User login
Managing hyperhidrosis, HS: Ask questions first
A wide variety of medications exists for treating hyperhidrosis, a dermatologist told colleagues, but before prescribing anything to a pediatric patient, he recommended, ask the patient a simple question: “What bothers you the most?”
The answer will provide guidance for developing a step-by-step treatment strategy and help provide the patient “a set of realistic expectations in terms of what the response will look like,” George Hightower, MD, PhD, a pediatric dermatologist at Rady Children’s Hospital and the University of California, San Diego, said at MedscapeLive’s Women’s & Pediatric Dermatology Seminar.
A similar question-based approach will help guide therapy for patients with hidradenitis suppurativa (HS), he said.
With regards to hyperhidrosis, Dr. Hightower said that patients most commonly complain that their underarms are too smelly, too sweaty, and red, itchy, or painful. Causes, he said, can include irritation/contact dermatitis, folliculitis, and seborrheic dermatitis, as well as hyperhidrosis or HS.
Primary focal axillary hyperhidrosis is defined as focal, visible, excessive sweating for at least 6 months without an apparent cause plus at least two of the following characteristics: Sweating is bilateral and relatively symmetric, it impairs daily activities, it starts before the age of 25 with at least one episode per week (many patients have it daily), a family history of idiopathic hyperhidrosis is present, and focal sweating does not occur during sleep.
Secondary hyperhidrosis can be linked to other conditions, such as a spinal column injury, Dr. Hightower noted.
The first step on the treatment ladder is topical 20% aluminum chloride, which is available over the counter. This should be applied nightly for 1 week then every 1-2 weeks, Dr. Hightower recommended. All of his patients with hyperhidrosis have had at least one trial of this treatment.
The next option is daily topical treatment with 2.4% glycopyrronium tosylate (Qbrexza) cloths, approved by the Food and Drug Administration in 2018 for primary axillary hyperhidrosis in patients aged 9 and older. According to the prescribing information, dry mouth was by far the most common treatment-associated adverse effect in clinical trials (24% versus almost 6% among those on vehicle). As for skin reactions, erythema occurred in about 17% of both the intervention and vehicle groups, and burning/stinging occurred in 14% of those on treatment and almost 17% of those on vehicle.
“If they’re not able to get access to the cloths due to [insurance] coverage issues, or they don’t allow them to reach the clinical endpoint desired, then I use an oral daily glycopyrrolate pill,” Dr. Hightower said.
He recommends 1 mg to 6 mg daily of the anticholinergic drug, which has been used off-label for hyperhidrosis for several years. A 2012 study of 31 children with hyperhidrosis, he noted, supported the use of the drug. The retrospective study found that 90% of the patients, at a mean daily dose of 2 mg, experienced improvements, reported as major in 71%. In addition, patients experienced improvement within hours of taking the medication, and benefits disappeared within a day of stopping the medication. In the study, patients were on the treatment for an average of 2.1 years, and 29% experienced side effects, which were dose related; the most common were dry mouth in 26% and dry eyes in 10%.
According to goodrx.com, a month’s supply of 2 mg of the drug costs as little as $13 with a discount or coupon.
The next steps in treatment are procedural interventions such as microwave-based therapies.
Dr. Hightower said that patients should be advised that treatment may take years, and to encourage them to return for follow-up. He suggested this helpful message: “We’re still trying to find the best treatment for you, and we’ll need to see you back in the office.”
Hidradenitis suppurativa
Dr. Hightower said that too often, HS goes undiagnosed for a significant period of time, preventing patients from seeing a dermatologist for treatment. Hallmarks of HS include inflammatory nodules, abscesses, and scarring, he said. “It can be disfiguring, painful, embarrassing, and associated with significantly decreased quality of life. Early recognition in terms of making and solidifying the diagnosis is important so we can prevent further worsening of the disease.”
The goal of treatment include preventing scars and unnecessary emergency department visits, and stopping flares from worsening, Dr. Hightower said. For specifics, he pointed to clinical management guidelines released by the United States and Canadian hidradenitis suppurativa foundations in 2019.
Make sure to set individualized treatment goals and understand the impact of treatment on the patient’s interactions with family, school, and peers, he said. And keep in mind that “parent-defined goals may be different from patient-defined goals.”
Dr. Hightower reported no relevant disclosures. MedscapeLive and this news organization are owned by the same parent company
A wide variety of medications exists for treating hyperhidrosis, a dermatologist told colleagues, but before prescribing anything to a pediatric patient, he recommended, ask the patient a simple question: “What bothers you the most?”
The answer will provide guidance for developing a step-by-step treatment strategy and help provide the patient “a set of realistic expectations in terms of what the response will look like,” George Hightower, MD, PhD, a pediatric dermatologist at Rady Children’s Hospital and the University of California, San Diego, said at MedscapeLive’s Women’s & Pediatric Dermatology Seminar.
A similar question-based approach will help guide therapy for patients with hidradenitis suppurativa (HS), he said.
With regards to hyperhidrosis, Dr. Hightower said that patients most commonly complain that their underarms are too smelly, too sweaty, and red, itchy, or painful. Causes, he said, can include irritation/contact dermatitis, folliculitis, and seborrheic dermatitis, as well as hyperhidrosis or HS.
Primary focal axillary hyperhidrosis is defined as focal, visible, excessive sweating for at least 6 months without an apparent cause plus at least two of the following characteristics: Sweating is bilateral and relatively symmetric, it impairs daily activities, it starts before the age of 25 with at least one episode per week (many patients have it daily), a family history of idiopathic hyperhidrosis is present, and focal sweating does not occur during sleep.
Secondary hyperhidrosis can be linked to other conditions, such as a spinal column injury, Dr. Hightower noted.
The first step on the treatment ladder is topical 20% aluminum chloride, which is available over the counter. This should be applied nightly for 1 week then every 1-2 weeks, Dr. Hightower recommended. All of his patients with hyperhidrosis have had at least one trial of this treatment.
The next option is daily topical treatment with 2.4% glycopyrronium tosylate (Qbrexza) cloths, approved by the Food and Drug Administration in 2018 for primary axillary hyperhidrosis in patients aged 9 and older. According to the prescribing information, dry mouth was by far the most common treatment-associated adverse effect in clinical trials (24% versus almost 6% among those on vehicle). As for skin reactions, erythema occurred in about 17% of both the intervention and vehicle groups, and burning/stinging occurred in 14% of those on treatment and almost 17% of those on vehicle.
“If they’re not able to get access to the cloths due to [insurance] coverage issues, or they don’t allow them to reach the clinical endpoint desired, then I use an oral daily glycopyrrolate pill,” Dr. Hightower said.
He recommends 1 mg to 6 mg daily of the anticholinergic drug, which has been used off-label for hyperhidrosis for several years. A 2012 study of 31 children with hyperhidrosis, he noted, supported the use of the drug. The retrospective study found that 90% of the patients, at a mean daily dose of 2 mg, experienced improvements, reported as major in 71%. In addition, patients experienced improvement within hours of taking the medication, and benefits disappeared within a day of stopping the medication. In the study, patients were on the treatment for an average of 2.1 years, and 29% experienced side effects, which were dose related; the most common were dry mouth in 26% and dry eyes in 10%.
According to goodrx.com, a month’s supply of 2 mg of the drug costs as little as $13 with a discount or coupon.
The next steps in treatment are procedural interventions such as microwave-based therapies.
Dr. Hightower said that patients should be advised that treatment may take years, and to encourage them to return for follow-up. He suggested this helpful message: “We’re still trying to find the best treatment for you, and we’ll need to see you back in the office.”
Hidradenitis suppurativa
Dr. Hightower said that too often, HS goes undiagnosed for a significant period of time, preventing patients from seeing a dermatologist for treatment. Hallmarks of HS include inflammatory nodules, abscesses, and scarring, he said. “It can be disfiguring, painful, embarrassing, and associated with significantly decreased quality of life. Early recognition in terms of making and solidifying the diagnosis is important so we can prevent further worsening of the disease.”
The goal of treatment include preventing scars and unnecessary emergency department visits, and stopping flares from worsening, Dr. Hightower said. For specifics, he pointed to clinical management guidelines released by the United States and Canadian hidradenitis suppurativa foundations in 2019.
Make sure to set individualized treatment goals and understand the impact of treatment on the patient’s interactions with family, school, and peers, he said. And keep in mind that “parent-defined goals may be different from patient-defined goals.”
Dr. Hightower reported no relevant disclosures. MedscapeLive and this news organization are owned by the same parent company
A wide variety of medications exists for treating hyperhidrosis, a dermatologist told colleagues, but before prescribing anything to a pediatric patient, he recommended, ask the patient a simple question: “What bothers you the most?”
The answer will provide guidance for developing a step-by-step treatment strategy and help provide the patient “a set of realistic expectations in terms of what the response will look like,” George Hightower, MD, PhD, a pediatric dermatologist at Rady Children’s Hospital and the University of California, San Diego, said at MedscapeLive’s Women’s & Pediatric Dermatology Seminar.
A similar question-based approach will help guide therapy for patients with hidradenitis suppurativa (HS), he said.
With regards to hyperhidrosis, Dr. Hightower said that patients most commonly complain that their underarms are too smelly, too sweaty, and red, itchy, or painful. Causes, he said, can include irritation/contact dermatitis, folliculitis, and seborrheic dermatitis, as well as hyperhidrosis or HS.
Primary focal axillary hyperhidrosis is defined as focal, visible, excessive sweating for at least 6 months without an apparent cause plus at least two of the following characteristics: Sweating is bilateral and relatively symmetric, it impairs daily activities, it starts before the age of 25 with at least one episode per week (many patients have it daily), a family history of idiopathic hyperhidrosis is present, and focal sweating does not occur during sleep.
Secondary hyperhidrosis can be linked to other conditions, such as a spinal column injury, Dr. Hightower noted.
The first step on the treatment ladder is topical 20% aluminum chloride, which is available over the counter. This should be applied nightly for 1 week then every 1-2 weeks, Dr. Hightower recommended. All of his patients with hyperhidrosis have had at least one trial of this treatment.
The next option is daily topical treatment with 2.4% glycopyrronium tosylate (Qbrexza) cloths, approved by the Food and Drug Administration in 2018 for primary axillary hyperhidrosis in patients aged 9 and older. According to the prescribing information, dry mouth was by far the most common treatment-associated adverse effect in clinical trials (24% versus almost 6% among those on vehicle). As for skin reactions, erythema occurred in about 17% of both the intervention and vehicle groups, and burning/stinging occurred in 14% of those on treatment and almost 17% of those on vehicle.
“If they’re not able to get access to the cloths due to [insurance] coverage issues, or they don’t allow them to reach the clinical endpoint desired, then I use an oral daily glycopyrrolate pill,” Dr. Hightower said.
He recommends 1 mg to 6 mg daily of the anticholinergic drug, which has been used off-label for hyperhidrosis for several years. A 2012 study of 31 children with hyperhidrosis, he noted, supported the use of the drug. The retrospective study found that 90% of the patients, at a mean daily dose of 2 mg, experienced improvements, reported as major in 71%. In addition, patients experienced improvement within hours of taking the medication, and benefits disappeared within a day of stopping the medication. In the study, patients were on the treatment for an average of 2.1 years, and 29% experienced side effects, which were dose related; the most common were dry mouth in 26% and dry eyes in 10%.
According to goodrx.com, a month’s supply of 2 mg of the drug costs as little as $13 with a discount or coupon.
The next steps in treatment are procedural interventions such as microwave-based therapies.
Dr. Hightower said that patients should be advised that treatment may take years, and to encourage them to return for follow-up. He suggested this helpful message: “We’re still trying to find the best treatment for you, and we’ll need to see you back in the office.”
Hidradenitis suppurativa
Dr. Hightower said that too often, HS goes undiagnosed for a significant period of time, preventing patients from seeing a dermatologist for treatment. Hallmarks of HS include inflammatory nodules, abscesses, and scarring, he said. “It can be disfiguring, painful, embarrassing, and associated with significantly decreased quality of life. Early recognition in terms of making and solidifying the diagnosis is important so we can prevent further worsening of the disease.”
The goal of treatment include preventing scars and unnecessary emergency department visits, and stopping flares from worsening, Dr. Hightower said. For specifics, he pointed to clinical management guidelines released by the United States and Canadian hidradenitis suppurativa foundations in 2019.
Make sure to set individualized treatment goals and understand the impact of treatment on the patient’s interactions with family, school, and peers, he said. And keep in mind that “parent-defined goals may be different from patient-defined goals.”
Dr. Hightower reported no relevant disclosures. MedscapeLive and this news organization are owned by the same parent company
FROM MEDSCAPELIVE WOMEN’S & PEDIATRIC DERMATOLOGY SEMINAR
Psychological difficulties persist among patients with IBD
Psychological issues in patients with inflammatory bowel disease should be addressed at both personal and systemic levels, according to a review of current literature.
In a review published in the Journal of Clinical Gastroenterology, researchers highlighted data on the burden of mental disorders in inflammatory bowel disease (IBD) patients and presented several strategies for addressing them.
“From a systems perspective, underrecognized and/or suboptimally treated mental health problems in patients with IBD are associated with increased disability, poorer adherence, and more admissions and surgeries, driving increased health care utilization and costs,” Maia S. Kredentser, PhD, of the University of Manitoba, Winnipeg, and colleagues wrote, citing a 2018 study’s findings.
“There is ample evidence for a higher prevalence of mental disorders in IBD, in particular depression and anxiety, compared with the general population,” the authors wrote.
They cited a recent population-based study in which the incident rate ratios were significantly higher for IBD patients, compared with matched controls for depression (IRR, 1.58), anxiety disorder (IRR, 1.39), bipolar disorder (IRR, 1.82), and schizophrenia (IRR, 1.64).
Mental disorders associated with IBD also include issues of body image and sexuality. Although research on the impact of disease activity on sexual function is inconsistent, one study suggested that body image “may be an important target of treatment in women reporting poor quality of life and psychological distress,” the researchers noted. A French study from 2017 published in the Journal of Crohn’s and Colitis showed that approximately half of men and women reported problems with erectile or sexual dysfunction.
Issues related to environmental stressors may contribute to IBD by promoting chronic inflammation, the researchers wrote. For example, data from longitudinal, population-based research suggest that adverse childhood experiences can promote proinflammatory states across inflammatory illnesses. Research has also suggested that people with IBD have higher rates of these adverse childhood experiences than the general population. However, data also show that many are able to cope and adapt: “Many patients with IBD are resilient, experience growth, and in fact, thrive,” the researchers added. One longitudinal study suggested that patients with IBD who identified with “thriving” had “stronger coping efficacy (the perceived ability to meet illness demands), illness acceptance, and social support and lower depression” and that this was associated with life satisfaction 6 months later.
Fatigue also has been shown to be a factor for patients with IBD. The researchers cited one population-based study showing fatigue in 57%-72% of IBD patients with active disease. IBD patients with quiescent disease also report fatigue. The psychological and behavioral factors driving fatigue could be related to mental disorders or other factors such as suboptimal sleep, stress, and use of caffeine and alcohol, they noted. Management strategies include improving sleep hygiene and evaluation of mental health concerns.
Seek complete picture before treatment
“Addressing psychological comorbidity in IBD requires individual and systemic approaches focused on both the prevention and treatment of mental health concerns,” the researchers wrote. “Because of the pervasiveness of psychological comorbidities in IBD, and recent evidence that they may be part of the disease process itself, assessment of psychological functioning in IBD is considered an essential aspect of disease management.”
Evidence-based psychological interventions include cognitive-behavioral therapy, which includes training in relaxation; treatment with clinical hypnosis; and encouraging mindfulness through acceptance and commitment therapy, which focuses on developing psychological flexibility to cope with suffering. In addition, a small but evolving body of research shows some benefit to motivational interviewing (a strategy focused on behavior change) for IBD patients. Notably, one review of four studies showed benefits of motivational interviewing for improving medication adherence and advice seeking, the researchers reported.
Although several psychological treatment options exist for addressing mental health issues in IBD, randomized trials are needed. “To facilitate this important research and optimize patient care, the integration of psychologists and other mental health providers into IBD care is considered best practice and provides exciting opportunities for improving patient care and outcomes,” the researchers concluded.
Address mental health to ease disease burden
“There is a large burden of mental health issues in patients with inflammatory bowel disease, with depression and anxiety leading the way,” Kim L. Isaacs, MD, of the University of North Carolina at Chapel Hill, said in an interview.
“There are multiple reasons for this, including dealing with chronic pain, social concerns around using the bathroom, body-image issues due to surgery, and drug side effects. There is increasing evidence that the inflammatory process in IBD may be driving some of the changes in the brain which lead to further mental health dysfunction,” she noted.
“Addressing depression, anxiety, [and] sleep disturbance in patients will not only improve quality of life from a mental health perspective but has been shown to improve control of disease,” Dr. Isaacs emphasized.
“Small things like increased medication compliance have a large impact on disease management and decreased need for hospitalization and hospitalization,” said Dr. Isaacs. “As gastroenterologists we need to expand our focus beyond the gut and address the emotional needs of our patients – identifying those patients who need increased mental health support.”
Barriers to better care
The greatest barriers to treating mental health issues in IBD patients are time and knowledge, said Dr. Isaacs. “Many gastroenterologists have limited time in the office to do more than address the acute issues of the patients such as rectal bleeding and worsening diarrhea. It takes time and trust to explore what is going on in a patient’s life. Is the patient anxious and depressed? How are they coping with their current disease manifestations? Simple screening tools may help with this, but then there need to be resources to support interventions.”
Some IBD practices, especially academic ones, have a psychologist in the IBD center or one that’s readily available for consultation. “This is an investment for the practice that may reduce significantly disease burden. The IBD specialty home model includes resources for management of psychiatric issues and nutritional concerns as well as disease management,” she added.
More research in several areas can help reduce the mental health burden of IBD. “On the immunology/biology side, understanding how the microbiome affects the brain/gut may allow for more directed mental health treatment. On the disease management side, larger trials directed at psychiatric interventions may help to determine which therapy is best for each patient,” Dr. Isaacs said. “Further work developing health care systems, such as the medical home, that allow for maximum disease management and decreased system costs will go far in implementation of models of care that address the needs of the entire patient with inflammatory bowel disease.”
The review received no outside funding. The researchers had no financial conflicts to disclose. Dr. Isaacs disclosed consulting on the data safety monitoring board for Janssen.
Psychological issues in patients with inflammatory bowel disease should be addressed at both personal and systemic levels, according to a review of current literature.
In a review published in the Journal of Clinical Gastroenterology, researchers highlighted data on the burden of mental disorders in inflammatory bowel disease (IBD) patients and presented several strategies for addressing them.
“From a systems perspective, underrecognized and/or suboptimally treated mental health problems in patients with IBD are associated with increased disability, poorer adherence, and more admissions and surgeries, driving increased health care utilization and costs,” Maia S. Kredentser, PhD, of the University of Manitoba, Winnipeg, and colleagues wrote, citing a 2018 study’s findings.
“There is ample evidence for a higher prevalence of mental disorders in IBD, in particular depression and anxiety, compared with the general population,” the authors wrote.
They cited a recent population-based study in which the incident rate ratios were significantly higher for IBD patients, compared with matched controls for depression (IRR, 1.58), anxiety disorder (IRR, 1.39), bipolar disorder (IRR, 1.82), and schizophrenia (IRR, 1.64).
Mental disorders associated with IBD also include issues of body image and sexuality. Although research on the impact of disease activity on sexual function is inconsistent, one study suggested that body image “may be an important target of treatment in women reporting poor quality of life and psychological distress,” the researchers noted. A French study from 2017 published in the Journal of Crohn’s and Colitis showed that approximately half of men and women reported problems with erectile or sexual dysfunction.
Issues related to environmental stressors may contribute to IBD by promoting chronic inflammation, the researchers wrote. For example, data from longitudinal, population-based research suggest that adverse childhood experiences can promote proinflammatory states across inflammatory illnesses. Research has also suggested that people with IBD have higher rates of these adverse childhood experiences than the general population. However, data also show that many are able to cope and adapt: “Many patients with IBD are resilient, experience growth, and in fact, thrive,” the researchers added. One longitudinal study suggested that patients with IBD who identified with “thriving” had “stronger coping efficacy (the perceived ability to meet illness demands), illness acceptance, and social support and lower depression” and that this was associated with life satisfaction 6 months later.
Fatigue also has been shown to be a factor for patients with IBD. The researchers cited one population-based study showing fatigue in 57%-72% of IBD patients with active disease. IBD patients with quiescent disease also report fatigue. The psychological and behavioral factors driving fatigue could be related to mental disorders or other factors such as suboptimal sleep, stress, and use of caffeine and alcohol, they noted. Management strategies include improving sleep hygiene and evaluation of mental health concerns.
Seek complete picture before treatment
“Addressing psychological comorbidity in IBD requires individual and systemic approaches focused on both the prevention and treatment of mental health concerns,” the researchers wrote. “Because of the pervasiveness of psychological comorbidities in IBD, and recent evidence that they may be part of the disease process itself, assessment of psychological functioning in IBD is considered an essential aspect of disease management.”
Evidence-based psychological interventions include cognitive-behavioral therapy, which includes training in relaxation; treatment with clinical hypnosis; and encouraging mindfulness through acceptance and commitment therapy, which focuses on developing psychological flexibility to cope with suffering. In addition, a small but evolving body of research shows some benefit to motivational interviewing (a strategy focused on behavior change) for IBD patients. Notably, one review of four studies showed benefits of motivational interviewing for improving medication adherence and advice seeking, the researchers reported.
Although several psychological treatment options exist for addressing mental health issues in IBD, randomized trials are needed. “To facilitate this important research and optimize patient care, the integration of psychologists and other mental health providers into IBD care is considered best practice and provides exciting opportunities for improving patient care and outcomes,” the researchers concluded.
Address mental health to ease disease burden
“There is a large burden of mental health issues in patients with inflammatory bowel disease, with depression and anxiety leading the way,” Kim L. Isaacs, MD, of the University of North Carolina at Chapel Hill, said in an interview.
“There are multiple reasons for this, including dealing with chronic pain, social concerns around using the bathroom, body-image issues due to surgery, and drug side effects. There is increasing evidence that the inflammatory process in IBD may be driving some of the changes in the brain which lead to further mental health dysfunction,” she noted.
“Addressing depression, anxiety, [and] sleep disturbance in patients will not only improve quality of life from a mental health perspective but has been shown to improve control of disease,” Dr. Isaacs emphasized.
“Small things like increased medication compliance have a large impact on disease management and decreased need for hospitalization and hospitalization,” said Dr. Isaacs. “As gastroenterologists we need to expand our focus beyond the gut and address the emotional needs of our patients – identifying those patients who need increased mental health support.”
Barriers to better care
The greatest barriers to treating mental health issues in IBD patients are time and knowledge, said Dr. Isaacs. “Many gastroenterologists have limited time in the office to do more than address the acute issues of the patients such as rectal bleeding and worsening diarrhea. It takes time and trust to explore what is going on in a patient’s life. Is the patient anxious and depressed? How are they coping with their current disease manifestations? Simple screening tools may help with this, but then there need to be resources to support interventions.”
Some IBD practices, especially academic ones, have a psychologist in the IBD center or one that’s readily available for consultation. “This is an investment for the practice that may reduce significantly disease burden. The IBD specialty home model includes resources for management of psychiatric issues and nutritional concerns as well as disease management,” she added.
More research in several areas can help reduce the mental health burden of IBD. “On the immunology/biology side, understanding how the microbiome affects the brain/gut may allow for more directed mental health treatment. On the disease management side, larger trials directed at psychiatric interventions may help to determine which therapy is best for each patient,” Dr. Isaacs said. “Further work developing health care systems, such as the medical home, that allow for maximum disease management and decreased system costs will go far in implementation of models of care that address the needs of the entire patient with inflammatory bowel disease.”
The review received no outside funding. The researchers had no financial conflicts to disclose. Dr. Isaacs disclosed consulting on the data safety monitoring board for Janssen.
Psychological issues in patients with inflammatory bowel disease should be addressed at both personal and systemic levels, according to a review of current literature.
In a review published in the Journal of Clinical Gastroenterology, researchers highlighted data on the burden of mental disorders in inflammatory bowel disease (IBD) patients and presented several strategies for addressing them.
“From a systems perspective, underrecognized and/or suboptimally treated mental health problems in patients with IBD are associated with increased disability, poorer adherence, and more admissions and surgeries, driving increased health care utilization and costs,” Maia S. Kredentser, PhD, of the University of Manitoba, Winnipeg, and colleagues wrote, citing a 2018 study’s findings.
“There is ample evidence for a higher prevalence of mental disorders in IBD, in particular depression and anxiety, compared with the general population,” the authors wrote.
They cited a recent population-based study in which the incident rate ratios were significantly higher for IBD patients, compared with matched controls for depression (IRR, 1.58), anxiety disorder (IRR, 1.39), bipolar disorder (IRR, 1.82), and schizophrenia (IRR, 1.64).
Mental disorders associated with IBD also include issues of body image and sexuality. Although research on the impact of disease activity on sexual function is inconsistent, one study suggested that body image “may be an important target of treatment in women reporting poor quality of life and psychological distress,” the researchers noted. A French study from 2017 published in the Journal of Crohn’s and Colitis showed that approximately half of men and women reported problems with erectile or sexual dysfunction.
Issues related to environmental stressors may contribute to IBD by promoting chronic inflammation, the researchers wrote. For example, data from longitudinal, population-based research suggest that adverse childhood experiences can promote proinflammatory states across inflammatory illnesses. Research has also suggested that people with IBD have higher rates of these adverse childhood experiences than the general population. However, data also show that many are able to cope and adapt: “Many patients with IBD are resilient, experience growth, and in fact, thrive,” the researchers added. One longitudinal study suggested that patients with IBD who identified with “thriving” had “stronger coping efficacy (the perceived ability to meet illness demands), illness acceptance, and social support and lower depression” and that this was associated with life satisfaction 6 months later.
Fatigue also has been shown to be a factor for patients with IBD. The researchers cited one population-based study showing fatigue in 57%-72% of IBD patients with active disease. IBD patients with quiescent disease also report fatigue. The psychological and behavioral factors driving fatigue could be related to mental disorders or other factors such as suboptimal sleep, stress, and use of caffeine and alcohol, they noted. Management strategies include improving sleep hygiene and evaluation of mental health concerns.
Seek complete picture before treatment
“Addressing psychological comorbidity in IBD requires individual and systemic approaches focused on both the prevention and treatment of mental health concerns,” the researchers wrote. “Because of the pervasiveness of psychological comorbidities in IBD, and recent evidence that they may be part of the disease process itself, assessment of psychological functioning in IBD is considered an essential aspect of disease management.”
Evidence-based psychological interventions include cognitive-behavioral therapy, which includes training in relaxation; treatment with clinical hypnosis; and encouraging mindfulness through acceptance and commitment therapy, which focuses on developing psychological flexibility to cope with suffering. In addition, a small but evolving body of research shows some benefit to motivational interviewing (a strategy focused on behavior change) for IBD patients. Notably, one review of four studies showed benefits of motivational interviewing for improving medication adherence and advice seeking, the researchers reported.
Although several psychological treatment options exist for addressing mental health issues in IBD, randomized trials are needed. “To facilitate this important research and optimize patient care, the integration of psychologists and other mental health providers into IBD care is considered best practice and provides exciting opportunities for improving patient care and outcomes,” the researchers concluded.
Address mental health to ease disease burden
“There is a large burden of mental health issues in patients with inflammatory bowel disease, with depression and anxiety leading the way,” Kim L. Isaacs, MD, of the University of North Carolina at Chapel Hill, said in an interview.
“There are multiple reasons for this, including dealing with chronic pain, social concerns around using the bathroom, body-image issues due to surgery, and drug side effects. There is increasing evidence that the inflammatory process in IBD may be driving some of the changes in the brain which lead to further mental health dysfunction,” she noted.
“Addressing depression, anxiety, [and] sleep disturbance in patients will not only improve quality of life from a mental health perspective but has been shown to improve control of disease,” Dr. Isaacs emphasized.
“Small things like increased medication compliance have a large impact on disease management and decreased need for hospitalization and hospitalization,” said Dr. Isaacs. “As gastroenterologists we need to expand our focus beyond the gut and address the emotional needs of our patients – identifying those patients who need increased mental health support.”
Barriers to better care
The greatest barriers to treating mental health issues in IBD patients are time and knowledge, said Dr. Isaacs. “Many gastroenterologists have limited time in the office to do more than address the acute issues of the patients such as rectal bleeding and worsening diarrhea. It takes time and trust to explore what is going on in a patient’s life. Is the patient anxious and depressed? How are they coping with their current disease manifestations? Simple screening tools may help with this, but then there need to be resources to support interventions.”
Some IBD practices, especially academic ones, have a psychologist in the IBD center or one that’s readily available for consultation. “This is an investment for the practice that may reduce significantly disease burden. The IBD specialty home model includes resources for management of psychiatric issues and nutritional concerns as well as disease management,” she added.
More research in several areas can help reduce the mental health burden of IBD. “On the immunology/biology side, understanding how the microbiome affects the brain/gut may allow for more directed mental health treatment. On the disease management side, larger trials directed at psychiatric interventions may help to determine which therapy is best for each patient,” Dr. Isaacs said. “Further work developing health care systems, such as the medical home, that allow for maximum disease management and decreased system costs will go far in implementation of models of care that address the needs of the entire patient with inflammatory bowel disease.”
The review received no outside funding. The researchers had no financial conflicts to disclose. Dr. Isaacs disclosed consulting on the data safety monitoring board for Janssen.
FROM THE JOURNAL OF CLINICAL GASTROENTEROLOGY
New ‘minimal monitoring’ approach to HCV treatment may simplify care
A novel minimal monitoring (MINMON) approach to hepatitis C virus (HCV) treatment was safe and achieved sustained virology response (SVR) compared to current clinical standards in treatment-naive patients without evidence of decompensated cirrhosis, according to a recent study.
“This model may allow for HCV elimination, while minimizing resource use and face-to-face contact,” said investigator Sunil S. Solomon, MBBS, PhD, of Johns Hopkins University in Baltimore. “The COVID-19 pandemic has highlighted the urgent need for simple and safe models of HCV [care] delivery.”
Dr. Solomon described the new approach to HCV treatment during a presentation at this year’s Conference on Retroviruses and Opportunistic Infections virtual meeting.
Study design
ACTG A5360 was an international, single-arm, open-label, phase 4 trial that enrolled 400 patients across 38 treatment sites.
The researchers evaluated the efficacy and safety of the MINMON approach in treatment-naive individuals who had no evidence of decompensated cirrhosis. Study participants received a fixed-dose, single-tablet regimen of sofosbuvir 400 mg/velpatasvir 100 mg once daily for 12 weeks.
The MINMON approach comprised four key elements: no pretreatment genotyping, all tablets dispensed at study entry, no scheduled on-treatment clinic visits/labs, and two remote contacts at weeks 4 (adherence evaluation) and 22 (scheduled SVR visit). Unplanned visits for patients concerns were permitted.
Key eligibility criteria included active HCV infection (HCV RNA > 1,000 IU/mL) and no prior HCV treatment history. Persons with HIV coinfection (50% or less of sample) and compensated cirrhosis (20% or less of sample) were also eligible. Persons with chronic hepatitis B virus (HBV) infection and decompensated cirrhosis were excluded.
The primary efficacy endpoint was SVR, defined as HCV RNA less than the lower limit of quantification in the first sample at least 22 weeks post treatment initiation. The primary safety endpoint was any serious adverse events (AEs) occurring between treatment initiation and week 28.
Results
Among 400 patients enrolled, 399 (99.8%) were included in the primary efficacy analysis and 397 (99.3%) were included in the safety analysis. The median age of participants was 47 years, and 35% were female sex at birth. At baseline, 166 (42%) patients had HIV coinfection and 34 (9%) had compensated cirrhosis.
After analysis, the researchers found that remote contact was successful at weeks 4 and 22 for 394 (98.7%) and 335 (84.0%) participants, respectively.
In total, 15 (3.8%) participants recorded 21 unplanned visits, 3 (14.3%) of which were due to AEs, none of which were treatment related. Three participants reported losing study medications and one participant prematurely discontinued therapy due to an AE.
HCV RNA data at SVR were available for 396 participants. Overall, 379 patients (95.0%) achieved SVR (95% confidence interval [CI], 92.4%-96.7%).
“The study was not powered for SVR by subgroups, which explains why we observed wide confidence intervals in our forest plot,” Dr. Solomon said.
With respect to safety, serious AEs were reported in 14 (3.5%) participants through week 24 visit, none of which were treatment related or resulted in death.
Dr. Solomon acknowledged that a key limitation of the study was the single-arm design. As a result, there was no direct comparison to standard monitoring practices. In addition, these results may not be generalizable to all nonresearch treatment sites.
“The COVID-19 pandemic has required us to pivot clinical programs to minimize in-person contact, and promote more remote approaches, which is really the essence of the MINMON approach,” Dr. Solomon explained.
“There are really wonderful results in the population that was studied, but may reflect a more adherent patient population,” said moderator Robert T. Schooley, MD, of the University of California, San Diego.
During a discussion, Dr. Solomon noted that the MINMON approach may be further explored in patients who are actively injecting drugs, as these patients were not well represented in the present study.
Dr. Solomon disclosed financial relationships with Gilead Sciences and Abbott Diagnostics. The study was funded by the National Institutes of Health and Gilead Sciences.
A novel minimal monitoring (MINMON) approach to hepatitis C virus (HCV) treatment was safe and achieved sustained virology response (SVR) compared to current clinical standards in treatment-naive patients without evidence of decompensated cirrhosis, according to a recent study.
“This model may allow for HCV elimination, while minimizing resource use and face-to-face contact,” said investigator Sunil S. Solomon, MBBS, PhD, of Johns Hopkins University in Baltimore. “The COVID-19 pandemic has highlighted the urgent need for simple and safe models of HCV [care] delivery.”
Dr. Solomon described the new approach to HCV treatment during a presentation at this year’s Conference on Retroviruses and Opportunistic Infections virtual meeting.
Study design
ACTG A5360 was an international, single-arm, open-label, phase 4 trial that enrolled 400 patients across 38 treatment sites.
The researchers evaluated the efficacy and safety of the MINMON approach in treatment-naive individuals who had no evidence of decompensated cirrhosis. Study participants received a fixed-dose, single-tablet regimen of sofosbuvir 400 mg/velpatasvir 100 mg once daily for 12 weeks.
The MINMON approach comprised four key elements: no pretreatment genotyping, all tablets dispensed at study entry, no scheduled on-treatment clinic visits/labs, and two remote contacts at weeks 4 (adherence evaluation) and 22 (scheduled SVR visit). Unplanned visits for patients concerns were permitted.
Key eligibility criteria included active HCV infection (HCV RNA > 1,000 IU/mL) and no prior HCV treatment history. Persons with HIV coinfection (50% or less of sample) and compensated cirrhosis (20% or less of sample) were also eligible. Persons with chronic hepatitis B virus (HBV) infection and decompensated cirrhosis were excluded.
The primary efficacy endpoint was SVR, defined as HCV RNA less than the lower limit of quantification in the first sample at least 22 weeks post treatment initiation. The primary safety endpoint was any serious adverse events (AEs) occurring between treatment initiation and week 28.
Results
Among 400 patients enrolled, 399 (99.8%) were included in the primary efficacy analysis and 397 (99.3%) were included in the safety analysis. The median age of participants was 47 years, and 35% were female sex at birth. At baseline, 166 (42%) patients had HIV coinfection and 34 (9%) had compensated cirrhosis.
After analysis, the researchers found that remote contact was successful at weeks 4 and 22 for 394 (98.7%) and 335 (84.0%) participants, respectively.
In total, 15 (3.8%) participants recorded 21 unplanned visits, 3 (14.3%) of which were due to AEs, none of which were treatment related. Three participants reported losing study medications and one participant prematurely discontinued therapy due to an AE.
HCV RNA data at SVR were available for 396 participants. Overall, 379 patients (95.0%) achieved SVR (95% confidence interval [CI], 92.4%-96.7%).
“The study was not powered for SVR by subgroups, which explains why we observed wide confidence intervals in our forest plot,” Dr. Solomon said.
With respect to safety, serious AEs were reported in 14 (3.5%) participants through week 24 visit, none of which were treatment related or resulted in death.
Dr. Solomon acknowledged that a key limitation of the study was the single-arm design. As a result, there was no direct comparison to standard monitoring practices. In addition, these results may not be generalizable to all nonresearch treatment sites.
“The COVID-19 pandemic has required us to pivot clinical programs to minimize in-person contact, and promote more remote approaches, which is really the essence of the MINMON approach,” Dr. Solomon explained.
“There are really wonderful results in the population that was studied, but may reflect a more adherent patient population,” said moderator Robert T. Schooley, MD, of the University of California, San Diego.
During a discussion, Dr. Solomon noted that the MINMON approach may be further explored in patients who are actively injecting drugs, as these patients were not well represented in the present study.
Dr. Solomon disclosed financial relationships with Gilead Sciences and Abbott Diagnostics. The study was funded by the National Institutes of Health and Gilead Sciences.
A novel minimal monitoring (MINMON) approach to hepatitis C virus (HCV) treatment was safe and achieved sustained virology response (SVR) compared to current clinical standards in treatment-naive patients without evidence of decompensated cirrhosis, according to a recent study.
“This model may allow for HCV elimination, while minimizing resource use and face-to-face contact,” said investigator Sunil S. Solomon, MBBS, PhD, of Johns Hopkins University in Baltimore. “The COVID-19 pandemic has highlighted the urgent need for simple and safe models of HCV [care] delivery.”
Dr. Solomon described the new approach to HCV treatment during a presentation at this year’s Conference on Retroviruses and Opportunistic Infections virtual meeting.
Study design
ACTG A5360 was an international, single-arm, open-label, phase 4 trial that enrolled 400 patients across 38 treatment sites.
The researchers evaluated the efficacy and safety of the MINMON approach in treatment-naive individuals who had no evidence of decompensated cirrhosis. Study participants received a fixed-dose, single-tablet regimen of sofosbuvir 400 mg/velpatasvir 100 mg once daily for 12 weeks.
The MINMON approach comprised four key elements: no pretreatment genotyping, all tablets dispensed at study entry, no scheduled on-treatment clinic visits/labs, and two remote contacts at weeks 4 (adherence evaluation) and 22 (scheduled SVR visit). Unplanned visits for patients concerns were permitted.
Key eligibility criteria included active HCV infection (HCV RNA > 1,000 IU/mL) and no prior HCV treatment history. Persons with HIV coinfection (50% or less of sample) and compensated cirrhosis (20% or less of sample) were also eligible. Persons with chronic hepatitis B virus (HBV) infection and decompensated cirrhosis were excluded.
The primary efficacy endpoint was SVR, defined as HCV RNA less than the lower limit of quantification in the first sample at least 22 weeks post treatment initiation. The primary safety endpoint was any serious adverse events (AEs) occurring between treatment initiation and week 28.
Results
Among 400 patients enrolled, 399 (99.8%) were included in the primary efficacy analysis and 397 (99.3%) were included in the safety analysis. The median age of participants was 47 years, and 35% were female sex at birth. At baseline, 166 (42%) patients had HIV coinfection and 34 (9%) had compensated cirrhosis.
After analysis, the researchers found that remote contact was successful at weeks 4 and 22 for 394 (98.7%) and 335 (84.0%) participants, respectively.
In total, 15 (3.8%) participants recorded 21 unplanned visits, 3 (14.3%) of which were due to AEs, none of which were treatment related. Three participants reported losing study medications and one participant prematurely discontinued therapy due to an AE.
HCV RNA data at SVR were available for 396 participants. Overall, 379 patients (95.0%) achieved SVR (95% confidence interval [CI], 92.4%-96.7%).
“The study was not powered for SVR by subgroups, which explains why we observed wide confidence intervals in our forest plot,” Dr. Solomon said.
With respect to safety, serious AEs were reported in 14 (3.5%) participants through week 24 visit, none of which were treatment related or resulted in death.
Dr. Solomon acknowledged that a key limitation of the study was the single-arm design. As a result, there was no direct comparison to standard monitoring practices. In addition, these results may not be generalizable to all nonresearch treatment sites.
“The COVID-19 pandemic has required us to pivot clinical programs to minimize in-person contact, and promote more remote approaches, which is really the essence of the MINMON approach,” Dr. Solomon explained.
“There are really wonderful results in the population that was studied, but may reflect a more adherent patient population,” said moderator Robert T. Schooley, MD, of the University of California, San Diego.
During a discussion, Dr. Solomon noted that the MINMON approach may be further explored in patients who are actively injecting drugs, as these patients were not well represented in the present study.
Dr. Solomon disclosed financial relationships with Gilead Sciences and Abbott Diagnostics. The study was funded by the National Institutes of Health and Gilead Sciences.
FROM CROI 2021
Nearly 20% of lupus patients have severe infection in first decade after diagnosis
People with systemic lupus erythematosus (SLE) experienced significantly higher rates of first severe infections, a higher number of severe infections overall, and greater infection-related mortality, compared with controls, based on data from a population-based cohort study of more than 30,000 individuals.
Infections remain a leading cause of morbidity and early mortality in patients with SLE, wrote Kai Zhao, MSc, of Arthritis Research Canada, Richmond, and colleagues. However, “limitations from existing studies including selected samples, small sizes, and prevalent cohorts can negatively affect the accuracy of both the absolute and relative risk estimates of infections in SLE at the population level,” they said.
In a study published in Rheumatology, the researchers identified 5,169 people newly diagnosed with SLE between Jan. 1, 1997, and March 31, 2015, and matched them with 25,845 non-SLE controls using an administrative health database of all health care services funded in British Columbia during the time period. The investigators said the study is the first “to evaluate the risk of severe infections in a large population-based and incident SLE cohort.”
The average age of the patients was 46.9 at the time of their index SLE diagnosis, and 86% were women. The average follow-up period was approximately 10 years.
The primary outcome was the first severe infection after the onset of SLE that required hospitalization or occurred in the hospital setting. A total of 955 (18.5%) first severe infections occurred in the SLE group, compared with 1,988 (7.7%) in the controls, for incidence rates of 19.7 events per 1,000 person-years and 7.6 events per 1,000 person-years, respectively, yielding an 82% increased risk of severe infection for SLE patients after adjustment for confounding baseline factors.
Secondary outcomes of the total number of severe infections and infection-related mortality both showed significant increases in SLE patients, compared with controls. The total number of severe infections in the SLE and control groups was 1,898 and 3,114, respectively, with an adjusted risk ratio of 2.07.
As for mortality, a total of 539 deaths occurred in SLE patients during the study period, and 114 (21%) were related to severe infection. A total of 1,495 deaths occurred in the control group, including 269 (18%) related to severe infection. The adjusted hazard ratio was 1.61 after adjustment for confounding baseline variables.
The risks for first severe infection, total number of severe infections, and infection-related mortality were “independent of traditional risk factors for infection and the results remain robust in the presence of an unmeasured confounder (smoking) and competing risk of death,” the researchers said. Reasons for the increased risk are uncertain, but likely result from intrinsic factors such as immune system dysfunction and extrinsic factors such as the impact of immunosuppressive medications. “Future research can focus on quantifying the relative contributions of these intrinsic and extrinsic factors on the increased infection risk in SLE patients,” they added.
The study findings were limited by several factors linked to the observational design, including possible misdiagnosis of SLE and inaccurate measure of SLE onset, the researchers noted. In addition, no data were available for certain confounders such as smoking and nonhospitalized infections, they said.
However, the results were strengthened by the large size and general population and the use of sensitivity analyses, they noted. For SLE patients, “increased awareness of the risk of infections can identify their early signs and potentially prevent hospitalizations,” and clinicians can promote infection prevention strategies, including vaccinations when appropriate, they added.
Based on their findings, “we recommend a closer surveillance for severe infections in SLE patients and risk assessment for severe infections for SLE patients after diagnosis,” the researchers emphasized. “Further studies are warranted to further identify risk factors for infections in SLE patients to develop personalized treatment regimens and to select treatment in practice by synthesizing patient information,” they concluded.
The study was supported by the Canadian Institutes for Health Research. The researchers had no financial conflicts to disclose.
People with systemic lupus erythematosus (SLE) experienced significantly higher rates of first severe infections, a higher number of severe infections overall, and greater infection-related mortality, compared with controls, based on data from a population-based cohort study of more than 30,000 individuals.
Infections remain a leading cause of morbidity and early mortality in patients with SLE, wrote Kai Zhao, MSc, of Arthritis Research Canada, Richmond, and colleagues. However, “limitations from existing studies including selected samples, small sizes, and prevalent cohorts can negatively affect the accuracy of both the absolute and relative risk estimates of infections in SLE at the population level,” they said.
In a study published in Rheumatology, the researchers identified 5,169 people newly diagnosed with SLE between Jan. 1, 1997, and March 31, 2015, and matched them with 25,845 non-SLE controls using an administrative health database of all health care services funded in British Columbia during the time period. The investigators said the study is the first “to evaluate the risk of severe infections in a large population-based and incident SLE cohort.”
The average age of the patients was 46.9 at the time of their index SLE diagnosis, and 86% were women. The average follow-up period was approximately 10 years.
The primary outcome was the first severe infection after the onset of SLE that required hospitalization or occurred in the hospital setting. A total of 955 (18.5%) first severe infections occurred in the SLE group, compared with 1,988 (7.7%) in the controls, for incidence rates of 19.7 events per 1,000 person-years and 7.6 events per 1,000 person-years, respectively, yielding an 82% increased risk of severe infection for SLE patients after adjustment for confounding baseline factors.
Secondary outcomes of the total number of severe infections and infection-related mortality both showed significant increases in SLE patients, compared with controls. The total number of severe infections in the SLE and control groups was 1,898 and 3,114, respectively, with an adjusted risk ratio of 2.07.
As for mortality, a total of 539 deaths occurred in SLE patients during the study period, and 114 (21%) were related to severe infection. A total of 1,495 deaths occurred in the control group, including 269 (18%) related to severe infection. The adjusted hazard ratio was 1.61 after adjustment for confounding baseline variables.
The risks for first severe infection, total number of severe infections, and infection-related mortality were “independent of traditional risk factors for infection and the results remain robust in the presence of an unmeasured confounder (smoking) and competing risk of death,” the researchers said. Reasons for the increased risk are uncertain, but likely result from intrinsic factors such as immune system dysfunction and extrinsic factors such as the impact of immunosuppressive medications. “Future research can focus on quantifying the relative contributions of these intrinsic and extrinsic factors on the increased infection risk in SLE patients,” they added.
The study findings were limited by several factors linked to the observational design, including possible misdiagnosis of SLE and inaccurate measure of SLE onset, the researchers noted. In addition, no data were available for certain confounders such as smoking and nonhospitalized infections, they said.
However, the results were strengthened by the large size and general population and the use of sensitivity analyses, they noted. For SLE patients, “increased awareness of the risk of infections can identify their early signs and potentially prevent hospitalizations,” and clinicians can promote infection prevention strategies, including vaccinations when appropriate, they added.
Based on their findings, “we recommend a closer surveillance for severe infections in SLE patients and risk assessment for severe infections for SLE patients after diagnosis,” the researchers emphasized. “Further studies are warranted to further identify risk factors for infections in SLE patients to develop personalized treatment regimens and to select treatment in practice by synthesizing patient information,” they concluded.
The study was supported by the Canadian Institutes for Health Research. The researchers had no financial conflicts to disclose.
People with systemic lupus erythematosus (SLE) experienced significantly higher rates of first severe infections, a higher number of severe infections overall, and greater infection-related mortality, compared with controls, based on data from a population-based cohort study of more than 30,000 individuals.
Infections remain a leading cause of morbidity and early mortality in patients with SLE, wrote Kai Zhao, MSc, of Arthritis Research Canada, Richmond, and colleagues. However, “limitations from existing studies including selected samples, small sizes, and prevalent cohorts can negatively affect the accuracy of both the absolute and relative risk estimates of infections in SLE at the population level,” they said.
In a study published in Rheumatology, the researchers identified 5,169 people newly diagnosed with SLE between Jan. 1, 1997, and March 31, 2015, and matched them with 25,845 non-SLE controls using an administrative health database of all health care services funded in British Columbia during the time period. The investigators said the study is the first “to evaluate the risk of severe infections in a large population-based and incident SLE cohort.”
The average age of the patients was 46.9 at the time of their index SLE diagnosis, and 86% were women. The average follow-up period was approximately 10 years.
The primary outcome was the first severe infection after the onset of SLE that required hospitalization or occurred in the hospital setting. A total of 955 (18.5%) first severe infections occurred in the SLE group, compared with 1,988 (7.7%) in the controls, for incidence rates of 19.7 events per 1,000 person-years and 7.6 events per 1,000 person-years, respectively, yielding an 82% increased risk of severe infection for SLE patients after adjustment for confounding baseline factors.
Secondary outcomes of the total number of severe infections and infection-related mortality both showed significant increases in SLE patients, compared with controls. The total number of severe infections in the SLE and control groups was 1,898 and 3,114, respectively, with an adjusted risk ratio of 2.07.
As for mortality, a total of 539 deaths occurred in SLE patients during the study period, and 114 (21%) were related to severe infection. A total of 1,495 deaths occurred in the control group, including 269 (18%) related to severe infection. The adjusted hazard ratio was 1.61 after adjustment for confounding baseline variables.
The risks for first severe infection, total number of severe infections, and infection-related mortality were “independent of traditional risk factors for infection and the results remain robust in the presence of an unmeasured confounder (smoking) and competing risk of death,” the researchers said. Reasons for the increased risk are uncertain, but likely result from intrinsic factors such as immune system dysfunction and extrinsic factors such as the impact of immunosuppressive medications. “Future research can focus on quantifying the relative contributions of these intrinsic and extrinsic factors on the increased infection risk in SLE patients,” they added.
The study findings were limited by several factors linked to the observational design, including possible misdiagnosis of SLE and inaccurate measure of SLE onset, the researchers noted. In addition, no data were available for certain confounders such as smoking and nonhospitalized infections, they said.
However, the results were strengthened by the large size and general population and the use of sensitivity analyses, they noted. For SLE patients, “increased awareness of the risk of infections can identify their early signs and potentially prevent hospitalizations,” and clinicians can promote infection prevention strategies, including vaccinations when appropriate, they added.
Based on their findings, “we recommend a closer surveillance for severe infections in SLE patients and risk assessment for severe infections for SLE patients after diagnosis,” the researchers emphasized. “Further studies are warranted to further identify risk factors for infections in SLE patients to develop personalized treatment regimens and to select treatment in practice by synthesizing patient information,” they concluded.
The study was supported by the Canadian Institutes for Health Research. The researchers had no financial conflicts to disclose.
FROM RHEUMATOLOGY
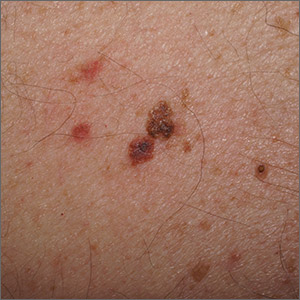
Outlier lesion on the back
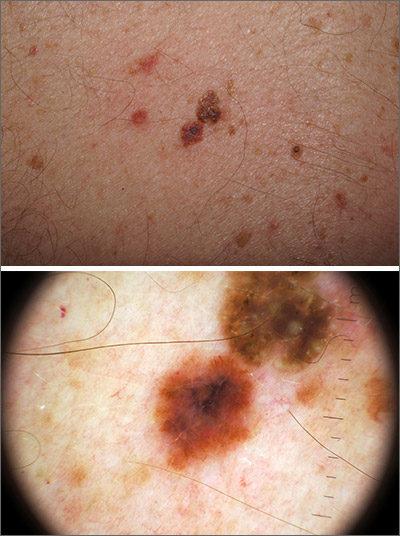
In addition to the patient’s SK, the second finding was diagnosed as a thin melanoma. The clinical appearance of SKs and nevi or melanoma can overlap. Dermoscopy is a helpful tool in distinguishing between them, even when juxtaposed in a collision lesion such as this.1
Dermoscopy of the superior portion of the lesion demonstrated a well-demarcated brown, waxy papule with milia-like cysts, consistent with an SK. Inferiorly, the dermoscopic features included atypical pigment network, asymmetrical streaks, and blue-white veil, suggestive of melanoma or an atypical melanocytic neoplasm. A deep-shave biopsy was performed of the lower section, aiming for a narrow margin (1-3 mm) of normal skin. The biopsy confirmed a superficial spreading melanoma with a Breslow depth of 0.5 mm with 0 mitoses per high-power field.
A deep-shave biopsy was chosen over a punch biopsy because the latter would be unlikely to sample the entire lesion.
One month after the initial biopsy, a wide local excision with a 1-cm margin was performed. The planned follow-up for the patient was skin exams every 3 months for the first year, every 6 months for the next 4 years, and then annually for life.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Blum A, Siggs G, Marghoob AA, et al. Collision skin lesions-results of a multicenter study of the International Dermoscopy Society (IDS). Dermatol Pract Concept. 2017;7:51-62. doi:10.5826/dpc.0704a12

In addition to the patient’s SK, the second finding was diagnosed as a thin melanoma. The clinical appearance of SKs and nevi or melanoma can overlap. Dermoscopy is a helpful tool in distinguishing between them, even when juxtaposed in a collision lesion such as this.1
Dermoscopy of the superior portion of the lesion demonstrated a well-demarcated brown, waxy papule with milia-like cysts, consistent with an SK. Inferiorly, the dermoscopic features included atypical pigment network, asymmetrical streaks, and blue-white veil, suggestive of melanoma or an atypical melanocytic neoplasm. A deep-shave biopsy was performed of the lower section, aiming for a narrow margin (1-3 mm) of normal skin. The biopsy confirmed a superficial spreading melanoma with a Breslow depth of 0.5 mm with 0 mitoses per high-power field.
A deep-shave biopsy was chosen over a punch biopsy because the latter would be unlikely to sample the entire lesion.
One month after the initial biopsy, a wide local excision with a 1-cm margin was performed. The planned follow-up for the patient was skin exams every 3 months for the first year, every 6 months for the next 4 years, and then annually for life.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).

In addition to the patient’s SK, the second finding was diagnosed as a thin melanoma. The clinical appearance of SKs and nevi or melanoma can overlap. Dermoscopy is a helpful tool in distinguishing between them, even when juxtaposed in a collision lesion such as this.1
Dermoscopy of the superior portion of the lesion demonstrated a well-demarcated brown, waxy papule with milia-like cysts, consistent with an SK. Inferiorly, the dermoscopic features included atypical pigment network, asymmetrical streaks, and blue-white veil, suggestive of melanoma or an atypical melanocytic neoplasm. A deep-shave biopsy was performed of the lower section, aiming for a narrow margin (1-3 mm) of normal skin. The biopsy confirmed a superficial spreading melanoma with a Breslow depth of 0.5 mm with 0 mitoses per high-power field.
A deep-shave biopsy was chosen over a punch biopsy because the latter would be unlikely to sample the entire lesion.
One month after the initial biopsy, a wide local excision with a 1-cm margin was performed. The planned follow-up for the patient was skin exams every 3 months for the first year, every 6 months for the next 4 years, and then annually for life.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Blum A, Siggs G, Marghoob AA, et al. Collision skin lesions-results of a multicenter study of the International Dermoscopy Society (IDS). Dermatol Pract Concept. 2017;7:51-62. doi:10.5826/dpc.0704a12
1. Blum A, Siggs G, Marghoob AA, et al. Collision skin lesions-results of a multicenter study of the International Dermoscopy Society (IDS). Dermatol Pract Concept. 2017;7:51-62. doi:10.5826/dpc.0704a12
Microaggressions, racism, and antiracism: The role of gastroenterology
On a busy call day, Oviea (a second-year gastroenterology fellow), paused in the hallway to listen to a conversation between an endoscopy nurse and a patient. The nurse was requesting the patient’s permission for a gastroenterology fellow to participate in their care and the patient, well acquainted with the role from prior procedures, immediately agreed. Oviea entered the patient’s room, introduced himself as “Dr. Akpotaire, the gastroenterology fellow,” as he had with hundreds of other patients during his fellowship, and completed the informed consent. The interaction was brief but pleasant. As Oviea was leaving the room, the patient asked: “When will I meet the doctor”?
This question was familiar to Oviea. Despite always introducing himself by title and wearing matching identification, many patients had dismissed his credentials since graduating from medical school. His answer was equally familiar: “I am a doctor, and Dr. X, the supervising physician, will meet you soon.” With the patient seemingly placated, Oviea delivered the consent form to the procedure room. Minutes later, he was surprised to learn that the patient specifically requested that he not be allowed to participate in their care. This in combination with the patient’s initial dismissal of Oviea’s credentials, left a sting. While none of the other team members outwardly questioned the reason for the patient’s change of heart, Oviea continued to wonder if the patient’s decision was because of his race.
Beyond gastroenterology, similar experiences are common in other spheres. The Twitter thread #BlackintheIvory recounts stories of microaggressions and structural racism in medicine and academia. The cumulative toll of these experiences leads to departures of Black physicians including Uché Blackstock, MD;1 Aysha Khoury, MD, MPH;2 Ben Danielson, MD;3 Princess Dennar, MD;4 and others.
Microaggressions as proxy for bias
The term microaggression was coined by Chester Pierce, MD, the first Black tenured professor at Massachusetts General Hospital in the 1970’s, to describe the frequent, yet subtle dismissals Black Americans experienced in society. Over time, the term has been expanded to include “brief and commonplace daily verbal, behavioral, or environmental indignities, intentional or unintentional, that communicate hostile, derogatory, or negative slights and insults” to any marginalized group.5
While the term microaggressions is useful in contextualizing individual experiences, it narrowly focuses on conscious or unconscious interpersonal prejudices. In medicine, this misdirects attention away from the policies and practices that create and reinforce prejudices; these policies and practices do so by systematically excluding underrepresented minority (URM) physicians,6 defined by the American Association of Medical Colleges as physicians who are Black, Hispanic, Native Americans, and Alaska Natives,7 from the medical workforce. Ultimately, this leads to and exacerbates poor health outcomes for racial and ethnic minority patients.
Microaggressions represent our society’s deepest and oldest biases and are rooted in structural racism, as well as misogyny, homophobia, transphobia, xenophobia, ableism, and other prejudices.8 For URM physicians, experiences like the example above are frequently caused by structural racism.
Structural racism in medicine
Structural racism refers to the policies, practices, cultural representations, and norms that reinforce inequities by providing privileges to White people at the disadvantage of non-White people.9 In 1910, Abraham Flexner, commissioned by the Carnegie Foundation and the American Medical Association, wrote that African American physicians should be trained in hygiene rather than surgery and should primarily serve as “sanitarians” whose purpose was to “protect Whites” from common diseases like tuberculosis.10 The 1910 Flexner Report also emphasized the importance of prerequisite basic sciences education and recommended that only two of the seven existing Black medical schools remain open because Flexner believed that only these schools had the potential to meet the new requirements for medical education.11 A recent analysis found that, had the other five medical schools affiliated with historically Black colleges and universities remained open, this would have resulted in an additional 33,315 Black medical school graduates by 2019.12 Structural racism explains why the majority of practicing physicians, medical educators, National Institutes of Health–funded researchers, and hospital executives are White and, similarly, why White patients are overrepresented in clinical trials, have better health outcomes, and live longer lives than several racial and ethnic minority groups.13
The murders of Ahmaud Arbery, Breonna Taylor, and George Floyd and the inequitable toll of the COVID-19 pandemic on Black, Hispanic, and Native American people renewed the dialogue regarding structural racism in America. Beyond criminal justice and police reform, the current social justice movement demands that structural racism is examined in all spheres. In medicine and health care, acknowledging the history of exclusion and exploitation of Black people and other URM groups is an important first step, but this must be followed by a commitment to an antiracist future for the benefit of all medical professionals and patients.14,15
Antiracism as a path forward
Antiracism refers to actions and policies that seek to dismantle structural racism. While individuals can and should engage in antiracist actions, it is equally important for organizations and government to actively participate in this process as well.
Individual and interpersonal levels
Gastroenterologists should advocate an end to racist practices within their organizations (e.g., unjustified use of race-based corrections in diagnostic algorithms and practice guidelines),16 and interrupt microaggressions and racist actions in real time (e.g., overpolicing of underrepresented groups in health care settings).17 Gastroenterologists from underrepresented groups may also need to unlearn internalized racism, which is defined as acceptance by members of disadvantaged races of the negative messages about their own abilities and intrinsic worth.18
Organizational level
Gastroenterology divisions and practices must ensure that the entire workforce, including leadership, reflects the diversity of our country. Underrepresented groups represent 33% of the U.S. population, but only 9.1% of gastroenterology fellows and 10% of gastroenterology faculty are from underrepresented groups.19 In addition to diversifying the field of gastroenterology through financial and operational support of pipeline educational programs, organizations should also promote the scholarship of URM groups, whose work is often undervalued, and redistribute power by elevating voices that have been historically absent.20 Gastroenterology practices should also collect high-quality patient data disaggregated by demographic factors. Doing so will enable rapid identification of disparate health outcomes by demographic variables and inform interventions to eliminate identified disparities.
Government level
The “Executive Order On Advancing Racial Equity and Support for Underserved Communities Through the Federal Government” issued by President Biden on Jan. 20, 2021, is an example of how government can promote antiracism.21 The executive order states that domestic policies cause group inequities and calls for the removal of systemic barriers in current and future domestic policies. The executive order outlines several additional ways to improve equity in current and future policy, including engagement, consultation, and coordination with members of underserved communities. The details outlined in the executive order should serve as the foundation for establishing new standards at the state, county, and city levels as well. Gastroenterologists can influence government by voting for officials at all levels that support and promote these standards.
Conclusion
Beyond calling out microaggressions in real time, we must also interrogate the biases, policies, and practices that support them in medicine and beyond. As Black gastroenterologists who have experienced microaggressions and overt acts of racism, we ground Oviea’s experience in structural racism and offer strategies that individuals, organizations, and governing institutions can adopt toward an antiracist future. This model can be applied to experiences rooted in misogyny, homophobia, transphobia, xenophobia, ableism, and other prejudices.
As a nation, we must make an active and collective choice to address structural racism. In health care, doing so will strengthen communities, enhance the lived experiences of URM physician colleagues, and save patient lives. Gastroenterologists, as trusted health care providers, are uniquely positioned to lead the way.
Dr. Akpotaire is a second-year GI fellow in the division of gastroenterology at the University of Washington, Seattle. Dr. Issaka is an assistant professor with both the Fred Hutchinson Cancer Research Center, Seattle, and the division of gastroenterology at the University of Washington.
References
1. Blackstock U. “Why Black doctors like me are leaving faculty positions in academic medical centers.” STAT News, 2020.
2. Asare JG. “One Doctor Shares Her Story of Racism in Medicine.” Forbes. 2021 Feb 1.
3. Kroman D. “Revered doctor steps down, accusing Seattle Children’s Hospital of racism.” Crosscut. 2020 Dec 31.
4. United States District Court Eastern District of Louisiana. Princess Dennar, M.D. v. The Administrators of the Tulane Educational Fund, 2020.
5. Sue DW. Microaggressions in Everyday Life: Race, Gender, and Sexual Orientation. Hoboken, N.J.: Wiley, 2010.
6. Boyd RW. Lancet. 2019 Jun 22;393(10190):2484-5.
7. AAMC. Diversity in Medicine Facts and Figures 2019. Washington, D.C., 2019.
8. Overland MK et al. PM R. 2019 Sep;11(9):1004-12.
9. Jones CP. Ethn Dis. 2018 Aug 9;28(Suppl 1):231-4.
10. Hlavinka E. “Racial Bias in Flexner Report Permeates Medical Education Today.” Medpage Today. 2020 Jun 18.
11. Flexner A. Medical Education in the United States and Canada. New York: 1910. Republished: Bull World Health Organ. 2002;80(7):594-602.
12. Campbell KM et al. JAMA Netw Open. 2020 Aug 3;3(8):e2015220.
13. Malat J et al. Soc Sci Med. 2018 Feb;199:148-56.
14. Kendi IX. How to be an antiracist. New York: Random House Books, 2019.
15. Gray DM 2nd et al. Nat Rev Gastroenterol Hepatol. 2020 Oct;17(10):589-90.
16. Vyas DA et al. N Engl J Med. 2020 Aug 27;383(9):874-82.
17. Green CR et al. J Natl Med Assoc. 2018 Feb;110(1):37-43.
18. Jones CP. Am J Public Health. 2000 Aug;90(8):1212-5.
19. Anyane-Yeboa A et al. Am J Gastroenterol. 2020 Aug;115(8):1147-9.
20. Issaka RB. JAMA. 2020 Aug 11;324(6):556-7.
21. Biden JR. Executive Order On Advancing Racial Equity and Support for Underserved Communities Through the Federal Government. Washington, D.C.: The White House, 2021.
On a busy call day, Oviea (a second-year gastroenterology fellow), paused in the hallway to listen to a conversation between an endoscopy nurse and a patient. The nurse was requesting the patient’s permission for a gastroenterology fellow to participate in their care and the patient, well acquainted with the role from prior procedures, immediately agreed. Oviea entered the patient’s room, introduced himself as “Dr. Akpotaire, the gastroenterology fellow,” as he had with hundreds of other patients during his fellowship, and completed the informed consent. The interaction was brief but pleasant. As Oviea was leaving the room, the patient asked: “When will I meet the doctor”?
This question was familiar to Oviea. Despite always introducing himself by title and wearing matching identification, many patients had dismissed his credentials since graduating from medical school. His answer was equally familiar: “I am a doctor, and Dr. X, the supervising physician, will meet you soon.” With the patient seemingly placated, Oviea delivered the consent form to the procedure room. Minutes later, he was surprised to learn that the patient specifically requested that he not be allowed to participate in their care. This in combination with the patient’s initial dismissal of Oviea’s credentials, left a sting. While none of the other team members outwardly questioned the reason for the patient’s change of heart, Oviea continued to wonder if the patient’s decision was because of his race.
Beyond gastroenterology, similar experiences are common in other spheres. The Twitter thread #BlackintheIvory recounts stories of microaggressions and structural racism in medicine and academia. The cumulative toll of these experiences leads to departures of Black physicians including Uché Blackstock, MD;1 Aysha Khoury, MD, MPH;2 Ben Danielson, MD;3 Princess Dennar, MD;4 and others.
Microaggressions as proxy for bias
The term microaggression was coined by Chester Pierce, MD, the first Black tenured professor at Massachusetts General Hospital in the 1970’s, to describe the frequent, yet subtle dismissals Black Americans experienced in society. Over time, the term has been expanded to include “brief and commonplace daily verbal, behavioral, or environmental indignities, intentional or unintentional, that communicate hostile, derogatory, or negative slights and insults” to any marginalized group.5
While the term microaggressions is useful in contextualizing individual experiences, it narrowly focuses on conscious or unconscious interpersonal prejudices. In medicine, this misdirects attention away from the policies and practices that create and reinforce prejudices; these policies and practices do so by systematically excluding underrepresented minority (URM) physicians,6 defined by the American Association of Medical Colleges as physicians who are Black, Hispanic, Native Americans, and Alaska Natives,7 from the medical workforce. Ultimately, this leads to and exacerbates poor health outcomes for racial and ethnic minority patients.
Microaggressions represent our society’s deepest and oldest biases and are rooted in structural racism, as well as misogyny, homophobia, transphobia, xenophobia, ableism, and other prejudices.8 For URM physicians, experiences like the example above are frequently caused by structural racism.
Structural racism in medicine
Structural racism refers to the policies, practices, cultural representations, and norms that reinforce inequities by providing privileges to White people at the disadvantage of non-White people.9 In 1910, Abraham Flexner, commissioned by the Carnegie Foundation and the American Medical Association, wrote that African American physicians should be trained in hygiene rather than surgery and should primarily serve as “sanitarians” whose purpose was to “protect Whites” from common diseases like tuberculosis.10 The 1910 Flexner Report also emphasized the importance of prerequisite basic sciences education and recommended that only two of the seven existing Black medical schools remain open because Flexner believed that only these schools had the potential to meet the new requirements for medical education.11 A recent analysis found that, had the other five medical schools affiliated with historically Black colleges and universities remained open, this would have resulted in an additional 33,315 Black medical school graduates by 2019.12 Structural racism explains why the majority of practicing physicians, medical educators, National Institutes of Health–funded researchers, and hospital executives are White and, similarly, why White patients are overrepresented in clinical trials, have better health outcomes, and live longer lives than several racial and ethnic minority groups.13
The murders of Ahmaud Arbery, Breonna Taylor, and George Floyd and the inequitable toll of the COVID-19 pandemic on Black, Hispanic, and Native American people renewed the dialogue regarding structural racism in America. Beyond criminal justice and police reform, the current social justice movement demands that structural racism is examined in all spheres. In medicine and health care, acknowledging the history of exclusion and exploitation of Black people and other URM groups is an important first step, but this must be followed by a commitment to an antiracist future for the benefit of all medical professionals and patients.14,15
Antiracism as a path forward
Antiracism refers to actions and policies that seek to dismantle structural racism. While individuals can and should engage in antiracist actions, it is equally important for organizations and government to actively participate in this process as well.
Individual and interpersonal levels
Gastroenterologists should advocate an end to racist practices within their organizations (e.g., unjustified use of race-based corrections in diagnostic algorithms and practice guidelines),16 and interrupt microaggressions and racist actions in real time (e.g., overpolicing of underrepresented groups in health care settings).17 Gastroenterologists from underrepresented groups may also need to unlearn internalized racism, which is defined as acceptance by members of disadvantaged races of the negative messages about their own abilities and intrinsic worth.18
Organizational level
Gastroenterology divisions and practices must ensure that the entire workforce, including leadership, reflects the diversity of our country. Underrepresented groups represent 33% of the U.S. population, but only 9.1% of gastroenterology fellows and 10% of gastroenterology faculty are from underrepresented groups.19 In addition to diversifying the field of gastroenterology through financial and operational support of pipeline educational programs, organizations should also promote the scholarship of URM groups, whose work is often undervalued, and redistribute power by elevating voices that have been historically absent.20 Gastroenterology practices should also collect high-quality patient data disaggregated by demographic factors. Doing so will enable rapid identification of disparate health outcomes by demographic variables and inform interventions to eliminate identified disparities.
Government level
The “Executive Order On Advancing Racial Equity and Support for Underserved Communities Through the Federal Government” issued by President Biden on Jan. 20, 2021, is an example of how government can promote antiracism.21 The executive order states that domestic policies cause group inequities and calls for the removal of systemic barriers in current and future domestic policies. The executive order outlines several additional ways to improve equity in current and future policy, including engagement, consultation, and coordination with members of underserved communities. The details outlined in the executive order should serve as the foundation for establishing new standards at the state, county, and city levels as well. Gastroenterologists can influence government by voting for officials at all levels that support and promote these standards.
Conclusion
Beyond calling out microaggressions in real time, we must also interrogate the biases, policies, and practices that support them in medicine and beyond. As Black gastroenterologists who have experienced microaggressions and overt acts of racism, we ground Oviea’s experience in structural racism and offer strategies that individuals, organizations, and governing institutions can adopt toward an antiracist future. This model can be applied to experiences rooted in misogyny, homophobia, transphobia, xenophobia, ableism, and other prejudices.
As a nation, we must make an active and collective choice to address structural racism. In health care, doing so will strengthen communities, enhance the lived experiences of URM physician colleagues, and save patient lives. Gastroenterologists, as trusted health care providers, are uniquely positioned to lead the way.
Dr. Akpotaire is a second-year GI fellow in the division of gastroenterology at the University of Washington, Seattle. Dr. Issaka is an assistant professor with both the Fred Hutchinson Cancer Research Center, Seattle, and the division of gastroenterology at the University of Washington.
References
1. Blackstock U. “Why Black doctors like me are leaving faculty positions in academic medical centers.” STAT News, 2020.
2. Asare JG. “One Doctor Shares Her Story of Racism in Medicine.” Forbes. 2021 Feb 1.
3. Kroman D. “Revered doctor steps down, accusing Seattle Children’s Hospital of racism.” Crosscut. 2020 Dec 31.
4. United States District Court Eastern District of Louisiana. Princess Dennar, M.D. v. The Administrators of the Tulane Educational Fund, 2020.
5. Sue DW. Microaggressions in Everyday Life: Race, Gender, and Sexual Orientation. Hoboken, N.J.: Wiley, 2010.
6. Boyd RW. Lancet. 2019 Jun 22;393(10190):2484-5.
7. AAMC. Diversity in Medicine Facts and Figures 2019. Washington, D.C., 2019.
8. Overland MK et al. PM R. 2019 Sep;11(9):1004-12.
9. Jones CP. Ethn Dis. 2018 Aug 9;28(Suppl 1):231-4.
10. Hlavinka E. “Racial Bias in Flexner Report Permeates Medical Education Today.” Medpage Today. 2020 Jun 18.
11. Flexner A. Medical Education in the United States and Canada. New York: 1910. Republished: Bull World Health Organ. 2002;80(7):594-602.
12. Campbell KM et al. JAMA Netw Open. 2020 Aug 3;3(8):e2015220.
13. Malat J et al. Soc Sci Med. 2018 Feb;199:148-56.
14. Kendi IX. How to be an antiracist. New York: Random House Books, 2019.
15. Gray DM 2nd et al. Nat Rev Gastroenterol Hepatol. 2020 Oct;17(10):589-90.
16. Vyas DA et al. N Engl J Med. 2020 Aug 27;383(9):874-82.
17. Green CR et al. J Natl Med Assoc. 2018 Feb;110(1):37-43.
18. Jones CP. Am J Public Health. 2000 Aug;90(8):1212-5.
19. Anyane-Yeboa A et al. Am J Gastroenterol. 2020 Aug;115(8):1147-9.
20. Issaka RB. JAMA. 2020 Aug 11;324(6):556-7.
21. Biden JR. Executive Order On Advancing Racial Equity and Support for Underserved Communities Through the Federal Government. Washington, D.C.: The White House, 2021.
On a busy call day, Oviea (a second-year gastroenterology fellow), paused in the hallway to listen to a conversation between an endoscopy nurse and a patient. The nurse was requesting the patient’s permission for a gastroenterology fellow to participate in their care and the patient, well acquainted with the role from prior procedures, immediately agreed. Oviea entered the patient’s room, introduced himself as “Dr. Akpotaire, the gastroenterology fellow,” as he had with hundreds of other patients during his fellowship, and completed the informed consent. The interaction was brief but pleasant. As Oviea was leaving the room, the patient asked: “When will I meet the doctor”?
This question was familiar to Oviea. Despite always introducing himself by title and wearing matching identification, many patients had dismissed his credentials since graduating from medical school. His answer was equally familiar: “I am a doctor, and Dr. X, the supervising physician, will meet you soon.” With the patient seemingly placated, Oviea delivered the consent form to the procedure room. Minutes later, he was surprised to learn that the patient specifically requested that he not be allowed to participate in their care. This in combination with the patient’s initial dismissal of Oviea’s credentials, left a sting. While none of the other team members outwardly questioned the reason for the patient’s change of heart, Oviea continued to wonder if the patient’s decision was because of his race.
Beyond gastroenterology, similar experiences are common in other spheres. The Twitter thread #BlackintheIvory recounts stories of microaggressions and structural racism in medicine and academia. The cumulative toll of these experiences leads to departures of Black physicians including Uché Blackstock, MD;1 Aysha Khoury, MD, MPH;2 Ben Danielson, MD;3 Princess Dennar, MD;4 and others.
Microaggressions as proxy for bias
The term microaggression was coined by Chester Pierce, MD, the first Black tenured professor at Massachusetts General Hospital in the 1970’s, to describe the frequent, yet subtle dismissals Black Americans experienced in society. Over time, the term has been expanded to include “brief and commonplace daily verbal, behavioral, or environmental indignities, intentional or unintentional, that communicate hostile, derogatory, or negative slights and insults” to any marginalized group.5
While the term microaggressions is useful in contextualizing individual experiences, it narrowly focuses on conscious or unconscious interpersonal prejudices. In medicine, this misdirects attention away from the policies and practices that create and reinforce prejudices; these policies and practices do so by systematically excluding underrepresented minority (URM) physicians,6 defined by the American Association of Medical Colleges as physicians who are Black, Hispanic, Native Americans, and Alaska Natives,7 from the medical workforce. Ultimately, this leads to and exacerbates poor health outcomes for racial and ethnic minority patients.
Microaggressions represent our society’s deepest and oldest biases and are rooted in structural racism, as well as misogyny, homophobia, transphobia, xenophobia, ableism, and other prejudices.8 For URM physicians, experiences like the example above are frequently caused by structural racism.
Structural racism in medicine
Structural racism refers to the policies, practices, cultural representations, and norms that reinforce inequities by providing privileges to White people at the disadvantage of non-White people.9 In 1910, Abraham Flexner, commissioned by the Carnegie Foundation and the American Medical Association, wrote that African American physicians should be trained in hygiene rather than surgery and should primarily serve as “sanitarians” whose purpose was to “protect Whites” from common diseases like tuberculosis.10 The 1910 Flexner Report also emphasized the importance of prerequisite basic sciences education and recommended that only two of the seven existing Black medical schools remain open because Flexner believed that only these schools had the potential to meet the new requirements for medical education.11 A recent analysis found that, had the other five medical schools affiliated with historically Black colleges and universities remained open, this would have resulted in an additional 33,315 Black medical school graduates by 2019.12 Structural racism explains why the majority of practicing physicians, medical educators, National Institutes of Health–funded researchers, and hospital executives are White and, similarly, why White patients are overrepresented in clinical trials, have better health outcomes, and live longer lives than several racial and ethnic minority groups.13
The murders of Ahmaud Arbery, Breonna Taylor, and George Floyd and the inequitable toll of the COVID-19 pandemic on Black, Hispanic, and Native American people renewed the dialogue regarding structural racism in America. Beyond criminal justice and police reform, the current social justice movement demands that structural racism is examined in all spheres. In medicine and health care, acknowledging the history of exclusion and exploitation of Black people and other URM groups is an important first step, but this must be followed by a commitment to an antiracist future for the benefit of all medical professionals and patients.14,15
Antiracism as a path forward
Antiracism refers to actions and policies that seek to dismantle structural racism. While individuals can and should engage in antiracist actions, it is equally important for organizations and government to actively participate in this process as well.
Individual and interpersonal levels
Gastroenterologists should advocate an end to racist practices within their organizations (e.g., unjustified use of race-based corrections in diagnostic algorithms and practice guidelines),16 and interrupt microaggressions and racist actions in real time (e.g., overpolicing of underrepresented groups in health care settings).17 Gastroenterologists from underrepresented groups may also need to unlearn internalized racism, which is defined as acceptance by members of disadvantaged races of the negative messages about their own abilities and intrinsic worth.18
Organizational level
Gastroenterology divisions and practices must ensure that the entire workforce, including leadership, reflects the diversity of our country. Underrepresented groups represent 33% of the U.S. population, but only 9.1% of gastroenterology fellows and 10% of gastroenterology faculty are from underrepresented groups.19 In addition to diversifying the field of gastroenterology through financial and operational support of pipeline educational programs, organizations should also promote the scholarship of URM groups, whose work is often undervalued, and redistribute power by elevating voices that have been historically absent.20 Gastroenterology practices should also collect high-quality patient data disaggregated by demographic factors. Doing so will enable rapid identification of disparate health outcomes by demographic variables and inform interventions to eliminate identified disparities.
Government level
The “Executive Order On Advancing Racial Equity and Support for Underserved Communities Through the Federal Government” issued by President Biden on Jan. 20, 2021, is an example of how government can promote antiracism.21 The executive order states that domestic policies cause group inequities and calls for the removal of systemic barriers in current and future domestic policies. The executive order outlines several additional ways to improve equity in current and future policy, including engagement, consultation, and coordination with members of underserved communities. The details outlined in the executive order should serve as the foundation for establishing new standards at the state, county, and city levels as well. Gastroenterologists can influence government by voting for officials at all levels that support and promote these standards.
Conclusion
Beyond calling out microaggressions in real time, we must also interrogate the biases, policies, and practices that support them in medicine and beyond. As Black gastroenterologists who have experienced microaggressions and overt acts of racism, we ground Oviea’s experience in structural racism and offer strategies that individuals, organizations, and governing institutions can adopt toward an antiracist future. This model can be applied to experiences rooted in misogyny, homophobia, transphobia, xenophobia, ableism, and other prejudices.
As a nation, we must make an active and collective choice to address structural racism. In health care, doing so will strengthen communities, enhance the lived experiences of URM physician colleagues, and save patient lives. Gastroenterologists, as trusted health care providers, are uniquely positioned to lead the way.
Dr. Akpotaire is a second-year GI fellow in the division of gastroenterology at the University of Washington, Seattle. Dr. Issaka is an assistant professor with both the Fred Hutchinson Cancer Research Center, Seattle, and the division of gastroenterology at the University of Washington.
References
1. Blackstock U. “Why Black doctors like me are leaving faculty positions in academic medical centers.” STAT News, 2020.
2. Asare JG. “One Doctor Shares Her Story of Racism in Medicine.” Forbes. 2021 Feb 1.
3. Kroman D. “Revered doctor steps down, accusing Seattle Children’s Hospital of racism.” Crosscut. 2020 Dec 31.
4. United States District Court Eastern District of Louisiana. Princess Dennar, M.D. v. The Administrators of the Tulane Educational Fund, 2020.
5. Sue DW. Microaggressions in Everyday Life: Race, Gender, and Sexual Orientation. Hoboken, N.J.: Wiley, 2010.
6. Boyd RW. Lancet. 2019 Jun 22;393(10190):2484-5.
7. AAMC. Diversity in Medicine Facts and Figures 2019. Washington, D.C., 2019.
8. Overland MK et al. PM R. 2019 Sep;11(9):1004-12.
9. Jones CP. Ethn Dis. 2018 Aug 9;28(Suppl 1):231-4.
10. Hlavinka E. “Racial Bias in Flexner Report Permeates Medical Education Today.” Medpage Today. 2020 Jun 18.
11. Flexner A. Medical Education in the United States and Canada. New York: 1910. Republished: Bull World Health Organ. 2002;80(7):594-602.
12. Campbell KM et al. JAMA Netw Open. 2020 Aug 3;3(8):e2015220.
13. Malat J et al. Soc Sci Med. 2018 Feb;199:148-56.
14. Kendi IX. How to be an antiracist. New York: Random House Books, 2019.
15. Gray DM 2nd et al. Nat Rev Gastroenterol Hepatol. 2020 Oct;17(10):589-90.
16. Vyas DA et al. N Engl J Med. 2020 Aug 27;383(9):874-82.
17. Green CR et al. J Natl Med Assoc. 2018 Feb;110(1):37-43.
18. Jones CP. Am J Public Health. 2000 Aug;90(8):1212-5.
19. Anyane-Yeboa A et al. Am J Gastroenterol. 2020 Aug;115(8):1147-9.
20. Issaka RB. JAMA. 2020 Aug 11;324(6):556-7.
21. Biden JR. Executive Order On Advancing Racial Equity and Support for Underserved Communities Through the Federal Government. Washington, D.C.: The White House, 2021.
This month in the journal CHEST®
Editor’s picks
Adherence to Asthma Biologics: Implications for Patient Selection, Step Therapy and Outcomes. By Dr. Rank, et al.
Long-term Benefits of Pulmonary Rehabilitation in COPD Patients: A 2-Year Follow-up Study. By Dr. A. Yohannes, et al.
Impact of Corticosteroids in COVID-19 Outcomes: Systematic Review and Meta-Analysis. By Dr. E. Cano, et al.
Leadership Essentials for the Chest Physician: Models, Attributes, and Styles. By Dr. J. K. Stoller.
Incidence of Venous Thromboembolism and Bleeding Among Hospitalized Patients With COVID-19: A Systematic Review and Meta-Analysis. By Dr. D. Jiménez, et al.
Disparities in Sleep Health and Potential Intervention Models: A Focused Review. By Dr. S. Sharma, et al.
Editor’s picks
Editor’s picks
Adherence to Asthma Biologics: Implications for Patient Selection, Step Therapy and Outcomes. By Dr. Rank, et al.
Long-term Benefits of Pulmonary Rehabilitation in COPD Patients: A 2-Year Follow-up Study. By Dr. A. Yohannes, et al.
Impact of Corticosteroids in COVID-19 Outcomes: Systematic Review and Meta-Analysis. By Dr. E. Cano, et al.
Leadership Essentials for the Chest Physician: Models, Attributes, and Styles. By Dr. J. K. Stoller.
Incidence of Venous Thromboembolism and Bleeding Among Hospitalized Patients With COVID-19: A Systematic Review and Meta-Analysis. By Dr. D. Jiménez, et al.
Disparities in Sleep Health and Potential Intervention Models: A Focused Review. By Dr. S. Sharma, et al.
Adherence to Asthma Biologics: Implications for Patient Selection, Step Therapy and Outcomes. By Dr. Rank, et al.
Long-term Benefits of Pulmonary Rehabilitation in COPD Patients: A 2-Year Follow-up Study. By Dr. A. Yohannes, et al.
Impact of Corticosteroids in COVID-19 Outcomes: Systematic Review and Meta-Analysis. By Dr. E. Cano, et al.
Leadership Essentials for the Chest Physician: Models, Attributes, and Styles. By Dr. J. K. Stoller.
Incidence of Venous Thromboembolism and Bleeding Among Hospitalized Patients With COVID-19: A Systematic Review and Meta-Analysis. By Dr. D. Jiménez, et al.
Disparities in Sleep Health and Potential Intervention Models: A Focused Review. By Dr. S. Sharma, et al.
CHEST 2021 moves to Orlando and online – the choice is yours
CHEST is excited to announce that CHEST 2021 will be held in Orlando, Florida, from October 17-21 at the Orange County Convention Center. CHEST 2021 will be offered as both an in-person and online experience. Since travel restrictions remain unknown, CHEST is working to ensure that everyone has access to the same top-tier learning – wherever they are.
“Learning together as a community is an important aspect of the CHEST annual meeting. Whether we are face-to-face or online, the knowledge gained from expert presenters, simulations and games, and talking with one another can’t be duplicated elsewhere. In whatever way you can attend, join us at CHEST 2021 to discuss the critically relevant topics affecting our patients and chest medicine,” said CHEST President Steve Simpson, MD, FCCP.
It is also essential that those who cannot travel can still avail themselves of the engaging and interactive learning offered at the CHEST conference. Everyone – whether online or in-person – will be able to experience the meeting in real-time, including expert faculty presentations, simulated learning experiences, gaming, and more.
What to expect
Through bite-sized, immersive learning, experts in the field will cover the latest updates in pulmonary, critical care, and sleep medicine. CHEST 2021 offers you the opportunity to learn from a diverse set of knowledgeable educators representing different viewpoints and experiences.
Team-based learning is an indispensable component of the annual meeting. The activities support collaborative discovery and help you build relationships with your peers. Known for its development of simulation courses, at CHEST 2021, you can take part in the latest in “hands-on” learning. In addition, gaming will allow for friendly competition among colleagues, whether playing from home or on-site.
Getting involved
Make your mark by submitting your original abstracts and case reports to be presented at CHEST 2021. Because of the past year’s challenges, new discoveries were made in the treatment and approaches to managing chest medicine diseases. This work is important and will inform the way patients receive care in the future.
Showcase COVID-19 research, among other topics you are working on, for a chance to share your findings with colleagues, gain feedback from expert faculty, collaborate with other professionals in the field, and expand your professional portfolio. The deadline to submit is April 28. [link]
Keeping safe
It’s been a long time since in-person conferences were possible. CHEST is closely monitoring the status of the pandemic throughout the planning process. The Orange County Convention Center was selected because the venue is large enough to support social distancing. The CHEST team is establishing protocols that limit the number of individuals in a space, promote good traffic flow, require the wearing of masks, and other safety measures. All on-site participants and CHEST support staff will be required to attest to having received a COVID-19 vaccination to attend.
Continue to watch for more information. Registration for CHEST 2021 will open in May. We’ve missed you, and we look forward to seeing you in Orlando, Florida, October 17-20.
CHEST is excited to announce that CHEST 2021 will be held in Orlando, Florida, from October 17-21 at the Orange County Convention Center. CHEST 2021 will be offered as both an in-person and online experience. Since travel restrictions remain unknown, CHEST is working to ensure that everyone has access to the same top-tier learning – wherever they are.
“Learning together as a community is an important aspect of the CHEST annual meeting. Whether we are face-to-face or online, the knowledge gained from expert presenters, simulations and games, and talking with one another can’t be duplicated elsewhere. In whatever way you can attend, join us at CHEST 2021 to discuss the critically relevant topics affecting our patients and chest medicine,” said CHEST President Steve Simpson, MD, FCCP.
It is also essential that those who cannot travel can still avail themselves of the engaging and interactive learning offered at the CHEST conference. Everyone – whether online or in-person – will be able to experience the meeting in real-time, including expert faculty presentations, simulated learning experiences, gaming, and more.
What to expect
Through bite-sized, immersive learning, experts in the field will cover the latest updates in pulmonary, critical care, and sleep medicine. CHEST 2021 offers you the opportunity to learn from a diverse set of knowledgeable educators representing different viewpoints and experiences.
Team-based learning is an indispensable component of the annual meeting. The activities support collaborative discovery and help you build relationships with your peers. Known for its development of simulation courses, at CHEST 2021, you can take part in the latest in “hands-on” learning. In addition, gaming will allow for friendly competition among colleagues, whether playing from home or on-site.
Getting involved
Make your mark by submitting your original abstracts and case reports to be presented at CHEST 2021. Because of the past year’s challenges, new discoveries were made in the treatment and approaches to managing chest medicine diseases. This work is important and will inform the way patients receive care in the future.
Showcase COVID-19 research, among other topics you are working on, for a chance to share your findings with colleagues, gain feedback from expert faculty, collaborate with other professionals in the field, and expand your professional portfolio. The deadline to submit is April 28. [link]
Keeping safe
It’s been a long time since in-person conferences were possible. CHEST is closely monitoring the status of the pandemic throughout the planning process. The Orange County Convention Center was selected because the venue is large enough to support social distancing. The CHEST team is establishing protocols that limit the number of individuals in a space, promote good traffic flow, require the wearing of masks, and other safety measures. All on-site participants and CHEST support staff will be required to attest to having received a COVID-19 vaccination to attend.
Continue to watch for more information. Registration for CHEST 2021 will open in May. We’ve missed you, and we look forward to seeing you in Orlando, Florida, October 17-20.
CHEST is excited to announce that CHEST 2021 will be held in Orlando, Florida, from October 17-21 at the Orange County Convention Center. CHEST 2021 will be offered as both an in-person and online experience. Since travel restrictions remain unknown, CHEST is working to ensure that everyone has access to the same top-tier learning – wherever they are.
“Learning together as a community is an important aspect of the CHEST annual meeting. Whether we are face-to-face or online, the knowledge gained from expert presenters, simulations and games, and talking with one another can’t be duplicated elsewhere. In whatever way you can attend, join us at CHEST 2021 to discuss the critically relevant topics affecting our patients and chest medicine,” said CHEST President Steve Simpson, MD, FCCP.
It is also essential that those who cannot travel can still avail themselves of the engaging and interactive learning offered at the CHEST conference. Everyone – whether online or in-person – will be able to experience the meeting in real-time, including expert faculty presentations, simulated learning experiences, gaming, and more.
What to expect
Through bite-sized, immersive learning, experts in the field will cover the latest updates in pulmonary, critical care, and sleep medicine. CHEST 2021 offers you the opportunity to learn from a diverse set of knowledgeable educators representing different viewpoints and experiences.
Team-based learning is an indispensable component of the annual meeting. The activities support collaborative discovery and help you build relationships with your peers. Known for its development of simulation courses, at CHEST 2021, you can take part in the latest in “hands-on” learning. In addition, gaming will allow for friendly competition among colleagues, whether playing from home or on-site.
Getting involved
Make your mark by submitting your original abstracts and case reports to be presented at CHEST 2021. Because of the past year’s challenges, new discoveries were made in the treatment and approaches to managing chest medicine diseases. This work is important and will inform the way patients receive care in the future.
Showcase COVID-19 research, among other topics you are working on, for a chance to share your findings with colleagues, gain feedback from expert faculty, collaborate with other professionals in the field, and expand your professional portfolio. The deadline to submit is April 28. [link]
Keeping safe
It’s been a long time since in-person conferences were possible. CHEST is closely monitoring the status of the pandemic throughout the planning process. The Orange County Convention Center was selected because the venue is large enough to support social distancing. The CHEST team is establishing protocols that limit the number of individuals in a space, promote good traffic flow, require the wearing of masks, and other safety measures. All on-site participants and CHEST support staff will be required to attest to having received a COVID-19 vaccination to attend.
Continue to watch for more information. Registration for CHEST 2021 will open in May. We’ve missed you, and we look forward to seeing you in Orlando, Florida, October 17-20.
Home noninvasive ventilation in hypercapnic COPD: Progress but important unanswered questions
Patients with COPD may develop sustained hypercapnia, often defined as an awake arterial PCO2 of >45 mm Hg. Other synonymous terms include alveolar hypoventilation or chronic hypercapnic respiratory failure, noting that the specific terminology used may reflect local practice, an assessment of patient severity, or specific insurance requirements. Regardless, available data suggest that hypercapnic COPD patients are at high risk for adverse health outcomes (Yang H, et al. BMJ Open. 2015;5[12]:e008909). Moreover, there appears to have been a growing interest in this population driven by a focus on reducing COPD hospitalizations, increasing recognition of sleep disordered breathing, and progress in potential therapeutic strategies.
There are a number of factors that might drive COPD patients to develop hypercapnia. Lower airway obstruction, expiratory flow limitation and air trapping cause mechanical load on breathing, as well as a trade-off between time spent in inspiration vs prolonged expiration. The function of the diaphragm is impacted by hyperinflation leading to mal-positioning, as well as possibly by local and/or systemic myopathy. The net result is often decreased overall minute ventilation. In terms of gas exchange, increased dead space and ventilation-perfusion mismatching leads to reduced efficiency of ventilation towards CO2 removal. Breathing changes during sleep play an important role, as evidenced by worsened hypercapnia during sleep that can drive chronic CO2 retention (O’Donoghue FJ, et al. Eur Respir J. 2003;21[6]:977). The pathogenesis includes reduced central respiratory drive, increased upper airway resistance and/or obstructive hypopneas and apneas, and respiratory muscle atonia, particularly during REM sleep. The extent to which each of these factors contributes to hypercapnia varies across individual patients, in accordance with the known substantial heterogeneity of COPD. Regardless of underlying traits, patients with COPD who develop hypercapnia have sufficiently severe perturbations to disrupt the normally tight control over CO2 homeostasis.
Nocturnal home noninvasive ventilation (NIV) has been examined as a potential therapeutic strategy for patients with hypercapnic COPD. While older studies have not shown consistent benefits, more recent evidence suggests that NIV can reduce hospitalizations, improve quality of life, and potentially reduce mortality among those with hypercapnic COPD. Accordingly, the American Thoracic Society recently released a clinical practice guideline regarding the use of NIV in patients with chronic stable hypercapnic COPD (Macrea M, et al. Am J Respir Crit Care Med. 2020;202[4]:e74-e87). Recommendations from the guideline included:
1) The use of nocturnal NIV for patients with chronic stable hypercapnic COPD
2) Screening for OSA before initiation of long-term NIV
3) Not using in-hospital initiation of long-term NIV after an episode of acute or chronic hypercapnic respiratory failure, favoring instead reassessment for NIV at 2–4 weeks after resolution
4) Not using an in-laboratory overnight PSG to initially titrate NIV
5) Targeting normalization of PaCO2.
Although it now seems clear that efforts should be made to use NIV in COPD to decrease chronic hypercapnia, there are a number of important questions that remain, particularly surrounding the topic of concurrent OSA, titration, and devices:
• What is the appropriate approach towards patients with suspected concurrent OSA? Most studies of NIV have excluded patients with OSA, or otherwise at higher risk of OSA. Nonetheless, such patients may be common, both based on continued high prevalence of obesity, as well as the potential role that upper airway obstructive events may play towards elevations in CO2 (Resta O., et al. Sleep Breath. 2002;6[1]:11-8). COPD epidemiological studies indicate obesity as a risk factor for several poor outcomes, including severe COPD exacerbation (Lambert AA, et al. Chest. 2017;151[1]:68-77), while studies of COPD and OSA suggest that the presence of hypercapnia defines a high-risk group Jaoude P., Lung. 2014;192:215). Recognizing the potential importance of OSA in this group, ATS guidelines recommend that a general questionnaire-based screening be performed. If screening is positive, the implication would be to perform diagnostic polysomnography to confirm the diagnosis of OSA. However, this may be a challenge for chronically ill patients, and likely would result in delays in NIV initiation. Of note, emerging evidence suggests that home sleep apnea testing (HSAT) might have reasonable accuracy in this group, which may facilitate formal diagnosis. Other concerns in this area include the lack of questionnaire validation in COPD patients.
• Should patients with OSA be managed differently than those without OSA? A diagnosis of OSA might impact several subsequent management decisions related to appropriate NIV therapy and titration. Patients with OSA have increased upper airway collapsibility, which might necessitate higher EPAP support than the minimal EPAP used in NIV trials with non-OSA patients (often fixed at 4 cm water). Potential strategies for optimizing EPAP include use of an NIV device with auto-titrating EPAP, titration in the sleep laboratory (discussed below), or outpatient titration based on clinical parameters and subsequent device download follow-up. On the other hand, one might consider all patients to be at risk for upper airway obstruction and need for additional EPAP titration, which would obviate the need for OSA diagnostic testing.
• What is the role of the sleep laboratory towards successful titration? The inpatient hospital setting has been the traditional site to initiate home NIV in some institutions but is highly resource intensive and increasingly impractical in many health systems. On the other hand, advances in home remote device monitoring now provide the clinician with the ability to examine daily usage, estimated leak, tidal volumes, respiratory rate, and other parameters – often reported as recently as the prior night. In addition, setting changes can be made via these remote monitoring tools (for nonventilator devices), allowing titration to be performed over time on outpatients. Several studies support the effectiveness of this approach over hospital titration in neuromuscular disease and now in COPD (Duiverman ML, et al. Thorax. 2020;75[3]:244-52). Similarly, data suggest that titration under polysomnographic guidance might not be necessary (Patout M, Arbane G, Cuvelier A, Muir JF, Hart N, Murphy PB. Polysomnography versus limited respiratory monitoring and nurse-led titration to optimize non-invasive ventilation set-up: a pilot randomised clinical trial. Thorax. 2019;74:83-86).
Limitations towards the sleep lab as the site of initial titration include waiting time, cost and insurance coverage, and the need to accommodate issues such as impaired mobility or reliance on a caretaker. In addition, titration goals must be clearly outlined in protocols and via staff training specific to NIV. The sleep laboratory may be most appropriately utilized in the minority of patients in whom outpatient titration is unsuccessful. Relatively common issues that might be best addressed in the lab setting include excessive mask leaks, residual apneas and hypopneas, failure to control CO2, or other sleep complaints. In general, studies should probably be focused primarily on titrating EPAP to alleviate upper airway obstructive events. The goals in terms of IPAP titration (or ventilation titration, in the case of “VAPS” modes) are less clear, and overly aggressive increases may complicate the picture with excessive leaks or airway obstruction due to glottic closure. Attempting to accomplish “too much” often leads to a study with limited utility. In contrast, simply performing the study in the patient’s home settings can provide useful diagnostic information regarding the problem one is trying to solve.
• When and where should one initiate NIV following a severe COPD exacerbation? In contrast to the ATS guidelines, the European Respiratory Society guidelines suggest that patients recovering from severe COPD exacerbations be initiated on NIV during that hospitalization, noting that this is a group at high risk for early rehospitalization and mortality (Ergan B, et al. Eur Respir J. 2019;54[3]:1901003). ATS guidelines had the concern of unnecessary start of NIV in those who might normalize their CO2 after recovery, and the possibility of prolonging hospitalizations for titration. For the clinician, the decision will probably be individualized based on risk and available resources. For patients with frequent ICU admissions and/or difficulty with close outpatient follow-up, earlier NIV initiation is certainly a reasonable approach, but adherence and effectiveness remains a concern and, thus, more data are needed.
• Which patients should receive a bedside respiratory assist device (RAD, i.e., BIPAP machine) vs. a noninvasive ventilator? Two classes of devices can be used for home NIV. While both can provide similar positive pressure ventilation, ventilators are designed as life support with alarms and batteries, and may have modes not otherwise available (e.g., auto-titrating EPAP). On the other hand, RAD devices are more convenient for patients and less expensive, but difficult qualification requirements (particularly for devices capable of Bilevel ST or VAPS) have likely resulted in their underutilization. CHEST is spearheading an effort to reconsider Medicare coverage determinations (current rules are from 1998), which will hopefully better align device qualification requirements with emerging evidence regarding patient needs and preferences.
Home non-invasive ventilation can improve outcomes in these high-risk patients with hypercapnic COPD, and the new clinical practice guidelines are an important step in outlining appropriate management. Further progress is needed to delineate an individualized approach based on underlying patient pathophysiology, COPD manifestations/phenotypes, and systems-based practice considerations.
Dr. Orr is Assistant Professor, Division of Pulmonary, Critical Care, and Sleep Medicine, UC San Diego.
Patients with COPD may develop sustained hypercapnia, often defined as an awake arterial PCO2 of >45 mm Hg. Other synonymous terms include alveolar hypoventilation or chronic hypercapnic respiratory failure, noting that the specific terminology used may reflect local practice, an assessment of patient severity, or specific insurance requirements. Regardless, available data suggest that hypercapnic COPD patients are at high risk for adverse health outcomes (Yang H, et al. BMJ Open. 2015;5[12]:e008909). Moreover, there appears to have been a growing interest in this population driven by a focus on reducing COPD hospitalizations, increasing recognition of sleep disordered breathing, and progress in potential therapeutic strategies.
There are a number of factors that might drive COPD patients to develop hypercapnia. Lower airway obstruction, expiratory flow limitation and air trapping cause mechanical load on breathing, as well as a trade-off between time spent in inspiration vs prolonged expiration. The function of the diaphragm is impacted by hyperinflation leading to mal-positioning, as well as possibly by local and/or systemic myopathy. The net result is often decreased overall minute ventilation. In terms of gas exchange, increased dead space and ventilation-perfusion mismatching leads to reduced efficiency of ventilation towards CO2 removal. Breathing changes during sleep play an important role, as evidenced by worsened hypercapnia during sleep that can drive chronic CO2 retention (O’Donoghue FJ, et al. Eur Respir J. 2003;21[6]:977). The pathogenesis includes reduced central respiratory drive, increased upper airway resistance and/or obstructive hypopneas and apneas, and respiratory muscle atonia, particularly during REM sleep. The extent to which each of these factors contributes to hypercapnia varies across individual patients, in accordance with the known substantial heterogeneity of COPD. Regardless of underlying traits, patients with COPD who develop hypercapnia have sufficiently severe perturbations to disrupt the normally tight control over CO2 homeostasis.
Nocturnal home noninvasive ventilation (NIV) has been examined as a potential therapeutic strategy for patients with hypercapnic COPD. While older studies have not shown consistent benefits, more recent evidence suggests that NIV can reduce hospitalizations, improve quality of life, and potentially reduce mortality among those with hypercapnic COPD. Accordingly, the American Thoracic Society recently released a clinical practice guideline regarding the use of NIV in patients with chronic stable hypercapnic COPD (Macrea M, et al. Am J Respir Crit Care Med. 2020;202[4]:e74-e87). Recommendations from the guideline included:
1) The use of nocturnal NIV for patients with chronic stable hypercapnic COPD
2) Screening for OSA before initiation of long-term NIV
3) Not using in-hospital initiation of long-term NIV after an episode of acute or chronic hypercapnic respiratory failure, favoring instead reassessment for NIV at 2–4 weeks after resolution
4) Not using an in-laboratory overnight PSG to initially titrate NIV
5) Targeting normalization of PaCO2.
Although it now seems clear that efforts should be made to use NIV in COPD to decrease chronic hypercapnia, there are a number of important questions that remain, particularly surrounding the topic of concurrent OSA, titration, and devices:
• What is the appropriate approach towards patients with suspected concurrent OSA? Most studies of NIV have excluded patients with OSA, or otherwise at higher risk of OSA. Nonetheless, such patients may be common, both based on continued high prevalence of obesity, as well as the potential role that upper airway obstructive events may play towards elevations in CO2 (Resta O., et al. Sleep Breath. 2002;6[1]:11-8). COPD epidemiological studies indicate obesity as a risk factor for several poor outcomes, including severe COPD exacerbation (Lambert AA, et al. Chest. 2017;151[1]:68-77), while studies of COPD and OSA suggest that the presence of hypercapnia defines a high-risk group Jaoude P., Lung. 2014;192:215). Recognizing the potential importance of OSA in this group, ATS guidelines recommend that a general questionnaire-based screening be performed. If screening is positive, the implication would be to perform diagnostic polysomnography to confirm the diagnosis of OSA. However, this may be a challenge for chronically ill patients, and likely would result in delays in NIV initiation. Of note, emerging evidence suggests that home sleep apnea testing (HSAT) might have reasonable accuracy in this group, which may facilitate formal diagnosis. Other concerns in this area include the lack of questionnaire validation in COPD patients.
• Should patients with OSA be managed differently than those without OSA? A diagnosis of OSA might impact several subsequent management decisions related to appropriate NIV therapy and titration. Patients with OSA have increased upper airway collapsibility, which might necessitate higher EPAP support than the minimal EPAP used in NIV trials with non-OSA patients (often fixed at 4 cm water). Potential strategies for optimizing EPAP include use of an NIV device with auto-titrating EPAP, titration in the sleep laboratory (discussed below), or outpatient titration based on clinical parameters and subsequent device download follow-up. On the other hand, one might consider all patients to be at risk for upper airway obstruction and need for additional EPAP titration, which would obviate the need for OSA diagnostic testing.
• What is the role of the sleep laboratory towards successful titration? The inpatient hospital setting has been the traditional site to initiate home NIV in some institutions but is highly resource intensive and increasingly impractical in many health systems. On the other hand, advances in home remote device monitoring now provide the clinician with the ability to examine daily usage, estimated leak, tidal volumes, respiratory rate, and other parameters – often reported as recently as the prior night. In addition, setting changes can be made via these remote monitoring tools (for nonventilator devices), allowing titration to be performed over time on outpatients. Several studies support the effectiveness of this approach over hospital titration in neuromuscular disease and now in COPD (Duiverman ML, et al. Thorax. 2020;75[3]:244-52). Similarly, data suggest that titration under polysomnographic guidance might not be necessary (Patout M, Arbane G, Cuvelier A, Muir JF, Hart N, Murphy PB. Polysomnography versus limited respiratory monitoring and nurse-led titration to optimize non-invasive ventilation set-up: a pilot randomised clinical trial. Thorax. 2019;74:83-86).
Limitations towards the sleep lab as the site of initial titration include waiting time, cost and insurance coverage, and the need to accommodate issues such as impaired mobility or reliance on a caretaker. In addition, titration goals must be clearly outlined in protocols and via staff training specific to NIV. The sleep laboratory may be most appropriately utilized in the minority of patients in whom outpatient titration is unsuccessful. Relatively common issues that might be best addressed in the lab setting include excessive mask leaks, residual apneas and hypopneas, failure to control CO2, or other sleep complaints. In general, studies should probably be focused primarily on titrating EPAP to alleviate upper airway obstructive events. The goals in terms of IPAP titration (or ventilation titration, in the case of “VAPS” modes) are less clear, and overly aggressive increases may complicate the picture with excessive leaks or airway obstruction due to glottic closure. Attempting to accomplish “too much” often leads to a study with limited utility. In contrast, simply performing the study in the patient’s home settings can provide useful diagnostic information regarding the problem one is trying to solve.
• When and where should one initiate NIV following a severe COPD exacerbation? In contrast to the ATS guidelines, the European Respiratory Society guidelines suggest that patients recovering from severe COPD exacerbations be initiated on NIV during that hospitalization, noting that this is a group at high risk for early rehospitalization and mortality (Ergan B, et al. Eur Respir J. 2019;54[3]:1901003). ATS guidelines had the concern of unnecessary start of NIV in those who might normalize their CO2 after recovery, and the possibility of prolonging hospitalizations for titration. For the clinician, the decision will probably be individualized based on risk and available resources. For patients with frequent ICU admissions and/or difficulty with close outpatient follow-up, earlier NIV initiation is certainly a reasonable approach, but adherence and effectiveness remains a concern and, thus, more data are needed.
• Which patients should receive a bedside respiratory assist device (RAD, i.e., BIPAP machine) vs. a noninvasive ventilator? Two classes of devices can be used for home NIV. While both can provide similar positive pressure ventilation, ventilators are designed as life support with alarms and batteries, and may have modes not otherwise available (e.g., auto-titrating EPAP). On the other hand, RAD devices are more convenient for patients and less expensive, but difficult qualification requirements (particularly for devices capable of Bilevel ST or VAPS) have likely resulted in their underutilization. CHEST is spearheading an effort to reconsider Medicare coverage determinations (current rules are from 1998), which will hopefully better align device qualification requirements with emerging evidence regarding patient needs and preferences.
Home non-invasive ventilation can improve outcomes in these high-risk patients with hypercapnic COPD, and the new clinical practice guidelines are an important step in outlining appropriate management. Further progress is needed to delineate an individualized approach based on underlying patient pathophysiology, COPD manifestations/phenotypes, and systems-based practice considerations.
Dr. Orr is Assistant Professor, Division of Pulmonary, Critical Care, and Sleep Medicine, UC San Diego.
Patients with COPD may develop sustained hypercapnia, often defined as an awake arterial PCO2 of >45 mm Hg. Other synonymous terms include alveolar hypoventilation or chronic hypercapnic respiratory failure, noting that the specific terminology used may reflect local practice, an assessment of patient severity, or specific insurance requirements. Regardless, available data suggest that hypercapnic COPD patients are at high risk for adverse health outcomes (Yang H, et al. BMJ Open. 2015;5[12]:e008909). Moreover, there appears to have been a growing interest in this population driven by a focus on reducing COPD hospitalizations, increasing recognition of sleep disordered breathing, and progress in potential therapeutic strategies.
There are a number of factors that might drive COPD patients to develop hypercapnia. Lower airway obstruction, expiratory flow limitation and air trapping cause mechanical load on breathing, as well as a trade-off between time spent in inspiration vs prolonged expiration. The function of the diaphragm is impacted by hyperinflation leading to mal-positioning, as well as possibly by local and/or systemic myopathy. The net result is often decreased overall minute ventilation. In terms of gas exchange, increased dead space and ventilation-perfusion mismatching leads to reduced efficiency of ventilation towards CO2 removal. Breathing changes during sleep play an important role, as evidenced by worsened hypercapnia during sleep that can drive chronic CO2 retention (O’Donoghue FJ, et al. Eur Respir J. 2003;21[6]:977). The pathogenesis includes reduced central respiratory drive, increased upper airway resistance and/or obstructive hypopneas and apneas, and respiratory muscle atonia, particularly during REM sleep. The extent to which each of these factors contributes to hypercapnia varies across individual patients, in accordance with the known substantial heterogeneity of COPD. Regardless of underlying traits, patients with COPD who develop hypercapnia have sufficiently severe perturbations to disrupt the normally tight control over CO2 homeostasis.
Nocturnal home noninvasive ventilation (NIV) has been examined as a potential therapeutic strategy for patients with hypercapnic COPD. While older studies have not shown consistent benefits, more recent evidence suggests that NIV can reduce hospitalizations, improve quality of life, and potentially reduce mortality among those with hypercapnic COPD. Accordingly, the American Thoracic Society recently released a clinical practice guideline regarding the use of NIV in patients with chronic stable hypercapnic COPD (Macrea M, et al. Am J Respir Crit Care Med. 2020;202[4]:e74-e87). Recommendations from the guideline included:
1) The use of nocturnal NIV for patients with chronic stable hypercapnic COPD
2) Screening for OSA before initiation of long-term NIV
3) Not using in-hospital initiation of long-term NIV after an episode of acute or chronic hypercapnic respiratory failure, favoring instead reassessment for NIV at 2–4 weeks after resolution
4) Not using an in-laboratory overnight PSG to initially titrate NIV
5) Targeting normalization of PaCO2.
Although it now seems clear that efforts should be made to use NIV in COPD to decrease chronic hypercapnia, there are a number of important questions that remain, particularly surrounding the topic of concurrent OSA, titration, and devices:
• What is the appropriate approach towards patients with suspected concurrent OSA? Most studies of NIV have excluded patients with OSA, or otherwise at higher risk of OSA. Nonetheless, such patients may be common, both based on continued high prevalence of obesity, as well as the potential role that upper airway obstructive events may play towards elevations in CO2 (Resta O., et al. Sleep Breath. 2002;6[1]:11-8). COPD epidemiological studies indicate obesity as a risk factor for several poor outcomes, including severe COPD exacerbation (Lambert AA, et al. Chest. 2017;151[1]:68-77), while studies of COPD and OSA suggest that the presence of hypercapnia defines a high-risk group Jaoude P., Lung. 2014;192:215). Recognizing the potential importance of OSA in this group, ATS guidelines recommend that a general questionnaire-based screening be performed. If screening is positive, the implication would be to perform diagnostic polysomnography to confirm the diagnosis of OSA. However, this may be a challenge for chronically ill patients, and likely would result in delays in NIV initiation. Of note, emerging evidence suggests that home sleep apnea testing (HSAT) might have reasonable accuracy in this group, which may facilitate formal diagnosis. Other concerns in this area include the lack of questionnaire validation in COPD patients.
• Should patients with OSA be managed differently than those without OSA? A diagnosis of OSA might impact several subsequent management decisions related to appropriate NIV therapy and titration. Patients with OSA have increased upper airway collapsibility, which might necessitate higher EPAP support than the minimal EPAP used in NIV trials with non-OSA patients (often fixed at 4 cm water). Potential strategies for optimizing EPAP include use of an NIV device with auto-titrating EPAP, titration in the sleep laboratory (discussed below), or outpatient titration based on clinical parameters and subsequent device download follow-up. On the other hand, one might consider all patients to be at risk for upper airway obstruction and need for additional EPAP titration, which would obviate the need for OSA diagnostic testing.
• What is the role of the sleep laboratory towards successful titration? The inpatient hospital setting has been the traditional site to initiate home NIV in some institutions but is highly resource intensive and increasingly impractical in many health systems. On the other hand, advances in home remote device monitoring now provide the clinician with the ability to examine daily usage, estimated leak, tidal volumes, respiratory rate, and other parameters – often reported as recently as the prior night. In addition, setting changes can be made via these remote monitoring tools (for nonventilator devices), allowing titration to be performed over time on outpatients. Several studies support the effectiveness of this approach over hospital titration in neuromuscular disease and now in COPD (Duiverman ML, et al. Thorax. 2020;75[3]:244-52). Similarly, data suggest that titration under polysomnographic guidance might not be necessary (Patout M, Arbane G, Cuvelier A, Muir JF, Hart N, Murphy PB. Polysomnography versus limited respiratory monitoring and nurse-led titration to optimize non-invasive ventilation set-up: a pilot randomised clinical trial. Thorax. 2019;74:83-86).
Limitations towards the sleep lab as the site of initial titration include waiting time, cost and insurance coverage, and the need to accommodate issues such as impaired mobility or reliance on a caretaker. In addition, titration goals must be clearly outlined in protocols and via staff training specific to NIV. The sleep laboratory may be most appropriately utilized in the minority of patients in whom outpatient titration is unsuccessful. Relatively common issues that might be best addressed in the lab setting include excessive mask leaks, residual apneas and hypopneas, failure to control CO2, or other sleep complaints. In general, studies should probably be focused primarily on titrating EPAP to alleviate upper airway obstructive events. The goals in terms of IPAP titration (or ventilation titration, in the case of “VAPS” modes) are less clear, and overly aggressive increases may complicate the picture with excessive leaks or airway obstruction due to glottic closure. Attempting to accomplish “too much” often leads to a study with limited utility. In contrast, simply performing the study in the patient’s home settings can provide useful diagnostic information regarding the problem one is trying to solve.
• When and where should one initiate NIV following a severe COPD exacerbation? In contrast to the ATS guidelines, the European Respiratory Society guidelines suggest that patients recovering from severe COPD exacerbations be initiated on NIV during that hospitalization, noting that this is a group at high risk for early rehospitalization and mortality (Ergan B, et al. Eur Respir J. 2019;54[3]:1901003). ATS guidelines had the concern of unnecessary start of NIV in those who might normalize their CO2 after recovery, and the possibility of prolonging hospitalizations for titration. For the clinician, the decision will probably be individualized based on risk and available resources. For patients with frequent ICU admissions and/or difficulty with close outpatient follow-up, earlier NIV initiation is certainly a reasonable approach, but adherence and effectiveness remains a concern and, thus, more data are needed.
• Which patients should receive a bedside respiratory assist device (RAD, i.e., BIPAP machine) vs. a noninvasive ventilator? Two classes of devices can be used for home NIV. While both can provide similar positive pressure ventilation, ventilators are designed as life support with alarms and batteries, and may have modes not otherwise available (e.g., auto-titrating EPAP). On the other hand, RAD devices are more convenient for patients and less expensive, but difficult qualification requirements (particularly for devices capable of Bilevel ST or VAPS) have likely resulted in their underutilization. CHEST is spearheading an effort to reconsider Medicare coverage determinations (current rules are from 1998), which will hopefully better align device qualification requirements with emerging evidence regarding patient needs and preferences.
Home non-invasive ventilation can improve outcomes in these high-risk patients with hypercapnic COPD, and the new clinical practice guidelines are an important step in outlining appropriate management. Further progress is needed to delineate an individualized approach based on underlying patient pathophysiology, COPD manifestations/phenotypes, and systems-based practice considerations.
Dr. Orr is Assistant Professor, Division of Pulmonary, Critical Care, and Sleep Medicine, UC San Diego.
Disaster response and global health. Interstitial and diffuse lung disease. Practice operations. Transplant. Women’s lung health.
Disaster response and global health
One step forward, two back…
No adult alive today will live to see global gender parity. The 2020 World Economic Forum Global Gender Gap Report, published December 2019, assessed four dimensions of gender inequality – health, economic opportunities, educational advancement, and political empowerment.
The report stated that despite some advances, overall global gender parity would not be reached for 99 years. The gender gap is not solely a developing nation’s problem. The US standing as the 51st in gender parity fell to 53rd during the previous 2-year period. And these numbers were before Covid COVID-19.
Disasters, including pandemics, negatively affect female subjects disproportionately. Covid COVID-19 has unmasked and exacerbated both gender and minority disparity. Global health care workers (HCW) are overwhelmingly female, exposing them to a higher risk of contagion. This risk was exceptionally high among Black, Asian, and minority ethnic HCW (Nguyen et al. Lancet Public Health. 2020;5[9]:E475). The gender pay gap, where women are paid 80% of their male counterparts and women of color make 63%, has led to a greater financial burden among female HCW during Covid COVID-19. Women, including HCW, provide the majority of the unpaid work, i.e., childcare, elder care, and home care. 2020 saw an unprecedented loss of women in the workplace, including health care. Both clinical practice and research have been affected. The long- term effect on women HCW careers is unknown at present. Global gross domestic product growth loss due to this decline in the female workforce is estimated at 1 trillion USD over the next decade.
Disaster and gender parity are entwined. Covid COVID-19 has revealed the persistence of inequalities that nees to be considered in future disaster planning.
Mary Jane Reed, MD, FCCP
Steering Committee Ex-Officio
Interstitial and diffuse lung disease
Emergence and benefits of home monitoring and telemedicine for patients with ILD
Patients with interstitial lung disease (ILD) require regular monitoring with outpatient clinic visits and pulmonary function tests.
The emergence of COVID-19 forced an unprecedented transition to telemedicine and a new reliance on home monitoring. Home spirometry enables quick detection of rapidly progressive disease and is more sensitive than hospital-based spirometry in predicting prognosis (Russel, et al. Am J Respir Crit Care Med. 2016;194[8]:989). Patients with idiopathic pulmonary fibrosis randomized to a home monitoring program had improved psychological wellbeing and higher patient satisfaction with individually tailored treatment decisions (Moor, et al. Am J Respir Crit Care Med. 2020;202[3]:393). However, there are some inaccuracies in home monitoring. For instance, pulse oximetry is less reliable in African American patients receiving supplemental oxygen (Sjoding, et al. N Engl J Med. 2020;383:2477). It is critical to protect ILD patients from potential COVID-19 exposure given the high risk of serious complications. Telemedicine should be offered to all patients and may actually increase access to care in ILD patients, a population with disabling dyspnea and supplemental oxygen needs that requires specialist care unavailable in many geographic regions. African American patients, those older than 65, and patients with lower socioeconomic status are less willing to engage in videoconferencing (Fischer, et al. JAMA Netw Open. 2020;3[10]:e2022302). It is essential that telephone visits be offered to minimize disparities in access to care. Many telemedicine platforms enable caregivers and family members to attend visits from separate locations and provide a unique opportunity to address advance care planning. In-person visits should be arranged for patients with no access to internet or telephone or those with poor medical literacy or insufficient social support to conduct a productive remote visit. Telemedicine and home monitoring have proved invaluable during the COVID-19 pandemic and have the potential to continually increase access to and quality of care.
Rebecca Anna Gersten, MD
Steering Committee Member
Practice operations
Use of media platforms to eliminate the COVID-19 infodemic
We were shocked when we read a tweet in December 2020 from a health care worker stating, “My biggest concern is the lack of data and the quick development time. Feels like we are a bunch of guinea pigs” in reference to the new COVID-19 vaccine.
I reflected back on the last pandemic in 2009, H1N1, and remembered when the new vaccine developed in 174 days was first released to pregnant women and children after phase 3 trials. How did we get here? What do we do to fix it?
This misinformation is labeled as the “COVID-19 infodemic.” In the last year, we have seen the media, more specifically social platforms, quickly spread medical misinformation. In the book “Made to Stick: Why Some Ideas Survive and Some Die,” the authors described core elements that make an idea “sticky.” Use of those exact same sticky techniques can be used to circulate accurate information and to halt the spread of this infodemic. Although, numerous media companies, including Twitter, are making an effort to remove the false content from their platforms, their efforts require a lengthy process and are delayed. Therefore, it is crucial for the public health figures and community at large in partnership with various national organizations to establish a robust connection with the social platforms in a dynamic and timely fashion to help spread the verified information across social media, digital and traditional media outlets.
The UN has launched an initiate called “Verified.” This is a worldwide effort to help individuals spread reliable information regarding COVID-19 to their friends and families via social platforms as various media platforms and businesses have partnered with Verified. Also, we encourage our members to access the CHEST COVID-19 resource center and benefit from the various clinical and practice management tools along with validated patient information materials.
Roozera Khan, DO, FCCP
Steering Committee Member
Humayun Anjum, MD, FCCP
Chair
References
1. The Lancet Infectious Diseases-Editorial. The COVID-19 infodemic. Lancet Infect Dis. 2020;20(8):875.
2. Tangcharoensathien V, et al. Framework for managing the COVID-19 infodemic: methods and results of an online, crowdsourced WHO technical consultation. J Med Internet Res. 2020;22e19659.
3. Verified. https://shareverified.com/en/about. Accessed Feb 18, 2021.
Transplant
COVID-19 + lung transplant
The COVID-19 pandemic has created a dilemma for lung transplantation, with a new group of patients with refractory respiratory failure secondary to the viral illness. As transplant centers worldwide receive referrals for COVID-19 related respiratory failure, information regarding evaluation, listing, and posttransplant care continues to be published, but further research will be needed to care for this complex population.
The first lung transplant for COVID-19 in the United States occurred at Northwestern Hospital on June 5th, 2020,and was publicized for its innovativeness. Information from their three lung transplants completed thus far includes information regarding pathologic findings of the explanted lung tissue; pulmonary fibrosis was the dominant feature, suggesting COVID-19-induced acute respiratory distress syndrome with prolonged time supported by mechanical support may only be survivable with the use of lung transplant (Bharat, et al. Sci Transl Med. 2020;12(574):eabe4282).
Lung transplant in the setting of COVID-19 fibrosis increases surgical complexity as well, with case reports of dense adhesions and distortion of regular surgical planes (Bharat, et al. Sci. Transl. Med. 2020; Lang, et al. Lancet Respir Med. 2020;8:1057). Recognizing the difficulty with deciding to use transplantation after an infectious disease, The International Society for Heart and Lung Transplant (ISHLT) has created guidelines regarding indications for transplantation (ISHLT.org). Continued research will be necessary to identify those at the highest likelihood for success from transplantation, preparation for the increased complexity, and long-term outcomes. Further information is available in a CHEST webinar titled “Lung Transplantation in the Era of COVID-19” .
Clauden Louis, MD
Grant Turner, MD
Fellows-in-Training NetWork Members
Women’s lung health
Pregnancy in cystic fibrosis
The newest in the line of modulator therapy, Trikafta (elexacaftor/tezacaftor/ivacaftor and ivacaftor), is expected to improve life expectancy and quality of life for patients with cystic fibrosis (CF). This evolution in therapy will shape how providers care for their patients, particularly women of reproductive age. Conventionally, women with significantly impaired lung function due to CF have been advised to avoid pregnancy due to potential complications for mother and baby. It is likely that now, with improved lung function while receiving Trikafta, more women will feel better equipped to attempt pregnancy.
There are several considerations in this setting, including the need for careful drug safety and monitoring, creating a plan of action for possible decline in lung function while off certain CF-related medications, and counseling on drug interactions during lactation. In our experience with women becoming pregnant while receiving Trikafta or contemplating pregnancy, all have opted to discontinue modulator therapy with declines in lung function. Trikafta does not report teratogenicity based on animal studies of the individual components of the drug; however, ivacaftor is known to cause impairment in fertility and reproductive indices, including nonviable embryos and implantation failure in a rat model at five times the maximum recommended human dose, dosed prior to and during early embryogenesis. Small mammal models have decreased birth weight at high doses of elexacaftor, tezacaftor and ivacaftor administered individually. There is evidence of placental transfer of ivacaftor and breast milk concentrations of tezacaftor and ivacaftor are higher than plasma concentrations in rats. There are no human data in parturient or lactating women or infants. Three women became pregnant during the phase 3 clinical study of Trikafta, one with elective termination, one pregnancy was carried to full term with normal birth outcome, and one ended in a spontaneous abortion, which was deemed not to be related to the study drug. Translating this information into recommendations for patients has important implications.
Debasree Banerjee, MD, MS
Steering Committee Member
Disaster response and global health
One step forward, two back…
No adult alive today will live to see global gender parity. The 2020 World Economic Forum Global Gender Gap Report, published December 2019, assessed four dimensions of gender inequality – health, economic opportunities, educational advancement, and political empowerment.
The report stated that despite some advances, overall global gender parity would not be reached for 99 years. The gender gap is not solely a developing nation’s problem. The US standing as the 51st in gender parity fell to 53rd during the previous 2-year period. And these numbers were before Covid COVID-19.
Disasters, including pandemics, negatively affect female subjects disproportionately. Covid COVID-19 has unmasked and exacerbated both gender and minority disparity. Global health care workers (HCW) are overwhelmingly female, exposing them to a higher risk of contagion. This risk was exceptionally high among Black, Asian, and minority ethnic HCW (Nguyen et al. Lancet Public Health. 2020;5[9]:E475). The gender pay gap, where women are paid 80% of their male counterparts and women of color make 63%, has led to a greater financial burden among female HCW during Covid COVID-19. Women, including HCW, provide the majority of the unpaid work, i.e., childcare, elder care, and home care. 2020 saw an unprecedented loss of women in the workplace, including health care. Both clinical practice and research have been affected. The long- term effect on women HCW careers is unknown at present. Global gross domestic product growth loss due to this decline in the female workforce is estimated at 1 trillion USD over the next decade.
Disaster and gender parity are entwined. Covid COVID-19 has revealed the persistence of inequalities that nees to be considered in future disaster planning.
Mary Jane Reed, MD, FCCP
Steering Committee Ex-Officio
Interstitial and diffuse lung disease
Emergence and benefits of home monitoring and telemedicine for patients with ILD
Patients with interstitial lung disease (ILD) require regular monitoring with outpatient clinic visits and pulmonary function tests.
The emergence of COVID-19 forced an unprecedented transition to telemedicine and a new reliance on home monitoring. Home spirometry enables quick detection of rapidly progressive disease and is more sensitive than hospital-based spirometry in predicting prognosis (Russel, et al. Am J Respir Crit Care Med. 2016;194[8]:989). Patients with idiopathic pulmonary fibrosis randomized to a home monitoring program had improved psychological wellbeing and higher patient satisfaction with individually tailored treatment decisions (Moor, et al. Am J Respir Crit Care Med. 2020;202[3]:393). However, there are some inaccuracies in home monitoring. For instance, pulse oximetry is less reliable in African American patients receiving supplemental oxygen (Sjoding, et al. N Engl J Med. 2020;383:2477). It is critical to protect ILD patients from potential COVID-19 exposure given the high risk of serious complications. Telemedicine should be offered to all patients and may actually increase access to care in ILD patients, a population with disabling dyspnea and supplemental oxygen needs that requires specialist care unavailable in many geographic regions. African American patients, those older than 65, and patients with lower socioeconomic status are less willing to engage in videoconferencing (Fischer, et al. JAMA Netw Open. 2020;3[10]:e2022302). It is essential that telephone visits be offered to minimize disparities in access to care. Many telemedicine platforms enable caregivers and family members to attend visits from separate locations and provide a unique opportunity to address advance care planning. In-person visits should be arranged for patients with no access to internet or telephone or those with poor medical literacy or insufficient social support to conduct a productive remote visit. Telemedicine and home monitoring have proved invaluable during the COVID-19 pandemic and have the potential to continually increase access to and quality of care.
Rebecca Anna Gersten, MD
Steering Committee Member
Practice operations
Use of media platforms to eliminate the COVID-19 infodemic
We were shocked when we read a tweet in December 2020 from a health care worker stating, “My biggest concern is the lack of data and the quick development time. Feels like we are a bunch of guinea pigs” in reference to the new COVID-19 vaccine.
I reflected back on the last pandemic in 2009, H1N1, and remembered when the new vaccine developed in 174 days was first released to pregnant women and children after phase 3 trials. How did we get here? What do we do to fix it?
This misinformation is labeled as the “COVID-19 infodemic.” In the last year, we have seen the media, more specifically social platforms, quickly spread medical misinformation. In the book “Made to Stick: Why Some Ideas Survive and Some Die,” the authors described core elements that make an idea “sticky.” Use of those exact same sticky techniques can be used to circulate accurate information and to halt the spread of this infodemic. Although, numerous media companies, including Twitter, are making an effort to remove the false content from their platforms, their efforts require a lengthy process and are delayed. Therefore, it is crucial for the public health figures and community at large in partnership with various national organizations to establish a robust connection with the social platforms in a dynamic and timely fashion to help spread the verified information across social media, digital and traditional media outlets.
The UN has launched an initiate called “Verified.” This is a worldwide effort to help individuals spread reliable information regarding COVID-19 to their friends and families via social platforms as various media platforms and businesses have partnered with Verified. Also, we encourage our members to access the CHEST COVID-19 resource center and benefit from the various clinical and practice management tools along with validated patient information materials.
Roozera Khan, DO, FCCP
Steering Committee Member
Humayun Anjum, MD, FCCP
Chair
References
1. The Lancet Infectious Diseases-Editorial. The COVID-19 infodemic. Lancet Infect Dis. 2020;20(8):875.
2. Tangcharoensathien V, et al. Framework for managing the COVID-19 infodemic: methods and results of an online, crowdsourced WHO technical consultation. J Med Internet Res. 2020;22e19659.
3. Verified. https://shareverified.com/en/about. Accessed Feb 18, 2021.
Transplant
COVID-19 + lung transplant
The COVID-19 pandemic has created a dilemma for lung transplantation, with a new group of patients with refractory respiratory failure secondary to the viral illness. As transplant centers worldwide receive referrals for COVID-19 related respiratory failure, information regarding evaluation, listing, and posttransplant care continues to be published, but further research will be needed to care for this complex population.
The first lung transplant for COVID-19 in the United States occurred at Northwestern Hospital on June 5th, 2020,and was publicized for its innovativeness. Information from their three lung transplants completed thus far includes information regarding pathologic findings of the explanted lung tissue; pulmonary fibrosis was the dominant feature, suggesting COVID-19-induced acute respiratory distress syndrome with prolonged time supported by mechanical support may only be survivable with the use of lung transplant (Bharat, et al. Sci Transl Med. 2020;12(574):eabe4282).
Lung transplant in the setting of COVID-19 fibrosis increases surgical complexity as well, with case reports of dense adhesions and distortion of regular surgical planes (Bharat, et al. Sci. Transl. Med. 2020; Lang, et al. Lancet Respir Med. 2020;8:1057). Recognizing the difficulty with deciding to use transplantation after an infectious disease, The International Society for Heart and Lung Transplant (ISHLT) has created guidelines regarding indications for transplantation (ISHLT.org). Continued research will be necessary to identify those at the highest likelihood for success from transplantation, preparation for the increased complexity, and long-term outcomes. Further information is available in a CHEST webinar titled “Lung Transplantation in the Era of COVID-19” .
Clauden Louis, MD
Grant Turner, MD
Fellows-in-Training NetWork Members
Women’s lung health
Pregnancy in cystic fibrosis
The newest in the line of modulator therapy, Trikafta (elexacaftor/tezacaftor/ivacaftor and ivacaftor), is expected to improve life expectancy and quality of life for patients with cystic fibrosis (CF). This evolution in therapy will shape how providers care for their patients, particularly women of reproductive age. Conventionally, women with significantly impaired lung function due to CF have been advised to avoid pregnancy due to potential complications for mother and baby. It is likely that now, with improved lung function while receiving Trikafta, more women will feel better equipped to attempt pregnancy.
There are several considerations in this setting, including the need for careful drug safety and monitoring, creating a plan of action for possible decline in lung function while off certain CF-related medications, and counseling on drug interactions during lactation. In our experience with women becoming pregnant while receiving Trikafta or contemplating pregnancy, all have opted to discontinue modulator therapy with declines in lung function. Trikafta does not report teratogenicity based on animal studies of the individual components of the drug; however, ivacaftor is known to cause impairment in fertility and reproductive indices, including nonviable embryos and implantation failure in a rat model at five times the maximum recommended human dose, dosed prior to and during early embryogenesis. Small mammal models have decreased birth weight at high doses of elexacaftor, tezacaftor and ivacaftor administered individually. There is evidence of placental transfer of ivacaftor and breast milk concentrations of tezacaftor and ivacaftor are higher than plasma concentrations in rats. There are no human data in parturient or lactating women or infants. Three women became pregnant during the phase 3 clinical study of Trikafta, one with elective termination, one pregnancy was carried to full term with normal birth outcome, and one ended in a spontaneous abortion, which was deemed not to be related to the study drug. Translating this information into recommendations for patients has important implications.
Debasree Banerjee, MD, MS
Steering Committee Member
Disaster response and global health
One step forward, two back…
No adult alive today will live to see global gender parity. The 2020 World Economic Forum Global Gender Gap Report, published December 2019, assessed four dimensions of gender inequality – health, economic opportunities, educational advancement, and political empowerment.
The report stated that despite some advances, overall global gender parity would not be reached for 99 years. The gender gap is not solely a developing nation’s problem. The US standing as the 51st in gender parity fell to 53rd during the previous 2-year period. And these numbers were before Covid COVID-19.
Disasters, including pandemics, negatively affect female subjects disproportionately. Covid COVID-19 has unmasked and exacerbated both gender and minority disparity. Global health care workers (HCW) are overwhelmingly female, exposing them to a higher risk of contagion. This risk was exceptionally high among Black, Asian, and minority ethnic HCW (Nguyen et al. Lancet Public Health. 2020;5[9]:E475). The gender pay gap, where women are paid 80% of their male counterparts and women of color make 63%, has led to a greater financial burden among female HCW during Covid COVID-19. Women, including HCW, provide the majority of the unpaid work, i.e., childcare, elder care, and home care. 2020 saw an unprecedented loss of women in the workplace, including health care. Both clinical practice and research have been affected. The long- term effect on women HCW careers is unknown at present. Global gross domestic product growth loss due to this decline in the female workforce is estimated at 1 trillion USD over the next decade.
Disaster and gender parity are entwined. Covid COVID-19 has revealed the persistence of inequalities that nees to be considered in future disaster planning.
Mary Jane Reed, MD, FCCP
Steering Committee Ex-Officio
Interstitial and diffuse lung disease
Emergence and benefits of home monitoring and telemedicine for patients with ILD
Patients with interstitial lung disease (ILD) require regular monitoring with outpatient clinic visits and pulmonary function tests.
The emergence of COVID-19 forced an unprecedented transition to telemedicine and a new reliance on home monitoring. Home spirometry enables quick detection of rapidly progressive disease and is more sensitive than hospital-based spirometry in predicting prognosis (Russel, et al. Am J Respir Crit Care Med. 2016;194[8]:989). Patients with idiopathic pulmonary fibrosis randomized to a home monitoring program had improved psychological wellbeing and higher patient satisfaction with individually tailored treatment decisions (Moor, et al. Am J Respir Crit Care Med. 2020;202[3]:393). However, there are some inaccuracies in home monitoring. For instance, pulse oximetry is less reliable in African American patients receiving supplemental oxygen (Sjoding, et al. N Engl J Med. 2020;383:2477). It is critical to protect ILD patients from potential COVID-19 exposure given the high risk of serious complications. Telemedicine should be offered to all patients and may actually increase access to care in ILD patients, a population with disabling dyspnea and supplemental oxygen needs that requires specialist care unavailable in many geographic regions. African American patients, those older than 65, and patients with lower socioeconomic status are less willing to engage in videoconferencing (Fischer, et al. JAMA Netw Open. 2020;3[10]:e2022302). It is essential that telephone visits be offered to minimize disparities in access to care. Many telemedicine platforms enable caregivers and family members to attend visits from separate locations and provide a unique opportunity to address advance care planning. In-person visits should be arranged for patients with no access to internet or telephone or those with poor medical literacy or insufficient social support to conduct a productive remote visit. Telemedicine and home monitoring have proved invaluable during the COVID-19 pandemic and have the potential to continually increase access to and quality of care.
Rebecca Anna Gersten, MD
Steering Committee Member
Practice operations
Use of media platforms to eliminate the COVID-19 infodemic
We were shocked when we read a tweet in December 2020 from a health care worker stating, “My biggest concern is the lack of data and the quick development time. Feels like we are a bunch of guinea pigs” in reference to the new COVID-19 vaccine.
I reflected back on the last pandemic in 2009, H1N1, and remembered when the new vaccine developed in 174 days was first released to pregnant women and children after phase 3 trials. How did we get here? What do we do to fix it?
This misinformation is labeled as the “COVID-19 infodemic.” In the last year, we have seen the media, more specifically social platforms, quickly spread medical misinformation. In the book “Made to Stick: Why Some Ideas Survive and Some Die,” the authors described core elements that make an idea “sticky.” Use of those exact same sticky techniques can be used to circulate accurate information and to halt the spread of this infodemic. Although, numerous media companies, including Twitter, are making an effort to remove the false content from their platforms, their efforts require a lengthy process and are delayed. Therefore, it is crucial for the public health figures and community at large in partnership with various national organizations to establish a robust connection with the social platforms in a dynamic and timely fashion to help spread the verified information across social media, digital and traditional media outlets.
The UN has launched an initiate called “Verified.” This is a worldwide effort to help individuals spread reliable information regarding COVID-19 to their friends and families via social platforms as various media platforms and businesses have partnered with Verified. Also, we encourage our members to access the CHEST COVID-19 resource center and benefit from the various clinical and practice management tools along with validated patient information materials.
Roozera Khan, DO, FCCP
Steering Committee Member
Humayun Anjum, MD, FCCP
Chair
References
1. The Lancet Infectious Diseases-Editorial. The COVID-19 infodemic. Lancet Infect Dis. 2020;20(8):875.
2. Tangcharoensathien V, et al. Framework for managing the COVID-19 infodemic: methods and results of an online, crowdsourced WHO technical consultation. J Med Internet Res. 2020;22e19659.
3. Verified. https://shareverified.com/en/about. Accessed Feb 18, 2021.
Transplant
COVID-19 + lung transplant
The COVID-19 pandemic has created a dilemma for lung transplantation, with a new group of patients with refractory respiratory failure secondary to the viral illness. As transplant centers worldwide receive referrals for COVID-19 related respiratory failure, information regarding evaluation, listing, and posttransplant care continues to be published, but further research will be needed to care for this complex population.
The first lung transplant for COVID-19 in the United States occurred at Northwestern Hospital on June 5th, 2020,and was publicized for its innovativeness. Information from their three lung transplants completed thus far includes information regarding pathologic findings of the explanted lung tissue; pulmonary fibrosis was the dominant feature, suggesting COVID-19-induced acute respiratory distress syndrome with prolonged time supported by mechanical support may only be survivable with the use of lung transplant (Bharat, et al. Sci Transl Med. 2020;12(574):eabe4282).
Lung transplant in the setting of COVID-19 fibrosis increases surgical complexity as well, with case reports of dense adhesions and distortion of regular surgical planes (Bharat, et al. Sci. Transl. Med. 2020; Lang, et al. Lancet Respir Med. 2020;8:1057). Recognizing the difficulty with deciding to use transplantation after an infectious disease, The International Society for Heart and Lung Transplant (ISHLT) has created guidelines regarding indications for transplantation (ISHLT.org). Continued research will be necessary to identify those at the highest likelihood for success from transplantation, preparation for the increased complexity, and long-term outcomes. Further information is available in a CHEST webinar titled “Lung Transplantation in the Era of COVID-19” .
Clauden Louis, MD
Grant Turner, MD
Fellows-in-Training NetWork Members
Women’s lung health
Pregnancy in cystic fibrosis
The newest in the line of modulator therapy, Trikafta (elexacaftor/tezacaftor/ivacaftor and ivacaftor), is expected to improve life expectancy and quality of life for patients with cystic fibrosis (CF). This evolution in therapy will shape how providers care for their patients, particularly women of reproductive age. Conventionally, women with significantly impaired lung function due to CF have been advised to avoid pregnancy due to potential complications for mother and baby. It is likely that now, with improved lung function while receiving Trikafta, more women will feel better equipped to attempt pregnancy.
There are several considerations in this setting, including the need for careful drug safety and monitoring, creating a plan of action for possible decline in lung function while off certain CF-related medications, and counseling on drug interactions during lactation. In our experience with women becoming pregnant while receiving Trikafta or contemplating pregnancy, all have opted to discontinue modulator therapy with declines in lung function. Trikafta does not report teratogenicity based on animal studies of the individual components of the drug; however, ivacaftor is known to cause impairment in fertility and reproductive indices, including nonviable embryos and implantation failure in a rat model at five times the maximum recommended human dose, dosed prior to and during early embryogenesis. Small mammal models have decreased birth weight at high doses of elexacaftor, tezacaftor and ivacaftor administered individually. There is evidence of placental transfer of ivacaftor and breast milk concentrations of tezacaftor and ivacaftor are higher than plasma concentrations in rats. There are no human data in parturient or lactating women or infants. Three women became pregnant during the phase 3 clinical study of Trikafta, one with elective termination, one pregnancy was carried to full term with normal birth outcome, and one ended in a spontaneous abortion, which was deemed not to be related to the study drug. Translating this information into recommendations for patients has important implications.
Debasree Banerjee, MD, MS
Steering Committee Member