User login
Growing Periumbilical Plaque: A Case of Perforating Calcific Elastosis
To the Editor:
Pseudoxanthoma elasticum (PXE) is a genetic perforating dermatosis characterized by fragmentation and calcification of elastic fibers that most commonly manifests on the skin, eyes, gastrointestinal tract, or cardiovascular system.1 Classic skin findings include multiple symmetric yellowish papules favoring the flexural surfaces of the body and neck as well as the periumbilical and inguinal regions.1,2 Many life-threatening complications from this disease can occur due to calcification of elastic fibers in other parts of the body, such as the internal elastic lamina of arteries, which can cause gastrointestinal tract bleeding and accelerated cardiovascular disease including valvular disease.2,3 If PXE is localized to the skin only without systemic involvement or a family history, a diagnosis of perforating calcific elastosis (PCE) can be made. We report a case of PCE in a patient with a growing umbilical lesion.
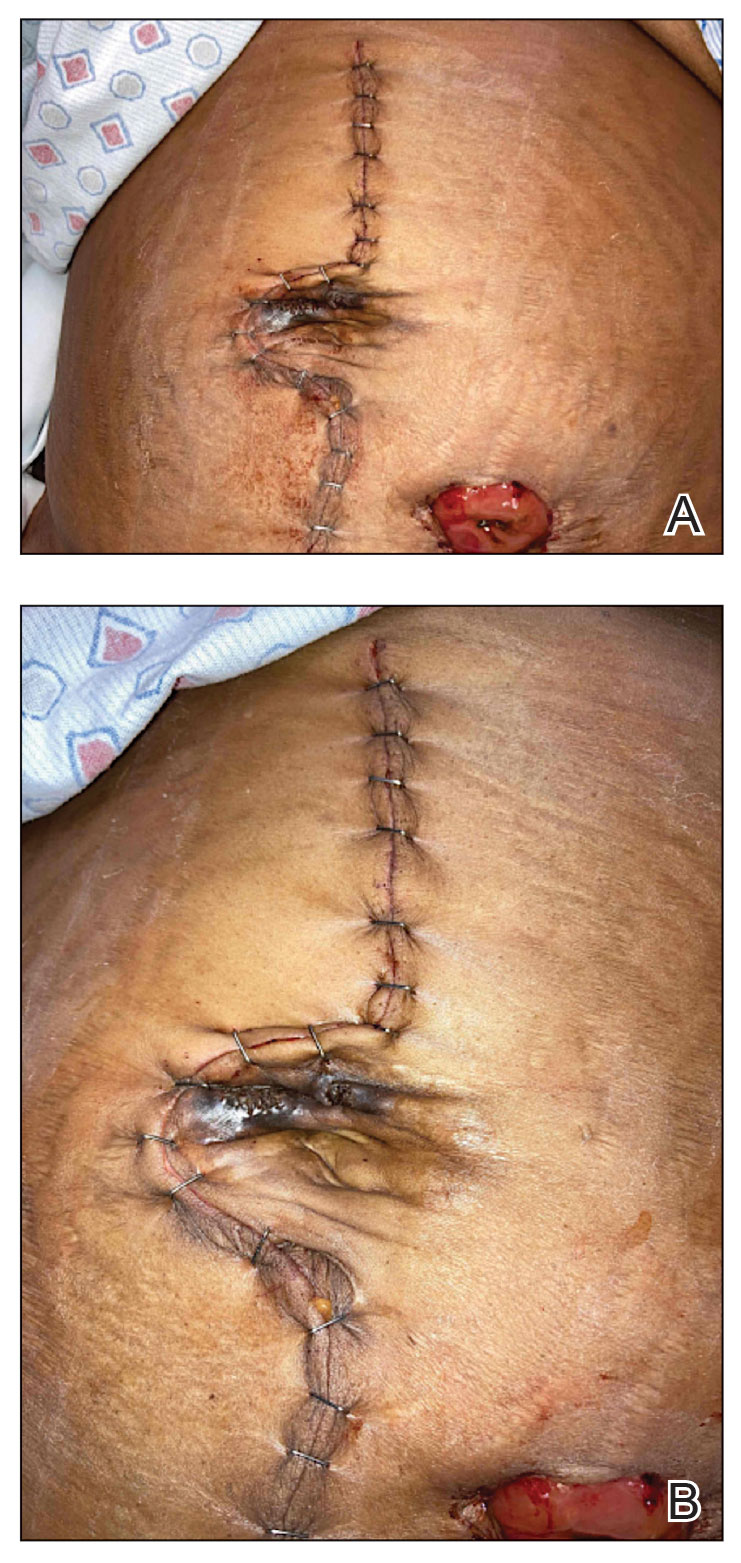
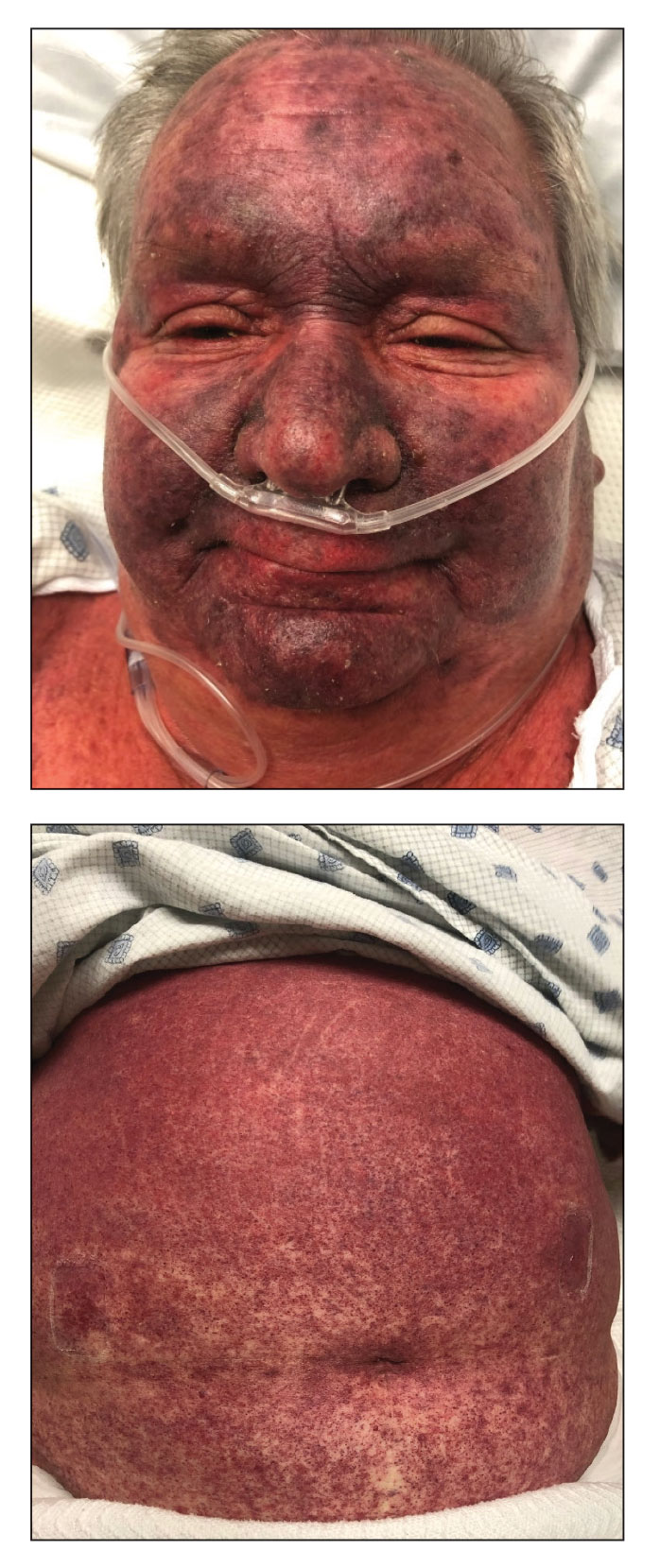
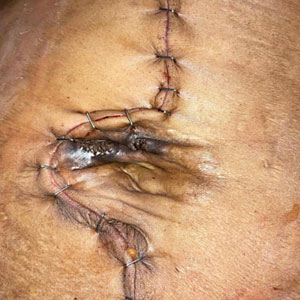
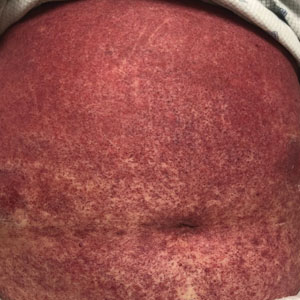
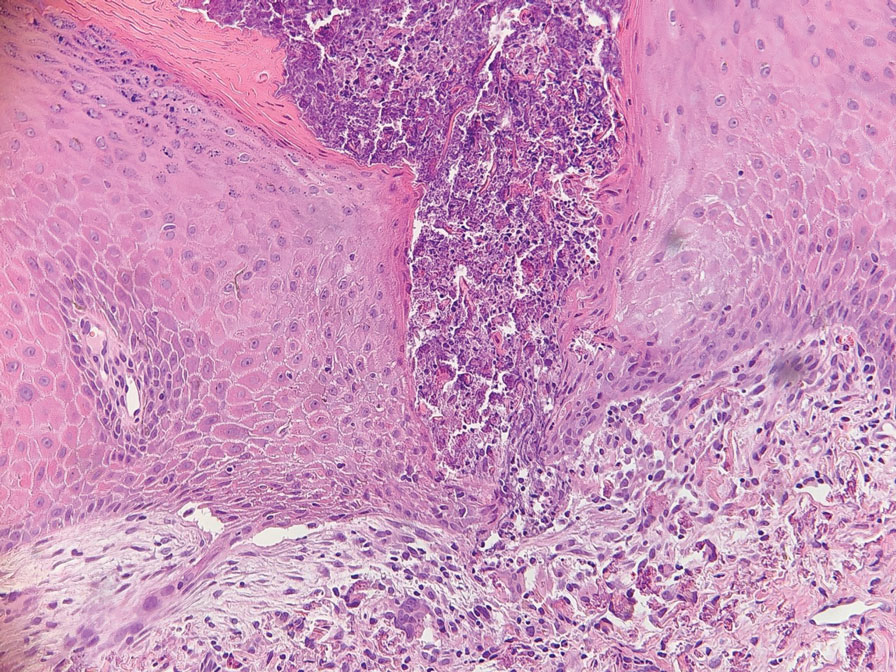
A 49-year-old multiparous (gravida 3, para 3) woman presented for evaluation of an evolving periumbilical lesion of 4 months’ duration. She denied pain, bleeding, or drainage from the area, as well as any systemic symptoms. The patient had a surgical history of a laparoscopic hysterectomy 7 years prior to the current presentation due to uterine fibroids, which resulted in a periumbilical scar. At the current presentation, physical examination revealed 2 hyperpigmented to violaceous periumbilical papules coalescing into a plaque with overlying hyperkeratosis and crusting (Figure 1). A punch biopsy was performed and histopathology showed diffuse dermal collections of degenerated eosinophilic distorted elastic fibers with calcification (Figure 2). Further sections showed a transepidermal channel in which the elastic fibers extruded from the dermis through the epidermis (Figure 3). The diagnosis of acquired PCE was made based on the clinical presentation, relevant medical history, and lack of underlying medical conditions or family history of PXE. No further workup was needed, and the patient reported no further progression and rather some improvement (decrease in size) of the lesion at 3-month follow-up.
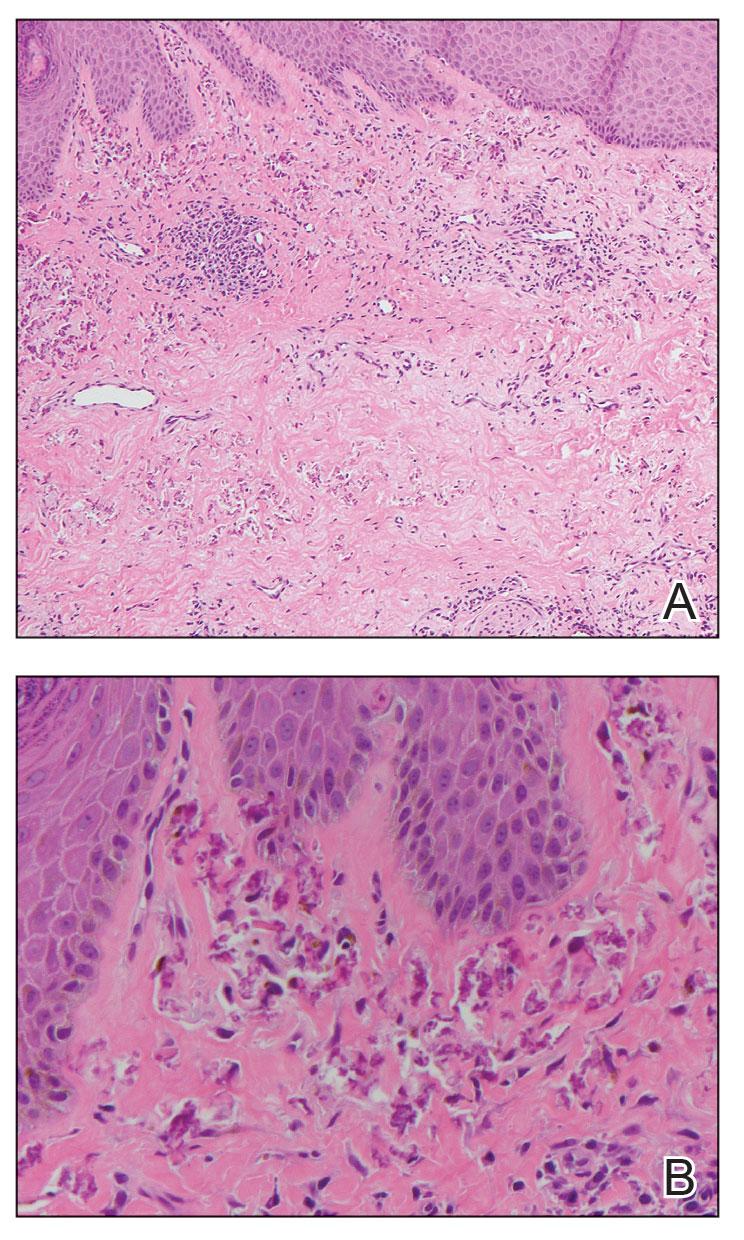
Perforating calcific elastosis (also known as periumbilical perforating PXE) is a rare acquired condition that is seen predominantly in multiparous middle-aged women.4-6 This diagnosis consists of degenerated calcified elastic fibers that may perforate the skin of the abdominal or periumbilical region. It clinically manifests as multiple painless hyperkeratotic papules surrounding the periumbilical region.4-6
The etiology and pathogenesis of PCE have not been defined but have been attributed to recurrent stressing of elastic fibers due to repeat traumas,1 which is proposed to lead to degeneration of elastic fibers and calcification of damaged tissue.4-7 As a result, PCE most commonly manifests in multiparous, obese, middle-aged women and patients with multiple abdominal surgeries or ascites.1 It also has been reported in patients with renal failure due to deposition of abnormal calcium phosphate products onto elastic fibers.4 In our patient, the development of PCE was related to both multiparity and trauma from prior surgery.
The histopathologic findings of PCE and PXE are similar, warranting differentiation via thorough clinical examination as well as further investigation of the patient’s medical and family history. Both show degenerated, fragmented, curly elastic fibers with calcium deposition throughout the dermis and a transepidermal channel extruding these elastic fibers.7,8 The biopsies stain positive for elastic fibers and calcium deposition. Calcium staining can help to differentiate these entities from elastosis perforans serpiginosa, which lacks the presence of calcium staining.7
There are no definitive treatments for PCE. A single case report of a patient with PCE and renal failure showed regression with hemodialysis.9 In a study evaluating patients with inherited PXE, notable improvement was seen in skin lesions treated with bisphosphonates, possibly suggesting that regulating serum calcium may contribute to improvement of the disease.3 Most cases spontaneously resolve with atrophic plaques. Our patient required no additional treatment with no further progression and reported improvement of the lesion with spontaneous decrease in size.
- Jha AK, Zheeshan MD, Sinha BK, et al. Periumbilical perforating pseudoxanthoma elasticum: a rare case report. Dermatol Pract Concept. 2018;8:75-77. doi:10.5826/dpc.0802a02
- Ko JH, Shih YC, Huang YC, et al. Pseudoxanthoma elasticum. Lancet. 2013;381:565.
- Sherer DW, Singer G, Uribarri J, et al. Oral phosphate binders in the treatment of pseudoxanthoma elasticum. J Am Acad Dermatol. 2005;53:610-615.
- Lal NR, Bandyopadhyay D, Verma R, et al. Perforating calcific elastosis: revisiting a rare entity. Indian J Dermatol. 2018;63:186-188. doi:10.4103/ijd.IJD_111_17
- Kocatürk E, Kavala M, Zindanci I, et al. Periumbilical perforating pseudoxanthoma elasticum. Indian J Dermatol Venereol Leprol. 2009;75:329.
- Bressan AL, Vasconcelos BN, Silva RDS, et al. Periumbilical and periareolar perforating pseudoxanthoma elasticum. An Bras Dermatol. 2010;85:705-707. doi:10.1590/s0365-05962010000500018
- Hosen MJ, Lamoen A, De Paepe A, et al. Histopathology of pseudoxanthoma elasticum and related disorders: histological hallmarks and diagnostic clues. Scientifica (Cairo). 2012;2012:598262.
- Bathina M, Hedge SP, Shanavaz AA, et al. Pruritic periumbilical plaque as a presentation of rare perforating dermatosis. Indian Dermatol Online J. 2020;11:68-71. doi:10.4103/idoj.IDOJ_95_19
- Sapadin AN, Lebwohl MG, Teich SA, et al. Periumbilical pseudoxanthoma elasticum associated with chronic renal failure and angioid streaks—apparent regression with hemodialysis. J Am Acad Dermatol. 1998;39:338-344.
To the Editor:
Pseudoxanthoma elasticum (PXE) is a genetic perforating dermatosis characterized by fragmentation and calcification of elastic fibers that most commonly manifests on the skin, eyes, gastrointestinal tract, or cardiovascular system.1 Classic skin findings include multiple symmetric yellowish papules favoring the flexural surfaces of the body and neck as well as the periumbilical and inguinal regions.1,2 Many life-threatening complications from this disease can occur due to calcification of elastic fibers in other parts of the body, such as the internal elastic lamina of arteries, which can cause gastrointestinal tract bleeding and accelerated cardiovascular disease including valvular disease.2,3 If PXE is localized to the skin only without systemic involvement or a family history, a diagnosis of perforating calcific elastosis (PCE) can be made. We report a case of PCE in a patient with a growing umbilical lesion.

A 49-year-old multiparous (gravida 3, para 3) woman presented for evaluation of an evolving periumbilical lesion of 4 months’ duration. She denied pain, bleeding, or drainage from the area, as well as any systemic symptoms. The patient had a surgical history of a laparoscopic hysterectomy 7 years prior to the current presentation due to uterine fibroids, which resulted in a periumbilical scar. At the current presentation, physical examination revealed 2 hyperpigmented to violaceous periumbilical papules coalescing into a plaque with overlying hyperkeratosis and crusting (Figure 1). A punch biopsy was performed and histopathology showed diffuse dermal collections of degenerated eosinophilic distorted elastic fibers with calcification (Figure 2). Further sections showed a transepidermal channel in which the elastic fibers extruded from the dermis through the epidermis (Figure 3). The diagnosis of acquired PCE was made based on the clinical presentation, relevant medical history, and lack of underlying medical conditions or family history of PXE. No further workup was needed, and the patient reported no further progression and rather some improvement (decrease in size) of the lesion at 3-month follow-up.

Perforating calcific elastosis (also known as periumbilical perforating PXE) is a rare acquired condition that is seen predominantly in multiparous middle-aged women.4-6 This diagnosis consists of degenerated calcified elastic fibers that may perforate the skin of the abdominal or periumbilical region. It clinically manifests as multiple painless hyperkeratotic papules surrounding the periumbilical region.4-6

The etiology and pathogenesis of PCE have not been defined but have been attributed to recurrent stressing of elastic fibers due to repeat traumas,1 which is proposed to lead to degeneration of elastic fibers and calcification of damaged tissue.4-7 As a result, PCE most commonly manifests in multiparous, obese, middle-aged women and patients with multiple abdominal surgeries or ascites.1 It also has been reported in patients with renal failure due to deposition of abnormal calcium phosphate products onto elastic fibers.4 In our patient, the development of PCE was related to both multiparity and trauma from prior surgery.
The histopathologic findings of PCE and PXE are similar, warranting differentiation via thorough clinical examination as well as further investigation of the patient’s medical and family history. Both show degenerated, fragmented, curly elastic fibers with calcium deposition throughout the dermis and a transepidermal channel extruding these elastic fibers.7,8 The biopsies stain positive for elastic fibers and calcium deposition. Calcium staining can help to differentiate these entities from elastosis perforans serpiginosa, which lacks the presence of calcium staining.7
There are no definitive treatments for PCE. A single case report of a patient with PCE and renal failure showed regression with hemodialysis.9 In a study evaluating patients with inherited PXE, notable improvement was seen in skin lesions treated with bisphosphonates, possibly suggesting that regulating serum calcium may contribute to improvement of the disease.3 Most cases spontaneously resolve with atrophic plaques. Our patient required no additional treatment with no further progression and reported improvement of the lesion with spontaneous decrease in size.
To the Editor:
Pseudoxanthoma elasticum (PXE) is a genetic perforating dermatosis characterized by fragmentation and calcification of elastic fibers that most commonly manifests on the skin, eyes, gastrointestinal tract, or cardiovascular system.1 Classic skin findings include multiple symmetric yellowish papules favoring the flexural surfaces of the body and neck as well as the periumbilical and inguinal regions.1,2 Many life-threatening complications from this disease can occur due to calcification of elastic fibers in other parts of the body, such as the internal elastic lamina of arteries, which can cause gastrointestinal tract bleeding and accelerated cardiovascular disease including valvular disease.2,3 If PXE is localized to the skin only without systemic involvement or a family history, a diagnosis of perforating calcific elastosis (PCE) can be made. We report a case of PCE in a patient with a growing umbilical lesion.

A 49-year-old multiparous (gravida 3, para 3) woman presented for evaluation of an evolving periumbilical lesion of 4 months’ duration. She denied pain, bleeding, or drainage from the area, as well as any systemic symptoms. The patient had a surgical history of a laparoscopic hysterectomy 7 years prior to the current presentation due to uterine fibroids, which resulted in a periumbilical scar. At the current presentation, physical examination revealed 2 hyperpigmented to violaceous periumbilical papules coalescing into a plaque with overlying hyperkeratosis and crusting (Figure 1). A punch biopsy was performed and histopathology showed diffuse dermal collections of degenerated eosinophilic distorted elastic fibers with calcification (Figure 2). Further sections showed a transepidermal channel in which the elastic fibers extruded from the dermis through the epidermis (Figure 3). The diagnosis of acquired PCE was made based on the clinical presentation, relevant medical history, and lack of underlying medical conditions or family history of PXE. No further workup was needed, and the patient reported no further progression and rather some improvement (decrease in size) of the lesion at 3-month follow-up.

Perforating calcific elastosis (also known as periumbilical perforating PXE) is a rare acquired condition that is seen predominantly in multiparous middle-aged women.4-6 This diagnosis consists of degenerated calcified elastic fibers that may perforate the skin of the abdominal or periumbilical region. It clinically manifests as multiple painless hyperkeratotic papules surrounding the periumbilical region.4-6

The etiology and pathogenesis of PCE have not been defined but have been attributed to recurrent stressing of elastic fibers due to repeat traumas,1 which is proposed to lead to degeneration of elastic fibers and calcification of damaged tissue.4-7 As a result, PCE most commonly manifests in multiparous, obese, middle-aged women and patients with multiple abdominal surgeries or ascites.1 It also has been reported in patients with renal failure due to deposition of abnormal calcium phosphate products onto elastic fibers.4 In our patient, the development of PCE was related to both multiparity and trauma from prior surgery.
The histopathologic findings of PCE and PXE are similar, warranting differentiation via thorough clinical examination as well as further investigation of the patient’s medical and family history. Both show degenerated, fragmented, curly elastic fibers with calcium deposition throughout the dermis and a transepidermal channel extruding these elastic fibers.7,8 The biopsies stain positive for elastic fibers and calcium deposition. Calcium staining can help to differentiate these entities from elastosis perforans serpiginosa, which lacks the presence of calcium staining.7
There are no definitive treatments for PCE. A single case report of a patient with PCE and renal failure showed regression with hemodialysis.9 In a study evaluating patients with inherited PXE, notable improvement was seen in skin lesions treated with bisphosphonates, possibly suggesting that regulating serum calcium may contribute to improvement of the disease.3 Most cases spontaneously resolve with atrophic plaques. Our patient required no additional treatment with no further progression and reported improvement of the lesion with spontaneous decrease in size.
- Jha AK, Zheeshan MD, Sinha BK, et al. Periumbilical perforating pseudoxanthoma elasticum: a rare case report. Dermatol Pract Concept. 2018;8:75-77. doi:10.5826/dpc.0802a02
- Ko JH, Shih YC, Huang YC, et al. Pseudoxanthoma elasticum. Lancet. 2013;381:565.
- Sherer DW, Singer G, Uribarri J, et al. Oral phosphate binders in the treatment of pseudoxanthoma elasticum. J Am Acad Dermatol. 2005;53:610-615.
- Lal NR, Bandyopadhyay D, Verma R, et al. Perforating calcific elastosis: revisiting a rare entity. Indian J Dermatol. 2018;63:186-188. doi:10.4103/ijd.IJD_111_17
- Kocatürk E, Kavala M, Zindanci I, et al. Periumbilical perforating pseudoxanthoma elasticum. Indian J Dermatol Venereol Leprol. 2009;75:329.
- Bressan AL, Vasconcelos BN, Silva RDS, et al. Periumbilical and periareolar perforating pseudoxanthoma elasticum. An Bras Dermatol. 2010;85:705-707. doi:10.1590/s0365-05962010000500018
- Hosen MJ, Lamoen A, De Paepe A, et al. Histopathology of pseudoxanthoma elasticum and related disorders: histological hallmarks and diagnostic clues. Scientifica (Cairo). 2012;2012:598262.
- Bathina M, Hedge SP, Shanavaz AA, et al. Pruritic periumbilical plaque as a presentation of rare perforating dermatosis. Indian Dermatol Online J. 2020;11:68-71. doi:10.4103/idoj.IDOJ_95_19
- Sapadin AN, Lebwohl MG, Teich SA, et al. Periumbilical pseudoxanthoma elasticum associated with chronic renal failure and angioid streaks—apparent regression with hemodialysis. J Am Acad Dermatol. 1998;39:338-344.
- Jha AK, Zheeshan MD, Sinha BK, et al. Periumbilical perforating pseudoxanthoma elasticum: a rare case report. Dermatol Pract Concept. 2018;8:75-77. doi:10.5826/dpc.0802a02
- Ko JH, Shih YC, Huang YC, et al. Pseudoxanthoma elasticum. Lancet. 2013;381:565.
- Sherer DW, Singer G, Uribarri J, et al. Oral phosphate binders in the treatment of pseudoxanthoma elasticum. J Am Acad Dermatol. 2005;53:610-615.
- Lal NR, Bandyopadhyay D, Verma R, et al. Perforating calcific elastosis: revisiting a rare entity. Indian J Dermatol. 2018;63:186-188. doi:10.4103/ijd.IJD_111_17
- Kocatürk E, Kavala M, Zindanci I, et al. Periumbilical perforating pseudoxanthoma elasticum. Indian J Dermatol Venereol Leprol. 2009;75:329.
- Bressan AL, Vasconcelos BN, Silva RDS, et al. Periumbilical and periareolar perforating pseudoxanthoma elasticum. An Bras Dermatol. 2010;85:705-707. doi:10.1590/s0365-05962010000500018
- Hosen MJ, Lamoen A, De Paepe A, et al. Histopathology of pseudoxanthoma elasticum and related disorders: histological hallmarks and diagnostic clues. Scientifica (Cairo). 2012;2012:598262.
- Bathina M, Hedge SP, Shanavaz AA, et al. Pruritic periumbilical plaque as a presentation of rare perforating dermatosis. Indian Dermatol Online J. 2020;11:68-71. doi:10.4103/idoj.IDOJ_95_19
- Sapadin AN, Lebwohl MG, Teich SA, et al. Periumbilical pseudoxanthoma elasticum associated with chronic renal failure and angioid streaks—apparent regression with hemodialysis. J Am Acad Dermatol. 1998;39:338-344.
PRACTICE POINTS
- Perforating calcific elastosis (PCE) is a rare, localized, acquired variant of the inherited connective tissue disorder pseudoxanthoma elasticum (PXE).
- Histopathologic findings are identical for PCE and PXE, warranting differentiation via thorough clinical examination as well as further investigation of the patient’s medical and family history.
- Although there are no definitive treatments, most cases of PCE resolve spontaneously.
- Dermatologists should be aware of the importance of clinically differentiating PCE from PXE to prevent extensive workup, which can lead to unnecessary testing and increased morbidity in patients.
Early Treatment of Lyme Disease Prompted by Histopathologic Analysis of the Abdomen of an Engorged Tick
To the Editor:
Lyme disease is caused by spirochetes of the Borrelia burgdorferi sensu lato species complex and transmitted to humans by the bite of the Ixodes scapularis tick. It was first classified as a nationally notifiable disease in 1991, and the incidence has risen remarkably since then.1 More than 63,000 cases are reported annually to the Centers for Disease Control and Prevention; however, this number reflects severe underreporting, as the true incidence of the disease is projected to be closer to 476,000 cases per year.2 Additionally, 95% of US cases occur in the Northeast and upper Midwest.3 Given the pervasiveness of Lyme disease, early and reliable diagnostic methodology is critical, especially in cases in which the timeline of inoculation is unclear. We present a case of Lyme disease that was discovered during a routine dermatologic visit.
A 77-year-old White man with no relevant medical history presented to a dermatology clinic in west-central Virginia for a routine skin check. Physical examination revealed a well-appearing patient without overt skin abnormalities. However, on closer evaluation, a 0.2×0.1-cm engorged black I scapularis tick was visualized on the left lateral upper back. There was a surrounding zone of erythema that measured less than the 5-cm-diameter criterion for erythema migrans.1
Upon questioning, the patient reported that he was unaware of the tick and could not provide a timeline for inoculation. To ensure proper treatment, the tick was removed in the office and a specimen was sent for histopathology. The arthropod was formalin fixed and paraffin embedded, and it was examined using hematoxylin and eosin and Warthin-Starry stains. Histopathology of the specimen revealed a blood-engorged arthropod. Warthin-Starry stain of the abdomen of the tick highlighted tiny strandlike spirochetes within the gut that were compatible with B burgdorferi (Figure). This finding prompted treatment with a 3-week course of doxycycline. Following treatment, erythema resolved. The patient experienced no sequelae.
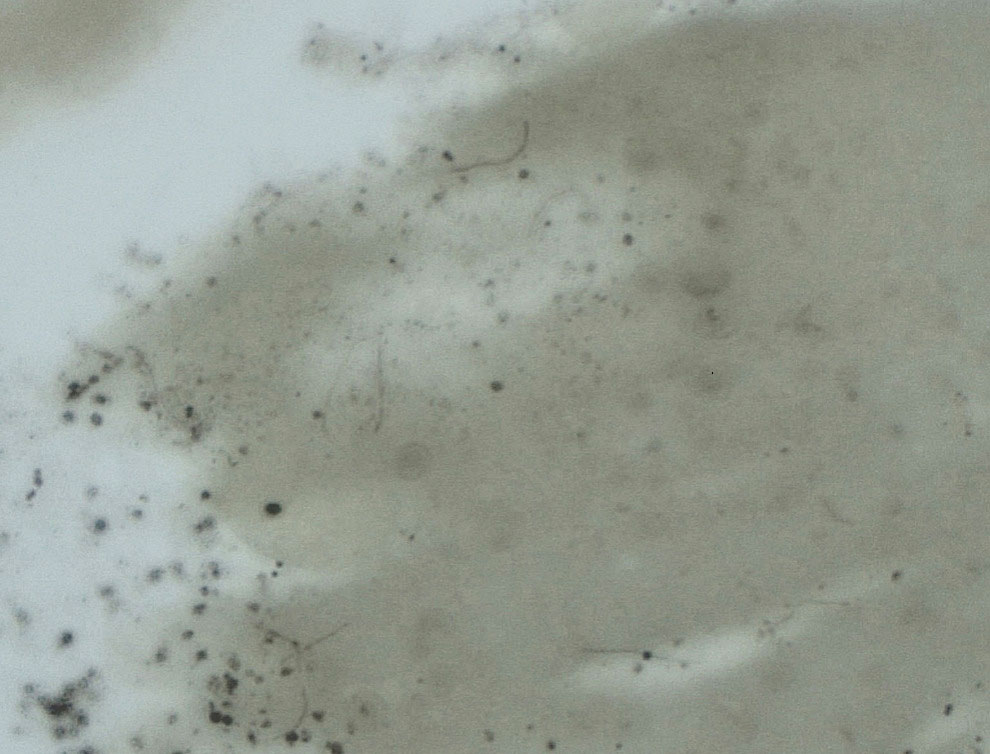
Lyme disease can cause a range of serious complications if left untreated, including arthritis, neurologic deficits, and heart block. During the early stages of disease, the sensitivity and specificity of diagnostic methods such as serologic testing are limited.4 The gold standard for the diagnosis of Lyme disease comprises culture and subsequent confirmation by polymerase chain reaction.1 However, cultivation of B burgdorferi is challenging.5 The Centers for Disease Control and Prevention recommends 2-tiered serologic antibody analysis, which has 27% sensitivity during the first week of cutaneous symptoms, and involves an enzyme-linked immunoassay followed by reflexive immunoblotting for positive or indeterminate cases.2,6 The precision of this method is limited by several variables; for example, seroconversion fails to occur in approximately 40% of cases, even after proven exposure to the spirochete.7 Furthermore, the sensitivity of the test is particularly low during the first 4 to 6 weeks of infection—before the body mounts a proper immune response; fewer than 50% of patients exhibit a positive response to the test at initial presentation.3
Clinical diagnosis of Lyme disease is possible, though the pathognomonic erythema migrans rash can be delayed for as long as 30 days and remains absent in 20% to 30% of patients.1 Prophylactic treatment can be offered to individuals who reside in a hyperendemic area and have a rash or have had an engorged Ixodes tick attached for longer than 36 hours.8
More definitive techniques for early diagnosis are needed to enable selective and accurate treatment. The standard of care for Lyme disease includes a 10-day course of doxycycline or a 14-day course of cefuroxime axetil or amoxicillin.9 Many patients tolerate treatment and achieve resolution of disease, but antibiotics are not benign, as some patients experience drug-related adverse effects such as photosensitivity, urticaria, diarrhea, nausea, vomiting, esophagitis, hepatotoxicity, and the Jarisch-Herxheimer reaction (fever, chills, rigors, nausea and vomiting, headache, tachycardia, hypotension, hyperventilation, flushing, myalgia, and exacerbation of lesions).10,11 In a group of 123 patients with Lyme disease, 30% treated with cefuroxime axetil and 32% treated with doxycycline had 1 or more drug-related adverse events.10 Additionally, avoidable antibiotic use is associated with increasing antibiotic resistance.12 Improved diagnostic accuracy would prevent unnecessary treatment. Galan and colleagues7 reported that Warthin-Starry staining of prepared sections of the abdomen of a tick allowed for detection of B burgdorferi with a sensitivity of 71% and specificity of 83%. This technique did not delay the final biopsy report and may be a promising adjunct to the diagnosis of early Lyme disease.7
Anecdotally, many patients who present with an attached and engorged tick are unaware of the timeline of their exposure. Histologic analysis of a removed tick could aid in early clinical decision-making—ie, when the diagnosis is unclear and treatment guidelines vary by region and circumstance. Improved sensitivity and specificity of diagnosis can prevent unnecessary antibiotic treatment, which is associated with adverse effects and escalation of antibiotic resistance.
- Borchers AT, Keen CL, Huntley AC, et al. Lyme disease: a rigorous review of diagnostic criteria and treatment. J Autoimmun. 2015;57:82-115. doi:10.1016/j.jaut.2014.09.004
- Centers for Disease Control and Prevention. Lyme disease: data and surveillance. February 14, 2024. Accessed March 5, 2024. https://www.cdc.gov/lyme/datasurveillance/index.html
- Marques AR. Laboratory diagnosis of Lyme disease. Infect Dis Clin North Am. 2015;29:295-307. doi:10.1016/j.idc.2015.02.005
- Bratton RL, Whiteside JW, Hovan MJ, et al. Diagnosis and treatment of Lyme disease. Mayo Clin Proc. 2008;83:566-571. doi:10.4065/83.5.566
- Berger B, Johnson R, Kodner C. Cultivation of Borrelia burgdorferi from human tick bite sites: a guide to the risk of infection. J Am Acad Dermatol. 1995;32(2 pt 1):184-187. doi:10.1016/0190-9622(95)90123-x
- Branda JA, Linskey K, Kim YA, et al. Two-tiered antibody testing for Lyme disease with use of 2 enzyme immunoassays, a whole-cell sonicate enzyme immunoassay followed by a VlsE C6 peptide enzyme immunoassay. Clin Infect Dis. 2011;53:541-547. doi:10.1093/cid/cir464
- Galan A, Kupernik P, Cowper SE. Detection of Borrelia in Ixodes scapularis ticks by silver stain, immunohistochemical and direct immunofluorescent methods. J Cutan Pathol. 2018;45:473-477. doi:10.1111/cup.13143
- Nadelman RB, Nowakowski J, Fish D, et al; Prophylaxis with single-dose doxycycline for the prevention of Lyme disease after an Ixodes scapularis tick bite. N Engl J Med. 2001;345:79-84. doi:10.1056/NEJM200107123450201
- Lantos PM, Rumbaugh J, Bockenstedt LK, et al. Clinical practice guidelines by the Infectious Diseases Society of America (IDSA), American Academy of Neurology (AAN), and American College of Rheumatology (ACR): 2020 guidelines for the prevention, diagnosis, and treatment of Lyme disease. Arthritis Rheumatol. 2021;73:12-20. doi:10.1002/art.41562
- Nadelman RB, Luger SW, Frank E, et al. Comparison of cefuroxime axetil and doxycycline in the treatment of early Lyme disease. Ann Intern Med. 1992;117:273-280. doi:10.7326/0003-4819-117-4-273
- Gresser U. Amoxicillin–clavulanic acid therapy may be associated with severe side effects—review of the literature. Eur J Med Res. 2001;6:139-149.
- Nathan C, Cars O. Antibiotic resistance—problems, progress, and prospects. N Engl J Med. 2014;371:1761-1763. doi:10.1056/NEJMp1408040
To the Editor:
Lyme disease is caused by spirochetes of the Borrelia burgdorferi sensu lato species complex and transmitted to humans by the bite of the Ixodes scapularis tick. It was first classified as a nationally notifiable disease in 1991, and the incidence has risen remarkably since then.1 More than 63,000 cases are reported annually to the Centers for Disease Control and Prevention; however, this number reflects severe underreporting, as the true incidence of the disease is projected to be closer to 476,000 cases per year.2 Additionally, 95% of US cases occur in the Northeast and upper Midwest.3 Given the pervasiveness of Lyme disease, early and reliable diagnostic methodology is critical, especially in cases in which the timeline of inoculation is unclear. We present a case of Lyme disease that was discovered during a routine dermatologic visit.
A 77-year-old White man with no relevant medical history presented to a dermatology clinic in west-central Virginia for a routine skin check. Physical examination revealed a well-appearing patient without overt skin abnormalities. However, on closer evaluation, a 0.2×0.1-cm engorged black I scapularis tick was visualized on the left lateral upper back. There was a surrounding zone of erythema that measured less than the 5-cm-diameter criterion for erythema migrans.1
Upon questioning, the patient reported that he was unaware of the tick and could not provide a timeline for inoculation. To ensure proper treatment, the tick was removed in the office and a specimen was sent for histopathology. The arthropod was formalin fixed and paraffin embedded, and it was examined using hematoxylin and eosin and Warthin-Starry stains. Histopathology of the specimen revealed a blood-engorged arthropod. Warthin-Starry stain of the abdomen of the tick highlighted tiny strandlike spirochetes within the gut that were compatible with B burgdorferi (Figure). This finding prompted treatment with a 3-week course of doxycycline. Following treatment, erythema resolved. The patient experienced no sequelae.

Lyme disease can cause a range of serious complications if left untreated, including arthritis, neurologic deficits, and heart block. During the early stages of disease, the sensitivity and specificity of diagnostic methods such as serologic testing are limited.4 The gold standard for the diagnosis of Lyme disease comprises culture and subsequent confirmation by polymerase chain reaction.1 However, cultivation of B burgdorferi is challenging.5 The Centers for Disease Control and Prevention recommends 2-tiered serologic antibody analysis, which has 27% sensitivity during the first week of cutaneous symptoms, and involves an enzyme-linked immunoassay followed by reflexive immunoblotting for positive or indeterminate cases.2,6 The precision of this method is limited by several variables; for example, seroconversion fails to occur in approximately 40% of cases, even after proven exposure to the spirochete.7 Furthermore, the sensitivity of the test is particularly low during the first 4 to 6 weeks of infection—before the body mounts a proper immune response; fewer than 50% of patients exhibit a positive response to the test at initial presentation.3
Clinical diagnosis of Lyme disease is possible, though the pathognomonic erythema migrans rash can be delayed for as long as 30 days and remains absent in 20% to 30% of patients.1 Prophylactic treatment can be offered to individuals who reside in a hyperendemic area and have a rash or have had an engorged Ixodes tick attached for longer than 36 hours.8
More definitive techniques for early diagnosis are needed to enable selective and accurate treatment. The standard of care for Lyme disease includes a 10-day course of doxycycline or a 14-day course of cefuroxime axetil or amoxicillin.9 Many patients tolerate treatment and achieve resolution of disease, but antibiotics are not benign, as some patients experience drug-related adverse effects such as photosensitivity, urticaria, diarrhea, nausea, vomiting, esophagitis, hepatotoxicity, and the Jarisch-Herxheimer reaction (fever, chills, rigors, nausea and vomiting, headache, tachycardia, hypotension, hyperventilation, flushing, myalgia, and exacerbation of lesions).10,11 In a group of 123 patients with Lyme disease, 30% treated with cefuroxime axetil and 32% treated with doxycycline had 1 or more drug-related adverse events.10 Additionally, avoidable antibiotic use is associated with increasing antibiotic resistance.12 Improved diagnostic accuracy would prevent unnecessary treatment. Galan and colleagues7 reported that Warthin-Starry staining of prepared sections of the abdomen of a tick allowed for detection of B burgdorferi with a sensitivity of 71% and specificity of 83%. This technique did not delay the final biopsy report and may be a promising adjunct to the diagnosis of early Lyme disease.7
Anecdotally, many patients who present with an attached and engorged tick are unaware of the timeline of their exposure. Histologic analysis of a removed tick could aid in early clinical decision-making—ie, when the diagnosis is unclear and treatment guidelines vary by region and circumstance. Improved sensitivity and specificity of diagnosis can prevent unnecessary antibiotic treatment, which is associated with adverse effects and escalation of antibiotic resistance.
To the Editor:
Lyme disease is caused by spirochetes of the Borrelia burgdorferi sensu lato species complex and transmitted to humans by the bite of the Ixodes scapularis tick. It was first classified as a nationally notifiable disease in 1991, and the incidence has risen remarkably since then.1 More than 63,000 cases are reported annually to the Centers for Disease Control and Prevention; however, this number reflects severe underreporting, as the true incidence of the disease is projected to be closer to 476,000 cases per year.2 Additionally, 95% of US cases occur in the Northeast and upper Midwest.3 Given the pervasiveness of Lyme disease, early and reliable diagnostic methodology is critical, especially in cases in which the timeline of inoculation is unclear. We present a case of Lyme disease that was discovered during a routine dermatologic visit.
A 77-year-old White man with no relevant medical history presented to a dermatology clinic in west-central Virginia for a routine skin check. Physical examination revealed a well-appearing patient without overt skin abnormalities. However, on closer evaluation, a 0.2×0.1-cm engorged black I scapularis tick was visualized on the left lateral upper back. There was a surrounding zone of erythema that measured less than the 5-cm-diameter criterion for erythema migrans.1
Upon questioning, the patient reported that he was unaware of the tick and could not provide a timeline for inoculation. To ensure proper treatment, the tick was removed in the office and a specimen was sent for histopathology. The arthropod was formalin fixed and paraffin embedded, and it was examined using hematoxylin and eosin and Warthin-Starry stains. Histopathology of the specimen revealed a blood-engorged arthropod. Warthin-Starry stain of the abdomen of the tick highlighted tiny strandlike spirochetes within the gut that were compatible with B burgdorferi (Figure). This finding prompted treatment with a 3-week course of doxycycline. Following treatment, erythema resolved. The patient experienced no sequelae.

Lyme disease can cause a range of serious complications if left untreated, including arthritis, neurologic deficits, and heart block. During the early stages of disease, the sensitivity and specificity of diagnostic methods such as serologic testing are limited.4 The gold standard for the diagnosis of Lyme disease comprises culture and subsequent confirmation by polymerase chain reaction.1 However, cultivation of B burgdorferi is challenging.5 The Centers for Disease Control and Prevention recommends 2-tiered serologic antibody analysis, which has 27% sensitivity during the first week of cutaneous symptoms, and involves an enzyme-linked immunoassay followed by reflexive immunoblotting for positive or indeterminate cases.2,6 The precision of this method is limited by several variables; for example, seroconversion fails to occur in approximately 40% of cases, even after proven exposure to the spirochete.7 Furthermore, the sensitivity of the test is particularly low during the first 4 to 6 weeks of infection—before the body mounts a proper immune response; fewer than 50% of patients exhibit a positive response to the test at initial presentation.3
Clinical diagnosis of Lyme disease is possible, though the pathognomonic erythema migrans rash can be delayed for as long as 30 days and remains absent in 20% to 30% of patients.1 Prophylactic treatment can be offered to individuals who reside in a hyperendemic area and have a rash or have had an engorged Ixodes tick attached for longer than 36 hours.8
More definitive techniques for early diagnosis are needed to enable selective and accurate treatment. The standard of care for Lyme disease includes a 10-day course of doxycycline or a 14-day course of cefuroxime axetil or amoxicillin.9 Many patients tolerate treatment and achieve resolution of disease, but antibiotics are not benign, as some patients experience drug-related adverse effects such as photosensitivity, urticaria, diarrhea, nausea, vomiting, esophagitis, hepatotoxicity, and the Jarisch-Herxheimer reaction (fever, chills, rigors, nausea and vomiting, headache, tachycardia, hypotension, hyperventilation, flushing, myalgia, and exacerbation of lesions).10,11 In a group of 123 patients with Lyme disease, 30% treated with cefuroxime axetil and 32% treated with doxycycline had 1 or more drug-related adverse events.10 Additionally, avoidable antibiotic use is associated with increasing antibiotic resistance.12 Improved diagnostic accuracy would prevent unnecessary treatment. Galan and colleagues7 reported that Warthin-Starry staining of prepared sections of the abdomen of a tick allowed for detection of B burgdorferi with a sensitivity of 71% and specificity of 83%. This technique did not delay the final biopsy report and may be a promising adjunct to the diagnosis of early Lyme disease.7
Anecdotally, many patients who present with an attached and engorged tick are unaware of the timeline of their exposure. Histologic analysis of a removed tick could aid in early clinical decision-making—ie, when the diagnosis is unclear and treatment guidelines vary by region and circumstance. Improved sensitivity and specificity of diagnosis can prevent unnecessary antibiotic treatment, which is associated with adverse effects and escalation of antibiotic resistance.
- Borchers AT, Keen CL, Huntley AC, et al. Lyme disease: a rigorous review of diagnostic criteria and treatment. J Autoimmun. 2015;57:82-115. doi:10.1016/j.jaut.2014.09.004
- Centers for Disease Control and Prevention. Lyme disease: data and surveillance. February 14, 2024. Accessed March 5, 2024. https://www.cdc.gov/lyme/datasurveillance/index.html
- Marques AR. Laboratory diagnosis of Lyme disease. Infect Dis Clin North Am. 2015;29:295-307. doi:10.1016/j.idc.2015.02.005
- Bratton RL, Whiteside JW, Hovan MJ, et al. Diagnosis and treatment of Lyme disease. Mayo Clin Proc. 2008;83:566-571. doi:10.4065/83.5.566
- Berger B, Johnson R, Kodner C. Cultivation of Borrelia burgdorferi from human tick bite sites: a guide to the risk of infection. J Am Acad Dermatol. 1995;32(2 pt 1):184-187. doi:10.1016/0190-9622(95)90123-x
- Branda JA, Linskey K, Kim YA, et al. Two-tiered antibody testing for Lyme disease with use of 2 enzyme immunoassays, a whole-cell sonicate enzyme immunoassay followed by a VlsE C6 peptide enzyme immunoassay. Clin Infect Dis. 2011;53:541-547. doi:10.1093/cid/cir464
- Galan A, Kupernik P, Cowper SE. Detection of Borrelia in Ixodes scapularis ticks by silver stain, immunohistochemical and direct immunofluorescent methods. J Cutan Pathol. 2018;45:473-477. doi:10.1111/cup.13143
- Nadelman RB, Nowakowski J, Fish D, et al; Prophylaxis with single-dose doxycycline for the prevention of Lyme disease after an Ixodes scapularis tick bite. N Engl J Med. 2001;345:79-84. doi:10.1056/NEJM200107123450201
- Lantos PM, Rumbaugh J, Bockenstedt LK, et al. Clinical practice guidelines by the Infectious Diseases Society of America (IDSA), American Academy of Neurology (AAN), and American College of Rheumatology (ACR): 2020 guidelines for the prevention, diagnosis, and treatment of Lyme disease. Arthritis Rheumatol. 2021;73:12-20. doi:10.1002/art.41562
- Nadelman RB, Luger SW, Frank E, et al. Comparison of cefuroxime axetil and doxycycline in the treatment of early Lyme disease. Ann Intern Med. 1992;117:273-280. doi:10.7326/0003-4819-117-4-273
- Gresser U. Amoxicillin–clavulanic acid therapy may be associated with severe side effects—review of the literature. Eur J Med Res. 2001;6:139-149.
- Nathan C, Cars O. Antibiotic resistance—problems, progress, and prospects. N Engl J Med. 2014;371:1761-1763. doi:10.1056/NEJMp1408040
- Borchers AT, Keen CL, Huntley AC, et al. Lyme disease: a rigorous review of diagnostic criteria and treatment. J Autoimmun. 2015;57:82-115. doi:10.1016/j.jaut.2014.09.004
- Centers for Disease Control and Prevention. Lyme disease: data and surveillance. February 14, 2024. Accessed March 5, 2024. https://www.cdc.gov/lyme/datasurveillance/index.html
- Marques AR. Laboratory diagnosis of Lyme disease. Infect Dis Clin North Am. 2015;29:295-307. doi:10.1016/j.idc.2015.02.005
- Bratton RL, Whiteside JW, Hovan MJ, et al. Diagnosis and treatment of Lyme disease. Mayo Clin Proc. 2008;83:566-571. doi:10.4065/83.5.566
- Berger B, Johnson R, Kodner C. Cultivation of Borrelia burgdorferi from human tick bite sites: a guide to the risk of infection. J Am Acad Dermatol. 1995;32(2 pt 1):184-187. doi:10.1016/0190-9622(95)90123-x
- Branda JA, Linskey K, Kim YA, et al. Two-tiered antibody testing for Lyme disease with use of 2 enzyme immunoassays, a whole-cell sonicate enzyme immunoassay followed by a VlsE C6 peptide enzyme immunoassay. Clin Infect Dis. 2011;53:541-547. doi:10.1093/cid/cir464
- Galan A, Kupernik P, Cowper SE. Detection of Borrelia in Ixodes scapularis ticks by silver stain, immunohistochemical and direct immunofluorescent methods. J Cutan Pathol. 2018;45:473-477. doi:10.1111/cup.13143
- Nadelman RB, Nowakowski J, Fish D, et al; Prophylaxis with single-dose doxycycline for the prevention of Lyme disease after an Ixodes scapularis tick bite. N Engl J Med. 2001;345:79-84. doi:10.1056/NEJM200107123450201
- Lantos PM, Rumbaugh J, Bockenstedt LK, et al. Clinical practice guidelines by the Infectious Diseases Society of America (IDSA), American Academy of Neurology (AAN), and American College of Rheumatology (ACR): 2020 guidelines for the prevention, diagnosis, and treatment of Lyme disease. Arthritis Rheumatol. 2021;73:12-20. doi:10.1002/art.41562
- Nadelman RB, Luger SW, Frank E, et al. Comparison of cefuroxime axetil and doxycycline in the treatment of early Lyme disease. Ann Intern Med. 1992;117:273-280. doi:10.7326/0003-4819-117-4-273
- Gresser U. Amoxicillin–clavulanic acid therapy may be associated with severe side effects—review of the literature. Eur J Med Res. 2001;6:139-149.
- Nathan C, Cars O. Antibiotic resistance—problems, progress, and prospects. N Engl J Med. 2014;371:1761-1763. doi:10.1056/NEJMp1408040
PRACTICE POINTS
- Lyme disease is increasingly common in the United States.
- Lyme disease can cause debilitating sequelae if left untreated, including arthritis, neurologic deficits, and heart block.
- Diagnostic methods for identifying early Lyme disease have limited sensitivity and specificity, necessitating alternative strategies for making an accurate diagnosis and initiating treatment.
Genes May Govern Intestinal Sites of Pediatric Crohn’s
, a small analysis in Cellular and Molecular Gastroenterology and Hepatology suggests.
Richard Kellermayer, MD, PhD, professor of pediatrics in the Section of Pediatric Gastroenterology, Hepatology and Nutrition at Baylor College of Medicine in Houston, Texas, and colleagues compared the genetic makeup of patients based on their Crohn’s disease location — predominantly in the small bowel (L4) or predominantly in the colon (L2 and/or L3). They then generated bipartite networks of susceptibility genes to study the polygenic background of the disease subtypes. They hypothesize that such networks may govern where a patient develops Crohn’s disease.
According to current understanding, as Dr. Kellermayer told GI & Hepatology News, most autoimmune disorders, CD included, develop in people with a genetic predisposition after serial environmental insults between conception and young adulthood. “As opposed to single-gene-associated genetic disorders, autoimmune diseases are linked to several hundred genes in which subtle anomalies can work in concert to predispose someone to a certain disorder,” he said. “We hope our findings will guide the development of personalized treatments based on the disease location at diagnosis to advance precision medicine.”
CD cases
Eight cases of SB-CD and 11 of C-CD met the inclusion criteria. Mean age at CD diagnosis was about 11 years for both subtypes, while 36.3% of patients with C-CD were female vs 25% of those with SB-CD. Ethnicity was 72.2% White in the C-CD group and 87.5% in the SB-CD group.
As to the main ileocolonic locations according to the Paris Classification of pediatric inflammatory bowel disease, 54.5% in the C-CD group had involvement at L2 and 45.5% at L3. In SB-CD cases, 100% had disease at L4b, 37.5% at L4, and 50% at L1.
The researchers identified 115 single-nucleotide polymorphisms (SNPs) with a combined annotation-dependent depletion (CADD) score on Phil’s Read Editor (PHRED) of >10 that was associated with 97 genes. PHRED is a computer program measuring the quality of the identification of nucleobases generated by automated DNA sequencing and scores the deleteriousness of single-nucleotide variants. The identified genes in this study had a significantly (P < .01) different allele variation between C-CD and SB-CD.
Among the top 28 candidates was an SNP in the EFNA3 gene with a CADD score > 20 for differentiating between the two phenotypically distinct CD groups. Furthermore, the EFNA3 rs17723260 (predicted to be deleterious) was found to have a significantly lower allele frequency (4.5%) in C-CD compared with its allele frequency of 37.5% in SB-CD (chi square P = .0097).
“This finding indicates that EFNA3 might play a role in modulating colonic inflammation, in which a deleterious genetic defect might provide protection against colitis (and direct autoimmunity against the proximal small bowel) in the polygenic background of CD,” the investigators wrote.
EFNA3 has been linked to both CD and ulcerative colitis. Another four genes associated with the top five SNP candidates had already been connected with IBD or mammalian intestinal inflammation.
According to the authors, the biomedical literature and mouse model findings “implicate the translational relevance of our candidate gene compendium for directing colon- vs small-bowel–predominant CD development.” They hope the findings will be replicated in larger CD cohorts differentiated by disease location. “Our work may set the nidus for CD subtype–based precision medicine by guiding individualized treatment strategies,” they wrote.
This study was supported by the ProKIIDS Network of the Crohn’s and Colitis Foundation and the Public Health Service. It was also supported by the Wagner, Frugoni, and Klaasmeyer families’ Gutsy Kids Fund and by the DR and GL Laws Fund. The authors disclosed no conflicts of interest.
, a small analysis in Cellular and Molecular Gastroenterology and Hepatology suggests.
Richard Kellermayer, MD, PhD, professor of pediatrics in the Section of Pediatric Gastroenterology, Hepatology and Nutrition at Baylor College of Medicine in Houston, Texas, and colleagues compared the genetic makeup of patients based on their Crohn’s disease location — predominantly in the small bowel (L4) or predominantly in the colon (L2 and/or L3). They then generated bipartite networks of susceptibility genes to study the polygenic background of the disease subtypes. They hypothesize that such networks may govern where a patient develops Crohn’s disease.
According to current understanding, as Dr. Kellermayer told GI & Hepatology News, most autoimmune disorders, CD included, develop in people with a genetic predisposition after serial environmental insults between conception and young adulthood. “As opposed to single-gene-associated genetic disorders, autoimmune diseases are linked to several hundred genes in which subtle anomalies can work in concert to predispose someone to a certain disorder,” he said. “We hope our findings will guide the development of personalized treatments based on the disease location at diagnosis to advance precision medicine.”
CD cases
Eight cases of SB-CD and 11 of C-CD met the inclusion criteria. Mean age at CD diagnosis was about 11 years for both subtypes, while 36.3% of patients with C-CD were female vs 25% of those with SB-CD. Ethnicity was 72.2% White in the C-CD group and 87.5% in the SB-CD group.
As to the main ileocolonic locations according to the Paris Classification of pediatric inflammatory bowel disease, 54.5% in the C-CD group had involvement at L2 and 45.5% at L3. In SB-CD cases, 100% had disease at L4b, 37.5% at L4, and 50% at L1.
The researchers identified 115 single-nucleotide polymorphisms (SNPs) with a combined annotation-dependent depletion (CADD) score on Phil’s Read Editor (PHRED) of >10 that was associated with 97 genes. PHRED is a computer program measuring the quality of the identification of nucleobases generated by automated DNA sequencing and scores the deleteriousness of single-nucleotide variants. The identified genes in this study had a significantly (P < .01) different allele variation between C-CD and SB-CD.
Among the top 28 candidates was an SNP in the EFNA3 gene with a CADD score > 20 for differentiating between the two phenotypically distinct CD groups. Furthermore, the EFNA3 rs17723260 (predicted to be deleterious) was found to have a significantly lower allele frequency (4.5%) in C-CD compared with its allele frequency of 37.5% in SB-CD (chi square P = .0097).
“This finding indicates that EFNA3 might play a role in modulating colonic inflammation, in which a deleterious genetic defect might provide protection against colitis (and direct autoimmunity against the proximal small bowel) in the polygenic background of CD,” the investigators wrote.
EFNA3 has been linked to both CD and ulcerative colitis. Another four genes associated with the top five SNP candidates had already been connected with IBD or mammalian intestinal inflammation.
According to the authors, the biomedical literature and mouse model findings “implicate the translational relevance of our candidate gene compendium for directing colon- vs small-bowel–predominant CD development.” They hope the findings will be replicated in larger CD cohorts differentiated by disease location. “Our work may set the nidus for CD subtype–based precision medicine by guiding individualized treatment strategies,” they wrote.
This study was supported by the ProKIIDS Network of the Crohn’s and Colitis Foundation and the Public Health Service. It was also supported by the Wagner, Frugoni, and Klaasmeyer families’ Gutsy Kids Fund and by the DR and GL Laws Fund. The authors disclosed no conflicts of interest.
, a small analysis in Cellular and Molecular Gastroenterology and Hepatology suggests.
Richard Kellermayer, MD, PhD, professor of pediatrics in the Section of Pediatric Gastroenterology, Hepatology and Nutrition at Baylor College of Medicine in Houston, Texas, and colleagues compared the genetic makeup of patients based on their Crohn’s disease location — predominantly in the small bowel (L4) or predominantly in the colon (L2 and/or L3). They then generated bipartite networks of susceptibility genes to study the polygenic background of the disease subtypes. They hypothesize that such networks may govern where a patient develops Crohn’s disease.
According to current understanding, as Dr. Kellermayer told GI & Hepatology News, most autoimmune disorders, CD included, develop in people with a genetic predisposition after serial environmental insults between conception and young adulthood. “As opposed to single-gene-associated genetic disorders, autoimmune diseases are linked to several hundred genes in which subtle anomalies can work in concert to predispose someone to a certain disorder,” he said. “We hope our findings will guide the development of personalized treatments based on the disease location at diagnosis to advance precision medicine.”
CD cases
Eight cases of SB-CD and 11 of C-CD met the inclusion criteria. Mean age at CD diagnosis was about 11 years for both subtypes, while 36.3% of patients with C-CD were female vs 25% of those with SB-CD. Ethnicity was 72.2% White in the C-CD group and 87.5% in the SB-CD group.
As to the main ileocolonic locations according to the Paris Classification of pediatric inflammatory bowel disease, 54.5% in the C-CD group had involvement at L2 and 45.5% at L3. In SB-CD cases, 100% had disease at L4b, 37.5% at L4, and 50% at L1.
The researchers identified 115 single-nucleotide polymorphisms (SNPs) with a combined annotation-dependent depletion (CADD) score on Phil’s Read Editor (PHRED) of >10 that was associated with 97 genes. PHRED is a computer program measuring the quality of the identification of nucleobases generated by automated DNA sequencing and scores the deleteriousness of single-nucleotide variants. The identified genes in this study had a significantly (P < .01) different allele variation between C-CD and SB-CD.
Among the top 28 candidates was an SNP in the EFNA3 gene with a CADD score > 20 for differentiating between the two phenotypically distinct CD groups. Furthermore, the EFNA3 rs17723260 (predicted to be deleterious) was found to have a significantly lower allele frequency (4.5%) in C-CD compared with its allele frequency of 37.5% in SB-CD (chi square P = .0097).
“This finding indicates that EFNA3 might play a role in modulating colonic inflammation, in which a deleterious genetic defect might provide protection against colitis (and direct autoimmunity against the proximal small bowel) in the polygenic background of CD,” the investigators wrote.
EFNA3 has been linked to both CD and ulcerative colitis. Another four genes associated with the top five SNP candidates had already been connected with IBD or mammalian intestinal inflammation.
According to the authors, the biomedical literature and mouse model findings “implicate the translational relevance of our candidate gene compendium for directing colon- vs small-bowel–predominant CD development.” They hope the findings will be replicated in larger CD cohorts differentiated by disease location. “Our work may set the nidus for CD subtype–based precision medicine by guiding individualized treatment strategies,” they wrote.
This study was supported by the ProKIIDS Network of the Crohn’s and Colitis Foundation and the Public Health Service. It was also supported by the Wagner, Frugoni, and Klaasmeyer families’ Gutsy Kids Fund and by the DR and GL Laws Fund. The authors disclosed no conflicts of interest.
FROM CELLULAR AND MOLECULAR GASTROENTEROLOGY AND HEPATOLOGY
Negative Colonoscopy? 15-Year Screening Interval May Be Safe
TOPLINE:
a population-based study suggests.
METHODOLOGY:
- Using Swedish nationwide registry data, researchers compared 110,074 individuals who had a first colonoscopy with negative findings for CRC at age 45-69 years (exposed group) with more than 1.9 million matched controls who either did not have a colonoscopy during the study period or underwent colonoscopy that led to a CRC diagnosis.
- They calculated 10-year standardized incidence ratio (SIR) and standardized mortality ratio (SMR) to compare risks for CRC and CRC-specific death in the exposed and control groups based on different follow-up screening intervals.
TAKEAWAY:
- During up to 29 years of follow-up, 484 incident CRCs and 112 CRC deaths occurred in the group with a negative initial colonoscopy.
- Up to 15 years after negative colonoscopy, the 10-year cumulative risk for CRC and CRC mortality was lower than in the control group, with an SIR of 0.72 and SMR of 0.55, respectively.
- Extending the screening interval from 10 to 15 years would miss early detection of only two CRC cases and prevention of only one CRC death per 1000 individuals, while potentially avoiding 1000 colonoscopies.
IN PRACTICE:
“This study provides evidence for recommending a longer colonoscopy screening interval than what is currently recommended in most guidelines for populations with no familial risk of CRC,” the authors wrote. “A longer interval between colonoscopy screenings could be beneficial in avoiding unnecessary invasive examinations.”
SOURCE:
The study, with first author Qunfeng Liang, MSc, with the German Cancer Research Center, Heidelberg, Germany, was published online on May 2 in JAMA Oncology.
LIMITATIONS:
The study population primarily included White individuals, particularly ethnic Swedish individuals, so external validation would be necessary to generalize the recommendation to other populations. The researchers lacked data on non-endoscopic tests, such as fecal occult blood tests, which could have been performed as a substitution for colonoscopy during the interval between colonoscopy screenings.
DISCLOSURES:
The study had no specific funding. The authors had no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
a population-based study suggests.
METHODOLOGY:
- Using Swedish nationwide registry data, researchers compared 110,074 individuals who had a first colonoscopy with negative findings for CRC at age 45-69 years (exposed group) with more than 1.9 million matched controls who either did not have a colonoscopy during the study period or underwent colonoscopy that led to a CRC diagnosis.
- They calculated 10-year standardized incidence ratio (SIR) and standardized mortality ratio (SMR) to compare risks for CRC and CRC-specific death in the exposed and control groups based on different follow-up screening intervals.
TAKEAWAY:
- During up to 29 years of follow-up, 484 incident CRCs and 112 CRC deaths occurred in the group with a negative initial colonoscopy.
- Up to 15 years after negative colonoscopy, the 10-year cumulative risk for CRC and CRC mortality was lower than in the control group, with an SIR of 0.72 and SMR of 0.55, respectively.
- Extending the screening interval from 10 to 15 years would miss early detection of only two CRC cases and prevention of only one CRC death per 1000 individuals, while potentially avoiding 1000 colonoscopies.
IN PRACTICE:
“This study provides evidence for recommending a longer colonoscopy screening interval than what is currently recommended in most guidelines for populations with no familial risk of CRC,” the authors wrote. “A longer interval between colonoscopy screenings could be beneficial in avoiding unnecessary invasive examinations.”
SOURCE:
The study, with first author Qunfeng Liang, MSc, with the German Cancer Research Center, Heidelberg, Germany, was published online on May 2 in JAMA Oncology.
LIMITATIONS:
The study population primarily included White individuals, particularly ethnic Swedish individuals, so external validation would be necessary to generalize the recommendation to other populations. The researchers lacked data on non-endoscopic tests, such as fecal occult blood tests, which could have been performed as a substitution for colonoscopy during the interval between colonoscopy screenings.
DISCLOSURES:
The study had no specific funding. The authors had no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
a population-based study suggests.
METHODOLOGY:
- Using Swedish nationwide registry data, researchers compared 110,074 individuals who had a first colonoscopy with negative findings for CRC at age 45-69 years (exposed group) with more than 1.9 million matched controls who either did not have a colonoscopy during the study period or underwent colonoscopy that led to a CRC diagnosis.
- They calculated 10-year standardized incidence ratio (SIR) and standardized mortality ratio (SMR) to compare risks for CRC and CRC-specific death in the exposed and control groups based on different follow-up screening intervals.
TAKEAWAY:
- During up to 29 years of follow-up, 484 incident CRCs and 112 CRC deaths occurred in the group with a negative initial colonoscopy.
- Up to 15 years after negative colonoscopy, the 10-year cumulative risk for CRC and CRC mortality was lower than in the control group, with an SIR of 0.72 and SMR of 0.55, respectively.
- Extending the screening interval from 10 to 15 years would miss early detection of only two CRC cases and prevention of only one CRC death per 1000 individuals, while potentially avoiding 1000 colonoscopies.
IN PRACTICE:
“This study provides evidence for recommending a longer colonoscopy screening interval than what is currently recommended in most guidelines for populations with no familial risk of CRC,” the authors wrote. “A longer interval between colonoscopy screenings could be beneficial in avoiding unnecessary invasive examinations.”
SOURCE:
The study, with first author Qunfeng Liang, MSc, with the German Cancer Research Center, Heidelberg, Germany, was published online on May 2 in JAMA Oncology.
LIMITATIONS:
The study population primarily included White individuals, particularly ethnic Swedish individuals, so external validation would be necessary to generalize the recommendation to other populations. The researchers lacked data on non-endoscopic tests, such as fecal occult blood tests, which could have been performed as a substitution for colonoscopy during the interval between colonoscopy screenings.
DISCLOSURES:
The study had no specific funding. The authors had no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
Clinical Guidelines: Start Screening at Age 50 for Age-Related Hearing Loss
Clinical guidelines on age-related hearing loss (ARHL), published in Otolaryngology–Head and Neck Surgery, highlight referral recommendations for all clinicians, including primary care doctors, who often are the first clinicians to screen for and address the condition.
Betty S. Tsai Do, MD, with the department of head & neck surgery at Kaiser Permanente in Walnut Creek, California, is the first author for the guidelines, which recommend screening patients 50 years or older at the time of a healthcare encounter. They also detail when to test and refer.
Three ‘Strong Recommendations’
Three of the action points are labeled “strong recommendations.” They are:
- If screening suggests hearing loss, clinicians should conduct an audiogram or refer to a clinician who can conduct one.
- Clinicians should offer, or refer to a specialist who can offer, appropriately fit amplification, such as hearing aids.
- If patients have appropriately fit amplification and still have trouble with hearing and understanding speech, clinicians should refer patients to see if they are good candidates for a cochlear implant.
The authors note that ARHL is the most common sensory deficit seen in older patients, but it is underdiagnosed and undertreated. “Between ages 65 and 74, one in three adults experience hearing loss and almost 50% of those 75 years of age or older will report hearing loss according to the National Institute on Deafness and Other Communication Disorders.” Consequences of the untreated deficit, in addition to limiting ability to communicate, include higher risk of dementia, cardiovascular disease, depression, falls, and workplace marginalization.
Until now, there have been no evidence-based clinical guidelines on when to screen, test, and refer. Though previously proposed quality improvement measures have defined ARHL as starting at age 60, these guidelines include those 50 and older to promote earlier detection.
Guidelines Only Part of the Solution
While the guidelines are a step in the right direction, they won’t address some persistent barriers to changing practice, said Michael McKee, MD, MPH, a family medicine physician and co-director of the Center for Disability Health and Wellness at the University of Michigan in Ann Arbor, who was not part of the guideline team.
“I think [the guidelines] will raise the awareness on why it’s important to address hearing loss,” he says. “Many primary care providers don’t elevate hearing loss as a priority topic. The problem is that we’re struggling with getting things in place to have a more supportive system to carry out those recommendations.”
Lack of Training and Support
The problems include lack of training on hearing loss for physicians, starting with medical school. Another complication is time: A conversation about hearing loss adds to the multitude of conversations a primary care provider is expected to have with their patients in a short visit.
Additionally, when hearing loss is suspected, an audiologist may be hard to find to perform the audiogram, Dr. McKee says. If patients agree to see an audiologist and that specialist finds hearing loss, patients may not want to wear a device due to stigma or may not be able to afford a device that will fit properly and truly benefit them because Medicare does not cover hearing aids.
“Only about 20-plus percent of those eligible for hearing aids get them,” he said. Hearing aids available over the counter help some people, but may be difficult to fit properly and may be hard for some to use correctly, he added.
“That comes back to the primary care provider, so it’s unfortunately a very unsatisfying course,” he said.
‘Primary Care Providers Do Value Guidelines’
However, “Primary care providers do value guidelines. They do value strong recommendations,” he said. We are trying to figure out how we can support people with unaddressed hearing loss in the primary care setting, Dr. McKee said. “Once we get there, we need to advocate for an expansion of coverage,” he said.
The authors note that the messages in the guidelines are important for all clinicians.
“The impact of hearing loss and screening should not be the sole responsibility of an audiologist, an otolaryngologist, nor primary care provider. Any time and place that a patient interacts with the healthcare system is an opportunity for preventive healthcare, such as hearing screening, to occur,” they write.
Funding for this research was provided by the American Academy of Otolaryngology–Head and Neck Surgery Foundation. Dr. Do and Dr. McKee report no relevant financial relationships. Full disclosures of the co-authors are listed with the full text of the paper.
Clinical guidelines on age-related hearing loss (ARHL), published in Otolaryngology–Head and Neck Surgery, highlight referral recommendations for all clinicians, including primary care doctors, who often are the first clinicians to screen for and address the condition.
Betty S. Tsai Do, MD, with the department of head & neck surgery at Kaiser Permanente in Walnut Creek, California, is the first author for the guidelines, which recommend screening patients 50 years or older at the time of a healthcare encounter. They also detail when to test and refer.
Three ‘Strong Recommendations’
Three of the action points are labeled “strong recommendations.” They are:
- If screening suggests hearing loss, clinicians should conduct an audiogram or refer to a clinician who can conduct one.
- Clinicians should offer, or refer to a specialist who can offer, appropriately fit amplification, such as hearing aids.
- If patients have appropriately fit amplification and still have trouble with hearing and understanding speech, clinicians should refer patients to see if they are good candidates for a cochlear implant.
The authors note that ARHL is the most common sensory deficit seen in older patients, but it is underdiagnosed and undertreated. “Between ages 65 and 74, one in three adults experience hearing loss and almost 50% of those 75 years of age or older will report hearing loss according to the National Institute on Deafness and Other Communication Disorders.” Consequences of the untreated deficit, in addition to limiting ability to communicate, include higher risk of dementia, cardiovascular disease, depression, falls, and workplace marginalization.
Until now, there have been no evidence-based clinical guidelines on when to screen, test, and refer. Though previously proposed quality improvement measures have defined ARHL as starting at age 60, these guidelines include those 50 and older to promote earlier detection.
Guidelines Only Part of the Solution
While the guidelines are a step in the right direction, they won’t address some persistent barriers to changing practice, said Michael McKee, MD, MPH, a family medicine physician and co-director of the Center for Disability Health and Wellness at the University of Michigan in Ann Arbor, who was not part of the guideline team.
“I think [the guidelines] will raise the awareness on why it’s important to address hearing loss,” he says. “Many primary care providers don’t elevate hearing loss as a priority topic. The problem is that we’re struggling with getting things in place to have a more supportive system to carry out those recommendations.”
Lack of Training and Support
The problems include lack of training on hearing loss for physicians, starting with medical school. Another complication is time: A conversation about hearing loss adds to the multitude of conversations a primary care provider is expected to have with their patients in a short visit.
Additionally, when hearing loss is suspected, an audiologist may be hard to find to perform the audiogram, Dr. McKee says. If patients agree to see an audiologist and that specialist finds hearing loss, patients may not want to wear a device due to stigma or may not be able to afford a device that will fit properly and truly benefit them because Medicare does not cover hearing aids.
“Only about 20-plus percent of those eligible for hearing aids get them,” he said. Hearing aids available over the counter help some people, but may be difficult to fit properly and may be hard for some to use correctly, he added.
“That comes back to the primary care provider, so it’s unfortunately a very unsatisfying course,” he said.
‘Primary Care Providers Do Value Guidelines’
However, “Primary care providers do value guidelines. They do value strong recommendations,” he said. We are trying to figure out how we can support people with unaddressed hearing loss in the primary care setting, Dr. McKee said. “Once we get there, we need to advocate for an expansion of coverage,” he said.
The authors note that the messages in the guidelines are important for all clinicians.
“The impact of hearing loss and screening should not be the sole responsibility of an audiologist, an otolaryngologist, nor primary care provider. Any time and place that a patient interacts with the healthcare system is an opportunity for preventive healthcare, such as hearing screening, to occur,” they write.
Funding for this research was provided by the American Academy of Otolaryngology–Head and Neck Surgery Foundation. Dr. Do and Dr. McKee report no relevant financial relationships. Full disclosures of the co-authors are listed with the full text of the paper.
Clinical guidelines on age-related hearing loss (ARHL), published in Otolaryngology–Head and Neck Surgery, highlight referral recommendations for all clinicians, including primary care doctors, who often are the first clinicians to screen for and address the condition.
Betty S. Tsai Do, MD, with the department of head & neck surgery at Kaiser Permanente in Walnut Creek, California, is the first author for the guidelines, which recommend screening patients 50 years or older at the time of a healthcare encounter. They also detail when to test and refer.
Three ‘Strong Recommendations’
Three of the action points are labeled “strong recommendations.” They are:
- If screening suggests hearing loss, clinicians should conduct an audiogram or refer to a clinician who can conduct one.
- Clinicians should offer, or refer to a specialist who can offer, appropriately fit amplification, such as hearing aids.
- If patients have appropriately fit amplification and still have trouble with hearing and understanding speech, clinicians should refer patients to see if they are good candidates for a cochlear implant.
The authors note that ARHL is the most common sensory deficit seen in older patients, but it is underdiagnosed and undertreated. “Between ages 65 and 74, one in three adults experience hearing loss and almost 50% of those 75 years of age or older will report hearing loss according to the National Institute on Deafness and Other Communication Disorders.” Consequences of the untreated deficit, in addition to limiting ability to communicate, include higher risk of dementia, cardiovascular disease, depression, falls, and workplace marginalization.
Until now, there have been no evidence-based clinical guidelines on when to screen, test, and refer. Though previously proposed quality improvement measures have defined ARHL as starting at age 60, these guidelines include those 50 and older to promote earlier detection.
Guidelines Only Part of the Solution
While the guidelines are a step in the right direction, they won’t address some persistent barriers to changing practice, said Michael McKee, MD, MPH, a family medicine physician and co-director of the Center for Disability Health and Wellness at the University of Michigan in Ann Arbor, who was not part of the guideline team.
“I think [the guidelines] will raise the awareness on why it’s important to address hearing loss,” he says. “Many primary care providers don’t elevate hearing loss as a priority topic. The problem is that we’re struggling with getting things in place to have a more supportive system to carry out those recommendations.”
Lack of Training and Support
The problems include lack of training on hearing loss for physicians, starting with medical school. Another complication is time: A conversation about hearing loss adds to the multitude of conversations a primary care provider is expected to have with their patients in a short visit.
Additionally, when hearing loss is suspected, an audiologist may be hard to find to perform the audiogram, Dr. McKee says. If patients agree to see an audiologist and that specialist finds hearing loss, patients may not want to wear a device due to stigma or may not be able to afford a device that will fit properly and truly benefit them because Medicare does not cover hearing aids.
“Only about 20-plus percent of those eligible for hearing aids get them,” he said. Hearing aids available over the counter help some people, but may be difficult to fit properly and may be hard for some to use correctly, he added.
“That comes back to the primary care provider, so it’s unfortunately a very unsatisfying course,” he said.
‘Primary Care Providers Do Value Guidelines’
However, “Primary care providers do value guidelines. They do value strong recommendations,” he said. We are trying to figure out how we can support people with unaddressed hearing loss in the primary care setting, Dr. McKee said. “Once we get there, we need to advocate for an expansion of coverage,” he said.
The authors note that the messages in the guidelines are important for all clinicians.
“The impact of hearing loss and screening should not be the sole responsibility of an audiologist, an otolaryngologist, nor primary care provider. Any time and place that a patient interacts with the healthcare system is an opportunity for preventive healthcare, such as hearing screening, to occur,” they write.
Funding for this research was provided by the American Academy of Otolaryngology–Head and Neck Surgery Foundation. Dr. Do and Dr. McKee report no relevant financial relationships. Full disclosures of the co-authors are listed with the full text of the paper.
FROM OTOLARYNGOLOGY–HEAD AND NECK SURGERY
Pediatrician Credibility Remains Intact in Midst of Health Misinformation
TORONTO —
Despite acknowledging that health misinformation is on the rise, “nearly all the pediatricians we surveyed agreed or strongly agreed that their patients consider them a trusted information source,” reported Elizabeth A. Gottschlich, MA, a senior research associate with the American Academy of Pediatrics, Itasca, Illinois.
These data were generated by an ongoing cohort analysis called the Pediatricians Life and Career Experience Study (PLACES). Each year, two surveys are conducted with three groups of pediatricians in this cohort. They are defined by years in which they graduated from residency (2002-2004, 2009-2011, or 2016-2018).
While the longer survey of the two captures an array of issues regarding life and practice, the shorter “checkpoint” survey addresses a high-priority topic. In 2023, it was health misinformation. The data from this survey were presented at the Pediatric Academic Societies annual meeting.
About 40% of the 2706 pediatricians who completed this particular survey (just over 65% of the participants in PLACES) were general pediatricians, 50% were pediatric subspecialists, and 10% were hospitalists.
Almost all of the survey questions were answered on a five-point Likert scale.
A Matter of Trust
According to Ms. Gottschlich, approximately 80% of pediatricians agreed or strongly agreed that misinformation is a clinical issue for them. About one third of these strongly agreed, and only 6% disagreed.
There was also strong consensus that the problem has grown worse since the start of the COVID-19 epidemic. To this statement, 70% agreed or strongly agreed and 24% did not agree or disagree. Only 4% disagreed.
However, relatively few respondents appeared to be concerned about the ability of pediatricians to address the problem of misinformation, Ms. Gottschlich reported.
When asked to respond to the statement that the “community recognizes and uses pediatricians as trusted source for health information,” 87% agreed or strongly agreed. Of the remaining, 9% did not agree or disagree, leaving just 4% that disagreed or strongly disagreed.
For a similar but slightly different question, the consensus was even greater. To the statement “patients/families in your practice seek your input as a trusted source for health information,” 94% agreed or strongly agreed.
Encountering Misinformation
The survey went on to ask pediatricians about encounters with misinformation for seven specific issues. On the five-point Likert scale, the choices ranged from a few times per year to every day.
For reproductive health, gender-affirming care, and firearm injury prevention, about 80% of respondents answered at the very low end of the scale, meaning no more than about once per month. Encounters with misinformation was slightly greater with autism; nearly one third responded that they encountered misinformation once a week or more frequently.
For all three questions regarding vaccines, the proportions climbed substantially. Of these, the COVID-19 vaccine was the most common topic of misinformation, with more than half reporting that they addressed incorrect information once a week or more. Seven percent reported this occurs daily.
Nearly 40% of pediatricians responded that they dealt with misinformation about the HPV vaccine once per week or more, while 35% reported that they encountered misinformation this frequently about routine childhood vaccines. There was a small but not necessarily trivial proportion for each of these categories of vaccine who reported that they encountered misinformation on a daily basis.
When stratified by clinical focus, the encounters varied. For the COVID-19 vaccine, general pediatricians (67%) were far more likely to report addressing misinformation on a weekly or more frequent basis than hospitalists (39%) or subspecialists (46%). They were more than twice as likely to encounter misinformation about the HPV vaccine than hospitalists or pediatric subspecialists (46%, 17%, and 19%, respectively).
When stratified by urban, suburban, or rural practice areas, differences were relatively modest. Pediatricians in urban practices were less likely to face misinformation about HPV vaccine (29% vs 44% and 48% for suburban and rural areas, respectively), while pediatricians in rural practice were more likely to face misinformation about routine childhood vaccines (60% vs 33% and 35% for urban and suburban practices, respectively).
Differences were even narrower when misinformation encounters were compared among the West, Midwest, South, and Northeast. For the threshold of once per week or more commonly, misinformation about the COVID-19 vaccine was less common in the South (50% vs 55%-58% in the other areas), while misinformation about routine childhood vaccines was more commonly encountered in the West (41% vs 32%-35% in the other areas).
A Growing Problem
The confidence among pediatricians that their knowledge is valued is reassuring, according to Ms. Gottschlich, who noted that the U.S. Surgeon General declared health misinformation a serious threat to public health in 2021, but the problem of misinformation is growing, according to several sources.
One of these sources, at least in regard to adolescent health, appears to be social media, according to a recently published review article in JAMA Pediatrics. The lead author of that article, Monica L. Wang, DSc, has dual academic appointments at the Boston University School of Public Health and Harvard University’s T.H. Chan School of Public Health, Boston. Asked for a comment on this issue, she suggested that it might not be enough to just respond to misinformation but rather might be better to develop a dialogue that will reveal misconceptions.
“Just as they screen for preventive issues like seat belt use, sunscreen, and safe sex practices, [pediatricians should integrate] questions about health misinformation into visits, which can be a natural and effective way to encourage dialogue, proactively share accurate information, and promote well-being,” she said.
Agreeing with the premise that pediatricians are a credible source of information for parents and children, Dr. Wang very much endorses the principle that “pediatricians can play a critical role in addressing health misinformation.”
Ms. Gottschlich and Dr. Wang report no potential conflicts of interest.
TORONTO —
Despite acknowledging that health misinformation is on the rise, “nearly all the pediatricians we surveyed agreed or strongly agreed that their patients consider them a trusted information source,” reported Elizabeth A. Gottschlich, MA, a senior research associate with the American Academy of Pediatrics, Itasca, Illinois.
These data were generated by an ongoing cohort analysis called the Pediatricians Life and Career Experience Study (PLACES). Each year, two surveys are conducted with three groups of pediatricians in this cohort. They are defined by years in which they graduated from residency (2002-2004, 2009-2011, or 2016-2018).
While the longer survey of the two captures an array of issues regarding life and practice, the shorter “checkpoint” survey addresses a high-priority topic. In 2023, it was health misinformation. The data from this survey were presented at the Pediatric Academic Societies annual meeting.
About 40% of the 2706 pediatricians who completed this particular survey (just over 65% of the participants in PLACES) were general pediatricians, 50% were pediatric subspecialists, and 10% were hospitalists.
Almost all of the survey questions were answered on a five-point Likert scale.
A Matter of Trust
According to Ms. Gottschlich, approximately 80% of pediatricians agreed or strongly agreed that misinformation is a clinical issue for them. About one third of these strongly agreed, and only 6% disagreed.
There was also strong consensus that the problem has grown worse since the start of the COVID-19 epidemic. To this statement, 70% agreed or strongly agreed and 24% did not agree or disagree. Only 4% disagreed.
However, relatively few respondents appeared to be concerned about the ability of pediatricians to address the problem of misinformation, Ms. Gottschlich reported.
When asked to respond to the statement that the “community recognizes and uses pediatricians as trusted source for health information,” 87% agreed or strongly agreed. Of the remaining, 9% did not agree or disagree, leaving just 4% that disagreed or strongly disagreed.
For a similar but slightly different question, the consensus was even greater. To the statement “patients/families in your practice seek your input as a trusted source for health information,” 94% agreed or strongly agreed.
Encountering Misinformation
The survey went on to ask pediatricians about encounters with misinformation for seven specific issues. On the five-point Likert scale, the choices ranged from a few times per year to every day.
For reproductive health, gender-affirming care, and firearm injury prevention, about 80% of respondents answered at the very low end of the scale, meaning no more than about once per month. Encounters with misinformation was slightly greater with autism; nearly one third responded that they encountered misinformation once a week or more frequently.
For all three questions regarding vaccines, the proportions climbed substantially. Of these, the COVID-19 vaccine was the most common topic of misinformation, with more than half reporting that they addressed incorrect information once a week or more. Seven percent reported this occurs daily.
Nearly 40% of pediatricians responded that they dealt with misinformation about the HPV vaccine once per week or more, while 35% reported that they encountered misinformation this frequently about routine childhood vaccines. There was a small but not necessarily trivial proportion for each of these categories of vaccine who reported that they encountered misinformation on a daily basis.
When stratified by clinical focus, the encounters varied. For the COVID-19 vaccine, general pediatricians (67%) were far more likely to report addressing misinformation on a weekly or more frequent basis than hospitalists (39%) or subspecialists (46%). They were more than twice as likely to encounter misinformation about the HPV vaccine than hospitalists or pediatric subspecialists (46%, 17%, and 19%, respectively).
When stratified by urban, suburban, or rural practice areas, differences were relatively modest. Pediatricians in urban practices were less likely to face misinformation about HPV vaccine (29% vs 44% and 48% for suburban and rural areas, respectively), while pediatricians in rural practice were more likely to face misinformation about routine childhood vaccines (60% vs 33% and 35% for urban and suburban practices, respectively).
Differences were even narrower when misinformation encounters were compared among the West, Midwest, South, and Northeast. For the threshold of once per week or more commonly, misinformation about the COVID-19 vaccine was less common in the South (50% vs 55%-58% in the other areas), while misinformation about routine childhood vaccines was more commonly encountered in the West (41% vs 32%-35% in the other areas).
A Growing Problem
The confidence among pediatricians that their knowledge is valued is reassuring, according to Ms. Gottschlich, who noted that the U.S. Surgeon General declared health misinformation a serious threat to public health in 2021, but the problem of misinformation is growing, according to several sources.
One of these sources, at least in regard to adolescent health, appears to be social media, according to a recently published review article in JAMA Pediatrics. The lead author of that article, Monica L. Wang, DSc, has dual academic appointments at the Boston University School of Public Health and Harvard University’s T.H. Chan School of Public Health, Boston. Asked for a comment on this issue, she suggested that it might not be enough to just respond to misinformation but rather might be better to develop a dialogue that will reveal misconceptions.
“Just as they screen for preventive issues like seat belt use, sunscreen, and safe sex practices, [pediatricians should integrate] questions about health misinformation into visits, which can be a natural and effective way to encourage dialogue, proactively share accurate information, and promote well-being,” she said.
Agreeing with the premise that pediatricians are a credible source of information for parents and children, Dr. Wang very much endorses the principle that “pediatricians can play a critical role in addressing health misinformation.”
Ms. Gottschlich and Dr. Wang report no potential conflicts of interest.
TORONTO —
Despite acknowledging that health misinformation is on the rise, “nearly all the pediatricians we surveyed agreed or strongly agreed that their patients consider them a trusted information source,” reported Elizabeth A. Gottschlich, MA, a senior research associate with the American Academy of Pediatrics, Itasca, Illinois.
These data were generated by an ongoing cohort analysis called the Pediatricians Life and Career Experience Study (PLACES). Each year, two surveys are conducted with three groups of pediatricians in this cohort. They are defined by years in which they graduated from residency (2002-2004, 2009-2011, or 2016-2018).
While the longer survey of the two captures an array of issues regarding life and practice, the shorter “checkpoint” survey addresses a high-priority topic. In 2023, it was health misinformation. The data from this survey were presented at the Pediatric Academic Societies annual meeting.
About 40% of the 2706 pediatricians who completed this particular survey (just over 65% of the participants in PLACES) were general pediatricians, 50% were pediatric subspecialists, and 10% were hospitalists.
Almost all of the survey questions were answered on a five-point Likert scale.
A Matter of Trust
According to Ms. Gottschlich, approximately 80% of pediatricians agreed or strongly agreed that misinformation is a clinical issue for them. About one third of these strongly agreed, and only 6% disagreed.
There was also strong consensus that the problem has grown worse since the start of the COVID-19 epidemic. To this statement, 70% agreed or strongly agreed and 24% did not agree or disagree. Only 4% disagreed.
However, relatively few respondents appeared to be concerned about the ability of pediatricians to address the problem of misinformation, Ms. Gottschlich reported.
When asked to respond to the statement that the “community recognizes and uses pediatricians as trusted source for health information,” 87% agreed or strongly agreed. Of the remaining, 9% did not agree or disagree, leaving just 4% that disagreed or strongly disagreed.
For a similar but slightly different question, the consensus was even greater. To the statement “patients/families in your practice seek your input as a trusted source for health information,” 94% agreed or strongly agreed.
Encountering Misinformation
The survey went on to ask pediatricians about encounters with misinformation for seven specific issues. On the five-point Likert scale, the choices ranged from a few times per year to every day.
For reproductive health, gender-affirming care, and firearm injury prevention, about 80% of respondents answered at the very low end of the scale, meaning no more than about once per month. Encounters with misinformation was slightly greater with autism; nearly one third responded that they encountered misinformation once a week or more frequently.
For all three questions regarding vaccines, the proportions climbed substantially. Of these, the COVID-19 vaccine was the most common topic of misinformation, with more than half reporting that they addressed incorrect information once a week or more. Seven percent reported this occurs daily.
Nearly 40% of pediatricians responded that they dealt with misinformation about the HPV vaccine once per week or more, while 35% reported that they encountered misinformation this frequently about routine childhood vaccines. There was a small but not necessarily trivial proportion for each of these categories of vaccine who reported that they encountered misinformation on a daily basis.
When stratified by clinical focus, the encounters varied. For the COVID-19 vaccine, general pediatricians (67%) were far more likely to report addressing misinformation on a weekly or more frequent basis than hospitalists (39%) or subspecialists (46%). They were more than twice as likely to encounter misinformation about the HPV vaccine than hospitalists or pediatric subspecialists (46%, 17%, and 19%, respectively).
When stratified by urban, suburban, or rural practice areas, differences were relatively modest. Pediatricians in urban practices were less likely to face misinformation about HPV vaccine (29% vs 44% and 48% for suburban and rural areas, respectively), while pediatricians in rural practice were more likely to face misinformation about routine childhood vaccines (60% vs 33% and 35% for urban and suburban practices, respectively).
Differences were even narrower when misinformation encounters were compared among the West, Midwest, South, and Northeast. For the threshold of once per week or more commonly, misinformation about the COVID-19 vaccine was less common in the South (50% vs 55%-58% in the other areas), while misinformation about routine childhood vaccines was more commonly encountered in the West (41% vs 32%-35% in the other areas).
A Growing Problem
The confidence among pediatricians that their knowledge is valued is reassuring, according to Ms. Gottschlich, who noted that the U.S. Surgeon General declared health misinformation a serious threat to public health in 2021, but the problem of misinformation is growing, according to several sources.
One of these sources, at least in regard to adolescent health, appears to be social media, according to a recently published review article in JAMA Pediatrics. The lead author of that article, Monica L. Wang, DSc, has dual academic appointments at the Boston University School of Public Health and Harvard University’s T.H. Chan School of Public Health, Boston. Asked for a comment on this issue, she suggested that it might not be enough to just respond to misinformation but rather might be better to develop a dialogue that will reveal misconceptions.
“Just as they screen for preventive issues like seat belt use, sunscreen, and safe sex practices, [pediatricians should integrate] questions about health misinformation into visits, which can be a natural and effective way to encourage dialogue, proactively share accurate information, and promote well-being,” she said.
Agreeing with the premise that pediatricians are a credible source of information for parents and children, Dr. Wang very much endorses the principle that “pediatricians can play a critical role in addressing health misinformation.”
Ms. Gottschlich and Dr. Wang report no potential conflicts of interest.
FROM PAS 2024
The Importance of Family Therapy for Transgender Youth
Recent newspaper headlines have focused almost exclusively on gender-affirming medical interventions for transgender youth (eg, puberty blockers and gender-affirming hormones like estrogen and testosterone). It is true that these are important treatments that are consistently tied to improvements in mental health. However, an additional powerful predictor of good mental health outcomes for transgender youth is parental support and acceptance.
It is essential that clinicians consider this when creating treatment plans for transgender youth. Sadly, they are often afraid to share their own struggles, despite working through these being essential for their children’s thriving and well-being. I have a few key tips for combating this issue. My upcoming book Free to Be: Understanding Kids & Gender Identity provides much more context for parents and providers, but I will highlight a few big takeaways here.
Give Parents Their Own Space
Many parents have never encountered a transgender person in their life and have a lot of questions. At times, they may be “thinking out loud” and say things in passing that aren’t their final thoughts or opinions on a matter. This can, unfortunately, be damaging to their children. I often speak with adult transgender people whose parents said something they no longer believe (eg, “being trans is just a mental illness – you need therapy to fix it”), but these comments stick in the person’s mind and drive shame and self-esteem challenges later in life, sometimes for decades. Parents need to have a safe space, with a trained professional with expertise in gender, to work through their concerns and questions away from their children, so that when they talk to their kids about gender, they are presenting their fully formed thoughts.
Validate Parents’ Difficult Experiences
As pediatric providers, we are often focused on the difficult experiences of our transgender pediatric patients. However, their parents tend to be struggling as well, and that struggling predicts adverse mental health outcomes for their children.
The most common reaction a parent has upon learning their child is transgender is fear. It’s important to validate this fear (and other feelings that come out), so that parents know they can share with you what’s really going on in their minds.
There are some common themes we see for parents. Some are big fears: fear that their child will be victimized or fear that their child will later regret taking gender-affirming hormones and blame the parents for giving permission to take them. Parents often say they had a gendered vision for what their child’s future would be like, and their child coming out as transgender changes that (it can be helpful to gently remind parents that children almost never grow up exactly how we predict).
Some themes are more mundane but nonetheless distressing for parents, such as not wanting to throw away meaningful souvenirs from past vacations that have their child’s birth name on them. Clinicians can and should validate these thoughts and feelings, while also providing additional context and education. I often recommend the book Found in Transition by Pariah Hassouri, a pediatrician who goes through many of these common struggles after her daughter comes out as transgender.
Take a Three-Stage Approach When Adolescents Are Considering Gender-Affirming Medical Interventions
We recently outlined our process for conducting a biopsychosocial assessment for adolescents considering pubertal suppression for adolescent gender dysphoria in The Journal of the American Academy of Child & Adolescent Psychiatry, for those who want more detail on how to conduct these assessments. On the theme of supporting parents, I would highlight the value of taking a three-stage approach. In the first stage, a clinician meets with an adolescent alone to collect their gender history and discuss important considerations regarding the medical intervention. In stage two, the same information about the medical intervention is shared with parents, along with a summary of what the adolescent shared with the clinician (with the adolescent’s consent, of course). Often there will be some areas of disconnect. We make a list of these areas of disconnect that are addressed in stage three, in which the full family is brought together to get everyone on the same page and understanding each other’s perspectives.
Common disconnects include gender dysphoria seeming to “come out of nowhere” from the parents’ perspective, necessitating the young person to recount an early life experience in which they were harassed for expressing gender nonconformity, leading them to act stereotypically in line with their sex assigned at birth for years to avoid being “outed” and harassed more. Conversations around fertility preservation can be particularly complex. Young people and their parents also sometimes have different conceptualizations of gender identity and require a shared framework for talking about gender identity (which I offer in my forthcoming book). This list of family therapy topics can be diverse and highly dependent on the family. An additional resource for this phase of the family therapy is The Family Acceptance Project, which has created culturally tailored materials to help parents understand their sexual and gender minority children.
In summary, fostering healthy family functioning is essential for the care of transgender and gender diverse youth, and parents require support in addition to their children needing support. I encourage all gender providers to incorporate the vital element of family therapy into their practice.
Dr. Turban is director of the Gender Psychiatry Program at the University of California, San Francisco, where he is an assistant professor of child & adolescent psychiatry and affiliate faculty at the Philip R. Lee Institute for Health Policy Studies. He is on X @jack_turban.
Recent newspaper headlines have focused almost exclusively on gender-affirming medical interventions for transgender youth (eg, puberty blockers and gender-affirming hormones like estrogen and testosterone). It is true that these are important treatments that are consistently tied to improvements in mental health. However, an additional powerful predictor of good mental health outcomes for transgender youth is parental support and acceptance.
It is essential that clinicians consider this when creating treatment plans for transgender youth. Sadly, they are often afraid to share their own struggles, despite working through these being essential for their children’s thriving and well-being. I have a few key tips for combating this issue. My upcoming book Free to Be: Understanding Kids & Gender Identity provides much more context for parents and providers, but I will highlight a few big takeaways here.
Give Parents Their Own Space
Many parents have never encountered a transgender person in their life and have a lot of questions. At times, they may be “thinking out loud” and say things in passing that aren’t their final thoughts or opinions on a matter. This can, unfortunately, be damaging to their children. I often speak with adult transgender people whose parents said something they no longer believe (eg, “being trans is just a mental illness – you need therapy to fix it”), but these comments stick in the person’s mind and drive shame and self-esteem challenges later in life, sometimes for decades. Parents need to have a safe space, with a trained professional with expertise in gender, to work through their concerns and questions away from their children, so that when they talk to their kids about gender, they are presenting their fully formed thoughts.
Validate Parents’ Difficult Experiences
As pediatric providers, we are often focused on the difficult experiences of our transgender pediatric patients. However, their parents tend to be struggling as well, and that struggling predicts adverse mental health outcomes for their children.
The most common reaction a parent has upon learning their child is transgender is fear. It’s important to validate this fear (and other feelings that come out), so that parents know they can share with you what’s really going on in their minds.
There are some common themes we see for parents. Some are big fears: fear that their child will be victimized or fear that their child will later regret taking gender-affirming hormones and blame the parents for giving permission to take them. Parents often say they had a gendered vision for what their child’s future would be like, and their child coming out as transgender changes that (it can be helpful to gently remind parents that children almost never grow up exactly how we predict).
Some themes are more mundane but nonetheless distressing for parents, such as not wanting to throw away meaningful souvenirs from past vacations that have their child’s birth name on them. Clinicians can and should validate these thoughts and feelings, while also providing additional context and education. I often recommend the book Found in Transition by Pariah Hassouri, a pediatrician who goes through many of these common struggles after her daughter comes out as transgender.
Take a Three-Stage Approach When Adolescents Are Considering Gender-Affirming Medical Interventions
We recently outlined our process for conducting a biopsychosocial assessment for adolescents considering pubertal suppression for adolescent gender dysphoria in The Journal of the American Academy of Child & Adolescent Psychiatry, for those who want more detail on how to conduct these assessments. On the theme of supporting parents, I would highlight the value of taking a three-stage approach. In the first stage, a clinician meets with an adolescent alone to collect their gender history and discuss important considerations regarding the medical intervention. In stage two, the same information about the medical intervention is shared with parents, along with a summary of what the adolescent shared with the clinician (with the adolescent’s consent, of course). Often there will be some areas of disconnect. We make a list of these areas of disconnect that are addressed in stage three, in which the full family is brought together to get everyone on the same page and understanding each other’s perspectives.
Common disconnects include gender dysphoria seeming to “come out of nowhere” from the parents’ perspective, necessitating the young person to recount an early life experience in which they were harassed for expressing gender nonconformity, leading them to act stereotypically in line with their sex assigned at birth for years to avoid being “outed” and harassed more. Conversations around fertility preservation can be particularly complex. Young people and their parents also sometimes have different conceptualizations of gender identity and require a shared framework for talking about gender identity (which I offer in my forthcoming book). This list of family therapy topics can be diverse and highly dependent on the family. An additional resource for this phase of the family therapy is The Family Acceptance Project, which has created culturally tailored materials to help parents understand their sexual and gender minority children.
In summary, fostering healthy family functioning is essential for the care of transgender and gender diverse youth, and parents require support in addition to their children needing support. I encourage all gender providers to incorporate the vital element of family therapy into their practice.
Dr. Turban is director of the Gender Psychiatry Program at the University of California, San Francisco, where he is an assistant professor of child & adolescent psychiatry and affiliate faculty at the Philip R. Lee Institute for Health Policy Studies. He is on X @jack_turban.
Recent newspaper headlines have focused almost exclusively on gender-affirming medical interventions for transgender youth (eg, puberty blockers and gender-affirming hormones like estrogen and testosterone). It is true that these are important treatments that are consistently tied to improvements in mental health. However, an additional powerful predictor of good mental health outcomes for transgender youth is parental support and acceptance.
It is essential that clinicians consider this when creating treatment plans for transgender youth. Sadly, they are often afraid to share their own struggles, despite working through these being essential for their children’s thriving and well-being. I have a few key tips for combating this issue. My upcoming book Free to Be: Understanding Kids & Gender Identity provides much more context for parents and providers, but I will highlight a few big takeaways here.
Give Parents Their Own Space
Many parents have never encountered a transgender person in their life and have a lot of questions. At times, they may be “thinking out loud” and say things in passing that aren’t their final thoughts or opinions on a matter. This can, unfortunately, be damaging to their children. I often speak with adult transgender people whose parents said something they no longer believe (eg, “being trans is just a mental illness – you need therapy to fix it”), but these comments stick in the person’s mind and drive shame and self-esteem challenges later in life, sometimes for decades. Parents need to have a safe space, with a trained professional with expertise in gender, to work through their concerns and questions away from their children, so that when they talk to their kids about gender, they are presenting their fully formed thoughts.
Validate Parents’ Difficult Experiences
As pediatric providers, we are often focused on the difficult experiences of our transgender pediatric patients. However, their parents tend to be struggling as well, and that struggling predicts adverse mental health outcomes for their children.
The most common reaction a parent has upon learning their child is transgender is fear. It’s important to validate this fear (and other feelings that come out), so that parents know they can share with you what’s really going on in their minds.
There are some common themes we see for parents. Some are big fears: fear that their child will be victimized or fear that their child will later regret taking gender-affirming hormones and blame the parents for giving permission to take them. Parents often say they had a gendered vision for what their child’s future would be like, and their child coming out as transgender changes that (it can be helpful to gently remind parents that children almost never grow up exactly how we predict).
Some themes are more mundane but nonetheless distressing for parents, such as not wanting to throw away meaningful souvenirs from past vacations that have their child’s birth name on them. Clinicians can and should validate these thoughts and feelings, while also providing additional context and education. I often recommend the book Found in Transition by Pariah Hassouri, a pediatrician who goes through many of these common struggles after her daughter comes out as transgender.
Take a Three-Stage Approach When Adolescents Are Considering Gender-Affirming Medical Interventions
We recently outlined our process for conducting a biopsychosocial assessment for adolescents considering pubertal suppression for adolescent gender dysphoria in The Journal of the American Academy of Child & Adolescent Psychiatry, for those who want more detail on how to conduct these assessments. On the theme of supporting parents, I would highlight the value of taking a three-stage approach. In the first stage, a clinician meets with an adolescent alone to collect their gender history and discuss important considerations regarding the medical intervention. In stage two, the same information about the medical intervention is shared with parents, along with a summary of what the adolescent shared with the clinician (with the adolescent’s consent, of course). Often there will be some areas of disconnect. We make a list of these areas of disconnect that are addressed in stage three, in which the full family is brought together to get everyone on the same page and understanding each other’s perspectives.
Common disconnects include gender dysphoria seeming to “come out of nowhere” from the parents’ perspective, necessitating the young person to recount an early life experience in which they were harassed for expressing gender nonconformity, leading them to act stereotypically in line with their sex assigned at birth for years to avoid being “outed” and harassed more. Conversations around fertility preservation can be particularly complex. Young people and their parents also sometimes have different conceptualizations of gender identity and require a shared framework for talking about gender identity (which I offer in my forthcoming book). This list of family therapy topics can be diverse and highly dependent on the family. An additional resource for this phase of the family therapy is The Family Acceptance Project, which has created culturally tailored materials to help parents understand their sexual and gender minority children.
In summary, fostering healthy family functioning is essential for the care of transgender and gender diverse youth, and parents require support in addition to their children needing support. I encourage all gender providers to incorporate the vital element of family therapy into their practice.
Dr. Turban is director of the Gender Psychiatry Program at the University of California, San Francisco, where he is an assistant professor of child & adolescent psychiatry and affiliate faculty at the Philip R. Lee Institute for Health Policy Studies. He is on X @jack_turban.
Vast Majority of Adults At Risk for Cardiovascular-Kidney-Metabolic Syndrome
TOPLINE:
Nearly 90% of adults were at risk of developing cardiovascular-kidney-metabolic (CKM) syndrome between 2011 and 2020, according to new research published in JAMA.
METHODOLOGY:
- In 2023, the American Heart Association defined to acknowledge how heart and kidney diseases, diabetes, and obesity interact and are increasingly co-occurring conditions.
- Researchers used data from the National Health and Nutrition Examination Survey between 2011 and 2020.
- More than 10,000 adults over age 20 years were included; all of them received a physical and fasting laboratory measurements and self-reported their cardiovascular disease (CVD) status.
- Researchers created categories for risk, ranging from 0 (no risk factors) to 4, using factors such as kidney disease, obesity, and hypertension.
TAKEAWAY:
- (having metabolic risk factors like hypertension or moderate- to high-risk chronic kidney disease).
- 14.6% met the criteria for advanced stage 3 (very high-risk chronic kidney disease or a high risk for 10-year CVD) and stage 4 CKM syndrome (established CVD) combined.
- Men, adults over age 65 years, and Black individuals were at a greater risk for advanced stages of the CKM syndrome.
- Almost half of people met the criteria for stage 2 (having metabolic risk factors like hypertension or moderate- to high-risk chronic kidney disease).
- 14.6% met the criteria for advanced stage 3 (very high-risk chronic kidney disease or a high risk for 10-year CVD) and stage 4 CKM syndrome (established CVD) combined.
- Men, adults over age 65 years, and Black individuals were at a greater risk for advanced stages of the CKM syndrome.
IN PRACTICE:
“Equitable health care approaches prioritizing CKM health are urgently needed,” the study authors wrote.
SOURCE:
The study was led by Muthiah Vaduganathan, MD, MPH, cardiologist and researcher at Brigham and Women’s Hospital, Harvard Medical School, Boston.
LIMITATIONS:
Established CVD statuses were self-reported. Some data that would indicate advanced CKM stages were not available (eg, cardiac biomarkers, echocardiography, and coronary angiography), which may have led to an underestimation of rates.
DISCLOSURES:
One author received grants from Bristol Myers Squibb–Pfizer outside the submitted work. Dr. Vaduganathan received grants from and was an adviser and committee trial member for various pharmaceutical companies outside the submitted work. The authors reported no other disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
Nearly 90% of adults were at risk of developing cardiovascular-kidney-metabolic (CKM) syndrome between 2011 and 2020, according to new research published in JAMA.
METHODOLOGY:
- In 2023, the American Heart Association defined to acknowledge how heart and kidney diseases, diabetes, and obesity interact and are increasingly co-occurring conditions.
- Researchers used data from the National Health and Nutrition Examination Survey between 2011 and 2020.
- More than 10,000 adults over age 20 years were included; all of them received a physical and fasting laboratory measurements and self-reported their cardiovascular disease (CVD) status.
- Researchers created categories for risk, ranging from 0 (no risk factors) to 4, using factors such as kidney disease, obesity, and hypertension.
TAKEAWAY:
- (having metabolic risk factors like hypertension or moderate- to high-risk chronic kidney disease).
- 14.6% met the criteria for advanced stage 3 (very high-risk chronic kidney disease or a high risk for 10-year CVD) and stage 4 CKM syndrome (established CVD) combined.
- Men, adults over age 65 years, and Black individuals were at a greater risk for advanced stages of the CKM syndrome.
- Almost half of people met the criteria for stage 2 (having metabolic risk factors like hypertension or moderate- to high-risk chronic kidney disease).
- 14.6% met the criteria for advanced stage 3 (very high-risk chronic kidney disease or a high risk for 10-year CVD) and stage 4 CKM syndrome (established CVD) combined.
- Men, adults over age 65 years, and Black individuals were at a greater risk for advanced stages of the CKM syndrome.
IN PRACTICE:
“Equitable health care approaches prioritizing CKM health are urgently needed,” the study authors wrote.
SOURCE:
The study was led by Muthiah Vaduganathan, MD, MPH, cardiologist and researcher at Brigham and Women’s Hospital, Harvard Medical School, Boston.
LIMITATIONS:
Established CVD statuses were self-reported. Some data that would indicate advanced CKM stages were not available (eg, cardiac biomarkers, echocardiography, and coronary angiography), which may have led to an underestimation of rates.
DISCLOSURES:
One author received grants from Bristol Myers Squibb–Pfizer outside the submitted work. Dr. Vaduganathan received grants from and was an adviser and committee trial member for various pharmaceutical companies outside the submitted work. The authors reported no other disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
Nearly 90% of adults were at risk of developing cardiovascular-kidney-metabolic (CKM) syndrome between 2011 and 2020, according to new research published in JAMA.
METHODOLOGY:
- In 2023, the American Heart Association defined to acknowledge how heart and kidney diseases, diabetes, and obesity interact and are increasingly co-occurring conditions.
- Researchers used data from the National Health and Nutrition Examination Survey between 2011 and 2020.
- More than 10,000 adults over age 20 years were included; all of them received a physical and fasting laboratory measurements and self-reported their cardiovascular disease (CVD) status.
- Researchers created categories for risk, ranging from 0 (no risk factors) to 4, using factors such as kidney disease, obesity, and hypertension.
TAKEAWAY:
- (having metabolic risk factors like hypertension or moderate- to high-risk chronic kidney disease).
- 14.6% met the criteria for advanced stage 3 (very high-risk chronic kidney disease or a high risk for 10-year CVD) and stage 4 CKM syndrome (established CVD) combined.
- Men, adults over age 65 years, and Black individuals were at a greater risk for advanced stages of the CKM syndrome.
- Almost half of people met the criteria for stage 2 (having metabolic risk factors like hypertension or moderate- to high-risk chronic kidney disease).
- 14.6% met the criteria for advanced stage 3 (very high-risk chronic kidney disease or a high risk for 10-year CVD) and stage 4 CKM syndrome (established CVD) combined.
- Men, adults over age 65 years, and Black individuals were at a greater risk for advanced stages of the CKM syndrome.
IN PRACTICE:
“Equitable health care approaches prioritizing CKM health are urgently needed,” the study authors wrote.
SOURCE:
The study was led by Muthiah Vaduganathan, MD, MPH, cardiologist and researcher at Brigham and Women’s Hospital, Harvard Medical School, Boston.
LIMITATIONS:
Established CVD statuses were self-reported. Some data that would indicate advanced CKM stages were not available (eg, cardiac biomarkers, echocardiography, and coronary angiography), which may have led to an underestimation of rates.
DISCLOSURES:
One author received grants from Bristol Myers Squibb–Pfizer outside the submitted work. Dr. Vaduganathan received grants from and was an adviser and committee trial member for various pharmaceutical companies outside the submitted work. The authors reported no other disclosures.
A version of this article appeared on Medscape.com.
For Pediatric LGS, Cenobamate Shows Promise
DENVER — The conclusions were reached by comparing outcomes to patient historical data, though they were not analyzed statistically.
A Proof-of-Concept Study
In an interview, Karen Keough, MD, who presented the study during a poster session at the 2024 annual meeting of the American Academy of Neurology, conceded the key limitation was that the researchers were not able to perform statistical analysis due to the nature of the data. “It’s just showing trends. It’s proof of concept that cenobamate can be effective in one of the most refractory forms of epilepsy, which always includes focal seizures. That’s what led to the initial FDA indication, but we also know that this medication has a lot of promise and is probably going to be effective in other forms of epilepsy and probably in other seizure types,” said Dr. Keough, who is a neurologist at Pediatrix Child Neurology Consultants of Austin, Texas.
Although she has seen significant improvements in many patients, Dr. Keough reported that most patients become refractory again. “Unfortunately, honeymoons are probably real in cenobamate. Continuing to follow those patients as I do, since they’re my own patients, I have quite a few who had more than a year of seizure freedom, [but] their seizures are back, though not as bad as they were before cenobamate. I just can’t get them back to that 100% control category. I do have a few that are still in the 100% control category, but not very many,” she said.
She also presented a retrospective chart review of 36 LGS patients between the ages of 1 and 27 years at the American Epilepsy Society in December 2023, which showed that addition of cenobamate was associated with a reduction in seizure frequency in 85% of patients. “It was a profound number of patients who had long periods of seizure freedom,” she said.
A Promising Treatment Option
Dr. Keough is considering moving cenobamate up in the treatment sequence of new LGS patients. “I don’t use cenobamate first line in anyone because we have good first-line agents for simple epilepsy in Lennox-Gastaut, but I’m bringing out cenobamate pretty early in the course, because I do think it has superior efficacy compared with most other drugs that we have available. Most of my patients are very established and they’ve seen lots of other drugs. For many patients, there are only a couple of drugs left on the list that [they] have never tried, but we’re going to put cenobamate at the top of that. For my newer diagnoses, I’m going to bring it out much earlier,” she said, though other drugs such as clobazam (Onfi) would still rank ahead of cenobamate.
The study was drawn from records of the HealthVerity Marketplace Database, which includes more than 150 commercial, Medicare, and Medicaid payers. It included 76 patients aged 17 or under who took at least one antiseizure medication between May 2020 and December 2022, and who had filled at least two prescriptions of cenobamate and had 180 or more days of medical and pharmacy enrollment. The mean age was 13.4 years (5.3% 0-5 years, 15.8% 6-11 years, 78.9% 12-17 years), and 40.8% were female. Seizure types included absence (17.1%), focal (75.0%), and generalized tonic-clonic (86.8%). All patients had a history of intractable seizures, 80.3% had a history of status epilepticus, and 28.9% had a history of infantile seizures. A little more than one fourth (27.6%) of patients had commercial insurance. In the previous 90 days, 21.1% had had an emergency room visit or in-patient hospital stay.
Common antiseizure medications taken with cenobamate included cannabidiol (n = 14), clobazam (n = 8), and levetiracetam (n = 18).
During the cenobamate treatment period, patients had a lower incidence of epilepsy-related inpatient days per year (3.36 vs 3.94), epilepsy-related ER visits per year (0.66 vs 1.19), and likelihood of requiring a new line of epilepsy therapy (35.5% vs 100%).
‘Promising’ Results, but More Research Is Needed
The fact that cenobamate was used in combination with other therapies, plus the lack of a control group, makes it difficult to determine if cenobamate was actually responsible for the improvements, according to Nassim Zecavati, MD, who was asked for comment on the study. “I think the results are promising, but there’s obviously a need for a randomized, controlled trial to understand whether it was this medication or the combination of cenobamate with [other medications]. How do we know that this isn’t a compound effect, that it’s multifactorial, and a combination of multiple medications versus the cenobamate?” she said.
Still, she noted that LGS patients are highly vulnerable to hospital admissions and status epilepticus, making the parameters examined in the study valid and important. “I think that this drug likely has a role in the treatment of patients with LGS, particularly pediatric patients. I think we just need more data,” said Dr. Zecavati, who is director of Epilepsy at the Children’s Hospital of Richmond in Virginia and associate professor of Neurology at Virginia Commonwealth University.
Dr. Keough is a speaker for SK Life Sciences. Dr. Zecavati has no relevant financial disclosures.
DENVER — The conclusions were reached by comparing outcomes to patient historical data, though they were not analyzed statistically.
A Proof-of-Concept Study
In an interview, Karen Keough, MD, who presented the study during a poster session at the 2024 annual meeting of the American Academy of Neurology, conceded the key limitation was that the researchers were not able to perform statistical analysis due to the nature of the data. “It’s just showing trends. It’s proof of concept that cenobamate can be effective in one of the most refractory forms of epilepsy, which always includes focal seizures. That’s what led to the initial FDA indication, but we also know that this medication has a lot of promise and is probably going to be effective in other forms of epilepsy and probably in other seizure types,” said Dr. Keough, who is a neurologist at Pediatrix Child Neurology Consultants of Austin, Texas.
Although she has seen significant improvements in many patients, Dr. Keough reported that most patients become refractory again. “Unfortunately, honeymoons are probably real in cenobamate. Continuing to follow those patients as I do, since they’re my own patients, I have quite a few who had more than a year of seizure freedom, [but] their seizures are back, though not as bad as they were before cenobamate. I just can’t get them back to that 100% control category. I do have a few that are still in the 100% control category, but not very many,” she said.
She also presented a retrospective chart review of 36 LGS patients between the ages of 1 and 27 years at the American Epilepsy Society in December 2023, which showed that addition of cenobamate was associated with a reduction in seizure frequency in 85% of patients. “It was a profound number of patients who had long periods of seizure freedom,” she said.
A Promising Treatment Option
Dr. Keough is considering moving cenobamate up in the treatment sequence of new LGS patients. “I don’t use cenobamate first line in anyone because we have good first-line agents for simple epilepsy in Lennox-Gastaut, but I’m bringing out cenobamate pretty early in the course, because I do think it has superior efficacy compared with most other drugs that we have available. Most of my patients are very established and they’ve seen lots of other drugs. For many patients, there are only a couple of drugs left on the list that [they] have never tried, but we’re going to put cenobamate at the top of that. For my newer diagnoses, I’m going to bring it out much earlier,” she said, though other drugs such as clobazam (Onfi) would still rank ahead of cenobamate.
The study was drawn from records of the HealthVerity Marketplace Database, which includes more than 150 commercial, Medicare, and Medicaid payers. It included 76 patients aged 17 or under who took at least one antiseizure medication between May 2020 and December 2022, and who had filled at least two prescriptions of cenobamate and had 180 or more days of medical and pharmacy enrollment. The mean age was 13.4 years (5.3% 0-5 years, 15.8% 6-11 years, 78.9% 12-17 years), and 40.8% were female. Seizure types included absence (17.1%), focal (75.0%), and generalized tonic-clonic (86.8%). All patients had a history of intractable seizures, 80.3% had a history of status epilepticus, and 28.9% had a history of infantile seizures. A little more than one fourth (27.6%) of patients had commercial insurance. In the previous 90 days, 21.1% had had an emergency room visit or in-patient hospital stay.
Common antiseizure medications taken with cenobamate included cannabidiol (n = 14), clobazam (n = 8), and levetiracetam (n = 18).
During the cenobamate treatment period, patients had a lower incidence of epilepsy-related inpatient days per year (3.36 vs 3.94), epilepsy-related ER visits per year (0.66 vs 1.19), and likelihood of requiring a new line of epilepsy therapy (35.5% vs 100%).
‘Promising’ Results, but More Research Is Needed
The fact that cenobamate was used in combination with other therapies, plus the lack of a control group, makes it difficult to determine if cenobamate was actually responsible for the improvements, according to Nassim Zecavati, MD, who was asked for comment on the study. “I think the results are promising, but there’s obviously a need for a randomized, controlled trial to understand whether it was this medication or the combination of cenobamate with [other medications]. How do we know that this isn’t a compound effect, that it’s multifactorial, and a combination of multiple medications versus the cenobamate?” she said.
Still, she noted that LGS patients are highly vulnerable to hospital admissions and status epilepticus, making the parameters examined in the study valid and important. “I think that this drug likely has a role in the treatment of patients with LGS, particularly pediatric patients. I think we just need more data,” said Dr. Zecavati, who is director of Epilepsy at the Children’s Hospital of Richmond in Virginia and associate professor of Neurology at Virginia Commonwealth University.
Dr. Keough is a speaker for SK Life Sciences. Dr. Zecavati has no relevant financial disclosures.
DENVER — The conclusions were reached by comparing outcomes to patient historical data, though they were not analyzed statistically.
A Proof-of-Concept Study
In an interview, Karen Keough, MD, who presented the study during a poster session at the 2024 annual meeting of the American Academy of Neurology, conceded the key limitation was that the researchers were not able to perform statistical analysis due to the nature of the data. “It’s just showing trends. It’s proof of concept that cenobamate can be effective in one of the most refractory forms of epilepsy, which always includes focal seizures. That’s what led to the initial FDA indication, but we also know that this medication has a lot of promise and is probably going to be effective in other forms of epilepsy and probably in other seizure types,” said Dr. Keough, who is a neurologist at Pediatrix Child Neurology Consultants of Austin, Texas.
Although she has seen significant improvements in many patients, Dr. Keough reported that most patients become refractory again. “Unfortunately, honeymoons are probably real in cenobamate. Continuing to follow those patients as I do, since they’re my own patients, I have quite a few who had more than a year of seizure freedom, [but] their seizures are back, though not as bad as they were before cenobamate. I just can’t get them back to that 100% control category. I do have a few that are still in the 100% control category, but not very many,” she said.
She also presented a retrospective chart review of 36 LGS patients between the ages of 1 and 27 years at the American Epilepsy Society in December 2023, which showed that addition of cenobamate was associated with a reduction in seizure frequency in 85% of patients. “It was a profound number of patients who had long periods of seizure freedom,” she said.
A Promising Treatment Option
Dr. Keough is considering moving cenobamate up in the treatment sequence of new LGS patients. “I don’t use cenobamate first line in anyone because we have good first-line agents for simple epilepsy in Lennox-Gastaut, but I’m bringing out cenobamate pretty early in the course, because I do think it has superior efficacy compared with most other drugs that we have available. Most of my patients are very established and they’ve seen lots of other drugs. For many patients, there are only a couple of drugs left on the list that [they] have never tried, but we’re going to put cenobamate at the top of that. For my newer diagnoses, I’m going to bring it out much earlier,” she said, though other drugs such as clobazam (Onfi) would still rank ahead of cenobamate.
The study was drawn from records of the HealthVerity Marketplace Database, which includes more than 150 commercial, Medicare, and Medicaid payers. It included 76 patients aged 17 or under who took at least one antiseizure medication between May 2020 and December 2022, and who had filled at least two prescriptions of cenobamate and had 180 or more days of medical and pharmacy enrollment. The mean age was 13.4 years (5.3% 0-5 years, 15.8% 6-11 years, 78.9% 12-17 years), and 40.8% were female. Seizure types included absence (17.1%), focal (75.0%), and generalized tonic-clonic (86.8%). All patients had a history of intractable seizures, 80.3% had a history of status epilepticus, and 28.9% had a history of infantile seizures. A little more than one fourth (27.6%) of patients had commercial insurance. In the previous 90 days, 21.1% had had an emergency room visit or in-patient hospital stay.
Common antiseizure medications taken with cenobamate included cannabidiol (n = 14), clobazam (n = 8), and levetiracetam (n = 18).
During the cenobamate treatment period, patients had a lower incidence of epilepsy-related inpatient days per year (3.36 vs 3.94), epilepsy-related ER visits per year (0.66 vs 1.19), and likelihood of requiring a new line of epilepsy therapy (35.5% vs 100%).
‘Promising’ Results, but More Research Is Needed
The fact that cenobamate was used in combination with other therapies, plus the lack of a control group, makes it difficult to determine if cenobamate was actually responsible for the improvements, according to Nassim Zecavati, MD, who was asked for comment on the study. “I think the results are promising, but there’s obviously a need for a randomized, controlled trial to understand whether it was this medication or the combination of cenobamate with [other medications]. How do we know that this isn’t a compound effect, that it’s multifactorial, and a combination of multiple medications versus the cenobamate?” she said.
Still, she noted that LGS patients are highly vulnerable to hospital admissions and status epilepticus, making the parameters examined in the study valid and important. “I think that this drug likely has a role in the treatment of patients with LGS, particularly pediatric patients. I think we just need more data,” said Dr. Zecavati, who is director of Epilepsy at the Children’s Hospital of Richmond in Virginia and associate professor of Neurology at Virginia Commonwealth University.
Dr. Keough is a speaker for SK Life Sciences. Dr. Zecavati has no relevant financial disclosures.
FROM AAN 2024
Purpuric Eruption in a Patient With Hairy Cell Leukemia
The Diagnosis: Purpuric Drug Eruption
Histopathology revealed interface dermatitis, spongiosis, and a perivascular lymphocytic infiltrate with extravasated red blood cells consistent with a purpuric drug eruption. Our patient achieved remission of hairy cell leukemia after receiving only 2 of 5 expected doses of cladribine. The rash resolved completely in 3 weeks following a prednisone taper (Figure).
Hairy cell leukemia is a rare indolent lymphoproliferative disorder of B cells that accounts for approximately 2% of adult leukemias in the United States. Cladribine, a purine nucleoside analog that impairs DNA synthesis and repair, has become the mainstay of therapy, demonstrating a 95% complete response rate.1 Although few reports have addressed the cutaneous reactions seen with cladribine therapy, they can occur in more than 50% of patients.1,2 The most common skin manifestation associated with cladribine therapy is a morbilliform rash, but Stevens-Johnson syndrome and toxic epidermal necrolysis (TEN) have been reported.1
Few cases of purpuric eruption secondary to cladribine treatment have been described, and nearly all reports involve concomitant medications such as allopurinol, which our patient was taking, and antibiotics including trimethoprim-sulfamethoxazole and penicillins.1,3,4 In a cohort of 35 patients receiving cladribine,1 only concomitant treatment with cladribine and allopurinol caused cutaneous reactions, further supporting the hypothesis of cladribine-induced drug sensitivity. Allopurinol often is prescribed during induction therapy for prophylaxis against tumor lysis syndrome; similarly, antibiotics frequently are given prophylactically and therapeutically for neutropenic fever. It is believed that T-cell imbalance and profound lymphopenia induced by cladribine increase susceptibility to drug hypersensitivity reactions.1,3
The typical purpuric eruption develops within 2 days of starting cladribine therapy. Diascopy will reveal petechiae, and biopsy should be performed to rule out other serious drug-induced reactions, such as erythema multiforme, Stevens-Johnson syndrome, and TEN. A cladribine-induced purpuric eruption typically is self-resolving and carries a favorable prognosis, though high-dose corticosteroids often are prescribed to hasten recovery. The rare reports of serious cutaneous reactions secondary to cladribine therapy have been with maculopapular, not purpuric eruptions.2 Based on limited available data, cladribine-induced purpura should not be a limitation to continued treatment in patients who need it.1 Careful consideration of concomitant drug use is necessary, as the current literature demonstrates resolution of rash with withdrawal of other therapies, namely allopurinol.2-4 Future studies are needed to examine the safety of withholding offending medications and to further elucidate the mechanisms contributing to drug hypersensitivity due to cladribine.
Widespread purpura and petechiae can pose a wide differential; the patient’s recent history of cladribine administration pointed to a classic purpuric eruption. Other diagnoses such as toxic erythema of chemotherapy (TEC) and TEN are not purpuric, though plaques can be violaceous. Lack of bullae, blisters, and facial or mucosal surface involvement suggest TEN.5 Thrombotic thrombocytopenic purpura and disseminated intravascular coagulation do manifest with petechiae and purpura, though such a robust eruption in the context of recent cladribine therapy is less likely. The classic retiform purpura and necrosis were not present to suggest purpura fulminans from disseminated intravascular coagulation.
Several of the proposed diagnoses as well as a purpuric drug eruption would demonstrate extravasated red blood cells on histopathology, but the presence of interface dermatitis narrows the differential to a purpuric drug eruption. Necrotic keratinocytes and full-thickness necrosis were not present on biopsy to support a diagnosis of TEN in our patient. Characteristic features of TEC—including eccrine squamous syringometaplasia, dermal edema, and keratinocyte atypia—were not present on biopsy.6 Finally, although TEN should resolve with steroid treatment, TEC is self-limited and thrombotic thrombocytopenic purpura and disseminated intravascular coagulation would not resolve with use of steroids alone.
- Ganzel C, Gatt ME, Maly A, et al. High incidence of skin rash in patients with hairy cell leukemia treated with cladribine. Leuk Lymphoma. 2012;53:1169-1173. doi:10.3109/10428194.2011.635864
- Chubar Y, Bennett M. Cutaneous reactions in hairy cell leukaemia treated with 2-chlorodeoxyadenosine and allopurinol. Br J Haematol. 2003;122:768-770. doi:10.1046/j.1365-2141.2003.04506.x
- Espinosa Lara P, Quirós Redondo V, Aguado Lobo M, et al. Purpuric exanthema in a patient with hairy cell leukemia treated with cladribine and allopurinol. Ann Hematol. 2017;96:1209-1210. doi:10.1007 /s00277-017-2992-z
- Hendrick A. Purpuric rash following treatment with 2-chlorodeoxyadenosine. Clin Lab Haematol. 2001;23:67-68. doi:10.1046 /j.1365-2257.2001.0346b.x
- Kang S, Amagai M, Bruckner AL, et al, eds. Fitzpatrick’s Dermatology. 9th ed. McGraw-Hill Education; 2019.
- Bolognia JL, Cooper DL, Glusac EJ. Toxic erythema of chemotherapy: a useful clinical term. J Am Acad Dermatol. 2008;59:524-529.
The Diagnosis: Purpuric Drug Eruption
Histopathology revealed interface dermatitis, spongiosis, and a perivascular lymphocytic infiltrate with extravasated red blood cells consistent with a purpuric drug eruption. Our patient achieved remission of hairy cell leukemia after receiving only 2 of 5 expected doses of cladribine. The rash resolved completely in 3 weeks following a prednisone taper (Figure).

Hairy cell leukemia is a rare indolent lymphoproliferative disorder of B cells that accounts for approximately 2% of adult leukemias in the United States. Cladribine, a purine nucleoside analog that impairs DNA synthesis and repair, has become the mainstay of therapy, demonstrating a 95% complete response rate.1 Although few reports have addressed the cutaneous reactions seen with cladribine therapy, they can occur in more than 50% of patients.1,2 The most common skin manifestation associated with cladribine therapy is a morbilliform rash, but Stevens-Johnson syndrome and toxic epidermal necrolysis (TEN) have been reported.1
Few cases of purpuric eruption secondary to cladribine treatment have been described, and nearly all reports involve concomitant medications such as allopurinol, which our patient was taking, and antibiotics including trimethoprim-sulfamethoxazole and penicillins.1,3,4 In a cohort of 35 patients receiving cladribine,1 only concomitant treatment with cladribine and allopurinol caused cutaneous reactions, further supporting the hypothesis of cladribine-induced drug sensitivity. Allopurinol often is prescribed during induction therapy for prophylaxis against tumor lysis syndrome; similarly, antibiotics frequently are given prophylactically and therapeutically for neutropenic fever. It is believed that T-cell imbalance and profound lymphopenia induced by cladribine increase susceptibility to drug hypersensitivity reactions.1,3
The typical purpuric eruption develops within 2 days of starting cladribine therapy. Diascopy will reveal petechiae, and biopsy should be performed to rule out other serious drug-induced reactions, such as erythema multiforme, Stevens-Johnson syndrome, and TEN. A cladribine-induced purpuric eruption typically is self-resolving and carries a favorable prognosis, though high-dose corticosteroids often are prescribed to hasten recovery. The rare reports of serious cutaneous reactions secondary to cladribine therapy have been with maculopapular, not purpuric eruptions.2 Based on limited available data, cladribine-induced purpura should not be a limitation to continued treatment in patients who need it.1 Careful consideration of concomitant drug use is necessary, as the current literature demonstrates resolution of rash with withdrawal of other therapies, namely allopurinol.2-4 Future studies are needed to examine the safety of withholding offending medications and to further elucidate the mechanisms contributing to drug hypersensitivity due to cladribine.
Widespread purpura and petechiae can pose a wide differential; the patient’s recent history of cladribine administration pointed to a classic purpuric eruption. Other diagnoses such as toxic erythema of chemotherapy (TEC) and TEN are not purpuric, though plaques can be violaceous. Lack of bullae, blisters, and facial or mucosal surface involvement suggest TEN.5 Thrombotic thrombocytopenic purpura and disseminated intravascular coagulation do manifest with petechiae and purpura, though such a robust eruption in the context of recent cladribine therapy is less likely. The classic retiform purpura and necrosis were not present to suggest purpura fulminans from disseminated intravascular coagulation.
Several of the proposed diagnoses as well as a purpuric drug eruption would demonstrate extravasated red blood cells on histopathology, but the presence of interface dermatitis narrows the differential to a purpuric drug eruption. Necrotic keratinocytes and full-thickness necrosis were not present on biopsy to support a diagnosis of TEN in our patient. Characteristic features of TEC—including eccrine squamous syringometaplasia, dermal edema, and keratinocyte atypia—were not present on biopsy.6 Finally, although TEN should resolve with steroid treatment, TEC is self-limited and thrombotic thrombocytopenic purpura and disseminated intravascular coagulation would not resolve with use of steroids alone.
The Diagnosis: Purpuric Drug Eruption
Histopathology revealed interface dermatitis, spongiosis, and a perivascular lymphocytic infiltrate with extravasated red blood cells consistent with a purpuric drug eruption. Our patient achieved remission of hairy cell leukemia after receiving only 2 of 5 expected doses of cladribine. The rash resolved completely in 3 weeks following a prednisone taper (Figure).

Hairy cell leukemia is a rare indolent lymphoproliferative disorder of B cells that accounts for approximately 2% of adult leukemias in the United States. Cladribine, a purine nucleoside analog that impairs DNA synthesis and repair, has become the mainstay of therapy, demonstrating a 95% complete response rate.1 Although few reports have addressed the cutaneous reactions seen with cladribine therapy, they can occur in more than 50% of patients.1,2 The most common skin manifestation associated with cladribine therapy is a morbilliform rash, but Stevens-Johnson syndrome and toxic epidermal necrolysis (TEN) have been reported.1
Few cases of purpuric eruption secondary to cladribine treatment have been described, and nearly all reports involve concomitant medications such as allopurinol, which our patient was taking, and antibiotics including trimethoprim-sulfamethoxazole and penicillins.1,3,4 In a cohort of 35 patients receiving cladribine,1 only concomitant treatment with cladribine and allopurinol caused cutaneous reactions, further supporting the hypothesis of cladribine-induced drug sensitivity. Allopurinol often is prescribed during induction therapy for prophylaxis against tumor lysis syndrome; similarly, antibiotics frequently are given prophylactically and therapeutically for neutropenic fever. It is believed that T-cell imbalance and profound lymphopenia induced by cladribine increase susceptibility to drug hypersensitivity reactions.1,3
The typical purpuric eruption develops within 2 days of starting cladribine therapy. Diascopy will reveal petechiae, and biopsy should be performed to rule out other serious drug-induced reactions, such as erythema multiforme, Stevens-Johnson syndrome, and TEN. A cladribine-induced purpuric eruption typically is self-resolving and carries a favorable prognosis, though high-dose corticosteroids often are prescribed to hasten recovery. The rare reports of serious cutaneous reactions secondary to cladribine therapy have been with maculopapular, not purpuric eruptions.2 Based on limited available data, cladribine-induced purpura should not be a limitation to continued treatment in patients who need it.1 Careful consideration of concomitant drug use is necessary, as the current literature demonstrates resolution of rash with withdrawal of other therapies, namely allopurinol.2-4 Future studies are needed to examine the safety of withholding offending medications and to further elucidate the mechanisms contributing to drug hypersensitivity due to cladribine.
Widespread purpura and petechiae can pose a wide differential; the patient’s recent history of cladribine administration pointed to a classic purpuric eruption. Other diagnoses such as toxic erythema of chemotherapy (TEC) and TEN are not purpuric, though plaques can be violaceous. Lack of bullae, blisters, and facial or mucosal surface involvement suggest TEN.5 Thrombotic thrombocytopenic purpura and disseminated intravascular coagulation do manifest with petechiae and purpura, though such a robust eruption in the context of recent cladribine therapy is less likely. The classic retiform purpura and necrosis were not present to suggest purpura fulminans from disseminated intravascular coagulation.
Several of the proposed diagnoses as well as a purpuric drug eruption would demonstrate extravasated red blood cells on histopathology, but the presence of interface dermatitis narrows the differential to a purpuric drug eruption. Necrotic keratinocytes and full-thickness necrosis were not present on biopsy to support a diagnosis of TEN in our patient. Characteristic features of TEC—including eccrine squamous syringometaplasia, dermal edema, and keratinocyte atypia—were not present on biopsy.6 Finally, although TEN should resolve with steroid treatment, TEC is self-limited and thrombotic thrombocytopenic purpura and disseminated intravascular coagulation would not resolve with use of steroids alone.
- Ganzel C, Gatt ME, Maly A, et al. High incidence of skin rash in patients with hairy cell leukemia treated with cladribine. Leuk Lymphoma. 2012;53:1169-1173. doi:10.3109/10428194.2011.635864
- Chubar Y, Bennett M. Cutaneous reactions in hairy cell leukaemia treated with 2-chlorodeoxyadenosine and allopurinol. Br J Haematol. 2003;122:768-770. doi:10.1046/j.1365-2141.2003.04506.x
- Espinosa Lara P, Quirós Redondo V, Aguado Lobo M, et al. Purpuric exanthema in a patient with hairy cell leukemia treated with cladribine and allopurinol. Ann Hematol. 2017;96:1209-1210. doi:10.1007 /s00277-017-2992-z
- Hendrick A. Purpuric rash following treatment with 2-chlorodeoxyadenosine. Clin Lab Haematol. 2001;23:67-68. doi:10.1046 /j.1365-2257.2001.0346b.x
- Kang S, Amagai M, Bruckner AL, et al, eds. Fitzpatrick’s Dermatology. 9th ed. McGraw-Hill Education; 2019.
- Bolognia JL, Cooper DL, Glusac EJ. Toxic erythema of chemotherapy: a useful clinical term. J Am Acad Dermatol. 2008;59:524-529.
- Ganzel C, Gatt ME, Maly A, et al. High incidence of skin rash in patients with hairy cell leukemia treated with cladribine. Leuk Lymphoma. 2012;53:1169-1173. doi:10.3109/10428194.2011.635864
- Chubar Y, Bennett M. Cutaneous reactions in hairy cell leukaemia treated with 2-chlorodeoxyadenosine and allopurinol. Br J Haematol. 2003;122:768-770. doi:10.1046/j.1365-2141.2003.04506.x
- Espinosa Lara P, Quirós Redondo V, Aguado Lobo M, et al. Purpuric exanthema in a patient with hairy cell leukemia treated with cladribine and allopurinol. Ann Hematol. 2017;96:1209-1210. doi:10.1007 /s00277-017-2992-z
- Hendrick A. Purpuric rash following treatment with 2-chlorodeoxyadenosine. Clin Lab Haematol. 2001;23:67-68. doi:10.1046 /j.1365-2257.2001.0346b.x
- Kang S, Amagai M, Bruckner AL, et al, eds. Fitzpatrick’s Dermatology. 9th ed. McGraw-Hill Education; 2019.
- Bolognia JL, Cooper DL, Glusac EJ. Toxic erythema of chemotherapy: a useful clinical term. J Am Acad Dermatol. 2008;59:524-529.
A 68-year-old woman presented to the emergency department with neutropenic fever and a rash over the body after receiving 2 doses of cladribine therapy for hairy cell leukemia. Physical examination demonstrated marked facial (top), lip, and tongue swelling, as well as a diffuse dusky nonpalpable purpuric rash on the abdomen (bottom) and back involving 90% of the body surface area. Bilateral ear edema was appreciated with accentuation of the earlobe crease. The patient exhibited subconjunctival hemorrhage, ectropion, and scleral injection. A punch biopsy of the thigh was performed.