User login
Freezing breast cancer to death avoids surgery: Why not further along?
In the United States, cryoablation or freezing tissue to death is a primary treatment option for a variety of cancers, including those originating in or spread to the bone, cervix, eye, kidney, liver, lung, pancreas, prostate, and skin.
Cryoablation for prostate cancer, one of the most common cancers in men, was first approved in the 1990s.
But unlike in Europe, this nonsurgical approach is not approved for breast cancer in the United States; it is one of the most common cancers in women.
So why is this approach still experimental for breast cancer?
“I don’t know,” answered cryoablation researcher Richard Fine, MD, of West Cancer Center in Germantown, Tenn., when asked by this news organization.
“It’s very interesting how slow the [Food and Drug Administration] is in approving devices for breast cancer [when compared with] other cancers,” he said.
New clinical data
Perhaps new clinical data will eventually lead to approval of this nonsurgical technique for use in low-risk breast cancer. However, the related trial had a controversial design that might discourage uptake by practitioners if it is approved, said an expert not involved in the study.
Nevertheless, the new data show that cryoablation can be an effective treatment for small, low-risk, early-stage breast cancers in older patients.
The findings come from ICE-3, a multicenter single-arm study of cryoablation in 194 such patients with mean follow-up of roughly 3 years.
It used liquid nitrogen-based cryoablation technology from IceCure Medical Ltd., an Israeli company and the study sponsor.
The results show that 2.06% (n = 4) of patients had a recurrence in the same breast, which is “basically the same” as lumpectomy, the surgical standard for this patient group, said Dr. Fine, the lead investigator on the trial.
These are interim data, Dr. Fine said at the American Society of Breast Surgeons annual meeting, held virtually.
The primary outcome is the 5-year recurrence rate, and this is the first-ever cryoablation trial that does not involve follow-up surgery, he said.
Cryoablation, which delivers a gas to a tumor via a thin needle-like probe that is guided by ultrasound, has multiple advantages over surgery, Dr. Fine said.
“The noninvasive procedure is fast, painless, and can be delivered under local anesthesia in a doctor’s office. Recovery time is minimal and cosmetic outcomes are excellent with little loss of breast tissue and no scarring,” he said in a meeting press statement.
The potential market for cryoablation in breast cancer is large, as it is intended for tumors ≤1.5 cm, which comprise approximately 60%-70% of stage 1 breast cancers that are hormone receptor–positive (HR+), and HER2-negative (HER2–), Dr. Fine said in an interview.
Cryoablation is part of a logical, de-escalation of breast cancer care, he added. “We have moved from radical mastectomy to modified mastectomy to lumpectomy – so the next step in that evolution is ablative technology, which is ‘nonsurgical.’ ”
There are other experimental ablative treatments for breast cancer including high-frequency ultrasound and laser, but cryoablation is the furthest along in development.
Cryoablation as a primary cancer treatment was first approved for coverage by the Centers for Medicare & Medicaid Services for localized prostate cancer in 1999.
But the concept extends back to 1845, when English physician James Arnott first used iced salt solutions (about –20 °C or – 4 °F) to induce tissue necrosis, reducing tumor size and ameliorating pain. Because the crude cryogen needed to be applied topically, the pioneering technique was limited to breast and cervical cancers because of their accessibility.
Not likely to show superiority
The new study’s population was composed of women aged 60 years or older (mean of 75 years) with unifocal invasive ductal cancers measuring ≤1.5 cm or less that were all low-grade, HR+, and HER2–, as noted.
The liquid nitrogen–based cryoablation consisted of a freeze-thaw-freeze cycle that totals 20-40 minutes, with freezing temperatures targeting the tumor area and turning it into an “ice ball.”
That ice ball eventually surrounds the tumor, creating a “lethal zone,” and thus a margin in which no cancer exists, akin to surgery, said Dr. Fine.
There were no significant device-related adverse events or complications reported, say the investigators. Most of the adverse events were minor and included bruising, localized edema, minor skin freeze burn, rash, minor bleeding from needle insertion, minor local hematoma, skin induration, minor infection, and pruritis.
Two of 15 patients who underwent sentinel lymph node biopsies had a positive sentinel node. At the discretion of their treating physician, 27 patients underwent adjuvant radiation, 1 patient received chemotherapy, and 148 began endocrine therapy. More than 95% of the patients and 98% of physicians reported satisfaction from the cosmetic results during follow-up visits.
Because not all patients underwent sentinel lymph node biopsy and adjuvant radiation, there is likely to be controversy about this approach, suggested Deanna J. Attai, MD, a breast surgeon at the University of California, Los Angeles, and past president of the American Society of Breast Surgeons, who was asked for comment.
“We have studies that [indicate that] these treatments don’t add significant benefit [in this patient population] but there still is this hesitation [to forgo them],” she told this news organization.
“The patients in this study were exceedingly low risk,” she emphasized.
“Is 5 years enough to assess recurrence rates? The answer is probably no. Recurrences or distant metastases are more likely to happen 10-20 years later.”
Thus, it will be difficult to show that cryoablation is superior to surgery, she said.
“You can show that cryoablation is not inferior to lumpectomy alone – which allows patients to avoid the operating room,” Dr. Attai summarized.
The surgical mindset and breast cancer
Dr. Attai, who was not involved in the current trial, was an investigator in an earlier single-arm cooperative group study of cryoablation for breast cancer, which had the rate of complete tumor ablation as the primary outcome. The study, known as the American College of Surgeons Oncology Group Z1072 trial, enrolled 99 patients, all of whom underwent ablation followed by surgery. The study reported results in 2014 but was very slow to develop, she observed.
“I did my first training in 2004 and I don’t think the study opened for several years after that. I think there’s been a lot of hesitation to change the mindset that every cancer needs to be removed surgically,” Dr. Attai stated.
“When you put breast cancer in the context of the other organs, we are lagging behind a bit [with cryoablation],” she added.
“I don’t want to go there but … the innovation for male diseases and procedures sometimes surpasses that of women’s diseases,” she said.
But she also defended her fellow practitioners. “There’s been tremendous changes in management over the 27 years I’ve been in practice,” she said, citing the movement from mastectomy to lumpectomy as one of multiple big changes.
The disparity between the development of cryoablation for breast and prostate cancer is a mystery when you contemplate the potential side effects, Dr. Fine observed. “There’s not a lot of vital structures inside the breast, so you don’t have risks that you have with the prostate, including urinary incontinence and impotence.”
As a next move, the American Society of Breast Surgeons is planning to establish a cryoablation registry and aims to enroll 50 sites and 500 patients who are aged 55-85 years; for those aged 65-70, radiation therapy will be required, said Dr. Fine.
Currently, cryoablation for breast cancer is allowed only in a clinical trial, so a registry would expand usage considerably, he said.
However, cryoablation, including from IceCure, has FDA clearance for ablating cancerous tissue in general (but not breast cancer specifically).
Dr. Attai hopes the field is ready for the nonsurgical approach.
“Halsted died in 1922 and the Halsted radical mastectomy really didn’t start to fall out of favor until the 1950s, 1960,” said Dr. Attai, referring to Dr William Halsted, who pioneered the procedure in the 1890s. “I would hope we are better at speeding up our progress. Changing the surgical mindset takes time,” she said.
Dr. Fine was an investigator in the ICE3 trial, which is funded by IceCure Medical. Dr. Attai has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In the United States, cryoablation or freezing tissue to death is a primary treatment option for a variety of cancers, including those originating in or spread to the bone, cervix, eye, kidney, liver, lung, pancreas, prostate, and skin.
Cryoablation for prostate cancer, one of the most common cancers in men, was first approved in the 1990s.
But unlike in Europe, this nonsurgical approach is not approved for breast cancer in the United States; it is one of the most common cancers in women.
So why is this approach still experimental for breast cancer?
“I don’t know,” answered cryoablation researcher Richard Fine, MD, of West Cancer Center in Germantown, Tenn., when asked by this news organization.
“It’s very interesting how slow the [Food and Drug Administration] is in approving devices for breast cancer [when compared with] other cancers,” he said.
New clinical data
Perhaps new clinical data will eventually lead to approval of this nonsurgical technique for use in low-risk breast cancer. However, the related trial had a controversial design that might discourage uptake by practitioners if it is approved, said an expert not involved in the study.
Nevertheless, the new data show that cryoablation can be an effective treatment for small, low-risk, early-stage breast cancers in older patients.
The findings come from ICE-3, a multicenter single-arm study of cryoablation in 194 such patients with mean follow-up of roughly 3 years.
It used liquid nitrogen-based cryoablation technology from IceCure Medical Ltd., an Israeli company and the study sponsor.
The results show that 2.06% (n = 4) of patients had a recurrence in the same breast, which is “basically the same” as lumpectomy, the surgical standard for this patient group, said Dr. Fine, the lead investigator on the trial.
These are interim data, Dr. Fine said at the American Society of Breast Surgeons annual meeting, held virtually.
The primary outcome is the 5-year recurrence rate, and this is the first-ever cryoablation trial that does not involve follow-up surgery, he said.
Cryoablation, which delivers a gas to a tumor via a thin needle-like probe that is guided by ultrasound, has multiple advantages over surgery, Dr. Fine said.
“The noninvasive procedure is fast, painless, and can be delivered under local anesthesia in a doctor’s office. Recovery time is minimal and cosmetic outcomes are excellent with little loss of breast tissue and no scarring,” he said in a meeting press statement.
The potential market for cryoablation in breast cancer is large, as it is intended for tumors ≤1.5 cm, which comprise approximately 60%-70% of stage 1 breast cancers that are hormone receptor–positive (HR+), and HER2-negative (HER2–), Dr. Fine said in an interview.
Cryoablation is part of a logical, de-escalation of breast cancer care, he added. “We have moved from radical mastectomy to modified mastectomy to lumpectomy – so the next step in that evolution is ablative technology, which is ‘nonsurgical.’ ”
There are other experimental ablative treatments for breast cancer including high-frequency ultrasound and laser, but cryoablation is the furthest along in development.
Cryoablation as a primary cancer treatment was first approved for coverage by the Centers for Medicare & Medicaid Services for localized prostate cancer in 1999.
But the concept extends back to 1845, when English physician James Arnott first used iced salt solutions (about –20 °C or – 4 °F) to induce tissue necrosis, reducing tumor size and ameliorating pain. Because the crude cryogen needed to be applied topically, the pioneering technique was limited to breast and cervical cancers because of their accessibility.
Not likely to show superiority
The new study’s population was composed of women aged 60 years or older (mean of 75 years) with unifocal invasive ductal cancers measuring ≤1.5 cm or less that were all low-grade, HR+, and HER2–, as noted.
The liquid nitrogen–based cryoablation consisted of a freeze-thaw-freeze cycle that totals 20-40 minutes, with freezing temperatures targeting the tumor area and turning it into an “ice ball.”
That ice ball eventually surrounds the tumor, creating a “lethal zone,” and thus a margin in which no cancer exists, akin to surgery, said Dr. Fine.
There were no significant device-related adverse events or complications reported, say the investigators. Most of the adverse events were minor and included bruising, localized edema, minor skin freeze burn, rash, minor bleeding from needle insertion, minor local hematoma, skin induration, minor infection, and pruritis.
Two of 15 patients who underwent sentinel lymph node biopsies had a positive sentinel node. At the discretion of their treating physician, 27 patients underwent adjuvant radiation, 1 patient received chemotherapy, and 148 began endocrine therapy. More than 95% of the patients and 98% of physicians reported satisfaction from the cosmetic results during follow-up visits.
Because not all patients underwent sentinel lymph node biopsy and adjuvant radiation, there is likely to be controversy about this approach, suggested Deanna J. Attai, MD, a breast surgeon at the University of California, Los Angeles, and past president of the American Society of Breast Surgeons, who was asked for comment.
“We have studies that [indicate that] these treatments don’t add significant benefit [in this patient population] but there still is this hesitation [to forgo them],” she told this news organization.
“The patients in this study were exceedingly low risk,” she emphasized.
“Is 5 years enough to assess recurrence rates? The answer is probably no. Recurrences or distant metastases are more likely to happen 10-20 years later.”
Thus, it will be difficult to show that cryoablation is superior to surgery, she said.
“You can show that cryoablation is not inferior to lumpectomy alone – which allows patients to avoid the operating room,” Dr. Attai summarized.
The surgical mindset and breast cancer
Dr. Attai, who was not involved in the current trial, was an investigator in an earlier single-arm cooperative group study of cryoablation for breast cancer, which had the rate of complete tumor ablation as the primary outcome. The study, known as the American College of Surgeons Oncology Group Z1072 trial, enrolled 99 patients, all of whom underwent ablation followed by surgery. The study reported results in 2014 but was very slow to develop, she observed.
“I did my first training in 2004 and I don’t think the study opened for several years after that. I think there’s been a lot of hesitation to change the mindset that every cancer needs to be removed surgically,” Dr. Attai stated.
“When you put breast cancer in the context of the other organs, we are lagging behind a bit [with cryoablation],” she added.
“I don’t want to go there but … the innovation for male diseases and procedures sometimes surpasses that of women’s diseases,” she said.
But she also defended her fellow practitioners. “There’s been tremendous changes in management over the 27 years I’ve been in practice,” she said, citing the movement from mastectomy to lumpectomy as one of multiple big changes.
The disparity between the development of cryoablation for breast and prostate cancer is a mystery when you contemplate the potential side effects, Dr. Fine observed. “There’s not a lot of vital structures inside the breast, so you don’t have risks that you have with the prostate, including urinary incontinence and impotence.”
As a next move, the American Society of Breast Surgeons is planning to establish a cryoablation registry and aims to enroll 50 sites and 500 patients who are aged 55-85 years; for those aged 65-70, radiation therapy will be required, said Dr. Fine.
Currently, cryoablation for breast cancer is allowed only in a clinical trial, so a registry would expand usage considerably, he said.
However, cryoablation, including from IceCure, has FDA clearance for ablating cancerous tissue in general (but not breast cancer specifically).
Dr. Attai hopes the field is ready for the nonsurgical approach.
“Halsted died in 1922 and the Halsted radical mastectomy really didn’t start to fall out of favor until the 1950s, 1960,” said Dr. Attai, referring to Dr William Halsted, who pioneered the procedure in the 1890s. “I would hope we are better at speeding up our progress. Changing the surgical mindset takes time,” she said.
Dr. Fine was an investigator in the ICE3 trial, which is funded by IceCure Medical. Dr. Attai has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In the United States, cryoablation or freezing tissue to death is a primary treatment option for a variety of cancers, including those originating in or spread to the bone, cervix, eye, kidney, liver, lung, pancreas, prostate, and skin.
Cryoablation for prostate cancer, one of the most common cancers in men, was first approved in the 1990s.
But unlike in Europe, this nonsurgical approach is not approved for breast cancer in the United States; it is one of the most common cancers in women.
So why is this approach still experimental for breast cancer?
“I don’t know,” answered cryoablation researcher Richard Fine, MD, of West Cancer Center in Germantown, Tenn., when asked by this news organization.
“It’s very interesting how slow the [Food and Drug Administration] is in approving devices for breast cancer [when compared with] other cancers,” he said.
New clinical data
Perhaps new clinical data will eventually lead to approval of this nonsurgical technique for use in low-risk breast cancer. However, the related trial had a controversial design that might discourage uptake by practitioners if it is approved, said an expert not involved in the study.
Nevertheless, the new data show that cryoablation can be an effective treatment for small, low-risk, early-stage breast cancers in older patients.
The findings come from ICE-3, a multicenter single-arm study of cryoablation in 194 such patients with mean follow-up of roughly 3 years.
It used liquid nitrogen-based cryoablation technology from IceCure Medical Ltd., an Israeli company and the study sponsor.
The results show that 2.06% (n = 4) of patients had a recurrence in the same breast, which is “basically the same” as lumpectomy, the surgical standard for this patient group, said Dr. Fine, the lead investigator on the trial.
These are interim data, Dr. Fine said at the American Society of Breast Surgeons annual meeting, held virtually.
The primary outcome is the 5-year recurrence rate, and this is the first-ever cryoablation trial that does not involve follow-up surgery, he said.
Cryoablation, which delivers a gas to a tumor via a thin needle-like probe that is guided by ultrasound, has multiple advantages over surgery, Dr. Fine said.
“The noninvasive procedure is fast, painless, and can be delivered under local anesthesia in a doctor’s office. Recovery time is minimal and cosmetic outcomes are excellent with little loss of breast tissue and no scarring,” he said in a meeting press statement.
The potential market for cryoablation in breast cancer is large, as it is intended for tumors ≤1.5 cm, which comprise approximately 60%-70% of stage 1 breast cancers that are hormone receptor–positive (HR+), and HER2-negative (HER2–), Dr. Fine said in an interview.
Cryoablation is part of a logical, de-escalation of breast cancer care, he added. “We have moved from radical mastectomy to modified mastectomy to lumpectomy – so the next step in that evolution is ablative technology, which is ‘nonsurgical.’ ”
There are other experimental ablative treatments for breast cancer including high-frequency ultrasound and laser, but cryoablation is the furthest along in development.
Cryoablation as a primary cancer treatment was first approved for coverage by the Centers for Medicare & Medicaid Services for localized prostate cancer in 1999.
But the concept extends back to 1845, when English physician James Arnott first used iced salt solutions (about –20 °C or – 4 °F) to induce tissue necrosis, reducing tumor size and ameliorating pain. Because the crude cryogen needed to be applied topically, the pioneering technique was limited to breast and cervical cancers because of their accessibility.
Not likely to show superiority
The new study’s population was composed of women aged 60 years or older (mean of 75 years) with unifocal invasive ductal cancers measuring ≤1.5 cm or less that were all low-grade, HR+, and HER2–, as noted.
The liquid nitrogen–based cryoablation consisted of a freeze-thaw-freeze cycle that totals 20-40 minutes, with freezing temperatures targeting the tumor area and turning it into an “ice ball.”
That ice ball eventually surrounds the tumor, creating a “lethal zone,” and thus a margin in which no cancer exists, akin to surgery, said Dr. Fine.
There were no significant device-related adverse events or complications reported, say the investigators. Most of the adverse events were minor and included bruising, localized edema, minor skin freeze burn, rash, minor bleeding from needle insertion, minor local hematoma, skin induration, minor infection, and pruritis.
Two of 15 patients who underwent sentinel lymph node biopsies had a positive sentinel node. At the discretion of their treating physician, 27 patients underwent adjuvant radiation, 1 patient received chemotherapy, and 148 began endocrine therapy. More than 95% of the patients and 98% of physicians reported satisfaction from the cosmetic results during follow-up visits.
Because not all patients underwent sentinel lymph node biopsy and adjuvant radiation, there is likely to be controversy about this approach, suggested Deanna J. Attai, MD, a breast surgeon at the University of California, Los Angeles, and past president of the American Society of Breast Surgeons, who was asked for comment.
“We have studies that [indicate that] these treatments don’t add significant benefit [in this patient population] but there still is this hesitation [to forgo them],” she told this news organization.
“The patients in this study were exceedingly low risk,” she emphasized.
“Is 5 years enough to assess recurrence rates? The answer is probably no. Recurrences or distant metastases are more likely to happen 10-20 years later.”
Thus, it will be difficult to show that cryoablation is superior to surgery, she said.
“You can show that cryoablation is not inferior to lumpectomy alone – which allows patients to avoid the operating room,” Dr. Attai summarized.
The surgical mindset and breast cancer
Dr. Attai, who was not involved in the current trial, was an investigator in an earlier single-arm cooperative group study of cryoablation for breast cancer, which had the rate of complete tumor ablation as the primary outcome. The study, known as the American College of Surgeons Oncology Group Z1072 trial, enrolled 99 patients, all of whom underwent ablation followed by surgery. The study reported results in 2014 but was very slow to develop, she observed.
“I did my first training in 2004 and I don’t think the study opened for several years after that. I think there’s been a lot of hesitation to change the mindset that every cancer needs to be removed surgically,” Dr. Attai stated.
“When you put breast cancer in the context of the other organs, we are lagging behind a bit [with cryoablation],” she added.
“I don’t want to go there but … the innovation for male diseases and procedures sometimes surpasses that of women’s diseases,” she said.
But she also defended her fellow practitioners. “There’s been tremendous changes in management over the 27 years I’ve been in practice,” she said, citing the movement from mastectomy to lumpectomy as one of multiple big changes.
The disparity between the development of cryoablation for breast and prostate cancer is a mystery when you contemplate the potential side effects, Dr. Fine observed. “There’s not a lot of vital structures inside the breast, so you don’t have risks that you have with the prostate, including urinary incontinence and impotence.”
As a next move, the American Society of Breast Surgeons is planning to establish a cryoablation registry and aims to enroll 50 sites and 500 patients who are aged 55-85 years; for those aged 65-70, radiation therapy will be required, said Dr. Fine.
Currently, cryoablation for breast cancer is allowed only in a clinical trial, so a registry would expand usage considerably, he said.
However, cryoablation, including from IceCure, has FDA clearance for ablating cancerous tissue in general (but not breast cancer specifically).
Dr. Attai hopes the field is ready for the nonsurgical approach.
“Halsted died in 1922 and the Halsted radical mastectomy really didn’t start to fall out of favor until the 1950s, 1960,” said Dr. Attai, referring to Dr William Halsted, who pioneered the procedure in the 1890s. “I would hope we are better at speeding up our progress. Changing the surgical mindset takes time,” she said.
Dr. Fine was an investigator in the ICE3 trial, which is funded by IceCure Medical. Dr. Attai has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Converging to build for tomorrow
Last month we converged virtually for our annual conference, SHM Converge – the second time since the start of the coronavirus pandemic. We are thankful for innovations and advancements in technology that have allowed the world, including SHM, to continue connecting us all together. And yet, 18 months in, having forged new roads, experienced unique and life-changing events, we long for the in-person human connection that allows us to share a common experience. At a time of imperatives in our world – a global pandemic, systemic racism, and deep geopolitical divides – more than ever, we need to converge. Isolation only festers, deepening our divisions and conflicts.
In high school, I read Robert Frost’s poem “The Road Not Taken” and clung to the notion of diverging roads and choosing the road less traveled. Like most young people, my years since reading the poem were filled with attempts at forging new paths and experiencing great things – and yet, always feeling unaccomplished. Was Oscar Wilde right when he wrote: “Life imitates Art far more than Art imitates Life?” After all, these past 18 months, we have shared in the traumas of our times, and still, we remain isolated and alone. Our diverse experiences have been real, both tragic and heroic, from east to west, city to country, black to white, and red to blue.
At SHM, it’s time to converge and face the great challenges of our lifetime. A deadly pandemic continues to rage around the world, bringing unprecedented human suffering and loss of lives. In its wake, this pandemic also laid bare the ugly face of systemic racism, brought our deepest divisions to the surface – all threatening the very fabric of our society. This pandemic has been a stress test for health care systems, revealing our vulnerabilities and expanding the chasm of care between urban and rural communities, all in turn worsening our growing health disparities. This moment needs convergence to rekindle connection and solidarity.
Scholars do not interpret “The Road Not Taken” as a recommendation to take the road less traveled. Instead, it is a suggestion that the diverging roads lead to a common place having been “worn about the same” as they “equally lay.” It is true that our roads are unique and shape our lives, but so, too, does the destination and common place our roads lead us to. At that common place, during these taxing times, SHM enables hospitalists to tackle these great challenges.
For over 2 decades of dynamic changes in health care, SHM has been the workshop where hospitalists converged to sharpen clinical skills, improve quality and safety, develop acute care models inside and outside of hospitals, advocate for better health policy and blaze new trails. Though the issues evolved, and new ones emerge, today is no different.
Indeed, this is an historic time. This weighted moment meets us at the crossroads. A moment that demands synergy, cooperation, and creativity. A dynamic change to health care policy, advances in care innovation, renewed prioritization of public health, and rich national discourse on our social fabric; hospitalists are essential to every one of those conversations. SHM has evolved to meet our growing needs, equipping hospitalists with tools to engage at every level, and most importantly, enabled us to find our common place.
Where do we go now? I suggest we continue to take the roads not taken and at the destination, build the map of tomorrow, together.
Dr. Siy is division medical director, hospital specialties, in the departments of hospital medicine and community senior and palliative care at HealthPartners in Bloomington, Minn. He is the new president of SHM.
Last month we converged virtually for our annual conference, SHM Converge – the second time since the start of the coronavirus pandemic. We are thankful for innovations and advancements in technology that have allowed the world, including SHM, to continue connecting us all together. And yet, 18 months in, having forged new roads, experienced unique and life-changing events, we long for the in-person human connection that allows us to share a common experience. At a time of imperatives in our world – a global pandemic, systemic racism, and deep geopolitical divides – more than ever, we need to converge. Isolation only festers, deepening our divisions and conflicts.
In high school, I read Robert Frost’s poem “The Road Not Taken” and clung to the notion of diverging roads and choosing the road less traveled. Like most young people, my years since reading the poem were filled with attempts at forging new paths and experiencing great things – and yet, always feeling unaccomplished. Was Oscar Wilde right when he wrote: “Life imitates Art far more than Art imitates Life?” After all, these past 18 months, we have shared in the traumas of our times, and still, we remain isolated and alone. Our diverse experiences have been real, both tragic and heroic, from east to west, city to country, black to white, and red to blue.
At SHM, it’s time to converge and face the great challenges of our lifetime. A deadly pandemic continues to rage around the world, bringing unprecedented human suffering and loss of lives. In its wake, this pandemic also laid bare the ugly face of systemic racism, brought our deepest divisions to the surface – all threatening the very fabric of our society. This pandemic has been a stress test for health care systems, revealing our vulnerabilities and expanding the chasm of care between urban and rural communities, all in turn worsening our growing health disparities. This moment needs convergence to rekindle connection and solidarity.
Scholars do not interpret “The Road Not Taken” as a recommendation to take the road less traveled. Instead, it is a suggestion that the diverging roads lead to a common place having been “worn about the same” as they “equally lay.” It is true that our roads are unique and shape our lives, but so, too, does the destination and common place our roads lead us to. At that common place, during these taxing times, SHM enables hospitalists to tackle these great challenges.
For over 2 decades of dynamic changes in health care, SHM has been the workshop where hospitalists converged to sharpen clinical skills, improve quality and safety, develop acute care models inside and outside of hospitals, advocate for better health policy and blaze new trails. Though the issues evolved, and new ones emerge, today is no different.
Indeed, this is an historic time. This weighted moment meets us at the crossroads. A moment that demands synergy, cooperation, and creativity. A dynamic change to health care policy, advances in care innovation, renewed prioritization of public health, and rich national discourse on our social fabric; hospitalists are essential to every one of those conversations. SHM has evolved to meet our growing needs, equipping hospitalists with tools to engage at every level, and most importantly, enabled us to find our common place.
Where do we go now? I suggest we continue to take the roads not taken and at the destination, build the map of tomorrow, together.
Dr. Siy is division medical director, hospital specialties, in the departments of hospital medicine and community senior and palliative care at HealthPartners in Bloomington, Minn. He is the new president of SHM.
Last month we converged virtually for our annual conference, SHM Converge – the second time since the start of the coronavirus pandemic. We are thankful for innovations and advancements in technology that have allowed the world, including SHM, to continue connecting us all together. And yet, 18 months in, having forged new roads, experienced unique and life-changing events, we long for the in-person human connection that allows us to share a common experience. At a time of imperatives in our world – a global pandemic, systemic racism, and deep geopolitical divides – more than ever, we need to converge. Isolation only festers, deepening our divisions and conflicts.
In high school, I read Robert Frost’s poem “The Road Not Taken” and clung to the notion of diverging roads and choosing the road less traveled. Like most young people, my years since reading the poem were filled with attempts at forging new paths and experiencing great things – and yet, always feeling unaccomplished. Was Oscar Wilde right when he wrote: “Life imitates Art far more than Art imitates Life?” After all, these past 18 months, we have shared in the traumas of our times, and still, we remain isolated and alone. Our diverse experiences have been real, both tragic and heroic, from east to west, city to country, black to white, and red to blue.
At SHM, it’s time to converge and face the great challenges of our lifetime. A deadly pandemic continues to rage around the world, bringing unprecedented human suffering and loss of lives. In its wake, this pandemic also laid bare the ugly face of systemic racism, brought our deepest divisions to the surface – all threatening the very fabric of our society. This pandemic has been a stress test for health care systems, revealing our vulnerabilities and expanding the chasm of care between urban and rural communities, all in turn worsening our growing health disparities. This moment needs convergence to rekindle connection and solidarity.
Scholars do not interpret “The Road Not Taken” as a recommendation to take the road less traveled. Instead, it is a suggestion that the diverging roads lead to a common place having been “worn about the same” as they “equally lay.” It is true that our roads are unique and shape our lives, but so, too, does the destination and common place our roads lead us to. At that common place, during these taxing times, SHM enables hospitalists to tackle these great challenges.
For over 2 decades of dynamic changes in health care, SHM has been the workshop where hospitalists converged to sharpen clinical skills, improve quality and safety, develop acute care models inside and outside of hospitals, advocate for better health policy and blaze new trails. Though the issues evolved, and new ones emerge, today is no different.
Indeed, this is an historic time. This weighted moment meets us at the crossroads. A moment that demands synergy, cooperation, and creativity. A dynamic change to health care policy, advances in care innovation, renewed prioritization of public health, and rich national discourse on our social fabric; hospitalists are essential to every one of those conversations. SHM has evolved to meet our growing needs, equipping hospitalists with tools to engage at every level, and most importantly, enabled us to find our common place.
Where do we go now? I suggest we continue to take the roads not taken and at the destination, build the map of tomorrow, together.
Dr. Siy is division medical director, hospital specialties, in the departments of hospital medicine and community senior and palliative care at HealthPartners in Bloomington, Minn. He is the new president of SHM.
New biomarkers may predict interstitial lung disease progression in patients with systemic sclerosis
Quantitative assessment of the extent of interstitial lung disease in patients with systemic sclerosis and levels of certain proteins in bronchoalveolar lavage samples have potential for predicting mortality and disease progression, according to two analyses of data from the Scleroderma Lung Study I and II.
The analyses, presented at the annual European Congress of Rheumatology, aim to improve current prognostic abilities in patients with systemic sclerosis–interstitial lung disease (SSc-ILD). Although forced vital capacity is commonly used as a biomarker for survival in many SSc-ILD trials, other factors can affect FVC, such as respiratory muscle weakness and skin fibrosis. Further, FVC correlates poorly with patient-reported outcomes, explained first author Elizabeth Volkmann, MD, director of the scleroderma program at the University of California, Los Angeles, and the founder and codirector of the UCLA connective tissue disease–related interstitial lung disease program.
Dr. Volkmann presented two studies that investigated the potential of radiographic and protein biomarkers for predicting mortality and identifying patients at risk for ILD progression. The biomarkers may also help to identify patients who would benefit most from immunosuppressive therapy.
The first study found that tracking the quantitative extent of ILD (QILD) over time with high-resolution CT (HRCT) predicted poorer outcomes and could therefore act as a surrogate endpoint for mortality among patients with SSc-ILD. The other study identified associations between specific proteins from bronchoalveolar lavage (BAL) and the likelihood of ILD progression, although some associations were treatment dependent.
Jacob M. van Laar, MD, PhD, professor of rheumatology at the University Medical Center Utrecht (the Netherlands), who was not involved in the study, found the results intriguing and noted the importance of further validation in research before these biomarkers are considered for clinical use.
“It would be wonderful if we can tailor therapy based on BAL biomarkers in the future, as clinicians often struggle to decide on selection, timing, and duration of immunosuppressive treatment,” Dr. van Laar told this news organization. “This has become even more relevant with the introduction of new drugs such as nintedanib.”
Extent of ILD progression as a surrogate for mortality
Scleroderma Lung Study I involved 158 patients with SSc-ILD who were randomly assigned to receive either cyclophosphamide or placebo for 12 months. Scleroderma Lung Study II included 142 patients with SSc-ILD who were randomly assigned to receive either mycophenolate for 24 months or cyclophosphamide for 12 months followed by placebo for 12 months.
The researchers calculated QILD in the whole lung at baseline, at 12 months in the first trial, and at 24 months in the second trial. However, only 82 participants from the first trial and 90 participants from the second trial underwent HRCT. Demographic and disease characteristics were similar between the two groups on follow-up scans.
Follow-up continued for 12 years for patients in the first trial and 8 years in the second. The researchers compared survival rates between the 41% of participants from the first study and 31% of participants from the second study who had poorer QILD scores (at least a 2% increase) with the participants who had stable or improved scores (less than 2% increase).
Participants from both trials had significantly poorer long-term survival if their QILD scores had increased by at least 2% at follow-up (P = .01 for I; P = .019 for II). The association was no longer significant after adjustment for baseline FVC, age, and modified Rodnan skin score in the first trial (hazard ratio, 1.98; P = .089), but it remained significant for participants of the second trial (HR, 3.86; P = .014).
“Data from two independent trial cohorts demonstrated that radiographic progression of SSc-ILD at 1 and 2 years is associated with worse long-term survival,” Dr. Volkmann told attendees.
However, FVC did not significantly predict risk of mortality in either trial.
“To me, the most striking finding from the first study was that change in QILD performed better as a predictor of survival than change in FVC,” Dr. van Laar said in an interview. “This indicates QILD is fit for purpose and worth including in future clinical trials.”
Limitations of the study included lack of HRCT for all participants in the trials and the difference in timing (1 year and 2 years) of HRCT assessment between the two trials. The greater hazard ratio for worsened QILD in the second trial may suggest that assessment at 2 years provides more reliable data as a biomarker, Dr. Volkmann said.
“QILD may represent a better proxy for how a patient feels, functions, and survives than FVC,” she said.
Treatment-dependent biomarkers for worsening lung fibrosis
In the second study, the researchers looked for any associations between changes in the radiographic extent of SSc-ILD and 68 proteins from BAL.
“Being able to risk-stratify patients with interstitial lung disease at the time of diagnosis and predict which patients are likely to have a stable versus progressive disease course is critical for making important treatment decisions for these patients,” Dr. Volkmann told attendees.
The second study she presented involved Scleroderma Lung Study I. Of the 158 participants, 144 underwent a bronchoscopy, yielding BAL protein samples from 103 participants. The researchers determined the extent of radiographic fibrosis in the whole lung with quantitative imaging analysis of HRCT of the chest at baseline and 12 months.
Although the researchers identified several statistically significant associations between certain proteins and changes in radiographic fibrosis, “baseline protein levels were differentially associated with the course of ILD based on treatment status,” she told attendees.
For example, increased levels of the following proteins were linked to poor radiographic fibrosis scores for patients who received placebo:
- Granulocyte-macrophage colony-stimulating factor
- Interleukin-1
- Monocyte chemoattractant protein–3
- Chemokine ligand–5
- Transforming growth factor–beta
- Hepatocyte growth factor
- Stem cell factor
- IL-4
- TGF-alpha
Yet increases in these proteins predicted improvement in radiographic fibrosis in patients who had taken cyclophosphamide.
Independently of treatment, the researchers also identified an association between higher levels of fractalkine and poorer radiographic fibrosis scores and between higher IL-7 levels and improved radiographic fibrosis scores.
After adjusting for treatment arm and baseline severity of ILD, significant associations remained between change in radiographic fibrosis score and IL-1, MCP-3, surfactant protein C, IL-7 and CCL-5 levels.
“Biomarker discovery is really central to our ability to risk stratify patients with SSc-ILD,” Dr. Volkmann told attendees. “Understanding how biomarkers predict outcomes in treated and untreated patients may improve personalized medicine to patients with SSc-ILD and could also reveal novel treatment targets.”
Dr. van Laar said in an interview that this study’s biggest strength lay in its large sample size and in the comprehensiveness of the biomarkers studied.
“The findings are interesting from a research perspective and potentially relevant for clinical practice, but the utility of measuring biomarkers in BAL should be further studied for predictive value on clinical endpoints,” Dr. van Laar said. “BAL is an invasive procedure [that] is not routinely done.”
The research was funded by the National Institutes of Health. Dr. Volkmann has consulted for Boehringer Ingelheim and received grant funding from Corbus, Forbius, and Kadmon. Dr. van Laar has received grant funding or personal fees from Arthrogen, Arxx Therapeutics, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Gesynta, Leadiant, Merck Sharp & Dohme, Roche, Sanofi, and Thermofisher.
A version of this article first appeared on Medscape.com.
Quantitative assessment of the extent of interstitial lung disease in patients with systemic sclerosis and levels of certain proteins in bronchoalveolar lavage samples have potential for predicting mortality and disease progression, according to two analyses of data from the Scleroderma Lung Study I and II.
The analyses, presented at the annual European Congress of Rheumatology, aim to improve current prognostic abilities in patients with systemic sclerosis–interstitial lung disease (SSc-ILD). Although forced vital capacity is commonly used as a biomarker for survival in many SSc-ILD trials, other factors can affect FVC, such as respiratory muscle weakness and skin fibrosis. Further, FVC correlates poorly with patient-reported outcomes, explained first author Elizabeth Volkmann, MD, director of the scleroderma program at the University of California, Los Angeles, and the founder and codirector of the UCLA connective tissue disease–related interstitial lung disease program.
Dr. Volkmann presented two studies that investigated the potential of radiographic and protein biomarkers for predicting mortality and identifying patients at risk for ILD progression. The biomarkers may also help to identify patients who would benefit most from immunosuppressive therapy.
The first study found that tracking the quantitative extent of ILD (QILD) over time with high-resolution CT (HRCT) predicted poorer outcomes and could therefore act as a surrogate endpoint for mortality among patients with SSc-ILD. The other study identified associations between specific proteins from bronchoalveolar lavage (BAL) and the likelihood of ILD progression, although some associations were treatment dependent.
Jacob M. van Laar, MD, PhD, professor of rheumatology at the University Medical Center Utrecht (the Netherlands), who was not involved in the study, found the results intriguing and noted the importance of further validation in research before these biomarkers are considered for clinical use.
“It would be wonderful if we can tailor therapy based on BAL biomarkers in the future, as clinicians often struggle to decide on selection, timing, and duration of immunosuppressive treatment,” Dr. van Laar told this news organization. “This has become even more relevant with the introduction of new drugs such as nintedanib.”
Extent of ILD progression as a surrogate for mortality
Scleroderma Lung Study I involved 158 patients with SSc-ILD who were randomly assigned to receive either cyclophosphamide or placebo for 12 months. Scleroderma Lung Study II included 142 patients with SSc-ILD who were randomly assigned to receive either mycophenolate for 24 months or cyclophosphamide for 12 months followed by placebo for 12 months.
The researchers calculated QILD in the whole lung at baseline, at 12 months in the first trial, and at 24 months in the second trial. However, only 82 participants from the first trial and 90 participants from the second trial underwent HRCT. Demographic and disease characteristics were similar between the two groups on follow-up scans.
Follow-up continued for 12 years for patients in the first trial and 8 years in the second. The researchers compared survival rates between the 41% of participants from the first study and 31% of participants from the second study who had poorer QILD scores (at least a 2% increase) with the participants who had stable or improved scores (less than 2% increase).
Participants from both trials had significantly poorer long-term survival if their QILD scores had increased by at least 2% at follow-up (P = .01 for I; P = .019 for II). The association was no longer significant after adjustment for baseline FVC, age, and modified Rodnan skin score in the first trial (hazard ratio, 1.98; P = .089), but it remained significant for participants of the second trial (HR, 3.86; P = .014).
“Data from two independent trial cohorts demonstrated that radiographic progression of SSc-ILD at 1 and 2 years is associated with worse long-term survival,” Dr. Volkmann told attendees.
However, FVC did not significantly predict risk of mortality in either trial.
“To me, the most striking finding from the first study was that change in QILD performed better as a predictor of survival than change in FVC,” Dr. van Laar said in an interview. “This indicates QILD is fit for purpose and worth including in future clinical trials.”
Limitations of the study included lack of HRCT for all participants in the trials and the difference in timing (1 year and 2 years) of HRCT assessment between the two trials. The greater hazard ratio for worsened QILD in the second trial may suggest that assessment at 2 years provides more reliable data as a biomarker, Dr. Volkmann said.
“QILD may represent a better proxy for how a patient feels, functions, and survives than FVC,” she said.
Treatment-dependent biomarkers for worsening lung fibrosis
In the second study, the researchers looked for any associations between changes in the radiographic extent of SSc-ILD and 68 proteins from BAL.
“Being able to risk-stratify patients with interstitial lung disease at the time of diagnosis and predict which patients are likely to have a stable versus progressive disease course is critical for making important treatment decisions for these patients,” Dr. Volkmann told attendees.
The second study she presented involved Scleroderma Lung Study I. Of the 158 participants, 144 underwent a bronchoscopy, yielding BAL protein samples from 103 participants. The researchers determined the extent of radiographic fibrosis in the whole lung with quantitative imaging analysis of HRCT of the chest at baseline and 12 months.
Although the researchers identified several statistically significant associations between certain proteins and changes in radiographic fibrosis, “baseline protein levels were differentially associated with the course of ILD based on treatment status,” she told attendees.
For example, increased levels of the following proteins were linked to poor radiographic fibrosis scores for patients who received placebo:
- Granulocyte-macrophage colony-stimulating factor
- Interleukin-1
- Monocyte chemoattractant protein–3
- Chemokine ligand–5
- Transforming growth factor–beta
- Hepatocyte growth factor
- Stem cell factor
- IL-4
- TGF-alpha
Yet increases in these proteins predicted improvement in radiographic fibrosis in patients who had taken cyclophosphamide.
Independently of treatment, the researchers also identified an association between higher levels of fractalkine and poorer radiographic fibrosis scores and between higher IL-7 levels and improved radiographic fibrosis scores.
After adjusting for treatment arm and baseline severity of ILD, significant associations remained between change in radiographic fibrosis score and IL-1, MCP-3, surfactant protein C, IL-7 and CCL-5 levels.
“Biomarker discovery is really central to our ability to risk stratify patients with SSc-ILD,” Dr. Volkmann told attendees. “Understanding how biomarkers predict outcomes in treated and untreated patients may improve personalized medicine to patients with SSc-ILD and could also reveal novel treatment targets.”
Dr. van Laar said in an interview that this study’s biggest strength lay in its large sample size and in the comprehensiveness of the biomarkers studied.
“The findings are interesting from a research perspective and potentially relevant for clinical practice, but the utility of measuring biomarkers in BAL should be further studied for predictive value on clinical endpoints,” Dr. van Laar said. “BAL is an invasive procedure [that] is not routinely done.”
The research was funded by the National Institutes of Health. Dr. Volkmann has consulted for Boehringer Ingelheim and received grant funding from Corbus, Forbius, and Kadmon. Dr. van Laar has received grant funding or personal fees from Arthrogen, Arxx Therapeutics, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Gesynta, Leadiant, Merck Sharp & Dohme, Roche, Sanofi, and Thermofisher.
A version of this article first appeared on Medscape.com.
Quantitative assessment of the extent of interstitial lung disease in patients with systemic sclerosis and levels of certain proteins in bronchoalveolar lavage samples have potential for predicting mortality and disease progression, according to two analyses of data from the Scleroderma Lung Study I and II.
The analyses, presented at the annual European Congress of Rheumatology, aim to improve current prognostic abilities in patients with systemic sclerosis–interstitial lung disease (SSc-ILD). Although forced vital capacity is commonly used as a biomarker for survival in many SSc-ILD trials, other factors can affect FVC, such as respiratory muscle weakness and skin fibrosis. Further, FVC correlates poorly with patient-reported outcomes, explained first author Elizabeth Volkmann, MD, director of the scleroderma program at the University of California, Los Angeles, and the founder and codirector of the UCLA connective tissue disease–related interstitial lung disease program.
Dr. Volkmann presented two studies that investigated the potential of radiographic and protein biomarkers for predicting mortality and identifying patients at risk for ILD progression. The biomarkers may also help to identify patients who would benefit most from immunosuppressive therapy.
The first study found that tracking the quantitative extent of ILD (QILD) over time with high-resolution CT (HRCT) predicted poorer outcomes and could therefore act as a surrogate endpoint for mortality among patients with SSc-ILD. The other study identified associations between specific proteins from bronchoalveolar lavage (BAL) and the likelihood of ILD progression, although some associations were treatment dependent.
Jacob M. van Laar, MD, PhD, professor of rheumatology at the University Medical Center Utrecht (the Netherlands), who was not involved in the study, found the results intriguing and noted the importance of further validation in research before these biomarkers are considered for clinical use.
“It would be wonderful if we can tailor therapy based on BAL biomarkers in the future, as clinicians often struggle to decide on selection, timing, and duration of immunosuppressive treatment,” Dr. van Laar told this news organization. “This has become even more relevant with the introduction of new drugs such as nintedanib.”
Extent of ILD progression as a surrogate for mortality
Scleroderma Lung Study I involved 158 patients with SSc-ILD who were randomly assigned to receive either cyclophosphamide or placebo for 12 months. Scleroderma Lung Study II included 142 patients with SSc-ILD who were randomly assigned to receive either mycophenolate for 24 months or cyclophosphamide for 12 months followed by placebo for 12 months.
The researchers calculated QILD in the whole lung at baseline, at 12 months in the first trial, and at 24 months in the second trial. However, only 82 participants from the first trial and 90 participants from the second trial underwent HRCT. Demographic and disease characteristics were similar between the two groups on follow-up scans.
Follow-up continued for 12 years for patients in the first trial and 8 years in the second. The researchers compared survival rates between the 41% of participants from the first study and 31% of participants from the second study who had poorer QILD scores (at least a 2% increase) with the participants who had stable or improved scores (less than 2% increase).
Participants from both trials had significantly poorer long-term survival if their QILD scores had increased by at least 2% at follow-up (P = .01 for I; P = .019 for II). The association was no longer significant after adjustment for baseline FVC, age, and modified Rodnan skin score in the first trial (hazard ratio, 1.98; P = .089), but it remained significant for participants of the second trial (HR, 3.86; P = .014).
“Data from two independent trial cohorts demonstrated that radiographic progression of SSc-ILD at 1 and 2 years is associated with worse long-term survival,” Dr. Volkmann told attendees.
However, FVC did not significantly predict risk of mortality in either trial.
“To me, the most striking finding from the first study was that change in QILD performed better as a predictor of survival than change in FVC,” Dr. van Laar said in an interview. “This indicates QILD is fit for purpose and worth including in future clinical trials.”
Limitations of the study included lack of HRCT for all participants in the trials and the difference in timing (1 year and 2 years) of HRCT assessment between the two trials. The greater hazard ratio for worsened QILD in the second trial may suggest that assessment at 2 years provides more reliable data as a biomarker, Dr. Volkmann said.
“QILD may represent a better proxy for how a patient feels, functions, and survives than FVC,” she said.
Treatment-dependent biomarkers for worsening lung fibrosis
In the second study, the researchers looked for any associations between changes in the radiographic extent of SSc-ILD and 68 proteins from BAL.
“Being able to risk-stratify patients with interstitial lung disease at the time of diagnosis and predict which patients are likely to have a stable versus progressive disease course is critical for making important treatment decisions for these patients,” Dr. Volkmann told attendees.
The second study she presented involved Scleroderma Lung Study I. Of the 158 participants, 144 underwent a bronchoscopy, yielding BAL protein samples from 103 participants. The researchers determined the extent of radiographic fibrosis in the whole lung with quantitative imaging analysis of HRCT of the chest at baseline and 12 months.
Although the researchers identified several statistically significant associations between certain proteins and changes in radiographic fibrosis, “baseline protein levels were differentially associated with the course of ILD based on treatment status,” she told attendees.
For example, increased levels of the following proteins were linked to poor radiographic fibrosis scores for patients who received placebo:
- Granulocyte-macrophage colony-stimulating factor
- Interleukin-1
- Monocyte chemoattractant protein–3
- Chemokine ligand–5
- Transforming growth factor–beta
- Hepatocyte growth factor
- Stem cell factor
- IL-4
- TGF-alpha
Yet increases in these proteins predicted improvement in radiographic fibrosis in patients who had taken cyclophosphamide.
Independently of treatment, the researchers also identified an association between higher levels of fractalkine and poorer radiographic fibrosis scores and between higher IL-7 levels and improved radiographic fibrosis scores.
After adjusting for treatment arm and baseline severity of ILD, significant associations remained between change in radiographic fibrosis score and IL-1, MCP-3, surfactant protein C, IL-7 and CCL-5 levels.
“Biomarker discovery is really central to our ability to risk stratify patients with SSc-ILD,” Dr. Volkmann told attendees. “Understanding how biomarkers predict outcomes in treated and untreated patients may improve personalized medicine to patients with SSc-ILD and could also reveal novel treatment targets.”
Dr. van Laar said in an interview that this study’s biggest strength lay in its large sample size and in the comprehensiveness of the biomarkers studied.
“The findings are interesting from a research perspective and potentially relevant for clinical practice, but the utility of measuring biomarkers in BAL should be further studied for predictive value on clinical endpoints,” Dr. van Laar said. “BAL is an invasive procedure [that] is not routinely done.”
The research was funded by the National Institutes of Health. Dr. Volkmann has consulted for Boehringer Ingelheim and received grant funding from Corbus, Forbius, and Kadmon. Dr. van Laar has received grant funding or personal fees from Arthrogen, Arxx Therapeutics, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Gesynta, Leadiant, Merck Sharp & Dohme, Roche, Sanofi, and Thermofisher.
A version of this article first appeared on Medscape.com.
Atopic dermatitis
THE COMPARISON
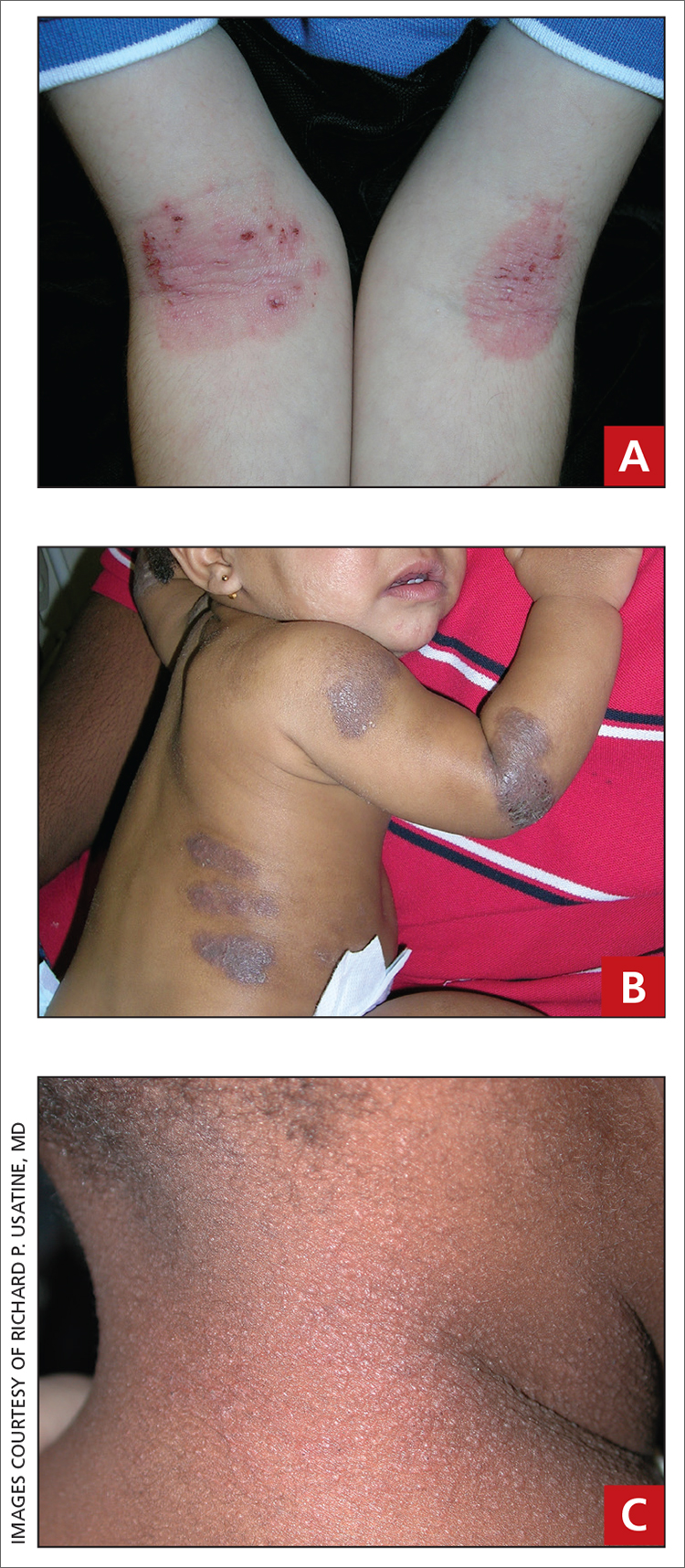
A Pink scaling plaques and erythematous erosions in the antecubital fossae of a 6-year-old White boy.
B Violaceous, hyperpigmented, nummular plaques on the back and extensor surface of the right arm of a 16-month-old Black girl.
C Atopic dermatitis and follicular prominence/accentuation on the neck of a young Black girl.
Epidemiology
People of African descent have the highest atopic dermatitis prevalence and severity.
Key clinical features in people with darker skin tones include:
- follicular prominence
- papular morphology
- prurigo nodules
- hyperpigmented, violaceous-brown or gray plaques instead of erythematous plaques
- lichenification
- treatment resistant.1,2
Worth noting
Postinflammatory hyperpigmentation and postinflammatory hypopigmentation may be more distressing to the patient/family than the atopic dermatitis itself.
Health disparity highlight
In the United States, patients with skin of color are more likely to be hospitalized with severe atopic dermatitis, have more substantial out-of-pocket costs, be underinsured, and have an increased number of missed days of work. Limited access to outpatient health care plays a role in exacerbating this health disparity.3,4
1. McKenzie C, Silverberg JI. The prevalence and persistence of atopic dermatitis in urban United States children. Ann Allergy Asthma Immunol. 2019;123:173-178.e1. doi:10.1016/j.anai.2019.05.014
2. Kim Y, Bloomberg M, Rifas-Shiman SL, et al. Racial/ethnic differences in incidence and persistence of childhood atopic dermatitis. J Invest Dermatol. 2019;139:827-834. doi:10.1016/j.jid.2018.10.029
3. Narla S, Hsu DY, Thyssen JP, et al. Predictors of hospitalization, length of stay, and costs of care among adult and pediatric inpatients with atopic dermatitis in the United States. Dermatitis. 2018;29:22-31. doi:10.1097/DER.0000000000000323
4. Silverberg JI. Health care utilization, patient costs, and access to care in US adults with eczema. JAMA Dermatol. 2015;151:743-752. doi:10.1001/jamadermatol.2014.5432
THE COMPARISON
A Pink scaling plaques and erythematous erosions in the antecubital fossae of a 6-year-old White boy.
B Violaceous, hyperpigmented, nummular plaques on the back and extensor surface of the right arm of a 16-month-old Black girl.
C Atopic dermatitis and follicular prominence/accentuation on the neck of a young Black girl.

Epidemiology
People of African descent have the highest atopic dermatitis prevalence and severity.
Key clinical features in people with darker skin tones include:
- follicular prominence
- papular morphology
- prurigo nodules
- hyperpigmented, violaceous-brown or gray plaques instead of erythematous plaques
- lichenification
- treatment resistant.1,2
Worth noting
Postinflammatory hyperpigmentation and postinflammatory hypopigmentation may be more distressing to the patient/family than the atopic dermatitis itself.
Health disparity highlight
In the United States, patients with skin of color are more likely to be hospitalized with severe atopic dermatitis, have more substantial out-of-pocket costs, be underinsured, and have an increased number of missed days of work. Limited access to outpatient health care plays a role in exacerbating this health disparity.3,4
THE COMPARISON
A Pink scaling plaques and erythematous erosions in the antecubital fossae of a 6-year-old White boy.
B Violaceous, hyperpigmented, nummular plaques on the back and extensor surface of the right arm of a 16-month-old Black girl.
C Atopic dermatitis and follicular prominence/accentuation on the neck of a young Black girl.

Epidemiology
People of African descent have the highest atopic dermatitis prevalence and severity.
Key clinical features in people with darker skin tones include:
- follicular prominence
- papular morphology
- prurigo nodules
- hyperpigmented, violaceous-brown or gray plaques instead of erythematous plaques
- lichenification
- treatment resistant.1,2
Worth noting
Postinflammatory hyperpigmentation and postinflammatory hypopigmentation may be more distressing to the patient/family than the atopic dermatitis itself.
Health disparity highlight
In the United States, patients with skin of color are more likely to be hospitalized with severe atopic dermatitis, have more substantial out-of-pocket costs, be underinsured, and have an increased number of missed days of work. Limited access to outpatient health care plays a role in exacerbating this health disparity.3,4
1. McKenzie C, Silverberg JI. The prevalence and persistence of atopic dermatitis in urban United States children. Ann Allergy Asthma Immunol. 2019;123:173-178.e1. doi:10.1016/j.anai.2019.05.014
2. Kim Y, Bloomberg M, Rifas-Shiman SL, et al. Racial/ethnic differences in incidence and persistence of childhood atopic dermatitis. J Invest Dermatol. 2019;139:827-834. doi:10.1016/j.jid.2018.10.029
3. Narla S, Hsu DY, Thyssen JP, et al. Predictors of hospitalization, length of stay, and costs of care among adult and pediatric inpatients with atopic dermatitis in the United States. Dermatitis. 2018;29:22-31. doi:10.1097/DER.0000000000000323
4. Silverberg JI. Health care utilization, patient costs, and access to care in US adults with eczema. JAMA Dermatol. 2015;151:743-752. doi:10.1001/jamadermatol.2014.5432
1. McKenzie C, Silverberg JI. The prevalence and persistence of atopic dermatitis in urban United States children. Ann Allergy Asthma Immunol. 2019;123:173-178.e1. doi:10.1016/j.anai.2019.05.014
2. Kim Y, Bloomberg M, Rifas-Shiman SL, et al. Racial/ethnic differences in incidence and persistence of childhood atopic dermatitis. J Invest Dermatol. 2019;139:827-834. doi:10.1016/j.jid.2018.10.029
3. Narla S, Hsu DY, Thyssen JP, et al. Predictors of hospitalization, length of stay, and costs of care among adult and pediatric inpatients with atopic dermatitis in the United States. Dermatitis. 2018;29:22-31. doi:10.1097/DER.0000000000000323
4. Silverberg JI. Health care utilization, patient costs, and access to care in US adults with eczema. JAMA Dermatol. 2015;151:743-752. doi:10.1001/jamadermatol.2014.5432
Argyria From a Topical Home Remedy
To the Editor:
Argyria is a rare disease caused by chronic exposure to products with high silver content (eg, oral ingestion, inhalation, percutaneous absorption). With time, the blood levels of silver surpass the body’s renal and hepatic excretory capacities that lead to silver granules being deposited in the skin and internal organs, including the liver, spleen, adrenal glands, and bone marrow.1 The cutaneous deposition results in a blue or blue-gray pigmentation of the skin, mucous membranes, and nails. Intervals of exposure that span from 8 months to 5 years prior to symptom onset have been described in the literature.2 The discoloration that results often is permanent, with no established way of effectively removing silver deposits from the tissue.3
A 22-year-old autistic man, who was completely dependent on his mother’s care, presented to the emergency department with a primary concern of abdominal pain. The mother reported that he was indicating abdominal pain by motioning to his stomach for the last 5 days. The mother also reported he did not have a bowel movement during this time, and she noticed his hands were shaking. Prior to presentation, the mother had given him 2 enemas and had him on a 3-day strict liquid fast consisting of water, lemon juice, cayenne pepper, honey, and orange juice. Notably, the mother had a strong history of using naturopathic remedies for treatment of her son’s ailments.
On admission, the patient was stable. There was a 2-point decrease in the patient’s body mass index over the last month. Initial serum electrolytes were highly abnormal with a serum sodium level of 124 mEq/L (reference range, 135–145 mEq/L), blood urea nitrogen of 3 mg/dL (reference range, 7–20 mg/dL), creatinine of 0.77 mg/dL (reference range, 0.74–1.35 mg/dL), and lactic acid of 2.1 mEq/L (reference range, 0.5–1 mEq/L). Serum osmolality was 272 mOsm/kg (reference range, 275–295 mOsm/kg). Urine osmolality was 114 mOsm/kg (reference range, 500–850 mOsm/kg) with a low-normal urine sodium level of 41 mmol/24 hr (reference range, 40–220 mmol/24 hr). Abnormalities were felt to be secondary to malnutrition from the strict liquid diet (blood urea nitrogen and creatinine ratio of 3:1 suggestive of notable protein calorie malnutrition). The patient was given 1 L of normal saline in the emergency department, with further fluids held so as not to increase serum sodium level too rapidly. A regular diet was started.
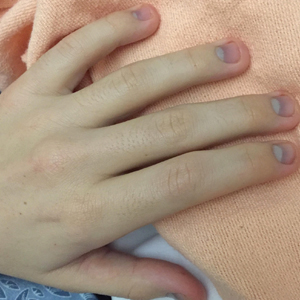
Physical examination revealed dry mucosal membranes but otherwise was unremarkable. Active bowel sounds were noted, as well as a soft, nontender, and nondistended abdomen; however, when examining the patient’s hands for reported shaking, a distinct abnormality of the nails was noticed. The patient had slate blue discoloration of the lunula, along with hyperpigmented violaceous discoloration of the proximal nail bed on all 10 fingernails (Figure 1). No abnormalities were seen on the toenails. The mother had a distinct bluish gray discoloration of the face as well as similar nail findings (Figure 2), strongly suggestive of colloidal silver use. An urgent serum silver level was ordered on the patient as well as a heavy metal panel. The mother was found applying numerous “natural remedies” to the patient’s skin while in the hospital, including a liquid spray and lotion, both in unmarked bottles. At that time, the mother was informed that no external supplements should be applied to her son. The serum silver level was elevated substantially at 94.3 ng/mL (reference range, <1.0 ng/mL). When the mother was confronted, she initially denied use of silver but later admitted to notable silver content in the cream she was applying to her son’s skin. The mother reported that she read online that colloidal silver had been historically used to cure numerous ailments and she was ordering products from an online company. She was counseled on the dangers of both topical application and ingestion of silver, and all supplements were removed from the home.
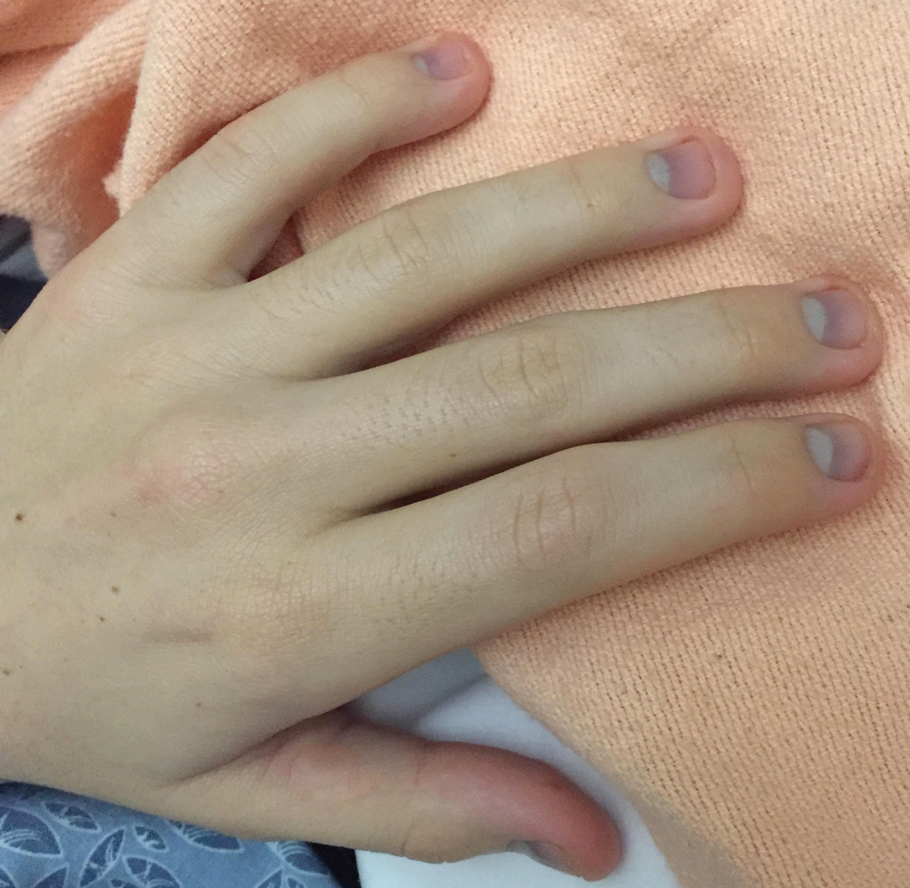
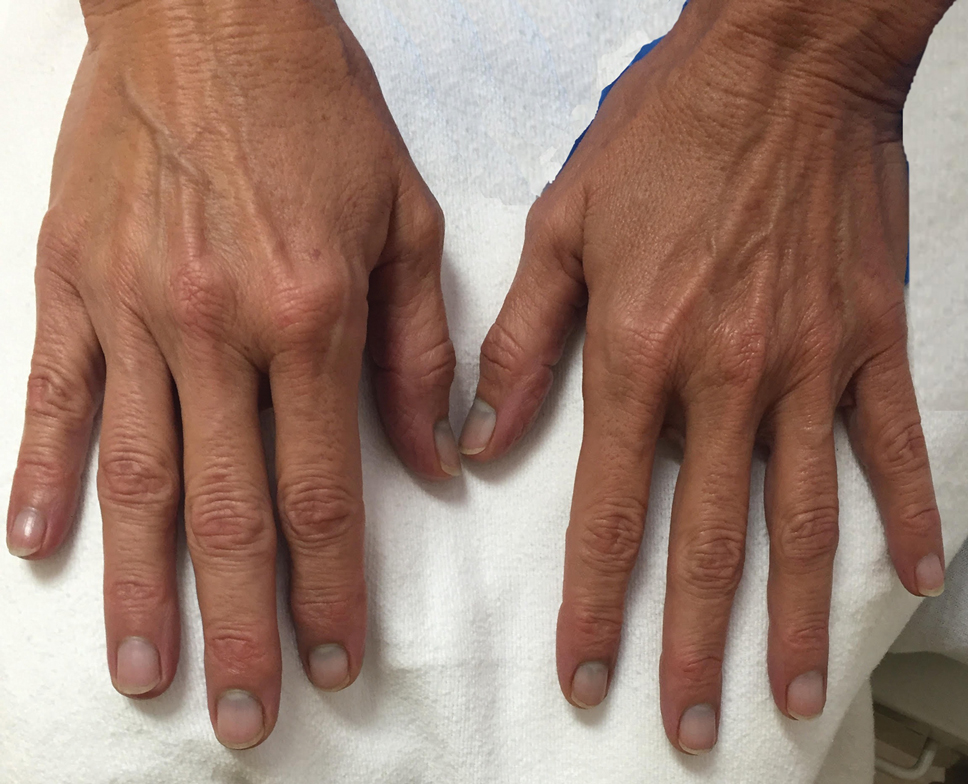
Argyria is a rare condition caused by chronic exposure to silver and is characterized by a blue-gray pigmentation in the skin and appendages, mucous membranes, and internal organs.4 Clinically, argyria is classified as generalized or localized. Generalized argyria results from ingestion or inhalation of silver compounds, where granules deposit preferentially in sun-exposed areas of skin as well as internal organs, with the highest concentration in the liver, spleen, and adrenal glands; discoloration often is permanent.5 On the contrary, localized argyria results from direct external contact with silver and granules deposited in the hands, eyes, and mucosa.5 Although the exact mechanism of penetration from topical silver remains unknown, it is thought to enter via the eccrine sweat ducts, as histopathology reveals silver granules found in highest concentration surrounding sweat glands in the dermis.6
Initial differential diagnoses for altered nail pigmentation include drug-induced causes, systemic diseases, cyanosis, and exposure to metals.7 The most commonly indicated medications resulting in blue nail pigment changes include antimalarials, minocycline, zidovudine, and phenothiazine. Systemic diseases that may cause blue nail color change include Wilson disease, hemochromatosis, Addison disease, methemoglobinemia, and alkaptonuria.7 Metals include gold, mercury, arsenic, bismuth, lead, and silver.4 After a thorough review of the patient’s medications and lack of support for any underlying disease process, contact with metals, particularly silver, was ranked highly on our differential list. In support of this theory, the mother’s bluish gray facial skin led to high clinical suspicion that she was ingesting colloidal silver and also was exposing her son to silver.
Treatment of argyria is challenging but first and foremost involves discontinuation of the source of chronic silver exposure. Unfortunately, the discoloration of generalized argyria often is permanent. Sunscreen can be used to help prevent any further darkening of pigment. The pigment in localized argyria has been reported to slowly fade with time, and there also have been reports of successful treatment using a low-fluence Q-switched 1064-nm Nd:YAG laser.8
- Molina-Hernandez AI, Diaz-Gonzalez JM, Saeb-Lima M, et al. Argyria after silver nitrate intake: case report and brief review of literature. Indian J Dermatol. 2015;60:520.
- Lencastre A, Lobo M, João A. Argyria—case report. An Bras Dermatol. 2013;88:413-416.
- Park S-W, Kim J-H, Shin H-T, et al. An effective modality for argyria treatment: Q-switched 1,064-nm Nd:YAG laser. Ann Dermatol. 2013;25:511-512.
- Molina-Hernandez AI, Diaz-Gonzalez JM, Saeb-Lima M, et al. Argyria after silver nitrate intake: case report and brief review of literature. Indian J Dermatol. 2015;60:520.
- Garcias-Ladaria J, Hernandez-Bel P, Torregrosa-Calatayud JL, et al. Localized cutaneous argyria: a report of 2 cases. Actas Dermosifiliogr. 2013;104:253-254.
- Kapur N, Landon G, Yu RC. Localized argyria in an antique restorer. Br J Dermatol. 2001;144:191-192.
- Kubba A, Kubba R, Batrani M, Pal T. Argyria an unrecognized cause of cutaneous pigmentation in Indian patients: a case series and review of the literature. Indian J Dermatol Venereol Leprol. 2013;79:805-811.
- Han TY, Chang HS, Lee HK, et al. Successful treatment of argyria using a low-fluence Q-switched 1064-nm Nd:YAG laser. Int J Dermatol. 2011;50:751-753.
To the Editor:
Argyria is a rare disease caused by chronic exposure to products with high silver content (eg, oral ingestion, inhalation, percutaneous absorption). With time, the blood levels of silver surpass the body’s renal and hepatic excretory capacities that lead to silver granules being deposited in the skin and internal organs, including the liver, spleen, adrenal glands, and bone marrow.1 The cutaneous deposition results in a blue or blue-gray pigmentation of the skin, mucous membranes, and nails. Intervals of exposure that span from 8 months to 5 years prior to symptom onset have been described in the literature.2 The discoloration that results often is permanent, with no established way of effectively removing silver deposits from the tissue.3
A 22-year-old autistic man, who was completely dependent on his mother’s care, presented to the emergency department with a primary concern of abdominal pain. The mother reported that he was indicating abdominal pain by motioning to his stomach for the last 5 days. The mother also reported he did not have a bowel movement during this time, and she noticed his hands were shaking. Prior to presentation, the mother had given him 2 enemas and had him on a 3-day strict liquid fast consisting of water, lemon juice, cayenne pepper, honey, and orange juice. Notably, the mother had a strong history of using naturopathic remedies for treatment of her son’s ailments.
On admission, the patient was stable. There was a 2-point decrease in the patient’s body mass index over the last month. Initial serum electrolytes were highly abnormal with a serum sodium level of 124 mEq/L (reference range, 135–145 mEq/L), blood urea nitrogen of 3 mg/dL (reference range, 7–20 mg/dL), creatinine of 0.77 mg/dL (reference range, 0.74–1.35 mg/dL), and lactic acid of 2.1 mEq/L (reference range, 0.5–1 mEq/L). Serum osmolality was 272 mOsm/kg (reference range, 275–295 mOsm/kg). Urine osmolality was 114 mOsm/kg (reference range, 500–850 mOsm/kg) with a low-normal urine sodium level of 41 mmol/24 hr (reference range, 40–220 mmol/24 hr). Abnormalities were felt to be secondary to malnutrition from the strict liquid diet (blood urea nitrogen and creatinine ratio of 3:1 suggestive of notable protein calorie malnutrition). The patient was given 1 L of normal saline in the emergency department, with further fluids held so as not to increase serum sodium level too rapidly. A regular diet was started.
Physical examination revealed dry mucosal membranes but otherwise was unremarkable. Active bowel sounds were noted, as well as a soft, nontender, and nondistended abdomen; however, when examining the patient’s hands for reported shaking, a distinct abnormality of the nails was noticed. The patient had slate blue discoloration of the lunula, along with hyperpigmented violaceous discoloration of the proximal nail bed on all 10 fingernails (Figure 1). No abnormalities were seen on the toenails. The mother had a distinct bluish gray discoloration of the face as well as similar nail findings (Figure 2), strongly suggestive of colloidal silver use. An urgent serum silver level was ordered on the patient as well as a heavy metal panel. The mother was found applying numerous “natural remedies” to the patient’s skin while in the hospital, including a liquid spray and lotion, both in unmarked bottles. At that time, the mother was informed that no external supplements should be applied to her son. The serum silver level was elevated substantially at 94.3 ng/mL (reference range, <1.0 ng/mL). When the mother was confronted, she initially denied use of silver but later admitted to notable silver content in the cream she was applying to her son’s skin. The mother reported that she read online that colloidal silver had been historically used to cure numerous ailments and she was ordering products from an online company. She was counseled on the dangers of both topical application and ingestion of silver, and all supplements were removed from the home.


Argyria is a rare condition caused by chronic exposure to silver and is characterized by a blue-gray pigmentation in the skin and appendages, mucous membranes, and internal organs.4 Clinically, argyria is classified as generalized or localized. Generalized argyria results from ingestion or inhalation of silver compounds, where granules deposit preferentially in sun-exposed areas of skin as well as internal organs, with the highest concentration in the liver, spleen, and adrenal glands; discoloration often is permanent.5 On the contrary, localized argyria results from direct external contact with silver and granules deposited in the hands, eyes, and mucosa.5 Although the exact mechanism of penetration from topical silver remains unknown, it is thought to enter via the eccrine sweat ducts, as histopathology reveals silver granules found in highest concentration surrounding sweat glands in the dermis.6
Initial differential diagnoses for altered nail pigmentation include drug-induced causes, systemic diseases, cyanosis, and exposure to metals.7 The most commonly indicated medications resulting in blue nail pigment changes include antimalarials, minocycline, zidovudine, and phenothiazine. Systemic diseases that may cause blue nail color change include Wilson disease, hemochromatosis, Addison disease, methemoglobinemia, and alkaptonuria.7 Metals include gold, mercury, arsenic, bismuth, lead, and silver.4 After a thorough review of the patient’s medications and lack of support for any underlying disease process, contact with metals, particularly silver, was ranked highly on our differential list. In support of this theory, the mother’s bluish gray facial skin led to high clinical suspicion that she was ingesting colloidal silver and also was exposing her son to silver.
Treatment of argyria is challenging but first and foremost involves discontinuation of the source of chronic silver exposure. Unfortunately, the discoloration of generalized argyria often is permanent. Sunscreen can be used to help prevent any further darkening of pigment. The pigment in localized argyria has been reported to slowly fade with time, and there also have been reports of successful treatment using a low-fluence Q-switched 1064-nm Nd:YAG laser.8
To the Editor:
Argyria is a rare disease caused by chronic exposure to products with high silver content (eg, oral ingestion, inhalation, percutaneous absorption). With time, the blood levels of silver surpass the body’s renal and hepatic excretory capacities that lead to silver granules being deposited in the skin and internal organs, including the liver, spleen, adrenal glands, and bone marrow.1 The cutaneous deposition results in a blue or blue-gray pigmentation of the skin, mucous membranes, and nails. Intervals of exposure that span from 8 months to 5 years prior to symptom onset have been described in the literature.2 The discoloration that results often is permanent, with no established way of effectively removing silver deposits from the tissue.3
A 22-year-old autistic man, who was completely dependent on his mother’s care, presented to the emergency department with a primary concern of abdominal pain. The mother reported that he was indicating abdominal pain by motioning to his stomach for the last 5 days. The mother also reported he did not have a bowel movement during this time, and she noticed his hands were shaking. Prior to presentation, the mother had given him 2 enemas and had him on a 3-day strict liquid fast consisting of water, lemon juice, cayenne pepper, honey, and orange juice. Notably, the mother had a strong history of using naturopathic remedies for treatment of her son’s ailments.
On admission, the patient was stable. There was a 2-point decrease in the patient’s body mass index over the last month. Initial serum electrolytes were highly abnormal with a serum sodium level of 124 mEq/L (reference range, 135–145 mEq/L), blood urea nitrogen of 3 mg/dL (reference range, 7–20 mg/dL), creatinine of 0.77 mg/dL (reference range, 0.74–1.35 mg/dL), and lactic acid of 2.1 mEq/L (reference range, 0.5–1 mEq/L). Serum osmolality was 272 mOsm/kg (reference range, 275–295 mOsm/kg). Urine osmolality was 114 mOsm/kg (reference range, 500–850 mOsm/kg) with a low-normal urine sodium level of 41 mmol/24 hr (reference range, 40–220 mmol/24 hr). Abnormalities were felt to be secondary to malnutrition from the strict liquid diet (blood urea nitrogen and creatinine ratio of 3:1 suggestive of notable protein calorie malnutrition). The patient was given 1 L of normal saline in the emergency department, with further fluids held so as not to increase serum sodium level too rapidly. A regular diet was started.
Physical examination revealed dry mucosal membranes but otherwise was unremarkable. Active bowel sounds were noted, as well as a soft, nontender, and nondistended abdomen; however, when examining the patient’s hands for reported shaking, a distinct abnormality of the nails was noticed. The patient had slate blue discoloration of the lunula, along with hyperpigmented violaceous discoloration of the proximal nail bed on all 10 fingernails (Figure 1). No abnormalities were seen on the toenails. The mother had a distinct bluish gray discoloration of the face as well as similar nail findings (Figure 2), strongly suggestive of colloidal silver use. An urgent serum silver level was ordered on the patient as well as a heavy metal panel. The mother was found applying numerous “natural remedies” to the patient’s skin while in the hospital, including a liquid spray and lotion, both in unmarked bottles. At that time, the mother was informed that no external supplements should be applied to her son. The serum silver level was elevated substantially at 94.3 ng/mL (reference range, <1.0 ng/mL). When the mother was confronted, she initially denied use of silver but later admitted to notable silver content in the cream she was applying to her son’s skin. The mother reported that she read online that colloidal silver had been historically used to cure numerous ailments and she was ordering products from an online company. She was counseled on the dangers of both topical application and ingestion of silver, and all supplements were removed from the home.


Argyria is a rare condition caused by chronic exposure to silver and is characterized by a blue-gray pigmentation in the skin and appendages, mucous membranes, and internal organs.4 Clinically, argyria is classified as generalized or localized. Generalized argyria results from ingestion or inhalation of silver compounds, where granules deposit preferentially in sun-exposed areas of skin as well as internal organs, with the highest concentration in the liver, spleen, and adrenal glands; discoloration often is permanent.5 On the contrary, localized argyria results from direct external contact with silver and granules deposited in the hands, eyes, and mucosa.5 Although the exact mechanism of penetration from topical silver remains unknown, it is thought to enter via the eccrine sweat ducts, as histopathology reveals silver granules found in highest concentration surrounding sweat glands in the dermis.6
Initial differential diagnoses for altered nail pigmentation include drug-induced causes, systemic diseases, cyanosis, and exposure to metals.7 The most commonly indicated medications resulting in blue nail pigment changes include antimalarials, minocycline, zidovudine, and phenothiazine. Systemic diseases that may cause blue nail color change include Wilson disease, hemochromatosis, Addison disease, methemoglobinemia, and alkaptonuria.7 Metals include gold, mercury, arsenic, bismuth, lead, and silver.4 After a thorough review of the patient’s medications and lack of support for any underlying disease process, contact with metals, particularly silver, was ranked highly on our differential list. In support of this theory, the mother’s bluish gray facial skin led to high clinical suspicion that she was ingesting colloidal silver and also was exposing her son to silver.
Treatment of argyria is challenging but first and foremost involves discontinuation of the source of chronic silver exposure. Unfortunately, the discoloration of generalized argyria often is permanent. Sunscreen can be used to help prevent any further darkening of pigment. The pigment in localized argyria has been reported to slowly fade with time, and there also have been reports of successful treatment using a low-fluence Q-switched 1064-nm Nd:YAG laser.8
- Molina-Hernandez AI, Diaz-Gonzalez JM, Saeb-Lima M, et al. Argyria after silver nitrate intake: case report and brief review of literature. Indian J Dermatol. 2015;60:520.
- Lencastre A, Lobo M, João A. Argyria—case report. An Bras Dermatol. 2013;88:413-416.
- Park S-W, Kim J-H, Shin H-T, et al. An effective modality for argyria treatment: Q-switched 1,064-nm Nd:YAG laser. Ann Dermatol. 2013;25:511-512.
- Molina-Hernandez AI, Diaz-Gonzalez JM, Saeb-Lima M, et al. Argyria after silver nitrate intake: case report and brief review of literature. Indian J Dermatol. 2015;60:520.
- Garcias-Ladaria J, Hernandez-Bel P, Torregrosa-Calatayud JL, et al. Localized cutaneous argyria: a report of 2 cases. Actas Dermosifiliogr. 2013;104:253-254.
- Kapur N, Landon G, Yu RC. Localized argyria in an antique restorer. Br J Dermatol. 2001;144:191-192.
- Kubba A, Kubba R, Batrani M, Pal T. Argyria an unrecognized cause of cutaneous pigmentation in Indian patients: a case series and review of the literature. Indian J Dermatol Venereol Leprol. 2013;79:805-811.
- Han TY, Chang HS, Lee HK, et al. Successful treatment of argyria using a low-fluence Q-switched 1064-nm Nd:YAG laser. Int J Dermatol. 2011;50:751-753.
- Molina-Hernandez AI, Diaz-Gonzalez JM, Saeb-Lima M, et al. Argyria after silver nitrate intake: case report and brief review of literature. Indian J Dermatol. 2015;60:520.
- Lencastre A, Lobo M, João A. Argyria—case report. An Bras Dermatol. 2013;88:413-416.
- Park S-W, Kim J-H, Shin H-T, et al. An effective modality for argyria treatment: Q-switched 1,064-nm Nd:YAG laser. Ann Dermatol. 2013;25:511-512.
- Molina-Hernandez AI, Diaz-Gonzalez JM, Saeb-Lima M, et al. Argyria after silver nitrate intake: case report and brief review of literature. Indian J Dermatol. 2015;60:520.
- Garcias-Ladaria J, Hernandez-Bel P, Torregrosa-Calatayud JL, et al. Localized cutaneous argyria: a report of 2 cases. Actas Dermosifiliogr. 2013;104:253-254.
- Kapur N, Landon G, Yu RC. Localized argyria in an antique restorer. Br J Dermatol. 2001;144:191-192.
- Kubba A, Kubba R, Batrani M, Pal T. Argyria an unrecognized cause of cutaneous pigmentation in Indian patients: a case series and review of the literature. Indian J Dermatol Venereol Leprol. 2013;79:805-811.
- Han TY, Chang HS, Lee HK, et al. Successful treatment of argyria using a low-fluence Q-switched 1064-nm Nd:YAG laser. Int J Dermatol. 2011;50:751-753.
Practice Points
- Argyria results from chronic exposure to products with a high silver content and may result in abnormalities of the skin and internal organs.
- Examination of the fingernails can provide important clues to underlying systemic conditions or external exposures.
Squamoid Eccrine Ductal Carcinoma
Squamoid eccrine ductal carcinoma (SEDC) is an aggressive underrecognized cutaneous malignancy of unknown etiology.1 It is most likely to occur in sun-exposed areas of the body, most commonly the head and neck. Risk factors include male sex, increased age, and chronic immunosuppression.1-4 Current reports suggest that SEDC is likely a high-grade subtype of squamous cell carcinoma (SCC) with a high risk for local recurrence (25%) and metastasis (13%).1,3,5,6 There are as few as 56 cases of SEDC reported in the literature; however, the number of cases may be closer to 100 due to SEDC being classified as either adenosquamous carcinoma of the skin or ductal eccrine carcinoma with squamous differentiation.1
Clinically, SEDC mimics keratinocyte carcinomas. Histologically, SEDC is biphasic, with a superficial portion resembling well-differentiated SCC and a deeply invasive portion having infiltrative irregular cords with ductal differentiation. Perineural invasion (PNI) frequently is present. Multiple connections to the overlying epidermis also can be seen, serving as a subtle clue to the diagnosis on broad superficial specimens.1-3 Due to superficial sampling, approximately 50% of reported cases are misdiagnosed as SCC during the initial biopsy.4 The diagnosis of SEDC often is made during complete excision when deeper tissue is sampled. Establishing an accurate diagnosis is important given the more aggressive nature of SEDC compared with SCC and its proclivity for PNI.1,3,6 The purpose of this review is to increase awareness of this underrecognized entity and describe the histologic findings that help distinguish SEDC from SCC.
Patient Chart Review
We reviewed chart notes as well as frozen and formalin-fixed paraffin-embedded tissue sections from all 5 patients diagnosed with SEDC at a single institution between November 2018 and May 2020. The mean age of patients was 81 years, and 4 were male. Four of the patients presented for MMS with a preoperative diagnosis of SCC per the original biopsy results. Only 1 patient had a preoperative diagnosis of SEDC. The details of each case are recorded in the Table. All tumors were greater than 2 cm in diameter on initial presentation, were located on the head, and clinically resembled keratinocyte carcinoma with either a nodular or plaquelike appearance (Figure 1).
Intraoperative histologic examination of the excised tissue revealed a biphasic pattern consisting of superficial SCC features overlying deeper dermal and subcutaneous infiltrative malignant ductal elements with gland formation in all 5 patients (Figures 2–4). Immunohistochemical staining with cytokeratin AE1/AE3 revealed thin strands of carcinoma in the mid to deeper dermis with squamous differentiation and eccrine ductal differentiation (Figure 5), thus confirming the diagnosis in all 5 patients.
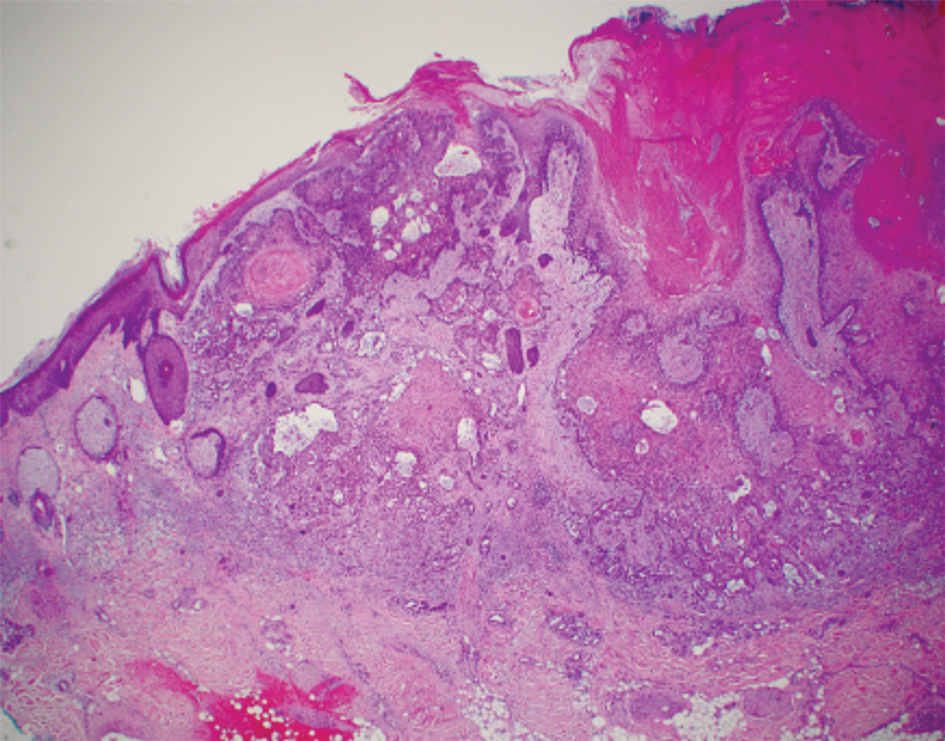


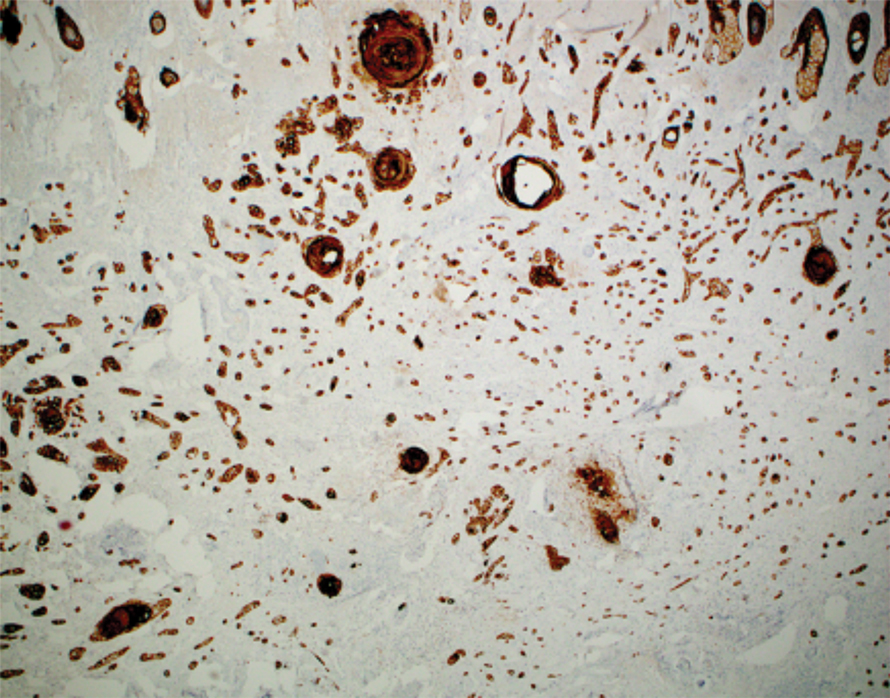
The median depth of tumor invasion was 4.1 mm (range, 2.2–5.45 mm). Ulceration was seen in 3 of the patients, and PNI of large-caliber nerves was observed in all 5 patients. A connection with the overlying epidermis was present in all 5 patients. All 5 patients required more than 1 Mohs stage for complete tumor clearance (Table).
In 4 of the patients, nodal imaging performed at the time of diagnosis revealed no evidence of metastasis. Two patients received adjuvant radiation therapy, and none demonstrated evidence of recurrence. The mean follow-up time was 11 months (range, 6.5–18 months) for the 4 cases with available follow-up data (Table).
Literature Review
A PubMed review of the literature using the search term squamoid eccrine ductal carcinoma resulted in 28 articles, 19 of which were included in the review based on inclusion criteria (original articles available in English, in full text, and pertained to SEDC). Our review yielded 56 cases of SEDC.1-19 The mean age of patients with SEDC was 72 years. The number of male and female cases was 52% (29/56) and 48% (27/56), respectively. The most common location of SEDC was on the head or neck (71% [40/56]), followed by the extremities (19% [11/56]). Immunosuppression was noted in 9% (5/56) of cases. Wide local excision was the most commonly employed treatment modality (91% [51/56]), with MMS being used in 4 patients (7%). Adjuvant radiation was reported in 5% (3/56) of cases. Perineural invasion was reported in 34% (19/56) of cases. Recurrence was seen in 23% (13/56) of cases, with a mean time to recurrence of 10.4 months. Metastasis to regional lymph nodes was observed in 13% (7/56) of cases, with 7% (4/56) of those cases having distant metastases.
Comment
Squamoid eccrine ductal carcinoma was successfully treated with MMS in all 5 of the patients we reviewed. Recognition of a distinct biphasic pattern consisting of squamous differentiation superficially with epidermal connection overlying deeper dermal and subcutaneous infiltrative malignant ductal elements with gland formation should lead to consideration of this diagnosis. A thorough inspection for PNI also should be performed, as this finding was present in all of 5 cases and in 34% of reported cases in our literature review.
The differential diagnosis for SEDC includes SCC, metastatic adenocarcinoma with squamoid features, and eccrine tumors, including eccrine poroma, microcystic adnexal carcinoma (MAC), and porocarcinoma with squamous differentiation. The combination of histologic features with the immunoexpression profile of carcinoembryonic antigen (CEA), epithelial membrane antigen (EMA), cytokeratin (CK) 5/6, and p63 can effectively exclude the other entities in the differential and confirm the diagnosis of SEDC.1,3,4 While the diagnosis of SEDC relies on the specific histologic features of multiple surface attachments and superficial squamoid changes with deep ductular elements, immunohistochemistry can nonetheless be adjunctive in difficult cases. Positive immunohistochemical staining for CEA and EMA can help to highlight and delineate true glandular elements, whereas CK5/6 highlights the overall contour of the tumor, displaying more clearly the multiple epidermal attachments and the subtle infiltrative nature of the deeper components of invasive cords and ducts. In addition, the combination of CK5/6 and p63 positivity supports the primary cutaneous nature of the lesion rather than metastatic adenocarcinoma.13,20 Other markers of eccrine secretory coils, such as CK7, CAM5.2, and S100, also are sometimes used for confirmation, some of which can aid in distinction from noneccrine sweat gland differentiation, as CK7 and CAM5.2 are negative in both luminal and basal cells of the dermal duct while being positive within the secretory coil, and S100 protein is expressed within eccrine secretory coil but negative within the apocrine sweat glands.2,4,21
The clinical findings from our chart review corroborated those reported in the literature. The mean age of SEDC in the 5 patients we reviewed was 81 years, and all cases presented on the head, consistent with the findings observed in the literature. Although 4 of our cases were male, there may not be a difference in risk based on sex as previously thought.1 Our literature review revealed an almost equivalent percentage of male and female cases, with 52% being male.
Immunosuppression has been associated with an increased risk for SEDC. Our literature review revealed that approximately 9% (5/56) of cases occurred in immunosuppressed individuals. Two of these reported cases were in the setting of underlying chronic lymphocytic leukemia, 2 in individuals with a history of organ transplant, and 1 treated with azathioprine for myasthenia gravis.2,4,10,12,13 Our chart review supported this correlation, as all 5 patients had a medical history potentially consistent with being in an immunocompromised state (Table). Notably, patient 5 represents a unique case of SEDC occurring in the setting of HIV. The patient had HIV for 33 years, with his most recent CD4+ count of 794 mm3 and HIV-1 RNA load of 35 copies/mL. Given that HIV-positive individuals may have more than a 2-fold increased risk of SCC, a greater degree of suspicion for SEDC should be maintained for these patients.22,23
The etiology of SEDC is controversial but is thought to be either an SCC arising from eccrine glands or a variant of eccrine carcinoma with extensive squamoid differentiation.4,6,13,14,17,24 While SEDC certainly appears to share the proclivity for PNI with the malignant eccrine tumor MAC, it is simultaneously quite distinct, demonstrating nuclear pleomorphism and mitotic activity, both of which are lacking in the bland nature of MACs.12,25
The exact prevalence of SEDC is difficult to ascertain because of its frequent misdiagnosis and variable nomenclature used within the literature. Most reported cases of SEDC are mistakenly diagnosed as SCC on the initial shave or punch biopsy because of superficial sampling. This also was the case in 4 of the patients we reviewed. In addition, there are reported cases of SEDC that were referred to by the investigators as cutaneous adenosquamous carcinoma (cASC), among other descriptors, such as ductal eccrine carcinoma with squamous differentiation, adnexal carcinoma with squamous and ductal differentiation, and syringoid eccrine carcinoma.26-32 While the World Health Organization classifies SEDC as a distinct variant of cASC, which is a rare variant of SCC in itself, the 2 can be differentiated. Despite the similar clinical and histologic features shared between cASC and SEDC, the neoplastic aggregates in SEDC exhibit ductal differentiation containing lumina positive for CEA and EMA.4 Overall, we favor the term squamoid eccrine ductal carcinoma, as there has recently been more uniformity for the designation of this disease entity as such.
It is unclear whether the high incidence of local recurrence (23% [13/56]) of SEDC reported in the literature is related to the treatment modality employed (ie, wide local excision) or due to the innate aggressiveness of SEDC.1,3,5 The literature has shown that MMS has lower recurrence rates than other treatments at 5-year follow-up for SCC (3.1%–5%) and eccrine carcinomas (0%–5%).33,34 Although studies assessing tumor behavior or comparing treatment modalities are limited because of the rarity and underrecognition of SEDC, MMS has been used several times for SEDC with only 1 recurrence reported.4,13,17,24 Given that all 5 of the patients we reviewed required more than 1 Mohs stage for complete tumor clearance and none demonstrated evidence of recurrence or metastasis (Table), we recommend MMS as the treatment of choice for SEDC.
Conclusion
Squamoid eccrine ductal carcinoma is a rare but likely underdiagnosed cutaneous tumor of uncertain etiology. Because of its propensity for recurrence and metastasis, excision of SEDC with complete circumferential peripheral and deep margin assessment with close follow-up is recommended.
- van der Horst MP, Garcia-Herrera A, Markiewicz D, et al. Squamoid eccrine ductal carcinoma: a clinicopathologic study of 30 cases. Am J Surg Pathol. 2016;40:755-760.
- Jacob J, Kugelman L. Squamoid eccrine ductal carcinoma. Cutis. 2018;101:378-380, 385.
- Yim S, Lee YH, Chae SW, et al. Squamoid eccrine ductal carcinoma of the ear helix. Clin Case Rep. 2019;7:1409-1411.
- Terushkin E, Leffell DJ, Futoryan T, et al. Squamoid eccrine ductal carcinoma: a case report and review of the literature. Am J Dermatopathol. 2010;32:287-292.
- Jung YH, Jo HJ, Kang MS. Squamoid eccrine ductal carcinoma of the scalp. Korean J Pathol. 2012;46:278-281.
- Saraiva MI, Vieira MA, Portocarrero LK, et al. Squamoid eccrine ductal carcinoma. An Bras Dermatol. 2016;91:799-802.
- Phan K, Kim L, Lim P, et al. A case report of temple squamoid eccrine ductal carcinoma: a diagnostic challenge beneath the tip of the iceberg. Dermatol Ther. 2020;33:E13213.
- McKissack SS, Wohltmann W, Dalton SR, et al. Squamoid eccrine ductal carcinoma: an aggressive mimicker of squamous cell carcinoma. Am J Dermatopathol. 2019;41:140-143.
- Lobo-Jardim MM, Souza BdCE, Kakizaki P, et al. Dermoscopy of squamoid eccrine ductal carcinoma: an aid for early diagnosis. An Bras Dermatol. 2018;93:893-895.
- Chan H, Howard V, Moir D, et al. Squamoid eccrine ductal carcinoma of the scalp. Aust J Dermatol. 2016;57:E117-E119.
- Wang B, Jarell AD, Bingham JL, et al. PET/CT imaging of squamoid eccrine ductal carcinoma. Clin Nucl Med. 2015;40:322-324.
- Frouin E, Vignon-Pennamen MD, Balme B, et al. Anatomoclinical study of 30 cases of sclerosing sweat duct carcinomas (microcystic adnexal carcinoma, syringomatous carcinoma and squamoid eccrine ductal carcinoma). J Eur Acad Dermatol Venereol. 2015;29:1978-1994.
- Clark S, Young A, Piatigorsky E, et al. Mohs micrographic surgery in the setting of squamoid eccrine ductal carcinoma: addressing a diagnostic and therapeutic challenge. J Clin Aesthet Dermatol. 2013;6:33-36.
- Pusiol T, Morichetti D, Zorzi MG, et al. Squamoid eccrine ductal carcinoma: inappropriate diagnosis. Dermatol Surg. 2011;37:1819-1820.
- Kavand S, Cassarino DS. “Squamoid eccrine ductal carcinoma”: an unusual low-grade case with follicular differentiation. are these tumors squamoid variants of microcystic adnexal carcinoma? Am J Dermatopathol. 2009;31:849-852.
- Wasserman DI, Sack J, Gonzalez-Serva A, et al. Sentinel lymph node biopsy for a squamoid eccrine carcinoma with lymphatic invasion. Dermatol Surg. 2007;33:1126-1129.
- Kim YJ, Kim AR, Yu DS. Mohs micrographic surgery for squamoid eccrine ductal carcinoma. Dermatol Surg. 2005;31:1462-1464.
- Herrero J, Monteagudo C, Jorda E, et al. Squamoid eccrine ductal carcinoma. Histopathology. 1998;32:478-480.
- Wong TY, Suster S, Mihm MC. Squamoid eccrine ductal carcinoma. Histopathology. 1997;30:288-293.
- Qureshi HS, Ormsby AH, Lee MW, et al. The diagnostic utility of p63, CK5/6, CK 7, and CK 20 in distinguishing primary cutaneous adnexal neoplasms from metastatic carcinomas. J Cutan Pathol. 2004;31:145-152.
- Dabbs DJ. Diagnostic Immunohistochemistry: Theranostic and Genomic Applications. 4th ed. Elsevier/Saunders; 2014.
- Silverberg MJ, Leyden W, Warton EM, et al. HIV infection status, immunodeficiency, and the incidence of non-melanoma skin cancer. J Natl Cancer Inst. 2013;105:350-360.
- Asgari MM, Ray GT, Quesenberry CP Jr, et al. Association of multiple primary skin cancers with human immunodeficiency virus infection, CD4 count, and viral load. JAMA Dermatol. 2017;153:892-896.
- Tolkachjov SN. Adnexal carcinomas treated with Mohs micrographic surgery: a comprehensive review. Dermatol Surg. 2017;43:1199-1207.
- Kazakov DV. Cutaneous Adnexal Tumors. Wolters Kluwer Health/ Lippincott Williams & Wilkins; 2012.
- Weidner N, Foucar E. Adenosquamous carcinoma of the skin. an aggressive mucin- and gland-forming squamous carcinoma. Arch Dermatol. 1985;121:775-779.
- Banks ER, Cooper PH. Adenosquamous carcinoma of the skin: a report of 10 cases. J Cutan Pathol. 1991;18:227-234.
- Ko CJ, Leffell DJ, McNiff JM. Adenosquamous carcinoma: a report of nine cases with p63 and cytokeratin 5/6 staining. J Cutan Pathol. 2009;36:448-452.
- Patel V, Squires SM, Liu DY, et al. Cutaneous adenosquamous carcinoma: a rare neoplasm with biphasic differentiation. Cutis. 2014;94:231-233.
- Chhibber V, Lyle S, Mahalingam M. Ductal eccrine carcinoma with squamous differentiation: apropos a case. J Cutan Pathol. 2007;34:503-507.
- Sidiropoulos M, Sade S, Al-Habeeb A, et al. Syringoid eccrine carcinoma: a clinicopathological and immunohistochemical study of four cases. J Clin Pathol. 2011;64:788-792.
- Azorín D, López-Ríos F, Ballestín C, et al. Primary cutaneous adenosquamous carcinoma: a case report and review of the literature. J Cutan Pathol. 2001;28:542-545.
- Wildemore JK, Lee JB, Humphreys TR. Mohs surgery for malignant eccrine neoplasms. Dermatol Surg. 2004;30(12 pt 2):1574-1579.
- Garcia-Zuazaga J, Olbricht SM. Cutaneous squamous cell carcinoma. Adv Dermatol. 2008;24:33-57.
Squamoid eccrine ductal carcinoma (SEDC) is an aggressive underrecognized cutaneous malignancy of unknown etiology.1 It is most likely to occur in sun-exposed areas of the body, most commonly the head and neck. Risk factors include male sex, increased age, and chronic immunosuppression.1-4 Current reports suggest that SEDC is likely a high-grade subtype of squamous cell carcinoma (SCC) with a high risk for local recurrence (25%) and metastasis (13%).1,3,5,6 There are as few as 56 cases of SEDC reported in the literature; however, the number of cases may be closer to 100 due to SEDC being classified as either adenosquamous carcinoma of the skin or ductal eccrine carcinoma with squamous differentiation.1
Clinically, SEDC mimics keratinocyte carcinomas. Histologically, SEDC is biphasic, with a superficial portion resembling well-differentiated SCC and a deeply invasive portion having infiltrative irregular cords with ductal differentiation. Perineural invasion (PNI) frequently is present. Multiple connections to the overlying epidermis also can be seen, serving as a subtle clue to the diagnosis on broad superficial specimens.1-3 Due to superficial sampling, approximately 50% of reported cases are misdiagnosed as SCC during the initial biopsy.4 The diagnosis of SEDC often is made during complete excision when deeper tissue is sampled. Establishing an accurate diagnosis is important given the more aggressive nature of SEDC compared with SCC and its proclivity for PNI.1,3,6 The purpose of this review is to increase awareness of this underrecognized entity and describe the histologic findings that help distinguish SEDC from SCC.
Patient Chart Review
We reviewed chart notes as well as frozen and formalin-fixed paraffin-embedded tissue sections from all 5 patients diagnosed with SEDC at a single institution between November 2018 and May 2020. The mean age of patients was 81 years, and 4 were male. Four of the patients presented for MMS with a preoperative diagnosis of SCC per the original biopsy results. Only 1 patient had a preoperative diagnosis of SEDC. The details of each case are recorded in the Table. All tumors were greater than 2 cm in diameter on initial presentation, were located on the head, and clinically resembled keratinocyte carcinoma with either a nodular or plaquelike appearance (Figure 1).

Intraoperative histologic examination of the excised tissue revealed a biphasic pattern consisting of superficial SCC features overlying deeper dermal and subcutaneous infiltrative malignant ductal elements with gland formation in all 5 patients (Figures 2–4). Immunohistochemical staining with cytokeratin AE1/AE3 revealed thin strands of carcinoma in the mid to deeper dermis with squamous differentiation and eccrine ductal differentiation (Figure 5), thus confirming the diagnosis in all 5 patients.




The median depth of tumor invasion was 4.1 mm (range, 2.2–5.45 mm). Ulceration was seen in 3 of the patients, and PNI of large-caliber nerves was observed in all 5 patients. A connection with the overlying epidermis was present in all 5 patients. All 5 patients required more than 1 Mohs stage for complete tumor clearance (Table).
In 4 of the patients, nodal imaging performed at the time of diagnosis revealed no evidence of metastasis. Two patients received adjuvant radiation therapy, and none demonstrated evidence of recurrence. The mean follow-up time was 11 months (range, 6.5–18 months) for the 4 cases with available follow-up data (Table).
Literature Review
A PubMed review of the literature using the search term squamoid eccrine ductal carcinoma resulted in 28 articles, 19 of which were included in the review based on inclusion criteria (original articles available in English, in full text, and pertained to SEDC). Our review yielded 56 cases of SEDC.1-19 The mean age of patients with SEDC was 72 years. The number of male and female cases was 52% (29/56) and 48% (27/56), respectively. The most common location of SEDC was on the head or neck (71% [40/56]), followed by the extremities (19% [11/56]). Immunosuppression was noted in 9% (5/56) of cases. Wide local excision was the most commonly employed treatment modality (91% [51/56]), with MMS being used in 4 patients (7%). Adjuvant radiation was reported in 5% (3/56) of cases. Perineural invasion was reported in 34% (19/56) of cases. Recurrence was seen in 23% (13/56) of cases, with a mean time to recurrence of 10.4 months. Metastasis to regional lymph nodes was observed in 13% (7/56) of cases, with 7% (4/56) of those cases having distant metastases.
Comment
Squamoid eccrine ductal carcinoma was successfully treated with MMS in all 5 of the patients we reviewed. Recognition of a distinct biphasic pattern consisting of squamous differentiation superficially with epidermal connection overlying deeper dermal and subcutaneous infiltrative malignant ductal elements with gland formation should lead to consideration of this diagnosis. A thorough inspection for PNI also should be performed, as this finding was present in all of 5 cases and in 34% of reported cases in our literature review.
The differential diagnosis for SEDC includes SCC, metastatic adenocarcinoma with squamoid features, and eccrine tumors, including eccrine poroma, microcystic adnexal carcinoma (MAC), and porocarcinoma with squamous differentiation. The combination of histologic features with the immunoexpression profile of carcinoembryonic antigen (CEA), epithelial membrane antigen (EMA), cytokeratin (CK) 5/6, and p63 can effectively exclude the other entities in the differential and confirm the diagnosis of SEDC.1,3,4 While the diagnosis of SEDC relies on the specific histologic features of multiple surface attachments and superficial squamoid changes with deep ductular elements, immunohistochemistry can nonetheless be adjunctive in difficult cases. Positive immunohistochemical staining for CEA and EMA can help to highlight and delineate true glandular elements, whereas CK5/6 highlights the overall contour of the tumor, displaying more clearly the multiple epidermal attachments and the subtle infiltrative nature of the deeper components of invasive cords and ducts. In addition, the combination of CK5/6 and p63 positivity supports the primary cutaneous nature of the lesion rather than metastatic adenocarcinoma.13,20 Other markers of eccrine secretory coils, such as CK7, CAM5.2, and S100, also are sometimes used for confirmation, some of which can aid in distinction from noneccrine sweat gland differentiation, as CK7 and CAM5.2 are negative in both luminal and basal cells of the dermal duct while being positive within the secretory coil, and S100 protein is expressed within eccrine secretory coil but negative within the apocrine sweat glands.2,4,21
The clinical findings from our chart review corroborated those reported in the literature. The mean age of SEDC in the 5 patients we reviewed was 81 years, and all cases presented on the head, consistent with the findings observed in the literature. Although 4 of our cases were male, there may not be a difference in risk based on sex as previously thought.1 Our literature review revealed an almost equivalent percentage of male and female cases, with 52% being male.
Immunosuppression has been associated with an increased risk for SEDC. Our literature review revealed that approximately 9% (5/56) of cases occurred in immunosuppressed individuals. Two of these reported cases were in the setting of underlying chronic lymphocytic leukemia, 2 in individuals with a history of organ transplant, and 1 treated with azathioprine for myasthenia gravis.2,4,10,12,13 Our chart review supported this correlation, as all 5 patients had a medical history potentially consistent with being in an immunocompromised state (Table). Notably, patient 5 represents a unique case of SEDC occurring in the setting of HIV. The patient had HIV for 33 years, with his most recent CD4+ count of 794 mm3 and HIV-1 RNA load of 35 copies/mL. Given that HIV-positive individuals may have more than a 2-fold increased risk of SCC, a greater degree of suspicion for SEDC should be maintained for these patients.22,23
The etiology of SEDC is controversial but is thought to be either an SCC arising from eccrine glands or a variant of eccrine carcinoma with extensive squamoid differentiation.4,6,13,14,17,24 While SEDC certainly appears to share the proclivity for PNI with the malignant eccrine tumor MAC, it is simultaneously quite distinct, demonstrating nuclear pleomorphism and mitotic activity, both of which are lacking in the bland nature of MACs.12,25
The exact prevalence of SEDC is difficult to ascertain because of its frequent misdiagnosis and variable nomenclature used within the literature. Most reported cases of SEDC are mistakenly diagnosed as SCC on the initial shave or punch biopsy because of superficial sampling. This also was the case in 4 of the patients we reviewed. In addition, there are reported cases of SEDC that were referred to by the investigators as cutaneous adenosquamous carcinoma (cASC), among other descriptors, such as ductal eccrine carcinoma with squamous differentiation, adnexal carcinoma with squamous and ductal differentiation, and syringoid eccrine carcinoma.26-32 While the World Health Organization classifies SEDC as a distinct variant of cASC, which is a rare variant of SCC in itself, the 2 can be differentiated. Despite the similar clinical and histologic features shared between cASC and SEDC, the neoplastic aggregates in SEDC exhibit ductal differentiation containing lumina positive for CEA and EMA.4 Overall, we favor the term squamoid eccrine ductal carcinoma, as there has recently been more uniformity for the designation of this disease entity as such.
It is unclear whether the high incidence of local recurrence (23% [13/56]) of SEDC reported in the literature is related to the treatment modality employed (ie, wide local excision) or due to the innate aggressiveness of SEDC.1,3,5 The literature has shown that MMS has lower recurrence rates than other treatments at 5-year follow-up for SCC (3.1%–5%) and eccrine carcinomas (0%–5%).33,34 Although studies assessing tumor behavior or comparing treatment modalities are limited because of the rarity and underrecognition of SEDC, MMS has been used several times for SEDC with only 1 recurrence reported.4,13,17,24 Given that all 5 of the patients we reviewed required more than 1 Mohs stage for complete tumor clearance and none demonstrated evidence of recurrence or metastasis (Table), we recommend MMS as the treatment of choice for SEDC.
Conclusion
Squamoid eccrine ductal carcinoma is a rare but likely underdiagnosed cutaneous tumor of uncertain etiology. Because of its propensity for recurrence and metastasis, excision of SEDC with complete circumferential peripheral and deep margin assessment with close follow-up is recommended.
Squamoid eccrine ductal carcinoma (SEDC) is an aggressive underrecognized cutaneous malignancy of unknown etiology.1 It is most likely to occur in sun-exposed areas of the body, most commonly the head and neck. Risk factors include male sex, increased age, and chronic immunosuppression.1-4 Current reports suggest that SEDC is likely a high-grade subtype of squamous cell carcinoma (SCC) with a high risk for local recurrence (25%) and metastasis (13%).1,3,5,6 There are as few as 56 cases of SEDC reported in the literature; however, the number of cases may be closer to 100 due to SEDC being classified as either adenosquamous carcinoma of the skin or ductal eccrine carcinoma with squamous differentiation.1
Clinically, SEDC mimics keratinocyte carcinomas. Histologically, SEDC is biphasic, with a superficial portion resembling well-differentiated SCC and a deeply invasive portion having infiltrative irregular cords with ductal differentiation. Perineural invasion (PNI) frequently is present. Multiple connections to the overlying epidermis also can be seen, serving as a subtle clue to the diagnosis on broad superficial specimens.1-3 Due to superficial sampling, approximately 50% of reported cases are misdiagnosed as SCC during the initial biopsy.4 The diagnosis of SEDC often is made during complete excision when deeper tissue is sampled. Establishing an accurate diagnosis is important given the more aggressive nature of SEDC compared with SCC and its proclivity for PNI.1,3,6 The purpose of this review is to increase awareness of this underrecognized entity and describe the histologic findings that help distinguish SEDC from SCC.
Patient Chart Review
We reviewed chart notes as well as frozen and formalin-fixed paraffin-embedded tissue sections from all 5 patients diagnosed with SEDC at a single institution between November 2018 and May 2020. The mean age of patients was 81 years, and 4 were male. Four of the patients presented for MMS with a preoperative diagnosis of SCC per the original biopsy results. Only 1 patient had a preoperative diagnosis of SEDC. The details of each case are recorded in the Table. All tumors were greater than 2 cm in diameter on initial presentation, were located on the head, and clinically resembled keratinocyte carcinoma with either a nodular or plaquelike appearance (Figure 1).

Intraoperative histologic examination of the excised tissue revealed a biphasic pattern consisting of superficial SCC features overlying deeper dermal and subcutaneous infiltrative malignant ductal elements with gland formation in all 5 patients (Figures 2–4). Immunohistochemical staining with cytokeratin AE1/AE3 revealed thin strands of carcinoma in the mid to deeper dermis with squamous differentiation and eccrine ductal differentiation (Figure 5), thus confirming the diagnosis in all 5 patients.




The median depth of tumor invasion was 4.1 mm (range, 2.2–5.45 mm). Ulceration was seen in 3 of the patients, and PNI of large-caliber nerves was observed in all 5 patients. A connection with the overlying epidermis was present in all 5 patients. All 5 patients required more than 1 Mohs stage for complete tumor clearance (Table).
In 4 of the patients, nodal imaging performed at the time of diagnosis revealed no evidence of metastasis. Two patients received adjuvant radiation therapy, and none demonstrated evidence of recurrence. The mean follow-up time was 11 months (range, 6.5–18 months) for the 4 cases with available follow-up data (Table).
Literature Review
A PubMed review of the literature using the search term squamoid eccrine ductal carcinoma resulted in 28 articles, 19 of which were included in the review based on inclusion criteria (original articles available in English, in full text, and pertained to SEDC). Our review yielded 56 cases of SEDC.1-19 The mean age of patients with SEDC was 72 years. The number of male and female cases was 52% (29/56) and 48% (27/56), respectively. The most common location of SEDC was on the head or neck (71% [40/56]), followed by the extremities (19% [11/56]). Immunosuppression was noted in 9% (5/56) of cases. Wide local excision was the most commonly employed treatment modality (91% [51/56]), with MMS being used in 4 patients (7%). Adjuvant radiation was reported in 5% (3/56) of cases. Perineural invasion was reported in 34% (19/56) of cases. Recurrence was seen in 23% (13/56) of cases, with a mean time to recurrence of 10.4 months. Metastasis to regional lymph nodes was observed in 13% (7/56) of cases, with 7% (4/56) of those cases having distant metastases.
Comment
Squamoid eccrine ductal carcinoma was successfully treated with MMS in all 5 of the patients we reviewed. Recognition of a distinct biphasic pattern consisting of squamous differentiation superficially with epidermal connection overlying deeper dermal and subcutaneous infiltrative malignant ductal elements with gland formation should lead to consideration of this diagnosis. A thorough inspection for PNI also should be performed, as this finding was present in all of 5 cases and in 34% of reported cases in our literature review.
The differential diagnosis for SEDC includes SCC, metastatic adenocarcinoma with squamoid features, and eccrine tumors, including eccrine poroma, microcystic adnexal carcinoma (MAC), and porocarcinoma with squamous differentiation. The combination of histologic features with the immunoexpression profile of carcinoembryonic antigen (CEA), epithelial membrane antigen (EMA), cytokeratin (CK) 5/6, and p63 can effectively exclude the other entities in the differential and confirm the diagnosis of SEDC.1,3,4 While the diagnosis of SEDC relies on the specific histologic features of multiple surface attachments and superficial squamoid changes with deep ductular elements, immunohistochemistry can nonetheless be adjunctive in difficult cases. Positive immunohistochemical staining for CEA and EMA can help to highlight and delineate true glandular elements, whereas CK5/6 highlights the overall contour of the tumor, displaying more clearly the multiple epidermal attachments and the subtle infiltrative nature of the deeper components of invasive cords and ducts. In addition, the combination of CK5/6 and p63 positivity supports the primary cutaneous nature of the lesion rather than metastatic adenocarcinoma.13,20 Other markers of eccrine secretory coils, such as CK7, CAM5.2, and S100, also are sometimes used for confirmation, some of which can aid in distinction from noneccrine sweat gland differentiation, as CK7 and CAM5.2 are negative in both luminal and basal cells of the dermal duct while being positive within the secretory coil, and S100 protein is expressed within eccrine secretory coil but negative within the apocrine sweat glands.2,4,21
The clinical findings from our chart review corroborated those reported in the literature. The mean age of SEDC in the 5 patients we reviewed was 81 years, and all cases presented on the head, consistent with the findings observed in the literature. Although 4 of our cases were male, there may not be a difference in risk based on sex as previously thought.1 Our literature review revealed an almost equivalent percentage of male and female cases, with 52% being male.
Immunosuppression has been associated with an increased risk for SEDC. Our literature review revealed that approximately 9% (5/56) of cases occurred in immunosuppressed individuals. Two of these reported cases were in the setting of underlying chronic lymphocytic leukemia, 2 in individuals with a history of organ transplant, and 1 treated with azathioprine for myasthenia gravis.2,4,10,12,13 Our chart review supported this correlation, as all 5 patients had a medical history potentially consistent with being in an immunocompromised state (Table). Notably, patient 5 represents a unique case of SEDC occurring in the setting of HIV. The patient had HIV for 33 years, with his most recent CD4+ count of 794 mm3 and HIV-1 RNA load of 35 copies/mL. Given that HIV-positive individuals may have more than a 2-fold increased risk of SCC, a greater degree of suspicion for SEDC should be maintained for these patients.22,23
The etiology of SEDC is controversial but is thought to be either an SCC arising from eccrine glands or a variant of eccrine carcinoma with extensive squamoid differentiation.4,6,13,14,17,24 While SEDC certainly appears to share the proclivity for PNI with the malignant eccrine tumor MAC, it is simultaneously quite distinct, demonstrating nuclear pleomorphism and mitotic activity, both of which are lacking in the bland nature of MACs.12,25
The exact prevalence of SEDC is difficult to ascertain because of its frequent misdiagnosis and variable nomenclature used within the literature. Most reported cases of SEDC are mistakenly diagnosed as SCC on the initial shave or punch biopsy because of superficial sampling. This also was the case in 4 of the patients we reviewed. In addition, there are reported cases of SEDC that were referred to by the investigators as cutaneous adenosquamous carcinoma (cASC), among other descriptors, such as ductal eccrine carcinoma with squamous differentiation, adnexal carcinoma with squamous and ductal differentiation, and syringoid eccrine carcinoma.26-32 While the World Health Organization classifies SEDC as a distinct variant of cASC, which is a rare variant of SCC in itself, the 2 can be differentiated. Despite the similar clinical and histologic features shared between cASC and SEDC, the neoplastic aggregates in SEDC exhibit ductal differentiation containing lumina positive for CEA and EMA.4 Overall, we favor the term squamoid eccrine ductal carcinoma, as there has recently been more uniformity for the designation of this disease entity as such.
It is unclear whether the high incidence of local recurrence (23% [13/56]) of SEDC reported in the literature is related to the treatment modality employed (ie, wide local excision) or due to the innate aggressiveness of SEDC.1,3,5 The literature has shown that MMS has lower recurrence rates than other treatments at 5-year follow-up for SCC (3.1%–5%) and eccrine carcinomas (0%–5%).33,34 Although studies assessing tumor behavior or comparing treatment modalities are limited because of the rarity and underrecognition of SEDC, MMS has been used several times for SEDC with only 1 recurrence reported.4,13,17,24 Given that all 5 of the patients we reviewed required more than 1 Mohs stage for complete tumor clearance and none demonstrated evidence of recurrence or metastasis (Table), we recommend MMS as the treatment of choice for SEDC.
Conclusion
Squamoid eccrine ductal carcinoma is a rare but likely underdiagnosed cutaneous tumor of uncertain etiology. Because of its propensity for recurrence and metastasis, excision of SEDC with complete circumferential peripheral and deep margin assessment with close follow-up is recommended.
- van der Horst MP, Garcia-Herrera A, Markiewicz D, et al. Squamoid eccrine ductal carcinoma: a clinicopathologic study of 30 cases. Am J Surg Pathol. 2016;40:755-760.
- Jacob J, Kugelman L. Squamoid eccrine ductal carcinoma. Cutis. 2018;101:378-380, 385.
- Yim S, Lee YH, Chae SW, et al. Squamoid eccrine ductal carcinoma of the ear helix. Clin Case Rep. 2019;7:1409-1411.
- Terushkin E, Leffell DJ, Futoryan T, et al. Squamoid eccrine ductal carcinoma: a case report and review of the literature. Am J Dermatopathol. 2010;32:287-292.
- Jung YH, Jo HJ, Kang MS. Squamoid eccrine ductal carcinoma of the scalp. Korean J Pathol. 2012;46:278-281.
- Saraiva MI, Vieira MA, Portocarrero LK, et al. Squamoid eccrine ductal carcinoma. An Bras Dermatol. 2016;91:799-802.
- Phan K, Kim L, Lim P, et al. A case report of temple squamoid eccrine ductal carcinoma: a diagnostic challenge beneath the tip of the iceberg. Dermatol Ther. 2020;33:E13213.
- McKissack SS, Wohltmann W, Dalton SR, et al. Squamoid eccrine ductal carcinoma: an aggressive mimicker of squamous cell carcinoma. Am J Dermatopathol. 2019;41:140-143.
- Lobo-Jardim MM, Souza BdCE, Kakizaki P, et al. Dermoscopy of squamoid eccrine ductal carcinoma: an aid for early diagnosis. An Bras Dermatol. 2018;93:893-895.
- Chan H, Howard V, Moir D, et al. Squamoid eccrine ductal carcinoma of the scalp. Aust J Dermatol. 2016;57:E117-E119.
- Wang B, Jarell AD, Bingham JL, et al. PET/CT imaging of squamoid eccrine ductal carcinoma. Clin Nucl Med. 2015;40:322-324.
- Frouin E, Vignon-Pennamen MD, Balme B, et al. Anatomoclinical study of 30 cases of sclerosing sweat duct carcinomas (microcystic adnexal carcinoma, syringomatous carcinoma and squamoid eccrine ductal carcinoma). J Eur Acad Dermatol Venereol. 2015;29:1978-1994.
- Clark S, Young A, Piatigorsky E, et al. Mohs micrographic surgery in the setting of squamoid eccrine ductal carcinoma: addressing a diagnostic and therapeutic challenge. J Clin Aesthet Dermatol. 2013;6:33-36.
- Pusiol T, Morichetti D, Zorzi MG, et al. Squamoid eccrine ductal carcinoma: inappropriate diagnosis. Dermatol Surg. 2011;37:1819-1820.
- Kavand S, Cassarino DS. “Squamoid eccrine ductal carcinoma”: an unusual low-grade case with follicular differentiation. are these tumors squamoid variants of microcystic adnexal carcinoma? Am J Dermatopathol. 2009;31:849-852.
- Wasserman DI, Sack J, Gonzalez-Serva A, et al. Sentinel lymph node biopsy for a squamoid eccrine carcinoma with lymphatic invasion. Dermatol Surg. 2007;33:1126-1129.
- Kim YJ, Kim AR, Yu DS. Mohs micrographic surgery for squamoid eccrine ductal carcinoma. Dermatol Surg. 2005;31:1462-1464.
- Herrero J, Monteagudo C, Jorda E, et al. Squamoid eccrine ductal carcinoma. Histopathology. 1998;32:478-480.
- Wong TY, Suster S, Mihm MC. Squamoid eccrine ductal carcinoma. Histopathology. 1997;30:288-293.
- Qureshi HS, Ormsby AH, Lee MW, et al. The diagnostic utility of p63, CK5/6, CK 7, and CK 20 in distinguishing primary cutaneous adnexal neoplasms from metastatic carcinomas. J Cutan Pathol. 2004;31:145-152.
- Dabbs DJ. Diagnostic Immunohistochemistry: Theranostic and Genomic Applications. 4th ed. Elsevier/Saunders; 2014.
- Silverberg MJ, Leyden W, Warton EM, et al. HIV infection status, immunodeficiency, and the incidence of non-melanoma skin cancer. J Natl Cancer Inst. 2013;105:350-360.
- Asgari MM, Ray GT, Quesenberry CP Jr, et al. Association of multiple primary skin cancers with human immunodeficiency virus infection, CD4 count, and viral load. JAMA Dermatol. 2017;153:892-896.
- Tolkachjov SN. Adnexal carcinomas treated with Mohs micrographic surgery: a comprehensive review. Dermatol Surg. 2017;43:1199-1207.
- Kazakov DV. Cutaneous Adnexal Tumors. Wolters Kluwer Health/ Lippincott Williams & Wilkins; 2012.
- Weidner N, Foucar E. Adenosquamous carcinoma of the skin. an aggressive mucin- and gland-forming squamous carcinoma. Arch Dermatol. 1985;121:775-779.
- Banks ER, Cooper PH. Adenosquamous carcinoma of the skin: a report of 10 cases. J Cutan Pathol. 1991;18:227-234.
- Ko CJ, Leffell DJ, McNiff JM. Adenosquamous carcinoma: a report of nine cases with p63 and cytokeratin 5/6 staining. J Cutan Pathol. 2009;36:448-452.
- Patel V, Squires SM, Liu DY, et al. Cutaneous adenosquamous carcinoma: a rare neoplasm with biphasic differentiation. Cutis. 2014;94:231-233.
- Chhibber V, Lyle S, Mahalingam M. Ductal eccrine carcinoma with squamous differentiation: apropos a case. J Cutan Pathol. 2007;34:503-507.
- Sidiropoulos M, Sade S, Al-Habeeb A, et al. Syringoid eccrine carcinoma: a clinicopathological and immunohistochemical study of four cases. J Clin Pathol. 2011;64:788-792.
- Azorín D, López-Ríos F, Ballestín C, et al. Primary cutaneous adenosquamous carcinoma: a case report and review of the literature. J Cutan Pathol. 2001;28:542-545.
- Wildemore JK, Lee JB, Humphreys TR. Mohs surgery for malignant eccrine neoplasms. Dermatol Surg. 2004;30(12 pt 2):1574-1579.
- Garcia-Zuazaga J, Olbricht SM. Cutaneous squamous cell carcinoma. Adv Dermatol. 2008;24:33-57.
- van der Horst MP, Garcia-Herrera A, Markiewicz D, et al. Squamoid eccrine ductal carcinoma: a clinicopathologic study of 30 cases. Am J Surg Pathol. 2016;40:755-760.
- Jacob J, Kugelman L. Squamoid eccrine ductal carcinoma. Cutis. 2018;101:378-380, 385.
- Yim S, Lee YH, Chae SW, et al. Squamoid eccrine ductal carcinoma of the ear helix. Clin Case Rep. 2019;7:1409-1411.
- Terushkin E, Leffell DJ, Futoryan T, et al. Squamoid eccrine ductal carcinoma: a case report and review of the literature. Am J Dermatopathol. 2010;32:287-292.
- Jung YH, Jo HJ, Kang MS. Squamoid eccrine ductal carcinoma of the scalp. Korean J Pathol. 2012;46:278-281.
- Saraiva MI, Vieira MA, Portocarrero LK, et al. Squamoid eccrine ductal carcinoma. An Bras Dermatol. 2016;91:799-802.
- Phan K, Kim L, Lim P, et al. A case report of temple squamoid eccrine ductal carcinoma: a diagnostic challenge beneath the tip of the iceberg. Dermatol Ther. 2020;33:E13213.
- McKissack SS, Wohltmann W, Dalton SR, et al. Squamoid eccrine ductal carcinoma: an aggressive mimicker of squamous cell carcinoma. Am J Dermatopathol. 2019;41:140-143.
- Lobo-Jardim MM, Souza BdCE, Kakizaki P, et al. Dermoscopy of squamoid eccrine ductal carcinoma: an aid for early diagnosis. An Bras Dermatol. 2018;93:893-895.
- Chan H, Howard V, Moir D, et al. Squamoid eccrine ductal carcinoma of the scalp. Aust J Dermatol. 2016;57:E117-E119.
- Wang B, Jarell AD, Bingham JL, et al. PET/CT imaging of squamoid eccrine ductal carcinoma. Clin Nucl Med. 2015;40:322-324.
- Frouin E, Vignon-Pennamen MD, Balme B, et al. Anatomoclinical study of 30 cases of sclerosing sweat duct carcinomas (microcystic adnexal carcinoma, syringomatous carcinoma and squamoid eccrine ductal carcinoma). J Eur Acad Dermatol Venereol. 2015;29:1978-1994.
- Clark S, Young A, Piatigorsky E, et al. Mohs micrographic surgery in the setting of squamoid eccrine ductal carcinoma: addressing a diagnostic and therapeutic challenge. J Clin Aesthet Dermatol. 2013;6:33-36.
- Pusiol T, Morichetti D, Zorzi MG, et al. Squamoid eccrine ductal carcinoma: inappropriate diagnosis. Dermatol Surg. 2011;37:1819-1820.
- Kavand S, Cassarino DS. “Squamoid eccrine ductal carcinoma”: an unusual low-grade case with follicular differentiation. are these tumors squamoid variants of microcystic adnexal carcinoma? Am J Dermatopathol. 2009;31:849-852.
- Wasserman DI, Sack J, Gonzalez-Serva A, et al. Sentinel lymph node biopsy for a squamoid eccrine carcinoma with lymphatic invasion. Dermatol Surg. 2007;33:1126-1129.
- Kim YJ, Kim AR, Yu DS. Mohs micrographic surgery for squamoid eccrine ductal carcinoma. Dermatol Surg. 2005;31:1462-1464.
- Herrero J, Monteagudo C, Jorda E, et al. Squamoid eccrine ductal carcinoma. Histopathology. 1998;32:478-480.
- Wong TY, Suster S, Mihm MC. Squamoid eccrine ductal carcinoma. Histopathology. 1997;30:288-293.
- Qureshi HS, Ormsby AH, Lee MW, et al. The diagnostic utility of p63, CK5/6, CK 7, and CK 20 in distinguishing primary cutaneous adnexal neoplasms from metastatic carcinomas. J Cutan Pathol. 2004;31:145-152.
- Dabbs DJ. Diagnostic Immunohistochemistry: Theranostic and Genomic Applications. 4th ed. Elsevier/Saunders; 2014.
- Silverberg MJ, Leyden W, Warton EM, et al. HIV infection status, immunodeficiency, and the incidence of non-melanoma skin cancer. J Natl Cancer Inst. 2013;105:350-360.
- Asgari MM, Ray GT, Quesenberry CP Jr, et al. Association of multiple primary skin cancers with human immunodeficiency virus infection, CD4 count, and viral load. JAMA Dermatol. 2017;153:892-896.
- Tolkachjov SN. Adnexal carcinomas treated with Mohs micrographic surgery: a comprehensive review. Dermatol Surg. 2017;43:1199-1207.
- Kazakov DV. Cutaneous Adnexal Tumors. Wolters Kluwer Health/ Lippincott Williams & Wilkins; 2012.
- Weidner N, Foucar E. Adenosquamous carcinoma of the skin. an aggressive mucin- and gland-forming squamous carcinoma. Arch Dermatol. 1985;121:775-779.
- Banks ER, Cooper PH. Adenosquamous carcinoma of the skin: a report of 10 cases. J Cutan Pathol. 1991;18:227-234.
- Ko CJ, Leffell DJ, McNiff JM. Adenosquamous carcinoma: a report of nine cases with p63 and cytokeratin 5/6 staining. J Cutan Pathol. 2009;36:448-452.
- Patel V, Squires SM, Liu DY, et al. Cutaneous adenosquamous carcinoma: a rare neoplasm with biphasic differentiation. Cutis. 2014;94:231-233.
- Chhibber V, Lyle S, Mahalingam M. Ductal eccrine carcinoma with squamous differentiation: apropos a case. J Cutan Pathol. 2007;34:503-507.
- Sidiropoulos M, Sade S, Al-Habeeb A, et al. Syringoid eccrine carcinoma: a clinicopathological and immunohistochemical study of four cases. J Clin Pathol. 2011;64:788-792.
- Azorín D, López-Ríos F, Ballestín C, et al. Primary cutaneous adenosquamous carcinoma: a case report and review of the literature. J Cutan Pathol. 2001;28:542-545.
- Wildemore JK, Lee JB, Humphreys TR. Mohs surgery for malignant eccrine neoplasms. Dermatol Surg. 2004;30(12 pt 2):1574-1579.
- Garcia-Zuazaga J, Olbricht SM. Cutaneous squamous cell carcinoma. Adv Dermatol. 2008;24:33-57.
PRACTICE POINTS
- Squamoid eccrine ductal carcinoma is an aggressive underrecognized cutaneous malignancy that often is misdiagnosed as squamous cell carcinoma (SCC) during initial biopsy.
- Squamoid eccrine ductal carcinoma has a biphasic histologic appearance with a superficial portion resembling well-differentiated SCC and a deeply invasive portion comprised of infiltrative irregular cords with ductal differentiation.
- Excision with complete circumferential peripheral and deep margin assessment with close follow-up is recommended for these patients because of the high risk for recurrence and metastasis.
Nivolumab-Induced Granuloma Annulare
Granuloma annulare (GA) is a benign, cutaneous, granulomatous disease of unclear etiology. Typically, GA presents in young adults as asymptomatic, annular, flesh-colored to pink papules and plaques, commonly on the upper and lower extremities. Histologically, GA is characterized by mucin deposition, palisading or an interstitial granulomatous pattern, and collagen and elastic fiber degeneration.1
Granuloma annulare has been associated with various medications and medical conditions, including diabetes mellitus, hyperlipidemia, thyroid disease, and HIV.1 More recently, immune-checkpoint inhibitors (ICIs) have been reported to trigger GA.2 We report a case of nivolumab-induced GA in a 54-year-old woman.
Case Report
A 54-year-old woman presented with an itchy rash on the upper extremities, face, and chest of 4 months’ duration. The patient noted that the rash started on the hands and progressed to include the arms, face, and chest. She also reported associated mild tenderness. She had a history of stage IV non–small-cell lung carcinoma with metastases to the ribs and adrenal glands. She had been started on biweekly intravenous infusions of the ICI nivolumab by her oncologist approximately 1 year prior to the current presentation after failing a course of conventional chemotherapy. The most recent positron emission tomography–computed tomography scan 1 month prior to presentation showed a stable lung mass with radiologic disappearance of metastases, indicating a favorable response to nivolumab. The patient also had a history of hypothyroidism and depression, which were treated with oral levothyroxine 75 μg once daily and oral sertraline 50 mg once daily, respectively, both for longer than 5 years.
Physical examination revealed annular, erythematous, flat-topped papules, some with surmounting fine scale, coalescing into larger plaques along the dorsal surface of the hands and arms (Figure 1) as well as the forehead and chest. A biopsy of a papule on the dorsal aspect of the left hand revealed nodules of histiocytes admixed with Langerhans giant cells within the dermis; mucin was noted centrally within some nodules (Figure 2). Periodic acid–Schiff staining was negative for fungal elements compared to control. Polarization of the specimen was negative for foreign bodies. The biopsy findings therefore were consistent with a diagnosis of GA.
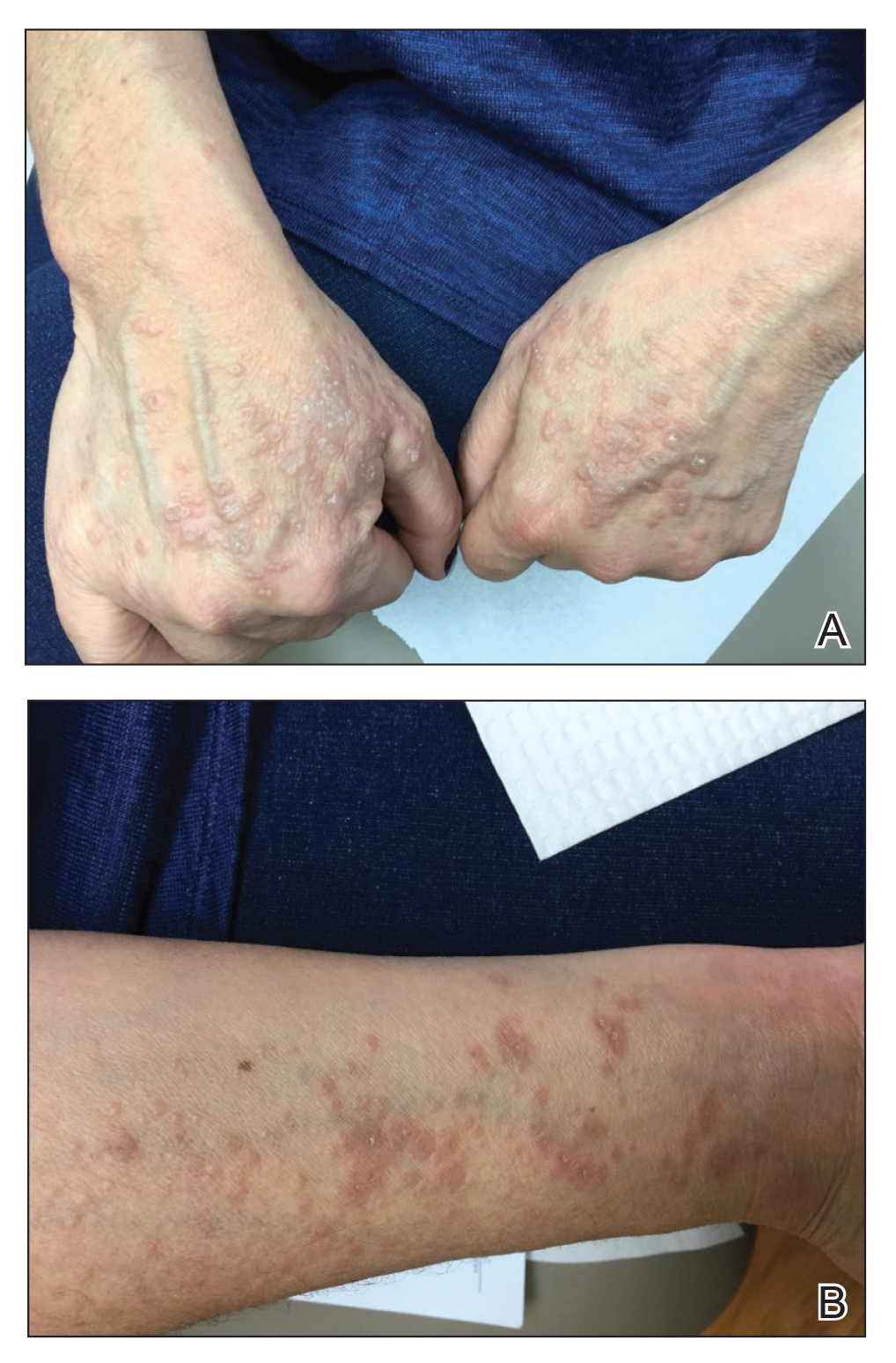
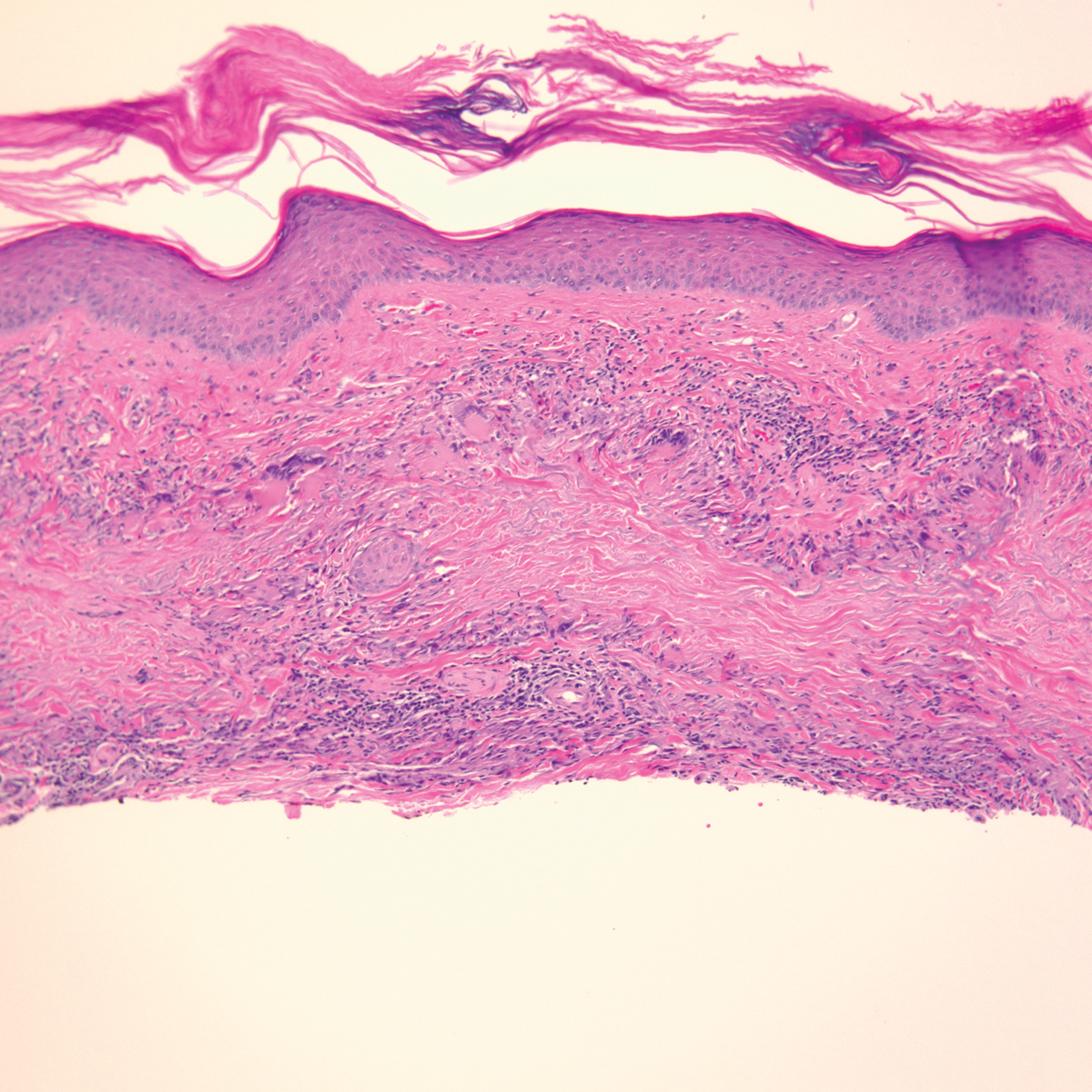
A 3-month treatment course of betamethasone dipropionate 0.05% cream twice daily failed. Narrowband UVB phototherapy was then initiated at 3 sessions weekly. The eruption of GA improved after 3 months of phototherapy. Subsequently, the patient was lost to follow-up.
Comment
Discovery of specific immune checkpoints in tumor-induced immunosuppression revolutionized oncologic therapy. An example is the programmed cell-death protein 1 (PD-1) receptor that is expressed on activated immune cells, including T cells and macrophages.3,4 Upon binding to the PD-1 ligand (PD-L1), T-cell proliferation is inhibited, resulting in downregulation of the immune response. As a result, tumor cells have evolved to overexpress PD-L1 to evade immunologic detection.3 Nivolumab, a fully human IgG4 antibody to PD-1, has emerged along with other ICIs as effective treatments for numerous cancers, including melanoma and non–small-cell lung cancer. By disrupting downregulation of T cells, ICIs improve immune-mediated antitumor activity.3
However, the resulting immunologic disturbance by ICIs has been reported to induce various cutaneous and systemic immune-mediated adverse reactions, including granulomatous reactions such as sarcoidosis, GA, and a cutaneous sarcoidlike granulomatous reaction.1,2,5,6 Our patient represents a rare case of nivolumab-induced GA.
Recent evidence suggests that GA might be caused in part by a cell-mediated hypersensitivity reaction that is regulated by a helper T cell subset 1 inflammatory reaction. Through release of cytokines by activated CD4+ T cells, macrophages are recruited, forming the granulomatous pattern and secreting enzymes that can degrade connective tissue. Nivolumab and other ICIs can thus trigger this reaction because their blockade of PD-1 enhances T cell–mediated immune reactions.2 In addition, because macrophages themselves also express PD-1, ICIs can directly enhance macrophage recruitment and proliferation, further increasing the risk of a granulomatous reaction.4
Interestingly, cutaneous adverse reactions to nivolumab have been associated with improved survival in melanoma patients.7 The nature of this association with granulomatous reactions in general and with GA specifically remains to be determined.
Conclusion
Since the approval of the first PD-1 inhibitors, pembrolizumab and nivolumab, in 2014, other ICIs targeting the immune checkpoint pathway have been developed. Newer agents targeting PD-L1 (avelumab, atezolizumab, and durvalumab) were recently approved. Additionally, cemiplimab, another PD-1 inhibitor, was approved by the US Food and Drug Administration in 2018 for the treatment of advanced cutaneous squamous cell carcinoma.8 Indications for all ICIs also have expanded considerably.3 Therefore, the incidence of immune-mediated adverse reactions, including GA, is bound to increase. Physicians should be cognizant of this association to accurately diagnose and effectively treat adverse reactions in patients who are taking ICIs.
- Piette EW, Rosenbach M. Granuloma annulare: pathogenesis, disease associations and triggers, and therapeutic options. J Am Acad Dermatol. 2016;75:467-479. doi:10.1016/j.jaad.2015.03.055
- Wu J, Kwong BY, Martires KJ, et al. Granuloma annulare associated with immune checkpoint inhibitors. J Eur Acad Dermatol. 2018;32:E124-E126. doi:10.1111/jdv.14617
- Gong J, Chehrazi-Raffle A, Reddi S, et al. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J Immunother Cancer. 2018;6:8. doi:10.1186/s40425-018-0316-z
- Gordon SR, Maute RL, Dulken BW, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545:495-499. doi:10.1038/nature22396
- Birnbaum MR, Ma MW, Fleisig S, et al. Nivolumab-related cutaneous sarcoidosis in a patient with lung adenocarcinoma. JAAD Case Rep. 2017;3:208-211. doi:10.1016/j.jdcr.2017.02.015
- Danlos F-X, Pagès C, Baroudjian B, et al. Nivolumab-induced sarcoid-like granulomatous reaction in a patient with advanced melanoma. Chest. 2016;149:E133-E136. doi:10.1016/j.chest.2015.10.082
- Freeman-Keller M, Kim Y, Cronin H, et al. Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res. 2016;22:886-894. doi:10.1158/1078-0432.CCR-15-1136
- Migden MR, Rischin D, Schmults CD, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018;379:341-351. doi:10.1056/NEJMoa1805131
Granuloma annulare (GA) is a benign, cutaneous, granulomatous disease of unclear etiology. Typically, GA presents in young adults as asymptomatic, annular, flesh-colored to pink papules and plaques, commonly on the upper and lower extremities. Histologically, GA is characterized by mucin deposition, palisading or an interstitial granulomatous pattern, and collagen and elastic fiber degeneration.1
Granuloma annulare has been associated with various medications and medical conditions, including diabetes mellitus, hyperlipidemia, thyroid disease, and HIV.1 More recently, immune-checkpoint inhibitors (ICIs) have been reported to trigger GA.2 We report a case of nivolumab-induced GA in a 54-year-old woman.
Case Report
A 54-year-old woman presented with an itchy rash on the upper extremities, face, and chest of 4 months’ duration. The patient noted that the rash started on the hands and progressed to include the arms, face, and chest. She also reported associated mild tenderness. She had a history of stage IV non–small-cell lung carcinoma with metastases to the ribs and adrenal glands. She had been started on biweekly intravenous infusions of the ICI nivolumab by her oncologist approximately 1 year prior to the current presentation after failing a course of conventional chemotherapy. The most recent positron emission tomography–computed tomography scan 1 month prior to presentation showed a stable lung mass with radiologic disappearance of metastases, indicating a favorable response to nivolumab. The patient also had a history of hypothyroidism and depression, which were treated with oral levothyroxine 75 μg once daily and oral sertraline 50 mg once daily, respectively, both for longer than 5 years.
Physical examination revealed annular, erythematous, flat-topped papules, some with surmounting fine scale, coalescing into larger plaques along the dorsal surface of the hands and arms (Figure 1) as well as the forehead and chest. A biopsy of a papule on the dorsal aspect of the left hand revealed nodules of histiocytes admixed with Langerhans giant cells within the dermis; mucin was noted centrally within some nodules (Figure 2). Periodic acid–Schiff staining was negative for fungal elements compared to control. Polarization of the specimen was negative for foreign bodies. The biopsy findings therefore were consistent with a diagnosis of GA.


A 3-month treatment course of betamethasone dipropionate 0.05% cream twice daily failed. Narrowband UVB phototherapy was then initiated at 3 sessions weekly. The eruption of GA improved after 3 months of phototherapy. Subsequently, the patient was lost to follow-up.
Comment
Discovery of specific immune checkpoints in tumor-induced immunosuppression revolutionized oncologic therapy. An example is the programmed cell-death protein 1 (PD-1) receptor that is expressed on activated immune cells, including T cells and macrophages.3,4 Upon binding to the PD-1 ligand (PD-L1), T-cell proliferation is inhibited, resulting in downregulation of the immune response. As a result, tumor cells have evolved to overexpress PD-L1 to evade immunologic detection.3 Nivolumab, a fully human IgG4 antibody to PD-1, has emerged along with other ICIs as effective treatments for numerous cancers, including melanoma and non–small-cell lung cancer. By disrupting downregulation of T cells, ICIs improve immune-mediated antitumor activity.3
However, the resulting immunologic disturbance by ICIs has been reported to induce various cutaneous and systemic immune-mediated adverse reactions, including granulomatous reactions such as sarcoidosis, GA, and a cutaneous sarcoidlike granulomatous reaction.1,2,5,6 Our patient represents a rare case of nivolumab-induced GA.
Recent evidence suggests that GA might be caused in part by a cell-mediated hypersensitivity reaction that is regulated by a helper T cell subset 1 inflammatory reaction. Through release of cytokines by activated CD4+ T cells, macrophages are recruited, forming the granulomatous pattern and secreting enzymes that can degrade connective tissue. Nivolumab and other ICIs can thus trigger this reaction because their blockade of PD-1 enhances T cell–mediated immune reactions.2 In addition, because macrophages themselves also express PD-1, ICIs can directly enhance macrophage recruitment and proliferation, further increasing the risk of a granulomatous reaction.4
Interestingly, cutaneous adverse reactions to nivolumab have been associated with improved survival in melanoma patients.7 The nature of this association with granulomatous reactions in general and with GA specifically remains to be determined.
Conclusion
Since the approval of the first PD-1 inhibitors, pembrolizumab and nivolumab, in 2014, other ICIs targeting the immune checkpoint pathway have been developed. Newer agents targeting PD-L1 (avelumab, atezolizumab, and durvalumab) were recently approved. Additionally, cemiplimab, another PD-1 inhibitor, was approved by the US Food and Drug Administration in 2018 for the treatment of advanced cutaneous squamous cell carcinoma.8 Indications for all ICIs also have expanded considerably.3 Therefore, the incidence of immune-mediated adverse reactions, including GA, is bound to increase. Physicians should be cognizant of this association to accurately diagnose and effectively treat adverse reactions in patients who are taking ICIs.
Granuloma annulare (GA) is a benign, cutaneous, granulomatous disease of unclear etiology. Typically, GA presents in young adults as asymptomatic, annular, flesh-colored to pink papules and plaques, commonly on the upper and lower extremities. Histologically, GA is characterized by mucin deposition, palisading or an interstitial granulomatous pattern, and collagen and elastic fiber degeneration.1
Granuloma annulare has been associated with various medications and medical conditions, including diabetes mellitus, hyperlipidemia, thyroid disease, and HIV.1 More recently, immune-checkpoint inhibitors (ICIs) have been reported to trigger GA.2 We report a case of nivolumab-induced GA in a 54-year-old woman.
Case Report
A 54-year-old woman presented with an itchy rash on the upper extremities, face, and chest of 4 months’ duration. The patient noted that the rash started on the hands and progressed to include the arms, face, and chest. She also reported associated mild tenderness. She had a history of stage IV non–small-cell lung carcinoma with metastases to the ribs and adrenal glands. She had been started on biweekly intravenous infusions of the ICI nivolumab by her oncologist approximately 1 year prior to the current presentation after failing a course of conventional chemotherapy. The most recent positron emission tomography–computed tomography scan 1 month prior to presentation showed a stable lung mass with radiologic disappearance of metastases, indicating a favorable response to nivolumab. The patient also had a history of hypothyroidism and depression, which were treated with oral levothyroxine 75 μg once daily and oral sertraline 50 mg once daily, respectively, both for longer than 5 years.
Physical examination revealed annular, erythematous, flat-topped papules, some with surmounting fine scale, coalescing into larger plaques along the dorsal surface of the hands and arms (Figure 1) as well as the forehead and chest. A biopsy of a papule on the dorsal aspect of the left hand revealed nodules of histiocytes admixed with Langerhans giant cells within the dermis; mucin was noted centrally within some nodules (Figure 2). Periodic acid–Schiff staining was negative for fungal elements compared to control. Polarization of the specimen was negative for foreign bodies. The biopsy findings therefore were consistent with a diagnosis of GA.


A 3-month treatment course of betamethasone dipropionate 0.05% cream twice daily failed. Narrowband UVB phototherapy was then initiated at 3 sessions weekly. The eruption of GA improved after 3 months of phototherapy. Subsequently, the patient was lost to follow-up.
Comment
Discovery of specific immune checkpoints in tumor-induced immunosuppression revolutionized oncologic therapy. An example is the programmed cell-death protein 1 (PD-1) receptor that is expressed on activated immune cells, including T cells and macrophages.3,4 Upon binding to the PD-1 ligand (PD-L1), T-cell proliferation is inhibited, resulting in downregulation of the immune response. As a result, tumor cells have evolved to overexpress PD-L1 to evade immunologic detection.3 Nivolumab, a fully human IgG4 antibody to PD-1, has emerged along with other ICIs as effective treatments for numerous cancers, including melanoma and non–small-cell lung cancer. By disrupting downregulation of T cells, ICIs improve immune-mediated antitumor activity.3
However, the resulting immunologic disturbance by ICIs has been reported to induce various cutaneous and systemic immune-mediated adverse reactions, including granulomatous reactions such as sarcoidosis, GA, and a cutaneous sarcoidlike granulomatous reaction.1,2,5,6 Our patient represents a rare case of nivolumab-induced GA.
Recent evidence suggests that GA might be caused in part by a cell-mediated hypersensitivity reaction that is regulated by a helper T cell subset 1 inflammatory reaction. Through release of cytokines by activated CD4+ T cells, macrophages are recruited, forming the granulomatous pattern and secreting enzymes that can degrade connective tissue. Nivolumab and other ICIs can thus trigger this reaction because their blockade of PD-1 enhances T cell–mediated immune reactions.2 In addition, because macrophages themselves also express PD-1, ICIs can directly enhance macrophage recruitment and proliferation, further increasing the risk of a granulomatous reaction.4
Interestingly, cutaneous adverse reactions to nivolumab have been associated with improved survival in melanoma patients.7 The nature of this association with granulomatous reactions in general and with GA specifically remains to be determined.
Conclusion
Since the approval of the first PD-1 inhibitors, pembrolizumab and nivolumab, in 2014, other ICIs targeting the immune checkpoint pathway have been developed. Newer agents targeting PD-L1 (avelumab, atezolizumab, and durvalumab) were recently approved. Additionally, cemiplimab, another PD-1 inhibitor, was approved by the US Food and Drug Administration in 2018 for the treatment of advanced cutaneous squamous cell carcinoma.8 Indications for all ICIs also have expanded considerably.3 Therefore, the incidence of immune-mediated adverse reactions, including GA, is bound to increase. Physicians should be cognizant of this association to accurately diagnose and effectively treat adverse reactions in patients who are taking ICIs.
- Piette EW, Rosenbach M. Granuloma annulare: pathogenesis, disease associations and triggers, and therapeutic options. J Am Acad Dermatol. 2016;75:467-479. doi:10.1016/j.jaad.2015.03.055
- Wu J, Kwong BY, Martires KJ, et al. Granuloma annulare associated with immune checkpoint inhibitors. J Eur Acad Dermatol. 2018;32:E124-E126. doi:10.1111/jdv.14617
- Gong J, Chehrazi-Raffle A, Reddi S, et al. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J Immunother Cancer. 2018;6:8. doi:10.1186/s40425-018-0316-z
- Gordon SR, Maute RL, Dulken BW, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545:495-499. doi:10.1038/nature22396
- Birnbaum MR, Ma MW, Fleisig S, et al. Nivolumab-related cutaneous sarcoidosis in a patient with lung adenocarcinoma. JAAD Case Rep. 2017;3:208-211. doi:10.1016/j.jdcr.2017.02.015
- Danlos F-X, Pagès C, Baroudjian B, et al. Nivolumab-induced sarcoid-like granulomatous reaction in a patient with advanced melanoma. Chest. 2016;149:E133-E136. doi:10.1016/j.chest.2015.10.082
- Freeman-Keller M, Kim Y, Cronin H, et al. Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res. 2016;22:886-894. doi:10.1158/1078-0432.CCR-15-1136
- Migden MR, Rischin D, Schmults CD, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018;379:341-351. doi:10.1056/NEJMoa1805131
- Piette EW, Rosenbach M. Granuloma annulare: pathogenesis, disease associations and triggers, and therapeutic options. J Am Acad Dermatol. 2016;75:467-479. doi:10.1016/j.jaad.2015.03.055
- Wu J, Kwong BY, Martires KJ, et al. Granuloma annulare associated with immune checkpoint inhibitors. J Eur Acad Dermatol. 2018;32:E124-E126. doi:10.1111/jdv.14617
- Gong J, Chehrazi-Raffle A, Reddi S, et al. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J Immunother Cancer. 2018;6:8. doi:10.1186/s40425-018-0316-z
- Gordon SR, Maute RL, Dulken BW, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545:495-499. doi:10.1038/nature22396
- Birnbaum MR, Ma MW, Fleisig S, et al. Nivolumab-related cutaneous sarcoidosis in a patient with lung adenocarcinoma. JAAD Case Rep. 2017;3:208-211. doi:10.1016/j.jdcr.2017.02.015
- Danlos F-X, Pagès C, Baroudjian B, et al. Nivolumab-induced sarcoid-like granulomatous reaction in a patient with advanced melanoma. Chest. 2016;149:E133-E136. doi:10.1016/j.chest.2015.10.082
- Freeman-Keller M, Kim Y, Cronin H, et al. Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res. 2016;22:886-894. doi:10.1158/1078-0432.CCR-15-1136
- Migden MR, Rischin D, Schmults CD, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018;379:341-351. doi:10.1056/NEJMoa1805131
Practice Points
- Immune-related adverse events (irAEs) frequently occur in patients on immunotherapy, with the skin representing the most common site of involvement.
- Although rare, granulomatous reactions such as granuloma annulare increasingly are recognized as potential irAEs.
- Clinicians should be aware of this novel association to accurately diagnose and effectively treat adverse reactions in patients receiving immunotherapy.
20-year-old woman • 2 syncopal episodes • nausea • dizziness • Dx?
THE CASE
A 20-year-old woman presented to clinic with a chief complaint of 2 syncopal episodes within 10 minutes of each other. She reported that in both cases, she felt nauseated and dizzy before losing consciousness. She lost consciousness for a few seconds during the first episode and a few minutes during the second episode. Both episodes were unwitnessed.
The patient denied any fasting, vomiting, diarrhea, palpitations, chest pain, incontinence, oral trauma, headaches, fevers, chills, or tremors. Her last menstrual period started 3 days prior to presentation. The patient was taking sertraline 25 mg once daily for anxiety and depression and norethindrone acetate–ethinyl estradiol tablets 20 µg daily for birth control. She also was finishing a 7-day course of metronidazole for bacterial vaginosis. She reported having started the sertraline about 10 days prior to the syncopal episodes. She denied any personal history of drug or alcohol use, syncope, seizures, or any other medical conditions. Family history was negative for any cardiac or neurologic conditions.
The patient appeared euvolemic on exam. Overall, the review of the respiratory, cardiac, and neurologic systems was unremarkable. An electrocardiogram, obtained in clinic, showed a normal sinus rhythm and QT interval. Orthostatic blood pressure and heart rate measurements were as follows: supine, 122/83 mm Hg and 67 beats/min; seated, 118/87 mm Hg and 60 beats/min; and standing, 123/83 mm Hg and 95 beats/min. In addition to the increase in pulse between sitting and standing, the patient reported feeling nauseated when transitioning to a standing position.
Laboratory work-up included a comprehensive metabolic panel, complete blood count, and thyroid-stimulating hormone test. The results showed mild erythrocytosis with a hematocrit and hemoglobin of 46.1% and 15.6 g/dL respectively, as well as mild hypercalcemia (10.4 mg/dL).
THE DIAGNOSIS
An increase in heart rate of more than 30 beats/min when the patient went from a sitting to a standing position pointed to a diagnosis of postural orthostatic tachycardia syndrome (POTS). This prompted us to stop the sertraline.
DISCUSSION
POTS is a type of intolerance to orthostasis related to a significant increase in pulse without resulting hypotension upon standing. Other symptoms that accompany this change in position include dizziness, lightheadedness, blurry vision, and fatigue. Syncope occurs in about 40% of patients with POTS, which may be more frequent than for patients with orthostatic hypotension.1
The overall prevalence of POTS is 0.2% to 1%; however, it is generally seen in a 5:1 female-to-male ratio.2,3 POTS is often idiopathic. That said, it can also be caused by medication adverse effects, hypovolemia, and stressors, including vaccinations, viral infections, trauma, and emotional triggers. On physical exam, this patient did not appear to be hypovolemic, and she reported normal oral intake prior to this visit. Since the patient had started taking sertraline about 10 days prior to her syncopal episodes, we suspected POTS secondary to sertraline use was the likely etiology in this otherwise healthy young woman.
Continue to: Syncope could indicate a larger cardiovascular problem
Syncope could indicate a larger cardiovascular problem
The differential diagnosis of dizziness with loss of consciousness includes anemia, vasovagal syncope, orthostatic hypotension, dehydration, electrolyte imbalance, arrhythmia, prolonged QT syndrome, cardiac valve or structure abnormality, and seizure. Most of these differentials can be ruled out from basic laboratory tests or cardiac imaging. In POTS, the diagnostic work-up is essentially normal compared to other causes of syncope. Orthostatic hypotension, for example, is similar; however, there is an additional change in the arterial blood pressure.
Unintended adverse effects
Selective serotonin reuptake inhibitors (SSRIs), such as sertraline, are known to have fewer cardiovascular adverse effects compared to older antidepressants such as tricyclic antidepressants and monoamine oxidase inhibitors.4 However, case reports have shown an association between SSRIs and syncope.4-6 SSRIs have also been tied to increased heart rate variability.7
Nearly 2 weeks after stopping sertraline, our patient presented to clinic and was given a diagnosis of streptococcal pharyngitis. She said she’d had no additional syncopal episodes. Twenty days after sertraline cessation, the patient returned for follow-up. Her blood pressure and heart rate were as follows: supine, 112/68 mm Hg and 61 beats/min; seated, 113/74 mm Hg and 87 beats/min; and standing, 108/74 mm Hg and 78 beats/min.
Thus, after cessation of sertraline, her orthostatic heart rate changes were smaller than when she was first examined. Her vital signs showed an increase in pulse of 26 beats/min between lying and sitting, without any reports of nausea. She had no further complaints of dizziness or syncopal episodes.
THE TAKEAWAY
We don’t always know how a patient will respond to a newly prescribed medication or lifestyle change. A proper review of a patient’s history and medication use is a pivotal first step in making any diagnosis.
CORRESPONDENCE
Courtney Lynn Dominguez, MD, 4220 North Roxboro Street, Durham, NC 27704; [email protected]
1. Ojha A, McNeeley K, Heller E, et al. Orthostatic syndromes differ in syncope frequency. Am J Med. 2010;123:245-249. doi: 10.1016/j.amjmed.2009.09.018
2. Arnold AC, Ng J, Raj SR. Postural tachycardia syndrome—diagnosis, physiology, and prognosis. Auton Neurosci. 2018;215:3-11. doi: 10.1016/j.autneu.2018.02.005
3. Fedorowski A. Postural orthostatic tachycardia syndrome: clinical presentation, aetiology and management. J Intern Med. 2018;285:352-366. doi:10.1111/joim.12852
4. Pacher P, Ungvari Z, Kecskemeti V, et al. Review of cardiovascular effects of fluoxetine, a selective serotonin reuptake inhibitor, compared to tricyclic antidepressants. Curr Med Chem. 1998;5:381-390.
5. Feder R. Bradycardia and syncope induced by fluoxetine. J Clin Psychiatry. 1991;52:139.
6. Ellison JM, Milofsky JE, Ely E. Fluoxetine-induced bradycardia and syncope in two patients. J Clin Psychiatry. 1990;51:385-386.
7. Tucker P, Adamson P, Miranda R Jr, et al. Paroxetine increases heart rate variability in panic disorder. J Clin Psychopharmacol. 1997;17:370-376. doi: 10.1097/00004714-199710000-00006
THE CASE
A 20-year-old woman presented to clinic with a chief complaint of 2 syncopal episodes within 10 minutes of each other. She reported that in both cases, she felt nauseated and dizzy before losing consciousness. She lost consciousness for a few seconds during the first episode and a few minutes during the second episode. Both episodes were unwitnessed.
The patient denied any fasting, vomiting, diarrhea, palpitations, chest pain, incontinence, oral trauma, headaches, fevers, chills, or tremors. Her last menstrual period started 3 days prior to presentation. The patient was taking sertraline 25 mg once daily for anxiety and depression and norethindrone acetate–ethinyl estradiol tablets 20 µg daily for birth control. She also was finishing a 7-day course of metronidazole for bacterial vaginosis. She reported having started the sertraline about 10 days prior to the syncopal episodes. She denied any personal history of drug or alcohol use, syncope, seizures, or any other medical conditions. Family history was negative for any cardiac or neurologic conditions.
The patient appeared euvolemic on exam. Overall, the review of the respiratory, cardiac, and neurologic systems was unremarkable. An electrocardiogram, obtained in clinic, showed a normal sinus rhythm and QT interval. Orthostatic blood pressure and heart rate measurements were as follows: supine, 122/83 mm Hg and 67 beats/min; seated, 118/87 mm Hg and 60 beats/min; and standing, 123/83 mm Hg and 95 beats/min. In addition to the increase in pulse between sitting and standing, the patient reported feeling nauseated when transitioning to a standing position.
Laboratory work-up included a comprehensive metabolic panel, complete blood count, and thyroid-stimulating hormone test. The results showed mild erythrocytosis with a hematocrit and hemoglobin of 46.1% and 15.6 g/dL respectively, as well as mild hypercalcemia (10.4 mg/dL).
THE DIAGNOSIS
An increase in heart rate of more than 30 beats/min when the patient went from a sitting to a standing position pointed to a diagnosis of postural orthostatic tachycardia syndrome (POTS). This prompted us to stop the sertraline.
DISCUSSION
POTS is a type of intolerance to orthostasis related to a significant increase in pulse without resulting hypotension upon standing. Other symptoms that accompany this change in position include dizziness, lightheadedness, blurry vision, and fatigue. Syncope occurs in about 40% of patients with POTS, which may be more frequent than for patients with orthostatic hypotension.1
The overall prevalence of POTS is 0.2% to 1%; however, it is generally seen in a 5:1 female-to-male ratio.2,3 POTS is often idiopathic. That said, it can also be caused by medication adverse effects, hypovolemia, and stressors, including vaccinations, viral infections, trauma, and emotional triggers. On physical exam, this patient did not appear to be hypovolemic, and she reported normal oral intake prior to this visit. Since the patient had started taking sertraline about 10 days prior to her syncopal episodes, we suspected POTS secondary to sertraline use was the likely etiology in this otherwise healthy young woman.
Continue to: Syncope could indicate a larger cardiovascular problem
Syncope could indicate a larger cardiovascular problem
The differential diagnosis of dizziness with loss of consciousness includes anemia, vasovagal syncope, orthostatic hypotension, dehydration, electrolyte imbalance, arrhythmia, prolonged QT syndrome, cardiac valve or structure abnormality, and seizure. Most of these differentials can be ruled out from basic laboratory tests or cardiac imaging. In POTS, the diagnostic work-up is essentially normal compared to other causes of syncope. Orthostatic hypotension, for example, is similar; however, there is an additional change in the arterial blood pressure.
Unintended adverse effects
Selective serotonin reuptake inhibitors (SSRIs), such as sertraline, are known to have fewer cardiovascular adverse effects compared to older antidepressants such as tricyclic antidepressants and monoamine oxidase inhibitors.4 However, case reports have shown an association between SSRIs and syncope.4-6 SSRIs have also been tied to increased heart rate variability.7
Nearly 2 weeks after stopping sertraline, our patient presented to clinic and was given a diagnosis of streptococcal pharyngitis. She said she’d had no additional syncopal episodes. Twenty days after sertraline cessation, the patient returned for follow-up. Her blood pressure and heart rate were as follows: supine, 112/68 mm Hg and 61 beats/min; seated, 113/74 mm Hg and 87 beats/min; and standing, 108/74 mm Hg and 78 beats/min.
Thus, after cessation of sertraline, her orthostatic heart rate changes were smaller than when she was first examined. Her vital signs showed an increase in pulse of 26 beats/min between lying and sitting, without any reports of nausea. She had no further complaints of dizziness or syncopal episodes.
THE TAKEAWAY
We don’t always know how a patient will respond to a newly prescribed medication or lifestyle change. A proper review of a patient’s history and medication use is a pivotal first step in making any diagnosis.
CORRESPONDENCE
Courtney Lynn Dominguez, MD, 4220 North Roxboro Street, Durham, NC 27704; [email protected]
THE CASE
A 20-year-old woman presented to clinic with a chief complaint of 2 syncopal episodes within 10 minutes of each other. She reported that in both cases, she felt nauseated and dizzy before losing consciousness. She lost consciousness for a few seconds during the first episode and a few minutes during the second episode. Both episodes were unwitnessed.
The patient denied any fasting, vomiting, diarrhea, palpitations, chest pain, incontinence, oral trauma, headaches, fevers, chills, or tremors. Her last menstrual period started 3 days prior to presentation. The patient was taking sertraline 25 mg once daily for anxiety and depression and norethindrone acetate–ethinyl estradiol tablets 20 µg daily for birth control. She also was finishing a 7-day course of metronidazole for bacterial vaginosis. She reported having started the sertraline about 10 days prior to the syncopal episodes. She denied any personal history of drug or alcohol use, syncope, seizures, or any other medical conditions. Family history was negative for any cardiac or neurologic conditions.
The patient appeared euvolemic on exam. Overall, the review of the respiratory, cardiac, and neurologic systems was unremarkable. An electrocardiogram, obtained in clinic, showed a normal sinus rhythm and QT interval. Orthostatic blood pressure and heart rate measurements were as follows: supine, 122/83 mm Hg and 67 beats/min; seated, 118/87 mm Hg and 60 beats/min; and standing, 123/83 mm Hg and 95 beats/min. In addition to the increase in pulse between sitting and standing, the patient reported feeling nauseated when transitioning to a standing position.
Laboratory work-up included a comprehensive metabolic panel, complete blood count, and thyroid-stimulating hormone test. The results showed mild erythrocytosis with a hematocrit and hemoglobin of 46.1% and 15.6 g/dL respectively, as well as mild hypercalcemia (10.4 mg/dL).
THE DIAGNOSIS
An increase in heart rate of more than 30 beats/min when the patient went from a sitting to a standing position pointed to a diagnosis of postural orthostatic tachycardia syndrome (POTS). This prompted us to stop the sertraline.
DISCUSSION
POTS is a type of intolerance to orthostasis related to a significant increase in pulse without resulting hypotension upon standing. Other symptoms that accompany this change in position include dizziness, lightheadedness, blurry vision, and fatigue. Syncope occurs in about 40% of patients with POTS, which may be more frequent than for patients with orthostatic hypotension.1
The overall prevalence of POTS is 0.2% to 1%; however, it is generally seen in a 5:1 female-to-male ratio.2,3 POTS is often idiopathic. That said, it can also be caused by medication adverse effects, hypovolemia, and stressors, including vaccinations, viral infections, trauma, and emotional triggers. On physical exam, this patient did not appear to be hypovolemic, and she reported normal oral intake prior to this visit. Since the patient had started taking sertraline about 10 days prior to her syncopal episodes, we suspected POTS secondary to sertraline use was the likely etiology in this otherwise healthy young woman.
Continue to: Syncope could indicate a larger cardiovascular problem
Syncope could indicate a larger cardiovascular problem
The differential diagnosis of dizziness with loss of consciousness includes anemia, vasovagal syncope, orthostatic hypotension, dehydration, electrolyte imbalance, arrhythmia, prolonged QT syndrome, cardiac valve or structure abnormality, and seizure. Most of these differentials can be ruled out from basic laboratory tests or cardiac imaging. In POTS, the diagnostic work-up is essentially normal compared to other causes of syncope. Orthostatic hypotension, for example, is similar; however, there is an additional change in the arterial blood pressure.
Unintended adverse effects
Selective serotonin reuptake inhibitors (SSRIs), such as sertraline, are known to have fewer cardiovascular adverse effects compared to older antidepressants such as tricyclic antidepressants and monoamine oxidase inhibitors.4 However, case reports have shown an association between SSRIs and syncope.4-6 SSRIs have also been tied to increased heart rate variability.7
Nearly 2 weeks after stopping sertraline, our patient presented to clinic and was given a diagnosis of streptococcal pharyngitis. She said she’d had no additional syncopal episodes. Twenty days after sertraline cessation, the patient returned for follow-up. Her blood pressure and heart rate were as follows: supine, 112/68 mm Hg and 61 beats/min; seated, 113/74 mm Hg and 87 beats/min; and standing, 108/74 mm Hg and 78 beats/min.
Thus, after cessation of sertraline, her orthostatic heart rate changes were smaller than when she was first examined. Her vital signs showed an increase in pulse of 26 beats/min between lying and sitting, without any reports of nausea. She had no further complaints of dizziness or syncopal episodes.
THE TAKEAWAY
We don’t always know how a patient will respond to a newly prescribed medication or lifestyle change. A proper review of a patient’s history and medication use is a pivotal first step in making any diagnosis.
CORRESPONDENCE
Courtney Lynn Dominguez, MD, 4220 North Roxboro Street, Durham, NC 27704; [email protected]
1. Ojha A, McNeeley K, Heller E, et al. Orthostatic syndromes differ in syncope frequency. Am J Med. 2010;123:245-249. doi: 10.1016/j.amjmed.2009.09.018
2. Arnold AC, Ng J, Raj SR. Postural tachycardia syndrome—diagnosis, physiology, and prognosis. Auton Neurosci. 2018;215:3-11. doi: 10.1016/j.autneu.2018.02.005
3. Fedorowski A. Postural orthostatic tachycardia syndrome: clinical presentation, aetiology and management. J Intern Med. 2018;285:352-366. doi:10.1111/joim.12852
4. Pacher P, Ungvari Z, Kecskemeti V, et al. Review of cardiovascular effects of fluoxetine, a selective serotonin reuptake inhibitor, compared to tricyclic antidepressants. Curr Med Chem. 1998;5:381-390.
5. Feder R. Bradycardia and syncope induced by fluoxetine. J Clin Psychiatry. 1991;52:139.
6. Ellison JM, Milofsky JE, Ely E. Fluoxetine-induced bradycardia and syncope in two patients. J Clin Psychiatry. 1990;51:385-386.
7. Tucker P, Adamson P, Miranda R Jr, et al. Paroxetine increases heart rate variability in panic disorder. J Clin Psychopharmacol. 1997;17:370-376. doi: 10.1097/00004714-199710000-00006
1. Ojha A, McNeeley K, Heller E, et al. Orthostatic syndromes differ in syncope frequency. Am J Med. 2010;123:245-249. doi: 10.1016/j.amjmed.2009.09.018
2. Arnold AC, Ng J, Raj SR. Postural tachycardia syndrome—diagnosis, physiology, and prognosis. Auton Neurosci. 2018;215:3-11. doi: 10.1016/j.autneu.2018.02.005
3. Fedorowski A. Postural orthostatic tachycardia syndrome: clinical presentation, aetiology and management. J Intern Med. 2018;285:352-366. doi:10.1111/joim.12852
4. Pacher P, Ungvari Z, Kecskemeti V, et al. Review of cardiovascular effects of fluoxetine, a selective serotonin reuptake inhibitor, compared to tricyclic antidepressants. Curr Med Chem. 1998;5:381-390.
5. Feder R. Bradycardia and syncope induced by fluoxetine. J Clin Psychiatry. 1991;52:139.
6. Ellison JM, Milofsky JE, Ely E. Fluoxetine-induced bradycardia and syncope in two patients. J Clin Psychiatry. 1990;51:385-386.
7. Tucker P, Adamson P, Miranda R Jr, et al. Paroxetine increases heart rate variability in panic disorder. J Clin Psychopharmacol. 1997;17:370-376. doi: 10.1097/00004714-199710000-00006
A guide to the Tx of cellulitis and other soft-tissue infections
Skin and soft-tissue infections, frequently encountered in primary care, range from the uncomplicated erysipelas to the life-threatening necrotizing fasciitis. This review draws from the latest evidence and guidelines to help guide the care you provide to patients with cellulitis, orbital cellulitis, erysipelas, folliculitis, furuncles, carbuncles, abscesses, and necrotizing fasciitis.
Cellulitis
Cellulitis, an infection of the deep dermal and subcutaneous layers of the skin, has become increasingly common in recent years, with both incidence and hospitalization rates rising.1 Cellulitis occurs when pathogens enter the dermis through breaks in the skin barrier due to cutaneous fungal infections, trauma, pressure sores, venous stasis, or inflammation. The diagnosis is often made clinically based on characteristic skin findings—classically an acute, poorly demarcated area of erythema, warmth, swelling, and tenderness. Lymphangitic streaking and local lymphadenopathy may also be present. Infection often occurs on an extremity (although it can be found on other areas of the body) and is usually unilateral. Fever may or may not be present.2
Likely responsible microorganisms. Staphylococcus aureus and Group A streptococci (often Streptococcus pyogenes) are common culprits. One systematic review that examined cultures taken of intact skin in cellulitis patients found S aureus to be about twice as common as S pyogenes, with both bacteria accounting for a little more than 70% of cases. Of the remaining positive cultures, the most common organisms were alpha-hemolytic streptococcus, group B streptococcus, Pseudomonas aeruginosa, Clostridium perfringens, Escherichia coli, Pasteurella multocida, and Proteus mirabilis.3 Similarly, a systematic review of bacteremia in patients with cellulitis and erysipelas found that S pyogenes, other beta-hemolytic strep, and S aureus account for about 70% of cases (although S aureus was responsible for just 14%), with the remainder of cases caused by gram-negative organisms such as E coli and P aeruginosa.4
Treatment considerations. Strict treatment guidelines for cellulitis are lacking, but general consensus encourages the use of antibiotics and occasionally surgery. For mild and moderate cases of cellulitis, prescribe oral and parenteral antibiotics to cover for streptococci and methicillin-susceptible S aureus, respectively. Expand coverage to include vancomycin if nasal colonization shows methicillin-resistant S aureus (MRSA) or if you otherwise suspect prior MRSA exposure. Expanded coverage will also be needed if there is severe nonpurulent infection associated with penetrating trauma or a history of intravenous drug use, or the patient meets criteria for systemic inflammatory response syndrome. If patients are severely compromised (eg, neutropenic), it is reasonable to further add broad-spectrum coverage (eg, intravenous piperacillin-tazobactam or carbapenem). Typical duration of treatment is 5 to 7 days, although this should be extended if there is no clinical improvement.
Generally, cellulitis can be managed in the outpatient setting, although hospitalization is recommended if there are concerns for deep or necrotizing infection, if patients are nonadherent to therapy or are immunocompromised, or if outpatient therapy has failed.5 Furthermore, in an observational study of 606 adult patients, prior episodes of cellulitis, venous insufficiency, and immunosuppression were all independently associated with poorer clinical outcomes.2 Also treat underlying predisposing factors such as edema, obesity, eczema, venous insufficiency, and toe web abnormalities such as fissures, scaling, or maceration.5 Consider the use of prophylactic antibiotics for patients who have had 3 to 4 episodes of cellulitis despite attempts to treat predisposing conditions. Prophylactic antibiotic regimens include penicillin or erythromycin orally and penicillin G benzathine intramuscularly.5 Antibiotic regimens are summarized in the TABLE.5
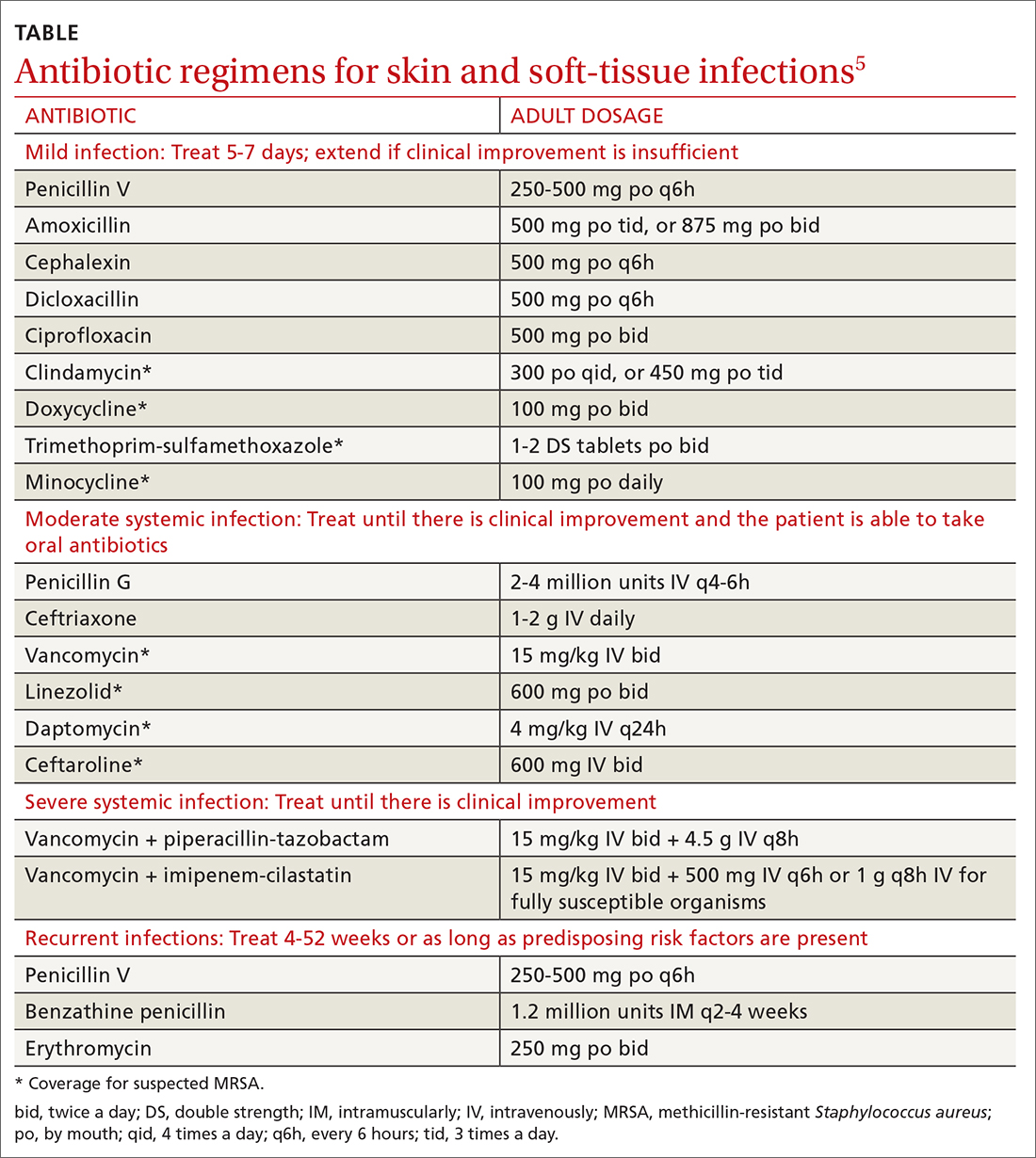
Orbital cellulitis
Orbital cellulitis is an infection of the tissues posterior to the orbital septum.6,7 Periorbital, or preseptal, cellulitis occurs anterior to the orbital septum and is the more common of the 2 infections—84% compared with 16% for orbital cellulitis.6 However, orbital cellulitis, which affects mainly children at a median age of 7 years,6 must be detected and treated early due to the potential for serious complications such as cavernous sinus thrombosis, meningitis, intracranial abscess, and vision loss.7 Chemosis (conjunctival edema) and diplopia are more commonly associated with orbital cellulitis and are seldom seen with preseptal cellulitis.
Predominant causative organisms are S pneumoniae, Moraxella catarrhalis, non-typeable Haemophilus influenzae, and group A streptococcus. The most common mechanism of infection is tracking from periorbital structures (eg, paranasal and ethmoid sinusitis). Other causes include orbital trauma/fracture, periorbital surgery, and bacterial endocarditis. Clinically, patients present with limited ocular motility and proptosis associated with inflamed conjunctiva, orbital pain, headache, malaise, fever, eyelid edema, and possible decrease in visual acuity. The diagnosis is often made clinically and confirmed with orbital computed tomography (CT) with contrast, which can assist in ruling out intracranial involvement such as abscess.
Continue to: Antibiotic therapy
Antibiotic therapy, generally administered intravenously, is recommended for at least 3 days or until orbital symptoms begin to resolve. Choose antibiotics effective against sinusitis-related pathogens (eg, S pneumoniae, H influenzae, M catarrhalis), S aureus, and anaerobes.8 For instance, a regimen may include vancomycin for MRSA coverage, a third-generation cephalosporin, or metronidazole for anaerobic coverage if there is concern about intracranial involvement. Surgical intervention is often reserved for patients with inadequate response to antibiotic therapy, necessitating biopsy for pathogen identification, as well as drainage of large abscesses refractory to antibiotics.
Erysipelas
Erysipelas, a related yet distinct form of cellulitis, is a bacterial infection of the superficial dermis and hypodermis and is commonly caused by group A streptococcus.5,9 Other less common organisms include S aureus, P aeruginosa, and enterobacteria. Erysipelas predominantly affects the lower extremities unilaterally (~90%); the arms and the face are the next most common locations. In addition to the rapid onset of well-demarcated erythema, pain, and swelling, patients may have fever and regional lymphadenopathy. Risk factors include portal of entry (eg, tinea pedis, ulceration), lymphedema, and diabetes. Complications of erysipelas include bullae from edema, abscess formation, and, rarely, bacteremia.
Antibiotic treatment regimens include penicillin G, macrolides (reserved for those with penicillin allergies), fluoroquinolones, and cephalosporins, with duration of treatment ranging from 10 to 14 days depending on infection severity. Fever, pain, and erythema generally improve within 48 to 72 hours of antibiotic therapy. If there is no improvement, consider alternative diagnoses, such as necrotizing fasciitis. Recurrence rates following the initial episode of erysipelas are estimated at 10% of patients at 6 months and 30% at 3 years.10
Folliculitis
Inflammation of hair follicles is characterized by superficial inflammation with the development of perifollicular papules or pustules on an erythematous base.11,12 Folliculitis most commonly affects the face, scalp, thighs, buttocks, axillae, and inguinal areas.13 It may be caused by infection, an inflammatory reaction, or physical injury. Diagnosis is typically based on the patient’s history and physical examination.
Bacteria are the most common cause of infection, although fungi, viruses, and other entities can cause folliculitis. S aureus (methicillin sensitive or methicillin resistant) is the most common pathogen; in the past, superficial pustular folliculitis attributed to S aureus was referred to as Bockhart impetigo. Folliculitis secondary to P aeruginosa, often seen after exposure to contaminated water or hot tubs, is frequently referred to as “hot tub folliculitis.” Malassezia, a reported cause of fungal folliculitis, tends to occur in adolescents of either sex and men with high sebum production, is common in tropical climates, and can be associated with HIV or immunosuppression.11,12,14
Continue to: Differential diagnosis...
Differential diagnosis of folliculitis includes pseudofolliculitis barbae, eosinophilic folliculitis, keratosis pilaris, acne vulgaris, candidiasis, contact dermatitis, impetigo, and miliaria.13 Pseudofolliculitis barbae is an inflammatory reaction to shaving, more commonly seen in darkly pigmented skin. Pseudofolliculitis develops when the hair shaft penetrates the wall of the follicle or directly enters the epidermis.
Initial treatment for mild disease includes the elimination of predisposing factors such as occlusion, moisture, and abrasion. The area should be kept clean and dry, avoiding friction. For localized disease, prescribe topical clindamycin, mupirocin ointment, or benzoyl peroxide. If symptoms fail to respond, prescribe a 7-day course of antibiotic that targets methicillin-sensitive S aureus—eg, cephalexin or dicloxacillin. Also consider doxycycline, which has anti-inflammatory effects and is effective against MRSA. For refractory lesions, trimethoprim-sulfamethoxazole, clindamycin, or minocycline may be useful. If you suspect pseudomonas, consider giving ciprofloxacin for 10 to 14 days for persistent lesions or if the patient is immunocompromised.13,15 Consider obtaining bacterial, fungal, or viral cultures for lesions that fail to respond to initial treatment.
Furuncles/carbuncles/abscesses
A furuncle, commonly referred to as a boil, is an infected hair follicle that becomes enclosed, creating a collection of pus. A carbuncle is a collection of furuncles that converge and drain through a single opening. An abscess is a localized collection of pus arising from within the dermis that can extend within deeper tissues.5 Furuncles, carbuncles, and abscesses are managed similarly with drainage and consideration for MRSA risk factors.
S aureus is the most common cause of these infections; 59% of skin abscesses are due to community-acquired MRSA.16 Anaerobes may contribute to the polymicrobial flora of skin abscesses.17 Risk factors for MRSA infection include a history of previous MRSA infection, diabetes, dialysis or renal failure, placement of an indwelling catheter or medical device, injection drug use, incarceration, close contact with a person with known MRSA infection or colonization, long-term care residence, hospitalization or surgery within the past 12 months, and high prevalence of MRSA in the community.5
Ultrasound improves diagnostic accuracy. One study showed that when a clinical exam alone was inconclusive in evaluating skin and soft-tissue infections in children and adolescents, an ultrasound-assisted examination improved diagnostic accuracy.18 Sensitivity of the clinical examination was 43.7%, compared with 77.6% for the clinical examination plus ultrasound.18
Continue to: Incision and drainage first
Incision and drainage first. Ultrasound-guided needle aspiration, however, has not improved treatment efficacy compared with incision and drainage,19 the mainstay approach for abscesses.17 The procedure to drain a furuncle, carbuncle, or abscess should include the expression of all purulent material and the removal of all loculations if possible. Wound culture is recommended during incision and drainage per current guidelines.5 Simple dry dressings are convenient and effective, although some wounds may require packing. Tap water (that is potable) is suitable for wound cleansing. However, there is no strong evidence that irrigating wounds increases healing or reduces infection.20
Routine use of antibiotics is not recommended for simple cutaneous abscesses.5,17,21 Evidence has been conflicting regarding empiric antibiotic coverage of MRSA following incision and drainage.22-25 Guidelines recommend considering the initiation of antibiotics if there are multiple abscesses, gangrene, surrounding cellulitis, or systemic signs of infection, or if the host is immunocompromised.5
If MRSA is suspected, recommended antibiotic coverage includes trimethoprim-sulfamethoxazole, clindamycin, doxycycline, or minocycline.5 If MRSA is identified, treatment options include dicloxacillin or cephalexin. For severe infections persisting after incision and drainage, in addition to oral antibiotic therapy, consider intravenous antibiotic options for MRSA: cefazolin, clindamycin, linezolid, nafcillin, telavancin, or vancomycin.5
Necrotizing fasciitis
Necrotizing fasciitis is a rare but potentially deadly infection of the skin and soft tissue. It progresses rapidly and spreads along fascial planes, leading to the necrosis of the superficial fascia. The infection often is more extensive than is indicated by superficial signs. Prompt diagnosis is imperative as necrotizing fasciitis is a surgical emergency.5,26 In the United States, 500 to 1500 cases of necrotizing fasciitis occur each year.27 Risk factors for necrotizing fasciitis include diabetes, peripheral vascular disease, malignancy, obesity, cirrhosis, renal failure, injection drug use, chronic corticosteroid therapy, alcohol abuse, malnutrition, and iatrogenic immunosuppression.26,28
Necrotizing fasciitis may be polymicrobial or monomicrobial. Polymicrobial infection, also referred to as type I, is often due to multiple bacteria that originate from the bowel flora, typically including a mix of anaerobic and aerobic organisms. On average, there can be 5 infecting organisms identified per wound, although in some cases up to 15 organisms have been identified in a single wound.5 Type I infection is often associated with tissue injury, abscess, or abdominal surgery. The majority of cases of necrotizing fasciitis are polymicrobial.27,28
Continue to: Monomicrobial infection...
Monomicrobial infection, also referred to as type II, is often due to group A streptococcus, S aureus, vibrio spp, Aeromonas hyrophilio, or an anaerobic streptococci like peptostreptococcus spp. Typically monomicrobial infections, which account for 20% to 30% of cases of necrotizing fasciitis, are community acquired.5,26,29,30
Clinical presentation. In the early stages of disease, patients commonly complain of flu-like symptoms and extreme pain that is out of proportion to findings on the exam. Additional warning signs include fevers and other symptoms of toxicity such as tachycardia, hypotension, nausea, vomiting, and diarrhea. Later in the course, symptoms may localize to the affected area and include erythema, tense swelling, development of blisters or bullae, blackish blue discoloration of the skin, severe pain, and loss of sensation. In some cases involving gas-forming bacteria, tissue crepitus may be noted on exam.5,27-31
Rely on clinical judgment to hasten surgical intervention. Laboratory or imaging findings may augment clinical judgment. But if you suspect necrotizing fasciitis, obtaining blood tests and imaging should not delay surgery. Blood tests that may aid in the diagnosis of necrotizing fasciitis include a complete blood count with differential; coagulation studies; a comprehensive metabolic panel; assays of lactate, C-reactive protein (CRP), and creatinine kinase; and blood cultures. Most often, patients with necrotizing fasciitis will have leukocytosis or leukopenia, evidence of hemolysis, thrombocytopenia, acute renal failure, and significantly elevated CRP.
On any imaging modality, indications of necrotizing fasciitis are inflammatory infiltration of the deep fascia on the affected side that is absent on the contralateral side, and the presence of subcutaneous air (which is a specific but rare finding). Imaging modalities may include CT or magnetic resonance imaging. A definitive diagnosis can only be made with surgical exploration of the involved area. Definitive microbiologic diagnosis will require culture of organisms from affected tissue or blood.5,26,30,31
First address any hemodynamic instability (hypotension is frequently encountered), followed by urgent surgical exploration, debridement of the wound, and antimicrobial therapy. Antibiotic treatment should align with probable pathogens and treatment should be continued until repeated surgical debridement is no longer necessary, clinical improvement is evident, and 48 to 72 hours have passed since defervescence. A reasonable initial empiric regimen in adults would include an agent that is effective against group A streptococcus, gram-negative pathogens, and anaerobes, such as a carbapenem or a beta-lactam-beta-lactamase inhibitor such as piperacillin-tazobactam. Additionally, include an agent that targets MRSA, such as vancomycin, linezolid, or clindamycin.5
CORRESPONDENCE
Karl T. Clebak, MD, Department of Family and Community Medicine Residency Program, Penn State Health M.S. Hershey Medical Center, 500 University Drive, H154/C1613, Hershey, PA 17033; [email protected]
1. Raff AB, Kroshinsky D. Cellulitis: a review. JAMA. 2016;316:325-337.
2. Collazos J, de la Fuente B, García A, et al. Cellulitis in adult patients: a large, multicenter, observational, prospective study of 606 episodes and analysis of the factors related to the response to treatment. PLoS One. 2018;13:e0204036.
3. Chira S, Miller LG. Staphylococcus aureus is the most common identified cause of cellulitis: a systematic review. Epidemiol Infect. 2010;138:313-317.
4. Gunderson CG, Martinello RA. A systematic review of bacteremias in cellulitis and erysipelas. J Infect. 2012;64:148-155.
5. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59:147-159.
6. Jain A, Rubin PA. Orbital cellulitis in children. Int Ophthalmol Clin. 2001;41:71-86.
7. Seltz LB, Smith J, Durairaj VD, et al. Microbiology and antibiotic management of orbital cellulitis. Pediatrics. 2011;127:e566-e572.
8. Nageswaran S, Woods CR, Benjamin DK, et al. Orbital cellulitis in children. Pediatr Infect Dis J. 2006;25:695-699.
9. Bonnetblanc J-M, Bédane C. Erysipelas. Am J Clin Dermatol. 2003;4:157-163.
10. Jorup-Rönström C, Britton S. Recurrent erysipelas: predisposing factors and costs of prophylaxis. Infection. 1987;15:105-106.
11. Clebak KT, Malone MA. Skin Infections. Prim Care. 2018;45:433-454.
12. Luelmo-Aguilar J, Santandreu MS. Folliculitis: recognition and management. Am J Clin Dermatol. 2004;5:301-310.
13. Mengesha YM, Bennett ML. Pustular skin disorders: diagnosis and treatment. Am J Clin Dermatol. 2002;3:389-400.
14. Akaza N, Akamatsu H, Sasaki Y, et al. Malassezia folliculitis is caused by cutaneous resident Malassezia species. Med Mycol. 2009;47:618-624.
15. Berger RS, Seifert MR. Whirlpool folliculitis: a review of its cause, treatment, and prevention. Cutis. 1990;45:97-98.
16. Fridkin SK, Hageman JC, Morrison M, et al. Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005;352:1436-1444.
17. Meislin HW, Lerner SA, Graves MH, et al. Cutaneous abscesses: anaerobic and aerobic bacteriology and outpatient management. Ann Intern Med. 1977;87:145-149.
18. Marin JR, Dean AJ, Bilker WB, et al. Emergency ultrasound-assisted examination of skin and soft tissue infections in the pediatric emergency department. Acad Emerg Med. 2013;20:545-553.
19. Gaspari RJ, Resop D, Mendoza M, et al. A randomized controlled trial of incision and drainage versus ultrasonographically guided needle aspiration for skin abscesses and the effect of methicillin-resistant Staphylococcus aureus. Ann Emerg Med. 2011;57:483-491.
20. Fernandez R, Griffiths R, Ussia C. Water for wound cleansing. Cochrane Database Syst Rev. 2002: CD003861.
21. Llera JL, Levy RC. Treatment of cutaneous abscess: a double-blind clinical study. Ann Emerg Med. 1985;14:15-19.
22. Talan DA, Mower WR, Krishnadasan A, et al. Trimethoprim-sulfamethoxazole versus placebo for uncomplicated skin abscess. N Engl J Med. 2016;374:823-832.
23. Korownyk C, Allan GM. Evidence-based approach to abscess management. Can Fam Physician. 2007;53:1680-1684.
24. Schmitz GR, Bruner D, Pitotti R, et al. Randomized controlled trial of trimethoprim-sulfamethoxazole for uncomplicated skin abscesses in patients at risk for community-associated methicillin-resistant Staphylococcus aureus infection. Ann Emerg Med. 2010;56:283-287.
25. Rajendran PM, Young D, Maurer T, et al. Randomized, double-blind, placebo-controlled trial of cephalexin for treatment of uncomplicated skin abscesses in a population at risk for community-acquired methicillin-resistant Staphylococcus aureus infection. Antimicrob Agents Chemother. 2007;51:4044-4048.
26. Hunter J, Quarterman C, Waseem M, et al. Diagnosis and management of necrotizing fasciitis. Br J Hosp Med. 2011;72:391-395.
27. Hussein QA, Anaya DA. Necrotizing soft tissue infections. Crit Care Clin. 2013;29:795-806.
28. Puvanendran R, Huey JCM, Pasupathy S. Necrotizing fasciitis. Can Fam Physician. 2009;55:981-987.
29. Raven MC, Billings JC, Goldfrank LR, et al. Medicaid patients at high risk for frequent hospital admission: real-time identification and remediable risks. J Urban Health. 2009;86:230-241.
30. Ustin JS, Malangoni MA. Necrotizing soft-tissue infections: Crit Care Med. 2011;39:2156-2162.
31. Bystritsky R, Chambers H. Cellulitis and soft tissue infections. Ann Intern Med. 2018;168:ITC17- ITC32.
Skin and soft-tissue infections, frequently encountered in primary care, range from the uncomplicated erysipelas to the life-threatening necrotizing fasciitis. This review draws from the latest evidence and guidelines to help guide the care you provide to patients with cellulitis, orbital cellulitis, erysipelas, folliculitis, furuncles, carbuncles, abscesses, and necrotizing fasciitis.
Cellulitis
Cellulitis, an infection of the deep dermal and subcutaneous layers of the skin, has become increasingly common in recent years, with both incidence and hospitalization rates rising.1 Cellulitis occurs when pathogens enter the dermis through breaks in the skin barrier due to cutaneous fungal infections, trauma, pressure sores, venous stasis, or inflammation. The diagnosis is often made clinically based on characteristic skin findings—classically an acute, poorly demarcated area of erythema, warmth, swelling, and tenderness. Lymphangitic streaking and local lymphadenopathy may also be present. Infection often occurs on an extremity (although it can be found on other areas of the body) and is usually unilateral. Fever may or may not be present.2
Likely responsible microorganisms. Staphylococcus aureus and Group A streptococci (often Streptococcus pyogenes) are common culprits. One systematic review that examined cultures taken of intact skin in cellulitis patients found S aureus to be about twice as common as S pyogenes, with both bacteria accounting for a little more than 70% of cases. Of the remaining positive cultures, the most common organisms were alpha-hemolytic streptococcus, group B streptococcus, Pseudomonas aeruginosa, Clostridium perfringens, Escherichia coli, Pasteurella multocida, and Proteus mirabilis.3 Similarly, a systematic review of bacteremia in patients with cellulitis and erysipelas found that S pyogenes, other beta-hemolytic strep, and S aureus account for about 70% of cases (although S aureus was responsible for just 14%), with the remainder of cases caused by gram-negative organisms such as E coli and P aeruginosa.4
Treatment considerations. Strict treatment guidelines for cellulitis are lacking, but general consensus encourages the use of antibiotics and occasionally surgery. For mild and moderate cases of cellulitis, prescribe oral and parenteral antibiotics to cover for streptococci and methicillin-susceptible S aureus, respectively. Expand coverage to include vancomycin if nasal colonization shows methicillin-resistant S aureus (MRSA) or if you otherwise suspect prior MRSA exposure. Expanded coverage will also be needed if there is severe nonpurulent infection associated with penetrating trauma or a history of intravenous drug use, or the patient meets criteria for systemic inflammatory response syndrome. If patients are severely compromised (eg, neutropenic), it is reasonable to further add broad-spectrum coverage (eg, intravenous piperacillin-tazobactam or carbapenem). Typical duration of treatment is 5 to 7 days, although this should be extended if there is no clinical improvement.
Generally, cellulitis can be managed in the outpatient setting, although hospitalization is recommended if there are concerns for deep or necrotizing infection, if patients are nonadherent to therapy or are immunocompromised, or if outpatient therapy has failed.5 Furthermore, in an observational study of 606 adult patients, prior episodes of cellulitis, venous insufficiency, and immunosuppression were all independently associated with poorer clinical outcomes.2 Also treat underlying predisposing factors such as edema, obesity, eczema, venous insufficiency, and toe web abnormalities such as fissures, scaling, or maceration.5 Consider the use of prophylactic antibiotics for patients who have had 3 to 4 episodes of cellulitis despite attempts to treat predisposing conditions. Prophylactic antibiotic regimens include penicillin or erythromycin orally and penicillin G benzathine intramuscularly.5 Antibiotic regimens are summarized in the TABLE.5

Orbital cellulitis
Orbital cellulitis is an infection of the tissues posterior to the orbital septum.6,7 Periorbital, or preseptal, cellulitis occurs anterior to the orbital septum and is the more common of the 2 infections—84% compared with 16% for orbital cellulitis.6 However, orbital cellulitis, which affects mainly children at a median age of 7 years,6 must be detected and treated early due to the potential for serious complications such as cavernous sinus thrombosis, meningitis, intracranial abscess, and vision loss.7 Chemosis (conjunctival edema) and diplopia are more commonly associated with orbital cellulitis and are seldom seen with preseptal cellulitis.
Predominant causative organisms are S pneumoniae, Moraxella catarrhalis, non-typeable Haemophilus influenzae, and group A streptococcus. The most common mechanism of infection is tracking from periorbital structures (eg, paranasal and ethmoid sinusitis). Other causes include orbital trauma/fracture, periorbital surgery, and bacterial endocarditis. Clinically, patients present with limited ocular motility and proptosis associated with inflamed conjunctiva, orbital pain, headache, malaise, fever, eyelid edema, and possible decrease in visual acuity. The diagnosis is often made clinically and confirmed with orbital computed tomography (CT) with contrast, which can assist in ruling out intracranial involvement such as abscess.
Continue to: Antibiotic therapy
Antibiotic therapy, generally administered intravenously, is recommended for at least 3 days or until orbital symptoms begin to resolve. Choose antibiotics effective against sinusitis-related pathogens (eg, S pneumoniae, H influenzae, M catarrhalis), S aureus, and anaerobes.8 For instance, a regimen may include vancomycin for MRSA coverage, a third-generation cephalosporin, or metronidazole for anaerobic coverage if there is concern about intracranial involvement. Surgical intervention is often reserved for patients with inadequate response to antibiotic therapy, necessitating biopsy for pathogen identification, as well as drainage of large abscesses refractory to antibiotics.
Erysipelas
Erysipelas, a related yet distinct form of cellulitis, is a bacterial infection of the superficial dermis and hypodermis and is commonly caused by group A streptococcus.5,9 Other less common organisms include S aureus, P aeruginosa, and enterobacteria. Erysipelas predominantly affects the lower extremities unilaterally (~90%); the arms and the face are the next most common locations. In addition to the rapid onset of well-demarcated erythema, pain, and swelling, patients may have fever and regional lymphadenopathy. Risk factors include portal of entry (eg, tinea pedis, ulceration), lymphedema, and diabetes. Complications of erysipelas include bullae from edema, abscess formation, and, rarely, bacteremia.
Antibiotic treatment regimens include penicillin G, macrolides (reserved for those with penicillin allergies), fluoroquinolones, and cephalosporins, with duration of treatment ranging from 10 to 14 days depending on infection severity. Fever, pain, and erythema generally improve within 48 to 72 hours of antibiotic therapy. If there is no improvement, consider alternative diagnoses, such as necrotizing fasciitis. Recurrence rates following the initial episode of erysipelas are estimated at 10% of patients at 6 months and 30% at 3 years.10
Folliculitis
Inflammation of hair follicles is characterized by superficial inflammation with the development of perifollicular papules or pustules on an erythematous base.11,12 Folliculitis most commonly affects the face, scalp, thighs, buttocks, axillae, and inguinal areas.13 It may be caused by infection, an inflammatory reaction, or physical injury. Diagnosis is typically based on the patient’s history and physical examination.
Bacteria are the most common cause of infection, although fungi, viruses, and other entities can cause folliculitis. S aureus (methicillin sensitive or methicillin resistant) is the most common pathogen; in the past, superficial pustular folliculitis attributed to S aureus was referred to as Bockhart impetigo. Folliculitis secondary to P aeruginosa, often seen after exposure to contaminated water or hot tubs, is frequently referred to as “hot tub folliculitis.” Malassezia, a reported cause of fungal folliculitis, tends to occur in adolescents of either sex and men with high sebum production, is common in tropical climates, and can be associated with HIV or immunosuppression.11,12,14
Continue to: Differential diagnosis...
Differential diagnosis of folliculitis includes pseudofolliculitis barbae, eosinophilic folliculitis, keratosis pilaris, acne vulgaris, candidiasis, contact dermatitis, impetigo, and miliaria.13 Pseudofolliculitis barbae is an inflammatory reaction to shaving, more commonly seen in darkly pigmented skin. Pseudofolliculitis develops when the hair shaft penetrates the wall of the follicle or directly enters the epidermis.
Initial treatment for mild disease includes the elimination of predisposing factors such as occlusion, moisture, and abrasion. The area should be kept clean and dry, avoiding friction. For localized disease, prescribe topical clindamycin, mupirocin ointment, or benzoyl peroxide. If symptoms fail to respond, prescribe a 7-day course of antibiotic that targets methicillin-sensitive S aureus—eg, cephalexin or dicloxacillin. Also consider doxycycline, which has anti-inflammatory effects and is effective against MRSA. For refractory lesions, trimethoprim-sulfamethoxazole, clindamycin, or minocycline may be useful. If you suspect pseudomonas, consider giving ciprofloxacin for 10 to 14 days for persistent lesions or if the patient is immunocompromised.13,15 Consider obtaining bacterial, fungal, or viral cultures for lesions that fail to respond to initial treatment.
Furuncles/carbuncles/abscesses
A furuncle, commonly referred to as a boil, is an infected hair follicle that becomes enclosed, creating a collection of pus. A carbuncle is a collection of furuncles that converge and drain through a single opening. An abscess is a localized collection of pus arising from within the dermis that can extend within deeper tissues.5 Furuncles, carbuncles, and abscesses are managed similarly with drainage and consideration for MRSA risk factors.
S aureus is the most common cause of these infections; 59% of skin abscesses are due to community-acquired MRSA.16 Anaerobes may contribute to the polymicrobial flora of skin abscesses.17 Risk factors for MRSA infection include a history of previous MRSA infection, diabetes, dialysis or renal failure, placement of an indwelling catheter or medical device, injection drug use, incarceration, close contact with a person with known MRSA infection or colonization, long-term care residence, hospitalization or surgery within the past 12 months, and high prevalence of MRSA in the community.5
Ultrasound improves diagnostic accuracy. One study showed that when a clinical exam alone was inconclusive in evaluating skin and soft-tissue infections in children and adolescents, an ultrasound-assisted examination improved diagnostic accuracy.18 Sensitivity of the clinical examination was 43.7%, compared with 77.6% for the clinical examination plus ultrasound.18
Continue to: Incision and drainage first
Incision and drainage first. Ultrasound-guided needle aspiration, however, has not improved treatment efficacy compared with incision and drainage,19 the mainstay approach for abscesses.17 The procedure to drain a furuncle, carbuncle, or abscess should include the expression of all purulent material and the removal of all loculations if possible. Wound culture is recommended during incision and drainage per current guidelines.5 Simple dry dressings are convenient and effective, although some wounds may require packing. Tap water (that is potable) is suitable for wound cleansing. However, there is no strong evidence that irrigating wounds increases healing or reduces infection.20
Routine use of antibiotics is not recommended for simple cutaneous abscesses.5,17,21 Evidence has been conflicting regarding empiric antibiotic coverage of MRSA following incision and drainage.22-25 Guidelines recommend considering the initiation of antibiotics if there are multiple abscesses, gangrene, surrounding cellulitis, or systemic signs of infection, or if the host is immunocompromised.5
If MRSA is suspected, recommended antibiotic coverage includes trimethoprim-sulfamethoxazole, clindamycin, doxycycline, or minocycline.5 If MRSA is identified, treatment options include dicloxacillin or cephalexin. For severe infections persisting after incision and drainage, in addition to oral antibiotic therapy, consider intravenous antibiotic options for MRSA: cefazolin, clindamycin, linezolid, nafcillin, telavancin, or vancomycin.5
Necrotizing fasciitis
Necrotizing fasciitis is a rare but potentially deadly infection of the skin and soft tissue. It progresses rapidly and spreads along fascial planes, leading to the necrosis of the superficial fascia. The infection often is more extensive than is indicated by superficial signs. Prompt diagnosis is imperative as necrotizing fasciitis is a surgical emergency.5,26 In the United States, 500 to 1500 cases of necrotizing fasciitis occur each year.27 Risk factors for necrotizing fasciitis include diabetes, peripheral vascular disease, malignancy, obesity, cirrhosis, renal failure, injection drug use, chronic corticosteroid therapy, alcohol abuse, malnutrition, and iatrogenic immunosuppression.26,28
Necrotizing fasciitis may be polymicrobial or monomicrobial. Polymicrobial infection, also referred to as type I, is often due to multiple bacteria that originate from the bowel flora, typically including a mix of anaerobic and aerobic organisms. On average, there can be 5 infecting organisms identified per wound, although in some cases up to 15 organisms have been identified in a single wound.5 Type I infection is often associated with tissue injury, abscess, or abdominal surgery. The majority of cases of necrotizing fasciitis are polymicrobial.27,28
Continue to: Monomicrobial infection...
Monomicrobial infection, also referred to as type II, is often due to group A streptococcus, S aureus, vibrio spp, Aeromonas hyrophilio, or an anaerobic streptococci like peptostreptococcus spp. Typically monomicrobial infections, which account for 20% to 30% of cases of necrotizing fasciitis, are community acquired.5,26,29,30
Clinical presentation. In the early stages of disease, patients commonly complain of flu-like symptoms and extreme pain that is out of proportion to findings on the exam. Additional warning signs include fevers and other symptoms of toxicity such as tachycardia, hypotension, nausea, vomiting, and diarrhea. Later in the course, symptoms may localize to the affected area and include erythema, tense swelling, development of blisters or bullae, blackish blue discoloration of the skin, severe pain, and loss of sensation. In some cases involving gas-forming bacteria, tissue crepitus may be noted on exam.5,27-31
Rely on clinical judgment to hasten surgical intervention. Laboratory or imaging findings may augment clinical judgment. But if you suspect necrotizing fasciitis, obtaining blood tests and imaging should not delay surgery. Blood tests that may aid in the diagnosis of necrotizing fasciitis include a complete blood count with differential; coagulation studies; a comprehensive metabolic panel; assays of lactate, C-reactive protein (CRP), and creatinine kinase; and blood cultures. Most often, patients with necrotizing fasciitis will have leukocytosis or leukopenia, evidence of hemolysis, thrombocytopenia, acute renal failure, and significantly elevated CRP.
On any imaging modality, indications of necrotizing fasciitis are inflammatory infiltration of the deep fascia on the affected side that is absent on the contralateral side, and the presence of subcutaneous air (which is a specific but rare finding). Imaging modalities may include CT or magnetic resonance imaging. A definitive diagnosis can only be made with surgical exploration of the involved area. Definitive microbiologic diagnosis will require culture of organisms from affected tissue or blood.5,26,30,31
First address any hemodynamic instability (hypotension is frequently encountered), followed by urgent surgical exploration, debridement of the wound, and antimicrobial therapy. Antibiotic treatment should align with probable pathogens and treatment should be continued until repeated surgical debridement is no longer necessary, clinical improvement is evident, and 48 to 72 hours have passed since defervescence. A reasonable initial empiric regimen in adults would include an agent that is effective against group A streptococcus, gram-negative pathogens, and anaerobes, such as a carbapenem or a beta-lactam-beta-lactamase inhibitor such as piperacillin-tazobactam. Additionally, include an agent that targets MRSA, such as vancomycin, linezolid, or clindamycin.5
CORRESPONDENCE
Karl T. Clebak, MD, Department of Family and Community Medicine Residency Program, Penn State Health M.S. Hershey Medical Center, 500 University Drive, H154/C1613, Hershey, PA 17033; [email protected]
Skin and soft-tissue infections, frequently encountered in primary care, range from the uncomplicated erysipelas to the life-threatening necrotizing fasciitis. This review draws from the latest evidence and guidelines to help guide the care you provide to patients with cellulitis, orbital cellulitis, erysipelas, folliculitis, furuncles, carbuncles, abscesses, and necrotizing fasciitis.
Cellulitis
Cellulitis, an infection of the deep dermal and subcutaneous layers of the skin, has become increasingly common in recent years, with both incidence and hospitalization rates rising.1 Cellulitis occurs when pathogens enter the dermis through breaks in the skin barrier due to cutaneous fungal infections, trauma, pressure sores, venous stasis, or inflammation. The diagnosis is often made clinically based on characteristic skin findings—classically an acute, poorly demarcated area of erythema, warmth, swelling, and tenderness. Lymphangitic streaking and local lymphadenopathy may also be present. Infection often occurs on an extremity (although it can be found on other areas of the body) and is usually unilateral. Fever may or may not be present.2
Likely responsible microorganisms. Staphylococcus aureus and Group A streptococci (often Streptococcus pyogenes) are common culprits. One systematic review that examined cultures taken of intact skin in cellulitis patients found S aureus to be about twice as common as S pyogenes, with both bacteria accounting for a little more than 70% of cases. Of the remaining positive cultures, the most common organisms were alpha-hemolytic streptococcus, group B streptococcus, Pseudomonas aeruginosa, Clostridium perfringens, Escherichia coli, Pasteurella multocida, and Proteus mirabilis.3 Similarly, a systematic review of bacteremia in patients with cellulitis and erysipelas found that S pyogenes, other beta-hemolytic strep, and S aureus account for about 70% of cases (although S aureus was responsible for just 14%), with the remainder of cases caused by gram-negative organisms such as E coli and P aeruginosa.4
Treatment considerations. Strict treatment guidelines for cellulitis are lacking, but general consensus encourages the use of antibiotics and occasionally surgery. For mild and moderate cases of cellulitis, prescribe oral and parenteral antibiotics to cover for streptococci and methicillin-susceptible S aureus, respectively. Expand coverage to include vancomycin if nasal colonization shows methicillin-resistant S aureus (MRSA) or if you otherwise suspect prior MRSA exposure. Expanded coverage will also be needed if there is severe nonpurulent infection associated with penetrating trauma or a history of intravenous drug use, or the patient meets criteria for systemic inflammatory response syndrome. If patients are severely compromised (eg, neutropenic), it is reasonable to further add broad-spectrum coverage (eg, intravenous piperacillin-tazobactam or carbapenem). Typical duration of treatment is 5 to 7 days, although this should be extended if there is no clinical improvement.
Generally, cellulitis can be managed in the outpatient setting, although hospitalization is recommended if there are concerns for deep or necrotizing infection, if patients are nonadherent to therapy or are immunocompromised, or if outpatient therapy has failed.5 Furthermore, in an observational study of 606 adult patients, prior episodes of cellulitis, venous insufficiency, and immunosuppression were all independently associated with poorer clinical outcomes.2 Also treat underlying predisposing factors such as edema, obesity, eczema, venous insufficiency, and toe web abnormalities such as fissures, scaling, or maceration.5 Consider the use of prophylactic antibiotics for patients who have had 3 to 4 episodes of cellulitis despite attempts to treat predisposing conditions. Prophylactic antibiotic regimens include penicillin or erythromycin orally and penicillin G benzathine intramuscularly.5 Antibiotic regimens are summarized in the TABLE.5

Orbital cellulitis
Orbital cellulitis is an infection of the tissues posterior to the orbital septum.6,7 Periorbital, or preseptal, cellulitis occurs anterior to the orbital septum and is the more common of the 2 infections—84% compared with 16% for orbital cellulitis.6 However, orbital cellulitis, which affects mainly children at a median age of 7 years,6 must be detected and treated early due to the potential for serious complications such as cavernous sinus thrombosis, meningitis, intracranial abscess, and vision loss.7 Chemosis (conjunctival edema) and diplopia are more commonly associated with orbital cellulitis and are seldom seen with preseptal cellulitis.
Predominant causative organisms are S pneumoniae, Moraxella catarrhalis, non-typeable Haemophilus influenzae, and group A streptococcus. The most common mechanism of infection is tracking from periorbital structures (eg, paranasal and ethmoid sinusitis). Other causes include orbital trauma/fracture, periorbital surgery, and bacterial endocarditis. Clinically, patients present with limited ocular motility and proptosis associated with inflamed conjunctiva, orbital pain, headache, malaise, fever, eyelid edema, and possible decrease in visual acuity. The diagnosis is often made clinically and confirmed with orbital computed tomography (CT) with contrast, which can assist in ruling out intracranial involvement such as abscess.
Continue to: Antibiotic therapy
Antibiotic therapy, generally administered intravenously, is recommended for at least 3 days or until orbital symptoms begin to resolve. Choose antibiotics effective against sinusitis-related pathogens (eg, S pneumoniae, H influenzae, M catarrhalis), S aureus, and anaerobes.8 For instance, a regimen may include vancomycin for MRSA coverage, a third-generation cephalosporin, or metronidazole for anaerobic coverage if there is concern about intracranial involvement. Surgical intervention is often reserved for patients with inadequate response to antibiotic therapy, necessitating biopsy for pathogen identification, as well as drainage of large abscesses refractory to antibiotics.
Erysipelas
Erysipelas, a related yet distinct form of cellulitis, is a bacterial infection of the superficial dermis and hypodermis and is commonly caused by group A streptococcus.5,9 Other less common organisms include S aureus, P aeruginosa, and enterobacteria. Erysipelas predominantly affects the lower extremities unilaterally (~90%); the arms and the face are the next most common locations. In addition to the rapid onset of well-demarcated erythema, pain, and swelling, patients may have fever and regional lymphadenopathy. Risk factors include portal of entry (eg, tinea pedis, ulceration), lymphedema, and diabetes. Complications of erysipelas include bullae from edema, abscess formation, and, rarely, bacteremia.
Antibiotic treatment regimens include penicillin G, macrolides (reserved for those with penicillin allergies), fluoroquinolones, and cephalosporins, with duration of treatment ranging from 10 to 14 days depending on infection severity. Fever, pain, and erythema generally improve within 48 to 72 hours of antibiotic therapy. If there is no improvement, consider alternative diagnoses, such as necrotizing fasciitis. Recurrence rates following the initial episode of erysipelas are estimated at 10% of patients at 6 months and 30% at 3 years.10
Folliculitis
Inflammation of hair follicles is characterized by superficial inflammation with the development of perifollicular papules or pustules on an erythematous base.11,12 Folliculitis most commonly affects the face, scalp, thighs, buttocks, axillae, and inguinal areas.13 It may be caused by infection, an inflammatory reaction, or physical injury. Diagnosis is typically based on the patient’s history and physical examination.
Bacteria are the most common cause of infection, although fungi, viruses, and other entities can cause folliculitis. S aureus (methicillin sensitive or methicillin resistant) is the most common pathogen; in the past, superficial pustular folliculitis attributed to S aureus was referred to as Bockhart impetigo. Folliculitis secondary to P aeruginosa, often seen after exposure to contaminated water or hot tubs, is frequently referred to as “hot tub folliculitis.” Malassezia, a reported cause of fungal folliculitis, tends to occur in adolescents of either sex and men with high sebum production, is common in tropical climates, and can be associated with HIV or immunosuppression.11,12,14
Continue to: Differential diagnosis...
Differential diagnosis of folliculitis includes pseudofolliculitis barbae, eosinophilic folliculitis, keratosis pilaris, acne vulgaris, candidiasis, contact dermatitis, impetigo, and miliaria.13 Pseudofolliculitis barbae is an inflammatory reaction to shaving, more commonly seen in darkly pigmented skin. Pseudofolliculitis develops when the hair shaft penetrates the wall of the follicle or directly enters the epidermis.
Initial treatment for mild disease includes the elimination of predisposing factors such as occlusion, moisture, and abrasion. The area should be kept clean and dry, avoiding friction. For localized disease, prescribe topical clindamycin, mupirocin ointment, or benzoyl peroxide. If symptoms fail to respond, prescribe a 7-day course of antibiotic that targets methicillin-sensitive S aureus—eg, cephalexin or dicloxacillin. Also consider doxycycline, which has anti-inflammatory effects and is effective against MRSA. For refractory lesions, trimethoprim-sulfamethoxazole, clindamycin, or minocycline may be useful. If you suspect pseudomonas, consider giving ciprofloxacin for 10 to 14 days for persistent lesions or if the patient is immunocompromised.13,15 Consider obtaining bacterial, fungal, or viral cultures for lesions that fail to respond to initial treatment.
Furuncles/carbuncles/abscesses
A furuncle, commonly referred to as a boil, is an infected hair follicle that becomes enclosed, creating a collection of pus. A carbuncle is a collection of furuncles that converge and drain through a single opening. An abscess is a localized collection of pus arising from within the dermis that can extend within deeper tissues.5 Furuncles, carbuncles, and abscesses are managed similarly with drainage and consideration for MRSA risk factors.
S aureus is the most common cause of these infections; 59% of skin abscesses are due to community-acquired MRSA.16 Anaerobes may contribute to the polymicrobial flora of skin abscesses.17 Risk factors for MRSA infection include a history of previous MRSA infection, diabetes, dialysis or renal failure, placement of an indwelling catheter or medical device, injection drug use, incarceration, close contact with a person with known MRSA infection or colonization, long-term care residence, hospitalization or surgery within the past 12 months, and high prevalence of MRSA in the community.5
Ultrasound improves diagnostic accuracy. One study showed that when a clinical exam alone was inconclusive in evaluating skin and soft-tissue infections in children and adolescents, an ultrasound-assisted examination improved diagnostic accuracy.18 Sensitivity of the clinical examination was 43.7%, compared with 77.6% for the clinical examination plus ultrasound.18
Continue to: Incision and drainage first
Incision and drainage first. Ultrasound-guided needle aspiration, however, has not improved treatment efficacy compared with incision and drainage,19 the mainstay approach for abscesses.17 The procedure to drain a furuncle, carbuncle, or abscess should include the expression of all purulent material and the removal of all loculations if possible. Wound culture is recommended during incision and drainage per current guidelines.5 Simple dry dressings are convenient and effective, although some wounds may require packing. Tap water (that is potable) is suitable for wound cleansing. However, there is no strong evidence that irrigating wounds increases healing or reduces infection.20
Routine use of antibiotics is not recommended for simple cutaneous abscesses.5,17,21 Evidence has been conflicting regarding empiric antibiotic coverage of MRSA following incision and drainage.22-25 Guidelines recommend considering the initiation of antibiotics if there are multiple abscesses, gangrene, surrounding cellulitis, or systemic signs of infection, or if the host is immunocompromised.5
If MRSA is suspected, recommended antibiotic coverage includes trimethoprim-sulfamethoxazole, clindamycin, doxycycline, or minocycline.5 If MRSA is identified, treatment options include dicloxacillin or cephalexin. For severe infections persisting after incision and drainage, in addition to oral antibiotic therapy, consider intravenous antibiotic options for MRSA: cefazolin, clindamycin, linezolid, nafcillin, telavancin, or vancomycin.5
Necrotizing fasciitis
Necrotizing fasciitis is a rare but potentially deadly infection of the skin and soft tissue. It progresses rapidly and spreads along fascial planes, leading to the necrosis of the superficial fascia. The infection often is more extensive than is indicated by superficial signs. Prompt diagnosis is imperative as necrotizing fasciitis is a surgical emergency.5,26 In the United States, 500 to 1500 cases of necrotizing fasciitis occur each year.27 Risk factors for necrotizing fasciitis include diabetes, peripheral vascular disease, malignancy, obesity, cirrhosis, renal failure, injection drug use, chronic corticosteroid therapy, alcohol abuse, malnutrition, and iatrogenic immunosuppression.26,28
Necrotizing fasciitis may be polymicrobial or monomicrobial. Polymicrobial infection, also referred to as type I, is often due to multiple bacteria that originate from the bowel flora, typically including a mix of anaerobic and aerobic organisms. On average, there can be 5 infecting organisms identified per wound, although in some cases up to 15 organisms have been identified in a single wound.5 Type I infection is often associated with tissue injury, abscess, or abdominal surgery. The majority of cases of necrotizing fasciitis are polymicrobial.27,28
Continue to: Monomicrobial infection...
Monomicrobial infection, also referred to as type II, is often due to group A streptococcus, S aureus, vibrio spp, Aeromonas hyrophilio, or an anaerobic streptococci like peptostreptococcus spp. Typically monomicrobial infections, which account for 20% to 30% of cases of necrotizing fasciitis, are community acquired.5,26,29,30
Clinical presentation. In the early stages of disease, patients commonly complain of flu-like symptoms and extreme pain that is out of proportion to findings on the exam. Additional warning signs include fevers and other symptoms of toxicity such as tachycardia, hypotension, nausea, vomiting, and diarrhea. Later in the course, symptoms may localize to the affected area and include erythema, tense swelling, development of blisters or bullae, blackish blue discoloration of the skin, severe pain, and loss of sensation. In some cases involving gas-forming bacteria, tissue crepitus may be noted on exam.5,27-31
Rely on clinical judgment to hasten surgical intervention. Laboratory or imaging findings may augment clinical judgment. But if you suspect necrotizing fasciitis, obtaining blood tests and imaging should not delay surgery. Blood tests that may aid in the diagnosis of necrotizing fasciitis include a complete blood count with differential; coagulation studies; a comprehensive metabolic panel; assays of lactate, C-reactive protein (CRP), and creatinine kinase; and blood cultures. Most often, patients with necrotizing fasciitis will have leukocytosis or leukopenia, evidence of hemolysis, thrombocytopenia, acute renal failure, and significantly elevated CRP.
On any imaging modality, indications of necrotizing fasciitis are inflammatory infiltration of the deep fascia on the affected side that is absent on the contralateral side, and the presence of subcutaneous air (which is a specific but rare finding). Imaging modalities may include CT or magnetic resonance imaging. A definitive diagnosis can only be made with surgical exploration of the involved area. Definitive microbiologic diagnosis will require culture of organisms from affected tissue or blood.5,26,30,31
First address any hemodynamic instability (hypotension is frequently encountered), followed by urgent surgical exploration, debridement of the wound, and antimicrobial therapy. Antibiotic treatment should align with probable pathogens and treatment should be continued until repeated surgical debridement is no longer necessary, clinical improvement is evident, and 48 to 72 hours have passed since defervescence. A reasonable initial empiric regimen in adults would include an agent that is effective against group A streptococcus, gram-negative pathogens, and anaerobes, such as a carbapenem or a beta-lactam-beta-lactamase inhibitor such as piperacillin-tazobactam. Additionally, include an agent that targets MRSA, such as vancomycin, linezolid, or clindamycin.5
CORRESPONDENCE
Karl T. Clebak, MD, Department of Family and Community Medicine Residency Program, Penn State Health M.S. Hershey Medical Center, 500 University Drive, H154/C1613, Hershey, PA 17033; [email protected]
1. Raff AB, Kroshinsky D. Cellulitis: a review. JAMA. 2016;316:325-337.
2. Collazos J, de la Fuente B, García A, et al. Cellulitis in adult patients: a large, multicenter, observational, prospective study of 606 episodes and analysis of the factors related to the response to treatment. PLoS One. 2018;13:e0204036.
3. Chira S, Miller LG. Staphylococcus aureus is the most common identified cause of cellulitis: a systematic review. Epidemiol Infect. 2010;138:313-317.
4. Gunderson CG, Martinello RA. A systematic review of bacteremias in cellulitis and erysipelas. J Infect. 2012;64:148-155.
5. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59:147-159.
6. Jain A, Rubin PA. Orbital cellulitis in children. Int Ophthalmol Clin. 2001;41:71-86.
7. Seltz LB, Smith J, Durairaj VD, et al. Microbiology and antibiotic management of orbital cellulitis. Pediatrics. 2011;127:e566-e572.
8. Nageswaran S, Woods CR, Benjamin DK, et al. Orbital cellulitis in children. Pediatr Infect Dis J. 2006;25:695-699.
9. Bonnetblanc J-M, Bédane C. Erysipelas. Am J Clin Dermatol. 2003;4:157-163.
10. Jorup-Rönström C, Britton S. Recurrent erysipelas: predisposing factors and costs of prophylaxis. Infection. 1987;15:105-106.
11. Clebak KT, Malone MA. Skin Infections. Prim Care. 2018;45:433-454.
12. Luelmo-Aguilar J, Santandreu MS. Folliculitis: recognition and management. Am J Clin Dermatol. 2004;5:301-310.
13. Mengesha YM, Bennett ML. Pustular skin disorders: diagnosis and treatment. Am J Clin Dermatol. 2002;3:389-400.
14. Akaza N, Akamatsu H, Sasaki Y, et al. Malassezia folliculitis is caused by cutaneous resident Malassezia species. Med Mycol. 2009;47:618-624.
15. Berger RS, Seifert MR. Whirlpool folliculitis: a review of its cause, treatment, and prevention. Cutis. 1990;45:97-98.
16. Fridkin SK, Hageman JC, Morrison M, et al. Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005;352:1436-1444.
17. Meislin HW, Lerner SA, Graves MH, et al. Cutaneous abscesses: anaerobic and aerobic bacteriology and outpatient management. Ann Intern Med. 1977;87:145-149.
18. Marin JR, Dean AJ, Bilker WB, et al. Emergency ultrasound-assisted examination of skin and soft tissue infections in the pediatric emergency department. Acad Emerg Med. 2013;20:545-553.
19. Gaspari RJ, Resop D, Mendoza M, et al. A randomized controlled trial of incision and drainage versus ultrasonographically guided needle aspiration for skin abscesses and the effect of methicillin-resistant Staphylococcus aureus. Ann Emerg Med. 2011;57:483-491.
20. Fernandez R, Griffiths R, Ussia C. Water for wound cleansing. Cochrane Database Syst Rev. 2002: CD003861.
21. Llera JL, Levy RC. Treatment of cutaneous abscess: a double-blind clinical study. Ann Emerg Med. 1985;14:15-19.
22. Talan DA, Mower WR, Krishnadasan A, et al. Trimethoprim-sulfamethoxazole versus placebo for uncomplicated skin abscess. N Engl J Med. 2016;374:823-832.
23. Korownyk C, Allan GM. Evidence-based approach to abscess management. Can Fam Physician. 2007;53:1680-1684.
24. Schmitz GR, Bruner D, Pitotti R, et al. Randomized controlled trial of trimethoprim-sulfamethoxazole for uncomplicated skin abscesses in patients at risk for community-associated methicillin-resistant Staphylococcus aureus infection. Ann Emerg Med. 2010;56:283-287.
25. Rajendran PM, Young D, Maurer T, et al. Randomized, double-blind, placebo-controlled trial of cephalexin for treatment of uncomplicated skin abscesses in a population at risk for community-acquired methicillin-resistant Staphylococcus aureus infection. Antimicrob Agents Chemother. 2007;51:4044-4048.
26. Hunter J, Quarterman C, Waseem M, et al. Diagnosis and management of necrotizing fasciitis. Br J Hosp Med. 2011;72:391-395.
27. Hussein QA, Anaya DA. Necrotizing soft tissue infections. Crit Care Clin. 2013;29:795-806.
28. Puvanendran R, Huey JCM, Pasupathy S. Necrotizing fasciitis. Can Fam Physician. 2009;55:981-987.
29. Raven MC, Billings JC, Goldfrank LR, et al. Medicaid patients at high risk for frequent hospital admission: real-time identification and remediable risks. J Urban Health. 2009;86:230-241.
30. Ustin JS, Malangoni MA. Necrotizing soft-tissue infections: Crit Care Med. 2011;39:2156-2162.
31. Bystritsky R, Chambers H. Cellulitis and soft tissue infections. Ann Intern Med. 2018;168:ITC17- ITC32.
1. Raff AB, Kroshinsky D. Cellulitis: a review. JAMA. 2016;316:325-337.
2. Collazos J, de la Fuente B, García A, et al. Cellulitis in adult patients: a large, multicenter, observational, prospective study of 606 episodes and analysis of the factors related to the response to treatment. PLoS One. 2018;13:e0204036.
3. Chira S, Miller LG. Staphylococcus aureus is the most common identified cause of cellulitis: a systematic review. Epidemiol Infect. 2010;138:313-317.
4. Gunderson CG, Martinello RA. A systematic review of bacteremias in cellulitis and erysipelas. J Infect. 2012;64:148-155.
5. Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59:147-159.
6. Jain A, Rubin PA. Orbital cellulitis in children. Int Ophthalmol Clin. 2001;41:71-86.
7. Seltz LB, Smith J, Durairaj VD, et al. Microbiology and antibiotic management of orbital cellulitis. Pediatrics. 2011;127:e566-e572.
8. Nageswaran S, Woods CR, Benjamin DK, et al. Orbital cellulitis in children. Pediatr Infect Dis J. 2006;25:695-699.
9. Bonnetblanc J-M, Bédane C. Erysipelas. Am J Clin Dermatol. 2003;4:157-163.
10. Jorup-Rönström C, Britton S. Recurrent erysipelas: predisposing factors and costs of prophylaxis. Infection. 1987;15:105-106.
11. Clebak KT, Malone MA. Skin Infections. Prim Care. 2018;45:433-454.
12. Luelmo-Aguilar J, Santandreu MS. Folliculitis: recognition and management. Am J Clin Dermatol. 2004;5:301-310.
13. Mengesha YM, Bennett ML. Pustular skin disorders: diagnosis and treatment. Am J Clin Dermatol. 2002;3:389-400.
14. Akaza N, Akamatsu H, Sasaki Y, et al. Malassezia folliculitis is caused by cutaneous resident Malassezia species. Med Mycol. 2009;47:618-624.
15. Berger RS, Seifert MR. Whirlpool folliculitis: a review of its cause, treatment, and prevention. Cutis. 1990;45:97-98.
16. Fridkin SK, Hageman JC, Morrison M, et al. Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005;352:1436-1444.
17. Meislin HW, Lerner SA, Graves MH, et al. Cutaneous abscesses: anaerobic and aerobic bacteriology and outpatient management. Ann Intern Med. 1977;87:145-149.
18. Marin JR, Dean AJ, Bilker WB, et al. Emergency ultrasound-assisted examination of skin and soft tissue infections in the pediatric emergency department. Acad Emerg Med. 2013;20:545-553.
19. Gaspari RJ, Resop D, Mendoza M, et al. A randomized controlled trial of incision and drainage versus ultrasonographically guided needle aspiration for skin abscesses and the effect of methicillin-resistant Staphylococcus aureus. Ann Emerg Med. 2011;57:483-491.
20. Fernandez R, Griffiths R, Ussia C. Water for wound cleansing. Cochrane Database Syst Rev. 2002: CD003861.
21. Llera JL, Levy RC. Treatment of cutaneous abscess: a double-blind clinical study. Ann Emerg Med. 1985;14:15-19.
22. Talan DA, Mower WR, Krishnadasan A, et al. Trimethoprim-sulfamethoxazole versus placebo for uncomplicated skin abscess. N Engl J Med. 2016;374:823-832.
23. Korownyk C, Allan GM. Evidence-based approach to abscess management. Can Fam Physician. 2007;53:1680-1684.
24. Schmitz GR, Bruner D, Pitotti R, et al. Randomized controlled trial of trimethoprim-sulfamethoxazole for uncomplicated skin abscesses in patients at risk for community-associated methicillin-resistant Staphylococcus aureus infection. Ann Emerg Med. 2010;56:283-287.
25. Rajendran PM, Young D, Maurer T, et al. Randomized, double-blind, placebo-controlled trial of cephalexin for treatment of uncomplicated skin abscesses in a population at risk for community-acquired methicillin-resistant Staphylococcus aureus infection. Antimicrob Agents Chemother. 2007;51:4044-4048.
26. Hunter J, Quarterman C, Waseem M, et al. Diagnosis and management of necrotizing fasciitis. Br J Hosp Med. 2011;72:391-395.
27. Hussein QA, Anaya DA. Necrotizing soft tissue infections. Crit Care Clin. 2013;29:795-806.
28. Puvanendran R, Huey JCM, Pasupathy S. Necrotizing fasciitis. Can Fam Physician. 2009;55:981-987.
29. Raven MC, Billings JC, Goldfrank LR, et al. Medicaid patients at high risk for frequent hospital admission: real-time identification and remediable risks. J Urban Health. 2009;86:230-241.
30. Ustin JS, Malangoni MA. Necrotizing soft-tissue infections: Crit Care Med. 2011;39:2156-2162.
31. Bystritsky R, Chambers H. Cellulitis and soft tissue infections. Ann Intern Med. 2018;168:ITC17- ITC32.
PRACTICE RECOMMENDATIONS
› Start trimethoprim-sulfamethoxazole, clindamycin, doxycycline, minocycline, or a third- or fourth-generation fluoroquinolone for patients with cellulitis likely caused by community acquired methicillin-resistant Staphylococcus aureus (MRSA). A
› Consider culturing for MRSA and treating with oral doxycycline or trimethoprim-sulfamethoxazole for resistant cases of folliculitis. C
› Perform complete surgical debridement promptly if necrotizing fasciitis is suspected. C
› Prescribe broad-spectrum antibiotics for necrotizing fasciitis, covering both anaerobes and aerobes including MRSA. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Getting hypertension under control in the youngest of patients
Hypertension and elevated blood pressure (BP) in children and adolescents correlate to hypertension in adults, insofar as complications and medical therapy increase with age.1,2 Untreated, hypertension in children and adolescents can result in multiple harmful physiologic changes, including left ventricular hypertrophy, left atrial enlargement, diastolic dysfunction, arterial stiffening, endothelial dysfunction, and neurocognitive deficits.3-5
In 2017, the American Academy of Pediatrics (AAP) published clinical practice guidelines for the diagnosis and management of elevated BP and hypertension in children and adolescentsa (TABLE 16). Applying the definition of elevated BP set out in these guidelines yielded a 13% prevalence of hypertension in a cohort of subjects 10 to 18 years of age with comorbid obesity and diabetes mellitus (DM). AAP guideline definitions also improved the sensitivity for identifying hypertensive end-organ damage.7
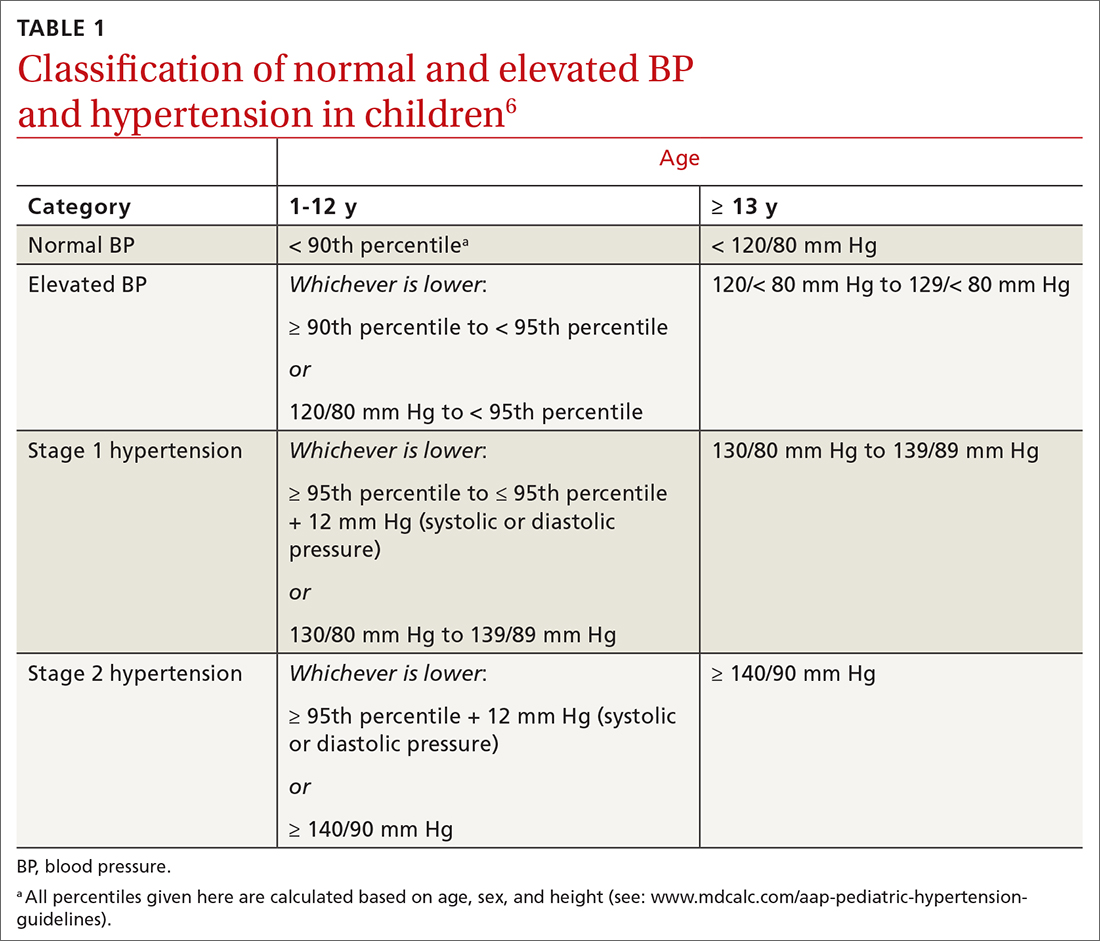
As the prevalence of hypertension increases, screening for and accurate diagnosis of this condition in children are becoming more important. Recognition and management remain a vital part of primary care. In this article, we review the updated guidance on diagnosis and treatment, including lifestyle modification and pharmacotherapy.
First step: Identifying hypertension
Risk factors
Risk factors for pediatric hypertension are similar to those in adults. These include obesity (body mass index ≥ 95th percentile for age), types 1 and 2 DM, elevated sodium intake, sleep-disordered breathing, and chronic kidney disease (CKD). Some risk factors, such as premature birth and coarctation of the aorta, are specific to the pediatric population.8-14 Pediatric obesity strongly correlates with both pediatric and adult hypertension, and accelerated weight gain might increase the risk of elevated BP in adulthood.15,16

Intervening early to mitigate or eliminate some of these modifiable risk factors can prevent or treat hypertension.17 Alternatively, having been breastfed as an infant has been reliably shown to reduce the risk of elevated BP in children.13
Recommendations for screening and measuring BP
The optimal age to start measuring BP is not clearly defined. AAP recommends measurement:
- annually in all children ≥ 3 years of age
- at every encounter in patients who have a specific comorbid condition, including obesity, DM, renal disease, and aortic-arch abnormalities (obstruction and coarctation) and in those who are taking medication known to increase BP.6
Protocol. Measure BP in the right arm for consistency and comparison with reference values. The width of the cuff bladder should be at least 40%, and the length, 80% to 100%, of arm circumference. Position the cuff bladder midway between the olecranon and acromion. Obtain the measurement in a quiet and comfortable environment after the patient has rested for 3 to 5 minutes. The patient should be seated, preferably with feet on the floor; elbows should be supported at the level of the heart.
Continue to: When an initial reading...
When an initial reading is elevated, whether by oscillometric or auscultatory measurement, 2 more auscultatory BP measurements should be taken during the same visit; these measurements are averaged to determine the BP category.18
TABLE 16 defines BP categories based on age, sex, and height. We recommend using the free resource MD Calc (www.mdcalc.com/aap-pediatric-hypertension-guidelines) to assist in calculating the BP category.
TABLE 26 describes the timing of follow-up based on the initial BP reading and diagnosis.
Ambulatory BP monitoring (ABPM) is a validated device that measures BP every 20 to 30 minutes throughout the day and night. ABPM should be performed initially in all patients with persistently elevated BP and routinely in children and adolescents with a high-risk comorbidity (TABLE 26). Note: Insurance coverage of ABPM is limited.
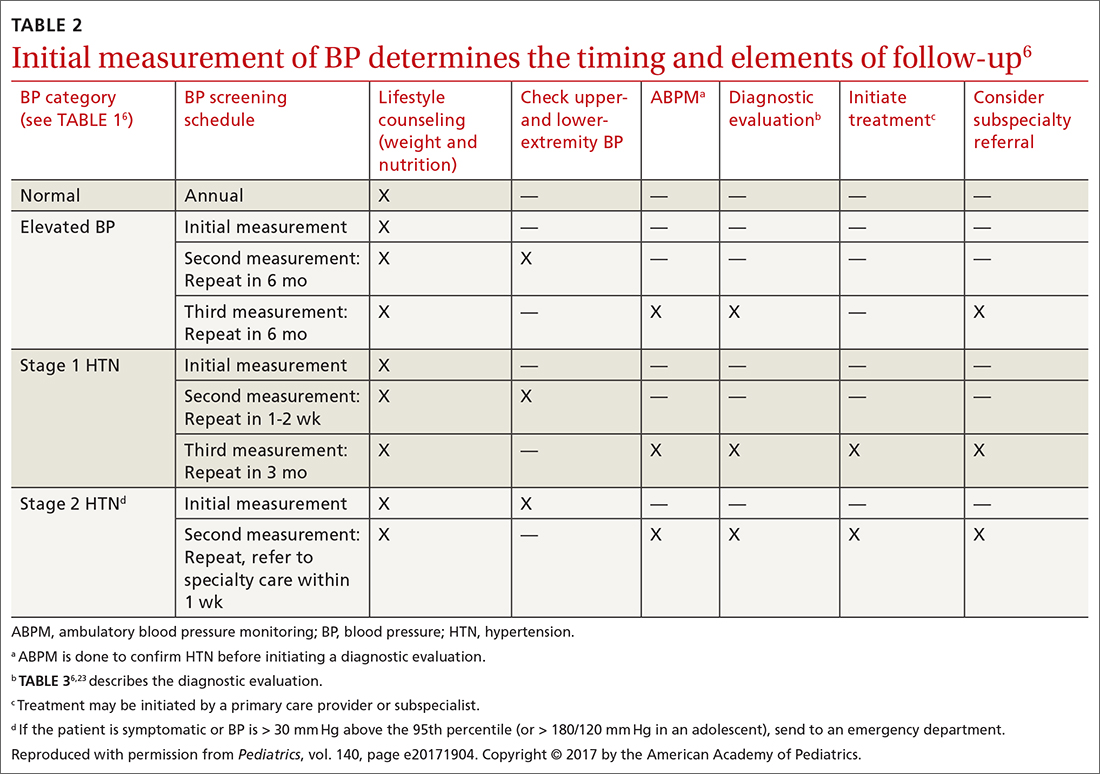
ABPM is also used to diagnose so-called white-coat hypertension, defined as BP ≥ 95th percentile for age, sex, and height in the clinic setting but < 95th percentile during ABPM. This phenomenon can be challenging to diagnose.
Continue to: Home monitoring
Home monitoring. Do not use home BP monitoring to establish a diagnosis of hypertension, although one of these devices can be used as an adjunct to office and ambulatory BP monitoring after the diagnosis has been made.6
Evaluating hypertension in children and adolescents
Once a diagnosis of hypertension has been made, undertake a thorough history, physical examination, and diagnostic testing to evaluate for possible causes, comorbidities, and any evidence of end-organ damage.
Comprehensive history. Pertinent aspects include perinatal, nutritional, physical activity, psychosocial, family, medication—and of course, medical—histories.6
Maternal elevated BP or hypertension is related to an offspring’s elevated BP in childhood and adolescence.19 Other pertinent aspects of the perinatal history include complications of pregnancy, gestational age, birth weight, and neonatal complications.6
Nutritional and physical activity histories can highlight contributing factors in the development of hypertension and can be a guide to recommending lifestyle modifications.6 Sodium intake, which influences BP, should be part of the nutritional history.20
Continue to: Important aspects...
Important aspects of the psychosocial history include feelings of depression or anxiety, bullying, and body perception. Children older than 10 years should be asked about smoking, alcohol, and other substance use.
The family history should include notation of first- and second-degree relatives with hypertension.6
Inquire about medications that can raise BP, including oral contraceptives, which are commonly prescribed in this population.21,22
The physical exam should include measured height and weight, with calculation of the body mass index percentile for age; of note, obesity is strongly associated with hypertension, and poor growth might signal underlying chronic disease. Once elevated BP has been confirmed, the exam should include measurement of BP in both arms and in a leg (TABLE 26). BP that is lower in the leg than in the arms (in any given patient, BP readings in the legs are usually higher than in the arms), or weak or absent femoral pulses, suggest coarctation of the aorta.6
Focus the balance of the physical exam on physical findings that suggest secondary causes of hypertension or evidence of end-organ damage.
Continue to: Testing
Testing. TABLE 36,23 summarizes the diagnostic testing recommended for all children and for specific populations; TABLE 26 indicates when to obtain diagnostic testing.
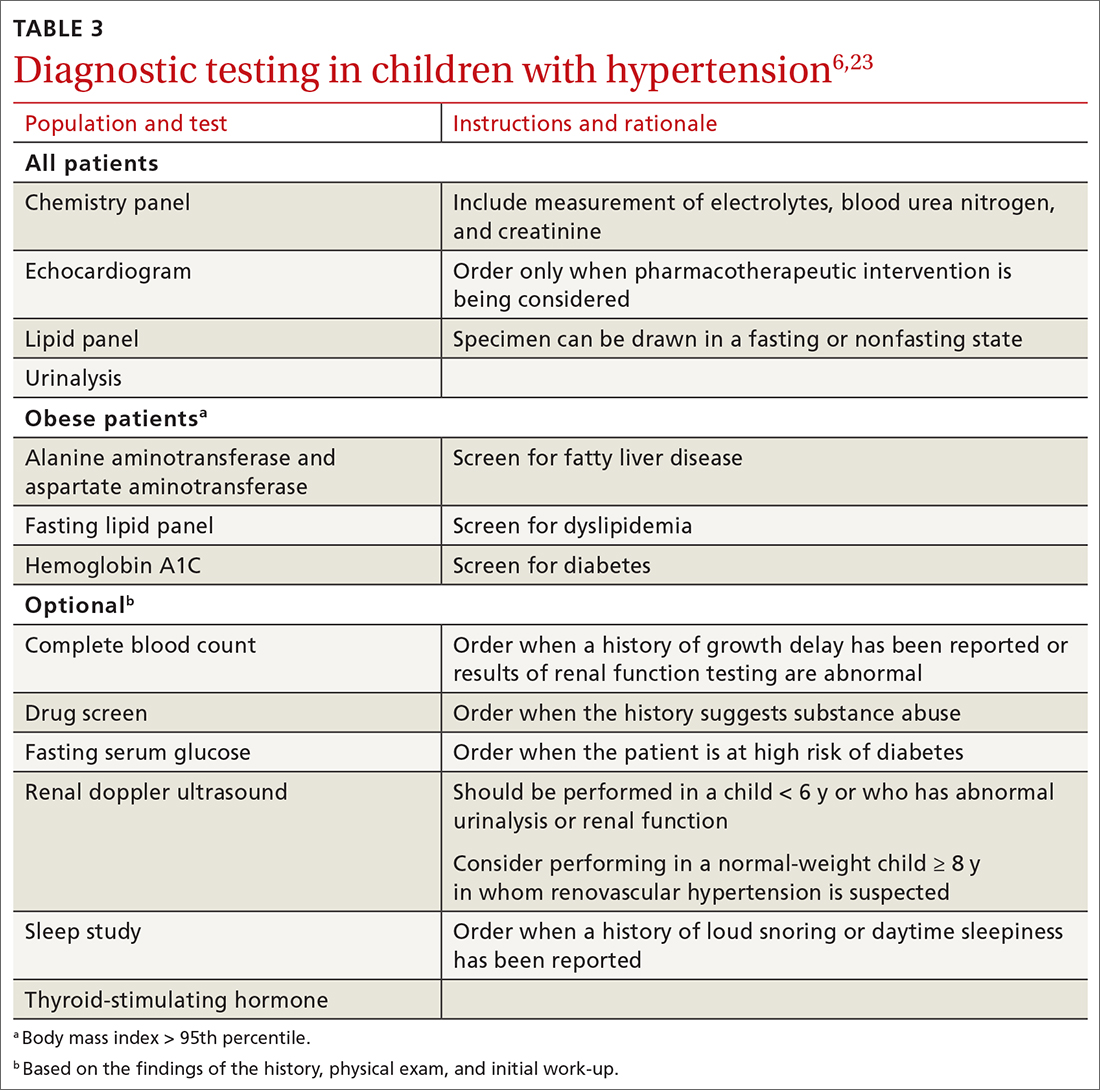
TABLE 42,12,13,24 outlines the basis of primary and of secondary hypertension and common historical and physical findings that suggest a secondary cause.

Mapping out the treatment plan
Pediatric hypertension should be treated in patients with stage 1 or higher hypertension.6 This threshold for therapy is based on evidence that reducing BP below a goal of (1) the 90th percentile (calculated based on age, sex, and height) in children up to 12 years of age or (2) of < 130/80 mm Hg for children ≥ 13 years reduces short- and long-term morbidity and mortality.5,6,25
Choice of initial treatment depends on the severity of BP elevation and the presence of comorbidities (FIGURE6,20,25-28). The initial, fundamental treatment recommendation is lifestyle modification,6,29 including regular physical exercise, a change in nutritional habits, weight loss (because obesity is a common comorbid condition), elimination of tobacco and substance use, and stress reduction.25,26 Medications can be used as well, along with other treatments for specific causes of secondary hypertension.
Referral to a specialist can be considered if consultation for assistance with treatment is preferred (TABLE 26) or if the patient has:
- treatment-resistant hypertension
- stage 2 hypertension that is not quickly responsive to initial treatment
- an identified secondary cause of hypertension.
Continue to: Lifestyle modification can make a big difference
Lifestyle modification can make a big difference
Exercise. “Regular” physical exercise for children to reduce BP is defined as ≥ 30 to 60 minutes of active play daily.6,29 Studies have shown significant improvement not only in BP but also in other cardiovascular disease risk parameters with regular physical exercise.27 A study found that the reduction in systolic BP is, on average, approximately 6 mm Hg with physical activity alone.30
Nutrition. DASH—Dietary Approaches to Stop Hypertension—is an evidence-based program to reduce BP. This nutritional guideline focuses on a diet rich in natural foods, including fruits, vegetables, minimally processed carbohydrates and whole grains, and low-fat dairy and meats. It also emphasizes the importance of avoiding foods high in processed sugars and reducing sodium intake.31 Higher-than-recommended sodium intake, based on age and sex (and established as part of dietary recommendations for children on the US Department of Health and Human Services’ website health.gov) directly correlates with the risk of prehypertension and hypertension—especially in overweight and obese children.20,32 DASH has been shown to reliably reduce the incidence of hypertension in children; other studies have supported increased intake of fruits, vegetables, and legumes as strategies to reduce BP.33,34
Other interventions. Techniques to improve adherence to exercise and nutritional modifications for children include motivational interviewing, community programs and education, and family counseling.27,35 A recent study showed that a community-based lifestyle modification program that is focused on weight loss in obese children resulted in a significant reduction in BP values at higher stages of obesity.36 There is evidence that techniques such as controlled breathing and meditation can reduce BP.37 Last, screening and counseling to encourage tobacco and substance use discontinuation are recommended for children and adolescents to improve health outcomes.25
Proceed with pharmacotherapy when these criteria are met
Medical therapy is recommended when certain criteria are met, although this decision should be individualized and made in agreement by the treating physician, patient, and family. These criteria (FIGURE6,20,25-28) are6,29:
- once a diagnosis of stage 1 hypertension has been established, failure to meet a BP goal after 3 to 6 months of attempting lifestyle modifications
- stage 2 hypertension without a modifiable risk factor, such as obesity
- any stage of hypertension with comorbid CKD, DM, or proteinuria
- target-organ damage, such as left ventricular hypertrophy
- symptomatic hypertension.6,29
There are circumstances in which one or another specific antihypertensive agent is recommended for children; however, for most patients with primary hypertension, the following classes are recommended for first-line use6,22:
- angiotensin-converting enzyme (ACE) inhibitors
- angiotensin receptor blockers (ARBs)
- calcium-channel blockers (CCBs)
- thiazide diuretics.
Continue to: For a child with known CKD...
For a child with known CKD, DM, or proteinuria, an ACE inhibitor or ARB is beneficial as first-line therapy.38 Because ACE inhibitors and ARBs have teratogenic effects, however, a thorough review of fertility status is recommended for female patients before any of these agents are started. CCBs and thiazides are typically recommended as first-line agents for Black patients.6,28 Beta-blockers are typically avoided in the first line because of their adverse effect profile.
Most antihypertensive medications can be titrated every 1 or 2 weeks; the patient’s BP can be monitored with a home BP cuff to track the effect of titration. In general, the patient should be seen for follow-up every 4 to 6 weeks for a BP recheck and review of medication tolerance and adverse effects. Once the treatment goal is achieved, it is reasonable to have the patient return every 3 to 6 months to reassess the treatment plan.
If the BP goal is difficult to achieve despite titration of medication and lifestyle changes, consider repeat ABPM assessment, a specialty referral, or both. It is reasonable for children who have been started on medication and have adhered to lifestyle modifications to practice a “step-down” approach to discontinuing medication; this approach can also be considered once any secondary cause has been corrected. Any target-organ abnormalities identified at diagnosis (eg, proteinuria, CKD, left ventricular hypertrophy) need to be reexamined at follow-up.6
Restrict activities—or not?
There is evidence that a child with stage 1 or well-controlled stage 2 hypertension without evidence of end-organ damage should not have restrictions on sports or activity. However, in uncontrolled stage 2 hypertension or when evidence of target end-organ damage is present, you should advise against participation in highly competitive sports and highly static sports (eg, weightlifting, wrestling), based on expert opinion6,25 (FIGURE6,20,25-28).
aAAP guidelines on the management of pediatric hypertension vary from those of the US Preventive Services Task Force. See the Practice Alert, “A review of the latest USPSTF recommendations,” in the May 2021 issue.
CORRESPONDENCE
Dustin K. Smith, MD, Family Medicine Department, 2080 Child Street, Jacksonville, FL, 32214; [email protected]
1. Theodore RF, Broadbent J, Nagin D, et al. Childhood to early-midlife systolic blood pressure trajectories: early-life predictors, effect modifiers, and adult cardiovascular outcomes. Hypertension. 2015;66:1108-1115. doi: 10.1161/HYPERTENSIONAHA.115.05831
2. Lurbe E, Agabiti-Rosei E, Cruickshank JK, et al. 2016 European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J Hypertens. 2016;34:1887-1920. doi: 10.1097/HJH.0000000000001039
3. Weaver DJ, Mitsnefes MM. Effects of systemic hypertension on the cardiovascular system. Prog Pediatr Cardiol. 2016;41:59-65. https://doi.org/10.1016/j.ppedcard.2015.11.005
4. Ippisch HM, Daniels SR. Hypertension in overweight and obese children. Prog Pediatr Cardiol. 2008;25:177-182. doi: org/10.1016/j.ppedcard.2008.05.002
5. Urbina EM, Lande MB, Hooper SR, et al. Target organ abnormalities in pediatric hypertension. J Pediatr. 2018;202:14-22. doi: 10.1016/j.jpeds.2018.07.026
6. Flynn JT, Kaelber DC, Baker-Smith CM, et al; . Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140:e20171904. doi: 10.1542/peds.2017-1904
7. Khoury M, Khoury PR, Dolan LM, et al. Clinical implications of the revised AAP pediatric hypertension guidelines. Pediatrics. 2018;142:e20180245. doi: 10.1542/peds.2018-0245
8. Falkner B, Gidding SS, Ramirez-Garnica G, et al. The relationship of body mass index and blood pressure in primary care pediatric patients. J Pediatr. 2006;148:195-200. doi: 10.1016/j.jpeds.2005.10.030
9. Rodriguez BL, Dabelea D, Liese AD, et al; SEARCH Study Group. Prevalence and correlates of elevated blood pressure in youth with diabetes mellitus: the SEARCH for diabetes in youth study. J Pediatr. 2010;157:245-251.e1. doi: 10.1016/j.jpeds.2010.02.021
10. Shay CM, Ning H, Daniels SR, et al. Status of cardiovascular health in US adolescents: prevalence estimates from the National Health and Nutrition Examination Surveys (NHANES) 2005-2010. Circulation. 2013;127:1369-1376. doi: 10.1161/CIRCULATIONAHA.113.001559
11. Archbold KH, Vasquez MM, Goodwin JL, et al. Effects of sleep patterns and obesity on increases in blood pressure in a 5-year period: report from the Tucson Children’s Assessment of Sleep Apnea Study. J Pediatr. 2012;161:26-30. doi: 10.1016/j.jpeds.2011.12.034
12. Flynn JT, Mitsnefes M, Pierce C, et al; . Blood pressure in children with chronic kidney disease: a report from the Chronic Kidney Disease in Children study. Hypertension. 2008;52:631-637. doi: 10.1161/HYPERTENSIONAHA.108.110635
13. Martin RM, Ness AR, Gunnell D, et al; ALSPAC Study Team. Does breast-feeding in infancy lower blood pressure in childhood? The Avon Longitudinal Study of Parents and Children (ALSPAC). Circulation. 2004;109:1259-1266. doi: 10.1161/01.CIR.0000118468.76447.CE
14. Brickner ME, Hillis LD, Lange RA. Congenital heart disease in adults. N Engl J Med. 2000;342:256-263. doi: 10.1056/NEJM200001273420407
15. Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: a systematic review and meta-regression analysis. Circulation. 2008;117:3171-3180. doi: 10.1161/CIRCULATIONAHA.107.730366
16. Sun SS, Grave GD, Siervogel RM, et al. Systolic blood pressure in childhood predicts hypertension and metabolic syndrome later in life. Pediatrics. 2007;119:237-246. doi: 10.1542/peds.2006-2543
17. Parker ED, Sinaiko AR, Kharbanda EO, et al. Change in weight status and development of hypertension. Pediatrics. 2016; 137:e20151662. doi: 10.1542/peds.2015-1662
18. Pickering TG, Hall JE, Appel LJ, et al; . Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142-161. doi: 10.1161/01.HYP.0000150859.47929.8e
19. Staley JR, Bradley J, Silverwood RJ, et al. Associations of blood pressure in pregnancy with offspring blood pressure trajectories during childhood and adolescence: findings from a prospective study. J Am Heart Assoc. 2015;4:e001422. doi: 10.1161/JAHA.114.001422
20. Yang Q, Zhang Z, Zuklina EV, et al. Sodium intake and blood pressure among US children and adolescents. Pediatrics. 2012;130:611-619. doi: 10.1542/peds.2011-3870
21. Le-Ha C, Beilin LJ, Burrows S, et al. Oral contraceptive use in girls and alcohol consumption in boys are associated with increased blood pressure in late adolescence. Eur J Prev Cardiol. 2013;20:947-955. doi: 10.1177/2047487312452966
22. Samuels JA, Franco K, Wan F, Sorof JM. Effect of stimulants on 24-h ambulatory blood pressure in children with ADHD: a double-blind, randomized, cross-over trial. Pediatr Nephrol. 2006;21:92-95. doi: 10.1007/s00467-005-2051-1
23. Wiesen J, Adkins M, Fortune S, et al. Evaluation of pediatric patients with mild-to-moderate hypertension: yield of diagnostic testing. Pediatrics. 2008;122:e988-993. doi: 10.1542/peds.2008-0365
24. Kapur G, Ahmed M, Pan C, et al. Secondary hypertension in overweight and stage 1 hypertensive children: a Midwest Pediatric Nephrology Consortium report. J Clin Hypertens (Greenwich). 2010;12:34-39. doi: 10.1111/j.1751-7176.2009.00195.x
25. Anyaegbu EI, Dharnidharka VR. Hypertension in the teenager. Pediatr Clin North Am. 2014;61:131-151. doi: 10.1016/j.pcl.2013.09.011
26. Gandhi B, Cheek S, Campo JV. Anxiety in the pediatric medical setting. Child Adolesc Psychiatr Clin N Am. 2012;21:643-653. doi: 10.1016/j.chc.2012.05.013
27. Farpour-Lambert NJ, Aggoun Y, Marchand LM, et al. Physical activity reduces systemic blood pressure and improves early markers of atherosclerosis in pre-pubertal obese children. J Am Coll Cardiol. 2009;54:2396-2406. doi: 10.1016/j.jacc.2009.08.030
28. Li JS, Baker-Smith CM, Smith PB, et al. Racial differences in blood pressure response to angiotensin-converting enzyme inhibitors in children: a meta-analysis. Clin Pharmacol Ther. 2008;84:315-319. doi: 10.1038/clpt.2008.113
29. Singer PS. Updates on hypertension and new guidelines. Adv Pediatr. 2019;66:177-187. doi: 10.1016/j.yapd.2019.03.009
30. Torrance B, McGuire KA, Lewanczuk R, et al. Overweight, physical activity and high blood pressure in children: a review of the literature. Vasc Health Risk Manag. 2007;3:139-149.
31. DASH eating plan. National Heart, Lung, and Blood Institute. Accessed April 26, 2021. www.nhlbi.nih.gov/health-topics/dash-eating-plan
32. Nutritional goals for age-sex groups based on dietary reference intakes and dietary guidelines recommendations (Appendix 7). In: US Department of Agriculture. Dietary guidelines for Americans, 2015-2020. 8th ed. December 2015;97-98. Accessed April 26, 2021. https://health.gov/sites/default/files/2019-09/2015-2020_Dietary_Guidelines.pdf
33. Asghari G, Yuzbashian E, Mirmiran P, et al. Dietary Approaches to Stop Hypertension (DASH) dietary pattern is associated with reduced incidence of metabolic syndrome in children and adolescents. J Pediatr. 2016;174:178-184.e1. doi: 10.1016/j.jpeds.2016.03.077
34. Damasceno MMC, de Araújo MFM, de Freitas RWJF, et al. The association between blood pressure in adolescents and the consumption of fruits, vegetables and fruit juice–an exploratory study. J Clin Nurs. 2011;20:1553-1560. doi: 10.1111/j.1365-2702.2010.03608.x
35. Anderson KL. A review of the prevention and medical management of childhood obesity. Child Adolesc Psychiatr Clin N Am. 2018;27:63-76. doi: 10.1016/j.chc.2017.08.003
36. Kumar S, King EC, Christison, et al; POWER Work Group. Health outcomes of youth in clinical pediatric weight management programs in POWER. J Pediatr. 2019;208:57-65.e4. doi: 10.1016/j.jpeds.2018.12.049
37. Gregoski MJ, Barnes VA, Tingen MS, et al. Breathing awareness meditation and LifeSkills® Training programs influence upon ambulatory blood pressure and sodium excretion among African American adolescents. J Adolesc Health. 2011;48:59-64. doi: 10.1016/j.jadohealth.2010.05.019
38. Escape Trial Group; E, Trivelli A, Picca S, et al. Strict blood-pressure control and progression of renal failure in children. N Engl J Med. 2009;361:1639-1650. doi: 10.1056/NEJMoa0902066
Hypertension and elevated blood pressure (BP) in children and adolescents correlate to hypertension in adults, insofar as complications and medical therapy increase with age.1,2 Untreated, hypertension in children and adolescents can result in multiple harmful physiologic changes, including left ventricular hypertrophy, left atrial enlargement, diastolic dysfunction, arterial stiffening, endothelial dysfunction, and neurocognitive deficits.3-5
In 2017, the American Academy of Pediatrics (AAP) published clinical practice guidelines for the diagnosis and management of elevated BP and hypertension in children and adolescentsa (TABLE 16). Applying the definition of elevated BP set out in these guidelines yielded a 13% prevalence of hypertension in a cohort of subjects 10 to 18 years of age with comorbid obesity and diabetes mellitus (DM). AAP guideline definitions also improved the sensitivity for identifying hypertensive end-organ damage.7

As the prevalence of hypertension increases, screening for and accurate diagnosis of this condition in children are becoming more important. Recognition and management remain a vital part of primary care. In this article, we review the updated guidance on diagnosis and treatment, including lifestyle modification and pharmacotherapy.
First step: Identifying hypertension
Risk factors
Risk factors for pediatric hypertension are similar to those in adults. These include obesity (body mass index ≥ 95th percentile for age), types 1 and 2 DM, elevated sodium intake, sleep-disordered breathing, and chronic kidney disease (CKD). Some risk factors, such as premature birth and coarctation of the aorta, are specific to the pediatric population.8-14 Pediatric obesity strongly correlates with both pediatric and adult hypertension, and accelerated weight gain might increase the risk of elevated BP in adulthood.15,16

Intervening early to mitigate or eliminate some of these modifiable risk factors can prevent or treat hypertension.17 Alternatively, having been breastfed as an infant has been reliably shown to reduce the risk of elevated BP in children.13
Recommendations for screening and measuring BP
The optimal age to start measuring BP is not clearly defined. AAP recommends measurement:
- annually in all children ≥ 3 years of age
- at every encounter in patients who have a specific comorbid condition, including obesity, DM, renal disease, and aortic-arch abnormalities (obstruction and coarctation) and in those who are taking medication known to increase BP.6
Protocol. Measure BP in the right arm for consistency and comparison with reference values. The width of the cuff bladder should be at least 40%, and the length, 80% to 100%, of arm circumference. Position the cuff bladder midway between the olecranon and acromion. Obtain the measurement in a quiet and comfortable environment after the patient has rested for 3 to 5 minutes. The patient should be seated, preferably with feet on the floor; elbows should be supported at the level of the heart.
Continue to: When an initial reading...
When an initial reading is elevated, whether by oscillometric or auscultatory measurement, 2 more auscultatory BP measurements should be taken during the same visit; these measurements are averaged to determine the BP category.18
TABLE 16 defines BP categories based on age, sex, and height. We recommend using the free resource MD Calc (www.mdcalc.com/aap-pediatric-hypertension-guidelines) to assist in calculating the BP category.
TABLE 26 describes the timing of follow-up based on the initial BP reading and diagnosis.
Ambulatory BP monitoring (ABPM) is a validated device that measures BP every 20 to 30 minutes throughout the day and night. ABPM should be performed initially in all patients with persistently elevated BP and routinely in children and adolescents with a high-risk comorbidity (TABLE 26). Note: Insurance coverage of ABPM is limited.

ABPM is also used to diagnose so-called white-coat hypertension, defined as BP ≥ 95th percentile for age, sex, and height in the clinic setting but < 95th percentile during ABPM. This phenomenon can be challenging to diagnose.
Continue to: Home monitoring
Home monitoring. Do not use home BP monitoring to establish a diagnosis of hypertension, although one of these devices can be used as an adjunct to office and ambulatory BP monitoring after the diagnosis has been made.6
Evaluating hypertension in children and adolescents
Once a diagnosis of hypertension has been made, undertake a thorough history, physical examination, and diagnostic testing to evaluate for possible causes, comorbidities, and any evidence of end-organ damage.
Comprehensive history. Pertinent aspects include perinatal, nutritional, physical activity, psychosocial, family, medication—and of course, medical—histories.6
Maternal elevated BP or hypertension is related to an offspring’s elevated BP in childhood and adolescence.19 Other pertinent aspects of the perinatal history include complications of pregnancy, gestational age, birth weight, and neonatal complications.6
Nutritional and physical activity histories can highlight contributing factors in the development of hypertension and can be a guide to recommending lifestyle modifications.6 Sodium intake, which influences BP, should be part of the nutritional history.20
Continue to: Important aspects...
Important aspects of the psychosocial history include feelings of depression or anxiety, bullying, and body perception. Children older than 10 years should be asked about smoking, alcohol, and other substance use.
The family history should include notation of first- and second-degree relatives with hypertension.6
Inquire about medications that can raise BP, including oral contraceptives, which are commonly prescribed in this population.21,22
The physical exam should include measured height and weight, with calculation of the body mass index percentile for age; of note, obesity is strongly associated with hypertension, and poor growth might signal underlying chronic disease. Once elevated BP has been confirmed, the exam should include measurement of BP in both arms and in a leg (TABLE 26). BP that is lower in the leg than in the arms (in any given patient, BP readings in the legs are usually higher than in the arms), or weak or absent femoral pulses, suggest coarctation of the aorta.6
Focus the balance of the physical exam on physical findings that suggest secondary causes of hypertension or evidence of end-organ damage.
Continue to: Testing
Testing. TABLE 36,23 summarizes the diagnostic testing recommended for all children and for specific populations; TABLE 26 indicates when to obtain diagnostic testing.

TABLE 42,12,13,24 outlines the basis of primary and of secondary hypertension and common historical and physical findings that suggest a secondary cause.

Mapping out the treatment plan
Pediatric hypertension should be treated in patients with stage 1 or higher hypertension.6 This threshold for therapy is based on evidence that reducing BP below a goal of (1) the 90th percentile (calculated based on age, sex, and height) in children up to 12 years of age or (2) of < 130/80 mm Hg for children ≥ 13 years reduces short- and long-term morbidity and mortality.5,6,25
Choice of initial treatment depends on the severity of BP elevation and the presence of comorbidities (FIGURE6,20,25-28). The initial, fundamental treatment recommendation is lifestyle modification,6,29 including regular physical exercise, a change in nutritional habits, weight loss (because obesity is a common comorbid condition), elimination of tobacco and substance use, and stress reduction.25,26 Medications can be used as well, along with other treatments for specific causes of secondary hypertension.

Referral to a specialist can be considered if consultation for assistance with treatment is preferred (TABLE 26) or if the patient has:
- treatment-resistant hypertension
- stage 2 hypertension that is not quickly responsive to initial treatment
- an identified secondary cause of hypertension.
Continue to: Lifestyle modification can make a big difference
Lifestyle modification can make a big difference
Exercise. “Regular” physical exercise for children to reduce BP is defined as ≥ 30 to 60 minutes of active play daily.6,29 Studies have shown significant improvement not only in BP but also in other cardiovascular disease risk parameters with regular physical exercise.27 A study found that the reduction in systolic BP is, on average, approximately 6 mm Hg with physical activity alone.30
Nutrition. DASH—Dietary Approaches to Stop Hypertension—is an evidence-based program to reduce BP. This nutritional guideline focuses on a diet rich in natural foods, including fruits, vegetables, minimally processed carbohydrates and whole grains, and low-fat dairy and meats. It also emphasizes the importance of avoiding foods high in processed sugars and reducing sodium intake.31 Higher-than-recommended sodium intake, based on age and sex (and established as part of dietary recommendations for children on the US Department of Health and Human Services’ website health.gov) directly correlates with the risk of prehypertension and hypertension—especially in overweight and obese children.20,32 DASH has been shown to reliably reduce the incidence of hypertension in children; other studies have supported increased intake of fruits, vegetables, and legumes as strategies to reduce BP.33,34
Other interventions. Techniques to improve adherence to exercise and nutritional modifications for children include motivational interviewing, community programs and education, and family counseling.27,35 A recent study showed that a community-based lifestyle modification program that is focused on weight loss in obese children resulted in a significant reduction in BP values at higher stages of obesity.36 There is evidence that techniques such as controlled breathing and meditation can reduce BP.37 Last, screening and counseling to encourage tobacco and substance use discontinuation are recommended for children and adolescents to improve health outcomes.25
Proceed with pharmacotherapy when these criteria are met
Medical therapy is recommended when certain criteria are met, although this decision should be individualized and made in agreement by the treating physician, patient, and family. These criteria (FIGURE6,20,25-28) are6,29:
- once a diagnosis of stage 1 hypertension has been established, failure to meet a BP goal after 3 to 6 months of attempting lifestyle modifications
- stage 2 hypertension without a modifiable risk factor, such as obesity
- any stage of hypertension with comorbid CKD, DM, or proteinuria
- target-organ damage, such as left ventricular hypertrophy
- symptomatic hypertension.6,29
There are circumstances in which one or another specific antihypertensive agent is recommended for children; however, for most patients with primary hypertension, the following classes are recommended for first-line use6,22:
- angiotensin-converting enzyme (ACE) inhibitors
- angiotensin receptor blockers (ARBs)
- calcium-channel blockers (CCBs)
- thiazide diuretics.
Continue to: For a child with known CKD...
For a child with known CKD, DM, or proteinuria, an ACE inhibitor or ARB is beneficial as first-line therapy.38 Because ACE inhibitors and ARBs have teratogenic effects, however, a thorough review of fertility status is recommended for female patients before any of these agents are started. CCBs and thiazides are typically recommended as first-line agents for Black patients.6,28 Beta-blockers are typically avoided in the first line because of their adverse effect profile.
Most antihypertensive medications can be titrated every 1 or 2 weeks; the patient’s BP can be monitored with a home BP cuff to track the effect of titration. In general, the patient should be seen for follow-up every 4 to 6 weeks for a BP recheck and review of medication tolerance and adverse effects. Once the treatment goal is achieved, it is reasonable to have the patient return every 3 to 6 months to reassess the treatment plan.
If the BP goal is difficult to achieve despite titration of medication and lifestyle changes, consider repeat ABPM assessment, a specialty referral, or both. It is reasonable for children who have been started on medication and have adhered to lifestyle modifications to practice a “step-down” approach to discontinuing medication; this approach can also be considered once any secondary cause has been corrected. Any target-organ abnormalities identified at diagnosis (eg, proteinuria, CKD, left ventricular hypertrophy) need to be reexamined at follow-up.6
Restrict activities—or not?
There is evidence that a child with stage 1 or well-controlled stage 2 hypertension without evidence of end-organ damage should not have restrictions on sports or activity. However, in uncontrolled stage 2 hypertension or when evidence of target end-organ damage is present, you should advise against participation in highly competitive sports and highly static sports (eg, weightlifting, wrestling), based on expert opinion6,25 (FIGURE6,20,25-28).
aAAP guidelines on the management of pediatric hypertension vary from those of the US Preventive Services Task Force. See the Practice Alert, “A review of the latest USPSTF recommendations,” in the May 2021 issue.
CORRESPONDENCE
Dustin K. Smith, MD, Family Medicine Department, 2080 Child Street, Jacksonville, FL, 32214; [email protected]
Hypertension and elevated blood pressure (BP) in children and adolescents correlate to hypertension in adults, insofar as complications and medical therapy increase with age.1,2 Untreated, hypertension in children and adolescents can result in multiple harmful physiologic changes, including left ventricular hypertrophy, left atrial enlargement, diastolic dysfunction, arterial stiffening, endothelial dysfunction, and neurocognitive deficits.3-5
In 2017, the American Academy of Pediatrics (AAP) published clinical practice guidelines for the diagnosis and management of elevated BP and hypertension in children and adolescentsa (TABLE 16). Applying the definition of elevated BP set out in these guidelines yielded a 13% prevalence of hypertension in a cohort of subjects 10 to 18 years of age with comorbid obesity and diabetes mellitus (DM). AAP guideline definitions also improved the sensitivity for identifying hypertensive end-organ damage.7

As the prevalence of hypertension increases, screening for and accurate diagnosis of this condition in children are becoming more important. Recognition and management remain a vital part of primary care. In this article, we review the updated guidance on diagnosis and treatment, including lifestyle modification and pharmacotherapy.
First step: Identifying hypertension
Risk factors
Risk factors for pediatric hypertension are similar to those in adults. These include obesity (body mass index ≥ 95th percentile for age), types 1 and 2 DM, elevated sodium intake, sleep-disordered breathing, and chronic kidney disease (CKD). Some risk factors, such as premature birth and coarctation of the aorta, are specific to the pediatric population.8-14 Pediatric obesity strongly correlates with both pediatric and adult hypertension, and accelerated weight gain might increase the risk of elevated BP in adulthood.15,16

Intervening early to mitigate or eliminate some of these modifiable risk factors can prevent or treat hypertension.17 Alternatively, having been breastfed as an infant has been reliably shown to reduce the risk of elevated BP in children.13
Recommendations for screening and measuring BP
The optimal age to start measuring BP is not clearly defined. AAP recommends measurement:
- annually in all children ≥ 3 years of age
- at every encounter in patients who have a specific comorbid condition, including obesity, DM, renal disease, and aortic-arch abnormalities (obstruction and coarctation) and in those who are taking medication known to increase BP.6
Protocol. Measure BP in the right arm for consistency and comparison with reference values. The width of the cuff bladder should be at least 40%, and the length, 80% to 100%, of arm circumference. Position the cuff bladder midway between the olecranon and acromion. Obtain the measurement in a quiet and comfortable environment after the patient has rested for 3 to 5 minutes. The patient should be seated, preferably with feet on the floor; elbows should be supported at the level of the heart.
Continue to: When an initial reading...
When an initial reading is elevated, whether by oscillometric or auscultatory measurement, 2 more auscultatory BP measurements should be taken during the same visit; these measurements are averaged to determine the BP category.18
TABLE 16 defines BP categories based on age, sex, and height. We recommend using the free resource MD Calc (www.mdcalc.com/aap-pediatric-hypertension-guidelines) to assist in calculating the BP category.
TABLE 26 describes the timing of follow-up based on the initial BP reading and diagnosis.
Ambulatory BP monitoring (ABPM) is a validated device that measures BP every 20 to 30 minutes throughout the day and night. ABPM should be performed initially in all patients with persistently elevated BP and routinely in children and adolescents with a high-risk comorbidity (TABLE 26). Note: Insurance coverage of ABPM is limited.

ABPM is also used to diagnose so-called white-coat hypertension, defined as BP ≥ 95th percentile for age, sex, and height in the clinic setting but < 95th percentile during ABPM. This phenomenon can be challenging to diagnose.
Continue to: Home monitoring
Home monitoring. Do not use home BP monitoring to establish a diagnosis of hypertension, although one of these devices can be used as an adjunct to office and ambulatory BP monitoring after the diagnosis has been made.6
Evaluating hypertension in children and adolescents
Once a diagnosis of hypertension has been made, undertake a thorough history, physical examination, and diagnostic testing to evaluate for possible causes, comorbidities, and any evidence of end-organ damage.
Comprehensive history. Pertinent aspects include perinatal, nutritional, physical activity, psychosocial, family, medication—and of course, medical—histories.6
Maternal elevated BP or hypertension is related to an offspring’s elevated BP in childhood and adolescence.19 Other pertinent aspects of the perinatal history include complications of pregnancy, gestational age, birth weight, and neonatal complications.6
Nutritional and physical activity histories can highlight contributing factors in the development of hypertension and can be a guide to recommending lifestyle modifications.6 Sodium intake, which influences BP, should be part of the nutritional history.20
Continue to: Important aspects...
Important aspects of the psychosocial history include feelings of depression or anxiety, bullying, and body perception. Children older than 10 years should be asked about smoking, alcohol, and other substance use.
The family history should include notation of first- and second-degree relatives with hypertension.6
Inquire about medications that can raise BP, including oral contraceptives, which are commonly prescribed in this population.21,22
The physical exam should include measured height and weight, with calculation of the body mass index percentile for age; of note, obesity is strongly associated with hypertension, and poor growth might signal underlying chronic disease. Once elevated BP has been confirmed, the exam should include measurement of BP in both arms and in a leg (TABLE 26). BP that is lower in the leg than in the arms (in any given patient, BP readings in the legs are usually higher than in the arms), or weak or absent femoral pulses, suggest coarctation of the aorta.6
Focus the balance of the physical exam on physical findings that suggest secondary causes of hypertension or evidence of end-organ damage.
Continue to: Testing
Testing. TABLE 36,23 summarizes the diagnostic testing recommended for all children and for specific populations; TABLE 26 indicates when to obtain diagnostic testing.

TABLE 42,12,13,24 outlines the basis of primary and of secondary hypertension and common historical and physical findings that suggest a secondary cause.

Mapping out the treatment plan
Pediatric hypertension should be treated in patients with stage 1 or higher hypertension.6 This threshold for therapy is based on evidence that reducing BP below a goal of (1) the 90th percentile (calculated based on age, sex, and height) in children up to 12 years of age or (2) of < 130/80 mm Hg for children ≥ 13 years reduces short- and long-term morbidity and mortality.5,6,25
Choice of initial treatment depends on the severity of BP elevation and the presence of comorbidities (FIGURE6,20,25-28). The initial, fundamental treatment recommendation is lifestyle modification,6,29 including regular physical exercise, a change in nutritional habits, weight loss (because obesity is a common comorbid condition), elimination of tobacco and substance use, and stress reduction.25,26 Medications can be used as well, along with other treatments for specific causes of secondary hypertension.

Referral to a specialist can be considered if consultation for assistance with treatment is preferred (TABLE 26) or if the patient has:
- treatment-resistant hypertension
- stage 2 hypertension that is not quickly responsive to initial treatment
- an identified secondary cause of hypertension.
Continue to: Lifestyle modification can make a big difference
Lifestyle modification can make a big difference
Exercise. “Regular” physical exercise for children to reduce BP is defined as ≥ 30 to 60 minutes of active play daily.6,29 Studies have shown significant improvement not only in BP but also in other cardiovascular disease risk parameters with regular physical exercise.27 A study found that the reduction in systolic BP is, on average, approximately 6 mm Hg with physical activity alone.30
Nutrition. DASH—Dietary Approaches to Stop Hypertension—is an evidence-based program to reduce BP. This nutritional guideline focuses on a diet rich in natural foods, including fruits, vegetables, minimally processed carbohydrates and whole grains, and low-fat dairy and meats. It also emphasizes the importance of avoiding foods high in processed sugars and reducing sodium intake.31 Higher-than-recommended sodium intake, based on age and sex (and established as part of dietary recommendations for children on the US Department of Health and Human Services’ website health.gov) directly correlates with the risk of prehypertension and hypertension—especially in overweight and obese children.20,32 DASH has been shown to reliably reduce the incidence of hypertension in children; other studies have supported increased intake of fruits, vegetables, and legumes as strategies to reduce BP.33,34
Other interventions. Techniques to improve adherence to exercise and nutritional modifications for children include motivational interviewing, community programs and education, and family counseling.27,35 A recent study showed that a community-based lifestyle modification program that is focused on weight loss in obese children resulted in a significant reduction in BP values at higher stages of obesity.36 There is evidence that techniques such as controlled breathing and meditation can reduce BP.37 Last, screening and counseling to encourage tobacco and substance use discontinuation are recommended for children and adolescents to improve health outcomes.25
Proceed with pharmacotherapy when these criteria are met
Medical therapy is recommended when certain criteria are met, although this decision should be individualized and made in agreement by the treating physician, patient, and family. These criteria (FIGURE6,20,25-28) are6,29:
- once a diagnosis of stage 1 hypertension has been established, failure to meet a BP goal after 3 to 6 months of attempting lifestyle modifications
- stage 2 hypertension without a modifiable risk factor, such as obesity
- any stage of hypertension with comorbid CKD, DM, or proteinuria
- target-organ damage, such as left ventricular hypertrophy
- symptomatic hypertension.6,29
There are circumstances in which one or another specific antihypertensive agent is recommended for children; however, for most patients with primary hypertension, the following classes are recommended for first-line use6,22:
- angiotensin-converting enzyme (ACE) inhibitors
- angiotensin receptor blockers (ARBs)
- calcium-channel blockers (CCBs)
- thiazide diuretics.
Continue to: For a child with known CKD...
For a child with known CKD, DM, or proteinuria, an ACE inhibitor or ARB is beneficial as first-line therapy.38 Because ACE inhibitors and ARBs have teratogenic effects, however, a thorough review of fertility status is recommended for female patients before any of these agents are started. CCBs and thiazides are typically recommended as first-line agents for Black patients.6,28 Beta-blockers are typically avoided in the first line because of their adverse effect profile.
Most antihypertensive medications can be titrated every 1 or 2 weeks; the patient’s BP can be monitored with a home BP cuff to track the effect of titration. In general, the patient should be seen for follow-up every 4 to 6 weeks for a BP recheck and review of medication tolerance and adverse effects. Once the treatment goal is achieved, it is reasonable to have the patient return every 3 to 6 months to reassess the treatment plan.
If the BP goal is difficult to achieve despite titration of medication and lifestyle changes, consider repeat ABPM assessment, a specialty referral, or both. It is reasonable for children who have been started on medication and have adhered to lifestyle modifications to practice a “step-down” approach to discontinuing medication; this approach can also be considered once any secondary cause has been corrected. Any target-organ abnormalities identified at diagnosis (eg, proteinuria, CKD, left ventricular hypertrophy) need to be reexamined at follow-up.6
Restrict activities—or not?
There is evidence that a child with stage 1 or well-controlled stage 2 hypertension without evidence of end-organ damage should not have restrictions on sports or activity. However, in uncontrolled stage 2 hypertension or when evidence of target end-organ damage is present, you should advise against participation in highly competitive sports and highly static sports (eg, weightlifting, wrestling), based on expert opinion6,25 (FIGURE6,20,25-28).
aAAP guidelines on the management of pediatric hypertension vary from those of the US Preventive Services Task Force. See the Practice Alert, “A review of the latest USPSTF recommendations,” in the May 2021 issue.
CORRESPONDENCE
Dustin K. Smith, MD, Family Medicine Department, 2080 Child Street, Jacksonville, FL, 32214; [email protected]
1. Theodore RF, Broadbent J, Nagin D, et al. Childhood to early-midlife systolic blood pressure trajectories: early-life predictors, effect modifiers, and adult cardiovascular outcomes. Hypertension. 2015;66:1108-1115. doi: 10.1161/HYPERTENSIONAHA.115.05831
2. Lurbe E, Agabiti-Rosei E, Cruickshank JK, et al. 2016 European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J Hypertens. 2016;34:1887-1920. doi: 10.1097/HJH.0000000000001039
3. Weaver DJ, Mitsnefes MM. Effects of systemic hypertension on the cardiovascular system. Prog Pediatr Cardiol. 2016;41:59-65. https://doi.org/10.1016/j.ppedcard.2015.11.005
4. Ippisch HM, Daniels SR. Hypertension in overweight and obese children. Prog Pediatr Cardiol. 2008;25:177-182. doi: org/10.1016/j.ppedcard.2008.05.002
5. Urbina EM, Lande MB, Hooper SR, et al. Target organ abnormalities in pediatric hypertension. J Pediatr. 2018;202:14-22. doi: 10.1016/j.jpeds.2018.07.026
6. Flynn JT, Kaelber DC, Baker-Smith CM, et al; . Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140:e20171904. doi: 10.1542/peds.2017-1904
7. Khoury M, Khoury PR, Dolan LM, et al. Clinical implications of the revised AAP pediatric hypertension guidelines. Pediatrics. 2018;142:e20180245. doi: 10.1542/peds.2018-0245
8. Falkner B, Gidding SS, Ramirez-Garnica G, et al. The relationship of body mass index and blood pressure in primary care pediatric patients. J Pediatr. 2006;148:195-200. doi: 10.1016/j.jpeds.2005.10.030
9. Rodriguez BL, Dabelea D, Liese AD, et al; SEARCH Study Group. Prevalence and correlates of elevated blood pressure in youth with diabetes mellitus: the SEARCH for diabetes in youth study. J Pediatr. 2010;157:245-251.e1. doi: 10.1016/j.jpeds.2010.02.021
10. Shay CM, Ning H, Daniels SR, et al. Status of cardiovascular health in US adolescents: prevalence estimates from the National Health and Nutrition Examination Surveys (NHANES) 2005-2010. Circulation. 2013;127:1369-1376. doi: 10.1161/CIRCULATIONAHA.113.001559
11. Archbold KH, Vasquez MM, Goodwin JL, et al. Effects of sleep patterns and obesity on increases in blood pressure in a 5-year period: report from the Tucson Children’s Assessment of Sleep Apnea Study. J Pediatr. 2012;161:26-30. doi: 10.1016/j.jpeds.2011.12.034
12. Flynn JT, Mitsnefes M, Pierce C, et al; . Blood pressure in children with chronic kidney disease: a report from the Chronic Kidney Disease in Children study. Hypertension. 2008;52:631-637. doi: 10.1161/HYPERTENSIONAHA.108.110635
13. Martin RM, Ness AR, Gunnell D, et al; ALSPAC Study Team. Does breast-feeding in infancy lower blood pressure in childhood? The Avon Longitudinal Study of Parents and Children (ALSPAC). Circulation. 2004;109:1259-1266. doi: 10.1161/01.CIR.0000118468.76447.CE
14. Brickner ME, Hillis LD, Lange RA. Congenital heart disease in adults. N Engl J Med. 2000;342:256-263. doi: 10.1056/NEJM200001273420407
15. Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: a systematic review and meta-regression analysis. Circulation. 2008;117:3171-3180. doi: 10.1161/CIRCULATIONAHA.107.730366
16. Sun SS, Grave GD, Siervogel RM, et al. Systolic blood pressure in childhood predicts hypertension and metabolic syndrome later in life. Pediatrics. 2007;119:237-246. doi: 10.1542/peds.2006-2543
17. Parker ED, Sinaiko AR, Kharbanda EO, et al. Change in weight status and development of hypertension. Pediatrics. 2016; 137:e20151662. doi: 10.1542/peds.2015-1662
18. Pickering TG, Hall JE, Appel LJ, et al; . Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142-161. doi: 10.1161/01.HYP.0000150859.47929.8e
19. Staley JR, Bradley J, Silverwood RJ, et al. Associations of blood pressure in pregnancy with offspring blood pressure trajectories during childhood and adolescence: findings from a prospective study. J Am Heart Assoc. 2015;4:e001422. doi: 10.1161/JAHA.114.001422
20. Yang Q, Zhang Z, Zuklina EV, et al. Sodium intake and blood pressure among US children and adolescents. Pediatrics. 2012;130:611-619. doi: 10.1542/peds.2011-3870
21. Le-Ha C, Beilin LJ, Burrows S, et al. Oral contraceptive use in girls and alcohol consumption in boys are associated with increased blood pressure in late adolescence. Eur J Prev Cardiol. 2013;20:947-955. doi: 10.1177/2047487312452966
22. Samuels JA, Franco K, Wan F, Sorof JM. Effect of stimulants on 24-h ambulatory blood pressure in children with ADHD: a double-blind, randomized, cross-over trial. Pediatr Nephrol. 2006;21:92-95. doi: 10.1007/s00467-005-2051-1
23. Wiesen J, Adkins M, Fortune S, et al. Evaluation of pediatric patients with mild-to-moderate hypertension: yield of diagnostic testing. Pediatrics. 2008;122:e988-993. doi: 10.1542/peds.2008-0365
24. Kapur G, Ahmed M, Pan C, et al. Secondary hypertension in overweight and stage 1 hypertensive children: a Midwest Pediatric Nephrology Consortium report. J Clin Hypertens (Greenwich). 2010;12:34-39. doi: 10.1111/j.1751-7176.2009.00195.x
25. Anyaegbu EI, Dharnidharka VR. Hypertension in the teenager. Pediatr Clin North Am. 2014;61:131-151. doi: 10.1016/j.pcl.2013.09.011
26. Gandhi B, Cheek S, Campo JV. Anxiety in the pediatric medical setting. Child Adolesc Psychiatr Clin N Am. 2012;21:643-653. doi: 10.1016/j.chc.2012.05.013
27. Farpour-Lambert NJ, Aggoun Y, Marchand LM, et al. Physical activity reduces systemic blood pressure and improves early markers of atherosclerosis in pre-pubertal obese children. J Am Coll Cardiol. 2009;54:2396-2406. doi: 10.1016/j.jacc.2009.08.030
28. Li JS, Baker-Smith CM, Smith PB, et al. Racial differences in blood pressure response to angiotensin-converting enzyme inhibitors in children: a meta-analysis. Clin Pharmacol Ther. 2008;84:315-319. doi: 10.1038/clpt.2008.113
29. Singer PS. Updates on hypertension and new guidelines. Adv Pediatr. 2019;66:177-187. doi: 10.1016/j.yapd.2019.03.009
30. Torrance B, McGuire KA, Lewanczuk R, et al. Overweight, physical activity and high blood pressure in children: a review of the literature. Vasc Health Risk Manag. 2007;3:139-149.
31. DASH eating plan. National Heart, Lung, and Blood Institute. Accessed April 26, 2021. www.nhlbi.nih.gov/health-topics/dash-eating-plan
32. Nutritional goals for age-sex groups based on dietary reference intakes and dietary guidelines recommendations (Appendix 7). In: US Department of Agriculture. Dietary guidelines for Americans, 2015-2020. 8th ed. December 2015;97-98. Accessed April 26, 2021. https://health.gov/sites/default/files/2019-09/2015-2020_Dietary_Guidelines.pdf
33. Asghari G, Yuzbashian E, Mirmiran P, et al. Dietary Approaches to Stop Hypertension (DASH) dietary pattern is associated with reduced incidence of metabolic syndrome in children and adolescents. J Pediatr. 2016;174:178-184.e1. doi: 10.1016/j.jpeds.2016.03.077
34. Damasceno MMC, de Araújo MFM, de Freitas RWJF, et al. The association between blood pressure in adolescents and the consumption of fruits, vegetables and fruit juice–an exploratory study. J Clin Nurs. 2011;20:1553-1560. doi: 10.1111/j.1365-2702.2010.03608.x
35. Anderson KL. A review of the prevention and medical management of childhood obesity. Child Adolesc Psychiatr Clin N Am. 2018;27:63-76. doi: 10.1016/j.chc.2017.08.003
36. Kumar S, King EC, Christison, et al; POWER Work Group. Health outcomes of youth in clinical pediatric weight management programs in POWER. J Pediatr. 2019;208:57-65.e4. doi: 10.1016/j.jpeds.2018.12.049
37. Gregoski MJ, Barnes VA, Tingen MS, et al. Breathing awareness meditation and LifeSkills® Training programs influence upon ambulatory blood pressure and sodium excretion among African American adolescents. J Adolesc Health. 2011;48:59-64. doi: 10.1016/j.jadohealth.2010.05.019
38. Escape Trial Group; E, Trivelli A, Picca S, et al. Strict blood-pressure control and progression of renal failure in children. N Engl J Med. 2009;361:1639-1650. doi: 10.1056/NEJMoa0902066
1. Theodore RF, Broadbent J, Nagin D, et al. Childhood to early-midlife systolic blood pressure trajectories: early-life predictors, effect modifiers, and adult cardiovascular outcomes. Hypertension. 2015;66:1108-1115. doi: 10.1161/HYPERTENSIONAHA.115.05831
2. Lurbe E, Agabiti-Rosei E, Cruickshank JK, et al. 2016 European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J Hypertens. 2016;34:1887-1920. doi: 10.1097/HJH.0000000000001039
3. Weaver DJ, Mitsnefes MM. Effects of systemic hypertension on the cardiovascular system. Prog Pediatr Cardiol. 2016;41:59-65. https://doi.org/10.1016/j.ppedcard.2015.11.005
4. Ippisch HM, Daniels SR. Hypertension in overweight and obese children. Prog Pediatr Cardiol. 2008;25:177-182. doi: org/10.1016/j.ppedcard.2008.05.002
5. Urbina EM, Lande MB, Hooper SR, et al. Target organ abnormalities in pediatric hypertension. J Pediatr. 2018;202:14-22. doi: 10.1016/j.jpeds.2018.07.026
6. Flynn JT, Kaelber DC, Baker-Smith CM, et al; . Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140:e20171904. doi: 10.1542/peds.2017-1904
7. Khoury M, Khoury PR, Dolan LM, et al. Clinical implications of the revised AAP pediatric hypertension guidelines. Pediatrics. 2018;142:e20180245. doi: 10.1542/peds.2018-0245
8. Falkner B, Gidding SS, Ramirez-Garnica G, et al. The relationship of body mass index and blood pressure in primary care pediatric patients. J Pediatr. 2006;148:195-200. doi: 10.1016/j.jpeds.2005.10.030
9. Rodriguez BL, Dabelea D, Liese AD, et al; SEARCH Study Group. Prevalence and correlates of elevated blood pressure in youth with diabetes mellitus: the SEARCH for diabetes in youth study. J Pediatr. 2010;157:245-251.e1. doi: 10.1016/j.jpeds.2010.02.021
10. Shay CM, Ning H, Daniels SR, et al. Status of cardiovascular health in US adolescents: prevalence estimates from the National Health and Nutrition Examination Surveys (NHANES) 2005-2010. Circulation. 2013;127:1369-1376. doi: 10.1161/CIRCULATIONAHA.113.001559
11. Archbold KH, Vasquez MM, Goodwin JL, et al. Effects of sleep patterns and obesity on increases in blood pressure in a 5-year period: report from the Tucson Children’s Assessment of Sleep Apnea Study. J Pediatr. 2012;161:26-30. doi: 10.1016/j.jpeds.2011.12.034
12. Flynn JT, Mitsnefes M, Pierce C, et al; . Blood pressure in children with chronic kidney disease: a report from the Chronic Kidney Disease in Children study. Hypertension. 2008;52:631-637. doi: 10.1161/HYPERTENSIONAHA.108.110635
13. Martin RM, Ness AR, Gunnell D, et al; ALSPAC Study Team. Does breast-feeding in infancy lower blood pressure in childhood? The Avon Longitudinal Study of Parents and Children (ALSPAC). Circulation. 2004;109:1259-1266. doi: 10.1161/01.CIR.0000118468.76447.CE
14. Brickner ME, Hillis LD, Lange RA. Congenital heart disease in adults. N Engl J Med. 2000;342:256-263. doi: 10.1056/NEJM200001273420407
15. Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: a systematic review and meta-regression analysis. Circulation. 2008;117:3171-3180. doi: 10.1161/CIRCULATIONAHA.107.730366
16. Sun SS, Grave GD, Siervogel RM, et al. Systolic blood pressure in childhood predicts hypertension and metabolic syndrome later in life. Pediatrics. 2007;119:237-246. doi: 10.1542/peds.2006-2543
17. Parker ED, Sinaiko AR, Kharbanda EO, et al. Change in weight status and development of hypertension. Pediatrics. 2016; 137:e20151662. doi: 10.1542/peds.2015-1662
18. Pickering TG, Hall JE, Appel LJ, et al; . Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142-161. doi: 10.1161/01.HYP.0000150859.47929.8e
19. Staley JR, Bradley J, Silverwood RJ, et al. Associations of blood pressure in pregnancy with offspring blood pressure trajectories during childhood and adolescence: findings from a prospective study. J Am Heart Assoc. 2015;4:e001422. doi: 10.1161/JAHA.114.001422
20. Yang Q, Zhang Z, Zuklina EV, et al. Sodium intake and blood pressure among US children and adolescents. Pediatrics. 2012;130:611-619. doi: 10.1542/peds.2011-3870
21. Le-Ha C, Beilin LJ, Burrows S, et al. Oral contraceptive use in girls and alcohol consumption in boys are associated with increased blood pressure in late adolescence. Eur J Prev Cardiol. 2013;20:947-955. doi: 10.1177/2047487312452966
22. Samuels JA, Franco K, Wan F, Sorof JM. Effect of stimulants on 24-h ambulatory blood pressure in children with ADHD: a double-blind, randomized, cross-over trial. Pediatr Nephrol. 2006;21:92-95. doi: 10.1007/s00467-005-2051-1
23. Wiesen J, Adkins M, Fortune S, et al. Evaluation of pediatric patients with mild-to-moderate hypertension: yield of diagnostic testing. Pediatrics. 2008;122:e988-993. doi: 10.1542/peds.2008-0365
24. Kapur G, Ahmed M, Pan C, et al. Secondary hypertension in overweight and stage 1 hypertensive children: a Midwest Pediatric Nephrology Consortium report. J Clin Hypertens (Greenwich). 2010;12:34-39. doi: 10.1111/j.1751-7176.2009.00195.x
25. Anyaegbu EI, Dharnidharka VR. Hypertension in the teenager. Pediatr Clin North Am. 2014;61:131-151. doi: 10.1016/j.pcl.2013.09.011
26. Gandhi B, Cheek S, Campo JV. Anxiety in the pediatric medical setting. Child Adolesc Psychiatr Clin N Am. 2012;21:643-653. doi: 10.1016/j.chc.2012.05.013
27. Farpour-Lambert NJ, Aggoun Y, Marchand LM, et al. Physical activity reduces systemic blood pressure and improves early markers of atherosclerosis in pre-pubertal obese children. J Am Coll Cardiol. 2009;54:2396-2406. doi: 10.1016/j.jacc.2009.08.030
28. Li JS, Baker-Smith CM, Smith PB, et al. Racial differences in blood pressure response to angiotensin-converting enzyme inhibitors in children: a meta-analysis. Clin Pharmacol Ther. 2008;84:315-319. doi: 10.1038/clpt.2008.113
29. Singer PS. Updates on hypertension and new guidelines. Adv Pediatr. 2019;66:177-187. doi: 10.1016/j.yapd.2019.03.009
30. Torrance B, McGuire KA, Lewanczuk R, et al. Overweight, physical activity and high blood pressure in children: a review of the literature. Vasc Health Risk Manag. 2007;3:139-149.
31. DASH eating plan. National Heart, Lung, and Blood Institute. Accessed April 26, 2021. www.nhlbi.nih.gov/health-topics/dash-eating-plan
32. Nutritional goals for age-sex groups based on dietary reference intakes and dietary guidelines recommendations (Appendix 7). In: US Department of Agriculture. Dietary guidelines for Americans, 2015-2020. 8th ed. December 2015;97-98. Accessed April 26, 2021. https://health.gov/sites/default/files/2019-09/2015-2020_Dietary_Guidelines.pdf
33. Asghari G, Yuzbashian E, Mirmiran P, et al. Dietary Approaches to Stop Hypertension (DASH) dietary pattern is associated with reduced incidence of metabolic syndrome in children and adolescents. J Pediatr. 2016;174:178-184.e1. doi: 10.1016/j.jpeds.2016.03.077
34. Damasceno MMC, de Araújo MFM, de Freitas RWJF, et al. The association between blood pressure in adolescents and the consumption of fruits, vegetables and fruit juice–an exploratory study. J Clin Nurs. 2011;20:1553-1560. doi: 10.1111/j.1365-2702.2010.03608.x
35. Anderson KL. A review of the prevention and medical management of childhood obesity. Child Adolesc Psychiatr Clin N Am. 2018;27:63-76. doi: 10.1016/j.chc.2017.08.003
36. Kumar S, King EC, Christison, et al; POWER Work Group. Health outcomes of youth in clinical pediatric weight management programs in POWER. J Pediatr. 2019;208:57-65.e4. doi: 10.1016/j.jpeds.2018.12.049
37. Gregoski MJ, Barnes VA, Tingen MS, et al. Breathing awareness meditation and LifeSkills® Training programs influence upon ambulatory blood pressure and sodium excretion among African American adolescents. J Adolesc Health. 2011;48:59-64. doi: 10.1016/j.jadohealth.2010.05.019
38. Escape Trial Group; E, Trivelli A, Picca S, et al. Strict blood-pressure control and progression of renal failure in children. N Engl J Med. 2009;361:1639-1650. doi: 10.1056/NEJMoa0902066
PRACTICE RECOMMENDATIONS
› Measure the blood pressure (BP) of all children 3 years and older annually; those who have a specific comorbid condition (eg, obesity, diabetes, renal disease, or an aortic-arch abnormality) or who are taking medication known to elevate BP should have their BP checked at every health care visit. C
› Encourage lifestyle modification as the initial treatment for elevated BP or hypertension in children. A
› Utilize pharmacotherapy for (1) children with stage 1 hypertension who have failed to meet BP goals after 3 to 6 months of lifestyle modification and (2) children with stage 2 hypertension who do not have a modifiable risk factor, such as obesity. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series