User login
ACG: CRC screening should start at age 45
The starting age was previously 50 years for most patients. However, for Black patients, the starting age was lowered to 45 years in 2005.
The new guidance brings the ACG in line with recommendations of the American Cancer Society, which lowered the starting age to 45 years for average-risk individuals in 2018.
However, the U.S. Preventive Services Task Force, the Multi-Specialty Task Force, and the American College of Physicians still recommend that CRC screening begin at the age of 50.
The new ACG guideline were published in March 2021 in the American Journal of Gastroenterology. The last time they were updated was in 2009.
The ACG said that the move was made in light of reports of an increase in the incidence of CRC in adults younger than 50.
“It has been estimated that [in the United States] persons born around 1990 have twice the risk of colon cancer and four times the risk of rectal cancer, compared with those born around 1950,” guideline author Aasma Shaukat, MD, MPH, University of Minnesota, Minneapolis, and colleagues pointed out.
“The fact that other developed countries are reporting similar increases in early-onset CRC and birth-cohort effects suggests that the Western lifestyle (especially exemplified by the obesity epidemic) is a significant contributor,” the authors added.
The new ACG guideline emphasize the importance of initiating CRC screening for average-risk patients aged 50-75 years. “Given that current rates of screening uptake are close to 60% (57.9% ages 50-64 and 62.4% ages 50-75), expanding the population to be screened may reduce these rates as emphasis shifts to screening 45- to 49-year-olds at the expense of efforts to screen the unscreened 50- to 75-year-olds,” the authors commented.
Now, however, the guideline suggests that the decision to continue screening after age 75 should be individualized. It notes that the benefits of screening are limited for those who are not expected to live for another 7-10 years. For patients with a family history of CRC, the guideline authors recommended initiating CRC screening at the age of 40 for patients with one or two first-degree relatives with either CRC or advanced colorectal polyps.
They also recommend screening colonoscopy over any other screening modality if the first-degree relative is younger than 60 or if two or more first-degree relatives of any age have CRC or advanced colorectal polyps. For such patients, screening should be repeated every 5 years.
For screening average-risk individuals, either colonoscopy or fecal immunochemical testing (FIT) is recommended. If colonoscopy is used, it should be repeated every 10 years. FIT should be conducted on an annual basis.
This is somewhat in contrast to recent changes proposed by the American Gastroenterological Association. The AGA recommends greater use of noninvasive testing, such as with fecal occult blood tests, initially. It recommends that initial colonoscopy be used only for patients at high risk for CRC.
For individuals unwilling or unable to undergo colonoscopy or FIT, the ACG suggests flexible sigmoidoscopy, multitarget stool DNA testing, CT colonography, or colon capsule. Only colonoscopy is a single-step test; all other screening modalities require a follow-up colonoscopy if test results are positive.
“We recommend against the use of aspirin as a substitute for CRC screening,” the ACG members emphasized. Rather, they suggest that the use of low-dose aspirin be considered only for patients aged 50-69 years whose risk for cardiovascular disease over the next 10 years is at least 10% and who are at low risk for bleeding.
To reduce their risk for CRC, patients need to take aspirin for at least 10 years, they pointed out.
Quality indicators
For endoscopists who perform colonoscopy, the ACG recommended that all operators determine their individual cecal intubation rates, adenoma detection rates, and withdrawal times. They also recommended that endoscopists spend at least 6 minutes inspecting the mucosa during withdrawal and achieve a cecal intubation rate of at least 95% for all patients screened.
The ACG recommended remedial training for any provider whose adenoma detection rate is less than 25%.
Screening rates dropped during pandemic
The authors of the new recommendations also pointed out that, despite public health initiatives to boost CRC screening in the United States and the availability of multiple screening modalities, almost one-third of individuals who are eligible for CRC screening do not undergo screening.
Moreover, the proportion of individuals not being screened has reportedly increased during the pandemic. In one report, claims data for colonoscopies dropped by 90% during April. “Colorectal cancer screening rates must be optimized to reach the aspirational target of >80%,” the authors emphasized.
“A recommendation to be screened by a PCP [primary care provider] – who is known and trusted by the person – is clearly effective in raising participation,” they added.
Dr. Shaukat has served as a scientific consultant for Iterative Scopes and Freenome. Other ACG guideline authors reported numerous financial relationships.
A version of this article first appeared on Medscape.com.
The starting age was previously 50 years for most patients. However, for Black patients, the starting age was lowered to 45 years in 2005.
The new guidance brings the ACG in line with recommendations of the American Cancer Society, which lowered the starting age to 45 years for average-risk individuals in 2018.
However, the U.S. Preventive Services Task Force, the Multi-Specialty Task Force, and the American College of Physicians still recommend that CRC screening begin at the age of 50.
The new ACG guideline were published in March 2021 in the American Journal of Gastroenterology. The last time they were updated was in 2009.
The ACG said that the move was made in light of reports of an increase in the incidence of CRC in adults younger than 50.
“It has been estimated that [in the United States] persons born around 1990 have twice the risk of colon cancer and four times the risk of rectal cancer, compared with those born around 1950,” guideline author Aasma Shaukat, MD, MPH, University of Minnesota, Minneapolis, and colleagues pointed out.
“The fact that other developed countries are reporting similar increases in early-onset CRC and birth-cohort effects suggests that the Western lifestyle (especially exemplified by the obesity epidemic) is a significant contributor,” the authors added.
The new ACG guideline emphasize the importance of initiating CRC screening for average-risk patients aged 50-75 years. “Given that current rates of screening uptake are close to 60% (57.9% ages 50-64 and 62.4% ages 50-75), expanding the population to be screened may reduce these rates as emphasis shifts to screening 45- to 49-year-olds at the expense of efforts to screen the unscreened 50- to 75-year-olds,” the authors commented.
Now, however, the guideline suggests that the decision to continue screening after age 75 should be individualized. It notes that the benefits of screening are limited for those who are not expected to live for another 7-10 years. For patients with a family history of CRC, the guideline authors recommended initiating CRC screening at the age of 40 for patients with one or two first-degree relatives with either CRC or advanced colorectal polyps.
They also recommend screening colonoscopy over any other screening modality if the first-degree relative is younger than 60 or if two or more first-degree relatives of any age have CRC or advanced colorectal polyps. For such patients, screening should be repeated every 5 years.
For screening average-risk individuals, either colonoscopy or fecal immunochemical testing (FIT) is recommended. If colonoscopy is used, it should be repeated every 10 years. FIT should be conducted on an annual basis.
This is somewhat in contrast to recent changes proposed by the American Gastroenterological Association. The AGA recommends greater use of noninvasive testing, such as with fecal occult blood tests, initially. It recommends that initial colonoscopy be used only for patients at high risk for CRC.
For individuals unwilling or unable to undergo colonoscopy or FIT, the ACG suggests flexible sigmoidoscopy, multitarget stool DNA testing, CT colonography, or colon capsule. Only colonoscopy is a single-step test; all other screening modalities require a follow-up colonoscopy if test results are positive.
“We recommend against the use of aspirin as a substitute for CRC screening,” the ACG members emphasized. Rather, they suggest that the use of low-dose aspirin be considered only for patients aged 50-69 years whose risk for cardiovascular disease over the next 10 years is at least 10% and who are at low risk for bleeding.
To reduce their risk for CRC, patients need to take aspirin for at least 10 years, they pointed out.
Quality indicators
For endoscopists who perform colonoscopy, the ACG recommended that all operators determine their individual cecal intubation rates, adenoma detection rates, and withdrawal times. They also recommended that endoscopists spend at least 6 minutes inspecting the mucosa during withdrawal and achieve a cecal intubation rate of at least 95% for all patients screened.
The ACG recommended remedial training for any provider whose adenoma detection rate is less than 25%.
Screening rates dropped during pandemic
The authors of the new recommendations also pointed out that, despite public health initiatives to boost CRC screening in the United States and the availability of multiple screening modalities, almost one-third of individuals who are eligible for CRC screening do not undergo screening.
Moreover, the proportion of individuals not being screened has reportedly increased during the pandemic. In one report, claims data for colonoscopies dropped by 90% during April. “Colorectal cancer screening rates must be optimized to reach the aspirational target of >80%,” the authors emphasized.
“A recommendation to be screened by a PCP [primary care provider] – who is known and trusted by the person – is clearly effective in raising participation,” they added.
Dr. Shaukat has served as a scientific consultant for Iterative Scopes and Freenome. Other ACG guideline authors reported numerous financial relationships.
A version of this article first appeared on Medscape.com.
The starting age was previously 50 years for most patients. However, for Black patients, the starting age was lowered to 45 years in 2005.
The new guidance brings the ACG in line with recommendations of the American Cancer Society, which lowered the starting age to 45 years for average-risk individuals in 2018.
However, the U.S. Preventive Services Task Force, the Multi-Specialty Task Force, and the American College of Physicians still recommend that CRC screening begin at the age of 50.
The new ACG guideline were published in March 2021 in the American Journal of Gastroenterology. The last time they were updated was in 2009.
The ACG said that the move was made in light of reports of an increase in the incidence of CRC in adults younger than 50.
“It has been estimated that [in the United States] persons born around 1990 have twice the risk of colon cancer and four times the risk of rectal cancer, compared with those born around 1950,” guideline author Aasma Shaukat, MD, MPH, University of Minnesota, Minneapolis, and colleagues pointed out.
“The fact that other developed countries are reporting similar increases in early-onset CRC and birth-cohort effects suggests that the Western lifestyle (especially exemplified by the obesity epidemic) is a significant contributor,” the authors added.
The new ACG guideline emphasize the importance of initiating CRC screening for average-risk patients aged 50-75 years. “Given that current rates of screening uptake are close to 60% (57.9% ages 50-64 and 62.4% ages 50-75), expanding the population to be screened may reduce these rates as emphasis shifts to screening 45- to 49-year-olds at the expense of efforts to screen the unscreened 50- to 75-year-olds,” the authors commented.
Now, however, the guideline suggests that the decision to continue screening after age 75 should be individualized. It notes that the benefits of screening are limited for those who are not expected to live for another 7-10 years. For patients with a family history of CRC, the guideline authors recommended initiating CRC screening at the age of 40 for patients with one or two first-degree relatives with either CRC or advanced colorectal polyps.
They also recommend screening colonoscopy over any other screening modality if the first-degree relative is younger than 60 or if two or more first-degree relatives of any age have CRC or advanced colorectal polyps. For such patients, screening should be repeated every 5 years.
For screening average-risk individuals, either colonoscopy or fecal immunochemical testing (FIT) is recommended. If colonoscopy is used, it should be repeated every 10 years. FIT should be conducted on an annual basis.
This is somewhat in contrast to recent changes proposed by the American Gastroenterological Association. The AGA recommends greater use of noninvasive testing, such as with fecal occult blood tests, initially. It recommends that initial colonoscopy be used only for patients at high risk for CRC.
For individuals unwilling or unable to undergo colonoscopy or FIT, the ACG suggests flexible sigmoidoscopy, multitarget stool DNA testing, CT colonography, or colon capsule. Only colonoscopy is a single-step test; all other screening modalities require a follow-up colonoscopy if test results are positive.
“We recommend against the use of aspirin as a substitute for CRC screening,” the ACG members emphasized. Rather, they suggest that the use of low-dose aspirin be considered only for patients aged 50-69 years whose risk for cardiovascular disease over the next 10 years is at least 10% and who are at low risk for bleeding.
To reduce their risk for CRC, patients need to take aspirin for at least 10 years, they pointed out.
Quality indicators
For endoscopists who perform colonoscopy, the ACG recommended that all operators determine their individual cecal intubation rates, adenoma detection rates, and withdrawal times. They also recommended that endoscopists spend at least 6 minutes inspecting the mucosa during withdrawal and achieve a cecal intubation rate of at least 95% for all patients screened.
The ACG recommended remedial training for any provider whose adenoma detection rate is less than 25%.
Screening rates dropped during pandemic
The authors of the new recommendations also pointed out that, despite public health initiatives to boost CRC screening in the United States and the availability of multiple screening modalities, almost one-third of individuals who are eligible for CRC screening do not undergo screening.
Moreover, the proportion of individuals not being screened has reportedly increased during the pandemic. In one report, claims data for colonoscopies dropped by 90% during April. “Colorectal cancer screening rates must be optimized to reach the aspirational target of >80%,” the authors emphasized.
“A recommendation to be screened by a PCP [primary care provider] – who is known and trusted by the person – is clearly effective in raising participation,” they added.
Dr. Shaukat has served as a scientific consultant for Iterative Scopes and Freenome. Other ACG guideline authors reported numerous financial relationships.
A version of this article first appeared on Medscape.com.
Mitochondrial DNA variant increases gallstone risk
A mitochondrial DNA variant may increase the risk of gallstone disease more than fourfold, according to investigators.
Mitochondrial DNA 827A>G disrupts mitochondrial function and leads to abnormal cholesterol transport, which increases gallstone development, reported Dayan Sun, of Fudan University, Shanghai, China, and colleagues.
The investigators noted that the findings add support to a genetic role in gallstone development, which could allow for identification of at-risk individuals and implementation of preventive measures.
“The etiology of gallstone disease is multifactorial; age, sex, pregnancy, diet (macronutrients, alcohol, and coffee), and other factors are involved,” the investigators wrote in Cellular and Molecular Gastroenterology and Hepatology. “Moreover, the significant familial predisposition and ethnic differences in prevalence of this disease indicate the potential influences of genetic factors.”
In 2002, Nakeeb and colleagues reported that at least 30% of gallstone disease cases stemmed from genetic factors. And genetics may play an even greater role in certain populations, such as Native Americans, among whom more than 70% of women have gallstone disease, based on a study by Everhart and colleagues.
According to Ms. Sun and colleagues, a variety of genetic drivers of gallstone disease have been identified, such as ABCG8, identified as the most common genetic risk factor by at least one study, along with a list of other rare mutations, such as one affecting CFTR that leads to altered bile composition.
Based on previous research that linked mitochondrial DNA variants with metabolic defects and, more specifically, aberrations in lipid metabolism, as well as an observed “maternal bias in the maternal transmission of gallstone disease” that suggest mitochondrial influence, the investigators looked for patterns specifically in mitochondrial DNA variants among patients with gallstones.
The study enrolled 104 probands with confirmed gallstone disease and 300 unrelated controls. After collecting DNA samples from all participants, the investigators sequenced mitochondrial DNA HVS1 regions. A comparison of haplogroups showed that B4b’d’e’j was more common among patients with gallstone disease than among controls (odds ratio, 4.428; P = .00012), and further analysis pinpointed 827A>G, a variant in 12S ribosomal RNA.
“During the evolutionary history of modern humans, haplogroup B4 might have originated in East Asia approximately 40,000 years ago,” the investigators wrote, noting that B2, a subhaplogroup of B4, “was a founder haplogroup and expanded in the Americas after the Last Glacial Maximum (approximately 20,000 years ago).”
According to the investigators, this may explain why Native Americans have a higher prevalence of gallstones than East Asians (14%-35% vs. 3%-12%) because they are more often carriers of B4 (14%-44% vs. 2%-8%).
The investigators sought to characterize the impact that the 827A>G variant has on mitochondrial function and found effects ranging from lower respiratory chain complex activity, diminished mitochondrial function, activated mitochondrial protein quality control and retrograde signaling pathways, abnormal lipid metabolism, and abnormal cholesterol transport processes.
For example, the investigators investigated respiratory chain complex activity by creating two sister branch haplogroup cell models, including six cybrids for 827A and six more for 827G, which is how they detected the lower activity. Another step the investigators took was corroborating this finding by detecting OXPHOS function in the 827A and 827G cybrids to determine mitochondrial function.
“In summary, our study demonstrates a potential link between mitochondrial DNA 827A>G and gallstone disease,” the investigators wrote. “Our findings provide a significant biological basis for the clinical diagnosis and prevention of gallstone disease in the future.”
The study was funded by the National Natural Science Foundation of China, the 111 Project, the Shanghai Municipal Science and Technology Major Project, the Scientific and Technology Committee of Shanghai Municipality, and the CAMS Innovation Fund for Medical Sciences. The investigators reported no conflicts of interest.
Cholesterol gallstone disease results from imbalances in cholesterol metabolism. Other than the well-known lifestyle risk factors, there is also a strong genetic predisposition to gallstone formation. This study by Sun and colleagues examined the possible association between mitochondrial DNA (mtDNA) variants and cholesterol gallstone development because of the importance of the mitochondria in cellular metabolism and the increased maternal transmission of gallstone disease.
This study highlighted gallstone disease as a multifactorial condition that results from complex interaction between genetic and environmental factors. Interestingly, the allele frequency of the 827A>G mtDNA variant was noted to be higher in Native Americans, which may partially explain the high prevalence of gallstones in this population. Further studies are needed to identify additional genetic risk factors in ethnic groups that also have a significant burden of cholelithiasis.
Xiao Zhao, MD, is an assistant professor of medicine of division of digestive diseases in the department of medicine at Columbia University, New York. She reported having no conflicts of interest.
Cholesterol gallstone disease results from imbalances in cholesterol metabolism. Other than the well-known lifestyle risk factors, there is also a strong genetic predisposition to gallstone formation. This study by Sun and colleagues examined the possible association between mitochondrial DNA (mtDNA) variants and cholesterol gallstone development because of the importance of the mitochondria in cellular metabolism and the increased maternal transmission of gallstone disease.
This study highlighted gallstone disease as a multifactorial condition that results from complex interaction between genetic and environmental factors. Interestingly, the allele frequency of the 827A>G mtDNA variant was noted to be higher in Native Americans, which may partially explain the high prevalence of gallstones in this population. Further studies are needed to identify additional genetic risk factors in ethnic groups that also have a significant burden of cholelithiasis.
Xiao Zhao, MD, is an assistant professor of medicine of division of digestive diseases in the department of medicine at Columbia University, New York. She reported having no conflicts of interest.
Cholesterol gallstone disease results from imbalances in cholesterol metabolism. Other than the well-known lifestyle risk factors, there is also a strong genetic predisposition to gallstone formation. This study by Sun and colleagues examined the possible association between mitochondrial DNA (mtDNA) variants and cholesterol gallstone development because of the importance of the mitochondria in cellular metabolism and the increased maternal transmission of gallstone disease.
This study highlighted gallstone disease as a multifactorial condition that results from complex interaction between genetic and environmental factors. Interestingly, the allele frequency of the 827A>G mtDNA variant was noted to be higher in Native Americans, which may partially explain the high prevalence of gallstones in this population. Further studies are needed to identify additional genetic risk factors in ethnic groups that also have a significant burden of cholelithiasis.
Xiao Zhao, MD, is an assistant professor of medicine of division of digestive diseases in the department of medicine at Columbia University, New York. She reported having no conflicts of interest.
A mitochondrial DNA variant may increase the risk of gallstone disease more than fourfold, according to investigators.
Mitochondrial DNA 827A>G disrupts mitochondrial function and leads to abnormal cholesterol transport, which increases gallstone development, reported Dayan Sun, of Fudan University, Shanghai, China, and colleagues.
The investigators noted that the findings add support to a genetic role in gallstone development, which could allow for identification of at-risk individuals and implementation of preventive measures.
“The etiology of gallstone disease is multifactorial; age, sex, pregnancy, diet (macronutrients, alcohol, and coffee), and other factors are involved,” the investigators wrote in Cellular and Molecular Gastroenterology and Hepatology. “Moreover, the significant familial predisposition and ethnic differences in prevalence of this disease indicate the potential influences of genetic factors.”
In 2002, Nakeeb and colleagues reported that at least 30% of gallstone disease cases stemmed from genetic factors. And genetics may play an even greater role in certain populations, such as Native Americans, among whom more than 70% of women have gallstone disease, based on a study by Everhart and colleagues.
According to Ms. Sun and colleagues, a variety of genetic drivers of gallstone disease have been identified, such as ABCG8, identified as the most common genetic risk factor by at least one study, along with a list of other rare mutations, such as one affecting CFTR that leads to altered bile composition.
Based on previous research that linked mitochondrial DNA variants with metabolic defects and, more specifically, aberrations in lipid metabolism, as well as an observed “maternal bias in the maternal transmission of gallstone disease” that suggest mitochondrial influence, the investigators looked for patterns specifically in mitochondrial DNA variants among patients with gallstones.
The study enrolled 104 probands with confirmed gallstone disease and 300 unrelated controls. After collecting DNA samples from all participants, the investigators sequenced mitochondrial DNA HVS1 regions. A comparison of haplogroups showed that B4b’d’e’j was more common among patients with gallstone disease than among controls (odds ratio, 4.428; P = .00012), and further analysis pinpointed 827A>G, a variant in 12S ribosomal RNA.
“During the evolutionary history of modern humans, haplogroup B4 might have originated in East Asia approximately 40,000 years ago,” the investigators wrote, noting that B2, a subhaplogroup of B4, “was a founder haplogroup and expanded in the Americas after the Last Glacial Maximum (approximately 20,000 years ago).”
According to the investigators, this may explain why Native Americans have a higher prevalence of gallstones than East Asians (14%-35% vs. 3%-12%) because they are more often carriers of B4 (14%-44% vs. 2%-8%).
The investigators sought to characterize the impact that the 827A>G variant has on mitochondrial function and found effects ranging from lower respiratory chain complex activity, diminished mitochondrial function, activated mitochondrial protein quality control and retrograde signaling pathways, abnormal lipid metabolism, and abnormal cholesterol transport processes.
For example, the investigators investigated respiratory chain complex activity by creating two sister branch haplogroup cell models, including six cybrids for 827A and six more for 827G, which is how they detected the lower activity. Another step the investigators took was corroborating this finding by detecting OXPHOS function in the 827A and 827G cybrids to determine mitochondrial function.
“In summary, our study demonstrates a potential link between mitochondrial DNA 827A>G and gallstone disease,” the investigators wrote. “Our findings provide a significant biological basis for the clinical diagnosis and prevention of gallstone disease in the future.”
The study was funded by the National Natural Science Foundation of China, the 111 Project, the Shanghai Municipal Science and Technology Major Project, the Scientific and Technology Committee of Shanghai Municipality, and the CAMS Innovation Fund for Medical Sciences. The investigators reported no conflicts of interest.
A mitochondrial DNA variant may increase the risk of gallstone disease more than fourfold, according to investigators.
Mitochondrial DNA 827A>G disrupts mitochondrial function and leads to abnormal cholesterol transport, which increases gallstone development, reported Dayan Sun, of Fudan University, Shanghai, China, and colleagues.
The investigators noted that the findings add support to a genetic role in gallstone development, which could allow for identification of at-risk individuals and implementation of preventive measures.
“The etiology of gallstone disease is multifactorial; age, sex, pregnancy, diet (macronutrients, alcohol, and coffee), and other factors are involved,” the investigators wrote in Cellular and Molecular Gastroenterology and Hepatology. “Moreover, the significant familial predisposition and ethnic differences in prevalence of this disease indicate the potential influences of genetic factors.”
In 2002, Nakeeb and colleagues reported that at least 30% of gallstone disease cases stemmed from genetic factors. And genetics may play an even greater role in certain populations, such as Native Americans, among whom more than 70% of women have gallstone disease, based on a study by Everhart and colleagues.
According to Ms. Sun and colleagues, a variety of genetic drivers of gallstone disease have been identified, such as ABCG8, identified as the most common genetic risk factor by at least one study, along with a list of other rare mutations, such as one affecting CFTR that leads to altered bile composition.
Based on previous research that linked mitochondrial DNA variants with metabolic defects and, more specifically, aberrations in lipid metabolism, as well as an observed “maternal bias in the maternal transmission of gallstone disease” that suggest mitochondrial influence, the investigators looked for patterns specifically in mitochondrial DNA variants among patients with gallstones.
The study enrolled 104 probands with confirmed gallstone disease and 300 unrelated controls. After collecting DNA samples from all participants, the investigators sequenced mitochondrial DNA HVS1 regions. A comparison of haplogroups showed that B4b’d’e’j was more common among patients with gallstone disease than among controls (odds ratio, 4.428; P = .00012), and further analysis pinpointed 827A>G, a variant in 12S ribosomal RNA.
“During the evolutionary history of modern humans, haplogroup B4 might have originated in East Asia approximately 40,000 years ago,” the investigators wrote, noting that B2, a subhaplogroup of B4, “was a founder haplogroup and expanded in the Americas after the Last Glacial Maximum (approximately 20,000 years ago).”
According to the investigators, this may explain why Native Americans have a higher prevalence of gallstones than East Asians (14%-35% vs. 3%-12%) because they are more often carriers of B4 (14%-44% vs. 2%-8%).
The investigators sought to characterize the impact that the 827A>G variant has on mitochondrial function and found effects ranging from lower respiratory chain complex activity, diminished mitochondrial function, activated mitochondrial protein quality control and retrograde signaling pathways, abnormal lipid metabolism, and abnormal cholesterol transport processes.
For example, the investigators investigated respiratory chain complex activity by creating two sister branch haplogroup cell models, including six cybrids for 827A and six more for 827G, which is how they detected the lower activity. Another step the investigators took was corroborating this finding by detecting OXPHOS function in the 827A and 827G cybrids to determine mitochondrial function.
“In summary, our study demonstrates a potential link between mitochondrial DNA 827A>G and gallstone disease,” the investigators wrote. “Our findings provide a significant biological basis for the clinical diagnosis and prevention of gallstone disease in the future.”
The study was funded by the National Natural Science Foundation of China, the 111 Project, the Shanghai Municipal Science and Technology Major Project, the Scientific and Technology Committee of Shanghai Municipality, and the CAMS Innovation Fund for Medical Sciences. The investigators reported no conflicts of interest.
FROM CELLULAR AND MOLECULAR GASTROENTEROLOGY AND HEPATOLOGY
COVID-related immunization gaps portend return of preventable infections
Because of significant reduction in delivery of recommended childhood immunization during the pandemic, there is a risk for resurgence of vaccine preventable infections, including measles, pertussis, and polio, which can result in significant morbidity and mortality in children, reported Amy G. Feldman, MD, of Children’s Hospital Colorado, Aurora, and associates.
Will loss of herd immunity lead to vaccine deserts?
When asked to comment, pediatric infectious disease specialist Christopher J. Harrison, MD, said, “My concern is that we may see expansion of what I call ‘vaccine deserts.’ Vaccine deserts occur in underserved communities, areas with pockets of vaccine-hesitant families or among selected groups with difficult access to health care. These vaccine deserts have held a higher density of vulnerables due to low vaccine uptake, often giving rise to outbreaks of vaccine-preventable diseases, e.g., measles, mumps, pertussis. They are usually due to an index case arriving from another vaccine desert (a developing country or a developed country, U.S. or foreign) where the disease is still endemic or pockets of vaccine hesitancy/refusal exist. When detected, local outbreaks result in rapid responses from public/private health collaborations that limit the outbreak. But what if vaccine deserts became more generalized in the U.S. because of loss of vaccine-induced herd immunity in many more or larger areas of our communities because of pandemic-driven lack of vaccinations? That pandemic-driven indirect damage would further stress the health care system and the economy. And it may first show up in the older children whose vaccines were deferred in the first 4-6 months of the pandemic.”
Dr. Feldman and associates cited findings from a collaborative survey conducted by UNICEF, the World Health Organization, Gavi the Vaccine Alliance, the CDC, the Sabin Vaccine Institute, and the Johns Hopkins Bloomberg School of Public Health, which found that immunization programs experienced moderate to severe disruptions or terminations in at least 68 of 129 low and middle-income countries surveyed. According to the WHO, CDC, Red Cross, and GAVI, 94 million people presently are estimated to be at risk as a consequence of not receiving their measles vaccines following the suspensions.
“These national and international declines in routine immunizations have placed the global community at significant risk for outbreaks of vaccine-preventable infections (VPIs) including measles, polio, and pertussis, diseases which are more deadly, more contagious and have a higher reproductive factor (R0) amongst children than COVID-19,” the authors observed.
Dr. Feldman and associates outlined the horrible devastation that these VPI can cause in children, including significantly higher morbidity and mortality than adults, especially among those with immunodeficiencies. Neurologic deficits, paralysis, intellectual disabilities, and vision and hearing loss are just some of the permanent effects conveyed. “It is concerning to imagine how measles could spread across the United States when social distancing restriction[s] are relaxed and unvaccinated children return to school and usual community engagement,” they noted.
Collaborative engagement key to course correction
The authors found that primary care providers and public health communities are working not only to restore vaccine administration but also to restore confidence that vaccine delivery is safe in spite of COVID. In addition to recommending specific risk mitigation strategies for clinicians, they also suggested individual practitioners use electronic health records to identify patients with COVID-related lapses in vaccination, employ electronic health record–based parent notification of overdue immunizations, and offer distance-friendly vaccination options that include parking lot or drive-up window vaccine delivery.
Additionally, Dr. Feldman and colleagues recommended that local, state, regional, and national health systems use public service announcements via television and digital as well as social media platforms to convey important messages about the considerable health risks associated with vaccine avoidance and the availability of free or reduced-cost vaccination programs through the federally funded Vaccines For Children program for parents out of work or without insurance. Equally important is messaging around encouraging vaccine opportunities during all health care visits, whether they be subspecialty, urgent care, emergency room, or inpatient visits. In areas where access to clinics is limited, they urged the use of mobile clinics as well as additional focus on providing medical homes to children with poor access to care.
“A partial but expanding safety net may be developing spontaneously, i.e., practices and clinics based on a patient-centered medical home (PCMH) model,” noted Dr. Harrison, professor of pediatrics, University of Missouri-Kansas City, in an interview. “When lagging vaccinations were reported in mid-2020, we checked with a local hospital–based urban clinic and two suburban private practices modeled on PCMH. Each had noted a drastic drop in well checks in the first months of the pandemic. But with ill visits nearly nonexistent, they doubled down on maintaining health maintenance visits. Even though staff and provider work hours were limited, and families were less enthusiastic about well checks, momentum appears to have grown so that, by later in 2020, vaccine uptake rates were again comparable to 2019. So, some already seem to have answered the call, but practices/clinics remain hampered by months of reduced revenue needed to support staff, providers, PPE supplies, and added infection control needs,” he said.The study was funded by the Agency for Healthcare Research Quality. Dr. Isakov disclosed relationships with various pharmaceutical companies outside the submitted work. The other authors had no relevant disclosures. Dr. Harrison’s institution receives grant funding from GSK, Merck, and Pfizer for pediatric vaccine trials and pneumococcal seroprevalence studies on which he is an investigator.
Because of significant reduction in delivery of recommended childhood immunization during the pandemic, there is a risk for resurgence of vaccine preventable infections, including measles, pertussis, and polio, which can result in significant morbidity and mortality in children, reported Amy G. Feldman, MD, of Children’s Hospital Colorado, Aurora, and associates.
Will loss of herd immunity lead to vaccine deserts?
When asked to comment, pediatric infectious disease specialist Christopher J. Harrison, MD, said, “My concern is that we may see expansion of what I call ‘vaccine deserts.’ Vaccine deserts occur in underserved communities, areas with pockets of vaccine-hesitant families or among selected groups with difficult access to health care. These vaccine deserts have held a higher density of vulnerables due to low vaccine uptake, often giving rise to outbreaks of vaccine-preventable diseases, e.g., measles, mumps, pertussis. They are usually due to an index case arriving from another vaccine desert (a developing country or a developed country, U.S. or foreign) where the disease is still endemic or pockets of vaccine hesitancy/refusal exist. When detected, local outbreaks result in rapid responses from public/private health collaborations that limit the outbreak. But what if vaccine deserts became more generalized in the U.S. because of loss of vaccine-induced herd immunity in many more or larger areas of our communities because of pandemic-driven lack of vaccinations? That pandemic-driven indirect damage would further stress the health care system and the economy. And it may first show up in the older children whose vaccines were deferred in the first 4-6 months of the pandemic.”
Dr. Feldman and associates cited findings from a collaborative survey conducted by UNICEF, the World Health Organization, Gavi the Vaccine Alliance, the CDC, the Sabin Vaccine Institute, and the Johns Hopkins Bloomberg School of Public Health, which found that immunization programs experienced moderate to severe disruptions or terminations in at least 68 of 129 low and middle-income countries surveyed. According to the WHO, CDC, Red Cross, and GAVI, 94 million people presently are estimated to be at risk as a consequence of not receiving their measles vaccines following the suspensions.
“These national and international declines in routine immunizations have placed the global community at significant risk for outbreaks of vaccine-preventable infections (VPIs) including measles, polio, and pertussis, diseases which are more deadly, more contagious and have a higher reproductive factor (R0) amongst children than COVID-19,” the authors observed.
Dr. Feldman and associates outlined the horrible devastation that these VPI can cause in children, including significantly higher morbidity and mortality than adults, especially among those with immunodeficiencies. Neurologic deficits, paralysis, intellectual disabilities, and vision and hearing loss are just some of the permanent effects conveyed. “It is concerning to imagine how measles could spread across the United States when social distancing restriction[s] are relaxed and unvaccinated children return to school and usual community engagement,” they noted.
Collaborative engagement key to course correction
The authors found that primary care providers and public health communities are working not only to restore vaccine administration but also to restore confidence that vaccine delivery is safe in spite of COVID. In addition to recommending specific risk mitigation strategies for clinicians, they also suggested individual practitioners use electronic health records to identify patients with COVID-related lapses in vaccination, employ electronic health record–based parent notification of overdue immunizations, and offer distance-friendly vaccination options that include parking lot or drive-up window vaccine delivery.
Additionally, Dr. Feldman and colleagues recommended that local, state, regional, and national health systems use public service announcements via television and digital as well as social media platforms to convey important messages about the considerable health risks associated with vaccine avoidance and the availability of free or reduced-cost vaccination programs through the federally funded Vaccines For Children program for parents out of work or without insurance. Equally important is messaging around encouraging vaccine opportunities during all health care visits, whether they be subspecialty, urgent care, emergency room, or inpatient visits. In areas where access to clinics is limited, they urged the use of mobile clinics as well as additional focus on providing medical homes to children with poor access to care.
“A partial but expanding safety net may be developing spontaneously, i.e., practices and clinics based on a patient-centered medical home (PCMH) model,” noted Dr. Harrison, professor of pediatrics, University of Missouri-Kansas City, in an interview. “When lagging vaccinations were reported in mid-2020, we checked with a local hospital–based urban clinic and two suburban private practices modeled on PCMH. Each had noted a drastic drop in well checks in the first months of the pandemic. But with ill visits nearly nonexistent, they doubled down on maintaining health maintenance visits. Even though staff and provider work hours were limited, and families were less enthusiastic about well checks, momentum appears to have grown so that, by later in 2020, vaccine uptake rates were again comparable to 2019. So, some already seem to have answered the call, but practices/clinics remain hampered by months of reduced revenue needed to support staff, providers, PPE supplies, and added infection control needs,” he said.The study was funded by the Agency for Healthcare Research Quality. Dr. Isakov disclosed relationships with various pharmaceutical companies outside the submitted work. The other authors had no relevant disclosures. Dr. Harrison’s institution receives grant funding from GSK, Merck, and Pfizer for pediatric vaccine trials and pneumococcal seroprevalence studies on which he is an investigator.
Because of significant reduction in delivery of recommended childhood immunization during the pandemic, there is a risk for resurgence of vaccine preventable infections, including measles, pertussis, and polio, which can result in significant morbidity and mortality in children, reported Amy G. Feldman, MD, of Children’s Hospital Colorado, Aurora, and associates.
Will loss of herd immunity lead to vaccine deserts?
When asked to comment, pediatric infectious disease specialist Christopher J. Harrison, MD, said, “My concern is that we may see expansion of what I call ‘vaccine deserts.’ Vaccine deserts occur in underserved communities, areas with pockets of vaccine-hesitant families or among selected groups with difficult access to health care. These vaccine deserts have held a higher density of vulnerables due to low vaccine uptake, often giving rise to outbreaks of vaccine-preventable diseases, e.g., measles, mumps, pertussis. They are usually due to an index case arriving from another vaccine desert (a developing country or a developed country, U.S. or foreign) where the disease is still endemic or pockets of vaccine hesitancy/refusal exist. When detected, local outbreaks result in rapid responses from public/private health collaborations that limit the outbreak. But what if vaccine deserts became more generalized in the U.S. because of loss of vaccine-induced herd immunity in many more or larger areas of our communities because of pandemic-driven lack of vaccinations? That pandemic-driven indirect damage would further stress the health care system and the economy. And it may first show up in the older children whose vaccines were deferred in the first 4-6 months of the pandemic.”
Dr. Feldman and associates cited findings from a collaborative survey conducted by UNICEF, the World Health Organization, Gavi the Vaccine Alliance, the CDC, the Sabin Vaccine Institute, and the Johns Hopkins Bloomberg School of Public Health, which found that immunization programs experienced moderate to severe disruptions or terminations in at least 68 of 129 low and middle-income countries surveyed. According to the WHO, CDC, Red Cross, and GAVI, 94 million people presently are estimated to be at risk as a consequence of not receiving their measles vaccines following the suspensions.
“These national and international declines in routine immunizations have placed the global community at significant risk for outbreaks of vaccine-preventable infections (VPIs) including measles, polio, and pertussis, diseases which are more deadly, more contagious and have a higher reproductive factor (R0) amongst children than COVID-19,” the authors observed.
Dr. Feldman and associates outlined the horrible devastation that these VPI can cause in children, including significantly higher morbidity and mortality than adults, especially among those with immunodeficiencies. Neurologic deficits, paralysis, intellectual disabilities, and vision and hearing loss are just some of the permanent effects conveyed. “It is concerning to imagine how measles could spread across the United States when social distancing restriction[s] are relaxed and unvaccinated children return to school and usual community engagement,” they noted.
Collaborative engagement key to course correction
The authors found that primary care providers and public health communities are working not only to restore vaccine administration but also to restore confidence that vaccine delivery is safe in spite of COVID. In addition to recommending specific risk mitigation strategies for clinicians, they also suggested individual practitioners use electronic health records to identify patients with COVID-related lapses in vaccination, employ electronic health record–based parent notification of overdue immunizations, and offer distance-friendly vaccination options that include parking lot or drive-up window vaccine delivery.
Additionally, Dr. Feldman and colleagues recommended that local, state, regional, and national health systems use public service announcements via television and digital as well as social media platforms to convey important messages about the considerable health risks associated with vaccine avoidance and the availability of free or reduced-cost vaccination programs through the federally funded Vaccines For Children program for parents out of work or without insurance. Equally important is messaging around encouraging vaccine opportunities during all health care visits, whether they be subspecialty, urgent care, emergency room, or inpatient visits. In areas where access to clinics is limited, they urged the use of mobile clinics as well as additional focus on providing medical homes to children with poor access to care.
“A partial but expanding safety net may be developing spontaneously, i.e., practices and clinics based on a patient-centered medical home (PCMH) model,” noted Dr. Harrison, professor of pediatrics, University of Missouri-Kansas City, in an interview. “When lagging vaccinations were reported in mid-2020, we checked with a local hospital–based urban clinic and two suburban private practices modeled on PCMH. Each had noted a drastic drop in well checks in the first months of the pandemic. But with ill visits nearly nonexistent, they doubled down on maintaining health maintenance visits. Even though staff and provider work hours were limited, and families were less enthusiastic about well checks, momentum appears to have grown so that, by later in 2020, vaccine uptake rates were again comparable to 2019. So, some already seem to have answered the call, but practices/clinics remain hampered by months of reduced revenue needed to support staff, providers, PPE supplies, and added infection control needs,” he said.The study was funded by the Agency for Healthcare Research Quality. Dr. Isakov disclosed relationships with various pharmaceutical companies outside the submitted work. The other authors had no relevant disclosures. Dr. Harrison’s institution receives grant funding from GSK, Merck, and Pfizer for pediatric vaccine trials and pneumococcal seroprevalence studies on which he is an investigator.
FROM CLINICAL INFECTIOUS DISEASES
Progressive Telangiectatic Rash
The Diagnosis: Cutaneous Collagenous Vasculopathy
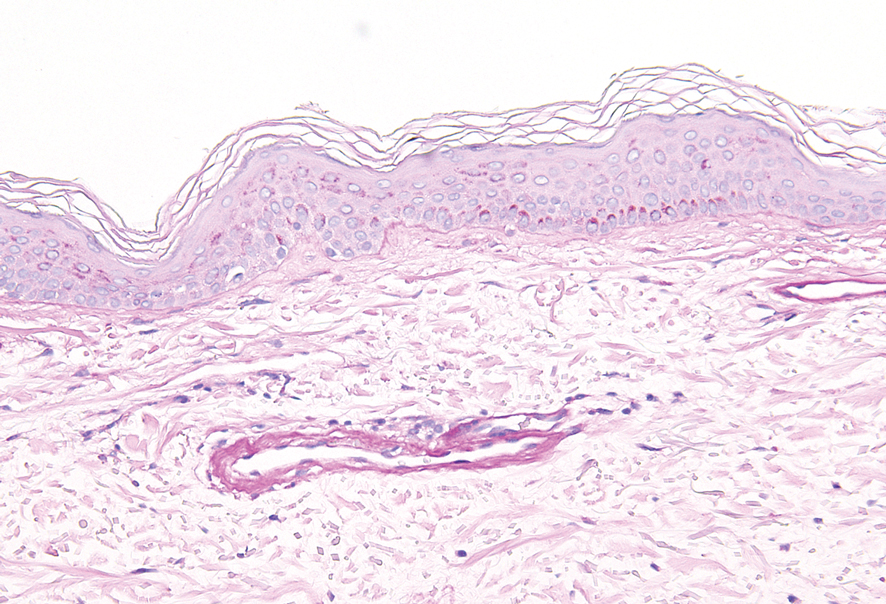
Cutaneous collagenous vasculopathy (CCV) is an idiopathic microangiopathy of the small vessels in the superficial dermis. A condition first identified by Salama and Rosenthal1 in 2000, CCV likely is underreported, as its clinical mimickers are not routinely biopsied.2 It presents as asymptomatic telangiectatic macules, initially on the lower extremities and often spreading to the trunk. Cutaneous collagenous vasculopathy often is seen in middle-aged adults, and most patients have comorbidities such as hypertension, diabetes mellitus, or cardiovascular disease. The exact etiology of this disease is unknown.3,4
Histopathologically, CCV is characterized by dilated superficial vessels with thickened eosinophilic walls. The eosinophilic material is composed of hyalinized type IV collagen, which is periodic acid-Schiff positive and diastase resistant (Figure 1).3,4 Stains for amyloid are negative.
Generalized essential telangiectasia (GET) is a condition that presents with symmetric, blanchable, erythematous telangiectases.5 These lesions can occur alone or can accompany systemic diseases. Similar to CCV, the telangiectases tend to begin on the legs before gradually spreading to the trunk; however, this process more often is seen in females and occurs at an earlier age. Unlike CCV, GET can occur on mucosal surfaces, with cases of conjunctival and oral involvement reported.6 Generalized essential telangiectasia usually is a diagnosis of exclusion.7,8 It is thought that many CCV lesions have been misclassified clinically as GET, which highlights the importance of biopsy. Microscopically, GET is distinct from CCV in that the superficial dermis lacks thick-walled vessels.5,7 Although usually not associated with systemic diseases or progressive morbidity, treatment options are limited.8
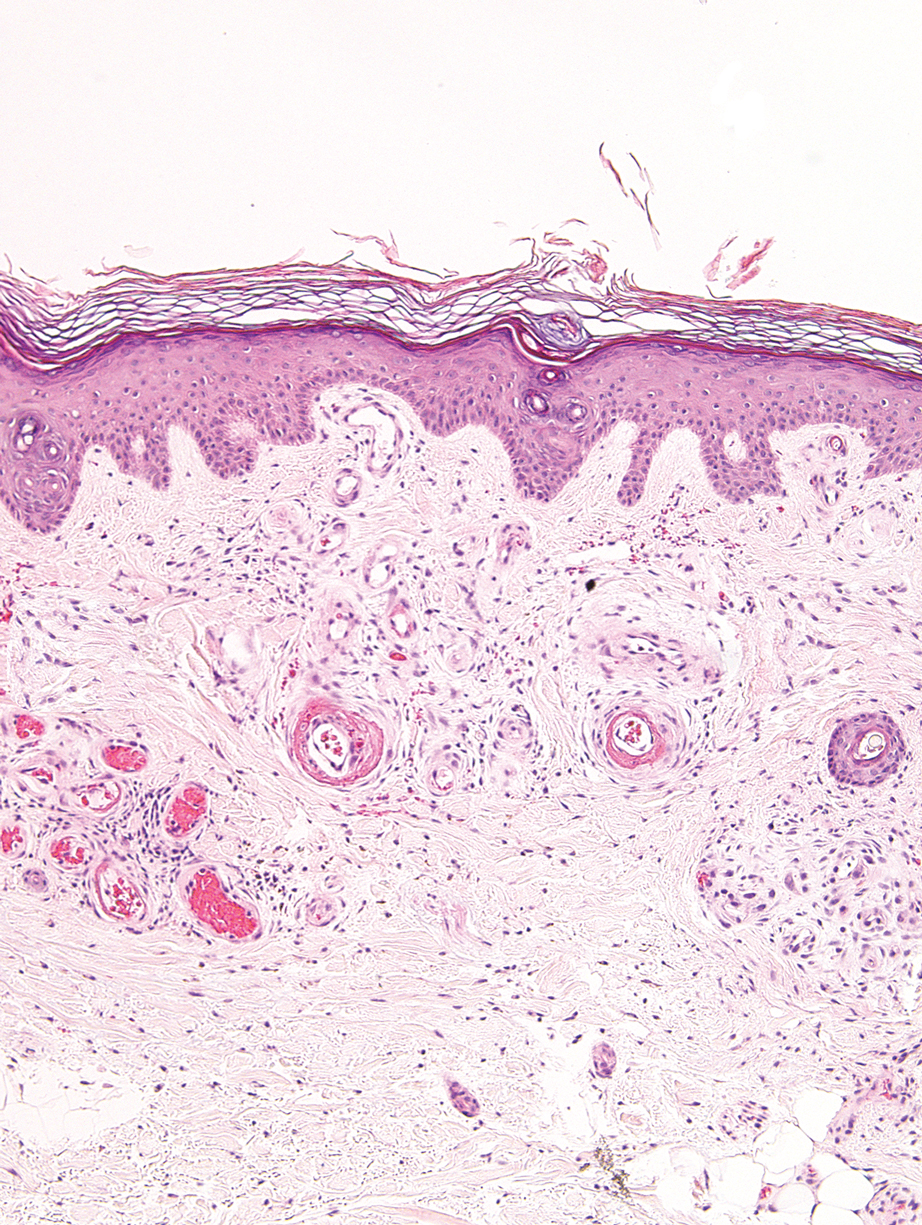
Livedoid vasculopathy, also known as atrophie blanche, is caused by fibrin thrombi occlusion of dermal vessels. Clinically, patients have recurrent telangiectatic papules and painful ulcers on the lower extremities that gradually heal, leaving behind white stellate scars. It is caused by an underlying prothrombotic state with a superimposed inflammatory response.9 Livedoid vasculopathy primarily affects middle-aged women, and many patients have comorbidities such as scleroderma or systemic lupus erythematosus. Histologically, the epidermis often is ulcerated, and thrombi are visualized within small vessels. Eosinophilic fibrinoid material is deposited in vessel walls, including but not confined to vessels at the base of the epidermal ulcer (Figure 2). The fibrinoid material is periodic acid-Schiff positive and diastase resistant and can be highlighted with immunofluorescence, which may help to distinguish this entity from CCV.1,9 As the disease progresses, vessels are diffusely hyalinized, and there is epidermal atrophy and dermal sclerosis. Treatment options include antiplatelet and fibrinolytic drugs with a multidisciplinary approach to resolve pain and scarring.9
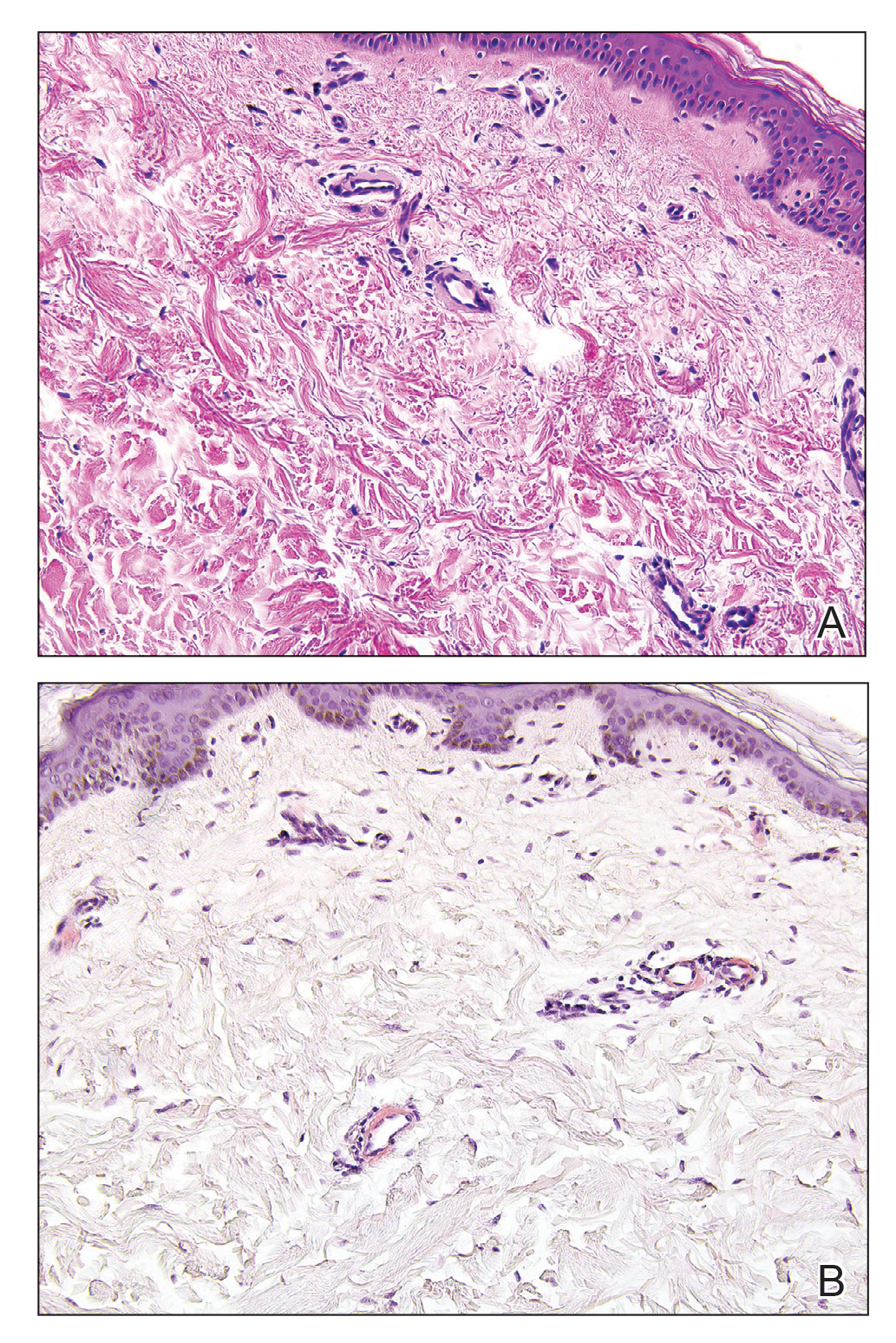
Primary systemic amyloidosis is a rare condition, and cutaneous manifestations are seen in approximately one-third of affected individuals. Amyloid deposition results in waxy papules that predominantly affect the face and periorbital areas but also may occur on the neck, flexural areas, and genitalia.5 Because the amyloid deposits also can be found within vessel walls, hemorrhagic lesions may occur. Microscopically, amorphous eosinophilic material can be found within the vessel walls, similar to CCV (Figure 3A); however, when stained with Congo red, cutaneous amyloidosis shows waxy red-orange material involving the vessel walls and exhibits apple green birefringence under polarization (Figure 3B).10 Amyloid also will be negative for type IV collagen, fibronectin, and laminin, whereas CCV will be positive.5
- Salama S, Rosenthal D. Cutaneous collagenous vasculopathy with generalized telangiectasia: an immunohistochemical and ultrastructural study. J Cutan Pathol. 2000;27:40-48.
- Bondier L, Tardieu M, Leveque P, et al. Cutaneous collagenous vasculopathy: report of two cases presenting as disseminated telangiectasias and review of the literature. Am J Dermatopathol. 2017;39:682-688.
- Sartori DS, Almeida HL Jr, Dorn TV, et al. Cutaneous collagenous vasculopathy: light and transmission electron microscopy. An Bras Dermatol. 2019;94:211-213.
- Brady BG, Ortleb M, Boyd AS, et al. Cutaneous collagenous vasculopathy. J Clin Aesthet Dermatol. 2015;8:49-52.
- Patterson JW, ed. Vascular tumors. Weedon's Skin Pathology. 4th ed. Churchill Livingstone/Elsevier; 2016:1069-1115.
- Knöpfel N, Martín-Santiago A, Saus C, et al. Extensive acquired telangiectasias: comparison of generalized essential telangiectasia and cutaneous collagenous vasculopathy. Actas Dermosifiliogr. 2017;108:E21-E26.
- Karimkhani C, Boyers LN, Olivere J, et al. Cutaneous collagenous vasculopathy. Cutis. 2019;103:E7-E8.
- McGrae JD, Winkelmann RK. Generalized essential telangiectasia: report of a clinical and histochemical study of 13 patients with acquired cutaneous lesions. JAMA. 1963;185:909-913.
- Vasudeva B, Neema S, Verma R. Livedoid vasculopathy: a review of pathogenesis and principles of management. Indian J Dermatol Venereol Leprol. 2016;82:478.
- Ko CJ, Barr RJ. Color--pink. In: Ko CJ, Barr RJ, eds. Dermatopathology: Diagnosis by First Impression. 3rd ed. Wiley; 2016:303-322.
- Clark ML, McGuinness AE, Vidal CI. Cutaneous collagenous vasculopathy: a unique case with positive direct immunofluorescence findings. Am J Dermatopathol. 2019;41:77-79.
The Diagnosis: Cutaneous Collagenous Vasculopathy
Cutaneous collagenous vasculopathy (CCV) is an idiopathic microangiopathy of the small vessels in the superficial dermis. A condition first identified by Salama and Rosenthal1 in 2000, CCV likely is underreported, as its clinical mimickers are not routinely biopsied.2 It presents as asymptomatic telangiectatic macules, initially on the lower extremities and often spreading to the trunk. Cutaneous collagenous vasculopathy often is seen in middle-aged adults, and most patients have comorbidities such as hypertension, diabetes mellitus, or cardiovascular disease. The exact etiology of this disease is unknown.3,4
Histopathologically, CCV is characterized by dilated superficial vessels with thickened eosinophilic walls. The eosinophilic material is composed of hyalinized type IV collagen, which is periodic acid-Schiff positive and diastase resistant (Figure 1).3,4 Stains for amyloid are negative.

Generalized essential telangiectasia (GET) is a condition that presents with symmetric, blanchable, erythematous telangiectases.5 These lesions can occur alone or can accompany systemic diseases. Similar to CCV, the telangiectases tend to begin on the legs before gradually spreading to the trunk; however, this process more often is seen in females and occurs at an earlier age. Unlike CCV, GET can occur on mucosal surfaces, with cases of conjunctival and oral involvement reported.6 Generalized essential telangiectasia usually is a diagnosis of exclusion.7,8 It is thought that many CCV lesions have been misclassified clinically as GET, which highlights the importance of biopsy. Microscopically, GET is distinct from CCV in that the superficial dermis lacks thick-walled vessels.5,7 Although usually not associated with systemic diseases or progressive morbidity, treatment options are limited.8
Livedoid vasculopathy, also known as atrophie blanche, is caused by fibrin thrombi occlusion of dermal vessels. Clinically, patients have recurrent telangiectatic papules and painful ulcers on the lower extremities that gradually heal, leaving behind white stellate scars. It is caused by an underlying prothrombotic state with a superimposed inflammatory response.9 Livedoid vasculopathy primarily affects middle-aged women, and many patients have comorbidities such as scleroderma or systemic lupus erythematosus. Histologically, the epidermis often is ulcerated, and thrombi are visualized within small vessels. Eosinophilic fibrinoid material is deposited in vessel walls, including but not confined to vessels at the base of the epidermal ulcer (Figure 2). The fibrinoid material is periodic acid-Schiff positive and diastase resistant and can be highlighted with immunofluorescence, which may help to distinguish this entity from CCV.1,9 As the disease progresses, vessels are diffusely hyalinized, and there is epidermal atrophy and dermal sclerosis. Treatment options include antiplatelet and fibrinolytic drugs with a multidisciplinary approach to resolve pain and scarring.9

Primary systemic amyloidosis is a rare condition, and cutaneous manifestations are seen in approximately one-third of affected individuals. Amyloid deposition results in waxy papules that predominantly affect the face and periorbital areas but also may occur on the neck, flexural areas, and genitalia.5 Because the amyloid deposits also can be found within vessel walls, hemorrhagic lesions may occur. Microscopically, amorphous eosinophilic material can be found within the vessel walls, similar to CCV (Figure 3A); however, when stained with Congo red, cutaneous amyloidosis shows waxy red-orange material involving the vessel walls and exhibits apple green birefringence under polarization (Figure 3B).10 Amyloid also will be negative for type IV collagen, fibronectin, and laminin, whereas CCV will be positive.5


The Diagnosis: Cutaneous Collagenous Vasculopathy
Cutaneous collagenous vasculopathy (CCV) is an idiopathic microangiopathy of the small vessels in the superficial dermis. A condition first identified by Salama and Rosenthal1 in 2000, CCV likely is underreported, as its clinical mimickers are not routinely biopsied.2 It presents as asymptomatic telangiectatic macules, initially on the lower extremities and often spreading to the trunk. Cutaneous collagenous vasculopathy often is seen in middle-aged adults, and most patients have comorbidities such as hypertension, diabetes mellitus, or cardiovascular disease. The exact etiology of this disease is unknown.3,4
Histopathologically, CCV is characterized by dilated superficial vessels with thickened eosinophilic walls. The eosinophilic material is composed of hyalinized type IV collagen, which is periodic acid-Schiff positive and diastase resistant (Figure 1).3,4 Stains for amyloid are negative.

Generalized essential telangiectasia (GET) is a condition that presents with symmetric, blanchable, erythematous telangiectases.5 These lesions can occur alone or can accompany systemic diseases. Similar to CCV, the telangiectases tend to begin on the legs before gradually spreading to the trunk; however, this process more often is seen in females and occurs at an earlier age. Unlike CCV, GET can occur on mucosal surfaces, with cases of conjunctival and oral involvement reported.6 Generalized essential telangiectasia usually is a diagnosis of exclusion.7,8 It is thought that many CCV lesions have been misclassified clinically as GET, which highlights the importance of biopsy. Microscopically, GET is distinct from CCV in that the superficial dermis lacks thick-walled vessels.5,7 Although usually not associated with systemic diseases or progressive morbidity, treatment options are limited.8
Livedoid vasculopathy, also known as atrophie blanche, is caused by fibrin thrombi occlusion of dermal vessels. Clinically, patients have recurrent telangiectatic papules and painful ulcers on the lower extremities that gradually heal, leaving behind white stellate scars. It is caused by an underlying prothrombotic state with a superimposed inflammatory response.9 Livedoid vasculopathy primarily affects middle-aged women, and many patients have comorbidities such as scleroderma or systemic lupus erythematosus. Histologically, the epidermis often is ulcerated, and thrombi are visualized within small vessels. Eosinophilic fibrinoid material is deposited in vessel walls, including but not confined to vessels at the base of the epidermal ulcer (Figure 2). The fibrinoid material is periodic acid-Schiff positive and diastase resistant and can be highlighted with immunofluorescence, which may help to distinguish this entity from CCV.1,9 As the disease progresses, vessels are diffusely hyalinized, and there is epidermal atrophy and dermal sclerosis. Treatment options include antiplatelet and fibrinolytic drugs with a multidisciplinary approach to resolve pain and scarring.9

Primary systemic amyloidosis is a rare condition, and cutaneous manifestations are seen in approximately one-third of affected individuals. Amyloid deposition results in waxy papules that predominantly affect the face and periorbital areas but also may occur on the neck, flexural areas, and genitalia.5 Because the amyloid deposits also can be found within vessel walls, hemorrhagic lesions may occur. Microscopically, amorphous eosinophilic material can be found within the vessel walls, similar to CCV (Figure 3A); however, when stained with Congo red, cutaneous amyloidosis shows waxy red-orange material involving the vessel walls and exhibits apple green birefringence under polarization (Figure 3B).10 Amyloid also will be negative for type IV collagen, fibronectin, and laminin, whereas CCV will be positive.5


- Salama S, Rosenthal D. Cutaneous collagenous vasculopathy with generalized telangiectasia: an immunohistochemical and ultrastructural study. J Cutan Pathol. 2000;27:40-48.
- Bondier L, Tardieu M, Leveque P, et al. Cutaneous collagenous vasculopathy: report of two cases presenting as disseminated telangiectasias and review of the literature. Am J Dermatopathol. 2017;39:682-688.
- Sartori DS, Almeida HL Jr, Dorn TV, et al. Cutaneous collagenous vasculopathy: light and transmission electron microscopy. An Bras Dermatol. 2019;94:211-213.
- Brady BG, Ortleb M, Boyd AS, et al. Cutaneous collagenous vasculopathy. J Clin Aesthet Dermatol. 2015;8:49-52.
- Patterson JW, ed. Vascular tumors. Weedon's Skin Pathology. 4th ed. Churchill Livingstone/Elsevier; 2016:1069-1115.
- Knöpfel N, Martín-Santiago A, Saus C, et al. Extensive acquired telangiectasias: comparison of generalized essential telangiectasia and cutaneous collagenous vasculopathy. Actas Dermosifiliogr. 2017;108:E21-E26.
- Karimkhani C, Boyers LN, Olivere J, et al. Cutaneous collagenous vasculopathy. Cutis. 2019;103:E7-E8.
- McGrae JD, Winkelmann RK. Generalized essential telangiectasia: report of a clinical and histochemical study of 13 patients with acquired cutaneous lesions. JAMA. 1963;185:909-913.
- Vasudeva B, Neema S, Verma R. Livedoid vasculopathy: a review of pathogenesis and principles of management. Indian J Dermatol Venereol Leprol. 2016;82:478.
- Ko CJ, Barr RJ. Color--pink. In: Ko CJ, Barr RJ, eds. Dermatopathology: Diagnosis by First Impression. 3rd ed. Wiley; 2016:303-322.
- Clark ML, McGuinness AE, Vidal CI. Cutaneous collagenous vasculopathy: a unique case with positive direct immunofluorescence findings. Am J Dermatopathol. 2019;41:77-79.
- Salama S, Rosenthal D. Cutaneous collagenous vasculopathy with generalized telangiectasia: an immunohistochemical and ultrastructural study. J Cutan Pathol. 2000;27:40-48.
- Bondier L, Tardieu M, Leveque P, et al. Cutaneous collagenous vasculopathy: report of two cases presenting as disseminated telangiectasias and review of the literature. Am J Dermatopathol. 2017;39:682-688.
- Sartori DS, Almeida HL Jr, Dorn TV, et al. Cutaneous collagenous vasculopathy: light and transmission electron microscopy. An Bras Dermatol. 2019;94:211-213.
- Brady BG, Ortleb M, Boyd AS, et al. Cutaneous collagenous vasculopathy. J Clin Aesthet Dermatol. 2015;8:49-52.
- Patterson JW, ed. Vascular tumors. Weedon's Skin Pathology. 4th ed. Churchill Livingstone/Elsevier; 2016:1069-1115.
- Knöpfel N, Martín-Santiago A, Saus C, et al. Extensive acquired telangiectasias: comparison of generalized essential telangiectasia and cutaneous collagenous vasculopathy. Actas Dermosifiliogr. 2017;108:E21-E26.
- Karimkhani C, Boyers LN, Olivere J, et al. Cutaneous collagenous vasculopathy. Cutis. 2019;103:E7-E8.
- McGrae JD, Winkelmann RK. Generalized essential telangiectasia: report of a clinical and histochemical study of 13 patients with acquired cutaneous lesions. JAMA. 1963;185:909-913.
- Vasudeva B, Neema S, Verma R. Livedoid vasculopathy: a review of pathogenesis and principles of management. Indian J Dermatol Venereol Leprol. 2016;82:478.
- Ko CJ, Barr RJ. Color--pink. In: Ko CJ, Barr RJ, eds. Dermatopathology: Diagnosis by First Impression. 3rd ed. Wiley; 2016:303-322.
- Clark ML, McGuinness AE, Vidal CI. Cutaneous collagenous vasculopathy: a unique case with positive direct immunofluorescence findings. Am J Dermatopathol. 2019;41:77-79.
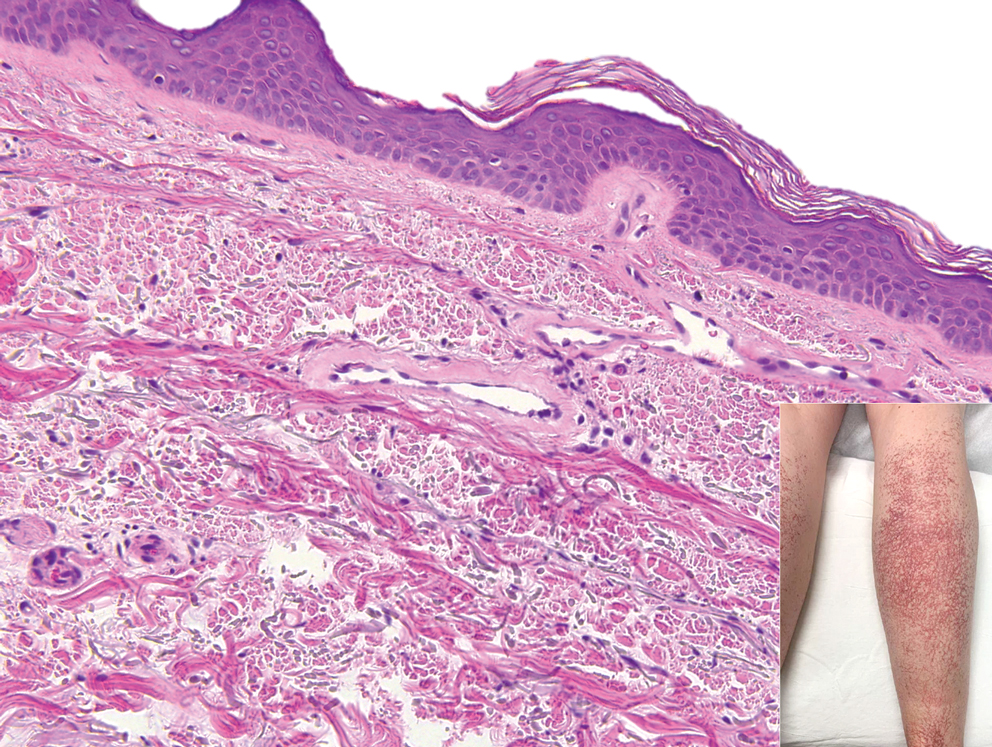
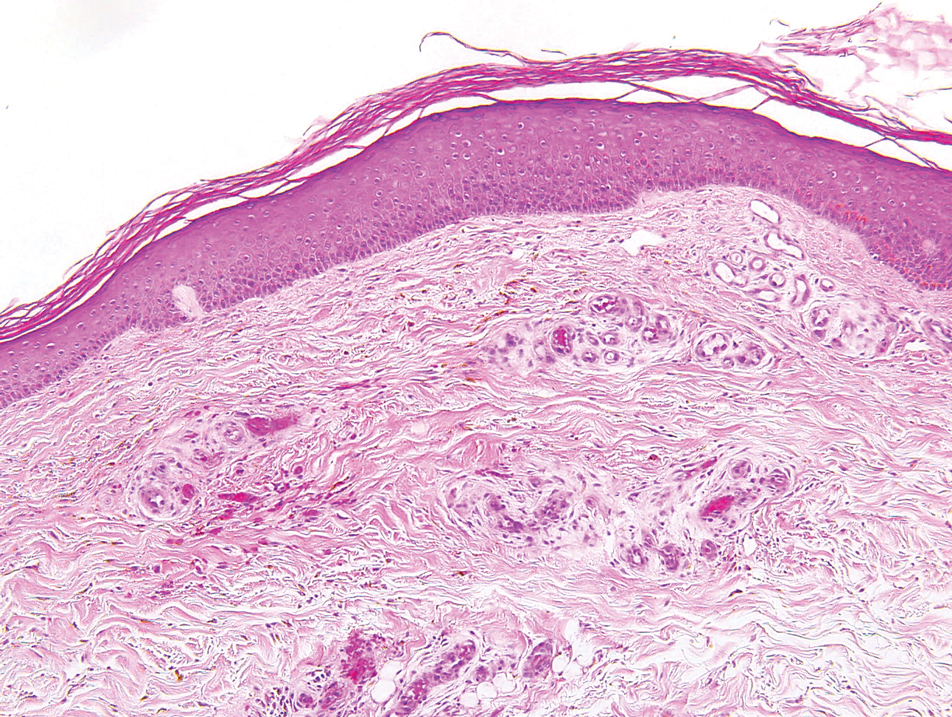
A 54-year-old woman presented with purple-red vessels on the lower legs of 15 years’ duration with gradual proximal progression to involve the thighs, breasts, and forearms. A punch biopsy of the inner thigh was obtained for histopathologic evaluation.
To improve psoriatic arthritis outcomes, address common comorbidities
Only about 30% or fewer of patients with psoriatic arthritis (PsA) on therapy achieve disease remission by any definition. One reason for this may be inadequate attention to common comorbid conditions, Alexis Ogdie, MD, MSCE, declared at the 2021 Rheumatology Winter Clinical Symposium.
“I believe that addressing off-target aspects of disease is really important to improving the patient experience of their disease. We might need to target these directly in order to improve outcomes,” said Dr. Ogdie, a rheumatologist and epidemiologist at the University of Pennsylvania, Philadelphia, who coauthored the current American College of Rheumatology/National Psoriasis Foundation PsA guidelines.
Since rheumatologists are by now well informed about the increased cardiovascular risk associated with PsA, she focused on two common comorbidities that get less attention, both of which are associated with worse clinical outcomes in PsA: obesity and mental health issues.
Anxiety and depression
Dr. Ogdie was first author of a large, population-based, longitudinal cohort study of cause-specific mortality in 8,706 U.K. patients with PsA, 41,752 with RA, and more than 81,000 controls. Particularly striking was the finding of elevated mortality because of suicide in the rheumatic disease patients: a 203% increased risk in the PsA population, compared with the general population, and a 147% greater risk in patients with RA.
Overall, 30%-40% of PsA patients have comorbid depression and/or anxiety.
“That’s pretty striking. It’s also true for rheumatoid arthritis and axial spondyloarthritis. And if you’re depressed, you’re much less likely to respond to therapy in the way that we are measuring response to therapy,” Dr. Ogdie said.
Her approach to screening for depression and anxiety in her PsA patients, and indeed in all her other patients, is to begin by normalizing the topic, explaining to them that these affective disorders are common among patients with these disorders. She lets her patients know they can talk to her about it. And she informs them that, while effective treatment of their rheumatic disease may improve their depression or anxiety, managing those is also important for improving their disease. Additionally, understanding whether depression is present is important prior to prescribing certain medications. Apremilast (Otezla), for example, can worsen preexisting depression.
“Ask about signs and symptoms of depression,” Dr. Ogdie urged her colleagues. “I do this at every single visit in my review of symptoms. This is one I don’t skip. I ask: ‘Do you have any symptoms of depression or anxiety?’ ”
Structured evidence-based screening tools, many of which are well suited for completion during a patient’s preappointment check-in survey, include the Patient Health Questionnaire–2, the PHQ-9, the Patient-Reported Outcomes Measure Information System–10, PROMIS–Depression, and Routine Assessment of Patient Index Data 3.
“I also really like the PROMIS-29. It covers many domains of interest: depression and anxiety, sleep, fatigue, pain, physical function. It gives a lot of information about what’s going on in a patient’s life right now,” according to the rheumatologist.
The main thing is to regularly screen for anxiety and depression and then refer symptomatic patients for further assessment and treatment. This is not something that all rheumatologists have been trained to do.
Obesity
Dr. Ogdie was lead author of a national CORRONA Registry study which concluded that obese patients with PsA were only half as likely to achieve remission on a tumor necrosis factor (TNF) inhibitor, compared with nonobese patients. She believes the same holds true for all other types of therapy: Across the board, obesity is associated with a poor response. And obesity is much more common in PsA patients than the general population in every age group. Moreover, obesity is associated with risk factors for cardiovascular disease and is associated with fatty liver disease, two other major comorbid conditions in the PsA population.
The CORRONA Registry findings are supportive of an earlier Italian prospective, observational study of 135 obese and an equal number of normal-weight PsA patients, all of whom started on a TNF inhibitor and were followed for 24 months. In a multivariate-adjusted analysis, obesity was independently associated with a 390% higher risk of not achieving minimal disease activity.
The same Italian group subsequently conducted a prospective dietary intervention study in 138 overweight or obese patients with PsA starting anti-TNF therapy. A total of 59% of participants randomized to either of the two dietary interventions experienced at least a 5% weight loss at 6 months. The key study finding: Compared with the subjects with less than 5% weight loss, those with 5%-10% weight loss were 275% more likely to achieve minimal disease activity at 6 months, and in those with greater than 10% weight loss the likelihood of attaining minimal disease activity increased by 567%.
“We’re talking about a disease where treatments tested in clinical trials have odds ratios in the 1.2 range, compared with other therapies, so this is a really striking difference,” she observed.
Several studies have demonstrated that obesity in psoriasis patients is a risk factor for developing PsA. Recently, U.K. investigators took things a step further, reporting in a huge observational study that obese or overweight psoriasis patients who reduced their body mass index over a 10-year period had a corresponding reduction in the risk of developing PsA, compared with overweight or obese psoriasis patients whose BMI remained steady over the same period.
What’s needed now is access to programs to help patients with PsA lose weight. Health insurers are often unwilling to provide coverage. “We have a really tough time getting the patients in to see a nutritionist unless they’re willing to pay out of pocket,” Dr. Ogdie said.
Physical activity is an important element in successful weight loss. It also is recommended in practice guidelines for patients with inflammatory arthritis because of its salutary effects on disease activity scores, pain and stiffness, sleep, and quality of life. But a recent survey conducted by Dr. Ogdie and coworkers concluded that patients with PsA and other forms of inflammatory arthritis don’t receive much exercise guidance from their rheumatologists. About 60% of subjects were inactive. Those who were physically active typically engaged in aerobic exercise but were much less likely to do the other guideline-recommended forms of exercise, namely flexibility, balance, and resistance training. The patients’ report of low engagement of their physicians “suggests an opportunity for more prescriptive exercise discussions,” according to the investigators.
Diabetes, a critical risk factor for cardiovascular disease, occurs at an increased incidence in PsA. This was demonstrated in a U.K. cohort study coauthored by Dr. Ogdie. The study, which included nearly 4,200 individuals with PsA, concluded that they had a 43% greater incidence of diabetes than the general population in an analysis adjusted for body mass index, smoking, alcohol use, and demographics.
New-onset diabetes can be readily picked up by rheumatologists based upon the laboratory work they often order at patient office visits, or during their review of symptoms, she noted, and added that the U.S. Preventive Services Task Force recommends ordering a hemoglobin A1c test every 3 years.
Dr. Ogdie reported receiving research grants and/or consulting fees from numerous pharmaceutical companies. Her research is also funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the Rheumatology Research Foundation, and the National Psoriasis Foundation.
Only about 30% or fewer of patients with psoriatic arthritis (PsA) on therapy achieve disease remission by any definition. One reason for this may be inadequate attention to common comorbid conditions, Alexis Ogdie, MD, MSCE, declared at the 2021 Rheumatology Winter Clinical Symposium.
“I believe that addressing off-target aspects of disease is really important to improving the patient experience of their disease. We might need to target these directly in order to improve outcomes,” said Dr. Ogdie, a rheumatologist and epidemiologist at the University of Pennsylvania, Philadelphia, who coauthored the current American College of Rheumatology/National Psoriasis Foundation PsA guidelines.
Since rheumatologists are by now well informed about the increased cardiovascular risk associated with PsA, she focused on two common comorbidities that get less attention, both of which are associated with worse clinical outcomes in PsA: obesity and mental health issues.
Anxiety and depression
Dr. Ogdie was first author of a large, population-based, longitudinal cohort study of cause-specific mortality in 8,706 U.K. patients with PsA, 41,752 with RA, and more than 81,000 controls. Particularly striking was the finding of elevated mortality because of suicide in the rheumatic disease patients: a 203% increased risk in the PsA population, compared with the general population, and a 147% greater risk in patients with RA.
Overall, 30%-40% of PsA patients have comorbid depression and/or anxiety.
“That’s pretty striking. It’s also true for rheumatoid arthritis and axial spondyloarthritis. And if you’re depressed, you’re much less likely to respond to therapy in the way that we are measuring response to therapy,” Dr. Ogdie said.
Her approach to screening for depression and anxiety in her PsA patients, and indeed in all her other patients, is to begin by normalizing the topic, explaining to them that these affective disorders are common among patients with these disorders. She lets her patients know they can talk to her about it. And she informs them that, while effective treatment of their rheumatic disease may improve their depression or anxiety, managing those is also important for improving their disease. Additionally, understanding whether depression is present is important prior to prescribing certain medications. Apremilast (Otezla), for example, can worsen preexisting depression.
“Ask about signs and symptoms of depression,” Dr. Ogdie urged her colleagues. “I do this at every single visit in my review of symptoms. This is one I don’t skip. I ask: ‘Do you have any symptoms of depression or anxiety?’ ”
Structured evidence-based screening tools, many of which are well suited for completion during a patient’s preappointment check-in survey, include the Patient Health Questionnaire–2, the PHQ-9, the Patient-Reported Outcomes Measure Information System–10, PROMIS–Depression, and Routine Assessment of Patient Index Data 3.
“I also really like the PROMIS-29. It covers many domains of interest: depression and anxiety, sleep, fatigue, pain, physical function. It gives a lot of information about what’s going on in a patient’s life right now,” according to the rheumatologist.
The main thing is to regularly screen for anxiety and depression and then refer symptomatic patients for further assessment and treatment. This is not something that all rheumatologists have been trained to do.
Obesity
Dr. Ogdie was lead author of a national CORRONA Registry study which concluded that obese patients with PsA were only half as likely to achieve remission on a tumor necrosis factor (TNF) inhibitor, compared with nonobese patients. She believes the same holds true for all other types of therapy: Across the board, obesity is associated with a poor response. And obesity is much more common in PsA patients than the general population in every age group. Moreover, obesity is associated with risk factors for cardiovascular disease and is associated with fatty liver disease, two other major comorbid conditions in the PsA population.
The CORRONA Registry findings are supportive of an earlier Italian prospective, observational study of 135 obese and an equal number of normal-weight PsA patients, all of whom started on a TNF inhibitor and were followed for 24 months. In a multivariate-adjusted analysis, obesity was independently associated with a 390% higher risk of not achieving minimal disease activity.
The same Italian group subsequently conducted a prospective dietary intervention study in 138 overweight or obese patients with PsA starting anti-TNF therapy. A total of 59% of participants randomized to either of the two dietary interventions experienced at least a 5% weight loss at 6 months. The key study finding: Compared with the subjects with less than 5% weight loss, those with 5%-10% weight loss were 275% more likely to achieve minimal disease activity at 6 months, and in those with greater than 10% weight loss the likelihood of attaining minimal disease activity increased by 567%.
“We’re talking about a disease where treatments tested in clinical trials have odds ratios in the 1.2 range, compared with other therapies, so this is a really striking difference,” she observed.
Several studies have demonstrated that obesity in psoriasis patients is a risk factor for developing PsA. Recently, U.K. investigators took things a step further, reporting in a huge observational study that obese or overweight psoriasis patients who reduced their body mass index over a 10-year period had a corresponding reduction in the risk of developing PsA, compared with overweight or obese psoriasis patients whose BMI remained steady over the same period.
What’s needed now is access to programs to help patients with PsA lose weight. Health insurers are often unwilling to provide coverage. “We have a really tough time getting the patients in to see a nutritionist unless they’re willing to pay out of pocket,” Dr. Ogdie said.
Physical activity is an important element in successful weight loss. It also is recommended in practice guidelines for patients with inflammatory arthritis because of its salutary effects on disease activity scores, pain and stiffness, sleep, and quality of life. But a recent survey conducted by Dr. Ogdie and coworkers concluded that patients with PsA and other forms of inflammatory arthritis don’t receive much exercise guidance from their rheumatologists. About 60% of subjects were inactive. Those who were physically active typically engaged in aerobic exercise but were much less likely to do the other guideline-recommended forms of exercise, namely flexibility, balance, and resistance training. The patients’ report of low engagement of their physicians “suggests an opportunity for more prescriptive exercise discussions,” according to the investigators.
Diabetes, a critical risk factor for cardiovascular disease, occurs at an increased incidence in PsA. This was demonstrated in a U.K. cohort study coauthored by Dr. Ogdie. The study, which included nearly 4,200 individuals with PsA, concluded that they had a 43% greater incidence of diabetes than the general population in an analysis adjusted for body mass index, smoking, alcohol use, and demographics.
New-onset diabetes can be readily picked up by rheumatologists based upon the laboratory work they often order at patient office visits, or during their review of symptoms, she noted, and added that the U.S. Preventive Services Task Force recommends ordering a hemoglobin A1c test every 3 years.
Dr. Ogdie reported receiving research grants and/or consulting fees from numerous pharmaceutical companies. Her research is also funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the Rheumatology Research Foundation, and the National Psoriasis Foundation.
Only about 30% or fewer of patients with psoriatic arthritis (PsA) on therapy achieve disease remission by any definition. One reason for this may be inadequate attention to common comorbid conditions, Alexis Ogdie, MD, MSCE, declared at the 2021 Rheumatology Winter Clinical Symposium.
“I believe that addressing off-target aspects of disease is really important to improving the patient experience of their disease. We might need to target these directly in order to improve outcomes,” said Dr. Ogdie, a rheumatologist and epidemiologist at the University of Pennsylvania, Philadelphia, who coauthored the current American College of Rheumatology/National Psoriasis Foundation PsA guidelines.
Since rheumatologists are by now well informed about the increased cardiovascular risk associated with PsA, she focused on two common comorbidities that get less attention, both of which are associated with worse clinical outcomes in PsA: obesity and mental health issues.
Anxiety and depression
Dr. Ogdie was first author of a large, population-based, longitudinal cohort study of cause-specific mortality in 8,706 U.K. patients with PsA, 41,752 with RA, and more than 81,000 controls. Particularly striking was the finding of elevated mortality because of suicide in the rheumatic disease patients: a 203% increased risk in the PsA population, compared with the general population, and a 147% greater risk in patients with RA.
Overall, 30%-40% of PsA patients have comorbid depression and/or anxiety.
“That’s pretty striking. It’s also true for rheumatoid arthritis and axial spondyloarthritis. And if you’re depressed, you’re much less likely to respond to therapy in the way that we are measuring response to therapy,” Dr. Ogdie said.
Her approach to screening for depression and anxiety in her PsA patients, and indeed in all her other patients, is to begin by normalizing the topic, explaining to them that these affective disorders are common among patients with these disorders. She lets her patients know they can talk to her about it. And she informs them that, while effective treatment of their rheumatic disease may improve their depression or anxiety, managing those is also important for improving their disease. Additionally, understanding whether depression is present is important prior to prescribing certain medications. Apremilast (Otezla), for example, can worsen preexisting depression.
“Ask about signs and symptoms of depression,” Dr. Ogdie urged her colleagues. “I do this at every single visit in my review of symptoms. This is one I don’t skip. I ask: ‘Do you have any symptoms of depression or anxiety?’ ”
Structured evidence-based screening tools, many of which are well suited for completion during a patient’s preappointment check-in survey, include the Patient Health Questionnaire–2, the PHQ-9, the Patient-Reported Outcomes Measure Information System–10, PROMIS–Depression, and Routine Assessment of Patient Index Data 3.
“I also really like the PROMIS-29. It covers many domains of interest: depression and anxiety, sleep, fatigue, pain, physical function. It gives a lot of information about what’s going on in a patient’s life right now,” according to the rheumatologist.
The main thing is to regularly screen for anxiety and depression and then refer symptomatic patients for further assessment and treatment. This is not something that all rheumatologists have been trained to do.
Obesity
Dr. Ogdie was lead author of a national CORRONA Registry study which concluded that obese patients with PsA were only half as likely to achieve remission on a tumor necrosis factor (TNF) inhibitor, compared with nonobese patients. She believes the same holds true for all other types of therapy: Across the board, obesity is associated with a poor response. And obesity is much more common in PsA patients than the general population in every age group. Moreover, obesity is associated with risk factors for cardiovascular disease and is associated with fatty liver disease, two other major comorbid conditions in the PsA population.
The CORRONA Registry findings are supportive of an earlier Italian prospective, observational study of 135 obese and an equal number of normal-weight PsA patients, all of whom started on a TNF inhibitor and were followed for 24 months. In a multivariate-adjusted analysis, obesity was independently associated with a 390% higher risk of not achieving minimal disease activity.
The same Italian group subsequently conducted a prospective dietary intervention study in 138 overweight or obese patients with PsA starting anti-TNF therapy. A total of 59% of participants randomized to either of the two dietary interventions experienced at least a 5% weight loss at 6 months. The key study finding: Compared with the subjects with less than 5% weight loss, those with 5%-10% weight loss were 275% more likely to achieve minimal disease activity at 6 months, and in those with greater than 10% weight loss the likelihood of attaining minimal disease activity increased by 567%.
“We’re talking about a disease where treatments tested in clinical trials have odds ratios in the 1.2 range, compared with other therapies, so this is a really striking difference,” she observed.
Several studies have demonstrated that obesity in psoriasis patients is a risk factor for developing PsA. Recently, U.K. investigators took things a step further, reporting in a huge observational study that obese or overweight psoriasis patients who reduced their body mass index over a 10-year period had a corresponding reduction in the risk of developing PsA, compared with overweight or obese psoriasis patients whose BMI remained steady over the same period.
What’s needed now is access to programs to help patients with PsA lose weight. Health insurers are often unwilling to provide coverage. “We have a really tough time getting the patients in to see a nutritionist unless they’re willing to pay out of pocket,” Dr. Ogdie said.
Physical activity is an important element in successful weight loss. It also is recommended in practice guidelines for patients with inflammatory arthritis because of its salutary effects on disease activity scores, pain and stiffness, sleep, and quality of life. But a recent survey conducted by Dr. Ogdie and coworkers concluded that patients with PsA and other forms of inflammatory arthritis don’t receive much exercise guidance from their rheumatologists. About 60% of subjects were inactive. Those who were physically active typically engaged in aerobic exercise but were much less likely to do the other guideline-recommended forms of exercise, namely flexibility, balance, and resistance training. The patients’ report of low engagement of their physicians “suggests an opportunity for more prescriptive exercise discussions,” according to the investigators.
Diabetes, a critical risk factor for cardiovascular disease, occurs at an increased incidence in PsA. This was demonstrated in a U.K. cohort study coauthored by Dr. Ogdie. The study, which included nearly 4,200 individuals with PsA, concluded that they had a 43% greater incidence of diabetes than the general population in an analysis adjusted for body mass index, smoking, alcohol use, and demographics.
New-onset diabetes can be readily picked up by rheumatologists based upon the laboratory work they often order at patient office visits, or during their review of symptoms, she noted, and added that the U.S. Preventive Services Task Force recommends ordering a hemoglobin A1c test every 3 years.
Dr. Ogdie reported receiving research grants and/or consulting fees from numerous pharmaceutical companies. Her research is also funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the Rheumatology Research Foundation, and the National Psoriasis Foundation.
FROM RWCS 2021
Close joint health monitoring essential with new hemophilia therapies
Novel therapies have transformed the treatment of hemophilia in recent decades, but these new approaches also raise new challenges for clinicians who monitor joint health in persons with hemophilia, a specialist said.
“Patient-reported outcomes should be combined with other, more objective outcome measures for joint health monitoring, and joint ultrasound is a promising tool for objective joint health monitoring, although, due to its relatively recent introduction in clinical practice, we lack objective data and standardization,” said Roberta Gualtierotti, MD, PhD, from the Università Degli Studi of Milan.
She reviewed the challenges and approaches to monitoring joint health in persons with hemophilia during the annual congress of the European Association for Haemophilia and Allied Disorders.
Over the last decades the target of hemophilia treatment has shifted from prolonging survival to improving joint health and quality of life, and care has improved with the introduction of novel therapies such as extended half-life replacement products, nonreplacement therapies, and gene therapy, she noted.
However, “due to different pharmacodynamics and pharmacokinetics profiles, the currently available therapies cannot be compared to each other on several levels,” Dr. Gualtierotti said.
Laboratory monitoring of replacement therapies with standard coagulation assays may be unreliable, and depending on the mechanism of action and type of administration of nonreplacement agents, patients may experience breakthrough bleeding, especially after traumatic injury, she said.
Until the specific noncoagulatory effects of factor VIII on bone and joint health is better understood, close monitoring of patients will be required, she added.
Outcome measures
Subjective measures of joint health include patient-reported bleeding rates and health-related quality of life. These are practical for home management, but patients may not be able to distinguish symptoms of acute joint bleeding from chronic arthritis pain, with the potential for either under- or overtreatment, and subjective reporting is likely to miss subclinical bleeding that can occur even when patients are on prophylaxis.
Health-related quality of life tools, whether generic or specific for hemophilia, are not sensitive to small improvements, and they are not always used in routine clinical practice.
Objective measures include physical examination with scoring according to the World Federation of Hemophilia (Gilbert Scale) score or Hemophilia Joint Health Score, but these measures have limited ability to identify early or subclinical joint abnormalities.
“Therefore, joint physical examination on its own is not a sufficient measure of treatment efficacy, and it should be used in combination with other tools more objective, such as imaging,” Dr. Gualtierotti said.
Get the picture?
Imaging with x-rays, MRI, and, recently in some centers, point-of-care ultrasound can provide clinicians with important real-time information about the joint stability and health.
Point-of-care ultrasound in particular offers promise as a practical tool, with no ionizing radiation and high sensitivity for synovial hyperplasia subclinical joint effusion. It’s relatively inexpensive, can be used to image multiple joints, and allows for ease of follow-up, she said. The technique requires specialized training, however, and there is a lack of prospective data about its utility in hemophilia.
Various ultrasound scoring systems have been proposed, and home-based ultrasound is currently being explored in several clinical trials, Dr. Gualtierotti noted.
Other avenues for remote joint health monitoring under consideration are serum or synovial biomarkers for joint bleeding and arthropathy that could be employed at bedside or at home, smartphone apps for breakthrough bleeding and patient-reported outcomes, and sensors for detecting abnormalities in gait that may signal joint dysfunction, she said.
Best practice
In the question-and-answer session following the talk, Fernando Zikan, MD, from the Federal University of Rio de Janeiro noted that, “in underdeveloped countries, we still find it very difficult to guide good practices for joint health control by the patient and family members. Which strategy do you think is fundamental for the patient to feel safe to notice changes in his body?”
“It would be useful to educate patients to come to the center whenever the patient has trauma and whenever an increase in his physical activity occurs. If this is far, a bedside ultrasound evaluation by the general practitioner could help avoid joint damage. Finally, a correct rehabilitation is fundamental,” Dr. Gualtierotti replied.
Asked by several others whether she used ultrasound in her daily practice, Dr. Gualtierotti said that “we use joint ultrasound in our clinical practice in the regular annual check-up examination and whenever the patient suspects and reports hemarthrosis.”
Dr. Gualtierotti reported participation in advisory boards for Biomarin, Pfizer, Bayer, and Takeda, and in educational seminars sponsored by Pfizer, Sobi, and Roche. She has received support for congress travel and/or attendance by Bayer and Pfizer. Dr. Zikan reported no relevant disclosures.
Novel therapies have transformed the treatment of hemophilia in recent decades, but these new approaches also raise new challenges for clinicians who monitor joint health in persons with hemophilia, a specialist said.
“Patient-reported outcomes should be combined with other, more objective outcome measures for joint health monitoring, and joint ultrasound is a promising tool for objective joint health monitoring, although, due to its relatively recent introduction in clinical practice, we lack objective data and standardization,” said Roberta Gualtierotti, MD, PhD, from the Università Degli Studi of Milan.
She reviewed the challenges and approaches to monitoring joint health in persons with hemophilia during the annual congress of the European Association for Haemophilia and Allied Disorders.
Over the last decades the target of hemophilia treatment has shifted from prolonging survival to improving joint health and quality of life, and care has improved with the introduction of novel therapies such as extended half-life replacement products, nonreplacement therapies, and gene therapy, she noted.
However, “due to different pharmacodynamics and pharmacokinetics profiles, the currently available therapies cannot be compared to each other on several levels,” Dr. Gualtierotti said.
Laboratory monitoring of replacement therapies with standard coagulation assays may be unreliable, and depending on the mechanism of action and type of administration of nonreplacement agents, patients may experience breakthrough bleeding, especially after traumatic injury, she said.
Until the specific noncoagulatory effects of factor VIII on bone and joint health is better understood, close monitoring of patients will be required, she added.
Outcome measures
Subjective measures of joint health include patient-reported bleeding rates and health-related quality of life. These are practical for home management, but patients may not be able to distinguish symptoms of acute joint bleeding from chronic arthritis pain, with the potential for either under- or overtreatment, and subjective reporting is likely to miss subclinical bleeding that can occur even when patients are on prophylaxis.
Health-related quality of life tools, whether generic or specific for hemophilia, are not sensitive to small improvements, and they are not always used in routine clinical practice.
Objective measures include physical examination with scoring according to the World Federation of Hemophilia (Gilbert Scale) score or Hemophilia Joint Health Score, but these measures have limited ability to identify early or subclinical joint abnormalities.
“Therefore, joint physical examination on its own is not a sufficient measure of treatment efficacy, and it should be used in combination with other tools more objective, such as imaging,” Dr. Gualtierotti said.
Get the picture?
Imaging with x-rays, MRI, and, recently in some centers, point-of-care ultrasound can provide clinicians with important real-time information about the joint stability and health.
Point-of-care ultrasound in particular offers promise as a practical tool, with no ionizing radiation and high sensitivity for synovial hyperplasia subclinical joint effusion. It’s relatively inexpensive, can be used to image multiple joints, and allows for ease of follow-up, she said. The technique requires specialized training, however, and there is a lack of prospective data about its utility in hemophilia.
Various ultrasound scoring systems have been proposed, and home-based ultrasound is currently being explored in several clinical trials, Dr. Gualtierotti noted.
Other avenues for remote joint health monitoring under consideration are serum or synovial biomarkers for joint bleeding and arthropathy that could be employed at bedside or at home, smartphone apps for breakthrough bleeding and patient-reported outcomes, and sensors for detecting abnormalities in gait that may signal joint dysfunction, she said.
Best practice
In the question-and-answer session following the talk, Fernando Zikan, MD, from the Federal University of Rio de Janeiro noted that, “in underdeveloped countries, we still find it very difficult to guide good practices for joint health control by the patient and family members. Which strategy do you think is fundamental for the patient to feel safe to notice changes in his body?”
“It would be useful to educate patients to come to the center whenever the patient has trauma and whenever an increase in his physical activity occurs. If this is far, a bedside ultrasound evaluation by the general practitioner could help avoid joint damage. Finally, a correct rehabilitation is fundamental,” Dr. Gualtierotti replied.
Asked by several others whether she used ultrasound in her daily practice, Dr. Gualtierotti said that “we use joint ultrasound in our clinical practice in the regular annual check-up examination and whenever the patient suspects and reports hemarthrosis.”
Dr. Gualtierotti reported participation in advisory boards for Biomarin, Pfizer, Bayer, and Takeda, and in educational seminars sponsored by Pfizer, Sobi, and Roche. She has received support for congress travel and/or attendance by Bayer and Pfizer. Dr. Zikan reported no relevant disclosures.
Novel therapies have transformed the treatment of hemophilia in recent decades, but these new approaches also raise new challenges for clinicians who monitor joint health in persons with hemophilia, a specialist said.
“Patient-reported outcomes should be combined with other, more objective outcome measures for joint health monitoring, and joint ultrasound is a promising tool for objective joint health monitoring, although, due to its relatively recent introduction in clinical practice, we lack objective data and standardization,” said Roberta Gualtierotti, MD, PhD, from the Università Degli Studi of Milan.
She reviewed the challenges and approaches to monitoring joint health in persons with hemophilia during the annual congress of the European Association for Haemophilia and Allied Disorders.
Over the last decades the target of hemophilia treatment has shifted from prolonging survival to improving joint health and quality of life, and care has improved with the introduction of novel therapies such as extended half-life replacement products, nonreplacement therapies, and gene therapy, she noted.
However, “due to different pharmacodynamics and pharmacokinetics profiles, the currently available therapies cannot be compared to each other on several levels,” Dr. Gualtierotti said.
Laboratory monitoring of replacement therapies with standard coagulation assays may be unreliable, and depending on the mechanism of action and type of administration of nonreplacement agents, patients may experience breakthrough bleeding, especially after traumatic injury, she said.
Until the specific noncoagulatory effects of factor VIII on bone and joint health is better understood, close monitoring of patients will be required, she added.
Outcome measures
Subjective measures of joint health include patient-reported bleeding rates and health-related quality of life. These are practical for home management, but patients may not be able to distinguish symptoms of acute joint bleeding from chronic arthritis pain, with the potential for either under- or overtreatment, and subjective reporting is likely to miss subclinical bleeding that can occur even when patients are on prophylaxis.
Health-related quality of life tools, whether generic or specific for hemophilia, are not sensitive to small improvements, and they are not always used in routine clinical practice.
Objective measures include physical examination with scoring according to the World Federation of Hemophilia (Gilbert Scale) score or Hemophilia Joint Health Score, but these measures have limited ability to identify early or subclinical joint abnormalities.
“Therefore, joint physical examination on its own is not a sufficient measure of treatment efficacy, and it should be used in combination with other tools more objective, such as imaging,” Dr. Gualtierotti said.
Get the picture?
Imaging with x-rays, MRI, and, recently in some centers, point-of-care ultrasound can provide clinicians with important real-time information about the joint stability and health.
Point-of-care ultrasound in particular offers promise as a practical tool, with no ionizing radiation and high sensitivity for synovial hyperplasia subclinical joint effusion. It’s relatively inexpensive, can be used to image multiple joints, and allows for ease of follow-up, she said. The technique requires specialized training, however, and there is a lack of prospective data about its utility in hemophilia.
Various ultrasound scoring systems have been proposed, and home-based ultrasound is currently being explored in several clinical trials, Dr. Gualtierotti noted.
Other avenues for remote joint health monitoring under consideration are serum or synovial biomarkers for joint bleeding and arthropathy that could be employed at bedside or at home, smartphone apps for breakthrough bleeding and patient-reported outcomes, and sensors for detecting abnormalities in gait that may signal joint dysfunction, she said.
Best practice
In the question-and-answer session following the talk, Fernando Zikan, MD, from the Federal University of Rio de Janeiro noted that, “in underdeveloped countries, we still find it very difficult to guide good practices for joint health control by the patient and family members. Which strategy do you think is fundamental for the patient to feel safe to notice changes in his body?”
“It would be useful to educate patients to come to the center whenever the patient has trauma and whenever an increase in his physical activity occurs. If this is far, a bedside ultrasound evaluation by the general practitioner could help avoid joint damage. Finally, a correct rehabilitation is fundamental,” Dr. Gualtierotti replied.
Asked by several others whether she used ultrasound in her daily practice, Dr. Gualtierotti said that “we use joint ultrasound in our clinical practice in the regular annual check-up examination and whenever the patient suspects and reports hemarthrosis.”
Dr. Gualtierotti reported participation in advisory boards for Biomarin, Pfizer, Bayer, and Takeda, and in educational seminars sponsored by Pfizer, Sobi, and Roche. She has received support for congress travel and/or attendance by Bayer and Pfizer. Dr. Zikan reported no relevant disclosures.
FROM EAHAD 2021
SHM Fellowship Class of 2021
The Society of Hospital Medicine has announced its 2021 class of Master Fellows, Senior Fellows, and Fellows in Hospital Medicine.
All Fellowship classes are listed in alphabetical order.
Master Fellows Class of 2021
Nasim Afsar, MD, MBA, MHM
Shaun D. Frost, MD, MHM
Jeffrey L. Schnipper, MD, MPH, MHM
Senior Fellows Class of 2021
Akindele Adaramola, MD, MPH, SFHM
Ramesh Adhikari, MD, SFHM
Pankaj Agrawal, MD, SFHM
Robert L. Anderson, MD, SFHM
Glenda B. Atilano, MD, SFHM
Bi A. Awosika, MD, FACP, SFHM
David N. Aymond, MD, SFHM
Paula Bailey, MD, SFHM
Amit B. Bansal, MD, MBA, SFHM
Jamie K. Bartley, DO, FACP, SFHM
Stephen J. Behnke, MD, SFHM
Christina A. Beyer, MD, SFHM
Vinil K. Bhuma, MD, SFHM
John P. Biebelhausen, MD, MBA, SFHM
Matthew T. Calestino, MD, FACP, SFHM
Domingo Caparas Jr., MD, FAAFP, SFHM
Darren Caudill, DO, FACP, SFHM
Julie M. Cernanec, MD, FAAP, SFHM
Will Cushing, PA-C, SFHM
Douglas A. Dodds II, MD, FAAP, SFHM
Coley B. Duncan, MD, CPE, MMM, SFHM
Noah Finkel, MD, SFHM
Justin Glasgow, MD, PhD, SFHM
Taylor Goot, MD, SFHM
Craig G. Gunderson, MD, SFHM
Alan Hall, MD, SFHM
Vivian Hamlett, MD, SFHM
Kathrin Harrington, MD, SFHM
Hossan Hassan, MD, SFHM
Anand D. Hongalgi, MD, FACP, SFHM
Akshata Hopkins, MD, FAAP, SFHM
Neelima Kamineni, MD, SFHM
Sudheer R. Kantharajpur, MBBS, MD, MHA, SFHM
Prakash Karki, MD, SFHM
Susrutha Kotwal, MD, SFHM
Ethan Kuperman, MD, SFHM
Rumman A. Langah, MD, FACP, SFHM
Sean LaVine, MD, FACP, SFHM
Don S. Lee, MD, FACP, SFHM
Charmaine A. Lewis, MD, MPH, CLHM, SFHM
Rishi Likhi, MD, SFHM
Lenny Lopez, MD, MPH, SFHM
Anthony Macchiavelli, MD, SFHM
Brian McGillen, MD, FACP, SFHM
Parth H. Mehta, MBBS, MD, MPH, SFHM
Anuj Mehta, MBBS, MD, MBA, SFHM
Prem Nair, MD, FACP, SFHM
Don J. Neer, MD, FACP, SFHM
Shyam Odeti, MD, FAAFP, SFHM
Amy T. O’Linn, DO, SFHM
Mihir Patel, MD, FACP, MBA, MPH, SFHM
Kimberly S. Pedram, MD, FACP, SFHM
Thomas Pineo, DO, SFHM
Mauricio Pinto, MD, SFHM
Lakmali C. Ranathunga, MBBS, SFHM
Matthew Reuter, MD, SFHM
Erik P. Rufa, MD, SFHM
Dipali Ruby Sahoo, DO, MBA, SFHM
Chady Sarraf, MD, SFHM
Suchita S. Sata, MD, SFHM
Klint Schwenk, MD, FAAP, MBA, SFHM
Aaron M. Sebach, CRNP, DNP, MBA, PhD, SFHM
Kevin Sowti, MD, MBA, SFHM
Joseph G. Surber, DO, FAAFP, SFHM
Bright Thilagar, MD, SFHM
Thomas S. Trawick Jr., MD, SFHM
Rehman Usmani, MD, SFHM
Arash Velayati, MD, SFHM
Jose A. Ventura, MD, FAAFP, SFHM
Andre Wajner, MD, PhD, SFHM
Phillip D. Warr, MD, SFHM
Virginia E. Watson, MD, SFHM
Kristin R. Wise, MD, SFHM
Elham A. Yousef, MD, FACP, MBA, MSc, SFHM
Fellows Class of 2021
Elizabeth M. Aarons, MD, FHM
Suhail A. Abbasi, MD, FACP, FHM
Waqas Adeel, MD, FHM
Rajender K. Agarwal, MD, MBA, MPH, FHM
Khaalisha Ajala, MD, MBA, FHM
Faraz S. Alam, MD, FHM
Amee Amin, MD, FHM
Muhammad W. Amir, MD, FACP, FHM
Saba Asad, MD, FHM
Logan Atkins, MD, FHM
Navneet Attri, MD, FHM
Jennifer Barnett, PA, FHM
Karyn Baum, MD, FHM
Prabhjot Bedi, MD, FHM
Nicolle R. Benz, DO, FHM
Ricky Bhimani, MD, FHM
Elizabeth Blankenship, PA-C, FHM
Rahul Borsadia, MD, FHM
Kalpana Chalasani, MD, FHM
Rani Chikkanna, MD, FHM
Venu Chippa, MBBS, MD, FHM
Lisa M. Coontz, FNP, FHM
Christie Crawford, MD, FHM
Rene Daniel, MD, PhD, FHM
Elda Dede, FHM
Radha Denmark, CNP, FHM
Alvine N. Nwehla Desamours, PA-C, FHM
Satyendra Dhar, MD, FHM
Manuel Jose Diaz, MD, FHM
Tiffany Egbe, MD, FHM
Chinwe Egbo, MD, FHM
Mohammad A. Farkhondehpour, MD, FACP, FHM
Shaheen Faruque, MBBS, FHM
Chris W. Fellin, MD, FACP, FHM
Juan Carlos Fuentes-Rosales, MD, FACP, MPH, FHM
Evelyn W. Gathecha, MD, FHM
Benjamin P. Geisler, MD, FACP, MPH, FHM
Matthew George, MD, FHM
Sonia George, DO, FHM
Mirna Giordano, MD, FHM
Rebecca Gomez, MD, FHM
David Gonzales, MD, FHM
Maria A. Guevara Hernandez, MD, FACP, FHM
Shubhra Gupta, MBBS, FHM
Rohini Harvey, MD, FHM
Allison Heinen, DO, FHM
Hollie L. Hurner, PA-C, FHM
Doug Hutcheon, MD, FHM
Varalakshmi Janamanchi, MD, FHM
Brian Keegan, MD, FACP, FHM
Qasim Khalil, MD, FHM
Irfana Khan, MD, FHM
Muhammad O. Khan, MD, FAAFP, MBA, FHM
Smita Kohli, MD, FHM
Julie Kolinski, MD, FAAP, FHM
Ewa Kontny, MD, FHM
Sungmi Lian, MD, FHM
Brian Lichtenstein, MD, FHM
Fernando Madero Gorostieta, MD, FHM
Vipul Mahajan, MD, FACP, FHM
Neetu Mahendraker, MD, FHM
Victoria McCurry, MD, FHM
Bridget McGrath, PA-C, FHM
Evan Meadors, MD, FHM
Kapil Mehta, MD, FACP, MBA, FHM
Waseem Mohamed, MD, FHM
Ernest Murray, MD, FHM
Murali K. Nagubandi, MD, FHM
Jessica Nave Allen, MD, FHM
Peter Nwafor, MD, FACP, FHM
Ike Anthony Nwaobi, MBBS, MBA, FHM
Olugbenga B. Ojo, MD, FACP, MBA, FHM
Jacqueline Okere, MD, FHM
Ifedolapo S. Olanrewaju, MD, MBchB, FHM
Mobolaji Olulade, MD, FHM
Elizabeth H. Papetti, MBA, FHM
Love Patel, MBBS, FHM
Kamakshya P. Patra, MD, MMM, FHM
Charles Pizanis, MD, FHM
Rajat Prakash, MD, FHM
Chris Pribula, MD, FHM
Michael Puchaev, MD, FHM
Ryan Punsalan, MD, FHM
Bhavya Rajanna, MD, FHM
Miguel A. Ramirez, MD, FHM
Raymund Ramirez, MD, FHM
Sandeep Randhawa, MBBS, FHM
Rohit Rattan, MD, FHM
Denisha Powell Rawlings, MD, FHM
Praveen K. Reddy, MD, MPH, FHM
Michael Ree, DO, MPH, FHM
Patrick Rendon, MD, FHM
David J. Rizk, MD, FAAFP, MBA, FHM
Michael Roberts, MD, FHM
Edwin Rosas, MD, FHM
Devjit Roy, MD, FHM
Sabyasachi Roy, MD, FHM
Paul Sandroni, CMPE, MSM, FHM
Vijairam Selvaraj, MD, MPH, FHM
Megha Shah, MD, MMM, FHM
Edie Shen, MD, FHM
Gurpinder Singh, MD, FACP, FHM
Vishwas A. Singh, MD, FHM
Karen Slatkovsky, MD, FHM
Sean M. Snyder, MD, FHM
Jaclyn Spiegel, MD, FHM
Dale Stapler Jr., MD, FHM
Christina E. Stovall, MD, FHM
Daniel Suders, DO, FHM
Clayton Swalstad, CMPE, MSM, FHM
Harshil Swaminarayan, MD, FHM
Keniesha Thompson, MD, FHM
Tet Toe, MD, FACP, FHM
Christine Tsai, MD, FHM
Ajay Vaikuntam, MD, FHM
Valerie Vaughn, MD, FHM
Jane N. Wainaina, FACP, MBchB, FHM
Neshahthari Wijeyakuhan, MD, FACP, FHM
Chia-Shing Yang, MD, FHM
Jennifer Zagursky, MD, FHM
The Society of Hospital Medicine has announced its 2021 class of Master Fellows, Senior Fellows, and Fellows in Hospital Medicine.
All Fellowship classes are listed in alphabetical order.
Master Fellows Class of 2021
Nasim Afsar, MD, MBA, MHM
Shaun D. Frost, MD, MHM
Jeffrey L. Schnipper, MD, MPH, MHM
Senior Fellows Class of 2021
Akindele Adaramola, MD, MPH, SFHM
Ramesh Adhikari, MD, SFHM
Pankaj Agrawal, MD, SFHM
Robert L. Anderson, MD, SFHM
Glenda B. Atilano, MD, SFHM
Bi A. Awosika, MD, FACP, SFHM
David N. Aymond, MD, SFHM
Paula Bailey, MD, SFHM
Amit B. Bansal, MD, MBA, SFHM
Jamie K. Bartley, DO, FACP, SFHM
Stephen J. Behnke, MD, SFHM
Christina A. Beyer, MD, SFHM
Vinil K. Bhuma, MD, SFHM
John P. Biebelhausen, MD, MBA, SFHM
Matthew T. Calestino, MD, FACP, SFHM
Domingo Caparas Jr., MD, FAAFP, SFHM
Darren Caudill, DO, FACP, SFHM
Julie M. Cernanec, MD, FAAP, SFHM
Will Cushing, PA-C, SFHM
Douglas A. Dodds II, MD, FAAP, SFHM
Coley B. Duncan, MD, CPE, MMM, SFHM
Noah Finkel, MD, SFHM
Justin Glasgow, MD, PhD, SFHM
Taylor Goot, MD, SFHM
Craig G. Gunderson, MD, SFHM
Alan Hall, MD, SFHM
Vivian Hamlett, MD, SFHM
Kathrin Harrington, MD, SFHM
Hossan Hassan, MD, SFHM
Anand D. Hongalgi, MD, FACP, SFHM
Akshata Hopkins, MD, FAAP, SFHM
Neelima Kamineni, MD, SFHM
Sudheer R. Kantharajpur, MBBS, MD, MHA, SFHM
Prakash Karki, MD, SFHM
Susrutha Kotwal, MD, SFHM
Ethan Kuperman, MD, SFHM
Rumman A. Langah, MD, FACP, SFHM
Sean LaVine, MD, FACP, SFHM
Don S. Lee, MD, FACP, SFHM
Charmaine A. Lewis, MD, MPH, CLHM, SFHM
Rishi Likhi, MD, SFHM
Lenny Lopez, MD, MPH, SFHM
Anthony Macchiavelli, MD, SFHM
Brian McGillen, MD, FACP, SFHM
Parth H. Mehta, MBBS, MD, MPH, SFHM
Anuj Mehta, MBBS, MD, MBA, SFHM
Prem Nair, MD, FACP, SFHM
Don J. Neer, MD, FACP, SFHM
Shyam Odeti, MD, FAAFP, SFHM
Amy T. O’Linn, DO, SFHM
Mihir Patel, MD, FACP, MBA, MPH, SFHM
Kimberly S. Pedram, MD, FACP, SFHM
Thomas Pineo, DO, SFHM
Mauricio Pinto, MD, SFHM
Lakmali C. Ranathunga, MBBS, SFHM
Matthew Reuter, MD, SFHM
Erik P. Rufa, MD, SFHM
Dipali Ruby Sahoo, DO, MBA, SFHM
Chady Sarraf, MD, SFHM
Suchita S. Sata, MD, SFHM
Klint Schwenk, MD, FAAP, MBA, SFHM
Aaron M. Sebach, CRNP, DNP, MBA, PhD, SFHM
Kevin Sowti, MD, MBA, SFHM
Joseph G. Surber, DO, FAAFP, SFHM
Bright Thilagar, MD, SFHM
Thomas S. Trawick Jr., MD, SFHM
Rehman Usmani, MD, SFHM
Arash Velayati, MD, SFHM
Jose A. Ventura, MD, FAAFP, SFHM
Andre Wajner, MD, PhD, SFHM
Phillip D. Warr, MD, SFHM
Virginia E. Watson, MD, SFHM
Kristin R. Wise, MD, SFHM
Elham A. Yousef, MD, FACP, MBA, MSc, SFHM
Fellows Class of 2021
Elizabeth M. Aarons, MD, FHM
Suhail A. Abbasi, MD, FACP, FHM
Waqas Adeel, MD, FHM
Rajender K. Agarwal, MD, MBA, MPH, FHM
Khaalisha Ajala, MD, MBA, FHM
Faraz S. Alam, MD, FHM
Amee Amin, MD, FHM
Muhammad W. Amir, MD, FACP, FHM
Saba Asad, MD, FHM
Logan Atkins, MD, FHM
Navneet Attri, MD, FHM
Jennifer Barnett, PA, FHM
Karyn Baum, MD, FHM
Prabhjot Bedi, MD, FHM
Nicolle R. Benz, DO, FHM
Ricky Bhimani, MD, FHM
Elizabeth Blankenship, PA-C, FHM
Rahul Borsadia, MD, FHM
Kalpana Chalasani, MD, FHM
Rani Chikkanna, MD, FHM
Venu Chippa, MBBS, MD, FHM
Lisa M. Coontz, FNP, FHM
Christie Crawford, MD, FHM
Rene Daniel, MD, PhD, FHM
Elda Dede, FHM
Radha Denmark, CNP, FHM
Alvine N. Nwehla Desamours, PA-C, FHM
Satyendra Dhar, MD, FHM
Manuel Jose Diaz, MD, FHM
Tiffany Egbe, MD, FHM
Chinwe Egbo, MD, FHM
Mohammad A. Farkhondehpour, MD, FACP, FHM
Shaheen Faruque, MBBS, FHM
Chris W. Fellin, MD, FACP, FHM
Juan Carlos Fuentes-Rosales, MD, FACP, MPH, FHM
Evelyn W. Gathecha, MD, FHM
Benjamin P. Geisler, MD, FACP, MPH, FHM
Matthew George, MD, FHM
Sonia George, DO, FHM
Mirna Giordano, MD, FHM
Rebecca Gomez, MD, FHM
David Gonzales, MD, FHM
Maria A. Guevara Hernandez, MD, FACP, FHM
Shubhra Gupta, MBBS, FHM
Rohini Harvey, MD, FHM
Allison Heinen, DO, FHM
Hollie L. Hurner, PA-C, FHM
Doug Hutcheon, MD, FHM
Varalakshmi Janamanchi, MD, FHM
Brian Keegan, MD, FACP, FHM
Qasim Khalil, MD, FHM
Irfana Khan, MD, FHM
Muhammad O. Khan, MD, FAAFP, MBA, FHM
Smita Kohli, MD, FHM
Julie Kolinski, MD, FAAP, FHM
Ewa Kontny, MD, FHM
Sungmi Lian, MD, FHM
Brian Lichtenstein, MD, FHM
Fernando Madero Gorostieta, MD, FHM
Vipul Mahajan, MD, FACP, FHM
Neetu Mahendraker, MD, FHM
Victoria McCurry, MD, FHM
Bridget McGrath, PA-C, FHM
Evan Meadors, MD, FHM
Kapil Mehta, MD, FACP, MBA, FHM
Waseem Mohamed, MD, FHM
Ernest Murray, MD, FHM
Murali K. Nagubandi, MD, FHM
Jessica Nave Allen, MD, FHM
Peter Nwafor, MD, FACP, FHM
Ike Anthony Nwaobi, MBBS, MBA, FHM
Olugbenga B. Ojo, MD, FACP, MBA, FHM
Jacqueline Okere, MD, FHM
Ifedolapo S. Olanrewaju, MD, MBchB, FHM
Mobolaji Olulade, MD, FHM
Elizabeth H. Papetti, MBA, FHM
Love Patel, MBBS, FHM
Kamakshya P. Patra, MD, MMM, FHM
Charles Pizanis, MD, FHM
Rajat Prakash, MD, FHM
Chris Pribula, MD, FHM
Michael Puchaev, MD, FHM
Ryan Punsalan, MD, FHM
Bhavya Rajanna, MD, FHM
Miguel A. Ramirez, MD, FHM
Raymund Ramirez, MD, FHM
Sandeep Randhawa, MBBS, FHM
Rohit Rattan, MD, FHM
Denisha Powell Rawlings, MD, FHM
Praveen K. Reddy, MD, MPH, FHM
Michael Ree, DO, MPH, FHM
Patrick Rendon, MD, FHM
David J. Rizk, MD, FAAFP, MBA, FHM
Michael Roberts, MD, FHM
Edwin Rosas, MD, FHM
Devjit Roy, MD, FHM
Sabyasachi Roy, MD, FHM
Paul Sandroni, CMPE, MSM, FHM
Vijairam Selvaraj, MD, MPH, FHM
Megha Shah, MD, MMM, FHM
Edie Shen, MD, FHM
Gurpinder Singh, MD, FACP, FHM
Vishwas A. Singh, MD, FHM
Karen Slatkovsky, MD, FHM
Sean M. Snyder, MD, FHM
Jaclyn Spiegel, MD, FHM
Dale Stapler Jr., MD, FHM
Christina E. Stovall, MD, FHM
Daniel Suders, DO, FHM
Clayton Swalstad, CMPE, MSM, FHM
Harshil Swaminarayan, MD, FHM
Keniesha Thompson, MD, FHM
Tet Toe, MD, FACP, FHM
Christine Tsai, MD, FHM
Ajay Vaikuntam, MD, FHM
Valerie Vaughn, MD, FHM
Jane N. Wainaina, FACP, MBchB, FHM
Neshahthari Wijeyakuhan, MD, FACP, FHM
Chia-Shing Yang, MD, FHM
Jennifer Zagursky, MD, FHM
The Society of Hospital Medicine has announced its 2021 class of Master Fellows, Senior Fellows, and Fellows in Hospital Medicine.
All Fellowship classes are listed in alphabetical order.
Master Fellows Class of 2021
Nasim Afsar, MD, MBA, MHM
Shaun D. Frost, MD, MHM
Jeffrey L. Schnipper, MD, MPH, MHM
Senior Fellows Class of 2021
Akindele Adaramola, MD, MPH, SFHM
Ramesh Adhikari, MD, SFHM
Pankaj Agrawal, MD, SFHM
Robert L. Anderson, MD, SFHM
Glenda B. Atilano, MD, SFHM
Bi A. Awosika, MD, FACP, SFHM
David N. Aymond, MD, SFHM
Paula Bailey, MD, SFHM
Amit B. Bansal, MD, MBA, SFHM
Jamie K. Bartley, DO, FACP, SFHM
Stephen J. Behnke, MD, SFHM
Christina A. Beyer, MD, SFHM
Vinil K. Bhuma, MD, SFHM
John P. Biebelhausen, MD, MBA, SFHM
Matthew T. Calestino, MD, FACP, SFHM
Domingo Caparas Jr., MD, FAAFP, SFHM
Darren Caudill, DO, FACP, SFHM
Julie M. Cernanec, MD, FAAP, SFHM
Will Cushing, PA-C, SFHM
Douglas A. Dodds II, MD, FAAP, SFHM
Coley B. Duncan, MD, CPE, MMM, SFHM
Noah Finkel, MD, SFHM
Justin Glasgow, MD, PhD, SFHM
Taylor Goot, MD, SFHM
Craig G. Gunderson, MD, SFHM
Alan Hall, MD, SFHM
Vivian Hamlett, MD, SFHM
Kathrin Harrington, MD, SFHM
Hossan Hassan, MD, SFHM
Anand D. Hongalgi, MD, FACP, SFHM
Akshata Hopkins, MD, FAAP, SFHM
Neelima Kamineni, MD, SFHM
Sudheer R. Kantharajpur, MBBS, MD, MHA, SFHM
Prakash Karki, MD, SFHM
Susrutha Kotwal, MD, SFHM
Ethan Kuperman, MD, SFHM
Rumman A. Langah, MD, FACP, SFHM
Sean LaVine, MD, FACP, SFHM
Don S. Lee, MD, FACP, SFHM
Charmaine A. Lewis, MD, MPH, CLHM, SFHM
Rishi Likhi, MD, SFHM
Lenny Lopez, MD, MPH, SFHM
Anthony Macchiavelli, MD, SFHM
Brian McGillen, MD, FACP, SFHM
Parth H. Mehta, MBBS, MD, MPH, SFHM
Anuj Mehta, MBBS, MD, MBA, SFHM
Prem Nair, MD, FACP, SFHM
Don J. Neer, MD, FACP, SFHM
Shyam Odeti, MD, FAAFP, SFHM
Amy T. O’Linn, DO, SFHM
Mihir Patel, MD, FACP, MBA, MPH, SFHM
Kimberly S. Pedram, MD, FACP, SFHM
Thomas Pineo, DO, SFHM
Mauricio Pinto, MD, SFHM
Lakmali C. Ranathunga, MBBS, SFHM
Matthew Reuter, MD, SFHM
Erik P. Rufa, MD, SFHM
Dipali Ruby Sahoo, DO, MBA, SFHM
Chady Sarraf, MD, SFHM
Suchita S. Sata, MD, SFHM
Klint Schwenk, MD, FAAP, MBA, SFHM
Aaron M. Sebach, CRNP, DNP, MBA, PhD, SFHM
Kevin Sowti, MD, MBA, SFHM
Joseph G. Surber, DO, FAAFP, SFHM
Bright Thilagar, MD, SFHM
Thomas S. Trawick Jr., MD, SFHM
Rehman Usmani, MD, SFHM
Arash Velayati, MD, SFHM
Jose A. Ventura, MD, FAAFP, SFHM
Andre Wajner, MD, PhD, SFHM
Phillip D. Warr, MD, SFHM
Virginia E. Watson, MD, SFHM
Kristin R. Wise, MD, SFHM
Elham A. Yousef, MD, FACP, MBA, MSc, SFHM
Fellows Class of 2021
Elizabeth M. Aarons, MD, FHM
Suhail A. Abbasi, MD, FACP, FHM
Waqas Adeel, MD, FHM
Rajender K. Agarwal, MD, MBA, MPH, FHM
Khaalisha Ajala, MD, MBA, FHM
Faraz S. Alam, MD, FHM
Amee Amin, MD, FHM
Muhammad W. Amir, MD, FACP, FHM
Saba Asad, MD, FHM
Logan Atkins, MD, FHM
Navneet Attri, MD, FHM
Jennifer Barnett, PA, FHM
Karyn Baum, MD, FHM
Prabhjot Bedi, MD, FHM
Nicolle R. Benz, DO, FHM
Ricky Bhimani, MD, FHM
Elizabeth Blankenship, PA-C, FHM
Rahul Borsadia, MD, FHM
Kalpana Chalasani, MD, FHM
Rani Chikkanna, MD, FHM
Venu Chippa, MBBS, MD, FHM
Lisa M. Coontz, FNP, FHM
Christie Crawford, MD, FHM
Rene Daniel, MD, PhD, FHM
Elda Dede, FHM
Radha Denmark, CNP, FHM
Alvine N. Nwehla Desamours, PA-C, FHM
Satyendra Dhar, MD, FHM
Manuel Jose Diaz, MD, FHM
Tiffany Egbe, MD, FHM
Chinwe Egbo, MD, FHM
Mohammad A. Farkhondehpour, MD, FACP, FHM
Shaheen Faruque, MBBS, FHM
Chris W. Fellin, MD, FACP, FHM
Juan Carlos Fuentes-Rosales, MD, FACP, MPH, FHM
Evelyn W. Gathecha, MD, FHM
Benjamin P. Geisler, MD, FACP, MPH, FHM
Matthew George, MD, FHM
Sonia George, DO, FHM
Mirna Giordano, MD, FHM
Rebecca Gomez, MD, FHM
David Gonzales, MD, FHM
Maria A. Guevara Hernandez, MD, FACP, FHM
Shubhra Gupta, MBBS, FHM
Rohini Harvey, MD, FHM
Allison Heinen, DO, FHM
Hollie L. Hurner, PA-C, FHM
Doug Hutcheon, MD, FHM
Varalakshmi Janamanchi, MD, FHM
Brian Keegan, MD, FACP, FHM
Qasim Khalil, MD, FHM
Irfana Khan, MD, FHM
Muhammad O. Khan, MD, FAAFP, MBA, FHM
Smita Kohli, MD, FHM
Julie Kolinski, MD, FAAP, FHM
Ewa Kontny, MD, FHM
Sungmi Lian, MD, FHM
Brian Lichtenstein, MD, FHM
Fernando Madero Gorostieta, MD, FHM
Vipul Mahajan, MD, FACP, FHM
Neetu Mahendraker, MD, FHM
Victoria McCurry, MD, FHM
Bridget McGrath, PA-C, FHM
Evan Meadors, MD, FHM
Kapil Mehta, MD, FACP, MBA, FHM
Waseem Mohamed, MD, FHM
Ernest Murray, MD, FHM
Murali K. Nagubandi, MD, FHM
Jessica Nave Allen, MD, FHM
Peter Nwafor, MD, FACP, FHM
Ike Anthony Nwaobi, MBBS, MBA, FHM
Olugbenga B. Ojo, MD, FACP, MBA, FHM
Jacqueline Okere, MD, FHM
Ifedolapo S. Olanrewaju, MD, MBchB, FHM
Mobolaji Olulade, MD, FHM
Elizabeth H. Papetti, MBA, FHM
Love Patel, MBBS, FHM
Kamakshya P. Patra, MD, MMM, FHM
Charles Pizanis, MD, FHM
Rajat Prakash, MD, FHM
Chris Pribula, MD, FHM
Michael Puchaev, MD, FHM
Ryan Punsalan, MD, FHM
Bhavya Rajanna, MD, FHM
Miguel A. Ramirez, MD, FHM
Raymund Ramirez, MD, FHM
Sandeep Randhawa, MBBS, FHM
Rohit Rattan, MD, FHM
Denisha Powell Rawlings, MD, FHM
Praveen K. Reddy, MD, MPH, FHM
Michael Ree, DO, MPH, FHM
Patrick Rendon, MD, FHM
David J. Rizk, MD, FAAFP, MBA, FHM
Michael Roberts, MD, FHM
Edwin Rosas, MD, FHM
Devjit Roy, MD, FHM
Sabyasachi Roy, MD, FHM
Paul Sandroni, CMPE, MSM, FHM
Vijairam Selvaraj, MD, MPH, FHM
Megha Shah, MD, MMM, FHM
Edie Shen, MD, FHM
Gurpinder Singh, MD, FACP, FHM
Vishwas A. Singh, MD, FHM
Karen Slatkovsky, MD, FHM
Sean M. Snyder, MD, FHM
Jaclyn Spiegel, MD, FHM
Dale Stapler Jr., MD, FHM
Christina E. Stovall, MD, FHM
Daniel Suders, DO, FHM
Clayton Swalstad, CMPE, MSM, FHM
Harshil Swaminarayan, MD, FHM
Keniesha Thompson, MD, FHM
Tet Toe, MD, FACP, FHM
Christine Tsai, MD, FHM
Ajay Vaikuntam, MD, FHM
Valerie Vaughn, MD, FHM
Jane N. Wainaina, FACP, MBchB, FHM
Neshahthari Wijeyakuhan, MD, FACP, FHM
Chia-Shing Yang, MD, FHM
Jennifer Zagursky, MD, FHM
Doxorubicin-pomalidomide combo shows promise for Kaposi sarcoma
Liposomal doxorubicin (Dox) plus pomalidomide (Pom) was safe and active in heavily pretreated patients with Kaposi sarcoma, according to results from a phase 1/2 trial.
“The results of our phase 1/2 study suggest pomalidomide and liposomal doxorubicin is safe with evidence of activity among patients with Kaposi sarcoma,” said investigator Ramya Ramaswami, MBBS, MPH, of the HIV & AIDS malignancy branch at the National Cancer Institute. The results were presented at the Conference on Retroviruses and Opportunistic Infections.
The researchers evaluated the safety and tolerability of Pom/Dox in two groups of patients with Kaposi sarcoma: group 1 included patients with Kaposi sarcoma alone and group 2 included patients with Kaposi sarcoma–associated herpesvirus and concurrent multicentric Castleman disease (KSHV-MCD) and KSHV inflammatory cytokine syndrome (KICS).
“Kaposi sarcoma can be challenging to treat when it co-occurs with KSHV-MCD or KICS, resulting in high mortality rates,” Dr. Ramaswami explained.
Study participants received IV liposomal Dox at 20 mg/m2 on day 1 of a 28-day cycle, in addition to oral Pom once daily on days 1-21 at three escalating dose levels (2 mg, 3 mg, or 4 mg, respectively) using a standard 3 + 3 design until plateau of response, progression, dose-limiting toxicities (DLTs) or patient preference. Some eligibility criteria differed between groups 1 and 2. Participants in group 1 were required to be on antiretroviral therapy for at least 1 month and had a performance status of 2 or less, while those in group 2 had a performance status of 3 or less and could be antiretroviral therapy naive.
All participants received oral aspirin thromboprophylaxis (81 mg daily) and could have received prior Kaposi sarcoma therapy.
With respect to outcomes, Kaposi sarcoma responses were assessed using the modified AIDS Clinical Trial Group criteria and KICS and KSHV-MCD responses were evaluated using an NCI clinical benefit criteria.
Results
Overall, 34 cisgender men were enrolled in the study: 21 (62%) in group 1 and 13 (38%) in group 2. All participants had severe (T1) Kaposi sarcoma; 32 (94%) participants were HIV-infected and 22 (65%) had prior chemotherapy for Kaposi sarcoma.
While the HIV viral load was largely controlled in both groups, the CD4 count differed, with median CD4 counts of 286 and 92 cells/mcL in groups 1 and 2, respectively.
With respect to safety, no DLTs were observed in group 1. As a result, 12 participants were treated at the maximum tolerated dose (MTD) of 4 mg of Pom. However, two DLTs (grade 3 rash and pharyngeal edema) were observed in group 2 at the 3 mg dose level.
A median of six cycles were administered for all participants and the most common grade 3/4 toxicity was neutropenia; nine patients with grade 3 neutropenia required dose reduction and three patients had febrile neutropenia requiring hospitalization. Other Pom-related adverse events were rash, constipation, and fatigue.
Among evaluable participants receiving two or more cycles, 17 (81%) patients in group 1 had a response (95% confidence interval, 58-95%; 16 partial response and 1 complete response) and 5 (50%) patients in group 2 had a response (95% CI, 19-81%; 4 PR and 1 CR).
“Our waterfall plots indicated that the vast majority of patients in group 1 had a positive change in nodular lesions at baseline,” Dr. Ramaswami said. “Participants in group 2 showed some decrease in nodular lesions, but this was usually temporary.”
Among seven participants with KICS responses, four participants (57%) experienced a CR or PR in symptoms and lab abnormalities associated with KICS; three of six participants (50%) with KSHV-MCD responses experienced a PR as per response criteria.
“While activity was noted, the combination was less well tolerated in patients with Kaposi sarcoma and concurrent KSHV-MCD or KICS,” Dr. Ramaswami said.
During a live discussion, Ronald T. Mitsuyasu, MD, of the University of California, Los Angeles, asked Dr. Ramaswami about the use of liposomal doxorubicin alone in patients with Kaposi sarcoma and concurrent KSHV-MCD or KICS.
While there is currently no data on the use of doxorubicin alone in this population, Dr. Ramaswami noted that she was more confident administering Pom/Dox combination therapy for these patients.
Dr. Ramaswami disclosed financial relationships with the National Cancer Institute, Celgene/Bristol-Myers Squibb, EMD Serono, Merck, CTI Biopharma, and Janssen. The study was funded by a cooperative research and drug development agreement between the National Cancer Institute and Celgene/BMS, EMD Serono, Merck, CTI Biopharma, and Janssen.
Liposomal doxorubicin (Dox) plus pomalidomide (Pom) was safe and active in heavily pretreated patients with Kaposi sarcoma, according to results from a phase 1/2 trial.
“The results of our phase 1/2 study suggest pomalidomide and liposomal doxorubicin is safe with evidence of activity among patients with Kaposi sarcoma,” said investigator Ramya Ramaswami, MBBS, MPH, of the HIV & AIDS malignancy branch at the National Cancer Institute. The results were presented at the Conference on Retroviruses and Opportunistic Infections.
The researchers evaluated the safety and tolerability of Pom/Dox in two groups of patients with Kaposi sarcoma: group 1 included patients with Kaposi sarcoma alone and group 2 included patients with Kaposi sarcoma–associated herpesvirus and concurrent multicentric Castleman disease (KSHV-MCD) and KSHV inflammatory cytokine syndrome (KICS).
“Kaposi sarcoma can be challenging to treat when it co-occurs with KSHV-MCD or KICS, resulting in high mortality rates,” Dr. Ramaswami explained.
Study participants received IV liposomal Dox at 20 mg/m2 on day 1 of a 28-day cycle, in addition to oral Pom once daily on days 1-21 at three escalating dose levels (2 mg, 3 mg, or 4 mg, respectively) using a standard 3 + 3 design until plateau of response, progression, dose-limiting toxicities (DLTs) or patient preference. Some eligibility criteria differed between groups 1 and 2. Participants in group 1 were required to be on antiretroviral therapy for at least 1 month and had a performance status of 2 or less, while those in group 2 had a performance status of 3 or less and could be antiretroviral therapy naive.
All participants received oral aspirin thromboprophylaxis (81 mg daily) and could have received prior Kaposi sarcoma therapy.
With respect to outcomes, Kaposi sarcoma responses were assessed using the modified AIDS Clinical Trial Group criteria and KICS and KSHV-MCD responses were evaluated using an NCI clinical benefit criteria.
Results
Overall, 34 cisgender men were enrolled in the study: 21 (62%) in group 1 and 13 (38%) in group 2. All participants had severe (T1) Kaposi sarcoma; 32 (94%) participants were HIV-infected and 22 (65%) had prior chemotherapy for Kaposi sarcoma.
While the HIV viral load was largely controlled in both groups, the CD4 count differed, with median CD4 counts of 286 and 92 cells/mcL in groups 1 and 2, respectively.
With respect to safety, no DLTs were observed in group 1. As a result, 12 participants were treated at the maximum tolerated dose (MTD) of 4 mg of Pom. However, two DLTs (grade 3 rash and pharyngeal edema) were observed in group 2 at the 3 mg dose level.
A median of six cycles were administered for all participants and the most common grade 3/4 toxicity was neutropenia; nine patients with grade 3 neutropenia required dose reduction and three patients had febrile neutropenia requiring hospitalization. Other Pom-related adverse events were rash, constipation, and fatigue.
Among evaluable participants receiving two or more cycles, 17 (81%) patients in group 1 had a response (95% confidence interval, 58-95%; 16 partial response and 1 complete response) and 5 (50%) patients in group 2 had a response (95% CI, 19-81%; 4 PR and 1 CR).
“Our waterfall plots indicated that the vast majority of patients in group 1 had a positive change in nodular lesions at baseline,” Dr. Ramaswami said. “Participants in group 2 showed some decrease in nodular lesions, but this was usually temporary.”
Among seven participants with KICS responses, four participants (57%) experienced a CR or PR in symptoms and lab abnormalities associated with KICS; three of six participants (50%) with KSHV-MCD responses experienced a PR as per response criteria.
“While activity was noted, the combination was less well tolerated in patients with Kaposi sarcoma and concurrent KSHV-MCD or KICS,” Dr. Ramaswami said.
During a live discussion, Ronald T. Mitsuyasu, MD, of the University of California, Los Angeles, asked Dr. Ramaswami about the use of liposomal doxorubicin alone in patients with Kaposi sarcoma and concurrent KSHV-MCD or KICS.
While there is currently no data on the use of doxorubicin alone in this population, Dr. Ramaswami noted that she was more confident administering Pom/Dox combination therapy for these patients.
Dr. Ramaswami disclosed financial relationships with the National Cancer Institute, Celgene/Bristol-Myers Squibb, EMD Serono, Merck, CTI Biopharma, and Janssen. The study was funded by a cooperative research and drug development agreement between the National Cancer Institute and Celgene/BMS, EMD Serono, Merck, CTI Biopharma, and Janssen.
Liposomal doxorubicin (Dox) plus pomalidomide (Pom) was safe and active in heavily pretreated patients with Kaposi sarcoma, according to results from a phase 1/2 trial.
“The results of our phase 1/2 study suggest pomalidomide and liposomal doxorubicin is safe with evidence of activity among patients with Kaposi sarcoma,” said investigator Ramya Ramaswami, MBBS, MPH, of the HIV & AIDS malignancy branch at the National Cancer Institute. The results were presented at the Conference on Retroviruses and Opportunistic Infections.
The researchers evaluated the safety and tolerability of Pom/Dox in two groups of patients with Kaposi sarcoma: group 1 included patients with Kaposi sarcoma alone and group 2 included patients with Kaposi sarcoma–associated herpesvirus and concurrent multicentric Castleman disease (KSHV-MCD) and KSHV inflammatory cytokine syndrome (KICS).
“Kaposi sarcoma can be challenging to treat when it co-occurs with KSHV-MCD or KICS, resulting in high mortality rates,” Dr. Ramaswami explained.
Study participants received IV liposomal Dox at 20 mg/m2 on day 1 of a 28-day cycle, in addition to oral Pom once daily on days 1-21 at three escalating dose levels (2 mg, 3 mg, or 4 mg, respectively) using a standard 3 + 3 design until plateau of response, progression, dose-limiting toxicities (DLTs) or patient preference. Some eligibility criteria differed between groups 1 and 2. Participants in group 1 were required to be on antiretroviral therapy for at least 1 month and had a performance status of 2 or less, while those in group 2 had a performance status of 3 or less and could be antiretroviral therapy naive.
All participants received oral aspirin thromboprophylaxis (81 mg daily) and could have received prior Kaposi sarcoma therapy.
With respect to outcomes, Kaposi sarcoma responses were assessed using the modified AIDS Clinical Trial Group criteria and KICS and KSHV-MCD responses were evaluated using an NCI clinical benefit criteria.
Results
Overall, 34 cisgender men were enrolled in the study: 21 (62%) in group 1 and 13 (38%) in group 2. All participants had severe (T1) Kaposi sarcoma; 32 (94%) participants were HIV-infected and 22 (65%) had prior chemotherapy for Kaposi sarcoma.
While the HIV viral load was largely controlled in both groups, the CD4 count differed, with median CD4 counts of 286 and 92 cells/mcL in groups 1 and 2, respectively.
With respect to safety, no DLTs were observed in group 1. As a result, 12 participants were treated at the maximum tolerated dose (MTD) of 4 mg of Pom. However, two DLTs (grade 3 rash and pharyngeal edema) were observed in group 2 at the 3 mg dose level.
A median of six cycles were administered for all participants and the most common grade 3/4 toxicity was neutropenia; nine patients with grade 3 neutropenia required dose reduction and three patients had febrile neutropenia requiring hospitalization. Other Pom-related adverse events were rash, constipation, and fatigue.
Among evaluable participants receiving two or more cycles, 17 (81%) patients in group 1 had a response (95% confidence interval, 58-95%; 16 partial response and 1 complete response) and 5 (50%) patients in group 2 had a response (95% CI, 19-81%; 4 PR and 1 CR).
“Our waterfall plots indicated that the vast majority of patients in group 1 had a positive change in nodular lesions at baseline,” Dr. Ramaswami said. “Participants in group 2 showed some decrease in nodular lesions, but this was usually temporary.”
Among seven participants with KICS responses, four participants (57%) experienced a CR or PR in symptoms and lab abnormalities associated with KICS; three of six participants (50%) with KSHV-MCD responses experienced a PR as per response criteria.
“While activity was noted, the combination was less well tolerated in patients with Kaposi sarcoma and concurrent KSHV-MCD or KICS,” Dr. Ramaswami said.
During a live discussion, Ronald T. Mitsuyasu, MD, of the University of California, Los Angeles, asked Dr. Ramaswami about the use of liposomal doxorubicin alone in patients with Kaposi sarcoma and concurrent KSHV-MCD or KICS.
While there is currently no data on the use of doxorubicin alone in this population, Dr. Ramaswami noted that she was more confident administering Pom/Dox combination therapy for these patients.
Dr. Ramaswami disclosed financial relationships with the National Cancer Institute, Celgene/Bristol-Myers Squibb, EMD Serono, Merck, CTI Biopharma, and Janssen. The study was funded by a cooperative research and drug development agreement between the National Cancer Institute and Celgene/BMS, EMD Serono, Merck, CTI Biopharma, and Janssen.
FROM CROI 2021
Eating fish tied to fewer CVD events in high-risk people
People with cardiovascular disease who regularly ate fish had significantly fewer major CVD events and there were fewer total deaths, compared with similar individuals who didn’t eat fish, but there was no beneficial link from eating fish among the general population in prospective data collected from more than 191,000 people from 58 countries.
Despite the neutral finding among people without CVD, the finding that eating fish was associated with significant benefit for those with CVD or who were at high risk for CVD confirms the public health importance of regular fish or fish oil consumption, said one expert.
A little over a quarter of those included in the new study had a history of CVD or were at high risk for CVD. In this subgroup of more than 51,000 people, those who consumed on average at least two servings of fish weekly (at least 175 g, or about 6.2 ounces per week) had a significant 16% lower rate of major CVD events during a median follow-up of about 7.5 years.
The rate of all-cause death was a significant 18% lower among people who ate two or more fish portions weekly, compared with those who didn’t, Deepa Mohan, PhD, and associates wrote in their report in JAMA Internal Medicine.
The researchers saw no additional benefit when people regularly ate greater amounts of fish.
“There is a significant protective benefit of fish consumption in people with cardiovascular disease,” said Andrew Mente, PhD, a senior investigator on the study and an epidemiologist at McMaster University, Hamilton, Ont..
“This study has important implications for guidelines on fish intake globally. It indicates that increasing fish consumption and particularly oily fish in vascular patients may produce a modest cardiovascular benefit,” he said in a statement released by McMaster.
‘A large body of evidence’ for CVD benefit
The neutral finding of no significant benefit (as well as no harm) regarding either CVD events or total mortality among people without CVD “does not alter the large body of prior observational evidence supporting the cardiac benefits of fish intake in general populations,” noted Dariush Mozaffarian, MD, DrPH, in a commentary that accompanies the report by Dr. Mohan and colleagues.
Although the new analysis failed to show a significant association between regular fish consumption and fewer CVD events for people without established CVD or CVD risk, “based on the cumulative evidence from prospective observational studies, randomized clinical trials, and mechanistic and experimental studies, modest fish consumption appears to have some cardiac benefits,” he added.
“Adults should aim to consume about two servings of fish per week, and larger benefits may accrue from nonfried oily (dark meat) fish,” wrote Dr. Mozaffarian, a professor of medicine and nutrition at Tufts University, Boston.
Oily, dark fishes include salmon, tuna steak, mackerel, herring, and sardines. Species such as these contain the highest levels of long-chain omega-3 fatty acids, eicosapentaenoic acid, and docosapentaenoic acid; these nutrients likely underlie the CVD benefits from fish, Dr. Mozaffarian said in an interview with JAMA Internal Medicine that accompanied his commentary. (Dr. Mente also participated.)
“Fish oil lowers heart rate, blood pressure, and triglycerides (at high dosages), increases adiponectin, improves endothelial function, and in some studies improves oxygen consumption in myocardium. If there is benefit from fish it’s from the omega 3s, and all in all the evidence supports this,” but because the evidence is primarily observational, it can only show linkage and cannot prove causation, he explained.
Given the potential benefit and limited risk, “I think everyone should aim to eat two servings of fish each week, preferentially oily fish. That’s very solid,” said Dr. Mozaffarian, who is also a cardiologist and dean of the Tufts Friedman School of Nutrition Science.
The investigators did not have adequate data to compare the associations between outcomes and a diet with oily fish versus less oily fish.
OTC fish oil capsules are ‘very reasonable’
For people who either can’t consume two fish meals a week or want to ensure their omega 3 intake is adequate, “it’s very reasonable for the average person to take one OTC [over-the-counter] fish oil capsule a day,” Dr. Mozaffarian added.
He acknowledged that several studies of fish oil supplements failed to show benefit, but several others have. “It’s a confusing field, but the evidence supports benefit from omega 3s,” he concluded.
He discounted the new finding that only people with established CVD or who are at high-risk benefit. “I’m not sure we should make too much of this, because many prior studies showed a lower CVD risk in fish-eating people without prevalent CVD,” he said. The new study “provides important information given its worldwide breadth.”
The new report used data regarding 191,558 people enrolled prospectively in any of four studies. The average age of the participants was 54 years, and 52% were women.
During follow-up, death from any cause occurred in 6% of those without CVD or CVD risk and in 13% of those with these factors. Major CVD events occurred in 5% and 17% of these two subgroups, respectively. To calculate the relative risks between those who ate fish and those who did not, the investigators used standard multivariate adjustment for potential confounders and adjusted for several dietary variables, Dr. Mente said.
Dr. Mohan and Dr. Mente disclosed no relevant financial relationships. Dr. Mozaffarian has received personal fees from Acasti Pharma, Amarin, America’s Test Kitchen, Barilla, Danone, GEOD, and Motif Food Works, and he has been an adviser to numerous companies.
A version of this article first appeared on Medscape.com.
People with cardiovascular disease who regularly ate fish had significantly fewer major CVD events and there were fewer total deaths, compared with similar individuals who didn’t eat fish, but there was no beneficial link from eating fish among the general population in prospective data collected from more than 191,000 people from 58 countries.
Despite the neutral finding among people without CVD, the finding that eating fish was associated with significant benefit for those with CVD or who were at high risk for CVD confirms the public health importance of regular fish or fish oil consumption, said one expert.
A little over a quarter of those included in the new study had a history of CVD or were at high risk for CVD. In this subgroup of more than 51,000 people, those who consumed on average at least two servings of fish weekly (at least 175 g, or about 6.2 ounces per week) had a significant 16% lower rate of major CVD events during a median follow-up of about 7.5 years.
The rate of all-cause death was a significant 18% lower among people who ate two or more fish portions weekly, compared with those who didn’t, Deepa Mohan, PhD, and associates wrote in their report in JAMA Internal Medicine.
The researchers saw no additional benefit when people regularly ate greater amounts of fish.
“There is a significant protective benefit of fish consumption in people with cardiovascular disease,” said Andrew Mente, PhD, a senior investigator on the study and an epidemiologist at McMaster University, Hamilton, Ont..
“This study has important implications for guidelines on fish intake globally. It indicates that increasing fish consumption and particularly oily fish in vascular patients may produce a modest cardiovascular benefit,” he said in a statement released by McMaster.
‘A large body of evidence’ for CVD benefit
The neutral finding of no significant benefit (as well as no harm) regarding either CVD events or total mortality among people without CVD “does not alter the large body of prior observational evidence supporting the cardiac benefits of fish intake in general populations,” noted Dariush Mozaffarian, MD, DrPH, in a commentary that accompanies the report by Dr. Mohan and colleagues.
Although the new analysis failed to show a significant association between regular fish consumption and fewer CVD events for people without established CVD or CVD risk, “based on the cumulative evidence from prospective observational studies, randomized clinical trials, and mechanistic and experimental studies, modest fish consumption appears to have some cardiac benefits,” he added.
“Adults should aim to consume about two servings of fish per week, and larger benefits may accrue from nonfried oily (dark meat) fish,” wrote Dr. Mozaffarian, a professor of medicine and nutrition at Tufts University, Boston.
Oily, dark fishes include salmon, tuna steak, mackerel, herring, and sardines. Species such as these contain the highest levels of long-chain omega-3 fatty acids, eicosapentaenoic acid, and docosapentaenoic acid; these nutrients likely underlie the CVD benefits from fish, Dr. Mozaffarian said in an interview with JAMA Internal Medicine that accompanied his commentary. (Dr. Mente also participated.)
“Fish oil lowers heart rate, blood pressure, and triglycerides (at high dosages), increases adiponectin, improves endothelial function, and in some studies improves oxygen consumption in myocardium. If there is benefit from fish it’s from the omega 3s, and all in all the evidence supports this,” but because the evidence is primarily observational, it can only show linkage and cannot prove causation, he explained.
Given the potential benefit and limited risk, “I think everyone should aim to eat two servings of fish each week, preferentially oily fish. That’s very solid,” said Dr. Mozaffarian, who is also a cardiologist and dean of the Tufts Friedman School of Nutrition Science.
The investigators did not have adequate data to compare the associations between outcomes and a diet with oily fish versus less oily fish.
OTC fish oil capsules are ‘very reasonable’
For people who either can’t consume two fish meals a week or want to ensure their omega 3 intake is adequate, “it’s very reasonable for the average person to take one OTC [over-the-counter] fish oil capsule a day,” Dr. Mozaffarian added.
He acknowledged that several studies of fish oil supplements failed to show benefit, but several others have. “It’s a confusing field, but the evidence supports benefit from omega 3s,” he concluded.
He discounted the new finding that only people with established CVD or who are at high-risk benefit. “I’m not sure we should make too much of this, because many prior studies showed a lower CVD risk in fish-eating people without prevalent CVD,” he said. The new study “provides important information given its worldwide breadth.”
The new report used data regarding 191,558 people enrolled prospectively in any of four studies. The average age of the participants was 54 years, and 52% were women.
During follow-up, death from any cause occurred in 6% of those without CVD or CVD risk and in 13% of those with these factors. Major CVD events occurred in 5% and 17% of these two subgroups, respectively. To calculate the relative risks between those who ate fish and those who did not, the investigators used standard multivariate adjustment for potential confounders and adjusted for several dietary variables, Dr. Mente said.
Dr. Mohan and Dr. Mente disclosed no relevant financial relationships. Dr. Mozaffarian has received personal fees from Acasti Pharma, Amarin, America’s Test Kitchen, Barilla, Danone, GEOD, and Motif Food Works, and he has been an adviser to numerous companies.
A version of this article first appeared on Medscape.com.
People with cardiovascular disease who regularly ate fish had significantly fewer major CVD events and there were fewer total deaths, compared with similar individuals who didn’t eat fish, but there was no beneficial link from eating fish among the general population in prospective data collected from more than 191,000 people from 58 countries.
Despite the neutral finding among people without CVD, the finding that eating fish was associated with significant benefit for those with CVD or who were at high risk for CVD confirms the public health importance of regular fish or fish oil consumption, said one expert.
A little over a quarter of those included in the new study had a history of CVD or were at high risk for CVD. In this subgroup of more than 51,000 people, those who consumed on average at least two servings of fish weekly (at least 175 g, or about 6.2 ounces per week) had a significant 16% lower rate of major CVD events during a median follow-up of about 7.5 years.
The rate of all-cause death was a significant 18% lower among people who ate two or more fish portions weekly, compared with those who didn’t, Deepa Mohan, PhD, and associates wrote in their report in JAMA Internal Medicine.
The researchers saw no additional benefit when people regularly ate greater amounts of fish.
“There is a significant protective benefit of fish consumption in people with cardiovascular disease,” said Andrew Mente, PhD, a senior investigator on the study and an epidemiologist at McMaster University, Hamilton, Ont..
“This study has important implications for guidelines on fish intake globally. It indicates that increasing fish consumption and particularly oily fish in vascular patients may produce a modest cardiovascular benefit,” he said in a statement released by McMaster.
‘A large body of evidence’ for CVD benefit
The neutral finding of no significant benefit (as well as no harm) regarding either CVD events or total mortality among people without CVD “does not alter the large body of prior observational evidence supporting the cardiac benefits of fish intake in general populations,” noted Dariush Mozaffarian, MD, DrPH, in a commentary that accompanies the report by Dr. Mohan and colleagues.
Although the new analysis failed to show a significant association between regular fish consumption and fewer CVD events for people without established CVD or CVD risk, “based on the cumulative evidence from prospective observational studies, randomized clinical trials, and mechanistic and experimental studies, modest fish consumption appears to have some cardiac benefits,” he added.
“Adults should aim to consume about two servings of fish per week, and larger benefits may accrue from nonfried oily (dark meat) fish,” wrote Dr. Mozaffarian, a professor of medicine and nutrition at Tufts University, Boston.
Oily, dark fishes include salmon, tuna steak, mackerel, herring, and sardines. Species such as these contain the highest levels of long-chain omega-3 fatty acids, eicosapentaenoic acid, and docosapentaenoic acid; these nutrients likely underlie the CVD benefits from fish, Dr. Mozaffarian said in an interview with JAMA Internal Medicine that accompanied his commentary. (Dr. Mente also participated.)
“Fish oil lowers heart rate, blood pressure, and triglycerides (at high dosages), increases adiponectin, improves endothelial function, and in some studies improves oxygen consumption in myocardium. If there is benefit from fish it’s from the omega 3s, and all in all the evidence supports this,” but because the evidence is primarily observational, it can only show linkage and cannot prove causation, he explained.
Given the potential benefit and limited risk, “I think everyone should aim to eat two servings of fish each week, preferentially oily fish. That’s very solid,” said Dr. Mozaffarian, who is also a cardiologist and dean of the Tufts Friedman School of Nutrition Science.
The investigators did not have adequate data to compare the associations between outcomes and a diet with oily fish versus less oily fish.
OTC fish oil capsules are ‘very reasonable’
For people who either can’t consume two fish meals a week or want to ensure their omega 3 intake is adequate, “it’s very reasonable for the average person to take one OTC [over-the-counter] fish oil capsule a day,” Dr. Mozaffarian added.
He acknowledged that several studies of fish oil supplements failed to show benefit, but several others have. “It’s a confusing field, but the evidence supports benefit from omega 3s,” he concluded.
He discounted the new finding that only people with established CVD or who are at high-risk benefit. “I’m not sure we should make too much of this, because many prior studies showed a lower CVD risk in fish-eating people without prevalent CVD,” he said. The new study “provides important information given its worldwide breadth.”
The new report used data regarding 191,558 people enrolled prospectively in any of four studies. The average age of the participants was 54 years, and 52% were women.
During follow-up, death from any cause occurred in 6% of those without CVD or CVD risk and in 13% of those with these factors. Major CVD events occurred in 5% and 17% of these two subgroups, respectively. To calculate the relative risks between those who ate fish and those who did not, the investigators used standard multivariate adjustment for potential confounders and adjusted for several dietary variables, Dr. Mente said.
Dr. Mohan and Dr. Mente disclosed no relevant financial relationships. Dr. Mozaffarian has received personal fees from Acasti Pharma, Amarin, America’s Test Kitchen, Barilla, Danone, GEOD, and Motif Food Works, and he has been an adviser to numerous companies.
A version of this article first appeared on Medscape.com.
Erythropoietin falls short of neuroprotection in optic neuritis
“EPO conveyed neither functional nor structural neuroprotection in the visual pathways after optic neuritis as a clinically isolated syndrome,” said first author Wolf A. Lagreze, MD, of the University of Freiburg (Germany), in presenting the results at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
There are currently no treatments that provide neuroprotection for patients with optic neuritis, which can result in the degeneration of retinal ganglion cells, the axons of which form the optic nerve.
Although methylprednisolone, the standard treatment, can be of benefit, it has no effect in preventing neurodegeneration or subsequent vision impairment.
Importantly, optic neuritis, which can be a first sign of multiple sclerosis (MS), is considered an ideal model for an acute inflammatory attack on the nervous system and resulting neurodegeneration. Therefore, any treatment that provides neuroprotection for patients with optic neuritis could have potentially exciting broader implications.
The TONE trial
Preclinical studies have shown that EPO provides a small, potential degree of neuroprotection. To further evaluate EPO in this setting, Dr. Lagreze and colleagues conducted the TONE trial (Treatment of Optic Neuritis With Erythropoietin) in Germany between 2014 and 2017, in which they enrolled 108 patients with optic neuritis.
Inclusion criteria were having only unilateral optic neuritis as a clinically isolated syndrome that presented within 10 days of the first symptoms and having moderate to severe loss of visual acuity.
Persons with known MS were excluded; however, patients who were diagnosed with MS at the beginning of the study during the workup evaluation were included. Hence, about 20% of patients did have newly diagnosed MS, Dr. Lagreze noted.
The participants were randomly assigned in double-blind 1:1 ratio to receive treatment with either 33,000 IU EPO or placebo intravenously for 3 days as an adjunct to high-dose intravenous methylprednisolone (1,000 mg/day).
The final analysis included 52 patients who received EPO and 51 patients who received placebo. There were no significant differences between the groups in the first primary outcome of retinal nerve fiber layer atrophy, assessed with optic coherence tomography at week 26 (P = .76).
Likewise, no significant difference between groups was observed in the second primary outcome of low-contrast visual acuity at week 26, assessed using the 2.5% Sloan chart score of the affected eye (P = .38).
In addition, there were no significant differences between the groups in the rates of optic neuritis relapse.
In terms of safety measures, one patient in the EPO group developed sinus venous thrombosis, which was treated with anticoagulants and resolved without complications.
Reduced conversion to MS?
Interestingly, after 6 months, significantly fewer patients in the EPO arm (36%) had converted from clinically isolated syndrome to MS, compared with 57% in the placebo arm (P = .032). The difference became apparent as early as week 4.
Although those findings suggest that EPO provided some neuroprotection, there are notable caveats, Philippe Albrecht, MD, of the department of neurology at the University Hospital Dusseldorf (Germany), and a coauthor on the study, said.
“The significant separation of EPO and placebo group regarding MS conversion was observed very early on in the course and did not change thereafter,” Dr. Albrecht noted.
“One would expect a true disease-modifying effect of EPO on MS conversion to take longer to develop, and this early separation may very well have been due to an imbalance in the treatment groups, [for example] regarding MRI imaging findings such as gadolinium enhancement at baseline,” he said.
Dr. Lagreze said that it was a surprise to see no benefit from the drug, and a closer look at certain subgroups may still be worthwhile. Factors that could have a bearing on results include a shorter time interval for inclusion, having no concomitant use of steroids, and longer duration of treatment with EPO.
“If I could do the study again, I would do the treatment for longer than 3 days – that was based on experiences in previous EPO trials,” he said. “I would also love to do the trial without the concomitant methylprednisolone, but that is not possible from an ethical point of view.”
Trial nevertheless important
Commenting on the study, E. Anne Yeh, MD, of the division of neurology at the Hospital for Sick Children, Toronto, agreed that a challenge in evaluating therapies for optic neuritis is the potential for confounding from existing therapies that patients need to take.
“This agent could not be evaluated alone for its protective effect in comparison to no treatment at all,” she said.
In addition, improved metrics for gauging outcomes are needed to better determine the true effects, she added.
“The development of newer vision-related outcome metrics is important for future studies, and many are hard at work on both structural and functional metrics that may help us to understand the benefits of any protective therapies in a more nuanced manner than we are currently able to,” she said.
However, results of any kind – negative or positive – are valuable in improving understanding, Dr. Yeh underscored.
“Negative results can be disappointing in any trial, especially one in which alternative therapeutic pathways are being sought,” Dr. Yeh said. “I want to emphasize, however, that the fact that we are even considering and completing trials in this area is important.”
Dr. Yeh noted that she is currently involved in a trial that is evaluating the diabetes drug metformin for its remyelinating potential. “We hope to have some pilot data on MS in a few years,” she said.
Dr. Lagreze, Dr. Albrecht, and Dr. Yeh have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“EPO conveyed neither functional nor structural neuroprotection in the visual pathways after optic neuritis as a clinically isolated syndrome,” said first author Wolf A. Lagreze, MD, of the University of Freiburg (Germany), in presenting the results at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
There are currently no treatments that provide neuroprotection for patients with optic neuritis, which can result in the degeneration of retinal ganglion cells, the axons of which form the optic nerve.
Although methylprednisolone, the standard treatment, can be of benefit, it has no effect in preventing neurodegeneration or subsequent vision impairment.
Importantly, optic neuritis, which can be a first sign of multiple sclerosis (MS), is considered an ideal model for an acute inflammatory attack on the nervous system and resulting neurodegeneration. Therefore, any treatment that provides neuroprotection for patients with optic neuritis could have potentially exciting broader implications.
The TONE trial
Preclinical studies have shown that EPO provides a small, potential degree of neuroprotection. To further evaluate EPO in this setting, Dr. Lagreze and colleagues conducted the TONE trial (Treatment of Optic Neuritis With Erythropoietin) in Germany between 2014 and 2017, in which they enrolled 108 patients with optic neuritis.
Inclusion criteria were having only unilateral optic neuritis as a clinically isolated syndrome that presented within 10 days of the first symptoms and having moderate to severe loss of visual acuity.
Persons with known MS were excluded; however, patients who were diagnosed with MS at the beginning of the study during the workup evaluation were included. Hence, about 20% of patients did have newly diagnosed MS, Dr. Lagreze noted.
The participants were randomly assigned in double-blind 1:1 ratio to receive treatment with either 33,000 IU EPO or placebo intravenously for 3 days as an adjunct to high-dose intravenous methylprednisolone (1,000 mg/day).
The final analysis included 52 patients who received EPO and 51 patients who received placebo. There were no significant differences between the groups in the first primary outcome of retinal nerve fiber layer atrophy, assessed with optic coherence tomography at week 26 (P = .76).
Likewise, no significant difference between groups was observed in the second primary outcome of low-contrast visual acuity at week 26, assessed using the 2.5% Sloan chart score of the affected eye (P = .38).
In addition, there were no significant differences between the groups in the rates of optic neuritis relapse.
In terms of safety measures, one patient in the EPO group developed sinus venous thrombosis, which was treated with anticoagulants and resolved without complications.
Reduced conversion to MS?
Interestingly, after 6 months, significantly fewer patients in the EPO arm (36%) had converted from clinically isolated syndrome to MS, compared with 57% in the placebo arm (P = .032). The difference became apparent as early as week 4.
Although those findings suggest that EPO provided some neuroprotection, there are notable caveats, Philippe Albrecht, MD, of the department of neurology at the University Hospital Dusseldorf (Germany), and a coauthor on the study, said.
“The significant separation of EPO and placebo group regarding MS conversion was observed very early on in the course and did not change thereafter,” Dr. Albrecht noted.
“One would expect a true disease-modifying effect of EPO on MS conversion to take longer to develop, and this early separation may very well have been due to an imbalance in the treatment groups, [for example] regarding MRI imaging findings such as gadolinium enhancement at baseline,” he said.
Dr. Lagreze said that it was a surprise to see no benefit from the drug, and a closer look at certain subgroups may still be worthwhile. Factors that could have a bearing on results include a shorter time interval for inclusion, having no concomitant use of steroids, and longer duration of treatment with EPO.
“If I could do the study again, I would do the treatment for longer than 3 days – that was based on experiences in previous EPO trials,” he said. “I would also love to do the trial without the concomitant methylprednisolone, but that is not possible from an ethical point of view.”
Trial nevertheless important
Commenting on the study, E. Anne Yeh, MD, of the division of neurology at the Hospital for Sick Children, Toronto, agreed that a challenge in evaluating therapies for optic neuritis is the potential for confounding from existing therapies that patients need to take.
“This agent could not be evaluated alone for its protective effect in comparison to no treatment at all,” she said.
In addition, improved metrics for gauging outcomes are needed to better determine the true effects, she added.
“The development of newer vision-related outcome metrics is important for future studies, and many are hard at work on both structural and functional metrics that may help us to understand the benefits of any protective therapies in a more nuanced manner than we are currently able to,” she said.
However, results of any kind – negative or positive – are valuable in improving understanding, Dr. Yeh underscored.
“Negative results can be disappointing in any trial, especially one in which alternative therapeutic pathways are being sought,” Dr. Yeh said. “I want to emphasize, however, that the fact that we are even considering and completing trials in this area is important.”
Dr. Yeh noted that she is currently involved in a trial that is evaluating the diabetes drug metformin for its remyelinating potential. “We hope to have some pilot data on MS in a few years,” she said.
Dr. Lagreze, Dr. Albrecht, and Dr. Yeh have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“EPO conveyed neither functional nor structural neuroprotection in the visual pathways after optic neuritis as a clinically isolated syndrome,” said first author Wolf A. Lagreze, MD, of the University of Freiburg (Germany), in presenting the results at the meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
There are currently no treatments that provide neuroprotection for patients with optic neuritis, which can result in the degeneration of retinal ganglion cells, the axons of which form the optic nerve.
Although methylprednisolone, the standard treatment, can be of benefit, it has no effect in preventing neurodegeneration or subsequent vision impairment.
Importantly, optic neuritis, which can be a first sign of multiple sclerosis (MS), is considered an ideal model for an acute inflammatory attack on the nervous system and resulting neurodegeneration. Therefore, any treatment that provides neuroprotection for patients with optic neuritis could have potentially exciting broader implications.
The TONE trial
Preclinical studies have shown that EPO provides a small, potential degree of neuroprotection. To further evaluate EPO in this setting, Dr. Lagreze and colleagues conducted the TONE trial (Treatment of Optic Neuritis With Erythropoietin) in Germany between 2014 and 2017, in which they enrolled 108 patients with optic neuritis.
Inclusion criteria were having only unilateral optic neuritis as a clinically isolated syndrome that presented within 10 days of the first symptoms and having moderate to severe loss of visual acuity.
Persons with known MS were excluded; however, patients who were diagnosed with MS at the beginning of the study during the workup evaluation were included. Hence, about 20% of patients did have newly diagnosed MS, Dr. Lagreze noted.
The participants were randomly assigned in double-blind 1:1 ratio to receive treatment with either 33,000 IU EPO or placebo intravenously for 3 days as an adjunct to high-dose intravenous methylprednisolone (1,000 mg/day).
The final analysis included 52 patients who received EPO and 51 patients who received placebo. There were no significant differences between the groups in the first primary outcome of retinal nerve fiber layer atrophy, assessed with optic coherence tomography at week 26 (P = .76).
Likewise, no significant difference between groups was observed in the second primary outcome of low-contrast visual acuity at week 26, assessed using the 2.5% Sloan chart score of the affected eye (P = .38).
In addition, there were no significant differences between the groups in the rates of optic neuritis relapse.
In terms of safety measures, one patient in the EPO group developed sinus venous thrombosis, which was treated with anticoagulants and resolved without complications.
Reduced conversion to MS?
Interestingly, after 6 months, significantly fewer patients in the EPO arm (36%) had converted from clinically isolated syndrome to MS, compared with 57% in the placebo arm (P = .032). The difference became apparent as early as week 4.
Although those findings suggest that EPO provided some neuroprotection, there are notable caveats, Philippe Albrecht, MD, of the department of neurology at the University Hospital Dusseldorf (Germany), and a coauthor on the study, said.
“The significant separation of EPO and placebo group regarding MS conversion was observed very early on in the course and did not change thereafter,” Dr. Albrecht noted.
“One would expect a true disease-modifying effect of EPO on MS conversion to take longer to develop, and this early separation may very well have been due to an imbalance in the treatment groups, [for example] regarding MRI imaging findings such as gadolinium enhancement at baseline,” he said.
Dr. Lagreze said that it was a surprise to see no benefit from the drug, and a closer look at certain subgroups may still be worthwhile. Factors that could have a bearing on results include a shorter time interval for inclusion, having no concomitant use of steroids, and longer duration of treatment with EPO.
“If I could do the study again, I would do the treatment for longer than 3 days – that was based on experiences in previous EPO trials,” he said. “I would also love to do the trial without the concomitant methylprednisolone, but that is not possible from an ethical point of view.”
Trial nevertheless important
Commenting on the study, E. Anne Yeh, MD, of the division of neurology at the Hospital for Sick Children, Toronto, agreed that a challenge in evaluating therapies for optic neuritis is the potential for confounding from existing therapies that patients need to take.
“This agent could not be evaluated alone for its protective effect in comparison to no treatment at all,” she said.
In addition, improved metrics for gauging outcomes are needed to better determine the true effects, she added.
“The development of newer vision-related outcome metrics is important for future studies, and many are hard at work on both structural and functional metrics that may help us to understand the benefits of any protective therapies in a more nuanced manner than we are currently able to,” she said.
However, results of any kind – negative or positive – are valuable in improving understanding, Dr. Yeh underscored.
“Negative results can be disappointing in any trial, especially one in which alternative therapeutic pathways are being sought,” Dr. Yeh said. “I want to emphasize, however, that the fact that we are even considering and completing trials in this area is important.”
Dr. Yeh noted that she is currently involved in a trial that is evaluating the diabetes drug metformin for its remyelinating potential. “We hope to have some pilot data on MS in a few years,” she said.
Dr. Lagreze, Dr. Albrecht, and Dr. Yeh have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ACTRIMS FORUM 2021