User login
Aerobic exercise may mitigate age-related cognitive decline
published in Neurology.
“The effect of aerobic exercise on executive function was more pronounced as age increased, suggesting that it may mitigate age-related declines,” wrote Yaakov Stern, PhD, chief of cognitive neuroscience in the department of neurology at Columbia University, New York, and his research colleagues.
Research indicates that aerobic exercise provides cognitive benefits across the lifespan, but controlled exercise studies have been limited to elderly individuals, the researchers wrote. To examine the effects of aerobic exercise on cognitive function in younger, healthy adults, they conducted a randomized, parallel-group, observer-masked, community-based clinical trial. The investigators enrolled 132 cognitively normal people aged 20-67 years with aerobic capacity below the median. About 70% were women, and participants’ mean age was about 40 years.
“We hypothesized that aerobic exercise would have cognitive benefits, even in this younger age range, but that age might moderate the nature or degree of the benefit,” Dr. Stern and his colleagues wrote.
Participants were nonsmoking, habitual nonexercisers with below-average fitness by American Heart Association standards. The investigators used baseline aerobic capacity testing to establish safe exercise measures and heart rate targets.
The investigators randomly assigned participants to a group that performed aerobic exercise or to a control group that performed stretching and toning four times per week for 6 months. Outcome measures included domains of cognitive function (such as executive function, episodic memory, processing speed, language, and attention), everyday function, aerobic capacity, body mass index, and cortical thickness.
During a 2-week run-in period, participants went to their choice of five YMCA of New York City fitness centers three times per week. They had to attend at least five of these sessions to stay in the study. In both study arms, training sessions consisted of 10-15 minutes of warm-up and cooldown and 30-40 minutes of workout. Coaches contacted participants weekly to monitor their progress, and participants wore heart rate monitors during each session. Exercises in the control group were designed to promote flexibility and improve core strength. In the aerobic exercise group, participants had a choice of exercises such as walking on a treadmill, cycling on a stationary bike, or using an elliptical machine, and they gradually increased their exercise intensity to 75% of maximum heart rate by week 5. A total of 94 participants – 50 in the control group and 44 in the aerobic exercise group – completed the 6-month trial.
Executive function, but not other cognitive measures, improved significantly in the aerobic exercise group. The effect on executive function was greater in older participants. For example, at age 40 years, the executive function measure increased by 0.228 standard deviation units from baseline; at age 60, it increased by 0.596 standard deviation units.
In addition, cortical thickness increased significantly in the aerobic exercise group in the left caudal middle frontal cortex Brodmann area; this effect did not differ by age. Improvement on executive function in the aerobic exercise group was greater among participants without an APOE E4 allele, contrasting with the findings of prior studies.
“Since a difference of 0.5 standard deviations is equivalent to 20 years of age-related difference in performance on these tests, the people who exercised were testing as if they were about 10 years younger at age 40 and about 20 years younger at age 60,” Dr. Stern said in a press release. “Since thinking skills at the start of the study were poorer for participants who were older, our findings suggest that aerobic exercise is more likely to improve age-related declines in thinking skills rather than improve performance in those without a decline.”
Furthermore, aerobic exercise significantly increased aerobic capacity and significantly decreased body mass index, whereas stretching and toning did not.
“Participants in this trial scheduled their exercise sessions on their own and exercised by themselves,” the authors noted. “In addition, they were allowed to choose whatever aerobic exercise modality they preferred, so long as they reached target heart rates, enhancing the flexibility of the intervention.” Limitations of the study include its relatively small sample size and the large number of participants who dropped out of the study between consenting to participate and randomization.
The trial was funded by the National Institutes of Health. Dr. Stern reported receiving a grant from the California Walnut Commission and consulting with Eli Lilly, Axovant Sciences, Takeda, and AbbVie. A coauthor reported grant support from AposTherapy, LIH Medical, and the Everest Foundation.
SOURCE: Stern Y et al. Neurology. 2019 Jan 30. doi: 10.1212/WNL.0000000000007003.
published in Neurology.
“The effect of aerobic exercise on executive function was more pronounced as age increased, suggesting that it may mitigate age-related declines,” wrote Yaakov Stern, PhD, chief of cognitive neuroscience in the department of neurology at Columbia University, New York, and his research colleagues.
Research indicates that aerobic exercise provides cognitive benefits across the lifespan, but controlled exercise studies have been limited to elderly individuals, the researchers wrote. To examine the effects of aerobic exercise on cognitive function in younger, healthy adults, they conducted a randomized, parallel-group, observer-masked, community-based clinical trial. The investigators enrolled 132 cognitively normal people aged 20-67 years with aerobic capacity below the median. About 70% were women, and participants’ mean age was about 40 years.
“We hypothesized that aerobic exercise would have cognitive benefits, even in this younger age range, but that age might moderate the nature or degree of the benefit,” Dr. Stern and his colleagues wrote.
Participants were nonsmoking, habitual nonexercisers with below-average fitness by American Heart Association standards. The investigators used baseline aerobic capacity testing to establish safe exercise measures and heart rate targets.
The investigators randomly assigned participants to a group that performed aerobic exercise or to a control group that performed stretching and toning four times per week for 6 months. Outcome measures included domains of cognitive function (such as executive function, episodic memory, processing speed, language, and attention), everyday function, aerobic capacity, body mass index, and cortical thickness.
During a 2-week run-in period, participants went to their choice of five YMCA of New York City fitness centers three times per week. They had to attend at least five of these sessions to stay in the study. In both study arms, training sessions consisted of 10-15 minutes of warm-up and cooldown and 30-40 minutes of workout. Coaches contacted participants weekly to monitor their progress, and participants wore heart rate monitors during each session. Exercises in the control group were designed to promote flexibility and improve core strength. In the aerobic exercise group, participants had a choice of exercises such as walking on a treadmill, cycling on a stationary bike, or using an elliptical machine, and they gradually increased their exercise intensity to 75% of maximum heart rate by week 5. A total of 94 participants – 50 in the control group and 44 in the aerobic exercise group – completed the 6-month trial.
Executive function, but not other cognitive measures, improved significantly in the aerobic exercise group. The effect on executive function was greater in older participants. For example, at age 40 years, the executive function measure increased by 0.228 standard deviation units from baseline; at age 60, it increased by 0.596 standard deviation units.
In addition, cortical thickness increased significantly in the aerobic exercise group in the left caudal middle frontal cortex Brodmann area; this effect did not differ by age. Improvement on executive function in the aerobic exercise group was greater among participants without an APOE E4 allele, contrasting with the findings of prior studies.
“Since a difference of 0.5 standard deviations is equivalent to 20 years of age-related difference in performance on these tests, the people who exercised were testing as if they were about 10 years younger at age 40 and about 20 years younger at age 60,” Dr. Stern said in a press release. “Since thinking skills at the start of the study were poorer for participants who were older, our findings suggest that aerobic exercise is more likely to improve age-related declines in thinking skills rather than improve performance in those without a decline.”
Furthermore, aerobic exercise significantly increased aerobic capacity and significantly decreased body mass index, whereas stretching and toning did not.
“Participants in this trial scheduled their exercise sessions on their own and exercised by themselves,” the authors noted. “In addition, they were allowed to choose whatever aerobic exercise modality they preferred, so long as they reached target heart rates, enhancing the flexibility of the intervention.” Limitations of the study include its relatively small sample size and the large number of participants who dropped out of the study between consenting to participate and randomization.
The trial was funded by the National Institutes of Health. Dr. Stern reported receiving a grant from the California Walnut Commission and consulting with Eli Lilly, Axovant Sciences, Takeda, and AbbVie. A coauthor reported grant support from AposTherapy, LIH Medical, and the Everest Foundation.
SOURCE: Stern Y et al. Neurology. 2019 Jan 30. doi: 10.1212/WNL.0000000000007003.
published in Neurology.
“The effect of aerobic exercise on executive function was more pronounced as age increased, suggesting that it may mitigate age-related declines,” wrote Yaakov Stern, PhD, chief of cognitive neuroscience in the department of neurology at Columbia University, New York, and his research colleagues.
Research indicates that aerobic exercise provides cognitive benefits across the lifespan, but controlled exercise studies have been limited to elderly individuals, the researchers wrote. To examine the effects of aerobic exercise on cognitive function in younger, healthy adults, they conducted a randomized, parallel-group, observer-masked, community-based clinical trial. The investigators enrolled 132 cognitively normal people aged 20-67 years with aerobic capacity below the median. About 70% were women, and participants’ mean age was about 40 years.
“We hypothesized that aerobic exercise would have cognitive benefits, even in this younger age range, but that age might moderate the nature or degree of the benefit,” Dr. Stern and his colleagues wrote.
Participants were nonsmoking, habitual nonexercisers with below-average fitness by American Heart Association standards. The investigators used baseline aerobic capacity testing to establish safe exercise measures and heart rate targets.
The investigators randomly assigned participants to a group that performed aerobic exercise or to a control group that performed stretching and toning four times per week for 6 months. Outcome measures included domains of cognitive function (such as executive function, episodic memory, processing speed, language, and attention), everyday function, aerobic capacity, body mass index, and cortical thickness.
During a 2-week run-in period, participants went to their choice of five YMCA of New York City fitness centers three times per week. They had to attend at least five of these sessions to stay in the study. In both study arms, training sessions consisted of 10-15 minutes of warm-up and cooldown and 30-40 minutes of workout. Coaches contacted participants weekly to monitor their progress, and participants wore heart rate monitors during each session. Exercises in the control group were designed to promote flexibility and improve core strength. In the aerobic exercise group, participants had a choice of exercises such as walking on a treadmill, cycling on a stationary bike, or using an elliptical machine, and they gradually increased their exercise intensity to 75% of maximum heart rate by week 5. A total of 94 participants – 50 in the control group and 44 in the aerobic exercise group – completed the 6-month trial.
Executive function, but not other cognitive measures, improved significantly in the aerobic exercise group. The effect on executive function was greater in older participants. For example, at age 40 years, the executive function measure increased by 0.228 standard deviation units from baseline; at age 60, it increased by 0.596 standard deviation units.
In addition, cortical thickness increased significantly in the aerobic exercise group in the left caudal middle frontal cortex Brodmann area; this effect did not differ by age. Improvement on executive function in the aerobic exercise group was greater among participants without an APOE E4 allele, contrasting with the findings of prior studies.
“Since a difference of 0.5 standard deviations is equivalent to 20 years of age-related difference in performance on these tests, the people who exercised were testing as if they were about 10 years younger at age 40 and about 20 years younger at age 60,” Dr. Stern said in a press release. “Since thinking skills at the start of the study were poorer for participants who were older, our findings suggest that aerobic exercise is more likely to improve age-related declines in thinking skills rather than improve performance in those without a decline.”
Furthermore, aerobic exercise significantly increased aerobic capacity and significantly decreased body mass index, whereas stretching and toning did not.
“Participants in this trial scheduled their exercise sessions on their own and exercised by themselves,” the authors noted. “In addition, they were allowed to choose whatever aerobic exercise modality they preferred, so long as they reached target heart rates, enhancing the flexibility of the intervention.” Limitations of the study include its relatively small sample size and the large number of participants who dropped out of the study between consenting to participate and randomization.
The trial was funded by the National Institutes of Health. Dr. Stern reported receiving a grant from the California Walnut Commission and consulting with Eli Lilly, Axovant Sciences, Takeda, and AbbVie. A coauthor reported grant support from AposTherapy, LIH Medical, and the Everest Foundation.
SOURCE: Stern Y et al. Neurology. 2019 Jan 30. doi: 10.1212/WNL.0000000000007003.
FROM NEUROLOGY
Key clinical point: Among adults with below-average fitness, a 6-month aerobic exercise program significantly improves executive function.
Major finding: The effect is more pronounced as age increases.
Study details: A randomized, parallel-group, observer-masked, community-based clinical trial of 132 cognitively normal adults aged 20-67 years.
Disclosures: The study was funded by the National Institutes of Health. Dr. Stern reported receiving a grant from the California Walnut Commission and consulted with Eli Lilly, Axovant Sciences, Takeda, and AbbVie. Another reported grant support from AposTherapy, LIH Medical, and the Everest Foundation.
Source: Stern Y et al. Neurology. 2019 Jan 30. doi: 10.1212/WNL.0000000000007003.
Lifetime cost of tobacco use tops $1.9 million per smoker
according to the personal financial website WalletHub.
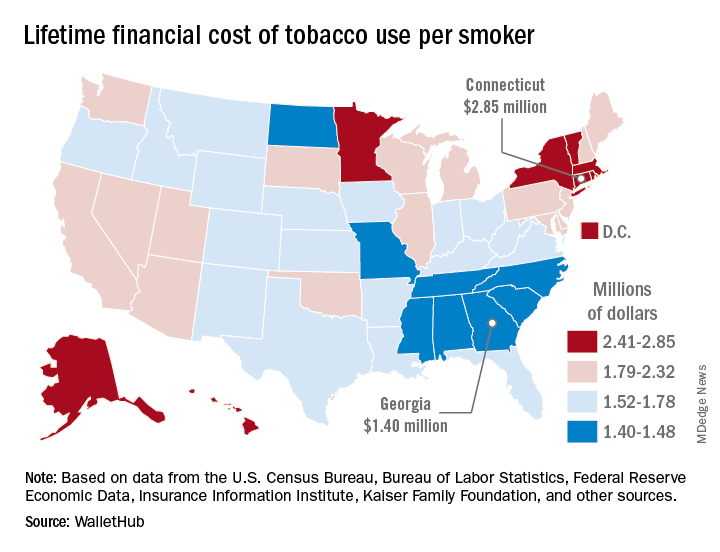
Economic and societal losses related to 37.8 million U.S. tobacco users – including out-of-pocket spending for cigarettes, health care expenses, and lost income – top $300 billion annually, but those costs vary considerably by state, WalletHub said in a recent report.
The state with the highest lifetime cost per smoker is Connecticut, with an estimated total of $2.85 million. That works out to just under $56,000 a year for 51 years because lifetime use was defined as one pack a day starting at age 18 years and continuing until age 69 years. New York has the second-highest lifetime cost, which also rounds off to $2.85 million, followed by the District of Columbia ($2.81 million), Massachusetts ($2.76 million), and Rhode Island ($2.68 million), WalletHub said.
Georgia has the lowest lifetime cost of any state – $1.40 million per smoker – followed by Missouri at $1.41 million, North Carolina at $1.42 million, Mississippi at $1.43 million, and South Carolina at $1.44 million, according to the report.
WalletHub’s formula for total lifetime cost has five components: out-of-pocket cost (one pack of cigarettes per day for 51 years), financial opportunity cost (defined as “the amount of return a person would have earned by instead investing that money in the stock market”), health care cost (spending on treatment for smoking-related health complications), income loss (an average 8% decrease caused by absenteeism and lost productivity), and other costs (loss of a homeowner’s insurance credit and costs of secondhand exposure).
The analysis was based on data from the U.S. Census Bureau, Bureau of Labor Statistics, Centers for Disease Control and Prevention, Insurance Information Institute, Campaign for Tobacco-Free Kids, NYsmokefree.com, Federal Reserve Economic Data, Kaiser Family Foundation, and the Independent Insurance Agents & Brokers of America.
according to the personal financial website WalletHub.

Economic and societal losses related to 37.8 million U.S. tobacco users – including out-of-pocket spending for cigarettes, health care expenses, and lost income – top $300 billion annually, but those costs vary considerably by state, WalletHub said in a recent report.
The state with the highest lifetime cost per smoker is Connecticut, with an estimated total of $2.85 million. That works out to just under $56,000 a year for 51 years because lifetime use was defined as one pack a day starting at age 18 years and continuing until age 69 years. New York has the second-highest lifetime cost, which also rounds off to $2.85 million, followed by the District of Columbia ($2.81 million), Massachusetts ($2.76 million), and Rhode Island ($2.68 million), WalletHub said.
Georgia has the lowest lifetime cost of any state – $1.40 million per smoker – followed by Missouri at $1.41 million, North Carolina at $1.42 million, Mississippi at $1.43 million, and South Carolina at $1.44 million, according to the report.
WalletHub’s formula for total lifetime cost has five components: out-of-pocket cost (one pack of cigarettes per day for 51 years), financial opportunity cost (defined as “the amount of return a person would have earned by instead investing that money in the stock market”), health care cost (spending on treatment for smoking-related health complications), income loss (an average 8% decrease caused by absenteeism and lost productivity), and other costs (loss of a homeowner’s insurance credit and costs of secondhand exposure).
The analysis was based on data from the U.S. Census Bureau, Bureau of Labor Statistics, Centers for Disease Control and Prevention, Insurance Information Institute, Campaign for Tobacco-Free Kids, NYsmokefree.com, Federal Reserve Economic Data, Kaiser Family Foundation, and the Independent Insurance Agents & Brokers of America.
according to the personal financial website WalletHub.

Economic and societal losses related to 37.8 million U.S. tobacco users – including out-of-pocket spending for cigarettes, health care expenses, and lost income – top $300 billion annually, but those costs vary considerably by state, WalletHub said in a recent report.
The state with the highest lifetime cost per smoker is Connecticut, with an estimated total of $2.85 million. That works out to just under $56,000 a year for 51 years because lifetime use was defined as one pack a day starting at age 18 years and continuing until age 69 years. New York has the second-highest lifetime cost, which also rounds off to $2.85 million, followed by the District of Columbia ($2.81 million), Massachusetts ($2.76 million), and Rhode Island ($2.68 million), WalletHub said.
Georgia has the lowest lifetime cost of any state – $1.40 million per smoker – followed by Missouri at $1.41 million, North Carolina at $1.42 million, Mississippi at $1.43 million, and South Carolina at $1.44 million, according to the report.
WalletHub’s formula for total lifetime cost has five components: out-of-pocket cost (one pack of cigarettes per day for 51 years), financial opportunity cost (defined as “the amount of return a person would have earned by instead investing that money in the stock market”), health care cost (spending on treatment for smoking-related health complications), income loss (an average 8% decrease caused by absenteeism and lost productivity), and other costs (loss of a homeowner’s insurance credit and costs of secondhand exposure).
The analysis was based on data from the U.S. Census Bureau, Bureau of Labor Statistics, Centers for Disease Control and Prevention, Insurance Information Institute, Campaign for Tobacco-Free Kids, NYsmokefree.com, Federal Reserve Economic Data, Kaiser Family Foundation, and the Independent Insurance Agents & Brokers of America.
Pulmonary hypertension linked to complications after head and neck procedures
SAN DIEGO – Patients with , compared with their counterparts who do not have the condition. They also face an increase in total charges and length of stay.
The findings come from what is believed to be the first study of its kind to investigate the impact of pulmonary hypertension (PHTN) on major head and neck procedures. “PHTN is a common condition which affects the caliber of lung vasculature, with varied symptom presentation from shortness of breath to syncope, with an estimated prevalence of 2.5 to 7.1 million new cases each year worldwide,” one of the study authors, Nirali M. Patel, said at the Triological Society’s Combined Sections Meeting. “Due to improved therapeutic options, there is an enhanced survival of PHTN patients and higher prevalence of this disease. PHTN has some significant systemic implications. Therefore, cardiopulmonary clearance and a clear understanding of postoperative complications are very important.”
Previous studies have shown PHTN to be an independent predictor of morbidity and mortality in noncardiac procedures, said Ms. Patel, a fourth-year medical student at New Jersey Medical School, Newark. Furthermore, the rate of pulmonary complications is reported to be between 11% and 44.8% following radical head and neck cancer resection. “Despite these findings, there is currently a lack of information regarding perioperative morbidity and mortality of patients with PHTN undergoing major head and neck procedures,” she said.
The researchers queried the National Inpatient Survey from 2002 to 2013 for all cases of major head and neck surgery based on the ICD-9 codes for esophagectomy, glossectomy, laryngectomy, mandibulectomy, and pharyngectomy. They divided patients into two groups: those who had PHTN and those who did not, and performed demographic analyses as well as univariate and multivariate regression analyses.
Ms. Patel reported findings from a cohort of 46,311 patients. Of these, 46,073 had PHTN and 238 did not. The two groups were similar in age (a mean of 69 vs. 60 years in those with and without PHTN, respectively) and race (80% white vs. 79% white, respectively), but there were significantly fewer male patients in the PHTN group (57% vs. 70%; P less than .0001).
Several patient comorbidities were increased in the PHTN group, compared with the non-PHTN group, including coagulopathy (8.4% vs. 3.1%; P less than .0001), chronic heart failure (22.4% vs. 4.1%; P less than .0001), complicated diabetes (4.6% vs. 1.2%; P less than .0001), fluid and electrolyte disorders (30% vs. 18.3%; P less than .0001), and hypertension (63.3% vs. 43.7%; P less than .0001).
Postoperatively, patients with PHTN had a longer length of stay (a mean of 15.80 vs. 11.50 days, respectively; P less than .0001) as well as significantly higher total charges (a mean of $162,021.06 vs. $107,309.46, respectively; P less than .0001). When the researchers evaluated postoperative outcomes between the two cohorts, patients with PHTN had significantly higher rates of cardiac complications (9.2% vs. 3.5%; P less than .0001), iatrogenic pulmonary embolism (2.1% vs. 0.3%; P = .001), pulmonary edema (2.1% vs. 0.4%; P = .003), venous thrombotic events (4.6% vs. 1%; P less than. 0001), pulmonary insufficiency (17.2% vs. 9.7%; P less than .0001), and pneumonia (9.7% vs. 6%; P = .027), as well as higher rates of postoperative tracheostomy (8.4% vs. 4.5%; P = .007).
Multivariate analysis revealed that the following factors predicted in-hospital mortality: coagulopathy, chronic heart failure, fluid and electrolyte disorders, hyperlipidemia, hypertension, hypothyroidism, liver disease, obesity, paralysis, and renal failure. Despite these findings, there was no significant change in overall hospital mortality for those with PHTN, with an odds ratio of 1.055.
“Our study is not without its limitations, many of which are inherent to the use of a database, which is subject to errors in coding and sampling,” Ms. Patel noted at the meeting, which was jointly sponsored by the Triological Society and the American College of Surgeons. “Despite these limitations, the study provides valuable information on the impact of PHTN on major head and neck procedures.
She reported having no financial disclosures.
SOURCE: Patel N et al. Triological CSM, Abstracts.
SAN DIEGO – Patients with , compared with their counterparts who do not have the condition. They also face an increase in total charges and length of stay.
The findings come from what is believed to be the first study of its kind to investigate the impact of pulmonary hypertension (PHTN) on major head and neck procedures. “PHTN is a common condition which affects the caliber of lung vasculature, with varied symptom presentation from shortness of breath to syncope, with an estimated prevalence of 2.5 to 7.1 million new cases each year worldwide,” one of the study authors, Nirali M. Patel, said at the Triological Society’s Combined Sections Meeting. “Due to improved therapeutic options, there is an enhanced survival of PHTN patients and higher prevalence of this disease. PHTN has some significant systemic implications. Therefore, cardiopulmonary clearance and a clear understanding of postoperative complications are very important.”
Previous studies have shown PHTN to be an independent predictor of morbidity and mortality in noncardiac procedures, said Ms. Patel, a fourth-year medical student at New Jersey Medical School, Newark. Furthermore, the rate of pulmonary complications is reported to be between 11% and 44.8% following radical head and neck cancer resection. “Despite these findings, there is currently a lack of information regarding perioperative morbidity and mortality of patients with PHTN undergoing major head and neck procedures,” she said.
The researchers queried the National Inpatient Survey from 2002 to 2013 for all cases of major head and neck surgery based on the ICD-9 codes for esophagectomy, glossectomy, laryngectomy, mandibulectomy, and pharyngectomy. They divided patients into two groups: those who had PHTN and those who did not, and performed demographic analyses as well as univariate and multivariate regression analyses.
Ms. Patel reported findings from a cohort of 46,311 patients. Of these, 46,073 had PHTN and 238 did not. The two groups were similar in age (a mean of 69 vs. 60 years in those with and without PHTN, respectively) and race (80% white vs. 79% white, respectively), but there were significantly fewer male patients in the PHTN group (57% vs. 70%; P less than .0001).
Several patient comorbidities were increased in the PHTN group, compared with the non-PHTN group, including coagulopathy (8.4% vs. 3.1%; P less than .0001), chronic heart failure (22.4% vs. 4.1%; P less than .0001), complicated diabetes (4.6% vs. 1.2%; P less than .0001), fluid and electrolyte disorders (30% vs. 18.3%; P less than .0001), and hypertension (63.3% vs. 43.7%; P less than .0001).
Postoperatively, patients with PHTN had a longer length of stay (a mean of 15.80 vs. 11.50 days, respectively; P less than .0001) as well as significantly higher total charges (a mean of $162,021.06 vs. $107,309.46, respectively; P less than .0001). When the researchers evaluated postoperative outcomes between the two cohorts, patients with PHTN had significantly higher rates of cardiac complications (9.2% vs. 3.5%; P less than .0001), iatrogenic pulmonary embolism (2.1% vs. 0.3%; P = .001), pulmonary edema (2.1% vs. 0.4%; P = .003), venous thrombotic events (4.6% vs. 1%; P less than. 0001), pulmonary insufficiency (17.2% vs. 9.7%; P less than .0001), and pneumonia (9.7% vs. 6%; P = .027), as well as higher rates of postoperative tracheostomy (8.4% vs. 4.5%; P = .007).
Multivariate analysis revealed that the following factors predicted in-hospital mortality: coagulopathy, chronic heart failure, fluid and electrolyte disorders, hyperlipidemia, hypertension, hypothyroidism, liver disease, obesity, paralysis, and renal failure. Despite these findings, there was no significant change in overall hospital mortality for those with PHTN, with an odds ratio of 1.055.
“Our study is not without its limitations, many of which are inherent to the use of a database, which is subject to errors in coding and sampling,” Ms. Patel noted at the meeting, which was jointly sponsored by the Triological Society and the American College of Surgeons. “Despite these limitations, the study provides valuable information on the impact of PHTN on major head and neck procedures.
She reported having no financial disclosures.
SOURCE: Patel N et al. Triological CSM, Abstracts.
SAN DIEGO – Patients with , compared with their counterparts who do not have the condition. They also face an increase in total charges and length of stay.
The findings come from what is believed to be the first study of its kind to investigate the impact of pulmonary hypertension (PHTN) on major head and neck procedures. “PHTN is a common condition which affects the caliber of lung vasculature, with varied symptom presentation from shortness of breath to syncope, with an estimated prevalence of 2.5 to 7.1 million new cases each year worldwide,” one of the study authors, Nirali M. Patel, said at the Triological Society’s Combined Sections Meeting. “Due to improved therapeutic options, there is an enhanced survival of PHTN patients and higher prevalence of this disease. PHTN has some significant systemic implications. Therefore, cardiopulmonary clearance and a clear understanding of postoperative complications are very important.”
Previous studies have shown PHTN to be an independent predictor of morbidity and mortality in noncardiac procedures, said Ms. Patel, a fourth-year medical student at New Jersey Medical School, Newark. Furthermore, the rate of pulmonary complications is reported to be between 11% and 44.8% following radical head and neck cancer resection. “Despite these findings, there is currently a lack of information regarding perioperative morbidity and mortality of patients with PHTN undergoing major head and neck procedures,” she said.
The researchers queried the National Inpatient Survey from 2002 to 2013 for all cases of major head and neck surgery based on the ICD-9 codes for esophagectomy, glossectomy, laryngectomy, mandibulectomy, and pharyngectomy. They divided patients into two groups: those who had PHTN and those who did not, and performed demographic analyses as well as univariate and multivariate regression analyses.
Ms. Patel reported findings from a cohort of 46,311 patients. Of these, 46,073 had PHTN and 238 did not. The two groups were similar in age (a mean of 69 vs. 60 years in those with and without PHTN, respectively) and race (80% white vs. 79% white, respectively), but there were significantly fewer male patients in the PHTN group (57% vs. 70%; P less than .0001).
Several patient comorbidities were increased in the PHTN group, compared with the non-PHTN group, including coagulopathy (8.4% vs. 3.1%; P less than .0001), chronic heart failure (22.4% vs. 4.1%; P less than .0001), complicated diabetes (4.6% vs. 1.2%; P less than .0001), fluid and electrolyte disorders (30% vs. 18.3%; P less than .0001), and hypertension (63.3% vs. 43.7%; P less than .0001).
Postoperatively, patients with PHTN had a longer length of stay (a mean of 15.80 vs. 11.50 days, respectively; P less than .0001) as well as significantly higher total charges (a mean of $162,021.06 vs. $107,309.46, respectively; P less than .0001). When the researchers evaluated postoperative outcomes between the two cohorts, patients with PHTN had significantly higher rates of cardiac complications (9.2% vs. 3.5%; P less than .0001), iatrogenic pulmonary embolism (2.1% vs. 0.3%; P = .001), pulmonary edema (2.1% vs. 0.4%; P = .003), venous thrombotic events (4.6% vs. 1%; P less than. 0001), pulmonary insufficiency (17.2% vs. 9.7%; P less than .0001), and pneumonia (9.7% vs. 6%; P = .027), as well as higher rates of postoperative tracheostomy (8.4% vs. 4.5%; P = .007).
Multivariate analysis revealed that the following factors predicted in-hospital mortality: coagulopathy, chronic heart failure, fluid and electrolyte disorders, hyperlipidemia, hypertension, hypothyroidism, liver disease, obesity, paralysis, and renal failure. Despite these findings, there was no significant change in overall hospital mortality for those with PHTN, with an odds ratio of 1.055.
“Our study is not without its limitations, many of which are inherent to the use of a database, which is subject to errors in coding and sampling,” Ms. Patel noted at the meeting, which was jointly sponsored by the Triological Society and the American College of Surgeons. “Despite these limitations, the study provides valuable information on the impact of PHTN on major head and neck procedures.
She reported having no financial disclosures.
SOURCE: Patel N et al. Triological CSM, Abstracts.
REPORTING FROM THE TRIOLOGICAL CSM
Key clinical point: Pulmonary hypertension (PHTN) is linked with certain head and neck surgical complications.
Major finding: For patients undergoing head and neck surgery, PHTN is associated with an increased risk of cardiac complications (9.2% vs. 3.5%) and iatrogenic pulmonary embolism (2.1% vs. 0.3%).
Study details: A retrospective analysis of 46,311 patient records from the National Inpatient Survey.
Disclosures: The researchers reported having no financial disclosures.
Source: Patel N et al. Triological CSM, Abstracts.
Guardian angel or watchdog? Pills of capecitabine contain sensors
Recently, a woman with advanced colorectal cancer at the University of Minnesota, Minneapolis, was taking capecitabine (Xeloda) in the morning but skipping her evening dose.
Her hands hurt, and she couldn’t open the childproof cap. Her daughter had been doing it for her in the morning but wasn’t around to help out at night.
It was the kind of problem that might have gone on for days or weeks until the next clinic visit, and, even then, be addressed only if the woman remembered to mention it.
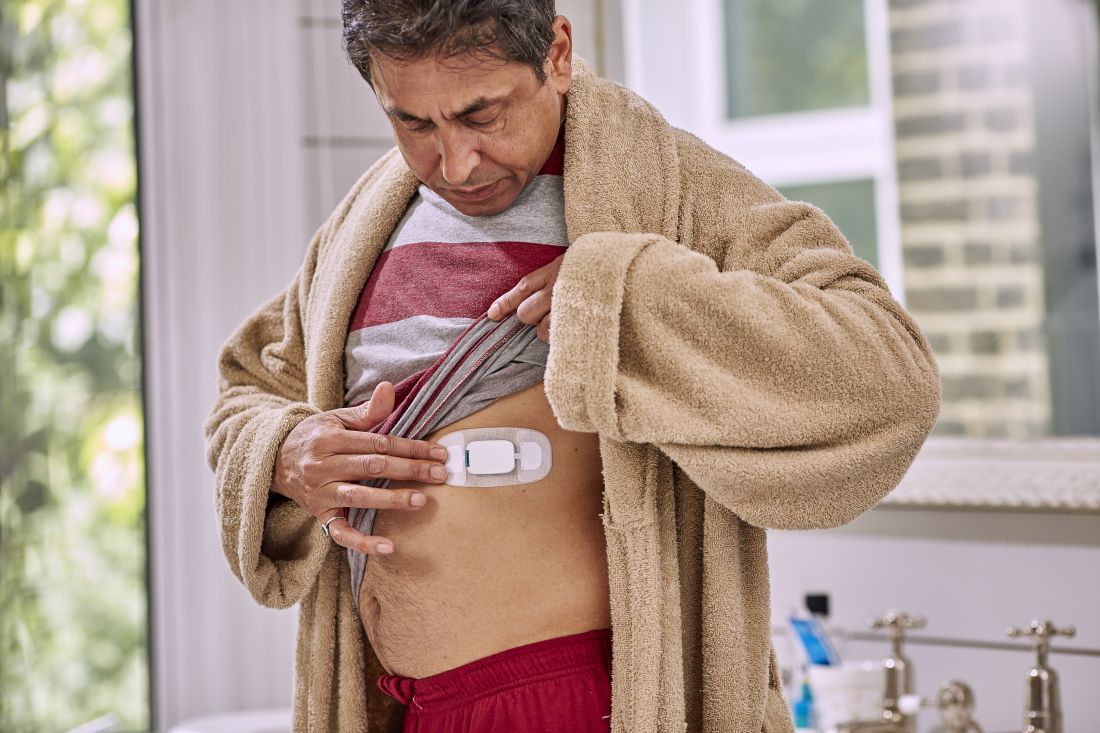
But that’s not what happened. The care team realized pretty much right away that she was skipping the p.m. dose because the woman was taking her capecitabine in a gel cap with an adherence sensor.
The sensor, a sandwich of copper, silicon, and magnesium in a millimeter square, sent out an electric ping when she took her dose, activated by stomach acid; the ping was picked up by an adhesive bandage patch the woman wore, which relayed the signal to an app on her smartphone; the phone passed it on to a server cloud that the woman had given her providers permission to access.
They monitored her adherence on a Web portal, along with heart rate and activity data, also captured by the patch. The system is called Proteus Discover, from Proteus Digital Health.
Instead of taking days or weeks, the care team quickly realized that she wasn’t taking her evening dose of capecitabine. They contacted her, replaced the childproof cap, and twice-daily dosing resumed.
Expanded use in oncology?
Seven other advanced colorectal cancer patients have participated in the University of Minnesota (UM) pilot project, the first use of the device in oncology. “It’s gone so well that it’s annoying to me to not have this for all my patients. I’m already feeling frustrated that I can’t just put this in all the drugs that I give orally,” said Edward Greeno, MD, an oncologist/hematologist at the university, and the Proteus point man.
“You would assume that cancer patients would be hypercompliant, but it turns out they have the same compliance problems” as other patients, plus additional hurdles, he said, including complex regimens and drug toxicity.
“Every patient we have approached so far has been enthusiastic. They might be a little bit annoyed that I know they haven’t been taking their pills, but they are also glad that I am there to hold them responsible and help them. My intention is to roll this out much more broadly,” he said. That just might happen. Dr. Greeno is working with Proteus to roll the sensor out at UM and oncology programs elsewhere.
A slow, careful rollout
The device was cleared by the Food and Drug Administration in 2012. After more than 180,000 ingestions, there have been no safety issues, besides occasional skin irritation from the patch, which is waterproof and meant to be worn for a week, then replaced. It pings if it’s taken off. The sensor is passes through the body like food.
Patients can communicate with providers over the phone app, which also sends reminders when it’s time to take the next pill.
So far, Proteus has worked with ten health systems in the United States, and more are in the works. Commercialization efforts have focused mostly on blood pressure, cholesterol, and type 2 diabetes drugs, the bad boys of drug adherence, but the system has also been piloted for hepatitis C treatment, and trials are underway for HIV preexposure prophylaxis.
Among the company’s many favorable studies, the system has already been demonstrated to be a viable alternative to directly observed therapy in tuberculosis, the current gold-standard, but hugely labor and resource intensive (PLoS One. 2013;8[1]:e53373. doi: 10.1371/journal.pone.0053373).
Proteus can’t be picked up at the local Walgreens. The company works closely with clients and is being careful in its rollout. For one thing, each sensor has to be programed for the specific drug it’s being used with, but also, and as with any new technology, business and payment models are still being worked out.
UM’s partner in the oncology project, Fairview Health Services, pays Proteus when patients hit an adherence rate of 80%, but how much they pay is a proprietary secret. “Most of the cost issue is still not in the public domain,” said Scooter Plowman, MD, the company’s medical director.
In 2017, FDA approved a version of the antipsychotic aripiprazole embedded with the Proteus sensor. The rollout of “Abilify MyCite” by Otsuka Pharmaceuticals has been similarly cautious, under contract with health systems.
“Otsuka has been very smart in the approach they are taking,” Dr. Plowman said.
He wouldn’t give details, but Proteus is in talks with other pharmaceutical makers to bring pills with sensors to the market.
A new fix for an old problem
Proteus isn’t alone in the ingestible event marker (IEM) market. The FDA is reviewing a rival sensor from etectRx, in Gainesville, Fla.
The technology is a little different; the etectRx sensor is a microchip made out of magnesium and silver chloride that’s embedded on the inside of a gel cap. Instead of an electric blip, it sends out a radio wave when activated by stomach acid. It’s larger than the Proteus offering, but still has room to spare in a gel cap.
The signal is stronger, so patients wear a neck pendant instead of a patch to pick it up. The pendant does not capture heart rate or activity data. It’s not meant to be worn continuously and can come off after it pings the system.
The two systems are otherwise similar; etectRx also uses a phone app to relay adherence data to a server cloud clinicians can access, with patient permission. As with Proteus, everything works as long as the phone is on. President and CEO Harry Travis anticipates clearance in 2019.
Adherence is a huge and well-known problem in medicine; only about half of patients take medications as they are prescribed. People end up in the ED or the hospital with problems that might have been avoided. Providers and payers want solutions.
Industry is bringing technology to bear on the problem. The payoff will be huge for the winners; analysts project multiple billion dollar growth in the adherence technology sector.
Most companies, however, are pinning their hopes on indirect approaches, bottle caps that ping when opened, for instance, or coaching apps for smart phones. IEMs seem to be ahead of the curve.
Guardian angel or watchdog?
Whether that’s a good thing or bad thing depends on who you talk to, but patients do seem more likely to take their medications if a sensor is on board.
Dr. Plowman said he thinks IEMs improve adherence because, with their own health at stake, patients want to do better, and IEM systems provide the extra help they need, complete with positive feedback.
But patients also know they are being watched. The technology is barely off the ground, but concerns have already been raised about surveillance. It’s not hard to imagine insurance companies demanding proof of adherence before paying for expensive drugs. There are privacy concerns as well; everything is encrypted with IEMs, but hackers are clever.
Dr. Plowman and Mr. Travis acknowledged the concerns, and also that there’s no way to know how IEMs – if they take off – will play out in coming decades; it’s a lot like the Internet in 1992.
The intent is for the systems to remain voluntary, as they are now, perhaps with inducements for patients to use them, maybe lower insurance premiums.
“There is something inherently personal about swallowable data,” Dr. Plowman said. “It’s something we take tremendous efforts to protect.” As for compulsory use, “we take enormous strides to prevent that. It’s a major priority.”
“Always, there will be an opt-out” option, said Mr. Travis.
It’s important to consider the potential for IEMs to move medicine forward. When patients with acute bone fractures in one study, for instance, were sent home with the usual handful of oxycodone tablets, it turned out that they only took a median of six. Researchers knew that because the subjects took their oxycodone in an etectRx capsule. It’s was an important insight in the midst of an opioid epidemic (Anesth Analg. 2017 Dec;125[6]:2105-12).
Dr. Greeno is an adviser for Proteus; the company covers his travel costs.
Recently, a woman with advanced colorectal cancer at the University of Minnesota, Minneapolis, was taking capecitabine (Xeloda) in the morning but skipping her evening dose.
Her hands hurt, and she couldn’t open the childproof cap. Her daughter had been doing it for her in the morning but wasn’t around to help out at night.
It was the kind of problem that might have gone on for days or weeks until the next clinic visit, and, even then, be addressed only if the woman remembered to mention it.
But that’s not what happened. The care team realized pretty much right away that she was skipping the p.m. dose because the woman was taking her capecitabine in a gel cap with an adherence sensor.
The sensor, a sandwich of copper, silicon, and magnesium in a millimeter square, sent out an electric ping when she took her dose, activated by stomach acid; the ping was picked up by an adhesive bandage patch the woman wore, which relayed the signal to an app on her smartphone; the phone passed it on to a server cloud that the woman had given her providers permission to access.
They monitored her adherence on a Web portal, along with heart rate and activity data, also captured by the patch. The system is called Proteus Discover, from Proteus Digital Health.
Instead of taking days or weeks, the care team quickly realized that she wasn’t taking her evening dose of capecitabine. They contacted her, replaced the childproof cap, and twice-daily dosing resumed.
Expanded use in oncology?
Seven other advanced colorectal cancer patients have participated in the University of Minnesota (UM) pilot project, the first use of the device in oncology. “It’s gone so well that it’s annoying to me to not have this for all my patients. I’m already feeling frustrated that I can’t just put this in all the drugs that I give orally,” said Edward Greeno, MD, an oncologist/hematologist at the university, and the Proteus point man.
“You would assume that cancer patients would be hypercompliant, but it turns out they have the same compliance problems” as other patients, plus additional hurdles, he said, including complex regimens and drug toxicity.
“Every patient we have approached so far has been enthusiastic. They might be a little bit annoyed that I know they haven’t been taking their pills, but they are also glad that I am there to hold them responsible and help them. My intention is to roll this out much more broadly,” he said. That just might happen. Dr. Greeno is working with Proteus to roll the sensor out at UM and oncology programs elsewhere.
A slow, careful rollout
The device was cleared by the Food and Drug Administration in 2012. After more than 180,000 ingestions, there have been no safety issues, besides occasional skin irritation from the patch, which is waterproof and meant to be worn for a week, then replaced. It pings if it’s taken off. The sensor is passes through the body like food.
Patients can communicate with providers over the phone app, which also sends reminders when it’s time to take the next pill.
So far, Proteus has worked with ten health systems in the United States, and more are in the works. Commercialization efforts have focused mostly on blood pressure, cholesterol, and type 2 diabetes drugs, the bad boys of drug adherence, but the system has also been piloted for hepatitis C treatment, and trials are underway for HIV preexposure prophylaxis.
Among the company’s many favorable studies, the system has already been demonstrated to be a viable alternative to directly observed therapy in tuberculosis, the current gold-standard, but hugely labor and resource intensive (PLoS One. 2013;8[1]:e53373. doi: 10.1371/journal.pone.0053373).
Proteus can’t be picked up at the local Walgreens. The company works closely with clients and is being careful in its rollout. For one thing, each sensor has to be programed for the specific drug it’s being used with, but also, and as with any new technology, business and payment models are still being worked out.
UM’s partner in the oncology project, Fairview Health Services, pays Proteus when patients hit an adherence rate of 80%, but how much they pay is a proprietary secret. “Most of the cost issue is still not in the public domain,” said Scooter Plowman, MD, the company’s medical director.
In 2017, FDA approved a version of the antipsychotic aripiprazole embedded with the Proteus sensor. The rollout of “Abilify MyCite” by Otsuka Pharmaceuticals has been similarly cautious, under contract with health systems.
“Otsuka has been very smart in the approach they are taking,” Dr. Plowman said.
He wouldn’t give details, but Proteus is in talks with other pharmaceutical makers to bring pills with sensors to the market.
A new fix for an old problem
Proteus isn’t alone in the ingestible event marker (IEM) market. The FDA is reviewing a rival sensor from etectRx, in Gainesville, Fla.
The technology is a little different; the etectRx sensor is a microchip made out of magnesium and silver chloride that’s embedded on the inside of a gel cap. Instead of an electric blip, it sends out a radio wave when activated by stomach acid. It’s larger than the Proteus offering, but still has room to spare in a gel cap.
The signal is stronger, so patients wear a neck pendant instead of a patch to pick it up. The pendant does not capture heart rate or activity data. It’s not meant to be worn continuously and can come off after it pings the system.
The two systems are otherwise similar; etectRx also uses a phone app to relay adherence data to a server cloud clinicians can access, with patient permission. As with Proteus, everything works as long as the phone is on. President and CEO Harry Travis anticipates clearance in 2019.
Adherence is a huge and well-known problem in medicine; only about half of patients take medications as they are prescribed. People end up in the ED or the hospital with problems that might have been avoided. Providers and payers want solutions.
Industry is bringing technology to bear on the problem. The payoff will be huge for the winners; analysts project multiple billion dollar growth in the adherence technology sector.
Most companies, however, are pinning their hopes on indirect approaches, bottle caps that ping when opened, for instance, or coaching apps for smart phones. IEMs seem to be ahead of the curve.
Guardian angel or watchdog?
Whether that’s a good thing or bad thing depends on who you talk to, but patients do seem more likely to take their medications if a sensor is on board.
Dr. Plowman said he thinks IEMs improve adherence because, with their own health at stake, patients want to do better, and IEM systems provide the extra help they need, complete with positive feedback.
But patients also know they are being watched. The technology is barely off the ground, but concerns have already been raised about surveillance. It’s not hard to imagine insurance companies demanding proof of adherence before paying for expensive drugs. There are privacy concerns as well; everything is encrypted with IEMs, but hackers are clever.
Dr. Plowman and Mr. Travis acknowledged the concerns, and also that there’s no way to know how IEMs – if they take off – will play out in coming decades; it’s a lot like the Internet in 1992.
The intent is for the systems to remain voluntary, as they are now, perhaps with inducements for patients to use them, maybe lower insurance premiums.
“There is something inherently personal about swallowable data,” Dr. Plowman said. “It’s something we take tremendous efforts to protect.” As for compulsory use, “we take enormous strides to prevent that. It’s a major priority.”
“Always, there will be an opt-out” option, said Mr. Travis.
It’s important to consider the potential for IEMs to move medicine forward. When patients with acute bone fractures in one study, for instance, were sent home with the usual handful of oxycodone tablets, it turned out that they only took a median of six. Researchers knew that because the subjects took their oxycodone in an etectRx capsule. It’s was an important insight in the midst of an opioid epidemic (Anesth Analg. 2017 Dec;125[6]:2105-12).
Dr. Greeno is an adviser for Proteus; the company covers his travel costs.
Recently, a woman with advanced colorectal cancer at the University of Minnesota, Minneapolis, was taking capecitabine (Xeloda) in the morning but skipping her evening dose.
Her hands hurt, and she couldn’t open the childproof cap. Her daughter had been doing it for her in the morning but wasn’t around to help out at night.
It was the kind of problem that might have gone on for days or weeks until the next clinic visit, and, even then, be addressed only if the woman remembered to mention it.
But that’s not what happened. The care team realized pretty much right away that she was skipping the p.m. dose because the woman was taking her capecitabine in a gel cap with an adherence sensor.
The sensor, a sandwich of copper, silicon, and magnesium in a millimeter square, sent out an electric ping when she took her dose, activated by stomach acid; the ping was picked up by an adhesive bandage patch the woman wore, which relayed the signal to an app on her smartphone; the phone passed it on to a server cloud that the woman had given her providers permission to access.
They monitored her adherence on a Web portal, along with heart rate and activity data, also captured by the patch. The system is called Proteus Discover, from Proteus Digital Health.
Instead of taking days or weeks, the care team quickly realized that she wasn’t taking her evening dose of capecitabine. They contacted her, replaced the childproof cap, and twice-daily dosing resumed.
Expanded use in oncology?
Seven other advanced colorectal cancer patients have participated in the University of Minnesota (UM) pilot project, the first use of the device in oncology. “It’s gone so well that it’s annoying to me to not have this for all my patients. I’m already feeling frustrated that I can’t just put this in all the drugs that I give orally,” said Edward Greeno, MD, an oncologist/hematologist at the university, and the Proteus point man.
“You would assume that cancer patients would be hypercompliant, but it turns out they have the same compliance problems” as other patients, plus additional hurdles, he said, including complex regimens and drug toxicity.
“Every patient we have approached so far has been enthusiastic. They might be a little bit annoyed that I know they haven’t been taking their pills, but they are also glad that I am there to hold them responsible and help them. My intention is to roll this out much more broadly,” he said. That just might happen. Dr. Greeno is working with Proteus to roll the sensor out at UM and oncology programs elsewhere.
A slow, careful rollout
The device was cleared by the Food and Drug Administration in 2012. After more than 180,000 ingestions, there have been no safety issues, besides occasional skin irritation from the patch, which is waterproof and meant to be worn for a week, then replaced. It pings if it’s taken off. The sensor is passes through the body like food.
Patients can communicate with providers over the phone app, which also sends reminders when it’s time to take the next pill.
So far, Proteus has worked with ten health systems in the United States, and more are in the works. Commercialization efforts have focused mostly on blood pressure, cholesterol, and type 2 diabetes drugs, the bad boys of drug adherence, but the system has also been piloted for hepatitis C treatment, and trials are underway for HIV preexposure prophylaxis.
Among the company’s many favorable studies, the system has already been demonstrated to be a viable alternative to directly observed therapy in tuberculosis, the current gold-standard, but hugely labor and resource intensive (PLoS One. 2013;8[1]:e53373. doi: 10.1371/journal.pone.0053373).
Proteus can’t be picked up at the local Walgreens. The company works closely with clients and is being careful in its rollout. For one thing, each sensor has to be programed for the specific drug it’s being used with, but also, and as with any new technology, business and payment models are still being worked out.
UM’s partner in the oncology project, Fairview Health Services, pays Proteus when patients hit an adherence rate of 80%, but how much they pay is a proprietary secret. “Most of the cost issue is still not in the public domain,” said Scooter Plowman, MD, the company’s medical director.
In 2017, FDA approved a version of the antipsychotic aripiprazole embedded with the Proteus sensor. The rollout of “Abilify MyCite” by Otsuka Pharmaceuticals has been similarly cautious, under contract with health systems.
“Otsuka has been very smart in the approach they are taking,” Dr. Plowman said.
He wouldn’t give details, but Proteus is in talks with other pharmaceutical makers to bring pills with sensors to the market.
A new fix for an old problem
Proteus isn’t alone in the ingestible event marker (IEM) market. The FDA is reviewing a rival sensor from etectRx, in Gainesville, Fla.
The technology is a little different; the etectRx sensor is a microchip made out of magnesium and silver chloride that’s embedded on the inside of a gel cap. Instead of an electric blip, it sends out a radio wave when activated by stomach acid. It’s larger than the Proteus offering, but still has room to spare in a gel cap.
The signal is stronger, so patients wear a neck pendant instead of a patch to pick it up. The pendant does not capture heart rate or activity data. It’s not meant to be worn continuously and can come off after it pings the system.
The two systems are otherwise similar; etectRx also uses a phone app to relay adherence data to a server cloud clinicians can access, with patient permission. As with Proteus, everything works as long as the phone is on. President and CEO Harry Travis anticipates clearance in 2019.
Adherence is a huge and well-known problem in medicine; only about half of patients take medications as they are prescribed. People end up in the ED or the hospital with problems that might have been avoided. Providers and payers want solutions.
Industry is bringing technology to bear on the problem. The payoff will be huge for the winners; analysts project multiple billion dollar growth in the adherence technology sector.
Most companies, however, are pinning their hopes on indirect approaches, bottle caps that ping when opened, for instance, or coaching apps for smart phones. IEMs seem to be ahead of the curve.
Guardian angel or watchdog?
Whether that’s a good thing or bad thing depends on who you talk to, but patients do seem more likely to take their medications if a sensor is on board.
Dr. Plowman said he thinks IEMs improve adherence because, with their own health at stake, patients want to do better, and IEM systems provide the extra help they need, complete with positive feedback.
But patients also know they are being watched. The technology is barely off the ground, but concerns have already been raised about surveillance. It’s not hard to imagine insurance companies demanding proof of adherence before paying for expensive drugs. There are privacy concerns as well; everything is encrypted with IEMs, but hackers are clever.
Dr. Plowman and Mr. Travis acknowledged the concerns, and also that there’s no way to know how IEMs – if they take off – will play out in coming decades; it’s a lot like the Internet in 1992.
The intent is for the systems to remain voluntary, as they are now, perhaps with inducements for patients to use them, maybe lower insurance premiums.
“There is something inherently personal about swallowable data,” Dr. Plowman said. “It’s something we take tremendous efforts to protect.” As for compulsory use, “we take enormous strides to prevent that. It’s a major priority.”
“Always, there will be an opt-out” option, said Mr. Travis.
It’s important to consider the potential for IEMs to move medicine forward. When patients with acute bone fractures in one study, for instance, were sent home with the usual handful of oxycodone tablets, it turned out that they only took a median of six. Researchers knew that because the subjects took their oxycodone in an etectRx capsule. It’s was an important insight in the midst of an opioid epidemic (Anesth Analg. 2017 Dec;125[6]:2105-12).
Dr. Greeno is an adviser for Proteus; the company covers his travel costs.
Penicillin allergy
A 75-year-old man presents with fever, chills, and facial pain. He had an upper respiratory infection 3 weeks ago and has had persistent sinus drainage since. He has tried nasal irrigation and nasal steroids without improvement.
Over the past 5 days, he has had thicker postnasal drip, the development of facial pain, and today fevers as high as 102 degrees. He has a history of giant cell arteritis, for which he takes 30 mg of prednisone daily; coronary artery disease; and hypertension. He has a penicillin allergy (rash on chest, back, and arms 25 years ago). Exam reveals temperature of 101.5 and tenderness over left maxillary sinus.
What treatment do you recommend?
A. Amoxicillin/clavulanate.
B. Cefpodoxime.
C. Levofloxacin.
D. Trimethoprim/sulfamethoxazole.
I think cefpodoxime is probably the best of these choices to treat sinusitis in this patient. Choosing amoxicillin /clavulanate is an option only if you could give the patient a test dose in a controlled setting. I think giving this patient levofloxacin poses greater risk than a penicillin rechallenge. This patient is elderly and on prednisone, both of which increase his risk of tendon rupture if given a quinolone. Also, the Food and Drug Administration released a warning recently regarding increased risk of aortic disease in patients with cardiovascular risk factors who receive fluoroquinolones.1
Merin Kuruvilla, MD, and colleagues described oral amoxicillin challenge for patients with a history of low-risk penicillin allergy (described as benign rash, benign somatic symptoms, or unknown history with penicillin exposure more than 12 months prior).2 The study was done in a single allergy practice where 38 of 50 patients with penicillin allergy histories qualified for the study. Of the 38 eligible patients, 20 consented to oral rechallenge in clinic, and none of them developed immediate or delayed hypersensitivity reactions.
Melissa Iammatteo, MD, et al. studied 155 patients with a history of non–life-threatening penicillin reactions.3 Study participants received placebo followed by a two-step graded challenge to amoxicillin. No reaction occurred in 77% of patients, while 20% of patients had nonallergic reactions, which were equal between placebo and amoxicillin. Only 2.6 % had allergic reactions, all of which were classified as mild.
Reported penicillin allergy occurs in about 10% of community patients, but 90% of these patients can tolerate penicillins.4 Patients reporting a penicillin allergy have increased risk for drug resistance and prolonged hospital stays.5
The American Academy of Allergy, Asthma & Immunology recommended more widespread and routine performance of penicillin allergy testing in patients with a history of allergy to penicillin or other beta-lactam antibiotics.6 Patients who have penicillin allergy histories are more likely to receive drugs, such as clindamycin or a fluoroquinolone, that may carry much greater risks than a beta-lactam antibiotic. It also leads to more vancomycin use, which increases risk of vancomycin resistance.
Allergic reactions to cephalosporins are very infrequent in patients with a penicillin allergy. Eric Macy, MD, and colleagues studied all members of Kaiser Permanente Southern California health plan who had received cephalosporins over a 2-year period.7 More than 275,000 courses were given to patients with penicillin allergy, with only about 1% having an allergic reaction and only three cases of anaphylaxis.
Pearl: Most patients with a history of penicillin allergy will tolerate penicillins and cephalosporins. Penicillin allergy testing should be done to assess if they have a penicillin allergy, and in low-risk patients (patients who do not recall the allergy or had a maculopapular rash), consideration for oral rechallenge in a controlled setting may be an option. Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Food and Drug Administration. “FDA warns about increased risk of ruptures or tears in the aorta blood vessel with fluoroquinolone antibiotics in certain patients,” 2018 Dec 20.
2. Ann Allergy Asthma Immunol. 2018 Nov;121(5):627-8.
3. J Allergy Clin Immunol Pract. 2019 Jan;7(1):236-43.
4. Immunol Allergy Clin North Am. 2017 Nov;37(4):643-62.
5. J Allergy Clin Immunol. 2014 Mar;133(3):790-6.
6. J Allergy Clin Immunol Pract. 2017 Mar - Apr;5(2):333-4.
7. J Allergy Clin Immunol. 2015 Mar;135(3):745-52.e5.
A 75-year-old man presents with fever, chills, and facial pain. He had an upper respiratory infection 3 weeks ago and has had persistent sinus drainage since. He has tried nasal irrigation and nasal steroids without improvement.
Over the past 5 days, he has had thicker postnasal drip, the development of facial pain, and today fevers as high as 102 degrees. He has a history of giant cell arteritis, for which he takes 30 mg of prednisone daily; coronary artery disease; and hypertension. He has a penicillin allergy (rash on chest, back, and arms 25 years ago). Exam reveals temperature of 101.5 and tenderness over left maxillary sinus.
What treatment do you recommend?
A. Amoxicillin/clavulanate.
B. Cefpodoxime.
C. Levofloxacin.
D. Trimethoprim/sulfamethoxazole.
I think cefpodoxime is probably the best of these choices to treat sinusitis in this patient. Choosing amoxicillin /clavulanate is an option only if you could give the patient a test dose in a controlled setting. I think giving this patient levofloxacin poses greater risk than a penicillin rechallenge. This patient is elderly and on prednisone, both of which increase his risk of tendon rupture if given a quinolone. Also, the Food and Drug Administration released a warning recently regarding increased risk of aortic disease in patients with cardiovascular risk factors who receive fluoroquinolones.1
Merin Kuruvilla, MD, and colleagues described oral amoxicillin challenge for patients with a history of low-risk penicillin allergy (described as benign rash, benign somatic symptoms, or unknown history with penicillin exposure more than 12 months prior).2 The study was done in a single allergy practice where 38 of 50 patients with penicillin allergy histories qualified for the study. Of the 38 eligible patients, 20 consented to oral rechallenge in clinic, and none of them developed immediate or delayed hypersensitivity reactions.
Melissa Iammatteo, MD, et al. studied 155 patients with a history of non–life-threatening penicillin reactions.3 Study participants received placebo followed by a two-step graded challenge to amoxicillin. No reaction occurred in 77% of patients, while 20% of patients had nonallergic reactions, which were equal between placebo and amoxicillin. Only 2.6 % had allergic reactions, all of which were classified as mild.
Reported penicillin allergy occurs in about 10% of community patients, but 90% of these patients can tolerate penicillins.4 Patients reporting a penicillin allergy have increased risk for drug resistance and prolonged hospital stays.5
The American Academy of Allergy, Asthma & Immunology recommended more widespread and routine performance of penicillin allergy testing in patients with a history of allergy to penicillin or other beta-lactam antibiotics.6 Patients who have penicillin allergy histories are more likely to receive drugs, such as clindamycin or a fluoroquinolone, that may carry much greater risks than a beta-lactam antibiotic. It also leads to more vancomycin use, which increases risk of vancomycin resistance.
Allergic reactions to cephalosporins are very infrequent in patients with a penicillin allergy. Eric Macy, MD, and colleagues studied all members of Kaiser Permanente Southern California health plan who had received cephalosporins over a 2-year period.7 More than 275,000 courses were given to patients with penicillin allergy, with only about 1% having an allergic reaction and only three cases of anaphylaxis.
Pearl: Most patients with a history of penicillin allergy will tolerate penicillins and cephalosporins. Penicillin allergy testing should be done to assess if they have a penicillin allergy, and in low-risk patients (patients who do not recall the allergy or had a maculopapular rash), consideration for oral rechallenge in a controlled setting may be an option. Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Food and Drug Administration. “FDA warns about increased risk of ruptures or tears in the aorta blood vessel with fluoroquinolone antibiotics in certain patients,” 2018 Dec 20.
2. Ann Allergy Asthma Immunol. 2018 Nov;121(5):627-8.
3. J Allergy Clin Immunol Pract. 2019 Jan;7(1):236-43.
4. Immunol Allergy Clin North Am. 2017 Nov;37(4):643-62.
5. J Allergy Clin Immunol. 2014 Mar;133(3):790-6.
6. J Allergy Clin Immunol Pract. 2017 Mar - Apr;5(2):333-4.
7. J Allergy Clin Immunol. 2015 Mar;135(3):745-52.e5.
A 75-year-old man presents with fever, chills, and facial pain. He had an upper respiratory infection 3 weeks ago and has had persistent sinus drainage since. He has tried nasal irrigation and nasal steroids without improvement.
Over the past 5 days, he has had thicker postnasal drip, the development of facial pain, and today fevers as high as 102 degrees. He has a history of giant cell arteritis, for which he takes 30 mg of prednisone daily; coronary artery disease; and hypertension. He has a penicillin allergy (rash on chest, back, and arms 25 years ago). Exam reveals temperature of 101.5 and tenderness over left maxillary sinus.
What treatment do you recommend?
A. Amoxicillin/clavulanate.
B. Cefpodoxime.
C. Levofloxacin.
D. Trimethoprim/sulfamethoxazole.
I think cefpodoxime is probably the best of these choices to treat sinusitis in this patient. Choosing amoxicillin /clavulanate is an option only if you could give the patient a test dose in a controlled setting. I think giving this patient levofloxacin poses greater risk than a penicillin rechallenge. This patient is elderly and on prednisone, both of which increase his risk of tendon rupture if given a quinolone. Also, the Food and Drug Administration released a warning recently regarding increased risk of aortic disease in patients with cardiovascular risk factors who receive fluoroquinolones.1
Merin Kuruvilla, MD, and colleagues described oral amoxicillin challenge for patients with a history of low-risk penicillin allergy (described as benign rash, benign somatic symptoms, or unknown history with penicillin exposure more than 12 months prior).2 The study was done in a single allergy practice where 38 of 50 patients with penicillin allergy histories qualified for the study. Of the 38 eligible patients, 20 consented to oral rechallenge in clinic, and none of them developed immediate or delayed hypersensitivity reactions.
Melissa Iammatteo, MD, et al. studied 155 patients with a history of non–life-threatening penicillin reactions.3 Study participants received placebo followed by a two-step graded challenge to amoxicillin. No reaction occurred in 77% of patients, while 20% of patients had nonallergic reactions, which were equal between placebo and amoxicillin. Only 2.6 % had allergic reactions, all of which were classified as mild.
Reported penicillin allergy occurs in about 10% of community patients, but 90% of these patients can tolerate penicillins.4 Patients reporting a penicillin allergy have increased risk for drug resistance and prolonged hospital stays.5
The American Academy of Allergy, Asthma & Immunology recommended more widespread and routine performance of penicillin allergy testing in patients with a history of allergy to penicillin or other beta-lactam antibiotics.6 Patients who have penicillin allergy histories are more likely to receive drugs, such as clindamycin or a fluoroquinolone, that may carry much greater risks than a beta-lactam antibiotic. It also leads to more vancomycin use, which increases risk of vancomycin resistance.
Allergic reactions to cephalosporins are very infrequent in patients with a penicillin allergy. Eric Macy, MD, and colleagues studied all members of Kaiser Permanente Southern California health plan who had received cephalosporins over a 2-year period.7 More than 275,000 courses were given to patients with penicillin allergy, with only about 1% having an allergic reaction and only three cases of anaphylaxis.
Pearl: Most patients with a history of penicillin allergy will tolerate penicillins and cephalosporins. Penicillin allergy testing should be done to assess if they have a penicillin allergy, and in low-risk patients (patients who do not recall the allergy or had a maculopapular rash), consideration for oral rechallenge in a controlled setting may be an option. Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Food and Drug Administration. “FDA warns about increased risk of ruptures or tears in the aorta blood vessel with fluoroquinolone antibiotics in certain patients,” 2018 Dec 20.
2. Ann Allergy Asthma Immunol. 2018 Nov;121(5):627-8.
3. J Allergy Clin Immunol Pract. 2019 Jan;7(1):236-43.
4. Immunol Allergy Clin North Am. 2017 Nov;37(4):643-62.
5. J Allergy Clin Immunol. 2014 Mar;133(3):790-6.
6. J Allergy Clin Immunol Pract. 2017 Mar - Apr;5(2):333-4.
7. J Allergy Clin Immunol. 2015 Mar;135(3):745-52.e5.
The personal cancer vaccine NEO-PV-01 shows promise in metastatic cancers
WASHINGTON – according to findings from the ongoing phase 1b NT-001study of patients with metastatic melanoma, smoking-associated non–small cell lung cancer (NSCLC), and bladder cancer.
No vaccine-related serious adverse events occurred in 34 patients in a per-protocol set who were treated with the regimen, Siwen Hu-Lieskovan, MD, PhD, reported at the annual meeting of the Society for Immunotherapy of Cancer.
“We found that NEO-PV-01 in combination with nivolumab was very safe; we did not see any grade 3 to grade 4 toxicity associated with the combination,” said Dr. Hu-Lieskovan, a medical oncologist at the University of California, Los Angeles.
Most adverse events that occurred were mild and related to the local injection, she noted.
Although safety was the primary endpoint of the study, Dr. Hu-Lieskovan and her colleagues also looked at immune responses and treatment efficacy, however, with respect to translational data her presentation addressed only the findings in the melanoma and lung cancer patients.
All patients exhibited an immune response to the vaccine, with 56% of the epitopes generating CD4- and/or CD8-positive T cell responses.
“These immune responses were very durable and still could be detected 52 weeks into the treatment,” she said. Additionally, epitope spreading – increased immune response targeting nonvaccine epitopes (which is indirect evidence of vaccine-induced tumor toxicity) – was observed in 8 of 10 patients tested.
The study subjects, including 16 adults with melanoma, 11 with NSCLC, and 7 with bladder cancer, were treated with nivolumab every 2 weeks for 12 weeks prior to vaccination (while their personalized vaccine was being developed). NEO-PV-01 – which included up to 20 unique peptides plus the immunostimulant poly-ICLC – was then administered subcutaneously in five priming doses followed by two booster doses over the next 12 weeks. Of note, very few patients had programmed cell death protein 1 expression of 50% or greater, including only 13.3%, 28.6%, and 0% of the melanoma, NSCLC, and bladder cancer patients, respectively, and tumor mutation burden was consistent with published reports, she said.
As for efficacy, 11 of 16 melanoma patients (68.6%) had either a partial response (8 pre vaccination and an additional 3 post vaccination) or stable disease. One (6.3%) had a postvaccination complete response, Dr. Hu-Lieskovan said.
“[This is] much better than the historical data,” she noted, adding that 12 patients (75%) are still on the study and continuing treatment with response duration of at least 39.7 weeks.
Of the 11 NSCLC patients, 5 (45.5%) had a partial response (3 pre vaccination and 2 post vaccination), and none had a complete response. Seven (63.6%) remained on the study and were continuing treatment, and response duration was at least 30.6 weeks.
An exploratory analysis of tumor responses after vaccination showed that the majority of melanoma patients and half of the lung cancer patients had further tumor shrinkage after vaccination, and some patients were converted to responders. Most – including some with stable or progressive disease – stayed on treatment, she said.
The findings demonstrate that NEO-PV-01 is very well tolerated and associated with post vaccine responses observed after week 24.
“We saw evidence of vaccination-induced immune response specific to the injected vaccine, as well as epitope spreading, and the T cells induced by these neoantigens can traffic into the tumor and they seem to be functional,” she concluded.
Dr. Hu-Lieskovan reported receiving consulting fees and/or research support from Amgen, BMS, Genmab, Merck, and Vaccinex. She is the UCLA site principal investigator for the NT-001 study and has conducted contracted research for Astellas, F Star, Genentech, Nektar Therapeutics, Neon Therapeutics, Pfizer, Plexxikon, and Xencor.
SOURCE: Hu-Lieskovan S et al. SITC 2018, Abstract 07.
WASHINGTON – according to findings from the ongoing phase 1b NT-001study of patients with metastatic melanoma, smoking-associated non–small cell lung cancer (NSCLC), and bladder cancer.
No vaccine-related serious adverse events occurred in 34 patients in a per-protocol set who were treated with the regimen, Siwen Hu-Lieskovan, MD, PhD, reported at the annual meeting of the Society for Immunotherapy of Cancer.
“We found that NEO-PV-01 in combination with nivolumab was very safe; we did not see any grade 3 to grade 4 toxicity associated with the combination,” said Dr. Hu-Lieskovan, a medical oncologist at the University of California, Los Angeles.
Most adverse events that occurred were mild and related to the local injection, she noted.
Although safety was the primary endpoint of the study, Dr. Hu-Lieskovan and her colleagues also looked at immune responses and treatment efficacy, however, with respect to translational data her presentation addressed only the findings in the melanoma and lung cancer patients.
All patients exhibited an immune response to the vaccine, with 56% of the epitopes generating CD4- and/or CD8-positive T cell responses.
“These immune responses were very durable and still could be detected 52 weeks into the treatment,” she said. Additionally, epitope spreading – increased immune response targeting nonvaccine epitopes (which is indirect evidence of vaccine-induced tumor toxicity) – was observed in 8 of 10 patients tested.
The study subjects, including 16 adults with melanoma, 11 with NSCLC, and 7 with bladder cancer, were treated with nivolumab every 2 weeks for 12 weeks prior to vaccination (while their personalized vaccine was being developed). NEO-PV-01 – which included up to 20 unique peptides plus the immunostimulant poly-ICLC – was then administered subcutaneously in five priming doses followed by two booster doses over the next 12 weeks. Of note, very few patients had programmed cell death protein 1 expression of 50% or greater, including only 13.3%, 28.6%, and 0% of the melanoma, NSCLC, and bladder cancer patients, respectively, and tumor mutation burden was consistent with published reports, she said.
As for efficacy, 11 of 16 melanoma patients (68.6%) had either a partial response (8 pre vaccination and an additional 3 post vaccination) or stable disease. One (6.3%) had a postvaccination complete response, Dr. Hu-Lieskovan said.
“[This is] much better than the historical data,” she noted, adding that 12 patients (75%) are still on the study and continuing treatment with response duration of at least 39.7 weeks.
Of the 11 NSCLC patients, 5 (45.5%) had a partial response (3 pre vaccination and 2 post vaccination), and none had a complete response. Seven (63.6%) remained on the study and were continuing treatment, and response duration was at least 30.6 weeks.
An exploratory analysis of tumor responses after vaccination showed that the majority of melanoma patients and half of the lung cancer patients had further tumor shrinkage after vaccination, and some patients were converted to responders. Most – including some with stable or progressive disease – stayed on treatment, she said.
The findings demonstrate that NEO-PV-01 is very well tolerated and associated with post vaccine responses observed after week 24.
“We saw evidence of vaccination-induced immune response specific to the injected vaccine, as well as epitope spreading, and the T cells induced by these neoantigens can traffic into the tumor and they seem to be functional,” she concluded.
Dr. Hu-Lieskovan reported receiving consulting fees and/or research support from Amgen, BMS, Genmab, Merck, and Vaccinex. She is the UCLA site principal investigator for the NT-001 study and has conducted contracted research for Astellas, F Star, Genentech, Nektar Therapeutics, Neon Therapeutics, Pfizer, Plexxikon, and Xencor.
SOURCE: Hu-Lieskovan S et al. SITC 2018, Abstract 07.
WASHINGTON – according to findings from the ongoing phase 1b NT-001study of patients with metastatic melanoma, smoking-associated non–small cell lung cancer (NSCLC), and bladder cancer.
No vaccine-related serious adverse events occurred in 34 patients in a per-protocol set who were treated with the regimen, Siwen Hu-Lieskovan, MD, PhD, reported at the annual meeting of the Society for Immunotherapy of Cancer.
“We found that NEO-PV-01 in combination with nivolumab was very safe; we did not see any grade 3 to grade 4 toxicity associated with the combination,” said Dr. Hu-Lieskovan, a medical oncologist at the University of California, Los Angeles.
Most adverse events that occurred were mild and related to the local injection, she noted.
Although safety was the primary endpoint of the study, Dr. Hu-Lieskovan and her colleagues also looked at immune responses and treatment efficacy, however, with respect to translational data her presentation addressed only the findings in the melanoma and lung cancer patients.
All patients exhibited an immune response to the vaccine, with 56% of the epitopes generating CD4- and/or CD8-positive T cell responses.
“These immune responses were very durable and still could be detected 52 weeks into the treatment,” she said. Additionally, epitope spreading – increased immune response targeting nonvaccine epitopes (which is indirect evidence of vaccine-induced tumor toxicity) – was observed in 8 of 10 patients tested.
The study subjects, including 16 adults with melanoma, 11 with NSCLC, and 7 with bladder cancer, were treated with nivolumab every 2 weeks for 12 weeks prior to vaccination (while their personalized vaccine was being developed). NEO-PV-01 – which included up to 20 unique peptides plus the immunostimulant poly-ICLC – was then administered subcutaneously in five priming doses followed by two booster doses over the next 12 weeks. Of note, very few patients had programmed cell death protein 1 expression of 50% or greater, including only 13.3%, 28.6%, and 0% of the melanoma, NSCLC, and bladder cancer patients, respectively, and tumor mutation burden was consistent with published reports, she said.
As for efficacy, 11 of 16 melanoma patients (68.6%) had either a partial response (8 pre vaccination and an additional 3 post vaccination) or stable disease. One (6.3%) had a postvaccination complete response, Dr. Hu-Lieskovan said.
“[This is] much better than the historical data,” she noted, adding that 12 patients (75%) are still on the study and continuing treatment with response duration of at least 39.7 weeks.
Of the 11 NSCLC patients, 5 (45.5%) had a partial response (3 pre vaccination and 2 post vaccination), and none had a complete response. Seven (63.6%) remained on the study and were continuing treatment, and response duration was at least 30.6 weeks.
An exploratory analysis of tumor responses after vaccination showed that the majority of melanoma patients and half of the lung cancer patients had further tumor shrinkage after vaccination, and some patients were converted to responders. Most – including some with stable or progressive disease – stayed on treatment, she said.
The findings demonstrate that NEO-PV-01 is very well tolerated and associated with post vaccine responses observed after week 24.
“We saw evidence of vaccination-induced immune response specific to the injected vaccine, as well as epitope spreading, and the T cells induced by these neoantigens can traffic into the tumor and they seem to be functional,” she concluded.
Dr. Hu-Lieskovan reported receiving consulting fees and/or research support from Amgen, BMS, Genmab, Merck, and Vaccinex. She is the UCLA site principal investigator for the NT-001 study and has conducted contracted research for Astellas, F Star, Genentech, Nektar Therapeutics, Neon Therapeutics, Pfizer, Plexxikon, and Xencor.
SOURCE: Hu-Lieskovan S et al. SITC 2018, Abstract 07.
REPORTING FROM SITC 2018
Key clinical point: The NEO-PV-01 personalized cancer vaccine shows good tolerability and safety and appears to have clinical efficacy.
Major finding: There were no vaccine-related serious adverse events, and all patients exhibited an immune response to the vaccine, with 56% of the epitopes generating CD4- and/or CD8-positive T-cell responses.
Study details: A study of the NEO-PV-01 personalized cancer vaccine in 34 patients.
Disclosures: Dr. Hu-Lieskovan reported receiving consulting fees and/or research support from Amgen, BMS, Genmab, Merck, and Vaccinex. She is the UCLA site principal investigator for the NT-001 study and has conducted contracted research for Astellas, F Star, Genentech, Nektar Therapeutics, Neon Therapeutics, Pfizer, Plexxikon, and Xencor.
Source: Hu-Lieskovan S et al. SITC 2018, Abstract 07.
Smoking has small effect on postop hernia wound morbidity
Smoking before elective clean open ventral hernia repair (OVHR) was not associated with significant differences in postoperative wound morbidity or all 30-day morbidity, according to results published in Surgery.
The question of the impact of smoking on infection risk and wound healing has been studied in a variety of surgical procedures. While some associations have been established (JAMA Surg. 2017;152[5]:476-83; Infect Immun. 2015;83[6]:2443-52), the data have not been strong enough to lead to recommendations that surgeons delay procedures until a patient stops smoking.
In a study of 418 active smokers and 418 matched patients who had never smoked, the rate of surgical site infection (SSI) was 4.1% in both groups (P = .98). The total 30-day complication rates were 7.5% for current smokers and 6.6% for nonsmokers (P = .60), reported Clayton C. Petro, MD, of the department of general surgery at the Cleveland Clinic Comprehensive Hernia Center, and his coauthors.
Patient data were obtained from the Americas Hernia Society Quality Collaborative (AHSQC), and included those who had undergone OVHR in a Centers for Disease Control and Prevention class 1 wound with 30-day follow-up.
Participants were grouped according to smoking status: those who had never smoked, those who had smoked within 30 days of operation (“current smokers”), and patients who had quit smoking more than 1 month before operation (“former smokers”). Current smokers were matched with never smokers by characteristics including age, sex, and body mass index, and conditions such as diabetes and hypertension. Former smokers were excluded.
The investigators collected data from both groups on SSI, surgical site occurrence (SSO), surgical site occurrence requiring a procedural intervention (SSOPI), and all 30-day morbidity (any morbidity event entered into the AHSQC database within 30 days of operation), the authors said.
Rates of SSI were 4.1% for both smokers and nonsmokers (P = .98). Rates of SSO were greater in current smokers (12.0% vs. 7.4%, P = .03). SSOPI and reoperation rates were similar in current and never smokers (6.2% vs. 5.0%, P = .43; 1.9% vs. 1.2%, P = .39, respectively).
The results suggest that “active smoking before an elective OVHR in a CDC class I wound has a clinically negligible impact on postoperative wound morbidity and all 30-day morbidity,” wrote Dr. Petro and his colleagues.
“Surgeons allowing perioperative smoking should monitor their outcomes to [ensure that] these findings are replicable in their own practice,” they concluded.
The study authors disclosed financial relationships with Medtronic, Ariste Medical, and other companies.
SOURCE: Petro CC et al. Surgery. 2019 Feb;165(2):406-11.
Smoking before elective clean open ventral hernia repair (OVHR) was not associated with significant differences in postoperative wound morbidity or all 30-day morbidity, according to results published in Surgery.
The question of the impact of smoking on infection risk and wound healing has been studied in a variety of surgical procedures. While some associations have been established (JAMA Surg. 2017;152[5]:476-83; Infect Immun. 2015;83[6]:2443-52), the data have not been strong enough to lead to recommendations that surgeons delay procedures until a patient stops smoking.
In a study of 418 active smokers and 418 matched patients who had never smoked, the rate of surgical site infection (SSI) was 4.1% in both groups (P = .98). The total 30-day complication rates were 7.5% for current smokers and 6.6% for nonsmokers (P = .60), reported Clayton C. Petro, MD, of the department of general surgery at the Cleveland Clinic Comprehensive Hernia Center, and his coauthors.
Patient data were obtained from the Americas Hernia Society Quality Collaborative (AHSQC), and included those who had undergone OVHR in a Centers for Disease Control and Prevention class 1 wound with 30-day follow-up.
Participants were grouped according to smoking status: those who had never smoked, those who had smoked within 30 days of operation (“current smokers”), and patients who had quit smoking more than 1 month before operation (“former smokers”). Current smokers were matched with never smokers by characteristics including age, sex, and body mass index, and conditions such as diabetes and hypertension. Former smokers were excluded.
The investigators collected data from both groups on SSI, surgical site occurrence (SSO), surgical site occurrence requiring a procedural intervention (SSOPI), and all 30-day morbidity (any morbidity event entered into the AHSQC database within 30 days of operation), the authors said.
Rates of SSI were 4.1% for both smokers and nonsmokers (P = .98). Rates of SSO were greater in current smokers (12.0% vs. 7.4%, P = .03). SSOPI and reoperation rates were similar in current and never smokers (6.2% vs. 5.0%, P = .43; 1.9% vs. 1.2%, P = .39, respectively).
The results suggest that “active smoking before an elective OVHR in a CDC class I wound has a clinically negligible impact on postoperative wound morbidity and all 30-day morbidity,” wrote Dr. Petro and his colleagues.
“Surgeons allowing perioperative smoking should monitor their outcomes to [ensure that] these findings are replicable in their own practice,” they concluded.
The study authors disclosed financial relationships with Medtronic, Ariste Medical, and other companies.
SOURCE: Petro CC et al. Surgery. 2019 Feb;165(2):406-11.
Smoking before elective clean open ventral hernia repair (OVHR) was not associated with significant differences in postoperative wound morbidity or all 30-day morbidity, according to results published in Surgery.
The question of the impact of smoking on infection risk and wound healing has been studied in a variety of surgical procedures. While some associations have been established (JAMA Surg. 2017;152[5]:476-83; Infect Immun. 2015;83[6]:2443-52), the data have not been strong enough to lead to recommendations that surgeons delay procedures until a patient stops smoking.
In a study of 418 active smokers and 418 matched patients who had never smoked, the rate of surgical site infection (SSI) was 4.1% in both groups (P = .98). The total 30-day complication rates were 7.5% for current smokers and 6.6% for nonsmokers (P = .60), reported Clayton C. Petro, MD, of the department of general surgery at the Cleveland Clinic Comprehensive Hernia Center, and his coauthors.
Patient data were obtained from the Americas Hernia Society Quality Collaborative (AHSQC), and included those who had undergone OVHR in a Centers for Disease Control and Prevention class 1 wound with 30-day follow-up.
Participants were grouped according to smoking status: those who had never smoked, those who had smoked within 30 days of operation (“current smokers”), and patients who had quit smoking more than 1 month before operation (“former smokers”). Current smokers were matched with never smokers by characteristics including age, sex, and body mass index, and conditions such as diabetes and hypertension. Former smokers were excluded.
The investigators collected data from both groups on SSI, surgical site occurrence (SSO), surgical site occurrence requiring a procedural intervention (SSOPI), and all 30-day morbidity (any morbidity event entered into the AHSQC database within 30 days of operation), the authors said.
Rates of SSI were 4.1% for both smokers and nonsmokers (P = .98). Rates of SSO were greater in current smokers (12.0% vs. 7.4%, P = .03). SSOPI and reoperation rates were similar in current and never smokers (6.2% vs. 5.0%, P = .43; 1.9% vs. 1.2%, P = .39, respectively).
The results suggest that “active smoking before an elective OVHR in a CDC class I wound has a clinically negligible impact on postoperative wound morbidity and all 30-day morbidity,” wrote Dr. Petro and his colleagues.
“Surgeons allowing perioperative smoking should monitor their outcomes to [ensure that] these findings are replicable in their own practice,” they concluded.
The study authors disclosed financial relationships with Medtronic, Ariste Medical, and other companies.
SOURCE: Petro CC et al. Surgery. 2019 Feb;165(2):406-11.
FROM SURGERY
Key clinical point: Smoking did not appear to increase the risk of wound morbidity after open ventral hernia repair surgery.
Major finding: Rates of postop surgical site infection were 4.1% for both smokers and nonsmokers.
Study details: Data from matched hernia repair patients (418 smokers and 418 nonsmokers) obtained from the Americas Hernia Society Quality Collaborative.
Disclosures: The study authors disclosed financial relationships with Medtronic, Ariste Medical, and other companies.
Source: Petro CC et al. Surgery. 2019 Feb;165(2):406-11.
FDA grants BI-1206 orphan designation for MCL
The Food and Drug Administration has granted orphan designation to BI-1206 for the treatment of mantle cell lymphoma (MCL).
BI-1206 is a monoclonal antibody being developed by BioInvent International.
The company says BI-1206 works by inhibiting FcgRIIB (CD32B), which is associated with poor prognosis in MCL and other non-Hodgkin lymphomas. By inhibiting FcgRIIB, BI-1206 is expected to enhance the activity of rituximab or other anti-CD20 monoclonal antibodies.
BioInvent is conducting a phase 1/2a study (NCT03571568) of BI-1206 in combination with rituximab in patients with indolent, relapsed/refractory B-cell non-Hodgkin lymphomas, including MCL. The first patient began receiving treatment with BI-1206 in September 2018.
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases or disorders that affect fewer than 200,000 people in the United States. Orphan designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
The Food and Drug Administration has granted orphan designation to BI-1206 for the treatment of mantle cell lymphoma (MCL).
BI-1206 is a monoclonal antibody being developed by BioInvent International.
The company says BI-1206 works by inhibiting FcgRIIB (CD32B), which is associated with poor prognosis in MCL and other non-Hodgkin lymphomas. By inhibiting FcgRIIB, BI-1206 is expected to enhance the activity of rituximab or other anti-CD20 monoclonal antibodies.
BioInvent is conducting a phase 1/2a study (NCT03571568) of BI-1206 in combination with rituximab in patients with indolent, relapsed/refractory B-cell non-Hodgkin lymphomas, including MCL. The first patient began receiving treatment with BI-1206 in September 2018.
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases or disorders that affect fewer than 200,000 people in the United States. Orphan designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
The Food and Drug Administration has granted orphan designation to BI-1206 for the treatment of mantle cell lymphoma (MCL).
BI-1206 is a monoclonal antibody being developed by BioInvent International.
The company says BI-1206 works by inhibiting FcgRIIB (CD32B), which is associated with poor prognosis in MCL and other non-Hodgkin lymphomas. By inhibiting FcgRIIB, BI-1206 is expected to enhance the activity of rituximab or other anti-CD20 monoclonal antibodies.
BioInvent is conducting a phase 1/2a study (NCT03571568) of BI-1206 in combination with rituximab in patients with indolent, relapsed/refractory B-cell non-Hodgkin lymphomas, including MCL. The first patient began receiving treatment with BI-1206 in September 2018.
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases or disorders that affect fewer than 200,000 people in the United States. Orphan designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
Female radiation oncologists receive less funds from industry
In the year 2016, female radiation oncologists received less money than did male radiation oncologists from industry across every type of compensation evaluated, according to an analysis of data from the Centers for Medicare & Medicated Services Open Payments program.
These sex disparities included a median $1,000 less for consulting (P = .005), $500 less for honoraria (P = .005) and $135 less for research payments, although the difference in this latter category fell short of statistical significance (P = .08), reported Julius K. Weng, department of radiation oncology, University of California, Los Angeles, and his colleagues, in JAMA Network Open.
The CMS Open Payments Program, which is part of the Physician Payments Sunshine Act, made the study possible. The CMS data were intended to allow evaluation of potential conflicts of interest, but they were amenable to compare payments by gender. For this purpose, the investigators determined gender by first name, which was confirmed by Internet search when ambiguous.
At least one industry payment in 2016 was made to 61.4% of 1,164 female and 70.4% of 3,319 male radiation oncologists included in this retrospective cross-sectional study.
Of research funding, only 20.7% went to women even though they represented 25.9% of radiation oncologists in the United States at that time. In other categories, such as honoraria or consulting, the proportion of compensation going to females was even lower, never exceeding 15%.
In the United States, about one-third of radiation oncologists are women, according to the authors of this study. This is substantially lower than the proportion in many other specialties and is far below that of current medical school enrollment, where women are now in a slight majority.
It cannot be ascertained from these data why industry compensation was lower for women, but the authors offered numerous potential explanations including the possibility that more female than male radiation oncologists do not elect to pursue relationships with industry. They labeled such relationships as “controversial” due to potential conflicts of interest.
Among the theories put forth are those that have been proposed to explain other sex disparities, including lower salaries and slower promotion, in medicine and elsewhere.
For one, it has been suggested that “agentic traits” of men might propel them to seek opportunities more aggressively, compared with women, who have “historically been associated with communal qualities,” according to the authors.
If due to gender bias, disparities may also accumulate over time as “downstream consequences of sex gaps experienced early in a female physician’s career,” the authors stated. They noted that women have a lower proportion of leadership positions in radiation oncology than predicted by their numbers in the specialty.
The disparity in industry partnerships and compensation is a relevant measure of sex disparity because these are associated with “substantial career advantages,” according to the authors of this study. In addition to the advantages of research funding, they believe these include association with important signs of success in academic clinical medicine, such being identified as a key opinion leader.
One limitation of the CMS data regarding industry payments is that the information is derived from self-reports. In 2016, the CMS Open Payments Program was in its fourth year, which the authors suggested had more complete information on industry payments than prior years because of initiatives to improve reporting compliance.
The lower payments from compensation are likely to be related to other gender disparities in radiation oncology, such as lower publication productivity and fewer patents held by women. It is unclear how non–career oriented activities, whether alone or together, particularly raising children, might interfere with both career advancement and compensation from industry, according to the authors.
Coauthor Ann C. Raldow, MD, also of the department of radiation oncology at UCLA, acknowledged in an interview that any of the potential explanations, such as the choice not to choose to pursue industry relationships, might be valid. However, she suggested that this issue deserves further exploration.
“Of greater concern is the possibility that this observed disparity may be a proxy for the systemic inequalities that female physicians have in radiation oncology. A first step in clarifying the origin of this gap could be incorporating industry-related questions into a workforce survey,” Dr. Raldow said.
If this step demonstrates a true disparity, “the most relevant metric may be female physicians receiving a percentage of total industry funding that corresponds to their representation in the field,” she added.
SOURCE: Weng JK et al. JAMA Netw Open. 2019 Jan. 25. doi: 10.1001/jamanetworkopen.2018.7377.
In the year 2016, female radiation oncologists received less money than did male radiation oncologists from industry across every type of compensation evaluated, according to an analysis of data from the Centers for Medicare & Medicated Services Open Payments program.
These sex disparities included a median $1,000 less for consulting (P = .005), $500 less for honoraria (P = .005) and $135 less for research payments, although the difference in this latter category fell short of statistical significance (P = .08), reported Julius K. Weng, department of radiation oncology, University of California, Los Angeles, and his colleagues, in JAMA Network Open.
The CMS Open Payments Program, which is part of the Physician Payments Sunshine Act, made the study possible. The CMS data were intended to allow evaluation of potential conflicts of interest, but they were amenable to compare payments by gender. For this purpose, the investigators determined gender by first name, which was confirmed by Internet search when ambiguous.
At least one industry payment in 2016 was made to 61.4% of 1,164 female and 70.4% of 3,319 male radiation oncologists included in this retrospective cross-sectional study.
Of research funding, only 20.7% went to women even though they represented 25.9% of radiation oncologists in the United States at that time. In other categories, such as honoraria or consulting, the proportion of compensation going to females was even lower, never exceeding 15%.
In the United States, about one-third of radiation oncologists are women, according to the authors of this study. This is substantially lower than the proportion in many other specialties and is far below that of current medical school enrollment, where women are now in a slight majority.
It cannot be ascertained from these data why industry compensation was lower for women, but the authors offered numerous potential explanations including the possibility that more female than male radiation oncologists do not elect to pursue relationships with industry. They labeled such relationships as “controversial” due to potential conflicts of interest.
Among the theories put forth are those that have been proposed to explain other sex disparities, including lower salaries and slower promotion, in medicine and elsewhere.
For one, it has been suggested that “agentic traits” of men might propel them to seek opportunities more aggressively, compared with women, who have “historically been associated with communal qualities,” according to the authors.
If due to gender bias, disparities may also accumulate over time as “downstream consequences of sex gaps experienced early in a female physician’s career,” the authors stated. They noted that women have a lower proportion of leadership positions in radiation oncology than predicted by their numbers in the specialty.
The disparity in industry partnerships and compensation is a relevant measure of sex disparity because these are associated with “substantial career advantages,” according to the authors of this study. In addition to the advantages of research funding, they believe these include association with important signs of success in academic clinical medicine, such being identified as a key opinion leader.
One limitation of the CMS data regarding industry payments is that the information is derived from self-reports. In 2016, the CMS Open Payments Program was in its fourth year, which the authors suggested had more complete information on industry payments than prior years because of initiatives to improve reporting compliance.
The lower payments from compensation are likely to be related to other gender disparities in radiation oncology, such as lower publication productivity and fewer patents held by women. It is unclear how non–career oriented activities, whether alone or together, particularly raising children, might interfere with both career advancement and compensation from industry, according to the authors.
Coauthor Ann C. Raldow, MD, also of the department of radiation oncology at UCLA, acknowledged in an interview that any of the potential explanations, such as the choice not to choose to pursue industry relationships, might be valid. However, she suggested that this issue deserves further exploration.
“Of greater concern is the possibility that this observed disparity may be a proxy for the systemic inequalities that female physicians have in radiation oncology. A first step in clarifying the origin of this gap could be incorporating industry-related questions into a workforce survey,” Dr. Raldow said.
If this step demonstrates a true disparity, “the most relevant metric may be female physicians receiving a percentage of total industry funding that corresponds to their representation in the field,” she added.
SOURCE: Weng JK et al. JAMA Netw Open. 2019 Jan. 25. doi: 10.1001/jamanetworkopen.2018.7377.
In the year 2016, female radiation oncologists received less money than did male radiation oncologists from industry across every type of compensation evaluated, according to an analysis of data from the Centers for Medicare & Medicated Services Open Payments program.
These sex disparities included a median $1,000 less for consulting (P = .005), $500 less for honoraria (P = .005) and $135 less for research payments, although the difference in this latter category fell short of statistical significance (P = .08), reported Julius K. Weng, department of radiation oncology, University of California, Los Angeles, and his colleagues, in JAMA Network Open.
The CMS Open Payments Program, which is part of the Physician Payments Sunshine Act, made the study possible. The CMS data were intended to allow evaluation of potential conflicts of interest, but they were amenable to compare payments by gender. For this purpose, the investigators determined gender by first name, which was confirmed by Internet search when ambiguous.
At least one industry payment in 2016 was made to 61.4% of 1,164 female and 70.4% of 3,319 male radiation oncologists included in this retrospective cross-sectional study.
Of research funding, only 20.7% went to women even though they represented 25.9% of radiation oncologists in the United States at that time. In other categories, such as honoraria or consulting, the proportion of compensation going to females was even lower, never exceeding 15%.
In the United States, about one-third of radiation oncologists are women, according to the authors of this study. This is substantially lower than the proportion in many other specialties and is far below that of current medical school enrollment, where women are now in a slight majority.
It cannot be ascertained from these data why industry compensation was lower for women, but the authors offered numerous potential explanations including the possibility that more female than male radiation oncologists do not elect to pursue relationships with industry. They labeled such relationships as “controversial” due to potential conflicts of interest.
Among the theories put forth are those that have been proposed to explain other sex disparities, including lower salaries and slower promotion, in medicine and elsewhere.
For one, it has been suggested that “agentic traits” of men might propel them to seek opportunities more aggressively, compared with women, who have “historically been associated with communal qualities,” according to the authors.
If due to gender bias, disparities may also accumulate over time as “downstream consequences of sex gaps experienced early in a female physician’s career,” the authors stated. They noted that women have a lower proportion of leadership positions in radiation oncology than predicted by their numbers in the specialty.
The disparity in industry partnerships and compensation is a relevant measure of sex disparity because these are associated with “substantial career advantages,” according to the authors of this study. In addition to the advantages of research funding, they believe these include association with important signs of success in academic clinical medicine, such being identified as a key opinion leader.
One limitation of the CMS data regarding industry payments is that the information is derived from self-reports. In 2016, the CMS Open Payments Program was in its fourth year, which the authors suggested had more complete information on industry payments than prior years because of initiatives to improve reporting compliance.
The lower payments from compensation are likely to be related to other gender disparities in radiation oncology, such as lower publication productivity and fewer patents held by women. It is unclear how non–career oriented activities, whether alone or together, particularly raising children, might interfere with both career advancement and compensation from industry, according to the authors.
Coauthor Ann C. Raldow, MD, also of the department of radiation oncology at UCLA, acknowledged in an interview that any of the potential explanations, such as the choice not to choose to pursue industry relationships, might be valid. However, she suggested that this issue deserves further exploration.
“Of greater concern is the possibility that this observed disparity may be a proxy for the systemic inequalities that female physicians have in radiation oncology. A first step in clarifying the origin of this gap could be incorporating industry-related questions into a workforce survey,” Dr. Raldow said.
If this step demonstrates a true disparity, “the most relevant metric may be female physicians receiving a percentage of total industry funding that corresponds to their representation in the field,” she added.
SOURCE: Weng JK et al. JAMA Netw Open. 2019 Jan. 25. doi: 10.1001/jamanetworkopen.2018.7377.
FROM JAMA NETWORK OPEN
Key clinical point: Sex disparity has been found for payments made by industry to radiation oncologists.
Major finding: Females were less likely to receive any industry money (61.4% vs. 70.4%) and received smaller amounts in all categories.
Study details: Retrospective cross-sectional study.
Disclosures: No conflicts of interest were reported.
Source: Weng JK et al. JAMA Netw Open. 2019 Jan. 25. doi: 10.1001/jamanetworkopen.2018.7377.
Things We Do for No Reason: Prescribing Docusate for Constipation in Hospitalized Adults
The “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
Click here for the Choosing Wisely website.

CASE PRESENTATION
An 80-year-old woman with no significant past medical history presents with a mechanical fall. X-rays are notable for a right hip fracture. She is treated with morphine for analgesia and evaluated by orthopedic surgery for surgical repair. The hospitalist recognizes that this patient is at high risk for constipation and orders docusate for prevention of constipation.
BACKGROUND
Constipation is a highly prevalent problem in all practice settings, especially in the hospital, affecting two out of five hospitalized patients.1 Multiple factors in the inpatient setting contribute to constipation, including decreased mobility, medical comorbidities, postsurgical ileus, anesthetics, and medications such as opioid analgesics. Furthermore, the inpatient population is aging in parallel with the general population and constipation is more common in the elderly, likely owing to a combination of decreased muscle mass and impaired function of autonomic nerves.2 Consequently, inpatient providers frequently treat constipation or try to prevent it using stool softeners or laxatives.
One of the most commonly prescribed agents, regardless of medical specialty, is docusate, also known as dioctyl sulfosuccinate or by its brand name, Colace. A study from McGill University Health Centre in Montreal, Canada reported that docusate was the most frequently prescribed laxative, accounting for 64% of laxative medication doses, with associated costs approaching $60,000 per year.3 Direct drug costs accounted for a quarter of the expenses, and the remaining three quarters were estimated labor costs for administration. Medical and surgical admissions shared similar proportions of usage, with an average of 10 doses of docusate per admission across 17,064 admissions. Furthermore, half of the patients were prescribed docusate upon discharge. The authors extrapolated their data to suggest that total healthcare spending in North America on docusate products likely exceeds $100,000,000 yearly. A second study from Toronto found that 15% of all hospitalized patients are prescribed at least one dose of docusate, and that one-third of all new inpatient prescriptions are continued at discharge.4
WHY YOU THINK DOCUSATE MIGHT BE HELPFUL FOR CONSTIPATION
Docusate is thought to act as a detergent to retain water in the stool, thereby acting as a stool softener to facilitate stool passage. Physicians have prescribed docusate for decades, and attendings have passed down the practice of prescribing docusate for constipation to medical trainees for generations. The initial docusate studies showed promise, as it softened the stool by increasing its water content and made it easier to pass through the intestines.5 One of the earliest human studies compared docusate to an unspecified placebo in 35 elderly patients with chronic atonic constipation and found a decreased need for enemas.6 Some other observational studies also reported a decreased need for manual disimpactions and enemas in elderly populations.7,8 One randomized, controlled trial from 1968 showed an increased frequency of bowel movements compared to placebo, but it excluded half of the enrolled patients because they had a positive placebo response.9 Since those early studies from the 1950s and 1960s, docusate remains widely accepted as an effective stool softener with positive endorsements from hospital formularies and order sets and patient information sheets such as the JAMA Patient Page.10 Furthermore, the World Health Organization lists docusate as an “essential medicine,” reinforcing the notion that it is effective.11
WHY THERE IS NO REASON TO PRESCRIBE DOCUSATE FOR CONSTIPATION
Despite common practice, the efficacy of docusate as a stool softener has not been borne out by rigorous scientific data. On the contrary, multiple randomized controlled trials have failed to show any significant efficacy of this drug over placebo (Table).
The initial trial in 1976 studied 34 elderly patients on a general medical ward for prophylaxis of constipation.12 They randomized patients to 100 mg twice daily of docusate sodium versus a control group that did not receive any type of laxative. The number of bowel movements and their character served as the measured outcomes. The study demonstrated no statistically significant differences in the frequency and character of bowel movements between the docusate and placebo groups. Even at that time, the authors questioned whether docusate had any efficacy at all: “[w]hether the drug actually offers anything beyond a placebo effect in preventing constipation is in doubt.”
Another trial in 1978 studied 46 elderly, institutionalized patients with chronic functional constipation.13 All patients underwent a two-week placebo period followed by a three-week treatment period with three arms of randomization: docusate sodium 100 mg daily, docusate sodium 100 mg twice daily, or docusate calcium 240 mg daily. Patients received enemas or suppositories if required. All three arms showed an increase in the average number of natural bowel movements when compared to each patient’s own placebo period, but only the arm with docusate calcium reached statistical significance (P < .02). According to the authors, none of the therapies appeared to have a significant effect on stool consistency. The authors hypothesized that the higher dose given to the docusate calcium arm may have been the reason for the apparent efficacy in this cohort. As such, studies with higher doses of docusate calcium would be reasonable.
A third study in 1985 compared docusate sodium 100 mg three times daily versus placebo in six healthy patients with ileostomies and six healthy volunteers.14 Therapy with docusate “had no effect on stool weight, stool frequency, stool water, or mean transit time.”
Another study in 1991 evaluated 15 elderly nursing home residents with a randomized, double-blind crossover design.15 Subjects received 240 mg twice daily of docusate calcium versus placebo for three weeks and then crossed over to other arm after a two-week wash-out period. The investigators found no difference in the number of bowel movements per week or in the need for additional laxatives between the two study periods. There were also no differences in the patients’ subjective experience of constipation or discomfort with defecation.
Larger studies were subsequently initiated in more recent years. In 1998, a randomized controlled trial in 170 subjects with chronic idiopathic constipation compared psyllium 5.1 g twice daily and docusate sodium 100 mg twice daily with a corresponding placebo in each arm for a treatment duration of two weeks after a two-week placebo baseline period.16 Psyllium was found to increase stool water content and stool water weight over the baseline period, while docusate essentially had no effect on stool water content or water weight. Furthermore, by treatment week 2, psyllium demonstrated an increase in the frequency of bowel movements, whereas docusate did not. It should be noted that this study was funded by Procter & Gamble, which manufactures Metamucil, a popular brand of psyllium.
Lastly, the most recent randomized controlled trial was published in 2013. It included 74 hospice patients in Canada, comparing docusate 200 mg and sennosides twice daily versus placebo and sennosides for 10 days. The study found no difference in stool frequency, volume, or consistency between docusate and placebo.17
A number of systematic reviews have studied the literature on bowel regimens and have noted the paucity of high-quality data supporting the efficacy of docusate, despite its widespread use.18-22 With these weak data, multiple authors have advocated for removing docusate from hospital formularies and using hospitalizations as an opportunity to deprescribe this medication to reduce polypharmacy. 3,4,23
Although docusate is considered a benign therapy, there is certainly potential for harm to the patient and detrimental effects on the healthcare system. Patients commonly complain about the unpleasant taste and lingering aftertaste, which may lead to decreased oral intake and worsening nutritional status.23 Furthermore, docusate may impact the absorption and effectiveness of other proven treatments.23 Perhaps the most important harm is that providers needlessly wait for docusate to fail before prescribing effective therapies for constipation. This process negatively impacts patient satisfaction and potentially increases healthcare costs if hospital length of stay is increased. Another important consideration is that patients may refuse truly necessary medications due to the excessive pill burden.
Costs to the healthcare system are increased needlessly when medications that do not improve outcomes are prescribed. Although the individual pill cost is low, the widespread use and the associated pharmacy and nursing resources required for administration create an estimated cost for docusate over $100,000,000 per year for North America alone.3 The staff time required for administration may prevent healthcare personnel from engaging in other more valuable tasks. Additionally, every medication order creates an opportunity for medical error. Lastly, bacteria were recently found contaminating the liquid formulation, which carries its own obvious implications if patients develop iatrogenic infections.24
WHAT YOU SHOULD DO INSTEAD
Instead of using docusate, prescribe agents with established efficacy. In 2006, a systematic review published in the American Journal of Gastroenterology graded the evidence behind different therapies for chronic constipation.21 They found good evidence (Grade A) to support the use of polyethylene glycol (PEG), while psyllium and lactulose had moderate evidence (Grade B) to support their use. All other currently available agents that were reviewed had poor evidence to support their use. A more recent study in people prescribed opioids similarly found evidence to support the use of polyethylene glycol, lactulose, and sennosides.25 Lastly, the 2016 guidelines from the American Society of Colon and Rectal Surgeons do not mention docusate, though they comment on the paucity of data on stool softeners. Their recommendations for laxative therapy are similar to those of the previously discussed reviews.26 Ultimately, the choice of therapy, pharmacological and nonpharmacological, should be individualized for each patient based on the clinical context and cause of constipation. Nonpharmacologic treatments include dietary modification, mobilization, chewing gum, and biofeedback. If pharmacotherapy is required, use laxatives with the strongest evidence.
RECOMMENDATIONS
- In patients with constipation or at risk for constipation, use laxatives with proven efficacy (such as polyethylene glycol, lactulose, psyllium, or sennosides) for treatment or prophylaxis of constipation instead of using docusate.
- Discuss de-prescription for patients using docusate prior to admission.
- Remove docusate from your hospital formulary.
CONCLUSION
Docusate is commonly used for the treatment and prevention of constipation in hospitalized patients, with significant associated costs. This common practice continues despite little evidence supporting its efficacy and many trials failing to show benefits over placebo. Decreased utilization of ineffective therapies such as docusate is recommended. Returning to the case presentation, the hospitalist should start the patient on alternative therapies, instead of docusate, such as polyethylene glycol, lactulose, psyllium, or sennosides, which have better evidence supporting their use.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing [email protected].
Disclosures
All authors deny any relevant conflict of interest with the attached manuscript.
1. Noiesen E, Trosborg I, Bager L, Herning M, Lyngby C, Konradsen H. Constipation--prevalence and incidence among medical patients acutely admitted to hospital with a medical condition. J Clin Nurs. 2014;23(15-16):2295-2302. doi: 10.1111/jocn.12511.
2. De Giorgio R, Ruggeri E, Stanghellini V, Eusebi LH, Bazzoli F, Chiarioni G. Chronic constipation in the elderly: a primer for the gastroenterologist. BMC Gastroenterol. 2015;15:130. doi: 10.1186/s12876-015-0366-3.
3. Lee TC, McDonald EG, Bonnici A, Tamblyn R. Pattern of inpatient laxative use: waste not, want not. JAMA Intern Med. 2016;176(8):1216-1217. doi: 10.1001/jamainternmed.2016.2775.
4. MacMillan TE, Kamali R, Cavalcanti RB. Missed opportunity to deprescribe: docusate for constipation in medical inpatients. Am J Med. 2016;129(9):1001 e1001-1007. doi: 10.1016/j.amjmed.2016.04.008.
5. Spiesman MG, Malow L. New fecal softener (doxinate) in the treatment of constipation. J Lancet. 1956;76(6):164-167.
6. Harris R. Constipation in geriatrics; management with dioctyl sodium sulfosuccinate. Am J Dig Dis. Sep 1957;2(9):487-492.
7. Smigel JO, Lowe KJ, Hosp PH, Gibson JH. Constipation in elderly patients; treatment with dioctyl sodium sulfosuccinate and dioctyl sodium sulfosuccinate plus peristim. Med Times. 1958;86(12):1521-1526.
8. Wilson JL, Dickinson DG. Use of dioctyl sodium sulfosuccinate (aerosol O.T.) for severe constipation. J Am Med Assoc. 1955;158(4):261-263. doi: 10.1001/jama.1955.02960040019006a.
9. Hyland CM, Foran JD. Dioctyl sodium sulphosuccinate as a laxative in the elderly. Practitioner. 1968;200(199):698-699.
10. Jin J. JAMA patient page. Over-the-counter laxatives. JAMA. 2014;312(11):1167. doi: 10.1001/jama.2014.2078.
11. 19th WHO Model List of Essential Medicines (April 2015). 2015; http://www.who.int/medicines/publications/essentialmedicines/en/.
12. Goodman J, Pang J, Bessman AN. Dioctyl sodium sulfosuccinate- an ineffective prophylactic laxative. J Chronic Dis. 1976;29(1):59-63. doi: 10.1016/0021-9681(76)90068-0.
13. Fain AM, Susat R, Herring M, Dorton K. Treatment of constipation in geriatric and chronically ill patients: a comparison. South Med J. 1978;71(6):677-680.
14. Chapman RW, Sillery J, Fontana DD, Matthys C, Saunders DR. Effect of oral dioctyl sodium sulfosuccinate on intake-output studies of human small and large intestine. Gastroenterology. 1985;89(3):489-493. doi: 10.1016/0016-5085(85)90441-X.
15. Castle SC, Cantrell M, Israel DS, Samuelson MJ. Constipation prevention: empiric use of stool softeners questioned. Geriatrics. 1991;46(11):84-86.
16. McRorie JW, Daggy BP, Morel JG, Diersing PS, Miner PB, Robinson M. Psyllium is superior to docusate sodium for treatment of chronic constipation. Aliment Pharmacol Ther. 1998;12(5):491-497. doi: 10.1046/j.1365-2036.1998.00336.x.
17. Tarumi Y, Wilson MP, Szafran O, Spooner GR. Randomized, double-blind, placebo-controlled trial of oral docusate in the management of constipation in hospice patients. J Pain Symptom Manage. 2013;45(1):2-13. doi: 10.1016/j.jpainsymman.2012.02.008.
18. Candy B, Jones L, Larkin PJ, Vickerstaff V, Tookman A, Stone P. Laxatives for the management of constipation in people receiving palliative care. Cochrane Database Syst Rev. 2015(5):CD003448.
19. Hurdon V, Viola R, Schroder C. How useful is docusate in patients at risk for constipation? A systematic review of the evidence in the chronically ill. J Pain Symptom Manage. 2000;19(2):130-136. doi: 10.1016/S0885-3924(99)00157-8.
20. Pare P, Fedorak RN. Systematic review of stimulant and nonstimulant laxatives for the treatment of functional constipation. Can J Gastroenterol Hepatol. 2014;28(10):549-557.
21. Ramkumar D, Rao SS. Efficacy and safety of traditional medical therapies for chronic constipation: systematic review. Am J Gastroenterol. 2005;100(4):936-971. doi: 10.1111/j.1572-0241.2005.40925.x
22. Health CAfDaTi. Dioctyl sulfosuccinate or docusate (calcium or sodium) for the prevention or management of constipation: a review of the clinical effectiveness. Ottawa (ON)2014.
23. McKee KY, Widera E. Habitual prescribing of laxatives-it’s time to flush outdated protocols down the drain. JAMA Intern Med. 2016;176(8):1217-1219. doi: 10.1001/jamainternmed.2016.2780.
24. Marquez L, Jones KN, Whaley EM, et al. An outbreak of burkholderia cepacia complex infections associated with contaminated liquid docusate. Infect Control Hosp Epidemiol. 2017;38(5):567-573. doi: 10.1017/ice.2017.11.
25. Ahmedzai SH, Boland J. Constipation in people prescribed opioids. BMJ Clin Evid. 2010;2010.
26. Paquette IM, Varma M, Ternent C, et al. The American society of colon and rectal surgeons’ clinical practice guideline for the evaluation and management of constipation. Dis Colon Rectum. 2016;59(6):479-492. doi: 10.1097/DCR.0000000000000599
The “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
Click here for the Choosing Wisely website.

CASE PRESENTATION
An 80-year-old woman with no significant past medical history presents with a mechanical fall. X-rays are notable for a right hip fracture. She is treated with morphine for analgesia and evaluated by orthopedic surgery for surgical repair. The hospitalist recognizes that this patient is at high risk for constipation and orders docusate for prevention of constipation.
BACKGROUND
Constipation is a highly prevalent problem in all practice settings, especially in the hospital, affecting two out of five hospitalized patients.1 Multiple factors in the inpatient setting contribute to constipation, including decreased mobility, medical comorbidities, postsurgical ileus, anesthetics, and medications such as opioid analgesics. Furthermore, the inpatient population is aging in parallel with the general population and constipation is more common in the elderly, likely owing to a combination of decreased muscle mass and impaired function of autonomic nerves.2 Consequently, inpatient providers frequently treat constipation or try to prevent it using stool softeners or laxatives.
One of the most commonly prescribed agents, regardless of medical specialty, is docusate, also known as dioctyl sulfosuccinate or by its brand name, Colace. A study from McGill University Health Centre in Montreal, Canada reported that docusate was the most frequently prescribed laxative, accounting for 64% of laxative medication doses, with associated costs approaching $60,000 per year.3 Direct drug costs accounted for a quarter of the expenses, and the remaining three quarters were estimated labor costs for administration. Medical and surgical admissions shared similar proportions of usage, with an average of 10 doses of docusate per admission across 17,064 admissions. Furthermore, half of the patients were prescribed docusate upon discharge. The authors extrapolated their data to suggest that total healthcare spending in North America on docusate products likely exceeds $100,000,000 yearly. A second study from Toronto found that 15% of all hospitalized patients are prescribed at least one dose of docusate, and that one-third of all new inpatient prescriptions are continued at discharge.4
WHY YOU THINK DOCUSATE MIGHT BE HELPFUL FOR CONSTIPATION
Docusate is thought to act as a detergent to retain water in the stool, thereby acting as a stool softener to facilitate stool passage. Physicians have prescribed docusate for decades, and attendings have passed down the practice of prescribing docusate for constipation to medical trainees for generations. The initial docusate studies showed promise, as it softened the stool by increasing its water content and made it easier to pass through the intestines.5 One of the earliest human studies compared docusate to an unspecified placebo in 35 elderly patients with chronic atonic constipation and found a decreased need for enemas.6 Some other observational studies also reported a decreased need for manual disimpactions and enemas in elderly populations.7,8 One randomized, controlled trial from 1968 showed an increased frequency of bowel movements compared to placebo, but it excluded half of the enrolled patients because they had a positive placebo response.9 Since those early studies from the 1950s and 1960s, docusate remains widely accepted as an effective stool softener with positive endorsements from hospital formularies and order sets and patient information sheets such as the JAMA Patient Page.10 Furthermore, the World Health Organization lists docusate as an “essential medicine,” reinforcing the notion that it is effective.11
WHY THERE IS NO REASON TO PRESCRIBE DOCUSATE FOR CONSTIPATION
Despite common practice, the efficacy of docusate as a stool softener has not been borne out by rigorous scientific data. On the contrary, multiple randomized controlled trials have failed to show any significant efficacy of this drug over placebo (Table).
The initial trial in 1976 studied 34 elderly patients on a general medical ward for prophylaxis of constipation.12 They randomized patients to 100 mg twice daily of docusate sodium versus a control group that did not receive any type of laxative. The number of bowel movements and their character served as the measured outcomes. The study demonstrated no statistically significant differences in the frequency and character of bowel movements between the docusate and placebo groups. Even at that time, the authors questioned whether docusate had any efficacy at all: “[w]hether the drug actually offers anything beyond a placebo effect in preventing constipation is in doubt.”
Another trial in 1978 studied 46 elderly, institutionalized patients with chronic functional constipation.13 All patients underwent a two-week placebo period followed by a three-week treatment period with three arms of randomization: docusate sodium 100 mg daily, docusate sodium 100 mg twice daily, or docusate calcium 240 mg daily. Patients received enemas or suppositories if required. All three arms showed an increase in the average number of natural bowel movements when compared to each patient’s own placebo period, but only the arm with docusate calcium reached statistical significance (P < .02). According to the authors, none of the therapies appeared to have a significant effect on stool consistency. The authors hypothesized that the higher dose given to the docusate calcium arm may have been the reason for the apparent efficacy in this cohort. As such, studies with higher doses of docusate calcium would be reasonable.
A third study in 1985 compared docusate sodium 100 mg three times daily versus placebo in six healthy patients with ileostomies and six healthy volunteers.14 Therapy with docusate “had no effect on stool weight, stool frequency, stool water, or mean transit time.”
Another study in 1991 evaluated 15 elderly nursing home residents with a randomized, double-blind crossover design.15 Subjects received 240 mg twice daily of docusate calcium versus placebo for three weeks and then crossed over to other arm after a two-week wash-out period. The investigators found no difference in the number of bowel movements per week or in the need for additional laxatives between the two study periods. There were also no differences in the patients’ subjective experience of constipation or discomfort with defecation.
Larger studies were subsequently initiated in more recent years. In 1998, a randomized controlled trial in 170 subjects with chronic idiopathic constipation compared psyllium 5.1 g twice daily and docusate sodium 100 mg twice daily with a corresponding placebo in each arm for a treatment duration of two weeks after a two-week placebo baseline period.16 Psyllium was found to increase stool water content and stool water weight over the baseline period, while docusate essentially had no effect on stool water content or water weight. Furthermore, by treatment week 2, psyllium demonstrated an increase in the frequency of bowel movements, whereas docusate did not. It should be noted that this study was funded by Procter & Gamble, which manufactures Metamucil, a popular brand of psyllium.
Lastly, the most recent randomized controlled trial was published in 2013. It included 74 hospice patients in Canada, comparing docusate 200 mg and sennosides twice daily versus placebo and sennosides for 10 days. The study found no difference in stool frequency, volume, or consistency between docusate and placebo.17
A number of systematic reviews have studied the literature on bowel regimens and have noted the paucity of high-quality data supporting the efficacy of docusate, despite its widespread use.18-22 With these weak data, multiple authors have advocated for removing docusate from hospital formularies and using hospitalizations as an opportunity to deprescribe this medication to reduce polypharmacy. 3,4,23
Although docusate is considered a benign therapy, there is certainly potential for harm to the patient and detrimental effects on the healthcare system. Patients commonly complain about the unpleasant taste and lingering aftertaste, which may lead to decreased oral intake and worsening nutritional status.23 Furthermore, docusate may impact the absorption and effectiveness of other proven treatments.23 Perhaps the most important harm is that providers needlessly wait for docusate to fail before prescribing effective therapies for constipation. This process negatively impacts patient satisfaction and potentially increases healthcare costs if hospital length of stay is increased. Another important consideration is that patients may refuse truly necessary medications due to the excessive pill burden.
Costs to the healthcare system are increased needlessly when medications that do not improve outcomes are prescribed. Although the individual pill cost is low, the widespread use and the associated pharmacy and nursing resources required for administration create an estimated cost for docusate over $100,000,000 per year for North America alone.3 The staff time required for administration may prevent healthcare personnel from engaging in other more valuable tasks. Additionally, every medication order creates an opportunity for medical error. Lastly, bacteria were recently found contaminating the liquid formulation, which carries its own obvious implications if patients develop iatrogenic infections.24
WHAT YOU SHOULD DO INSTEAD
Instead of using docusate, prescribe agents with established efficacy. In 2006, a systematic review published in the American Journal of Gastroenterology graded the evidence behind different therapies for chronic constipation.21 They found good evidence (Grade A) to support the use of polyethylene glycol (PEG), while psyllium and lactulose had moderate evidence (Grade B) to support their use. All other currently available agents that were reviewed had poor evidence to support their use. A more recent study in people prescribed opioids similarly found evidence to support the use of polyethylene glycol, lactulose, and sennosides.25 Lastly, the 2016 guidelines from the American Society of Colon and Rectal Surgeons do not mention docusate, though they comment on the paucity of data on stool softeners. Their recommendations for laxative therapy are similar to those of the previously discussed reviews.26 Ultimately, the choice of therapy, pharmacological and nonpharmacological, should be individualized for each patient based on the clinical context and cause of constipation. Nonpharmacologic treatments include dietary modification, mobilization, chewing gum, and biofeedback. If pharmacotherapy is required, use laxatives with the strongest evidence.
RECOMMENDATIONS
- In patients with constipation or at risk for constipation, use laxatives with proven efficacy (such as polyethylene glycol, lactulose, psyllium, or sennosides) for treatment or prophylaxis of constipation instead of using docusate.
- Discuss de-prescription for patients using docusate prior to admission.
- Remove docusate from your hospital formulary.
CONCLUSION
Docusate is commonly used for the treatment and prevention of constipation in hospitalized patients, with significant associated costs. This common practice continues despite little evidence supporting its efficacy and many trials failing to show benefits over placebo. Decreased utilization of ineffective therapies such as docusate is recommended. Returning to the case presentation, the hospitalist should start the patient on alternative therapies, instead of docusate, such as polyethylene glycol, lactulose, psyllium, or sennosides, which have better evidence supporting their use.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing [email protected].
Disclosures
All authors deny any relevant conflict of interest with the attached manuscript.
The “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
Click here for the Choosing Wisely website.

CASE PRESENTATION
An 80-year-old woman with no significant past medical history presents with a mechanical fall. X-rays are notable for a right hip fracture. She is treated with morphine for analgesia and evaluated by orthopedic surgery for surgical repair. The hospitalist recognizes that this patient is at high risk for constipation and orders docusate for prevention of constipation.
BACKGROUND
Constipation is a highly prevalent problem in all practice settings, especially in the hospital, affecting two out of five hospitalized patients.1 Multiple factors in the inpatient setting contribute to constipation, including decreased mobility, medical comorbidities, postsurgical ileus, anesthetics, and medications such as opioid analgesics. Furthermore, the inpatient population is aging in parallel with the general population and constipation is more common in the elderly, likely owing to a combination of decreased muscle mass and impaired function of autonomic nerves.2 Consequently, inpatient providers frequently treat constipation or try to prevent it using stool softeners or laxatives.
One of the most commonly prescribed agents, regardless of medical specialty, is docusate, also known as dioctyl sulfosuccinate or by its brand name, Colace. A study from McGill University Health Centre in Montreal, Canada reported that docusate was the most frequently prescribed laxative, accounting for 64% of laxative medication doses, with associated costs approaching $60,000 per year.3 Direct drug costs accounted for a quarter of the expenses, and the remaining three quarters were estimated labor costs for administration. Medical and surgical admissions shared similar proportions of usage, with an average of 10 doses of docusate per admission across 17,064 admissions. Furthermore, half of the patients were prescribed docusate upon discharge. The authors extrapolated their data to suggest that total healthcare spending in North America on docusate products likely exceeds $100,000,000 yearly. A second study from Toronto found that 15% of all hospitalized patients are prescribed at least one dose of docusate, and that one-third of all new inpatient prescriptions are continued at discharge.4
WHY YOU THINK DOCUSATE MIGHT BE HELPFUL FOR CONSTIPATION
Docusate is thought to act as a detergent to retain water in the stool, thereby acting as a stool softener to facilitate stool passage. Physicians have prescribed docusate for decades, and attendings have passed down the practice of prescribing docusate for constipation to medical trainees for generations. The initial docusate studies showed promise, as it softened the stool by increasing its water content and made it easier to pass through the intestines.5 One of the earliest human studies compared docusate to an unspecified placebo in 35 elderly patients with chronic atonic constipation and found a decreased need for enemas.6 Some other observational studies also reported a decreased need for manual disimpactions and enemas in elderly populations.7,8 One randomized, controlled trial from 1968 showed an increased frequency of bowel movements compared to placebo, but it excluded half of the enrolled patients because they had a positive placebo response.9 Since those early studies from the 1950s and 1960s, docusate remains widely accepted as an effective stool softener with positive endorsements from hospital formularies and order sets and patient information sheets such as the JAMA Patient Page.10 Furthermore, the World Health Organization lists docusate as an “essential medicine,” reinforcing the notion that it is effective.11
WHY THERE IS NO REASON TO PRESCRIBE DOCUSATE FOR CONSTIPATION
Despite common practice, the efficacy of docusate as a stool softener has not been borne out by rigorous scientific data. On the contrary, multiple randomized controlled trials have failed to show any significant efficacy of this drug over placebo (Table).
The initial trial in 1976 studied 34 elderly patients on a general medical ward for prophylaxis of constipation.12 They randomized patients to 100 mg twice daily of docusate sodium versus a control group that did not receive any type of laxative. The number of bowel movements and their character served as the measured outcomes. The study demonstrated no statistically significant differences in the frequency and character of bowel movements between the docusate and placebo groups. Even at that time, the authors questioned whether docusate had any efficacy at all: “[w]hether the drug actually offers anything beyond a placebo effect in preventing constipation is in doubt.”
Another trial in 1978 studied 46 elderly, institutionalized patients with chronic functional constipation.13 All patients underwent a two-week placebo period followed by a three-week treatment period with three arms of randomization: docusate sodium 100 mg daily, docusate sodium 100 mg twice daily, or docusate calcium 240 mg daily. Patients received enemas or suppositories if required. All three arms showed an increase in the average number of natural bowel movements when compared to each patient’s own placebo period, but only the arm with docusate calcium reached statistical significance (P < .02). According to the authors, none of the therapies appeared to have a significant effect on stool consistency. The authors hypothesized that the higher dose given to the docusate calcium arm may have been the reason for the apparent efficacy in this cohort. As such, studies with higher doses of docusate calcium would be reasonable.
A third study in 1985 compared docusate sodium 100 mg three times daily versus placebo in six healthy patients with ileostomies and six healthy volunteers.14 Therapy with docusate “had no effect on stool weight, stool frequency, stool water, or mean transit time.”
Another study in 1991 evaluated 15 elderly nursing home residents with a randomized, double-blind crossover design.15 Subjects received 240 mg twice daily of docusate calcium versus placebo for three weeks and then crossed over to other arm after a two-week wash-out period. The investigators found no difference in the number of bowel movements per week or in the need for additional laxatives between the two study periods. There were also no differences in the patients’ subjective experience of constipation or discomfort with defecation.
Larger studies were subsequently initiated in more recent years. In 1998, a randomized controlled trial in 170 subjects with chronic idiopathic constipation compared psyllium 5.1 g twice daily and docusate sodium 100 mg twice daily with a corresponding placebo in each arm for a treatment duration of two weeks after a two-week placebo baseline period.16 Psyllium was found to increase stool water content and stool water weight over the baseline period, while docusate essentially had no effect on stool water content or water weight. Furthermore, by treatment week 2, psyllium demonstrated an increase in the frequency of bowel movements, whereas docusate did not. It should be noted that this study was funded by Procter & Gamble, which manufactures Metamucil, a popular brand of psyllium.
Lastly, the most recent randomized controlled trial was published in 2013. It included 74 hospice patients in Canada, comparing docusate 200 mg and sennosides twice daily versus placebo and sennosides for 10 days. The study found no difference in stool frequency, volume, or consistency between docusate and placebo.17
A number of systematic reviews have studied the literature on bowel regimens and have noted the paucity of high-quality data supporting the efficacy of docusate, despite its widespread use.18-22 With these weak data, multiple authors have advocated for removing docusate from hospital formularies and using hospitalizations as an opportunity to deprescribe this medication to reduce polypharmacy. 3,4,23
Although docusate is considered a benign therapy, there is certainly potential for harm to the patient and detrimental effects on the healthcare system. Patients commonly complain about the unpleasant taste and lingering aftertaste, which may lead to decreased oral intake and worsening nutritional status.23 Furthermore, docusate may impact the absorption and effectiveness of other proven treatments.23 Perhaps the most important harm is that providers needlessly wait for docusate to fail before prescribing effective therapies for constipation. This process negatively impacts patient satisfaction and potentially increases healthcare costs if hospital length of stay is increased. Another important consideration is that patients may refuse truly necessary medications due to the excessive pill burden.
Costs to the healthcare system are increased needlessly when medications that do not improve outcomes are prescribed. Although the individual pill cost is low, the widespread use and the associated pharmacy and nursing resources required for administration create an estimated cost for docusate over $100,000,000 per year for North America alone.3 The staff time required for administration may prevent healthcare personnel from engaging in other more valuable tasks. Additionally, every medication order creates an opportunity for medical error. Lastly, bacteria were recently found contaminating the liquid formulation, which carries its own obvious implications if patients develop iatrogenic infections.24
WHAT YOU SHOULD DO INSTEAD
Instead of using docusate, prescribe agents with established efficacy. In 2006, a systematic review published in the American Journal of Gastroenterology graded the evidence behind different therapies for chronic constipation.21 They found good evidence (Grade A) to support the use of polyethylene glycol (PEG), while psyllium and lactulose had moderate evidence (Grade B) to support their use. All other currently available agents that were reviewed had poor evidence to support their use. A more recent study in people prescribed opioids similarly found evidence to support the use of polyethylene glycol, lactulose, and sennosides.25 Lastly, the 2016 guidelines from the American Society of Colon and Rectal Surgeons do not mention docusate, though they comment on the paucity of data on stool softeners. Their recommendations for laxative therapy are similar to those of the previously discussed reviews.26 Ultimately, the choice of therapy, pharmacological and nonpharmacological, should be individualized for each patient based on the clinical context and cause of constipation. Nonpharmacologic treatments include dietary modification, mobilization, chewing gum, and biofeedback. If pharmacotherapy is required, use laxatives with the strongest evidence.
RECOMMENDATIONS
- In patients with constipation or at risk for constipation, use laxatives with proven efficacy (such as polyethylene glycol, lactulose, psyllium, or sennosides) for treatment or prophylaxis of constipation instead of using docusate.
- Discuss de-prescription for patients using docusate prior to admission.
- Remove docusate from your hospital formulary.
CONCLUSION
Docusate is commonly used for the treatment and prevention of constipation in hospitalized patients, with significant associated costs. This common practice continues despite little evidence supporting its efficacy and many trials failing to show benefits over placebo. Decreased utilization of ineffective therapies such as docusate is recommended. Returning to the case presentation, the hospitalist should start the patient on alternative therapies, instead of docusate, such as polyethylene glycol, lactulose, psyllium, or sennosides, which have better evidence supporting their use.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing [email protected].
Disclosures
All authors deny any relevant conflict of interest with the attached manuscript.
1. Noiesen E, Trosborg I, Bager L, Herning M, Lyngby C, Konradsen H. Constipation--prevalence and incidence among medical patients acutely admitted to hospital with a medical condition. J Clin Nurs. 2014;23(15-16):2295-2302. doi: 10.1111/jocn.12511.
2. De Giorgio R, Ruggeri E, Stanghellini V, Eusebi LH, Bazzoli F, Chiarioni G. Chronic constipation in the elderly: a primer for the gastroenterologist. BMC Gastroenterol. 2015;15:130. doi: 10.1186/s12876-015-0366-3.
3. Lee TC, McDonald EG, Bonnici A, Tamblyn R. Pattern of inpatient laxative use: waste not, want not. JAMA Intern Med. 2016;176(8):1216-1217. doi: 10.1001/jamainternmed.2016.2775.
4. MacMillan TE, Kamali R, Cavalcanti RB. Missed opportunity to deprescribe: docusate for constipation in medical inpatients. Am J Med. 2016;129(9):1001 e1001-1007. doi: 10.1016/j.amjmed.2016.04.008.
5. Spiesman MG, Malow L. New fecal softener (doxinate) in the treatment of constipation. J Lancet. 1956;76(6):164-167.
6. Harris R. Constipation in geriatrics; management with dioctyl sodium sulfosuccinate. Am J Dig Dis. Sep 1957;2(9):487-492.
7. Smigel JO, Lowe KJ, Hosp PH, Gibson JH. Constipation in elderly patients; treatment with dioctyl sodium sulfosuccinate and dioctyl sodium sulfosuccinate plus peristim. Med Times. 1958;86(12):1521-1526.
8. Wilson JL, Dickinson DG. Use of dioctyl sodium sulfosuccinate (aerosol O.T.) for severe constipation. J Am Med Assoc. 1955;158(4):261-263. doi: 10.1001/jama.1955.02960040019006a.
9. Hyland CM, Foran JD. Dioctyl sodium sulphosuccinate as a laxative in the elderly. Practitioner. 1968;200(199):698-699.
10. Jin J. JAMA patient page. Over-the-counter laxatives. JAMA. 2014;312(11):1167. doi: 10.1001/jama.2014.2078.
11. 19th WHO Model List of Essential Medicines (April 2015). 2015; http://www.who.int/medicines/publications/essentialmedicines/en/.
12. Goodman J, Pang J, Bessman AN. Dioctyl sodium sulfosuccinate- an ineffective prophylactic laxative. J Chronic Dis. 1976;29(1):59-63. doi: 10.1016/0021-9681(76)90068-0.
13. Fain AM, Susat R, Herring M, Dorton K. Treatment of constipation in geriatric and chronically ill patients: a comparison. South Med J. 1978;71(6):677-680.
14. Chapman RW, Sillery J, Fontana DD, Matthys C, Saunders DR. Effect of oral dioctyl sodium sulfosuccinate on intake-output studies of human small and large intestine. Gastroenterology. 1985;89(3):489-493. doi: 10.1016/0016-5085(85)90441-X.
15. Castle SC, Cantrell M, Israel DS, Samuelson MJ. Constipation prevention: empiric use of stool softeners questioned. Geriatrics. 1991;46(11):84-86.
16. McRorie JW, Daggy BP, Morel JG, Diersing PS, Miner PB, Robinson M. Psyllium is superior to docusate sodium for treatment of chronic constipation. Aliment Pharmacol Ther. 1998;12(5):491-497. doi: 10.1046/j.1365-2036.1998.00336.x.
17. Tarumi Y, Wilson MP, Szafran O, Spooner GR. Randomized, double-blind, placebo-controlled trial of oral docusate in the management of constipation in hospice patients. J Pain Symptom Manage. 2013;45(1):2-13. doi: 10.1016/j.jpainsymman.2012.02.008.
18. Candy B, Jones L, Larkin PJ, Vickerstaff V, Tookman A, Stone P. Laxatives for the management of constipation in people receiving palliative care. Cochrane Database Syst Rev. 2015(5):CD003448.
19. Hurdon V, Viola R, Schroder C. How useful is docusate in patients at risk for constipation? A systematic review of the evidence in the chronically ill. J Pain Symptom Manage. 2000;19(2):130-136. doi: 10.1016/S0885-3924(99)00157-8.
20. Pare P, Fedorak RN. Systematic review of stimulant and nonstimulant laxatives for the treatment of functional constipation. Can J Gastroenterol Hepatol. 2014;28(10):549-557.
21. Ramkumar D, Rao SS. Efficacy and safety of traditional medical therapies for chronic constipation: systematic review. Am J Gastroenterol. 2005;100(4):936-971. doi: 10.1111/j.1572-0241.2005.40925.x
22. Health CAfDaTi. Dioctyl sulfosuccinate or docusate (calcium or sodium) for the prevention or management of constipation: a review of the clinical effectiveness. Ottawa (ON)2014.
23. McKee KY, Widera E. Habitual prescribing of laxatives-it’s time to flush outdated protocols down the drain. JAMA Intern Med. 2016;176(8):1217-1219. doi: 10.1001/jamainternmed.2016.2780.
24. Marquez L, Jones KN, Whaley EM, et al. An outbreak of burkholderia cepacia complex infections associated with contaminated liquid docusate. Infect Control Hosp Epidemiol. 2017;38(5):567-573. doi: 10.1017/ice.2017.11.
25. Ahmedzai SH, Boland J. Constipation in people prescribed opioids. BMJ Clin Evid. 2010;2010.
26. Paquette IM, Varma M, Ternent C, et al. The American society of colon and rectal surgeons’ clinical practice guideline for the evaluation and management of constipation. Dis Colon Rectum. 2016;59(6):479-492. doi: 10.1097/DCR.0000000000000599
1. Noiesen E, Trosborg I, Bager L, Herning M, Lyngby C, Konradsen H. Constipation--prevalence and incidence among medical patients acutely admitted to hospital with a medical condition. J Clin Nurs. 2014;23(15-16):2295-2302. doi: 10.1111/jocn.12511.
2. De Giorgio R, Ruggeri E, Stanghellini V, Eusebi LH, Bazzoli F, Chiarioni G. Chronic constipation in the elderly: a primer for the gastroenterologist. BMC Gastroenterol. 2015;15:130. doi: 10.1186/s12876-015-0366-3.
3. Lee TC, McDonald EG, Bonnici A, Tamblyn R. Pattern of inpatient laxative use: waste not, want not. JAMA Intern Med. 2016;176(8):1216-1217. doi: 10.1001/jamainternmed.2016.2775.
4. MacMillan TE, Kamali R, Cavalcanti RB. Missed opportunity to deprescribe: docusate for constipation in medical inpatients. Am J Med. 2016;129(9):1001 e1001-1007. doi: 10.1016/j.amjmed.2016.04.008.
5. Spiesman MG, Malow L. New fecal softener (doxinate) in the treatment of constipation. J Lancet. 1956;76(6):164-167.
6. Harris R. Constipation in geriatrics; management with dioctyl sodium sulfosuccinate. Am J Dig Dis. Sep 1957;2(9):487-492.
7. Smigel JO, Lowe KJ, Hosp PH, Gibson JH. Constipation in elderly patients; treatment with dioctyl sodium sulfosuccinate and dioctyl sodium sulfosuccinate plus peristim. Med Times. 1958;86(12):1521-1526.
8. Wilson JL, Dickinson DG. Use of dioctyl sodium sulfosuccinate (aerosol O.T.) for severe constipation. J Am Med Assoc. 1955;158(4):261-263. doi: 10.1001/jama.1955.02960040019006a.
9. Hyland CM, Foran JD. Dioctyl sodium sulphosuccinate as a laxative in the elderly. Practitioner. 1968;200(199):698-699.
10. Jin J. JAMA patient page. Over-the-counter laxatives. JAMA. 2014;312(11):1167. doi: 10.1001/jama.2014.2078.
11. 19th WHO Model List of Essential Medicines (April 2015). 2015; http://www.who.int/medicines/publications/essentialmedicines/en/.
12. Goodman J, Pang J, Bessman AN. Dioctyl sodium sulfosuccinate- an ineffective prophylactic laxative. J Chronic Dis. 1976;29(1):59-63. doi: 10.1016/0021-9681(76)90068-0.
13. Fain AM, Susat R, Herring M, Dorton K. Treatment of constipation in geriatric and chronically ill patients: a comparison. South Med J. 1978;71(6):677-680.
14. Chapman RW, Sillery J, Fontana DD, Matthys C, Saunders DR. Effect of oral dioctyl sodium sulfosuccinate on intake-output studies of human small and large intestine. Gastroenterology. 1985;89(3):489-493. doi: 10.1016/0016-5085(85)90441-X.
15. Castle SC, Cantrell M, Israel DS, Samuelson MJ. Constipation prevention: empiric use of stool softeners questioned. Geriatrics. 1991;46(11):84-86.
16. McRorie JW, Daggy BP, Morel JG, Diersing PS, Miner PB, Robinson M. Psyllium is superior to docusate sodium for treatment of chronic constipation. Aliment Pharmacol Ther. 1998;12(5):491-497. doi: 10.1046/j.1365-2036.1998.00336.x.
17. Tarumi Y, Wilson MP, Szafran O, Spooner GR. Randomized, double-blind, placebo-controlled trial of oral docusate in the management of constipation in hospice patients. J Pain Symptom Manage. 2013;45(1):2-13. doi: 10.1016/j.jpainsymman.2012.02.008.
18. Candy B, Jones L, Larkin PJ, Vickerstaff V, Tookman A, Stone P. Laxatives for the management of constipation in people receiving palliative care. Cochrane Database Syst Rev. 2015(5):CD003448.
19. Hurdon V, Viola R, Schroder C. How useful is docusate in patients at risk for constipation? A systematic review of the evidence in the chronically ill. J Pain Symptom Manage. 2000;19(2):130-136. doi: 10.1016/S0885-3924(99)00157-8.
20. Pare P, Fedorak RN. Systematic review of stimulant and nonstimulant laxatives for the treatment of functional constipation. Can J Gastroenterol Hepatol. 2014;28(10):549-557.
21. Ramkumar D, Rao SS. Efficacy and safety of traditional medical therapies for chronic constipation: systematic review. Am J Gastroenterol. 2005;100(4):936-971. doi: 10.1111/j.1572-0241.2005.40925.x
22. Health CAfDaTi. Dioctyl sulfosuccinate or docusate (calcium or sodium) for the prevention or management of constipation: a review of the clinical effectiveness. Ottawa (ON)2014.
23. McKee KY, Widera E. Habitual prescribing of laxatives-it’s time to flush outdated protocols down the drain. JAMA Intern Med. 2016;176(8):1217-1219. doi: 10.1001/jamainternmed.2016.2780.
24. Marquez L, Jones KN, Whaley EM, et al. An outbreak of burkholderia cepacia complex infections associated with contaminated liquid docusate. Infect Control Hosp Epidemiol. 2017;38(5):567-573. doi: 10.1017/ice.2017.11.
25. Ahmedzai SH, Boland J. Constipation in people prescribed opioids. BMJ Clin Evid. 2010;2010.
26. Paquette IM, Varma M, Ternent C, et al. The American society of colon and rectal surgeons’ clinical practice guideline for the evaluation and management of constipation. Dis Colon Rectum. 2016;59(6):479-492. doi: 10.1097/DCR.0000000000000599
© 2019 Society of Hospital Medicine