User login
COVID-19 tied to spike in suspected suicide attempts by girls
Suspected suicide attempts by teenage girls have increased significantly during the COVID-19 pandemic, according to data released today by the U.S. Centers for Disease Control and Prevention.
Among children and adolescents aged 12-17 years, the average weekly number of emergency department visits for suspected suicide attempts was 22.3% higher during summer 2020 and 39.1% higher during winter 2021 than during the corresponding periods in 2019.
The increase was most evident among young girls.
Between Feb. 21 and March 20, 2021, the number of ED visits for suspected suicide attempts was about 51% higher among girls aged 12-17 years than during the same period in 2019. Among boys aged 12-17 years, ED visits for suspected suicide attempts increased 4%, the CDC reports.
“Young persons might represent a group at high risk because they might have been particularly affected by mitigation measures, such as physical distancing (including a lack of connectedness to schools, teachers, and peers); barriers to mental health treatment; increases in substance use; and anxiety about family health and economic problems, which are all risk factors for suicide,” write the authors, led by Ellen Yard, PhD, with the CDC’s National Center for Injury Prevention and Control.
In addition, the findings from this study suggest there has been “more severe distress among young females than has been identified in previous reports during the pandemic, reinforcing the need for increased attention to, and prevention for, this population,” they point out.
The results were published June 11 in Morbidity and Mortality Weekly Report.
The findings are based on data for ED visits for suspected suicide from the National Syndromic Surveillance Program, which includes about 71% of the nation’s EDs in 49 states (all except Hawaii) and the District of Columbia.
Earlier data reported by the CDC showed that the proportion of mental health–related ED visits among children and adolescents aged 12-17 years increased by 31% during 2020, compared with 2019.
‘Time for action is now’
These new findings underscore the “enormous impact the COVID-19 pandemic is having on our country’s overall emotional wellbeing, especially among young people,” the National Action Alliance for Suicide Prevention (Action Alliance) Media Messaging Work Group said in a statement responding to the newly released data.
“Just as we have taken steps to protect our physical health throughout the pandemic, we must also take steps to protect our mental and emotional health,” the group says.
The data, the group says, specifically speak to the importance of improving suicide care both during and after ED visits by scaling up the adoption of best practices, such as the Recommended Standard Care for People with Suicide Risk: Making Health Care Suicide Safe and Best Practices in Care Transitions for Individuals with Suicide Risk: Inpatient Care to Outpatient Care.
“These and other evidence-based best practices must be adopted by health care systems nationwide to ensure safe, effective suicide care for all,” the group says.
“However, health care systems cannot address this issue alone. Suicide is a complex public health issue that also requires a comprehensive, community-based approach to addressing it. We must ensure suicide prevention is infused into a variety of community-based settings – such as schools, workplaces, and places of worship – to ensure people are connected with prevention activities and resources before a crisis occurs,” the group says.
It also highlights the crucial role of social connectedness as a protective factor against suicide.
“Research indicates that a sense of belonging and social connectedness improves physical, mental, and emotional wellbeing. Everyone can play a role in being there for each other and helping to build resiliency. Having real, honest conversations about our own mental health opens the door for connection and social support,” the group says.
It calls on leaders from all sectors and industries to make suicide prevention “a national priority by becoming engaged in the issue and bringing resources to bear. The time for action is now.”
A version of this article first appeared on Medscape.com.
Suspected suicide attempts by teenage girls have increased significantly during the COVID-19 pandemic, according to data released today by the U.S. Centers for Disease Control and Prevention.
Among children and adolescents aged 12-17 years, the average weekly number of emergency department visits for suspected suicide attempts was 22.3% higher during summer 2020 and 39.1% higher during winter 2021 than during the corresponding periods in 2019.
The increase was most evident among young girls.
Between Feb. 21 and March 20, 2021, the number of ED visits for suspected suicide attempts was about 51% higher among girls aged 12-17 years than during the same period in 2019. Among boys aged 12-17 years, ED visits for suspected suicide attempts increased 4%, the CDC reports.
“Young persons might represent a group at high risk because they might have been particularly affected by mitigation measures, such as physical distancing (including a lack of connectedness to schools, teachers, and peers); barriers to mental health treatment; increases in substance use; and anxiety about family health and economic problems, which are all risk factors for suicide,” write the authors, led by Ellen Yard, PhD, with the CDC’s National Center for Injury Prevention and Control.
In addition, the findings from this study suggest there has been “more severe distress among young females than has been identified in previous reports during the pandemic, reinforcing the need for increased attention to, and prevention for, this population,” they point out.
The results were published June 11 in Morbidity and Mortality Weekly Report.
The findings are based on data for ED visits for suspected suicide from the National Syndromic Surveillance Program, which includes about 71% of the nation’s EDs in 49 states (all except Hawaii) and the District of Columbia.
Earlier data reported by the CDC showed that the proportion of mental health–related ED visits among children and adolescents aged 12-17 years increased by 31% during 2020, compared with 2019.
‘Time for action is now’
These new findings underscore the “enormous impact the COVID-19 pandemic is having on our country’s overall emotional wellbeing, especially among young people,” the National Action Alliance for Suicide Prevention (Action Alliance) Media Messaging Work Group said in a statement responding to the newly released data.
“Just as we have taken steps to protect our physical health throughout the pandemic, we must also take steps to protect our mental and emotional health,” the group says.
The data, the group says, specifically speak to the importance of improving suicide care both during and after ED visits by scaling up the adoption of best practices, such as the Recommended Standard Care for People with Suicide Risk: Making Health Care Suicide Safe and Best Practices in Care Transitions for Individuals with Suicide Risk: Inpatient Care to Outpatient Care.
“These and other evidence-based best practices must be adopted by health care systems nationwide to ensure safe, effective suicide care for all,” the group says.
“However, health care systems cannot address this issue alone. Suicide is a complex public health issue that also requires a comprehensive, community-based approach to addressing it. We must ensure suicide prevention is infused into a variety of community-based settings – such as schools, workplaces, and places of worship – to ensure people are connected with prevention activities and resources before a crisis occurs,” the group says.
It also highlights the crucial role of social connectedness as a protective factor against suicide.
“Research indicates that a sense of belonging and social connectedness improves physical, mental, and emotional wellbeing. Everyone can play a role in being there for each other and helping to build resiliency. Having real, honest conversations about our own mental health opens the door for connection and social support,” the group says.
It calls on leaders from all sectors and industries to make suicide prevention “a national priority by becoming engaged in the issue and bringing resources to bear. The time for action is now.”
A version of this article first appeared on Medscape.com.
Suspected suicide attempts by teenage girls have increased significantly during the COVID-19 pandemic, according to data released today by the U.S. Centers for Disease Control and Prevention.
Among children and adolescents aged 12-17 years, the average weekly number of emergency department visits for suspected suicide attempts was 22.3% higher during summer 2020 and 39.1% higher during winter 2021 than during the corresponding periods in 2019.
The increase was most evident among young girls.
Between Feb. 21 and March 20, 2021, the number of ED visits for suspected suicide attempts was about 51% higher among girls aged 12-17 years than during the same period in 2019. Among boys aged 12-17 years, ED visits for suspected suicide attempts increased 4%, the CDC reports.
“Young persons might represent a group at high risk because they might have been particularly affected by mitigation measures, such as physical distancing (including a lack of connectedness to schools, teachers, and peers); barriers to mental health treatment; increases in substance use; and anxiety about family health and economic problems, which are all risk factors for suicide,” write the authors, led by Ellen Yard, PhD, with the CDC’s National Center for Injury Prevention and Control.
In addition, the findings from this study suggest there has been “more severe distress among young females than has been identified in previous reports during the pandemic, reinforcing the need for increased attention to, and prevention for, this population,” they point out.
The results were published June 11 in Morbidity and Mortality Weekly Report.
The findings are based on data for ED visits for suspected suicide from the National Syndromic Surveillance Program, which includes about 71% of the nation’s EDs in 49 states (all except Hawaii) and the District of Columbia.
Earlier data reported by the CDC showed that the proportion of mental health–related ED visits among children and adolescents aged 12-17 years increased by 31% during 2020, compared with 2019.
‘Time for action is now’
These new findings underscore the “enormous impact the COVID-19 pandemic is having on our country’s overall emotional wellbeing, especially among young people,” the National Action Alliance for Suicide Prevention (Action Alliance) Media Messaging Work Group said in a statement responding to the newly released data.
“Just as we have taken steps to protect our physical health throughout the pandemic, we must also take steps to protect our mental and emotional health,” the group says.
The data, the group says, specifically speak to the importance of improving suicide care both during and after ED visits by scaling up the adoption of best practices, such as the Recommended Standard Care for People with Suicide Risk: Making Health Care Suicide Safe and Best Practices in Care Transitions for Individuals with Suicide Risk: Inpatient Care to Outpatient Care.
“These and other evidence-based best practices must be adopted by health care systems nationwide to ensure safe, effective suicide care for all,” the group says.
“However, health care systems cannot address this issue alone. Suicide is a complex public health issue that also requires a comprehensive, community-based approach to addressing it. We must ensure suicide prevention is infused into a variety of community-based settings – such as schools, workplaces, and places of worship – to ensure people are connected with prevention activities and resources before a crisis occurs,” the group says.
It also highlights the crucial role of social connectedness as a protective factor against suicide.
“Research indicates that a sense of belonging and social connectedness improves physical, mental, and emotional wellbeing. Everyone can play a role in being there for each other and helping to build resiliency. Having real, honest conversations about our own mental health opens the door for connection and social support,” the group says.
It calls on leaders from all sectors and industries to make suicide prevention “a national priority by becoming engaged in the issue and bringing resources to bear. The time for action is now.”
A version of this article first appeared on Medscape.com.
Remove sex designation from public part of birth certificates, AMA advises
Requiring the designation can lead to discrimination and unnecessary burden on individuals whose current gender identity does not align with their designation at birth when they register for school or sports, adopt, get married, or request personal records.
A person’s sex designation at birth would still be submitted to the U.S. Standard Certificate of Live Birth for medical, public health, and statistical use only, report authors note.
Willie Underwood III, MD, MSc, author of Board Report 15, explained in reference committee testimony that a standard certificate of live birth is critical for uniformly collecting and processing data, but birth certificates are issued by the government to individuals.
Ten states allow gender-neutral designation
According to the report, 48 states (Tennessee and Ohio are the exceptions) and the District of Columbia allow people to amend their sex designation on their birth certificate to reflect their gender identities, but only 10 states allow for a gender-neutral designation, usually “X,” on birth certificates. The U.S. Department of State does not currently offer an option for a gender-neutral designation on U.S. passports.
“Assigning sex using binary variables in the public portion of the birth certificate fails to recognize the medical spectrum of gender identity,” Dr. Underwood said, and it can be used to discriminate.
Jeremy Toler, MD, a delegate from GLMA: Health Professionals Advancing LGBTQ Equality, testified that there is precedent for information to be removed from the public portion of the birth certificates. And much data is collected for each live birth that doesn’t show up on individuals’ birth certificates, he noted.
Dr. Toler said transgender, gender nonbinary, and individuals with differences in sex development can be placed at a disadvantage by the sex label on the birth certificate.
“We unfortunately still live in a world where it is unsafe in many cases for one’s gender to vary from the sex assigned at birth,” Dr. Toler said.
Not having this data on the widely used form will reduce unnecessary reliance on sex as a stand-in for gender, he said, and would “serve as an equalizer” since policies differ by state.
Robert Jackson, MD, an alternate delegate from the American Academy of Cosmetic Surgery, spoke against the measure.
“We as physicians need to report things accurately,” Dr. Jackson said. “All through medical school, residency, and specialty training we were supposed to delegate all of the physical findings of the patient we’re taking care of. I think when the child is born, they do have physical characteristics either male or female, and I think that probably should be on the public record. That’s just my personal opinion.”
Sarah Mae Smith, MD, delegate from California, speaking on behalf of the Women Physicians Section, said removing the sex designation is important for moving toward gender equity.
“We need to recognize [that] gender is not a binary but a spectrum,” she said. “Obligating our patients to jump through numerous administrative hoops to identify as who they are based on a sex assigned at birth primarily on genitalia is not only unnecessary but actively deleterious to their health.”
Race was once public on birth certificates
She noted that the report mentions that previously, information on the race of a person’s parents was included on the public portion of the birth certificate and that information was recognized to facilitate discrimination.
“Thankfully, a change was made to obviate at least that avenue for discriminatory practices,” she said. “Now, likewise, the information on sex assigned at birth is being used to undermine the rights of our transgender, intersex, and nonbinary patients.”
Arlene Seid, MD, MPH, an alternate delegate from the American Association of Public Health Physicians, said the resolution protects the aggregate data “without the discrimination associated with the individual data.”
Sex no longer has a role to play in the jobs people do, she noted, and the designation shouldn’t have to be evaluated for something like a job interview.
“Our society doesn’t need it on an individual basis for most of what occurs in public life,” Dr. Seid said.
Dr. Underwood, Dr. Toler, Dr. Jackson, Dr. Smith, and Dr. Seid declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Requiring the designation can lead to discrimination and unnecessary burden on individuals whose current gender identity does not align with their designation at birth when they register for school or sports, adopt, get married, or request personal records.
A person’s sex designation at birth would still be submitted to the U.S. Standard Certificate of Live Birth for medical, public health, and statistical use only, report authors note.
Willie Underwood III, MD, MSc, author of Board Report 15, explained in reference committee testimony that a standard certificate of live birth is critical for uniformly collecting and processing data, but birth certificates are issued by the government to individuals.
Ten states allow gender-neutral designation
According to the report, 48 states (Tennessee and Ohio are the exceptions) and the District of Columbia allow people to amend their sex designation on their birth certificate to reflect their gender identities, but only 10 states allow for a gender-neutral designation, usually “X,” on birth certificates. The U.S. Department of State does not currently offer an option for a gender-neutral designation on U.S. passports.
“Assigning sex using binary variables in the public portion of the birth certificate fails to recognize the medical spectrum of gender identity,” Dr. Underwood said, and it can be used to discriminate.
Jeremy Toler, MD, a delegate from GLMA: Health Professionals Advancing LGBTQ Equality, testified that there is precedent for information to be removed from the public portion of the birth certificates. And much data is collected for each live birth that doesn’t show up on individuals’ birth certificates, he noted.
Dr. Toler said transgender, gender nonbinary, and individuals with differences in sex development can be placed at a disadvantage by the sex label on the birth certificate.
“We unfortunately still live in a world where it is unsafe in many cases for one’s gender to vary from the sex assigned at birth,” Dr. Toler said.
Not having this data on the widely used form will reduce unnecessary reliance on sex as a stand-in for gender, he said, and would “serve as an equalizer” since policies differ by state.
Robert Jackson, MD, an alternate delegate from the American Academy of Cosmetic Surgery, spoke against the measure.
“We as physicians need to report things accurately,” Dr. Jackson said. “All through medical school, residency, and specialty training we were supposed to delegate all of the physical findings of the patient we’re taking care of. I think when the child is born, they do have physical characteristics either male or female, and I think that probably should be on the public record. That’s just my personal opinion.”
Sarah Mae Smith, MD, delegate from California, speaking on behalf of the Women Physicians Section, said removing the sex designation is important for moving toward gender equity.
“We need to recognize [that] gender is not a binary but a spectrum,” she said. “Obligating our patients to jump through numerous administrative hoops to identify as who they are based on a sex assigned at birth primarily on genitalia is not only unnecessary but actively deleterious to their health.”
Race was once public on birth certificates
She noted that the report mentions that previously, information on the race of a person’s parents was included on the public portion of the birth certificate and that information was recognized to facilitate discrimination.
“Thankfully, a change was made to obviate at least that avenue for discriminatory practices,” she said. “Now, likewise, the information on sex assigned at birth is being used to undermine the rights of our transgender, intersex, and nonbinary patients.”
Arlene Seid, MD, MPH, an alternate delegate from the American Association of Public Health Physicians, said the resolution protects the aggregate data “without the discrimination associated with the individual data.”
Sex no longer has a role to play in the jobs people do, she noted, and the designation shouldn’t have to be evaluated for something like a job interview.
“Our society doesn’t need it on an individual basis for most of what occurs in public life,” Dr. Seid said.
Dr. Underwood, Dr. Toler, Dr. Jackson, Dr. Smith, and Dr. Seid declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Requiring the designation can lead to discrimination and unnecessary burden on individuals whose current gender identity does not align with their designation at birth when they register for school or sports, adopt, get married, or request personal records.
A person’s sex designation at birth would still be submitted to the U.S. Standard Certificate of Live Birth for medical, public health, and statistical use only, report authors note.
Willie Underwood III, MD, MSc, author of Board Report 15, explained in reference committee testimony that a standard certificate of live birth is critical for uniformly collecting and processing data, but birth certificates are issued by the government to individuals.
Ten states allow gender-neutral designation
According to the report, 48 states (Tennessee and Ohio are the exceptions) and the District of Columbia allow people to amend their sex designation on their birth certificate to reflect their gender identities, but only 10 states allow for a gender-neutral designation, usually “X,” on birth certificates. The U.S. Department of State does not currently offer an option for a gender-neutral designation on U.S. passports.
“Assigning sex using binary variables in the public portion of the birth certificate fails to recognize the medical spectrum of gender identity,” Dr. Underwood said, and it can be used to discriminate.
Jeremy Toler, MD, a delegate from GLMA: Health Professionals Advancing LGBTQ Equality, testified that there is precedent for information to be removed from the public portion of the birth certificates. And much data is collected for each live birth that doesn’t show up on individuals’ birth certificates, he noted.
Dr. Toler said transgender, gender nonbinary, and individuals with differences in sex development can be placed at a disadvantage by the sex label on the birth certificate.
“We unfortunately still live in a world where it is unsafe in many cases for one’s gender to vary from the sex assigned at birth,” Dr. Toler said.
Not having this data on the widely used form will reduce unnecessary reliance on sex as a stand-in for gender, he said, and would “serve as an equalizer” since policies differ by state.
Robert Jackson, MD, an alternate delegate from the American Academy of Cosmetic Surgery, spoke against the measure.
“We as physicians need to report things accurately,” Dr. Jackson said. “All through medical school, residency, and specialty training we were supposed to delegate all of the physical findings of the patient we’re taking care of. I think when the child is born, they do have physical characteristics either male or female, and I think that probably should be on the public record. That’s just my personal opinion.”
Sarah Mae Smith, MD, delegate from California, speaking on behalf of the Women Physicians Section, said removing the sex designation is important for moving toward gender equity.
“We need to recognize [that] gender is not a binary but a spectrum,” she said. “Obligating our patients to jump through numerous administrative hoops to identify as who they are based on a sex assigned at birth primarily on genitalia is not only unnecessary but actively deleterious to their health.”
Race was once public on birth certificates
She noted that the report mentions that previously, information on the race of a person’s parents was included on the public portion of the birth certificate and that information was recognized to facilitate discrimination.
“Thankfully, a change was made to obviate at least that avenue for discriminatory practices,” she said. “Now, likewise, the information on sex assigned at birth is being used to undermine the rights of our transgender, intersex, and nonbinary patients.”
Arlene Seid, MD, MPH, an alternate delegate from the American Association of Public Health Physicians, said the resolution protects the aggregate data “without the discrimination associated with the individual data.”
Sex no longer has a role to play in the jobs people do, she noted, and the designation shouldn’t have to be evaluated for something like a job interview.
“Our society doesn’t need it on an individual basis for most of what occurs in public life,” Dr. Seid said.
Dr. Underwood, Dr. Toler, Dr. Jackson, Dr. Smith, and Dr. Seid declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Toxic chemicals found in many cosmetics
People may be absorbing and ingesting potentially toxic chemicals from their cosmetic products, a new study suggests.
– per- and polyfluoroalkyl substances. Many of these chemicals were not included on the product labels, making it difficult for consumers to consciously avoid them.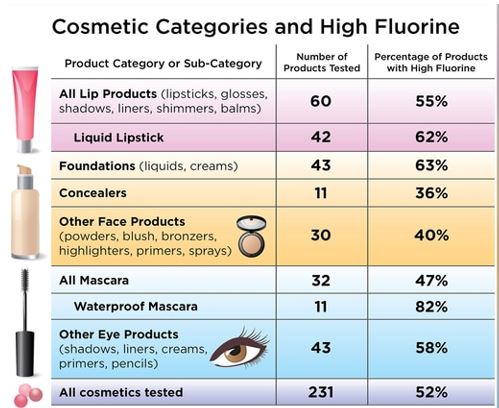
“This study is very helpful for elucidating the PFAS content of different types of cosmetics in the U.S. and Canadian markets,” said Elsie Sunderland, PhD, an environmental scientist who was not involved with the study.
“Previously, all the data had been collected in Europe, and this study shows we are dealing with similar problems in the North American marketplace,” said Dr. Sunderland, a professor of environmental chemistry at the Harvard School of Public Health, Boston.
PFAS are a class of chemicals used in a variety of consumer products, such as nonstick cookware, stain-resistant carpeting, and water-repellent clothing, according to the Centers for Disease Control and Prevention. They are added to cosmetics to make the products more durable and spreadable, researchers said in the study.
“[PFAS] are added to change the properties of surfaces, to make them nonstick or resistant to stay in water or oils,” said study coauthor Tom Bruton, PhD, senior scientist at the Green Science Policy Institute in Berkeley, Calif. “The concerning thing about cosmetics is that these are products that you’re applying to your skin and face every day, so there’s the skin absorption route that’s of concern, but also incidental ingestion of cosmetics is also a concern as well.”
The CDC says some of the potential health effects of PFAS exposure includes increased cholesterol levels, increased risk of kidney and testicular cancer, changes in liver enzymes, decreased vaccine response in children, and a higher risk of high blood pressure or preeclampsia in pregnant women.
“PFAS are a large class of chemicals. In humans, exposure to some of these chemicals has been associated with impaired immune function, certain cancers, increased risks of diabetes, obesity and endocrine disruption,” Dr. Sunderland said. “They appear to be harmful to every major organ system in the human body.”
For the current study, published online in Environmental Science & Technology Letters, Dr. Bruton and colleagues purchased 231 cosmetic products in the United States and Canada from retailers such as Ulta Beauty, Sephora, Target, and Bed Bath & Beyond. They then screened them for fluorine.Three-quarters of waterproof mascara samples contained high fluorine concentrations, as did nearly two-thirds of foundations and liquid lipsticks, and more than half of the eye and lip products tested.
The authors found that different categories of makeup tended to have higher or lower fluorine concentrations. “High fluorine levels were found in products commonly advertised as ‘wear-resistant’ to water and oils or ‘long-lasting,’ including foundations, liquid lipsticks, and waterproof mascaras,” Dr. Bruton and colleagues wrote.
When they further analyzed a subset of 29 products to determine what types of chemicals were present, they found that each cosmetic product contained at least 4 PFAS, with one product containing 13.The PFAS substances found included some that break down into other chemicals that are known to be highly toxic and environmentally harmful.
“It’s concerning that some of the products we tested appear to be intentionally using PFAS, but not listing those ingredients on the label,” Dr. Bruton said. “I do think that it is helpful for consumers to read labels, but beyond that, there’s not a lot of ways that consumers themselves can solve this problem. ... We think that the industry needs to be more proactive about moving away from this group of chemicals.”
Dr. Sunderland said a resource people can use when trying to avoid PFAS is the Environmental Working Group, a nonprofit organization that maintains an extensive database of cosmetics and personal care products.
“At this point, there is very little regulatory activity related to PFAS in cosmetics,” Dr. Sunderland said. “The best thing to happen now would be for consumers to indicate that they prefer products without PFAS and to demand better transparency in product ingredient lists.”
A similar study done in 2018 by the Danish Environmental Protection Agency found high levels of PFAS in nearly one-third of the cosmetics products it tested.
People can also be exposed to PFAS by eating or drinking contaminated food or water and through food packaging. Dr. Sunderland said some wild foods like seafood are known to accumulate these compounds in the environment.
“There are examples of contaminated biosolids leading to accumulation of PFAS in vegetables and milk,” Dr. Sunderland explained. “Food packaging is another concern because it can also result in PFAS accumulation in the foods we eat.”
Although it’s difficult to avoid PFAS altogether, the CDC suggests lowering exposure rates by avoiding contaminated water and food. If you’re not sure if your water is contaminated, you should ask your local or state health and environmental quality departments for fish or water advisories in your area.
A version of this article first appeared on WebMD.com.
People may be absorbing and ingesting potentially toxic chemicals from their cosmetic products, a new study suggests.
– per- and polyfluoroalkyl substances. Many of these chemicals were not included on the product labels, making it difficult for consumers to consciously avoid them.
“This study is very helpful for elucidating the PFAS content of different types of cosmetics in the U.S. and Canadian markets,” said Elsie Sunderland, PhD, an environmental scientist who was not involved with the study.
“Previously, all the data had been collected in Europe, and this study shows we are dealing with similar problems in the North American marketplace,” said Dr. Sunderland, a professor of environmental chemistry at the Harvard School of Public Health, Boston.
PFAS are a class of chemicals used in a variety of consumer products, such as nonstick cookware, stain-resistant carpeting, and water-repellent clothing, according to the Centers for Disease Control and Prevention. They are added to cosmetics to make the products more durable and spreadable, researchers said in the study.
“[PFAS] are added to change the properties of surfaces, to make them nonstick or resistant to stay in water or oils,” said study coauthor Tom Bruton, PhD, senior scientist at the Green Science Policy Institute in Berkeley, Calif. “The concerning thing about cosmetics is that these are products that you’re applying to your skin and face every day, so there’s the skin absorption route that’s of concern, but also incidental ingestion of cosmetics is also a concern as well.”
The CDC says some of the potential health effects of PFAS exposure includes increased cholesterol levels, increased risk of kidney and testicular cancer, changes in liver enzymes, decreased vaccine response in children, and a higher risk of high blood pressure or preeclampsia in pregnant women.
“PFAS are a large class of chemicals. In humans, exposure to some of these chemicals has been associated with impaired immune function, certain cancers, increased risks of diabetes, obesity and endocrine disruption,” Dr. Sunderland said. “They appear to be harmful to every major organ system in the human body.”
For the current study, published online in Environmental Science & Technology Letters, Dr. Bruton and colleagues purchased 231 cosmetic products in the United States and Canada from retailers such as Ulta Beauty, Sephora, Target, and Bed Bath & Beyond. They then screened them for fluorine.Three-quarters of waterproof mascara samples contained high fluorine concentrations, as did nearly two-thirds of foundations and liquid lipsticks, and more than half of the eye and lip products tested.
The authors found that different categories of makeup tended to have higher or lower fluorine concentrations. “High fluorine levels were found in products commonly advertised as ‘wear-resistant’ to water and oils or ‘long-lasting,’ including foundations, liquid lipsticks, and waterproof mascaras,” Dr. Bruton and colleagues wrote.
When they further analyzed a subset of 29 products to determine what types of chemicals were present, they found that each cosmetic product contained at least 4 PFAS, with one product containing 13.The PFAS substances found included some that break down into other chemicals that are known to be highly toxic and environmentally harmful.
“It’s concerning that some of the products we tested appear to be intentionally using PFAS, but not listing those ingredients on the label,” Dr. Bruton said. “I do think that it is helpful for consumers to read labels, but beyond that, there’s not a lot of ways that consumers themselves can solve this problem. ... We think that the industry needs to be more proactive about moving away from this group of chemicals.”
Dr. Sunderland said a resource people can use when trying to avoid PFAS is the Environmental Working Group, a nonprofit organization that maintains an extensive database of cosmetics and personal care products.
“At this point, there is very little regulatory activity related to PFAS in cosmetics,” Dr. Sunderland said. “The best thing to happen now would be for consumers to indicate that they prefer products without PFAS and to demand better transparency in product ingredient lists.”
A similar study done in 2018 by the Danish Environmental Protection Agency found high levels of PFAS in nearly one-third of the cosmetics products it tested.
People can also be exposed to PFAS by eating or drinking contaminated food or water and through food packaging. Dr. Sunderland said some wild foods like seafood are known to accumulate these compounds in the environment.
“There are examples of contaminated biosolids leading to accumulation of PFAS in vegetables and milk,” Dr. Sunderland explained. “Food packaging is another concern because it can also result in PFAS accumulation in the foods we eat.”
Although it’s difficult to avoid PFAS altogether, the CDC suggests lowering exposure rates by avoiding contaminated water and food. If you’re not sure if your water is contaminated, you should ask your local or state health and environmental quality departments for fish or water advisories in your area.
A version of this article first appeared on WebMD.com.
People may be absorbing and ingesting potentially toxic chemicals from their cosmetic products, a new study suggests.
– per- and polyfluoroalkyl substances. Many of these chemicals were not included on the product labels, making it difficult for consumers to consciously avoid them.
“This study is very helpful for elucidating the PFAS content of different types of cosmetics in the U.S. and Canadian markets,” said Elsie Sunderland, PhD, an environmental scientist who was not involved with the study.
“Previously, all the data had been collected in Europe, and this study shows we are dealing with similar problems in the North American marketplace,” said Dr. Sunderland, a professor of environmental chemistry at the Harvard School of Public Health, Boston.
PFAS are a class of chemicals used in a variety of consumer products, such as nonstick cookware, stain-resistant carpeting, and water-repellent clothing, according to the Centers for Disease Control and Prevention. They are added to cosmetics to make the products more durable and spreadable, researchers said in the study.
“[PFAS] are added to change the properties of surfaces, to make them nonstick or resistant to stay in water or oils,” said study coauthor Tom Bruton, PhD, senior scientist at the Green Science Policy Institute in Berkeley, Calif. “The concerning thing about cosmetics is that these are products that you’re applying to your skin and face every day, so there’s the skin absorption route that’s of concern, but also incidental ingestion of cosmetics is also a concern as well.”
The CDC says some of the potential health effects of PFAS exposure includes increased cholesterol levels, increased risk of kidney and testicular cancer, changes in liver enzymes, decreased vaccine response in children, and a higher risk of high blood pressure or preeclampsia in pregnant women.
“PFAS are a large class of chemicals. In humans, exposure to some of these chemicals has been associated with impaired immune function, certain cancers, increased risks of diabetes, obesity and endocrine disruption,” Dr. Sunderland said. “They appear to be harmful to every major organ system in the human body.”
For the current study, published online in Environmental Science & Technology Letters, Dr. Bruton and colleagues purchased 231 cosmetic products in the United States and Canada from retailers such as Ulta Beauty, Sephora, Target, and Bed Bath & Beyond. They then screened them for fluorine.Three-quarters of waterproof mascara samples contained high fluorine concentrations, as did nearly two-thirds of foundations and liquid lipsticks, and more than half of the eye and lip products tested.
The authors found that different categories of makeup tended to have higher or lower fluorine concentrations. “High fluorine levels were found in products commonly advertised as ‘wear-resistant’ to water and oils or ‘long-lasting,’ including foundations, liquid lipsticks, and waterproof mascaras,” Dr. Bruton and colleagues wrote.
When they further analyzed a subset of 29 products to determine what types of chemicals were present, they found that each cosmetic product contained at least 4 PFAS, with one product containing 13.The PFAS substances found included some that break down into other chemicals that are known to be highly toxic and environmentally harmful.
“It’s concerning that some of the products we tested appear to be intentionally using PFAS, but not listing those ingredients on the label,” Dr. Bruton said. “I do think that it is helpful for consumers to read labels, but beyond that, there’s not a lot of ways that consumers themselves can solve this problem. ... We think that the industry needs to be more proactive about moving away from this group of chemicals.”
Dr. Sunderland said a resource people can use when trying to avoid PFAS is the Environmental Working Group, a nonprofit organization that maintains an extensive database of cosmetics and personal care products.
“At this point, there is very little regulatory activity related to PFAS in cosmetics,” Dr. Sunderland said. “The best thing to happen now would be for consumers to indicate that they prefer products without PFAS and to demand better transparency in product ingredient lists.”
A similar study done in 2018 by the Danish Environmental Protection Agency found high levels of PFAS in nearly one-third of the cosmetics products it tested.
People can also be exposed to PFAS by eating or drinking contaminated food or water and through food packaging. Dr. Sunderland said some wild foods like seafood are known to accumulate these compounds in the environment.
“There are examples of contaminated biosolids leading to accumulation of PFAS in vegetables and milk,” Dr. Sunderland explained. “Food packaging is another concern because it can also result in PFAS accumulation in the foods we eat.”
Although it’s difficult to avoid PFAS altogether, the CDC suggests lowering exposure rates by avoiding contaminated water and food. If you’re not sure if your water is contaminated, you should ask your local or state health and environmental quality departments for fish or water advisories in your area.
A version of this article first appeared on WebMD.com.
Watchdog group demands removal of FDA leaders after aducanumab approval
In a letter to the U.S. Department of Health and Human Services Secretary Xavier Becerra, Michael A. Carome, MD, director of Public Citizen’s Health Research Group, said: “The FDA’s decision to approve aducanumab for anyone with Alzheimer’s disease, regardless of severity, showed a stunning disregard for science, eviscerated the agency’s standards for approving new drugs, and ranks as one of the most irresponsible and egregious decisions in the history of the agency.”
Public Citizen urged Mr. Becerra to seek the resignations or the removal of the three FDA officials it said were most responsible for the approval – Acting FDA Commissioner Janet Woodcock, MD; Center for Drug Evaluation and Research (CDER) Director Patrizia Cavazzoni, MD; and CDER’s Office of Neuroscience Director Billy Dunn, MD.
“This decision is a disastrous blow to the agency’s credibility, public health, and the financial sustainability of the Medicare program,” writes Dr. Carome, noting that Biogen said it would charge $56,000 annually for the infusion.
Aaron Kesselheim, MD, one of three FDA Peripheral and Central Nervous System Drugs advisory committee members who resigned in the wake of the approval, agreed with Public Citizen that the agency’s credibility is suffering.
“The aducanumab decision is the worst example yet of the FDA’s movement away from its high standards,” Dr. Kesselheim, a professor of medicine at Harvard Medical School, Boston, and Harvard colleague Jerry Avorn, MD, wrote in the New York Times on June 15.
“As physicians, we know well that Alzheimer’s disease is a terrible condition,” they wrote. However, they added, “approving a drug that has such poor evidence that it works and causes such worrisome side effects is not the solution.”
In his resignation letter, Dr. Kesselheim said he had also been dismayed by the agency’s 2016 approval of eteplirsen (Exondys 51, Sarepta Therapeutics) for Duchenne muscular dystrophy. In both the eteplirsen and aducanumab approvals, the agency went against its advisers’ recommendations, Dr. Kesselheim said.
Advocates who backed approval decry cost
Aducanumab had a rocky road to approval but had unwavering backing from the Alzheimer’s Association and at least one other organization, UsAgainstAlzheimer’s.
The Alzheimer’s Association was particularly outspoken in its support and, in March, was accused of potential conflict of interest by Public Citizen and several neurologists because the association accepted at least $1.4 million from Biogen and its partner Eisai since fiscal year 2018.
The association applauded the FDA approval but, a few days later, expressed outrage over the $56,000-a-year price tag.
“This price is simply unacceptable,” the Alzheimer’s Association said in the statement. “For many, this price will pose an insurmountable barrier to access, it complicates and jeopardizes sustainable access to this treatment, and may further deepen issues of health equity,” the association said, adding, “We call on Biogen to change this price.”
UsAgainstAlzheimer’s also expressed concerns about access, even before it knew aducanumab’s price.
“Shockingly, Medicare does not reimburse patients for the expensive PET scans important to determine whether someone is appropriate for this drug,” noted George Vradenburg, chairman and cofounder of the group, in a June 7 statement. “We intend to work with Biogen and Medicare to make access to this drug affordable for every American who needs it,” Mr. Vradenburg said.
Dr. Carome said the advocates’ complaints were hard to fathom.
“This should not have come as a surprise to anyone,” Dr. Carome said, adding that “it’s essentially the ballpark figure the company threw out weeks ago.”
“Fifty-six-thousand-dollars is particularly egregiously overpriced for a drug that doesn’t work,” Dr. Carome said. “If the [Alzheimer’s Association] truly finds this objectionable, hopefully they’ll stop accepting money from Biogen and its partner Eisai,” he added.
“The Alzheimer’s Association is recognizing that the genie is out of the bottle and that they are going to have trouble reining in the inevitable run-away costs,” said Mike Greicius, MD, MPH, associate professor of neurology at Stanford University’s Wu Tsai Neurosciences Institute, Stanford, California.
“In addition to the eye-popping annual cost that Biogen has invented, I hope the Alzheimer’s Association is also concerned about the dangerously loose and broad FDA labeling which does not require screening for amyloid-positivity and does not restrict use to the milder forms of disease studied in the Phase 3 trials,” Dr. Greicius said.
Another advocacy group, Patients For Affordable Drugs, commended the Alzheimer’s Association. Its statement “was nothing short of courageous, especially in light of the Alzheimer’s Association’s reliance on funding from drug corporations, including Biogen,” said David Mitchell, a cancer patient and founder of Patients For Affordable Drugs, in a statement.
Mr. Mitchell said his members “stand with the Alzheimer’s Association in its denunciation of the price set by Biogen” and called for a new law that would allow Medicare to negotiate drug prices.
A version of this article first appeared on Medscape.com.
In a letter to the U.S. Department of Health and Human Services Secretary Xavier Becerra, Michael A. Carome, MD, director of Public Citizen’s Health Research Group, said: “The FDA’s decision to approve aducanumab for anyone with Alzheimer’s disease, regardless of severity, showed a stunning disregard for science, eviscerated the agency’s standards for approving new drugs, and ranks as one of the most irresponsible and egregious decisions in the history of the agency.”
Public Citizen urged Mr. Becerra to seek the resignations or the removal of the three FDA officials it said were most responsible for the approval – Acting FDA Commissioner Janet Woodcock, MD; Center for Drug Evaluation and Research (CDER) Director Patrizia Cavazzoni, MD; and CDER’s Office of Neuroscience Director Billy Dunn, MD.
“This decision is a disastrous blow to the agency’s credibility, public health, and the financial sustainability of the Medicare program,” writes Dr. Carome, noting that Biogen said it would charge $56,000 annually for the infusion.
Aaron Kesselheim, MD, one of three FDA Peripheral and Central Nervous System Drugs advisory committee members who resigned in the wake of the approval, agreed with Public Citizen that the agency’s credibility is suffering.
“The aducanumab decision is the worst example yet of the FDA’s movement away from its high standards,” Dr. Kesselheim, a professor of medicine at Harvard Medical School, Boston, and Harvard colleague Jerry Avorn, MD, wrote in the New York Times on June 15.
“As physicians, we know well that Alzheimer’s disease is a terrible condition,” they wrote. However, they added, “approving a drug that has such poor evidence that it works and causes such worrisome side effects is not the solution.”
In his resignation letter, Dr. Kesselheim said he had also been dismayed by the agency’s 2016 approval of eteplirsen (Exondys 51, Sarepta Therapeutics) for Duchenne muscular dystrophy. In both the eteplirsen and aducanumab approvals, the agency went against its advisers’ recommendations, Dr. Kesselheim said.
Advocates who backed approval decry cost
Aducanumab had a rocky road to approval but had unwavering backing from the Alzheimer’s Association and at least one other organization, UsAgainstAlzheimer’s.
The Alzheimer’s Association was particularly outspoken in its support and, in March, was accused of potential conflict of interest by Public Citizen and several neurologists because the association accepted at least $1.4 million from Biogen and its partner Eisai since fiscal year 2018.
The association applauded the FDA approval but, a few days later, expressed outrage over the $56,000-a-year price tag.
“This price is simply unacceptable,” the Alzheimer’s Association said in the statement. “For many, this price will pose an insurmountable barrier to access, it complicates and jeopardizes sustainable access to this treatment, and may further deepen issues of health equity,” the association said, adding, “We call on Biogen to change this price.”
UsAgainstAlzheimer’s also expressed concerns about access, even before it knew aducanumab’s price.
“Shockingly, Medicare does not reimburse patients for the expensive PET scans important to determine whether someone is appropriate for this drug,” noted George Vradenburg, chairman and cofounder of the group, in a June 7 statement. “We intend to work with Biogen and Medicare to make access to this drug affordable for every American who needs it,” Mr. Vradenburg said.
Dr. Carome said the advocates’ complaints were hard to fathom.
“This should not have come as a surprise to anyone,” Dr. Carome said, adding that “it’s essentially the ballpark figure the company threw out weeks ago.”
“Fifty-six-thousand-dollars is particularly egregiously overpriced for a drug that doesn’t work,” Dr. Carome said. “If the [Alzheimer’s Association] truly finds this objectionable, hopefully they’ll stop accepting money from Biogen and its partner Eisai,” he added.
“The Alzheimer’s Association is recognizing that the genie is out of the bottle and that they are going to have trouble reining in the inevitable run-away costs,” said Mike Greicius, MD, MPH, associate professor of neurology at Stanford University’s Wu Tsai Neurosciences Institute, Stanford, California.
“In addition to the eye-popping annual cost that Biogen has invented, I hope the Alzheimer’s Association is also concerned about the dangerously loose and broad FDA labeling which does not require screening for amyloid-positivity and does not restrict use to the milder forms of disease studied in the Phase 3 trials,” Dr. Greicius said.
Another advocacy group, Patients For Affordable Drugs, commended the Alzheimer’s Association. Its statement “was nothing short of courageous, especially in light of the Alzheimer’s Association’s reliance on funding from drug corporations, including Biogen,” said David Mitchell, a cancer patient and founder of Patients For Affordable Drugs, in a statement.
Mr. Mitchell said his members “stand with the Alzheimer’s Association in its denunciation of the price set by Biogen” and called for a new law that would allow Medicare to negotiate drug prices.
A version of this article first appeared on Medscape.com.
In a letter to the U.S. Department of Health and Human Services Secretary Xavier Becerra, Michael A. Carome, MD, director of Public Citizen’s Health Research Group, said: “The FDA’s decision to approve aducanumab for anyone with Alzheimer’s disease, regardless of severity, showed a stunning disregard for science, eviscerated the agency’s standards for approving new drugs, and ranks as one of the most irresponsible and egregious decisions in the history of the agency.”
Public Citizen urged Mr. Becerra to seek the resignations or the removal of the three FDA officials it said were most responsible for the approval – Acting FDA Commissioner Janet Woodcock, MD; Center for Drug Evaluation and Research (CDER) Director Patrizia Cavazzoni, MD; and CDER’s Office of Neuroscience Director Billy Dunn, MD.
“This decision is a disastrous blow to the agency’s credibility, public health, and the financial sustainability of the Medicare program,” writes Dr. Carome, noting that Biogen said it would charge $56,000 annually for the infusion.
Aaron Kesselheim, MD, one of three FDA Peripheral and Central Nervous System Drugs advisory committee members who resigned in the wake of the approval, agreed with Public Citizen that the agency’s credibility is suffering.
“The aducanumab decision is the worst example yet of the FDA’s movement away from its high standards,” Dr. Kesselheim, a professor of medicine at Harvard Medical School, Boston, and Harvard colleague Jerry Avorn, MD, wrote in the New York Times on June 15.
“As physicians, we know well that Alzheimer’s disease is a terrible condition,” they wrote. However, they added, “approving a drug that has such poor evidence that it works and causes such worrisome side effects is not the solution.”
In his resignation letter, Dr. Kesselheim said he had also been dismayed by the agency’s 2016 approval of eteplirsen (Exondys 51, Sarepta Therapeutics) for Duchenne muscular dystrophy. In both the eteplirsen and aducanumab approvals, the agency went against its advisers’ recommendations, Dr. Kesselheim said.
Advocates who backed approval decry cost
Aducanumab had a rocky road to approval but had unwavering backing from the Alzheimer’s Association and at least one other organization, UsAgainstAlzheimer’s.
The Alzheimer’s Association was particularly outspoken in its support and, in March, was accused of potential conflict of interest by Public Citizen and several neurologists because the association accepted at least $1.4 million from Biogen and its partner Eisai since fiscal year 2018.
The association applauded the FDA approval but, a few days later, expressed outrage over the $56,000-a-year price tag.
“This price is simply unacceptable,” the Alzheimer’s Association said in the statement. “For many, this price will pose an insurmountable barrier to access, it complicates and jeopardizes sustainable access to this treatment, and may further deepen issues of health equity,” the association said, adding, “We call on Biogen to change this price.”
UsAgainstAlzheimer’s also expressed concerns about access, even before it knew aducanumab’s price.
“Shockingly, Medicare does not reimburse patients for the expensive PET scans important to determine whether someone is appropriate for this drug,” noted George Vradenburg, chairman and cofounder of the group, in a June 7 statement. “We intend to work with Biogen and Medicare to make access to this drug affordable for every American who needs it,” Mr. Vradenburg said.
Dr. Carome said the advocates’ complaints were hard to fathom.
“This should not have come as a surprise to anyone,” Dr. Carome said, adding that “it’s essentially the ballpark figure the company threw out weeks ago.”
“Fifty-six-thousand-dollars is particularly egregiously overpriced for a drug that doesn’t work,” Dr. Carome said. “If the [Alzheimer’s Association] truly finds this objectionable, hopefully they’ll stop accepting money from Biogen and its partner Eisai,” he added.
“The Alzheimer’s Association is recognizing that the genie is out of the bottle and that they are going to have trouble reining in the inevitable run-away costs,” said Mike Greicius, MD, MPH, associate professor of neurology at Stanford University’s Wu Tsai Neurosciences Institute, Stanford, California.
“In addition to the eye-popping annual cost that Biogen has invented, I hope the Alzheimer’s Association is also concerned about the dangerously loose and broad FDA labeling which does not require screening for amyloid-positivity and does not restrict use to the milder forms of disease studied in the Phase 3 trials,” Dr. Greicius said.
Another advocacy group, Patients For Affordable Drugs, commended the Alzheimer’s Association. Its statement “was nothing short of courageous, especially in light of the Alzheimer’s Association’s reliance on funding from drug corporations, including Biogen,” said David Mitchell, a cancer patient and founder of Patients For Affordable Drugs, in a statement.
Mr. Mitchell said his members “stand with the Alzheimer’s Association in its denunciation of the price set by Biogen” and called for a new law that would allow Medicare to negotiate drug prices.
A version of this article first appeared on Medscape.com.
Incorporating self-care, wellness into routines can prevent doctors’ burnout
Gradually, we are emerging from the chaos, isolation, and anxiety of COVID-19. As the Centers for Disease Control and Prevention adjusts its recommendations and vaccinations become more widely available, our communities are beginning to return to normalcy. We are encouraged to put aside our masks if vaccinated and rejoin society, to venture out with less hesitancy and anxiety. As family and friends reunite, memories of confusion, frustration, and fear are beginning to fade to black. Despite the prevailing belief that we should move on, look forward, and remember the past to safeguard our future, remnants of the pandemic remain.
Unvaccinated individuals, notably children under the age of 12, are quite significant in number. The use of telehealth is now standard practice.
For several years, we were warned about the looming “mental health crisis.” The past year has demonstrated that a crisis no longer looms – it has arrived. Our patients can reveal the vulnerability COVID-19 has wrought – from the devastation of lives lost, supply shortages, loss of employment and financial stability – to a lack of access to computers and thereby, the risk of educational decline. Those factors, coupled with isolation and uncertainty about the future, have led to an influx of individuals with anxiety, depression, and other mood disorders seeking mental health treatment.
Doctors, others suffering
As result of a medical culture guided by the sacred oath to which care, compassion, and dedication held as true in ancient Greece as it does today, the focus centers on those around us – while signs of our own weariness are waved away as “a bad day.” Even though several support groups are readily available to offer a listening ear and mental health physicians who focus on the treatment of health care professionals are becoming more ubiquitous, the vestiges of past doctrine remain.
In this modern age of medical training, there is often as much sacrifice as there is attainment of knowledge. This philosophy is so ingrained that throughout training and practice one may come across colleagues experiencing an abundance of guilt when leave is needed for personal reasons. We are quick to recommend such steps for our patients, family, and friends, but hesitant to consider such for ourselves. Yet, of all the lessons this past year has wrought, the importance of mental health and self-care cannot be overstated. This raises the question:
It is vital to accept our humanity as something not to repair, treat, or overcome but to understand. There is strength and power in vulnerability. If we do not perceive and validate this process within ourselves, how can we do so for others? In other words, the oxygen mask must be placed on us first before we can place it on anyone else – patients or otherwise.
Chiefly and above all else, the importance of identifying individual signs of stress is essential. Where do you hold tension? Are you prone to GI distress or headaches when taxed? Do you tend toward irritability, apathy, or exhaustion?
Once this is determined, it is important to assess your stress on a numerical scale, such as those used for pain. Are you a 5 or an 8? Finally, are there identifiable triggers or reliable alleviators? Is there a time of day or day of the week that is most difficult to manage? Can you anticipate potential stressors? Understanding your triggers, listening to your body, and practicing the language of self is the first step toward wellness.
Following introspection and observation, the next step is inventory. Take stock of your reserves. What replenishes? What depletes? What brings joy? What brings dread? Are there certain activities that mitigate stress? If so, how much time do they entail? Identify your number on a scale and associate that number with specific strategies or techniques. Remember that decompression for a 6 might be excessive for a 4. Furthermore, what is the duration of these feelings? Chronic stressors may incur gradual change verses sudden impact if acute. Through identifying personal signs, devising and using a scale, as well as escalating or de-escalating factors, individuals become more in tune with their bodies and therefore, more likely to intervene before burnout takes hold.
With this process well integrated, one can now consider stylized approaches for stress management. For example, those inclined toward mindfulness practices may find yoga, meditation, and relaxation exercises beneficial. Others may thrive on positive affirmations, gratitude, and thankfulness. While some might find relief in physical activity, be it strenuous or casual, the creative arts might appeal to those who find joy in painting, writing, or doing crafts. In addition, baking, reading, dancing, and/or listening to music might help lift stress.
Along with those discoveries, or in some cases, rediscoveries, basic needs such as dietary habits and nutrition, hydration, and sleep are vital toward emotional regulation, physiological homeostasis, and stress modulation. Remember HALT: Hungry, Angry, Lonely, Tired, Too hot, Too cold, Sad or Stressed. Those strategies are meant to guide self-care and highlight the importance of allowing time for self-awareness. Imagine yourself as if you are meeting a new patient. Establish rapport, identify symptoms, and explore options for treatment. When we give time to ourselves, we can give time more freely to others. With this in mind, try following the 5-minute wellness check that I formulated:
1. How am I feeling? What am I feeling?
2. Assess HALTS.
3. Identify the number on your scale.
4. Methods of quick de-escalation:
- Designate and schedule personal time.
- Write down daily goals.
- Repeat positive affirmations or write down words of gratitude.
- Use deep breathing exercises.
- Stretch or take a brief walk.
- Engage in mindfulness practices, such as meditation.
Once we develop a habit of monitoring, assessing, and practicing self-care, the process becomes more efficient and effective. Think of the way a seasoned attending can manage workflow with ease, compared with an intern. Recognizing signs and using these strategies routinely can become a quick daily measure of well-being.
Dr. Thomas is a board-certified adult psychiatrist with interests in chronic illness, women’s behavioral health, and minority mental health. She currently practices in North Kingstown and East Providence, R.I. Dr. Thomas has no conflicts of interest.
Gradually, we are emerging from the chaos, isolation, and anxiety of COVID-19. As the Centers for Disease Control and Prevention adjusts its recommendations and vaccinations become more widely available, our communities are beginning to return to normalcy. We are encouraged to put aside our masks if vaccinated and rejoin society, to venture out with less hesitancy and anxiety. As family and friends reunite, memories of confusion, frustration, and fear are beginning to fade to black. Despite the prevailing belief that we should move on, look forward, and remember the past to safeguard our future, remnants of the pandemic remain.
Unvaccinated individuals, notably children under the age of 12, are quite significant in number. The use of telehealth is now standard practice.
For several years, we were warned about the looming “mental health crisis.” The past year has demonstrated that a crisis no longer looms – it has arrived. Our patients can reveal the vulnerability COVID-19 has wrought – from the devastation of lives lost, supply shortages, loss of employment and financial stability – to a lack of access to computers and thereby, the risk of educational decline. Those factors, coupled with isolation and uncertainty about the future, have led to an influx of individuals with anxiety, depression, and other mood disorders seeking mental health treatment.
Doctors, others suffering
As result of a medical culture guided by the sacred oath to which care, compassion, and dedication held as true in ancient Greece as it does today, the focus centers on those around us – while signs of our own weariness are waved away as “a bad day.” Even though several support groups are readily available to offer a listening ear and mental health physicians who focus on the treatment of health care professionals are becoming more ubiquitous, the vestiges of past doctrine remain.
In this modern age of medical training, there is often as much sacrifice as there is attainment of knowledge. This philosophy is so ingrained that throughout training and practice one may come across colleagues experiencing an abundance of guilt when leave is needed for personal reasons. We are quick to recommend such steps for our patients, family, and friends, but hesitant to consider such for ourselves. Yet, of all the lessons this past year has wrought, the importance of mental health and self-care cannot be overstated. This raises the question:
It is vital to accept our humanity as something not to repair, treat, or overcome but to understand. There is strength and power in vulnerability. If we do not perceive and validate this process within ourselves, how can we do so for others? In other words, the oxygen mask must be placed on us first before we can place it on anyone else – patients or otherwise.
Chiefly and above all else, the importance of identifying individual signs of stress is essential. Where do you hold tension? Are you prone to GI distress or headaches when taxed? Do you tend toward irritability, apathy, or exhaustion?
Once this is determined, it is important to assess your stress on a numerical scale, such as those used for pain. Are you a 5 or an 8? Finally, are there identifiable triggers or reliable alleviators? Is there a time of day or day of the week that is most difficult to manage? Can you anticipate potential stressors? Understanding your triggers, listening to your body, and practicing the language of self is the first step toward wellness.
Following introspection and observation, the next step is inventory. Take stock of your reserves. What replenishes? What depletes? What brings joy? What brings dread? Are there certain activities that mitigate stress? If so, how much time do they entail? Identify your number on a scale and associate that number with specific strategies or techniques. Remember that decompression for a 6 might be excessive for a 4. Furthermore, what is the duration of these feelings? Chronic stressors may incur gradual change verses sudden impact if acute. Through identifying personal signs, devising and using a scale, as well as escalating or de-escalating factors, individuals become more in tune with their bodies and therefore, more likely to intervene before burnout takes hold.
With this process well integrated, one can now consider stylized approaches for stress management. For example, those inclined toward mindfulness practices may find yoga, meditation, and relaxation exercises beneficial. Others may thrive on positive affirmations, gratitude, and thankfulness. While some might find relief in physical activity, be it strenuous or casual, the creative arts might appeal to those who find joy in painting, writing, or doing crafts. In addition, baking, reading, dancing, and/or listening to music might help lift stress.
Along with those discoveries, or in some cases, rediscoveries, basic needs such as dietary habits and nutrition, hydration, and sleep are vital toward emotional regulation, physiological homeostasis, and stress modulation. Remember HALT: Hungry, Angry, Lonely, Tired, Too hot, Too cold, Sad or Stressed. Those strategies are meant to guide self-care and highlight the importance of allowing time for self-awareness. Imagine yourself as if you are meeting a new patient. Establish rapport, identify symptoms, and explore options for treatment. When we give time to ourselves, we can give time more freely to others. With this in mind, try following the 5-minute wellness check that I formulated:
1. How am I feeling? What am I feeling?
2. Assess HALTS.
3. Identify the number on your scale.
4. Methods of quick de-escalation:
- Designate and schedule personal time.
- Write down daily goals.
- Repeat positive affirmations or write down words of gratitude.
- Use deep breathing exercises.
- Stretch or take a brief walk.
- Engage in mindfulness practices, such as meditation.
Once we develop a habit of monitoring, assessing, and practicing self-care, the process becomes more efficient and effective. Think of the way a seasoned attending can manage workflow with ease, compared with an intern. Recognizing signs and using these strategies routinely can become a quick daily measure of well-being.
Dr. Thomas is a board-certified adult psychiatrist with interests in chronic illness, women’s behavioral health, and minority mental health. She currently practices in North Kingstown and East Providence, R.I. Dr. Thomas has no conflicts of interest.
Gradually, we are emerging from the chaos, isolation, and anxiety of COVID-19. As the Centers for Disease Control and Prevention adjusts its recommendations and vaccinations become more widely available, our communities are beginning to return to normalcy. We are encouraged to put aside our masks if vaccinated and rejoin society, to venture out with less hesitancy and anxiety. As family and friends reunite, memories of confusion, frustration, and fear are beginning to fade to black. Despite the prevailing belief that we should move on, look forward, and remember the past to safeguard our future, remnants of the pandemic remain.
Unvaccinated individuals, notably children under the age of 12, are quite significant in number. The use of telehealth is now standard practice.
For several years, we were warned about the looming “mental health crisis.” The past year has demonstrated that a crisis no longer looms – it has arrived. Our patients can reveal the vulnerability COVID-19 has wrought – from the devastation of lives lost, supply shortages, loss of employment and financial stability – to a lack of access to computers and thereby, the risk of educational decline. Those factors, coupled with isolation and uncertainty about the future, have led to an influx of individuals with anxiety, depression, and other mood disorders seeking mental health treatment.
Doctors, others suffering
As result of a medical culture guided by the sacred oath to which care, compassion, and dedication held as true in ancient Greece as it does today, the focus centers on those around us – while signs of our own weariness are waved away as “a bad day.” Even though several support groups are readily available to offer a listening ear and mental health physicians who focus on the treatment of health care professionals are becoming more ubiquitous, the vestiges of past doctrine remain.
In this modern age of medical training, there is often as much sacrifice as there is attainment of knowledge. This philosophy is so ingrained that throughout training and practice one may come across colleagues experiencing an abundance of guilt when leave is needed for personal reasons. We are quick to recommend such steps for our patients, family, and friends, but hesitant to consider such for ourselves. Yet, of all the lessons this past year has wrought, the importance of mental health and self-care cannot be overstated. This raises the question:
It is vital to accept our humanity as something not to repair, treat, or overcome but to understand. There is strength and power in vulnerability. If we do not perceive and validate this process within ourselves, how can we do so for others? In other words, the oxygen mask must be placed on us first before we can place it on anyone else – patients or otherwise.
Chiefly and above all else, the importance of identifying individual signs of stress is essential. Where do you hold tension? Are you prone to GI distress or headaches when taxed? Do you tend toward irritability, apathy, or exhaustion?
Once this is determined, it is important to assess your stress on a numerical scale, such as those used for pain. Are you a 5 or an 8? Finally, are there identifiable triggers or reliable alleviators? Is there a time of day or day of the week that is most difficult to manage? Can you anticipate potential stressors? Understanding your triggers, listening to your body, and practicing the language of self is the first step toward wellness.
Following introspection and observation, the next step is inventory. Take stock of your reserves. What replenishes? What depletes? What brings joy? What brings dread? Are there certain activities that mitigate stress? If so, how much time do they entail? Identify your number on a scale and associate that number with specific strategies or techniques. Remember that decompression for a 6 might be excessive for a 4. Furthermore, what is the duration of these feelings? Chronic stressors may incur gradual change verses sudden impact if acute. Through identifying personal signs, devising and using a scale, as well as escalating or de-escalating factors, individuals become more in tune with their bodies and therefore, more likely to intervene before burnout takes hold.
With this process well integrated, one can now consider stylized approaches for stress management. For example, those inclined toward mindfulness practices may find yoga, meditation, and relaxation exercises beneficial. Others may thrive on positive affirmations, gratitude, and thankfulness. While some might find relief in physical activity, be it strenuous or casual, the creative arts might appeal to those who find joy in painting, writing, or doing crafts. In addition, baking, reading, dancing, and/or listening to music might help lift stress.
Along with those discoveries, or in some cases, rediscoveries, basic needs such as dietary habits and nutrition, hydration, and sleep are vital toward emotional regulation, physiological homeostasis, and stress modulation. Remember HALT: Hungry, Angry, Lonely, Tired, Too hot, Too cold, Sad or Stressed. Those strategies are meant to guide self-care and highlight the importance of allowing time for self-awareness. Imagine yourself as if you are meeting a new patient. Establish rapport, identify symptoms, and explore options for treatment. When we give time to ourselves, we can give time more freely to others. With this in mind, try following the 5-minute wellness check that I formulated:
1. How am I feeling? What am I feeling?
2. Assess HALTS.
3. Identify the number on your scale.
4. Methods of quick de-escalation:
- Designate and schedule personal time.
- Write down daily goals.
- Repeat positive affirmations or write down words of gratitude.
- Use deep breathing exercises.
- Stretch or take a brief walk.
- Engage in mindfulness practices, such as meditation.
Once we develop a habit of monitoring, assessing, and practicing self-care, the process becomes more efficient and effective. Think of the way a seasoned attending can manage workflow with ease, compared with an intern. Recognizing signs and using these strategies routinely can become a quick daily measure of well-being.
Dr. Thomas is a board-certified adult psychiatrist with interests in chronic illness, women’s behavioral health, and minority mental health. She currently practices in North Kingstown and East Providence, R.I. Dr. Thomas has no conflicts of interest.
AMA acknowledges medical education racism of past, vows better future
The report received overwhelming support at the House of Delegates, the AMA’s legislative policy making body, during an online meeting held June 13.
The Council on Medical Education’s report recommends that the AMA acknowledge the harm caused by the Flexner Report, which was issued in 1910 and has since shaped medical education. The Flexner Report caused harm not only to historically Black medical schools, but also to physician workforce diversity and to the clinical outcomes of minority and marginalized patients, according to the medical education advisory body.
The council also recommended conducting a study on medical education with a focus on health equity and racial justice, improving diversity among healthcare workers, and fixing inequitable outcomes from minorities and marginalized patient populations.
The report comes on the heels of the resignation of JAMA editor-in-chief Howard Bauchner, MD, and another high-ranking editor following a February podcast on systemic racism in medicine. The AMA has since released a strategic plan addressing racism and health inequity that has divided membership.
Flexner Report’s effect on physician diversity
The Council on Medical Education’s report observed that as a result of the Flexner Report’s recommendations, 89 medical schools, including 5 of the 7 existing medical schools training Black physicians, were closed because they didn’t meet the report’s standards. In addition, the report created a limited role for Black physicians while “hint[ing] that Black physicians possessed less potential and ability than their White counterparts,” read the Council’s report.
In addition to consigning the role of the Black physician to “educating the [Black] race to know and to practice fundamental hygienic principles,” the Flexner Report also observed that “a well-taught negro sanitarian will be immensely useful,” per the Council’s report.
The impact of the closure of medical schools training Black physicians was dramatic. According to the Council’s report, in 1964, 93% of medical students in the United States were men and 97% of those students were non-Hispanic White.
Today, 56% of physicians identify as White, 17% as Asian, 6% as Hispanic, and 5% as Black or African American, per the Association of American Medical Colleges; nearly 14% of active physicians didn’t report their race in the survey. By means of contrast, the U.S. population in 2019 was 60% White, 19% Latino/Hispanic, 13% Black or African American, and 6% Asian American, according to the Brookings Institute.
Abraham Flexner, who wrote the Flexner Report, is often referred to as the “father of modern medical education,” according to the AAMC. In November, the AAMC observed that the Flexner Report contained racist and sexist ideas and that his work contributed to the closure of historically Black medical schools. Both statements were included in AAMC’s announcement about the removal of Flexner’s name from its most prestigious award. As of January, the award is now called the AAMC Award for Excellence in Medical Education.
Pathway programs can increase diversity
Pathway programs, which leverage targeted milestones along the journey to becoming a physician in order to increase diversity, were an area of focus in the council’s report. These programs “can exert a meaningful, positive effect on student outcomes and increase diversity across various levels of educational settings,” according to its report.
Centers of Excellence, which provides grants for mentorship and training programs, is one of many pathway programs. During the 2018-2019 academic year, Centers of Excellence supported more than 1,300 trainees – 99% of them were underrepresented minorities and 64% came from financially or educationally disadvantaged backgrounds. In 2006, federal funding was cut to these programs and the number of Centers of Excellence fell.
Still, the report cites the passage of federal funding in 2020 of $50 million for public institutions of higher education that train physicians; educational institutions in states with a projected primary care shortage in 2025 are given priority in the grant-funding process.
AMA council’s report garners support from delegates
Delegates voiced overwhelming support of the council’s report during the June 13 meeting. Lou Edje, MD, a Perrysburgh, Ohio–based family physician, voiced strong support for the council’s report, in particular its recommendations that recognize the harm caused by the Flexner Report. Dr. Edje observed that the Flexner Report, with its elimination of five of seven Black medical schools, “[set] back admissions of Black students into medicine by 50 years.”
“Empathy is what we are called to have as physicians. I implore you to simply substitute your ethnicity into these quotes to help understand the historic need for health equity in medicine today. This CME report is part of the antidote to Flexner. We support [it] fully,” concluded Dr. Edje, who spoke for the Great Lakes States Coalition of the AMA.
Rohan Khazanchi, a medical student at the University of Nebraska, Omaha, and a member of the council, said, “Our broad attempt with this report was twofold: to fill gaps in AMA policy with evidence-based recommendations which could improve diversity in our health workforce and, second, to enhance our organization’s vision for truth, reconciliation, and healing to redress the historic marginalization of minoritized physicians in medicine.”
According to an AMA spokesperson, the House of Delegates will vote on this and other policies this week, after which the policies are considered final.
A version of this article first appeared on Medscape.com.
The report received overwhelming support at the House of Delegates, the AMA’s legislative policy making body, during an online meeting held June 13.
The Council on Medical Education’s report recommends that the AMA acknowledge the harm caused by the Flexner Report, which was issued in 1910 and has since shaped medical education. The Flexner Report caused harm not only to historically Black medical schools, but also to physician workforce diversity and to the clinical outcomes of minority and marginalized patients, according to the medical education advisory body.
The council also recommended conducting a study on medical education with a focus on health equity and racial justice, improving diversity among healthcare workers, and fixing inequitable outcomes from minorities and marginalized patient populations.
The report comes on the heels of the resignation of JAMA editor-in-chief Howard Bauchner, MD, and another high-ranking editor following a February podcast on systemic racism in medicine. The AMA has since released a strategic plan addressing racism and health inequity that has divided membership.
Flexner Report’s effect on physician diversity
The Council on Medical Education’s report observed that as a result of the Flexner Report’s recommendations, 89 medical schools, including 5 of the 7 existing medical schools training Black physicians, were closed because they didn’t meet the report’s standards. In addition, the report created a limited role for Black physicians while “hint[ing] that Black physicians possessed less potential and ability than their White counterparts,” read the Council’s report.
In addition to consigning the role of the Black physician to “educating the [Black] race to know and to practice fundamental hygienic principles,” the Flexner Report also observed that “a well-taught negro sanitarian will be immensely useful,” per the Council’s report.
The impact of the closure of medical schools training Black physicians was dramatic. According to the Council’s report, in 1964, 93% of medical students in the United States were men and 97% of those students were non-Hispanic White.
Today, 56% of physicians identify as White, 17% as Asian, 6% as Hispanic, and 5% as Black or African American, per the Association of American Medical Colleges; nearly 14% of active physicians didn’t report their race in the survey. By means of contrast, the U.S. population in 2019 was 60% White, 19% Latino/Hispanic, 13% Black or African American, and 6% Asian American, according to the Brookings Institute.
Abraham Flexner, who wrote the Flexner Report, is often referred to as the “father of modern medical education,” according to the AAMC. In November, the AAMC observed that the Flexner Report contained racist and sexist ideas and that his work contributed to the closure of historically Black medical schools. Both statements were included in AAMC’s announcement about the removal of Flexner’s name from its most prestigious award. As of January, the award is now called the AAMC Award for Excellence in Medical Education.
Pathway programs can increase diversity
Pathway programs, which leverage targeted milestones along the journey to becoming a physician in order to increase diversity, were an area of focus in the council’s report. These programs “can exert a meaningful, positive effect on student outcomes and increase diversity across various levels of educational settings,” according to its report.
Centers of Excellence, which provides grants for mentorship and training programs, is one of many pathway programs. During the 2018-2019 academic year, Centers of Excellence supported more than 1,300 trainees – 99% of them were underrepresented minorities and 64% came from financially or educationally disadvantaged backgrounds. In 2006, federal funding was cut to these programs and the number of Centers of Excellence fell.
Still, the report cites the passage of federal funding in 2020 of $50 million for public institutions of higher education that train physicians; educational institutions in states with a projected primary care shortage in 2025 are given priority in the grant-funding process.
AMA council’s report garners support from delegates
Delegates voiced overwhelming support of the council’s report during the June 13 meeting. Lou Edje, MD, a Perrysburgh, Ohio–based family physician, voiced strong support for the council’s report, in particular its recommendations that recognize the harm caused by the Flexner Report. Dr. Edje observed that the Flexner Report, with its elimination of five of seven Black medical schools, “[set] back admissions of Black students into medicine by 50 years.”
“Empathy is what we are called to have as physicians. I implore you to simply substitute your ethnicity into these quotes to help understand the historic need for health equity in medicine today. This CME report is part of the antidote to Flexner. We support [it] fully,” concluded Dr. Edje, who spoke for the Great Lakes States Coalition of the AMA.
Rohan Khazanchi, a medical student at the University of Nebraska, Omaha, and a member of the council, said, “Our broad attempt with this report was twofold: to fill gaps in AMA policy with evidence-based recommendations which could improve diversity in our health workforce and, second, to enhance our organization’s vision for truth, reconciliation, and healing to redress the historic marginalization of minoritized physicians in medicine.”
According to an AMA spokesperson, the House of Delegates will vote on this and other policies this week, after which the policies are considered final.
A version of this article first appeared on Medscape.com.
The report received overwhelming support at the House of Delegates, the AMA’s legislative policy making body, during an online meeting held June 13.
The Council on Medical Education’s report recommends that the AMA acknowledge the harm caused by the Flexner Report, which was issued in 1910 and has since shaped medical education. The Flexner Report caused harm not only to historically Black medical schools, but also to physician workforce diversity and to the clinical outcomes of minority and marginalized patients, according to the medical education advisory body.
The council also recommended conducting a study on medical education with a focus on health equity and racial justice, improving diversity among healthcare workers, and fixing inequitable outcomes from minorities and marginalized patient populations.
The report comes on the heels of the resignation of JAMA editor-in-chief Howard Bauchner, MD, and another high-ranking editor following a February podcast on systemic racism in medicine. The AMA has since released a strategic plan addressing racism and health inequity that has divided membership.
Flexner Report’s effect on physician diversity
The Council on Medical Education’s report observed that as a result of the Flexner Report’s recommendations, 89 medical schools, including 5 of the 7 existing medical schools training Black physicians, were closed because they didn’t meet the report’s standards. In addition, the report created a limited role for Black physicians while “hint[ing] that Black physicians possessed less potential and ability than their White counterparts,” read the Council’s report.
In addition to consigning the role of the Black physician to “educating the [Black] race to know and to practice fundamental hygienic principles,” the Flexner Report also observed that “a well-taught negro sanitarian will be immensely useful,” per the Council’s report.
The impact of the closure of medical schools training Black physicians was dramatic. According to the Council’s report, in 1964, 93% of medical students in the United States were men and 97% of those students were non-Hispanic White.
Today, 56% of physicians identify as White, 17% as Asian, 6% as Hispanic, and 5% as Black or African American, per the Association of American Medical Colleges; nearly 14% of active physicians didn’t report their race in the survey. By means of contrast, the U.S. population in 2019 was 60% White, 19% Latino/Hispanic, 13% Black or African American, and 6% Asian American, according to the Brookings Institute.
Abraham Flexner, who wrote the Flexner Report, is often referred to as the “father of modern medical education,” according to the AAMC. In November, the AAMC observed that the Flexner Report contained racist and sexist ideas and that his work contributed to the closure of historically Black medical schools. Both statements were included in AAMC’s announcement about the removal of Flexner’s name from its most prestigious award. As of January, the award is now called the AAMC Award for Excellence in Medical Education.
Pathway programs can increase diversity
Pathway programs, which leverage targeted milestones along the journey to becoming a physician in order to increase diversity, were an area of focus in the council’s report. These programs “can exert a meaningful, positive effect on student outcomes and increase diversity across various levels of educational settings,” according to its report.
Centers of Excellence, which provides grants for mentorship and training programs, is one of many pathway programs. During the 2018-2019 academic year, Centers of Excellence supported more than 1,300 trainees – 99% of them were underrepresented minorities and 64% came from financially or educationally disadvantaged backgrounds. In 2006, federal funding was cut to these programs and the number of Centers of Excellence fell.
Still, the report cites the passage of federal funding in 2020 of $50 million for public institutions of higher education that train physicians; educational institutions in states with a projected primary care shortage in 2025 are given priority in the grant-funding process.
AMA council’s report garners support from delegates
Delegates voiced overwhelming support of the council’s report during the June 13 meeting. Lou Edje, MD, a Perrysburgh, Ohio–based family physician, voiced strong support for the council’s report, in particular its recommendations that recognize the harm caused by the Flexner Report. Dr. Edje observed that the Flexner Report, with its elimination of five of seven Black medical schools, “[set] back admissions of Black students into medicine by 50 years.”
“Empathy is what we are called to have as physicians. I implore you to simply substitute your ethnicity into these quotes to help understand the historic need for health equity in medicine today. This CME report is part of the antidote to Flexner. We support [it] fully,” concluded Dr. Edje, who spoke for the Great Lakes States Coalition of the AMA.
Rohan Khazanchi, a medical student at the University of Nebraska, Omaha, and a member of the council, said, “Our broad attempt with this report was twofold: to fill gaps in AMA policy with evidence-based recommendations which could improve diversity in our health workforce and, second, to enhance our organization’s vision for truth, reconciliation, and healing to redress the historic marginalization of minoritized physicians in medicine.”
According to an AMA spokesperson, the House of Delegates will vote on this and other policies this week, after which the policies are considered final.
A version of this article first appeared on Medscape.com.
Photobiomodulation: Evaluation in a wide range of medical specialties underway
according to Juanita J. Anders, PhD.
During the annual conference of the American Society for Laser Medicine and Surgery, Dr. Anders, professor of anatomy, physiology, and genetics at the Uniformed Services University of the Health Sciences, Bethesda, Md., defined photobiomodulation (PBM) as the mechanism by which nonionizing optical radiation in the visible and near-infrared spectral range is absorbed by endogenous chromophores to elicit photophysical and photochemical events at various biological scales. Photobiomodulation therapy (PBMT) involves the use of light sources including lasers, LEDs, and broadband light, that emit visible and/or near-infrared light to cause physiological changes in cells and tissues and result in therapeutic benefits.
In dermatology, LED light therapy devices are commonly used for PBMT in wavelengths that range from blue (415 nm) and red (633 nm) to near infrared (830 nm). “Often, when PBMT is referred to by dermatologists it’s called LED therapy or LED light therapy,” Dr. Anders noted. “Some people are under the impression that this is different from PBMT. But remember: It’s not the device that’s producing the photons that is clinically relevant, but it’s the photons themselves. In both cases, the same radiances and fluence ranges are being used and the mechanisms are the same, so it’s all PBMT.”
The therapy is used to treat a wide variety of medical and aesthetic disorders including acne vulgaris, psoriasis, burns, and wound healing. It has also been used in conjunction with surgical aesthetic and resurfacing procedures and has been reported to reduce erythema, edema, bruising, and days to healing. It’s been shown that PBMT stimulates fibroblast proliferation, collagen synthesis, and extracellular matrix resulting in lifting and tightening lax skin.
According to Dr. Anders, French dermatologists Linda Fouque, MD, and Michele Pelletier, MD, performed a series of in vivo and in vitro studies in which they tested the effects of yellow and red light for skin rejuvenation when used individually or in combination. “They found that fibroblasts and keratinocytes in vitro had great improvement in their morphology both with the yellow and red light, but the best improvement was seen with combination therapy,” Dr. Anders said. “This held true in their work looking at epidermal and dermal markers in the skin, where they found the best up-regulation in protein synthesis of such markers as collagens and fibronectin were produced when a combination wavelength light was used.”
Oral mucositis and pain
PBMT is also being used to treat oral mucositis (OM), a common adverse response to chemotherapy and/or radiation therapy, which causes pain, difficulty in swallowing and eating, and oral ulceration, and often interrupts the course of treatments. Authors of a recently published review on the risks and benefits of PBMT concluded that there is consistent evidence from a small number of high-quality studies that PBMT can help prevent the development of cancer therapy–induced OM, reduce pain intensity, as well as promote healing, and enhance patient quality of life.
“They also cautioned that, due to the limited long-term follow-up of patients, there is still concern for the potential long-term risks of PBMT in cancer cell mutation and amplification,” Dr. Anders said. “They advised that PBMT should be used carefully when the irradiation beam is in the direction of the tumor zone.”
Using PBMT for modulation of pain is another area of active research. Based on work from the laboratory of Dr. Anders and others, there are two methods to modulate pain. The first is to target tissue at irradiances below 100 mW/cm2.
“In my laboratory, based on in vivo preclinical animal models of neuropathic pain, we used a 980-nm wavelength laser at 43.25 mW/cm2 transcutaneously delivered to the level of the nerve for 20 seconds,” said Dr. Anders, who is a past president of the ASLMS. “Essentially, we found that the pain was modulated by reducing sensitivity to mechanical stimulation and also by causing an anti-inflammatory shift in microglial and macrophage phenotype in the dorsal root ganglion and spinal cord of affected segments.”
The second way to modulate pain, she continued, is to target tissue at irradiances above 250 mW/cm2. She and her colleagues have conducted in vitro and in vivo studies, which indicate that treatment with an irradiance/fluence rate at 270 mW/cm2 or higher at the nerve can rapidly block pain transmission.
“In vitro, we found that if we used an 810-nm wavelength light at 300 mW/cm2, we got a disruption of microtubules in the DRG neurons in culture, specifically the small neurons, the nociceptive fibers, but we did not affect the proprioceptive fibers unless we increased the length of the treatment,” she said. “We essentially found the same thing in vivo in a rodent model of neuropathic pain.”
In a pilot study, Dr. Anders and coauthors examined the efficacy of laser irradiation of the dorsal root ganglion of the second lumbar spinal nerve for patients with chronic back pain.
They found that PBMT effectively reduced back pain equal to the effects of lidocaine.
Based on these two irradiation approaches of targeting tissue, Dr. Anders recommends that a combination therapy be used to modulate neuropathic pain going forward. “This approach would involve the initial use of a high-irradiance treatment [at least 250 mW/cm2] at the nerve to block the pain transmission,” she said. “That treatment would be followed by a series of low-irradiance treatments [10-100 mW/cm2] along the course of the involved nerve to alter chronic pathology and inflammation.”
Potential applications in neurology
Dr. Anders also discussed research efforts under way involving transcranial PBMT: the delivery of near-infrared light through the tissues of the scalp and skull to targeted brain regions to treat neurologic injuries and disorders. “There have been some exciting results in preclinical animal work and in small clinical pilot work that show that there could be possible beneficial effects in Parkinson’s disease, Alzheimer’s disease, depression, and improvement in cognition and memory after a brain injury, such as a TBI,” she said.
“Initially, though, there were a lot of questions about whether you could really deliver light to the brain through the scalp. In my laboratory, we used slices of nonfixed brain and found that the sulci within the human brain act as light-wave guides. We used an 808-nm near-infrared wavelength of light, so that the light could penetrate more deeply.” Using nonfixed cadaver heads, where the light was applied at the scalp surface, Dr. Anders and colleagues were able to measure photons down to the depth of 4 cm. “It’s generally agreed now, though, that it’s to a maximum depth of 2.5-3 cm that enough photons are delivered that would cause a beneficial therapeutic effect,” she said.
Dr. Anders disclosed that she has received equipment from LiteCure, grant funding from the Department of Defense, and that she holds advisory board roles with LiteCure and Neurothera. She has also served in leadership roles for the Optical Society and holds intellectual property rights for the Henry M. Jackson Foundation for the Advancement of Military Medicine.
according to Juanita J. Anders, PhD.
During the annual conference of the American Society for Laser Medicine and Surgery, Dr. Anders, professor of anatomy, physiology, and genetics at the Uniformed Services University of the Health Sciences, Bethesda, Md., defined photobiomodulation (PBM) as the mechanism by which nonionizing optical radiation in the visible and near-infrared spectral range is absorbed by endogenous chromophores to elicit photophysical and photochemical events at various biological scales. Photobiomodulation therapy (PBMT) involves the use of light sources including lasers, LEDs, and broadband light, that emit visible and/or near-infrared light to cause physiological changes in cells and tissues and result in therapeutic benefits.
In dermatology, LED light therapy devices are commonly used for PBMT in wavelengths that range from blue (415 nm) and red (633 nm) to near infrared (830 nm). “Often, when PBMT is referred to by dermatologists it’s called LED therapy or LED light therapy,” Dr. Anders noted. “Some people are under the impression that this is different from PBMT. But remember: It’s not the device that’s producing the photons that is clinically relevant, but it’s the photons themselves. In both cases, the same radiances and fluence ranges are being used and the mechanisms are the same, so it’s all PBMT.”
The therapy is used to treat a wide variety of medical and aesthetic disorders including acne vulgaris, psoriasis, burns, and wound healing. It has also been used in conjunction with surgical aesthetic and resurfacing procedures and has been reported to reduce erythema, edema, bruising, and days to healing. It’s been shown that PBMT stimulates fibroblast proliferation, collagen synthesis, and extracellular matrix resulting in lifting and tightening lax skin.
According to Dr. Anders, French dermatologists Linda Fouque, MD, and Michele Pelletier, MD, performed a series of in vivo and in vitro studies in which they tested the effects of yellow and red light for skin rejuvenation when used individually or in combination. “They found that fibroblasts and keratinocytes in vitro had great improvement in their morphology both with the yellow and red light, but the best improvement was seen with combination therapy,” Dr. Anders said. “This held true in their work looking at epidermal and dermal markers in the skin, where they found the best up-regulation in protein synthesis of such markers as collagens and fibronectin were produced when a combination wavelength light was used.”
Oral mucositis and pain
PBMT is also being used to treat oral mucositis (OM), a common adverse response to chemotherapy and/or radiation therapy, which causes pain, difficulty in swallowing and eating, and oral ulceration, and often interrupts the course of treatments. Authors of a recently published review on the risks and benefits of PBMT concluded that there is consistent evidence from a small number of high-quality studies that PBMT can help prevent the development of cancer therapy–induced OM, reduce pain intensity, as well as promote healing, and enhance patient quality of life.
“They also cautioned that, due to the limited long-term follow-up of patients, there is still concern for the potential long-term risks of PBMT in cancer cell mutation and amplification,” Dr. Anders said. “They advised that PBMT should be used carefully when the irradiation beam is in the direction of the tumor zone.”
Using PBMT for modulation of pain is another area of active research. Based on work from the laboratory of Dr. Anders and others, there are two methods to modulate pain. The first is to target tissue at irradiances below 100 mW/cm2.
“In my laboratory, based on in vivo preclinical animal models of neuropathic pain, we used a 980-nm wavelength laser at 43.25 mW/cm2 transcutaneously delivered to the level of the nerve for 20 seconds,” said Dr. Anders, who is a past president of the ASLMS. “Essentially, we found that the pain was modulated by reducing sensitivity to mechanical stimulation and also by causing an anti-inflammatory shift in microglial and macrophage phenotype in the dorsal root ganglion and spinal cord of affected segments.”
The second way to modulate pain, she continued, is to target tissue at irradiances above 250 mW/cm2. She and her colleagues have conducted in vitro and in vivo studies, which indicate that treatment with an irradiance/fluence rate at 270 mW/cm2 or higher at the nerve can rapidly block pain transmission.
“In vitro, we found that if we used an 810-nm wavelength light at 300 mW/cm2, we got a disruption of microtubules in the DRG neurons in culture, specifically the small neurons, the nociceptive fibers, but we did not affect the proprioceptive fibers unless we increased the length of the treatment,” she said. “We essentially found the same thing in vivo in a rodent model of neuropathic pain.”
In a pilot study, Dr. Anders and coauthors examined the efficacy of laser irradiation of the dorsal root ganglion of the second lumbar spinal nerve for patients with chronic back pain.
They found that PBMT effectively reduced back pain equal to the effects of lidocaine.
Based on these two irradiation approaches of targeting tissue, Dr. Anders recommends that a combination therapy be used to modulate neuropathic pain going forward. “This approach would involve the initial use of a high-irradiance treatment [at least 250 mW/cm2] at the nerve to block the pain transmission,” she said. “That treatment would be followed by a series of low-irradiance treatments [10-100 mW/cm2] along the course of the involved nerve to alter chronic pathology and inflammation.”
Potential applications in neurology
Dr. Anders also discussed research efforts under way involving transcranial PBMT: the delivery of near-infrared light through the tissues of the scalp and skull to targeted brain regions to treat neurologic injuries and disorders. “There have been some exciting results in preclinical animal work and in small clinical pilot work that show that there could be possible beneficial effects in Parkinson’s disease, Alzheimer’s disease, depression, and improvement in cognition and memory after a brain injury, such as a TBI,” she said.
“Initially, though, there were a lot of questions about whether you could really deliver light to the brain through the scalp. In my laboratory, we used slices of nonfixed brain and found that the sulci within the human brain act as light-wave guides. We used an 808-nm near-infrared wavelength of light, so that the light could penetrate more deeply.” Using nonfixed cadaver heads, where the light was applied at the scalp surface, Dr. Anders and colleagues were able to measure photons down to the depth of 4 cm. “It’s generally agreed now, though, that it’s to a maximum depth of 2.5-3 cm that enough photons are delivered that would cause a beneficial therapeutic effect,” she said.
Dr. Anders disclosed that she has received equipment from LiteCure, grant funding from the Department of Defense, and that she holds advisory board roles with LiteCure and Neurothera. She has also served in leadership roles for the Optical Society and holds intellectual property rights for the Henry M. Jackson Foundation for the Advancement of Military Medicine.
according to Juanita J. Anders, PhD.
During the annual conference of the American Society for Laser Medicine and Surgery, Dr. Anders, professor of anatomy, physiology, and genetics at the Uniformed Services University of the Health Sciences, Bethesda, Md., defined photobiomodulation (PBM) as the mechanism by which nonionizing optical radiation in the visible and near-infrared spectral range is absorbed by endogenous chromophores to elicit photophysical and photochemical events at various biological scales. Photobiomodulation therapy (PBMT) involves the use of light sources including lasers, LEDs, and broadband light, that emit visible and/or near-infrared light to cause physiological changes in cells and tissues and result in therapeutic benefits.
In dermatology, LED light therapy devices are commonly used for PBMT in wavelengths that range from blue (415 nm) and red (633 nm) to near infrared (830 nm). “Often, when PBMT is referred to by dermatologists it’s called LED therapy or LED light therapy,” Dr. Anders noted. “Some people are under the impression that this is different from PBMT. But remember: It’s not the device that’s producing the photons that is clinically relevant, but it’s the photons themselves. In both cases, the same radiances and fluence ranges are being used and the mechanisms are the same, so it’s all PBMT.”
The therapy is used to treat a wide variety of medical and aesthetic disorders including acne vulgaris, psoriasis, burns, and wound healing. It has also been used in conjunction with surgical aesthetic and resurfacing procedures and has been reported to reduce erythema, edema, bruising, and days to healing. It’s been shown that PBMT stimulates fibroblast proliferation, collagen synthesis, and extracellular matrix resulting in lifting and tightening lax skin.
According to Dr. Anders, French dermatologists Linda Fouque, MD, and Michele Pelletier, MD, performed a series of in vivo and in vitro studies in which they tested the effects of yellow and red light for skin rejuvenation when used individually or in combination. “They found that fibroblasts and keratinocytes in vitro had great improvement in their morphology both with the yellow and red light, but the best improvement was seen with combination therapy,” Dr. Anders said. “This held true in their work looking at epidermal and dermal markers in the skin, where they found the best up-regulation in protein synthesis of such markers as collagens and fibronectin were produced when a combination wavelength light was used.”
Oral mucositis and pain
PBMT is also being used to treat oral mucositis (OM), a common adverse response to chemotherapy and/or radiation therapy, which causes pain, difficulty in swallowing and eating, and oral ulceration, and often interrupts the course of treatments. Authors of a recently published review on the risks and benefits of PBMT concluded that there is consistent evidence from a small number of high-quality studies that PBMT can help prevent the development of cancer therapy–induced OM, reduce pain intensity, as well as promote healing, and enhance patient quality of life.
“They also cautioned that, due to the limited long-term follow-up of patients, there is still concern for the potential long-term risks of PBMT in cancer cell mutation and amplification,” Dr. Anders said. “They advised that PBMT should be used carefully when the irradiation beam is in the direction of the tumor zone.”
Using PBMT for modulation of pain is another area of active research. Based on work from the laboratory of Dr. Anders and others, there are two methods to modulate pain. The first is to target tissue at irradiances below 100 mW/cm2.
“In my laboratory, based on in vivo preclinical animal models of neuropathic pain, we used a 980-nm wavelength laser at 43.25 mW/cm2 transcutaneously delivered to the level of the nerve for 20 seconds,” said Dr. Anders, who is a past president of the ASLMS. “Essentially, we found that the pain was modulated by reducing sensitivity to mechanical stimulation and also by causing an anti-inflammatory shift in microglial and macrophage phenotype in the dorsal root ganglion and spinal cord of affected segments.”
The second way to modulate pain, she continued, is to target tissue at irradiances above 250 mW/cm2. She and her colleagues have conducted in vitro and in vivo studies, which indicate that treatment with an irradiance/fluence rate at 270 mW/cm2 or higher at the nerve can rapidly block pain transmission.
“In vitro, we found that if we used an 810-nm wavelength light at 300 mW/cm2, we got a disruption of microtubules in the DRG neurons in culture, specifically the small neurons, the nociceptive fibers, but we did not affect the proprioceptive fibers unless we increased the length of the treatment,” she said. “We essentially found the same thing in vivo in a rodent model of neuropathic pain.”
In a pilot study, Dr. Anders and coauthors examined the efficacy of laser irradiation of the dorsal root ganglion of the second lumbar spinal nerve for patients with chronic back pain.
They found that PBMT effectively reduced back pain equal to the effects of lidocaine.
Based on these two irradiation approaches of targeting tissue, Dr. Anders recommends that a combination therapy be used to modulate neuropathic pain going forward. “This approach would involve the initial use of a high-irradiance treatment [at least 250 mW/cm2] at the nerve to block the pain transmission,” she said. “That treatment would be followed by a series of low-irradiance treatments [10-100 mW/cm2] along the course of the involved nerve to alter chronic pathology and inflammation.”
Potential applications in neurology
Dr. Anders also discussed research efforts under way involving transcranial PBMT: the delivery of near-infrared light through the tissues of the scalp and skull to targeted brain regions to treat neurologic injuries and disorders. “There have been some exciting results in preclinical animal work and in small clinical pilot work that show that there could be possible beneficial effects in Parkinson’s disease, Alzheimer’s disease, depression, and improvement in cognition and memory after a brain injury, such as a TBI,” she said.
“Initially, though, there were a lot of questions about whether you could really deliver light to the brain through the scalp. In my laboratory, we used slices of nonfixed brain and found that the sulci within the human brain act as light-wave guides. We used an 808-nm near-infrared wavelength of light, so that the light could penetrate more deeply.” Using nonfixed cadaver heads, where the light was applied at the scalp surface, Dr. Anders and colleagues were able to measure photons down to the depth of 4 cm. “It’s generally agreed now, though, that it’s to a maximum depth of 2.5-3 cm that enough photons are delivered that would cause a beneficial therapeutic effect,” she said.
Dr. Anders disclosed that she has received equipment from LiteCure, grant funding from the Department of Defense, and that she holds advisory board roles with LiteCure and Neurothera. She has also served in leadership roles for the Optical Society and holds intellectual property rights for the Henry M. Jackson Foundation for the Advancement of Military Medicine.
FROM ASLMS 2021
‘COVID toes’ chilblain-like lesions not related to COVID-19
from Italy.
These lesions are “most likely are benign” and resolve on their own after 2-6 weeks, Valentina Discepolo, MD, PhD, University of Naples Federico II, told this news organization.
“They do not seem to be the manifestation of systemic inflammatory or autoimmune phenomena. According to our experience, they should not require a SARS-CoV-2–specific molecular or serological test since in all cases in our series they were negative,” said Dr. Discepolo.
The study was published online June 10, 2021, in JAMA Network Open.
‘COVID toes’ a fallacy?
The temporal association between the COVID-19 pandemic and the increasing number of chilblain-like lesions has led some in the media to call it “COVID toes,” the investigators wrote. However, data on the association with SARS-CoV-2 are controversial.
For this report, Dr. Discepolo and colleagues evaluated 17 adolescents who presented with chilblain-like lesions of the toes during the first wave of the pandemic in southern Italy.
None had evidence of current, past, or local SARS-CoV-2 infection.
“In our experience, chilblain-like lesions are not a manifestation of COVID-19, as shown by negative serological and molecular specific for SARS-CoV2,” Dr. Discepolo said in an interview.
The lesions were bilaterally distributed in 16 adolescents (94.1%) and heel skin was involved in 7 (41.2%). Ulceration complicated one patient during the active phase of the disease, and desquamation developed over time in three patients (17.6%). Only two patients (11.8%) had concurrent involvement of the fingers.
Self-administered therapies included topical antibiotics and/or corticosteroids, disinfectants, and antifungal agents; systemic antibiotics or corticosteroids were used rarely.
None of the therapies substantially changed the course of the lesions. Duration was “extremely variable,” ranging from 49 to 145 days; however, at follow-up, all patients had full resolution.
Almost invariably, the lesions were characterized by a triad of red dots, white rosettes, and white streaks on an erythematous background, the investigators reported.
In more than half the patients (56%), red dots often appeared as dotted and comma-shaped congested vessels that surrounded the rosettes in the early stage of the lesions. In later stages, red dots were still present, but the rosettes had disappeared.
Although found inconsistently in inflammatory cutaneous conditions, these three signs do not characterize the dermoscopic picture of perniosis, suggesting a distinct disease process, the investigators said.
Don’t blame it on ischemia, clots
Histologic analysis revealed “remodeling of the dermal blood vessels with a lobular arrangement, wall thickening, and a mild perivascular lymphocytic infiltrate,” they noted.
Punch biopsy of the involved skin mostly showed endothelial hyperplasia, mild lymphocytic infiltrate, and vessels’ architecture disruption with no papillary dermal edema or eosinophilic or neutrophilic infiltrate.
Pathology did not reveal any ischemic changes, which argues against systemic vasculopathy, Farzam Gorouhi, MD, from Kaiser Permanente, South Sacramento Medical Center, noted in a linked editorial. “Thus, this study provides further evidence against the thromboembolic nature of the presented pattern in adolescents during the COVID-19 pandemic.”
Results of capillaroscopy, used to investigate structural changes in peripheral microcirculation, were either completely normal or showed rare ectasias, supporting a lack of systemic inflammatory process.
“The lack of capillaroscopic features of a major vasculopathic event in the study by Discepolo et al. argues against the ischemic nature of this disease and, thus, indicates that this presentation is not associated with systemic ischemia or an embolic event,” Dr. Gorouhi noted.
Chilblain-like lesions have been one of the most commonly described cutaneous manifestations during the COVID-19 pandemic, but their etiopathogenesis, including the role of SARS-CoV-2, has remained elusive, the investigators wrote.
The findings in this case series do not support the association of the lesions with SARS-CoV-2 infection, they concluded.
The fact that only three new cases of chilblain-like lesions were reported during the highest peaks of the pandemic further supports a lack of association with SARS-CoV-2 infection, they noted.
In addition, none of these patients tested positive for SARS-CoV-2 and all three cases during the second wave occurred in the winter months, suggesting that exposure to the cold might, at least in some cases, trigger the skin lesions, the investigators said.
In line with this hypothesis, seven of the adolescents in this case series (41.2%) relapsed during the winter months while again testing negative for SARS-CoV-2.
“We believe that lifestyle modifications [reduced physical activity, microtraumatisms caused by walking barefoot at home] during the first strict lockdown played a role, likely promoting a local inflammatory process promoted by vascular stasis that led in genetically susceptible individuals to the onset of these lesions,” Dr. Discepolo said in an interview.
This research had no specific funding. The investigators and Dr. Gorouhi declared no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
from Italy.
These lesions are “most likely are benign” and resolve on their own after 2-6 weeks, Valentina Discepolo, MD, PhD, University of Naples Federico II, told this news organization.
“They do not seem to be the manifestation of systemic inflammatory or autoimmune phenomena. According to our experience, they should not require a SARS-CoV-2–specific molecular or serological test since in all cases in our series they were negative,” said Dr. Discepolo.
The study was published online June 10, 2021, in JAMA Network Open.
‘COVID toes’ a fallacy?
The temporal association between the COVID-19 pandemic and the increasing number of chilblain-like lesions has led some in the media to call it “COVID toes,” the investigators wrote. However, data on the association with SARS-CoV-2 are controversial.
For this report, Dr. Discepolo and colleagues evaluated 17 adolescents who presented with chilblain-like lesions of the toes during the first wave of the pandemic in southern Italy.
None had evidence of current, past, or local SARS-CoV-2 infection.
“In our experience, chilblain-like lesions are not a manifestation of COVID-19, as shown by negative serological and molecular specific for SARS-CoV2,” Dr. Discepolo said in an interview.
The lesions were bilaterally distributed in 16 adolescents (94.1%) and heel skin was involved in 7 (41.2%). Ulceration complicated one patient during the active phase of the disease, and desquamation developed over time in three patients (17.6%). Only two patients (11.8%) had concurrent involvement of the fingers.
Self-administered therapies included topical antibiotics and/or corticosteroids, disinfectants, and antifungal agents; systemic antibiotics or corticosteroids were used rarely.
None of the therapies substantially changed the course of the lesions. Duration was “extremely variable,” ranging from 49 to 145 days; however, at follow-up, all patients had full resolution.
Almost invariably, the lesions were characterized by a triad of red dots, white rosettes, and white streaks on an erythematous background, the investigators reported.
In more than half the patients (56%), red dots often appeared as dotted and comma-shaped congested vessels that surrounded the rosettes in the early stage of the lesions. In later stages, red dots were still present, but the rosettes had disappeared.
Although found inconsistently in inflammatory cutaneous conditions, these three signs do not characterize the dermoscopic picture of perniosis, suggesting a distinct disease process, the investigators said.
Don’t blame it on ischemia, clots
Histologic analysis revealed “remodeling of the dermal blood vessels with a lobular arrangement, wall thickening, and a mild perivascular lymphocytic infiltrate,” they noted.
Punch biopsy of the involved skin mostly showed endothelial hyperplasia, mild lymphocytic infiltrate, and vessels’ architecture disruption with no papillary dermal edema or eosinophilic or neutrophilic infiltrate.
Pathology did not reveal any ischemic changes, which argues against systemic vasculopathy, Farzam Gorouhi, MD, from Kaiser Permanente, South Sacramento Medical Center, noted in a linked editorial. “Thus, this study provides further evidence against the thromboembolic nature of the presented pattern in adolescents during the COVID-19 pandemic.”
Results of capillaroscopy, used to investigate structural changes in peripheral microcirculation, were either completely normal or showed rare ectasias, supporting a lack of systemic inflammatory process.
“The lack of capillaroscopic features of a major vasculopathic event in the study by Discepolo et al. argues against the ischemic nature of this disease and, thus, indicates that this presentation is not associated with systemic ischemia or an embolic event,” Dr. Gorouhi noted.
Chilblain-like lesions have been one of the most commonly described cutaneous manifestations during the COVID-19 pandemic, but their etiopathogenesis, including the role of SARS-CoV-2, has remained elusive, the investigators wrote.
The findings in this case series do not support the association of the lesions with SARS-CoV-2 infection, they concluded.
The fact that only three new cases of chilblain-like lesions were reported during the highest peaks of the pandemic further supports a lack of association with SARS-CoV-2 infection, they noted.
In addition, none of these patients tested positive for SARS-CoV-2 and all three cases during the second wave occurred in the winter months, suggesting that exposure to the cold might, at least in some cases, trigger the skin lesions, the investigators said.
In line with this hypothesis, seven of the adolescents in this case series (41.2%) relapsed during the winter months while again testing negative for SARS-CoV-2.
“We believe that lifestyle modifications [reduced physical activity, microtraumatisms caused by walking barefoot at home] during the first strict lockdown played a role, likely promoting a local inflammatory process promoted by vascular stasis that led in genetically susceptible individuals to the onset of these lesions,” Dr. Discepolo said in an interview.
This research had no specific funding. The investigators and Dr. Gorouhi declared no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
from Italy.
These lesions are “most likely are benign” and resolve on their own after 2-6 weeks, Valentina Discepolo, MD, PhD, University of Naples Federico II, told this news organization.
“They do not seem to be the manifestation of systemic inflammatory or autoimmune phenomena. According to our experience, they should not require a SARS-CoV-2–specific molecular or serological test since in all cases in our series they were negative,” said Dr. Discepolo.
The study was published online June 10, 2021, in JAMA Network Open.
‘COVID toes’ a fallacy?
The temporal association between the COVID-19 pandemic and the increasing number of chilblain-like lesions has led some in the media to call it “COVID toes,” the investigators wrote. However, data on the association with SARS-CoV-2 are controversial.
For this report, Dr. Discepolo and colleagues evaluated 17 adolescents who presented with chilblain-like lesions of the toes during the first wave of the pandemic in southern Italy.
None had evidence of current, past, or local SARS-CoV-2 infection.
“In our experience, chilblain-like lesions are not a manifestation of COVID-19, as shown by negative serological and molecular specific for SARS-CoV2,” Dr. Discepolo said in an interview.
The lesions were bilaterally distributed in 16 adolescents (94.1%) and heel skin was involved in 7 (41.2%). Ulceration complicated one patient during the active phase of the disease, and desquamation developed over time in three patients (17.6%). Only two patients (11.8%) had concurrent involvement of the fingers.
Self-administered therapies included topical antibiotics and/or corticosteroids, disinfectants, and antifungal agents; systemic antibiotics or corticosteroids were used rarely.
None of the therapies substantially changed the course of the lesions. Duration was “extremely variable,” ranging from 49 to 145 days; however, at follow-up, all patients had full resolution.
Almost invariably, the lesions were characterized by a triad of red dots, white rosettes, and white streaks on an erythematous background, the investigators reported.
In more than half the patients (56%), red dots often appeared as dotted and comma-shaped congested vessels that surrounded the rosettes in the early stage of the lesions. In later stages, red dots were still present, but the rosettes had disappeared.
Although found inconsistently in inflammatory cutaneous conditions, these three signs do not characterize the dermoscopic picture of perniosis, suggesting a distinct disease process, the investigators said.
Don’t blame it on ischemia, clots
Histologic analysis revealed “remodeling of the dermal blood vessels with a lobular arrangement, wall thickening, and a mild perivascular lymphocytic infiltrate,” they noted.
Punch biopsy of the involved skin mostly showed endothelial hyperplasia, mild lymphocytic infiltrate, and vessels’ architecture disruption with no papillary dermal edema or eosinophilic or neutrophilic infiltrate.
Pathology did not reveal any ischemic changes, which argues against systemic vasculopathy, Farzam Gorouhi, MD, from Kaiser Permanente, South Sacramento Medical Center, noted in a linked editorial. “Thus, this study provides further evidence against the thromboembolic nature of the presented pattern in adolescents during the COVID-19 pandemic.”
Results of capillaroscopy, used to investigate structural changes in peripheral microcirculation, were either completely normal or showed rare ectasias, supporting a lack of systemic inflammatory process.
“The lack of capillaroscopic features of a major vasculopathic event in the study by Discepolo et al. argues against the ischemic nature of this disease and, thus, indicates that this presentation is not associated with systemic ischemia or an embolic event,” Dr. Gorouhi noted.
Chilblain-like lesions have been one of the most commonly described cutaneous manifestations during the COVID-19 pandemic, but their etiopathogenesis, including the role of SARS-CoV-2, has remained elusive, the investigators wrote.
The findings in this case series do not support the association of the lesions with SARS-CoV-2 infection, they concluded.
The fact that only three new cases of chilblain-like lesions were reported during the highest peaks of the pandemic further supports a lack of association with SARS-CoV-2 infection, they noted.
In addition, none of these patients tested positive for SARS-CoV-2 and all three cases during the second wave occurred in the winter months, suggesting that exposure to the cold might, at least in some cases, trigger the skin lesions, the investigators said.
In line with this hypothesis, seven of the adolescents in this case series (41.2%) relapsed during the winter months while again testing negative for SARS-CoV-2.
“We believe that lifestyle modifications [reduced physical activity, microtraumatisms caused by walking barefoot at home] during the first strict lockdown played a role, likely promoting a local inflammatory process promoted by vascular stasis that led in genetically susceptible individuals to the onset of these lesions,” Dr. Discepolo said in an interview.
This research had no specific funding. The investigators and Dr. Gorouhi declared no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Focus on cardiovascular disease
Cardiovascular disease is the leading cause of women’s death in the United States, accounting for 1 in every 5 female deaths per year according to the Centers for Disease Control and Prevention. Most risk factors for cardiovascular disease are modifiable. With a disease so prevalent in women’s health, obstetricians and gynecologists can assist patients in modifying these risk factors. This, however, is easier said than done.
One of the rate-limiting steps in assisting patients is the process of identifying an individual’s risk factors. This can be a time-consuming task in a women’s health appointment that is already busy with ObGyn-specific concerns, but technology can assist us.
Cardiovascular health app considerations
Many smartphone applications and websites are available that can alleviate the time constraints for identifying these individual modifiable risk factors. When evaluating cardiovascular risk factor apps for patients, keep these qualities (as outlined in the ACOG-recommended rubric) in mind: design, functionality, usefulness, and accuracy.
The patient-centered resources that assist women in identifying cardiovascular risk factors and that provide tools to positively impact these risk factors through lifestyle changes can help women achieve improved cardiovascular health. Recommendations include 1) manage blood pressure, 2) control cholesterol, 3) reduce blood sugar, 4) get active, 5) eat better, 6) lose weight, and 7) stop smoking.
National organizations’ smartphone apps guide the patient through a handful of questions about their current lifestyle, gender, age, and basic laboratory values. Their individual “heart health” results of these questions distribute the 7 risk factors to 3 categories based on the need to focus, improve, or celebrate. Through nonthreatening videos, patients can improve their scores themselves and bring the areas they need to focus, or improve, to their ObGyn’s attention for further assistance.
While adding one more task to an already busy practice can seem daunting, there are great technology resources that can be leveraged to successfully address this important health metric. By using the ACOG-recommended rubric and focusing on the app characteristics identified above, you can find one that works best for you and your patients and incorporate it into your practice today. ●
Cardiovascular disease is the leading cause of women’s death in the United States, accounting for 1 in every 5 female deaths per year according to the Centers for Disease Control and Prevention. Most risk factors for cardiovascular disease are modifiable. With a disease so prevalent in women’s health, obstetricians and gynecologists can assist patients in modifying these risk factors. This, however, is easier said than done.
One of the rate-limiting steps in assisting patients is the process of identifying an individual’s risk factors. This can be a time-consuming task in a women’s health appointment that is already busy with ObGyn-specific concerns, but technology can assist us.
Cardiovascular health app considerations
Many smartphone applications and websites are available that can alleviate the time constraints for identifying these individual modifiable risk factors. When evaluating cardiovascular risk factor apps for patients, keep these qualities (as outlined in the ACOG-recommended rubric) in mind: design, functionality, usefulness, and accuracy.
The patient-centered resources that assist women in identifying cardiovascular risk factors and that provide tools to positively impact these risk factors through lifestyle changes can help women achieve improved cardiovascular health. Recommendations include 1) manage blood pressure, 2) control cholesterol, 3) reduce blood sugar, 4) get active, 5) eat better, 6) lose weight, and 7) stop smoking.
National organizations’ smartphone apps guide the patient through a handful of questions about their current lifestyle, gender, age, and basic laboratory values. Their individual “heart health” results of these questions distribute the 7 risk factors to 3 categories based on the need to focus, improve, or celebrate. Through nonthreatening videos, patients can improve their scores themselves and bring the areas they need to focus, or improve, to their ObGyn’s attention for further assistance.
While adding one more task to an already busy practice can seem daunting, there are great technology resources that can be leveraged to successfully address this important health metric. By using the ACOG-recommended rubric and focusing on the app characteristics identified above, you can find one that works best for you and your patients and incorporate it into your practice today. ●
Cardiovascular disease is the leading cause of women’s death in the United States, accounting for 1 in every 5 female deaths per year according to the Centers for Disease Control and Prevention. Most risk factors for cardiovascular disease are modifiable. With a disease so prevalent in women’s health, obstetricians and gynecologists can assist patients in modifying these risk factors. This, however, is easier said than done.
One of the rate-limiting steps in assisting patients is the process of identifying an individual’s risk factors. This can be a time-consuming task in a women’s health appointment that is already busy with ObGyn-specific concerns, but technology can assist us.
Cardiovascular health app considerations
Many smartphone applications and websites are available that can alleviate the time constraints for identifying these individual modifiable risk factors. When evaluating cardiovascular risk factor apps for patients, keep these qualities (as outlined in the ACOG-recommended rubric) in mind: design, functionality, usefulness, and accuracy.
The patient-centered resources that assist women in identifying cardiovascular risk factors and that provide tools to positively impact these risk factors through lifestyle changes can help women achieve improved cardiovascular health. Recommendations include 1) manage blood pressure, 2) control cholesterol, 3) reduce blood sugar, 4) get active, 5) eat better, 6) lose weight, and 7) stop smoking.
National organizations’ smartphone apps guide the patient through a handful of questions about their current lifestyle, gender, age, and basic laboratory values. Their individual “heart health” results of these questions distribute the 7 risk factors to 3 categories based on the need to focus, improve, or celebrate. Through nonthreatening videos, patients can improve their scores themselves and bring the areas they need to focus, or improve, to their ObGyn’s attention for further assistance.
While adding one more task to an already busy practice can seem daunting, there are great technology resources that can be leveraged to successfully address this important health metric. By using the ACOG-recommended rubric and focusing on the app characteristics identified above, you can find one that works best for you and your patients and incorporate it into your practice today. ●
Focus on diabetes mellitus
Diabetes mellitus affects 10% of the US population, and as many as one-third of US adults have prediabetes, according to the National Diabetes Statistics Report 2020 from the Centers for Disease Control and Prevention. While diabetes is associated with significant long-term morbidity and mortality, with early identification and interventions, lifestyle modifications can significantly improve long-term health.
As with obesity (see “Focus on obesity” in OBG Management, May 2021), it is difficult to address lifestyle modifications with patients who have diabetes. However, many apps can be leveraged to aid physicians in this effort.
Diabetes app considerations
Obstetrician-gynecologists can play a pivotal role in helping to screen women for diabetes. When applying the ACOG-recommended rubric to evaluate the quality of an app that is targeted to address screening and diagnosing diabetes, it’s important to consider the app’s timeliness, authority, usefulness, and design.
There are point-of-care apps that include a few simple questions that can quickly identify which women should be screened. Some apps combine screening questions with testing results to streamline screening and diagnosis of diabetes and prediabetes. These apps also provide clinical content to help physicians educate, initiate, and even treat diabetes if they desire.
A wealth of patient-centered apps are available to help patients address a diagnosis of diabetes. Apps that provide real-time feedback, motivational features to engage the user, and links to nutritional, fitness, and diabetic goals provide a woman with a comprehensive and personalized experience that can considerably improve health.
By incorporating apps and engaging with our patients on app technology, ObGyns can successfully partner with women to decrease morbidity with respect to diabetes mellitus and its long-term implications. ●
Diabetes mellitus affects 10% of the US population, and as many as one-third of US adults have prediabetes, according to the National Diabetes Statistics Report 2020 from the Centers for Disease Control and Prevention. While diabetes is associated with significant long-term morbidity and mortality, with early identification and interventions, lifestyle modifications can significantly improve long-term health.
As with obesity (see “Focus on obesity” in OBG Management, May 2021), it is difficult to address lifestyle modifications with patients who have diabetes. However, many apps can be leveraged to aid physicians in this effort.
Diabetes app considerations
Obstetrician-gynecologists can play a pivotal role in helping to screen women for diabetes. When applying the ACOG-recommended rubric to evaluate the quality of an app that is targeted to address screening and diagnosing diabetes, it’s important to consider the app’s timeliness, authority, usefulness, and design.
There are point-of-care apps that include a few simple questions that can quickly identify which women should be screened. Some apps combine screening questions with testing results to streamline screening and diagnosis of diabetes and prediabetes. These apps also provide clinical content to help physicians educate, initiate, and even treat diabetes if they desire.
A wealth of patient-centered apps are available to help patients address a diagnosis of diabetes. Apps that provide real-time feedback, motivational features to engage the user, and links to nutritional, fitness, and diabetic goals provide a woman with a comprehensive and personalized experience that can considerably improve health.
By incorporating apps and engaging with our patients on app technology, ObGyns can successfully partner with women to decrease morbidity with respect to diabetes mellitus and its long-term implications. ●
Diabetes mellitus affects 10% of the US population, and as many as one-third of US adults have prediabetes, according to the National Diabetes Statistics Report 2020 from the Centers for Disease Control and Prevention. While diabetes is associated with significant long-term morbidity and mortality, with early identification and interventions, lifestyle modifications can significantly improve long-term health.
As with obesity (see “Focus on obesity” in OBG Management, May 2021), it is difficult to address lifestyle modifications with patients who have diabetes. However, many apps can be leveraged to aid physicians in this effort.
Diabetes app considerations
Obstetrician-gynecologists can play a pivotal role in helping to screen women for diabetes. When applying the ACOG-recommended rubric to evaluate the quality of an app that is targeted to address screening and diagnosing diabetes, it’s important to consider the app’s timeliness, authority, usefulness, and design.
There are point-of-care apps that include a few simple questions that can quickly identify which women should be screened. Some apps combine screening questions with testing results to streamline screening and diagnosis of diabetes and prediabetes. These apps also provide clinical content to help physicians educate, initiate, and even treat diabetes if they desire.
A wealth of patient-centered apps are available to help patients address a diagnosis of diabetes. Apps that provide real-time feedback, motivational features to engage the user, and links to nutritional, fitness, and diabetic goals provide a woman with a comprehensive and personalized experience that can considerably improve health.
By incorporating apps and engaging with our patients on app technology, ObGyns can successfully partner with women to decrease morbidity with respect to diabetes mellitus and its long-term implications. ●








