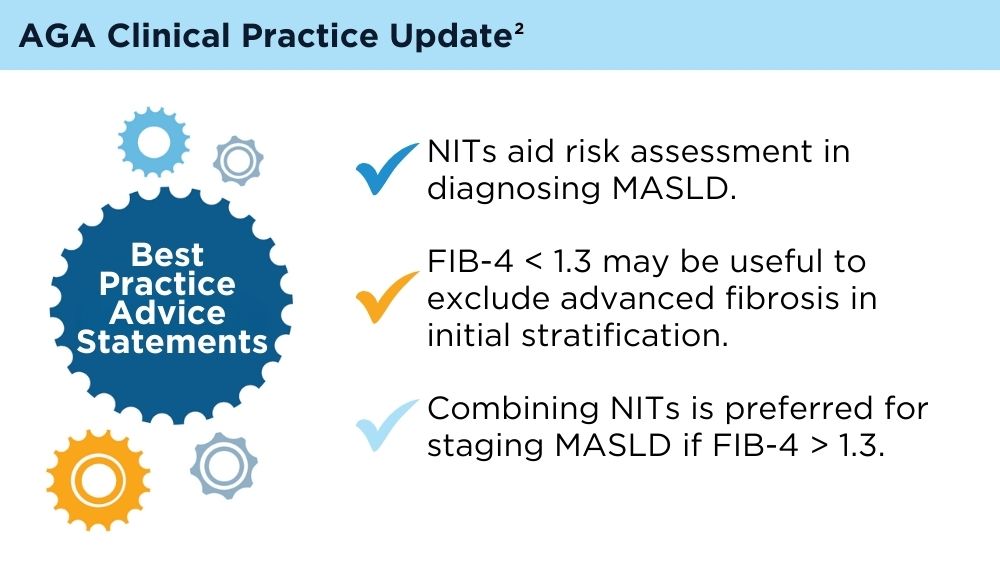
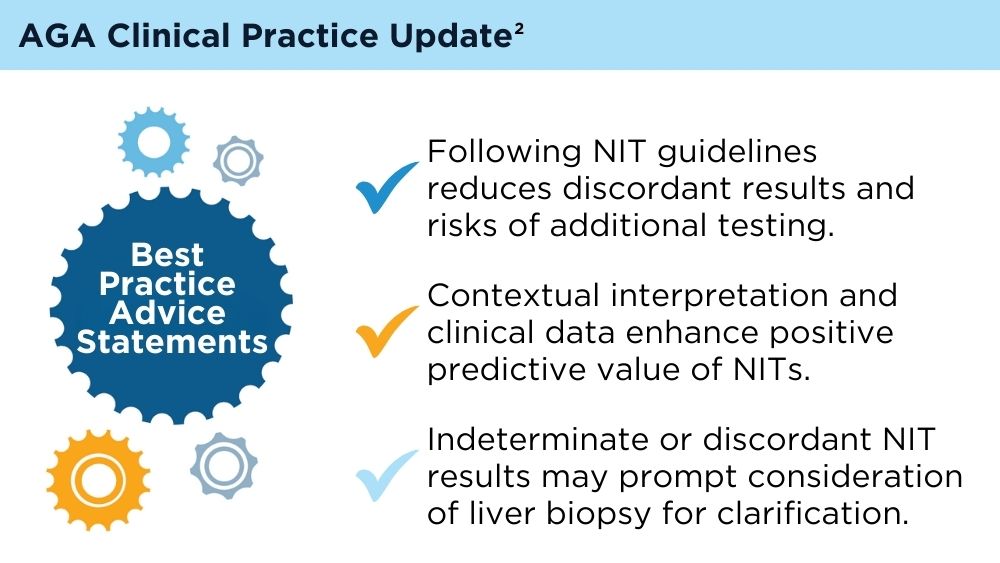
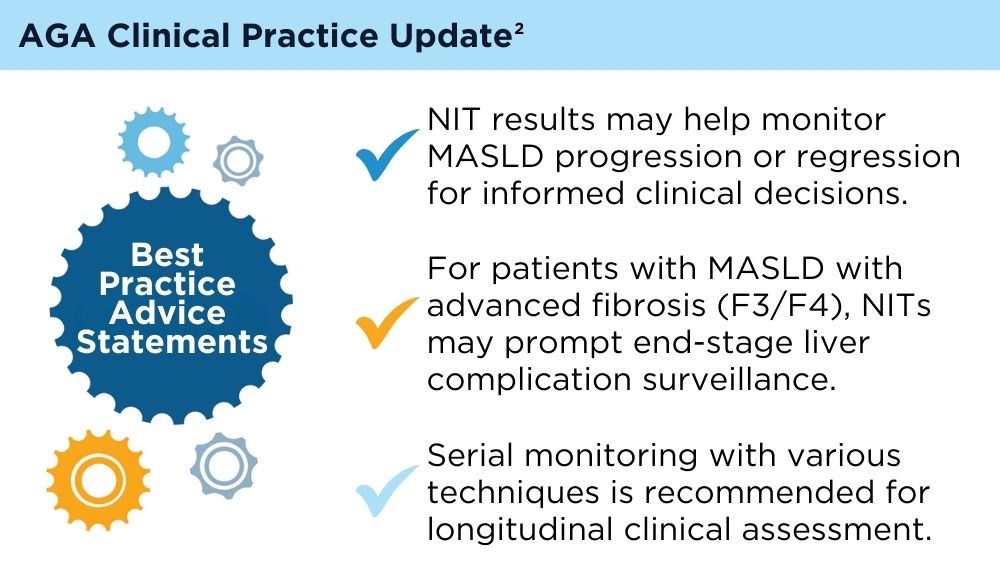
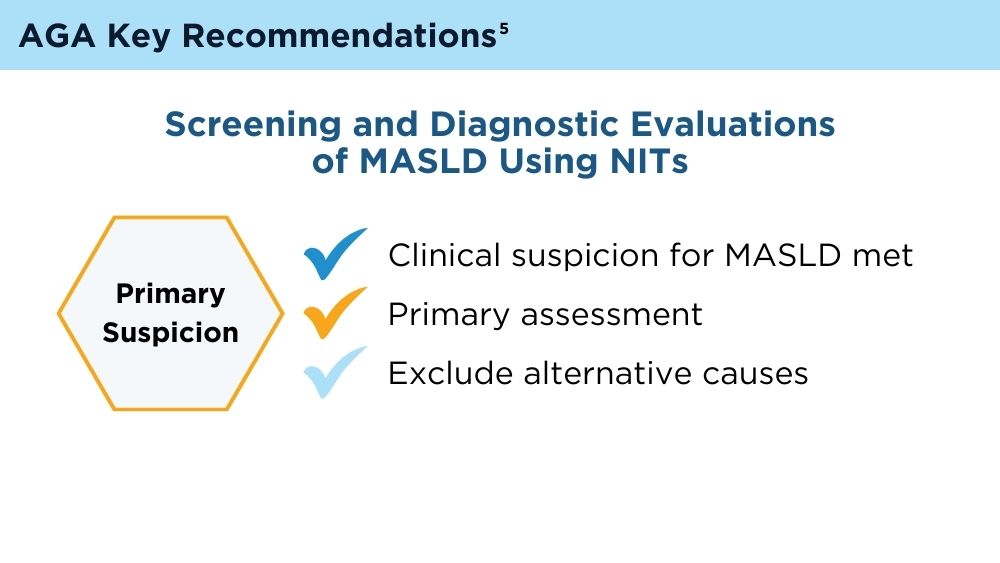
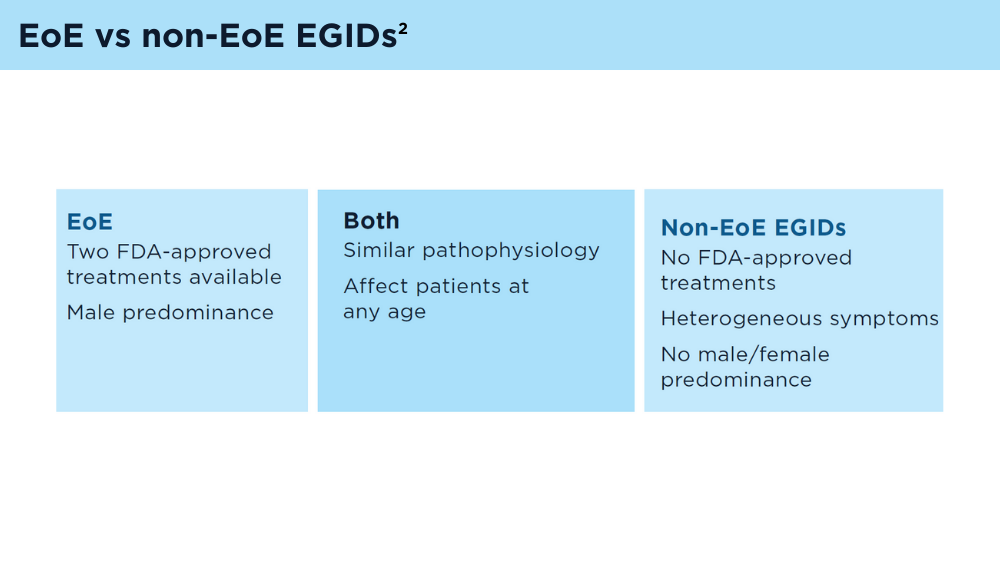
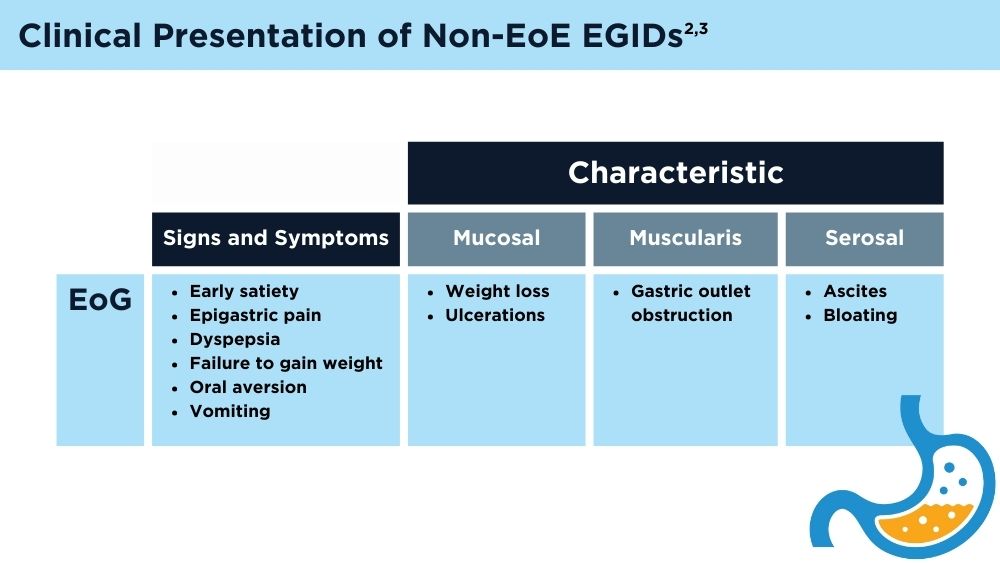
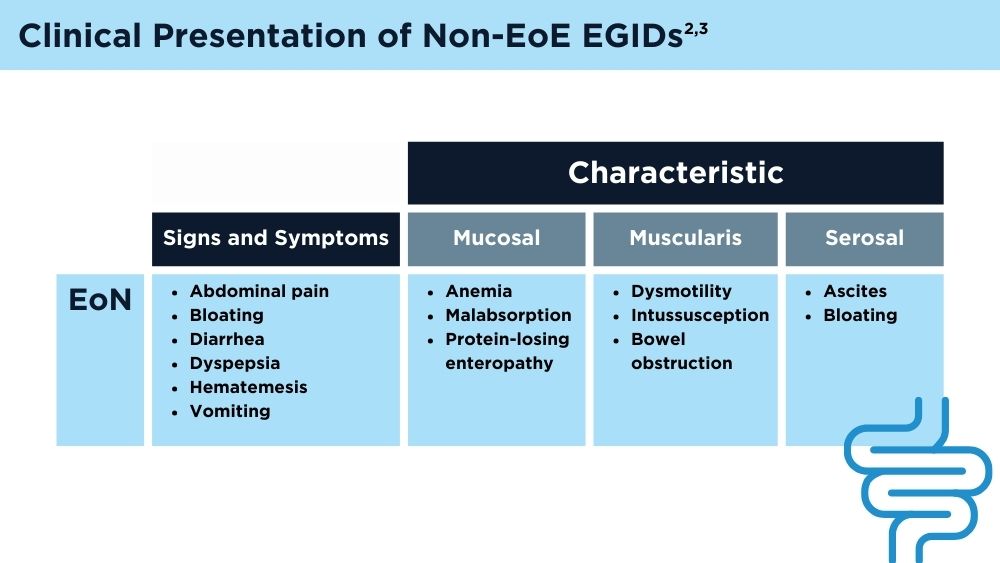
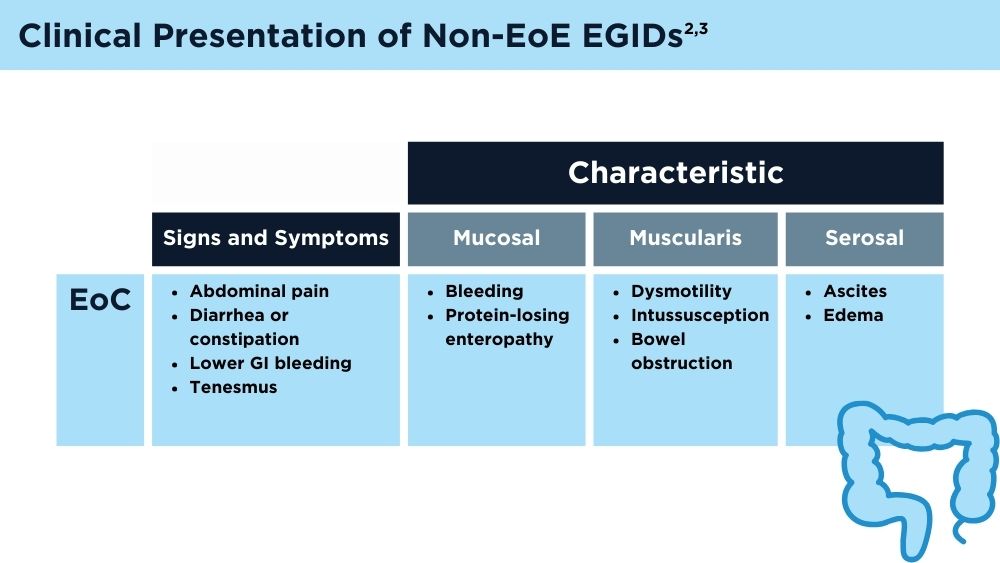
User login
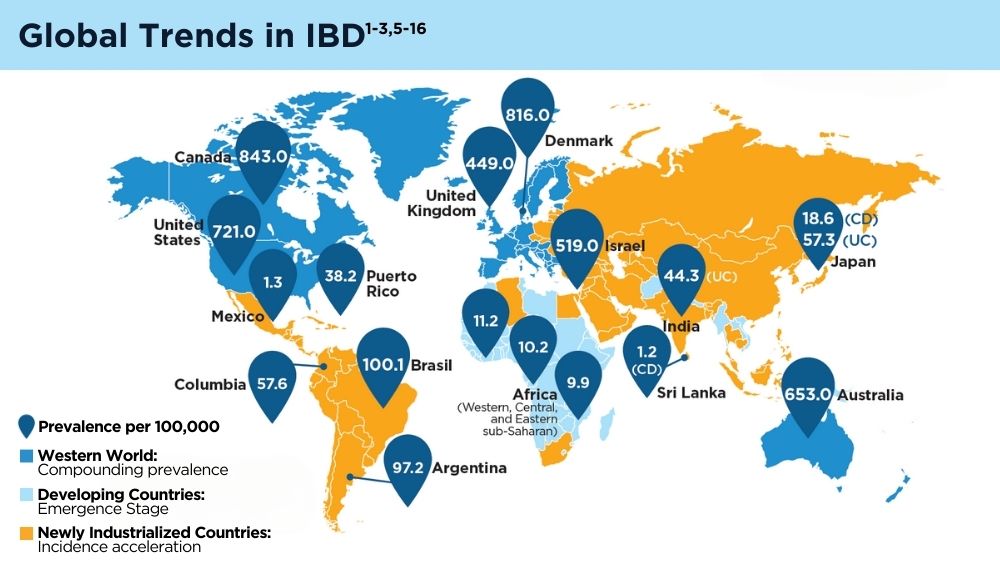
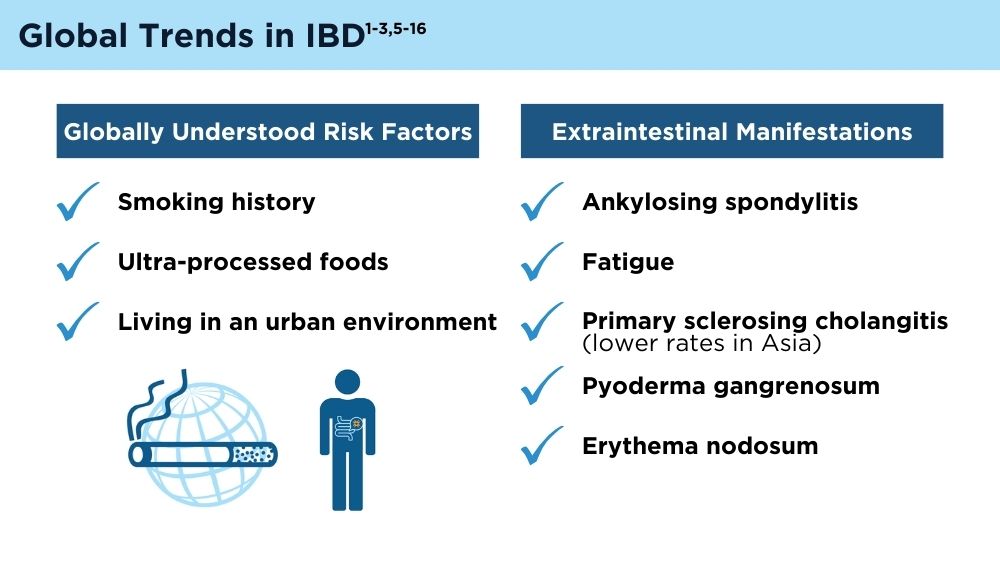
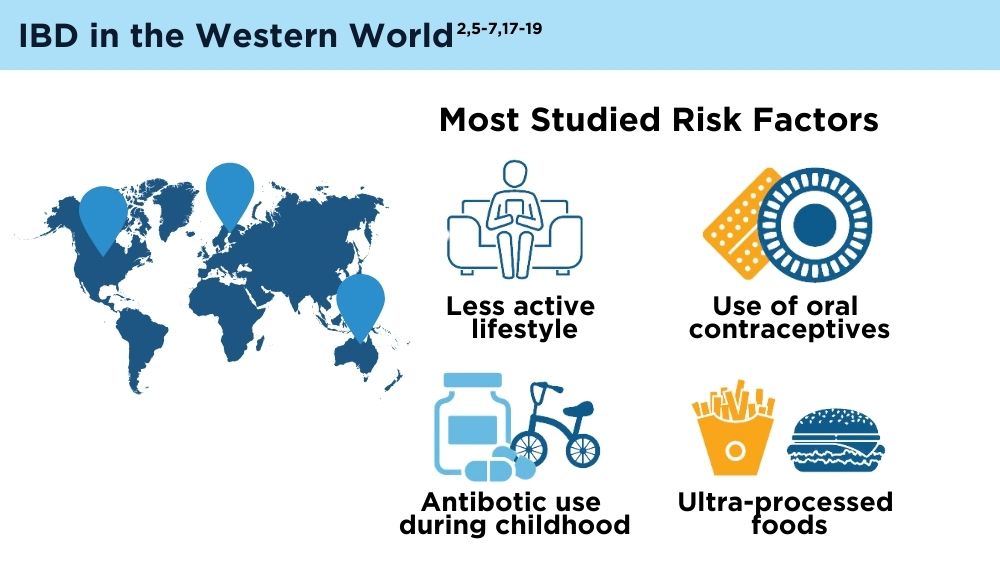
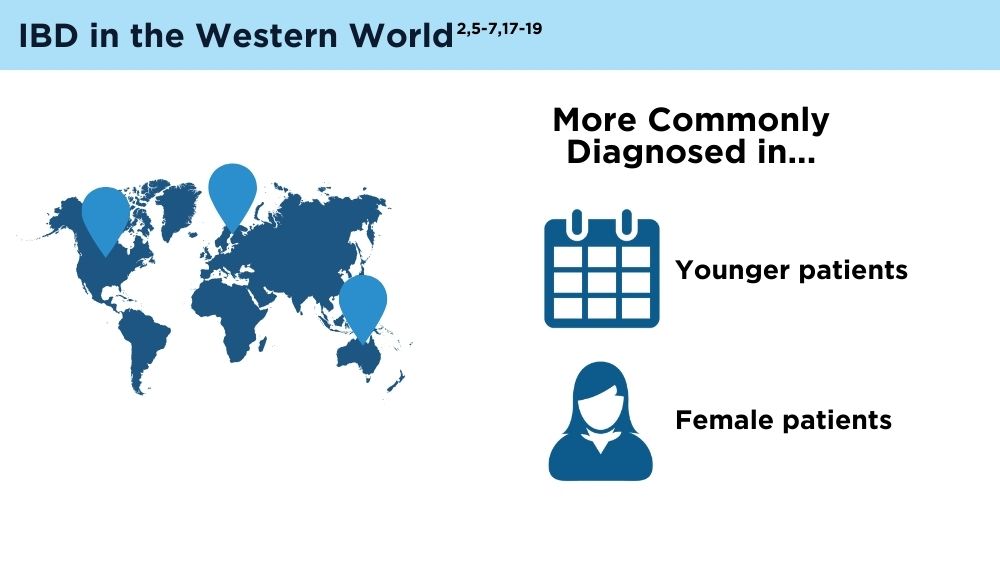
The Changing Face of IBD: Beyond the Western World
- Kaplan GG, Windsor JW. The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2021;18(1):56-66. doi:10.1038/s41575-020-00360-x
- Kaplan GG, Ng SC. Understanding and preventing the global increase of inflammatory bowel disease [published correction appears in Gastroenterology. 2017;152(8):2084]. Gastroenterology. 2017;152(2):313-321.e2. doi:10.1053/j.gastro.2016.10.020
- Balderramo D, Quaresma AB, Olivera PA, et al. Challenges in diagnosis and treatment of inflammatory bowel disease in Latin America. Lancet Gastroenterol Hepatol. 2024; 9(3):263-272. doi:10.1016/S2468-1253(23)00284-4
- Song EM, Na SY, Hong SN, Ng SC, Hisamatsu T, Ye BD. Treatment of inflammatory bowel disease–Asian perspectives: the results of a multinational web-based survey in the 8th Asian Organization for Crohn’s and Colitis meeting. Intest Res. 2023;21(3):339-352. doi:10.5217/ir.2022.00135
- GBD 2017 Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5(1):17-30. doi:10.1016/S2468-1253(19)30333-4
- Chen X, Xiang X, Xia W, et al. Evolving trends and burden of inflammatory bowel disease in Asia, 1990-2019: a comprehensive analysis based on the Global Burden of Disease Study. J Epidemiol Glob Health. 2023;13(4):725-739. doi:10.1007/s44197-023-00145-w
- Zhao M, Feng R, Ben-Horin S, et al. Systematic review with meta-analysis: environmental and dietary differences of inflammatory bowel disease in Eastern and Western populations. Aliment Pharmacol Ther. 2022;55(3):266-276. doi:10.1111/apt.16703
- Lewis JD, Parlett LE, Jonsson Funk ML, et al. Incidence, prevalence, and racial and ethnic distribution of inflammatory bowel disease in the United States. Gastroenterology. 2023;165(5):1197-1205.e2. doi:10.1053/j.gastro.2023.07.003
- Quaresma AB, Damiao AOMC, Coy CSR, et al. Temporal trends in the epidemiology of inflammatory bowel diseases in the public healthcare system in Brazil: a large population-based study. Lancet Reg Health Am. 2022;13:100298. doi:10.1016/j.lana.2022.100298
- Gordon H, Burisch J, Ellul P, et al. ECCO guidelines on extraintestinal manifestations in inflammatory bowel disease. J Crohns Colitis. 2024;18(1):1-37. doi:10.1093/ecco-jcc/jjad108
- Coward S, Benchimol EI, Bernstein CN, et al; Canadian Gastro-Intestinal Epidemiology Consortium (CanGIEC). Forecasting the Incidence and Prevalence of Inflammatory Bowel Disease: A Canadian Nationwide Analysis. Am J Gastroenterol. 2024 Mar 18. doi:10.14309/ajg.0000000000002687. Epub ahead of print. PMID: 38299598.
- Dorn-Rasmussen M, Lo B, Zhao M, Kaplan GG, Malham M, Wewer V, Burisch J. The Incidence and Prevalence of Paediatric- and Adult-Onset Inflammatory Bowel Disease in Denmark During a 37-Year Period: A Nationwide Cohort Study (1980-2017). J Crohns Colitis. 2023;17(2):259- 268. doi:10.1093/ecco-jcc/jjac138. PMID: 36125076.
- Watermeyer G, Katsidzira L, Setshedi M, et al. Inflammatory bowel disease in sub-Saharan Africa: epidemiology, risk factors, and challenges in diagnosis. Lancet Gastroenterol Hepatol. 2022;7(10):952-961. doi:10.1016/S2468-1253(22)00047-4
- Stulman MY, Asayag N, Focht G, et al. Epidemiology of Inflammatory Bowel Diseases in Israel: A Nationwide Epi-Israeli IBD Research Nucleus Study. Inflamm Bowel Dis. 2021;27(11):1784-1794. doi:10.1093/ibd/izaa341
- Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies [published correction appears in Lancet. 2020;396(10256):e56]. Lancet. 2017;390(10114):2769-2778. doi:10.1016/S0140-6736(17)32448-0
- Busingye D, Pollack A, Chidwick K. Prevalence of inflammatory bowel disease in the Australian general practice population: A cross-sectional study. PLoS One. 2021;16(5):e0252458. Published 2021 May 27. doi:10.1371/ journal.pone.0252458
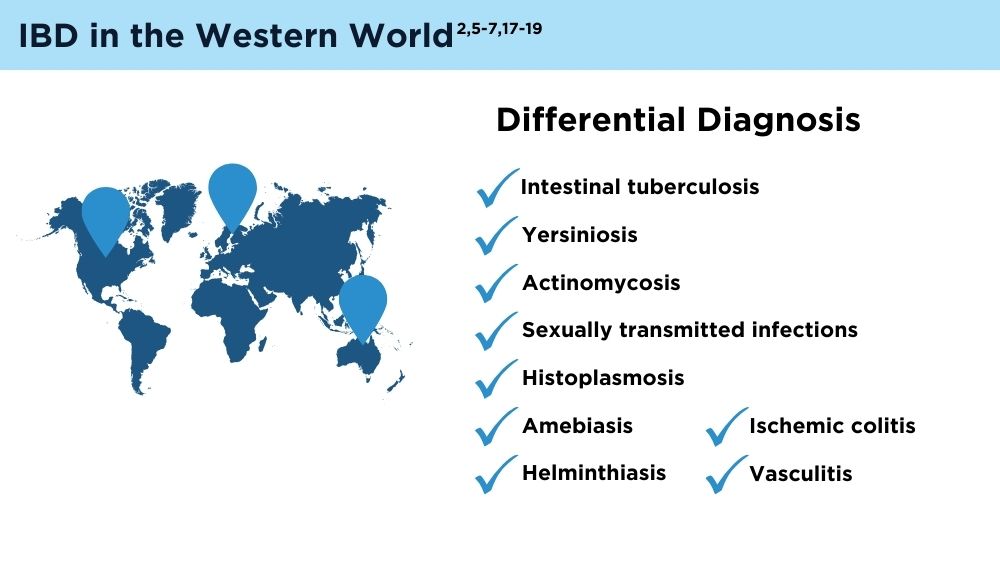
- Gecse KB, Vermeire S. Differential diagnosis of inflammatory bowel disease: imitations and complications. Lancet Gastroenterol Hepatol. 2018;3(9):644-653. doi:10.1016/S2468-1253(18)30159-6
- Inflammatory bowel disease (IBD): comorbidities. Centers for Disease Control and Prevention. Last reviewed April 14, 2022. Accessed February 21, 2024. https://www.cdc.gov/ibd/data-and-statistics/comorbidities.html
- Mosli MH, Alsahafi M, Alsanea MN, Alhasani F, Ahmed M, Saadah O. Multimorbidity among inflammatory bowel disease patients in a tertiary care center: a retrospective study. BMC Gastroenterol. 2022;22(1):487. doi:10.1186/s12876-022-02578-2
- Inflammatory bowel disease (IBD). Mayo Clinic. September 3, 2022. Accessed February 21, 2024. https://www.mayoclinic.org/diseases-conditions/inflammatory-bowel-disease/diagnosis-treatment/drc-20353320
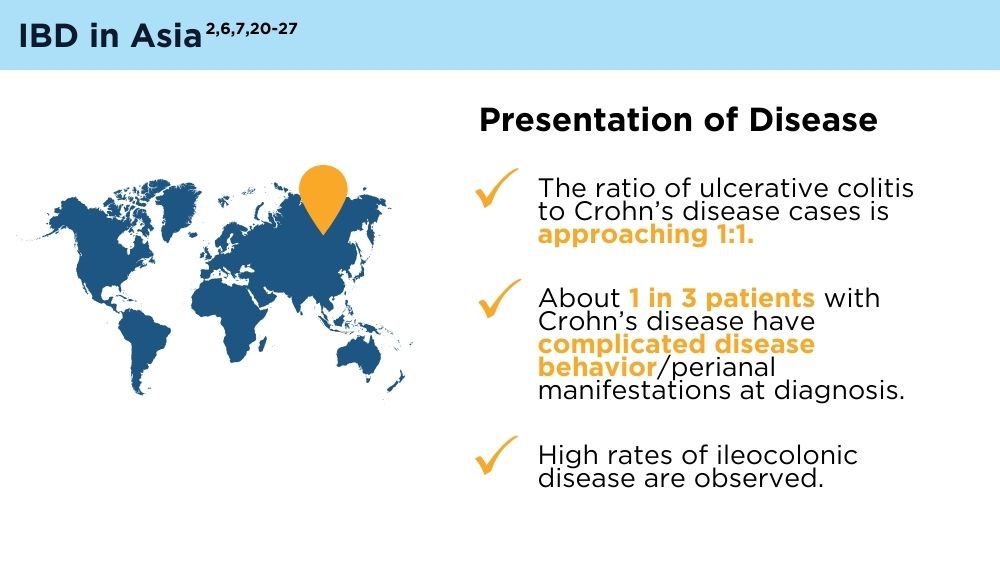
- Ng SC, Tang W, Ching JY, et al. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-pacific Crohn’s and Colitis Epidemiology Study. Gastroenterology. 2013;145(1):158-165.e2. doi:10.1053/j.gastro.2013.04.007
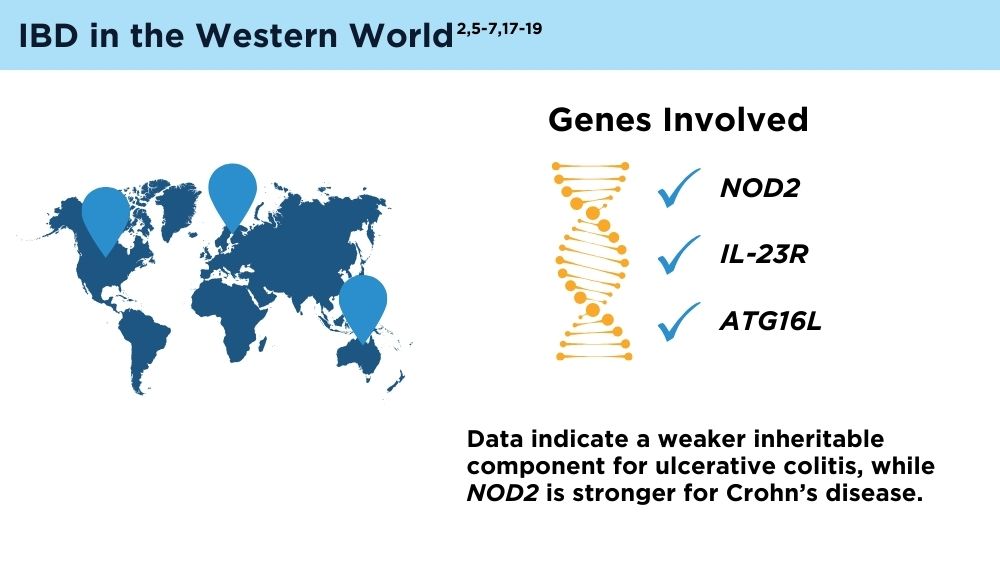
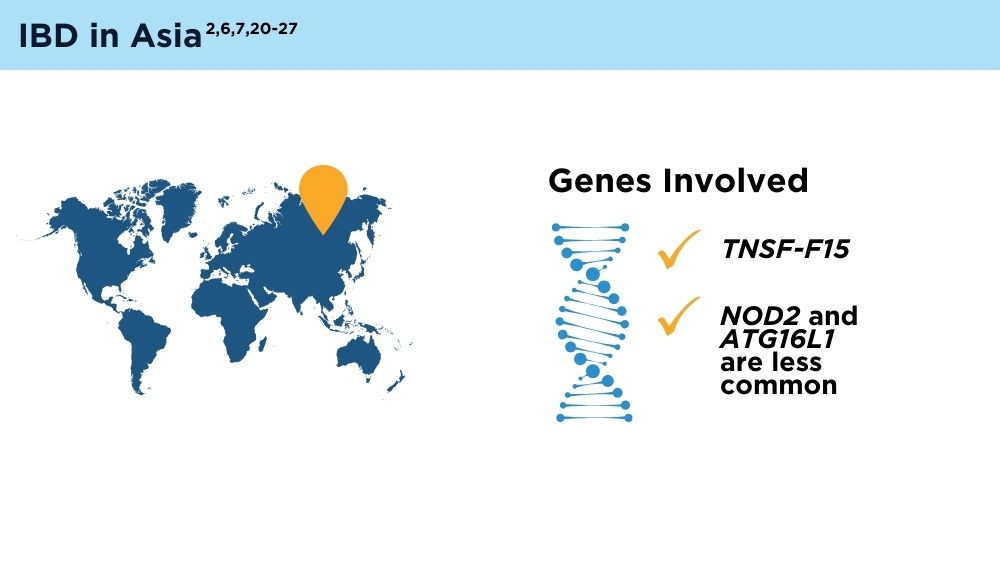
- Ng SC, Tsoi KK, Kamm MA, et al. Genetics of inflammatory bowel disease in Asia: systematic review and meta-analysis. Inflamm Bowel Dis. 2012;18(6):1164-1176. doi:10.1002/ibd.21845
- Banerjee R, Pal P, Mak JWY, Ng SC. Challenges in the diagnosis and management of inflammatory bowel disease in resource-limited settings in Asia. Lancet Gastroenterol Hepatol. 2020;5(12):1076-1088. doi:10.1016/S2468-1253(20)30299-5
- Ng SC, Mak JWY, Pal P, Banerjee R. Optimising management strategies of inflammatory bowel disease in resource-limited settings in Asia. Lancet Gastroenterol Hepatol. 2020;5(12):1089-1100. 10.1016/S2468-1253(20)30298-3
- Ng SC. Emerging trends of inflammatory bowel disease in Asia. Gastroenterol Hepatol (N Y). 2016;12(3):193-196. PMID: 27231449
- Ran Z, Wu K, Matsuoka K, et al. Asian Organization for Crohn’s and Colitis and Asia Pacific Association of Gastroenterology practice recommendations for medical management and monitoring of inflammatory bowel disease in Asia. J Gastroenterol Hepatol. 2021;36(3):637-645. doi:10.1111/jgh.15185
- Liu JZ, van Sommeren S, Huang H, et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet. 2015;47(9):979-986. doi:10.1038/ng.3359
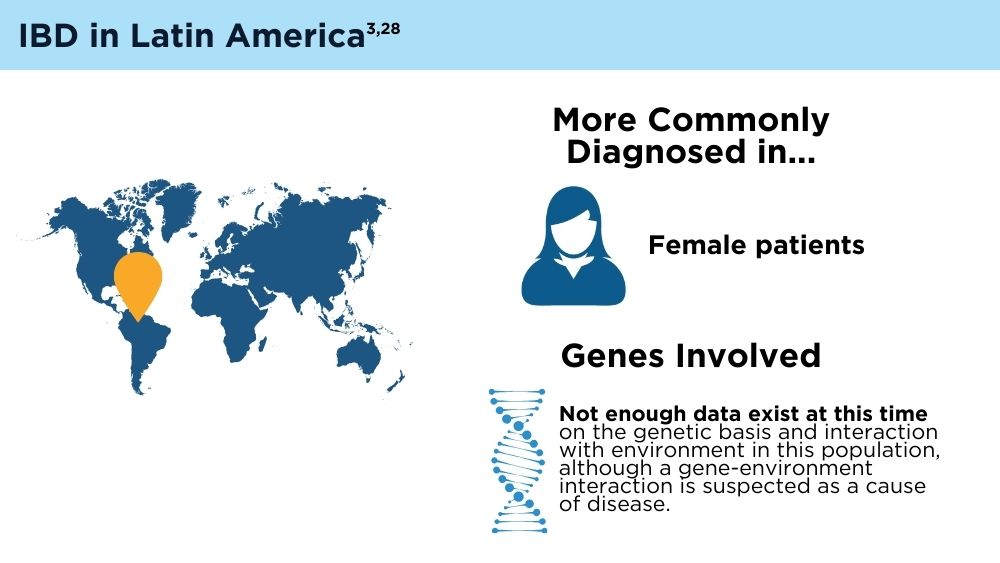
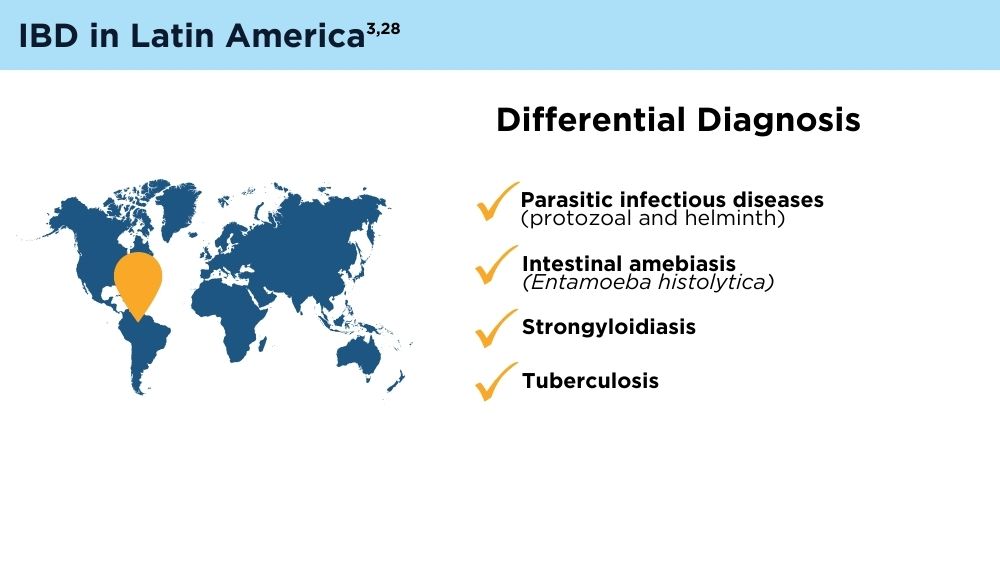
- Yamamoto-Furusho JK, Parra-Holguín NN, Juliao-Baños F, et al; for the EPILATAM study group. Clinical differentiation of inflammatory bowel disease (IBD) in Latin America and the Caribbean. Medicine (Baltimore). 2022;101(3):e28624. doi:10.1097/MD.0000000000028624
- Kaplan GG, Windsor JW. The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2021;18(1):56-66. doi:10.1038/s41575-020-00360-x
- Kaplan GG, Ng SC. Understanding and preventing the global increase of inflammatory bowel disease [published correction appears in Gastroenterology. 2017;152(8):2084]. Gastroenterology. 2017;152(2):313-321.e2. doi:10.1053/j.gastro.2016.10.020
- Balderramo D, Quaresma AB, Olivera PA, et al. Challenges in diagnosis and treatment of inflammatory bowel disease in Latin America. Lancet Gastroenterol Hepatol. 2024; 9(3):263-272. doi:10.1016/S2468-1253(23)00284-4
- Song EM, Na SY, Hong SN, Ng SC, Hisamatsu T, Ye BD. Treatment of inflammatory bowel disease–Asian perspectives: the results of a multinational web-based survey in the 8th Asian Organization for Crohn’s and Colitis meeting. Intest Res. 2023;21(3):339-352. doi:10.5217/ir.2022.00135
- GBD 2017 Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5(1):17-30. doi:10.1016/S2468-1253(19)30333-4
- Chen X, Xiang X, Xia W, et al. Evolving trends and burden of inflammatory bowel disease in Asia, 1990-2019: a comprehensive analysis based on the Global Burden of Disease Study. J Epidemiol Glob Health. 2023;13(4):725-739. doi:10.1007/s44197-023-00145-w
- Zhao M, Feng R, Ben-Horin S, et al. Systematic review with meta-analysis: environmental and dietary differences of inflammatory bowel disease in Eastern and Western populations. Aliment Pharmacol Ther. 2022;55(3):266-276. doi:10.1111/apt.16703
- Lewis JD, Parlett LE, Jonsson Funk ML, et al. Incidence, prevalence, and racial and ethnic distribution of inflammatory bowel disease in the United States. Gastroenterology. 2023;165(5):1197-1205.e2. doi:10.1053/j.gastro.2023.07.003
- Quaresma AB, Damiao AOMC, Coy CSR, et al. Temporal trends in the epidemiology of inflammatory bowel diseases in the public healthcare system in Brazil: a large population-based study. Lancet Reg Health Am. 2022;13:100298. doi:10.1016/j.lana.2022.100298
- Gordon H, Burisch J, Ellul P, et al. ECCO guidelines on extraintestinal manifestations in inflammatory bowel disease. J Crohns Colitis. 2024;18(1):1-37. doi:10.1093/ecco-jcc/jjad108
- Coward S, Benchimol EI, Bernstein CN, et al; Canadian Gastro-Intestinal Epidemiology Consortium (CanGIEC). Forecasting the Incidence and Prevalence of Inflammatory Bowel Disease: A Canadian Nationwide Analysis. Am J Gastroenterol. 2024 Mar 18. doi:10.14309/ajg.0000000000002687. Epub ahead of print. PMID: 38299598.
- Dorn-Rasmussen M, Lo B, Zhao M, Kaplan GG, Malham M, Wewer V, Burisch J. The Incidence and Prevalence of Paediatric- and Adult-Onset Inflammatory Bowel Disease in Denmark During a 37-Year Period: A Nationwide Cohort Study (1980-2017). J Crohns Colitis. 2023;17(2):259- 268. doi:10.1093/ecco-jcc/jjac138. PMID: 36125076.
- Watermeyer G, Katsidzira L, Setshedi M, et al. Inflammatory bowel disease in sub-Saharan Africa: epidemiology, risk factors, and challenges in diagnosis. Lancet Gastroenterol Hepatol. 2022;7(10):952-961. doi:10.1016/S2468-1253(22)00047-4
- Stulman MY, Asayag N, Focht G, et al. Epidemiology of Inflammatory Bowel Diseases in Israel: A Nationwide Epi-Israeli IBD Research Nucleus Study. Inflamm Bowel Dis. 2021;27(11):1784-1794. doi:10.1093/ibd/izaa341
- Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies [published correction appears in Lancet. 2020;396(10256):e56]. Lancet. 2017;390(10114):2769-2778. doi:10.1016/S0140-6736(17)32448-0
- Busingye D, Pollack A, Chidwick K. Prevalence of inflammatory bowel disease in the Australian general practice population: A cross-sectional study. PLoS One. 2021;16(5):e0252458. Published 2021 May 27. doi:10.1371/ journal.pone.0252458
- Gecse KB, Vermeire S. Differential diagnosis of inflammatory bowel disease: imitations and complications. Lancet Gastroenterol Hepatol. 2018;3(9):644-653. doi:10.1016/S2468-1253(18)30159-6
- Inflammatory bowel disease (IBD): comorbidities. Centers for Disease Control and Prevention. Last reviewed April 14, 2022. Accessed February 21, 2024. https://www.cdc.gov/ibd/data-and-statistics/comorbidities.html
- Mosli MH, Alsahafi M, Alsanea MN, Alhasani F, Ahmed M, Saadah O. Multimorbidity among inflammatory bowel disease patients in a tertiary care center: a retrospective study. BMC Gastroenterol. 2022;22(1):487. doi:10.1186/s12876-022-02578-2
- Inflammatory bowel disease (IBD). Mayo Clinic. September 3, 2022. Accessed February 21, 2024. https://www.mayoclinic.org/diseases-conditions/inflammatory-bowel-disease/diagnosis-treatment/drc-20353320
- Ng SC, Tang W, Ching JY, et al. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-pacific Crohn’s and Colitis Epidemiology Study. Gastroenterology. 2013;145(1):158-165.e2. doi:10.1053/j.gastro.2013.04.007
- Ng SC, Tsoi KK, Kamm MA, et al. Genetics of inflammatory bowel disease in Asia: systematic review and meta-analysis. Inflamm Bowel Dis. 2012;18(6):1164-1176. doi:10.1002/ibd.21845
- Banerjee R, Pal P, Mak JWY, Ng SC. Challenges in the diagnosis and management of inflammatory bowel disease in resource-limited settings in Asia. Lancet Gastroenterol Hepatol. 2020;5(12):1076-1088. doi:10.1016/S2468-1253(20)30299-5
- Ng SC, Mak JWY, Pal P, Banerjee R. Optimising management strategies of inflammatory bowel disease in resource-limited settings in Asia. Lancet Gastroenterol Hepatol. 2020;5(12):1089-1100. 10.1016/S2468-1253(20)30298-3
- Ng SC. Emerging trends of inflammatory bowel disease in Asia. Gastroenterol Hepatol (N Y). 2016;12(3):193-196. PMID: 27231449
- Ran Z, Wu K, Matsuoka K, et al. Asian Organization for Crohn’s and Colitis and Asia Pacific Association of Gastroenterology practice recommendations for medical management and monitoring of inflammatory bowel disease in Asia. J Gastroenterol Hepatol. 2021;36(3):637-645. doi:10.1111/jgh.15185
- Liu JZ, van Sommeren S, Huang H, et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet. 2015;47(9):979-986. doi:10.1038/ng.3359
- Yamamoto-Furusho JK, Parra-Holguín NN, Juliao-Baños F, et al; for the EPILATAM study group. Clinical differentiation of inflammatory bowel disease (IBD) in Latin America and the Caribbean. Medicine (Baltimore). 2022;101(3):e28624. doi:10.1097/MD.0000000000028624
- Kaplan GG, Windsor JW. The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2021;18(1):56-66. doi:10.1038/s41575-020-00360-x
- Kaplan GG, Ng SC. Understanding and preventing the global increase of inflammatory bowel disease [published correction appears in Gastroenterology. 2017;152(8):2084]. Gastroenterology. 2017;152(2):313-321.e2. doi:10.1053/j.gastro.2016.10.020
- Balderramo D, Quaresma AB, Olivera PA, et al. Challenges in diagnosis and treatment of inflammatory bowel disease in Latin America. Lancet Gastroenterol Hepatol. 2024; 9(3):263-272. doi:10.1016/S2468-1253(23)00284-4
- Song EM, Na SY, Hong SN, Ng SC, Hisamatsu T, Ye BD. Treatment of inflammatory bowel disease–Asian perspectives: the results of a multinational web-based survey in the 8th Asian Organization for Crohn’s and Colitis meeting. Intest Res. 2023;21(3):339-352. doi:10.5217/ir.2022.00135
- GBD 2017 Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5(1):17-30. doi:10.1016/S2468-1253(19)30333-4
- Chen X, Xiang X, Xia W, et al. Evolving trends and burden of inflammatory bowel disease in Asia, 1990-2019: a comprehensive analysis based on the Global Burden of Disease Study. J Epidemiol Glob Health. 2023;13(4):725-739. doi:10.1007/s44197-023-00145-w
- Zhao M, Feng R, Ben-Horin S, et al. Systematic review with meta-analysis: environmental and dietary differences of inflammatory bowel disease in Eastern and Western populations. Aliment Pharmacol Ther. 2022;55(3):266-276. doi:10.1111/apt.16703
- Lewis JD, Parlett LE, Jonsson Funk ML, et al. Incidence, prevalence, and racial and ethnic distribution of inflammatory bowel disease in the United States. Gastroenterology. 2023;165(5):1197-1205.e2. doi:10.1053/j.gastro.2023.07.003
- Quaresma AB, Damiao AOMC, Coy CSR, et al. Temporal trends in the epidemiology of inflammatory bowel diseases in the public healthcare system in Brazil: a large population-based study. Lancet Reg Health Am. 2022;13:100298. doi:10.1016/j.lana.2022.100298
- Gordon H, Burisch J, Ellul P, et al. ECCO guidelines on extraintestinal manifestations in inflammatory bowel disease. J Crohns Colitis. 2024;18(1):1-37. doi:10.1093/ecco-jcc/jjad108
- Coward S, Benchimol EI, Bernstein CN, et al; Canadian Gastro-Intestinal Epidemiology Consortium (CanGIEC). Forecasting the Incidence and Prevalence of Inflammatory Bowel Disease: A Canadian Nationwide Analysis. Am J Gastroenterol. 2024 Mar 18. doi:10.14309/ajg.0000000000002687. Epub ahead of print. PMID: 38299598.
- Dorn-Rasmussen M, Lo B, Zhao M, Kaplan GG, Malham M, Wewer V, Burisch J. The Incidence and Prevalence of Paediatric- and Adult-Onset Inflammatory Bowel Disease in Denmark During a 37-Year Period: A Nationwide Cohort Study (1980-2017). J Crohns Colitis. 2023;17(2):259- 268. doi:10.1093/ecco-jcc/jjac138. PMID: 36125076.
- Watermeyer G, Katsidzira L, Setshedi M, et al. Inflammatory bowel disease in sub-Saharan Africa: epidemiology, risk factors, and challenges in diagnosis. Lancet Gastroenterol Hepatol. 2022;7(10):952-961. doi:10.1016/S2468-1253(22)00047-4
- Stulman MY, Asayag N, Focht G, et al. Epidemiology of Inflammatory Bowel Diseases in Israel: A Nationwide Epi-Israeli IBD Research Nucleus Study. Inflamm Bowel Dis. 2021;27(11):1784-1794. doi:10.1093/ibd/izaa341
- Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies [published correction appears in Lancet. 2020;396(10256):e56]. Lancet. 2017;390(10114):2769-2778. doi:10.1016/S0140-6736(17)32448-0
- Busingye D, Pollack A, Chidwick K. Prevalence of inflammatory bowel disease in the Australian general practice population: A cross-sectional study. PLoS One. 2021;16(5):e0252458. Published 2021 May 27. doi:10.1371/ journal.pone.0252458
- Gecse KB, Vermeire S. Differential diagnosis of inflammatory bowel disease: imitations and complications. Lancet Gastroenterol Hepatol. 2018;3(9):644-653. doi:10.1016/S2468-1253(18)30159-6
- Inflammatory bowel disease (IBD): comorbidities. Centers for Disease Control and Prevention. Last reviewed April 14, 2022. Accessed February 21, 2024. https://www.cdc.gov/ibd/data-and-statistics/comorbidities.html
- Mosli MH, Alsahafi M, Alsanea MN, Alhasani F, Ahmed M, Saadah O. Multimorbidity among inflammatory bowel disease patients in a tertiary care center: a retrospective study. BMC Gastroenterol. 2022;22(1):487. doi:10.1186/s12876-022-02578-2
- Inflammatory bowel disease (IBD). Mayo Clinic. September 3, 2022. Accessed February 21, 2024. https://www.mayoclinic.org/diseases-conditions/inflammatory-bowel-disease/diagnosis-treatment/drc-20353320
- Ng SC, Tang W, Ching JY, et al. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-pacific Crohn’s and Colitis Epidemiology Study. Gastroenterology. 2013;145(1):158-165.e2. doi:10.1053/j.gastro.2013.04.007
- Ng SC, Tsoi KK, Kamm MA, et al. Genetics of inflammatory bowel disease in Asia: systematic review and meta-analysis. Inflamm Bowel Dis. 2012;18(6):1164-1176. doi:10.1002/ibd.21845
- Banerjee R, Pal P, Mak JWY, Ng SC. Challenges in the diagnosis and management of inflammatory bowel disease in resource-limited settings in Asia. Lancet Gastroenterol Hepatol. 2020;5(12):1076-1088. doi:10.1016/S2468-1253(20)30299-5
- Ng SC, Mak JWY, Pal P, Banerjee R. Optimising management strategies of inflammatory bowel disease in resource-limited settings in Asia. Lancet Gastroenterol Hepatol. 2020;5(12):1089-1100. 10.1016/S2468-1253(20)30298-3
- Ng SC. Emerging trends of inflammatory bowel disease in Asia. Gastroenterol Hepatol (N Y). 2016;12(3):193-196. PMID: 27231449
- Ran Z, Wu K, Matsuoka K, et al. Asian Organization for Crohn’s and Colitis and Asia Pacific Association of Gastroenterology practice recommendations for medical management and monitoring of inflammatory bowel disease in Asia. J Gastroenterol Hepatol. 2021;36(3):637-645. doi:10.1111/jgh.15185
- Liu JZ, van Sommeren S, Huang H, et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet. 2015;47(9):979-986. doi:10.1038/ng.3359
- Yamamoto-Furusho JK, Parra-Holguín NN, Juliao-Baños F, et al; for the EPILATAM study group. Clinical differentiation of inflammatory bowel disease (IBD) in Latin America and the Caribbean. Medicine (Baltimore). 2022;101(3):e28624. doi:10.1097/MD.0000000000028624
Investigational MS Med Nearly Eliminates Disease Activity on MRI
NASHVILLE, TENNESSEE — , new trial data suggested.
Researchers found a near absence of new brain lesions at 48 weeks in patients on the highest dose. At this level of disease suppression, there was no evidence of increased infection risk, which investigators said might relate to its mechanism of action. In addition, there were no thrombotic events, which is what defeated a first-generation drug in this same class.
Among those initially randomly assigned to receive 1200 mg every 4 weeks, 96% were free of new gadolinium-positive (Gd+ T1) lesions at 48 weeks, reported investigator Yang Mao-Draayer, MD, PhD, director of Clinical and Experimental Therapeutics at the Oklahoma Medical Research Foundation’s Multiple Sclerosis Center of Excellence, Oklahoma City. Annual relapse rates were also low.
The findings were presented at the annual meeting of the Consortium of Multiple Sclerosis Centers.
No Effect on Lymphocyte Count
As previously reported, 12-week frexalimab results were noteworthy because they provided validation for CD40L as a target in the control of MS. One of the unique features of this therapy relative to many other immunomodulatory therapies is that it has shown little, if any, effect on lymphocyte counts or immunoglobulin levels.
In the double-blind randomized phase 2 trial, 125 patients with MS of all other MS therapy were randomized in a 4:4:4:1 ratio to 1200-mg frexalimab administered intravenously every 4 weeks after a loading dose, to 300-mg frexalimab administered subcutaneously every 2 weeks after a loading dose, or to one of the two matching placebo arms.
For the primary endpoint of new Gd+ T1 lesions at the end of the blinded study, the rates at week 12 were 0.2 and 0.3 in the higher- and lower-dose treatment groups, respectively, and 1.4 in the pooled placebo groups.
At 48 weeks, the results were even better. From 12 weeks, the rate of Gd+ T1 lesions in the high-dose group continued to fall, reaching 0.1 at week 24 and 0.0 at week 48. In the lower-dose group, there was also a stepwise decline over time with a value of 0.2 at week 48. The annual relapse rate at week 48 was 0.4.
Reengineered Agent
In the placebo groups, the same type of suppression of disease activity was observed after they were switched to active therapy at the end of 12 weeks.
By 24 weeks, the number of new Gd+ T1 lesions had fallen to 0.3 in placebo patients switched to the higher dose and 1.0 in those switched to the lower dose.
By week 48, the rates were 0.2 in both of the switch arms.
The proportions of patients free of new Gd+ T1 lesions at 48 weeks were 96% in the group started and maintained on the highest dose of frexalimab, 87% in those started and maintained on the lower dose, 90% in those started on placebo and switched to the highest dose of frexalimab, and 92% of placebo patients switched to the lower dose.
“T2 lesion volume from baseline through week 48 was stable in patients who continued receiving frexalimab and decreased in placebo participants after switching to frexalimab at week 12,” Dr. Mao-Draayer reported.
The CD40-CD40L co-stimulatory pathway that regulates both adaptive and innate immune responses has been pursued as a target for MS therapies for decades, Dr. Mao-Draayer said.
A first-generation monoclonal antibody directed at elevated levels of CD40L, which is implicated in the inflammation that drives MS, showed promise but was abandoned after it was associated with an increased risk for thromboembolic events in a phase 1 trial, she said.
However, the second-generation agent was engineered to avoid an interaction with platelets, which played a role in the risk for thrombosis associated with the failure of the earlier drug.
As with the first-generation agent, frexalimab had little or no impact on lymphocyte count or immunoglobulin G and immunoglobulin M levels. Both remained stable during the 12-week controlled trial and through the ongoing open-label extension, Dr. Mao-Draayer said.
This might be a factor in the low level of adverse events. Most importantly, there have been no thromboembolic events associated with frexalimab so far, but the follow-up data also show rates of infection and other events, such as nasopharyngitis, that were comparable with placebo in the 12-week controlled trial and have not increased over longer-term monitoring.
Such adverse events as headache and COVID-19 infection have also occurred at rates similar to placebo.
Two phase 3 trials are underway. FREXALT is being conducted in relapsing-remitting MS. FREVIV has enrolled patients with nonrelapsing secondary progressive MS.
Impressively Low New Lesion Count
Commenting on the findings, Jeffrey Cohen, MD, director of the Mellen Center for Multiple Sclerosis, Cleveland Clinic, who was not involved in the research, said that over the course of the extended follow-up, MS activity in the central nervous system as measured with new Gd+ T1 lesions was impressively low.
He noted that the phase 2 open-label follow-up continues to support the promise of frexalimab. But Dr. Cohen cautioned that this does not obviate the need for phase 3 data.
In particular, he said that an immunomodulatory agent that does not affect the lymphocyte count has a theoretical advantage, but pointed out that the benefit is still presumably mediated by blocking pathways that mediate autoimmune activity.
Even if lymphocyte count is unaffected, the immunomodulatory pathway by which frexalimab does exert its benefit might pose a different set of risks, he said.
“We will not have sufficient data to judge the promise of this agent until the phase 3 trials are completed,” he said.
Dr. Mao-Draayer reported financial relationships with Acorda, Bayer, Biogen, Bristol Myers Squibb, Celgene, EMD Serono, Genentech, Horizon, Janssen, Novartis, Questor, Teva, and Sanofi, which provided funding for the phase 2 frexalimab trial. Dr. Cohen reported financial relationships with Astoria, Convelo, EMD Serono, FiND, INmune, and Sandoz.
A version of this article appeared on Medscape.com.
NASHVILLE, TENNESSEE — , new trial data suggested.
Researchers found a near absence of new brain lesions at 48 weeks in patients on the highest dose. At this level of disease suppression, there was no evidence of increased infection risk, which investigators said might relate to its mechanism of action. In addition, there were no thrombotic events, which is what defeated a first-generation drug in this same class.
Among those initially randomly assigned to receive 1200 mg every 4 weeks, 96% were free of new gadolinium-positive (Gd+ T1) lesions at 48 weeks, reported investigator Yang Mao-Draayer, MD, PhD, director of Clinical and Experimental Therapeutics at the Oklahoma Medical Research Foundation’s Multiple Sclerosis Center of Excellence, Oklahoma City. Annual relapse rates were also low.
The findings were presented at the annual meeting of the Consortium of Multiple Sclerosis Centers.
No Effect on Lymphocyte Count
As previously reported, 12-week frexalimab results were noteworthy because they provided validation for CD40L as a target in the control of MS. One of the unique features of this therapy relative to many other immunomodulatory therapies is that it has shown little, if any, effect on lymphocyte counts or immunoglobulin levels.
In the double-blind randomized phase 2 trial, 125 patients with MS of all other MS therapy were randomized in a 4:4:4:1 ratio to 1200-mg frexalimab administered intravenously every 4 weeks after a loading dose, to 300-mg frexalimab administered subcutaneously every 2 weeks after a loading dose, or to one of the two matching placebo arms.
For the primary endpoint of new Gd+ T1 lesions at the end of the blinded study, the rates at week 12 were 0.2 and 0.3 in the higher- and lower-dose treatment groups, respectively, and 1.4 in the pooled placebo groups.
At 48 weeks, the results were even better. From 12 weeks, the rate of Gd+ T1 lesions in the high-dose group continued to fall, reaching 0.1 at week 24 and 0.0 at week 48. In the lower-dose group, there was also a stepwise decline over time with a value of 0.2 at week 48. The annual relapse rate at week 48 was 0.4.
Reengineered Agent
In the placebo groups, the same type of suppression of disease activity was observed after they were switched to active therapy at the end of 12 weeks.
By 24 weeks, the number of new Gd+ T1 lesions had fallen to 0.3 in placebo patients switched to the higher dose and 1.0 in those switched to the lower dose.
By week 48, the rates were 0.2 in both of the switch arms.
The proportions of patients free of new Gd+ T1 lesions at 48 weeks were 96% in the group started and maintained on the highest dose of frexalimab, 87% in those started and maintained on the lower dose, 90% in those started on placebo and switched to the highest dose of frexalimab, and 92% of placebo patients switched to the lower dose.
“T2 lesion volume from baseline through week 48 was stable in patients who continued receiving frexalimab and decreased in placebo participants after switching to frexalimab at week 12,” Dr. Mao-Draayer reported.
The CD40-CD40L co-stimulatory pathway that regulates both adaptive and innate immune responses has been pursued as a target for MS therapies for decades, Dr. Mao-Draayer said.
A first-generation monoclonal antibody directed at elevated levels of CD40L, which is implicated in the inflammation that drives MS, showed promise but was abandoned after it was associated with an increased risk for thromboembolic events in a phase 1 trial, she said.
However, the second-generation agent was engineered to avoid an interaction with platelets, which played a role in the risk for thrombosis associated with the failure of the earlier drug.
As with the first-generation agent, frexalimab had little or no impact on lymphocyte count or immunoglobulin G and immunoglobulin M levels. Both remained stable during the 12-week controlled trial and through the ongoing open-label extension, Dr. Mao-Draayer said.
This might be a factor in the low level of adverse events. Most importantly, there have been no thromboembolic events associated with frexalimab so far, but the follow-up data also show rates of infection and other events, such as nasopharyngitis, that were comparable with placebo in the 12-week controlled trial and have not increased over longer-term monitoring.
Such adverse events as headache and COVID-19 infection have also occurred at rates similar to placebo.
Two phase 3 trials are underway. FREXALT is being conducted in relapsing-remitting MS. FREVIV has enrolled patients with nonrelapsing secondary progressive MS.
Impressively Low New Lesion Count
Commenting on the findings, Jeffrey Cohen, MD, director of the Mellen Center for Multiple Sclerosis, Cleveland Clinic, who was not involved in the research, said that over the course of the extended follow-up, MS activity in the central nervous system as measured with new Gd+ T1 lesions was impressively low.
He noted that the phase 2 open-label follow-up continues to support the promise of frexalimab. But Dr. Cohen cautioned that this does not obviate the need for phase 3 data.
In particular, he said that an immunomodulatory agent that does not affect the lymphocyte count has a theoretical advantage, but pointed out that the benefit is still presumably mediated by blocking pathways that mediate autoimmune activity.
Even if lymphocyte count is unaffected, the immunomodulatory pathway by which frexalimab does exert its benefit might pose a different set of risks, he said.
“We will not have sufficient data to judge the promise of this agent until the phase 3 trials are completed,” he said.
Dr. Mao-Draayer reported financial relationships with Acorda, Bayer, Biogen, Bristol Myers Squibb, Celgene, EMD Serono, Genentech, Horizon, Janssen, Novartis, Questor, Teva, and Sanofi, which provided funding for the phase 2 frexalimab trial. Dr. Cohen reported financial relationships with Astoria, Convelo, EMD Serono, FiND, INmune, and Sandoz.
A version of this article appeared on Medscape.com.
NASHVILLE, TENNESSEE — , new trial data suggested.
Researchers found a near absence of new brain lesions at 48 weeks in patients on the highest dose. At this level of disease suppression, there was no evidence of increased infection risk, which investigators said might relate to its mechanism of action. In addition, there were no thrombotic events, which is what defeated a first-generation drug in this same class.
Among those initially randomly assigned to receive 1200 mg every 4 weeks, 96% were free of new gadolinium-positive (Gd+ T1) lesions at 48 weeks, reported investigator Yang Mao-Draayer, MD, PhD, director of Clinical and Experimental Therapeutics at the Oklahoma Medical Research Foundation’s Multiple Sclerosis Center of Excellence, Oklahoma City. Annual relapse rates were also low.
The findings were presented at the annual meeting of the Consortium of Multiple Sclerosis Centers.
No Effect on Lymphocyte Count
As previously reported, 12-week frexalimab results were noteworthy because they provided validation for CD40L as a target in the control of MS. One of the unique features of this therapy relative to many other immunomodulatory therapies is that it has shown little, if any, effect on lymphocyte counts or immunoglobulin levels.
In the double-blind randomized phase 2 trial, 125 patients with MS of all other MS therapy were randomized in a 4:4:4:1 ratio to 1200-mg frexalimab administered intravenously every 4 weeks after a loading dose, to 300-mg frexalimab administered subcutaneously every 2 weeks after a loading dose, or to one of the two matching placebo arms.
For the primary endpoint of new Gd+ T1 lesions at the end of the blinded study, the rates at week 12 were 0.2 and 0.3 in the higher- and lower-dose treatment groups, respectively, and 1.4 in the pooled placebo groups.
At 48 weeks, the results were even better. From 12 weeks, the rate of Gd+ T1 lesions in the high-dose group continued to fall, reaching 0.1 at week 24 and 0.0 at week 48. In the lower-dose group, there was also a stepwise decline over time with a value of 0.2 at week 48. The annual relapse rate at week 48 was 0.4.
Reengineered Agent
In the placebo groups, the same type of suppression of disease activity was observed after they were switched to active therapy at the end of 12 weeks.
By 24 weeks, the number of new Gd+ T1 lesions had fallen to 0.3 in placebo patients switched to the higher dose and 1.0 in those switched to the lower dose.
By week 48, the rates were 0.2 in both of the switch arms.
The proportions of patients free of new Gd+ T1 lesions at 48 weeks were 96% in the group started and maintained on the highest dose of frexalimab, 87% in those started and maintained on the lower dose, 90% in those started on placebo and switched to the highest dose of frexalimab, and 92% of placebo patients switched to the lower dose.
“T2 lesion volume from baseline through week 48 was stable in patients who continued receiving frexalimab and decreased in placebo participants after switching to frexalimab at week 12,” Dr. Mao-Draayer reported.
The CD40-CD40L co-stimulatory pathway that regulates both adaptive and innate immune responses has been pursued as a target for MS therapies for decades, Dr. Mao-Draayer said.
A first-generation monoclonal antibody directed at elevated levels of CD40L, which is implicated in the inflammation that drives MS, showed promise but was abandoned after it was associated with an increased risk for thromboembolic events in a phase 1 trial, she said.
However, the second-generation agent was engineered to avoid an interaction with platelets, which played a role in the risk for thrombosis associated with the failure of the earlier drug.
As with the first-generation agent, frexalimab had little or no impact on lymphocyte count or immunoglobulin G and immunoglobulin M levels. Both remained stable during the 12-week controlled trial and through the ongoing open-label extension, Dr. Mao-Draayer said.
This might be a factor in the low level of adverse events. Most importantly, there have been no thromboembolic events associated with frexalimab so far, but the follow-up data also show rates of infection and other events, such as nasopharyngitis, that were comparable with placebo in the 12-week controlled trial and have not increased over longer-term monitoring.
Such adverse events as headache and COVID-19 infection have also occurred at rates similar to placebo.
Two phase 3 trials are underway. FREXALT is being conducted in relapsing-remitting MS. FREVIV has enrolled patients with nonrelapsing secondary progressive MS.
Impressively Low New Lesion Count
Commenting on the findings, Jeffrey Cohen, MD, director of the Mellen Center for Multiple Sclerosis, Cleveland Clinic, who was not involved in the research, said that over the course of the extended follow-up, MS activity in the central nervous system as measured with new Gd+ T1 lesions was impressively low.
He noted that the phase 2 open-label follow-up continues to support the promise of frexalimab. But Dr. Cohen cautioned that this does not obviate the need for phase 3 data.
In particular, he said that an immunomodulatory agent that does not affect the lymphocyte count has a theoretical advantage, but pointed out that the benefit is still presumably mediated by blocking pathways that mediate autoimmune activity.
Even if lymphocyte count is unaffected, the immunomodulatory pathway by which frexalimab does exert its benefit might pose a different set of risks, he said.
“We will not have sufficient data to judge the promise of this agent until the phase 3 trials are completed,” he said.
Dr. Mao-Draayer reported financial relationships with Acorda, Bayer, Biogen, Bristol Myers Squibb, Celgene, EMD Serono, Genentech, Horizon, Janssen, Novartis, Questor, Teva, and Sanofi, which provided funding for the phase 2 frexalimab trial. Dr. Cohen reported financial relationships with Astoria, Convelo, EMD Serono, FiND, INmune, and Sandoz.
A version of this article appeared on Medscape.com.
FROM CMSC 2024
Strategies for MS Fatigue and Sleep Issues
NASHVILLE, TENNESSEE —
Fatigue related to MS is complex, but it often follows a pattern. “Oftentimes when I meet with patients for the first time, they’re not always sure [what their own pattern is]. They know that the fatigue is present, and it’s limiting their activities. It’s important for us to break down and see that pattern for [the patient] specifically, and what are some ways that we can intervene to perhaps make that pattern something that improves quality of life and day-to-day living,” said Grace Tworek, PsyD, during a presentation at the annual meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
A cycle may start on a day that a patient has lots of energy. They are ambitious that day and get a lot done on their “to do” list while they have the energy. Unfortunately, they commonly overdo it, leading to fatigue the next day. Over ensuing days, the patient might feel unable to engage in everyday tasks and begin to feel they are falling behind. This in turn can affect mood, resulting in increased symptoms of depression and anxiety. That leads to days of inactivity and rest, which leads to recovery. Then comes a day with better mood and increased energy, where the cycle can begin again.
It’s an addressable problem. “What we really want to do is break this cycle, get out of those peaks and valleys of high energy days and very low energy days to try to create more sustainable patterns” said Dr. Tworek, who is a staff health psychologist at Cleveland Clinic’s Mellen Center for Multiple Sclerosis, Cleveland, Ohio.
Fatigue
When addressing fatigue in MS patients, Dr. Tworek and her colleagues begin with a fatigue diary that includes typical activities engaged in throughout the day. It also distinguishes between activities the patient feels are important and activities that give them satisfaction.
“If we can find ways to include these [satisfying] activities, and not focus only on those important activities. This is where that quality of life really comes into play. But I always say to folks, we are not striving for perfection at first. I want you to write down what’s actually happening so we can use this data to later inform how we are going to make changes,” said Dr. Tworek.
It’s also important to encourage patients to seek help. Activities that are neither important nor satisfying may not need doing at all, and they encourage patients to seek help in other tasks. As for tasks that are important in their day-to-day lives, “How can we break those down? We break those down by pacing activities,” said Dr. Tworek.
A simple way to pace yourself is to use “The rule of two.” It asks: How long can I do a task before I experience a two-point increase on a 1-10 fatigue scale. “At that time, is when we want to start inserting breaks. We want to find activities we can do that will reduce [fatigue] or get us back to baseline. Or if that’s not realistic, keep us where we are at rather than increasing fatigue,” said Dr. Tworek.
Another way to think about it is spoon theory, sometimes referred to as coin theory. The idea is that you wake up each morning with ten spoons. Each task on a given day will cost a certain number of spoons. “You might start your day, you go downstairs, you have breakfast, and you’re already down to seven points, the next day, you might still be at 10. So it’s really about monitoring where you’re at in terms of how many coins or spoons you’re spending so that we can then reflect on how many coins or spoons do I have left?” said Dr. Tworek.
The strategy can aid communication with partners or family members who may have difficulty understanding MS fatigue. “Sometimes putting a number to it can really open up the doors to having these difficult conversations with friends and family,” said Dr. Tworek.
Sleep
Fatigue and sleep are naturally intertwined, and sleep problems are also common in MS, with 30%-56% reporting problems, depending on the estimate.
One concept to think about is sleep drive. “From the moment we wake up, we are building sleep pressure, just like from the moment you stop eating, your body starts building pressure to eat again,” said Dr. Tworek.
Naps can interfere with that drive, much like a snack can rob you of a meal-time appetite. “A nap is going to curb that appetite for sleep, making it more difficult potentially to fall asleep,” said Dr. Tworek. If a nap is absolutely necessary, it’s better to do it earlier in the day to allow time to build sleep pressure again.
As with fatigue, Dr. Tworek has patients fill out a sleep diary that documents difficulty falling or staying asleep, timing and length of awakenings, quality of sleep, length and timing of any naps, and other factors. It sometimes reveals patterns, like difficulty falling asleep on specific days of the week. Such rhythms may be attributable to regular stressors, like anticipating some event the next morning. Then it might be possible to tie in other techniques like stress management to reduce accompanying anxiety.
Sleep hygiene is an important factor, employing strategies like staying off screens or social media while in bed. “About 1 hour before bedtime, we want to try to create some relaxation time,” said Dr. Tworek.
Her clinic also emphasizes consistent wake time. “If we are waking every day in about the same half hour period, we are able to build that sleep pressure consistently. [Then] your body is going to let you know when it is time for bed. You’re going to feel sleepiness,” said Dr. Tworek.
Dr. Tworek did not report any disclosures or conflicts of interest.
NASHVILLE, TENNESSEE —
Fatigue related to MS is complex, but it often follows a pattern. “Oftentimes when I meet with patients for the first time, they’re not always sure [what their own pattern is]. They know that the fatigue is present, and it’s limiting their activities. It’s important for us to break down and see that pattern for [the patient] specifically, and what are some ways that we can intervene to perhaps make that pattern something that improves quality of life and day-to-day living,” said Grace Tworek, PsyD, during a presentation at the annual meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
A cycle may start on a day that a patient has lots of energy. They are ambitious that day and get a lot done on their “to do” list while they have the energy. Unfortunately, they commonly overdo it, leading to fatigue the next day. Over ensuing days, the patient might feel unable to engage in everyday tasks and begin to feel they are falling behind. This in turn can affect mood, resulting in increased symptoms of depression and anxiety. That leads to days of inactivity and rest, which leads to recovery. Then comes a day with better mood and increased energy, where the cycle can begin again.
It’s an addressable problem. “What we really want to do is break this cycle, get out of those peaks and valleys of high energy days and very low energy days to try to create more sustainable patterns” said Dr. Tworek, who is a staff health psychologist at Cleveland Clinic’s Mellen Center for Multiple Sclerosis, Cleveland, Ohio.
Fatigue
When addressing fatigue in MS patients, Dr. Tworek and her colleagues begin with a fatigue diary that includes typical activities engaged in throughout the day. It also distinguishes between activities the patient feels are important and activities that give them satisfaction.
“If we can find ways to include these [satisfying] activities, and not focus only on those important activities. This is where that quality of life really comes into play. But I always say to folks, we are not striving for perfection at first. I want you to write down what’s actually happening so we can use this data to later inform how we are going to make changes,” said Dr. Tworek.
It’s also important to encourage patients to seek help. Activities that are neither important nor satisfying may not need doing at all, and they encourage patients to seek help in other tasks. As for tasks that are important in their day-to-day lives, “How can we break those down? We break those down by pacing activities,” said Dr. Tworek.
A simple way to pace yourself is to use “The rule of two.” It asks: How long can I do a task before I experience a two-point increase on a 1-10 fatigue scale. “At that time, is when we want to start inserting breaks. We want to find activities we can do that will reduce [fatigue] or get us back to baseline. Or if that’s not realistic, keep us where we are at rather than increasing fatigue,” said Dr. Tworek.
Another way to think about it is spoon theory, sometimes referred to as coin theory. The idea is that you wake up each morning with ten spoons. Each task on a given day will cost a certain number of spoons. “You might start your day, you go downstairs, you have breakfast, and you’re already down to seven points, the next day, you might still be at 10. So it’s really about monitoring where you’re at in terms of how many coins or spoons you’re spending so that we can then reflect on how many coins or spoons do I have left?” said Dr. Tworek.
The strategy can aid communication with partners or family members who may have difficulty understanding MS fatigue. “Sometimes putting a number to it can really open up the doors to having these difficult conversations with friends and family,” said Dr. Tworek.
Sleep
Fatigue and sleep are naturally intertwined, and sleep problems are also common in MS, with 30%-56% reporting problems, depending on the estimate.
One concept to think about is sleep drive. “From the moment we wake up, we are building sleep pressure, just like from the moment you stop eating, your body starts building pressure to eat again,” said Dr. Tworek.
Naps can interfere with that drive, much like a snack can rob you of a meal-time appetite. “A nap is going to curb that appetite for sleep, making it more difficult potentially to fall asleep,” said Dr. Tworek. If a nap is absolutely necessary, it’s better to do it earlier in the day to allow time to build sleep pressure again.
As with fatigue, Dr. Tworek has patients fill out a sleep diary that documents difficulty falling or staying asleep, timing and length of awakenings, quality of sleep, length and timing of any naps, and other factors. It sometimes reveals patterns, like difficulty falling asleep on specific days of the week. Such rhythms may be attributable to regular stressors, like anticipating some event the next morning. Then it might be possible to tie in other techniques like stress management to reduce accompanying anxiety.
Sleep hygiene is an important factor, employing strategies like staying off screens or social media while in bed. “About 1 hour before bedtime, we want to try to create some relaxation time,” said Dr. Tworek.
Her clinic also emphasizes consistent wake time. “If we are waking every day in about the same half hour period, we are able to build that sleep pressure consistently. [Then] your body is going to let you know when it is time for bed. You’re going to feel sleepiness,” said Dr. Tworek.
Dr. Tworek did not report any disclosures or conflicts of interest.
NASHVILLE, TENNESSEE —
Fatigue related to MS is complex, but it often follows a pattern. “Oftentimes when I meet with patients for the first time, they’re not always sure [what their own pattern is]. They know that the fatigue is present, and it’s limiting their activities. It’s important for us to break down and see that pattern for [the patient] specifically, and what are some ways that we can intervene to perhaps make that pattern something that improves quality of life and day-to-day living,” said Grace Tworek, PsyD, during a presentation at the annual meeting of the Consortium of Multiple Sclerosis Centers (CMSC).
A cycle may start on a day that a patient has lots of energy. They are ambitious that day and get a lot done on their “to do” list while they have the energy. Unfortunately, they commonly overdo it, leading to fatigue the next day. Over ensuing days, the patient might feel unable to engage in everyday tasks and begin to feel they are falling behind. This in turn can affect mood, resulting in increased symptoms of depression and anxiety. That leads to days of inactivity and rest, which leads to recovery. Then comes a day with better mood and increased energy, where the cycle can begin again.
It’s an addressable problem. “What we really want to do is break this cycle, get out of those peaks and valleys of high energy days and very low energy days to try to create more sustainable patterns” said Dr. Tworek, who is a staff health psychologist at Cleveland Clinic’s Mellen Center for Multiple Sclerosis, Cleveland, Ohio.
Fatigue
When addressing fatigue in MS patients, Dr. Tworek and her colleagues begin with a fatigue diary that includes typical activities engaged in throughout the day. It also distinguishes between activities the patient feels are important and activities that give them satisfaction.
“If we can find ways to include these [satisfying] activities, and not focus only on those important activities. This is where that quality of life really comes into play. But I always say to folks, we are not striving for perfection at first. I want you to write down what’s actually happening so we can use this data to later inform how we are going to make changes,” said Dr. Tworek.
It’s also important to encourage patients to seek help. Activities that are neither important nor satisfying may not need doing at all, and they encourage patients to seek help in other tasks. As for tasks that are important in their day-to-day lives, “How can we break those down? We break those down by pacing activities,” said Dr. Tworek.
A simple way to pace yourself is to use “The rule of two.” It asks: How long can I do a task before I experience a two-point increase on a 1-10 fatigue scale. “At that time, is when we want to start inserting breaks. We want to find activities we can do that will reduce [fatigue] or get us back to baseline. Or if that’s not realistic, keep us where we are at rather than increasing fatigue,” said Dr. Tworek.
Another way to think about it is spoon theory, sometimes referred to as coin theory. The idea is that you wake up each morning with ten spoons. Each task on a given day will cost a certain number of spoons. “You might start your day, you go downstairs, you have breakfast, and you’re already down to seven points, the next day, you might still be at 10. So it’s really about monitoring where you’re at in terms of how many coins or spoons you’re spending so that we can then reflect on how many coins or spoons do I have left?” said Dr. Tworek.
The strategy can aid communication with partners or family members who may have difficulty understanding MS fatigue. “Sometimes putting a number to it can really open up the doors to having these difficult conversations with friends and family,” said Dr. Tworek.
Sleep
Fatigue and sleep are naturally intertwined, and sleep problems are also common in MS, with 30%-56% reporting problems, depending on the estimate.
One concept to think about is sleep drive. “From the moment we wake up, we are building sleep pressure, just like from the moment you stop eating, your body starts building pressure to eat again,” said Dr. Tworek.
Naps can interfere with that drive, much like a snack can rob you of a meal-time appetite. “A nap is going to curb that appetite for sleep, making it more difficult potentially to fall asleep,” said Dr. Tworek. If a nap is absolutely necessary, it’s better to do it earlier in the day to allow time to build sleep pressure again.
As with fatigue, Dr. Tworek has patients fill out a sleep diary that documents difficulty falling or staying asleep, timing and length of awakenings, quality of sleep, length and timing of any naps, and other factors. It sometimes reveals patterns, like difficulty falling asleep on specific days of the week. Such rhythms may be attributable to regular stressors, like anticipating some event the next morning. Then it might be possible to tie in other techniques like stress management to reduce accompanying anxiety.
Sleep hygiene is an important factor, employing strategies like staying off screens or social media while in bed. “About 1 hour before bedtime, we want to try to create some relaxation time,” said Dr. Tworek.
Her clinic also emphasizes consistent wake time. “If we are waking every day in about the same half hour period, we are able to build that sleep pressure consistently. [Then] your body is going to let you know when it is time for bed. You’re going to feel sleepiness,” said Dr. Tworek.
Dr. Tworek did not report any disclosures or conflicts of interest.
FROM CMSC 2024
Is Semaglutide the ‘New Statin’? Not So Fast
There has been much hyperbole since the presentation of results from the SELECT cardiovascular outcomes trial (CVOT) at this year’s European Congress on Obesity, which led many to herald semaglutide as the “new statin.”
In the SELECT CVOT, participants with overweight or obesity (body mass index [BMI] ≥ 27), established cardiovascular disease (CVD), and no history of type 2 diabetes were administered the injectable glucagon-like peptide 1 (GLP-1) receptor agonist semaglutide (Wegovy) at a 2.4-mg dose weekly. Treatment resulted in a significant 20% relative risk reduction in major adverse CV events (a composite endpoint comprising CV death, nonfatal myocardial infarction, or nonfatal stroke). Importantly, SELECT was a trial on secondary prevention of CVD.
The CV benefits of semaglutide were notably independent of baseline weight or amount of weight lost. This suggests that the underlying driver of improved CV outcomes with semaglutide extends beyond simple reduction in obesity and perhaps indicates a direct effect on vasculature and reduction in atherosclerosis, although this remains unproven.
Not All Risk Reduction Is Equal
Much of the sensationalist coverage in the lay press focused on the 20% relative risk reduction figure. This endpoint is often more impressive and headline-grabbing than the absolute risk reduction, which provides a clearer view of a treatment’s real-world impact.
In SELECT, the absolute risk reduction was 1.5 percentage points, which translated into a number needed to treat (NNT) of 67 over 34 months to prevent one primary outcome of a major adverse CV event.
Lower NNTs suggest more effective treatments because fewer people need to be treated to prevent one clinical event, such as the major adverse CV events used in SELECT.
Semaglutide vs Statins
How does the clinical effectiveness observed in the SELECT trial compare with that observed in statin trials when it comes to the secondary prevention of CVD?
The seminal 4S study published in 1994 explored the impact of simvastatin on all-cause mortality among people with previous myocardial infarction or angina and hyperlipidemia (mean baseline BMI, 26). After 5.4 years of follow-up, the trial was stopped early owing to a 3.3-percentage point absolute risk reduction in all-cause mortality (NNT, 30; relative risk reduction, 28%). The NNT to prevent one death from CV causes was 31, and the NNT to prevent one major coronary event was lower, at 15.
Other statin secondary prevention trials, such as the LIPID and MIRACL studies, demonstrated similarly low NNTs.
So, you can see that the NNTs for statins in secondary prevention are much lower than with semaglutide in SELECT. Furthermore, the benefits of semaglutide in preventing CVD in people living with overweight/obesity have yet to be elucidated.
In contrast, we already have published evidence showing the benefits of statins in the primary prevention of CVD, albeit with higher and more variable NNTs than in the statin secondary prevention studies.
The benefits of statins are also postulated to extend beyond their impact on lowering low-density lipoprotein cholesterol. Statins have been suggested to have anti-inflammatory and plaque-stabilizing effects, among other pleiotropic benefits.
We also currently lack evidence for the cost-effectiveness of semaglutide for CV risk reduction. Assessing economic viability and use in health care systems, such as the UK’s National Health Service, involves comparing the cost of semaglutide against the health care savings from prevented CV events. Health economic studies are vital to determine whether the benefits justify the expense. In contrast, the cost-effectiveness of statins is well established, particularly for high-risk individuals.
Advantages of GLP-1s Should Not Be Overlooked
Of course, statins don’t provide the significant weight loss benefits of semaglutide.
Additional data from SELECT presented at the 2024 European Congress on Obesity demonstrated that participants lost a mean of 10.2% body weight and 7.7 cm from their waist circumference after 4 years. Moreover, after 2 years, 12% of individuals randomized to semaglutide had returned to a normal BMI, and nearly half were no longer living with obesity.
Although the CV benefits of semaglutide were independent of weight reduction, this level of weight loss is clinically meaningful and will reduce the risk of many other cardiometabolic conditions including type 2 diabetes, metabolic dysfunction–associated steatotic liver disease, and obstructive sleep apnea/hypopnea syndrome, as well as improve low mood, depression, and overall quality of life. Additionally, obesity is now a risk factor for 13 different types of cancer, including bowel, breast, and pancreatic cancer, so facilitating a return to a healthier body weight will also mitigate future risk for cancer.
Sticking With Our Cornerstone Therapy, For Now
In conclusion, I do not believe that semaglutide is the “new statin.” Statins are the cornerstone of primary and secondary prevention of CVD in a wide range of comorbidities, as evidenced in multiple large and high-quality trials dating back over 30 years.
However, there is no doubt that the GLP-1 receptor agonist class is the most significant therapeutic advance for the management of obesity and comorbidities to date.
The SELECT CVOT data uniquely position semaglutide as a secondary CVD prevention agent on top of guideline-driven management for people living with overweight/obesity and established CVD. Additionally, the clinically meaningful weight loss achieved with semaglutide will impact the risk of developing many other cardiometabolic conditions, as well as improve mental health and overall quality of life.
Dr. Fernando, GP Partner, North Berwick Health Centre, North Berwick, Scotland, creates concise clinical aide-mémoire for primary and secondary care to make life easier for health care professionals and ultimately to improve the lives of patients. He is very active on social media (X handle @drkevinfernando), where he posts hot topics in type 2 diabetes and CVRM. He recently has forayed into YouTube (@DrKevinFernando) and TikTok (@drkevinfernando) with patient-facing video content. Dr. Fernando has been elected to Fellowship of the Royal College of General Practitioners, the Royal College of Physicians of Edinburgh, and the Academy of Medical Educators for his work in diabetes and medical education. He has disclosed the following relevant financial relationships: Serve(d) as a speaker or a member of a speakers bureau for AstraZeneca; Boehringer Ingelheim; Lilly; Menarini; Bayer; Dexcom; Novartis; Novo Nordisk; Amgen; and Daiichi Sankyo; received income in an amount equal to or greater than $250 from AstraZeneca; Boehringer Ingelheim; Lilly; Menarini; Bayer; Dexcom; Novartis; Novo Nordisk; Amgen; and Daiichi Sankyo.
A version of this article first appeared on Medscape.com.
There has been much hyperbole since the presentation of results from the SELECT cardiovascular outcomes trial (CVOT) at this year’s European Congress on Obesity, which led many to herald semaglutide as the “new statin.”
In the SELECT CVOT, participants with overweight or obesity (body mass index [BMI] ≥ 27), established cardiovascular disease (CVD), and no history of type 2 diabetes were administered the injectable glucagon-like peptide 1 (GLP-1) receptor agonist semaglutide (Wegovy) at a 2.4-mg dose weekly. Treatment resulted in a significant 20% relative risk reduction in major adverse CV events (a composite endpoint comprising CV death, nonfatal myocardial infarction, or nonfatal stroke). Importantly, SELECT was a trial on secondary prevention of CVD.
The CV benefits of semaglutide were notably independent of baseline weight or amount of weight lost. This suggests that the underlying driver of improved CV outcomes with semaglutide extends beyond simple reduction in obesity and perhaps indicates a direct effect on vasculature and reduction in atherosclerosis, although this remains unproven.
Not All Risk Reduction Is Equal
Much of the sensationalist coverage in the lay press focused on the 20% relative risk reduction figure. This endpoint is often more impressive and headline-grabbing than the absolute risk reduction, which provides a clearer view of a treatment’s real-world impact.
In SELECT, the absolute risk reduction was 1.5 percentage points, which translated into a number needed to treat (NNT) of 67 over 34 months to prevent one primary outcome of a major adverse CV event.
Lower NNTs suggest more effective treatments because fewer people need to be treated to prevent one clinical event, such as the major adverse CV events used in SELECT.
Semaglutide vs Statins
How does the clinical effectiveness observed in the SELECT trial compare with that observed in statin trials when it comes to the secondary prevention of CVD?
The seminal 4S study published in 1994 explored the impact of simvastatin on all-cause mortality among people with previous myocardial infarction or angina and hyperlipidemia (mean baseline BMI, 26). After 5.4 years of follow-up, the trial was stopped early owing to a 3.3-percentage point absolute risk reduction in all-cause mortality (NNT, 30; relative risk reduction, 28%). The NNT to prevent one death from CV causes was 31, and the NNT to prevent one major coronary event was lower, at 15.
Other statin secondary prevention trials, such as the LIPID and MIRACL studies, demonstrated similarly low NNTs.
So, you can see that the NNTs for statins in secondary prevention are much lower than with semaglutide in SELECT. Furthermore, the benefits of semaglutide in preventing CVD in people living with overweight/obesity have yet to be elucidated.
In contrast, we already have published evidence showing the benefits of statins in the primary prevention of CVD, albeit with higher and more variable NNTs than in the statin secondary prevention studies.
The benefits of statins are also postulated to extend beyond their impact on lowering low-density lipoprotein cholesterol. Statins have been suggested to have anti-inflammatory and plaque-stabilizing effects, among other pleiotropic benefits.
We also currently lack evidence for the cost-effectiveness of semaglutide for CV risk reduction. Assessing economic viability and use in health care systems, such as the UK’s National Health Service, involves comparing the cost of semaglutide against the health care savings from prevented CV events. Health economic studies are vital to determine whether the benefits justify the expense. In contrast, the cost-effectiveness of statins is well established, particularly for high-risk individuals.
Advantages of GLP-1s Should Not Be Overlooked
Of course, statins don’t provide the significant weight loss benefits of semaglutide.
Additional data from SELECT presented at the 2024 European Congress on Obesity demonstrated that participants lost a mean of 10.2% body weight and 7.7 cm from their waist circumference after 4 years. Moreover, after 2 years, 12% of individuals randomized to semaglutide had returned to a normal BMI, and nearly half were no longer living with obesity.
Although the CV benefits of semaglutide were independent of weight reduction, this level of weight loss is clinically meaningful and will reduce the risk of many other cardiometabolic conditions including type 2 diabetes, metabolic dysfunction–associated steatotic liver disease, and obstructive sleep apnea/hypopnea syndrome, as well as improve low mood, depression, and overall quality of life. Additionally, obesity is now a risk factor for 13 different types of cancer, including bowel, breast, and pancreatic cancer, so facilitating a return to a healthier body weight will also mitigate future risk for cancer.
Sticking With Our Cornerstone Therapy, For Now
In conclusion, I do not believe that semaglutide is the “new statin.” Statins are the cornerstone of primary and secondary prevention of CVD in a wide range of comorbidities, as evidenced in multiple large and high-quality trials dating back over 30 years.
However, there is no doubt that the GLP-1 receptor agonist class is the most significant therapeutic advance for the management of obesity and comorbidities to date.
The SELECT CVOT data uniquely position semaglutide as a secondary CVD prevention agent on top of guideline-driven management for people living with overweight/obesity and established CVD. Additionally, the clinically meaningful weight loss achieved with semaglutide will impact the risk of developing many other cardiometabolic conditions, as well as improve mental health and overall quality of life.
Dr. Fernando, GP Partner, North Berwick Health Centre, North Berwick, Scotland, creates concise clinical aide-mémoire for primary and secondary care to make life easier for health care professionals and ultimately to improve the lives of patients. He is very active on social media (X handle @drkevinfernando), where he posts hot topics in type 2 diabetes and CVRM. He recently has forayed into YouTube (@DrKevinFernando) and TikTok (@drkevinfernando) with patient-facing video content. Dr. Fernando has been elected to Fellowship of the Royal College of General Practitioners, the Royal College of Physicians of Edinburgh, and the Academy of Medical Educators for his work in diabetes and medical education. He has disclosed the following relevant financial relationships: Serve(d) as a speaker or a member of a speakers bureau for AstraZeneca; Boehringer Ingelheim; Lilly; Menarini; Bayer; Dexcom; Novartis; Novo Nordisk; Amgen; and Daiichi Sankyo; received income in an amount equal to or greater than $250 from AstraZeneca; Boehringer Ingelheim; Lilly; Menarini; Bayer; Dexcom; Novartis; Novo Nordisk; Amgen; and Daiichi Sankyo.
A version of this article first appeared on Medscape.com.
There has been much hyperbole since the presentation of results from the SELECT cardiovascular outcomes trial (CVOT) at this year’s European Congress on Obesity, which led many to herald semaglutide as the “new statin.”
In the SELECT CVOT, participants with overweight or obesity (body mass index [BMI] ≥ 27), established cardiovascular disease (CVD), and no history of type 2 diabetes were administered the injectable glucagon-like peptide 1 (GLP-1) receptor agonist semaglutide (Wegovy) at a 2.4-mg dose weekly. Treatment resulted in a significant 20% relative risk reduction in major adverse CV events (a composite endpoint comprising CV death, nonfatal myocardial infarction, or nonfatal stroke). Importantly, SELECT was a trial on secondary prevention of CVD.
The CV benefits of semaglutide were notably independent of baseline weight or amount of weight lost. This suggests that the underlying driver of improved CV outcomes with semaglutide extends beyond simple reduction in obesity and perhaps indicates a direct effect on vasculature and reduction in atherosclerosis, although this remains unproven.
Not All Risk Reduction Is Equal
Much of the sensationalist coverage in the lay press focused on the 20% relative risk reduction figure. This endpoint is often more impressive and headline-grabbing than the absolute risk reduction, which provides a clearer view of a treatment’s real-world impact.
In SELECT, the absolute risk reduction was 1.5 percentage points, which translated into a number needed to treat (NNT) of 67 over 34 months to prevent one primary outcome of a major adverse CV event.
Lower NNTs suggest more effective treatments because fewer people need to be treated to prevent one clinical event, such as the major adverse CV events used in SELECT.
Semaglutide vs Statins
How does the clinical effectiveness observed in the SELECT trial compare with that observed in statin trials when it comes to the secondary prevention of CVD?
The seminal 4S study published in 1994 explored the impact of simvastatin on all-cause mortality among people with previous myocardial infarction or angina and hyperlipidemia (mean baseline BMI, 26). After 5.4 years of follow-up, the trial was stopped early owing to a 3.3-percentage point absolute risk reduction in all-cause mortality (NNT, 30; relative risk reduction, 28%). The NNT to prevent one death from CV causes was 31, and the NNT to prevent one major coronary event was lower, at 15.
Other statin secondary prevention trials, such as the LIPID and MIRACL studies, demonstrated similarly low NNTs.
So, you can see that the NNTs for statins in secondary prevention are much lower than with semaglutide in SELECT. Furthermore, the benefits of semaglutide in preventing CVD in people living with overweight/obesity have yet to be elucidated.
In contrast, we already have published evidence showing the benefits of statins in the primary prevention of CVD, albeit with higher and more variable NNTs than in the statin secondary prevention studies.
The benefits of statins are also postulated to extend beyond their impact on lowering low-density lipoprotein cholesterol. Statins have been suggested to have anti-inflammatory and plaque-stabilizing effects, among other pleiotropic benefits.
We also currently lack evidence for the cost-effectiveness of semaglutide for CV risk reduction. Assessing economic viability and use in health care systems, such as the UK’s National Health Service, involves comparing the cost of semaglutide against the health care savings from prevented CV events. Health economic studies are vital to determine whether the benefits justify the expense. In contrast, the cost-effectiveness of statins is well established, particularly for high-risk individuals.
Advantages of GLP-1s Should Not Be Overlooked
Of course, statins don’t provide the significant weight loss benefits of semaglutide.
Additional data from SELECT presented at the 2024 European Congress on Obesity demonstrated that participants lost a mean of 10.2% body weight and 7.7 cm from their waist circumference after 4 years. Moreover, after 2 years, 12% of individuals randomized to semaglutide had returned to a normal BMI, and nearly half were no longer living with obesity.
Although the CV benefits of semaglutide were independent of weight reduction, this level of weight loss is clinically meaningful and will reduce the risk of many other cardiometabolic conditions including type 2 diabetes, metabolic dysfunction–associated steatotic liver disease, and obstructive sleep apnea/hypopnea syndrome, as well as improve low mood, depression, and overall quality of life. Additionally, obesity is now a risk factor for 13 different types of cancer, including bowel, breast, and pancreatic cancer, so facilitating a return to a healthier body weight will also mitigate future risk for cancer.
Sticking With Our Cornerstone Therapy, For Now
In conclusion, I do not believe that semaglutide is the “new statin.” Statins are the cornerstone of primary and secondary prevention of CVD in a wide range of comorbidities, as evidenced in multiple large and high-quality trials dating back over 30 years.
However, there is no doubt that the GLP-1 receptor agonist class is the most significant therapeutic advance for the management of obesity and comorbidities to date.
The SELECT CVOT data uniquely position semaglutide as a secondary CVD prevention agent on top of guideline-driven management for people living with overweight/obesity and established CVD. Additionally, the clinically meaningful weight loss achieved with semaglutide will impact the risk of developing many other cardiometabolic conditions, as well as improve mental health and overall quality of life.
Dr. Fernando, GP Partner, North Berwick Health Centre, North Berwick, Scotland, creates concise clinical aide-mémoire for primary and secondary care to make life easier for health care professionals and ultimately to improve the lives of patients. He is very active on social media (X handle @drkevinfernando), where he posts hot topics in type 2 diabetes and CVRM. He recently has forayed into YouTube (@DrKevinFernando) and TikTok (@drkevinfernando) with patient-facing video content. Dr. Fernando has been elected to Fellowship of the Royal College of General Practitioners, the Royal College of Physicians of Edinburgh, and the Academy of Medical Educators for his work in diabetes and medical education. He has disclosed the following relevant financial relationships: Serve(d) as a speaker or a member of a speakers bureau for AstraZeneca; Boehringer Ingelheim; Lilly; Menarini; Bayer; Dexcom; Novartis; Novo Nordisk; Amgen; and Daiichi Sankyo; received income in an amount equal to or greater than $250 from AstraZeneca; Boehringer Ingelheim; Lilly; Menarini; Bayer; Dexcom; Novartis; Novo Nordisk; Amgen; and Daiichi Sankyo.
A version of this article first appeared on Medscape.com.
5 Vaccinations Adults Need Beyond COVID and Flu
Many adults are complacent about vaccinations, believing that annual COVID and flu shots aside, they had all the immunizations they need as children and teens. But adults need vaccines as well, especially if they have missed earlier doses. And older and health-compromised adults, in particular, can benefit from newer vaccines that were not part of the childhood schedule.
“The question is whether adults had the vaccinations they need in the first place,” Sandra Adamson Fryhofer, MD, an internist in Atlanta and the American Medical Association’s liaison to the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention, said in an interview. “Many do not even have reliable records of vaccination.”
Primary care physicians are ideally positioned to get adult patients to update their vaccination status on older vaccines and obtain newer ones as needed. “ACIP recommendations for adult vaccines are getting longer and more complicated and the way they’re administered is more complex, too, in that they’re not all given in the primary care office but sometimes in pharmacies,” Dr. Fryhofer said.
Not all adult patients want to update their vaccinations. “Vaccine hesitancy among many adults is accelerated by the several new vaccines that have been recommended in recent years,” Lauren Block, MD, MPH, an internist at Northwell Health and assistant professor in the Institute of Health System Science at the Feinstein Institutes for Medical Research in metropolitan New York City, said in an interview.
Physicians are rightly concerned about the lagging rates of adult vaccination, Dr. Block said. “Given the prevalence of conditions like pneumonia and shingles and the morbidity associated with them, healthcare providers should take every opportunity to discuss vaccination with patients, from well visits to hospital visits,” Dr. Block added.
She pointed to several obstacles to broader uptake, including product shortages, financial barriers, and, increasingly, the negative vocal messaging from media outlets and social media.
Current Recommendations
The main vaccines recommended for adults, besides flu and COVID shots, are for respiratory syncytial virus (RVS); shingles; pneumococcal disease; measles, mumps, and rubella (MMR); and tetanus, diphtheria, and pertussis (Tdap). Less commonly, booster vaccines for MM, and hepatitis are recommended when titers are proven to be low.
ACIP’s updated 2024 Adult Immunization Schedule can be downloaded from the website of the CDC.
The newest additions to the schedule include RSV vaccines, the mpox vaccine (Jynneos), a new MenACWY-MenB combo vaccine (Penbraya), and the new 2023-2024 formulation of updated COVID vaccines (both mRNA and protein-based adjuvanted versions).
1. Respiratory Syncytial Virus Vaccines
There are two licensed RSV vaccines, Arexvy and Abrysvo. The CDC schedule recommends a single-dose RSV vaccine for adults age 60 years and older, especially those at high risk of contracting the virus — but after shared decision-making based on a discussion of the risk-harm balance since this vaccine carries a small increased chance of developing the neurological symptoms of Guillain-Barré syndrome.
Chronic health conditions associated with a higher risk of severe RVS include cardiopulmonary disease, diabetes, and kidney, liver, and hematologic disorders, as well as compromised immunity, older age, and frailty.
2. Shingles Vaccines
This painful disease carries the potential complication of postherpetic neuralgia (PHN), which leads to long-term nerve pain in 10%-18% of patients, especially those over age 40. ACIP recommends two doses of the recombinant zoster vaccine (Shingrix) for individuals 50 years and older. Those 19 years and older with weakened immune systems due to disease or medical treatments should get two doses of the recombinant vaccine, as they have a higher risk of getting shingles and its complications, including neurological problems and skin and eye infections.
3 Pneumococcal Vaccines
There are three approved pneumococcal vaccines: PCV15 (Vaxneuvance), PCV20 (Prevnar20), and PPSV23 (Pneumovax23).
“The pneumococcal vaccine schedule is the most complicated one as higher-valent products continue to become available,” Dr. Fryhofer said.
The two types are pneumococcal conjugate vaccines (PCVs, specifically PCV15 and PCV20) and the pneumococcal polysaccharide vaccine (PPSV23). “While PPSV23 covers 23 strains, it doesn’t give the long-term immunity of the conjugate vaccine,” said Dr. Fryhofer. “A patient may have completed their vaccination with the polysaccharide vaccine but 5 years out may no longer be protected. So we offer the option of getting a dose of PCV20 to round out the protection and confer greater immune memory.”
The ACIP schedule recommends immunization against the Streptococcus pneumoniae pathogen for all older and all at-risk adults. Routine administration of PCV15 or PCV20 is advised for those 65 years or older who have never received any pneumococcal conjugate vaccine or whose previous vaccination history is unknown. If PCV15 is used, it should be followed by PPSV23. Those 65 years or older should get PPSV23 even if they already had one or more doses of pneumococcal vaccine before turning 65.
Further vaccination is recommended for younger at-risk adults aged 19-64 years who have received both PCV13 and PPSV23 but have incomplete vaccination status. These individuals are advised to complete their pneumococcal series by receiving either a single dose of PCV20 at an interval of at least 5 years after the last pneumococcal vaccine dose or more than one dose of PPSV23.
See Pneumococcal Vaccination: Summary of Who and When to Vaccinate for CDC guidance on vaccination options for adults who have previously received a pneumococcal conjugate vaccine. Or, to sort out quickly who gets what and when based on their age, concurrent conditions, and vaccination history, the CDC offers a type-in app called the PneumoRecs VaxAdvisor.
4. Measles, Mumps, and Rubella, and Varicella Vaccines
The two approved MMR vaccines are M-M-R II and PRIORIX. A third vaccine, ProQuad, adds varicella.
Adults lacking presumptive evidence of immunity should get at least one dose of the MMR combination vaccine.
Those born before 1957 are deemed to be immune, Dr. Fryhofer noted.
Two doses are recommended for adults entering high-risk settings for measles or mumps transmission such as healthcare personnel, students away at college, and international travelers. The two doses should be separated by at least 28 days. It’s no secret that measles, though preventable, is making a comeback, with 146 reported cases (48 in adults) across 21 states as of May 31 — most linked to international travel.
Women who plan to get pregnant should be vaccinated before but not during each pregnancy. (The vaccine is safe during lactation.) And those of childbearing age with no presumptive evidence of immunity are advised to get at least one dose of the MMR vaccine.
5. Tetanus, Diphtheria, and Pertussis Vaccine
Adults with no previous Tdap vaccination should receive a single dose of Adacel or Boostrix followed by a booster every 10 years. Boostrix is recommended for adults over 64 years.
During every pregnancy, women should have a single dose of Tdap, preferably in gestational weeks 27 through 36.
As to the immediate postpartum period, Tdap is recommended only for mothers who did not receive it during their current pregnancy and never received a prior dose. If a woman did not receive Tdap during her current pregnancy but did receive a prior dose of Tdap, she does not need Tdap postpartum.
The Challenges
According to Dr. Fryhofer, widespread disinformation about the risks of immunization against vaccine-preventable diseases has brought us to a flashpoint. “It’s now more important than ever to keep telling patients that vaccination is one of the most effective tools for preventing individual illness and protecting public health.”
She recommends that doctors follow the National Institutes of Health’s AIMS method to broach the subject of adult vaccination and increase participation in an inquiring, reassuring, and low-pressure way. Standing for Announce, Inquire, Mirror, and Secure, AIMS structures a nonjudgmental, patient-friendly conversation around immunization to elicit and acknowledge the reasons for hesitancy while explaining the safety and efficacy of vaccines.
Dr. Fryhofer frequently uses AIMS to bring inoculation-averse patients around. “Keep the conversation open with reluctant patients but leave them where they are. They need to see you as a reliable source and nonjudgmental source of information,” she said.
Dr. Block recommends outlining the diseases that have been eliminated through vaccines, from polio to measles, as well as the dangers of vaccine refusal, as indicated by recent outbreaks of vaccine-preventable diseases in areas with low immunization rates. “This approach highlights the opportunity we all have to get vaccinated to protect ourselves and our communities,” she said.
In Dr. Fryhofer’s view, the situation is urgent and doctors need to be proactive. “We’re now at a public-health tipping point where we may see a sliding back and a reversing of many years of progress.”
Dr. Fryhofer and Dr. Block disclosed no competing interests relevant to their comments.
Many adults are complacent about vaccinations, believing that annual COVID and flu shots aside, they had all the immunizations they need as children and teens. But adults need vaccines as well, especially if they have missed earlier doses. And older and health-compromised adults, in particular, can benefit from newer vaccines that were not part of the childhood schedule.
“The question is whether adults had the vaccinations they need in the first place,” Sandra Adamson Fryhofer, MD, an internist in Atlanta and the American Medical Association’s liaison to the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention, said in an interview. “Many do not even have reliable records of vaccination.”
Primary care physicians are ideally positioned to get adult patients to update their vaccination status on older vaccines and obtain newer ones as needed. “ACIP recommendations for adult vaccines are getting longer and more complicated and the way they’re administered is more complex, too, in that they’re not all given in the primary care office but sometimes in pharmacies,” Dr. Fryhofer said.
Not all adult patients want to update their vaccinations. “Vaccine hesitancy among many adults is accelerated by the several new vaccines that have been recommended in recent years,” Lauren Block, MD, MPH, an internist at Northwell Health and assistant professor in the Institute of Health System Science at the Feinstein Institutes for Medical Research in metropolitan New York City, said in an interview.
Physicians are rightly concerned about the lagging rates of adult vaccination, Dr. Block said. “Given the prevalence of conditions like pneumonia and shingles and the morbidity associated with them, healthcare providers should take every opportunity to discuss vaccination with patients, from well visits to hospital visits,” Dr. Block added.
She pointed to several obstacles to broader uptake, including product shortages, financial barriers, and, increasingly, the negative vocal messaging from media outlets and social media.
Current Recommendations
The main vaccines recommended for adults, besides flu and COVID shots, are for respiratory syncytial virus (RVS); shingles; pneumococcal disease; measles, mumps, and rubella (MMR); and tetanus, diphtheria, and pertussis (Tdap). Less commonly, booster vaccines for MM, and hepatitis are recommended when titers are proven to be low.
ACIP’s updated 2024 Adult Immunization Schedule can be downloaded from the website of the CDC.
The newest additions to the schedule include RSV vaccines, the mpox vaccine (Jynneos), a new MenACWY-MenB combo vaccine (Penbraya), and the new 2023-2024 formulation of updated COVID vaccines (both mRNA and protein-based adjuvanted versions).
1. Respiratory Syncytial Virus Vaccines
There are two licensed RSV vaccines, Arexvy and Abrysvo. The CDC schedule recommends a single-dose RSV vaccine for adults age 60 years and older, especially those at high risk of contracting the virus — but after shared decision-making based on a discussion of the risk-harm balance since this vaccine carries a small increased chance of developing the neurological symptoms of Guillain-Barré syndrome.
Chronic health conditions associated with a higher risk of severe RVS include cardiopulmonary disease, diabetes, and kidney, liver, and hematologic disorders, as well as compromised immunity, older age, and frailty.
2. Shingles Vaccines
This painful disease carries the potential complication of postherpetic neuralgia (PHN), which leads to long-term nerve pain in 10%-18% of patients, especially those over age 40. ACIP recommends two doses of the recombinant zoster vaccine (Shingrix) for individuals 50 years and older. Those 19 years and older with weakened immune systems due to disease or medical treatments should get two doses of the recombinant vaccine, as they have a higher risk of getting shingles and its complications, including neurological problems and skin and eye infections.
3 Pneumococcal Vaccines
There are three approved pneumococcal vaccines: PCV15 (Vaxneuvance), PCV20 (Prevnar20), and PPSV23 (Pneumovax23).
“The pneumococcal vaccine schedule is the most complicated one as higher-valent products continue to become available,” Dr. Fryhofer said.
The two types are pneumococcal conjugate vaccines (PCVs, specifically PCV15 and PCV20) and the pneumococcal polysaccharide vaccine (PPSV23). “While PPSV23 covers 23 strains, it doesn’t give the long-term immunity of the conjugate vaccine,” said Dr. Fryhofer. “A patient may have completed their vaccination with the polysaccharide vaccine but 5 years out may no longer be protected. So we offer the option of getting a dose of PCV20 to round out the protection and confer greater immune memory.”
The ACIP schedule recommends immunization against the Streptococcus pneumoniae pathogen for all older and all at-risk adults. Routine administration of PCV15 or PCV20 is advised for those 65 years or older who have never received any pneumococcal conjugate vaccine or whose previous vaccination history is unknown. If PCV15 is used, it should be followed by PPSV23. Those 65 years or older should get PPSV23 even if they already had one or more doses of pneumococcal vaccine before turning 65.
Further vaccination is recommended for younger at-risk adults aged 19-64 years who have received both PCV13 and PPSV23 but have incomplete vaccination status. These individuals are advised to complete their pneumococcal series by receiving either a single dose of PCV20 at an interval of at least 5 years after the last pneumococcal vaccine dose or more than one dose of PPSV23.
See Pneumococcal Vaccination: Summary of Who and When to Vaccinate for CDC guidance on vaccination options for adults who have previously received a pneumococcal conjugate vaccine. Or, to sort out quickly who gets what and when based on their age, concurrent conditions, and vaccination history, the CDC offers a type-in app called the PneumoRecs VaxAdvisor.
4. Measles, Mumps, and Rubella, and Varicella Vaccines
The two approved MMR vaccines are M-M-R II and PRIORIX. A third vaccine, ProQuad, adds varicella.
Adults lacking presumptive evidence of immunity should get at least one dose of the MMR combination vaccine.
Those born before 1957 are deemed to be immune, Dr. Fryhofer noted.
Two doses are recommended for adults entering high-risk settings for measles or mumps transmission such as healthcare personnel, students away at college, and international travelers. The two doses should be separated by at least 28 days. It’s no secret that measles, though preventable, is making a comeback, with 146 reported cases (48 in adults) across 21 states as of May 31 — most linked to international travel.
Women who plan to get pregnant should be vaccinated before but not during each pregnancy. (The vaccine is safe during lactation.) And those of childbearing age with no presumptive evidence of immunity are advised to get at least one dose of the MMR vaccine.
5. Tetanus, Diphtheria, and Pertussis Vaccine
Adults with no previous Tdap vaccination should receive a single dose of Adacel or Boostrix followed by a booster every 10 years. Boostrix is recommended for adults over 64 years.
During every pregnancy, women should have a single dose of Tdap, preferably in gestational weeks 27 through 36.
As to the immediate postpartum period, Tdap is recommended only for mothers who did not receive it during their current pregnancy and never received a prior dose. If a woman did not receive Tdap during her current pregnancy but did receive a prior dose of Tdap, she does not need Tdap postpartum.
The Challenges
According to Dr. Fryhofer, widespread disinformation about the risks of immunization against vaccine-preventable diseases has brought us to a flashpoint. “It’s now more important than ever to keep telling patients that vaccination is one of the most effective tools for preventing individual illness and protecting public health.”
She recommends that doctors follow the National Institutes of Health’s AIMS method to broach the subject of adult vaccination and increase participation in an inquiring, reassuring, and low-pressure way. Standing for Announce, Inquire, Mirror, and Secure, AIMS structures a nonjudgmental, patient-friendly conversation around immunization to elicit and acknowledge the reasons for hesitancy while explaining the safety and efficacy of vaccines.
Dr. Fryhofer frequently uses AIMS to bring inoculation-averse patients around. “Keep the conversation open with reluctant patients but leave them where they are. They need to see you as a reliable source and nonjudgmental source of information,” she said.
Dr. Block recommends outlining the diseases that have been eliminated through vaccines, from polio to measles, as well as the dangers of vaccine refusal, as indicated by recent outbreaks of vaccine-preventable diseases in areas with low immunization rates. “This approach highlights the opportunity we all have to get vaccinated to protect ourselves and our communities,” she said.
In Dr. Fryhofer’s view, the situation is urgent and doctors need to be proactive. “We’re now at a public-health tipping point where we may see a sliding back and a reversing of many years of progress.”
Dr. Fryhofer and Dr. Block disclosed no competing interests relevant to their comments.
Many adults are complacent about vaccinations, believing that annual COVID and flu shots aside, they had all the immunizations they need as children and teens. But adults need vaccines as well, especially if they have missed earlier doses. And older and health-compromised adults, in particular, can benefit from newer vaccines that were not part of the childhood schedule.
“The question is whether adults had the vaccinations they need in the first place,” Sandra Adamson Fryhofer, MD, an internist in Atlanta and the American Medical Association’s liaison to the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention, said in an interview. “Many do not even have reliable records of vaccination.”
Primary care physicians are ideally positioned to get adult patients to update their vaccination status on older vaccines and obtain newer ones as needed. “ACIP recommendations for adult vaccines are getting longer and more complicated and the way they’re administered is more complex, too, in that they’re not all given in the primary care office but sometimes in pharmacies,” Dr. Fryhofer said.
Not all adult patients want to update their vaccinations. “Vaccine hesitancy among many adults is accelerated by the several new vaccines that have been recommended in recent years,” Lauren Block, MD, MPH, an internist at Northwell Health and assistant professor in the Institute of Health System Science at the Feinstein Institutes for Medical Research in metropolitan New York City, said in an interview.
Physicians are rightly concerned about the lagging rates of adult vaccination, Dr. Block said. “Given the prevalence of conditions like pneumonia and shingles and the morbidity associated with them, healthcare providers should take every opportunity to discuss vaccination with patients, from well visits to hospital visits,” Dr. Block added.
She pointed to several obstacles to broader uptake, including product shortages, financial barriers, and, increasingly, the negative vocal messaging from media outlets and social media.
Current Recommendations
The main vaccines recommended for adults, besides flu and COVID shots, are for respiratory syncytial virus (RVS); shingles; pneumococcal disease; measles, mumps, and rubella (MMR); and tetanus, diphtheria, and pertussis (Tdap). Less commonly, booster vaccines for MM, and hepatitis are recommended when titers are proven to be low.
ACIP’s updated 2024 Adult Immunization Schedule can be downloaded from the website of the CDC.
The newest additions to the schedule include RSV vaccines, the mpox vaccine (Jynneos), a new MenACWY-MenB combo vaccine (Penbraya), and the new 2023-2024 formulation of updated COVID vaccines (both mRNA and protein-based adjuvanted versions).
1. Respiratory Syncytial Virus Vaccines
There are two licensed RSV vaccines, Arexvy and Abrysvo. The CDC schedule recommends a single-dose RSV vaccine for adults age 60 years and older, especially those at high risk of contracting the virus — but after shared decision-making based on a discussion of the risk-harm balance since this vaccine carries a small increased chance of developing the neurological symptoms of Guillain-Barré syndrome.
Chronic health conditions associated with a higher risk of severe RVS include cardiopulmonary disease, diabetes, and kidney, liver, and hematologic disorders, as well as compromised immunity, older age, and frailty.
2. Shingles Vaccines
This painful disease carries the potential complication of postherpetic neuralgia (PHN), which leads to long-term nerve pain in 10%-18% of patients, especially those over age 40. ACIP recommends two doses of the recombinant zoster vaccine (Shingrix) for individuals 50 years and older. Those 19 years and older with weakened immune systems due to disease or medical treatments should get two doses of the recombinant vaccine, as they have a higher risk of getting shingles and its complications, including neurological problems and skin and eye infections.
3 Pneumococcal Vaccines
There are three approved pneumococcal vaccines: PCV15 (Vaxneuvance), PCV20 (Prevnar20), and PPSV23 (Pneumovax23).
“The pneumococcal vaccine schedule is the most complicated one as higher-valent products continue to become available,” Dr. Fryhofer said.
The two types are pneumococcal conjugate vaccines (PCVs, specifically PCV15 and PCV20) and the pneumococcal polysaccharide vaccine (PPSV23). “While PPSV23 covers 23 strains, it doesn’t give the long-term immunity of the conjugate vaccine,” said Dr. Fryhofer. “A patient may have completed their vaccination with the polysaccharide vaccine but 5 years out may no longer be protected. So we offer the option of getting a dose of PCV20 to round out the protection and confer greater immune memory.”
The ACIP schedule recommends immunization against the Streptococcus pneumoniae pathogen for all older and all at-risk adults. Routine administration of PCV15 or PCV20 is advised for those 65 years or older who have never received any pneumococcal conjugate vaccine or whose previous vaccination history is unknown. If PCV15 is used, it should be followed by PPSV23. Those 65 years or older should get PPSV23 even if they already had one or more doses of pneumococcal vaccine before turning 65.
Further vaccination is recommended for younger at-risk adults aged 19-64 years who have received both PCV13 and PPSV23 but have incomplete vaccination status. These individuals are advised to complete their pneumococcal series by receiving either a single dose of PCV20 at an interval of at least 5 years after the last pneumococcal vaccine dose or more than one dose of PPSV23.
See Pneumococcal Vaccination: Summary of Who and When to Vaccinate for CDC guidance on vaccination options for adults who have previously received a pneumococcal conjugate vaccine. Or, to sort out quickly who gets what and when based on their age, concurrent conditions, and vaccination history, the CDC offers a type-in app called the PneumoRecs VaxAdvisor.
4. Measles, Mumps, and Rubella, and Varicella Vaccines
The two approved MMR vaccines are M-M-R II and PRIORIX. A third vaccine, ProQuad, adds varicella.
Adults lacking presumptive evidence of immunity should get at least one dose of the MMR combination vaccine.
Those born before 1957 are deemed to be immune, Dr. Fryhofer noted.
Two doses are recommended for adults entering high-risk settings for measles or mumps transmission such as healthcare personnel, students away at college, and international travelers. The two doses should be separated by at least 28 days. It’s no secret that measles, though preventable, is making a comeback, with 146 reported cases (48 in adults) across 21 states as of May 31 — most linked to international travel.
Women who plan to get pregnant should be vaccinated before but not during each pregnancy. (The vaccine is safe during lactation.) And those of childbearing age with no presumptive evidence of immunity are advised to get at least one dose of the MMR vaccine.
5. Tetanus, Diphtheria, and Pertussis Vaccine
Adults with no previous Tdap vaccination should receive a single dose of Adacel or Boostrix followed by a booster every 10 years. Boostrix is recommended for adults over 64 years.
During every pregnancy, women should have a single dose of Tdap, preferably in gestational weeks 27 through 36.
As to the immediate postpartum period, Tdap is recommended only for mothers who did not receive it during their current pregnancy and never received a prior dose. If a woman did not receive Tdap during her current pregnancy but did receive a prior dose of Tdap, she does not need Tdap postpartum.
The Challenges
According to Dr. Fryhofer, widespread disinformation about the risks of immunization against vaccine-preventable diseases has brought us to a flashpoint. “It’s now more important than ever to keep telling patients that vaccination is one of the most effective tools for preventing individual illness and protecting public health.”
She recommends that doctors follow the National Institutes of Health’s AIMS method to broach the subject of adult vaccination and increase participation in an inquiring, reassuring, and low-pressure way. Standing for Announce, Inquire, Mirror, and Secure, AIMS structures a nonjudgmental, patient-friendly conversation around immunization to elicit and acknowledge the reasons for hesitancy while explaining the safety and efficacy of vaccines.
Dr. Fryhofer frequently uses AIMS to bring inoculation-averse patients around. “Keep the conversation open with reluctant patients but leave them where they are. They need to see you as a reliable source and nonjudgmental source of information,” she said.
Dr. Block recommends outlining the diseases that have been eliminated through vaccines, from polio to measles, as well as the dangers of vaccine refusal, as indicated by recent outbreaks of vaccine-preventable diseases in areas with low immunization rates. “This approach highlights the opportunity we all have to get vaccinated to protect ourselves and our communities,” she said.
In Dr. Fryhofer’s view, the situation is urgent and doctors need to be proactive. “We’re now at a public-health tipping point where we may see a sliding back and a reversing of many years of progress.”
Dr. Fryhofer and Dr. Block disclosed no competing interests relevant to their comments.
Vaginal Ring Use Raises Risk for Certain STIs
Use of combined contraceptive vaginal rings was associated with an increased risk for several types of sexually transmitted infections (STIs), based on data from a pair of studies presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists (ACOG).
Previous research has shown that the use of a combined contraceptive vaginal ring (CCVR) may promote changes in immunity in the female genital tract by upregulating immune-related genes in the endocervix and immune mediators within the cervicovaginal fluid, wrote Amy Arceneaux, BS, a medical student at the University of Texas Medical Branch John Sealy School of Medicine, Galveston, and colleagues.
The infection rates in the female genital tract can vary according to hormones in the local environment and continued safety analysis is needed as the use of CCVR continues to rise, the researchers noted.
In a retrospective chart review, the researchers assessed de-identified data from TriNetX, a patient database, including 30,796 women who received etonogestrel and ethinyl estradiol CCVRs without segesterone and an equal number who were using oral contraceptive pills (OCP) without vaginal hormones. Patients were matched for age, race, and ethnicity.
Overall use of CCVRs was significantly associated with an increased risk for Herpes simplex virus 2 (HSV-2; relative risk [RR], 1.790), acute vaginitis (RR, 1.722), subacute/chronic vaginitis (RR, 1.904), subacute/chronic vulvitis (RR, 1.969), acute vulvitis (RR, 1.894), candidiasis (RR, 1.464), trichomoniasis (RR, 2.162), and pelvic inflammatory disease (RR, 2.984; P < .0005 for all).
By contrast, use of CCVRs was significantly associated with a decreased risk for chlamydia (RR, 0.760; P = .047). No differences in risk appeared for gonorrhea, syphilis, HIV, or anogenital warts between the CCVR and OCP groups.
Another study presented at the meeting, led by Kathleen Karam, BS, also a medical student at the University of Texas Medical Branch John Sealy School of Medicine, Galveston, Texas, focused on outcomes on vaginal health and infection risk in women who used CCVRs compared with women who did not use hormones.
The study by Ms. Karam and colleagues included de-identified TriNetX data for two cohorts of 274,743 women.
Overall, the researchers found a significantly increased risk for gonorrhea, HSV-2, vaginitis, vulvitis, pelvic inflammatory disease, anogenital warts, and candidiasis in women using CCVR compared with those using no hormonal contraception, while the risk for chlamydia, syphilis, and HIV was decreased in women using CCVR compared with those using no hormonal contraception.
“I was pleasantly surprised by the finding that the group of women using the hormonal contraception vaginal ring had decreased risk for HIV and syphilis infections,” said Kathleen L. Vincent, MD, of the University of Texas Medical Branch John Sealy School of Medicine, Galveston, Texas, and senior author on both studies, in an interview. She hypothesized that the estrogen released from the ring might have contributed to the decreased risk for those infections.
The findings of both studies were limited primarily by the retrospective design, but the results suggest a need for further study of the effect of local hormone delivery on the vaginal mucosa, the researchers wrote.
Although the study population was large, the lack of randomization can allow for differences in the behaviors or risk-taking of the groups, Dr. Vincent said in an interview.
“The fact that there were STIs that were increased and some that were decreased with use of the vaginal ring tells us that there were women with similar behaviors in both groups, or we might have seen STIs only in one group,” she said. “Additional research could be done to look at varying time courses of outcomes after initiation of the vaginal ring or to go more in-depth with matching the groups at baseline based on a history of risky behaviors,” she noted.
Data Inform Multipurpose Prevention Technology
Dr. Vincent and her colleague, Richard Pyles, PhD, have a 15-year history of studying vaginal drug and hormone effects on the vaginal mucosa in women and preclinical and cell models. “Based on that work, it was plausible for estrogen to be protective for several types of infections,” she said. The availability of TriNetX allowed the researchers to explore these relationships in a large database of women in the studies presented at the meeting. “We began with a basic science observation in an animal model and grew it into this clinical study because of the available TriNetX system that supported extensive medical record review,” Dr. Pyles noted.
The take-home messages from the current research remain that vaginal rings delivering hormones are indicated only for contraception or birth control, not for protection against STIs or HIV, and women at an increased risk for these infections should protect themselves by using condoms, Dr. Vincent said.
However, “the real clinical implication is for the future for the drugs that we call MPTs or multi-purpose prevention technologies,” Dr. Vincent said.
“This could be a vaginal ring that releases medications for birth control and prevention of HIV or an STI,” she explained.
The findings from the studies presented at the meeting have great potential for an MPT on which Dr. Vincent and Dr. Pyles are working that would provide protection against both HIV and pregnancy. “For HIV prevention, the hormonal vaginal ring components have potential to work synergistically with the HIV prevention drug rather than working against each other, and this could be realized as a need for less HIV prevention drug, and subsequently fewer potential side effects from that drug,” said Dr. Vincent.
The studies received no outside funding. The researchers had no financial conflicts to disclose.
Use of combined contraceptive vaginal rings was associated with an increased risk for several types of sexually transmitted infections (STIs), based on data from a pair of studies presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists (ACOG).
Previous research has shown that the use of a combined contraceptive vaginal ring (CCVR) may promote changes in immunity in the female genital tract by upregulating immune-related genes in the endocervix and immune mediators within the cervicovaginal fluid, wrote Amy Arceneaux, BS, a medical student at the University of Texas Medical Branch John Sealy School of Medicine, Galveston, and colleagues.
The infection rates in the female genital tract can vary according to hormones in the local environment and continued safety analysis is needed as the use of CCVR continues to rise, the researchers noted.
In a retrospective chart review, the researchers assessed de-identified data from TriNetX, a patient database, including 30,796 women who received etonogestrel and ethinyl estradiol CCVRs without segesterone and an equal number who were using oral contraceptive pills (OCP) without vaginal hormones. Patients were matched for age, race, and ethnicity.
Overall use of CCVRs was significantly associated with an increased risk for Herpes simplex virus 2 (HSV-2; relative risk [RR], 1.790), acute vaginitis (RR, 1.722), subacute/chronic vaginitis (RR, 1.904), subacute/chronic vulvitis (RR, 1.969), acute vulvitis (RR, 1.894), candidiasis (RR, 1.464), trichomoniasis (RR, 2.162), and pelvic inflammatory disease (RR, 2.984; P < .0005 for all).
By contrast, use of CCVRs was significantly associated with a decreased risk for chlamydia (RR, 0.760; P = .047). No differences in risk appeared for gonorrhea, syphilis, HIV, or anogenital warts between the CCVR and OCP groups.
Another study presented at the meeting, led by Kathleen Karam, BS, also a medical student at the University of Texas Medical Branch John Sealy School of Medicine, Galveston, Texas, focused on outcomes on vaginal health and infection risk in women who used CCVRs compared with women who did not use hormones.
The study by Ms. Karam and colleagues included de-identified TriNetX data for two cohorts of 274,743 women.
Overall, the researchers found a significantly increased risk for gonorrhea, HSV-2, vaginitis, vulvitis, pelvic inflammatory disease, anogenital warts, and candidiasis in women using CCVR compared with those using no hormonal contraception, while the risk for chlamydia, syphilis, and HIV was decreased in women using CCVR compared with those using no hormonal contraception.
“I was pleasantly surprised by the finding that the group of women using the hormonal contraception vaginal ring had decreased risk for HIV and syphilis infections,” said Kathleen L. Vincent, MD, of the University of Texas Medical Branch John Sealy School of Medicine, Galveston, Texas, and senior author on both studies, in an interview. She hypothesized that the estrogen released from the ring might have contributed to the decreased risk for those infections.
The findings of both studies were limited primarily by the retrospective design, but the results suggest a need for further study of the effect of local hormone delivery on the vaginal mucosa, the researchers wrote.
Although the study population was large, the lack of randomization can allow for differences in the behaviors or risk-taking of the groups, Dr. Vincent said in an interview.
“The fact that there were STIs that were increased and some that were decreased with use of the vaginal ring tells us that there were women with similar behaviors in both groups, or we might have seen STIs only in one group,” she said. “Additional research could be done to look at varying time courses of outcomes after initiation of the vaginal ring or to go more in-depth with matching the groups at baseline based on a history of risky behaviors,” she noted.
Data Inform Multipurpose Prevention Technology
Dr. Vincent and her colleague, Richard Pyles, PhD, have a 15-year history of studying vaginal drug and hormone effects on the vaginal mucosa in women and preclinical and cell models. “Based on that work, it was plausible for estrogen to be protective for several types of infections,” she said. The availability of TriNetX allowed the researchers to explore these relationships in a large database of women in the studies presented at the meeting. “We began with a basic science observation in an animal model and grew it into this clinical study because of the available TriNetX system that supported extensive medical record review,” Dr. Pyles noted.
The take-home messages from the current research remain that vaginal rings delivering hormones are indicated only for contraception or birth control, not for protection against STIs or HIV, and women at an increased risk for these infections should protect themselves by using condoms, Dr. Vincent said.
However, “the real clinical implication is for the future for the drugs that we call MPTs or multi-purpose prevention technologies,” Dr. Vincent said.
“This could be a vaginal ring that releases medications for birth control and prevention of HIV or an STI,” she explained.
The findings from the studies presented at the meeting have great potential for an MPT on which Dr. Vincent and Dr. Pyles are working that would provide protection against both HIV and pregnancy. “For HIV prevention, the hormonal vaginal ring components have potential to work synergistically with the HIV prevention drug rather than working against each other, and this could be realized as a need for less HIV prevention drug, and subsequently fewer potential side effects from that drug,” said Dr. Vincent.
The studies received no outside funding. The researchers had no financial conflicts to disclose.
Use of combined contraceptive vaginal rings was associated with an increased risk for several types of sexually transmitted infections (STIs), based on data from a pair of studies presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists (ACOG).
Previous research has shown that the use of a combined contraceptive vaginal ring (CCVR) may promote changes in immunity in the female genital tract by upregulating immune-related genes in the endocervix and immune mediators within the cervicovaginal fluid, wrote Amy Arceneaux, BS, a medical student at the University of Texas Medical Branch John Sealy School of Medicine, Galveston, and colleagues.
The infection rates in the female genital tract can vary according to hormones in the local environment and continued safety analysis is needed as the use of CCVR continues to rise, the researchers noted.
In a retrospective chart review, the researchers assessed de-identified data from TriNetX, a patient database, including 30,796 women who received etonogestrel and ethinyl estradiol CCVRs without segesterone and an equal number who were using oral contraceptive pills (OCP) without vaginal hormones. Patients were matched for age, race, and ethnicity.
Overall use of CCVRs was significantly associated with an increased risk for Herpes simplex virus 2 (HSV-2; relative risk [RR], 1.790), acute vaginitis (RR, 1.722), subacute/chronic vaginitis (RR, 1.904), subacute/chronic vulvitis (RR, 1.969), acute vulvitis (RR, 1.894), candidiasis (RR, 1.464), trichomoniasis (RR, 2.162), and pelvic inflammatory disease (RR, 2.984; P < .0005 for all).
By contrast, use of CCVRs was significantly associated with a decreased risk for chlamydia (RR, 0.760; P = .047). No differences in risk appeared for gonorrhea, syphilis, HIV, or anogenital warts between the CCVR and OCP groups.
Another study presented at the meeting, led by Kathleen Karam, BS, also a medical student at the University of Texas Medical Branch John Sealy School of Medicine, Galveston, Texas, focused on outcomes on vaginal health and infection risk in women who used CCVRs compared with women who did not use hormones.
The study by Ms. Karam and colleagues included de-identified TriNetX data for two cohorts of 274,743 women.
Overall, the researchers found a significantly increased risk for gonorrhea, HSV-2, vaginitis, vulvitis, pelvic inflammatory disease, anogenital warts, and candidiasis in women using CCVR compared with those using no hormonal contraception, while the risk for chlamydia, syphilis, and HIV was decreased in women using CCVR compared with those using no hormonal contraception.
“I was pleasantly surprised by the finding that the group of women using the hormonal contraception vaginal ring had decreased risk for HIV and syphilis infections,” said Kathleen L. Vincent, MD, of the University of Texas Medical Branch John Sealy School of Medicine, Galveston, Texas, and senior author on both studies, in an interview. She hypothesized that the estrogen released from the ring might have contributed to the decreased risk for those infections.
The findings of both studies were limited primarily by the retrospective design, but the results suggest a need for further study of the effect of local hormone delivery on the vaginal mucosa, the researchers wrote.
Although the study population was large, the lack of randomization can allow for differences in the behaviors or risk-taking of the groups, Dr. Vincent said in an interview.
“The fact that there were STIs that were increased and some that were decreased with use of the vaginal ring tells us that there were women with similar behaviors in both groups, or we might have seen STIs only in one group,” she said. “Additional research could be done to look at varying time courses of outcomes after initiation of the vaginal ring or to go more in-depth with matching the groups at baseline based on a history of risky behaviors,” she noted.
Data Inform Multipurpose Prevention Technology
Dr. Vincent and her colleague, Richard Pyles, PhD, have a 15-year history of studying vaginal drug and hormone effects on the vaginal mucosa in women and preclinical and cell models. “Based on that work, it was plausible for estrogen to be protective for several types of infections,” she said. The availability of TriNetX allowed the researchers to explore these relationships in a large database of women in the studies presented at the meeting. “We began with a basic science observation in an animal model and grew it into this clinical study because of the available TriNetX system that supported extensive medical record review,” Dr. Pyles noted.
The take-home messages from the current research remain that vaginal rings delivering hormones are indicated only for contraception or birth control, not for protection against STIs or HIV, and women at an increased risk for these infections should protect themselves by using condoms, Dr. Vincent said.
However, “the real clinical implication is for the future for the drugs that we call MPTs or multi-purpose prevention technologies,” Dr. Vincent said.
“This could be a vaginal ring that releases medications for birth control and prevention of HIV or an STI,” she explained.
The findings from the studies presented at the meeting have great potential for an MPT on which Dr. Vincent and Dr. Pyles are working that would provide protection against both HIV and pregnancy. “For HIV prevention, the hormonal vaginal ring components have potential to work synergistically with the HIV prevention drug rather than working against each other, and this could be realized as a need for less HIV prevention drug, and subsequently fewer potential side effects from that drug,” said Dr. Vincent.
The studies received no outside funding. The researchers had no financial conflicts to disclose.
FROM ACOG 2024
Role of Non-invasive Biomarkers in the Evaluation and Management of MASLD
Rinella ME, Lazarus JV, Ratziu V, et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology. 2023;78(6):1966-1986. doi:10.1097/HEP.0000000000000520
Wattacheril JJ, Abdelmalek MF, Lim JK, Sanyal AJ. AGA Clinical Practice Update on the Role of Noninvasive Biomarkers in the Evaluation and Management of Nonalcoholic Fatty Liver Disease: Expert Review. Gastroenterology. 2023;165(4):1080-1088. doi:10.1053/j.gastro.2023.06.013
Di Mauro S, Scamporrino A, Filippello A, et al. Clinical and Molecular Biomarkers for Diagnosis and Staging of NAFLD. Int J Mol Sci. 2021;22(21):11905. Published 2021 Nov 2. doi:10.3390/ijms222111905
Hsu C, Caussy C, Imajo K, et al. Magnetic Resonance vs Transient Elastography Analysis of Patients With Nonalcoholic Fatty Liver Disease: A Systematic Review and Pooled Analysis of Individual Participants. Clin Gastroenterol Hepatol. 2019;17(4):630-637.e8. doi:10.1016/j.cgh.2018.05.059
Ilagan-Ying YC, Banini BA, Do A, Lam R, Lim JK. Screening, Diagnosis, and Staging of Non-Alcoholic Fatty Liver Disease (NAFLD): Application of Society Guidelines to Clinical Practice. Curr Gastroenterol Rep. 2023;25(10):213-224. doi:10.1007/s11894-023-00883-8
Chen W, Gao Y, Xie W, et al. Genome-wide association analyses provide genetic and biochemical insights into natural variation in rice metabolism. Nat Genet. 2014;46(7):714-721. doi:10.1038/ng.3007
Wu YL, Kumar R, Wang MF, et al. Validation of conventional non-invasive fibrosis scoring systems in patients with metabolic associated fatty liver disease. World J Gastroenterol. 2021;27(34):5753-5763. doi:10.3748/wjg.v27.i34.5753
Kaneva AM, Bojko ER. Fatty liver index (FLI): more than a marker of hepatic steatosis. J Physiol Biochem. Published online October 25, 2023. doi:10.1007/s13105-023-00991-z
Rinella ME, Lazarus JV, Ratziu V, et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology. 2023;78(6):1966-1986. doi:10.1097/HEP.0000000000000520
Wattacheril JJ, Abdelmalek MF, Lim JK, Sanyal AJ. AGA Clinical Practice Update on the Role of Noninvasive Biomarkers in the Evaluation and Management of Nonalcoholic Fatty Liver Disease: Expert Review. Gastroenterology. 2023;165(4):1080-1088. doi:10.1053/j.gastro.2023.06.013
Di Mauro S, Scamporrino A, Filippello A, et al. Clinical and Molecular Biomarkers for Diagnosis and Staging of NAFLD. Int J Mol Sci. 2021;22(21):11905. Published 2021 Nov 2. doi:10.3390/ijms222111905
Hsu C, Caussy C, Imajo K, et al. Magnetic Resonance vs Transient Elastography Analysis of Patients With Nonalcoholic Fatty Liver Disease: A Systematic Review and Pooled Analysis of Individual Participants. Clin Gastroenterol Hepatol. 2019;17(4):630-637.e8. doi:10.1016/j.cgh.2018.05.059
Ilagan-Ying YC, Banini BA, Do A, Lam R, Lim JK. Screening, Diagnosis, and Staging of Non-Alcoholic Fatty Liver Disease (NAFLD): Application of Society Guidelines to Clinical Practice. Curr Gastroenterol Rep. 2023;25(10):213-224. doi:10.1007/s11894-023-00883-8
Chen W, Gao Y, Xie W, et al. Genome-wide association analyses provide genetic and biochemical insights into natural variation in rice metabolism. Nat Genet. 2014;46(7):714-721. doi:10.1038/ng.3007
Wu YL, Kumar R, Wang MF, et al. Validation of conventional non-invasive fibrosis scoring systems in patients with metabolic associated fatty liver disease. World J Gastroenterol. 2021;27(34):5753-5763. doi:10.3748/wjg.v27.i34.5753
Kaneva AM, Bojko ER. Fatty liver index (FLI): more than a marker of hepatic steatosis. J Physiol Biochem. Published online October 25, 2023. doi:10.1007/s13105-023-00991-z
Rinella ME, Lazarus JV, Ratziu V, et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology. 2023;78(6):1966-1986. doi:10.1097/HEP.0000000000000520
Wattacheril JJ, Abdelmalek MF, Lim JK, Sanyal AJ. AGA Clinical Practice Update on the Role of Noninvasive Biomarkers in the Evaluation and Management of Nonalcoholic Fatty Liver Disease: Expert Review. Gastroenterology. 2023;165(4):1080-1088. doi:10.1053/j.gastro.2023.06.013
Di Mauro S, Scamporrino A, Filippello A, et al. Clinical and Molecular Biomarkers for Diagnosis and Staging of NAFLD. Int J Mol Sci. 2021;22(21):11905. Published 2021 Nov 2. doi:10.3390/ijms222111905
Hsu C, Caussy C, Imajo K, et al. Magnetic Resonance vs Transient Elastography Analysis of Patients With Nonalcoholic Fatty Liver Disease: A Systematic Review and Pooled Analysis of Individual Participants. Clin Gastroenterol Hepatol. 2019;17(4):630-637.e8. doi:10.1016/j.cgh.2018.05.059
Ilagan-Ying YC, Banini BA, Do A, Lam R, Lim JK. Screening, Diagnosis, and Staging of Non-Alcoholic Fatty Liver Disease (NAFLD): Application of Society Guidelines to Clinical Practice. Curr Gastroenterol Rep. 2023;25(10):213-224. doi:10.1007/s11894-023-00883-8
Chen W, Gao Y, Xie W, et al. Genome-wide association analyses provide genetic and biochemical insights into natural variation in rice metabolism. Nat Genet. 2014;46(7):714-721. doi:10.1038/ng.3007
Wu YL, Kumar R, Wang MF, et al. Validation of conventional non-invasive fibrosis scoring systems in patients with metabolic associated fatty liver disease. World J Gastroenterol. 2021;27(34):5753-5763. doi:10.3748/wjg.v27.i34.5753
Kaneva AM, Bojko ER. Fatty liver index (FLI): more than a marker of hepatic steatosis. J Physiol Biochem. Published online October 25, 2023. doi:10.1007/s13105-023-00991-z
Fluid Management in Acute Pancreatitis
Tenner S, Baillie J, DeWitt J, Vege SS; American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis [published correction appears in Am J Gastroenterol. 2014;109(2):302]. Am J Gastroenterol. 2013;108(9):1400-1415. doi:10.1038/ajg.2013.218
de-Madaria E, Buxbaum JL, Maisonneuve P, et al. Aggressive or moderate fluid resuscitation in acute pancreatitis. N Engl J Med. 2022;387(11):989-1000. doi:10.1056/NEJMoa2202884
Zhao G, Zhang JG, Wu HS, et al. Effects of different resuscitation fluid on severe acute pancreatitis. World J Gastroenterol. 2013;19(13):2044-2052. doi:10.3748/wjg.v19.i13.2044
Guzmán-Calderón E, Diaz-Arocutipa C, Monge E. Lactate Ringer's versus normal saline in the management of acute pancreatitis: a systematic review and meta-analysis of randomized controlled trials. Dig Dis Sci. 2022;67(8):4131-4139. doi:10.1007/s10620-021-07269-8
Hoste EA, Maitland K, Brudney CS, et al; ADQI XII Investigators Group. Four phases of intravenous fluid therapy: a conceptual model. Br J Anaesth. 2014;113(5):740-747. doi:10.1093/bja/aeu300
Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13(4 suppl 2):e1-e15. doi:10.1016/j.pan.2013.07.063
Machicado JD, Papachristou GI. Pharmacologic management and prevention of acute pancreatitis. Curr Opin Gastroenterol. 2019;35(5):460-467. doi:10.1097/MOG.0000000000000563
Tenner S, Baillie J, DeWitt J, Vege SS; American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis [published correction appears in Am J Gastroenterol. 2014;109(2):302]. Am J Gastroenterol. 2013;108(9):1400-1415. doi:10.1038/ajg.2013.218
de-Madaria E, Buxbaum JL, Maisonneuve P, et al. Aggressive or moderate fluid resuscitation in acute pancreatitis. N Engl J Med. 2022;387(11):989-1000. doi:10.1056/NEJMoa2202884
Zhao G, Zhang JG, Wu HS, et al. Effects of different resuscitation fluid on severe acute pancreatitis. World J Gastroenterol. 2013;19(13):2044-2052. doi:10.3748/wjg.v19.i13.2044
Guzmán-Calderón E, Diaz-Arocutipa C, Monge E. Lactate Ringer's versus normal saline in the management of acute pancreatitis: a systematic review and meta-analysis of randomized controlled trials. Dig Dis Sci. 2022;67(8):4131-4139. doi:10.1007/s10620-021-07269-8
Hoste EA, Maitland K, Brudney CS, et al; ADQI XII Investigators Group. Four phases of intravenous fluid therapy: a conceptual model. Br J Anaesth. 2014;113(5):740-747. doi:10.1093/bja/aeu300
Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13(4 suppl 2):e1-e15. doi:10.1016/j.pan.2013.07.063
Machicado JD, Papachristou GI. Pharmacologic management and prevention of acute pancreatitis. Curr Opin Gastroenterol. 2019;35(5):460-467. doi:10.1097/MOG.0000000000000563
Tenner S, Baillie J, DeWitt J, Vege SS; American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis [published correction appears in Am J Gastroenterol. 2014;109(2):302]. Am J Gastroenterol. 2013;108(9):1400-1415. doi:10.1038/ajg.2013.218
de-Madaria E, Buxbaum JL, Maisonneuve P, et al. Aggressive or moderate fluid resuscitation in acute pancreatitis. N Engl J Med. 2022;387(11):989-1000. doi:10.1056/NEJMoa2202884
Zhao G, Zhang JG, Wu HS, et al. Effects of different resuscitation fluid on severe acute pancreatitis. World J Gastroenterol. 2013;19(13):2044-2052. doi:10.3748/wjg.v19.i13.2044
Guzmán-Calderón E, Diaz-Arocutipa C, Monge E. Lactate Ringer's versus normal saline in the management of acute pancreatitis: a systematic review and meta-analysis of randomized controlled trials. Dig Dis Sci. 2022;67(8):4131-4139. doi:10.1007/s10620-021-07269-8
Hoste EA, Maitland K, Brudney CS, et al; ADQI XII Investigators Group. Four phases of intravenous fluid therapy: a conceptual model. Br J Anaesth. 2014;113(5):740-747. doi:10.1093/bja/aeu300
Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13(4 suppl 2):e1-e15. doi:10.1016/j.pan.2013.07.063
Machicado JD, Papachristou GI. Pharmacologic management and prevention of acute pancreatitis. Curr Opin Gastroenterol. 2019;35(5):460-467. doi:10.1097/MOG.0000000000000563
The Emerging Role of Liquid Biopsy in the Diagnosis and Management of CRC
Key statistics for colorectal cancer. American Cancer Society. Revised January 13, 2023. Accessed November 30, 2023. https://www.cancer.org/cancer/types/colon-rectal-cancer/about/key-statistics.html
Mazouji O, Ouhajjou A, Incitti R, Mansour H. Updates on clinical use of liquid biopsy in colorectal cancer screening, diagnosis, follow-up, and treatment guidance. Front Cell Dev Biol. 2021;9:660924. doi:10.3389/fcell.2021.660924
Vacante M, Ciuni R, Basile F, Biondi A. The liquid biopsy in the management of colorectal cancer: an overview. Biomedicines. 2020;8(9):308. doi:10.3390/biomedicines8090308
American Cancer Society. Colorectal cancer facts & figures 2020-2022. Published 2022. Accessed November 30, 2023. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/colorectal-cancer-facts-and-figures/colorectal-cancer-facts-and-figures-2020-2022.pdf
Johnson & Johnson. FDA clears Cellsearch™ circulating tumor cell test [news release]. Published February 27, 2008. Accessed November 30, 2023. https://johnsonandjohnson.gcs-web.com/news-releases/news-release-details/fda-clears-cellsearchtm-circulating-tumor-cell-test
US Food and Drug Administration. Summary of safety and effectiveness data, Epi proColon®. PMA number P130001. Published April 12, 2016. Accessed November 30, 2023. https://www.accessdata.fda.gov/cdrh_docs/pdf13/p130001b.pdf
FDA approves blood tests that can help guide cancer treatment. National Institutes of Health, National Cancer Institute. Published October 15, 2020. Accessed November 30, 2023. https://www.cancer.gov/news-events/cancer-currents-blog/2020/fda-guardant-360-foundation-one-cancer-liquid-biopsy
Foundation Medicine. US Food and Drug Administration (FDA) approves FoundationOne®LiquidCDx as a companion diagnostic for Pfizer’s BRAFTOVI® (encorafenib) in combination with cetuximab to identify patients with BRAF V600E alterations in metastatic colorectal cancer [press release]. Published June 10, 2023. Accessed November 30, 2023. https://www.foundationmedicine.com/press-releases/f9b285eb-db6d-4f61-856c-3f1edb803937
Key statistics for colorectal cancer. American Cancer Society. Revised January 13, 2023. Accessed November 30, 2023. https://www.cancer.org/cancer/types/colon-rectal-cancer/about/key-statistics.html
Mazouji O, Ouhajjou A, Incitti R, Mansour H. Updates on clinical use of liquid biopsy in colorectal cancer screening, diagnosis, follow-up, and treatment guidance. Front Cell Dev Biol. 2021;9:660924. doi:10.3389/fcell.2021.660924
Vacante M, Ciuni R, Basile F, Biondi A. The liquid biopsy in the management of colorectal cancer: an overview. Biomedicines. 2020;8(9):308. doi:10.3390/biomedicines8090308
American Cancer Society. Colorectal cancer facts & figures 2020-2022. Published 2022. Accessed November 30, 2023. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/colorectal-cancer-facts-and-figures/colorectal-cancer-facts-and-figures-2020-2022.pdf
Johnson & Johnson. FDA clears Cellsearch™ circulating tumor cell test [news release]. Published February 27, 2008. Accessed November 30, 2023. https://johnsonandjohnson.gcs-web.com/news-releases/news-release-details/fda-clears-cellsearchtm-circulating-tumor-cell-test
US Food and Drug Administration. Summary of safety and effectiveness data, Epi proColon®. PMA number P130001. Published April 12, 2016. Accessed November 30, 2023. https://www.accessdata.fda.gov/cdrh_docs/pdf13/p130001b.pdf
FDA approves blood tests that can help guide cancer treatment. National Institutes of Health, National Cancer Institute. Published October 15, 2020. Accessed November 30, 2023. https://www.cancer.gov/news-events/cancer-currents-blog/2020/fda-guardant-360-foundation-one-cancer-liquid-biopsy
Foundation Medicine. US Food and Drug Administration (FDA) approves FoundationOne®LiquidCDx as a companion diagnostic for Pfizer’s BRAFTOVI® (encorafenib) in combination with cetuximab to identify patients with BRAF V600E alterations in metastatic colorectal cancer [press release]. Published June 10, 2023. Accessed November 30, 2023. https://www.foundationmedicine.com/press-releases/f9b285eb-db6d-4f61-856c-3f1edb803937
Key statistics for colorectal cancer. American Cancer Society. Revised January 13, 2023. Accessed November 30, 2023. https://www.cancer.org/cancer/types/colon-rectal-cancer/about/key-statistics.html
Mazouji O, Ouhajjou A, Incitti R, Mansour H. Updates on clinical use of liquid biopsy in colorectal cancer screening, diagnosis, follow-up, and treatment guidance. Front Cell Dev Biol. 2021;9:660924. doi:10.3389/fcell.2021.660924
Vacante M, Ciuni R, Basile F, Biondi A. The liquid biopsy in the management of colorectal cancer: an overview. Biomedicines. 2020;8(9):308. doi:10.3390/biomedicines8090308
American Cancer Society. Colorectal cancer facts & figures 2020-2022. Published 2022. Accessed November 30, 2023. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/colorectal-cancer-facts-and-figures/colorectal-cancer-facts-and-figures-2020-2022.pdf
Johnson & Johnson. FDA clears Cellsearch™ circulating tumor cell test [news release]. Published February 27, 2008. Accessed November 30, 2023. https://johnsonandjohnson.gcs-web.com/news-releases/news-release-details/fda-clears-cellsearchtm-circulating-tumor-cell-test
US Food and Drug Administration. Summary of safety and effectiveness data, Epi proColon®. PMA number P130001. Published April 12, 2016. Accessed November 30, 2023. https://www.accessdata.fda.gov/cdrh_docs/pdf13/p130001b.pdf
FDA approves blood tests that can help guide cancer treatment. National Institutes of Health, National Cancer Institute. Published October 15, 2020. Accessed November 30, 2023. https://www.cancer.gov/news-events/cancer-currents-blog/2020/fda-guardant-360-foundation-one-cancer-liquid-biopsy
Foundation Medicine. US Food and Drug Administration (FDA) approves FoundationOne®LiquidCDx as a companion diagnostic for Pfizer’s BRAFTOVI® (encorafenib) in combination with cetuximab to identify patients with BRAF V600E alterations in metastatic colorectal cancer [press release]. Published June 10, 2023. Accessed November 30, 2023. https://www.foundationmedicine.com/press-releases/f9b285eb-db6d-4f61-856c-3f1edb803937
Eosinophilic Gastrointestinal Diseases: Beyond EoE
- Dellon ES, Gonsalves N, Abonia JP, et al. International consensus recommendations for eosinophilic gastrointestinal disease nomenclature. Clin Gastroenterol Hepatol. 2022;20(11):2474-2484.e3. doi:10.1016/j.cgh.2022.02.017
- Naramore S, Gupta SK. Nonesophageal eosinophilic gastrointestinal disorders: clinical care and future directions. J Pediatr Gastroenterol Nutr. 2018;67(3):318-321. doi:10.1097/MPG.0000000000002040
- Kinoshita Y, Sanuki T. Review of non-eosinophilic esophagitis-eosinophilic gastrointestinal disease (non-EoE-EGID) and a case series of twenty-eight affected patients. Biomolecules. 2023;13(9):1417. doi:10.3390/biom13091417
- Gonsalves N, Doerfler B, Zalewski A, et al. Prospective study of an amino acid-based elemental diet in an eosinophilic gastritis and gastroenteritis nutrition trial. J Allergy Clin Immunol. 2023;152(3):676-688. doi:10.1016/j.jaci.2023.05.024
- Oshima T. Biologic therapies targeting eosinophilic gastrointestinal diseases. Intern Med. 2023;62(23):3429-3430. doi:10.2169/internalmedicine.1911-23
- Pineton de Chambrun G, Gonzalez F, Canva JY, et al. Natural history of eosinophilic gastroenteritis. Clin Gastroenterol Hepatol. 2011;9(11):950-956.e1. doi:10.1016/j.cgh.2011.07.017
- Hirano I, Collins MH, King E, et al; CEGIR Investigators. Prospective endoscopic activity assessment for eosinophilic gastritis in a multi-site cohort. Am J Gastroenterol. 2022;117(3):413-423. doi:10.14309/ajg.0000000000001625
- Pesek RD, Reed CC, Muir AB, et al; Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR). Increasing rates of diagnosis, substantial co-occurrence, and variable treatment patterns of eosinophilic gastritis, gastroenteritis, and colitis based on 10-year data across a multicenter consortium. Am J Gastroenterol. 2019;114(6):984-994. doi:10.14309/ajg.0000000000000228
- Dellon ES, Gonsalves N, Abonia JP, et al. International consensus recommendations for eosinophilic gastrointestinal disease nomenclature. Clin Gastroenterol Hepatol. 2022;20(11):2474-2484.e3. doi:10.1016/j.cgh.2022.02.017
- Naramore S, Gupta SK. Nonesophageal eosinophilic gastrointestinal disorders: clinical care and future directions. J Pediatr Gastroenterol Nutr. 2018;67(3):318-321. doi:10.1097/MPG.0000000000002040
- Kinoshita Y, Sanuki T. Review of non-eosinophilic esophagitis-eosinophilic gastrointestinal disease (non-EoE-EGID) and a case series of twenty-eight affected patients. Biomolecules. 2023;13(9):1417. doi:10.3390/biom13091417
- Gonsalves N, Doerfler B, Zalewski A, et al. Prospective study of an amino acid-based elemental diet in an eosinophilic gastritis and gastroenteritis nutrition trial. J Allergy Clin Immunol. 2023;152(3):676-688. doi:10.1016/j.jaci.2023.05.024
- Oshima T. Biologic therapies targeting eosinophilic gastrointestinal diseases. Intern Med. 2023;62(23):3429-3430. doi:10.2169/internalmedicine.1911-23
- Pineton de Chambrun G, Gonzalez F, Canva JY, et al. Natural history of eosinophilic gastroenteritis. Clin Gastroenterol Hepatol. 2011;9(11):950-956.e1. doi:10.1016/j.cgh.2011.07.017
- Hirano I, Collins MH, King E, et al; CEGIR Investigators. Prospective endoscopic activity assessment for eosinophilic gastritis in a multi-site cohort. Am J Gastroenterol. 2022;117(3):413-423. doi:10.14309/ajg.0000000000001625
- Pesek RD, Reed CC, Muir AB, et al; Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR). Increasing rates of diagnosis, substantial co-occurrence, and variable treatment patterns of eosinophilic gastritis, gastroenteritis, and colitis based on 10-year data across a multicenter consortium. Am J Gastroenterol. 2019;114(6):984-994. doi:10.14309/ajg.0000000000000228
- Dellon ES, Gonsalves N, Abonia JP, et al. International consensus recommendations for eosinophilic gastrointestinal disease nomenclature. Clin Gastroenterol Hepatol. 2022;20(11):2474-2484.e3. doi:10.1016/j.cgh.2022.02.017
- Naramore S, Gupta SK. Nonesophageal eosinophilic gastrointestinal disorders: clinical care and future directions. J Pediatr Gastroenterol Nutr. 2018;67(3):318-321. doi:10.1097/MPG.0000000000002040
- Kinoshita Y, Sanuki T. Review of non-eosinophilic esophagitis-eosinophilic gastrointestinal disease (non-EoE-EGID) and a case series of twenty-eight affected patients. Biomolecules. 2023;13(9):1417. doi:10.3390/biom13091417
- Gonsalves N, Doerfler B, Zalewski A, et al. Prospective study of an amino acid-based elemental diet in an eosinophilic gastritis and gastroenteritis nutrition trial. J Allergy Clin Immunol. 2023;152(3):676-688. doi:10.1016/j.jaci.2023.05.024
- Oshima T. Biologic therapies targeting eosinophilic gastrointestinal diseases. Intern Med. 2023;62(23):3429-3430. doi:10.2169/internalmedicine.1911-23
- Pineton de Chambrun G, Gonzalez F, Canva JY, et al. Natural history of eosinophilic gastroenteritis. Clin Gastroenterol Hepatol. 2011;9(11):950-956.e1. doi:10.1016/j.cgh.2011.07.017
- Hirano I, Collins MH, King E, et al; CEGIR Investigators. Prospective endoscopic activity assessment for eosinophilic gastritis in a multi-site cohort. Am J Gastroenterol. 2022;117(3):413-423. doi:10.14309/ajg.0000000000001625
- Pesek RD, Reed CC, Muir AB, et al; Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR). Increasing rates of diagnosis, substantial co-occurrence, and variable treatment patterns of eosinophilic gastritis, gastroenteritis, and colitis based on 10-year data across a multicenter consortium. Am J Gastroenterol. 2019;114(6):984-994. doi:10.14309/ajg.0000000000000228