide, 50% will require antimicrobials at some time during their life cycle. Diabetic foot infections are difficult to treat because of their resistance to conventional therapies, often leading to severe complications, including amputations.
To address this issue, the antimicrobial peptide PL-5 spray was developed with a novel mechanism of action to potentially improve treatment outcomes. The study aimed to assess the clinical efficacy and safety of the PL-5 spray combined with standard debridement procedures in treating mild to moderate diabetic foot ulcers.
This multicenter, randomized, double-blind, placebo-controlled clinical trial was conducted in four hospitals across China. Participants with mild to moderate diabetic foot ulcers were randomly assigned in a 2:1 ratio to either the PL-5 group or the placebo group, both receiving standard debridement. The primary endpoint was clinical efficacy at day 1 after the end of treatment (EOT1). Secondary endpoints included clinical efficacy at day 7 (EOT7), microbiological efficacy, drug-resistant bacteria clearance rate, wound healing rate, and safety outcomes evaluated at both EOT1 and EOT7.
The study included 47 participants, with 32 in the PL-5 group and 15 in the placebo group. Both groups had statistically comparable demographic and clinical characteristics. The primary endpoint showed a higher clinical efficacy (cure/improvement ratio) in the PL-5 group, compared with the control group (1.33 vs 0.55; P =.0764), suggesting a positive trend but not reaching statistical significance in this population.
Among the secondary endpoints, clinical efficacy at EOT7 was significantly higher in the PL-5 group than in the control group (1.6 vs 0.86). Microbial eradication rates were notably better in the PL-5 group at both EOT1 (57.89% vs 33.33%) and EOT7 (64.71% vs 40.00%). The clearance rates of drug-resistant bacteria were also higher in the PL-5 group at EOT1 (71.43% vs 50%).
Of importance, safety parameters showed no significant differences between the two groups (24.24% vs 33.33%), highlighting the favorable safety profile of PL-5 spray.
The study presented at the ADA Scientific Sessions provides a glint of promising evidence supporting the potential efficacy and safety of PL-5 spray in treating mild to moderate diabetic foot infections. Despite the limited sample size, the results suggest that PL-5 spray may enhance the recovery speed of diabetic foot wounds, particularly in clearing drug-resistant bacterial infections. These findings justify further investigation with larger sample sizes to confirm or refute the efficacy and potentially establish PL-5 spray as a standard treatment option in diabetic foot care.
The novel antimicrobial peptide PL-5 spray shows potential in addressing the challenging issue of diabetic foot infections. This recent ADA presentation sparked significant interest and discussions about the future of diabetic foot ulcer treatments, emphasizing the importance of innovative approaches in managing complex diabetic complications.
Dr. Armstrong is a professor of surgery and director of limb preservation at the University of Southern California, Los Angeles. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Don’t Fear Hormone Therapy, but Prescribe It Correctly
This transcript has been edited for clarity.
Rachel S. Rubin, MD: As a sexual medicine specialist, I treat a lot of menopause. Why? Because menopausal complaints are not just hot flashes and night sweats; we see so many sexual health problems: genital urinary syndrome of menopause (GUSM), low libido, pain with sex, arousal disorders, orgasm disorders. I am joined today with a superstar in the menopause field, Dr. Stephanie Faubion. Introduce yourself to our amazing listeners.
Stephanie S. Faubion, MD, MBA: I am Stephanie Faubion, director of the Mayo Clinic Center for Women’s Health and medical director for the Menopause Society.
Dr. Rubin: That is a very short introduction for a very impressive person who really is an authority, if you’ve ever read an article about menopause. I asked Dr. Faubion if she spends all her time talking to reporters. But it’s very important because menopause is having a moment. We can’t go a day without seeing a headline, an Instagram story, or something; my feed is full of menopause information. Why do you think menopause is having a moment right now?
Dr. Faubion: It’s a well-deserved moment and should have happened a long time ago. It’s having a moment for several reasons. The generation of women experiencing perimenopause and menopause is different now; they are less willing to suffer in silence, which is a great thing. We’ve also created a little bit of a care vacuum. The Women’s Health Initiative (WHI) study came out in 2002, and after that, we really left women with few choices about what to take to manage their symptoms. That created a vacuum.
After that, clinicians decided they no longer needed to worry about being educated about menopause because there was really nothing to do for menopause if we weren’t going to use hormone therapy. Where we’ve come to now is women are having symptoms; they’re having a problem. It’s affecting all aspects of their lives: their relationships, their quality of life, their ability to work. And they’re saying, “Hey, this isn’t right. We need to do something about this.” There’s still very little research in this area. We have a lot more to do. They’re demanding answers, as they should.
Dr. Rubin: We have quite a lot of tools in our toolbox that are evidence based, that really work and help people. I always say to my patients, “You have a generation of clinicians who were not taught how to do this well. Hormones are not all good or all bad, all right or all wrong, but they require some understanding of when to use them and how to safely use them.” That way, you can avoid your patients going to the snake oil salesmen down the street selling non–evidence-based treatments.
One article that came out this year that I thought was really fascinating was about what we are calling NFLM: not feeling like myself. I will tell you, I think it’s brilliant because there is not a woman aged 40 or above who doesn’t deeply connect with the idea of NFLM. Can you speak to the symptoms of perimenopause and menopause beyond hot flashes and night sweats? I named a few sexual symptoms earlier. We’re really learning about all these new areas to understand, what is perimenopause?
Dr. Faubion: Very rarely does a woman come in and say, “I have hot flashes” and I say, “Well, is that all you have?” “Yep. That’s all I have. I just have a couple of hot flashes.” That almost never happens, as you know. Menopause is not just about hot flashes, although that’s one of the most common symptoms. Hot flashes also occur at night. We call them night sweats when that happens. But there’s the sleep disturbance, which is probably not just related to night sweats but a lot of other things as well. Mood symptoms can be crazy. A lot of women come in with descriptions of irritability, just not feeling right, or feeling anxious. Another common symptom that we’re learning about is joint aches.
It’s important to remember when we’re talking about these symptoms that estrogen affects every tissue and organ system in the body. And when you lose it, you have effects in pretty much every tissue and organ system in the body. So, it’s not just about hot flashes and night sweats. We’ve also learned recently that women in perimenopause can have the same symptoms that women have after menopause. It’s not just that it starts at menopause.
Dr. Rubin: This is really important because we are speaking to the primary care world. The way medicine is set up, you’re allowed to have one problem. If you have more than one problem, I don’t know what to do. You go in the crazy bucket of we’re not interested or we don’t have time to take care of you. But menopause is never one problem. So, the disaster here is that these women are getting diagnosed with a mental health condition, with fibromyalgia, with dry eye, with sexual dysfunction, with depression or anxiety. They’re getting 10 diagnoses for what is actually one underlying hypogonadal problem.
Dr. Faubion: That’s exactly right. I’ve seen a woman at the Mayo Clinic, who came to me as a general internist, not even knowing I did menopause. She traveled across the country to see me. She’s gaining weight, she’s losing her hair, she’s sweating. She thinks there is something horribly wrong with her, like she must have cancer or something. When you put it all together — the palpitations and the rest — it was all menopause. Think of the expense to come to the Mayo Clinic and be evaluated for that. But no one, including her, had put together the fact that all of these symptoms were related to menopause. You’re exactly right. Sometimes women don’t even recognize that it’s all related.
Dr. Rubin: For the primary care viewers, we were raised on the idea that hormones cause cancer. Can you speak to that? What are the data in 2024? Am I going to die if I take hormone therapy? Am I going to risk blood clots and horrible cancers?
Dr. Faubion: To be brief, we now know who the best candidates are for hormone therapy, and we can really minimize risk. We also know that there are differences between the formulations that we use, the route of delivery, and the dose. We can really individualize this for the woman.
When it comes down to cancer risk, the WHI found that if you have a uterus and you’re taking both an estrogen and a progestogen (specifically conjugated equine estrogen and medroxyprogesterone acetate), the risk for breast cancer was increased slightly. When I say “slightly,” I’m talking the same as the increase in breast cancer risk of drinking one to two glasses of wine a night, or being overweight, or being inactive. We are really talking about less than one case per thousand women per year after about 5 years of hormone therapy. So, it’s a very small increased risk.
In contrast, the data showed that the risk for breast cancer did not appear to be increased in women who did not have a uterus and were using conjugated equine estrogen alone, either during the study or in the 18-year follow-up. The blood clot risk associated with estrogen-containing hormone therapy can be minimized with transdermal preparations of estrogen, particularly with lower doses. Overall, we don’t see that these risks are prohibitive for most women, and if they are having bothersome symptoms, they can use an estrogen-containing product safely.
Dr. Rubin: We can learn new things, right? For example, the new GLP-1 drugs, which is also very fascinating — using those in perimenopause and menopause. A GLP-1 deficiency may be increased as you go to perimenopause and menopause. By adding back hormones, maybe we can help keep muscle around, keep mental health better, and keep bones stronger, because osteoporosis and fractures kill more people than breast cancer does.
So, as a primary care clinician, how do we learn to write prescriptions for hormone therapy? How do we learn how to counsel patients properly? Do we have to go back and take a fellowship? How do I learn how to integrate the evidence into my practice?
Dr. Faubion: An easy thing to do to gain confidence is take a course. The North American Menopause Society has an annual meeting in Chicago in September, and we do a Menopause 101 course for clinicians there. It’s also available online. There are ways to get this information in a digestible way to where you can learn the basics: Here’s where I start; here’s how I need to follow it up. It’s really not that difficult to get into this.
As to your point about the GLP-1 drugs, we all have to learn new things every day because treatments change, drugs change, etc. Although hormones have been out there for a long time, many clinicians haven’t had the experience of treating menopausal women. I would put a plea out to my primary care colleagues in internal medicine and family medicine that you need to be doing this. Think about it — you already are the expert on brain health and bone health and heart health. You should be the most comfortable in dealing with hormone therapy that has effects throughout the entire body. It’s important for us as primary care providers to really have a handle on this and to be the owners of managing menopause for women in midlife.
Dr. Rubin: I couldn’t agree more. As a sexual medicine doctor, treating menopausal women is actually what fuels my soul and stops all burnout because they get better. My clinic is full of a fifty-something-year-old people who come back and they say sex is good. “My relationship is good.” “I’m kicking butt at work.” I have a patient who just started law school because she feels good, and she says, “I’m keeping up with the 20-year-olds.” It is incredible to see people who feel terrible and then watch them blossom and get better. There’s nothing that fuels my soul more than these patients.
What is exciting you in the menopause world? What are you hopeful for down the road with some of these new initiatives coming out?
Dr. Faubion: The fact that we have a president of the United States and a National Institutes of Health who are more interested in looking at menopause is amazing. It’s an exciting time; there’s more interest, and more research funding seems to be available for the United States.
In terms of clinical management, we now have so many options available to women. We’ve been talking about hormone therapy, but we now have nonhormonal medications out there as well that are on the market, such as fezolinetant, a neurokinin 3 inhibitor that came out last year. There’s probably another one coming out in the next year or so. So, women have lots of options, and for the first time, we can really individualize treatment for women and look at what symptoms are bothering them, and how best to get them back to where they should be.
We’re also starting a menopause-in-the-workplace initiative with the Menopause Society and really kind of tackling that one. We know that a lot of women are missing work, not taking a promotion, or avoiding a leadership role because of their menopause symptoms. Women should never be in the position of compromising their work lives because of menopause symptoms. This is something we can help women with.
Dr. Rubin: Our big takeaway today is: Believe your patients when they come to you, and they’ve driven and parked and arranged childcare, and showed up to your office and waited to see you. When they’re telling you that they have all these symptoms and they’re not feeling like themselves, maybe before you jump straight to the SSRI or just say, “Do some yoga and deep breathing,” maybe really dive into the menopause literature and understand the pros and cons, and the risks and benefits of hormone therapy. We do it with so many other things. We can do it with hormone therapy as well. It is not a one-size-fits-all. We do need to talk to our patients, customize their care, and really figure out what they care about and what they want. Patients are able to understand risks and benefits and can make good decisions for themselves.
Dr. Rubin is an assistant clinical professor, Department of Urology, at Georgetown University, Washington, DC. She reported conflicts of interest with Sprout, Maternal Medical, Absorption Pharmaceuticals, GSK, and Endo.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Rachel S. Rubin, MD: As a sexual medicine specialist, I treat a lot of menopause. Why? Because menopausal complaints are not just hot flashes and night sweats; we see so many sexual health problems: genital urinary syndrome of menopause (GUSM), low libido, pain with sex, arousal disorders, orgasm disorders. I am joined today with a superstar in the menopause field, Dr. Stephanie Faubion. Introduce yourself to our amazing listeners.
Stephanie S. Faubion, MD, MBA: I am Stephanie Faubion, director of the Mayo Clinic Center for Women’s Health and medical director for the Menopause Society.
Dr. Rubin: That is a very short introduction for a very impressive person who really is an authority, if you’ve ever read an article about menopause. I asked Dr. Faubion if she spends all her time talking to reporters. But it’s very important because menopause is having a moment. We can’t go a day without seeing a headline, an Instagram story, or something; my feed is full of menopause information. Why do you think menopause is having a moment right now?
Dr. Faubion: It’s a well-deserved moment and should have happened a long time ago. It’s having a moment for several reasons. The generation of women experiencing perimenopause and menopause is different now; they are less willing to suffer in silence, which is a great thing. We’ve also created a little bit of a care vacuum. The Women’s Health Initiative (WHI) study came out in 2002, and after that, we really left women with few choices about what to take to manage their symptoms. That created a vacuum.
After that, clinicians decided they no longer needed to worry about being educated about menopause because there was really nothing to do for menopause if we weren’t going to use hormone therapy. Where we’ve come to now is women are having symptoms; they’re having a problem. It’s affecting all aspects of their lives: their relationships, their quality of life, their ability to work. And they’re saying, “Hey, this isn’t right. We need to do something about this.” There’s still very little research in this area. We have a lot more to do. They’re demanding answers, as they should.
Dr. Rubin: We have quite a lot of tools in our toolbox that are evidence based, that really work and help people. I always say to my patients, “You have a generation of clinicians who were not taught how to do this well. Hormones are not all good or all bad, all right or all wrong, but they require some understanding of when to use them and how to safely use them.” That way, you can avoid your patients going to the snake oil salesmen down the street selling non–evidence-based treatments.
One article that came out this year that I thought was really fascinating was about what we are calling NFLM: not feeling like myself. I will tell you, I think it’s brilliant because there is not a woman aged 40 or above who doesn’t deeply connect with the idea of NFLM. Can you speak to the symptoms of perimenopause and menopause beyond hot flashes and night sweats? I named a few sexual symptoms earlier. We’re really learning about all these new areas to understand, what is perimenopause?
Dr. Faubion: Very rarely does a woman come in and say, “I have hot flashes” and I say, “Well, is that all you have?” “Yep. That’s all I have. I just have a couple of hot flashes.” That almost never happens, as you know. Menopause is not just about hot flashes, although that’s one of the most common symptoms. Hot flashes also occur at night. We call them night sweats when that happens. But there’s the sleep disturbance, which is probably not just related to night sweats but a lot of other things as well. Mood symptoms can be crazy. A lot of women come in with descriptions of irritability, just not feeling right, or feeling anxious. Another common symptom that we’re learning about is joint aches.
It’s important to remember when we’re talking about these symptoms that estrogen affects every tissue and organ system in the body. And when you lose it, you have effects in pretty much every tissue and organ system in the body. So, it’s not just about hot flashes and night sweats. We’ve also learned recently that women in perimenopause can have the same symptoms that women have after menopause. It’s not just that it starts at menopause.
Dr. Rubin: This is really important because we are speaking to the primary care world. The way medicine is set up, you’re allowed to have one problem. If you have more than one problem, I don’t know what to do. You go in the crazy bucket of we’re not interested or we don’t have time to take care of you. But menopause is never one problem. So, the disaster here is that these women are getting diagnosed with a mental health condition, with fibromyalgia, with dry eye, with sexual dysfunction, with depression or anxiety. They’re getting 10 diagnoses for what is actually one underlying hypogonadal problem.
Dr. Faubion: That’s exactly right. I’ve seen a woman at the Mayo Clinic, who came to me as a general internist, not even knowing I did menopause. She traveled across the country to see me. She’s gaining weight, she’s losing her hair, she’s sweating. She thinks there is something horribly wrong with her, like she must have cancer or something. When you put it all together — the palpitations and the rest — it was all menopause. Think of the expense to come to the Mayo Clinic and be evaluated for that. But no one, including her, had put together the fact that all of these symptoms were related to menopause. You’re exactly right. Sometimes women don’t even recognize that it’s all related.
Dr. Rubin: For the primary care viewers, we were raised on the idea that hormones cause cancer. Can you speak to that? What are the data in 2024? Am I going to die if I take hormone therapy? Am I going to risk blood clots and horrible cancers?
Dr. Faubion: To be brief, we now know who the best candidates are for hormone therapy, and we can really minimize risk. We also know that there are differences between the formulations that we use, the route of delivery, and the dose. We can really individualize this for the woman.
When it comes down to cancer risk, the WHI found that if you have a uterus and you’re taking both an estrogen and a progestogen (specifically conjugated equine estrogen and medroxyprogesterone acetate), the risk for breast cancer was increased slightly. When I say “slightly,” I’m talking the same as the increase in breast cancer risk of drinking one to two glasses of wine a night, or being overweight, or being inactive. We are really talking about less than one case per thousand women per year after about 5 years of hormone therapy. So, it’s a very small increased risk.
In contrast, the data showed that the risk for breast cancer did not appear to be increased in women who did not have a uterus and were using conjugated equine estrogen alone, either during the study or in the 18-year follow-up. The blood clot risk associated with estrogen-containing hormone therapy can be minimized with transdermal preparations of estrogen, particularly with lower doses. Overall, we don’t see that these risks are prohibitive for most women, and if they are having bothersome symptoms, they can use an estrogen-containing product safely.
Dr. Rubin: We can learn new things, right? For example, the new GLP-1 drugs, which is also very fascinating — using those in perimenopause and menopause. A GLP-1 deficiency may be increased as you go to perimenopause and menopause. By adding back hormones, maybe we can help keep muscle around, keep mental health better, and keep bones stronger, because osteoporosis and fractures kill more people than breast cancer does.
So, as a primary care clinician, how do we learn to write prescriptions for hormone therapy? How do we learn how to counsel patients properly? Do we have to go back and take a fellowship? How do I learn how to integrate the evidence into my practice?
Dr. Faubion: An easy thing to do to gain confidence is take a course. The North American Menopause Society has an annual meeting in Chicago in September, and we do a Menopause 101 course for clinicians there. It’s also available online. There are ways to get this information in a digestible way to where you can learn the basics: Here’s where I start; here’s how I need to follow it up. It’s really not that difficult to get into this.
As to your point about the GLP-1 drugs, we all have to learn new things every day because treatments change, drugs change, etc. Although hormones have been out there for a long time, many clinicians haven’t had the experience of treating menopausal women. I would put a plea out to my primary care colleagues in internal medicine and family medicine that you need to be doing this. Think about it — you already are the expert on brain health and bone health and heart health. You should be the most comfortable in dealing with hormone therapy that has effects throughout the entire body. It’s important for us as primary care providers to really have a handle on this and to be the owners of managing menopause for women in midlife.
Dr. Rubin: I couldn’t agree more. As a sexual medicine doctor, treating menopausal women is actually what fuels my soul and stops all burnout because they get better. My clinic is full of a fifty-something-year-old people who come back and they say sex is good. “My relationship is good.” “I’m kicking butt at work.” I have a patient who just started law school because she feels good, and she says, “I’m keeping up with the 20-year-olds.” It is incredible to see people who feel terrible and then watch them blossom and get better. There’s nothing that fuels my soul more than these patients.
What is exciting you in the menopause world? What are you hopeful for down the road with some of these new initiatives coming out?
Dr. Faubion: The fact that we have a president of the United States and a National Institutes of Health who are more interested in looking at menopause is amazing. It’s an exciting time; there’s more interest, and more research funding seems to be available for the United States.
In terms of clinical management, we now have so many options available to women. We’ve been talking about hormone therapy, but we now have nonhormonal medications out there as well that are on the market, such as fezolinetant, a neurokinin 3 inhibitor that came out last year. There’s probably another one coming out in the next year or so. So, women have lots of options, and for the first time, we can really individualize treatment for women and look at what symptoms are bothering them, and how best to get them back to where they should be.
We’re also starting a menopause-in-the-workplace initiative with the Menopause Society and really kind of tackling that one. We know that a lot of women are missing work, not taking a promotion, or avoiding a leadership role because of their menopause symptoms. Women should never be in the position of compromising their work lives because of menopause symptoms. This is something we can help women with.
Dr. Rubin: Our big takeaway today is: Believe your patients when they come to you, and they’ve driven and parked and arranged childcare, and showed up to your office and waited to see you. When they’re telling you that they have all these symptoms and they’re not feeling like themselves, maybe before you jump straight to the SSRI or just say, “Do some yoga and deep breathing,” maybe really dive into the menopause literature and understand the pros and cons, and the risks and benefits of hormone therapy. We do it with so many other things. We can do it with hormone therapy as well. It is not a one-size-fits-all. We do need to talk to our patients, customize their care, and really figure out what they care about and what they want. Patients are able to understand risks and benefits and can make good decisions for themselves.
Dr. Rubin is an assistant clinical professor, Department of Urology, at Georgetown University, Washington, DC. She reported conflicts of interest with Sprout, Maternal Medical, Absorption Pharmaceuticals, GSK, and Endo.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Rachel S. Rubin, MD: As a sexual medicine specialist, I treat a lot of menopause. Why? Because menopausal complaints are not just hot flashes and night sweats; we see so many sexual health problems: genital urinary syndrome of menopause (GUSM), low libido, pain with sex, arousal disorders, orgasm disorders. I am joined today with a superstar in the menopause field, Dr. Stephanie Faubion. Introduce yourself to our amazing listeners.
Stephanie S. Faubion, MD, MBA: I am Stephanie Faubion, director of the Mayo Clinic Center for Women’s Health and medical director for the Menopause Society.
Dr. Rubin: That is a very short introduction for a very impressive person who really is an authority, if you’ve ever read an article about menopause. I asked Dr. Faubion if she spends all her time talking to reporters. But it’s very important because menopause is having a moment. We can’t go a day without seeing a headline, an Instagram story, or something; my feed is full of menopause information. Why do you think menopause is having a moment right now?
Dr. Faubion: It’s a well-deserved moment and should have happened a long time ago. It’s having a moment for several reasons. The generation of women experiencing perimenopause and menopause is different now; they are less willing to suffer in silence, which is a great thing. We’ve also created a little bit of a care vacuum. The Women’s Health Initiative (WHI) study came out in 2002, and after that, we really left women with few choices about what to take to manage their symptoms. That created a vacuum.
After that, clinicians decided they no longer needed to worry about being educated about menopause because there was really nothing to do for menopause if we weren’t going to use hormone therapy. Where we’ve come to now is women are having symptoms; they’re having a problem. It’s affecting all aspects of their lives: their relationships, their quality of life, their ability to work. And they’re saying, “Hey, this isn’t right. We need to do something about this.” There’s still very little research in this area. We have a lot more to do. They’re demanding answers, as they should.
Dr. Rubin: We have quite a lot of tools in our toolbox that are evidence based, that really work and help people. I always say to my patients, “You have a generation of clinicians who were not taught how to do this well. Hormones are not all good or all bad, all right or all wrong, but they require some understanding of when to use them and how to safely use them.” That way, you can avoid your patients going to the snake oil salesmen down the street selling non–evidence-based treatments.
One article that came out this year that I thought was really fascinating was about what we are calling NFLM: not feeling like myself. I will tell you, I think it’s brilliant because there is not a woman aged 40 or above who doesn’t deeply connect with the idea of NFLM. Can you speak to the symptoms of perimenopause and menopause beyond hot flashes and night sweats? I named a few sexual symptoms earlier. We’re really learning about all these new areas to understand, what is perimenopause?
Dr. Faubion: Very rarely does a woman come in and say, “I have hot flashes” and I say, “Well, is that all you have?” “Yep. That’s all I have. I just have a couple of hot flashes.” That almost never happens, as you know. Menopause is not just about hot flashes, although that’s one of the most common symptoms. Hot flashes also occur at night. We call them night sweats when that happens. But there’s the sleep disturbance, which is probably not just related to night sweats but a lot of other things as well. Mood symptoms can be crazy. A lot of women come in with descriptions of irritability, just not feeling right, or feeling anxious. Another common symptom that we’re learning about is joint aches.
It’s important to remember when we’re talking about these symptoms that estrogen affects every tissue and organ system in the body. And when you lose it, you have effects in pretty much every tissue and organ system in the body. So, it’s not just about hot flashes and night sweats. We’ve also learned recently that women in perimenopause can have the same symptoms that women have after menopause. It’s not just that it starts at menopause.
Dr. Rubin: This is really important because we are speaking to the primary care world. The way medicine is set up, you’re allowed to have one problem. If you have more than one problem, I don’t know what to do. You go in the crazy bucket of we’re not interested or we don’t have time to take care of you. But menopause is never one problem. So, the disaster here is that these women are getting diagnosed with a mental health condition, with fibromyalgia, with dry eye, with sexual dysfunction, with depression or anxiety. They’re getting 10 diagnoses for what is actually one underlying hypogonadal problem.
Dr. Faubion: That’s exactly right. I’ve seen a woman at the Mayo Clinic, who came to me as a general internist, not even knowing I did menopause. She traveled across the country to see me. She’s gaining weight, she’s losing her hair, she’s sweating. She thinks there is something horribly wrong with her, like she must have cancer or something. When you put it all together — the palpitations and the rest — it was all menopause. Think of the expense to come to the Mayo Clinic and be evaluated for that. But no one, including her, had put together the fact that all of these symptoms were related to menopause. You’re exactly right. Sometimes women don’t even recognize that it’s all related.
Dr. Rubin: For the primary care viewers, we were raised on the idea that hormones cause cancer. Can you speak to that? What are the data in 2024? Am I going to die if I take hormone therapy? Am I going to risk blood clots and horrible cancers?
Dr. Faubion: To be brief, we now know who the best candidates are for hormone therapy, and we can really minimize risk. We also know that there are differences between the formulations that we use, the route of delivery, and the dose. We can really individualize this for the woman.
When it comes down to cancer risk, the WHI found that if you have a uterus and you’re taking both an estrogen and a progestogen (specifically conjugated equine estrogen and medroxyprogesterone acetate), the risk for breast cancer was increased slightly. When I say “slightly,” I’m talking the same as the increase in breast cancer risk of drinking one to two glasses of wine a night, or being overweight, or being inactive. We are really talking about less than one case per thousand women per year after about 5 years of hormone therapy. So, it’s a very small increased risk.
In contrast, the data showed that the risk for breast cancer did not appear to be increased in women who did not have a uterus and were using conjugated equine estrogen alone, either during the study or in the 18-year follow-up. The blood clot risk associated with estrogen-containing hormone therapy can be minimized with transdermal preparations of estrogen, particularly with lower doses. Overall, we don’t see that these risks are prohibitive for most women, and if they are having bothersome symptoms, they can use an estrogen-containing product safely.
Dr. Rubin: We can learn new things, right? For example, the new GLP-1 drugs, which is also very fascinating — using those in perimenopause and menopause. A GLP-1 deficiency may be increased as you go to perimenopause and menopause. By adding back hormones, maybe we can help keep muscle around, keep mental health better, and keep bones stronger, because osteoporosis and fractures kill more people than breast cancer does.
So, as a primary care clinician, how do we learn to write prescriptions for hormone therapy? How do we learn how to counsel patients properly? Do we have to go back and take a fellowship? How do I learn how to integrate the evidence into my practice?
Dr. Faubion: An easy thing to do to gain confidence is take a course. The North American Menopause Society has an annual meeting in Chicago in September, and we do a Menopause 101 course for clinicians there. It’s also available online. There are ways to get this information in a digestible way to where you can learn the basics: Here’s where I start; here’s how I need to follow it up. It’s really not that difficult to get into this.
As to your point about the GLP-1 drugs, we all have to learn new things every day because treatments change, drugs change, etc. Although hormones have been out there for a long time, many clinicians haven’t had the experience of treating menopausal women. I would put a plea out to my primary care colleagues in internal medicine and family medicine that you need to be doing this. Think about it — you already are the expert on brain health and bone health and heart health. You should be the most comfortable in dealing with hormone therapy that has effects throughout the entire body. It’s important for us as primary care providers to really have a handle on this and to be the owners of managing menopause for women in midlife.
Dr. Rubin: I couldn’t agree more. As a sexual medicine doctor, treating menopausal women is actually what fuels my soul and stops all burnout because they get better. My clinic is full of a fifty-something-year-old people who come back and they say sex is good. “My relationship is good.” “I’m kicking butt at work.” I have a patient who just started law school because she feels good, and she says, “I’m keeping up with the 20-year-olds.” It is incredible to see people who feel terrible and then watch them blossom and get better. There’s nothing that fuels my soul more than these patients.
What is exciting you in the menopause world? What are you hopeful for down the road with some of these new initiatives coming out?
Dr. Faubion: The fact that we have a president of the United States and a National Institutes of Health who are more interested in looking at menopause is amazing. It’s an exciting time; there’s more interest, and more research funding seems to be available for the United States.
In terms of clinical management, we now have so many options available to women. We’ve been talking about hormone therapy, but we now have nonhormonal medications out there as well that are on the market, such as fezolinetant, a neurokinin 3 inhibitor that came out last year. There’s probably another one coming out in the next year or so. So, women have lots of options, and for the first time, we can really individualize treatment for women and look at what symptoms are bothering them, and how best to get them back to where they should be.
We’re also starting a menopause-in-the-workplace initiative with the Menopause Society and really kind of tackling that one. We know that a lot of women are missing work, not taking a promotion, or avoiding a leadership role because of their menopause symptoms. Women should never be in the position of compromising their work lives because of menopause symptoms. This is something we can help women with.
Dr. Rubin: Our big takeaway today is: Believe your patients when they come to you, and they’ve driven and parked and arranged childcare, and showed up to your office and waited to see you. When they’re telling you that they have all these symptoms and they’re not feeling like themselves, maybe before you jump straight to the SSRI or just say, “Do some yoga and deep breathing,” maybe really dive into the menopause literature and understand the pros and cons, and the risks and benefits of hormone therapy. We do it with so many other things. We can do it with hormone therapy as well. It is not a one-size-fits-all. We do need to talk to our patients, customize their care, and really figure out what they care about and what they want. Patients are able to understand risks and benefits and can make good decisions for themselves.
Dr. Rubin is an assistant clinical professor, Department of Urology, at Georgetown University, Washington, DC. She reported conflicts of interest with Sprout, Maternal Medical, Absorption Pharmaceuticals, GSK, and Endo.
A version of this article first appeared on Medscape.com.
Online Diagnosis of Sexually Transmitted Infections? Ethicist Says We Are Nowhere Close
This transcript has been edited for clarity.
There has been a large amount of news lately about dating online and dating apps. Probably the most common way younger people find potential partners is to go online and see who’s there that they might want to meet.
Online dating is also notorious for being full of scammers. There are all kinds of people out there that you have to be careful of, who are trying to rip you off by saying, “Send me money, I’m in trouble,” or “Now that we have a relationship, will you support my particular entrepreneurial idea?” Certainly, dangers are there.
Another danger we don’t talk much about is meeting people who have sexually transmitted diseases. That’s been a problem before websites and before dating apps. I think the opportunity of meeting more people — strangers, people you don’t really know — who may not tell you the truth about their health, and particularly their sexual health, is really out there.
It’s always good medical advice to tell people to practice safe sex, and that often involves a man wearing a condom. It certainly is the case that we want to attend not just to the prevention of unwanted pregnancy but also to the transmission of diseases. I think it’s very important to tell women of reproductive age to get their HPV shot to try to reduce cancers in their reproductive systems, or sometimes in men — anal cancers, or even being a transmitter of disease.
Even then, certainly one wants to recommend that, in an age where some people are going to meet many partners that they don’t know well or don’t have much background with, it’s wise to try to prevent diseases using the vaccines we’ve got, using the contraceptive methods that will prevent disease transmission, and reminding people to ask about sex life.
I did come across a website that just startled me. It’s called HeHealth, and basically it says to men, if you are conscientious about your sex life, take a picture of your penis, send it to us, and we have doctors — I presume they’re US doctors but I don’t know — who will diagnose venereal diseases based on that picture. I presume women could also say, “Before we have sex, or now that we’re approaching that possibility, I want you to send a picture to this company on this website.”
Now, a couple of reminders. I think we all know this, but just because you’re not manifesting symptoms on your reproductive organs doesn’t mean you don’t have a sexual disease. It’s not a reliable measure. Yes, maybe you could have somebody say: “Oh, that looks nasty. I’m not sure you ought to have sex right now, and maybe you should go get some treatment.” This is going to miss many cases and is not a reliable indicator that your partner is safe in terms of not transmitting diseases to you.
It also isn’t clear what they do with these images. Do they keep them? Who can see them? Could they resell them? What sort of privacy protection have you got if you decide to use this?
There’s another issue here, which is, if they misdiagnose someone and you do catch a sexual disease, who’s liable? Can you go after them for using doctors who weren’t competent or transmitting images that weren’t really adequate because you didn’t know how to take that picture properly when you sent that off to them? There are many unknowns.
The bottom line is that we’re in a different world, I think, of romance. We’re in a world where some people are going to meet more partners. Some people are going to meet more strangers. One approach is to have us take pictures of ourselves, send them off to who knows where, and ask for a green light to go ahead and have sexual relations. I don’t think we’re anywhere close to being able to rely on that as a way to avoid the risks of unprotected sexual behavior.
We do know what to do in dealing with patients who are sexually active. First, we have to ask them. Then we’ve got to recommend available vaccinations to prevent the transmission of some cancers, the HPV vaccine. Then they need that reminder about safe sexual practices not only to protect against unwanted pregnancy, but still, in this day and age, to protect against syphilis, which is on the rise, plus HIV, gonorrhea, chlamydia, and other sexually transmissible diseases.
I’m not going to rely on the penis picture to make the world safe for sex. I think we have to still use the old-fashioned techniques of education and prevention to do the best we can.
Dr. Caplan is director of the Division of Medical Ethics at New York University Langone Medical Center, New York City. He reported conflicts of interest with Johnson & Johnson’s Panel for Compassionate Drug Use and Medscape.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
There has been a large amount of news lately about dating online and dating apps. Probably the most common way younger people find potential partners is to go online and see who’s there that they might want to meet.
Online dating is also notorious for being full of scammers. There are all kinds of people out there that you have to be careful of, who are trying to rip you off by saying, “Send me money, I’m in trouble,” or “Now that we have a relationship, will you support my particular entrepreneurial idea?” Certainly, dangers are there.
Another danger we don’t talk much about is meeting people who have sexually transmitted diseases. That’s been a problem before websites and before dating apps. I think the opportunity of meeting more people — strangers, people you don’t really know — who may not tell you the truth about their health, and particularly their sexual health, is really out there.
It’s always good medical advice to tell people to practice safe sex, and that often involves a man wearing a condom. It certainly is the case that we want to attend not just to the prevention of unwanted pregnancy but also to the transmission of diseases. I think it’s very important to tell women of reproductive age to get their HPV shot to try to reduce cancers in their reproductive systems, or sometimes in men — anal cancers, or even being a transmitter of disease.
Even then, certainly one wants to recommend that, in an age where some people are going to meet many partners that they don’t know well or don’t have much background with, it’s wise to try to prevent diseases using the vaccines we’ve got, using the contraceptive methods that will prevent disease transmission, and reminding people to ask about sex life.
I did come across a website that just startled me. It’s called HeHealth, and basically it says to men, if you are conscientious about your sex life, take a picture of your penis, send it to us, and we have doctors — I presume they’re US doctors but I don’t know — who will diagnose venereal diseases based on that picture. I presume women could also say, “Before we have sex, or now that we’re approaching that possibility, I want you to send a picture to this company on this website.”
Now, a couple of reminders. I think we all know this, but just because you’re not manifesting symptoms on your reproductive organs doesn’t mean you don’t have a sexual disease. It’s not a reliable measure. Yes, maybe you could have somebody say: “Oh, that looks nasty. I’m not sure you ought to have sex right now, and maybe you should go get some treatment.” This is going to miss many cases and is not a reliable indicator that your partner is safe in terms of not transmitting diseases to you.
It also isn’t clear what they do with these images. Do they keep them? Who can see them? Could they resell them? What sort of privacy protection have you got if you decide to use this?
There’s another issue here, which is, if they misdiagnose someone and you do catch a sexual disease, who’s liable? Can you go after them for using doctors who weren’t competent or transmitting images that weren’t really adequate because you didn’t know how to take that picture properly when you sent that off to them? There are many unknowns.
The bottom line is that we’re in a different world, I think, of romance. We’re in a world where some people are going to meet more partners. Some people are going to meet more strangers. One approach is to have us take pictures of ourselves, send them off to who knows where, and ask for a green light to go ahead and have sexual relations. I don’t think we’re anywhere close to being able to rely on that as a way to avoid the risks of unprotected sexual behavior.
We do know what to do in dealing with patients who are sexually active. First, we have to ask them. Then we’ve got to recommend available vaccinations to prevent the transmission of some cancers, the HPV vaccine. Then they need that reminder about safe sexual practices not only to protect against unwanted pregnancy, but still, in this day and age, to protect against syphilis, which is on the rise, plus HIV, gonorrhea, chlamydia, and other sexually transmissible diseases.
I’m not going to rely on the penis picture to make the world safe for sex. I think we have to still use the old-fashioned techniques of education and prevention to do the best we can.
Dr. Caplan is director of the Division of Medical Ethics at New York University Langone Medical Center, New York City. He reported conflicts of interest with Johnson & Johnson’s Panel for Compassionate Drug Use and Medscape.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
There has been a large amount of news lately about dating online and dating apps. Probably the most common way younger people find potential partners is to go online and see who’s there that they might want to meet.
Online dating is also notorious for being full of scammers. There are all kinds of people out there that you have to be careful of, who are trying to rip you off by saying, “Send me money, I’m in trouble,” or “Now that we have a relationship, will you support my particular entrepreneurial idea?” Certainly, dangers are there.
Another danger we don’t talk much about is meeting people who have sexually transmitted diseases. That’s been a problem before websites and before dating apps. I think the opportunity of meeting more people — strangers, people you don’t really know — who may not tell you the truth about their health, and particularly their sexual health, is really out there.
It’s always good medical advice to tell people to practice safe sex, and that often involves a man wearing a condom. It certainly is the case that we want to attend not just to the prevention of unwanted pregnancy but also to the transmission of diseases. I think it’s very important to tell women of reproductive age to get their HPV shot to try to reduce cancers in their reproductive systems, or sometimes in men — anal cancers, or even being a transmitter of disease.
Even then, certainly one wants to recommend that, in an age where some people are going to meet many partners that they don’t know well or don’t have much background with, it’s wise to try to prevent diseases using the vaccines we’ve got, using the contraceptive methods that will prevent disease transmission, and reminding people to ask about sex life.
I did come across a website that just startled me. It’s called HeHealth, and basically it says to men, if you are conscientious about your sex life, take a picture of your penis, send it to us, and we have doctors — I presume they’re US doctors but I don’t know — who will diagnose venereal diseases based on that picture. I presume women could also say, “Before we have sex, or now that we’re approaching that possibility, I want you to send a picture to this company on this website.”
Now, a couple of reminders. I think we all know this, but just because you’re not manifesting symptoms on your reproductive organs doesn’t mean you don’t have a sexual disease. It’s not a reliable measure. Yes, maybe you could have somebody say: “Oh, that looks nasty. I’m not sure you ought to have sex right now, and maybe you should go get some treatment.” This is going to miss many cases and is not a reliable indicator that your partner is safe in terms of not transmitting diseases to you.
It also isn’t clear what they do with these images. Do they keep them? Who can see them? Could they resell them? What sort of privacy protection have you got if you decide to use this?
There’s another issue here, which is, if they misdiagnose someone and you do catch a sexual disease, who’s liable? Can you go after them for using doctors who weren’t competent or transmitting images that weren’t really adequate because you didn’t know how to take that picture properly when you sent that off to them? There are many unknowns.
The bottom line is that we’re in a different world, I think, of romance. We’re in a world where some people are going to meet more partners. Some people are going to meet more strangers. One approach is to have us take pictures of ourselves, send them off to who knows where, and ask for a green light to go ahead and have sexual relations. I don’t think we’re anywhere close to being able to rely on that as a way to avoid the risks of unprotected sexual behavior.
We do know what to do in dealing with patients who are sexually active. First, we have to ask them. Then we’ve got to recommend available vaccinations to prevent the transmission of some cancers, the HPV vaccine. Then they need that reminder about safe sexual practices not only to protect against unwanted pregnancy, but still, in this day and age, to protect against syphilis, which is on the rise, plus HIV, gonorrhea, chlamydia, and other sexually transmissible diseases.
I’m not going to rely on the penis picture to make the world safe for sex. I think we have to still use the old-fashioned techniques of education and prevention to do the best we can.
Dr. Caplan is director of the Division of Medical Ethics at New York University Langone Medical Center, New York City. He reported conflicts of interest with Johnson & Johnson’s Panel for Compassionate Drug Use and Medscape.
A version of this article first appeared on Medscape.com.
FDA Approves Adagrasib for KRAS G12C–Mutated CRC
More specifically, the highly selective and potent small-molecule KRAS G12C inhibitor is now indicated for patients with locally advanced or metastatic KRAS G12C–mutated CRC — as determined by an FDA-approved test — who previously received fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapy and, if eligible, a vascular endothelial growth factor inhibitor, according to an FDA press release.
The agent is the first KRAS inhibitor approved for CRC. Adagrasib was previously granted accelerated approval for KRAS G12C–mutated non–small cell lung cancer, based on findings from the KRYSTAL-12 trial.
The CRC approval was based on findings from the KRYSTAL-1 multicenter, single-arm expansion cohort trial, which reported an overall response rate of 34% among 94 enrolled patients.
All responses were partial responses, and the median duration of response was 5.8 months, with 31% of responding patients experiencing a duration of response of at least 6 months.
Patients received 600 mg of adagrasib twice daily plus cetuximab administered in either a biweekly 500 mg/m2 dose or an initial dose of 400 mg/m2 followed by weekly doses of 250 mg/m2. Those who discontinued adagrasib also had to discontinue cetuximab, but adagrasib could be continued if cetuximab was discontinued.
The recommended adagrasib dose is 600 mg given orally twice daily until disease progression or unacceptable toxicity, according to the prescribing information.
Adverse reactions occurring in at least 20% of treated patients included rash, nausea, diarrhea, vomiting, fatigue, musculoskeletal pain, hepatotoxicity, headache, dry skin, abdominal pain, decreased appetite, edema, anemia, cough, dizziness, constipation, and peripheral neuropathy.
“Patients with KRAS G12C–mutated colorectal cancer have historically faced poor prognoses and remain in need of additional treatment options,” Scott Kopetz, MD, PhD, of the University of Texas MD Anderson Cancer Center, Houston, stated earlier this year in a press release announcing the FDA’s decision to accept the drug application for priority review.
“Although KRAS had previously been considered ‘undruggable,’ these data from KRYSTAL-1 reinforce the potential benefit of adagrasib for these specific patients,” Dr. Kopetz said in the statement from Bristol Myers Squibb, which acquired Mirati Therapeutics in 2023.
A version of this article first appeared on Medscape.com.
More specifically, the highly selective and potent small-molecule KRAS G12C inhibitor is now indicated for patients with locally advanced or metastatic KRAS G12C–mutated CRC — as determined by an FDA-approved test — who previously received fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapy and, if eligible, a vascular endothelial growth factor inhibitor, according to an FDA press release.
The agent is the first KRAS inhibitor approved for CRC. Adagrasib was previously granted accelerated approval for KRAS G12C–mutated non–small cell lung cancer, based on findings from the KRYSTAL-12 trial.
The CRC approval was based on findings from the KRYSTAL-1 multicenter, single-arm expansion cohort trial, which reported an overall response rate of 34% among 94 enrolled patients.
All responses were partial responses, and the median duration of response was 5.8 months, with 31% of responding patients experiencing a duration of response of at least 6 months.
Patients received 600 mg of adagrasib twice daily plus cetuximab administered in either a biweekly 500 mg/m2 dose or an initial dose of 400 mg/m2 followed by weekly doses of 250 mg/m2. Those who discontinued adagrasib also had to discontinue cetuximab, but adagrasib could be continued if cetuximab was discontinued.
The recommended adagrasib dose is 600 mg given orally twice daily until disease progression or unacceptable toxicity, according to the prescribing information.
Adverse reactions occurring in at least 20% of treated patients included rash, nausea, diarrhea, vomiting, fatigue, musculoskeletal pain, hepatotoxicity, headache, dry skin, abdominal pain, decreased appetite, edema, anemia, cough, dizziness, constipation, and peripheral neuropathy.
“Patients with KRAS G12C–mutated colorectal cancer have historically faced poor prognoses and remain in need of additional treatment options,” Scott Kopetz, MD, PhD, of the University of Texas MD Anderson Cancer Center, Houston, stated earlier this year in a press release announcing the FDA’s decision to accept the drug application for priority review.
“Although KRAS had previously been considered ‘undruggable,’ these data from KRYSTAL-1 reinforce the potential benefit of adagrasib for these specific patients,” Dr. Kopetz said in the statement from Bristol Myers Squibb, which acquired Mirati Therapeutics in 2023.
A version of this article first appeared on Medscape.com.
More specifically, the highly selective and potent small-molecule KRAS G12C inhibitor is now indicated for patients with locally advanced or metastatic KRAS G12C–mutated CRC — as determined by an FDA-approved test — who previously received fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapy and, if eligible, a vascular endothelial growth factor inhibitor, according to an FDA press release.
The agent is the first KRAS inhibitor approved for CRC. Adagrasib was previously granted accelerated approval for KRAS G12C–mutated non–small cell lung cancer, based on findings from the KRYSTAL-12 trial.
The CRC approval was based on findings from the KRYSTAL-1 multicenter, single-arm expansion cohort trial, which reported an overall response rate of 34% among 94 enrolled patients.
All responses were partial responses, and the median duration of response was 5.8 months, with 31% of responding patients experiencing a duration of response of at least 6 months.
Patients received 600 mg of adagrasib twice daily plus cetuximab administered in either a biweekly 500 mg/m2 dose or an initial dose of 400 mg/m2 followed by weekly doses of 250 mg/m2. Those who discontinued adagrasib also had to discontinue cetuximab, but adagrasib could be continued if cetuximab was discontinued.
The recommended adagrasib dose is 600 mg given orally twice daily until disease progression or unacceptable toxicity, according to the prescribing information.
Adverse reactions occurring in at least 20% of treated patients included rash, nausea, diarrhea, vomiting, fatigue, musculoskeletal pain, hepatotoxicity, headache, dry skin, abdominal pain, decreased appetite, edema, anemia, cough, dizziness, constipation, and peripheral neuropathy.
“Patients with KRAS G12C–mutated colorectal cancer have historically faced poor prognoses and remain in need of additional treatment options,” Scott Kopetz, MD, PhD, of the University of Texas MD Anderson Cancer Center, Houston, stated earlier this year in a press release announcing the FDA’s decision to accept the drug application for priority review.
“Although KRAS had previously been considered ‘undruggable,’ these data from KRYSTAL-1 reinforce the potential benefit of adagrasib for these specific patients,” Dr. Kopetz said in the statement from Bristol Myers Squibb, which acquired Mirati Therapeutics in 2023.
A version of this article first appeared on Medscape.com.
FDA Approves Topical Anticholinergic for Axillary Hyperhidrosis
The .
According to a press release from Botanix Pharmaceuticals, which developed the product and will market it under the brand name Sofdra, approval was based on results from two phase 3 studies that enrolled 710 patients with primary axillary hyperhidrosis. In the trials, patients treated with sofpironium topical gel, 12.45%, experienced “clinically and statistically meaningful changes” from baseline in the Gravimetric Sweat Production and the Hyperhidrosis Disease Severity Measure–Axillary seven-item score, according to the company.
Botanix plans to enable qualified patients to gain early access to the product in the third quarter of 2024, with commercial sales expected in the fourth quarter of 2024.
A version of this article first appeared on Medscape.com.
The .
According to a press release from Botanix Pharmaceuticals, which developed the product and will market it under the brand name Sofdra, approval was based on results from two phase 3 studies that enrolled 710 patients with primary axillary hyperhidrosis. In the trials, patients treated with sofpironium topical gel, 12.45%, experienced “clinically and statistically meaningful changes” from baseline in the Gravimetric Sweat Production and the Hyperhidrosis Disease Severity Measure–Axillary seven-item score, according to the company.
Botanix plans to enable qualified patients to gain early access to the product in the third quarter of 2024, with commercial sales expected in the fourth quarter of 2024.
A version of this article first appeared on Medscape.com.
The .
According to a press release from Botanix Pharmaceuticals, which developed the product and will market it under the brand name Sofdra, approval was based on results from two phase 3 studies that enrolled 710 patients with primary axillary hyperhidrosis. In the trials, patients treated with sofpironium topical gel, 12.45%, experienced “clinically and statistically meaningful changes” from baseline in the Gravimetric Sweat Production and the Hyperhidrosis Disease Severity Measure–Axillary seven-item score, according to the company.
Botanix plans to enable qualified patients to gain early access to the product in the third quarter of 2024, with commercial sales expected in the fourth quarter of 2024.
A version of this article first appeared on Medscape.com.
Should ctDNA guide clinical decisions in GI cancers?
CHICAGO – Circulating tumor DNA (ctDNA), or DNA shed from tumors that is detected in the bloodstream, has shown increasing promise as a prognostic tool in gastrointestinal cancers, allowing investigators to make real-time assessments of treatment response and the likelihood of recurrence.
Depending on the type of assay and analysis used, ctDNA can provide a wealth of information about cancer genetic variants. ctDNA assays can be used for primary screening, to track tumor burden, or to detect minimal residual disease (MRD) after cancer surgery.
However, ctDNA’s role in guiding clinical decisions is still being defined.
The same group also presented exploratory findings showing that positive ctDNA is a significant predictor of recurrence in people with early-stage pancreatic cancer following surgery. However, the investigators concluded, ctDNA status should not be used to inform treatment decisions concerning duration of adjuvant chemotherapy in these patients.
DYNAMIC Trial Results
Jeanne Tie, MD, of the Peter MacCallum Cancer Centre in Melbourne, presented 5-year survival results at ASCO from the DYNAMIC randomized controlled trial, whose 2-year findings had already shown ctDNA to be helpful in stratifying stage II colon cancer patients for adjuvant chemotherapy or no treatment.
Because surgery is curative in 80% of these patients, it is important to identify the minority that will need chemotherapy, Dr. Tie said.
At 5 years’ follow-up, Dr. Tie reported, patients randomized to a ctDNA-guided approach (negative ctDNA post surgery resulted in no treatment, and positive ctDNA led to adjuvant chemotherapy) did not see differences in overall survival compared with conventionally managed patients, who received chemotherapy at the clinician’s discretion.
Among ctDNA-guided patients in the study (n = 302), 5-year overall survival was 93.8%. For conventionally managed patients (n = 153), overall survival was 93.3% at 5 years (hazard ratio [HR], 1.05; 95% CI, 0.47-2.37; P = .887).
Further, the researchers found that a high ctDNA clearance rate was achieved with adjuvant chemotherapy in postoperative patients who were ctDNA positive. And 5-year recurrence rates were markedly lower in patients who achieved ctDNA clearance, compared with those who did not: 85.2% vs 20% (HR, 15.4; 95% CI, 3.91-61.0; P < .001).
“This approach of only treating patients with a positive ctDNA achieved excellent survival outcomes, including in patients with T4 disease. A high ctDNA clearance rate can be achieved with adjuvant chemotherapy, and this in turn was associated with favorable outcomes,” Dr. Tie said during the meeting. “And finally, the precision of the ctDNA approach may be further refined by increasing [the number of genetic variants] tracked and by incorporating ctDNA molecular burden. However, these findings will require further validation.”
DYNAMIC-Pancreas Study Results
In a separate presentation during the same session, Belinda Lee, MD, also of the Peter MacCallum Cancer Centre, showed results from the DYNAMIC-Pancreas study, which looked at ctDNA testing a median 5 weeks after surgery in 102 people with early-stage (Eastern Cooperative Oncology Group 0-1) pancreatic cancer. Patients who were ctDNA positive received 6 months of adjuvant chemotherapy of the physician’s choice (FOLFIRINOX or gemcitabine/capecitabine) while those who were ctDNA negative after surgery had the option to de-escalate to 3 months of chemotherapy treatment at the physician’s discretion.
At a median 3 years’ follow-up, Dr. Lee and colleagues found that the median recurrence-free survival was 13 months for patients who were ctDNA positive after surgery and 22 months for those who were ctDNA negative (HR, 0.52; P = .003), showing that positive ctDNA is prognostic of earlier recurrence independent of other factors.
Dr. Lee said that, given the high recurrence risk also seen in ctDNA-negative patients, reducing duration of chemotherapy was not recommended based on ctDNA-negative status.
In an interview, Stacey Cohen, MD, of Fred Hutch Cancer Center in Seattle, Washington, the discussant on the two presentations at ASCO, said that, until these results are further validated in stage II colon cancer patients,t it is unlikely that they will change clinical practice guidelines.
“They did an amazing job,” Dr. Cohen said of the researchers. “They’re at the forefront of the field of actually doing prospective analysis. And yet there are still some gaps that are missing in our understanding.”
The assays used in both studies, Dr. Cohen noted, are used only in research and are not available commercially in the United States. That, plus the fact that physicians were allowed to choose between chemotherapy regimens, made it harder to parse the results.
“Provider choice increases bias,” Dr. Cohen said. “And I think that’s the problem of having two chemo regimens to choose from, or in the case of the colon cancer trial, not selecting whether patients got a single chemotherapy agent or a doublet. These are pretty big differences.”
But the field is moving quickly, “and it is an exciting time to improve patient selection for chemotherapy treatment,” she continued.
Allowing physicians to choose chemotherapy regimens reflected real-world clinical practice, “especially given that this study is designed to test a strategy rather than a specific treatment, said Dr. Tie in an interview. “More work will need to be done to specifically address the question of which chemotherapy regimen is more effective to treat ctDNA-positive disease.”
Dr. Cohen noted that, while evidence is mounting to support the value of ctDNA in colon cancer, there is far less evidence for pancreatic cancer.
Dr. Lee and colleagues’ study “adds to the literature, and I think what it teaches us is that ctDNA remains a prognostic risk factor,” she said. “But we saw that even patients who are negative have a high recurrence risk. So we’re not ready to act on it yet. As with the colon cancer study, different chemotherapy regimens were used, and for different time lengths.”
Whether in colon cancer or pancreatic cancer, ctDNA results, “are highly tied to which assay you’re using and which scenario you’re testing them in,” Dr. Cohen said.
Dr. Tie and colleagues’ study was sponsored by her institution, with additional funding received from the Australian government, the National Institutes of Health, and other foundations. She disclosed speaking and/or consulting fees from Haystack Oncology, Amgen, Novartis, Bristol-Myers Squibb, Merck, AstraZeneca, and others. Dr. Lee’s study was sponsored by the Marcus Foundation. She disclosed receiving honoraria from Roche. Dr. Cohen reported no conflicts of interest.
CHICAGO – Circulating tumor DNA (ctDNA), or DNA shed from tumors that is detected in the bloodstream, has shown increasing promise as a prognostic tool in gastrointestinal cancers, allowing investigators to make real-time assessments of treatment response and the likelihood of recurrence.
Depending on the type of assay and analysis used, ctDNA can provide a wealth of information about cancer genetic variants. ctDNA assays can be used for primary screening, to track tumor burden, or to detect minimal residual disease (MRD) after cancer surgery.
However, ctDNA’s role in guiding clinical decisions is still being defined.
The same group also presented exploratory findings showing that positive ctDNA is a significant predictor of recurrence in people with early-stage pancreatic cancer following surgery. However, the investigators concluded, ctDNA status should not be used to inform treatment decisions concerning duration of adjuvant chemotherapy in these patients.
DYNAMIC Trial Results
Jeanne Tie, MD, of the Peter MacCallum Cancer Centre in Melbourne, presented 5-year survival results at ASCO from the DYNAMIC randomized controlled trial, whose 2-year findings had already shown ctDNA to be helpful in stratifying stage II colon cancer patients for adjuvant chemotherapy or no treatment.
Because surgery is curative in 80% of these patients, it is important to identify the minority that will need chemotherapy, Dr. Tie said.
At 5 years’ follow-up, Dr. Tie reported, patients randomized to a ctDNA-guided approach (negative ctDNA post surgery resulted in no treatment, and positive ctDNA led to adjuvant chemotherapy) did not see differences in overall survival compared with conventionally managed patients, who received chemotherapy at the clinician’s discretion.
Among ctDNA-guided patients in the study (n = 302), 5-year overall survival was 93.8%. For conventionally managed patients (n = 153), overall survival was 93.3% at 5 years (hazard ratio [HR], 1.05; 95% CI, 0.47-2.37; P = .887).
Further, the researchers found that a high ctDNA clearance rate was achieved with adjuvant chemotherapy in postoperative patients who were ctDNA positive. And 5-year recurrence rates were markedly lower in patients who achieved ctDNA clearance, compared with those who did not: 85.2% vs 20% (HR, 15.4; 95% CI, 3.91-61.0; P < .001).
“This approach of only treating patients with a positive ctDNA achieved excellent survival outcomes, including in patients with T4 disease. A high ctDNA clearance rate can be achieved with adjuvant chemotherapy, and this in turn was associated with favorable outcomes,” Dr. Tie said during the meeting. “And finally, the precision of the ctDNA approach may be further refined by increasing [the number of genetic variants] tracked and by incorporating ctDNA molecular burden. However, these findings will require further validation.”
DYNAMIC-Pancreas Study Results
In a separate presentation during the same session, Belinda Lee, MD, also of the Peter MacCallum Cancer Centre, showed results from the DYNAMIC-Pancreas study, which looked at ctDNA testing a median 5 weeks after surgery in 102 people with early-stage (Eastern Cooperative Oncology Group 0-1) pancreatic cancer. Patients who were ctDNA positive received 6 months of adjuvant chemotherapy of the physician’s choice (FOLFIRINOX or gemcitabine/capecitabine) while those who were ctDNA negative after surgery had the option to de-escalate to 3 months of chemotherapy treatment at the physician’s discretion.
At a median 3 years’ follow-up, Dr. Lee and colleagues found that the median recurrence-free survival was 13 months for patients who were ctDNA positive after surgery and 22 months for those who were ctDNA negative (HR, 0.52; P = .003), showing that positive ctDNA is prognostic of earlier recurrence independent of other factors.
Dr. Lee said that, given the high recurrence risk also seen in ctDNA-negative patients, reducing duration of chemotherapy was not recommended based on ctDNA-negative status.
In an interview, Stacey Cohen, MD, of Fred Hutch Cancer Center in Seattle, Washington, the discussant on the two presentations at ASCO, said that, until these results are further validated in stage II colon cancer patients,t it is unlikely that they will change clinical practice guidelines.
“They did an amazing job,” Dr. Cohen said of the researchers. “They’re at the forefront of the field of actually doing prospective analysis. And yet there are still some gaps that are missing in our understanding.”
The assays used in both studies, Dr. Cohen noted, are used only in research and are not available commercially in the United States. That, plus the fact that physicians were allowed to choose between chemotherapy regimens, made it harder to parse the results.
“Provider choice increases bias,” Dr. Cohen said. “And I think that’s the problem of having two chemo regimens to choose from, or in the case of the colon cancer trial, not selecting whether patients got a single chemotherapy agent or a doublet. These are pretty big differences.”
But the field is moving quickly, “and it is an exciting time to improve patient selection for chemotherapy treatment,” she continued.
Allowing physicians to choose chemotherapy regimens reflected real-world clinical practice, “especially given that this study is designed to test a strategy rather than a specific treatment, said Dr. Tie in an interview. “More work will need to be done to specifically address the question of which chemotherapy regimen is more effective to treat ctDNA-positive disease.”
Dr. Cohen noted that, while evidence is mounting to support the value of ctDNA in colon cancer, there is far less evidence for pancreatic cancer.
Dr. Lee and colleagues’ study “adds to the literature, and I think what it teaches us is that ctDNA remains a prognostic risk factor,” she said. “But we saw that even patients who are negative have a high recurrence risk. So we’re not ready to act on it yet. As with the colon cancer study, different chemotherapy regimens were used, and for different time lengths.”
Whether in colon cancer or pancreatic cancer, ctDNA results, “are highly tied to which assay you’re using and which scenario you’re testing them in,” Dr. Cohen said.
Dr. Tie and colleagues’ study was sponsored by her institution, with additional funding received from the Australian government, the National Institutes of Health, and other foundations. She disclosed speaking and/or consulting fees from Haystack Oncology, Amgen, Novartis, Bristol-Myers Squibb, Merck, AstraZeneca, and others. Dr. Lee’s study was sponsored by the Marcus Foundation. She disclosed receiving honoraria from Roche. Dr. Cohen reported no conflicts of interest.
CHICAGO – Circulating tumor DNA (ctDNA), or DNA shed from tumors that is detected in the bloodstream, has shown increasing promise as a prognostic tool in gastrointestinal cancers, allowing investigators to make real-time assessments of treatment response and the likelihood of recurrence.
Depending on the type of assay and analysis used, ctDNA can provide a wealth of information about cancer genetic variants. ctDNA assays can be used for primary screening, to track tumor burden, or to detect minimal residual disease (MRD) after cancer surgery.
However, ctDNA’s role in guiding clinical decisions is still being defined.
The same group also presented exploratory findings showing that positive ctDNA is a significant predictor of recurrence in people with early-stage pancreatic cancer following surgery. However, the investigators concluded, ctDNA status should not be used to inform treatment decisions concerning duration of adjuvant chemotherapy in these patients.
DYNAMIC Trial Results
Jeanne Tie, MD, of the Peter MacCallum Cancer Centre in Melbourne, presented 5-year survival results at ASCO from the DYNAMIC randomized controlled trial, whose 2-year findings had already shown ctDNA to be helpful in stratifying stage II colon cancer patients for adjuvant chemotherapy or no treatment.
Because surgery is curative in 80% of these patients, it is important to identify the minority that will need chemotherapy, Dr. Tie said.
At 5 years’ follow-up, Dr. Tie reported, patients randomized to a ctDNA-guided approach (negative ctDNA post surgery resulted in no treatment, and positive ctDNA led to adjuvant chemotherapy) did not see differences in overall survival compared with conventionally managed patients, who received chemotherapy at the clinician’s discretion.
Among ctDNA-guided patients in the study (n = 302), 5-year overall survival was 93.8%. For conventionally managed patients (n = 153), overall survival was 93.3% at 5 years (hazard ratio [HR], 1.05; 95% CI, 0.47-2.37; P = .887).
Further, the researchers found that a high ctDNA clearance rate was achieved with adjuvant chemotherapy in postoperative patients who were ctDNA positive. And 5-year recurrence rates were markedly lower in patients who achieved ctDNA clearance, compared with those who did not: 85.2% vs 20% (HR, 15.4; 95% CI, 3.91-61.0; P < .001).
“This approach of only treating patients with a positive ctDNA achieved excellent survival outcomes, including in patients with T4 disease. A high ctDNA clearance rate can be achieved with adjuvant chemotherapy, and this in turn was associated with favorable outcomes,” Dr. Tie said during the meeting. “And finally, the precision of the ctDNA approach may be further refined by increasing [the number of genetic variants] tracked and by incorporating ctDNA molecular burden. However, these findings will require further validation.”
DYNAMIC-Pancreas Study Results
In a separate presentation during the same session, Belinda Lee, MD, also of the Peter MacCallum Cancer Centre, showed results from the DYNAMIC-Pancreas study, which looked at ctDNA testing a median 5 weeks after surgery in 102 people with early-stage (Eastern Cooperative Oncology Group 0-1) pancreatic cancer. Patients who were ctDNA positive received 6 months of adjuvant chemotherapy of the physician’s choice (FOLFIRINOX or gemcitabine/capecitabine) while those who were ctDNA negative after surgery had the option to de-escalate to 3 months of chemotherapy treatment at the physician’s discretion.
At a median 3 years’ follow-up, Dr. Lee and colleagues found that the median recurrence-free survival was 13 months for patients who were ctDNA positive after surgery and 22 months for those who were ctDNA negative (HR, 0.52; P = .003), showing that positive ctDNA is prognostic of earlier recurrence independent of other factors.
Dr. Lee said that, given the high recurrence risk also seen in ctDNA-negative patients, reducing duration of chemotherapy was not recommended based on ctDNA-negative status.
In an interview, Stacey Cohen, MD, of Fred Hutch Cancer Center in Seattle, Washington, the discussant on the two presentations at ASCO, said that, until these results are further validated in stage II colon cancer patients,t it is unlikely that they will change clinical practice guidelines.
“They did an amazing job,” Dr. Cohen said of the researchers. “They’re at the forefront of the field of actually doing prospective analysis. And yet there are still some gaps that are missing in our understanding.”
The assays used in both studies, Dr. Cohen noted, are used only in research and are not available commercially in the United States. That, plus the fact that physicians were allowed to choose between chemotherapy regimens, made it harder to parse the results.
“Provider choice increases bias,” Dr. Cohen said. “And I think that’s the problem of having two chemo regimens to choose from, or in the case of the colon cancer trial, not selecting whether patients got a single chemotherapy agent or a doublet. These are pretty big differences.”
But the field is moving quickly, “and it is an exciting time to improve patient selection for chemotherapy treatment,” she continued.
Allowing physicians to choose chemotherapy regimens reflected real-world clinical practice, “especially given that this study is designed to test a strategy rather than a specific treatment, said Dr. Tie in an interview. “More work will need to be done to specifically address the question of which chemotherapy regimen is more effective to treat ctDNA-positive disease.”
Dr. Cohen noted that, while evidence is mounting to support the value of ctDNA in colon cancer, there is far less evidence for pancreatic cancer.
Dr. Lee and colleagues’ study “adds to the literature, and I think what it teaches us is that ctDNA remains a prognostic risk factor,” she said. “But we saw that even patients who are negative have a high recurrence risk. So we’re not ready to act on it yet. As with the colon cancer study, different chemotherapy regimens were used, and for different time lengths.”
Whether in colon cancer or pancreatic cancer, ctDNA results, “are highly tied to which assay you’re using and which scenario you’re testing them in,” Dr. Cohen said.
Dr. Tie and colleagues’ study was sponsored by her institution, with additional funding received from the Australian government, the National Institutes of Health, and other foundations. She disclosed speaking and/or consulting fees from Haystack Oncology, Amgen, Novartis, Bristol-Myers Squibb, Merck, AstraZeneca, and others. Dr. Lee’s study was sponsored by the Marcus Foundation. She disclosed receiving honoraria from Roche. Dr. Cohen reported no conflicts of interest.
FROM ASCO 2024