User login
Screening programs can improve disease-free interval outcomes in BC
Key clinical point: Detection of breast cancer (BC) by screening vs clinical or other non-screening procedures led to significantly improved disease-free interval outcomes.
Major finding: After correcting for lead time bias, the 10-year disease-free interval was improved significantly in women with screen-detected vs clinically-detected cancer (adjusted hazard ratio [aHR] 0.77; 95% CI 0.68-0.87), with similar improvements observed in 5-year disease-free interval in women with screen-detected vs non-screen-related cancer (aHR 0.76; 95% CI 0.66-0.88).
Study details: Findings are from an analysis of two cohorts including 6215 and 15,176 women with invasive, non-metastatic BC who underwent surgery and were followed for 10 and 5 years, respectively, of which 55.8% of women in either of the cohorts had a screen-detected cancer.
Disclosures: This study did not declare any specific funding. S Siesling declared receiving support and serving as an advisor for various sources. The other authors declared no conflicts of interest.
Source: de Munck L et al. Method of primary breast cancer detection and the disease-free interval, adjusting for lead time. J Natl Cancer Inst. 2023 (Nov 3). doi: 10.1093/jnci/djad230
Key clinical point: Detection of breast cancer (BC) by screening vs clinical or other non-screening procedures led to significantly improved disease-free interval outcomes.
Major finding: After correcting for lead time bias, the 10-year disease-free interval was improved significantly in women with screen-detected vs clinically-detected cancer (adjusted hazard ratio [aHR] 0.77; 95% CI 0.68-0.87), with similar improvements observed in 5-year disease-free interval in women with screen-detected vs non-screen-related cancer (aHR 0.76; 95% CI 0.66-0.88).
Study details: Findings are from an analysis of two cohorts including 6215 and 15,176 women with invasive, non-metastatic BC who underwent surgery and were followed for 10 and 5 years, respectively, of which 55.8% of women in either of the cohorts had a screen-detected cancer.
Disclosures: This study did not declare any specific funding. S Siesling declared receiving support and serving as an advisor for various sources. The other authors declared no conflicts of interest.
Source: de Munck L et al. Method of primary breast cancer detection and the disease-free interval, adjusting for lead time. J Natl Cancer Inst. 2023 (Nov 3). doi: 10.1093/jnci/djad230
Key clinical point: Detection of breast cancer (BC) by screening vs clinical or other non-screening procedures led to significantly improved disease-free interval outcomes.
Major finding: After correcting for lead time bias, the 10-year disease-free interval was improved significantly in women with screen-detected vs clinically-detected cancer (adjusted hazard ratio [aHR] 0.77; 95% CI 0.68-0.87), with similar improvements observed in 5-year disease-free interval in women with screen-detected vs non-screen-related cancer (aHR 0.76; 95% CI 0.66-0.88).
Study details: Findings are from an analysis of two cohorts including 6215 and 15,176 women with invasive, non-metastatic BC who underwent surgery and were followed for 10 and 5 years, respectively, of which 55.8% of women in either of the cohorts had a screen-detected cancer.
Disclosures: This study did not declare any specific funding. S Siesling declared receiving support and serving as an advisor for various sources. The other authors declared no conflicts of interest.
Source: de Munck L et al. Method of primary breast cancer detection and the disease-free interval, adjusting for lead time. J Natl Cancer Inst. 2023 (Nov 3). doi: 10.1093/jnci/djad230
Is gel tamoxifen noninferior to oral tamoxifen in DCIS of the breast?
Key clinical point: Although local transdermal therapy with 4-hydroxytamoxifen was associated with low systemic exposure, it was not as effective as oral tamoxifen in suppressing proliferation in the ductal carcinoma in situ (DCIS) lesions of the breast.
Major finding: Posttreatment Ki67 labelling index was significantly higher in the transdermal 4-hydroxytamoxifen gel vs oral tamoxifen treatment group (3.3%; 80% CI 2.1%-4.6%), with the value exceeding the noninferiority margin of 2.6%. Grade 2 adverse events were reported by five patients in both groups.
Study details: Findings are from a phase 2 study including 107 patients with DCIS of the breast who were randomly assigned to receive oral tamoxifen or 4-hydroxytamoxifen gel treatment for 4-10 weeks, of which 90 patients completed the treatment and underwent surgery.
Disclosures: This trial was sponsored by the US National Cancer Institute. Some authors declared receiving grant funding from various sources or holding a patent.
Source: Khan SA et al. Presurgical oral tamoxifen vs transdermal 4-hydroxytamoxifen in women with ductal carcinoma in situ: A randomized clinical trial. JAMA Surg. 2023 (Oct 23). doi: 10.1001/jamasurg.2023.5113
Key clinical point: Although local transdermal therapy with 4-hydroxytamoxifen was associated with low systemic exposure, it was not as effective as oral tamoxifen in suppressing proliferation in the ductal carcinoma in situ (DCIS) lesions of the breast.
Major finding: Posttreatment Ki67 labelling index was significantly higher in the transdermal 4-hydroxytamoxifen gel vs oral tamoxifen treatment group (3.3%; 80% CI 2.1%-4.6%), with the value exceeding the noninferiority margin of 2.6%. Grade 2 adverse events were reported by five patients in both groups.
Study details: Findings are from a phase 2 study including 107 patients with DCIS of the breast who were randomly assigned to receive oral tamoxifen or 4-hydroxytamoxifen gel treatment for 4-10 weeks, of which 90 patients completed the treatment and underwent surgery.
Disclosures: This trial was sponsored by the US National Cancer Institute. Some authors declared receiving grant funding from various sources or holding a patent.
Source: Khan SA et al. Presurgical oral tamoxifen vs transdermal 4-hydroxytamoxifen in women with ductal carcinoma in situ: A randomized clinical trial. JAMA Surg. 2023 (Oct 23). doi: 10.1001/jamasurg.2023.5113
Key clinical point: Although local transdermal therapy with 4-hydroxytamoxifen was associated with low systemic exposure, it was not as effective as oral tamoxifen in suppressing proliferation in the ductal carcinoma in situ (DCIS) lesions of the breast.
Major finding: Posttreatment Ki67 labelling index was significantly higher in the transdermal 4-hydroxytamoxifen gel vs oral tamoxifen treatment group (3.3%; 80% CI 2.1%-4.6%), with the value exceeding the noninferiority margin of 2.6%. Grade 2 adverse events were reported by five patients in both groups.
Study details: Findings are from a phase 2 study including 107 patients with DCIS of the breast who were randomly assigned to receive oral tamoxifen or 4-hydroxytamoxifen gel treatment for 4-10 weeks, of which 90 patients completed the treatment and underwent surgery.
Disclosures: This trial was sponsored by the US National Cancer Institute. Some authors declared receiving grant funding from various sources or holding a patent.
Source: Khan SA et al. Presurgical oral tamoxifen vs transdermal 4-hydroxytamoxifen in women with ductal carcinoma in situ: A randomized clinical trial. JAMA Surg. 2023 (Oct 23). doi: 10.1001/jamasurg.2023.5113
Dual HER2 inhibition with pyrotinib-trastuzumab-docetaxel confers survival benefits in untreated HER2+ metastatic BC
Key clinical point: Pyrotinib+trastuzumab+docetaxel was more effective than placebo+trastuzumab+docetaxel in improving progression-free survival (PFS) outcomes and showed a manageable safety profile in patients with untreated human epidermal growth factor receptor 2-positive (HER2+) metastatic breast cancer (BC).
Major finding: PFS improved by 59% with pyrotinib+trastuzumab+docetaxel vs placebo+trastuzumab+docetaxel treatment (24.3 vs 10.4 months; hazard ratio 0.41; stratified 1-sided P < .001). The most frequently reported grade ≥3 adverse events in the pyrotinib vs placebo treatment arm were decreased neutrophil count (63% vs 65%), decreased white blood cell count (53% vs 51%), and diarrhea (46% vs 3%).
Study details: Findings are from the phase 3 PHILA trial including 590 female patients with untreated HER2+ metastatic BC who were randomly assigned to receive pyrotinib or placebo, both in combination with trastuzumab and docetaxel.
Disclosures: This study was funded by Jiangsu Hengrui Pharmaceuticals, China, and other sources. Three authors declared being employees of Jiangsu Hengrui Pharmaceuticals, and two other authors reported ties with various sources.
Source: Ma F et al, on behalf of the PHILA Investigators. Pyrotinib versus placebo in combination with trastuzumab and docetaxel as first line treatment in patients with HER2 positive metastatic breast cancer (PHILA): Randomised, double blind, multicentre, phase 3 trial. BMJ. 2023;383:e076065 (Oct 31). doi: 10.1136/bmj-2023-076065
Key clinical point: Pyrotinib+trastuzumab+docetaxel was more effective than placebo+trastuzumab+docetaxel in improving progression-free survival (PFS) outcomes and showed a manageable safety profile in patients with untreated human epidermal growth factor receptor 2-positive (HER2+) metastatic breast cancer (BC).
Major finding: PFS improved by 59% with pyrotinib+trastuzumab+docetaxel vs placebo+trastuzumab+docetaxel treatment (24.3 vs 10.4 months; hazard ratio 0.41; stratified 1-sided P < .001). The most frequently reported grade ≥3 adverse events in the pyrotinib vs placebo treatment arm were decreased neutrophil count (63% vs 65%), decreased white blood cell count (53% vs 51%), and diarrhea (46% vs 3%).
Study details: Findings are from the phase 3 PHILA trial including 590 female patients with untreated HER2+ metastatic BC who were randomly assigned to receive pyrotinib or placebo, both in combination with trastuzumab and docetaxel.
Disclosures: This study was funded by Jiangsu Hengrui Pharmaceuticals, China, and other sources. Three authors declared being employees of Jiangsu Hengrui Pharmaceuticals, and two other authors reported ties with various sources.
Source: Ma F et al, on behalf of the PHILA Investigators. Pyrotinib versus placebo in combination with trastuzumab and docetaxel as first line treatment in patients with HER2 positive metastatic breast cancer (PHILA): Randomised, double blind, multicentre, phase 3 trial. BMJ. 2023;383:e076065 (Oct 31). doi: 10.1136/bmj-2023-076065
Key clinical point: Pyrotinib+trastuzumab+docetaxel was more effective than placebo+trastuzumab+docetaxel in improving progression-free survival (PFS) outcomes and showed a manageable safety profile in patients with untreated human epidermal growth factor receptor 2-positive (HER2+) metastatic breast cancer (BC).
Major finding: PFS improved by 59% with pyrotinib+trastuzumab+docetaxel vs placebo+trastuzumab+docetaxel treatment (24.3 vs 10.4 months; hazard ratio 0.41; stratified 1-sided P < .001). The most frequently reported grade ≥3 adverse events in the pyrotinib vs placebo treatment arm were decreased neutrophil count (63% vs 65%), decreased white blood cell count (53% vs 51%), and diarrhea (46% vs 3%).
Study details: Findings are from the phase 3 PHILA trial including 590 female patients with untreated HER2+ metastatic BC who were randomly assigned to receive pyrotinib or placebo, both in combination with trastuzumab and docetaxel.
Disclosures: This study was funded by Jiangsu Hengrui Pharmaceuticals, China, and other sources. Three authors declared being employees of Jiangsu Hengrui Pharmaceuticals, and two other authors reported ties with various sources.
Source: Ma F et al, on behalf of the PHILA Investigators. Pyrotinib versus placebo in combination with trastuzumab and docetaxel as first line treatment in patients with HER2 positive metastatic breast cancer (PHILA): Randomised, double blind, multicentre, phase 3 trial. BMJ. 2023;383:e076065 (Oct 31). doi: 10.1136/bmj-2023-076065
Meta-analysis shows benefits of regional node irradiation in early BC
Key clinical point: Regional node radiotherapy improved breast cancer (BC) mortality rates in women with early-stage BC who had received other effective local and systemic therapies.
Major finding: Regional node radiotherapy reduced the risk for overall BC recurrence and mortality by 10% (rate ratio [RR] 0.90; P = .0020) and 9% (RR 0.91; P = .012), respectively. In the newer trials that assessed more tailored radiotherapy approaches, a more prominent reduction was observed in the overall BC recurrence (RR 0.88; P = .00083) and mortality (RR 0.87; P = .0010) rates along with 10% improvement in overall mortality (P = .0022).
Study details: Findings are from a meta-analysis of 16 trials including 14,324 patients with early BC, with 8 of the 16 trials being newer and including 12,167 patients.
Disclosures: This study was funded by Cancer Research UK and the UK Medical Research Council. Some authors declared receiving institutional grants, honoraria, payments, or research funding from and having other ties with several sources.
Source: Early Breast Cancer Trialists' Collaborative Group. Radiotherapy to regional nodes in early breast cancer: An individual patient data meta-analysis of 14 324 women in 16 trials. Lancet. 2023 (Nov 3). doi: 10.1016/S0140-6736(23)01082-6
Key clinical point: Regional node radiotherapy improved breast cancer (BC) mortality rates in women with early-stage BC who had received other effective local and systemic therapies.
Major finding: Regional node radiotherapy reduced the risk for overall BC recurrence and mortality by 10% (rate ratio [RR] 0.90; P = .0020) and 9% (RR 0.91; P = .012), respectively. In the newer trials that assessed more tailored radiotherapy approaches, a more prominent reduction was observed in the overall BC recurrence (RR 0.88; P = .00083) and mortality (RR 0.87; P = .0010) rates along with 10% improvement in overall mortality (P = .0022).
Study details: Findings are from a meta-analysis of 16 trials including 14,324 patients with early BC, with 8 of the 16 trials being newer and including 12,167 patients.
Disclosures: This study was funded by Cancer Research UK and the UK Medical Research Council. Some authors declared receiving institutional grants, honoraria, payments, or research funding from and having other ties with several sources.
Source: Early Breast Cancer Trialists' Collaborative Group. Radiotherapy to regional nodes in early breast cancer: An individual patient data meta-analysis of 14 324 women in 16 trials. Lancet. 2023 (Nov 3). doi: 10.1016/S0140-6736(23)01082-6
Key clinical point: Regional node radiotherapy improved breast cancer (BC) mortality rates in women with early-stage BC who had received other effective local and systemic therapies.
Major finding: Regional node radiotherapy reduced the risk for overall BC recurrence and mortality by 10% (rate ratio [RR] 0.90; P = .0020) and 9% (RR 0.91; P = .012), respectively. In the newer trials that assessed more tailored radiotherapy approaches, a more prominent reduction was observed in the overall BC recurrence (RR 0.88; P = .00083) and mortality (RR 0.87; P = .0010) rates along with 10% improvement in overall mortality (P = .0022).
Study details: Findings are from a meta-analysis of 16 trials including 14,324 patients with early BC, with 8 of the 16 trials being newer and including 12,167 patients.
Disclosures: This study was funded by Cancer Research UK and the UK Medical Research Council. Some authors declared receiving institutional grants, honoraria, payments, or research funding from and having other ties with several sources.
Source: Early Breast Cancer Trialists' Collaborative Group. Radiotherapy to regional nodes in early breast cancer: An individual patient data meta-analysis of 14 324 women in 16 trials. Lancet. 2023 (Nov 3). doi: 10.1016/S0140-6736(23)01082-6
Obesity is a risk factor for recurrence in aromatase inhibitor-treated HR+ BC
Key clinical point: Obesity increased the risk for recurrence in postmenopausal women with early-stage hormone receptor-positive (HR+) breast cancer (BC) who were treated with aromatase inhibitors (AI).
Major finding: Among patients with AI-treated HR+ BC, the risk for BC recurrence was significantly higher in those with obesity (adjusted hazard ratio [aHR] 1.18; 95% CI 1.01-1.37) and severe obesity (aHR 1.32; 95% CI 1.08-1.62) than in those with a healthy weight.
Study details: Findings are from a nationwide cohort study including 13,230 postmenopausal women with stages I-III HR+ BC who received AI.
Disclosures: This study was supported by the Jeppe Juhl Memorial Foundation, Denmark, and other Danish sources. Jensen MR declared receiving from Novartis meeting expenses and personal fees unrelated to this study.
Source: Harborg S et al. Obesity and risk of recurrence in patients with breast cancer treated with aromatase inhibitors. JAMA Netw Open. 2023;6(10):e2337780 (Oct 13). doi: 10.1001/jamanetworkopen.2023.37780
Key clinical point: Obesity increased the risk for recurrence in postmenopausal women with early-stage hormone receptor-positive (HR+) breast cancer (BC) who were treated with aromatase inhibitors (AI).
Major finding: Among patients with AI-treated HR+ BC, the risk for BC recurrence was significantly higher in those with obesity (adjusted hazard ratio [aHR] 1.18; 95% CI 1.01-1.37) and severe obesity (aHR 1.32; 95% CI 1.08-1.62) than in those with a healthy weight.
Study details: Findings are from a nationwide cohort study including 13,230 postmenopausal women with stages I-III HR+ BC who received AI.
Disclosures: This study was supported by the Jeppe Juhl Memorial Foundation, Denmark, and other Danish sources. Jensen MR declared receiving from Novartis meeting expenses and personal fees unrelated to this study.
Source: Harborg S et al. Obesity and risk of recurrence in patients with breast cancer treated with aromatase inhibitors. JAMA Netw Open. 2023;6(10):e2337780 (Oct 13). doi: 10.1001/jamanetworkopen.2023.37780
Key clinical point: Obesity increased the risk for recurrence in postmenopausal women with early-stage hormone receptor-positive (HR+) breast cancer (BC) who were treated with aromatase inhibitors (AI).
Major finding: Among patients with AI-treated HR+ BC, the risk for BC recurrence was significantly higher in those with obesity (adjusted hazard ratio [aHR] 1.18; 95% CI 1.01-1.37) and severe obesity (aHR 1.32; 95% CI 1.08-1.62) than in those with a healthy weight.
Study details: Findings are from a nationwide cohort study including 13,230 postmenopausal women with stages I-III HR+ BC who received AI.
Disclosures: This study was supported by the Jeppe Juhl Memorial Foundation, Denmark, and other Danish sources. Jensen MR declared receiving from Novartis meeting expenses and personal fees unrelated to this study.
Source: Harborg S et al. Obesity and risk of recurrence in patients with breast cancer treated with aromatase inhibitors. JAMA Netw Open. 2023;6(10):e2337780 (Oct 13). doi: 10.1001/jamanetworkopen.2023.37780
The future of medicine is RNA
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
Every once in a while, medicine changes in a fundamental way, and we may not realize it while it’s happening. I wasn’t around in 1928 when Fleming discovered penicillin; or in 1953 when Watson, Crick, and Franklin characterized the double-helical structure of DNA.
But looking at medicine today, there are essentially two places where I think we will see, in retrospect, that we were at a fundamental turning point. One is artificial intelligence, which gets so much attention and hype that I will simply say yes, this will change things, stay tuned.
The other is a bit more obscure, but I suspect it may be just as impactful. That other thing is
I want to start with the idea that many diseases are, fundamentally, a problem of proteins. In some cases, like hypercholesterolemia, the body produces too much protein; in others, like hemophilia, too little.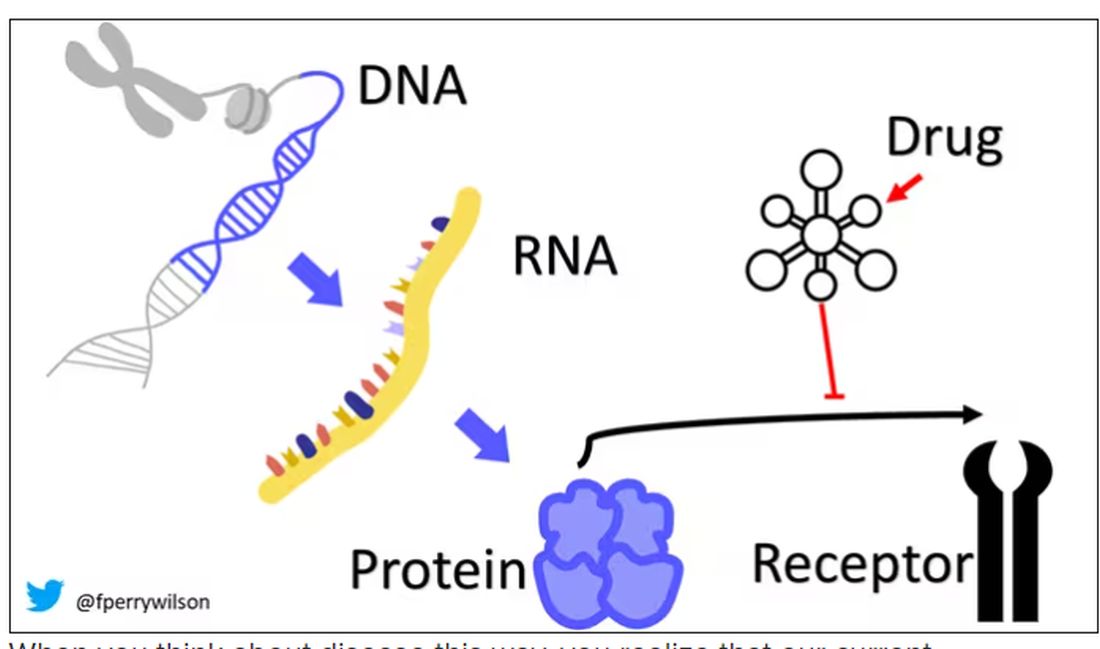
When you think about disease this way, you realize that our current medications take effect late in the disease game. We have these molecules that try to block a protein from its receptor, prevent a protein from cleaving another protein, or increase the rate that a protein is broken down. It’s all distal to the fundamental problem: the production of the bad protein in the first place.
Enter small inhibitory RNAs, or siRNAs for short, discovered in 1998 by Andrew Fire and Craig Mello at UMass Worcester. The two won the Nobel prize in medicine just 8 years later; that’s a really short time, highlighting just how important this discovery was. In contrast, Karikó and Weissman won the Nobel for mRNA vaccines this year, after inventing them 18 years ago.
siRNAs are the body’s way of targeting proteins for destruction before they are ever created. About 20 base pairs long, siRNAs seek out a complementary target mRNA, attach to it, and call in a group of proteins to destroy it. With the target mRNA gone, no protein can be created.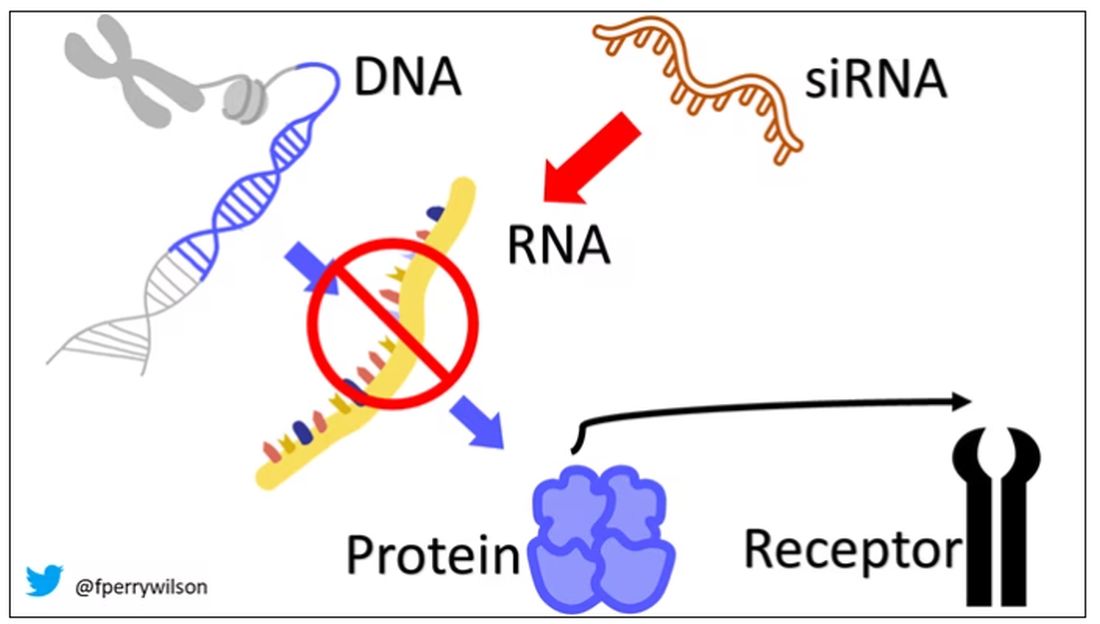
You see where this is going, right? How does high cholesterol kill you? Proteins. How does Staphylococcus aureus kill you? Proteins. Even viruses can’t replicate if their RNA is prevented from being turned into proteins.
So, how do we use siRNAs? A new paper appearing in JAMA describes a fairly impressive use case.
The background here is that higher levels of lipoprotein(a), an LDL-like protein, are associated with cardiovascular disease, heart attack, and stroke. But unfortunately, statins really don’t have any effect on lipoprotein(a) levels. Neither does diet. Your lipoprotein(a) level seems to be more or less hard-coded genetically.
So, what if we stop the genetic machinery from working? Enter lepodisiran, a drug from Eli Lilly. Unlike so many other medications, which are usually found in nature, purified, and synthesized, lepodisiran was created from scratch. It’s not hard. Thanks to the Human Genome Project, we know the genetic code for lipoprotein(a), so inventing an siRNA to target it specifically is trivial. That’s one of the key features of siRNA – you don’t have to find a chemical that binds strongly to some protein receptor, and worry about the off-target effects and all that nonsense. You just pick a protein you want to suppress and you suppress it.
Okay, it’s not that simple. siRNA is broken down very quickly by the body, so it needs to be targeted to the organ of interest – in this case, the liver, since that is where lipoprotein(a) is synthesized. Lepodisiran is targeted to the liver by this special targeting label here.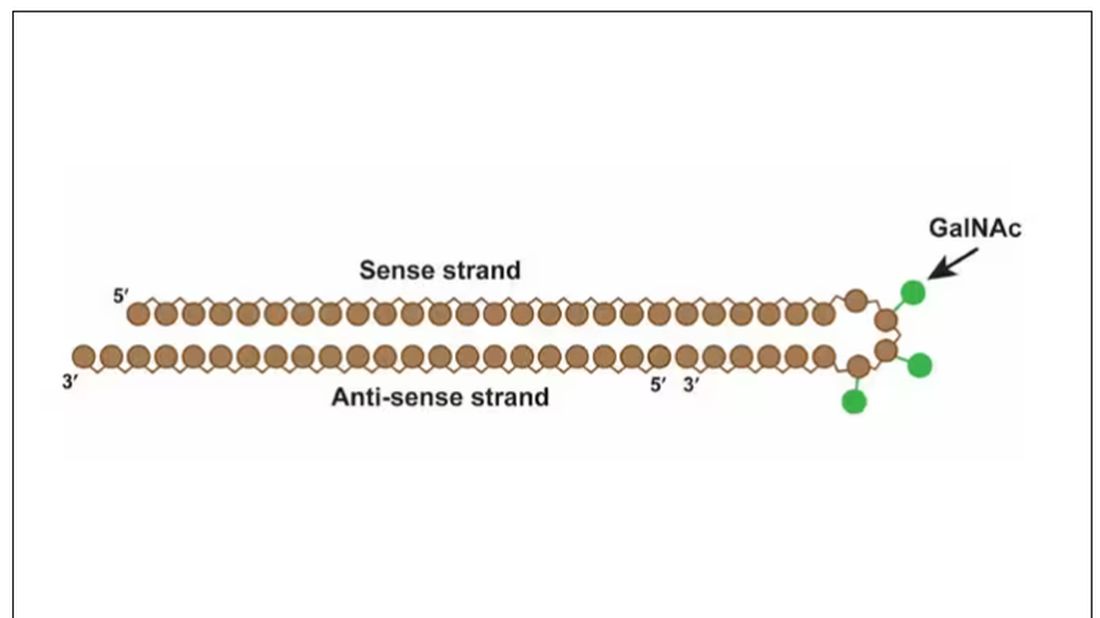
The report is a standard dose-escalation trial. Six patients, all with elevated lipoprotein(a) levels, were started with a 4-mg dose (two additional individuals got placebo). They were intensely monitored, spending 3 days in a research unit for multiple blood draws followed by weekly, and then biweekly outpatient visits. Once they had done well, the next group of six people received a higher dose (two more got placebo), and the process was repeated – six times total – until the highest dose, 608 mg, was reached.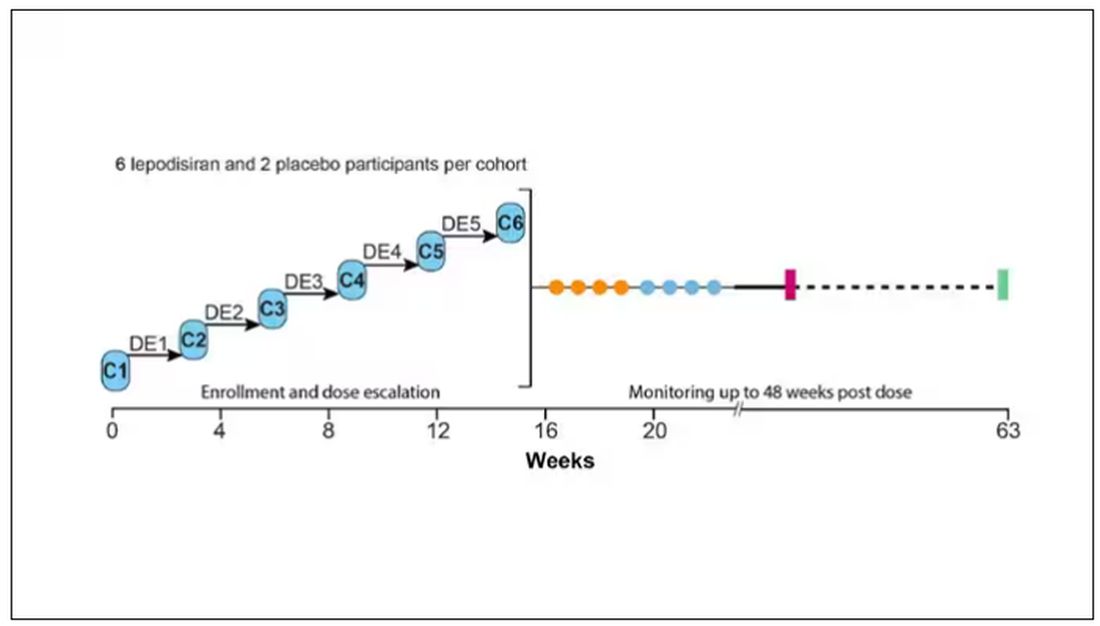
This is an injection, of course; siRNA wouldn’t withstand the harshness of the digestive system. And it’s only one injection. You can see from the blood concentration curves that within about 48 hours, circulating lepodisiran was not detectable.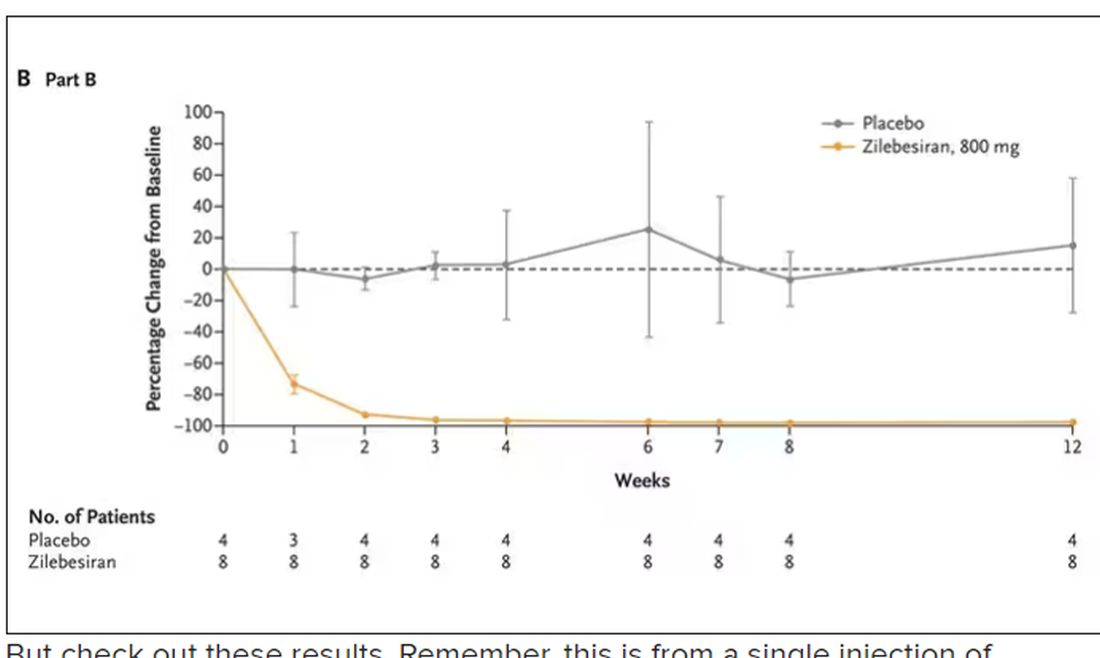
But check out these results. Remember, this is from a single injection of lepodisiran.
Lipoprotein(a) levels start to drop within a week of administration, and they stay down. In the higher-dose groups, levels are nearly undetectable a year after that injection.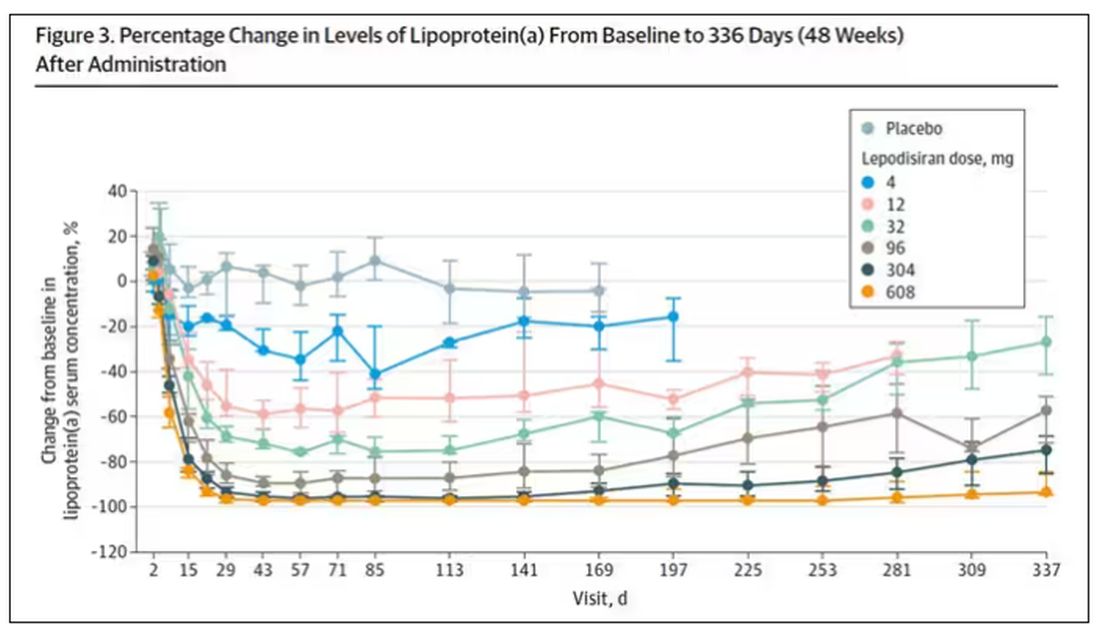
It was this graph that made me sit back and think that there might be something new under the sun. A single injection that can suppress protein synthesis for an entire year? If it really works, it changes the game.
Of course, this study wasn’t powered to look at important outcomes like heart attacks and strokes. It was primarily designed to assess safety, and the drug was pretty well tolerated, with similar rates of adverse events in the drug and placebo groups.
As crazy as it sounds, the real concern here might be that this drug is too good; is it safe to drop your lipoprotein(a) levels to zero for a year? I don’t know. But lower doses don’t have quite as strong an effect.
Trust me, these drugs are going to change things. They already are. In July, The New England Journal of Medicine published a study of zilebesiran, an siRNA that inhibits the production of angiotensinogen, to control blood pressure. Similar story: One injection led to a basically complete suppression of angiotensinogen and a sustained decrease in blood pressure.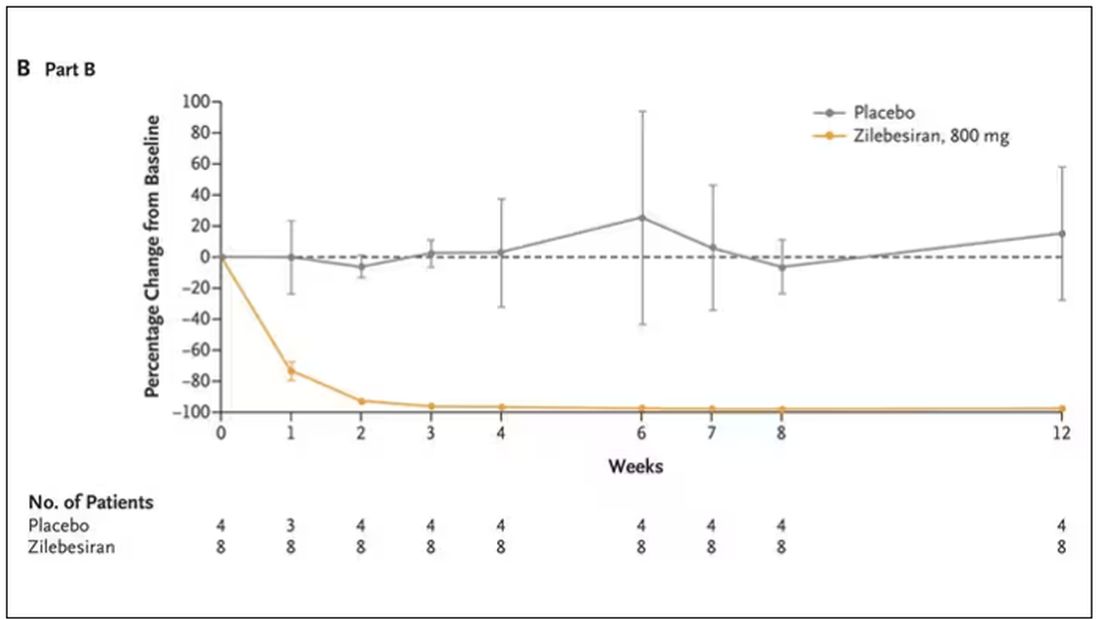
I’m not exaggerating when I say that there may come a time when you go to your doctor once a year, get your RNA shots, and don’t have to take any other medication from that point on. And that time may be, like, 5 years from now. It’s wild.
Seems to me that that rapid Nobel Prize was very well deserved.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships. This transcript has been edited for clarity.
A version of this article appeared on Medscape.com.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
Every once in a while, medicine changes in a fundamental way, and we may not realize it while it’s happening. I wasn’t around in 1928 when Fleming discovered penicillin; or in 1953 when Watson, Crick, and Franklin characterized the double-helical structure of DNA.
But looking at medicine today, there are essentially two places where I think we will see, in retrospect, that we were at a fundamental turning point. One is artificial intelligence, which gets so much attention and hype that I will simply say yes, this will change things, stay tuned.
The other is a bit more obscure, but I suspect it may be just as impactful. That other thing is
I want to start with the idea that many diseases are, fundamentally, a problem of proteins. In some cases, like hypercholesterolemia, the body produces too much protein; in others, like hemophilia, too little.
When you think about disease this way, you realize that our current medications take effect late in the disease game. We have these molecules that try to block a protein from its receptor, prevent a protein from cleaving another protein, or increase the rate that a protein is broken down. It’s all distal to the fundamental problem: the production of the bad protein in the first place.
Enter small inhibitory RNAs, or siRNAs for short, discovered in 1998 by Andrew Fire and Craig Mello at UMass Worcester. The two won the Nobel prize in medicine just 8 years later; that’s a really short time, highlighting just how important this discovery was. In contrast, Karikó and Weissman won the Nobel for mRNA vaccines this year, after inventing them 18 years ago.
siRNAs are the body’s way of targeting proteins for destruction before they are ever created. About 20 base pairs long, siRNAs seek out a complementary target mRNA, attach to it, and call in a group of proteins to destroy it. With the target mRNA gone, no protein can be created.
You see where this is going, right? How does high cholesterol kill you? Proteins. How does Staphylococcus aureus kill you? Proteins. Even viruses can’t replicate if their RNA is prevented from being turned into proteins.
So, how do we use siRNAs? A new paper appearing in JAMA describes a fairly impressive use case.
The background here is that higher levels of lipoprotein(a), an LDL-like protein, are associated with cardiovascular disease, heart attack, and stroke. But unfortunately, statins really don’t have any effect on lipoprotein(a) levels. Neither does diet. Your lipoprotein(a) level seems to be more or less hard-coded genetically.
So, what if we stop the genetic machinery from working? Enter lepodisiran, a drug from Eli Lilly. Unlike so many other medications, which are usually found in nature, purified, and synthesized, lepodisiran was created from scratch. It’s not hard. Thanks to the Human Genome Project, we know the genetic code for lipoprotein(a), so inventing an siRNA to target it specifically is trivial. That’s one of the key features of siRNA – you don’t have to find a chemical that binds strongly to some protein receptor, and worry about the off-target effects and all that nonsense. You just pick a protein you want to suppress and you suppress it.
Okay, it’s not that simple. siRNA is broken down very quickly by the body, so it needs to be targeted to the organ of interest – in this case, the liver, since that is where lipoprotein(a) is synthesized. Lepodisiran is targeted to the liver by this special targeting label here.
The report is a standard dose-escalation trial. Six patients, all with elevated lipoprotein(a) levels, were started with a 4-mg dose (two additional individuals got placebo). They were intensely monitored, spending 3 days in a research unit for multiple blood draws followed by weekly, and then biweekly outpatient visits. Once they had done well, the next group of six people received a higher dose (two more got placebo), and the process was repeated – six times total – until the highest dose, 608 mg, was reached.
This is an injection, of course; siRNA wouldn’t withstand the harshness of the digestive system. And it’s only one injection. You can see from the blood concentration curves that within about 48 hours, circulating lepodisiran was not detectable.
But check out these results. Remember, this is from a single injection of lepodisiran.
Lipoprotein(a) levels start to drop within a week of administration, and they stay down. In the higher-dose groups, levels are nearly undetectable a year after that injection.
It was this graph that made me sit back and think that there might be something new under the sun. A single injection that can suppress protein synthesis for an entire year? If it really works, it changes the game.
Of course, this study wasn’t powered to look at important outcomes like heart attacks and strokes. It was primarily designed to assess safety, and the drug was pretty well tolerated, with similar rates of adverse events in the drug and placebo groups.
As crazy as it sounds, the real concern here might be that this drug is too good; is it safe to drop your lipoprotein(a) levels to zero for a year? I don’t know. But lower doses don’t have quite as strong an effect.
Trust me, these drugs are going to change things. They already are. In July, The New England Journal of Medicine published a study of zilebesiran, an siRNA that inhibits the production of angiotensinogen, to control blood pressure. Similar story: One injection led to a basically complete suppression of angiotensinogen and a sustained decrease in blood pressure.
I’m not exaggerating when I say that there may come a time when you go to your doctor once a year, get your RNA shots, and don’t have to take any other medication from that point on. And that time may be, like, 5 years from now. It’s wild.
Seems to me that that rapid Nobel Prize was very well deserved.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships. This transcript has been edited for clarity.
A version of this article appeared on Medscape.com.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
Every once in a while, medicine changes in a fundamental way, and we may not realize it while it’s happening. I wasn’t around in 1928 when Fleming discovered penicillin; or in 1953 when Watson, Crick, and Franklin characterized the double-helical structure of DNA.
But looking at medicine today, there are essentially two places where I think we will see, in retrospect, that we were at a fundamental turning point. One is artificial intelligence, which gets so much attention and hype that I will simply say yes, this will change things, stay tuned.
The other is a bit more obscure, but I suspect it may be just as impactful. That other thing is
I want to start with the idea that many diseases are, fundamentally, a problem of proteins. In some cases, like hypercholesterolemia, the body produces too much protein; in others, like hemophilia, too little.
When you think about disease this way, you realize that our current medications take effect late in the disease game. We have these molecules that try to block a protein from its receptor, prevent a protein from cleaving another protein, or increase the rate that a protein is broken down. It’s all distal to the fundamental problem: the production of the bad protein in the first place.
Enter small inhibitory RNAs, or siRNAs for short, discovered in 1998 by Andrew Fire and Craig Mello at UMass Worcester. The two won the Nobel prize in medicine just 8 years later; that’s a really short time, highlighting just how important this discovery was. In contrast, Karikó and Weissman won the Nobel for mRNA vaccines this year, after inventing them 18 years ago.
siRNAs are the body’s way of targeting proteins for destruction before they are ever created. About 20 base pairs long, siRNAs seek out a complementary target mRNA, attach to it, and call in a group of proteins to destroy it. With the target mRNA gone, no protein can be created.
You see where this is going, right? How does high cholesterol kill you? Proteins. How does Staphylococcus aureus kill you? Proteins. Even viruses can’t replicate if their RNA is prevented from being turned into proteins.
So, how do we use siRNAs? A new paper appearing in JAMA describes a fairly impressive use case.
The background here is that higher levels of lipoprotein(a), an LDL-like protein, are associated with cardiovascular disease, heart attack, and stroke. But unfortunately, statins really don’t have any effect on lipoprotein(a) levels. Neither does diet. Your lipoprotein(a) level seems to be more or less hard-coded genetically.
So, what if we stop the genetic machinery from working? Enter lepodisiran, a drug from Eli Lilly. Unlike so many other medications, which are usually found in nature, purified, and synthesized, lepodisiran was created from scratch. It’s not hard. Thanks to the Human Genome Project, we know the genetic code for lipoprotein(a), so inventing an siRNA to target it specifically is trivial. That’s one of the key features of siRNA – you don’t have to find a chemical that binds strongly to some protein receptor, and worry about the off-target effects and all that nonsense. You just pick a protein you want to suppress and you suppress it.
Okay, it’s not that simple. siRNA is broken down very quickly by the body, so it needs to be targeted to the organ of interest – in this case, the liver, since that is where lipoprotein(a) is synthesized. Lepodisiran is targeted to the liver by this special targeting label here.
The report is a standard dose-escalation trial. Six patients, all with elevated lipoprotein(a) levels, were started with a 4-mg dose (two additional individuals got placebo). They were intensely monitored, spending 3 days in a research unit for multiple blood draws followed by weekly, and then biweekly outpatient visits. Once they had done well, the next group of six people received a higher dose (two more got placebo), and the process was repeated – six times total – until the highest dose, 608 mg, was reached.
This is an injection, of course; siRNA wouldn’t withstand the harshness of the digestive system. And it’s only one injection. You can see from the blood concentration curves that within about 48 hours, circulating lepodisiran was not detectable.
But check out these results. Remember, this is from a single injection of lepodisiran.
Lipoprotein(a) levels start to drop within a week of administration, and they stay down. In the higher-dose groups, levels are nearly undetectable a year after that injection.
It was this graph that made me sit back and think that there might be something new under the sun. A single injection that can suppress protein synthesis for an entire year? If it really works, it changes the game.
Of course, this study wasn’t powered to look at important outcomes like heart attacks and strokes. It was primarily designed to assess safety, and the drug was pretty well tolerated, with similar rates of adverse events in the drug and placebo groups.
As crazy as it sounds, the real concern here might be that this drug is too good; is it safe to drop your lipoprotein(a) levels to zero for a year? I don’t know. But lower doses don’t have quite as strong an effect.
Trust me, these drugs are going to change things. They already are. In July, The New England Journal of Medicine published a study of zilebesiran, an siRNA that inhibits the production of angiotensinogen, to control blood pressure. Similar story: One injection led to a basically complete suppression of angiotensinogen and a sustained decrease in blood pressure.
I’m not exaggerating when I say that there may come a time when you go to your doctor once a year, get your RNA shots, and don’t have to take any other medication from that point on. And that time may be, like, 5 years from now. It’s wild.
Seems to me that that rapid Nobel Prize was very well deserved.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships. This transcript has been edited for clarity.
A version of this article appeared on Medscape.com.
Meta-analysis shows benefits of capecitabine-based chemo in early TNBC
Key clinical point: Capecitabine-based chemotherapy improved prognostic outcomes in patients with early-stage triple-negative breast cancer (TNBC).
Major finding: Capecitabine-based chemotherapy vs capecitabine-free regimens improved disease-free survival (DFS; hazard ratio [HR] 0.81; P < .001) and overall survival (HR 0.75; P < .001) outcomes. DFS benefits were particularly observed in the adjuvant setting (HR 0.79; P < .001) and in the subgroup of patients with lymph node-negative TNBC (HR 0.68; P = .006) and in those who received capecitabine for ≥ 6 cycles (HR 0.71; P < .001).
Study details: Findings are from a meta-analysis of 12 randomized controlled trials including 5390 patients with TNBC who were treated with capecitabine-based chemotherapy or capecitabine-free regimens.
Disclosures: This study was supported by the National Natural Science Foundation of China and the Natural Science Foundation of Chongqing. The authors declared no conflicts of interest.
Source: Bai J et al. Capecitabine-based chemotherapy in early-stage triple-negative breast cancer: A meta-analysis. Front Oncol. 2023;13:1245650 (Oct 25). doi: 10.3389/fonc.2023.1245650
Key clinical point: Capecitabine-based chemotherapy improved prognostic outcomes in patients with early-stage triple-negative breast cancer (TNBC).
Major finding: Capecitabine-based chemotherapy vs capecitabine-free regimens improved disease-free survival (DFS; hazard ratio [HR] 0.81; P < .001) and overall survival (HR 0.75; P < .001) outcomes. DFS benefits were particularly observed in the adjuvant setting (HR 0.79; P < .001) and in the subgroup of patients with lymph node-negative TNBC (HR 0.68; P = .006) and in those who received capecitabine for ≥ 6 cycles (HR 0.71; P < .001).
Study details: Findings are from a meta-analysis of 12 randomized controlled trials including 5390 patients with TNBC who were treated with capecitabine-based chemotherapy or capecitabine-free regimens.
Disclosures: This study was supported by the National Natural Science Foundation of China and the Natural Science Foundation of Chongqing. The authors declared no conflicts of interest.
Source: Bai J et al. Capecitabine-based chemotherapy in early-stage triple-negative breast cancer: A meta-analysis. Front Oncol. 2023;13:1245650 (Oct 25). doi: 10.3389/fonc.2023.1245650
Key clinical point: Capecitabine-based chemotherapy improved prognostic outcomes in patients with early-stage triple-negative breast cancer (TNBC).
Major finding: Capecitabine-based chemotherapy vs capecitabine-free regimens improved disease-free survival (DFS; hazard ratio [HR] 0.81; P < .001) and overall survival (HR 0.75; P < .001) outcomes. DFS benefits were particularly observed in the adjuvant setting (HR 0.79; P < .001) and in the subgroup of patients with lymph node-negative TNBC (HR 0.68; P = .006) and in those who received capecitabine for ≥ 6 cycles (HR 0.71; P < .001).
Study details: Findings are from a meta-analysis of 12 randomized controlled trials including 5390 patients with TNBC who were treated with capecitabine-based chemotherapy or capecitabine-free regimens.
Disclosures: This study was supported by the National Natural Science Foundation of China and the Natural Science Foundation of Chongqing. The authors declared no conflicts of interest.
Source: Bai J et al. Capecitabine-based chemotherapy in early-stage triple-negative breast cancer: A meta-analysis. Front Oncol. 2023;13:1245650 (Oct 25). doi: 10.3389/fonc.2023.1245650
Regional nodal irradiation may not be needed after preoperative systemic therapy in HER2+ BC
Key clinical point: Patients with human epidermal growth factor receptor 2-positive (HER2+) breast cancer (BC) who received docetaxel/carboplatin/trastuzumab/pertuzumab (TCHP)-based preoperative systemic therapy experienced no extra clinical benefits with postoperative regional nodal irradiation (RNI).
Major finding: Patients who did vs did not receive RNI had comparable locoregional recurrence frequency (2.6% vs 1.0%; P = .651) and disease-free survival outcomes (hazard ratio 0.72; P = .638); however, pathological complete response was achieved by a significantly higher proportion of patients in the no-RNI vs RNI group (72.5% vs 44.4%; P < .001).
Study details: This retrospective study included 255 patients with HER2+ BC who received six cycles of TCHP, of which 60% of patients received RNI.
Disclosures: This study did not declare the source of funding or conflicts of interest.
Source: Kim N, Kim J-Y, et al. Benefit of postoperative regional nodal irradiation in patients receiving preoperative systemic therapy with docetaxel/carboplatin/trastuzumab/pertuzumab for HER2-positive breast cancer. Breast. 2023;72:103594 (Oct 30). doi: 10.1016/j.breast.2023.103594
Key clinical point: Patients with human epidermal growth factor receptor 2-positive (HER2+) breast cancer (BC) who received docetaxel/carboplatin/trastuzumab/pertuzumab (TCHP)-based preoperative systemic therapy experienced no extra clinical benefits with postoperative regional nodal irradiation (RNI).
Major finding: Patients who did vs did not receive RNI had comparable locoregional recurrence frequency (2.6% vs 1.0%; P = .651) and disease-free survival outcomes (hazard ratio 0.72; P = .638); however, pathological complete response was achieved by a significantly higher proportion of patients in the no-RNI vs RNI group (72.5% vs 44.4%; P < .001).
Study details: This retrospective study included 255 patients with HER2+ BC who received six cycles of TCHP, of which 60% of patients received RNI.
Disclosures: This study did not declare the source of funding or conflicts of interest.
Source: Kim N, Kim J-Y, et al. Benefit of postoperative regional nodal irradiation in patients receiving preoperative systemic therapy with docetaxel/carboplatin/trastuzumab/pertuzumab for HER2-positive breast cancer. Breast. 2023;72:103594 (Oct 30). doi: 10.1016/j.breast.2023.103594
Key clinical point: Patients with human epidermal growth factor receptor 2-positive (HER2+) breast cancer (BC) who received docetaxel/carboplatin/trastuzumab/pertuzumab (TCHP)-based preoperative systemic therapy experienced no extra clinical benefits with postoperative regional nodal irradiation (RNI).
Major finding: Patients who did vs did not receive RNI had comparable locoregional recurrence frequency (2.6% vs 1.0%; P = .651) and disease-free survival outcomes (hazard ratio 0.72; P = .638); however, pathological complete response was achieved by a significantly higher proportion of patients in the no-RNI vs RNI group (72.5% vs 44.4%; P < .001).
Study details: This retrospective study included 255 patients with HER2+ BC who received six cycles of TCHP, of which 60% of patients received RNI.
Disclosures: This study did not declare the source of funding or conflicts of interest.
Source: Kim N, Kim J-Y, et al. Benefit of postoperative regional nodal irradiation in patients receiving preoperative systemic therapy with docetaxel/carboplatin/trastuzumab/pertuzumab for HER2-positive breast cancer. Breast. 2023;72:103594 (Oct 30). doi: 10.1016/j.breast.2023.103594
Taxanes followed by PLD show promise in metastatic BC under real-world settings
Key clinical point: First-line treatment with taxanes followed by pegylated liposomal doxorubicin (PLD) was associated with improved prognostic outcomes in patients with human epidermal growth factor receptor 2-negative (HER2−) metastatic breast cancer (BC) than with PLD followed by taxanes.
Major finding: First-line taxane followed by PLD vs first-line PLD followed by taxane significantly improved time to next chemotherapy (9.9 vs 4.9 months; P = .006) and progression-free survival outcomes (9.0 vs 4.4 months; P = .005).
Study details: Findings are from a retrospective study including 42 patients with HER2− metastatic BC who received first-line PLD and later taxane (n = 23) or first-line taxane and later PLD (n = 19).
Disclosures: This study did not receive any specific grants. The authors declared no conflicts of interest.
Source: Wallrabenstein T et al. Upfront taxane could be superior to pegylated liposomal doxorubicin (PLD): A retrospective real-world analysis of treatment sequence taxane-PLD versus PLD-taxane in patients with metastatic breast cancer. Cancers (Basel). 2023;15(20):4953 (Oct 12). doi: 10.3390/cancers15204953
Key clinical point: First-line treatment with taxanes followed by pegylated liposomal doxorubicin (PLD) was associated with improved prognostic outcomes in patients with human epidermal growth factor receptor 2-negative (HER2−) metastatic breast cancer (BC) than with PLD followed by taxanes.
Major finding: First-line taxane followed by PLD vs first-line PLD followed by taxane significantly improved time to next chemotherapy (9.9 vs 4.9 months; P = .006) and progression-free survival outcomes (9.0 vs 4.4 months; P = .005).
Study details: Findings are from a retrospective study including 42 patients with HER2− metastatic BC who received first-line PLD and later taxane (n = 23) or first-line taxane and later PLD (n = 19).
Disclosures: This study did not receive any specific grants. The authors declared no conflicts of interest.
Source: Wallrabenstein T et al. Upfront taxane could be superior to pegylated liposomal doxorubicin (PLD): A retrospective real-world analysis of treatment sequence taxane-PLD versus PLD-taxane in patients with metastatic breast cancer. Cancers (Basel). 2023;15(20):4953 (Oct 12). doi: 10.3390/cancers15204953
Key clinical point: First-line treatment with taxanes followed by pegylated liposomal doxorubicin (PLD) was associated with improved prognostic outcomes in patients with human epidermal growth factor receptor 2-negative (HER2−) metastatic breast cancer (BC) than with PLD followed by taxanes.
Major finding: First-line taxane followed by PLD vs first-line PLD followed by taxane significantly improved time to next chemotherapy (9.9 vs 4.9 months; P = .006) and progression-free survival outcomes (9.0 vs 4.4 months; P = .005).
Study details: Findings are from a retrospective study including 42 patients with HER2− metastatic BC who received first-line PLD and later taxane (n = 23) or first-line taxane and later PLD (n = 19).
Disclosures: This study did not receive any specific grants. The authors declared no conflicts of interest.
Source: Wallrabenstein T et al. Upfront taxane could be superior to pegylated liposomal doxorubicin (PLD): A retrospective real-world analysis of treatment sequence taxane-PLD versus PLD-taxane in patients with metastatic breast cancer. Cancers (Basel). 2023;15(20):4953 (Oct 12). doi: 10.3390/cancers15204953
Better efficacy-safety with 3-week vs 4-week nab-paclitaxel in HER2− metastatic BC
Key clinical point: A 3-week vs 4-week nab-paclitaxel schedule showed more effective anti-tumor activity and a more manageable safety profile in patients with human epidermal growth factor receptor 2-negative (HER2−) metastatic breast cancer (BC).
Major finding: Compared with a 4-week paclitaxel regimen, a 3-week regimen led to a 56% improvement in progression-free survival outcomes (hazard ratio 0.44; P = .029) and was associated with a lower rate of grade ≥ 3 adverse events (14.9% vs 42.6%).
Study details: Findings are from a phase 2 study including 94 patients with HER2− metastatic BC who were randomly assigned to receive nab-paclitaxel for either a 3-week or 4-week schedule.
Disclosures: This study was sponsored by CSPC Ouyi Pharmaceutical Co., Ltd, China. The authors declared no conflicts of interest.
Source: Liu Y et al. Three-week versus 4-week schedule of nab-paclitaxel in patients with metastatic breast cancer: A randomized phase II study. Oncologist. 2023 (Oct 26). doi: 10.1093/oncolo/oyad288
Key clinical point: A 3-week vs 4-week nab-paclitaxel schedule showed more effective anti-tumor activity and a more manageable safety profile in patients with human epidermal growth factor receptor 2-negative (HER2−) metastatic breast cancer (BC).
Major finding: Compared with a 4-week paclitaxel regimen, a 3-week regimen led to a 56% improvement in progression-free survival outcomes (hazard ratio 0.44; P = .029) and was associated with a lower rate of grade ≥ 3 adverse events (14.9% vs 42.6%).
Study details: Findings are from a phase 2 study including 94 patients with HER2− metastatic BC who were randomly assigned to receive nab-paclitaxel for either a 3-week or 4-week schedule.
Disclosures: This study was sponsored by CSPC Ouyi Pharmaceutical Co., Ltd, China. The authors declared no conflicts of interest.
Source: Liu Y et al. Three-week versus 4-week schedule of nab-paclitaxel in patients with metastatic breast cancer: A randomized phase II study. Oncologist. 2023 (Oct 26). doi: 10.1093/oncolo/oyad288
Key clinical point: A 3-week vs 4-week nab-paclitaxel schedule showed more effective anti-tumor activity and a more manageable safety profile in patients with human epidermal growth factor receptor 2-negative (HER2−) metastatic breast cancer (BC).
Major finding: Compared with a 4-week paclitaxel regimen, a 3-week regimen led to a 56% improvement in progression-free survival outcomes (hazard ratio 0.44; P = .029) and was associated with a lower rate of grade ≥ 3 adverse events (14.9% vs 42.6%).
Study details: Findings are from a phase 2 study including 94 patients with HER2− metastatic BC who were randomly assigned to receive nab-paclitaxel for either a 3-week or 4-week schedule.
Disclosures: This study was sponsored by CSPC Ouyi Pharmaceutical Co., Ltd, China. The authors declared no conflicts of interest.
Source: Liu Y et al. Three-week versus 4-week schedule of nab-paclitaxel in patients with metastatic breast cancer: A randomized phase II study. Oncologist. 2023 (Oct 26). doi: 10.1093/oncolo/oyad288

