User login
AAN publishes position statement on brain death
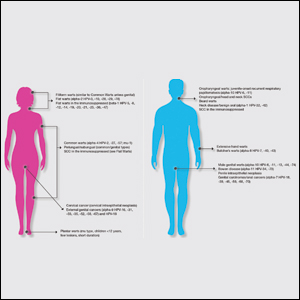
In a position statement published online ahead of print Jan. 2 in Neurology, Such uniformity would reduce uncertainty and improve patient care, according to the authors. The statement, which was drafted by the AAN’s Brain Death Working Group, also supports the development of uniform policies regarding brain death and its determination within American medical institutions. Finally, the document provides neurologists with guidance for responding to requests for accommodation, including objections to the determination of brain death and to the withdrawal of organ-sustaining technology.
The AAN defines brain death as death resulting from irreversible loss of function of the entire brain. The Uniform Determination of Death Act of 1981 held that brain death and circulatory death (that is, death resulting from irreversible loss of function of the circulatory system) are equivalent, and the AAN acknowledges this equivalence.
The two current medical standards for brain death are the AAN’s 2010 Evidence-Based Guideline Update: Determining Brain Death in Adults and the 2011 Guidelines for the Determination of Brain Death in Infants and Children, which was published by the pediatric section of the Society of Critical Care Medicine, the sections of neurology and critical care of the American Academy of Pediatrics, and the Child Neurology Society. “The AAN is unaware of any cases in which compliant application of the brain death guidelines led to inaccurate determination of death with return of any brain function, including consciousness, brainstem reflexes, or ventilatory effort,” according to their 2019 statement.
The only jurisdiction with laws that specifically defer to these standards, however, is Nevada. The vagueness of most states’ laws has contributed to divergent legal interpretations and idiosyncratic standards for determining brain death, according to the statement.
“The AAN believes that a specific, uniform standard for the determination of brain death is critically important to provide the highest quality patient-centered neurologic and end-of-life care,” said James Russell, DO, MS, a neurologist at Lahey Hospital and Medical Center in Burlington, Mass., and lead author of the position statement. “The AAN supports the development of legislation in every state modeled after the Nevada statute, which specifically defers to these current adult and pediatric brain death guidelines and any future updates.”
In addition to uniform institutional policies for determining brain death within U.S. medical facilities, the AAN calls for the development of training programs and credentialing mechanisms for physicians who determine brain death, regardless of their specialties. The association also supports research that enhances understanding of brain death and enhanced professional and public education.
While expressing respect and sympathy for requests for limited accommodation, the AAN asserts that these requests “must be based on the values of the patient, and not those of loved ones or other surrogate decision makers.” The association further observes that physicians have no ethical obligation to provide medical treatment to a deceased patient. New Jersey is the only state that legally obliges physicians to provide indefinite accommodation and continued application of organ-sustaining technology.
“The AAN believes that its members have both the moral authority and professional responsibility, when lawful, to perform a brain death evaluation, including apnea testing, after informing a patient’s loved ones or lawful surrogates of that intention, but without obligation to obtain informed consent,” according to the statement. “This position is analogous to the authority and responsibility historically granted to the medical profession to determine circulatory death without the requirement for additional informed consent.”
If a dispute about indefinite accommodation cannot be resolved, it is acceptable for a physician to withdraw organ-sustaining technology unilaterally over the objection of loved ones when legally permitted, according to the AAN. Such unilateral action is a measure of last resort and does not apply when the patient is a pregnant woman, said the authors. In the latter case, the ethical analysis should focus mainly on the welfare of the fetus.
The AAN provided financial support for the Brain Death Working Group’s efforts. The statement’s authors reported no relevant disclosures. The American Neurological Association and the Child Neurology Society have endorsed the AAN’s position statement.
SOURCE: Russell JA et al. Neurology. 2018 Jan 2. doi: 10.1212/WNL.0000000000006750.
In a position statement published online ahead of print Jan. 2 in Neurology, Such uniformity would reduce uncertainty and improve patient care, according to the authors. The statement, which was drafted by the AAN’s Brain Death Working Group, also supports the development of uniform policies regarding brain death and its determination within American medical institutions. Finally, the document provides neurologists with guidance for responding to requests for accommodation, including objections to the determination of brain death and to the withdrawal of organ-sustaining technology.
The AAN defines brain death as death resulting from irreversible loss of function of the entire brain. The Uniform Determination of Death Act of 1981 held that brain death and circulatory death (that is, death resulting from irreversible loss of function of the circulatory system) are equivalent, and the AAN acknowledges this equivalence.
The two current medical standards for brain death are the AAN’s 2010 Evidence-Based Guideline Update: Determining Brain Death in Adults and the 2011 Guidelines for the Determination of Brain Death in Infants and Children, which was published by the pediatric section of the Society of Critical Care Medicine, the sections of neurology and critical care of the American Academy of Pediatrics, and the Child Neurology Society. “The AAN is unaware of any cases in which compliant application of the brain death guidelines led to inaccurate determination of death with return of any brain function, including consciousness, brainstem reflexes, or ventilatory effort,” according to their 2019 statement.
The only jurisdiction with laws that specifically defer to these standards, however, is Nevada. The vagueness of most states’ laws has contributed to divergent legal interpretations and idiosyncratic standards for determining brain death, according to the statement.
“The AAN believes that a specific, uniform standard for the determination of brain death is critically important to provide the highest quality patient-centered neurologic and end-of-life care,” said James Russell, DO, MS, a neurologist at Lahey Hospital and Medical Center in Burlington, Mass., and lead author of the position statement. “The AAN supports the development of legislation in every state modeled after the Nevada statute, which specifically defers to these current adult and pediatric brain death guidelines and any future updates.”
In addition to uniform institutional policies for determining brain death within U.S. medical facilities, the AAN calls for the development of training programs and credentialing mechanisms for physicians who determine brain death, regardless of their specialties. The association also supports research that enhances understanding of brain death and enhanced professional and public education.
While expressing respect and sympathy for requests for limited accommodation, the AAN asserts that these requests “must be based on the values of the patient, and not those of loved ones or other surrogate decision makers.” The association further observes that physicians have no ethical obligation to provide medical treatment to a deceased patient. New Jersey is the only state that legally obliges physicians to provide indefinite accommodation and continued application of organ-sustaining technology.
“The AAN believes that its members have both the moral authority and professional responsibility, when lawful, to perform a brain death evaluation, including apnea testing, after informing a patient’s loved ones or lawful surrogates of that intention, but without obligation to obtain informed consent,” according to the statement. “This position is analogous to the authority and responsibility historically granted to the medical profession to determine circulatory death without the requirement for additional informed consent.”
If a dispute about indefinite accommodation cannot be resolved, it is acceptable for a physician to withdraw organ-sustaining technology unilaterally over the objection of loved ones when legally permitted, according to the AAN. Such unilateral action is a measure of last resort and does not apply when the patient is a pregnant woman, said the authors. In the latter case, the ethical analysis should focus mainly on the welfare of the fetus.
The AAN provided financial support for the Brain Death Working Group’s efforts. The statement’s authors reported no relevant disclosures. The American Neurological Association and the Child Neurology Society have endorsed the AAN’s position statement.
SOURCE: Russell JA et al. Neurology. 2018 Jan 2. doi: 10.1212/WNL.0000000000006750.
In a position statement published online ahead of print Jan. 2 in Neurology, Such uniformity would reduce uncertainty and improve patient care, according to the authors. The statement, which was drafted by the AAN’s Brain Death Working Group, also supports the development of uniform policies regarding brain death and its determination within American medical institutions. Finally, the document provides neurologists with guidance for responding to requests for accommodation, including objections to the determination of brain death and to the withdrawal of organ-sustaining technology.
The AAN defines brain death as death resulting from irreversible loss of function of the entire brain. The Uniform Determination of Death Act of 1981 held that brain death and circulatory death (that is, death resulting from irreversible loss of function of the circulatory system) are equivalent, and the AAN acknowledges this equivalence.
The two current medical standards for brain death are the AAN’s 2010 Evidence-Based Guideline Update: Determining Brain Death in Adults and the 2011 Guidelines for the Determination of Brain Death in Infants and Children, which was published by the pediatric section of the Society of Critical Care Medicine, the sections of neurology and critical care of the American Academy of Pediatrics, and the Child Neurology Society. “The AAN is unaware of any cases in which compliant application of the brain death guidelines led to inaccurate determination of death with return of any brain function, including consciousness, brainstem reflexes, or ventilatory effort,” according to their 2019 statement.
The only jurisdiction with laws that specifically defer to these standards, however, is Nevada. The vagueness of most states’ laws has contributed to divergent legal interpretations and idiosyncratic standards for determining brain death, according to the statement.
“The AAN believes that a specific, uniform standard for the determination of brain death is critically important to provide the highest quality patient-centered neurologic and end-of-life care,” said James Russell, DO, MS, a neurologist at Lahey Hospital and Medical Center in Burlington, Mass., and lead author of the position statement. “The AAN supports the development of legislation in every state modeled after the Nevada statute, which specifically defers to these current adult and pediatric brain death guidelines and any future updates.”
In addition to uniform institutional policies for determining brain death within U.S. medical facilities, the AAN calls for the development of training programs and credentialing mechanisms for physicians who determine brain death, regardless of their specialties. The association also supports research that enhances understanding of brain death and enhanced professional and public education.
While expressing respect and sympathy for requests for limited accommodation, the AAN asserts that these requests “must be based on the values of the patient, and not those of loved ones or other surrogate decision makers.” The association further observes that physicians have no ethical obligation to provide medical treatment to a deceased patient. New Jersey is the only state that legally obliges physicians to provide indefinite accommodation and continued application of organ-sustaining technology.
“The AAN believes that its members have both the moral authority and professional responsibility, when lawful, to perform a brain death evaluation, including apnea testing, after informing a patient’s loved ones or lawful surrogates of that intention, but without obligation to obtain informed consent,” according to the statement. “This position is analogous to the authority and responsibility historically granted to the medical profession to determine circulatory death without the requirement for additional informed consent.”
If a dispute about indefinite accommodation cannot be resolved, it is acceptable for a physician to withdraw organ-sustaining technology unilaterally over the objection of loved ones when legally permitted, according to the AAN. Such unilateral action is a measure of last resort and does not apply when the patient is a pregnant woman, said the authors. In the latter case, the ethical analysis should focus mainly on the welfare of the fetus.
The AAN provided financial support for the Brain Death Working Group’s efforts. The statement’s authors reported no relevant disclosures. The American Neurological Association and the Child Neurology Society have endorsed the AAN’s position statement.
SOURCE: Russell JA et al. Neurology. 2018 Jan 2. doi: 10.1212/WNL.0000000000006750.
FROM NEUROLOGY
Key clinical point: The AAN calls for uniform brain death laws, policies, and practices.
Major finding: The association published a position statement online on January 2.
Study details: The AAN’s Brain Death Working Group drafted the statement.
Disclosures: The authors reported no relevant disclosures, and the American Academy of Neurology funded their work.
Source: Russell JA et al. Neurology. 2018 Jan 2. doi: 10.1212/WNL.0000000000006750.
Spending on medical marketing increased by $12.2 billion over the last 2 decades
Total spending on medical marketing in the United States increased from $17.7 billion in 1997 to $29.9 billion in 2016, according to an analysis of direct-to-consumer (DTC) and professional marketing for prescription drugs, disease awareness campaigns, health services, and laboratory tests.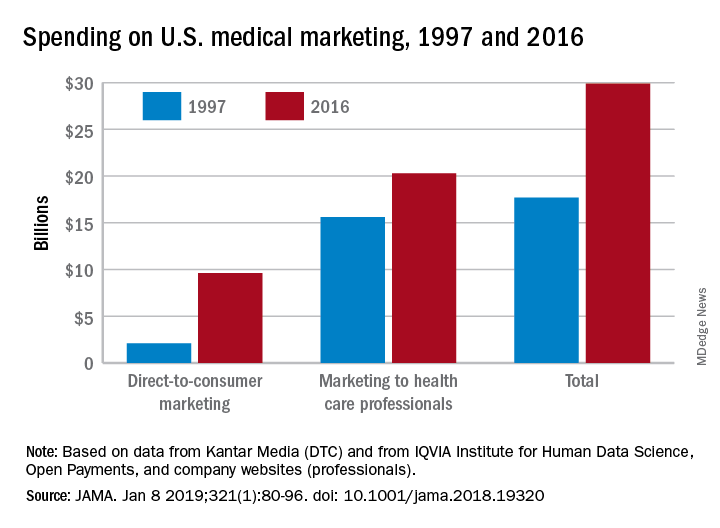
“Increased medical marketing reflects a convergence of scientific, economic, legal, and social forces,” wrote Lisa M. Schwartz, MD, and her coauthor, adding that “although marketing expanded over 20 years, regulatory oversight remains relatively limited.” Dr. Schwartz, then codirector of the Center for Medicine and Media at The Dartmouth Institute in Lebanon, N.H., died in November of 2018, after her work was accepted for publication in JAMA.
Dr. Schwartz and her coauthor, David Woloshin, MD, also of Dartmouth, reviewed consumer advertising and professional marketing data, along with searches of medical literature and business journals, to ascertain the quantity and impact of spending. The most money was spent on marketing to medical professionals, which increased from $15.6 billion in 1997 to $20.3 billion in 2016. In terms of percentages, the biggest increase was seen in DTC advertising: $2.1 billion in 1997 (11.9% of total spending) ballooned to $9.6 billion (32.1% of total spending).
These increases were not accompanied by corresponding regulatory efforts to limit influence or protect patients and consumers. In 2016, the Food and Drug Administration’s Office of Prescription Drug Promotion received 97,252 promotional materials that drug companies submitted for review, compared with 34,182 in 1997, but violation letters for prescription drug advertising decreased from 156 to 11. In the same year, the FDA reviewed 41% of core materials – such as risk disclosures and key messages – for new drugs or indications prior to launch, a performance measure the coauthors called “critically important.”
In regard to disease awareness campaigns, 2004 guidance from the FDA on awareness advertising – including standards for unbranded campaigns and recommendations to avoid encouraging self-diagnosis and self-treatment – was withdrawn in 2015 and never replaced. The Federal Trade Commission, which has jurisdiction over unbranded advertising, has not taken regulatory action of its own; any FDA requests for investigation are unknown. In addition, these 2 decades have not seen state attorneys general initiate any action against deceptive consumer advertising, nor has the FTC acted against misleading laboratory test promotion.
“The FDA and FTC should establish and enforce standards for responsible disease awareness campaigns,” the coauthors wrote, “including criteria to validate symptom quizzes (or banning them) and evidence-based strategies to minimize misconceptions that a drug can treat all symptoms of disease.”
Overall, spending on medical marketing actually increased faster than did spending on health services overall. Marketing saw a remarkable 430% increase ($542 million to $2.9 billion) over the 2 decades, while health services spending increased by 90% ($1.2 trillion to $2.2 trillion).
One of the rare similarities from 1997 to 2016 was spending on marketing prescription drugs to physicians, typically through face-to-face meetings and hospital visits; this held steady at approximately $5 billion. However, spending on drug samples increased from $8.9 billion to $13.5 billion, while medical journal advertising declined drastically from $744 million to $119 million.
Spending on DTC marketing of prescription drugs increased across all therapeutic categories but three: cholesterol, allergy, and osteoporosis, each of which saw top-selling drugs either become over-the-counter or lose patent protection. Spending on drugs for diabetes/endocrine disease went from $27 million in 1997 to a whopping $725 million in 2016, followed by dermatology drugs ($67 million to $605 million) and pain/central nervous system drugs ($56 million to $542 million).
The coauthors shared potential limitations of their study, including the likelihood that they underestimated how much is actually spent on medical marketing. “Data on professional marketing (e.g., detailing) of laboratory tests, health services or devices, and pharmaceutical company spending on coupons or rebates, online promotion, and meetings and events could not be obtained,” they noted. In addition, company marketing budgets often do not include additional expenses that should count toward this total, and any published literature on medical marketing’s return on investment is largely based on observational data and cannot be fully relied upon.
The two coauthors previously served as medical experts in testosterone litigation and were cofounders of a company that provided data about the benefits and harms of prescription drugs, which ceased operations in December 2016. No other conflicts of interest were reported.
SOURCE: Schwartz LM et al. JAMA. 2019 Jan 8. doi: 10.1001/jama.2018.19320.
Total spending on medical marketing in the United States increased from $17.7 billion in 1997 to $29.9 billion in 2016, according to an analysis of direct-to-consumer (DTC) and professional marketing for prescription drugs, disease awareness campaigns, health services, and laboratory tests.
“Increased medical marketing reflects a convergence of scientific, economic, legal, and social forces,” wrote Lisa M. Schwartz, MD, and her coauthor, adding that “although marketing expanded over 20 years, regulatory oversight remains relatively limited.” Dr. Schwartz, then codirector of the Center for Medicine and Media at The Dartmouth Institute in Lebanon, N.H., died in November of 2018, after her work was accepted for publication in JAMA.
Dr. Schwartz and her coauthor, David Woloshin, MD, also of Dartmouth, reviewed consumer advertising and professional marketing data, along with searches of medical literature and business journals, to ascertain the quantity and impact of spending. The most money was spent on marketing to medical professionals, which increased from $15.6 billion in 1997 to $20.3 billion in 2016. In terms of percentages, the biggest increase was seen in DTC advertising: $2.1 billion in 1997 (11.9% of total spending) ballooned to $9.6 billion (32.1% of total spending).
These increases were not accompanied by corresponding regulatory efforts to limit influence or protect patients and consumers. In 2016, the Food and Drug Administration’s Office of Prescription Drug Promotion received 97,252 promotional materials that drug companies submitted for review, compared with 34,182 in 1997, but violation letters for prescription drug advertising decreased from 156 to 11. In the same year, the FDA reviewed 41% of core materials – such as risk disclosures and key messages – for new drugs or indications prior to launch, a performance measure the coauthors called “critically important.”
In regard to disease awareness campaigns, 2004 guidance from the FDA on awareness advertising – including standards for unbranded campaigns and recommendations to avoid encouraging self-diagnosis and self-treatment – was withdrawn in 2015 and never replaced. The Federal Trade Commission, which has jurisdiction over unbranded advertising, has not taken regulatory action of its own; any FDA requests for investigation are unknown. In addition, these 2 decades have not seen state attorneys general initiate any action against deceptive consumer advertising, nor has the FTC acted against misleading laboratory test promotion.
“The FDA and FTC should establish and enforce standards for responsible disease awareness campaigns,” the coauthors wrote, “including criteria to validate symptom quizzes (or banning them) and evidence-based strategies to minimize misconceptions that a drug can treat all symptoms of disease.”
Overall, spending on medical marketing actually increased faster than did spending on health services overall. Marketing saw a remarkable 430% increase ($542 million to $2.9 billion) over the 2 decades, while health services spending increased by 90% ($1.2 trillion to $2.2 trillion).
One of the rare similarities from 1997 to 2016 was spending on marketing prescription drugs to physicians, typically through face-to-face meetings and hospital visits; this held steady at approximately $5 billion. However, spending on drug samples increased from $8.9 billion to $13.5 billion, while medical journal advertising declined drastically from $744 million to $119 million.
Spending on DTC marketing of prescription drugs increased across all therapeutic categories but three: cholesterol, allergy, and osteoporosis, each of which saw top-selling drugs either become over-the-counter or lose patent protection. Spending on drugs for diabetes/endocrine disease went from $27 million in 1997 to a whopping $725 million in 2016, followed by dermatology drugs ($67 million to $605 million) and pain/central nervous system drugs ($56 million to $542 million).
The coauthors shared potential limitations of their study, including the likelihood that they underestimated how much is actually spent on medical marketing. “Data on professional marketing (e.g., detailing) of laboratory tests, health services or devices, and pharmaceutical company spending on coupons or rebates, online promotion, and meetings and events could not be obtained,” they noted. In addition, company marketing budgets often do not include additional expenses that should count toward this total, and any published literature on medical marketing’s return on investment is largely based on observational data and cannot be fully relied upon.
The two coauthors previously served as medical experts in testosterone litigation and were cofounders of a company that provided data about the benefits and harms of prescription drugs, which ceased operations in December 2016. No other conflicts of interest were reported.
SOURCE: Schwartz LM et al. JAMA. 2019 Jan 8. doi: 10.1001/jama.2018.19320.
Total spending on medical marketing in the United States increased from $17.7 billion in 1997 to $29.9 billion in 2016, according to an analysis of direct-to-consumer (DTC) and professional marketing for prescription drugs, disease awareness campaigns, health services, and laboratory tests.
“Increased medical marketing reflects a convergence of scientific, economic, legal, and social forces,” wrote Lisa M. Schwartz, MD, and her coauthor, adding that “although marketing expanded over 20 years, regulatory oversight remains relatively limited.” Dr. Schwartz, then codirector of the Center for Medicine and Media at The Dartmouth Institute in Lebanon, N.H., died in November of 2018, after her work was accepted for publication in JAMA.
Dr. Schwartz and her coauthor, David Woloshin, MD, also of Dartmouth, reviewed consumer advertising and professional marketing data, along with searches of medical literature and business journals, to ascertain the quantity and impact of spending. The most money was spent on marketing to medical professionals, which increased from $15.6 billion in 1997 to $20.3 billion in 2016. In terms of percentages, the biggest increase was seen in DTC advertising: $2.1 billion in 1997 (11.9% of total spending) ballooned to $9.6 billion (32.1% of total spending).
These increases were not accompanied by corresponding regulatory efforts to limit influence or protect patients and consumers. In 2016, the Food and Drug Administration’s Office of Prescription Drug Promotion received 97,252 promotional materials that drug companies submitted for review, compared with 34,182 in 1997, but violation letters for prescription drug advertising decreased from 156 to 11. In the same year, the FDA reviewed 41% of core materials – such as risk disclosures and key messages – for new drugs or indications prior to launch, a performance measure the coauthors called “critically important.”
In regard to disease awareness campaigns, 2004 guidance from the FDA on awareness advertising – including standards for unbranded campaigns and recommendations to avoid encouraging self-diagnosis and self-treatment – was withdrawn in 2015 and never replaced. The Federal Trade Commission, which has jurisdiction over unbranded advertising, has not taken regulatory action of its own; any FDA requests for investigation are unknown. In addition, these 2 decades have not seen state attorneys general initiate any action against deceptive consumer advertising, nor has the FTC acted against misleading laboratory test promotion.
“The FDA and FTC should establish and enforce standards for responsible disease awareness campaigns,” the coauthors wrote, “including criteria to validate symptom quizzes (or banning them) and evidence-based strategies to minimize misconceptions that a drug can treat all symptoms of disease.”
Overall, spending on medical marketing actually increased faster than did spending on health services overall. Marketing saw a remarkable 430% increase ($542 million to $2.9 billion) over the 2 decades, while health services spending increased by 90% ($1.2 trillion to $2.2 trillion).
One of the rare similarities from 1997 to 2016 was spending on marketing prescription drugs to physicians, typically through face-to-face meetings and hospital visits; this held steady at approximately $5 billion. However, spending on drug samples increased from $8.9 billion to $13.5 billion, while medical journal advertising declined drastically from $744 million to $119 million.
Spending on DTC marketing of prescription drugs increased across all therapeutic categories but three: cholesterol, allergy, and osteoporosis, each of which saw top-selling drugs either become over-the-counter or lose patent protection. Spending on drugs for diabetes/endocrine disease went from $27 million in 1997 to a whopping $725 million in 2016, followed by dermatology drugs ($67 million to $605 million) and pain/central nervous system drugs ($56 million to $542 million).
The coauthors shared potential limitations of their study, including the likelihood that they underestimated how much is actually spent on medical marketing. “Data on professional marketing (e.g., detailing) of laboratory tests, health services or devices, and pharmaceutical company spending on coupons or rebates, online promotion, and meetings and events could not be obtained,” they noted. In addition, company marketing budgets often do not include additional expenses that should count toward this total, and any published literature on medical marketing’s return on investment is largely based on observational data and cannot be fully relied upon.
The two coauthors previously served as medical experts in testosterone litigation and were cofounders of a company that provided data about the benefits and harms of prescription drugs, which ceased operations in December 2016. No other conflicts of interest were reported.
SOURCE: Schwartz LM et al. JAMA. 2019 Jan 8. doi: 10.1001/jama.2018.19320.
FROM JAMA
Key clinical point: Medical marketing spending – especially on direct-to-consumer advertising for drugs and health services – increased exponentially over the last 2 decades.
Major finding: From 1997 through 2016, spending on medical marketing of drugs, disease awareness campaigns, health services, and laboratory testing increased from $17.7 to $29.9 billion.
Study details: An analysis of consumer advertising and professional marketing data, along with a review of regulations and legal actions undertaken by U.S. federal agencies.
Disclosures: The two coauthors previously served as medical experts in testosterone litigation and were cofounders of a company that provided data about the benefits and harms of prescription drugs, which ceased operations in December 2016. No other conflicts of interest were reported.
Source: Schwartz LM et al. JAMA. 2019 Jan 8. doi: 10.1001/jama.2018.19320.
Pediatric Warts: Update on Interventions
The definition of warts is variable, largely reflecting their manifold appearance, biologic potential, and public health concerns. One vernacular dictionary defines warts as:
Small, benign growths caused by a vital infection of the skin or mucous membrane. The virus infects the surface layer. The viruses that cause warts are members of the human papilloma virus (HPV) family. Warts are not cancerous but some strains of HPV, usually not associated with warts, have been linked with cancer formation. Warts are contagious from person to person and from one area of the body to another on the same person.1
The World Health Organization defines warts by their structural components as:
Human papillomavirus (HPV) is a small, non-enveloped deoxyribonucleic acid (DNA) virus that infects skin or mucosal cells. The circular, double-stranded viral genome is approximately 8-kb in length. The genome encodes for 6 early proteins responsible for virus replication and 2 late proteins, L1 and L2, which are the viral structural proteins.2
In pediatric and adolescent dermatology, warts often are defined by their location and morphology; for example, facial warts typically are flat, minimally hyperkeratotic, or filiform, wherein the base is narrow and the lesion is tall, growing at a 90° angle to the surface of the skin. On the arms and legs, warts usually present as round to oval papules with overlying thick hyperkeratosis and/or callosity.3,4 Common warts usually are flesh colored or lighter, and heavily pigmented lesions should be evaluated dermoscopically for a pigment network and biopsied when pigment is present.5
In this article, a successful paradigm for management of pediatric warts is provided with enhanced outcomes based on further insight into the disease course and patient selection.
Epidemiology of Pediatric Warts
There are more than 200 types of human papillomaviruses (HPV), with more than 100 oncogenic types. There is quite a bit of homology by species and genus that contributes to cross-immunity and similar behavior between certain types of HPV. The lifetime incidence of warts is very high. Approximately 30% of children develop a wart.6 A review of the 2007 National Health Interview Survey of 9417 children demonstrated a steady increase in prevalence of warts from 1 to 2 years of age to 7 to 8 years of age, with a peak at 9 to 10 years of age and a plateau at 11 to 17 years of age. Warts were most common in non-Hispanic white children and less common in black children.7 In an in-person survey of 12,370 individuals aged 18 to 74 years from 5 European countries, warts were the most common physician-diagnosed (27.3%) and self-reported (41.0%) dermatologic condition. Warts are more common in Northern countries (eg, Netherlands, Germany).8 Children with atopic dermatitis have a higher risk of developing warts and extracutaneous infections. In one study, children with warts and atopic dermatitis had a higher number of infections and food allergies and higher incidence of asthma and hay fever than either condition alone.9
Clinical Presentation of Warts
Warts usually present as common, palmoplantar, flat, or filiform in childhood, but variations by age are common (eFigure). The common and palmoplantar variants often are caused by HPV types 1 and 2.4,5 In infancy, vertically transmitted HPV infections can cause juvenile-onset respiratory papillomatosis or vertically transmitted condyloma. Juvenile-onset respiratory papillomatosis refers to upper respiratory papillomas that are difficult to eliminate and has been associated with exfoliated cervical cell testing with 18.1% (13/72) typed HPV-positive, which allows neonates to be exposed to HPV in the upper respiratory tract in utero.10
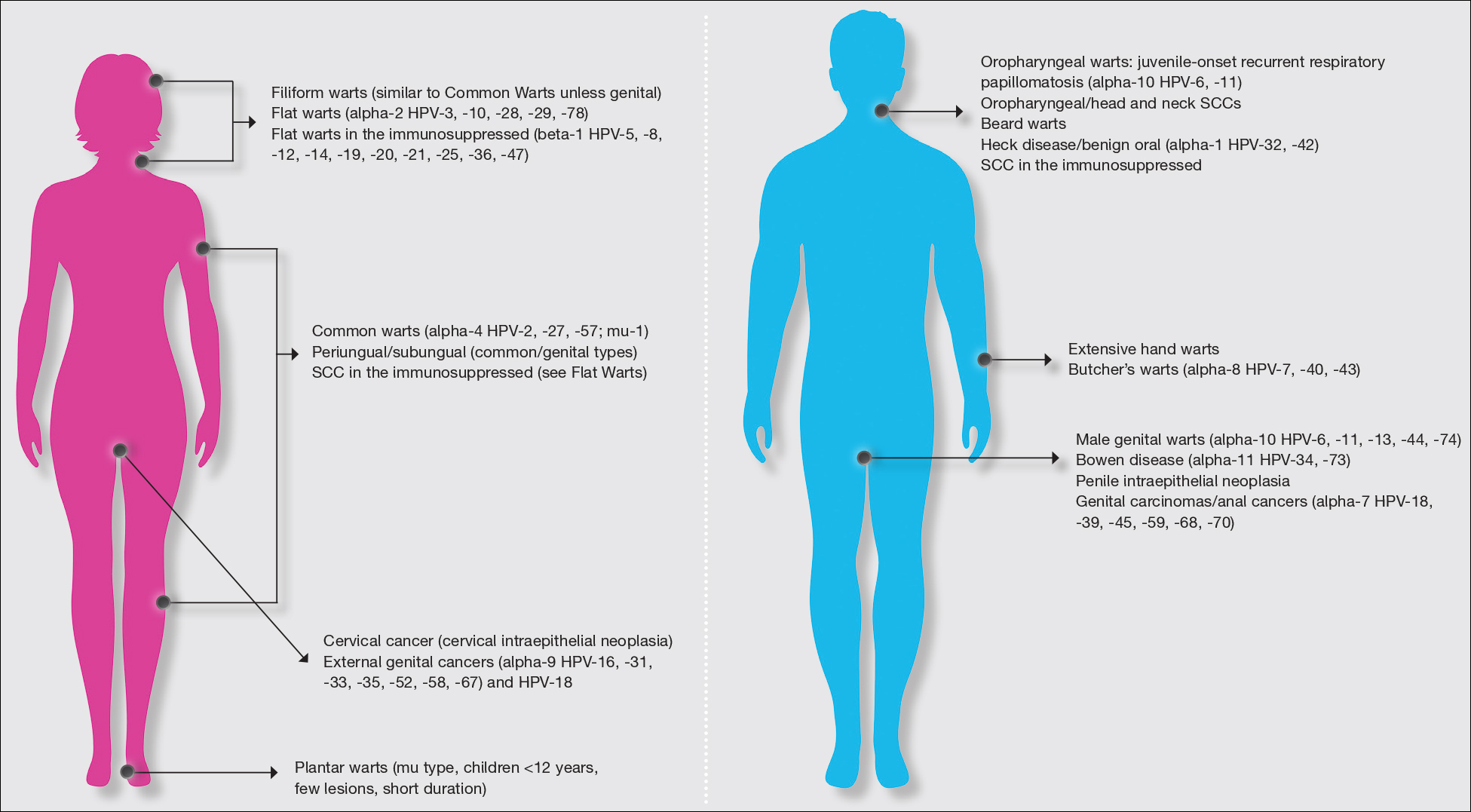
Vertically transmitted condyloma is a difficult topic. Much data supports the vertical transmission of condyloma as the leading cause of condyloma in small children; however, a reasonable amount of caution is needed in this patient population. In cases suspicious for sexual abuse as well as those presenting in children 4 years and older, formal household evaluation by a sexual abuse clinic and mandatory reporting is needed. Anywhere from 2.6% to 32% of cases of genital warts in children have been reported to be caused by sexual abuse.11-13 Therefore, most investigators have recommended careful review of the patient’s history and socioeconomic circumstances as well as a thorough physical examination. Mandatory reporting of suspected child sexual abuse is required in suspicious cases. Because HPV type 16 has been found in vertically transmitted cases, concern for long-term oncogenesis exists.11-13
Adolescents generally present with lesions on the hands and feet. Plantar warts often are caused by HPV types from the alpha genus. Subtypes noted in plantar warts include HPV types 1a, 2, 27, 57, and 65.14 By 15 years of age, genital HPV becomes a common adolescent infection, persisting into adulthood.15 When studied, genital HPV often is subclinical or latent and often is preventable through vaccination. High-risk oncogenic alpha-genus HPV types can immortalize human keratinocytes. When HPV types 11, 16, 18, and 31 are compared, HPV-18 has the highest oncogenic potential based on colony-stimulating potential.16 Vaccination with the 9-valent HPV vaccine is recommended in adolescence due to the concern for exposures to both low-potential (HPV types 6 and 11) and high-potential (HPV types 16 and 18) oncogenic HPV types. Data strongly support the benefit of 9-valent HPV vaccination in the prevention of sexually transmitted HPV in both males and females.17
Contagion of HPV is easy due to its excellent survival of fomites on surfaces, which generally is how warts are transferred in gym or pool settings where individuals who walk barefoot in changing rooms are almost twice as likely to contract plantar warts (odds ratio, 1.97 [95% CI, 1.39%-2.79%]).18 In another case series, walking barefoot, using a swimming pool, and having a household contact with warts were the leading risk factors for contraction of warts in children younger than 13 years.19 Children often transfer warts from site to site as well as to siblings and other close contacts. Skin-to-skin contact is responsible for sexual transmission of warts, and surface transmission occurs via fomites. Entry of the virus often occurs through small breaks in the skin. Other modes of transmission include orogenital.20
Therapeutic Options
Although the nuances of each available treatment for pediatric warts are beyond the scope of this article, the main core of therapy is 1 of 3 approaches: (1) observation, (2) over-the-counter salicylic acid therapy, and (3) in-office cryotherapy. Observation is an affirmed style of therapy for warts, as it is expected that two-thirds of warts will spontaneously resolve in 2 years and three-quarters will resolve in 3 years.4,5 Condyloma in children has been responsive to therapies such as cryotherapy and imiquimod,13 but spontaneous clearance in 5 years has been noted in 76% of children,21 which is linked to development of spontaneous immune response in most individuals.
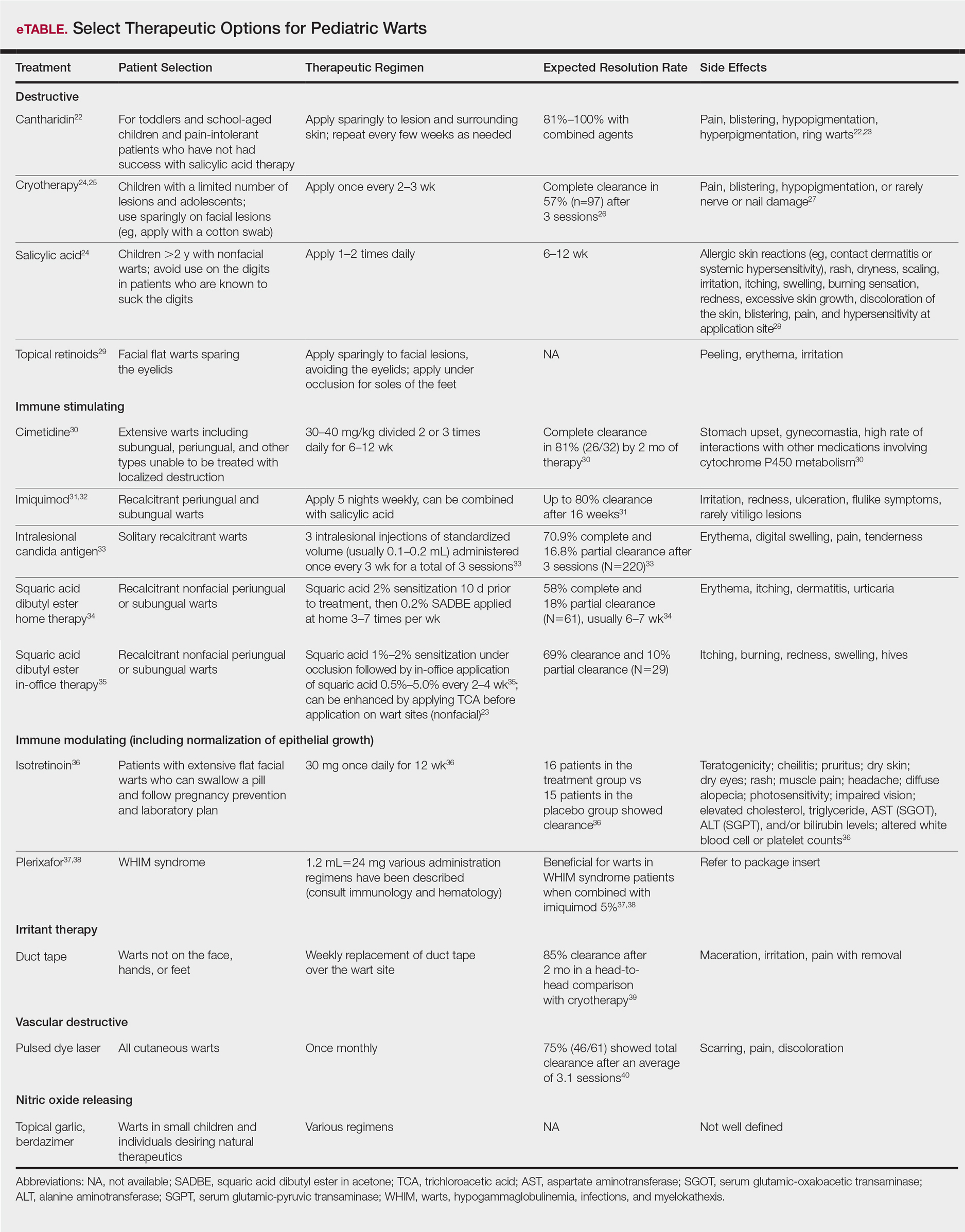
Therapies for pediatric warts are characterized according to 6 major categories: destructive; immune stimulating; immune modulating, including normalization of epithelial growth; irritant; vascular destructive; and nitric oxide releasing (eTable).
Destructive Therapies
Destructive therapies for warts often are implemented in cases of disfigurement, discomfort/pain, and/or spreading, as well as to control contagion. According to a 2001 Cochrane review, salicylic acid has the best evidence of all therapeutics for the clearance of warts compared to placebo.24 On the other hand, aggressive cryotherapy and combined salicylic acid and cryotherapy had the best evidence in their favor in a 2011 meta-analysis by Kwok et al.25 Both salicylic acid and cryotherapy are considered destructive therapies. A recent meta-analysis of cantharidin, another destructive therapy, showed that local cantharidin alone as well as in combination with salicylic acid and podophyllotoxin showed good efficacy for warts; however, increased caution should be exerted with the combination regimen in young children due to a potential increase in the side-effect profile (eg, severe blistering).22 Other destructive agents such as topical retinoids can only peel surface layers of the skin and therefore are limited to flat facial warts, which are not expected to have an extensive hyperkeratotic layer; however, with occlusion, agents such as adapalene gel 0.1% can be used even on plantar warts with some efficacy.29
Immune-Stimulating Therapies
Immune stimulants often are used to treat warts in children and adolescents who have many lesions, a prolonged disease course, disfigurement, and/or subungual localizations, as well as in those who have been treated with multiple destructive methods without success. Topical imiquimod and oral cimetidine are readily available, while squaric acid (at-home or in-office therapy) and intralesional candida antigen can be used in offices that carry these agents. Topical imiquimod has been reported to achieve success in genital warts in children,13 with good efficacy in recalcitrant, periungual, and subungual warts when used for up to 16 weeks.31 In one randomized clinical trial, imiquimod cream 5% combined with salicylic acid 15% was applied to warts for 6 to 10 hours for 5 consecutive days per week versus cryotherapy with liquid nitrogen every 2 weeks for a maximum of 3 months. At the end of the study period, 81.1% (30/37) of participants treated with imiquimod and salicylic acid showed clearance of their warts versus 67.3% (33/49) of those treated with cryotherapy.32
Oral cimetidine has been reported to be successful in treating recalcitrant warts in more than 80% of children when dosed at 30 to 40 mg/kg 3 times daily, requiring 6 to 12 weeks to achieve clearance. Side effects of oral cimetidine include many cytochrome P450 interactions; gynecomastia, which limits usage in teenaged males; and stomach upset.30
Treatment of recalcitrant pediatric warts with intralesional candida antigen has been associated with side effects consistent with delayed-type hypersensitivity reactions. Injections should be administered once monthly, with a minimum of 3 cycles if not effective and up to 6 cycles where partial efficacy is noted. In a retrospective review of 220 cases, 70.9% of children showed complete clearance and 16.8% had partial response.33 However, the treatment may be limited in children by fear of needles.
Squaric acid dibutyl ester is a universal allergen that is not mutagenic on Ames testing and causes milder allergy symptoms than the mutagenic dinitrochlorobenzene and less erythema and pruritus than diphencyclopropenone. Squaric acid dibutyl ester home therapy was evaluated in 61 children with at least one nonfacial wart.34 Application began with squaric acid dibutyl ester in acetone (SADBE) 2% sensitization on the arm followed by at-home application of SADBE 0.2% three to seven times weekly for a minimum of 2 months to determine benefit and for 3 to 4 months as needed; however, average response was 7 weeks. The average complete clearance was 58% and partial clearance was 18%. Side effects included erythema and mild itching as well as urticaria in one case.34 In-office SADBE also has been evaluated in children. In a case series that included 29 children sensitized with SADBE 1% to 2% under occlusion followed by once monthly application of SADBE 0.5% to 5.0% to their warts, 69% clearance and 10% partial clearance was noted after a little more than 4 months of treatment.35 One retrospective review compared combination SADBE, trichloroacetic acid (TCA), and cantharidin both alone and in combination as duos (eg, SADBE and TCA) or trios (SADBE, TCA, and cantharidin).23 Of the 74 children whose medical charts were reviewed, the addition of pretreatment of warts with TCA 50% prior to in-office sensitization and monthly in-office application of SADBE increased treatment response to 100% with an average 2.45 months of therapy, whereas no enhancement was noted with cantharidin. Therefore, it appears that there may be enhanced immune reactivity when TCA pretreatment of warts is performed.23
Immune-Modulating Therapies (Including Normalization of Epithelial Growth)
The most novel immunologic therapy for warts is plerixafor, an agent used to treat WHIM (warts, hypogammaglobulinemia, infections, and myelokathexis) syndrome, which has been linked to heterozygous gain of function mutations in the chemokine receptor CXCR4 (located on 2q22). In WHIM syndrome, the mutated CXCR4 is more sensitive to CXCL12 activation. Plerixafor is a selective reversible antagonist that blocks the capacity of the chemokine CXCL12 to sustain the permanent activation of CXCR4.37 Combination therapy with plerixafor and topical imiquimod has resulted in wart improvement in WHIM syndrome patients in a small series.38
Oral isotretinoin has been described to be efficacious over placebo at a dosage of 30 mg daily for 12 weeks and can be used in teenagers but requires standard monitoring.36
Irritant Therapies
Duct tape is a classic agent that produces maceration and irritation of warts. Application of duct tape over warts has been described in cycles of 6 days on, 1 day off with weekly repetition for a few months but usually not on the palms or soles due to difficulty maintaining occlusive tape in these locations over an extended period of time. In one trial, 85% (22/26) of duct tape–treated cases cleared versus 60% (15/25) of cryotherapy-treated cases over a 2-month maximum therapeutic period.39
Vascular Destructive Therapies
The pulsed dye laser is a classic modality that induces localized destruction of blood supply to warts in children. A case series of 61 children treated with the pulsed dye laser revealed 75% overall clearance in an average of 3.1 sessions. The usage of this therapy often is limited to institutions where the technology is readily available for usage.40
Nitric Oxide–Releasing Therapies
Nitric oxide release may increase local blood flow, thereby increasing immune response, or may have a primary mechanism of antimicrobial activity, which is why these agents have been investigated for wart treatment. Topical garlic has been described anecdotally as a therapy for thin childhood warts with the putative mechanism being nitric oxide release.42 A new investigational drug recently has had phase 2 data published. Berdazimer sodium plus carboxymethyl cellulose hydrogel has demonstrated benefit in adult warts, but data in children is lacking.41
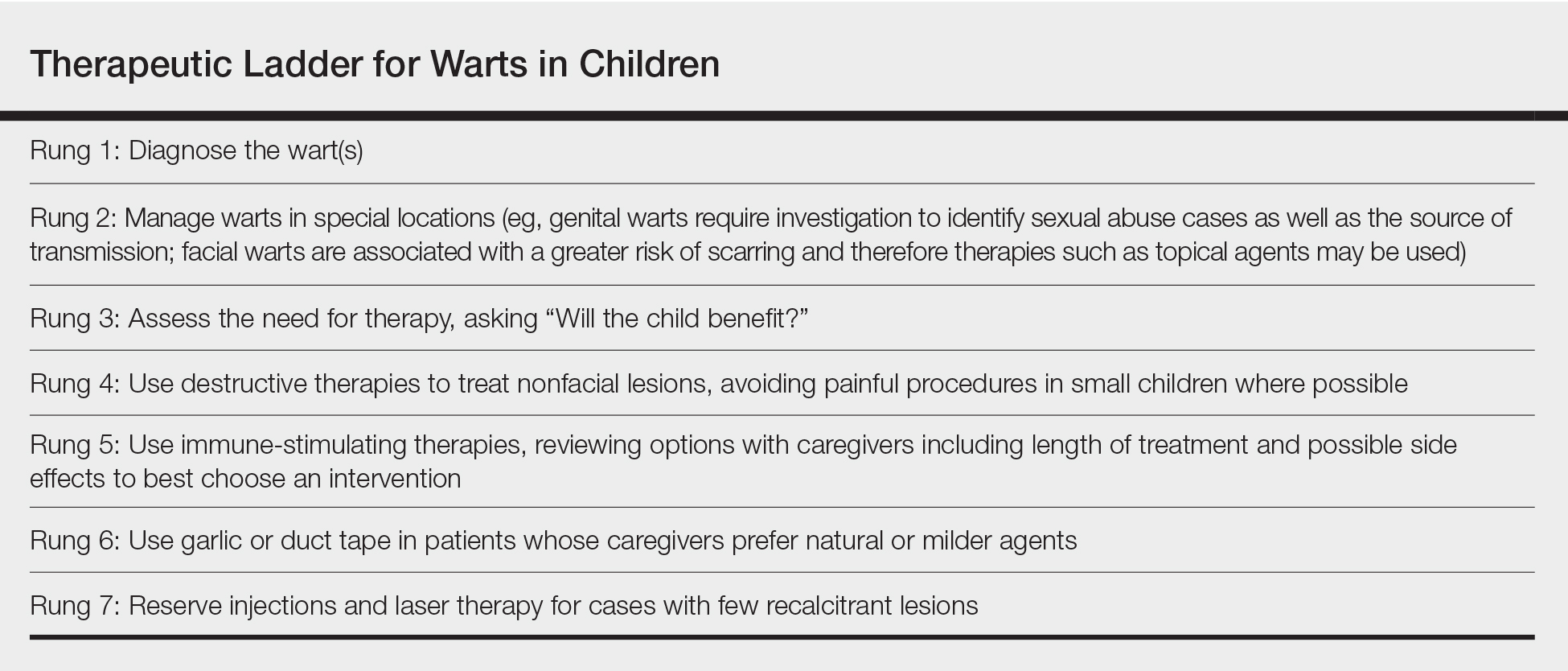
Therapeutic Ladder for Childhood Warts
The therapeutic ladder (Table) for childhood warts starts with first doing no harm. Although many parents are disturbed by their child’s condition, the natural history of resolution is spontaneous and therefore no therapy is required in many cases. The child and his/her caregivers should be engaged to determine if he/she is emotionally disturbed or uncomfortable with their lesions and to address any fears and concerns that some children may experience (eg, contagion risk, pain with ambulation, ostracism). For example, children with hand warts may report that other children will not hold their hand while in line at school. Prominent facial lesions can be particularly problematic for children due to teasing and bullying.
Conclusion
Warts are a common infection in childhood caused by the ubiquitous HPV virus. Therapeutic options abound, but most cases are either ignored or treated with over-the-counter salicylic acid or in-office cryotherapy. The decision to employ alternative therapeutic options requires agreement by the child, his/her caregiver, and the treating physician and can be tailored to suit the desires and needs of the child. Whether or not therapy is offered, spontaneous clearance is frequently seen in common warts. On the other hand, genital warts are associated with later conversion to malignancies of the genital tract; therefore, encouragement of HPV vaccination is needed in the adolescent population to best ensure long-term genital health.
1. Warts. https://medical-dictionary.thefreedictionary.com/warts. Accessed November 30, 2018.
2. Human papillomavirus. WHO website. http://www.who.int/biologicals/areas/human_papillomavirus/en. Accessed December 3, 2018.
3. Silverberg NB. Human papillomavirus infections in children. Curr Opin Pediatr. 2004;16:402-409.
4. Silverberg NB. Warts and molluscum in children. Adv Dermatol. 2004;20:23-73.
5. Silverberg NB, McCuaig CC. Melanoma in childhood: changing our mind-set. Cutis. 2013;92:217-218.
6. Bruggink SC, Eekhof JA, Egberts PF, et al. Warts transmitted in families and schools: a prospective cohort. Pediatrics. 2013;131:928-934.
7. Silverberg JI, Silverberg NB. The U.S. prevalence of common warts in childhood: a population-based study. J Invest Dermatol. 2013;133:2788-2790.
8. Svensson A, Ofenloch RF, Bruze M, et al. Br J Dermatol. 2018;178:1111-1118.
9. Silverberg JI, Silverberg NB. Childhood atopic dermatitis and warts are associated with increased risk of infection: a US population-based study. J Allergy Clin Immunol. 2014;133:1041-1047.
10. Smith EM, Johnson SR, Cripe TP, et al. Perinatal vertical transmission of human papillomavirus and subsequent development of respiratory tract papillomatosis. Ann Otol Rhinol Laryngol. 1991;100:479-483.
11. Costa-Silva M, Azevedo F, Lisboa C. Anogenital warts in children: analysis of a cohort of 34 prepubertal children. Pediatr Dermatol. 2018;35:E325-E327.
12. Marcoux D, Nadeau K, McCuaig C, et al. Pediatric anogenital warts: a 7-year review of children referred to a tertiary-care hospital in Montreal, Canada. Pediatr Dermatol. 2006;23:199-207.
13. Stefanaki C, Barkas G, Valari M, et al. Condylomata acuminata in children. Pediatr Infect Dis J. 2012;31:422-424.
14. dePlanell-Mas E, Martinez-Garriga B, Zalacain AJ, et al. Human papillomaviruses genotyping in plantar warts. J Med Virol. 2017;89:902-907.
15. Satterwhite CL, Torrone E, Meites E, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sex Transm Dis. 2013;40:187-193.
16. Lace MJ, Anson JR, Klingelhutz AJ, et al. Human papillomavirus (HPV) type 18 induces extended growth in primary human cervical, tonsillar, or foreskin keratinocytes more effectively than other high-risk mucosal HPVs. J Virol. 2009;83:11784-11794.
17. Sudenga SL, Ingles DJ, Pierce Campbell CM, et al. Genital human papillomavirus infection progression to external genital lesions: the HIM study. Eur Urol. 2016;69:166-173.
18. Rigo MV, Martínez Campillo F, Verdú M, et al. Risk factors linked to the transmission of papilloma virus in the school environment [in Spanish]. Alicante, 1999. Aten Primaria. 2003;31:415-420.
19. Al-Mutairi N, AlKhalaf M. Mucocutaneous warts in children: clinical presentations, risk factors, and response to treatment. Acta Dermatovenerol Alp Pannonica Adriat. 2012;21:69-72.
20. Clarke J, Terry RM, Lacey CJ. A study to estimate the prevalence of upper respiratory tract papillomatosis in patients with genital warts. Int J STD AIDS. 1991;2:114-115.
21. Allen AL, Siegfried EC. The natural history of condyloma in children. J Am Acad Dermatol. 1998;39:951-955.
22. Vakharia PP, Chopra R, Silverberg NB, et al. Efficacy and safety of topical cantharidin treatment for molluscum contagiosum and warts: a systematic review. Am J Clin Dermatol. 2018;19:791-803.
23. Silverberg JI, Silverberg NB. Adjunctive trichloroacetic acid therapy enhances squaric acid response to verruca vulgaris. J Drugs Dermatol. 2012;11:1228-1230.
24. Gibbs S, Harvey I, Sterling JC, et al. Local treatments for cutaneous warts. Cochrane Database Syst Rev. 2001:CD001781.
25. Kwok CS, Holland R, Gibbs S. Efficacy of topical treatments for cutaneous warts: a meta-analysis and pooled analysis of randomized controlled trials. Br J Dermatol. 2011;165:233-246.
26. Allington HV. Liquid nitrogen in the treatment of skin diseases. Calif Med. 1950;72:153-155.
27. Caravati CM Jr, Wood BT, Richardson DR. Onychodystrophies secondary to liquid nitrogen cryotherapy. Arch Dermatol. 1969;100:441-442.
28. Duofilm [package insert]. Sligo, Ireland: Stiefel Laboratories (Ireland) Ltd; 2016.
29. Gupta R, Gupta S. Topical adapalene in the treatment of plantar warts: randomized comparative open trial in comparison with cryo-therapy. Indian J Dermatol. 2015;60:102.
30. Orlow SJ, Paller A. Cimetidine therapy for multiple viral warts in children. J Am Acad Dermatol. 1993;28(5 pt 1):794-796.
31. Micali G, Dall’Oglio F, Nasca MR. An open label evaluation of the efficacy of imiquimod 5% cream in the treatment of recalcitrant subungual and periungual cutaneous warts. J Dermatolog Treat. 2003;14:233-236.
32. Stefanaki C, Lagogiani I, Kouris A, et al. Cryotherapy versus imiquimod 5% cream combined with a keratolytic lotion in cutaneous warts in children: a randomized study. J Dermatolog Treat. 2016;27:80-82.
33. Muñoz Garza FZ, Roé Crespo E, Torres Pradilla M, et al. Intralesional Candida antigen immunotherapy for the treatment of recalcitrant and multiple warts in children. Pediatr Dermatol. 2015;32:797-801.
34. Silverberg NB, Lim JK, Paller AS, et al. Squaric acid immunotherapy for warts in children. J Am Acad Dermatol. 2000;42(5 pt 1):803-808.
35. Lee AN, Mallory SB. Contact immunotherapy with squaric acid dibutylester for the treatment of recalcitrant warts. J Am Acad Dermatol. 1999;41:595-599.
36. Olguin-García MG, Jurado-Santa Cruz F, Peralta-Pedrero ML, et al. A double-blind, randomized, placebo-controlled trial of oral isotretinoin in the treatment of recalcitrant facial flat warts. J Dermatolog Treat. 2015;26:78-82.
37. Badolato R, Donadieu J; WHIM Research Group. How I treat warts, hypogammaglobulinemia, infections, and myelokathexis syndrome. Blood. 2017;130:2491-2498.
38. McDermott DH, Liu Q, Velez D, et al. A phase 1 clinical trial of long-term, low-dose treatment of WHIM syndrome with the CXCR4 antagonist plerixafor. Blood. 2014;123:2308-2316.
39. Focht DR 3rd, Spicer C, Fairchok MP. The efficacy of duct tape vs cryotherapy in the treatment of verruca vulgaris (the common wart). Arch Pediatr Adolesc Med. 2002;156:971-974.
40. Sethuraman G, Richards KA, Hiremagalore RN, et al. Effectiveness of pulsed dye laser in the treatment of recalcitrant warts in children. Dermatol Surg. 2010;36:58-65.
41. Tyring SK, Rosen T, Berman B, et al. A phase 2 controlled study of SB206, a topical nitric oxide-releasing drug for extragenital wart treatment. J Drugs Dermatol. 2018;17:1100-1105.
42. Silverberg NB. Garlic cloves for verruca vulgaris. Pediatr Dermatol. 2002;19:183.
The definition of warts is variable, largely reflecting their manifold appearance, biologic potential, and public health concerns. One vernacular dictionary defines warts as:
Small, benign growths caused by a vital infection of the skin or mucous membrane. The virus infects the surface layer. The viruses that cause warts are members of the human papilloma virus (HPV) family. Warts are not cancerous but some strains of HPV, usually not associated with warts, have been linked with cancer formation. Warts are contagious from person to person and from one area of the body to another on the same person.1
The World Health Organization defines warts by their structural components as:
Human papillomavirus (HPV) is a small, non-enveloped deoxyribonucleic acid (DNA) virus that infects skin or mucosal cells. The circular, double-stranded viral genome is approximately 8-kb in length. The genome encodes for 6 early proteins responsible for virus replication and 2 late proteins, L1 and L2, which are the viral structural proteins.2
In pediatric and adolescent dermatology, warts often are defined by their location and morphology; for example, facial warts typically are flat, minimally hyperkeratotic, or filiform, wherein the base is narrow and the lesion is tall, growing at a 90° angle to the surface of the skin. On the arms and legs, warts usually present as round to oval papules with overlying thick hyperkeratosis and/or callosity.3,4 Common warts usually are flesh colored or lighter, and heavily pigmented lesions should be evaluated dermoscopically for a pigment network and biopsied when pigment is present.5
In this article, a successful paradigm for management of pediatric warts is provided with enhanced outcomes based on further insight into the disease course and patient selection.
Epidemiology of Pediatric Warts
There are more than 200 types of human papillomaviruses (HPV), with more than 100 oncogenic types. There is quite a bit of homology by species and genus that contributes to cross-immunity and similar behavior between certain types of HPV. The lifetime incidence of warts is very high. Approximately 30% of children develop a wart.6 A review of the 2007 National Health Interview Survey of 9417 children demonstrated a steady increase in prevalence of warts from 1 to 2 years of age to 7 to 8 years of age, with a peak at 9 to 10 years of age and a plateau at 11 to 17 years of age. Warts were most common in non-Hispanic white children and less common in black children.7 In an in-person survey of 12,370 individuals aged 18 to 74 years from 5 European countries, warts were the most common physician-diagnosed (27.3%) and self-reported (41.0%) dermatologic condition. Warts are more common in Northern countries (eg, Netherlands, Germany).8 Children with atopic dermatitis have a higher risk of developing warts and extracutaneous infections. In one study, children with warts and atopic dermatitis had a higher number of infections and food allergies and higher incidence of asthma and hay fever than either condition alone.9
Clinical Presentation of Warts
Warts usually present as common, palmoplantar, flat, or filiform in childhood, but variations by age are common (eFigure). The common and palmoplantar variants often are caused by HPV types 1 and 2.4,5 In infancy, vertically transmitted HPV infections can cause juvenile-onset respiratory papillomatosis or vertically transmitted condyloma. Juvenile-onset respiratory papillomatosis refers to upper respiratory papillomas that are difficult to eliminate and has been associated with exfoliated cervical cell testing with 18.1% (13/72) typed HPV-positive, which allows neonates to be exposed to HPV in the upper respiratory tract in utero.10

Vertically transmitted condyloma is a difficult topic. Much data supports the vertical transmission of condyloma as the leading cause of condyloma in small children; however, a reasonable amount of caution is needed in this patient population. In cases suspicious for sexual abuse as well as those presenting in children 4 years and older, formal household evaluation by a sexual abuse clinic and mandatory reporting is needed. Anywhere from 2.6% to 32% of cases of genital warts in children have been reported to be caused by sexual abuse.11-13 Therefore, most investigators have recommended careful review of the patient’s history and socioeconomic circumstances as well as a thorough physical examination. Mandatory reporting of suspected child sexual abuse is required in suspicious cases. Because HPV type 16 has been found in vertically transmitted cases, concern for long-term oncogenesis exists.11-13
Adolescents generally present with lesions on the hands and feet. Plantar warts often are caused by HPV types from the alpha genus. Subtypes noted in plantar warts include HPV types 1a, 2, 27, 57, and 65.14 By 15 years of age, genital HPV becomes a common adolescent infection, persisting into adulthood.15 When studied, genital HPV often is subclinical or latent and often is preventable through vaccination. High-risk oncogenic alpha-genus HPV types can immortalize human keratinocytes. When HPV types 11, 16, 18, and 31 are compared, HPV-18 has the highest oncogenic potential based on colony-stimulating potential.16 Vaccination with the 9-valent HPV vaccine is recommended in adolescence due to the concern for exposures to both low-potential (HPV types 6 and 11) and high-potential (HPV types 16 and 18) oncogenic HPV types. Data strongly support the benefit of 9-valent HPV vaccination in the prevention of sexually transmitted HPV in both males and females.17
Contagion of HPV is easy due to its excellent survival of fomites on surfaces, which generally is how warts are transferred in gym or pool settings where individuals who walk barefoot in changing rooms are almost twice as likely to contract plantar warts (odds ratio, 1.97 [95% CI, 1.39%-2.79%]).18 In another case series, walking barefoot, using a swimming pool, and having a household contact with warts were the leading risk factors for contraction of warts in children younger than 13 years.19 Children often transfer warts from site to site as well as to siblings and other close contacts. Skin-to-skin contact is responsible for sexual transmission of warts, and surface transmission occurs via fomites. Entry of the virus often occurs through small breaks in the skin. Other modes of transmission include orogenital.20
Therapeutic Options
Although the nuances of each available treatment for pediatric warts are beyond the scope of this article, the main core of therapy is 1 of 3 approaches: (1) observation, (2) over-the-counter salicylic acid therapy, and (3) in-office cryotherapy. Observation is an affirmed style of therapy for warts, as it is expected that two-thirds of warts will spontaneously resolve in 2 years and three-quarters will resolve in 3 years.4,5 Condyloma in children has been responsive to therapies such as cryotherapy and imiquimod,13 but spontaneous clearance in 5 years has been noted in 76% of children,21 which is linked to development of spontaneous immune response in most individuals.
Therapies for pediatric warts are characterized according to 6 major categories: destructive; immune stimulating; immune modulating, including normalization of epithelial growth; irritant; vascular destructive; and nitric oxide releasing (eTable).

Destructive Therapies
Destructive therapies for warts often are implemented in cases of disfigurement, discomfort/pain, and/or spreading, as well as to control contagion. According to a 2001 Cochrane review, salicylic acid has the best evidence of all therapeutics for the clearance of warts compared to placebo.24 On the other hand, aggressive cryotherapy and combined salicylic acid and cryotherapy had the best evidence in their favor in a 2011 meta-analysis by Kwok et al.25 Both salicylic acid and cryotherapy are considered destructive therapies. A recent meta-analysis of cantharidin, another destructive therapy, showed that local cantharidin alone as well as in combination with salicylic acid and podophyllotoxin showed good efficacy for warts; however, increased caution should be exerted with the combination regimen in young children due to a potential increase in the side-effect profile (eg, severe blistering).22 Other destructive agents such as topical retinoids can only peel surface layers of the skin and therefore are limited to flat facial warts, which are not expected to have an extensive hyperkeratotic layer; however, with occlusion, agents such as adapalene gel 0.1% can be used even on plantar warts with some efficacy.29
Immune-Stimulating Therapies
Immune stimulants often are used to treat warts in children and adolescents who have many lesions, a prolonged disease course, disfigurement, and/or subungual localizations, as well as in those who have been treated with multiple destructive methods without success. Topical imiquimod and oral cimetidine are readily available, while squaric acid (at-home or in-office therapy) and intralesional candida antigen can be used in offices that carry these agents. Topical imiquimod has been reported to achieve success in genital warts in children,13 with good efficacy in recalcitrant, periungual, and subungual warts when used for up to 16 weeks.31 In one randomized clinical trial, imiquimod cream 5% combined with salicylic acid 15% was applied to warts for 6 to 10 hours for 5 consecutive days per week versus cryotherapy with liquid nitrogen every 2 weeks for a maximum of 3 months. At the end of the study period, 81.1% (30/37) of participants treated with imiquimod and salicylic acid showed clearance of their warts versus 67.3% (33/49) of those treated with cryotherapy.32
Oral cimetidine has been reported to be successful in treating recalcitrant warts in more than 80% of children when dosed at 30 to 40 mg/kg 3 times daily, requiring 6 to 12 weeks to achieve clearance. Side effects of oral cimetidine include many cytochrome P450 interactions; gynecomastia, which limits usage in teenaged males; and stomach upset.30
Treatment of recalcitrant pediatric warts with intralesional candida antigen has been associated with side effects consistent with delayed-type hypersensitivity reactions. Injections should be administered once monthly, with a minimum of 3 cycles if not effective and up to 6 cycles where partial efficacy is noted. In a retrospective review of 220 cases, 70.9% of children showed complete clearance and 16.8% had partial response.33 However, the treatment may be limited in children by fear of needles.
Squaric acid dibutyl ester is a universal allergen that is not mutagenic on Ames testing and causes milder allergy symptoms than the mutagenic dinitrochlorobenzene and less erythema and pruritus than diphencyclopropenone. Squaric acid dibutyl ester home therapy was evaluated in 61 children with at least one nonfacial wart.34 Application began with squaric acid dibutyl ester in acetone (SADBE) 2% sensitization on the arm followed by at-home application of SADBE 0.2% three to seven times weekly for a minimum of 2 months to determine benefit and for 3 to 4 months as needed; however, average response was 7 weeks. The average complete clearance was 58% and partial clearance was 18%. Side effects included erythema and mild itching as well as urticaria in one case.34 In-office SADBE also has been evaluated in children. In a case series that included 29 children sensitized with SADBE 1% to 2% under occlusion followed by once monthly application of SADBE 0.5% to 5.0% to their warts, 69% clearance and 10% partial clearance was noted after a little more than 4 months of treatment.35 One retrospective review compared combination SADBE, trichloroacetic acid (TCA), and cantharidin both alone and in combination as duos (eg, SADBE and TCA) or trios (SADBE, TCA, and cantharidin).23 Of the 74 children whose medical charts were reviewed, the addition of pretreatment of warts with TCA 50% prior to in-office sensitization and monthly in-office application of SADBE increased treatment response to 100% with an average 2.45 months of therapy, whereas no enhancement was noted with cantharidin. Therefore, it appears that there may be enhanced immune reactivity when TCA pretreatment of warts is performed.23
Immune-Modulating Therapies (Including Normalization of Epithelial Growth)
The most novel immunologic therapy for warts is plerixafor, an agent used to treat WHIM (warts, hypogammaglobulinemia, infections, and myelokathexis) syndrome, which has been linked to heterozygous gain of function mutations in the chemokine receptor CXCR4 (located on 2q22). In WHIM syndrome, the mutated CXCR4 is more sensitive to CXCL12 activation. Plerixafor is a selective reversible antagonist that blocks the capacity of the chemokine CXCL12 to sustain the permanent activation of CXCR4.37 Combination therapy with plerixafor and topical imiquimod has resulted in wart improvement in WHIM syndrome patients in a small series.38
Oral isotretinoin has been described to be efficacious over placebo at a dosage of 30 mg daily for 12 weeks and can be used in teenagers but requires standard monitoring.36
Irritant Therapies
Duct tape is a classic agent that produces maceration and irritation of warts. Application of duct tape over warts has been described in cycles of 6 days on, 1 day off with weekly repetition for a few months but usually not on the palms or soles due to difficulty maintaining occlusive tape in these locations over an extended period of time. In one trial, 85% (22/26) of duct tape–treated cases cleared versus 60% (15/25) of cryotherapy-treated cases over a 2-month maximum therapeutic period.39
Vascular Destructive Therapies
The pulsed dye laser is a classic modality that induces localized destruction of blood supply to warts in children. A case series of 61 children treated with the pulsed dye laser revealed 75% overall clearance in an average of 3.1 sessions. The usage of this therapy often is limited to institutions where the technology is readily available for usage.40
Nitric Oxide–Releasing Therapies
Nitric oxide release may increase local blood flow, thereby increasing immune response, or may have a primary mechanism of antimicrobial activity, which is why these agents have been investigated for wart treatment. Topical garlic has been described anecdotally as a therapy for thin childhood warts with the putative mechanism being nitric oxide release.42 A new investigational drug recently has had phase 2 data published. Berdazimer sodium plus carboxymethyl cellulose hydrogel has demonstrated benefit in adult warts, but data in children is lacking.41
Therapeutic Ladder for Childhood Warts
The therapeutic ladder (Table) for childhood warts starts with first doing no harm. Although many parents are disturbed by their child’s condition, the natural history of resolution is spontaneous and therefore no therapy is required in many cases. The child and his/her caregivers should be engaged to determine if he/she is emotionally disturbed or uncomfortable with their lesions and to address any fears and concerns that some children may experience (eg, contagion risk, pain with ambulation, ostracism). For example, children with hand warts may report that other children will not hold their hand while in line at school. Prominent facial lesions can be particularly problematic for children due to teasing and bullying.

Conclusion
Warts are a common infection in childhood caused by the ubiquitous HPV virus. Therapeutic options abound, but most cases are either ignored or treated with over-the-counter salicylic acid or in-office cryotherapy. The decision to employ alternative therapeutic options requires agreement by the child, his/her caregiver, and the treating physician and can be tailored to suit the desires and needs of the child. Whether or not therapy is offered, spontaneous clearance is frequently seen in common warts. On the other hand, genital warts are associated with later conversion to malignancies of the genital tract; therefore, encouragement of HPV vaccination is needed in the adolescent population to best ensure long-term genital health.
The definition of warts is variable, largely reflecting their manifold appearance, biologic potential, and public health concerns. One vernacular dictionary defines warts as:
Small, benign growths caused by a vital infection of the skin or mucous membrane. The virus infects the surface layer. The viruses that cause warts are members of the human papilloma virus (HPV) family. Warts are not cancerous but some strains of HPV, usually not associated with warts, have been linked with cancer formation. Warts are contagious from person to person and from one area of the body to another on the same person.1
The World Health Organization defines warts by their structural components as:
Human papillomavirus (HPV) is a small, non-enveloped deoxyribonucleic acid (DNA) virus that infects skin or mucosal cells. The circular, double-stranded viral genome is approximately 8-kb in length. The genome encodes for 6 early proteins responsible for virus replication and 2 late proteins, L1 and L2, which are the viral structural proteins.2
In pediatric and adolescent dermatology, warts often are defined by their location and morphology; for example, facial warts typically are flat, minimally hyperkeratotic, or filiform, wherein the base is narrow and the lesion is tall, growing at a 90° angle to the surface of the skin. On the arms and legs, warts usually present as round to oval papules with overlying thick hyperkeratosis and/or callosity.3,4 Common warts usually are flesh colored or lighter, and heavily pigmented lesions should be evaluated dermoscopically for a pigment network and biopsied when pigment is present.5
In this article, a successful paradigm for management of pediatric warts is provided with enhanced outcomes based on further insight into the disease course and patient selection.
Epidemiology of Pediatric Warts
There are more than 200 types of human papillomaviruses (HPV), with more than 100 oncogenic types. There is quite a bit of homology by species and genus that contributes to cross-immunity and similar behavior between certain types of HPV. The lifetime incidence of warts is very high. Approximately 30% of children develop a wart.6 A review of the 2007 National Health Interview Survey of 9417 children demonstrated a steady increase in prevalence of warts from 1 to 2 years of age to 7 to 8 years of age, with a peak at 9 to 10 years of age and a plateau at 11 to 17 years of age. Warts were most common in non-Hispanic white children and less common in black children.7 In an in-person survey of 12,370 individuals aged 18 to 74 years from 5 European countries, warts were the most common physician-diagnosed (27.3%) and self-reported (41.0%) dermatologic condition. Warts are more common in Northern countries (eg, Netherlands, Germany).8 Children with atopic dermatitis have a higher risk of developing warts and extracutaneous infections. In one study, children with warts and atopic dermatitis had a higher number of infections and food allergies and higher incidence of asthma and hay fever than either condition alone.9
Clinical Presentation of Warts
Warts usually present as common, palmoplantar, flat, or filiform in childhood, but variations by age are common (eFigure). The common and palmoplantar variants often are caused by HPV types 1 and 2.4,5 In infancy, vertically transmitted HPV infections can cause juvenile-onset respiratory papillomatosis or vertically transmitted condyloma. Juvenile-onset respiratory papillomatosis refers to upper respiratory papillomas that are difficult to eliminate and has been associated with exfoliated cervical cell testing with 18.1% (13/72) typed HPV-positive, which allows neonates to be exposed to HPV in the upper respiratory tract in utero.10

Vertically transmitted condyloma is a difficult topic. Much data supports the vertical transmission of condyloma as the leading cause of condyloma in small children; however, a reasonable amount of caution is needed in this patient population. In cases suspicious for sexual abuse as well as those presenting in children 4 years and older, formal household evaluation by a sexual abuse clinic and mandatory reporting is needed. Anywhere from 2.6% to 32% of cases of genital warts in children have been reported to be caused by sexual abuse.11-13 Therefore, most investigators have recommended careful review of the patient’s history and socioeconomic circumstances as well as a thorough physical examination. Mandatory reporting of suspected child sexual abuse is required in suspicious cases. Because HPV type 16 has been found in vertically transmitted cases, concern for long-term oncogenesis exists.11-13
Adolescents generally present with lesions on the hands and feet. Plantar warts often are caused by HPV types from the alpha genus. Subtypes noted in plantar warts include HPV types 1a, 2, 27, 57, and 65.14 By 15 years of age, genital HPV becomes a common adolescent infection, persisting into adulthood.15 When studied, genital HPV often is subclinical or latent and often is preventable through vaccination. High-risk oncogenic alpha-genus HPV types can immortalize human keratinocytes. When HPV types 11, 16, 18, and 31 are compared, HPV-18 has the highest oncogenic potential based on colony-stimulating potential.16 Vaccination with the 9-valent HPV vaccine is recommended in adolescence due to the concern for exposures to both low-potential (HPV types 6 and 11) and high-potential (HPV types 16 and 18) oncogenic HPV types. Data strongly support the benefit of 9-valent HPV vaccination in the prevention of sexually transmitted HPV in both males and females.17
Contagion of HPV is easy due to its excellent survival of fomites on surfaces, which generally is how warts are transferred in gym or pool settings where individuals who walk barefoot in changing rooms are almost twice as likely to contract plantar warts (odds ratio, 1.97 [95% CI, 1.39%-2.79%]).18 In another case series, walking barefoot, using a swimming pool, and having a household contact with warts were the leading risk factors for contraction of warts in children younger than 13 years.19 Children often transfer warts from site to site as well as to siblings and other close contacts. Skin-to-skin contact is responsible for sexual transmission of warts, and surface transmission occurs via fomites. Entry of the virus often occurs through small breaks in the skin. Other modes of transmission include orogenital.20
Therapeutic Options
Although the nuances of each available treatment for pediatric warts are beyond the scope of this article, the main core of therapy is 1 of 3 approaches: (1) observation, (2) over-the-counter salicylic acid therapy, and (3) in-office cryotherapy. Observation is an affirmed style of therapy for warts, as it is expected that two-thirds of warts will spontaneously resolve in 2 years and three-quarters will resolve in 3 years.4,5 Condyloma in children has been responsive to therapies such as cryotherapy and imiquimod,13 but spontaneous clearance in 5 years has been noted in 76% of children,21 which is linked to development of spontaneous immune response in most individuals.
Therapies for pediatric warts are characterized according to 6 major categories: destructive; immune stimulating; immune modulating, including normalization of epithelial growth; irritant; vascular destructive; and nitric oxide releasing (eTable).

Destructive Therapies
Destructive therapies for warts often are implemented in cases of disfigurement, discomfort/pain, and/or spreading, as well as to control contagion. According to a 2001 Cochrane review, salicylic acid has the best evidence of all therapeutics for the clearance of warts compared to placebo.24 On the other hand, aggressive cryotherapy and combined salicylic acid and cryotherapy had the best evidence in their favor in a 2011 meta-analysis by Kwok et al.25 Both salicylic acid and cryotherapy are considered destructive therapies. A recent meta-analysis of cantharidin, another destructive therapy, showed that local cantharidin alone as well as in combination with salicylic acid and podophyllotoxin showed good efficacy for warts; however, increased caution should be exerted with the combination regimen in young children due to a potential increase in the side-effect profile (eg, severe blistering).22 Other destructive agents such as topical retinoids can only peel surface layers of the skin and therefore are limited to flat facial warts, which are not expected to have an extensive hyperkeratotic layer; however, with occlusion, agents such as adapalene gel 0.1% can be used even on plantar warts with some efficacy.29
Immune-Stimulating Therapies
Immune stimulants often are used to treat warts in children and adolescents who have many lesions, a prolonged disease course, disfigurement, and/or subungual localizations, as well as in those who have been treated with multiple destructive methods without success. Topical imiquimod and oral cimetidine are readily available, while squaric acid (at-home or in-office therapy) and intralesional candida antigen can be used in offices that carry these agents. Topical imiquimod has been reported to achieve success in genital warts in children,13 with good efficacy in recalcitrant, periungual, and subungual warts when used for up to 16 weeks.31 In one randomized clinical trial, imiquimod cream 5% combined with salicylic acid 15% was applied to warts for 6 to 10 hours for 5 consecutive days per week versus cryotherapy with liquid nitrogen every 2 weeks for a maximum of 3 months. At the end of the study period, 81.1% (30/37) of participants treated with imiquimod and salicylic acid showed clearance of their warts versus 67.3% (33/49) of those treated with cryotherapy.32
Oral cimetidine has been reported to be successful in treating recalcitrant warts in more than 80% of children when dosed at 30 to 40 mg/kg 3 times daily, requiring 6 to 12 weeks to achieve clearance. Side effects of oral cimetidine include many cytochrome P450 interactions; gynecomastia, which limits usage in teenaged males; and stomach upset.30
Treatment of recalcitrant pediatric warts with intralesional candida antigen has been associated with side effects consistent with delayed-type hypersensitivity reactions. Injections should be administered once monthly, with a minimum of 3 cycles if not effective and up to 6 cycles where partial efficacy is noted. In a retrospective review of 220 cases, 70.9% of children showed complete clearance and 16.8% had partial response.33 However, the treatment may be limited in children by fear of needles.
Squaric acid dibutyl ester is a universal allergen that is not mutagenic on Ames testing and causes milder allergy symptoms than the mutagenic dinitrochlorobenzene and less erythema and pruritus than diphencyclopropenone. Squaric acid dibutyl ester home therapy was evaluated in 61 children with at least one nonfacial wart.34 Application began with squaric acid dibutyl ester in acetone (SADBE) 2% sensitization on the arm followed by at-home application of SADBE 0.2% three to seven times weekly for a minimum of 2 months to determine benefit and for 3 to 4 months as needed; however, average response was 7 weeks. The average complete clearance was 58% and partial clearance was 18%. Side effects included erythema and mild itching as well as urticaria in one case.34 In-office SADBE also has been evaluated in children. In a case series that included 29 children sensitized with SADBE 1% to 2% under occlusion followed by once monthly application of SADBE 0.5% to 5.0% to their warts, 69% clearance and 10% partial clearance was noted after a little more than 4 months of treatment.35 One retrospective review compared combination SADBE, trichloroacetic acid (TCA), and cantharidin both alone and in combination as duos (eg, SADBE and TCA) or trios (SADBE, TCA, and cantharidin).23 Of the 74 children whose medical charts were reviewed, the addition of pretreatment of warts with TCA 50% prior to in-office sensitization and monthly in-office application of SADBE increased treatment response to 100% with an average 2.45 months of therapy, whereas no enhancement was noted with cantharidin. Therefore, it appears that there may be enhanced immune reactivity when TCA pretreatment of warts is performed.23
Immune-Modulating Therapies (Including Normalization of Epithelial Growth)
The most novel immunologic therapy for warts is plerixafor, an agent used to treat WHIM (warts, hypogammaglobulinemia, infections, and myelokathexis) syndrome, which has been linked to heterozygous gain of function mutations in the chemokine receptor CXCR4 (located on 2q22). In WHIM syndrome, the mutated CXCR4 is more sensitive to CXCL12 activation. Plerixafor is a selective reversible antagonist that blocks the capacity of the chemokine CXCL12 to sustain the permanent activation of CXCR4.37 Combination therapy with plerixafor and topical imiquimod has resulted in wart improvement in WHIM syndrome patients in a small series.38
Oral isotretinoin has been described to be efficacious over placebo at a dosage of 30 mg daily for 12 weeks and can be used in teenagers but requires standard monitoring.36
Irritant Therapies
Duct tape is a classic agent that produces maceration and irritation of warts. Application of duct tape over warts has been described in cycles of 6 days on, 1 day off with weekly repetition for a few months but usually not on the palms or soles due to difficulty maintaining occlusive tape in these locations over an extended period of time. In one trial, 85% (22/26) of duct tape–treated cases cleared versus 60% (15/25) of cryotherapy-treated cases over a 2-month maximum therapeutic period.39
Vascular Destructive Therapies
The pulsed dye laser is a classic modality that induces localized destruction of blood supply to warts in children. A case series of 61 children treated with the pulsed dye laser revealed 75% overall clearance in an average of 3.1 sessions. The usage of this therapy often is limited to institutions where the technology is readily available for usage.40
Nitric Oxide–Releasing Therapies
Nitric oxide release may increase local blood flow, thereby increasing immune response, or may have a primary mechanism of antimicrobial activity, which is why these agents have been investigated for wart treatment. Topical garlic has been described anecdotally as a therapy for thin childhood warts with the putative mechanism being nitric oxide release.42 A new investigational drug recently has had phase 2 data published. Berdazimer sodium plus carboxymethyl cellulose hydrogel has demonstrated benefit in adult warts, but data in children is lacking.41
Therapeutic Ladder for Childhood Warts
The therapeutic ladder (Table) for childhood warts starts with first doing no harm. Although many parents are disturbed by their child’s condition, the natural history of resolution is spontaneous and therefore no therapy is required in many cases. The child and his/her caregivers should be engaged to determine if he/she is emotionally disturbed or uncomfortable with their lesions and to address any fears and concerns that some children may experience (eg, contagion risk, pain with ambulation, ostracism). For example, children with hand warts may report that other children will not hold their hand while in line at school. Prominent facial lesions can be particularly problematic for children due to teasing and bullying.

Conclusion
Warts are a common infection in childhood caused by the ubiquitous HPV virus. Therapeutic options abound, but most cases are either ignored or treated with over-the-counter salicylic acid or in-office cryotherapy. The decision to employ alternative therapeutic options requires agreement by the child, his/her caregiver, and the treating physician and can be tailored to suit the desires and needs of the child. Whether or not therapy is offered, spontaneous clearance is frequently seen in common warts. On the other hand, genital warts are associated with later conversion to malignancies of the genital tract; therefore, encouragement of HPV vaccination is needed in the adolescent population to best ensure long-term genital health.
1. Warts. https://medical-dictionary.thefreedictionary.com/warts. Accessed November 30, 2018.
2. Human papillomavirus. WHO website. http://www.who.int/biologicals/areas/human_papillomavirus/en. Accessed December 3, 2018.
3. Silverberg NB. Human papillomavirus infections in children. Curr Opin Pediatr. 2004;16:402-409.
4. Silverberg NB. Warts and molluscum in children. Adv Dermatol. 2004;20:23-73.
5. Silverberg NB, McCuaig CC. Melanoma in childhood: changing our mind-set. Cutis. 2013;92:217-218.
6. Bruggink SC, Eekhof JA, Egberts PF, et al. Warts transmitted in families and schools: a prospective cohort. Pediatrics. 2013;131:928-934.
7. Silverberg JI, Silverberg NB. The U.S. prevalence of common warts in childhood: a population-based study. J Invest Dermatol. 2013;133:2788-2790.
8. Svensson A, Ofenloch RF, Bruze M, et al. Br J Dermatol. 2018;178:1111-1118.
9. Silverberg JI, Silverberg NB. Childhood atopic dermatitis and warts are associated with increased risk of infection: a US population-based study. J Allergy Clin Immunol. 2014;133:1041-1047.
10. Smith EM, Johnson SR, Cripe TP, et al. Perinatal vertical transmission of human papillomavirus and subsequent development of respiratory tract papillomatosis. Ann Otol Rhinol Laryngol. 1991;100:479-483.
11. Costa-Silva M, Azevedo F, Lisboa C. Anogenital warts in children: analysis of a cohort of 34 prepubertal children. Pediatr Dermatol. 2018;35:E325-E327.
12. Marcoux D, Nadeau K, McCuaig C, et al. Pediatric anogenital warts: a 7-year review of children referred to a tertiary-care hospital in Montreal, Canada. Pediatr Dermatol. 2006;23:199-207.
13. Stefanaki C, Barkas G, Valari M, et al. Condylomata acuminata in children. Pediatr Infect Dis J. 2012;31:422-424.
14. dePlanell-Mas E, Martinez-Garriga B, Zalacain AJ, et al. Human papillomaviruses genotyping in plantar warts. J Med Virol. 2017;89:902-907.
15. Satterwhite CL, Torrone E, Meites E, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sex Transm Dis. 2013;40:187-193.
16. Lace MJ, Anson JR, Klingelhutz AJ, et al. Human papillomavirus (HPV) type 18 induces extended growth in primary human cervical, tonsillar, or foreskin keratinocytes more effectively than other high-risk mucosal HPVs. J Virol. 2009;83:11784-11794.
17. Sudenga SL, Ingles DJ, Pierce Campbell CM, et al. Genital human papillomavirus infection progression to external genital lesions: the HIM study. Eur Urol. 2016;69:166-173.
18. Rigo MV, Martínez Campillo F, Verdú M, et al. Risk factors linked to the transmission of papilloma virus in the school environment [in Spanish]. Alicante, 1999. Aten Primaria. 2003;31:415-420.
19. Al-Mutairi N, AlKhalaf M. Mucocutaneous warts in children: clinical presentations, risk factors, and response to treatment. Acta Dermatovenerol Alp Pannonica Adriat. 2012;21:69-72.
20. Clarke J, Terry RM, Lacey CJ. A study to estimate the prevalence of upper respiratory tract papillomatosis in patients with genital warts. Int J STD AIDS. 1991;2:114-115.
21. Allen AL, Siegfried EC. The natural history of condyloma in children. J Am Acad Dermatol. 1998;39:951-955.
22. Vakharia PP, Chopra R, Silverberg NB, et al. Efficacy and safety of topical cantharidin treatment for molluscum contagiosum and warts: a systematic review. Am J Clin Dermatol. 2018;19:791-803.
23. Silverberg JI, Silverberg NB. Adjunctive trichloroacetic acid therapy enhances squaric acid response to verruca vulgaris. J Drugs Dermatol. 2012;11:1228-1230.
24. Gibbs S, Harvey I, Sterling JC, et al. Local treatments for cutaneous warts. Cochrane Database Syst Rev. 2001:CD001781.
25. Kwok CS, Holland R, Gibbs S. Efficacy of topical treatments for cutaneous warts: a meta-analysis and pooled analysis of randomized controlled trials. Br J Dermatol. 2011;165:233-246.
26. Allington HV. Liquid nitrogen in the treatment of skin diseases. Calif Med. 1950;72:153-155.
27. Caravati CM Jr, Wood BT, Richardson DR. Onychodystrophies secondary to liquid nitrogen cryotherapy. Arch Dermatol. 1969;100:441-442.
28. Duofilm [package insert]. Sligo, Ireland: Stiefel Laboratories (Ireland) Ltd; 2016.
29. Gupta R, Gupta S. Topical adapalene in the treatment of plantar warts: randomized comparative open trial in comparison with cryo-therapy. Indian J Dermatol. 2015;60:102.
30. Orlow SJ, Paller A. Cimetidine therapy for multiple viral warts in children. J Am Acad Dermatol. 1993;28(5 pt 1):794-796.
31. Micali G, Dall’Oglio F, Nasca MR. An open label evaluation of the efficacy of imiquimod 5% cream in the treatment of recalcitrant subungual and periungual cutaneous warts. J Dermatolog Treat. 2003;14:233-236.
32. Stefanaki C, Lagogiani I, Kouris A, et al. Cryotherapy versus imiquimod 5% cream combined with a keratolytic lotion in cutaneous warts in children: a randomized study. J Dermatolog Treat. 2016;27:80-82.
33. Muñoz Garza FZ, Roé Crespo E, Torres Pradilla M, et al. Intralesional Candida antigen immunotherapy for the treatment of recalcitrant and multiple warts in children. Pediatr Dermatol. 2015;32:797-801.
34. Silverberg NB, Lim JK, Paller AS, et al. Squaric acid immunotherapy for warts in children. J Am Acad Dermatol. 2000;42(5 pt 1):803-808.
35. Lee AN, Mallory SB. Contact immunotherapy with squaric acid dibutylester for the treatment of recalcitrant warts. J Am Acad Dermatol. 1999;41:595-599.
36. Olguin-García MG, Jurado-Santa Cruz F, Peralta-Pedrero ML, et al. A double-blind, randomized, placebo-controlled trial of oral isotretinoin in the treatment of recalcitrant facial flat warts. J Dermatolog Treat. 2015;26:78-82.
37. Badolato R, Donadieu J; WHIM Research Group. How I treat warts, hypogammaglobulinemia, infections, and myelokathexis syndrome. Blood. 2017;130:2491-2498.
38. McDermott DH, Liu Q, Velez D, et al. A phase 1 clinical trial of long-term, low-dose treatment of WHIM syndrome with the CXCR4 antagonist plerixafor. Blood. 2014;123:2308-2316.
39. Focht DR 3rd, Spicer C, Fairchok MP. The efficacy of duct tape vs cryotherapy in the treatment of verruca vulgaris (the common wart). Arch Pediatr Adolesc Med. 2002;156:971-974.
40. Sethuraman G, Richards KA, Hiremagalore RN, et al. Effectiveness of pulsed dye laser in the treatment of recalcitrant warts in children. Dermatol Surg. 2010;36:58-65.
41. Tyring SK, Rosen T, Berman B, et al. A phase 2 controlled study of SB206, a topical nitric oxide-releasing drug for extragenital wart treatment. J Drugs Dermatol. 2018;17:1100-1105.
42. Silverberg NB. Garlic cloves for verruca vulgaris. Pediatr Dermatol. 2002;19:183.
1. Warts. https://medical-dictionary.thefreedictionary.com/warts. Accessed November 30, 2018.
2. Human papillomavirus. WHO website. http://www.who.int/biologicals/areas/human_papillomavirus/en. Accessed December 3, 2018.
3. Silverberg NB. Human papillomavirus infections in children. Curr Opin Pediatr. 2004;16:402-409.
4. Silverberg NB. Warts and molluscum in children. Adv Dermatol. 2004;20:23-73.
5. Silverberg NB, McCuaig CC. Melanoma in childhood: changing our mind-set. Cutis. 2013;92:217-218.
6. Bruggink SC, Eekhof JA, Egberts PF, et al. Warts transmitted in families and schools: a prospective cohort. Pediatrics. 2013;131:928-934.
7. Silverberg JI, Silverberg NB. The U.S. prevalence of common warts in childhood: a population-based study. J Invest Dermatol. 2013;133:2788-2790.
8. Svensson A, Ofenloch RF, Bruze M, et al. Br J Dermatol. 2018;178:1111-1118.
9. Silverberg JI, Silverberg NB. Childhood atopic dermatitis and warts are associated with increased risk of infection: a US population-based study. J Allergy Clin Immunol. 2014;133:1041-1047.
10. Smith EM, Johnson SR, Cripe TP, et al. Perinatal vertical transmission of human papillomavirus and subsequent development of respiratory tract papillomatosis. Ann Otol Rhinol Laryngol. 1991;100:479-483.
11. Costa-Silva M, Azevedo F, Lisboa C. Anogenital warts in children: analysis of a cohort of 34 prepubertal children. Pediatr Dermatol. 2018;35:E325-E327.
12. Marcoux D, Nadeau K, McCuaig C, et al. Pediatric anogenital warts: a 7-year review of children referred to a tertiary-care hospital in Montreal, Canada. Pediatr Dermatol. 2006;23:199-207.
13. Stefanaki C, Barkas G, Valari M, et al. Condylomata acuminata in children. Pediatr Infect Dis J. 2012;31:422-424.
14. dePlanell-Mas E, Martinez-Garriga B, Zalacain AJ, et al. Human papillomaviruses genotyping in plantar warts. J Med Virol. 2017;89:902-907.
15. Satterwhite CL, Torrone E, Meites E, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sex Transm Dis. 2013;40:187-193.
16. Lace MJ, Anson JR, Klingelhutz AJ, et al. Human papillomavirus (HPV) type 18 induces extended growth in primary human cervical, tonsillar, or foreskin keratinocytes more effectively than other high-risk mucosal HPVs. J Virol. 2009;83:11784-11794.
17. Sudenga SL, Ingles DJ, Pierce Campbell CM, et al. Genital human papillomavirus infection progression to external genital lesions: the HIM study. Eur Urol. 2016;69:166-173.
18. Rigo MV, Martínez Campillo F, Verdú M, et al. Risk factors linked to the transmission of papilloma virus in the school environment [in Spanish]. Alicante, 1999. Aten Primaria. 2003;31:415-420.
19. Al-Mutairi N, AlKhalaf M. Mucocutaneous warts in children: clinical presentations, risk factors, and response to treatment. Acta Dermatovenerol Alp Pannonica Adriat. 2012;21:69-72.
20. Clarke J, Terry RM, Lacey CJ. A study to estimate the prevalence of upper respiratory tract papillomatosis in patients with genital warts. Int J STD AIDS. 1991;2:114-115.
21. Allen AL, Siegfried EC. The natural history of condyloma in children. J Am Acad Dermatol. 1998;39:951-955.
22. Vakharia PP, Chopra R, Silverberg NB, et al. Efficacy and safety of topical cantharidin treatment for molluscum contagiosum and warts: a systematic review. Am J Clin Dermatol. 2018;19:791-803.
23. Silverberg JI, Silverberg NB. Adjunctive trichloroacetic acid therapy enhances squaric acid response to verruca vulgaris. J Drugs Dermatol. 2012;11:1228-1230.
24. Gibbs S, Harvey I, Sterling JC, et al. Local treatments for cutaneous warts. Cochrane Database Syst Rev. 2001:CD001781.
25. Kwok CS, Holland R, Gibbs S. Efficacy of topical treatments for cutaneous warts: a meta-analysis and pooled analysis of randomized controlled trials. Br J Dermatol. 2011;165:233-246.
26. Allington HV. Liquid nitrogen in the treatment of skin diseases. Calif Med. 1950;72:153-155.
27. Caravati CM Jr, Wood BT, Richardson DR. Onychodystrophies secondary to liquid nitrogen cryotherapy. Arch Dermatol. 1969;100:441-442.
28. Duofilm [package insert]. Sligo, Ireland: Stiefel Laboratories (Ireland) Ltd; 2016.
29. Gupta R, Gupta S. Topical adapalene in the treatment of plantar warts: randomized comparative open trial in comparison with cryo-therapy. Indian J Dermatol. 2015;60:102.
30. Orlow SJ, Paller A. Cimetidine therapy for multiple viral warts in children. J Am Acad Dermatol. 1993;28(5 pt 1):794-796.
31. Micali G, Dall’Oglio F, Nasca MR. An open label evaluation of the efficacy of imiquimod 5% cream in the treatment of recalcitrant subungual and periungual cutaneous warts. J Dermatolog Treat. 2003;14:233-236.
32. Stefanaki C, Lagogiani I, Kouris A, et al. Cryotherapy versus imiquimod 5% cream combined with a keratolytic lotion in cutaneous warts in children: a randomized study. J Dermatolog Treat. 2016;27:80-82.
33. Muñoz Garza FZ, Roé Crespo E, Torres Pradilla M, et al. Intralesional Candida antigen immunotherapy for the treatment of recalcitrant and multiple warts in children. Pediatr Dermatol. 2015;32:797-801.
34. Silverberg NB, Lim JK, Paller AS, et al. Squaric acid immunotherapy for warts in children. J Am Acad Dermatol. 2000;42(5 pt 1):803-808.
35. Lee AN, Mallory SB. Contact immunotherapy with squaric acid dibutylester for the treatment of recalcitrant warts. J Am Acad Dermatol. 1999;41:595-599.
36. Olguin-García MG, Jurado-Santa Cruz F, Peralta-Pedrero ML, et al. A double-blind, randomized, placebo-controlled trial of oral isotretinoin in the treatment of recalcitrant facial flat warts. J Dermatolog Treat. 2015;26:78-82.
37. Badolato R, Donadieu J; WHIM Research Group. How I treat warts, hypogammaglobulinemia, infections, and myelokathexis syndrome. Blood. 2017;130:2491-2498.
38. McDermott DH, Liu Q, Velez D, et al. A phase 1 clinical trial of long-term, low-dose treatment of WHIM syndrome with the CXCR4 antagonist plerixafor. Blood. 2014;123:2308-2316.
39. Focht DR 3rd, Spicer C, Fairchok MP. The efficacy of duct tape vs cryotherapy in the treatment of verruca vulgaris (the common wart). Arch Pediatr Adolesc Med. 2002;156:971-974.
40. Sethuraman G, Richards KA, Hiremagalore RN, et al. Effectiveness of pulsed dye laser in the treatment of recalcitrant warts in children. Dermatol Surg. 2010;36:58-65.
41. Tyring SK, Rosen T, Berman B, et al. A phase 2 controlled study of SB206, a topical nitric oxide-releasing drug for extragenital wart treatment. J Drugs Dermatol. 2018;17:1100-1105.
42. Silverberg NB. Garlic cloves for verruca vulgaris. Pediatr Dermatol. 2002;19:183.
Practice Points
- Warts are caused by infection with the human papillomavirus.
- Warts are extremely common in all age groups, but risk factors and types of lesions vary by age and location of lesions.
- Therapies for pediatric warts are characterized according to 6 major categories: destructive; immune stimulating; immune modulating, including normalization of epithelial growth; vascular destructive; irritant; and nitric oxide releasing.
The state of hospital medicine in 2018
Productivity, pay, and roles remain center stage
In a national health care environment undergoing unprecedented transformation, the specialty of hospital medicine appears to be an island of relative stability, a conclusion that is supported by the principal findings from SHM’s 2018 State of Hospital Medicine (SoHM) report.
The report of hospitalist group practice characteristics, as well as other key data defining the field’s current status, that the Society of Hospital Medicine puts out every 2 years reveals that overall salaries for hospitalist physicians are up by 3.8% since 2016. Although productivity, as measured by work relative value units (RVUs), remained largely flat over the same period, financial support per full-time equivalent (FTE) physician position to hospitalist groups from their hospitals and health systems is up significantly.
Total support per FTE averaged $176,657 in 2018, 12% higher than in 2016, noted Leslie Flores, MHA, SFHM, of Nelson Flores Hospital Medicine Consultants, and a member of SHM’s Practice Analysis Committee, which oversees the biennial survey. Compensation and productivity data were collected by the Medical Group Management Association and licensed by SHM for inclusion in its report.
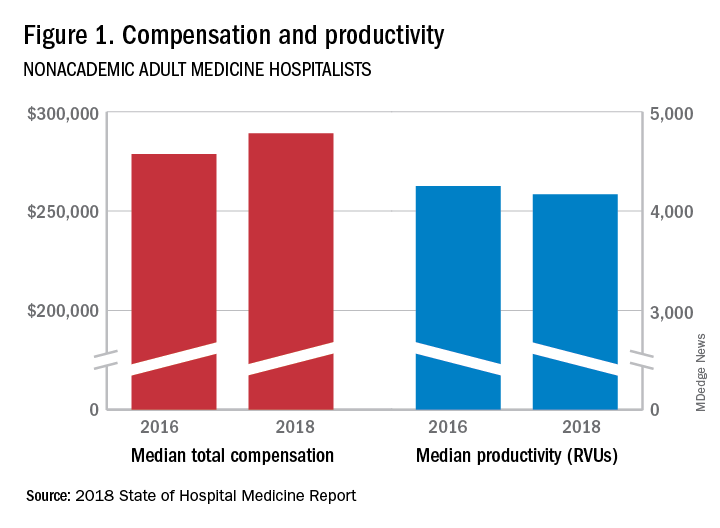
These findings – particularly the flat productivity – raise questions about long-term sustainability, Ms. Flores said. “What is going on? Do hospital administrators still recognize the value hospitalists bring to the operations and the quality of their hospitals? Or is paying the subsidy just a cost of doing business – a necessity for most hospitals in a setting where demand for hospitalist positions remains high?”
Andrew White, MD, FACP, SFHM, chair of SHM’s Practice Analysis Committee and director of the hospital medicine service at the University of Washington Medical Center, Seattle, said basic market forces dictate that it is “pretty much inconceivable” to run a modern hospital of any size without hospitalists.
“Clearly, demand outstrips supply, which drives up salaries and support, whether CEOs feel that the hospitalist group is earning that support or not,” Dr. White said. “The unfilled hospitalist positions we identified speak to ongoing projected greater demand than supply. That said, hospitalists and group leaders can’t be complacent and must collaborate effectively with hospitals to provide highly valuable services.” Turnover of hospitalist positions was up slightly, he noted, at 7.4% in 2018, from 6.9% in 2016, reversing a trend of previous years.
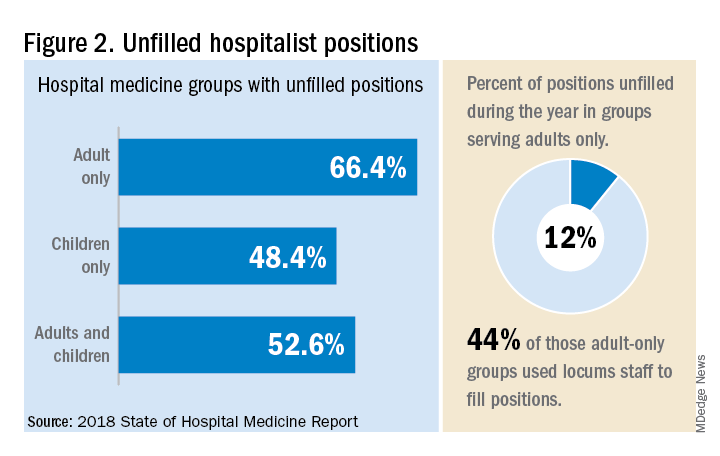
But will these trends continue at a time when hospitals face continued pressure to cut costs, as the hospital medicine subsidy may represent one of their largest cost centers? Because the size of hospitalist groups continues to grow, hospitals’ total subsidy for hospital medicine is going up faster than the percentage increase in support per FTE.
How do hospitalists use the SoHM report?
Dr. White called the 2018 SoHM report the “most representative and balanced sample to date” of hospitalist group practices, with some of the highest quality data, thanks to more robust participation in the survey by pediatric groups and improved distribution among hospitalist management companies and academic programs.
“Not that past reports had major flaws, but this version is more authoritative, reflecting an intentional effort by our Practice Analysis Committee to bring in more participants from key groups,” he said.
The biennial report has been around long enough to achieve brand recognition in the field as the most authoritative source of information regarding hospitalist practice, he added. “We worked hard this year to balance the participants, with more of our responses than in the past coming from multi-hospital groups, whether 4 to 5 sites, or 20 to 30.”
Surveys were conducted online in January and February of 2018 in response to invitations mailed and emailed to targeted hospital medicine group leaders. A total of 569 groups completed the survey, representing 8,889 hospitalist FTEs, approximately 16% of the total hospitalist workforce. Responses were presented in several categories, including by size of program, region and employment model. Groups that care for adults only represented 87.9% of the surveys, while groups that care for children only were 6.7% and groups that care for both adults and children were 5.4%.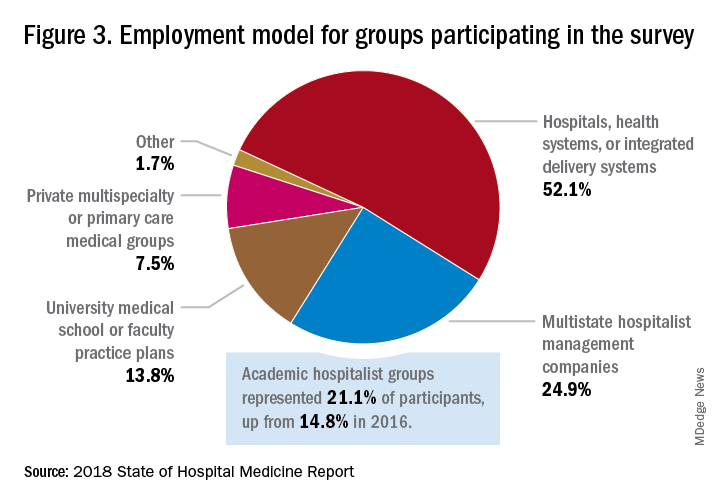
“This survey doesn’t tell us what should be best practice in hospital medicine,” Dr. White said, only what is actual current practice. He uses it in his own health system to not only contextualize and justify his group’s performance metrics for hospital administrators – relative to national and categorical averages – but also to see if the direction his group is following is consistent with what’s going on in the larger field.
“These data offer a very powerful resource regarding the trends in hospital medicine,” said Romil Chadha, MD, MPH, FACP, SFHM, associate division chief for operations in the division of hospital medicine at the University of Kentucky and UK Healthcare, Lexington. “It is my repository of data to go before my administrators for decisions that need to be made or to pilot new programs.”
Dr. Chadha also uses the data to help answer compensation, scheduling, and support questions from his group’s members.
Thomas McIlraith, MD, immediate past chairman of the hospital medicine department at Mercy Medical Group, Sacramento, Calif., said the report’s value is that it allows comparisons of salaries in different settings, and to see, for example, how night staffing is structured. “A lot of leaders I spoke to at SHM’s 2018 Leadership Academy in Vancouver were saying they didn’t feel up to parity with the national standards. You can use the report to look at the state of hospital medicine nationally and make comparisons,” he said.
Calls for more productivity
Roberta Himebaugh, MBA, SFHM, senior vice president of acute care services for the national hospitalist management company TeamHealth, and cochair of the SHM Practice Administrators Special Interest Group, said her company’s clients have traditionally asked for greater productivity from their hospitalist contracts as a way to decrease overall costs. Some markets are starting to see a change in that approach, she noted.
“Recently there’s been an increased focus on paying hospitalists to focus on quality rather than just productivity. Some of our clients are willing to pay for that, and we are trying to assign value to this non-billable time or adjust our productivity standards appropriately. I think hospitals definitely understand the value of non-billable services from hospitalists, but still will push us on the productivity targets,” Ms. Himebaugh said.
“I don’t believe hospital medicine can be sustainable long term on flat productivity or flat RVUs,” she added. “Yet the costs of burnout associated with pushing higher productivity are not sustainable, either.” So what are the answers? She said many inefficiencies are involved in responding to inquiries on the floor that could have been addressed another way, or waiting for the turnaround of diagnostic tests.
“Maybe we don’t need physicians to be in the hospital 24/7 if we have access to telehealth, or a partnership with the emergency department, or greater use of advanced care practice providers,” Ms. Himebaugh said. “Our hospitals are examining those options, and we have to look at how we can become more efficient and less costly. At TeamHealth, we are trying to staff for value – looking at patient flow patterns and adjusting our schedules accordingly. Is there a bolus of admissions tied to emergency department shift changes, or to certain days of the week? How can we move from the 12-hour shift that begins at 7 a.m. and ends at 7 p.m., and instead provide coverage for when the patients are there?”
Mark Williams, MD, MHM, chief of the division of hospital medicine at the University of Kentucky, Lexington, said he appreciates the volume of data in the report but wishes for even more survey participants, which could make the breakouts for subgroups such as academic hospitalists more robust. Other current sources of hospitalist salary data include the Association of American Medical Colleges (AAMC), which produces compensation reports to help medical schools and teaching hospitals with benchmarking, and the Faculty Practice Solution Center developed jointly by AAMC and Vizient to provide faculty practice plans with analytic tools. The Medical Group Management Association (MGMA) is another valuable source of information, some of which was licensed for inclusion in the SoHM report.
“There is no source of absolute truth that hospitalists can point to,” Dr. Williams said. “I will present my data and my administrators will reply: ‘We have our own data.’ Our institution has consistently ranked first or second nationwide for the sickest patients. We take more Medicaid and dually eligible patients, who have a lot of social issues. They take a lot of time to manage medically and the RVUs don’t reflect that. And yet I’m still judged by my RVUs generated per hospitalist. Hospital administrators understandably want to get the most productivity, and they are looking for their own data for average productivity numbers.”
Ryan Brown, MD, specialty medical director for hospital medicine with Atrium Health in Charlotte, N.C., said that hospital medicine’s flat productivity trends would be difficult to sustain in the business world. But there aren’t easy or obvious ways to increase hospitalists’ productivity. The SoHM report also shows that as productivity increases, total compensation increases but at a lower rate, resulting in a gradual decrease in compensation per RVU.
Pressures to increase productivity can be a double-edged sword, Dr. Williams added. Demanding that doctors make more billable visits faster to generate more RVUs can be a recipe for burnout and turnover, with huge costs associated with recruiting replacements.
“If there was recent turnover of hospitalists at the hospital, with the need to find replacements, there may be institutional memory about that,” he said. “But where are hospitals spending their money? Bottom line, we still need to learn to cut our costs.”
How is hospitalist practice evolving?
In addition to payment and productivity data, the SoHM report provides a current picture of the evolving state of hospitalist group practices. A key thread is how the work hospitalists are doing, and the way they do it, is changing, with new information about comanagement roles, dedicated admitters, night coverage, geographic rounding, and the like.
Making greater use of nurse practitioners and physician assistants (NPs/PAs), may be one way to change the flat productivity trends, Dr. Brown said. With a cost per RVU that’s roughly half that of a doctor’s, NPs/PAs could contribute to the bottom line. But he sees surprisingly large variation in how hospitalist groups are using them. Typically, they are deployed at a ratio of four doctors to one NP/PA, but that ratio could be two to one or even one to one, he said.
Use of NPs/PAs by academic hospitalist groups is up, from 52.1% in 2016 to 75.7% in 2018. For adult-only groups, 76.8% had NPs/PAs, with higher rates in hospitals and health systems and lower rates in the West region. But a lot of groups are using these practitioners for nonproductive work, and some are failing to generate any billing income, Dr. Brown said.
“The rate at which NPs/PAs performed billable services was higher in physician-owned practices, resulting in a lower cost per RVU, suggesting that many practices may be underutilizing their NPs/PAs or not sharing the work.” Not every NP or PA wants to or is able to care for very complex patients, Dr. Brown said, “but you want a system where the NP and PA can work at the highest level permitted by state law.”
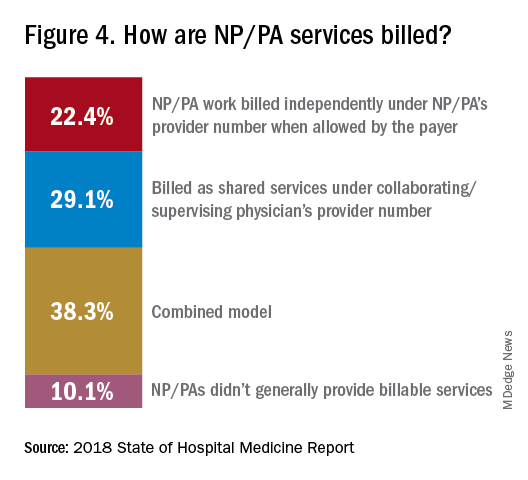
The predominant scheduling model of hospital medicine, 7 days on duty followed by 7 days off, has diminished somewhat in recent years. There appears to be some fluctuation and a gradual move away from 7 on/7 off toward some kind of variable approach, since the former may not be physically sustainable for the doctor over the long haul, Dr. Brown said. Some groups are experimenting with a combined approach.
“I think balancing workload with manpower has always been a challenge for our field. Maybe we should be working shorter shifts or fewer days and making sure our hospitalists aren’t ever sitting around idle,” he said. “And could we come in on nonclinical days to do administrative tasks? I think the solution is out there, but we haven’t created the algorithms to define that yet. If you could somehow use the data for volume, number of beds, nurse staffing, etc., by year and seasonally, you might be able to reliably predict census. This is about applying data hospitals already have in their electronic health records, but utilizing the data in ways that are more helpful.”
Dr. McIlraith added that a big driver of the future of hospital medicine will be the evolution of the EHR and the digitalization of health care, as hospitals learn how to leverage more of what’s in their EHRs. “The impact will grow for hospitalists through the creation and maturation of big data systems – and the learning that can be extracted from what’s contained in the electronic health record.”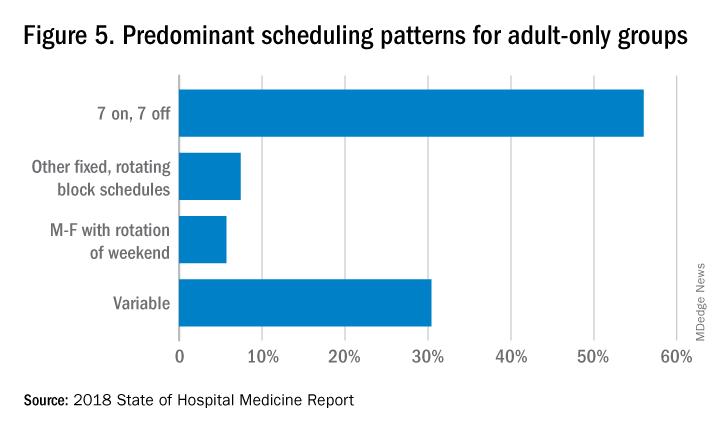
Another important question for hospitalist groups is their model of backup scheduling, to make sure there is a replacement available if a scheduled doctor calls in sick or if demand is unexpectedly high.
“In today’s world, this is how we have traditionally managed unpredictability,” Dr. Brown said. “You don’t know when you will need it, but if you need it, you want it immediately. So how do you pay for it – only when the doctor comes in, or also an amount just for being on call?” Some groups pay for both, he said, others for neither.
“We are a group of 70 hospitalists, and if someone is sick you can’t just shut down the service,” said Dr. Chadha. “We are one of the few to use incentives for both, which could include a 1-week decrease in clinical shifts in exchange for 2 weeks of backup. We have times with 25% usage of backup number 1, and 10% usage of backup number 2,” he noted. “But the goal is for our hospitalists to have assurances that there is a backup system and that it works.”
The presence of nocturnists in hospitals continues to rise, with 76.1% of adults-only groups having nocturnists, 27.6% of children-only groups, and 68.2% of adults and children groups. Geographic or unit-based hospital assignments have grown to 36.4% of adult-only groups.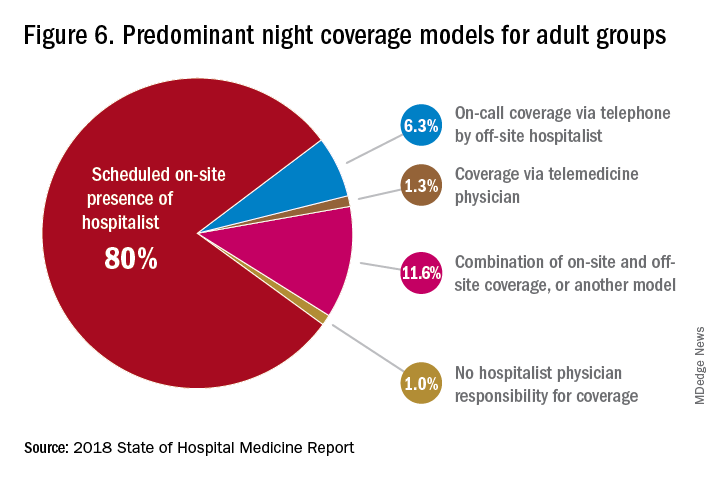
What are hospitalists’ other new roles?
“We have a large group of 50 doctors, with about 40 FTEs, and we are evolving from the traditional generalist role toward more subspecialty comanagement,” said Bryan Huang, MD, physician adviser and associate clinical professor in the division of hospital medicine at the University of California–San Diego. “Our hospitalists are asking what it means to be an academic hospitalist as our teaching roles have shrunk.”
Dr. Huang recently took on a new role as physician adviser for his hospital in such areas as utilization review, patient flow, and length of stay. “I’m spearheading a work group to address quality issues – all of which involve collaboration with other professionals. We also developed an admitting role here for a hospitalist whose sole role for the day is to admit patients.” Nationally up to 51.2% of hospitalist groups utilize a dedicated daytime admitter.
The report found that hospital services for which hospitalists are more likely to be attendings than consultants include GI/liver, 78.4%; palliative care, 77.3%; neurology/stroke, 73.6%; oncology, 67.8%; cardiology, 56.9%; and critical care, 50.7%. Conditions where hospitalists are more likely to consult rather than admit and attend include neurosurgery, orthopedics, general surgery, cardiovascular surgery, and other surgical subspecialties.
Other hospital services routinely provided by adult-only hospitalists include care of patients in an ICU setting (62.7%); primary responsibility for observation units (54.6%); primary clinical responsibility for rapid response teams (48.8%); primary responsibility for code blue or cardiac arrest teams (43.8%); nighttime admissions or tuck-in services (33.9%); and medical procedures (31.5%). For pediatric hospital medicine groups, care of healthy newborns and medical procedures were among the most common services provided, while for hospitalists serving adults and children, rapid response teams, ICUs, and specialty units were most common.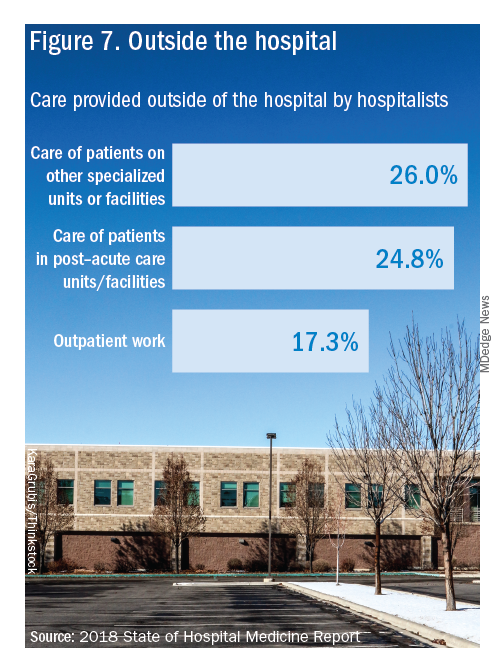
New models of payment for health care
As the larger health care system is being transformed by new payment models and benefit structures, including accountable care organizations (ACOs), value-based purchasing, bundled payments, and other forms of population-based coverage – which is described as a volume-to-value shift in health care – how are these new models affecting hospitalists?
Observers say penetration of these new models varies widely by locality but they haven’t had much direct impact on hospitalists’ practices – at least not yet. However, as hospitals and health systems find themselves needing to learn new ways to invest their resources differently in response to these trends, what matters to the hospital should be of great importance to the hospitalist group.
“I haven’t seen a lot of dramatic changes in how hospitalists engage with value-based purchasing,” Dr. White said. “If we know that someone is part of an ACO, the instinctual – and right – response is to treat them like any other patient. But we still need to be committed to not waste resources.”
Hospitalists are the best people to understand the intricacies of how the health care system works under value-based approaches, Dr. Huang said. “That’s why so many hospitalists have taken leadership positions in their hospitals. I think all of this translates to the practical, day-to-day work of hospitalists, reflected in our focus on readmissions and length of stay.”
Dr. Williams said the health care system still hasn’t turned the corner from fee-for-service to value-based purchasing. “It still represents a tiny fraction of the income of hospitalists. Hospitals still have to focus on the bottom line, as fee-for-service reimbursement for hospitalized patients continues to get squeezed, and ACOs aren’t exactly paying premium rates either. Ask almost any hospital CEO what drives their bottom line today and the answer is volume – along with optimizing productivity. Pretty much every place I look, the future does not look terribly rosy for hospitals.”
Ms. Himebaugh said she is bullish on hospital medicine, in the sense that it’s unlikely to go away anytime soon. “Hospitalists are needed and provide value. But I don’t think we have devised the right model yet. I’m not sure our current model is sustainable. We need to find new models we can afford that don’t require squeezing our providers.”
For more information about the 2018 State of Hospital Medicine Report, contact SHM’s Practice Management Department at: [email protected] or call 800-843-3360. See also: https://www.hospitalmedicine.org/practice-management/shms-state-of-hospital-medicine/.
Productivity, pay, and roles remain center stage
Productivity, pay, and roles remain center stage
In a national health care environment undergoing unprecedented transformation, the specialty of hospital medicine appears to be an island of relative stability, a conclusion that is supported by the principal findings from SHM’s 2018 State of Hospital Medicine (SoHM) report.
The report of hospitalist group practice characteristics, as well as other key data defining the field’s current status, that the Society of Hospital Medicine puts out every 2 years reveals that overall salaries for hospitalist physicians are up by 3.8% since 2016. Although productivity, as measured by work relative value units (RVUs), remained largely flat over the same period, financial support per full-time equivalent (FTE) physician position to hospitalist groups from their hospitals and health systems is up significantly.
Total support per FTE averaged $176,657 in 2018, 12% higher than in 2016, noted Leslie Flores, MHA, SFHM, of Nelson Flores Hospital Medicine Consultants, and a member of SHM’s Practice Analysis Committee, which oversees the biennial survey. Compensation and productivity data were collected by the Medical Group Management Association and licensed by SHM for inclusion in its report.

These findings – particularly the flat productivity – raise questions about long-term sustainability, Ms. Flores said. “What is going on? Do hospital administrators still recognize the value hospitalists bring to the operations and the quality of their hospitals? Or is paying the subsidy just a cost of doing business – a necessity for most hospitals in a setting where demand for hospitalist positions remains high?”
Andrew White, MD, FACP, SFHM, chair of SHM’s Practice Analysis Committee and director of the hospital medicine service at the University of Washington Medical Center, Seattle, said basic market forces dictate that it is “pretty much inconceivable” to run a modern hospital of any size without hospitalists.
“Clearly, demand outstrips supply, which drives up salaries and support, whether CEOs feel that the hospitalist group is earning that support or not,” Dr. White said. “The unfilled hospitalist positions we identified speak to ongoing projected greater demand than supply. That said, hospitalists and group leaders can’t be complacent and must collaborate effectively with hospitals to provide highly valuable services.” Turnover of hospitalist positions was up slightly, he noted, at 7.4% in 2018, from 6.9% in 2016, reversing a trend of previous years.

But will these trends continue at a time when hospitals face continued pressure to cut costs, as the hospital medicine subsidy may represent one of their largest cost centers? Because the size of hospitalist groups continues to grow, hospitals’ total subsidy for hospital medicine is going up faster than the percentage increase in support per FTE.
How do hospitalists use the SoHM report?
Dr. White called the 2018 SoHM report the “most representative and balanced sample to date” of hospitalist group practices, with some of the highest quality data, thanks to more robust participation in the survey by pediatric groups and improved distribution among hospitalist management companies and academic programs.
“Not that past reports had major flaws, but this version is more authoritative, reflecting an intentional effort by our Practice Analysis Committee to bring in more participants from key groups,” he said.
The biennial report has been around long enough to achieve brand recognition in the field as the most authoritative source of information regarding hospitalist practice, he added. “We worked hard this year to balance the participants, with more of our responses than in the past coming from multi-hospital groups, whether 4 to 5 sites, or 20 to 30.”
Surveys were conducted online in January and February of 2018 in response to invitations mailed and emailed to targeted hospital medicine group leaders. A total of 569 groups completed the survey, representing 8,889 hospitalist FTEs, approximately 16% of the total hospitalist workforce. Responses were presented in several categories, including by size of program, region and employment model. Groups that care for adults only represented 87.9% of the surveys, while groups that care for children only were 6.7% and groups that care for both adults and children were 5.4%.
“This survey doesn’t tell us what should be best practice in hospital medicine,” Dr. White said, only what is actual current practice. He uses it in his own health system to not only contextualize and justify his group’s performance metrics for hospital administrators – relative to national and categorical averages – but also to see if the direction his group is following is consistent with what’s going on in the larger field.
“These data offer a very powerful resource regarding the trends in hospital medicine,” said Romil Chadha, MD, MPH, FACP, SFHM, associate division chief for operations in the division of hospital medicine at the University of Kentucky and UK Healthcare, Lexington. “It is my repository of data to go before my administrators for decisions that need to be made or to pilot new programs.”
Dr. Chadha also uses the data to help answer compensation, scheduling, and support questions from his group’s members.
Thomas McIlraith, MD, immediate past chairman of the hospital medicine department at Mercy Medical Group, Sacramento, Calif., said the report’s value is that it allows comparisons of salaries in different settings, and to see, for example, how night staffing is structured. “A lot of leaders I spoke to at SHM’s 2018 Leadership Academy in Vancouver were saying they didn’t feel up to parity with the national standards. You can use the report to look at the state of hospital medicine nationally and make comparisons,” he said.
Calls for more productivity
Roberta Himebaugh, MBA, SFHM, senior vice president of acute care services for the national hospitalist management company TeamHealth, and cochair of the SHM Practice Administrators Special Interest Group, said her company’s clients have traditionally asked for greater productivity from their hospitalist contracts as a way to decrease overall costs. Some markets are starting to see a change in that approach, she noted.
“Recently there’s been an increased focus on paying hospitalists to focus on quality rather than just productivity. Some of our clients are willing to pay for that, and we are trying to assign value to this non-billable time or adjust our productivity standards appropriately. I think hospitals definitely understand the value of non-billable services from hospitalists, but still will push us on the productivity targets,” Ms. Himebaugh said.
“I don’t believe hospital medicine can be sustainable long term on flat productivity or flat RVUs,” she added. “Yet the costs of burnout associated with pushing higher productivity are not sustainable, either.” So what are the answers? She said many inefficiencies are involved in responding to inquiries on the floor that could have been addressed another way, or waiting for the turnaround of diagnostic tests.
“Maybe we don’t need physicians to be in the hospital 24/7 if we have access to telehealth, or a partnership with the emergency department, or greater use of advanced care practice providers,” Ms. Himebaugh said. “Our hospitals are examining those options, and we have to look at how we can become more efficient and less costly. At TeamHealth, we are trying to staff for value – looking at patient flow patterns and adjusting our schedules accordingly. Is there a bolus of admissions tied to emergency department shift changes, or to certain days of the week? How can we move from the 12-hour shift that begins at 7 a.m. and ends at 7 p.m., and instead provide coverage for when the patients are there?”
Mark Williams, MD, MHM, chief of the division of hospital medicine at the University of Kentucky, Lexington, said he appreciates the volume of data in the report but wishes for even more survey participants, which could make the breakouts for subgroups such as academic hospitalists more robust. Other current sources of hospitalist salary data include the Association of American Medical Colleges (AAMC), which produces compensation reports to help medical schools and teaching hospitals with benchmarking, and the Faculty Practice Solution Center developed jointly by AAMC and Vizient to provide faculty practice plans with analytic tools. The Medical Group Management Association (MGMA) is another valuable source of information, some of which was licensed for inclusion in the SoHM report.
“There is no source of absolute truth that hospitalists can point to,” Dr. Williams said. “I will present my data and my administrators will reply: ‘We have our own data.’ Our institution has consistently ranked first or second nationwide for the sickest patients. We take more Medicaid and dually eligible patients, who have a lot of social issues. They take a lot of time to manage medically and the RVUs don’t reflect that. And yet I’m still judged by my RVUs generated per hospitalist. Hospital administrators understandably want to get the most productivity, and they are looking for their own data for average productivity numbers.”
Ryan Brown, MD, specialty medical director for hospital medicine with Atrium Health in Charlotte, N.C., said that hospital medicine’s flat productivity trends would be difficult to sustain in the business world. But there aren’t easy or obvious ways to increase hospitalists’ productivity. The SoHM report also shows that as productivity increases, total compensation increases but at a lower rate, resulting in a gradual decrease in compensation per RVU.
Pressures to increase productivity can be a double-edged sword, Dr. Williams added. Demanding that doctors make more billable visits faster to generate more RVUs can be a recipe for burnout and turnover, with huge costs associated with recruiting replacements.
“If there was recent turnover of hospitalists at the hospital, with the need to find replacements, there may be institutional memory about that,” he said. “But where are hospitals spending their money? Bottom line, we still need to learn to cut our costs.”
How is hospitalist practice evolving?
In addition to payment and productivity data, the SoHM report provides a current picture of the evolving state of hospitalist group practices. A key thread is how the work hospitalists are doing, and the way they do it, is changing, with new information about comanagement roles, dedicated admitters, night coverage, geographic rounding, and the like.
Making greater use of nurse practitioners and physician assistants (NPs/PAs), may be one way to change the flat productivity trends, Dr. Brown said. With a cost per RVU that’s roughly half that of a doctor’s, NPs/PAs could contribute to the bottom line. But he sees surprisingly large variation in how hospitalist groups are using them. Typically, they are deployed at a ratio of four doctors to one NP/PA, but that ratio could be two to one or even one to one, he said.
Use of NPs/PAs by academic hospitalist groups is up, from 52.1% in 2016 to 75.7% in 2018. For adult-only groups, 76.8% had NPs/PAs, with higher rates in hospitals and health systems and lower rates in the West region. But a lot of groups are using these practitioners for nonproductive work, and some are failing to generate any billing income, Dr. Brown said.
“The rate at which NPs/PAs performed billable services was higher in physician-owned practices, resulting in a lower cost per RVU, suggesting that many practices may be underutilizing their NPs/PAs or not sharing the work.” Not every NP or PA wants to or is able to care for very complex patients, Dr. Brown said, “but you want a system where the NP and PA can work at the highest level permitted by state law.”

The predominant scheduling model of hospital medicine, 7 days on duty followed by 7 days off, has diminished somewhat in recent years. There appears to be some fluctuation and a gradual move away from 7 on/7 off toward some kind of variable approach, since the former may not be physically sustainable for the doctor over the long haul, Dr. Brown said. Some groups are experimenting with a combined approach.
“I think balancing workload with manpower has always been a challenge for our field. Maybe we should be working shorter shifts or fewer days and making sure our hospitalists aren’t ever sitting around idle,” he said. “And could we come in on nonclinical days to do administrative tasks? I think the solution is out there, but we haven’t created the algorithms to define that yet. If you could somehow use the data for volume, number of beds, nurse staffing, etc., by year and seasonally, you might be able to reliably predict census. This is about applying data hospitals already have in their electronic health records, but utilizing the data in ways that are more helpful.”
Dr. McIlraith added that a big driver of the future of hospital medicine will be the evolution of the EHR and the digitalization of health care, as hospitals learn how to leverage more of what’s in their EHRs. “The impact will grow for hospitalists through the creation and maturation of big data systems – and the learning that can be extracted from what’s contained in the electronic health record.”
Another important question for hospitalist groups is their model of backup scheduling, to make sure there is a replacement available if a scheduled doctor calls in sick or if demand is unexpectedly high.
“In today’s world, this is how we have traditionally managed unpredictability,” Dr. Brown said. “You don’t know when you will need it, but if you need it, you want it immediately. So how do you pay for it – only when the doctor comes in, or also an amount just for being on call?” Some groups pay for both, he said, others for neither.
“We are a group of 70 hospitalists, and if someone is sick you can’t just shut down the service,” said Dr. Chadha. “We are one of the few to use incentives for both, which could include a 1-week decrease in clinical shifts in exchange for 2 weeks of backup. We have times with 25% usage of backup number 1, and 10% usage of backup number 2,” he noted. “But the goal is for our hospitalists to have assurances that there is a backup system and that it works.”
The presence of nocturnists in hospitals continues to rise, with 76.1% of adults-only groups having nocturnists, 27.6% of children-only groups, and 68.2% of adults and children groups. Geographic or unit-based hospital assignments have grown to 36.4% of adult-only groups.
What are hospitalists’ other new roles?
“We have a large group of 50 doctors, with about 40 FTEs, and we are evolving from the traditional generalist role toward more subspecialty comanagement,” said Bryan Huang, MD, physician adviser and associate clinical professor in the division of hospital medicine at the University of California–San Diego. “Our hospitalists are asking what it means to be an academic hospitalist as our teaching roles have shrunk.”
Dr. Huang recently took on a new role as physician adviser for his hospital in such areas as utilization review, patient flow, and length of stay. “I’m spearheading a work group to address quality issues – all of which involve collaboration with other professionals. We also developed an admitting role here for a hospitalist whose sole role for the day is to admit patients.” Nationally up to 51.2% of hospitalist groups utilize a dedicated daytime admitter.
The report found that hospital services for which hospitalists are more likely to be attendings than consultants include GI/liver, 78.4%; palliative care, 77.3%; neurology/stroke, 73.6%; oncology, 67.8%; cardiology, 56.9%; and critical care, 50.7%. Conditions where hospitalists are more likely to consult rather than admit and attend include neurosurgery, orthopedics, general surgery, cardiovascular surgery, and other surgical subspecialties.
Other hospital services routinely provided by adult-only hospitalists include care of patients in an ICU setting (62.7%); primary responsibility for observation units (54.6%); primary clinical responsibility for rapid response teams (48.8%); primary responsibility for code blue or cardiac arrest teams (43.8%); nighttime admissions or tuck-in services (33.9%); and medical procedures (31.5%). For pediatric hospital medicine groups, care of healthy newborns and medical procedures were among the most common services provided, while for hospitalists serving adults and children, rapid response teams, ICUs, and specialty units were most common.
New models of payment for health care
As the larger health care system is being transformed by new payment models and benefit structures, including accountable care organizations (ACOs), value-based purchasing, bundled payments, and other forms of population-based coverage – which is described as a volume-to-value shift in health care – how are these new models affecting hospitalists?
Observers say penetration of these new models varies widely by locality but they haven’t had much direct impact on hospitalists’ practices – at least not yet. However, as hospitals and health systems find themselves needing to learn new ways to invest their resources differently in response to these trends, what matters to the hospital should be of great importance to the hospitalist group.
“I haven’t seen a lot of dramatic changes in how hospitalists engage with value-based purchasing,” Dr. White said. “If we know that someone is part of an ACO, the instinctual – and right – response is to treat them like any other patient. But we still need to be committed to not waste resources.”
Hospitalists are the best people to understand the intricacies of how the health care system works under value-based approaches, Dr. Huang said. “That’s why so many hospitalists have taken leadership positions in their hospitals. I think all of this translates to the practical, day-to-day work of hospitalists, reflected in our focus on readmissions and length of stay.”
Dr. Williams said the health care system still hasn’t turned the corner from fee-for-service to value-based purchasing. “It still represents a tiny fraction of the income of hospitalists. Hospitals still have to focus on the bottom line, as fee-for-service reimbursement for hospitalized patients continues to get squeezed, and ACOs aren’t exactly paying premium rates either. Ask almost any hospital CEO what drives their bottom line today and the answer is volume – along with optimizing productivity. Pretty much every place I look, the future does not look terribly rosy for hospitals.”
Ms. Himebaugh said she is bullish on hospital medicine, in the sense that it’s unlikely to go away anytime soon. “Hospitalists are needed and provide value. But I don’t think we have devised the right model yet. I’m not sure our current model is sustainable. We need to find new models we can afford that don’t require squeezing our providers.”
For more information about the 2018 State of Hospital Medicine Report, contact SHM’s Practice Management Department at: [email protected] or call 800-843-3360. See also: https://www.hospitalmedicine.org/practice-management/shms-state-of-hospital-medicine/.
In a national health care environment undergoing unprecedented transformation, the specialty of hospital medicine appears to be an island of relative stability, a conclusion that is supported by the principal findings from SHM’s 2018 State of Hospital Medicine (SoHM) report.
The report of hospitalist group practice characteristics, as well as other key data defining the field’s current status, that the Society of Hospital Medicine puts out every 2 years reveals that overall salaries for hospitalist physicians are up by 3.8% since 2016. Although productivity, as measured by work relative value units (RVUs), remained largely flat over the same period, financial support per full-time equivalent (FTE) physician position to hospitalist groups from their hospitals and health systems is up significantly.
Total support per FTE averaged $176,657 in 2018, 12% higher than in 2016, noted Leslie Flores, MHA, SFHM, of Nelson Flores Hospital Medicine Consultants, and a member of SHM’s Practice Analysis Committee, which oversees the biennial survey. Compensation and productivity data were collected by the Medical Group Management Association and licensed by SHM for inclusion in its report.

These findings – particularly the flat productivity – raise questions about long-term sustainability, Ms. Flores said. “What is going on? Do hospital administrators still recognize the value hospitalists bring to the operations and the quality of their hospitals? Or is paying the subsidy just a cost of doing business – a necessity for most hospitals in a setting where demand for hospitalist positions remains high?”
Andrew White, MD, FACP, SFHM, chair of SHM’s Practice Analysis Committee and director of the hospital medicine service at the University of Washington Medical Center, Seattle, said basic market forces dictate that it is “pretty much inconceivable” to run a modern hospital of any size without hospitalists.
“Clearly, demand outstrips supply, which drives up salaries and support, whether CEOs feel that the hospitalist group is earning that support or not,” Dr. White said. “The unfilled hospitalist positions we identified speak to ongoing projected greater demand than supply. That said, hospitalists and group leaders can’t be complacent and must collaborate effectively with hospitals to provide highly valuable services.” Turnover of hospitalist positions was up slightly, he noted, at 7.4% in 2018, from 6.9% in 2016, reversing a trend of previous years.

But will these trends continue at a time when hospitals face continued pressure to cut costs, as the hospital medicine subsidy may represent one of their largest cost centers? Because the size of hospitalist groups continues to grow, hospitals’ total subsidy for hospital medicine is going up faster than the percentage increase in support per FTE.
How do hospitalists use the SoHM report?
Dr. White called the 2018 SoHM report the “most representative and balanced sample to date” of hospitalist group practices, with some of the highest quality data, thanks to more robust participation in the survey by pediatric groups and improved distribution among hospitalist management companies and academic programs.
“Not that past reports had major flaws, but this version is more authoritative, reflecting an intentional effort by our Practice Analysis Committee to bring in more participants from key groups,” he said.
The biennial report has been around long enough to achieve brand recognition in the field as the most authoritative source of information regarding hospitalist practice, he added. “We worked hard this year to balance the participants, with more of our responses than in the past coming from multi-hospital groups, whether 4 to 5 sites, or 20 to 30.”
Surveys were conducted online in January and February of 2018 in response to invitations mailed and emailed to targeted hospital medicine group leaders. A total of 569 groups completed the survey, representing 8,889 hospitalist FTEs, approximately 16% of the total hospitalist workforce. Responses were presented in several categories, including by size of program, region and employment model. Groups that care for adults only represented 87.9% of the surveys, while groups that care for children only were 6.7% and groups that care for both adults and children were 5.4%.
“This survey doesn’t tell us what should be best practice in hospital medicine,” Dr. White said, only what is actual current practice. He uses it in his own health system to not only contextualize and justify his group’s performance metrics for hospital administrators – relative to national and categorical averages – but also to see if the direction his group is following is consistent with what’s going on in the larger field.
“These data offer a very powerful resource regarding the trends in hospital medicine,” said Romil Chadha, MD, MPH, FACP, SFHM, associate division chief for operations in the division of hospital medicine at the University of Kentucky and UK Healthcare, Lexington. “It is my repository of data to go before my administrators for decisions that need to be made or to pilot new programs.”
Dr. Chadha also uses the data to help answer compensation, scheduling, and support questions from his group’s members.
Thomas McIlraith, MD, immediate past chairman of the hospital medicine department at Mercy Medical Group, Sacramento, Calif., said the report’s value is that it allows comparisons of salaries in different settings, and to see, for example, how night staffing is structured. “A lot of leaders I spoke to at SHM’s 2018 Leadership Academy in Vancouver were saying they didn’t feel up to parity with the national standards. You can use the report to look at the state of hospital medicine nationally and make comparisons,” he said.
Calls for more productivity
Roberta Himebaugh, MBA, SFHM, senior vice president of acute care services for the national hospitalist management company TeamHealth, and cochair of the SHM Practice Administrators Special Interest Group, said her company’s clients have traditionally asked for greater productivity from their hospitalist contracts as a way to decrease overall costs. Some markets are starting to see a change in that approach, she noted.
“Recently there’s been an increased focus on paying hospitalists to focus on quality rather than just productivity. Some of our clients are willing to pay for that, and we are trying to assign value to this non-billable time or adjust our productivity standards appropriately. I think hospitals definitely understand the value of non-billable services from hospitalists, but still will push us on the productivity targets,” Ms. Himebaugh said.
“I don’t believe hospital medicine can be sustainable long term on flat productivity or flat RVUs,” she added. “Yet the costs of burnout associated with pushing higher productivity are not sustainable, either.” So what are the answers? She said many inefficiencies are involved in responding to inquiries on the floor that could have been addressed another way, or waiting for the turnaround of diagnostic tests.
“Maybe we don’t need physicians to be in the hospital 24/7 if we have access to telehealth, or a partnership with the emergency department, or greater use of advanced care practice providers,” Ms. Himebaugh said. “Our hospitals are examining those options, and we have to look at how we can become more efficient and less costly. At TeamHealth, we are trying to staff for value – looking at patient flow patterns and adjusting our schedules accordingly. Is there a bolus of admissions tied to emergency department shift changes, or to certain days of the week? How can we move from the 12-hour shift that begins at 7 a.m. and ends at 7 p.m., and instead provide coverage for when the patients are there?”
Mark Williams, MD, MHM, chief of the division of hospital medicine at the University of Kentucky, Lexington, said he appreciates the volume of data in the report but wishes for even more survey participants, which could make the breakouts for subgroups such as academic hospitalists more robust. Other current sources of hospitalist salary data include the Association of American Medical Colleges (AAMC), which produces compensation reports to help medical schools and teaching hospitals with benchmarking, and the Faculty Practice Solution Center developed jointly by AAMC and Vizient to provide faculty practice plans with analytic tools. The Medical Group Management Association (MGMA) is another valuable source of information, some of which was licensed for inclusion in the SoHM report.
“There is no source of absolute truth that hospitalists can point to,” Dr. Williams said. “I will present my data and my administrators will reply: ‘We have our own data.’ Our institution has consistently ranked first or second nationwide for the sickest patients. We take more Medicaid and dually eligible patients, who have a lot of social issues. They take a lot of time to manage medically and the RVUs don’t reflect that. And yet I’m still judged by my RVUs generated per hospitalist. Hospital administrators understandably want to get the most productivity, and they are looking for their own data for average productivity numbers.”
Ryan Brown, MD, specialty medical director for hospital medicine with Atrium Health in Charlotte, N.C., said that hospital medicine’s flat productivity trends would be difficult to sustain in the business world. But there aren’t easy or obvious ways to increase hospitalists’ productivity. The SoHM report also shows that as productivity increases, total compensation increases but at a lower rate, resulting in a gradual decrease in compensation per RVU.
Pressures to increase productivity can be a double-edged sword, Dr. Williams added. Demanding that doctors make more billable visits faster to generate more RVUs can be a recipe for burnout and turnover, with huge costs associated with recruiting replacements.
“If there was recent turnover of hospitalists at the hospital, with the need to find replacements, there may be institutional memory about that,” he said. “But where are hospitals spending their money? Bottom line, we still need to learn to cut our costs.”
How is hospitalist practice evolving?
In addition to payment and productivity data, the SoHM report provides a current picture of the evolving state of hospitalist group practices. A key thread is how the work hospitalists are doing, and the way they do it, is changing, with new information about comanagement roles, dedicated admitters, night coverage, geographic rounding, and the like.
Making greater use of nurse practitioners and physician assistants (NPs/PAs), may be one way to change the flat productivity trends, Dr. Brown said. With a cost per RVU that’s roughly half that of a doctor’s, NPs/PAs could contribute to the bottom line. But he sees surprisingly large variation in how hospitalist groups are using them. Typically, they are deployed at a ratio of four doctors to one NP/PA, but that ratio could be two to one or even one to one, he said.
Use of NPs/PAs by academic hospitalist groups is up, from 52.1% in 2016 to 75.7% in 2018. For adult-only groups, 76.8% had NPs/PAs, with higher rates in hospitals and health systems and lower rates in the West region. But a lot of groups are using these practitioners for nonproductive work, and some are failing to generate any billing income, Dr. Brown said.
“The rate at which NPs/PAs performed billable services was higher in physician-owned practices, resulting in a lower cost per RVU, suggesting that many practices may be underutilizing their NPs/PAs or not sharing the work.” Not every NP or PA wants to or is able to care for very complex patients, Dr. Brown said, “but you want a system where the NP and PA can work at the highest level permitted by state law.”

The predominant scheduling model of hospital medicine, 7 days on duty followed by 7 days off, has diminished somewhat in recent years. There appears to be some fluctuation and a gradual move away from 7 on/7 off toward some kind of variable approach, since the former may not be physically sustainable for the doctor over the long haul, Dr. Brown said. Some groups are experimenting with a combined approach.
“I think balancing workload with manpower has always been a challenge for our field. Maybe we should be working shorter shifts or fewer days and making sure our hospitalists aren’t ever sitting around idle,” he said. “And could we come in on nonclinical days to do administrative tasks? I think the solution is out there, but we haven’t created the algorithms to define that yet. If you could somehow use the data for volume, number of beds, nurse staffing, etc., by year and seasonally, you might be able to reliably predict census. This is about applying data hospitals already have in their electronic health records, but utilizing the data in ways that are more helpful.”
Dr. McIlraith added that a big driver of the future of hospital medicine will be the evolution of the EHR and the digitalization of health care, as hospitals learn how to leverage more of what’s in their EHRs. “The impact will grow for hospitalists through the creation and maturation of big data systems – and the learning that can be extracted from what’s contained in the electronic health record.”
Another important question for hospitalist groups is their model of backup scheduling, to make sure there is a replacement available if a scheduled doctor calls in sick or if demand is unexpectedly high.
“In today’s world, this is how we have traditionally managed unpredictability,” Dr. Brown said. “You don’t know when you will need it, but if you need it, you want it immediately. So how do you pay for it – only when the doctor comes in, or also an amount just for being on call?” Some groups pay for both, he said, others for neither.
“We are a group of 70 hospitalists, and if someone is sick you can’t just shut down the service,” said Dr. Chadha. “We are one of the few to use incentives for both, which could include a 1-week decrease in clinical shifts in exchange for 2 weeks of backup. We have times with 25% usage of backup number 1, and 10% usage of backup number 2,” he noted. “But the goal is for our hospitalists to have assurances that there is a backup system and that it works.”
The presence of nocturnists in hospitals continues to rise, with 76.1% of adults-only groups having nocturnists, 27.6% of children-only groups, and 68.2% of adults and children groups. Geographic or unit-based hospital assignments have grown to 36.4% of adult-only groups.
What are hospitalists’ other new roles?
“We have a large group of 50 doctors, with about 40 FTEs, and we are evolving from the traditional generalist role toward more subspecialty comanagement,” said Bryan Huang, MD, physician adviser and associate clinical professor in the division of hospital medicine at the University of California–San Diego. “Our hospitalists are asking what it means to be an academic hospitalist as our teaching roles have shrunk.”
Dr. Huang recently took on a new role as physician adviser for his hospital in such areas as utilization review, patient flow, and length of stay. “I’m spearheading a work group to address quality issues – all of which involve collaboration with other professionals. We also developed an admitting role here for a hospitalist whose sole role for the day is to admit patients.” Nationally up to 51.2% of hospitalist groups utilize a dedicated daytime admitter.
The report found that hospital services for which hospitalists are more likely to be attendings than consultants include GI/liver, 78.4%; palliative care, 77.3%; neurology/stroke, 73.6%; oncology, 67.8%; cardiology, 56.9%; and critical care, 50.7%. Conditions where hospitalists are more likely to consult rather than admit and attend include neurosurgery, orthopedics, general surgery, cardiovascular surgery, and other surgical subspecialties.
Other hospital services routinely provided by adult-only hospitalists include care of patients in an ICU setting (62.7%); primary responsibility for observation units (54.6%); primary clinical responsibility for rapid response teams (48.8%); primary responsibility for code blue or cardiac arrest teams (43.8%); nighttime admissions or tuck-in services (33.9%); and medical procedures (31.5%). For pediatric hospital medicine groups, care of healthy newborns and medical procedures were among the most common services provided, while for hospitalists serving adults and children, rapid response teams, ICUs, and specialty units were most common.
New models of payment for health care
As the larger health care system is being transformed by new payment models and benefit structures, including accountable care organizations (ACOs), value-based purchasing, bundled payments, and other forms of population-based coverage – which is described as a volume-to-value shift in health care – how are these new models affecting hospitalists?
Observers say penetration of these new models varies widely by locality but they haven’t had much direct impact on hospitalists’ practices – at least not yet. However, as hospitals and health systems find themselves needing to learn new ways to invest their resources differently in response to these trends, what matters to the hospital should be of great importance to the hospitalist group.
“I haven’t seen a lot of dramatic changes in how hospitalists engage with value-based purchasing,” Dr. White said. “If we know that someone is part of an ACO, the instinctual – and right – response is to treat them like any other patient. But we still need to be committed to not waste resources.”
Hospitalists are the best people to understand the intricacies of how the health care system works under value-based approaches, Dr. Huang said. “That’s why so many hospitalists have taken leadership positions in their hospitals. I think all of this translates to the practical, day-to-day work of hospitalists, reflected in our focus on readmissions and length of stay.”
Dr. Williams said the health care system still hasn’t turned the corner from fee-for-service to value-based purchasing. “It still represents a tiny fraction of the income of hospitalists. Hospitals still have to focus on the bottom line, as fee-for-service reimbursement for hospitalized patients continues to get squeezed, and ACOs aren’t exactly paying premium rates either. Ask almost any hospital CEO what drives their bottom line today and the answer is volume – along with optimizing productivity. Pretty much every place I look, the future does not look terribly rosy for hospitals.”
Ms. Himebaugh said she is bullish on hospital medicine, in the sense that it’s unlikely to go away anytime soon. “Hospitalists are needed and provide value. But I don’t think we have devised the right model yet. I’m not sure our current model is sustainable. We need to find new models we can afford that don’t require squeezing our providers.”
For more information about the 2018 State of Hospital Medicine Report, contact SHM’s Practice Management Department at: [email protected] or call 800-843-3360. See also: https://www.hospitalmedicine.org/practice-management/shms-state-of-hospital-medicine/.
Solitary Nodule on the Thigh
The Diagnosis: Ruptured Molluscum
Molluscum contagiosum (MC) is caused by a DNA virus (MC virus) belonging to the poxvirus family. Molluscum contagiosum is common and predominantly seen in children and young adults. In sexually active adults, the lesions commonly occur in the genital region, abdomen, and inner thighs. In immunocompromised individuals, including those with AIDS, the lesions are more extensive and may cause disfigurement.1 Molluscum contagiosum involving epidermoid cysts has been reported.2
Histopathologically, MC can be classified as noninflammatory or inflammatory. In noninflamed lesions, multiple large, intracytoplasmic, eosinophilic inclusions (Henderson-Paterson bodies) appear within the lobulated endophytic and hyperplastic epidermis. Ultrastructurally, these bodies show membrane-bound collections of MC virus.1 Replicating Henderson-Paterson bodies can result in rupture and inflammation. This case demonstrates a palisading granuloma containing keratin with few Henderson-Paterson bodies (quiz image) due to prior rupture of a molluscum or molluscoid cyst.
Rheumatoid nodules, the most characteristic histopathologic lesions of rheumatoid arthritis, are most commonly found in the subcutis at points of pressure and may occur in connective tissue of numerous organs. Rheumatoid nodules are firm, nontender, and mobile within the subcutaneous tissue but may be fixed to underlying structures including the periosteum, tendons, or bursae.3,4 Occasionally, superficial nodules may perforate the epidermis.5 The inner central necrobiotic zone appears as intensely eosinophilic, amorphous fibrin and other cellular debris. This central area is surrounded by histiocytes in a palisaded configuration (Figure 1). Multinucleated foreign body giant cells also may be present. Occasionally, mast cells, eosinophils, and neutrophils are present.6,7
Lupus miliaris disseminatus faciei presents with multiple discrete, smooth, yellow-brown to red, dome-shaped papules. The lesions typically are located on the central and lateral sides of the face and infrequently involve the neck. Other sites including the axillae, arms, hands, legs, and groin occasionally can be involved. Diascopy may reveal an apple jelly color.8,9 The histopathologic hallmark of lupus miliaris disseminatus faciei is an epithelioid cell granuloma with central necrosis (Figure 2).

Epithelioid sarcoma (ES) is a soft tissue tumor with a known propensity for local recurrence, regional lymph node involvement, sporotrichoid spread, and distant metastases.10 The name was coined by Enzinger11 in 1970 during a review of 62 cases of a “peculiar form of sarcoma that has repeatedly been confused with a chronic inflammatory process, a necrotizing granuloma, and a squamous cell carcinoma.” Epithelioid sarcoma tends to grow slowly in a nodular or multinodular manner along fascial structures and tendons, often with central necrosis and ulceration of the overlying skin. Histopathologically, classic ES shows nodular masses of uniform plump epithelioid cells with abundant eosinophilic cytoplasm and prominent central necrosis. A biphasic pattern is typical with spindle cells merging with epithelioid cells. Cellular atypia is relatively mild and mitoses are rare (Figure 3). Recurrent or metastatic lesions can show a greater degree of pleomorphism.12 Given the low-grade atypia in early lesions, this sarcoma is easily misdiagnosed as granulomatous dermatitis. Immunohistochemically, the majority of ES cases are positive for cytokeratins and epithelial membrane antigen; SMARCB1/INI-1 expression is characteristically lost.13
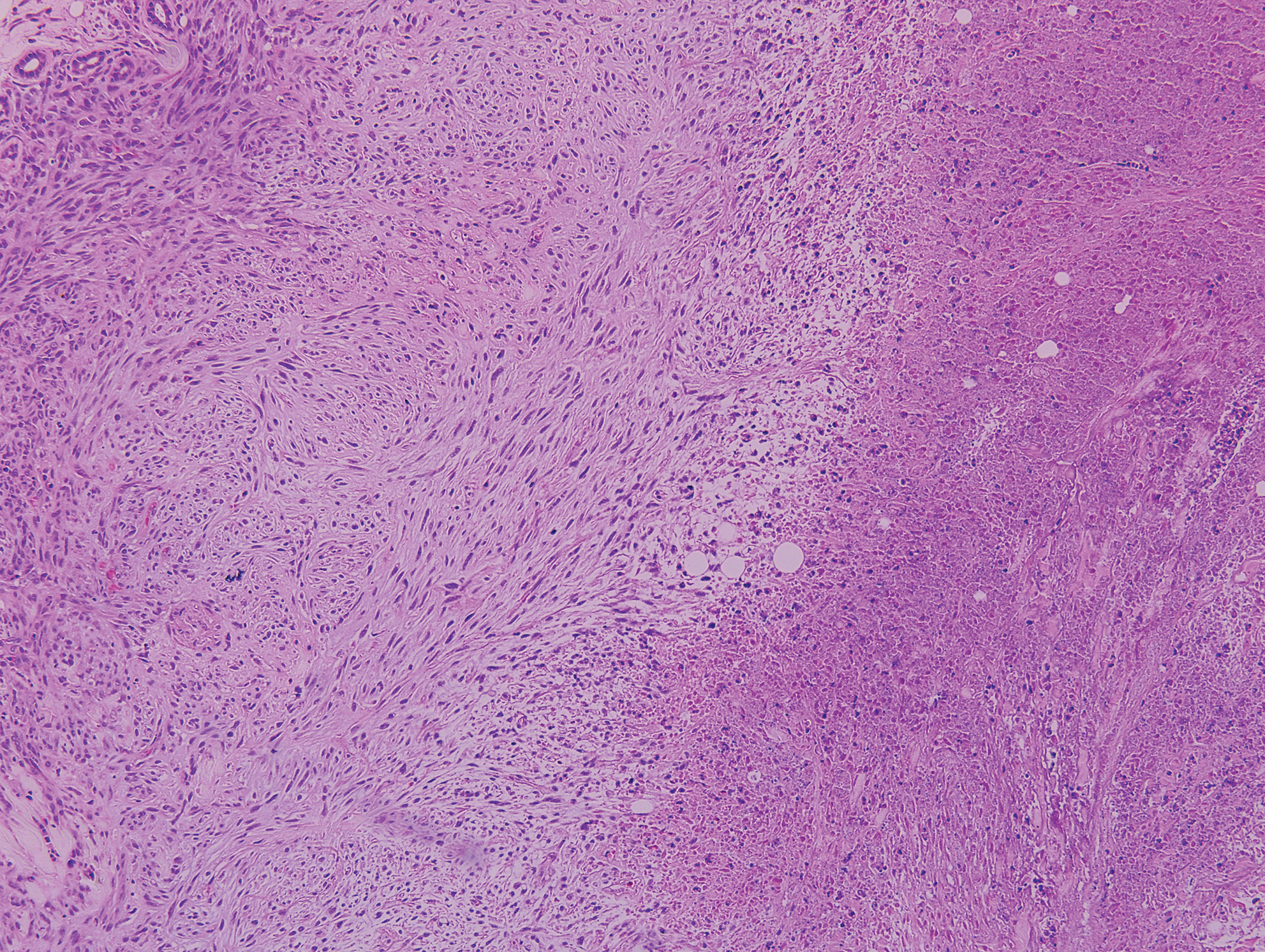
Granulomatosis with polyangiitis (formerly Wegener granulomatosis) is an autoimmune vasculitis highly associated with antineutrophil cytoplasmic antibodies. Clinical manifestations include systemic necrotizing vasculitis; necrotizing glomerulonephritis; and granulomatous inflammation, which predominantly involves the upper respiratory tract, skin, and mucosa.14,15 Skin involvement may be the initial manifestation of the disease and consists of palpable purpura, papules, ulcerations, vesicles, subcutaneous nodules, necrotizing ulcerations, papulonecrotic lesions, and petechiae. None of the findings are pathognomonic. The cutaneous histopathologic spectrum includes leukocytoclastic vasculitis, extravascular palisading granulomas, and granulomatous vasculitis.16 In the acute lesions of granulomatosis with polyangiitis, the predominant pattern of inflammation is not granulomatous but purulent with the appearance of an abscess. As it evolves, it develops a central zone of necrosis with extensive karyorrhectic debris and palisades of macrophages with scattered multinucleated giant cells (Figure 4).17
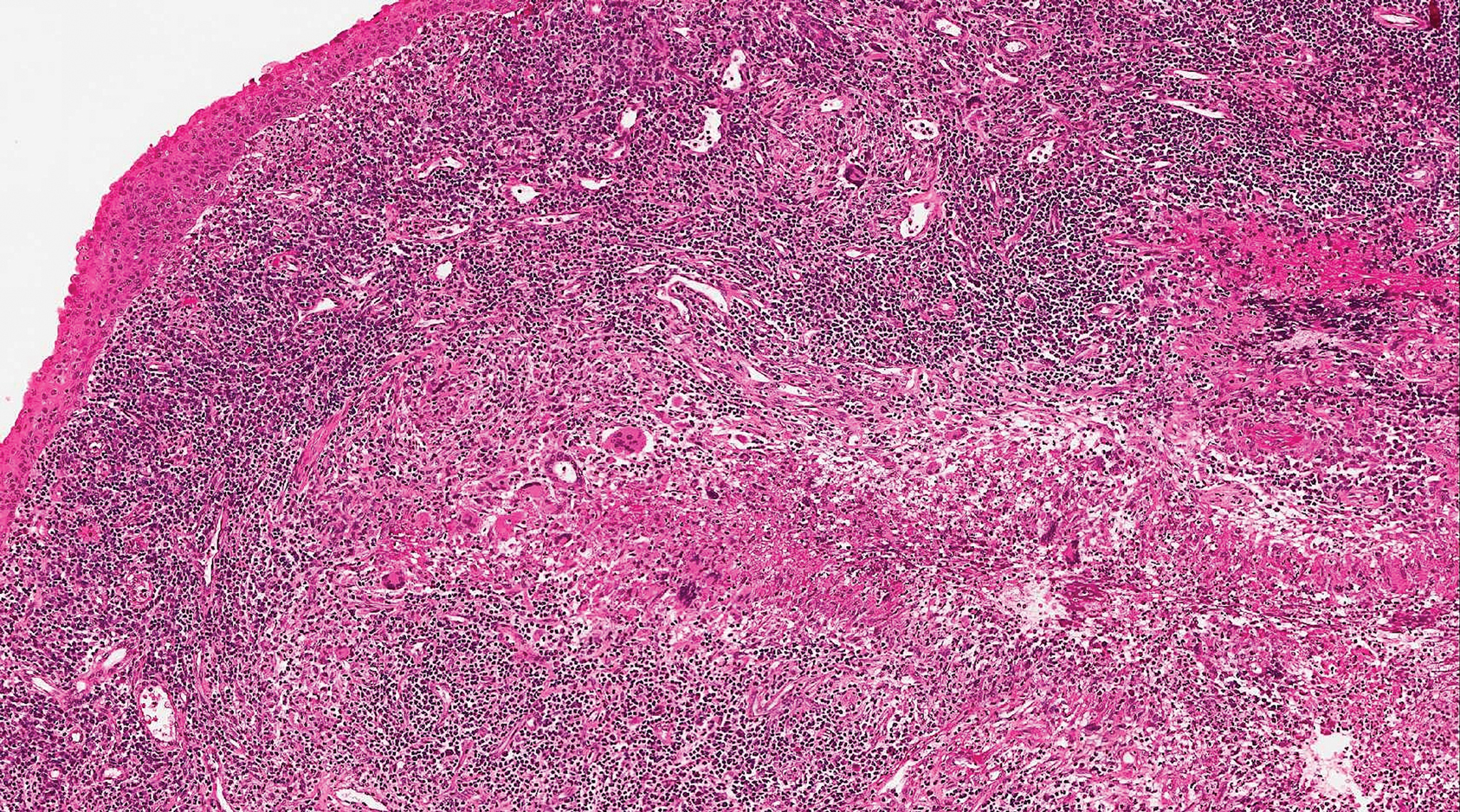
1. Nandhini G, Rajkumar K, Kanth KS, et al. Molluscum contagiosum in a 12-year-old child—report of a case and review of literature. J Int Oral Health. 2015;7:63-66.
2. Phelps A, Murphy M, Elaba Z, et al. Molluscum contagiosum virus infection in benign cutaneous epithelial cystic lesions-report of 2 cases with different pathogenesis? Am J Dermatopathol. 2010;32:740-742.
3. Sayah A, English JC 3rd. Rheumatoid arthritis: a review of the cutaneous manifestations. J Am Acad Dermatol. 2005;53:191-209; quiz 210-192.
4. Sibbitt WL Jr, Williams RC Jr. Cutaneous manifestations of rheumatoid arthritis. Int J Dermatol. 1982;21:563-572.
5. Barzilai A, Huszar M, Shpiro D, et al. Pseudorheumatoid nodules in adults: a juxta-articular form of nodular granuloma annulare. Am J Dermatopathol. 2005;27:1-5.
6. Garcia-Patos V. Rheumatoid nodule. Semin Cutan Med Surg. 2007;26:100-107.
7. Patterson JW. Rheumatoid nodule and subcutaneous granuloma annulare. a comparative histologic study. Am J Dermatopathol. 1988;10:1-8.
8. Sehgal VN, Srivastava G, Aggarwal AK, et al. Lupus miliaris disseminatus faciei part II: an overview. Skinmed. 2005;4:234-238.
9. Cymerman R, Rosenstein R, Shvartsbeyn M, et al. Lupus miliaris disseminatus faciei. Dermatol Online J. 2015;21. pii:13030/qt6b83q5gp.
10. Sobanko JF, Meijer L, Nigra TP. Epithelioid sarcoma: a review and update. J Clin Aesthet Dermatol. 2009;2:49-54.
11. Enzinger FM. Epitheloid sarcoma. a sarcoma simulating a granuloma or a carcinoma. Cancer. 1970;26:1029-1041.
12. Fisher C. Epithelioid sarcoma of Enzinger. Adv Anat Pathol. 2006;13:114-121.
13. Miettinen M, Fanburg-Smith JC, Virolainen M, et al. Epithelioid sarcoma: an immunohistochemical analysis of 112 classical and variant cases and a discussion of the differential diagnosis. Hum Pathol. 1999;30:934-942.
14. Lutalo PM, D’Cruz DP. Diagnosis and classification of granulomatosis with polyangiitis (aka Wegener’s granulomatosis)[published online January 29, 2014]. J Autoimmun. 2014;48-49:94-98.
15. Frances C, Du LT, Piette JC, et al. Wegener’s granulomatosis. dermatological manifestations in 75 cases with clinicopathologic correlation. Arch Dermatol. 1994;130:861-867.
16. Daoud MS, Gibson LE, DeRemee RA, et al. Cutaneous Wegener’s granulomatosis: clinical, histopathologic, and immunopathologic features of thirty patients. J Am Acad Dermatol. 1994;31:605-612.
17. Jennette JC. Nomenclature and classification of vasculitis: lessons learned from granulomatosis with polyangiitis (Wegener’s granulomatosis). Clin Exp Immunol. 2011;164 (suppl 1):7-10.
The Diagnosis: Ruptured Molluscum
Molluscum contagiosum (MC) is caused by a DNA virus (MC virus) belonging to the poxvirus family. Molluscum contagiosum is common and predominantly seen in children and young adults. In sexually active adults, the lesions commonly occur in the genital region, abdomen, and inner thighs. In immunocompromised individuals, including those with AIDS, the lesions are more extensive and may cause disfigurement.1 Molluscum contagiosum involving epidermoid cysts has been reported.2
Histopathologically, MC can be classified as noninflammatory or inflammatory. In noninflamed lesions, multiple large, intracytoplasmic, eosinophilic inclusions (Henderson-Paterson bodies) appear within the lobulated endophytic and hyperplastic epidermis. Ultrastructurally, these bodies show membrane-bound collections of MC virus.1 Replicating Henderson-Paterson bodies can result in rupture and inflammation. This case demonstrates a palisading granuloma containing keratin with few Henderson-Paterson bodies (quiz image) due to prior rupture of a molluscum or molluscoid cyst.
Rheumatoid nodules, the most characteristic histopathologic lesions of rheumatoid arthritis, are most commonly found in the subcutis at points of pressure and may occur in connective tissue of numerous organs. Rheumatoid nodules are firm, nontender, and mobile within the subcutaneous tissue but may be fixed to underlying structures including the periosteum, tendons, or bursae.3,4 Occasionally, superficial nodules may perforate the epidermis.5 The inner central necrobiotic zone appears as intensely eosinophilic, amorphous fibrin and other cellular debris. This central area is surrounded by histiocytes in a palisaded configuration (Figure 1). Multinucleated foreign body giant cells also may be present. Occasionally, mast cells, eosinophils, and neutrophils are present.6,7

Lupus miliaris disseminatus faciei presents with multiple discrete, smooth, yellow-brown to red, dome-shaped papules. The lesions typically are located on the central and lateral sides of the face and infrequently involve the neck. Other sites including the axillae, arms, hands, legs, and groin occasionally can be involved. Diascopy may reveal an apple jelly color.8,9 The histopathologic hallmark of lupus miliaris disseminatus faciei is an epithelioid cell granuloma with central necrosis (Figure 2).

Epithelioid sarcoma (ES) is a soft tissue tumor with a known propensity for local recurrence, regional lymph node involvement, sporotrichoid spread, and distant metastases.10 The name was coined by Enzinger11 in 1970 during a review of 62 cases of a “peculiar form of sarcoma that has repeatedly been confused with a chronic inflammatory process, a necrotizing granuloma, and a squamous cell carcinoma.” Epithelioid sarcoma tends to grow slowly in a nodular or multinodular manner along fascial structures and tendons, often with central necrosis and ulceration of the overlying skin. Histopathologically, classic ES shows nodular masses of uniform plump epithelioid cells with abundant eosinophilic cytoplasm and prominent central necrosis. A biphasic pattern is typical with spindle cells merging with epithelioid cells. Cellular atypia is relatively mild and mitoses are rare (Figure 3). Recurrent or metastatic lesions can show a greater degree of pleomorphism.12 Given the low-grade atypia in early lesions, this sarcoma is easily misdiagnosed as granulomatous dermatitis. Immunohistochemically, the majority of ES cases are positive for cytokeratins and epithelial membrane antigen; SMARCB1/INI-1 expression is characteristically lost.13

Granulomatosis with polyangiitis (formerly Wegener granulomatosis) is an autoimmune vasculitis highly associated with antineutrophil cytoplasmic antibodies. Clinical manifestations include systemic necrotizing vasculitis; necrotizing glomerulonephritis; and granulomatous inflammation, which predominantly involves the upper respiratory tract, skin, and mucosa.14,15 Skin involvement may be the initial manifestation of the disease and consists of palpable purpura, papules, ulcerations, vesicles, subcutaneous nodules, necrotizing ulcerations, papulonecrotic lesions, and petechiae. None of the findings are pathognomonic. The cutaneous histopathologic spectrum includes leukocytoclastic vasculitis, extravascular palisading granulomas, and granulomatous vasculitis.16 In the acute lesions of granulomatosis with polyangiitis, the predominant pattern of inflammation is not granulomatous but purulent with the appearance of an abscess. As it evolves, it develops a central zone of necrosis with extensive karyorrhectic debris and palisades of macrophages with scattered multinucleated giant cells (Figure 4).17

The Diagnosis: Ruptured Molluscum
Molluscum contagiosum (MC) is caused by a DNA virus (MC virus) belonging to the poxvirus family. Molluscum contagiosum is common and predominantly seen in children and young adults. In sexually active adults, the lesions commonly occur in the genital region, abdomen, and inner thighs. In immunocompromised individuals, including those with AIDS, the lesions are more extensive and may cause disfigurement.1 Molluscum contagiosum involving epidermoid cysts has been reported.2
Histopathologically, MC can be classified as noninflammatory or inflammatory. In noninflamed lesions, multiple large, intracytoplasmic, eosinophilic inclusions (Henderson-Paterson bodies) appear within the lobulated endophytic and hyperplastic epidermis. Ultrastructurally, these bodies show membrane-bound collections of MC virus.1 Replicating Henderson-Paterson bodies can result in rupture and inflammation. This case demonstrates a palisading granuloma containing keratin with few Henderson-Paterson bodies (quiz image) due to prior rupture of a molluscum or molluscoid cyst.
Rheumatoid nodules, the most characteristic histopathologic lesions of rheumatoid arthritis, are most commonly found in the subcutis at points of pressure and may occur in connective tissue of numerous organs. Rheumatoid nodules are firm, nontender, and mobile within the subcutaneous tissue but may be fixed to underlying structures including the periosteum, tendons, or bursae.3,4 Occasionally, superficial nodules may perforate the epidermis.5 The inner central necrobiotic zone appears as intensely eosinophilic, amorphous fibrin and other cellular debris. This central area is surrounded by histiocytes in a palisaded configuration (Figure 1). Multinucleated foreign body giant cells also may be present. Occasionally, mast cells, eosinophils, and neutrophils are present.6,7

Lupus miliaris disseminatus faciei presents with multiple discrete, smooth, yellow-brown to red, dome-shaped papules. The lesions typically are located on the central and lateral sides of the face and infrequently involve the neck. Other sites including the axillae, arms, hands, legs, and groin occasionally can be involved. Diascopy may reveal an apple jelly color.8,9 The histopathologic hallmark of lupus miliaris disseminatus faciei is an epithelioid cell granuloma with central necrosis (Figure 2).

Epithelioid sarcoma (ES) is a soft tissue tumor with a known propensity for local recurrence, regional lymph node involvement, sporotrichoid spread, and distant metastases.10 The name was coined by Enzinger11 in 1970 during a review of 62 cases of a “peculiar form of sarcoma that has repeatedly been confused with a chronic inflammatory process, a necrotizing granuloma, and a squamous cell carcinoma.” Epithelioid sarcoma tends to grow slowly in a nodular or multinodular manner along fascial structures and tendons, often with central necrosis and ulceration of the overlying skin. Histopathologically, classic ES shows nodular masses of uniform plump epithelioid cells with abundant eosinophilic cytoplasm and prominent central necrosis. A biphasic pattern is typical with spindle cells merging with epithelioid cells. Cellular atypia is relatively mild and mitoses are rare (Figure 3). Recurrent or metastatic lesions can show a greater degree of pleomorphism.12 Given the low-grade atypia in early lesions, this sarcoma is easily misdiagnosed as granulomatous dermatitis. Immunohistochemically, the majority of ES cases are positive for cytokeratins and epithelial membrane antigen; SMARCB1/INI-1 expression is characteristically lost.13

Granulomatosis with polyangiitis (formerly Wegener granulomatosis) is an autoimmune vasculitis highly associated with antineutrophil cytoplasmic antibodies. Clinical manifestations include systemic necrotizing vasculitis; necrotizing glomerulonephritis; and granulomatous inflammation, which predominantly involves the upper respiratory tract, skin, and mucosa.14,15 Skin involvement may be the initial manifestation of the disease and consists of palpable purpura, papules, ulcerations, vesicles, subcutaneous nodules, necrotizing ulcerations, papulonecrotic lesions, and petechiae. None of the findings are pathognomonic. The cutaneous histopathologic spectrum includes leukocytoclastic vasculitis, extravascular palisading granulomas, and granulomatous vasculitis.16 In the acute lesions of granulomatosis with polyangiitis, the predominant pattern of inflammation is not granulomatous but purulent with the appearance of an abscess. As it evolves, it develops a central zone of necrosis with extensive karyorrhectic debris and palisades of macrophages with scattered multinucleated giant cells (Figure 4).17

1. Nandhini G, Rajkumar K, Kanth KS, et al. Molluscum contagiosum in a 12-year-old child—report of a case and review of literature. J Int Oral Health. 2015;7:63-66.
2. Phelps A, Murphy M, Elaba Z, et al. Molluscum contagiosum virus infection in benign cutaneous epithelial cystic lesions-report of 2 cases with different pathogenesis? Am J Dermatopathol. 2010;32:740-742.
3. Sayah A, English JC 3rd. Rheumatoid arthritis: a review of the cutaneous manifestations. J Am Acad Dermatol. 2005;53:191-209; quiz 210-192.
4. Sibbitt WL Jr, Williams RC Jr. Cutaneous manifestations of rheumatoid arthritis. Int J Dermatol. 1982;21:563-572.
5. Barzilai A, Huszar M, Shpiro D, et al. Pseudorheumatoid nodules in adults: a juxta-articular form of nodular granuloma annulare. Am J Dermatopathol. 2005;27:1-5.
6. Garcia-Patos V. Rheumatoid nodule. Semin Cutan Med Surg. 2007;26:100-107.
7. Patterson JW. Rheumatoid nodule and subcutaneous granuloma annulare. a comparative histologic study. Am J Dermatopathol. 1988;10:1-8.
8. Sehgal VN, Srivastava G, Aggarwal AK, et al. Lupus miliaris disseminatus faciei part II: an overview. Skinmed. 2005;4:234-238.
9. Cymerman R, Rosenstein R, Shvartsbeyn M, et al. Lupus miliaris disseminatus faciei. Dermatol Online J. 2015;21. pii:13030/qt6b83q5gp.
10. Sobanko JF, Meijer L, Nigra TP. Epithelioid sarcoma: a review and update. J Clin Aesthet Dermatol. 2009;2:49-54.
11. Enzinger FM. Epitheloid sarcoma. a sarcoma simulating a granuloma or a carcinoma. Cancer. 1970;26:1029-1041.
12. Fisher C. Epithelioid sarcoma of Enzinger. Adv Anat Pathol. 2006;13:114-121.
13. Miettinen M, Fanburg-Smith JC, Virolainen M, et al. Epithelioid sarcoma: an immunohistochemical analysis of 112 classical and variant cases and a discussion of the differential diagnosis. Hum Pathol. 1999;30:934-942.
14. Lutalo PM, D’Cruz DP. Diagnosis and classification of granulomatosis with polyangiitis (aka Wegener’s granulomatosis)[published online January 29, 2014]. J Autoimmun. 2014;48-49:94-98.
15. Frances C, Du LT, Piette JC, et al. Wegener’s granulomatosis. dermatological manifestations in 75 cases with clinicopathologic correlation. Arch Dermatol. 1994;130:861-867.
16. Daoud MS, Gibson LE, DeRemee RA, et al. Cutaneous Wegener’s granulomatosis: clinical, histopathologic, and immunopathologic features of thirty patients. J Am Acad Dermatol. 1994;31:605-612.
17. Jennette JC. Nomenclature and classification of vasculitis: lessons learned from granulomatosis with polyangiitis (Wegener’s granulomatosis). Clin Exp Immunol. 2011;164 (suppl 1):7-10.
1. Nandhini G, Rajkumar K, Kanth KS, et al. Molluscum contagiosum in a 12-year-old child—report of a case and review of literature. J Int Oral Health. 2015;7:63-66.
2. Phelps A, Murphy M, Elaba Z, et al. Molluscum contagiosum virus infection in benign cutaneous epithelial cystic lesions-report of 2 cases with different pathogenesis? Am J Dermatopathol. 2010;32:740-742.
3. Sayah A, English JC 3rd. Rheumatoid arthritis: a review of the cutaneous manifestations. J Am Acad Dermatol. 2005;53:191-209; quiz 210-192.
4. Sibbitt WL Jr, Williams RC Jr. Cutaneous manifestations of rheumatoid arthritis. Int J Dermatol. 1982;21:563-572.
5. Barzilai A, Huszar M, Shpiro D, et al. Pseudorheumatoid nodules in adults: a juxta-articular form of nodular granuloma annulare. Am J Dermatopathol. 2005;27:1-5.
6. Garcia-Patos V. Rheumatoid nodule. Semin Cutan Med Surg. 2007;26:100-107.
7. Patterson JW. Rheumatoid nodule and subcutaneous granuloma annulare. a comparative histologic study. Am J Dermatopathol. 1988;10:1-8.
8. Sehgal VN, Srivastava G, Aggarwal AK, et al. Lupus miliaris disseminatus faciei part II: an overview. Skinmed. 2005;4:234-238.
9. Cymerman R, Rosenstein R, Shvartsbeyn M, et al. Lupus miliaris disseminatus faciei. Dermatol Online J. 2015;21. pii:13030/qt6b83q5gp.
10. Sobanko JF, Meijer L, Nigra TP. Epithelioid sarcoma: a review and update. J Clin Aesthet Dermatol. 2009;2:49-54.
11. Enzinger FM. Epitheloid sarcoma. a sarcoma simulating a granuloma or a carcinoma. Cancer. 1970;26:1029-1041.
12. Fisher C. Epithelioid sarcoma of Enzinger. Adv Anat Pathol. 2006;13:114-121.
13. Miettinen M, Fanburg-Smith JC, Virolainen M, et al. Epithelioid sarcoma: an immunohistochemical analysis of 112 classical and variant cases and a discussion of the differential diagnosis. Hum Pathol. 1999;30:934-942.
14. Lutalo PM, D’Cruz DP. Diagnosis and classification of granulomatosis with polyangiitis (aka Wegener’s granulomatosis)[published online January 29, 2014]. J Autoimmun. 2014;48-49:94-98.
15. Frances C, Du LT, Piette JC, et al. Wegener’s granulomatosis. dermatological manifestations in 75 cases with clinicopathologic correlation. Arch Dermatol. 1994;130:861-867.
16. Daoud MS, Gibson LE, DeRemee RA, et al. Cutaneous Wegener’s granulomatosis: clinical, histopathologic, and immunopathologic features of thirty patients. J Am Acad Dermatol. 1994;31:605-612.
17. Jennette JC. Nomenclature and classification of vasculitis: lessons learned from granulomatosis with polyangiitis (Wegener’s granulomatosis). Clin Exp Immunol. 2011;164 (suppl 1):7-10.
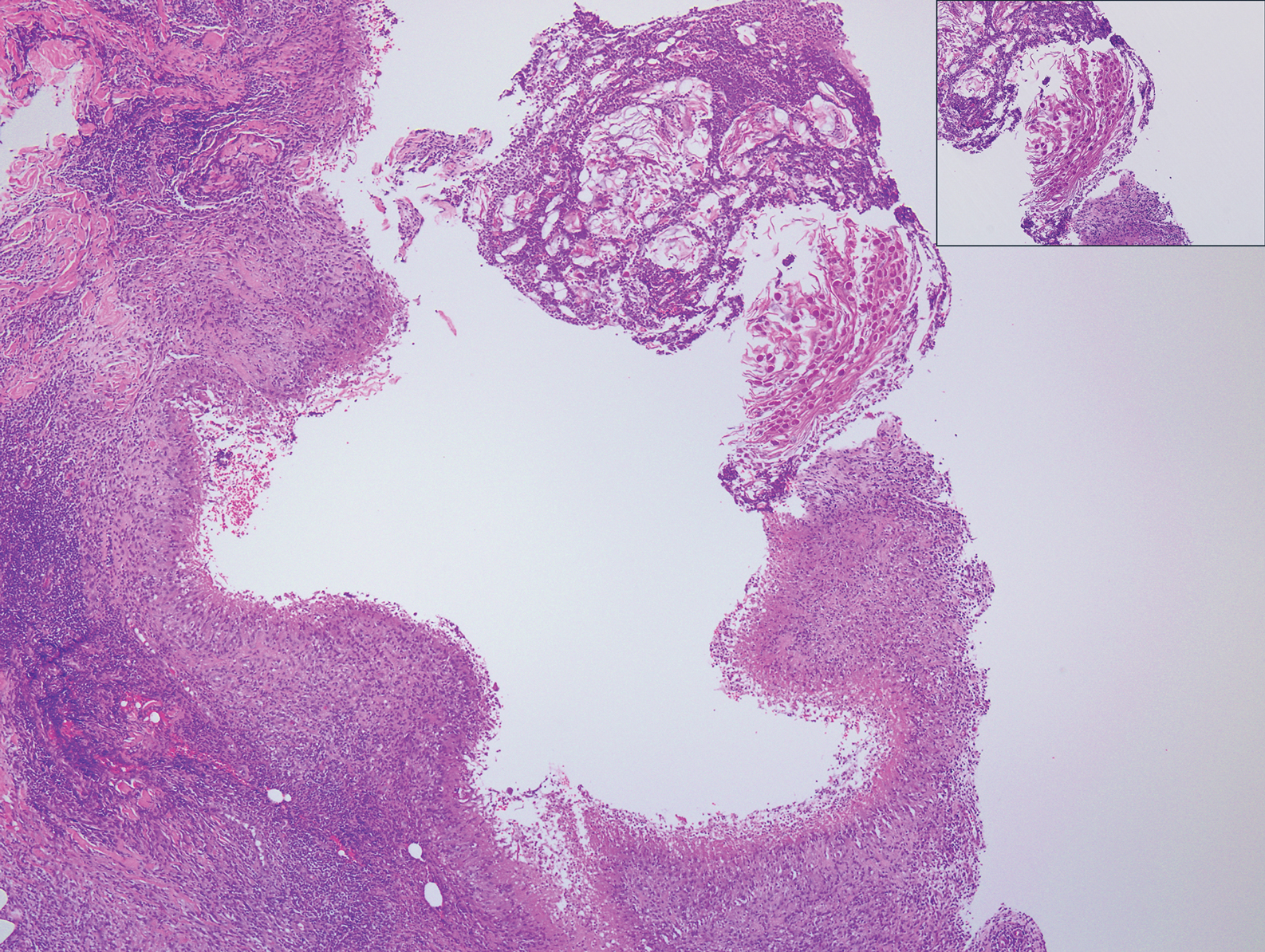
A 17-year-old adolescent girl presented with a discrete nodule on the thigh.
Researchers exploring ways to mitigate aging’s impact on diabetes
LOS ANGELES – When Derek LeRoith, MD, PhD, was a medical student, he remembers professors telling him that human tissue response to aging diminishes over time, and that individuals can develop insulin resistance purely from aging.
“Whether that was right or wrong I don’t know, but certainly it seems to be one of the major issues that leads to the increase in diabetes, with all of its associated aspects such as dyslipidemia and hypertension,” he said at the World Congress on Insulin Resistance, Diabetes & Cardiovascular Disease.
According to Dr. LeRoith, professor of medicine and director of research in the division of endocrinology at Icahn School of Medicine at Mount Sinai, New York, studies have demonstrated that the elderly have worse glucose tolerance, compared with younger adults. One such analysis found that the insulin secretion index and disposition index are lower in the elderly, compared with their younger patients (Diabetes 2003;52[7]:1738-48). “But it’s not just the insulin resistance per se,” he said. “It’s also a defect of the beta cell. .”
Another major issue for aging patients is the impact of diabetes on cognitive decline and the formation of Alzheimer’s disease. “There’s a suggestion that the brain has insulin resistance and that this may also affect cognitive decline and Alzheimer’s,” Dr. LeRoith said. “But there are other aspects: insulin insufficiency, hyperglycemia, and, of course ... hypoglycemia. There is a debate as to what the major causes are. Is it amyloid beta accumulation, or is it vascular damage?”
In collaboration with Israeli researchers, Dr. LeRoith and his associates have been evaluating patients that belong to the Maccabi Health System in Tel Aviv, which has a diabetes registry with complete hemoglobin A1c measurements since 1998. One study of 897 registry participants found a strong association between worse diabetes control and worse cognition (Am J Geriatr Psych 2014;22:1055-9). Specifically, an interaction of duration of type 2 diabetes with HbA1c was associated with executive functioning (P = .006), semantic categorization (P = .019), attention/working memory (P = .011), and overall cognition (P = .006), such that the associations between duration of type 2 diabetes and cognitive impairment increased as HbA1c levels increased – but not for episodic memory (P = .984).
In a separate analysis of patients from the same registry, Dr. LeRoith and his colleagues evaluated the relationships of long-term trajectories of glycemic control with cognitive performance in cognitively normal elderly with type 2 diabetes (PLoS ONE 9[6]:e97384 doi: 10.1371/journal.pone.0097384). They found that subjects with stable HbA1c over time had the lowest HbA1c at study entry and performed best on cognitive measures, “suggesting that the trajectile of HbA1c over 10 or 12 years can really influence the cognitive ability in these patients,” he said.
Another, unrelated study found that insulin in combination with other diabetes medication is associated with less Alzheimer’s neuropathology (Neurology 2008;71:750-7), while an Alzheimer’s mouse model from Dr. LeRoith and his colleagues demonstrated that high dietary advanced glycation end products are associated with poorer spatial learning and accelerated amyloid beta deposition (Aging Cell 2016;15:309-16). “From that study we conclude that high dietary advance glycation end (AGE) products may be neurotoxic and that a diet low in AGEs may decrease dementia risk, particularly in diabetic elderly who are at increased risk and have higher levels of AGEs,” he said.
Potential ways to mitigate some of aging’s effects on the course of diabetes include caloric restriction, exercise, and taking metformin, Dr. LeRoith said. “There is a correlation between fitness and cognitive function, so the implication for clinical practice in individuals with diabetes is to encourage them to engage in physical activity on most days of the week,” he said. “It’s also known that depression makes the diabetes worse and depression makes cognitive function worse. It’s been suggested that if you have patients who are depressed, you should treat them with antidepressants if necessary, because this may help with their cognitive function.”
Meanwhile, an ongoing trial first announced in 2016 known as Targeting Aging with Metformin (TAME) is exploring the effects of metformin in helping to delay the aging process (Cell Metab 2016;23[6]:1060-5). Early support exists that metformin may delay cognitive decline and Alzheimer’s, even in non–type 2 diabetes. “An intended consequence of this effort is to create a paradigm for evaluation of pharmacologic approaches to delay aging,” the researchers wrote in an article describing the project, which is funded by the National Institute on Aging. “The randomized, controlled clinical trial we have proposed, if successful, could profoundly change the approach to aging and its diseases and affect health care delivery and costs.”
Dr. LeRoith reported having no financial disclosures.
LOS ANGELES – When Derek LeRoith, MD, PhD, was a medical student, he remembers professors telling him that human tissue response to aging diminishes over time, and that individuals can develop insulin resistance purely from aging.
“Whether that was right or wrong I don’t know, but certainly it seems to be one of the major issues that leads to the increase in diabetes, with all of its associated aspects such as dyslipidemia and hypertension,” he said at the World Congress on Insulin Resistance, Diabetes & Cardiovascular Disease.
According to Dr. LeRoith, professor of medicine and director of research in the division of endocrinology at Icahn School of Medicine at Mount Sinai, New York, studies have demonstrated that the elderly have worse glucose tolerance, compared with younger adults. One such analysis found that the insulin secretion index and disposition index are lower in the elderly, compared with their younger patients (Diabetes 2003;52[7]:1738-48). “But it’s not just the insulin resistance per se,” he said. “It’s also a defect of the beta cell. .”
Another major issue for aging patients is the impact of diabetes on cognitive decline and the formation of Alzheimer’s disease. “There’s a suggestion that the brain has insulin resistance and that this may also affect cognitive decline and Alzheimer’s,” Dr. LeRoith said. “But there are other aspects: insulin insufficiency, hyperglycemia, and, of course ... hypoglycemia. There is a debate as to what the major causes are. Is it amyloid beta accumulation, or is it vascular damage?”
In collaboration with Israeli researchers, Dr. LeRoith and his associates have been evaluating patients that belong to the Maccabi Health System in Tel Aviv, which has a diabetes registry with complete hemoglobin A1c measurements since 1998. One study of 897 registry participants found a strong association between worse diabetes control and worse cognition (Am J Geriatr Psych 2014;22:1055-9). Specifically, an interaction of duration of type 2 diabetes with HbA1c was associated with executive functioning (P = .006), semantic categorization (P = .019), attention/working memory (P = .011), and overall cognition (P = .006), such that the associations between duration of type 2 diabetes and cognitive impairment increased as HbA1c levels increased – but not for episodic memory (P = .984).
In a separate analysis of patients from the same registry, Dr. LeRoith and his colleagues evaluated the relationships of long-term trajectories of glycemic control with cognitive performance in cognitively normal elderly with type 2 diabetes (PLoS ONE 9[6]:e97384 doi: 10.1371/journal.pone.0097384). They found that subjects with stable HbA1c over time had the lowest HbA1c at study entry and performed best on cognitive measures, “suggesting that the trajectile of HbA1c over 10 or 12 years can really influence the cognitive ability in these patients,” he said.
Another, unrelated study found that insulin in combination with other diabetes medication is associated with less Alzheimer’s neuropathology (Neurology 2008;71:750-7), while an Alzheimer’s mouse model from Dr. LeRoith and his colleagues demonstrated that high dietary advanced glycation end products are associated with poorer spatial learning and accelerated amyloid beta deposition (Aging Cell 2016;15:309-16). “From that study we conclude that high dietary advance glycation end (AGE) products may be neurotoxic and that a diet low in AGEs may decrease dementia risk, particularly in diabetic elderly who are at increased risk and have higher levels of AGEs,” he said.
Potential ways to mitigate some of aging’s effects on the course of diabetes include caloric restriction, exercise, and taking metformin, Dr. LeRoith said. “There is a correlation between fitness and cognitive function, so the implication for clinical practice in individuals with diabetes is to encourage them to engage in physical activity on most days of the week,” he said. “It’s also known that depression makes the diabetes worse and depression makes cognitive function worse. It’s been suggested that if you have patients who are depressed, you should treat them with antidepressants if necessary, because this may help with their cognitive function.”
Meanwhile, an ongoing trial first announced in 2016 known as Targeting Aging with Metformin (TAME) is exploring the effects of metformin in helping to delay the aging process (Cell Metab 2016;23[6]:1060-5). Early support exists that metformin may delay cognitive decline and Alzheimer’s, even in non–type 2 diabetes. “An intended consequence of this effort is to create a paradigm for evaluation of pharmacologic approaches to delay aging,” the researchers wrote in an article describing the project, which is funded by the National Institute on Aging. “The randomized, controlled clinical trial we have proposed, if successful, could profoundly change the approach to aging and its diseases and affect health care delivery and costs.”
Dr. LeRoith reported having no financial disclosures.
LOS ANGELES – When Derek LeRoith, MD, PhD, was a medical student, he remembers professors telling him that human tissue response to aging diminishes over time, and that individuals can develop insulin resistance purely from aging.
“Whether that was right or wrong I don’t know, but certainly it seems to be one of the major issues that leads to the increase in diabetes, with all of its associated aspects such as dyslipidemia and hypertension,” he said at the World Congress on Insulin Resistance, Diabetes & Cardiovascular Disease.
According to Dr. LeRoith, professor of medicine and director of research in the division of endocrinology at Icahn School of Medicine at Mount Sinai, New York, studies have demonstrated that the elderly have worse glucose tolerance, compared with younger adults. One such analysis found that the insulin secretion index and disposition index are lower in the elderly, compared with their younger patients (Diabetes 2003;52[7]:1738-48). “But it’s not just the insulin resistance per se,” he said. “It’s also a defect of the beta cell. .”
Another major issue for aging patients is the impact of diabetes on cognitive decline and the formation of Alzheimer’s disease. “There’s a suggestion that the brain has insulin resistance and that this may also affect cognitive decline and Alzheimer’s,” Dr. LeRoith said. “But there are other aspects: insulin insufficiency, hyperglycemia, and, of course ... hypoglycemia. There is a debate as to what the major causes are. Is it amyloid beta accumulation, or is it vascular damage?”
In collaboration with Israeli researchers, Dr. LeRoith and his associates have been evaluating patients that belong to the Maccabi Health System in Tel Aviv, which has a diabetes registry with complete hemoglobin A1c measurements since 1998. One study of 897 registry participants found a strong association between worse diabetes control and worse cognition (Am J Geriatr Psych 2014;22:1055-9). Specifically, an interaction of duration of type 2 diabetes with HbA1c was associated with executive functioning (P = .006), semantic categorization (P = .019), attention/working memory (P = .011), and overall cognition (P = .006), such that the associations between duration of type 2 diabetes and cognitive impairment increased as HbA1c levels increased – but not for episodic memory (P = .984).
In a separate analysis of patients from the same registry, Dr. LeRoith and his colleagues evaluated the relationships of long-term trajectories of glycemic control with cognitive performance in cognitively normal elderly with type 2 diabetes (PLoS ONE 9[6]:e97384 doi: 10.1371/journal.pone.0097384). They found that subjects with stable HbA1c over time had the lowest HbA1c at study entry and performed best on cognitive measures, “suggesting that the trajectile of HbA1c over 10 or 12 years can really influence the cognitive ability in these patients,” he said.
Another, unrelated study found that insulin in combination with other diabetes medication is associated with less Alzheimer’s neuropathology (Neurology 2008;71:750-7), while an Alzheimer’s mouse model from Dr. LeRoith and his colleagues demonstrated that high dietary advanced glycation end products are associated with poorer spatial learning and accelerated amyloid beta deposition (Aging Cell 2016;15:309-16). “From that study we conclude that high dietary advance glycation end (AGE) products may be neurotoxic and that a diet low in AGEs may decrease dementia risk, particularly in diabetic elderly who are at increased risk and have higher levels of AGEs,” he said.
Potential ways to mitigate some of aging’s effects on the course of diabetes include caloric restriction, exercise, and taking metformin, Dr. LeRoith said. “There is a correlation between fitness and cognitive function, so the implication for clinical practice in individuals with diabetes is to encourage them to engage in physical activity on most days of the week,” he said. “It’s also known that depression makes the diabetes worse and depression makes cognitive function worse. It’s been suggested that if you have patients who are depressed, you should treat them with antidepressants if necessary, because this may help with their cognitive function.”
Meanwhile, an ongoing trial first announced in 2016 known as Targeting Aging with Metformin (TAME) is exploring the effects of metformin in helping to delay the aging process (Cell Metab 2016;23[6]:1060-5). Early support exists that metformin may delay cognitive decline and Alzheimer’s, even in non–type 2 diabetes. “An intended consequence of this effort is to create a paradigm for evaluation of pharmacologic approaches to delay aging,” the researchers wrote in an article describing the project, which is funded by the National Institute on Aging. “The randomized, controlled clinical trial we have proposed, if successful, could profoundly change the approach to aging and its diseases and affect health care delivery and costs.”
Dr. LeRoith reported having no financial disclosures.
EXPERT ANALYSIS FROM WCIRDC 2018
Proposed triple I criteria may overlook febrile women at risk post partum
A large proportion of laboring febrile women are not meeting proposed criteria for intrauterine inflammation or infection or both (triple I), but still may be at risk, according to an analysis of expert recommendations for clinical diagnosis published in Obstetrics & Gynecology.
“Our data suggest caution in universal implementation of the triple I criteria to guide clinical management of febrile women in the intrapartum period,” according to lead author Samsiya Ona, MD, of Brigham and Women’s Hospital in Boston, and her coauthors.
In early 2015, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) established criteria for diagnosing triple I in an effort to “decrease overtreatment of intrapartum women and low-risk newborns.” To assess the validity of those criteria, Dr. Ona and her colleagues analyzed 339 women with a temperature taken of 100.4°F or greater (38.0°C) during labor or within 1 hour post partum from June 2015 to September 2017.
The women were split into two groups: 212 met criteria for suspected triple I (documented fever plus clinical signs of intrauterine infection such as maternal leukocytosis greater than 15,000 per mm3, fetal tachycardia greater than 160 beats per minute, and purulent amniotic fluid) and 127 met criteria for isolated maternal fever. Among the suspected triple I group, incidence of adverse clinical infectious outcomes was 12%, comparable with 10% in the isolated maternal fever group (P = .50). When it came to predicting confirmed triple I, the sensitivity and specificity of the suspected triple I criteria were 71% (95% confidence interval, 61.4%-80.1%) and 41% (95% CI, 33.6%-47.8%), respectively. For predicting adverse clinical infectious outcomes, the sensitivity and specificity of the suspected triple I criteria were 68% (95% CI, 50.2%-82.0%) and 38% (95% CI, 32.6%-43.8%).
The authors cited among study limitations their including only women who had blood cultures sent at initial fever and excluding women who did not have repeat febrile temperature taken within 45 minutes. However, they noted the benefits of working with “a unique, large database with physiologic, laboratory, and microbiological parameters” and emphasized the need for an improved method of diagnosis, suggesting “a simple bedside minimally invasive marker of infection may be ideal.”
The study was supported by an Expanding the Boundaries Faculty Grant from the department of obstetrics, gynecology, and reproductive biology at the Brigham and Women’s Hospital in Boston. No conflicts of interest were reported.
SOURCE: Ona S et al. Obstet Gynecol. 2019 Jan. doi: 10.1097/AOG.0000000000003008.
A large proportion of laboring febrile women are not meeting proposed criteria for intrauterine inflammation or infection or both (triple I), but still may be at risk, according to an analysis of expert recommendations for clinical diagnosis published in Obstetrics & Gynecology.
“Our data suggest caution in universal implementation of the triple I criteria to guide clinical management of febrile women in the intrapartum period,” according to lead author Samsiya Ona, MD, of Brigham and Women’s Hospital in Boston, and her coauthors.
In early 2015, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) established criteria for diagnosing triple I in an effort to “decrease overtreatment of intrapartum women and low-risk newborns.” To assess the validity of those criteria, Dr. Ona and her colleagues analyzed 339 women with a temperature taken of 100.4°F or greater (38.0°C) during labor or within 1 hour post partum from June 2015 to September 2017.
The women were split into two groups: 212 met criteria for suspected triple I (documented fever plus clinical signs of intrauterine infection such as maternal leukocytosis greater than 15,000 per mm3, fetal tachycardia greater than 160 beats per minute, and purulent amniotic fluid) and 127 met criteria for isolated maternal fever. Among the suspected triple I group, incidence of adverse clinical infectious outcomes was 12%, comparable with 10% in the isolated maternal fever group (P = .50). When it came to predicting confirmed triple I, the sensitivity and specificity of the suspected triple I criteria were 71% (95% confidence interval, 61.4%-80.1%) and 41% (95% CI, 33.6%-47.8%), respectively. For predicting adverse clinical infectious outcomes, the sensitivity and specificity of the suspected triple I criteria were 68% (95% CI, 50.2%-82.0%) and 38% (95% CI, 32.6%-43.8%).
The authors cited among study limitations their including only women who had blood cultures sent at initial fever and excluding women who did not have repeat febrile temperature taken within 45 minutes. However, they noted the benefits of working with “a unique, large database with physiologic, laboratory, and microbiological parameters” and emphasized the need for an improved method of diagnosis, suggesting “a simple bedside minimally invasive marker of infection may be ideal.”
The study was supported by an Expanding the Boundaries Faculty Grant from the department of obstetrics, gynecology, and reproductive biology at the Brigham and Women’s Hospital in Boston. No conflicts of interest were reported.
SOURCE: Ona S et al. Obstet Gynecol. 2019 Jan. doi: 10.1097/AOG.0000000000003008.
A large proportion of laboring febrile women are not meeting proposed criteria for intrauterine inflammation or infection or both (triple I), but still may be at risk, according to an analysis of expert recommendations for clinical diagnosis published in Obstetrics & Gynecology.
“Our data suggest caution in universal implementation of the triple I criteria to guide clinical management of febrile women in the intrapartum period,” according to lead author Samsiya Ona, MD, of Brigham and Women’s Hospital in Boston, and her coauthors.
In early 2015, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) established criteria for diagnosing triple I in an effort to “decrease overtreatment of intrapartum women and low-risk newborns.” To assess the validity of those criteria, Dr. Ona and her colleagues analyzed 339 women with a temperature taken of 100.4°F or greater (38.0°C) during labor or within 1 hour post partum from June 2015 to September 2017.
The women were split into two groups: 212 met criteria for suspected triple I (documented fever plus clinical signs of intrauterine infection such as maternal leukocytosis greater than 15,000 per mm3, fetal tachycardia greater than 160 beats per minute, and purulent amniotic fluid) and 127 met criteria for isolated maternal fever. Among the suspected triple I group, incidence of adverse clinical infectious outcomes was 12%, comparable with 10% in the isolated maternal fever group (P = .50). When it came to predicting confirmed triple I, the sensitivity and specificity of the suspected triple I criteria were 71% (95% confidence interval, 61.4%-80.1%) and 41% (95% CI, 33.6%-47.8%), respectively. For predicting adverse clinical infectious outcomes, the sensitivity and specificity of the suspected triple I criteria were 68% (95% CI, 50.2%-82.0%) and 38% (95% CI, 32.6%-43.8%).
The authors cited among study limitations their including only women who had blood cultures sent at initial fever and excluding women who did not have repeat febrile temperature taken within 45 minutes. However, they noted the benefits of working with “a unique, large database with physiologic, laboratory, and microbiological parameters” and emphasized the need for an improved method of diagnosis, suggesting “a simple bedside minimally invasive marker of infection may be ideal.”
The study was supported by an Expanding the Boundaries Faculty Grant from the department of obstetrics, gynecology, and reproductive biology at the Brigham and Women’s Hospital in Boston. No conflicts of interest were reported.
SOURCE: Ona S et al. Obstet Gynecol. 2019 Jan. doi: 10.1097/AOG.0000000000003008.
FROM OBSTETRICS & GYNECOLOGY
Key clinical point:
Major finding: The sensitivity and specificity of the suspected triple I criteria to predict an adverse clinical infectious outcome were 68% for the suspected triple I group and 38% for the isolated maternal fever group.
Study details: A retrospective cohort study of 339 women with intrapartum fever from June 2015 to September 2017.
Disclosures: The study was supported by an Expanding the Boundaries Faculty Grant from the department of obstetrics, gynecology, and reproductive biology at the Brigham and Women’s Hospital in Boston. No conflicts of interest were reported.
Source: Ona S et al. Obstet Gynecol. 2019 Jan. doi: 10.1097/AOG.0000000000003008.
2019 Update on Obstetrics
The past year was an exciting one in obstetrics. The landmark ARRIVE trial presented at the Society for Maternal-Fetal Medicine’s (SMFM) annual meeting and subsequently published in the New England Journal of Medicine contradicted a long-held belief about the safety of elective labor induction. In a large randomized trial, Cahill and colleagues took a controversial but practical clinical question about second-stage labor management and answered it for the practicing obstetrician in the trenches. Finally, the American College of Obstetricians and Gynecologists (ACOG) placed new emphasis on the oft overlooked but increasingly more complicated postpartum period, offering guidance to support improving care for women in this transitional period.
Ultimately, this was the year of the patient, as research, clinical guidelines, and education focused on how to achieve the best in safety and quality of care for delivery planning, the delivery itself, and the so-called fourth trimester.
ARRIVE: Labor induction at 39 weeks reduces CD rate with no difference in perinatal death or serious outcomes
Grobman WA, Rice MM, Reddy UM, et al; for the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
The term "elective induction of labor" has long had a negative connotation because of its association with increased CD rates and adverse perinatal outcomes. This view was based on results from older observational studies that compared outcomes for labor induction with those of spontaneous labor. In more recent observational studies that more appropriately compared labor induction with expectant management, however, elective induction of labor appears to be associated with similar CD rates and perinatal outcomes.
To test the hypothesis that elective induction would have a lower risk for perinatal death or severe neonatal complications than expectant management in low-risk nulliparous women, Grobman and colleagues conducted A Randomized Trial of Induction Versus Expectant Management (ARRIVE).1
Study population, timing of delivery, and trial outcomes
This randomized controlled trial included 6,106 women at 41 US centers in the Maternal-Fetal Medicine Units Network of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Study participants were low-risk nulliparous women with a singleton vertex fetus who were randomly assigned to induction of labor at 39 to 39 4/7 weeks (n = 3,062) or expectant management (n = 3,044) until 40 5/7 to 42 2/7 weeks.
"Low risk" was defined as having no maternal or fetal indication for delivery prior to 40 5/7 weeks. Reliable gestational dating was required.
While no specific protocol for induction of labor management was required, there were 2 requests: 1) Cervical ripening was requested for an unfavorable cervix (63% of participants had a modified Bishop score <5), and 2) a duration of at least 12 hours after cervical ripening, rupture of membranes, and use of uterine stimulant was requested before performing a CD for "failed induction" (if medically appropriate).
The primary outcome was a composite of perinatal death or serious neonatal complications. The main secondary outcome was CD.
Potentially game-changing findings
The investigators found that there was no statistically significant difference between the elective induction and expectant management groups for the primary composite perinatal outcome (4.3% vs 5.4%; P = .049, with P<.046 prespecified for significance). In addition, the rate of CD was significantly lower in the labor induction group than in the expectant management group (18.6% vs 22.2%; P<.001).
Other significant findings in secondary outcomes included the following:
- Hypertensive disorders of pregnancy were significantly lower in the labor induction group compared with the expectant management group (9.1% vs 14.1%; P<.001).
- The labor induction group had a longer length of stay in the labor and delivery unit but a shorter postpartum hospital stay.
- The labor induction group reported less pain and more control during labor.
Results refute negative notion of elective labor induction
The authors concluded that in a low-risk nulliparous patient population, elective induction of labor at 39 weeks does not increase the risk for adverse perinatal outcomes and decreases the rate of CD and hypertensive disorders of pregnancy. Additionally, they noted that induction at 39 weeks should not be avoided with the goal of preventing CD, as even women with an unfavorable cervix had a lower rate of CD in the induction group compared with the expectant management group.
After publication of the ARRIVE trial findings, both ACOG and SMFM released statements supporting elective labor induction at or beyond 39 weeks’ gestation in low-risk nulliparous women with good gestational dating.2,3 They cited the following as important issues: adherence to the trial inclusion criteria except for research purposes, shared decision-making with the patient, consideration of the logistics and impact on the health care facility, and the yet unknown impact on cost. Finally, it should be a priority to avoid the primary CD for a failed induction by allowing a longer latent phase of labor, as long as maternal and fetal conditions allow. In my practice, I actively offer induction of labor to most of my patients at 39 weeks after a discussion of the risks and benefits.
Continue to: Immediate pushing in second stage...
Immediate pushing in second stage offers benefits and is preferable to delayed pushing
Cahill AG, Srinivas SK, Tita AT, et al. Effect of immediate vs delayed pushing on rates of spontaneous vaginal delivery among nulliparous women receiving neuraxial analgesia: a randomized clinical trial. JAMA. 2018;320:1444-1454.
In a randomized trial of 2,414 women, Cahill and colleagues sought to answer a seemingly simple question: What is the best timing for pushing during the second stage of labor--immediate or delayed?
Practical management of the second stage of labor (defined as complete cervical dilation to the delivery of the infant) varies by provider and setting, and previous data on pushing efforts are conflicting. Delayed pushing, or "laboring down," has been suggested to allow passive fetal rotation and to conserve maternal energy for pushing. Older studies have shown that delayed pushing decreases the rate of operative delivery. More recent study data have not demonstrated a difference between immediate and delayed pushing techniques on vaginal delivery rates and have noted that increased maternal and neonatal morbidities are associated with a longer second stage of labor.
The recent trial by Cahill and colleagues was designed to determine the effect of these 2 techniques on spontaneous vaginal delivery rates and on maternal and neonatal morbidities.4
Large study population
This randomized pragmatic trial was conducted at 6 centers in the United States. Study participants (2,404 women completed the study) were nulliparous women at 37 or more weeks' gestation with neuraxial anesthesia who were randomly assigned at complete cervical dilation either to immediate pushing (n = 1,200) or to delayed pushing, that is, instructed to wait 60 minutes before starting to push (n = 1,204). The obstetric provider determined the rest of the labor management.
The primary outcome was the rate of spontaneous vaginal delivery. Secondary outcomes included duration of the second stage of labor, duration of active pushing, operative vaginal delivery, CD, and several maternal assessments (postpartum hemorrhage, chorioamnionitis, endometritis, and perineal lacerations).
Both groups had similar vaginal delivery rates, differences in some measures
There was no difference in the primary outcome between the 2 groups: The spontaneous vaginal delivery rate was 85.9% (n = 1,031) in the immediate pushing group and 86.5% (n = 1,041) in the delayed pushing group (P = .67).
Analysis of secondary outcomes revealed several significant differences:
- decreased total time for the second stage of labor in the immediate pushing group compared with the delayed pushing group (102.4 vs 134.2 minutes) but longer active pushing time (83.7 vs 74.5 minutes)
- a lower rate of postpartum hemorrhage, chorioamnionitis in the second stage, neonatal acidemia, and suspected neonatal sepsis in the immediate pushing group
- a higher rate of third-degree perineal lacerations in the immediate pushing group.
No difference was found between groups in rates of operative vaginal deliveries, CDs, endometritis, overall perineal lacerations, or spontaneous vaginal delivery by fetal station or occiput position.
Authors' takeaway
The authors concluded that since delayed pushing does not increase spontaneous vaginal delivery rates and increases the duration of the second stage of labor and both maternal and neonatal morbidity, immediate pushing may be preferred in this patient population.
After reviewing the available literature in light of this study’s findings, ACOG released a practice advisory in October 2018 stating that “it is reasonable to choose immediate over delayed pushing in nulliparous patients with neuraxial anesthesia.”5 Nulliparous patients with neuraxial anesthesia should be counseled that delayed pushing does not increase the rate of spontaneous vaginal birth and may increase both maternal and neonatal complications. As this may be a practice change for many obstetrics units, the obstetric nursing department should be included in this education and counseling. In my practice, I would recommend immediate pushing, but it is important to include both the patient and her nurse in the discussion.
ACOG aims to optimize postpartum care
American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 736. Optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150.
In May 2018, ACOG released "Optimizing postpartum care," a committee opinion that proposes a new model of comprehensive postpartum care focused on improving both short- and long-term health outcomes for women and infants. (This replaces the June 2016 committee opinion No. 666.) Described as "the fourth trimester," the postpartum period is a critical transitional period in which both pregnancy-related and pre-existing conditions may affect maternal, neonatal, and family status; half of pregnancy-related maternal deaths occur during the postpartum period.6
The postpartum visit: Often a lost opportunity
ACOG cites that up to 40% of women in the United States do not attend their postpartum visit.6 Many aspects of the postpartum visit, including follow-up for chronic diseases, mental health screening, and contraceptive counseling, provide opportunities for acute intervention as well as establishment of healthy behaviors. Some studies have shown that postpartum depression, breastfeeding, and patient satisfaction outcomes improve as a result of postpartum engagement.
Continue to: ACOG's recommendations...
ACOG's recommendations
Ongoing process. ACOG's first proposed change concerns the structure of the postpartum visit itself, which traditionally has been a single visit with a provider at approximately 6 weeks postpartum. Postpartum care plans actually should be started before birth, during regular prenatal care, and adjusted in the hospital as needed so that the provider can educate patients about the issues they may face and resources they may need during this time. This prenatal preparation hopefully will encourage more patients to attend their postpartum visits.
Increased provider contact. Another proposed change is that after delivery, the patient should have contact with a provider within the first 3 weeks postpartum. For high-risk patients, this may involve an in-person clinic visit as soon as 3 to 10 days postpartum (for hypertensive disorders of pregnancy) or at 1 to 2 weeks (for postpartum depression screening, incision checks, and lactation issues). For lower-risk patients, a phone call may be appropriate and/or preferred. Ongoing follow-up for all patients before the final postpartum visit should be individualized.
Postpartum visit and care transition. ACOG recommends a comprehensive postpartum visit at 4 to 12 weeks to fully evaluate the woman's physical, social, and psychologic well-being and to serve as a transition from pregnancy care to well-woman care. This is a large order and includes evaluation of the following:
- mood and emotional well-being
- infant care and feeding
- sexuality, contraception, and birth spacing
- sleep and fatigue
- physical recovery from birth
- chronic disease management and transition to primary care provider
- health maintenance
- review of labor and delivery course if needed
- review of risks and recommendations for future pregnancies.
After these components are addressed, it is expected that the patient will be transitioned to a primary care provider (who may continue to be the ObGyn, as appropriate) to coordinate her future care in the primary medical home.
Useful resource for adopting new paradigm
ACOG's recommendations are somewhat daunting, and these changes will require education and resources, a significant increase in obstetric provider time and effort, and consideration of policy change regarding such issues as parental leave and postpartum care reimbursement. As a start, ACOG has developed an online aid for health care providers called "Postpartum toolkit" (https://www.acog.org/About-ACOG/ACOG-Departments/Toolkits-for-Health-Care-Providers/Postpartum-Toolkit), which provides education and resources for all steps in the process and can be individualized for each practice and patient.7
Postpartum care should be seen as an ongoing process to address both short- and long-term health outcomes for the patient, her newborn, and their family. This process should begin with planning in the antenatal period, continue with close individualized follow-up within the first 3 weeks of birth, and conclude with a comprehensive postpartum evaluation and transition to well-woman care. Shifting the paradigm of postpartum care will take considerable commitment and resources on the part of obstetric providers and their practices. In my practice, we routinely see hypertensive patients within the first week postpartum and patients at risk for postpartum depression within the first 2 weeks in our clinics. We have a standard 6-week postpartum visit for all patients as well. Going forward, we need to further determine how and when we can implement ACOG’s extensive new recommendations for optimizing postpartum care.
- Grobman WA, Rice MM, Reddy UM, et al; for the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
- American College of Obstetricians and Gynecologists. Practice advisory: clinical guidance for integration of the findings of the ARRIVE trial: Labor induction versus expectant management in low-risk nulliparous women. August 2018. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Practice-Advisory-Clinical-guidance-for-integration-of-the-findings-of-The-ARRIVE-Trial. Accessed November 25, 2018.
- Society for Maternal-Fetal Medicine (SMFM) Publications Committee. SMFM statement on elective induction of labor in low-risk nulliparous women at term: the ARRIVE trial. Am J Obstet Gynecol. doi:10.1016/j.ajog.2018.08.009. In press.
- Cahill AG, Srinivas SK, Tita AT, et al. Effect of immediate vs delayed pushing on rates of spontaneous vaginal delivery among nulliparous women receiving neuraxial analgesia: a randomized clinical trial. JAMA. 2018;320:1444-1454.
- American College of Obstetricians and Gynecologists. Practice advisory: immediate versus delayed pushing in nulliparous women receiving neuraxial analgesia. October 2018. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Practice-Advisory-Immediate-vs-delayed-pushing-in-nulliparous-women-receiving-neuraxial-analgesia. Accessed November 25, 2018.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 736. Optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150.
- American College of Obstetricians and Gynecologists. ACOG Postpartum toolkit. https://www.acog.org/About-ACOG/ACOG-Departments/Toolkits-for-Health-Care-Providers/Postpartum-Toolkit. Accessed November 25, 2018.
The past year was an exciting one in obstetrics. The landmark ARRIVE trial presented at the Society for Maternal-Fetal Medicine’s (SMFM) annual meeting and subsequently published in the New England Journal of Medicine contradicted a long-held belief about the safety of elective labor induction. In a large randomized trial, Cahill and colleagues took a controversial but practical clinical question about second-stage labor management and answered it for the practicing obstetrician in the trenches. Finally, the American College of Obstetricians and Gynecologists (ACOG) placed new emphasis on the oft overlooked but increasingly more complicated postpartum period, offering guidance to support improving care for women in this transitional period.
Ultimately, this was the year of the patient, as research, clinical guidelines, and education focused on how to achieve the best in safety and quality of care for delivery planning, the delivery itself, and the so-called fourth trimester.
ARRIVE: Labor induction at 39 weeks reduces CD rate with no difference in perinatal death or serious outcomes
Grobman WA, Rice MM, Reddy UM, et al; for the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
The term "elective induction of labor" has long had a negative connotation because of its association with increased CD rates and adverse perinatal outcomes. This view was based on results from older observational studies that compared outcomes for labor induction with those of spontaneous labor. In more recent observational studies that more appropriately compared labor induction with expectant management, however, elective induction of labor appears to be associated with similar CD rates and perinatal outcomes.
To test the hypothesis that elective induction would have a lower risk for perinatal death or severe neonatal complications than expectant management in low-risk nulliparous women, Grobman and colleagues conducted A Randomized Trial of Induction Versus Expectant Management (ARRIVE).1

Study population, timing of delivery, and trial outcomes
This randomized controlled trial included 6,106 women at 41 US centers in the Maternal-Fetal Medicine Units Network of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Study participants were low-risk nulliparous women with a singleton vertex fetus who were randomly assigned to induction of labor at 39 to 39 4/7 weeks (n = 3,062) or expectant management (n = 3,044) until 40 5/7 to 42 2/7 weeks.
"Low risk" was defined as having no maternal or fetal indication for delivery prior to 40 5/7 weeks. Reliable gestational dating was required.
While no specific protocol for induction of labor management was required, there were 2 requests: 1) Cervical ripening was requested for an unfavorable cervix (63% of participants had a modified Bishop score <5), and 2) a duration of at least 12 hours after cervical ripening, rupture of membranes, and use of uterine stimulant was requested before performing a CD for "failed induction" (if medically appropriate).
The primary outcome was a composite of perinatal death or serious neonatal complications. The main secondary outcome was CD.
Potentially game-changing findings
The investigators found that there was no statistically significant difference between the elective induction and expectant management groups for the primary composite perinatal outcome (4.3% vs 5.4%; P = .049, with P<.046 prespecified for significance). In addition, the rate of CD was significantly lower in the labor induction group than in the expectant management group (18.6% vs 22.2%; P<.001).
Other significant findings in secondary outcomes included the following:
- Hypertensive disorders of pregnancy were significantly lower in the labor induction group compared with the expectant management group (9.1% vs 14.1%; P<.001).
- The labor induction group had a longer length of stay in the labor and delivery unit but a shorter postpartum hospital stay.
- The labor induction group reported less pain and more control during labor.
Results refute negative notion of elective labor induction
The authors concluded that in a low-risk nulliparous patient population, elective induction of labor at 39 weeks does not increase the risk for adverse perinatal outcomes and decreases the rate of CD and hypertensive disorders of pregnancy. Additionally, they noted that induction at 39 weeks should not be avoided with the goal of preventing CD, as even women with an unfavorable cervix had a lower rate of CD in the induction group compared with the expectant management group.
After publication of the ARRIVE trial findings, both ACOG and SMFM released statements supporting elective labor induction at or beyond 39 weeks’ gestation in low-risk nulliparous women with good gestational dating.2,3 They cited the following as important issues: adherence to the trial inclusion criteria except for research purposes, shared decision-making with the patient, consideration of the logistics and impact on the health care facility, and the yet unknown impact on cost. Finally, it should be a priority to avoid the primary CD for a failed induction by allowing a longer latent phase of labor, as long as maternal and fetal conditions allow. In my practice, I actively offer induction of labor to most of my patients at 39 weeks after a discussion of the risks and benefits.
Continue to: Immediate pushing in second stage...
Immediate pushing in second stage offers benefits and is preferable to delayed pushing
Cahill AG, Srinivas SK, Tita AT, et al. Effect of immediate vs delayed pushing on rates of spontaneous vaginal delivery among nulliparous women receiving neuraxial analgesia: a randomized clinical trial. JAMA. 2018;320:1444-1454.
In a randomized trial of 2,414 women, Cahill and colleagues sought to answer a seemingly simple question: What is the best timing for pushing during the second stage of labor--immediate or delayed?
Practical management of the second stage of labor (defined as complete cervical dilation to the delivery of the infant) varies by provider and setting, and previous data on pushing efforts are conflicting. Delayed pushing, or "laboring down," has been suggested to allow passive fetal rotation and to conserve maternal energy for pushing. Older studies have shown that delayed pushing decreases the rate of operative delivery. More recent study data have not demonstrated a difference between immediate and delayed pushing techniques on vaginal delivery rates and have noted that increased maternal and neonatal morbidities are associated with a longer second stage of labor.
The recent trial by Cahill and colleagues was designed to determine the effect of these 2 techniques on spontaneous vaginal delivery rates and on maternal and neonatal morbidities.4
Large study population
This randomized pragmatic trial was conducted at 6 centers in the United States. Study participants (2,404 women completed the study) were nulliparous women at 37 or more weeks' gestation with neuraxial anesthesia who were randomly assigned at complete cervical dilation either to immediate pushing (n = 1,200) or to delayed pushing, that is, instructed to wait 60 minutes before starting to push (n = 1,204). The obstetric provider determined the rest of the labor management.
The primary outcome was the rate of spontaneous vaginal delivery. Secondary outcomes included duration of the second stage of labor, duration of active pushing, operative vaginal delivery, CD, and several maternal assessments (postpartum hemorrhage, chorioamnionitis, endometritis, and perineal lacerations).
Both groups had similar vaginal delivery rates, differences in some measures
There was no difference in the primary outcome between the 2 groups: The spontaneous vaginal delivery rate was 85.9% (n = 1,031) in the immediate pushing group and 86.5% (n = 1,041) in the delayed pushing group (P = .67).
Analysis of secondary outcomes revealed several significant differences:
- decreased total time for the second stage of labor in the immediate pushing group compared with the delayed pushing group (102.4 vs 134.2 minutes) but longer active pushing time (83.7 vs 74.5 minutes)
- a lower rate of postpartum hemorrhage, chorioamnionitis in the second stage, neonatal acidemia, and suspected neonatal sepsis in the immediate pushing group
- a higher rate of third-degree perineal lacerations in the immediate pushing group.
No difference was found between groups in rates of operative vaginal deliveries, CDs, endometritis, overall perineal lacerations, or spontaneous vaginal delivery by fetal station or occiput position.
Authors' takeaway
The authors concluded that since delayed pushing does not increase spontaneous vaginal delivery rates and increases the duration of the second stage of labor and both maternal and neonatal morbidity, immediate pushing may be preferred in this patient population.
After reviewing the available literature in light of this study’s findings, ACOG released a practice advisory in October 2018 stating that “it is reasonable to choose immediate over delayed pushing in nulliparous patients with neuraxial anesthesia.”5 Nulliparous patients with neuraxial anesthesia should be counseled that delayed pushing does not increase the rate of spontaneous vaginal birth and may increase both maternal and neonatal complications. As this may be a practice change for many obstetrics units, the obstetric nursing department should be included in this education and counseling. In my practice, I would recommend immediate pushing, but it is important to include both the patient and her nurse in the discussion.
ACOG aims to optimize postpartum care
American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 736. Optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150.
In May 2018, ACOG released "Optimizing postpartum care," a committee opinion that proposes a new model of comprehensive postpartum care focused on improving both short- and long-term health outcomes for women and infants. (This replaces the June 2016 committee opinion No. 666.) Described as "the fourth trimester," the postpartum period is a critical transitional period in which both pregnancy-related and pre-existing conditions may affect maternal, neonatal, and family status; half of pregnancy-related maternal deaths occur during the postpartum period.6
The postpartum visit: Often a lost opportunity
ACOG cites that up to 40% of women in the United States do not attend their postpartum visit.6 Many aspects of the postpartum visit, including follow-up for chronic diseases, mental health screening, and contraceptive counseling, provide opportunities for acute intervention as well as establishment of healthy behaviors. Some studies have shown that postpartum depression, breastfeeding, and patient satisfaction outcomes improve as a result of postpartum engagement.
Continue to: ACOG's recommendations...
ACOG's recommendations
Ongoing process. ACOG's first proposed change concerns the structure of the postpartum visit itself, which traditionally has been a single visit with a provider at approximately 6 weeks postpartum. Postpartum care plans actually should be started before birth, during regular prenatal care, and adjusted in the hospital as needed so that the provider can educate patients about the issues they may face and resources they may need during this time. This prenatal preparation hopefully will encourage more patients to attend their postpartum visits.
Increased provider contact. Another proposed change is that after delivery, the patient should have contact with a provider within the first 3 weeks postpartum. For high-risk patients, this may involve an in-person clinic visit as soon as 3 to 10 days postpartum (for hypertensive disorders of pregnancy) or at 1 to 2 weeks (for postpartum depression screening, incision checks, and lactation issues). For lower-risk patients, a phone call may be appropriate and/or preferred. Ongoing follow-up for all patients before the final postpartum visit should be individualized.
Postpartum visit and care transition. ACOG recommends a comprehensive postpartum visit at 4 to 12 weeks to fully evaluate the woman's physical, social, and psychologic well-being and to serve as a transition from pregnancy care to well-woman care. This is a large order and includes evaluation of the following:
- mood and emotional well-being
- infant care and feeding
- sexuality, contraception, and birth spacing
- sleep and fatigue
- physical recovery from birth
- chronic disease management and transition to primary care provider
- health maintenance
- review of labor and delivery course if needed
- review of risks and recommendations for future pregnancies.
After these components are addressed, it is expected that the patient will be transitioned to a primary care provider (who may continue to be the ObGyn, as appropriate) to coordinate her future care in the primary medical home.
Useful resource for adopting new paradigm
ACOG's recommendations are somewhat daunting, and these changes will require education and resources, a significant increase in obstetric provider time and effort, and consideration of policy change regarding such issues as parental leave and postpartum care reimbursement. As a start, ACOG has developed an online aid for health care providers called "Postpartum toolkit" (https://www.acog.org/About-ACOG/ACOG-Departments/Toolkits-for-Health-Care-Providers/Postpartum-Toolkit), which provides education and resources for all steps in the process and can be individualized for each practice and patient.7
Postpartum care should be seen as an ongoing process to address both short- and long-term health outcomes for the patient, her newborn, and their family. This process should begin with planning in the antenatal period, continue with close individualized follow-up within the first 3 weeks of birth, and conclude with a comprehensive postpartum evaluation and transition to well-woman care. Shifting the paradigm of postpartum care will take considerable commitment and resources on the part of obstetric providers and their practices. In my practice, we routinely see hypertensive patients within the first week postpartum and patients at risk for postpartum depression within the first 2 weeks in our clinics. We have a standard 6-week postpartum visit for all patients as well. Going forward, we need to further determine how and when we can implement ACOG’s extensive new recommendations for optimizing postpartum care.
The past year was an exciting one in obstetrics. The landmark ARRIVE trial presented at the Society for Maternal-Fetal Medicine’s (SMFM) annual meeting and subsequently published in the New England Journal of Medicine contradicted a long-held belief about the safety of elective labor induction. In a large randomized trial, Cahill and colleagues took a controversial but practical clinical question about second-stage labor management and answered it for the practicing obstetrician in the trenches. Finally, the American College of Obstetricians and Gynecologists (ACOG) placed new emphasis on the oft overlooked but increasingly more complicated postpartum period, offering guidance to support improving care for women in this transitional period.
Ultimately, this was the year of the patient, as research, clinical guidelines, and education focused on how to achieve the best in safety and quality of care for delivery planning, the delivery itself, and the so-called fourth trimester.
ARRIVE: Labor induction at 39 weeks reduces CD rate with no difference in perinatal death or serious outcomes
Grobman WA, Rice MM, Reddy UM, et al; for the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
The term "elective induction of labor" has long had a negative connotation because of its association with increased CD rates and adverse perinatal outcomes. This view was based on results from older observational studies that compared outcomes for labor induction with those of spontaneous labor. In more recent observational studies that more appropriately compared labor induction with expectant management, however, elective induction of labor appears to be associated with similar CD rates and perinatal outcomes.
To test the hypothesis that elective induction would have a lower risk for perinatal death or severe neonatal complications than expectant management in low-risk nulliparous women, Grobman and colleagues conducted A Randomized Trial of Induction Versus Expectant Management (ARRIVE).1

Study population, timing of delivery, and trial outcomes
This randomized controlled trial included 6,106 women at 41 US centers in the Maternal-Fetal Medicine Units Network of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Study participants were low-risk nulliparous women with a singleton vertex fetus who were randomly assigned to induction of labor at 39 to 39 4/7 weeks (n = 3,062) or expectant management (n = 3,044) until 40 5/7 to 42 2/7 weeks.
"Low risk" was defined as having no maternal or fetal indication for delivery prior to 40 5/7 weeks. Reliable gestational dating was required.
While no specific protocol for induction of labor management was required, there were 2 requests: 1) Cervical ripening was requested for an unfavorable cervix (63% of participants had a modified Bishop score <5), and 2) a duration of at least 12 hours after cervical ripening, rupture of membranes, and use of uterine stimulant was requested before performing a CD for "failed induction" (if medically appropriate).
The primary outcome was a composite of perinatal death or serious neonatal complications. The main secondary outcome was CD.
Potentially game-changing findings
The investigators found that there was no statistically significant difference between the elective induction and expectant management groups for the primary composite perinatal outcome (4.3% vs 5.4%; P = .049, with P<.046 prespecified for significance). In addition, the rate of CD was significantly lower in the labor induction group than in the expectant management group (18.6% vs 22.2%; P<.001).
Other significant findings in secondary outcomes included the following:
- Hypertensive disorders of pregnancy were significantly lower in the labor induction group compared with the expectant management group (9.1% vs 14.1%; P<.001).
- The labor induction group had a longer length of stay in the labor and delivery unit but a shorter postpartum hospital stay.
- The labor induction group reported less pain and more control during labor.
Results refute negative notion of elective labor induction
The authors concluded that in a low-risk nulliparous patient population, elective induction of labor at 39 weeks does not increase the risk for adverse perinatal outcomes and decreases the rate of CD and hypertensive disorders of pregnancy. Additionally, they noted that induction at 39 weeks should not be avoided with the goal of preventing CD, as even women with an unfavorable cervix had a lower rate of CD in the induction group compared with the expectant management group.
After publication of the ARRIVE trial findings, both ACOG and SMFM released statements supporting elective labor induction at or beyond 39 weeks’ gestation in low-risk nulliparous women with good gestational dating.2,3 They cited the following as important issues: adherence to the trial inclusion criteria except for research purposes, shared decision-making with the patient, consideration of the logistics and impact on the health care facility, and the yet unknown impact on cost. Finally, it should be a priority to avoid the primary CD for a failed induction by allowing a longer latent phase of labor, as long as maternal and fetal conditions allow. In my practice, I actively offer induction of labor to most of my patients at 39 weeks after a discussion of the risks and benefits.
Continue to: Immediate pushing in second stage...
Immediate pushing in second stage offers benefits and is preferable to delayed pushing
Cahill AG, Srinivas SK, Tita AT, et al. Effect of immediate vs delayed pushing on rates of spontaneous vaginal delivery among nulliparous women receiving neuraxial analgesia: a randomized clinical trial. JAMA. 2018;320:1444-1454.
In a randomized trial of 2,414 women, Cahill and colleagues sought to answer a seemingly simple question: What is the best timing for pushing during the second stage of labor--immediate or delayed?
Practical management of the second stage of labor (defined as complete cervical dilation to the delivery of the infant) varies by provider and setting, and previous data on pushing efforts are conflicting. Delayed pushing, or "laboring down," has been suggested to allow passive fetal rotation and to conserve maternal energy for pushing. Older studies have shown that delayed pushing decreases the rate of operative delivery. More recent study data have not demonstrated a difference between immediate and delayed pushing techniques on vaginal delivery rates and have noted that increased maternal and neonatal morbidities are associated with a longer second stage of labor.
The recent trial by Cahill and colleagues was designed to determine the effect of these 2 techniques on spontaneous vaginal delivery rates and on maternal and neonatal morbidities.4
Large study population
This randomized pragmatic trial was conducted at 6 centers in the United States. Study participants (2,404 women completed the study) were nulliparous women at 37 or more weeks' gestation with neuraxial anesthesia who were randomly assigned at complete cervical dilation either to immediate pushing (n = 1,200) or to delayed pushing, that is, instructed to wait 60 minutes before starting to push (n = 1,204). The obstetric provider determined the rest of the labor management.
The primary outcome was the rate of spontaneous vaginal delivery. Secondary outcomes included duration of the second stage of labor, duration of active pushing, operative vaginal delivery, CD, and several maternal assessments (postpartum hemorrhage, chorioamnionitis, endometritis, and perineal lacerations).
Both groups had similar vaginal delivery rates, differences in some measures
There was no difference in the primary outcome between the 2 groups: The spontaneous vaginal delivery rate was 85.9% (n = 1,031) in the immediate pushing group and 86.5% (n = 1,041) in the delayed pushing group (P = .67).
Analysis of secondary outcomes revealed several significant differences:
- decreased total time for the second stage of labor in the immediate pushing group compared with the delayed pushing group (102.4 vs 134.2 minutes) but longer active pushing time (83.7 vs 74.5 minutes)
- a lower rate of postpartum hemorrhage, chorioamnionitis in the second stage, neonatal acidemia, and suspected neonatal sepsis in the immediate pushing group
- a higher rate of third-degree perineal lacerations in the immediate pushing group.
No difference was found between groups in rates of operative vaginal deliveries, CDs, endometritis, overall perineal lacerations, or spontaneous vaginal delivery by fetal station or occiput position.
Authors' takeaway
The authors concluded that since delayed pushing does not increase spontaneous vaginal delivery rates and increases the duration of the second stage of labor and both maternal and neonatal morbidity, immediate pushing may be preferred in this patient population.
After reviewing the available literature in light of this study’s findings, ACOG released a practice advisory in October 2018 stating that “it is reasonable to choose immediate over delayed pushing in nulliparous patients with neuraxial anesthesia.”5 Nulliparous patients with neuraxial anesthesia should be counseled that delayed pushing does not increase the rate of spontaneous vaginal birth and may increase both maternal and neonatal complications. As this may be a practice change for many obstetrics units, the obstetric nursing department should be included in this education and counseling. In my practice, I would recommend immediate pushing, but it is important to include both the patient and her nurse in the discussion.
ACOG aims to optimize postpartum care
American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 736. Optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150.
In May 2018, ACOG released "Optimizing postpartum care," a committee opinion that proposes a new model of comprehensive postpartum care focused on improving both short- and long-term health outcomes for women and infants. (This replaces the June 2016 committee opinion No. 666.) Described as "the fourth trimester," the postpartum period is a critical transitional period in which both pregnancy-related and pre-existing conditions may affect maternal, neonatal, and family status; half of pregnancy-related maternal deaths occur during the postpartum period.6
The postpartum visit: Often a lost opportunity
ACOG cites that up to 40% of women in the United States do not attend their postpartum visit.6 Many aspects of the postpartum visit, including follow-up for chronic diseases, mental health screening, and contraceptive counseling, provide opportunities for acute intervention as well as establishment of healthy behaviors. Some studies have shown that postpartum depression, breastfeeding, and patient satisfaction outcomes improve as a result of postpartum engagement.
Continue to: ACOG's recommendations...
ACOG's recommendations
Ongoing process. ACOG's first proposed change concerns the structure of the postpartum visit itself, which traditionally has been a single visit with a provider at approximately 6 weeks postpartum. Postpartum care plans actually should be started before birth, during regular prenatal care, and adjusted in the hospital as needed so that the provider can educate patients about the issues they may face and resources they may need during this time. This prenatal preparation hopefully will encourage more patients to attend their postpartum visits.
Increased provider contact. Another proposed change is that after delivery, the patient should have contact with a provider within the first 3 weeks postpartum. For high-risk patients, this may involve an in-person clinic visit as soon as 3 to 10 days postpartum (for hypertensive disorders of pregnancy) or at 1 to 2 weeks (for postpartum depression screening, incision checks, and lactation issues). For lower-risk patients, a phone call may be appropriate and/or preferred. Ongoing follow-up for all patients before the final postpartum visit should be individualized.
Postpartum visit and care transition. ACOG recommends a comprehensive postpartum visit at 4 to 12 weeks to fully evaluate the woman's physical, social, and psychologic well-being and to serve as a transition from pregnancy care to well-woman care. This is a large order and includes evaluation of the following:
- mood and emotional well-being
- infant care and feeding
- sexuality, contraception, and birth spacing
- sleep and fatigue
- physical recovery from birth
- chronic disease management and transition to primary care provider
- health maintenance
- review of labor and delivery course if needed
- review of risks and recommendations for future pregnancies.
After these components are addressed, it is expected that the patient will be transitioned to a primary care provider (who may continue to be the ObGyn, as appropriate) to coordinate her future care in the primary medical home.
Useful resource for adopting new paradigm
ACOG's recommendations are somewhat daunting, and these changes will require education and resources, a significant increase in obstetric provider time and effort, and consideration of policy change regarding such issues as parental leave and postpartum care reimbursement. As a start, ACOG has developed an online aid for health care providers called "Postpartum toolkit" (https://www.acog.org/About-ACOG/ACOG-Departments/Toolkits-for-Health-Care-Providers/Postpartum-Toolkit), which provides education and resources for all steps in the process and can be individualized for each practice and patient.7
Postpartum care should be seen as an ongoing process to address both short- and long-term health outcomes for the patient, her newborn, and their family. This process should begin with planning in the antenatal period, continue with close individualized follow-up within the first 3 weeks of birth, and conclude with a comprehensive postpartum evaluation and transition to well-woman care. Shifting the paradigm of postpartum care will take considerable commitment and resources on the part of obstetric providers and their practices. In my practice, we routinely see hypertensive patients within the first week postpartum and patients at risk for postpartum depression within the first 2 weeks in our clinics. We have a standard 6-week postpartum visit for all patients as well. Going forward, we need to further determine how and when we can implement ACOG’s extensive new recommendations for optimizing postpartum care.
- Grobman WA, Rice MM, Reddy UM, et al; for the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
- American College of Obstetricians and Gynecologists. Practice advisory: clinical guidance for integration of the findings of the ARRIVE trial: Labor induction versus expectant management in low-risk nulliparous women. August 2018. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Practice-Advisory-Clinical-guidance-for-integration-of-the-findings-of-The-ARRIVE-Trial. Accessed November 25, 2018.
- Society for Maternal-Fetal Medicine (SMFM) Publications Committee. SMFM statement on elective induction of labor in low-risk nulliparous women at term: the ARRIVE trial. Am J Obstet Gynecol. doi:10.1016/j.ajog.2018.08.009. In press.
- Cahill AG, Srinivas SK, Tita AT, et al. Effect of immediate vs delayed pushing on rates of spontaneous vaginal delivery among nulliparous women receiving neuraxial analgesia: a randomized clinical trial. JAMA. 2018;320:1444-1454.
- American College of Obstetricians and Gynecologists. Practice advisory: immediate versus delayed pushing in nulliparous women receiving neuraxial analgesia. October 2018. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Practice-Advisory-Immediate-vs-delayed-pushing-in-nulliparous-women-receiving-neuraxial-analgesia. Accessed November 25, 2018.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 736. Optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150.
- American College of Obstetricians and Gynecologists. ACOG Postpartum toolkit. https://www.acog.org/About-ACOG/ACOG-Departments/Toolkits-for-Health-Care-Providers/Postpartum-Toolkit. Accessed November 25, 2018.
- Grobman WA, Rice MM, Reddy UM, et al; for the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379:513-523.
- American College of Obstetricians and Gynecologists. Practice advisory: clinical guidance for integration of the findings of the ARRIVE trial: Labor induction versus expectant management in low-risk nulliparous women. August 2018. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Practice-Advisory-Clinical-guidance-for-integration-of-the-findings-of-The-ARRIVE-Trial. Accessed November 25, 2018.
- Society for Maternal-Fetal Medicine (SMFM) Publications Committee. SMFM statement on elective induction of labor in low-risk nulliparous women at term: the ARRIVE trial. Am J Obstet Gynecol. doi:10.1016/j.ajog.2018.08.009. In press.
- Cahill AG, Srinivas SK, Tita AT, et al. Effect of immediate vs delayed pushing on rates of spontaneous vaginal delivery among nulliparous women receiving neuraxial analgesia: a randomized clinical trial. JAMA. 2018;320:1444-1454.
- American College of Obstetricians and Gynecologists. Practice advisory: immediate versus delayed pushing in nulliparous women receiving neuraxial analgesia. October 2018. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Practice-Advisory-Immediate-vs-delayed-pushing-in-nulliparous-women-receiving-neuraxial-analgesia. Accessed November 25, 2018.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 736. Optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150.
- American College of Obstetricians and Gynecologists. ACOG Postpartum toolkit. https://www.acog.org/About-ACOG/ACOG-Departments/Toolkits-for-Health-Care-Providers/Postpartum-Toolkit. Accessed November 25, 2018.
How does HT in recent and 10+ years past menopause affect atherosclerosis progression?
Expert Commentary
Sriprasert I, Hodis HN, Karim R, et al. Differential effect of plasma estradiol on subclinical atherosclerosis progression in early versus late postmenopause. J Clin Endocrinol Metab. 2019;104:293-300. doi:10.1210/jc.2018-01600.
In 2016, the primary findings of the Early versus Late Intervention Trial with Estradiol (ELITE) demonstrated that oral E2 administered to women who were less than 6 years postmenopause slowed progression of subclinical atherosclerosis as assessed by carotid artery intima-media thickness (CIMT), while it had no effect in women who were at least 10 years postmenopause.1
That trial included 643 healthy women without cardiovascular disease who at enrollment had a median age of 55.4 years in the early postmenopause group (median 3.5 years since menopause) and 63.6 years in the late postmenopause group (median 14.3 years since menopause). The study medications were oral estradiol 1 mg daily plus progesterone vaginal gel for women with a uterus or placebo and placebo gel for a median of 5 years.
The investigators found also that, in contrast with CIMT, cardiac computed tomography (CT) measures of atherosclerosis did not differ significantly between the estradiol and placebo groups, regardless of age.1
Posttrial data analysis revealed a new finding
In a secondary analysis of data from the ELITE trial, Sriprasert and colleagues dug deeper to assess the impact of plasma E2 levels on progression of subclinical atherosclerosis.2
Among 596 women (69.6% white non-Hispanic, 8.7% black, 13.3% Hispanic, and 8.4% Asian/Pacific Islander), E2 levels were available in 248 women in early postmenopause (mean age, 54.7 years) and 348 women in late postmenopause (median age, 63.6 years).
For women in the estradiol-treated group, mean E2 levels during the trial as well as change of E2 levels from baseline were significantly higher in the early postmenopause group than in the late postmenopause group, even though both groups had similar adherence based on pill count. For those in the placebo group, mean E2 levels and change of E2 levels from baseline were equivalent in early and late menopause.
In the E2-treated group and the placebo group combined, the mixed effects analysis of the CIMT progression rate (based on the mean E2 level during the trial) demonstrated that a higher level of E2 was inversely associated with the CIMT progression rate in early postmenopausal women (beta coefficient = -0.04 [95% confidence interval (CI), -0.09 to -0.001] μm CIMT per year per 1 pg/mL estradiol; P = .04). However, a higher level of E2 was positively associated (beta coefficient = 0.063 [95% CI, 0.018 to 0.107] μm CIMT per year per 1 pg/mL estradiol; P = .006) with CIMT progression rate in the late postmenopausal women.
Continue to: Bottom line...
Bottom line. E2 levels resulting from administration of oral estradiol were inversely associated with atherosclerosis progression in women in early menopause, but they were positively associated with progression in late postmenopause participants.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
These new findings from a posttrial analysis of ELITE data provide yet further support for the hormone therapy (HT) “timing hypothesis,” which postulates that HT slows atherosclerosis progression in recently menopausal women but has neutral or adverse effects in women who are at least a decade past menopause onset. As the authors suggest, the favorable vascular effects of E2 appear limited to those women (most often in early menopause) who have not yet developed atherosclerosis. Whether or not HT should be considered for cardioprotection remains unresolved (and controversial). By contrast, these data, along with findings from the Women’s Health Initiative,3 provide reassurance regarding the cardiovascular safety of HT when prescribed for recently menopausal women with bothersome vasomotor symptoms.
ANDREW M. KAUNITZ, MD
References
1. Hodis HN, Mack WJ, Henderson VW, et al; for the ELITE Research Group. Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med. 2016;374;1221-1231.
2. Sriprasert I, Hodis HN, Karim R, et al. Differential effect of plasma estradiol on subclinical atherosclerosis progression in early versus late postmenopause. J Clin Endocrinol Metab. 2019;104:293-300. doi:10.1210/jc.2018-01600.
3. Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310:1353-1368.
Expert Commentary
Sriprasert I, Hodis HN, Karim R, et al. Differential effect of plasma estradiol on subclinical atherosclerosis progression in early versus late postmenopause. J Clin Endocrinol Metab. 2019;104:293-300. doi:10.1210/jc.2018-01600.
In 2016, the primary findings of the Early versus Late Intervention Trial with Estradiol (ELITE) demonstrated that oral E2 administered to women who were less than 6 years postmenopause slowed progression of subclinical atherosclerosis as assessed by carotid artery intima-media thickness (CIMT), while it had no effect in women who were at least 10 years postmenopause.1
That trial included 643 healthy women without cardiovascular disease who at enrollment had a median age of 55.4 years in the early postmenopause group (median 3.5 years since menopause) and 63.6 years in the late postmenopause group (median 14.3 years since menopause). The study medications were oral estradiol 1 mg daily plus progesterone vaginal gel for women with a uterus or placebo and placebo gel for a median of 5 years.
The investigators found also that, in contrast with CIMT, cardiac computed tomography (CT) measures of atherosclerosis did not differ significantly between the estradiol and placebo groups, regardless of age.1
Posttrial data analysis revealed a new finding
In a secondary analysis of data from the ELITE trial, Sriprasert and colleagues dug deeper to assess the impact of plasma E2 levels on progression of subclinical atherosclerosis.2
Among 596 women (69.6% white non-Hispanic, 8.7% black, 13.3% Hispanic, and 8.4% Asian/Pacific Islander), E2 levels were available in 248 women in early postmenopause (mean age, 54.7 years) and 348 women in late postmenopause (median age, 63.6 years).
For women in the estradiol-treated group, mean E2 levels during the trial as well as change of E2 levels from baseline were significantly higher in the early postmenopause group than in the late postmenopause group, even though both groups had similar adherence based on pill count. For those in the placebo group, mean E2 levels and change of E2 levels from baseline were equivalent in early and late menopause.
In the E2-treated group and the placebo group combined, the mixed effects analysis of the CIMT progression rate (based on the mean E2 level during the trial) demonstrated that a higher level of E2 was inversely associated with the CIMT progression rate in early postmenopausal women (beta coefficient = -0.04 [95% confidence interval (CI), -0.09 to -0.001] μm CIMT per year per 1 pg/mL estradiol; P = .04). However, a higher level of E2 was positively associated (beta coefficient = 0.063 [95% CI, 0.018 to 0.107] μm CIMT per year per 1 pg/mL estradiol; P = .006) with CIMT progression rate in the late postmenopausal women.
Continue to: Bottom line...
Bottom line. E2 levels resulting from administration of oral estradiol were inversely associated with atherosclerosis progression in women in early menopause, but they were positively associated with progression in late postmenopause participants.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
These new findings from a posttrial analysis of ELITE data provide yet further support for the hormone therapy (HT) “timing hypothesis,” which postulates that HT slows atherosclerosis progression in recently menopausal women but has neutral or adverse effects in women who are at least a decade past menopause onset. As the authors suggest, the favorable vascular effects of E2 appear limited to those women (most often in early menopause) who have not yet developed atherosclerosis. Whether or not HT should be considered for cardioprotection remains unresolved (and controversial). By contrast, these data, along with findings from the Women’s Health Initiative,3 provide reassurance regarding the cardiovascular safety of HT when prescribed for recently menopausal women with bothersome vasomotor symptoms.
ANDREW M. KAUNITZ, MD
References
1. Hodis HN, Mack WJ, Henderson VW, et al; for the ELITE Research Group. Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med. 2016;374;1221-1231.
2. Sriprasert I, Hodis HN, Karim R, et al. Differential effect of plasma estradiol on subclinical atherosclerosis progression in early versus late postmenopause. J Clin Endocrinol Metab. 2019;104:293-300. doi:10.1210/jc.2018-01600.
3. Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310:1353-1368.
Expert Commentary
Sriprasert I, Hodis HN, Karim R, et al. Differential effect of plasma estradiol on subclinical atherosclerosis progression in early versus late postmenopause. J Clin Endocrinol Metab. 2019;104:293-300. doi:10.1210/jc.2018-01600.
In 2016, the primary findings of the Early versus Late Intervention Trial with Estradiol (ELITE) demonstrated that oral E2 administered to women who were less than 6 years postmenopause slowed progression of subclinical atherosclerosis as assessed by carotid artery intima-media thickness (CIMT), while it had no effect in women who were at least 10 years postmenopause.1
That trial included 643 healthy women without cardiovascular disease who at enrollment had a median age of 55.4 years in the early postmenopause group (median 3.5 years since menopause) and 63.6 years in the late postmenopause group (median 14.3 years since menopause). The study medications were oral estradiol 1 mg daily plus progesterone vaginal gel for women with a uterus or placebo and placebo gel for a median of 5 years.
The investigators found also that, in contrast with CIMT, cardiac computed tomography (CT) measures of atherosclerosis did not differ significantly between the estradiol and placebo groups, regardless of age.1
Posttrial data analysis revealed a new finding
In a secondary analysis of data from the ELITE trial, Sriprasert and colleagues dug deeper to assess the impact of plasma E2 levels on progression of subclinical atherosclerosis.2
Among 596 women (69.6% white non-Hispanic, 8.7% black, 13.3% Hispanic, and 8.4% Asian/Pacific Islander), E2 levels were available in 248 women in early postmenopause (mean age, 54.7 years) and 348 women in late postmenopause (median age, 63.6 years).
For women in the estradiol-treated group, mean E2 levels during the trial as well as change of E2 levels from baseline were significantly higher in the early postmenopause group than in the late postmenopause group, even though both groups had similar adherence based on pill count. For those in the placebo group, mean E2 levels and change of E2 levels from baseline were equivalent in early and late menopause.
In the E2-treated group and the placebo group combined, the mixed effects analysis of the CIMT progression rate (based on the mean E2 level during the trial) demonstrated that a higher level of E2 was inversely associated with the CIMT progression rate in early postmenopausal women (beta coefficient = -0.04 [95% confidence interval (CI), -0.09 to -0.001] μm CIMT per year per 1 pg/mL estradiol; P = .04). However, a higher level of E2 was positively associated (beta coefficient = 0.063 [95% CI, 0.018 to 0.107] μm CIMT per year per 1 pg/mL estradiol; P = .006) with CIMT progression rate in the late postmenopausal women.
Continue to: Bottom line...
Bottom line. E2 levels resulting from administration of oral estradiol were inversely associated with atherosclerosis progression in women in early menopause, but they were positively associated with progression in late postmenopause participants.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
These new findings from a posttrial analysis of ELITE data provide yet further support for the hormone therapy (HT) “timing hypothesis,” which postulates that HT slows atherosclerosis progression in recently menopausal women but has neutral or adverse effects in women who are at least a decade past menopause onset. As the authors suggest, the favorable vascular effects of E2 appear limited to those women (most often in early menopause) who have not yet developed atherosclerosis. Whether or not HT should be considered for cardioprotection remains unresolved (and controversial). By contrast, these data, along with findings from the Women’s Health Initiative,3 provide reassurance regarding the cardiovascular safety of HT when prescribed for recently menopausal women with bothersome vasomotor symptoms.
ANDREW M. KAUNITZ, MD
References
1. Hodis HN, Mack WJ, Henderson VW, et al; for the ELITE Research Group. Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med. 2016;374;1221-1231.
2. Sriprasert I, Hodis HN, Karim R, et al. Differential effect of plasma estradiol on subclinical atherosclerosis progression in early versus late postmenopause. J Clin Endocrinol Metab. 2019;104:293-300. doi:10.1210/jc.2018-01600.
3. Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310:1353-1368.
Should we abandon minimally invasive surgery for cervical cancer?
A minimally invasive approach for gynecologic surgery increasingly has become the surgical modality of choice (vs open surgery) due to decreased perioperative and postoperative morbidity for many gynecologic cancers.1-3 This has included radical hysterectomy for cervical cancers. Until recently, retrospective evidence supported its use, suggesting decreased perioperative and postoperative complications with similar survival outcomes between patients undergoing minimally invasive and open radical hysterectomy.4,5 In November 2018, two new studies were published in the New England Journal of Medicine, and another study was presented at the American Society of Clinical Oncology (ASCO) annual meeting challenging this practice paradigm. These studies reveal a higher risk of disease recurrence and decreased overall survival with minimally invasive surgery (MIS) compared with open surgery for Stages IA–IB1 cervical cancer. These findings have resulted in a change in practice nationwide.
RCT findings astonish specialty
The first study, the Laparoscopic Approach to Cervical Cancer (LACC) trial, authored by Ramirez and colleagues was a noninferiority randomized controlled trial evaluating MIS versus open radical hysterectomy for patients with cervical cancer (Stage 1A–1B1) conducted from 2008–2017.6 The primary outcome was disease-free survival at 4.5 years. Secondary outcomes included recurrence and overall survival rates. Power analysis suggested a sample size of 740 patients to provide greater than 80% power with a noninferiority margin of -7.2% between disease-free rates of the two groups. However, the study was closed prematurely at enrollment of 631 patients (85% recruitment) by the Data Safety Monitoring Committee due to the astounding differences in survival between the two groups.
The rate of disease-free survival at 4.5 years was 86.0% with MIS and 96% with open surgery. There were 27 recurrences (8.5%) in the MIS group and only 7 (2.2%) in the open-surgery group, accounting for a hazard ratio (HR) for disease recurrence or death from cervical cancer of 3.74 (95% confidence interval [CI], 1.63–8.58). This difference remained after adjusting for confounding variables. There were 22 deaths—19 (5.9%) in the MIS group and 3 (0.1%) in the open-surgery group (HR, 6.56). Although patient characteristics between groups appeared to be similar, more than one-third of patients in each group had missing data regarding histology at the time of surgery, grade, tumor size, lymphovascular space invasion, and depth of invasion. Interestingly, intraoperative, perioperative, and postoperative complications between the two groups were similar (with rates of 11%, about 40%, and about 25%, respectively).
Surprising findings continue in NEJM
The second study, by Melamed and colleagues, was a retrospective cohort study using data from the National Cancer Database (NCDB) and the Surveillance, Epidemiology, and End Results (SEER) database evaluating women with stage IA2 or IB1 cervical cancer who underwent either minimally invasive or open radical hysterectomy between 2010 and 2013.7 The primary outcome was time to death.
Participant characteristics. A total of 2,461 women were included: 49.8% underwent MIS and 50.2% underwent open surgery. According to the raw data, patients undergoing MIS were more likely to be white, privately insured, reside in an area associated with higher income, undergo surgery at a nonacademic institution, have adenocarcinoma, and have smaller, lower-grade tumors. After propensity-score weighting, demographic and clinical characteristics were similar between groups. Median follow-up was 45 months.
Results. A total of 164 deaths occurred: 94 in the MIS and 70 in the open-surgery group. The risk of death during study follow-up was 9.1% in the MIS group versus 5.3% in the open-surgery group, and women who underwent MIS had shorter overall survival (P = .002; HR, 1.65; 95% CI, 1.22–2.22). Mortality rates remained higher in the MIS group after adjusting for adjuvant therapy (HR, 1.62; 95% CI, 1.2–2.19). However, the HR for death with MIS was not statistically significant in a subgroup analysis evaluating tumors 2 cm in size or less (HR, 1.46; 95% CI, 0.70–3.02). The authors demonstrated that the adoption of MIS for radical hysterectomy corresponded to a drop in the 4-year survival rate of 0.8% per year (P = .01).
Continue to: ASCO meeting data emphasize lower...
ASCO meeting data emphasize lower mortality and survival rates for MIS
A third important, but less publicized study, is a retrospective cohort study by Marguland and colleagues that was presented at the ASCO annual meeting and is pending publication. This study evaluated the 5-year survival of women with stage IB1 cervical cancer after MIS or open radical hysterectomy from 2010 to 2013.8 The findings demonstrated similar results to the above studies with decreased 5-year survival rates in patients with a tumor size of 2 cm or greater in the MIS group (81.3% vs 90.8; HR, 2.14; 95% CI, 1.36–3.38; P<.001). These results hold true when controlling for confounding clinical variables. Interestingly, in a subset analysis evaluating patients with tumors less than 2 cm, survival rates were similar between groups. This study confirms decreased morbidity and cost associated with MIS radical hysterectomy.
A consistent message emerges from 3 independent studies
We must take the study findings seriously and evaluate the quality of the evidence. There are many strengths to the above studies. First and most importantly, the LACC study is the only prospective randomized controlled trial (RCT) to evaluate this very important clinical question. RCTs are the gold standard for understanding the effectiveness and safety of an intervention compared with an established treatment. The study was well designed in that the study population was clearly defined with detailed inclusion and exclusion criteria. The intention to treat analysis was similar to the per-protocol analysis, and the study followed Consolidated Standards of Reporting Trials (CONSORT) guidelines. While the study was stopped early, there was still 84% power for the primary outcome. Therefore, when it comes to MIS for cervical cancer, this study provides the soundest data we have available. It is also extremely noteworthy that two additional large retrospective studies evaluating this question separately found similar results.
Criticisms remain, but older research has drawbacks
A main concern with these studies is that the findings challenge previously published research, which overall suggest similar survival outcomes between MIS and open surgical approaches. However, in evaluating the previously published retrospective data it is clear that the studies have considerable limitations.
Long-term survival not always evaluated in research. First, the majority of studies comparing MIS and open treatment modalities specifically evaluated perioperative complications and did not consider long-term survival.4,9,10 Of those studies that did consider survival outcomes, the groups often were not balanced and were skewed toward the open surgery patients having larger tumors and higher-stage disease.5
Difficult to compare “apples to apples.” These findings are complicated by the fact that open radical hysterectomies were essentially replaced by MIS radical hysterectomies, and therefore, the comparisons are not equivalent since they are comparing different treatment times. For instance, throughout the time period many of these studies were conducted, the treatment paradigm for early-stage cervical cancer changed regarding who received adjuvant therapy and imaging techniques. Therefore, these studies are not comparing apples to apples.11,12
Are we going to increase morbidity? Another common concern when considering abandoning MIS for cervical cancer is the increase in morbidity that our patients may incur immediately postoperatively due to open surgery. Multiple studies have associated minimally invasive radical hysterectomies with decreased blood loss, shorter hospital stay, lower transfusion rates, and decreased time until return of bowel function.4,10,13
Continue to: While we recognize that...
While we recognize that open surgery is associated with increased morbidity, we do argue that, with the almost-universal implementation of Enhanced Recovery Pathways (ERP) in gynecologic oncology, the disparities between the two groups will be minimized and likely are much smaller than that reported in historical literature.14 Notably, there were no differences in peri-, intra-, or postoperative complications between the two groups in the LACC study, indicating that MIS may not be saving our patients as much morbidity as we think.
Surgical ability differences. Despite the vast strengths associated with the studies we have discussed they certainly embody limitations as well. First, surgical aptitude is difficult to evaluate and tease out. This is extremely pertinent given perioperative, and postoperative, outcomes in cervical cancer, as well as survival outcomes, in multiple surgically managed cancers, which are directly associated with the volume and proficiency of the surgeon.15-19 Additionally, the mode of minimally invasive surgery that was most commonly utilized was different from practice in the United States. Eighty four percent of the patients in the MIS group of the LACC study underwent laparoscopic and 13.6% underwent robot-assisted radical hysterectomy. This is starkly different from US practice, where 75% of gynecologic oncologists report performing radical hysterectomies only robotically.20
Take-home points
Consider this latest evidence in your surgical planning. Most importantly, the evidence is the evidence. In other words, we can attempt to explain away the findings, but despite arguments against these studies, these data are the most reliable evidence we have to date regarding outcomes for cervical cancer with MIS versus open approaches. These data demonstrate that MIS may be harming our patients and so we must take this into careful consideration during surgical planning.
For small cancers, MIS may be the best option. MIS radical hysterectomy may still be the best approach for patients with tumors less than 2 cm in size. The LACC study is not powered to evaluate oncologic outcomes in this subset of patients and the two retrospective studies suggest no difference in survival in this cohort.
We must work to understand the driving force between the disparate outcomes. Are the increased rates due to the open surgical approach, the uterine manipulator, circulating CO2 gas, or tumor exposure to the intraperitoneal cavity as the authors suggest? Or is it due to surgical expertise, tumor biology, tumor size, or mode of MIS? At this point the impelling cause is unknown.
New NCCN guidelines are to come. Up to this point the National Comprehensive Cancer Network (NCCN) guidelines stated that “radical hysterectomy procedure may be performed either via laparotomy or laparoscopy.” Given these recent studies, however, new NCCN guidelines will be released cautioning the use of the MIS approach. In short, these data have transformed the standard of care.
At our institution, the majority of radical hysterectomies will be performed open. Continued discussion remains regarding small lesions, but even in these cases most surgeons will proceed with open surgery in an attempt to maximize survival.
As providers, it is our duty to honestly reflect on published data and comprehensively counsel patients about the risks and benefits associated with each approach, including the fact that recurrence may be higher with a minimally invasive approach. Patients and providers must then collectively decide what is best for each individual case.
- Walker JL, Piedmonte MR, Spirtos NM, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol. 2009;27:5331-5336.
- Zanagnolo V, Minig L, Rollo D, et al. Clinical and oncologic outcomes of robotic versus abdominal radical hysterectomy for women with cervical cancer: experience at a referral cancer center. Int J Gynecol Cancer. 2016;26:568-574.
- Wallin E, Floter Radestad A, et al. Introduction of robot-assisted radical hysterectomy for early stage cervical cancer: impact on complications, costs and oncologic outcome. Acta Obstet Gynecol Scand. 2017;96:536-542.
- Sert BM, Boggess JF, Ahmad S, et al. Robot-assisted versus open radical hysterectomy: a multi-institutional experience for early-stage cervical cancer. Euro J Surg Oncol. 2016;42:513-522.
- Shah CA, Beck T, Liao JB, et al. Surgical and oncologic outcomes after robotic radical hysterectomy as compared to open radical hysterectomy in the treatment of early cervical cancer. J Gynecol Oncol. 2017;28:e82.
- Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895-1904.
- Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early stage cervical cancer. N Engl J Med. 2018;379:1905-1914.
- Margul DJ, Yang J, Seagle BL, et al. Outcomes and costs of open, robotic, and laparoscopic radical hysterectomy for stage IB1 cervical cancer. J Clin Oncol. 2018;36(15 suppl):5502.
- Geetha P, Nair MK. Laparoscopic, robotic and open method of radical hysterectomy for cervical cancer: a systematic review. J Minim Access Surg. 2012;8:67-73.
- Jin YM, Liu SS, Chen J, et al. Robotic radical hysterectomy is superior to laparoscopic radical hysterectomy and open radical hysterectomy in the treatment of cervical cancer. PloS One. 2018;13:e0193033.
- Rotman M, Sedlis A, Piedmonte MR, et al. A phase III randomized trial of postoperative pelvic irradiation in Stage IB cervical carcinoma with poor prognostic features: follow-up of a gynecologic oncology group study. Int J Radiation Oncol, Biol, Phys. 2006;65:169-176.
- Peters WA 3rd, Liu PY, Barrett RJ 2nd, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18:1606-1613.
- Uppal S, Liu RJ, Reynolds KR, et al. Trends and comparative effectiveness of inpatient radical hysterectomy for cervical cancer in the United States (2012-2015). Gynecol Oncol. 2018. pii: S0090-8258(18)31246-0.
- Barber EL, Van Le L. Enhanced Recovery Pathways in Gynecology and Gynecologic Oncology. Obstetr Gynecol Surv. 2015;70:780-792.
- Morche J, Mathes T, Pieper D. Relationship between surgeon volume and outcomes: a systematic review of systematic reviews. Syst Rev. 2016;5:204.
- Persson J, Reynisson P, Borgfeldt C, et al. Robot assisted laparoscopic radical hysterectomy and pelvic lymphadenectomy with short and long term morbidity data. Gynecol Oncol. 2009;113:185-190.
- Woelk JL, Casiano ER, Weaver AL, et al. The learning curve of robotic hysterectomy. Obstetr Gynecol. 2013;121:87-95.
- Yim GW, Kim SW, Nam EJ, et al. Learning curve analysis of robot-assisted radical hysterectomy for cervical cancer: initial experience at a single institution. J Gynecol Oncol. 2013;24:303-312.
- Vickers AJ, Bianco FJ, Serio AM, et al. The surgical learning curve for prostate cancer control after radical prostatectomy. J Natl Can Inst. 2007;99:1171-1177.
- Conrad LB, Ramirez PT, Burke W, et al. Role of minimally invasive surgery in gynecologic oncology: an updated survey of members of the Society of Gynecologic Oncology. Int J Gynecol Cancer. 2015;25:1121-1127.
A minimally invasive approach for gynecologic surgery increasingly has become the surgical modality of choice (vs open surgery) due to decreased perioperative and postoperative morbidity for many gynecologic cancers.1-3 This has included radical hysterectomy for cervical cancers. Until recently, retrospective evidence supported its use, suggesting decreased perioperative and postoperative complications with similar survival outcomes between patients undergoing minimally invasive and open radical hysterectomy.4,5 In November 2018, two new studies were published in the New England Journal of Medicine, and another study was presented at the American Society of Clinical Oncology (ASCO) annual meeting challenging this practice paradigm. These studies reveal a higher risk of disease recurrence and decreased overall survival with minimally invasive surgery (MIS) compared with open surgery for Stages IA–IB1 cervical cancer. These findings have resulted in a change in practice nationwide.
RCT findings astonish specialty
The first study, the Laparoscopic Approach to Cervical Cancer (LACC) trial, authored by Ramirez and colleagues was a noninferiority randomized controlled trial evaluating MIS versus open radical hysterectomy for patients with cervical cancer (Stage 1A–1B1) conducted from 2008–2017.6 The primary outcome was disease-free survival at 4.5 years. Secondary outcomes included recurrence and overall survival rates. Power analysis suggested a sample size of 740 patients to provide greater than 80% power with a noninferiority margin of -7.2% between disease-free rates of the two groups. However, the study was closed prematurely at enrollment of 631 patients (85% recruitment) by the Data Safety Monitoring Committee due to the astounding differences in survival between the two groups.
The rate of disease-free survival at 4.5 years was 86.0% with MIS and 96% with open surgery. There were 27 recurrences (8.5%) in the MIS group and only 7 (2.2%) in the open-surgery group, accounting for a hazard ratio (HR) for disease recurrence or death from cervical cancer of 3.74 (95% confidence interval [CI], 1.63–8.58). This difference remained after adjusting for confounding variables. There were 22 deaths—19 (5.9%) in the MIS group and 3 (0.1%) in the open-surgery group (HR, 6.56). Although patient characteristics between groups appeared to be similar, more than one-third of patients in each group had missing data regarding histology at the time of surgery, grade, tumor size, lymphovascular space invasion, and depth of invasion. Interestingly, intraoperative, perioperative, and postoperative complications between the two groups were similar (with rates of 11%, about 40%, and about 25%, respectively).
Surprising findings continue in NEJM
The second study, by Melamed and colleagues, was a retrospective cohort study using data from the National Cancer Database (NCDB) and the Surveillance, Epidemiology, and End Results (SEER) database evaluating women with stage IA2 or IB1 cervical cancer who underwent either minimally invasive or open radical hysterectomy between 2010 and 2013.7 The primary outcome was time to death.
Participant characteristics. A total of 2,461 women were included: 49.8% underwent MIS and 50.2% underwent open surgery. According to the raw data, patients undergoing MIS were more likely to be white, privately insured, reside in an area associated with higher income, undergo surgery at a nonacademic institution, have adenocarcinoma, and have smaller, lower-grade tumors. After propensity-score weighting, demographic and clinical characteristics were similar between groups. Median follow-up was 45 months.
Results. A total of 164 deaths occurred: 94 in the MIS and 70 in the open-surgery group. The risk of death during study follow-up was 9.1% in the MIS group versus 5.3% in the open-surgery group, and women who underwent MIS had shorter overall survival (P = .002; HR, 1.65; 95% CI, 1.22–2.22). Mortality rates remained higher in the MIS group after adjusting for adjuvant therapy (HR, 1.62; 95% CI, 1.2–2.19). However, the HR for death with MIS was not statistically significant in a subgroup analysis evaluating tumors 2 cm in size or less (HR, 1.46; 95% CI, 0.70–3.02). The authors demonstrated that the adoption of MIS for radical hysterectomy corresponded to a drop in the 4-year survival rate of 0.8% per year (P = .01).
Continue to: ASCO meeting data emphasize lower...
ASCO meeting data emphasize lower mortality and survival rates for MIS
A third important, but less publicized study, is a retrospective cohort study by Marguland and colleagues that was presented at the ASCO annual meeting and is pending publication. This study evaluated the 5-year survival of women with stage IB1 cervical cancer after MIS or open radical hysterectomy from 2010 to 2013.8 The findings demonstrated similar results to the above studies with decreased 5-year survival rates in patients with a tumor size of 2 cm or greater in the MIS group (81.3% vs 90.8; HR, 2.14; 95% CI, 1.36–3.38; P<.001). These results hold true when controlling for confounding clinical variables. Interestingly, in a subset analysis evaluating patients with tumors less than 2 cm, survival rates were similar between groups. This study confirms decreased morbidity and cost associated with MIS radical hysterectomy.
A consistent message emerges from 3 independent studies
We must take the study findings seriously and evaluate the quality of the evidence. There are many strengths to the above studies. First and most importantly, the LACC study is the only prospective randomized controlled trial (RCT) to evaluate this very important clinical question. RCTs are the gold standard for understanding the effectiveness and safety of an intervention compared with an established treatment. The study was well designed in that the study population was clearly defined with detailed inclusion and exclusion criteria. The intention to treat analysis was similar to the per-protocol analysis, and the study followed Consolidated Standards of Reporting Trials (CONSORT) guidelines. While the study was stopped early, there was still 84% power for the primary outcome. Therefore, when it comes to MIS for cervical cancer, this study provides the soundest data we have available. It is also extremely noteworthy that two additional large retrospective studies evaluating this question separately found similar results.
Criticisms remain, but older research has drawbacks
A main concern with these studies is that the findings challenge previously published research, which overall suggest similar survival outcomes between MIS and open surgical approaches. However, in evaluating the previously published retrospective data it is clear that the studies have considerable limitations.
Long-term survival not always evaluated in research. First, the majority of studies comparing MIS and open treatment modalities specifically evaluated perioperative complications and did not consider long-term survival.4,9,10 Of those studies that did consider survival outcomes, the groups often were not balanced and were skewed toward the open surgery patients having larger tumors and higher-stage disease.5
Difficult to compare “apples to apples.” These findings are complicated by the fact that open radical hysterectomies were essentially replaced by MIS radical hysterectomies, and therefore, the comparisons are not equivalent since they are comparing different treatment times. For instance, throughout the time period many of these studies were conducted, the treatment paradigm for early-stage cervical cancer changed regarding who received adjuvant therapy and imaging techniques. Therefore, these studies are not comparing apples to apples.11,12
Are we going to increase morbidity? Another common concern when considering abandoning MIS for cervical cancer is the increase in morbidity that our patients may incur immediately postoperatively due to open surgery. Multiple studies have associated minimally invasive radical hysterectomies with decreased blood loss, shorter hospital stay, lower transfusion rates, and decreased time until return of bowel function.4,10,13
Continue to: While we recognize that...
While we recognize that open surgery is associated with increased morbidity, we do argue that, with the almost-universal implementation of Enhanced Recovery Pathways (ERP) in gynecologic oncology, the disparities between the two groups will be minimized and likely are much smaller than that reported in historical literature.14 Notably, there were no differences in peri-, intra-, or postoperative complications between the two groups in the LACC study, indicating that MIS may not be saving our patients as much morbidity as we think.
Surgical ability differences. Despite the vast strengths associated with the studies we have discussed they certainly embody limitations as well. First, surgical aptitude is difficult to evaluate and tease out. This is extremely pertinent given perioperative, and postoperative, outcomes in cervical cancer, as well as survival outcomes, in multiple surgically managed cancers, which are directly associated with the volume and proficiency of the surgeon.15-19 Additionally, the mode of minimally invasive surgery that was most commonly utilized was different from practice in the United States. Eighty four percent of the patients in the MIS group of the LACC study underwent laparoscopic and 13.6% underwent robot-assisted radical hysterectomy. This is starkly different from US practice, where 75% of gynecologic oncologists report performing radical hysterectomies only robotically.20
Take-home points
Consider this latest evidence in your surgical planning. Most importantly, the evidence is the evidence. In other words, we can attempt to explain away the findings, but despite arguments against these studies, these data are the most reliable evidence we have to date regarding outcomes for cervical cancer with MIS versus open approaches. These data demonstrate that MIS may be harming our patients and so we must take this into careful consideration during surgical planning.
For small cancers, MIS may be the best option. MIS radical hysterectomy may still be the best approach for patients with tumors less than 2 cm in size. The LACC study is not powered to evaluate oncologic outcomes in this subset of patients and the two retrospective studies suggest no difference in survival in this cohort.
We must work to understand the driving force between the disparate outcomes. Are the increased rates due to the open surgical approach, the uterine manipulator, circulating CO2 gas, or tumor exposure to the intraperitoneal cavity as the authors suggest? Or is it due to surgical expertise, tumor biology, tumor size, or mode of MIS? At this point the impelling cause is unknown.
New NCCN guidelines are to come. Up to this point the National Comprehensive Cancer Network (NCCN) guidelines stated that “radical hysterectomy procedure may be performed either via laparotomy or laparoscopy.” Given these recent studies, however, new NCCN guidelines will be released cautioning the use of the MIS approach. In short, these data have transformed the standard of care.
At our institution, the majority of radical hysterectomies will be performed open. Continued discussion remains regarding small lesions, but even in these cases most surgeons will proceed with open surgery in an attempt to maximize survival.
As providers, it is our duty to honestly reflect on published data and comprehensively counsel patients about the risks and benefits associated with each approach, including the fact that recurrence may be higher with a minimally invasive approach. Patients and providers must then collectively decide what is best for each individual case.
A minimally invasive approach for gynecologic surgery increasingly has become the surgical modality of choice (vs open surgery) due to decreased perioperative and postoperative morbidity for many gynecologic cancers.1-3 This has included radical hysterectomy for cervical cancers. Until recently, retrospective evidence supported its use, suggesting decreased perioperative and postoperative complications with similar survival outcomes between patients undergoing minimally invasive and open radical hysterectomy.4,5 In November 2018, two new studies were published in the New England Journal of Medicine, and another study was presented at the American Society of Clinical Oncology (ASCO) annual meeting challenging this practice paradigm. These studies reveal a higher risk of disease recurrence and decreased overall survival with minimally invasive surgery (MIS) compared with open surgery for Stages IA–IB1 cervical cancer. These findings have resulted in a change in practice nationwide.
RCT findings astonish specialty
The first study, the Laparoscopic Approach to Cervical Cancer (LACC) trial, authored by Ramirez and colleagues was a noninferiority randomized controlled trial evaluating MIS versus open radical hysterectomy for patients with cervical cancer (Stage 1A–1B1) conducted from 2008–2017.6 The primary outcome was disease-free survival at 4.5 years. Secondary outcomes included recurrence and overall survival rates. Power analysis suggested a sample size of 740 patients to provide greater than 80% power with a noninferiority margin of -7.2% between disease-free rates of the two groups. However, the study was closed prematurely at enrollment of 631 patients (85% recruitment) by the Data Safety Monitoring Committee due to the astounding differences in survival between the two groups.
The rate of disease-free survival at 4.5 years was 86.0% with MIS and 96% with open surgery. There were 27 recurrences (8.5%) in the MIS group and only 7 (2.2%) in the open-surgery group, accounting for a hazard ratio (HR) for disease recurrence or death from cervical cancer of 3.74 (95% confidence interval [CI], 1.63–8.58). This difference remained after adjusting for confounding variables. There were 22 deaths—19 (5.9%) in the MIS group and 3 (0.1%) in the open-surgery group (HR, 6.56). Although patient characteristics between groups appeared to be similar, more than one-third of patients in each group had missing data regarding histology at the time of surgery, grade, tumor size, lymphovascular space invasion, and depth of invasion. Interestingly, intraoperative, perioperative, and postoperative complications between the two groups were similar (with rates of 11%, about 40%, and about 25%, respectively).
Surprising findings continue in NEJM
The second study, by Melamed and colleagues, was a retrospective cohort study using data from the National Cancer Database (NCDB) and the Surveillance, Epidemiology, and End Results (SEER) database evaluating women with stage IA2 or IB1 cervical cancer who underwent either minimally invasive or open radical hysterectomy between 2010 and 2013.7 The primary outcome was time to death.
Participant characteristics. A total of 2,461 women were included: 49.8% underwent MIS and 50.2% underwent open surgery. According to the raw data, patients undergoing MIS were more likely to be white, privately insured, reside in an area associated with higher income, undergo surgery at a nonacademic institution, have adenocarcinoma, and have smaller, lower-grade tumors. After propensity-score weighting, demographic and clinical characteristics were similar between groups. Median follow-up was 45 months.
Results. A total of 164 deaths occurred: 94 in the MIS and 70 in the open-surgery group. The risk of death during study follow-up was 9.1% in the MIS group versus 5.3% in the open-surgery group, and women who underwent MIS had shorter overall survival (P = .002; HR, 1.65; 95% CI, 1.22–2.22). Mortality rates remained higher in the MIS group after adjusting for adjuvant therapy (HR, 1.62; 95% CI, 1.2–2.19). However, the HR for death with MIS was not statistically significant in a subgroup analysis evaluating tumors 2 cm in size or less (HR, 1.46; 95% CI, 0.70–3.02). The authors demonstrated that the adoption of MIS for radical hysterectomy corresponded to a drop in the 4-year survival rate of 0.8% per year (P = .01).
Continue to: ASCO meeting data emphasize lower...
ASCO meeting data emphasize lower mortality and survival rates for MIS
A third important, but less publicized study, is a retrospective cohort study by Marguland and colleagues that was presented at the ASCO annual meeting and is pending publication. This study evaluated the 5-year survival of women with stage IB1 cervical cancer after MIS or open radical hysterectomy from 2010 to 2013.8 The findings demonstrated similar results to the above studies with decreased 5-year survival rates in patients with a tumor size of 2 cm or greater in the MIS group (81.3% vs 90.8; HR, 2.14; 95% CI, 1.36–3.38; P<.001). These results hold true when controlling for confounding clinical variables. Interestingly, in a subset analysis evaluating patients with tumors less than 2 cm, survival rates were similar between groups. This study confirms decreased morbidity and cost associated with MIS radical hysterectomy.
A consistent message emerges from 3 independent studies
We must take the study findings seriously and evaluate the quality of the evidence. There are many strengths to the above studies. First and most importantly, the LACC study is the only prospective randomized controlled trial (RCT) to evaluate this very important clinical question. RCTs are the gold standard for understanding the effectiveness and safety of an intervention compared with an established treatment. The study was well designed in that the study population was clearly defined with detailed inclusion and exclusion criteria. The intention to treat analysis was similar to the per-protocol analysis, and the study followed Consolidated Standards of Reporting Trials (CONSORT) guidelines. While the study was stopped early, there was still 84% power for the primary outcome. Therefore, when it comes to MIS for cervical cancer, this study provides the soundest data we have available. It is also extremely noteworthy that two additional large retrospective studies evaluating this question separately found similar results.
Criticisms remain, but older research has drawbacks
A main concern with these studies is that the findings challenge previously published research, which overall suggest similar survival outcomes between MIS and open surgical approaches. However, in evaluating the previously published retrospective data it is clear that the studies have considerable limitations.
Long-term survival not always evaluated in research. First, the majority of studies comparing MIS and open treatment modalities specifically evaluated perioperative complications and did not consider long-term survival.4,9,10 Of those studies that did consider survival outcomes, the groups often were not balanced and were skewed toward the open surgery patients having larger tumors and higher-stage disease.5
Difficult to compare “apples to apples.” These findings are complicated by the fact that open radical hysterectomies were essentially replaced by MIS radical hysterectomies, and therefore, the comparisons are not equivalent since they are comparing different treatment times. For instance, throughout the time period many of these studies were conducted, the treatment paradigm for early-stage cervical cancer changed regarding who received adjuvant therapy and imaging techniques. Therefore, these studies are not comparing apples to apples.11,12
Are we going to increase morbidity? Another common concern when considering abandoning MIS for cervical cancer is the increase in morbidity that our patients may incur immediately postoperatively due to open surgery. Multiple studies have associated minimally invasive radical hysterectomies with decreased blood loss, shorter hospital stay, lower transfusion rates, and decreased time until return of bowel function.4,10,13
Continue to: While we recognize that...
While we recognize that open surgery is associated with increased morbidity, we do argue that, with the almost-universal implementation of Enhanced Recovery Pathways (ERP) in gynecologic oncology, the disparities between the two groups will be minimized and likely are much smaller than that reported in historical literature.14 Notably, there were no differences in peri-, intra-, or postoperative complications between the two groups in the LACC study, indicating that MIS may not be saving our patients as much morbidity as we think.
Surgical ability differences. Despite the vast strengths associated with the studies we have discussed they certainly embody limitations as well. First, surgical aptitude is difficult to evaluate and tease out. This is extremely pertinent given perioperative, and postoperative, outcomes in cervical cancer, as well as survival outcomes, in multiple surgically managed cancers, which are directly associated with the volume and proficiency of the surgeon.15-19 Additionally, the mode of minimally invasive surgery that was most commonly utilized was different from practice in the United States. Eighty four percent of the patients in the MIS group of the LACC study underwent laparoscopic and 13.6% underwent robot-assisted radical hysterectomy. This is starkly different from US practice, where 75% of gynecologic oncologists report performing radical hysterectomies only robotically.20
Take-home points
Consider this latest evidence in your surgical planning. Most importantly, the evidence is the evidence. In other words, we can attempt to explain away the findings, but despite arguments against these studies, these data are the most reliable evidence we have to date regarding outcomes for cervical cancer with MIS versus open approaches. These data demonstrate that MIS may be harming our patients and so we must take this into careful consideration during surgical planning.
For small cancers, MIS may be the best option. MIS radical hysterectomy may still be the best approach for patients with tumors less than 2 cm in size. The LACC study is not powered to evaluate oncologic outcomes in this subset of patients and the two retrospective studies suggest no difference in survival in this cohort.
We must work to understand the driving force between the disparate outcomes. Are the increased rates due to the open surgical approach, the uterine manipulator, circulating CO2 gas, or tumor exposure to the intraperitoneal cavity as the authors suggest? Or is it due to surgical expertise, tumor biology, tumor size, or mode of MIS? At this point the impelling cause is unknown.
New NCCN guidelines are to come. Up to this point the National Comprehensive Cancer Network (NCCN) guidelines stated that “radical hysterectomy procedure may be performed either via laparotomy or laparoscopy.” Given these recent studies, however, new NCCN guidelines will be released cautioning the use of the MIS approach. In short, these data have transformed the standard of care.
At our institution, the majority of radical hysterectomies will be performed open. Continued discussion remains regarding small lesions, but even in these cases most surgeons will proceed with open surgery in an attempt to maximize survival.
As providers, it is our duty to honestly reflect on published data and comprehensively counsel patients about the risks and benefits associated with each approach, including the fact that recurrence may be higher with a minimally invasive approach. Patients and providers must then collectively decide what is best for each individual case.
- Walker JL, Piedmonte MR, Spirtos NM, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol. 2009;27:5331-5336.
- Zanagnolo V, Minig L, Rollo D, et al. Clinical and oncologic outcomes of robotic versus abdominal radical hysterectomy for women with cervical cancer: experience at a referral cancer center. Int J Gynecol Cancer. 2016;26:568-574.
- Wallin E, Floter Radestad A, et al. Introduction of robot-assisted radical hysterectomy for early stage cervical cancer: impact on complications, costs and oncologic outcome. Acta Obstet Gynecol Scand. 2017;96:536-542.
- Sert BM, Boggess JF, Ahmad S, et al. Robot-assisted versus open radical hysterectomy: a multi-institutional experience for early-stage cervical cancer. Euro J Surg Oncol. 2016;42:513-522.
- Shah CA, Beck T, Liao JB, et al. Surgical and oncologic outcomes after robotic radical hysterectomy as compared to open radical hysterectomy in the treatment of early cervical cancer. J Gynecol Oncol. 2017;28:e82.
- Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895-1904.
- Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early stage cervical cancer. N Engl J Med. 2018;379:1905-1914.
- Margul DJ, Yang J, Seagle BL, et al. Outcomes and costs of open, robotic, and laparoscopic radical hysterectomy for stage IB1 cervical cancer. J Clin Oncol. 2018;36(15 suppl):5502.
- Geetha P, Nair MK. Laparoscopic, robotic and open method of radical hysterectomy for cervical cancer: a systematic review. J Minim Access Surg. 2012;8:67-73.
- Jin YM, Liu SS, Chen J, et al. Robotic radical hysterectomy is superior to laparoscopic radical hysterectomy and open radical hysterectomy in the treatment of cervical cancer. PloS One. 2018;13:e0193033.
- Rotman M, Sedlis A, Piedmonte MR, et al. A phase III randomized trial of postoperative pelvic irradiation in Stage IB cervical carcinoma with poor prognostic features: follow-up of a gynecologic oncology group study. Int J Radiation Oncol, Biol, Phys. 2006;65:169-176.
- Peters WA 3rd, Liu PY, Barrett RJ 2nd, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18:1606-1613.
- Uppal S, Liu RJ, Reynolds KR, et al. Trends and comparative effectiveness of inpatient radical hysterectomy for cervical cancer in the United States (2012-2015). Gynecol Oncol. 2018. pii: S0090-8258(18)31246-0.
- Barber EL, Van Le L. Enhanced Recovery Pathways in Gynecology and Gynecologic Oncology. Obstetr Gynecol Surv. 2015;70:780-792.
- Morche J, Mathes T, Pieper D. Relationship between surgeon volume and outcomes: a systematic review of systematic reviews. Syst Rev. 2016;5:204.
- Persson J, Reynisson P, Borgfeldt C, et al. Robot assisted laparoscopic radical hysterectomy and pelvic lymphadenectomy with short and long term morbidity data. Gynecol Oncol. 2009;113:185-190.
- Woelk JL, Casiano ER, Weaver AL, et al. The learning curve of robotic hysterectomy. Obstetr Gynecol. 2013;121:87-95.
- Yim GW, Kim SW, Nam EJ, et al. Learning curve analysis of robot-assisted radical hysterectomy for cervical cancer: initial experience at a single institution. J Gynecol Oncol. 2013;24:303-312.
- Vickers AJ, Bianco FJ, Serio AM, et al. The surgical learning curve for prostate cancer control after radical prostatectomy. J Natl Can Inst. 2007;99:1171-1177.
- Conrad LB, Ramirez PT, Burke W, et al. Role of minimally invasive surgery in gynecologic oncology: an updated survey of members of the Society of Gynecologic Oncology. Int J Gynecol Cancer. 2015;25:1121-1127.
- Walker JL, Piedmonte MR, Spirtos NM, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol. 2009;27:5331-5336.
- Zanagnolo V, Minig L, Rollo D, et al. Clinical and oncologic outcomes of robotic versus abdominal radical hysterectomy for women with cervical cancer: experience at a referral cancer center. Int J Gynecol Cancer. 2016;26:568-574.
- Wallin E, Floter Radestad A, et al. Introduction of robot-assisted radical hysterectomy for early stage cervical cancer: impact on complications, costs and oncologic outcome. Acta Obstet Gynecol Scand. 2017;96:536-542.
- Sert BM, Boggess JF, Ahmad S, et al. Robot-assisted versus open radical hysterectomy: a multi-institutional experience for early-stage cervical cancer. Euro J Surg Oncol. 2016;42:513-522.
- Shah CA, Beck T, Liao JB, et al. Surgical and oncologic outcomes after robotic radical hysterectomy as compared to open radical hysterectomy in the treatment of early cervical cancer. J Gynecol Oncol. 2017;28:e82.
- Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895-1904.
- Melamed A, Margul DJ, Chen L, et al. Survival after minimally invasive radical hysterectomy for early stage cervical cancer. N Engl J Med. 2018;379:1905-1914.
- Margul DJ, Yang J, Seagle BL, et al. Outcomes and costs of open, robotic, and laparoscopic radical hysterectomy for stage IB1 cervical cancer. J Clin Oncol. 2018;36(15 suppl):5502.
- Geetha P, Nair MK. Laparoscopic, robotic and open method of radical hysterectomy for cervical cancer: a systematic review. J Minim Access Surg. 2012;8:67-73.
- Jin YM, Liu SS, Chen J, et al. Robotic radical hysterectomy is superior to laparoscopic radical hysterectomy and open radical hysterectomy in the treatment of cervical cancer. PloS One. 2018;13:e0193033.
- Rotman M, Sedlis A, Piedmonte MR, et al. A phase III randomized trial of postoperative pelvic irradiation in Stage IB cervical carcinoma with poor prognostic features: follow-up of a gynecologic oncology group study. Int J Radiation Oncol, Biol, Phys. 2006;65:169-176.
- Peters WA 3rd, Liu PY, Barrett RJ 2nd, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18:1606-1613.
- Uppal S, Liu RJ, Reynolds KR, et al. Trends and comparative effectiveness of inpatient radical hysterectomy for cervical cancer in the United States (2012-2015). Gynecol Oncol. 2018. pii: S0090-8258(18)31246-0.
- Barber EL, Van Le L. Enhanced Recovery Pathways in Gynecology and Gynecologic Oncology. Obstetr Gynecol Surv. 2015;70:780-792.
- Morche J, Mathes T, Pieper D. Relationship between surgeon volume and outcomes: a systematic review of systematic reviews. Syst Rev. 2016;5:204.
- Persson J, Reynisson P, Borgfeldt C, et al. Robot assisted laparoscopic radical hysterectomy and pelvic lymphadenectomy with short and long term morbidity data. Gynecol Oncol. 2009;113:185-190.
- Woelk JL, Casiano ER, Weaver AL, et al. The learning curve of robotic hysterectomy. Obstetr Gynecol. 2013;121:87-95.
- Yim GW, Kim SW, Nam EJ, et al. Learning curve analysis of robot-assisted radical hysterectomy for cervical cancer: initial experience at a single institution. J Gynecol Oncol. 2013;24:303-312.
- Vickers AJ, Bianco FJ, Serio AM, et al. The surgical learning curve for prostate cancer control after radical prostatectomy. J Natl Can Inst. 2007;99:1171-1177.
- Conrad LB, Ramirez PT, Burke W, et al. Role of minimally invasive surgery in gynecologic oncology: an updated survey of members of the Society of Gynecologic Oncology. Int J Gynecol Cancer. 2015;25:1121-1127.