User login
AAP guidance: How to ask about military service
Knee pathologies predict accelerated knee osteoarthritis, patients with a poor-prognosis cancer have a higher risk of suicide in the first year, and Nuedexta is mainly being prescribed for dementia and Parkinson’s.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Knee pathologies predict accelerated knee osteoarthritis, patients with a poor-prognosis cancer have a higher risk of suicide in the first year, and Nuedexta is mainly being prescribed for dementia and Parkinson’s.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Knee pathologies predict accelerated knee osteoarthritis, patients with a poor-prognosis cancer have a higher risk of suicide in the first year, and Nuedexta is mainly being prescribed for dementia and Parkinson’s.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Necrobiosis Lipoidica With Superimposed Pyoderma Vegetans
Case Report
A 26-year-old woman with a medical history of newly diagnosed diabetes mellitus (DM), obesity, and asthma was evaluated as a hospital consultation with a vegetative plaque on the left lateral ankle of 13 months’ duration. The lesion first appeared as a red scaly rash that became purulent. The lesion had been treated with multiple rounds of topical antibiotics, oral antibiotics, topical antifungals, and corticosteroids without resolution. The patient denied pain or any decrease in ankle mobility. Review of systems was otherwise negative.
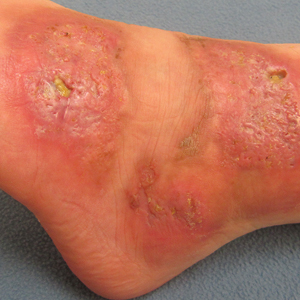
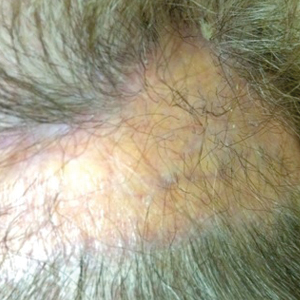
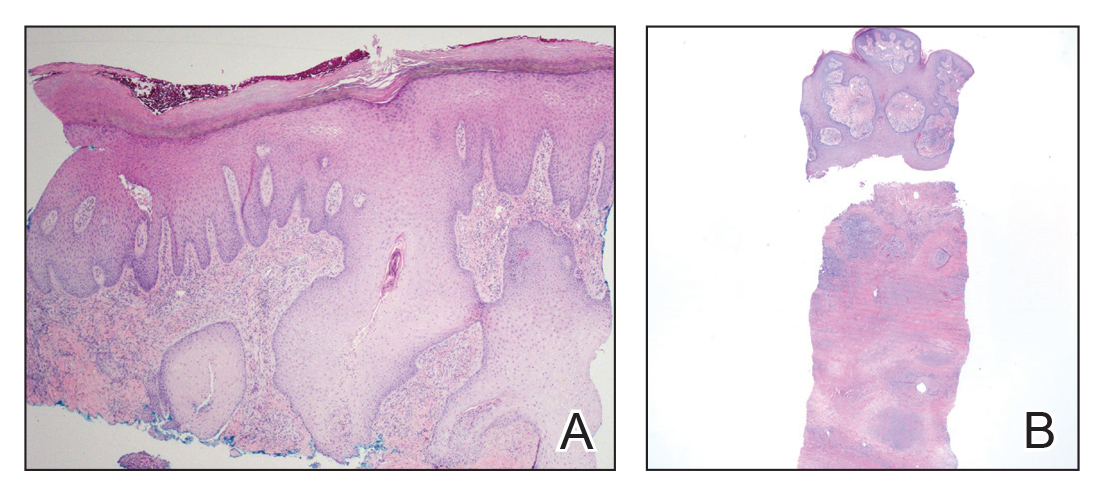
On physical examination, 3 large, pink, scaly, crusted plaques with surrounding erythema were observed (Figure 1A). On palpation, purulent drainage with a foul odor was noted in the area underlying the lesion. Initial punch biopsy demonstrated epidermal hyperplasia with neutrophil-rich sinus tracts consistent with pyoderma vegetans (PV)(Figure 2A). Tissue culture was positive for Staphylococcus aureus and Streptococcus anginosus. Cultures for both fungi and acid-fast bacilli were negative for growth.

The patient was treated with mupirocin ointment 2% and 3 months of cephalexin 250 mg twice daily, which cleared the purulent crust; however, serous drainage, ulceration, and erythema persisted. The patient needed an extended course of antibiotics, which had not been previously administered to clear the purulence. During this treatment regimen, the patient’s DM remained uncontrolled.
A second deeper punch biopsy revealed a layered granulomatous infiltrate with sclerosis throughout the dermis most consistent with necrobiosis lipoidica (NL)(Figure 2B). Direct immunofluorescence biopsy was negative. Once the PV was clear, betamethasone dipropionate ointment 0.05% was initiated to address the residual lesions (Figure 1B).
Physical examination combined with histopathologic findings and staphylococcal- and streptococcal-positive tissue cultures supported a diagnosis of NL with superimposed PV.
Comment
Necrobiosis lipoidica is a chronic granulomatous disease characterized by collagen degeneration, granulomatous formation, and endothelial wall thickening.1 The condition is most commonly seen in association with insulin-dependent DM, though it also has been described in other inflammatory conditions. A case of NL in monozygotic twins has been reported, suggesting a genetic component in nondiabetic patients with NL.2 Necrobiosis lipoidica affects females more often than males.
The pathogenesis of NL is not well understood but likely involves secondary microangiopathy because of glycoprotein deposition in vessel walls, leading to vascular thickening. Histopathology reveals palisading and necrobiotic granulomas comprising large confluent areas of necrobiosis throughout the dermis, giving a layered appearance.3
Clinically, NL presents with asymptomatic, well-circumscribed, violaceous papules and nodules that coalesce into plaques on the lower extremities, face, or trunk. The plaques have a central red-brown hue that progressively becomes more yellow and atrophic. The lesions can become eroded and ulcerated if left untreated.1
Clinical diagnosis of NL can be challenging due to the similar clinical findings of other granulomatous lesions, such as granuloma annulare and cutaneous sarcoidosis. As reported by Pellicano and colleagues,4 dermoscopy has proved to be an excellent tool for differentiating these granulomatous skin lesions. Necrobiosis lipoidica demonstrates elongated serpentine telangiectases overlying a white structureless background, whereas granuloma annulare reveals orange-red structureless peripheral borders.5
Treatment of NL is difficult; patients often are refractory. Tight control of blood glucose alone has not been proven to cure NL. The mainstay of treatment is topical and intralesional corticosteroids at the active borders of the lesions. Tumor necrosis factor α inhibitors have shown some success, though recurrence has been reported.6 Other treatments, such as topical tretinoin and topical tacrolimus, may be of some benefit for atrophic NL lesions. Studies also have shown that skin grafting can be of surgical benefit in ulcerative NL with a low rate of recurrence.6 Control and management of DM plus lifestyle modifications may play a role in decreasing the severity of NL.7 Topical psoralen plus UVA light therapy and other experimental treatments, such as antiplatelet medications,8 also have been utilized.
The case of NL presented here was complicated by a superimposed suppurative infection consistent with PV, a rare chronic bacterial infection of the skin that presents with vegetative plaques. Pyoderma vegetans is most commonly observed in patients with underlying immunosuppression, likely secondary to DM in this case. Pyoderma vegetans is most often caused by S aureus and β-hemolytic streptococci. The clinical presentation of PV reveals verrucous vegetative plaques with pustules and abscesses. The borders of the lesions may be elevated and have a granulomatous appearance, thus complicating clinical diagnosis. There often is foul-smelling, purulent discharge within the plaques.9
Histopathology reveals pseudoepitheliomatous hyperplasia with abscesses and sinus tracts. An acute or chronic granulomatous inflammatory infiltrate may be observed. Basophilic fungus like granules are not seen within specimens of PV, which helps differentiate the disease from botryomycosis.10
There is no standardized treatment of PV; topical and systemic antibiotics are mainstays.10 One reported case of PV responded well to acitretin.9 Our patient responded well to 3 months of oral antibiotic therapy, followed by topical corticosteroids.
1. Reid SD, Ladizinski B, Lee K, et al. Update on necrobiosis lipoidica: a review of etiology, diagnosis, and treatment options. J Am Acad Dermatol. 2013;69:783-791.
2. Shimanovich I, Erdmann H, Grabbe J, et al. Necrobiosis lipoidica in monozygotic twins. Arch Dermatol. 2008;144:119-120.
3. Ghazarian D, Al Habeeb A. Necrobiotic lesions of the skin: an approach and review of the literature. Diagn Histopathol. 2009;15:186-194.
4. Pellicano R, Caldarola G, Filabozzi P, et al. Dermoscopy of necrobiosis lipoidica and granuloma annulare. Dermatology. 2013;226:319-323.
5. Bakos RM, Cartell A, Bakos L. Dermatoscopy of early-onset necrobiosis lipoidica. J Am Acad Dermatol. 2012;66:143-144.
6. Feily A, Mehraban S. Treatment modalities of necrobiosis lipoidica: a concise systematic review. Dermatol Reports. 2015;7:5749.
7. Yigit S, Estrada E. Recurrent necrobiosis lipoidica diabeticorum associated with venous insufficiency in an adolescent with poorly controlled type 2 diabetes mellitus. J Pediatr. 2002;141:280-282.
8. Heng MC, Song MK, Heng MK. Healing of necrobiotic ulcers with antiplatelet therapy. Correlation with plasma thromboxane levels. Int J Dermatol. 1989;28:195-197.
9. Lee Y, Jung SW, Sim HS, et al. Blastomycosis-like pyoderma with good response to acitretin. Ann Dermatol. 2011;23:365-368.
10. Marschalko M, Preisz K, Harsing J, et al. Pyoderma vegetans. report on a case and review of data on pyoderma vegetans and cutaneous botryomycosis. Acta Dermatovenerol. 1995;95:55-59.
Case Report
A 26-year-old woman with a medical history of newly diagnosed diabetes mellitus (DM), obesity, and asthma was evaluated as a hospital consultation with a vegetative plaque on the left lateral ankle of 13 months’ duration. The lesion first appeared as a red scaly rash that became purulent. The lesion had been treated with multiple rounds of topical antibiotics, oral antibiotics, topical antifungals, and corticosteroids without resolution. The patient denied pain or any decrease in ankle mobility. Review of systems was otherwise negative.
On physical examination, 3 large, pink, scaly, crusted plaques with surrounding erythema were observed (Figure 1A). On palpation, purulent drainage with a foul odor was noted in the area underlying the lesion. Initial punch biopsy demonstrated epidermal hyperplasia with neutrophil-rich sinus tracts consistent with pyoderma vegetans (PV)(Figure 2A). Tissue culture was positive for Staphylococcus aureus and Streptococcus anginosus. Cultures for both fungi and acid-fast bacilli were negative for growth.


The patient was treated with mupirocin ointment 2% and 3 months of cephalexin 250 mg twice daily, which cleared the purulent crust; however, serous drainage, ulceration, and erythema persisted. The patient needed an extended course of antibiotics, which had not been previously administered to clear the purulence. During this treatment regimen, the patient’s DM remained uncontrolled.
A second deeper punch biopsy revealed a layered granulomatous infiltrate with sclerosis throughout the dermis most consistent with necrobiosis lipoidica (NL)(Figure 2B). Direct immunofluorescence biopsy was negative. Once the PV was clear, betamethasone dipropionate ointment 0.05% was initiated to address the residual lesions (Figure 1B).
Physical examination combined with histopathologic findings and staphylococcal- and streptococcal-positive tissue cultures supported a diagnosis of NL with superimposed PV.
Comment
Necrobiosis lipoidica is a chronic granulomatous disease characterized by collagen degeneration, granulomatous formation, and endothelial wall thickening.1 The condition is most commonly seen in association with insulin-dependent DM, though it also has been described in other inflammatory conditions. A case of NL in monozygotic twins has been reported, suggesting a genetic component in nondiabetic patients with NL.2 Necrobiosis lipoidica affects females more often than males.
The pathogenesis of NL is not well understood but likely involves secondary microangiopathy because of glycoprotein deposition in vessel walls, leading to vascular thickening. Histopathology reveals palisading and necrobiotic granulomas comprising large confluent areas of necrobiosis throughout the dermis, giving a layered appearance.3
Clinically, NL presents with asymptomatic, well-circumscribed, violaceous papules and nodules that coalesce into plaques on the lower extremities, face, or trunk. The plaques have a central red-brown hue that progressively becomes more yellow and atrophic. The lesions can become eroded and ulcerated if left untreated.1
Clinical diagnosis of NL can be challenging due to the similar clinical findings of other granulomatous lesions, such as granuloma annulare and cutaneous sarcoidosis. As reported by Pellicano and colleagues,4 dermoscopy has proved to be an excellent tool for differentiating these granulomatous skin lesions. Necrobiosis lipoidica demonstrates elongated serpentine telangiectases overlying a white structureless background, whereas granuloma annulare reveals orange-red structureless peripheral borders.5
Treatment of NL is difficult; patients often are refractory. Tight control of blood glucose alone has not been proven to cure NL. The mainstay of treatment is topical and intralesional corticosteroids at the active borders of the lesions. Tumor necrosis factor α inhibitors have shown some success, though recurrence has been reported.6 Other treatments, such as topical tretinoin and topical tacrolimus, may be of some benefit for atrophic NL lesions. Studies also have shown that skin grafting can be of surgical benefit in ulcerative NL with a low rate of recurrence.6 Control and management of DM plus lifestyle modifications may play a role in decreasing the severity of NL.7 Topical psoralen plus UVA light therapy and other experimental treatments, such as antiplatelet medications,8 also have been utilized.
The case of NL presented here was complicated by a superimposed suppurative infection consistent with PV, a rare chronic bacterial infection of the skin that presents with vegetative plaques. Pyoderma vegetans is most commonly observed in patients with underlying immunosuppression, likely secondary to DM in this case. Pyoderma vegetans is most often caused by S aureus and β-hemolytic streptococci. The clinical presentation of PV reveals verrucous vegetative plaques with pustules and abscesses. The borders of the lesions may be elevated and have a granulomatous appearance, thus complicating clinical diagnosis. There often is foul-smelling, purulent discharge within the plaques.9
Histopathology reveals pseudoepitheliomatous hyperplasia with abscesses and sinus tracts. An acute or chronic granulomatous inflammatory infiltrate may be observed. Basophilic fungus like granules are not seen within specimens of PV, which helps differentiate the disease from botryomycosis.10
There is no standardized treatment of PV; topical and systemic antibiotics are mainstays.10 One reported case of PV responded well to acitretin.9 Our patient responded well to 3 months of oral antibiotic therapy, followed by topical corticosteroids.
Case Report
A 26-year-old woman with a medical history of newly diagnosed diabetes mellitus (DM), obesity, and asthma was evaluated as a hospital consultation with a vegetative plaque on the left lateral ankle of 13 months’ duration. The lesion first appeared as a red scaly rash that became purulent. The lesion had been treated with multiple rounds of topical antibiotics, oral antibiotics, topical antifungals, and corticosteroids without resolution. The patient denied pain or any decrease in ankle mobility. Review of systems was otherwise negative.
On physical examination, 3 large, pink, scaly, crusted plaques with surrounding erythema were observed (Figure 1A). On palpation, purulent drainage with a foul odor was noted in the area underlying the lesion. Initial punch biopsy demonstrated epidermal hyperplasia with neutrophil-rich sinus tracts consistent with pyoderma vegetans (PV)(Figure 2A). Tissue culture was positive for Staphylococcus aureus and Streptococcus anginosus. Cultures for both fungi and acid-fast bacilli were negative for growth.


The patient was treated with mupirocin ointment 2% and 3 months of cephalexin 250 mg twice daily, which cleared the purulent crust; however, serous drainage, ulceration, and erythema persisted. The patient needed an extended course of antibiotics, which had not been previously administered to clear the purulence. During this treatment regimen, the patient’s DM remained uncontrolled.
A second deeper punch biopsy revealed a layered granulomatous infiltrate with sclerosis throughout the dermis most consistent with necrobiosis lipoidica (NL)(Figure 2B). Direct immunofluorescence biopsy was negative. Once the PV was clear, betamethasone dipropionate ointment 0.05% was initiated to address the residual lesions (Figure 1B).
Physical examination combined with histopathologic findings and staphylococcal- and streptococcal-positive tissue cultures supported a diagnosis of NL with superimposed PV.
Comment
Necrobiosis lipoidica is a chronic granulomatous disease characterized by collagen degeneration, granulomatous formation, and endothelial wall thickening.1 The condition is most commonly seen in association with insulin-dependent DM, though it also has been described in other inflammatory conditions. A case of NL in monozygotic twins has been reported, suggesting a genetic component in nondiabetic patients with NL.2 Necrobiosis lipoidica affects females more often than males.
The pathogenesis of NL is not well understood but likely involves secondary microangiopathy because of glycoprotein deposition in vessel walls, leading to vascular thickening. Histopathology reveals palisading and necrobiotic granulomas comprising large confluent areas of necrobiosis throughout the dermis, giving a layered appearance.3
Clinically, NL presents with asymptomatic, well-circumscribed, violaceous papules and nodules that coalesce into plaques on the lower extremities, face, or trunk. The plaques have a central red-brown hue that progressively becomes more yellow and atrophic. The lesions can become eroded and ulcerated if left untreated.1
Clinical diagnosis of NL can be challenging due to the similar clinical findings of other granulomatous lesions, such as granuloma annulare and cutaneous sarcoidosis. As reported by Pellicano and colleagues,4 dermoscopy has proved to be an excellent tool for differentiating these granulomatous skin lesions. Necrobiosis lipoidica demonstrates elongated serpentine telangiectases overlying a white structureless background, whereas granuloma annulare reveals orange-red structureless peripheral borders.5
Treatment of NL is difficult; patients often are refractory. Tight control of blood glucose alone has not been proven to cure NL. The mainstay of treatment is topical and intralesional corticosteroids at the active borders of the lesions. Tumor necrosis factor α inhibitors have shown some success, though recurrence has been reported.6 Other treatments, such as topical tretinoin and topical tacrolimus, may be of some benefit for atrophic NL lesions. Studies also have shown that skin grafting can be of surgical benefit in ulcerative NL with a low rate of recurrence.6 Control and management of DM plus lifestyle modifications may play a role in decreasing the severity of NL.7 Topical psoralen plus UVA light therapy and other experimental treatments, such as antiplatelet medications,8 also have been utilized.
The case of NL presented here was complicated by a superimposed suppurative infection consistent with PV, a rare chronic bacterial infection of the skin that presents with vegetative plaques. Pyoderma vegetans is most commonly observed in patients with underlying immunosuppression, likely secondary to DM in this case. Pyoderma vegetans is most often caused by S aureus and β-hemolytic streptococci. The clinical presentation of PV reveals verrucous vegetative plaques with pustules and abscesses. The borders of the lesions may be elevated and have a granulomatous appearance, thus complicating clinical diagnosis. There often is foul-smelling, purulent discharge within the plaques.9
Histopathology reveals pseudoepitheliomatous hyperplasia with abscesses and sinus tracts. An acute or chronic granulomatous inflammatory infiltrate may be observed. Basophilic fungus like granules are not seen within specimens of PV, which helps differentiate the disease from botryomycosis.10
There is no standardized treatment of PV; topical and systemic antibiotics are mainstays.10 One reported case of PV responded well to acitretin.9 Our patient responded well to 3 months of oral antibiotic therapy, followed by topical corticosteroids.
1. Reid SD, Ladizinski B, Lee K, et al. Update on necrobiosis lipoidica: a review of etiology, diagnosis, and treatment options. J Am Acad Dermatol. 2013;69:783-791.
2. Shimanovich I, Erdmann H, Grabbe J, et al. Necrobiosis lipoidica in monozygotic twins. Arch Dermatol. 2008;144:119-120.
3. Ghazarian D, Al Habeeb A. Necrobiotic lesions of the skin: an approach and review of the literature. Diagn Histopathol. 2009;15:186-194.
4. Pellicano R, Caldarola G, Filabozzi P, et al. Dermoscopy of necrobiosis lipoidica and granuloma annulare. Dermatology. 2013;226:319-323.
5. Bakos RM, Cartell A, Bakos L. Dermatoscopy of early-onset necrobiosis lipoidica. J Am Acad Dermatol. 2012;66:143-144.
6. Feily A, Mehraban S. Treatment modalities of necrobiosis lipoidica: a concise systematic review. Dermatol Reports. 2015;7:5749.
7. Yigit S, Estrada E. Recurrent necrobiosis lipoidica diabeticorum associated with venous insufficiency in an adolescent with poorly controlled type 2 diabetes mellitus. J Pediatr. 2002;141:280-282.
8. Heng MC, Song MK, Heng MK. Healing of necrobiotic ulcers with antiplatelet therapy. Correlation with plasma thromboxane levels. Int J Dermatol. 1989;28:195-197.
9. Lee Y, Jung SW, Sim HS, et al. Blastomycosis-like pyoderma with good response to acitretin. Ann Dermatol. 2011;23:365-368.
10. Marschalko M, Preisz K, Harsing J, et al. Pyoderma vegetans. report on a case and review of data on pyoderma vegetans and cutaneous botryomycosis. Acta Dermatovenerol. 1995;95:55-59.
1. Reid SD, Ladizinski B, Lee K, et al. Update on necrobiosis lipoidica: a review of etiology, diagnosis, and treatment options. J Am Acad Dermatol. 2013;69:783-791.
2. Shimanovich I, Erdmann H, Grabbe J, et al. Necrobiosis lipoidica in monozygotic twins. Arch Dermatol. 2008;144:119-120.
3. Ghazarian D, Al Habeeb A. Necrobiotic lesions of the skin: an approach and review of the literature. Diagn Histopathol. 2009;15:186-194.
4. Pellicano R, Caldarola G, Filabozzi P, et al. Dermoscopy of necrobiosis lipoidica and granuloma annulare. Dermatology. 2013;226:319-323.
5. Bakos RM, Cartell A, Bakos L. Dermatoscopy of early-onset necrobiosis lipoidica. J Am Acad Dermatol. 2012;66:143-144.
6. Feily A, Mehraban S. Treatment modalities of necrobiosis lipoidica: a concise systematic review. Dermatol Reports. 2015;7:5749.
7. Yigit S, Estrada E. Recurrent necrobiosis lipoidica diabeticorum associated with venous insufficiency in an adolescent with poorly controlled type 2 diabetes mellitus. J Pediatr. 2002;141:280-282.
8. Heng MC, Song MK, Heng MK. Healing of necrobiotic ulcers with antiplatelet therapy. Correlation with plasma thromboxane levels. Int J Dermatol. 1989;28:195-197.
9. Lee Y, Jung SW, Sim HS, et al. Blastomycosis-like pyoderma with good response to acitretin. Ann Dermatol. 2011;23:365-368.
10. Marschalko M, Preisz K, Harsing J, et al. Pyoderma vegetans. report on a case and review of data on pyoderma vegetans and cutaneous botryomycosis. Acta Dermatovenerol. 1995;95:55-59.
Practice Points
- Necrobiosis lipoidica (NL), a chronic granulomatous disease characterized by collagen degeneration, granulomatous formation, and endothelial-wall thickening, is most often seen in association with insulin-dependent diabetes mellitus (DM).
- Asymptomatic, well-circumscribed, violaceous papules and nodules coalesce into plaques on the lower extremities, face, or trunk in NL.
- Treatment mainstay is topical and intralesional corticosteroids at active borders of lesions. Other treatments used with some success include tumor necrosis factor 11α inhibitors, topical tretinoin, topical tacrolimus, and skin grafting. Control and management of DM can be helpful.
Daclizumab beta may be superior to interferon beta on MS disability progression
(MS), according to research published in the December 2018 issue of the Multiple Sclerosis Journal. The benefits are observed in the overall patient population, as well as in subgroups of patients based on demographic and disease characteristics.
Biogen and AbbVie, the manufacturers of daclizumab beta, voluntarily removed the therapy from the market in March 2018 because of safety concerns that included reports of severe liver damage and conditions associated with the immune system.
The phase 3 DECIDE study (NCT01064401) compared the safety and efficacy of subcutaneous daclizumab beta (150 mg) every 4 weeks with those of intramuscular interferon beta-1a (30 mcg) once weekly in patients with relapsing-remitting MS. Daclizumab beta reduced the risk of 24-week confirmed disability progression as assessed by the Expanded Disability Status Scale (EDSS) by 27%, compared with interferon beta-1a. Daclizumab beta also was associated with a greater median change from baseline to week 96 in MS Functional Composite (MSFC) score and a 24% reduction in the risk of clinically meaningful worsening on the physical impact subscale of the patient-reported 29-Item MS Impact Scale (MSIS-29 PHYS).
To shed light on the treatment’s effects in various demographic groups and in patients with specific clinical characteristics, Stanley L. Cohan, MD, PhD, medical director of Providence MS Center in Portland, Ore., and colleagues conducted a post hoc analysis of DECIDE data to examine the treatment effects of daclizumab beta and interferon beta-1a on patient disability or impairment in specific patient subgroups. The investigators examined results according to demographic characteristics, such as age (that is, 35 years or younger and older than 35 years) and sex. They also examined results in subgroups with the following baseline disease characteristics: disability (as defined by EDSS score), relapses in the previous 12 months, disease duration, presence of gadolinium enhancing lesions, T2 hyperintense lesion volume, disease activity, prior use of disease-modifying treatment, and prior use of interferon beta.
Dr. Cohan and colleagues focused on the following three outcome measures: 24-week confirmed disability progression (as measured by EDSS), 24-week sustained worsening on the MSFC, and the proportion of patients with clinically meaningful worsening in MSIS-29 PHYS at week 96. The researchers defined 24-week confirmed disability progression as an increase in the EDSS score of one or more points from a baseline score of 1 or higher or 1.5 points or more from a baseline score of 0 as confirmed after 24 weeks. They defined 24-week sustained worsening on the MSFC as worsening of 20% or more on the Timed 25-Foot Walk, worsening of 20% or more on Nine-Hole Peg Test, or a decrease of four or more points on the Symbol Digit Modalities Test sustained for 24 weeks.
Of the 1,841 patients enrolled in DECIDE, 922 were randomized to interferon beta-1a, and 919 were randomized to daclizumab beta. The treatment groups were well balanced in terms of demographic characteristics. Patients’ mean age was approximately 36 years, 68% of participants were female, and 90% of patients were white. Mean time since diagnosis at baseline was about 4 years, mean number of relapses in the previous year was 1.6, and mean baseline EDSS score was 2.5.
Daclizumab beta was associated with a lower risk of 24-week confirmed disability progression, compared with interferon beta-1a, in all subgroups. Patients aged 35 years or younger had the greatest risk reduction.
The proportion of patients who had 24-week sustained worsening on the MSFC at week 96 was 24% for daclizumab beta and 28% for interferon beta-1a. In the whole study population, daclizumab beta reduced the risk of this outcome by 20%, compared with interferon beta-1a. Daclizumab beta resulted in improved outcomes among all subgroups, compared with interferon beta-1a.
In addition, daclizumab beta reduced the risk of a clinically meaningful worsening of MSIS-29 PHYS at week 96 by 24%, compared with interferon beta-1a. The investigators observed trends favoring daclizumab beta in all subgroups.
“These analyses should be interpreted as exploratory and hypothesis-generating for future studies,” said Dr. Cohan and colleagues. They observed that some of the subgroups analyzed had small sample sizes and that no adjustments were made for multiple testing. Nevertheless, the results suggest that daclizumab beta has superior efficacy, compared with interferon beta-1a, regardless of patients’ demographic and disease characteristics, they concluded.
Biogen and AbbVie Biotherapeutics supported the study.
SOURCE: Cohan S et al. Mult Scler J. 2018. doi: 10.1177/1352458517735190.
This article was updated on 3/22/19.
(MS), according to research published in the December 2018 issue of the Multiple Sclerosis Journal. The benefits are observed in the overall patient population, as well as in subgroups of patients based on demographic and disease characteristics.
Biogen and AbbVie, the manufacturers of daclizumab beta, voluntarily removed the therapy from the market in March 2018 because of safety concerns that included reports of severe liver damage and conditions associated with the immune system.
The phase 3 DECIDE study (NCT01064401) compared the safety and efficacy of subcutaneous daclizumab beta (150 mg) every 4 weeks with those of intramuscular interferon beta-1a (30 mcg) once weekly in patients with relapsing-remitting MS. Daclizumab beta reduced the risk of 24-week confirmed disability progression as assessed by the Expanded Disability Status Scale (EDSS) by 27%, compared with interferon beta-1a. Daclizumab beta also was associated with a greater median change from baseline to week 96 in MS Functional Composite (MSFC) score and a 24% reduction in the risk of clinically meaningful worsening on the physical impact subscale of the patient-reported 29-Item MS Impact Scale (MSIS-29 PHYS).
To shed light on the treatment’s effects in various demographic groups and in patients with specific clinical characteristics, Stanley L. Cohan, MD, PhD, medical director of Providence MS Center in Portland, Ore., and colleagues conducted a post hoc analysis of DECIDE data to examine the treatment effects of daclizumab beta and interferon beta-1a on patient disability or impairment in specific patient subgroups. The investigators examined results according to demographic characteristics, such as age (that is, 35 years or younger and older than 35 years) and sex. They also examined results in subgroups with the following baseline disease characteristics: disability (as defined by EDSS score), relapses in the previous 12 months, disease duration, presence of gadolinium enhancing lesions, T2 hyperintense lesion volume, disease activity, prior use of disease-modifying treatment, and prior use of interferon beta.
Dr. Cohan and colleagues focused on the following three outcome measures: 24-week confirmed disability progression (as measured by EDSS), 24-week sustained worsening on the MSFC, and the proportion of patients with clinically meaningful worsening in MSIS-29 PHYS at week 96. The researchers defined 24-week confirmed disability progression as an increase in the EDSS score of one or more points from a baseline score of 1 or higher or 1.5 points or more from a baseline score of 0 as confirmed after 24 weeks. They defined 24-week sustained worsening on the MSFC as worsening of 20% or more on the Timed 25-Foot Walk, worsening of 20% or more on Nine-Hole Peg Test, or a decrease of four or more points on the Symbol Digit Modalities Test sustained for 24 weeks.
Of the 1,841 patients enrolled in DECIDE, 922 were randomized to interferon beta-1a, and 919 were randomized to daclizumab beta. The treatment groups were well balanced in terms of demographic characteristics. Patients’ mean age was approximately 36 years, 68% of participants were female, and 90% of patients were white. Mean time since diagnosis at baseline was about 4 years, mean number of relapses in the previous year was 1.6, and mean baseline EDSS score was 2.5.
Daclizumab beta was associated with a lower risk of 24-week confirmed disability progression, compared with interferon beta-1a, in all subgroups. Patients aged 35 years or younger had the greatest risk reduction.
The proportion of patients who had 24-week sustained worsening on the MSFC at week 96 was 24% for daclizumab beta and 28% for interferon beta-1a. In the whole study population, daclizumab beta reduced the risk of this outcome by 20%, compared with interferon beta-1a. Daclizumab beta resulted in improved outcomes among all subgroups, compared with interferon beta-1a.
In addition, daclizumab beta reduced the risk of a clinically meaningful worsening of MSIS-29 PHYS at week 96 by 24%, compared with interferon beta-1a. The investigators observed trends favoring daclizumab beta in all subgroups.
“These analyses should be interpreted as exploratory and hypothesis-generating for future studies,” said Dr. Cohan and colleagues. They observed that some of the subgroups analyzed had small sample sizes and that no adjustments were made for multiple testing. Nevertheless, the results suggest that daclizumab beta has superior efficacy, compared with interferon beta-1a, regardless of patients’ demographic and disease characteristics, they concluded.
Biogen and AbbVie Biotherapeutics supported the study.
SOURCE: Cohan S et al. Mult Scler J. 2018. doi: 10.1177/1352458517735190.
This article was updated on 3/22/19.
(MS), according to research published in the December 2018 issue of the Multiple Sclerosis Journal. The benefits are observed in the overall patient population, as well as in subgroups of patients based on demographic and disease characteristics.
Biogen and AbbVie, the manufacturers of daclizumab beta, voluntarily removed the therapy from the market in March 2018 because of safety concerns that included reports of severe liver damage and conditions associated with the immune system.
The phase 3 DECIDE study (NCT01064401) compared the safety and efficacy of subcutaneous daclizumab beta (150 mg) every 4 weeks with those of intramuscular interferon beta-1a (30 mcg) once weekly in patients with relapsing-remitting MS. Daclizumab beta reduced the risk of 24-week confirmed disability progression as assessed by the Expanded Disability Status Scale (EDSS) by 27%, compared with interferon beta-1a. Daclizumab beta also was associated with a greater median change from baseline to week 96 in MS Functional Composite (MSFC) score and a 24% reduction in the risk of clinically meaningful worsening on the physical impact subscale of the patient-reported 29-Item MS Impact Scale (MSIS-29 PHYS).
To shed light on the treatment’s effects in various demographic groups and in patients with specific clinical characteristics, Stanley L. Cohan, MD, PhD, medical director of Providence MS Center in Portland, Ore., and colleagues conducted a post hoc analysis of DECIDE data to examine the treatment effects of daclizumab beta and interferon beta-1a on patient disability or impairment in specific patient subgroups. The investigators examined results according to demographic characteristics, such as age (that is, 35 years or younger and older than 35 years) and sex. They also examined results in subgroups with the following baseline disease characteristics: disability (as defined by EDSS score), relapses in the previous 12 months, disease duration, presence of gadolinium enhancing lesions, T2 hyperintense lesion volume, disease activity, prior use of disease-modifying treatment, and prior use of interferon beta.
Dr. Cohan and colleagues focused on the following three outcome measures: 24-week confirmed disability progression (as measured by EDSS), 24-week sustained worsening on the MSFC, and the proportion of patients with clinically meaningful worsening in MSIS-29 PHYS at week 96. The researchers defined 24-week confirmed disability progression as an increase in the EDSS score of one or more points from a baseline score of 1 or higher or 1.5 points or more from a baseline score of 0 as confirmed after 24 weeks. They defined 24-week sustained worsening on the MSFC as worsening of 20% or more on the Timed 25-Foot Walk, worsening of 20% or more on Nine-Hole Peg Test, or a decrease of four or more points on the Symbol Digit Modalities Test sustained for 24 weeks.
Of the 1,841 patients enrolled in DECIDE, 922 were randomized to interferon beta-1a, and 919 were randomized to daclizumab beta. The treatment groups were well balanced in terms of demographic characteristics. Patients’ mean age was approximately 36 years, 68% of participants were female, and 90% of patients were white. Mean time since diagnosis at baseline was about 4 years, mean number of relapses in the previous year was 1.6, and mean baseline EDSS score was 2.5.
Daclizumab beta was associated with a lower risk of 24-week confirmed disability progression, compared with interferon beta-1a, in all subgroups. Patients aged 35 years or younger had the greatest risk reduction.
The proportion of patients who had 24-week sustained worsening on the MSFC at week 96 was 24% for daclizumab beta and 28% for interferon beta-1a. In the whole study population, daclizumab beta reduced the risk of this outcome by 20%, compared with interferon beta-1a. Daclizumab beta resulted in improved outcomes among all subgroups, compared with interferon beta-1a.
In addition, daclizumab beta reduced the risk of a clinically meaningful worsening of MSIS-29 PHYS at week 96 by 24%, compared with interferon beta-1a. The investigators observed trends favoring daclizumab beta in all subgroups.
“These analyses should be interpreted as exploratory and hypothesis-generating for future studies,” said Dr. Cohan and colleagues. They observed that some of the subgroups analyzed had small sample sizes and that no adjustments were made for multiple testing. Nevertheless, the results suggest that daclizumab beta has superior efficacy, compared with interferon beta-1a, regardless of patients’ demographic and disease characteristics, they concluded.
Biogen and AbbVie Biotherapeutics supported the study.
SOURCE: Cohan S et al. Mult Scler J. 2018. doi: 10.1177/1352458517735190.
This article was updated on 3/22/19.
FROM MULTIPLE SCLEROSIS JOURNAL
Key clinical point: Daclizumab beta reduces the risk of 24-week sustained worsening on the MSFC by 20%, compared with interferon beta-1a.
Major finding: Daclizumab appears superior to interferon beta-1a regardless of patients’ demographic or disease characteristics.
Study details: A post hoc analysis of the DECIDE study, which included 1,841 patients with relapsing-remitting MS.
Disclosures: Biogen and AbbVie Biotherapeutics supported the DECIDE study.
Source: Cohan S et al. Mult Scler J. 2018. doi: 10.1177/1352458517735190.
Doc groups pushing back on Part B drug reimbursement proposal
The Centers for Medicare & Medicaid Services in October 2018 issued an “advance notice of proposed rulemaking with comment” outlining a test that would pay for Part B drugs with price points more closely aligned with international rates through the use of private sector vendors that would negotiate drug prices, procure the products, distribute them to physicians and hospitals, and take on the responsibility of billing Medicare.
Although the so-called International Pricing Index (IPI) model is not fully fleshed out in the regulatory filing, one of the key details that has been announced is that the demonstration project would have mandatory participation. This did not sit well with medical societies offering feedback to CMS.
The American College of Rheumatology stated in comments filed with the agency that “we do not support mandatory demonstration projects.”
The American Society of Clinical Oncology echoed that sentiment in Dec. 31, 2018, comments filed with the agency. “ASCO cannot support a mandatory demonstration program,” the group noted. “We are concerned about losing access points to oncology care provided by oncology practices, especially in rural, underserved, and low-income areas that are already struggling to deliver care.”
And while the Community Oncology Alliance also spoke against making participation in the IPI model demonstration project mandatory, it went further with its criticism of the proposal.
“COA does not support the IPI Model as proposed in the pre-proposed rule published by [CMS] because we have serious concerns about its impact on cancer patient care and even its legality,” the group said in Dec. 31, 2018, comments filed with the agency, adding that “mandatory demonstration projects are clearly not in the charter of CMMI [Center for Medicare & Medicaid Innovation] as written into law by Congress. If CMS believes that CMMI has the power via statute to effectively amend Medicare law – in this case the Part B drug reimbursement rate for at least 50% of Part B providers – it (CMS representing the Executive branch) either has overstepped its constitutional boundaries separating the powers of government branches or Congress has effectively handed over its powers to the Executive branch. That would either be illegal or unconstitutional, with the latter case invalidating the section of the law that created and funded CMMI.”
While none of the groups offered support to the IPI demonstration project, all offered suggestions on what could be done to improve on the details outlined in the advance notice of proposed rulemaking.
The American Academy of Dermatology Association, which like other groups took exception to CMS’ “insinuation” in its regulatory pre-proposal that physicians select treatments based on reimbursement ahead of patient need, suggested in Dec. 19, 2018, comments to the agency that drugs on the Food and Drug Administration’s drug shortage list be excluded from the list.
It also expressed concerns regarding access if a drug goes without international reference pricing because a manufacturer chooses not to sell in certain countries.
“AADA is concerned that this model could result in patients losing access to some drugs when a distributor and manufacturer are unable to agree on a price,” the group said. Similarly, the lag in setting a reference price after a new product is introduced could also create access issues.
AADA also took issue with the fact that vendors could not offer physicians and hospitals volume-based incentive payments or rebates but did not have the same prohibition from vendors receiving such incentives. “Under this proposal, CMS should prohibit vendors from prioritizing drug availability or excluding some drugs from distribution to physicians based on discounts provided by manufacturers. CMS will need to monitor utilization to ensure access to necessary treatments is not delayed or impeded.”
AADA also wants more clarity in how providers will be selected to participate.
The American College of Rheumatology expressed concern that “the administrative difficulties that would be associated with utilizing vendors could lead some practices to lose the ability to provide infusion services. Specifically, we are concerned that the added administrative burden of proposed interactions with the vendors in the model exceeds any inherent benefits to practice.” It added that CMS needs to look at how a potentially mandatory participation could affect specialty physician shortages.
The ACR made a number of recommendations, including making the IPI model participation voluntary; allowing for an exit for participants if the program is not working for them; providing incentives that could increase gross reimbursement; increasing provider reimbursement to cover the expenses associated with dealing with vendors; and making sure the agency is adequately tracking the effect on patient access.
ASCO used its comments to reiterate previous comments provided to the agency on revising the competitive acquisition program (CAP), a failed program that used third-party vendors as suppliers for Part B drugs. Among its suggestions were making the program voluntary; ensuring it does not result in an aggregate reduction in payments to oncology practices; ensuring a CAP does not result in interruption in care; and restricting its burdensome utilization management requirements.
The Community Oncology Alliance said it is working on an alternative to the IPI model, one that could contain a number of provisions, such as tiered average sales price-based reimbursement; clinically appropriate utilization management techniques; and drug prices that are lowered without artificial international price indexing.
The American Medical Association in Dec. 20, 2018, comments to the agency outlined a number of principles that any new vendor-based program needs to include, such as being voluntary; providing supplemental payments to cover the cost of special handling and storage of Part B drugs; flexibility to ensure various ordering issues; preventing variation in treatments for patients; and prohibiting vendors from restricting access using utilization management techniques.
The AMA also offered a range of suggestions for the IPI model, including measuring timeliness of deliveries in hours, not days; prohibiting vendors from withholding shipments of subsequent treatments if the initial claim has not been processed; making all treatment options available, even for off-label use; and getting guarantees from vendors on the availability of next-day delivery to any location where the patient is being treated.
Likewise, the AMA suggested that CMS should not set unreasonable deadlines for claims submissions and should provide an adequate number of vendors to ensure choice and access.
“We are also concerned about the impact of the proposed IPI model and its overall impact on costs to physician practices,” the AMA said in its comment letter. “The Administration proposes to allow the vendors to charge administrative fees to physician practices as part of their agreement to provide drug products to those practices included in the model. While we understand that third-party vendors must have a financial incentive in order to participate in the program, allowing vendors to charge physician practices administrative fees would add new, potentially significant increased costs to physicians in acquiring and providing treatments to patients without adequate changes to the reimbursement model to compensate for these costs.”
The AMA said that lower reimbursement combined with administrative fees would likely make the model untenable for physician practices unless changes to the reimbursement model were made.
The AMA also took issue with the focus on single-source drugs and biologics indexed with international pricing, which could create access issues.
“We urge CMS to undertake modeling and simulation of the impact if vendors are unable to obtain these drugs at the reimbursement amount,” the AMA stated in its comment letter. “There is a distinct possibility of immediate adverse patient impact if none of the vendors are able to secure needed clinical treatments.”
The Centers for Medicare & Medicaid Services in October 2018 issued an “advance notice of proposed rulemaking with comment” outlining a test that would pay for Part B drugs with price points more closely aligned with international rates through the use of private sector vendors that would negotiate drug prices, procure the products, distribute them to physicians and hospitals, and take on the responsibility of billing Medicare.
Although the so-called International Pricing Index (IPI) model is not fully fleshed out in the regulatory filing, one of the key details that has been announced is that the demonstration project would have mandatory participation. This did not sit well with medical societies offering feedback to CMS.
The American College of Rheumatology stated in comments filed with the agency that “we do not support mandatory demonstration projects.”
The American Society of Clinical Oncology echoed that sentiment in Dec. 31, 2018, comments filed with the agency. “ASCO cannot support a mandatory demonstration program,” the group noted. “We are concerned about losing access points to oncology care provided by oncology practices, especially in rural, underserved, and low-income areas that are already struggling to deliver care.”
And while the Community Oncology Alliance also spoke against making participation in the IPI model demonstration project mandatory, it went further with its criticism of the proposal.
“COA does not support the IPI Model as proposed in the pre-proposed rule published by [CMS] because we have serious concerns about its impact on cancer patient care and even its legality,” the group said in Dec. 31, 2018, comments filed with the agency, adding that “mandatory demonstration projects are clearly not in the charter of CMMI [Center for Medicare & Medicaid Innovation] as written into law by Congress. If CMS believes that CMMI has the power via statute to effectively amend Medicare law – in this case the Part B drug reimbursement rate for at least 50% of Part B providers – it (CMS representing the Executive branch) either has overstepped its constitutional boundaries separating the powers of government branches or Congress has effectively handed over its powers to the Executive branch. That would either be illegal or unconstitutional, with the latter case invalidating the section of the law that created and funded CMMI.”
While none of the groups offered support to the IPI demonstration project, all offered suggestions on what could be done to improve on the details outlined in the advance notice of proposed rulemaking.
The American Academy of Dermatology Association, which like other groups took exception to CMS’ “insinuation” in its regulatory pre-proposal that physicians select treatments based on reimbursement ahead of patient need, suggested in Dec. 19, 2018, comments to the agency that drugs on the Food and Drug Administration’s drug shortage list be excluded from the list.
It also expressed concerns regarding access if a drug goes without international reference pricing because a manufacturer chooses not to sell in certain countries.
“AADA is concerned that this model could result in patients losing access to some drugs when a distributor and manufacturer are unable to agree on a price,” the group said. Similarly, the lag in setting a reference price after a new product is introduced could also create access issues.
AADA also took issue with the fact that vendors could not offer physicians and hospitals volume-based incentive payments or rebates but did not have the same prohibition from vendors receiving such incentives. “Under this proposal, CMS should prohibit vendors from prioritizing drug availability or excluding some drugs from distribution to physicians based on discounts provided by manufacturers. CMS will need to monitor utilization to ensure access to necessary treatments is not delayed or impeded.”
AADA also wants more clarity in how providers will be selected to participate.
The American College of Rheumatology expressed concern that “the administrative difficulties that would be associated with utilizing vendors could lead some practices to lose the ability to provide infusion services. Specifically, we are concerned that the added administrative burden of proposed interactions with the vendors in the model exceeds any inherent benefits to practice.” It added that CMS needs to look at how a potentially mandatory participation could affect specialty physician shortages.
The ACR made a number of recommendations, including making the IPI model participation voluntary; allowing for an exit for participants if the program is not working for them; providing incentives that could increase gross reimbursement; increasing provider reimbursement to cover the expenses associated with dealing with vendors; and making sure the agency is adequately tracking the effect on patient access.
ASCO used its comments to reiterate previous comments provided to the agency on revising the competitive acquisition program (CAP), a failed program that used third-party vendors as suppliers for Part B drugs. Among its suggestions were making the program voluntary; ensuring it does not result in an aggregate reduction in payments to oncology practices; ensuring a CAP does not result in interruption in care; and restricting its burdensome utilization management requirements.
The Community Oncology Alliance said it is working on an alternative to the IPI model, one that could contain a number of provisions, such as tiered average sales price-based reimbursement; clinically appropriate utilization management techniques; and drug prices that are lowered without artificial international price indexing.
The American Medical Association in Dec. 20, 2018, comments to the agency outlined a number of principles that any new vendor-based program needs to include, such as being voluntary; providing supplemental payments to cover the cost of special handling and storage of Part B drugs; flexibility to ensure various ordering issues; preventing variation in treatments for patients; and prohibiting vendors from restricting access using utilization management techniques.
The AMA also offered a range of suggestions for the IPI model, including measuring timeliness of deliveries in hours, not days; prohibiting vendors from withholding shipments of subsequent treatments if the initial claim has not been processed; making all treatment options available, even for off-label use; and getting guarantees from vendors on the availability of next-day delivery to any location where the patient is being treated.
Likewise, the AMA suggested that CMS should not set unreasonable deadlines for claims submissions and should provide an adequate number of vendors to ensure choice and access.
“We are also concerned about the impact of the proposed IPI model and its overall impact on costs to physician practices,” the AMA said in its comment letter. “The Administration proposes to allow the vendors to charge administrative fees to physician practices as part of their agreement to provide drug products to those practices included in the model. While we understand that third-party vendors must have a financial incentive in order to participate in the program, allowing vendors to charge physician practices administrative fees would add new, potentially significant increased costs to physicians in acquiring and providing treatments to patients without adequate changes to the reimbursement model to compensate for these costs.”
The AMA said that lower reimbursement combined with administrative fees would likely make the model untenable for physician practices unless changes to the reimbursement model were made.
The AMA also took issue with the focus on single-source drugs and biologics indexed with international pricing, which could create access issues.
“We urge CMS to undertake modeling and simulation of the impact if vendors are unable to obtain these drugs at the reimbursement amount,” the AMA stated in its comment letter. “There is a distinct possibility of immediate adverse patient impact if none of the vendors are able to secure needed clinical treatments.”
The Centers for Medicare & Medicaid Services in October 2018 issued an “advance notice of proposed rulemaking with comment” outlining a test that would pay for Part B drugs with price points more closely aligned with international rates through the use of private sector vendors that would negotiate drug prices, procure the products, distribute them to physicians and hospitals, and take on the responsibility of billing Medicare.
Although the so-called International Pricing Index (IPI) model is not fully fleshed out in the regulatory filing, one of the key details that has been announced is that the demonstration project would have mandatory participation. This did not sit well with medical societies offering feedback to CMS.
The American College of Rheumatology stated in comments filed with the agency that “we do not support mandatory demonstration projects.”
The American Society of Clinical Oncology echoed that sentiment in Dec. 31, 2018, comments filed with the agency. “ASCO cannot support a mandatory demonstration program,” the group noted. “We are concerned about losing access points to oncology care provided by oncology practices, especially in rural, underserved, and low-income areas that are already struggling to deliver care.”
And while the Community Oncology Alliance also spoke against making participation in the IPI model demonstration project mandatory, it went further with its criticism of the proposal.
“COA does not support the IPI Model as proposed in the pre-proposed rule published by [CMS] because we have serious concerns about its impact on cancer patient care and even its legality,” the group said in Dec. 31, 2018, comments filed with the agency, adding that “mandatory demonstration projects are clearly not in the charter of CMMI [Center for Medicare & Medicaid Innovation] as written into law by Congress. If CMS believes that CMMI has the power via statute to effectively amend Medicare law – in this case the Part B drug reimbursement rate for at least 50% of Part B providers – it (CMS representing the Executive branch) either has overstepped its constitutional boundaries separating the powers of government branches or Congress has effectively handed over its powers to the Executive branch. That would either be illegal or unconstitutional, with the latter case invalidating the section of the law that created and funded CMMI.”
While none of the groups offered support to the IPI demonstration project, all offered suggestions on what could be done to improve on the details outlined in the advance notice of proposed rulemaking.
The American Academy of Dermatology Association, which like other groups took exception to CMS’ “insinuation” in its regulatory pre-proposal that physicians select treatments based on reimbursement ahead of patient need, suggested in Dec. 19, 2018, comments to the agency that drugs on the Food and Drug Administration’s drug shortage list be excluded from the list.
It also expressed concerns regarding access if a drug goes without international reference pricing because a manufacturer chooses not to sell in certain countries.
“AADA is concerned that this model could result in patients losing access to some drugs when a distributor and manufacturer are unable to agree on a price,” the group said. Similarly, the lag in setting a reference price after a new product is introduced could also create access issues.
AADA also took issue with the fact that vendors could not offer physicians and hospitals volume-based incentive payments or rebates but did not have the same prohibition from vendors receiving such incentives. “Under this proposal, CMS should prohibit vendors from prioritizing drug availability or excluding some drugs from distribution to physicians based on discounts provided by manufacturers. CMS will need to monitor utilization to ensure access to necessary treatments is not delayed or impeded.”
AADA also wants more clarity in how providers will be selected to participate.
The American College of Rheumatology expressed concern that “the administrative difficulties that would be associated with utilizing vendors could lead some practices to lose the ability to provide infusion services. Specifically, we are concerned that the added administrative burden of proposed interactions with the vendors in the model exceeds any inherent benefits to practice.” It added that CMS needs to look at how a potentially mandatory participation could affect specialty physician shortages.
The ACR made a number of recommendations, including making the IPI model participation voluntary; allowing for an exit for participants if the program is not working for them; providing incentives that could increase gross reimbursement; increasing provider reimbursement to cover the expenses associated with dealing with vendors; and making sure the agency is adequately tracking the effect on patient access.
ASCO used its comments to reiterate previous comments provided to the agency on revising the competitive acquisition program (CAP), a failed program that used third-party vendors as suppliers for Part B drugs. Among its suggestions were making the program voluntary; ensuring it does not result in an aggregate reduction in payments to oncology practices; ensuring a CAP does not result in interruption in care; and restricting its burdensome utilization management requirements.
The Community Oncology Alliance said it is working on an alternative to the IPI model, one that could contain a number of provisions, such as tiered average sales price-based reimbursement; clinically appropriate utilization management techniques; and drug prices that are lowered without artificial international price indexing.
The American Medical Association in Dec. 20, 2018, comments to the agency outlined a number of principles that any new vendor-based program needs to include, such as being voluntary; providing supplemental payments to cover the cost of special handling and storage of Part B drugs; flexibility to ensure various ordering issues; preventing variation in treatments for patients; and prohibiting vendors from restricting access using utilization management techniques.
The AMA also offered a range of suggestions for the IPI model, including measuring timeliness of deliveries in hours, not days; prohibiting vendors from withholding shipments of subsequent treatments if the initial claim has not been processed; making all treatment options available, even for off-label use; and getting guarantees from vendors on the availability of next-day delivery to any location where the patient is being treated.
Likewise, the AMA suggested that CMS should not set unreasonable deadlines for claims submissions and should provide an adequate number of vendors to ensure choice and access.
“We are also concerned about the impact of the proposed IPI model and its overall impact on costs to physician practices,” the AMA said in its comment letter. “The Administration proposes to allow the vendors to charge administrative fees to physician practices as part of their agreement to provide drug products to those practices included in the model. While we understand that third-party vendors must have a financial incentive in order to participate in the program, allowing vendors to charge physician practices administrative fees would add new, potentially significant increased costs to physicians in acquiring and providing treatments to patients without adequate changes to the reimbursement model to compensate for these costs.”
The AMA said that lower reimbursement combined with administrative fees would likely make the model untenable for physician practices unless changes to the reimbursement model were made.
The AMA also took issue with the focus on single-source drugs and biologics indexed with international pricing, which could create access issues.
“We urge CMS to undertake modeling and simulation of the impact if vendors are unable to obtain these drugs at the reimbursement amount,” the AMA stated in its comment letter. “There is a distinct possibility of immediate adverse patient impact if none of the vendors are able to secure needed clinical treatments.”
Idiopathic Granulomatous Mastitis
Idiopathic granulomatous mastitis (IGM) is rare during pregnancy; it typically is seen in women of childbearing potential from 6 months to 6 years postpartum.1 Because of a temporal association with breastfeeding, it is believed that hyperprolactinemia2 or an immune response to local lobular secretions might play a role in pathogenesis. Early misdiagnosis as bacterial mastitis is common, prompting multiple antibiotic regimens. When antibiotics fail, patients are worked up for inflammatory breast cancer, given the nonhealing breast nodules. Mammography, ultrasonography, and fine-needle aspiration often are unable to rule out carcinoma, warranting excisional biopsies of nodules. The patient is then referred to rheumatology for potential sarcoidosis or to dermatology for IGM. In either case, the workup should be similar, but additional history focused on behavior and medications is essential in suspected IGM, given the association with hyperprolactinemia.
Because IGM is rare, there are no randomized, placebo-controlled trials of treatment efficacy. In many cases, patients undergo complete mastectomy, which is curative but may be psychologically and physically impactful in young women. In some cases, high-dose corticosteroids have been successful; however, because the IGM process can last longer than 2 years, patients treated in this manner are exposed to steroid morbidities.1
We report 3 cases of IGM that add to the literature on possible contributing factors, clinical presentations, and treatments for this disease. We also demonstrate that appropriate trigger identification and steroid-sparing agents, specifically methotrexate, can be breast-saving as they can alleviate this debilitating condition, obviating the need for radical surgical intervention.
CASE REPORTS
Patient 1
A 40-year-old woman with a 4-year history of breastfeeding noted a grape-sized nodule on the left breast that grew to the size of a grapefruit after 2 weeks. Ulceration and drainage periodically occurred, forming pink plaques along the lateral aspects of the breast after healing. Her primary care provider suspected infectious mastitis; she was given an oral antibiotic (cephalexin) and intravenous antibiotics without improvement.
Imaging
Subsequent magnetic resonance imaging revealed a large, irregular, enhancing mass within the outer left breast (6.5 cm at greatest dimension) with additional surrounding amorphous enhancement highly suspicious for malignancy. There also were multiple prominent left axillary lymph nodes, with the largest demonstrating a cortical thickness of 8 mm.
Biopsy
Core breast biopsy showed benign tissue with fat necrosis. Fine-needle aspiration revealed few benign ductal cells and rare histiocytes; because these findings were nondiagnostic and cancer was still a consideration, the patient underwent excisional biopsy.
Histologic sections of breast tissue showed extensive lobulocentric inflammation comprising histiocytes and lymphocytes, with neutrophils admixed and forming microabscesses (Figure 1A). Multinucleated giant cells and single-cell necrosis were seen, but true caseous necrosis was absent (Figure 1B). Duct spaces often contained inflammatory cells or secretions. Special stains for fungal and acid-fast bacterial microorganisms were negative.
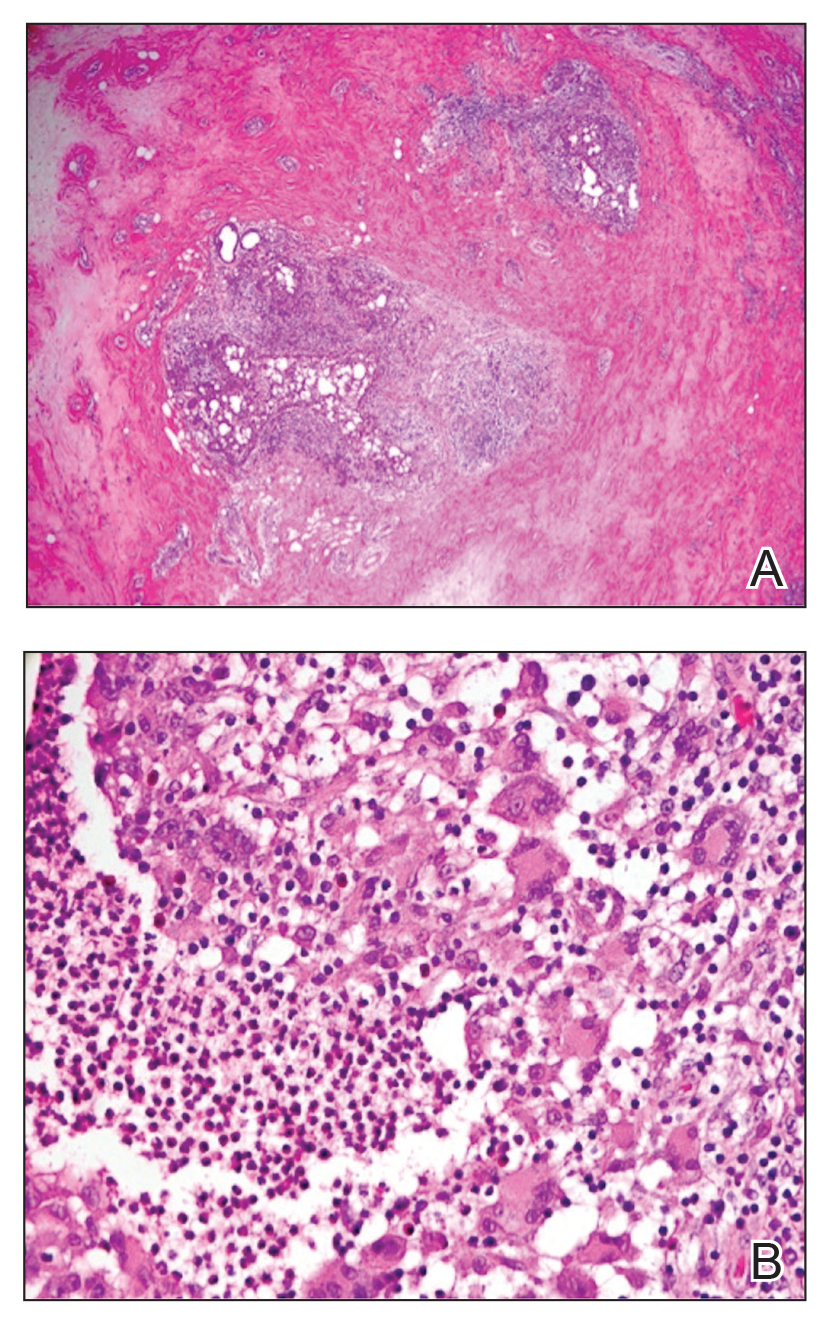
Referral to Dermatology
Granulomatous lobular mastitis was diagnosed, and the patient was referred to dermatology. On presentation to dermatology, the left breast showed a 6-cm area of firm induration and overlying peau d’orange change to the epidermis (Figure 2A). Based on pathologic analysis, she was worked up for a possible granulomatous etiology. Negative purified protein derivative (tuberculin)(PPD) and a normal chest radiograph ruled out tuberculosis. Normal chest radiography, serum Ca2+ and angiotensin-converting enzyme (ACE) levels, and ophthalmology examination ruled out sarcoidosis.
The patient reported she continued breastfeeding her 4-year-old son. Additionally, she had been started on trazadone and buspirone for alcohol abuse recovery, then switched to and maintained on fluoxetine 1 year before developing these symptoms.
Buspirone, fluoxetine, and prolonged breastfeeding all contribute to hyperprolactinemia, a possible trigger of IGM. The patient was therefore advised to stop breastfeeding and to be switched from fluoxetine to a medication that would not increase the prolactin level. She did not require methotrexate treatment because her condition resolved rapidly after breastfeeding and fluoxetine were discontinued (Figure 2B).
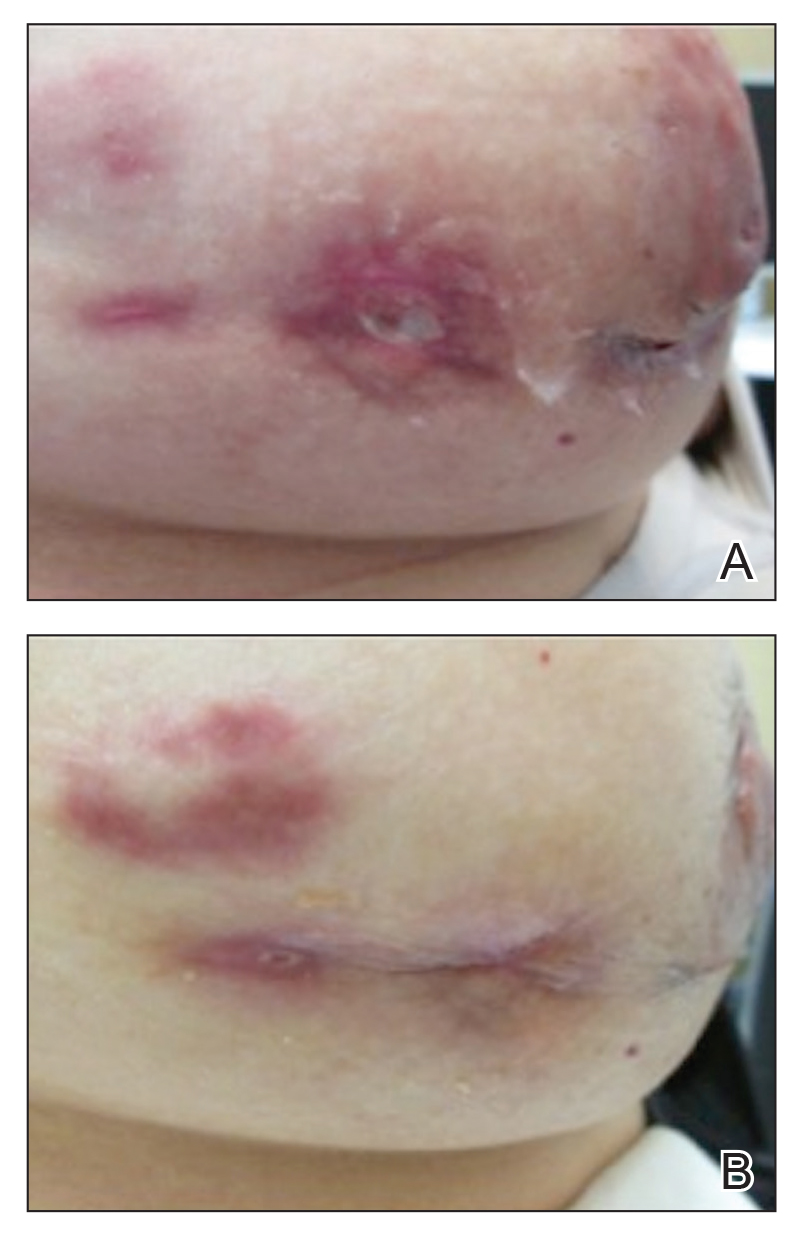
B, Resolution after discontinuation of breastfeeding and fluoxetine.
Patient 2
A 40-year-old woman with no history of breastfeeding who gave birth 4.5 years prior presented to her primary care provider with a painful breast lump and rash on the right breast of 2 months’ duration. Infectious mastitis was suspected; she was given cephalexin and clindamycin without improvement of symptoms.
Imaging
Mammography and ultrasonography were nondiagnostic.
Biopsy
Breast biopsy showed tissue with large expanses of histiocytes, neutrophils, lymphocytes, plasma cells, and multinucleated giant cells (Figure 3A). Many discrete granulomas were seen against this mixed inflammatory background, associated with focal fat necrosis (Figure 3B). Special stains were negative for microorganisms. Histologic findings were consistent with granulomatous mastitis.
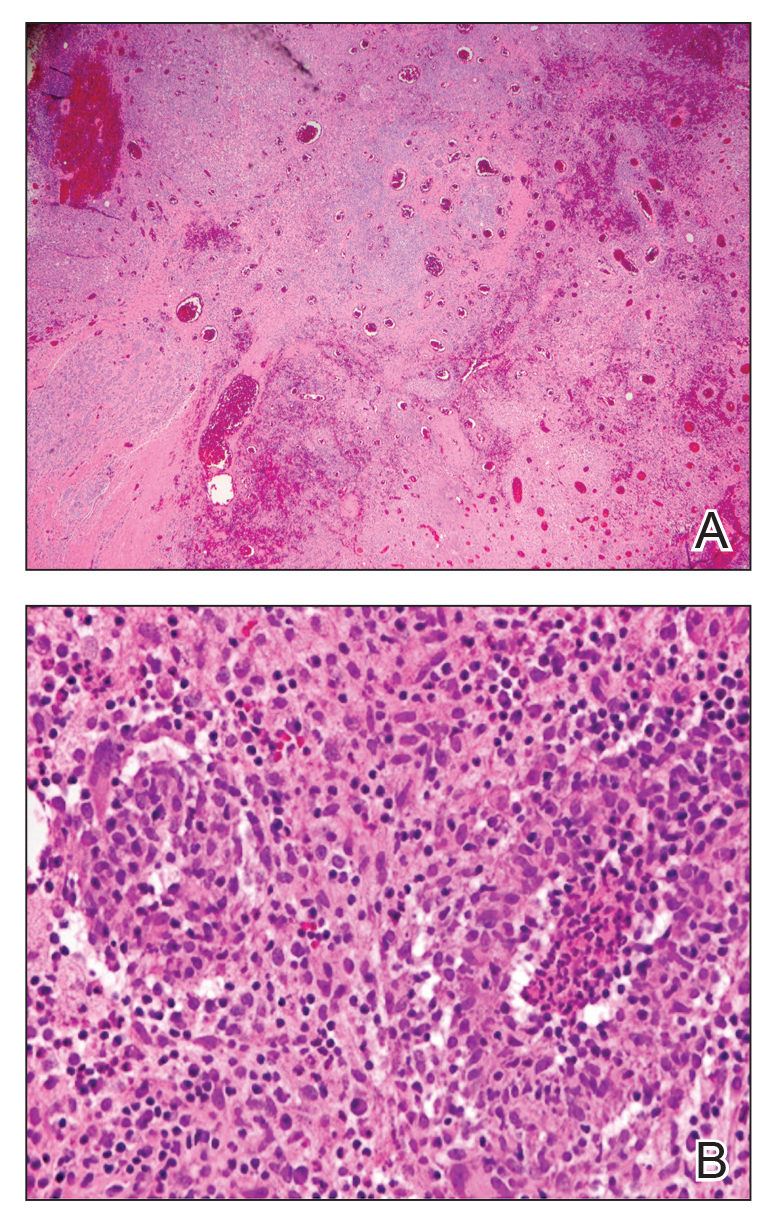
Referral to Dermatology
On presentation to dermatology, the patient was worked up for a possible granulomatous etiology, which included a negative PPD, as well as a normal chest radiograph, serum Ca2+ and ACE levels, and ophthalmology examination. Review of symptoms (ROS),medical history, and medication review were unremarkable.
By exclusion, the patient was given a diagnosis of IGM and started on methotrexate (15 mg weekly) with folic acid (1 mg daily). The condition of the right breast improved within 4 weeks of starting methotrexate; however, methotrexate was increased to 30 mg weekly because of occasional flares. The patient remained on methotrexate without further IGM flares for 8 months compared to prior unremitting pain and drainage. She was then tapered from methotrexate over 6 weeks without additional flares.
Patient 3
A 27-year-old woman who gave birth 2 years prior and discontinued breastfeeding 6 weeks after delivery noted bilateral breast rashes for several months. The lesions were growing in size, tender, and draining. Her primary care provider suspected infectious mastitis and prescribed antibiotics, which were ineffective.
Biopsy
Breast core biopsy showed histologic findings similar to patients 1 and 2, including lobulocentric mixed inflammation, neutrophilic microabscesses, and scattered discrete granulomas. Microorganisms were not found using special stains. Breast cancer was ruled out, and granulomatous mastitis was diagnosed.
Referral to Dermatology
Two years earlier, the patient tested positive for latent tuberculosis and was prescribed a 9-month regimen of isoniazid. At the current presentation, she did not have symptoms of active tuberculosis on ROS (ie, no cough, hemoptysis, weight loss, night sweats); a chest radiograph was normal. Additionally, serum Ca2+ and ACE levels as well as an ophthalmology examination were normal, and she was not taking any medications known to increase the prolactin level.
The patient was started on methotrexate (12.5 mg weekly) and folic acid (1 mg daily). She had 1 IGM flare and was given a tapering regimen of prednisone. She received methotrexate for 14 months, tapered during the final 3 months. She has been off methotrexate for 3 years without IGM flares and appears to be in complete remission.
COMMENT
We report 3 cases of IGM, which contribute to the literature on possible presentations, causes, and conservative treatment of this rare connective-tissue disorder.
Differential Diagnosis
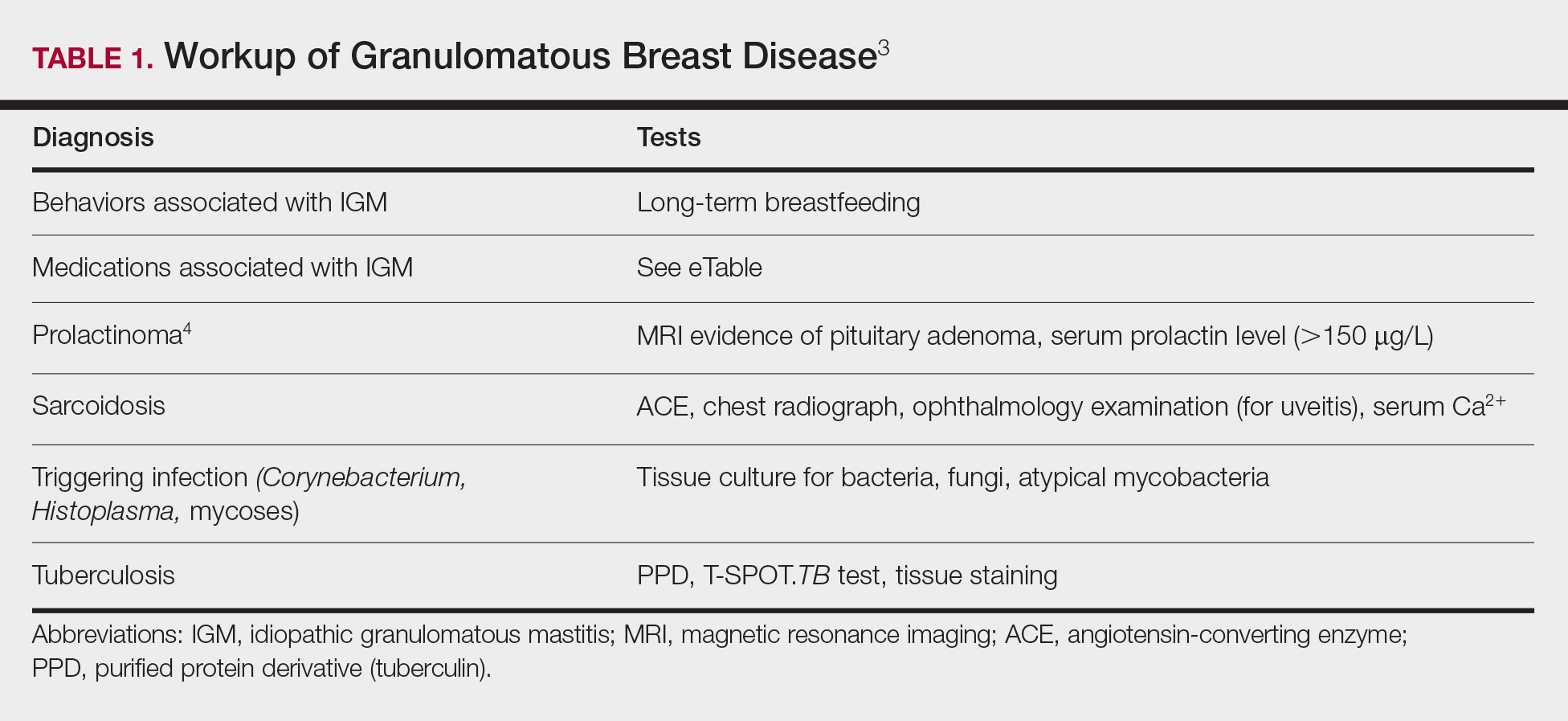
The time between recognition of symptoms and diagnosis and treatment of IGM often is prolonged because IGM can present similarly to other disorders, such as infection, breast cancer, tuberculosis, and sarcoidosis. Idiopathic granulomatous mastitis is a diagnosis of exclusion, made after obtaining evidence of granulomatous inflammation on breast biopsy and ruling out other granulomatous disorders, such as tuberculosis and sarcoidosis (Table 1).3,4
Tuberculosis
A full ROS and a PPD test or T-SPOT.TB test can be helpful in ruling out tuberculosis; because anergy occurs in some patients, tuberculosis should be evaluated in the context of known immunosuppression or human immunodeficiency virus status, or in the case of miliary tuberculosis.
Chest radiography findings classically showing upper lobe infiltrates with cavities in active tuberculosis also should be sought.3 Ziehl-Neelsen staining of 2 sputum specimens, assessed by conventional light microscopy at the time of tissue biopsy has 64% sensitivity and 98% specificity for detecting Mycobacterium tuberculosis; auramine O staining, examined with light-emitting diode fluorescence microscopy, has 73% sensitivity and 93% specificity.5
Sarcoidosis
Because more than 90% of sarcoid patients have lung disease, a chest radiograph is used to screen for hilar lymphadenopathy.3 An elevated serum ACE level also can be helpful in diagnosis, but patients do not always have increased ACE, which can occur in other diseases, such as hyperthyroidism and miliary tuberculosis. Sarcoid granulomas can increase active vitamin D production, which in turn increases serum Ca2+ in 10% of sarcoid patients. Last, an ophthalmology evaluation should be obtained to rule out anterior or posterior uveitis that can occur in sarcoidosis and initially remain asymptomatic.3 Once these other causes of granulomatous inflammation have been ruled out, a diagnosis of IGM can be made.
Prolactinoma
Prolactinoma is an important cause of hyperprolactinemia that can be screened for based on ROS and the serum prolactin level. Prolactinoma can cause oligomenorrhea or amenorrhea and galactorrhea in 90% and 80% of premenopausal women, respectively, as well as erectile dysfunction and decreased libido in men. Infertility, headache, and visual impairment may be experienced in both sexes.4
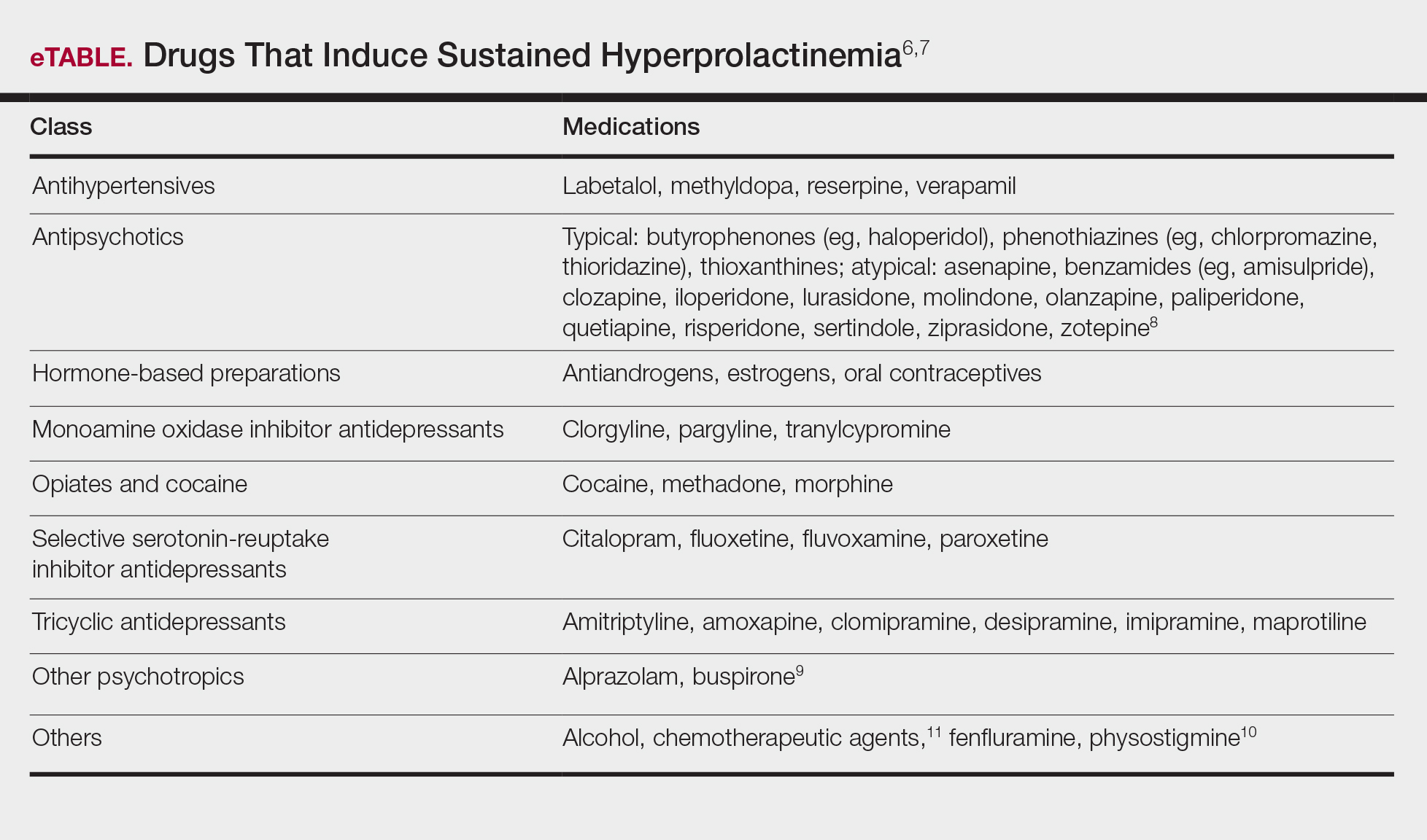
A normal prolactin level is less than 25 μg/L; more than 25 μg/L but less than 100 μg/L usually is due to certain drugs (eTable),6-11 estrogen, or idiopathic reasons; and more than 150 μg/L usually is due to prolactinoma.5 In many cases, removal of hyperprolactinemia-precipitating factors can resolve disease, as in patient 1. If symptoms continue or precipitating factors are absent, IGM symptom-based treatment should be administered.
Course and Management
Idiopathic granulomatous mastitis is self-limited and usually resolves within 2 years. Therefore, the goal of treatment is to suppress associated pain and drainage until the active inflammatory phase of IGM self-resolves. An established protocol for treating IGM does not exist, but common treatments include corticosteroids, methotrexate, and limited or wide surgical excision (Table 2).12-16 Before beginning any of these treatments, IGM triggers, such as breastfeeding and drugs that induce hyperprolactinemia, should be removed.
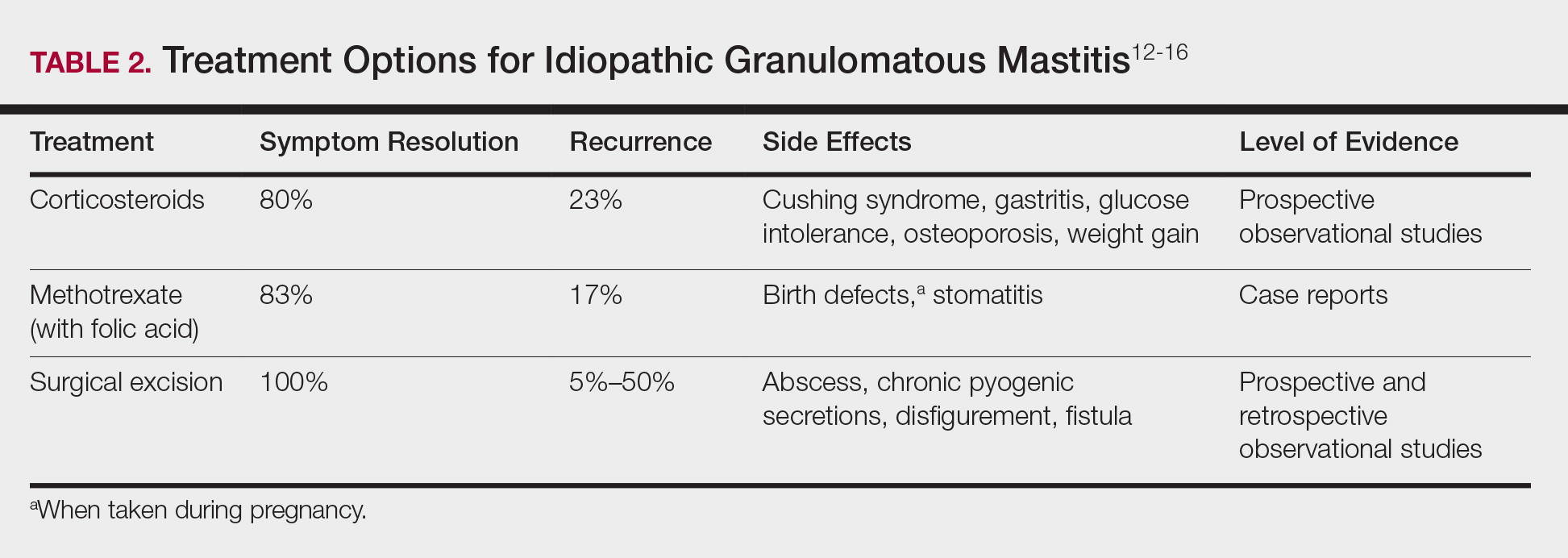
It is important to consider which treatment option is best for limiting disease recurrence and adverse effects (AEs). Keep in mind that the available data are limited, as there are no randomized controlled trials looking at these treatments. Nevertheless, we recommend methotrexate as first line because it resolves granulomatous inflammation symptoms without invasive surgery, while limiting corticosteroid AEs.12
With or without concurrent use of corticosteroids, surgical excision typically is the mainstay of treatment. However, surgical excision of IGM lesions can be complicated by abscess formation, fistula, and chronic pyogenic secretions, in addition to a 5% to 50% rate of recurrence of disease.12-14 Limited excision often is insufficient; therefore, wide local excision, in which negative margins around granulomatous inflammation are obtained, is the surgical modality of choice.14 Wide local excision can be disfiguring to the breast in young women affected by IGM, making it an undesirable treatment option.
Corticosteroids often have been used to treat IGM, but their efficacy is variable, symptoms can recur upon drug removal, and remarkable AEs can result from long-term use.12 Additionally, corticosteroid therapy often is used in combination with excision, making it difficult to determine the extent to which corticosteroids or excision are more beneficial. In a prospective observational study, corticosteroid therapy alone resolved 80% of IGM symptoms after 159 days on average. After complete symptom resolution, 23% of patients had disease recurrence.9 Observed AEs included gastritis, weight gain, osteoporosis, glucose intolerance, and Cushing syndrome.12,15
Methotrexate for IGM has not been reviewed in a randomized controlled trial; case reports have shown 83% symptom resolution, with 17% recurrence and limited long-term AEs.12 Because the active phase of IGM can persist for 2 years, immunosuppressive therapy with limited AEs is necessary. Many AEs can occur when high-dose methotrexate is given for cancer treatment. Low-dose methotrexate has been extensively studied in long-term treatment of rheumatoid arthritis. Adverse effects may include gastrointestinal tract upset and hepatic dysfunction, which are limited when given with folic acid.
Regardless of folic acid cotreatment, stomatitis may occur. Women should use an effective method of birth control because severe birth defects may occur on even low-dose methotrexate.16
Compared to corticosteroid or surgical treatment, we recommend low-dose methotrexate therapy based on its high efficacy with limited AEs. An occasional mild flare of IGM symptoms with methotrexate is not unusual. If it occurs, corticosteroids can be added and tapered for as long as 2 weeks to speed up resolution of flares while reducing long-term AEs of corticosteroids.
Surgical excision can be performed in cases refractory to all systemic therapies.
CONCLUSION
Idiopathic granulomatous mastitis is a rare granulomatous breast disorder that can have a prolonged time to diagnosis, delaying proper treatment. Many cases self-resolve, but more severe cases can persist for a long period before adequate symptomatic treatment is achieved by methotrexate, corticosteroids, or surgical excision. Before using these therapies, it is important to identify and remove contributing factors, such as long-term breastfeeding and drugs that induce hyperprolactinemia. Improving the rate of IGM diagnosis and treatment would greatly benefit these patients. We report 1 case in which removal of possible precipitating IGM factors led to symptom resolution and 2 cases in which methotrexate was an effective IGM treatment that limited the need for invasive procedures and corticosteroid AEs.
1. Patel RA, Strickland P, Sankara IR, et al. Idiopathic granulomatous mastitis: case reports and review of literature. J Gen Intern Med. 2010;25:270-273.
2. Bellavia M, Damiano G, Palumbo VD, et al. Granulomatous mastitis during chronic antidepressant therapy: is it possible a conservative therapeutic approach? J Breast Cancer. 2012;15:371-372.
3. Longo D, Fauci A, Kasper D, et al. Harrison’s Principles of Internal Medicine. 18th ed. New York, NY: McGraw-Hill; 2012.
4. Davis JL, Cattamanchi A, Cuevas LE, et al. Diagnostic accuracy of same-day microscopy versus standard microscopy for pulmonary tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2013;13:147-154.
5. Casanueva FF, Molitch ME, Schlechte JA, et al. Guidelines of the Pituitary Society for the diagnosis and management of prolactinomas. Clin Endocrinol (Oxf). 2006;65:265-273.
6. Akbulut S, Arikanoglu Z, Senol A, et al. Is methotrexate an acceptable treatment in the management of idiopathic granulomatous mastitis? Arch Gynecol Obstet. 2011;284:1189-1195.
7. Bani-Hani KE, Yaghan RJ, Matalka II, et al. Idiopathic granulomatous mastitis: time to avoid unnecessary mastectomies. Breast J. 2004;10:318-322.
8. Asoglu O, Ozmen V, Karanlik H, et al. Feasibility of surgical management in patients with granulomatous mastitis. Breast J. 2005;11:108-114.
9. Pandey TS, Mackinnon JC, Bressler L, et al. Idiopathic granulomatous mastitis—a prospective study of 49 women and treatment outcomes with steroid therapy. Breast J. 2014;20:258-266.
10. Shea B, Swinden MV, Tanjong Ghogomu E, et al. Folic acid and folinic acid for reducing side effects in patients receiving methotrexate for rheumatoid arthritis. Cochrane Database Syst Rev. 2013;5:CD000951.
11. Molitch ME. Drugs and prolactin. Pituitary. 2008;11:209-218.
12.
13.
14.
15.
16.
Idiopathic granulomatous mastitis (IGM) is rare during pregnancy; it typically is seen in women of childbearing potential from 6 months to 6 years postpartum.1 Because of a temporal association with breastfeeding, it is believed that hyperprolactinemia2 or an immune response to local lobular secretions might play a role in pathogenesis. Early misdiagnosis as bacterial mastitis is common, prompting multiple antibiotic regimens. When antibiotics fail, patients are worked up for inflammatory breast cancer, given the nonhealing breast nodules. Mammography, ultrasonography, and fine-needle aspiration often are unable to rule out carcinoma, warranting excisional biopsies of nodules. The patient is then referred to rheumatology for potential sarcoidosis or to dermatology for IGM. In either case, the workup should be similar, but additional history focused on behavior and medications is essential in suspected IGM, given the association with hyperprolactinemia.
Because IGM is rare, there are no randomized, placebo-controlled trials of treatment efficacy. In many cases, patients undergo complete mastectomy, which is curative but may be psychologically and physically impactful in young women. In some cases, high-dose corticosteroids have been successful; however, because the IGM process can last longer than 2 years, patients treated in this manner are exposed to steroid morbidities.1
We report 3 cases of IGM that add to the literature on possible contributing factors, clinical presentations, and treatments for this disease. We also demonstrate that appropriate trigger identification and steroid-sparing agents, specifically methotrexate, can be breast-saving as they can alleviate this debilitating condition, obviating the need for radical surgical intervention.
CASE REPORTS
Patient 1
A 40-year-old woman with a 4-year history of breastfeeding noted a grape-sized nodule on the left breast that grew to the size of a grapefruit after 2 weeks. Ulceration and drainage periodically occurred, forming pink plaques along the lateral aspects of the breast after healing. Her primary care provider suspected infectious mastitis; she was given an oral antibiotic (cephalexin) and intravenous antibiotics without improvement.
Imaging
Subsequent magnetic resonance imaging revealed a large, irregular, enhancing mass within the outer left breast (6.5 cm at greatest dimension) with additional surrounding amorphous enhancement highly suspicious for malignancy. There also were multiple prominent left axillary lymph nodes, with the largest demonstrating a cortical thickness of 8 mm.
Biopsy
Core breast biopsy showed benign tissue with fat necrosis. Fine-needle aspiration revealed few benign ductal cells and rare histiocytes; because these findings were nondiagnostic and cancer was still a consideration, the patient underwent excisional biopsy.
Histologic sections of breast tissue showed extensive lobulocentric inflammation comprising histiocytes and lymphocytes, with neutrophils admixed and forming microabscesses (Figure 1A). Multinucleated giant cells and single-cell necrosis were seen, but true caseous necrosis was absent (Figure 1B). Duct spaces often contained inflammatory cells or secretions. Special stains for fungal and acid-fast bacterial microorganisms were negative.

Referral to Dermatology
Granulomatous lobular mastitis was diagnosed, and the patient was referred to dermatology. On presentation to dermatology, the left breast showed a 6-cm area of firm induration and overlying peau d’orange change to the epidermis (Figure 2A). Based on pathologic analysis, she was worked up for a possible granulomatous etiology. Negative purified protein derivative (tuberculin)(PPD) and a normal chest radiograph ruled out tuberculosis. Normal chest radiography, serum Ca2+ and angiotensin-converting enzyme (ACE) levels, and ophthalmology examination ruled out sarcoidosis.
The patient reported she continued breastfeeding her 4-year-old son. Additionally, she had been started on trazadone and buspirone for alcohol abuse recovery, then switched to and maintained on fluoxetine 1 year before developing these symptoms.
Buspirone, fluoxetine, and prolonged breastfeeding all contribute to hyperprolactinemia, a possible trigger of IGM. The patient was therefore advised to stop breastfeeding and to be switched from fluoxetine to a medication that would not increase the prolactin level. She did not require methotrexate treatment because her condition resolved rapidly after breastfeeding and fluoxetine were discontinued (Figure 2B).

B, Resolution after discontinuation of breastfeeding and fluoxetine.
Patient 2
A 40-year-old woman with no history of breastfeeding who gave birth 4.5 years prior presented to her primary care provider with a painful breast lump and rash on the right breast of 2 months’ duration. Infectious mastitis was suspected; she was given cephalexin and clindamycin without improvement of symptoms.
Imaging
Mammography and ultrasonography were nondiagnostic.
Biopsy
Breast biopsy showed tissue with large expanses of histiocytes, neutrophils, lymphocytes, plasma cells, and multinucleated giant cells (Figure 3A). Many discrete granulomas were seen against this mixed inflammatory background, associated with focal fat necrosis (Figure 3B). Special stains were negative for microorganisms. Histologic findings were consistent with granulomatous mastitis.

Referral to Dermatology
On presentation to dermatology, the patient was worked up for a possible granulomatous etiology, which included a negative PPD, as well as a normal chest radiograph, serum Ca2+ and ACE levels, and ophthalmology examination. Review of symptoms (ROS),medical history, and medication review were unremarkable.
By exclusion, the patient was given a diagnosis of IGM and started on methotrexate (15 mg weekly) with folic acid (1 mg daily). The condition of the right breast improved within 4 weeks of starting methotrexate; however, methotrexate was increased to 30 mg weekly because of occasional flares. The patient remained on methotrexate without further IGM flares for 8 months compared to prior unremitting pain and drainage. She was then tapered from methotrexate over 6 weeks without additional flares.
Patient 3
A 27-year-old woman who gave birth 2 years prior and discontinued breastfeeding 6 weeks after delivery noted bilateral breast rashes for several months. The lesions were growing in size, tender, and draining. Her primary care provider suspected infectious mastitis and prescribed antibiotics, which were ineffective.
Biopsy
Breast core biopsy showed histologic findings similar to patients 1 and 2, including lobulocentric mixed inflammation, neutrophilic microabscesses, and scattered discrete granulomas. Microorganisms were not found using special stains. Breast cancer was ruled out, and granulomatous mastitis was diagnosed.
Referral to Dermatology
Two years earlier, the patient tested positive for latent tuberculosis and was prescribed a 9-month regimen of isoniazid. At the current presentation, she did not have symptoms of active tuberculosis on ROS (ie, no cough, hemoptysis, weight loss, night sweats); a chest radiograph was normal. Additionally, serum Ca2+ and ACE levels as well as an ophthalmology examination were normal, and she was not taking any medications known to increase the prolactin level.
The patient was started on methotrexate (12.5 mg weekly) and folic acid (1 mg daily). She had 1 IGM flare and was given a tapering regimen of prednisone. She received methotrexate for 14 months, tapered during the final 3 months. She has been off methotrexate for 3 years without IGM flares and appears to be in complete remission.
COMMENT
We report 3 cases of IGM, which contribute to the literature on possible presentations, causes, and conservative treatment of this rare connective-tissue disorder.
Differential Diagnosis
The time between recognition of symptoms and diagnosis and treatment of IGM often is prolonged because IGM can present similarly to other disorders, such as infection, breast cancer, tuberculosis, and sarcoidosis. Idiopathic granulomatous mastitis is a diagnosis of exclusion, made after obtaining evidence of granulomatous inflammation on breast biopsy and ruling out other granulomatous disorders, such as tuberculosis and sarcoidosis (Table 1).3,4

Tuberculosis
A full ROS and a PPD test or T-SPOT.TB test can be helpful in ruling out tuberculosis; because anergy occurs in some patients, tuberculosis should be evaluated in the context of known immunosuppression or human immunodeficiency virus status, or in the case of miliary tuberculosis.
Chest radiography findings classically showing upper lobe infiltrates with cavities in active tuberculosis also should be sought.3 Ziehl-Neelsen staining of 2 sputum specimens, assessed by conventional light microscopy at the time of tissue biopsy has 64% sensitivity and 98% specificity for detecting Mycobacterium tuberculosis; auramine O staining, examined with light-emitting diode fluorescence microscopy, has 73% sensitivity and 93% specificity.5
Sarcoidosis
Because more than 90% of sarcoid patients have lung disease, a chest radiograph is used to screen for hilar lymphadenopathy.3 An elevated serum ACE level also can be helpful in diagnosis, but patients do not always have increased ACE, which can occur in other diseases, such as hyperthyroidism and miliary tuberculosis. Sarcoid granulomas can increase active vitamin D production, which in turn increases serum Ca2+ in 10% of sarcoid patients. Last, an ophthalmology evaluation should be obtained to rule out anterior or posterior uveitis that can occur in sarcoidosis and initially remain asymptomatic.3 Once these other causes of granulomatous inflammation have been ruled out, a diagnosis of IGM can be made.
Prolactinoma
Prolactinoma is an important cause of hyperprolactinemia that can be screened for based on ROS and the serum prolactin level. Prolactinoma can cause oligomenorrhea or amenorrhea and galactorrhea in 90% and 80% of premenopausal women, respectively, as well as erectile dysfunction and decreased libido in men. Infertility, headache, and visual impairment may be experienced in both sexes.4
A normal prolactin level is less than 25 μg/L; more than 25 μg/L but less than 100 μg/L usually is due to certain drugs (eTable),6-11 estrogen, or idiopathic reasons; and more than 150 μg/L usually is due to prolactinoma.5 In many cases, removal of hyperprolactinemia-precipitating factors can resolve disease, as in patient 1. If symptoms continue or precipitating factors are absent, IGM symptom-based treatment should be administered.

Course and Management
Idiopathic granulomatous mastitis is self-limited and usually resolves within 2 years. Therefore, the goal of treatment is to suppress associated pain and drainage until the active inflammatory phase of IGM self-resolves. An established protocol for treating IGM does not exist, but common treatments include corticosteroids, methotrexate, and limited or wide surgical excision (Table 2).12-16 Before beginning any of these treatments, IGM triggers, such as breastfeeding and drugs that induce hyperprolactinemia, should be removed.

It is important to consider which treatment option is best for limiting disease recurrence and adverse effects (AEs). Keep in mind that the available data are limited, as there are no randomized controlled trials looking at these treatments. Nevertheless, we recommend methotrexate as first line because it resolves granulomatous inflammation symptoms without invasive surgery, while limiting corticosteroid AEs.12
With or without concurrent use of corticosteroids, surgical excision typically is the mainstay of treatment. However, surgical excision of IGM lesions can be complicated by abscess formation, fistula, and chronic pyogenic secretions, in addition to a 5% to 50% rate of recurrence of disease.12-14 Limited excision often is insufficient; therefore, wide local excision, in which negative margins around granulomatous inflammation are obtained, is the surgical modality of choice.14 Wide local excision can be disfiguring to the breast in young women affected by IGM, making it an undesirable treatment option.
Corticosteroids often have been used to treat IGM, but their efficacy is variable, symptoms can recur upon drug removal, and remarkable AEs can result from long-term use.12 Additionally, corticosteroid therapy often is used in combination with excision, making it difficult to determine the extent to which corticosteroids or excision are more beneficial. In a prospective observational study, corticosteroid therapy alone resolved 80% of IGM symptoms after 159 days on average. After complete symptom resolution, 23% of patients had disease recurrence.9 Observed AEs included gastritis, weight gain, osteoporosis, glucose intolerance, and Cushing syndrome.12,15
Methotrexate for IGM has not been reviewed in a randomized controlled trial; case reports have shown 83% symptom resolution, with 17% recurrence and limited long-term AEs.12 Because the active phase of IGM can persist for 2 years, immunosuppressive therapy with limited AEs is necessary. Many AEs can occur when high-dose methotrexate is given for cancer treatment. Low-dose methotrexate has been extensively studied in long-term treatment of rheumatoid arthritis. Adverse effects may include gastrointestinal tract upset and hepatic dysfunction, which are limited when given with folic acid.
Regardless of folic acid cotreatment, stomatitis may occur. Women should use an effective method of birth control because severe birth defects may occur on even low-dose methotrexate.16
Compared to corticosteroid or surgical treatment, we recommend low-dose methotrexate therapy based on its high efficacy with limited AEs. An occasional mild flare of IGM symptoms with methotrexate is not unusual. If it occurs, corticosteroids can be added and tapered for as long as 2 weeks to speed up resolution of flares while reducing long-term AEs of corticosteroids.
Surgical excision can be performed in cases refractory to all systemic therapies.
CONCLUSION
Idiopathic granulomatous mastitis is a rare granulomatous breast disorder that can have a prolonged time to diagnosis, delaying proper treatment. Many cases self-resolve, but more severe cases can persist for a long period before adequate symptomatic treatment is achieved by methotrexate, corticosteroids, or surgical excision. Before using these therapies, it is important to identify and remove contributing factors, such as long-term breastfeeding and drugs that induce hyperprolactinemia. Improving the rate of IGM diagnosis and treatment would greatly benefit these patients. We report 1 case in which removal of possible precipitating IGM factors led to symptom resolution and 2 cases in which methotrexate was an effective IGM treatment that limited the need for invasive procedures and corticosteroid AEs.
Idiopathic granulomatous mastitis (IGM) is rare during pregnancy; it typically is seen in women of childbearing potential from 6 months to 6 years postpartum.1 Because of a temporal association with breastfeeding, it is believed that hyperprolactinemia2 or an immune response to local lobular secretions might play a role in pathogenesis. Early misdiagnosis as bacterial mastitis is common, prompting multiple antibiotic regimens. When antibiotics fail, patients are worked up for inflammatory breast cancer, given the nonhealing breast nodules. Mammography, ultrasonography, and fine-needle aspiration often are unable to rule out carcinoma, warranting excisional biopsies of nodules. The patient is then referred to rheumatology for potential sarcoidosis or to dermatology for IGM. In either case, the workup should be similar, but additional history focused on behavior and medications is essential in suspected IGM, given the association with hyperprolactinemia.
Because IGM is rare, there are no randomized, placebo-controlled trials of treatment efficacy. In many cases, patients undergo complete mastectomy, which is curative but may be psychologically and physically impactful in young women. In some cases, high-dose corticosteroids have been successful; however, because the IGM process can last longer than 2 years, patients treated in this manner are exposed to steroid morbidities.1
We report 3 cases of IGM that add to the literature on possible contributing factors, clinical presentations, and treatments for this disease. We also demonstrate that appropriate trigger identification and steroid-sparing agents, specifically methotrexate, can be breast-saving as they can alleviate this debilitating condition, obviating the need for radical surgical intervention.
CASE REPORTS
Patient 1
A 40-year-old woman with a 4-year history of breastfeeding noted a grape-sized nodule on the left breast that grew to the size of a grapefruit after 2 weeks. Ulceration and drainage periodically occurred, forming pink plaques along the lateral aspects of the breast after healing. Her primary care provider suspected infectious mastitis; she was given an oral antibiotic (cephalexin) and intravenous antibiotics without improvement.
Imaging
Subsequent magnetic resonance imaging revealed a large, irregular, enhancing mass within the outer left breast (6.5 cm at greatest dimension) with additional surrounding amorphous enhancement highly suspicious for malignancy. There also were multiple prominent left axillary lymph nodes, with the largest demonstrating a cortical thickness of 8 mm.
Biopsy
Core breast biopsy showed benign tissue with fat necrosis. Fine-needle aspiration revealed few benign ductal cells and rare histiocytes; because these findings were nondiagnostic and cancer was still a consideration, the patient underwent excisional biopsy.
Histologic sections of breast tissue showed extensive lobulocentric inflammation comprising histiocytes and lymphocytes, with neutrophils admixed and forming microabscesses (Figure 1A). Multinucleated giant cells and single-cell necrosis were seen, but true caseous necrosis was absent (Figure 1B). Duct spaces often contained inflammatory cells or secretions. Special stains for fungal and acid-fast bacterial microorganisms were negative.

Referral to Dermatology
Granulomatous lobular mastitis was diagnosed, and the patient was referred to dermatology. On presentation to dermatology, the left breast showed a 6-cm area of firm induration and overlying peau d’orange change to the epidermis (Figure 2A). Based on pathologic analysis, she was worked up for a possible granulomatous etiology. Negative purified protein derivative (tuberculin)(PPD) and a normal chest radiograph ruled out tuberculosis. Normal chest radiography, serum Ca2+ and angiotensin-converting enzyme (ACE) levels, and ophthalmology examination ruled out sarcoidosis.
The patient reported she continued breastfeeding her 4-year-old son. Additionally, she had been started on trazadone and buspirone for alcohol abuse recovery, then switched to and maintained on fluoxetine 1 year before developing these symptoms.
Buspirone, fluoxetine, and prolonged breastfeeding all contribute to hyperprolactinemia, a possible trigger of IGM. The patient was therefore advised to stop breastfeeding and to be switched from fluoxetine to a medication that would not increase the prolactin level. She did not require methotrexate treatment because her condition resolved rapidly after breastfeeding and fluoxetine were discontinued (Figure 2B).

B, Resolution after discontinuation of breastfeeding and fluoxetine.
Patient 2
A 40-year-old woman with no history of breastfeeding who gave birth 4.5 years prior presented to her primary care provider with a painful breast lump and rash on the right breast of 2 months’ duration. Infectious mastitis was suspected; she was given cephalexin and clindamycin without improvement of symptoms.
Imaging
Mammography and ultrasonography were nondiagnostic.
Biopsy
Breast biopsy showed tissue with large expanses of histiocytes, neutrophils, lymphocytes, plasma cells, and multinucleated giant cells (Figure 3A). Many discrete granulomas were seen against this mixed inflammatory background, associated with focal fat necrosis (Figure 3B). Special stains were negative for microorganisms. Histologic findings were consistent with granulomatous mastitis.

Referral to Dermatology
On presentation to dermatology, the patient was worked up for a possible granulomatous etiology, which included a negative PPD, as well as a normal chest radiograph, serum Ca2+ and ACE levels, and ophthalmology examination. Review of symptoms (ROS),medical history, and medication review were unremarkable.
By exclusion, the patient was given a diagnosis of IGM and started on methotrexate (15 mg weekly) with folic acid (1 mg daily). The condition of the right breast improved within 4 weeks of starting methotrexate; however, methotrexate was increased to 30 mg weekly because of occasional flares. The patient remained on methotrexate without further IGM flares for 8 months compared to prior unremitting pain and drainage. She was then tapered from methotrexate over 6 weeks without additional flares.
Patient 3
A 27-year-old woman who gave birth 2 years prior and discontinued breastfeeding 6 weeks after delivery noted bilateral breast rashes for several months. The lesions were growing in size, tender, and draining. Her primary care provider suspected infectious mastitis and prescribed antibiotics, which were ineffective.
Biopsy
Breast core biopsy showed histologic findings similar to patients 1 and 2, including lobulocentric mixed inflammation, neutrophilic microabscesses, and scattered discrete granulomas. Microorganisms were not found using special stains. Breast cancer was ruled out, and granulomatous mastitis was diagnosed.
Referral to Dermatology
Two years earlier, the patient tested positive for latent tuberculosis and was prescribed a 9-month regimen of isoniazid. At the current presentation, she did not have symptoms of active tuberculosis on ROS (ie, no cough, hemoptysis, weight loss, night sweats); a chest radiograph was normal. Additionally, serum Ca2+ and ACE levels as well as an ophthalmology examination were normal, and she was not taking any medications known to increase the prolactin level.
The patient was started on methotrexate (12.5 mg weekly) and folic acid (1 mg daily). She had 1 IGM flare and was given a tapering regimen of prednisone. She received methotrexate for 14 months, tapered during the final 3 months. She has been off methotrexate for 3 years without IGM flares and appears to be in complete remission.
COMMENT
We report 3 cases of IGM, which contribute to the literature on possible presentations, causes, and conservative treatment of this rare connective-tissue disorder.
Differential Diagnosis
The time between recognition of symptoms and diagnosis and treatment of IGM often is prolonged because IGM can present similarly to other disorders, such as infection, breast cancer, tuberculosis, and sarcoidosis. Idiopathic granulomatous mastitis is a diagnosis of exclusion, made after obtaining evidence of granulomatous inflammation on breast biopsy and ruling out other granulomatous disorders, such as tuberculosis and sarcoidosis (Table 1).3,4

Tuberculosis
A full ROS and a PPD test or T-SPOT.TB test can be helpful in ruling out tuberculosis; because anergy occurs in some patients, tuberculosis should be evaluated in the context of known immunosuppression or human immunodeficiency virus status, or in the case of miliary tuberculosis.
Chest radiography findings classically showing upper lobe infiltrates with cavities in active tuberculosis also should be sought.3 Ziehl-Neelsen staining of 2 sputum specimens, assessed by conventional light microscopy at the time of tissue biopsy has 64% sensitivity and 98% specificity for detecting Mycobacterium tuberculosis; auramine O staining, examined with light-emitting diode fluorescence microscopy, has 73% sensitivity and 93% specificity.5
Sarcoidosis
Because more than 90% of sarcoid patients have lung disease, a chest radiograph is used to screen for hilar lymphadenopathy.3 An elevated serum ACE level also can be helpful in diagnosis, but patients do not always have increased ACE, which can occur in other diseases, such as hyperthyroidism and miliary tuberculosis. Sarcoid granulomas can increase active vitamin D production, which in turn increases serum Ca2+ in 10% of sarcoid patients. Last, an ophthalmology evaluation should be obtained to rule out anterior or posterior uveitis that can occur in sarcoidosis and initially remain asymptomatic.3 Once these other causes of granulomatous inflammation have been ruled out, a diagnosis of IGM can be made.
Prolactinoma
Prolactinoma is an important cause of hyperprolactinemia that can be screened for based on ROS and the serum prolactin level. Prolactinoma can cause oligomenorrhea or amenorrhea and galactorrhea in 90% and 80% of premenopausal women, respectively, as well as erectile dysfunction and decreased libido in men. Infertility, headache, and visual impairment may be experienced in both sexes.4
A normal prolactin level is less than 25 μg/L; more than 25 μg/L but less than 100 μg/L usually is due to certain drugs (eTable),6-11 estrogen, or idiopathic reasons; and more than 150 μg/L usually is due to prolactinoma.5 In many cases, removal of hyperprolactinemia-precipitating factors can resolve disease, as in patient 1. If symptoms continue or precipitating factors are absent, IGM symptom-based treatment should be administered.

Course and Management
Idiopathic granulomatous mastitis is self-limited and usually resolves within 2 years. Therefore, the goal of treatment is to suppress associated pain and drainage until the active inflammatory phase of IGM self-resolves. An established protocol for treating IGM does not exist, but common treatments include corticosteroids, methotrexate, and limited or wide surgical excision (Table 2).12-16 Before beginning any of these treatments, IGM triggers, such as breastfeeding and drugs that induce hyperprolactinemia, should be removed.

It is important to consider which treatment option is best for limiting disease recurrence and adverse effects (AEs). Keep in mind that the available data are limited, as there are no randomized controlled trials looking at these treatments. Nevertheless, we recommend methotrexate as first line because it resolves granulomatous inflammation symptoms without invasive surgery, while limiting corticosteroid AEs.12
With or without concurrent use of corticosteroids, surgical excision typically is the mainstay of treatment. However, surgical excision of IGM lesions can be complicated by abscess formation, fistula, and chronic pyogenic secretions, in addition to a 5% to 50% rate of recurrence of disease.12-14 Limited excision often is insufficient; therefore, wide local excision, in which negative margins around granulomatous inflammation are obtained, is the surgical modality of choice.14 Wide local excision can be disfiguring to the breast in young women affected by IGM, making it an undesirable treatment option.
Corticosteroids often have been used to treat IGM, but their efficacy is variable, symptoms can recur upon drug removal, and remarkable AEs can result from long-term use.12 Additionally, corticosteroid therapy often is used in combination with excision, making it difficult to determine the extent to which corticosteroids or excision are more beneficial. In a prospective observational study, corticosteroid therapy alone resolved 80% of IGM symptoms after 159 days on average. After complete symptom resolution, 23% of patients had disease recurrence.9 Observed AEs included gastritis, weight gain, osteoporosis, glucose intolerance, and Cushing syndrome.12,15
Methotrexate for IGM has not been reviewed in a randomized controlled trial; case reports have shown 83% symptom resolution, with 17% recurrence and limited long-term AEs.12 Because the active phase of IGM can persist for 2 years, immunosuppressive therapy with limited AEs is necessary. Many AEs can occur when high-dose methotrexate is given for cancer treatment. Low-dose methotrexate has been extensively studied in long-term treatment of rheumatoid arthritis. Adverse effects may include gastrointestinal tract upset and hepatic dysfunction, which are limited when given with folic acid.
Regardless of folic acid cotreatment, stomatitis may occur. Women should use an effective method of birth control because severe birth defects may occur on even low-dose methotrexate.16
Compared to corticosteroid or surgical treatment, we recommend low-dose methotrexate therapy based on its high efficacy with limited AEs. An occasional mild flare of IGM symptoms with methotrexate is not unusual. If it occurs, corticosteroids can be added and tapered for as long as 2 weeks to speed up resolution of flares while reducing long-term AEs of corticosteroids.
Surgical excision can be performed in cases refractory to all systemic therapies.
CONCLUSION
Idiopathic granulomatous mastitis is a rare granulomatous breast disorder that can have a prolonged time to diagnosis, delaying proper treatment. Many cases self-resolve, but more severe cases can persist for a long period before adequate symptomatic treatment is achieved by methotrexate, corticosteroids, or surgical excision. Before using these therapies, it is important to identify and remove contributing factors, such as long-term breastfeeding and drugs that induce hyperprolactinemia. Improving the rate of IGM diagnosis and treatment would greatly benefit these patients. We report 1 case in which removal of possible precipitating IGM factors led to symptom resolution and 2 cases in which methotrexate was an effective IGM treatment that limited the need for invasive procedures and corticosteroid AEs.
1. Patel RA, Strickland P, Sankara IR, et al. Idiopathic granulomatous mastitis: case reports and review of literature. J Gen Intern Med. 2010;25:270-273.
2. Bellavia M, Damiano G, Palumbo VD, et al. Granulomatous mastitis during chronic antidepressant therapy: is it possible a conservative therapeutic approach? J Breast Cancer. 2012;15:371-372.
3. Longo D, Fauci A, Kasper D, et al. Harrison’s Principles of Internal Medicine. 18th ed. New York, NY: McGraw-Hill; 2012.
4. Davis JL, Cattamanchi A, Cuevas LE, et al. Diagnostic accuracy of same-day microscopy versus standard microscopy for pulmonary tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2013;13:147-154.
5. Casanueva FF, Molitch ME, Schlechte JA, et al. Guidelines of the Pituitary Society for the diagnosis and management of prolactinomas. Clin Endocrinol (Oxf). 2006;65:265-273.
6. Akbulut S, Arikanoglu Z, Senol A, et al. Is methotrexate an acceptable treatment in the management of idiopathic granulomatous mastitis? Arch Gynecol Obstet. 2011;284:1189-1195.
7. Bani-Hani KE, Yaghan RJ, Matalka II, et al. Idiopathic granulomatous mastitis: time to avoid unnecessary mastectomies. Breast J. 2004;10:318-322.
8. Asoglu O, Ozmen V, Karanlik H, et al. Feasibility of surgical management in patients with granulomatous mastitis. Breast J. 2005;11:108-114.
9. Pandey TS, Mackinnon JC, Bressler L, et al. Idiopathic granulomatous mastitis—a prospective study of 49 women and treatment outcomes with steroid therapy. Breast J. 2014;20:258-266.
10. Shea B, Swinden MV, Tanjong Ghogomu E, et al. Folic acid and folinic acid for reducing side effects in patients receiving methotrexate for rheumatoid arthritis. Cochrane Database Syst Rev. 2013;5:CD000951.
11. Molitch ME. Drugs and prolactin. Pituitary. 2008;11:209-218.
12.
13.
14.
15.
16.
1. Patel RA, Strickland P, Sankara IR, et al. Idiopathic granulomatous mastitis: case reports and review of literature. J Gen Intern Med. 2010;25:270-273.
2. Bellavia M, Damiano G, Palumbo VD, et al. Granulomatous mastitis during chronic antidepressant therapy: is it possible a conservative therapeutic approach? J Breast Cancer. 2012;15:371-372.
3. Longo D, Fauci A, Kasper D, et al. Harrison’s Principles of Internal Medicine. 18th ed. New York, NY: McGraw-Hill; 2012.
4. Davis JL, Cattamanchi A, Cuevas LE, et al. Diagnostic accuracy of same-day microscopy versus standard microscopy for pulmonary tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2013;13:147-154.
5. Casanueva FF, Molitch ME, Schlechte JA, et al. Guidelines of the Pituitary Society for the diagnosis and management of prolactinomas. Clin Endocrinol (Oxf). 2006;65:265-273.
6. Akbulut S, Arikanoglu Z, Senol A, et al. Is methotrexate an acceptable treatment in the management of idiopathic granulomatous mastitis? Arch Gynecol Obstet. 2011;284:1189-1195.
7. Bani-Hani KE, Yaghan RJ, Matalka II, et al. Idiopathic granulomatous mastitis: time to avoid unnecessary mastectomies. Breast J. 2004;10:318-322.
8. Asoglu O, Ozmen V, Karanlik H, et al. Feasibility of surgical management in patients with granulomatous mastitis. Breast J. 2005;11:108-114.
9. Pandey TS, Mackinnon JC, Bressler L, et al. Idiopathic granulomatous mastitis—a prospective study of 49 women and treatment outcomes with steroid therapy. Breast J. 2014;20:258-266.
10. Shea B, Swinden MV, Tanjong Ghogomu E, et al. Folic acid and folinic acid for reducing side effects in patients receiving methotrexate for rheumatoid arthritis. Cochrane Database Syst Rev. 2013;5:CD000951.
11. Molitch ME. Drugs and prolactin. Pituitary. 2008;11:209-218.
12.
13.
14.
15.
16.
Practice Points
- Idiopathic granulomatous mastitis (IGM) is a painful and scarring rare granulomatous breast disorder that can have a prolonged time to diagnosis that delays proper treatment.
- The pathogenesis of IGM remains poorly understood. The temporal association of the disorder with breastfeeding suggests that hyperprolactinemia or an immune response to local lobular secretions might play a role.
- Although many cases of IGM resolve without treatment, more severe cases can persist for a long period before adequate symptomatic treatment is provided with methotrexate, corticosteroids, or surgical excision.
- Before any of these therapies are applied, however, contributing factors, such as long-term breastfeeding and drugs that induce hyperprolactinemia, should be identified and withdrawn.
En Coup de Sabre
En coup de sabre (ECDS) is a rare subtype of linear scleroderma that is limited to the hemiface in a unilateral distribution. The lesional skin first exhibits contraction and stiffness that lead to characteristic fibrotic plaques with associated linear alopecia.1 The pansclerotic plaques are ivory in color with hyperpigmented to violaceous borders extending as a paramedian band on the frontoparietal scalp.2,3 The skin lesions bear resemblance to the stroke of the sabre sword, giving the condition its unique name. Many patients initially present with concerns of frontal scalp alopecia.3 Linear morphea, including the ECDS subtype, is predominantly seen in children and women, usually presenting within the first 2 decades of life.1,4
The differential diagnoses of ECDS include focal dermal hypoplasia, steroid atrophy, localized morphea, and lupus profundus.5 En coup de sabre should be distinguished from progressive hemifacial atrophy (PHA)(also known as Parry-Romberg syndrome).6 Progressive hemifacial atrophy presents as unilateral atrophy of the face involving skin, subcutaneous tissue, muscle, and underlying bone in the distribution of the trigeminal nerve.1 Both PHA and ECDS exist on a spectrum of linear scleroderma and may coexist in the same patient.6
There is a strong association with extracutaneous neurologic involvement, including seizures, ocular abnormalities, trigeminal neuralgia, and headache.7-10 One study examining ECDS and PHA demonstrated that 44% (19/43) of patients who underwent central nervous system imaging had abnormal findings.11 The majority of patients had magnetic resonance imaging with or without contrast, computed tomography, or both. The most common findings on T2-weighted images were white matter hyperintensities, mostly in subcortical and periventricular regions. The findings were bilateral in 61% (11/18) of patients and ipsilateral to the lesion in 33% (6/18) of patients.11 We present a case of ECDS masquerading as alopecia in a 77-year-old woman.
Case Report
A 77-year-old white woman presented with a chief concern of hair loss on the scalp that had been present since 12 years of age. During her adult life, the scalp lesion remained unchanged with no associated symptoms. Her medical history was remarkable for hypertension and non–insulin-dependent diabetes mellitus. The patient denied any history of seizure disorders, facial paralysis, or neurologic deficits.
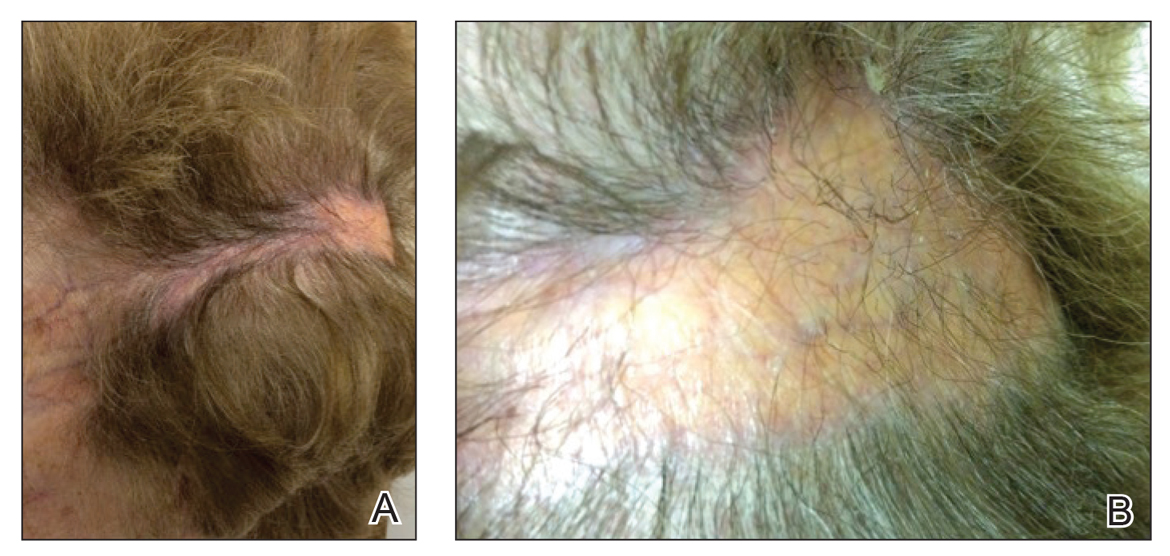
Comment
Etiology and Presentation
En coup de sabre is a rare subtype of linear morphea that involves the frontoparietal scalp and forehead.7,12,13 It manifests as a solitary, linear, fibrous plaque that involves the skin, underlying muscle, and bone.7 Although most cases present with a single lesion, multiple lesions can occur.8 The exact etiology of this disease remains to be determined but is characterized by thickening and hardening of the skin secondary to increased collagen production.7 The incidence of linear morphea ranges from 0.4 to 2.7 cases per 100,000 individuals and is more prevalent in white patients and women.14 Linear morphea is commonly found in children. Children are more likely to have linear morphea on the face, which can lead to lifelong disfigurement.2 Although the disease peaks in the fourth decade of life for adults, most pediatric cases are diagnosed between 2 and 14 years of age.14-16
Pathogenesis
Clinical and histopathological data suggest that a complex interaction among the vasculature, extracellular matrix, and immune system plays a role in the pathogenesis of the disease. Similar to scleroderma, the CD4 helper T cell may be involved in the fibrotic changes that occur within these lesions.17 Early in the disease process, TH1 and TH17 inflammatory pathways predominate. The late fibrotic changes seen in scleroderma are more associated with a shift to the TH2 inflammatory pathway.17 Infection with Borrelia burgdorferi has been implicated abroad, but a large-scale study confirming Borrelia as a pathologic factor within morphea lesions has not been completed to date.18-20 Some authors believe early lesions of ECDS mimic erythema chronica migrans, with the late lesions resembling acrodermatitis chronica atrophicans.20
Histopathology
Histopathologic findings of morphea tend to vary depending on the stage of the disease. The 2 stages of morphea can be differentiated by the degree of inflammation present histologically.14,21 The early phase of morphea primarily affects the connective and subcutaneous tissue surrounding eccrine sweat glands.14,21 A dense dermal and subcutaneous perivascular lymphocytic infiltrate with a mixture of lymphocytes, plasma cells, and histiocytes is commonly observed.5 Later stages of the disease demonstrate densely packed homogenous collagen with minimal inflammation and loss of eccrine glands and blood vessels.14,21 The adipose tissue is generally replaced by sclerotic collagen, giving the biopsy a squared-off appearance.5,14
Management
En coup de sabre presents a treatment challenge. In active lesions, topical or intralesional corticosteroids are considered treatment of choice.5 Methotrexate has proven useful in the treatment of acute and deep forms of linear morphea. A study examining methotrexate in juvenile localized scleroderma, with the majority of patients having the linear subtype, revealed that methotrexate is both efficacious and well tolerated.22 Other reports in the literature reveal efficacy with the use of intravenous corticosteroids and methotrexate combination therapy for treatment of morphea.23,24 A longitudinal prospective study examining the use of high-dose methotrexate and oral corticosteroids for the treatment of localized scleroderma yielded positive results, with patients showing clinical improvement within 2 months of initiation of combination therapy.25 Other treatments include excimer laser; calcipotriene and tacrolimus; and surgical approaches such as autologous fat grafting, grafting with muscle flaps, and tissue inserts.21,26-31 In addition, patients can choose to forego therapy, as was the case with our patient.
Conclusion
En coup de sabre is a rare subtype of linear scleroderma that is limited to the ipsilateral scalp and face predominately in children and women. Neurologic involvement is common and should prompt a comprehensive neurologic workup in patients suspected to have ECDS or PHA. Current treatment recommendations include topical, intralesional, and oral corticosteroids; methotrexate; and surgical grafts. Although ECDS is a rare entity, more intensive research is needed on the exact pathophysiology and effective treatment options that focus on improving the cosmetic outcome in these patients. Cosmesis is the primary concern in patients with ECDS and should be managed early and appropriately to prevent long-term psychological sequelae.
1. Careta MF, Romiti R. Localized scleroderma: clinical spectrum and therapeutic update. An Bras Dermatol. 2015;90:62-73.
2. Picket AJ, Carpentieri D, Price H, et al. Early morphea mimicking acquired port-wine stain. Pediatr Dermatol. 2014;31:591-594.
3. Holland KE, Steffes B, Nocton JJ, et al. Linear scleroderma en coup de sabre with associated neurologic abnormalities. Pediatrics. 2006;117:132-136.
4. Goh C, Biswas A, Goldberg LJ. Alopecia with perineural lymphocytes: a clue to linear scleroderma en coup de sabre. J Cutan Pathol. 2012;39:518-520.
5. Kreuter A. Localized scleroderma. Dermatol Ther. 2012;25:135-147.
6. Tolkachjov SN, Patel NG, Tollefson MM. Progressive hemifacial atrophy: a review. Orphanet J Rare Dis. 2015;10:39.
7. Amaral TN, Marques Neto JF, Lapa AT, et al. Neurologic involvement in scleroderma en coup de sabre [published online January 27, 2012]. Autoimmune Dis. 2012;2012:719685.
8. Tollefson MM, Witman PM. En coup de sabre morphea and Parry-Romberg syndrome: a retrospective review of 54 patients. J Am Acad Dermatol. 2007;56:257-263.
9. Zannin ME, Martini G, Athreya BH, et al. Ocular involvement in children with localized scleroderma: a multi-center study. Br J Ophthalmol. 2007;91:1311-1314.
10. Polcari I, Moon A, Mathes EF, et al. Headaches as a presenting symptom of linear morphea en coup de sabre. Pediatrics. 2014;134:1715-1719.
11. Doolittle DA, Lehman VT, Schwartz KM, et al. CNS imaging findings associated with Parry-Romberg syndrome and en coup de sabre: correlation to dermatologic and neurologic abnormalities. Neuroradiology. 2015;57:21-34.
12. Pierre-Louis M, Sperling LC, Wilke MS, et al. Distinctive histopathologic findings in linear morphea (en coup de sabre) alopecia. J Cutan Pathol. 2013;40:580-584.
13. Thareja SK, Sadhwani D, Alan Fenske N. En coup de sabre morphea treated with hyaluronic acid filler. Report of a case and review of the literature. Int J Dermatol. 2015;54:823-826.
14. Fett N, Werth VP. Update on morphea: part I. epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol. 2011;64:217-228.
15. Christen-Zaech S, Hakim MD, Afsar FS, et al. Pediatric morphea (localized scleroderma): review of 136 patients. J Am Acad Dermatol. 2008;59:385-396.
16. Leitenberger JJ, Cayce RL, Haley RW, et al. Distinct autoimmune syndromes in morphea: a review of 245 adult and pediatric cases. Arch Dermatol. 2009;145:545-550.
17. Kurzinski K, Torok KS. Cytokine profiles in localized scleroderma and relationship to clinical features. Cytokine. 2011;55:157-164.
18. Eisendle K, Grabner T, Zelger B. Morphoea: a manifestation of infection with Borrelia species? Br J Dermatol. 2007;157:1189-1198.
19. Gutiérrez-Gómez C, Godínez-Hana AL, García-Hernández M, et al. Lack of IgG antibody seropositivity to Borrelia burgdorferi in patients with Parry-Romberg syndrome and linear morphea en coup de sabre in Mexico. Int J Dermatol. 2014;53:947-951.
20. Miller K, Lehrhoff S, Fischer M, et al. Linear morphea of the forehead (en coup de sabre). Dermatol Online J. 2012;18:22.
21. Hanson AH, Fivenson DP, Schapiro B. Linear scleroderma in an adolescent woman treated with methotrexate and excimer laser. Dermatol Ther. 2014;27:203-205.
22. Zulian F, Martini G, Vallongo C, et al. Methotrexate treatment in juvenile localized scleroderma: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2011;63:1998-2006.
23. Kreuter A, Gambichler T, Breuckmann F, et al. Pulsed high-dose corticosteroids combined with low-dose methotrexate in severe localized scleroderma. Arch Dermatol. 2005;141:847-852.
24. Weibel L, Sampaio MC, Visentin MT, et al. Evaluation of methotrexate and corticosteroids for the treatment of localized scleroderma (morphoea) in children. Br J Dermatol. 2006;155:1013-1020.
25. Torok KS, Arkachaisri T. Methotrexate and corticosteroids in the treatment of localized scleroderma: a standardized prospective longitudinal single-center study. J Rheumatol. 2012;39:286-294.
26. Nisticò SP, Saraceno R, Schipani C, et al. Different applications of monochromatic excimer light in skin diseases. Photomed Laser Surg. 2009;27:647-654.
27. Zwischenberger BA, Jacobe HT. A systematic review of morphea treatments and therapeutic algorithm. J Am Acad Dermatol. 2011;65:925-941.
28. Karaaltin MV, Akpinar AC, Baghaki S, et al. Treatment of “en coup de sabre” deformity with adipose-derived regenerative cell-enriched fat graft. J Craniofac Surg. 2012;23:103-105.
29. Consorti G, Tieghi R, Clauser LC. Frontal linear scleroderma: long-term result in volumetric restoration of the fronto-orbital area by structural fat grafting. J Craniofac Surg. 2012;23:263-265.
30. Cavusoglu T, Yazici I, Vargel I, et al. Reconstruction of coup de sabre deformity (linear localized scleroderma) by using galeal frontalis muscle flap and demineralized bone matrix combination. J Craniofac Surg. 2011;22:257-258.
31. Robitschek J, Wang D, Hall D. Treatment of linear scleroderma “en coup de sabre” with AlloDerm tissue matrix. Otolaryngol Head Neck Surg. 2008;138:540-541.
En coup de sabre (ECDS) is a rare subtype of linear scleroderma that is limited to the hemiface in a unilateral distribution. The lesional skin first exhibits contraction and stiffness that lead to characteristic fibrotic plaques with associated linear alopecia.1 The pansclerotic plaques are ivory in color with hyperpigmented to violaceous borders extending as a paramedian band on the frontoparietal scalp.2,3 The skin lesions bear resemblance to the stroke of the sabre sword, giving the condition its unique name. Many patients initially present with concerns of frontal scalp alopecia.3 Linear morphea, including the ECDS subtype, is predominantly seen in children and women, usually presenting within the first 2 decades of life.1,4
The differential diagnoses of ECDS include focal dermal hypoplasia, steroid atrophy, localized morphea, and lupus profundus.5 En coup de sabre should be distinguished from progressive hemifacial atrophy (PHA)(also known as Parry-Romberg syndrome).6 Progressive hemifacial atrophy presents as unilateral atrophy of the face involving skin, subcutaneous tissue, muscle, and underlying bone in the distribution of the trigeminal nerve.1 Both PHA and ECDS exist on a spectrum of linear scleroderma and may coexist in the same patient.6
There is a strong association with extracutaneous neurologic involvement, including seizures, ocular abnormalities, trigeminal neuralgia, and headache.7-10 One study examining ECDS and PHA demonstrated that 44% (19/43) of patients who underwent central nervous system imaging had abnormal findings.11 The majority of patients had magnetic resonance imaging with or without contrast, computed tomography, or both. The most common findings on T2-weighted images were white matter hyperintensities, mostly in subcortical and periventricular regions. The findings were bilateral in 61% (11/18) of patients and ipsilateral to the lesion in 33% (6/18) of patients.11 We present a case of ECDS masquerading as alopecia in a 77-year-old woman.
Case Report
A 77-year-old white woman presented with a chief concern of hair loss on the scalp that had been present since 12 years of age. During her adult life, the scalp lesion remained unchanged with no associated symptoms. Her medical history was remarkable for hypertension and non–insulin-dependent diabetes mellitus. The patient denied any history of seizure disorders, facial paralysis, or neurologic deficits.

Comment
Etiology and Presentation
En coup de sabre is a rare subtype of linear morphea that involves the frontoparietal scalp and forehead.7,12,13 It manifests as a solitary, linear, fibrous plaque that involves the skin, underlying muscle, and bone.7 Although most cases present with a single lesion, multiple lesions can occur.8 The exact etiology of this disease remains to be determined but is characterized by thickening and hardening of the skin secondary to increased collagen production.7 The incidence of linear morphea ranges from 0.4 to 2.7 cases per 100,000 individuals and is more prevalent in white patients and women.14 Linear morphea is commonly found in children. Children are more likely to have linear morphea on the face, which can lead to lifelong disfigurement.2 Although the disease peaks in the fourth decade of life for adults, most pediatric cases are diagnosed between 2 and 14 years of age.14-16
Pathogenesis
Clinical and histopathological data suggest that a complex interaction among the vasculature, extracellular matrix, and immune system plays a role in the pathogenesis of the disease. Similar to scleroderma, the CD4 helper T cell may be involved in the fibrotic changes that occur within these lesions.17 Early in the disease process, TH1 and TH17 inflammatory pathways predominate. The late fibrotic changes seen in scleroderma are more associated with a shift to the TH2 inflammatory pathway.17 Infection with Borrelia burgdorferi has been implicated abroad, but a large-scale study confirming Borrelia as a pathologic factor within morphea lesions has not been completed to date.18-20 Some authors believe early lesions of ECDS mimic erythema chronica migrans, with the late lesions resembling acrodermatitis chronica atrophicans.20
Histopathology
Histopathologic findings of morphea tend to vary depending on the stage of the disease. The 2 stages of morphea can be differentiated by the degree of inflammation present histologically.14,21 The early phase of morphea primarily affects the connective and subcutaneous tissue surrounding eccrine sweat glands.14,21 A dense dermal and subcutaneous perivascular lymphocytic infiltrate with a mixture of lymphocytes, plasma cells, and histiocytes is commonly observed.5 Later stages of the disease demonstrate densely packed homogenous collagen with minimal inflammation and loss of eccrine glands and blood vessels.14,21 The adipose tissue is generally replaced by sclerotic collagen, giving the biopsy a squared-off appearance.5,14
Management
En coup de sabre presents a treatment challenge. In active lesions, topical or intralesional corticosteroids are considered treatment of choice.5 Methotrexate has proven useful in the treatment of acute and deep forms of linear morphea. A study examining methotrexate in juvenile localized scleroderma, with the majority of patients having the linear subtype, revealed that methotrexate is both efficacious and well tolerated.22 Other reports in the literature reveal efficacy with the use of intravenous corticosteroids and methotrexate combination therapy for treatment of morphea.23,24 A longitudinal prospective study examining the use of high-dose methotrexate and oral corticosteroids for the treatment of localized scleroderma yielded positive results, with patients showing clinical improvement within 2 months of initiation of combination therapy.25 Other treatments include excimer laser; calcipotriene and tacrolimus; and surgical approaches such as autologous fat grafting, grafting with muscle flaps, and tissue inserts.21,26-31 In addition, patients can choose to forego therapy, as was the case with our patient.
Conclusion
En coup de sabre is a rare subtype of linear scleroderma that is limited to the ipsilateral scalp and face predominately in children and women. Neurologic involvement is common and should prompt a comprehensive neurologic workup in patients suspected to have ECDS or PHA. Current treatment recommendations include topical, intralesional, and oral corticosteroids; methotrexate; and surgical grafts. Although ECDS is a rare entity, more intensive research is needed on the exact pathophysiology and effective treatment options that focus on improving the cosmetic outcome in these patients. Cosmesis is the primary concern in patients with ECDS and should be managed early and appropriately to prevent long-term psychological sequelae.
En coup de sabre (ECDS) is a rare subtype of linear scleroderma that is limited to the hemiface in a unilateral distribution. The lesional skin first exhibits contraction and stiffness that lead to characteristic fibrotic plaques with associated linear alopecia.1 The pansclerotic plaques are ivory in color with hyperpigmented to violaceous borders extending as a paramedian band on the frontoparietal scalp.2,3 The skin lesions bear resemblance to the stroke of the sabre sword, giving the condition its unique name. Many patients initially present with concerns of frontal scalp alopecia.3 Linear morphea, including the ECDS subtype, is predominantly seen in children and women, usually presenting within the first 2 decades of life.1,4
The differential diagnoses of ECDS include focal dermal hypoplasia, steroid atrophy, localized morphea, and lupus profundus.5 En coup de sabre should be distinguished from progressive hemifacial atrophy (PHA)(also known as Parry-Romberg syndrome).6 Progressive hemifacial atrophy presents as unilateral atrophy of the face involving skin, subcutaneous tissue, muscle, and underlying bone in the distribution of the trigeminal nerve.1 Both PHA and ECDS exist on a spectrum of linear scleroderma and may coexist in the same patient.6
There is a strong association with extracutaneous neurologic involvement, including seizures, ocular abnormalities, trigeminal neuralgia, and headache.7-10 One study examining ECDS and PHA demonstrated that 44% (19/43) of patients who underwent central nervous system imaging had abnormal findings.11 The majority of patients had magnetic resonance imaging with or without contrast, computed tomography, or both. The most common findings on T2-weighted images were white matter hyperintensities, mostly in subcortical and periventricular regions. The findings were bilateral in 61% (11/18) of patients and ipsilateral to the lesion in 33% (6/18) of patients.11 We present a case of ECDS masquerading as alopecia in a 77-year-old woman.
Case Report
A 77-year-old white woman presented with a chief concern of hair loss on the scalp that had been present since 12 years of age. During her adult life, the scalp lesion remained unchanged with no associated symptoms. Her medical history was remarkable for hypertension and non–insulin-dependent diabetes mellitus. The patient denied any history of seizure disorders, facial paralysis, or neurologic deficits.

Comment
Etiology and Presentation
En coup de sabre is a rare subtype of linear morphea that involves the frontoparietal scalp and forehead.7,12,13 It manifests as a solitary, linear, fibrous plaque that involves the skin, underlying muscle, and bone.7 Although most cases present with a single lesion, multiple lesions can occur.8 The exact etiology of this disease remains to be determined but is characterized by thickening and hardening of the skin secondary to increased collagen production.7 The incidence of linear morphea ranges from 0.4 to 2.7 cases per 100,000 individuals and is more prevalent in white patients and women.14 Linear morphea is commonly found in children. Children are more likely to have linear morphea on the face, which can lead to lifelong disfigurement.2 Although the disease peaks in the fourth decade of life for adults, most pediatric cases are diagnosed between 2 and 14 years of age.14-16
Pathogenesis
Clinical and histopathological data suggest that a complex interaction among the vasculature, extracellular matrix, and immune system plays a role in the pathogenesis of the disease. Similar to scleroderma, the CD4 helper T cell may be involved in the fibrotic changes that occur within these lesions.17 Early in the disease process, TH1 and TH17 inflammatory pathways predominate. The late fibrotic changes seen in scleroderma are more associated with a shift to the TH2 inflammatory pathway.17 Infection with Borrelia burgdorferi has been implicated abroad, but a large-scale study confirming Borrelia as a pathologic factor within morphea lesions has not been completed to date.18-20 Some authors believe early lesions of ECDS mimic erythema chronica migrans, with the late lesions resembling acrodermatitis chronica atrophicans.20
Histopathology
Histopathologic findings of morphea tend to vary depending on the stage of the disease. The 2 stages of morphea can be differentiated by the degree of inflammation present histologically.14,21 The early phase of morphea primarily affects the connective and subcutaneous tissue surrounding eccrine sweat glands.14,21 A dense dermal and subcutaneous perivascular lymphocytic infiltrate with a mixture of lymphocytes, plasma cells, and histiocytes is commonly observed.5 Later stages of the disease demonstrate densely packed homogenous collagen with minimal inflammation and loss of eccrine glands and blood vessels.14,21 The adipose tissue is generally replaced by sclerotic collagen, giving the biopsy a squared-off appearance.5,14
Management
En coup de sabre presents a treatment challenge. In active lesions, topical or intralesional corticosteroids are considered treatment of choice.5 Methotrexate has proven useful in the treatment of acute and deep forms of linear morphea. A study examining methotrexate in juvenile localized scleroderma, with the majority of patients having the linear subtype, revealed that methotrexate is both efficacious and well tolerated.22 Other reports in the literature reveal efficacy with the use of intravenous corticosteroids and methotrexate combination therapy for treatment of morphea.23,24 A longitudinal prospective study examining the use of high-dose methotrexate and oral corticosteroids for the treatment of localized scleroderma yielded positive results, with patients showing clinical improvement within 2 months of initiation of combination therapy.25 Other treatments include excimer laser; calcipotriene and tacrolimus; and surgical approaches such as autologous fat grafting, grafting with muscle flaps, and tissue inserts.21,26-31 In addition, patients can choose to forego therapy, as was the case with our patient.
Conclusion
En coup de sabre is a rare subtype of linear scleroderma that is limited to the ipsilateral scalp and face predominately in children and women. Neurologic involvement is common and should prompt a comprehensive neurologic workup in patients suspected to have ECDS or PHA. Current treatment recommendations include topical, intralesional, and oral corticosteroids; methotrexate; and surgical grafts. Although ECDS is a rare entity, more intensive research is needed on the exact pathophysiology and effective treatment options that focus on improving the cosmetic outcome in these patients. Cosmesis is the primary concern in patients with ECDS and should be managed early and appropriately to prevent long-term psychological sequelae.
1. Careta MF, Romiti R. Localized scleroderma: clinical spectrum and therapeutic update. An Bras Dermatol. 2015;90:62-73.
2. Picket AJ, Carpentieri D, Price H, et al. Early morphea mimicking acquired port-wine stain. Pediatr Dermatol. 2014;31:591-594.
3. Holland KE, Steffes B, Nocton JJ, et al. Linear scleroderma en coup de sabre with associated neurologic abnormalities. Pediatrics. 2006;117:132-136.
4. Goh C, Biswas A, Goldberg LJ. Alopecia with perineural lymphocytes: a clue to linear scleroderma en coup de sabre. J Cutan Pathol. 2012;39:518-520.
5. Kreuter A. Localized scleroderma. Dermatol Ther. 2012;25:135-147.
6. Tolkachjov SN, Patel NG, Tollefson MM. Progressive hemifacial atrophy: a review. Orphanet J Rare Dis. 2015;10:39.
7. Amaral TN, Marques Neto JF, Lapa AT, et al. Neurologic involvement in scleroderma en coup de sabre [published online January 27, 2012]. Autoimmune Dis. 2012;2012:719685.
8. Tollefson MM, Witman PM. En coup de sabre morphea and Parry-Romberg syndrome: a retrospective review of 54 patients. J Am Acad Dermatol. 2007;56:257-263.
9. Zannin ME, Martini G, Athreya BH, et al. Ocular involvement in children with localized scleroderma: a multi-center study. Br J Ophthalmol. 2007;91:1311-1314.
10. Polcari I, Moon A, Mathes EF, et al. Headaches as a presenting symptom of linear morphea en coup de sabre. Pediatrics. 2014;134:1715-1719.
11. Doolittle DA, Lehman VT, Schwartz KM, et al. CNS imaging findings associated with Parry-Romberg syndrome and en coup de sabre: correlation to dermatologic and neurologic abnormalities. Neuroradiology. 2015;57:21-34.
12. Pierre-Louis M, Sperling LC, Wilke MS, et al. Distinctive histopathologic findings in linear morphea (en coup de sabre) alopecia. J Cutan Pathol. 2013;40:580-584.
13. Thareja SK, Sadhwani D, Alan Fenske N. En coup de sabre morphea treated with hyaluronic acid filler. Report of a case and review of the literature. Int J Dermatol. 2015;54:823-826.
14. Fett N, Werth VP. Update on morphea: part I. epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol. 2011;64:217-228.
15. Christen-Zaech S, Hakim MD, Afsar FS, et al. Pediatric morphea (localized scleroderma): review of 136 patients. J Am Acad Dermatol. 2008;59:385-396.
16. Leitenberger JJ, Cayce RL, Haley RW, et al. Distinct autoimmune syndromes in morphea: a review of 245 adult and pediatric cases. Arch Dermatol. 2009;145:545-550.
17. Kurzinski K, Torok KS. Cytokine profiles in localized scleroderma and relationship to clinical features. Cytokine. 2011;55:157-164.
18. Eisendle K, Grabner T, Zelger B. Morphoea: a manifestation of infection with Borrelia species? Br J Dermatol. 2007;157:1189-1198.
19. Gutiérrez-Gómez C, Godínez-Hana AL, García-Hernández M, et al. Lack of IgG antibody seropositivity to Borrelia burgdorferi in patients with Parry-Romberg syndrome and linear morphea en coup de sabre in Mexico. Int J Dermatol. 2014;53:947-951.
20. Miller K, Lehrhoff S, Fischer M, et al. Linear morphea of the forehead (en coup de sabre). Dermatol Online J. 2012;18:22.
21. Hanson AH, Fivenson DP, Schapiro B. Linear scleroderma in an adolescent woman treated with methotrexate and excimer laser. Dermatol Ther. 2014;27:203-205.
22. Zulian F, Martini G, Vallongo C, et al. Methotrexate treatment in juvenile localized scleroderma: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2011;63:1998-2006.
23. Kreuter A, Gambichler T, Breuckmann F, et al. Pulsed high-dose corticosteroids combined with low-dose methotrexate in severe localized scleroderma. Arch Dermatol. 2005;141:847-852.
24. Weibel L, Sampaio MC, Visentin MT, et al. Evaluation of methotrexate and corticosteroids for the treatment of localized scleroderma (morphoea) in children. Br J Dermatol. 2006;155:1013-1020.
25. Torok KS, Arkachaisri T. Methotrexate and corticosteroids in the treatment of localized scleroderma: a standardized prospective longitudinal single-center study. J Rheumatol. 2012;39:286-294.
26. Nisticò SP, Saraceno R, Schipani C, et al. Different applications of monochromatic excimer light in skin diseases. Photomed Laser Surg. 2009;27:647-654.
27. Zwischenberger BA, Jacobe HT. A systematic review of morphea treatments and therapeutic algorithm. J Am Acad Dermatol. 2011;65:925-941.
28. Karaaltin MV, Akpinar AC, Baghaki S, et al. Treatment of “en coup de sabre” deformity with adipose-derived regenerative cell-enriched fat graft. J Craniofac Surg. 2012;23:103-105.
29. Consorti G, Tieghi R, Clauser LC. Frontal linear scleroderma: long-term result in volumetric restoration of the fronto-orbital area by structural fat grafting. J Craniofac Surg. 2012;23:263-265.
30. Cavusoglu T, Yazici I, Vargel I, et al. Reconstruction of coup de sabre deformity (linear localized scleroderma) by using galeal frontalis muscle flap and demineralized bone matrix combination. J Craniofac Surg. 2011;22:257-258.
31. Robitschek J, Wang D, Hall D. Treatment of linear scleroderma “en coup de sabre” with AlloDerm tissue matrix. Otolaryngol Head Neck Surg. 2008;138:540-541.
1. Careta MF, Romiti R. Localized scleroderma: clinical spectrum and therapeutic update. An Bras Dermatol. 2015;90:62-73.
2. Picket AJ, Carpentieri D, Price H, et al. Early morphea mimicking acquired port-wine stain. Pediatr Dermatol. 2014;31:591-594.
3. Holland KE, Steffes B, Nocton JJ, et al. Linear scleroderma en coup de sabre with associated neurologic abnormalities. Pediatrics. 2006;117:132-136.
4. Goh C, Biswas A, Goldberg LJ. Alopecia with perineural lymphocytes: a clue to linear scleroderma en coup de sabre. J Cutan Pathol. 2012;39:518-520.
5. Kreuter A. Localized scleroderma. Dermatol Ther. 2012;25:135-147.
6. Tolkachjov SN, Patel NG, Tollefson MM. Progressive hemifacial atrophy: a review. Orphanet J Rare Dis. 2015;10:39.
7. Amaral TN, Marques Neto JF, Lapa AT, et al. Neurologic involvement in scleroderma en coup de sabre [published online January 27, 2012]. Autoimmune Dis. 2012;2012:719685.
8. Tollefson MM, Witman PM. En coup de sabre morphea and Parry-Romberg syndrome: a retrospective review of 54 patients. J Am Acad Dermatol. 2007;56:257-263.
9. Zannin ME, Martini G, Athreya BH, et al. Ocular involvement in children with localized scleroderma: a multi-center study. Br J Ophthalmol. 2007;91:1311-1314.
10. Polcari I, Moon A, Mathes EF, et al. Headaches as a presenting symptom of linear morphea en coup de sabre. Pediatrics. 2014;134:1715-1719.
11. Doolittle DA, Lehman VT, Schwartz KM, et al. CNS imaging findings associated with Parry-Romberg syndrome and en coup de sabre: correlation to dermatologic and neurologic abnormalities. Neuroradiology. 2015;57:21-34.
12. Pierre-Louis M, Sperling LC, Wilke MS, et al. Distinctive histopathologic findings in linear morphea (en coup de sabre) alopecia. J Cutan Pathol. 2013;40:580-584.
13. Thareja SK, Sadhwani D, Alan Fenske N. En coup de sabre morphea treated with hyaluronic acid filler. Report of a case and review of the literature. Int J Dermatol. 2015;54:823-826.
14. Fett N, Werth VP. Update on morphea: part I. epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol. 2011;64:217-228.
15. Christen-Zaech S, Hakim MD, Afsar FS, et al. Pediatric morphea (localized scleroderma): review of 136 patients. J Am Acad Dermatol. 2008;59:385-396.
16. Leitenberger JJ, Cayce RL, Haley RW, et al. Distinct autoimmune syndromes in morphea: a review of 245 adult and pediatric cases. Arch Dermatol. 2009;145:545-550.
17. Kurzinski K, Torok KS. Cytokine profiles in localized scleroderma and relationship to clinical features. Cytokine. 2011;55:157-164.
18. Eisendle K, Grabner T, Zelger B. Morphoea: a manifestation of infection with Borrelia species? Br J Dermatol. 2007;157:1189-1198.
19. Gutiérrez-Gómez C, Godínez-Hana AL, García-Hernández M, et al. Lack of IgG antibody seropositivity to Borrelia burgdorferi in patients with Parry-Romberg syndrome and linear morphea en coup de sabre in Mexico. Int J Dermatol. 2014;53:947-951.
20. Miller K, Lehrhoff S, Fischer M, et al. Linear morphea of the forehead (en coup de sabre). Dermatol Online J. 2012;18:22.
21. Hanson AH, Fivenson DP, Schapiro B. Linear scleroderma in an adolescent woman treated with methotrexate and excimer laser. Dermatol Ther. 2014;27:203-205.
22. Zulian F, Martini G, Vallongo C, et al. Methotrexate treatment in juvenile localized scleroderma: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2011;63:1998-2006.
23. Kreuter A, Gambichler T, Breuckmann F, et al. Pulsed high-dose corticosteroids combined with low-dose methotrexate in severe localized scleroderma. Arch Dermatol. 2005;141:847-852.
24. Weibel L, Sampaio MC, Visentin MT, et al. Evaluation of methotrexate and corticosteroids for the treatment of localized scleroderma (morphoea) in children. Br J Dermatol. 2006;155:1013-1020.
25. Torok KS, Arkachaisri T. Methotrexate and corticosteroids in the treatment of localized scleroderma: a standardized prospective longitudinal single-center study. J Rheumatol. 2012;39:286-294.
26. Nisticò SP, Saraceno R, Schipani C, et al. Different applications of monochromatic excimer light in skin diseases. Photomed Laser Surg. 2009;27:647-654.
27. Zwischenberger BA, Jacobe HT. A systematic review of morphea treatments and therapeutic algorithm. J Am Acad Dermatol. 2011;65:925-941.
28. Karaaltin MV, Akpinar AC, Baghaki S, et al. Treatment of “en coup de sabre” deformity with adipose-derived regenerative cell-enriched fat graft. J Craniofac Surg. 2012;23:103-105.
29. Consorti G, Tieghi R, Clauser LC. Frontal linear scleroderma: long-term result in volumetric restoration of the fronto-orbital area by structural fat grafting. J Craniofac Surg. 2012;23:263-265.
30. Cavusoglu T, Yazici I, Vargel I, et al. Reconstruction of coup de sabre deformity (linear localized scleroderma) by using galeal frontalis muscle flap and demineralized bone matrix combination. J Craniofac Surg. 2011;22:257-258.
31. Robitschek J, Wang D, Hall D. Treatment of linear scleroderma “en coup de sabre” with AlloDerm tissue matrix. Otolaryngol Head Neck Surg. 2008;138:540-541.
Practice Points
• En coup de sabre (ECDS) is a rare subtype of linear
scleroderma that is limited to the hemiface in a
unilateral distribution.
• Neurologic involvement is common and should
prompt a comprehensive neurologic workup in
patients suspected to have ECDS or progressive
hemiface atrophy.
• Corticosteroids remain the treatment of choice, but
other modalities such as methotrexate, excimer laser,
and grafting have been used with varying success.
AGA Regional Practice Skills Workshops
The evolution of a free and accessible resource for trainees and early career gastroenterologists
The AGA Trainee and Early Career Committee was formed in 2013 to address the needs of those at the beginning of their careers in gastroenterology. The committee is composed of 12 trainee and early career members, whose mission is to develop and support programs relevant to the needs of young clinicians and researchers in the field of GI. In an initial needs assessment, a survey of GI fellows/trainees was undertaken, which revealed a gap in preparation for the transition from fellowship to practice. In particular, respondents expressed a desire to better understand issues related to practice skills, including health care economics, billing/coding, contract negotiation, and health policy. In addition, some trainees felt uncomfortable bringing questions about their private practice job search to academic faculty, who in turn may not have the necessary experience to provide answers regarding various private practice models and opportunities. Furthermore, fellows have little time and opportunity to learn about the rapidly shifting health care environment that will directly affect their future GI practice. To address these unmet needs, the AGA Trainee and Early Career Committee (in partnership with the Practice Management and Economics Committee as well as the Education and Training Committee) developed a workshop to educate fellows and early career GIs about practice and employment models, contracts and negotiations, compliance, health care policy, and other pertinent topics.
These workshops were designed with a half-day curriculum and based regionally to facilitate attendance as well as to capture the local practice patterns in different regions. They were launched during the 2014-2015 academic year in three cities – Boston, Los Angeles, and Chicago – and received extremely positive feedback from participants.
Since then, 16 additional workshops have been held in the following locations: Columbus, Ohio; Philadelphia; Houston; San Diego; New York; Stanford, Calif.; Pinehurst, N.C.; and Iowa City, Iowa (simulcast). At various times, workshops were held in partnership with local societies such as the New York Society of Gastrointestinal Endoscopy, the North Carolina Society of Gastroenterology, and the Texas Society of Gastrointestinal Endoscopy, which offered additional opportunities for networking. Overall, the 19 regional practice skills workshops held over the last 4 years have reached 420 fellows and early career GIs.
The workshop agenda is focused on issues related to transitioning to life as an independent practitioner, which may not be adequately covered during training. The agenda is similar across locations and includes sessions on career options in research and clinical practice, how to evaluate a job, contract negotiations, health care reform, and work-life balance. Additional topics have been added to certain workshops to tailor it for the region, such as sessions in California related to working at Kaiser Permanente. Local leaders in private practice and regional health systems are often invited as speakers, presenting great opportunities for networking and potential job interviews. The workshops were primarily designed for second- and third-year fellows who are embarking on the job search. However, our feedback shows that medical residents interested in GI as well as early career practitioners also find the material very relevant because it describes the breadth of job possibilities and practical tips for a successful career. As the workshops have evolved, additional topics have been added based on attendee feedback, including those on financial management (e.g., disability insurance, retirement planning), social media, and leadership. All workshops include catered meals and are free to both AGA members and nonmembers.
Workshop attendees highly value the opportunity to network with other participants and pose questions to the speakers in person. However, in the past year we have also explored digitally streaming sessions with great success. In California, the workshop was streamed live from UCLA to an audience in Stanford and Iowa, who were also able to interact with the speakers remotely. The live streaming was very well received, as it offered increased access with the opportunity for real-time interactions with speakers. Based on the positive feedback, we are expanding its use in this current cycle, with the workshop in Ohio on Feb. 16 slated to be the first to be streamed live across the country. We also anticipate making the stream of the upcoming workshop in Boston on March 30 available to all interested fellows and early career GIs in the United States, including Puerto Rico.
Recognizing that the content delivered in these workshops will not change significantly over short periods of time, the highest-rated sessions have been archived on the AGA website for viewing off-line. This allows select content to be viewed on demand by those who cannot attend the live workshops or those who want a refresher course prior to their actual job interview. The current library of 23 videos from various workshop presentations is available on the AGA website and social media platforms and have already generated 1,863 views. To view some of the more recent videos, click here.
Moving forward, we anticipate hosting ongoing workshops at large regional sites, in collaboration with local GI societies, while also continuing to offer live streaming for those who cannot attend in person. We will also expand our library of on-demand content for remote viewing. We look forward to reaching trainees and early career GIs across the country and providing the most relevant and up-to-date materials. Those interested in attending one of the workshops can find more information at http://www.gastro.org/trainees. The Trainee and Early Career committee is also looking to expand to additional cities in future years so that more trainees and early-career GIs can participate in these workshops. As the workshops evolve, we welcome your input regarding additional topics or new formats for presenting the material. If you are interested in having a workshop hosted in your city, please let us know! Contact Carol Brown, senior manager of constituency programs, at [email protected].
Dr. Ketwaroo is assistant professor, Baylor College of Medicine, and therapeutic endoscopist, Michael E DeBakey VA Medical Center, Houston. Dr. Liang is instructor of medicine, division of gastroenterology, NYU Langone Health, and staff physician, VA New York Harbor Health Care System. Ms. Brown is senior manager of constituency programs, AGA. Ms. NuQuay is senior director, member relations and constituency programs, AGA.
The evolution of a free and accessible resource for trainees and early career gastroenterologists
The evolution of a free and accessible resource for trainees and early career gastroenterologists
The AGA Trainee and Early Career Committee was formed in 2013 to address the needs of those at the beginning of their careers in gastroenterology. The committee is composed of 12 trainee and early career members, whose mission is to develop and support programs relevant to the needs of young clinicians and researchers in the field of GI. In an initial needs assessment, a survey of GI fellows/trainees was undertaken, which revealed a gap in preparation for the transition from fellowship to practice. In particular, respondents expressed a desire to better understand issues related to practice skills, including health care economics, billing/coding, contract negotiation, and health policy. In addition, some trainees felt uncomfortable bringing questions about their private practice job search to academic faculty, who in turn may not have the necessary experience to provide answers regarding various private practice models and opportunities. Furthermore, fellows have little time and opportunity to learn about the rapidly shifting health care environment that will directly affect their future GI practice. To address these unmet needs, the AGA Trainee and Early Career Committee (in partnership with the Practice Management and Economics Committee as well as the Education and Training Committee) developed a workshop to educate fellows and early career GIs about practice and employment models, contracts and negotiations, compliance, health care policy, and other pertinent topics.
These workshops were designed with a half-day curriculum and based regionally to facilitate attendance as well as to capture the local practice patterns in different regions. They were launched during the 2014-2015 academic year in three cities – Boston, Los Angeles, and Chicago – and received extremely positive feedback from participants.
Since then, 16 additional workshops have been held in the following locations: Columbus, Ohio; Philadelphia; Houston; San Diego; New York; Stanford, Calif.; Pinehurst, N.C.; and Iowa City, Iowa (simulcast). At various times, workshops were held in partnership with local societies such as the New York Society of Gastrointestinal Endoscopy, the North Carolina Society of Gastroenterology, and the Texas Society of Gastrointestinal Endoscopy, which offered additional opportunities for networking. Overall, the 19 regional practice skills workshops held over the last 4 years have reached 420 fellows and early career GIs.
The workshop agenda is focused on issues related to transitioning to life as an independent practitioner, which may not be adequately covered during training. The agenda is similar across locations and includes sessions on career options in research and clinical practice, how to evaluate a job, contract negotiations, health care reform, and work-life balance. Additional topics have been added to certain workshops to tailor it for the region, such as sessions in California related to working at Kaiser Permanente. Local leaders in private practice and regional health systems are often invited as speakers, presenting great opportunities for networking and potential job interviews. The workshops were primarily designed for second- and third-year fellows who are embarking on the job search. However, our feedback shows that medical residents interested in GI as well as early career practitioners also find the material very relevant because it describes the breadth of job possibilities and practical tips for a successful career. As the workshops have evolved, additional topics have been added based on attendee feedback, including those on financial management (e.g., disability insurance, retirement planning), social media, and leadership. All workshops include catered meals and are free to both AGA members and nonmembers.
Workshop attendees highly value the opportunity to network with other participants and pose questions to the speakers in person. However, in the past year we have also explored digitally streaming sessions with great success. In California, the workshop was streamed live from UCLA to an audience in Stanford and Iowa, who were also able to interact with the speakers remotely. The live streaming was very well received, as it offered increased access with the opportunity for real-time interactions with speakers. Based on the positive feedback, we are expanding its use in this current cycle, with the workshop in Ohio on Feb. 16 slated to be the first to be streamed live across the country. We also anticipate making the stream of the upcoming workshop in Boston on March 30 available to all interested fellows and early career GIs in the United States, including Puerto Rico.
Recognizing that the content delivered in these workshops will not change significantly over short periods of time, the highest-rated sessions have been archived on the AGA website for viewing off-line. This allows select content to be viewed on demand by those who cannot attend the live workshops or those who want a refresher course prior to their actual job interview. The current library of 23 videos from various workshop presentations is available on the AGA website and social media platforms and have already generated 1,863 views. To view some of the more recent videos, click here.
Moving forward, we anticipate hosting ongoing workshops at large regional sites, in collaboration with local GI societies, while also continuing to offer live streaming for those who cannot attend in person. We will also expand our library of on-demand content for remote viewing. We look forward to reaching trainees and early career GIs across the country and providing the most relevant and up-to-date materials. Those interested in attending one of the workshops can find more information at http://www.gastro.org/trainees. The Trainee and Early Career committee is also looking to expand to additional cities in future years so that more trainees and early-career GIs can participate in these workshops. As the workshops evolve, we welcome your input regarding additional topics or new formats for presenting the material. If you are interested in having a workshop hosted in your city, please let us know! Contact Carol Brown, senior manager of constituency programs, at [email protected].
Dr. Ketwaroo is assistant professor, Baylor College of Medicine, and therapeutic endoscopist, Michael E DeBakey VA Medical Center, Houston. Dr. Liang is instructor of medicine, division of gastroenterology, NYU Langone Health, and staff physician, VA New York Harbor Health Care System. Ms. Brown is senior manager of constituency programs, AGA. Ms. NuQuay is senior director, member relations and constituency programs, AGA.
The AGA Trainee and Early Career Committee was formed in 2013 to address the needs of those at the beginning of their careers in gastroenterology. The committee is composed of 12 trainee and early career members, whose mission is to develop and support programs relevant to the needs of young clinicians and researchers in the field of GI. In an initial needs assessment, a survey of GI fellows/trainees was undertaken, which revealed a gap in preparation for the transition from fellowship to practice. In particular, respondents expressed a desire to better understand issues related to practice skills, including health care economics, billing/coding, contract negotiation, and health policy. In addition, some trainees felt uncomfortable bringing questions about their private practice job search to academic faculty, who in turn may not have the necessary experience to provide answers regarding various private practice models and opportunities. Furthermore, fellows have little time and opportunity to learn about the rapidly shifting health care environment that will directly affect their future GI practice. To address these unmet needs, the AGA Trainee and Early Career Committee (in partnership with the Practice Management and Economics Committee as well as the Education and Training Committee) developed a workshop to educate fellows and early career GIs about practice and employment models, contracts and negotiations, compliance, health care policy, and other pertinent topics.
These workshops were designed with a half-day curriculum and based regionally to facilitate attendance as well as to capture the local practice patterns in different regions. They were launched during the 2014-2015 academic year in three cities – Boston, Los Angeles, and Chicago – and received extremely positive feedback from participants.
Since then, 16 additional workshops have been held in the following locations: Columbus, Ohio; Philadelphia; Houston; San Diego; New York; Stanford, Calif.; Pinehurst, N.C.; and Iowa City, Iowa (simulcast). At various times, workshops were held in partnership with local societies such as the New York Society of Gastrointestinal Endoscopy, the North Carolina Society of Gastroenterology, and the Texas Society of Gastrointestinal Endoscopy, which offered additional opportunities for networking. Overall, the 19 regional practice skills workshops held over the last 4 years have reached 420 fellows and early career GIs.
The workshop agenda is focused on issues related to transitioning to life as an independent practitioner, which may not be adequately covered during training. The agenda is similar across locations and includes sessions on career options in research and clinical practice, how to evaluate a job, contract negotiations, health care reform, and work-life balance. Additional topics have been added to certain workshops to tailor it for the region, such as sessions in California related to working at Kaiser Permanente. Local leaders in private practice and regional health systems are often invited as speakers, presenting great opportunities for networking and potential job interviews. The workshops were primarily designed for second- and third-year fellows who are embarking on the job search. However, our feedback shows that medical residents interested in GI as well as early career practitioners also find the material very relevant because it describes the breadth of job possibilities and practical tips for a successful career. As the workshops have evolved, additional topics have been added based on attendee feedback, including those on financial management (e.g., disability insurance, retirement planning), social media, and leadership. All workshops include catered meals and are free to both AGA members and nonmembers.
Workshop attendees highly value the opportunity to network with other participants and pose questions to the speakers in person. However, in the past year we have also explored digitally streaming sessions with great success. In California, the workshop was streamed live from UCLA to an audience in Stanford and Iowa, who were also able to interact with the speakers remotely. The live streaming was very well received, as it offered increased access with the opportunity for real-time interactions with speakers. Based on the positive feedback, we are expanding its use in this current cycle, with the workshop in Ohio on Feb. 16 slated to be the first to be streamed live across the country. We also anticipate making the stream of the upcoming workshop in Boston on March 30 available to all interested fellows and early career GIs in the United States, including Puerto Rico.
Recognizing that the content delivered in these workshops will not change significantly over short periods of time, the highest-rated sessions have been archived on the AGA website for viewing off-line. This allows select content to be viewed on demand by those who cannot attend the live workshops or those who want a refresher course prior to their actual job interview. The current library of 23 videos from various workshop presentations is available on the AGA website and social media platforms and have already generated 1,863 views. To view some of the more recent videos, click here.
Moving forward, we anticipate hosting ongoing workshops at large regional sites, in collaboration with local GI societies, while also continuing to offer live streaming for those who cannot attend in person. We will also expand our library of on-demand content for remote viewing. We look forward to reaching trainees and early career GIs across the country and providing the most relevant and up-to-date materials. Those interested in attending one of the workshops can find more information at http://www.gastro.org/trainees. The Trainee and Early Career committee is also looking to expand to additional cities in future years so that more trainees and early-career GIs can participate in these workshops. As the workshops evolve, we welcome your input regarding additional topics or new formats for presenting the material. If you are interested in having a workshop hosted in your city, please let us know! Contact Carol Brown, senior manager of constituency programs, at [email protected].
Dr. Ketwaroo is assistant professor, Baylor College of Medicine, and therapeutic endoscopist, Michael E DeBakey VA Medical Center, Houston. Dr. Liang is instructor of medicine, division of gastroenterology, NYU Langone Health, and staff physician, VA New York Harbor Health Care System. Ms. Brown is senior manager of constituency programs, AGA. Ms. NuQuay is senior director, member relations and constituency programs, AGA.
Treating OSA with positive airway pressure decreased amyloid levels in CSF
Soluble amyloid-beta in cerebrospinal fluid (CSF) decreased when subjects with obstructive sleep apnea used a positive airway pressure device with good adherence, suggesting that improving sleep could reduce the risk of Alzheimer’s disease in this population.
The small decrease in cerebrospinal amyloid-beta 40 (Ab40) and Ab42 hints at decreased neuronal release of the neurotoxic protein, wrote Yo-El S. Ju, MD, and her colleagues. The report was published online in Annals of Neurology.
Alzheimer’s disease (AD) biomarker studies typically find decreased CSF levels associated with increased Ab brain plaques. But before plaques form, increased soluble Ab in CSF is a risk factor for aggregation. Thus, higher soluble Ab levels in mid-life may suggest a risk of later Ab pathology, wrote Dr. Ju of Washington University, St. Louis.
“We tested individuals without any AD pathology as assessed by Ab42 [in CSF], a highly sensitive biomarker of amyloid plaques,” Dr. Ju and her coauthors wrote. “This means our study findings can be extrapolated to the large population of people with OSA [obstructive sleep apnea], many of whom are middle-aged or younger, and have many years to accrue benefit from AD risk reduction ... The effect of OSA on SWA [slow wave activity], Ab, and possibly tau, is a probable proximal step in a cascade whereby OSA increases the risk of AD.”
The researchers recruited 35 subjects with mild to severe OSA and without abnormal Ab levels in CSF. Subjects used auto-titrating positive airway pressure (PAP) for 1-4 months; 18 were sufficiently compliant to be included in the analysis (more than 4 hours on more than 70% of 30 preceding nights as recorded by the machine). CSF was obtained after a baseline polysomnogram and after the treatment period lasting 1-4 months.
Of the 18 analyzed patients, 7 had mild OSA and 11 had moderate to severe OSA. They were an average of nearly 57 years old with a mean body mass index of 30.4 kg/m2; 7 patients had hypertension.
PAP treatment was effective, indicated by a normalized apnea-hypopnea index and decreased time in hypoxemia. Total sleep time and sleep efficiency were unchanged, but slow-wave activity did increase. As expected, hourly arousals and time in hypoxemia decreased, and hypoxic nadir shifted from an oxygen saturation of 82.5% to 91%.
“As a group, there was no significant change in Ab with treatment,” the researchers wrote. But a correlational analysis found that “greater improvement in OSA was associated with greater decrease in Ab40 and Ab42. Additionally, we found that change in tau negatively correlated with OSA improvement.”
The team suggested a two-factor model to explain the relationship between OSA and Ab levels. “Due to decreased SWA, there would be relatively increased release of Ab into the [interstitial fluid]. However, as OSA severity worsens, pressure effects of obstructive respiratory events impede the clearance of Ab and tau out of the interstitial space, resulting in lower levels in the CSF and an inverse U-shaped curve. In this model, a small improvement in OSA may result in an increase in Ab or tau, whereas a larger improvement in OSA – that ameliorates both SWA and clearance mechanisms – will result in a decrease in Ab and tau.”
The project was funded in part by Philips-Respironics, which provided the devices, and by the National Institutes of Health. Philips-Respironics had no input or role in any other part of the study. The authors had no financial disclosures.
SOURCE: Ju YS et al. Ann Neurol. 2018 Dec 31. doi: 10.1002/ana.25408.
Soluble amyloid-beta in cerebrospinal fluid (CSF) decreased when subjects with obstructive sleep apnea used a positive airway pressure device with good adherence, suggesting that improving sleep could reduce the risk of Alzheimer’s disease in this population.
The small decrease in cerebrospinal amyloid-beta 40 (Ab40) and Ab42 hints at decreased neuronal release of the neurotoxic protein, wrote Yo-El S. Ju, MD, and her colleagues. The report was published online in Annals of Neurology.
Alzheimer’s disease (AD) biomarker studies typically find decreased CSF levels associated with increased Ab brain plaques. But before plaques form, increased soluble Ab in CSF is a risk factor for aggregation. Thus, higher soluble Ab levels in mid-life may suggest a risk of later Ab pathology, wrote Dr. Ju of Washington University, St. Louis.
“We tested individuals without any AD pathology as assessed by Ab42 [in CSF], a highly sensitive biomarker of amyloid plaques,” Dr. Ju and her coauthors wrote. “This means our study findings can be extrapolated to the large population of people with OSA [obstructive sleep apnea], many of whom are middle-aged or younger, and have many years to accrue benefit from AD risk reduction ... The effect of OSA on SWA [slow wave activity], Ab, and possibly tau, is a probable proximal step in a cascade whereby OSA increases the risk of AD.”
The researchers recruited 35 subjects with mild to severe OSA and without abnormal Ab levels in CSF. Subjects used auto-titrating positive airway pressure (PAP) for 1-4 months; 18 were sufficiently compliant to be included in the analysis (more than 4 hours on more than 70% of 30 preceding nights as recorded by the machine). CSF was obtained after a baseline polysomnogram and after the treatment period lasting 1-4 months.
Of the 18 analyzed patients, 7 had mild OSA and 11 had moderate to severe OSA. They were an average of nearly 57 years old with a mean body mass index of 30.4 kg/m2; 7 patients had hypertension.
PAP treatment was effective, indicated by a normalized apnea-hypopnea index and decreased time in hypoxemia. Total sleep time and sleep efficiency were unchanged, but slow-wave activity did increase. As expected, hourly arousals and time in hypoxemia decreased, and hypoxic nadir shifted from an oxygen saturation of 82.5% to 91%.
“As a group, there was no significant change in Ab with treatment,” the researchers wrote. But a correlational analysis found that “greater improvement in OSA was associated with greater decrease in Ab40 and Ab42. Additionally, we found that change in tau negatively correlated with OSA improvement.”
The team suggested a two-factor model to explain the relationship between OSA and Ab levels. “Due to decreased SWA, there would be relatively increased release of Ab into the [interstitial fluid]. However, as OSA severity worsens, pressure effects of obstructive respiratory events impede the clearance of Ab and tau out of the interstitial space, resulting in lower levels in the CSF and an inverse U-shaped curve. In this model, a small improvement in OSA may result in an increase in Ab or tau, whereas a larger improvement in OSA – that ameliorates both SWA and clearance mechanisms – will result in a decrease in Ab and tau.”
The project was funded in part by Philips-Respironics, which provided the devices, and by the National Institutes of Health. Philips-Respironics had no input or role in any other part of the study. The authors had no financial disclosures.
SOURCE: Ju YS et al. Ann Neurol. 2018 Dec 31. doi: 10.1002/ana.25408.
Soluble amyloid-beta in cerebrospinal fluid (CSF) decreased when subjects with obstructive sleep apnea used a positive airway pressure device with good adherence, suggesting that improving sleep could reduce the risk of Alzheimer’s disease in this population.
The small decrease in cerebrospinal amyloid-beta 40 (Ab40) and Ab42 hints at decreased neuronal release of the neurotoxic protein, wrote Yo-El S. Ju, MD, and her colleagues. The report was published online in Annals of Neurology.
Alzheimer’s disease (AD) biomarker studies typically find decreased CSF levels associated with increased Ab brain plaques. But before plaques form, increased soluble Ab in CSF is a risk factor for aggregation. Thus, higher soluble Ab levels in mid-life may suggest a risk of later Ab pathology, wrote Dr. Ju of Washington University, St. Louis.
“We tested individuals without any AD pathology as assessed by Ab42 [in CSF], a highly sensitive biomarker of amyloid plaques,” Dr. Ju and her coauthors wrote. “This means our study findings can be extrapolated to the large population of people with OSA [obstructive sleep apnea], many of whom are middle-aged or younger, and have many years to accrue benefit from AD risk reduction ... The effect of OSA on SWA [slow wave activity], Ab, and possibly tau, is a probable proximal step in a cascade whereby OSA increases the risk of AD.”
The researchers recruited 35 subjects with mild to severe OSA and without abnormal Ab levels in CSF. Subjects used auto-titrating positive airway pressure (PAP) for 1-4 months; 18 were sufficiently compliant to be included in the analysis (more than 4 hours on more than 70% of 30 preceding nights as recorded by the machine). CSF was obtained after a baseline polysomnogram and after the treatment period lasting 1-4 months.
Of the 18 analyzed patients, 7 had mild OSA and 11 had moderate to severe OSA. They were an average of nearly 57 years old with a mean body mass index of 30.4 kg/m2; 7 patients had hypertension.
PAP treatment was effective, indicated by a normalized apnea-hypopnea index and decreased time in hypoxemia. Total sleep time and sleep efficiency were unchanged, but slow-wave activity did increase. As expected, hourly arousals and time in hypoxemia decreased, and hypoxic nadir shifted from an oxygen saturation of 82.5% to 91%.
“As a group, there was no significant change in Ab with treatment,” the researchers wrote. But a correlational analysis found that “greater improvement in OSA was associated with greater decrease in Ab40 and Ab42. Additionally, we found that change in tau negatively correlated with OSA improvement.”
The team suggested a two-factor model to explain the relationship between OSA and Ab levels. “Due to decreased SWA, there would be relatively increased release of Ab into the [interstitial fluid]. However, as OSA severity worsens, pressure effects of obstructive respiratory events impede the clearance of Ab and tau out of the interstitial space, resulting in lower levels in the CSF and an inverse U-shaped curve. In this model, a small improvement in OSA may result in an increase in Ab or tau, whereas a larger improvement in OSA – that ameliorates both SWA and clearance mechanisms – will result in a decrease in Ab and tau.”
The project was funded in part by Philips-Respironics, which provided the devices, and by the National Institutes of Health. Philips-Respironics had no input or role in any other part of the study. The authors had no financial disclosures.
SOURCE: Ju YS et al. Ann Neurol. 2018 Dec 31. doi: 10.1002/ana.25408.
FROM ANNALS OF NEUROLOGY
Key clinical point:
Major finding: After treatment, a correlational analysis found decreases in amyloid-beta 40 and 42.
Study details: The prospective, interventional study comprised 18 subjects.
Disclosures: The project was funded in part by Philips-Respironics, which provided the devices, and by the National Institutes of Health. Philips-Respironics had no input or role in any other part of the study. The authors had no financial disclosures.
Source: Ju YS et al. Ann Neurol. 2018 Dec 31. doi: 10.1002/ana.25408.
What’s Eating You? Bedbugs
Bedbugs are common pests causing several health and economic consequences. With increased travel, pesticide resistance, and a lack of awareness about prevention, bedbugs have become even more difficult to control, especially within large population centers.1 The US Environmental Protection Agency considers bedbugs to be a considerable public health issue.2 Typically, they are found in private residences; however, there have been more reports of bedbugs discovered in the workplace within the last 20 years.3-5 Herein, we present a case of bedbugs presenting in this unusual environment.
Case Report
A 42-year-old man presented to our dermatology clinic with intensely itchy bumps over the bilateral posterior arms of 3 months’ duration. He had no other skin, hair, or nail concerns. Over the last 3 months prior to dermatologic evaluation, he was treated by an outside physician with topical steroids, systemic antibiotics, topical antifungals, and even systemic steroids with no improvement of the lesions or symptoms. On clinical examination at the current presentation, 8 to 10 pink dermal papules coalescing into 10-cm round patches were noted on the bilateral posterior arms (Figure 1). A punch biopsy of the posterior right arm was performed, and histologic analysis showed a dense superficial and deep infiltrate and a perivascular infiltrate of lymphocytes and eosinophils (Figure 2). No notable epidermal changes were observed.
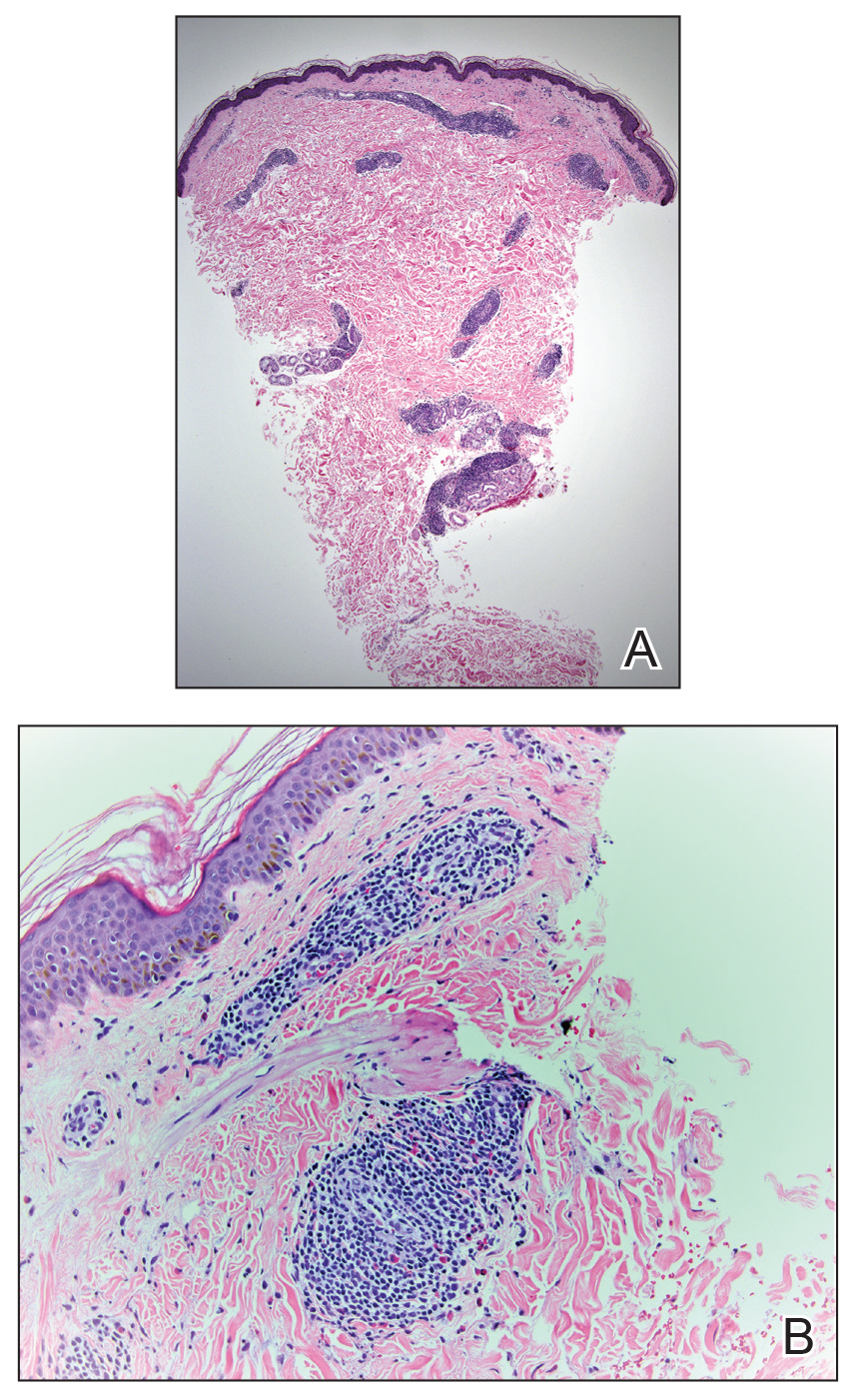
At this time, the patient was counseled that the most likely cause was some unknown arthropod exposure. Given the chronicity of the patient’s disease course, bedbugs were favored; however, an extensive search of the patient’s home failed to uncover any arthropods, let alone bedbugs. A few weeks later, the patient discovered insects emanating from the mesh backing of his office chair while at work (Figure 3). The location of the intruders corresponded exactly with the lesions on the posterior arms. The occupational health office at his workplace collected samples of the arthropods and confirmed they were bedbugs. The patient’s lesions resolved with topical clobetasol once eradication of the workplace was complete.

Discussion
Morphology and Epidemiology
Bedbugs are wingless arthropods that have flat, oval-shaped, reddish brown bodies. They are approximately 4.5-mm long and 2.5-mm wide (Figure 4). The 2 most common species of bedbugs that infect humans are Cimex lectularius and Cimex hemipterus. Bedbugs are most commonly found in hotels, apartments, and residential households near sleep locations. They reside in crevices, cracks, mattresses, cushions, dressers, and other structures proximal to the bed. During the day they remain hidden, but at night they emerge for a blood meal. The average lifespan of a bedbug is 6 to 12 months.6 Females lay more than 200 eggs that hatch in approximately 6 to 10 days.7 Bedbugs progress through 5 nymph stages before becoming adults; several blood meals are required to advance each stage.6

Although commonly attributed to the home, bedbugs are being increasingly seen in the office setting.3-5 In a survey given to pest management professionals in 2015, more than 45% reported that they were contracted by corporations for bedbug infestations in office settings, an increase from 18% in 2010 and 36% in 2013.3 Bedbugs are brought into offices through clothing, luggage, books, and other personal items. Unable to find hosts at night, bedbugs adapt to daytime hours and spread to more unpredictable locations, including chairs, office equipment, desks, and computers.4 Additionally, they frequently move around to find a suitable host.5 As a result, the growth rate of bedbugs in an office setting is much slower than in the home, with fewer insects. Our patient did not have bedbugs at home, but it is possible that other employees transported them to the office over time.
Clinical Manifestations
Bedbugs cause pruritic and nonpruritic skin rashes, often of the arms, legs, neck, and face. A common reaction is an erythematous papule with a hemorrhagic punctum caused by one bite.8 Other presentations include purpuric macules, bullae, and papular urticaria.8-10 Although bedbugs are suspected to transmit infectious diseases, no reports have substantiated that claim.11
Our patient had several coalescing dermal papules on the arms indicating multiple bites around the same area. Due to the stationary aspect of his job—with the arms resting on his chair while typing at his desk—our patient was an easy target for consistent blood meals.
Detection
Due to an overall smaller population of insects in an office setting, detection of bedbugs in the workplace can be difficult. Infestations can be primarily identified on visual inspection by pest control.12 The mesh backing on our patient’s chair was one site where bedbugs resided. It is important to check areas where employees congregate, such as lounges, lunch areas, conference rooms, and printers.4 It also is essential to examine coatracks and locker rooms, as employees may leave personal items that can serve as a source of transmission of the bugs from home. Additional detection tools provided by pest management professionals include canines, as well as devices that emit pheromones, carbon dioxide, or heat to ensnare the insects.12
Treatment
Treatment of bedbug bites is quite variable. For some patients, lesions may resolve on their own. Pruritic maculopapular eruptions can be treated with topical pramoxine or doxepin.8 Patients who develop allergic urticaria can use oral antihistamines. Systemic reactions such as anaphylaxis can be treated with a combination of intramuscular epinephrine, antihistamines, and corticosteroids.8 The etiology of our patient’s condition initially was unknown, and thus he was given unnecessary systemic steroids and antifungals until the source of the rash was identified and eradicated. Topical clobetasol was subsequently administered and was sufficient to resolve his symptoms.
Final Thoughts
Bedbugs continue to remain a nuisance in the home. This case provides an example of bedbugs in the office, a location that is not commonly associated with bedbug infestations. Bedbugs pose numerous psychological, economic, and health consequences.2 Productivity can be reduced, as patients with symptomatic lesions will be unable to work effectively, and those who are unaffected may be unwilling to work knowing their office environment poses a health risk. In addition, employees may worry about bringing the bedbugs home. It is important that employees be educated on the signs of a bedbug infestation and take preventive measures to stop spreading or introducing them to the office space. Due to the scattered habitation of bedbugs in offices, pest control managers need to be vigilant to identify sources of infestation and eradicate accordingly. Clinical manifestations can be nonspecific, resembling autoimmune disorders, fungal infections, or bites from other various arthropods; thus, treatment is highly dependent on the patient’s history and occupational exposure.
Bedbugs have successfully adapted to a new environment in the office space. Dermatologists and other health care professionals can no longer exclusively associate bedbugs with the home. When the clinical and histological presentation suggests an arthropod assault, we must counsel our patients to surveil their homes and work settings alike. If necessary, they should seek the assistance of occupational health professionals.
1. Ralph N, Jones HE, Thorpe LE. Self-reported bed bug infestation among New York City residents: prevalence and risk factors. J Environ Health; 2013;76:38-45.
2. US Environmental Protection Agency. Bed Bugs are public health pests. EPA website. https://www.epa.gov/bedbugs/bed-bugs-are-public-health-pests. Accessed December 6, 2018.
3. Potter MF, Haynes KF, Fredericks J. Bed bugs across America: 2015 Bugs Without Borders survey. Pestworld. 2015:4-14. https://www.npmapestworld.org/default/assets/File/newsroom/magazine/2015/nov-dec_2015.pdf. Accessed December 6, 2018.
4. Pinto LJ, Cooper R, Kraft SK. Bed bugs in office buildings: the ultimate challenge? MGK website. http://giecdn.blob.core.windows.net/fileuploads/file/bedbugs-office-buildings.pdf. Accessed December 6, 2018.
5. Baumblatt JA, Dunn JR, Schaffner W, et al. An outbreak of bed bug infestation in an office building. J Environ Health. 2014;76:16-19.
6. Parasites: bed bugs. Centers for Disease Control and Prevention website. www.cdc.gov/parasites/bedbugs/biology.html. Updated March 17, 2015. Accessed September 21, 2018.
7. Bed bugs. University of Minnesota Extension website. https://www.extension.umn.edu/garden/insects/find/bed-bugs-in-residences. Accessed September 21, 2018.
8. Goddard J, deShazo R. Bed bugs (Cimex lectularius) and clinical consequences of their bites. JAMA. 2009;301:1358-1366.
9. Scarupa, MD, Economides A. Bedbug bites masquerading as urticaria. J Allergy Clin Immunol. 2006;117:1508-1509.
10. Abdel-Naser MB, Lotfy RA, Al-Sherbiny MM, et al. Patients with papular urticaria have IgG antibodies to bedbug (Cimex lectularius) antigens. Parasitol Res. 2006;98:550-556.
11. Lai O, Ho D, Glick S, et al. Bed bugs and possible transmission of human pathogens: a systematic review. Arch Dermatol Res. 2016;308:531-538.
12. Vaidyanathan R, Feldlaufer MF. Bed bug detection: current technologies and future directions. Am J Trop Med Hyg. 2013;88:619-625.
Bedbugs are common pests causing several health and economic consequences. With increased travel, pesticide resistance, and a lack of awareness about prevention, bedbugs have become even more difficult to control, especially within large population centers.1 The US Environmental Protection Agency considers bedbugs to be a considerable public health issue.2 Typically, they are found in private residences; however, there have been more reports of bedbugs discovered in the workplace within the last 20 years.3-5 Herein, we present a case of bedbugs presenting in this unusual environment.
Case Report
A 42-year-old man presented to our dermatology clinic with intensely itchy bumps over the bilateral posterior arms of 3 months’ duration. He had no other skin, hair, or nail concerns. Over the last 3 months prior to dermatologic evaluation, he was treated by an outside physician with topical steroids, systemic antibiotics, topical antifungals, and even systemic steroids with no improvement of the lesions or symptoms. On clinical examination at the current presentation, 8 to 10 pink dermal papules coalescing into 10-cm round patches were noted on the bilateral posterior arms (Figure 1). A punch biopsy of the posterior right arm was performed, and histologic analysis showed a dense superficial and deep infiltrate and a perivascular infiltrate of lymphocytes and eosinophils (Figure 2). No notable epidermal changes were observed.


At this time, the patient was counseled that the most likely cause was some unknown arthropod exposure. Given the chronicity of the patient’s disease course, bedbugs were favored; however, an extensive search of the patient’s home failed to uncover any arthropods, let alone bedbugs. A few weeks later, the patient discovered insects emanating from the mesh backing of his office chair while at work (Figure 3). The location of the intruders corresponded exactly with the lesions on the posterior arms. The occupational health office at his workplace collected samples of the arthropods and confirmed they were bedbugs. The patient’s lesions resolved with topical clobetasol once eradication of the workplace was complete.

Discussion
Morphology and Epidemiology
Bedbugs are wingless arthropods that have flat, oval-shaped, reddish brown bodies. They are approximately 4.5-mm long and 2.5-mm wide (Figure 4). The 2 most common species of bedbugs that infect humans are Cimex lectularius and Cimex hemipterus. Bedbugs are most commonly found in hotels, apartments, and residential households near sleep locations. They reside in crevices, cracks, mattresses, cushions, dressers, and other structures proximal to the bed. During the day they remain hidden, but at night they emerge for a blood meal. The average lifespan of a bedbug is 6 to 12 months.6 Females lay more than 200 eggs that hatch in approximately 6 to 10 days.7 Bedbugs progress through 5 nymph stages before becoming adults; several blood meals are required to advance each stage.6

Although commonly attributed to the home, bedbugs are being increasingly seen in the office setting.3-5 In a survey given to pest management professionals in 2015, more than 45% reported that they were contracted by corporations for bedbug infestations in office settings, an increase from 18% in 2010 and 36% in 2013.3 Bedbugs are brought into offices through clothing, luggage, books, and other personal items. Unable to find hosts at night, bedbugs adapt to daytime hours and spread to more unpredictable locations, including chairs, office equipment, desks, and computers.4 Additionally, they frequently move around to find a suitable host.5 As a result, the growth rate of bedbugs in an office setting is much slower than in the home, with fewer insects. Our patient did not have bedbugs at home, but it is possible that other employees transported them to the office over time.
Clinical Manifestations
Bedbugs cause pruritic and nonpruritic skin rashes, often of the arms, legs, neck, and face. A common reaction is an erythematous papule with a hemorrhagic punctum caused by one bite.8 Other presentations include purpuric macules, bullae, and papular urticaria.8-10 Although bedbugs are suspected to transmit infectious diseases, no reports have substantiated that claim.11
Our patient had several coalescing dermal papules on the arms indicating multiple bites around the same area. Due to the stationary aspect of his job—with the arms resting on his chair while typing at his desk—our patient was an easy target for consistent blood meals.
Detection
Due to an overall smaller population of insects in an office setting, detection of bedbugs in the workplace can be difficult. Infestations can be primarily identified on visual inspection by pest control.12 The mesh backing on our patient’s chair was one site where bedbugs resided. It is important to check areas where employees congregate, such as lounges, lunch areas, conference rooms, and printers.4 It also is essential to examine coatracks and locker rooms, as employees may leave personal items that can serve as a source of transmission of the bugs from home. Additional detection tools provided by pest management professionals include canines, as well as devices that emit pheromones, carbon dioxide, or heat to ensnare the insects.12
Treatment
Treatment of bedbug bites is quite variable. For some patients, lesions may resolve on their own. Pruritic maculopapular eruptions can be treated with topical pramoxine or doxepin.8 Patients who develop allergic urticaria can use oral antihistamines. Systemic reactions such as anaphylaxis can be treated with a combination of intramuscular epinephrine, antihistamines, and corticosteroids.8 The etiology of our patient’s condition initially was unknown, and thus he was given unnecessary systemic steroids and antifungals until the source of the rash was identified and eradicated. Topical clobetasol was subsequently administered and was sufficient to resolve his symptoms.
Final Thoughts
Bedbugs continue to remain a nuisance in the home. This case provides an example of bedbugs in the office, a location that is not commonly associated with bedbug infestations. Bedbugs pose numerous psychological, economic, and health consequences.2 Productivity can be reduced, as patients with symptomatic lesions will be unable to work effectively, and those who are unaffected may be unwilling to work knowing their office environment poses a health risk. In addition, employees may worry about bringing the bedbugs home. It is important that employees be educated on the signs of a bedbug infestation and take preventive measures to stop spreading or introducing them to the office space. Due to the scattered habitation of bedbugs in offices, pest control managers need to be vigilant to identify sources of infestation and eradicate accordingly. Clinical manifestations can be nonspecific, resembling autoimmune disorders, fungal infections, or bites from other various arthropods; thus, treatment is highly dependent on the patient’s history and occupational exposure.
Bedbugs have successfully adapted to a new environment in the office space. Dermatologists and other health care professionals can no longer exclusively associate bedbugs with the home. When the clinical and histological presentation suggests an arthropod assault, we must counsel our patients to surveil their homes and work settings alike. If necessary, they should seek the assistance of occupational health professionals.
Bedbugs are common pests causing several health and economic consequences. With increased travel, pesticide resistance, and a lack of awareness about prevention, bedbugs have become even more difficult to control, especially within large population centers.1 The US Environmental Protection Agency considers bedbugs to be a considerable public health issue.2 Typically, they are found in private residences; however, there have been more reports of bedbugs discovered in the workplace within the last 20 years.3-5 Herein, we present a case of bedbugs presenting in this unusual environment.
Case Report
A 42-year-old man presented to our dermatology clinic with intensely itchy bumps over the bilateral posterior arms of 3 months’ duration. He had no other skin, hair, or nail concerns. Over the last 3 months prior to dermatologic evaluation, he was treated by an outside physician with topical steroids, systemic antibiotics, topical antifungals, and even systemic steroids with no improvement of the lesions or symptoms. On clinical examination at the current presentation, 8 to 10 pink dermal papules coalescing into 10-cm round patches were noted on the bilateral posterior arms (Figure 1). A punch biopsy of the posterior right arm was performed, and histologic analysis showed a dense superficial and deep infiltrate and a perivascular infiltrate of lymphocytes and eosinophils (Figure 2). No notable epidermal changes were observed.


At this time, the patient was counseled that the most likely cause was some unknown arthropod exposure. Given the chronicity of the patient’s disease course, bedbugs were favored; however, an extensive search of the patient’s home failed to uncover any arthropods, let alone bedbugs. A few weeks later, the patient discovered insects emanating from the mesh backing of his office chair while at work (Figure 3). The location of the intruders corresponded exactly with the lesions on the posterior arms. The occupational health office at his workplace collected samples of the arthropods and confirmed they were bedbugs. The patient’s lesions resolved with topical clobetasol once eradication of the workplace was complete.

Discussion
Morphology and Epidemiology
Bedbugs are wingless arthropods that have flat, oval-shaped, reddish brown bodies. They are approximately 4.5-mm long and 2.5-mm wide (Figure 4). The 2 most common species of bedbugs that infect humans are Cimex lectularius and Cimex hemipterus. Bedbugs are most commonly found in hotels, apartments, and residential households near sleep locations. They reside in crevices, cracks, mattresses, cushions, dressers, and other structures proximal to the bed. During the day they remain hidden, but at night they emerge for a blood meal. The average lifespan of a bedbug is 6 to 12 months.6 Females lay more than 200 eggs that hatch in approximately 6 to 10 days.7 Bedbugs progress through 5 nymph stages before becoming adults; several blood meals are required to advance each stage.6

Although commonly attributed to the home, bedbugs are being increasingly seen in the office setting.3-5 In a survey given to pest management professionals in 2015, more than 45% reported that they were contracted by corporations for bedbug infestations in office settings, an increase from 18% in 2010 and 36% in 2013.3 Bedbugs are brought into offices through clothing, luggage, books, and other personal items. Unable to find hosts at night, bedbugs adapt to daytime hours and spread to more unpredictable locations, including chairs, office equipment, desks, and computers.4 Additionally, they frequently move around to find a suitable host.5 As a result, the growth rate of bedbugs in an office setting is much slower than in the home, with fewer insects. Our patient did not have bedbugs at home, but it is possible that other employees transported them to the office over time.
Clinical Manifestations
Bedbugs cause pruritic and nonpruritic skin rashes, often of the arms, legs, neck, and face. A common reaction is an erythematous papule with a hemorrhagic punctum caused by one bite.8 Other presentations include purpuric macules, bullae, and papular urticaria.8-10 Although bedbugs are suspected to transmit infectious diseases, no reports have substantiated that claim.11
Our patient had several coalescing dermal papules on the arms indicating multiple bites around the same area. Due to the stationary aspect of his job—with the arms resting on his chair while typing at his desk—our patient was an easy target for consistent blood meals.
Detection
Due to an overall smaller population of insects in an office setting, detection of bedbugs in the workplace can be difficult. Infestations can be primarily identified on visual inspection by pest control.12 The mesh backing on our patient’s chair was one site where bedbugs resided. It is important to check areas where employees congregate, such as lounges, lunch areas, conference rooms, and printers.4 It also is essential to examine coatracks and locker rooms, as employees may leave personal items that can serve as a source of transmission of the bugs from home. Additional detection tools provided by pest management professionals include canines, as well as devices that emit pheromones, carbon dioxide, or heat to ensnare the insects.12
Treatment
Treatment of bedbug bites is quite variable. For some patients, lesions may resolve on their own. Pruritic maculopapular eruptions can be treated with topical pramoxine or doxepin.8 Patients who develop allergic urticaria can use oral antihistamines. Systemic reactions such as anaphylaxis can be treated with a combination of intramuscular epinephrine, antihistamines, and corticosteroids.8 The etiology of our patient’s condition initially was unknown, and thus he was given unnecessary systemic steroids and antifungals until the source of the rash was identified and eradicated. Topical clobetasol was subsequently administered and was sufficient to resolve his symptoms.
Final Thoughts
Bedbugs continue to remain a nuisance in the home. This case provides an example of bedbugs in the office, a location that is not commonly associated with bedbug infestations. Bedbugs pose numerous psychological, economic, and health consequences.2 Productivity can be reduced, as patients with symptomatic lesions will be unable to work effectively, and those who are unaffected may be unwilling to work knowing their office environment poses a health risk. In addition, employees may worry about bringing the bedbugs home. It is important that employees be educated on the signs of a bedbug infestation and take preventive measures to stop spreading or introducing them to the office space. Due to the scattered habitation of bedbugs in offices, pest control managers need to be vigilant to identify sources of infestation and eradicate accordingly. Clinical manifestations can be nonspecific, resembling autoimmune disorders, fungal infections, or bites from other various arthropods; thus, treatment is highly dependent on the patient’s history and occupational exposure.
Bedbugs have successfully adapted to a new environment in the office space. Dermatologists and other health care professionals can no longer exclusively associate bedbugs with the home. When the clinical and histological presentation suggests an arthropod assault, we must counsel our patients to surveil their homes and work settings alike. If necessary, they should seek the assistance of occupational health professionals.
1. Ralph N, Jones HE, Thorpe LE. Self-reported bed bug infestation among New York City residents: prevalence and risk factors. J Environ Health; 2013;76:38-45.
2. US Environmental Protection Agency. Bed Bugs are public health pests. EPA website. https://www.epa.gov/bedbugs/bed-bugs-are-public-health-pests. Accessed December 6, 2018.
3. Potter MF, Haynes KF, Fredericks J. Bed bugs across America: 2015 Bugs Without Borders survey. Pestworld. 2015:4-14. https://www.npmapestworld.org/default/assets/File/newsroom/magazine/2015/nov-dec_2015.pdf. Accessed December 6, 2018.
4. Pinto LJ, Cooper R, Kraft SK. Bed bugs in office buildings: the ultimate challenge? MGK website. http://giecdn.blob.core.windows.net/fileuploads/file/bedbugs-office-buildings.pdf. Accessed December 6, 2018.
5. Baumblatt JA, Dunn JR, Schaffner W, et al. An outbreak of bed bug infestation in an office building. J Environ Health. 2014;76:16-19.
6. Parasites: bed bugs. Centers for Disease Control and Prevention website. www.cdc.gov/parasites/bedbugs/biology.html. Updated March 17, 2015. Accessed September 21, 2018.
7. Bed bugs. University of Minnesota Extension website. https://www.extension.umn.edu/garden/insects/find/bed-bugs-in-residences. Accessed September 21, 2018.
8. Goddard J, deShazo R. Bed bugs (Cimex lectularius) and clinical consequences of their bites. JAMA. 2009;301:1358-1366.
9. Scarupa, MD, Economides A. Bedbug bites masquerading as urticaria. J Allergy Clin Immunol. 2006;117:1508-1509.
10. Abdel-Naser MB, Lotfy RA, Al-Sherbiny MM, et al. Patients with papular urticaria have IgG antibodies to bedbug (Cimex lectularius) antigens. Parasitol Res. 2006;98:550-556.
11. Lai O, Ho D, Glick S, et al. Bed bugs and possible transmission of human pathogens: a systematic review. Arch Dermatol Res. 2016;308:531-538.
12. Vaidyanathan R, Feldlaufer MF. Bed bug detection: current technologies and future directions. Am J Trop Med Hyg. 2013;88:619-625.
1. Ralph N, Jones HE, Thorpe LE. Self-reported bed bug infestation among New York City residents: prevalence and risk factors. J Environ Health; 2013;76:38-45.
2. US Environmental Protection Agency. Bed Bugs are public health pests. EPA website. https://www.epa.gov/bedbugs/bed-bugs-are-public-health-pests. Accessed December 6, 2018.
3. Potter MF, Haynes KF, Fredericks J. Bed bugs across America: 2015 Bugs Without Borders survey. Pestworld. 2015:4-14. https://www.npmapestworld.org/default/assets/File/newsroom/magazine/2015/nov-dec_2015.pdf. Accessed December 6, 2018.
4. Pinto LJ, Cooper R, Kraft SK. Bed bugs in office buildings: the ultimate challenge? MGK website. http://giecdn.blob.core.windows.net/fileuploads/file/bedbugs-office-buildings.pdf. Accessed December 6, 2018.
5. Baumblatt JA, Dunn JR, Schaffner W, et al. An outbreak of bed bug infestation in an office building. J Environ Health. 2014;76:16-19.
6. Parasites: bed bugs. Centers for Disease Control and Prevention website. www.cdc.gov/parasites/bedbugs/biology.html. Updated March 17, 2015. Accessed September 21, 2018.
7. Bed bugs. University of Minnesota Extension website. https://www.extension.umn.edu/garden/insects/find/bed-bugs-in-residences. Accessed September 21, 2018.
8. Goddard J, deShazo R. Bed bugs (Cimex lectularius) and clinical consequences of their bites. JAMA. 2009;301:1358-1366.
9. Scarupa, MD, Economides A. Bedbug bites masquerading as urticaria. J Allergy Clin Immunol. 2006;117:1508-1509.
10. Abdel-Naser MB, Lotfy RA, Al-Sherbiny MM, et al. Patients with papular urticaria have IgG antibodies to bedbug (Cimex lectularius) antigens. Parasitol Res. 2006;98:550-556.
11. Lai O, Ho D, Glick S, et al. Bed bugs and possible transmission of human pathogens: a systematic review. Arch Dermatol Res. 2016;308:531-538.
12. Vaidyanathan R, Feldlaufer MF. Bed bug detection: current technologies and future directions. Am J Trop Med Hyg. 2013;88:619-625.
Practice Points
- Bedbug exposures in the workplace are on the rise.
- High clinical suspicion is required when atypical dermatoses are not responding to therapy and histology suggests arthropod exposure.
- Once detected, partnership with occupational health and pest management experts is critical to eradicate bedbugs.
Finding your first job: Tips for picking the right practice
Editor’s Note: This is the second installment of the Private Practice Perspectives column, which is a collaboration between the AGA’s The New Gastroenterologist and the Digestive Health Physicians Association (DHPA). In this issue’s column, David Ramsay (Winston Salem, N.C.) provides valuable advice on the very important topic of picking the right practice.
Bryson W. Katona, MD, PhD
Editor in Chief, The New Gastroenterologist
Just 7 years ago, I faced the same difficult decisions many new gastroenterologists have. Like many physicians coming out of a residency and fellowship program, I had loans to repay and family to consider when evaluating the choices about where I would practice.
Looking back, there were several essential questions that helped guide my decision-making process. If you are early in your career as a GI, here are some questions to ask yourself and tips that I’ve learned along the way that may help make the decision about which practice is right for you.
What do you want to do with your training and skills? This may sound obvious, but it’s important to align your interests with the right practice. Did you receive extra training in endoscopic procedures, such as endoscopic ultrasonography and endoscopic retrograde cholangiopancreatography? Do you want to specialize in inflammatory bowel disease? Have a passion for hepatology? Look for a practice that has those specific opportunities available to match your interests.
In addition, some GI docs want to pursue their interest in research. Keep in mind that many independent practices have research arms and offer physicians the opportunity to continue on this path.
Lastly, consider whether you want to be involved in the business of medicine or take on a leadership role. Many practices offer (and even encourage) those opportunities, and you can winnow down your list of practices based on whether they allow you to take on those roles.
Where do you want to live? My wife and I completed our residencies and fellowships in Washington, but when it came time to find a place to practice medicine, we knew we wanted to be near family. We narrowed our search to Tennessee, Florida, and North Carolina, where we eventually ended up.
Of course, wherever you decide to go is a personal choice. Some people prefer living on the coasts or want to reside in a major city. This might come as a surprise to some, but very often you will command a higher salary in rural areas or smaller cities, which are traditionally underserved by our profession. That starts to matter when you think about paying off your student loan debt.
What is the long-term potential of each position? This is perhaps the most important question to ask. Does your new practice offer ownership potential? Are there opportunities to share in the various (ancillary) revenue streams, such as an ambulatory surgery center, anesthesia, or pathology? How soon might you have the opportunity to buy in and what is the buy in structure and cost? What are the practice rules around offering partnerships?
These are all questions that you should ask up front. Remember that the lifestyle you start out with may change over the course of your career. Find a practice that offers opportunities for growth because your long-term income potential is much more important than your starting salary or size of any sign-on bonus.
Once you’ve decided the answers to some of these questions, here are a few tips to help you land a job at the right medical practice.
Talk to your mentors and tap into your connections: Most GI physicians completing a fellowship will have mentors who have connections to practices. Speak with them about where to look. In addition, most medical societies and state-specific GI societies post classified job listings. Use these professional memberships.
Don’t be afraid of the cold call: If you know where you might want to live, you should consider cold calls to practices in the area to see what opportunities are available. That’s how I found my job. I started calling practices in North Carolina. Those that didn’t have openings knew of, and shared names of, practices in the state that did.
Call the local hospitals and ask to speak to the charge nurse in endoscopy: This is one the best tips I got to help narrow the field. These nurses are a great source of information with honest feedback about the reputation of the local GI practices.
Look for collegiality: This can be harder to spot, but it’s a good sign when the CEOs or practice administrators are engaging and take the time to answer questions.
Look for groups that don’t have a lot of turnover: This is another important sign. We call it the churn and burn: We all know of fellows who have joined a practice where they work long hours but never have the opportunity to make partner. You might ask the question directly: How many physicians have come here and left within the first 5 years of employment? A high turnover rate is a red flag. No matter what type of practice you choose, the key is to look at your long-term prospects, not just at short-term rewards. After all, that’s what will give you the greatest opportunities – and likely make you happiest in your career.
David Ramsay, MD, is treasurer of the Digestive Health Physicians Association. He is President of Digestive Health Specialists in Winston Salem, N.C., which he joined in 2012 after working in the Washington area.
Editor’s Note: This is the second installment of the Private Practice Perspectives column, which is a collaboration between the AGA’s The New Gastroenterologist and the Digestive Health Physicians Association (DHPA). In this issue’s column, David Ramsay (Winston Salem, N.C.) provides valuable advice on the very important topic of picking the right practice.
Bryson W. Katona, MD, PhD
Editor in Chief, The New Gastroenterologist
Just 7 years ago, I faced the same difficult decisions many new gastroenterologists have. Like many physicians coming out of a residency and fellowship program, I had loans to repay and family to consider when evaluating the choices about where I would practice.
Looking back, there were several essential questions that helped guide my decision-making process. If you are early in your career as a GI, here are some questions to ask yourself and tips that I’ve learned along the way that may help make the decision about which practice is right for you.
What do you want to do with your training and skills? This may sound obvious, but it’s important to align your interests with the right practice. Did you receive extra training in endoscopic procedures, such as endoscopic ultrasonography and endoscopic retrograde cholangiopancreatography? Do you want to specialize in inflammatory bowel disease? Have a passion for hepatology? Look for a practice that has those specific opportunities available to match your interests.
In addition, some GI docs want to pursue their interest in research. Keep in mind that many independent practices have research arms and offer physicians the opportunity to continue on this path.
Lastly, consider whether you want to be involved in the business of medicine or take on a leadership role. Many practices offer (and even encourage) those opportunities, and you can winnow down your list of practices based on whether they allow you to take on those roles.
Where do you want to live? My wife and I completed our residencies and fellowships in Washington, but when it came time to find a place to practice medicine, we knew we wanted to be near family. We narrowed our search to Tennessee, Florida, and North Carolina, where we eventually ended up.
Of course, wherever you decide to go is a personal choice. Some people prefer living on the coasts or want to reside in a major city. This might come as a surprise to some, but very often you will command a higher salary in rural areas or smaller cities, which are traditionally underserved by our profession. That starts to matter when you think about paying off your student loan debt.
What is the long-term potential of each position? This is perhaps the most important question to ask. Does your new practice offer ownership potential? Are there opportunities to share in the various (ancillary) revenue streams, such as an ambulatory surgery center, anesthesia, or pathology? How soon might you have the opportunity to buy in and what is the buy in structure and cost? What are the practice rules around offering partnerships?
These are all questions that you should ask up front. Remember that the lifestyle you start out with may change over the course of your career. Find a practice that offers opportunities for growth because your long-term income potential is much more important than your starting salary or size of any sign-on bonus.
Once you’ve decided the answers to some of these questions, here are a few tips to help you land a job at the right medical practice.
Talk to your mentors and tap into your connections: Most GI physicians completing a fellowship will have mentors who have connections to practices. Speak with them about where to look. In addition, most medical societies and state-specific GI societies post classified job listings. Use these professional memberships.
Don’t be afraid of the cold call: If you know where you might want to live, you should consider cold calls to practices in the area to see what opportunities are available. That’s how I found my job. I started calling practices in North Carolina. Those that didn’t have openings knew of, and shared names of, practices in the state that did.
Call the local hospitals and ask to speak to the charge nurse in endoscopy: This is one the best tips I got to help narrow the field. These nurses are a great source of information with honest feedback about the reputation of the local GI practices.
Look for collegiality: This can be harder to spot, but it’s a good sign when the CEOs or practice administrators are engaging and take the time to answer questions.
Look for groups that don’t have a lot of turnover: This is another important sign. We call it the churn and burn: We all know of fellows who have joined a practice where they work long hours but never have the opportunity to make partner. You might ask the question directly: How many physicians have come here and left within the first 5 years of employment? A high turnover rate is a red flag. No matter what type of practice you choose, the key is to look at your long-term prospects, not just at short-term rewards. After all, that’s what will give you the greatest opportunities – and likely make you happiest in your career.
David Ramsay, MD, is treasurer of the Digestive Health Physicians Association. He is President of Digestive Health Specialists in Winston Salem, N.C., which he joined in 2012 after working in the Washington area.
Editor’s Note: This is the second installment of the Private Practice Perspectives column, which is a collaboration between the AGA’s The New Gastroenterologist and the Digestive Health Physicians Association (DHPA). In this issue’s column, David Ramsay (Winston Salem, N.C.) provides valuable advice on the very important topic of picking the right practice.
Bryson W. Katona, MD, PhD
Editor in Chief, The New Gastroenterologist
Just 7 years ago, I faced the same difficult decisions many new gastroenterologists have. Like many physicians coming out of a residency and fellowship program, I had loans to repay and family to consider when evaluating the choices about where I would practice.
Looking back, there were several essential questions that helped guide my decision-making process. If you are early in your career as a GI, here are some questions to ask yourself and tips that I’ve learned along the way that may help make the decision about which practice is right for you.
What do you want to do with your training and skills? This may sound obvious, but it’s important to align your interests with the right practice. Did you receive extra training in endoscopic procedures, such as endoscopic ultrasonography and endoscopic retrograde cholangiopancreatography? Do you want to specialize in inflammatory bowel disease? Have a passion for hepatology? Look for a practice that has those specific opportunities available to match your interests.
In addition, some GI docs want to pursue their interest in research. Keep in mind that many independent practices have research arms and offer physicians the opportunity to continue on this path.
Lastly, consider whether you want to be involved in the business of medicine or take on a leadership role. Many practices offer (and even encourage) those opportunities, and you can winnow down your list of practices based on whether they allow you to take on those roles.
Where do you want to live? My wife and I completed our residencies and fellowships in Washington, but when it came time to find a place to practice medicine, we knew we wanted to be near family. We narrowed our search to Tennessee, Florida, and North Carolina, where we eventually ended up.
Of course, wherever you decide to go is a personal choice. Some people prefer living on the coasts or want to reside in a major city. This might come as a surprise to some, but very often you will command a higher salary in rural areas or smaller cities, which are traditionally underserved by our profession. That starts to matter when you think about paying off your student loan debt.
What is the long-term potential of each position? This is perhaps the most important question to ask. Does your new practice offer ownership potential? Are there opportunities to share in the various (ancillary) revenue streams, such as an ambulatory surgery center, anesthesia, or pathology? How soon might you have the opportunity to buy in and what is the buy in structure and cost? What are the practice rules around offering partnerships?
These are all questions that you should ask up front. Remember that the lifestyle you start out with may change over the course of your career. Find a practice that offers opportunities for growth because your long-term income potential is much more important than your starting salary or size of any sign-on bonus.
Once you’ve decided the answers to some of these questions, here are a few tips to help you land a job at the right medical practice.
Talk to your mentors and tap into your connections: Most GI physicians completing a fellowship will have mentors who have connections to practices. Speak with them about where to look. In addition, most medical societies and state-specific GI societies post classified job listings. Use these professional memberships.
Don’t be afraid of the cold call: If you know where you might want to live, you should consider cold calls to practices in the area to see what opportunities are available. That’s how I found my job. I started calling practices in North Carolina. Those that didn’t have openings knew of, and shared names of, practices in the state that did.
Call the local hospitals and ask to speak to the charge nurse in endoscopy: This is one the best tips I got to help narrow the field. These nurses are a great source of information with honest feedback about the reputation of the local GI practices.
Look for collegiality: This can be harder to spot, but it’s a good sign when the CEOs or practice administrators are engaging and take the time to answer questions.
Look for groups that don’t have a lot of turnover: This is another important sign. We call it the churn and burn: We all know of fellows who have joined a practice where they work long hours but never have the opportunity to make partner. You might ask the question directly: How many physicians have come here and left within the first 5 years of employment? A high turnover rate is a red flag. No matter what type of practice you choose, the key is to look at your long-term prospects, not just at short-term rewards. After all, that’s what will give you the greatest opportunities – and likely make you happiest in your career.
David Ramsay, MD, is treasurer of the Digestive Health Physicians Association. He is President of Digestive Health Specialists in Winston Salem, N.C., which he joined in 2012 after working in the Washington area.