User login
Emerging Evidence Supports Dietary Management of MASLD Through Gut-Liver Axis
WASHINGTON — , according to a study presented at the annual Digestive Disease Week® (DDW).
For instance, patients with MASLD had lower intake of fiber and omega-3 fatty acids but higher consumption of added sugars and ultraprocessed foods, which correlated with the associated bacterial species and functional pathways.
“MASLD is an escalating concern globally, which highlights the need for innovative targets for disease prevention and management,” said lead author Georgina Williams, PhD, a postdoctoral researcher in diet and gastroenterology at the University of Newcastle, Australia.
“Therapeutic options often rely on lifestyle modifications, with a focus on weight loss,” she said. “Diet is considered a key component of disease management.”
Although calorie restriction with a 3%-5% fat loss is associated with hepatic benefits in MASLD, Dr. Williams noted, researchers have considered whole dietary patterns and the best fit for patients. Aspects of the Mediterranean diet may be effective, as reflected in recommendations from the American Association for the Study of Liver Disease (AASLD), which highlight dietary components such as limited carbohydrates and saturated fat, along with high fiber and unsaturated fats. The gut microbiome may be essential to consider as well, she said, given MASLD-associated differences in bile acid metabolism, inflammation, and ethanol production.
Dr. Williams and colleagues conducted a retrospective case-control study in an outpatient liver clinic to understand diet and dysbiosis in MASLD, looking at differences in diet, gut microbiota composition, and functional pathways in those with and without MASLD. The researchers investigated daily average intake, serum, and stool samples among 50 people (25 per group) matched for age and gender, comparing fibrosis-4, MASLD severity scores, macronutrients, micronutrients, food groups, metagenomic sequencing, and inflammatory markers such as interleukin (IL)-1ß, IL-6, tumor necrosis factor (TNF)-α, cytokeratin (CK)-18, and high-sensitivity C-reactive protein (hsCRP).
Dietary Characteristics
At baseline, the groups differed by ethnicity, prescription medication use, and body mass index (BMI), where the MASLD group had greater ethnic diversity, medication use, and BMI. In addition, the MASLD group had a zero to mild score of fibrosis.
Overall, energy intake didn’t differ significantly between the two groups. The control group had higher alcohol intake, likely since the MASLD group was recommended to reduce alcohol intake, though the difference was about 5 grams per day. The MASLD group also had less caffeine intake than the control group, as well as slightly lower protein intake, though the differences weren’t statistically significant.
While consumption of total carbohydrates didn’t differ significantly between the groups, participants with MASLD consumed more calories from carbohydrates than did the controls. The MASLD group consumed more calories from added and free sugars and didn’t meet recommendations for dietary fiber.
With particular food groups, participants with MASLD ate significantly fewer whole grains, red and orange fruits, and leafy green vegetables. When consuming fruit, those with MASLD were more likely to drink juice than eat whole fruit. These findings could be relevant when considering high sugar intake and low dietary fiber, Dr. Williams said.
With dietary fat, there were no differences in total fat between the groups, but the fat profiles differed. The control group was significantly more likely to consume omega-3 fatty acids, including alpha-linolenic acid (ALA), eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA), and docosahexaenoic acid (DHA). The MASLD group was less likely to consume seafood, nuts, seeds, avocado, and olive oil.
With inflammatory markers, hsCRP and CK-18 were increased in MASLD, while IL-1ß was increased in controls, which was consistently associated with higher alcohol intake among the control group. IL-6 and TNF-α didn’t differ between the groups.
Notably, dietary fats were most consistently associated with inflammatory markers, Dr. Williams said, with inflammation being positively associated with saturated fats and negatively associated with unsaturated fats.
Looking at microbiota, the alpha diversity was no different, but the beta diversity was across 162 taxa. Per bacterial species, there was an inverse relationship between MASLD and associations with unsaturated fat, as well as positive indicators of high sugar and fructose intake and low unsaturated fat and dietary fiber intake.
Beyond that, the functional pathways enriched in MASLD were associated with increased sugar and carbohydrates, reduced fiber, and reduced unsaturated fat. Lower butyrate production in MASLD was associated with low intake of nuts, seeds, and unsaturated fat.
In Clinical Practice
Dr. Williams suggested reinforcing AASLD guidelines and looking at diet quality, not just diet quantity. Although an energy deficit remains relevant in MASLD, macronutrient consumption matters across dietary fats, fibers, and sugars.
Future avenues for research include metabolomic pathways related to bile acids and fatty acids, she said, as well as disentangling metabolic syndrome from MASLD outcomes.
Session moderator Olivier Barbier, PhD, professor of pharmacy at Laval University in Quebec, Canada, asked about microbiome differences across countries. Dr. Williams noted the limitations in this study of looking at differences across geography and ethnicity, particularly in Australia, but said the species identified were consistent with those found in most literature globally.
In response to other questions after the presentation, Dr. Williams said supplements (such as omega-3 fatty acids) were included in total intake, and those taking prebiotics or probiotics were excluded from the study. In an upcoming clinical trial, she and colleagues plan to control for household microbiomes as well.
“The premise is that microbiomes are shared between households, so when you’re doing these sorts of large-scale clinical studies, if you’re going to look at the microbiome, then you should control for one of the major confounding variables,” said Mark Sundrud, PhD, professor of medicine at the Dartmouth Center for Digestive Health in Lebanon, New Hampshire. Dr. Sundrud, who wasn’t involved with this study, presented on the role of bile acids in mucosal immune cell function at DDW.
“We’ve done a collaborative study looking at microbiomes and bile acids in inflammatory bowel disease (IBD) patients versus controls,” which included consideration of households, he said. “We were able to see more intrinsic disease-specific changes.”
Dr. Williams declared no relevant disclosures. Dr. Sundrud has served as a scientific adviser to Sage Therapeutics.
WASHINGTON — , according to a study presented at the annual Digestive Disease Week® (DDW).
For instance, patients with MASLD had lower intake of fiber and omega-3 fatty acids but higher consumption of added sugars and ultraprocessed foods, which correlated with the associated bacterial species and functional pathways.
“MASLD is an escalating concern globally, which highlights the need for innovative targets for disease prevention and management,” said lead author Georgina Williams, PhD, a postdoctoral researcher in diet and gastroenterology at the University of Newcastle, Australia.
“Therapeutic options often rely on lifestyle modifications, with a focus on weight loss,” she said. “Diet is considered a key component of disease management.”
Although calorie restriction with a 3%-5% fat loss is associated with hepatic benefits in MASLD, Dr. Williams noted, researchers have considered whole dietary patterns and the best fit for patients. Aspects of the Mediterranean diet may be effective, as reflected in recommendations from the American Association for the Study of Liver Disease (AASLD), which highlight dietary components such as limited carbohydrates and saturated fat, along with high fiber and unsaturated fats. The gut microbiome may be essential to consider as well, she said, given MASLD-associated differences in bile acid metabolism, inflammation, and ethanol production.
Dr. Williams and colleagues conducted a retrospective case-control study in an outpatient liver clinic to understand diet and dysbiosis in MASLD, looking at differences in diet, gut microbiota composition, and functional pathways in those with and without MASLD. The researchers investigated daily average intake, serum, and stool samples among 50 people (25 per group) matched for age and gender, comparing fibrosis-4, MASLD severity scores, macronutrients, micronutrients, food groups, metagenomic sequencing, and inflammatory markers such as interleukin (IL)-1ß, IL-6, tumor necrosis factor (TNF)-α, cytokeratin (CK)-18, and high-sensitivity C-reactive protein (hsCRP).
Dietary Characteristics
At baseline, the groups differed by ethnicity, prescription medication use, and body mass index (BMI), where the MASLD group had greater ethnic diversity, medication use, and BMI. In addition, the MASLD group had a zero to mild score of fibrosis.
Overall, energy intake didn’t differ significantly between the two groups. The control group had higher alcohol intake, likely since the MASLD group was recommended to reduce alcohol intake, though the difference was about 5 grams per day. The MASLD group also had less caffeine intake than the control group, as well as slightly lower protein intake, though the differences weren’t statistically significant.
While consumption of total carbohydrates didn’t differ significantly between the groups, participants with MASLD consumed more calories from carbohydrates than did the controls. The MASLD group consumed more calories from added and free sugars and didn’t meet recommendations for dietary fiber.
With particular food groups, participants with MASLD ate significantly fewer whole grains, red and orange fruits, and leafy green vegetables. When consuming fruit, those with MASLD were more likely to drink juice than eat whole fruit. These findings could be relevant when considering high sugar intake and low dietary fiber, Dr. Williams said.
With dietary fat, there were no differences in total fat between the groups, but the fat profiles differed. The control group was significantly more likely to consume omega-3 fatty acids, including alpha-linolenic acid (ALA), eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA), and docosahexaenoic acid (DHA). The MASLD group was less likely to consume seafood, nuts, seeds, avocado, and olive oil.
With inflammatory markers, hsCRP and CK-18 were increased in MASLD, while IL-1ß was increased in controls, which was consistently associated with higher alcohol intake among the control group. IL-6 and TNF-α didn’t differ between the groups.
Notably, dietary fats were most consistently associated with inflammatory markers, Dr. Williams said, with inflammation being positively associated with saturated fats and negatively associated with unsaturated fats.
Looking at microbiota, the alpha diversity was no different, but the beta diversity was across 162 taxa. Per bacterial species, there was an inverse relationship between MASLD and associations with unsaturated fat, as well as positive indicators of high sugar and fructose intake and low unsaturated fat and dietary fiber intake.
Beyond that, the functional pathways enriched in MASLD were associated with increased sugar and carbohydrates, reduced fiber, and reduced unsaturated fat. Lower butyrate production in MASLD was associated with low intake of nuts, seeds, and unsaturated fat.
In Clinical Practice
Dr. Williams suggested reinforcing AASLD guidelines and looking at diet quality, not just diet quantity. Although an energy deficit remains relevant in MASLD, macronutrient consumption matters across dietary fats, fibers, and sugars.
Future avenues for research include metabolomic pathways related to bile acids and fatty acids, she said, as well as disentangling metabolic syndrome from MASLD outcomes.
Session moderator Olivier Barbier, PhD, professor of pharmacy at Laval University in Quebec, Canada, asked about microbiome differences across countries. Dr. Williams noted the limitations in this study of looking at differences across geography and ethnicity, particularly in Australia, but said the species identified were consistent with those found in most literature globally.
In response to other questions after the presentation, Dr. Williams said supplements (such as omega-3 fatty acids) were included in total intake, and those taking prebiotics or probiotics were excluded from the study. In an upcoming clinical trial, she and colleagues plan to control for household microbiomes as well.
“The premise is that microbiomes are shared between households, so when you’re doing these sorts of large-scale clinical studies, if you’re going to look at the microbiome, then you should control for one of the major confounding variables,” said Mark Sundrud, PhD, professor of medicine at the Dartmouth Center for Digestive Health in Lebanon, New Hampshire. Dr. Sundrud, who wasn’t involved with this study, presented on the role of bile acids in mucosal immune cell function at DDW.
“We’ve done a collaborative study looking at microbiomes and bile acids in inflammatory bowel disease (IBD) patients versus controls,” which included consideration of households, he said. “We were able to see more intrinsic disease-specific changes.”
Dr. Williams declared no relevant disclosures. Dr. Sundrud has served as a scientific adviser to Sage Therapeutics.
WASHINGTON — , according to a study presented at the annual Digestive Disease Week® (DDW).
For instance, patients with MASLD had lower intake of fiber and omega-3 fatty acids but higher consumption of added sugars and ultraprocessed foods, which correlated with the associated bacterial species and functional pathways.
“MASLD is an escalating concern globally, which highlights the need for innovative targets for disease prevention and management,” said lead author Georgina Williams, PhD, a postdoctoral researcher in diet and gastroenterology at the University of Newcastle, Australia.
“Therapeutic options often rely on lifestyle modifications, with a focus on weight loss,” she said. “Diet is considered a key component of disease management.”
Although calorie restriction with a 3%-5% fat loss is associated with hepatic benefits in MASLD, Dr. Williams noted, researchers have considered whole dietary patterns and the best fit for patients. Aspects of the Mediterranean diet may be effective, as reflected in recommendations from the American Association for the Study of Liver Disease (AASLD), which highlight dietary components such as limited carbohydrates and saturated fat, along with high fiber and unsaturated fats. The gut microbiome may be essential to consider as well, she said, given MASLD-associated differences in bile acid metabolism, inflammation, and ethanol production.
Dr. Williams and colleagues conducted a retrospective case-control study in an outpatient liver clinic to understand diet and dysbiosis in MASLD, looking at differences in diet, gut microbiota composition, and functional pathways in those with and without MASLD. The researchers investigated daily average intake, serum, and stool samples among 50 people (25 per group) matched for age and gender, comparing fibrosis-4, MASLD severity scores, macronutrients, micronutrients, food groups, metagenomic sequencing, and inflammatory markers such as interleukin (IL)-1ß, IL-6, tumor necrosis factor (TNF)-α, cytokeratin (CK)-18, and high-sensitivity C-reactive protein (hsCRP).
Dietary Characteristics
At baseline, the groups differed by ethnicity, prescription medication use, and body mass index (BMI), where the MASLD group had greater ethnic diversity, medication use, and BMI. In addition, the MASLD group had a zero to mild score of fibrosis.
Overall, energy intake didn’t differ significantly between the two groups. The control group had higher alcohol intake, likely since the MASLD group was recommended to reduce alcohol intake, though the difference was about 5 grams per day. The MASLD group also had less caffeine intake than the control group, as well as slightly lower protein intake, though the differences weren’t statistically significant.
While consumption of total carbohydrates didn’t differ significantly between the groups, participants with MASLD consumed more calories from carbohydrates than did the controls. The MASLD group consumed more calories from added and free sugars and didn’t meet recommendations for dietary fiber.
With particular food groups, participants with MASLD ate significantly fewer whole grains, red and orange fruits, and leafy green vegetables. When consuming fruit, those with MASLD were more likely to drink juice than eat whole fruit. These findings could be relevant when considering high sugar intake and low dietary fiber, Dr. Williams said.
With dietary fat, there were no differences in total fat between the groups, but the fat profiles differed. The control group was significantly more likely to consume omega-3 fatty acids, including alpha-linolenic acid (ALA), eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA), and docosahexaenoic acid (DHA). The MASLD group was less likely to consume seafood, nuts, seeds, avocado, and olive oil.
With inflammatory markers, hsCRP and CK-18 were increased in MASLD, while IL-1ß was increased in controls, which was consistently associated with higher alcohol intake among the control group. IL-6 and TNF-α didn’t differ between the groups.
Notably, dietary fats were most consistently associated with inflammatory markers, Dr. Williams said, with inflammation being positively associated with saturated fats and negatively associated with unsaturated fats.
Looking at microbiota, the alpha diversity was no different, but the beta diversity was across 162 taxa. Per bacterial species, there was an inverse relationship between MASLD and associations with unsaturated fat, as well as positive indicators of high sugar and fructose intake and low unsaturated fat and dietary fiber intake.
Beyond that, the functional pathways enriched in MASLD were associated with increased sugar and carbohydrates, reduced fiber, and reduced unsaturated fat. Lower butyrate production in MASLD was associated with low intake of nuts, seeds, and unsaturated fat.
In Clinical Practice
Dr. Williams suggested reinforcing AASLD guidelines and looking at diet quality, not just diet quantity. Although an energy deficit remains relevant in MASLD, macronutrient consumption matters across dietary fats, fibers, and sugars.
Future avenues for research include metabolomic pathways related to bile acids and fatty acids, she said, as well as disentangling metabolic syndrome from MASLD outcomes.
Session moderator Olivier Barbier, PhD, professor of pharmacy at Laval University in Quebec, Canada, asked about microbiome differences across countries. Dr. Williams noted the limitations in this study of looking at differences across geography and ethnicity, particularly in Australia, but said the species identified were consistent with those found in most literature globally.
In response to other questions after the presentation, Dr. Williams said supplements (such as omega-3 fatty acids) were included in total intake, and those taking prebiotics or probiotics were excluded from the study. In an upcoming clinical trial, she and colleagues plan to control for household microbiomes as well.
“The premise is that microbiomes are shared between households, so when you’re doing these sorts of large-scale clinical studies, if you’re going to look at the microbiome, then you should control for one of the major confounding variables,” said Mark Sundrud, PhD, professor of medicine at the Dartmouth Center for Digestive Health in Lebanon, New Hampshire. Dr. Sundrud, who wasn’t involved with this study, presented on the role of bile acids in mucosal immune cell function at DDW.
“We’ve done a collaborative study looking at microbiomes and bile acids in inflammatory bowel disease (IBD) patients versus controls,” which included consideration of households, he said. “We were able to see more intrinsic disease-specific changes.”
Dr. Williams declared no relevant disclosures. Dr. Sundrud has served as a scientific adviser to Sage Therapeutics.
FROM DDW 2024
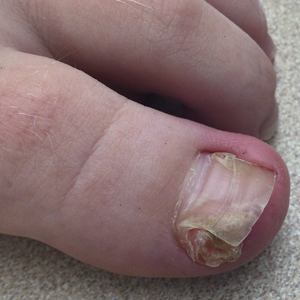
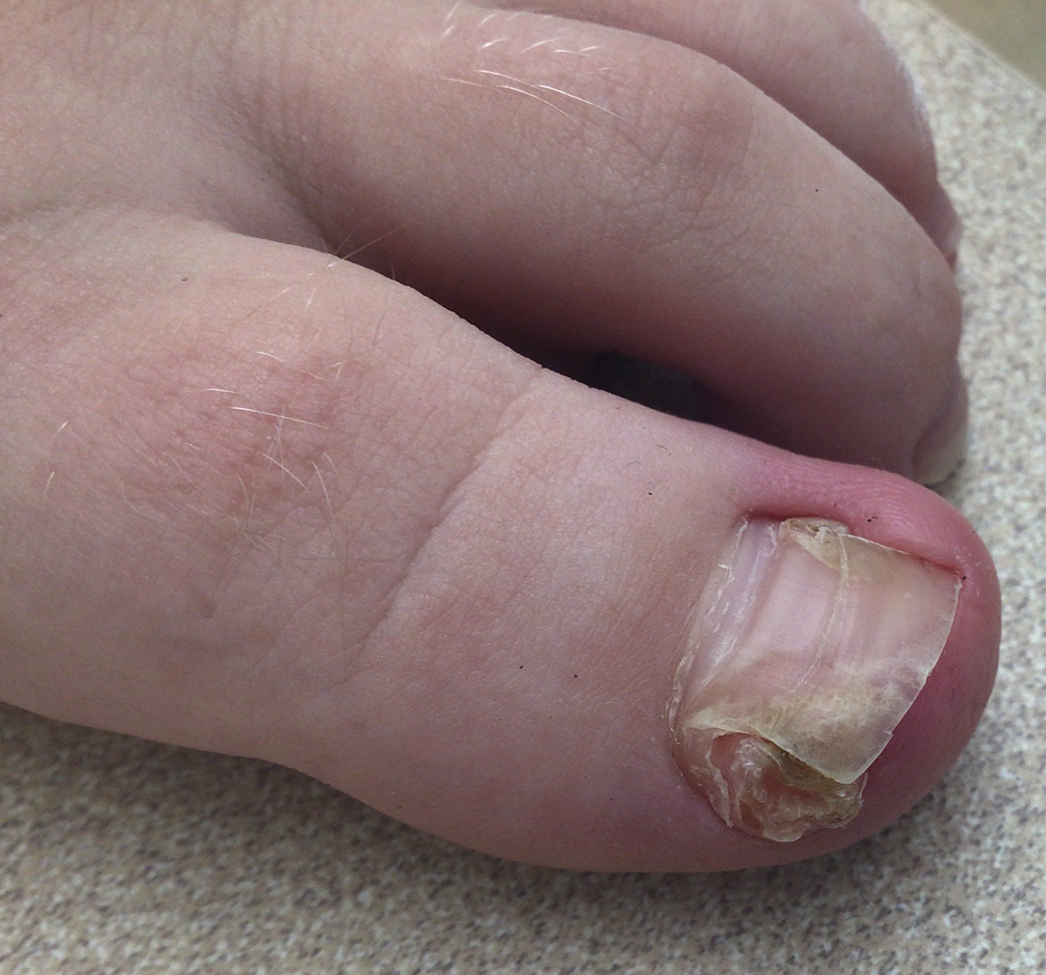
Subungual Nodule in a Pediatric Patient
The Diagnosis: Subungual Exostosis
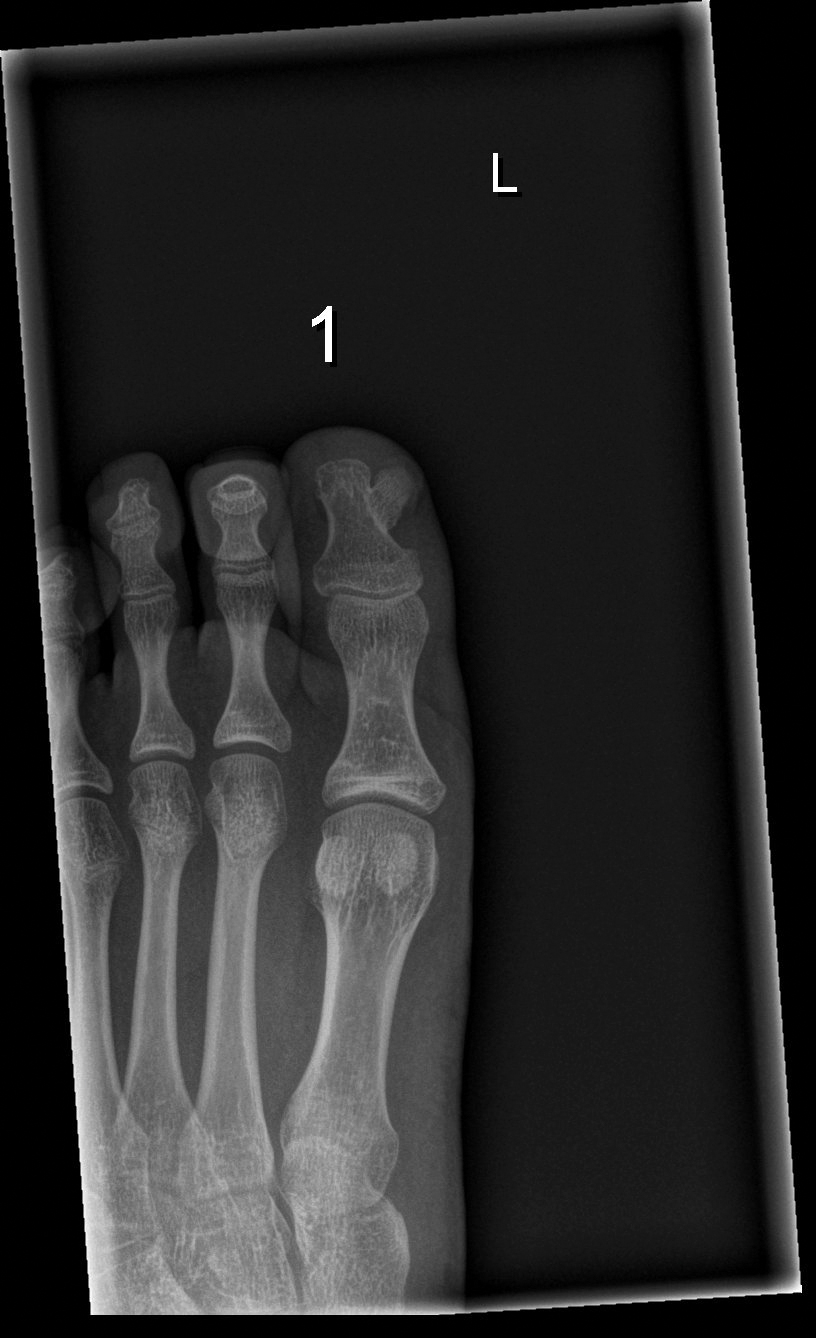
Subungual exostosis should be considered as a possible cause of an exophytic subungual nodule in a young active female. In our patient, the involvement of the great toe was a clue, as the hallux is the most common location of subungual exostosis. The patient’s age and sex also were supportive, as subungual exostosis is most common in female children and adolescents— particularly those who are active, as trauma is thought to play a possible role in development of this benign tumor.1-3 Radiography is the preferred modality for diagnosis; in our case, it showed a trabecular bony overgrowth (Figure 1), which confirmed the diagnosis. Subungual exostosis is a rare, benign, osteocartilaginous tumor of trabecular bone. The etiology is unknown but is hypothesized to be related to trauma, infection, or activation of a cartilaginous cyst.1,3 The subungual nodule may be asymptomatic or painful. Disruption and elevation of the nail plate is common.4 The differential diagnosis includes amelanotic melanoma, fibroma, fibrokeratoma, osteochondroma, pyogenic granuloma, squamous cell carcinoma, glomus tumor, and verruca vulgaris, among others.5
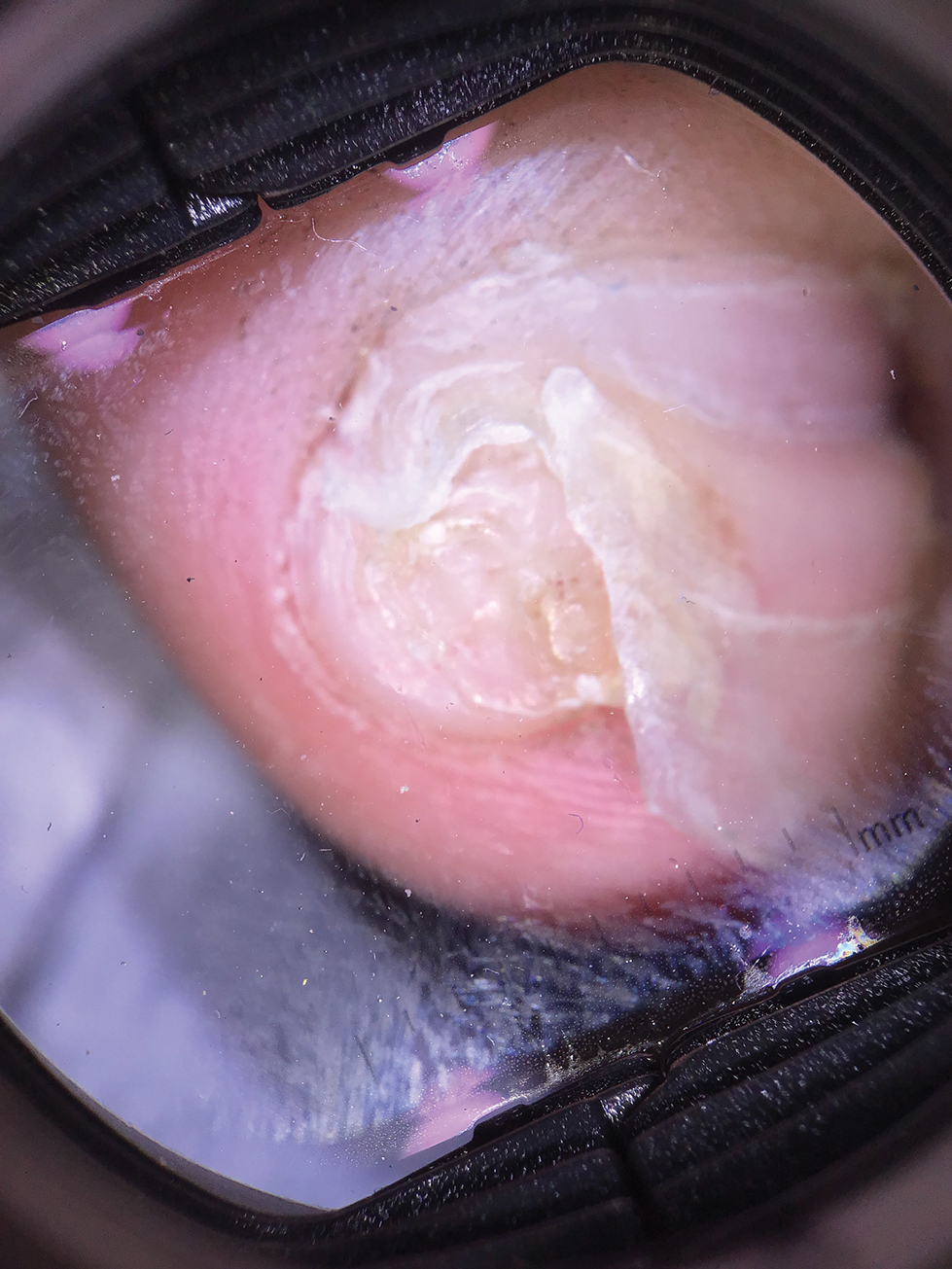
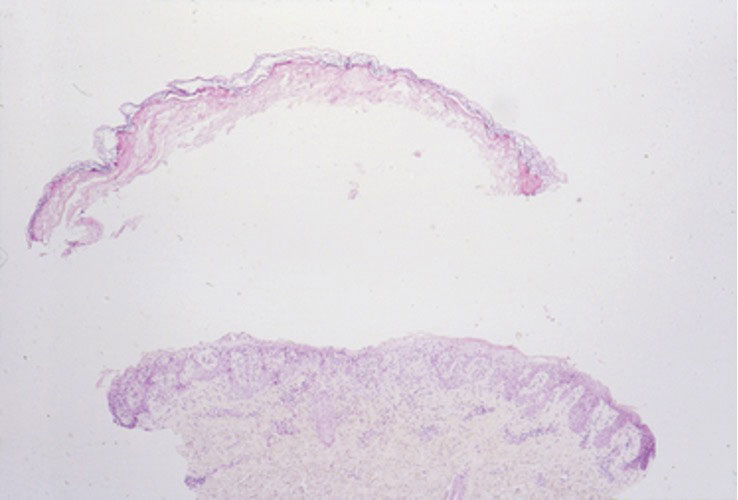
Physical examination demonstrates a firm, fixed, subungual nodule, often with an accompanying nail deformity. Further workup is required to confirm the benign nature of the lesion and exclude nail tumors such as melanoma or squamous cell carcinoma. Radiography is the gold standard for diagnosis, demonstrating a trabecular bony overgrowth.6 Performing a radiograph as the initial diagnostic test spares the patient from unnecessary procedures such as biopsy or expensive imaging techniques such as magnetic resonance imaging. Early lesions may not demonstrate sufficient bone formation shown on radiography. In these situations, a combination of dermoscopy and histopathologic examination may aid in diagnosis (Figure 2).4 Vascular ectasia, hyperkeratosis, onycholysis, and ulceration are the most common findings on dermoscopy (in ascending order).7 Histopathology typically demonstrates a base or stalk of normal-appearing trabecular bone with a fibrocartilage cap.8 However, initial clinical workup via radiography allows for the least-invasive and highest-yield intervention. Clinical suspicion for this condition is important, as it can be diagnosed with noninvasive inexpensive imaging rather than biopsy or more specialized imaging modalities. Appropriate recognition can save young patients from unnecessary and expensive procedures. Treatment typically involves surgical excision; to prevent regrowth, removal of the lesion at the base of the bone is recommended.2
Although amelanotic melanoma also can manifest as a subungual nail tumor, it would be unusual in a young child and would not be expected to show characteristic changes on radiography. A glomus tumor would be painful, is more common on the fingers than on the toes, and typically has a bluish hue.9 Verruca vulgaris can occur subungually but is more common around the nailfold and often has the characteristic dermoscopic finding of thrombosed capillaries. It also would not be expected to show characteristic radiographic findings. Osteochondroma can occur in young patients and can appear clinically similar to subungual exostosis; however, it typically is painful.10
- Pascoal D, Balaco I, Alves C, et al. Subungual exostosis—treatment results with preservation of the nail bed. J Pediatr Orthop B. 2020;29:382-386.
- Yousefian F, Davis B, Browning JC. Pediatric subungual exostosis. Cutis. 2021;108:256-257.
- Chiheb S, Slimani Y, Karam R, et al. Subungual exostosis: a case series of 48 patients. Skin Appendage Disord. 2021;7:475-479.
- Zhang W, Gu L, Fan H, et al. Subungual exostosis with an unusual dermoscopic feature. JAAD Case Rep. 2020;6:725-726.
- Demirdag HG, Tugrul Ayanoglu B, Akay BN. Dermoscopic features of subungual exostosis. Australas J Dermatol. 2019;60:E138-E141.
- Tritto M, Mirkin G, Hao X. Subungual exostosis on the right hallux. J Am Podiatr Med Assoc. 2021;111.
- Piccolo V, Argenziano G, Alessandrini AM, et al. Dermoscopy of subungual exostosis: a retrospective study of 10 patients. Dermatology. 2017;233:80-85.
- Lee SK, Jung MS, Lee YH, et al. Two distinctive subungual pathologies: subungual exostosis and subungual osteochondroma. Foot Ankle Int. 2007;28:595-601. doi:10.3113/FAI.2007.0595
- Samaniego E, Crespo A, Sanz A. Key diagnostic features and treatment of subungual glomus tumor. Actas Dermosifiliogr. 2009;100:875-882.
- Glick S. Subungual osteochondroma of the third toe. Consult.360. 2013;12.
The Diagnosis: Subungual Exostosis
Subungual exostosis should be considered as a possible cause of an exophytic subungual nodule in a young active female. In our patient, the involvement of the great toe was a clue, as the hallux is the most common location of subungual exostosis. The patient’s age and sex also were supportive, as subungual exostosis is most common in female children and adolescents— particularly those who are active, as trauma is thought to play a possible role in development of this benign tumor.1-3 Radiography is the preferred modality for diagnosis; in our case, it showed a trabecular bony overgrowth (Figure 1), which confirmed the diagnosis. Subungual exostosis is a rare, benign, osteocartilaginous tumor of trabecular bone. The etiology is unknown but is hypothesized to be related to trauma, infection, or activation of a cartilaginous cyst.1,3 The subungual nodule may be asymptomatic or painful. Disruption and elevation of the nail plate is common.4 The differential diagnosis includes amelanotic melanoma, fibroma, fibrokeratoma, osteochondroma, pyogenic granuloma, squamous cell carcinoma, glomus tumor, and verruca vulgaris, among others.5

Physical examination demonstrates a firm, fixed, subungual nodule, often with an accompanying nail deformity. Further workup is required to confirm the benign nature of the lesion and exclude nail tumors such as melanoma or squamous cell carcinoma. Radiography is the gold standard for diagnosis, demonstrating a trabecular bony overgrowth.6 Performing a radiograph as the initial diagnostic test spares the patient from unnecessary procedures such as biopsy or expensive imaging techniques such as magnetic resonance imaging. Early lesions may not demonstrate sufficient bone formation shown on radiography. In these situations, a combination of dermoscopy and histopathologic examination may aid in diagnosis (Figure 2).4 Vascular ectasia, hyperkeratosis, onycholysis, and ulceration are the most common findings on dermoscopy (in ascending order).7 Histopathology typically demonstrates a base or stalk of normal-appearing trabecular bone with a fibrocartilage cap.8 However, initial clinical workup via radiography allows for the least-invasive and highest-yield intervention. Clinical suspicion for this condition is important, as it can be diagnosed with noninvasive inexpensive imaging rather than biopsy or more specialized imaging modalities. Appropriate recognition can save young patients from unnecessary and expensive procedures. Treatment typically involves surgical excision; to prevent regrowth, removal of the lesion at the base of the bone is recommended.2

Although amelanotic melanoma also can manifest as a subungual nail tumor, it would be unusual in a young child and would not be expected to show characteristic changes on radiography. A glomus tumor would be painful, is more common on the fingers than on the toes, and typically has a bluish hue.9 Verruca vulgaris can occur subungually but is more common around the nailfold and often has the characteristic dermoscopic finding of thrombosed capillaries. It also would not be expected to show characteristic radiographic findings. Osteochondroma can occur in young patients and can appear clinically similar to subungual exostosis; however, it typically is painful.10
The Diagnosis: Subungual Exostosis
Subungual exostosis should be considered as a possible cause of an exophytic subungual nodule in a young active female. In our patient, the involvement of the great toe was a clue, as the hallux is the most common location of subungual exostosis. The patient’s age and sex also were supportive, as subungual exostosis is most common in female children and adolescents— particularly those who are active, as trauma is thought to play a possible role in development of this benign tumor.1-3 Radiography is the preferred modality for diagnosis; in our case, it showed a trabecular bony overgrowth (Figure 1), which confirmed the diagnosis. Subungual exostosis is a rare, benign, osteocartilaginous tumor of trabecular bone. The etiology is unknown but is hypothesized to be related to trauma, infection, or activation of a cartilaginous cyst.1,3 The subungual nodule may be asymptomatic or painful. Disruption and elevation of the nail plate is common.4 The differential diagnosis includes amelanotic melanoma, fibroma, fibrokeratoma, osteochondroma, pyogenic granuloma, squamous cell carcinoma, glomus tumor, and verruca vulgaris, among others.5

Physical examination demonstrates a firm, fixed, subungual nodule, often with an accompanying nail deformity. Further workup is required to confirm the benign nature of the lesion and exclude nail tumors such as melanoma or squamous cell carcinoma. Radiography is the gold standard for diagnosis, demonstrating a trabecular bony overgrowth.6 Performing a radiograph as the initial diagnostic test spares the patient from unnecessary procedures such as biopsy or expensive imaging techniques such as magnetic resonance imaging. Early lesions may not demonstrate sufficient bone formation shown on radiography. In these situations, a combination of dermoscopy and histopathologic examination may aid in diagnosis (Figure 2).4 Vascular ectasia, hyperkeratosis, onycholysis, and ulceration are the most common findings on dermoscopy (in ascending order).7 Histopathology typically demonstrates a base or stalk of normal-appearing trabecular bone with a fibrocartilage cap.8 However, initial clinical workup via radiography allows for the least-invasive and highest-yield intervention. Clinical suspicion for this condition is important, as it can be diagnosed with noninvasive inexpensive imaging rather than biopsy or more specialized imaging modalities. Appropriate recognition can save young patients from unnecessary and expensive procedures. Treatment typically involves surgical excision; to prevent regrowth, removal of the lesion at the base of the bone is recommended.2

Although amelanotic melanoma also can manifest as a subungual nail tumor, it would be unusual in a young child and would not be expected to show characteristic changes on radiography. A glomus tumor would be painful, is more common on the fingers than on the toes, and typically has a bluish hue.9 Verruca vulgaris can occur subungually but is more common around the nailfold and often has the characteristic dermoscopic finding of thrombosed capillaries. It also would not be expected to show characteristic radiographic findings. Osteochondroma can occur in young patients and can appear clinically similar to subungual exostosis; however, it typically is painful.10
- Pascoal D, Balaco I, Alves C, et al. Subungual exostosis—treatment results with preservation of the nail bed. J Pediatr Orthop B. 2020;29:382-386.
- Yousefian F, Davis B, Browning JC. Pediatric subungual exostosis. Cutis. 2021;108:256-257.
- Chiheb S, Slimani Y, Karam R, et al. Subungual exostosis: a case series of 48 patients. Skin Appendage Disord. 2021;7:475-479.
- Zhang W, Gu L, Fan H, et al. Subungual exostosis with an unusual dermoscopic feature. JAAD Case Rep. 2020;6:725-726.
- Demirdag HG, Tugrul Ayanoglu B, Akay BN. Dermoscopic features of subungual exostosis. Australas J Dermatol. 2019;60:E138-E141.
- Tritto M, Mirkin G, Hao X. Subungual exostosis on the right hallux. J Am Podiatr Med Assoc. 2021;111.
- Piccolo V, Argenziano G, Alessandrini AM, et al. Dermoscopy of subungual exostosis: a retrospective study of 10 patients. Dermatology. 2017;233:80-85.
- Lee SK, Jung MS, Lee YH, et al. Two distinctive subungual pathologies: subungual exostosis and subungual osteochondroma. Foot Ankle Int. 2007;28:595-601. doi:10.3113/FAI.2007.0595
- Samaniego E, Crespo A, Sanz A. Key diagnostic features and treatment of subungual glomus tumor. Actas Dermosifiliogr. 2009;100:875-882.
- Glick S. Subungual osteochondroma of the third toe. Consult.360. 2013;12.
- Pascoal D, Balaco I, Alves C, et al. Subungual exostosis—treatment results with preservation of the nail bed. J Pediatr Orthop B. 2020;29:382-386.
- Yousefian F, Davis B, Browning JC. Pediatric subungual exostosis. Cutis. 2021;108:256-257.
- Chiheb S, Slimani Y, Karam R, et al. Subungual exostosis: a case series of 48 patients. Skin Appendage Disord. 2021;7:475-479.
- Zhang W, Gu L, Fan H, et al. Subungual exostosis with an unusual dermoscopic feature. JAAD Case Rep. 2020;6:725-726.
- Demirdag HG, Tugrul Ayanoglu B, Akay BN. Dermoscopic features of subungual exostosis. Australas J Dermatol. 2019;60:E138-E141.
- Tritto M, Mirkin G, Hao X. Subungual exostosis on the right hallux. J Am Podiatr Med Assoc. 2021;111.
- Piccolo V, Argenziano G, Alessandrini AM, et al. Dermoscopy of subungual exostosis: a retrospective study of 10 patients. Dermatology. 2017;233:80-85.
- Lee SK, Jung MS, Lee YH, et al. Two distinctive subungual pathologies: subungual exostosis and subungual osteochondroma. Foot Ankle Int. 2007;28:595-601. doi:10.3113/FAI.2007.0595
- Samaniego E, Crespo A, Sanz A. Key diagnostic features and treatment of subungual glomus tumor. Actas Dermosifiliogr. 2009;100:875-882.
- Glick S. Subungual osteochondroma of the third toe. Consult.360. 2013;12.
A 13-year-old girl presented to her pediatrician with a small pink bump under the left great toenail of 8 months’ duration that was slowly growing. Months later, she developed an ingrown nail on the same toe, which was treated with partial nail avulsion by the pediatrician. Given continued nail dystrophy and a visible bump under the nail, the patient was referred to dermatology. Physical examination revealed a subungual, flesh-colored, sessile nodule causing distortion of the nail plate on the left great toe with associated intermittent redness and swelling. She denied wearing new shoes or experiencing any pain, pruritus, or purulent drainage or bleeding from the lesion. She reported being physically active and playing tennis.
Study Finds Mace Risk Remains High in Patients with Psoriasis, Dyslipidemia
Over a period of 5 years, the, even after adjusting for covariates, results from a large retrospective study showed.
“It is well-established that psoriasis is an independent risk factor for the development of MACE, with cardiometabolic risk factors being more prevalent and incident among patients with psoriasis,” the study’s first author Ana Ormaza Vera, MD, a dermatology research fellow at Eastern Virginia Medical School, Norfolk, said in an interview after the annual meeting of the Society for Investigational Dermatology, where the study was presented during a late-breaking abstract session.
Current guidelines from the joint American Academy of Dermatology/National Psoriasis Foundation and the American Academy of Cardiology/American Heart Association Task Force recommend statins, a lipid-lowering and anti-inflammatory therapy, “for patients with psoriasis who have additional risk-enhancing factors, similar to recommendations made for the general population without psoriasis,” she noted. But how the incidence of MACE differs between patients with and without psoriasis while on statin therapy “has not been explored in real-world settings,” she added.
To address this question, the researchers used real-world data from the TriNetX health research network to identify individuals aged 18-90 years with a diagnosis of both psoriasis and lipid disorders who were undergoing treatment with statins. Those with a prior history of MACE were excluded from the analysis. Patients with lipid disorders on statin therapy, but without psoriatic disease, were matched 1:1 by age, sex, race, ethnicity, common risk factors for MACE, and medications shown to reduce MACE risk. The researchers then assessed the cohorts 5 years following their first statin prescription and used the TriNetX analytics tool to calculate the odds ratio (OR) with 95% CI to evaluate the likelihood of MACE in the presence of statin therapy.
Dr. Ormaza Vera and colleagues identified 20,660 patients with psoriasis and 2,768,429 patients without psoriasis who met the criteria for analysis. After propensity score matching, each cohort included 20,660 patients with a mean age of 60 years. During the 5-year observation period, 2725 patients in the psoriasis cohort experienced MACE compared with 2203 patients in the non-psoriasis cohort (OR, 1.40; 95% CI, 1.317-1.488).
“This was an unexpected outcome that challenges the current understanding and highlights the need for further research into tailored treatments for cardiovascular risk in psoriasis patients,” Dr. Ormaza Vera told this news organization.
She acknowledged certain limitations of the study, including its retrospective design, the inherent limitations of an observational study, and the use of electronic medical record data.
Lawrence J. Green, MD, clinical professor of dermatology, George Washington University, Washington, who was asked to comment on the study results, said that the findings imply that there is more than statin use alone to protect someone with psoriasis from having an increased risk for MACE. “This is not really surprising because statin use alone is only part of a prevention strategy in someone with psoriasis who usually has multiple comorbidities,” Dr. Green said. “On the other hand, the study only went out for 5 years and cardiovascular disease is a long accumulating process, so it could also be too early to demonstrate MACE prevention.”
The study was funded by a grant from the American Skin Association. Dr. Ormaza Vera and her coauthors reported having no relevant disclosures. Dr. Green disclosed that he is a speaker, consultant, or investigator for many pharmaceutical companies.
A version of this article appeared on Medscape.com .
Over a period of 5 years, the, even after adjusting for covariates, results from a large retrospective study showed.
“It is well-established that psoriasis is an independent risk factor for the development of MACE, with cardiometabolic risk factors being more prevalent and incident among patients with psoriasis,” the study’s first author Ana Ormaza Vera, MD, a dermatology research fellow at Eastern Virginia Medical School, Norfolk, said in an interview after the annual meeting of the Society for Investigational Dermatology, where the study was presented during a late-breaking abstract session.
Current guidelines from the joint American Academy of Dermatology/National Psoriasis Foundation and the American Academy of Cardiology/American Heart Association Task Force recommend statins, a lipid-lowering and anti-inflammatory therapy, “for patients with psoriasis who have additional risk-enhancing factors, similar to recommendations made for the general population without psoriasis,” she noted. But how the incidence of MACE differs between patients with and without psoriasis while on statin therapy “has not been explored in real-world settings,” she added.
To address this question, the researchers used real-world data from the TriNetX health research network to identify individuals aged 18-90 years with a diagnosis of both psoriasis and lipid disorders who were undergoing treatment with statins. Those with a prior history of MACE were excluded from the analysis. Patients with lipid disorders on statin therapy, but without psoriatic disease, were matched 1:1 by age, sex, race, ethnicity, common risk factors for MACE, and medications shown to reduce MACE risk. The researchers then assessed the cohorts 5 years following their first statin prescription and used the TriNetX analytics tool to calculate the odds ratio (OR) with 95% CI to evaluate the likelihood of MACE in the presence of statin therapy.
Dr. Ormaza Vera and colleagues identified 20,660 patients with psoriasis and 2,768,429 patients without psoriasis who met the criteria for analysis. After propensity score matching, each cohort included 20,660 patients with a mean age of 60 years. During the 5-year observation period, 2725 patients in the psoriasis cohort experienced MACE compared with 2203 patients in the non-psoriasis cohort (OR, 1.40; 95% CI, 1.317-1.488).
“This was an unexpected outcome that challenges the current understanding and highlights the need for further research into tailored treatments for cardiovascular risk in psoriasis patients,” Dr. Ormaza Vera told this news organization.
She acknowledged certain limitations of the study, including its retrospective design, the inherent limitations of an observational study, and the use of electronic medical record data.
Lawrence J. Green, MD, clinical professor of dermatology, George Washington University, Washington, who was asked to comment on the study results, said that the findings imply that there is more than statin use alone to protect someone with psoriasis from having an increased risk for MACE. “This is not really surprising because statin use alone is only part of a prevention strategy in someone with psoriasis who usually has multiple comorbidities,” Dr. Green said. “On the other hand, the study only went out for 5 years and cardiovascular disease is a long accumulating process, so it could also be too early to demonstrate MACE prevention.”
The study was funded by a grant from the American Skin Association. Dr. Ormaza Vera and her coauthors reported having no relevant disclosures. Dr. Green disclosed that he is a speaker, consultant, or investigator for many pharmaceutical companies.
A version of this article appeared on Medscape.com .
Over a period of 5 years, the, even after adjusting for covariates, results from a large retrospective study showed.
“It is well-established that psoriasis is an independent risk factor for the development of MACE, with cardiometabolic risk factors being more prevalent and incident among patients with psoriasis,” the study’s first author Ana Ormaza Vera, MD, a dermatology research fellow at Eastern Virginia Medical School, Norfolk, said in an interview after the annual meeting of the Society for Investigational Dermatology, where the study was presented during a late-breaking abstract session.
Current guidelines from the joint American Academy of Dermatology/National Psoriasis Foundation and the American Academy of Cardiology/American Heart Association Task Force recommend statins, a lipid-lowering and anti-inflammatory therapy, “for patients with psoriasis who have additional risk-enhancing factors, similar to recommendations made for the general population without psoriasis,” she noted. But how the incidence of MACE differs between patients with and without psoriasis while on statin therapy “has not been explored in real-world settings,” she added.
To address this question, the researchers used real-world data from the TriNetX health research network to identify individuals aged 18-90 years with a diagnosis of both psoriasis and lipid disorders who were undergoing treatment with statins. Those with a prior history of MACE were excluded from the analysis. Patients with lipid disorders on statin therapy, but without psoriatic disease, were matched 1:1 by age, sex, race, ethnicity, common risk factors for MACE, and medications shown to reduce MACE risk. The researchers then assessed the cohorts 5 years following their first statin prescription and used the TriNetX analytics tool to calculate the odds ratio (OR) with 95% CI to evaluate the likelihood of MACE in the presence of statin therapy.
Dr. Ormaza Vera and colleagues identified 20,660 patients with psoriasis and 2,768,429 patients without psoriasis who met the criteria for analysis. After propensity score matching, each cohort included 20,660 patients with a mean age of 60 years. During the 5-year observation period, 2725 patients in the psoriasis cohort experienced MACE compared with 2203 patients in the non-psoriasis cohort (OR, 1.40; 95% CI, 1.317-1.488).
“This was an unexpected outcome that challenges the current understanding and highlights the need for further research into tailored treatments for cardiovascular risk in psoriasis patients,” Dr. Ormaza Vera told this news organization.
She acknowledged certain limitations of the study, including its retrospective design, the inherent limitations of an observational study, and the use of electronic medical record data.
Lawrence J. Green, MD, clinical professor of dermatology, George Washington University, Washington, who was asked to comment on the study results, said that the findings imply that there is more than statin use alone to protect someone with psoriasis from having an increased risk for MACE. “This is not really surprising because statin use alone is only part of a prevention strategy in someone with psoriasis who usually has multiple comorbidities,” Dr. Green said. “On the other hand, the study only went out for 5 years and cardiovascular disease is a long accumulating process, so it could also be too early to demonstrate MACE prevention.”
The study was funded by a grant from the American Skin Association. Dr. Ormaza Vera and her coauthors reported having no relevant disclosures. Dr. Green disclosed that he is a speaker, consultant, or investigator for many pharmaceutical companies.
A version of this article appeared on Medscape.com .
FROM SID 2024
Low-Field MRIs
Recently, “low field” MRIs have been in the news, with the promise that they’ll be safer and easier. People can go in them with their cell phones, car keys in pockets, no ear plugs needed for the noise, etc. They’re cheaper to build and can be plugged into a standard outlet.
That’s all well and good, but what about accuracy and image quality?
That’s a big question. Even proponents of the technology say it’s not as good as what we see with 3T MRI, so they’re trying to compensate by using AI and other software protocols to enhance the pictures. Allegedly it looks good, but so far only healthy volunteers have been scanned. How will it do with a small low-grade glioma or other subtle (but important) findings? We don’t know yet.
Personally, I think having to give up your iPhone and car keys for an hour, and put in foam ear plugs, are small trade-offs to get an accurate diagnosis.
Of course, I’m also approaching this as someone who deals with brain imaging. Maybe for other structures, like a knee, that kind of detail isn’t as necessary (or maybe it is. I’m definitely not in that field).
So, as with so many things that make it into the popular press, they likely have potential, but are still not ready for prime time.
This sort of stuff always gets my office phones ringing. Patients see a blurb about it on the news or Facebook and assume it’s available now, so they want one. They seem to think the new MRI is like Bones McCoy’s tricorder. I take the scanner off my belt, wave it over them, and the answer comes up on the screen. The fact that the unit still weighs over a ton is hidden at the bottom of the blurb, if it’s even mentioned at all.
There’s also the likelihood that this sort of thing is going to be taken to the public, in the same way carotid Dopplers have been. Marketed to the worried well with celebrity endorsements and taglines like “see what your doctor won’t look for.” Of course, MRIs are chock full of things like nonspecific white matter changes, disc bulges, tiny meningiomas, and a host of other incidental findings that cause panic in cyberchondriacs. Who then call us.
But that’s another story.
I understand that for some parts of the world a comparatively inexpensive, transportable, MRI that requires less shielding and power is a HUGE deal. Its availability can make the difference between life and death.
I’m not knocking the technology. I’m sure it will be useful. But, like so much in medicine, it’s not here yet.
Dr. Block has a solo neurology practice in Scottsdale, Arizona.
Recently, “low field” MRIs have been in the news, with the promise that they’ll be safer and easier. People can go in them with their cell phones, car keys in pockets, no ear plugs needed for the noise, etc. They’re cheaper to build and can be plugged into a standard outlet.
That’s all well and good, but what about accuracy and image quality?
That’s a big question. Even proponents of the technology say it’s not as good as what we see with 3T MRI, so they’re trying to compensate by using AI and other software protocols to enhance the pictures. Allegedly it looks good, but so far only healthy volunteers have been scanned. How will it do with a small low-grade glioma or other subtle (but important) findings? We don’t know yet.
Personally, I think having to give up your iPhone and car keys for an hour, and put in foam ear plugs, are small trade-offs to get an accurate diagnosis.
Of course, I’m also approaching this as someone who deals with brain imaging. Maybe for other structures, like a knee, that kind of detail isn’t as necessary (or maybe it is. I’m definitely not in that field).
So, as with so many things that make it into the popular press, they likely have potential, but are still not ready for prime time.
This sort of stuff always gets my office phones ringing. Patients see a blurb about it on the news or Facebook and assume it’s available now, so they want one. They seem to think the new MRI is like Bones McCoy’s tricorder. I take the scanner off my belt, wave it over them, and the answer comes up on the screen. The fact that the unit still weighs over a ton is hidden at the bottom of the blurb, if it’s even mentioned at all.
There’s also the likelihood that this sort of thing is going to be taken to the public, in the same way carotid Dopplers have been. Marketed to the worried well with celebrity endorsements and taglines like “see what your doctor won’t look for.” Of course, MRIs are chock full of things like nonspecific white matter changes, disc bulges, tiny meningiomas, and a host of other incidental findings that cause panic in cyberchondriacs. Who then call us.
But that’s another story.
I understand that for some parts of the world a comparatively inexpensive, transportable, MRI that requires less shielding and power is a HUGE deal. Its availability can make the difference between life and death.
I’m not knocking the technology. I’m sure it will be useful. But, like so much in medicine, it’s not here yet.
Dr. Block has a solo neurology practice in Scottsdale, Arizona.
Recently, “low field” MRIs have been in the news, with the promise that they’ll be safer and easier. People can go in them with their cell phones, car keys in pockets, no ear plugs needed for the noise, etc. They’re cheaper to build and can be plugged into a standard outlet.
That’s all well and good, but what about accuracy and image quality?
That’s a big question. Even proponents of the technology say it’s not as good as what we see with 3T MRI, so they’re trying to compensate by using AI and other software protocols to enhance the pictures. Allegedly it looks good, but so far only healthy volunteers have been scanned. How will it do with a small low-grade glioma or other subtle (but important) findings? We don’t know yet.
Personally, I think having to give up your iPhone and car keys for an hour, and put in foam ear plugs, are small trade-offs to get an accurate diagnosis.
Of course, I’m also approaching this as someone who deals with brain imaging. Maybe for other structures, like a knee, that kind of detail isn’t as necessary (or maybe it is. I’m definitely not in that field).
So, as with so many things that make it into the popular press, they likely have potential, but are still not ready for prime time.
This sort of stuff always gets my office phones ringing. Patients see a blurb about it on the news or Facebook and assume it’s available now, so they want one. They seem to think the new MRI is like Bones McCoy’s tricorder. I take the scanner off my belt, wave it over them, and the answer comes up on the screen. The fact that the unit still weighs over a ton is hidden at the bottom of the blurb, if it’s even mentioned at all.
There’s also the likelihood that this sort of thing is going to be taken to the public, in the same way carotid Dopplers have been. Marketed to the worried well with celebrity endorsements and taglines like “see what your doctor won’t look for.” Of course, MRIs are chock full of things like nonspecific white matter changes, disc bulges, tiny meningiomas, and a host of other incidental findings that cause panic in cyberchondriacs. Who then call us.
But that’s another story.
I understand that for some parts of the world a comparatively inexpensive, transportable, MRI that requires less shielding and power is a HUGE deal. Its availability can make the difference between life and death.
I’m not knocking the technology. I’m sure it will be useful. But, like so much in medicine, it’s not here yet.
Dr. Block has a solo neurology practice in Scottsdale, Arizona.
Aquatic Antagonists: Seaweed Dermatitis (Lyngbya majuscula)
Aquatic Antagonists: Seaweed Dermatitis (Lyngbya majuscula)
The filamentous cyanobacterium Lyngbya majuscula causes irritant contact dermatitis in beachgoers, fishers, and divers in tropical and subtropical marine environments worldwide.1 If fragments of L majuscula lodge in swimmers’ bathing suits, the toxins can become trapped against the skin and cause seaweed dermatitis.2 With climate change resulting in warmer oceans and more extreme storms, L majuscula blooms likely will become more frequent and widespread, thereby increasing the risk for human exposure.3,4 Herein, we describe the irritants that lead to dermatitis, clinical presentation, and prevention and management of seaweed dermatitis.
Identifying Features and Distribution of Plant
Lyngbya majuscula belongs to the family Oscillatoriaceae; these cyanobacteria grow as filaments and exhibit slow oscillating movements. Commonly referred to as blanketweed or mermaid’s hair due to its appearance, L majuscula grows fine hairlike clumps resembling a mass of olive-colored matted hair.1 Its thin filaments are 10- to 30-cm long and vary in color from red to white to brown.5 Microscopically, a rouleauxlike arrangement of discs provides the structure of each filament.6
First identified in Hawaii in 1912, L majuscula was not associated with seaweed dermatitis or dermatotoxicity by the medical community until the first outbreak occurred in Oahu in 1958, though fishermen and beachgoers previously had recognized a relationship between this particular seaweed and skin irritation.5,7 The first reporting included 125 confirmed cases, with many more mild unreported cases suspected.6 Now reported in about 100 locations worldwide, seaweed dermatitis outbreaks have occurred in Australia; Okinawa, Japan; Florida; and the Hawaiian and Marshall islands.1,2
Exposure to Seaweed
Lyngbya majuscula produces more than 70 biologically active compounds that irritate the skin, eyes, and respiratory system.2,8 It grows in marine and estuarine environments attached to seagrass, sand, and bedrock at depths of up to 30 m. Warm waters and maximal sunlight provide optimal growth conditions for L majuscula; therefore, the greatest risk for exposure occurs in the Northern and Southern hemispheres in the 1- to 2-month period following their summer solstices.5 Runoff during heavy rainfall, which is rich in soil extracts such as phosphorous, iron, and organic carbon, stimulates L majuscula growth and contributes to increased algal blooms.4
Dermatitis and Irritants
The dermatoxins Lyngbyatoxin A (LA) and debromoaplysiatoxin (DAT) cause the inflammatory and necrotic appearance of seaweed dermatitis.1,2,5,8 Lyngbyatoxin A is an indole alkaloid that is closely related to telocidin B, a poisonous compound associated with Streptomyces bacteria.9 Sampling of L majuscula and extraction of the dermatoxin, along with human and animal studies, confirmed DAT irritates the skin and induces dermatitis.5,6 Stylocheilus longicauda (sea hare) feeds on L majuscula and contains isolates of DAT in its digestive tract.
Samples of L majuscula taken from several Hawaiian Islands where seaweed dermatitis outbreaks have occurred were examined for differences in toxicities via 6-hour patch tests on human skin.6 The samples obtained from the windward side of Oahu contained DAT and aplysiatoxin, while those obtained from the leeward side and Kahala Beach primarily contained LA. Although DAT and LA are vastly different in their molecular structures, testing elicited the same biologic response and induced the same level of skin irritation.6 Interestingly, not all strands of L majuscula produced LA and DAT and caused seaweed dermatitis; those that did lead to irritation were more red in color than nontoxic blooms.5,9
Cutaneous Manifestations
Seaweed dermatitis resembles chemical and thermal burns, ranging from a mild skin rash to severe contact dermatitis with itchy, swollen, ulcerated lesions.1,7 Patients typically develop a burning or itching sensation beneath their bathing suit or wetsuit that progresses to an erythematous papulovesicular eruption 2 to 24 hours after exposure.2,6 Within a week, vesicles and bullae desquamate, leaving behind tender erosions.1,2,6,8 Inframammary lesions are common in females and scrotal swelling in males.1,6 There is no known association between length of time spent in the water and severity of symptoms.5
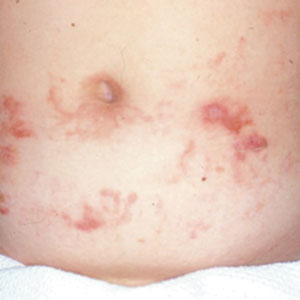
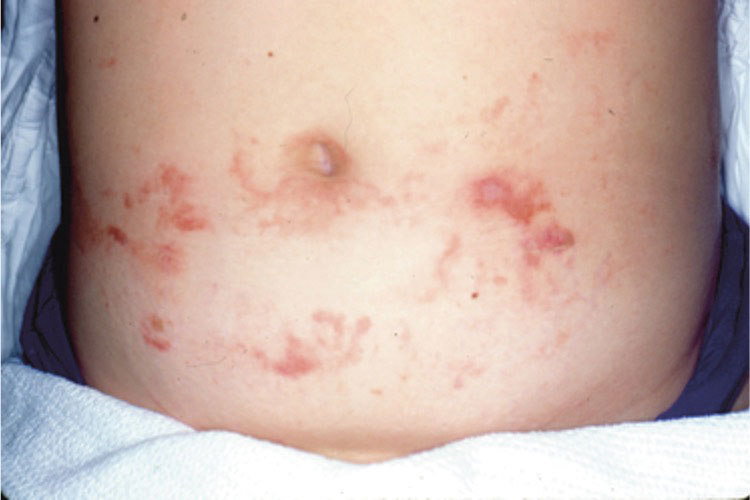
Most reactions to L majuscula occur from exposure in the water; however, particles that become aerosolized during strong winds or storms can cause seaweed dermatitis on the face. Inhalation of L majuscula may lead to mucous membrane ulceration and pulmonary edema.1,5,6 Noncutaneous manifestations of seaweed dermatitis include headache, fatigue, and swelling of the eyes, nose, and throat (Figures 1 and 2).1,5
Prevention and Management
To prevent seaweed dermatitis, avoid swimming in ocean water during L majuscula blooms,10 which frequently occur following the summer solstices in the Northern and Southern hemispheres.5 The National Centers for Coastal Ocean Science Harmful Algae Bloom Monitoring System provides real-time access to algae bloom locations.11 Although this monitoring system is not specific to L majuscula, it may be helpful in determining where potential blooms are. Wearing protective clothing such as coveralls may benefit individuals who enter the water during blooms, but it does not guarantee protection.10
magnification ×40). Photograph courtesy of Scott Norton, MD, MPH, MSc (Washington, DC).
Currently, there is no treatment for seaweed dermatitis, but symptom management may reduce discomfort and pain. Washing affected skin with soap and water within an hour of exposure may help reduce the severity of seaweed dermatitis, though studies have shown mixed results.6,7 Application of cool compresses and soothing ointments (eg, calamine) provide symptomatic relief and promote healing.7 The dermatitis typically self-resolves within 1 week.
- Werner K, Marquart L, Norton S. Lyngbya dermatitis (toxic seaweed dermatitis). Int J Dermatol. 2011;51:59-62. doi:10.1111/j.1365-4632.2011.05042.x
- Osborne N, Shaw G. Dermatitis associated with exposure to a marine cyanobacterium during recreational water exposure. BMC Dermatol. 2008;8:5. doi:10.1186/1471-5945-8-5
- Hays G, Richardson A, Robinson C. Climate change and marine plankton. Trends Ecol Evol. 2005;20:337-344. doi:10.1016/j.tree.2005.03.004
- Albert S, O’Neil J, Udy J, et al. Blooms of the cyanobacterium Lyngbya majuscula in costal Queensland, Australia: disparate sites, common factors. Mar Pollut Bull. 2004;51:428-437. doi:10.1016/j.marpolbul.2004.10.016
- Osborne N, Webb P, Shaw G. The toxins of Lyngbya majuscula and their human and ecological health effects. Environ Int. 2001;27:381-392. doi:10.1016/s0160-4120(01)00098-8
- Izumi A, Moore R. Seaweed ( Lyngbya majuscula ) dermatitis . Clin Dermatol . 1987;5:92-100. doi:10.1016/s0738-081x(87)80014-7
- Grauer F, Arnold H. Seaweed dermatitis: first report of a dermatitis-producing marine alga. Arch Dermatol. 1961; 84:720-732. doi:10.1001/archderm.1961.01580170014003
- Taylor M, Stahl-Timmins W, Redshaw C, et al. Toxic alkaloids in Lyngbya majuscula and related tropical marine cyanobacteria. Harmful Algae . 2014;31:1-8. doi:10.1016/j.hal.2013.09.003
- Cardellina J, Marner F, Moore R. Seaweed dermatitis: structure of lyngbyatoxin A. Science. 1979;204:193-195. doi:10.1126/science.107586
- Osborne N. Occupational dermatitis caused by Lyngbya majuscule in Australia. Int J Dermatol . 2012;5:122-123. doi:10.1111/j.1365-4632.2009.04455.x
- Harmful Algal Bloom Monitoring System. National Centers for Coastal Ocean Science. Accessed May 23, 2024. https://coastalscience.noaa.gov/research/stressor-impacts-mitigation/hab-monitoring-system/
The filamentous cyanobacterium Lyngbya majuscula causes irritant contact dermatitis in beachgoers, fishers, and divers in tropical and subtropical marine environments worldwide.1 If fragments of L majuscula lodge in swimmers’ bathing suits, the toxins can become trapped against the skin and cause seaweed dermatitis.2 With climate change resulting in warmer oceans and more extreme storms, L majuscula blooms likely will become more frequent and widespread, thereby increasing the risk for human exposure.3,4 Herein, we describe the irritants that lead to dermatitis, clinical presentation, and prevention and management of seaweed dermatitis.
Identifying Features and Distribution of Plant
Lyngbya majuscula belongs to the family Oscillatoriaceae; these cyanobacteria grow as filaments and exhibit slow oscillating movements. Commonly referred to as blanketweed or mermaid’s hair due to its appearance, L majuscula grows fine hairlike clumps resembling a mass of olive-colored matted hair.1 Its thin filaments are 10- to 30-cm long and vary in color from red to white to brown.5 Microscopically, a rouleauxlike arrangement of discs provides the structure of each filament.6
First identified in Hawaii in 1912, L majuscula was not associated with seaweed dermatitis or dermatotoxicity by the medical community until the first outbreak occurred in Oahu in 1958, though fishermen and beachgoers previously had recognized a relationship between this particular seaweed and skin irritation.5,7 The first reporting included 125 confirmed cases, with many more mild unreported cases suspected.6 Now reported in about 100 locations worldwide, seaweed dermatitis outbreaks have occurred in Australia; Okinawa, Japan; Florida; and the Hawaiian and Marshall islands.1,2
Exposure to Seaweed
Lyngbya majuscula produces more than 70 biologically active compounds that irritate the skin, eyes, and respiratory system.2,8 It grows in marine and estuarine environments attached to seagrass, sand, and bedrock at depths of up to 30 m. Warm waters and maximal sunlight provide optimal growth conditions for L majuscula; therefore, the greatest risk for exposure occurs in the Northern and Southern hemispheres in the 1- to 2-month period following their summer solstices.5 Runoff during heavy rainfall, which is rich in soil extracts such as phosphorous, iron, and organic carbon, stimulates L majuscula growth and contributes to increased algal blooms.4
Dermatitis and Irritants
The dermatoxins Lyngbyatoxin A (LA) and debromoaplysiatoxin (DAT) cause the inflammatory and necrotic appearance of seaweed dermatitis.1,2,5,8 Lyngbyatoxin A is an indole alkaloid that is closely related to telocidin B, a poisonous compound associated with Streptomyces bacteria.9 Sampling of L majuscula and extraction of the dermatoxin, along with human and animal studies, confirmed DAT irritates the skin and induces dermatitis.5,6 Stylocheilus longicauda (sea hare) feeds on L majuscula and contains isolates of DAT in its digestive tract.
Samples of L majuscula taken from several Hawaiian Islands where seaweed dermatitis outbreaks have occurred were examined for differences in toxicities via 6-hour patch tests on human skin.6 The samples obtained from the windward side of Oahu contained DAT and aplysiatoxin, while those obtained from the leeward side and Kahala Beach primarily contained LA. Although DAT and LA are vastly different in their molecular structures, testing elicited the same biologic response and induced the same level of skin irritation.6 Interestingly, not all strands of L majuscula produced LA and DAT and caused seaweed dermatitis; those that did lead to irritation were more red in color than nontoxic blooms.5,9
Cutaneous Manifestations
Seaweed dermatitis resembles chemical and thermal burns, ranging from a mild skin rash to severe contact dermatitis with itchy, swollen, ulcerated lesions.1,7 Patients typically develop a burning or itching sensation beneath their bathing suit or wetsuit that progresses to an erythematous papulovesicular eruption 2 to 24 hours after exposure.2,6 Within a week, vesicles and bullae desquamate, leaving behind tender erosions.1,2,6,8 Inframammary lesions are common in females and scrotal swelling in males.1,6 There is no known association between length of time spent in the water and severity of symptoms.5
Most reactions to L majuscula occur from exposure in the water; however, particles that become aerosolized during strong winds or storms can cause seaweed dermatitis on the face. Inhalation of L majuscula may lead to mucous membrane ulceration and pulmonary edema.1,5,6 Noncutaneous manifestations of seaweed dermatitis include headache, fatigue, and swelling of the eyes, nose, and throat (Figures 1 and 2).1,5
Prevention and Management
To prevent seaweed dermatitis, avoid swimming in ocean water during L majuscula blooms,10 which frequently occur following the summer solstices in the Northern and Southern hemispheres.5 The National Centers for Coastal Ocean Science Harmful Algae Bloom Monitoring System provides real-time access to algae bloom locations.11 Although this monitoring system is not specific to L majuscula, it may be helpful in determining where potential blooms are. Wearing protective clothing such as coveralls may benefit individuals who enter the water during blooms, but it does not guarantee protection.10

magnification ×40). Photograph courtesy of Scott Norton, MD, MPH, MSc (Washington, DC).

Currently, there is no treatment for seaweed dermatitis, but symptom management may reduce discomfort and pain. Washing affected skin with soap and water within an hour of exposure may help reduce the severity of seaweed dermatitis, though studies have shown mixed results.6,7 Application of cool compresses and soothing ointments (eg, calamine) provide symptomatic relief and promote healing.7 The dermatitis typically self-resolves within 1 week.
The filamentous cyanobacterium Lyngbya majuscula causes irritant contact dermatitis in beachgoers, fishers, and divers in tropical and subtropical marine environments worldwide.1 If fragments of L majuscula lodge in swimmers’ bathing suits, the toxins can become trapped against the skin and cause seaweed dermatitis.2 With climate change resulting in warmer oceans and more extreme storms, L majuscula blooms likely will become more frequent and widespread, thereby increasing the risk for human exposure.3,4 Herein, we describe the irritants that lead to dermatitis, clinical presentation, and prevention and management of seaweed dermatitis.
Identifying Features and Distribution of Plant
Lyngbya majuscula belongs to the family Oscillatoriaceae; these cyanobacteria grow as filaments and exhibit slow oscillating movements. Commonly referred to as blanketweed or mermaid’s hair due to its appearance, L majuscula grows fine hairlike clumps resembling a mass of olive-colored matted hair.1 Its thin filaments are 10- to 30-cm long and vary in color from red to white to brown.5 Microscopically, a rouleauxlike arrangement of discs provides the structure of each filament.6
First identified in Hawaii in 1912, L majuscula was not associated with seaweed dermatitis or dermatotoxicity by the medical community until the first outbreak occurred in Oahu in 1958, though fishermen and beachgoers previously had recognized a relationship between this particular seaweed and skin irritation.5,7 The first reporting included 125 confirmed cases, with many more mild unreported cases suspected.6 Now reported in about 100 locations worldwide, seaweed dermatitis outbreaks have occurred in Australia; Okinawa, Japan; Florida; and the Hawaiian and Marshall islands.1,2
Exposure to Seaweed
Lyngbya majuscula produces more than 70 biologically active compounds that irritate the skin, eyes, and respiratory system.2,8 It grows in marine and estuarine environments attached to seagrass, sand, and bedrock at depths of up to 30 m. Warm waters and maximal sunlight provide optimal growth conditions for L majuscula; therefore, the greatest risk for exposure occurs in the Northern and Southern hemispheres in the 1- to 2-month period following their summer solstices.5 Runoff during heavy rainfall, which is rich in soil extracts such as phosphorous, iron, and organic carbon, stimulates L majuscula growth and contributes to increased algal blooms.4
Dermatitis and Irritants
The dermatoxins Lyngbyatoxin A (LA) and debromoaplysiatoxin (DAT) cause the inflammatory and necrotic appearance of seaweed dermatitis.1,2,5,8 Lyngbyatoxin A is an indole alkaloid that is closely related to telocidin B, a poisonous compound associated with Streptomyces bacteria.9 Sampling of L majuscula and extraction of the dermatoxin, along with human and animal studies, confirmed DAT irritates the skin and induces dermatitis.5,6 Stylocheilus longicauda (sea hare) feeds on L majuscula and contains isolates of DAT in its digestive tract.
Samples of L majuscula taken from several Hawaiian Islands where seaweed dermatitis outbreaks have occurred were examined for differences in toxicities via 6-hour patch tests on human skin.6 The samples obtained from the windward side of Oahu contained DAT and aplysiatoxin, while those obtained from the leeward side and Kahala Beach primarily contained LA. Although DAT and LA are vastly different in their molecular structures, testing elicited the same biologic response and induced the same level of skin irritation.6 Interestingly, not all strands of L majuscula produced LA and DAT and caused seaweed dermatitis; those that did lead to irritation were more red in color than nontoxic blooms.5,9
Cutaneous Manifestations
Seaweed dermatitis resembles chemical and thermal burns, ranging from a mild skin rash to severe contact dermatitis with itchy, swollen, ulcerated lesions.1,7 Patients typically develop a burning or itching sensation beneath their bathing suit or wetsuit that progresses to an erythematous papulovesicular eruption 2 to 24 hours after exposure.2,6 Within a week, vesicles and bullae desquamate, leaving behind tender erosions.1,2,6,8 Inframammary lesions are common in females and scrotal swelling in males.1,6 There is no known association between length of time spent in the water and severity of symptoms.5
Most reactions to L majuscula occur from exposure in the water; however, particles that become aerosolized during strong winds or storms can cause seaweed dermatitis on the face. Inhalation of L majuscula may lead to mucous membrane ulceration and pulmonary edema.1,5,6 Noncutaneous manifestations of seaweed dermatitis include headache, fatigue, and swelling of the eyes, nose, and throat (Figures 1 and 2).1,5
Prevention and Management
To prevent seaweed dermatitis, avoid swimming in ocean water during L majuscula blooms,10 which frequently occur following the summer solstices in the Northern and Southern hemispheres.5 The National Centers for Coastal Ocean Science Harmful Algae Bloom Monitoring System provides real-time access to algae bloom locations.11 Although this monitoring system is not specific to L majuscula, it may be helpful in determining where potential blooms are. Wearing protective clothing such as coveralls may benefit individuals who enter the water during blooms, but it does not guarantee protection.10

magnification ×40). Photograph courtesy of Scott Norton, MD, MPH, MSc (Washington, DC).

Currently, there is no treatment for seaweed dermatitis, but symptom management may reduce discomfort and pain. Washing affected skin with soap and water within an hour of exposure may help reduce the severity of seaweed dermatitis, though studies have shown mixed results.6,7 Application of cool compresses and soothing ointments (eg, calamine) provide symptomatic relief and promote healing.7 The dermatitis typically self-resolves within 1 week.
- Werner K, Marquart L, Norton S. Lyngbya dermatitis (toxic seaweed dermatitis). Int J Dermatol. 2011;51:59-62. doi:10.1111/j.1365-4632.2011.05042.x
- Osborne N, Shaw G. Dermatitis associated with exposure to a marine cyanobacterium during recreational water exposure. BMC Dermatol. 2008;8:5. doi:10.1186/1471-5945-8-5
- Hays G, Richardson A, Robinson C. Climate change and marine plankton. Trends Ecol Evol. 2005;20:337-344. doi:10.1016/j.tree.2005.03.004
- Albert S, O’Neil J, Udy J, et al. Blooms of the cyanobacterium Lyngbya majuscula in costal Queensland, Australia: disparate sites, common factors. Mar Pollut Bull. 2004;51:428-437. doi:10.1016/j.marpolbul.2004.10.016
- Osborne N, Webb P, Shaw G. The toxins of Lyngbya majuscula and their human and ecological health effects. Environ Int. 2001;27:381-392. doi:10.1016/s0160-4120(01)00098-8
- Izumi A, Moore R. Seaweed ( Lyngbya majuscula ) dermatitis . Clin Dermatol . 1987;5:92-100. doi:10.1016/s0738-081x(87)80014-7
- Grauer F, Arnold H. Seaweed dermatitis: first report of a dermatitis-producing marine alga. Arch Dermatol. 1961; 84:720-732. doi:10.1001/archderm.1961.01580170014003
- Taylor M, Stahl-Timmins W, Redshaw C, et al. Toxic alkaloids in Lyngbya majuscula and related tropical marine cyanobacteria. Harmful Algae . 2014;31:1-8. doi:10.1016/j.hal.2013.09.003
- Cardellina J, Marner F, Moore R. Seaweed dermatitis: structure of lyngbyatoxin A. Science. 1979;204:193-195. doi:10.1126/science.107586
- Osborne N. Occupational dermatitis caused by Lyngbya majuscule in Australia. Int J Dermatol . 2012;5:122-123. doi:10.1111/j.1365-4632.2009.04455.x
- Harmful Algal Bloom Monitoring System. National Centers for Coastal Ocean Science. Accessed May 23, 2024. https://coastalscience.noaa.gov/research/stressor-impacts-mitigation/hab-monitoring-system/
- Werner K, Marquart L, Norton S. Lyngbya dermatitis (toxic seaweed dermatitis). Int J Dermatol. 2011;51:59-62. doi:10.1111/j.1365-4632.2011.05042.x
- Osborne N, Shaw G. Dermatitis associated with exposure to a marine cyanobacterium during recreational water exposure. BMC Dermatol. 2008;8:5. doi:10.1186/1471-5945-8-5
- Hays G, Richardson A, Robinson C. Climate change and marine plankton. Trends Ecol Evol. 2005;20:337-344. doi:10.1016/j.tree.2005.03.004
- Albert S, O’Neil J, Udy J, et al. Blooms of the cyanobacterium Lyngbya majuscula in costal Queensland, Australia: disparate sites, common factors. Mar Pollut Bull. 2004;51:428-437. doi:10.1016/j.marpolbul.2004.10.016
- Osborne N, Webb P, Shaw G. The toxins of Lyngbya majuscula and their human and ecological health effects. Environ Int. 2001;27:381-392. doi:10.1016/s0160-4120(01)00098-8
- Izumi A, Moore R. Seaweed ( Lyngbya majuscula ) dermatitis . Clin Dermatol . 1987;5:92-100. doi:10.1016/s0738-081x(87)80014-7
- Grauer F, Arnold H. Seaweed dermatitis: first report of a dermatitis-producing marine alga. Arch Dermatol. 1961; 84:720-732. doi:10.1001/archderm.1961.01580170014003
- Taylor M, Stahl-Timmins W, Redshaw C, et al. Toxic alkaloids in Lyngbya majuscula and related tropical marine cyanobacteria. Harmful Algae . 2014;31:1-8. doi:10.1016/j.hal.2013.09.003
- Cardellina J, Marner F, Moore R. Seaweed dermatitis: structure of lyngbyatoxin A. Science. 1979;204:193-195. doi:10.1126/science.107586
- Osborne N. Occupational dermatitis caused by Lyngbya majuscule in Australia. Int J Dermatol . 2012;5:122-123. doi:10.1111/j.1365-4632.2009.04455.x
- Harmful Algal Bloom Monitoring System. National Centers for Coastal Ocean Science. Accessed May 23, 2024. https://coastalscience.noaa.gov/research/stressor-impacts-mitigation/hab-monitoring-system/
Aquatic Antagonists: Seaweed Dermatitis (Lyngbya majuscula)
Aquatic Antagonists: Seaweed Dermatitis (Lyngbya majuscula)
PRACTICE POINTS
- Lyngbya majuscula causes seaweed dermatitis in swimmers and can be prevented by avoiding rough turbid waters in areas known to have L majuscula blooms.
- Seaweed dermatitis should be included in the differential diagnosis for erythematous papulovesicular rashes manifesting in patients who recently have spent time in the ocean.
Prodromal Parkinson’s Disease: Diagnostic Dilemma
As the availability of potential biomarkers for Parkinson’s disease drives the debate around diagnosing prodromal Parkinson’s disease (pPD) from theory to practice, said authors of a recent study, clinicians should weigh each patient’s preferences, circumstances, and goals against the potential benefits and harms of disclosure. The study and an accompanying editorial appeared online in Neurology.
Because markers such as SNCA, LRRK2, and GBA mutations impact small subgroups of patients at risk of developing monogenic forms of Parkinson’s disease, wrote Richard N. Rees, MBChB, MD, from the Department of Clinical and Movement Neurosciences at University College London Queen Square Institute of Neurology, and colleagues, researchers are working to identify people at risk of idiopathic Parkinson’s disease using models based on known risk and protective factors. The recent development of highly accurate cerebrospinal fluid (and potentially serum) alpha-synuclein seed amplification assays, which may show Parkinson’s disease’s signature before overt symptoms appear, will reinforce these efforts, authors added.
‘Tap the Brakes’
However, sources interviewed by Neurology Reviews counseled caution with potential prodromal Parkinson’s disease biomarkers. “As the science advances in Parkinson’s disease and related disorders,” said Michael S. Okun, MD, “our ability to predict who will and will not be diagnosed will improve. We should, however, tap the brakes and consider the consequences of making a diagnosis in someone at risk — especially someone without symptoms.” Dr. Okun is National Medical Advisor to the Parkinson’s Foundation and director of the Norman Fixel Institute for Neurological Diseases at University of Florida Health in Gainesville, Florida. He was not involved with the study.
Neurologists should ask themselves why they are testing for Parkinson’s disease biomarkers, said Dr. Okun, and what counseling and shared decision-making they provided beforehand. “This already complex scenario becomes even more complicated when we consider that many people with GBA gene mutations and some with LRRK2 mutations may never actually manifest Parkinson’s disease.”
Neurologists’ knowledge of Parkinson’s disease biomarkers remains in the research phase, said editorial co-author Colin Hoy, PhD, a postdoctoral researcher at the University of California, San Francisco, Weill Institute for Neurosciences in San Francisco, California. No one fully understands the relationships between potential biomarkers, what pathological risks they may carry, and how those risks eventually foment symptoms, he said.
The lack of disease-modifying therapies (DMTs) for Parkinson’s disease plays a critical role in whether patients want to know if they are at risk, added Dr. Hoy. In a survey of 101 patients with established Parkinson’s disease published in Neurology in 2020, 54% would have eschewed knowing about their risk in the absence of DMT.
Nevertheless, wrote Dr. Rees and colleagues, the earlier that patients with prodromal Parkinson’s disease know about it, the longer they might forestall Parkinson’s disease through nonpharmaceutical approaches. In a study published in Neurology in 2011, aerobic exercise reduced Parkinson’s disease risk. Similarly, techniques such as tai chi can significantly improve motor function, depression, and quality of life in Parkinson’s disease, according to a meta-analysis published in Parkinsonism & Related Disorders in 2017.
Having foreknowledge of Parkinson’s disease risk can empower people to manage comorbid conditions, seek evidence-based treatments, and enroll in clinical trials while their condition perhaps remains amenable to treatment, added Dr. Rees and colleagues. Patients also can proactively build support networks and address legal eventualities such as advance care directives, authors added.
A Holistic Approach to Shared Decision-Making
To avoid needlessly scaring patients, Dr. Hoy suggested broaching the topic of Parkinson’s disease biomarkers during advance care planning. “In the same conversation that you might talk about establishing surrogate decision-makers or potential do-not-resuscitate/intubate orders, you can talk about the potential of predictive testing, which is becoming more prevalent across domains of clinical practice.”
Understanding each patient’s values, preferences, and priorities requires a holistic approach, he said. “In the context of prodromal Parkinson’s disease, the benefits of enrolling in a new clinical trial or implementing lifestyle changes might vary depending on the person. Do you think this person would be likely to enroll in a clinical trial or implement those lifestyle changes?” Additionally, he recommended considering how a patient might react to a false diagnosis.
Whereas a diagnosis of mild cognitive impairment might not lead to Alzheimer’s disease or dementia, wrote Dr. Rees and colleagues, growing evidence including a review published in Neurology in 2022 supports the accuracy of alpha-synuclein seed amplification assays in detecting both established and prodromal Parkinson’s disease. For people thusly diagnosed, Dr. Rees and colleagues wrote, the psychosocial burden of inevitable progression could create feelings of helplessness, possibly undermining benefits of early knowledge.
Beyond patients’ reactions, said Dr. Hoy, a diagnosis of prodromal Parkinson’s disease could result in social stigma, changes to interpersonal relationships, or discrimination. “Understanding the implications and uncertainties of potential disclosure, relative to what a person would want to know or might be able to do about it, will be the key for deciding when is the right time,” he said.
Supporting Primary Care
As the shared decision-making burden likely will fall to primary care providers, Dr. Hoy added, neurologists should prioritize increasing these providers’ capacity to advise and refer patients appropriately. Although it is too soon to develop clinical guidelines, he said, neurologists could help educate such providers about pPD and the growing availability of promising biomarkers.
“Parkinson’s is thought of as a movement disorder first and foremost,” said Dr. Hoy. However, various non-motor symptoms including sleep problems, depression, anxiety, apathy, constipation, and gastrointestinal issues often appear before movement-related symptoms during the prodromal phase.
As potentially the first line of defense against prodromal Parkinson’s disease, primary care providers also should know the distinction between early and timely diagnosis, added Dr. Hoy. Introduced by Dr. Rees and colleagues in a 2018 review published in F1000Research, timely diagnosis balances patient preferences, the availability and efficacy of DMT, and health systems’ ability to support and manage individuals at every stage of disease.
The current study was funded by a Parkinson’s UK grant (which paid Dr. Rees’s salary). The editorial was supported by a National Institute of Mental Health Brain Research Through Advancing Innovative Neurotechnologies (BRAIN) Initiative award, a grant from the National Institute on Aging, and a Wellcome Discovery Award. Dr. Hoy reported no relevant disclosures.
As the availability of potential biomarkers for Parkinson’s disease drives the debate around diagnosing prodromal Parkinson’s disease (pPD) from theory to practice, said authors of a recent study, clinicians should weigh each patient’s preferences, circumstances, and goals against the potential benefits and harms of disclosure. The study and an accompanying editorial appeared online in Neurology.
Because markers such as SNCA, LRRK2, and GBA mutations impact small subgroups of patients at risk of developing monogenic forms of Parkinson’s disease, wrote Richard N. Rees, MBChB, MD, from the Department of Clinical and Movement Neurosciences at University College London Queen Square Institute of Neurology, and colleagues, researchers are working to identify people at risk of idiopathic Parkinson’s disease using models based on known risk and protective factors. The recent development of highly accurate cerebrospinal fluid (and potentially serum) alpha-synuclein seed amplification assays, which may show Parkinson’s disease’s signature before overt symptoms appear, will reinforce these efforts, authors added.
‘Tap the Brakes’
However, sources interviewed by Neurology Reviews counseled caution with potential prodromal Parkinson’s disease biomarkers. “As the science advances in Parkinson’s disease and related disorders,” said Michael S. Okun, MD, “our ability to predict who will and will not be diagnosed will improve. We should, however, tap the brakes and consider the consequences of making a diagnosis in someone at risk — especially someone without symptoms.” Dr. Okun is National Medical Advisor to the Parkinson’s Foundation and director of the Norman Fixel Institute for Neurological Diseases at University of Florida Health in Gainesville, Florida. He was not involved with the study.
Neurologists should ask themselves why they are testing for Parkinson’s disease biomarkers, said Dr. Okun, and what counseling and shared decision-making they provided beforehand. “This already complex scenario becomes even more complicated when we consider that many people with GBA gene mutations and some with LRRK2 mutations may never actually manifest Parkinson’s disease.”
Neurologists’ knowledge of Parkinson’s disease biomarkers remains in the research phase, said editorial co-author Colin Hoy, PhD, a postdoctoral researcher at the University of California, San Francisco, Weill Institute for Neurosciences in San Francisco, California. No one fully understands the relationships between potential biomarkers, what pathological risks they may carry, and how those risks eventually foment symptoms, he said.
The lack of disease-modifying therapies (DMTs) for Parkinson’s disease plays a critical role in whether patients want to know if they are at risk, added Dr. Hoy. In a survey of 101 patients with established Parkinson’s disease published in Neurology in 2020, 54% would have eschewed knowing about their risk in the absence of DMT.
Nevertheless, wrote Dr. Rees and colleagues, the earlier that patients with prodromal Parkinson’s disease know about it, the longer they might forestall Parkinson’s disease through nonpharmaceutical approaches. In a study published in Neurology in 2011, aerobic exercise reduced Parkinson’s disease risk. Similarly, techniques such as tai chi can significantly improve motor function, depression, and quality of life in Parkinson’s disease, according to a meta-analysis published in Parkinsonism & Related Disorders in 2017.
Having foreknowledge of Parkinson’s disease risk can empower people to manage comorbid conditions, seek evidence-based treatments, and enroll in clinical trials while their condition perhaps remains amenable to treatment, added Dr. Rees and colleagues. Patients also can proactively build support networks and address legal eventualities such as advance care directives, authors added.
A Holistic Approach to Shared Decision-Making
To avoid needlessly scaring patients, Dr. Hoy suggested broaching the topic of Parkinson’s disease biomarkers during advance care planning. “In the same conversation that you might talk about establishing surrogate decision-makers or potential do-not-resuscitate/intubate orders, you can talk about the potential of predictive testing, which is becoming more prevalent across domains of clinical practice.”
Understanding each patient’s values, preferences, and priorities requires a holistic approach, he said. “In the context of prodromal Parkinson’s disease, the benefits of enrolling in a new clinical trial or implementing lifestyle changes might vary depending on the person. Do you think this person would be likely to enroll in a clinical trial or implement those lifestyle changes?” Additionally, he recommended considering how a patient might react to a false diagnosis.
Whereas a diagnosis of mild cognitive impairment might not lead to Alzheimer’s disease or dementia, wrote Dr. Rees and colleagues, growing evidence including a review published in Neurology in 2022 supports the accuracy of alpha-synuclein seed amplification assays in detecting both established and prodromal Parkinson’s disease. For people thusly diagnosed, Dr. Rees and colleagues wrote, the psychosocial burden of inevitable progression could create feelings of helplessness, possibly undermining benefits of early knowledge.
Beyond patients’ reactions, said Dr. Hoy, a diagnosis of prodromal Parkinson’s disease could result in social stigma, changes to interpersonal relationships, or discrimination. “Understanding the implications and uncertainties of potential disclosure, relative to what a person would want to know or might be able to do about it, will be the key for deciding when is the right time,” he said.
Supporting Primary Care
As the shared decision-making burden likely will fall to primary care providers, Dr. Hoy added, neurologists should prioritize increasing these providers’ capacity to advise and refer patients appropriately. Although it is too soon to develop clinical guidelines, he said, neurologists could help educate such providers about pPD and the growing availability of promising biomarkers.
“Parkinson’s is thought of as a movement disorder first and foremost,” said Dr. Hoy. However, various non-motor symptoms including sleep problems, depression, anxiety, apathy, constipation, and gastrointestinal issues often appear before movement-related symptoms during the prodromal phase.
As potentially the first line of defense against prodromal Parkinson’s disease, primary care providers also should know the distinction between early and timely diagnosis, added Dr. Hoy. Introduced by Dr. Rees and colleagues in a 2018 review published in F1000Research, timely diagnosis balances patient preferences, the availability and efficacy of DMT, and health systems’ ability to support and manage individuals at every stage of disease.
The current study was funded by a Parkinson’s UK grant (which paid Dr. Rees’s salary). The editorial was supported by a National Institute of Mental Health Brain Research Through Advancing Innovative Neurotechnologies (BRAIN) Initiative award, a grant from the National Institute on Aging, and a Wellcome Discovery Award. Dr. Hoy reported no relevant disclosures.
As the availability of potential biomarkers for Parkinson’s disease drives the debate around diagnosing prodromal Parkinson’s disease (pPD) from theory to practice, said authors of a recent study, clinicians should weigh each patient’s preferences, circumstances, and goals against the potential benefits and harms of disclosure. The study and an accompanying editorial appeared online in Neurology.
Because markers such as SNCA, LRRK2, and GBA mutations impact small subgroups of patients at risk of developing monogenic forms of Parkinson’s disease, wrote Richard N. Rees, MBChB, MD, from the Department of Clinical and Movement Neurosciences at University College London Queen Square Institute of Neurology, and colleagues, researchers are working to identify people at risk of idiopathic Parkinson’s disease using models based on known risk and protective factors. The recent development of highly accurate cerebrospinal fluid (and potentially serum) alpha-synuclein seed amplification assays, which may show Parkinson’s disease’s signature before overt symptoms appear, will reinforce these efforts, authors added.
‘Tap the Brakes’
However, sources interviewed by Neurology Reviews counseled caution with potential prodromal Parkinson’s disease biomarkers. “As the science advances in Parkinson’s disease and related disorders,” said Michael S. Okun, MD, “our ability to predict who will and will not be diagnosed will improve. We should, however, tap the brakes and consider the consequences of making a diagnosis in someone at risk — especially someone without symptoms.” Dr. Okun is National Medical Advisor to the Parkinson’s Foundation and director of the Norman Fixel Institute for Neurological Diseases at University of Florida Health in Gainesville, Florida. He was not involved with the study.
Neurologists should ask themselves why they are testing for Parkinson’s disease biomarkers, said Dr. Okun, and what counseling and shared decision-making they provided beforehand. “This already complex scenario becomes even more complicated when we consider that many people with GBA gene mutations and some with LRRK2 mutations may never actually manifest Parkinson’s disease.”
Neurologists’ knowledge of Parkinson’s disease biomarkers remains in the research phase, said editorial co-author Colin Hoy, PhD, a postdoctoral researcher at the University of California, San Francisco, Weill Institute for Neurosciences in San Francisco, California. No one fully understands the relationships between potential biomarkers, what pathological risks they may carry, and how those risks eventually foment symptoms, he said.
The lack of disease-modifying therapies (DMTs) for Parkinson’s disease plays a critical role in whether patients want to know if they are at risk, added Dr. Hoy. In a survey of 101 patients with established Parkinson’s disease published in Neurology in 2020, 54% would have eschewed knowing about their risk in the absence of DMT.
Nevertheless, wrote Dr. Rees and colleagues, the earlier that patients with prodromal Parkinson’s disease know about it, the longer they might forestall Parkinson’s disease through nonpharmaceutical approaches. In a study published in Neurology in 2011, aerobic exercise reduced Parkinson’s disease risk. Similarly, techniques such as tai chi can significantly improve motor function, depression, and quality of life in Parkinson’s disease, according to a meta-analysis published in Parkinsonism & Related Disorders in 2017.
Having foreknowledge of Parkinson’s disease risk can empower people to manage comorbid conditions, seek evidence-based treatments, and enroll in clinical trials while their condition perhaps remains amenable to treatment, added Dr. Rees and colleagues. Patients also can proactively build support networks and address legal eventualities such as advance care directives, authors added.
A Holistic Approach to Shared Decision-Making
To avoid needlessly scaring patients, Dr. Hoy suggested broaching the topic of Parkinson’s disease biomarkers during advance care planning. “In the same conversation that you might talk about establishing surrogate decision-makers or potential do-not-resuscitate/intubate orders, you can talk about the potential of predictive testing, which is becoming more prevalent across domains of clinical practice.”
Understanding each patient’s values, preferences, and priorities requires a holistic approach, he said. “In the context of prodromal Parkinson’s disease, the benefits of enrolling in a new clinical trial or implementing lifestyle changes might vary depending on the person. Do you think this person would be likely to enroll in a clinical trial or implement those lifestyle changes?” Additionally, he recommended considering how a patient might react to a false diagnosis.
Whereas a diagnosis of mild cognitive impairment might not lead to Alzheimer’s disease or dementia, wrote Dr. Rees and colleagues, growing evidence including a review published in Neurology in 2022 supports the accuracy of alpha-synuclein seed amplification assays in detecting both established and prodromal Parkinson’s disease. For people thusly diagnosed, Dr. Rees and colleagues wrote, the psychosocial burden of inevitable progression could create feelings of helplessness, possibly undermining benefits of early knowledge.
Beyond patients’ reactions, said Dr. Hoy, a diagnosis of prodromal Parkinson’s disease could result in social stigma, changes to interpersonal relationships, or discrimination. “Understanding the implications and uncertainties of potential disclosure, relative to what a person would want to know or might be able to do about it, will be the key for deciding when is the right time,” he said.
Supporting Primary Care
As the shared decision-making burden likely will fall to primary care providers, Dr. Hoy added, neurologists should prioritize increasing these providers’ capacity to advise and refer patients appropriately. Although it is too soon to develop clinical guidelines, he said, neurologists could help educate such providers about pPD and the growing availability of promising biomarkers.
“Parkinson’s is thought of as a movement disorder first and foremost,” said Dr. Hoy. However, various non-motor symptoms including sleep problems, depression, anxiety, apathy, constipation, and gastrointestinal issues often appear before movement-related symptoms during the prodromal phase.
As potentially the first line of defense against prodromal Parkinson’s disease, primary care providers also should know the distinction between early and timely diagnosis, added Dr. Hoy. Introduced by Dr. Rees and colleagues in a 2018 review published in F1000Research, timely diagnosis balances patient preferences, the availability and efficacy of DMT, and health systems’ ability to support and manage individuals at every stage of disease.
The current study was funded by a Parkinson’s UK grant (which paid Dr. Rees’s salary). The editorial was supported by a National Institute of Mental Health Brain Research Through Advancing Innovative Neurotechnologies (BRAIN) Initiative award, a grant from the National Institute on Aging, and a Wellcome Discovery Award. Dr. Hoy reported no relevant disclosures.
FROM NEUROLOGY
Spondyloarthritis Screening Study Finds ‘High Burden of Need’ in Patients With Inflammatory Bowel Disease
More than 40% of patients with inflammatory bowel disease (IBD) screened positive for joint pain symptomatic of spondyloarthritis (SpA), according to a new study.
Of these patients, 75% did not have any history of arthritis.
“What we know is that a substantial proportion of patients with IBD do report musculoskeletal symptoms, and inflammatory back pain stands out as being one of the more frequent symptoms reported,” said Reem Jan, MBBS, a rheumatologist at the University of Chicago Medicine. She presented the study findings during the annual meeting of the Spondyloarthritis Research and Treatment Network (SPARTAN) in Cleveland.
“Yet a minority of these patients are evaluated by rheumatologists. So that suggests there’s a high burden of need in the IBD population to have this joint pain evaluated and addressed,” she said during her presentation.
She presented preliminary data from an ongoing project to better understand the prevalence of inflammatory arthritis in IBD — estimates range from 17% to 39%— and the risk factors for developing arthritis in this patient population.
Study Details
Researchers enrolled patients from outpatient gastroenterology clinics or procedure units at NYU Langone Health, New York City; Brigham and Women’s Hospital, Boston; University of Colorado Anschutz Medical Campus, Aurora, Colorado; Mayo Clinic, Rochester, Minnesota; University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago; and Icahn School of Medicine at Mount Sinai, New York City. Additional patients were recruited from Mercy Health, a community health system in Ohio.
Upon entry into the study, participants completed a survey documenting their history with joint pain. The survey combined questions from the DETAIL and the IBIS questionnaires.
Between January 2021 and December 2022, 669 patients joined the study. In total, 41% of patients (n = 275) screened positive.
“What really stood out to us was that of all the positive screens, only about a quarter of those patients were known to have SpA,” Dr. Jan said during her presentation. “[This] means 75% of the patients who screened positive were not known to have any type of arthritic disease.”
In addition, only 24% (n = 65) of all patients who screened positive — including those with a SpA diagnosis — had seen a rheumatologist in the previous year.
Among these patients, inflammatory back pain was the most commonly reported symptom, followed by painful swelling of peripheral joints and heel pain.
Excluding patients with a SpA diagnosis, researchers also investigated which characteristics were associated with a higher likelihood of screening positive in the questionnaire. The analysis, including 588 patients, identified the following risk factors:
- Female sex: Odds ratio (OR), 2.0; 95% CI, 1.4-2.9
- Older age: OR, 1.02; 95% CI, 1.01-1.4
- History of smoking: OR, 1.7; 95% CI, 1.1-2.6
- History of prior IBD-related surgery: OR, 1.60; 95% CI, 1.1-2.5
- History of biologic or small molecule therapy: OR, 2.3; 95% CI, 1.4-4.0
Future Directions
Commenting on the study, Mark Hwang, MD, a rheumatologist at UTHealth Houston, noted that it was “very interesting to see the fairly large, positive rates” of joint pain in patients with IBD, which certainly have clinical implications. However, it is not yet known if any of these patients went on to be diagnosed with SpA.
Jan noted that potential next steps include a follow-up analysis of patients who screened positive to see how many went on to see a rheumatologist and which patients were ultimately diagnosed with SpA or other inflammatory arthritis conditions.
These findings are a first step, Dr. Hwang said, and will likely “help further establish some of the validity of these questionnaires by testing in different patient populations,” he noted.
The ultimate goal is to “develop really good strategies to risk stratify IBD patients with the greatest need of rheumatologist consultation,” Dr. Jan said. “We certainly don’t want to see all these patients, so how can we figure out who really needs to be seen?”
Funding information was not available for this study. Dr. Hwang is conducting two clinical trials for psoriatic arthritis sponsored by Janssen and Eli Lilly. Dr. Jan reported no relevant disclosures.
A version of this article appeared on Medscape.com.
More than 40% of patients with inflammatory bowel disease (IBD) screened positive for joint pain symptomatic of spondyloarthritis (SpA), according to a new study.
Of these patients, 75% did not have any history of arthritis.
“What we know is that a substantial proportion of patients with IBD do report musculoskeletal symptoms, and inflammatory back pain stands out as being one of the more frequent symptoms reported,” said Reem Jan, MBBS, a rheumatologist at the University of Chicago Medicine. She presented the study findings during the annual meeting of the Spondyloarthritis Research and Treatment Network (SPARTAN) in Cleveland.
“Yet a minority of these patients are evaluated by rheumatologists. So that suggests there’s a high burden of need in the IBD population to have this joint pain evaluated and addressed,” she said during her presentation.
She presented preliminary data from an ongoing project to better understand the prevalence of inflammatory arthritis in IBD — estimates range from 17% to 39%— and the risk factors for developing arthritis in this patient population.
Study Details
Researchers enrolled patients from outpatient gastroenterology clinics or procedure units at NYU Langone Health, New York City; Brigham and Women’s Hospital, Boston; University of Colorado Anschutz Medical Campus, Aurora, Colorado; Mayo Clinic, Rochester, Minnesota; University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago; and Icahn School of Medicine at Mount Sinai, New York City. Additional patients were recruited from Mercy Health, a community health system in Ohio.
Upon entry into the study, participants completed a survey documenting their history with joint pain. The survey combined questions from the DETAIL and the IBIS questionnaires.
Between January 2021 and December 2022, 669 patients joined the study. In total, 41% of patients (n = 275) screened positive.
“What really stood out to us was that of all the positive screens, only about a quarter of those patients were known to have SpA,” Dr. Jan said during her presentation. “[This] means 75% of the patients who screened positive were not known to have any type of arthritic disease.”
In addition, only 24% (n = 65) of all patients who screened positive — including those with a SpA diagnosis — had seen a rheumatologist in the previous year.
Among these patients, inflammatory back pain was the most commonly reported symptom, followed by painful swelling of peripheral joints and heel pain.
Excluding patients with a SpA diagnosis, researchers also investigated which characteristics were associated with a higher likelihood of screening positive in the questionnaire. The analysis, including 588 patients, identified the following risk factors:
- Female sex: Odds ratio (OR), 2.0; 95% CI, 1.4-2.9
- Older age: OR, 1.02; 95% CI, 1.01-1.4
- History of smoking: OR, 1.7; 95% CI, 1.1-2.6
- History of prior IBD-related surgery: OR, 1.60; 95% CI, 1.1-2.5
- History of biologic or small molecule therapy: OR, 2.3; 95% CI, 1.4-4.0
Future Directions
Commenting on the study, Mark Hwang, MD, a rheumatologist at UTHealth Houston, noted that it was “very interesting to see the fairly large, positive rates” of joint pain in patients with IBD, which certainly have clinical implications. However, it is not yet known if any of these patients went on to be diagnosed with SpA.
Jan noted that potential next steps include a follow-up analysis of patients who screened positive to see how many went on to see a rheumatologist and which patients were ultimately diagnosed with SpA or other inflammatory arthritis conditions.
These findings are a first step, Dr. Hwang said, and will likely “help further establish some of the validity of these questionnaires by testing in different patient populations,” he noted.
The ultimate goal is to “develop really good strategies to risk stratify IBD patients with the greatest need of rheumatologist consultation,” Dr. Jan said. “We certainly don’t want to see all these patients, so how can we figure out who really needs to be seen?”
Funding information was not available for this study. Dr. Hwang is conducting two clinical trials for psoriatic arthritis sponsored by Janssen and Eli Lilly. Dr. Jan reported no relevant disclosures.
A version of this article appeared on Medscape.com.
More than 40% of patients with inflammatory bowel disease (IBD) screened positive for joint pain symptomatic of spondyloarthritis (SpA), according to a new study.
Of these patients, 75% did not have any history of arthritis.
“What we know is that a substantial proportion of patients with IBD do report musculoskeletal symptoms, and inflammatory back pain stands out as being one of the more frequent symptoms reported,” said Reem Jan, MBBS, a rheumatologist at the University of Chicago Medicine. She presented the study findings during the annual meeting of the Spondyloarthritis Research and Treatment Network (SPARTAN) in Cleveland.
“Yet a minority of these patients are evaluated by rheumatologists. So that suggests there’s a high burden of need in the IBD population to have this joint pain evaluated and addressed,” she said during her presentation.
She presented preliminary data from an ongoing project to better understand the prevalence of inflammatory arthritis in IBD — estimates range from 17% to 39%— and the risk factors for developing arthritis in this patient population.
Study Details
Researchers enrolled patients from outpatient gastroenterology clinics or procedure units at NYU Langone Health, New York City; Brigham and Women’s Hospital, Boston; University of Colorado Anschutz Medical Campus, Aurora, Colorado; Mayo Clinic, Rochester, Minnesota; University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago; and Icahn School of Medicine at Mount Sinai, New York City. Additional patients were recruited from Mercy Health, a community health system in Ohio.
Upon entry into the study, participants completed a survey documenting their history with joint pain. The survey combined questions from the DETAIL and the IBIS questionnaires.
Between January 2021 and December 2022, 669 patients joined the study. In total, 41% of patients (n = 275) screened positive.
“What really stood out to us was that of all the positive screens, only about a quarter of those patients were known to have SpA,” Dr. Jan said during her presentation. “[This] means 75% of the patients who screened positive were not known to have any type of arthritic disease.”
In addition, only 24% (n = 65) of all patients who screened positive — including those with a SpA diagnosis — had seen a rheumatologist in the previous year.
Among these patients, inflammatory back pain was the most commonly reported symptom, followed by painful swelling of peripheral joints and heel pain.
Excluding patients with a SpA diagnosis, researchers also investigated which characteristics were associated with a higher likelihood of screening positive in the questionnaire. The analysis, including 588 patients, identified the following risk factors:
- Female sex: Odds ratio (OR), 2.0; 95% CI, 1.4-2.9
- Older age: OR, 1.02; 95% CI, 1.01-1.4
- History of smoking: OR, 1.7; 95% CI, 1.1-2.6
- History of prior IBD-related surgery: OR, 1.60; 95% CI, 1.1-2.5
- History of biologic or small molecule therapy: OR, 2.3; 95% CI, 1.4-4.0
Future Directions
Commenting on the study, Mark Hwang, MD, a rheumatologist at UTHealth Houston, noted that it was “very interesting to see the fairly large, positive rates” of joint pain in patients with IBD, which certainly have clinical implications. However, it is not yet known if any of these patients went on to be diagnosed with SpA.
Jan noted that potential next steps include a follow-up analysis of patients who screened positive to see how many went on to see a rheumatologist and which patients were ultimately diagnosed with SpA or other inflammatory arthritis conditions.
These findings are a first step, Dr. Hwang said, and will likely “help further establish some of the validity of these questionnaires by testing in different patient populations,” he noted.
The ultimate goal is to “develop really good strategies to risk stratify IBD patients with the greatest need of rheumatologist consultation,” Dr. Jan said. “We certainly don’t want to see all these patients, so how can we figure out who really needs to be seen?”
Funding information was not available for this study. Dr. Hwang is conducting two clinical trials for psoriatic arthritis sponsored by Janssen and Eli Lilly. Dr. Jan reported no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM SPARTAN 2024
‘Don’t Screen’ for Vitamin D: New Endo Society Guideline
BOSTON —
The evidence-based document was presented on June 3, 2024, at the Endocrine Society annual meeting, and simultaneously published in The Journal of Clinical Endocrinology and Metabolism. It advises that people who may benefit from vitamin D supplementation include:
- Children aged 1-18 years to prevent rickets and to potentially lower the risk for respiratory tract infections
- Pregnant people to lower the risk for maternal and fetal or neonatal complications
- Adults older than 75 years to lower the risk for mortality
- Adults with prediabetes to lower the risk for type 2 diabetes
In those groups, the recommendation is for daily (rather than intermittent) empiric vitamin D supplementation of more than what was recommended in 2011 by the National Academy of Medicine (NAM), which was then called the Institute of Medicine (IOM): 600 IU/d for those aged 1-70 years and 800 IU/d for those older than 70 years. The document acknowledges that the optimal dose for these populations isn’t known, but it provides the dose ranges that were used in the trials cited as evidence for the recommendations.
In contrast, the document advises against more vitamin D than the recommended daily intake for most healthier adults younger than 75 years and recommends against testing for blood vitamin D levels in the general population, including those with obesity or darker complexions.
Guideline author Anastassios G. Pittas, MD, professor of medicine at Tufts University School of Medicine, Boston, told this news organization, “this guideline refers to people who are otherwise healthy, and there’s no clear indication for vitamin D, such as people with already established osteoporosis. This guideline is not relevant to them.”
Dr. Pittas also noted, “there’s no single question and single answer about the role of vitamin D in health and disease, which is what people often want to know. There are many questions, and we cannot answer all of them.”
Panel Chair Marie B. Demay, MD, professor of medicine at Harvard Medical School, Boston, told this news organization that indeed the panel was limited by lack of randomized clinical trial evidence to answer many important questions. “There is a paucity of data regarding definition of optimal levels and optimal intake of vitamin D for preventing specific diseases ... What we really need are large scale clinical trials and biomarkers so we can predict disease outcome before it happens.”
Overall, Dr. Demay said, “The recommendations are that populations adhere to the [NAM/IOM] dietary recommended intakes, and there are certain populations that will likely benefit from levels of intake above [those].”
Asked to comment, session moderator Clifford J. Rosen, MD, director of Clinical and Translational Research and senior scientist at Maine Medical Center Research Institute, Scarborough, Maine, noted that screening for vitamin D is quite common in clinical practice, but the recommendation against doing so makes sense.
“When clinicians measure vitamin D, then they’re forced to make a decision what to do about it. That’s where questions about the levels come in. And that’s a big problem. So what the panel’s saying is, don’t screen ... This really gets to the heart of the issue, because we have no data that there’s anything about screening that allows us to improve quality of life ... Screening is probably not worthwhile in any age group.”
Dr. Rosen, who was an author on the 2011 NAM/IOM dietary reference intakes, said that since then, new data have come out regarding the role of vitamin D in mortality in people older than 75 years, benefit in children with regard to respiratory illness, and the potential benefit of vitamin D in pregnancy. “Otherwise, I think we’re going over a lot of the same stuff that we’ve talked about since I was on the IOM panel 15 years ago ... But I think the level of evidence and rigor with which they did it is really impressive.”
However, Simeon I. Taylor, MD, professor of medicine at the University of Maryland, Baltimore, expressed disappointment that the document was limited to healthy people. “Although acknowledging challenges in managing vitamin D status in patients with several diseases, [such as] chronic kidney disease or inflammatory bowel disease, the new guidelines do not provide sufficient guidance for practicing physicians about how to manage these complex patients.”
In addition, Dr. Taylor said that the guidelines “do not explicitly consider the literature suggesting that alternative testing strategies may provide more relevant insights into vitamin D status. Just as variation in levels of thyroid-binding globulin have convinced endocrinologists not to rely on measurement of total thyroxine; interindividual variation in levels of vitamin D binding protein must be accounted for to interpret measurements of total levels of 25(OH)D. It would have been useful to explicitly consider the possible value of measuring vitamin D binding protein-independent indices of vitamin D status.”
Dr. Taylor also raised the same point as an audience member did during the Q&A period regarding patients with osteoporosis or osteopenia. “The value and utility of the new guidelines would be greatly strengthened by providing guidance for how to approach this important and very large group of individuals.”
Dr. Taylor did say that the document has “several strengths, including the fact that they acknowledge the major limitations of the quality of relevant evidence derived from clinical trials.”
In an accompanying commentary, the guideline authors delve into the issues of skin pigmentation and race as they pertain to vitamin D metabolism, writing:
The panel discovered that no randomized clinical trials have directly assessed vitamin D related patient-important outcomes based on participants’ skin pigmentation, although race and ethnicity often served as presumed proxies for skin pigmentation in the literature. In their deliberations, guideline panel members and selected Endocrine Society leaders underscored the critical need to distinguish between skin pigmentation as a biological variable and race and ethnicity as socially determined constructs. This differentiation is vital to maximize scientific rigor and, thus, the validity of resulting recommendations.
Dr. Pittas and Dr. Demay have no disclosures relevant to this clinical practice guideline. Dr. Rosen has no disclosures. Dr. Taylor serves as a consultant for Ionis Pharmaceuticals.
A version of this article appeared on Medscape.com.
BOSTON —
The evidence-based document was presented on June 3, 2024, at the Endocrine Society annual meeting, and simultaneously published in The Journal of Clinical Endocrinology and Metabolism. It advises that people who may benefit from vitamin D supplementation include:
- Children aged 1-18 years to prevent rickets and to potentially lower the risk for respiratory tract infections
- Pregnant people to lower the risk for maternal and fetal or neonatal complications
- Adults older than 75 years to lower the risk for mortality
- Adults with prediabetes to lower the risk for type 2 diabetes
In those groups, the recommendation is for daily (rather than intermittent) empiric vitamin D supplementation of more than what was recommended in 2011 by the National Academy of Medicine (NAM), which was then called the Institute of Medicine (IOM): 600 IU/d for those aged 1-70 years and 800 IU/d for those older than 70 years. The document acknowledges that the optimal dose for these populations isn’t known, but it provides the dose ranges that were used in the trials cited as evidence for the recommendations.
In contrast, the document advises against more vitamin D than the recommended daily intake for most healthier adults younger than 75 years and recommends against testing for blood vitamin D levels in the general population, including those with obesity or darker complexions.
Guideline author Anastassios G. Pittas, MD, professor of medicine at Tufts University School of Medicine, Boston, told this news organization, “this guideline refers to people who are otherwise healthy, and there’s no clear indication for vitamin D, such as people with already established osteoporosis. This guideline is not relevant to them.”
Dr. Pittas also noted, “there’s no single question and single answer about the role of vitamin D in health and disease, which is what people often want to know. There are many questions, and we cannot answer all of them.”
Panel Chair Marie B. Demay, MD, professor of medicine at Harvard Medical School, Boston, told this news organization that indeed the panel was limited by lack of randomized clinical trial evidence to answer many important questions. “There is a paucity of data regarding definition of optimal levels and optimal intake of vitamin D for preventing specific diseases ... What we really need are large scale clinical trials and biomarkers so we can predict disease outcome before it happens.”
Overall, Dr. Demay said, “The recommendations are that populations adhere to the [NAM/IOM] dietary recommended intakes, and there are certain populations that will likely benefit from levels of intake above [those].”
Asked to comment, session moderator Clifford J. Rosen, MD, director of Clinical and Translational Research and senior scientist at Maine Medical Center Research Institute, Scarborough, Maine, noted that screening for vitamin D is quite common in clinical practice, but the recommendation against doing so makes sense.
“When clinicians measure vitamin D, then they’re forced to make a decision what to do about it. That’s where questions about the levels come in. And that’s a big problem. So what the panel’s saying is, don’t screen ... This really gets to the heart of the issue, because we have no data that there’s anything about screening that allows us to improve quality of life ... Screening is probably not worthwhile in any age group.”
Dr. Rosen, who was an author on the 2011 NAM/IOM dietary reference intakes, said that since then, new data have come out regarding the role of vitamin D in mortality in people older than 75 years, benefit in children with regard to respiratory illness, and the potential benefit of vitamin D in pregnancy. “Otherwise, I think we’re going over a lot of the same stuff that we’ve talked about since I was on the IOM panel 15 years ago ... But I think the level of evidence and rigor with which they did it is really impressive.”
However, Simeon I. Taylor, MD, professor of medicine at the University of Maryland, Baltimore, expressed disappointment that the document was limited to healthy people. “Although acknowledging challenges in managing vitamin D status in patients with several diseases, [such as] chronic kidney disease or inflammatory bowel disease, the new guidelines do not provide sufficient guidance for practicing physicians about how to manage these complex patients.”
In addition, Dr. Taylor said that the guidelines “do not explicitly consider the literature suggesting that alternative testing strategies may provide more relevant insights into vitamin D status. Just as variation in levels of thyroid-binding globulin have convinced endocrinologists not to rely on measurement of total thyroxine; interindividual variation in levels of vitamin D binding protein must be accounted for to interpret measurements of total levels of 25(OH)D. It would have been useful to explicitly consider the possible value of measuring vitamin D binding protein-independent indices of vitamin D status.”
Dr. Taylor also raised the same point as an audience member did during the Q&A period regarding patients with osteoporosis or osteopenia. “The value and utility of the new guidelines would be greatly strengthened by providing guidance for how to approach this important and very large group of individuals.”
Dr. Taylor did say that the document has “several strengths, including the fact that they acknowledge the major limitations of the quality of relevant evidence derived from clinical trials.”
In an accompanying commentary, the guideline authors delve into the issues of skin pigmentation and race as they pertain to vitamin D metabolism, writing:
The panel discovered that no randomized clinical trials have directly assessed vitamin D related patient-important outcomes based on participants’ skin pigmentation, although race and ethnicity often served as presumed proxies for skin pigmentation in the literature. In their deliberations, guideline panel members and selected Endocrine Society leaders underscored the critical need to distinguish between skin pigmentation as a biological variable and race and ethnicity as socially determined constructs. This differentiation is vital to maximize scientific rigor and, thus, the validity of resulting recommendations.
Dr. Pittas and Dr. Demay have no disclosures relevant to this clinical practice guideline. Dr. Rosen has no disclosures. Dr. Taylor serves as a consultant for Ionis Pharmaceuticals.
A version of this article appeared on Medscape.com.
BOSTON —
The evidence-based document was presented on June 3, 2024, at the Endocrine Society annual meeting, and simultaneously published in The Journal of Clinical Endocrinology and Metabolism. It advises that people who may benefit from vitamin D supplementation include:
- Children aged 1-18 years to prevent rickets and to potentially lower the risk for respiratory tract infections
- Pregnant people to lower the risk for maternal and fetal or neonatal complications
- Adults older than 75 years to lower the risk for mortality
- Adults with prediabetes to lower the risk for type 2 diabetes
In those groups, the recommendation is for daily (rather than intermittent) empiric vitamin D supplementation of more than what was recommended in 2011 by the National Academy of Medicine (NAM), which was then called the Institute of Medicine (IOM): 600 IU/d for those aged 1-70 years and 800 IU/d for those older than 70 years. The document acknowledges that the optimal dose for these populations isn’t known, but it provides the dose ranges that were used in the trials cited as evidence for the recommendations.
In contrast, the document advises against more vitamin D than the recommended daily intake for most healthier adults younger than 75 years and recommends against testing for blood vitamin D levels in the general population, including those with obesity or darker complexions.
Guideline author Anastassios G. Pittas, MD, professor of medicine at Tufts University School of Medicine, Boston, told this news organization, “this guideline refers to people who are otherwise healthy, and there’s no clear indication for vitamin D, such as people with already established osteoporosis. This guideline is not relevant to them.”
Dr. Pittas also noted, “there’s no single question and single answer about the role of vitamin D in health and disease, which is what people often want to know. There are many questions, and we cannot answer all of them.”
Panel Chair Marie B. Demay, MD, professor of medicine at Harvard Medical School, Boston, told this news organization that indeed the panel was limited by lack of randomized clinical trial evidence to answer many important questions. “There is a paucity of data regarding definition of optimal levels and optimal intake of vitamin D for preventing specific diseases ... What we really need are large scale clinical trials and biomarkers so we can predict disease outcome before it happens.”
Overall, Dr. Demay said, “The recommendations are that populations adhere to the [NAM/IOM] dietary recommended intakes, and there are certain populations that will likely benefit from levels of intake above [those].”
Asked to comment, session moderator Clifford J. Rosen, MD, director of Clinical and Translational Research and senior scientist at Maine Medical Center Research Institute, Scarborough, Maine, noted that screening for vitamin D is quite common in clinical practice, but the recommendation against doing so makes sense.
“When clinicians measure vitamin D, then they’re forced to make a decision what to do about it. That’s where questions about the levels come in. And that’s a big problem. So what the panel’s saying is, don’t screen ... This really gets to the heart of the issue, because we have no data that there’s anything about screening that allows us to improve quality of life ... Screening is probably not worthwhile in any age group.”
Dr. Rosen, who was an author on the 2011 NAM/IOM dietary reference intakes, said that since then, new data have come out regarding the role of vitamin D in mortality in people older than 75 years, benefit in children with regard to respiratory illness, and the potential benefit of vitamin D in pregnancy. “Otherwise, I think we’re going over a lot of the same stuff that we’ve talked about since I was on the IOM panel 15 years ago ... But I think the level of evidence and rigor with which they did it is really impressive.”
However, Simeon I. Taylor, MD, professor of medicine at the University of Maryland, Baltimore, expressed disappointment that the document was limited to healthy people. “Although acknowledging challenges in managing vitamin D status in patients with several diseases, [such as] chronic kidney disease or inflammatory bowel disease, the new guidelines do not provide sufficient guidance for practicing physicians about how to manage these complex patients.”
In addition, Dr. Taylor said that the guidelines “do not explicitly consider the literature suggesting that alternative testing strategies may provide more relevant insights into vitamin D status. Just as variation in levels of thyroid-binding globulin have convinced endocrinologists not to rely on measurement of total thyroxine; interindividual variation in levels of vitamin D binding protein must be accounted for to interpret measurements of total levels of 25(OH)D. It would have been useful to explicitly consider the possible value of measuring vitamin D binding protein-independent indices of vitamin D status.”
Dr. Taylor also raised the same point as an audience member did during the Q&A period regarding patients with osteoporosis or osteopenia. “The value and utility of the new guidelines would be greatly strengthened by providing guidance for how to approach this important and very large group of individuals.”
Dr. Taylor did say that the document has “several strengths, including the fact that they acknowledge the major limitations of the quality of relevant evidence derived from clinical trials.”
In an accompanying commentary, the guideline authors delve into the issues of skin pigmentation and race as they pertain to vitamin D metabolism, writing:
The panel discovered that no randomized clinical trials have directly assessed vitamin D related patient-important outcomes based on participants’ skin pigmentation, although race and ethnicity often served as presumed proxies for skin pigmentation in the literature. In their deliberations, guideline panel members and selected Endocrine Society leaders underscored the critical need to distinguish between skin pigmentation as a biological variable and race and ethnicity as socially determined constructs. This differentiation is vital to maximize scientific rigor and, thus, the validity of resulting recommendations.
Dr. Pittas and Dr. Demay have no disclosures relevant to this clinical practice guideline. Dr. Rosen has no disclosures. Dr. Taylor serves as a consultant for Ionis Pharmaceuticals.
A version of this article appeared on Medscape.com.
Semaglutide Improves Taste Sensitivity in Women With Obesity
The glucagon-like peptide-1 (GLP-1) receptor agonist semaglutide (Ozempic, Wegovy) enhances taste sensitivity, changes brain responses to sweet tastes and may even alter expression of genes in the tongue associated with taste bud development, according to new research presented at the annual meeting of the Endocrine Society, held in Boston.
“Some studies have reported that individuals living with obesity often perceive tastes as less intense,” noted Mojca Jensterle Sever, PhD, of the University Medical Centre in Ljubljana, Slovenia, who presented the work. Research also suggests that “populations prone to obesity have an inherently elevated desire for sweet and energy-dense foods,” she continued.
Studies in animal models have also previously shown that GLP-1 plays an important role in taste sensitivity, but it was not known if this hormone also influenced human taste perception.
In this proof-of-concept study, researchers randomly assigned 30 women with polycystic ovary syndrome (PCOS) to either 1 mg of semaglutide, administered once a week, or placebo for 16 weeks. Participants were on average 34 years old with a body mass index (BMI) of 36.4. Participants with PCOS were selected with the “aim to reduce variability in taste perception across different phases of the menstrual cycle,” Dr. Sever said.
Prior to the intervention, researchers tested participants’ taste sensitivity using 16 taste strips infused with four different concentrations of sweet, sour, salty, and bitter substances. Participants were asked to identify the taste of each strip. Every correct answer counted as one point, with a possible total of 16 points overall. Tongue biopsies were conducted for gene expression analysis.
Researchers also used functional MRI (fMRI) to evaluate brain responses to a series of calorie-dense, low-calorie, and non-food visual cues as well as to sweet taste stimulus. A sweet solution was administered on the tongue 30 minutes before and after participants consumed a standardized meal: a high-protein enriched nutritional drink.
These tests were repeated after 16 weeks.
The semaglutide group also exhibited decreased activation of the putamen (a structure in the brain involved with the brain’s reward system) on fMRI in response to calorie-dense cues. In response to sweet taste stimulus, those taking semaglutide showed increased activation of angular gyrus on MRI compared with the placebo group. The angular gyrus is part of the brain’s parietal lobe and is involved with language, memory, reasoning, and attention.
Lastly, researchers identified differential mRNA expression in the genes EYA, PRMT8, CRLF1, and CYP1B1, which are associated with taste bud development, renewal, and differentiation.
The findings are “fascinating, because we think about all of the factors that this new class of agents are able to improve, but taste is often not something that we look at, though there have been very strong associations,” said Gitanjali Srivastava, MD, of Vanderbilt University, Nashville, Tennessee, who moderated the session.
“Is it possible that another mechanism of action for this class of agents is perhaps indirectly altering our taste perception,” she posited, and, because of that, “we have an altered sense of satiety and hunger?”
Dr. Sever noted Dr. Several limitations to the study, including that only specific tastes were evaluated in a controlled study environment, “which may not reflect everyday experience,” she said. Taste perception can also vary widely from person to person, and changes in mRNA expression do not necessarily reflect changes in protein levels or activity.
“Our study should be seen and interpreted as a proof-of-concept study,” Dr. Sever added, with additional research needed to explore the relationship between semaglutide and taste perception.
Dr. Srivastava consults for Novo Nordisk, Eli Lilly, and Rhythm Pharmaceuticals. She has received research grant support from Eli Lilly. Dr. Sever reports no relevant financial relationships.
A version of this article appeared on Medscape.com .
The glucagon-like peptide-1 (GLP-1) receptor agonist semaglutide (Ozempic, Wegovy) enhances taste sensitivity, changes brain responses to sweet tastes and may even alter expression of genes in the tongue associated with taste bud development, according to new research presented at the annual meeting of the Endocrine Society, held in Boston.
“Some studies have reported that individuals living with obesity often perceive tastes as less intense,” noted Mojca Jensterle Sever, PhD, of the University Medical Centre in Ljubljana, Slovenia, who presented the work. Research also suggests that “populations prone to obesity have an inherently elevated desire for sweet and energy-dense foods,” she continued.
Studies in animal models have also previously shown that GLP-1 plays an important role in taste sensitivity, but it was not known if this hormone also influenced human taste perception.
In this proof-of-concept study, researchers randomly assigned 30 women with polycystic ovary syndrome (PCOS) to either 1 mg of semaglutide, administered once a week, or placebo for 16 weeks. Participants were on average 34 years old with a body mass index (BMI) of 36.4. Participants with PCOS were selected with the “aim to reduce variability in taste perception across different phases of the menstrual cycle,” Dr. Sever said.
Prior to the intervention, researchers tested participants’ taste sensitivity using 16 taste strips infused with four different concentrations of sweet, sour, salty, and bitter substances. Participants were asked to identify the taste of each strip. Every correct answer counted as one point, with a possible total of 16 points overall. Tongue biopsies were conducted for gene expression analysis.
Researchers also used functional MRI (fMRI) to evaluate brain responses to a series of calorie-dense, low-calorie, and non-food visual cues as well as to sweet taste stimulus. A sweet solution was administered on the tongue 30 minutes before and after participants consumed a standardized meal: a high-protein enriched nutritional drink.
These tests were repeated after 16 weeks.
The semaglutide group also exhibited decreased activation of the putamen (a structure in the brain involved with the brain’s reward system) on fMRI in response to calorie-dense cues. In response to sweet taste stimulus, those taking semaglutide showed increased activation of angular gyrus on MRI compared with the placebo group. The angular gyrus is part of the brain’s parietal lobe and is involved with language, memory, reasoning, and attention.
Lastly, researchers identified differential mRNA expression in the genes EYA, PRMT8, CRLF1, and CYP1B1, which are associated with taste bud development, renewal, and differentiation.
The findings are “fascinating, because we think about all of the factors that this new class of agents are able to improve, but taste is often not something that we look at, though there have been very strong associations,” said Gitanjali Srivastava, MD, of Vanderbilt University, Nashville, Tennessee, who moderated the session.
“Is it possible that another mechanism of action for this class of agents is perhaps indirectly altering our taste perception,” she posited, and, because of that, “we have an altered sense of satiety and hunger?”
Dr. Sever noted Dr. Several limitations to the study, including that only specific tastes were evaluated in a controlled study environment, “which may not reflect everyday experience,” she said. Taste perception can also vary widely from person to person, and changes in mRNA expression do not necessarily reflect changes in protein levels or activity.
“Our study should be seen and interpreted as a proof-of-concept study,” Dr. Sever added, with additional research needed to explore the relationship between semaglutide and taste perception.
Dr. Srivastava consults for Novo Nordisk, Eli Lilly, and Rhythm Pharmaceuticals. She has received research grant support from Eli Lilly. Dr. Sever reports no relevant financial relationships.
A version of this article appeared on Medscape.com .
The glucagon-like peptide-1 (GLP-1) receptor agonist semaglutide (Ozempic, Wegovy) enhances taste sensitivity, changes brain responses to sweet tastes and may even alter expression of genes in the tongue associated with taste bud development, according to new research presented at the annual meeting of the Endocrine Society, held in Boston.
“Some studies have reported that individuals living with obesity often perceive tastes as less intense,” noted Mojca Jensterle Sever, PhD, of the University Medical Centre in Ljubljana, Slovenia, who presented the work. Research also suggests that “populations prone to obesity have an inherently elevated desire for sweet and energy-dense foods,” she continued.
Studies in animal models have also previously shown that GLP-1 plays an important role in taste sensitivity, but it was not known if this hormone also influenced human taste perception.
In this proof-of-concept study, researchers randomly assigned 30 women with polycystic ovary syndrome (PCOS) to either 1 mg of semaglutide, administered once a week, or placebo for 16 weeks. Participants were on average 34 years old with a body mass index (BMI) of 36.4. Participants with PCOS were selected with the “aim to reduce variability in taste perception across different phases of the menstrual cycle,” Dr. Sever said.
Prior to the intervention, researchers tested participants’ taste sensitivity using 16 taste strips infused with four different concentrations of sweet, sour, salty, and bitter substances. Participants were asked to identify the taste of each strip. Every correct answer counted as one point, with a possible total of 16 points overall. Tongue biopsies were conducted for gene expression analysis.
Researchers also used functional MRI (fMRI) to evaluate brain responses to a series of calorie-dense, low-calorie, and non-food visual cues as well as to sweet taste stimulus. A sweet solution was administered on the tongue 30 minutes before and after participants consumed a standardized meal: a high-protein enriched nutritional drink.
These tests were repeated after 16 weeks.
The semaglutide group also exhibited decreased activation of the putamen (a structure in the brain involved with the brain’s reward system) on fMRI in response to calorie-dense cues. In response to sweet taste stimulus, those taking semaglutide showed increased activation of angular gyrus on MRI compared with the placebo group. The angular gyrus is part of the brain’s parietal lobe and is involved with language, memory, reasoning, and attention.
Lastly, researchers identified differential mRNA expression in the genes EYA, PRMT8, CRLF1, and CYP1B1, which are associated with taste bud development, renewal, and differentiation.
The findings are “fascinating, because we think about all of the factors that this new class of agents are able to improve, but taste is often not something that we look at, though there have been very strong associations,” said Gitanjali Srivastava, MD, of Vanderbilt University, Nashville, Tennessee, who moderated the session.
“Is it possible that another mechanism of action for this class of agents is perhaps indirectly altering our taste perception,” she posited, and, because of that, “we have an altered sense of satiety and hunger?”
Dr. Sever noted Dr. Several limitations to the study, including that only specific tastes were evaluated in a controlled study environment, “which may not reflect everyday experience,” she said. Taste perception can also vary widely from person to person, and changes in mRNA expression do not necessarily reflect changes in protein levels or activity.
“Our study should be seen and interpreted as a proof-of-concept study,” Dr. Sever added, with additional research needed to explore the relationship between semaglutide and taste perception.
Dr. Srivastava consults for Novo Nordisk, Eli Lilly, and Rhythm Pharmaceuticals. She has received research grant support from Eli Lilly. Dr. Sever reports no relevant financial relationships.
A version of this article appeared on Medscape.com .
‘Ozempic Burgers’ Offer Indulgences to People With Obesity
My crystal ball says that Big Food’s ongoing development and marketing of products designed for the reduced appetites of people taking anti-obesity medications will simultaneously be welcomed by their target market and scorned by self-righteous, healthy-living, just-try-harder, isn’t-this-just-feeding-the-problem hypocrites.
For the privileged, self-righteous, healthy-living crowd, the right to enjoy dietary indulgences and conveniences is inversely proportional to your weight. Often, judgment isn’t cast on the less-than-perfect choices of those with so-called “normal” weight; that’s often not the case for those with obesity.
Think you’re free from this paradigm? If you are, good for you. But I’d wager that there are plenty of readers who state that they’re free from bias, but when standing in supermarket checkout lines, they scrutinize and silently pass judgment on the contents of the grocery carts of people with obesity or, similarly, on the orders of people with obesity in fast-food restaurants.
Yet, there are bags of chips and cookies in most of our weekly carts, and who among us doesn’t, at times, grab some greasy comfort or convenience?
Unfortunately, the fuel for these sorts of judgments — implicit weight bias — is not only pervasive but also durable. A recent study of temporal changes to implicit biases demonstrated that unlike biases about race, skin tone, sexuality, age, and disability — between 2007 and 2016, tested levels of these implicit bias were seen to be in decline —biases about weight remain stable.
As to the products themselves, according to the recent article, they’ll be smaller, lower in calories, and high in protein and fat. To put it another way, compared with their nonshrunken counterparts,
With that said, I’d be remiss if I didn’t assert that the discussion of the merits or lack thereof of these sorts of offerings is misguided and pointless in that the food industry’s job is not one of social service provision or preventive healthcare. As I’ve written in the past, the food industry is neither friend, foe, nor partner. The food industry’s one job is to sell food, and if they see a market opportunity, they’ll take it. In this case, that turns out to be refreshing in a sense in that unlike moral-panic scolds, the food industry doesn’t judge its customers’ right to buy its products on the basis of how much their customers weigh.
Whereas the food industry’s response to anti-obesity medications’ impact on appetite may be to embrace it, many others’, including in medicine, seem to involve some degree of judgment or scorn. Yes, our behavior has an impact on our weight, but intentional behavior change in the name of weight requires multiple layers of deep and perpetual privilege. And yes, our environment is indeed a tremendous contributor to the challenge of obesity, but the world is full of medical conditions influenced or caused by our environment. Yet, discussions around how medications fail to address obesity’s root cause are the only such root-cause discussions I ever see.
Put more plainly, “how dare we develop medications for conditions influenced by our environment” is an odd stance to take in a world full of conditions influenced by our environments and where our environments’ primary change-driver is sales. Products that support the use of medications that improve life’s quality while markedly reducing the risk for an ever-growing number of conditions should be celebrated.
Dr. Freedhoff has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, adviser, consultant, or trustee for Bariatric Medical Institute and Constant Health; received research grant from Novo Nordisk; publicly shared opinions via Weighty Matters and social media.
A version of this article appeared on Medscape.com.
My crystal ball says that Big Food’s ongoing development and marketing of products designed for the reduced appetites of people taking anti-obesity medications will simultaneously be welcomed by their target market and scorned by self-righteous, healthy-living, just-try-harder, isn’t-this-just-feeding-the-problem hypocrites.
For the privileged, self-righteous, healthy-living crowd, the right to enjoy dietary indulgences and conveniences is inversely proportional to your weight. Often, judgment isn’t cast on the less-than-perfect choices of those with so-called “normal” weight; that’s often not the case for those with obesity.
Think you’re free from this paradigm? If you are, good for you. But I’d wager that there are plenty of readers who state that they’re free from bias, but when standing in supermarket checkout lines, they scrutinize and silently pass judgment on the contents of the grocery carts of people with obesity or, similarly, on the orders of people with obesity in fast-food restaurants.
Yet, there are bags of chips and cookies in most of our weekly carts, and who among us doesn’t, at times, grab some greasy comfort or convenience?
Unfortunately, the fuel for these sorts of judgments — implicit weight bias — is not only pervasive but also durable. A recent study of temporal changes to implicit biases demonstrated that unlike biases about race, skin tone, sexuality, age, and disability — between 2007 and 2016, tested levels of these implicit bias were seen to be in decline —biases about weight remain stable.
As to the products themselves, according to the recent article, they’ll be smaller, lower in calories, and high in protein and fat. To put it another way, compared with their nonshrunken counterparts,
With that said, I’d be remiss if I didn’t assert that the discussion of the merits or lack thereof of these sorts of offerings is misguided and pointless in that the food industry’s job is not one of social service provision or preventive healthcare. As I’ve written in the past, the food industry is neither friend, foe, nor partner. The food industry’s one job is to sell food, and if they see a market opportunity, they’ll take it. In this case, that turns out to be refreshing in a sense in that unlike moral-panic scolds, the food industry doesn’t judge its customers’ right to buy its products on the basis of how much their customers weigh.
Whereas the food industry’s response to anti-obesity medications’ impact on appetite may be to embrace it, many others’, including in medicine, seem to involve some degree of judgment or scorn. Yes, our behavior has an impact on our weight, but intentional behavior change in the name of weight requires multiple layers of deep and perpetual privilege. And yes, our environment is indeed a tremendous contributor to the challenge of obesity, but the world is full of medical conditions influenced or caused by our environment. Yet, discussions around how medications fail to address obesity’s root cause are the only such root-cause discussions I ever see.
Put more plainly, “how dare we develop medications for conditions influenced by our environment” is an odd stance to take in a world full of conditions influenced by our environments and where our environments’ primary change-driver is sales. Products that support the use of medications that improve life’s quality while markedly reducing the risk for an ever-growing number of conditions should be celebrated.
Dr. Freedhoff has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, adviser, consultant, or trustee for Bariatric Medical Institute and Constant Health; received research grant from Novo Nordisk; publicly shared opinions via Weighty Matters and social media.
A version of this article appeared on Medscape.com.
My crystal ball says that Big Food’s ongoing development and marketing of products designed for the reduced appetites of people taking anti-obesity medications will simultaneously be welcomed by their target market and scorned by self-righteous, healthy-living, just-try-harder, isn’t-this-just-feeding-the-problem hypocrites.
For the privileged, self-righteous, healthy-living crowd, the right to enjoy dietary indulgences and conveniences is inversely proportional to your weight. Often, judgment isn’t cast on the less-than-perfect choices of those with so-called “normal” weight; that’s often not the case for those with obesity.
Think you’re free from this paradigm? If you are, good for you. But I’d wager that there are plenty of readers who state that they’re free from bias, but when standing in supermarket checkout lines, they scrutinize and silently pass judgment on the contents of the grocery carts of people with obesity or, similarly, on the orders of people with obesity in fast-food restaurants.
Yet, there are bags of chips and cookies in most of our weekly carts, and who among us doesn’t, at times, grab some greasy comfort or convenience?
Unfortunately, the fuel for these sorts of judgments — implicit weight bias — is not only pervasive but also durable. A recent study of temporal changes to implicit biases demonstrated that unlike biases about race, skin tone, sexuality, age, and disability — between 2007 and 2016, tested levels of these implicit bias were seen to be in decline —biases about weight remain stable.
As to the products themselves, according to the recent article, they’ll be smaller, lower in calories, and high in protein and fat. To put it another way, compared with their nonshrunken counterparts,
With that said, I’d be remiss if I didn’t assert that the discussion of the merits or lack thereof of these sorts of offerings is misguided and pointless in that the food industry’s job is not one of social service provision or preventive healthcare. As I’ve written in the past, the food industry is neither friend, foe, nor partner. The food industry’s one job is to sell food, and if they see a market opportunity, they’ll take it. In this case, that turns out to be refreshing in a sense in that unlike moral-panic scolds, the food industry doesn’t judge its customers’ right to buy its products on the basis of how much their customers weigh.
Whereas the food industry’s response to anti-obesity medications’ impact on appetite may be to embrace it, many others’, including in medicine, seem to involve some degree of judgment or scorn. Yes, our behavior has an impact on our weight, but intentional behavior change in the name of weight requires multiple layers of deep and perpetual privilege. And yes, our environment is indeed a tremendous contributor to the challenge of obesity, but the world is full of medical conditions influenced or caused by our environment. Yet, discussions around how medications fail to address obesity’s root cause are the only such root-cause discussions I ever see.
Put more plainly, “how dare we develop medications for conditions influenced by our environment” is an odd stance to take in a world full of conditions influenced by our environments and where our environments’ primary change-driver is sales. Products that support the use of medications that improve life’s quality while markedly reducing the risk for an ever-growing number of conditions should be celebrated.
Dr. Freedhoff has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, adviser, consultant, or trustee for Bariatric Medical Institute and Constant Health; received research grant from Novo Nordisk; publicly shared opinions via Weighty Matters and social media.
A version of this article appeared on Medscape.com.