User login
AGA Guideline: Treatment of mild to moderate ulcerative colitis
For patients with extensive mild to moderate ulcerative colitis, numerous randomized controlled trials support the use of either standard-dose mesalamine (2-3 grams per day) or diazo-bonded 5-aminosalicylic acid (ASA) instead of low-dose mesalamine, sulfasalazine, or no therapy, state new guidelines from the American Gastroenterological Association, published in Gastroenterology.
Sulfasalazine (2-4 grams per day) is less likely to be tolerated but remains a “reasonable option” for remitted patients who are already on it and for patients with prominent arthritis symptoms, especially if alternative treatments are cost prohibitive, wrote Cynthia W. Ko, MD, MS, of the University of Washington, Seattle, and her associates.
According to the guideline, patients with mild to moderate ulcerative colitis have less than four to six bowel movements per day, only mild or moderate rectal bleeding, no constitutional symptoms, and no high overall inflammatory burden or signs of high inflammatory activity on the Mayo Clinic score and Truelove and Witt’s criteria. These patients usually do not require colectomy, but this outcome is more likely when patients are diagnosed before age 40 years or have extensive disease or deep ulcers, extraintestinal manifestations, or elevated inflammatory markers. These higher-risk patients need more aggressive initial treatment and faster treatment intensification in cases of inadequate response, the guideline emphasizes. Even for cases of mild to moderate ulcerative colitis, treatment intensification is preferable to repeated courses of corticosteroids.
The guideline recommends adding rectal mesalamine to oral 5-ASA if patients have extensive or left-sided mild to moderate ulcerative colitis. In randomized controlled trials, this combination was significantly more likely to induce and maintain remission than was standard-dose oral mesalamine monotherapy, the authors noted. “In the maintenance trials, enemas were used twice per week or for 1 week per month. Both oral and topical mesalamine were well tolerated.”
For patients with moderate disease activity or a suboptimal response to standard-dose mesalamine or diazo-bonded 5-ASA, the guideline recommends adding rectal mesalamine to high-dose oral mesalamine (more than 3 grams daily). Combination therapy maximizes the delivery of mesalamine to the affected area of the colon, which optimizes the trial of 5-ASA before opting for treatment escalation, the authors noted. They recommend once-daily oral mesalamine dosing, since this is easier to adhere to and studies have found no benefit of more frequent dosing.
For inducing remission of mild to moderate ulcerative colitis, the guideline recommends standard-dose oral mesalamine or diazo-bonded 5-ASA over budesonide. “Overall, the budesonide preparations are not superior to mesalamine for induction of remission,” the authors wrote. Oral 5-ASAs are preferred, especially given the absence of data on the efficacy or safety of maintenance budesonide therapy.
For patients with mild to moderate ulcerative proctosigmoiditis or proctitis, the guideline conditionally recommends rectal mesalamine over oral mesalamine. Compared with placebo, rectal mesalamine suppositories were significantly more likely to induce remission in randomized trials of patients with mild to moderate ulcerative proctitis. If these patients cannot tolerate or are refractory to mesalamine suppositories, low-quality evidence supports rectal steroid therapy over no treatment, the guideline states. For patients with mild to moderate ulcerative proctosigmoiditis, moderate-quality evidence supports mesalamine enemas over rectal corticosteroids. If these patients want to avoid the difficulties of enemas, the guideline considers rectal corticosteroid foam a reasonable alternative.
Likewise, they cite low-quality evidence for adding oral prednisone or budesonide MMX to 5-ASA if patients are refractory to optimized 5-ASA therapy. No trials have directly compared rates of remission with budesonide MMX versus systemic corticosteroids. In just one placebo-controlled trial, adding budesonide MMX to 5-ASA slightly improved the chances of remission (risk ratio, 0.95; 95% confidence interval, 0.89-1.00). Furthermore, studies of other second-generation corticosteroids found they were better tolerated but no more likely to induce remission than oral prednisone or prednisolone.
Some patients with mild to moderate colitis respond inadequately to these recommended therapies and need systemic corticosteroids, immunomodulators, or biologic therapies to induce and maintain remission, the guideline authors noted. They make no recommendation on immunomodulators or biologics. Studies of probiotics, curcumin, and fecal microbiota transplantation are “urgently needed,” but for now, their use “risks delaying proven effective therapy, with the potential for worsening symptoms or complications,” they wrote. For patients without Clostridium difficile infections, they recommend against fecal microbiota transplantation except in the setting of a clinical trial.
The experts also noted the need for a tool to stratify patients with mild to moderate ulcerative colitis based on their risk of future progression and colectomy.
Finally, they call for studies on who will benefit most from high-dose mesalamine or topical mesalamine and on the relative safety and efficacy of budesonide and systemic corticosteroids in the event of an inadequate response to 5-ASAs.
All members were required to complete the disclosure statement. These statements are maintained at the American Gastroenterological Association headquarters in Bethesda, Maryland, and pertinent disclosures of conflict of interest are published with this report.
SOURCE: Crocket SD et al. Gastro 2019;156(2). doi: org/10.1053/j.gastro.2018.12.009.
For patients with extensive mild to moderate ulcerative colitis, numerous randomized controlled trials support the use of either standard-dose mesalamine (2-3 grams per day) or diazo-bonded 5-aminosalicylic acid (ASA) instead of low-dose mesalamine, sulfasalazine, or no therapy, state new guidelines from the American Gastroenterological Association, published in Gastroenterology.
Sulfasalazine (2-4 grams per day) is less likely to be tolerated but remains a “reasonable option” for remitted patients who are already on it and for patients with prominent arthritis symptoms, especially if alternative treatments are cost prohibitive, wrote Cynthia W. Ko, MD, MS, of the University of Washington, Seattle, and her associates.
According to the guideline, patients with mild to moderate ulcerative colitis have less than four to six bowel movements per day, only mild or moderate rectal bleeding, no constitutional symptoms, and no high overall inflammatory burden or signs of high inflammatory activity on the Mayo Clinic score and Truelove and Witt’s criteria. These patients usually do not require colectomy, but this outcome is more likely when patients are diagnosed before age 40 years or have extensive disease or deep ulcers, extraintestinal manifestations, or elevated inflammatory markers. These higher-risk patients need more aggressive initial treatment and faster treatment intensification in cases of inadequate response, the guideline emphasizes. Even for cases of mild to moderate ulcerative colitis, treatment intensification is preferable to repeated courses of corticosteroids.
The guideline recommends adding rectal mesalamine to oral 5-ASA if patients have extensive or left-sided mild to moderate ulcerative colitis. In randomized controlled trials, this combination was significantly more likely to induce and maintain remission than was standard-dose oral mesalamine monotherapy, the authors noted. “In the maintenance trials, enemas were used twice per week or for 1 week per month. Both oral and topical mesalamine were well tolerated.”
For patients with moderate disease activity or a suboptimal response to standard-dose mesalamine or diazo-bonded 5-ASA, the guideline recommends adding rectal mesalamine to high-dose oral mesalamine (more than 3 grams daily). Combination therapy maximizes the delivery of mesalamine to the affected area of the colon, which optimizes the trial of 5-ASA before opting for treatment escalation, the authors noted. They recommend once-daily oral mesalamine dosing, since this is easier to adhere to and studies have found no benefit of more frequent dosing.
For inducing remission of mild to moderate ulcerative colitis, the guideline recommends standard-dose oral mesalamine or diazo-bonded 5-ASA over budesonide. “Overall, the budesonide preparations are not superior to mesalamine for induction of remission,” the authors wrote. Oral 5-ASAs are preferred, especially given the absence of data on the efficacy or safety of maintenance budesonide therapy.
For patients with mild to moderate ulcerative proctosigmoiditis or proctitis, the guideline conditionally recommends rectal mesalamine over oral mesalamine. Compared with placebo, rectal mesalamine suppositories were significantly more likely to induce remission in randomized trials of patients with mild to moderate ulcerative proctitis. If these patients cannot tolerate or are refractory to mesalamine suppositories, low-quality evidence supports rectal steroid therapy over no treatment, the guideline states. For patients with mild to moderate ulcerative proctosigmoiditis, moderate-quality evidence supports mesalamine enemas over rectal corticosteroids. If these patients want to avoid the difficulties of enemas, the guideline considers rectal corticosteroid foam a reasonable alternative.
Likewise, they cite low-quality evidence for adding oral prednisone or budesonide MMX to 5-ASA if patients are refractory to optimized 5-ASA therapy. No trials have directly compared rates of remission with budesonide MMX versus systemic corticosteroids. In just one placebo-controlled trial, adding budesonide MMX to 5-ASA slightly improved the chances of remission (risk ratio, 0.95; 95% confidence interval, 0.89-1.00). Furthermore, studies of other second-generation corticosteroids found they were better tolerated but no more likely to induce remission than oral prednisone or prednisolone.
Some patients with mild to moderate colitis respond inadequately to these recommended therapies and need systemic corticosteroids, immunomodulators, or biologic therapies to induce and maintain remission, the guideline authors noted. They make no recommendation on immunomodulators or biologics. Studies of probiotics, curcumin, and fecal microbiota transplantation are “urgently needed,” but for now, their use “risks delaying proven effective therapy, with the potential for worsening symptoms or complications,” they wrote. For patients without Clostridium difficile infections, they recommend against fecal microbiota transplantation except in the setting of a clinical trial.
The experts also noted the need for a tool to stratify patients with mild to moderate ulcerative colitis based on their risk of future progression and colectomy.
Finally, they call for studies on who will benefit most from high-dose mesalamine or topical mesalamine and on the relative safety and efficacy of budesonide and systemic corticosteroids in the event of an inadequate response to 5-ASAs.
All members were required to complete the disclosure statement. These statements are maintained at the American Gastroenterological Association headquarters in Bethesda, Maryland, and pertinent disclosures of conflict of interest are published with this report.
SOURCE: Crocket SD et al. Gastro 2019;156(2). doi: org/10.1053/j.gastro.2018.12.009.
For patients with extensive mild to moderate ulcerative colitis, numerous randomized controlled trials support the use of either standard-dose mesalamine (2-3 grams per day) or diazo-bonded 5-aminosalicylic acid (ASA) instead of low-dose mesalamine, sulfasalazine, or no therapy, state new guidelines from the American Gastroenterological Association, published in Gastroenterology.
Sulfasalazine (2-4 grams per day) is less likely to be tolerated but remains a “reasonable option” for remitted patients who are already on it and for patients with prominent arthritis symptoms, especially if alternative treatments are cost prohibitive, wrote Cynthia W. Ko, MD, MS, of the University of Washington, Seattle, and her associates.
According to the guideline, patients with mild to moderate ulcerative colitis have less than four to six bowel movements per day, only mild or moderate rectal bleeding, no constitutional symptoms, and no high overall inflammatory burden or signs of high inflammatory activity on the Mayo Clinic score and Truelove and Witt’s criteria. These patients usually do not require colectomy, but this outcome is more likely when patients are diagnosed before age 40 years or have extensive disease or deep ulcers, extraintestinal manifestations, or elevated inflammatory markers. These higher-risk patients need more aggressive initial treatment and faster treatment intensification in cases of inadequate response, the guideline emphasizes. Even for cases of mild to moderate ulcerative colitis, treatment intensification is preferable to repeated courses of corticosteroids.
The guideline recommends adding rectal mesalamine to oral 5-ASA if patients have extensive or left-sided mild to moderate ulcerative colitis. In randomized controlled trials, this combination was significantly more likely to induce and maintain remission than was standard-dose oral mesalamine monotherapy, the authors noted. “In the maintenance trials, enemas were used twice per week or for 1 week per month. Both oral and topical mesalamine were well tolerated.”
For patients with moderate disease activity or a suboptimal response to standard-dose mesalamine or diazo-bonded 5-ASA, the guideline recommends adding rectal mesalamine to high-dose oral mesalamine (more than 3 grams daily). Combination therapy maximizes the delivery of mesalamine to the affected area of the colon, which optimizes the trial of 5-ASA before opting for treatment escalation, the authors noted. They recommend once-daily oral mesalamine dosing, since this is easier to adhere to and studies have found no benefit of more frequent dosing.
For inducing remission of mild to moderate ulcerative colitis, the guideline recommends standard-dose oral mesalamine or diazo-bonded 5-ASA over budesonide. “Overall, the budesonide preparations are not superior to mesalamine for induction of remission,” the authors wrote. Oral 5-ASAs are preferred, especially given the absence of data on the efficacy or safety of maintenance budesonide therapy.
For patients with mild to moderate ulcerative proctosigmoiditis or proctitis, the guideline conditionally recommends rectal mesalamine over oral mesalamine. Compared with placebo, rectal mesalamine suppositories were significantly more likely to induce remission in randomized trials of patients with mild to moderate ulcerative proctitis. If these patients cannot tolerate or are refractory to mesalamine suppositories, low-quality evidence supports rectal steroid therapy over no treatment, the guideline states. For patients with mild to moderate ulcerative proctosigmoiditis, moderate-quality evidence supports mesalamine enemas over rectal corticosteroids. If these patients want to avoid the difficulties of enemas, the guideline considers rectal corticosteroid foam a reasonable alternative.
Likewise, they cite low-quality evidence for adding oral prednisone or budesonide MMX to 5-ASA if patients are refractory to optimized 5-ASA therapy. No trials have directly compared rates of remission with budesonide MMX versus systemic corticosteroids. In just one placebo-controlled trial, adding budesonide MMX to 5-ASA slightly improved the chances of remission (risk ratio, 0.95; 95% confidence interval, 0.89-1.00). Furthermore, studies of other second-generation corticosteroids found they were better tolerated but no more likely to induce remission than oral prednisone or prednisolone.
Some patients with mild to moderate colitis respond inadequately to these recommended therapies and need systemic corticosteroids, immunomodulators, or biologic therapies to induce and maintain remission, the guideline authors noted. They make no recommendation on immunomodulators or biologics. Studies of probiotics, curcumin, and fecal microbiota transplantation are “urgently needed,” but for now, their use “risks delaying proven effective therapy, with the potential for worsening symptoms or complications,” they wrote. For patients without Clostridium difficile infections, they recommend against fecal microbiota transplantation except in the setting of a clinical trial.
The experts also noted the need for a tool to stratify patients with mild to moderate ulcerative colitis based on their risk of future progression and colectomy.
Finally, they call for studies on who will benefit most from high-dose mesalamine or topical mesalamine and on the relative safety and efficacy of budesonide and systemic corticosteroids in the event of an inadequate response to 5-ASAs.
All members were required to complete the disclosure statement. These statements are maintained at the American Gastroenterological Association headquarters in Bethesda, Maryland, and pertinent disclosures of conflict of interest are published with this report.
SOURCE: Crocket SD et al. Gastro 2019;156(2). doi: org/10.1053/j.gastro.2018.12.009.
FROM GASTROENTEROLOGY
Findings in seropositive arthralgia patients may help to predict RA
Findings from an ongoing study of individuals with seropositive arthralgia, as well as from numerous other ongoing research efforts, suggest that it will soon be possible to predict a future rheumatoid arthritis diagnosis, according to Douglas J. Veale, MD.
Evidence also suggests that disease onset can be delayed, and that there is potential for disease prevention in those cases, Dr. Veale, a professor and consultant rheumatologist at St. Vincent’s University Hospital in Dublin said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
Cellular and molecular profiling of RA risk
Dr. Veale’s current research focuses on patients presenting with joint pain but no joint swelling or clinical evidence of soft tissue swelling, who are found to be seropositive for anticitrullinated protein antibodies (ACPA) and/or rheumatoid factor (RF).
“We have termed these patients [as having] ‘seropositive arthralgia,’ and we started a study in our institution because we started seeing more of these patients being referred in by the general practitioners,” said Dr. Veale, who is also director of translational research at the Dublin Academic Medical Centre of University College Dublin.
The aim of the study is to biopsy synovial tissue obtained during knee joint arthroscopy (which has been shown in prior studies to provide the same synovial findings as can be obtained through wrist and ankle biopsies) and to assess cellular and molecular profiles and clinical outcomes in these subjects, he said.
Of 36 seropositive arthralgia patients recruited to date, 22 are women, and 19 developed RA by 2010 ACR criteria within 12 months; most of those did so within 2-3 months, he said.
Median swollen joint counts were zero, and tender joint counts were slightly raised (median = 0, interquartile range = 0-4) in the subjects at baseline. Overall, 82% were RF positive, 91% were ACPA positive, and 73% were both RF and ACPA positive.
“The median [C-reactive protein (CRP) level] was 3 [mg/dL] with a range of 2-7, so most of these are normal when they’re coming to see us,” he said.
The level of synovitis seen on knee arthroscopy was a median of 60 on a visual analog scale of 0-100.
“So the level of synovitis that we’re seeing is certainly over a median of 50%,” he added.
Of 22 patients who were followed for at least 1 year – including the 19 who developed RA – none were on therapy at baseline, and none had CRP over 5 mg/dL at baseline. Two of the 19 who later developed RA elected to begin treatment before they developed the disease – one with hydroxychloroquine and one with methotrexate – and treatment was initiated in the remaining 17 RA patients as soon as they met the ACR RA criteria. Currently, 14 are on synthetic disease-modifying antirheumatic drugs (DMARDs), and 5 are on biologic DMARDs. Overall, 13 of the 22 patients followed for 1 year have no disease activity, 5 have low disease activity, and 4 have moderate disease activity, he said.
One question addressed in this study is whether immunostaining predicts arthritis, Dr. Veale noted.
“The short answer is ‘no,’ ” he said, explaining that activated T and B cells are seen in the biopsies of subjects who remain as seropositive arthralgia patients, and also in patients who actually develop RA. “So the immunohistology of these biopsies is not telling us a great deal.”
Immunophenotyping to establish RA risk
Another finding of interest is an increase in the synovial tissue CD38 plasmablasts as detected by RNA sequencing in seropositive arthralgia subjects.
“Their pattern looks more like early RA or established RA,” he noted.
Similarly, the proportion of B cells is already increased in the seropositive arthralgia subjects, compared with healthy subjects, and is similar to that seen in established seropositive and seronegative RA patients.
After looking at “a whole range of immunophenotypes,” Dr. Veale and his colleagues found that several other genes (not just for CD38) are expressed at increased levels in the arthralgia patients.
“The pattern that we’re seeing is that the seropositive arthralgia patients look more like the early rheumatoid and the established rheumatoid patients when we actually analyze their gene signatures using immunophenotyping,” he said.
The patients in this study were all referred by general practitioners, but another study – the PRAIRI study – recruited seropositive arthralgia patients from the community and randomized them to receive a single rituximab infusion or placebo (Ann Rheum Dis. 2019;78:179-85).
“What they showed is that patients who received one dose of rituximab actually developed rheumatoid arthritis at a slower rate and at a later time than the patients who received placebo,” he said.
At 1-year follow-up, there was no difference in the rate of development of RA between the groups; rituximab had merely delayed the onset of RA, he said.
“They do discuss in this paper what the effect would be if you continued treatment in these patients: Would we actually prevent the onset of rheumatoid arthritis in a significant cohort of patients?” he said. “But we don’t know that.”
Could immune checkpoint inhibition reveal RA risk?
Recent findings from work with immune checkpoint therapy in the hematology/oncology arena, however, raise other interesting possibilities with respect to early treatment and prevention of RA.
A number of case reports have documented the development of autoimmune diseases in patients with cancer who have undergone treatment with checkpoint inhibitors.
“Essentially what happens in cancer is that the activated T cells upregulate immune checkpoint molecules. ... and what these molecules do is they make the T cells essentially resistant to attacking the tumor cells,” he explained, noting that the molecules include programmed death 1 (PD-1) and cytotoxic T-lymphocyte–associated protein 4 (CTLA-4).
Checkpoint inhibitors bind to these molecules and “free up these activated T cells to attack the tumor cells” both through cytokines (interferon release) and direct cell cytotoxicity, he said.
This is relevant for rheumatology patients, because the PD-1 checkpoint molecule is overexpressed in pathogenic T cells in RA and systemic lupus erythematosus.
A closer look at his own RA patients showed that those who were ACPA positive had higher levels of soluble PD-1 than did the ACPA-negative RA patients – a finding that has been replicated in two other cohorts, he noted.
“So we wanted to look at our seropositive arthralgia subjects and see if there is something in their gene signatures on immunophenotyping which actually would give us a clue in terms of this checkpoint inhibitor pathway,” he said. “What we found is that the anti–PD-1 signature is increased in our arthralgia patients, and again, the pattern of expression is more similar to early rheumatoid arthritis and established rheumatoid arthritis, and is significantly different from both healthy controls and patients with osteoarthritis.”
PD-1 expression was also found to be increased on CD4- and CD8-positive T cells taken from the synovial tissues in these patients, he said.
Immunostaining of the T cells showed, interestingly, that the ligand for PD-1 is “almost absent,” he noted.
“So there’s an overexpression of PD-1, but there’s a downregulation of the ligand for PD-1, so that means that the PD-1 pathway is not active in these patients because the PD-1 ... has no ligand to actually bind on to.”
This suggests that “something else may happen that will upregulate the ligand – maybe a second hit,” thereby allowing PD-1 to bind and become active, he said.
“I realize what I’ve been talking about is fairly controversial, but I think it may be possible soon to predict a diagnosis of rheumatoid arthritis before clinical arthritis develops, but not in everybody,” he said, noting that current diagnostic tools are often unreliable.
For example, CRP levels in most of his study subjects remained normal even after converting to meet RA criteria, he explained.
However, “the checkpoint inhibitor story is absolutely fascinating,” he said.
“It’s unmasked an RA phenotype in patients who are receiving these drugs, and we have identified that the PD-1 pathway is altered in the synovial tissue, not just in patients with established rheumatoid arthritis, but also in subjects before they have developed arthritis and they have circulating autoantibodies,” he said.
The CD38 plasmablasts found to be present in the synovial tissue before RA presents clinically may also represent a therapeutic target, he added.
Dr. Veale disclosed financial relationships (research grants, consulting fees, speaker’s bureau, and “other” relationships) with AbbVie, Pfizer, UCB, Roche, and Janssen.
Findings from an ongoing study of individuals with seropositive arthralgia, as well as from numerous other ongoing research efforts, suggest that it will soon be possible to predict a future rheumatoid arthritis diagnosis, according to Douglas J. Veale, MD.
Evidence also suggests that disease onset can be delayed, and that there is potential for disease prevention in those cases, Dr. Veale, a professor and consultant rheumatologist at St. Vincent’s University Hospital in Dublin said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
Cellular and molecular profiling of RA risk
Dr. Veale’s current research focuses on patients presenting with joint pain but no joint swelling or clinical evidence of soft tissue swelling, who are found to be seropositive for anticitrullinated protein antibodies (ACPA) and/or rheumatoid factor (RF).
“We have termed these patients [as having] ‘seropositive arthralgia,’ and we started a study in our institution because we started seeing more of these patients being referred in by the general practitioners,” said Dr. Veale, who is also director of translational research at the Dublin Academic Medical Centre of University College Dublin.
The aim of the study is to biopsy synovial tissue obtained during knee joint arthroscopy (which has been shown in prior studies to provide the same synovial findings as can be obtained through wrist and ankle biopsies) and to assess cellular and molecular profiles and clinical outcomes in these subjects, he said.
Of 36 seropositive arthralgia patients recruited to date, 22 are women, and 19 developed RA by 2010 ACR criteria within 12 months; most of those did so within 2-3 months, he said.
Median swollen joint counts were zero, and tender joint counts were slightly raised (median = 0, interquartile range = 0-4) in the subjects at baseline. Overall, 82% were RF positive, 91% were ACPA positive, and 73% were both RF and ACPA positive.
“The median [C-reactive protein (CRP) level] was 3 [mg/dL] with a range of 2-7, so most of these are normal when they’re coming to see us,” he said.
The level of synovitis seen on knee arthroscopy was a median of 60 on a visual analog scale of 0-100.
“So the level of synovitis that we’re seeing is certainly over a median of 50%,” he added.
Of 22 patients who were followed for at least 1 year – including the 19 who developed RA – none were on therapy at baseline, and none had CRP over 5 mg/dL at baseline. Two of the 19 who later developed RA elected to begin treatment before they developed the disease – one with hydroxychloroquine and one with methotrexate – and treatment was initiated in the remaining 17 RA patients as soon as they met the ACR RA criteria. Currently, 14 are on synthetic disease-modifying antirheumatic drugs (DMARDs), and 5 are on biologic DMARDs. Overall, 13 of the 22 patients followed for 1 year have no disease activity, 5 have low disease activity, and 4 have moderate disease activity, he said.
One question addressed in this study is whether immunostaining predicts arthritis, Dr. Veale noted.
“The short answer is ‘no,’ ” he said, explaining that activated T and B cells are seen in the biopsies of subjects who remain as seropositive arthralgia patients, and also in patients who actually develop RA. “So the immunohistology of these biopsies is not telling us a great deal.”
Immunophenotyping to establish RA risk
Another finding of interest is an increase in the synovial tissue CD38 plasmablasts as detected by RNA sequencing in seropositive arthralgia subjects.
“Their pattern looks more like early RA or established RA,” he noted.
Similarly, the proportion of B cells is already increased in the seropositive arthralgia subjects, compared with healthy subjects, and is similar to that seen in established seropositive and seronegative RA patients.
After looking at “a whole range of immunophenotypes,” Dr. Veale and his colleagues found that several other genes (not just for CD38) are expressed at increased levels in the arthralgia patients.
“The pattern that we’re seeing is that the seropositive arthralgia patients look more like the early rheumatoid and the established rheumatoid patients when we actually analyze their gene signatures using immunophenotyping,” he said.
The patients in this study were all referred by general practitioners, but another study – the PRAIRI study – recruited seropositive arthralgia patients from the community and randomized them to receive a single rituximab infusion or placebo (Ann Rheum Dis. 2019;78:179-85).
“What they showed is that patients who received one dose of rituximab actually developed rheumatoid arthritis at a slower rate and at a later time than the patients who received placebo,” he said.
At 1-year follow-up, there was no difference in the rate of development of RA between the groups; rituximab had merely delayed the onset of RA, he said.
“They do discuss in this paper what the effect would be if you continued treatment in these patients: Would we actually prevent the onset of rheumatoid arthritis in a significant cohort of patients?” he said. “But we don’t know that.”
Could immune checkpoint inhibition reveal RA risk?
Recent findings from work with immune checkpoint therapy in the hematology/oncology arena, however, raise other interesting possibilities with respect to early treatment and prevention of RA.
A number of case reports have documented the development of autoimmune diseases in patients with cancer who have undergone treatment with checkpoint inhibitors.
“Essentially what happens in cancer is that the activated T cells upregulate immune checkpoint molecules. ... and what these molecules do is they make the T cells essentially resistant to attacking the tumor cells,” he explained, noting that the molecules include programmed death 1 (PD-1) and cytotoxic T-lymphocyte–associated protein 4 (CTLA-4).
Checkpoint inhibitors bind to these molecules and “free up these activated T cells to attack the tumor cells” both through cytokines (interferon release) and direct cell cytotoxicity, he said.
This is relevant for rheumatology patients, because the PD-1 checkpoint molecule is overexpressed in pathogenic T cells in RA and systemic lupus erythematosus.
A closer look at his own RA patients showed that those who were ACPA positive had higher levels of soluble PD-1 than did the ACPA-negative RA patients – a finding that has been replicated in two other cohorts, he noted.
“So we wanted to look at our seropositive arthralgia subjects and see if there is something in their gene signatures on immunophenotyping which actually would give us a clue in terms of this checkpoint inhibitor pathway,” he said. “What we found is that the anti–PD-1 signature is increased in our arthralgia patients, and again, the pattern of expression is more similar to early rheumatoid arthritis and established rheumatoid arthritis, and is significantly different from both healthy controls and patients with osteoarthritis.”
PD-1 expression was also found to be increased on CD4- and CD8-positive T cells taken from the synovial tissues in these patients, he said.
Immunostaining of the T cells showed, interestingly, that the ligand for PD-1 is “almost absent,” he noted.
“So there’s an overexpression of PD-1, but there’s a downregulation of the ligand for PD-1, so that means that the PD-1 pathway is not active in these patients because the PD-1 ... has no ligand to actually bind on to.”
This suggests that “something else may happen that will upregulate the ligand – maybe a second hit,” thereby allowing PD-1 to bind and become active, he said.
“I realize what I’ve been talking about is fairly controversial, but I think it may be possible soon to predict a diagnosis of rheumatoid arthritis before clinical arthritis develops, but not in everybody,” he said, noting that current diagnostic tools are often unreliable.
For example, CRP levels in most of his study subjects remained normal even after converting to meet RA criteria, he explained.
However, “the checkpoint inhibitor story is absolutely fascinating,” he said.
“It’s unmasked an RA phenotype in patients who are receiving these drugs, and we have identified that the PD-1 pathway is altered in the synovial tissue, not just in patients with established rheumatoid arthritis, but also in subjects before they have developed arthritis and they have circulating autoantibodies,” he said.
The CD38 plasmablasts found to be present in the synovial tissue before RA presents clinically may also represent a therapeutic target, he added.
Dr. Veale disclosed financial relationships (research grants, consulting fees, speaker’s bureau, and “other” relationships) with AbbVie, Pfizer, UCB, Roche, and Janssen.
Findings from an ongoing study of individuals with seropositive arthralgia, as well as from numerous other ongoing research efforts, suggest that it will soon be possible to predict a future rheumatoid arthritis diagnosis, according to Douglas J. Veale, MD.
Evidence also suggests that disease onset can be delayed, and that there is potential for disease prevention in those cases, Dr. Veale, a professor and consultant rheumatologist at St. Vincent’s University Hospital in Dublin said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
Cellular and molecular profiling of RA risk
Dr. Veale’s current research focuses on patients presenting with joint pain but no joint swelling or clinical evidence of soft tissue swelling, who are found to be seropositive for anticitrullinated protein antibodies (ACPA) and/or rheumatoid factor (RF).
“We have termed these patients [as having] ‘seropositive arthralgia,’ and we started a study in our institution because we started seeing more of these patients being referred in by the general practitioners,” said Dr. Veale, who is also director of translational research at the Dublin Academic Medical Centre of University College Dublin.
The aim of the study is to biopsy synovial tissue obtained during knee joint arthroscopy (which has been shown in prior studies to provide the same synovial findings as can be obtained through wrist and ankle biopsies) and to assess cellular and molecular profiles and clinical outcomes in these subjects, he said.
Of 36 seropositive arthralgia patients recruited to date, 22 are women, and 19 developed RA by 2010 ACR criteria within 12 months; most of those did so within 2-3 months, he said.
Median swollen joint counts were zero, and tender joint counts were slightly raised (median = 0, interquartile range = 0-4) in the subjects at baseline. Overall, 82% were RF positive, 91% were ACPA positive, and 73% were both RF and ACPA positive.
“The median [C-reactive protein (CRP) level] was 3 [mg/dL] with a range of 2-7, so most of these are normal when they’re coming to see us,” he said.
The level of synovitis seen on knee arthroscopy was a median of 60 on a visual analog scale of 0-100.
“So the level of synovitis that we’re seeing is certainly over a median of 50%,” he added.
Of 22 patients who were followed for at least 1 year – including the 19 who developed RA – none were on therapy at baseline, and none had CRP over 5 mg/dL at baseline. Two of the 19 who later developed RA elected to begin treatment before they developed the disease – one with hydroxychloroquine and one with methotrexate – and treatment was initiated in the remaining 17 RA patients as soon as they met the ACR RA criteria. Currently, 14 are on synthetic disease-modifying antirheumatic drugs (DMARDs), and 5 are on biologic DMARDs. Overall, 13 of the 22 patients followed for 1 year have no disease activity, 5 have low disease activity, and 4 have moderate disease activity, he said.
One question addressed in this study is whether immunostaining predicts arthritis, Dr. Veale noted.
“The short answer is ‘no,’ ” he said, explaining that activated T and B cells are seen in the biopsies of subjects who remain as seropositive arthralgia patients, and also in patients who actually develop RA. “So the immunohistology of these biopsies is not telling us a great deal.”
Immunophenotyping to establish RA risk
Another finding of interest is an increase in the synovial tissue CD38 plasmablasts as detected by RNA sequencing in seropositive arthralgia subjects.
“Their pattern looks more like early RA or established RA,” he noted.
Similarly, the proportion of B cells is already increased in the seropositive arthralgia subjects, compared with healthy subjects, and is similar to that seen in established seropositive and seronegative RA patients.
After looking at “a whole range of immunophenotypes,” Dr. Veale and his colleagues found that several other genes (not just for CD38) are expressed at increased levels in the arthralgia patients.
“The pattern that we’re seeing is that the seropositive arthralgia patients look more like the early rheumatoid and the established rheumatoid patients when we actually analyze their gene signatures using immunophenotyping,” he said.
The patients in this study were all referred by general practitioners, but another study – the PRAIRI study – recruited seropositive arthralgia patients from the community and randomized them to receive a single rituximab infusion or placebo (Ann Rheum Dis. 2019;78:179-85).
“What they showed is that patients who received one dose of rituximab actually developed rheumatoid arthritis at a slower rate and at a later time than the patients who received placebo,” he said.
At 1-year follow-up, there was no difference in the rate of development of RA between the groups; rituximab had merely delayed the onset of RA, he said.
“They do discuss in this paper what the effect would be if you continued treatment in these patients: Would we actually prevent the onset of rheumatoid arthritis in a significant cohort of patients?” he said. “But we don’t know that.”
Could immune checkpoint inhibition reveal RA risk?
Recent findings from work with immune checkpoint therapy in the hematology/oncology arena, however, raise other interesting possibilities with respect to early treatment and prevention of RA.
A number of case reports have documented the development of autoimmune diseases in patients with cancer who have undergone treatment with checkpoint inhibitors.
“Essentially what happens in cancer is that the activated T cells upregulate immune checkpoint molecules. ... and what these molecules do is they make the T cells essentially resistant to attacking the tumor cells,” he explained, noting that the molecules include programmed death 1 (PD-1) and cytotoxic T-lymphocyte–associated protein 4 (CTLA-4).
Checkpoint inhibitors bind to these molecules and “free up these activated T cells to attack the tumor cells” both through cytokines (interferon release) and direct cell cytotoxicity, he said.
This is relevant for rheumatology patients, because the PD-1 checkpoint molecule is overexpressed in pathogenic T cells in RA and systemic lupus erythematosus.
A closer look at his own RA patients showed that those who were ACPA positive had higher levels of soluble PD-1 than did the ACPA-negative RA patients – a finding that has been replicated in two other cohorts, he noted.
“So we wanted to look at our seropositive arthralgia subjects and see if there is something in their gene signatures on immunophenotyping which actually would give us a clue in terms of this checkpoint inhibitor pathway,” he said. “What we found is that the anti–PD-1 signature is increased in our arthralgia patients, and again, the pattern of expression is more similar to early rheumatoid arthritis and established rheumatoid arthritis, and is significantly different from both healthy controls and patients with osteoarthritis.”
PD-1 expression was also found to be increased on CD4- and CD8-positive T cells taken from the synovial tissues in these patients, he said.
Immunostaining of the T cells showed, interestingly, that the ligand for PD-1 is “almost absent,” he noted.
“So there’s an overexpression of PD-1, but there’s a downregulation of the ligand for PD-1, so that means that the PD-1 pathway is not active in these patients because the PD-1 ... has no ligand to actually bind on to.”
This suggests that “something else may happen that will upregulate the ligand – maybe a second hit,” thereby allowing PD-1 to bind and become active, he said.
“I realize what I’ve been talking about is fairly controversial, but I think it may be possible soon to predict a diagnosis of rheumatoid arthritis before clinical arthritis develops, but not in everybody,” he said, noting that current diagnostic tools are often unreliable.
For example, CRP levels in most of his study subjects remained normal even after converting to meet RA criteria, he explained.
However, “the checkpoint inhibitor story is absolutely fascinating,” he said.
“It’s unmasked an RA phenotype in patients who are receiving these drugs, and we have identified that the PD-1 pathway is altered in the synovial tissue, not just in patients with established rheumatoid arthritis, but also in subjects before they have developed arthritis and they have circulating autoantibodies,” he said.
The CD38 plasmablasts found to be present in the synovial tissue before RA presents clinically may also represent a therapeutic target, he added.
Dr. Veale disclosed financial relationships (research grants, consulting fees, speaker’s bureau, and “other” relationships) with AbbVie, Pfizer, UCB, Roche, and Janssen.
EXPERT ANALYSIS FROM THE WINTER RHEUMATOLOGY SYMPOSIUM
Key clinical point:
Major finding: 19 of 36 patients developed RA within 12 months, and 13 of those had no disease activity with treatment initiated at RA onset.
Study details: A study of 36 seropositive arthralgia patients.
Disclosures: Dr. Veale disclosed financial relationships (research grants, consulting fees, speaker’s bureau, and “other” relationships) with AbbVie, Pfizer, UCB, Roche, and Janssen.
Case report: Longstanding actinic keratosis responds to kanuka honey
GRAND CAYMAN, CAYMAN ISLANDS – Not all honeys are created equal, Theodore Rosen, MD, said at the meeting provided by Global Academy for Medical Education.
“It seems that kanuka is the new manuka,” said Dr. Rosen, professor of dermatology at Baylor College of Medicine, Houston. These lesser-known New Zealand bush honeys may be something to watch because research and case reports continue to provide intriguing hints of how these honeys exert their immunomodulatory effects on skin, he commented, describing a recent case report describing the elimination of a large, long-standing actinic keratosis (AK) with application of kanuka honey.
Manuka (Leptospermum scoparium) is a large bush native to both Australia and New Zealand. Kanuka (Kunzea ericoides) is quite similar in size and appearance, but native only to New Zealand. Honey made from the flowers of these bushes possesses some unique properties that make it an attractive addition to wound healing regimens, according to a 2014 study (Int J Gen Med. 2014;7:149-58).
The study examined samples of manuka, kanuka, a manuka/kanuka blend, and clover honey. The investigators found that kanuka honey, and to a lesser extent manuka honey, exerted a potent anti-inflammatory effect in human embryonic kidney cells. The honeys interfered with toll-like receptor 1 and 2 signaling, which would reduce the production of proinflammatory cytokines.
Kanuka’s potency seems directly related to its unusually high level of arabinogalactan, according to Saras Mane, MD, primary author of the AK case report (Case Rep Dermatol Med. 2018 May 31;2018:4628971). Dr. Mane is with the Medical Research Institute of New Zealand in Wellington.
“The immunomodulatory properties of kanuka honey in particular are thought to be more potent than other New Zealand honeys due to the relatively high concentrations of arabinogalactan proteins present,” Dr. Mane and his coauthors wrote in the case report. “These proteins have been shown to stimulate release of TNF-alpha from monocytic cell lines in vitro.”
The report involved a 66-year-old man who was enrolled in a randomized trial of a commercialized medical-grade kanuka honey ointment (Honevo, 90% kanuka honey, 10% glycerin; Honeylab NZ) for rosacea.
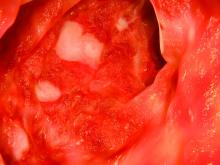
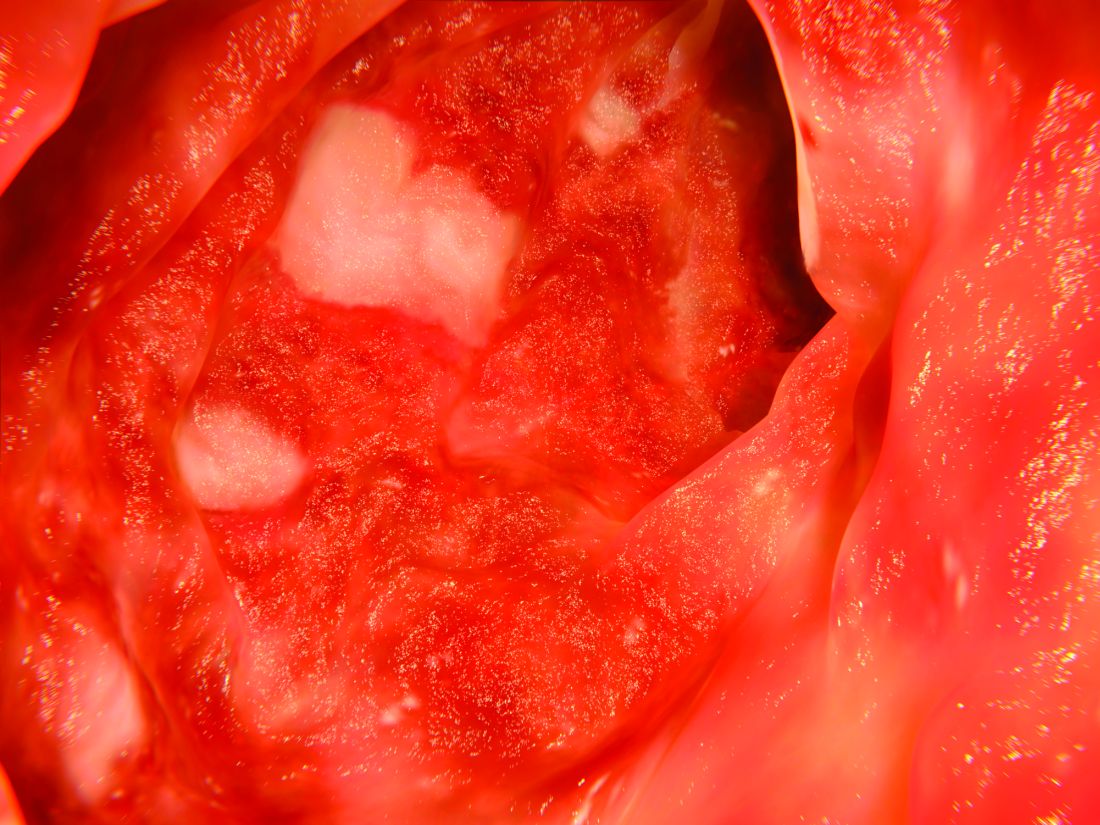
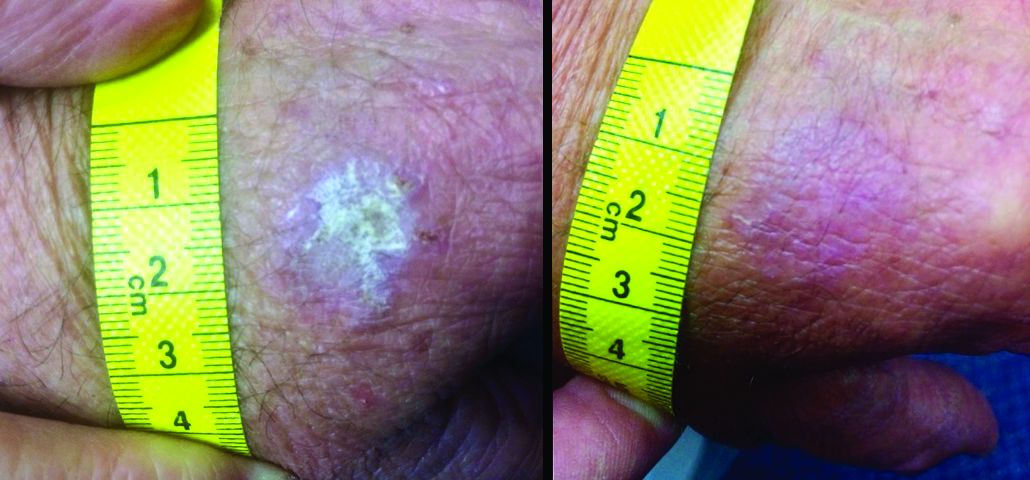
The patient also had multiple AKs, including a raised, crusted, scaly lesion measuring 20 mm by 21 mm with marginal erythema on the back of one hand. The lesion had been present and dormant for a number of years, but it had recently begun to grow.
“This gentleman decided he’d just try the honey on his AK, too,” Dr. Rosen said. The man reported applying a small amount to the lesion and erythematous area once a day, leaving it on for about 30 to 60 minutes. After 5 days, he stopped because the lesion became tender. During the next two days, the patient reported “picking at” the lesion, which was softening. He repeated this cycle of treatment for 3 months with no other therapy to the lesion.
“The lesion gradually reduced in size with an initial rapid reduction in its dry, crusted nature,” the authors reported. “After 3 months, residual appearance of the lesion was a 20 mm by 17 mm area of pink skin with no elements of hypertrophy, crusting, or loss of skin integrity,” they noted. “At 6 months, there were no signs of recurrence. At 9 months, the appearance of the skin had fully returned to normal. A telephone follow-up was conducted at 2 years after treatment, and the patient reported that his skin in the area was still completely normal and that there were no signs of recurrence.”
Dr. Mane noted that they had only clinical evidence, and no histology of the lesion either before or after its change. “The AK was diagnosed and treated in primary care, where it is not usual for AKs to be biopsied, and the decision to write up the case was made after the course of treatment had finished,” they said.
“Immunomodulatory topical agents are already widely used in the treatment of AK as an immune component is evident in its etiology,” they wrote. “Immunocompromised patients have 250 times the risk of developing an AK than the general population.”
Dr. Rosen said that kanuka honey is also being investigated in psoriasis, eczema, acne, herpes simplex virus, and diaper dermatitis. It is also being studied for rosacea.
Dr. Mane declared no conflicts of interest. Some coauthors disclosed that they have previously received funding from HoneyLab NZ. Dr. Rosen has no commercial interest in HoneyLab.
The meeting was sponsored by Global Academy for Medical Education; Global Academy and this news organization are owned by the same parent company.
GRAND CAYMAN, CAYMAN ISLANDS – Not all honeys are created equal, Theodore Rosen, MD, said at the meeting provided by Global Academy for Medical Education.
“It seems that kanuka is the new manuka,” said Dr. Rosen, professor of dermatology at Baylor College of Medicine, Houston. These lesser-known New Zealand bush honeys may be something to watch because research and case reports continue to provide intriguing hints of how these honeys exert their immunomodulatory effects on skin, he commented, describing a recent case report describing the elimination of a large, long-standing actinic keratosis (AK) with application of kanuka honey.
Manuka (Leptospermum scoparium) is a large bush native to both Australia and New Zealand. Kanuka (Kunzea ericoides) is quite similar in size and appearance, but native only to New Zealand. Honey made from the flowers of these bushes possesses some unique properties that make it an attractive addition to wound healing regimens, according to a 2014 study (Int J Gen Med. 2014;7:149-58).
The study examined samples of manuka, kanuka, a manuka/kanuka blend, and clover honey. The investigators found that kanuka honey, and to a lesser extent manuka honey, exerted a potent anti-inflammatory effect in human embryonic kidney cells. The honeys interfered with toll-like receptor 1 and 2 signaling, which would reduce the production of proinflammatory cytokines.
Kanuka’s potency seems directly related to its unusually high level of arabinogalactan, according to Saras Mane, MD, primary author of the AK case report (Case Rep Dermatol Med. 2018 May 31;2018:4628971). Dr. Mane is with the Medical Research Institute of New Zealand in Wellington.

“The immunomodulatory properties of kanuka honey in particular are thought to be more potent than other New Zealand honeys due to the relatively high concentrations of arabinogalactan proteins present,” Dr. Mane and his coauthors wrote in the case report. “These proteins have been shown to stimulate release of TNF-alpha from monocytic cell lines in vitro.”
The report involved a 66-year-old man who was enrolled in a randomized trial of a commercialized medical-grade kanuka honey ointment (Honevo, 90% kanuka honey, 10% glycerin; Honeylab NZ) for rosacea.
The patient also had multiple AKs, including a raised, crusted, scaly lesion measuring 20 mm by 21 mm with marginal erythema on the back of one hand. The lesion had been present and dormant for a number of years, but it had recently begun to grow.
“This gentleman decided he’d just try the honey on his AK, too,” Dr. Rosen said. The man reported applying a small amount to the lesion and erythematous area once a day, leaving it on for about 30 to 60 minutes. After 5 days, he stopped because the lesion became tender. During the next two days, the patient reported “picking at” the lesion, which was softening. He repeated this cycle of treatment for 3 months with no other therapy to the lesion.
“The lesion gradually reduced in size with an initial rapid reduction in its dry, crusted nature,” the authors reported. “After 3 months, residual appearance of the lesion was a 20 mm by 17 mm area of pink skin with no elements of hypertrophy, crusting, or loss of skin integrity,” they noted. “At 6 months, there were no signs of recurrence. At 9 months, the appearance of the skin had fully returned to normal. A telephone follow-up was conducted at 2 years after treatment, and the patient reported that his skin in the area was still completely normal and that there were no signs of recurrence.”
Dr. Mane noted that they had only clinical evidence, and no histology of the lesion either before or after its change. “The AK was diagnosed and treated in primary care, where it is not usual for AKs to be biopsied, and the decision to write up the case was made after the course of treatment had finished,” they said.
“Immunomodulatory topical agents are already widely used in the treatment of AK as an immune component is evident in its etiology,” they wrote. “Immunocompromised patients have 250 times the risk of developing an AK than the general population.”
Dr. Rosen said that kanuka honey is also being investigated in psoriasis, eczema, acne, herpes simplex virus, and diaper dermatitis. It is also being studied for rosacea.
Dr. Mane declared no conflicts of interest. Some coauthors disclosed that they have previously received funding from HoneyLab NZ. Dr. Rosen has no commercial interest in HoneyLab.
The meeting was sponsored by Global Academy for Medical Education; Global Academy and this news organization are owned by the same parent company.
GRAND CAYMAN, CAYMAN ISLANDS – Not all honeys are created equal, Theodore Rosen, MD, said at the meeting provided by Global Academy for Medical Education.
“It seems that kanuka is the new manuka,” said Dr. Rosen, professor of dermatology at Baylor College of Medicine, Houston. These lesser-known New Zealand bush honeys may be something to watch because research and case reports continue to provide intriguing hints of how these honeys exert their immunomodulatory effects on skin, he commented, describing a recent case report describing the elimination of a large, long-standing actinic keratosis (AK) with application of kanuka honey.
Manuka (Leptospermum scoparium) is a large bush native to both Australia and New Zealand. Kanuka (Kunzea ericoides) is quite similar in size and appearance, but native only to New Zealand. Honey made from the flowers of these bushes possesses some unique properties that make it an attractive addition to wound healing regimens, according to a 2014 study (Int J Gen Med. 2014;7:149-58).
The study examined samples of manuka, kanuka, a manuka/kanuka blend, and clover honey. The investigators found that kanuka honey, and to a lesser extent manuka honey, exerted a potent anti-inflammatory effect in human embryonic kidney cells. The honeys interfered with toll-like receptor 1 and 2 signaling, which would reduce the production of proinflammatory cytokines.
Kanuka’s potency seems directly related to its unusually high level of arabinogalactan, according to Saras Mane, MD, primary author of the AK case report (Case Rep Dermatol Med. 2018 May 31;2018:4628971). Dr. Mane is with the Medical Research Institute of New Zealand in Wellington.

“The immunomodulatory properties of kanuka honey in particular are thought to be more potent than other New Zealand honeys due to the relatively high concentrations of arabinogalactan proteins present,” Dr. Mane and his coauthors wrote in the case report. “These proteins have been shown to stimulate release of TNF-alpha from monocytic cell lines in vitro.”
The report involved a 66-year-old man who was enrolled in a randomized trial of a commercialized medical-grade kanuka honey ointment (Honevo, 90% kanuka honey, 10% glycerin; Honeylab NZ) for rosacea.
The patient also had multiple AKs, including a raised, crusted, scaly lesion measuring 20 mm by 21 mm with marginal erythema on the back of one hand. The lesion had been present and dormant for a number of years, but it had recently begun to grow.
“This gentleman decided he’d just try the honey on his AK, too,” Dr. Rosen said. The man reported applying a small amount to the lesion and erythematous area once a day, leaving it on for about 30 to 60 minutes. After 5 days, he stopped because the lesion became tender. During the next two days, the patient reported “picking at” the lesion, which was softening. He repeated this cycle of treatment for 3 months with no other therapy to the lesion.
“The lesion gradually reduced in size with an initial rapid reduction in its dry, crusted nature,” the authors reported. “After 3 months, residual appearance of the lesion was a 20 mm by 17 mm area of pink skin with no elements of hypertrophy, crusting, or loss of skin integrity,” they noted. “At 6 months, there were no signs of recurrence. At 9 months, the appearance of the skin had fully returned to normal. A telephone follow-up was conducted at 2 years after treatment, and the patient reported that his skin in the area was still completely normal and that there were no signs of recurrence.”
Dr. Mane noted that they had only clinical evidence, and no histology of the lesion either before or after its change. “The AK was diagnosed and treated in primary care, where it is not usual for AKs to be biopsied, and the decision to write up the case was made after the course of treatment had finished,” they said.
“Immunomodulatory topical agents are already widely used in the treatment of AK as an immune component is evident in its etiology,” they wrote. “Immunocompromised patients have 250 times the risk of developing an AK than the general population.”
Dr. Rosen said that kanuka honey is also being investigated in psoriasis, eczema, acne, herpes simplex virus, and diaper dermatitis. It is also being studied for rosacea.
Dr. Mane declared no conflicts of interest. Some coauthors disclosed that they have previously received funding from HoneyLab NZ. Dr. Rosen has no commercial interest in HoneyLab.
The meeting was sponsored by Global Academy for Medical Education; Global Academy and this news organization are owned by the same parent company.
REPORTING FROM THE ANNUAL CARIBBEAN DERMATOLOGY SYMPOSIUM
Multicentric Reticulohistiocytosis With Arthralgia and Red-Orange Papulonodules
To the Editor:
A 50-year-old woman presented with an asymptomatic eruption on the dorsal aspect of the hands, abdomen, and face of 6 months’ duration. The eruption was associated with generalized arthralgia and fatigue. Within several weeks of onset of the cutaneous eruption, the patient developed swelling in the hands as well as worsening arthralgia. She was treated for presumed Lyme borreliosis but reported no improvement in the symptoms. She was then referred to dermatology for further management.
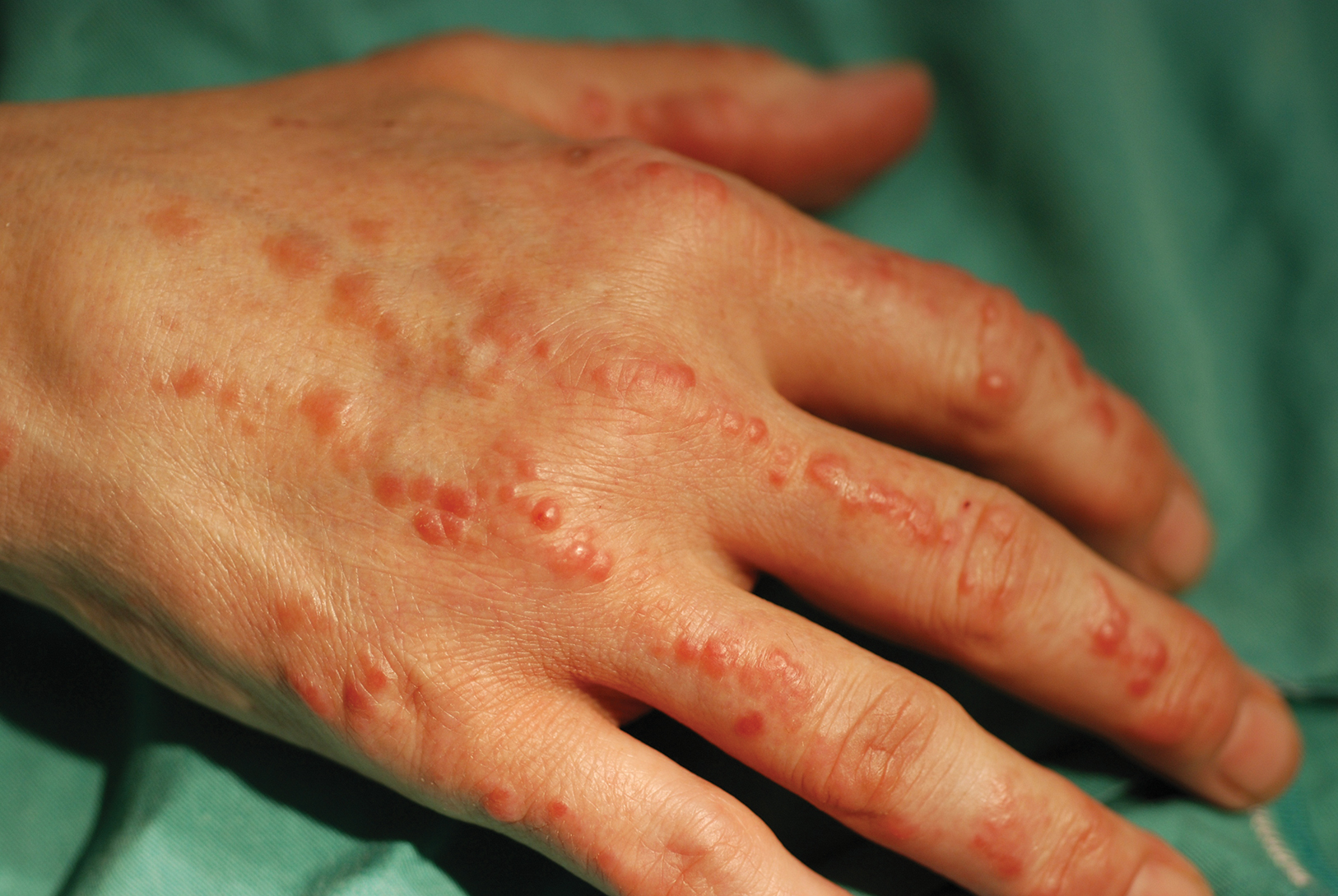
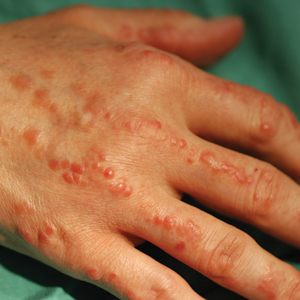
Physical examination revealed red-orange, edematous, monomorphic papulonodules scattered on the nasolabial folds, upper lip, and along the dorsal aspect of the hands and fingers (Figure 1). A brown rippled plaque was present on the left lower abdomen. The oral mucosa and nails were unremarkable. Laboratory studies showed elevated total cholesterol (244 mg/dL [reference range, <200 mg/dL]), low-density lipoproteins (130 mg/dL [reference range, 10–30 mg/dL]), aspartate aminotransferase (140 U/L [reference range, 10–30 U/L]), alanine aminotransferase (110 U/L [reference range, 10–40 U/L]), and total bilirubin (1.5 mg/dL [reference range, 0.3–1.2 mg/dL]). White blood cell count and C-reactive protein levels were within reference range. An antinuclear antibody titer of 1:80 with a homogenous pattern was found, and aldolase levels were elevated. Laboratory investigations for rheumatoid factor, Lyme disease, tuberculosis, hepatitis, and human immunodeficiency virus were negative. A chest radiograph was normal.
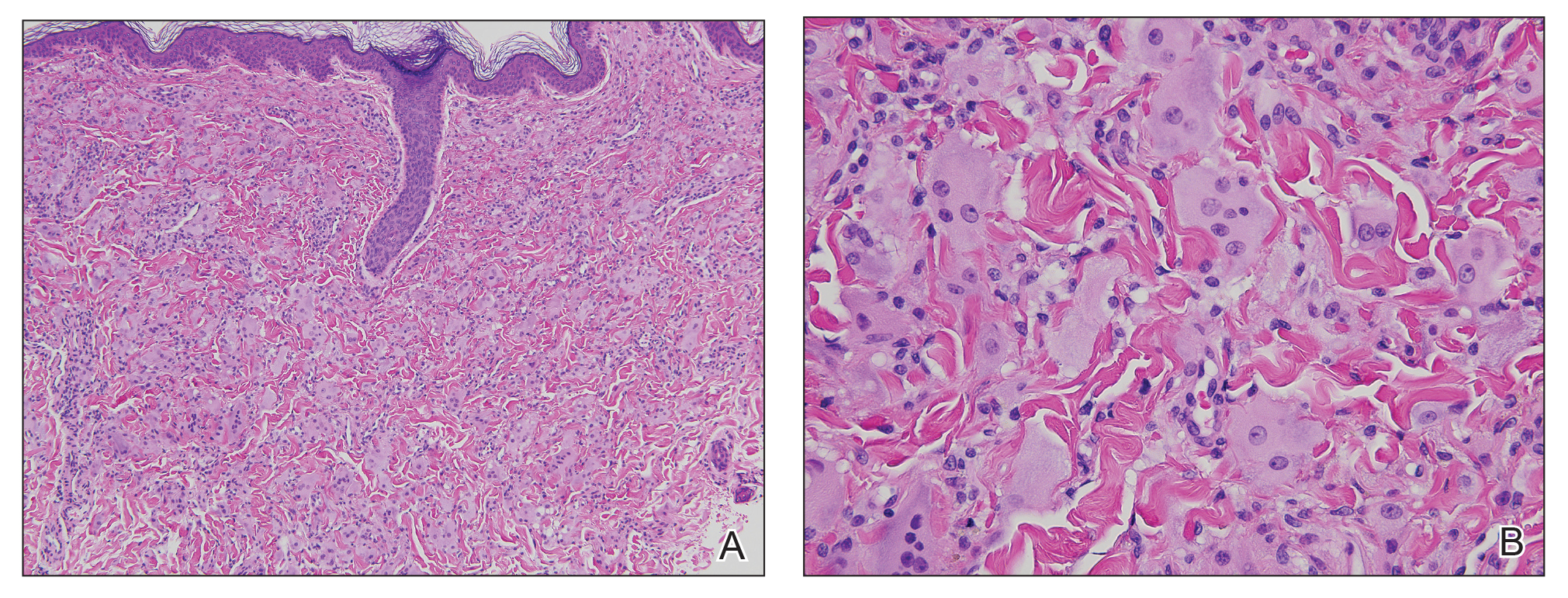
A punch biopsy from the right dorsal hand revealed a dermal proliferation of mononucleated and multinucleated epithelioid histiocytes with ample amounts of eosinophilic ground-glass cytoplasm (Figure 2). Immunohistochemistry revealed epithelioid histiocytes reactive for CD68, CD163, and factor XIIIA, and negative for S-100 and CD1a.
The patient was diagnosed with multicentric reticulohistiocytosis (MRH) and was initially treated with prednisone. Treatment was later augmented with etanercept and methotrexate with improvement in both the skin and joint symptoms.
Multicentric reticulohistiocytosis is a rare, non–Langerhans cell histiocytosis with both cutaneous and systemic features. Although case reports date back to the late 1800s, the term multicentric reticulohistiocytosis was first used in 1954.1 Multicentric reticulohistiocytosis is extremely uncommon and precludes thorough investigation of its etiology and management. The condition typically presents in the fifth to sixth decades of life and occurs more frequently in women with a female to male ratio estimated at 3 to 1.2,3 Pediatric cases have been reported but are exceedingly rare.4
Multicentric reticulohistiocytosis typically presents with a severe erosive arthropathy known as arthritis mutilans. Patients display a symmetric polyarthritis that commonly involves the elbows, wrists, and proximal and distal aspects of the interphalangeal joints. Onset and progression can be rapid, and the erosive nature leads to deformities in up to 45% of patients.2,5,6 Cutaneous findings arise an average of 3 years after the development of arthritis, though one-fifth of patients will initially present with cutaneous findings followed by the development of arthritis at any time.3,6 Clinical features include flesh-colored to reddish brown or yellow papulonodules that range in size from several millimeters to 2 cm. The lesions most commonly occur on the face (eg, ears, nose, paranasal cheeks), scalp, dorsal and lateral aspects of the hands and fingers, and overlying articular regions of the extremities. Characteristic periungual lesions classically are referred to as coral beads.4,6 Patients commonly report pruritus that may precede the development of the papules and nodules. Other cutaneous manifestations include xanthelasma, nail changes, and a photodistributed erythematous maculopapular eruption that may mimic dermatomyositis.6
Cutaneous findings of MRH can mimic rheumatoid nodules, gout, Gottron papules of dermatomyositis, lipoid proteinosis, sarcoidosis, lepromatous leprosy, granuloma annulare, xanthoma, xanthogranuloma, and fibroxanthoma.6,7 Histopathologic features may distinguish MRH from such entities. Findings include fairly well-circumscribed aggregates of large multinucleated giant cells with characteristic eosinophilic ground-glass cytoplasm. Histiocytes stain positively for CD68, HAM56, CD11b, and CD14, and variably for factor XIIIa. CD68, which is expressed by monocytes/macrophages, has been universally reported to be the most reliable marker of MRH. Negative staining for S-100 and CD1a supports a non-Langerhans origin for the involved histiocytes. If arthritic symptoms predominate, MRH must be distinguished from rheumatoid and psoriatic arthritis.6,7
Mucosal involvement occurs in approximately 50% of patients and includes the presence of nodules in the oral, nasal, and pharyngeal mucosae, as well as eye structures.2,3 Histiocytic infiltration has been documented in the heart, lungs, thyroid, liver, stomach, kidneys, muscle, bone marrow, and urogenital tract. Histiocytes also can invade the cartilage of the ears and nose causing disfigurement and characteristic leonine facies. Pathologic fractures may occur with bone involvement.5
Systemic features associated with MRH include hyperlipidemia, diabetes mellitus, thyroid disease, hypergammaglobulinemia, and various autoimmune diseases. Patients less frequently report fever and weight loss.2,5,6,8 Additionally, a positive tuberculin test occurs in 12% to 50% of patients.6 Various autoimmune diseases occur in 6% to 17% of cases including systemic lupus erythematosus, systemic sclerosis, rheumatoid arthritis, dermatomyositis, Sjögren syndrome, and primary biliary cirrhosis.2,5,6,8 The most clinically salient feature of MRH is its association with malignant conditions, which occur in up to 31% of patients. A variety of cancers have been reported in association with MRH, including breast, cervical, ovarian, stomach, penile, lymphoma, mesothelioma, and melanoma.7
The etiology of MRH is unclear. Although onset may precede the development of a malignant condition and regress with treatment, it cannot be considered a true paraneoplastic disorder, as it has no association with a specific cancer and does not typically parallel the disease course.6,9 Reports of increased levels of inflammatory mediators released from macrophages and endothelial cells, specifically IL-12, IL-1β, IL-6, and tumor necrosis factor α (TNF-α), have been thought to drive the destruction of bone and cartilage.6 In particular, TNF-α acts to indirectly induce destruction by stimulating proteolytic activity in macrophages, similar to the pathogenesis of joint damage in rheumatoid arthritis.8 Osteoclastic activity may play a role in the pathogenesis of MRH, as multinucleated giant cells in MRH can mature into osteoclasts by receptor activated nuclear factor–κB ligand signaling. In addition, patients treated with bisphosphonates have had decreased lacunar resorption.2,8
Initial management of MRH should include screening for hyperlipidemia, hypergammaglobulinemia, hyperglycemia, thyroid dysfunction, and autoimmune diseases, as well as age-appropriate cancer screening. Imaging studies should evaluate for the presence of erosive arthritis. There are no well-defined treatment algorithms for MRH due to the rarity of the disease, and recommendations largely rely on case reports. Although spontaneous remission typically occurs within 5 to 10 years, the risk for joint destruction argues for early pharmacologic intervention. Current management includes the use of nonsteroidal anti-inflammatory drugs and various immunosuppressants including oral glucocorticoids, cyclophosphamide, chlorambucil, methotrexate, or azathioprine.2 A combination of methotrexate with cyclophosphamide or glucocorticoids also has shown efficacy.10 Anti–TNF-α agents, such as etanercept, adalimumab, and infliximab, have been used with some success.2 Tumor necrosis factor α inhibitors used in combination with oral glucocorticoids and methotrexate may have an increased benefit.2,9,11 Evidence suggesting that TNF-α plays a role in the destruction of bone and cartilage led to the successful use of infliximab in combination with oral glucocorticoids and methotrexate, which prevented possible development of antibodies to infliximab and increased its efficacy.12 Bisphosphonate use in combination with glucocorticoids and methotrexate may prevent joint destruction without the serious adverse events associated with anti–TNF-α agents.2,9,13,14
- Goltz RW, Laymon CW. Multicentric reticulohistiocytosis of the skin and synovia; reticulohistiocytoma or ganglioneuroma. AMA Arch Derma Syphilol. 1954;69:717-731.
- Islam AD, Naguwa SM, Cheema GS, et al. Multicentric reticulohistiocytosis: a rare yet challenging disease. Clin Rev Allergy Immunol. 2013;45:281-289.
- West KL, Sporn T, Puri PK. Multicentric reticulohistiocytosis: a unique case with pulmonary fibrosis. Arch Dermatol. 2012;148:228-232.
- Outland JD, Keiran SJ, Schikler KN, et al. Multicentric reticulohistiocytosis in a 14-year-old girl. Pediatr Dermatol. 2002;19:527-531.
- Gorman JD, Danning C, Schumacher HR, et al. Multicentric reticulohistiocytosis: case report with immunohistochemical analysis and literature review. Arthritis Rheum. 2000;43:930-938.
- Tajirian AL, Malik MK, Robinson-Bostom L, et al. Multicentric reticulohistiocytosis. Clin Dermatol. 2006;24:486-492.
- Luz FB, Gaspar TAP, Kalil-Gaspar N, et al. Multicentric reticulohistiocytosis. J Eur Acad Dermatol Venereol. 2001;15:524-531.
- Trotta F, Castellino G, Lo Monaco A. Multicentric reticulohistiocytosis. Best Pract Res Clin Rheumatol. 2004;18:759-772.
- Kalajian AH, Callen JP. Multicentric reticulohistiocytosis successfully treated with infliximab: an illustrative case and evaluation of cytokine expression supporting anti-tumor necrosis factor therapy. Arch Derm. 2008;144:1360-1366.
- Liang GC, Granston AS. Complete remission of multicentric reticulohistiocytosis with combination therapy of steroid, cyclophosphamide, and low-dose pulse methotrexate. case report, review of the literature, and proposal for treatment. Arthritis Rheum. 1996;39:171-174.
- Lovelace K, Loyd A, Adelson D, et al. Etanercept and the treatment of multicentric reticulohistiocytosis. Arch Dermatol. 2005;141:1167-1168.
- Lee MW, Lee EY, Jeong YI, et al. Successful treatment of multicentric reticulohistiocytosis with a combination of infliximab, prednisolone and methotrexate. Acta Derm Venereol. 2004;84:478-479.
- Adamopoulos IE, Wordsworth PB, Edwards JR, et al. Osteoclast differentiation and bone resorption in multicentric reticulohistiocytosis. Hum Pathol. 2006;37:1176-1185.
- Satoh M, Oyama N, Yamada H, et al. Treatment trial of multicentric reticulohistiocytosis with a combination of predonisolone, methotrexate and alendronate. J Dermatol. 2008;35:168-171.
To the Editor:
A 50-year-old woman presented with an asymptomatic eruption on the dorsal aspect of the hands, abdomen, and face of 6 months’ duration. The eruption was associated with generalized arthralgia and fatigue. Within several weeks of onset of the cutaneous eruption, the patient developed swelling in the hands as well as worsening arthralgia. She was treated for presumed Lyme borreliosis but reported no improvement in the symptoms. She was then referred to dermatology for further management.
Physical examination revealed red-orange, edematous, monomorphic papulonodules scattered on the nasolabial folds, upper lip, and along the dorsal aspect of the hands and fingers (Figure 1). A brown rippled plaque was present on the left lower abdomen. The oral mucosa and nails were unremarkable. Laboratory studies showed elevated total cholesterol (244 mg/dL [reference range, <200 mg/dL]), low-density lipoproteins (130 mg/dL [reference range, 10–30 mg/dL]), aspartate aminotransferase (140 U/L [reference range, 10–30 U/L]), alanine aminotransferase (110 U/L [reference range, 10–40 U/L]), and total bilirubin (1.5 mg/dL [reference range, 0.3–1.2 mg/dL]). White blood cell count and C-reactive protein levels were within reference range. An antinuclear antibody titer of 1:80 with a homogenous pattern was found, and aldolase levels were elevated. Laboratory investigations for rheumatoid factor, Lyme disease, tuberculosis, hepatitis, and human immunodeficiency virus were negative. A chest radiograph was normal.
A punch biopsy from the right dorsal hand revealed a dermal proliferation of mononucleated and multinucleated epithelioid histiocytes with ample amounts of eosinophilic ground-glass cytoplasm (Figure 2). Immunohistochemistry revealed epithelioid histiocytes reactive for CD68, CD163, and factor XIIIA, and negative for S-100 and CD1a.
The patient was diagnosed with multicentric reticulohistiocytosis (MRH) and was initially treated with prednisone. Treatment was later augmented with etanercept and methotrexate with improvement in both the skin and joint symptoms.
Multicentric reticulohistiocytosis is a rare, non–Langerhans cell histiocytosis with both cutaneous and systemic features. Although case reports date back to the late 1800s, the term multicentric reticulohistiocytosis was first used in 1954.1 Multicentric reticulohistiocytosis is extremely uncommon and precludes thorough investigation of its etiology and management. The condition typically presents in the fifth to sixth decades of life and occurs more frequently in women with a female to male ratio estimated at 3 to 1.2,3 Pediatric cases have been reported but are exceedingly rare.4
Multicentric reticulohistiocytosis typically presents with a severe erosive arthropathy known as arthritis mutilans. Patients display a symmetric polyarthritis that commonly involves the elbows, wrists, and proximal and distal aspects of the interphalangeal joints. Onset and progression can be rapid, and the erosive nature leads to deformities in up to 45% of patients.2,5,6 Cutaneous findings arise an average of 3 years after the development of arthritis, though one-fifth of patients will initially present with cutaneous findings followed by the development of arthritis at any time.3,6 Clinical features include flesh-colored to reddish brown or yellow papulonodules that range in size from several millimeters to 2 cm. The lesions most commonly occur on the face (eg, ears, nose, paranasal cheeks), scalp, dorsal and lateral aspects of the hands and fingers, and overlying articular regions of the extremities. Characteristic periungual lesions classically are referred to as coral beads.4,6 Patients commonly report pruritus that may precede the development of the papules and nodules. Other cutaneous manifestations include xanthelasma, nail changes, and a photodistributed erythematous maculopapular eruption that may mimic dermatomyositis.6
Cutaneous findings of MRH can mimic rheumatoid nodules, gout, Gottron papules of dermatomyositis, lipoid proteinosis, sarcoidosis, lepromatous leprosy, granuloma annulare, xanthoma, xanthogranuloma, and fibroxanthoma.6,7 Histopathologic features may distinguish MRH from such entities. Findings include fairly well-circumscribed aggregates of large multinucleated giant cells with characteristic eosinophilic ground-glass cytoplasm. Histiocytes stain positively for CD68, HAM56, CD11b, and CD14, and variably for factor XIIIa. CD68, which is expressed by monocytes/macrophages, has been universally reported to be the most reliable marker of MRH. Negative staining for S-100 and CD1a supports a non-Langerhans origin for the involved histiocytes. If arthritic symptoms predominate, MRH must be distinguished from rheumatoid and psoriatic arthritis.6,7
Mucosal involvement occurs in approximately 50% of patients and includes the presence of nodules in the oral, nasal, and pharyngeal mucosae, as well as eye structures.2,3 Histiocytic infiltration has been documented in the heart, lungs, thyroid, liver, stomach, kidneys, muscle, bone marrow, and urogenital tract. Histiocytes also can invade the cartilage of the ears and nose causing disfigurement and characteristic leonine facies. Pathologic fractures may occur with bone involvement.5
Systemic features associated with MRH include hyperlipidemia, diabetes mellitus, thyroid disease, hypergammaglobulinemia, and various autoimmune diseases. Patients less frequently report fever and weight loss.2,5,6,8 Additionally, a positive tuberculin test occurs in 12% to 50% of patients.6 Various autoimmune diseases occur in 6% to 17% of cases including systemic lupus erythematosus, systemic sclerosis, rheumatoid arthritis, dermatomyositis, Sjögren syndrome, and primary biliary cirrhosis.2,5,6,8 The most clinically salient feature of MRH is its association with malignant conditions, which occur in up to 31% of patients. A variety of cancers have been reported in association with MRH, including breast, cervical, ovarian, stomach, penile, lymphoma, mesothelioma, and melanoma.7
The etiology of MRH is unclear. Although onset may precede the development of a malignant condition and regress with treatment, it cannot be considered a true paraneoplastic disorder, as it has no association with a specific cancer and does not typically parallel the disease course.6,9 Reports of increased levels of inflammatory mediators released from macrophages and endothelial cells, specifically IL-12, IL-1β, IL-6, and tumor necrosis factor α (TNF-α), have been thought to drive the destruction of bone and cartilage.6 In particular, TNF-α acts to indirectly induce destruction by stimulating proteolytic activity in macrophages, similar to the pathogenesis of joint damage in rheumatoid arthritis.8 Osteoclastic activity may play a role in the pathogenesis of MRH, as multinucleated giant cells in MRH can mature into osteoclasts by receptor activated nuclear factor–κB ligand signaling. In addition, patients treated with bisphosphonates have had decreased lacunar resorption.2,8
Initial management of MRH should include screening for hyperlipidemia, hypergammaglobulinemia, hyperglycemia, thyroid dysfunction, and autoimmune diseases, as well as age-appropriate cancer screening. Imaging studies should evaluate for the presence of erosive arthritis. There are no well-defined treatment algorithms for MRH due to the rarity of the disease, and recommendations largely rely on case reports. Although spontaneous remission typically occurs within 5 to 10 years, the risk for joint destruction argues for early pharmacologic intervention. Current management includes the use of nonsteroidal anti-inflammatory drugs and various immunosuppressants including oral glucocorticoids, cyclophosphamide, chlorambucil, methotrexate, or azathioprine.2 A combination of methotrexate with cyclophosphamide or glucocorticoids also has shown efficacy.10 Anti–TNF-α agents, such as etanercept, adalimumab, and infliximab, have been used with some success.2 Tumor necrosis factor α inhibitors used in combination with oral glucocorticoids and methotrexate may have an increased benefit.2,9,11 Evidence suggesting that TNF-α plays a role in the destruction of bone and cartilage led to the successful use of infliximab in combination with oral glucocorticoids and methotrexate, which prevented possible development of antibodies to infliximab and increased its efficacy.12 Bisphosphonate use in combination with glucocorticoids and methotrexate may prevent joint destruction without the serious adverse events associated with anti–TNF-α agents.2,9,13,14
To the Editor:
A 50-year-old woman presented with an asymptomatic eruption on the dorsal aspect of the hands, abdomen, and face of 6 months’ duration. The eruption was associated with generalized arthralgia and fatigue. Within several weeks of onset of the cutaneous eruption, the patient developed swelling in the hands as well as worsening arthralgia. She was treated for presumed Lyme borreliosis but reported no improvement in the symptoms. She was then referred to dermatology for further management.
Physical examination revealed red-orange, edematous, monomorphic papulonodules scattered on the nasolabial folds, upper lip, and along the dorsal aspect of the hands and fingers (Figure 1). A brown rippled plaque was present on the left lower abdomen. The oral mucosa and nails were unremarkable. Laboratory studies showed elevated total cholesterol (244 mg/dL [reference range, <200 mg/dL]), low-density lipoproteins (130 mg/dL [reference range, 10–30 mg/dL]), aspartate aminotransferase (140 U/L [reference range, 10–30 U/L]), alanine aminotransferase (110 U/L [reference range, 10–40 U/L]), and total bilirubin (1.5 mg/dL [reference range, 0.3–1.2 mg/dL]). White blood cell count and C-reactive protein levels were within reference range. An antinuclear antibody titer of 1:80 with a homogenous pattern was found, and aldolase levels were elevated. Laboratory investigations for rheumatoid factor, Lyme disease, tuberculosis, hepatitis, and human immunodeficiency virus were negative. A chest radiograph was normal.
A punch biopsy from the right dorsal hand revealed a dermal proliferation of mononucleated and multinucleated epithelioid histiocytes with ample amounts of eosinophilic ground-glass cytoplasm (Figure 2). Immunohistochemistry revealed epithelioid histiocytes reactive for CD68, CD163, and factor XIIIA, and negative for S-100 and CD1a.
The patient was diagnosed with multicentric reticulohistiocytosis (MRH) and was initially treated with prednisone. Treatment was later augmented with etanercept and methotrexate with improvement in both the skin and joint symptoms.
Multicentric reticulohistiocytosis is a rare, non–Langerhans cell histiocytosis with both cutaneous and systemic features. Although case reports date back to the late 1800s, the term multicentric reticulohistiocytosis was first used in 1954.1 Multicentric reticulohistiocytosis is extremely uncommon and precludes thorough investigation of its etiology and management. The condition typically presents in the fifth to sixth decades of life and occurs more frequently in women with a female to male ratio estimated at 3 to 1.2,3 Pediatric cases have been reported but are exceedingly rare.4
Multicentric reticulohistiocytosis typically presents with a severe erosive arthropathy known as arthritis mutilans. Patients display a symmetric polyarthritis that commonly involves the elbows, wrists, and proximal and distal aspects of the interphalangeal joints. Onset and progression can be rapid, and the erosive nature leads to deformities in up to 45% of patients.2,5,6 Cutaneous findings arise an average of 3 years after the development of arthritis, though one-fifth of patients will initially present with cutaneous findings followed by the development of arthritis at any time.3,6 Clinical features include flesh-colored to reddish brown or yellow papulonodules that range in size from several millimeters to 2 cm. The lesions most commonly occur on the face (eg, ears, nose, paranasal cheeks), scalp, dorsal and lateral aspects of the hands and fingers, and overlying articular regions of the extremities. Characteristic periungual lesions classically are referred to as coral beads.4,6 Patients commonly report pruritus that may precede the development of the papules and nodules. Other cutaneous manifestations include xanthelasma, nail changes, and a photodistributed erythematous maculopapular eruption that may mimic dermatomyositis.6
Cutaneous findings of MRH can mimic rheumatoid nodules, gout, Gottron papules of dermatomyositis, lipoid proteinosis, sarcoidosis, lepromatous leprosy, granuloma annulare, xanthoma, xanthogranuloma, and fibroxanthoma.6,7 Histopathologic features may distinguish MRH from such entities. Findings include fairly well-circumscribed aggregates of large multinucleated giant cells with characteristic eosinophilic ground-glass cytoplasm. Histiocytes stain positively for CD68, HAM56, CD11b, and CD14, and variably for factor XIIIa. CD68, which is expressed by monocytes/macrophages, has been universally reported to be the most reliable marker of MRH. Negative staining for S-100 and CD1a supports a non-Langerhans origin for the involved histiocytes. If arthritic symptoms predominate, MRH must be distinguished from rheumatoid and psoriatic arthritis.6,7
Mucosal involvement occurs in approximately 50% of patients and includes the presence of nodules in the oral, nasal, and pharyngeal mucosae, as well as eye structures.2,3 Histiocytic infiltration has been documented in the heart, lungs, thyroid, liver, stomach, kidneys, muscle, bone marrow, and urogenital tract. Histiocytes also can invade the cartilage of the ears and nose causing disfigurement and characteristic leonine facies. Pathologic fractures may occur with bone involvement.5
Systemic features associated with MRH include hyperlipidemia, diabetes mellitus, thyroid disease, hypergammaglobulinemia, and various autoimmune diseases. Patients less frequently report fever and weight loss.2,5,6,8 Additionally, a positive tuberculin test occurs in 12% to 50% of patients.6 Various autoimmune diseases occur in 6% to 17% of cases including systemic lupus erythematosus, systemic sclerosis, rheumatoid arthritis, dermatomyositis, Sjögren syndrome, and primary biliary cirrhosis.2,5,6,8 The most clinically salient feature of MRH is its association with malignant conditions, which occur in up to 31% of patients. A variety of cancers have been reported in association with MRH, including breast, cervical, ovarian, stomach, penile, lymphoma, mesothelioma, and melanoma.7
The etiology of MRH is unclear. Although onset may precede the development of a malignant condition and regress with treatment, it cannot be considered a true paraneoplastic disorder, as it has no association with a specific cancer and does not typically parallel the disease course.6,9 Reports of increased levels of inflammatory mediators released from macrophages and endothelial cells, specifically IL-12, IL-1β, IL-6, and tumor necrosis factor α (TNF-α), have been thought to drive the destruction of bone and cartilage.6 In particular, TNF-α acts to indirectly induce destruction by stimulating proteolytic activity in macrophages, similar to the pathogenesis of joint damage in rheumatoid arthritis.8 Osteoclastic activity may play a role in the pathogenesis of MRH, as multinucleated giant cells in MRH can mature into osteoclasts by receptor activated nuclear factor–κB ligand signaling. In addition, patients treated with bisphosphonates have had decreased lacunar resorption.2,8
Initial management of MRH should include screening for hyperlipidemia, hypergammaglobulinemia, hyperglycemia, thyroid dysfunction, and autoimmune diseases, as well as age-appropriate cancer screening. Imaging studies should evaluate for the presence of erosive arthritis. There are no well-defined treatment algorithms for MRH due to the rarity of the disease, and recommendations largely rely on case reports. Although spontaneous remission typically occurs within 5 to 10 years, the risk for joint destruction argues for early pharmacologic intervention. Current management includes the use of nonsteroidal anti-inflammatory drugs and various immunosuppressants including oral glucocorticoids, cyclophosphamide, chlorambucil, methotrexate, or azathioprine.2 A combination of methotrexate with cyclophosphamide or glucocorticoids also has shown efficacy.10 Anti–TNF-α agents, such as etanercept, adalimumab, and infliximab, have been used with some success.2 Tumor necrosis factor α inhibitors used in combination with oral glucocorticoids and methotrexate may have an increased benefit.2,9,11 Evidence suggesting that TNF-α plays a role in the destruction of bone and cartilage led to the successful use of infliximab in combination with oral glucocorticoids and methotrexate, which prevented possible development of antibodies to infliximab and increased its efficacy.12 Bisphosphonate use in combination with glucocorticoids and methotrexate may prevent joint destruction without the serious adverse events associated with anti–TNF-α agents.2,9,13,14
- Goltz RW, Laymon CW. Multicentric reticulohistiocytosis of the skin and synovia; reticulohistiocytoma or ganglioneuroma. AMA Arch Derma Syphilol. 1954;69:717-731.
- Islam AD, Naguwa SM, Cheema GS, et al. Multicentric reticulohistiocytosis: a rare yet challenging disease. Clin Rev Allergy Immunol. 2013;45:281-289.
- West KL, Sporn T, Puri PK. Multicentric reticulohistiocytosis: a unique case with pulmonary fibrosis. Arch Dermatol. 2012;148:228-232.
- Outland JD, Keiran SJ, Schikler KN, et al. Multicentric reticulohistiocytosis in a 14-year-old girl. Pediatr Dermatol. 2002;19:527-531.
- Gorman JD, Danning C, Schumacher HR, et al. Multicentric reticulohistiocytosis: case report with immunohistochemical analysis and literature review. Arthritis Rheum. 2000;43:930-938.
- Tajirian AL, Malik MK, Robinson-Bostom L, et al. Multicentric reticulohistiocytosis. Clin Dermatol. 2006;24:486-492.
- Luz FB, Gaspar TAP, Kalil-Gaspar N, et al. Multicentric reticulohistiocytosis. J Eur Acad Dermatol Venereol. 2001;15:524-531.
- Trotta F, Castellino G, Lo Monaco A. Multicentric reticulohistiocytosis. Best Pract Res Clin Rheumatol. 2004;18:759-772.
- Kalajian AH, Callen JP. Multicentric reticulohistiocytosis successfully treated with infliximab: an illustrative case and evaluation of cytokine expression supporting anti-tumor necrosis factor therapy. Arch Derm. 2008;144:1360-1366.
- Liang GC, Granston AS. Complete remission of multicentric reticulohistiocytosis with combination therapy of steroid, cyclophosphamide, and low-dose pulse methotrexate. case report, review of the literature, and proposal for treatment. Arthritis Rheum. 1996;39:171-174.
- Lovelace K, Loyd A, Adelson D, et al. Etanercept and the treatment of multicentric reticulohistiocytosis. Arch Dermatol. 2005;141:1167-1168.
- Lee MW, Lee EY, Jeong YI, et al. Successful treatment of multicentric reticulohistiocytosis with a combination of infliximab, prednisolone and methotrexate. Acta Derm Venereol. 2004;84:478-479.
- Adamopoulos IE, Wordsworth PB, Edwards JR, et al. Osteoclast differentiation and bone resorption in multicentric reticulohistiocytosis. Hum Pathol. 2006;37:1176-1185.
- Satoh M, Oyama N, Yamada H, et al. Treatment trial of multicentric reticulohistiocytosis with a combination of predonisolone, methotrexate and alendronate. J Dermatol. 2008;35:168-171.
- Goltz RW, Laymon CW. Multicentric reticulohistiocytosis of the skin and synovia; reticulohistiocytoma or ganglioneuroma. AMA Arch Derma Syphilol. 1954;69:717-731.
- Islam AD, Naguwa SM, Cheema GS, et al. Multicentric reticulohistiocytosis: a rare yet challenging disease. Clin Rev Allergy Immunol. 2013;45:281-289.
- West KL, Sporn T, Puri PK. Multicentric reticulohistiocytosis: a unique case with pulmonary fibrosis. Arch Dermatol. 2012;148:228-232.
- Outland JD, Keiran SJ, Schikler KN, et al. Multicentric reticulohistiocytosis in a 14-year-old girl. Pediatr Dermatol. 2002;19:527-531.
- Gorman JD, Danning C, Schumacher HR, et al. Multicentric reticulohistiocytosis: case report with immunohistochemical analysis and literature review. Arthritis Rheum. 2000;43:930-938.
- Tajirian AL, Malik MK, Robinson-Bostom L, et al. Multicentric reticulohistiocytosis. Clin Dermatol. 2006;24:486-492.
- Luz FB, Gaspar TAP, Kalil-Gaspar N, et al. Multicentric reticulohistiocytosis. J Eur Acad Dermatol Venereol. 2001;15:524-531.
- Trotta F, Castellino G, Lo Monaco A. Multicentric reticulohistiocytosis. Best Pract Res Clin Rheumatol. 2004;18:759-772.
- Kalajian AH, Callen JP. Multicentric reticulohistiocytosis successfully treated with infliximab: an illustrative case and evaluation of cytokine expression supporting anti-tumor necrosis factor therapy. Arch Derm. 2008;144:1360-1366.
- Liang GC, Granston AS. Complete remission of multicentric reticulohistiocytosis with combination therapy of steroid, cyclophosphamide, and low-dose pulse methotrexate. case report, review of the literature, and proposal for treatment. Arthritis Rheum. 1996;39:171-174.
- Lovelace K, Loyd A, Adelson D, et al. Etanercept and the treatment of multicentric reticulohistiocytosis. Arch Dermatol. 2005;141:1167-1168.
- Lee MW, Lee EY, Jeong YI, et al. Successful treatment of multicentric reticulohistiocytosis with a combination of infliximab, prednisolone and methotrexate. Acta Derm Venereol. 2004;84:478-479.
- Adamopoulos IE, Wordsworth PB, Edwards JR, et al. Osteoclast differentiation and bone resorption in multicentric reticulohistiocytosis. Hum Pathol. 2006;37:1176-1185.
- Satoh M, Oyama N, Yamada H, et al. Treatment trial of multicentric reticulohistiocytosis with a combination of predonisolone, methotrexate and alendronate. J Dermatol. 2008;35:168-171.
Practice Points
- Multicentric reticulohistiocytosis (MRH) is an important entity to recognize given its association with underlying malignancy and irreversible destructive arthritis.
- Diagnosis of MRH warrants extensive review of systems, age-appropriate cancer screening, and relevant systemic workup.
- Early pharmacologic intervention should be initiated with nonsteroidal anti-inflammatory agents or immunosuppressant agents.
2019 Ovarian Cancer Roundtable
According to the American Cancer Society, about 22,530 women will receive a new diagnosis of ovarian cancer this year. In this video series, Drs. Mark Einstein, Jenna Marcus, and Stuart Lichtman discuss new therapeutics, surgical innovations, and treatment options for primary and advanced/recurrent ovarian cancer.
This video roundtable was produced by the Custom Programs division. The faculty received modest honoraria from Custom Programs for participating in this roundtable.
The faculty was solely responsible for the content presented.
Disclosures
Dr. Einstein discloses that he has participated in educational speaking activties for Altum Pharma, Cynvec, Papivax, PDS Biotechnologies, and Photocure. He also was the overall or local primary investigator for clinical trials for AstraZeneca, Becton-Dickinson, Inovio, Johnson & Johnson, Pfizer, and PDS Biotechnologies.
Dr. Marcus has no conflicts to disclose.
Dr. Lichtman has no conflicts to disclose.
According to the American Cancer Society, about 22,530 women will receive a new diagnosis of ovarian cancer this year. In this video series, Drs. Mark Einstein, Jenna Marcus, and Stuart Lichtman discuss new therapeutics, surgical innovations, and treatment options for primary and advanced/recurrent ovarian cancer.
This video roundtable was produced by the Custom Programs division. The faculty received modest honoraria from Custom Programs for participating in this roundtable.
The faculty was solely responsible for the content presented.
Disclosures
Dr. Einstein discloses that he has participated in educational speaking activties for Altum Pharma, Cynvec, Papivax, PDS Biotechnologies, and Photocure. He also was the overall or local primary investigator for clinical trials for AstraZeneca, Becton-Dickinson, Inovio, Johnson & Johnson, Pfizer, and PDS Biotechnologies.
Dr. Marcus has no conflicts to disclose.
Dr. Lichtman has no conflicts to disclose.
According to the American Cancer Society, about 22,530 women will receive a new diagnosis of ovarian cancer this year. In this video series, Drs. Mark Einstein, Jenna Marcus, and Stuart Lichtman discuss new therapeutics, surgical innovations, and treatment options for primary and advanced/recurrent ovarian cancer.
This video roundtable was produced by the Custom Programs division. The faculty received modest honoraria from Custom Programs for participating in this roundtable.
The faculty was solely responsible for the content presented.
Disclosures
Dr. Einstein discloses that he has participated in educational speaking activties for Altum Pharma, Cynvec, Papivax, PDS Biotechnologies, and Photocure. He also was the overall or local primary investigator for clinical trials for AstraZeneca, Becton-Dickinson, Inovio, Johnson & Johnson, Pfizer, and PDS Biotechnologies.
Dr. Marcus has no conflicts to disclose.
Dr. Lichtman has no conflicts to disclose.
Atrial fib guidelines updated, SPRINT MIND published, and more
This week in cardiology news, revised atrial fibrillation guidelines revamp anticoagulation, the SPRINT MIND results showing that tight BP control staves off mild cognitive impairment are published, the FDA discovers that nitrosamine-contaminated ARBs have been on the market for years, and subclinical hypothyroidism boosts the immediate risk of heart failure.
Amazon Alexa
Apple Podcasts
Google Podcasts
TuneIn
This week in cardiology news, revised atrial fibrillation guidelines revamp anticoagulation, the SPRINT MIND results showing that tight BP control staves off mild cognitive impairment are published, the FDA discovers that nitrosamine-contaminated ARBs have been on the market for years, and subclinical hypothyroidism boosts the immediate risk of heart failure.
Amazon Alexa
Apple Podcasts
Google Podcasts
TuneIn
This week in cardiology news, revised atrial fibrillation guidelines revamp anticoagulation, the SPRINT MIND results showing that tight BP control staves off mild cognitive impairment are published, the FDA discovers that nitrosamine-contaminated ARBs have been on the market for years, and subclinical hypothyroidism boosts the immediate risk of heart failure.
Amazon Alexa
Apple Podcasts
Google Podcasts
TuneIn
GALLIUM: MRD response correlates with outcomes in follicular lymphoma
SAN DIEGO – Minimal residual disease (MRD) response at the end of induction correlates with outcomes in previously untreated follicular lymphoma patients who receive obinutuzumab- or rituximab-based immunochemotherapy, according to updated results from the phase 3 GALLIUM study.
After 57 months of follow-up, and regardless of treatment arm, 564 MRD-evaluable patients who were MRD negative at the end of induction had significantly greater probability of progression-free survival (PFS) than did 70 patients who were MRD positive at the end of induction (about 80% vs. 50%; hazard ratio, 0.38), Christiane Pott, MD, reported at the annual meeting of the American Society of Hematology.
GALLIUM participants were adults with follicular lymphoma requiring treatment. They were randomized to receive standard chemotherapy in combination with 6-8 cycles of either intravenous obinutuzumab at a dose of 1,000 mg on days 1, 8, and 15 of cycle 1 and on day 1 of the remaining cycles or intravenous rituximab at a dose of 375mg/m2 on day 1 of each cycle. Responders in each group received their assigned antibody as maintenance every 2 months for up to 2 years, said Dr. Pott, of University Hospital of Schleswig‐Holstein, Kiel, Germany.
Of 324 MRD-evaluable patients in the obinutuzumab arm who continued on maintenance treatment, 300 (92.6%) were MRD-negative at the end of induction, compared with 264 of 310 (85.2%) in the rituximab arm.
The majority of the MRD-negative patients remained negative during maintenance, including 67% of patients receiving obinutuzumab and 63.2% of patient receiving rituximab, she said. There was no difference seen in the relapse rate between groups – 6.3% vs. 6.1%, respectively.
The rate of disease progression or death was 11.4% in the obinutuzumab arm and 15.5% in the rituximab arm.
Additionally, 24 patients in the obinutuzumab arm and 46 in the rituximab arm were MRD positive at the end of induction but were eligible for maintenance therapy based on clinical response; of these, 22 (92%) and 36 (78%), respectively, achieved MRD negativity during maintenance, with 18 and 27 patients in the arms, respectively, achieving MRD negativity within the first 4 months of maintenance therapy, she said.
Of the 12 patients who never achieved an MRD response, 8 progressed or died within 7 months after the end of induction, 1 progressed after 15 months, 1 progressed after 26 months, and 2 remained MRD positive during maintenance up to month 8 and month 12, respectively, but had no documented tumor progression until day 1,348 and day 1,709.
“MRD status reflects the depth of response to treatment and provides insight regarding prognosis after first-line therapy in patients with follicular lymphoma,” Dr. Pott said in an interview, adding that “the findings of the current analysis demonstrate the prognostic value of MRD response assessments in previously untreated follicular lymphoma patients receiving immunochemotherapy.”
Further, the finding that a majority of patients who were MRD positive at the end of induction achieved MRD negativity during the first 4 months of maintenance is likely indicative of the efficacy of continued treatment, and it also suggests that response kinetics can be slower than in patients with an early MRD response at midinduction, she said.
“Also, responses that are beyond the sensitivity of the MRD assay may be less deep,” she added, noting that patients who failed to achieve MRD negativity at the end of induction or during early maintenance had a high chance of experiencing early progression or death.
The findings have implications for individualized treatment based on patient response, as well as for future clinical trial design, she said.
For example, MRD status could allow for earlier identification of patients with poor prognosis who aren’t likely to benefit from maintenance therapy. In clinical trials, it could be used to assess the efficiency of new treatments and to stratify patients based on the likelihood of response, allowing for the evaluation of different treatments in those groups, she explained.
“That would be a very important step in the direction of tailored therapies,” she said, adding that patients with follicular lymphoma tend to have very long PFS, and earlier outcomes parameters or tools beyond clinical parameters for assessing treatment efficiency are needed.
“I hope that future trials will address MRD-based treatment stratification as the adverse prognosis we detect by residual disease might be overcome by an MRD-based switch of patients to more effective and efficient treatments, including novel drugs,” she said.
The GALLIUM study is supported by F. Hoffmann–La Roche. Dr. Pott reported having no financial disclosures.
SOURCE: Pott C et al. ASH 2018, Abstract 396.
SAN DIEGO – Minimal residual disease (MRD) response at the end of induction correlates with outcomes in previously untreated follicular lymphoma patients who receive obinutuzumab- or rituximab-based immunochemotherapy, according to updated results from the phase 3 GALLIUM study.
After 57 months of follow-up, and regardless of treatment arm, 564 MRD-evaluable patients who were MRD negative at the end of induction had significantly greater probability of progression-free survival (PFS) than did 70 patients who were MRD positive at the end of induction (about 80% vs. 50%; hazard ratio, 0.38), Christiane Pott, MD, reported at the annual meeting of the American Society of Hematology.
GALLIUM participants were adults with follicular lymphoma requiring treatment. They were randomized to receive standard chemotherapy in combination with 6-8 cycles of either intravenous obinutuzumab at a dose of 1,000 mg on days 1, 8, and 15 of cycle 1 and on day 1 of the remaining cycles or intravenous rituximab at a dose of 375mg/m2 on day 1 of each cycle. Responders in each group received their assigned antibody as maintenance every 2 months for up to 2 years, said Dr. Pott, of University Hospital of Schleswig‐Holstein, Kiel, Germany.
Of 324 MRD-evaluable patients in the obinutuzumab arm who continued on maintenance treatment, 300 (92.6%) were MRD-negative at the end of induction, compared with 264 of 310 (85.2%) in the rituximab arm.
The majority of the MRD-negative patients remained negative during maintenance, including 67% of patients receiving obinutuzumab and 63.2% of patient receiving rituximab, she said. There was no difference seen in the relapse rate between groups – 6.3% vs. 6.1%, respectively.
The rate of disease progression or death was 11.4% in the obinutuzumab arm and 15.5% in the rituximab arm.
Additionally, 24 patients in the obinutuzumab arm and 46 in the rituximab arm were MRD positive at the end of induction but were eligible for maintenance therapy based on clinical response; of these, 22 (92%) and 36 (78%), respectively, achieved MRD negativity during maintenance, with 18 and 27 patients in the arms, respectively, achieving MRD negativity within the first 4 months of maintenance therapy, she said.
Of the 12 patients who never achieved an MRD response, 8 progressed or died within 7 months after the end of induction, 1 progressed after 15 months, 1 progressed after 26 months, and 2 remained MRD positive during maintenance up to month 8 and month 12, respectively, but had no documented tumor progression until day 1,348 and day 1,709.
“MRD status reflects the depth of response to treatment and provides insight regarding prognosis after first-line therapy in patients with follicular lymphoma,” Dr. Pott said in an interview, adding that “the findings of the current analysis demonstrate the prognostic value of MRD response assessments in previously untreated follicular lymphoma patients receiving immunochemotherapy.”
Further, the finding that a majority of patients who were MRD positive at the end of induction achieved MRD negativity during the first 4 months of maintenance is likely indicative of the efficacy of continued treatment, and it also suggests that response kinetics can be slower than in patients with an early MRD response at midinduction, she said.
“Also, responses that are beyond the sensitivity of the MRD assay may be less deep,” she added, noting that patients who failed to achieve MRD negativity at the end of induction or during early maintenance had a high chance of experiencing early progression or death.
The findings have implications for individualized treatment based on patient response, as well as for future clinical trial design, she said.
For example, MRD status could allow for earlier identification of patients with poor prognosis who aren’t likely to benefit from maintenance therapy. In clinical trials, it could be used to assess the efficiency of new treatments and to stratify patients based on the likelihood of response, allowing for the evaluation of different treatments in those groups, she explained.
“That would be a very important step in the direction of tailored therapies,” she said, adding that patients with follicular lymphoma tend to have very long PFS, and earlier outcomes parameters or tools beyond clinical parameters for assessing treatment efficiency are needed.
“I hope that future trials will address MRD-based treatment stratification as the adverse prognosis we detect by residual disease might be overcome by an MRD-based switch of patients to more effective and efficient treatments, including novel drugs,” she said.
The GALLIUM study is supported by F. Hoffmann–La Roche. Dr. Pott reported having no financial disclosures.
SOURCE: Pott C et al. ASH 2018, Abstract 396.
SAN DIEGO – Minimal residual disease (MRD) response at the end of induction correlates with outcomes in previously untreated follicular lymphoma patients who receive obinutuzumab- or rituximab-based immunochemotherapy, according to updated results from the phase 3 GALLIUM study.
After 57 months of follow-up, and regardless of treatment arm, 564 MRD-evaluable patients who were MRD negative at the end of induction had significantly greater probability of progression-free survival (PFS) than did 70 patients who were MRD positive at the end of induction (about 80% vs. 50%; hazard ratio, 0.38), Christiane Pott, MD, reported at the annual meeting of the American Society of Hematology.
GALLIUM participants were adults with follicular lymphoma requiring treatment. They were randomized to receive standard chemotherapy in combination with 6-8 cycles of either intravenous obinutuzumab at a dose of 1,000 mg on days 1, 8, and 15 of cycle 1 and on day 1 of the remaining cycles or intravenous rituximab at a dose of 375mg/m2 on day 1 of each cycle. Responders in each group received their assigned antibody as maintenance every 2 months for up to 2 years, said Dr. Pott, of University Hospital of Schleswig‐Holstein, Kiel, Germany.
Of 324 MRD-evaluable patients in the obinutuzumab arm who continued on maintenance treatment, 300 (92.6%) were MRD-negative at the end of induction, compared with 264 of 310 (85.2%) in the rituximab arm.
The majority of the MRD-negative patients remained negative during maintenance, including 67% of patients receiving obinutuzumab and 63.2% of patient receiving rituximab, she said. There was no difference seen in the relapse rate between groups – 6.3% vs. 6.1%, respectively.
The rate of disease progression or death was 11.4% in the obinutuzumab arm and 15.5% in the rituximab arm.
Additionally, 24 patients in the obinutuzumab arm and 46 in the rituximab arm were MRD positive at the end of induction but were eligible for maintenance therapy based on clinical response; of these, 22 (92%) and 36 (78%), respectively, achieved MRD negativity during maintenance, with 18 and 27 patients in the arms, respectively, achieving MRD negativity within the first 4 months of maintenance therapy, she said.
Of the 12 patients who never achieved an MRD response, 8 progressed or died within 7 months after the end of induction, 1 progressed after 15 months, 1 progressed after 26 months, and 2 remained MRD positive during maintenance up to month 8 and month 12, respectively, but had no documented tumor progression until day 1,348 and day 1,709.
“MRD status reflects the depth of response to treatment and provides insight regarding prognosis after first-line therapy in patients with follicular lymphoma,” Dr. Pott said in an interview, adding that “the findings of the current analysis demonstrate the prognostic value of MRD response assessments in previously untreated follicular lymphoma patients receiving immunochemotherapy.”
Further, the finding that a majority of patients who were MRD positive at the end of induction achieved MRD negativity during the first 4 months of maintenance is likely indicative of the efficacy of continued treatment, and it also suggests that response kinetics can be slower than in patients with an early MRD response at midinduction, she said.
“Also, responses that are beyond the sensitivity of the MRD assay may be less deep,” she added, noting that patients who failed to achieve MRD negativity at the end of induction or during early maintenance had a high chance of experiencing early progression or death.
The findings have implications for individualized treatment based on patient response, as well as for future clinical trial design, she said.
For example, MRD status could allow for earlier identification of patients with poor prognosis who aren’t likely to benefit from maintenance therapy. In clinical trials, it could be used to assess the efficiency of new treatments and to stratify patients based on the likelihood of response, allowing for the evaluation of different treatments in those groups, she explained.
“That would be a very important step in the direction of tailored therapies,” she said, adding that patients with follicular lymphoma tend to have very long PFS, and earlier outcomes parameters or tools beyond clinical parameters for assessing treatment efficiency are needed.
“I hope that future trials will address MRD-based treatment stratification as the adverse prognosis we detect by residual disease might be overcome by an MRD-based switch of patients to more effective and efficient treatments, including novel drugs,” she said.
The GALLIUM study is supported by F. Hoffmann–La Roche. Dr. Pott reported having no financial disclosures.
SOURCE: Pott C et al. ASH 2018, Abstract 396.
REPORTING FROM ASH 2018
Key clinical point:
Major finding: Progression-free survival (PFS) probability was about 80% in patients who were MRD negative at the end of induction, compared with about 50% in patients who were MRD positive (hazard ratio, 0.38).
Study details: An analysis of data from 634 patients in the phase 3 GALLIUM study.
Disclosures: The GALLIUM study is supported by F. Hoffmann–La Roche. Dr. Pott reported having no financial disclosures.
Source: Pott C et al. ASH 2018, Abstract 396.
Risk models fail to predict lower-GI bleeding outcomes
In cases of lower gastrointestinal bleeding (LGIB), albumin and hemoglobin levels are the best independent predictors of severe bleeding, according to investigators.
These findings came from a sobering look at LGIB risk-prediction models. While some models could predict specific outcomes with reasonable accuracy, none of the models demonstrated broad predictive power, reported Natalie Tapaskar, MD, of the department of medicine at the University of Chicago, and her colleagues.
LGIB requires intensive resource utilization and proves fatal in 5%-15% of patients, which means timely and appropriate interventions are essential, especially for those with severe bleeding.
“There are limited data on accurately predicting the risk of adverse outcomes for hospitalized patients with LGIB,” the investigators wrote in Gastrointestinal Endoscopy, “especially in comparison to patients with upper gastrointestinal bleeding (UGIB), where tools such as the Glasgow-Blatchford Bleeding Score have been validated to accurately predict important clinical outcomes.”
To assess existing risk models for LGIB, the investigators performed a prospective observational study involving 170 patients with LGIB who underwent colonoscopy during April 2016–September 2017 at the University of Chicago Medical Center. Data were collected through comprehensive medical record review.
The primary outcome was severe bleeding. This was defined by acute bleeding during the first 24 hours of admission that required a transfusion of 2 or more units of packed red blood cells, and/or caused a 20% or greater decrease in hematocrit; and/or recurrent bleeding 24 hours after clinical stability, involving rectal bleeding with an additional drop in hematocrit of 20% or more, and/or readmission for LGIB within 1 week of discharge. Secondary outcomes included blood transfusion requirements, in-hospital recurrent bleeding, length of stay, ICU admission, intervention (surgery, interventional radiology, endoscopy), and the comparative predictive ability of seven clinical risk stratification models: AIMS65, Charlson Comorbidity Index, Glasgow-Blatchford, NOBLADS, Oakland, Sengupta, and Strate. Area under the receiver operating characteristic curve (AUC) was used to compare model predictive power. Risk of adverse outcomes was calculated by univariable and multivariable logistic regression.
Results showed that median patient age was 70 years. Most of the patients (80%) were African American and slightly more than half were female (58%). These demographic factors were not predictive of severe bleeding, which occurred in about half of the cases (52%). Upon admission, patients with severe bleeding were more likely to have chronic renal failure (30% vs. 17%; P = .05), lower albumin (3.6 g/dL vs. 3.95 g/dL; P less than .0001), lower hemoglobin (8.6 g/dL vs. 11.1 g/dL; P = .0001), lower systolic blood pressure (118 mm Hg vs. 132 mm Hg; P = .01), and higher creatinine (1.3 mg/dL vs. 1 mg/dL; P = .04). After adjustment for confounding variables, the strongest independent predictors of severe bleeding were low albumin (odds ratio, 2.56 per 1-g/dL decrease; P = .02) and low hemoglobin (OR, 1.28 per 1-g/dL decrease; P = .0015).
On average, time between admission and colonoscopy was between 2 and 3 days (median, 62.2 hours). In 3 out of 4 patients (77%), etiology of LGIB was confirmed; diverticular bleeding was most common (39%), followed distantly by hemorrhoidal bleeding (15%).
Compared with milder cases, patients with severe bleeding were more likely to stay in the ICU (49% vs. 19%; P less than .0001), have a blood transfusion (85% vs 36%; P less than .0001), and need to remain in the hospital for a longer period of time (6 days vs. 4 days; P = .0009). These findings exemplify the high level of resource utilization required for LGIB and show how severe bleeding dramatically compounds intensity of care.
Further analysis showed that none of the seven risk models were predictive across all outcomes; however, some predicted specific outcomes better than others. Leaders were the Glasgow-Blatchford score for blood transfusion (AUC 0.87; P less than .0001), the Oakland score for severe bleeding (AUC 0.74; P less than .0001), the Sengupta score for ICU stay (AUC 0.74; P less than .0001), and the Strate score for both recurrent bleeding during hospital stay (AUC 0.66; P = .0008) and endoscopic intervention (AUC 0.62; P = .01).
The investigators noted that the Glasgow-Blatchford score, which also is used in cases of UGIB, has previously demonstrated accuracy in predicting blood transfusion, as it did in the present study, suggesting that, “[i]n instances where there may be uncertainty of the origin of the bleeding, the Blatchford score may be a preferential choice of risk score.”
“Overall, we found that no singular score performed best across all the outcomes studied nor did any score have an extremely strong discriminatory power for any individual variable,” the investigators wrote, concluding that “... simpler and more powerful prediction tools are required for better risk stratification in LGIB.”
The investigators reported no financial support or conflicts of interest.
*This story was updated on Jan. 31, 2019.
SOURCE: Tapaskar N et al. Gastrointest Endosc. 2018 Dec 18. doi: 10.1016/j.gie.2018.12.011.
In cases of lower gastrointestinal bleeding (LGIB), albumin and hemoglobin levels are the best independent predictors of severe bleeding, according to investigators.
These findings came from a sobering look at LGIB risk-prediction models. While some models could predict specific outcomes with reasonable accuracy, none of the models demonstrated broad predictive power, reported Natalie Tapaskar, MD, of the department of medicine at the University of Chicago, and her colleagues.
LGIB requires intensive resource utilization and proves fatal in 5%-15% of patients, which means timely and appropriate interventions are essential, especially for those with severe bleeding.
“There are limited data on accurately predicting the risk of adverse outcomes for hospitalized patients with LGIB,” the investigators wrote in Gastrointestinal Endoscopy, “especially in comparison to patients with upper gastrointestinal bleeding (UGIB), where tools such as the Glasgow-Blatchford Bleeding Score have been validated to accurately predict important clinical outcomes.”
To assess existing risk models for LGIB, the investigators performed a prospective observational study involving 170 patients with LGIB who underwent colonoscopy during April 2016–September 2017 at the University of Chicago Medical Center. Data were collected through comprehensive medical record review.
The primary outcome was severe bleeding. This was defined by acute bleeding during the first 24 hours of admission that required a transfusion of 2 or more units of packed red blood cells, and/or caused a 20% or greater decrease in hematocrit; and/or recurrent bleeding 24 hours after clinical stability, involving rectal bleeding with an additional drop in hematocrit of 20% or more, and/or readmission for LGIB within 1 week of discharge. Secondary outcomes included blood transfusion requirements, in-hospital recurrent bleeding, length of stay, ICU admission, intervention (surgery, interventional radiology, endoscopy), and the comparative predictive ability of seven clinical risk stratification models: AIMS65, Charlson Comorbidity Index, Glasgow-Blatchford, NOBLADS, Oakland, Sengupta, and Strate. Area under the receiver operating characteristic curve (AUC) was used to compare model predictive power. Risk of adverse outcomes was calculated by univariable and multivariable logistic regression.
Results showed that median patient age was 70 years. Most of the patients (80%) were African American and slightly more than half were female (58%). These demographic factors were not predictive of severe bleeding, which occurred in about half of the cases (52%). Upon admission, patients with severe bleeding were more likely to have chronic renal failure (30% vs. 17%; P = .05), lower albumin (3.6 g/dL vs. 3.95 g/dL; P less than .0001), lower hemoglobin (8.6 g/dL vs. 11.1 g/dL; P = .0001), lower systolic blood pressure (118 mm Hg vs. 132 mm Hg; P = .01), and higher creatinine (1.3 mg/dL vs. 1 mg/dL; P = .04). After adjustment for confounding variables, the strongest independent predictors of severe bleeding were low albumin (odds ratio, 2.56 per 1-g/dL decrease; P = .02) and low hemoglobin (OR, 1.28 per 1-g/dL decrease; P = .0015).
On average, time between admission and colonoscopy was between 2 and 3 days (median, 62.2 hours). In 3 out of 4 patients (77%), etiology of LGIB was confirmed; diverticular bleeding was most common (39%), followed distantly by hemorrhoidal bleeding (15%).
Compared with milder cases, patients with severe bleeding were more likely to stay in the ICU (49% vs. 19%; P less than .0001), have a blood transfusion (85% vs 36%; P less than .0001), and need to remain in the hospital for a longer period of time (6 days vs. 4 days; P = .0009). These findings exemplify the high level of resource utilization required for LGIB and show how severe bleeding dramatically compounds intensity of care.
Further analysis showed that none of the seven risk models were predictive across all outcomes; however, some predicted specific outcomes better than others. Leaders were the Glasgow-Blatchford score for blood transfusion (AUC 0.87; P less than .0001), the Oakland score for severe bleeding (AUC 0.74; P less than .0001), the Sengupta score for ICU stay (AUC 0.74; P less than .0001), and the Strate score for both recurrent bleeding during hospital stay (AUC 0.66; P = .0008) and endoscopic intervention (AUC 0.62; P = .01).
The investigators noted that the Glasgow-Blatchford score, which also is used in cases of UGIB, has previously demonstrated accuracy in predicting blood transfusion, as it did in the present study, suggesting that, “[i]n instances where there may be uncertainty of the origin of the bleeding, the Blatchford score may be a preferential choice of risk score.”
“Overall, we found that no singular score performed best across all the outcomes studied nor did any score have an extremely strong discriminatory power for any individual variable,” the investigators wrote, concluding that “... simpler and more powerful prediction tools are required for better risk stratification in LGIB.”
The investigators reported no financial support or conflicts of interest.
*This story was updated on Jan. 31, 2019.
SOURCE: Tapaskar N et al. Gastrointest Endosc. 2018 Dec 18. doi: 10.1016/j.gie.2018.12.011.
In cases of lower gastrointestinal bleeding (LGIB), albumin and hemoglobin levels are the best independent predictors of severe bleeding, according to investigators.
These findings came from a sobering look at LGIB risk-prediction models. While some models could predict specific outcomes with reasonable accuracy, none of the models demonstrated broad predictive power, reported Natalie Tapaskar, MD, of the department of medicine at the University of Chicago, and her colleagues.
LGIB requires intensive resource utilization and proves fatal in 5%-15% of patients, which means timely and appropriate interventions are essential, especially for those with severe bleeding.
“There are limited data on accurately predicting the risk of adverse outcomes for hospitalized patients with LGIB,” the investigators wrote in Gastrointestinal Endoscopy, “especially in comparison to patients with upper gastrointestinal bleeding (UGIB), where tools such as the Glasgow-Blatchford Bleeding Score have been validated to accurately predict important clinical outcomes.”
To assess existing risk models for LGIB, the investigators performed a prospective observational study involving 170 patients with LGIB who underwent colonoscopy during April 2016–September 2017 at the University of Chicago Medical Center. Data were collected through comprehensive medical record review.
The primary outcome was severe bleeding. This was defined by acute bleeding during the first 24 hours of admission that required a transfusion of 2 or more units of packed red blood cells, and/or caused a 20% or greater decrease in hematocrit; and/or recurrent bleeding 24 hours after clinical stability, involving rectal bleeding with an additional drop in hematocrit of 20% or more, and/or readmission for LGIB within 1 week of discharge. Secondary outcomes included blood transfusion requirements, in-hospital recurrent bleeding, length of stay, ICU admission, intervention (surgery, interventional radiology, endoscopy), and the comparative predictive ability of seven clinical risk stratification models: AIMS65, Charlson Comorbidity Index, Glasgow-Blatchford, NOBLADS, Oakland, Sengupta, and Strate. Area under the receiver operating characteristic curve (AUC) was used to compare model predictive power. Risk of adverse outcomes was calculated by univariable and multivariable logistic regression.
Results showed that median patient age was 70 years. Most of the patients (80%) were African American and slightly more than half were female (58%). These demographic factors were not predictive of severe bleeding, which occurred in about half of the cases (52%). Upon admission, patients with severe bleeding were more likely to have chronic renal failure (30% vs. 17%; P = .05), lower albumin (3.6 g/dL vs. 3.95 g/dL; P less than .0001), lower hemoglobin (8.6 g/dL vs. 11.1 g/dL; P = .0001), lower systolic blood pressure (118 mm Hg vs. 132 mm Hg; P = .01), and higher creatinine (1.3 mg/dL vs. 1 mg/dL; P = .04). After adjustment for confounding variables, the strongest independent predictors of severe bleeding were low albumin (odds ratio, 2.56 per 1-g/dL decrease; P = .02) and low hemoglobin (OR, 1.28 per 1-g/dL decrease; P = .0015).
On average, time between admission and colonoscopy was between 2 and 3 days (median, 62.2 hours). In 3 out of 4 patients (77%), etiology of LGIB was confirmed; diverticular bleeding was most common (39%), followed distantly by hemorrhoidal bleeding (15%).
Compared with milder cases, patients with severe bleeding were more likely to stay in the ICU (49% vs. 19%; P less than .0001), have a blood transfusion (85% vs 36%; P less than .0001), and need to remain in the hospital for a longer period of time (6 days vs. 4 days; P = .0009). These findings exemplify the high level of resource utilization required for LGIB and show how severe bleeding dramatically compounds intensity of care.
Further analysis showed that none of the seven risk models were predictive across all outcomes; however, some predicted specific outcomes better than others. Leaders were the Glasgow-Blatchford score for blood transfusion (AUC 0.87; P less than .0001), the Oakland score for severe bleeding (AUC 0.74; P less than .0001), the Sengupta score for ICU stay (AUC 0.74; P less than .0001), and the Strate score for both recurrent bleeding during hospital stay (AUC 0.66; P = .0008) and endoscopic intervention (AUC 0.62; P = .01).
The investigators noted that the Glasgow-Blatchford score, which also is used in cases of UGIB, has previously demonstrated accuracy in predicting blood transfusion, as it did in the present study, suggesting that, “[i]n instances where there may be uncertainty of the origin of the bleeding, the Blatchford score may be a preferential choice of risk score.”
“Overall, we found that no singular score performed best across all the outcomes studied nor did any score have an extremely strong discriminatory power for any individual variable,” the investigators wrote, concluding that “... simpler and more powerful prediction tools are required for better risk stratification in LGIB.”
The investigators reported no financial support or conflicts of interest.
*This story was updated on Jan. 31, 2019.
SOURCE: Tapaskar N et al. Gastrointest Endosc. 2018 Dec 18. doi: 10.1016/j.gie.2018.12.011.
FROM GASTROINTESTINAL ENDOSCOPY
Key clinical point: In cases of lower gastrointestinal bleeding (LGIB), albumin and hemoglobin levels are the best independent predictors of severe bleeding.
Major finding: After adjustment for confounding variables, low albumin upon admission was the strongest independent predictor of severe bleeding (OR, 2.56 per 1 g/dL decrease; P = .02).
Study details: A prospective, observational study of 170 patients with LGIB who underwent colonoscopy during April 2016–September 2017 at the University of Chicago Medical Center.
Disclosures: The investigators reported no financial support or conflicts of interest.
Source: Tapaskar N et al. Gastrointest Endosc. 2018 Dec 18. doi: 10.1016/j.gie.2018.12.011.
Clinical benefits persist 5 years after thymectomy for myasthenia gravis
Thymectomy may continue to benefit patients with myasthenia gravis 5 years after the procedure, according to an extension study published in Lancet Neurology.
The study evaluated the clinical status, medication requirements, and adverse events of patients with myasthenia gravis who completed a randomized controlled trial of thymectomy plus prednisone versus prednisone alone and agreed to participate in a rater-blinded 2-year extension.
“Thymectomy within the first few years of the disease course in addition to prednisone therapy confers benefits that persist for 5 years ... in patients with generalized nonthymomatous myasthenia gravis,” said lead study author Gil I. Wolfe, MD, chair of the department of neurology at the University at Buffalo in New York, and his research colleagues. “Results from the extension study provide further support for the use of thymectomy in management of myasthenia gravis and should encourage serious consideration of this treatment option in discussions between clinicians and their patients,” they wrote. “Our results should lead to revision of clinical guidelines in favor of thymectomy and could potentially reverse downward trends in the use of thymectomy in overall management of myasthenia gravis.”
The main 3-year results of the Thymectomy Trial in Nonthymomatous Myasthenia Gravis Patients Receiving Prednisone (MGTX) were reported in 2016; the international trial found that thymectomy plus prednisone was superior to prednisone alone at 3 years (N Engl J Med. 2016 Aug 11;375[6]:511-22). The extension study aimed to assess the durability of the treatment response.
MGTX enrolled patients aged 18-65 years who had generalized nonthymomatous myasthenia gravis of less than 5 years’ duration and Myasthenia Gravis Foundation of America Clinical Classification Class II-IV disease. Of 111 patients who completed MGTX, 68 entered the extension study, and 50 completed the 60-month assessment (24 patients in the prednisone alone group and 26 patients in the prednisone plus thymectomy group).
At 5 years, patients in the thymectomy plus prednisone group had significantly lower time-weighted average Quantitative Myasthenia Gravis (QMG) scores (5.47 vs. 9.34) and mean alternate-day prednisone doses (24 mg vs. 48 mg), compared with patients who received prednisone alone. Twelve of 35 patients in the thymectomy group and 14 of 33 patients in the prednisone group had at least one adverse event by month 60. No treatment-related deaths occurred in the extension phase.
At 5 years, significantly more patients who underwent thymectomy had minimal manifestation status (i.e., no functional limitations from the disease other than some muscle weakness) – 88% versus 58%. The corresponding figures at 3 years were 67% and 47%.
In addition, 3-year and 5-year data indicate that the need for hospitalization is reduced after surgery, compared with medical therapy alone, Dr. Wolfe said.
Two patients in each treatment arm had an increase of 2 points or more in the QMG score, indicating clinical worsening.
“Our current findings reinforce the benefit of thymectomy seen in [MGTX], dispelling doubts about the procedure’s benefits and how long those benefits last,” said Dr. Wolfe. “We do hope that the new findings help reverse the apparent reluctance to do thymectomy and that the proportion of patients with myasthenia gravis who undergo thymectomy will increase.”
The authors noted that the small sample size of the extension study may limit its generalizability.
The study received funding from the National Institutes of Health. Dr. Wolfe reported grants from the NIH, the Muscular Dystrophy Association, the Myasthenia Gravis Foundation of America, CSL-Behring, and ArgenX, as well as personal fees from Grifols, Shire, and Alexion Pharmaceuticals. Coauthors reported working with and receiving funds from agencies, foundations, and pharmaceutical companies.
SOURCE: Wolfe GI et al. Lancet Neurol. 2019 Jan 25. doi: 10.1016/S1474-4422(18)30392-2.
Thymectomy may continue to benefit patients with myasthenia gravis 5 years after the procedure, according to an extension study published in Lancet Neurology.
The study evaluated the clinical status, medication requirements, and adverse events of patients with myasthenia gravis who completed a randomized controlled trial of thymectomy plus prednisone versus prednisone alone and agreed to participate in a rater-blinded 2-year extension.
“Thymectomy within the first few years of the disease course in addition to prednisone therapy confers benefits that persist for 5 years ... in patients with generalized nonthymomatous myasthenia gravis,” said lead study author Gil I. Wolfe, MD, chair of the department of neurology at the University at Buffalo in New York, and his research colleagues. “Results from the extension study provide further support for the use of thymectomy in management of myasthenia gravis and should encourage serious consideration of this treatment option in discussions between clinicians and their patients,” they wrote. “Our results should lead to revision of clinical guidelines in favor of thymectomy and could potentially reverse downward trends in the use of thymectomy in overall management of myasthenia gravis.”
The main 3-year results of the Thymectomy Trial in Nonthymomatous Myasthenia Gravis Patients Receiving Prednisone (MGTX) were reported in 2016; the international trial found that thymectomy plus prednisone was superior to prednisone alone at 3 years (N Engl J Med. 2016 Aug 11;375[6]:511-22). The extension study aimed to assess the durability of the treatment response.
MGTX enrolled patients aged 18-65 years who had generalized nonthymomatous myasthenia gravis of less than 5 years’ duration and Myasthenia Gravis Foundation of America Clinical Classification Class II-IV disease. Of 111 patients who completed MGTX, 68 entered the extension study, and 50 completed the 60-month assessment (24 patients in the prednisone alone group and 26 patients in the prednisone plus thymectomy group).
At 5 years, patients in the thymectomy plus prednisone group had significantly lower time-weighted average Quantitative Myasthenia Gravis (QMG) scores (5.47 vs. 9.34) and mean alternate-day prednisone doses (24 mg vs. 48 mg), compared with patients who received prednisone alone. Twelve of 35 patients in the thymectomy group and 14 of 33 patients in the prednisone group had at least one adverse event by month 60. No treatment-related deaths occurred in the extension phase.
At 5 years, significantly more patients who underwent thymectomy had minimal manifestation status (i.e., no functional limitations from the disease other than some muscle weakness) – 88% versus 58%. The corresponding figures at 3 years were 67% and 47%.
In addition, 3-year and 5-year data indicate that the need for hospitalization is reduced after surgery, compared with medical therapy alone, Dr. Wolfe said.
Two patients in each treatment arm had an increase of 2 points or more in the QMG score, indicating clinical worsening.
“Our current findings reinforce the benefit of thymectomy seen in [MGTX], dispelling doubts about the procedure’s benefits and how long those benefits last,” said Dr. Wolfe. “We do hope that the new findings help reverse the apparent reluctance to do thymectomy and that the proportion of patients with myasthenia gravis who undergo thymectomy will increase.”
The authors noted that the small sample size of the extension study may limit its generalizability.
The study received funding from the National Institutes of Health. Dr. Wolfe reported grants from the NIH, the Muscular Dystrophy Association, the Myasthenia Gravis Foundation of America, CSL-Behring, and ArgenX, as well as personal fees from Grifols, Shire, and Alexion Pharmaceuticals. Coauthors reported working with and receiving funds from agencies, foundations, and pharmaceutical companies.
SOURCE: Wolfe GI et al. Lancet Neurol. 2019 Jan 25. doi: 10.1016/S1474-4422(18)30392-2.
Thymectomy may continue to benefit patients with myasthenia gravis 5 years after the procedure, according to an extension study published in Lancet Neurology.
The study evaluated the clinical status, medication requirements, and adverse events of patients with myasthenia gravis who completed a randomized controlled trial of thymectomy plus prednisone versus prednisone alone and agreed to participate in a rater-blinded 2-year extension.
“Thymectomy within the first few years of the disease course in addition to prednisone therapy confers benefits that persist for 5 years ... in patients with generalized nonthymomatous myasthenia gravis,” said lead study author Gil I. Wolfe, MD, chair of the department of neurology at the University at Buffalo in New York, and his research colleagues. “Results from the extension study provide further support for the use of thymectomy in management of myasthenia gravis and should encourage serious consideration of this treatment option in discussions between clinicians and their patients,” they wrote. “Our results should lead to revision of clinical guidelines in favor of thymectomy and could potentially reverse downward trends in the use of thymectomy in overall management of myasthenia gravis.”
The main 3-year results of the Thymectomy Trial in Nonthymomatous Myasthenia Gravis Patients Receiving Prednisone (MGTX) were reported in 2016; the international trial found that thymectomy plus prednisone was superior to prednisone alone at 3 years (N Engl J Med. 2016 Aug 11;375[6]:511-22). The extension study aimed to assess the durability of the treatment response.
MGTX enrolled patients aged 18-65 years who had generalized nonthymomatous myasthenia gravis of less than 5 years’ duration and Myasthenia Gravis Foundation of America Clinical Classification Class II-IV disease. Of 111 patients who completed MGTX, 68 entered the extension study, and 50 completed the 60-month assessment (24 patients in the prednisone alone group and 26 patients in the prednisone plus thymectomy group).
At 5 years, patients in the thymectomy plus prednisone group had significantly lower time-weighted average Quantitative Myasthenia Gravis (QMG) scores (5.47 vs. 9.34) and mean alternate-day prednisone doses (24 mg vs. 48 mg), compared with patients who received prednisone alone. Twelve of 35 patients in the thymectomy group and 14 of 33 patients in the prednisone group had at least one adverse event by month 60. No treatment-related deaths occurred in the extension phase.
At 5 years, significantly more patients who underwent thymectomy had minimal manifestation status (i.e., no functional limitations from the disease other than some muscle weakness) – 88% versus 58%. The corresponding figures at 3 years were 67% and 47%.
In addition, 3-year and 5-year data indicate that the need for hospitalization is reduced after surgery, compared with medical therapy alone, Dr. Wolfe said.
Two patients in each treatment arm had an increase of 2 points or more in the QMG score, indicating clinical worsening.
“Our current findings reinforce the benefit of thymectomy seen in [MGTX], dispelling doubts about the procedure’s benefits and how long those benefits last,” said Dr. Wolfe. “We do hope that the new findings help reverse the apparent reluctance to do thymectomy and that the proportion of patients with myasthenia gravis who undergo thymectomy will increase.”
The authors noted that the small sample size of the extension study may limit its generalizability.
The study received funding from the National Institutes of Health. Dr. Wolfe reported grants from the NIH, the Muscular Dystrophy Association, the Myasthenia Gravis Foundation of America, CSL-Behring, and ArgenX, as well as personal fees from Grifols, Shire, and Alexion Pharmaceuticals. Coauthors reported working with and receiving funds from agencies, foundations, and pharmaceutical companies.
SOURCE: Wolfe GI et al. Lancet Neurol. 2019 Jan 25. doi: 10.1016/S1474-4422(18)30392-2.
FROM LANCET NEUROLOGY
Key clinical point: The benefits of thymectomy for myasthenia gravis persist 5 years after the procedure.
Major finding: Patients who undergo thymectomy and receive prednisone have lower time-weighted average Quantitative Myasthenia Gravis scores (5.47 vs. 9.34) and mean alternate-day prednisone doses (24 mg vs. 48 mg), compared with patients who receive prednisone alone.
Study details: A rater-blinded 2-year extension study that enrolled 68 patients who had completed a 3-year randomized controlled trial.
Disclosures: The study received funding from the National Institutes of Health. Dr. Wolfe reported grants from the NIH, the Muscular Dystrophy Association, the Myasthenia Gravis Foundation of America, CSL-Behring, and ArgenX, as well as personal fees from Grifols, Shire, and Alexion Pharmaceuticals. Other authors reported working with and receiving funds from various agencies, foundations, and pharmaceutical companies.
Source: Wolfe GI et al. Lancet Neurol. 2019 Jan 25. doi: 10.1016/S1474-4422(18)30392-2.
Understanding the terminology of gender identity
Use vocabulary to reduce barriers
TRANSforming Gynecology is a column about the ways in which ob.gyns. can become leaders in addressing the needs of the transgender/gender-nonconforming population. We hope to provide readers with some basic tools to help open the door to this marginalized population. We will lay the groundwork with an article on terminology and the importance of language before moving onto more focused discussions of topics that intersect with medical care of gender-nonconforming individuals. Transgender individuals experience among the worst health care outcomes of any demographic, and we hope that this column can be a starting point for providers to continue affirming the needs of marginalized populations in their everyday practice.

We live in a society in which most people’s gender identities are congruent with the sex they were assigned at birth based on physical characteristics. Transgender and gender-nonconforming people – often referred to as trans people, broadly – feel that their gender identity does not match their sex assigned at birth. This gender nonconformity, or the extent to which someone’s gender identity or expression differs from the cultural norm assigned to people with certain sexual organs, is in fact a matter of diversity, not pathology.
To truly provide sensitive care to trans patients, medical providers must first gain familiarity with the terminology used when discussing gender diversity. Gender identity, for starters, refers to an individual’s own personal and internal experience of themselves as a man, woman, some of both, or neither gender.1 It is only possible to learn a person’s gender identity through direct communication because gender identity is not always signaled by a certain gender expression. Gender expression is an external display usually through clothing, attitudes, or body language, that may or may not fit into socially recognized masculine or feminine categories.1 A separate aspect of the human experience is sexuality, such as gay, straight, bisexual, lesbian, etc. Sexuality should not be confused with sexual practices, which can sometimes deviate from a person’s sexuality. Gender identity is distinct from sexuality and sexual practices because people of any gender identity can hold any sexuality and engage in any sexual practices. Tied up in all of these categories is sex assigned at birth, which is a process by which health care providers categorize babies into two buckets based on the appearance of the external genitalia at the time of birth. It bears mentioning that the assignment of sex based on the appearance of external genitalia at the time of birth is a biologically inconsistent method that can lead to the exclusion of and nonconsensual mutilation of intersex people, who are individuals born with ambiguous genitalia and/or discrepancies between sex chromosome genotype and phenotype (stay tuned for more on people who are intersex in a future article).
A simplified way of remembering the distinctions between these concepts is that gender identity is who you go to bed as; gender expression is what you were wearing before you went to bed; sexuality is whom you tell others/yourself you go to bed with; sexual practice is whom you actually go to bed with; and sex assigned at birth is what you have between your legs when you are born (generally in a bed).
While cisgender persons feel that their experience of gender – their gender identity – agrees with the cultural norms surrounding their sex assigned at birth, transgender/gender-nonconforming (GNC) persons feel that their experience of gender is incongruent with their sex assigned at birth.1 Specifically, a transgender man is a person born with a vagina and therefore assigned female at birth who experiences himself as a man. A transgender woman is a person born with a penis and therefore assigned male at birth who experiences herself as a woman. A gender nonbinary person is someone with any sexual assignment at birth whose gender experience cannot be described using a binary that includes only male and female concepts, and a gender fluid person is someone whose internal experience of gender can oscillate.1 While these examples represent only a few of the many facets of gender diversity, the general terms trans and gender nonconforming (GNC) are widely accepted as inclusive, umbrella terms to describe all persons whose experience of gender is not congruent with their sex assigned at birth.
It is pivotal that medical providers understand that a person’s sex assigned at birth, gender identity, gender expression, sexuality, sexual practice, and romantic attraction can vary widely along a spectrum in each distinct category.2 For example, the current social norm dictates that someone born with a penis and assigned male at birth (AMAB) will feel that he is male, dress in a masculine fashion, and be both sexually and romantically attracted to someone born with a vagina who was assigned female at birth (AFAB), feels that she is female, dresses femininely, and likewise is sexually and romantically attracted to him. One possible alternative reality is a person who was born with a vagina that was therefore AFAB that experiences a masculine gender identity while engaging in a feminine gender expression in order to conform to social norms. In addition, they may be sexually attracted to people whom they perceive to be feminine while engaging in sexual activity with people who were AMAB. Informed medical care for trans persons starts with the basic understanding that an individual’s gender identity may not necessarily align with their gender expression, sex assigned at birth, sexual attraction, or romantic attraction.
As obstetrician-gynecologists, we are tasked by the American College of Obstetricians and Gynecologists to provide nondiscriminatory care to all patients, regardless of gender identity.3 We must be careful not to assume that all of our patients are cisgender women who use “she/hers/her” pronouns. By simply asking patients what names or pronouns they would like us to use before initiating care, we become more sensitive to variations in gender identity. Many providers may feel uncertain about how to initiate or respond to this line of questioning. One way that health care practices can begin to respectfully access information around gender identity is to create intake forms that include more than two options for gender or to alter their office visit note templates to include a section that prompts the provider to include a discussion surrounding gender identity. By offering these opportunities for inclusion, we become more welcoming of gender minorities like transgender men seeking cervical cancer screening.
There are a number of reasons that trans persons have limited access to the health care system, but the greatest barrier reported by transgender patients is the paucity of knowledgeable providers.4 to an already marginalized patient population. Familiarity with this terminology normalizes the idea of gender diversity and subsequently reduces the risk of providers making assumptions about patients that contributes to suboptimal care.
Dr. Joyner is an assistant professor at Emory University, Atlanta, and is the director of gynecologic services in the Gender Center at Grady Memorial Hospital in Atlanta. Dr. Joyner identifies as a cisgender female and uses she/hers/her as her personal pronouns. Dr. Joey Bahng is a PGY-1 resident physician in Emory University’s gynecology & obstetrics residency program. Dr. Bahng identifies as nonbinary and uses they/them/their as their personal pronouns. Dr. Joyner and Dr. Bahng reported no financial disclosures.
1. Lancet. 2016 Jul 23;388(10042):390-400.
2. www.genderbread.org/resource/genderbread-person-v4-0.
3. Obstet Gynecol. 2011 Dec;118(6):1454-8.
4. Curr Opin Endocrinol Diabetes Obes. 2016 Apr 1;23(2):168-71.
Use vocabulary to reduce barriers
Use vocabulary to reduce barriers
TRANSforming Gynecology is a column about the ways in which ob.gyns. can become leaders in addressing the needs of the transgender/gender-nonconforming population. We hope to provide readers with some basic tools to help open the door to this marginalized population. We will lay the groundwork with an article on terminology and the importance of language before moving onto more focused discussions of topics that intersect with medical care of gender-nonconforming individuals. Transgender individuals experience among the worst health care outcomes of any demographic, and we hope that this column can be a starting point for providers to continue affirming the needs of marginalized populations in their everyday practice.

We live in a society in which most people’s gender identities are congruent with the sex they were assigned at birth based on physical characteristics. Transgender and gender-nonconforming people – often referred to as trans people, broadly – feel that their gender identity does not match their sex assigned at birth. This gender nonconformity, or the extent to which someone’s gender identity or expression differs from the cultural norm assigned to people with certain sexual organs, is in fact a matter of diversity, not pathology.
To truly provide sensitive care to trans patients, medical providers must first gain familiarity with the terminology used when discussing gender diversity. Gender identity, for starters, refers to an individual’s own personal and internal experience of themselves as a man, woman, some of both, or neither gender.1 It is only possible to learn a person’s gender identity through direct communication because gender identity is not always signaled by a certain gender expression. Gender expression is an external display usually through clothing, attitudes, or body language, that may or may not fit into socially recognized masculine or feminine categories.1 A separate aspect of the human experience is sexuality, such as gay, straight, bisexual, lesbian, etc. Sexuality should not be confused with sexual practices, which can sometimes deviate from a person’s sexuality. Gender identity is distinct from sexuality and sexual practices because people of any gender identity can hold any sexuality and engage in any sexual practices. Tied up in all of these categories is sex assigned at birth, which is a process by which health care providers categorize babies into two buckets based on the appearance of the external genitalia at the time of birth. It bears mentioning that the assignment of sex based on the appearance of external genitalia at the time of birth is a biologically inconsistent method that can lead to the exclusion of and nonconsensual mutilation of intersex people, who are individuals born with ambiguous genitalia and/or discrepancies between sex chromosome genotype and phenotype (stay tuned for more on people who are intersex in a future article).
A simplified way of remembering the distinctions between these concepts is that gender identity is who you go to bed as; gender expression is what you were wearing before you went to bed; sexuality is whom you tell others/yourself you go to bed with; sexual practice is whom you actually go to bed with; and sex assigned at birth is what you have between your legs when you are born (generally in a bed).
While cisgender persons feel that their experience of gender – their gender identity – agrees with the cultural norms surrounding their sex assigned at birth, transgender/gender-nonconforming (GNC) persons feel that their experience of gender is incongruent with their sex assigned at birth.1 Specifically, a transgender man is a person born with a vagina and therefore assigned female at birth who experiences himself as a man. A transgender woman is a person born with a penis and therefore assigned male at birth who experiences herself as a woman. A gender nonbinary person is someone with any sexual assignment at birth whose gender experience cannot be described using a binary that includes only male and female concepts, and a gender fluid person is someone whose internal experience of gender can oscillate.1 While these examples represent only a few of the many facets of gender diversity, the general terms trans and gender nonconforming (GNC) are widely accepted as inclusive, umbrella terms to describe all persons whose experience of gender is not congruent with their sex assigned at birth.
It is pivotal that medical providers understand that a person’s sex assigned at birth, gender identity, gender expression, sexuality, sexual practice, and romantic attraction can vary widely along a spectrum in each distinct category.2 For example, the current social norm dictates that someone born with a penis and assigned male at birth (AMAB) will feel that he is male, dress in a masculine fashion, and be both sexually and romantically attracted to someone born with a vagina who was assigned female at birth (AFAB), feels that she is female, dresses femininely, and likewise is sexually and romantically attracted to him. One possible alternative reality is a person who was born with a vagina that was therefore AFAB that experiences a masculine gender identity while engaging in a feminine gender expression in order to conform to social norms. In addition, they may be sexually attracted to people whom they perceive to be feminine while engaging in sexual activity with people who were AMAB. Informed medical care for trans persons starts with the basic understanding that an individual’s gender identity may not necessarily align with their gender expression, sex assigned at birth, sexual attraction, or romantic attraction.
As obstetrician-gynecologists, we are tasked by the American College of Obstetricians and Gynecologists to provide nondiscriminatory care to all patients, regardless of gender identity.3 We must be careful not to assume that all of our patients are cisgender women who use “she/hers/her” pronouns. By simply asking patients what names or pronouns they would like us to use before initiating care, we become more sensitive to variations in gender identity. Many providers may feel uncertain about how to initiate or respond to this line of questioning. One way that health care practices can begin to respectfully access information around gender identity is to create intake forms that include more than two options for gender or to alter their office visit note templates to include a section that prompts the provider to include a discussion surrounding gender identity. By offering these opportunities for inclusion, we become more welcoming of gender minorities like transgender men seeking cervical cancer screening.
There are a number of reasons that trans persons have limited access to the health care system, but the greatest barrier reported by transgender patients is the paucity of knowledgeable providers.4 to an already marginalized patient population. Familiarity with this terminology normalizes the idea of gender diversity and subsequently reduces the risk of providers making assumptions about patients that contributes to suboptimal care.
Dr. Joyner is an assistant professor at Emory University, Atlanta, and is the director of gynecologic services in the Gender Center at Grady Memorial Hospital in Atlanta. Dr. Joyner identifies as a cisgender female and uses she/hers/her as her personal pronouns. Dr. Joey Bahng is a PGY-1 resident physician in Emory University’s gynecology & obstetrics residency program. Dr. Bahng identifies as nonbinary and uses they/them/their as their personal pronouns. Dr. Joyner and Dr. Bahng reported no financial disclosures.
1. Lancet. 2016 Jul 23;388(10042):390-400.
2. www.genderbread.org/resource/genderbread-person-v4-0.
3. Obstet Gynecol. 2011 Dec;118(6):1454-8.
4. Curr Opin Endocrinol Diabetes Obes. 2016 Apr 1;23(2):168-71.
TRANSforming Gynecology is a column about the ways in which ob.gyns. can become leaders in addressing the needs of the transgender/gender-nonconforming population. We hope to provide readers with some basic tools to help open the door to this marginalized population. We will lay the groundwork with an article on terminology and the importance of language before moving onto more focused discussions of topics that intersect with medical care of gender-nonconforming individuals. Transgender individuals experience among the worst health care outcomes of any demographic, and we hope that this column can be a starting point for providers to continue affirming the needs of marginalized populations in their everyday practice.

We live in a society in which most people’s gender identities are congruent with the sex they were assigned at birth based on physical characteristics. Transgender and gender-nonconforming people – often referred to as trans people, broadly – feel that their gender identity does not match their sex assigned at birth. This gender nonconformity, or the extent to which someone’s gender identity or expression differs from the cultural norm assigned to people with certain sexual organs, is in fact a matter of diversity, not pathology.
To truly provide sensitive care to trans patients, medical providers must first gain familiarity with the terminology used when discussing gender diversity. Gender identity, for starters, refers to an individual’s own personal and internal experience of themselves as a man, woman, some of both, or neither gender.1 It is only possible to learn a person’s gender identity through direct communication because gender identity is not always signaled by a certain gender expression. Gender expression is an external display usually through clothing, attitudes, or body language, that may or may not fit into socially recognized masculine or feminine categories.1 A separate aspect of the human experience is sexuality, such as gay, straight, bisexual, lesbian, etc. Sexuality should not be confused with sexual practices, which can sometimes deviate from a person’s sexuality. Gender identity is distinct from sexuality and sexual practices because people of any gender identity can hold any sexuality and engage in any sexual practices. Tied up in all of these categories is sex assigned at birth, which is a process by which health care providers categorize babies into two buckets based on the appearance of the external genitalia at the time of birth. It bears mentioning that the assignment of sex based on the appearance of external genitalia at the time of birth is a biologically inconsistent method that can lead to the exclusion of and nonconsensual mutilation of intersex people, who are individuals born with ambiguous genitalia and/or discrepancies between sex chromosome genotype and phenotype (stay tuned for more on people who are intersex in a future article).
A simplified way of remembering the distinctions between these concepts is that gender identity is who you go to bed as; gender expression is what you were wearing before you went to bed; sexuality is whom you tell others/yourself you go to bed with; sexual practice is whom you actually go to bed with; and sex assigned at birth is what you have between your legs when you are born (generally in a bed).
While cisgender persons feel that their experience of gender – their gender identity – agrees with the cultural norms surrounding their sex assigned at birth, transgender/gender-nonconforming (GNC) persons feel that their experience of gender is incongruent with their sex assigned at birth.1 Specifically, a transgender man is a person born with a vagina and therefore assigned female at birth who experiences himself as a man. A transgender woman is a person born with a penis and therefore assigned male at birth who experiences herself as a woman. A gender nonbinary person is someone with any sexual assignment at birth whose gender experience cannot be described using a binary that includes only male and female concepts, and a gender fluid person is someone whose internal experience of gender can oscillate.1 While these examples represent only a few of the many facets of gender diversity, the general terms trans and gender nonconforming (GNC) are widely accepted as inclusive, umbrella terms to describe all persons whose experience of gender is not congruent with their sex assigned at birth.
It is pivotal that medical providers understand that a person’s sex assigned at birth, gender identity, gender expression, sexuality, sexual practice, and romantic attraction can vary widely along a spectrum in each distinct category.2 For example, the current social norm dictates that someone born with a penis and assigned male at birth (AMAB) will feel that he is male, dress in a masculine fashion, and be both sexually and romantically attracted to someone born with a vagina who was assigned female at birth (AFAB), feels that she is female, dresses femininely, and likewise is sexually and romantically attracted to him. One possible alternative reality is a person who was born with a vagina that was therefore AFAB that experiences a masculine gender identity while engaging in a feminine gender expression in order to conform to social norms. In addition, they may be sexually attracted to people whom they perceive to be feminine while engaging in sexual activity with people who were AMAB. Informed medical care for trans persons starts with the basic understanding that an individual’s gender identity may not necessarily align with their gender expression, sex assigned at birth, sexual attraction, or romantic attraction.
As obstetrician-gynecologists, we are tasked by the American College of Obstetricians and Gynecologists to provide nondiscriminatory care to all patients, regardless of gender identity.3 We must be careful not to assume that all of our patients are cisgender women who use “she/hers/her” pronouns. By simply asking patients what names or pronouns they would like us to use before initiating care, we become more sensitive to variations in gender identity. Many providers may feel uncertain about how to initiate or respond to this line of questioning. One way that health care practices can begin to respectfully access information around gender identity is to create intake forms that include more than two options for gender or to alter their office visit note templates to include a section that prompts the provider to include a discussion surrounding gender identity. By offering these opportunities for inclusion, we become more welcoming of gender minorities like transgender men seeking cervical cancer screening.
There are a number of reasons that trans persons have limited access to the health care system, but the greatest barrier reported by transgender patients is the paucity of knowledgeable providers.4 to an already marginalized patient population. Familiarity with this terminology normalizes the idea of gender diversity and subsequently reduces the risk of providers making assumptions about patients that contributes to suboptimal care.
Dr. Joyner is an assistant professor at Emory University, Atlanta, and is the director of gynecologic services in the Gender Center at Grady Memorial Hospital in Atlanta. Dr. Joyner identifies as a cisgender female and uses she/hers/her as her personal pronouns. Dr. Joey Bahng is a PGY-1 resident physician in Emory University’s gynecology & obstetrics residency program. Dr. Bahng identifies as nonbinary and uses they/them/their as their personal pronouns. Dr. Joyner and Dr. Bahng reported no financial disclosures.
1. Lancet. 2016 Jul 23;388(10042):390-400.
2. www.genderbread.org/resource/genderbread-person-v4-0.
3. Obstet Gynecol. 2011 Dec;118(6):1454-8.
4. Curr Opin Endocrinol Diabetes Obes. 2016 Apr 1;23(2):168-71.