User login
The most important meal of the day, with extra zinc
Busting the myth of skipping breakfast
Your mother told you that breakfast was the most important meal of the day. Cereal marketing teams banked on that, selling breakfast to millions of people based on a common turn of phrase like “an apple a day keeps the doctor away.” Well, what if the notion of breakfast’s importance isn’t just marketing BS?
A new study suggests that adults who don’t eat breakfast are setting themselves up for a nutritional gap. Common breakfast foods pack a ton of calcium, fiber, and vitamin C from milk, cereals, and fruit. Christopher Taylor, PhD, senior author of the study and professor of dietetics at the Ohio State University, Columbus, said that if you’re not getting those nutrients from foods at breakfast, there’s a tendency to skip them throughout the rest of your day.
Data from a sample of the National Health and Nutrition Examination Survey – 30,889 adults aged 19 and older who participated between 2005 and 2016 – showed that 15.2% of participants reported skipping breakfast.
The research team then estimated nutrient consumption using federal dietary studies and guidelines and compared it to Food and Nutrition Board of National Academies nutrient recommendations. The breakfast skippers, they determined, were missing out on pronounced levels of fiber, magnesium, iron, calcium, and vitamins A, B1, B2, B3, C, and D and were more likely to fall prey to lower-quality snacking. Cue those Oreos at 3 pm.
You may get more total calories within the day by eating breakfast, but your lunch, dinner, and snacks are much larger when you skip it. So the case of breakfast being the most important meal of the day checks out. Who knew that Tony the Tiger – and Mom – were actually on to something?
The bitter taste of a healthy liver
Alcohol and liver disease. They go together like, well, alcohol and liver disease. But alcohol isn’t the only reason people get liver disease, and now there’s a potential new treatment for people with hepatic steatosis on the way to becoming nonalcoholic fatty liver disease: beer.
Okay, not literally beer, but a pair of compounds derived from hops, the plant that gives beer its color and bitter flavor. In a study published in eLife, researchers from Oregon State University fed mice either a low-fat diet or a high-fat diet to induce hepatic steatosis, with some on the high-fat diet receiving either xanthohumol, a prenylated flavonoid from the hop plant, or tetrahydroxanthohumol, a hydrogenated derivative of xanthohumol.
Mice that received tetrahydroxanthohumol not only gained weight at a far slower rate than that of mice on the normal high-fat diet, their blood sugar remained stable; xanthohumol was similarly effective if the dosage was higher. The researchers noted that the two chemicals were effective because they acted as antagonists for the PPAR-gamma protein, which controls glucose metabolism and fatty cell activation. The chemicals bind to the protein but don’t activate it, meaning fat is unable to build up in the cells. No fat means no hepatic steatosis, which means no liver disease.
The researchers caution that more research is needed to determine the chemicals’ effectiveness in humans, but the classic line from a great animated philosopher still holds true: Alcohol may really be the source of, and solution to, all of life’s problems.
Life’s great mysteries, from A to zinc
Thanks to science, we now have answers to what were once unanswerable questions: Is Jello a solid or a liquid? If someone leads but no one follows, are they just out for a walk? Does zinc inhibit or promote the growth of kidney stones? How many licks does it take to get to the center of a Tootsie Pop? (Turns out science really did answer this one.)
If you’re anything like us, then you’ve been following the big debate on the two competing theories involving the role of zinc in kidney stone formation for years. One theory says that zinc stops the growth of calcium oxalate crystals that make up stones. The other says that zinc alters the surfaces of crystals, which encourages growth.
We can’t stand the suspense any longer, so here goes: The answer to “does zinc inhibit or promote the growth of kidney stones?” is … yes.
“What we see with zinc is something we haven’t seen before. It does slow down calcium oxalate crystal growth and at the same time it changes the surface of the crystals, causing defects in the form of intergrowths. These abnormalities create centers for new crystals to nucleate and grow,” said senior author Jeffrey Rimer, PhD, of the University of Houston.
In vitro experimentation, computational modeling, and atomic force microscopy don’t lie: Zinc ions have a unique ability “to alter the termination of crystal surfaces.” They tried alternative ions found in urine, including magnesium, and there was no effect on crystal formation.
With this one great mystery now solved, we contacted Dr. Rimer to ask him about the whole “sound of one hand clapping” business. He hasn’t cracked that one yet, but he did want to speak to our supervisor. So many of life’s unanswered questions, so little time. Oh well.
Babies’ ‘gut instinct’ to cry
At some point or another, you’ve probably been told not to “be such a baby” when you were scared of something. If you’ve been called a crybaby, it may be an indicator that you had a different gut microbiome as an infant.
Investigators from Michigan State University and the University of North Carolina say that babies who react more strongly to scary situations have different gut microbiomes compared with babies who don’t have such a strong reaction. The way babies react to scary situations can say a lot about their future, and there is even some evidence that gut microbiomes may have something to do with mental health.
Physicians who support neurologic development may one day be able to use this research on gut microbiomes to help monitor people’s neurological health. “This early developmental period is a time of tremendous opportunity for promoting healthy brain development. The microbiome is an exciting new target that can be potentially used for that,” said Rebecca Knickmeyer of MSU, leader of the study, which was published in Nature Communications. And loyal LOTME followers already know about the OpenBiome Microbiome Library, aka the “Amazon of bacteria.”
So the next time someone tells you not to be such a baby when you’re scared of something, tell them it’s not your fault. Blame it on your gut microbiome!
Busting the myth of skipping breakfast
Your mother told you that breakfast was the most important meal of the day. Cereal marketing teams banked on that, selling breakfast to millions of people based on a common turn of phrase like “an apple a day keeps the doctor away.” Well, what if the notion of breakfast’s importance isn’t just marketing BS?
A new study suggests that adults who don’t eat breakfast are setting themselves up for a nutritional gap. Common breakfast foods pack a ton of calcium, fiber, and vitamin C from milk, cereals, and fruit. Christopher Taylor, PhD, senior author of the study and professor of dietetics at the Ohio State University, Columbus, said that if you’re not getting those nutrients from foods at breakfast, there’s a tendency to skip them throughout the rest of your day.
Data from a sample of the National Health and Nutrition Examination Survey – 30,889 adults aged 19 and older who participated between 2005 and 2016 – showed that 15.2% of participants reported skipping breakfast.
The research team then estimated nutrient consumption using federal dietary studies and guidelines and compared it to Food and Nutrition Board of National Academies nutrient recommendations. The breakfast skippers, they determined, were missing out on pronounced levels of fiber, magnesium, iron, calcium, and vitamins A, B1, B2, B3, C, and D and were more likely to fall prey to lower-quality snacking. Cue those Oreos at 3 pm.
You may get more total calories within the day by eating breakfast, but your lunch, dinner, and snacks are much larger when you skip it. So the case of breakfast being the most important meal of the day checks out. Who knew that Tony the Tiger – and Mom – were actually on to something?
The bitter taste of a healthy liver
Alcohol and liver disease. They go together like, well, alcohol and liver disease. But alcohol isn’t the only reason people get liver disease, and now there’s a potential new treatment for people with hepatic steatosis on the way to becoming nonalcoholic fatty liver disease: beer.
Okay, not literally beer, but a pair of compounds derived from hops, the plant that gives beer its color and bitter flavor. In a study published in eLife, researchers from Oregon State University fed mice either a low-fat diet or a high-fat diet to induce hepatic steatosis, with some on the high-fat diet receiving either xanthohumol, a prenylated flavonoid from the hop plant, or tetrahydroxanthohumol, a hydrogenated derivative of xanthohumol.
Mice that received tetrahydroxanthohumol not only gained weight at a far slower rate than that of mice on the normal high-fat diet, their blood sugar remained stable; xanthohumol was similarly effective if the dosage was higher. The researchers noted that the two chemicals were effective because they acted as antagonists for the PPAR-gamma protein, which controls glucose metabolism and fatty cell activation. The chemicals bind to the protein but don’t activate it, meaning fat is unable to build up in the cells. No fat means no hepatic steatosis, which means no liver disease.
The researchers caution that more research is needed to determine the chemicals’ effectiveness in humans, but the classic line from a great animated philosopher still holds true: Alcohol may really be the source of, and solution to, all of life’s problems.
Life’s great mysteries, from A to zinc
Thanks to science, we now have answers to what were once unanswerable questions: Is Jello a solid or a liquid? If someone leads but no one follows, are they just out for a walk? Does zinc inhibit or promote the growth of kidney stones? How many licks does it take to get to the center of a Tootsie Pop? (Turns out science really did answer this one.)
If you’re anything like us, then you’ve been following the big debate on the two competing theories involving the role of zinc in kidney stone formation for years. One theory says that zinc stops the growth of calcium oxalate crystals that make up stones. The other says that zinc alters the surfaces of crystals, which encourages growth.
We can’t stand the suspense any longer, so here goes: The answer to “does zinc inhibit or promote the growth of kidney stones?” is … yes.
“What we see with zinc is something we haven’t seen before. It does slow down calcium oxalate crystal growth and at the same time it changes the surface of the crystals, causing defects in the form of intergrowths. These abnormalities create centers for new crystals to nucleate and grow,” said senior author Jeffrey Rimer, PhD, of the University of Houston.
In vitro experimentation, computational modeling, and atomic force microscopy don’t lie: Zinc ions have a unique ability “to alter the termination of crystal surfaces.” They tried alternative ions found in urine, including magnesium, and there was no effect on crystal formation.
With this one great mystery now solved, we contacted Dr. Rimer to ask him about the whole “sound of one hand clapping” business. He hasn’t cracked that one yet, but he did want to speak to our supervisor. So many of life’s unanswered questions, so little time. Oh well.
Babies’ ‘gut instinct’ to cry
At some point or another, you’ve probably been told not to “be such a baby” when you were scared of something. If you’ve been called a crybaby, it may be an indicator that you had a different gut microbiome as an infant.
Investigators from Michigan State University and the University of North Carolina say that babies who react more strongly to scary situations have different gut microbiomes compared with babies who don’t have such a strong reaction. The way babies react to scary situations can say a lot about their future, and there is even some evidence that gut microbiomes may have something to do with mental health.
Physicians who support neurologic development may one day be able to use this research on gut microbiomes to help monitor people’s neurological health. “This early developmental period is a time of tremendous opportunity for promoting healthy brain development. The microbiome is an exciting new target that can be potentially used for that,” said Rebecca Knickmeyer of MSU, leader of the study, which was published in Nature Communications. And loyal LOTME followers already know about the OpenBiome Microbiome Library, aka the “Amazon of bacteria.”
So the next time someone tells you not to be such a baby when you’re scared of something, tell them it’s not your fault. Blame it on your gut microbiome!
Busting the myth of skipping breakfast
Your mother told you that breakfast was the most important meal of the day. Cereal marketing teams banked on that, selling breakfast to millions of people based on a common turn of phrase like “an apple a day keeps the doctor away.” Well, what if the notion of breakfast’s importance isn’t just marketing BS?
A new study suggests that adults who don’t eat breakfast are setting themselves up for a nutritional gap. Common breakfast foods pack a ton of calcium, fiber, and vitamin C from milk, cereals, and fruit. Christopher Taylor, PhD, senior author of the study and professor of dietetics at the Ohio State University, Columbus, said that if you’re not getting those nutrients from foods at breakfast, there’s a tendency to skip them throughout the rest of your day.
Data from a sample of the National Health and Nutrition Examination Survey – 30,889 adults aged 19 and older who participated between 2005 and 2016 – showed that 15.2% of participants reported skipping breakfast.
The research team then estimated nutrient consumption using federal dietary studies and guidelines and compared it to Food and Nutrition Board of National Academies nutrient recommendations. The breakfast skippers, they determined, were missing out on pronounced levels of fiber, magnesium, iron, calcium, and vitamins A, B1, B2, B3, C, and D and were more likely to fall prey to lower-quality snacking. Cue those Oreos at 3 pm.
You may get more total calories within the day by eating breakfast, but your lunch, dinner, and snacks are much larger when you skip it. So the case of breakfast being the most important meal of the day checks out. Who knew that Tony the Tiger – and Mom – were actually on to something?
The bitter taste of a healthy liver
Alcohol and liver disease. They go together like, well, alcohol and liver disease. But alcohol isn’t the only reason people get liver disease, and now there’s a potential new treatment for people with hepatic steatosis on the way to becoming nonalcoholic fatty liver disease: beer.
Okay, not literally beer, but a pair of compounds derived from hops, the plant that gives beer its color and bitter flavor. In a study published in eLife, researchers from Oregon State University fed mice either a low-fat diet or a high-fat diet to induce hepatic steatosis, with some on the high-fat diet receiving either xanthohumol, a prenylated flavonoid from the hop plant, or tetrahydroxanthohumol, a hydrogenated derivative of xanthohumol.
Mice that received tetrahydroxanthohumol not only gained weight at a far slower rate than that of mice on the normal high-fat diet, their blood sugar remained stable; xanthohumol was similarly effective if the dosage was higher. The researchers noted that the two chemicals were effective because they acted as antagonists for the PPAR-gamma protein, which controls glucose metabolism and fatty cell activation. The chemicals bind to the protein but don’t activate it, meaning fat is unable to build up in the cells. No fat means no hepatic steatosis, which means no liver disease.
The researchers caution that more research is needed to determine the chemicals’ effectiveness in humans, but the classic line from a great animated philosopher still holds true: Alcohol may really be the source of, and solution to, all of life’s problems.
Life’s great mysteries, from A to zinc
Thanks to science, we now have answers to what were once unanswerable questions: Is Jello a solid or a liquid? If someone leads but no one follows, are they just out for a walk? Does zinc inhibit or promote the growth of kidney stones? How many licks does it take to get to the center of a Tootsie Pop? (Turns out science really did answer this one.)
If you’re anything like us, then you’ve been following the big debate on the two competing theories involving the role of zinc in kidney stone formation for years. One theory says that zinc stops the growth of calcium oxalate crystals that make up stones. The other says that zinc alters the surfaces of crystals, which encourages growth.
We can’t stand the suspense any longer, so here goes: The answer to “does zinc inhibit or promote the growth of kidney stones?” is … yes.
“What we see with zinc is something we haven’t seen before. It does slow down calcium oxalate crystal growth and at the same time it changes the surface of the crystals, causing defects in the form of intergrowths. These abnormalities create centers for new crystals to nucleate and grow,” said senior author Jeffrey Rimer, PhD, of the University of Houston.
In vitro experimentation, computational modeling, and atomic force microscopy don’t lie: Zinc ions have a unique ability “to alter the termination of crystal surfaces.” They tried alternative ions found in urine, including magnesium, and there was no effect on crystal formation.
With this one great mystery now solved, we contacted Dr. Rimer to ask him about the whole “sound of one hand clapping” business. He hasn’t cracked that one yet, but he did want to speak to our supervisor. So many of life’s unanswered questions, so little time. Oh well.
Babies’ ‘gut instinct’ to cry
At some point or another, you’ve probably been told not to “be such a baby” when you were scared of something. If you’ve been called a crybaby, it may be an indicator that you had a different gut microbiome as an infant.
Investigators from Michigan State University and the University of North Carolina say that babies who react more strongly to scary situations have different gut microbiomes compared with babies who don’t have such a strong reaction. The way babies react to scary situations can say a lot about their future, and there is even some evidence that gut microbiomes may have something to do with mental health.
Physicians who support neurologic development may one day be able to use this research on gut microbiomes to help monitor people’s neurological health. “This early developmental period is a time of tremendous opportunity for promoting healthy brain development. The microbiome is an exciting new target that can be potentially used for that,” said Rebecca Knickmeyer of MSU, leader of the study, which was published in Nature Communications. And loyal LOTME followers already know about the OpenBiome Microbiome Library, aka the “Amazon of bacteria.”
So the next time someone tells you not to be such a baby when you’re scared of something, tell them it’s not your fault. Blame it on your gut microbiome!
Lupus images fall short on diverse examples
Lupus images in medical resource materials underrepresent patients with skin of color, based on data from a review of more than 1,400 images published between 2014 and 2019 in materials from a university’s online medical library.
Patients with skin of color who develop lupus tend to present earlier and with more severe cases, and often experience worse outcomes, compared with other populations, wrote Amaad Rana, MD, of Washington University, St. Louis, and colleagues. Medical resources in general have historically underrepresented patients of color, and the researchers reviewed lupus materials for a similar publication bias.
In a study published in Arthritis Care & Research, the investigators identified 1,417 images in rheumatology, dermatology, and internal medicine resources, including 119 medical textbooks, 15 medical journals, 2 online image libraries, and the online image collections of Google and UpToDate. An additional 24 images came from skin of color atlases.
Excluding the skin of color atlases, 56.4% of the images represented light skin, 35.1% showed medium skin, and 8.5% showed dark skin. Overall, publishers were more than twice as likely to portray light skin tones and were significantly less likely to portray dark skin tones (odds ratios, 2.59 and 0.19, respectively), compared with an equal representation of skin tones; however, the difference was not significant for portrayal of medium skin tones (OR, 1.08).
By specialty, dermatology was more inclusive of skin of color images than rheumatology or internal medicine, although the internal medicine sample size was too small for comparable analysis, the researchers noted. Dermatology textbooks were 2.42 times more likely and rheumatology textbooks were 4.87 times more likely to depict light skin tones than an equal representation of light, medium, and dark skin tones.
The researchers rated the skin color in the images using the New Immigrant Survey Skin Color Scale and categorized the images as representing light (NISSCS scores, 1-2), medium (NISSCS scores, 3-5), or dark skin (NISSCS scores, 6-10). Medical journals had the most images of dark skin, excluding skin of color atlases. In a comparison of specialties, dermatology materials included the most images of medium and darker skin tones.
The underrepresentation of skin of color patients can contribute to a limited knowledge of lupus presentation that could lead to disparate health outcomes, the researchers noted.
The study findings were limited by several factors, including the review of only the online textbooks and journals available through the medical library of a single university, the researchers noted. In addition, definitions of light, medium, and dark skin tones were variable among studies, and the researchers did not distinguish among lupus pathologies.
“Further research is needed to quantitatively assess the influence these materials have on healthcare providers’ ability to care for patients with lupus and SOC, and new material and strategies will be required to correct this disparity and promote equitable representation,” the researchers emphasized. “Ultimately, this will arm practitioners with the resources to competently treat patients with any skin color and work towards reducing disparities in health outcomes.”
The study received no outside funding. The researchers had no financial conflicts to disclose.
Lupus images in medical resource materials underrepresent patients with skin of color, based on data from a review of more than 1,400 images published between 2014 and 2019 in materials from a university’s online medical library.
Patients with skin of color who develop lupus tend to present earlier and with more severe cases, and often experience worse outcomes, compared with other populations, wrote Amaad Rana, MD, of Washington University, St. Louis, and colleagues. Medical resources in general have historically underrepresented patients of color, and the researchers reviewed lupus materials for a similar publication bias.
In a study published in Arthritis Care & Research, the investigators identified 1,417 images in rheumatology, dermatology, and internal medicine resources, including 119 medical textbooks, 15 medical journals, 2 online image libraries, and the online image collections of Google and UpToDate. An additional 24 images came from skin of color atlases.
Excluding the skin of color atlases, 56.4% of the images represented light skin, 35.1% showed medium skin, and 8.5% showed dark skin. Overall, publishers were more than twice as likely to portray light skin tones and were significantly less likely to portray dark skin tones (odds ratios, 2.59 and 0.19, respectively), compared with an equal representation of skin tones; however, the difference was not significant for portrayal of medium skin tones (OR, 1.08).
By specialty, dermatology was more inclusive of skin of color images than rheumatology or internal medicine, although the internal medicine sample size was too small for comparable analysis, the researchers noted. Dermatology textbooks were 2.42 times more likely and rheumatology textbooks were 4.87 times more likely to depict light skin tones than an equal representation of light, medium, and dark skin tones.
The researchers rated the skin color in the images using the New Immigrant Survey Skin Color Scale and categorized the images as representing light (NISSCS scores, 1-2), medium (NISSCS scores, 3-5), or dark skin (NISSCS scores, 6-10). Medical journals had the most images of dark skin, excluding skin of color atlases. In a comparison of specialties, dermatology materials included the most images of medium and darker skin tones.
The underrepresentation of skin of color patients can contribute to a limited knowledge of lupus presentation that could lead to disparate health outcomes, the researchers noted.
The study findings were limited by several factors, including the review of only the online textbooks and journals available through the medical library of a single university, the researchers noted. In addition, definitions of light, medium, and dark skin tones were variable among studies, and the researchers did not distinguish among lupus pathologies.
“Further research is needed to quantitatively assess the influence these materials have on healthcare providers’ ability to care for patients with lupus and SOC, and new material and strategies will be required to correct this disparity and promote equitable representation,” the researchers emphasized. “Ultimately, this will arm practitioners with the resources to competently treat patients with any skin color and work towards reducing disparities in health outcomes.”
The study received no outside funding. The researchers had no financial conflicts to disclose.
Lupus images in medical resource materials underrepresent patients with skin of color, based on data from a review of more than 1,400 images published between 2014 and 2019 in materials from a university’s online medical library.
Patients with skin of color who develop lupus tend to present earlier and with more severe cases, and often experience worse outcomes, compared with other populations, wrote Amaad Rana, MD, of Washington University, St. Louis, and colleagues. Medical resources in general have historically underrepresented patients of color, and the researchers reviewed lupus materials for a similar publication bias.
In a study published in Arthritis Care & Research, the investigators identified 1,417 images in rheumatology, dermatology, and internal medicine resources, including 119 medical textbooks, 15 medical journals, 2 online image libraries, and the online image collections of Google and UpToDate. An additional 24 images came from skin of color atlases.
Excluding the skin of color atlases, 56.4% of the images represented light skin, 35.1% showed medium skin, and 8.5% showed dark skin. Overall, publishers were more than twice as likely to portray light skin tones and were significantly less likely to portray dark skin tones (odds ratios, 2.59 and 0.19, respectively), compared with an equal representation of skin tones; however, the difference was not significant for portrayal of medium skin tones (OR, 1.08).
By specialty, dermatology was more inclusive of skin of color images than rheumatology or internal medicine, although the internal medicine sample size was too small for comparable analysis, the researchers noted. Dermatology textbooks were 2.42 times more likely and rheumatology textbooks were 4.87 times more likely to depict light skin tones than an equal representation of light, medium, and dark skin tones.
The researchers rated the skin color in the images using the New Immigrant Survey Skin Color Scale and categorized the images as representing light (NISSCS scores, 1-2), medium (NISSCS scores, 3-5), or dark skin (NISSCS scores, 6-10). Medical journals had the most images of dark skin, excluding skin of color atlases. In a comparison of specialties, dermatology materials included the most images of medium and darker skin tones.
The underrepresentation of skin of color patients can contribute to a limited knowledge of lupus presentation that could lead to disparate health outcomes, the researchers noted.
The study findings were limited by several factors, including the review of only the online textbooks and journals available through the medical library of a single university, the researchers noted. In addition, definitions of light, medium, and dark skin tones were variable among studies, and the researchers did not distinguish among lupus pathologies.
“Further research is needed to quantitatively assess the influence these materials have on healthcare providers’ ability to care for patients with lupus and SOC, and new material and strategies will be required to correct this disparity and promote equitable representation,” the researchers emphasized. “Ultimately, this will arm practitioners with the resources to competently treat patients with any skin color and work towards reducing disparities in health outcomes.”
The study received no outside funding. The researchers had no financial conflicts to disclose.
FROM ARTHRITIS CARE & RESEARCH
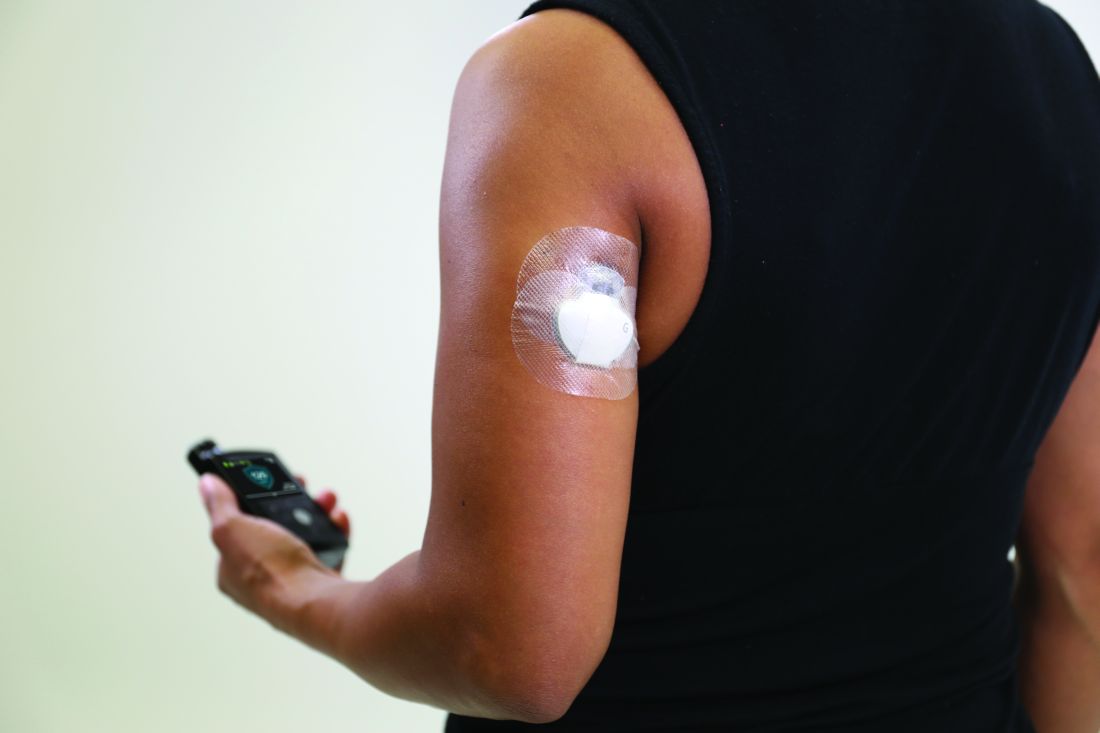
Medicare rule changes allow for broader CGM use
Beginning July 18, 2021, the Centers for Medicare & Medicaid Services will no longer require that beneficiaries test their blood sugar four times a day in order to qualify for CGM. In addition, the term “multiple daily injections” of insulin has been changed to multiple daily “administrations” in order to allow coverage for people who use inhaled insulin.
The changes are among those lobbied for by several organizations, including the American Diabetes Association and the Association of Diabetes Care and Education Specialists, which represents the professionals formerly known as “diabetes educators.”
The ADA tweeted on July 11 that “the removal of this criterion has been an effort long-led by the ADA, on which we have been actively engaged with CMS. People with diabetes on Medicare will now be able to more easily access this critical piece of technology, leading to better diabetes management and better health outcomes. A big win for the diabetes community!”
“After years of advocacy from the diabetes community and ADCES, Medicare has taken an important step to make [CGM] more accessible for Medicare beneficiaries with diabetes,” Kate Thomas, ADCES chief advocacy and external affairs officer, wrote in a blog post. “This updated [Local Coverage Determination] was a direct result of coordinated advocacy efforts among patient and provider groups, as well as industry partners, coalitions and other entities.”
It’s tough to test four times a day with only three strips
In a Jan. 29, 2021, letter to the Medicare Administrative Contractors, who oversee the policies for durable medical equipment, ADCES explained why the organization strongly supported removal of the four-daily fingerstick requirement, noting that “There is no evidence to suggest that requiring four or more fingerstick tests per day significantly impacts the outcomes of CGM therapy.”
Moreover, they pointed out that the requirement was particularly burdensome, considering the fact that Medicare only covers three test strips per day for insulin-using beneficiaries. “Removing this coverage requirement would allow for increased access to CGM systems and improved health outcomes for beneficiaries with diabetes by improving glycemic control. This also represents a step toward addressing the disparities that exist around diabetes technology under the Medicare program.”
As for the terminology change from “injection” to “administration,” ADCES said that, in addition to allowing CGM coverage for individuals who use rapid-acting inhaled insulin, “we also hope that updating this terminology will help to expedite coverage as future innovations in insulin delivery methods come to market.”
More changes needed, ADCES says
In that January 2021 letter, ADCES recommended several other changes, including covering CGM for anyone diagnosed with type 1 diabetes at any age and without having to meet other requirements except for twice-yearly clinician visits, and for anyone with type 2 diabetes who uses any type of insulin or who has had documented hypoglycemia regardless of therapy.
They also recommended that CGM coverage be considered for patients with chronic kidney disease, and that the required 6-month clinician visits be allowed to take place via telehealth. “ADCES believes that allowing the initiation of CGM therapy through a virtual visit will reduce barriers associated with travel and difficulty accessing a trained provider that are experienced by Medicare beneficiaries.”
In addition, ADCES requested that CMS eliminate the requirement that beneficiaries use insulin three times a day to qualify for CGM, noting that this creates a barrier for patients who can’t afford insulin at all but are at risk for hypoglycemia because they take sulfonylureas or other insulin secretagogues, or for those who use cheaper synthetic human insulins that are only taken twice a day, such as NPH.
“The existing CGM coverage criteria creates an unbalanced and disparate system that excludes from coverage beneficiaries who could greatly benefit from a CGM system, but do not qualify due to issues with insulin affordability,” ADCES wrote in the January letter.
Ms. Thomas wrote in the June 14th blog: “Our work is not done. We know there are more changes that must be made.”
Beginning July 18, 2021, the Centers for Medicare & Medicaid Services will no longer require that beneficiaries test their blood sugar four times a day in order to qualify for CGM. In addition, the term “multiple daily injections” of insulin has been changed to multiple daily “administrations” in order to allow coverage for people who use inhaled insulin.
The changes are among those lobbied for by several organizations, including the American Diabetes Association and the Association of Diabetes Care and Education Specialists, which represents the professionals formerly known as “diabetes educators.”
The ADA tweeted on July 11 that “the removal of this criterion has been an effort long-led by the ADA, on which we have been actively engaged with CMS. People with diabetes on Medicare will now be able to more easily access this critical piece of technology, leading to better diabetes management and better health outcomes. A big win for the diabetes community!”
“After years of advocacy from the diabetes community and ADCES, Medicare has taken an important step to make [CGM] more accessible for Medicare beneficiaries with diabetes,” Kate Thomas, ADCES chief advocacy and external affairs officer, wrote in a blog post. “This updated [Local Coverage Determination] was a direct result of coordinated advocacy efforts among patient and provider groups, as well as industry partners, coalitions and other entities.”
It’s tough to test four times a day with only three strips
In a Jan. 29, 2021, letter to the Medicare Administrative Contractors, who oversee the policies for durable medical equipment, ADCES explained why the organization strongly supported removal of the four-daily fingerstick requirement, noting that “There is no evidence to suggest that requiring four or more fingerstick tests per day significantly impacts the outcomes of CGM therapy.”
Moreover, they pointed out that the requirement was particularly burdensome, considering the fact that Medicare only covers three test strips per day for insulin-using beneficiaries. “Removing this coverage requirement would allow for increased access to CGM systems and improved health outcomes for beneficiaries with diabetes by improving glycemic control. This also represents a step toward addressing the disparities that exist around diabetes technology under the Medicare program.”
As for the terminology change from “injection” to “administration,” ADCES said that, in addition to allowing CGM coverage for individuals who use rapid-acting inhaled insulin, “we also hope that updating this terminology will help to expedite coverage as future innovations in insulin delivery methods come to market.”
More changes needed, ADCES says
In that January 2021 letter, ADCES recommended several other changes, including covering CGM for anyone diagnosed with type 1 diabetes at any age and without having to meet other requirements except for twice-yearly clinician visits, and for anyone with type 2 diabetes who uses any type of insulin or who has had documented hypoglycemia regardless of therapy.
They also recommended that CGM coverage be considered for patients with chronic kidney disease, and that the required 6-month clinician visits be allowed to take place via telehealth. “ADCES believes that allowing the initiation of CGM therapy through a virtual visit will reduce barriers associated with travel and difficulty accessing a trained provider that are experienced by Medicare beneficiaries.”
In addition, ADCES requested that CMS eliminate the requirement that beneficiaries use insulin three times a day to qualify for CGM, noting that this creates a barrier for patients who can’t afford insulin at all but are at risk for hypoglycemia because they take sulfonylureas or other insulin secretagogues, or for those who use cheaper synthetic human insulins that are only taken twice a day, such as NPH.
“The existing CGM coverage criteria creates an unbalanced and disparate system that excludes from coverage beneficiaries who could greatly benefit from a CGM system, but do not qualify due to issues with insulin affordability,” ADCES wrote in the January letter.
Ms. Thomas wrote in the June 14th blog: “Our work is not done. We know there are more changes that must be made.”
Beginning July 18, 2021, the Centers for Medicare & Medicaid Services will no longer require that beneficiaries test their blood sugar four times a day in order to qualify for CGM. In addition, the term “multiple daily injections” of insulin has been changed to multiple daily “administrations” in order to allow coverage for people who use inhaled insulin.
The changes are among those lobbied for by several organizations, including the American Diabetes Association and the Association of Diabetes Care and Education Specialists, which represents the professionals formerly known as “diabetes educators.”
The ADA tweeted on July 11 that “the removal of this criterion has been an effort long-led by the ADA, on which we have been actively engaged with CMS. People with diabetes on Medicare will now be able to more easily access this critical piece of technology, leading to better diabetes management and better health outcomes. A big win for the diabetes community!”
“After years of advocacy from the diabetes community and ADCES, Medicare has taken an important step to make [CGM] more accessible for Medicare beneficiaries with diabetes,” Kate Thomas, ADCES chief advocacy and external affairs officer, wrote in a blog post. “This updated [Local Coverage Determination] was a direct result of coordinated advocacy efforts among patient and provider groups, as well as industry partners, coalitions and other entities.”
It’s tough to test four times a day with only three strips
In a Jan. 29, 2021, letter to the Medicare Administrative Contractors, who oversee the policies for durable medical equipment, ADCES explained why the organization strongly supported removal of the four-daily fingerstick requirement, noting that “There is no evidence to suggest that requiring four or more fingerstick tests per day significantly impacts the outcomes of CGM therapy.”
Moreover, they pointed out that the requirement was particularly burdensome, considering the fact that Medicare only covers three test strips per day for insulin-using beneficiaries. “Removing this coverage requirement would allow for increased access to CGM systems and improved health outcomes for beneficiaries with diabetes by improving glycemic control. This also represents a step toward addressing the disparities that exist around diabetes technology under the Medicare program.”
As for the terminology change from “injection” to “administration,” ADCES said that, in addition to allowing CGM coverage for individuals who use rapid-acting inhaled insulin, “we also hope that updating this terminology will help to expedite coverage as future innovations in insulin delivery methods come to market.”
More changes needed, ADCES says
In that January 2021 letter, ADCES recommended several other changes, including covering CGM for anyone diagnosed with type 1 diabetes at any age and without having to meet other requirements except for twice-yearly clinician visits, and for anyone with type 2 diabetes who uses any type of insulin or who has had documented hypoglycemia regardless of therapy.
They also recommended that CGM coverage be considered for patients with chronic kidney disease, and that the required 6-month clinician visits be allowed to take place via telehealth. “ADCES believes that allowing the initiation of CGM therapy through a virtual visit will reduce barriers associated with travel and difficulty accessing a trained provider that are experienced by Medicare beneficiaries.”
In addition, ADCES requested that CMS eliminate the requirement that beneficiaries use insulin three times a day to qualify for CGM, noting that this creates a barrier for patients who can’t afford insulin at all but are at risk for hypoglycemia because they take sulfonylureas or other insulin secretagogues, or for those who use cheaper synthetic human insulins that are only taken twice a day, such as NPH.
“The existing CGM coverage criteria creates an unbalanced and disparate system that excludes from coverage beneficiaries who could greatly benefit from a CGM system, but do not qualify due to issues with insulin affordability,” ADCES wrote in the January letter.
Ms. Thomas wrote in the June 14th blog: “Our work is not done. We know there are more changes that must be made.”
Bariatric surgery cuts insulin needs in type 1 diabetes with severe obesity
While bariatric surgery does nothing to directly improve the disease of patients with type 1 diabetes, it can work indirectly by moderating severe obesity and improving insulin sensitivity to cut the total insulin needs of patients with type 1 diabetes and obesity, based on a single-center, retrospective chart review of 38 U.S. patients.
Two years following their bariatric surgery, these 38 patients with confirmed type 1 diabetes and an average body mass index of 43 kg/m2 before surgery saw their average daily insulin requirement nearly halved, dropping from 118 units/day to 60 units/day, a significant decrease, Brian J. Dessify, DO, said in a presentation at the annual meeting of the American Society for Metabolic and Bariatric Surgery.
Another measure of this effect showed that the percentage of patients who required more than one drug for treating their hyperglycemia fell from 66% before surgery to 52% 2 years after surgery, a change that was not statistically significant, said Dr. Dessify, a bariatric surgeon at Geisinger Medical Center in Danville, Pa.
Appropriate for patients with ‘double diabetes’
These results “provide good evidence for [using] bariatric surgery” in people with both obesity and type 1 diabetes,” he concluded. This includes people with what Dr. Dessify called “double diabetes,” meaning that they do not make endogenous insulin, and are also resistant to the effects of exogenous insulin and hence have features of both type 2 and type 1 diabetes.
“This is a really important study,” commented Ali Aminian, MD, director of the Bariatric and Metabolic Institute of the Cleveland Clinic. “For patients with type 1 diabetes, the primary goal of bariatric surgery is weight loss and improvement of obesity-related comorbidities. Patients with type 2 diabetes can be a candidate for bariatric surgery regardless of their weight,” Dr. Aminian said as designated discussant for the report.
“The goal of bariatric surgery in patients with type 1 diabetes is to promote sensitivity to the exogenous insulin they receive,” agreed Julie Kim, MD, a bariatric surgeon at Mount Auburn Hospital in Waltham, Mass., and a second discussant for the report. Patients with double diabetes “are probably a subclass of patients [with type 1 diabetes] who might benefit even more from bariatric surgery.”
Using gastric sleeves to avoid diabetic ketoacidosis
Dr. Aminian also noted that “at the Cleveland Clinic we consider a sleeve gastrectomy the procedure of choice” for patients with type 1 diabetes or type 2 diabetes with insulin insufficiency “unless the patient has an absolute contraindication” because of the increased risk for diabetic ketoacidosis in these patients “undergoing any surgery, including bariatric surgery.” Patients with insulin insufficiency “require intensive diabetes and insulin management preoperatively to reduce their risk for developing diabetic ketoacidosis,” and using a sleeve rather than bypass generally results in “more reliable absorption of carbohydrates and nutrients” while also reducing the risk for hypoglycemia, Dr. Aminian said.
In the series reported by Dr. Dessify, 33 patients underwent gastric bypass and 5 had sleeve gastrectomy. The decision to use bypass usually stemmed from its “marginal” improvement in weight loss, compared with a sleeve procedure, and an overall preference at Geisinger for bypass procedures. Dr. Dessify added that he had not yet run a comprehensive assessment of diabetic ketoacidosis complications among patients in his reported series.
Those 38 patients underwent their bariatric procedure during 2002-2019, constituting fewer than 1% of the 4,549 total bariatric surgeries done at Geisinger during that period. The 38 patients with type 1 diabetes averaged 41 years of age, 33 (87%) were women, and 37 (97%) were White. Dr. Dessify and associates undertook this review “to help provide supporting evidence for using bariatric surgery in people with obesity and type 1 diabetes,” he noted.
Dr. Dessify, Dr. Aminian, and Dr. Kim had no disclosures.
While bariatric surgery does nothing to directly improve the disease of patients with type 1 diabetes, it can work indirectly by moderating severe obesity and improving insulin sensitivity to cut the total insulin needs of patients with type 1 diabetes and obesity, based on a single-center, retrospective chart review of 38 U.S. patients.
Two years following their bariatric surgery, these 38 patients with confirmed type 1 diabetes and an average body mass index of 43 kg/m2 before surgery saw their average daily insulin requirement nearly halved, dropping from 118 units/day to 60 units/day, a significant decrease, Brian J. Dessify, DO, said in a presentation at the annual meeting of the American Society for Metabolic and Bariatric Surgery.
Another measure of this effect showed that the percentage of patients who required more than one drug for treating their hyperglycemia fell from 66% before surgery to 52% 2 years after surgery, a change that was not statistically significant, said Dr. Dessify, a bariatric surgeon at Geisinger Medical Center in Danville, Pa.
Appropriate for patients with ‘double diabetes’
These results “provide good evidence for [using] bariatric surgery” in people with both obesity and type 1 diabetes,” he concluded. This includes people with what Dr. Dessify called “double diabetes,” meaning that they do not make endogenous insulin, and are also resistant to the effects of exogenous insulin and hence have features of both type 2 and type 1 diabetes.
“This is a really important study,” commented Ali Aminian, MD, director of the Bariatric and Metabolic Institute of the Cleveland Clinic. “For patients with type 1 diabetes, the primary goal of bariatric surgery is weight loss and improvement of obesity-related comorbidities. Patients with type 2 diabetes can be a candidate for bariatric surgery regardless of their weight,” Dr. Aminian said as designated discussant for the report.
“The goal of bariatric surgery in patients with type 1 diabetes is to promote sensitivity to the exogenous insulin they receive,” agreed Julie Kim, MD, a bariatric surgeon at Mount Auburn Hospital in Waltham, Mass., and a second discussant for the report. Patients with double diabetes “are probably a subclass of patients [with type 1 diabetes] who might benefit even more from bariatric surgery.”
Using gastric sleeves to avoid diabetic ketoacidosis
Dr. Aminian also noted that “at the Cleveland Clinic we consider a sleeve gastrectomy the procedure of choice” for patients with type 1 diabetes or type 2 diabetes with insulin insufficiency “unless the patient has an absolute contraindication” because of the increased risk for diabetic ketoacidosis in these patients “undergoing any surgery, including bariatric surgery.” Patients with insulin insufficiency “require intensive diabetes and insulin management preoperatively to reduce their risk for developing diabetic ketoacidosis,” and using a sleeve rather than bypass generally results in “more reliable absorption of carbohydrates and nutrients” while also reducing the risk for hypoglycemia, Dr. Aminian said.
In the series reported by Dr. Dessify, 33 patients underwent gastric bypass and 5 had sleeve gastrectomy. The decision to use bypass usually stemmed from its “marginal” improvement in weight loss, compared with a sleeve procedure, and an overall preference at Geisinger for bypass procedures. Dr. Dessify added that he had not yet run a comprehensive assessment of diabetic ketoacidosis complications among patients in his reported series.
Those 38 patients underwent their bariatric procedure during 2002-2019, constituting fewer than 1% of the 4,549 total bariatric surgeries done at Geisinger during that period. The 38 patients with type 1 diabetes averaged 41 years of age, 33 (87%) were women, and 37 (97%) were White. Dr. Dessify and associates undertook this review “to help provide supporting evidence for using bariatric surgery in people with obesity and type 1 diabetes,” he noted.
Dr. Dessify, Dr. Aminian, and Dr. Kim had no disclosures.
While bariatric surgery does nothing to directly improve the disease of patients with type 1 diabetes, it can work indirectly by moderating severe obesity and improving insulin sensitivity to cut the total insulin needs of patients with type 1 diabetes and obesity, based on a single-center, retrospective chart review of 38 U.S. patients.
Two years following their bariatric surgery, these 38 patients with confirmed type 1 diabetes and an average body mass index of 43 kg/m2 before surgery saw their average daily insulin requirement nearly halved, dropping from 118 units/day to 60 units/day, a significant decrease, Brian J. Dessify, DO, said in a presentation at the annual meeting of the American Society for Metabolic and Bariatric Surgery.
Another measure of this effect showed that the percentage of patients who required more than one drug for treating their hyperglycemia fell from 66% before surgery to 52% 2 years after surgery, a change that was not statistically significant, said Dr. Dessify, a bariatric surgeon at Geisinger Medical Center in Danville, Pa.
Appropriate for patients with ‘double diabetes’
These results “provide good evidence for [using] bariatric surgery” in people with both obesity and type 1 diabetes,” he concluded. This includes people with what Dr. Dessify called “double diabetes,” meaning that they do not make endogenous insulin, and are also resistant to the effects of exogenous insulin and hence have features of both type 2 and type 1 diabetes.
“This is a really important study,” commented Ali Aminian, MD, director of the Bariatric and Metabolic Institute of the Cleveland Clinic. “For patients with type 1 diabetes, the primary goal of bariatric surgery is weight loss and improvement of obesity-related comorbidities. Patients with type 2 diabetes can be a candidate for bariatric surgery regardless of their weight,” Dr. Aminian said as designated discussant for the report.
“The goal of bariatric surgery in patients with type 1 diabetes is to promote sensitivity to the exogenous insulin they receive,” agreed Julie Kim, MD, a bariatric surgeon at Mount Auburn Hospital in Waltham, Mass., and a second discussant for the report. Patients with double diabetes “are probably a subclass of patients [with type 1 diabetes] who might benefit even more from bariatric surgery.”
Using gastric sleeves to avoid diabetic ketoacidosis
Dr. Aminian also noted that “at the Cleveland Clinic we consider a sleeve gastrectomy the procedure of choice” for patients with type 1 diabetes or type 2 diabetes with insulin insufficiency “unless the patient has an absolute contraindication” because of the increased risk for diabetic ketoacidosis in these patients “undergoing any surgery, including bariatric surgery.” Patients with insulin insufficiency “require intensive diabetes and insulin management preoperatively to reduce their risk for developing diabetic ketoacidosis,” and using a sleeve rather than bypass generally results in “more reliable absorption of carbohydrates and nutrients” while also reducing the risk for hypoglycemia, Dr. Aminian said.
In the series reported by Dr. Dessify, 33 patients underwent gastric bypass and 5 had sleeve gastrectomy. The decision to use bypass usually stemmed from its “marginal” improvement in weight loss, compared with a sleeve procedure, and an overall preference at Geisinger for bypass procedures. Dr. Dessify added that he had not yet run a comprehensive assessment of diabetic ketoacidosis complications among patients in his reported series.
Those 38 patients underwent their bariatric procedure during 2002-2019, constituting fewer than 1% of the 4,549 total bariatric surgeries done at Geisinger during that period. The 38 patients with type 1 diabetes averaged 41 years of age, 33 (87%) were women, and 37 (97%) were White. Dr. Dessify and associates undertook this review “to help provide supporting evidence for using bariatric surgery in people with obesity and type 1 diabetes,” he noted.
Dr. Dessify, Dr. Aminian, and Dr. Kim had no disclosures.
FROM ASMBS 2021
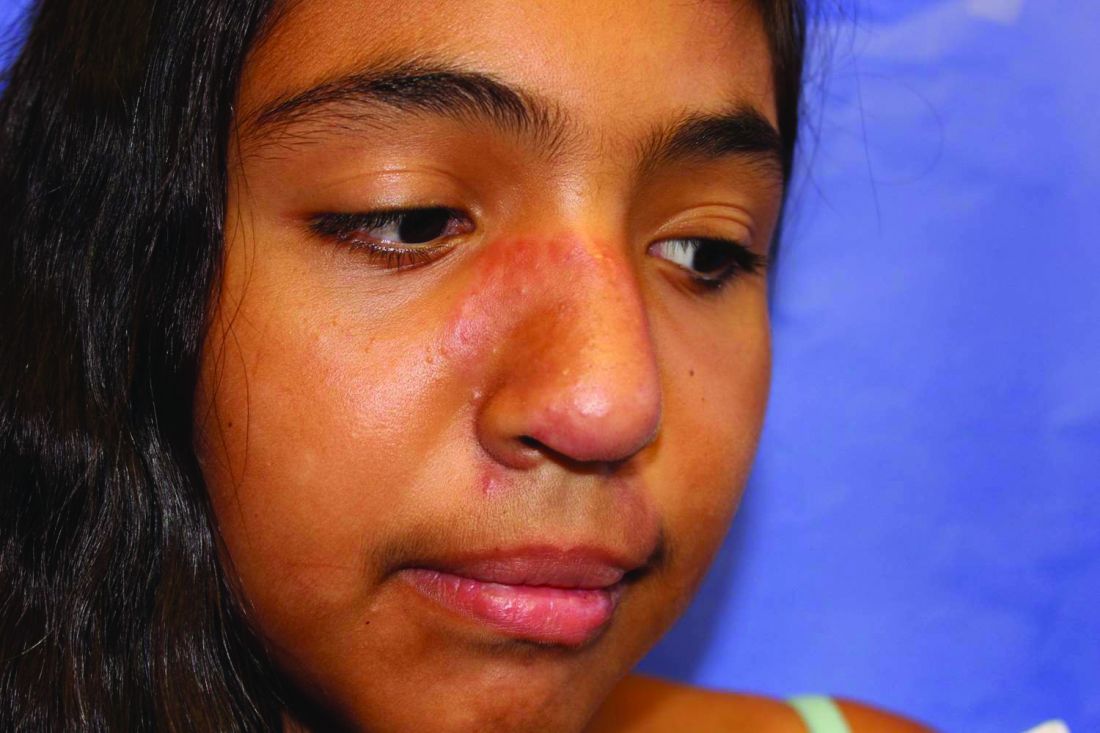
Rapidly growing hand nodule
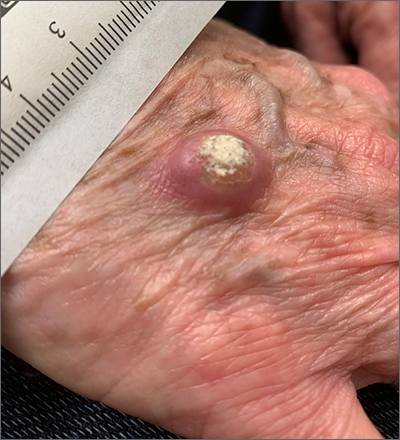
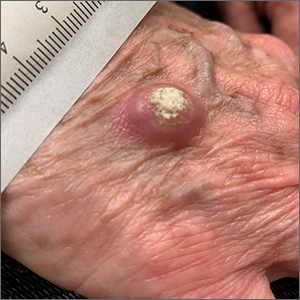
Large, firm, erythematous, nodular lesions with central keratin plugs that arise rapidly are the hallmark of keratoacanthomas (KAs).
KAs commonly manifest on the hands and face and can alarm patients who are unfamiliar with their rapid growth. Since KAs can involute spontaneously after their growth and scarring phase, they were previously regarded as a benign lesion. KAs have a characteristic central keratin plug; this contrasts with basal cell carcinomas, which have central ulceration or scabbing. Currently, KAs are considered a subtype of squamous cell carcinoma (SCC). KAs tend to grow much faster and are raised, unlike SCCs, which normally have scale surrounding macular or slightly raised erythematous tissue.
Treatments include surgical excision, cryosurgery, curettage with electrodesiccation, topical 5-fluorouracil, imiquimod, intralesional methotrexate, or bleomycin.1 The isolated form of KAs is most common, but there are syndromes of multiple KAs that can be treated with systemic retinoids.
For this patient, the KA was transected across the base level with the surrounding skin and sent for pathology. At the same visit, the base was curetted down to the dermis and treated with electrodesiccation. This was followed by a repeat cycle of curettage and electrodesiccation. The patient was advised that if the pathology report showed invasive SCC, excision with margins would be recommended; otherwise, she would be followed clinically. Pathology did confirm a KA type of SCC. At the 6-week follow-up, the area was healing well, with no evidence of recurrence.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque
Panagiotopoulos A, Kyriazis N, Polychronaki E, et al. The effectiveness of cryosurgery combined with curettage and electrodessication in the treatment of keratoacanthoma: a retrospective analysis of 90 cases. Indian J Dermatol. 2020;65:406-408. doi: 10.4103/ijd.IJD_202_18

Large, firm, erythematous, nodular lesions with central keratin plugs that arise rapidly are the hallmark of keratoacanthomas (KAs).
KAs commonly manifest on the hands and face and can alarm patients who are unfamiliar with their rapid growth. Since KAs can involute spontaneously after their growth and scarring phase, they were previously regarded as a benign lesion. KAs have a characteristic central keratin plug; this contrasts with basal cell carcinomas, which have central ulceration or scabbing. Currently, KAs are considered a subtype of squamous cell carcinoma (SCC). KAs tend to grow much faster and are raised, unlike SCCs, which normally have scale surrounding macular or slightly raised erythematous tissue.
Treatments include surgical excision, cryosurgery, curettage with electrodesiccation, topical 5-fluorouracil, imiquimod, intralesional methotrexate, or bleomycin.1 The isolated form of KAs is most common, but there are syndromes of multiple KAs that can be treated with systemic retinoids.
For this patient, the KA was transected across the base level with the surrounding skin and sent for pathology. At the same visit, the base was curetted down to the dermis and treated with electrodesiccation. This was followed by a repeat cycle of curettage and electrodesiccation. The patient was advised that if the pathology report showed invasive SCC, excision with margins would be recommended; otherwise, she would be followed clinically. Pathology did confirm a KA type of SCC. At the 6-week follow-up, the area was healing well, with no evidence of recurrence.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque

Large, firm, erythematous, nodular lesions with central keratin plugs that arise rapidly are the hallmark of keratoacanthomas (KAs).
KAs commonly manifest on the hands and face and can alarm patients who are unfamiliar with their rapid growth. Since KAs can involute spontaneously after their growth and scarring phase, they were previously regarded as a benign lesion. KAs have a characteristic central keratin plug; this contrasts with basal cell carcinomas, which have central ulceration or scabbing. Currently, KAs are considered a subtype of squamous cell carcinoma (SCC). KAs tend to grow much faster and are raised, unlike SCCs, which normally have scale surrounding macular or slightly raised erythematous tissue.
Treatments include surgical excision, cryosurgery, curettage with electrodesiccation, topical 5-fluorouracil, imiquimod, intralesional methotrexate, or bleomycin.1 The isolated form of KAs is most common, but there are syndromes of multiple KAs that can be treated with systemic retinoids.
For this patient, the KA was transected across the base level with the surrounding skin and sent for pathology. At the same visit, the base was curetted down to the dermis and treated with electrodesiccation. This was followed by a repeat cycle of curettage and electrodesiccation. The patient was advised that if the pathology report showed invasive SCC, excision with margins would be recommended; otherwise, she would be followed clinically. Pathology did confirm a KA type of SCC. At the 6-week follow-up, the area was healing well, with no evidence of recurrence.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque
Panagiotopoulos A, Kyriazis N, Polychronaki E, et al. The effectiveness of cryosurgery combined with curettage and electrodessication in the treatment of keratoacanthoma: a retrospective analysis of 90 cases. Indian J Dermatol. 2020;65:406-408. doi: 10.4103/ijd.IJD_202_18
Panagiotopoulos A, Kyriazis N, Polychronaki E, et al. The effectiveness of cryosurgery combined with curettage and electrodessication in the treatment of keratoacanthoma: a retrospective analysis of 90 cases. Indian J Dermatol. 2020;65:406-408. doi: 10.4103/ijd.IJD_202_18
The good old days
“It’s good to be in something from the ground floor. I came too late for that. ... But lately, I’m getting the feeling that I came in at the end. The best is over.” –Tony Soprano
For me, I’m unsure. Sometimes it feels like our best days are behind us. When I was a kid, we explored life in pond water, watching water fleas and hydra swim under our Child World toy microscopes. Today, kids learn to eat Tide Pods from TikTok. Back when I was young, a doctor’s appointment was a special occasion! My brothers and I had a bath and got dressed in our Sunday best for our appointment with Dr. Bellin, a genteel, gray-haired pediatrician who worked out of his Victorian office with wooden floors and crystal door handles. Contrast that with the appointment I had with a patient the other day, done by telephone while she was in line ordering at Starbucks. I waited patiently for her to give her order.
This ache I feel for the past is called nostalgia. At one time, it was a diagnosable condition. It was first used by Dr. Johannes Hofer in the 17th century to describe Swiss soldiers fighting in foreign lands. From the Greek, it means “homecoming pain.” Although over time nostalgia has lost its clinical meaning, the feeling of yearning for the past has dramatically gained in prevalence. The word “nostalgia” appears more in print now than at any point since 1800. We are most nostalgic during times of duress, it seems. This, no doubt, is because it’s comforting to think we’d be better off back in pastoral, idyll times, back when work ended at 5 p.m. and cotton balls were soaked in alcohol and office visits ended with a lollipop on a loop.
Of course, the good old days weren’t really better. We have a selective view of history – as many things were contemptible or bad then as now. Yes, Dr. Bellin was the consummate professional, but thank goodness, I didn’t have acute lymphocytic leukemia or Haemophilus influenzae type B or even suffocate under a pile of blankets while sleeping on my stomach. Without doubt, clinically we’re much better today. Also back then, there was hardly a consideration for atrocious racial disparities in care. We’ve not come far, but we are at least better off today than a few decades ago. And what about medicine as a profession? Although he had loads of autonomy and respect, Dr. Bellin also started every day of his 50-year career at 6 a.m. rounding in the newborn nursery before seeing patients in the office 6 days a week. Not many of us would trade our practice for his.
Yet, there’s reasons to be nostalgic. Chart notes might have been barely legible, but at least they served a purpose. The problem-oriented medical record was intended to logically capture and organize data. SOAP notes were invented to help us think better, to get diagnoses correct, to succinctly see progress. Today, notes are written for administrators and payers and patients. As a result, they’re often useless to us.
And although it may have been inconvenient to sit in the waiting room reading Highlights magazine, I’m unsure it was a worse user experience, compared with a chain pharmacy “virtual” doctor visit. (Particularly because you could always drop pennies down the large hot-air iron floor grate in the corner).
The thrumming undercurrent of progress promises artificial intelligence and genomics and wearable diagnostics in our future. But the assumption is the new things will be better suited to our needs than the old. Sometimes, they are not. Sometimes technology diminishes us instead of enhancing us.
I cannot count how many times I’ve hit my head or whacked my shin because our Tesla Model X doors open by magic and of their own accord. Back when I was young, we opened car doors by pulling on the door handle. I sometimes miss those days.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
“It’s good to be in something from the ground floor. I came too late for that. ... But lately, I’m getting the feeling that I came in at the end. The best is over.” –Tony Soprano
For me, I’m unsure. Sometimes it feels like our best days are behind us. When I was a kid, we explored life in pond water, watching water fleas and hydra swim under our Child World toy microscopes. Today, kids learn to eat Tide Pods from TikTok. Back when I was young, a doctor’s appointment was a special occasion! My brothers and I had a bath and got dressed in our Sunday best for our appointment with Dr. Bellin, a genteel, gray-haired pediatrician who worked out of his Victorian office with wooden floors and crystal door handles. Contrast that with the appointment I had with a patient the other day, done by telephone while she was in line ordering at Starbucks. I waited patiently for her to give her order.
This ache I feel for the past is called nostalgia. At one time, it was a diagnosable condition. It was first used by Dr. Johannes Hofer in the 17th century to describe Swiss soldiers fighting in foreign lands. From the Greek, it means “homecoming pain.” Although over time nostalgia has lost its clinical meaning, the feeling of yearning for the past has dramatically gained in prevalence. The word “nostalgia” appears more in print now than at any point since 1800. We are most nostalgic during times of duress, it seems. This, no doubt, is because it’s comforting to think we’d be better off back in pastoral, idyll times, back when work ended at 5 p.m. and cotton balls were soaked in alcohol and office visits ended with a lollipop on a loop.
Of course, the good old days weren’t really better. We have a selective view of history – as many things were contemptible or bad then as now. Yes, Dr. Bellin was the consummate professional, but thank goodness, I didn’t have acute lymphocytic leukemia or Haemophilus influenzae type B or even suffocate under a pile of blankets while sleeping on my stomach. Without doubt, clinically we’re much better today. Also back then, there was hardly a consideration for atrocious racial disparities in care. We’ve not come far, but we are at least better off today than a few decades ago. And what about medicine as a profession? Although he had loads of autonomy and respect, Dr. Bellin also started every day of his 50-year career at 6 a.m. rounding in the newborn nursery before seeing patients in the office 6 days a week. Not many of us would trade our practice for his.
Yet, there’s reasons to be nostalgic. Chart notes might have been barely legible, but at least they served a purpose. The problem-oriented medical record was intended to logically capture and organize data. SOAP notes were invented to help us think better, to get diagnoses correct, to succinctly see progress. Today, notes are written for administrators and payers and patients. As a result, they’re often useless to us.
And although it may have been inconvenient to sit in the waiting room reading Highlights magazine, I’m unsure it was a worse user experience, compared with a chain pharmacy “virtual” doctor visit. (Particularly because you could always drop pennies down the large hot-air iron floor grate in the corner).
The thrumming undercurrent of progress promises artificial intelligence and genomics and wearable diagnostics in our future. But the assumption is the new things will be better suited to our needs than the old. Sometimes, they are not. Sometimes technology diminishes us instead of enhancing us.
I cannot count how many times I’ve hit my head or whacked my shin because our Tesla Model X doors open by magic and of their own accord. Back when I was young, we opened car doors by pulling on the door handle. I sometimes miss those days.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
“It’s good to be in something from the ground floor. I came too late for that. ... But lately, I’m getting the feeling that I came in at the end. The best is over.” –Tony Soprano
For me, I’m unsure. Sometimes it feels like our best days are behind us. When I was a kid, we explored life in pond water, watching water fleas and hydra swim under our Child World toy microscopes. Today, kids learn to eat Tide Pods from TikTok. Back when I was young, a doctor’s appointment was a special occasion! My brothers and I had a bath and got dressed in our Sunday best for our appointment with Dr. Bellin, a genteel, gray-haired pediatrician who worked out of his Victorian office with wooden floors and crystal door handles. Contrast that with the appointment I had with a patient the other day, done by telephone while she was in line ordering at Starbucks. I waited patiently for her to give her order.
This ache I feel for the past is called nostalgia. At one time, it was a diagnosable condition. It was first used by Dr. Johannes Hofer in the 17th century to describe Swiss soldiers fighting in foreign lands. From the Greek, it means “homecoming pain.” Although over time nostalgia has lost its clinical meaning, the feeling of yearning for the past has dramatically gained in prevalence. The word “nostalgia” appears more in print now than at any point since 1800. We are most nostalgic during times of duress, it seems. This, no doubt, is because it’s comforting to think we’d be better off back in pastoral, idyll times, back when work ended at 5 p.m. and cotton balls were soaked in alcohol and office visits ended with a lollipop on a loop.
Of course, the good old days weren’t really better. We have a selective view of history – as many things were contemptible or bad then as now. Yes, Dr. Bellin was the consummate professional, but thank goodness, I didn’t have acute lymphocytic leukemia or Haemophilus influenzae type B or even suffocate under a pile of blankets while sleeping on my stomach. Without doubt, clinically we’re much better today. Also back then, there was hardly a consideration for atrocious racial disparities in care. We’ve not come far, but we are at least better off today than a few decades ago. And what about medicine as a profession? Although he had loads of autonomy and respect, Dr. Bellin also started every day of his 50-year career at 6 a.m. rounding in the newborn nursery before seeing patients in the office 6 days a week. Not many of us would trade our practice for his.
Yet, there’s reasons to be nostalgic. Chart notes might have been barely legible, but at least they served a purpose. The problem-oriented medical record was intended to logically capture and organize data. SOAP notes were invented to help us think better, to get diagnoses correct, to succinctly see progress. Today, notes are written for administrators and payers and patients. As a result, they’re often useless to us.
And although it may have been inconvenient to sit in the waiting room reading Highlights magazine, I’m unsure it was a worse user experience, compared with a chain pharmacy “virtual” doctor visit. (Particularly because you could always drop pennies down the large hot-air iron floor grate in the corner).
The thrumming undercurrent of progress promises artificial intelligence and genomics and wearable diagnostics in our future. But the assumption is the new things will be better suited to our needs than the old. Sometimes, they are not. Sometimes technology diminishes us instead of enhancing us.
I cannot count how many times I’ve hit my head or whacked my shin because our Tesla Model X doors open by magic and of their own accord. Back when I was young, we opened car doors by pulling on the door handle. I sometimes miss those days.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
Choosing a career as chief medical officer at a health technology startup
How did your career pathway lead you to working at a health tech startup?
I’ve always had an interest in technology – in fact, it was part of the reason I chose gastroenterology. When I finished GI fellowship, I decided to stay in academics because of an opportunity to lead clinical innovation efforts at my institution’s patient safety institute. This role provided protected time to foster external and internal partnerships around technology. It also gave me an opportunity to pursue clinical research and administrative experiences. While I enjoyed all three paths, it became clear that health technology was my passion. While the opportunity to join a startup was largely serendipitous – I met the founder of the company after presenting at a digital medicine conference – it also happened as a result of the steps outlined in a subsequent question. Not long after learning about the company, I made the transition to part-time faculty/clinical status and full-time chief medical officer (CMO).
What do you do as CMO?
There is no one answer to this question. It will depend on a number of variables, especially the type of business (for example, diagnostic, drug, digital, direct care management, and so on), stage of company (for example, concept, seed, series A/B/C, public), and the existing background of company founders (for example, technical, clinical, operations, and so on). Generally speaking, the earlier the stage of the company, the more hats you’ll wear (though this also means more risk; more on that later). An early-stage company was appealing to me because it gave me an opportunity to apply many of the same critical-thinking and problem-solving skills in clinical medicine to a host of other challenges. For example, as a practicing gastroenterologist, I know the pain points in the delivery of GI care and the challenges that my patients encounter. I then ask how can I develop our technology and product platform to address these issues. Also understanding how value and quality are measured in GI practice makes it easier to convey the effect of the solutions that are built and prioritize their development. In my current role I contribute to the following areas:
- Clinical strategy and vision. This means understanding the clinical need the company is trying to address at a fundamental level and designing how the technology or solution can address that need in a meaningful way. This includes working directly with technology and product teams to create a roadmap for how the technology/solution will continue to drive impact.
- Clinical care leadership. If the company employs or works with health professionals in any capacity, this usually involves developing clinical protocols and providing clinical direction.
- Clinical outcomes. This means being responsible for understanding and/or developing the metrics that will be used to demonstrate impact of the technology/solution. This includes designing clinical studies and being responsible for their execution.
- Stakeholder engagement. This means interfacing internally with nearly every aspect of the company and interacting externally with customers (usually medical peers and executives), investors, other companies, and key opinion leaders in the field.
- Regulatory. For companies pursuing Food and Drug Administration clearance or approval for their product, this entails developing a strategy and executing it.
- Research & development. This involves creating and executing a roadmap for integrating new technologies/ideas that generally complement the initial problem you are trying to solve.
What do you enjoy most about working at a startup?
The variety of experience, the flexibility, the fast pace, the ability to work creatively, and the potential to make a large-scale impact are all aspects of the job that I enjoy. The ability to continue clinical practice is important to me and is a major plus.
What do you find most challenging about working at a startup?
One of the biggest differences between a startup and a traditional clinical role is the degree of uncertainty that permeates the entire experience. It took some time for me to adjust to the relative volatility/risk associated with this type of work. Unlike an academic, administrative, or private practice job, things can change very quickly (as in a 24-hour period or less!). This can encompass a number of changes, such as funding, leadership, strategic direction, business model, and staffing, to name a few. What I’ve learned is that this doesn’t always mean changing for the worse, but it does mean things changing near constantly. Being mentally prepared to adapt quickly and frequently to big changes is part of the experience.
What are the ways that GIs can get involved in startups?
Gastroenterologists have more opportunities than most physicians due to the diversity of conditions we treat and the large corresponding number of unmet needs we encounter. There is also the inherent innovation potential associated with new applications in endoscopy, diagnostics, and drug therapies. As a result, there are a number of ways to get involved:
- This often takes the form of “spinning out” research from an academic institution but can also be done successfully from private practice, particularly in the context of new devices/services. Another related option is to license your technology to a company, which offloads the operational aspects of running a business.
- Provide consulting/advisory support. Many early-stage companies cannot afford to hire a full-time physician, but they are open to consulting arrangements (and of course volunteer work). Don’t hesitate to directly contact companies that are interesting to you. These opportunities are possible even while in clinical training.
- Work part time or full time. The majority of startups are supportive of physicians continuing to practice clinically. This makes engaging in a part-time position financially feasible for both parties. Given the relatively high remuneration for gastroenterologists working clinically, a full-time position at a startup may require a financial tradeoff (that is, lower short-term salary for a potential larger long-term gain – note the emphasis on “potential”).
- Invest in early-stage companies. Physicians can become angel investors for early-stage companies. Given the relatively time-intensive process of finding new opportunities and conducting due diligence, this often takes the form of pooling funds into angel networks that can distribute the execution of investments more efficiently.
How would a fellow or early-career GI who is interested in startups pursue this career pathway?
The first step I recommend is self-reflection – what about the startup experience is interesting to you? Not all aspects appeal to everyone, and not all options provide the same opportunities. Spending time deciding which specific aspects of the startup experience appeal to you will make it easier to find the right opportunity. A concurrent step is to build expertise. This can take many forms, including traditional basic science or clinical research, but also includes implementation, evaluation/analysis, design, education, regulation, policy, and so on. The next step is to proactively meet people who are doing what you are interested in doing. Reach out to mentors, alumni, faculty, and friends. Conferences and social media are also great places to network. Other potential paths can include developing expertise in an allied functional area that can be later leveraged into a startup role (for example, experience at pharma, payer, regulatory, and so on). Many of these organizations have programs specifically geared toward physicians making a transition. In addition, another potential option is to seek additional education through an MBA where internships, recruitment programs, and robust alumni networks can be helpful in finding placement.
What if I want to learn more about the health technology startup experience?
The AGA Center for GI Innovation and Technology (CGIT) has a number of programs throughout the year, including the annual Tech Summit where you can learn about new companies, ideas, and technologies from like-minded individuals. I also invite you to reach out to me directly via Twitter, LinkedIn, or email with specific questions. As gastroenterologists, we are fortunate to work in a field full of innovation and new ideas. As a result, there are many meaningful career paths available to those interested in gastroenterology and technology. Whether providing direct clinical care with the latest endoscopic techniques or developing the next digital therapy, the opportunities for gastroenterologists will only continue to grow.
Dr. Mathews is chief medical officer at Vivante Health and assistant professor of medicine at Johns Hopkins Medicine in Baltimore. He is an officer at Vivante Health with stock options, but he reports having nothing else to disclose.
How did your career pathway lead you to working at a health tech startup?
I’ve always had an interest in technology – in fact, it was part of the reason I chose gastroenterology. When I finished GI fellowship, I decided to stay in academics because of an opportunity to lead clinical innovation efforts at my institution’s patient safety institute. This role provided protected time to foster external and internal partnerships around technology. It also gave me an opportunity to pursue clinical research and administrative experiences. While I enjoyed all three paths, it became clear that health technology was my passion. While the opportunity to join a startup was largely serendipitous – I met the founder of the company after presenting at a digital medicine conference – it also happened as a result of the steps outlined in a subsequent question. Not long after learning about the company, I made the transition to part-time faculty/clinical status and full-time chief medical officer (CMO).
What do you do as CMO?
There is no one answer to this question. It will depend on a number of variables, especially the type of business (for example, diagnostic, drug, digital, direct care management, and so on), stage of company (for example, concept, seed, series A/B/C, public), and the existing background of company founders (for example, technical, clinical, operations, and so on). Generally speaking, the earlier the stage of the company, the more hats you’ll wear (though this also means more risk; more on that later). An early-stage company was appealing to me because it gave me an opportunity to apply many of the same critical-thinking and problem-solving skills in clinical medicine to a host of other challenges. For example, as a practicing gastroenterologist, I know the pain points in the delivery of GI care and the challenges that my patients encounter. I then ask how can I develop our technology and product platform to address these issues. Also understanding how value and quality are measured in GI practice makes it easier to convey the effect of the solutions that are built and prioritize their development. In my current role I contribute to the following areas:
- Clinical strategy and vision. This means understanding the clinical need the company is trying to address at a fundamental level and designing how the technology or solution can address that need in a meaningful way. This includes working directly with technology and product teams to create a roadmap for how the technology/solution will continue to drive impact.
- Clinical care leadership. If the company employs or works with health professionals in any capacity, this usually involves developing clinical protocols and providing clinical direction.
- Clinical outcomes. This means being responsible for understanding and/or developing the metrics that will be used to demonstrate impact of the technology/solution. This includes designing clinical studies and being responsible for their execution.
- Stakeholder engagement. This means interfacing internally with nearly every aspect of the company and interacting externally with customers (usually medical peers and executives), investors, other companies, and key opinion leaders in the field.
- Regulatory. For companies pursuing Food and Drug Administration clearance or approval for their product, this entails developing a strategy and executing it.
- Research & development. This involves creating and executing a roadmap for integrating new technologies/ideas that generally complement the initial problem you are trying to solve.
What do you enjoy most about working at a startup?
The variety of experience, the flexibility, the fast pace, the ability to work creatively, and the potential to make a large-scale impact are all aspects of the job that I enjoy. The ability to continue clinical practice is important to me and is a major plus.
What do you find most challenging about working at a startup?
One of the biggest differences between a startup and a traditional clinical role is the degree of uncertainty that permeates the entire experience. It took some time for me to adjust to the relative volatility/risk associated with this type of work. Unlike an academic, administrative, or private practice job, things can change very quickly (as in a 24-hour period or less!). This can encompass a number of changes, such as funding, leadership, strategic direction, business model, and staffing, to name a few. What I’ve learned is that this doesn’t always mean changing for the worse, but it does mean things changing near constantly. Being mentally prepared to adapt quickly and frequently to big changes is part of the experience.
What are the ways that GIs can get involved in startups?
Gastroenterologists have more opportunities than most physicians due to the diversity of conditions we treat and the large corresponding number of unmet needs we encounter. There is also the inherent innovation potential associated with new applications in endoscopy, diagnostics, and drug therapies. As a result, there are a number of ways to get involved:
- This often takes the form of “spinning out” research from an academic institution but can also be done successfully from private practice, particularly in the context of new devices/services. Another related option is to license your technology to a company, which offloads the operational aspects of running a business.
- Provide consulting/advisory support. Many early-stage companies cannot afford to hire a full-time physician, but they are open to consulting arrangements (and of course volunteer work). Don’t hesitate to directly contact companies that are interesting to you. These opportunities are possible even while in clinical training.
- Work part time or full time. The majority of startups are supportive of physicians continuing to practice clinically. This makes engaging in a part-time position financially feasible for both parties. Given the relatively high remuneration for gastroenterologists working clinically, a full-time position at a startup may require a financial tradeoff (that is, lower short-term salary for a potential larger long-term gain – note the emphasis on “potential”).
- Invest in early-stage companies. Physicians can become angel investors for early-stage companies. Given the relatively time-intensive process of finding new opportunities and conducting due diligence, this often takes the form of pooling funds into angel networks that can distribute the execution of investments more efficiently.
How would a fellow or early-career GI who is interested in startups pursue this career pathway?
The first step I recommend is self-reflection – what about the startup experience is interesting to you? Not all aspects appeal to everyone, and not all options provide the same opportunities. Spending time deciding which specific aspects of the startup experience appeal to you will make it easier to find the right opportunity. A concurrent step is to build expertise. This can take many forms, including traditional basic science or clinical research, but also includes implementation, evaluation/analysis, design, education, regulation, policy, and so on. The next step is to proactively meet people who are doing what you are interested in doing. Reach out to mentors, alumni, faculty, and friends. Conferences and social media are also great places to network. Other potential paths can include developing expertise in an allied functional area that can be later leveraged into a startup role (for example, experience at pharma, payer, regulatory, and so on). Many of these organizations have programs specifically geared toward physicians making a transition. In addition, another potential option is to seek additional education through an MBA where internships, recruitment programs, and robust alumni networks can be helpful in finding placement.
What if I want to learn more about the health technology startup experience?
The AGA Center for GI Innovation and Technology (CGIT) has a number of programs throughout the year, including the annual Tech Summit where you can learn about new companies, ideas, and technologies from like-minded individuals. I also invite you to reach out to me directly via Twitter, LinkedIn, or email with specific questions. As gastroenterologists, we are fortunate to work in a field full of innovation and new ideas. As a result, there are many meaningful career paths available to those interested in gastroenterology and technology. Whether providing direct clinical care with the latest endoscopic techniques or developing the next digital therapy, the opportunities for gastroenterologists will only continue to grow.
Dr. Mathews is chief medical officer at Vivante Health and assistant professor of medicine at Johns Hopkins Medicine in Baltimore. He is an officer at Vivante Health with stock options, but he reports having nothing else to disclose.
How did your career pathway lead you to working at a health tech startup?
I’ve always had an interest in technology – in fact, it was part of the reason I chose gastroenterology. When I finished GI fellowship, I decided to stay in academics because of an opportunity to lead clinical innovation efforts at my institution’s patient safety institute. This role provided protected time to foster external and internal partnerships around technology. It also gave me an opportunity to pursue clinical research and administrative experiences. While I enjoyed all three paths, it became clear that health technology was my passion. While the opportunity to join a startup was largely serendipitous – I met the founder of the company after presenting at a digital medicine conference – it also happened as a result of the steps outlined in a subsequent question. Not long after learning about the company, I made the transition to part-time faculty/clinical status and full-time chief medical officer (CMO).
What do you do as CMO?
There is no one answer to this question. It will depend on a number of variables, especially the type of business (for example, diagnostic, drug, digital, direct care management, and so on), stage of company (for example, concept, seed, series A/B/C, public), and the existing background of company founders (for example, technical, clinical, operations, and so on). Generally speaking, the earlier the stage of the company, the more hats you’ll wear (though this also means more risk; more on that later). An early-stage company was appealing to me because it gave me an opportunity to apply many of the same critical-thinking and problem-solving skills in clinical medicine to a host of other challenges. For example, as a practicing gastroenterologist, I know the pain points in the delivery of GI care and the challenges that my patients encounter. I then ask how can I develop our technology and product platform to address these issues. Also understanding how value and quality are measured in GI practice makes it easier to convey the effect of the solutions that are built and prioritize their development. In my current role I contribute to the following areas:
- Clinical strategy and vision. This means understanding the clinical need the company is trying to address at a fundamental level and designing how the technology or solution can address that need in a meaningful way. This includes working directly with technology and product teams to create a roadmap for how the technology/solution will continue to drive impact.
- Clinical care leadership. If the company employs or works with health professionals in any capacity, this usually involves developing clinical protocols and providing clinical direction.
- Clinical outcomes. This means being responsible for understanding and/or developing the metrics that will be used to demonstrate impact of the technology/solution. This includes designing clinical studies and being responsible for their execution.
- Stakeholder engagement. This means interfacing internally with nearly every aspect of the company and interacting externally with customers (usually medical peers and executives), investors, other companies, and key opinion leaders in the field.
- Regulatory. For companies pursuing Food and Drug Administration clearance or approval for their product, this entails developing a strategy and executing it.
- Research & development. This involves creating and executing a roadmap for integrating new technologies/ideas that generally complement the initial problem you are trying to solve.
What do you enjoy most about working at a startup?
The variety of experience, the flexibility, the fast pace, the ability to work creatively, and the potential to make a large-scale impact are all aspects of the job that I enjoy. The ability to continue clinical practice is important to me and is a major plus.
What do you find most challenging about working at a startup?
One of the biggest differences between a startup and a traditional clinical role is the degree of uncertainty that permeates the entire experience. It took some time for me to adjust to the relative volatility/risk associated with this type of work. Unlike an academic, administrative, or private practice job, things can change very quickly (as in a 24-hour period or less!). This can encompass a number of changes, such as funding, leadership, strategic direction, business model, and staffing, to name a few. What I’ve learned is that this doesn’t always mean changing for the worse, but it does mean things changing near constantly. Being mentally prepared to adapt quickly and frequently to big changes is part of the experience.
What are the ways that GIs can get involved in startups?
Gastroenterologists have more opportunities than most physicians due to the diversity of conditions we treat and the large corresponding number of unmet needs we encounter. There is also the inherent innovation potential associated with new applications in endoscopy, diagnostics, and drug therapies. As a result, there are a number of ways to get involved:
- This often takes the form of “spinning out” research from an academic institution but can also be done successfully from private practice, particularly in the context of new devices/services. Another related option is to license your technology to a company, which offloads the operational aspects of running a business.
- Provide consulting/advisory support. Many early-stage companies cannot afford to hire a full-time physician, but they are open to consulting arrangements (and of course volunteer work). Don’t hesitate to directly contact companies that are interesting to you. These opportunities are possible even while in clinical training.
- Work part time or full time. The majority of startups are supportive of physicians continuing to practice clinically. This makes engaging in a part-time position financially feasible for both parties. Given the relatively high remuneration for gastroenterologists working clinically, a full-time position at a startup may require a financial tradeoff (that is, lower short-term salary for a potential larger long-term gain – note the emphasis on “potential”).
- Invest in early-stage companies. Physicians can become angel investors for early-stage companies. Given the relatively time-intensive process of finding new opportunities and conducting due diligence, this often takes the form of pooling funds into angel networks that can distribute the execution of investments more efficiently.
How would a fellow or early-career GI who is interested in startups pursue this career pathway?
The first step I recommend is self-reflection – what about the startup experience is interesting to you? Not all aspects appeal to everyone, and not all options provide the same opportunities. Spending time deciding which specific aspects of the startup experience appeal to you will make it easier to find the right opportunity. A concurrent step is to build expertise. This can take many forms, including traditional basic science or clinical research, but also includes implementation, evaluation/analysis, design, education, regulation, policy, and so on. The next step is to proactively meet people who are doing what you are interested in doing. Reach out to mentors, alumni, faculty, and friends. Conferences and social media are also great places to network. Other potential paths can include developing expertise in an allied functional area that can be later leveraged into a startup role (for example, experience at pharma, payer, regulatory, and so on). Many of these organizations have programs specifically geared toward physicians making a transition. In addition, another potential option is to seek additional education through an MBA where internships, recruitment programs, and robust alumni networks can be helpful in finding placement.
What if I want to learn more about the health technology startup experience?
The AGA Center for GI Innovation and Technology (CGIT) has a number of programs throughout the year, including the annual Tech Summit where you can learn about new companies, ideas, and technologies from like-minded individuals. I also invite you to reach out to me directly via Twitter, LinkedIn, or email with specific questions. As gastroenterologists, we are fortunate to work in a field full of innovation and new ideas. As a result, there are many meaningful career paths available to those interested in gastroenterology and technology. Whether providing direct clinical care with the latest endoscopic techniques or developing the next digital therapy, the opportunities for gastroenterologists will only continue to grow.
Dr. Mathews is chief medical officer at Vivante Health and assistant professor of medicine at Johns Hopkins Medicine in Baltimore. He is an officer at Vivante Health with stock options, but he reports having nothing else to disclose.
Addressing an unmet need in IBD patients: Treatment of acute abdominal pain
In the acute care setting, providers of care for inflammatory bowel disease (IBD) patients are often faced with the dilemma of providing effective abdominal pain management in a population that has worse outcomes with both opioid and NSAID therapy. There is increased mortality associated with opioid use and risk of disease relapse with NSAID use in IBD patients.1,2 Due to this, patients often feel that their pain is inadequately addressed.3,4 There are multiple sources of abdominal pain in IBD, and understanding the mechanisms and presentations can help identify effective treatments. We will review pharmacologic and supportive therapies to optimize pain management in IBD.
Common pain presentations in IBD
Visceral pain is a dull, poorly localized, cramping pain from intestinal distension. It is associated with inflammation, dysmotility, obstruction, and visceral hypersensitivity. Somatic and parietal pain is sharp, intense, and often localizable. Somatic pain originates from surrounding skin or muscles, and parietal pain arises from irritation of the peritoneum.5 We will review two common pain presentations in IBD.
Case 1: Mr. A is a 32-year-old male with stricturing small bowel Crohn’s disease s/p small bowel resection, who presents to the ED with 3 days of abdominal pain, nausea, and vomiting. C-reactive protein is elevated to 6.8 mg/dL (normal 0.0 – 0.6 mg/dL), and CT is consistent with active small bowel inflammation, intraabdominal abscess at the anastomosis, and associated partial small bowel obstruction. He describes a sharp, intense abdominal pain with cramping. His exam is significant for diffuse abdominal tenderness and distension.
Case 2: Ms. B is a 28-year-old female with ulcerative colitis on mesalamine monotherapy who presents to the hospital for rectal bleeding and cramping abdominal pain. After 3 days of IV steroids her rectal bleeding has resolved, and CRP has normalized. However, she continues to have dull, cramping abdominal pain. Ibuprofen has improved this pain in the past.
Mr. A is having somatic pain from inflammation, abscess, and partial bowel obstruction. He also has visceral pain from luminal distension proximal to the obstruction. Ms. B is having visceral pain despite resolution of inflammation, which may be from postinflammatory visceral hypersensitivity.
Etiologies of pain
It’s best to group pain etiologies into inflammatory and noninflammatory causes. Inflammatory pain can be secondary to infection, such as abscess or enteric infection, active bowel inflammation, or disease complications (that is, enteric fistula). It is important to recognize that patients with active inflammation may also have noninflammatory pain. These include small bowel obstruction, strictures, adhesions, narcotic bowel syndrome, bacterial overgrowth, and visceral hypersensitivity. See figure 1.
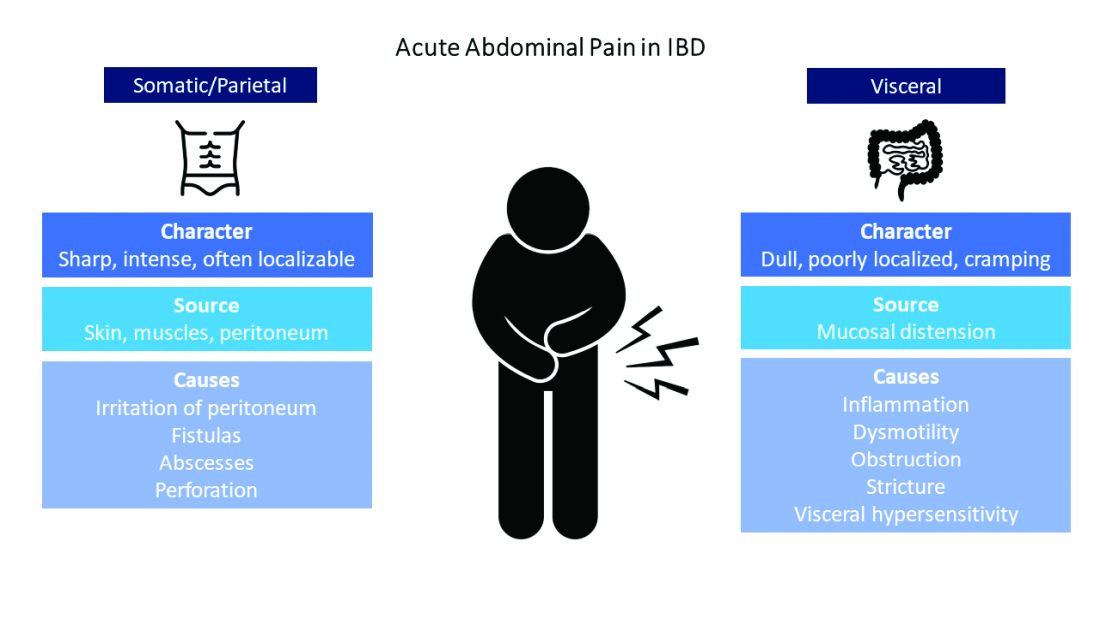
The brain-gut connection matters
Abdominal pain in IBD patients starts from painful stimuli in the gut. In addition to direct pain pathways, multiple areas of the brain modulate perception of pain.6 Patients with psychiatric comorbidities have increased perception of abdominal pain.7 In fact, high perceived stress is associated with disease relapse.8 Treatment of psychiatric disorders improves these symptoms with lasting effects.9 Addressing psychological and psychosocial needs is essential to successful pain management with long-term effect on quality of life and pain perception in IBD patients.
What are my options?
When IBD patients present with acute abdominal pain, it is important to directly address their pain as one of your primary concerns and provide them with a management plan. While this seems obvious, it is not routinely done.3-4
Next, it is important to identify the cause, whether it be infection, obstruction, active inflammation, or functional abdominal pain. In the case of active disease, in addition to steroids and optimization of IBD therapies, acetaminophen and antispasmodics can be used for initial pain management. Supportive therapies include sleep hygiene, physical activity, and psychotherapy. If initial treatments are unsuccessful in the acute setting, and presentation is consistent with somatic pain, it may be necessary to escalate to tramadol, opioid, or NSAID therapy. For visceral pain, a neuromodulator, such as a tricyclic antidepressant or gabapentin, may have greater effect. Bupropion, SNRIs, and SSRIs are options; however, they may not be effective in the acute setting. More recent focus in the IBD community has questioned the role of cannabinoids on pain in IBD patients. Cannabis has been shown in a few small studies to provide pain relief in IBD patients with active inflammation.10-11 In patients with mechanical causes for pain, management of obstruction is an important part of the treatment plan.
Let’s talk about opioids in IBD patients
Chronic narcotic use in IBD is associated with worse outcomes. So when is it okay to use opioid therapies in IBD patients? Postoperative patients, patients with severe perianal disease, or those who fail alternative pain management strategies may require opioid medications. The association with mortality and opioids in IBD is with patients who require moderate to heavy use, which is defined as being prescribed opioids more than once a year. Opioid use in IBD patients is also associated with increased risk of readmissions and poor surgical outcomes.12-13 Tramadol does not have increased mortality risk.1 If selecting opioid therapy in managing pain in IBD, it is important to define the course of therapy, with a clear goal of discontinuation after the acute episode. Opioids should be used in tandem with alternative strategies. Patients should be counseled on the synergistic effect of acetaminophen with opioids, which may allow lower effective doses of opioids.
What about NSAID use in IBD patients?
NSAIDs have negative effects in the gastrointestinal tract due to inhibition of protective prostaglandins. They also alter the gut microbiome, although clinical implications of this are unknown.14 A small study showed that IBD patients who used NSAIDs had increased risk of disease relapse.2 Symptoms of relapse would present within 2-9 days of exposure; however, most had resolution of symptoms within 2-11 days of discontinuation.2 Follow-up studies have not reliably found that NSAIDs are associated with disease relapse.8 and thus NSAIDs may be used sparingly if needed in the acute setting.
Case Review: How do we approach Mr. A and Ms. B?
Mr. A presented with a partial small bowel obstruction and abscess. His pain presentation was consistent with both visceral and somatic pain etiologies. In addition to treating active inflammation and infection, bowel rest, acetaminophen, and antispasmodics can be initiated for pain control. Concomitantly, gabapentin, TCA, or SNRI can be initiated for neurobiological pain but may have limited benefit in the acute hospitalized setting. Social work may identify needs that affect pain perception and assist in addressing those needs. If abdominal pain persists, tramadol or hydrocodone-acetaminophen can be considered.
Ms. B presented with disease relapse, but despite improving inflammatory markers she had continued cramping abdominal pain, which can be consistent with visceral hypersensitivity. Antispasmodic and neuromodulating agents, such as a TCA, could be effective. We can recommend discontinuation of chronic ibuprofen due to risk of intestinal inflammation. Patients may inquire about adjuvant cannabis in pain management. While cannabis can be considered, further research is needed to recommend its regular use.
Conclusion
Acute abdominal pain management in IBD can be challenging for providers when typical options are limited in this population. Addressing inflammatory, mechanical, neurobiological, and psychological influences is vital to appropriately address pain. Having a structured plan for pain management in IBD can improve outcomes by decreasing recurrent hospitalizations and use of opioids.15 Figure 2 presents an overview.
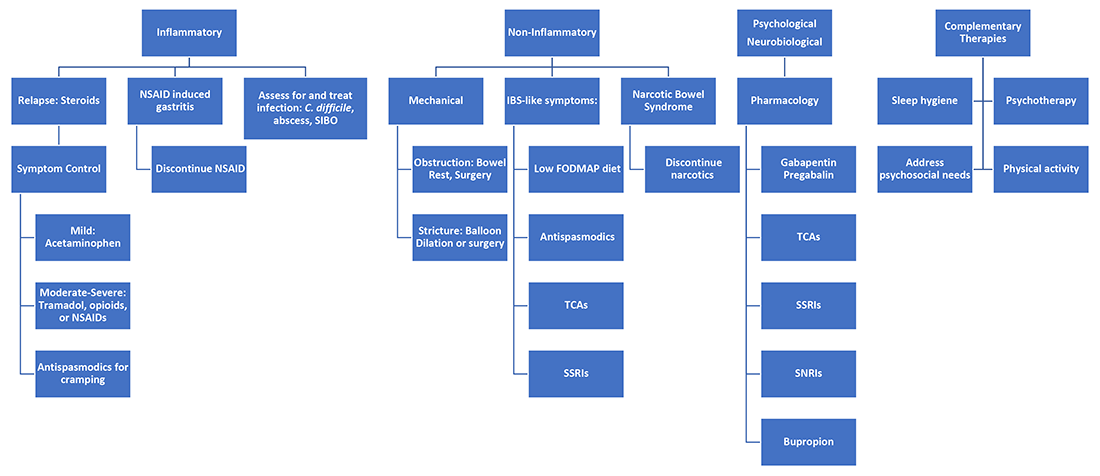
Dr. Ahmed is a second-year internal medicine resident at the University of Michigan, Ann Arbor. Dr. Kinnucan is with the department of internal medicine and the division of gastroenterology and hepatology and is an assistant professor of medicine in the division of gastroenterology, both at the University of Michigan. They have no conflicts of interest.
References
1. Burr NE et al. Clin Gastroenterol Hepatol. 2018 Apr;16(4):534-41.e6.
2. Takeuchi K et al. Clin Gastroenterol Hepatol. 2006 Feb;4(2):196-202.
3. Bernhofer EI et al. Gastroenterol Nurs. 2017 May/Jun;40(3):200-7.
4. Zeitz J et al. PLoS One. 2016 Jun 22;11(6):e0156666.
5. Srinath A et al. Inflamm Bowel Dis. 2014 Dec;20(12):2433-49.
6. Docherty MJ et al. Gastroenterol Hepatol (N Y). 2011 Sep;7(9):592-601.
7. Elsenbruch S et al. Gut. 2010 Apr;59(4):489-95.
8. Bernstein CN et al. Am J Gastroenterol. 2010 Sep;105(9):1994-2002.
9. Palsson OS and Whitehead WE. Clin Gastroenterol Hepatol. 2013 Mar;11(3):208-16; quiz e22-3.
10. Swaminath A et al. Inflamm Bowel Dis. 2019 Mar; 25(3):427-35.
11. Naftali T et al. Clin Gastroenterol Hepatol. 2013 Oct;11(10):1276-80.e1.
12. Sultan K and Swaminath A. J Crohns Colitis. 2020 Sep 16;14(9):1188-89.
13. Hirsch A et al. J Gastrointest Surg. 2015 Oct;19(10):1852-61.
14. Rogers MAM and Aronoff DM. Clin Microbiol Infect. 2016;22(2):178.e1-178.e9.
15. Kaimakliotis P et al. Int J Colorectal Dis. 2021 Jun;36(6):1193-200.
In the acute care setting, providers of care for inflammatory bowel disease (IBD) patients are often faced with the dilemma of providing effective abdominal pain management in a population that has worse outcomes with both opioid and NSAID therapy. There is increased mortality associated with opioid use and risk of disease relapse with NSAID use in IBD patients.1,2 Due to this, patients often feel that their pain is inadequately addressed.3,4 There are multiple sources of abdominal pain in IBD, and understanding the mechanisms and presentations can help identify effective treatments. We will review pharmacologic and supportive therapies to optimize pain management in IBD.
Common pain presentations in IBD
Visceral pain is a dull, poorly localized, cramping pain from intestinal distension. It is associated with inflammation, dysmotility, obstruction, and visceral hypersensitivity. Somatic and parietal pain is sharp, intense, and often localizable. Somatic pain originates from surrounding skin or muscles, and parietal pain arises from irritation of the peritoneum.5 We will review two common pain presentations in IBD.
Case 1: Mr. A is a 32-year-old male with stricturing small bowel Crohn’s disease s/p small bowel resection, who presents to the ED with 3 days of abdominal pain, nausea, and vomiting. C-reactive protein is elevated to 6.8 mg/dL (normal 0.0 – 0.6 mg/dL), and CT is consistent with active small bowel inflammation, intraabdominal abscess at the anastomosis, and associated partial small bowel obstruction. He describes a sharp, intense abdominal pain with cramping. His exam is significant for diffuse abdominal tenderness and distension.
Case 2: Ms. B is a 28-year-old female with ulcerative colitis on mesalamine monotherapy who presents to the hospital for rectal bleeding and cramping abdominal pain. After 3 days of IV steroids her rectal bleeding has resolved, and CRP has normalized. However, she continues to have dull, cramping abdominal pain. Ibuprofen has improved this pain in the past.
Mr. A is having somatic pain from inflammation, abscess, and partial bowel obstruction. He also has visceral pain from luminal distension proximal to the obstruction. Ms. B is having visceral pain despite resolution of inflammation, which may be from postinflammatory visceral hypersensitivity.
Etiologies of pain
It’s best to group pain etiologies into inflammatory and noninflammatory causes. Inflammatory pain can be secondary to infection, such as abscess or enteric infection, active bowel inflammation, or disease complications (that is, enteric fistula). It is important to recognize that patients with active inflammation may also have noninflammatory pain. These include small bowel obstruction, strictures, adhesions, narcotic bowel syndrome, bacterial overgrowth, and visceral hypersensitivity. See figure 1.

The brain-gut connection matters
Abdominal pain in IBD patients starts from painful stimuli in the gut. In addition to direct pain pathways, multiple areas of the brain modulate perception of pain.6 Patients with psychiatric comorbidities have increased perception of abdominal pain.7 In fact, high perceived stress is associated with disease relapse.8 Treatment of psychiatric disorders improves these symptoms with lasting effects.9 Addressing psychological and psychosocial needs is essential to successful pain management with long-term effect on quality of life and pain perception in IBD patients.
What are my options?
When IBD patients present with acute abdominal pain, it is important to directly address their pain as one of your primary concerns and provide them with a management plan. While this seems obvious, it is not routinely done.3-4
Next, it is important to identify the cause, whether it be infection, obstruction, active inflammation, or functional abdominal pain. In the case of active disease, in addition to steroids and optimization of IBD therapies, acetaminophen and antispasmodics can be used for initial pain management. Supportive therapies include sleep hygiene, physical activity, and psychotherapy. If initial treatments are unsuccessful in the acute setting, and presentation is consistent with somatic pain, it may be necessary to escalate to tramadol, opioid, or NSAID therapy. For visceral pain, a neuromodulator, such as a tricyclic antidepressant or gabapentin, may have greater effect. Bupropion, SNRIs, and SSRIs are options; however, they may not be effective in the acute setting. More recent focus in the IBD community has questioned the role of cannabinoids on pain in IBD patients. Cannabis has been shown in a few small studies to provide pain relief in IBD patients with active inflammation.10-11 In patients with mechanical causes for pain, management of obstruction is an important part of the treatment plan.
Let’s talk about opioids in IBD patients
Chronic narcotic use in IBD is associated with worse outcomes. So when is it okay to use opioid therapies in IBD patients? Postoperative patients, patients with severe perianal disease, or those who fail alternative pain management strategies may require opioid medications. The association with mortality and opioids in IBD is with patients who require moderate to heavy use, which is defined as being prescribed opioids more than once a year. Opioid use in IBD patients is also associated with increased risk of readmissions and poor surgical outcomes.12-13 Tramadol does not have increased mortality risk.1 If selecting opioid therapy in managing pain in IBD, it is important to define the course of therapy, with a clear goal of discontinuation after the acute episode. Opioids should be used in tandem with alternative strategies. Patients should be counseled on the synergistic effect of acetaminophen with opioids, which may allow lower effective doses of opioids.
What about NSAID use in IBD patients?
NSAIDs have negative effects in the gastrointestinal tract due to inhibition of protective prostaglandins. They also alter the gut microbiome, although clinical implications of this are unknown.14 A small study showed that IBD patients who used NSAIDs had increased risk of disease relapse.2 Symptoms of relapse would present within 2-9 days of exposure; however, most had resolution of symptoms within 2-11 days of discontinuation.2 Follow-up studies have not reliably found that NSAIDs are associated with disease relapse.8 and thus NSAIDs may be used sparingly if needed in the acute setting.
Case Review: How do we approach Mr. A and Ms. B?
Mr. A presented with a partial small bowel obstruction and abscess. His pain presentation was consistent with both visceral and somatic pain etiologies. In addition to treating active inflammation and infection, bowel rest, acetaminophen, and antispasmodics can be initiated for pain control. Concomitantly, gabapentin, TCA, or SNRI can be initiated for neurobiological pain but may have limited benefit in the acute hospitalized setting. Social work may identify needs that affect pain perception and assist in addressing those needs. If abdominal pain persists, tramadol or hydrocodone-acetaminophen can be considered.
Ms. B presented with disease relapse, but despite improving inflammatory markers she had continued cramping abdominal pain, which can be consistent with visceral hypersensitivity. Antispasmodic and neuromodulating agents, such as a TCA, could be effective. We can recommend discontinuation of chronic ibuprofen due to risk of intestinal inflammation. Patients may inquire about adjuvant cannabis in pain management. While cannabis can be considered, further research is needed to recommend its regular use.
Conclusion
Acute abdominal pain management in IBD can be challenging for providers when typical options are limited in this population. Addressing inflammatory, mechanical, neurobiological, and psychological influences is vital to appropriately address pain. Having a structured plan for pain management in IBD can improve outcomes by decreasing recurrent hospitalizations and use of opioids.15 Figure 2 presents an overview.

Dr. Ahmed is a second-year internal medicine resident at the University of Michigan, Ann Arbor. Dr. Kinnucan is with the department of internal medicine and the division of gastroenterology and hepatology and is an assistant professor of medicine in the division of gastroenterology, both at the University of Michigan. They have no conflicts of interest.
References
1. Burr NE et al. Clin Gastroenterol Hepatol. 2018 Apr;16(4):534-41.e6.
2. Takeuchi K et al. Clin Gastroenterol Hepatol. 2006 Feb;4(2):196-202.
3. Bernhofer EI et al. Gastroenterol Nurs. 2017 May/Jun;40(3):200-7.
4. Zeitz J et al. PLoS One. 2016 Jun 22;11(6):e0156666.
5. Srinath A et al. Inflamm Bowel Dis. 2014 Dec;20(12):2433-49.
6. Docherty MJ et al. Gastroenterol Hepatol (N Y). 2011 Sep;7(9):592-601.
7. Elsenbruch S et al. Gut. 2010 Apr;59(4):489-95.
8. Bernstein CN et al. Am J Gastroenterol. 2010 Sep;105(9):1994-2002.
9. Palsson OS and Whitehead WE. Clin Gastroenterol Hepatol. 2013 Mar;11(3):208-16; quiz e22-3.
10. Swaminath A et al. Inflamm Bowel Dis. 2019 Mar; 25(3):427-35.
11. Naftali T et al. Clin Gastroenterol Hepatol. 2013 Oct;11(10):1276-80.e1.
12. Sultan K and Swaminath A. J Crohns Colitis. 2020 Sep 16;14(9):1188-89.
13. Hirsch A et al. J Gastrointest Surg. 2015 Oct;19(10):1852-61.
14. Rogers MAM and Aronoff DM. Clin Microbiol Infect. 2016;22(2):178.e1-178.e9.
15. Kaimakliotis P et al. Int J Colorectal Dis. 2021 Jun;36(6):1193-200.
In the acute care setting, providers of care for inflammatory bowel disease (IBD) patients are often faced with the dilemma of providing effective abdominal pain management in a population that has worse outcomes with both opioid and NSAID therapy. There is increased mortality associated with opioid use and risk of disease relapse with NSAID use in IBD patients.1,2 Due to this, patients often feel that their pain is inadequately addressed.3,4 There are multiple sources of abdominal pain in IBD, and understanding the mechanisms and presentations can help identify effective treatments. We will review pharmacologic and supportive therapies to optimize pain management in IBD.
Common pain presentations in IBD
Visceral pain is a dull, poorly localized, cramping pain from intestinal distension. It is associated with inflammation, dysmotility, obstruction, and visceral hypersensitivity. Somatic and parietal pain is sharp, intense, and often localizable. Somatic pain originates from surrounding skin or muscles, and parietal pain arises from irritation of the peritoneum.5 We will review two common pain presentations in IBD.
Case 1: Mr. A is a 32-year-old male with stricturing small bowel Crohn’s disease s/p small bowel resection, who presents to the ED with 3 days of abdominal pain, nausea, and vomiting. C-reactive protein is elevated to 6.8 mg/dL (normal 0.0 – 0.6 mg/dL), and CT is consistent with active small bowel inflammation, intraabdominal abscess at the anastomosis, and associated partial small bowel obstruction. He describes a sharp, intense abdominal pain with cramping. His exam is significant for diffuse abdominal tenderness and distension.
Case 2: Ms. B is a 28-year-old female with ulcerative colitis on mesalamine monotherapy who presents to the hospital for rectal bleeding and cramping abdominal pain. After 3 days of IV steroids her rectal bleeding has resolved, and CRP has normalized. However, she continues to have dull, cramping abdominal pain. Ibuprofen has improved this pain in the past.
Mr. A is having somatic pain from inflammation, abscess, and partial bowel obstruction. He also has visceral pain from luminal distension proximal to the obstruction. Ms. B is having visceral pain despite resolution of inflammation, which may be from postinflammatory visceral hypersensitivity.
Etiologies of pain
It’s best to group pain etiologies into inflammatory and noninflammatory causes. Inflammatory pain can be secondary to infection, such as abscess or enteric infection, active bowel inflammation, or disease complications (that is, enteric fistula). It is important to recognize that patients with active inflammation may also have noninflammatory pain. These include small bowel obstruction, strictures, adhesions, narcotic bowel syndrome, bacterial overgrowth, and visceral hypersensitivity. See figure 1.

The brain-gut connection matters
Abdominal pain in IBD patients starts from painful stimuli in the gut. In addition to direct pain pathways, multiple areas of the brain modulate perception of pain.6 Patients with psychiatric comorbidities have increased perception of abdominal pain.7 In fact, high perceived stress is associated with disease relapse.8 Treatment of psychiatric disorders improves these symptoms with lasting effects.9 Addressing psychological and psychosocial needs is essential to successful pain management with long-term effect on quality of life and pain perception in IBD patients.
What are my options?
When IBD patients present with acute abdominal pain, it is important to directly address their pain as one of your primary concerns and provide them with a management plan. While this seems obvious, it is not routinely done.3-4
Next, it is important to identify the cause, whether it be infection, obstruction, active inflammation, or functional abdominal pain. In the case of active disease, in addition to steroids and optimization of IBD therapies, acetaminophen and antispasmodics can be used for initial pain management. Supportive therapies include sleep hygiene, physical activity, and psychotherapy. If initial treatments are unsuccessful in the acute setting, and presentation is consistent with somatic pain, it may be necessary to escalate to tramadol, opioid, or NSAID therapy. For visceral pain, a neuromodulator, such as a tricyclic antidepressant or gabapentin, may have greater effect. Bupropion, SNRIs, and SSRIs are options; however, they may not be effective in the acute setting. More recent focus in the IBD community has questioned the role of cannabinoids on pain in IBD patients. Cannabis has been shown in a few small studies to provide pain relief in IBD patients with active inflammation.10-11 In patients with mechanical causes for pain, management of obstruction is an important part of the treatment plan.
Let’s talk about opioids in IBD patients
Chronic narcotic use in IBD is associated with worse outcomes. So when is it okay to use opioid therapies in IBD patients? Postoperative patients, patients with severe perianal disease, or those who fail alternative pain management strategies may require opioid medications. The association with mortality and opioids in IBD is with patients who require moderate to heavy use, which is defined as being prescribed opioids more than once a year. Opioid use in IBD patients is also associated with increased risk of readmissions and poor surgical outcomes.12-13 Tramadol does not have increased mortality risk.1 If selecting opioid therapy in managing pain in IBD, it is important to define the course of therapy, with a clear goal of discontinuation after the acute episode. Opioids should be used in tandem with alternative strategies. Patients should be counseled on the synergistic effect of acetaminophen with opioids, which may allow lower effective doses of opioids.
What about NSAID use in IBD patients?
NSAIDs have negative effects in the gastrointestinal tract due to inhibition of protective prostaglandins. They also alter the gut microbiome, although clinical implications of this are unknown.14 A small study showed that IBD patients who used NSAIDs had increased risk of disease relapse.2 Symptoms of relapse would present within 2-9 days of exposure; however, most had resolution of symptoms within 2-11 days of discontinuation.2 Follow-up studies have not reliably found that NSAIDs are associated with disease relapse.8 and thus NSAIDs may be used sparingly if needed in the acute setting.
Case Review: How do we approach Mr. A and Ms. B?
Mr. A presented with a partial small bowel obstruction and abscess. His pain presentation was consistent with both visceral and somatic pain etiologies. In addition to treating active inflammation and infection, bowel rest, acetaminophen, and antispasmodics can be initiated for pain control. Concomitantly, gabapentin, TCA, or SNRI can be initiated for neurobiological pain but may have limited benefit in the acute hospitalized setting. Social work may identify needs that affect pain perception and assist in addressing those needs. If abdominal pain persists, tramadol or hydrocodone-acetaminophen can be considered.
Ms. B presented with disease relapse, but despite improving inflammatory markers she had continued cramping abdominal pain, which can be consistent with visceral hypersensitivity. Antispasmodic and neuromodulating agents, such as a TCA, could be effective. We can recommend discontinuation of chronic ibuprofen due to risk of intestinal inflammation. Patients may inquire about adjuvant cannabis in pain management. While cannabis can be considered, further research is needed to recommend its regular use.
Conclusion
Acute abdominal pain management in IBD can be challenging for providers when typical options are limited in this population. Addressing inflammatory, mechanical, neurobiological, and psychological influences is vital to appropriately address pain. Having a structured plan for pain management in IBD can improve outcomes by decreasing recurrent hospitalizations and use of opioids.15 Figure 2 presents an overview.

Dr. Ahmed is a second-year internal medicine resident at the University of Michigan, Ann Arbor. Dr. Kinnucan is with the department of internal medicine and the division of gastroenterology and hepatology and is an assistant professor of medicine in the division of gastroenterology, both at the University of Michigan. They have no conflicts of interest.
References
1. Burr NE et al. Clin Gastroenterol Hepatol. 2018 Apr;16(4):534-41.e6.
2. Takeuchi K et al. Clin Gastroenterol Hepatol. 2006 Feb;4(2):196-202.
3. Bernhofer EI et al. Gastroenterol Nurs. 2017 May/Jun;40(3):200-7.
4. Zeitz J et al. PLoS One. 2016 Jun 22;11(6):e0156666.
5. Srinath A et al. Inflamm Bowel Dis. 2014 Dec;20(12):2433-49.
6. Docherty MJ et al. Gastroenterol Hepatol (N Y). 2011 Sep;7(9):592-601.
7. Elsenbruch S et al. Gut. 2010 Apr;59(4):489-95.
8. Bernstein CN et al. Am J Gastroenterol. 2010 Sep;105(9):1994-2002.
9. Palsson OS and Whitehead WE. Clin Gastroenterol Hepatol. 2013 Mar;11(3):208-16; quiz e22-3.
10. Swaminath A et al. Inflamm Bowel Dis. 2019 Mar; 25(3):427-35.
11. Naftali T et al. Clin Gastroenterol Hepatol. 2013 Oct;11(10):1276-80.e1.
12. Sultan K and Swaminath A. J Crohns Colitis. 2020 Sep 16;14(9):1188-89.
13. Hirsch A et al. J Gastrointest Surg. 2015 Oct;19(10):1852-61.
14. Rogers MAM and Aronoff DM. Clin Microbiol Infect. 2016;22(2):178.e1-178.e9.
15. Kaimakliotis P et al. Int J Colorectal Dis. 2021 Jun;36(6):1193-200.
How a community-based program for SMI pivoted during the pandemic
For more than 70 years, Fountain House has offered a lifeline for people living with schizophrenia, bipolar disorder, major depression, and other serious mental illnesses through a community-based model of care. When he took the helm less than 2 years ago, CEO and President Ashwin Vasan, ScM, MD, PhD, wanted a greater focus on crisis-based solutions and a wider, public health approach.

That goal was put to the test in 2020, when SARS-CoV-2 shuttered all in-person activities. The nonprofit quickly rebounded, creating a digital platform, engaging with its members through online courses, face-to-face check-ins, and delivery services, and expanding partnerships to connect with individuals facing homelessness and involved in the criminal justice system. Those activities not only brought the community together – it expanded Fountain House’s footprint.
Among its membership of more than 2,000 people in New York City, about 70% connected to the digital platform. “We also enrolled more than 200 brand new members during the pandemic who had never set foot in the physical mental health “clubhouse.” They derived value as well,” Dr. Vasan said in an interview. Nationally, the program is replicated at more than 200 locations and serves about 60,000 people in almost 40 states. During the pandemic, Fountain House began formalizing affiliation opportunities with this network.
Now that the pandemic is showing signs of receding, Fountain House faces new challenges operating as a possible hybrid model. “More than three-quarters of our members say they want to continue to engage virtually as well as in person,” Dr. Vasan said. As of this writing, Fountain House is enjoying a soft reopening, slowly welcoming in-person activities. What this will look like in the coming weeks and months is a work in progress, he added. “We don’t know yet how people are going to prefer to engage.”
A role in the public policy conversation
Founded by a small group of former psychiatric patients in the late 1940s, Fountain House has since expanded from a single building in New York City to more than 300 replications in the United States and around the world. It originated the “clubhouse” model of mental health support: a community-based approach that helps members improve health, and break social and economic isolation by reclaiming social, educational, and work skills, and connecting with core services, including supportive housing and community-based primary and behavioral health care (Arts Psychother. 2012 Feb 39[1]:25-30).
Serious mental illness (SMI) is growing more pronounced as a crisis, not just in the people it affects, “but in all of the attendant and preventable social and economic crises that intersect with it, whether it’s increasing health care costs, homelessness, or criminalization,” Dr. Vasan said.
After 73 years, Fountain House is just beginning to gain relevance as a tool to help solve these intersecting public policy crises, he added.
“We’ve demonstrated through evaluation data that it reduces hospitalization rates, health care costs, reliance on emergency departments, homelessness, and recidivism to the criminal justice system,” he said. Health care costs for members are more than 20% lower than for others with mental illness, and recidivism rates among those with a criminal history are less than 5%.
Others familiar with Fountain House say the model delivers on its charge to improve quality of life for people with SMI.
It’s a great referral source for people who are under good mental health control, whether it’s therapy or a combination of therapy and medications, Robert T. London, MD, a practicing psychiatrist in New York who is not affiliated with Fountain House but has referred patients to the organization over the years, said in an interview.
“They can work with staff, learn skills regarding potential work, housekeeping, [and] social skills,” he said. One of the biggest problems facing people with SMI is they’re very isolated, Dr. London continued. “When you’re in a facility like Fountain House, you’re not isolated. You’re with fellow members, a very helpful educated staff, and you’re going to do well.” If a member is having some issues and losing touch with reality and needs to find treatment, Fountain House will provide that support.
“If you don’t have a treating person, they’re going to find you one. They’re not against traditional medical/psychiatric care,” he said.
Among those with unstable or no housing, 99% find housing within a year of joining Fountain House. While it does provide people with SMI with support to find a roof over their heads, Fountain House doesn’t necessarily fit a model of “housing first,” Stephanie Le Melle, MD, MS, director of public psychiatry education at department of psychiatry at Columbia University/New York State Psychiatric Institute, said in an interview.
“The housing first evidence-based model, as designed and implemented by Pathways to Housing program in New York in the early 90s, accepted people who were street homeless or in shelters, not involved in mental health treatment, and actively using substances into scatter-site apartments and wrapped services around them,” she said.
Dr. Le Melle, who is not affiliated with Fountain House, views it more as a supportive employment program that uses a recovery-oriented, community-based, jointly peer-run approach to engage members in vocational/educational programming. It also happens to have some supportive housing for its members, she added.
Dr. Vasan believes Fountain House could expand beyond a community model. The organization has been moving out from its history, evolving into a model that could be integrated as standard of care and standard of practice for community health, he said. Fountain House is part of Clubhouse International, an umbrella organization that received the American Psychiatric Association’s 2021 special presidential commendation award during its virtual annual meeting for the group’s use of “the evidence-based, cost-effective clubhouse model of psychosocial rehabilitation as a leading recovery resource for people living with mental illness around the world.”
How medication issues are handled
Fountain House doesn’t directly provide medication to its members. According to Dr. Vasan, psychiatric care is just one component of recovery for serious mental illness.
“We talk about Fountain House as a main vortex in a triangle of recovery. You need health care, housing, and community. The part that’s been neglected the most is community intervention, the social infrastructure for people who are deeply isolated and marginalized,” he said. “We know that people who have that infrastructure, and are stably housed, are then more likely to engage in community-based psychiatry and primary care. And in turn, people who are in stable clinical care can more durably engage in the community programming Fountain House offers.”
Health care and clinical care are changing. It’s becoming more person-centered and community based. “We need to move with the times and we have, in the last 2 decades,” he said.
Historically, Fountain House has owned and operated its own clinic in New York City. More recently, it partnered with Sun River Health and Ryan Health, two large federally qualified health center networks in New York, so that members receive access to psychiatric and medical care. It has also expanded similar partnerships with Columbia University, New York University, and other health care systems to ensure its members have access to sustainable clinical care as a part of the community conditions and resources needed to recover and thrive.
Those familiar with the organization don’t see the absence of a medication program as a negative factor. If Fountain House doesn’t provide psychiatric medications, “that tells me the patients are under control and able to function in a community setting” that focuses on rehabilitation, Dr. London said.
It’s true that psychiatric medication treatment is an essential part of a patient’s recovery journey, Dr. Le Melle said. “Treatment with medications can be done in a recovery-oriented way. However, the Fountain House model has been designed to keep these separate, and this model works well for most” of the members.
As long as members and staff are willing to collaborate with treatment providers outside of the clubhouse, when necessary, this model of separation between work and treatment can work really well, she added.
“There are some people who need a more integrated system of care. There is no ‘one size fits all’ program that can meet everyone’s needs,” said Dr. Le Melle. The absence of onsite treatment at Fountain House, to some extent “adds to the milieu and allows people to focus on other aspects of their lives besides their illness.”
This hasn’t always been the case in traditionally funded behavioral health programs, she continued. Most mental health clinics, because of fiscal structures, reimbursement, and staffing costs, focus more on psychotherapy and medication management than on other aspects of peoples’ lives, such as their recovery goals.
The bottom line is rehabilitation in medicine works – whether it’s for a mental health disorder, broken leg, arm, or stroke, Dr. London said. “Fountain House’s focus is integrating a person into society by helping them to think differently and interact socially in groups and learn some skills.”
Through cognitive-behavioral therapies, a person with mental illness can learn how to act differently. “The brain is always in a growing process where you learn and develop new ideas, make connections,” Dr. London said. “New protein molecules get created and stored; changes occur with the neurotransmitters.”
Overall, the Fountain House model is great for supporting and engaging people with serious mental illness, Dr. Le Melle said. “It provides a literal place, a community, and a safe environment that helps people to embrace their recovery journey. It is also great at supporting people in their engagement with vocational training and employment.”
Ideally, she would like Fountain House to grow and become more inclusive by engaging people who live with both mental illness and substance use.
COVID-19 changes the rules
The most difficult challenge for health care and other institutions is to keep individuals with SMI engaged and visible so that they can find access to health care and benefits – and avoid acute hospitalization or medical care. “That’s our goal, to prevent the worst effects and respond accordingly,” Dr. Vasan said.
SARS-CoV-2 forced the program to reevaluate its daily operations so that it could maintain crucial connections with its members.
Dr. Vasan and his staff immediately closed the clubhouse when COVID-19 first hit, transitioning to direct community-based services that provided one-on-one outreach, and meal, medication, and clothing delivery. “Even if people couldn’t visit our clubhouse, we wanted them to feel that sense of community connection, even if it was to drop off meals at their doorstep,” he said.
Donning personal protective equipment, his staff and interested program participants went out into the communities to do this personal outreach. At the height of pandemic in New York City, “we weren’t sure what to do,” as far as keeping safe, he admitted. Nevertheless, he believes this outreach work was lifesaving in that it kept people connected to the clubhouse.
As Fountain House worked to maintain in-person contact, it also built a digital community to keep the live community together. This wasn’t just about posting on a Facebook page – it was interactive, Dr. Vasan noted. An online group made masks for the community and sold them for people outside of Fountain House. Capacity building courses instructed members on writing resumes, looking for jobs, or filling out applications.
There’s an assumption that people with SMI lack the skills to navigate technology. Some of the hallmarks of SMI are demotivation and lack of confidence, and logging onto platforms and email can be challenging for some people, he acknowledged. Over the last 18 months, Fountain House’s virtual clubhouse proved this theory wrong, Dr. Vasan said. “There are a great number of people with serious mental illness who have basic digital skills and are already using technology, or are very eager to learn,” he said.
For the subset of members who did get discouraged by the virtual platform, Fountain House responded by giving them one-on-one home support and digital literacy training to help them stay motivated and engaged.
Fountain House also expanded partnerships during the pandemic, working with programs such as the Fortune Society to bring people with SMI from the criminal justice system into Fountain House. “We’re doing this either virtually or through outdoor, public park programs with groups such as the Times Square Alliance and Fort Greene Park Conservancy to ensure we’re meeting people where they are, at a time of a rising health crisis,” Dr. Vasan said.
Moving on to a hybrid model
At the height of the pandemic, it was easy to engage members through creative programming. People were craving socialization. Now that people are getting vaccinated and interacting inside and outside, some understandable apathy is forming toward digital platforms, Dr. Vasan said.
“The onus is on us now to look at that data and to design something new that can keep people engaged in a hybrid model,” he added.
June 14, 2021, marked Fountain House’s soft opening. “This was a big day for us, to work through the kinks,” he said. At press time, the plan was to fully reopen the clubhouse in a few weeks – if transmission and case rates stay low.
It’s unclear at this point how many people will engage with Fountain House on a daily, in-person basis. Some people might want to come to the clubhouse just a few days a week and use the online platform on other days.
“We’re doing a series of experiments to really understand what different offerings we need to make. For example, perhaps we need to have 24-7 programming on the digital platform. That way, you could access it on demand,” said Dr. Vasan. The goal is to create a menu of choices for members so that it becomes flexible and meets their needs.
Long term, Dr. Vasan hopes the digital platform will become a scalable technology. “We want this to be used not just by Fountain House, but for programs and in markets that don’t have clubhouses.” Health systems or insurance companies would benefit from software like this because it addresses one of the most difficult aspects for this population: keeping them engaged and visible to their systems, Dr. Vasan added.
“I think the most important lesson here is we’re designing for a group of people that no one designs for. No one’s paying attention to people with serious mental illness. Nor have they ever, really. Fountain House has always been their advocate and partner. It’s great that we can do this with them, and for them.”
Dr. Vasan, also an epidemiologist, serves as assistant professor of clinical population, and family health and medicine, at Columbia University. Dr. London and Dr. Le Melle have no conflicts of interest.
Two steps back, three steps forward
For some of its members, Fountain House provides more than just a sense of place. In an interview, longtime member and New York City native Rich Courage, 61, discussed his mental illness challenges and the role the organization played in reclaiming his life, leading to a new career as a counselor.
Question: What made you seek out Fountain House? Are you still a member?
Rich Courage: I’ve been a member since 2001. I was in a day program at Postgraduate, on West 36th Street. They had this huge theater program, and I was a part of that. But the program fell apart and I didn’t know what to do with myself. A friend of mine told me about Fountain House. I asked what it did, and the friend said that it puts people with mental health challenges back to life, to work, to school. I was making some art, some collages, and I heard they had an art gallery.
Seeing Fountain House, I was amazed. It was this very friendly, warm, cozy place. The staff was nice; the members were welcoming. The next thing you know it’s 2021, and here I am, a peer counselor at Fountain House. I work on “the warm line,” doing the evening shift. People call in who have crises, but a lot of them call in because they’re lonely and want someone to talk to. As a peer counselor, I don’t tell people what to do, but I do offer support. I encourage. I ask questions that enable them to figure out their own problems. And I tell stories anecdotally of people that I’ve known and about recovery.
I struggled with bad depression when I was in my 20s. My mother died, and I lost everything. Coming to Fountain House and being part of this community is unlike anything I’d ever experienced. People weren’t just sitting around and talking about their problems; they were doing something about it. They were going back to school, to work, to social engagements, and the world at large. And it wasn’t perfect or linear. It was two steps back, three steps forward.
That’s exactly what I was doing. I had a lot of self-esteem and confidence issues, and behavioral stuff. My mind was wired a certain way. I had hospitalizations; I was in psychiatric wards. I had a suicide attempt in 2006, which was nearly successful. I was feeling social, mental, and emotional pain for so long. The community has been invaluable for me. Hearing other people’s stories, being accepted, has been wonderful.
I’ve been down and now I’m up, on an upward trajectory.
Question: How else has Fountain House made a difference in your life?
RC: I’m in a Fountain House residence in a one-bedroom, and it’s the most stable housing I’ve ever had in 61 years. So I’ve gotten housing and I’ve gotten a job, which is all great, because it’s aided me in becoming a full human being. But it’s really eased my suffering and enabled me to feel some joy and have some life instead of this shadow existence that I had been living for 30 years.
Fountain House has different units, and I’ve been in the communications unit – we put out the weekly paper and handle all the mail. The unit has computers, and I was able to work on my writing. I wrote a play called "The Very Last Dance of Homeless Joe." We’ve had staged readings at Fountain House, and 200 people have seen it over 2 years. We Zoomed it through the virtual community. It was very successful. A recording of the staged reading won third place at a festival in Florida.
In September, it will be an off-Broadway show. It’s a play about the homeless, but it’s not depressing; it’s very uplifting.
Question: Did you stay connected to Fountain House during the pandemic, either through the digital community or through services they provided? What was this experience like for you?
RC: Ashwin [Vasan] had been here 6 months, and he saw the pandemic coming. During a programming meeting he said, “We need a virtual community, and we need it now.” None of us knew what Zoom was, how the mute button worked. But it’s been wonderful for me. I’m a performer, so I was able to get on to Facebook every day and post a song. Some of it was spoofs about COVID; some were dedications to members. I ended up connecting with a member in Minnesota who used to be a neighbor of mine. We had lost contact, and we reconnected through Fountain House.
Question: What would you tell someone who might need this service?
RC: We’ve partially reopened the clubhouse. In July we’ll be doing tours again. I’d say, come take a tour and see the different social, economic, housing, and educational opportunities. We have a home and garden unit that decorates the place. We have a gym, a wellness unit. But these are just things. The real heart is the people.
As a unit leader recently told me, “We’re not a clinic. We’re not a revolving door. We forge relationships with members that last in our hearts and minds for a lifetime. Even if it’s not in my job description, if there’s anything in my power that I could do to help a member ease their suffering, I will do it.”
For more than 70 years, Fountain House has offered a lifeline for people living with schizophrenia, bipolar disorder, major depression, and other serious mental illnesses through a community-based model of care. When he took the helm less than 2 years ago, CEO and President Ashwin Vasan, ScM, MD, PhD, wanted a greater focus on crisis-based solutions and a wider, public health approach.

That goal was put to the test in 2020, when SARS-CoV-2 shuttered all in-person activities. The nonprofit quickly rebounded, creating a digital platform, engaging with its members through online courses, face-to-face check-ins, and delivery services, and expanding partnerships to connect with individuals facing homelessness and involved in the criminal justice system. Those activities not only brought the community together – it expanded Fountain House’s footprint.
Among its membership of more than 2,000 people in New York City, about 70% connected to the digital platform. “We also enrolled more than 200 brand new members during the pandemic who had never set foot in the physical mental health “clubhouse.” They derived value as well,” Dr. Vasan said in an interview. Nationally, the program is replicated at more than 200 locations and serves about 60,000 people in almost 40 states. During the pandemic, Fountain House began formalizing affiliation opportunities with this network.
Now that the pandemic is showing signs of receding, Fountain House faces new challenges operating as a possible hybrid model. “More than three-quarters of our members say they want to continue to engage virtually as well as in person,” Dr. Vasan said. As of this writing, Fountain House is enjoying a soft reopening, slowly welcoming in-person activities. What this will look like in the coming weeks and months is a work in progress, he added. “We don’t know yet how people are going to prefer to engage.”
A role in the public policy conversation
Founded by a small group of former psychiatric patients in the late 1940s, Fountain House has since expanded from a single building in New York City to more than 300 replications in the United States and around the world. It originated the “clubhouse” model of mental health support: a community-based approach that helps members improve health, and break social and economic isolation by reclaiming social, educational, and work skills, and connecting with core services, including supportive housing and community-based primary and behavioral health care (Arts Psychother. 2012 Feb 39[1]:25-30).
Serious mental illness (SMI) is growing more pronounced as a crisis, not just in the people it affects, “but in all of the attendant and preventable social and economic crises that intersect with it, whether it’s increasing health care costs, homelessness, or criminalization,” Dr. Vasan said.
After 73 years, Fountain House is just beginning to gain relevance as a tool to help solve these intersecting public policy crises, he added.
“We’ve demonstrated through evaluation data that it reduces hospitalization rates, health care costs, reliance on emergency departments, homelessness, and recidivism to the criminal justice system,” he said. Health care costs for members are more than 20% lower than for others with mental illness, and recidivism rates among those with a criminal history are less than 5%.
Others familiar with Fountain House say the model delivers on its charge to improve quality of life for people with SMI.
It’s a great referral source for people who are under good mental health control, whether it’s therapy or a combination of therapy and medications, Robert T. London, MD, a practicing psychiatrist in New York who is not affiliated with Fountain House but has referred patients to the organization over the years, said in an interview.
“They can work with staff, learn skills regarding potential work, housekeeping, [and] social skills,” he said. One of the biggest problems facing people with SMI is they’re very isolated, Dr. London continued. “When you’re in a facility like Fountain House, you’re not isolated. You’re with fellow members, a very helpful educated staff, and you’re going to do well.” If a member is having some issues and losing touch with reality and needs to find treatment, Fountain House will provide that support.
“If you don’t have a treating person, they’re going to find you one. They’re not against traditional medical/psychiatric care,” he said.
Among those with unstable or no housing, 99% find housing within a year of joining Fountain House. While it does provide people with SMI with support to find a roof over their heads, Fountain House doesn’t necessarily fit a model of “housing first,” Stephanie Le Melle, MD, MS, director of public psychiatry education at department of psychiatry at Columbia University/New York State Psychiatric Institute, said in an interview.
“The housing first evidence-based model, as designed and implemented by Pathways to Housing program in New York in the early 90s, accepted people who were street homeless or in shelters, not involved in mental health treatment, and actively using substances into scatter-site apartments and wrapped services around them,” she said.
Dr. Le Melle, who is not affiliated with Fountain House, views it more as a supportive employment program that uses a recovery-oriented, community-based, jointly peer-run approach to engage members in vocational/educational programming. It also happens to have some supportive housing for its members, she added.
Dr. Vasan believes Fountain House could expand beyond a community model. The organization has been moving out from its history, evolving into a model that could be integrated as standard of care and standard of practice for community health, he said. Fountain House is part of Clubhouse International, an umbrella organization that received the American Psychiatric Association’s 2021 special presidential commendation award during its virtual annual meeting for the group’s use of “the evidence-based, cost-effective clubhouse model of psychosocial rehabilitation as a leading recovery resource for people living with mental illness around the world.”
How medication issues are handled
Fountain House doesn’t directly provide medication to its members. According to Dr. Vasan, psychiatric care is just one component of recovery for serious mental illness.
“We talk about Fountain House as a main vortex in a triangle of recovery. You need health care, housing, and community. The part that’s been neglected the most is community intervention, the social infrastructure for people who are deeply isolated and marginalized,” he said. “We know that people who have that infrastructure, and are stably housed, are then more likely to engage in community-based psychiatry and primary care. And in turn, people who are in stable clinical care can more durably engage in the community programming Fountain House offers.”
Health care and clinical care are changing. It’s becoming more person-centered and community based. “We need to move with the times and we have, in the last 2 decades,” he said.
Historically, Fountain House has owned and operated its own clinic in New York City. More recently, it partnered with Sun River Health and Ryan Health, two large federally qualified health center networks in New York, so that members receive access to psychiatric and medical care. It has also expanded similar partnerships with Columbia University, New York University, and other health care systems to ensure its members have access to sustainable clinical care as a part of the community conditions and resources needed to recover and thrive.
Those familiar with the organization don’t see the absence of a medication program as a negative factor. If Fountain House doesn’t provide psychiatric medications, “that tells me the patients are under control and able to function in a community setting” that focuses on rehabilitation, Dr. London said.
It’s true that psychiatric medication treatment is an essential part of a patient’s recovery journey, Dr. Le Melle said. “Treatment with medications can be done in a recovery-oriented way. However, the Fountain House model has been designed to keep these separate, and this model works well for most” of the members.
As long as members and staff are willing to collaborate with treatment providers outside of the clubhouse, when necessary, this model of separation between work and treatment can work really well, she added.
“There are some people who need a more integrated system of care. There is no ‘one size fits all’ program that can meet everyone’s needs,” said Dr. Le Melle. The absence of onsite treatment at Fountain House, to some extent “adds to the milieu and allows people to focus on other aspects of their lives besides their illness.”
This hasn’t always been the case in traditionally funded behavioral health programs, she continued. Most mental health clinics, because of fiscal structures, reimbursement, and staffing costs, focus more on psychotherapy and medication management than on other aspects of peoples’ lives, such as their recovery goals.
The bottom line is rehabilitation in medicine works – whether it’s for a mental health disorder, broken leg, arm, or stroke, Dr. London said. “Fountain House’s focus is integrating a person into society by helping them to think differently and interact socially in groups and learn some skills.”
Through cognitive-behavioral therapies, a person with mental illness can learn how to act differently. “The brain is always in a growing process where you learn and develop new ideas, make connections,” Dr. London said. “New protein molecules get created and stored; changes occur with the neurotransmitters.”
Overall, the Fountain House model is great for supporting and engaging people with serious mental illness, Dr. Le Melle said. “It provides a literal place, a community, and a safe environment that helps people to embrace their recovery journey. It is also great at supporting people in their engagement with vocational training and employment.”
Ideally, she would like Fountain House to grow and become more inclusive by engaging people who live with both mental illness and substance use.
COVID-19 changes the rules
The most difficult challenge for health care and other institutions is to keep individuals with SMI engaged and visible so that they can find access to health care and benefits – and avoid acute hospitalization or medical care. “That’s our goal, to prevent the worst effects and respond accordingly,” Dr. Vasan said.
SARS-CoV-2 forced the program to reevaluate its daily operations so that it could maintain crucial connections with its members.
Dr. Vasan and his staff immediately closed the clubhouse when COVID-19 first hit, transitioning to direct community-based services that provided one-on-one outreach, and meal, medication, and clothing delivery. “Even if people couldn’t visit our clubhouse, we wanted them to feel that sense of community connection, even if it was to drop off meals at their doorstep,” he said.
Donning personal protective equipment, his staff and interested program participants went out into the communities to do this personal outreach. At the height of pandemic in New York City, “we weren’t sure what to do,” as far as keeping safe, he admitted. Nevertheless, he believes this outreach work was lifesaving in that it kept people connected to the clubhouse.
As Fountain House worked to maintain in-person contact, it also built a digital community to keep the live community together. This wasn’t just about posting on a Facebook page – it was interactive, Dr. Vasan noted. An online group made masks for the community and sold them for people outside of Fountain House. Capacity building courses instructed members on writing resumes, looking for jobs, or filling out applications.
There’s an assumption that people with SMI lack the skills to navigate technology. Some of the hallmarks of SMI are demotivation and lack of confidence, and logging onto platforms and email can be challenging for some people, he acknowledged. Over the last 18 months, Fountain House’s virtual clubhouse proved this theory wrong, Dr. Vasan said. “There are a great number of people with serious mental illness who have basic digital skills and are already using technology, or are very eager to learn,” he said.
For the subset of members who did get discouraged by the virtual platform, Fountain House responded by giving them one-on-one home support and digital literacy training to help them stay motivated and engaged.
Fountain House also expanded partnerships during the pandemic, working with programs such as the Fortune Society to bring people with SMI from the criminal justice system into Fountain House. “We’re doing this either virtually or through outdoor, public park programs with groups such as the Times Square Alliance and Fort Greene Park Conservancy to ensure we’re meeting people where they are, at a time of a rising health crisis,” Dr. Vasan said.
Moving on to a hybrid model
At the height of the pandemic, it was easy to engage members through creative programming. People were craving socialization. Now that people are getting vaccinated and interacting inside and outside, some understandable apathy is forming toward digital platforms, Dr. Vasan said.
“The onus is on us now to look at that data and to design something new that can keep people engaged in a hybrid model,” he added.
June 14, 2021, marked Fountain House’s soft opening. “This was a big day for us, to work through the kinks,” he said. At press time, the plan was to fully reopen the clubhouse in a few weeks – if transmission and case rates stay low.
It’s unclear at this point how many people will engage with Fountain House on a daily, in-person basis. Some people might want to come to the clubhouse just a few days a week and use the online platform on other days.
“We’re doing a series of experiments to really understand what different offerings we need to make. For example, perhaps we need to have 24-7 programming on the digital platform. That way, you could access it on demand,” said Dr. Vasan. The goal is to create a menu of choices for members so that it becomes flexible and meets their needs.
Long term, Dr. Vasan hopes the digital platform will become a scalable technology. “We want this to be used not just by Fountain House, but for programs and in markets that don’t have clubhouses.” Health systems or insurance companies would benefit from software like this because it addresses one of the most difficult aspects for this population: keeping them engaged and visible to their systems, Dr. Vasan added.
“I think the most important lesson here is we’re designing for a group of people that no one designs for. No one’s paying attention to people with serious mental illness. Nor have they ever, really. Fountain House has always been their advocate and partner. It’s great that we can do this with them, and for them.”
Dr. Vasan, also an epidemiologist, serves as assistant professor of clinical population, and family health and medicine, at Columbia University. Dr. London and Dr. Le Melle have no conflicts of interest.
Two steps back, three steps forward
For some of its members, Fountain House provides more than just a sense of place. In an interview, longtime member and New York City native Rich Courage, 61, discussed his mental illness challenges and the role the organization played in reclaiming his life, leading to a new career as a counselor.
Question: What made you seek out Fountain House? Are you still a member?
Rich Courage: I’ve been a member since 2001. I was in a day program at Postgraduate, on West 36th Street. They had this huge theater program, and I was a part of that. But the program fell apart and I didn’t know what to do with myself. A friend of mine told me about Fountain House. I asked what it did, and the friend said that it puts people with mental health challenges back to life, to work, to school. I was making some art, some collages, and I heard they had an art gallery.
Seeing Fountain House, I was amazed. It was this very friendly, warm, cozy place. The staff was nice; the members were welcoming. The next thing you know it’s 2021, and here I am, a peer counselor at Fountain House. I work on “the warm line,” doing the evening shift. People call in who have crises, but a lot of them call in because they’re lonely and want someone to talk to. As a peer counselor, I don’t tell people what to do, but I do offer support. I encourage. I ask questions that enable them to figure out their own problems. And I tell stories anecdotally of people that I’ve known and about recovery.
I struggled with bad depression when I was in my 20s. My mother died, and I lost everything. Coming to Fountain House and being part of this community is unlike anything I’d ever experienced. People weren’t just sitting around and talking about their problems; they were doing something about it. They were going back to school, to work, to social engagements, and the world at large. And it wasn’t perfect or linear. It was two steps back, three steps forward.
That’s exactly what I was doing. I had a lot of self-esteem and confidence issues, and behavioral stuff. My mind was wired a certain way. I had hospitalizations; I was in psychiatric wards. I had a suicide attempt in 2006, which was nearly successful. I was feeling social, mental, and emotional pain for so long. The community has been invaluable for me. Hearing other people’s stories, being accepted, has been wonderful.
I’ve been down and now I’m up, on an upward trajectory.
Question: How else has Fountain House made a difference in your life?
RC: I’m in a Fountain House residence in a one-bedroom, and it’s the most stable housing I’ve ever had in 61 years. So I’ve gotten housing and I’ve gotten a job, which is all great, because it’s aided me in becoming a full human being. But it’s really eased my suffering and enabled me to feel some joy and have some life instead of this shadow existence that I had been living for 30 years.
Fountain House has different units, and I’ve been in the communications unit – we put out the weekly paper and handle all the mail. The unit has computers, and I was able to work on my writing. I wrote a play called "The Very Last Dance of Homeless Joe." We’ve had staged readings at Fountain House, and 200 people have seen it over 2 years. We Zoomed it through the virtual community. It was very successful. A recording of the staged reading won third place at a festival in Florida.
In September, it will be an off-Broadway show. It’s a play about the homeless, but it’s not depressing; it’s very uplifting.
Question: Did you stay connected to Fountain House during the pandemic, either through the digital community or through services they provided? What was this experience like for you?
RC: Ashwin [Vasan] had been here 6 months, and he saw the pandemic coming. During a programming meeting he said, “We need a virtual community, and we need it now.” None of us knew what Zoom was, how the mute button worked. But it’s been wonderful for me. I’m a performer, so I was able to get on to Facebook every day and post a song. Some of it was spoofs about COVID; some were dedications to members. I ended up connecting with a member in Minnesota who used to be a neighbor of mine. We had lost contact, and we reconnected through Fountain House.
Question: What would you tell someone who might need this service?
RC: We’ve partially reopened the clubhouse. In July we’ll be doing tours again. I’d say, come take a tour and see the different social, economic, housing, and educational opportunities. We have a home and garden unit that decorates the place. We have a gym, a wellness unit. But these are just things. The real heart is the people.
As a unit leader recently told me, “We’re not a clinic. We’re not a revolving door. We forge relationships with members that last in our hearts and minds for a lifetime. Even if it’s not in my job description, if there’s anything in my power that I could do to help a member ease their suffering, I will do it.”
For more than 70 years, Fountain House has offered a lifeline for people living with schizophrenia, bipolar disorder, major depression, and other serious mental illnesses through a community-based model of care. When he took the helm less than 2 years ago, CEO and President Ashwin Vasan, ScM, MD, PhD, wanted a greater focus on crisis-based solutions and a wider, public health approach.

That goal was put to the test in 2020, when SARS-CoV-2 shuttered all in-person activities. The nonprofit quickly rebounded, creating a digital platform, engaging with its members through online courses, face-to-face check-ins, and delivery services, and expanding partnerships to connect with individuals facing homelessness and involved in the criminal justice system. Those activities not only brought the community together – it expanded Fountain House’s footprint.
Among its membership of more than 2,000 people in New York City, about 70% connected to the digital platform. “We also enrolled more than 200 brand new members during the pandemic who had never set foot in the physical mental health “clubhouse.” They derived value as well,” Dr. Vasan said in an interview. Nationally, the program is replicated at more than 200 locations and serves about 60,000 people in almost 40 states. During the pandemic, Fountain House began formalizing affiliation opportunities with this network.
Now that the pandemic is showing signs of receding, Fountain House faces new challenges operating as a possible hybrid model. “More than three-quarters of our members say they want to continue to engage virtually as well as in person,” Dr. Vasan said. As of this writing, Fountain House is enjoying a soft reopening, slowly welcoming in-person activities. What this will look like in the coming weeks and months is a work in progress, he added. “We don’t know yet how people are going to prefer to engage.”
A role in the public policy conversation
Founded by a small group of former psychiatric patients in the late 1940s, Fountain House has since expanded from a single building in New York City to more than 300 replications in the United States and around the world. It originated the “clubhouse” model of mental health support: a community-based approach that helps members improve health, and break social and economic isolation by reclaiming social, educational, and work skills, and connecting with core services, including supportive housing and community-based primary and behavioral health care (Arts Psychother. 2012 Feb 39[1]:25-30).
Serious mental illness (SMI) is growing more pronounced as a crisis, not just in the people it affects, “but in all of the attendant and preventable social and economic crises that intersect with it, whether it’s increasing health care costs, homelessness, or criminalization,” Dr. Vasan said.
After 73 years, Fountain House is just beginning to gain relevance as a tool to help solve these intersecting public policy crises, he added.
“We’ve demonstrated through evaluation data that it reduces hospitalization rates, health care costs, reliance on emergency departments, homelessness, and recidivism to the criminal justice system,” he said. Health care costs for members are more than 20% lower than for others with mental illness, and recidivism rates among those with a criminal history are less than 5%.
Others familiar with Fountain House say the model delivers on its charge to improve quality of life for people with SMI.
It’s a great referral source for people who are under good mental health control, whether it’s therapy or a combination of therapy and medications, Robert T. London, MD, a practicing psychiatrist in New York who is not affiliated with Fountain House but has referred patients to the organization over the years, said in an interview.
“They can work with staff, learn skills regarding potential work, housekeeping, [and] social skills,” he said. One of the biggest problems facing people with SMI is they’re very isolated, Dr. London continued. “When you’re in a facility like Fountain House, you’re not isolated. You’re with fellow members, a very helpful educated staff, and you’re going to do well.” If a member is having some issues and losing touch with reality and needs to find treatment, Fountain House will provide that support.
“If you don’t have a treating person, they’re going to find you one. They’re not against traditional medical/psychiatric care,” he said.
Among those with unstable or no housing, 99% find housing within a year of joining Fountain House. While it does provide people with SMI with support to find a roof over their heads, Fountain House doesn’t necessarily fit a model of “housing first,” Stephanie Le Melle, MD, MS, director of public psychiatry education at department of psychiatry at Columbia University/New York State Psychiatric Institute, said in an interview.
“The housing first evidence-based model, as designed and implemented by Pathways to Housing program in New York in the early 90s, accepted people who were street homeless or in shelters, not involved in mental health treatment, and actively using substances into scatter-site apartments and wrapped services around them,” she said.
Dr. Le Melle, who is not affiliated with Fountain House, views it more as a supportive employment program that uses a recovery-oriented, community-based, jointly peer-run approach to engage members in vocational/educational programming. It also happens to have some supportive housing for its members, she added.
Dr. Vasan believes Fountain House could expand beyond a community model. The organization has been moving out from its history, evolving into a model that could be integrated as standard of care and standard of practice for community health, he said. Fountain House is part of Clubhouse International, an umbrella organization that received the American Psychiatric Association’s 2021 special presidential commendation award during its virtual annual meeting for the group’s use of “the evidence-based, cost-effective clubhouse model of psychosocial rehabilitation as a leading recovery resource for people living with mental illness around the world.”
How medication issues are handled
Fountain House doesn’t directly provide medication to its members. According to Dr. Vasan, psychiatric care is just one component of recovery for serious mental illness.
“We talk about Fountain House as a main vortex in a triangle of recovery. You need health care, housing, and community. The part that’s been neglected the most is community intervention, the social infrastructure for people who are deeply isolated and marginalized,” he said. “We know that people who have that infrastructure, and are stably housed, are then more likely to engage in community-based psychiatry and primary care. And in turn, people who are in stable clinical care can more durably engage in the community programming Fountain House offers.”
Health care and clinical care are changing. It’s becoming more person-centered and community based. “We need to move with the times and we have, in the last 2 decades,” he said.
Historically, Fountain House has owned and operated its own clinic in New York City. More recently, it partnered with Sun River Health and Ryan Health, two large federally qualified health center networks in New York, so that members receive access to psychiatric and medical care. It has also expanded similar partnerships with Columbia University, New York University, and other health care systems to ensure its members have access to sustainable clinical care as a part of the community conditions and resources needed to recover and thrive.
Those familiar with the organization don’t see the absence of a medication program as a negative factor. If Fountain House doesn’t provide psychiatric medications, “that tells me the patients are under control and able to function in a community setting” that focuses on rehabilitation, Dr. London said.
It’s true that psychiatric medication treatment is an essential part of a patient’s recovery journey, Dr. Le Melle said. “Treatment with medications can be done in a recovery-oriented way. However, the Fountain House model has been designed to keep these separate, and this model works well for most” of the members.
As long as members and staff are willing to collaborate with treatment providers outside of the clubhouse, when necessary, this model of separation between work and treatment can work really well, she added.
“There are some people who need a more integrated system of care. There is no ‘one size fits all’ program that can meet everyone’s needs,” said Dr. Le Melle. The absence of onsite treatment at Fountain House, to some extent “adds to the milieu and allows people to focus on other aspects of their lives besides their illness.”
This hasn’t always been the case in traditionally funded behavioral health programs, she continued. Most mental health clinics, because of fiscal structures, reimbursement, and staffing costs, focus more on psychotherapy and medication management than on other aspects of peoples’ lives, such as their recovery goals.
The bottom line is rehabilitation in medicine works – whether it’s for a mental health disorder, broken leg, arm, or stroke, Dr. London said. “Fountain House’s focus is integrating a person into society by helping them to think differently and interact socially in groups and learn some skills.”
Through cognitive-behavioral therapies, a person with mental illness can learn how to act differently. “The brain is always in a growing process where you learn and develop new ideas, make connections,” Dr. London said. “New protein molecules get created and stored; changes occur with the neurotransmitters.”
Overall, the Fountain House model is great for supporting and engaging people with serious mental illness, Dr. Le Melle said. “It provides a literal place, a community, and a safe environment that helps people to embrace their recovery journey. It is also great at supporting people in their engagement with vocational training and employment.”
Ideally, she would like Fountain House to grow and become more inclusive by engaging people who live with both mental illness and substance use.
COVID-19 changes the rules
The most difficult challenge for health care and other institutions is to keep individuals with SMI engaged and visible so that they can find access to health care and benefits – and avoid acute hospitalization or medical care. “That’s our goal, to prevent the worst effects and respond accordingly,” Dr. Vasan said.
SARS-CoV-2 forced the program to reevaluate its daily operations so that it could maintain crucial connections with its members.
Dr. Vasan and his staff immediately closed the clubhouse when COVID-19 first hit, transitioning to direct community-based services that provided one-on-one outreach, and meal, medication, and clothing delivery. “Even if people couldn’t visit our clubhouse, we wanted them to feel that sense of community connection, even if it was to drop off meals at their doorstep,” he said.
Donning personal protective equipment, his staff and interested program participants went out into the communities to do this personal outreach. At the height of pandemic in New York City, “we weren’t sure what to do,” as far as keeping safe, he admitted. Nevertheless, he believes this outreach work was lifesaving in that it kept people connected to the clubhouse.
As Fountain House worked to maintain in-person contact, it also built a digital community to keep the live community together. This wasn’t just about posting on a Facebook page – it was interactive, Dr. Vasan noted. An online group made masks for the community and sold them for people outside of Fountain House. Capacity building courses instructed members on writing resumes, looking for jobs, or filling out applications.
There’s an assumption that people with SMI lack the skills to navigate technology. Some of the hallmarks of SMI are demotivation and lack of confidence, and logging onto platforms and email can be challenging for some people, he acknowledged. Over the last 18 months, Fountain House’s virtual clubhouse proved this theory wrong, Dr. Vasan said. “There are a great number of people with serious mental illness who have basic digital skills and are already using technology, or are very eager to learn,” he said.
For the subset of members who did get discouraged by the virtual platform, Fountain House responded by giving them one-on-one home support and digital literacy training to help them stay motivated and engaged.
Fountain House also expanded partnerships during the pandemic, working with programs such as the Fortune Society to bring people with SMI from the criminal justice system into Fountain House. “We’re doing this either virtually or through outdoor, public park programs with groups such as the Times Square Alliance and Fort Greene Park Conservancy to ensure we’re meeting people where they are, at a time of a rising health crisis,” Dr. Vasan said.
Moving on to a hybrid model
At the height of the pandemic, it was easy to engage members through creative programming. People were craving socialization. Now that people are getting vaccinated and interacting inside and outside, some understandable apathy is forming toward digital platforms, Dr. Vasan said.
“The onus is on us now to look at that data and to design something new that can keep people engaged in a hybrid model,” he added.
June 14, 2021, marked Fountain House’s soft opening. “This was a big day for us, to work through the kinks,” he said. At press time, the plan was to fully reopen the clubhouse in a few weeks – if transmission and case rates stay low.
It’s unclear at this point how many people will engage with Fountain House on a daily, in-person basis. Some people might want to come to the clubhouse just a few days a week and use the online platform on other days.
“We’re doing a series of experiments to really understand what different offerings we need to make. For example, perhaps we need to have 24-7 programming on the digital platform. That way, you could access it on demand,” said Dr. Vasan. The goal is to create a menu of choices for members so that it becomes flexible and meets their needs.
Long term, Dr. Vasan hopes the digital platform will become a scalable technology. “We want this to be used not just by Fountain House, but for programs and in markets that don’t have clubhouses.” Health systems or insurance companies would benefit from software like this because it addresses one of the most difficult aspects for this population: keeping them engaged and visible to their systems, Dr. Vasan added.
“I think the most important lesson here is we’re designing for a group of people that no one designs for. No one’s paying attention to people with serious mental illness. Nor have they ever, really. Fountain House has always been their advocate and partner. It’s great that we can do this with them, and for them.”
Dr. Vasan, also an epidemiologist, serves as assistant professor of clinical population, and family health and medicine, at Columbia University. Dr. London and Dr. Le Melle have no conflicts of interest.
Two steps back, three steps forward
For some of its members, Fountain House provides more than just a sense of place. In an interview, longtime member and New York City native Rich Courage, 61, discussed his mental illness challenges and the role the organization played in reclaiming his life, leading to a new career as a counselor.
Question: What made you seek out Fountain House? Are you still a member?
Rich Courage: I’ve been a member since 2001. I was in a day program at Postgraduate, on West 36th Street. They had this huge theater program, and I was a part of that. But the program fell apart and I didn’t know what to do with myself. A friend of mine told me about Fountain House. I asked what it did, and the friend said that it puts people with mental health challenges back to life, to work, to school. I was making some art, some collages, and I heard they had an art gallery.
Seeing Fountain House, I was amazed. It was this very friendly, warm, cozy place. The staff was nice; the members were welcoming. The next thing you know it’s 2021, and here I am, a peer counselor at Fountain House. I work on “the warm line,” doing the evening shift. People call in who have crises, but a lot of them call in because they’re lonely and want someone to talk to. As a peer counselor, I don’t tell people what to do, but I do offer support. I encourage. I ask questions that enable them to figure out their own problems. And I tell stories anecdotally of people that I’ve known and about recovery.
I struggled with bad depression when I was in my 20s. My mother died, and I lost everything. Coming to Fountain House and being part of this community is unlike anything I’d ever experienced. People weren’t just sitting around and talking about their problems; they were doing something about it. They were going back to school, to work, to social engagements, and the world at large. And it wasn’t perfect or linear. It was two steps back, three steps forward.
That’s exactly what I was doing. I had a lot of self-esteem and confidence issues, and behavioral stuff. My mind was wired a certain way. I had hospitalizations; I was in psychiatric wards. I had a suicide attempt in 2006, which was nearly successful. I was feeling social, mental, and emotional pain for so long. The community has been invaluable for me. Hearing other people’s stories, being accepted, has been wonderful.
I’ve been down and now I’m up, on an upward trajectory.
Question: How else has Fountain House made a difference in your life?
RC: I’m in a Fountain House residence in a one-bedroom, and it’s the most stable housing I’ve ever had in 61 years. So I’ve gotten housing and I’ve gotten a job, which is all great, because it’s aided me in becoming a full human being. But it’s really eased my suffering and enabled me to feel some joy and have some life instead of this shadow existence that I had been living for 30 years.
Fountain House has different units, and I’ve been in the communications unit – we put out the weekly paper and handle all the mail. The unit has computers, and I was able to work on my writing. I wrote a play called "The Very Last Dance of Homeless Joe." We’ve had staged readings at Fountain House, and 200 people have seen it over 2 years. We Zoomed it through the virtual community. It was very successful. A recording of the staged reading won third place at a festival in Florida.
In September, it will be an off-Broadway show. It’s a play about the homeless, but it’s not depressing; it’s very uplifting.
Question: Did you stay connected to Fountain House during the pandemic, either through the digital community or through services they provided? What was this experience like for you?
RC: Ashwin [Vasan] had been here 6 months, and he saw the pandemic coming. During a programming meeting he said, “We need a virtual community, and we need it now.” None of us knew what Zoom was, how the mute button worked. But it’s been wonderful for me. I’m a performer, so I was able to get on to Facebook every day and post a song. Some of it was spoofs about COVID; some were dedications to members. I ended up connecting with a member in Minnesota who used to be a neighbor of mine. We had lost contact, and we reconnected through Fountain House.
Question: What would you tell someone who might need this service?
RC: We’ve partially reopened the clubhouse. In July we’ll be doing tours again. I’d say, come take a tour and see the different social, economic, housing, and educational opportunities. We have a home and garden unit that decorates the place. We have a gym, a wellness unit. But these are just things. The real heart is the people.
As a unit leader recently told me, “We’re not a clinic. We’re not a revolving door. We forge relationships with members that last in our hearts and minds for a lifetime. Even if it’s not in my job description, if there’s anything in my power that I could do to help a member ease their suffering, I will do it.”
Back-to-school threat: Missed vaccinations in children, teens
U.S. children and adolescents may be at higher risk for vaccine-preventable diseases this fall as vaccination levels have not caught up with prepandemic coverage, according to a study published in Morbidity and Mortality Weekly Report from the Centers for Disease Control and Prevention.
“Pediatric outbreaks of vaccine-preventable diseases have the potential to derail efforts to reopen schools for the 2021-22 academic year and further delay nationwide efforts to return students to the classroom,” wrote Bhavini Patel Murthy, MD, with the immunization services division, National Center for Immunization and Respiratory Diseases, and colleagues.
The number of children getting routine vaccinations plummeted between March and May 2020, compared with the same months in 2019. Although vaccination rates increased again from June 2020 to September 2020, the rebound was not enough to reach prepandemic levels, according to the study.
At the beginning of the June–September 2020 period, the news was good, the authors wrote. After most stay-at-home orders were lifted, the number of weekly routine pediatric vaccinations started to approach, and even surpass, baseline prepandemic levels in most of the 10 jurisdictions studied.
“However,” the authors wrote, “across all age groups and across all vaccine types, none of the jurisdictions demonstrated a sustained or prolonged increase in the number of weekly doses administered above prepandemic administration levels, which would have been necessary to catch up children and adolescents who missed routine vaccinations.”
To overcome the gap, the authors said that clinicians should take the initiative. “Health care providers should assess the vaccination status of all pediatric patients, including adolescents, and contact those who are behind schedule to ensure that all children are fully vaccinated.”
As COVID-19 vaccinations become more readily available to children, the CDC recommends that providers consider giving COVID-19 shots along with other routinely recommended vaccines.
Martha Perry, MD, associate professor and medical director at the University of North Carolina Children’s Primary Care Clinic, Chapel Hill, said in an interview that getting the message out about the need to get children and adolescents caught up may require a national messaging campaign similar to that for COVID-19 vaccinations, as well as opening mass vaccination sites rather than families seeking vaccinations from individual providers.
She noted that, although schools may offer a checks and balances system for required vaccinations, children who are not yet school age depend on families getting individual appointments.
Size of the gaps
The MMWR article shows that the shortfall in vaccinations in June–September 2020, compared with those months the year before are striking.
For children younger than 2 years old and aged 2-6 years, diphtheria, tetanus, and acellular pertussis (DtaP) vaccinations declined an average of 9.1% and 6.7%, respectively.
Among children aged 12-23 months and 2-8 years, MMR vaccinations decreased 8.8% and 11.3%, respectively.
Among children aged 9-12 years and adolescents 13-17 years, human papillomavirus vaccinations decreased an average 12.2% and 28.1%, respectively. Among the same age groups, Tdap vaccinations dropped 21.3% and 30.0%, respectively.
Dr. Perry said that, although all the shortfalls are important, lags in vaccinations for measles and pertussis are particularly alarming in light of outbreaks in recent years.
Additionally, she said, as COVID-19 restrictions are lifting, some of the mitigation strategies, such as mask wearing, that kept other diseases at bay will not be in place, heightening the risk for infection.
The authors chose to measure weekly doses in March–May 2020, and June–September 2020 because many jurisdictions imposed and then lifted stay-at-home orders during these times. They analyzed data from 10 jurisdictions with high-performing information systems (Idaho, Iowa, Louisiana, Michigan, Minnesota, New York City, North Dakota, Oregon, Washington, and Wisconsin).
Adults missing vaccinations as well
Another analysis, commissioned by GlaxoSmithKline and conducted by Avalere Health, calculated 8.8 million missed adolescent vaccine doses and 17.2 million missed adult vaccine doses as a result of the pandemic and ongoing government restrictions and public health measures.
That study examined claims for CDC-recommended vaccines across commercial, managed Medicaid, Medicare Advantage, and Medicare fee-for-service Part B for January–November 2020, compared with the same period in 2019.
It also found that vaccine claims remain well below 2019 levels. Total noninfluenza vaccine claims submissions were down by between 13% and 35% among adolescents and 17% and 40% among adults, compared with the same period in 2019.
Dr. Perry said it will be critical for schools across the nation to enforce their policies on requiring up-to-date vaccinations even if online attendance is offered.
The workforce needed for this will be challenging, she noted.
“We’ve lost a lot of workforce in the health care field in the pandemic for a variety of reasons and it may be challenging to fill those positions,” she said.
She also said the study underlines the importance of each state having a vaccine registry so each provider can determine what vaccinations a child needs.
The study authors and Dr. Perry reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
U.S. children and adolescents may be at higher risk for vaccine-preventable diseases this fall as vaccination levels have not caught up with prepandemic coverage, according to a study published in Morbidity and Mortality Weekly Report from the Centers for Disease Control and Prevention.
“Pediatric outbreaks of vaccine-preventable diseases have the potential to derail efforts to reopen schools for the 2021-22 academic year and further delay nationwide efforts to return students to the classroom,” wrote Bhavini Patel Murthy, MD, with the immunization services division, National Center for Immunization and Respiratory Diseases, and colleagues.
The number of children getting routine vaccinations plummeted between March and May 2020, compared with the same months in 2019. Although vaccination rates increased again from June 2020 to September 2020, the rebound was not enough to reach prepandemic levels, according to the study.
At the beginning of the June–September 2020 period, the news was good, the authors wrote. After most stay-at-home orders were lifted, the number of weekly routine pediatric vaccinations started to approach, and even surpass, baseline prepandemic levels in most of the 10 jurisdictions studied.
“However,” the authors wrote, “across all age groups and across all vaccine types, none of the jurisdictions demonstrated a sustained or prolonged increase in the number of weekly doses administered above prepandemic administration levels, which would have been necessary to catch up children and adolescents who missed routine vaccinations.”
To overcome the gap, the authors said that clinicians should take the initiative. “Health care providers should assess the vaccination status of all pediatric patients, including adolescents, and contact those who are behind schedule to ensure that all children are fully vaccinated.”
As COVID-19 vaccinations become more readily available to children, the CDC recommends that providers consider giving COVID-19 shots along with other routinely recommended vaccines.
Martha Perry, MD, associate professor and medical director at the University of North Carolina Children’s Primary Care Clinic, Chapel Hill, said in an interview that getting the message out about the need to get children and adolescents caught up may require a national messaging campaign similar to that for COVID-19 vaccinations, as well as opening mass vaccination sites rather than families seeking vaccinations from individual providers.
She noted that, although schools may offer a checks and balances system for required vaccinations, children who are not yet school age depend on families getting individual appointments.
Size of the gaps
The MMWR article shows that the shortfall in vaccinations in June–September 2020, compared with those months the year before are striking.
For children younger than 2 years old and aged 2-6 years, diphtheria, tetanus, and acellular pertussis (DtaP) vaccinations declined an average of 9.1% and 6.7%, respectively.
Among children aged 12-23 months and 2-8 years, MMR vaccinations decreased 8.8% and 11.3%, respectively.
Among children aged 9-12 years and adolescents 13-17 years, human papillomavirus vaccinations decreased an average 12.2% and 28.1%, respectively. Among the same age groups, Tdap vaccinations dropped 21.3% and 30.0%, respectively.
Dr. Perry said that, although all the shortfalls are important, lags in vaccinations for measles and pertussis are particularly alarming in light of outbreaks in recent years.
Additionally, she said, as COVID-19 restrictions are lifting, some of the mitigation strategies, such as mask wearing, that kept other diseases at bay will not be in place, heightening the risk for infection.
The authors chose to measure weekly doses in March–May 2020, and June–September 2020 because many jurisdictions imposed and then lifted stay-at-home orders during these times. They analyzed data from 10 jurisdictions with high-performing information systems (Idaho, Iowa, Louisiana, Michigan, Minnesota, New York City, North Dakota, Oregon, Washington, and Wisconsin).
Adults missing vaccinations as well
Another analysis, commissioned by GlaxoSmithKline and conducted by Avalere Health, calculated 8.8 million missed adolescent vaccine doses and 17.2 million missed adult vaccine doses as a result of the pandemic and ongoing government restrictions and public health measures.
That study examined claims for CDC-recommended vaccines across commercial, managed Medicaid, Medicare Advantage, and Medicare fee-for-service Part B for January–November 2020, compared with the same period in 2019.
It also found that vaccine claims remain well below 2019 levels. Total noninfluenza vaccine claims submissions were down by between 13% and 35% among adolescents and 17% and 40% among adults, compared with the same period in 2019.
Dr. Perry said it will be critical for schools across the nation to enforce their policies on requiring up-to-date vaccinations even if online attendance is offered.
The workforce needed for this will be challenging, she noted.
“We’ve lost a lot of workforce in the health care field in the pandemic for a variety of reasons and it may be challenging to fill those positions,” she said.
She also said the study underlines the importance of each state having a vaccine registry so each provider can determine what vaccinations a child needs.
The study authors and Dr. Perry reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
U.S. children and adolescents may be at higher risk for vaccine-preventable diseases this fall as vaccination levels have not caught up with prepandemic coverage, according to a study published in Morbidity and Mortality Weekly Report from the Centers for Disease Control and Prevention.
“Pediatric outbreaks of vaccine-preventable diseases have the potential to derail efforts to reopen schools for the 2021-22 academic year and further delay nationwide efforts to return students to the classroom,” wrote Bhavini Patel Murthy, MD, with the immunization services division, National Center for Immunization and Respiratory Diseases, and colleagues.
The number of children getting routine vaccinations plummeted between March and May 2020, compared with the same months in 2019. Although vaccination rates increased again from June 2020 to September 2020, the rebound was not enough to reach prepandemic levels, according to the study.
At the beginning of the June–September 2020 period, the news was good, the authors wrote. After most stay-at-home orders were lifted, the number of weekly routine pediatric vaccinations started to approach, and even surpass, baseline prepandemic levels in most of the 10 jurisdictions studied.
“However,” the authors wrote, “across all age groups and across all vaccine types, none of the jurisdictions demonstrated a sustained or prolonged increase in the number of weekly doses administered above prepandemic administration levels, which would have been necessary to catch up children and adolescents who missed routine vaccinations.”
To overcome the gap, the authors said that clinicians should take the initiative. “Health care providers should assess the vaccination status of all pediatric patients, including adolescents, and contact those who are behind schedule to ensure that all children are fully vaccinated.”
As COVID-19 vaccinations become more readily available to children, the CDC recommends that providers consider giving COVID-19 shots along with other routinely recommended vaccines.
Martha Perry, MD, associate professor and medical director at the University of North Carolina Children’s Primary Care Clinic, Chapel Hill, said in an interview that getting the message out about the need to get children and adolescents caught up may require a national messaging campaign similar to that for COVID-19 vaccinations, as well as opening mass vaccination sites rather than families seeking vaccinations from individual providers.
She noted that, although schools may offer a checks and balances system for required vaccinations, children who are not yet school age depend on families getting individual appointments.
Size of the gaps
The MMWR article shows that the shortfall in vaccinations in June–September 2020, compared with those months the year before are striking.
For children younger than 2 years old and aged 2-6 years, diphtheria, tetanus, and acellular pertussis (DtaP) vaccinations declined an average of 9.1% and 6.7%, respectively.
Among children aged 12-23 months and 2-8 years, MMR vaccinations decreased 8.8% and 11.3%, respectively.
Among children aged 9-12 years and adolescents 13-17 years, human papillomavirus vaccinations decreased an average 12.2% and 28.1%, respectively. Among the same age groups, Tdap vaccinations dropped 21.3% and 30.0%, respectively.
Dr. Perry said that, although all the shortfalls are important, lags in vaccinations for measles and pertussis are particularly alarming in light of outbreaks in recent years.
Additionally, she said, as COVID-19 restrictions are lifting, some of the mitigation strategies, such as mask wearing, that kept other diseases at bay will not be in place, heightening the risk for infection.
The authors chose to measure weekly doses in March–May 2020, and June–September 2020 because many jurisdictions imposed and then lifted stay-at-home orders during these times. They analyzed data from 10 jurisdictions with high-performing information systems (Idaho, Iowa, Louisiana, Michigan, Minnesota, New York City, North Dakota, Oregon, Washington, and Wisconsin).
Adults missing vaccinations as well
Another analysis, commissioned by GlaxoSmithKline and conducted by Avalere Health, calculated 8.8 million missed adolescent vaccine doses and 17.2 million missed adult vaccine doses as a result of the pandemic and ongoing government restrictions and public health measures.
That study examined claims for CDC-recommended vaccines across commercial, managed Medicaid, Medicare Advantage, and Medicare fee-for-service Part B for January–November 2020, compared with the same period in 2019.
It also found that vaccine claims remain well below 2019 levels. Total noninfluenza vaccine claims submissions were down by between 13% and 35% among adolescents and 17% and 40% among adults, compared with the same period in 2019.
Dr. Perry said it will be critical for schools across the nation to enforce their policies on requiring up-to-date vaccinations even if online attendance is offered.
The workforce needed for this will be challenging, she noted.
“We’ve lost a lot of workforce in the health care field in the pandemic for a variety of reasons and it may be challenging to fill those positions,” she said.
She also said the study underlines the importance of each state having a vaccine registry so each provider can determine what vaccinations a child needs.
The study authors and Dr. Perry reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.