User login
Team recommends melanoma screening in CLL
Patients with chronic lymphocytic leukemia (CLL) should be routinely monitored for melanoma, according to researchers.
A study of 470 CLL patients showed they have a significantly higher risk of invasive melanoma than the general population.
Most of the melanomas reported in this study were detected via routine surveillance, and most were discovered before they reached an advanced stage.
Clive Zent, MD, of Wilmot Cancer Institute at the University of Rochester Medical Center in Rochester, New York, and his colleagues described this study in Leukemia Research.
The researchers analyzed data on 470 CLL patients followed for 2849 person-years. Eighteen of these patients developed 22 melanomas. This included 14 cases of invasive melanoma in 13 patients.
The rate of invasive melanoma was significantly higher in this CLL cohort than the rate observed in the age- and sex-matched general population. The standardized incidence ratio was 6.32.
“We do not for sure know why CLL patients are more susceptible to melanoma, but the most likely cause is a suppressed immune system,” Dr Zent noted.
“Normally, in people with healthy immune systems, malignant skin cells might be detected and destroyed before they become a problem. But in CLL patients, failure of this control system increases the rate at which cancer cells can grow into tumors and also the likelihood that they will become invasive or spread to distant sites.”
Detection and management
Fifteen of the 22 melanomas (68.2%) in the CLL cohort were detected via surveillance in a dermatology clinic, and 2 (9.1%) were detected at the CLL/lymphoma clinic.
Three cases of melanoma (14.3%) were detected within the first year of a patient’s CLL diagnosis.
Seven melanomas (33.3%) were detected at pathologic stage 0, 8 (38.1%) at stage I, 2 (9.5%) at stage II, 3 (14.3%) at stage III, and 1 (4.8%) at stage IV. Detailed data were not available for the remaining case.
Melanomas were managed with wide local excision (n=19), sentinel node biopsies (n=6), Mohs surgery (n=1), drugs (n=2), palliative care (n=1), and comfort care (n=1).
The 4 patients who received drugs, palliative care, or comfort care had advanced melanoma.
The patient who received palliative care was still alive at 2.4 years of follow-up. The patient who received comfort care died of metastatic melanoma 1.4 years after diagnosis.
The third patient with advanced melanoma received 2 cycles of dacarbazine and palliative radiation to lung and brain metastases. This patient died 3.6 years after melanoma diagnosis.
The fourth patient received ipilimumab for the melanoma while also receiving ibrutinib to treat her CLL. When the ipilimumab failed, the patient proceeded to pembrolizumab and achieved a near-complete response within 3 months. Then, an intensely hypermetabolic abdominal node was detected and successfully treated with radiation.
The patient continued on pembrolizumab, and her melanoma was in sustained remission at last follow-up, after 23 cycles of pembrolizumab. Her CLL was still responding to ibrutinib at that point as well.
Based on these data, Dr Zent and his colleagues recommend routine melanoma screening for CLL patients. The team believes such surveillance might decrease morbidity and mortality in these patients, although more research is needed to confirm this theory.
Patients with chronic lymphocytic leukemia (CLL) should be routinely monitored for melanoma, according to researchers.
A study of 470 CLL patients showed they have a significantly higher risk of invasive melanoma than the general population.
Most of the melanomas reported in this study were detected via routine surveillance, and most were discovered before they reached an advanced stage.
Clive Zent, MD, of Wilmot Cancer Institute at the University of Rochester Medical Center in Rochester, New York, and his colleagues described this study in Leukemia Research.
The researchers analyzed data on 470 CLL patients followed for 2849 person-years. Eighteen of these patients developed 22 melanomas. This included 14 cases of invasive melanoma in 13 patients.
The rate of invasive melanoma was significantly higher in this CLL cohort than the rate observed in the age- and sex-matched general population. The standardized incidence ratio was 6.32.
“We do not for sure know why CLL patients are more susceptible to melanoma, but the most likely cause is a suppressed immune system,” Dr Zent noted.
“Normally, in people with healthy immune systems, malignant skin cells might be detected and destroyed before they become a problem. But in CLL patients, failure of this control system increases the rate at which cancer cells can grow into tumors and also the likelihood that they will become invasive or spread to distant sites.”
Detection and management
Fifteen of the 22 melanomas (68.2%) in the CLL cohort were detected via surveillance in a dermatology clinic, and 2 (9.1%) were detected at the CLL/lymphoma clinic.
Three cases of melanoma (14.3%) were detected within the first year of a patient’s CLL diagnosis.
Seven melanomas (33.3%) were detected at pathologic stage 0, 8 (38.1%) at stage I, 2 (9.5%) at stage II, 3 (14.3%) at stage III, and 1 (4.8%) at stage IV. Detailed data were not available for the remaining case.
Melanomas were managed with wide local excision (n=19), sentinel node biopsies (n=6), Mohs surgery (n=1), drugs (n=2), palliative care (n=1), and comfort care (n=1).
The 4 patients who received drugs, palliative care, or comfort care had advanced melanoma.
The patient who received palliative care was still alive at 2.4 years of follow-up. The patient who received comfort care died of metastatic melanoma 1.4 years after diagnosis.
The third patient with advanced melanoma received 2 cycles of dacarbazine and palliative radiation to lung and brain metastases. This patient died 3.6 years after melanoma diagnosis.
The fourth patient received ipilimumab for the melanoma while also receiving ibrutinib to treat her CLL. When the ipilimumab failed, the patient proceeded to pembrolizumab and achieved a near-complete response within 3 months. Then, an intensely hypermetabolic abdominal node was detected and successfully treated with radiation.
The patient continued on pembrolizumab, and her melanoma was in sustained remission at last follow-up, after 23 cycles of pembrolizumab. Her CLL was still responding to ibrutinib at that point as well.
Based on these data, Dr Zent and his colleagues recommend routine melanoma screening for CLL patients. The team believes such surveillance might decrease morbidity and mortality in these patients, although more research is needed to confirm this theory.
Patients with chronic lymphocytic leukemia (CLL) should be routinely monitored for melanoma, according to researchers.
A study of 470 CLL patients showed they have a significantly higher risk of invasive melanoma than the general population.
Most of the melanomas reported in this study were detected via routine surveillance, and most were discovered before they reached an advanced stage.
Clive Zent, MD, of Wilmot Cancer Institute at the University of Rochester Medical Center in Rochester, New York, and his colleagues described this study in Leukemia Research.
The researchers analyzed data on 470 CLL patients followed for 2849 person-years. Eighteen of these patients developed 22 melanomas. This included 14 cases of invasive melanoma in 13 patients.
The rate of invasive melanoma was significantly higher in this CLL cohort than the rate observed in the age- and sex-matched general population. The standardized incidence ratio was 6.32.
“We do not for sure know why CLL patients are more susceptible to melanoma, but the most likely cause is a suppressed immune system,” Dr Zent noted.
“Normally, in people with healthy immune systems, malignant skin cells might be detected and destroyed before they become a problem. But in CLL patients, failure of this control system increases the rate at which cancer cells can grow into tumors and also the likelihood that they will become invasive or spread to distant sites.”
Detection and management
Fifteen of the 22 melanomas (68.2%) in the CLL cohort were detected via surveillance in a dermatology clinic, and 2 (9.1%) were detected at the CLL/lymphoma clinic.
Three cases of melanoma (14.3%) were detected within the first year of a patient’s CLL diagnosis.
Seven melanomas (33.3%) were detected at pathologic stage 0, 8 (38.1%) at stage I, 2 (9.5%) at stage II, 3 (14.3%) at stage III, and 1 (4.8%) at stage IV. Detailed data were not available for the remaining case.
Melanomas were managed with wide local excision (n=19), sentinel node biopsies (n=6), Mohs surgery (n=1), drugs (n=2), palliative care (n=1), and comfort care (n=1).
The 4 patients who received drugs, palliative care, or comfort care had advanced melanoma.
The patient who received palliative care was still alive at 2.4 years of follow-up. The patient who received comfort care died of metastatic melanoma 1.4 years after diagnosis.
The third patient with advanced melanoma received 2 cycles of dacarbazine and palliative radiation to lung and brain metastases. This patient died 3.6 years after melanoma diagnosis.
The fourth patient received ipilimumab for the melanoma while also receiving ibrutinib to treat her CLL. When the ipilimumab failed, the patient proceeded to pembrolizumab and achieved a near-complete response within 3 months. Then, an intensely hypermetabolic abdominal node was detected and successfully treated with radiation.
The patient continued on pembrolizumab, and her melanoma was in sustained remission at last follow-up, after 23 cycles of pembrolizumab. Her CLL was still responding to ibrutinib at that point as well.
Based on these data, Dr Zent and his colleagues recommend routine melanoma screening for CLL patients. The team believes such surveillance might decrease morbidity and mortality in these patients, although more research is needed to confirm this theory.
Larger vegetation size associated with increased risk of embolism and mortality in infective endocarditis
Clinical question: In patients with infective endocarditis, does a vegetation size greater than 10 mm impart a greater embolic risk?
Background: A vegetation size greater than 10 mm has historically been used as the cutoff for increased risk of embolization in infective endocarditis, and this cutoff forms a key part of the American Heart Association guidelines for early surgical intervention. However, this cutoff is derived primarily from observational data from small studies.
Study design: Meta-analysis of observational studies and randomized clinical trials.
Setting: An English-language literature search from PubMed and EMBASE performed May 2017.
Synopsis: The authors identified 21 unique studies evaluating the association of vegetation size greater than 10 mm with embolic events in adult patients with infective endocarditis. This accounted for a total of 6,646 unique patients and 5,116 vegetations. Analysis of these data found that patients with a vegetation size greater than 10 mm had significantly increased odds of embolic events (odds ratio, 2.28; P less than .001) and mortality (OR, 1.63; P = .009), compared with those with a vegetation size less than 10 mm.
Limitations of this research include the potential for selection bias in the original studies, and the inability to incorporate information relating to microbiologic results, antibiotic use, and the location of systemic embolization. Interestingly, as vegetations with a size of exactly 10 mm were variably categorized in the original studies, this meta-analysis was unable to reach a conclusion on the risk of embolic events for instances when vegetation size is equal to 10 mm.
Bottom line: Patients with infective endocarditis and a vegetation size greater than 10 mm may have significantly increased odds of embolic events and mortality, compared with those with vegetation size less than 10 mm.
Citation: Mohananey D et al. Association of vegetation size with embolic risk in patients with infective endocarditis: A systematic review and meta-analysis. JAMA Intern Med. Apr 1;178(4):502-10.
Dr. Winters is a hospitalist at Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, Boston.
Clinical question: In patients with infective endocarditis, does a vegetation size greater than 10 mm impart a greater embolic risk?
Background: A vegetation size greater than 10 mm has historically been used as the cutoff for increased risk of embolization in infective endocarditis, and this cutoff forms a key part of the American Heart Association guidelines for early surgical intervention. However, this cutoff is derived primarily from observational data from small studies.
Study design: Meta-analysis of observational studies and randomized clinical trials.
Setting: An English-language literature search from PubMed and EMBASE performed May 2017.
Synopsis: The authors identified 21 unique studies evaluating the association of vegetation size greater than 10 mm with embolic events in adult patients with infective endocarditis. This accounted for a total of 6,646 unique patients and 5,116 vegetations. Analysis of these data found that patients with a vegetation size greater than 10 mm had significantly increased odds of embolic events (odds ratio, 2.28; P less than .001) and mortality (OR, 1.63; P = .009), compared with those with a vegetation size less than 10 mm.
Limitations of this research include the potential for selection bias in the original studies, and the inability to incorporate information relating to microbiologic results, antibiotic use, and the location of systemic embolization. Interestingly, as vegetations with a size of exactly 10 mm were variably categorized in the original studies, this meta-analysis was unable to reach a conclusion on the risk of embolic events for instances when vegetation size is equal to 10 mm.
Bottom line: Patients with infective endocarditis and a vegetation size greater than 10 mm may have significantly increased odds of embolic events and mortality, compared with those with vegetation size less than 10 mm.
Citation: Mohananey D et al. Association of vegetation size with embolic risk in patients with infective endocarditis: A systematic review and meta-analysis. JAMA Intern Med. Apr 1;178(4):502-10.
Dr. Winters is a hospitalist at Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, Boston.
Clinical question: In patients with infective endocarditis, does a vegetation size greater than 10 mm impart a greater embolic risk?
Background: A vegetation size greater than 10 mm has historically been used as the cutoff for increased risk of embolization in infective endocarditis, and this cutoff forms a key part of the American Heart Association guidelines for early surgical intervention. However, this cutoff is derived primarily from observational data from small studies.
Study design: Meta-analysis of observational studies and randomized clinical trials.
Setting: An English-language literature search from PubMed and EMBASE performed May 2017.
Synopsis: The authors identified 21 unique studies evaluating the association of vegetation size greater than 10 mm with embolic events in adult patients with infective endocarditis. This accounted for a total of 6,646 unique patients and 5,116 vegetations. Analysis of these data found that patients with a vegetation size greater than 10 mm had significantly increased odds of embolic events (odds ratio, 2.28; P less than .001) and mortality (OR, 1.63; P = .009), compared with those with a vegetation size less than 10 mm.
Limitations of this research include the potential for selection bias in the original studies, and the inability to incorporate information relating to microbiologic results, antibiotic use, and the location of systemic embolization. Interestingly, as vegetations with a size of exactly 10 mm were variably categorized in the original studies, this meta-analysis was unable to reach a conclusion on the risk of embolic events for instances when vegetation size is equal to 10 mm.
Bottom line: Patients with infective endocarditis and a vegetation size greater than 10 mm may have significantly increased odds of embolic events and mortality, compared with those with vegetation size less than 10 mm.
Citation: Mohananey D et al. Association of vegetation size with embolic risk in patients with infective endocarditis: A systematic review and meta-analysis. JAMA Intern Med. Apr 1;178(4):502-10.
Dr. Winters is a hospitalist at Beth Israel Deaconess Medical Center, and instructor in medicine, Harvard Medical School, Boston.
VEGF inhibitor shows promise in platinum resistant/refractory ovarian cancer
Combining the vascular endothelial growth factor (VEGF) receptor–targeting tyrosine kinase inhibitor apatinib with oral etoposide in people with platinum resistant or refractory ovarian cancer has shown promising efficacy and manageable toxicity in a Phase 2 study.
Angiogenesis was a “hallmark” process in cancer, and antiangiogenic therapy, including anti-VEGF antibodies and multireceptor tyrosine kinase inhibitors, has been shown to be an attractive therapeutic strategy for ovarian cancer.
“Increasing evidence suggests that the combination of antiangiogenic therapy and single-agent chemotherapy improves the outcome of platinum-resistant ovarian cancer,” Chun-Yan Lan, MD, of the Collaborative Innovation Center for Cancer Medicine at Sun Yat-sen University Cancer Center in Guangzhou, China, and colleagues wrote in Lancet Oncology.
The investigators chose apatinib because it has shown encouraging antitumor activities and tolerable toxicities in several malignant tumors and was available in mainland China, they said.
The single-arm prospective study enrolled 35 women aged 18-70 years with heavily pretreated ovarian cancer that was refractory to platinum (defined as progression during the initial platinum-based treatment) or resistant to platinum (defined as progression within 6 months after the last platinum treatment).
Women were treated with apatinib at an initial dose of 500 mg once daily on a continuous basis and with oral etoposide at a dose of 50 mg once daily on days 1-14 of a 21-day cycle. Oral etoposide was administered for a maximum of six cycles. Dose modifications, including dose interruptions, were allowed in order to manage adverse events.
Treatment was continued until disease progression, patient withdrawal, or unacceptable toxic effects. The primary endpoint of the study was the proportion of patients achieving an objective response according to Response Evaluation Criteria in Solid Tumors (RECIST). The study authors analyzed efficacy data using three populations: intention-to-treat, per-protocol, and safety populations.
Results showed that 19 of 35 patients achieved an objective response (54%; 95% confidence interval, 36.6%-71.2%) in an intention-to-treat analysis. In the per-protocol population, 19 of 31 patients (61%; 95% CI, 42.2%-78.2%) achieved an objective response.
Median progression-free survival was 8.1 months (interquartile range, 4.3-14.6; 95% CI, 2.8-13.4), and the median duration of response was 7.4 months (IQR, 4.0-13.0; 95% CI, 2.3-12.0).
The most common grade 3 or 4 adverse events reported were neutropenia (n = 17), fatigue (n = 11), anemia n = 10), and mucositis (n = 8). Serious adverse events were reported in two patients who were admitted to the hospital.
Dose reductions were required in 82% (n = 28) of 34 patients on apatinib and 77% (n = 26) for etoposide. The authors said they would suggest future studies use a lower starting dose of apatinib and give etoposide for 10 days rather than 14.
“Our study showed that the combination therapy of apatinib with oral etoposide shows promising efficacy and manageable toxicities in patients with platinum-resistant or platinum-refractory recurrent ovarian cancer and further study in phase 3 trials is warranted,” the study authors concluded.
They said that a strength of their study was that both apatinib and oral etoposide were able to be given orally without the need for hospital admission or an infusion pump, factors that could improve adherence and cost effectiveness for patients.
However, they noted that one limitation was that it was a single-arm study with no control group, which meant the selection bias could not be ruled out.
Source: Lan CY et al. Lancet Oncol. 2018 Aug 3. doi: 10.1016/ S1470-2045(18)30349-8.
In the study by Chun-Yan Lan and colleagues, an “impressive” number of patients in the intention to treat analysis achieved an objective response (54%; 95% confidence interval, 36.6%-71.2%). Given that the response to standard cytotoxic therapies in ovarian cancer is reported to be between 0-30%, the combination of apatinib with etoposide should be studied further.
The median progression-free survival of 8.1 months indicates the responses observed are durable, particularly against the backdrop of survival rates seen in other studies, such as 6.7 months in AURELIA and 6.4 months in MITO 11. However, the dangers of comparing results across trials when patient populations are different should be acknowledged.
The levels of neutropenia and anemia and fatigue seen in study participants is acceptable, but the grade 3 or 4 mucositis in 8 of 34 patients is of concern given the need for patients to take oral medications long term.
It is noteworthy that the number of patients requiring a dose reduction of apatinib was high – at 82% of patients, with 76% needing a dose reduction of etoposide. Although the authors do suggest that future studies should use a lower starting dose. Further studies should also include patient reported outcomes in order to ensure that the convenience of this combination of apatinib with etoposide is not at the expense of toxicity.
These comments were excerpted from an accompanying commentary (Lancet Oncol. 2018 Aug 3. doi: 10.1016/S1470-2045[18]30444-3) by Charlie Gourley, MD, of University of Edinburgh, U.K.
In the study by Chun-Yan Lan and colleagues, an “impressive” number of patients in the intention to treat analysis achieved an objective response (54%; 95% confidence interval, 36.6%-71.2%). Given that the response to standard cytotoxic therapies in ovarian cancer is reported to be between 0-30%, the combination of apatinib with etoposide should be studied further.
The median progression-free survival of 8.1 months indicates the responses observed are durable, particularly against the backdrop of survival rates seen in other studies, such as 6.7 months in AURELIA and 6.4 months in MITO 11. However, the dangers of comparing results across trials when patient populations are different should be acknowledged.
The levels of neutropenia and anemia and fatigue seen in study participants is acceptable, but the grade 3 or 4 mucositis in 8 of 34 patients is of concern given the need for patients to take oral medications long term.
It is noteworthy that the number of patients requiring a dose reduction of apatinib was high – at 82% of patients, with 76% needing a dose reduction of etoposide. Although the authors do suggest that future studies should use a lower starting dose. Further studies should also include patient reported outcomes in order to ensure that the convenience of this combination of apatinib with etoposide is not at the expense of toxicity.
These comments were excerpted from an accompanying commentary (Lancet Oncol. 2018 Aug 3. doi: 10.1016/S1470-2045[18]30444-3) by Charlie Gourley, MD, of University of Edinburgh, U.K.
In the study by Chun-Yan Lan and colleagues, an “impressive” number of patients in the intention to treat analysis achieved an objective response (54%; 95% confidence interval, 36.6%-71.2%). Given that the response to standard cytotoxic therapies in ovarian cancer is reported to be between 0-30%, the combination of apatinib with etoposide should be studied further.
The median progression-free survival of 8.1 months indicates the responses observed are durable, particularly against the backdrop of survival rates seen in other studies, such as 6.7 months in AURELIA and 6.4 months in MITO 11. However, the dangers of comparing results across trials when patient populations are different should be acknowledged.
The levels of neutropenia and anemia and fatigue seen in study participants is acceptable, but the grade 3 or 4 mucositis in 8 of 34 patients is of concern given the need for patients to take oral medications long term.
It is noteworthy that the number of patients requiring a dose reduction of apatinib was high – at 82% of patients, with 76% needing a dose reduction of etoposide. Although the authors do suggest that future studies should use a lower starting dose. Further studies should also include patient reported outcomes in order to ensure that the convenience of this combination of apatinib with etoposide is not at the expense of toxicity.
These comments were excerpted from an accompanying commentary (Lancet Oncol. 2018 Aug 3. doi: 10.1016/S1470-2045[18]30444-3) by Charlie Gourley, MD, of University of Edinburgh, U.K.
Combining the vascular endothelial growth factor (VEGF) receptor–targeting tyrosine kinase inhibitor apatinib with oral etoposide in people with platinum resistant or refractory ovarian cancer has shown promising efficacy and manageable toxicity in a Phase 2 study.
Angiogenesis was a “hallmark” process in cancer, and antiangiogenic therapy, including anti-VEGF antibodies and multireceptor tyrosine kinase inhibitors, has been shown to be an attractive therapeutic strategy for ovarian cancer.
“Increasing evidence suggests that the combination of antiangiogenic therapy and single-agent chemotherapy improves the outcome of platinum-resistant ovarian cancer,” Chun-Yan Lan, MD, of the Collaborative Innovation Center for Cancer Medicine at Sun Yat-sen University Cancer Center in Guangzhou, China, and colleagues wrote in Lancet Oncology.
The investigators chose apatinib because it has shown encouraging antitumor activities and tolerable toxicities in several malignant tumors and was available in mainland China, they said.
The single-arm prospective study enrolled 35 women aged 18-70 years with heavily pretreated ovarian cancer that was refractory to platinum (defined as progression during the initial platinum-based treatment) or resistant to platinum (defined as progression within 6 months after the last platinum treatment).
Women were treated with apatinib at an initial dose of 500 mg once daily on a continuous basis and with oral etoposide at a dose of 50 mg once daily on days 1-14 of a 21-day cycle. Oral etoposide was administered for a maximum of six cycles. Dose modifications, including dose interruptions, were allowed in order to manage adverse events.
Treatment was continued until disease progression, patient withdrawal, or unacceptable toxic effects. The primary endpoint of the study was the proportion of patients achieving an objective response according to Response Evaluation Criteria in Solid Tumors (RECIST). The study authors analyzed efficacy data using three populations: intention-to-treat, per-protocol, and safety populations.
Results showed that 19 of 35 patients achieved an objective response (54%; 95% confidence interval, 36.6%-71.2%) in an intention-to-treat analysis. In the per-protocol population, 19 of 31 patients (61%; 95% CI, 42.2%-78.2%) achieved an objective response.
Median progression-free survival was 8.1 months (interquartile range, 4.3-14.6; 95% CI, 2.8-13.4), and the median duration of response was 7.4 months (IQR, 4.0-13.0; 95% CI, 2.3-12.0).
The most common grade 3 or 4 adverse events reported were neutropenia (n = 17), fatigue (n = 11), anemia n = 10), and mucositis (n = 8). Serious adverse events were reported in two patients who were admitted to the hospital.
Dose reductions were required in 82% (n = 28) of 34 patients on apatinib and 77% (n = 26) for etoposide. The authors said they would suggest future studies use a lower starting dose of apatinib and give etoposide for 10 days rather than 14.
“Our study showed that the combination therapy of apatinib with oral etoposide shows promising efficacy and manageable toxicities in patients with platinum-resistant or platinum-refractory recurrent ovarian cancer and further study in phase 3 trials is warranted,” the study authors concluded.
They said that a strength of their study was that both apatinib and oral etoposide were able to be given orally without the need for hospital admission or an infusion pump, factors that could improve adherence and cost effectiveness for patients.
However, they noted that one limitation was that it was a single-arm study with no control group, which meant the selection bias could not be ruled out.
Source: Lan CY et al. Lancet Oncol. 2018 Aug 3. doi: 10.1016/ S1470-2045(18)30349-8.
Combining the vascular endothelial growth factor (VEGF) receptor–targeting tyrosine kinase inhibitor apatinib with oral etoposide in people with platinum resistant or refractory ovarian cancer has shown promising efficacy and manageable toxicity in a Phase 2 study.
Angiogenesis was a “hallmark” process in cancer, and antiangiogenic therapy, including anti-VEGF antibodies and multireceptor tyrosine kinase inhibitors, has been shown to be an attractive therapeutic strategy for ovarian cancer.
“Increasing evidence suggests that the combination of antiangiogenic therapy and single-agent chemotherapy improves the outcome of platinum-resistant ovarian cancer,” Chun-Yan Lan, MD, of the Collaborative Innovation Center for Cancer Medicine at Sun Yat-sen University Cancer Center in Guangzhou, China, and colleagues wrote in Lancet Oncology.
The investigators chose apatinib because it has shown encouraging antitumor activities and tolerable toxicities in several malignant tumors and was available in mainland China, they said.
The single-arm prospective study enrolled 35 women aged 18-70 years with heavily pretreated ovarian cancer that was refractory to platinum (defined as progression during the initial platinum-based treatment) or resistant to platinum (defined as progression within 6 months after the last platinum treatment).
Women were treated with apatinib at an initial dose of 500 mg once daily on a continuous basis and with oral etoposide at a dose of 50 mg once daily on days 1-14 of a 21-day cycle. Oral etoposide was administered for a maximum of six cycles. Dose modifications, including dose interruptions, were allowed in order to manage adverse events.
Treatment was continued until disease progression, patient withdrawal, or unacceptable toxic effects. The primary endpoint of the study was the proportion of patients achieving an objective response according to Response Evaluation Criteria in Solid Tumors (RECIST). The study authors analyzed efficacy data using three populations: intention-to-treat, per-protocol, and safety populations.
Results showed that 19 of 35 patients achieved an objective response (54%; 95% confidence interval, 36.6%-71.2%) in an intention-to-treat analysis. In the per-protocol population, 19 of 31 patients (61%; 95% CI, 42.2%-78.2%) achieved an objective response.
Median progression-free survival was 8.1 months (interquartile range, 4.3-14.6; 95% CI, 2.8-13.4), and the median duration of response was 7.4 months (IQR, 4.0-13.0; 95% CI, 2.3-12.0).
The most common grade 3 or 4 adverse events reported were neutropenia (n = 17), fatigue (n = 11), anemia n = 10), and mucositis (n = 8). Serious adverse events were reported in two patients who were admitted to the hospital.
Dose reductions were required in 82% (n = 28) of 34 patients on apatinib and 77% (n = 26) for etoposide. The authors said they would suggest future studies use a lower starting dose of apatinib and give etoposide for 10 days rather than 14.
“Our study showed that the combination therapy of apatinib with oral etoposide shows promising efficacy and manageable toxicities in patients with platinum-resistant or platinum-refractory recurrent ovarian cancer and further study in phase 3 trials is warranted,” the study authors concluded.
They said that a strength of their study was that both apatinib and oral etoposide were able to be given orally without the need for hospital admission or an infusion pump, factors that could improve adherence and cost effectiveness for patients.
However, they noted that one limitation was that it was a single-arm study with no control group, which meant the selection bias could not be ruled out.
Source: Lan CY et al. Lancet Oncol. 2018 Aug 3. doi: 10.1016/ S1470-2045(18)30349-8.
FROM LANCET ONCOLOGY
Key clinical point: The VEGF receptor tyrosine kinase inhibitor apatinib in combination with oral etoposide in people with platinum resistant or refractory ovarian cancer has shown promising efficacy and manageable toxicity.
Major finding: Nineteen of 35 patients with heavily pretreated ovarian cancer refractory or resistant to platinum achieved an objective response (54%; 95% CI 36.6-71.2) in an intention-to-treat analysis.
Study details: A single arm prospective phase 2 study of 35 women aged 18-70 years with heavily pretreated platinum resistant or refractory ovarian cancer.
Disclosures: Jiangsu Hengrui Pharmaceuticals discounted apatinib to patients enrolled in the study.
Source: Lan CY et al. Lancet Oncol. 2018 Aug 3. doi: 10.1016/ S1470-2045(18)30349-8.
ACP roadmap to raise adult immunization rates
NEW ORLEANS – carried out in seven rural southwestern Georgia counties.
“We saw increases of 52%-93% in our pneumococcal vaccination rates in our nine adult medicine clinics,” Frances E. Ferguson, MD, said at the annual meeting of the American College of Physicians.
The project, conducted through the ACP’s quality improvement program, known as Quality Connect, with funding from the Centers for Disease Control and Prevention, has as its ultimate goal making adult immunization standard practice across the country in general internal medicine and primary care. One of the initial pilot projects was conducted at Albany (Ga.) Area Primary Health Care, a federally qualified community health center with 28 service delivery sites in seven mainly rural southwestern Georgia counties. The majority of the center’s patients are at or below the poverty level. The lessons learned from this and other successful local projects will be disseminated nationally, according to Dr. Ferguson, a general internist at the health center.
The project utilizes the ACP Quality Connect “Plan, Do, Study, Act” approach to implementing constructive changes in medical practice, coupled with an abundance of resources readily available from the nonprofit Immunization Action Coalition, which has been funded by the CDC for more than 20 years.
The coalition’s website includes easily downloadable and custom-modifiable standing orders, plain-language information handouts on pneumococcal pneumonia and other vaccine-preventable illnesses and possible vaccine side effects for patients to read in the waiting room before their office visit, concise CDC-standardized talking points for providers to use in convincing patients to get vaccinated, as well as a detailed 10-point action plan for medical practice leadership to make it all happen.
“You don’t have to reinvent the wheel with this. You just go to their website,” Dr. Ferguson explained.
She cited her personal experience as representative of that of her fellow general internists who got on board with the project’s goal. As of April 2016, just before rollout of the pilot project, Dr. Ferguson’s pneumococcal vaccination rate stood at 22.5%. One month into the project, her vaccination rate had zoomed to 60%. When the formal pilot project ended in February 2017, her rate was 88.2%, an absolute 65.7% increase in 10 months. And since the pilot project’s conclusion, adult pneumococcal vaccination rates across Albany Area Primary Health Care have continued to climb.
“We have continued to improve because our champions are still in place, and our standing orders are, too,” she noted.
Standing orders are at the core of the project’s success, according to Dr. Ferguson. They provide nurses with the legal authority to determine if a patient is eligible for a vaccine and to go ahead and give it before seeing the physician.
“The most important thing for me is that nurses are an essential and invaluable asset to your practice, especially when you empower them to function fully within the scope of their practice. They can do amazing things. I just feel like the nurses are the winners here. We are winners because of our nurses,” she said.
It wasn’t easy at first, Dr. Ferguson recalled.
“We had pushback from nurses because they were afraid to do anything without the doctor directly telling them to do it. And we had physicians who didn’t want to have standing orders because they didn’t want anybody to do anything until they told them to. But we managed to get all that straightened out in the first month,” according to the internist.
Other keys to the program’s success included designating champions of the project at every clinic. Often this was the director of nursing, who was appointed to be in charge of the standing orders program. This “champions” concept is detailed in the ACP Quality Connect website. The champion – Dr. Ferguson was one – gets staff buy in on promoting the importance of immunizations, teaches strategies, and engages in community outreach.
“They lead the practice in the quality improvement project. We found that was very important, to have someone at each site who could keep the fire under the staff, keep the project going even when it’s very busy and everybody’s running around. There has to be someone there who’s encouraging people to remember to keep giving immunizations in spite of everything that’s going on that day,” she explained.
A central principle of successful quality improvement programs is accurate performance data gathering and dissemination. “Doctors are very competitive people,” Dr. Ferguson observed. “When I tell you you’re not doing as well as your colleagues and I can show you a graph that shows how far you are lagging behind, you get the fire under you and get moving.”
Audience members were agog at the community health center’s stratospheric vaccination rates. What about all the vaccine skeptics? they asked.
“I think for us it’s a matter of trust,” she replied. “We are a community health center and many of our patients and our providers have been with us for a long time. The nursing staff live in those communities, so the patients know them well. The CDC’s cards with talking points are a big help. I take the time to talk to patients and explain how the vaccine is going to build an army of immunologic protection. There are always going to be the diehards who say, ‘My cousin’s foot fell off when he got the pneumonia vaccine,’ though. You can’t get past those people. There’s just nothing you can say to them that will change their mind.”
She reported having no financial conflicts regarding her presentation or the adult immunization initiative.
[email protected]
NEW ORLEANS – carried out in seven rural southwestern Georgia counties.
“We saw increases of 52%-93% in our pneumococcal vaccination rates in our nine adult medicine clinics,” Frances E. Ferguson, MD, said at the annual meeting of the American College of Physicians.
The project, conducted through the ACP’s quality improvement program, known as Quality Connect, with funding from the Centers for Disease Control and Prevention, has as its ultimate goal making adult immunization standard practice across the country in general internal medicine and primary care. One of the initial pilot projects was conducted at Albany (Ga.) Area Primary Health Care, a federally qualified community health center with 28 service delivery sites in seven mainly rural southwestern Georgia counties. The majority of the center’s patients are at or below the poverty level. The lessons learned from this and other successful local projects will be disseminated nationally, according to Dr. Ferguson, a general internist at the health center.
The project utilizes the ACP Quality Connect “Plan, Do, Study, Act” approach to implementing constructive changes in medical practice, coupled with an abundance of resources readily available from the nonprofit Immunization Action Coalition, which has been funded by the CDC for more than 20 years.
The coalition’s website includes easily downloadable and custom-modifiable standing orders, plain-language information handouts on pneumococcal pneumonia and other vaccine-preventable illnesses and possible vaccine side effects for patients to read in the waiting room before their office visit, concise CDC-standardized talking points for providers to use in convincing patients to get vaccinated, as well as a detailed 10-point action plan for medical practice leadership to make it all happen.
“You don’t have to reinvent the wheel with this. You just go to their website,” Dr. Ferguson explained.
She cited her personal experience as representative of that of her fellow general internists who got on board with the project’s goal. As of April 2016, just before rollout of the pilot project, Dr. Ferguson’s pneumococcal vaccination rate stood at 22.5%. One month into the project, her vaccination rate had zoomed to 60%. When the formal pilot project ended in February 2017, her rate was 88.2%, an absolute 65.7% increase in 10 months. And since the pilot project’s conclusion, adult pneumococcal vaccination rates across Albany Area Primary Health Care have continued to climb.
“We have continued to improve because our champions are still in place, and our standing orders are, too,” she noted.
Standing orders are at the core of the project’s success, according to Dr. Ferguson. They provide nurses with the legal authority to determine if a patient is eligible for a vaccine and to go ahead and give it before seeing the physician.
“The most important thing for me is that nurses are an essential and invaluable asset to your practice, especially when you empower them to function fully within the scope of their practice. They can do amazing things. I just feel like the nurses are the winners here. We are winners because of our nurses,” she said.
It wasn’t easy at first, Dr. Ferguson recalled.
“We had pushback from nurses because they were afraid to do anything without the doctor directly telling them to do it. And we had physicians who didn’t want to have standing orders because they didn’t want anybody to do anything until they told them to. But we managed to get all that straightened out in the first month,” according to the internist.
Other keys to the program’s success included designating champions of the project at every clinic. Often this was the director of nursing, who was appointed to be in charge of the standing orders program. This “champions” concept is detailed in the ACP Quality Connect website. The champion – Dr. Ferguson was one – gets staff buy in on promoting the importance of immunizations, teaches strategies, and engages in community outreach.
“They lead the practice in the quality improvement project. We found that was very important, to have someone at each site who could keep the fire under the staff, keep the project going even when it’s very busy and everybody’s running around. There has to be someone there who’s encouraging people to remember to keep giving immunizations in spite of everything that’s going on that day,” she explained.
A central principle of successful quality improvement programs is accurate performance data gathering and dissemination. “Doctors are very competitive people,” Dr. Ferguson observed. “When I tell you you’re not doing as well as your colleagues and I can show you a graph that shows how far you are lagging behind, you get the fire under you and get moving.”
Audience members were agog at the community health center’s stratospheric vaccination rates. What about all the vaccine skeptics? they asked.
“I think for us it’s a matter of trust,” she replied. “We are a community health center and many of our patients and our providers have been with us for a long time. The nursing staff live in those communities, so the patients know them well. The CDC’s cards with talking points are a big help. I take the time to talk to patients and explain how the vaccine is going to build an army of immunologic protection. There are always going to be the diehards who say, ‘My cousin’s foot fell off when he got the pneumonia vaccine,’ though. You can’t get past those people. There’s just nothing you can say to them that will change their mind.”
She reported having no financial conflicts regarding her presentation or the adult immunization initiative.
[email protected]
NEW ORLEANS – carried out in seven rural southwestern Georgia counties.
“We saw increases of 52%-93% in our pneumococcal vaccination rates in our nine adult medicine clinics,” Frances E. Ferguson, MD, said at the annual meeting of the American College of Physicians.
The project, conducted through the ACP’s quality improvement program, known as Quality Connect, with funding from the Centers for Disease Control and Prevention, has as its ultimate goal making adult immunization standard practice across the country in general internal medicine and primary care. One of the initial pilot projects was conducted at Albany (Ga.) Area Primary Health Care, a federally qualified community health center with 28 service delivery sites in seven mainly rural southwestern Georgia counties. The majority of the center’s patients are at or below the poverty level. The lessons learned from this and other successful local projects will be disseminated nationally, according to Dr. Ferguson, a general internist at the health center.
The project utilizes the ACP Quality Connect “Plan, Do, Study, Act” approach to implementing constructive changes in medical practice, coupled with an abundance of resources readily available from the nonprofit Immunization Action Coalition, which has been funded by the CDC for more than 20 years.
The coalition’s website includes easily downloadable and custom-modifiable standing orders, plain-language information handouts on pneumococcal pneumonia and other vaccine-preventable illnesses and possible vaccine side effects for patients to read in the waiting room before their office visit, concise CDC-standardized talking points for providers to use in convincing patients to get vaccinated, as well as a detailed 10-point action plan for medical practice leadership to make it all happen.
“You don’t have to reinvent the wheel with this. You just go to their website,” Dr. Ferguson explained.
She cited her personal experience as representative of that of her fellow general internists who got on board with the project’s goal. As of April 2016, just before rollout of the pilot project, Dr. Ferguson’s pneumococcal vaccination rate stood at 22.5%. One month into the project, her vaccination rate had zoomed to 60%. When the formal pilot project ended in February 2017, her rate was 88.2%, an absolute 65.7% increase in 10 months. And since the pilot project’s conclusion, adult pneumococcal vaccination rates across Albany Area Primary Health Care have continued to climb.
“We have continued to improve because our champions are still in place, and our standing orders are, too,” she noted.
Standing orders are at the core of the project’s success, according to Dr. Ferguson. They provide nurses with the legal authority to determine if a patient is eligible for a vaccine and to go ahead and give it before seeing the physician.
“The most important thing for me is that nurses are an essential and invaluable asset to your practice, especially when you empower them to function fully within the scope of their practice. They can do amazing things. I just feel like the nurses are the winners here. We are winners because of our nurses,” she said.
It wasn’t easy at first, Dr. Ferguson recalled.
“We had pushback from nurses because they were afraid to do anything without the doctor directly telling them to do it. And we had physicians who didn’t want to have standing orders because they didn’t want anybody to do anything until they told them to. But we managed to get all that straightened out in the first month,” according to the internist.
Other keys to the program’s success included designating champions of the project at every clinic. Often this was the director of nursing, who was appointed to be in charge of the standing orders program. This “champions” concept is detailed in the ACP Quality Connect website. The champion – Dr. Ferguson was one – gets staff buy in on promoting the importance of immunizations, teaches strategies, and engages in community outreach.
“They lead the practice in the quality improvement project. We found that was very important, to have someone at each site who could keep the fire under the staff, keep the project going even when it’s very busy and everybody’s running around. There has to be someone there who’s encouraging people to remember to keep giving immunizations in spite of everything that’s going on that day,” she explained.
A central principle of successful quality improvement programs is accurate performance data gathering and dissemination. “Doctors are very competitive people,” Dr. Ferguson observed. “When I tell you you’re not doing as well as your colleagues and I can show you a graph that shows how far you are lagging behind, you get the fire under you and get moving.”
Audience members were agog at the community health center’s stratospheric vaccination rates. What about all the vaccine skeptics? they asked.
“I think for us it’s a matter of trust,” she replied. “We are a community health center and many of our patients and our providers have been with us for a long time. The nursing staff live in those communities, so the patients know them well. The CDC’s cards with talking points are a big help. I take the time to talk to patients and explain how the vaccine is going to build an army of immunologic protection. There are always going to be the diehards who say, ‘My cousin’s foot fell off when he got the pneumonia vaccine,’ though. You can’t get past those people. There’s just nothing you can say to them that will change their mind.”
She reported having no financial conflicts regarding her presentation or the adult immunization initiative.
[email protected]
REPORTING FROM ACP INTERNAL MEDICINE
Doctors decry inaction on physician-focused APMs
Doctors have expressed their displeasure at the lack of response by the Centers for Medicare & Medicaid Services to launch physician-focused advanced alternative payment models (APMs).
As part of the MACRA law, Congress created a process by which physicians could seek to implement specialty-specific APMs that they had developed and tested. The purpose was to provide more avenues for specialist participation in the Quality Payment Program’s APM track.
The process goes like this: Doctors create and implement an APM that focuses on providing value-based care in their particular specialty arena. They submit the program and early outcomes to the Physician-Focused Payment Model Technical Advisory Committee or PTAC. The committee reviews the APM and, if it has merit, forwards it to the CMS. The CMS can either approve the APM or ask for additional testing.
So far, PTAC has sent at least 10 APMs to the CMS. To date, not a single one has been approved or even tested on a limited scale.
“Physicians want to be engaged and involved in this process,” David Barbe, MD, immediate past president of the American Medical Association, told members of the House Energy and Commerce Health Subcommittee during a July 26 hearing. “PTAC was created for that very reason. They have received dozens of proposals that come from the ground level. Physicians that are practicing know what will work in their practices and perhaps in their specialty. And yet, none of these have been adopted by CMS or really, we think, given serious consideration.”
Frank Opelka, MD, medical director for quality and health policy at the American College of Surgeons, noted that a proposal they had submitted to PTAC appears to be the one that has gotten furthest along in the process.
The model was “accepted in a letter by the Secretary for consideration by the [CMS Innovation Center],” Dr. Opelka testified at the hearing. “The innovation center had a few conference calls with us and one 2-hour in-person meeting on a product that we’d developed that took almost 5 years in the making. There are no resources and no capability in the innovation center to complete a design and then to create an implementation and have a sandbox or pilot area in which to test. The PTAC has done a fantastic job. The Secretary vetted us. I think [ours was] the only one that went from the Secretary and was recommended to the innovation center and it died in there because [the Center] is just not wired to really innovate and we really need to turn that on.”
The CMS issued a letter on June 13 essentially rejecting eight of the models that PTAC recommended. The AMA asked the agency to reconsider at least four of the proposals in a June 21 letter.
AMA leadership does not think that the CMS gave serious consideration to any of the PTAC recommendations, Dr. Barbe said. “These span from very focused proposals in GI medicine to reduce rehospitalization in Crohn’s patients all the way up to the end-stage renal disease that could have very broad effect on improving care and reducing cost for dialysis patients. We think there is great opportunity there if CMS will listen to us.”
The AMA is “especially concerned because the statute to reform Medicare physician payment provided only 6 years of bonus payments to facilitate physicians’ migration to APMs,” according to the group’s letter to the CMS. “We are approaching the 3-year mark for the initial implementation and there is still not a robust APM pathway for physicians.”
Dr. Barbe also expressed concern that physicians’ taste for innovation could wane, given 3 years without successful implementation or testing of a physician-focused APM.
The CMS “seems to be interested in coming up with ideas on their own and I think that’s not only reinventing the wheel potentially, but it is not taking advantage of some very creative and innovated proposals that have come forward,” Dr. Barbe said.
The AMA recognizes “that the APMs recommended by PTAC needed some refinement. Data and pilot test experience likely would help in addressing some of the concerns raised by both PTAC and HHS,” according to the letter. “PTAC has indicated in its recommendations to HHS that it felt the issues it had identified could be resolved with assistance from CMS. Moreover, PTAC concluded that the positive attributes of the APM proposals outweigh the concerns they had identified, but the department does not seem to agree.”
Dr. Opelka, in his written testimony to the subcommittee, suggested that “it may be invaluable to commission a study on these challenges, including CMS’ ability to measure the true quality of care provided by physicians of all specialties, the availability of cost measures that are meaningful and actionable in concert with these quality measures, physicians’ ability to access patient health information when they need it and in a standardized predictable format, and the availability of APMs that grant physicians of all specialties the opportunity to be creative in using their expertise to increase quality and value of care to the patient.”
Doctors have expressed their displeasure at the lack of response by the Centers for Medicare & Medicaid Services to launch physician-focused advanced alternative payment models (APMs).
As part of the MACRA law, Congress created a process by which physicians could seek to implement specialty-specific APMs that they had developed and tested. The purpose was to provide more avenues for specialist participation in the Quality Payment Program’s APM track.
The process goes like this: Doctors create and implement an APM that focuses on providing value-based care in their particular specialty arena. They submit the program and early outcomes to the Physician-Focused Payment Model Technical Advisory Committee or PTAC. The committee reviews the APM and, if it has merit, forwards it to the CMS. The CMS can either approve the APM or ask for additional testing.
So far, PTAC has sent at least 10 APMs to the CMS. To date, not a single one has been approved or even tested on a limited scale.
“Physicians want to be engaged and involved in this process,” David Barbe, MD, immediate past president of the American Medical Association, told members of the House Energy and Commerce Health Subcommittee during a July 26 hearing. “PTAC was created for that very reason. They have received dozens of proposals that come from the ground level. Physicians that are practicing know what will work in their practices and perhaps in their specialty. And yet, none of these have been adopted by CMS or really, we think, given serious consideration.”
Frank Opelka, MD, medical director for quality and health policy at the American College of Surgeons, noted that a proposal they had submitted to PTAC appears to be the one that has gotten furthest along in the process.
The model was “accepted in a letter by the Secretary for consideration by the [CMS Innovation Center],” Dr. Opelka testified at the hearing. “The innovation center had a few conference calls with us and one 2-hour in-person meeting on a product that we’d developed that took almost 5 years in the making. There are no resources and no capability in the innovation center to complete a design and then to create an implementation and have a sandbox or pilot area in which to test. The PTAC has done a fantastic job. The Secretary vetted us. I think [ours was] the only one that went from the Secretary and was recommended to the innovation center and it died in there because [the Center] is just not wired to really innovate and we really need to turn that on.”
The CMS issued a letter on June 13 essentially rejecting eight of the models that PTAC recommended. The AMA asked the agency to reconsider at least four of the proposals in a June 21 letter.
AMA leadership does not think that the CMS gave serious consideration to any of the PTAC recommendations, Dr. Barbe said. “These span from very focused proposals in GI medicine to reduce rehospitalization in Crohn’s patients all the way up to the end-stage renal disease that could have very broad effect on improving care and reducing cost for dialysis patients. We think there is great opportunity there if CMS will listen to us.”
The AMA is “especially concerned because the statute to reform Medicare physician payment provided only 6 years of bonus payments to facilitate physicians’ migration to APMs,” according to the group’s letter to the CMS. “We are approaching the 3-year mark for the initial implementation and there is still not a robust APM pathway for physicians.”
Dr. Barbe also expressed concern that physicians’ taste for innovation could wane, given 3 years without successful implementation or testing of a physician-focused APM.
The CMS “seems to be interested in coming up with ideas on their own and I think that’s not only reinventing the wheel potentially, but it is not taking advantage of some very creative and innovated proposals that have come forward,” Dr. Barbe said.
The AMA recognizes “that the APMs recommended by PTAC needed some refinement. Data and pilot test experience likely would help in addressing some of the concerns raised by both PTAC and HHS,” according to the letter. “PTAC has indicated in its recommendations to HHS that it felt the issues it had identified could be resolved with assistance from CMS. Moreover, PTAC concluded that the positive attributes of the APM proposals outweigh the concerns they had identified, but the department does not seem to agree.”
Dr. Opelka, in his written testimony to the subcommittee, suggested that “it may be invaluable to commission a study on these challenges, including CMS’ ability to measure the true quality of care provided by physicians of all specialties, the availability of cost measures that are meaningful and actionable in concert with these quality measures, physicians’ ability to access patient health information when they need it and in a standardized predictable format, and the availability of APMs that grant physicians of all specialties the opportunity to be creative in using their expertise to increase quality and value of care to the patient.”
Doctors have expressed their displeasure at the lack of response by the Centers for Medicare & Medicaid Services to launch physician-focused advanced alternative payment models (APMs).
As part of the MACRA law, Congress created a process by which physicians could seek to implement specialty-specific APMs that they had developed and tested. The purpose was to provide more avenues for specialist participation in the Quality Payment Program’s APM track.
The process goes like this: Doctors create and implement an APM that focuses on providing value-based care in their particular specialty arena. They submit the program and early outcomes to the Physician-Focused Payment Model Technical Advisory Committee or PTAC. The committee reviews the APM and, if it has merit, forwards it to the CMS. The CMS can either approve the APM or ask for additional testing.
So far, PTAC has sent at least 10 APMs to the CMS. To date, not a single one has been approved or even tested on a limited scale.
“Physicians want to be engaged and involved in this process,” David Barbe, MD, immediate past president of the American Medical Association, told members of the House Energy and Commerce Health Subcommittee during a July 26 hearing. “PTAC was created for that very reason. They have received dozens of proposals that come from the ground level. Physicians that are practicing know what will work in their practices and perhaps in their specialty. And yet, none of these have been adopted by CMS or really, we think, given serious consideration.”
Frank Opelka, MD, medical director for quality and health policy at the American College of Surgeons, noted that a proposal they had submitted to PTAC appears to be the one that has gotten furthest along in the process.
The model was “accepted in a letter by the Secretary for consideration by the [CMS Innovation Center],” Dr. Opelka testified at the hearing. “The innovation center had a few conference calls with us and one 2-hour in-person meeting on a product that we’d developed that took almost 5 years in the making. There are no resources and no capability in the innovation center to complete a design and then to create an implementation and have a sandbox or pilot area in which to test. The PTAC has done a fantastic job. The Secretary vetted us. I think [ours was] the only one that went from the Secretary and was recommended to the innovation center and it died in there because [the Center] is just not wired to really innovate and we really need to turn that on.”
The CMS issued a letter on June 13 essentially rejecting eight of the models that PTAC recommended. The AMA asked the agency to reconsider at least four of the proposals in a June 21 letter.
AMA leadership does not think that the CMS gave serious consideration to any of the PTAC recommendations, Dr. Barbe said. “These span from very focused proposals in GI medicine to reduce rehospitalization in Crohn’s patients all the way up to the end-stage renal disease that could have very broad effect on improving care and reducing cost for dialysis patients. We think there is great opportunity there if CMS will listen to us.”
The AMA is “especially concerned because the statute to reform Medicare physician payment provided only 6 years of bonus payments to facilitate physicians’ migration to APMs,” according to the group’s letter to the CMS. “We are approaching the 3-year mark for the initial implementation and there is still not a robust APM pathway for physicians.”
Dr. Barbe also expressed concern that physicians’ taste for innovation could wane, given 3 years without successful implementation or testing of a physician-focused APM.
The CMS “seems to be interested in coming up with ideas on their own and I think that’s not only reinventing the wheel potentially, but it is not taking advantage of some very creative and innovated proposals that have come forward,” Dr. Barbe said.
The AMA recognizes “that the APMs recommended by PTAC needed some refinement. Data and pilot test experience likely would help in addressing some of the concerns raised by both PTAC and HHS,” according to the letter. “PTAC has indicated in its recommendations to HHS that it felt the issues it had identified could be resolved with assistance from CMS. Moreover, PTAC concluded that the positive attributes of the APM proposals outweigh the concerns they had identified, but the department does not seem to agree.”
Dr. Opelka, in his written testimony to the subcommittee, suggested that “it may be invaluable to commission a study on these challenges, including CMS’ ability to measure the true quality of care provided by physicians of all specialties, the availability of cost measures that are meaningful and actionable in concert with these quality measures, physicians’ ability to access patient health information when they need it and in a standardized predictable format, and the availability of APMs that grant physicians of all specialties the opportunity to be creative in using their expertise to increase quality and value of care to the patient.”
Worst comedy audience: far right or far left?
Comedy can be about pushing the limits of topics explored and questioning societal norms. For those comedians bold enough to push hard, the result can be societal pushback. As just one example, consider the arrest of George Carlin by Milwaukee police for allegedly violating public obscenity laws for uttering those seven naughty words not to be said on television (the charge was subsequently dismissed).
David Sedaris is a present-day boundary pusher. His sources of humor have ranged from his early life growing up in a family with five siblings to the challenges of getting older with aging parents, and from American attitudes about race to his own ambivalence about guns.
Pushback against his humor has come from both ends of the political spectrum. “I don’t know which is worse: a far-right audience or a far-left audience. Each of them is a hand around my throat slowly choking the life out of me,” Mr. Sedaris said in an interview with The Economist correspondent Anne McElvoy.
Mr. Sedaris’s take on the oddities of everyday life are side splitting to some, to the tune of millions of book sales and accolades as a giant of humor – and deeply offensive to others.
His career in making people laugh began in art school with monologues on the paintings of fellow students. What has followed is a life of keeping his eyes and ears open, and a notebook at the ready to record the goings-on of daily life.
Often, something bad can happen that can prove to be funny, at least in hindsight, Mr. Sedaris explains. He cites the example of his medical examination for a kidney stone that went sideways, with him ending up in the hospital’s waiting area clad only in his underwear.
“The thought came that someday, this will be funny to me. Today it’s not, but someday it will be,” Mr. Sedaris says.
Such humor seems global. Laughs at his stories may come at different parts, based on the language and cultural interpretations. But the basic premise of the story can hit home in far-flung places.
“It’s so hard to talk about race in the United States. ... The audience thinks: ‘Wait a minute, if I laugh at this, does it mean I’m racist?’ You can feel them getting snagged there, so oftentimes I just leave that element out of the story because I want the story to move,” Mr. Sedaris says.
Does that mean humor has a limit that should not be crossed? Not to Mr. Sedaris.
Click here to listen to the interview.
Comedy can be about pushing the limits of topics explored and questioning societal norms. For those comedians bold enough to push hard, the result can be societal pushback. As just one example, consider the arrest of George Carlin by Milwaukee police for allegedly violating public obscenity laws for uttering those seven naughty words not to be said on television (the charge was subsequently dismissed).
David Sedaris is a present-day boundary pusher. His sources of humor have ranged from his early life growing up in a family with five siblings to the challenges of getting older with aging parents, and from American attitudes about race to his own ambivalence about guns.
Pushback against his humor has come from both ends of the political spectrum. “I don’t know which is worse: a far-right audience or a far-left audience. Each of them is a hand around my throat slowly choking the life out of me,” Mr. Sedaris said in an interview with The Economist correspondent Anne McElvoy.
Mr. Sedaris’s take on the oddities of everyday life are side splitting to some, to the tune of millions of book sales and accolades as a giant of humor – and deeply offensive to others.
His career in making people laugh began in art school with monologues on the paintings of fellow students. What has followed is a life of keeping his eyes and ears open, and a notebook at the ready to record the goings-on of daily life.
Often, something bad can happen that can prove to be funny, at least in hindsight, Mr. Sedaris explains. He cites the example of his medical examination for a kidney stone that went sideways, with him ending up in the hospital’s waiting area clad only in his underwear.
“The thought came that someday, this will be funny to me. Today it’s not, but someday it will be,” Mr. Sedaris says.
Such humor seems global. Laughs at his stories may come at different parts, based on the language and cultural interpretations. But the basic premise of the story can hit home in far-flung places.
“It’s so hard to talk about race in the United States. ... The audience thinks: ‘Wait a minute, if I laugh at this, does it mean I’m racist?’ You can feel them getting snagged there, so oftentimes I just leave that element out of the story because I want the story to move,” Mr. Sedaris says.
Does that mean humor has a limit that should not be crossed? Not to Mr. Sedaris.
Click here to listen to the interview.
Comedy can be about pushing the limits of topics explored and questioning societal norms. For those comedians bold enough to push hard, the result can be societal pushback. As just one example, consider the arrest of George Carlin by Milwaukee police for allegedly violating public obscenity laws for uttering those seven naughty words not to be said on television (the charge was subsequently dismissed).
David Sedaris is a present-day boundary pusher. His sources of humor have ranged from his early life growing up in a family with five siblings to the challenges of getting older with aging parents, and from American attitudes about race to his own ambivalence about guns.
Pushback against his humor has come from both ends of the political spectrum. “I don’t know which is worse: a far-right audience or a far-left audience. Each of them is a hand around my throat slowly choking the life out of me,” Mr. Sedaris said in an interview with The Economist correspondent Anne McElvoy.
Mr. Sedaris’s take on the oddities of everyday life are side splitting to some, to the tune of millions of book sales and accolades as a giant of humor – and deeply offensive to others.
His career in making people laugh began in art school with monologues on the paintings of fellow students. What has followed is a life of keeping his eyes and ears open, and a notebook at the ready to record the goings-on of daily life.
Often, something bad can happen that can prove to be funny, at least in hindsight, Mr. Sedaris explains. He cites the example of his medical examination for a kidney stone that went sideways, with him ending up in the hospital’s waiting area clad only in his underwear.
“The thought came that someday, this will be funny to me. Today it’s not, but someday it will be,” Mr. Sedaris says.
Such humor seems global. Laughs at his stories may come at different parts, based on the language and cultural interpretations. But the basic premise of the story can hit home in far-flung places.
“It’s so hard to talk about race in the United States. ... The audience thinks: ‘Wait a minute, if I laugh at this, does it mean I’m racist?’ You can feel them getting snagged there, so oftentimes I just leave that element out of the story because I want the story to move,” Mr. Sedaris says.
Does that mean humor has a limit that should not be crossed? Not to Mr. Sedaris.
Click here to listen to the interview.
AS inflammatory back pain criteria fall short in PsA
Established criteria for identifying inflammatory back pain in people with ankylosing spondylitis do not perform well in identifying axial involvement in people with psoriatic arthritis and neither does clinical judgment, a study shows.
There’s reason to believe that the natural history of patients with psoriatic arthritis (PsA) who have axial disease could differ from those without it, and there are differences in how well criteria that are currently used to identify inflammatory back pain (IBP) in people with ankylosing spondylitis (AS) perform in people with PsA, study first author Kristy S. Yap, MBBS, and her colleagues at the University of Toronto Psoriatic Arthritis Clinic wrote in Annals of the Rheumatic Diseases.
“Axial involvement in PsA is a marker of disease severity, and those with axial disease often have worse outcomes, compared with peripheral arthritis alone,” they wrote.
This is backed up by European League Against Rheumatism recommendations that advise clinicians to consider prescribing tumor necrosis factor inhibitors for people with PsA who have active axial involvement.
“Thus, an important question when evaluating a patient with PsA is to determine if axial PsA is present,” they wrote, noting that it was currently unclear whether the three sets of criteria that exist for defining inflammatory back pain in AS – Calin, Rudwaleit, and Assessment of Spondyloarthritis International Society (ASAS) – were useful for screening for axial involvement in people with PsA.
The researchers therefore set out to determine the agreement between rheumatologist judgment of the presence of IBP as well as the presence of IBP according to the three criteria in 171 patients with PsA (52% male, average age 46.6 years), 96 of whom reported chronic back pain, including 65 with IBP and 31 with nonspecific back pain.
Radiology data from these patients showed that 27 with baseline x-rays fulfilled the New York radiographic criteria for AS, and 45 had radiographic sacroiliitis not satisfying NY criteria (excluding grade 1) and/or syndesmophytes. Nine out of 31 patients with no axial disease on x-ray had evidence of axial disease on MRI. Eighteen out of 54 patients had axial involvement without back pain.
Results showed that agreement (kappa coefficient) between rheumatologist judgment of IBP and IBP criteria in patients with back pain was moderate and was highest for the Calin criteria (0.70; 95% confidence interval, 0.56-0.85), followed by the ASAS criteria (0.61; 95% CI, 0.46-0.76) and the Rudwaleit criteria (0.59; 95% CI, 0.44-0.74).
When x-ray or MRI change was considered “gold standard” for axial involvement for all patients, the specificity was high for rheumatologist judgment of IBP as well as Calin, Rudwaleit, and ASAS criteria, but their sensitivity was low, the researchers reported.
When the investigators compared positive likelihood ratios (LRs) for the presence of back pain, the Rudwaleit criteria (2.17) performed the best in ruling in axial disease, whereas the LRs were 1.75 for Calin and 1.86 for ASAS criteria. Rheumatologist-reported back pain (0.68) performed the best for ruling out axial disease when comparing negative LRs.
“The low positive LRs of the Calin, Rudwaleit, and ASAS criteria as well as that of rheumatologist report of back pain or judgment of IBP for [axial] PsA defined as any axial radiological change found in our study suggests that none of these criteria performed well in detecting axial disease in patients with PsA,” the study authors wrote.
The authors also conducted an exploratory analysis within patients with PsA with back involvement (defined by x-rays or MRI) and compared those with back pain (n = 36) or without (n = 18). The back pain group had a significantly higher Bath Ankylosing Spondylitis Disease Activity Index score (5.72 vs. 4.27), a finding that the authors said they expected because it is a patient-reported measure.
The back pain group also had a lower prevalence of human leukocyte antigen-B*38 (2.78 vs. 27.78), a finding that the authors said was interesting but would need to be replicated in future studies.
The prevalence of HLA-B*27, HLA-B*08, and HLA-C*06 was similar between patients with and without back pain, indicating “that the two groups are largely similar and hence, for the purpose of defining axial disease in PsA, symptoms (back pain) may not be important.”
“The findings of this study suggest that rheumatologist-judged IBP or the criteria for IBP developed for AS may not perform well when ascertaining axial involvement in PsA,” the study authors concluded.
“Moreover, patients with axial radiological changes without back pain were similar to those with back pain. ... In order to stratify patients with poorer prognosis, rheumatologists should consider conducting axial imaging in all patients with PsA regardless of the presence or the nature of back pain,” they added.
The study was funded by the University of Toronto Psoriatic Arthritis Program, which is supported by the Krembil Foundation.
SOURCE: Yap KS et al. Ann Rheum Dis. 2018 Aug 4. doi: 10.1136/annrheumdis-2018-213334.
Identifying psoriatic arthritis with axial disease (AxPsA) is important because it changes the treatment selection and also may be associated with a more severe disease course. In a recent paper by Yap et al, the investigators underscore the challenges in identifying the prevalence of axial disease in PsA. Many of our patients with PsA report back pain at some point in their disease course, and as the rheumatologist, we must grapple with whether their symptoms represent inflammatory disease that requires a change in therapy.
In this study, the authors examined the correlation of three definitions of inflammatory back pain (IBP) with both the rheumatologist’s assessment of whether the patient has IBP and with the presence of imaging findings such as x-ray or MRI abnormalities in the sacroiliac joints or lumbar spine. Of the 171 patients studied, 38% were reported to have IBP per the rheumatologist, 18% were thought to have noninflammatory back pain, and 32% had imaging findings consistent with AxSpA. The agreement between the rheumatologist and the inflammatory back pain criteria was reasonable (kappa 0.6-0.7). Rheumatologists and IBP criteria had moderate sensitivity (0.73-0.82) for having x-ray or MRI changes consistent with axial disease but low specificity (0.33-0.46). Surprisingly, HLA markers were not good markers of having axial disease in this population, aside from HLA-B38, which was protective but relatively uncommon.
The bottom line is that using IBP criteria or our general gestalt is still not as good as getting appropriate imaging and further underscores the potential need to screen patients with PsA, particularly those reporting back pain, for axial involvement.
Alexis R. Ogdie, MD, is director of the Penn Psoriatic Arthritis Clinic at the University of Pennsylvania, Philadelphia, and is a member of the steering committee for the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
Identifying psoriatic arthritis with axial disease (AxPsA) is important because it changes the treatment selection and also may be associated with a more severe disease course. In a recent paper by Yap et al, the investigators underscore the challenges in identifying the prevalence of axial disease in PsA. Many of our patients with PsA report back pain at some point in their disease course, and as the rheumatologist, we must grapple with whether their symptoms represent inflammatory disease that requires a change in therapy.
In this study, the authors examined the correlation of three definitions of inflammatory back pain (IBP) with both the rheumatologist’s assessment of whether the patient has IBP and with the presence of imaging findings such as x-ray or MRI abnormalities in the sacroiliac joints or lumbar spine. Of the 171 patients studied, 38% were reported to have IBP per the rheumatologist, 18% were thought to have noninflammatory back pain, and 32% had imaging findings consistent with AxSpA. The agreement between the rheumatologist and the inflammatory back pain criteria was reasonable (kappa 0.6-0.7). Rheumatologists and IBP criteria had moderate sensitivity (0.73-0.82) for having x-ray or MRI changes consistent with axial disease but low specificity (0.33-0.46). Surprisingly, HLA markers were not good markers of having axial disease in this population, aside from HLA-B38, which was protective but relatively uncommon.
The bottom line is that using IBP criteria or our general gestalt is still not as good as getting appropriate imaging and further underscores the potential need to screen patients with PsA, particularly those reporting back pain, for axial involvement.
Alexis R. Ogdie, MD, is director of the Penn Psoriatic Arthritis Clinic at the University of Pennsylvania, Philadelphia, and is a member of the steering committee for the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
Identifying psoriatic arthritis with axial disease (AxPsA) is important because it changes the treatment selection and also may be associated with a more severe disease course. In a recent paper by Yap et al, the investigators underscore the challenges in identifying the prevalence of axial disease in PsA. Many of our patients with PsA report back pain at some point in their disease course, and as the rheumatologist, we must grapple with whether their symptoms represent inflammatory disease that requires a change in therapy.
In this study, the authors examined the correlation of three definitions of inflammatory back pain (IBP) with both the rheumatologist’s assessment of whether the patient has IBP and with the presence of imaging findings such as x-ray or MRI abnormalities in the sacroiliac joints or lumbar spine. Of the 171 patients studied, 38% were reported to have IBP per the rheumatologist, 18% were thought to have noninflammatory back pain, and 32% had imaging findings consistent with AxSpA. The agreement between the rheumatologist and the inflammatory back pain criteria was reasonable (kappa 0.6-0.7). Rheumatologists and IBP criteria had moderate sensitivity (0.73-0.82) for having x-ray or MRI changes consistent with axial disease but low specificity (0.33-0.46). Surprisingly, HLA markers were not good markers of having axial disease in this population, aside from HLA-B38, which was protective but relatively uncommon.
The bottom line is that using IBP criteria or our general gestalt is still not as good as getting appropriate imaging and further underscores the potential need to screen patients with PsA, particularly those reporting back pain, for axial involvement.
Alexis R. Ogdie, MD, is director of the Penn Psoriatic Arthritis Clinic at the University of Pennsylvania, Philadelphia, and is a member of the steering committee for the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
Established criteria for identifying inflammatory back pain in people with ankylosing spondylitis do not perform well in identifying axial involvement in people with psoriatic arthritis and neither does clinical judgment, a study shows.
There’s reason to believe that the natural history of patients with psoriatic arthritis (PsA) who have axial disease could differ from those without it, and there are differences in how well criteria that are currently used to identify inflammatory back pain (IBP) in people with ankylosing spondylitis (AS) perform in people with PsA, study first author Kristy S. Yap, MBBS, and her colleagues at the University of Toronto Psoriatic Arthritis Clinic wrote in Annals of the Rheumatic Diseases.
“Axial involvement in PsA is a marker of disease severity, and those with axial disease often have worse outcomes, compared with peripheral arthritis alone,” they wrote.
This is backed up by European League Against Rheumatism recommendations that advise clinicians to consider prescribing tumor necrosis factor inhibitors for people with PsA who have active axial involvement.
“Thus, an important question when evaluating a patient with PsA is to determine if axial PsA is present,” they wrote, noting that it was currently unclear whether the three sets of criteria that exist for defining inflammatory back pain in AS – Calin, Rudwaleit, and Assessment of Spondyloarthritis International Society (ASAS) – were useful for screening for axial involvement in people with PsA.
The researchers therefore set out to determine the agreement between rheumatologist judgment of the presence of IBP as well as the presence of IBP according to the three criteria in 171 patients with PsA (52% male, average age 46.6 years), 96 of whom reported chronic back pain, including 65 with IBP and 31 with nonspecific back pain.
Radiology data from these patients showed that 27 with baseline x-rays fulfilled the New York radiographic criteria for AS, and 45 had radiographic sacroiliitis not satisfying NY criteria (excluding grade 1) and/or syndesmophytes. Nine out of 31 patients with no axial disease on x-ray had evidence of axial disease on MRI. Eighteen out of 54 patients had axial involvement without back pain.
Results showed that agreement (kappa coefficient) between rheumatologist judgment of IBP and IBP criteria in patients with back pain was moderate and was highest for the Calin criteria (0.70; 95% confidence interval, 0.56-0.85), followed by the ASAS criteria (0.61; 95% CI, 0.46-0.76) and the Rudwaleit criteria (0.59; 95% CI, 0.44-0.74).
When x-ray or MRI change was considered “gold standard” for axial involvement for all patients, the specificity was high for rheumatologist judgment of IBP as well as Calin, Rudwaleit, and ASAS criteria, but their sensitivity was low, the researchers reported.
When the investigators compared positive likelihood ratios (LRs) for the presence of back pain, the Rudwaleit criteria (2.17) performed the best in ruling in axial disease, whereas the LRs were 1.75 for Calin and 1.86 for ASAS criteria. Rheumatologist-reported back pain (0.68) performed the best for ruling out axial disease when comparing negative LRs.
“The low positive LRs of the Calin, Rudwaleit, and ASAS criteria as well as that of rheumatologist report of back pain or judgment of IBP for [axial] PsA defined as any axial radiological change found in our study suggests that none of these criteria performed well in detecting axial disease in patients with PsA,” the study authors wrote.
The authors also conducted an exploratory analysis within patients with PsA with back involvement (defined by x-rays or MRI) and compared those with back pain (n = 36) or without (n = 18). The back pain group had a significantly higher Bath Ankylosing Spondylitis Disease Activity Index score (5.72 vs. 4.27), a finding that the authors said they expected because it is a patient-reported measure.
The back pain group also had a lower prevalence of human leukocyte antigen-B*38 (2.78 vs. 27.78), a finding that the authors said was interesting but would need to be replicated in future studies.
The prevalence of HLA-B*27, HLA-B*08, and HLA-C*06 was similar between patients with and without back pain, indicating “that the two groups are largely similar and hence, for the purpose of defining axial disease in PsA, symptoms (back pain) may not be important.”
“The findings of this study suggest that rheumatologist-judged IBP or the criteria for IBP developed for AS may not perform well when ascertaining axial involvement in PsA,” the study authors concluded.
“Moreover, patients with axial radiological changes without back pain were similar to those with back pain. ... In order to stratify patients with poorer prognosis, rheumatologists should consider conducting axial imaging in all patients with PsA regardless of the presence or the nature of back pain,” they added.
The study was funded by the University of Toronto Psoriatic Arthritis Program, which is supported by the Krembil Foundation.
SOURCE: Yap KS et al. Ann Rheum Dis. 2018 Aug 4. doi: 10.1136/annrheumdis-2018-213334.
Established criteria for identifying inflammatory back pain in people with ankylosing spondylitis do not perform well in identifying axial involvement in people with psoriatic arthritis and neither does clinical judgment, a study shows.
There’s reason to believe that the natural history of patients with psoriatic arthritis (PsA) who have axial disease could differ from those without it, and there are differences in how well criteria that are currently used to identify inflammatory back pain (IBP) in people with ankylosing spondylitis (AS) perform in people with PsA, study first author Kristy S. Yap, MBBS, and her colleagues at the University of Toronto Psoriatic Arthritis Clinic wrote in Annals of the Rheumatic Diseases.
“Axial involvement in PsA is a marker of disease severity, and those with axial disease often have worse outcomes, compared with peripheral arthritis alone,” they wrote.
This is backed up by European League Against Rheumatism recommendations that advise clinicians to consider prescribing tumor necrosis factor inhibitors for people with PsA who have active axial involvement.
“Thus, an important question when evaluating a patient with PsA is to determine if axial PsA is present,” they wrote, noting that it was currently unclear whether the three sets of criteria that exist for defining inflammatory back pain in AS – Calin, Rudwaleit, and Assessment of Spondyloarthritis International Society (ASAS) – were useful for screening for axial involvement in people with PsA.
The researchers therefore set out to determine the agreement between rheumatologist judgment of the presence of IBP as well as the presence of IBP according to the three criteria in 171 patients with PsA (52% male, average age 46.6 years), 96 of whom reported chronic back pain, including 65 with IBP and 31 with nonspecific back pain.
Radiology data from these patients showed that 27 with baseline x-rays fulfilled the New York radiographic criteria for AS, and 45 had radiographic sacroiliitis not satisfying NY criteria (excluding grade 1) and/or syndesmophytes. Nine out of 31 patients with no axial disease on x-ray had evidence of axial disease on MRI. Eighteen out of 54 patients had axial involvement without back pain.
Results showed that agreement (kappa coefficient) between rheumatologist judgment of IBP and IBP criteria in patients with back pain was moderate and was highest for the Calin criteria (0.70; 95% confidence interval, 0.56-0.85), followed by the ASAS criteria (0.61; 95% CI, 0.46-0.76) and the Rudwaleit criteria (0.59; 95% CI, 0.44-0.74).
When x-ray or MRI change was considered “gold standard” for axial involvement for all patients, the specificity was high for rheumatologist judgment of IBP as well as Calin, Rudwaleit, and ASAS criteria, but their sensitivity was low, the researchers reported.
When the investigators compared positive likelihood ratios (LRs) for the presence of back pain, the Rudwaleit criteria (2.17) performed the best in ruling in axial disease, whereas the LRs were 1.75 for Calin and 1.86 for ASAS criteria. Rheumatologist-reported back pain (0.68) performed the best for ruling out axial disease when comparing negative LRs.
“The low positive LRs of the Calin, Rudwaleit, and ASAS criteria as well as that of rheumatologist report of back pain or judgment of IBP for [axial] PsA defined as any axial radiological change found in our study suggests that none of these criteria performed well in detecting axial disease in patients with PsA,” the study authors wrote.
The authors also conducted an exploratory analysis within patients with PsA with back involvement (defined by x-rays or MRI) and compared those with back pain (n = 36) or without (n = 18). The back pain group had a significantly higher Bath Ankylosing Spondylitis Disease Activity Index score (5.72 vs. 4.27), a finding that the authors said they expected because it is a patient-reported measure.
The back pain group also had a lower prevalence of human leukocyte antigen-B*38 (2.78 vs. 27.78), a finding that the authors said was interesting but would need to be replicated in future studies.
The prevalence of HLA-B*27, HLA-B*08, and HLA-C*06 was similar between patients with and without back pain, indicating “that the two groups are largely similar and hence, for the purpose of defining axial disease in PsA, symptoms (back pain) may not be important.”
“The findings of this study suggest that rheumatologist-judged IBP or the criteria for IBP developed for AS may not perform well when ascertaining axial involvement in PsA,” the study authors concluded.
“Moreover, patients with axial radiological changes without back pain were similar to those with back pain. ... In order to stratify patients with poorer prognosis, rheumatologists should consider conducting axial imaging in all patients with PsA regardless of the presence or the nature of back pain,” they added.
The study was funded by the University of Toronto Psoriatic Arthritis Program, which is supported by the Krembil Foundation.
SOURCE: Yap KS et al. Ann Rheum Dis. 2018 Aug 4. doi: 10.1136/annrheumdis-2018-213334.
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point:
Major finding: Agreement as measured by kappa coefficient between rheumatologist judgment of inflammatory back pain and IBP criteria in patients with back pain was moderate and was highest for the Calin criteria (0.70; 95% confidence interval, 0.56-0.85), followed by the ASAS criteria (0.61; 95% CI, 0.46-0.76) and the Rudwaleit criteria (0.59; 95% CI, 0.44-0.74).
Study details: Prospectively collected data from 171 patients attending a PsA clinic
Disclosures: The study was funded by the University of Toronto Psoriatic Arthritis Program, which is supported by the Krembil Foundation.
Source: Yap KS et al. Ann Rheum Dis. 2018 Aug 4. doi: 10.1136/annrheumdis-2018-213334.
Janssen seeks approval for split dosing of daratumumab
Janssen has applied to the Food and Drug Administration and the European Medicines Agency to allow splitting of the first infusion of daratumumab (Darzalex) in multiple myeloma patients over 2 consecutive days.
The goal is to improve the treatment experience for patients and physicians, according to the announcement from Janssen.
The regulatory submissions are based on the global, multi-arm, phase 1b MMY1001 study (NCT01998971). The study evaluated daratumumab in combination with various other treatments in 240 patients with multiple myeloma. It found that both the safety profile and the pharmacokinetics concentrations seen with either single dosing or split dosing were similar.
Daratumumab is the first approved monoclonal antibody that targets CD38, which is expressed across multiple myeloma cells regardless of disease stage. Daratumumab is currently approved for treatment of multiple myeloma in both the United States and the European Union either as monotherapy or in conjunction with other treatments.
Daratumumab is known to sometimes cause severe/serious infusion reactions, such as anaphylactic reactions; interfere with serological testing; and cause neutropenia or thrombocytopenia.
Janssen has applied to the Food and Drug Administration and the European Medicines Agency to allow splitting of the first infusion of daratumumab (Darzalex) in multiple myeloma patients over 2 consecutive days.
The goal is to improve the treatment experience for patients and physicians, according to the announcement from Janssen.
The regulatory submissions are based on the global, multi-arm, phase 1b MMY1001 study (NCT01998971). The study evaluated daratumumab in combination with various other treatments in 240 patients with multiple myeloma. It found that both the safety profile and the pharmacokinetics concentrations seen with either single dosing or split dosing were similar.
Daratumumab is the first approved monoclonal antibody that targets CD38, which is expressed across multiple myeloma cells regardless of disease stage. Daratumumab is currently approved for treatment of multiple myeloma in both the United States and the European Union either as monotherapy or in conjunction with other treatments.
Daratumumab is known to sometimes cause severe/serious infusion reactions, such as anaphylactic reactions; interfere with serological testing; and cause neutropenia or thrombocytopenia.
Janssen has applied to the Food and Drug Administration and the European Medicines Agency to allow splitting of the first infusion of daratumumab (Darzalex) in multiple myeloma patients over 2 consecutive days.
The goal is to improve the treatment experience for patients and physicians, according to the announcement from Janssen.
The regulatory submissions are based on the global, multi-arm, phase 1b MMY1001 study (NCT01998971). The study evaluated daratumumab in combination with various other treatments in 240 patients with multiple myeloma. It found that both the safety profile and the pharmacokinetics concentrations seen with either single dosing or split dosing were similar.
Daratumumab is the first approved monoclonal antibody that targets CD38, which is expressed across multiple myeloma cells regardless of disease stage. Daratumumab is currently approved for treatment of multiple myeloma in both the United States and the European Union either as monotherapy or in conjunction with other treatments.
Daratumumab is known to sometimes cause severe/serious infusion reactions, such as anaphylactic reactions; interfere with serological testing; and cause neutropenia or thrombocytopenia.
Surgical outcomes for UC worse since introduction of biologics
Since the approval of more UC patients are having multiple operations to manage their disease and their surgical outcomes tend to be worse, according to a study published in Annals of Surgery.
“Encouragingly, early randomized controlled trials demonstrated that infliximab may reduce the short-term need for surgery,” wrote Jonathan Abelson, MD, of the department of surgery, Cornell University, New York, and his coauthors. “However, even after the development and approval of several other biologic agents to treat UC, 30%-66% of patients treated with biologic agents still ultimately require surgical intervention.”
The study reviewed records of 7,070 patients with UC in a New York State Department of Health database who had colorectal surgery in two comparative time periods: 3,803 from 1995 to 2005, before biologics were available, and 3,267 from 2006 to 2013, after infliximab was approved. Dr. Abelson and coauthors said this is the first study to look at long-term surgical outcomes in a large group of patients with UC over an extended time period. Previous studies have reported conflicting results of how biologic agents for UC can impact surgical outcomes. The researchers set out to explore two hypotheses: whether staged procedures increased after 2005 and whether UC patients had worse outcomes over the past decade. The study results validated both hypotheses. Up until 2005, the proportion of patients who underwent at least three procedures after the index hospitalization was 9%; after 2006, that proportion was 14% (P less than .01).
A potential explanation for trends in postsurgery death may be higher rates of Clostridium difficile after 2005 (10.6% vs. 5.8%; P less than .01), but that was accounted for in the adjusted analysis and is probably not a major factor, the researchers said. After 2006 patients were slightly older and more likely to be on Medicare and nonwhite; they also were sicker, with 28% having two or more comorbidities vs. 10% before 2006.
The investigators offered another explanation: “It is also possible that the immunosuppressive effect of biologic agents ... predisposes patients to worse postoperative outcomes. In addition, it is possible that patients are referred for surgery too late in their disease course because of prolonged medical therapy.”
Dr. Abelson and coauthors reported having no financial relationships.
SOURCE: Abelson JS et al. Ann Surg. 2018:268;311-7.
Since the approval of more UC patients are having multiple operations to manage their disease and their surgical outcomes tend to be worse, according to a study published in Annals of Surgery.
“Encouragingly, early randomized controlled trials demonstrated that infliximab may reduce the short-term need for surgery,” wrote Jonathan Abelson, MD, of the department of surgery, Cornell University, New York, and his coauthors. “However, even after the development and approval of several other biologic agents to treat UC, 30%-66% of patients treated with biologic agents still ultimately require surgical intervention.”
The study reviewed records of 7,070 patients with UC in a New York State Department of Health database who had colorectal surgery in two comparative time periods: 3,803 from 1995 to 2005, before biologics were available, and 3,267 from 2006 to 2013, after infliximab was approved. Dr. Abelson and coauthors said this is the first study to look at long-term surgical outcomes in a large group of patients with UC over an extended time period. Previous studies have reported conflicting results of how biologic agents for UC can impact surgical outcomes. The researchers set out to explore two hypotheses: whether staged procedures increased after 2005 and whether UC patients had worse outcomes over the past decade. The study results validated both hypotheses. Up until 2005, the proportion of patients who underwent at least three procedures after the index hospitalization was 9%; after 2006, that proportion was 14% (P less than .01).
A potential explanation for trends in postsurgery death may be higher rates of Clostridium difficile after 2005 (10.6% vs. 5.8%; P less than .01), but that was accounted for in the adjusted analysis and is probably not a major factor, the researchers said. After 2006 patients were slightly older and more likely to be on Medicare and nonwhite; they also were sicker, with 28% having two or more comorbidities vs. 10% before 2006.
The investigators offered another explanation: “It is also possible that the immunosuppressive effect of biologic agents ... predisposes patients to worse postoperative outcomes. In addition, it is possible that patients are referred for surgery too late in their disease course because of prolonged medical therapy.”
Dr. Abelson and coauthors reported having no financial relationships.
SOURCE: Abelson JS et al. Ann Surg. 2018:268;311-7.
Since the approval of more UC patients are having multiple operations to manage their disease and their surgical outcomes tend to be worse, according to a study published in Annals of Surgery.
“Encouragingly, early randomized controlled trials demonstrated that infliximab may reduce the short-term need for surgery,” wrote Jonathan Abelson, MD, of the department of surgery, Cornell University, New York, and his coauthors. “However, even after the development and approval of several other biologic agents to treat UC, 30%-66% of patients treated with biologic agents still ultimately require surgical intervention.”
The study reviewed records of 7,070 patients with UC in a New York State Department of Health database who had colorectal surgery in two comparative time periods: 3,803 from 1995 to 2005, before biologics were available, and 3,267 from 2006 to 2013, after infliximab was approved. Dr. Abelson and coauthors said this is the first study to look at long-term surgical outcomes in a large group of patients with UC over an extended time period. Previous studies have reported conflicting results of how biologic agents for UC can impact surgical outcomes. The researchers set out to explore two hypotheses: whether staged procedures increased after 2005 and whether UC patients had worse outcomes over the past decade. The study results validated both hypotheses. Up until 2005, the proportion of patients who underwent at least three procedures after the index hospitalization was 9%; after 2006, that proportion was 14% (P less than .01).
A potential explanation for trends in postsurgery death may be higher rates of Clostridium difficile after 2005 (10.6% vs. 5.8%; P less than .01), but that was accounted for in the adjusted analysis and is probably not a major factor, the researchers said. After 2006 patients were slightly older and more likely to be on Medicare and nonwhite; they also were sicker, with 28% having two or more comorbidities vs. 10% before 2006.
The investigators offered another explanation: “It is also possible that the immunosuppressive effect of biologic agents ... predisposes patients to worse postoperative outcomes. In addition, it is possible that patients are referred for surgery too late in their disease course because of prolonged medical therapy.”
Dr. Abelson and coauthors reported having no financial relationships.
SOURCE: Abelson JS et al. Ann Surg. 2018:268;311-7.
FROM ANNALS OF SURGERY
Key clinical point: Rates of multiple surgeries for ulcerative colitis have increased since biologic agents were introduced.
Major finding: Fourteen percent of patients have had multiple operations since 2006 vs. 9% before that.
Study details: A longitudinal analysis of 7,070 patients in the New York State Department of Health of Health Statewide Planning and Research Cooperative System database who had surgery for UC from 1995 to 2013.
Disclosures: Dr. Abelson and coauthors reported having no financial relationships.
Source: Abelson JS et al. Ann Surg. 2018;268:311-7.
COPD opposites: Utah and West Virginia
New estimates of chronic obstructive pulmonary disease (COPD) may have Utah residents breathing a sigh of relief. West Virginians, not so much.
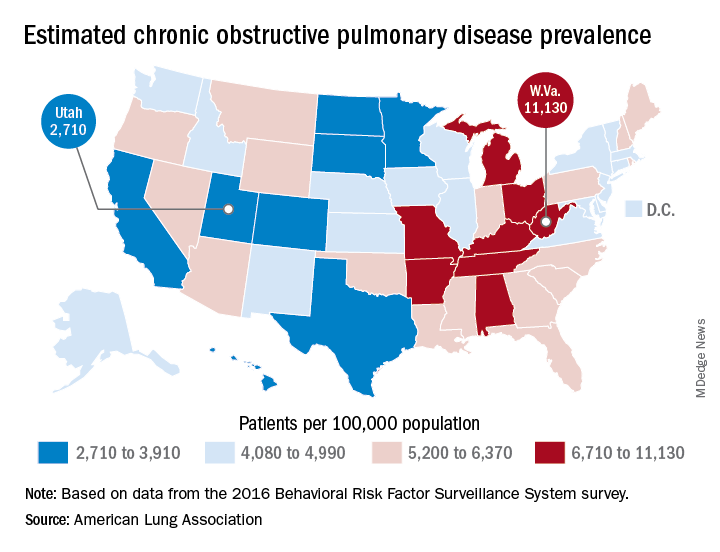
The Beehive State has the lowest prevalence of COPD in the country at 2,710 per 100,000 population, while the Mountain State tops the charts at 11,130 per 100,000, according to estimates from the American Lung Association. (Crude rates were calculated by MDedge News using the ALA’s estimates for total persons with COPD in each state and Census Bureau estimates for population.)
Other states with freer-breathing residents include Minnesota, which was just behind Utah with an estimated rate of 3,000 per 100,000 population, Hawaii (3,182), Colorado (3,334), and California (3,409). West Virginia’s rate, however, seems to be an outlier. The state with the next-highest rate, Kentucky, has a calculated prevalence of 8,890 per 100,000 population, followed by Tennessee at 7,880, Alabama at 7,400, and Arkansas at 7,330, using the ALA’s estimates, which were based on data from the 2016 Behavioral Risk Factor Surveillance System survey.
New estimates of chronic obstructive pulmonary disease (COPD) may have Utah residents breathing a sigh of relief. West Virginians, not so much.

The Beehive State has the lowest prevalence of COPD in the country at 2,710 per 100,000 population, while the Mountain State tops the charts at 11,130 per 100,000, according to estimates from the American Lung Association. (Crude rates were calculated by MDedge News using the ALA’s estimates for total persons with COPD in each state and Census Bureau estimates for population.)
Other states with freer-breathing residents include Minnesota, which was just behind Utah with an estimated rate of 3,000 per 100,000 population, Hawaii (3,182), Colorado (3,334), and California (3,409). West Virginia’s rate, however, seems to be an outlier. The state with the next-highest rate, Kentucky, has a calculated prevalence of 8,890 per 100,000 population, followed by Tennessee at 7,880, Alabama at 7,400, and Arkansas at 7,330, using the ALA’s estimates, which were based on data from the 2016 Behavioral Risk Factor Surveillance System survey.
New estimates of chronic obstructive pulmonary disease (COPD) may have Utah residents breathing a sigh of relief. West Virginians, not so much.

The Beehive State has the lowest prevalence of COPD in the country at 2,710 per 100,000 population, while the Mountain State tops the charts at 11,130 per 100,000, according to estimates from the American Lung Association. (Crude rates were calculated by MDedge News using the ALA’s estimates for total persons with COPD in each state and Census Bureau estimates for population.)
Other states with freer-breathing residents include Minnesota, which was just behind Utah with an estimated rate of 3,000 per 100,000 population, Hawaii (3,182), Colorado (3,334), and California (3,409). West Virginia’s rate, however, seems to be an outlier. The state with the next-highest rate, Kentucky, has a calculated prevalence of 8,890 per 100,000 population, followed by Tennessee at 7,880, Alabama at 7,400, and Arkansas at 7,330, using the ALA’s estimates, which were based on data from the 2016 Behavioral Risk Factor Surveillance System survey.