User login
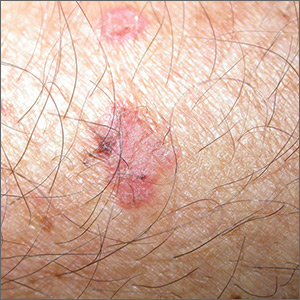
Scaling lesions on arm
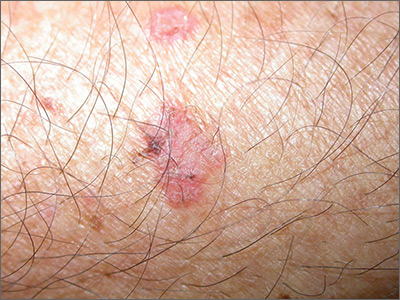
The FP also was concerned about a possible skin cancer, especially for the larger of the 2 lesions. The FP recommended 2 shave biopsies, which the patient agreed to. (See the Watch & Learn video on “Shave biopsy.”) The patient was directed to apply petrolatum once or twice daily to the biopsy sites and to cover them with dressings for the next 1 to 2 weeks. On the 2-week follow-up, the physician diagnosed the larger lesion as squamous cell carcinoma in situ (Bowen disease) and the top lesion as actinic keratosis.
The physician explained that the actinic keratosis did not need further treatment; however, the options for treating Bowen disease included cryosurgery, electrodesiccation and curettage, or elliptical excision. The patient chose an elliptical excision, which was performed without complications at the following visit.
The margins were clear and the surgery site healed without any problems. The patient said that he planned to wear long sleeves more often and use sunscreen when his arms were exposed to the sun.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R, Wah Y. Actinic keratosis and Bowen disease. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:969-976.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.

The FP also was concerned about a possible skin cancer, especially for the larger of the 2 lesions. The FP recommended 2 shave biopsies, which the patient agreed to. (See the Watch & Learn video on “Shave biopsy.”) The patient was directed to apply petrolatum once or twice daily to the biopsy sites and to cover them with dressings for the next 1 to 2 weeks. On the 2-week follow-up, the physician diagnosed the larger lesion as squamous cell carcinoma in situ (Bowen disease) and the top lesion as actinic keratosis.
The physician explained that the actinic keratosis did not need further treatment; however, the options for treating Bowen disease included cryosurgery, electrodesiccation and curettage, or elliptical excision. The patient chose an elliptical excision, which was performed without complications at the following visit.
The margins were clear and the surgery site healed without any problems. The patient said that he planned to wear long sleeves more often and use sunscreen when his arms were exposed to the sun.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R, Wah Y. Actinic keratosis and Bowen disease. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:969-976.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.

The FP also was concerned about a possible skin cancer, especially for the larger of the 2 lesions. The FP recommended 2 shave biopsies, which the patient agreed to. (See the Watch & Learn video on “Shave biopsy.”) The patient was directed to apply petrolatum once or twice daily to the biopsy sites and to cover them with dressings for the next 1 to 2 weeks. On the 2-week follow-up, the physician diagnosed the larger lesion as squamous cell carcinoma in situ (Bowen disease) and the top lesion as actinic keratosis.
The physician explained that the actinic keratosis did not need further treatment; however, the options for treating Bowen disease included cryosurgery, electrodesiccation and curettage, or elliptical excision. The patient chose an elliptical excision, which was performed without complications at the following visit.
The margins were clear and the surgery site healed without any problems. The patient said that he planned to wear long sleeves more often and use sunscreen when his arms were exposed to the sun.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R, Wah Y. Actinic keratosis and Bowen disease. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:969-976.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.
FAST and RAPID: Acronyms to prevent brain damage in stroke and psychosis
Psychosis and stroke are arguably the most serious acute threats to the integrity of the brain, and consequently the mind. Both are unquestionably associated with a grave outcome the longer their treatment is delayed.1,2
While the management of stroke has been elevated to the highest emergent priority because of the progressively deleterious impact of thrombotic ischemia in one of the cerebral arteries, rapid intervention for acute psychosis has never been regarded as an urgent neurologic condition with severe threats to the brain’s structure and function.3,4 There is extensive literature on the serious consequences of a long duration of untreated psychosis (DUP), including treatment resistance, frequent re-hospitalizations, more negative symptoms, and greater disability.5
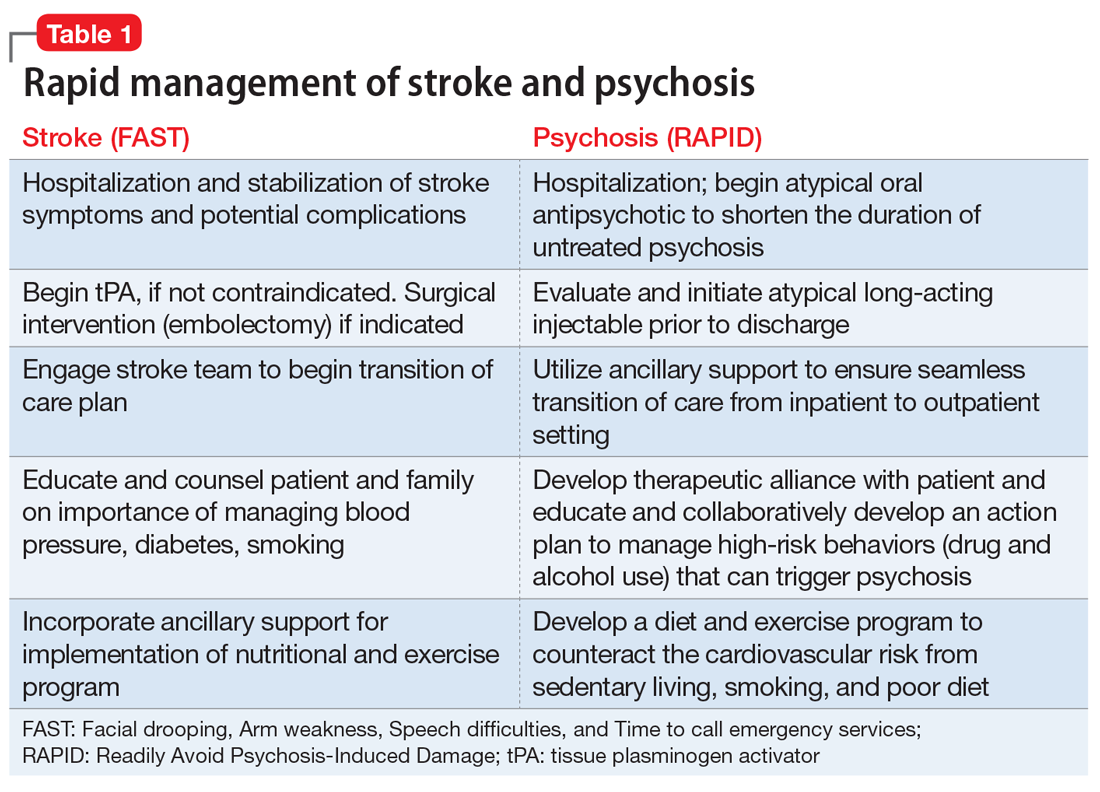
Physical paralysis from a stroke receives much more attention than “mental paralysis” of psychosis. Both must be rapidly treated, whether for the regional ischemia to brain tissue following a stroke or for the neurotoxicity of neuroinflammation and oxidative stress that lead to widespread neurodegeneration during psychosis.6 While an acronym for the quick recognition of a stroke (FAST: Facial drooping, Arm weakness, Speech difficulties, and Time to call emergency services) is well established, no acronym for the urgency to treat psychosis has been developed. We propose the acronym RAPID (Readily Avoid Psychosis-Induced Damage). The acronym RAPID would hopefully expedite the urgently needed pharmacotherapeutic and psychosocial intervention in psychosis to halt ongoing brain tissue loss.
It is ironic that the legal obstacles for immediate treatment, which do not exist for stroke, often delay administering antipsychotic medication to patients with anosognosia (a neurologic delusional belief that one is not ill, leading to refusal of treatment) for their psychosis and end up harming patients by prolonging their DUP until a court order is obtained to force brain-saving treatment. Frequent psychotic relapses due to nonadherence with medications are also a very common cause for prolonged DUP due to the inexplicable reluctance of some psychiatric practitioners to employ a long-acting injectable antipsychotic (LAI) medication as soon as possible after the onset of psychosis to circumvent subsequent relapses due to the very high risk of poor adherence. Table 1 describes the optimal management of both stroke and acute psychosis once they are rapidly diagnosed, thanks to the FAST and RAPID reminders.
Tragically, the treatments of the mind have become falsely disengaged from the brain, the physical organ whose neurons, electrical impulses, synapses, and neurotransmitters generate the mind with its advanced human functions, such as self-awareness, will, thoughts, mood, speech, executive functions, memories, and social cognition. Recent editorials have challenged psychiatric practitioners to behave like cardiologists7 and oncologists8 by aggressively treating first-episode psychosis to prevent ongoing neurodegeneration due to recurrences. The brain loses 1% of its brain volume (~11 ml) after the first psychotic episode,8 which represents hundreds of millions of cells, billions of synapses, and substantial myelin. A second psychotic episode causes significant additional neuropil and white matter fiber damage and represents a different stage of schizophrenia9 with more severe tissue loss and disruption of neural pathways that trigger the process of treatment resistance and functional disability. Ensuring adherence with LAI antipsychotic formulations immediately after the first psychotic episode may allow many patients with schizophrenia to achieve a relapse-free remission and to return to their baseline functioning.10
In addition to significant brain tissue loss during psychotic episodes, mortality is also a very high risk following discharge from the first hospitalization for psychosis.11 LAI second-generation antipsychotic medications have been shown to be associated with lower mortality and neuroprotective effects,12 compared with oral or injectable first-generation antipsychotics. The highest mortality rate was reported to be associated with the lack of any antipsychotic medication,12 underscoring how untreated psychosis can be fatal.
Bottom line: Rapid treatment of stroke and psychosis is an absolute imperative for minimizing brain damage that respectively leads to physical or mental disability. The acronyms FAST and RAPID are essential reminders of the urgency needed to halt progressive neurodegeneration in those 2 devastating acute threats to the integrity of brain and mind. Intensive physical, psychological, and social rehabilitation must follow the acute treatment of stroke and psychosis, and the prevention of any recurrence is an absolute must. For psychosis, the use of a LAI second-generation antipsychotic before hospital discharge from a first episode of psychosis can be disease-modifying, with a more benign illness trajectory and outcome than the devastating deterioration that follows repetitive psychotic relapse, most often due to nonadherence with oral medications.
Continue to: Psychosis should be conceptualized as...
Psychosis should be conceptualized as a “stroke of the mind,” and it can be prevented in most patients with schizophrenia by adopting injectable antipsychotics as early after the onset of psychosis as possible. Yet, starting a LAI antipsychotic drug in first-episode psychosis before hospital discharge is rarely done, and the few patients who currently receive LAIs (10% of U.S. patients) generally receive them after multiple episodes and a protracted DUP. That’s like calling the fire department when much of the house has turned to ashes, instead of calling them when the first small flame is noticed. It makes so much sense, but the decades-old practice of postponing the use of LAIs continues to ruin the lives of young persons in the prime of life. By changing our practice habits to early use of LAIs, we have nothing to lose and our patients with psychosis may be spared a lifetime of suffering, poverty, stigma, incarceration, and functional disability. Wouldn’t we want to avoid that atrocious outcome for our own family members if they develop schizophrenia?
1. Benjamin EJ, Virani SS, Callaway CW, et al; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2018 Update: a report from the American Heart Association. Circulation. 2018;137(12):e67-e492.
2. Cechnicki A, Cichocki Ł, Kalisz A, et al. Duration of untreated psychosis (DUP) and the course of schizophrenia in a 20-year follow-up study. Psychiatry Res. 2014;219(3):420-425.
3. Davis J, Moylan S, Harvey BH, et al. Neuroprogression in schizophrenia: pathways underpinning clinical staging and therapeutic corollaries. Aust N Z J Psychiatry. 2014;48(6):512-529.
4. Olabi B, Ellison-Wright I, McIntosh AM, et al. Are there progressive brain changes in schizophrenia? A meta-analysis of structural magnetic resonance imaging studies. Biol Psychiatry. 2011;70(1):88-96.
5. Kane JM, Robinson DG, Schooler NR, et al. Comprehensive versus usual community care for first-episode psychosis: 2-year outcomes from the NIMH RAISE Early Treatment Program. Am J Psychiatry. 2016;173(4):362-372.
6. Najjar S, Pearlman DM. Neuroinflammation and white matter pathology in schizophrenia: systematic review. Schizophr Res. 2015;161(1):102-112.
7. Nasrallah HA. For first-episode psychosis, psychiatrists should behave like cardiologists. Current Psychiatry. 2017;16(8):4-7.
8. Cahn W, Hulshoff Pol HE, Lems EB, et al. Brain volume changes in first-episode schizophrenia: a 1-year follow-up study. Arch Gen Psychiatry. 2002;59(11):1002-1010.
9. McGorry P, Nelson B. Why we need a transdiagnostic staging approach to emerging psychopathology, early diagnosis, and treatment. JAMA Psychiatry. 2016;73(3):191-192.
10. Emsley R, Oosthuizen P, Koen L, et al. Remission in patients with first-episode schizophrenia receiving assured antipsychotic medication: a study with risperidone long-acting injection. Int Clin Psychopharmacol. 2008;23(6):325-331.
11. Nasrallah HA. The crisis of poor physical health and early mortality of psychiatric patients. Current Psychiatry. 2018;17(4):7-8,11.
12. Nasrallah HA. Triple advantages of injectable long acting second generation antipsychotics: relapse prevention, neuroprotection, and lower mortality. Schizophr Res. 2018;197:69-70.
Psychosis and stroke are arguably the most serious acute threats to the integrity of the brain, and consequently the mind. Both are unquestionably associated with a grave outcome the longer their treatment is delayed.1,2
While the management of stroke has been elevated to the highest emergent priority because of the progressively deleterious impact of thrombotic ischemia in one of the cerebral arteries, rapid intervention for acute psychosis has never been regarded as an urgent neurologic condition with severe threats to the brain’s structure and function.3,4 There is extensive literature on the serious consequences of a long duration of untreated psychosis (DUP), including treatment resistance, frequent re-hospitalizations, more negative symptoms, and greater disability.5
Physical paralysis from a stroke receives much more attention than “mental paralysis” of psychosis. Both must be rapidly treated, whether for the regional ischemia to brain tissue following a stroke or for the neurotoxicity of neuroinflammation and oxidative stress that lead to widespread neurodegeneration during psychosis.6 While an acronym for the quick recognition of a stroke (FAST: Facial drooping, Arm weakness, Speech difficulties, and Time to call emergency services) is well established, no acronym for the urgency to treat psychosis has been developed. We propose the acronym RAPID (Readily Avoid Psychosis-Induced Damage). The acronym RAPID would hopefully expedite the urgently needed pharmacotherapeutic and psychosocial intervention in psychosis to halt ongoing brain tissue loss.
It is ironic that the legal obstacles for immediate treatment, which do not exist for stroke, often delay administering antipsychotic medication to patients with anosognosia (a neurologic delusional belief that one is not ill, leading to refusal of treatment) for their psychosis and end up harming patients by prolonging their DUP until a court order is obtained to force brain-saving treatment. Frequent psychotic relapses due to nonadherence with medications are also a very common cause for prolonged DUP due to the inexplicable reluctance of some psychiatric practitioners to employ a long-acting injectable antipsychotic (LAI) medication as soon as possible after the onset of psychosis to circumvent subsequent relapses due to the very high risk of poor adherence. Table 1 describes the optimal management of both stroke and acute psychosis once they are rapidly diagnosed, thanks to the FAST and RAPID reminders.

Tragically, the treatments of the mind have become falsely disengaged from the brain, the physical organ whose neurons, electrical impulses, synapses, and neurotransmitters generate the mind with its advanced human functions, such as self-awareness, will, thoughts, mood, speech, executive functions, memories, and social cognition. Recent editorials have challenged psychiatric practitioners to behave like cardiologists7 and oncologists8 by aggressively treating first-episode psychosis to prevent ongoing neurodegeneration due to recurrences. The brain loses 1% of its brain volume (~11 ml) after the first psychotic episode,8 which represents hundreds of millions of cells, billions of synapses, and substantial myelin. A second psychotic episode causes significant additional neuropil and white matter fiber damage and represents a different stage of schizophrenia9 with more severe tissue loss and disruption of neural pathways that trigger the process of treatment resistance and functional disability. Ensuring adherence with LAI antipsychotic formulations immediately after the first psychotic episode may allow many patients with schizophrenia to achieve a relapse-free remission and to return to their baseline functioning.10
In addition to significant brain tissue loss during psychotic episodes, mortality is also a very high risk following discharge from the first hospitalization for psychosis.11 LAI second-generation antipsychotic medications have been shown to be associated with lower mortality and neuroprotective effects,12 compared with oral or injectable first-generation antipsychotics. The highest mortality rate was reported to be associated with the lack of any antipsychotic medication,12 underscoring how untreated psychosis can be fatal.
Bottom line: Rapid treatment of stroke and psychosis is an absolute imperative for minimizing brain damage that respectively leads to physical or mental disability. The acronyms FAST and RAPID are essential reminders of the urgency needed to halt progressive neurodegeneration in those 2 devastating acute threats to the integrity of brain and mind. Intensive physical, psychological, and social rehabilitation must follow the acute treatment of stroke and psychosis, and the prevention of any recurrence is an absolute must. For psychosis, the use of a LAI second-generation antipsychotic before hospital discharge from a first episode of psychosis can be disease-modifying, with a more benign illness trajectory and outcome than the devastating deterioration that follows repetitive psychotic relapse, most often due to nonadherence with oral medications.
Continue to: Psychosis should be conceptualized as...
Psychosis should be conceptualized as a “stroke of the mind,” and it can be prevented in most patients with schizophrenia by adopting injectable antipsychotics as early after the onset of psychosis as possible. Yet, starting a LAI antipsychotic drug in first-episode psychosis before hospital discharge is rarely done, and the few patients who currently receive LAIs (10% of U.S. patients) generally receive them after multiple episodes and a protracted DUP. That’s like calling the fire department when much of the house has turned to ashes, instead of calling them when the first small flame is noticed. It makes so much sense, but the decades-old practice of postponing the use of LAIs continues to ruin the lives of young persons in the prime of life. By changing our practice habits to early use of LAIs, we have nothing to lose and our patients with psychosis may be spared a lifetime of suffering, poverty, stigma, incarceration, and functional disability. Wouldn’t we want to avoid that atrocious outcome for our own family members if they develop schizophrenia?
Psychosis and stroke are arguably the most serious acute threats to the integrity of the brain, and consequently the mind. Both are unquestionably associated with a grave outcome the longer their treatment is delayed.1,2
While the management of stroke has been elevated to the highest emergent priority because of the progressively deleterious impact of thrombotic ischemia in one of the cerebral arteries, rapid intervention for acute psychosis has never been regarded as an urgent neurologic condition with severe threats to the brain’s structure and function.3,4 There is extensive literature on the serious consequences of a long duration of untreated psychosis (DUP), including treatment resistance, frequent re-hospitalizations, more negative symptoms, and greater disability.5
Physical paralysis from a stroke receives much more attention than “mental paralysis” of psychosis. Both must be rapidly treated, whether for the regional ischemia to brain tissue following a stroke or for the neurotoxicity of neuroinflammation and oxidative stress that lead to widespread neurodegeneration during psychosis.6 While an acronym for the quick recognition of a stroke (FAST: Facial drooping, Arm weakness, Speech difficulties, and Time to call emergency services) is well established, no acronym for the urgency to treat psychosis has been developed. We propose the acronym RAPID (Readily Avoid Psychosis-Induced Damage). The acronym RAPID would hopefully expedite the urgently needed pharmacotherapeutic and psychosocial intervention in psychosis to halt ongoing brain tissue loss.
It is ironic that the legal obstacles for immediate treatment, which do not exist for stroke, often delay administering antipsychotic medication to patients with anosognosia (a neurologic delusional belief that one is not ill, leading to refusal of treatment) for their psychosis and end up harming patients by prolonging their DUP until a court order is obtained to force brain-saving treatment. Frequent psychotic relapses due to nonadherence with medications are also a very common cause for prolonged DUP due to the inexplicable reluctance of some psychiatric practitioners to employ a long-acting injectable antipsychotic (LAI) medication as soon as possible after the onset of psychosis to circumvent subsequent relapses due to the very high risk of poor adherence. Table 1 describes the optimal management of both stroke and acute psychosis once they are rapidly diagnosed, thanks to the FAST and RAPID reminders.

Tragically, the treatments of the mind have become falsely disengaged from the brain, the physical organ whose neurons, electrical impulses, synapses, and neurotransmitters generate the mind with its advanced human functions, such as self-awareness, will, thoughts, mood, speech, executive functions, memories, and social cognition. Recent editorials have challenged psychiatric practitioners to behave like cardiologists7 and oncologists8 by aggressively treating first-episode psychosis to prevent ongoing neurodegeneration due to recurrences. The brain loses 1% of its brain volume (~11 ml) after the first psychotic episode,8 which represents hundreds of millions of cells, billions of synapses, and substantial myelin. A second psychotic episode causes significant additional neuropil and white matter fiber damage and represents a different stage of schizophrenia9 with more severe tissue loss and disruption of neural pathways that trigger the process of treatment resistance and functional disability. Ensuring adherence with LAI antipsychotic formulations immediately after the first psychotic episode may allow many patients with schizophrenia to achieve a relapse-free remission and to return to their baseline functioning.10
In addition to significant brain tissue loss during psychotic episodes, mortality is also a very high risk following discharge from the first hospitalization for psychosis.11 LAI second-generation antipsychotic medications have been shown to be associated with lower mortality and neuroprotective effects,12 compared with oral or injectable first-generation antipsychotics. The highest mortality rate was reported to be associated with the lack of any antipsychotic medication,12 underscoring how untreated psychosis can be fatal.
Bottom line: Rapid treatment of stroke and psychosis is an absolute imperative for minimizing brain damage that respectively leads to physical or mental disability. The acronyms FAST and RAPID are essential reminders of the urgency needed to halt progressive neurodegeneration in those 2 devastating acute threats to the integrity of brain and mind. Intensive physical, psychological, and social rehabilitation must follow the acute treatment of stroke and psychosis, and the prevention of any recurrence is an absolute must. For psychosis, the use of a LAI second-generation antipsychotic before hospital discharge from a first episode of psychosis can be disease-modifying, with a more benign illness trajectory and outcome than the devastating deterioration that follows repetitive psychotic relapse, most often due to nonadherence with oral medications.
Continue to: Psychosis should be conceptualized as...
Psychosis should be conceptualized as a “stroke of the mind,” and it can be prevented in most patients with schizophrenia by adopting injectable antipsychotics as early after the onset of psychosis as possible. Yet, starting a LAI antipsychotic drug in first-episode psychosis before hospital discharge is rarely done, and the few patients who currently receive LAIs (10% of U.S. patients) generally receive them after multiple episodes and a protracted DUP. That’s like calling the fire department when much of the house has turned to ashes, instead of calling them when the first small flame is noticed. It makes so much sense, but the decades-old practice of postponing the use of LAIs continues to ruin the lives of young persons in the prime of life. By changing our practice habits to early use of LAIs, we have nothing to lose and our patients with psychosis may be spared a lifetime of suffering, poverty, stigma, incarceration, and functional disability. Wouldn’t we want to avoid that atrocious outcome for our own family members if they develop schizophrenia?
1. Benjamin EJ, Virani SS, Callaway CW, et al; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2018 Update: a report from the American Heart Association. Circulation. 2018;137(12):e67-e492.
2. Cechnicki A, Cichocki Ł, Kalisz A, et al. Duration of untreated psychosis (DUP) and the course of schizophrenia in a 20-year follow-up study. Psychiatry Res. 2014;219(3):420-425.
3. Davis J, Moylan S, Harvey BH, et al. Neuroprogression in schizophrenia: pathways underpinning clinical staging and therapeutic corollaries. Aust N Z J Psychiatry. 2014;48(6):512-529.
4. Olabi B, Ellison-Wright I, McIntosh AM, et al. Are there progressive brain changes in schizophrenia? A meta-analysis of structural magnetic resonance imaging studies. Biol Psychiatry. 2011;70(1):88-96.
5. Kane JM, Robinson DG, Schooler NR, et al. Comprehensive versus usual community care for first-episode psychosis: 2-year outcomes from the NIMH RAISE Early Treatment Program. Am J Psychiatry. 2016;173(4):362-372.
6. Najjar S, Pearlman DM. Neuroinflammation and white matter pathology in schizophrenia: systematic review. Schizophr Res. 2015;161(1):102-112.
7. Nasrallah HA. For first-episode psychosis, psychiatrists should behave like cardiologists. Current Psychiatry. 2017;16(8):4-7.
8. Cahn W, Hulshoff Pol HE, Lems EB, et al. Brain volume changes in first-episode schizophrenia: a 1-year follow-up study. Arch Gen Psychiatry. 2002;59(11):1002-1010.
9. McGorry P, Nelson B. Why we need a transdiagnostic staging approach to emerging psychopathology, early diagnosis, and treatment. JAMA Psychiatry. 2016;73(3):191-192.
10. Emsley R, Oosthuizen P, Koen L, et al. Remission in patients with first-episode schizophrenia receiving assured antipsychotic medication: a study with risperidone long-acting injection. Int Clin Psychopharmacol. 2008;23(6):325-331.
11. Nasrallah HA. The crisis of poor physical health and early mortality of psychiatric patients. Current Psychiatry. 2018;17(4):7-8,11.
12. Nasrallah HA. Triple advantages of injectable long acting second generation antipsychotics: relapse prevention, neuroprotection, and lower mortality. Schizophr Res. 2018;197:69-70.
1. Benjamin EJ, Virani SS, Callaway CW, et al; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2018 Update: a report from the American Heart Association. Circulation. 2018;137(12):e67-e492.
2. Cechnicki A, Cichocki Ł, Kalisz A, et al. Duration of untreated psychosis (DUP) and the course of schizophrenia in a 20-year follow-up study. Psychiatry Res. 2014;219(3):420-425.
3. Davis J, Moylan S, Harvey BH, et al. Neuroprogression in schizophrenia: pathways underpinning clinical staging and therapeutic corollaries. Aust N Z J Psychiatry. 2014;48(6):512-529.
4. Olabi B, Ellison-Wright I, McIntosh AM, et al. Are there progressive brain changes in schizophrenia? A meta-analysis of structural magnetic resonance imaging studies. Biol Psychiatry. 2011;70(1):88-96.
5. Kane JM, Robinson DG, Schooler NR, et al. Comprehensive versus usual community care for first-episode psychosis: 2-year outcomes from the NIMH RAISE Early Treatment Program. Am J Psychiatry. 2016;173(4):362-372.
6. Najjar S, Pearlman DM. Neuroinflammation and white matter pathology in schizophrenia: systematic review. Schizophr Res. 2015;161(1):102-112.
7. Nasrallah HA. For first-episode psychosis, psychiatrists should behave like cardiologists. Current Psychiatry. 2017;16(8):4-7.
8. Cahn W, Hulshoff Pol HE, Lems EB, et al. Brain volume changes in first-episode schizophrenia: a 1-year follow-up study. Arch Gen Psychiatry. 2002;59(11):1002-1010.
9. McGorry P, Nelson B. Why we need a transdiagnostic staging approach to emerging psychopathology, early diagnosis, and treatment. JAMA Psychiatry. 2016;73(3):191-192.
10. Emsley R, Oosthuizen P, Koen L, et al. Remission in patients with first-episode schizophrenia receiving assured antipsychotic medication: a study with risperidone long-acting injection. Int Clin Psychopharmacol. 2008;23(6):325-331.
11. Nasrallah HA. The crisis of poor physical health and early mortality of psychiatric patients. Current Psychiatry. 2018;17(4):7-8,11.
12. Nasrallah HA. Triple advantages of injectable long acting second generation antipsychotics: relapse prevention, neuroprotection, and lower mortality. Schizophr Res. 2018;197:69-70.
Prophylactic haloperidol does not improve survival in critically ill patients
Clinical question: Does prophylactic use of haloperidol in critically ill patients at high risk of delirium improve survival at 28 days?
Background: Delirium occurs frequently in critically ill patients and can lead to increased ICU length of stay, hospital length of stay, duration of mechanical ventilation, and mortality. Prior research into the use of prophylactic antipsychotic administration has yielded inconsistent results.
Study design: Double-blind, randomized, controlled trial.
Setting: 21 ICUs in the Netherlands, from July 2013 to March 2017.
Synopsis: A total of 1,789 critically ill adults with an anticipated ICU stay of at least 2 days were randomized to receive 1 mg of haloperidol, 2 mg of haloperidol, or a placebo three times daily. All study sites used “best practice” delirium prevention (for example, early mobilization, noise reduction, protocols aiming to prevent oversedation). The primary outcome was defined as the number of days patients survived in the 28 days following inclusion, and secondary outcome measures included number of days survived in 90 days, delirium incidence, number of delirium-free and coma-free days, duration of mechanical ventilation, and length of ICU and hospital stay. The 1-mg haloperidol group was stopped early because of futility. There was no significant difference between the 2-mg haloperidol group and the placebo group for the primary outcome (P = .93), or any of the secondary outcomes.Bottom line: In a population of critically ill patients at high risk of delirium, prophylactic haloperidol did not significantly improve 28-day survival, nor did it significantly reduce the incidence of delirium or length of stay.
Citation: van den Boogaard M et al. Effect of haloperidol on survival among critically ill adults with a high risk of delirium: The REDUCE randomized clinical trial. JAMA. 2018 Feb 20;319(7):680-90.
Dr. Winters is a hospitalist at Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, Boston.
Clinical question: Does prophylactic use of haloperidol in critically ill patients at high risk of delirium improve survival at 28 days?
Background: Delirium occurs frequently in critically ill patients and can lead to increased ICU length of stay, hospital length of stay, duration of mechanical ventilation, and mortality. Prior research into the use of prophylactic antipsychotic administration has yielded inconsistent results.
Study design: Double-blind, randomized, controlled trial.
Setting: 21 ICUs in the Netherlands, from July 2013 to March 2017.
Synopsis: A total of 1,789 critically ill adults with an anticipated ICU stay of at least 2 days were randomized to receive 1 mg of haloperidol, 2 mg of haloperidol, or a placebo three times daily. All study sites used “best practice” delirium prevention (for example, early mobilization, noise reduction, protocols aiming to prevent oversedation). The primary outcome was defined as the number of days patients survived in the 28 days following inclusion, and secondary outcome measures included number of days survived in 90 days, delirium incidence, number of delirium-free and coma-free days, duration of mechanical ventilation, and length of ICU and hospital stay. The 1-mg haloperidol group was stopped early because of futility. There was no significant difference between the 2-mg haloperidol group and the placebo group for the primary outcome (P = .93), or any of the secondary outcomes.Bottom line: In a population of critically ill patients at high risk of delirium, prophylactic haloperidol did not significantly improve 28-day survival, nor did it significantly reduce the incidence of delirium or length of stay.
Citation: van den Boogaard M et al. Effect of haloperidol on survival among critically ill adults with a high risk of delirium: The REDUCE randomized clinical trial. JAMA. 2018 Feb 20;319(7):680-90.
Dr. Winters is a hospitalist at Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, Boston.
Clinical question: Does prophylactic use of haloperidol in critically ill patients at high risk of delirium improve survival at 28 days?
Background: Delirium occurs frequently in critically ill patients and can lead to increased ICU length of stay, hospital length of stay, duration of mechanical ventilation, and mortality. Prior research into the use of prophylactic antipsychotic administration has yielded inconsistent results.
Study design: Double-blind, randomized, controlled trial.
Setting: 21 ICUs in the Netherlands, from July 2013 to March 2017.
Synopsis: A total of 1,789 critically ill adults with an anticipated ICU stay of at least 2 days were randomized to receive 1 mg of haloperidol, 2 mg of haloperidol, or a placebo three times daily. All study sites used “best practice” delirium prevention (for example, early mobilization, noise reduction, protocols aiming to prevent oversedation). The primary outcome was defined as the number of days patients survived in the 28 days following inclusion, and secondary outcome measures included number of days survived in 90 days, delirium incidence, number of delirium-free and coma-free days, duration of mechanical ventilation, and length of ICU and hospital stay. The 1-mg haloperidol group was stopped early because of futility. There was no significant difference between the 2-mg haloperidol group and the placebo group for the primary outcome (P = .93), or any of the secondary outcomes.Bottom line: In a population of critically ill patients at high risk of delirium, prophylactic haloperidol did not significantly improve 28-day survival, nor did it significantly reduce the incidence of delirium or length of stay.
Citation: van den Boogaard M et al. Effect of haloperidol on survival among critically ill adults with a high risk of delirium: The REDUCE randomized clinical trial. JAMA. 2018 Feb 20;319(7):680-90.
Dr. Winters is a hospitalist at Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, Boston.
Novel TKI PLX9486 showed efficacy against KIT mutations in GIST
CHICAGO – A combination of the investigational agent PLX9486 with another novel tyrosine kinase inhibitor (TKI) showed some efficacy against a range of primary and secondary KIT mutations in patients with gastrointestinal stromal tumor (GIST), the results of a phase 1 dose escalation study have suggested.
Among 39 patients with GIST who had progressed on imatinib and other TKIs, the rates of clinical benefit at 16 weeks were 64% for 11 patients treated with PLX8486 monotherapy at a dose of 1,000 mg daily and 67% for 9 patients treated with PLX9486 and the investigational TKI pexidartinib.
One patient in the 1,000 mg monotherapy group had a partial response on interim analysis. The median progression-free survival in this dose group was 6 months, which was “significantly better than at lower doses,” reported Andrew J. Wagner, MD, PhD, from the Dana-Farber Cancer Institute in Boston and his colleagues.
“The combination of PLX9486 with either pexidartinib or sunitinib is generally well tolerated and toxicities are typically grade 1 or 2 in nature and reversible,” they wrote in a poster presented at the annual meeting of the American Society of Clinical Oncology.
PLX9486 is an inhibitor of KIT primary mutations in exons 9 and 11 and secondary resistance mutations in exons 17 and 18. Compared with other KIT-targeted TKIs, PLX9486 has complementary selectivity for mutant forms of KIT with a greater than 150-fold selectivity for mutant versus wild-type KIT, the investigators explained.
“Combinations of PLX9486 with either pexidartinib (PLX3397) or sunitinib potentially inhibit and address all common primary and secondary KIT mutations,” they wrote.
The investigators conducted a phase 1, open-label, dose-escalation study with two parts. The first part was designed to study the safety and pharmacokinetics of single-agent PLX9486 and established a maximum tolerated dose (MTD) for phase 2 studies. The second part was designed to study the drug as a single agent at the recommended phase 2 dose in GIST and other solid tumors with KIT mutations and also in combination with either pexidartinib or sunitinib in patients with GIST.
They found that single-agent PLX9486 was well tolerated at all doses tested (250, 300, 350, 500, and 1,000 mg daily) and that it selectively inhibited a spectrum of KIT mutations, “including difficult to treat exon 17/18 activation loop variants.”
The combination of PLX9486 at 500 mg and pexidartinib 600 mg was associated with three partial responses and a clinical benefit rate of 67%, with a PFS on interim analysis of 6 months.
The efficacy of single agent PLX9486 was suggested by circulating tumor DNA studies, which showed reductions in circulating tumor DNA levels of exons 11 and 17/18, which reflected the selectivity profile of the TKI.
In the PLX9486 dose escalation phase, there were three cases of grade 3 or 4 toxicities, including one case each of fatigue, creatinine phosphokinase increase, and hypophosphatemia.
The combination of PLX9486 and pexidartinib was associated with grade 1 or 2 adverse events, including hair color changes in five patients; fatigue and decreased appetite in four patients each; anemia, diarrhea, nausea, alanine aminotransferase increase, and aspartate aminotransferase increase in three patients each; and weight loss, maculopapular rash, and hypertension in two patients each.
At the time of the poster presentation, the sunitinib cohort was still accruing, and interim efficacy data were not available.
“Given these interim results, it is anticipated that the selectivity profile and potency of PLX9486 + sunitinib combination will achieve broader and more durable coverage of primary and secondary KIT mutations,” Dr. Wagner and his associates wrote.
SOURCE: Wagner AJ et al. ASCO 2018, Abstract 11509.
CHICAGO – A combination of the investigational agent PLX9486 with another novel tyrosine kinase inhibitor (TKI) showed some efficacy against a range of primary and secondary KIT mutations in patients with gastrointestinal stromal tumor (GIST), the results of a phase 1 dose escalation study have suggested.
Among 39 patients with GIST who had progressed on imatinib and other TKIs, the rates of clinical benefit at 16 weeks were 64% for 11 patients treated with PLX8486 monotherapy at a dose of 1,000 mg daily and 67% for 9 patients treated with PLX9486 and the investigational TKI pexidartinib.
One patient in the 1,000 mg monotherapy group had a partial response on interim analysis. The median progression-free survival in this dose group was 6 months, which was “significantly better than at lower doses,” reported Andrew J. Wagner, MD, PhD, from the Dana-Farber Cancer Institute in Boston and his colleagues.
“The combination of PLX9486 with either pexidartinib or sunitinib is generally well tolerated and toxicities are typically grade 1 or 2 in nature and reversible,” they wrote in a poster presented at the annual meeting of the American Society of Clinical Oncology.
PLX9486 is an inhibitor of KIT primary mutations in exons 9 and 11 and secondary resistance mutations in exons 17 and 18. Compared with other KIT-targeted TKIs, PLX9486 has complementary selectivity for mutant forms of KIT with a greater than 150-fold selectivity for mutant versus wild-type KIT, the investigators explained.
“Combinations of PLX9486 with either pexidartinib (PLX3397) or sunitinib potentially inhibit and address all common primary and secondary KIT mutations,” they wrote.
The investigators conducted a phase 1, open-label, dose-escalation study with two parts. The first part was designed to study the safety and pharmacokinetics of single-agent PLX9486 and established a maximum tolerated dose (MTD) for phase 2 studies. The second part was designed to study the drug as a single agent at the recommended phase 2 dose in GIST and other solid tumors with KIT mutations and also in combination with either pexidartinib or sunitinib in patients with GIST.
They found that single-agent PLX9486 was well tolerated at all doses tested (250, 300, 350, 500, and 1,000 mg daily) and that it selectively inhibited a spectrum of KIT mutations, “including difficult to treat exon 17/18 activation loop variants.”
The combination of PLX9486 at 500 mg and pexidartinib 600 mg was associated with three partial responses and a clinical benefit rate of 67%, with a PFS on interim analysis of 6 months.
The efficacy of single agent PLX9486 was suggested by circulating tumor DNA studies, which showed reductions in circulating tumor DNA levels of exons 11 and 17/18, which reflected the selectivity profile of the TKI.
In the PLX9486 dose escalation phase, there were three cases of grade 3 or 4 toxicities, including one case each of fatigue, creatinine phosphokinase increase, and hypophosphatemia.
The combination of PLX9486 and pexidartinib was associated with grade 1 or 2 adverse events, including hair color changes in five patients; fatigue and decreased appetite in four patients each; anemia, diarrhea, nausea, alanine aminotransferase increase, and aspartate aminotransferase increase in three patients each; and weight loss, maculopapular rash, and hypertension in two patients each.
At the time of the poster presentation, the sunitinib cohort was still accruing, and interim efficacy data were not available.
“Given these interim results, it is anticipated that the selectivity profile and potency of PLX9486 + sunitinib combination will achieve broader and more durable coverage of primary and secondary KIT mutations,” Dr. Wagner and his associates wrote.
SOURCE: Wagner AJ et al. ASCO 2018, Abstract 11509.
CHICAGO – A combination of the investigational agent PLX9486 with another novel tyrosine kinase inhibitor (TKI) showed some efficacy against a range of primary and secondary KIT mutations in patients with gastrointestinal stromal tumor (GIST), the results of a phase 1 dose escalation study have suggested.
Among 39 patients with GIST who had progressed on imatinib and other TKIs, the rates of clinical benefit at 16 weeks were 64% for 11 patients treated with PLX8486 monotherapy at a dose of 1,000 mg daily and 67% for 9 patients treated with PLX9486 and the investigational TKI pexidartinib.
One patient in the 1,000 mg monotherapy group had a partial response on interim analysis. The median progression-free survival in this dose group was 6 months, which was “significantly better than at lower doses,” reported Andrew J. Wagner, MD, PhD, from the Dana-Farber Cancer Institute in Boston and his colleagues.
“The combination of PLX9486 with either pexidartinib or sunitinib is generally well tolerated and toxicities are typically grade 1 or 2 in nature and reversible,” they wrote in a poster presented at the annual meeting of the American Society of Clinical Oncology.
PLX9486 is an inhibitor of KIT primary mutations in exons 9 and 11 and secondary resistance mutations in exons 17 and 18. Compared with other KIT-targeted TKIs, PLX9486 has complementary selectivity for mutant forms of KIT with a greater than 150-fold selectivity for mutant versus wild-type KIT, the investigators explained.
“Combinations of PLX9486 with either pexidartinib (PLX3397) or sunitinib potentially inhibit and address all common primary and secondary KIT mutations,” they wrote.
The investigators conducted a phase 1, open-label, dose-escalation study with two parts. The first part was designed to study the safety and pharmacokinetics of single-agent PLX9486 and established a maximum tolerated dose (MTD) for phase 2 studies. The second part was designed to study the drug as a single agent at the recommended phase 2 dose in GIST and other solid tumors with KIT mutations and also in combination with either pexidartinib or sunitinib in patients with GIST.
They found that single-agent PLX9486 was well tolerated at all doses tested (250, 300, 350, 500, and 1,000 mg daily) and that it selectively inhibited a spectrum of KIT mutations, “including difficult to treat exon 17/18 activation loop variants.”
The combination of PLX9486 at 500 mg and pexidartinib 600 mg was associated with three partial responses and a clinical benefit rate of 67%, with a PFS on interim analysis of 6 months.
The efficacy of single agent PLX9486 was suggested by circulating tumor DNA studies, which showed reductions in circulating tumor DNA levels of exons 11 and 17/18, which reflected the selectivity profile of the TKI.
In the PLX9486 dose escalation phase, there were three cases of grade 3 or 4 toxicities, including one case each of fatigue, creatinine phosphokinase increase, and hypophosphatemia.
The combination of PLX9486 and pexidartinib was associated with grade 1 or 2 adverse events, including hair color changes in five patients; fatigue and decreased appetite in four patients each; anemia, diarrhea, nausea, alanine aminotransferase increase, and aspartate aminotransferase increase in three patients each; and weight loss, maculopapular rash, and hypertension in two patients each.
At the time of the poster presentation, the sunitinib cohort was still accruing, and interim efficacy data were not available.
“Given these interim results, it is anticipated that the selectivity profile and potency of PLX9486 + sunitinib combination will achieve broader and more durable coverage of primary and secondary KIT mutations,” Dr. Wagner and his associates wrote.
SOURCE: Wagner AJ et al. ASCO 2018, Abstract 11509.
PRESENTED AT ASCO 2018
Key clinical point: The novel tyrosine kinase inhibitor PLX9486 showed activity against resistance mutations in gastrointestinal stromal tumors.
Major finding: The combination of PLX9486 at 500 mg and pexidartinib 600 mg was associated with three partial responses and a clinical benefit rate of 67% with a PFS on interim analysis of 6 months.
Study details: Phase 1 dose-escalation, safety and pharmacokinetics study in 39 patients with GIST, four with adenocarcinomas, and one with follicular lymphoma.
Disclosures: The study was sponsored by Plexxikon. Dr. Wagner disclosed consulting or advisory roles with Prime Therapeutics, Lilly, and Loxo Oncology, as well as having received institutional research funding from AADi, Celldex Therapeutics, Daiichi Sankyo, Karyopharm Therapeutics, Lilly, and Plexxikon.
Source: Wagner AJ et al. ASCO 2018, Abstract 11509.
Rising U.S. PrEP use linked with dropping HIV infections
AMSTERDAM – Preexposure prophylaxis (PrEP) against HIV infection by U.S. residents appears to be paying off: The number of new U.S. HIV infections among those at least 13 years old dropped during 2012-2016, and this decline showed a statistically significant link with growth in drug prophylaxis among U.S. residents during the same time.
Uptake of HIV PrEP “was significantly associated with declines in HIV diagnoses in the United States, independent of levels of viral suppression,” Patrick S. Sullivan, Ph.D., and his associates said in a poster presented at the 22nd International AIDS Conference.
Their analysis of nationwide U.S. data showed that during 2012-2016 new HIV diagnoses in residents at least 13 years old fell by an estimated annual percent change of 4.65 among the 10 states with the greatest rate of PrEP use by residents, compared with increases in the estimated annual percent change in new HIV diagnoses of about 1-1.5 in the 14 states with the lowest PrEP use. This statistically significant link remained after adjusting for variations in levels of viral suppression among HIV-infected residents in each state, another factor driving reduced infection rates, Dr. Sullivan and his associates reported..
In the ten-state subgroup with the greatest PrEP uptake, use of PrEP in people at risk for HIV rose from 12/1,000 people in 2012, the year that the Food and Drug Administration first approved a PrEP regimen, to 110/1,000 at-risk people in 2016, a ninefold increase. PrEP use jumped by about the same relative amount in the seven states with the lowest PrEP use, but because it was only 3/1,000 people in 2012 it reached only 35/1,000 in 2016, less than a third of the rate in the states that administered the most PrEP.
In absolute, unadjusted numbers the rate of new HIV diagnoses in the 10 states with the greatest PrEP use fell from 19.4 cases/100,000 population to 13.6/100,000 in 2016. Total U.S. HIV diagnosis rates in people at least 13 years old fell from 15.7/100,000 in 2012 to 14.5/100,000 in 2016, reported Dr. Sullivan, a professor of epidemiology at Emory University in Atlanta.
Dr. Sullivan and his coauthors cautioned that these associations do not allow inference of a causal relationship, and their data did not allow them to estimate the relative contributions of PrEP uptake and HIV suppression to the declining trend in diagnosed HIV infections. However PrEP and suppressive HIV treatment act in a complimentary way to potentially drop the rate of new HIV transmissions, he said.
In the years since 2012, when the Food and Drug Administration approved PrEP as an indication for 200-mg emtricitabine (Emtriva) and 300 mg tenofovir (Viread) – formulated into a single pill and marketed as Truvada, the idea of PrEP for people at increased risk for HIV exposure has gained traction.
Awareness of, knowledge about, and uptake of PrEP have all increased among U.S. residents since a PrEP formulation became available, Dr. Sullivan said. It’s become a cultural norm in at least some communities, he said in an interview. The cost for daily PrEP has posed a barrier to some potential users, but in many U.S. settings people can find ways to at least partially subsidize the cost even when lacking insurance coverage for the drug, he noted.
To determine rates of new U.S. HIV diagnoses Dr. Sullivan and his associates used data collected by the National HIV Surveillance System. To estimate rates of PrEP uptake they used data from prescriptions filled for the emtricitabine and tenofovir formulation that they then adjusted to rule out use for indications other than PrEP (Ann Epidemiol. 2018 Jun 22. doi: 10.1016/j.annepidem.2018.06.009).
SOURCE: Sullivan PS et al. AIDS 2018, Abstract 13004.
AMSTERDAM – Preexposure prophylaxis (PrEP) against HIV infection by U.S. residents appears to be paying off: The number of new U.S. HIV infections among those at least 13 years old dropped during 2012-2016, and this decline showed a statistically significant link with growth in drug prophylaxis among U.S. residents during the same time.
Uptake of HIV PrEP “was significantly associated with declines in HIV diagnoses in the United States, independent of levels of viral suppression,” Patrick S. Sullivan, Ph.D., and his associates said in a poster presented at the 22nd International AIDS Conference.
Their analysis of nationwide U.S. data showed that during 2012-2016 new HIV diagnoses in residents at least 13 years old fell by an estimated annual percent change of 4.65 among the 10 states with the greatest rate of PrEP use by residents, compared with increases in the estimated annual percent change in new HIV diagnoses of about 1-1.5 in the 14 states with the lowest PrEP use. This statistically significant link remained after adjusting for variations in levels of viral suppression among HIV-infected residents in each state, another factor driving reduced infection rates, Dr. Sullivan and his associates reported..
In the ten-state subgroup with the greatest PrEP uptake, use of PrEP in people at risk for HIV rose from 12/1,000 people in 2012, the year that the Food and Drug Administration first approved a PrEP regimen, to 110/1,000 at-risk people in 2016, a ninefold increase. PrEP use jumped by about the same relative amount in the seven states with the lowest PrEP use, but because it was only 3/1,000 people in 2012 it reached only 35/1,000 in 2016, less than a third of the rate in the states that administered the most PrEP.
In absolute, unadjusted numbers the rate of new HIV diagnoses in the 10 states with the greatest PrEP use fell from 19.4 cases/100,000 population to 13.6/100,000 in 2016. Total U.S. HIV diagnosis rates in people at least 13 years old fell from 15.7/100,000 in 2012 to 14.5/100,000 in 2016, reported Dr. Sullivan, a professor of epidemiology at Emory University in Atlanta.
Dr. Sullivan and his coauthors cautioned that these associations do not allow inference of a causal relationship, and their data did not allow them to estimate the relative contributions of PrEP uptake and HIV suppression to the declining trend in diagnosed HIV infections. However PrEP and suppressive HIV treatment act in a complimentary way to potentially drop the rate of new HIV transmissions, he said.
In the years since 2012, when the Food and Drug Administration approved PrEP as an indication for 200-mg emtricitabine (Emtriva) and 300 mg tenofovir (Viread) – formulated into a single pill and marketed as Truvada, the idea of PrEP for people at increased risk for HIV exposure has gained traction.
Awareness of, knowledge about, and uptake of PrEP have all increased among U.S. residents since a PrEP formulation became available, Dr. Sullivan said. It’s become a cultural norm in at least some communities, he said in an interview. The cost for daily PrEP has posed a barrier to some potential users, but in many U.S. settings people can find ways to at least partially subsidize the cost even when lacking insurance coverage for the drug, he noted.
To determine rates of new U.S. HIV diagnoses Dr. Sullivan and his associates used data collected by the National HIV Surveillance System. To estimate rates of PrEP uptake they used data from prescriptions filled for the emtricitabine and tenofovir formulation that they then adjusted to rule out use for indications other than PrEP (Ann Epidemiol. 2018 Jun 22. doi: 10.1016/j.annepidem.2018.06.009).
SOURCE: Sullivan PS et al. AIDS 2018, Abstract 13004.
AMSTERDAM – Preexposure prophylaxis (PrEP) against HIV infection by U.S. residents appears to be paying off: The number of new U.S. HIV infections among those at least 13 years old dropped during 2012-2016, and this decline showed a statistically significant link with growth in drug prophylaxis among U.S. residents during the same time.
Uptake of HIV PrEP “was significantly associated with declines in HIV diagnoses in the United States, independent of levels of viral suppression,” Patrick S. Sullivan, Ph.D., and his associates said in a poster presented at the 22nd International AIDS Conference.
Their analysis of nationwide U.S. data showed that during 2012-2016 new HIV diagnoses in residents at least 13 years old fell by an estimated annual percent change of 4.65 among the 10 states with the greatest rate of PrEP use by residents, compared with increases in the estimated annual percent change in new HIV diagnoses of about 1-1.5 in the 14 states with the lowest PrEP use. This statistically significant link remained after adjusting for variations in levels of viral suppression among HIV-infected residents in each state, another factor driving reduced infection rates, Dr. Sullivan and his associates reported..
In the ten-state subgroup with the greatest PrEP uptake, use of PrEP in people at risk for HIV rose from 12/1,000 people in 2012, the year that the Food and Drug Administration first approved a PrEP regimen, to 110/1,000 at-risk people in 2016, a ninefold increase. PrEP use jumped by about the same relative amount in the seven states with the lowest PrEP use, but because it was only 3/1,000 people in 2012 it reached only 35/1,000 in 2016, less than a third of the rate in the states that administered the most PrEP.
In absolute, unadjusted numbers the rate of new HIV diagnoses in the 10 states with the greatest PrEP use fell from 19.4 cases/100,000 population to 13.6/100,000 in 2016. Total U.S. HIV diagnosis rates in people at least 13 years old fell from 15.7/100,000 in 2012 to 14.5/100,000 in 2016, reported Dr. Sullivan, a professor of epidemiology at Emory University in Atlanta.
Dr. Sullivan and his coauthors cautioned that these associations do not allow inference of a causal relationship, and their data did not allow them to estimate the relative contributions of PrEP uptake and HIV suppression to the declining trend in diagnosed HIV infections. However PrEP and suppressive HIV treatment act in a complimentary way to potentially drop the rate of new HIV transmissions, he said.
In the years since 2012, when the Food and Drug Administration approved PrEP as an indication for 200-mg emtricitabine (Emtriva) and 300 mg tenofovir (Viread) – formulated into a single pill and marketed as Truvada, the idea of PrEP for people at increased risk for HIV exposure has gained traction.
Awareness of, knowledge about, and uptake of PrEP have all increased among U.S. residents since a PrEP formulation became available, Dr. Sullivan said. It’s become a cultural norm in at least some communities, he said in an interview. The cost for daily PrEP has posed a barrier to some potential users, but in many U.S. settings people can find ways to at least partially subsidize the cost even when lacking insurance coverage for the drug, he noted.
To determine rates of new U.S. HIV diagnoses Dr. Sullivan and his associates used data collected by the National HIV Surveillance System. To estimate rates of PrEP uptake they used data from prescriptions filled for the emtricitabine and tenofovir formulation that they then adjusted to rule out use for indications other than PrEP (Ann Epidemiol. 2018 Jun 22. doi: 10.1016/j.annepidem.2018.06.009).
SOURCE: Sullivan PS et al. AIDS 2018, Abstract 13004.
REPORTING FROM AIDS 2018
Key clinical Rising use of PrEP to prevent HIV infection since 2012 is linked with a drop in new U.S. HIV infections.
Major finding: In 10 states with the highest PrEP use new HIV infections fell by an estimated annual percent change of 4.65.
Study details: Analysis of U.S. national data during 2012-2016.
Disclosures: The study was funded by Gilead, the company that markets emtricitabine and tenofovir (Truvada). Dr. Sullivan had no disclosures. Two coauthors on the study were Gilead employees.
Source: Sullivan PS et al. AIDS 2018, Abstract 13004.
Low response rate with trofosfamide for advanced STS in elderly
CHICAGO – In elderly patients with previously untreated metastatic soft-tissue sarcomas (STSs), the oral alkylating agent trofosfamide was associated with a lower overall response rate but long-lasting remissions among patients who had complete responses, investigators reported.
In a randomized phase 2 trial that compared trofosfamide with doxorubicin (Adriamycin), the 6-month progression-free survival (PFS) rate with trofosfamide, the primary endpoint, was 27. 6% versus 35.9% in the doxorubicin arm, said Joerg Thomas Hartmann, MD, from Franziskus Hospital in Bielefeld, Germany.
“Median age was 70 years, which means that the population included [patients] 10-15 years older as compared to other trials in metastatic adult sarcoma. The trial met its predefined endpoint, demonstrating that patients treated with trofosfamide attained a 6-month progression-free rate of more than 20%,” he said at the annual meeting of the American Society of Clinical Oncology.
Trofosfamide is an oral alkylating agent chemically related to cyclophosphamide and ifosfamide. It has been evaluated in a variety of hematologic and solid malignancies and has shown particular activity in patients with chemotherapy-naive and treatment-refractory adult STSs.
Dr. Hartmann and his colleagues conducted the phase 2 study to determine whether oral continuous or “metronomic” therapy with trofosfamide could produce a 6-month PFS rate of at least 20% in patients older than 60 years with previously untreated STSs. They selected this rate of 20% or higher based on the European Organisation for Research and Treatment of Cancer (EORTC) target criterion for doxorubicin of 25%.
They also compared grade 3 or greater toxicities of the two regimens, as well as overall response rate according to Response Evaluation Criteria in Solid Tumors (RECIST) 1,0, and overall survival.
A total of 120 patients with histologically confirmed STSs with no prior first-line chemotherapy and with adequate bone marrow, renal, and liver function were enrolled. The histologies included pleomorphic sarcoma not otherwise specified, leiomyosarcoma, liposarcoma, and others not specified by Dr. Hartmann.
The patients were randomly assigned on a 1:2 basis to receive either intravenous doxorubicin 60 mg/m2 on day 1 of each 21-day cycle for a total of 6 cycles (40 patients) or oral trofosfamide 300 mg/day for days 1 through 7 followed by 150 mg/day until disease progression or unacceptable toxicities (80 patients).
The median patient age in each arm was 70 years.
After a median follow-up of 18.4 months, the trial met its primary endpoint of a 6-months PFS with trofosfamide exceeding 20% (27.6%).
Overall response rates were 7.7% in the doxorubicin arm and 6.6% in the trofosfamide arm.
All three responses in the doxorubicin arm were partial. In the trofosfamide arm there were five responses, including two complete responses and three PR.
The duration of responses in the patients treated with trofosfamide who achieved a complete response were 8.8 and 46.6 months (median, 27.7 months). The median duration of response for trofosfamide-treated patients with a partial response was 8.2 months (range, 1.4-14.9 months).
In contrast, the median duration of response in the patients treated with doxorubicin who achieved a partial response was 4.3 months (range, 2.2-5.6 months).
Grade 3 or 4 adverse events occurred in significantly more patients treated with doxorubicin than they did in patients treated with trofosfamide (61.5% vs. 38.2%, respectively; P = .01). However, deaths within 30 or 60 days of starting on the assigned study drug were higher in the trofosfamide arm (zero vs. two and three vs. six, respectively).
Rates of anemia, leukocytopenia, nausea, and asthenia were similar between the groups, but trofosfamide was significantly associated with higher rates of dyspnea (P = .0148) and fatigue (P = .0264) and with lower rates of neutropenia (P less than .0001) and mucositis (P = .0008).
The trial was supported by Baxter Oncology of Germany. Dr. Hartmann reported having no conflicts of interest to disclose.
SOURCE: Hartman JT et al. ASCO 2018, Abstract 11507.
CHICAGO – In elderly patients with previously untreated metastatic soft-tissue sarcomas (STSs), the oral alkylating agent trofosfamide was associated with a lower overall response rate but long-lasting remissions among patients who had complete responses, investigators reported.
In a randomized phase 2 trial that compared trofosfamide with doxorubicin (Adriamycin), the 6-month progression-free survival (PFS) rate with trofosfamide, the primary endpoint, was 27. 6% versus 35.9% in the doxorubicin arm, said Joerg Thomas Hartmann, MD, from Franziskus Hospital in Bielefeld, Germany.
“Median age was 70 years, which means that the population included [patients] 10-15 years older as compared to other trials in metastatic adult sarcoma. The trial met its predefined endpoint, demonstrating that patients treated with trofosfamide attained a 6-month progression-free rate of more than 20%,” he said at the annual meeting of the American Society of Clinical Oncology.
Trofosfamide is an oral alkylating agent chemically related to cyclophosphamide and ifosfamide. It has been evaluated in a variety of hematologic and solid malignancies and has shown particular activity in patients with chemotherapy-naive and treatment-refractory adult STSs.
Dr. Hartmann and his colleagues conducted the phase 2 study to determine whether oral continuous or “metronomic” therapy with trofosfamide could produce a 6-month PFS rate of at least 20% in patients older than 60 years with previously untreated STSs. They selected this rate of 20% or higher based on the European Organisation for Research and Treatment of Cancer (EORTC) target criterion for doxorubicin of 25%.
They also compared grade 3 or greater toxicities of the two regimens, as well as overall response rate according to Response Evaluation Criteria in Solid Tumors (RECIST) 1,0, and overall survival.
A total of 120 patients with histologically confirmed STSs with no prior first-line chemotherapy and with adequate bone marrow, renal, and liver function were enrolled. The histologies included pleomorphic sarcoma not otherwise specified, leiomyosarcoma, liposarcoma, and others not specified by Dr. Hartmann.
The patients were randomly assigned on a 1:2 basis to receive either intravenous doxorubicin 60 mg/m2 on day 1 of each 21-day cycle for a total of 6 cycles (40 patients) or oral trofosfamide 300 mg/day for days 1 through 7 followed by 150 mg/day until disease progression or unacceptable toxicities (80 patients).
The median patient age in each arm was 70 years.
After a median follow-up of 18.4 months, the trial met its primary endpoint of a 6-months PFS with trofosfamide exceeding 20% (27.6%).
Overall response rates were 7.7% in the doxorubicin arm and 6.6% in the trofosfamide arm.
All three responses in the doxorubicin arm were partial. In the trofosfamide arm there were five responses, including two complete responses and three PR.
The duration of responses in the patients treated with trofosfamide who achieved a complete response were 8.8 and 46.6 months (median, 27.7 months). The median duration of response for trofosfamide-treated patients with a partial response was 8.2 months (range, 1.4-14.9 months).
In contrast, the median duration of response in the patients treated with doxorubicin who achieved a partial response was 4.3 months (range, 2.2-5.6 months).
Grade 3 or 4 adverse events occurred in significantly more patients treated with doxorubicin than they did in patients treated with trofosfamide (61.5% vs. 38.2%, respectively; P = .01). However, deaths within 30 or 60 days of starting on the assigned study drug were higher in the trofosfamide arm (zero vs. two and three vs. six, respectively).
Rates of anemia, leukocytopenia, nausea, and asthenia were similar between the groups, but trofosfamide was significantly associated with higher rates of dyspnea (P = .0148) and fatigue (P = .0264) and with lower rates of neutropenia (P less than .0001) and mucositis (P = .0008).
The trial was supported by Baxter Oncology of Germany. Dr. Hartmann reported having no conflicts of interest to disclose.
SOURCE: Hartman JT et al. ASCO 2018, Abstract 11507.
CHICAGO – In elderly patients with previously untreated metastatic soft-tissue sarcomas (STSs), the oral alkylating agent trofosfamide was associated with a lower overall response rate but long-lasting remissions among patients who had complete responses, investigators reported.
In a randomized phase 2 trial that compared trofosfamide with doxorubicin (Adriamycin), the 6-month progression-free survival (PFS) rate with trofosfamide, the primary endpoint, was 27. 6% versus 35.9% in the doxorubicin arm, said Joerg Thomas Hartmann, MD, from Franziskus Hospital in Bielefeld, Germany.
“Median age was 70 years, which means that the population included [patients] 10-15 years older as compared to other trials in metastatic adult sarcoma. The trial met its predefined endpoint, demonstrating that patients treated with trofosfamide attained a 6-month progression-free rate of more than 20%,” he said at the annual meeting of the American Society of Clinical Oncology.
Trofosfamide is an oral alkylating agent chemically related to cyclophosphamide and ifosfamide. It has been evaluated in a variety of hematologic and solid malignancies and has shown particular activity in patients with chemotherapy-naive and treatment-refractory adult STSs.
Dr. Hartmann and his colleagues conducted the phase 2 study to determine whether oral continuous or “metronomic” therapy with trofosfamide could produce a 6-month PFS rate of at least 20% in patients older than 60 years with previously untreated STSs. They selected this rate of 20% or higher based on the European Organisation for Research and Treatment of Cancer (EORTC) target criterion for doxorubicin of 25%.
They also compared grade 3 or greater toxicities of the two regimens, as well as overall response rate according to Response Evaluation Criteria in Solid Tumors (RECIST) 1,0, and overall survival.
A total of 120 patients with histologically confirmed STSs with no prior first-line chemotherapy and with adequate bone marrow, renal, and liver function were enrolled. The histologies included pleomorphic sarcoma not otherwise specified, leiomyosarcoma, liposarcoma, and others not specified by Dr. Hartmann.
The patients were randomly assigned on a 1:2 basis to receive either intravenous doxorubicin 60 mg/m2 on day 1 of each 21-day cycle for a total of 6 cycles (40 patients) or oral trofosfamide 300 mg/day for days 1 through 7 followed by 150 mg/day until disease progression or unacceptable toxicities (80 patients).
The median patient age in each arm was 70 years.
After a median follow-up of 18.4 months, the trial met its primary endpoint of a 6-months PFS with trofosfamide exceeding 20% (27.6%).
Overall response rates were 7.7% in the doxorubicin arm and 6.6% in the trofosfamide arm.
All three responses in the doxorubicin arm were partial. In the trofosfamide arm there were five responses, including two complete responses and three PR.
The duration of responses in the patients treated with trofosfamide who achieved a complete response were 8.8 and 46.6 months (median, 27.7 months). The median duration of response for trofosfamide-treated patients with a partial response was 8.2 months (range, 1.4-14.9 months).
In contrast, the median duration of response in the patients treated with doxorubicin who achieved a partial response was 4.3 months (range, 2.2-5.6 months).
Grade 3 or 4 adverse events occurred in significantly more patients treated with doxorubicin than they did in patients treated with trofosfamide (61.5% vs. 38.2%, respectively; P = .01). However, deaths within 30 or 60 days of starting on the assigned study drug were higher in the trofosfamide arm (zero vs. two and three vs. six, respectively).
Rates of anemia, leukocytopenia, nausea, and asthenia were similar between the groups, but trofosfamide was significantly associated with higher rates of dyspnea (P = .0148) and fatigue (P = .0264) and with lower rates of neutropenia (P less than .0001) and mucositis (P = .0008).
The trial was supported by Baxter Oncology of Germany. Dr. Hartmann reported having no conflicts of interest to disclose.
SOURCE: Hartman JT et al. ASCO 2018, Abstract 11507.
PRESENTED AT ASCO 2018
Key clinical point: The oral alkylating agent trofosfamide showed efficacy in a small number of elderly patients with untreated metastatic soft-tissue sarcomas (STS).
Major finding: The trial met its primary endpoint with a 6-month progression-free survival with trofosfamide of 27.6%
Study details: Randomized phase 2 trial comparing trofosfamide with doxorubicin in elderly patients with previously untreated metastatic STS.
Disclosures: The trial was supported by Baxter Oncology of Germany. Dr. Hartmann reported having no conflicts of interest to disclose.
Source: Hartman JT et al. ASCO 2018, Abstract 11507.
FDA approves biologic for mycosis fungoides, Sézary syndrome
The Food and Drug Administration has approved mogamulizumab-kpkc (Poteligeo) for the treatment of adults with relapsed or refractory mycosis fungoides (MF) or Sézary syndrome (SS) who have received at least one prior systemic therapy.
Mogamulizumab is a humanized monoclonal antibody directed against CC chemokine receptor 4 (CCR4). It is the first biologic agent targeting CCR4 to be approved for patients in the United States.
Mogamulizumab is expected to be commercially available in the fourth quarter of 2018.
The FDA previously granted mogamulizumab breakthrough therapy and orphan drug designations, as well as priority review.
The approval is supported by the phase 3 MAVORIC trial. Results from this trial were presented at the 10th Annual T-cell Lymphoma Forum in February 2018.
MAVORIC enrolled 372 adults with histologically confirmed MF or SS who had failed at least one systemic therapy. They were randomized to receive mogamulizumab at 1.0 mg/kg (weekly for the first 4-week cycle and then every 2 weeks) or vorinostat at 400 mg daily. Patients were treated until disease progression or unacceptable toxicity. Those receiving vorinostat could cross over to mogamulizumab if they progressed or experienced intolerable toxicity. Baseline characteristics were similar between the treatment arms. The study’s primary endpoint was progression-free survival. The median progression-free survival was 7.7 months with mogamulizumab and 3.1 months with vorinostat (hazard ratio, 0.53; P less than .0001).
The global overall response rate was 28% (52/189) in the mogamulizumab arm and 5% (9/186) in the vorinostat arm (P less than .0001). For patients with MF, the ORR was 21% with mogamulizumab and 7% with vorinostat; for patients with SS, the ORR was 37% and 2%, respectively. After crossover, the ORR in the mogamulizumab arm was 30% (41/136).
The median duration of response (DOR) was 14 months in the mogamulizumab arm and 9 months in the vorinostat arm. For MF patients, the median DOR was 13 months with mogamulizumab and 9 months with vorinostat; for SS patients, the median DOR was 17 months and 7 months, respectively.
The most common treatment-emergent adverse events (AEs), which occurred in at least 20% of patients in either arm (mogamulizumab and vorinostat, respectively), included the following:
- Infusion-related reactions (33.2% vs. 0.5%).
- Drug eruptions (23.9% vs. 0.5%).
- Diarrhea (23.4% vs. 61.8%).
- Nausea (15.2% vs. 42.5%).
- Thrombocytopenia (11.4% vs. 30.6%).
- Dysgeusia (3.3% vs. 28.0%).
- Increased blood creatinine (3.3% vs. 28.0%).
- Decreased appetite (7.6% vs. 24.7%).
There were no grade 4 AEs in the mogamulizumab arm. Grade 3 AEs in mogamulizumab recipients included drug eruptions (n = 8), infusion-related reactions (n = 3), fatigue (n = 3), decreased appetite (n = 2), nausea (n = 1), pyrexia (n = 1), and diarrhea (n = 1).
The drug is marketed by Kyowa Kirin.
The Food and Drug Administration has approved mogamulizumab-kpkc (Poteligeo) for the treatment of adults with relapsed or refractory mycosis fungoides (MF) or Sézary syndrome (SS) who have received at least one prior systemic therapy.
Mogamulizumab is a humanized monoclonal antibody directed against CC chemokine receptor 4 (CCR4). It is the first biologic agent targeting CCR4 to be approved for patients in the United States.
Mogamulizumab is expected to be commercially available in the fourth quarter of 2018.
The FDA previously granted mogamulizumab breakthrough therapy and orphan drug designations, as well as priority review.
The approval is supported by the phase 3 MAVORIC trial. Results from this trial were presented at the 10th Annual T-cell Lymphoma Forum in February 2018.
MAVORIC enrolled 372 adults with histologically confirmed MF or SS who had failed at least one systemic therapy. They were randomized to receive mogamulizumab at 1.0 mg/kg (weekly for the first 4-week cycle and then every 2 weeks) or vorinostat at 400 mg daily. Patients were treated until disease progression or unacceptable toxicity. Those receiving vorinostat could cross over to mogamulizumab if they progressed or experienced intolerable toxicity. Baseline characteristics were similar between the treatment arms. The study’s primary endpoint was progression-free survival. The median progression-free survival was 7.7 months with mogamulizumab and 3.1 months with vorinostat (hazard ratio, 0.53; P less than .0001).
The global overall response rate was 28% (52/189) in the mogamulizumab arm and 5% (9/186) in the vorinostat arm (P less than .0001). For patients with MF, the ORR was 21% with mogamulizumab and 7% with vorinostat; for patients with SS, the ORR was 37% and 2%, respectively. After crossover, the ORR in the mogamulizumab arm was 30% (41/136).
The median duration of response (DOR) was 14 months in the mogamulizumab arm and 9 months in the vorinostat arm. For MF patients, the median DOR was 13 months with mogamulizumab and 9 months with vorinostat; for SS patients, the median DOR was 17 months and 7 months, respectively.
The most common treatment-emergent adverse events (AEs), which occurred in at least 20% of patients in either arm (mogamulizumab and vorinostat, respectively), included the following:
- Infusion-related reactions (33.2% vs. 0.5%).
- Drug eruptions (23.9% vs. 0.5%).
- Diarrhea (23.4% vs. 61.8%).
- Nausea (15.2% vs. 42.5%).
- Thrombocytopenia (11.4% vs. 30.6%).
- Dysgeusia (3.3% vs. 28.0%).
- Increased blood creatinine (3.3% vs. 28.0%).
- Decreased appetite (7.6% vs. 24.7%).
There were no grade 4 AEs in the mogamulizumab arm. Grade 3 AEs in mogamulizumab recipients included drug eruptions (n = 8), infusion-related reactions (n = 3), fatigue (n = 3), decreased appetite (n = 2), nausea (n = 1), pyrexia (n = 1), and diarrhea (n = 1).
The drug is marketed by Kyowa Kirin.
The Food and Drug Administration has approved mogamulizumab-kpkc (Poteligeo) for the treatment of adults with relapsed or refractory mycosis fungoides (MF) or Sézary syndrome (SS) who have received at least one prior systemic therapy.
Mogamulizumab is a humanized monoclonal antibody directed against CC chemokine receptor 4 (CCR4). It is the first biologic agent targeting CCR4 to be approved for patients in the United States.
Mogamulizumab is expected to be commercially available in the fourth quarter of 2018.
The FDA previously granted mogamulizumab breakthrough therapy and orphan drug designations, as well as priority review.
The approval is supported by the phase 3 MAVORIC trial. Results from this trial were presented at the 10th Annual T-cell Lymphoma Forum in February 2018.
MAVORIC enrolled 372 adults with histologically confirmed MF or SS who had failed at least one systemic therapy. They were randomized to receive mogamulizumab at 1.0 mg/kg (weekly for the first 4-week cycle and then every 2 weeks) or vorinostat at 400 mg daily. Patients were treated until disease progression or unacceptable toxicity. Those receiving vorinostat could cross over to mogamulizumab if they progressed or experienced intolerable toxicity. Baseline characteristics were similar between the treatment arms. The study’s primary endpoint was progression-free survival. The median progression-free survival was 7.7 months with mogamulizumab and 3.1 months with vorinostat (hazard ratio, 0.53; P less than .0001).
The global overall response rate was 28% (52/189) in the mogamulizumab arm and 5% (9/186) in the vorinostat arm (P less than .0001). For patients with MF, the ORR was 21% with mogamulizumab and 7% with vorinostat; for patients with SS, the ORR was 37% and 2%, respectively. After crossover, the ORR in the mogamulizumab arm was 30% (41/136).
The median duration of response (DOR) was 14 months in the mogamulizumab arm and 9 months in the vorinostat arm. For MF patients, the median DOR was 13 months with mogamulizumab and 9 months with vorinostat; for SS patients, the median DOR was 17 months and 7 months, respectively.
The most common treatment-emergent adverse events (AEs), which occurred in at least 20% of patients in either arm (mogamulizumab and vorinostat, respectively), included the following:
- Infusion-related reactions (33.2% vs. 0.5%).
- Drug eruptions (23.9% vs. 0.5%).
- Diarrhea (23.4% vs. 61.8%).
- Nausea (15.2% vs. 42.5%).
- Thrombocytopenia (11.4% vs. 30.6%).
- Dysgeusia (3.3% vs. 28.0%).
- Increased blood creatinine (3.3% vs. 28.0%).
- Decreased appetite (7.6% vs. 24.7%).
There were no grade 4 AEs in the mogamulizumab arm. Grade 3 AEs in mogamulizumab recipients included drug eruptions (n = 8), infusion-related reactions (n = 3), fatigue (n = 3), decreased appetite (n = 2), nausea (n = 1), pyrexia (n = 1), and diarrhea (n = 1).
The drug is marketed by Kyowa Kirin.
Can celebrity stories about anxiety reduce stigma?
A public figure who chooses to share his or her private burdens can help others cope with a similar circumstance. Or, such actions might inspire feelings of annoyance and ridicule over the human foibles of someone who is rich and successful – and get airtime for something that many others struggle with alone.
Jessica Gold, MD, is with the department in psychiatry at Washington University in St. Louis. As a practicing psychiatrist who treats patients for anxiety-related conditions, she says she has been both grateful and annoyed at celebrity admissions of conditions like anxiety.
“People with mental health issues are still too often stigmatized in our culture, wrongly portrayed as weak or emotional, and this deters people from seeking care. So any increased awareness of what it’s really like to live with a mental health condition is obviously beneficial and extremely needed. I should be happy that anyone – celebrity or not – is speaking up about these topics. So why do I occasionally have a similar “here we go again” reaction when a celebrity talks about dealing with anxiety?” she wrote in an article for Self magazine.
One reason for the absence of empathy can be the perception that, since celebrities’ bread-and-butter is publicity, any pronouncement that makes the entertainment section can be good for their bank accounts.
For others,
Being annoyed with a celebrity proclamation is natural, according to Dr. Gold. But she advises people to think about why they are feeling that way.
“And in the back of your mind, remember that stigma attached to mental illness discourages people from seeking a diagnosis and treatment. So it’s a fantastic thing to see people with a voice and huge platform willingly open up about a mental health issue and help normalize it. This is especially the case when disclosures could uniquely target younger adults who consume media at high rates, and whose long delay to receiving treatment leads to worse outcomes or disability. Seeing a public figure disclose something so personal could save a life – or at least improve the quality of it.”
Click here to read the article.
A public figure who chooses to share his or her private burdens can help others cope with a similar circumstance. Or, such actions might inspire feelings of annoyance and ridicule over the human foibles of someone who is rich and successful – and get airtime for something that many others struggle with alone.
Jessica Gold, MD, is with the department in psychiatry at Washington University in St. Louis. As a practicing psychiatrist who treats patients for anxiety-related conditions, she says she has been both grateful and annoyed at celebrity admissions of conditions like anxiety.
“People with mental health issues are still too often stigmatized in our culture, wrongly portrayed as weak or emotional, and this deters people from seeking care. So any increased awareness of what it’s really like to live with a mental health condition is obviously beneficial and extremely needed. I should be happy that anyone – celebrity or not – is speaking up about these topics. So why do I occasionally have a similar “here we go again” reaction when a celebrity talks about dealing with anxiety?” she wrote in an article for Self magazine.
One reason for the absence of empathy can be the perception that, since celebrities’ bread-and-butter is publicity, any pronouncement that makes the entertainment section can be good for their bank accounts.
For others,
Being annoyed with a celebrity proclamation is natural, according to Dr. Gold. But she advises people to think about why they are feeling that way.
“And in the back of your mind, remember that stigma attached to mental illness discourages people from seeking a diagnosis and treatment. So it’s a fantastic thing to see people with a voice and huge platform willingly open up about a mental health issue and help normalize it. This is especially the case when disclosures could uniquely target younger adults who consume media at high rates, and whose long delay to receiving treatment leads to worse outcomes or disability. Seeing a public figure disclose something so personal could save a life – or at least improve the quality of it.”
Click here to read the article.
A public figure who chooses to share his or her private burdens can help others cope with a similar circumstance. Or, such actions might inspire feelings of annoyance and ridicule over the human foibles of someone who is rich and successful – and get airtime for something that many others struggle with alone.
Jessica Gold, MD, is with the department in psychiatry at Washington University in St. Louis. As a practicing psychiatrist who treats patients for anxiety-related conditions, she says she has been both grateful and annoyed at celebrity admissions of conditions like anxiety.
“People with mental health issues are still too often stigmatized in our culture, wrongly portrayed as weak or emotional, and this deters people from seeking care. So any increased awareness of what it’s really like to live with a mental health condition is obviously beneficial and extremely needed. I should be happy that anyone – celebrity or not – is speaking up about these topics. So why do I occasionally have a similar “here we go again” reaction when a celebrity talks about dealing with anxiety?” she wrote in an article for Self magazine.
One reason for the absence of empathy can be the perception that, since celebrities’ bread-and-butter is publicity, any pronouncement that makes the entertainment section can be good for their bank accounts.
For others,
Being annoyed with a celebrity proclamation is natural, according to Dr. Gold. But she advises people to think about why they are feeling that way.
“And in the back of your mind, remember that stigma attached to mental illness discourages people from seeking a diagnosis and treatment. So it’s a fantastic thing to see people with a voice and huge platform willingly open up about a mental health issue and help normalize it. This is especially the case when disclosures could uniquely target younger adults who consume media at high rates, and whose long delay to receiving treatment leads to worse outcomes or disability. Seeing a public figure disclose something so personal could save a life – or at least improve the quality of it.”
Click here to read the article.
Increased B-cell lymphoma risk with JAK1/2 inhibitors
Patients with myeloproliferative neoplasms treated with Janus-kinase (JAK) 1/2 inhibitors may be at significantly increased risk of aggressive B cell non-Hodgkin lymphomas, according to a study published in Blood.
A retrospective cohort study of 626 Viennese patients with myeloproliferative neoplasms – 69 of whom were treated with JAK1/2 inhibitors – found that 4 of the 69 patients (5.8%) developed aggressive B-cell lymphoma, compared with just 2 patients (0.36%) in the rest of the group. This represented a significant, 16-fold higher risk of aggressive B cell lymphoma associated with JAK1/2 inhibitor therapy (P = .0017).
The lymphoma was diagnosed within 13-35 months of starting JAK1/2 inhibitors. In three patients, the disease was in the bone marrow and peripheral blood, one patient had it in mammary tissue, and another had it in mucosal tissue. All four lymphomas showed positive MYC and p53 staining.
All four patients had been treated with ruxolitinib, one was also treated with fedratinib, and three of the four had been pretreated with alkylating agents.
Meanwhile, a second retrospective cohort study in Paris of 929 patients with myeloproliferative neoplasms, reported in the same paper, found that 3.51% of those treated with ruxolitinib developed lymphoma, compared with 0.23% of conventionally-treated patients.
Using archived bone marrow samples from 54 of the 69 patients treated with JAK1/2 inhibitors, researchers discovered that 15.9% of them – including three of the B-cell lymphoma patients (the fourth was not tested) – had a preexisting B cell clone. This was present as early as 47-70 months before the lymphoma diagnosis.
“In patients, the clonal B-cell population was present as long as 6 years before overt lymphoma and preceded JAK1/2 inhibition which offers the opportunity to determine patients at risk,” wrote Edit Porpaczy, MD, of the Comprehensive Cancer Center at the Medical University of Vienna, and her coauthors. “Targeted inhibition of JAK-STAT signaling appears to be required to trigger the appearance of the B-cell clone as other treatments eliminating the myeloid cell load in men do not exert a comparable effect.”
In the Viennese cohort, three of the lymphomas were aggressive CD19+ B-cell type, and the fourth was a nonspecified high-grade B-cell lymphoma.
Researchers also looked at the effects of JAK1/2 inhibition in STAT1-/- mice, and found that two-thirds developed a spontaneous myeloid hyperplasia with the concomitant presence of aberrant B-cells.
“Upon STAT1-deficiency myeloid hyperplasia is paralleled by the occurrence of a malignant B-cell clone, which evolves into disease upon bone-marrow transplantation and gives rise to a leukemic lymphoma phenotype,” the authors wrote.
The study was supported by the Austrian Science Fund, the Anniversary Fund of the Austrian National Bank and the WWTF Precision Medicine Program. Several authors reported support, funding or advisory board positions with the pharmaceutical industry.
SOURCE: Porpaczy E et al. Blood. 2018 Jun 14. doi: 10.1182/blood-2017-10-810739.
Patients with myeloproliferative neoplasms treated with Janus-kinase (JAK) 1/2 inhibitors may be at significantly increased risk of aggressive B cell non-Hodgkin lymphomas, according to a study published in Blood.
A retrospective cohort study of 626 Viennese patients with myeloproliferative neoplasms – 69 of whom were treated with JAK1/2 inhibitors – found that 4 of the 69 patients (5.8%) developed aggressive B-cell lymphoma, compared with just 2 patients (0.36%) in the rest of the group. This represented a significant, 16-fold higher risk of aggressive B cell lymphoma associated with JAK1/2 inhibitor therapy (P = .0017).
The lymphoma was diagnosed within 13-35 months of starting JAK1/2 inhibitors. In three patients, the disease was in the bone marrow and peripheral blood, one patient had it in mammary tissue, and another had it in mucosal tissue. All four lymphomas showed positive MYC and p53 staining.
All four patients had been treated with ruxolitinib, one was also treated with fedratinib, and three of the four had been pretreated with alkylating agents.
Meanwhile, a second retrospective cohort study in Paris of 929 patients with myeloproliferative neoplasms, reported in the same paper, found that 3.51% of those treated with ruxolitinib developed lymphoma, compared with 0.23% of conventionally-treated patients.
Using archived bone marrow samples from 54 of the 69 patients treated with JAK1/2 inhibitors, researchers discovered that 15.9% of them – including three of the B-cell lymphoma patients (the fourth was not tested) – had a preexisting B cell clone. This was present as early as 47-70 months before the lymphoma diagnosis.
“In patients, the clonal B-cell population was present as long as 6 years before overt lymphoma and preceded JAK1/2 inhibition which offers the opportunity to determine patients at risk,” wrote Edit Porpaczy, MD, of the Comprehensive Cancer Center at the Medical University of Vienna, and her coauthors. “Targeted inhibition of JAK-STAT signaling appears to be required to trigger the appearance of the B-cell clone as other treatments eliminating the myeloid cell load in men do not exert a comparable effect.”
In the Viennese cohort, three of the lymphomas were aggressive CD19+ B-cell type, and the fourth was a nonspecified high-grade B-cell lymphoma.
Researchers also looked at the effects of JAK1/2 inhibition in STAT1-/- mice, and found that two-thirds developed a spontaneous myeloid hyperplasia with the concomitant presence of aberrant B-cells.
“Upon STAT1-deficiency myeloid hyperplasia is paralleled by the occurrence of a malignant B-cell clone, which evolves into disease upon bone-marrow transplantation and gives rise to a leukemic lymphoma phenotype,” the authors wrote.
The study was supported by the Austrian Science Fund, the Anniversary Fund of the Austrian National Bank and the WWTF Precision Medicine Program. Several authors reported support, funding or advisory board positions with the pharmaceutical industry.
SOURCE: Porpaczy E et al. Blood. 2018 Jun 14. doi: 10.1182/blood-2017-10-810739.
Patients with myeloproliferative neoplasms treated with Janus-kinase (JAK) 1/2 inhibitors may be at significantly increased risk of aggressive B cell non-Hodgkin lymphomas, according to a study published in Blood.
A retrospective cohort study of 626 Viennese patients with myeloproliferative neoplasms – 69 of whom were treated with JAK1/2 inhibitors – found that 4 of the 69 patients (5.8%) developed aggressive B-cell lymphoma, compared with just 2 patients (0.36%) in the rest of the group. This represented a significant, 16-fold higher risk of aggressive B cell lymphoma associated with JAK1/2 inhibitor therapy (P = .0017).
The lymphoma was diagnosed within 13-35 months of starting JAK1/2 inhibitors. In three patients, the disease was in the bone marrow and peripheral blood, one patient had it in mammary tissue, and another had it in mucosal tissue. All four lymphomas showed positive MYC and p53 staining.
All four patients had been treated with ruxolitinib, one was also treated with fedratinib, and three of the four had been pretreated with alkylating agents.
Meanwhile, a second retrospective cohort study in Paris of 929 patients with myeloproliferative neoplasms, reported in the same paper, found that 3.51% of those treated with ruxolitinib developed lymphoma, compared with 0.23% of conventionally-treated patients.
Using archived bone marrow samples from 54 of the 69 patients treated with JAK1/2 inhibitors, researchers discovered that 15.9% of them – including three of the B-cell lymphoma patients (the fourth was not tested) – had a preexisting B cell clone. This was present as early as 47-70 months before the lymphoma diagnosis.
“In patients, the clonal B-cell population was present as long as 6 years before overt lymphoma and preceded JAK1/2 inhibition which offers the opportunity to determine patients at risk,” wrote Edit Porpaczy, MD, of the Comprehensive Cancer Center at the Medical University of Vienna, and her coauthors. “Targeted inhibition of JAK-STAT signaling appears to be required to trigger the appearance of the B-cell clone as other treatments eliminating the myeloid cell load in men do not exert a comparable effect.”
In the Viennese cohort, three of the lymphomas were aggressive CD19+ B-cell type, and the fourth was a nonspecified high-grade B-cell lymphoma.
Researchers also looked at the effects of JAK1/2 inhibition in STAT1-/- mice, and found that two-thirds developed a spontaneous myeloid hyperplasia with the concomitant presence of aberrant B-cells.
“Upon STAT1-deficiency myeloid hyperplasia is paralleled by the occurrence of a malignant B-cell clone, which evolves into disease upon bone-marrow transplantation and gives rise to a leukemic lymphoma phenotype,” the authors wrote.
The study was supported by the Austrian Science Fund, the Anniversary Fund of the Austrian National Bank and the WWTF Precision Medicine Program. Several authors reported support, funding or advisory board positions with the pharmaceutical industry.
SOURCE: Porpaczy E et al. Blood. 2018 Jun 14. doi: 10.1182/blood-2017-10-810739.
FROM BLOOD
Key clinical point:
Major finding: Patients with myeloproliferative neoplasms treated with JAK1/2 inhibitors have a 16-fold higher incidence of lymphoma.
Study details: A retrospective cohort study of 626 patients with myeloproliferative neoplasms.
Disclosures: The study was supported by the Austrian Science Fund, the Anniversary Fund of the Austrian National Bank, and the WWTF Precision Medicine Program. Several authors reported support, funding, or advisory board positions with the pharmaceutical industry.
Source: Porpaczy E et al. Blood. 2018 Jun 14. doi: 10.1182/blood-2017-10-810739.
‘Undetectable’ HIV means ‘untransmissible’ confirmed in larger sex study
AMSTERDAM – One maxim of current HIV management is U=U; Undetectable equals Untransmissible, and it received new backing for the specific question of whether HIV treated to an undetectable level can transmit from a man who engages in condomless anal sex to an uninfected man. The answer was it effectively could not.
In nearly 77,000 sexual encounters of this type performed during a median 1.6 years of follow-up of each participating couple, no episodes resulted in HIV transmission. When researchers combined the new findings with numbers from a smaller, earlier study they ran of similar male couples the statistics showed a worst case of at most one HIV transmission for every 435 person years of follow-up, or “effectively zero,” Alison Rodger, MD, said at the 22nd International AIDS Conference.
“The evidence is very robust. Transmission simply does not occur,” said Dr. Rodger, an infectious disease physician and clinical director of public health at the Royal Free Hospital in London. She cautioned, however, that the finding occurred in couples where the HIV-infected partner consistently had a serum viral load of less than 200 copies of HIV RNA/mL when tested at the start and the end of follow-up and at various times in between. The researchers censored data from couples when the infected partner had a detectable viral load.
The PARTNER-2 (Partners of People on ART – A New Evaluation of the Risks) study followed the PARTNER-1 study that had examined the same question of HIV transmissibility when undetectable during sexual encounters but with a much smaller number of men who have sex with men (JAMA. 2016 July 12;316[2]:171-81). PARTNER-2 enrolled 783 male couples from any of 12 European countries that contributed 1,596 years of couple follow-up. During the median 1.6 years of follow-up per couple, one or both partners had a different sexually transmitted disease 23%-27% of the time, and 37% of the HIV-negative partners had condomless sex with a different partner. The couples averaged 43 sexual encounters a year that produced an overall total of 76,991. During follow-up, 15 of the HIV-negative partners become HIV positive, but in all 15 cases the source of the infection was genetically proven to have come from someone other than the index partner.
Based on the number of sex events studies Dr. Rodger and her associates calculated the 95% confidence interval around the zero transmissions they observed during gay male sex events with undetectable HIV in both PARTNER-2 and PARTNER-1. The upper bound of this confidence interval was a rate of 0.23 transmissions per 100 person years of follow-up,
In other words, the scope of the two combined studies cannot statistically rule out a worst-case scenario of one transmission for every 435 person years of follow-up, or for every 18,696 sex events of the type studied. However, “we’ve made the confidence much more robust. We can’t say it’s zero risk, but it’s effectively zero,” Dr. Rodger said in an interview. “You could keep studying this forever” to further rule out the risk for transmission of undetectable HIV, “but I think the question is now settled,” she declared. She also stressed that condom use remained important to prevent other sexually transmitted diseases.
SOURCE: Rodger A et al. AIDS 2018, Abstract 13470.
AMSTERDAM – One maxim of current HIV management is U=U; Undetectable equals Untransmissible, and it received new backing for the specific question of whether HIV treated to an undetectable level can transmit from a man who engages in condomless anal sex to an uninfected man. The answer was it effectively could not.
In nearly 77,000 sexual encounters of this type performed during a median 1.6 years of follow-up of each participating couple, no episodes resulted in HIV transmission. When researchers combined the new findings with numbers from a smaller, earlier study they ran of similar male couples the statistics showed a worst case of at most one HIV transmission for every 435 person years of follow-up, or “effectively zero,” Alison Rodger, MD, said at the 22nd International AIDS Conference.
“The evidence is very robust. Transmission simply does not occur,” said Dr. Rodger, an infectious disease physician and clinical director of public health at the Royal Free Hospital in London. She cautioned, however, that the finding occurred in couples where the HIV-infected partner consistently had a serum viral load of less than 200 copies of HIV RNA/mL when tested at the start and the end of follow-up and at various times in between. The researchers censored data from couples when the infected partner had a detectable viral load.
The PARTNER-2 (Partners of People on ART – A New Evaluation of the Risks) study followed the PARTNER-1 study that had examined the same question of HIV transmissibility when undetectable during sexual encounters but with a much smaller number of men who have sex with men (JAMA. 2016 July 12;316[2]:171-81). PARTNER-2 enrolled 783 male couples from any of 12 European countries that contributed 1,596 years of couple follow-up. During the median 1.6 years of follow-up per couple, one or both partners had a different sexually transmitted disease 23%-27% of the time, and 37% of the HIV-negative partners had condomless sex with a different partner. The couples averaged 43 sexual encounters a year that produced an overall total of 76,991. During follow-up, 15 of the HIV-negative partners become HIV positive, but in all 15 cases the source of the infection was genetically proven to have come from someone other than the index partner.
Based on the number of sex events studies Dr. Rodger and her associates calculated the 95% confidence interval around the zero transmissions they observed during gay male sex events with undetectable HIV in both PARTNER-2 and PARTNER-1. The upper bound of this confidence interval was a rate of 0.23 transmissions per 100 person years of follow-up,
In other words, the scope of the two combined studies cannot statistically rule out a worst-case scenario of one transmission for every 435 person years of follow-up, or for every 18,696 sex events of the type studied. However, “we’ve made the confidence much more robust. We can’t say it’s zero risk, but it’s effectively zero,” Dr. Rodger said in an interview. “You could keep studying this forever” to further rule out the risk for transmission of undetectable HIV, “but I think the question is now settled,” she declared. She also stressed that condom use remained important to prevent other sexually transmitted diseases.
SOURCE: Rodger A et al. AIDS 2018, Abstract 13470.
AMSTERDAM – One maxim of current HIV management is U=U; Undetectable equals Untransmissible, and it received new backing for the specific question of whether HIV treated to an undetectable level can transmit from a man who engages in condomless anal sex to an uninfected man. The answer was it effectively could not.
In nearly 77,000 sexual encounters of this type performed during a median 1.6 years of follow-up of each participating couple, no episodes resulted in HIV transmission. When researchers combined the new findings with numbers from a smaller, earlier study they ran of similar male couples the statistics showed a worst case of at most one HIV transmission for every 435 person years of follow-up, or “effectively zero,” Alison Rodger, MD, said at the 22nd International AIDS Conference.
“The evidence is very robust. Transmission simply does not occur,” said Dr. Rodger, an infectious disease physician and clinical director of public health at the Royal Free Hospital in London. She cautioned, however, that the finding occurred in couples where the HIV-infected partner consistently had a serum viral load of less than 200 copies of HIV RNA/mL when tested at the start and the end of follow-up and at various times in between. The researchers censored data from couples when the infected partner had a detectable viral load.
The PARTNER-2 (Partners of People on ART – A New Evaluation of the Risks) study followed the PARTNER-1 study that had examined the same question of HIV transmissibility when undetectable during sexual encounters but with a much smaller number of men who have sex with men (JAMA. 2016 July 12;316[2]:171-81). PARTNER-2 enrolled 783 male couples from any of 12 European countries that contributed 1,596 years of couple follow-up. During the median 1.6 years of follow-up per couple, one or both partners had a different sexually transmitted disease 23%-27% of the time, and 37% of the HIV-negative partners had condomless sex with a different partner. The couples averaged 43 sexual encounters a year that produced an overall total of 76,991. During follow-up, 15 of the HIV-negative partners become HIV positive, but in all 15 cases the source of the infection was genetically proven to have come from someone other than the index partner.
Based on the number of sex events studies Dr. Rodger and her associates calculated the 95% confidence interval around the zero transmissions they observed during gay male sex events with undetectable HIV in both PARTNER-2 and PARTNER-1. The upper bound of this confidence interval was a rate of 0.23 transmissions per 100 person years of follow-up,
In other words, the scope of the two combined studies cannot statistically rule out a worst-case scenario of one transmission for every 435 person years of follow-up, or for every 18,696 sex events of the type studied. However, “we’ve made the confidence much more robust. We can’t say it’s zero risk, but it’s effectively zero,” Dr. Rodger said in an interview. “You could keep studying this forever” to further rule out the risk for transmission of undetectable HIV, “but I think the question is now settled,” she declared. She also stressed that condom use remained important to prevent other sexually transmitted diseases.
SOURCE: Rodger A et al. AIDS 2018, Abstract 13470.
REPORTING FROM AIDS 2018
Key clinical point: Undetectable HIV did not produce any transmissions during gay male sexual activity.
Major finding: During 76,991 unprotected gay male sex events no HIV transmission occurred from men with an undetectable viral load.
Study details: PARTNER-2, a prospective, multicenter study of 783 sexually active male couples.
Disclosures: PARTNER-2 received partial funding from unrestricted grants from Viiv and Gilead. Dr. Rodger had no disclosures.
Source: Rodger A et al. AIDS 2018, Abstract 13470.