User login
12-month follow-up shows monthly maintenance dose of tralokinumab maintains response in some AD patients
without the use of rescue medication including topical corticosteroids, results from a pooled analysis of two trials found.
“The interesting thing here is that there weren’t major differences in the maintenance dosing, which really allows us some flexibility with maintenance dosing for this particular drug,” lead study investigator Andrew Blauvelt, MD, MBA, said during the Revolutionizing Atopic Dermatitis symposium.
Administered subcutaneously, tralokinumab is a fully human IgG4 monoclonal antibody that specifically binds to interleukin-13, a key driver of underlying inflammation in AD. In two of the drug’s pivotal phase 3 trials, ECZTRA 1 and ECZTRA 2, tralokinumab monotherapy was superior to placebo at week 16 for all primary and secondary endpoints.
The purpose of the current trial was to investigate the maintenance of efficacy after 16 weeks of tralokinumab in those who were initial responders and to assess the efficacy of reduced dosing frequency from 300 mg every 2 weeks to 300 mg every 4 weeks after a 36-week maintenance phase. Patients who used rescue medication, including topical corticosteroids, were considered to be nonresponders.
Dr. Blauvelt reported results from 1,596 adult patients with a mean age of 38 years who were randomized to tralokinumab 300 mg every 2 weeks or placebo in the initial treatment period. At baseline, the mean duration of AD was 28.2 years, 50% had severe disease based on their IGA score, and their mean Dermatology Life Quality Index score was 17.
Of these patients, 412 achieved an IGA score of 0 or 1 and/or an EASI 75 at week 16 with tralokinumab every 2 weeks and were rerandomized (2:2:1) to continue tralokinumab 300 mg every 2 weeks, tralokinumab 300 mg every 4 weeks, or placebo for 36 weeks.
The researchers found that 56%-57% of patients in the tralokinumab every 2-week dosing group maintained their IGA 0/1 and EASI 75 response at week 52, compared with 42%-50% of those who received the drug every 4 weeks. “So, there may be a population of patients who require drug every 4 weeks after initially receiving the drug every 2 weeks for the first 16 weeks,” said Dr. Blauvelt, a dermatologist who is president of Oregon Medical Research Center, Portland. “Interestingly, 26%-34% of patients on placebo maintained their IGA 0/1 and EASI 75 response a response to week 52. Perhaps those are patients who have more mild disease or more episodic disease when they started this trial.”
He also noted that time to relapse based on their IGA 0/1 and EASI 75 was prolonged with tralokinumab treatment, compared with placebo, and adverse event frequency was similar among all treatment groups (73% among those who received tralokinumab every 2 weeks, 66% among those who received tralokinumab every 4 weeks, and 70% in the placebo group).
Dr. Blauvelt concluded that a step-down in tralokinumab dosing to every 4 weeks may be an option for some patients achieving clear or almost clear skin after an initial dosing schedule of every 2 weeks.
LEO Pharma, which is developing tralokinumab, sponsored the analysis. Dr. Blauvelt reported that he is an investigator and a scientific adviser for LEO Pharma and for several other pharmaceutical companies developing treatments for AD.
without the use of rescue medication including topical corticosteroids, results from a pooled analysis of two trials found.
“The interesting thing here is that there weren’t major differences in the maintenance dosing, which really allows us some flexibility with maintenance dosing for this particular drug,” lead study investigator Andrew Blauvelt, MD, MBA, said during the Revolutionizing Atopic Dermatitis symposium.
Administered subcutaneously, tralokinumab is a fully human IgG4 monoclonal antibody that specifically binds to interleukin-13, a key driver of underlying inflammation in AD. In two of the drug’s pivotal phase 3 trials, ECZTRA 1 and ECZTRA 2, tralokinumab monotherapy was superior to placebo at week 16 for all primary and secondary endpoints.
The purpose of the current trial was to investigate the maintenance of efficacy after 16 weeks of tralokinumab in those who were initial responders and to assess the efficacy of reduced dosing frequency from 300 mg every 2 weeks to 300 mg every 4 weeks after a 36-week maintenance phase. Patients who used rescue medication, including topical corticosteroids, were considered to be nonresponders.
Dr. Blauvelt reported results from 1,596 adult patients with a mean age of 38 years who were randomized to tralokinumab 300 mg every 2 weeks or placebo in the initial treatment period. At baseline, the mean duration of AD was 28.2 years, 50% had severe disease based on their IGA score, and their mean Dermatology Life Quality Index score was 17.
Of these patients, 412 achieved an IGA score of 0 or 1 and/or an EASI 75 at week 16 with tralokinumab every 2 weeks and were rerandomized (2:2:1) to continue tralokinumab 300 mg every 2 weeks, tralokinumab 300 mg every 4 weeks, or placebo for 36 weeks.
The researchers found that 56%-57% of patients in the tralokinumab every 2-week dosing group maintained their IGA 0/1 and EASI 75 response at week 52, compared with 42%-50% of those who received the drug every 4 weeks. “So, there may be a population of patients who require drug every 4 weeks after initially receiving the drug every 2 weeks for the first 16 weeks,” said Dr. Blauvelt, a dermatologist who is president of Oregon Medical Research Center, Portland. “Interestingly, 26%-34% of patients on placebo maintained their IGA 0/1 and EASI 75 response a response to week 52. Perhaps those are patients who have more mild disease or more episodic disease when they started this trial.”
He also noted that time to relapse based on their IGA 0/1 and EASI 75 was prolonged with tralokinumab treatment, compared with placebo, and adverse event frequency was similar among all treatment groups (73% among those who received tralokinumab every 2 weeks, 66% among those who received tralokinumab every 4 weeks, and 70% in the placebo group).
Dr. Blauvelt concluded that a step-down in tralokinumab dosing to every 4 weeks may be an option for some patients achieving clear or almost clear skin after an initial dosing schedule of every 2 weeks.
LEO Pharma, which is developing tralokinumab, sponsored the analysis. Dr. Blauvelt reported that he is an investigator and a scientific adviser for LEO Pharma and for several other pharmaceutical companies developing treatments for AD.
without the use of rescue medication including topical corticosteroids, results from a pooled analysis of two trials found.
“The interesting thing here is that there weren’t major differences in the maintenance dosing, which really allows us some flexibility with maintenance dosing for this particular drug,” lead study investigator Andrew Blauvelt, MD, MBA, said during the Revolutionizing Atopic Dermatitis symposium.
Administered subcutaneously, tralokinumab is a fully human IgG4 monoclonal antibody that specifically binds to interleukin-13, a key driver of underlying inflammation in AD. In two of the drug’s pivotal phase 3 trials, ECZTRA 1 and ECZTRA 2, tralokinumab monotherapy was superior to placebo at week 16 for all primary and secondary endpoints.
The purpose of the current trial was to investigate the maintenance of efficacy after 16 weeks of tralokinumab in those who were initial responders and to assess the efficacy of reduced dosing frequency from 300 mg every 2 weeks to 300 mg every 4 weeks after a 36-week maintenance phase. Patients who used rescue medication, including topical corticosteroids, were considered to be nonresponders.
Dr. Blauvelt reported results from 1,596 adult patients with a mean age of 38 years who were randomized to tralokinumab 300 mg every 2 weeks or placebo in the initial treatment period. At baseline, the mean duration of AD was 28.2 years, 50% had severe disease based on their IGA score, and their mean Dermatology Life Quality Index score was 17.
Of these patients, 412 achieved an IGA score of 0 or 1 and/or an EASI 75 at week 16 with tralokinumab every 2 weeks and were rerandomized (2:2:1) to continue tralokinumab 300 mg every 2 weeks, tralokinumab 300 mg every 4 weeks, or placebo for 36 weeks.
The researchers found that 56%-57% of patients in the tralokinumab every 2-week dosing group maintained their IGA 0/1 and EASI 75 response at week 52, compared with 42%-50% of those who received the drug every 4 weeks. “So, there may be a population of patients who require drug every 4 weeks after initially receiving the drug every 2 weeks for the first 16 weeks,” said Dr. Blauvelt, a dermatologist who is president of Oregon Medical Research Center, Portland. “Interestingly, 26%-34% of patients on placebo maintained their IGA 0/1 and EASI 75 response a response to week 52. Perhaps those are patients who have more mild disease or more episodic disease when they started this trial.”
He also noted that time to relapse based on their IGA 0/1 and EASI 75 was prolonged with tralokinumab treatment, compared with placebo, and adverse event frequency was similar among all treatment groups (73% among those who received tralokinumab every 2 weeks, 66% among those who received tralokinumab every 4 weeks, and 70% in the placebo group).
Dr. Blauvelt concluded that a step-down in tralokinumab dosing to every 4 weeks may be an option for some patients achieving clear or almost clear skin after an initial dosing schedule of every 2 weeks.
LEO Pharma, which is developing tralokinumab, sponsored the analysis. Dr. Blauvelt reported that he is an investigator and a scientific adviser for LEO Pharma and for several other pharmaceutical companies developing treatments for AD.
FROM REVOLUTIONIZING AD 2021
What’s behind brain fog in treated hypothyroidism?
The phenomenon of brain fog, as described by some patients with hypothyroidism despite treatment, is often associated with fatigue and cognitive symptoms and may be relieved by a variety of pharmacologic and nonpharmacologic approaches, new research suggests.
The findings come from a survey of more than 700 patients with hypothyroidism due to thyroid surgery and/or radioactive iodine therapy (RAI) or Hashimoto’s who reported having brain fog.
The survey results were presented May 29 at the American Association of Clinical Endocrinology Virtual Annual Meeting 2021 by investigators Matthew D. Ettleson, MD, and Ava Raine, of the University of Chicago, Illinois.
Many patients with hypothyroidism continue to experience symptoms despite taking thyroid hormone replacement therapy and having normal thyroid function test results.
These symptoms can include quantifiable cognitive, quality of life, and metabolic abnormalities. However, “some patients also experience vague and difficult to quantify symptoms, which they describe as brain fog,” Ms. Raine said.
The brain fog phenomenon has been described with somewhat varying features in several different chronic conditions, including postural orthostatic tachycardia syndrome, myalgic encephalomyelitis/chronic fatigue syndrome, fibromyalgia, post-menopausal syndrome, and recently, among people with “long haul” COVID-19 symptoms.
However, brain fog associated with treated hypothyroidism has not been explored in-depth, despite the fact that patients often report it, Ms. Raine noted.
Results will help clinicians assist patients with brain fog
Fatigue was the most prominent brain fog symptom reported in the survey, followed by forgetfulness and difficulty focusing. On the other hand, rest and relaxation were the most reported factors that alleviated symptoms, followed by thyroid hormone adjustment.
“Hopefully these findings will help clinicians to recognize and treat the symptoms of brain fog and shed light on a condition which up until now has not been very well understood,” Dr. Ettleson said.
Asked to comment, session moderator Jad G. Sfeir, MD, of the Mayo Medical School, Rochester, Minn., told this news organization: “We do see patients complain a lot about this brain fog. The question is how can I help, and what has worked for them in the past?”
“When you have symptoms that are vague, like brain fog, you don’t have a lot of objective tools to [measure], so you can’t really develop a study to see how a certain medication affects the symptoms. Relying on subjective information from patients saying what worked for them and what did not, you can draw a lot of implications to clinical practice.”
The survey results, Dr. Sfeir said, “will help direct clinicians to know what type of questions to ask patients based on the survey responses and how to make some recommendations that may help.”
Fatigue, memory problems, difficulty focusing characterize brain fog
The online survey was distributed to hypothyroidism support groups and through the American Thyroid Association. Of the 5,282 respondents with hypothyroidism and symptoms of brain fog, 46% (2,453) reported having experienced brain fog symptoms prior to their diagnosis of hypothyroidism.
The population analyzed for the study was the 17% (731) who reported experiencing brain fog weeks to months following a diagnosis of hypothyroidism. Of those, 33% had Hashimoto’s, 21% thyroid surgery, 11% RAI therapy, and 15.6% had both thyroid surgery and RAI.
Brain fog symptoms were reported as occurring “frequently” by 44.5% and “all the time” by 37.0%. The composite symptom score was 22.9 out of 30.
Fatigue, or lack of energy, was the most commonly named symptom, reported by over 90% of both the thyroid surgery/RAI and Hashimoto’s groups, and as occurring “all the time” by about half in each group. Others reported by at least half of both groups included memory problems, difficulty focusing, sleep problems, and difficulties with decision-making. Other symptoms frequently cited included confusion, mood disturbance, and anxiety.
“Each ... domain was reported with some frequency by at least 85% of respondents, regardless of etiology of hypothyroidism, so it really was a high symptom burden that we were seeing, even in those whose symptoms were the least frequent,” Ms. Raine noted.
Symptom scores generally correlated with patient satisfaction scores, particularly with those of cognitive signs and difficulty focusing.
Lifting the fog: What do patients say helps them?
The survey asked patients what factors improved or worsened their brain fog symptoms. By far, the most frequent answer was rest/relaxation, endorsed by 58.5%. Another 10.5% listed exercise/outdoor time, but 1.5% said exercise worsened their symptoms.
Unspecified adjustments of thyroid medications were said to improve symptoms for 13.9%. Specific thyroid hormones reported to improve symptoms were liothyronine in 8.8%, desiccated thyroid extract in 3.1%, and levothyroxine in 2.7%. However, another 4.2% said thyroxine worsened their symptoms.
Healthy/nutritious diets were reported to improve symptoms by 6.3%, while consuming gluten, a high-sugar diet, and consuming alcohol were reported to worsen symptoms for 1.3%, 3.2%, and 1.3%, respectively. Caffeine was said to help for 3.1% and to harm by 0.6%.
Small numbers of patients reported improvements in symptoms with vitamins B12 and D, Adderall, or other stimulant medications, antidepressants, naltrexone, sun exposure, and blood glucose stability.
Other factors reported to worsen symptoms included menstruation, infection or other acute illness, pain, and “loud noise.”
Dr. Ettleson pointed out, “For many of these patients [the brain fog] may have nothing to do with their thyroid. We saw a large proportion of patients who said they had symptoms well before they were ever diagnosed with hypothyroidism, and yet many patients have linked these brain fog symptoms to their thyroid.”
Nonetheless, he said, “I think it’s imperative for the clinician to at least engage in these conversations and not just stop when the thyroid function tests are normal. We have many lifestyle suggestions that have emerged from this study that I think physicians can put forward to patients who are dealing with this ... early in the process in addition to thyroid hormone adjustment, which may help some patients.”
Dr. Ettleson, Ms. Raine, and Dr. Sfeir have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The phenomenon of brain fog, as described by some patients with hypothyroidism despite treatment, is often associated with fatigue and cognitive symptoms and may be relieved by a variety of pharmacologic and nonpharmacologic approaches, new research suggests.
The findings come from a survey of more than 700 patients with hypothyroidism due to thyroid surgery and/or radioactive iodine therapy (RAI) or Hashimoto’s who reported having brain fog.
The survey results were presented May 29 at the American Association of Clinical Endocrinology Virtual Annual Meeting 2021 by investigators Matthew D. Ettleson, MD, and Ava Raine, of the University of Chicago, Illinois.
Many patients with hypothyroidism continue to experience symptoms despite taking thyroid hormone replacement therapy and having normal thyroid function test results.
These symptoms can include quantifiable cognitive, quality of life, and metabolic abnormalities. However, “some patients also experience vague and difficult to quantify symptoms, which they describe as brain fog,” Ms. Raine said.
The brain fog phenomenon has been described with somewhat varying features in several different chronic conditions, including postural orthostatic tachycardia syndrome, myalgic encephalomyelitis/chronic fatigue syndrome, fibromyalgia, post-menopausal syndrome, and recently, among people with “long haul” COVID-19 symptoms.
However, brain fog associated with treated hypothyroidism has not been explored in-depth, despite the fact that patients often report it, Ms. Raine noted.
Results will help clinicians assist patients with brain fog
Fatigue was the most prominent brain fog symptom reported in the survey, followed by forgetfulness and difficulty focusing. On the other hand, rest and relaxation were the most reported factors that alleviated symptoms, followed by thyroid hormone adjustment.
“Hopefully these findings will help clinicians to recognize and treat the symptoms of brain fog and shed light on a condition which up until now has not been very well understood,” Dr. Ettleson said.
Asked to comment, session moderator Jad G. Sfeir, MD, of the Mayo Medical School, Rochester, Minn., told this news organization: “We do see patients complain a lot about this brain fog. The question is how can I help, and what has worked for them in the past?”
“When you have symptoms that are vague, like brain fog, you don’t have a lot of objective tools to [measure], so you can’t really develop a study to see how a certain medication affects the symptoms. Relying on subjective information from patients saying what worked for them and what did not, you can draw a lot of implications to clinical practice.”
The survey results, Dr. Sfeir said, “will help direct clinicians to know what type of questions to ask patients based on the survey responses and how to make some recommendations that may help.”
Fatigue, memory problems, difficulty focusing characterize brain fog
The online survey was distributed to hypothyroidism support groups and through the American Thyroid Association. Of the 5,282 respondents with hypothyroidism and symptoms of brain fog, 46% (2,453) reported having experienced brain fog symptoms prior to their diagnosis of hypothyroidism.
The population analyzed for the study was the 17% (731) who reported experiencing brain fog weeks to months following a diagnosis of hypothyroidism. Of those, 33% had Hashimoto’s, 21% thyroid surgery, 11% RAI therapy, and 15.6% had both thyroid surgery and RAI.
Brain fog symptoms were reported as occurring “frequently” by 44.5% and “all the time” by 37.0%. The composite symptom score was 22.9 out of 30.
Fatigue, or lack of energy, was the most commonly named symptom, reported by over 90% of both the thyroid surgery/RAI and Hashimoto’s groups, and as occurring “all the time” by about half in each group. Others reported by at least half of both groups included memory problems, difficulty focusing, sleep problems, and difficulties with decision-making. Other symptoms frequently cited included confusion, mood disturbance, and anxiety.
“Each ... domain was reported with some frequency by at least 85% of respondents, regardless of etiology of hypothyroidism, so it really was a high symptom burden that we were seeing, even in those whose symptoms were the least frequent,” Ms. Raine noted.
Symptom scores generally correlated with patient satisfaction scores, particularly with those of cognitive signs and difficulty focusing.
Lifting the fog: What do patients say helps them?
The survey asked patients what factors improved or worsened their brain fog symptoms. By far, the most frequent answer was rest/relaxation, endorsed by 58.5%. Another 10.5% listed exercise/outdoor time, but 1.5% said exercise worsened their symptoms.
Unspecified adjustments of thyroid medications were said to improve symptoms for 13.9%. Specific thyroid hormones reported to improve symptoms were liothyronine in 8.8%, desiccated thyroid extract in 3.1%, and levothyroxine in 2.7%. However, another 4.2% said thyroxine worsened their symptoms.
Healthy/nutritious diets were reported to improve symptoms by 6.3%, while consuming gluten, a high-sugar diet, and consuming alcohol were reported to worsen symptoms for 1.3%, 3.2%, and 1.3%, respectively. Caffeine was said to help for 3.1% and to harm by 0.6%.
Small numbers of patients reported improvements in symptoms with vitamins B12 and D, Adderall, or other stimulant medications, antidepressants, naltrexone, sun exposure, and blood glucose stability.
Other factors reported to worsen symptoms included menstruation, infection or other acute illness, pain, and “loud noise.”
Dr. Ettleson pointed out, “For many of these patients [the brain fog] may have nothing to do with their thyroid. We saw a large proportion of patients who said they had symptoms well before they were ever diagnosed with hypothyroidism, and yet many patients have linked these brain fog symptoms to their thyroid.”
Nonetheless, he said, “I think it’s imperative for the clinician to at least engage in these conversations and not just stop when the thyroid function tests are normal. We have many lifestyle suggestions that have emerged from this study that I think physicians can put forward to patients who are dealing with this ... early in the process in addition to thyroid hormone adjustment, which may help some patients.”
Dr. Ettleson, Ms. Raine, and Dr. Sfeir have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The phenomenon of brain fog, as described by some patients with hypothyroidism despite treatment, is often associated with fatigue and cognitive symptoms and may be relieved by a variety of pharmacologic and nonpharmacologic approaches, new research suggests.
The findings come from a survey of more than 700 patients with hypothyroidism due to thyroid surgery and/or radioactive iodine therapy (RAI) or Hashimoto’s who reported having brain fog.
The survey results were presented May 29 at the American Association of Clinical Endocrinology Virtual Annual Meeting 2021 by investigators Matthew D. Ettleson, MD, and Ava Raine, of the University of Chicago, Illinois.
Many patients with hypothyroidism continue to experience symptoms despite taking thyroid hormone replacement therapy and having normal thyroid function test results.
These symptoms can include quantifiable cognitive, quality of life, and metabolic abnormalities. However, “some patients also experience vague and difficult to quantify symptoms, which they describe as brain fog,” Ms. Raine said.
The brain fog phenomenon has been described with somewhat varying features in several different chronic conditions, including postural orthostatic tachycardia syndrome, myalgic encephalomyelitis/chronic fatigue syndrome, fibromyalgia, post-menopausal syndrome, and recently, among people with “long haul” COVID-19 symptoms.
However, brain fog associated with treated hypothyroidism has not been explored in-depth, despite the fact that patients often report it, Ms. Raine noted.
Results will help clinicians assist patients with brain fog
Fatigue was the most prominent brain fog symptom reported in the survey, followed by forgetfulness and difficulty focusing. On the other hand, rest and relaxation were the most reported factors that alleviated symptoms, followed by thyroid hormone adjustment.
“Hopefully these findings will help clinicians to recognize and treat the symptoms of brain fog and shed light on a condition which up until now has not been very well understood,” Dr. Ettleson said.
Asked to comment, session moderator Jad G. Sfeir, MD, of the Mayo Medical School, Rochester, Minn., told this news organization: “We do see patients complain a lot about this brain fog. The question is how can I help, and what has worked for them in the past?”
“When you have symptoms that are vague, like brain fog, you don’t have a lot of objective tools to [measure], so you can’t really develop a study to see how a certain medication affects the symptoms. Relying on subjective information from patients saying what worked for them and what did not, you can draw a lot of implications to clinical practice.”
The survey results, Dr. Sfeir said, “will help direct clinicians to know what type of questions to ask patients based on the survey responses and how to make some recommendations that may help.”
Fatigue, memory problems, difficulty focusing characterize brain fog
The online survey was distributed to hypothyroidism support groups and through the American Thyroid Association. Of the 5,282 respondents with hypothyroidism and symptoms of brain fog, 46% (2,453) reported having experienced brain fog symptoms prior to their diagnosis of hypothyroidism.
The population analyzed for the study was the 17% (731) who reported experiencing brain fog weeks to months following a diagnosis of hypothyroidism. Of those, 33% had Hashimoto’s, 21% thyroid surgery, 11% RAI therapy, and 15.6% had both thyroid surgery and RAI.
Brain fog symptoms were reported as occurring “frequently” by 44.5% and “all the time” by 37.0%. The composite symptom score was 22.9 out of 30.
Fatigue, or lack of energy, was the most commonly named symptom, reported by over 90% of both the thyroid surgery/RAI and Hashimoto’s groups, and as occurring “all the time” by about half in each group. Others reported by at least half of both groups included memory problems, difficulty focusing, sleep problems, and difficulties with decision-making. Other symptoms frequently cited included confusion, mood disturbance, and anxiety.
“Each ... domain was reported with some frequency by at least 85% of respondents, regardless of etiology of hypothyroidism, so it really was a high symptom burden that we were seeing, even in those whose symptoms were the least frequent,” Ms. Raine noted.
Symptom scores generally correlated with patient satisfaction scores, particularly with those of cognitive signs and difficulty focusing.
Lifting the fog: What do patients say helps them?
The survey asked patients what factors improved or worsened their brain fog symptoms. By far, the most frequent answer was rest/relaxation, endorsed by 58.5%. Another 10.5% listed exercise/outdoor time, but 1.5% said exercise worsened their symptoms.
Unspecified adjustments of thyroid medications were said to improve symptoms for 13.9%. Specific thyroid hormones reported to improve symptoms were liothyronine in 8.8%, desiccated thyroid extract in 3.1%, and levothyroxine in 2.7%. However, another 4.2% said thyroxine worsened their symptoms.
Healthy/nutritious diets were reported to improve symptoms by 6.3%, while consuming gluten, a high-sugar diet, and consuming alcohol were reported to worsen symptoms for 1.3%, 3.2%, and 1.3%, respectively. Caffeine was said to help for 3.1% and to harm by 0.6%.
Small numbers of patients reported improvements in symptoms with vitamins B12 and D, Adderall, or other stimulant medications, antidepressants, naltrexone, sun exposure, and blood glucose stability.
Other factors reported to worsen symptoms included menstruation, infection or other acute illness, pain, and “loud noise.”
Dr. Ettleson pointed out, “For many of these patients [the brain fog] may have nothing to do with their thyroid. We saw a large proportion of patients who said they had symptoms well before they were ever diagnosed with hypothyroidism, and yet many patients have linked these brain fog symptoms to their thyroid.”
Nonetheless, he said, “I think it’s imperative for the clinician to at least engage in these conversations and not just stop when the thyroid function tests are normal. We have many lifestyle suggestions that have emerged from this study that I think physicians can put forward to patients who are dealing with this ... early in the process in addition to thyroid hormone adjustment, which may help some patients.”
Dr. Ettleson, Ms. Raine, and Dr. Sfeir have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Medically suspect criterion can determine bariatric surgery coverage
A delaying tactic used by some U.S. health insurers to limit coverage of bariatric surgery does not jibe with the clinical experience at one U.S. center with 461 patients who underwent primary or revisional bariatric surgery.
The tactic applies to patients with a baseline body mass index (BMI) of 35-39 kg/m2 who usually also need at least one comorbidity to qualify for insurance coverage for bariatric surgery, and specifically to the subgroup for whom hypertension is the qualifying comorbidity.
Some insurers limit surgery coverage to patients with hypertension who fail to reach their goal blood pressure on agents from three different drug classes, a policy that is “extremely frustrating and dangerous,” said Yannis Raftopoulos, MD, PhD, in his presentation at the annual meeting of the American Society for Metabolic and Bariatric Surgery.
Using number of antihypertensive drugs ‘is not correct’
“Using the number of antihypertensive medications to justify surgery is not correct because blood pressure control is not [always] better when patients take two or three medications, compared with when they are taking one. This harms patients because the more severe their hypertension, the worse their control,” said Dr. Raftopoulos, director of the weight management program at Holyoke (Mass.) Medical Center.
He presented findings from a retrospective study of 461 patients who underwent either sleeve gastrectomy or laparoscopic Roux-en-Y gastric bypass at his center, including 213 (46%) diagnosed with hypertension at the time of their surgery. Within this group were 68 patients with a BMI of 35-39, which meant that they could get insurance coverage for bariatric surgery only if they also had a relevant comorbidity such as hypertension, diabetes, or severe sleep apnea.
Among these patients, 36 (17% of those with hypertension) had only hypertension as their relevant comorbidity and would not have qualified for bariatric surgery under the strictest criteria applied by some insurers that require patients to remain hypertensive despite treatment with at least three different antihypertensive medications. (These 36 patients underwent bariatric surgery because their insurance coverage did not have this restriction.)
The analyses Dr. Raftopoulos presented also documented the rate of hypertension resolution among patients in the series who had hypertension at baseline and 1-year follow-up results. Among 65 patients on one antihypertensive drug at baseline, 43 (66%) had complete resolution of their hypertension after 1 year, defined as blood pressure of less than 130/90 mm Hg while completely off antihypertensive treatment. In contrast, among 55 patients on two antihypertensive medications at baseline, 28 (51%) had complete resolution after 1 year, and among 24 patients on three or more antihypertensive medications at baseline, 3 (13%) had complete resolution 1 year after bariatric surgery, he reported.
“Patients who were treated with one oral antihypertensive medication preoperatively had a higher likelihood of postoperative hypertension resolution,” concluded Dr. Raftopoulos.
Restricting access to bariatric surgery to patients with a BMI of less than 40 based on the preoperative intensity of their antihypertensive treatment “is not supported by our data, and can be potentially harmful,” he declared.
“This study was the result of discussions about this problem with multiple insurers in my area,” he added. “This affects a good number of patients.”
Waiting for hypertension to become less treatable
The results Dr. Raftopoulos presented “are not surprising, because they confirm the hypothesis that earlier intervention in the course of a disease like hypertension is more likely to be successful,” commented Bruce D. Schirmer, MD, a professor of surgery at the University of Virginia, Charlottesville, and designated discussant for the report.
The policy followed by some health insurers to delay coverage for bariatric surgery until patients fail three medications “forces patients with more treatable hypertension to wait until their disease worsens and becomes less treatable before they can receive appropriate treatment,” he said.
Dr. Schirmer attributed the motivation for this approach to a “despicable” and “reprehensible” reason: “Actuarial calculations that show paying for curative therapy is not cost effective in the short term. The duration of a patient’s policy may not be long enough to yield a positive financial outcome, so it becomes more appropriate to deny optimal care and have patients become sicker from their disease.”
“I applaud the authors for accumulating the data that point out this unfortunate rule of some insurance companies,” Dr. Schirmer added.
The practice is comparable with an insurer requiring that a patient’s cancer must be metastatic before allowing coverage for treatment, commented Ann M. Rogers, MD, professor and director of the Penn State University surgical weight loss program in Hershey, Penn., and a moderator of the session.
Dr. Raftopoulos, Dr. Schirmer, and Dr. Rogers had no disclosures.
A delaying tactic used by some U.S. health insurers to limit coverage of bariatric surgery does not jibe with the clinical experience at one U.S. center with 461 patients who underwent primary or revisional bariatric surgery.
The tactic applies to patients with a baseline body mass index (BMI) of 35-39 kg/m2 who usually also need at least one comorbidity to qualify for insurance coverage for bariatric surgery, and specifically to the subgroup for whom hypertension is the qualifying comorbidity.
Some insurers limit surgery coverage to patients with hypertension who fail to reach their goal blood pressure on agents from three different drug classes, a policy that is “extremely frustrating and dangerous,” said Yannis Raftopoulos, MD, PhD, in his presentation at the annual meeting of the American Society for Metabolic and Bariatric Surgery.
Using number of antihypertensive drugs ‘is not correct’
“Using the number of antihypertensive medications to justify surgery is not correct because blood pressure control is not [always] better when patients take two or three medications, compared with when they are taking one. This harms patients because the more severe their hypertension, the worse their control,” said Dr. Raftopoulos, director of the weight management program at Holyoke (Mass.) Medical Center.
He presented findings from a retrospective study of 461 patients who underwent either sleeve gastrectomy or laparoscopic Roux-en-Y gastric bypass at his center, including 213 (46%) diagnosed with hypertension at the time of their surgery. Within this group were 68 patients with a BMI of 35-39, which meant that they could get insurance coverage for bariatric surgery only if they also had a relevant comorbidity such as hypertension, diabetes, or severe sleep apnea.
Among these patients, 36 (17% of those with hypertension) had only hypertension as their relevant comorbidity and would not have qualified for bariatric surgery under the strictest criteria applied by some insurers that require patients to remain hypertensive despite treatment with at least three different antihypertensive medications. (These 36 patients underwent bariatric surgery because their insurance coverage did not have this restriction.)
The analyses Dr. Raftopoulos presented also documented the rate of hypertension resolution among patients in the series who had hypertension at baseline and 1-year follow-up results. Among 65 patients on one antihypertensive drug at baseline, 43 (66%) had complete resolution of their hypertension after 1 year, defined as blood pressure of less than 130/90 mm Hg while completely off antihypertensive treatment. In contrast, among 55 patients on two antihypertensive medications at baseline, 28 (51%) had complete resolution after 1 year, and among 24 patients on three or more antihypertensive medications at baseline, 3 (13%) had complete resolution 1 year after bariatric surgery, he reported.
“Patients who were treated with one oral antihypertensive medication preoperatively had a higher likelihood of postoperative hypertension resolution,” concluded Dr. Raftopoulos.
Restricting access to bariatric surgery to patients with a BMI of less than 40 based on the preoperative intensity of their antihypertensive treatment “is not supported by our data, and can be potentially harmful,” he declared.
“This study was the result of discussions about this problem with multiple insurers in my area,” he added. “This affects a good number of patients.”
Waiting for hypertension to become less treatable
The results Dr. Raftopoulos presented “are not surprising, because they confirm the hypothesis that earlier intervention in the course of a disease like hypertension is more likely to be successful,” commented Bruce D. Schirmer, MD, a professor of surgery at the University of Virginia, Charlottesville, and designated discussant for the report.
The policy followed by some health insurers to delay coverage for bariatric surgery until patients fail three medications “forces patients with more treatable hypertension to wait until their disease worsens and becomes less treatable before they can receive appropriate treatment,” he said.
Dr. Schirmer attributed the motivation for this approach to a “despicable” and “reprehensible” reason: “Actuarial calculations that show paying for curative therapy is not cost effective in the short term. The duration of a patient’s policy may not be long enough to yield a positive financial outcome, so it becomes more appropriate to deny optimal care and have patients become sicker from their disease.”
“I applaud the authors for accumulating the data that point out this unfortunate rule of some insurance companies,” Dr. Schirmer added.
The practice is comparable with an insurer requiring that a patient’s cancer must be metastatic before allowing coverage for treatment, commented Ann M. Rogers, MD, professor and director of the Penn State University surgical weight loss program in Hershey, Penn., and a moderator of the session.
Dr. Raftopoulos, Dr. Schirmer, and Dr. Rogers had no disclosures.
A delaying tactic used by some U.S. health insurers to limit coverage of bariatric surgery does not jibe with the clinical experience at one U.S. center with 461 patients who underwent primary or revisional bariatric surgery.
The tactic applies to patients with a baseline body mass index (BMI) of 35-39 kg/m2 who usually also need at least one comorbidity to qualify for insurance coverage for bariatric surgery, and specifically to the subgroup for whom hypertension is the qualifying comorbidity.
Some insurers limit surgery coverage to patients with hypertension who fail to reach their goal blood pressure on agents from three different drug classes, a policy that is “extremely frustrating and dangerous,” said Yannis Raftopoulos, MD, PhD, in his presentation at the annual meeting of the American Society for Metabolic and Bariatric Surgery.
Using number of antihypertensive drugs ‘is not correct’
“Using the number of antihypertensive medications to justify surgery is not correct because blood pressure control is not [always] better when patients take two or three medications, compared with when they are taking one. This harms patients because the more severe their hypertension, the worse their control,” said Dr. Raftopoulos, director of the weight management program at Holyoke (Mass.) Medical Center.
He presented findings from a retrospective study of 461 patients who underwent either sleeve gastrectomy or laparoscopic Roux-en-Y gastric bypass at his center, including 213 (46%) diagnosed with hypertension at the time of their surgery. Within this group were 68 patients with a BMI of 35-39, which meant that they could get insurance coverage for bariatric surgery only if they also had a relevant comorbidity such as hypertension, diabetes, or severe sleep apnea.
Among these patients, 36 (17% of those with hypertension) had only hypertension as their relevant comorbidity and would not have qualified for bariatric surgery under the strictest criteria applied by some insurers that require patients to remain hypertensive despite treatment with at least three different antihypertensive medications. (These 36 patients underwent bariatric surgery because their insurance coverage did not have this restriction.)
The analyses Dr. Raftopoulos presented also documented the rate of hypertension resolution among patients in the series who had hypertension at baseline and 1-year follow-up results. Among 65 patients on one antihypertensive drug at baseline, 43 (66%) had complete resolution of their hypertension after 1 year, defined as blood pressure of less than 130/90 mm Hg while completely off antihypertensive treatment. In contrast, among 55 patients on two antihypertensive medications at baseline, 28 (51%) had complete resolution after 1 year, and among 24 patients on three or more antihypertensive medications at baseline, 3 (13%) had complete resolution 1 year after bariatric surgery, he reported.
“Patients who were treated with one oral antihypertensive medication preoperatively had a higher likelihood of postoperative hypertension resolution,” concluded Dr. Raftopoulos.
Restricting access to bariatric surgery to patients with a BMI of less than 40 based on the preoperative intensity of their antihypertensive treatment “is not supported by our data, and can be potentially harmful,” he declared.
“This study was the result of discussions about this problem with multiple insurers in my area,” he added. “This affects a good number of patients.”
Waiting for hypertension to become less treatable
The results Dr. Raftopoulos presented “are not surprising, because they confirm the hypothesis that earlier intervention in the course of a disease like hypertension is more likely to be successful,” commented Bruce D. Schirmer, MD, a professor of surgery at the University of Virginia, Charlottesville, and designated discussant for the report.
The policy followed by some health insurers to delay coverage for bariatric surgery until patients fail three medications “forces patients with more treatable hypertension to wait until their disease worsens and becomes less treatable before they can receive appropriate treatment,” he said.
Dr. Schirmer attributed the motivation for this approach to a “despicable” and “reprehensible” reason: “Actuarial calculations that show paying for curative therapy is not cost effective in the short term. The duration of a patient’s policy may not be long enough to yield a positive financial outcome, so it becomes more appropriate to deny optimal care and have patients become sicker from their disease.”
“I applaud the authors for accumulating the data that point out this unfortunate rule of some insurance companies,” Dr. Schirmer added.
The practice is comparable with an insurer requiring that a patient’s cancer must be metastatic before allowing coverage for treatment, commented Ann M. Rogers, MD, professor and director of the Penn State University surgical weight loss program in Hershey, Penn., and a moderator of the session.
Dr. Raftopoulos, Dr. Schirmer, and Dr. Rogers had no disclosures.
FROM ASMBS 2021
Foot rash and joint pain
A 21-year-old man presented to the emergency department (ED) with a 2-month history of joint pain, swelling, and difficulty walking that began with swelling of his right knee (FIGURE 1A). The patient said that over the course of several weeks, the swelling and joint pain spread to his left knee, followed by bilateral elbows and ankles. Nonsteroidal anti-inflammatory drugs (NSAIDs) and aspirin produced only modest improvement.
Two weeks prior to presentation, the patient also experienced widespread pruritus and conjunctivitis. His past medical history was significant for a sexual encounter that resulted in urinary tract infection (UTI)–like symptoms approximately 1 month prior to the onset of his joint symptoms. He did not seek care for the UTI-like symptoms.
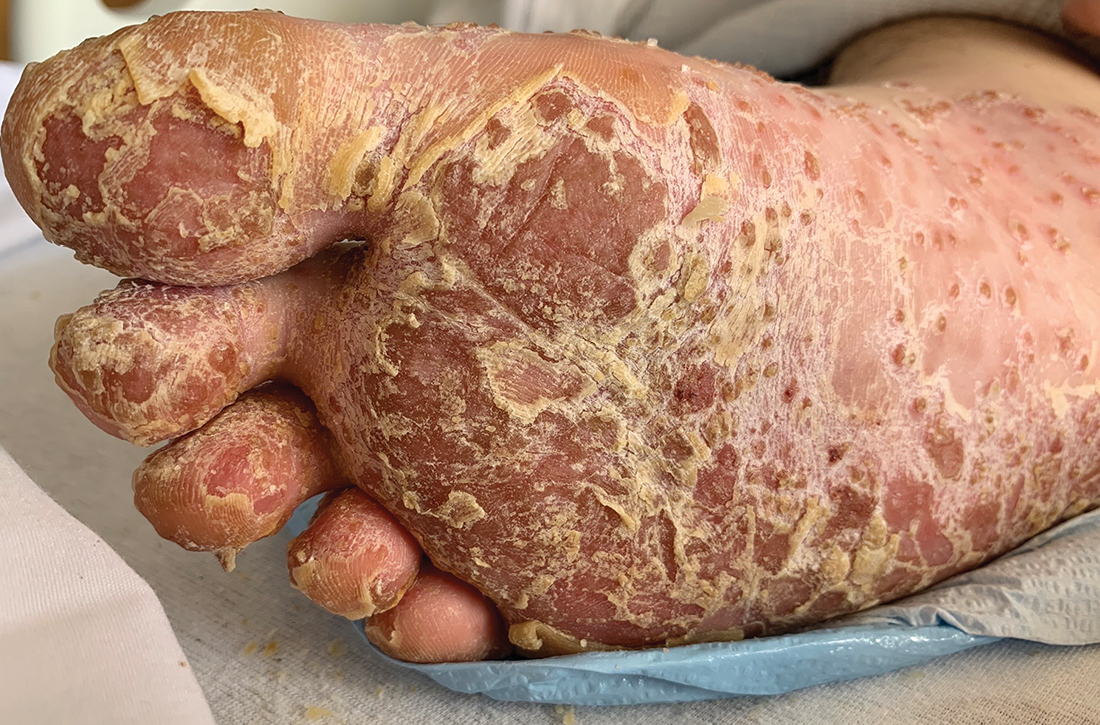
In the ED, the patient was febrile (102.1 °F) and tachycardic. Skin examination revealed erythematous papules, intact vesicles, and pustules with background hyperkeratosis and desquamation on his right foot (FIGURE 1B). The patient had spotty erythema on his palate and a 4-mm superficial erosion on the right penile shaft. Swelling and tenderness were noted over the elbows, knees, hands, and ankles. No inguinal lymphadenopathy was noted.

An arthrocentesis was performed on the right knee that demonstrated no organisms on Gram stain and a normal joint fluid cell count. A complete blood count (CBC), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and urinalysis were ordered. A punch biopsy was performed on a scaly patch on the right elbow.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Keratoderma blenorrhagicum
The patient’s history, clinical findings, and lab results, including a positive Chlamydia trachomatis polymerase chain reaction (PCR) test from a urethral swab, pointed to a diagnosis of keratoderma blenorrhagicum in association with reactive arthritis (following infection with C trachomatis).
Relevant diagnostic findings included an elevated CRP of 26.5 mg/L (normal range, < 10 mg/L), an elevated ESR of 116 mm/h (normal range, < 15 mm/h) and as noted, a positive C trachomatis PCR test. The patient’s white blood cell count was 9.7/μL (normal range, 4.5-11 μL) and the rest of the CBC was within normal limits. Urinalysis was positive for leukocytes and rare bacteria. A treponemal antibody test was negative.
Additionally, the punch biopsy from the right elbow revealed acanthosis, intercellular spongiosis, and subcorneal pustules consistent with localized pustular psoriasis or keratoderma blenorrhagicum. After the diagnosis was made, human leukocyte antigen B27 allele (HLA-B27) testing was conducted and was positive.
A predisposition exacerbates the infection
Reactive arthritis, a type of spondyloarthropathy, features a triad of conjunctivitis, urethritis, and arthritis that follows either gastrointestinal or urogenital infection.1 Reactive arthritis occurs with a male predominance of 3:1, and the worldwide prevalence is 1 in 3000.1 Causative bacteria include C trachomatis, Yersinia, Salmonella, Shigella, and Campylobacter, Escherichia coli, Clostridioides (formerly Clostridium) difficile, and C pneumoniae.2 Patients with the HLA-B27 allele are 50 times more likely to develop reactive arthritis following infection with the aforementioned bacteria.1
Findings consistent with a diagnosis of reactive arthritis include a recent history of gastrointestinal or urogenital illness, joint pain, conjunctivitis, oral lesions, cutaneous changes, and genital lesions.3 Diagnostic tests should include arthrocentesis with cultures or PCR and cell count, ESR, CRP, CBC, and urinalysis. HLA-B27 can be used to support the diagnosis but is not routinely recommended.2
Pustules and psoriasiform scaling characterize this diagnosis
The differential diagnosis for the signs and symptoms seen in this patient include disseminated gonococcal arthritis, psoriatic arthritis, rheumatoid arthritis, and secondary syphilis.
Gonococcal arthritis manifests with painful, sterile joints as well as pustules on the palms and soles, but not with the psoriasiform scaling and desquamation that was seen in this case. A culture or PCR from urethral discharge or pustules on the palms and soles could be used to confirm this diagnosis.3
Continue to: Psoriasis in association with psoriatic arthritis
Psoriasis in association with psoriatic arthritis and the psoriasiform rashing of reactive arthritis (keratoderma blenorrhagicum) show similar histopathology; however, patients with psoriatic arthritis generally exhibit fewer constitutional symptoms.4
Rheumatoid arthritis also manifests with joint pain and swelling, especially in the hands, wrists, and knees. This diagnosis was unlikely in this patient, where small joints were largely uninvolved.4
Secondary syphilis also manifests with papular, scaly, erythematous lesions on the palms and soles along with pityriasis rosea–like rashing on the trunk. However, it rarely produces pustules or hyperkeratotic keratoderma.5 As noted earlier, a treponemal antibody test in this patient was negative.
Drug therapy is the best option
First-line therapy for reactive arthritis consists of NSAIDs. If the patient exhibits an inadequate response after a 2-week trial, intra-articular or systemic glucocorticoids may be considered.3 If the patient fails to respond to the steroids, disease-modifying antirheumatic drugs (DMARDs) may be considered. Reactive arthritis is considered chronic if the disease lasts longer than 6 months, at which point, DMARDs or tumor necrosis factor-α inhibitors may be utilized.3 For cutaneous manifestations, such as keratoderma blenorrhagicum, topical glucocorticoids twice daily may be used along with keratolytic agents.
Our patient received 2 doses of azithromycin (500 mg IV) and 1 dose of ceftriaxone (2 g IV) to treat his infection while in the ED. Over the course of his hospital stay, he received ceftriaxone (1 g IV daily) for 6 days and naproxen (500 mg tid po) which was tapered. Additionally, he received a week of methylprednisolone (60 mg IM daily) before tapering to oral prednisone. His taper consisted of 40 mg po for 1 week and was decreased by 10 mg each week. Augmented betamethasone dipropionate 0.05% cream and urea 20% cream were prescribed for twice-daily application for the hyperkeratotic scale on both of his feet.
1. Hayes KM, Hayes RJP, Turk MA, et al. Evolving patterns of reactive arthritis. Clin Rheumatol. 2019;38:2083-2088. doi: 10.1007/s10067-019-04522-4
2. Duba AS, Mathew SD. The seronegative spondyloarthropathies. Prim Care. 2018;45:271-287. doi: 10.1016/j.pop.2018.02.005
3. Yu DT, van Tubergen A. Reactive arthritis. In: Joachim S, Romain PL, eds. UpToDate. Updated April 28, 2021. Accessed June 3, 2021. https://www.uptodate.com/contents/reactive-arthritis?search=reactive%20arthritis&topicRef=5571&source=see_link#H9
4. Barth WF, Segal K. Reactive arthritis (Reiter’s Syndrome). Am Fam Physician. 1999;60:499-503, 507.
5. Coleman E, Fiahlo A, Brateanu A. Secondary syphilis. Cleve Clin J Med. 2017;84:510-511. doi: 10.3949/ccjm.84a.16089
A 21-year-old man presented to the emergency department (ED) with a 2-month history of joint pain, swelling, and difficulty walking that began with swelling of his right knee (FIGURE 1A). The patient said that over the course of several weeks, the swelling and joint pain spread to his left knee, followed by bilateral elbows and ankles. Nonsteroidal anti-inflammatory drugs (NSAIDs) and aspirin produced only modest improvement.
Two weeks prior to presentation, the patient also experienced widespread pruritus and conjunctivitis. His past medical history was significant for a sexual encounter that resulted in urinary tract infection (UTI)–like symptoms approximately 1 month prior to the onset of his joint symptoms. He did not seek care for the UTI-like symptoms.
In the ED, the patient was febrile (102.1 °F) and tachycardic. Skin examination revealed erythematous papules, intact vesicles, and pustules with background hyperkeratosis and desquamation on his right foot (FIGURE 1B). The patient had spotty erythema on his palate and a 4-mm superficial erosion on the right penile shaft. Swelling and tenderness were noted over the elbows, knees, hands, and ankles. No inguinal lymphadenopathy was noted.

An arthrocentesis was performed on the right knee that demonstrated no organisms on Gram stain and a normal joint fluid cell count. A complete blood count (CBC), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and urinalysis were ordered. A punch biopsy was performed on a scaly patch on the right elbow.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Keratoderma blenorrhagicum
The patient’s history, clinical findings, and lab results, including a positive Chlamydia trachomatis polymerase chain reaction (PCR) test from a urethral swab, pointed to a diagnosis of keratoderma blenorrhagicum in association with reactive arthritis (following infection with C trachomatis).
Relevant diagnostic findings included an elevated CRP of 26.5 mg/L (normal range, < 10 mg/L), an elevated ESR of 116 mm/h (normal range, < 15 mm/h) and as noted, a positive C trachomatis PCR test. The patient’s white blood cell count was 9.7/μL (normal range, 4.5-11 μL) and the rest of the CBC was within normal limits. Urinalysis was positive for leukocytes and rare bacteria. A treponemal antibody test was negative.
Additionally, the punch biopsy from the right elbow revealed acanthosis, intercellular spongiosis, and subcorneal pustules consistent with localized pustular psoriasis or keratoderma blenorrhagicum. After the diagnosis was made, human leukocyte antigen B27 allele (HLA-B27) testing was conducted and was positive.
A predisposition exacerbates the infection
Reactive arthritis, a type of spondyloarthropathy, features a triad of conjunctivitis, urethritis, and arthritis that follows either gastrointestinal or urogenital infection.1 Reactive arthritis occurs with a male predominance of 3:1, and the worldwide prevalence is 1 in 3000.1 Causative bacteria include C trachomatis, Yersinia, Salmonella, Shigella, and Campylobacter, Escherichia coli, Clostridioides (formerly Clostridium) difficile, and C pneumoniae.2 Patients with the HLA-B27 allele are 50 times more likely to develop reactive arthritis following infection with the aforementioned bacteria.1
Findings consistent with a diagnosis of reactive arthritis include a recent history of gastrointestinal or urogenital illness, joint pain, conjunctivitis, oral lesions, cutaneous changes, and genital lesions.3 Diagnostic tests should include arthrocentesis with cultures or PCR and cell count, ESR, CRP, CBC, and urinalysis. HLA-B27 can be used to support the diagnosis but is not routinely recommended.2
Pustules and psoriasiform scaling characterize this diagnosis
The differential diagnosis for the signs and symptoms seen in this patient include disseminated gonococcal arthritis, psoriatic arthritis, rheumatoid arthritis, and secondary syphilis.
Gonococcal arthritis manifests with painful, sterile joints as well as pustules on the palms and soles, but not with the psoriasiform scaling and desquamation that was seen in this case. A culture or PCR from urethral discharge or pustules on the palms and soles could be used to confirm this diagnosis.3
Continue to: Psoriasis in association with psoriatic arthritis
Psoriasis in association with psoriatic arthritis and the psoriasiform rashing of reactive arthritis (keratoderma blenorrhagicum) show similar histopathology; however, patients with psoriatic arthritis generally exhibit fewer constitutional symptoms.4
Rheumatoid arthritis also manifests with joint pain and swelling, especially in the hands, wrists, and knees. This diagnosis was unlikely in this patient, where small joints were largely uninvolved.4
Secondary syphilis also manifests with papular, scaly, erythematous lesions on the palms and soles along with pityriasis rosea–like rashing on the trunk. However, it rarely produces pustules or hyperkeratotic keratoderma.5 As noted earlier, a treponemal antibody test in this patient was negative.
Drug therapy is the best option
First-line therapy for reactive arthritis consists of NSAIDs. If the patient exhibits an inadequate response after a 2-week trial, intra-articular or systemic glucocorticoids may be considered.3 If the patient fails to respond to the steroids, disease-modifying antirheumatic drugs (DMARDs) may be considered. Reactive arthritis is considered chronic if the disease lasts longer than 6 months, at which point, DMARDs or tumor necrosis factor-α inhibitors may be utilized.3 For cutaneous manifestations, such as keratoderma blenorrhagicum, topical glucocorticoids twice daily may be used along with keratolytic agents.
Our patient received 2 doses of azithromycin (500 mg IV) and 1 dose of ceftriaxone (2 g IV) to treat his infection while in the ED. Over the course of his hospital stay, he received ceftriaxone (1 g IV daily) for 6 days and naproxen (500 mg tid po) which was tapered. Additionally, he received a week of methylprednisolone (60 mg IM daily) before tapering to oral prednisone. His taper consisted of 40 mg po for 1 week and was decreased by 10 mg each week. Augmented betamethasone dipropionate 0.05% cream and urea 20% cream were prescribed for twice-daily application for the hyperkeratotic scale on both of his feet.
A 21-year-old man presented to the emergency department (ED) with a 2-month history of joint pain, swelling, and difficulty walking that began with swelling of his right knee (FIGURE 1A). The patient said that over the course of several weeks, the swelling and joint pain spread to his left knee, followed by bilateral elbows and ankles. Nonsteroidal anti-inflammatory drugs (NSAIDs) and aspirin produced only modest improvement.
Two weeks prior to presentation, the patient also experienced widespread pruritus and conjunctivitis. His past medical history was significant for a sexual encounter that resulted in urinary tract infection (UTI)–like symptoms approximately 1 month prior to the onset of his joint symptoms. He did not seek care for the UTI-like symptoms.
In the ED, the patient was febrile (102.1 °F) and tachycardic. Skin examination revealed erythematous papules, intact vesicles, and pustules with background hyperkeratosis and desquamation on his right foot (FIGURE 1B). The patient had spotty erythema on his palate and a 4-mm superficial erosion on the right penile shaft. Swelling and tenderness were noted over the elbows, knees, hands, and ankles. No inguinal lymphadenopathy was noted.

An arthrocentesis was performed on the right knee that demonstrated no organisms on Gram stain and a normal joint fluid cell count. A complete blood count (CBC), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and urinalysis were ordered. A punch biopsy was performed on a scaly patch on the right elbow.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Dx: Keratoderma blenorrhagicum
The patient’s history, clinical findings, and lab results, including a positive Chlamydia trachomatis polymerase chain reaction (PCR) test from a urethral swab, pointed to a diagnosis of keratoderma blenorrhagicum in association with reactive arthritis (following infection with C trachomatis).
Relevant diagnostic findings included an elevated CRP of 26.5 mg/L (normal range, < 10 mg/L), an elevated ESR of 116 mm/h (normal range, < 15 mm/h) and as noted, a positive C trachomatis PCR test. The patient’s white blood cell count was 9.7/μL (normal range, 4.5-11 μL) and the rest of the CBC was within normal limits. Urinalysis was positive for leukocytes and rare bacteria. A treponemal antibody test was negative.
Additionally, the punch biopsy from the right elbow revealed acanthosis, intercellular spongiosis, and subcorneal pustules consistent with localized pustular psoriasis or keratoderma blenorrhagicum. After the diagnosis was made, human leukocyte antigen B27 allele (HLA-B27) testing was conducted and was positive.
A predisposition exacerbates the infection
Reactive arthritis, a type of spondyloarthropathy, features a triad of conjunctivitis, urethritis, and arthritis that follows either gastrointestinal or urogenital infection.1 Reactive arthritis occurs with a male predominance of 3:1, and the worldwide prevalence is 1 in 3000.1 Causative bacteria include C trachomatis, Yersinia, Salmonella, Shigella, and Campylobacter, Escherichia coli, Clostridioides (formerly Clostridium) difficile, and C pneumoniae.2 Patients with the HLA-B27 allele are 50 times more likely to develop reactive arthritis following infection with the aforementioned bacteria.1
Findings consistent with a diagnosis of reactive arthritis include a recent history of gastrointestinal or urogenital illness, joint pain, conjunctivitis, oral lesions, cutaneous changes, and genital lesions.3 Diagnostic tests should include arthrocentesis with cultures or PCR and cell count, ESR, CRP, CBC, and urinalysis. HLA-B27 can be used to support the diagnosis but is not routinely recommended.2
Pustules and psoriasiform scaling characterize this diagnosis
The differential diagnosis for the signs and symptoms seen in this patient include disseminated gonococcal arthritis, psoriatic arthritis, rheumatoid arthritis, and secondary syphilis.
Gonococcal arthritis manifests with painful, sterile joints as well as pustules on the palms and soles, but not with the psoriasiform scaling and desquamation that was seen in this case. A culture or PCR from urethral discharge or pustules on the palms and soles could be used to confirm this diagnosis.3
Continue to: Psoriasis in association with psoriatic arthritis
Psoriasis in association with psoriatic arthritis and the psoriasiform rashing of reactive arthritis (keratoderma blenorrhagicum) show similar histopathology; however, patients with psoriatic arthritis generally exhibit fewer constitutional symptoms.4
Rheumatoid arthritis also manifests with joint pain and swelling, especially in the hands, wrists, and knees. This diagnosis was unlikely in this patient, where small joints were largely uninvolved.4
Secondary syphilis also manifests with papular, scaly, erythematous lesions on the palms and soles along with pityriasis rosea–like rashing on the trunk. However, it rarely produces pustules or hyperkeratotic keratoderma.5 As noted earlier, a treponemal antibody test in this patient was negative.
Drug therapy is the best option
First-line therapy for reactive arthritis consists of NSAIDs. If the patient exhibits an inadequate response after a 2-week trial, intra-articular or systemic glucocorticoids may be considered.3 If the patient fails to respond to the steroids, disease-modifying antirheumatic drugs (DMARDs) may be considered. Reactive arthritis is considered chronic if the disease lasts longer than 6 months, at which point, DMARDs or tumor necrosis factor-α inhibitors may be utilized.3 For cutaneous manifestations, such as keratoderma blenorrhagicum, topical glucocorticoids twice daily may be used along with keratolytic agents.
Our patient received 2 doses of azithromycin (500 mg IV) and 1 dose of ceftriaxone (2 g IV) to treat his infection while in the ED. Over the course of his hospital stay, he received ceftriaxone (1 g IV daily) for 6 days and naproxen (500 mg tid po) which was tapered. Additionally, he received a week of methylprednisolone (60 mg IM daily) before tapering to oral prednisone. His taper consisted of 40 mg po for 1 week and was decreased by 10 mg each week. Augmented betamethasone dipropionate 0.05% cream and urea 20% cream were prescribed for twice-daily application for the hyperkeratotic scale on both of his feet.
1. Hayes KM, Hayes RJP, Turk MA, et al. Evolving patterns of reactive arthritis. Clin Rheumatol. 2019;38:2083-2088. doi: 10.1007/s10067-019-04522-4
2. Duba AS, Mathew SD. The seronegative spondyloarthropathies. Prim Care. 2018;45:271-287. doi: 10.1016/j.pop.2018.02.005
3. Yu DT, van Tubergen A. Reactive arthritis. In: Joachim S, Romain PL, eds. UpToDate. Updated April 28, 2021. Accessed June 3, 2021. https://www.uptodate.com/contents/reactive-arthritis?search=reactive%20arthritis&topicRef=5571&source=see_link#H9
4. Barth WF, Segal K. Reactive arthritis (Reiter’s Syndrome). Am Fam Physician. 1999;60:499-503, 507.
5. Coleman E, Fiahlo A, Brateanu A. Secondary syphilis. Cleve Clin J Med. 2017;84:510-511. doi: 10.3949/ccjm.84a.16089
1. Hayes KM, Hayes RJP, Turk MA, et al. Evolving patterns of reactive arthritis. Clin Rheumatol. 2019;38:2083-2088. doi: 10.1007/s10067-019-04522-4
2. Duba AS, Mathew SD. The seronegative spondyloarthropathies. Prim Care. 2018;45:271-287. doi: 10.1016/j.pop.2018.02.005
3. Yu DT, van Tubergen A. Reactive arthritis. In: Joachim S, Romain PL, eds. UpToDate. Updated April 28, 2021. Accessed June 3, 2021. https://www.uptodate.com/contents/reactive-arthritis?search=reactive%20arthritis&topicRef=5571&source=see_link#H9
4. Barth WF, Segal K. Reactive arthritis (Reiter’s Syndrome). Am Fam Physician. 1999;60:499-503, 507.
5. Coleman E, Fiahlo A, Brateanu A. Secondary syphilis. Cleve Clin J Med. 2017;84:510-511. doi: 10.3949/ccjm.84a.16089
1 in 15 patients who start dupilumab may develop conjunctivitis, large analysis finds
showed.
“About 4 years after dupilumab’s approval, we’re interested in how conjunctivitis has played out in our daily clinical practice,” lead study investigator Maria C. Schneeweiss, MD, said during the Revolutionizing Atopic Dermatitis symposium.
Drawing from two nationwide U.S. databases, MarketScan and Optum, Dr. Schneeweiss, of the department of dermatology at Brigham and Women’s Hospital, Boston, and colleagues sought to characterize the incidence of bacterial and nonbacterial conjunctivitis among 6,730 patients with AD who started treatment with either dupilumab, methotrexate, mycophenolate, or cyclosporine between March 2017 and January 2020. They also wanted to identify patient subgroups at increased or decreased risk of dupilumab-related conjunctivitis in clinical practice.
Of the 6,730 patients, 3,755 started treatment with dupilumab, while 2,010 started with methotrexate, 536 started with mycophenolate, and 429 started with cyclosporine. Using a new-user, active-comparator study design, the researchers identified patients with AD from both databases and selected three dupilumab cohorts: dupilumab versus methotrexate (MTX), dupilumab versus mycophenolate (MMF), and dupilumab versus cyclosporine (CsA). Follow-up lasted 6 months and 1:1 propensity score matching was used to account for conjunctivitis risk factor differences. Patients with a history of conjunctivitis were excluded from the study, except one subgroup limited to those with prior conjunctivitis.
Dr. Schneeweiss reported that the overall incidence rate of conjunctivitis within 6 months of treatment initiation was 6.6% in dupilumab users, or 1 in 15 patients, compared with 3.3% in MTX users, 4.2% in MMF users, and 2.8% in CsA users. The incidence rates for the different types of conjunctivitis were as follows:
- Bacterial conjunctivitis: 1.5% in dupilumab users versus 0.95% in MTX, 0.4% in MMF, and 0.7% in CsA users.
- Allergic conjunctivitis: 2.2% in dupilumab users versus 0.8% in MTX, 0.2% in MMF, and 1.6% in CsA users.
- Keratoconjunctivitis: 0.8% in dupilumab users versus 1.1% in MTX, 1.5% in MMF, and 0.5% in CsA users.
In addition, the rate of conjunctivitis requiring ophthalmic medication was 2.6% in dupilumab users versus 0.7% in MTX, 1% in MMF, and 0.5% in CsA users.
After the researchers applied 1:1 propensity score matching, they observed that the risk of conjunctivitis within 6 months of starting treatment was increased in dupilumab users versus MTX users (relative risk, 2.12), dupilumab versus MMF users (RR, 2.43), and dupilumab versus CsA users (RR, 1.83). Among dupilumab users, the risk of conjunctivitis requiring ophthalmic medication was increased six to eightfold, compared with those who used MTX, MMF or CsA. In addition, bacterial conjunctivitis was increased 1.6- to 4.0-fold, compared with those who used MTX, MMF or CsA, but the confidence intervals were wide and included the null, while allergic conjunctivitis was increased 2.7- to 7-fold when compared with those who used MTX and MMF.
In other findings, the risk of allergic conjunctivitis was similar between dupilumab and CsA users (RR, 1.14), and there was no increased risk of keratoconjunctivitis in dupilumab users, compared with those who used MTX, MMF, or CsA. The relative risk of conjunctivitis in those who used dupilumab was further increased when the analysis was limited to AD patients with comorbid asthma (RR, 2.86), those who used systemic glucocorticoids fewer than 30 days prior (RR, 2.88), and those age 65 and older (RR, 2.57), compared with those who used methotrexate.
“Compared to AD patients who received treatment with other systemic agents, dupilumab treatment doubled the risk of conjunctivitis in clinical practice,” Dr. Schneeweiss concluded. “Risk factors that further increase the risk include comorbid asthma, use of systemic corticosteroids, and older age. It should be noted that conjunctivitis does not require treatment discontinuation and is manageable with ophthalmic medications.”
Lawrence J. Green, MD, clinical professor of dermatology at George Washington University, Washington, who was asked to comment on the study, said that the work “verifies what we see clinically: that conjunctivitis is increased among dupilumab users even when it is compared to immunosuppressive agents used to treat other conditions. Because the study is retrospective, one cannot assume all diagnosis of types of conjunctivitis or even of skin disease is entirely accurate. But, with the large numbers of claims looked at and compared, one would think its conclusions are accurate.”
Dr. Schneeweiss reported having no relevant financial disclosures. Dr. Green disclosed that he is a speaker, consultant, or investigator for Amgen, AbbVie, Arcutis, Brickell, Candescent, Cassiopeia, Dermavant, Galderma, Janssen, Forte, Incyte, MC-2, Lilly, Novartis, Novan, Ortho Dermatologics, Revance, Sun Pharma, UCB, and Vyne.
showed.
“About 4 years after dupilumab’s approval, we’re interested in how conjunctivitis has played out in our daily clinical practice,” lead study investigator Maria C. Schneeweiss, MD, said during the Revolutionizing Atopic Dermatitis symposium.
Drawing from two nationwide U.S. databases, MarketScan and Optum, Dr. Schneeweiss, of the department of dermatology at Brigham and Women’s Hospital, Boston, and colleagues sought to characterize the incidence of bacterial and nonbacterial conjunctivitis among 6,730 patients with AD who started treatment with either dupilumab, methotrexate, mycophenolate, or cyclosporine between March 2017 and January 2020. They also wanted to identify patient subgroups at increased or decreased risk of dupilumab-related conjunctivitis in clinical practice.
Of the 6,730 patients, 3,755 started treatment with dupilumab, while 2,010 started with methotrexate, 536 started with mycophenolate, and 429 started with cyclosporine. Using a new-user, active-comparator study design, the researchers identified patients with AD from both databases and selected three dupilumab cohorts: dupilumab versus methotrexate (MTX), dupilumab versus mycophenolate (MMF), and dupilumab versus cyclosporine (CsA). Follow-up lasted 6 months and 1:1 propensity score matching was used to account for conjunctivitis risk factor differences. Patients with a history of conjunctivitis were excluded from the study, except one subgroup limited to those with prior conjunctivitis.
Dr. Schneeweiss reported that the overall incidence rate of conjunctivitis within 6 months of treatment initiation was 6.6% in dupilumab users, or 1 in 15 patients, compared with 3.3% in MTX users, 4.2% in MMF users, and 2.8% in CsA users. The incidence rates for the different types of conjunctivitis were as follows:
- Bacterial conjunctivitis: 1.5% in dupilumab users versus 0.95% in MTX, 0.4% in MMF, and 0.7% in CsA users.
- Allergic conjunctivitis: 2.2% in dupilumab users versus 0.8% in MTX, 0.2% in MMF, and 1.6% in CsA users.
- Keratoconjunctivitis: 0.8% in dupilumab users versus 1.1% in MTX, 1.5% in MMF, and 0.5% in CsA users.
In addition, the rate of conjunctivitis requiring ophthalmic medication was 2.6% in dupilumab users versus 0.7% in MTX, 1% in MMF, and 0.5% in CsA users.
After the researchers applied 1:1 propensity score matching, they observed that the risk of conjunctivitis within 6 months of starting treatment was increased in dupilumab users versus MTX users (relative risk, 2.12), dupilumab versus MMF users (RR, 2.43), and dupilumab versus CsA users (RR, 1.83). Among dupilumab users, the risk of conjunctivitis requiring ophthalmic medication was increased six to eightfold, compared with those who used MTX, MMF or CsA. In addition, bacterial conjunctivitis was increased 1.6- to 4.0-fold, compared with those who used MTX, MMF or CsA, but the confidence intervals were wide and included the null, while allergic conjunctivitis was increased 2.7- to 7-fold when compared with those who used MTX and MMF.
In other findings, the risk of allergic conjunctivitis was similar between dupilumab and CsA users (RR, 1.14), and there was no increased risk of keratoconjunctivitis in dupilumab users, compared with those who used MTX, MMF, or CsA. The relative risk of conjunctivitis in those who used dupilumab was further increased when the analysis was limited to AD patients with comorbid asthma (RR, 2.86), those who used systemic glucocorticoids fewer than 30 days prior (RR, 2.88), and those age 65 and older (RR, 2.57), compared with those who used methotrexate.
“Compared to AD patients who received treatment with other systemic agents, dupilumab treatment doubled the risk of conjunctivitis in clinical practice,” Dr. Schneeweiss concluded. “Risk factors that further increase the risk include comorbid asthma, use of systemic corticosteroids, and older age. It should be noted that conjunctivitis does not require treatment discontinuation and is manageable with ophthalmic medications.”
Lawrence J. Green, MD, clinical professor of dermatology at George Washington University, Washington, who was asked to comment on the study, said that the work “verifies what we see clinically: that conjunctivitis is increased among dupilumab users even when it is compared to immunosuppressive agents used to treat other conditions. Because the study is retrospective, one cannot assume all diagnosis of types of conjunctivitis or even of skin disease is entirely accurate. But, with the large numbers of claims looked at and compared, one would think its conclusions are accurate.”
Dr. Schneeweiss reported having no relevant financial disclosures. Dr. Green disclosed that he is a speaker, consultant, or investigator for Amgen, AbbVie, Arcutis, Brickell, Candescent, Cassiopeia, Dermavant, Galderma, Janssen, Forte, Incyte, MC-2, Lilly, Novartis, Novan, Ortho Dermatologics, Revance, Sun Pharma, UCB, and Vyne.
showed.
“About 4 years after dupilumab’s approval, we’re interested in how conjunctivitis has played out in our daily clinical practice,” lead study investigator Maria C. Schneeweiss, MD, said during the Revolutionizing Atopic Dermatitis symposium.
Drawing from two nationwide U.S. databases, MarketScan and Optum, Dr. Schneeweiss, of the department of dermatology at Brigham and Women’s Hospital, Boston, and colleagues sought to characterize the incidence of bacterial and nonbacterial conjunctivitis among 6,730 patients with AD who started treatment with either dupilumab, methotrexate, mycophenolate, or cyclosporine between March 2017 and January 2020. They also wanted to identify patient subgroups at increased or decreased risk of dupilumab-related conjunctivitis in clinical practice.
Of the 6,730 patients, 3,755 started treatment with dupilumab, while 2,010 started with methotrexate, 536 started with mycophenolate, and 429 started with cyclosporine. Using a new-user, active-comparator study design, the researchers identified patients with AD from both databases and selected three dupilumab cohorts: dupilumab versus methotrexate (MTX), dupilumab versus mycophenolate (MMF), and dupilumab versus cyclosporine (CsA). Follow-up lasted 6 months and 1:1 propensity score matching was used to account for conjunctivitis risk factor differences. Patients with a history of conjunctivitis were excluded from the study, except one subgroup limited to those with prior conjunctivitis.
Dr. Schneeweiss reported that the overall incidence rate of conjunctivitis within 6 months of treatment initiation was 6.6% in dupilumab users, or 1 in 15 patients, compared with 3.3% in MTX users, 4.2% in MMF users, and 2.8% in CsA users. The incidence rates for the different types of conjunctivitis were as follows:
- Bacterial conjunctivitis: 1.5% in dupilumab users versus 0.95% in MTX, 0.4% in MMF, and 0.7% in CsA users.
- Allergic conjunctivitis: 2.2% in dupilumab users versus 0.8% in MTX, 0.2% in MMF, and 1.6% in CsA users.
- Keratoconjunctivitis: 0.8% in dupilumab users versus 1.1% in MTX, 1.5% in MMF, and 0.5% in CsA users.
In addition, the rate of conjunctivitis requiring ophthalmic medication was 2.6% in dupilumab users versus 0.7% in MTX, 1% in MMF, and 0.5% in CsA users.
After the researchers applied 1:1 propensity score matching, they observed that the risk of conjunctivitis within 6 months of starting treatment was increased in dupilumab users versus MTX users (relative risk, 2.12), dupilumab versus MMF users (RR, 2.43), and dupilumab versus CsA users (RR, 1.83). Among dupilumab users, the risk of conjunctivitis requiring ophthalmic medication was increased six to eightfold, compared with those who used MTX, MMF or CsA. In addition, bacterial conjunctivitis was increased 1.6- to 4.0-fold, compared with those who used MTX, MMF or CsA, but the confidence intervals were wide and included the null, while allergic conjunctivitis was increased 2.7- to 7-fold when compared with those who used MTX and MMF.
In other findings, the risk of allergic conjunctivitis was similar between dupilumab and CsA users (RR, 1.14), and there was no increased risk of keratoconjunctivitis in dupilumab users, compared with those who used MTX, MMF, or CsA. The relative risk of conjunctivitis in those who used dupilumab was further increased when the analysis was limited to AD patients with comorbid asthma (RR, 2.86), those who used systemic glucocorticoids fewer than 30 days prior (RR, 2.88), and those age 65 and older (RR, 2.57), compared with those who used methotrexate.
“Compared to AD patients who received treatment with other systemic agents, dupilumab treatment doubled the risk of conjunctivitis in clinical practice,” Dr. Schneeweiss concluded. “Risk factors that further increase the risk include comorbid asthma, use of systemic corticosteroids, and older age. It should be noted that conjunctivitis does not require treatment discontinuation and is manageable with ophthalmic medications.”
Lawrence J. Green, MD, clinical professor of dermatology at George Washington University, Washington, who was asked to comment on the study, said that the work “verifies what we see clinically: that conjunctivitis is increased among dupilumab users even when it is compared to immunosuppressive agents used to treat other conditions. Because the study is retrospective, one cannot assume all diagnosis of types of conjunctivitis or even of skin disease is entirely accurate. But, with the large numbers of claims looked at and compared, one would think its conclusions are accurate.”
Dr. Schneeweiss reported having no relevant financial disclosures. Dr. Green disclosed that he is a speaker, consultant, or investigator for Amgen, AbbVie, Arcutis, Brickell, Candescent, Cassiopeia, Dermavant, Galderma, Janssen, Forte, Incyte, MC-2, Lilly, Novartis, Novan, Ortho Dermatologics, Revance, Sun Pharma, UCB, and Vyne.
FROM REVOLUTIONIZING AD 2021
Trial offers first look at how tralokinumab-treated patients weather COVID-19
and all patients continued tralokinumab treatment following their diagnosis.
“This is a great first look at COVID-19 outcomes in this population,” lead study investigator Andrew Blauvelt, MD, MBA, said during the Revolutionizing Atopic Dermatitis symposium. “This suggests that tralokinumab does not significantly impact the ability to respond to SARS-CoV-2, the virus that causes COVID-19. It’s encouraging and promising.”
Tralokinumab is a fully human IgG4 monoclonal antibody that specifically binds to interleukin-13, which is a key driver of underlying inflammation in AD. An ongoing, open-label extension trial called ECZTEND is investigating the long-term safety and efficacy of tralokinumab in patients with AD who participated in previous tralokinumab trials. The purpose of the current case series is to describe the outcomes of patients diagnosed with COVID-19 while participating in ECZTEND, which is a 5-year study.
“Patients are receiving tralokinumab 300 mg every 2 weeks,” said Dr. Blauvelt, a dermatologist who is president of Oregon Medical Research Center, Portland. “They’re allowed to use topical steroids, but they’re not allowed to use other AD treatments. We do regular clinical and safety assessments throughout the study.”
As of Feb. 26, 2021, there were 51 adults with moderate to severe AD who had confirmed COVID-19 infection during treatment with tralokinumab every 2 weeks. “Patients were not required to discontinue tralokinumab treatment following a COVID-19 diagnosis, if continuation was deemed appropriate by the investigator,” Dr. Blauvelt said. Of the 51 patients, 22 were male, 29 were female, their mean age was 38 years, and their baseline body mass index was 27.6 kg/m2. Most of the patients (36, or 71%) were from Europe, 15 (29%) were from North America, and 30 (59%) had a history of asthma.
The average duration of COVID-19 infection was 15 days and severity of disease was mild in 35 patients (69%), moderate in 14 (27%), and severe in 2 (4%). According to the study abstract, those two patients had multiple risk factors and comorbidities, including obesity, chronic obstructive pulmonary disease, and cardiovascular disease. They were hospitalized for a mean of 7 days, but subsequently recovered – one with sequelae. None of the patients died.
Of the 51 COVID-19 cases, 2 were deemed to be possibly related to tralokinumab treatment by the investigator, Dr. Blauvelt said. Both were mild or moderate cases that occurred in patients younger than age 30. “Interestingly, 75% of the COVID-19 patients had no dose interruption; they continued dosing their tralokinumab every 2 weeks during and around the time they had COVID-19,” he said. “However, 25% of patients did interrupt their dosing during COVID-19 infection. That means that they either delayed or stopped dosing while they were sick.”
Of the 51 patients, 19 (37%) had received their first dose of the COVID-19 vaccine and 6 (12%) had received their second dose. “So, 12% of patients were fully vaccinated,” Dr. Blauvelt said. “We do know that the mRNA vaccines are about 95% effective in preventing COVID-19. Currently in Oregon, about 98% of our cases are in unvaccinated patients and about 2% of COVID-19 patients are fully vaccinated.”
In addition, the recently published ECZTRA5 vaccine study showed that nonlive vaccines (tetanus, diphtheria, and pertussis; and meningococcal vaccines) could be safely administered and can elicit normal immune responses in patients treated with tralokinumab.
“We sorely need COVID-19–related safety data for all of our current and emerging systemic and biologic therapies used to treat atopic dermatitis,” said Jonathan I. Silverberg, MD, PhD, MPH, director of clinical research in the division of dermatology at George Washington University, Washington, who was asked to comment about these results. “This study is important because it shows that tralokinumab was not associated with any obvious safety signals with respect to COVID-19 infections. The major limitation is that it is not a prospective study designed to assess tralokinumab efficacy in COVID-19 patients per se. However, this post hoc study provides reassuring data. We need similar or even more robust studies for other systemic therapies in AD.”
Dr. Blauvelt reported that he is an investigator and a scientific advisor for LEO Pharma, which is developing tralokinumab, and for several other pharmaceutical companies developing treatments for AD. Dr. Silverberg reported that he is a consultant to and/or an advisory board member for several pharmaceutical companies, including LEO Pharma.
and all patients continued tralokinumab treatment following their diagnosis.
“This is a great first look at COVID-19 outcomes in this population,” lead study investigator Andrew Blauvelt, MD, MBA, said during the Revolutionizing Atopic Dermatitis symposium. “This suggests that tralokinumab does not significantly impact the ability to respond to SARS-CoV-2, the virus that causes COVID-19. It’s encouraging and promising.”
Tralokinumab is a fully human IgG4 monoclonal antibody that specifically binds to interleukin-13, which is a key driver of underlying inflammation in AD. An ongoing, open-label extension trial called ECZTEND is investigating the long-term safety and efficacy of tralokinumab in patients with AD who participated in previous tralokinumab trials. The purpose of the current case series is to describe the outcomes of patients diagnosed with COVID-19 while participating in ECZTEND, which is a 5-year study.
“Patients are receiving tralokinumab 300 mg every 2 weeks,” said Dr. Blauvelt, a dermatologist who is president of Oregon Medical Research Center, Portland. “They’re allowed to use topical steroids, but they’re not allowed to use other AD treatments. We do regular clinical and safety assessments throughout the study.”
As of Feb. 26, 2021, there were 51 adults with moderate to severe AD who had confirmed COVID-19 infection during treatment with tralokinumab every 2 weeks. “Patients were not required to discontinue tralokinumab treatment following a COVID-19 diagnosis, if continuation was deemed appropriate by the investigator,” Dr. Blauvelt said. Of the 51 patients, 22 were male, 29 were female, their mean age was 38 years, and their baseline body mass index was 27.6 kg/m2. Most of the patients (36, or 71%) were from Europe, 15 (29%) were from North America, and 30 (59%) had a history of asthma.
The average duration of COVID-19 infection was 15 days and severity of disease was mild in 35 patients (69%), moderate in 14 (27%), and severe in 2 (4%). According to the study abstract, those two patients had multiple risk factors and comorbidities, including obesity, chronic obstructive pulmonary disease, and cardiovascular disease. They were hospitalized for a mean of 7 days, but subsequently recovered – one with sequelae. None of the patients died.
Of the 51 COVID-19 cases, 2 were deemed to be possibly related to tralokinumab treatment by the investigator, Dr. Blauvelt said. Both were mild or moderate cases that occurred in patients younger than age 30. “Interestingly, 75% of the COVID-19 patients had no dose interruption; they continued dosing their tralokinumab every 2 weeks during and around the time they had COVID-19,” he said. “However, 25% of patients did interrupt their dosing during COVID-19 infection. That means that they either delayed or stopped dosing while they were sick.”
Of the 51 patients, 19 (37%) had received their first dose of the COVID-19 vaccine and 6 (12%) had received their second dose. “So, 12% of patients were fully vaccinated,” Dr. Blauvelt said. “We do know that the mRNA vaccines are about 95% effective in preventing COVID-19. Currently in Oregon, about 98% of our cases are in unvaccinated patients and about 2% of COVID-19 patients are fully vaccinated.”
In addition, the recently published ECZTRA5 vaccine study showed that nonlive vaccines (tetanus, diphtheria, and pertussis; and meningococcal vaccines) could be safely administered and can elicit normal immune responses in patients treated with tralokinumab.
“We sorely need COVID-19–related safety data for all of our current and emerging systemic and biologic therapies used to treat atopic dermatitis,” said Jonathan I. Silverberg, MD, PhD, MPH, director of clinical research in the division of dermatology at George Washington University, Washington, who was asked to comment about these results. “This study is important because it shows that tralokinumab was not associated with any obvious safety signals with respect to COVID-19 infections. The major limitation is that it is not a prospective study designed to assess tralokinumab efficacy in COVID-19 patients per se. However, this post hoc study provides reassuring data. We need similar or even more robust studies for other systemic therapies in AD.”
Dr. Blauvelt reported that he is an investigator and a scientific advisor for LEO Pharma, which is developing tralokinumab, and for several other pharmaceutical companies developing treatments for AD. Dr. Silverberg reported that he is a consultant to and/or an advisory board member for several pharmaceutical companies, including LEO Pharma.
and all patients continued tralokinumab treatment following their diagnosis.
“This is a great first look at COVID-19 outcomes in this population,” lead study investigator Andrew Blauvelt, MD, MBA, said during the Revolutionizing Atopic Dermatitis symposium. “This suggests that tralokinumab does not significantly impact the ability to respond to SARS-CoV-2, the virus that causes COVID-19. It’s encouraging and promising.”
Tralokinumab is a fully human IgG4 monoclonal antibody that specifically binds to interleukin-13, which is a key driver of underlying inflammation in AD. An ongoing, open-label extension trial called ECZTEND is investigating the long-term safety and efficacy of tralokinumab in patients with AD who participated in previous tralokinumab trials. The purpose of the current case series is to describe the outcomes of patients diagnosed with COVID-19 while participating in ECZTEND, which is a 5-year study.
“Patients are receiving tralokinumab 300 mg every 2 weeks,” said Dr. Blauvelt, a dermatologist who is president of Oregon Medical Research Center, Portland. “They’re allowed to use topical steroids, but they’re not allowed to use other AD treatments. We do regular clinical and safety assessments throughout the study.”
As of Feb. 26, 2021, there were 51 adults with moderate to severe AD who had confirmed COVID-19 infection during treatment with tralokinumab every 2 weeks. “Patients were not required to discontinue tralokinumab treatment following a COVID-19 diagnosis, if continuation was deemed appropriate by the investigator,” Dr. Blauvelt said. Of the 51 patients, 22 were male, 29 were female, their mean age was 38 years, and their baseline body mass index was 27.6 kg/m2. Most of the patients (36, or 71%) were from Europe, 15 (29%) were from North America, and 30 (59%) had a history of asthma.
The average duration of COVID-19 infection was 15 days and severity of disease was mild in 35 patients (69%), moderate in 14 (27%), and severe in 2 (4%). According to the study abstract, those two patients had multiple risk factors and comorbidities, including obesity, chronic obstructive pulmonary disease, and cardiovascular disease. They were hospitalized for a mean of 7 days, but subsequently recovered – one with sequelae. None of the patients died.
Of the 51 COVID-19 cases, 2 were deemed to be possibly related to tralokinumab treatment by the investigator, Dr. Blauvelt said. Both were mild or moderate cases that occurred in patients younger than age 30. “Interestingly, 75% of the COVID-19 patients had no dose interruption; they continued dosing their tralokinumab every 2 weeks during and around the time they had COVID-19,” he said. “However, 25% of patients did interrupt their dosing during COVID-19 infection. That means that they either delayed or stopped dosing while they were sick.”
Of the 51 patients, 19 (37%) had received their first dose of the COVID-19 vaccine and 6 (12%) had received their second dose. “So, 12% of patients were fully vaccinated,” Dr. Blauvelt said. “We do know that the mRNA vaccines are about 95% effective in preventing COVID-19. Currently in Oregon, about 98% of our cases are in unvaccinated patients and about 2% of COVID-19 patients are fully vaccinated.”
In addition, the recently published ECZTRA5 vaccine study showed that nonlive vaccines (tetanus, diphtheria, and pertussis; and meningococcal vaccines) could be safely administered and can elicit normal immune responses in patients treated with tralokinumab.
“We sorely need COVID-19–related safety data for all of our current and emerging systemic and biologic therapies used to treat atopic dermatitis,” said Jonathan I. Silverberg, MD, PhD, MPH, director of clinical research in the division of dermatology at George Washington University, Washington, who was asked to comment about these results. “This study is important because it shows that tralokinumab was not associated with any obvious safety signals with respect to COVID-19 infections. The major limitation is that it is not a prospective study designed to assess tralokinumab efficacy in COVID-19 patients per se. However, this post hoc study provides reassuring data. We need similar or even more robust studies for other systemic therapies in AD.”
Dr. Blauvelt reported that he is an investigator and a scientific advisor for LEO Pharma, which is developing tralokinumab, and for several other pharmaceutical companies developing treatments for AD. Dr. Silverberg reported that he is a consultant to and/or an advisory board member for several pharmaceutical companies, including LEO Pharma.
FROM REVOLUTIONIZING AD 2021
Performance matters in adenoma detection
Low adenoma detection rates (ADRs) were associated with a greater risk of death in colorectal cancer (CRC) patients, especially among those with high-risk adenomas, based on a review of more than 250,000 colonoscopies.
“Both performance quality of the endoscopist as well as specific characteristics of resected adenomas at colonoscopy are associated with colorectal cancer mortality,” but the impact of these combined factors on colorectal cancer mortality has not been examined on a large scale, according to Elisabeth A. Waldmann, MD, of the Medical University of Vienna and colleagues.
In a study published in Clinical Gastroenterology & Hepatology, the researchers reviewed 259,885 colonoscopies performed by 361 endoscopists. Over an average follow-up period of 59 months, 165 CRC-related deaths occurred.
Across all risk groups, CRC mortality was higher among patients whose colonoscopies yielded an ADR of less than 25%, although this was not statistically significant in all groups.
The researchers then stratified patients into those with a negative colonoscopy, those with low-risk adenomas (one to two adenomas less than 10 mm), and those with high-risk adenomas (advanced adenomas or at least three adenomas), with the negative colonoscopy group used as the reference group for comparisons. The average age of the patients was 61 years, and approximately half were women.
Endoscopists were classified as having an ADR of less than 25% or 25% and higher.
Among individuals with low-risk adenomas, CRC mortality was similar whether the ADR on a negative colonoscopy was less than 25% or 25% or higher (adjusted hazard ratios, 1.25 and 1.22, respectively). CRC mortality also remained unaffected by ADR in patients with negatively colonoscopies (aHR, 1.27).
By contrast, individuals with high-risk adenomas had a significantly increased risk of CRC death if their colonoscopy was performed by an endoscopist with an ADR of less than 25%, compared with those whose endoscopists had ADRs of 25% or higher (aHR, 2.25 and 1.35, respectively).
“Our study demonstrated that adding ADR to the risk stratification model improved risk assessment in all risk groups,” the researchers noted. “Importantly, stratification improved most for individuals with high-risk adenomas, the group demanding most resources in health care systems.”
The study findings were limited by several factors including the focus on only screening and surveillance colonoscopies, not including diagnostic colonoscopies, and the inability to adjust for comorbidities and lifestyle factors that might impact CRC mortality, the researchers noted. The 22.4% average ADR in the current study was low, compared with other studies, and could be a limitation as well, although previous guidelines recommend a target ADR of at least 20%.
“Despite the extensive body of literature supporting the importance of ADR in terms of CRC prevention, its implementation into clinical surveillance is challenging,” as physicians under pressure might try to game their ADRs, the researchers wrote.
The findings support the value of mandatory assessment of performance quality, the researchers added. However, “because of the potential possibility of gaming one’s ADR one conclusion drawn by the study results should be that endoscopists’ quality parameters should be monitored and those not meeting the standards trained to improve rather than requiring minimum ADRs as premise for offering screening colonoscopy.”
Improve performance, but don’t discount patient factors
The study is important at this time because colorectal cancer is the third-leading cause of cancer death in the United States, Atsushi Sakuraba, MD, of the University of Chicago said in an interview.
“Screening colonoscopy has been shown to decrease CRC mortality, but factors influencing outcomes after screening colonoscopies remain to be determined,” he said.
“It was expected that high-quality colonoscopy performed by an endoscopist with ADR of 25% or greater was associated with a lower risk for CRC death,” Dr. Sakuraba said. “The strength of the study is that the authors demonstrated that high-quality colonoscopy was more important in individuals with high-risk adenomas, such as advanced adenomas or at least three adenomas.”
The study findings have implications for practice in that they show the importance of monitoring performance quality in screening colonoscopy, Dr. Sakuraba said, “especially when patients have high-risk adenomas.” However, “the authors included only age and sex as variables, but the influence of other factors, such as smoking, [body mass index], and race, need to be studied.”
The researchers had no financial conflicts to disclose. Dr. Sakuraba had no financial conflicts to disclose.
Low adenoma detection rates (ADRs) were associated with a greater risk of death in colorectal cancer (CRC) patients, especially among those with high-risk adenomas, based on a review of more than 250,000 colonoscopies.
“Both performance quality of the endoscopist as well as specific characteristics of resected adenomas at colonoscopy are associated with colorectal cancer mortality,” but the impact of these combined factors on colorectal cancer mortality has not been examined on a large scale, according to Elisabeth A. Waldmann, MD, of the Medical University of Vienna and colleagues.
In a study published in Clinical Gastroenterology & Hepatology, the researchers reviewed 259,885 colonoscopies performed by 361 endoscopists. Over an average follow-up period of 59 months, 165 CRC-related deaths occurred.
Across all risk groups, CRC mortality was higher among patients whose colonoscopies yielded an ADR of less than 25%, although this was not statistically significant in all groups.
The researchers then stratified patients into those with a negative colonoscopy, those with low-risk adenomas (one to two adenomas less than 10 mm), and those with high-risk adenomas (advanced adenomas or at least three adenomas), with the negative colonoscopy group used as the reference group for comparisons. The average age of the patients was 61 years, and approximately half were women.
Endoscopists were classified as having an ADR of less than 25% or 25% and higher.
Among individuals with low-risk adenomas, CRC mortality was similar whether the ADR on a negative colonoscopy was less than 25% or 25% or higher (adjusted hazard ratios, 1.25 and 1.22, respectively). CRC mortality also remained unaffected by ADR in patients with negatively colonoscopies (aHR, 1.27).
By contrast, individuals with high-risk adenomas had a significantly increased risk of CRC death if their colonoscopy was performed by an endoscopist with an ADR of less than 25%, compared with those whose endoscopists had ADRs of 25% or higher (aHR, 2.25 and 1.35, respectively).
“Our study demonstrated that adding ADR to the risk stratification model improved risk assessment in all risk groups,” the researchers noted. “Importantly, stratification improved most for individuals with high-risk adenomas, the group demanding most resources in health care systems.”
The study findings were limited by several factors including the focus on only screening and surveillance colonoscopies, not including diagnostic colonoscopies, and the inability to adjust for comorbidities and lifestyle factors that might impact CRC mortality, the researchers noted. The 22.4% average ADR in the current study was low, compared with other studies, and could be a limitation as well, although previous guidelines recommend a target ADR of at least 20%.
“Despite the extensive body of literature supporting the importance of ADR in terms of CRC prevention, its implementation into clinical surveillance is challenging,” as physicians under pressure might try to game their ADRs, the researchers wrote.
The findings support the value of mandatory assessment of performance quality, the researchers added. However, “because of the potential possibility of gaming one’s ADR one conclusion drawn by the study results should be that endoscopists’ quality parameters should be monitored and those not meeting the standards trained to improve rather than requiring minimum ADRs as premise for offering screening colonoscopy.”
Improve performance, but don’t discount patient factors
The study is important at this time because colorectal cancer is the third-leading cause of cancer death in the United States, Atsushi Sakuraba, MD, of the University of Chicago said in an interview.
“Screening colonoscopy has been shown to decrease CRC mortality, but factors influencing outcomes after screening colonoscopies remain to be determined,” he said.
“It was expected that high-quality colonoscopy performed by an endoscopist with ADR of 25% or greater was associated with a lower risk for CRC death,” Dr. Sakuraba said. “The strength of the study is that the authors demonstrated that high-quality colonoscopy was more important in individuals with high-risk adenomas, such as advanced adenomas or at least three adenomas.”
The study findings have implications for practice in that they show the importance of monitoring performance quality in screening colonoscopy, Dr. Sakuraba said, “especially when patients have high-risk adenomas.” However, “the authors included only age and sex as variables, but the influence of other factors, such as smoking, [body mass index], and race, need to be studied.”
The researchers had no financial conflicts to disclose. Dr. Sakuraba had no financial conflicts to disclose.
Low adenoma detection rates (ADRs) were associated with a greater risk of death in colorectal cancer (CRC) patients, especially among those with high-risk adenomas, based on a review of more than 250,000 colonoscopies.
“Both performance quality of the endoscopist as well as specific characteristics of resected adenomas at colonoscopy are associated with colorectal cancer mortality,” but the impact of these combined factors on colorectal cancer mortality has not been examined on a large scale, according to Elisabeth A. Waldmann, MD, of the Medical University of Vienna and colleagues.
In a study published in Clinical Gastroenterology & Hepatology, the researchers reviewed 259,885 colonoscopies performed by 361 endoscopists. Over an average follow-up period of 59 months, 165 CRC-related deaths occurred.
Across all risk groups, CRC mortality was higher among patients whose colonoscopies yielded an ADR of less than 25%, although this was not statistically significant in all groups.
The researchers then stratified patients into those with a negative colonoscopy, those with low-risk adenomas (one to two adenomas less than 10 mm), and those with high-risk adenomas (advanced adenomas or at least three adenomas), with the negative colonoscopy group used as the reference group for comparisons. The average age of the patients was 61 years, and approximately half were women.
Endoscopists were classified as having an ADR of less than 25% or 25% and higher.
Among individuals with low-risk adenomas, CRC mortality was similar whether the ADR on a negative colonoscopy was less than 25% or 25% or higher (adjusted hazard ratios, 1.25 and 1.22, respectively). CRC mortality also remained unaffected by ADR in patients with negatively colonoscopies (aHR, 1.27).
By contrast, individuals with high-risk adenomas had a significantly increased risk of CRC death if their colonoscopy was performed by an endoscopist with an ADR of less than 25%, compared with those whose endoscopists had ADRs of 25% or higher (aHR, 2.25 and 1.35, respectively).
“Our study demonstrated that adding ADR to the risk stratification model improved risk assessment in all risk groups,” the researchers noted. “Importantly, stratification improved most for individuals with high-risk adenomas, the group demanding most resources in health care systems.”
The study findings were limited by several factors including the focus on only screening and surveillance colonoscopies, not including diagnostic colonoscopies, and the inability to adjust for comorbidities and lifestyle factors that might impact CRC mortality, the researchers noted. The 22.4% average ADR in the current study was low, compared with other studies, and could be a limitation as well, although previous guidelines recommend a target ADR of at least 20%.
“Despite the extensive body of literature supporting the importance of ADR in terms of CRC prevention, its implementation into clinical surveillance is challenging,” as physicians under pressure might try to game their ADRs, the researchers wrote.
The findings support the value of mandatory assessment of performance quality, the researchers added. However, “because of the potential possibility of gaming one’s ADR one conclusion drawn by the study results should be that endoscopists’ quality parameters should be monitored and those not meeting the standards trained to improve rather than requiring minimum ADRs as premise for offering screening colonoscopy.”
Improve performance, but don’t discount patient factors
The study is important at this time because colorectal cancer is the third-leading cause of cancer death in the United States, Atsushi Sakuraba, MD, of the University of Chicago said in an interview.
“Screening colonoscopy has been shown to decrease CRC mortality, but factors influencing outcomes after screening colonoscopies remain to be determined,” he said.
“It was expected that high-quality colonoscopy performed by an endoscopist with ADR of 25% or greater was associated with a lower risk for CRC death,” Dr. Sakuraba said. “The strength of the study is that the authors demonstrated that high-quality colonoscopy was more important in individuals with high-risk adenomas, such as advanced adenomas or at least three adenomas.”
The study findings have implications for practice in that they show the importance of monitoring performance quality in screening colonoscopy, Dr. Sakuraba said, “especially when patients have high-risk adenomas.” However, “the authors included only age and sex as variables, but the influence of other factors, such as smoking, [body mass index], and race, need to be studied.”
The researchers had no financial conflicts to disclose. Dr. Sakuraba had no financial conflicts to disclose.
FROM CLINICAL GASTROENTEROLOGY & HEPATOLOGY
Fewer dangerous COPD flare-ups during COVID-19
Public health precautions meant to reduce the spread of COVID-19 may have had an unintended but happy side effect.
They may also have benefited individuals who have chronic obstructive pulmonary disease (COPD), according to a new study.
During the pandemic, admissions for COPD flare-ups dropped dramatically – by 53% – at University of Maryland Medical System hospitals.
Researchers at the university suspect this was the result of a drop in circulating seasonal respiratory viruses, such as influenza. They theorized that stay-at-home orders, social distancing, mask mandates, and strict limits on large gatherings reduced exposure not only to COVID but also to other respiratory infections.
“Our study shows there’s a silver lining to the behavior changes beyond protecting against COVID-19,” said senior author Robert Reed, MD, a pulmonologist and professor of medicine.
COPD is a group of lung diseases that worsen over time and make it hard to breathe. Before the pandemic, they were the fourth-leading cause of death worldwide, commonly triggered by tobacco smoke and dirty air. Nearly half of flare-ups are caused by seasonal respiratory viruses.
For the study, the researchers analyzed data from 13 UMMS hospitals, comparing weekly admissions for COPD in 2018 and 2019, with admissions after April 1, 2020, when COVID-19 public health measures were introduced. Investigators chose the same six-month period in each year for comparison – April 1 to Sept. 30.
The findings were matched against U.S. federal data on respiratory viral trends between Jan. 1, 2018, and Oct. 1, 2020.
As significant as was the system’s 53% drop in COPD admissions during the pandemic, there was also a 36% decline in weekly admissions for such serious conditions as congestive heart failure, diabetes and heart attack, said co–lead author Jennifer So, MD. She’s an assistant professor of medicine and COPD specialist.
The researchers warned that a full return to normal may again expose COPD patients to the familiar seasonal triggers.
“If we completely eliminate masks and distancing during cold and flu season, we’ll allow all those viruses that have been effectively suppressed to come raging back,” Dr. Reed said in a university news release. “There could be a lot of illness.”
He noted that the study did not assess which measures tamed seasonal viruses. But, Dr. Reed added, “a simple thing like wearing a mask while riding on public transit or working from home when you’re sick with a cold could go a long way to reduce virus exposure.”
Dr. So said it is a cultural norm in her native South Korea to wear masks during the winter.
“The COVID-19 pandemic has helped a lot of people around the world become more aware of the role of masking and social distancing to reduce the spread of disease,” she said in the release.
The findings were recently published in the preprint server medRxiv and have not yet been peer reviewed.
The U.S. Centers for Disease Control and Prevention has more information on COVID-19 and chronic lung diseases.
A version of this article first appeared on WebMD.com.
Public health precautions meant to reduce the spread of COVID-19 may have had an unintended but happy side effect.
They may also have benefited individuals who have chronic obstructive pulmonary disease (COPD), according to a new study.
During the pandemic, admissions for COPD flare-ups dropped dramatically – by 53% – at University of Maryland Medical System hospitals.
Researchers at the university suspect this was the result of a drop in circulating seasonal respiratory viruses, such as influenza. They theorized that stay-at-home orders, social distancing, mask mandates, and strict limits on large gatherings reduced exposure not only to COVID but also to other respiratory infections.
“Our study shows there’s a silver lining to the behavior changes beyond protecting against COVID-19,” said senior author Robert Reed, MD, a pulmonologist and professor of medicine.
COPD is a group of lung diseases that worsen over time and make it hard to breathe. Before the pandemic, they were the fourth-leading cause of death worldwide, commonly triggered by tobacco smoke and dirty air. Nearly half of flare-ups are caused by seasonal respiratory viruses.
For the study, the researchers analyzed data from 13 UMMS hospitals, comparing weekly admissions for COPD in 2018 and 2019, with admissions after April 1, 2020, when COVID-19 public health measures were introduced. Investigators chose the same six-month period in each year for comparison – April 1 to Sept. 30.
The findings were matched against U.S. federal data on respiratory viral trends between Jan. 1, 2018, and Oct. 1, 2020.
As significant as was the system’s 53% drop in COPD admissions during the pandemic, there was also a 36% decline in weekly admissions for such serious conditions as congestive heart failure, diabetes and heart attack, said co–lead author Jennifer So, MD. She’s an assistant professor of medicine and COPD specialist.
The researchers warned that a full return to normal may again expose COPD patients to the familiar seasonal triggers.
“If we completely eliminate masks and distancing during cold and flu season, we’ll allow all those viruses that have been effectively suppressed to come raging back,” Dr. Reed said in a university news release. “There could be a lot of illness.”
He noted that the study did not assess which measures tamed seasonal viruses. But, Dr. Reed added, “a simple thing like wearing a mask while riding on public transit or working from home when you’re sick with a cold could go a long way to reduce virus exposure.”
Dr. So said it is a cultural norm in her native South Korea to wear masks during the winter.
“The COVID-19 pandemic has helped a lot of people around the world become more aware of the role of masking and social distancing to reduce the spread of disease,” she said in the release.
The findings were recently published in the preprint server medRxiv and have not yet been peer reviewed.
The U.S. Centers for Disease Control and Prevention has more information on COVID-19 and chronic lung diseases.
A version of this article first appeared on WebMD.com.
Public health precautions meant to reduce the spread of COVID-19 may have had an unintended but happy side effect.
They may also have benefited individuals who have chronic obstructive pulmonary disease (COPD), according to a new study.
During the pandemic, admissions for COPD flare-ups dropped dramatically – by 53% – at University of Maryland Medical System hospitals.
Researchers at the university suspect this was the result of a drop in circulating seasonal respiratory viruses, such as influenza. They theorized that stay-at-home orders, social distancing, mask mandates, and strict limits on large gatherings reduced exposure not only to COVID but also to other respiratory infections.
“Our study shows there’s a silver lining to the behavior changes beyond protecting against COVID-19,” said senior author Robert Reed, MD, a pulmonologist and professor of medicine.
COPD is a group of lung diseases that worsen over time and make it hard to breathe. Before the pandemic, they were the fourth-leading cause of death worldwide, commonly triggered by tobacco smoke and dirty air. Nearly half of flare-ups are caused by seasonal respiratory viruses.
For the study, the researchers analyzed data from 13 UMMS hospitals, comparing weekly admissions for COPD in 2018 and 2019, with admissions after April 1, 2020, when COVID-19 public health measures were introduced. Investigators chose the same six-month period in each year for comparison – April 1 to Sept. 30.
The findings were matched against U.S. federal data on respiratory viral trends between Jan. 1, 2018, and Oct. 1, 2020.
As significant as was the system’s 53% drop in COPD admissions during the pandemic, there was also a 36% decline in weekly admissions for such serious conditions as congestive heart failure, diabetes and heart attack, said co–lead author Jennifer So, MD. She’s an assistant professor of medicine and COPD specialist.
The researchers warned that a full return to normal may again expose COPD patients to the familiar seasonal triggers.
“If we completely eliminate masks and distancing during cold and flu season, we’ll allow all those viruses that have been effectively suppressed to come raging back,” Dr. Reed said in a university news release. “There could be a lot of illness.”
He noted that the study did not assess which measures tamed seasonal viruses. But, Dr. Reed added, “a simple thing like wearing a mask while riding on public transit or working from home when you’re sick with a cold could go a long way to reduce virus exposure.”
Dr. So said it is a cultural norm in her native South Korea to wear masks during the winter.
“The COVID-19 pandemic has helped a lot of people around the world become more aware of the role of masking and social distancing to reduce the spread of disease,” she said in the release.
The findings were recently published in the preprint server medRxiv and have not yet been peer reviewed.
The U.S. Centers for Disease Control and Prevention has more information on COVID-19 and chronic lung diseases.
A version of this article first appeared on WebMD.com.
Key strategies for managing breast cancer brain metastases
Brain metastases remain a frequent and often fatal consequence of metastatic breast cancer (MBC). MBC carries a median survival of about 3 years, but that rate drops significantly when cancer cells move to the brain. A recent analysis estimates median survival in patients with brain metastases ranges from 6 months in triple-negative breast cancer (TNBC) to 21 months in human epidermal growth factor receptor 2 (HER2)–positive disease.
This news organization spoke to Kevin M. Kalinsky, MD, acting associate professor in the department of hematology and medical oncology at Emory University School of Medicine in Atlanta and director of the Glenn Family Breast Center at the Winship Cancer Institute of Emory University, about the risk for brain metastases in patients with MBC, strategies for screening and treatment, and the work being done to achieve a better understanding of the disease.
Question: Before we dig into strategies to manage MBC brain metastasis, let’s talk about the risks. When and how often do brain metastases present in patients with MBC? What factors increase the likelihood of developing brain metastasis?
Dr. Kalinsky: The biggest risk factor for MBC spreading to the central nervous system (CNS), which includes the brain and spine, is breast cancer subtype. For patients with metastatic TNBC, the risk for brain metastasis can be more than 50%. For patients with HER2-positive disease, the risk may be slightly lower, with estimates in the range of 25%-50%, whereas the likelihood of brain metastasis in patients with hormone receptor–positive MBC is significantly lower at close to 14%. In addition, patients with metastatic TNBC may have brain metastases a little earlier in their disease progression compared with patients with HER2-positive or estrogen receptor–positive breast cancers, where brain metastases generally develop a little later in the disease course.
At what point is it recommended to screen patients with MBC for brain metastasis?
Current guidelines suggest that we scan for brain metastasis in the presence of new neurologic symptoms, such as headache, dizziness, or weakness in the arms or legs. MRI, in particular, is useful for evaluating brain metastasis, especially for smaller lesions, but lesions are sometimes detected through CT imaging of the head, too.
That’s where the guidelines are now. But as our systemic agents improve, there’s always the possibility these recommendations will be revisited and potentially include imaging as screening tools in asymptomatic patients, as well.
How do you assess which patients with MBC should receive local therapy?
Increasingly, because our systemic therapies in breast cancer are getting better in terms of crossing the blood-brain barrier, we think about local therapy on a case-by-case basis. We think about it with the question of whether we delay surgery or radiation — whole brain radiation, in particular — given concerns surrounding the side effects of these modalities, namely cognitive dysfunction for radiation and increased risk of bleeding and infection for surgery.
Giving a patient-directed local therapy, such as Gamma Knife radiosurgery or whole-brain radiotherapy, ultimately depends on the burden of brain metastasis, the status of systemic disease outside of the brain, and the number and size of the lesions seen on imaging. If, for instance, a patient has a large lesion that will immediately impact their neurologic status, we may opt to resect the lesion. If there are innumerable lesions, some of which are large, we may do whole-brain radiotherapy. If, however, a patient has systemic disease that is largely under control but is experiencing local progression in the brain, we may use local radiotherapy while continuing systemic therapy.
What about systemic therapies that cross the blood-brain barrier? What’s available now and how do you choose among the options?The subtype of breast cancer informs treatment with systemic therapies. For instance, patients with HER2-positive disease may receive oral tyrosine kinase inhibitors, such as tucatinib, neratinib, and lapatinib, which have strong CNS penetration. For patients with estrogen receptor–positive, HER2-negative MBC, estrogen therapies including aromatase inhibitors, as well as targeted therapies such as the mTOR inhibitor everolimus, have good CNS penetration. For patients with metastatic TNBC, we have chemotherapies that cross the blood-brain barrier, such as capecitabine and platinum-based chemotherapy.
Evidence suggests that tumors in the brain may harbor different genetic abnormalities from tumors in the breast. How do you consider the potential genetic heterogeneity in CNS tumors vs. the primary breast tumor?When a patient’s disease has spread to the brain, we may preferentially use agents we know cross the blood-brain barrier, so we can obtain systemic control both intracranially and extracranially. If we have already resected or biopsied cancerous brain tissue, it’s good to check the tumor’s estrogen receptor, progesterone receptor, and HER2 status and do next-generation sequencing to see if the tumor has any other targetable mutations, such as PIK3CA mutations.
But when a patient has multiple lesions, we don’t go in and biopsy all of them to check for heterogeneity. We have to make decisions based on samples we have. In cases where we start systemic therapy and notice one lesion is not responding to these agents while others are, the nonresponsive lesion may be an outlier in terms of its biologic characteristics. It may be worth targeting that lesion for biopsy and further sequencing to determine the next best systemic approach.
How do quality of life considerations factor into the management of patients with MBC brain metastases?
We use a multidisciplinary approach when treating patients. This means patient care involves a team of experts, which can include medical oncologists, radiation oncologists, and neuro-oncologists who help determine a treatment plan that takes factors such as survival and quality of life into account.
This is why, for example, we try to delay whole brain radiotherapy when we can. The HER2CLIMB study, which led to the approval of tucatinib as a treatment option for patients with HER2-positive MBC, showed us that patients with treated or untreated brain metastases receiving systemic therapy before local therapy could benefit from the combination of tucatinib, trastuzumab, and capecitabine. These patients exhibited a median progression-free survival of 7.6 months compared with 5.4 months in the placebo group.
HER2CLIMB has been practice changing because it showed us that tucatinib has good CNS activity in patients with brain metastases. The HER2CLIMB findings raise an important question: As our systemic therapies improve, how aggressive do we need to be with local therapy? Can we push off modalities like whole-brain radiotherapy, which are associated with toxicity?
This study also highlights how important it is for patients with metastatic disease to seek clinical trials. Although some trials exclude patients with brain metastases and others may have criteria that require the stability of brain metastasis for a certain amount of time, the knowledge gained can be invaluable.
Where are some of the main gaps in our understanding of brain metastases in patients with MBC?
One issue is our understanding of tropism to the brain. In other words, why does MBC spread to the brain? Once we understand this key piece, we can work on developing more effective therapies and therapeutic combinations to block brain metastasis.
For hormone receptor–positive disease, in particular, a central question is whether the current antiestrogen therapies — such as selective estrogen receptor degraders like fulvestrant, as well as targeted AKT inhibitors — have the potential to affect brain tumor activity. The same holds true for TNBC, where antibody drug conjugates and immunotherapies are being evaluated for treatment of brain tumors. For patients with HER2-positive MBC that has spread to the brain, understanding the continued role for tyrosine kinase inhibitors, such as tucatinib and neratinib, as well as whether antibody drug conjugates, including trastuzumab deruxtecan and trastuzumab emtansine, have CNS activity are important areas to explore further.
The CompassHER2 trial, going on now, is randomizing patients with residual HER2-positive disease after neoadjuvant chemotherapy and HER2-targeted therapy to receive trastuzumab emtansine with or without tucatinib. One of the core questions of this study is whether trastuzumab emtansine/tucatinib lowers the rate of brain metastasis and the incidence of systemic metastasis.
Another area in MBC that requires greater scrutiny is patients who develop leptomeningeal disease, which is when cancer cells spread to the cerebrospinal fluid. These patients have a particularly poor prognosis, and it would be helpful to evaluate the efficacy of existing therapies, but these patients are often excluded from clinical trials.
Overall, the ultimate goal in these endeavors is to decrease the rate of metastasis to the brain and improve survival and quality of life in patients with MBC who do experience brain metastases.
A version of this article first appeared on Medscape.com.
Brain metastases remain a frequent and often fatal consequence of metastatic breast cancer (MBC). MBC carries a median survival of about 3 years, but that rate drops significantly when cancer cells move to the brain. A recent analysis estimates median survival in patients with brain metastases ranges from 6 months in triple-negative breast cancer (TNBC) to 21 months in human epidermal growth factor receptor 2 (HER2)–positive disease.
This news organization spoke to Kevin M. Kalinsky, MD, acting associate professor in the department of hematology and medical oncology at Emory University School of Medicine in Atlanta and director of the Glenn Family Breast Center at the Winship Cancer Institute of Emory University, about the risk for brain metastases in patients with MBC, strategies for screening and treatment, and the work being done to achieve a better understanding of the disease.
Question: Before we dig into strategies to manage MBC brain metastasis, let’s talk about the risks. When and how often do brain metastases present in patients with MBC? What factors increase the likelihood of developing brain metastasis?
Dr. Kalinsky: The biggest risk factor for MBC spreading to the central nervous system (CNS), which includes the brain and spine, is breast cancer subtype. For patients with metastatic TNBC, the risk for brain metastasis can be more than 50%. For patients with HER2-positive disease, the risk may be slightly lower, with estimates in the range of 25%-50%, whereas the likelihood of brain metastasis in patients with hormone receptor–positive MBC is significantly lower at close to 14%. In addition, patients with metastatic TNBC may have brain metastases a little earlier in their disease progression compared with patients with HER2-positive or estrogen receptor–positive breast cancers, where brain metastases generally develop a little later in the disease course.
At what point is it recommended to screen patients with MBC for brain metastasis?
Current guidelines suggest that we scan for brain metastasis in the presence of new neurologic symptoms, such as headache, dizziness, or weakness in the arms or legs. MRI, in particular, is useful for evaluating brain metastasis, especially for smaller lesions, but lesions are sometimes detected through CT imaging of the head, too.
That’s where the guidelines are now. But as our systemic agents improve, there’s always the possibility these recommendations will be revisited and potentially include imaging as screening tools in asymptomatic patients, as well.
How do you assess which patients with MBC should receive local therapy?
Increasingly, because our systemic therapies in breast cancer are getting better in terms of crossing the blood-brain barrier, we think about local therapy on a case-by-case basis. We think about it with the question of whether we delay surgery or radiation — whole brain radiation, in particular — given concerns surrounding the side effects of these modalities, namely cognitive dysfunction for radiation and increased risk of bleeding and infection for surgery.
Giving a patient-directed local therapy, such as Gamma Knife radiosurgery or whole-brain radiotherapy, ultimately depends on the burden of brain metastasis, the status of systemic disease outside of the brain, and the number and size of the lesions seen on imaging. If, for instance, a patient has a large lesion that will immediately impact their neurologic status, we may opt to resect the lesion. If there are innumerable lesions, some of which are large, we may do whole-brain radiotherapy. If, however, a patient has systemic disease that is largely under control but is experiencing local progression in the brain, we may use local radiotherapy while continuing systemic therapy.
What about systemic therapies that cross the blood-brain barrier? What’s available now and how do you choose among the options?The subtype of breast cancer informs treatment with systemic therapies. For instance, patients with HER2-positive disease may receive oral tyrosine kinase inhibitors, such as tucatinib, neratinib, and lapatinib, which have strong CNS penetration. For patients with estrogen receptor–positive, HER2-negative MBC, estrogen therapies including aromatase inhibitors, as well as targeted therapies such as the mTOR inhibitor everolimus, have good CNS penetration. For patients with metastatic TNBC, we have chemotherapies that cross the blood-brain barrier, such as capecitabine and platinum-based chemotherapy.
Evidence suggests that tumors in the brain may harbor different genetic abnormalities from tumors in the breast. How do you consider the potential genetic heterogeneity in CNS tumors vs. the primary breast tumor?When a patient’s disease has spread to the brain, we may preferentially use agents we know cross the blood-brain barrier, so we can obtain systemic control both intracranially and extracranially. If we have already resected or biopsied cancerous brain tissue, it’s good to check the tumor’s estrogen receptor, progesterone receptor, and HER2 status and do next-generation sequencing to see if the tumor has any other targetable mutations, such as PIK3CA mutations.
But when a patient has multiple lesions, we don’t go in and biopsy all of them to check for heterogeneity. We have to make decisions based on samples we have. In cases where we start systemic therapy and notice one lesion is not responding to these agents while others are, the nonresponsive lesion may be an outlier in terms of its biologic characteristics. It may be worth targeting that lesion for biopsy and further sequencing to determine the next best systemic approach.
How do quality of life considerations factor into the management of patients with MBC brain metastases?
We use a multidisciplinary approach when treating patients. This means patient care involves a team of experts, which can include medical oncologists, radiation oncologists, and neuro-oncologists who help determine a treatment plan that takes factors such as survival and quality of life into account.
This is why, for example, we try to delay whole brain radiotherapy when we can. The HER2CLIMB study, which led to the approval of tucatinib as a treatment option for patients with HER2-positive MBC, showed us that patients with treated or untreated brain metastases receiving systemic therapy before local therapy could benefit from the combination of tucatinib, trastuzumab, and capecitabine. These patients exhibited a median progression-free survival of 7.6 months compared with 5.4 months in the placebo group.
HER2CLIMB has been practice changing because it showed us that tucatinib has good CNS activity in patients with brain metastases. The HER2CLIMB findings raise an important question: As our systemic therapies improve, how aggressive do we need to be with local therapy? Can we push off modalities like whole-brain radiotherapy, which are associated with toxicity?
This study also highlights how important it is for patients with metastatic disease to seek clinical trials. Although some trials exclude patients with brain metastases and others may have criteria that require the stability of brain metastasis for a certain amount of time, the knowledge gained can be invaluable.
Where are some of the main gaps in our understanding of brain metastases in patients with MBC?
One issue is our understanding of tropism to the brain. In other words, why does MBC spread to the brain? Once we understand this key piece, we can work on developing more effective therapies and therapeutic combinations to block brain metastasis.
For hormone receptor–positive disease, in particular, a central question is whether the current antiestrogen therapies — such as selective estrogen receptor degraders like fulvestrant, as well as targeted AKT inhibitors — have the potential to affect brain tumor activity. The same holds true for TNBC, where antibody drug conjugates and immunotherapies are being evaluated for treatment of brain tumors. For patients with HER2-positive MBC that has spread to the brain, understanding the continued role for tyrosine kinase inhibitors, such as tucatinib and neratinib, as well as whether antibody drug conjugates, including trastuzumab deruxtecan and trastuzumab emtansine, have CNS activity are important areas to explore further.
The CompassHER2 trial, going on now, is randomizing patients with residual HER2-positive disease after neoadjuvant chemotherapy and HER2-targeted therapy to receive trastuzumab emtansine with or without tucatinib. One of the core questions of this study is whether trastuzumab emtansine/tucatinib lowers the rate of brain metastasis and the incidence of systemic metastasis.
Another area in MBC that requires greater scrutiny is patients who develop leptomeningeal disease, which is when cancer cells spread to the cerebrospinal fluid. These patients have a particularly poor prognosis, and it would be helpful to evaluate the efficacy of existing therapies, but these patients are often excluded from clinical trials.
Overall, the ultimate goal in these endeavors is to decrease the rate of metastasis to the brain and improve survival and quality of life in patients with MBC who do experience brain metastases.
A version of this article first appeared on Medscape.com.
Brain metastases remain a frequent and often fatal consequence of metastatic breast cancer (MBC). MBC carries a median survival of about 3 years, but that rate drops significantly when cancer cells move to the brain. A recent analysis estimates median survival in patients with brain metastases ranges from 6 months in triple-negative breast cancer (TNBC) to 21 months in human epidermal growth factor receptor 2 (HER2)–positive disease.
This news organization spoke to Kevin M. Kalinsky, MD, acting associate professor in the department of hematology and medical oncology at Emory University School of Medicine in Atlanta and director of the Glenn Family Breast Center at the Winship Cancer Institute of Emory University, about the risk for brain metastases in patients with MBC, strategies for screening and treatment, and the work being done to achieve a better understanding of the disease.
Question: Before we dig into strategies to manage MBC brain metastasis, let’s talk about the risks. When and how often do brain metastases present in patients with MBC? What factors increase the likelihood of developing brain metastasis?
Dr. Kalinsky: The biggest risk factor for MBC spreading to the central nervous system (CNS), which includes the brain and spine, is breast cancer subtype. For patients with metastatic TNBC, the risk for brain metastasis can be more than 50%. For patients with HER2-positive disease, the risk may be slightly lower, with estimates in the range of 25%-50%, whereas the likelihood of brain metastasis in patients with hormone receptor–positive MBC is significantly lower at close to 14%. In addition, patients with metastatic TNBC may have brain metastases a little earlier in their disease progression compared with patients with HER2-positive or estrogen receptor–positive breast cancers, where brain metastases generally develop a little later in the disease course.
At what point is it recommended to screen patients with MBC for brain metastasis?
Current guidelines suggest that we scan for brain metastasis in the presence of new neurologic symptoms, such as headache, dizziness, or weakness in the arms or legs. MRI, in particular, is useful for evaluating brain metastasis, especially for smaller lesions, but lesions are sometimes detected through CT imaging of the head, too.
That’s where the guidelines are now. But as our systemic agents improve, there’s always the possibility these recommendations will be revisited and potentially include imaging as screening tools in asymptomatic patients, as well.
How do you assess which patients with MBC should receive local therapy?
Increasingly, because our systemic therapies in breast cancer are getting better in terms of crossing the blood-brain barrier, we think about local therapy on a case-by-case basis. We think about it with the question of whether we delay surgery or radiation — whole brain radiation, in particular — given concerns surrounding the side effects of these modalities, namely cognitive dysfunction for radiation and increased risk of bleeding and infection for surgery.
Giving a patient-directed local therapy, such as Gamma Knife radiosurgery or whole-brain radiotherapy, ultimately depends on the burden of brain metastasis, the status of systemic disease outside of the brain, and the number and size of the lesions seen on imaging. If, for instance, a patient has a large lesion that will immediately impact their neurologic status, we may opt to resect the lesion. If there are innumerable lesions, some of which are large, we may do whole-brain radiotherapy. If, however, a patient has systemic disease that is largely under control but is experiencing local progression in the brain, we may use local radiotherapy while continuing systemic therapy.
What about systemic therapies that cross the blood-brain barrier? What’s available now and how do you choose among the options?The subtype of breast cancer informs treatment with systemic therapies. For instance, patients with HER2-positive disease may receive oral tyrosine kinase inhibitors, such as tucatinib, neratinib, and lapatinib, which have strong CNS penetration. For patients with estrogen receptor–positive, HER2-negative MBC, estrogen therapies including aromatase inhibitors, as well as targeted therapies such as the mTOR inhibitor everolimus, have good CNS penetration. For patients with metastatic TNBC, we have chemotherapies that cross the blood-brain barrier, such as capecitabine and platinum-based chemotherapy.
Evidence suggests that tumors in the brain may harbor different genetic abnormalities from tumors in the breast. How do you consider the potential genetic heterogeneity in CNS tumors vs. the primary breast tumor?When a patient’s disease has spread to the brain, we may preferentially use agents we know cross the blood-brain barrier, so we can obtain systemic control both intracranially and extracranially. If we have already resected or biopsied cancerous brain tissue, it’s good to check the tumor’s estrogen receptor, progesterone receptor, and HER2 status and do next-generation sequencing to see if the tumor has any other targetable mutations, such as PIK3CA mutations.
But when a patient has multiple lesions, we don’t go in and biopsy all of them to check for heterogeneity. We have to make decisions based on samples we have. In cases where we start systemic therapy and notice one lesion is not responding to these agents while others are, the nonresponsive lesion may be an outlier in terms of its biologic characteristics. It may be worth targeting that lesion for biopsy and further sequencing to determine the next best systemic approach.
How do quality of life considerations factor into the management of patients with MBC brain metastases?
We use a multidisciplinary approach when treating patients. This means patient care involves a team of experts, which can include medical oncologists, radiation oncologists, and neuro-oncologists who help determine a treatment plan that takes factors such as survival and quality of life into account.
This is why, for example, we try to delay whole brain radiotherapy when we can. The HER2CLIMB study, which led to the approval of tucatinib as a treatment option for patients with HER2-positive MBC, showed us that patients with treated or untreated brain metastases receiving systemic therapy before local therapy could benefit from the combination of tucatinib, trastuzumab, and capecitabine. These patients exhibited a median progression-free survival of 7.6 months compared with 5.4 months in the placebo group.
HER2CLIMB has been practice changing because it showed us that tucatinib has good CNS activity in patients with brain metastases. The HER2CLIMB findings raise an important question: As our systemic therapies improve, how aggressive do we need to be with local therapy? Can we push off modalities like whole-brain radiotherapy, which are associated with toxicity?
This study also highlights how important it is for patients with metastatic disease to seek clinical trials. Although some trials exclude patients with brain metastases and others may have criteria that require the stability of brain metastasis for a certain amount of time, the knowledge gained can be invaluable.
Where are some of the main gaps in our understanding of brain metastases in patients with MBC?
One issue is our understanding of tropism to the brain. In other words, why does MBC spread to the brain? Once we understand this key piece, we can work on developing more effective therapies and therapeutic combinations to block brain metastasis.
For hormone receptor–positive disease, in particular, a central question is whether the current antiestrogen therapies — such as selective estrogen receptor degraders like fulvestrant, as well as targeted AKT inhibitors — have the potential to affect brain tumor activity. The same holds true for TNBC, where antibody drug conjugates and immunotherapies are being evaluated for treatment of brain tumors. For patients with HER2-positive MBC that has spread to the brain, understanding the continued role for tyrosine kinase inhibitors, such as tucatinib and neratinib, as well as whether antibody drug conjugates, including trastuzumab deruxtecan and trastuzumab emtansine, have CNS activity are important areas to explore further.
The CompassHER2 trial, going on now, is randomizing patients with residual HER2-positive disease after neoadjuvant chemotherapy and HER2-targeted therapy to receive trastuzumab emtansine with or without tucatinib. One of the core questions of this study is whether trastuzumab emtansine/tucatinib lowers the rate of brain metastasis and the incidence of systemic metastasis.
Another area in MBC that requires greater scrutiny is patients who develop leptomeningeal disease, which is when cancer cells spread to the cerebrospinal fluid. These patients have a particularly poor prognosis, and it would be helpful to evaluate the efficacy of existing therapies, but these patients are often excluded from clinical trials.
Overall, the ultimate goal in these endeavors is to decrease the rate of metastasis to the brain and improve survival and quality of life in patients with MBC who do experience brain metastases.
A version of this article first appeared on Medscape.com.
Hormone pellet safety data ‘not very reassuring at all’ for women
Women who receive pellet hormonal therapy may be significantly more likely to have side effects such as mood swings, anxiety, breast tenderness, hair pattern change, acne, and weight gain, compared with women who receive hormonal treatments that have been approved by the Food and Drug Administration, a study indicates.
In addition, abnormal uterine bleeding may be significantly more common in women who receive pellets than it is in women who receive Food and Drug Administration–approved options, according to the retrospective study, which was published online in Menopause.
Women receiving pellets also were more likely to undergo hysterectomy while on hormonal therapy, and they had higher supraphysiological levels of estradiol and total testosterone during treatment, compared with women on conventional therapy, the study of 539 women shows.
The findings, which had been presented at the North American Menopause Society annual meeting, were highlighted during a lecture at the 2021 virtual meeting of the American College of Obstetricians and Gynecologists.
The data are “not very reassuring at all,” said Robert P. Kauffman, MD, a professor of obstetrics and gynecology at Texas Tech University, Amarillo, who was not involved in the study.
Dr. Kauffman commented on the research during a review of concerns surrounding non–FDA-approved hormone replacement therapies at the ACOG meeting. Concerns include variations in compounded products, a lack of randomized, controlled trial data supporting their use, and ethical dilemmas that may exist if clinicians have financial incentives to provide compounded bioidentical hormone therapy over FDA-approved treatments.
No peer-reviewed studies show that compounded hormone creams or pellets are safer, more efficacious, or less likely to cause adverse effects, compared with FDA-approved products, Dr. Kauffman said.
Data from Pennsylvania
For the retrospective study, Xuezhi (Daniel) Jiang, MD, PhD, and colleagues identified postmenopausal patients in the Reading Hospital Electronic Medical Record System, including 10,801 on FDA-approved hormonal therapy and 1,061 on pellet hormonal therapy. Their analysis focused on data from the medical records of 384 women on pellet hormonal therapy and 155 women on FDA-approved hormonal therapy. Dr. Jiang is affiliated with the department of obstetrics and gynecology at Reading (Pa.) Hospital and Sidney Kimmel Medical College, Philadelphia.
The researchers examined data from 2005 to 2017 for patients in the pellet therapy group, and from 1985 to 2017 for patients in the conventional therapy group.
Patients in the conventional therapy group received 24 brands of FDA-approved hormone products; 4.5% received testosterone or methyltestosterone in addition to estrogen. Patients in the pellet therapy group had pellets prescribed by clinicians at two private practices in the hospital system that use this treatment approach. Patients in the pellet group received compounded estradiol and testosterone pellets made at a pharmacy in Tennessee.* Almost all of the patients in the pellet group received testosterone and estradiol pellets.
Low libido was listed as a reason why women started treatment for 83.5% of the pellet group versus 4.5% of the conventional therapy group.
In all, 57.6% of patients on pellet therapy had side effects, versus 14.8% on FDA-approved therapy, the researchers found. Patients on pellet hormonal therapy reported higher incidence of mood swings (7% vs. 1.9%), anxiety (18.5% vs. 5.8%), breast tenderness (10.1% vs. 2.6%), hair pattern change (13.5% vs. 2.6%), acne (8.6% vs. 1.3%), and weight gain (34.4% vs 4.5%), relative to patients on FDA-approved options.
Among those with an intact uterus when starting therapy (246 of those on pellets and 133 of those on FDA-approved treatments), abnormal uterine bleeding occurred in 55.3% on pellets, compared with 15.2% on FDA-approved treatments (adjusted odds ratio, 7.9). Hysterectomy secondary to abnormal uterine bleeding occurred in 20.3% of the patients on pellets versus 6.3% on FDA-approved treatments (aOR, 3.2).
In many cases, records show that patients chose to have a hysterectomy so they could continue pellet therapy without worrying about abnormal uterine bleeding, Dr. Jiang said in an interview.
Dr. Kauffman has seen patients on pellet therapy, usually implanted by family physicians, develop postmenopausal bleeding because of high levels of estrogen. “Our experience has been too that, if you have pellets, you are more likely to get a hysterectomy for bleeding issues. And I think these are the safety issues that need to be looked at on a broader scope,” he said in an interview.
Although hysterectomy may stop the bleeding, other safety risks may remain with pellet therapy, noted Sharon Winer, MD, MPH, an obstetrician and gynecologist with a subspecialty in reproductive endocrinology and infertility who practices in Beverly Hills, Calif.
Pellets, which are about the size of a grain of rice, typically are implanted in the hip, lower abdomen, or buttock and release hormones over 3-6 months. The pellets are not retrievable. “The question becomes, what if she has a new breast cancer diagnosis or a diagnosis where estrogen is contraindicated? She has got that estrogen already in her system,” Dr. Winer said.
“The hysterectomy may solve the bleeding problem ... but it doesn’t solve the safety problem overall,” said Dr. Winer, who also is a professor of obstetrics and gynecology and codirector of the reproductive endocrinology and infertility clinic at the University of Southern California, Los Angeles.
Elevated levels
Average peak serum estradiol was significantly higher in the pellet treatment group than in the conventional therapy group (237.70 pg/mL vs. 93.45 pg/mL), as was average peak serum testosterone (192.84 ng/dL vs. 15.59 ng/dL), the researchers reported. Patients on FDA-approved treatments were less likely to have had their hormone levels measured. How concentrations of hormone levels correlate with side effects is unclear, Dr. Jiang said.
The study was limited by its single-institution, retrospective design, and some patient characteristics differed between the treatment groups, the authors noted.
Still, “clinicians ought to be mindful of fully counseling patients on side effects identified in the current study,” Dr. Jiang and coauthors concluded. Clinicians also need to discuss potential risks of breast cancer, endometrial cancer, and cardiovascular disease with patients.
Many primary care clinicians rely on outdated information from the Women’s Health Initiative, published in 2002 and 2004, in their understanding of postmenopausal hormonal therapy and its risks and benefits, Dr. Jiang said. And some patients consider custom-compounded hormone therapy to be safer and more natural, “which is totally misleading.”
Pellets and other custom-compounded medicine containing testosterone may make patients feel better and more energetic, Dr. Jiang acknowledged. “That’s a reason why patients ... tend to stay on, even though they have side effects. The only issue is the safety.”
Additional questions remain. The researchers recently started to examine rates of breast cancer and abnormal breast pathology and mammogram results. “It’s a long journey,” he said.
Plenty of approved options
Custom-compounded medicines are not FDA approved and are not recommended by medical menopause societies, Dr. Jiang said. Meanwhile, plenty of approved hormone therapies, including bioidentical treatments, have safety data and are available.
A 2020 consensus study report from the National Academies of Sciences, Engineering, and Medicine that examined the use of compounded hormonal therapy and provides guidance for clinicians is a good start in addressing this major issue, he added.
A committee determined “there is insufficient evidence to support the overall clinical utility of [compounded bioidentical hormone therapies] as treatment for menopause and male hypogonadism symptoms.”
If an FDA-approved option is available, “I would always go with an FDA-approved product before I would go with a compounded product,” Dr. Winer said. A 2012 fungal meningitis outbreak linked to a compounding pharmacy highlighted risks associated with poor quality compounded drugs.
“I think at least now it is recognized that compounding is an issue that has got to be dealt with,” Dr. Winer said. “It is just that it is so widespread and it is sometimes under the radar ... that I think it is really hard for the FDA to get a handle on it.”
Dr. Winer has seen patients on compounded treatments who are underdosed and patients who are overdosed. “I’ve also seen patients who do quite well with it, but I’m not happy continuing it because tomorrow there may be inconsistency in potency or quality resulting in a different clinical response,” she said.
Nevertheless, compounded pharmacies are needed, Dr. Winer said. If she wants to give natural progesterone that is FDA approved but happens to be made with peanut oil, she will have a compounding pharmacy make it with canola oil instead if a patient has a peanut allergy, for example. Other patients need dosages that are so low that they are not available as FDA-approved products.
Dr. Jiang and Dr. Kauffman had no relevant financial disclosures. Dr. Winer has done work with AbbVie (related to endometriosis), TherapeuticsMD (related to a menopause bioidentical hormonal pill and vaginal estrogen product), and Biogix (related to an antioxidant supplement for menopause symptoms).
*This story was updated on 6/22/2021.
Women who receive pellet hormonal therapy may be significantly more likely to have side effects such as mood swings, anxiety, breast tenderness, hair pattern change, acne, and weight gain, compared with women who receive hormonal treatments that have been approved by the Food and Drug Administration, a study indicates.
In addition, abnormal uterine bleeding may be significantly more common in women who receive pellets than it is in women who receive Food and Drug Administration–approved options, according to the retrospective study, which was published online in Menopause.
Women receiving pellets also were more likely to undergo hysterectomy while on hormonal therapy, and they had higher supraphysiological levels of estradiol and total testosterone during treatment, compared with women on conventional therapy, the study of 539 women shows.
The findings, which had been presented at the North American Menopause Society annual meeting, were highlighted during a lecture at the 2021 virtual meeting of the American College of Obstetricians and Gynecologists.
The data are “not very reassuring at all,” said Robert P. Kauffman, MD, a professor of obstetrics and gynecology at Texas Tech University, Amarillo, who was not involved in the study.
Dr. Kauffman commented on the research during a review of concerns surrounding non–FDA-approved hormone replacement therapies at the ACOG meeting. Concerns include variations in compounded products, a lack of randomized, controlled trial data supporting their use, and ethical dilemmas that may exist if clinicians have financial incentives to provide compounded bioidentical hormone therapy over FDA-approved treatments.
No peer-reviewed studies show that compounded hormone creams or pellets are safer, more efficacious, or less likely to cause adverse effects, compared with FDA-approved products, Dr. Kauffman said.
Data from Pennsylvania
For the retrospective study, Xuezhi (Daniel) Jiang, MD, PhD, and colleagues identified postmenopausal patients in the Reading Hospital Electronic Medical Record System, including 10,801 on FDA-approved hormonal therapy and 1,061 on pellet hormonal therapy. Their analysis focused on data from the medical records of 384 women on pellet hormonal therapy and 155 women on FDA-approved hormonal therapy. Dr. Jiang is affiliated with the department of obstetrics and gynecology at Reading (Pa.) Hospital and Sidney Kimmel Medical College, Philadelphia.
The researchers examined data from 2005 to 2017 for patients in the pellet therapy group, and from 1985 to 2017 for patients in the conventional therapy group.
Patients in the conventional therapy group received 24 brands of FDA-approved hormone products; 4.5% received testosterone or methyltestosterone in addition to estrogen. Patients in the pellet therapy group had pellets prescribed by clinicians at two private practices in the hospital system that use this treatment approach. Patients in the pellet group received compounded estradiol and testosterone pellets made at a pharmacy in Tennessee.* Almost all of the patients in the pellet group received testosterone and estradiol pellets.
Low libido was listed as a reason why women started treatment for 83.5% of the pellet group versus 4.5% of the conventional therapy group.
In all, 57.6% of patients on pellet therapy had side effects, versus 14.8% on FDA-approved therapy, the researchers found. Patients on pellet hormonal therapy reported higher incidence of mood swings (7% vs. 1.9%), anxiety (18.5% vs. 5.8%), breast tenderness (10.1% vs. 2.6%), hair pattern change (13.5% vs. 2.6%), acne (8.6% vs. 1.3%), and weight gain (34.4% vs 4.5%), relative to patients on FDA-approved options.
Among those with an intact uterus when starting therapy (246 of those on pellets and 133 of those on FDA-approved treatments), abnormal uterine bleeding occurred in 55.3% on pellets, compared with 15.2% on FDA-approved treatments (adjusted odds ratio, 7.9). Hysterectomy secondary to abnormal uterine bleeding occurred in 20.3% of the patients on pellets versus 6.3% on FDA-approved treatments (aOR, 3.2).
In many cases, records show that patients chose to have a hysterectomy so they could continue pellet therapy without worrying about abnormal uterine bleeding, Dr. Jiang said in an interview.
Dr. Kauffman has seen patients on pellet therapy, usually implanted by family physicians, develop postmenopausal bleeding because of high levels of estrogen. “Our experience has been too that, if you have pellets, you are more likely to get a hysterectomy for bleeding issues. And I think these are the safety issues that need to be looked at on a broader scope,” he said in an interview.
Although hysterectomy may stop the bleeding, other safety risks may remain with pellet therapy, noted Sharon Winer, MD, MPH, an obstetrician and gynecologist with a subspecialty in reproductive endocrinology and infertility who practices in Beverly Hills, Calif.
Pellets, which are about the size of a grain of rice, typically are implanted in the hip, lower abdomen, or buttock and release hormones over 3-6 months. The pellets are not retrievable. “The question becomes, what if she has a new breast cancer diagnosis or a diagnosis where estrogen is contraindicated? She has got that estrogen already in her system,” Dr. Winer said.
“The hysterectomy may solve the bleeding problem ... but it doesn’t solve the safety problem overall,” said Dr. Winer, who also is a professor of obstetrics and gynecology and codirector of the reproductive endocrinology and infertility clinic at the University of Southern California, Los Angeles.
Elevated levels
Average peak serum estradiol was significantly higher in the pellet treatment group than in the conventional therapy group (237.70 pg/mL vs. 93.45 pg/mL), as was average peak serum testosterone (192.84 ng/dL vs. 15.59 ng/dL), the researchers reported. Patients on FDA-approved treatments were less likely to have had their hormone levels measured. How concentrations of hormone levels correlate with side effects is unclear, Dr. Jiang said.
The study was limited by its single-institution, retrospective design, and some patient characteristics differed between the treatment groups, the authors noted.
Still, “clinicians ought to be mindful of fully counseling patients on side effects identified in the current study,” Dr. Jiang and coauthors concluded. Clinicians also need to discuss potential risks of breast cancer, endometrial cancer, and cardiovascular disease with patients.
Many primary care clinicians rely on outdated information from the Women’s Health Initiative, published in 2002 and 2004, in their understanding of postmenopausal hormonal therapy and its risks and benefits, Dr. Jiang said. And some patients consider custom-compounded hormone therapy to be safer and more natural, “which is totally misleading.”
Pellets and other custom-compounded medicine containing testosterone may make patients feel better and more energetic, Dr. Jiang acknowledged. “That’s a reason why patients ... tend to stay on, even though they have side effects. The only issue is the safety.”
Additional questions remain. The researchers recently started to examine rates of breast cancer and abnormal breast pathology and mammogram results. “It’s a long journey,” he said.
Plenty of approved options
Custom-compounded medicines are not FDA approved and are not recommended by medical menopause societies, Dr. Jiang said. Meanwhile, plenty of approved hormone therapies, including bioidentical treatments, have safety data and are available.
A 2020 consensus study report from the National Academies of Sciences, Engineering, and Medicine that examined the use of compounded hormonal therapy and provides guidance for clinicians is a good start in addressing this major issue, he added.
A committee determined “there is insufficient evidence to support the overall clinical utility of [compounded bioidentical hormone therapies] as treatment for menopause and male hypogonadism symptoms.”
If an FDA-approved option is available, “I would always go with an FDA-approved product before I would go with a compounded product,” Dr. Winer said. A 2012 fungal meningitis outbreak linked to a compounding pharmacy highlighted risks associated with poor quality compounded drugs.
“I think at least now it is recognized that compounding is an issue that has got to be dealt with,” Dr. Winer said. “It is just that it is so widespread and it is sometimes under the radar ... that I think it is really hard for the FDA to get a handle on it.”
Dr. Winer has seen patients on compounded treatments who are underdosed and patients who are overdosed. “I’ve also seen patients who do quite well with it, but I’m not happy continuing it because tomorrow there may be inconsistency in potency or quality resulting in a different clinical response,” she said.
Nevertheless, compounded pharmacies are needed, Dr. Winer said. If she wants to give natural progesterone that is FDA approved but happens to be made with peanut oil, she will have a compounding pharmacy make it with canola oil instead if a patient has a peanut allergy, for example. Other patients need dosages that are so low that they are not available as FDA-approved products.
Dr. Jiang and Dr. Kauffman had no relevant financial disclosures. Dr. Winer has done work with AbbVie (related to endometriosis), TherapeuticsMD (related to a menopause bioidentical hormonal pill and vaginal estrogen product), and Biogix (related to an antioxidant supplement for menopause symptoms).
*This story was updated on 6/22/2021.
Women who receive pellet hormonal therapy may be significantly more likely to have side effects such as mood swings, anxiety, breast tenderness, hair pattern change, acne, and weight gain, compared with women who receive hormonal treatments that have been approved by the Food and Drug Administration, a study indicates.
In addition, abnormal uterine bleeding may be significantly more common in women who receive pellets than it is in women who receive Food and Drug Administration–approved options, according to the retrospective study, which was published online in Menopause.
Women receiving pellets also were more likely to undergo hysterectomy while on hormonal therapy, and they had higher supraphysiological levels of estradiol and total testosterone during treatment, compared with women on conventional therapy, the study of 539 women shows.
The findings, which had been presented at the North American Menopause Society annual meeting, were highlighted during a lecture at the 2021 virtual meeting of the American College of Obstetricians and Gynecologists.
The data are “not very reassuring at all,” said Robert P. Kauffman, MD, a professor of obstetrics and gynecology at Texas Tech University, Amarillo, who was not involved in the study.
Dr. Kauffman commented on the research during a review of concerns surrounding non–FDA-approved hormone replacement therapies at the ACOG meeting. Concerns include variations in compounded products, a lack of randomized, controlled trial data supporting their use, and ethical dilemmas that may exist if clinicians have financial incentives to provide compounded bioidentical hormone therapy over FDA-approved treatments.
No peer-reviewed studies show that compounded hormone creams or pellets are safer, more efficacious, or less likely to cause adverse effects, compared with FDA-approved products, Dr. Kauffman said.
Data from Pennsylvania
For the retrospective study, Xuezhi (Daniel) Jiang, MD, PhD, and colleagues identified postmenopausal patients in the Reading Hospital Electronic Medical Record System, including 10,801 on FDA-approved hormonal therapy and 1,061 on pellet hormonal therapy. Their analysis focused on data from the medical records of 384 women on pellet hormonal therapy and 155 women on FDA-approved hormonal therapy. Dr. Jiang is affiliated with the department of obstetrics and gynecology at Reading (Pa.) Hospital and Sidney Kimmel Medical College, Philadelphia.
The researchers examined data from 2005 to 2017 for patients in the pellet therapy group, and from 1985 to 2017 for patients in the conventional therapy group.
Patients in the conventional therapy group received 24 brands of FDA-approved hormone products; 4.5% received testosterone or methyltestosterone in addition to estrogen. Patients in the pellet therapy group had pellets prescribed by clinicians at two private practices in the hospital system that use this treatment approach. Patients in the pellet group received compounded estradiol and testosterone pellets made at a pharmacy in Tennessee.* Almost all of the patients in the pellet group received testosterone and estradiol pellets.
Low libido was listed as a reason why women started treatment for 83.5% of the pellet group versus 4.5% of the conventional therapy group.
In all, 57.6% of patients on pellet therapy had side effects, versus 14.8% on FDA-approved therapy, the researchers found. Patients on pellet hormonal therapy reported higher incidence of mood swings (7% vs. 1.9%), anxiety (18.5% vs. 5.8%), breast tenderness (10.1% vs. 2.6%), hair pattern change (13.5% vs. 2.6%), acne (8.6% vs. 1.3%), and weight gain (34.4% vs 4.5%), relative to patients on FDA-approved options.
Among those with an intact uterus when starting therapy (246 of those on pellets and 133 of those on FDA-approved treatments), abnormal uterine bleeding occurred in 55.3% on pellets, compared with 15.2% on FDA-approved treatments (adjusted odds ratio, 7.9). Hysterectomy secondary to abnormal uterine bleeding occurred in 20.3% of the patients on pellets versus 6.3% on FDA-approved treatments (aOR, 3.2).
In many cases, records show that patients chose to have a hysterectomy so they could continue pellet therapy without worrying about abnormal uterine bleeding, Dr. Jiang said in an interview.
Dr. Kauffman has seen patients on pellet therapy, usually implanted by family physicians, develop postmenopausal bleeding because of high levels of estrogen. “Our experience has been too that, if you have pellets, you are more likely to get a hysterectomy for bleeding issues. And I think these are the safety issues that need to be looked at on a broader scope,” he said in an interview.
Although hysterectomy may stop the bleeding, other safety risks may remain with pellet therapy, noted Sharon Winer, MD, MPH, an obstetrician and gynecologist with a subspecialty in reproductive endocrinology and infertility who practices in Beverly Hills, Calif.
Pellets, which are about the size of a grain of rice, typically are implanted in the hip, lower abdomen, or buttock and release hormones over 3-6 months. The pellets are not retrievable. “The question becomes, what if she has a new breast cancer diagnosis or a diagnosis where estrogen is contraindicated? She has got that estrogen already in her system,” Dr. Winer said.
“The hysterectomy may solve the bleeding problem ... but it doesn’t solve the safety problem overall,” said Dr. Winer, who also is a professor of obstetrics and gynecology and codirector of the reproductive endocrinology and infertility clinic at the University of Southern California, Los Angeles.
Elevated levels
Average peak serum estradiol was significantly higher in the pellet treatment group than in the conventional therapy group (237.70 pg/mL vs. 93.45 pg/mL), as was average peak serum testosterone (192.84 ng/dL vs. 15.59 ng/dL), the researchers reported. Patients on FDA-approved treatments were less likely to have had their hormone levels measured. How concentrations of hormone levels correlate with side effects is unclear, Dr. Jiang said.
The study was limited by its single-institution, retrospective design, and some patient characteristics differed between the treatment groups, the authors noted.
Still, “clinicians ought to be mindful of fully counseling patients on side effects identified in the current study,” Dr. Jiang and coauthors concluded. Clinicians also need to discuss potential risks of breast cancer, endometrial cancer, and cardiovascular disease with patients.
Many primary care clinicians rely on outdated information from the Women’s Health Initiative, published in 2002 and 2004, in their understanding of postmenopausal hormonal therapy and its risks and benefits, Dr. Jiang said. And some patients consider custom-compounded hormone therapy to be safer and more natural, “which is totally misleading.”
Pellets and other custom-compounded medicine containing testosterone may make patients feel better and more energetic, Dr. Jiang acknowledged. “That’s a reason why patients ... tend to stay on, even though they have side effects. The only issue is the safety.”
Additional questions remain. The researchers recently started to examine rates of breast cancer and abnormal breast pathology and mammogram results. “It’s a long journey,” he said.
Plenty of approved options
Custom-compounded medicines are not FDA approved and are not recommended by medical menopause societies, Dr. Jiang said. Meanwhile, plenty of approved hormone therapies, including bioidentical treatments, have safety data and are available.
A 2020 consensus study report from the National Academies of Sciences, Engineering, and Medicine that examined the use of compounded hormonal therapy and provides guidance for clinicians is a good start in addressing this major issue, he added.
A committee determined “there is insufficient evidence to support the overall clinical utility of [compounded bioidentical hormone therapies] as treatment for menopause and male hypogonadism symptoms.”
If an FDA-approved option is available, “I would always go with an FDA-approved product before I would go with a compounded product,” Dr. Winer said. A 2012 fungal meningitis outbreak linked to a compounding pharmacy highlighted risks associated with poor quality compounded drugs.
“I think at least now it is recognized that compounding is an issue that has got to be dealt with,” Dr. Winer said. “It is just that it is so widespread and it is sometimes under the radar ... that I think it is really hard for the FDA to get a handle on it.”
Dr. Winer has seen patients on compounded treatments who are underdosed and patients who are overdosed. “I’ve also seen patients who do quite well with it, but I’m not happy continuing it because tomorrow there may be inconsistency in potency or quality resulting in a different clinical response,” she said.
Nevertheless, compounded pharmacies are needed, Dr. Winer said. If she wants to give natural progesterone that is FDA approved but happens to be made with peanut oil, she will have a compounding pharmacy make it with canola oil instead if a patient has a peanut allergy, for example. Other patients need dosages that are so low that they are not available as FDA-approved products.
Dr. Jiang and Dr. Kauffman had no relevant financial disclosures. Dr. Winer has done work with AbbVie (related to endometriosis), TherapeuticsMD (related to a menopause bioidentical hormonal pill and vaginal estrogen product), and Biogix (related to an antioxidant supplement for menopause symptoms).
*This story was updated on 6/22/2021.
FROM ACOG 2021