User login
Knee pain on walking
Overall, persons with schizophrenia are more likely than the general population to be overweight and have cardiovascular risk factors before starting treatment with antipsychotics, and such treatment generally worsens these measures. Weight gain and associated morbidity and mortality are common side effects of antipsychotic medications. Olanzapine is associated with significant weight gain of 7% or more, higher than other second-generation antipsychotics. Olanzapine treatment is the major contributor to this patient's additional weight gain over the past 2 years. This added weight has translated to excess wear and tear on her joints, leading to evidence of osteoarthritis. Treatment with olanzapine is also independently associated with detrimental changes in cardiometabolic parameters.
Interventions to prevent or mitigate weight gain with antipsychotics are limited. In general, the American Psychiatric Association does not recommend switching antipsychotics for patients whose schizophrenia is well managed. However, there is increasing evidence that metformin may have a role in mitigating weight gain as well as beneficially modifying cardiometabolic factors in patients with schizophrenia being treated with olanzapine. A systematic review of emerging evidence with metformin in patients with schizophrenia suggests that metformin may also improve some cognitive symptoms of the illness, although further research is needed. The randomized, double-blind MELIA trial of metformin plus lifestyle intervention in antipsychotic-induced weight gain is ongoing. Starting metformin as a preventive measure at the same time as antipsychotic therapy may help to limit excess weight gain.
Research continues on the potential benefit of adding weight loss medications, including glucagon-like peptide-1 (GLP-1) receptor agonists, to antipsychotics. Daily liraglutide is most widely studied, but a published case series with weekly semaglutide also demonstrated weight loss in this setting. Liraglutide also has shown beneficial cardiometabolic effects in patients using antipsychotic medications. More studies of these drugs and of GLP-1/glucose-dependent insulinotropic polypeptide agonists are needed to elucidate the optimal use of these therapies for patients with schizophrenia.
There are few other effective ways to mitigate weight gain with olanzapine. Patients should be counseled on nutrition and lifestyle modifications. Evidence supports improvement with structured lifestyle modifications across a range of patients with less severe mental health issues, and structured programs combined with motivational interviewing were associated with reductions in antipsychotic-induced weight gain in patients with severe mental illness. As with any patient with obesity, however, the success of lifestyle modifications is heavily dependent on the individual's ability and motivation to comply with recommended interventions.
Nonpharmacologic interventions to address joint pain include heat or cold compresses, physical therapy, and strength and resistance training to improve the strength of muscles supporting the joints. If these measures are ineffective, nonsteroidal anti-inflammatory drugs (NSAIDs), including ibuprofen, naproxen, meloxicam, diclofenac, or celecoxib may be used with regular follow-up to assess cardiovascular and gastrointestinal health. Topical NSAIDs also may be useful. For more intractable joint pain, options include injecting a corticosteroid or sodium hyaluronate into the affected joints or joint replacement.
Carolyn Newberry, MD, Assistant Professor of Medicine, Director of GI Nutrition, Innovative Center for Health and Nutrition in Gastroenterology (ICHANGE), Division of Gastroenterology, Weill Cornell Medical Center, New York, NY.
Disclosure: Carolyn Newberry, MD, has disclosed the following relevant financial relationships:
Serve(d) as a speaker or a member of a speakers bureau for: Baster International; InBody.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Overall, persons with schizophrenia are more likely than the general population to be overweight and have cardiovascular risk factors before starting treatment with antipsychotics, and such treatment generally worsens these measures. Weight gain and associated morbidity and mortality are common side effects of antipsychotic medications. Olanzapine is associated with significant weight gain of 7% or more, higher than other second-generation antipsychotics. Olanzapine treatment is the major contributor to this patient's additional weight gain over the past 2 years. This added weight has translated to excess wear and tear on her joints, leading to evidence of osteoarthritis. Treatment with olanzapine is also independently associated with detrimental changes in cardiometabolic parameters.
Interventions to prevent or mitigate weight gain with antipsychotics are limited. In general, the American Psychiatric Association does not recommend switching antipsychotics for patients whose schizophrenia is well managed. However, there is increasing evidence that metformin may have a role in mitigating weight gain as well as beneficially modifying cardiometabolic factors in patients with schizophrenia being treated with olanzapine. A systematic review of emerging evidence with metformin in patients with schizophrenia suggests that metformin may also improve some cognitive symptoms of the illness, although further research is needed. The randomized, double-blind MELIA trial of metformin plus lifestyle intervention in antipsychotic-induced weight gain is ongoing. Starting metformin as a preventive measure at the same time as antipsychotic therapy may help to limit excess weight gain.
Research continues on the potential benefit of adding weight loss medications, including glucagon-like peptide-1 (GLP-1) receptor agonists, to antipsychotics. Daily liraglutide is most widely studied, but a published case series with weekly semaglutide also demonstrated weight loss in this setting. Liraglutide also has shown beneficial cardiometabolic effects in patients using antipsychotic medications. More studies of these drugs and of GLP-1/glucose-dependent insulinotropic polypeptide agonists are needed to elucidate the optimal use of these therapies for patients with schizophrenia.
There are few other effective ways to mitigate weight gain with olanzapine. Patients should be counseled on nutrition and lifestyle modifications. Evidence supports improvement with structured lifestyle modifications across a range of patients with less severe mental health issues, and structured programs combined with motivational interviewing were associated with reductions in antipsychotic-induced weight gain in patients with severe mental illness. As with any patient with obesity, however, the success of lifestyle modifications is heavily dependent on the individual's ability and motivation to comply with recommended interventions.
Nonpharmacologic interventions to address joint pain include heat or cold compresses, physical therapy, and strength and resistance training to improve the strength of muscles supporting the joints. If these measures are ineffective, nonsteroidal anti-inflammatory drugs (NSAIDs), including ibuprofen, naproxen, meloxicam, diclofenac, or celecoxib may be used with regular follow-up to assess cardiovascular and gastrointestinal health. Topical NSAIDs also may be useful. For more intractable joint pain, options include injecting a corticosteroid or sodium hyaluronate into the affected joints or joint replacement.
Carolyn Newberry, MD, Assistant Professor of Medicine, Director of GI Nutrition, Innovative Center for Health and Nutrition in Gastroenterology (ICHANGE), Division of Gastroenterology, Weill Cornell Medical Center, New York, NY.
Disclosure: Carolyn Newberry, MD, has disclosed the following relevant financial relationships:
Serve(d) as a speaker or a member of a speakers bureau for: Baster International; InBody.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Overall, persons with schizophrenia are more likely than the general population to be overweight and have cardiovascular risk factors before starting treatment with antipsychotics, and such treatment generally worsens these measures. Weight gain and associated morbidity and mortality are common side effects of antipsychotic medications. Olanzapine is associated with significant weight gain of 7% or more, higher than other second-generation antipsychotics. Olanzapine treatment is the major contributor to this patient's additional weight gain over the past 2 years. This added weight has translated to excess wear and tear on her joints, leading to evidence of osteoarthritis. Treatment with olanzapine is also independently associated with detrimental changes in cardiometabolic parameters.
Interventions to prevent or mitigate weight gain with antipsychotics are limited. In general, the American Psychiatric Association does not recommend switching antipsychotics for patients whose schizophrenia is well managed. However, there is increasing evidence that metformin may have a role in mitigating weight gain as well as beneficially modifying cardiometabolic factors in patients with schizophrenia being treated with olanzapine. A systematic review of emerging evidence with metformin in patients with schizophrenia suggests that metformin may also improve some cognitive symptoms of the illness, although further research is needed. The randomized, double-blind MELIA trial of metformin plus lifestyle intervention in antipsychotic-induced weight gain is ongoing. Starting metformin as a preventive measure at the same time as antipsychotic therapy may help to limit excess weight gain.
Research continues on the potential benefit of adding weight loss medications, including glucagon-like peptide-1 (GLP-1) receptor agonists, to antipsychotics. Daily liraglutide is most widely studied, but a published case series with weekly semaglutide also demonstrated weight loss in this setting. Liraglutide also has shown beneficial cardiometabolic effects in patients using antipsychotic medications. More studies of these drugs and of GLP-1/glucose-dependent insulinotropic polypeptide agonists are needed to elucidate the optimal use of these therapies for patients with schizophrenia.
There are few other effective ways to mitigate weight gain with olanzapine. Patients should be counseled on nutrition and lifestyle modifications. Evidence supports improvement with structured lifestyle modifications across a range of patients with less severe mental health issues, and structured programs combined with motivational interviewing were associated with reductions in antipsychotic-induced weight gain in patients with severe mental illness. As with any patient with obesity, however, the success of lifestyle modifications is heavily dependent on the individual's ability and motivation to comply with recommended interventions.
Nonpharmacologic interventions to address joint pain include heat or cold compresses, physical therapy, and strength and resistance training to improve the strength of muscles supporting the joints. If these measures are ineffective, nonsteroidal anti-inflammatory drugs (NSAIDs), including ibuprofen, naproxen, meloxicam, diclofenac, or celecoxib may be used with regular follow-up to assess cardiovascular and gastrointestinal health. Topical NSAIDs also may be useful. For more intractable joint pain, options include injecting a corticosteroid or sodium hyaluronate into the affected joints or joint replacement.
Carolyn Newberry, MD, Assistant Professor of Medicine, Director of GI Nutrition, Innovative Center for Health and Nutrition in Gastroenterology (ICHANGE), Division of Gastroenterology, Weill Cornell Medical Center, New York, NY.
Disclosure: Carolyn Newberry, MD, has disclosed the following relevant financial relationships:
Serve(d) as a speaker or a member of a speakers bureau for: Baster International; InBody.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
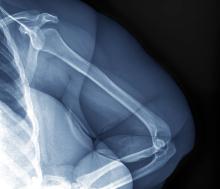
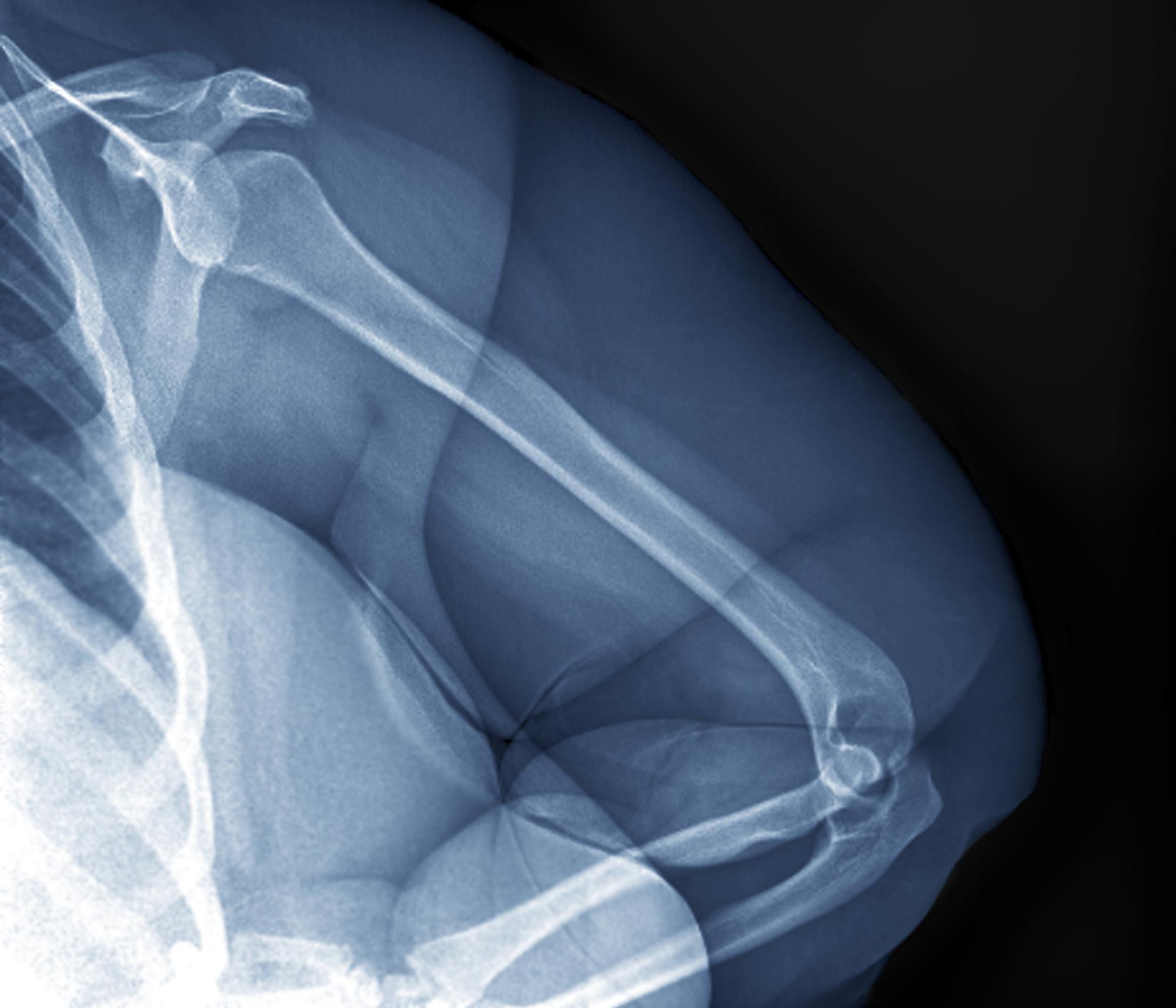
A 32-year-old woman presents with knee pain on walking and elbow pain. She is 5 ft 6 in tall and weighs 187 lb (BMI 30.2). She was diagnosed with schizophrenia 2 years ago and began treatment with olanzapine at diagnosis; her symptoms currently are controlled, and she has tolerated the medication well.
The patient says that she has been overweight since her teenage years and weighed 170 lb (BMI ~27) at age 30. However, she remained physically active until development of painful joints over the past 18 months. She works remotely full time and lives alone. She describes her long-standing diet as heavy on meat protein and light on vegetables and snacks and says it hasn't changed; she denies binge eating or other disordered eating.
Physical exam reveals tender joints at knees and elbows and central obesity (waist circumference, 42 in). Blood pressure is 135/90 mm Hg. Lab results indicate a fasting glucose level of 115 mg/dL and a triglyceride level of 170 mg/dL. She is negative for rheumatoid factor. Radiography shows premature joint erosion at the knees and elbows.
APL: Should Chemo-Free Regimen Become New Standard?
“First-line therapy with ATRA-ATO with two initial doses of idarubicin results in superior event-free survival, compared to conventional ATRA-chemotherapy in patients with high-risk APL,” said first author Uwe Platzbecker, MD, of the University Hospital Leipzig, department for hematology, cellular therapy, hemostaseology, and infectious diseases, in Leipzig, Germany, at the annual meeting of the European Hematology Association (EHA) in Madrid, Spain.
“We believe that the trial may support the implementation of this regimen as a new standard of care in all patients with high-risk APL,” he said.
In the treatment of low and intermediate risk APL, a subtype of acute myeloid leukemia (AML), the combination of ATRA and ATO has become standard since being shown in a pivotal 2013 study to be superior versus ATRA and chemotherapy. The approach is approved by the Food and Drug Administration in the treatment of adults with newly diagnosed low-risk APL.
Importantly, the improved survival with ATRA/ATO approach may result from “reduced severe hematologic toxicity together with similar antileukemic efficacy,” compared with the regimen that include chemotherapy, the authors of the 2013 study speculated.
However, the treatment regimen has not been evaluated in randomized trials in patients with high-risk APL, defined as having a white blood cell count of more than 10,000 cells per μL.
For those patients, the conventional treatment remains ATRA with a chemotherapy backbone, Dr. Platzbecker explained.
To evaluate if the improvements extend to high-risk APL patients without compromising safety, Dr. Platzbecker and colleagues conducted the open-label, prospective APOLLO trial, involving newly diagnosed high-risk APL who were enrolled between 2016 and 2022 at 143 sites in six European countries.
The patients were randomized into one of two groups: ATRA/ATO, involving treatment consisting of two doses of idarubicin (12 mg/m2) on days 1 and 3 at the time of induction therapy, in addition to ATO 0.15 mg/kg and ATRA 45 mg/m2, daily until complete remission, or the ATRA-chemotherapy arm, involving standard ATRA also with idarubicin induction, followed by three cycles of chemotherapy-based consolidation as well as 2 years of maintenance treatment.
While the study was prematurely discontinued in August 2022 because of COVID-19–related recruitment delays and expiration of the study drug, the maintenance and observational periods are ongoing.
Of 131 patients with high-risk APL who were evaluable for the outcome analysis, 68 were in the ATRA/ATO group and 63 in the ATRA-chemotherapy arm.
Overall, participants had a mean age of 46, 50% were female, their median Eastern Cooperative Oncology Group score was 1. Their median white blood cell count was 36 × 109/L, with 39% having a white blood cell count greater than 50 × 109/L.
Molecular resistance occurred in 1.7% in the ATRA/ATO arm vs 5.5% in the ATRA chemotherapy arm, which was not statistically significant (P = .268); however, the incidence of molecular relapse was much lower without chemotherapy, at 1.6% with ATRA/ATO vs 14% with ATRA and chemotherapy.
For the primary endpoint, with a median follow-up of 31 months, the 2-year rate of event-free survival those in the ATRA/ATO arm was 88% vs 70% in the ATRA plus chemotherapy regimen (P = .02). The 5-year event-free survival continued to favor ATRA-ATO (87% vs 55%; P = .0034).
The estimated 5-year overall survival was 93% vs 82% for ATRA/ATO vs ATRA-chemotherapy, respectively, which was not significantly different (P = .17).
There were no significant differences between the arms in complete response (93% with ATRA/ATO vs 91% with ATRA-chemotherapy; P = .65), and rates of early death (within the first 30 days) were also similar across arms, at 7% vs 10%, respectively.
Death while in complete remission occurred in zero patients in the ATRA/ATO arm and three in the ATRA chemotherapy arm.
In terms of toxicities, the ATRA/ATO group had significantly lower rates of hematologic toxicity versus ATRA-chemotherapy, including rates of thrombocytopenia grade 1-4 and neutropenia grade 3-4 (P < .001), while there were no significant differences between the groups in hepatic toxicities (11.8% and 14.3%, respectively; P = .08) or differentiation syndrome (1.5% vs 4.8%; P = .27).
QTc prolongation grade 3-4 occurred in 4.4 patients receiving ATRA/ATO, compared with 0 in the ATRA-chemotherapy group; however, Dr. Platzbecker said the cases had no clinical implications.
Asked to elaborate on the regimens’ toxicities in the press briefing, Dr. Platzbecker noted that “what is very important especially for patients, is [lower rates] of issues such as hair loss and constipation that are much less common with the ATRO/ATO regimen.”
“In addition, we know from the early experiences with this that younger patients are being cured by this regimen,” hence improving pregnancy prospects for women.
A take-home message from the overall results is that the ATRO/ATO regimen for high-risk APL patients should represent “a new treatment paradigm” that will “hopefully soon” be reflected in guideline recommendations, Dr. Platzbecker said in an interview.
Concerns Included Relapse, Differentiation Syndrome
Commenting on the research, Mikkael A. Sekeres, MD, explained that, while the “less is more” non-chemotherapy approach was adopted in widespread utilization in low-risk APL because of superior outcomes, a variety of concerns surrounded its use in high-risk patients.
“In high-risk patients, there were concerns that a durable response would be lower and that relapse would be higher for patients receiving ATRA and ATO than those receiving standard chemotherapy,” Dr. Sekeres, who is chief of the division of hematology, department of medicine, Sylvester Comprehensive Cancer Center, University of Miami, Miami, Florida, said in an interview.
“In addition, it was theoretically possible that patients receiving the differentiating agents ATRA and ATO could suffer higher rates of differentiation syndrome, which could contribute to early death,” he explained. “These fears were simply not realized in the trial.”
Caveats of the trial “include the relatively small sample size and that the trial was stopped prematurely due to low enrollment during the COVID pandemic,” he noted.
Another limitation was the median follow-up of about 2.5 years.
However, Dr. Sekeres said he agreed that, “with further follow-up and continued superiority of the idarubicin, ATRA, and ATO combination, this could become a new standard of care for high-risk patients with APL.”
Dr. Platzbecker’s disclosures include ties with Teva, BMS, Curis, Janssen, AbbVie, and Takeda. Dr. Sekeres had no disclosures.
“First-line therapy with ATRA-ATO with two initial doses of idarubicin results in superior event-free survival, compared to conventional ATRA-chemotherapy in patients with high-risk APL,” said first author Uwe Platzbecker, MD, of the University Hospital Leipzig, department for hematology, cellular therapy, hemostaseology, and infectious diseases, in Leipzig, Germany, at the annual meeting of the European Hematology Association (EHA) in Madrid, Spain.
“We believe that the trial may support the implementation of this regimen as a new standard of care in all patients with high-risk APL,” he said.
In the treatment of low and intermediate risk APL, a subtype of acute myeloid leukemia (AML), the combination of ATRA and ATO has become standard since being shown in a pivotal 2013 study to be superior versus ATRA and chemotherapy. The approach is approved by the Food and Drug Administration in the treatment of adults with newly diagnosed low-risk APL.
Importantly, the improved survival with ATRA/ATO approach may result from “reduced severe hematologic toxicity together with similar antileukemic efficacy,” compared with the regimen that include chemotherapy, the authors of the 2013 study speculated.
However, the treatment regimen has not been evaluated in randomized trials in patients with high-risk APL, defined as having a white blood cell count of more than 10,000 cells per μL.
For those patients, the conventional treatment remains ATRA with a chemotherapy backbone, Dr. Platzbecker explained.
To evaluate if the improvements extend to high-risk APL patients without compromising safety, Dr. Platzbecker and colleagues conducted the open-label, prospective APOLLO trial, involving newly diagnosed high-risk APL who were enrolled between 2016 and 2022 at 143 sites in six European countries.
The patients were randomized into one of two groups: ATRA/ATO, involving treatment consisting of two doses of idarubicin (12 mg/m2) on days 1 and 3 at the time of induction therapy, in addition to ATO 0.15 mg/kg and ATRA 45 mg/m2, daily until complete remission, or the ATRA-chemotherapy arm, involving standard ATRA also with idarubicin induction, followed by three cycles of chemotherapy-based consolidation as well as 2 years of maintenance treatment.
While the study was prematurely discontinued in August 2022 because of COVID-19–related recruitment delays and expiration of the study drug, the maintenance and observational periods are ongoing.
Of 131 patients with high-risk APL who were evaluable for the outcome analysis, 68 were in the ATRA/ATO group and 63 in the ATRA-chemotherapy arm.
Overall, participants had a mean age of 46, 50% were female, their median Eastern Cooperative Oncology Group score was 1. Their median white blood cell count was 36 × 109/L, with 39% having a white blood cell count greater than 50 × 109/L.
Molecular resistance occurred in 1.7% in the ATRA/ATO arm vs 5.5% in the ATRA chemotherapy arm, which was not statistically significant (P = .268); however, the incidence of molecular relapse was much lower without chemotherapy, at 1.6% with ATRA/ATO vs 14% with ATRA and chemotherapy.
For the primary endpoint, with a median follow-up of 31 months, the 2-year rate of event-free survival those in the ATRA/ATO arm was 88% vs 70% in the ATRA plus chemotherapy regimen (P = .02). The 5-year event-free survival continued to favor ATRA-ATO (87% vs 55%; P = .0034).
The estimated 5-year overall survival was 93% vs 82% for ATRA/ATO vs ATRA-chemotherapy, respectively, which was not significantly different (P = .17).
There were no significant differences between the arms in complete response (93% with ATRA/ATO vs 91% with ATRA-chemotherapy; P = .65), and rates of early death (within the first 30 days) were also similar across arms, at 7% vs 10%, respectively.
Death while in complete remission occurred in zero patients in the ATRA/ATO arm and three in the ATRA chemotherapy arm.
In terms of toxicities, the ATRA/ATO group had significantly lower rates of hematologic toxicity versus ATRA-chemotherapy, including rates of thrombocytopenia grade 1-4 and neutropenia grade 3-4 (P < .001), while there were no significant differences between the groups in hepatic toxicities (11.8% and 14.3%, respectively; P = .08) or differentiation syndrome (1.5% vs 4.8%; P = .27).
QTc prolongation grade 3-4 occurred in 4.4 patients receiving ATRA/ATO, compared with 0 in the ATRA-chemotherapy group; however, Dr. Platzbecker said the cases had no clinical implications.
Asked to elaborate on the regimens’ toxicities in the press briefing, Dr. Platzbecker noted that “what is very important especially for patients, is [lower rates] of issues such as hair loss and constipation that are much less common with the ATRO/ATO regimen.”
“In addition, we know from the early experiences with this that younger patients are being cured by this regimen,” hence improving pregnancy prospects for women.
A take-home message from the overall results is that the ATRO/ATO regimen for high-risk APL patients should represent “a new treatment paradigm” that will “hopefully soon” be reflected in guideline recommendations, Dr. Platzbecker said in an interview.
Concerns Included Relapse, Differentiation Syndrome
Commenting on the research, Mikkael A. Sekeres, MD, explained that, while the “less is more” non-chemotherapy approach was adopted in widespread utilization in low-risk APL because of superior outcomes, a variety of concerns surrounded its use in high-risk patients.
“In high-risk patients, there were concerns that a durable response would be lower and that relapse would be higher for patients receiving ATRA and ATO than those receiving standard chemotherapy,” Dr. Sekeres, who is chief of the division of hematology, department of medicine, Sylvester Comprehensive Cancer Center, University of Miami, Miami, Florida, said in an interview.
“In addition, it was theoretically possible that patients receiving the differentiating agents ATRA and ATO could suffer higher rates of differentiation syndrome, which could contribute to early death,” he explained. “These fears were simply not realized in the trial.”
Caveats of the trial “include the relatively small sample size and that the trial was stopped prematurely due to low enrollment during the COVID pandemic,” he noted.
Another limitation was the median follow-up of about 2.5 years.
However, Dr. Sekeres said he agreed that, “with further follow-up and continued superiority of the idarubicin, ATRA, and ATO combination, this could become a new standard of care for high-risk patients with APL.”
Dr. Platzbecker’s disclosures include ties with Teva, BMS, Curis, Janssen, AbbVie, and Takeda. Dr. Sekeres had no disclosures.
“First-line therapy with ATRA-ATO with two initial doses of idarubicin results in superior event-free survival, compared to conventional ATRA-chemotherapy in patients with high-risk APL,” said first author Uwe Platzbecker, MD, of the University Hospital Leipzig, department for hematology, cellular therapy, hemostaseology, and infectious diseases, in Leipzig, Germany, at the annual meeting of the European Hematology Association (EHA) in Madrid, Spain.
“We believe that the trial may support the implementation of this regimen as a new standard of care in all patients with high-risk APL,” he said.
In the treatment of low and intermediate risk APL, a subtype of acute myeloid leukemia (AML), the combination of ATRA and ATO has become standard since being shown in a pivotal 2013 study to be superior versus ATRA and chemotherapy. The approach is approved by the Food and Drug Administration in the treatment of adults with newly diagnosed low-risk APL.
Importantly, the improved survival with ATRA/ATO approach may result from “reduced severe hematologic toxicity together with similar antileukemic efficacy,” compared with the regimen that include chemotherapy, the authors of the 2013 study speculated.
However, the treatment regimen has not been evaluated in randomized trials in patients with high-risk APL, defined as having a white blood cell count of more than 10,000 cells per μL.
For those patients, the conventional treatment remains ATRA with a chemotherapy backbone, Dr. Platzbecker explained.
To evaluate if the improvements extend to high-risk APL patients without compromising safety, Dr. Platzbecker and colleagues conducted the open-label, prospective APOLLO trial, involving newly diagnosed high-risk APL who were enrolled between 2016 and 2022 at 143 sites in six European countries.
The patients were randomized into one of two groups: ATRA/ATO, involving treatment consisting of two doses of idarubicin (12 mg/m2) on days 1 and 3 at the time of induction therapy, in addition to ATO 0.15 mg/kg and ATRA 45 mg/m2, daily until complete remission, or the ATRA-chemotherapy arm, involving standard ATRA also with idarubicin induction, followed by three cycles of chemotherapy-based consolidation as well as 2 years of maintenance treatment.
While the study was prematurely discontinued in August 2022 because of COVID-19–related recruitment delays and expiration of the study drug, the maintenance and observational periods are ongoing.
Of 131 patients with high-risk APL who were evaluable for the outcome analysis, 68 were in the ATRA/ATO group and 63 in the ATRA-chemotherapy arm.
Overall, participants had a mean age of 46, 50% were female, their median Eastern Cooperative Oncology Group score was 1. Their median white blood cell count was 36 × 109/L, with 39% having a white blood cell count greater than 50 × 109/L.
Molecular resistance occurred in 1.7% in the ATRA/ATO arm vs 5.5% in the ATRA chemotherapy arm, which was not statistically significant (P = .268); however, the incidence of molecular relapse was much lower without chemotherapy, at 1.6% with ATRA/ATO vs 14% with ATRA and chemotherapy.
For the primary endpoint, with a median follow-up of 31 months, the 2-year rate of event-free survival those in the ATRA/ATO arm was 88% vs 70% in the ATRA plus chemotherapy regimen (P = .02). The 5-year event-free survival continued to favor ATRA-ATO (87% vs 55%; P = .0034).
The estimated 5-year overall survival was 93% vs 82% for ATRA/ATO vs ATRA-chemotherapy, respectively, which was not significantly different (P = .17).
There were no significant differences between the arms in complete response (93% with ATRA/ATO vs 91% with ATRA-chemotherapy; P = .65), and rates of early death (within the first 30 days) were also similar across arms, at 7% vs 10%, respectively.
Death while in complete remission occurred in zero patients in the ATRA/ATO arm and three in the ATRA chemotherapy arm.
In terms of toxicities, the ATRA/ATO group had significantly lower rates of hematologic toxicity versus ATRA-chemotherapy, including rates of thrombocytopenia grade 1-4 and neutropenia grade 3-4 (P < .001), while there were no significant differences between the groups in hepatic toxicities (11.8% and 14.3%, respectively; P = .08) or differentiation syndrome (1.5% vs 4.8%; P = .27).
QTc prolongation grade 3-4 occurred in 4.4 patients receiving ATRA/ATO, compared with 0 in the ATRA-chemotherapy group; however, Dr. Platzbecker said the cases had no clinical implications.
Asked to elaborate on the regimens’ toxicities in the press briefing, Dr. Platzbecker noted that “what is very important especially for patients, is [lower rates] of issues such as hair loss and constipation that are much less common with the ATRO/ATO regimen.”
“In addition, we know from the early experiences with this that younger patients are being cured by this regimen,” hence improving pregnancy prospects for women.
A take-home message from the overall results is that the ATRO/ATO regimen for high-risk APL patients should represent “a new treatment paradigm” that will “hopefully soon” be reflected in guideline recommendations, Dr. Platzbecker said in an interview.
Concerns Included Relapse, Differentiation Syndrome
Commenting on the research, Mikkael A. Sekeres, MD, explained that, while the “less is more” non-chemotherapy approach was adopted in widespread utilization in low-risk APL because of superior outcomes, a variety of concerns surrounded its use in high-risk patients.
“In high-risk patients, there were concerns that a durable response would be lower and that relapse would be higher for patients receiving ATRA and ATO than those receiving standard chemotherapy,” Dr. Sekeres, who is chief of the division of hematology, department of medicine, Sylvester Comprehensive Cancer Center, University of Miami, Miami, Florida, said in an interview.
“In addition, it was theoretically possible that patients receiving the differentiating agents ATRA and ATO could suffer higher rates of differentiation syndrome, which could contribute to early death,” he explained. “These fears were simply not realized in the trial.”
Caveats of the trial “include the relatively small sample size and that the trial was stopped prematurely due to low enrollment during the COVID pandemic,” he noted.
Another limitation was the median follow-up of about 2.5 years.
However, Dr. Sekeres said he agreed that, “with further follow-up and continued superiority of the idarubicin, ATRA, and ATO combination, this could become a new standard of care for high-risk patients with APL.”
Dr. Platzbecker’s disclosures include ties with Teva, BMS, Curis, Janssen, AbbVie, and Takeda. Dr. Sekeres had no disclosures.
FROM EHA 2024
Latest Izokibep Trial for PsA Shows Promise But Misses on Enthesitis
VIENNA — The investigational interleukin (IL)-17 inhibitor izokibep hit its mark when it came to improving overall disease activity in people with active psoriatic arthritis (PsA) in a phase 2b/3 trial, but it was no better than placebo at reducing inflammation of the entheses.
This apparent and unexpected lack of effect in the entheses was a key talking point after Philip J. Mease, MD, presented the late-breaking trial findings at the annual European Congress of Rheumatology.
At just 18.6 kilodaltons in size, izokibep is just “one tenth the size of a standard monoclonal antibody” and is classed as a small protein therapeutic, Dr. Mease said. It has a “very tight” binding affinity for IL-17A, and because it also binds to albumin, it has a prolonged half-life compared with other IL-17 inhibitors. Potentially, it should be able to “penetrate into difficult areas,” such as the entheses, he said.
Prespecified Enthesitis Analysis
However, results of a prespecified secondary analysis conducted in 209 of the 343 trial participants who had received treatment showed no significant difference in the proportions with enthesis resolution at 16 weeks, defined as a Leeds Enthesitis Index (LEI) of 0.
Comparing two dosing regimens of izokibep 160 mg once weekly (QW) vs every other week (Q2W) with placebo, enthesitis resolution was seen in 45%, 56%, and 47%, respectively, of patients.
The LEI is “sometimes subject to problems with evaluation because of placebo response, which is what we see here,” noted Dr. Mease, director of rheumatology research at the Providence Swedish Medical Center and a rheumatology professor at the University of Washington School of Medicine in Seattle.
An exploratory analysis showed that there was a better response for izokibep vs placebo if the analysis included only patients with higher LEI scores at baseline, at 8.0% (n = 12) for placebo, 22.0% (n = 9) for izokibep 160 mg QW, and 50.0% (n = 12) for izokibep 160 mg Q2W.
Main Efficacy Data
The primary endpoint for the trial was the proportion of patients who had 50% improvement in American College of Rheumatology response criteria (ACR50) at 16 weeks. This showed a clear advantage for treatment with izokibep 160 QW and Q2W compared with placebo, with a respective 40%, 43%, and 15% of patients meeting this endpoint.
Corresponding ACR20 response rates were 59%, 64%, and 35%, respectively; ACR70 response rates were a respective 25%, 23%, and 5%.
In addition to ACR70, izokibep 160 QW and Q2W met a number of other “high hurdle” efficacy endpoints better than did placebo, Dr. Mease reported. A 90% reduction from baseline in the Psoriasis Area and Severity Index (PASI90) was achieved by a respective 64%, 58%, and 12% of patients, and a 100% reduction in this index (PASI100) was achieved by a respective 51%, 47%, and 12%. And 41%, 42%, and 14% of patients, respectively, met the criteria for minimal disease activity.
Patient Population
Mease pointed out during his presentation that the trial included patients with adult-onset PsA that had been ongoing for ≥ 6 months. Patients had to have at least three tender or swollen joints and an inadequate response, intolerance, or contraindication to commonly used front-line therapies such as nonsteroidal anti-inflammatory drugs, conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), and tumor necrosis factor inhibitors (TNFi).
In fact, around half of the participants across the three treatment arms had received prior csDMARDs, and almost a quarter had received a TNFi.
The mean duration of disease was around 7 years, the average age was about 50 years, and the majority of the participants were White individuals. There were more women than men in the placebo vs the izokibep arms (43.4% vs about 60.0%).
Adverse Events
Injection site reactions were the most common adverse events, most of which were mild to moderate. Very few (< 1% to 4%) led to any need to discontinue the drug.
Serious adverse events occurred at low rates in all study arms: 0.8% for placebo, 2.7% for izokibep QW, and 1.8% for izokibep Q2W.
One patient each (0.9%) in the izokibep arms developed ulcerative colitis, whereas none in the placebo group did. Only two patients developed candidiasis. One was in the placebo group and had a skin infection, and the other was an oral infection in the QW izokibep arm.
There were no cases of uveitis, suicidal ideation, or deaths reported.
Comments on the Study
During the discussion that followed the presentation, Walter P. Maksymowych, MBChB, of the University of Alberta in Edmonton, Alberta, Canada, addressed the dosing regimens used.
“Looking at the side effect profile and then looking at the response rate, comparing the weekly dosing and every 2 weeks, do you think, in hindsight, you might be remiss that there wasn’t an additional dosing on a monthly basis, especially since this is a construct that is meant to prolong the half-life of the molecule?” he asked, adding that perhaps this should be something to consider in future studies.
Mease responded that there had been a fourth dosing arm in the trial — izokibep 80 mg once a month — but because there were only eight patients, the data were not sufficiently robust to analyze.
Commenting on the study, Laura C. Coates, MBChB, PhD, said: “It’s a pretty standard phase 2b/3 study,” and the outcomes were not wildly different from what has been seen with other IL-17A inhibitors.
“In phase 2, the enthesitis data looked really good; in phase 3, the enthesitis data looks the same as for any other IL-17 inhibitor,” Dr. Coates said.
More and longer-term data are needed to see if “the theoretical biological difference in the drug design translates to a different clinical outcome or whether it’s another IL-17,” added Dr. Coates, a clinician scientist and senior clinical research fellow at the University of Oxford in England.
Dennis McGonagle, MB MCH BAO, PhD, of the University of Leeds, England, also picked up on the enthesitis data, echoing the conclusion that the phase 2 enthesitis data were “spectacular” and noting that “it’s a real inversion of what was expected, given the small molecule.”
The study was funded by Acelyrin. Dr. Mease disclosed ties with Acelyrin and other pharmaceutical companies. Dr. Maksymowych, Dr. Coates, and Dr. McGonagle reported having a variety of financial relationships with pharmaceutical companies outside of this study.
A version of this article appeared on Medscape.com.
VIENNA — The investigational interleukin (IL)-17 inhibitor izokibep hit its mark when it came to improving overall disease activity in people with active psoriatic arthritis (PsA) in a phase 2b/3 trial, but it was no better than placebo at reducing inflammation of the entheses.
This apparent and unexpected lack of effect in the entheses was a key talking point after Philip J. Mease, MD, presented the late-breaking trial findings at the annual European Congress of Rheumatology.
At just 18.6 kilodaltons in size, izokibep is just “one tenth the size of a standard monoclonal antibody” and is classed as a small protein therapeutic, Dr. Mease said. It has a “very tight” binding affinity for IL-17A, and because it also binds to albumin, it has a prolonged half-life compared with other IL-17 inhibitors. Potentially, it should be able to “penetrate into difficult areas,” such as the entheses, he said.
Prespecified Enthesitis Analysis
However, results of a prespecified secondary analysis conducted in 209 of the 343 trial participants who had received treatment showed no significant difference in the proportions with enthesis resolution at 16 weeks, defined as a Leeds Enthesitis Index (LEI) of 0.
Comparing two dosing regimens of izokibep 160 mg once weekly (QW) vs every other week (Q2W) with placebo, enthesitis resolution was seen in 45%, 56%, and 47%, respectively, of patients.
The LEI is “sometimes subject to problems with evaluation because of placebo response, which is what we see here,” noted Dr. Mease, director of rheumatology research at the Providence Swedish Medical Center and a rheumatology professor at the University of Washington School of Medicine in Seattle.
An exploratory analysis showed that there was a better response for izokibep vs placebo if the analysis included only patients with higher LEI scores at baseline, at 8.0% (n = 12) for placebo, 22.0% (n = 9) for izokibep 160 mg QW, and 50.0% (n = 12) for izokibep 160 mg Q2W.
Main Efficacy Data
The primary endpoint for the trial was the proportion of patients who had 50% improvement in American College of Rheumatology response criteria (ACR50) at 16 weeks. This showed a clear advantage for treatment with izokibep 160 QW and Q2W compared with placebo, with a respective 40%, 43%, and 15% of patients meeting this endpoint.
Corresponding ACR20 response rates were 59%, 64%, and 35%, respectively; ACR70 response rates were a respective 25%, 23%, and 5%.
In addition to ACR70, izokibep 160 QW and Q2W met a number of other “high hurdle” efficacy endpoints better than did placebo, Dr. Mease reported. A 90% reduction from baseline in the Psoriasis Area and Severity Index (PASI90) was achieved by a respective 64%, 58%, and 12% of patients, and a 100% reduction in this index (PASI100) was achieved by a respective 51%, 47%, and 12%. And 41%, 42%, and 14% of patients, respectively, met the criteria for minimal disease activity.
Patient Population
Mease pointed out during his presentation that the trial included patients with adult-onset PsA that had been ongoing for ≥ 6 months. Patients had to have at least three tender or swollen joints and an inadequate response, intolerance, or contraindication to commonly used front-line therapies such as nonsteroidal anti-inflammatory drugs, conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), and tumor necrosis factor inhibitors (TNFi).
In fact, around half of the participants across the three treatment arms had received prior csDMARDs, and almost a quarter had received a TNFi.
The mean duration of disease was around 7 years, the average age was about 50 years, and the majority of the participants were White individuals. There were more women than men in the placebo vs the izokibep arms (43.4% vs about 60.0%).
Adverse Events
Injection site reactions were the most common adverse events, most of which were mild to moderate. Very few (< 1% to 4%) led to any need to discontinue the drug.
Serious adverse events occurred at low rates in all study arms: 0.8% for placebo, 2.7% for izokibep QW, and 1.8% for izokibep Q2W.
One patient each (0.9%) in the izokibep arms developed ulcerative colitis, whereas none in the placebo group did. Only two patients developed candidiasis. One was in the placebo group and had a skin infection, and the other was an oral infection in the QW izokibep arm.
There were no cases of uveitis, suicidal ideation, or deaths reported.
Comments on the Study
During the discussion that followed the presentation, Walter P. Maksymowych, MBChB, of the University of Alberta in Edmonton, Alberta, Canada, addressed the dosing regimens used.
“Looking at the side effect profile and then looking at the response rate, comparing the weekly dosing and every 2 weeks, do you think, in hindsight, you might be remiss that there wasn’t an additional dosing on a monthly basis, especially since this is a construct that is meant to prolong the half-life of the molecule?” he asked, adding that perhaps this should be something to consider in future studies.
Mease responded that there had been a fourth dosing arm in the trial — izokibep 80 mg once a month — but because there were only eight patients, the data were not sufficiently robust to analyze.
Commenting on the study, Laura C. Coates, MBChB, PhD, said: “It’s a pretty standard phase 2b/3 study,” and the outcomes were not wildly different from what has been seen with other IL-17A inhibitors.
“In phase 2, the enthesitis data looked really good; in phase 3, the enthesitis data looks the same as for any other IL-17 inhibitor,” Dr. Coates said.
More and longer-term data are needed to see if “the theoretical biological difference in the drug design translates to a different clinical outcome or whether it’s another IL-17,” added Dr. Coates, a clinician scientist and senior clinical research fellow at the University of Oxford in England.
Dennis McGonagle, MB MCH BAO, PhD, of the University of Leeds, England, also picked up on the enthesitis data, echoing the conclusion that the phase 2 enthesitis data were “spectacular” and noting that “it’s a real inversion of what was expected, given the small molecule.”
The study was funded by Acelyrin. Dr. Mease disclosed ties with Acelyrin and other pharmaceutical companies. Dr. Maksymowych, Dr. Coates, and Dr. McGonagle reported having a variety of financial relationships with pharmaceutical companies outside of this study.
A version of this article appeared on Medscape.com.
VIENNA — The investigational interleukin (IL)-17 inhibitor izokibep hit its mark when it came to improving overall disease activity in people with active psoriatic arthritis (PsA) in a phase 2b/3 trial, but it was no better than placebo at reducing inflammation of the entheses.
This apparent and unexpected lack of effect in the entheses was a key talking point after Philip J. Mease, MD, presented the late-breaking trial findings at the annual European Congress of Rheumatology.
At just 18.6 kilodaltons in size, izokibep is just “one tenth the size of a standard monoclonal antibody” and is classed as a small protein therapeutic, Dr. Mease said. It has a “very tight” binding affinity for IL-17A, and because it also binds to albumin, it has a prolonged half-life compared with other IL-17 inhibitors. Potentially, it should be able to “penetrate into difficult areas,” such as the entheses, he said.
Prespecified Enthesitis Analysis
However, results of a prespecified secondary analysis conducted in 209 of the 343 trial participants who had received treatment showed no significant difference in the proportions with enthesis resolution at 16 weeks, defined as a Leeds Enthesitis Index (LEI) of 0.
Comparing two dosing regimens of izokibep 160 mg once weekly (QW) vs every other week (Q2W) with placebo, enthesitis resolution was seen in 45%, 56%, and 47%, respectively, of patients.
The LEI is “sometimes subject to problems with evaluation because of placebo response, which is what we see here,” noted Dr. Mease, director of rheumatology research at the Providence Swedish Medical Center and a rheumatology professor at the University of Washington School of Medicine in Seattle.
An exploratory analysis showed that there was a better response for izokibep vs placebo if the analysis included only patients with higher LEI scores at baseline, at 8.0% (n = 12) for placebo, 22.0% (n = 9) for izokibep 160 mg QW, and 50.0% (n = 12) for izokibep 160 mg Q2W.
Main Efficacy Data
The primary endpoint for the trial was the proportion of patients who had 50% improvement in American College of Rheumatology response criteria (ACR50) at 16 weeks. This showed a clear advantage for treatment with izokibep 160 QW and Q2W compared with placebo, with a respective 40%, 43%, and 15% of patients meeting this endpoint.
Corresponding ACR20 response rates were 59%, 64%, and 35%, respectively; ACR70 response rates were a respective 25%, 23%, and 5%.
In addition to ACR70, izokibep 160 QW and Q2W met a number of other “high hurdle” efficacy endpoints better than did placebo, Dr. Mease reported. A 90% reduction from baseline in the Psoriasis Area and Severity Index (PASI90) was achieved by a respective 64%, 58%, and 12% of patients, and a 100% reduction in this index (PASI100) was achieved by a respective 51%, 47%, and 12%. And 41%, 42%, and 14% of patients, respectively, met the criteria for minimal disease activity.
Patient Population
Mease pointed out during his presentation that the trial included patients with adult-onset PsA that had been ongoing for ≥ 6 months. Patients had to have at least three tender or swollen joints and an inadequate response, intolerance, or contraindication to commonly used front-line therapies such as nonsteroidal anti-inflammatory drugs, conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), and tumor necrosis factor inhibitors (TNFi).
In fact, around half of the participants across the three treatment arms had received prior csDMARDs, and almost a quarter had received a TNFi.
The mean duration of disease was around 7 years, the average age was about 50 years, and the majority of the participants were White individuals. There were more women than men in the placebo vs the izokibep arms (43.4% vs about 60.0%).
Adverse Events
Injection site reactions were the most common adverse events, most of which were mild to moderate. Very few (< 1% to 4%) led to any need to discontinue the drug.
Serious adverse events occurred at low rates in all study arms: 0.8% for placebo, 2.7% for izokibep QW, and 1.8% for izokibep Q2W.
One patient each (0.9%) in the izokibep arms developed ulcerative colitis, whereas none in the placebo group did. Only two patients developed candidiasis. One was in the placebo group and had a skin infection, and the other was an oral infection in the QW izokibep arm.
There were no cases of uveitis, suicidal ideation, or deaths reported.
Comments on the Study
During the discussion that followed the presentation, Walter P. Maksymowych, MBChB, of the University of Alberta in Edmonton, Alberta, Canada, addressed the dosing regimens used.
“Looking at the side effect profile and then looking at the response rate, comparing the weekly dosing and every 2 weeks, do you think, in hindsight, you might be remiss that there wasn’t an additional dosing on a monthly basis, especially since this is a construct that is meant to prolong the half-life of the molecule?” he asked, adding that perhaps this should be something to consider in future studies.
Mease responded that there had been a fourth dosing arm in the trial — izokibep 80 mg once a month — but because there were only eight patients, the data were not sufficiently robust to analyze.
Commenting on the study, Laura C. Coates, MBChB, PhD, said: “It’s a pretty standard phase 2b/3 study,” and the outcomes were not wildly different from what has been seen with other IL-17A inhibitors.
“In phase 2, the enthesitis data looked really good; in phase 3, the enthesitis data looks the same as for any other IL-17 inhibitor,” Dr. Coates said.
More and longer-term data are needed to see if “the theoretical biological difference in the drug design translates to a different clinical outcome or whether it’s another IL-17,” added Dr. Coates, a clinician scientist and senior clinical research fellow at the University of Oxford in England.
Dennis McGonagle, MB MCH BAO, PhD, of the University of Leeds, England, also picked up on the enthesitis data, echoing the conclusion that the phase 2 enthesitis data were “spectacular” and noting that “it’s a real inversion of what was expected, given the small molecule.”
The study was funded by Acelyrin. Dr. Mease disclosed ties with Acelyrin and other pharmaceutical companies. Dr. Maksymowych, Dr. Coates, and Dr. McGonagle reported having a variety of financial relationships with pharmaceutical companies outside of this study.
A version of this article appeared on Medscape.com.
FROM EULAR 2024
Selective JAK 1 Inhibitor for RA Proves Promising in Phase 3 Trial
VIENNA — The highly selective oral Janus kinase (JAK) inhibitor SHR0302 (ivarmacitinib) enables more patients with active rheumatoid arthritis to meet American College of Rheumatology (ACR) response criteria than placebo, the results of a phase 3 trial showed.
After 24 weeks of daily treatment, the primary endpoint of an ACR20 response was met by 40.4% of those who had been given placebo, 70.4% who had received a 4-mg dose, and 75.1% given an 8-mg dose. At the same time point, ACR50 responses were a respective 15.4%, 46.0%, and 57.1%, and ACR70 responses were 6.9%, 22.2%, and 31.7%. All analyses comparing SHR0302 vs placebo were highly significant (P < .0001).
First Phase 3 Trial in China
“This is the first highly selective JAK inhibitor originally developed, and a phase 3 clinical trial conducted, [exclusively] in China,” Jinjing Liu, from the department of rheumatology at Peking Union Medical College Hospital in Beijing, China, said in an interview.
Ms. Liu presented the results at the European Alliance of Associations for Rheumatology (EULAR) 2024 Annual Meeting, during the Abstract Plenary, which highlights the best-scored abstracts of the meeting.
“We are working our best to provide more choices for Chinese patients,” Ms. Liu said, which includes lowering the financial cost of treatments. A locally developed JAK inhibitor could potentially be a much cheaper option than other alternatives that are currently available, she said.
But it is more than that, Ms. Liu said. “The selectivity of SHR0302 for JAK 1 is nine times greater than for JAK 2, so it surpasses either tofacitinib or baricitinib.” The theory is that this higher selectivity for JAK 1 over JAK 2 could lead to fewer adverse events (AEs).
“Maybe it will result in lower JAK 2–associated hematologic side effects,” Ms. Liu said.
“We have noticed that, throughout the clinical trial, the most commonly reported AEs in the drug groups were upper extremity infection [21.7%-22.8% vs 13.8% for placebo] and hyperlipidemia [12.2%-15.3% vs 5.3%].” And for the control group, she said that anemia was the second highest reported AE, at 11.7% vs 6.3% and 7.4% for SHR0302 4 and 8 mg, respectively.
Standard Design
The trial design was typical for a phase 3 study: Multicenter, randomized, placebo controlled, and double blind for the first 24 weeks, followed by an extension period out to 52 weeks. For inclusion in the study, patients had to be aged 18-75 years and have active rheumatoid arthritis and an inadequate response to previous treatment with conventional synthetic disease-modifying antirheumatic drugs.
Of 1085 patients who were initially screened, 566 were randomly allocated to receive placebo (n = 188), SHR0302 4 mg (n = 189), or SHR0302 8 mg (n = 189). The average age of patients was 51 years, and 13.3% of patients were older than 65 years.
Additional Results
Alongside improvements in ACR responses, Ms Liu reported that a significantly higher proportion of patients treated with SHR0302 vs placebo achieved a Disease Activity Score in 28 joints based on C-reactive protein less than 2.6 (29.6% with 4 mg and 39.2% with 8 mg vs 4.2% with placebo; both P < .0001) and at least 3.2 (57.1% and 46.0% vs 15.4%; both P < .0001) at 24 weeks.
There were also greater improvements seen in Health Assessment Questionnaire-Disability Index, 36-item Short-Form (SF36) physical component summary, and SF36 mental component summary scores for active vs placebo treatment.
As for AEs, there were no surprises. During the main 24-week trial period, 81.5%, 90.5%, and 79.3% of patients treated with SHR0302 4 and 8 mg and placebo, respectively, experienced any AE.
Infection-related treatment-emergent adverse effects occurred slightly more often in the SHR0302-treated groups (40.2% for 4 mg and 40.7% for 8 mg) than in the placebo group (34.0%). There was a single case of serious infection that required treatment in the SHR0302 8 mg–treated group but no cases of systemic opportunistic infection.
There was one thromboembolic event and one major cardiovascular event in the 24-week period, both occurring in patients treated with SHR0302 8 mg. There were also single cases of each reported during the extension phase of the trial, but both were in the placebo arm.
Two cases of liver function abnormality — one each in the SHR0302 4- and 8-mg groups — were recorded during the main part of the trial and two cases — both in the SHR0302 4-mg group — during the extension phase.
As for malignancy, there was a single, newly diagnosed case in the SHR0302 4 mg group in the first part of the trial and two cases, both in the SHR0302 4-mg group, during the extension phase.
“We hope this [JAK inhibitor] will be for everybody. But, you know, if it’s for patients, globally, more clinical trials would be required,” Ms. Liu said in an interview. The future, she added, was to start accumulating some real-world data and perhaps do a trial comparing SHR0302 with another JAK inhibitor or a tumor necrosis factor inhibitor.
Another JAK in the Box?
Following her presentation, Ms. Liu at EULAR 2024 was quizzed as to why there were so many screening failures. She responded that she did not have the full data to answer the question but noted that some patients in her center had been worried about being randomized to a placebo. This trial has also been conducted during the COVID-19 pandemic, so that may have been a contributing factor with patients unable to get to their follow-up appointments.
Iain B. McInnes, MD, PhD, vice principal, professor of rheumatology, and head of the College of Medical, Veterinary & Life Sciences at the University of Glasgow, Glasgow, Scotland, commented on the study, saying: “The JAK field is in evolution. We need to understand the broader toxicities. There is an unexplained mechanism driving potential cardiovascular and malignant risk in a small proportion of patients receiving the drugs.”
Dr. McInnes added, “It’s really unclear whether the solution is going to be greater selectivity and potency, or whether we need to think really about selecting the right patients for a JAK inhibitor.”
The study was funded by Jiangsu Hengrui Pharmaceuticals. Two of the 18 authors of the abstract were employees of the sponsoring company, but Ms. Liu reported having no conflicts of interest. Dr. McInnes reported serving on speaker’s bureaus for AbbVie and UCB; receiving consulting fees received from AbbVie, Amgen, AstraZeneca, Bristol-Myers Squibb, Causeway Therapeutics, Cabaletta Bio, Compugen, Eli Lilly, Evelo, Gilead, Janssen, Novartis, MoonLake Immunotherapeutics, Pfizer, Sanofi Regeneron, and UCB; and receiving grant/research support from GlaxoSmithKline, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Janssen, Novartis, and UCB.
A version of this article appeared on Medscape.com.
VIENNA — The highly selective oral Janus kinase (JAK) inhibitor SHR0302 (ivarmacitinib) enables more patients with active rheumatoid arthritis to meet American College of Rheumatology (ACR) response criteria than placebo, the results of a phase 3 trial showed.
After 24 weeks of daily treatment, the primary endpoint of an ACR20 response was met by 40.4% of those who had been given placebo, 70.4% who had received a 4-mg dose, and 75.1% given an 8-mg dose. At the same time point, ACR50 responses were a respective 15.4%, 46.0%, and 57.1%, and ACR70 responses were 6.9%, 22.2%, and 31.7%. All analyses comparing SHR0302 vs placebo were highly significant (P < .0001).
First Phase 3 Trial in China
“This is the first highly selective JAK inhibitor originally developed, and a phase 3 clinical trial conducted, [exclusively] in China,” Jinjing Liu, from the department of rheumatology at Peking Union Medical College Hospital in Beijing, China, said in an interview.
Ms. Liu presented the results at the European Alliance of Associations for Rheumatology (EULAR) 2024 Annual Meeting, during the Abstract Plenary, which highlights the best-scored abstracts of the meeting.
“We are working our best to provide more choices for Chinese patients,” Ms. Liu said, which includes lowering the financial cost of treatments. A locally developed JAK inhibitor could potentially be a much cheaper option than other alternatives that are currently available, she said.
But it is more than that, Ms. Liu said. “The selectivity of SHR0302 for JAK 1 is nine times greater than for JAK 2, so it surpasses either tofacitinib or baricitinib.” The theory is that this higher selectivity for JAK 1 over JAK 2 could lead to fewer adverse events (AEs).
“Maybe it will result in lower JAK 2–associated hematologic side effects,” Ms. Liu said.
“We have noticed that, throughout the clinical trial, the most commonly reported AEs in the drug groups were upper extremity infection [21.7%-22.8% vs 13.8% for placebo] and hyperlipidemia [12.2%-15.3% vs 5.3%].” And for the control group, she said that anemia was the second highest reported AE, at 11.7% vs 6.3% and 7.4% for SHR0302 4 and 8 mg, respectively.
Standard Design
The trial design was typical for a phase 3 study: Multicenter, randomized, placebo controlled, and double blind for the first 24 weeks, followed by an extension period out to 52 weeks. For inclusion in the study, patients had to be aged 18-75 years and have active rheumatoid arthritis and an inadequate response to previous treatment with conventional synthetic disease-modifying antirheumatic drugs.
Of 1085 patients who were initially screened, 566 were randomly allocated to receive placebo (n = 188), SHR0302 4 mg (n = 189), or SHR0302 8 mg (n = 189). The average age of patients was 51 years, and 13.3% of patients were older than 65 years.
Additional Results
Alongside improvements in ACR responses, Ms Liu reported that a significantly higher proportion of patients treated with SHR0302 vs placebo achieved a Disease Activity Score in 28 joints based on C-reactive protein less than 2.6 (29.6% with 4 mg and 39.2% with 8 mg vs 4.2% with placebo; both P < .0001) and at least 3.2 (57.1% and 46.0% vs 15.4%; both P < .0001) at 24 weeks.
There were also greater improvements seen in Health Assessment Questionnaire-Disability Index, 36-item Short-Form (SF36) physical component summary, and SF36 mental component summary scores for active vs placebo treatment.
As for AEs, there were no surprises. During the main 24-week trial period, 81.5%, 90.5%, and 79.3% of patients treated with SHR0302 4 and 8 mg and placebo, respectively, experienced any AE.
Infection-related treatment-emergent adverse effects occurred slightly more often in the SHR0302-treated groups (40.2% for 4 mg and 40.7% for 8 mg) than in the placebo group (34.0%). There was a single case of serious infection that required treatment in the SHR0302 8 mg–treated group but no cases of systemic opportunistic infection.
There was one thromboembolic event and one major cardiovascular event in the 24-week period, both occurring in patients treated with SHR0302 8 mg. There were also single cases of each reported during the extension phase of the trial, but both were in the placebo arm.
Two cases of liver function abnormality — one each in the SHR0302 4- and 8-mg groups — were recorded during the main part of the trial and two cases — both in the SHR0302 4-mg group — during the extension phase.
As for malignancy, there was a single, newly diagnosed case in the SHR0302 4 mg group in the first part of the trial and two cases, both in the SHR0302 4-mg group, during the extension phase.
“We hope this [JAK inhibitor] will be for everybody. But, you know, if it’s for patients, globally, more clinical trials would be required,” Ms. Liu said in an interview. The future, she added, was to start accumulating some real-world data and perhaps do a trial comparing SHR0302 with another JAK inhibitor or a tumor necrosis factor inhibitor.
Another JAK in the Box?
Following her presentation, Ms. Liu at EULAR 2024 was quizzed as to why there were so many screening failures. She responded that she did not have the full data to answer the question but noted that some patients in her center had been worried about being randomized to a placebo. This trial has also been conducted during the COVID-19 pandemic, so that may have been a contributing factor with patients unable to get to their follow-up appointments.
Iain B. McInnes, MD, PhD, vice principal, professor of rheumatology, and head of the College of Medical, Veterinary & Life Sciences at the University of Glasgow, Glasgow, Scotland, commented on the study, saying: “The JAK field is in evolution. We need to understand the broader toxicities. There is an unexplained mechanism driving potential cardiovascular and malignant risk in a small proportion of patients receiving the drugs.”
Dr. McInnes added, “It’s really unclear whether the solution is going to be greater selectivity and potency, or whether we need to think really about selecting the right patients for a JAK inhibitor.”
The study was funded by Jiangsu Hengrui Pharmaceuticals. Two of the 18 authors of the abstract were employees of the sponsoring company, but Ms. Liu reported having no conflicts of interest. Dr. McInnes reported serving on speaker’s bureaus for AbbVie and UCB; receiving consulting fees received from AbbVie, Amgen, AstraZeneca, Bristol-Myers Squibb, Causeway Therapeutics, Cabaletta Bio, Compugen, Eli Lilly, Evelo, Gilead, Janssen, Novartis, MoonLake Immunotherapeutics, Pfizer, Sanofi Regeneron, and UCB; and receiving grant/research support from GlaxoSmithKline, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Janssen, Novartis, and UCB.
A version of this article appeared on Medscape.com.
VIENNA — The highly selective oral Janus kinase (JAK) inhibitor SHR0302 (ivarmacitinib) enables more patients with active rheumatoid arthritis to meet American College of Rheumatology (ACR) response criteria than placebo, the results of a phase 3 trial showed.
After 24 weeks of daily treatment, the primary endpoint of an ACR20 response was met by 40.4% of those who had been given placebo, 70.4% who had received a 4-mg dose, and 75.1% given an 8-mg dose. At the same time point, ACR50 responses were a respective 15.4%, 46.0%, and 57.1%, and ACR70 responses were 6.9%, 22.2%, and 31.7%. All analyses comparing SHR0302 vs placebo were highly significant (P < .0001).
First Phase 3 Trial in China
“This is the first highly selective JAK inhibitor originally developed, and a phase 3 clinical trial conducted, [exclusively] in China,” Jinjing Liu, from the department of rheumatology at Peking Union Medical College Hospital in Beijing, China, said in an interview.
Ms. Liu presented the results at the European Alliance of Associations for Rheumatology (EULAR) 2024 Annual Meeting, during the Abstract Plenary, which highlights the best-scored abstracts of the meeting.
“We are working our best to provide more choices for Chinese patients,” Ms. Liu said, which includes lowering the financial cost of treatments. A locally developed JAK inhibitor could potentially be a much cheaper option than other alternatives that are currently available, she said.
But it is more than that, Ms. Liu said. “The selectivity of SHR0302 for JAK 1 is nine times greater than for JAK 2, so it surpasses either tofacitinib or baricitinib.” The theory is that this higher selectivity for JAK 1 over JAK 2 could lead to fewer adverse events (AEs).
“Maybe it will result in lower JAK 2–associated hematologic side effects,” Ms. Liu said.
“We have noticed that, throughout the clinical trial, the most commonly reported AEs in the drug groups were upper extremity infection [21.7%-22.8% vs 13.8% for placebo] and hyperlipidemia [12.2%-15.3% vs 5.3%].” And for the control group, she said that anemia was the second highest reported AE, at 11.7% vs 6.3% and 7.4% for SHR0302 4 and 8 mg, respectively.
Standard Design
The trial design was typical for a phase 3 study: Multicenter, randomized, placebo controlled, and double blind for the first 24 weeks, followed by an extension period out to 52 weeks. For inclusion in the study, patients had to be aged 18-75 years and have active rheumatoid arthritis and an inadequate response to previous treatment with conventional synthetic disease-modifying antirheumatic drugs.
Of 1085 patients who were initially screened, 566 were randomly allocated to receive placebo (n = 188), SHR0302 4 mg (n = 189), or SHR0302 8 mg (n = 189). The average age of patients was 51 years, and 13.3% of patients were older than 65 years.
Additional Results
Alongside improvements in ACR responses, Ms Liu reported that a significantly higher proportion of patients treated with SHR0302 vs placebo achieved a Disease Activity Score in 28 joints based on C-reactive protein less than 2.6 (29.6% with 4 mg and 39.2% with 8 mg vs 4.2% with placebo; both P < .0001) and at least 3.2 (57.1% and 46.0% vs 15.4%; both P < .0001) at 24 weeks.
There were also greater improvements seen in Health Assessment Questionnaire-Disability Index, 36-item Short-Form (SF36) physical component summary, and SF36 mental component summary scores for active vs placebo treatment.
As for AEs, there were no surprises. During the main 24-week trial period, 81.5%, 90.5%, and 79.3% of patients treated with SHR0302 4 and 8 mg and placebo, respectively, experienced any AE.
Infection-related treatment-emergent adverse effects occurred slightly more often in the SHR0302-treated groups (40.2% for 4 mg and 40.7% for 8 mg) than in the placebo group (34.0%). There was a single case of serious infection that required treatment in the SHR0302 8 mg–treated group but no cases of systemic opportunistic infection.
There was one thromboembolic event and one major cardiovascular event in the 24-week period, both occurring in patients treated with SHR0302 8 mg. There were also single cases of each reported during the extension phase of the trial, but both were in the placebo arm.
Two cases of liver function abnormality — one each in the SHR0302 4- and 8-mg groups — were recorded during the main part of the trial and two cases — both in the SHR0302 4-mg group — during the extension phase.
As for malignancy, there was a single, newly diagnosed case in the SHR0302 4 mg group in the first part of the trial and two cases, both in the SHR0302 4-mg group, during the extension phase.
“We hope this [JAK inhibitor] will be for everybody. But, you know, if it’s for patients, globally, more clinical trials would be required,” Ms. Liu said in an interview. The future, she added, was to start accumulating some real-world data and perhaps do a trial comparing SHR0302 with another JAK inhibitor or a tumor necrosis factor inhibitor.
Another JAK in the Box?
Following her presentation, Ms. Liu at EULAR 2024 was quizzed as to why there were so many screening failures. She responded that she did not have the full data to answer the question but noted that some patients in her center had been worried about being randomized to a placebo. This trial has also been conducted during the COVID-19 pandemic, so that may have been a contributing factor with patients unable to get to their follow-up appointments.
Iain B. McInnes, MD, PhD, vice principal, professor of rheumatology, and head of the College of Medical, Veterinary & Life Sciences at the University of Glasgow, Glasgow, Scotland, commented on the study, saying: “The JAK field is in evolution. We need to understand the broader toxicities. There is an unexplained mechanism driving potential cardiovascular and malignant risk in a small proportion of patients receiving the drugs.”
Dr. McInnes added, “It’s really unclear whether the solution is going to be greater selectivity and potency, or whether we need to think really about selecting the right patients for a JAK inhibitor.”
The study was funded by Jiangsu Hengrui Pharmaceuticals. Two of the 18 authors of the abstract were employees of the sponsoring company, but Ms. Liu reported having no conflicts of interest. Dr. McInnes reported serving on speaker’s bureaus for AbbVie and UCB; receiving consulting fees received from AbbVie, Amgen, AstraZeneca, Bristol-Myers Squibb, Causeway Therapeutics, Cabaletta Bio, Compugen, Eli Lilly, Evelo, Gilead, Janssen, Novartis, MoonLake Immunotherapeutics, Pfizer, Sanofi Regeneron, and UCB; and receiving grant/research support from GlaxoSmithKline, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Janssen, Novartis, and UCB.
A version of this article appeared on Medscape.com.
FROM EULAR 2024
Obesity and Pregnancy
Do Artificial Sweeteners Really Help People With Diabetes?
It seems intuitive that, because people with type 2 diabetes (T2D) generally need to avoid sugar, clinicians should recommend eating foods and using recipes containing artificial sweeteners such as sucralose instead.
Splenda, which produces sucralose and other non-sugar sweeteners (NSS), is a sponsor of the American Diabetes Association (ADA) Diabetes Food Hub. Earlier in 2024, the ADA settled a lawsuit regarding its former director of nutrition’s refusal to approve recipes containing sucralose (Splenda), which she believed “flew in the face of the ADA’s mission.”
“There’s not a lot of evidence that sweeteners like sucralose provide significant benefits, especially over the long term,” said Susan Swithers, PhD, professor, department of psychological sciences and associate dean for faculty affairs at Purdue University, West Lafayette, Indiana.
Dr. Swithers authored an article several years ago cautioning that consuming nonnutritive sweeteners in beverages not only fails to prevent disease but also is associated with an increase in risks for the same health outcomes associated with sugar-sweetened beverages, including T2D, cardiovascular disease, hypertension, and stroke.
“At this point, we have pretty good evidence that these chemicals that were once touted as being completely inert are, in fact, not inert,” she said. “We know that they’re unlikely to be toxic in the short term, but they are not benign, and they have consequences. Right now, we have little understanding of the outcomes of consumption of these products chronically.”
What the Science Says
In 2023, the World Health Organization (WHO) released a guideline on NSS that recommended against their use for weight control or to reduce the risk for noncommunicable diseases.
The systematic review and meta-analysis upon which the guideline is based found that high intakes of NSS were associated with increases in body mass index and, as Dr. Swithers found, risks of developing T2D, cardiovascular events, and any type of stroke, as well as hypertension, bladder cancer, and all-cause mortality.
In a press release announcing the guideline, Francesco Branca, WHO director for Nutrition and Food Safety, said, “NSS are not essential dietary factors and have no nutritional value. People should reduce the sweetness of the diet altogether, starting early in life, to improve their health.”
The “common” NSS named by WHO included sucralose, as well as acesulfame K, aspartame, advantame, cyclamates, neotame, saccharin, stevia, and stevia derivatives.
If NSS consumption can increase T2D risk, what about people who already have T2D?
Some research suggests that NSS may affect people with and without T2D differently, said Dr. Swithers. For example, one small study showed that sucralose enhanced glucagon-like peptide 1 release and lowered blood glucose in healthy patients but not in patients with newly diagnosed T2D.
Similarly, Jotham Suez, PhD, an assistant professor in the department of molecular microbiology and immunology at Johns Hopkins Bloomberg School of Public Health in Baltimore, Maryland, said in an interview that his group “showed for the first time in 2014 that disruption of the microbiome by artificial sweeteners is causally linked to disrupted glycemic control.”
Recently, the team studied the impact of sucralose, aspartame, saccharin, and stevia in healthy adults and “were surprised to discover that all four sweeteners altered gut bacteria and the molecules they secrete,” he said. However, subsequent glucose tolerance tests in healthy humans showed varying results, “suggesting that human microbiome responses to the nonnutritive sweeteners we assessed are highly personalized and may lead to glycemic alterations in some, but not all, consumers depending on their microbes and the sweeteners they consume.”
Nevertheless, a recent review led by researchers in Mexico concluded that sucralose consumption “is associated with various adverse health effects. Despite being considered safe following previous studies, recent research suggests possible links to systemic inflammation, metabolic diseases, disruptions in gut microbiota, liver damage, and toxic effects at the cellular level.”
In addition, they wrote, “it is crucial to highlight the persistence of sucralose in the body, its ability to cross the placenta, and its presence in breast milk, raising concerns about prenatal and neonatal exposure.”
Sabyasachi Sen, MD, a professor of biochemistry and molecular medicine at George Washington School of Medicine & Health Sciences, Washington, DC, has led and coauthored preclinical and clinical studies demonstrating the potential ill effects of sucralose and other artificial sweeteners. One showed that sucralose and acesulfame potassium–containing diet soda altered microbial taxa in two pilot studies in healthy young adults; another showed a connection between artificial sweeteners and inflammation.
But Dr. Sen’s current work is directed at his team’s finding that sucralose promotes the accumulation of reactive oxygen species and adipogenesis in human stem cells, he said in an interview. “It is essentially an additive that is clearly harmful to cells. Our concern is that stem cells are going to remain in the system for a long period of time. If it is causing inflammation in these cells, then that may lead to adverse outcomes.”
Ruchi Mathur, MD, director of the Diabetes Outpatient Treatment & Education Center at Cedars-Sinai in Los Angeles, California, is the principal investigator of a recent study suggesting that non-aspartame NSS and aspartame alone may alter the structure and function of the stool and duodenal microbiomes. Levels of circulating inflammatory markers were also altered in participants who consumed artificial sweeteners, compared with control participants who did not.
In addition to these potential adverse effects, “we have to think about the fact that patients with diabetes often have other comorbidities like obesity and are at higher risk for cardiovascular disease and other conditions,” she said in an interview. “If you’re taking a patient who’s already at risk for those things and you don’t have a detailed discussion with them about pros and cons, you’re doing them a disservice.”
Industry Interests
Addressing the largely negative but varying findings, Dr. Swithers said, “one of the difficulties with getting clear answers about the science is that the food and beverage industry has an interest in confusing the picture. If people are selling or using a product, the best thing is for them not have a clear reason to change their behavior. All that needs to happen is for them to be able say, ‘well, it’s not clear, and we don’t really know what’s going on, so I’m just going to keep doing what I’m doing.’ Then the producers and sellers of that product have won.”
“As Upton Sinclair said,” she added, “‘It is difficult to get a man to understand something when his salary depends on his not understanding it.’ When organizations like ADA appear to be promoting a product like sucralose, and they’re not always being clear about disclosing the funding, I think that’s problematic.”
In fact, some recipes in the ADA’s hub that contain Splenda are marked sponsored, such as the four-ingredient peanut butter cookies; others, such as gluten-free brownies, are not — even though the latter contains “1/4 cup plus 1 tbsp” of Splenda Sugar Blend (Splenda produces several nonnutritive sweeteners, not all of which contain sucralose). Splenda is a sponsor of the ADA’s hub.
Consume in Moderation?
Regarding the use of Splenda products, Robert Gabbay, MD, PhD, the ADA’s chief scientific and medical officer, said in an interview that “some people with diabetes are accustomed to regularly consuming sugar-sweetened products, which can make management of their diabetes more challenging. As highlighted in the ADA’s Standards of Care, nonnutritive sweeteners (containing few or no calories) may be an acceptable substitute for sweeteners that contain sugar and calories when consumed in moderation. By providing a diabetes-friendly way to prepare foods people are used to eating, we can meet people where they are in offering support to effectively manage their diabetes.”
Of course, “moderation” means different things to different people. “With sucralose in particular, you can bake with it, you can cook with it, and beverages and packaged foods contain it, so it’s easy to end up overconsuming foods that may be fine if they’re occasional treats but aren’t healthy choices to have every single day,” Dr. Swithers said. “If you’re having a cookie containing sucralose once a week, it’s not a big deal, but if you’re having a cookie or a brownie every day, that’s something different.”
“I think ‘everything in moderation’ is a very reasonable approach here,” Dr. Mathur said. “Anything too much is probably not good, and that includes sweeteners like sucralose and others.”
Dr. Suez, whose team is currently exploring the mechanisms through which gut bacteria interact with nonnutritive sweeteners in the pathogenesis of cardiometabolic diseases, was more circumspect.
“We believe that additional, long-term, and non–industry-sponsored studies in humans are needed before we can make a recommendation in favor or against the use of nonnutritive sweeteners,” he said.
“However, our results demonstrating that nonnutritive sweeteners are not inert, when taken together with a growing body of evidence on potential harms of these sweeteners, merit caution until additional studies are completed,” he added. “Our findings do not imply in any way that sugar consumption, shown to be harmful to human health in many studies, is superior to nonnutritive sweeteners. Sugar consumption should be minimized, especially in individuals with obesity or diabetes. Of all the options, unsweetened beverages, specifically water, seem to be the safest and best options.”
Dr. Sen, who also “tries to convince patients to have sparkling or cold bottled water,” instead of artificially sweetened soda, agreed. “If a diabetes patient is trying to choose between sugar and sucralose, I’m not sure which one is worse.”
Dr. Swithers, Dr. Mathur, Dr. Sen, and Dr. Suez declared no competing interests.
A version of this article first appeared on Medscape.com.
It seems intuitive that, because people with type 2 diabetes (T2D) generally need to avoid sugar, clinicians should recommend eating foods and using recipes containing artificial sweeteners such as sucralose instead.
Splenda, which produces sucralose and other non-sugar sweeteners (NSS), is a sponsor of the American Diabetes Association (ADA) Diabetes Food Hub. Earlier in 2024, the ADA settled a lawsuit regarding its former director of nutrition’s refusal to approve recipes containing sucralose (Splenda), which she believed “flew in the face of the ADA’s mission.”
“There’s not a lot of evidence that sweeteners like sucralose provide significant benefits, especially over the long term,” said Susan Swithers, PhD, professor, department of psychological sciences and associate dean for faculty affairs at Purdue University, West Lafayette, Indiana.
Dr. Swithers authored an article several years ago cautioning that consuming nonnutritive sweeteners in beverages not only fails to prevent disease but also is associated with an increase in risks for the same health outcomes associated with sugar-sweetened beverages, including T2D, cardiovascular disease, hypertension, and stroke.
“At this point, we have pretty good evidence that these chemicals that were once touted as being completely inert are, in fact, not inert,” she said. “We know that they’re unlikely to be toxic in the short term, but they are not benign, and they have consequences. Right now, we have little understanding of the outcomes of consumption of these products chronically.”
What the Science Says
In 2023, the World Health Organization (WHO) released a guideline on NSS that recommended against their use for weight control or to reduce the risk for noncommunicable diseases.
The systematic review and meta-analysis upon which the guideline is based found that high intakes of NSS were associated with increases in body mass index and, as Dr. Swithers found, risks of developing T2D, cardiovascular events, and any type of stroke, as well as hypertension, bladder cancer, and all-cause mortality.
In a press release announcing the guideline, Francesco Branca, WHO director for Nutrition and Food Safety, said, “NSS are not essential dietary factors and have no nutritional value. People should reduce the sweetness of the diet altogether, starting early in life, to improve their health.”
The “common” NSS named by WHO included sucralose, as well as acesulfame K, aspartame, advantame, cyclamates, neotame, saccharin, stevia, and stevia derivatives.
If NSS consumption can increase T2D risk, what about people who already have T2D?
Some research suggests that NSS may affect people with and without T2D differently, said Dr. Swithers. For example, one small study showed that sucralose enhanced glucagon-like peptide 1 release and lowered blood glucose in healthy patients but not in patients with newly diagnosed T2D.
Similarly, Jotham Suez, PhD, an assistant professor in the department of molecular microbiology and immunology at Johns Hopkins Bloomberg School of Public Health in Baltimore, Maryland, said in an interview that his group “showed for the first time in 2014 that disruption of the microbiome by artificial sweeteners is causally linked to disrupted glycemic control.”
Recently, the team studied the impact of sucralose, aspartame, saccharin, and stevia in healthy adults and “were surprised to discover that all four sweeteners altered gut bacteria and the molecules they secrete,” he said. However, subsequent glucose tolerance tests in healthy humans showed varying results, “suggesting that human microbiome responses to the nonnutritive sweeteners we assessed are highly personalized and may lead to glycemic alterations in some, but not all, consumers depending on their microbes and the sweeteners they consume.”
Nevertheless, a recent review led by researchers in Mexico concluded that sucralose consumption “is associated with various adverse health effects. Despite being considered safe following previous studies, recent research suggests possible links to systemic inflammation, metabolic diseases, disruptions in gut microbiota, liver damage, and toxic effects at the cellular level.”
In addition, they wrote, “it is crucial to highlight the persistence of sucralose in the body, its ability to cross the placenta, and its presence in breast milk, raising concerns about prenatal and neonatal exposure.”
Sabyasachi Sen, MD, a professor of biochemistry and molecular medicine at George Washington School of Medicine & Health Sciences, Washington, DC, has led and coauthored preclinical and clinical studies demonstrating the potential ill effects of sucralose and other artificial sweeteners. One showed that sucralose and acesulfame potassium–containing diet soda altered microbial taxa in two pilot studies in healthy young adults; another showed a connection between artificial sweeteners and inflammation.
But Dr. Sen’s current work is directed at his team’s finding that sucralose promotes the accumulation of reactive oxygen species and adipogenesis in human stem cells, he said in an interview. “It is essentially an additive that is clearly harmful to cells. Our concern is that stem cells are going to remain in the system for a long period of time. If it is causing inflammation in these cells, then that may lead to adverse outcomes.”
Ruchi Mathur, MD, director of the Diabetes Outpatient Treatment & Education Center at Cedars-Sinai in Los Angeles, California, is the principal investigator of a recent study suggesting that non-aspartame NSS and aspartame alone may alter the structure and function of the stool and duodenal microbiomes. Levels of circulating inflammatory markers were also altered in participants who consumed artificial sweeteners, compared with control participants who did not.
In addition to these potential adverse effects, “we have to think about the fact that patients with diabetes often have other comorbidities like obesity and are at higher risk for cardiovascular disease and other conditions,” she said in an interview. “If you’re taking a patient who’s already at risk for those things and you don’t have a detailed discussion with them about pros and cons, you’re doing them a disservice.”
Industry Interests
Addressing the largely negative but varying findings, Dr. Swithers said, “one of the difficulties with getting clear answers about the science is that the food and beverage industry has an interest in confusing the picture. If people are selling or using a product, the best thing is for them not have a clear reason to change their behavior. All that needs to happen is for them to be able say, ‘well, it’s not clear, and we don’t really know what’s going on, so I’m just going to keep doing what I’m doing.’ Then the producers and sellers of that product have won.”
“As Upton Sinclair said,” she added, “‘It is difficult to get a man to understand something when his salary depends on his not understanding it.’ When organizations like ADA appear to be promoting a product like sucralose, and they’re not always being clear about disclosing the funding, I think that’s problematic.”
In fact, some recipes in the ADA’s hub that contain Splenda are marked sponsored, such as the four-ingredient peanut butter cookies; others, such as gluten-free brownies, are not — even though the latter contains “1/4 cup plus 1 tbsp” of Splenda Sugar Blend (Splenda produces several nonnutritive sweeteners, not all of which contain sucralose). Splenda is a sponsor of the ADA’s hub.
Consume in Moderation?
Regarding the use of Splenda products, Robert Gabbay, MD, PhD, the ADA’s chief scientific and medical officer, said in an interview that “some people with diabetes are accustomed to regularly consuming sugar-sweetened products, which can make management of their diabetes more challenging. As highlighted in the ADA’s Standards of Care, nonnutritive sweeteners (containing few or no calories) may be an acceptable substitute for sweeteners that contain sugar and calories when consumed in moderation. By providing a diabetes-friendly way to prepare foods people are used to eating, we can meet people where they are in offering support to effectively manage their diabetes.”
Of course, “moderation” means different things to different people. “With sucralose in particular, you can bake with it, you can cook with it, and beverages and packaged foods contain it, so it’s easy to end up overconsuming foods that may be fine if they’re occasional treats but aren’t healthy choices to have every single day,” Dr. Swithers said. “If you’re having a cookie containing sucralose once a week, it’s not a big deal, but if you’re having a cookie or a brownie every day, that’s something different.”
“I think ‘everything in moderation’ is a very reasonable approach here,” Dr. Mathur said. “Anything too much is probably not good, and that includes sweeteners like sucralose and others.”
Dr. Suez, whose team is currently exploring the mechanisms through which gut bacteria interact with nonnutritive sweeteners in the pathogenesis of cardiometabolic diseases, was more circumspect.
“We believe that additional, long-term, and non–industry-sponsored studies in humans are needed before we can make a recommendation in favor or against the use of nonnutritive sweeteners,” he said.
“However, our results demonstrating that nonnutritive sweeteners are not inert, when taken together with a growing body of evidence on potential harms of these sweeteners, merit caution until additional studies are completed,” he added. “Our findings do not imply in any way that sugar consumption, shown to be harmful to human health in many studies, is superior to nonnutritive sweeteners. Sugar consumption should be minimized, especially in individuals with obesity or diabetes. Of all the options, unsweetened beverages, specifically water, seem to be the safest and best options.”
Dr. Sen, who also “tries to convince patients to have sparkling or cold bottled water,” instead of artificially sweetened soda, agreed. “If a diabetes patient is trying to choose between sugar and sucralose, I’m not sure which one is worse.”
Dr. Swithers, Dr. Mathur, Dr. Sen, and Dr. Suez declared no competing interests.
A version of this article first appeared on Medscape.com.
It seems intuitive that, because people with type 2 diabetes (T2D) generally need to avoid sugar, clinicians should recommend eating foods and using recipes containing artificial sweeteners such as sucralose instead.
Splenda, which produces sucralose and other non-sugar sweeteners (NSS), is a sponsor of the American Diabetes Association (ADA) Diabetes Food Hub. Earlier in 2024, the ADA settled a lawsuit regarding its former director of nutrition’s refusal to approve recipes containing sucralose (Splenda), which she believed “flew in the face of the ADA’s mission.”
“There’s not a lot of evidence that sweeteners like sucralose provide significant benefits, especially over the long term,” said Susan Swithers, PhD, professor, department of psychological sciences and associate dean for faculty affairs at Purdue University, West Lafayette, Indiana.
Dr. Swithers authored an article several years ago cautioning that consuming nonnutritive sweeteners in beverages not only fails to prevent disease but also is associated with an increase in risks for the same health outcomes associated with sugar-sweetened beverages, including T2D, cardiovascular disease, hypertension, and stroke.
“At this point, we have pretty good evidence that these chemicals that were once touted as being completely inert are, in fact, not inert,” she said. “We know that they’re unlikely to be toxic in the short term, but they are not benign, and they have consequences. Right now, we have little understanding of the outcomes of consumption of these products chronically.”
What the Science Says
In 2023, the World Health Organization (WHO) released a guideline on NSS that recommended against their use for weight control or to reduce the risk for noncommunicable diseases.
The systematic review and meta-analysis upon which the guideline is based found that high intakes of NSS were associated with increases in body mass index and, as Dr. Swithers found, risks of developing T2D, cardiovascular events, and any type of stroke, as well as hypertension, bladder cancer, and all-cause mortality.
In a press release announcing the guideline, Francesco Branca, WHO director for Nutrition and Food Safety, said, “NSS are not essential dietary factors and have no nutritional value. People should reduce the sweetness of the diet altogether, starting early in life, to improve their health.”
The “common” NSS named by WHO included sucralose, as well as acesulfame K, aspartame, advantame, cyclamates, neotame, saccharin, stevia, and stevia derivatives.
If NSS consumption can increase T2D risk, what about people who already have T2D?
Some research suggests that NSS may affect people with and without T2D differently, said Dr. Swithers. For example, one small study showed that sucralose enhanced glucagon-like peptide 1 release and lowered blood glucose in healthy patients but not in patients with newly diagnosed T2D.
Similarly, Jotham Suez, PhD, an assistant professor in the department of molecular microbiology and immunology at Johns Hopkins Bloomberg School of Public Health in Baltimore, Maryland, said in an interview that his group “showed for the first time in 2014 that disruption of the microbiome by artificial sweeteners is causally linked to disrupted glycemic control.”
Recently, the team studied the impact of sucralose, aspartame, saccharin, and stevia in healthy adults and “were surprised to discover that all four sweeteners altered gut bacteria and the molecules they secrete,” he said. However, subsequent glucose tolerance tests in healthy humans showed varying results, “suggesting that human microbiome responses to the nonnutritive sweeteners we assessed are highly personalized and may lead to glycemic alterations in some, but not all, consumers depending on their microbes and the sweeteners they consume.”
Nevertheless, a recent review led by researchers in Mexico concluded that sucralose consumption “is associated with various adverse health effects. Despite being considered safe following previous studies, recent research suggests possible links to systemic inflammation, metabolic diseases, disruptions in gut microbiota, liver damage, and toxic effects at the cellular level.”
In addition, they wrote, “it is crucial to highlight the persistence of sucralose in the body, its ability to cross the placenta, and its presence in breast milk, raising concerns about prenatal and neonatal exposure.”
Sabyasachi Sen, MD, a professor of biochemistry and molecular medicine at George Washington School of Medicine & Health Sciences, Washington, DC, has led and coauthored preclinical and clinical studies demonstrating the potential ill effects of sucralose and other artificial sweeteners. One showed that sucralose and acesulfame potassium–containing diet soda altered microbial taxa in two pilot studies in healthy young adults; another showed a connection between artificial sweeteners and inflammation.
But Dr. Sen’s current work is directed at his team’s finding that sucralose promotes the accumulation of reactive oxygen species and adipogenesis in human stem cells, he said in an interview. “It is essentially an additive that is clearly harmful to cells. Our concern is that stem cells are going to remain in the system for a long period of time. If it is causing inflammation in these cells, then that may lead to adverse outcomes.”
Ruchi Mathur, MD, director of the Diabetes Outpatient Treatment & Education Center at Cedars-Sinai in Los Angeles, California, is the principal investigator of a recent study suggesting that non-aspartame NSS and aspartame alone may alter the structure and function of the stool and duodenal microbiomes. Levels of circulating inflammatory markers were also altered in participants who consumed artificial sweeteners, compared with control participants who did not.
In addition to these potential adverse effects, “we have to think about the fact that patients with diabetes often have other comorbidities like obesity and are at higher risk for cardiovascular disease and other conditions,” she said in an interview. “If you’re taking a patient who’s already at risk for those things and you don’t have a detailed discussion with them about pros and cons, you’re doing them a disservice.”
Industry Interests
Addressing the largely negative but varying findings, Dr. Swithers said, “one of the difficulties with getting clear answers about the science is that the food and beverage industry has an interest in confusing the picture. If people are selling or using a product, the best thing is for them not have a clear reason to change their behavior. All that needs to happen is for them to be able say, ‘well, it’s not clear, and we don’t really know what’s going on, so I’m just going to keep doing what I’m doing.’ Then the producers and sellers of that product have won.”
“As Upton Sinclair said,” she added, “‘It is difficult to get a man to understand something when his salary depends on his not understanding it.’ When organizations like ADA appear to be promoting a product like sucralose, and they’re not always being clear about disclosing the funding, I think that’s problematic.”
In fact, some recipes in the ADA’s hub that contain Splenda are marked sponsored, such as the four-ingredient peanut butter cookies; others, such as gluten-free brownies, are not — even though the latter contains “1/4 cup plus 1 tbsp” of Splenda Sugar Blend (Splenda produces several nonnutritive sweeteners, not all of which contain sucralose). Splenda is a sponsor of the ADA’s hub.
Consume in Moderation?
Regarding the use of Splenda products, Robert Gabbay, MD, PhD, the ADA’s chief scientific and medical officer, said in an interview that “some people with diabetes are accustomed to regularly consuming sugar-sweetened products, which can make management of their diabetes more challenging. As highlighted in the ADA’s Standards of Care, nonnutritive sweeteners (containing few or no calories) may be an acceptable substitute for sweeteners that contain sugar and calories when consumed in moderation. By providing a diabetes-friendly way to prepare foods people are used to eating, we can meet people where they are in offering support to effectively manage their diabetes.”
Of course, “moderation” means different things to different people. “With sucralose in particular, you can bake with it, you can cook with it, and beverages and packaged foods contain it, so it’s easy to end up overconsuming foods that may be fine if they’re occasional treats but aren’t healthy choices to have every single day,” Dr. Swithers said. “If you’re having a cookie containing sucralose once a week, it’s not a big deal, but if you’re having a cookie or a brownie every day, that’s something different.”
“I think ‘everything in moderation’ is a very reasonable approach here,” Dr. Mathur said. “Anything too much is probably not good, and that includes sweeteners like sucralose and others.”
Dr. Suez, whose team is currently exploring the mechanisms through which gut bacteria interact with nonnutritive sweeteners in the pathogenesis of cardiometabolic diseases, was more circumspect.
“We believe that additional, long-term, and non–industry-sponsored studies in humans are needed before we can make a recommendation in favor or against the use of nonnutritive sweeteners,” he said.
“However, our results demonstrating that nonnutritive sweeteners are not inert, when taken together with a growing body of evidence on potential harms of these sweeteners, merit caution until additional studies are completed,” he added. “Our findings do not imply in any way that sugar consumption, shown to be harmful to human health in many studies, is superior to nonnutritive sweeteners. Sugar consumption should be minimized, especially in individuals with obesity or diabetes. Of all the options, unsweetened beverages, specifically water, seem to be the safest and best options.”
Dr. Sen, who also “tries to convince patients to have sparkling or cold bottled water,” instead of artificially sweetened soda, agreed. “If a diabetes patient is trying to choose between sugar and sucralose, I’m not sure which one is worse.”
Dr. Swithers, Dr. Mathur, Dr. Sen, and Dr. Suez declared no competing interests.
A version of this article first appeared on Medscape.com.
Ultraprocessed Food Linked to Constipation
TOPLINE:
METHODOLOGY:
- Excess consumption of UPF has been linked to disturbed intestinal motility.
- Using data from the National Health and Nutrition Examination Survey (2005-2010), researchers performed a cross-sectional study to assess the association between UPF and MPF intake and bowel habits.
- They used two 24-hour dietary recalls to capture the participants’ dietary intake and subsequently categorized food items into MPF, processed culinary ingredients, processed food, and UPF, according to the Nova classification.
- The Bowel Health Questionnaire was used to assess bowel habits, with constipation and diarrhea being defined according to the Bristol Stool Form Scale and stool frequency.
- The odds ratios for constipation and diarrhea were calculated by comparing the quartiles of UPF and MPF consumption using survey-weighted logistic regressions adjusted for potential confounding factors.
TAKEAWAY:
- Researchers included 12,716 US adults, of whom 1290 and 1067 had constipation and diarrhea, respectively.
- Increased consumption of UPF was associated with more than two times increased odds of constipation; the association held after adjusting for diet quality, water intake, and fiber intake.
- Conversely, increased intake of MPF was associated with reduced odds of constipation; the association held after adjustment.
- Substituting 10% of UPF with an equivalent proportion of MPF was associated with 10% lower odds of constipation.
- Neither MPF nor UPF consumption was associated with increased odds of diarrhea.
IN PRACTICE:
“The persistently strong associations with [UPF] and MPF consumption despite adjustment for diet quality suggest that food processing plays a unique role in constipation,” the authors wrote.
SOURCE:
The study, led by Chun-Han Lo, MD, MPH, Department of Internal Medicine, Kirk Kerkorian School of Medicine at the University of Nevada, Las Vegas, was published online in Clinical Gastroenterology and Hepatology.
LIMITATIONS:
The evaluation of dietary intake using two 24-hour dietary recalls did not allow for the assessment of dietary changes over time. Misclassification bias could be present due to varying degrees of food processing across different brands. The authors could not fully account for unmeasured confounders owing to the observational nature of this study.
DISCLOSURES:
This study did not receive any funding. Some authors declared serving as consultants, being on advisory boards, or receiving research funding from various sources.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Excess consumption of UPF has been linked to disturbed intestinal motility.
- Using data from the National Health and Nutrition Examination Survey (2005-2010), researchers performed a cross-sectional study to assess the association between UPF and MPF intake and bowel habits.
- They used two 24-hour dietary recalls to capture the participants’ dietary intake and subsequently categorized food items into MPF, processed culinary ingredients, processed food, and UPF, according to the Nova classification.
- The Bowel Health Questionnaire was used to assess bowel habits, with constipation and diarrhea being defined according to the Bristol Stool Form Scale and stool frequency.
- The odds ratios for constipation and diarrhea were calculated by comparing the quartiles of UPF and MPF consumption using survey-weighted logistic regressions adjusted for potential confounding factors.
TAKEAWAY:
- Researchers included 12,716 US adults, of whom 1290 and 1067 had constipation and diarrhea, respectively.
- Increased consumption of UPF was associated with more than two times increased odds of constipation; the association held after adjusting for diet quality, water intake, and fiber intake.
- Conversely, increased intake of MPF was associated with reduced odds of constipation; the association held after adjustment.
- Substituting 10% of UPF with an equivalent proportion of MPF was associated with 10% lower odds of constipation.
- Neither MPF nor UPF consumption was associated with increased odds of diarrhea.
IN PRACTICE:
“The persistently strong associations with [UPF] and MPF consumption despite adjustment for diet quality suggest that food processing plays a unique role in constipation,” the authors wrote.
SOURCE:
The study, led by Chun-Han Lo, MD, MPH, Department of Internal Medicine, Kirk Kerkorian School of Medicine at the University of Nevada, Las Vegas, was published online in Clinical Gastroenterology and Hepatology.
LIMITATIONS:
The evaluation of dietary intake using two 24-hour dietary recalls did not allow for the assessment of dietary changes over time. Misclassification bias could be present due to varying degrees of food processing across different brands. The authors could not fully account for unmeasured confounders owing to the observational nature of this study.
DISCLOSURES:
This study did not receive any funding. Some authors declared serving as consultants, being on advisory boards, or receiving research funding from various sources.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Excess consumption of UPF has been linked to disturbed intestinal motility.
- Using data from the National Health and Nutrition Examination Survey (2005-2010), researchers performed a cross-sectional study to assess the association between UPF and MPF intake and bowel habits.
- They used two 24-hour dietary recalls to capture the participants’ dietary intake and subsequently categorized food items into MPF, processed culinary ingredients, processed food, and UPF, according to the Nova classification.
- The Bowel Health Questionnaire was used to assess bowel habits, with constipation and diarrhea being defined according to the Bristol Stool Form Scale and stool frequency.
- The odds ratios for constipation and diarrhea were calculated by comparing the quartiles of UPF and MPF consumption using survey-weighted logistic regressions adjusted for potential confounding factors.
TAKEAWAY:
- Researchers included 12,716 US adults, of whom 1290 and 1067 had constipation and diarrhea, respectively.
- Increased consumption of UPF was associated with more than two times increased odds of constipation; the association held after adjusting for diet quality, water intake, and fiber intake.
- Conversely, increased intake of MPF was associated with reduced odds of constipation; the association held after adjustment.
- Substituting 10% of UPF with an equivalent proportion of MPF was associated with 10% lower odds of constipation.
- Neither MPF nor UPF consumption was associated with increased odds of diarrhea.
IN PRACTICE:
“The persistently strong associations with [UPF] and MPF consumption despite adjustment for diet quality suggest that food processing plays a unique role in constipation,” the authors wrote.
SOURCE:
The study, led by Chun-Han Lo, MD, MPH, Department of Internal Medicine, Kirk Kerkorian School of Medicine at the University of Nevada, Las Vegas, was published online in Clinical Gastroenterology and Hepatology.
LIMITATIONS:
The evaluation of dietary intake using two 24-hour dietary recalls did not allow for the assessment of dietary changes over time. Misclassification bias could be present due to varying degrees of food processing across different brands. The authors could not fully account for unmeasured confounders owing to the observational nature of this study.
DISCLOSURES:
This study did not receive any funding. Some authors declared serving as consultants, being on advisory boards, or receiving research funding from various sources.
A version of this article first appeared on Medscape.com.
Experts Expect New Human Cases of Avian Flu
With avian influenza spreading quickly around the globe, the virus has more opportunities to mutate and cause problems for people. By some calculations, H5N1 bird flu is still at least two mutations away from widespread human infections, but experts warn that new flu symptoms in individuals at high risk are likely to start turning up in health systems this summer.
“Some of this will not be obvious or at the forefront of our minds.”
Dr. Dugan is leading the team of CDC scientists that is working with partners from the US Department of Agriculture, the US Food and Drug Administration (FDA), and state and local health departments to track and respond to the H5N1 bird flu outbreak currently sweeping through the United States.
Since 2022, avian influenza A viruses have been detected in more than 9300 wild birds in 50 states and territories and in commercial and backyard flocks.
“It’s a bad situation,” said Florian Krammer, PhD, professor of vaccinology at the Icahn School of Medicine at Mount Sinai in New York. “Globally, we’ve seen tons of exposure in cities around the world and even in the birds here in New York City where I am.”
Birds shed the virus in their saliva, mucous, and feces, so people or other animals with close, unprotected contact with infected birds or their contaminated environments can be infected.
And for the first time in March 2024, H5N1 bird flu was reported in dairy cows. The US Department of Agriculture said that at last count, 101 dairy herds in 12 states had been infected, with several cases also found in dairy workers.
From Birds to Cattle and Farm Workers
The National Veterinary Services Laboratories confirmed the infections were highly pathogenic avian influenza H5N1 clade 2.3.4.4b of Eurasian lineage. Also known as the goose, Guangdong clade from China, phylogenetic analysis and epidemiology suggests a single introduction into cows followed by onward transmission.
“I was surprised when H5 was introduced to dairy cattle in this way,” Dr. Dugan said. “Influenza viruses are always surprising us and it reminds me to stay humble and keep an open mind when dealing with them.”
People rarely inhale or get sufficient virus in their eyes or mouth to get sick, Dr. Dugan said, but those in close contact with animals are still at risk for infection, which could lead to upper respiratory tract symptoms such as shortness of breath, cough, sore throat, or runny or stuffy nose.
Like with other viruses, people can also experience muscle or body aches, headache, fatigue, fever or, as was seen in farm workers, conjunctivitis.
But there are less-common symptoms too like diarrhea, nausea, and vomiting — and sometimes, even seizures.
The risk to the general public is still low, Dr. Dugan said, but authorities recommend that people working with animals wash their hands with soap and water and wear personal protective equipment that includes fluid-resistant coveralls, a waterproof apron, a safety-approved respirator, properly fitted goggles or face shield, a head or hair cover, gloves, and boots.
Dr. Dugan said that health care providers often don’t take a history of occupational exposures when a patient presents with flu. But with rising rates of bird flu in new animal hosts, “this will be an important next step.”
Asking Unusual Questions
This approach is not standardized on most electronic health records, so these are questions that clinicians will need to initiate themselves.
“Physicians should ask about work,” said Meghan Davis, PhD, associate professor at the Johns Hopkins Bloomberg School of Public Health in Baltimore. “If it’s not already on the radar, asking about any direct contact with dairy cows, poultry, pigs, wild birds, or wild mammals is important.”
Dr. Davis says she’s worried about a new study tracking risk factors for farm-to-farm transmission because it shows that farms testing positive for avian influenza often have workers with a family member also employed on another farm. “This suggests that we might need to be on the lookout for possible transmission within families,” she said. Now, we have to ask “not just if the person with symptoms has contact with or works on a dairy farm, milk processing plant, or slaughterhouse, but also if any family member does.”
Dr. Davis said that it’s important to bear in mind when taking these histories that there may be younger workers on farms and in slaughter and processing facilities due to exemptions or illegal work.
What is important now is to get the situation under control this season in dairy cattle, Dr. Krammer said. “This will be easier to stop in cows than humans, so this is the time to stop moving dairy cattle and start vaccinating them.”
Spotting New Cases
Since April 2024, there have been three human cases of avian influenza after exposure to dairy cows reported. “And what we don’t want to see this summer is an unusual human cluster of influenza. It’s important we keep a close, watchful eye for this,” Dr. Krammer said.
“Influenza viruses do very interesting things and as we head into fall and winter flu season, we don’t want new human co-infections that could cause major problems for us,” he said.
If people become mixing vessels of a seasonal cocktail of multiple viruses, that could empower H5N1 to mutate again into something more dangerous, sparking a new pandemic.
“It wasn’t all that long ago that we were asking China difficult questions about the steps Chinese authorities took to protect human lives from SARS-CoV-2 in the COVID pandemic. Now, we must ask ourselves many of these questions,” Dr. Krammer said. “We are at a crucial crossroad where we will either elude a new pandemic or see one take off, risking 10 to 20 million lives.”
There is a precedent for safely evading more trouble, Dr. Krammer pointed out. Government agencies have already been working with the poultry industry for a couple of years now. “And here, we have successfully stopped H5N1 with new regulations and policies.”
But moving from poultry farms to cattle has not been an easy transition, Dr. Dugan said. Cattle farms have no experience with bird flu or tactics to contain it with regulations, and officials too are working in new, unfamiliar terrain.
“What we have now isn’t a science problem, it’s a policy issue, and it hasn’t always been clear who is in charge,” Dr. Krammer said.
“Agencies are working together at the state, federal, and global level,” said Dr. Dugan. “We are increasing our transparency and are working to share what we know, when we know it.”
The infrastructure built during the COVID pandemic has helped teams prepare for this new crisis, Dr. Dugan said. Year-round, layered monitoring has clinical labs reporting seasonal influenza and novel cases.
“Laboratories are ready to help with testing,” Dr. Dugan said.
Specimens should be collected as soon as possible from patients with flu symptoms. A nasopharyngeal swab is recommended with a nasal swab combined with an oropharyngeal swab. If a patient has conjunctivitis with or without respiratory symptoms, both a conjunctival swab and a nasopharyngeal swab should be collected.
People with severe respiratory disease should also have lower respiratory tract specimens collected.
Standard, contact, and airborne precautions are recommended for patients presenting for medical care who have illness consistent with influenza and recent exposure to birds or other animals.
Antiviral Drugs
There are four FDA-approved antivirals for influenza: Oseltamivir phosphate (available as a generic drug or by the trade name Tamiflu), zanamivir (Relenza), peramivir (Rapivab) , and baloxavir (Xofluza).
For people with suspected or confirmed avian influenza, treatment is recommended as soon as possible.
There are no clinical trials measuring the outcome of antivirals in people infected with avian influenza. However, data from animal models and human observational studies suggest a benefit.
“We can’t afford to wait this summer,” Dr. Krammer said. “We have an opportunity right now to stop this in cows before we risk infecting more people. I hope we do.”
A version of this article first appeared on Medscape.com.
With avian influenza spreading quickly around the globe, the virus has more opportunities to mutate and cause problems for people. By some calculations, H5N1 bird flu is still at least two mutations away from widespread human infections, but experts warn that new flu symptoms in individuals at high risk are likely to start turning up in health systems this summer.
“Some of this will not be obvious or at the forefront of our minds.”
Dr. Dugan is leading the team of CDC scientists that is working with partners from the US Department of Agriculture, the US Food and Drug Administration (FDA), and state and local health departments to track and respond to the H5N1 bird flu outbreak currently sweeping through the United States.
Since 2022, avian influenza A viruses have been detected in more than 9300 wild birds in 50 states and territories and in commercial and backyard flocks.
“It’s a bad situation,” said Florian Krammer, PhD, professor of vaccinology at the Icahn School of Medicine at Mount Sinai in New York. “Globally, we’ve seen tons of exposure in cities around the world and even in the birds here in New York City where I am.”
Birds shed the virus in their saliva, mucous, and feces, so people or other animals with close, unprotected contact with infected birds or their contaminated environments can be infected.
And for the first time in March 2024, H5N1 bird flu was reported in dairy cows. The US Department of Agriculture said that at last count, 101 dairy herds in 12 states had been infected, with several cases also found in dairy workers.
From Birds to Cattle and Farm Workers
The National Veterinary Services Laboratories confirmed the infections were highly pathogenic avian influenza H5N1 clade 2.3.4.4b of Eurasian lineage. Also known as the goose, Guangdong clade from China, phylogenetic analysis and epidemiology suggests a single introduction into cows followed by onward transmission.
“I was surprised when H5 was introduced to dairy cattle in this way,” Dr. Dugan said. “Influenza viruses are always surprising us and it reminds me to stay humble and keep an open mind when dealing with them.”
People rarely inhale or get sufficient virus in their eyes or mouth to get sick, Dr. Dugan said, but those in close contact with animals are still at risk for infection, which could lead to upper respiratory tract symptoms such as shortness of breath, cough, sore throat, or runny or stuffy nose.
Like with other viruses, people can also experience muscle or body aches, headache, fatigue, fever or, as was seen in farm workers, conjunctivitis.
But there are less-common symptoms too like diarrhea, nausea, and vomiting — and sometimes, even seizures.
The risk to the general public is still low, Dr. Dugan said, but authorities recommend that people working with animals wash their hands with soap and water and wear personal protective equipment that includes fluid-resistant coveralls, a waterproof apron, a safety-approved respirator, properly fitted goggles or face shield, a head or hair cover, gloves, and boots.
Dr. Dugan said that health care providers often don’t take a history of occupational exposures when a patient presents with flu. But with rising rates of bird flu in new animal hosts, “this will be an important next step.”
Asking Unusual Questions
This approach is not standardized on most electronic health records, so these are questions that clinicians will need to initiate themselves.
“Physicians should ask about work,” said Meghan Davis, PhD, associate professor at the Johns Hopkins Bloomberg School of Public Health in Baltimore. “If it’s not already on the radar, asking about any direct contact with dairy cows, poultry, pigs, wild birds, or wild mammals is important.”
Dr. Davis says she’s worried about a new study tracking risk factors for farm-to-farm transmission because it shows that farms testing positive for avian influenza often have workers with a family member also employed on another farm. “This suggests that we might need to be on the lookout for possible transmission within families,” she said. Now, we have to ask “not just if the person with symptoms has contact with or works on a dairy farm, milk processing plant, or slaughterhouse, but also if any family member does.”
Dr. Davis said that it’s important to bear in mind when taking these histories that there may be younger workers on farms and in slaughter and processing facilities due to exemptions or illegal work.
What is important now is to get the situation under control this season in dairy cattle, Dr. Krammer said. “This will be easier to stop in cows than humans, so this is the time to stop moving dairy cattle and start vaccinating them.”
Spotting New Cases
Since April 2024, there have been three human cases of avian influenza after exposure to dairy cows reported. “And what we don’t want to see this summer is an unusual human cluster of influenza. It’s important we keep a close, watchful eye for this,” Dr. Krammer said.
“Influenza viruses do very interesting things and as we head into fall and winter flu season, we don’t want new human co-infections that could cause major problems for us,” he said.
If people become mixing vessels of a seasonal cocktail of multiple viruses, that could empower H5N1 to mutate again into something more dangerous, sparking a new pandemic.
“It wasn’t all that long ago that we were asking China difficult questions about the steps Chinese authorities took to protect human lives from SARS-CoV-2 in the COVID pandemic. Now, we must ask ourselves many of these questions,” Dr. Krammer said. “We are at a crucial crossroad where we will either elude a new pandemic or see one take off, risking 10 to 20 million lives.”
There is a precedent for safely evading more trouble, Dr. Krammer pointed out. Government agencies have already been working with the poultry industry for a couple of years now. “And here, we have successfully stopped H5N1 with new regulations and policies.”
But moving from poultry farms to cattle has not been an easy transition, Dr. Dugan said. Cattle farms have no experience with bird flu or tactics to contain it with regulations, and officials too are working in new, unfamiliar terrain.
“What we have now isn’t a science problem, it’s a policy issue, and it hasn’t always been clear who is in charge,” Dr. Krammer said.
“Agencies are working together at the state, federal, and global level,” said Dr. Dugan. “We are increasing our transparency and are working to share what we know, when we know it.”
The infrastructure built during the COVID pandemic has helped teams prepare for this new crisis, Dr. Dugan said. Year-round, layered monitoring has clinical labs reporting seasonal influenza and novel cases.
“Laboratories are ready to help with testing,” Dr. Dugan said.
Specimens should be collected as soon as possible from patients with flu symptoms. A nasopharyngeal swab is recommended with a nasal swab combined with an oropharyngeal swab. If a patient has conjunctivitis with or without respiratory symptoms, both a conjunctival swab and a nasopharyngeal swab should be collected.
People with severe respiratory disease should also have lower respiratory tract specimens collected.
Standard, contact, and airborne precautions are recommended for patients presenting for medical care who have illness consistent with influenza and recent exposure to birds or other animals.
Antiviral Drugs
There are four FDA-approved antivirals for influenza: Oseltamivir phosphate (available as a generic drug or by the trade name Tamiflu), zanamivir (Relenza), peramivir (Rapivab) , and baloxavir (Xofluza).
For people with suspected or confirmed avian influenza, treatment is recommended as soon as possible.
There are no clinical trials measuring the outcome of antivirals in people infected with avian influenza. However, data from animal models and human observational studies suggest a benefit.
“We can’t afford to wait this summer,” Dr. Krammer said. “We have an opportunity right now to stop this in cows before we risk infecting more people. I hope we do.”
A version of this article first appeared on Medscape.com.
With avian influenza spreading quickly around the globe, the virus has more opportunities to mutate and cause problems for people. By some calculations, H5N1 bird flu is still at least two mutations away from widespread human infections, but experts warn that new flu symptoms in individuals at high risk are likely to start turning up in health systems this summer.
“Some of this will not be obvious or at the forefront of our minds.”
Dr. Dugan is leading the team of CDC scientists that is working with partners from the US Department of Agriculture, the US Food and Drug Administration (FDA), and state and local health departments to track and respond to the H5N1 bird flu outbreak currently sweeping through the United States.
Since 2022, avian influenza A viruses have been detected in more than 9300 wild birds in 50 states and territories and in commercial and backyard flocks.
“It’s a bad situation,” said Florian Krammer, PhD, professor of vaccinology at the Icahn School of Medicine at Mount Sinai in New York. “Globally, we’ve seen tons of exposure in cities around the world and even in the birds here in New York City where I am.”
Birds shed the virus in their saliva, mucous, and feces, so people or other animals with close, unprotected contact with infected birds or their contaminated environments can be infected.
And for the first time in March 2024, H5N1 bird flu was reported in dairy cows. The US Department of Agriculture said that at last count, 101 dairy herds in 12 states had been infected, with several cases also found in dairy workers.
From Birds to Cattle and Farm Workers
The National Veterinary Services Laboratories confirmed the infections were highly pathogenic avian influenza H5N1 clade 2.3.4.4b of Eurasian lineage. Also known as the goose, Guangdong clade from China, phylogenetic analysis and epidemiology suggests a single introduction into cows followed by onward transmission.
“I was surprised when H5 was introduced to dairy cattle in this way,” Dr. Dugan said. “Influenza viruses are always surprising us and it reminds me to stay humble and keep an open mind when dealing with them.”
People rarely inhale or get sufficient virus in their eyes or mouth to get sick, Dr. Dugan said, but those in close contact with animals are still at risk for infection, which could lead to upper respiratory tract symptoms such as shortness of breath, cough, sore throat, or runny or stuffy nose.
Like with other viruses, people can also experience muscle or body aches, headache, fatigue, fever or, as was seen in farm workers, conjunctivitis.
But there are less-common symptoms too like diarrhea, nausea, and vomiting — and sometimes, even seizures.
The risk to the general public is still low, Dr. Dugan said, but authorities recommend that people working with animals wash their hands with soap and water and wear personal protective equipment that includes fluid-resistant coveralls, a waterproof apron, a safety-approved respirator, properly fitted goggles or face shield, a head or hair cover, gloves, and boots.
Dr. Dugan said that health care providers often don’t take a history of occupational exposures when a patient presents with flu. But with rising rates of bird flu in new animal hosts, “this will be an important next step.”
Asking Unusual Questions
This approach is not standardized on most electronic health records, so these are questions that clinicians will need to initiate themselves.
“Physicians should ask about work,” said Meghan Davis, PhD, associate professor at the Johns Hopkins Bloomberg School of Public Health in Baltimore. “If it’s not already on the radar, asking about any direct contact with dairy cows, poultry, pigs, wild birds, or wild mammals is important.”
Dr. Davis says she’s worried about a new study tracking risk factors for farm-to-farm transmission because it shows that farms testing positive for avian influenza often have workers with a family member also employed on another farm. “This suggests that we might need to be on the lookout for possible transmission within families,” she said. Now, we have to ask “not just if the person with symptoms has contact with or works on a dairy farm, milk processing plant, or slaughterhouse, but also if any family member does.”
Dr. Davis said that it’s important to bear in mind when taking these histories that there may be younger workers on farms and in slaughter and processing facilities due to exemptions or illegal work.
What is important now is to get the situation under control this season in dairy cattle, Dr. Krammer said. “This will be easier to stop in cows than humans, so this is the time to stop moving dairy cattle and start vaccinating them.”
Spotting New Cases
Since April 2024, there have been three human cases of avian influenza after exposure to dairy cows reported. “And what we don’t want to see this summer is an unusual human cluster of influenza. It’s important we keep a close, watchful eye for this,” Dr. Krammer said.
“Influenza viruses do very interesting things and as we head into fall and winter flu season, we don’t want new human co-infections that could cause major problems for us,” he said.
If people become mixing vessels of a seasonal cocktail of multiple viruses, that could empower H5N1 to mutate again into something more dangerous, sparking a new pandemic.
“It wasn’t all that long ago that we were asking China difficult questions about the steps Chinese authorities took to protect human lives from SARS-CoV-2 in the COVID pandemic. Now, we must ask ourselves many of these questions,” Dr. Krammer said. “We are at a crucial crossroad where we will either elude a new pandemic or see one take off, risking 10 to 20 million lives.”
There is a precedent for safely evading more trouble, Dr. Krammer pointed out. Government agencies have already been working with the poultry industry for a couple of years now. “And here, we have successfully stopped H5N1 with new regulations and policies.”
But moving from poultry farms to cattle has not been an easy transition, Dr. Dugan said. Cattle farms have no experience with bird flu or tactics to contain it with regulations, and officials too are working in new, unfamiliar terrain.
“What we have now isn’t a science problem, it’s a policy issue, and it hasn’t always been clear who is in charge,” Dr. Krammer said.
“Agencies are working together at the state, federal, and global level,” said Dr. Dugan. “We are increasing our transparency and are working to share what we know, when we know it.”
The infrastructure built during the COVID pandemic has helped teams prepare for this new crisis, Dr. Dugan said. Year-round, layered monitoring has clinical labs reporting seasonal influenza and novel cases.
“Laboratories are ready to help with testing,” Dr. Dugan said.
Specimens should be collected as soon as possible from patients with flu symptoms. A nasopharyngeal swab is recommended with a nasal swab combined with an oropharyngeal swab. If a patient has conjunctivitis with or without respiratory symptoms, both a conjunctival swab and a nasopharyngeal swab should be collected.
People with severe respiratory disease should also have lower respiratory tract specimens collected.
Standard, contact, and airborne precautions are recommended for patients presenting for medical care who have illness consistent with influenza and recent exposure to birds or other animals.
Antiviral Drugs
There are four FDA-approved antivirals for influenza: Oseltamivir phosphate (available as a generic drug or by the trade name Tamiflu), zanamivir (Relenza), peramivir (Rapivab) , and baloxavir (Xofluza).
For people with suspected or confirmed avian influenza, treatment is recommended as soon as possible.
There are no clinical trials measuring the outcome of antivirals in people infected with avian influenza. However, data from animal models and human observational studies suggest a benefit.
“We can’t afford to wait this summer,” Dr. Krammer said. “We have an opportunity right now to stop this in cows before we risk infecting more people. I hope we do.”
A version of this article first appeared on Medscape.com.
Migraine Differential Diagnosis
VEXAS Syndrome: Study Highlights Cutaneous Symptoms
Additionally, the most common histologic findings include leukocytoclastic vasculitis, neutrophilic dermatosis, and perivascular dermatitis; different variants in the UBA1 gene are associated with specific skin manifestations.
Those are key findings from a cohort study of 112 patients with VEXAS published online in JAMA Dermatology. The study, conducted by researchers at the National Institutes of Health (NIH) and several other institutions, aimed to define the spectrum of cutaneous manifestations in VEXAS in association with genetic, histologic, and other clinical findings.
First described in 2020, VEXAS syndrome is an adult-onset multisystem disease that can pose a diagnostic challenge to clinicians, the study’s corresponding author, Edward W. Cowen, MD, MHSc, of the dermatology branch at the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), said in an interview. The disease is caused by pathogenic variants in the UBA1 gene, located on the X chromosome. Affected individuals exhibit a wide range of manifestations, including cytopenia/myelodysplasia, multiorgan systemic inflammation, and cutaneous involvement.
“Patients may present to a variety of disease specialists depending on their symptoms and providers may not immediately consider a genetic etiology in an older individual,” Dr. Cowen said in an interview. “Although skin involvement occurs in more than 80% of patients, it is pleomorphic and may resemble a variety of other conditions such as vasculitis and Sweet syndrome.”
To better understand the cutaneous manifestations of VEXAS syndrome, the researchers evaluated data from 112 patients with VEXAS-defining genetic variants in the UBA1 gene between 2019 and 2023. Of the 112 patients, 73 underwent medical record review only, and 39 were prospectively evaluated at NIH. All but one of the patients were men, 94% were White individuals, and their mean age was 64 years. Skin involvement occurred in 83% of cases and was the most common presenting feature of VEXAS in 61% of cases.
Of the 64 histopathologic reports available from 60 patients, the main skin histopathologic findings were leukocytoclastic vasculitis in 23 patients (36%), neutrophilic dermatosis in 22 patients (34%), and perivascular dermatitis in 19 patients (30%). According to Dr. Cowen, one key histologic finding was a distinct pattern of “histiocytoid” dermal neutrophilic inflammation, which was present in 13 of 15 specimens (86%) that underwent central re-review. “This pattern can occasionally also be seen in patients with Sweet syndrome, unrelated to VEXAS, but was a hallmark feature found in the majority of skin biopsies of patients with VEXAS,” he said.
“Together with another pathologic finding, leukocytoclasia, these features can be useful clues to alert the pathologist to a potential diagnosis of VEXAS. This myeloid predominant pattern of skin inflammation was also most strongly associated with the leucine pathogenic variant of the UBA1 gene.” In contrast, cutaneous vasculitis was most strongly associated with the valine pathogenic variant of UBA1. “This is important because the valine variant has been previously independently linked to decreased survival,” he said.
In findings related to pathogenic genetic variants, the researchers observed that the p.Met41Leu variant was most frequently associated with neutrophilic dermal infiltrates in 14 of 17 patients (82%) with this variant and often resembled histiocytoid Sweet syndrome. In addition, the p.Met41Val variant was associated with vasculitic lesions in 11 of 20 patients (55%) with this variant and with a mixed leukocytic infiltrate in 17 of these 20 patients (85%).
Treatment Outcomes
In the realm of therapies, skin manifestations improved in 67 of 73 patients (92%) treated with oral prednisone, while treatment with the interleukin-1 receptor antagonist anakinra improved cutaneous disease in 9 of the 16 (56%) who received it. However, 12 (75%) of those who received anakinra developed severe injection-site reactions, including ulceration in two patients and abscess formation in one patient.
Dr. Cowen noted that VEXAS is associated with high mortality (22% in this cohort), and a high degree of suspicion is required to diagnose patients with VEXAS before significant end organ damage has occurred. “This diagnosis should be considered in all older male patients who present with neutrophilic dermatosis — particularly histiocytoid Sweet syndrome, vasculitis, or leukocytoclasia without vasculitis. Patients who appear to have isolated skin involvement may have cytopenias and acute phase reactants. Therefore, complete blood count with differential and ESR and CRP should be considered to investigate for macrocytosis, cytopenias, and systemic inflammation.”
He acknowledged certain limitations of the study, including the fact that many patients were first evaluated at the NIH after having disease symptoms for many months or years. “It is possible that patients with VEXAS referred to the NIH, either for genetic testing or in person evaluation, represent a population with more aggressive disease.”
Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Connecticut, who was asked to comment on the study, emphasized the importance of the UBA1 mutation in the diagnosis of this complex syndrome. “Dermatologists should be aware of VEXAS syndrome as the majority of patients present with skin lesions, which can range from urticarial to Sweet syndrome–like to palpable purpura,” Dr. Ko said.
“Chondritis and periorbital edema, sometimes unilateral, are also associated. Histopathologic clues include a predominantly histiocytoid infiltrate,” she noted. In addition, “the prominent myxoid stroma around blood vessels and adnexal structures as a clue to VEXAS syndrome surprised me; I had not read that before.”
The study was supported by the Intramural Research Program of NIAMS. One of the study authors reported personal fees from Alexion, Novartis, and Sobi outside of the submitted work. No other disclosures were reported. Dr. Ko reported having no disclosures.
A version of this article appeared on Medscape.com .
Additionally, the most common histologic findings include leukocytoclastic vasculitis, neutrophilic dermatosis, and perivascular dermatitis; different variants in the UBA1 gene are associated with specific skin manifestations.
Those are key findings from a cohort study of 112 patients with VEXAS published online in JAMA Dermatology. The study, conducted by researchers at the National Institutes of Health (NIH) and several other institutions, aimed to define the spectrum of cutaneous manifestations in VEXAS in association with genetic, histologic, and other clinical findings.
First described in 2020, VEXAS syndrome is an adult-onset multisystem disease that can pose a diagnostic challenge to clinicians, the study’s corresponding author, Edward W. Cowen, MD, MHSc, of the dermatology branch at the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), said in an interview. The disease is caused by pathogenic variants in the UBA1 gene, located on the X chromosome. Affected individuals exhibit a wide range of manifestations, including cytopenia/myelodysplasia, multiorgan systemic inflammation, and cutaneous involvement.
“Patients may present to a variety of disease specialists depending on their symptoms and providers may not immediately consider a genetic etiology in an older individual,” Dr. Cowen said in an interview. “Although skin involvement occurs in more than 80% of patients, it is pleomorphic and may resemble a variety of other conditions such as vasculitis and Sweet syndrome.”
To better understand the cutaneous manifestations of VEXAS syndrome, the researchers evaluated data from 112 patients with VEXAS-defining genetic variants in the UBA1 gene between 2019 and 2023. Of the 112 patients, 73 underwent medical record review only, and 39 were prospectively evaluated at NIH. All but one of the patients were men, 94% were White individuals, and their mean age was 64 years. Skin involvement occurred in 83% of cases and was the most common presenting feature of VEXAS in 61% of cases.
Of the 64 histopathologic reports available from 60 patients, the main skin histopathologic findings were leukocytoclastic vasculitis in 23 patients (36%), neutrophilic dermatosis in 22 patients (34%), and perivascular dermatitis in 19 patients (30%). According to Dr. Cowen, one key histologic finding was a distinct pattern of “histiocytoid” dermal neutrophilic inflammation, which was present in 13 of 15 specimens (86%) that underwent central re-review. “This pattern can occasionally also be seen in patients with Sweet syndrome, unrelated to VEXAS, but was a hallmark feature found in the majority of skin biopsies of patients with VEXAS,” he said.
“Together with another pathologic finding, leukocytoclasia, these features can be useful clues to alert the pathologist to a potential diagnosis of VEXAS. This myeloid predominant pattern of skin inflammation was also most strongly associated with the leucine pathogenic variant of the UBA1 gene.” In contrast, cutaneous vasculitis was most strongly associated with the valine pathogenic variant of UBA1. “This is important because the valine variant has been previously independently linked to decreased survival,” he said.
In findings related to pathogenic genetic variants, the researchers observed that the p.Met41Leu variant was most frequently associated with neutrophilic dermal infiltrates in 14 of 17 patients (82%) with this variant and often resembled histiocytoid Sweet syndrome. In addition, the p.Met41Val variant was associated with vasculitic lesions in 11 of 20 patients (55%) with this variant and with a mixed leukocytic infiltrate in 17 of these 20 patients (85%).
Treatment Outcomes
In the realm of therapies, skin manifestations improved in 67 of 73 patients (92%) treated with oral prednisone, while treatment with the interleukin-1 receptor antagonist anakinra improved cutaneous disease in 9 of the 16 (56%) who received it. However, 12 (75%) of those who received anakinra developed severe injection-site reactions, including ulceration in two patients and abscess formation in one patient.
Dr. Cowen noted that VEXAS is associated with high mortality (22% in this cohort), and a high degree of suspicion is required to diagnose patients with VEXAS before significant end organ damage has occurred. “This diagnosis should be considered in all older male patients who present with neutrophilic dermatosis — particularly histiocytoid Sweet syndrome, vasculitis, or leukocytoclasia without vasculitis. Patients who appear to have isolated skin involvement may have cytopenias and acute phase reactants. Therefore, complete blood count with differential and ESR and CRP should be considered to investigate for macrocytosis, cytopenias, and systemic inflammation.”
He acknowledged certain limitations of the study, including the fact that many patients were first evaluated at the NIH after having disease symptoms for many months or years. “It is possible that patients with VEXAS referred to the NIH, either for genetic testing or in person evaluation, represent a population with more aggressive disease.”
Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Connecticut, who was asked to comment on the study, emphasized the importance of the UBA1 mutation in the diagnosis of this complex syndrome. “Dermatologists should be aware of VEXAS syndrome as the majority of patients present with skin lesions, which can range from urticarial to Sweet syndrome–like to palpable purpura,” Dr. Ko said.
“Chondritis and periorbital edema, sometimes unilateral, are also associated. Histopathologic clues include a predominantly histiocytoid infiltrate,” she noted. In addition, “the prominent myxoid stroma around blood vessels and adnexal structures as a clue to VEXAS syndrome surprised me; I had not read that before.”
The study was supported by the Intramural Research Program of NIAMS. One of the study authors reported personal fees from Alexion, Novartis, and Sobi outside of the submitted work. No other disclosures were reported. Dr. Ko reported having no disclosures.
A version of this article appeared on Medscape.com .
Additionally, the most common histologic findings include leukocytoclastic vasculitis, neutrophilic dermatosis, and perivascular dermatitis; different variants in the UBA1 gene are associated with specific skin manifestations.
Those are key findings from a cohort study of 112 patients with VEXAS published online in JAMA Dermatology. The study, conducted by researchers at the National Institutes of Health (NIH) and several other institutions, aimed to define the spectrum of cutaneous manifestations in VEXAS in association with genetic, histologic, and other clinical findings.
First described in 2020, VEXAS syndrome is an adult-onset multisystem disease that can pose a diagnostic challenge to clinicians, the study’s corresponding author, Edward W. Cowen, MD, MHSc, of the dermatology branch at the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), said in an interview. The disease is caused by pathogenic variants in the UBA1 gene, located on the X chromosome. Affected individuals exhibit a wide range of manifestations, including cytopenia/myelodysplasia, multiorgan systemic inflammation, and cutaneous involvement.
“Patients may present to a variety of disease specialists depending on their symptoms and providers may not immediately consider a genetic etiology in an older individual,” Dr. Cowen said in an interview. “Although skin involvement occurs in more than 80% of patients, it is pleomorphic and may resemble a variety of other conditions such as vasculitis and Sweet syndrome.”
To better understand the cutaneous manifestations of VEXAS syndrome, the researchers evaluated data from 112 patients with VEXAS-defining genetic variants in the UBA1 gene between 2019 and 2023. Of the 112 patients, 73 underwent medical record review only, and 39 were prospectively evaluated at NIH. All but one of the patients were men, 94% were White individuals, and their mean age was 64 years. Skin involvement occurred in 83% of cases and was the most common presenting feature of VEXAS in 61% of cases.
Of the 64 histopathologic reports available from 60 patients, the main skin histopathologic findings were leukocytoclastic vasculitis in 23 patients (36%), neutrophilic dermatosis in 22 patients (34%), and perivascular dermatitis in 19 patients (30%). According to Dr. Cowen, one key histologic finding was a distinct pattern of “histiocytoid” dermal neutrophilic inflammation, which was present in 13 of 15 specimens (86%) that underwent central re-review. “This pattern can occasionally also be seen in patients with Sweet syndrome, unrelated to VEXAS, but was a hallmark feature found in the majority of skin biopsies of patients with VEXAS,” he said.
“Together with another pathologic finding, leukocytoclasia, these features can be useful clues to alert the pathologist to a potential diagnosis of VEXAS. This myeloid predominant pattern of skin inflammation was also most strongly associated with the leucine pathogenic variant of the UBA1 gene.” In contrast, cutaneous vasculitis was most strongly associated with the valine pathogenic variant of UBA1. “This is important because the valine variant has been previously independently linked to decreased survival,” he said.
In findings related to pathogenic genetic variants, the researchers observed that the p.Met41Leu variant was most frequently associated with neutrophilic dermal infiltrates in 14 of 17 patients (82%) with this variant and often resembled histiocytoid Sweet syndrome. In addition, the p.Met41Val variant was associated with vasculitic lesions in 11 of 20 patients (55%) with this variant and with a mixed leukocytic infiltrate in 17 of these 20 patients (85%).
Treatment Outcomes
In the realm of therapies, skin manifestations improved in 67 of 73 patients (92%) treated with oral prednisone, while treatment with the interleukin-1 receptor antagonist anakinra improved cutaneous disease in 9 of the 16 (56%) who received it. However, 12 (75%) of those who received anakinra developed severe injection-site reactions, including ulceration in two patients and abscess formation in one patient.
Dr. Cowen noted that VEXAS is associated with high mortality (22% in this cohort), and a high degree of suspicion is required to diagnose patients with VEXAS before significant end organ damage has occurred. “This diagnosis should be considered in all older male patients who present with neutrophilic dermatosis — particularly histiocytoid Sweet syndrome, vasculitis, or leukocytoclasia without vasculitis. Patients who appear to have isolated skin involvement may have cytopenias and acute phase reactants. Therefore, complete blood count with differential and ESR and CRP should be considered to investigate for macrocytosis, cytopenias, and systemic inflammation.”
He acknowledged certain limitations of the study, including the fact that many patients were first evaluated at the NIH after having disease symptoms for many months or years. “It is possible that patients with VEXAS referred to the NIH, either for genetic testing or in person evaluation, represent a population with more aggressive disease.”
Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Connecticut, who was asked to comment on the study, emphasized the importance of the UBA1 mutation in the diagnosis of this complex syndrome. “Dermatologists should be aware of VEXAS syndrome as the majority of patients present with skin lesions, which can range from urticarial to Sweet syndrome–like to palpable purpura,” Dr. Ko said.
“Chondritis and periorbital edema, sometimes unilateral, are also associated. Histopathologic clues include a predominantly histiocytoid infiltrate,” she noted. In addition, “the prominent myxoid stroma around blood vessels and adnexal structures as a clue to VEXAS syndrome surprised me; I had not read that before.”
The study was supported by the Intramural Research Program of NIAMS. One of the study authors reported personal fees from Alexion, Novartis, and Sobi outside of the submitted work. No other disclosures were reported. Dr. Ko reported having no disclosures.
A version of this article appeared on Medscape.com .
FROM JAMA DERMATOLOGY