User login
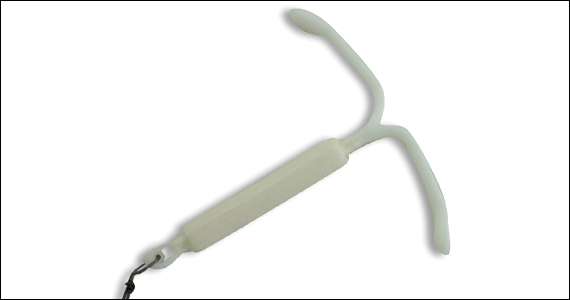
For heavy menstrual bleeding, are long-term outcomes similar for treatment with the LNG-IUS and radiofrequency endometrial ablation?
Beelen P, van den Brink MJ, Herman MC, et al. Levonorgestrel-releasing intrauterine system versus endometrial ablation for heavy menstrual bleeding. Am J Obstet Gynecol. 2021;224:187.e1-187.e10.
EXPERT COMMENTARY
Counseling patients regarding treatment of HMB requires a realistic discussion about the risks of intervention and the expected outcomes. In addition to decreasing menstrual blood loss, treatment benefits of the LNG-IUS include a reversible form of intervention, minimal discomfort with placement in an office environment with an awake patient, and a reliable form of contraception. Abnormal uterine bleeding (AUB) and progesterone-related adverse effects historically have been associated with LNG-IUS use and can lead to patient desires for device removal or additional intervention.
Similarly, in addition to endometrial ablation (EA) decreasing menstrual blood loss, its benefits include avoiding a hysterectomy with an outpatient procedure. Endometrial ablation does require a desire for no future pregnancies while using a reliable form of contraception. Risks of EA include failure to improve HMB or worsening pelvic pain that requires additional intervention, such as hysterectomy. Historically, clinical data suggest failure is more likely for women less than 40 years of age or with adenomyosis at the time of ablation.
Results of a long-term RCT by Beelen and colleagues may aid gynecologists in counseling patients on the risks and benefits of these 2 treatment options.
Details of the study
Performed between 2012 and 2016, this multicenter RCT evaluated primary intervention of the LNG-IUS in 132 women versus EA in 138 women. The women were older than age 34, did not want a future pregnancy, and had other etiologies of AUB eliminated.
The primary outcome was blood loss after 24 months as assessed with a Pictorial Blood Loss Assessment Chart (PBAC) score.
Secondary outcomes included controlled bleeding, defined as a PBAC score not exceeding 75 points; complications and reinterventions within 24 months; amenorrhea; spotting; dysmenorrhea; presence of clots; duration of blood loss; satisfaction with treatment; QoL; and sexual function.
The statistical null hypothesis of the trial was noninferiority of LNG-IUS treatment compared with EA treatment.
Results. Regarding the primary outcome, the mean PBAC score at 2 years was 64.8 for the LNG-IUS treatment group and 14.2 for the EA group. Importantly, however, the authors could not demonstrate noninferiority of the LNG-IUS compared with EA as a primary intervention for HMB.
For the secondary outcomes, there was no significant difference between groups, with both groups having a significant decrease in HMB at 3 months with PBAC scores that did not exceed 75 points: 60% in the LNG-IUS group and 83% in the EA group. In the LNG-IUS group, 35% of women received additional medical or surgical intervention versus 20% in the EA group.
Study strengths and limitations
Strengths of this study include its multicenter design, with 26 hospitals, and the long-term follow-up of 24 months. During the follow-up period, women were allowed to receive a reintervention as clinically indicated; thus, outcomes reflect results that are not from only a single designated intervention. For example, of the women in the LNG-IUS group, 34 received a surgical intervention, 31 (24%) underwent EA, and 9 (7%) underwent a hysterectomy. However, 6 of the 9 who underwent hysterectomy had a preceding EA, and these 6 women are not reported as surgical intervention of EA since the original designation for intervention was the LNG-IUS.
Notably, the patients and physicians were not blinded to the intervention, and the study excluded patients who wanted a future pregnancy. ●
Counseling patients regarding the LNG-IUS and EA for management of HMB requires a discussion balanced by information regarding the risks and the foreseeable benefits of these interventions. This study suggests that long-term primary and secondary outcomes are similar. Therefore, in choosing between the 2, a patient may rely more on her values, her age, and her consideration of future pregnancy and uterine preservation.
AMY L. GARCIA, MD
Beelen P, van den Brink MJ, Herman MC, et al. Levonorgestrel-releasing intrauterine system versus endometrial ablation for heavy menstrual bleeding. Am J Obstet Gynecol. 2021;224:187.e1-187.e10.
EXPERT COMMENTARY
Counseling patients regarding treatment of HMB requires a realistic discussion about the risks of intervention and the expected outcomes. In addition to decreasing menstrual blood loss, treatment benefits of the LNG-IUS include a reversible form of intervention, minimal discomfort with placement in an office environment with an awake patient, and a reliable form of contraception. Abnormal uterine bleeding (AUB) and progesterone-related adverse effects historically have been associated with LNG-IUS use and can lead to patient desires for device removal or additional intervention.
Similarly, in addition to endometrial ablation (EA) decreasing menstrual blood loss, its benefits include avoiding a hysterectomy with an outpatient procedure. Endometrial ablation does require a desire for no future pregnancies while using a reliable form of contraception. Risks of EA include failure to improve HMB or worsening pelvic pain that requires additional intervention, such as hysterectomy. Historically, clinical data suggest failure is more likely for women less than 40 years of age or with adenomyosis at the time of ablation.
Results of a long-term RCT by Beelen and colleagues may aid gynecologists in counseling patients on the risks and benefits of these 2 treatment options.
Details of the study
Performed between 2012 and 2016, this multicenter RCT evaluated primary intervention of the LNG-IUS in 132 women versus EA in 138 women. The women were older than age 34, did not want a future pregnancy, and had other etiologies of AUB eliminated.
The primary outcome was blood loss after 24 months as assessed with a Pictorial Blood Loss Assessment Chart (PBAC) score.
Secondary outcomes included controlled bleeding, defined as a PBAC score not exceeding 75 points; complications and reinterventions within 24 months; amenorrhea; spotting; dysmenorrhea; presence of clots; duration of blood loss; satisfaction with treatment; QoL; and sexual function.
The statistical null hypothesis of the trial was noninferiority of LNG-IUS treatment compared with EA treatment.
Results. Regarding the primary outcome, the mean PBAC score at 2 years was 64.8 for the LNG-IUS treatment group and 14.2 for the EA group. Importantly, however, the authors could not demonstrate noninferiority of the LNG-IUS compared with EA as a primary intervention for HMB.
For the secondary outcomes, there was no significant difference between groups, with both groups having a significant decrease in HMB at 3 months with PBAC scores that did not exceed 75 points: 60% in the LNG-IUS group and 83% in the EA group. In the LNG-IUS group, 35% of women received additional medical or surgical intervention versus 20% in the EA group.
Study strengths and limitations
Strengths of this study include its multicenter design, with 26 hospitals, and the long-term follow-up of 24 months. During the follow-up period, women were allowed to receive a reintervention as clinically indicated; thus, outcomes reflect results that are not from only a single designated intervention. For example, of the women in the LNG-IUS group, 34 received a surgical intervention, 31 (24%) underwent EA, and 9 (7%) underwent a hysterectomy. However, 6 of the 9 who underwent hysterectomy had a preceding EA, and these 6 women are not reported as surgical intervention of EA since the original designation for intervention was the LNG-IUS.
Notably, the patients and physicians were not blinded to the intervention, and the study excluded patients who wanted a future pregnancy. ●
Counseling patients regarding the LNG-IUS and EA for management of HMB requires a discussion balanced by information regarding the risks and the foreseeable benefits of these interventions. This study suggests that long-term primary and secondary outcomes are similar. Therefore, in choosing between the 2, a patient may rely more on her values, her age, and her consideration of future pregnancy and uterine preservation.
AMY L. GARCIA, MD
Beelen P, van den Brink MJ, Herman MC, et al. Levonorgestrel-releasing intrauterine system versus endometrial ablation for heavy menstrual bleeding. Am J Obstet Gynecol. 2021;224:187.e1-187.e10.
EXPERT COMMENTARY
Counseling patients regarding treatment of HMB requires a realistic discussion about the risks of intervention and the expected outcomes. In addition to decreasing menstrual blood loss, treatment benefits of the LNG-IUS include a reversible form of intervention, minimal discomfort with placement in an office environment with an awake patient, and a reliable form of contraception. Abnormal uterine bleeding (AUB) and progesterone-related adverse effects historically have been associated with LNG-IUS use and can lead to patient desires for device removal or additional intervention.
Similarly, in addition to endometrial ablation (EA) decreasing menstrual blood loss, its benefits include avoiding a hysterectomy with an outpatient procedure. Endometrial ablation does require a desire for no future pregnancies while using a reliable form of contraception. Risks of EA include failure to improve HMB or worsening pelvic pain that requires additional intervention, such as hysterectomy. Historically, clinical data suggest failure is more likely for women less than 40 years of age or with adenomyosis at the time of ablation.
Results of a long-term RCT by Beelen and colleagues may aid gynecologists in counseling patients on the risks and benefits of these 2 treatment options.
Details of the study
Performed between 2012 and 2016, this multicenter RCT evaluated primary intervention of the LNG-IUS in 132 women versus EA in 138 women. The women were older than age 34, did not want a future pregnancy, and had other etiologies of AUB eliminated.
The primary outcome was blood loss after 24 months as assessed with a Pictorial Blood Loss Assessment Chart (PBAC) score.
Secondary outcomes included controlled bleeding, defined as a PBAC score not exceeding 75 points; complications and reinterventions within 24 months; amenorrhea; spotting; dysmenorrhea; presence of clots; duration of blood loss; satisfaction with treatment; QoL; and sexual function.
The statistical null hypothesis of the trial was noninferiority of LNG-IUS treatment compared with EA treatment.
Results. Regarding the primary outcome, the mean PBAC score at 2 years was 64.8 for the LNG-IUS treatment group and 14.2 for the EA group. Importantly, however, the authors could not demonstrate noninferiority of the LNG-IUS compared with EA as a primary intervention for HMB.
For the secondary outcomes, there was no significant difference between groups, with both groups having a significant decrease in HMB at 3 months with PBAC scores that did not exceed 75 points: 60% in the LNG-IUS group and 83% in the EA group. In the LNG-IUS group, 35% of women received additional medical or surgical intervention versus 20% in the EA group.
Study strengths and limitations
Strengths of this study include its multicenter design, with 26 hospitals, and the long-term follow-up of 24 months. During the follow-up period, women were allowed to receive a reintervention as clinically indicated; thus, outcomes reflect results that are not from only a single designated intervention. For example, of the women in the LNG-IUS group, 34 received a surgical intervention, 31 (24%) underwent EA, and 9 (7%) underwent a hysterectomy. However, 6 of the 9 who underwent hysterectomy had a preceding EA, and these 6 women are not reported as surgical intervention of EA since the original designation for intervention was the LNG-IUS.
Notably, the patients and physicians were not blinded to the intervention, and the study excluded patients who wanted a future pregnancy. ●
Counseling patients regarding the LNG-IUS and EA for management of HMB requires a discussion balanced by information regarding the risks and the foreseeable benefits of these interventions. This study suggests that long-term primary and secondary outcomes are similar. Therefore, in choosing between the 2, a patient may rely more on her values, her age, and her consideration of future pregnancy and uterine preservation.
AMY L. GARCIA, MD
Office-based ambulatory cervical ripening prior to inpatient induction of labor
For women with a Bishop score ≤6, CR is an important first step in planned induction of labor (IOL). CR is believed to reduce the length of labor induction and increase the probability of a vaginal delivery. Historically, CR has been undertaken on a labor unit. However, with an increased rate of labor induction, the resources of the modern labor unit are incredibly stressed. Compounding the problem is the nursing shortage caused by the COVID-19 pandemic, which has resulted in staff being unavailable as they recover from a respiratory infection or are quarantined after an exposure. The COVID-19 pandemic also has motivated many patients to avoid the hospital as much as possible.
Office-based ambulatory CR is an alternative to inpatient CR and has the potential to reduce the use of labor unit resources. When CR is initiated in the office, the patient either is sent home overnight to return to the labor unit for IOL in the morning or is sent home in the morning to return for IOL in the evening or at night. A secondary benefit of office- and home-based CR is that it may increase patient satisfaction with the process of CR. This editorial summarizes the literature supporting office-based ambulatory CR.
Mechanical methods of CR
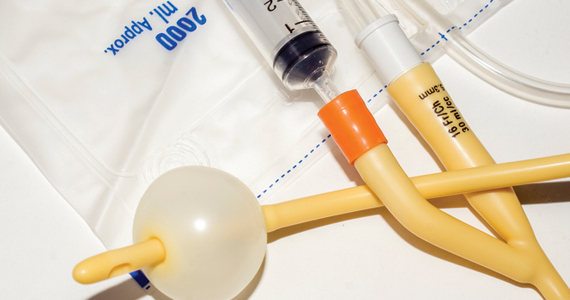
Contemporary mechanical methods of CR include the transcervical insertion of a Foley catheter, Cook double-balloon CR catheter, Dilapan-S, or laminaria. There are many publications reporting the feasibility of office-based ambulatory CR with transcervical balloon catheters and very few publications reporting on the use of Dilapan-S or laminaria for ambulatory CR.
Foley catheter
Many studies have investigated the effectiveness of transcervical Foley catheter for ambulatory CR. Policiano and colleagues compared the effectiveness of ambulatory versus inpatient Foley catheter CR.1 A total of 130 women with a Bishop score <6 at ≥41 weeks’ gestation were randomly assigned to outpatient or inpatient CR with a transcervical Foley catheter (Covidian Dover Silicon coated latex Foley catheter 16 Fr/5.3 mm diameter). The Foley catheter bulb was distended with 40 mL of a sterile saline solution. The end of the Foley was taped to the patient’s inner thigh. Manual traction was gently applied to the catheter every 6 hours. If the catheter was extruded, the Bishop score was assessed. For a Bishop score <6, the patient was given additional inpatient misoprostol (25 µg vaginally every 4 hours for up to 5 doses). For a Bishop score ≥6, intravenous oxytocin IOL was initiated. At 24 hours if the Foley catheter was still in situ, it was removed. Women were excluded from the study for the following factors: noncephalic presentation, spontaneous labor, hydramnios, nonreassuring cardiotocography (CTG), multiple pregnancy, ruptured membranes, active vaginal bleeding, Streptococcus group B infection, and HIV infection. Prostaglandin CR was not used if the woman had a previous cesarean delivery. No prophylactic antibiotics were administered. After placement of the Foley catheter, reassuring CTG was documented prior to sending the patient home.
Outpatient, compared with inpatient, CR resulted in a mean reduction of 10 hours in the time from admission to delivery. The time from insertion of the Foley catheter to delivery in the outpatient group was 38.2 hours, and 44.9 hours for the inpatient group (P<.01). The cesarean delivery rates were similar in both groups—28% and 38%, respectively. Three cases of chorioamnionitis occurred in each group. These study results support the feasibility of office-based ambulatory CR with a transcervical Foley.
Ausbeck and colleagues randomly assigned 126 nulliparous women with a Bishop score <5, at a gestational age ranging from 39 weeks and 0 days through 41 weeks and 6 days, to outpatient overnight CR or inpatient CR with a transcervical Foley catheter.2 Breech presentation and multiple gestation pregnancies were excluded from the study. The investigators utilized a 16 French Foley catheter and filled the balloon with 30 mL of sterile water. The Foley was taped to the woman’s inner thigh on slight tension. After placement of the Foley catheter at least 20 minutes of CTG monitoring was performed. The women in the outpatient group were given the contact number for the labor unit and advised that they could take acetaminophen for pain. They were advised that they could stay at home if the Foley catheter was expelled. They were admitted to the labor unit at the time scheduled for their IOL.
The mean time from admission to delivery was reduced by 4.3 hours in the outpatient compared with the inpatient CR group (17.4 vs 21.7 hours; P<.01). In the outpatient CR group, 22% of the women were admitted to labor before the time of the scheduled IOL. The cesarean delivery rates were similar in the outpatient and inpatient CR groups (24% vs 33%, P = .32). In the outpatient and inpatient groups, chorioamnionitis was diagnosed in 22% and 13% (P = .16) of the women. The authors concluded that outpatient CR with a transcervical Foley catheter reduced the time from admission to delivery.
Other research groups also have confirmed the feasibility of outpatient CR with a transcervical Foley catheter.3-5
Placement of the Foley catheter can be performed digitally without direct visualization of the cervix or by direct visualization using a vaginal speculum. After placement of the speculum, the cervix is cleansed with a povidone-iodine solution and a sterile ring forceps is used to grasp the catheter and guide it through the cervical os. In one small study, self-reported pain was similar for both digital and direct visualization methods for placement of the balloon catheter.6 When using Foley catheter CR, filling the standard Foley catheter balloon with 60 mL of fluid, rather than 30 to 40 mL of fluid, is rarely associated with balloon rupture and may result in more effective CR.6,7
Continue to: Double-balloon catheter...
Double-balloon catheter
The Cook double-balloon catheter for CR is meant to create pressure on both sides of the cervix, facilitating CR. Studies have reported that the Cook double-balloon catheter can be used for outpatient CR. In one study, 48 women with a low-risk pregnancy, at 37 to 42 weeks’ gestation and a Bishop score <7 were randomly assigned to outpatient or inpatient double-balloon CR.8 Both balloons were filled with 70 to 80 mL of sterile water. CTG monitoring was performed for 20 minutes before and after balloon placement. The women in the outpatient CR group were instructed to return to the labor unit the next day at 8 AM for IOL or earlier if they had regular uterine contractions, rupture of membranes, or vaginal bleeding. Seven percent of the women in the outpatient group returned to the labor unit before 8 AM. After removal of the balloon catheter, women in the outpatient and inpatient groups needed additional misoprostol CR in 12% and 13% of cases, respectively. Outcomes were similar in the two groups, but the study was not powered to identify small differences between the groups.
In another study of outpatient CR with the Cook double-balloon catheter, 695 women with a Bishop score <7, at ≥37 weeks’ gestation, were randomly assigned to outpatient CR with a double-balloon catheter or inpatient CR with dinoprostone (PGE2) (2 mg dinoprostone vaginal gel [Prostin] or dinoprostone 10 mg controlled-release tape (Cervidil).9 Women assigned to dinoprostone CR had CTG monitoring prior to commencing PGE2 CR and at least 30 min of CTG monitoring after insertion of the vaginal PGE2. Women assigned to balloon CR were not admitted to the hospital. CTG was performed prior to insertion of the balloon. After insertion, the two balloons on the catheter were each filled with 80 mL of saline. After catheter insertion CTG monitoring was not routinely performed. The women in the double-balloon catheter group returned to the labor unit 12 hours after insertion to initiate IOL. The primary outcome was composite neonatal morbidity and mortality, including admission to a neonatal intensive care unit (NICU), intubation, cardiac compressions, acidemia, hypoxic ischemic encephalopathy, seizure, infection, pulmonary hypertension, stillbirth, or death.
There was no significant difference in the rate of the primary outcome in the catheter versus the PGE2 group (18.6% and 25.8%; P = .07). Admission to the NICU occurred at rates of 12.6% and 15.5% in the catheter and PGE2 groups. Umbilical cord arterial pH <7.00 at birth occurred at a rate of 3.5% in the catheter group and 9.2% in the PGE2 group. The cesarean delivery rates in the catheter and PGE groups were 32.6% and 25.8%, respectively (P = .24). The investigators concluded that outpatient CR using a double-balloon catheter is safe and feasible for nulliparous women.
Two systematic reviews and meta-analyses reported that outcomes were similar when using the Foley or double-balloon catheter for CR.10,11 The Cook double-balloon CR kit includes a stylet, which can facilitate passing the catheter through the cervix.
Continue to: Dilapan-S and laminaria...
Dilapan-S and laminaria
There are many published studies using Dilapan-S and laminaria for cervical preparation prior to uterine evacuation.12 There are few published studies using Dilapan-S or laminaria for CR prior to IOL. In a pilot study, 21 patients were randomly assigned to outpatient versus inpatient Dilapan-S for CR the night prior to scheduled oxytocin IOL.13 The length of time from initiation of oxytocin to delivery in the outpatient and inpatient groups was similar (11 vs 14 hours, respectively). The outpatient compared with the inpatient group had a shorter length of hospitalization until delivery (51 vs 70 hours).
In other studies of Dilapan-S for CR, the patients remained in the hospital once the dilators were inserted. In one small trial, 41 women were randomized to CR with Dilapan-S or laminaria. As many dilators as could be comfortably tolerated by the patient were inserted.14 The mean numbers of Dilapan-S and laminaria dilators inserted were 4.3 and 9.7, respectively. The morning after the insertion of the dilators, oxytocin IOL was initiated. The times from initiation of oxytocin to delivery for the women in the Dilapan-S and laminaria groups were 11.6 and 15.5 hours, respectively.
An observational study reported on outcomes with Dilapan-S for CR on inpatients.15 In the study 444 women scheduled for IOL at 37 to 40 weeks’ gestation, with a mean baseline Bishop score of 2.9, had Dilapan-S placed for approximately 15 hours prior to oxytocin IOL. The mean number of Dilapan-S dilators that were inserted was 3.8. The study protocol prohibited placing more than 5 cervical dilator devices. The mean Bishop score after removal of the dilators was 6.5. The most common adverse effects of Dilapan-S CR were bleeding (2.7%) and pain (0.2%). The cesarean delivery rate in the cohort was 30.1%. An Apgar score <7 at 5 minutes was recorded for 3 newborns. An umbilical artery pH of <7.10 was observed in 8 newborns.
In a randomized trial performed on inpatients, 419 women undergoing CR were assigned to a Foley balloon or Dilapan-S.16 The vaginal delivery rates were similar in the groups—76% for Foley and 81% for Dilapan-S. Maternal and neonatal adverse effects were similar between the two groups. Compared with Foley catheter, women assigned to Dilapan-S reported greater satisfaction with their CR experience, more sleep, and more ability to perform daily activities.
Misoprostol and dinoprostone
Both misoprostol and dinoprostone are effective for outpatient CR. However, a Cochrane systematic review and meta-analysis concluded that balloon CR, compared with prostaglandin CR, is probably associated with a lower risk of uterine hyperstimulation with concerning fetal heart rate changes.17 Because misoprostol and dinoprostone occasionally can cause uterine hyperstimulation with fetal heart changes, many experts recommend CTG monitoring both before and after administration of misoprostol or dinoprostone for CR.
In a trial of outpatient versus inpatient vaginal PGE2 CR, 425 women at 37 to 42 weeks’ gestation were assigned randomly to outpatient or inpatient CR.18 All women had CTG monitoring for 20 minutes before and after vaginal placement of the PGE2 gel. The PGE2 dose was 2 mg for nulliparous and 1 mg for parous women. The cesarean delivery rates were similar in the outpatient and inpatient groups—22.3% and 22.9%, respectively. Among the women randomized to outpatient CR, 27 women (13%) could not be discharged home after administration of the vaginal PGE2 because of frequent uterine contractions or an abnormal fetal heart rate pattern. In addition, 64 women (30%) in the outpatient group returned to the hospital before scheduled induction because of frequent contractions. Maternal and neonatal complications were similar in the two groups. The investigators concluded that, at the dose and route of prostaglandin utilized in this study, the resultant rates of abnormal fetal heart rate pattern and frequent contractions might reduce the clinical utility of outpatient vaginal prostaglandin CR.
Another study also reported a greater rate of uterine tachysystole with vaginal PGE2 compared with a Foley catheter for CR (9% vs 0%).19 In a Cochrane systematic review of vaginal prostaglandin for CR, compared with placebo, vaginal prostaglandins were associated with a significantly greater rate of uterine hyperstimulation with fetal heart rate changes (4.8% vs 1.0%).20 Other studies also reported the feasibility of outpatient CR with vaginal prostaglandin.21,22
Both oral and vaginal misoprostol have been utilized for outpatient CR. In one study, 87 women with singleton pregnancy at 40 to 42 weeks’ gestation with a Bishop score <6 were randomized to outpatient CR with oral misoprostol (100 µg) or placebo.23 Following administration of the oral misoprostol, the women had 2 hours of CTG monitoring. The treatment was repeated daily for up to 3 days if there was no change in the cervix. If labor occurred, the patient was admitted to the labor unit for oxytocin IOL. The times from first dose of misoprostol or placebo to delivery were 46 and 84 hours (P<.001), respectively.
In another study, 49 women ≥40 weeks’ gestation with a Bishop score <5 were randomly assigned to receive outpatient oral misoprostol 25 µg or 50 µg.24 The dose could be repeated every 3 days over 9 days if ripening or labor had not been achieved. The women had CTG before administration of oral misoprostol. After the misoprostol dose, they had 2 hours of CTG monitoring. The number of doses received by the women assigned to the 50 µg group were 83%, 13%, and 4% for 1, 2, and 3 doses, respectively. The number of doses received by the women assigned to the 25 µg group were 58%, 26%, and 16% for 1, 2, and 3 doses, respectively. The mean intervals from initiation of CR to delivery in the 25 µg and the 50 µg groups were 3.9 and 2.5 days, respectively. The investigators reported no maternal or newborn adverse events, although the study was not powered to detect infrequent events.
Many studies have reported on the feasibility of outpatient CR with vaginal misoprostol.25-30 In one study, 77 women at 40 weeks’ gestation and a Bishop score ≤8 were randomized to a single dose of vaginal misoprostol 25 µg or gentle cervical examination (control).25 The women had 1 hour of CTG monitoring after the intervention. If they had regular contractions they were admitted to the birthing unit. If they had no regular contractions they were discharged home. For nulliparous women, the time from intervention to delivery in the misoprostol group was 4.9 days, and 8.1 days in the control group. For parous women, the times from intervention to delivery in the two groups were 3.8 and 6.9 days, respectively.
Continue to: Inclusion and exclusion criteria for outpatient CR...
Inclusion and exclusion criteria for outpatient CR
Outpatient CR should be limited to low-risk women with a singleton gestation, who have reliable access to transportation from home to the labor unit and have a clear understanding of the instructions for outpatient CR. Patient characteristics that may be utilized to offer office-based CR include:
- singleton pregnancy at 39 weeks’ and 0 days’ gestation through 40 weeks’ and 6 days’ gestation
- cephalic presentation
- Bishop score ≤6.
Women who should be excluded from outpatient CR include those with:
- contraindications to vaginal delivery
- fetal growth restriction
- abnormal umbilical artery Doppler results
- oligo- or polyhydramnios
- multiple gestation
- major fetal anomaly
- recent nonreactive fetal heart rate tracing
- maternal report of decreased fetal movement
- abnormal biophysical profile
- prior cesarean delivery
- recent vaginal bleeding
- gestational diabetes requiring medication treatment
- significant hypertension.
Practices should establish their own inclusion and exclusion criteria for ambulatory CR.
Safety of office-based ambulatory CR among low-risk women
Safety is a complex concept with experts often disagreeing on what level of safety is required to accept a new medical procedure. Establishing the safety of office-based ambulatory CR among low-risk women would require a very large cohort or randomized studies with at least a thousand participants. Only a few large studies focused on the safety of CR have been reported. Sciscione and colleagues reported a large observational study of inpatient transcervical Foley catheter for CR involving 1,905 women.31 They reported no adverse outcomes among term, singleton, uncomplicated pregnancies. They calculated that the 95% confidence interval (CI) for an adverse event was between 0.0% and 0.2%. In a meta-analysis of 26 studies including 5,563 women, the risk of chorioamnionitis during IOL was equivalent with pre-IOL Foley catheter CR (7.2%) or prostaglandin CR (7.2%) (relative risk, 0.96; 95% CI, 0.66–1.38).32
Two systematic reviews have reported that, compared with balloon CR, misoprostol CR is associated with an increased risk of uterine tachysystole.33-34 In a large retrospective study, compared with inpatient CR, outpatient CR with dinoprostone vaginal insert was not associated with an increased risk of newborn admission to the neonatal intensive care unit or a low Apgar score at 5 minutes after birth.35
Will you consider office-based CR in your obstetric practice?
As reviewed in this editorial, evolving data suggest that it is feasible to initiate CR in the office ambulatory setting prior to admission to the labor unit for additional CR or IOL. Many women prefer to complete CR at home after initiation in the office, rather than have CR in a labor unit or hospital setting.36 The transcervical balloon catheter has the most published data supporting the feasibility of ambulatory CR. Compared with misoprostol, the transcervical balloon catheter is associated with a low rate of uterine tachysystole. It may be a preferred method for outpatient CR. If placement of a transcervical balloon catheter is challenging, for example when the patient has a tightly closed cervix, oral misoprostol ambulatory CR may be an option if CTG monitoring is available in the office.
During the COVID pandemic, many in-person office visits have transitioned to virtual visits with the patient in their home. Historically, most cases of CR have been performed on labor and delivery units. It may be time for your practice to consider office-based ambulatory CR for low-risk women planning an IOL. Office-based ambulatory CR is a win for labor nurses who generally prefer to manage laboring patients rather than patients undergoing prolonged in-hospital CR. Outpatient CR is also a win for low-risk patients who prefer to be at home rather than in a labor unit. ●
- Policiano C, Pimenta M, Martins D, et al. Outpatient versus inpatient cervix priming with Foley catheter: a randomized trial. Eur J Obstet Gynecol Repro Biol. 2017;210:1-6.
- Ausbeck EB, Jauk VC, Xue Y, et al. Outpatient Foley catheter for induction of labor in nulliparous women. Obstet Gynecol. 2020;136:597-606.
- Wilkinson C, Adelson P, Turnbull D. A comparison of inpatient with outpatient balloon catheter cervical ripening: a pilot randomized controlled trial. BMC Pregnancy Childbirth. 2015;15:126.
- Sciscione AC, Muench M, Pollock M, et al. Transcervical Foley catheter for preinduction cervical ripening in an outpatient versus inpatient setting. Obstet Gynecol. 2001;98:751-756.
- Henry A, Madan A, Reid R, et al. Outpatient Foley catheter versus inpatient prostaglandin E2 gel for induction of labour: a randomised trial. BMC Pregnancy Childbirth. 2013;13:25.
- Kuhlmann MJ, Spencer N, Garcia-Jasso C, et al. Foley bulb insertion by blind placement compared with direct visualization. Obstet Gynecol. 2021;137:139-145.
- Delaney S, Shaffer BL, Chen YW, et al. Labor induction with a Foley balloon inflated to 30 mL compared with 60 mL. Obstet Gynecol. 2015;115:1239-1245.
- Wilkinson C, Adelson P, Turnbull D. A comparison of inpatient with outpatient balloon catheter cervical ripening: a pilot randomized controlled trial. BMC Pregnancy Childbirth. 2015;15:126.
- Beckmann M, Gibbons K, Flenady V, et al. Induction of labor using prostaglandin E2 as an inpatient versus balloon catheter as an outpatient: a multicenter randomised controlled trial. BJOG. 2020;127:571-579.
- Liu X, Wang Y, Zhange F, et al. Double- versus single-balloon catheters for labour induction and cervical ripening: a meta-analysis. BMC Pregnancy Childbirth. 2019;19:358.
- Yang F, Huan S, Long Y, et al. Double-balloon versus single-balloon catheter for cervical ripening and labor induction: a systematic review and meta-analysis. J Obstet Gynaecol Res. 2018;44: 27-34.
- Goldberg AB, Fortin JA, Drey EA, et al. Cervical preparation before dilation and evacuation using adjunctive misoprostol and mifepristone compared with overnight osmotic dilators alone: a randomized controlled trial. Obstet Gynecol. 2015;126:599-609.
- Upadhyaya NB, Childs KD, Neiger R, et al. Ambulatory cervical ripening in term pregnancy. J Reprod Med. 1999;44:363-366.
- Blumenthal PD, Rmanauskas R. Randomized trial of Dilapan and Laminaria as cervical ripening agents before induction of labor. Obstet Gynecol. 1990;75:365-368.
- Gupta J, Chodankar R, Baev O, et al. Synthetic osmotic dilators in the induction of labour—an international multicenter observational study. Eur J Obstet Gynecol Repro Biol. 2018;229:70-75.
- Saad AF, Villarreal J, Eid J, et al. A randomized controlled trial of Dilapan-S vs Foley balloon for preinduction cervical ripening (DILAFOL trial). Am J Obstet Gynecol. 2019;220:275.e1-e9.
- de Vaan MD, Eikleder MLT, Jozwiak M, et al. Mechanical methods for induction of labour. Cochrane Database Syst Rev. 2019;CD001233.
- Wilkinson C, Bryce R, Adelson P, et al. A randomized controlled trial of outpatient compared with inpatient cervical ripening with prostaglandin E2 (OPRA study). BJOG. 2015;122:94-104.
- Blair R, Harvey MA, Pudwell J, et al. Retrospective comparison of PGE2 vaginal insert and Foley catheter for outpatient cervical ripening. J Obstet Gynaecol Can. 2020;42:1103-1110.
- Thomas J, Fairclough A, Kavanagh J, et al. Vaginal prostaglandin (PGE2 or PGF2alpha) for induction of labour at term. Cochrane Database Syst Rev. 2014;CD003101.
- O’Brien JM, Mercer BM, Cleary NT, et al. Efficacy of outpatient induction with low-dose intravaginal prostaglandin E2: a randomized, doubleblind, placebo controlled trial. Am J Obstet Gynecol. 1995;173:1855-1859.
- Biem SR, Turnell RW, Olatunbosun O, et al. A randomized controlled trial of outpatient versus inpatient labour induction with vaginal controlled-release prostaglandin-E2: effectiveness and satisfaction. J Obstet Gynaecol Can. 2003;25:23-31.
- Gaffaney CA, Saul LL, Rumney PJ, et al. Outpatient oral misoprostol for prolonged pregnancies: a pilot investigation. Am J Perinatol. 2009;26: 673-677.
- Kipikasa JH, Adair CD, Williamson J, et al. Use of misoprostol on an outpatient basis for postdate pregnancy. Int J Gynaecol Obstet. 2005;88:108-111.
- Oboro VO, Tabowei TO. Outpatient misoprostol cervical ripening without subsequent induction of labor to prevent post-term pregnancy. Acta Obstet Gynecol Scand. 2005;84:628-631.
- Stitely ML, Browning J, Fowler M, et al. Outpatient cervical ripening with intravaginal misoprostol. Obstet Gynecol. 2000;96:684-688.
- McKenna DS, Ester JB, Proffitt M, et al. Misoprostol outpatient cervical ripening without subsequent induction of labor: a randomized trial. Obstet Gynecol. 2004;104:579-584.
- PonMalar J, Benjamin SJ, Abraham A, et al. Randomized double-blind placebo controlled study of preinduction cervical priming with 25 µg of misoprostol in the outpatient setting to prevent formal induction of labor. Arch Gynecol Obstet. 2017;295:33-38.
- Chang DW, Velazquez MD, Colyer M, et al. Vaginal misoprostol for cervical ripening at term: comparison of outpatient vs inpatient administration. Obstet Gynecol Surv. 2006;61:167-168.
- Meyer M, Pflum J, Howard D. Outpatient misoprostol compared with dinoprostone gel for preinduction cervical ripening: a randomized controlled trial. Obstet Gynecol. 2005;105:466-472.
- Sciscione AC, Bedder CL, Hoffman MK, et al. The timing of adverse events with Foley catheter preinduction cervical ripening; implications for outpatient use. Am J Perinatol. 2014;31:781-786.
- McMaster K, Sanchez-Ramos L, Kaunitz AM. Evaluation of a transcervical Foley catheter as a source of infection. Obstet Gynecol. 2015;126:539-551.
- Fox NS, Saltzman DH, Roman AS, et al. Intravaginal misoprostol versus Foley catheter for labour induction: a meta-analysis. BJOG. 2011;118: 647-654.
- Hofmeyr GJ, Gulmezoglu AM, Pileggi C. Vaginal misoprostol for cervical ripening and induction of labour. Cochrane Database Syst Rev. 2010:CD000941.
- Salvador SC, Simpson ML, Cundiff GW. Dinoprostone vaginal insert for labour induction: a comparison of outpatient and inpatient settings. J Obstet Gynaecol Can. 2009;31:1028-1034.
- Sutton C, Harding J, Griffin C. Patient attitudes towards outpatient cervical ripening prior to induction of labour at an Australian tertiary hospital. J Obstet Gynaecol. 2016;36:921-928.
For women with a Bishop score ≤6, CR is an important first step in planned induction of labor (IOL). CR is believed to reduce the length of labor induction and increase the probability of a vaginal delivery. Historically, CR has been undertaken on a labor unit. However, with an increased rate of labor induction, the resources of the modern labor unit are incredibly stressed. Compounding the problem is the nursing shortage caused by the COVID-19 pandemic, which has resulted in staff being unavailable as they recover from a respiratory infection or are quarantined after an exposure. The COVID-19 pandemic also has motivated many patients to avoid the hospital as much as possible.
Office-based ambulatory CR is an alternative to inpatient CR and has the potential to reduce the use of labor unit resources. When CR is initiated in the office, the patient either is sent home overnight to return to the labor unit for IOL in the morning or is sent home in the morning to return for IOL in the evening or at night. A secondary benefit of office- and home-based CR is that it may increase patient satisfaction with the process of CR. This editorial summarizes the literature supporting office-based ambulatory CR.
Mechanical methods of CR
Contemporary mechanical methods of CR include the transcervical insertion of a Foley catheter, Cook double-balloon CR catheter, Dilapan-S, or laminaria. There are many publications reporting the feasibility of office-based ambulatory CR with transcervical balloon catheters and very few publications reporting on the use of Dilapan-S or laminaria for ambulatory CR.
Foley catheter
Many studies have investigated the effectiveness of transcervical Foley catheter for ambulatory CR. Policiano and colleagues compared the effectiveness of ambulatory versus inpatient Foley catheter CR.1 A total of 130 women with a Bishop score <6 at ≥41 weeks’ gestation were randomly assigned to outpatient or inpatient CR with a transcervical Foley catheter (Covidian Dover Silicon coated latex Foley catheter 16 Fr/5.3 mm diameter). The Foley catheter bulb was distended with 40 mL of a sterile saline solution. The end of the Foley was taped to the patient’s inner thigh. Manual traction was gently applied to the catheter every 6 hours. If the catheter was extruded, the Bishop score was assessed. For a Bishop score <6, the patient was given additional inpatient misoprostol (25 µg vaginally every 4 hours for up to 5 doses). For a Bishop score ≥6, intravenous oxytocin IOL was initiated. At 24 hours if the Foley catheter was still in situ, it was removed. Women were excluded from the study for the following factors: noncephalic presentation, spontaneous labor, hydramnios, nonreassuring cardiotocography (CTG), multiple pregnancy, ruptured membranes, active vaginal bleeding, Streptococcus group B infection, and HIV infection. Prostaglandin CR was not used if the woman had a previous cesarean delivery. No prophylactic antibiotics were administered. After placement of the Foley catheter, reassuring CTG was documented prior to sending the patient home.
Outpatient, compared with inpatient, CR resulted in a mean reduction of 10 hours in the time from admission to delivery. The time from insertion of the Foley catheter to delivery in the outpatient group was 38.2 hours, and 44.9 hours for the inpatient group (P<.01). The cesarean delivery rates were similar in both groups—28% and 38%, respectively. Three cases of chorioamnionitis occurred in each group. These study results support the feasibility of office-based ambulatory CR with a transcervical Foley.
Ausbeck and colleagues randomly assigned 126 nulliparous women with a Bishop score <5, at a gestational age ranging from 39 weeks and 0 days through 41 weeks and 6 days, to outpatient overnight CR or inpatient CR with a transcervical Foley catheter.2 Breech presentation and multiple gestation pregnancies were excluded from the study. The investigators utilized a 16 French Foley catheter and filled the balloon with 30 mL of sterile water. The Foley was taped to the woman’s inner thigh on slight tension. After placement of the Foley catheter at least 20 minutes of CTG monitoring was performed. The women in the outpatient group were given the contact number for the labor unit and advised that they could take acetaminophen for pain. They were advised that they could stay at home if the Foley catheter was expelled. They were admitted to the labor unit at the time scheduled for their IOL.
The mean time from admission to delivery was reduced by 4.3 hours in the outpatient compared with the inpatient CR group (17.4 vs 21.7 hours; P<.01). In the outpatient CR group, 22% of the women were admitted to labor before the time of the scheduled IOL. The cesarean delivery rates were similar in the outpatient and inpatient CR groups (24% vs 33%, P = .32). In the outpatient and inpatient groups, chorioamnionitis was diagnosed in 22% and 13% (P = .16) of the women. The authors concluded that outpatient CR with a transcervical Foley catheter reduced the time from admission to delivery.
Other research groups also have confirmed the feasibility of outpatient CR with a transcervical Foley catheter.3-5
Placement of the Foley catheter can be performed digitally without direct visualization of the cervix or by direct visualization using a vaginal speculum. After placement of the speculum, the cervix is cleansed with a povidone-iodine solution and a sterile ring forceps is used to grasp the catheter and guide it through the cervical os. In one small study, self-reported pain was similar for both digital and direct visualization methods for placement of the balloon catheter.6 When using Foley catheter CR, filling the standard Foley catheter balloon with 60 mL of fluid, rather than 30 to 40 mL of fluid, is rarely associated with balloon rupture and may result in more effective CR.6,7
Continue to: Double-balloon catheter...
Double-balloon catheter
The Cook double-balloon catheter for CR is meant to create pressure on both sides of the cervix, facilitating CR. Studies have reported that the Cook double-balloon catheter can be used for outpatient CR. In one study, 48 women with a low-risk pregnancy, at 37 to 42 weeks’ gestation and a Bishop score <7 were randomly assigned to outpatient or inpatient double-balloon CR.8 Both balloons were filled with 70 to 80 mL of sterile water. CTG monitoring was performed for 20 minutes before and after balloon placement. The women in the outpatient CR group were instructed to return to the labor unit the next day at 8 AM for IOL or earlier if they had regular uterine contractions, rupture of membranes, or vaginal bleeding. Seven percent of the women in the outpatient group returned to the labor unit before 8 AM. After removal of the balloon catheter, women in the outpatient and inpatient groups needed additional misoprostol CR in 12% and 13% of cases, respectively. Outcomes were similar in the two groups, but the study was not powered to identify small differences between the groups.
In another study of outpatient CR with the Cook double-balloon catheter, 695 women with a Bishop score <7, at ≥37 weeks’ gestation, were randomly assigned to outpatient CR with a double-balloon catheter or inpatient CR with dinoprostone (PGE2) (2 mg dinoprostone vaginal gel [Prostin] or dinoprostone 10 mg controlled-release tape (Cervidil).9 Women assigned to dinoprostone CR had CTG monitoring prior to commencing PGE2 CR and at least 30 min of CTG monitoring after insertion of the vaginal PGE2. Women assigned to balloon CR were not admitted to the hospital. CTG was performed prior to insertion of the balloon. After insertion, the two balloons on the catheter were each filled with 80 mL of saline. After catheter insertion CTG monitoring was not routinely performed. The women in the double-balloon catheter group returned to the labor unit 12 hours after insertion to initiate IOL. The primary outcome was composite neonatal morbidity and mortality, including admission to a neonatal intensive care unit (NICU), intubation, cardiac compressions, acidemia, hypoxic ischemic encephalopathy, seizure, infection, pulmonary hypertension, stillbirth, or death.
There was no significant difference in the rate of the primary outcome in the catheter versus the PGE2 group (18.6% and 25.8%; P = .07). Admission to the NICU occurred at rates of 12.6% and 15.5% in the catheter and PGE2 groups. Umbilical cord arterial pH <7.00 at birth occurred at a rate of 3.5% in the catheter group and 9.2% in the PGE2 group. The cesarean delivery rates in the catheter and PGE groups were 32.6% and 25.8%, respectively (P = .24). The investigators concluded that outpatient CR using a double-balloon catheter is safe and feasible for nulliparous women.
Two systematic reviews and meta-analyses reported that outcomes were similar when using the Foley or double-balloon catheter for CR.10,11 The Cook double-balloon CR kit includes a stylet, which can facilitate passing the catheter through the cervix.
Continue to: Dilapan-S and laminaria...
Dilapan-S and laminaria
There are many published studies using Dilapan-S and laminaria for cervical preparation prior to uterine evacuation.12 There are few published studies using Dilapan-S or laminaria for CR prior to IOL. In a pilot study, 21 patients were randomly assigned to outpatient versus inpatient Dilapan-S for CR the night prior to scheduled oxytocin IOL.13 The length of time from initiation of oxytocin to delivery in the outpatient and inpatient groups was similar (11 vs 14 hours, respectively). The outpatient compared with the inpatient group had a shorter length of hospitalization until delivery (51 vs 70 hours).
In other studies of Dilapan-S for CR, the patients remained in the hospital once the dilators were inserted. In one small trial, 41 women were randomized to CR with Dilapan-S or laminaria. As many dilators as could be comfortably tolerated by the patient were inserted.14 The mean numbers of Dilapan-S and laminaria dilators inserted were 4.3 and 9.7, respectively. The morning after the insertion of the dilators, oxytocin IOL was initiated. The times from initiation of oxytocin to delivery for the women in the Dilapan-S and laminaria groups were 11.6 and 15.5 hours, respectively.
An observational study reported on outcomes with Dilapan-S for CR on inpatients.15 In the study 444 women scheduled for IOL at 37 to 40 weeks’ gestation, with a mean baseline Bishop score of 2.9, had Dilapan-S placed for approximately 15 hours prior to oxytocin IOL. The mean number of Dilapan-S dilators that were inserted was 3.8. The study protocol prohibited placing more than 5 cervical dilator devices. The mean Bishop score after removal of the dilators was 6.5. The most common adverse effects of Dilapan-S CR were bleeding (2.7%) and pain (0.2%). The cesarean delivery rate in the cohort was 30.1%. An Apgar score <7 at 5 minutes was recorded for 3 newborns. An umbilical artery pH of <7.10 was observed in 8 newborns.
In a randomized trial performed on inpatients, 419 women undergoing CR were assigned to a Foley balloon or Dilapan-S.16 The vaginal delivery rates were similar in the groups—76% for Foley and 81% for Dilapan-S. Maternal and neonatal adverse effects were similar between the two groups. Compared with Foley catheter, women assigned to Dilapan-S reported greater satisfaction with their CR experience, more sleep, and more ability to perform daily activities.
Misoprostol and dinoprostone
Both misoprostol and dinoprostone are effective for outpatient CR. However, a Cochrane systematic review and meta-analysis concluded that balloon CR, compared with prostaglandin CR, is probably associated with a lower risk of uterine hyperstimulation with concerning fetal heart rate changes.17 Because misoprostol and dinoprostone occasionally can cause uterine hyperstimulation with fetal heart changes, many experts recommend CTG monitoring both before and after administration of misoprostol or dinoprostone for CR.
In a trial of outpatient versus inpatient vaginal PGE2 CR, 425 women at 37 to 42 weeks’ gestation were assigned randomly to outpatient or inpatient CR.18 All women had CTG monitoring for 20 minutes before and after vaginal placement of the PGE2 gel. The PGE2 dose was 2 mg for nulliparous and 1 mg for parous women. The cesarean delivery rates were similar in the outpatient and inpatient groups—22.3% and 22.9%, respectively. Among the women randomized to outpatient CR, 27 women (13%) could not be discharged home after administration of the vaginal PGE2 because of frequent uterine contractions or an abnormal fetal heart rate pattern. In addition, 64 women (30%) in the outpatient group returned to the hospital before scheduled induction because of frequent contractions. Maternal and neonatal complications were similar in the two groups. The investigators concluded that, at the dose and route of prostaglandin utilized in this study, the resultant rates of abnormal fetal heart rate pattern and frequent contractions might reduce the clinical utility of outpatient vaginal prostaglandin CR.
Another study also reported a greater rate of uterine tachysystole with vaginal PGE2 compared with a Foley catheter for CR (9% vs 0%).19 In a Cochrane systematic review of vaginal prostaglandin for CR, compared with placebo, vaginal prostaglandins were associated with a significantly greater rate of uterine hyperstimulation with fetal heart rate changes (4.8% vs 1.0%).20 Other studies also reported the feasibility of outpatient CR with vaginal prostaglandin.21,22
Both oral and vaginal misoprostol have been utilized for outpatient CR. In one study, 87 women with singleton pregnancy at 40 to 42 weeks’ gestation with a Bishop score <6 were randomized to outpatient CR with oral misoprostol (100 µg) or placebo.23 Following administration of the oral misoprostol, the women had 2 hours of CTG monitoring. The treatment was repeated daily for up to 3 days if there was no change in the cervix. If labor occurred, the patient was admitted to the labor unit for oxytocin IOL. The times from first dose of misoprostol or placebo to delivery were 46 and 84 hours (P<.001), respectively.
In another study, 49 women ≥40 weeks’ gestation with a Bishop score <5 were randomly assigned to receive outpatient oral misoprostol 25 µg or 50 µg.24 The dose could be repeated every 3 days over 9 days if ripening or labor had not been achieved. The women had CTG before administration of oral misoprostol. After the misoprostol dose, they had 2 hours of CTG monitoring. The number of doses received by the women assigned to the 50 µg group were 83%, 13%, and 4% for 1, 2, and 3 doses, respectively. The number of doses received by the women assigned to the 25 µg group were 58%, 26%, and 16% for 1, 2, and 3 doses, respectively. The mean intervals from initiation of CR to delivery in the 25 µg and the 50 µg groups were 3.9 and 2.5 days, respectively. The investigators reported no maternal or newborn adverse events, although the study was not powered to detect infrequent events.
Many studies have reported on the feasibility of outpatient CR with vaginal misoprostol.25-30 In one study, 77 women at 40 weeks’ gestation and a Bishop score ≤8 were randomized to a single dose of vaginal misoprostol 25 µg or gentle cervical examination (control).25 The women had 1 hour of CTG monitoring after the intervention. If they had regular contractions they were admitted to the birthing unit. If they had no regular contractions they were discharged home. For nulliparous women, the time from intervention to delivery in the misoprostol group was 4.9 days, and 8.1 days in the control group. For parous women, the times from intervention to delivery in the two groups were 3.8 and 6.9 days, respectively.
Continue to: Inclusion and exclusion criteria for outpatient CR...
Inclusion and exclusion criteria for outpatient CR
Outpatient CR should be limited to low-risk women with a singleton gestation, who have reliable access to transportation from home to the labor unit and have a clear understanding of the instructions for outpatient CR. Patient characteristics that may be utilized to offer office-based CR include:
- singleton pregnancy at 39 weeks’ and 0 days’ gestation through 40 weeks’ and 6 days’ gestation
- cephalic presentation
- Bishop score ≤6.
Women who should be excluded from outpatient CR include those with:
- contraindications to vaginal delivery
- fetal growth restriction
- abnormal umbilical artery Doppler results
- oligo- or polyhydramnios
- multiple gestation
- major fetal anomaly
- recent nonreactive fetal heart rate tracing
- maternal report of decreased fetal movement
- abnormal biophysical profile
- prior cesarean delivery
- recent vaginal bleeding
- gestational diabetes requiring medication treatment
- significant hypertension.
Practices should establish their own inclusion and exclusion criteria for ambulatory CR.
Safety of office-based ambulatory CR among low-risk women
Safety is a complex concept with experts often disagreeing on what level of safety is required to accept a new medical procedure. Establishing the safety of office-based ambulatory CR among low-risk women would require a very large cohort or randomized studies with at least a thousand participants. Only a few large studies focused on the safety of CR have been reported. Sciscione and colleagues reported a large observational study of inpatient transcervical Foley catheter for CR involving 1,905 women.31 They reported no adverse outcomes among term, singleton, uncomplicated pregnancies. They calculated that the 95% confidence interval (CI) for an adverse event was between 0.0% and 0.2%. In a meta-analysis of 26 studies including 5,563 women, the risk of chorioamnionitis during IOL was equivalent with pre-IOL Foley catheter CR (7.2%) or prostaglandin CR (7.2%) (relative risk, 0.96; 95% CI, 0.66–1.38).32
Two systematic reviews have reported that, compared with balloon CR, misoprostol CR is associated with an increased risk of uterine tachysystole.33-34 In a large retrospective study, compared with inpatient CR, outpatient CR with dinoprostone vaginal insert was not associated with an increased risk of newborn admission to the neonatal intensive care unit or a low Apgar score at 5 minutes after birth.35
Will you consider office-based CR in your obstetric practice?
As reviewed in this editorial, evolving data suggest that it is feasible to initiate CR in the office ambulatory setting prior to admission to the labor unit for additional CR or IOL. Many women prefer to complete CR at home after initiation in the office, rather than have CR in a labor unit or hospital setting.36 The transcervical balloon catheter has the most published data supporting the feasibility of ambulatory CR. Compared with misoprostol, the transcervical balloon catheter is associated with a low rate of uterine tachysystole. It may be a preferred method for outpatient CR. If placement of a transcervical balloon catheter is challenging, for example when the patient has a tightly closed cervix, oral misoprostol ambulatory CR may be an option if CTG monitoring is available in the office.
During the COVID pandemic, many in-person office visits have transitioned to virtual visits with the patient in their home. Historically, most cases of CR have been performed on labor and delivery units. It may be time for your practice to consider office-based ambulatory CR for low-risk women planning an IOL. Office-based ambulatory CR is a win for labor nurses who generally prefer to manage laboring patients rather than patients undergoing prolonged in-hospital CR. Outpatient CR is also a win for low-risk patients who prefer to be at home rather than in a labor unit. ●
For women with a Bishop score ≤6, CR is an important first step in planned induction of labor (IOL). CR is believed to reduce the length of labor induction and increase the probability of a vaginal delivery. Historically, CR has been undertaken on a labor unit. However, with an increased rate of labor induction, the resources of the modern labor unit are incredibly stressed. Compounding the problem is the nursing shortage caused by the COVID-19 pandemic, which has resulted in staff being unavailable as they recover from a respiratory infection or are quarantined after an exposure. The COVID-19 pandemic also has motivated many patients to avoid the hospital as much as possible.
Office-based ambulatory CR is an alternative to inpatient CR and has the potential to reduce the use of labor unit resources. When CR is initiated in the office, the patient either is sent home overnight to return to the labor unit for IOL in the morning or is sent home in the morning to return for IOL in the evening or at night. A secondary benefit of office- and home-based CR is that it may increase patient satisfaction with the process of CR. This editorial summarizes the literature supporting office-based ambulatory CR.
Mechanical methods of CR
Contemporary mechanical methods of CR include the transcervical insertion of a Foley catheter, Cook double-balloon CR catheter, Dilapan-S, or laminaria. There are many publications reporting the feasibility of office-based ambulatory CR with transcervical balloon catheters and very few publications reporting on the use of Dilapan-S or laminaria for ambulatory CR.
Foley catheter
Many studies have investigated the effectiveness of transcervical Foley catheter for ambulatory CR. Policiano and colleagues compared the effectiveness of ambulatory versus inpatient Foley catheter CR.1 A total of 130 women with a Bishop score <6 at ≥41 weeks’ gestation were randomly assigned to outpatient or inpatient CR with a transcervical Foley catheter (Covidian Dover Silicon coated latex Foley catheter 16 Fr/5.3 mm diameter). The Foley catheter bulb was distended with 40 mL of a sterile saline solution. The end of the Foley was taped to the patient’s inner thigh. Manual traction was gently applied to the catheter every 6 hours. If the catheter was extruded, the Bishop score was assessed. For a Bishop score <6, the patient was given additional inpatient misoprostol (25 µg vaginally every 4 hours for up to 5 doses). For a Bishop score ≥6, intravenous oxytocin IOL was initiated. At 24 hours if the Foley catheter was still in situ, it was removed. Women were excluded from the study for the following factors: noncephalic presentation, spontaneous labor, hydramnios, nonreassuring cardiotocography (CTG), multiple pregnancy, ruptured membranes, active vaginal bleeding, Streptococcus group B infection, and HIV infection. Prostaglandin CR was not used if the woman had a previous cesarean delivery. No prophylactic antibiotics were administered. After placement of the Foley catheter, reassuring CTG was documented prior to sending the patient home.
Outpatient, compared with inpatient, CR resulted in a mean reduction of 10 hours in the time from admission to delivery. The time from insertion of the Foley catheter to delivery in the outpatient group was 38.2 hours, and 44.9 hours for the inpatient group (P<.01). The cesarean delivery rates were similar in both groups—28% and 38%, respectively. Three cases of chorioamnionitis occurred in each group. These study results support the feasibility of office-based ambulatory CR with a transcervical Foley.
Ausbeck and colleagues randomly assigned 126 nulliparous women with a Bishop score <5, at a gestational age ranging from 39 weeks and 0 days through 41 weeks and 6 days, to outpatient overnight CR or inpatient CR with a transcervical Foley catheter.2 Breech presentation and multiple gestation pregnancies were excluded from the study. The investigators utilized a 16 French Foley catheter and filled the balloon with 30 mL of sterile water. The Foley was taped to the woman’s inner thigh on slight tension. After placement of the Foley catheter at least 20 minutes of CTG monitoring was performed. The women in the outpatient group were given the contact number for the labor unit and advised that they could take acetaminophen for pain. They were advised that they could stay at home if the Foley catheter was expelled. They were admitted to the labor unit at the time scheduled for their IOL.
The mean time from admission to delivery was reduced by 4.3 hours in the outpatient compared with the inpatient CR group (17.4 vs 21.7 hours; P<.01). In the outpatient CR group, 22% of the women were admitted to labor before the time of the scheduled IOL. The cesarean delivery rates were similar in the outpatient and inpatient CR groups (24% vs 33%, P = .32). In the outpatient and inpatient groups, chorioamnionitis was diagnosed in 22% and 13% (P = .16) of the women. The authors concluded that outpatient CR with a transcervical Foley catheter reduced the time from admission to delivery.
Other research groups also have confirmed the feasibility of outpatient CR with a transcervical Foley catheter.3-5
Placement of the Foley catheter can be performed digitally without direct visualization of the cervix or by direct visualization using a vaginal speculum. After placement of the speculum, the cervix is cleansed with a povidone-iodine solution and a sterile ring forceps is used to grasp the catheter and guide it through the cervical os. In one small study, self-reported pain was similar for both digital and direct visualization methods for placement of the balloon catheter.6 When using Foley catheter CR, filling the standard Foley catheter balloon with 60 mL of fluid, rather than 30 to 40 mL of fluid, is rarely associated with balloon rupture and may result in more effective CR.6,7
Continue to: Double-balloon catheter...
Double-balloon catheter
The Cook double-balloon catheter for CR is meant to create pressure on both sides of the cervix, facilitating CR. Studies have reported that the Cook double-balloon catheter can be used for outpatient CR. In one study, 48 women with a low-risk pregnancy, at 37 to 42 weeks’ gestation and a Bishop score <7 were randomly assigned to outpatient or inpatient double-balloon CR.8 Both balloons were filled with 70 to 80 mL of sterile water. CTG monitoring was performed for 20 minutes before and after balloon placement. The women in the outpatient CR group were instructed to return to the labor unit the next day at 8 AM for IOL or earlier if they had regular uterine contractions, rupture of membranes, or vaginal bleeding. Seven percent of the women in the outpatient group returned to the labor unit before 8 AM. After removal of the balloon catheter, women in the outpatient and inpatient groups needed additional misoprostol CR in 12% and 13% of cases, respectively. Outcomes were similar in the two groups, but the study was not powered to identify small differences between the groups.
In another study of outpatient CR with the Cook double-balloon catheter, 695 women with a Bishop score <7, at ≥37 weeks’ gestation, were randomly assigned to outpatient CR with a double-balloon catheter or inpatient CR with dinoprostone (PGE2) (2 mg dinoprostone vaginal gel [Prostin] or dinoprostone 10 mg controlled-release tape (Cervidil).9 Women assigned to dinoprostone CR had CTG monitoring prior to commencing PGE2 CR and at least 30 min of CTG monitoring after insertion of the vaginal PGE2. Women assigned to balloon CR were not admitted to the hospital. CTG was performed prior to insertion of the balloon. After insertion, the two balloons on the catheter were each filled with 80 mL of saline. After catheter insertion CTG monitoring was not routinely performed. The women in the double-balloon catheter group returned to the labor unit 12 hours after insertion to initiate IOL. The primary outcome was composite neonatal morbidity and mortality, including admission to a neonatal intensive care unit (NICU), intubation, cardiac compressions, acidemia, hypoxic ischemic encephalopathy, seizure, infection, pulmonary hypertension, stillbirth, or death.
There was no significant difference in the rate of the primary outcome in the catheter versus the PGE2 group (18.6% and 25.8%; P = .07). Admission to the NICU occurred at rates of 12.6% and 15.5% in the catheter and PGE2 groups. Umbilical cord arterial pH <7.00 at birth occurred at a rate of 3.5% in the catheter group and 9.2% in the PGE2 group. The cesarean delivery rates in the catheter and PGE groups were 32.6% and 25.8%, respectively (P = .24). The investigators concluded that outpatient CR using a double-balloon catheter is safe and feasible for nulliparous women.
Two systematic reviews and meta-analyses reported that outcomes were similar when using the Foley or double-balloon catheter for CR.10,11 The Cook double-balloon CR kit includes a stylet, which can facilitate passing the catheter through the cervix.
Continue to: Dilapan-S and laminaria...
Dilapan-S and laminaria
There are many published studies using Dilapan-S and laminaria for cervical preparation prior to uterine evacuation.12 There are few published studies using Dilapan-S or laminaria for CR prior to IOL. In a pilot study, 21 patients were randomly assigned to outpatient versus inpatient Dilapan-S for CR the night prior to scheduled oxytocin IOL.13 The length of time from initiation of oxytocin to delivery in the outpatient and inpatient groups was similar (11 vs 14 hours, respectively). The outpatient compared with the inpatient group had a shorter length of hospitalization until delivery (51 vs 70 hours).
In other studies of Dilapan-S for CR, the patients remained in the hospital once the dilators were inserted. In one small trial, 41 women were randomized to CR with Dilapan-S or laminaria. As many dilators as could be comfortably tolerated by the patient were inserted.14 The mean numbers of Dilapan-S and laminaria dilators inserted were 4.3 and 9.7, respectively. The morning after the insertion of the dilators, oxytocin IOL was initiated. The times from initiation of oxytocin to delivery for the women in the Dilapan-S and laminaria groups were 11.6 and 15.5 hours, respectively.
An observational study reported on outcomes with Dilapan-S for CR on inpatients.15 In the study 444 women scheduled for IOL at 37 to 40 weeks’ gestation, with a mean baseline Bishop score of 2.9, had Dilapan-S placed for approximately 15 hours prior to oxytocin IOL. The mean number of Dilapan-S dilators that were inserted was 3.8. The study protocol prohibited placing more than 5 cervical dilator devices. The mean Bishop score after removal of the dilators was 6.5. The most common adverse effects of Dilapan-S CR were bleeding (2.7%) and pain (0.2%). The cesarean delivery rate in the cohort was 30.1%. An Apgar score <7 at 5 minutes was recorded for 3 newborns. An umbilical artery pH of <7.10 was observed in 8 newborns.
In a randomized trial performed on inpatients, 419 women undergoing CR were assigned to a Foley balloon or Dilapan-S.16 The vaginal delivery rates were similar in the groups—76% for Foley and 81% for Dilapan-S. Maternal and neonatal adverse effects were similar between the two groups. Compared with Foley catheter, women assigned to Dilapan-S reported greater satisfaction with their CR experience, more sleep, and more ability to perform daily activities.
Misoprostol and dinoprostone
Both misoprostol and dinoprostone are effective for outpatient CR. However, a Cochrane systematic review and meta-analysis concluded that balloon CR, compared with prostaglandin CR, is probably associated with a lower risk of uterine hyperstimulation with concerning fetal heart rate changes.17 Because misoprostol and dinoprostone occasionally can cause uterine hyperstimulation with fetal heart changes, many experts recommend CTG monitoring both before and after administration of misoprostol or dinoprostone for CR.
In a trial of outpatient versus inpatient vaginal PGE2 CR, 425 women at 37 to 42 weeks’ gestation were assigned randomly to outpatient or inpatient CR.18 All women had CTG monitoring for 20 minutes before and after vaginal placement of the PGE2 gel. The PGE2 dose was 2 mg for nulliparous and 1 mg for parous women. The cesarean delivery rates were similar in the outpatient and inpatient groups—22.3% and 22.9%, respectively. Among the women randomized to outpatient CR, 27 women (13%) could not be discharged home after administration of the vaginal PGE2 because of frequent uterine contractions or an abnormal fetal heart rate pattern. In addition, 64 women (30%) in the outpatient group returned to the hospital before scheduled induction because of frequent contractions. Maternal and neonatal complications were similar in the two groups. The investigators concluded that, at the dose and route of prostaglandin utilized in this study, the resultant rates of abnormal fetal heart rate pattern and frequent contractions might reduce the clinical utility of outpatient vaginal prostaglandin CR.
Another study also reported a greater rate of uterine tachysystole with vaginal PGE2 compared with a Foley catheter for CR (9% vs 0%).19 In a Cochrane systematic review of vaginal prostaglandin for CR, compared with placebo, vaginal prostaglandins were associated with a significantly greater rate of uterine hyperstimulation with fetal heart rate changes (4.8% vs 1.0%).20 Other studies also reported the feasibility of outpatient CR with vaginal prostaglandin.21,22
Both oral and vaginal misoprostol have been utilized for outpatient CR. In one study, 87 women with singleton pregnancy at 40 to 42 weeks’ gestation with a Bishop score <6 were randomized to outpatient CR with oral misoprostol (100 µg) or placebo.23 Following administration of the oral misoprostol, the women had 2 hours of CTG monitoring. The treatment was repeated daily for up to 3 days if there was no change in the cervix. If labor occurred, the patient was admitted to the labor unit for oxytocin IOL. The times from first dose of misoprostol or placebo to delivery were 46 and 84 hours (P<.001), respectively.
In another study, 49 women ≥40 weeks’ gestation with a Bishop score <5 were randomly assigned to receive outpatient oral misoprostol 25 µg or 50 µg.24 The dose could be repeated every 3 days over 9 days if ripening or labor had not been achieved. The women had CTG before administration of oral misoprostol. After the misoprostol dose, they had 2 hours of CTG monitoring. The number of doses received by the women assigned to the 50 µg group were 83%, 13%, and 4% for 1, 2, and 3 doses, respectively. The number of doses received by the women assigned to the 25 µg group were 58%, 26%, and 16% for 1, 2, and 3 doses, respectively. The mean intervals from initiation of CR to delivery in the 25 µg and the 50 µg groups were 3.9 and 2.5 days, respectively. The investigators reported no maternal or newborn adverse events, although the study was not powered to detect infrequent events.
Many studies have reported on the feasibility of outpatient CR with vaginal misoprostol.25-30 In one study, 77 women at 40 weeks’ gestation and a Bishop score ≤8 were randomized to a single dose of vaginal misoprostol 25 µg or gentle cervical examination (control).25 The women had 1 hour of CTG monitoring after the intervention. If they had regular contractions they were admitted to the birthing unit. If they had no regular contractions they were discharged home. For nulliparous women, the time from intervention to delivery in the misoprostol group was 4.9 days, and 8.1 days in the control group. For parous women, the times from intervention to delivery in the two groups were 3.8 and 6.9 days, respectively.
Continue to: Inclusion and exclusion criteria for outpatient CR...
Inclusion and exclusion criteria for outpatient CR
Outpatient CR should be limited to low-risk women with a singleton gestation, who have reliable access to transportation from home to the labor unit and have a clear understanding of the instructions for outpatient CR. Patient characteristics that may be utilized to offer office-based CR include:
- singleton pregnancy at 39 weeks’ and 0 days’ gestation through 40 weeks’ and 6 days’ gestation
- cephalic presentation
- Bishop score ≤6.
Women who should be excluded from outpatient CR include those with:
- contraindications to vaginal delivery
- fetal growth restriction
- abnormal umbilical artery Doppler results
- oligo- or polyhydramnios
- multiple gestation
- major fetal anomaly
- recent nonreactive fetal heart rate tracing
- maternal report of decreased fetal movement
- abnormal biophysical profile
- prior cesarean delivery
- recent vaginal bleeding
- gestational diabetes requiring medication treatment
- significant hypertension.
Practices should establish their own inclusion and exclusion criteria for ambulatory CR.
Safety of office-based ambulatory CR among low-risk women
Safety is a complex concept with experts often disagreeing on what level of safety is required to accept a new medical procedure. Establishing the safety of office-based ambulatory CR among low-risk women would require a very large cohort or randomized studies with at least a thousand participants. Only a few large studies focused on the safety of CR have been reported. Sciscione and colleagues reported a large observational study of inpatient transcervical Foley catheter for CR involving 1,905 women.31 They reported no adverse outcomes among term, singleton, uncomplicated pregnancies. They calculated that the 95% confidence interval (CI) for an adverse event was between 0.0% and 0.2%. In a meta-analysis of 26 studies including 5,563 women, the risk of chorioamnionitis during IOL was equivalent with pre-IOL Foley catheter CR (7.2%) or prostaglandin CR (7.2%) (relative risk, 0.96; 95% CI, 0.66–1.38).32
Two systematic reviews have reported that, compared with balloon CR, misoprostol CR is associated with an increased risk of uterine tachysystole.33-34 In a large retrospective study, compared with inpatient CR, outpatient CR with dinoprostone vaginal insert was not associated with an increased risk of newborn admission to the neonatal intensive care unit or a low Apgar score at 5 minutes after birth.35
Will you consider office-based CR in your obstetric practice?
As reviewed in this editorial, evolving data suggest that it is feasible to initiate CR in the office ambulatory setting prior to admission to the labor unit for additional CR or IOL. Many women prefer to complete CR at home after initiation in the office, rather than have CR in a labor unit or hospital setting.36 The transcervical balloon catheter has the most published data supporting the feasibility of ambulatory CR. Compared with misoprostol, the transcervical balloon catheter is associated with a low rate of uterine tachysystole. It may be a preferred method for outpatient CR. If placement of a transcervical balloon catheter is challenging, for example when the patient has a tightly closed cervix, oral misoprostol ambulatory CR may be an option if CTG monitoring is available in the office.
During the COVID pandemic, many in-person office visits have transitioned to virtual visits with the patient in their home. Historically, most cases of CR have been performed on labor and delivery units. It may be time for your practice to consider office-based ambulatory CR for low-risk women planning an IOL. Office-based ambulatory CR is a win for labor nurses who generally prefer to manage laboring patients rather than patients undergoing prolonged in-hospital CR. Outpatient CR is also a win for low-risk patients who prefer to be at home rather than in a labor unit. ●
- Policiano C, Pimenta M, Martins D, et al. Outpatient versus inpatient cervix priming with Foley catheter: a randomized trial. Eur J Obstet Gynecol Repro Biol. 2017;210:1-6.
- Ausbeck EB, Jauk VC, Xue Y, et al. Outpatient Foley catheter for induction of labor in nulliparous women. Obstet Gynecol. 2020;136:597-606.
- Wilkinson C, Adelson P, Turnbull D. A comparison of inpatient with outpatient balloon catheter cervical ripening: a pilot randomized controlled trial. BMC Pregnancy Childbirth. 2015;15:126.
- Sciscione AC, Muench M, Pollock M, et al. Transcervical Foley catheter for preinduction cervical ripening in an outpatient versus inpatient setting. Obstet Gynecol. 2001;98:751-756.
- Henry A, Madan A, Reid R, et al. Outpatient Foley catheter versus inpatient prostaglandin E2 gel for induction of labour: a randomised trial. BMC Pregnancy Childbirth. 2013;13:25.
- Kuhlmann MJ, Spencer N, Garcia-Jasso C, et al. Foley bulb insertion by blind placement compared with direct visualization. Obstet Gynecol. 2021;137:139-145.
- Delaney S, Shaffer BL, Chen YW, et al. Labor induction with a Foley balloon inflated to 30 mL compared with 60 mL. Obstet Gynecol. 2015;115:1239-1245.
- Wilkinson C, Adelson P, Turnbull D. A comparison of inpatient with outpatient balloon catheter cervical ripening: a pilot randomized controlled trial. BMC Pregnancy Childbirth. 2015;15:126.
- Beckmann M, Gibbons K, Flenady V, et al. Induction of labor using prostaglandin E2 as an inpatient versus balloon catheter as an outpatient: a multicenter randomised controlled trial. BJOG. 2020;127:571-579.
- Liu X, Wang Y, Zhange F, et al. Double- versus single-balloon catheters for labour induction and cervical ripening: a meta-analysis. BMC Pregnancy Childbirth. 2019;19:358.
- Yang F, Huan S, Long Y, et al. Double-balloon versus single-balloon catheter for cervical ripening and labor induction: a systematic review and meta-analysis. J Obstet Gynaecol Res. 2018;44: 27-34.
- Goldberg AB, Fortin JA, Drey EA, et al. Cervical preparation before dilation and evacuation using adjunctive misoprostol and mifepristone compared with overnight osmotic dilators alone: a randomized controlled trial. Obstet Gynecol. 2015;126:599-609.
- Upadhyaya NB, Childs KD, Neiger R, et al. Ambulatory cervical ripening in term pregnancy. J Reprod Med. 1999;44:363-366.
- Blumenthal PD, Rmanauskas R. Randomized trial of Dilapan and Laminaria as cervical ripening agents before induction of labor. Obstet Gynecol. 1990;75:365-368.
- Gupta J, Chodankar R, Baev O, et al. Synthetic osmotic dilators in the induction of labour—an international multicenter observational study. Eur J Obstet Gynecol Repro Biol. 2018;229:70-75.
- Saad AF, Villarreal J, Eid J, et al. A randomized controlled trial of Dilapan-S vs Foley balloon for preinduction cervical ripening (DILAFOL trial). Am J Obstet Gynecol. 2019;220:275.e1-e9.
- de Vaan MD, Eikleder MLT, Jozwiak M, et al. Mechanical methods for induction of labour. Cochrane Database Syst Rev. 2019;CD001233.
- Wilkinson C, Bryce R, Adelson P, et al. A randomized controlled trial of outpatient compared with inpatient cervical ripening with prostaglandin E2 (OPRA study). BJOG. 2015;122:94-104.
- Blair R, Harvey MA, Pudwell J, et al. Retrospective comparison of PGE2 vaginal insert and Foley catheter for outpatient cervical ripening. J Obstet Gynaecol Can. 2020;42:1103-1110.
- Thomas J, Fairclough A, Kavanagh J, et al. Vaginal prostaglandin (PGE2 or PGF2alpha) for induction of labour at term. Cochrane Database Syst Rev. 2014;CD003101.
- O’Brien JM, Mercer BM, Cleary NT, et al. Efficacy of outpatient induction with low-dose intravaginal prostaglandin E2: a randomized, doubleblind, placebo controlled trial. Am J Obstet Gynecol. 1995;173:1855-1859.
- Biem SR, Turnell RW, Olatunbosun O, et al. A randomized controlled trial of outpatient versus inpatient labour induction with vaginal controlled-release prostaglandin-E2: effectiveness and satisfaction. J Obstet Gynaecol Can. 2003;25:23-31.
- Gaffaney CA, Saul LL, Rumney PJ, et al. Outpatient oral misoprostol for prolonged pregnancies: a pilot investigation. Am J Perinatol. 2009;26: 673-677.
- Kipikasa JH, Adair CD, Williamson J, et al. Use of misoprostol on an outpatient basis for postdate pregnancy. Int J Gynaecol Obstet. 2005;88:108-111.
- Oboro VO, Tabowei TO. Outpatient misoprostol cervical ripening without subsequent induction of labor to prevent post-term pregnancy. Acta Obstet Gynecol Scand. 2005;84:628-631.
- Stitely ML, Browning J, Fowler M, et al. Outpatient cervical ripening with intravaginal misoprostol. Obstet Gynecol. 2000;96:684-688.
- McKenna DS, Ester JB, Proffitt M, et al. Misoprostol outpatient cervical ripening without subsequent induction of labor: a randomized trial. Obstet Gynecol. 2004;104:579-584.
- PonMalar J, Benjamin SJ, Abraham A, et al. Randomized double-blind placebo controlled study of preinduction cervical priming with 25 µg of misoprostol in the outpatient setting to prevent formal induction of labor. Arch Gynecol Obstet. 2017;295:33-38.
- Chang DW, Velazquez MD, Colyer M, et al. Vaginal misoprostol for cervical ripening at term: comparison of outpatient vs inpatient administration. Obstet Gynecol Surv. 2006;61:167-168.
- Meyer M, Pflum J, Howard D. Outpatient misoprostol compared with dinoprostone gel for preinduction cervical ripening: a randomized controlled trial. Obstet Gynecol. 2005;105:466-472.
- Sciscione AC, Bedder CL, Hoffman MK, et al. The timing of adverse events with Foley catheter preinduction cervical ripening; implications for outpatient use. Am J Perinatol. 2014;31:781-786.
- McMaster K, Sanchez-Ramos L, Kaunitz AM. Evaluation of a transcervical Foley catheter as a source of infection. Obstet Gynecol. 2015;126:539-551.
- Fox NS, Saltzman DH, Roman AS, et al. Intravaginal misoprostol versus Foley catheter for labour induction: a meta-analysis. BJOG. 2011;118: 647-654.
- Hofmeyr GJ, Gulmezoglu AM, Pileggi C. Vaginal misoprostol for cervical ripening and induction of labour. Cochrane Database Syst Rev. 2010:CD000941.
- Salvador SC, Simpson ML, Cundiff GW. Dinoprostone vaginal insert for labour induction: a comparison of outpatient and inpatient settings. J Obstet Gynaecol Can. 2009;31:1028-1034.
- Sutton C, Harding J, Griffin C. Patient attitudes towards outpatient cervical ripening prior to induction of labour at an Australian tertiary hospital. J Obstet Gynaecol. 2016;36:921-928.
- Policiano C, Pimenta M, Martins D, et al. Outpatient versus inpatient cervix priming with Foley catheter: a randomized trial. Eur J Obstet Gynecol Repro Biol. 2017;210:1-6.
- Ausbeck EB, Jauk VC, Xue Y, et al. Outpatient Foley catheter for induction of labor in nulliparous women. Obstet Gynecol. 2020;136:597-606.
- Wilkinson C, Adelson P, Turnbull D. A comparison of inpatient with outpatient balloon catheter cervical ripening: a pilot randomized controlled trial. BMC Pregnancy Childbirth. 2015;15:126.
- Sciscione AC, Muench M, Pollock M, et al. Transcervical Foley catheter for preinduction cervical ripening in an outpatient versus inpatient setting. Obstet Gynecol. 2001;98:751-756.
- Henry A, Madan A, Reid R, et al. Outpatient Foley catheter versus inpatient prostaglandin E2 gel for induction of labour: a randomised trial. BMC Pregnancy Childbirth. 2013;13:25.
- Kuhlmann MJ, Spencer N, Garcia-Jasso C, et al. Foley bulb insertion by blind placement compared with direct visualization. Obstet Gynecol. 2021;137:139-145.
- Delaney S, Shaffer BL, Chen YW, et al. Labor induction with a Foley balloon inflated to 30 mL compared with 60 mL. Obstet Gynecol. 2015;115:1239-1245.
- Wilkinson C, Adelson P, Turnbull D. A comparison of inpatient with outpatient balloon catheter cervical ripening: a pilot randomized controlled trial. BMC Pregnancy Childbirth. 2015;15:126.
- Beckmann M, Gibbons K, Flenady V, et al. Induction of labor using prostaglandin E2 as an inpatient versus balloon catheter as an outpatient: a multicenter randomised controlled trial. BJOG. 2020;127:571-579.
- Liu X, Wang Y, Zhange F, et al. Double- versus single-balloon catheters for labour induction and cervical ripening: a meta-analysis. BMC Pregnancy Childbirth. 2019;19:358.
- Yang F, Huan S, Long Y, et al. Double-balloon versus single-balloon catheter for cervical ripening and labor induction: a systematic review and meta-analysis. J Obstet Gynaecol Res. 2018;44: 27-34.
- Goldberg AB, Fortin JA, Drey EA, et al. Cervical preparation before dilation and evacuation using adjunctive misoprostol and mifepristone compared with overnight osmotic dilators alone: a randomized controlled trial. Obstet Gynecol. 2015;126:599-609.
- Upadhyaya NB, Childs KD, Neiger R, et al. Ambulatory cervical ripening in term pregnancy. J Reprod Med. 1999;44:363-366.
- Blumenthal PD, Rmanauskas R. Randomized trial of Dilapan and Laminaria as cervical ripening agents before induction of labor. Obstet Gynecol. 1990;75:365-368.
- Gupta J, Chodankar R, Baev O, et al. Synthetic osmotic dilators in the induction of labour—an international multicenter observational study. Eur J Obstet Gynecol Repro Biol. 2018;229:70-75.
- Saad AF, Villarreal J, Eid J, et al. A randomized controlled trial of Dilapan-S vs Foley balloon for preinduction cervical ripening (DILAFOL trial). Am J Obstet Gynecol. 2019;220:275.e1-e9.
- de Vaan MD, Eikleder MLT, Jozwiak M, et al. Mechanical methods for induction of labour. Cochrane Database Syst Rev. 2019;CD001233.
- Wilkinson C, Bryce R, Adelson P, et al. A randomized controlled trial of outpatient compared with inpatient cervical ripening with prostaglandin E2 (OPRA study). BJOG. 2015;122:94-104.
- Blair R, Harvey MA, Pudwell J, et al. Retrospective comparison of PGE2 vaginal insert and Foley catheter for outpatient cervical ripening. J Obstet Gynaecol Can. 2020;42:1103-1110.
- Thomas J, Fairclough A, Kavanagh J, et al. Vaginal prostaglandin (PGE2 or PGF2alpha) for induction of labour at term. Cochrane Database Syst Rev. 2014;CD003101.
- O’Brien JM, Mercer BM, Cleary NT, et al. Efficacy of outpatient induction with low-dose intravaginal prostaglandin E2: a randomized, doubleblind, placebo controlled trial. Am J Obstet Gynecol. 1995;173:1855-1859.
- Biem SR, Turnell RW, Olatunbosun O, et al. A randomized controlled trial of outpatient versus inpatient labour induction with vaginal controlled-release prostaglandin-E2: effectiveness and satisfaction. J Obstet Gynaecol Can. 2003;25:23-31.
- Gaffaney CA, Saul LL, Rumney PJ, et al. Outpatient oral misoprostol for prolonged pregnancies: a pilot investigation. Am J Perinatol. 2009;26: 673-677.
- Kipikasa JH, Adair CD, Williamson J, et al. Use of misoprostol on an outpatient basis for postdate pregnancy. Int J Gynaecol Obstet. 2005;88:108-111.
- Oboro VO, Tabowei TO. Outpatient misoprostol cervical ripening without subsequent induction of labor to prevent post-term pregnancy. Acta Obstet Gynecol Scand. 2005;84:628-631.
- Stitely ML, Browning J, Fowler M, et al. Outpatient cervical ripening with intravaginal misoprostol. Obstet Gynecol. 2000;96:684-688.
- McKenna DS, Ester JB, Proffitt M, et al. Misoprostol outpatient cervical ripening without subsequent induction of labor: a randomized trial. Obstet Gynecol. 2004;104:579-584.
- PonMalar J, Benjamin SJ, Abraham A, et al. Randomized double-blind placebo controlled study of preinduction cervical priming with 25 µg of misoprostol in the outpatient setting to prevent formal induction of labor. Arch Gynecol Obstet. 2017;295:33-38.
- Chang DW, Velazquez MD, Colyer M, et al. Vaginal misoprostol for cervical ripening at term: comparison of outpatient vs inpatient administration. Obstet Gynecol Surv. 2006;61:167-168.
- Meyer M, Pflum J, Howard D. Outpatient misoprostol compared with dinoprostone gel for preinduction cervical ripening: a randomized controlled trial. Obstet Gynecol. 2005;105:466-472.
- Sciscione AC, Bedder CL, Hoffman MK, et al. The timing of adverse events with Foley catheter preinduction cervical ripening; implications for outpatient use. Am J Perinatol. 2014;31:781-786.
- McMaster K, Sanchez-Ramos L, Kaunitz AM. Evaluation of a transcervical Foley catheter as a source of infection. Obstet Gynecol. 2015;126:539-551.
- Fox NS, Saltzman DH, Roman AS, et al. Intravaginal misoprostol versus Foley catheter for labour induction: a meta-analysis. BJOG. 2011;118: 647-654.
- Hofmeyr GJ, Gulmezoglu AM, Pileggi C. Vaginal misoprostol for cervical ripening and induction of labour. Cochrane Database Syst Rev. 2010:CD000941.
- Salvador SC, Simpson ML, Cundiff GW. Dinoprostone vaginal insert for labour induction: a comparison of outpatient and inpatient settings. J Obstet Gynaecol Can. 2009;31:1028-1034.
- Sutton C, Harding J, Griffin C. Patient attitudes towards outpatient cervical ripening prior to induction of labour at an Australian tertiary hospital. J Obstet Gynaecol. 2016;36:921-928.
COMMENT & CONTROVERSY
PHYSICIAN LEADERSHIP: RACIAL DISPARITIES AND RACISM. WHERE DO WE GO FROM HERE?
BIFTU MENGESHA, MD, MAS; KAVITA SHAH ARORA, MD, MBE, MS; AND BARBARA LEVY, MD
(COMMENTARY; AUGUST 2020)
Political diatribe paints a huge swath
I read the above referenced article with equal measure of angst and offense, amazement and incredulity, irritation and, some would say, typical white male denial. The authors have succumbed to the zeitgeist currently enveloping our country and painted us all with one huge swath of the same proverbial brush—inappropriately.
No doubt certain reforms are needed in our society and perhaps within areas of medicine. I am for anything that improves all peoples’ lives and health. The country is rightfully clamoring for equality. That means equality for all, however. But by way of one small example of systemic overreaction, many articles now are replete with comments about “Black people and Brown people and white people.” Adjectives have been turned into proper nouns, but applied only to some groups, not all. That foments continued inequity, not equality.
The authors implore us to strive for “engaged, passionate, and innovative leadership deliberately aimed toward antiracism and equity.” In my view, the best way I can do that is not by words but actions, and that is to do what I am trained to do, which is take care of patients the best I can regardless of their color or creed. I have always done that, and everyone I work with does as well. For the authors to imply I (we) don’t is at a minimum offensive and pejorative, and flat wrong.
I agree to a certain extent that our health care contributes to poor, or at least less desirable, outcomes. But so do the actions or inactions of our patients. I do not agree that it is a racial issue. It affects all people. I see it every day. I am probably in the minority of physicians who think we should go to a single-payor system (note I did not say free). But to state that the system creates poor outcomes only for “Blacks, Indigenous, and Latinx,” I disagree. Tennessee has TennCare (Medicaid) and almost anyone can get it, and if they are pregnant they certainly can. Access is not an issue. All people have to do is avail themselves of it. It does not matter the race!
The authors call for the implementation “of system-wide intersectional and antiracist practices” to address “racism, sexism, gender discrimination, economic and social injustice.” They are preaching to the wrong crowd. If I (we) pursued all these lofty goals, I (we) would not have time to care for the very patients they are now lamenting don’t have enough care or proper care.
I facilitate conversations on a regular basis with my black patients as well as my white patients. I recently asked a patient if she distrusted me because I am a white male. It is enlightening to hear patient comments, which are mostly along the lines of “the world has gone crazy.” That is a polite interpretation of their comments. Maybe they say what they think I want to hear, but I don’t think so.
“Repair what we have broken…” by “uplifting their voices and redistributing our power to them.” I don’t see how I (we) have broken anything. If taking care of all comers as best as one can has broken something, then I am guilty. Regarding that I need to examine how “we have eroded the trust of the very communities we care for” and that we are guilty of “medical experimentation on and exploitation of Black and Brown bodies,” what are we to examine? How have I (we) eroded the trust? Present-day physicians had nothing to do with things like the Tuskegee Institute experiment, and I hardly see how blaming us today for that abominable episode in our HISTORY is a valid point. Implying that we add to that distrust by not giving the same pain relief to certain people because of race is just preposterous. Further, asking to let others lead, for example, on the medical executive committee or in positions such as chief of service or chief of staff usually are not something one “gets” to be, but something one usually is “talked into.” There is not a racial barrier to those roles, at least at my institution, and once again the brush has inappropriately painted us all.
I understand the general gestalt of this article and agree with its basic premises. But while well meaning, this political diatribe belongs in the halls of Congress, not in the halls of our hospitals or the pages of a medical journal. I am tired of being told, directly or indirectly, that I am a racist by TV news, newspapers, social media, professional sports teams, and now by my medical journals. I would ask the authors to be careful who they throw under the bus.
Scott Peters, MD
Oak Ridge, Tennessee
Continue to: Drs. Mengesha, Arora, and Levy respond...
Drs. Mengesha, Arora, and Levy respond
We appreciate the opportunity to respond to Dr. Peters. Our article brings long overdue awareness to systemic and structural problems that result in disproportionately inequitable outcomes for people of color. We are not debating the morality of individuals or talking about racism as an inherently “bad” trait that some people have, but rather recognizing the impact of social structures on health and well-being in which we all—Black, Brown, and White—live. We all have inherent biases, recognized or unrecognized, that impact our actions, decisions, and behaviors. We are humans with upbringing, backgrounds, and learned frameworks influenced by our sociocultural context that conditions our responses to a given situation. This is also woven into our hospitals, exam rooms, and even our medical journals. It constantly influences the health, well-being, and livelihood of patients—nothing that we do in medicine is in isolation of this greater context. Our health care system is steeped in this sociocultural context and impacts all patients in intersecting ways, whether that be by race, class, gender, or other social identities. And while we did not create the system in which we operate, we now have abundant evidence that shows it continually delivers inequitable outcomes particularly for people of color.
We are very clear that physicians and health care professionals strive to provide the very best care for each and every patient. We do not discount the hard work and good intentions of our colleagues. And while some individual patient behaviors may somewhat modify outcomes, we also strongly disagree with the premise that patients are to blame for poor or less desirable outcomes they face. Instead, our position is focused on the impact that the systems we work in are creating barriers to equitable care at levels of influence above a single individual, and that it is our collective professional responsibility to acknowledge and take action to lessen those barriers.
System-wide changes would not be at the expense of patient care, and physicians cannot and in fact should not shoulder these changes alone. Our current paradigm of training does not give us the capacity to do so, and a single individual cannot make such a large system change alone. The change we are advocating for requires collaboration within multidisciplinary and interprofessional teams, long-term planning, and incremental but intentional change. This is not dissimilar to the recognition over 20 years ago by the Institute of Medicine (now the National Academy of Medicine) that “to err is human.” Our eyes were opened to the structural issues resulting in medical errors, and very slowly our profession has acknowledged the necessity to recognize, report, and analyze the root causes of those errors. We do so because it is critically important to ensure that the same error never happens again. It is part of the commitment to honor our oath to “do no harm.” Similarly, racial inequities in health outcomes should also be “never events” as there is no biological basis or individual blame for these inequities, but rather systemic and structural processes (which is de facto racism) that contribute to disproportionately worse outcomes.
Disparities in COVID-19 vaccination rates for people of color is a current example that illustrates the deep distrust in our health care system that historical events, like Tuskegee, have created. In Tennessee, for example, 7% of COVID-19 vaccines have been administered to Black people despite the fact that they make up 15% of cases, 18% of deaths, and 16% of the total population.1 There are other ongoing systemic issues, including inequities in distribution, prioritization, and access, that are contributing to the lower vaccination rates among people of color; however, as physicians and advocates for our patients, it is crucial for us to acknowledge the fear of, and resistance to, government-sponsored health programs which has resulted from events like Tuskegee.
We are advocating for building our systems to help support all of the social and societal determinants of health our patients are faced with, including racism, while they are receiving care from us. Patients are faced with undue morbidity and mortality because of our health care system’s ineffectiveness in incorporating this as a part of systemic care delivery to all. We must work together alongside other health care professionals, public health and policy agencies, and community advocates to stop this deadly cycle. There will be no improvement and no end in sight unless we work together toward this common goal.
Reference
- Ndugga N, Pham O, Hill L, et al. Latest data on COVID-19 vaccinations: race/ethnicity. Kaiser Family Foundation website. February 1, 2021. https://www.kff.org/coronavirus-covid-19/issue-brief/latest-data-covid-19-vaccinations-cases-deaths-race-ethnicity/. Accessed February 11, 2021.
PHYSICIAN LEADERSHIP: RACIAL DISPARITIES AND RACISM. WHERE DO WE GO FROM HERE?
BIFTU MENGESHA, MD, MAS; KAVITA SHAH ARORA, MD, MBE, MS; AND BARBARA LEVY, MD
(COMMENTARY; AUGUST 2020)
Political diatribe paints a huge swath
I read the above referenced article with equal measure of angst and offense, amazement and incredulity, irritation and, some would say, typical white male denial. The authors have succumbed to the zeitgeist currently enveloping our country and painted us all with one huge swath of the same proverbial brush—inappropriately.
No doubt certain reforms are needed in our society and perhaps within areas of medicine. I am for anything that improves all peoples’ lives and health. The country is rightfully clamoring for equality. That means equality for all, however. But by way of one small example of systemic overreaction, many articles now are replete with comments about “Black people and Brown people and white people.” Adjectives have been turned into proper nouns, but applied only to some groups, not all. That foments continued inequity, not equality.
The authors implore us to strive for “engaged, passionate, and innovative leadership deliberately aimed toward antiracism and equity.” In my view, the best way I can do that is not by words but actions, and that is to do what I am trained to do, which is take care of patients the best I can regardless of their color or creed. I have always done that, and everyone I work with does as well. For the authors to imply I (we) don’t is at a minimum offensive and pejorative, and flat wrong.
I agree to a certain extent that our health care contributes to poor, or at least less desirable, outcomes. But so do the actions or inactions of our patients. I do not agree that it is a racial issue. It affects all people. I see it every day. I am probably in the minority of physicians who think we should go to a single-payor system (note I did not say free). But to state that the system creates poor outcomes only for “Blacks, Indigenous, and Latinx,” I disagree. Tennessee has TennCare (Medicaid) and almost anyone can get it, and if they are pregnant they certainly can. Access is not an issue. All people have to do is avail themselves of it. It does not matter the race!
The authors call for the implementation “of system-wide intersectional and antiracist practices” to address “racism, sexism, gender discrimination, economic and social injustice.” They are preaching to the wrong crowd. If I (we) pursued all these lofty goals, I (we) would not have time to care for the very patients they are now lamenting don’t have enough care or proper care.
I facilitate conversations on a regular basis with my black patients as well as my white patients. I recently asked a patient if she distrusted me because I am a white male. It is enlightening to hear patient comments, which are mostly along the lines of “the world has gone crazy.” That is a polite interpretation of their comments. Maybe they say what they think I want to hear, but I don’t think so.
“Repair what we have broken…” by “uplifting their voices and redistributing our power to them.” I don’t see how I (we) have broken anything. If taking care of all comers as best as one can has broken something, then I am guilty. Regarding that I need to examine how “we have eroded the trust of the very communities we care for” and that we are guilty of “medical experimentation on and exploitation of Black and Brown bodies,” what are we to examine? How have I (we) eroded the trust? Present-day physicians had nothing to do with things like the Tuskegee Institute experiment, and I hardly see how blaming us today for that abominable episode in our HISTORY is a valid point. Implying that we add to that distrust by not giving the same pain relief to certain people because of race is just preposterous. Further, asking to let others lead, for example, on the medical executive committee or in positions such as chief of service or chief of staff usually are not something one “gets” to be, but something one usually is “talked into.” There is not a racial barrier to those roles, at least at my institution, and once again the brush has inappropriately painted us all.
I understand the general gestalt of this article and agree with its basic premises. But while well meaning, this political diatribe belongs in the halls of Congress, not in the halls of our hospitals or the pages of a medical journal. I am tired of being told, directly or indirectly, that I am a racist by TV news, newspapers, social media, professional sports teams, and now by my medical journals. I would ask the authors to be careful who they throw under the bus.
Scott Peters, MD
Oak Ridge, Tennessee
Continue to: Drs. Mengesha, Arora, and Levy respond...
Drs. Mengesha, Arora, and Levy respond
We appreciate the opportunity to respond to Dr. Peters. Our article brings long overdue awareness to systemic and structural problems that result in disproportionately inequitable outcomes for people of color. We are not debating the morality of individuals or talking about racism as an inherently “bad” trait that some people have, but rather recognizing the impact of social structures on health and well-being in which we all—Black, Brown, and White—live. We all have inherent biases, recognized or unrecognized, that impact our actions, decisions, and behaviors. We are humans with upbringing, backgrounds, and learned frameworks influenced by our sociocultural context that conditions our responses to a given situation. This is also woven into our hospitals, exam rooms, and even our medical journals. It constantly influences the health, well-being, and livelihood of patients—nothing that we do in medicine is in isolation of this greater context. Our health care system is steeped in this sociocultural context and impacts all patients in intersecting ways, whether that be by race, class, gender, or other social identities. And while we did not create the system in which we operate, we now have abundant evidence that shows it continually delivers inequitable outcomes particularly for people of color.
We are very clear that physicians and health care professionals strive to provide the very best care for each and every patient. We do not discount the hard work and good intentions of our colleagues. And while some individual patient behaviors may somewhat modify outcomes, we also strongly disagree with the premise that patients are to blame for poor or less desirable outcomes they face. Instead, our position is focused on the impact that the systems we work in are creating barriers to equitable care at levels of influence above a single individual, and that it is our collective professional responsibility to acknowledge and take action to lessen those barriers.
System-wide changes would not be at the expense of patient care, and physicians cannot and in fact should not shoulder these changes alone. Our current paradigm of training does not give us the capacity to do so, and a single individual cannot make such a large system change alone. The change we are advocating for requires collaboration within multidisciplinary and interprofessional teams, long-term planning, and incremental but intentional change. This is not dissimilar to the recognition over 20 years ago by the Institute of Medicine (now the National Academy of Medicine) that “to err is human.” Our eyes were opened to the structural issues resulting in medical errors, and very slowly our profession has acknowledged the necessity to recognize, report, and analyze the root causes of those errors. We do so because it is critically important to ensure that the same error never happens again. It is part of the commitment to honor our oath to “do no harm.” Similarly, racial inequities in health outcomes should also be “never events” as there is no biological basis or individual blame for these inequities, but rather systemic and structural processes (which is de facto racism) that contribute to disproportionately worse outcomes.
Disparities in COVID-19 vaccination rates for people of color is a current example that illustrates the deep distrust in our health care system that historical events, like Tuskegee, have created. In Tennessee, for example, 7% of COVID-19 vaccines have been administered to Black people despite the fact that they make up 15% of cases, 18% of deaths, and 16% of the total population.1 There are other ongoing systemic issues, including inequities in distribution, prioritization, and access, that are contributing to the lower vaccination rates among people of color; however, as physicians and advocates for our patients, it is crucial for us to acknowledge the fear of, and resistance to, government-sponsored health programs which has resulted from events like Tuskegee.
We are advocating for building our systems to help support all of the social and societal determinants of health our patients are faced with, including racism, while they are receiving care from us. Patients are faced with undue morbidity and mortality because of our health care system’s ineffectiveness in incorporating this as a part of systemic care delivery to all. We must work together alongside other health care professionals, public health and policy agencies, and community advocates to stop this deadly cycle. There will be no improvement and no end in sight unless we work together toward this common goal.
Reference
- Ndugga N, Pham O, Hill L, et al. Latest data on COVID-19 vaccinations: race/ethnicity. Kaiser Family Foundation website. February 1, 2021. https://www.kff.org/coronavirus-covid-19/issue-brief/latest-data-covid-19-vaccinations-cases-deaths-race-ethnicity/. Accessed February 11, 2021.
PHYSICIAN LEADERSHIP: RACIAL DISPARITIES AND RACISM. WHERE DO WE GO FROM HERE?
BIFTU MENGESHA, MD, MAS; KAVITA SHAH ARORA, MD, MBE, MS; AND BARBARA LEVY, MD
(COMMENTARY; AUGUST 2020)
Political diatribe paints a huge swath
I read the above referenced article with equal measure of angst and offense, amazement and incredulity, irritation and, some would say, typical white male denial. The authors have succumbed to the zeitgeist currently enveloping our country and painted us all with one huge swath of the same proverbial brush—inappropriately.
No doubt certain reforms are needed in our society and perhaps within areas of medicine. I am for anything that improves all peoples’ lives and health. The country is rightfully clamoring for equality. That means equality for all, however. But by way of one small example of systemic overreaction, many articles now are replete with comments about “Black people and Brown people and white people.” Adjectives have been turned into proper nouns, but applied only to some groups, not all. That foments continued inequity, not equality.
The authors implore us to strive for “engaged, passionate, and innovative leadership deliberately aimed toward antiracism and equity.” In my view, the best way I can do that is not by words but actions, and that is to do what I am trained to do, which is take care of patients the best I can regardless of their color or creed. I have always done that, and everyone I work with does as well. For the authors to imply I (we) don’t is at a minimum offensive and pejorative, and flat wrong.
I agree to a certain extent that our health care contributes to poor, or at least less desirable, outcomes. But so do the actions or inactions of our patients. I do not agree that it is a racial issue. It affects all people. I see it every day. I am probably in the minority of physicians who think we should go to a single-payor system (note I did not say free). But to state that the system creates poor outcomes only for “Blacks, Indigenous, and Latinx,” I disagree. Tennessee has TennCare (Medicaid) and almost anyone can get it, and if they are pregnant they certainly can. Access is not an issue. All people have to do is avail themselves of it. It does not matter the race!
The authors call for the implementation “of system-wide intersectional and antiracist practices” to address “racism, sexism, gender discrimination, economic and social injustice.” They are preaching to the wrong crowd. If I (we) pursued all these lofty goals, I (we) would not have time to care for the very patients they are now lamenting don’t have enough care or proper care.
I facilitate conversations on a regular basis with my black patients as well as my white patients. I recently asked a patient if she distrusted me because I am a white male. It is enlightening to hear patient comments, which are mostly along the lines of “the world has gone crazy.” That is a polite interpretation of their comments. Maybe they say what they think I want to hear, but I don’t think so.
“Repair what we have broken…” by “uplifting their voices and redistributing our power to them.” I don’t see how I (we) have broken anything. If taking care of all comers as best as one can has broken something, then I am guilty. Regarding that I need to examine how “we have eroded the trust of the very communities we care for” and that we are guilty of “medical experimentation on and exploitation of Black and Brown bodies,” what are we to examine? How have I (we) eroded the trust? Present-day physicians had nothing to do with things like the Tuskegee Institute experiment, and I hardly see how blaming us today for that abominable episode in our HISTORY is a valid point. Implying that we add to that distrust by not giving the same pain relief to certain people because of race is just preposterous. Further, asking to let others lead, for example, on the medical executive committee or in positions such as chief of service or chief of staff usually are not something one “gets” to be, but something one usually is “talked into.” There is not a racial barrier to those roles, at least at my institution, and once again the brush has inappropriately painted us all.
I understand the general gestalt of this article and agree with its basic premises. But while well meaning, this political diatribe belongs in the halls of Congress, not in the halls of our hospitals or the pages of a medical journal. I am tired of being told, directly or indirectly, that I am a racist by TV news, newspapers, social media, professional sports teams, and now by my medical journals. I would ask the authors to be careful who they throw under the bus.
Scott Peters, MD
Oak Ridge, Tennessee
Continue to: Drs. Mengesha, Arora, and Levy respond...
Drs. Mengesha, Arora, and Levy respond
We appreciate the opportunity to respond to Dr. Peters. Our article brings long overdue awareness to systemic and structural problems that result in disproportionately inequitable outcomes for people of color. We are not debating the morality of individuals or talking about racism as an inherently “bad” trait that some people have, but rather recognizing the impact of social structures on health and well-being in which we all—Black, Brown, and White—live. We all have inherent biases, recognized or unrecognized, that impact our actions, decisions, and behaviors. We are humans with upbringing, backgrounds, and learned frameworks influenced by our sociocultural context that conditions our responses to a given situation. This is also woven into our hospitals, exam rooms, and even our medical journals. It constantly influences the health, well-being, and livelihood of patients—nothing that we do in medicine is in isolation of this greater context. Our health care system is steeped in this sociocultural context and impacts all patients in intersecting ways, whether that be by race, class, gender, or other social identities. And while we did not create the system in which we operate, we now have abundant evidence that shows it continually delivers inequitable outcomes particularly for people of color.
We are very clear that physicians and health care professionals strive to provide the very best care for each and every patient. We do not discount the hard work and good intentions of our colleagues. And while some individual patient behaviors may somewhat modify outcomes, we also strongly disagree with the premise that patients are to blame for poor or less desirable outcomes they face. Instead, our position is focused on the impact that the systems we work in are creating barriers to equitable care at levels of influence above a single individual, and that it is our collective professional responsibility to acknowledge and take action to lessen those barriers.
System-wide changes would not be at the expense of patient care, and physicians cannot and in fact should not shoulder these changes alone. Our current paradigm of training does not give us the capacity to do so, and a single individual cannot make such a large system change alone. The change we are advocating for requires collaboration within multidisciplinary and interprofessional teams, long-term planning, and incremental but intentional change. This is not dissimilar to the recognition over 20 years ago by the Institute of Medicine (now the National Academy of Medicine) that “to err is human.” Our eyes were opened to the structural issues resulting in medical errors, and very slowly our profession has acknowledged the necessity to recognize, report, and analyze the root causes of those errors. We do so because it is critically important to ensure that the same error never happens again. It is part of the commitment to honor our oath to “do no harm.” Similarly, racial inequities in health outcomes should also be “never events” as there is no biological basis or individual blame for these inequities, but rather systemic and structural processes (which is de facto racism) that contribute to disproportionately worse outcomes.
Disparities in COVID-19 vaccination rates for people of color is a current example that illustrates the deep distrust in our health care system that historical events, like Tuskegee, have created. In Tennessee, for example, 7% of COVID-19 vaccines have been administered to Black people despite the fact that they make up 15% of cases, 18% of deaths, and 16% of the total population.1 There are other ongoing systemic issues, including inequities in distribution, prioritization, and access, that are contributing to the lower vaccination rates among people of color; however, as physicians and advocates for our patients, it is crucial for us to acknowledge the fear of, and resistance to, government-sponsored health programs which has resulted from events like Tuskegee.
We are advocating for building our systems to help support all of the social and societal determinants of health our patients are faced with, including racism, while they are receiving care from us. Patients are faced with undue morbidity and mortality because of our health care system’s ineffectiveness in incorporating this as a part of systemic care delivery to all. We must work together alongside other health care professionals, public health and policy agencies, and community advocates to stop this deadly cycle. There will be no improvement and no end in sight unless we work together toward this common goal.
Reference
- Ndugga N, Pham O, Hill L, et al. Latest data on COVID-19 vaccinations: race/ethnicity. Kaiser Family Foundation website. February 1, 2021. https://www.kff.org/coronavirus-covid-19/issue-brief/latest-data-covid-19-vaccinations-cases-deaths-race-ethnicity/. Accessed February 11, 2021.
HBV viremia linked to HCC risk in HIV/HBV coinfection
Any level of hepatitis B virus (HBV) viremia was associated with increased hepatocellular carcinoma (HCC) risk in adults with HIV/HBV coinfection, according to new research presented at the Conference on Retroviruses and Opportunistic Infections (Abstract 136).
“Chronic HBV coinfection is common among people with HIV, but the determinants of HBV-associated HCC are not well characterized,” said presenter H. Nina Kim MD, MSc, of the University of Washington, Seattle. “We sought to identify factors that contribute to HCC development in persons with HIV/HBV coinfection to guide early detection and prevention measures.”
The researchers conducted a longitudinal cohort study within the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD), a collaboration of single-site and multisite cohorts throughout the United States and Canada; 22 cohorts from NA-ACCORD were included in the analysis.
Potential HIV and HBV risk factors were examined, including viremia and CD4 percentage, as well as HBV DNA levels. Traditional risk factors for liver disease progression, including age, sex, and heavy alcohol use, were also assessed.
Eligible patients were 18 years of age or older who were followed for at least 6 months, had evidence of chronic HBV, and had HIV RNA or CD4+ cell measurement during this period. Persons with prevalent HCC at baseline were excluded.
The primary outcome was first occurrence of HCC, which was adjudicated by medical chart review and/or cancer registry. Multivariable Cox regression was used to determine adjusted hazard ratios of risk factors.
Results
Among 9,383 HIV/HBV-coinfected individuals identified, 8,354 (89%) were included in the analysis. The median age of participants was 43 years and 93.1% were male. Heavy alcohol use (35.3%) and chronic hepatitis C virus (HCV) coinfection (21.6%) were common among participants.
Among 8,354 eligible participants, 115 developed HCC over a median 6.9 years of follow-up (incidence rate, 1.8 events per 1,000 person-years; 95% confidence interval [CI], 1.5-2.1).
Independent risk factors for HCC were chronic HCV coinfection (adjusted hazard ratio [aHR], 1.60 [95% confidence interval, 1.07-2.39]), age 40 years and older (aHR, 2.14 [1.36-3.37]), and heavy alcohol use (aHR, 1.51 [1.03-2.21]); however, time-updated CD4+ percentage less than 14% (aHR, 1.03 [0.56-1.90]) and time-updated HIV RNA level over 500 copies/mL (aHR, 0.88 [0.55-1.41]) were not associated with HCC risk.
In a second model, among 3,054 patients who had HBV DNA measured, the risk of HCC was higher with HBV DNA levels greater than 200 IU/mL (aHR, 2.70 [1.23-5.93]), and the risk was particularly elevated at levels greater than 200,000 IU/mL (aHR, 4.34 [1.72-10.94]).
The researchers also found that the risk of HCC was significantly lower in patients with HBV DNA suppression less than 200 IU/mL receiving HBV-active ART for 1 year or more (aHR, 0.42 [0.24-0.73]). In addition, a dose-response relationship was observed between the duration of suppression and this protective effect.
Dr. Nina Kim acknowledged that a key limitation of the study was inconsistent monitoring of HBV DNA level while patients were on treatment. Furthermore, given the demographics of the cohort, these results may not be generalizable outside of North America.
“Our study was the first to show that any level of HBV viremia using 200 as a threshold of detection was associated with HCC risk in a large regionally diverse cohort of adults outside of Asia,” Dr. Kim said. “To gain maximal protective benefit from antiviral therapy for HCC prevention, sustained and ideally uninterrupted suppression of HBV may be necessary over years.”
“HIV/HBV coinfected patients can take much longer than a year to achieve less than 200 copies on HBV DNA due to their baseline levels, but we still don’t know if HBV therapy intensification could hasten this process,” said moderator Robert T. Schooley, MD, of the University of California, San Diego.
Dr. Kim disclosed no conflicts of interest. The study was supported by multiple sources, including the National Institutes of Health, the Centers for Disease Control and Prevention, and the National Cancer Institute.
Any level of hepatitis B virus (HBV) viremia was associated with increased hepatocellular carcinoma (HCC) risk in adults with HIV/HBV coinfection, according to new research presented at the Conference on Retroviruses and Opportunistic Infections (Abstract 136).
“Chronic HBV coinfection is common among people with HIV, but the determinants of HBV-associated HCC are not well characterized,” said presenter H. Nina Kim MD, MSc, of the University of Washington, Seattle. “We sought to identify factors that contribute to HCC development in persons with HIV/HBV coinfection to guide early detection and prevention measures.”
The researchers conducted a longitudinal cohort study within the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD), a collaboration of single-site and multisite cohorts throughout the United States and Canada; 22 cohorts from NA-ACCORD were included in the analysis.
Potential HIV and HBV risk factors were examined, including viremia and CD4 percentage, as well as HBV DNA levels. Traditional risk factors for liver disease progression, including age, sex, and heavy alcohol use, were also assessed.
Eligible patients were 18 years of age or older who were followed for at least 6 months, had evidence of chronic HBV, and had HIV RNA or CD4+ cell measurement during this period. Persons with prevalent HCC at baseline were excluded.
The primary outcome was first occurrence of HCC, which was adjudicated by medical chart review and/or cancer registry. Multivariable Cox regression was used to determine adjusted hazard ratios of risk factors.
Results
Among 9,383 HIV/HBV-coinfected individuals identified, 8,354 (89%) were included in the analysis. The median age of participants was 43 years and 93.1% were male. Heavy alcohol use (35.3%) and chronic hepatitis C virus (HCV) coinfection (21.6%) were common among participants.
Among 8,354 eligible participants, 115 developed HCC over a median 6.9 years of follow-up (incidence rate, 1.8 events per 1,000 person-years; 95% confidence interval [CI], 1.5-2.1).
Independent risk factors for HCC were chronic HCV coinfection (adjusted hazard ratio [aHR], 1.60 [95% confidence interval, 1.07-2.39]), age 40 years and older (aHR, 2.14 [1.36-3.37]), and heavy alcohol use (aHR, 1.51 [1.03-2.21]); however, time-updated CD4+ percentage less than 14% (aHR, 1.03 [0.56-1.90]) and time-updated HIV RNA level over 500 copies/mL (aHR, 0.88 [0.55-1.41]) were not associated with HCC risk.
In a second model, among 3,054 patients who had HBV DNA measured, the risk of HCC was higher with HBV DNA levels greater than 200 IU/mL (aHR, 2.70 [1.23-5.93]), and the risk was particularly elevated at levels greater than 200,000 IU/mL (aHR, 4.34 [1.72-10.94]).
The researchers also found that the risk of HCC was significantly lower in patients with HBV DNA suppression less than 200 IU/mL receiving HBV-active ART for 1 year or more (aHR, 0.42 [0.24-0.73]). In addition, a dose-response relationship was observed between the duration of suppression and this protective effect.
Dr. Nina Kim acknowledged that a key limitation of the study was inconsistent monitoring of HBV DNA level while patients were on treatment. Furthermore, given the demographics of the cohort, these results may not be generalizable outside of North America.
“Our study was the first to show that any level of HBV viremia using 200 as a threshold of detection was associated with HCC risk in a large regionally diverse cohort of adults outside of Asia,” Dr. Kim said. “To gain maximal protective benefit from antiviral therapy for HCC prevention, sustained and ideally uninterrupted suppression of HBV may be necessary over years.”
“HIV/HBV coinfected patients can take much longer than a year to achieve less than 200 copies on HBV DNA due to their baseline levels, but we still don’t know if HBV therapy intensification could hasten this process,” said moderator Robert T. Schooley, MD, of the University of California, San Diego.
Dr. Kim disclosed no conflicts of interest. The study was supported by multiple sources, including the National Institutes of Health, the Centers for Disease Control and Prevention, and the National Cancer Institute.
Any level of hepatitis B virus (HBV) viremia was associated with increased hepatocellular carcinoma (HCC) risk in adults with HIV/HBV coinfection, according to new research presented at the Conference on Retroviruses and Opportunistic Infections (Abstract 136).
“Chronic HBV coinfection is common among people with HIV, but the determinants of HBV-associated HCC are not well characterized,” said presenter H. Nina Kim MD, MSc, of the University of Washington, Seattle. “We sought to identify factors that contribute to HCC development in persons with HIV/HBV coinfection to guide early detection and prevention measures.”
The researchers conducted a longitudinal cohort study within the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD), a collaboration of single-site and multisite cohorts throughout the United States and Canada; 22 cohorts from NA-ACCORD were included in the analysis.
Potential HIV and HBV risk factors were examined, including viremia and CD4 percentage, as well as HBV DNA levels. Traditional risk factors for liver disease progression, including age, sex, and heavy alcohol use, were also assessed.
Eligible patients were 18 years of age or older who were followed for at least 6 months, had evidence of chronic HBV, and had HIV RNA or CD4+ cell measurement during this period. Persons with prevalent HCC at baseline were excluded.
The primary outcome was first occurrence of HCC, which was adjudicated by medical chart review and/or cancer registry. Multivariable Cox regression was used to determine adjusted hazard ratios of risk factors.
Results
Among 9,383 HIV/HBV-coinfected individuals identified, 8,354 (89%) were included in the analysis. The median age of participants was 43 years and 93.1% were male. Heavy alcohol use (35.3%) and chronic hepatitis C virus (HCV) coinfection (21.6%) were common among participants.
Among 8,354 eligible participants, 115 developed HCC over a median 6.9 years of follow-up (incidence rate, 1.8 events per 1,000 person-years; 95% confidence interval [CI], 1.5-2.1).
Independent risk factors for HCC were chronic HCV coinfection (adjusted hazard ratio [aHR], 1.60 [95% confidence interval, 1.07-2.39]), age 40 years and older (aHR, 2.14 [1.36-3.37]), and heavy alcohol use (aHR, 1.51 [1.03-2.21]); however, time-updated CD4+ percentage less than 14% (aHR, 1.03 [0.56-1.90]) and time-updated HIV RNA level over 500 copies/mL (aHR, 0.88 [0.55-1.41]) were not associated with HCC risk.
In a second model, among 3,054 patients who had HBV DNA measured, the risk of HCC was higher with HBV DNA levels greater than 200 IU/mL (aHR, 2.70 [1.23-5.93]), and the risk was particularly elevated at levels greater than 200,000 IU/mL (aHR, 4.34 [1.72-10.94]).
The researchers also found that the risk of HCC was significantly lower in patients with HBV DNA suppression less than 200 IU/mL receiving HBV-active ART for 1 year or more (aHR, 0.42 [0.24-0.73]). In addition, a dose-response relationship was observed between the duration of suppression and this protective effect.
Dr. Nina Kim acknowledged that a key limitation of the study was inconsistent monitoring of HBV DNA level while patients were on treatment. Furthermore, given the demographics of the cohort, these results may not be generalizable outside of North America.
“Our study was the first to show that any level of HBV viremia using 200 as a threshold of detection was associated with HCC risk in a large regionally diverse cohort of adults outside of Asia,” Dr. Kim said. “To gain maximal protective benefit from antiviral therapy for HCC prevention, sustained and ideally uninterrupted suppression of HBV may be necessary over years.”
“HIV/HBV coinfected patients can take much longer than a year to achieve less than 200 copies on HBV DNA due to their baseline levels, but we still don’t know if HBV therapy intensification could hasten this process,” said moderator Robert T. Schooley, MD, of the University of California, San Diego.
Dr. Kim disclosed no conflicts of interest. The study was supported by multiple sources, including the National Institutes of Health, the Centers for Disease Control and Prevention, and the National Cancer Institute.
FROM CROI 2021
PPIs improve functional dyspepsia via anti-inflammatory effects
Proton pump inhibitors (PPIs) improve functional dyspepsia (FD) by reducing duodenal eosinophils and mast cells, according to a prospective study.
This suggests that the anti-inflammatory effects of PPIs are responsible for symptom improvement, and not barrier-protective or acid-suppressive effects, a finding that may guide future therapies and biomarkers, reported lead author Lucas Wauters, PhD, of University Hospitals Leuven (Belgium), and colleagues reported in Gastroenterology.
“FD is a common and unexplained disorder with unknown pathophysiology, hampering a conclusive diagnosis and the development of effective drugs,” the investigators wrote.
Although PPIs are currently used as first-line FD therapy, ostensibly for acid suppression, “the exact mechanism of action of PPIs in FD is unknown,” the investigators noted.
According to Dr. Wauters and colleagues, previous FD studies, such as a 2020 study published in Gut, have reported a variety of pathophysiological findings in the duodenum, including increased eosinophils and mast cells, as well as activation of duodenogastric reflexes, which suggests “a primary role for duodenal pathology in FD symptom generation.” Several drivers of this pathology have been proposed. Some, such as aberrations in bile salts and acidity, point to local, luminal changes, whereas others, such as dysregulated hypothalamic-pituitary-adrenal axis responsiveness and psychosocial factors, implicate a broader set of drivers, the investigators wrote.
The present study explored this landscape through a prospective trial that enrolled 30 healthy volunteers and 47 patients with FD (2 patients with FD did not complete the study).
Patients with FD were subgrouped into “FD-starters” who had not taken PPIs and/or acid suppression for at least 3 months leading up to the trial (n = 28) and “FD-stoppers” who had refractory symptoms after at least 1 month of daily PPI usage (n = 19). Among participants with FD, 25 had postprandial distress syndrome (PDS), 9 had epigastric pain syndrome (EPS), and 13 had subtype overlap.
For the trial, FD-starters and healthy volunteers took 4 weeks of pantoprazole 40 mg once daily, whereas FD-stoppers ceased PPI therapy for 8 weeks. Before and after these respective periods, certain study procedures were conducted, including duodenal biopsy collection, duodenal fluid aspiration, and questionnaires for symptoms and stress. The study also included use of Ussing chambers for biopsies, immunohistochemistry, and bile salt measurements.
FD-starters were significantly more symptomatic than healthy volunteers were at baseline. After starting PPIs, those with FD had symptom improvements, confirming “clinical efficacy of a standard course of PPIs in all FD subtypes,” whereas healthy volunteers showed no significant change in symptoms.
Similarly, baseline duodenal eosinophil counts were higher in FD-starters than in healthy volunteers. On starting PPIs, however, eosinophil counts in these two groups moved in opposite directions: FD-starters’ counts dropped from a mean of 331 to 183 eosinophils/mm2, whereas healthy volunteers’ counts rose from a mean of 115 to 229 eosinophils/mm2 (P < .0001). Changes in mast cells and paracellular passage followed the same pattern, falling in FD-starters and rising in healthy volunteers. On the other hand, symptoms actually improved in the FD-stoppers after they went off PPIs, although they did not reach symptom levels of the healthy volunteers.
“Differential effects of PPIs in healthy volunteers point to the role of luminal changes in determining low-grade mucosal immune activation in the duodenum, which can also occur in FD after long-term use and provide arguments against continued use in refractory patients,” the investigators wrote.
Dr. Wauters and colleagues suggested that their findings could guide future approaches to FD management.
“Our results suggest that quantification of duodenal eosinophils has the potential to become part of diagnostic workup and guide therapeutic decisions in FD,” they wrote. “Additional study of the underlying mediators might lead to the discovery of new potential biomarkers or novel therapeutic targets, potentially allowing the identification of subgroups responding to biologically targeted rather than symptom-based treatments.”
The study was supported by the clinical research fund of the University Hospitals Leuven. The investigators reported no conflicts of interest.
Proton pump inhibitors (PPIs) improve functional dyspepsia (FD) by reducing duodenal eosinophils and mast cells, according to a prospective study.
This suggests that the anti-inflammatory effects of PPIs are responsible for symptom improvement, and not barrier-protective or acid-suppressive effects, a finding that may guide future therapies and biomarkers, reported lead author Lucas Wauters, PhD, of University Hospitals Leuven (Belgium), and colleagues reported in Gastroenterology.
“FD is a common and unexplained disorder with unknown pathophysiology, hampering a conclusive diagnosis and the development of effective drugs,” the investigators wrote.
Although PPIs are currently used as first-line FD therapy, ostensibly for acid suppression, “the exact mechanism of action of PPIs in FD is unknown,” the investigators noted.
According to Dr. Wauters and colleagues, previous FD studies, such as a 2020 study published in Gut, have reported a variety of pathophysiological findings in the duodenum, including increased eosinophils and mast cells, as well as activation of duodenogastric reflexes, which suggests “a primary role for duodenal pathology in FD symptom generation.” Several drivers of this pathology have been proposed. Some, such as aberrations in bile salts and acidity, point to local, luminal changes, whereas others, such as dysregulated hypothalamic-pituitary-adrenal axis responsiveness and psychosocial factors, implicate a broader set of drivers, the investigators wrote.
The present study explored this landscape through a prospective trial that enrolled 30 healthy volunteers and 47 patients with FD (2 patients with FD did not complete the study).
Patients with FD were subgrouped into “FD-starters” who had not taken PPIs and/or acid suppression for at least 3 months leading up to the trial (n = 28) and “FD-stoppers” who had refractory symptoms after at least 1 month of daily PPI usage (n = 19). Among participants with FD, 25 had postprandial distress syndrome (PDS), 9 had epigastric pain syndrome (EPS), and 13 had subtype overlap.
For the trial, FD-starters and healthy volunteers took 4 weeks of pantoprazole 40 mg once daily, whereas FD-stoppers ceased PPI therapy for 8 weeks. Before and after these respective periods, certain study procedures were conducted, including duodenal biopsy collection, duodenal fluid aspiration, and questionnaires for symptoms and stress. The study also included use of Ussing chambers for biopsies, immunohistochemistry, and bile salt measurements.
FD-starters were significantly more symptomatic than healthy volunteers were at baseline. After starting PPIs, those with FD had symptom improvements, confirming “clinical efficacy of a standard course of PPIs in all FD subtypes,” whereas healthy volunteers showed no significant change in symptoms.
Similarly, baseline duodenal eosinophil counts were higher in FD-starters than in healthy volunteers. On starting PPIs, however, eosinophil counts in these two groups moved in opposite directions: FD-starters’ counts dropped from a mean of 331 to 183 eosinophils/mm2, whereas healthy volunteers’ counts rose from a mean of 115 to 229 eosinophils/mm2 (P < .0001). Changes in mast cells and paracellular passage followed the same pattern, falling in FD-starters and rising in healthy volunteers. On the other hand, symptoms actually improved in the FD-stoppers after they went off PPIs, although they did not reach symptom levels of the healthy volunteers.
“Differential effects of PPIs in healthy volunteers point to the role of luminal changes in determining low-grade mucosal immune activation in the duodenum, which can also occur in FD after long-term use and provide arguments against continued use in refractory patients,” the investigators wrote.
Dr. Wauters and colleagues suggested that their findings could guide future approaches to FD management.
“Our results suggest that quantification of duodenal eosinophils has the potential to become part of diagnostic workup and guide therapeutic decisions in FD,” they wrote. “Additional study of the underlying mediators might lead to the discovery of new potential biomarkers or novel therapeutic targets, potentially allowing the identification of subgroups responding to biologically targeted rather than symptom-based treatments.”
The study was supported by the clinical research fund of the University Hospitals Leuven. The investigators reported no conflicts of interest.
Proton pump inhibitors (PPIs) improve functional dyspepsia (FD) by reducing duodenal eosinophils and mast cells, according to a prospective study.
This suggests that the anti-inflammatory effects of PPIs are responsible for symptom improvement, and not barrier-protective or acid-suppressive effects, a finding that may guide future therapies and biomarkers, reported lead author Lucas Wauters, PhD, of University Hospitals Leuven (Belgium), and colleagues reported in Gastroenterology.
“FD is a common and unexplained disorder with unknown pathophysiology, hampering a conclusive diagnosis and the development of effective drugs,” the investigators wrote.
Although PPIs are currently used as first-line FD therapy, ostensibly for acid suppression, “the exact mechanism of action of PPIs in FD is unknown,” the investigators noted.
According to Dr. Wauters and colleagues, previous FD studies, such as a 2020 study published in Gut, have reported a variety of pathophysiological findings in the duodenum, including increased eosinophils and mast cells, as well as activation of duodenogastric reflexes, which suggests “a primary role for duodenal pathology in FD symptom generation.” Several drivers of this pathology have been proposed. Some, such as aberrations in bile salts and acidity, point to local, luminal changes, whereas others, such as dysregulated hypothalamic-pituitary-adrenal axis responsiveness and psychosocial factors, implicate a broader set of drivers, the investigators wrote.
The present study explored this landscape through a prospective trial that enrolled 30 healthy volunteers and 47 patients with FD (2 patients with FD did not complete the study).
Patients with FD were subgrouped into “FD-starters” who had not taken PPIs and/or acid suppression for at least 3 months leading up to the trial (n = 28) and “FD-stoppers” who had refractory symptoms after at least 1 month of daily PPI usage (n = 19). Among participants with FD, 25 had postprandial distress syndrome (PDS), 9 had epigastric pain syndrome (EPS), and 13 had subtype overlap.
For the trial, FD-starters and healthy volunteers took 4 weeks of pantoprazole 40 mg once daily, whereas FD-stoppers ceased PPI therapy for 8 weeks. Before and after these respective periods, certain study procedures were conducted, including duodenal biopsy collection, duodenal fluid aspiration, and questionnaires for symptoms and stress. The study also included use of Ussing chambers for biopsies, immunohistochemistry, and bile salt measurements.
FD-starters were significantly more symptomatic than healthy volunteers were at baseline. After starting PPIs, those with FD had symptom improvements, confirming “clinical efficacy of a standard course of PPIs in all FD subtypes,” whereas healthy volunteers showed no significant change in symptoms.
Similarly, baseline duodenal eosinophil counts were higher in FD-starters than in healthy volunteers. On starting PPIs, however, eosinophil counts in these two groups moved in opposite directions: FD-starters’ counts dropped from a mean of 331 to 183 eosinophils/mm2, whereas healthy volunteers’ counts rose from a mean of 115 to 229 eosinophils/mm2 (P < .0001). Changes in mast cells and paracellular passage followed the same pattern, falling in FD-starters and rising in healthy volunteers. On the other hand, symptoms actually improved in the FD-stoppers after they went off PPIs, although they did not reach symptom levels of the healthy volunteers.
“Differential effects of PPIs in healthy volunteers point to the role of luminal changes in determining low-grade mucosal immune activation in the duodenum, which can also occur in FD after long-term use and provide arguments against continued use in refractory patients,” the investigators wrote.
Dr. Wauters and colleagues suggested that their findings could guide future approaches to FD management.
“Our results suggest that quantification of duodenal eosinophils has the potential to become part of diagnostic workup and guide therapeutic decisions in FD,” they wrote. “Additional study of the underlying mediators might lead to the discovery of new potential biomarkers or novel therapeutic targets, potentially allowing the identification of subgroups responding to biologically targeted rather than symptom-based treatments.”
The study was supported by the clinical research fund of the University Hospitals Leuven. The investigators reported no conflicts of interest.
FROM GASTROENTEROLOGY
Bone loss common in kidney stone patients, yet rarely detected
Almost one in four men and women diagnosed with kidney stones have osteoporosis or a history of fracture at the time of their diagnosis, yet fewer than 10% undergo bone mineral density (BMD) screening, a retrospective analysis of a Veterans Health Administration database shows.
Because the majority of those analyzed in the VA dataset were men, this means that middle-aged and older men with kidney stones have about the same risk for osteoporosis as postmenopausal women do, but BMD screening for such men is not currently recommended, the study notes.
“These findings suggest that the risk of osteoporosis or fractures in patients with kidney stone disease is not restricted to postmenopausal women but is also observed in men, a group that is less well recognized to be at risk,” Calyani Ganesan, MD, of Stanford (Calif.) University and colleagues say in their article, published online March 3 in the Journal of Bone and Mineral Research.
“We hope this work raises awareness regarding the possibility of reduced bone strength in patients with kidney stones, [and] in our future work, we hope to identify which patients with kidney stones are at higher risk for osteoporosis or fracture to help guide bone density screening efforts by clinicians in this population,” Dr. Ganesan added in a statement.
VA dataset: Just 9.1% had DXA after kidney stone diagnosed
A total of 531,431 patients with a history of kidney stone disease were identified in the VA dataset. Of these, 23.6% either had been diagnosed with osteoporosis or had a history of fracture around the time of their kidney stone diagnosis. The most common diagnosis was a non-hip fracture, seen in 19% of patients, Dr. Ganesan and colleagues note, followed by osteoporosis in 6.1%, and hip fracture in 2.1%.
The mean age of the patients who concurrently had received a diagnosis of kidney stone disease and osteoporosis or had a fracture history was 64.2 years. In this cohort, more than 91% were men. The majority of the patients were White.
Among some 462,681 patients who had no prior history of either osteoporosis or fracture before their diagnosis of kidney stones, only 9.1% had undergone dual-energy x-ray absorptiometry (DXA) screening for BMD in the 5 years after their kidney stone diagnosis.
“Of those who completed DXA ... 20% were subsequently diagnosed with osteoporosis,” the authors note – 19% with non-hip fracture, and 2.4% with hip fracture.
Importantly, 85% of patients with kidney stone disease who were screened with DXA and were later diagnosed with osteoporosis were men.
“Given that almost 20% of patients in our cohort had a non-hip fracture, we contend that osteoporosis is underdiagnosed and undertreated in older men with kidney stone disease,” the authors stress.
Perform DXA screen in older men, even in absence of hypercalciuria
The authors also explain that the most common metabolic abnormality associated with kidney stones is high urine calcium excretion, or hypercalciuria.
“In a subset of patients with kidney stones, dysregulated calcium homeostasis may be present in which calcium is resorbed from bone and excreted into the urine, which can lead to osteoporosis and the formation of calcium stones,” they explain.
However, when they carried out a 24-hour assessment of urine calcium excretion on a small subset of patients with kidney stones, “we found no correlation between osteoporosis and the level of 24-hour urine calcium excretion,” they point out.
Even when the authors excluded patients who were taking a thiazide diuretic – a class of drugs that decreases urine calcium excretion – there was no correlation between osteoporosis and the level of 24-hour urine calcium excretion.
The investigators suggest it is possible that, in the majority of patients with kidney stones, the cause of hypercalciuria is more closely related to overabsorption of calcium from the gut, not to overresorption of calcium from the bone.
“Nonetheless, our findings indicate that patients with kidney stone disease could benefit from DXA screening even in the absence of hypercalciuria,” they state.
“And our findings provide support for wider use of bone mineral density screening in patients with kidney stone disease, including middle-aged and older men, for whom efforts to mitigate risks of osteoporosis and fractures are not commonly emphasized,” they reaffirm.
The study was funded by the VA Merit Review and the National Institute of Diabetes and Digestive and Kidney Diseases. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Almost one in four men and women diagnosed with kidney stones have osteoporosis or a history of fracture at the time of their diagnosis, yet fewer than 10% undergo bone mineral density (BMD) screening, a retrospective analysis of a Veterans Health Administration database shows.
Because the majority of those analyzed in the VA dataset were men, this means that middle-aged and older men with kidney stones have about the same risk for osteoporosis as postmenopausal women do, but BMD screening for such men is not currently recommended, the study notes.
“These findings suggest that the risk of osteoporosis or fractures in patients with kidney stone disease is not restricted to postmenopausal women but is also observed in men, a group that is less well recognized to be at risk,” Calyani Ganesan, MD, of Stanford (Calif.) University and colleagues say in their article, published online March 3 in the Journal of Bone and Mineral Research.
“We hope this work raises awareness regarding the possibility of reduced bone strength in patients with kidney stones, [and] in our future work, we hope to identify which patients with kidney stones are at higher risk for osteoporosis or fracture to help guide bone density screening efforts by clinicians in this population,” Dr. Ganesan added in a statement.
VA dataset: Just 9.1% had DXA after kidney stone diagnosed
A total of 531,431 patients with a history of kidney stone disease were identified in the VA dataset. Of these, 23.6% either had been diagnosed with osteoporosis or had a history of fracture around the time of their kidney stone diagnosis. The most common diagnosis was a non-hip fracture, seen in 19% of patients, Dr. Ganesan and colleagues note, followed by osteoporosis in 6.1%, and hip fracture in 2.1%.
The mean age of the patients who concurrently had received a diagnosis of kidney stone disease and osteoporosis or had a fracture history was 64.2 years. In this cohort, more than 91% were men. The majority of the patients were White.
Among some 462,681 patients who had no prior history of either osteoporosis or fracture before their diagnosis of kidney stones, only 9.1% had undergone dual-energy x-ray absorptiometry (DXA) screening for BMD in the 5 years after their kidney stone diagnosis.
“Of those who completed DXA ... 20% were subsequently diagnosed with osteoporosis,” the authors note – 19% with non-hip fracture, and 2.4% with hip fracture.
Importantly, 85% of patients with kidney stone disease who were screened with DXA and were later diagnosed with osteoporosis were men.
“Given that almost 20% of patients in our cohort had a non-hip fracture, we contend that osteoporosis is underdiagnosed and undertreated in older men with kidney stone disease,” the authors stress.
Perform DXA screen in older men, even in absence of hypercalciuria
The authors also explain that the most common metabolic abnormality associated with kidney stones is high urine calcium excretion, or hypercalciuria.
“In a subset of patients with kidney stones, dysregulated calcium homeostasis may be present in which calcium is resorbed from bone and excreted into the urine, which can lead to osteoporosis and the formation of calcium stones,” they explain.
However, when they carried out a 24-hour assessment of urine calcium excretion on a small subset of patients with kidney stones, “we found no correlation between osteoporosis and the level of 24-hour urine calcium excretion,” they point out.
Even when the authors excluded patients who were taking a thiazide diuretic – a class of drugs that decreases urine calcium excretion – there was no correlation between osteoporosis and the level of 24-hour urine calcium excretion.
The investigators suggest it is possible that, in the majority of patients with kidney stones, the cause of hypercalciuria is more closely related to overabsorption of calcium from the gut, not to overresorption of calcium from the bone.
“Nonetheless, our findings indicate that patients with kidney stone disease could benefit from DXA screening even in the absence of hypercalciuria,” they state.
“And our findings provide support for wider use of bone mineral density screening in patients with kidney stone disease, including middle-aged and older men, for whom efforts to mitigate risks of osteoporosis and fractures are not commonly emphasized,” they reaffirm.
The study was funded by the VA Merit Review and the National Institute of Diabetes and Digestive and Kidney Diseases. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Almost one in four men and women diagnosed with kidney stones have osteoporosis or a history of fracture at the time of their diagnosis, yet fewer than 10% undergo bone mineral density (BMD) screening, a retrospective analysis of a Veterans Health Administration database shows.
Because the majority of those analyzed in the VA dataset were men, this means that middle-aged and older men with kidney stones have about the same risk for osteoporosis as postmenopausal women do, but BMD screening for such men is not currently recommended, the study notes.
“These findings suggest that the risk of osteoporosis or fractures in patients with kidney stone disease is not restricted to postmenopausal women but is also observed in men, a group that is less well recognized to be at risk,” Calyani Ganesan, MD, of Stanford (Calif.) University and colleagues say in their article, published online March 3 in the Journal of Bone and Mineral Research.
“We hope this work raises awareness regarding the possibility of reduced bone strength in patients with kidney stones, [and] in our future work, we hope to identify which patients with kidney stones are at higher risk for osteoporosis or fracture to help guide bone density screening efforts by clinicians in this population,” Dr. Ganesan added in a statement.
VA dataset: Just 9.1% had DXA after kidney stone diagnosed
A total of 531,431 patients with a history of kidney stone disease were identified in the VA dataset. Of these, 23.6% either had been diagnosed with osteoporosis or had a history of fracture around the time of their kidney stone diagnosis. The most common diagnosis was a non-hip fracture, seen in 19% of patients, Dr. Ganesan and colleagues note, followed by osteoporosis in 6.1%, and hip fracture in 2.1%.
The mean age of the patients who concurrently had received a diagnosis of kidney stone disease and osteoporosis or had a fracture history was 64.2 years. In this cohort, more than 91% were men. The majority of the patients were White.
Among some 462,681 patients who had no prior history of either osteoporosis or fracture before their diagnosis of kidney stones, only 9.1% had undergone dual-energy x-ray absorptiometry (DXA) screening for BMD in the 5 years after their kidney stone diagnosis.
“Of those who completed DXA ... 20% were subsequently diagnosed with osteoporosis,” the authors note – 19% with non-hip fracture, and 2.4% with hip fracture.
Importantly, 85% of patients with kidney stone disease who were screened with DXA and were later diagnosed with osteoporosis were men.
“Given that almost 20% of patients in our cohort had a non-hip fracture, we contend that osteoporosis is underdiagnosed and undertreated in older men with kidney stone disease,” the authors stress.
Perform DXA screen in older men, even in absence of hypercalciuria
The authors also explain that the most common metabolic abnormality associated with kidney stones is high urine calcium excretion, or hypercalciuria.
“In a subset of patients with kidney stones, dysregulated calcium homeostasis may be present in which calcium is resorbed from bone and excreted into the urine, which can lead to osteoporosis and the formation of calcium stones,” they explain.
However, when they carried out a 24-hour assessment of urine calcium excretion on a small subset of patients with kidney stones, “we found no correlation between osteoporosis and the level of 24-hour urine calcium excretion,” they point out.
Even when the authors excluded patients who were taking a thiazide diuretic – a class of drugs that decreases urine calcium excretion – there was no correlation between osteoporosis and the level of 24-hour urine calcium excretion.
The investigators suggest it is possible that, in the majority of patients with kidney stones, the cause of hypercalciuria is more closely related to overabsorption of calcium from the gut, not to overresorption of calcium from the bone.
“Nonetheless, our findings indicate that patients with kidney stone disease could benefit from DXA screening even in the absence of hypercalciuria,” they state.
“And our findings provide support for wider use of bone mineral density screening in patients with kidney stone disease, including middle-aged and older men, for whom efforts to mitigate risks of osteoporosis and fractures are not commonly emphasized,” they reaffirm.
The study was funded by the VA Merit Review and the National Institute of Diabetes and Digestive and Kidney Diseases. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Palliative care for patients with dementia: When to refer?
Palliative care for people with dementia is increasingly recognized as a way to improve quality of life and provide relief from the myriad physical and psychological symptoms of advancing neurodegenerative disease. But unlike in cancer,
A new literature review has found these referrals to be all over the map among patients with dementia – with many occurring very late in the disease process – and do not reflect any consistent criteria based on patient needs.
For their research, published March 2 in the Journal of the American Geriatrics Society, Li Mo, MD, of the University of Texas MD Anderson Cancer Center in Houston, and colleagues looked at nearly 60 studies dating back to the early 1990s that contained information on referrals to palliative care for patients with dementia. While a palliative care approach can be provided by nonspecialists, all the included studies dealt at least in part with specialist care.
Standardized criteria is lacking
The investigators found advanced or late-stage dementia to be the most common reason cited for referral, with three quarters of the studies recommending palliative care for late-stage or advanced dementia, generally without qualifying what symptoms or needs were present. Patients received palliative care across a range of settings, including nursing homes, hospitals, and their own homes, though many articles did not include information on where patients received care.
A fifth of the articles suggested that medical complications of dementia including falls, pneumonia, and ulcers should trigger referrals to palliative care, while another fifth cited poor prognosis, defined varyingly as having between 2 years and 6 months likely left to live. Poor nutrition status was identified in 10% of studies as meriting referral.
Only 20% of the studies identified patient needs – evidence of psychological distress or functional decline, for example – as criteria for referral, despite these being ubiquitous in dementia. The authors said they were surprised by this finding, which could possibly be explained, they wrote, by “the interest among geriatrician, neurologist, and primary care teams to provide good symptom management,” reflecting a de facto palliative care approach. “There is also significant stigma associated with a specialist palliative care referral,” the authors noted.
Curiously, the researchers noted, a new diagnosis of dementia in more than a quarter of the studies triggered referral, a finding that possibly reflected delayed diagnoses.
The findings revealed “heterogeneity in the literature in reasons for involving specialist palliative care, which may partly explain the variation in patterns of palliative care referral,” Dr. Mo and colleagues wrote, stressing that more standardized criteria are urgently needed to bring dementia in line with cancer in terms of providing timely palliative care.
Patients with advancing dementia have little chance to self-report symptoms, meaning that more attention to patient complaints earlier in the disease course, and greater sensitivity to patient distress, are required. By routinely screening symptoms, clinicians could use specific cutoffs “as triggers to initiate automatic timely palliative care referral,” the authors concluded, noting that more research was needed before these cutoffs, whether based on symptom intensity or other measures, could be calculated.
Dr. Mo and colleagues acknowledged as weaknesses of their study the fact that a third of the articles in the review were based on expert consensus, while others did not distinguish clearly between primary and specialist palliative care.
A starting point for further discussion
Asked to comment on the findings, Elizabeth Sampson, MD, a palliative care researcher at University College London, praised Dr. Mo and colleagues’ study as “starting to pull together the strands” of a systematic approach to referrals and access to palliative care in dementia.
“Sometimes you need a paper like this to kick off the discussion to say look, this is where we are,” Dr. Sampson said, noting that the focus on need-based criteria dovetailed with a “general feeling in the field that we need to really think about needs, and what palliative care needs might be. What the threshold for referral should be we don’t know yet. Should it be three unmet needs? Or five? We’re still a long way from knowing.”
Dr. Sampson’s group is leading a UK-government funded research effort that aims to develop cost-effective palliative care interventions in dementia, in part through a tool that uses caregiver reports to assess symptom burden and patient needs. The research program “is founded on a needs-based approach, which aims to look at people’s individual needs and responding to them in a proactive way,” she said.
One of the obstacles to timely palliative care in dementia, Dr. Sampson said, is weighing resource allocation against what can be wildly varying prognoses. “Hospices understand when someone has terminal cancer and [is] likely to die within a few weeks, but it’s not unheard of for someone in very advanced stages of dementia to live another year,” she said. “There are concerns that a rapid increase in people with dementia being moved to palliative care could overwhelm already limited hospice capacity. We would argue that the best approach is to get palliative care out to where people with dementia live, which is usually the care home.”
Dr. Mo and colleagues’ study received funding from the National Institutes of Health, and its authors disclosed no financial conflicts of interest. Dr. Sampson’s work is supported by the UK’s Economic and Social Research Council and National Institute for Health Research. She disclosed no conflicts of interest.
Palliative care for people with dementia is increasingly recognized as a way to improve quality of life and provide relief from the myriad physical and psychological symptoms of advancing neurodegenerative disease. But unlike in cancer,
A new literature review has found these referrals to be all over the map among patients with dementia – with many occurring very late in the disease process – and do not reflect any consistent criteria based on patient needs.
For their research, published March 2 in the Journal of the American Geriatrics Society, Li Mo, MD, of the University of Texas MD Anderson Cancer Center in Houston, and colleagues looked at nearly 60 studies dating back to the early 1990s that contained information on referrals to palliative care for patients with dementia. While a palliative care approach can be provided by nonspecialists, all the included studies dealt at least in part with specialist care.
Standardized criteria is lacking
The investigators found advanced or late-stage dementia to be the most common reason cited for referral, with three quarters of the studies recommending palliative care for late-stage or advanced dementia, generally without qualifying what symptoms or needs were present. Patients received palliative care across a range of settings, including nursing homes, hospitals, and their own homes, though many articles did not include information on where patients received care.
A fifth of the articles suggested that medical complications of dementia including falls, pneumonia, and ulcers should trigger referrals to palliative care, while another fifth cited poor prognosis, defined varyingly as having between 2 years and 6 months likely left to live. Poor nutrition status was identified in 10% of studies as meriting referral.
Only 20% of the studies identified patient needs – evidence of psychological distress or functional decline, for example – as criteria for referral, despite these being ubiquitous in dementia. The authors said they were surprised by this finding, which could possibly be explained, they wrote, by “the interest among geriatrician, neurologist, and primary care teams to provide good symptom management,” reflecting a de facto palliative care approach. “There is also significant stigma associated with a specialist palliative care referral,” the authors noted.
Curiously, the researchers noted, a new diagnosis of dementia in more than a quarter of the studies triggered referral, a finding that possibly reflected delayed diagnoses.
The findings revealed “heterogeneity in the literature in reasons for involving specialist palliative care, which may partly explain the variation in patterns of palliative care referral,” Dr. Mo and colleagues wrote, stressing that more standardized criteria are urgently needed to bring dementia in line with cancer in terms of providing timely palliative care.
Patients with advancing dementia have little chance to self-report symptoms, meaning that more attention to patient complaints earlier in the disease course, and greater sensitivity to patient distress, are required. By routinely screening symptoms, clinicians could use specific cutoffs “as triggers to initiate automatic timely palliative care referral,” the authors concluded, noting that more research was needed before these cutoffs, whether based on symptom intensity or other measures, could be calculated.
Dr. Mo and colleagues acknowledged as weaknesses of their study the fact that a third of the articles in the review were based on expert consensus, while others did not distinguish clearly between primary and specialist palliative care.
A starting point for further discussion
Asked to comment on the findings, Elizabeth Sampson, MD, a palliative care researcher at University College London, praised Dr. Mo and colleagues’ study as “starting to pull together the strands” of a systematic approach to referrals and access to palliative care in dementia.
“Sometimes you need a paper like this to kick off the discussion to say look, this is where we are,” Dr. Sampson said, noting that the focus on need-based criteria dovetailed with a “general feeling in the field that we need to really think about needs, and what palliative care needs might be. What the threshold for referral should be we don’t know yet. Should it be three unmet needs? Or five? We’re still a long way from knowing.”
Dr. Sampson’s group is leading a UK-government funded research effort that aims to develop cost-effective palliative care interventions in dementia, in part through a tool that uses caregiver reports to assess symptom burden and patient needs. The research program “is founded on a needs-based approach, which aims to look at people’s individual needs and responding to them in a proactive way,” she said.
One of the obstacles to timely palliative care in dementia, Dr. Sampson said, is weighing resource allocation against what can be wildly varying prognoses. “Hospices understand when someone has terminal cancer and [is] likely to die within a few weeks, but it’s not unheard of for someone in very advanced stages of dementia to live another year,” she said. “There are concerns that a rapid increase in people with dementia being moved to palliative care could overwhelm already limited hospice capacity. We would argue that the best approach is to get palliative care out to where people with dementia live, which is usually the care home.”
Dr. Mo and colleagues’ study received funding from the National Institutes of Health, and its authors disclosed no financial conflicts of interest. Dr. Sampson’s work is supported by the UK’s Economic and Social Research Council and National Institute for Health Research. She disclosed no conflicts of interest.
Palliative care for people with dementia is increasingly recognized as a way to improve quality of life and provide relief from the myriad physical and psychological symptoms of advancing neurodegenerative disease. But unlike in cancer,
A new literature review has found these referrals to be all over the map among patients with dementia – with many occurring very late in the disease process – and do not reflect any consistent criteria based on patient needs.
For their research, published March 2 in the Journal of the American Geriatrics Society, Li Mo, MD, of the University of Texas MD Anderson Cancer Center in Houston, and colleagues looked at nearly 60 studies dating back to the early 1990s that contained information on referrals to palliative care for patients with dementia. While a palliative care approach can be provided by nonspecialists, all the included studies dealt at least in part with specialist care.
Standardized criteria is lacking
The investigators found advanced or late-stage dementia to be the most common reason cited for referral, with three quarters of the studies recommending palliative care for late-stage or advanced dementia, generally without qualifying what symptoms or needs were present. Patients received palliative care across a range of settings, including nursing homes, hospitals, and their own homes, though many articles did not include information on where patients received care.
A fifth of the articles suggested that medical complications of dementia including falls, pneumonia, and ulcers should trigger referrals to palliative care, while another fifth cited poor prognosis, defined varyingly as having between 2 years and 6 months likely left to live. Poor nutrition status was identified in 10% of studies as meriting referral.
Only 20% of the studies identified patient needs – evidence of psychological distress or functional decline, for example – as criteria for referral, despite these being ubiquitous in dementia. The authors said they were surprised by this finding, which could possibly be explained, they wrote, by “the interest among geriatrician, neurologist, and primary care teams to provide good symptom management,” reflecting a de facto palliative care approach. “There is also significant stigma associated with a specialist palliative care referral,” the authors noted.
Curiously, the researchers noted, a new diagnosis of dementia in more than a quarter of the studies triggered referral, a finding that possibly reflected delayed diagnoses.
The findings revealed “heterogeneity in the literature in reasons for involving specialist palliative care, which may partly explain the variation in patterns of palliative care referral,” Dr. Mo and colleagues wrote, stressing that more standardized criteria are urgently needed to bring dementia in line with cancer in terms of providing timely palliative care.
Patients with advancing dementia have little chance to self-report symptoms, meaning that more attention to patient complaints earlier in the disease course, and greater sensitivity to patient distress, are required. By routinely screening symptoms, clinicians could use specific cutoffs “as triggers to initiate automatic timely palliative care referral,” the authors concluded, noting that more research was needed before these cutoffs, whether based on symptom intensity or other measures, could be calculated.
Dr. Mo and colleagues acknowledged as weaknesses of their study the fact that a third of the articles in the review were based on expert consensus, while others did not distinguish clearly between primary and specialist palliative care.
A starting point for further discussion
Asked to comment on the findings, Elizabeth Sampson, MD, a palliative care researcher at University College London, praised Dr. Mo and colleagues’ study as “starting to pull together the strands” of a systematic approach to referrals and access to palliative care in dementia.
“Sometimes you need a paper like this to kick off the discussion to say look, this is where we are,” Dr. Sampson said, noting that the focus on need-based criteria dovetailed with a “general feeling in the field that we need to really think about needs, and what palliative care needs might be. What the threshold for referral should be we don’t know yet. Should it be three unmet needs? Or five? We’re still a long way from knowing.”
Dr. Sampson’s group is leading a UK-government funded research effort that aims to develop cost-effective palliative care interventions in dementia, in part through a tool that uses caregiver reports to assess symptom burden and patient needs. The research program “is founded on a needs-based approach, which aims to look at people’s individual needs and responding to them in a proactive way,” she said.
One of the obstacles to timely palliative care in dementia, Dr. Sampson said, is weighing resource allocation against what can be wildly varying prognoses. “Hospices understand when someone has terminal cancer and [is] likely to die within a few weeks, but it’s not unheard of for someone in very advanced stages of dementia to live another year,” she said. “There are concerns that a rapid increase in people with dementia being moved to palliative care could overwhelm already limited hospice capacity. We would argue that the best approach is to get palliative care out to where people with dementia live, which is usually the care home.”
Dr. Mo and colleagues’ study received funding from the National Institutes of Health, and its authors disclosed no financial conflicts of interest. Dr. Sampson’s work is supported by the UK’s Economic and Social Research Council and National Institute for Health Research. She disclosed no conflicts of interest.
FROM THE JOURNAL OF THE AMERICAN GERIATRICS SOCIETY
Disease progression and therapy response vary in MS by ethnicity
a new study finds, and there are big gaps in how they respond to disease-modifying therapies (DMTs).
“Hispanics and African Americans develop a more severe disease course and accumulate more MS-related disability over time despite similar sociodemographic backgrounds and similar patterns of DMT use throughout their disease, suggesting that socioeconomic status and access to health care may not be the main determinants of health,” said neurologist Carlos Pérez, MD, of the University of Texas Health Science Center, Houston. He spoke at the annual meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis and in a follow-up interview.
“In addition,” Dr. Pérez said, “therapeutic responses to individual DMTs, as well as tolerance and side-effect profiles, are also variable among racial/ethnic groups.”
The researchers tracked 150 patients with MS at the University of Texas Health Science Center – 50 Whites, 50 African American, and 50 Hispanic – who were age and gender matched. The average age of the subjects was 45, and 74% of those in each group were women.
While educational levels between the groups were similar, African Americans had a much higher rate of lost employment because of disability (38%) than Hispanics (19%) and Whites (15%, P = .02). Fifty-seven patients (38%) needed escalation of therapy, and 63% were African American.
About 30% of subjects switched DMTs because of intolerance/adverse events, and 47% of those were African American. Interferons most commonly caused adverse effects in African Americans (61%), and Whites were the most likely to not tolerate glatiramer acetate (39%) than Hispanics (8%) and African Americans (13%).
What might be behind the disparities? “It is possible that genetic factors may play a greater role than previously thought. A recent study reported that Hispanic and African American patients with MS have higher levels of peripheral blood plasmablasts, which may provide indirect evidence for potential biological mechanisms underlying racial and clinical disparities in MS,” Dr. Pérez said. “These mechanisms appear to involve higher degrees of inflammation in the central nervous system. This may explain why African Americans may respond better to higher-efficacy therapies initially, when inflammatory processes predominate MS-related pathology, rather than at later stages of the disease when inflammation plays a less prominent role. Neurologists should consider higher-efficacy DMT as first line. We have begun to do this in our practice.”
Dr. Pérez said the findings offer other lessons. “Neurologists should consider that Caucasian patients tolerate glatiramer acetate less frequently, compared with other racial groups, and potentially consider using alternative DMTs unless the benefits outweigh the risks, such as during pregnancy.”
He also noted that African Americans treated with oral DMTs at baseline were more likely to develop worsening disability over time. “This argues in favor of infusion therapies as first-line treatment,” he said, adding that more Hispanics with MS were not on treatment – or discontinued treatment – compared with Whites and African Americans.
Close patient monitoring is key
Atlanta-area neurologist Mitzi Joi Williams, MD, who was asked to comment on the study findings, said in an interview that it “adds to the body of real-world evidence to assist understanding of MS in minority populations.”
She noted that African American patients who started on infusions appeared to be more stable. “There are a great deal of questions surrounding starting patients on injectables versus higher-efficacy therapy initially to prevent disability and this may lend credence to the need for closer examination of initial therapy for these patients. It is important to closely monitor patients and consider a switch in DMT if there is any clinical or radiologic progression, especially for African American and Hispanic patients since there is a great deal of data to suggest they may have more aggressive disease.”
Moving forward, more research like this is needed, she said. “Patients did all have insurance and were largely educated, but there could be other social determinants of health – i.e., transportation, lapses in insurance, or technology barriers – that may have led to worse outcomes.”
No study funding was reported, and Dr. Pérez reported no disclosures. Dr. Williams disclosed research support from EMD Serono, Genentech, and Novartis and advisory committee/consultant relationships with AbbVie, Biogen Idec, Bristol-Myers Squibb, EMD Serono, Genentech, Novartis, and Sanofi Genzyme.
a new study finds, and there are big gaps in how they respond to disease-modifying therapies (DMTs).
“Hispanics and African Americans develop a more severe disease course and accumulate more MS-related disability over time despite similar sociodemographic backgrounds and similar patterns of DMT use throughout their disease, suggesting that socioeconomic status and access to health care may not be the main determinants of health,” said neurologist Carlos Pérez, MD, of the University of Texas Health Science Center, Houston. He spoke at the annual meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis and in a follow-up interview.
“In addition,” Dr. Pérez said, “therapeutic responses to individual DMTs, as well as tolerance and side-effect profiles, are also variable among racial/ethnic groups.”
The researchers tracked 150 patients with MS at the University of Texas Health Science Center – 50 Whites, 50 African American, and 50 Hispanic – who were age and gender matched. The average age of the subjects was 45, and 74% of those in each group were women.
While educational levels between the groups were similar, African Americans had a much higher rate of lost employment because of disability (38%) than Hispanics (19%) and Whites (15%, P = .02). Fifty-seven patients (38%) needed escalation of therapy, and 63% were African American.
About 30% of subjects switched DMTs because of intolerance/adverse events, and 47% of those were African American. Interferons most commonly caused adverse effects in African Americans (61%), and Whites were the most likely to not tolerate glatiramer acetate (39%) than Hispanics (8%) and African Americans (13%).
What might be behind the disparities? “It is possible that genetic factors may play a greater role than previously thought. A recent study reported that Hispanic and African American patients with MS have higher levels of peripheral blood plasmablasts, which may provide indirect evidence for potential biological mechanisms underlying racial and clinical disparities in MS,” Dr. Pérez said. “These mechanisms appear to involve higher degrees of inflammation in the central nervous system. This may explain why African Americans may respond better to higher-efficacy therapies initially, when inflammatory processes predominate MS-related pathology, rather than at later stages of the disease when inflammation plays a less prominent role. Neurologists should consider higher-efficacy DMT as first line. We have begun to do this in our practice.”
Dr. Pérez said the findings offer other lessons. “Neurologists should consider that Caucasian patients tolerate glatiramer acetate less frequently, compared with other racial groups, and potentially consider using alternative DMTs unless the benefits outweigh the risks, such as during pregnancy.”
He also noted that African Americans treated with oral DMTs at baseline were more likely to develop worsening disability over time. “This argues in favor of infusion therapies as first-line treatment,” he said, adding that more Hispanics with MS were not on treatment – or discontinued treatment – compared with Whites and African Americans.
Close patient monitoring is key
Atlanta-area neurologist Mitzi Joi Williams, MD, who was asked to comment on the study findings, said in an interview that it “adds to the body of real-world evidence to assist understanding of MS in minority populations.”
She noted that African American patients who started on infusions appeared to be more stable. “There are a great deal of questions surrounding starting patients on injectables versus higher-efficacy therapy initially to prevent disability and this may lend credence to the need for closer examination of initial therapy for these patients. It is important to closely monitor patients and consider a switch in DMT if there is any clinical or radiologic progression, especially for African American and Hispanic patients since there is a great deal of data to suggest they may have more aggressive disease.”
Moving forward, more research like this is needed, she said. “Patients did all have insurance and were largely educated, but there could be other social determinants of health – i.e., transportation, lapses in insurance, or technology barriers – that may have led to worse outcomes.”
No study funding was reported, and Dr. Pérez reported no disclosures. Dr. Williams disclosed research support from EMD Serono, Genentech, and Novartis and advisory committee/consultant relationships with AbbVie, Biogen Idec, Bristol-Myers Squibb, EMD Serono, Genentech, Novartis, and Sanofi Genzyme.
a new study finds, and there are big gaps in how they respond to disease-modifying therapies (DMTs).
“Hispanics and African Americans develop a more severe disease course and accumulate more MS-related disability over time despite similar sociodemographic backgrounds and similar patterns of DMT use throughout their disease, suggesting that socioeconomic status and access to health care may not be the main determinants of health,” said neurologist Carlos Pérez, MD, of the University of Texas Health Science Center, Houston. He spoke at the annual meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis and in a follow-up interview.
“In addition,” Dr. Pérez said, “therapeutic responses to individual DMTs, as well as tolerance and side-effect profiles, are also variable among racial/ethnic groups.”
The researchers tracked 150 patients with MS at the University of Texas Health Science Center – 50 Whites, 50 African American, and 50 Hispanic – who were age and gender matched. The average age of the subjects was 45, and 74% of those in each group were women.
While educational levels between the groups were similar, African Americans had a much higher rate of lost employment because of disability (38%) than Hispanics (19%) and Whites (15%, P = .02). Fifty-seven patients (38%) needed escalation of therapy, and 63% were African American.
About 30% of subjects switched DMTs because of intolerance/adverse events, and 47% of those were African American. Interferons most commonly caused adverse effects in African Americans (61%), and Whites were the most likely to not tolerate glatiramer acetate (39%) than Hispanics (8%) and African Americans (13%).
What might be behind the disparities? “It is possible that genetic factors may play a greater role than previously thought. A recent study reported that Hispanic and African American patients with MS have higher levels of peripheral blood plasmablasts, which may provide indirect evidence for potential biological mechanisms underlying racial and clinical disparities in MS,” Dr. Pérez said. “These mechanisms appear to involve higher degrees of inflammation in the central nervous system. This may explain why African Americans may respond better to higher-efficacy therapies initially, when inflammatory processes predominate MS-related pathology, rather than at later stages of the disease when inflammation plays a less prominent role. Neurologists should consider higher-efficacy DMT as first line. We have begun to do this in our practice.”
Dr. Pérez said the findings offer other lessons. “Neurologists should consider that Caucasian patients tolerate glatiramer acetate less frequently, compared with other racial groups, and potentially consider using alternative DMTs unless the benefits outweigh the risks, such as during pregnancy.”
He also noted that African Americans treated with oral DMTs at baseline were more likely to develop worsening disability over time. “This argues in favor of infusion therapies as first-line treatment,” he said, adding that more Hispanics with MS were not on treatment – or discontinued treatment – compared with Whites and African Americans.
Close patient monitoring is key
Atlanta-area neurologist Mitzi Joi Williams, MD, who was asked to comment on the study findings, said in an interview that it “adds to the body of real-world evidence to assist understanding of MS in minority populations.”
She noted that African American patients who started on infusions appeared to be more stable. “There are a great deal of questions surrounding starting patients on injectables versus higher-efficacy therapy initially to prevent disability and this may lend credence to the need for closer examination of initial therapy for these patients. It is important to closely monitor patients and consider a switch in DMT if there is any clinical or radiologic progression, especially for African American and Hispanic patients since there is a great deal of data to suggest they may have more aggressive disease.”
Moving forward, more research like this is needed, she said. “Patients did all have insurance and were largely educated, but there could be other social determinants of health – i.e., transportation, lapses in insurance, or technology barriers – that may have led to worse outcomes.”
No study funding was reported, and Dr. Pérez reported no disclosures. Dr. Williams disclosed research support from EMD Serono, Genentech, and Novartis and advisory committee/consultant relationships with AbbVie, Biogen Idec, Bristol-Myers Squibb, EMD Serono, Genentech, Novartis, and Sanofi Genzyme.
FROM ACTRIMS FORUM 2021
Newly approved drugs offer new hope in NMOSD
a neurologist told colleagues.
“Patients have a choice of different options with different types of action. It’s good news,” said Sean J. Pittock, MD, of the Mayo Clinic in Rochester, Minn. “If you don’t stop the clinical attacks, patients can become very disabled very quickly. These medications have a significant impact in reducing the likelihood of having a clinical relapse. If you can stop the relapses, you certainly can eventually stop most – if not all – of the disability accrual.”
Dr. Pittock spoke at the annual meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis and answered follow-up questions in an interview.
Treatment advances for NMOSD
NMOSD, also known as NMO, is a relapsing autoimmune inflammatory disorder that causes recurrent optic neuritis and myelitis. It’s a rare disease, affecting 0.5-10 people per 100,000, mostly women.
Several recent trials have supported the use of the drugs eculizumab (Soliris), satralizumab (Enspryng) and inebilizumab (Uplizna) in NMOSD, Dr. Pittock said, and all have received Food and Drug Administration approval to treat the condition over the past 2 years. Dr. Pittock led the PREVENT trial of eculizumab, which showed a 94% reduction of relapse risk versus placebo.
The newly approved drugs are stunningly expensive. According to Dr. Pittock, eculizumab costs $710,000 a year, while inebilizumab runs $393,000 the first year, then $262,000 a year. Satralizumab is $219,000 the first year, then $190,000 a year. Assistance programs are available, Dr. Pittock said, “and we’ve not had any major problems in terms of initiation.”
The cost of rituximab (Rituxan), which has a history of use as an off-label treatment option, is $18,000 a year and dropping, according to Dr. Pittock. There’s also new research on rituximab: In 2020, a small Japanese trial (n = 38) reported prevention of relapses compared with placebo, but Dr. Pittock cautioned that “the placebo patients seem to have a more benign course or more benign phenotypes” than the intervention group.
“There’s no doubt that rituximab works, but does it work as well as the other medications that have been through more of a robust trial process?” he asked. Keep in mind, he added, that perhaps 20%-50% of patients will relapse on rituximab.
Dr. Pittock advised colleagues to consider factors like patient schedules and compliance when choosing a drug. Satralizumab is self-administered monthly, while inebilizumab and rituximab are infused every 6 months.
Progress in anti-MOG disease
The trials in NMOSD should spur studies of the drugs in anti–myelin oligodendrocyte glycoprotein (MOG) disease, he said. “I think we’ll see a more rapid move toward phase 3 trials because of the experience with NMO. We will just have to wait and see which medications enter trial.”
Anti-MOG disease, also known as MOG antibody disease (MOGAD) and anti-MOG–associated encephalomyelitis, is caused by anti-MOG antibodies. Optic neuritis is very common, and transverse myelitis can occur.
The condition “actually responds to different drugs than MS and has a different immune pathophysiology,” Dr. Pittock said.
He cautioned colleagues to be aware that “the ability of the antibody to tell you whether or not the patient has the disease is less clear for MOGAD than it is for other diseases. If your patient has a low titer of MOG antibody, and their phenotype really doesn’t look like [MOGAD], you really need to interpret that with significant caution.”
He also highlighted a 2018 report that offers guidance about diagnosis and when MOG-IgC antibody tests are appropriate in CNS demyelinating disease.
Dr. Pittock reported numerous disclosures plus patents issued or pending.
a neurologist told colleagues.
“Patients have a choice of different options with different types of action. It’s good news,” said Sean J. Pittock, MD, of the Mayo Clinic in Rochester, Minn. “If you don’t stop the clinical attacks, patients can become very disabled very quickly. These medications have a significant impact in reducing the likelihood of having a clinical relapse. If you can stop the relapses, you certainly can eventually stop most – if not all – of the disability accrual.”
Dr. Pittock spoke at the annual meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis and answered follow-up questions in an interview.
Treatment advances for NMOSD
NMOSD, also known as NMO, is a relapsing autoimmune inflammatory disorder that causes recurrent optic neuritis and myelitis. It’s a rare disease, affecting 0.5-10 people per 100,000, mostly women.
Several recent trials have supported the use of the drugs eculizumab (Soliris), satralizumab (Enspryng) and inebilizumab (Uplizna) in NMOSD, Dr. Pittock said, and all have received Food and Drug Administration approval to treat the condition over the past 2 years. Dr. Pittock led the PREVENT trial of eculizumab, which showed a 94% reduction of relapse risk versus placebo.
The newly approved drugs are stunningly expensive. According to Dr. Pittock, eculizumab costs $710,000 a year, while inebilizumab runs $393,000 the first year, then $262,000 a year. Satralizumab is $219,000 the first year, then $190,000 a year. Assistance programs are available, Dr. Pittock said, “and we’ve not had any major problems in terms of initiation.”
The cost of rituximab (Rituxan), which has a history of use as an off-label treatment option, is $18,000 a year and dropping, according to Dr. Pittock. There’s also new research on rituximab: In 2020, a small Japanese trial (n = 38) reported prevention of relapses compared with placebo, but Dr. Pittock cautioned that “the placebo patients seem to have a more benign course or more benign phenotypes” than the intervention group.
“There’s no doubt that rituximab works, but does it work as well as the other medications that have been through more of a robust trial process?” he asked. Keep in mind, he added, that perhaps 20%-50% of patients will relapse on rituximab.
Dr. Pittock advised colleagues to consider factors like patient schedules and compliance when choosing a drug. Satralizumab is self-administered monthly, while inebilizumab and rituximab are infused every 6 months.
Progress in anti-MOG disease
The trials in NMOSD should spur studies of the drugs in anti–myelin oligodendrocyte glycoprotein (MOG) disease, he said. “I think we’ll see a more rapid move toward phase 3 trials because of the experience with NMO. We will just have to wait and see which medications enter trial.”
Anti-MOG disease, also known as MOG antibody disease (MOGAD) and anti-MOG–associated encephalomyelitis, is caused by anti-MOG antibodies. Optic neuritis is very common, and transverse myelitis can occur.
The condition “actually responds to different drugs than MS and has a different immune pathophysiology,” Dr. Pittock said.
He cautioned colleagues to be aware that “the ability of the antibody to tell you whether or not the patient has the disease is less clear for MOGAD than it is for other diseases. If your patient has a low titer of MOG antibody, and their phenotype really doesn’t look like [MOGAD], you really need to interpret that with significant caution.”
He also highlighted a 2018 report that offers guidance about diagnosis and when MOG-IgC antibody tests are appropriate in CNS demyelinating disease.
Dr. Pittock reported numerous disclosures plus patents issued or pending.
a neurologist told colleagues.
“Patients have a choice of different options with different types of action. It’s good news,” said Sean J. Pittock, MD, of the Mayo Clinic in Rochester, Minn. “If you don’t stop the clinical attacks, patients can become very disabled very quickly. These medications have a significant impact in reducing the likelihood of having a clinical relapse. If you can stop the relapses, you certainly can eventually stop most – if not all – of the disability accrual.”
Dr. Pittock spoke at the annual meeting held by the Americas Committee for Treatment and Research in Multiple Sclerosis and answered follow-up questions in an interview.
Treatment advances for NMOSD
NMOSD, also known as NMO, is a relapsing autoimmune inflammatory disorder that causes recurrent optic neuritis and myelitis. It’s a rare disease, affecting 0.5-10 people per 100,000, mostly women.
Several recent trials have supported the use of the drugs eculizumab (Soliris), satralizumab (Enspryng) and inebilizumab (Uplizna) in NMOSD, Dr. Pittock said, and all have received Food and Drug Administration approval to treat the condition over the past 2 years. Dr. Pittock led the PREVENT trial of eculizumab, which showed a 94% reduction of relapse risk versus placebo.
The newly approved drugs are stunningly expensive. According to Dr. Pittock, eculizumab costs $710,000 a year, while inebilizumab runs $393,000 the first year, then $262,000 a year. Satralizumab is $219,000 the first year, then $190,000 a year. Assistance programs are available, Dr. Pittock said, “and we’ve not had any major problems in terms of initiation.”
The cost of rituximab (Rituxan), which has a history of use as an off-label treatment option, is $18,000 a year and dropping, according to Dr. Pittock. There’s also new research on rituximab: In 2020, a small Japanese trial (n = 38) reported prevention of relapses compared with placebo, but Dr. Pittock cautioned that “the placebo patients seem to have a more benign course or more benign phenotypes” than the intervention group.
“There’s no doubt that rituximab works, but does it work as well as the other medications that have been through more of a robust trial process?” he asked. Keep in mind, he added, that perhaps 20%-50% of patients will relapse on rituximab.
Dr. Pittock advised colleagues to consider factors like patient schedules and compliance when choosing a drug. Satralizumab is self-administered monthly, while inebilizumab and rituximab are infused every 6 months.
Progress in anti-MOG disease
The trials in NMOSD should spur studies of the drugs in anti–myelin oligodendrocyte glycoprotein (MOG) disease, he said. “I think we’ll see a more rapid move toward phase 3 trials because of the experience with NMO. We will just have to wait and see which medications enter trial.”
Anti-MOG disease, also known as MOG antibody disease (MOGAD) and anti-MOG–associated encephalomyelitis, is caused by anti-MOG antibodies. Optic neuritis is very common, and transverse myelitis can occur.
The condition “actually responds to different drugs than MS and has a different immune pathophysiology,” Dr. Pittock said.
He cautioned colleagues to be aware that “the ability of the antibody to tell you whether or not the patient has the disease is less clear for MOGAD than it is for other diseases. If your patient has a low titer of MOG antibody, and their phenotype really doesn’t look like [MOGAD], you really need to interpret that with significant caution.”
He also highlighted a 2018 report that offers guidance about diagnosis and when MOG-IgC antibody tests are appropriate in CNS demyelinating disease.
Dr. Pittock reported numerous disclosures plus patents issued or pending.
FROM ACTRIMS FORUM 2021
Is it possible to classify dermatologists and internists into different patterns of prescribing behavior?
An exploratory analysis recently published in the Journal of the American Academy of Dermatology examines whether it is possible to classify dermatologists and internists into different patterns of prescribing behavior for patients with acne.
“Prior research has highlighted that prescribing for acne may not be aligned with guideline recommendations, including the overuse of oral antibiotics and lack of use of concomitant topical medications such as topical retinoids,” the study’s corresponding author, John S. Barbieri, MD, MBA, of the department of dermatology at the University of Pennsylvania, Philadelphia, said in an interview.
“In addition, there is substantial variation in prescribing practices among clinicians. . By identifying such groups, it would facilitate future qualitative interviews to understand factors that might contribute to clinicians having certain prescribing patterns, which could help guide implementation science work to better align practices with evidence and guidelines.”
For the study, which appeared online on March 1, Dr. Barbieri and colleague David J. Margolis, MD, PhD, professor of dermatology and epidemiology at the University of Pennsylvania, evaluated all clinical encounters associated with an ICD-9 or ICD-10 code for acne that occurred in the university’s departments of dermatology and internal medicine between Jan. 1, 2011, and Dec. 31, 2019. They used a machine-learning method known as k-means clustering to cluster clinicians based on their relative use of acne medications, as well as the ratio of spironolactone versus tetracycline use among female patients and stratified their analyses by specialty.
Of the 116 dermatologists included in the analysis, the researchers identified three clusters. The first cluster included 17 dermatologists (14.7%) and was characterized by low use of topical retinoids, high use of oral tetracycline, and low use of spironolactone, compared with oral antibiotics, among women with acne. Physicians in this cluster were more likely to be male and to have more years in practice.
The second cluster included 46 dermatologists (39.6%) and was marked by high use of spironolactone and low use of isotretinoin. The third cluster included 53 dermatologists (45.7%) and was characterized by high use of topical retinoids and frequent use of systemic medications.
Of the 86 internists included in the study, the researchers identified three clusters. The first cluster included 39 internists (45.4%) and was characterized by low use of topical retinoids, high use of oral tetracycline, and limited use of spironolactone. The second cluster included 34 internists (39.5%) and was marked by low use of topical retinoids and systemic medications. The third cluster included 13 clinicians (15.1%), most of whom were nurse practitioners, physician assistants, and other advanced practice providers. This cluster was characterized by high use of topical retinoids and relatively high use of spironolactone.
“There are likely opportunities to improve the use of topical retinoids by internists caring for patients with acne, since these are a first-line treatment option that may be underutilized by internists,” Dr. Barbieri said in the interview. “Future work is needed to identify underlying factors associated with different prescribing phenotypes among both dermatologists and internists. By understanding these factors, we can develop implementation science efforts to align prescribing behavior with best practices based on the guidelines and available evidence.”
He acknowledged certain limitations of the analysis, including its single-center design and the lack of data on patient characteristics. “Future studies are needed to examine whether our results generalize to other settings,” he said.
Dr. Barbieri disclosed that he receives partial salary support through a Pfizer Fellowship in Dermatology Patient Oriented Research grant to the Trustees of the University of Pennsylvania. The authors had no other disclosures.
An exploratory analysis recently published in the Journal of the American Academy of Dermatology examines whether it is possible to classify dermatologists and internists into different patterns of prescribing behavior for patients with acne.
“Prior research has highlighted that prescribing for acne may not be aligned with guideline recommendations, including the overuse of oral antibiotics and lack of use of concomitant topical medications such as topical retinoids,” the study’s corresponding author, John S. Barbieri, MD, MBA, of the department of dermatology at the University of Pennsylvania, Philadelphia, said in an interview.
“In addition, there is substantial variation in prescribing practices among clinicians. . By identifying such groups, it would facilitate future qualitative interviews to understand factors that might contribute to clinicians having certain prescribing patterns, which could help guide implementation science work to better align practices with evidence and guidelines.”
For the study, which appeared online on March 1, Dr. Barbieri and colleague David J. Margolis, MD, PhD, professor of dermatology and epidemiology at the University of Pennsylvania, evaluated all clinical encounters associated with an ICD-9 or ICD-10 code for acne that occurred in the university’s departments of dermatology and internal medicine between Jan. 1, 2011, and Dec. 31, 2019. They used a machine-learning method known as k-means clustering to cluster clinicians based on their relative use of acne medications, as well as the ratio of spironolactone versus tetracycline use among female patients and stratified their analyses by specialty.
Of the 116 dermatologists included in the analysis, the researchers identified three clusters. The first cluster included 17 dermatologists (14.7%) and was characterized by low use of topical retinoids, high use of oral tetracycline, and low use of spironolactone, compared with oral antibiotics, among women with acne. Physicians in this cluster were more likely to be male and to have more years in practice.
The second cluster included 46 dermatologists (39.6%) and was marked by high use of spironolactone and low use of isotretinoin. The third cluster included 53 dermatologists (45.7%) and was characterized by high use of topical retinoids and frequent use of systemic medications.
Of the 86 internists included in the study, the researchers identified three clusters. The first cluster included 39 internists (45.4%) and was characterized by low use of topical retinoids, high use of oral tetracycline, and limited use of spironolactone. The second cluster included 34 internists (39.5%) and was marked by low use of topical retinoids and systemic medications. The third cluster included 13 clinicians (15.1%), most of whom were nurse practitioners, physician assistants, and other advanced practice providers. This cluster was characterized by high use of topical retinoids and relatively high use of spironolactone.
“There are likely opportunities to improve the use of topical retinoids by internists caring for patients with acne, since these are a first-line treatment option that may be underutilized by internists,” Dr. Barbieri said in the interview. “Future work is needed to identify underlying factors associated with different prescribing phenotypes among both dermatologists and internists. By understanding these factors, we can develop implementation science efforts to align prescribing behavior with best practices based on the guidelines and available evidence.”
He acknowledged certain limitations of the analysis, including its single-center design and the lack of data on patient characteristics. “Future studies are needed to examine whether our results generalize to other settings,” he said.
Dr. Barbieri disclosed that he receives partial salary support through a Pfizer Fellowship in Dermatology Patient Oriented Research grant to the Trustees of the University of Pennsylvania. The authors had no other disclosures.
An exploratory analysis recently published in the Journal of the American Academy of Dermatology examines whether it is possible to classify dermatologists and internists into different patterns of prescribing behavior for patients with acne.
“Prior research has highlighted that prescribing for acne may not be aligned with guideline recommendations, including the overuse of oral antibiotics and lack of use of concomitant topical medications such as topical retinoids,” the study’s corresponding author, John S. Barbieri, MD, MBA, of the department of dermatology at the University of Pennsylvania, Philadelphia, said in an interview.
“In addition, there is substantial variation in prescribing practices among clinicians. . By identifying such groups, it would facilitate future qualitative interviews to understand factors that might contribute to clinicians having certain prescribing patterns, which could help guide implementation science work to better align practices with evidence and guidelines.”
For the study, which appeared online on March 1, Dr. Barbieri and colleague David J. Margolis, MD, PhD, professor of dermatology and epidemiology at the University of Pennsylvania, evaluated all clinical encounters associated with an ICD-9 or ICD-10 code for acne that occurred in the university’s departments of dermatology and internal medicine between Jan. 1, 2011, and Dec. 31, 2019. They used a machine-learning method known as k-means clustering to cluster clinicians based on their relative use of acne medications, as well as the ratio of spironolactone versus tetracycline use among female patients and stratified their analyses by specialty.
Of the 116 dermatologists included in the analysis, the researchers identified three clusters. The first cluster included 17 dermatologists (14.7%) and was characterized by low use of topical retinoids, high use of oral tetracycline, and low use of spironolactone, compared with oral antibiotics, among women with acne. Physicians in this cluster were more likely to be male and to have more years in practice.
The second cluster included 46 dermatologists (39.6%) and was marked by high use of spironolactone and low use of isotretinoin. The third cluster included 53 dermatologists (45.7%) and was characterized by high use of topical retinoids and frequent use of systemic medications.
Of the 86 internists included in the study, the researchers identified three clusters. The first cluster included 39 internists (45.4%) and was characterized by low use of topical retinoids, high use of oral tetracycline, and limited use of spironolactone. The second cluster included 34 internists (39.5%) and was marked by low use of topical retinoids and systemic medications. The third cluster included 13 clinicians (15.1%), most of whom were nurse practitioners, physician assistants, and other advanced practice providers. This cluster was characterized by high use of topical retinoids and relatively high use of spironolactone.
“There are likely opportunities to improve the use of topical retinoids by internists caring for patients with acne, since these are a first-line treatment option that may be underutilized by internists,” Dr. Barbieri said in the interview. “Future work is needed to identify underlying factors associated with different prescribing phenotypes among both dermatologists and internists. By understanding these factors, we can develop implementation science efforts to align prescribing behavior with best practices based on the guidelines and available evidence.”
He acknowledged certain limitations of the analysis, including its single-center design and the lack of data on patient characteristics. “Future studies are needed to examine whether our results generalize to other settings,” he said.
Dr. Barbieri disclosed that he receives partial salary support through a Pfizer Fellowship in Dermatology Patient Oriented Research grant to the Trustees of the University of Pennsylvania. The authors had no other disclosures.