User login
Actinic keratoses may predict skin cancers in older adults
TOPLINE:
.
METHODOLOGY:
- AKs have been associated with a small risk for cutaneous SCC, but associations with risk for other skin cancers have not been well studied.
- AKs may be a marker of overall skin cancer risk, but guidelines for AK management lack recommendations for follow-up cancer surveillance.
- The researchers reviewed data from a random sample of 5 million fee-for-service Medicare beneficiaries treated for AKs from 2009 through 2018 in the United States. Patients with seborrheic keratoses (SKs) were included as comparators, and patients with a history of skin cancer were excluded.
- The primary outcome was the first surgically treated skin cancer, including SCC, BCC, and melanoma.
TAKEAWAY:
- A total of 555,945 adults with AKs and 481,024 with SKs were included. The mean age was approximately 74.0 years. More than half were female. Most were non-Hispanic White.
- Among patients with AKs, the absolute risk for any skin cancer after the first AK was 6.3%, 18.4%, and 28.5% at 1, 3, and 5 years, respectively.
- Patients with AKs had a significantly increased relative risk for any skin cancer compared with those with SKs (adjusted hazard ratio [aHR], 2.17) and separately for keratinocyte carcinoma (aHR, 2.20), SCC (aHR, 2.63), BCC (aHR, 1.85), and melanoma (aHR, 1.67).
- Although AKs are not considered a biological precursor of melanoma or BCC, the results suggest that AKs may be clinical indicators of increased UV exposure that subsequently increases the risk for skin cancer.
IN PRACTICE:
“The present results highlight the importance of developing evidence-based guidelines for follow-up skin cancer surveillance in patients with AKs, optimally including measures of AK burden,” the researchers wrote.
SOURCE:
The lead author on the study was Cassandra Mohr, BS, with corresponding author Mackenzie R. Wehner, MD, MPhil, of The University of Texas MD Anderson Cancer Center, Houston. The study was published online in JAMA Dermatology .
LIMITATIONS:
The study population of Medicare beneficiaries aged 65 years or older may not be a nationally representative sample, and surveillance bias may contribute to the increased risk for skin cancer in patients with AKs. The use of both ICD and CPT codes may underestimate the number of skin cancers because of cases that were treated nonsurgically.
DISCLOSURES:
The study was supported by the National Cancer Institute of the National Institutes of Health, the Cancer Prevention and Research Institute of Texas, and The University of Texas Rising STARS program. The researchers had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
.
METHODOLOGY:
- AKs have been associated with a small risk for cutaneous SCC, but associations with risk for other skin cancers have not been well studied.
- AKs may be a marker of overall skin cancer risk, but guidelines for AK management lack recommendations for follow-up cancer surveillance.
- The researchers reviewed data from a random sample of 5 million fee-for-service Medicare beneficiaries treated for AKs from 2009 through 2018 in the United States. Patients with seborrheic keratoses (SKs) were included as comparators, and patients with a history of skin cancer were excluded.
- The primary outcome was the first surgically treated skin cancer, including SCC, BCC, and melanoma.
TAKEAWAY:
- A total of 555,945 adults with AKs and 481,024 with SKs were included. The mean age was approximately 74.0 years. More than half were female. Most were non-Hispanic White.
- Among patients with AKs, the absolute risk for any skin cancer after the first AK was 6.3%, 18.4%, and 28.5% at 1, 3, and 5 years, respectively.
- Patients with AKs had a significantly increased relative risk for any skin cancer compared with those with SKs (adjusted hazard ratio [aHR], 2.17) and separately for keratinocyte carcinoma (aHR, 2.20), SCC (aHR, 2.63), BCC (aHR, 1.85), and melanoma (aHR, 1.67).
- Although AKs are not considered a biological precursor of melanoma or BCC, the results suggest that AKs may be clinical indicators of increased UV exposure that subsequently increases the risk for skin cancer.
IN PRACTICE:
“The present results highlight the importance of developing evidence-based guidelines for follow-up skin cancer surveillance in patients with AKs, optimally including measures of AK burden,” the researchers wrote.
SOURCE:
The lead author on the study was Cassandra Mohr, BS, with corresponding author Mackenzie R. Wehner, MD, MPhil, of The University of Texas MD Anderson Cancer Center, Houston. The study was published online in JAMA Dermatology .
LIMITATIONS:
The study population of Medicare beneficiaries aged 65 years or older may not be a nationally representative sample, and surveillance bias may contribute to the increased risk for skin cancer in patients with AKs. The use of both ICD and CPT codes may underestimate the number of skin cancers because of cases that were treated nonsurgically.
DISCLOSURES:
The study was supported by the National Cancer Institute of the National Institutes of Health, the Cancer Prevention and Research Institute of Texas, and The University of Texas Rising STARS program. The researchers had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
.
METHODOLOGY:
- AKs have been associated with a small risk for cutaneous SCC, but associations with risk for other skin cancers have not been well studied.
- AKs may be a marker of overall skin cancer risk, but guidelines for AK management lack recommendations for follow-up cancer surveillance.
- The researchers reviewed data from a random sample of 5 million fee-for-service Medicare beneficiaries treated for AKs from 2009 through 2018 in the United States. Patients with seborrheic keratoses (SKs) were included as comparators, and patients with a history of skin cancer were excluded.
- The primary outcome was the first surgically treated skin cancer, including SCC, BCC, and melanoma.
TAKEAWAY:
- A total of 555,945 adults with AKs and 481,024 with SKs were included. The mean age was approximately 74.0 years. More than half were female. Most were non-Hispanic White.
- Among patients with AKs, the absolute risk for any skin cancer after the first AK was 6.3%, 18.4%, and 28.5% at 1, 3, and 5 years, respectively.
- Patients with AKs had a significantly increased relative risk for any skin cancer compared with those with SKs (adjusted hazard ratio [aHR], 2.17) and separately for keratinocyte carcinoma (aHR, 2.20), SCC (aHR, 2.63), BCC (aHR, 1.85), and melanoma (aHR, 1.67).
- Although AKs are not considered a biological precursor of melanoma or BCC, the results suggest that AKs may be clinical indicators of increased UV exposure that subsequently increases the risk for skin cancer.
IN PRACTICE:
“The present results highlight the importance of developing evidence-based guidelines for follow-up skin cancer surveillance in patients with AKs, optimally including measures of AK burden,” the researchers wrote.
SOURCE:
The lead author on the study was Cassandra Mohr, BS, with corresponding author Mackenzie R. Wehner, MD, MPhil, of The University of Texas MD Anderson Cancer Center, Houston. The study was published online in JAMA Dermatology .
LIMITATIONS:
The study population of Medicare beneficiaries aged 65 years or older may not be a nationally representative sample, and surveillance bias may contribute to the increased risk for skin cancer in patients with AKs. The use of both ICD and CPT codes may underestimate the number of skin cancers because of cases that were treated nonsurgically.
DISCLOSURES:
The study was supported by the National Cancer Institute of the National Institutes of Health, the Cancer Prevention and Research Institute of Texas, and The University of Texas Rising STARS program. The researchers had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
Incipient ulceration may affect prognosis in primary melanoma
TOPLINE:
Incipient ulceration in primary cutaneous melanoma may represent a more biologically aggressive disease population than truly nonulcerated tumors.
METHODOLOGY:
- The final cohort included 40 cases of incipient ulceration that were matched 1:2 with 80 nonulcerated controls and 80 ulcerated controls.
- The prognostic significance of incipient ulceration in cutaneous melanoma is unclear.
- Current American Joint Committee on Cancer (AJCC) guidelines classify incipient ulceration as nonulcerated.
- In a retrospective case-control study, researchers drew from the Melanoma Institute Australia database to identify resected primary cutaneous melanomas diagnosed between 2005 and 2015 that had slides available at Royal Prince Alfred Hospital in Sydney and a Breslow thickness greater than 0 mm.
- Clinical outcomes compared between cases and controls were recurrence-free survival (RFS), melanoma-specific survival (MSS), and overall survival (OS).
TAKEAWAY:
- The median Breslow depth was 2.8 mm for incipient cases, compared with 1.0 mm for nonulcerated melanomas and 5.3 mm for ulcerated melanomas, while the median tumor mitotic rate was 5.0 per mm2 for incipient cases, compared with 1 per mm2 in nonulcerated controls and 9 per mm2 in ulcerated controls.
- On univariable analyses, compared with patients with incipiently ulcerated cases, patients with nonulcerated tumors had significantly better OS (hazard ratio [HR], 0.49) and RFS (HR, 0.37), while patients with ulcerated tumors showed worse RFS (HR, 1.67).
- On multivariable analyses, no differences in survival outcomes were observed, perhaps due to the moderate number of incipient ulceration cases included in the study, the authors wrote.
IN PRACTICE:
“Future editions of the AJCC staging system should consider acknowledging this interpretive challenge and provide guidance on how primary melanomas with incipient ulceration should be classified,” the researchers wrote.
SOURCE:
Richard A. Scolyer, MD, a pathologist at Royal Prince Alfred Hospital, Camperdown, Australia, is the senior author on the study, which was published online in JAMA Dermatology.
LIMITATIONS:
Limitations of the study include its retrospective design and the relatively small number of cases that met criteria for inclusion.
DISCLOSURES:
Dr. Scolyer disclosed that he has received grants from the Australian National Health and Medical Research Council and personal fees from MetaOptima, F. Hoffmann-La Roche, Evaxion, Provectus, QBiotics, Novartis, Merck Sharp & Dohme, NeraCare, Amgen, Bristol-Myers Squibb, Myriad Genetics, and GlaxoSmithKline, all outside the submitted work. Four coauthors reported having received financial support outside of the submitted work.
A version of this article appeared on Medscape.com.
TOPLINE:
Incipient ulceration in primary cutaneous melanoma may represent a more biologically aggressive disease population than truly nonulcerated tumors.
METHODOLOGY:
- The final cohort included 40 cases of incipient ulceration that were matched 1:2 with 80 nonulcerated controls and 80 ulcerated controls.
- The prognostic significance of incipient ulceration in cutaneous melanoma is unclear.
- Current American Joint Committee on Cancer (AJCC) guidelines classify incipient ulceration as nonulcerated.
- In a retrospective case-control study, researchers drew from the Melanoma Institute Australia database to identify resected primary cutaneous melanomas diagnosed between 2005 and 2015 that had slides available at Royal Prince Alfred Hospital in Sydney and a Breslow thickness greater than 0 mm.
- Clinical outcomes compared between cases and controls were recurrence-free survival (RFS), melanoma-specific survival (MSS), and overall survival (OS).
TAKEAWAY:
- The median Breslow depth was 2.8 mm for incipient cases, compared with 1.0 mm for nonulcerated melanomas and 5.3 mm for ulcerated melanomas, while the median tumor mitotic rate was 5.0 per mm2 for incipient cases, compared with 1 per mm2 in nonulcerated controls and 9 per mm2 in ulcerated controls.
- On univariable analyses, compared with patients with incipiently ulcerated cases, patients with nonulcerated tumors had significantly better OS (hazard ratio [HR], 0.49) and RFS (HR, 0.37), while patients with ulcerated tumors showed worse RFS (HR, 1.67).
- On multivariable analyses, no differences in survival outcomes were observed, perhaps due to the moderate number of incipient ulceration cases included in the study, the authors wrote.
IN PRACTICE:
“Future editions of the AJCC staging system should consider acknowledging this interpretive challenge and provide guidance on how primary melanomas with incipient ulceration should be classified,” the researchers wrote.
SOURCE:
Richard A. Scolyer, MD, a pathologist at Royal Prince Alfred Hospital, Camperdown, Australia, is the senior author on the study, which was published online in JAMA Dermatology.
LIMITATIONS:
Limitations of the study include its retrospective design and the relatively small number of cases that met criteria for inclusion.
DISCLOSURES:
Dr. Scolyer disclosed that he has received grants from the Australian National Health and Medical Research Council and personal fees from MetaOptima, F. Hoffmann-La Roche, Evaxion, Provectus, QBiotics, Novartis, Merck Sharp & Dohme, NeraCare, Amgen, Bristol-Myers Squibb, Myriad Genetics, and GlaxoSmithKline, all outside the submitted work. Four coauthors reported having received financial support outside of the submitted work.
A version of this article appeared on Medscape.com.
TOPLINE:
Incipient ulceration in primary cutaneous melanoma may represent a more biologically aggressive disease population than truly nonulcerated tumors.
METHODOLOGY:
- The final cohort included 40 cases of incipient ulceration that were matched 1:2 with 80 nonulcerated controls and 80 ulcerated controls.
- The prognostic significance of incipient ulceration in cutaneous melanoma is unclear.
- Current American Joint Committee on Cancer (AJCC) guidelines classify incipient ulceration as nonulcerated.
- In a retrospective case-control study, researchers drew from the Melanoma Institute Australia database to identify resected primary cutaneous melanomas diagnosed between 2005 and 2015 that had slides available at Royal Prince Alfred Hospital in Sydney and a Breslow thickness greater than 0 mm.
- Clinical outcomes compared between cases and controls were recurrence-free survival (RFS), melanoma-specific survival (MSS), and overall survival (OS).
TAKEAWAY:
- The median Breslow depth was 2.8 mm for incipient cases, compared with 1.0 mm for nonulcerated melanomas and 5.3 mm for ulcerated melanomas, while the median tumor mitotic rate was 5.0 per mm2 for incipient cases, compared with 1 per mm2 in nonulcerated controls and 9 per mm2 in ulcerated controls.
- On univariable analyses, compared with patients with incipiently ulcerated cases, patients with nonulcerated tumors had significantly better OS (hazard ratio [HR], 0.49) and RFS (HR, 0.37), while patients with ulcerated tumors showed worse RFS (HR, 1.67).
- On multivariable analyses, no differences in survival outcomes were observed, perhaps due to the moderate number of incipient ulceration cases included in the study, the authors wrote.
IN PRACTICE:
“Future editions of the AJCC staging system should consider acknowledging this interpretive challenge and provide guidance on how primary melanomas with incipient ulceration should be classified,” the researchers wrote.
SOURCE:
Richard A. Scolyer, MD, a pathologist at Royal Prince Alfred Hospital, Camperdown, Australia, is the senior author on the study, which was published online in JAMA Dermatology.
LIMITATIONS:
Limitations of the study include its retrospective design and the relatively small number of cases that met criteria for inclusion.
DISCLOSURES:
Dr. Scolyer disclosed that he has received grants from the Australian National Health and Medical Research Council and personal fees from MetaOptima, F. Hoffmann-La Roche, Evaxion, Provectus, QBiotics, Novartis, Merck Sharp & Dohme, NeraCare, Amgen, Bristol-Myers Squibb, Myriad Genetics, and GlaxoSmithKline, all outside the submitted work. Four coauthors reported having received financial support outside of the submitted work.
A version of this article appeared on Medscape.com.
For AFib cardioversion in obesity, dual energy might be the answer
PHILADELPHIA – , a multicenter randomized trial shows.
When treated with dual direct current cardioversion (DCCV), only 2% of patients with obesity failed to cardiovert on the first shock versus 14% (P = .002) of those treated with a conventional single DCCV, reported Joshua D. Aymond, MD, a fellow in electrophysiology at Ochsner Health, New Orleans.
Of the 14 patients in the single DCCV arm who did not convert on the first shock, 12 cardioverted when switched to dual energy. The remaining two cardioverted on the second dual shock.
In the dual DCCV group, of the two patients who did not cardiovert on the first dual shock, one did on the second. The other also cardioverted on a second shock, but this second shock was not delivered for 2 weeks, during which time the patient received a course of amiodarone-based anti-arrhythmic therapy.
No disadvantages seen with dual energy
The greater efficacy of a first shock with dual DCCV was achieved with no apparent disadvantages. There were no differences in post-procedure chest discomfort and no procedure-related adverse events in either arm, Dr. Aymond said.
The rising prevalence of obesity in the United States has created the need for a more effective first-line strategy for AF, noted Dr. Aymond, who presented the results of this study at the annual scientific sessions of the American Heart Association.
Cardioversion, which he characterized as the treatment of choice for AF, “fails to restore sinus rhythm in 20% to 35% of obese patients versus less than 10% of non-obese patients,” he said. The higher failure rate in patients with obesity is becoming a more common clinical issue not only due to the rising rates of obesity but a corresponding rise in AF, which is a related phenomenon.
“The risk of atrial fibrillation is increased by 50% relative to those who are not obese,” Dr. Aymond explained.
In this study, 200 patients at three participating centers were randomized to single DCCV or double DCCV after exclusions that included ventricular tachycardia and respiratory instability. The baseline characteristics were comparable. All 101 patients in the single DCCV group and 99 patients in the dual DCCV group were available for the intention-to-treat analysis.
200 vs. 400 joules delivered across the heart
In the study protocol, patients were fitted with four chest pads, two located adjacent but above the heart and two adjacent but below the heart. For single DCCV, 200 joules of energy were delivered from the upper right pad to the lower left pad across the heart. For dual DCCV, another 200 joules were delivered simultaneously from the upper left to the lower right across the heart. The total dose in the dual DCCV group was 400 joules.
The primary outcome was restoration of sinus rhythm of any duration immediately after DCCV. Safety, including clinical events, was a secondary outcome. Only the patients were blinded to the energy they received.
On univariate analysis, the odds ratio for successful cardioversion with dual DCCV was nearly eightfold higher (OR 7.8; P = .008) than single DCCV. On a simple multivariable analysis, when the researchers controlled for just age, sex, and body mass index, the odds ratio rose (OR 8.5; P = .007).
On a comprehensive multivariable analysis adding control for such characteristics as left ventricular ejection fraction (LVEF), obstructive sleep apnea, and antiarrhythmic drugs, the advantage of dual DCCV climbed above 12-fold (OR 12.6; P = .03).
The study is addressing a relevant and persistent question, said the AHA-invited discussant Jose A. Joglar, MD, program director, Clinical Cardiac Electrophysiology Fellowship, University of Texas Southwestern Medical Center, Dallas.
Dr. Joglar pointed out that alternatives to single DCCV for patients more difficult to cardiovert have been “sought for decades.” He noted that a variety of techniques, including dual DCCV, have been evaluated in small studies and case reports.
Alternatives for obese outlined
Several have shown promise, Dr. Joglar said. As one of several examples, he cited a 20-patient study that randomized patients to adhesive patches, like those employed in the Aymond trial, or handheld paddles. Both patches and paddles were applied with manual pressure while a 200-joule shock was delivered. The proportion of patients who cardioverted on the first shock was almost two times higher in the group after the first shock with the paddles (50% vs. 27%; P = .01). Dr. Joglar said the study supports the principle that 200 joules delivered by adhesive patches is inadequate for treatment of AF in many patients with obesity.
Dr. Joglar also cited studies suggesting that single DCCV delivered with higher energy than 200 joules appears to improve cardioversion success rates, but he indicated that this study with dual DCCV in the front-line setting provides evidence for another alternative.
“This is the first such trial with dual defibrillators as an initial strategy,” he said, calling the groups well matched and the superiority of dual DCCV “impressive.” He cautioned that the study size was well powered for the endpoint but perhaps small for evaluating relative safety.
Yet, “the study adds credibility and confidence for the use of dual DCCV, especially in difficult or refractory patients,” he said. He is less certain that it establishes dual DCCV as a standard first-line therapy in all patients with obesity. This would require additional studies to compare it to other types of strategies such as those he mentioned.
As an option for improving cardioversion in first-line treatment, dual DCCV “can be added to a list of other techniques, such as manual pressure or a higher initial dose with single DCCV,” he said.
Dr. Aymond and Dr. Joglar report no potential conflicts of interest.
PHILADELPHIA – , a multicenter randomized trial shows.
When treated with dual direct current cardioversion (DCCV), only 2% of patients with obesity failed to cardiovert on the first shock versus 14% (P = .002) of those treated with a conventional single DCCV, reported Joshua D. Aymond, MD, a fellow in electrophysiology at Ochsner Health, New Orleans.
Of the 14 patients in the single DCCV arm who did not convert on the first shock, 12 cardioverted when switched to dual energy. The remaining two cardioverted on the second dual shock.
In the dual DCCV group, of the two patients who did not cardiovert on the first dual shock, one did on the second. The other also cardioverted on a second shock, but this second shock was not delivered for 2 weeks, during which time the patient received a course of amiodarone-based anti-arrhythmic therapy.
No disadvantages seen with dual energy
The greater efficacy of a first shock with dual DCCV was achieved with no apparent disadvantages. There were no differences in post-procedure chest discomfort and no procedure-related adverse events in either arm, Dr. Aymond said.
The rising prevalence of obesity in the United States has created the need for a more effective first-line strategy for AF, noted Dr. Aymond, who presented the results of this study at the annual scientific sessions of the American Heart Association.
Cardioversion, which he characterized as the treatment of choice for AF, “fails to restore sinus rhythm in 20% to 35% of obese patients versus less than 10% of non-obese patients,” he said. The higher failure rate in patients with obesity is becoming a more common clinical issue not only due to the rising rates of obesity but a corresponding rise in AF, which is a related phenomenon.
“The risk of atrial fibrillation is increased by 50% relative to those who are not obese,” Dr. Aymond explained.
In this study, 200 patients at three participating centers were randomized to single DCCV or double DCCV after exclusions that included ventricular tachycardia and respiratory instability. The baseline characteristics were comparable. All 101 patients in the single DCCV group and 99 patients in the dual DCCV group were available for the intention-to-treat analysis.
200 vs. 400 joules delivered across the heart
In the study protocol, patients were fitted with four chest pads, two located adjacent but above the heart and two adjacent but below the heart. For single DCCV, 200 joules of energy were delivered from the upper right pad to the lower left pad across the heart. For dual DCCV, another 200 joules were delivered simultaneously from the upper left to the lower right across the heart. The total dose in the dual DCCV group was 400 joules.
The primary outcome was restoration of sinus rhythm of any duration immediately after DCCV. Safety, including clinical events, was a secondary outcome. Only the patients were blinded to the energy they received.
On univariate analysis, the odds ratio for successful cardioversion with dual DCCV was nearly eightfold higher (OR 7.8; P = .008) than single DCCV. On a simple multivariable analysis, when the researchers controlled for just age, sex, and body mass index, the odds ratio rose (OR 8.5; P = .007).
On a comprehensive multivariable analysis adding control for such characteristics as left ventricular ejection fraction (LVEF), obstructive sleep apnea, and antiarrhythmic drugs, the advantage of dual DCCV climbed above 12-fold (OR 12.6; P = .03).
The study is addressing a relevant and persistent question, said the AHA-invited discussant Jose A. Joglar, MD, program director, Clinical Cardiac Electrophysiology Fellowship, University of Texas Southwestern Medical Center, Dallas.
Dr. Joglar pointed out that alternatives to single DCCV for patients more difficult to cardiovert have been “sought for decades.” He noted that a variety of techniques, including dual DCCV, have been evaluated in small studies and case reports.
Alternatives for obese outlined
Several have shown promise, Dr. Joglar said. As one of several examples, he cited a 20-patient study that randomized patients to adhesive patches, like those employed in the Aymond trial, or handheld paddles. Both patches and paddles were applied with manual pressure while a 200-joule shock was delivered. The proportion of patients who cardioverted on the first shock was almost two times higher in the group after the first shock with the paddles (50% vs. 27%; P = .01). Dr. Joglar said the study supports the principle that 200 joules delivered by adhesive patches is inadequate for treatment of AF in many patients with obesity.
Dr. Joglar also cited studies suggesting that single DCCV delivered with higher energy than 200 joules appears to improve cardioversion success rates, but he indicated that this study with dual DCCV in the front-line setting provides evidence for another alternative.
“This is the first such trial with dual defibrillators as an initial strategy,” he said, calling the groups well matched and the superiority of dual DCCV “impressive.” He cautioned that the study size was well powered for the endpoint but perhaps small for evaluating relative safety.
Yet, “the study adds credibility and confidence for the use of dual DCCV, especially in difficult or refractory patients,” he said. He is less certain that it establishes dual DCCV as a standard first-line therapy in all patients with obesity. This would require additional studies to compare it to other types of strategies such as those he mentioned.
As an option for improving cardioversion in first-line treatment, dual DCCV “can be added to a list of other techniques, such as manual pressure or a higher initial dose with single DCCV,” he said.
Dr. Aymond and Dr. Joglar report no potential conflicts of interest.
PHILADELPHIA – , a multicenter randomized trial shows.
When treated with dual direct current cardioversion (DCCV), only 2% of patients with obesity failed to cardiovert on the first shock versus 14% (P = .002) of those treated with a conventional single DCCV, reported Joshua D. Aymond, MD, a fellow in electrophysiology at Ochsner Health, New Orleans.
Of the 14 patients in the single DCCV arm who did not convert on the first shock, 12 cardioverted when switched to dual energy. The remaining two cardioverted on the second dual shock.
In the dual DCCV group, of the two patients who did not cardiovert on the first dual shock, one did on the second. The other also cardioverted on a second shock, but this second shock was not delivered for 2 weeks, during which time the patient received a course of amiodarone-based anti-arrhythmic therapy.
No disadvantages seen with dual energy
The greater efficacy of a first shock with dual DCCV was achieved with no apparent disadvantages. There were no differences in post-procedure chest discomfort and no procedure-related adverse events in either arm, Dr. Aymond said.
The rising prevalence of obesity in the United States has created the need for a more effective first-line strategy for AF, noted Dr. Aymond, who presented the results of this study at the annual scientific sessions of the American Heart Association.
Cardioversion, which he characterized as the treatment of choice for AF, “fails to restore sinus rhythm in 20% to 35% of obese patients versus less than 10% of non-obese patients,” he said. The higher failure rate in patients with obesity is becoming a more common clinical issue not only due to the rising rates of obesity but a corresponding rise in AF, which is a related phenomenon.
“The risk of atrial fibrillation is increased by 50% relative to those who are not obese,” Dr. Aymond explained.
In this study, 200 patients at three participating centers were randomized to single DCCV or double DCCV after exclusions that included ventricular tachycardia and respiratory instability. The baseline characteristics were comparable. All 101 patients in the single DCCV group and 99 patients in the dual DCCV group were available for the intention-to-treat analysis.
200 vs. 400 joules delivered across the heart
In the study protocol, patients were fitted with four chest pads, two located adjacent but above the heart and two adjacent but below the heart. For single DCCV, 200 joules of energy were delivered from the upper right pad to the lower left pad across the heart. For dual DCCV, another 200 joules were delivered simultaneously from the upper left to the lower right across the heart. The total dose in the dual DCCV group was 400 joules.
The primary outcome was restoration of sinus rhythm of any duration immediately after DCCV. Safety, including clinical events, was a secondary outcome. Only the patients were blinded to the energy they received.
On univariate analysis, the odds ratio for successful cardioversion with dual DCCV was nearly eightfold higher (OR 7.8; P = .008) than single DCCV. On a simple multivariable analysis, when the researchers controlled for just age, sex, and body mass index, the odds ratio rose (OR 8.5; P = .007).
On a comprehensive multivariable analysis adding control for such characteristics as left ventricular ejection fraction (LVEF), obstructive sleep apnea, and antiarrhythmic drugs, the advantage of dual DCCV climbed above 12-fold (OR 12.6; P = .03).
The study is addressing a relevant and persistent question, said the AHA-invited discussant Jose A. Joglar, MD, program director, Clinical Cardiac Electrophysiology Fellowship, University of Texas Southwestern Medical Center, Dallas.
Dr. Joglar pointed out that alternatives to single DCCV for patients more difficult to cardiovert have been “sought for decades.” He noted that a variety of techniques, including dual DCCV, have been evaluated in small studies and case reports.
Alternatives for obese outlined
Several have shown promise, Dr. Joglar said. As one of several examples, he cited a 20-patient study that randomized patients to adhesive patches, like those employed in the Aymond trial, or handheld paddles. Both patches and paddles were applied with manual pressure while a 200-joule shock was delivered. The proportion of patients who cardioverted on the first shock was almost two times higher in the group after the first shock with the paddles (50% vs. 27%; P = .01). Dr. Joglar said the study supports the principle that 200 joules delivered by adhesive patches is inadequate for treatment of AF in many patients with obesity.
Dr. Joglar also cited studies suggesting that single DCCV delivered with higher energy than 200 joules appears to improve cardioversion success rates, but he indicated that this study with dual DCCV in the front-line setting provides evidence for another alternative.
“This is the first such trial with dual defibrillators as an initial strategy,” he said, calling the groups well matched and the superiority of dual DCCV “impressive.” He cautioned that the study size was well powered for the endpoint but perhaps small for evaluating relative safety.
Yet, “the study adds credibility and confidence for the use of dual DCCV, especially in difficult or refractory patients,” he said. He is less certain that it establishes dual DCCV as a standard first-line therapy in all patients with obesity. This would require additional studies to compare it to other types of strategies such as those he mentioned.
As an option for improving cardioversion in first-line treatment, dual DCCV “can be added to a list of other techniques, such as manual pressure or a higher initial dose with single DCCV,” he said.
Dr. Aymond and Dr. Joglar report no potential conflicts of interest.
AT AHA 2023
Blood pressure lowering reduces dementia risk
Results of a trial using an intensive, 4-year program aimed at blood pressure lowering showed that intervention reduced not only blood pressure, but also significantly reduced the risk of total dementia over that period.
and cognitive impairment no dementia (CIND), a secondary outcome, was also significantly reduced by 16%.
“Blood pressure reduction is effective in reducing the risk of dementia in patients with hypertension,” concluded Jiang He, MD, PhD, professor of epidemiology and medicine and director of Tulane University’s Translational Science Institute, New Orleans. “This proven, effective intervention should be widely scaled up to reduce the global burden of dementia.”
He presented these results from the China Rural Hypertension Control Project (CRHCP) at the annual scientific sessions of the American Heart Association.
Target organ damage
Keith Ferdinand, MD, also from Tulane University, commented on the findings during a press conference at the meeting, noting that the result “opens our opportunity to recognize that the target organ damage of hypertension also now includes dementia.”
The researchers were able to “rigorously lower blood pressure from 157 to 127.6 in the intervention, 155 to 147 in the controls – 22 mg Hg – and if you look at the P values for all the various outcomes, they were very robust,” Dr. Ferdinand said.
Another interesting feature about the strategy used in this trial is that “this was true team-based care,” he pointed out. The trained interventionists in the study, called village doctors, collaborated with primary care physicians and initiated medications. “They stayed on a simple treatment protocol, and they were able to assist patients to ensure they had free medications, health coaching for lifestyle, home blood pressure measurement, and ensuring adherence.”
So, Dr. Ferdinand added, “one of the questions is whether this is a model we can use in other places around the globe, in places with low resources, and in the United States in disadvantaged populations.”
Public health priority
It’s estimated that the global number of those living with dementia will increase from 57.4 million in 2019 to 152.8 million by 2050, Dr. He said. “In the absence of curative treatment, the primary prevention of dementia through risk factor reduction, such as blood pressure lowering, becomes a public health priority.”
Previous randomized trials have lacked sample size and duration but have reported a nonsignificant reduction in dementia associated with antihypertensive treatment in patients with hypertension or a history of stroke, Dr. He noted.
This new trial aimed to test the effectiveness of intensive BP intervention to reduce the risk of all-cause dementia and cognitive impairment over a 48-month intervention period versus usual care.
It was an open-label, blinded-endpoint, cluster-randomized trial, and included 33,995 individual patients from 325 villages in China, aged 40 years and older, with untreated hypertension. The villages were randomly assigned to an intervention group or usual care, stratified by province, county, and township.
Patients were eligible if they had mean untreated systolic BP greater than 140 mm Hg and/or diastolic BP greater than 90 mm Hg or mean treated systolic BP of greater than 130 and/or diastolic greater than 80 mm Hg. Patients with a history of cardiovascular disease, chronic kidney disease, or diabetes and a mean systolic BP greater than 130 mm Hg and/or diastolic BP greater than 80 mm Hg from six measures on two different days were also eligible.
All were enrolled in the China New Rural Cooperative Medical Scheme, which covers 99% of rural residents for health care services, Dr. He noted.
The intervention was a simple stepped-care protocol for hypertension treatment, aimed at achieving a target systolic BP of less than 130 mm Hg and diastolic of less than 80 mm Hg.
Village doctors started and titrated antihypertensive treatment based on a protocol and were able to deliver discounted and free medications to patients. They also did health coaching on lifestyle modification and adherence to medication, and instructed patients on home BP monitoring.
Patients were provided training, supervision, and consultation by primary care physicians and hypertension specialists.
At the month 48 follow-up visit, the participants were assessed by neurologists who were blinded to randomization assignments. Neurologists did a variety of tests and assessments including collecting data on the patient’s medical and psychiatric history and risk factors for dementia, as well as neurologic assessment using the Mini-Mental State Examination, the Functional Activities Questionnaire, and the Quick Dementia Rating System.
The primary outcome was all-cause dementia, defined according to recommendations from the National Institute on Aging–Alzheimer’s Association work groups on diagnostic guidelines for Alzheimer’s disease.
Secondary outcomes included CIND, a composite outcome of dementia or CIND, and a composite of dementia or deaths.
The final diagnosis of all-cause dementia or CIND was made by an expert adjudication panel blinded to the intervention assignment.
At 48 months, 91.3% of patients completed the follow-up for clinical outcomes. Participants were an average of 63 years of age, 61% were female, and 23% had less than a primary school education, Dr. He noted.
The net group differences in systolic and diastolic BP reduction were 22 and 9.3 mm Hg, respectively (P < .0001).
Significant differences were also seen between the groups in the primary outcome of all-cause dementia, as well as secondary outcomes of CIND, dementia or cognitive impairment, or dementia or deaths.
Serious adverse events were more common in the usual care group, and there was no difference between groups in the occurrence of falls or syncope.
The effect was consistent across subgroups, Dr. He said, including age, sex, education, cigarette smoking, body mass index, systolic BP, and fasting plasma glucose at baseline.
First definitive evidence
Invited discussant for the trial, Daniel W. Jones, MD, University of Mississippi Medical Center, Jackson, and past president of the AHA, pointed out that previous results from CRHCP on cardiovascular outcomes, reported earlier in 2023 in The Lancet, showed that, similar to results of the large SPRINT trial, lowering systolic BP to a goal of less than 130 mm Hg reduced a composite endpoint of MI, stroke, heart failure requiring hospitalization, and cardiovascular disease death over the 36-month follow-up.
The SPRINT findings also suggested a possible reduction in dementia, Dr. Jones said.
Now, in these new CRHCP results, “there was a clear benefit for intensive BP control in reducing risk for dementia and cognitive dysfunction,” he said. “This is, importantly, the first definitive evidence of dementia risk reduction demonstrated in a randomized controlled clinical trial. This outcome supports observational data that shows a strong relationship between BP and dementia.”
Since it is the first of its kind though, replication of the results will be important, he noted.
The study also showed that the intervention, using minimally trained village doctors, sustained BP control for 48 months. “This model could be used in any setting with modifications, including in the United States,” Dr. Jones said.
The study was supported by the Ministry of Science and Technology of China; U.S. investigators did not receive financial support from this study. The researchers and Dr. Jones disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Results of a trial using an intensive, 4-year program aimed at blood pressure lowering showed that intervention reduced not only blood pressure, but also significantly reduced the risk of total dementia over that period.
and cognitive impairment no dementia (CIND), a secondary outcome, was also significantly reduced by 16%.
“Blood pressure reduction is effective in reducing the risk of dementia in patients with hypertension,” concluded Jiang He, MD, PhD, professor of epidemiology and medicine and director of Tulane University’s Translational Science Institute, New Orleans. “This proven, effective intervention should be widely scaled up to reduce the global burden of dementia.”
He presented these results from the China Rural Hypertension Control Project (CRHCP) at the annual scientific sessions of the American Heart Association.
Target organ damage
Keith Ferdinand, MD, also from Tulane University, commented on the findings during a press conference at the meeting, noting that the result “opens our opportunity to recognize that the target organ damage of hypertension also now includes dementia.”
The researchers were able to “rigorously lower blood pressure from 157 to 127.6 in the intervention, 155 to 147 in the controls – 22 mg Hg – and if you look at the P values for all the various outcomes, they were very robust,” Dr. Ferdinand said.
Another interesting feature about the strategy used in this trial is that “this was true team-based care,” he pointed out. The trained interventionists in the study, called village doctors, collaborated with primary care physicians and initiated medications. “They stayed on a simple treatment protocol, and they were able to assist patients to ensure they had free medications, health coaching for lifestyle, home blood pressure measurement, and ensuring adherence.”
So, Dr. Ferdinand added, “one of the questions is whether this is a model we can use in other places around the globe, in places with low resources, and in the United States in disadvantaged populations.”
Public health priority
It’s estimated that the global number of those living with dementia will increase from 57.4 million in 2019 to 152.8 million by 2050, Dr. He said. “In the absence of curative treatment, the primary prevention of dementia through risk factor reduction, such as blood pressure lowering, becomes a public health priority.”
Previous randomized trials have lacked sample size and duration but have reported a nonsignificant reduction in dementia associated with antihypertensive treatment in patients with hypertension or a history of stroke, Dr. He noted.
This new trial aimed to test the effectiveness of intensive BP intervention to reduce the risk of all-cause dementia and cognitive impairment over a 48-month intervention period versus usual care.
It was an open-label, blinded-endpoint, cluster-randomized trial, and included 33,995 individual patients from 325 villages in China, aged 40 years and older, with untreated hypertension. The villages were randomly assigned to an intervention group or usual care, stratified by province, county, and township.
Patients were eligible if they had mean untreated systolic BP greater than 140 mm Hg and/or diastolic BP greater than 90 mm Hg or mean treated systolic BP of greater than 130 and/or diastolic greater than 80 mm Hg. Patients with a history of cardiovascular disease, chronic kidney disease, or diabetes and a mean systolic BP greater than 130 mm Hg and/or diastolic BP greater than 80 mm Hg from six measures on two different days were also eligible.
All were enrolled in the China New Rural Cooperative Medical Scheme, which covers 99% of rural residents for health care services, Dr. He noted.
The intervention was a simple stepped-care protocol for hypertension treatment, aimed at achieving a target systolic BP of less than 130 mm Hg and diastolic of less than 80 mm Hg.
Village doctors started and titrated antihypertensive treatment based on a protocol and were able to deliver discounted and free medications to patients. They also did health coaching on lifestyle modification and adherence to medication, and instructed patients on home BP monitoring.
Patients were provided training, supervision, and consultation by primary care physicians and hypertension specialists.
At the month 48 follow-up visit, the participants were assessed by neurologists who were blinded to randomization assignments. Neurologists did a variety of tests and assessments including collecting data on the patient’s medical and psychiatric history and risk factors for dementia, as well as neurologic assessment using the Mini-Mental State Examination, the Functional Activities Questionnaire, and the Quick Dementia Rating System.
The primary outcome was all-cause dementia, defined according to recommendations from the National Institute on Aging–Alzheimer’s Association work groups on diagnostic guidelines for Alzheimer’s disease.
Secondary outcomes included CIND, a composite outcome of dementia or CIND, and a composite of dementia or deaths.
The final diagnosis of all-cause dementia or CIND was made by an expert adjudication panel blinded to the intervention assignment.
At 48 months, 91.3% of patients completed the follow-up for clinical outcomes. Participants were an average of 63 years of age, 61% were female, and 23% had less than a primary school education, Dr. He noted.
The net group differences in systolic and diastolic BP reduction were 22 and 9.3 mm Hg, respectively (P < .0001).
Significant differences were also seen between the groups in the primary outcome of all-cause dementia, as well as secondary outcomes of CIND, dementia or cognitive impairment, or dementia or deaths.
Serious adverse events were more common in the usual care group, and there was no difference between groups in the occurrence of falls or syncope.
The effect was consistent across subgroups, Dr. He said, including age, sex, education, cigarette smoking, body mass index, systolic BP, and fasting plasma glucose at baseline.
First definitive evidence
Invited discussant for the trial, Daniel W. Jones, MD, University of Mississippi Medical Center, Jackson, and past president of the AHA, pointed out that previous results from CRHCP on cardiovascular outcomes, reported earlier in 2023 in The Lancet, showed that, similar to results of the large SPRINT trial, lowering systolic BP to a goal of less than 130 mm Hg reduced a composite endpoint of MI, stroke, heart failure requiring hospitalization, and cardiovascular disease death over the 36-month follow-up.
The SPRINT findings also suggested a possible reduction in dementia, Dr. Jones said.
Now, in these new CRHCP results, “there was a clear benefit for intensive BP control in reducing risk for dementia and cognitive dysfunction,” he said. “This is, importantly, the first definitive evidence of dementia risk reduction demonstrated in a randomized controlled clinical trial. This outcome supports observational data that shows a strong relationship between BP and dementia.”
Since it is the first of its kind though, replication of the results will be important, he noted.
The study also showed that the intervention, using minimally trained village doctors, sustained BP control for 48 months. “This model could be used in any setting with modifications, including in the United States,” Dr. Jones said.
The study was supported by the Ministry of Science and Technology of China; U.S. investigators did not receive financial support from this study. The researchers and Dr. Jones disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Results of a trial using an intensive, 4-year program aimed at blood pressure lowering showed that intervention reduced not only blood pressure, but also significantly reduced the risk of total dementia over that period.
and cognitive impairment no dementia (CIND), a secondary outcome, was also significantly reduced by 16%.
“Blood pressure reduction is effective in reducing the risk of dementia in patients with hypertension,” concluded Jiang He, MD, PhD, professor of epidemiology and medicine and director of Tulane University’s Translational Science Institute, New Orleans. “This proven, effective intervention should be widely scaled up to reduce the global burden of dementia.”
He presented these results from the China Rural Hypertension Control Project (CRHCP) at the annual scientific sessions of the American Heart Association.
Target organ damage
Keith Ferdinand, MD, also from Tulane University, commented on the findings during a press conference at the meeting, noting that the result “opens our opportunity to recognize that the target organ damage of hypertension also now includes dementia.”
The researchers were able to “rigorously lower blood pressure from 157 to 127.6 in the intervention, 155 to 147 in the controls – 22 mg Hg – and if you look at the P values for all the various outcomes, they were very robust,” Dr. Ferdinand said.
Another interesting feature about the strategy used in this trial is that “this was true team-based care,” he pointed out. The trained interventionists in the study, called village doctors, collaborated with primary care physicians and initiated medications. “They stayed on a simple treatment protocol, and they were able to assist patients to ensure they had free medications, health coaching for lifestyle, home blood pressure measurement, and ensuring adherence.”
So, Dr. Ferdinand added, “one of the questions is whether this is a model we can use in other places around the globe, in places with low resources, and in the United States in disadvantaged populations.”
Public health priority
It’s estimated that the global number of those living with dementia will increase from 57.4 million in 2019 to 152.8 million by 2050, Dr. He said. “In the absence of curative treatment, the primary prevention of dementia through risk factor reduction, such as blood pressure lowering, becomes a public health priority.”
Previous randomized trials have lacked sample size and duration but have reported a nonsignificant reduction in dementia associated with antihypertensive treatment in patients with hypertension or a history of stroke, Dr. He noted.
This new trial aimed to test the effectiveness of intensive BP intervention to reduce the risk of all-cause dementia and cognitive impairment over a 48-month intervention period versus usual care.
It was an open-label, blinded-endpoint, cluster-randomized trial, and included 33,995 individual patients from 325 villages in China, aged 40 years and older, with untreated hypertension. The villages were randomly assigned to an intervention group or usual care, stratified by province, county, and township.
Patients were eligible if they had mean untreated systolic BP greater than 140 mm Hg and/or diastolic BP greater than 90 mm Hg or mean treated systolic BP of greater than 130 and/or diastolic greater than 80 mm Hg. Patients with a history of cardiovascular disease, chronic kidney disease, or diabetes and a mean systolic BP greater than 130 mm Hg and/or diastolic BP greater than 80 mm Hg from six measures on two different days were also eligible.
All were enrolled in the China New Rural Cooperative Medical Scheme, which covers 99% of rural residents for health care services, Dr. He noted.
The intervention was a simple stepped-care protocol for hypertension treatment, aimed at achieving a target systolic BP of less than 130 mm Hg and diastolic of less than 80 mm Hg.
Village doctors started and titrated antihypertensive treatment based on a protocol and were able to deliver discounted and free medications to patients. They also did health coaching on lifestyle modification and adherence to medication, and instructed patients on home BP monitoring.
Patients were provided training, supervision, and consultation by primary care physicians and hypertension specialists.
At the month 48 follow-up visit, the participants were assessed by neurologists who were blinded to randomization assignments. Neurologists did a variety of tests and assessments including collecting data on the patient’s medical and psychiatric history and risk factors for dementia, as well as neurologic assessment using the Mini-Mental State Examination, the Functional Activities Questionnaire, and the Quick Dementia Rating System.
The primary outcome was all-cause dementia, defined according to recommendations from the National Institute on Aging–Alzheimer’s Association work groups on diagnostic guidelines for Alzheimer’s disease.
Secondary outcomes included CIND, a composite outcome of dementia or CIND, and a composite of dementia or deaths.
The final diagnosis of all-cause dementia or CIND was made by an expert adjudication panel blinded to the intervention assignment.
At 48 months, 91.3% of patients completed the follow-up for clinical outcomes. Participants were an average of 63 years of age, 61% were female, and 23% had less than a primary school education, Dr. He noted.
The net group differences in systolic and diastolic BP reduction were 22 and 9.3 mm Hg, respectively (P < .0001).
Significant differences were also seen between the groups in the primary outcome of all-cause dementia, as well as secondary outcomes of CIND, dementia or cognitive impairment, or dementia or deaths.
Serious adverse events were more common in the usual care group, and there was no difference between groups in the occurrence of falls or syncope.
The effect was consistent across subgroups, Dr. He said, including age, sex, education, cigarette smoking, body mass index, systolic BP, and fasting plasma glucose at baseline.
First definitive evidence
Invited discussant for the trial, Daniel W. Jones, MD, University of Mississippi Medical Center, Jackson, and past president of the AHA, pointed out that previous results from CRHCP on cardiovascular outcomes, reported earlier in 2023 in The Lancet, showed that, similar to results of the large SPRINT trial, lowering systolic BP to a goal of less than 130 mm Hg reduced a composite endpoint of MI, stroke, heart failure requiring hospitalization, and cardiovascular disease death over the 36-month follow-up.
The SPRINT findings also suggested a possible reduction in dementia, Dr. Jones said.
Now, in these new CRHCP results, “there was a clear benefit for intensive BP control in reducing risk for dementia and cognitive dysfunction,” he said. “This is, importantly, the first definitive evidence of dementia risk reduction demonstrated in a randomized controlled clinical trial. This outcome supports observational data that shows a strong relationship between BP and dementia.”
Since it is the first of its kind though, replication of the results will be important, he noted.
The study also showed that the intervention, using minimally trained village doctors, sustained BP control for 48 months. “This model could be used in any setting with modifications, including in the United States,” Dr. Jones said.
The study was supported by the Ministry of Science and Technology of China; U.S. investigators did not receive financial support from this study. The researchers and Dr. Jones disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM AHA 2023
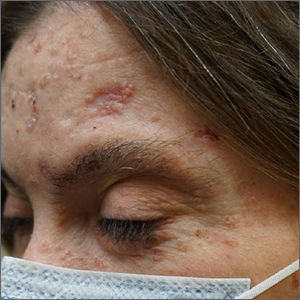
Multiple basal cell carcinomas
These skin findings were the latest manifestation of a condition that the patient had been diagnosed with at age 32: basal cell nevus syndrome (BCNS), also called Gorlin syndrome. This syndrome is characterized by multiple biopsy-proven BCCs, palmar pitting, frontal bossing, scoliosis, and gum cysts. This patient had had gum cysts since she was 8 years old; her sister and mother had similar gum cysts and her mother had at least 1 BCC. BCNS is caused by an inheritable defect in the Patched 1 (PTCH1) gene, leading to various findings—including numerous BCCs at a young age.1
The Oncology team started the patient on the oral small-molecule chemotherapy agent vismodegib, 150 mg/d. The patient was also referred to Medical Genetics and Wound Care. Although her diagnosis had been made clinically years earlier, genetic testing was performed and confirmed a defect in the PTCH1 gene. This helped with surveillance plans. A computed tomography scan of the head revealed a nasal dermoid cyst of the ethmoid sinus that the Ear, Nose, & Throat and Neurology teams felt safe to observe.
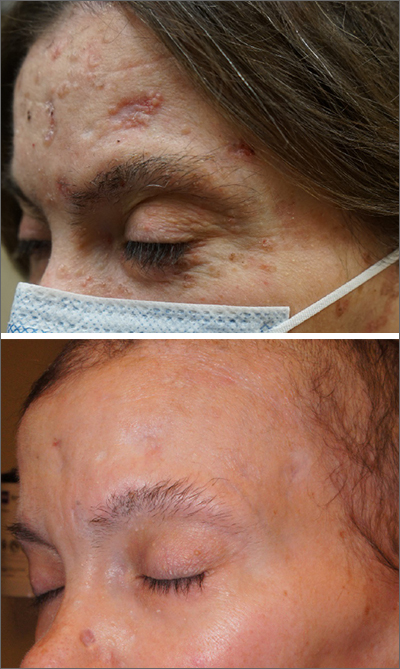
After 3 months of therapy with vismodegib, the patient had significant improvement of facial lesions and significant re-epithelialization of the crown.
Patients on vismodegib often deal with adverse effects, but adjusted dosing regimens have proved to improve tolerability. This patient had substantial adverse effects including fatigue, hair loss, loss of taste, and weight loss (26 lbs). Because of these adverse effects, her regimen was adjusted to 1 month of every other day active treatment and 2 months off treatment, cycled continuously. With this regimen, her weight returned to normal and her sense of taste returned for most days in the treatment cycle.
She has been tolerating this regimen for 3 years with continued control of BCCs.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Yang X, Dinehart SM. Intermittent vismodegib therapy in basal cell nevus syndrome. JAMA Dermatol. 2016;152:223-224. doi:10.1001/jamadermatol.2015.3210

These skin findings were the latest manifestation of a condition that the patient had been diagnosed with at age 32: basal cell nevus syndrome (BCNS), also called Gorlin syndrome. This syndrome is characterized by multiple biopsy-proven BCCs, palmar pitting, frontal bossing, scoliosis, and gum cysts. This patient had had gum cysts since she was 8 years old; her sister and mother had similar gum cysts and her mother had at least 1 BCC. BCNS is caused by an inheritable defect in the Patched 1 (PTCH1) gene, leading to various findings—including numerous BCCs at a young age.1
The Oncology team started the patient on the oral small-molecule chemotherapy agent vismodegib, 150 mg/d. The patient was also referred to Medical Genetics and Wound Care. Although her diagnosis had been made clinically years earlier, genetic testing was performed and confirmed a defect in the PTCH1 gene. This helped with surveillance plans. A computed tomography scan of the head revealed a nasal dermoid cyst of the ethmoid sinus that the Ear, Nose, & Throat and Neurology teams felt safe to observe.
After 3 months of therapy with vismodegib, the patient had significant improvement of facial lesions and significant re-epithelialization of the crown.
Patients on vismodegib often deal with adverse effects, but adjusted dosing regimens have proved to improve tolerability. This patient had substantial adverse effects including fatigue, hair loss, loss of taste, and weight loss (26 lbs). Because of these adverse effects, her regimen was adjusted to 1 month of every other day active treatment and 2 months off treatment, cycled continuously. With this regimen, her weight returned to normal and her sense of taste returned for most days in the treatment cycle.
She has been tolerating this regimen for 3 years with continued control of BCCs.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

These skin findings were the latest manifestation of a condition that the patient had been diagnosed with at age 32: basal cell nevus syndrome (BCNS), also called Gorlin syndrome. This syndrome is characterized by multiple biopsy-proven BCCs, palmar pitting, frontal bossing, scoliosis, and gum cysts. This patient had had gum cysts since she was 8 years old; her sister and mother had similar gum cysts and her mother had at least 1 BCC. BCNS is caused by an inheritable defect in the Patched 1 (PTCH1) gene, leading to various findings—including numerous BCCs at a young age.1
The Oncology team started the patient on the oral small-molecule chemotherapy agent vismodegib, 150 mg/d. The patient was also referred to Medical Genetics and Wound Care. Although her diagnosis had been made clinically years earlier, genetic testing was performed and confirmed a defect in the PTCH1 gene. This helped with surveillance plans. A computed tomography scan of the head revealed a nasal dermoid cyst of the ethmoid sinus that the Ear, Nose, & Throat and Neurology teams felt safe to observe.
After 3 months of therapy with vismodegib, the patient had significant improvement of facial lesions and significant re-epithelialization of the crown.
Patients on vismodegib often deal with adverse effects, but adjusted dosing regimens have proved to improve tolerability. This patient had substantial adverse effects including fatigue, hair loss, loss of taste, and weight loss (26 lbs). Because of these adverse effects, her regimen was adjusted to 1 month of every other day active treatment and 2 months off treatment, cycled continuously. With this regimen, her weight returned to normal and her sense of taste returned for most days in the treatment cycle.
She has been tolerating this regimen for 3 years with continued control of BCCs.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Yang X, Dinehart SM. Intermittent vismodegib therapy in basal cell nevus syndrome. JAMA Dermatol. 2016;152:223-224. doi:10.1001/jamadermatol.2015.3210
1. Yang X, Dinehart SM. Intermittent vismodegib therapy in basal cell nevus syndrome. JAMA Dermatol. 2016;152:223-224. doi:10.1001/jamadermatol.2015.3210
Highlights in Early Breast Cancer From ESMO 2023
Developments in early breast cancer reported at the European Society for Medical Oncology (ESMO) 2023 Congress are discussed by Dr Lisa Carey of University of North Carolina, Chapel Hill.
Dr Carey begins with 5-year results from the KEYNOTE-522 study in patients with early triple-negative breast cancer in which the immune checkpoint inhibitor pembrolizumab was incorporated into combination therapy both pre- and postoperatively. The new findings were consistent with earlier results, showing that pembrolizumab improved pathologic complete response (pCR) and event-free survival.
Turning to human epidermal growth factor receptor 2–positive (HER2+) disease, Dr Carey discusses the PHERGain trial's use of a genomic assay to define risk and predict pCR. She suggests that such assays could lead to tailored therapy for HER2+ patients.
On estrogen receptor–positive (ER+)/HER2- disease, Dr Carey reports first on KEYNOTE-756, which examined the addition of pembrolizumab to combination therapy for high-risk patients in both the neoadjuvant and adjuvant settings. Pembrolizumab improved pCR compared with placebo.
Dr Carey closes by discussing another study in high-risk ER+/HER2- disease. Similar in design to KEYNOTE-756, CheckMate 7FL found that nivolumab added to combination therapy again augmented pCR results.
--
Lisa A. Carey, MD, Distinguished Professor or Breast Cancer Research, University of North Carolina Lineberger Comprehensive Cancer Center; Professor, Division of Medical Oncology, Bassnight North Carolina Cancer Hospital, Chapel Hill, North Carolina
Lisa A. Carey, MD, has disclosed no relevant financial relationships.
Developments in early breast cancer reported at the European Society for Medical Oncology (ESMO) 2023 Congress are discussed by Dr Lisa Carey of University of North Carolina, Chapel Hill.
Dr Carey begins with 5-year results from the KEYNOTE-522 study in patients with early triple-negative breast cancer in which the immune checkpoint inhibitor pembrolizumab was incorporated into combination therapy both pre- and postoperatively. The new findings were consistent with earlier results, showing that pembrolizumab improved pathologic complete response (pCR) and event-free survival.
Turning to human epidermal growth factor receptor 2–positive (HER2+) disease, Dr Carey discusses the PHERGain trial's use of a genomic assay to define risk and predict pCR. She suggests that such assays could lead to tailored therapy for HER2+ patients.
On estrogen receptor–positive (ER+)/HER2- disease, Dr Carey reports first on KEYNOTE-756, which examined the addition of pembrolizumab to combination therapy for high-risk patients in both the neoadjuvant and adjuvant settings. Pembrolizumab improved pCR compared with placebo.
Dr Carey closes by discussing another study in high-risk ER+/HER2- disease. Similar in design to KEYNOTE-756, CheckMate 7FL found that nivolumab added to combination therapy again augmented pCR results.
--
Lisa A. Carey, MD, Distinguished Professor or Breast Cancer Research, University of North Carolina Lineberger Comprehensive Cancer Center; Professor, Division of Medical Oncology, Bassnight North Carolina Cancer Hospital, Chapel Hill, North Carolina
Lisa A. Carey, MD, has disclosed no relevant financial relationships.
Developments in early breast cancer reported at the European Society for Medical Oncology (ESMO) 2023 Congress are discussed by Dr Lisa Carey of University of North Carolina, Chapel Hill.
Dr Carey begins with 5-year results from the KEYNOTE-522 study in patients with early triple-negative breast cancer in which the immune checkpoint inhibitor pembrolizumab was incorporated into combination therapy both pre- and postoperatively. The new findings were consistent with earlier results, showing that pembrolizumab improved pathologic complete response (pCR) and event-free survival.
Turning to human epidermal growth factor receptor 2–positive (HER2+) disease, Dr Carey discusses the PHERGain trial's use of a genomic assay to define risk and predict pCR. She suggests that such assays could lead to tailored therapy for HER2+ patients.
On estrogen receptor–positive (ER+)/HER2- disease, Dr Carey reports first on KEYNOTE-756, which examined the addition of pembrolizumab to combination therapy for high-risk patients in both the neoadjuvant and adjuvant settings. Pembrolizumab improved pCR compared with placebo.
Dr Carey closes by discussing another study in high-risk ER+/HER2- disease. Similar in design to KEYNOTE-756, CheckMate 7FL found that nivolumab added to combination therapy again augmented pCR results.
--
Lisa A. Carey, MD, Distinguished Professor or Breast Cancer Research, University of North Carolina Lineberger Comprehensive Cancer Center; Professor, Division of Medical Oncology, Bassnight North Carolina Cancer Hospital, Chapel Hill, North Carolina
Lisa A. Carey, MD, has disclosed no relevant financial relationships.

Marketing the meds
I am not a marketing person. I never will be. I don’t think like one.
A current article on FiercePharma talked about Boehringer Ingelheim’s recent “rebranding,” which involved (among other things) changing the blues in its logo and ads to greens.
Maybe someone else out there would notice that change, but I wouldn’t have if I hadn’t read about it. Nor am I sure what affect it would have on me, if any. But I’m sure they paid psychologists and marketing teams quite a bit to make sure it was a good idea.
Likewise, when AbbVie repackaged Ubrelvy from 10 to a package to 16, the company felt the need to change the design of the sample boxes (which are also now green). I’m pretty sure none of my patients noticed. The only reason I did is because I’m the one who stocks my sample shelf here.
Abbvie and Boehringer aren’t alone in this, of course. Pharmaceutical marketing is big business. I understand the companies want doctors and patients to know about their products. In that respect they’re no different from General Motors or Kellogg’s.
But pharmaceuticals fall into a different area. Kellogg’s products don’t require a middleman handing you a script allowing you to buy corn flakes, so although the products are sold to the public, they also have to be sold to a person who isn’t buying them – the prescriber.
Not all these ads are bad, of course. At best they raise public awareness of different health conditions and the options to treat them. At worst ... well, currently there are several movies out there about the results of marketing done by the Sackler family and Purdue.
To me, most pharmaceutical ads look the same. They show happy people going about their lives, with the impression being that they couldn’t have done this without the benefit of the drug being marketed.
To a large extent I can’t knock that. Pharmaceuticals are amazing things. They’ve contributed dramatically to human health, life quality, and longevity.
But would I, or most people, notice if the lettering in the ads were blue, green, or yellow? Probably not. Someone with a background in the psychology of marketing would be able to show me data on how different colors affect our perceptions, but I still look at this and wonder if the money could have been better spent.
Maybe that’s why I’m not in marketing. I tend to be on the practical side. The idea of hiring a celebrity to endorse a migraine (or pretty much any) medication would never have occurred to me. I have no idea how much Pfizer paid Lady Gaga to sell Nurtec, but I’m pretty sure it’s a lot more than I’ll earn this year. Probably ever.
Like most neurologists I’m hopelessly left-brained. But I still wonder how much things like this really make a difference.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I am not a marketing person. I never will be. I don’t think like one.
A current article on FiercePharma talked about Boehringer Ingelheim’s recent “rebranding,” which involved (among other things) changing the blues in its logo and ads to greens.
Maybe someone else out there would notice that change, but I wouldn’t have if I hadn’t read about it. Nor am I sure what affect it would have on me, if any. But I’m sure they paid psychologists and marketing teams quite a bit to make sure it was a good idea.
Likewise, when AbbVie repackaged Ubrelvy from 10 to a package to 16, the company felt the need to change the design of the sample boxes (which are also now green). I’m pretty sure none of my patients noticed. The only reason I did is because I’m the one who stocks my sample shelf here.
Abbvie and Boehringer aren’t alone in this, of course. Pharmaceutical marketing is big business. I understand the companies want doctors and patients to know about their products. In that respect they’re no different from General Motors or Kellogg’s.
But pharmaceuticals fall into a different area. Kellogg’s products don’t require a middleman handing you a script allowing you to buy corn flakes, so although the products are sold to the public, they also have to be sold to a person who isn’t buying them – the prescriber.
Not all these ads are bad, of course. At best they raise public awareness of different health conditions and the options to treat them. At worst ... well, currently there are several movies out there about the results of marketing done by the Sackler family and Purdue.
To me, most pharmaceutical ads look the same. They show happy people going about their lives, with the impression being that they couldn’t have done this without the benefit of the drug being marketed.
To a large extent I can’t knock that. Pharmaceuticals are amazing things. They’ve contributed dramatically to human health, life quality, and longevity.
But would I, or most people, notice if the lettering in the ads were blue, green, or yellow? Probably not. Someone with a background in the psychology of marketing would be able to show me data on how different colors affect our perceptions, but I still look at this and wonder if the money could have been better spent.
Maybe that’s why I’m not in marketing. I tend to be on the practical side. The idea of hiring a celebrity to endorse a migraine (or pretty much any) medication would never have occurred to me. I have no idea how much Pfizer paid Lady Gaga to sell Nurtec, but I’m pretty sure it’s a lot more than I’ll earn this year. Probably ever.
Like most neurologists I’m hopelessly left-brained. But I still wonder how much things like this really make a difference.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I am not a marketing person. I never will be. I don’t think like one.
A current article on FiercePharma talked about Boehringer Ingelheim’s recent “rebranding,” which involved (among other things) changing the blues in its logo and ads to greens.
Maybe someone else out there would notice that change, but I wouldn’t have if I hadn’t read about it. Nor am I sure what affect it would have on me, if any. But I’m sure they paid psychologists and marketing teams quite a bit to make sure it was a good idea.
Likewise, when AbbVie repackaged Ubrelvy from 10 to a package to 16, the company felt the need to change the design of the sample boxes (which are also now green). I’m pretty sure none of my patients noticed. The only reason I did is because I’m the one who stocks my sample shelf here.
Abbvie and Boehringer aren’t alone in this, of course. Pharmaceutical marketing is big business. I understand the companies want doctors and patients to know about their products. In that respect they’re no different from General Motors or Kellogg’s.
But pharmaceuticals fall into a different area. Kellogg’s products don’t require a middleman handing you a script allowing you to buy corn flakes, so although the products are sold to the public, they also have to be sold to a person who isn’t buying them – the prescriber.
Not all these ads are bad, of course. At best they raise public awareness of different health conditions and the options to treat them. At worst ... well, currently there are several movies out there about the results of marketing done by the Sackler family and Purdue.
To me, most pharmaceutical ads look the same. They show happy people going about their lives, with the impression being that they couldn’t have done this without the benefit of the drug being marketed.
To a large extent I can’t knock that. Pharmaceuticals are amazing things. They’ve contributed dramatically to human health, life quality, and longevity.
But would I, or most people, notice if the lettering in the ads were blue, green, or yellow? Probably not. Someone with a background in the psychology of marketing would be able to show me data on how different colors affect our perceptions, but I still look at this and wonder if the money could have been better spent.
Maybe that’s why I’m not in marketing. I tend to be on the practical side. The idea of hiring a celebrity to endorse a migraine (or pretty much any) medication would never have occurred to me. I have no idea how much Pfizer paid Lady Gaga to sell Nurtec, but I’m pretty sure it’s a lot more than I’ll earn this year. Probably ever.
Like most neurologists I’m hopelessly left-brained. But I still wonder how much things like this really make a difference.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
FDA approves first tx for rare, deadly clotting disorder
Congenital TTP affects fewer than 1,000 people in the United States and is caused by a mutation in the ADAMTS13 gene, which makes an enzyme that regulates blood clotting. Patients with the congenital TTP typically receive prophylactic plasma-based therapy to replenish the ADAMTS13 enzyme and reduce the risk for clotting and bleeding. The condition, however, can be fatal if left untreated.
The new agent is a purified recombinant form of the ADAMTS13 enzyme that works by replacing low levels of the deficient enzyme in patients with congenital TTP. Adzynma is given prophylactically to reduce the risk for disease symptoms and on demand when a patient is experiencing an acute event, according to the FDA approval announcement.
The approval was based on a global randomized phase 3 study comparing the product with plasma-based therapies in 46 patients with congenital TTP. Patients in the trial were randomized to receive 6 months of treatment with either intravenous Adzynma — given once every other week as prophylactic enzyme replacement therapy or once daily as on-demand enzyme replacement therapy — or plasma-based therapies. The patients then crossed over to the other treatment for 6 months.
Interim findings from the study showed that Adzynma reduced the incidence of thrombocytopenia — the most common symptom of congenital TTP — by 60% compared with plasma-based therapy (rate ratio, 0.40). No patients experienced an acute TTP event during Adzynma prophylaxis, Takeda said.
Significantly more patients receiving plasma-based therapies experienced treatment-emergent adverse events compared with those receiving the biologic.
The most common side effects associated with the biologic were headache (31.3%), diarrhea (16.7%), migraine (14.6%), abdominal pain (12.5%), nausea (12.5%), upper respiratory tract infection (12.5%), dizziness (10.4%), and vomiting (10.4%). No treatment-related adverse events, including allergic reactions, were observed during administration.
“The FDA remains deeply committed in our efforts to help facilitate the development and approval of safe and effective therapies for patients with rare diseases,” Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, stated. The “approval reflects important progress in the development of much-needed treatment options for patients affected by this life-threatening disorder.”
A version of this article first appeared on Medscape.com.
Congenital TTP affects fewer than 1,000 people in the United States and is caused by a mutation in the ADAMTS13 gene, which makes an enzyme that regulates blood clotting. Patients with the congenital TTP typically receive prophylactic plasma-based therapy to replenish the ADAMTS13 enzyme and reduce the risk for clotting and bleeding. The condition, however, can be fatal if left untreated.
The new agent is a purified recombinant form of the ADAMTS13 enzyme that works by replacing low levels of the deficient enzyme in patients with congenital TTP. Adzynma is given prophylactically to reduce the risk for disease symptoms and on demand when a patient is experiencing an acute event, according to the FDA approval announcement.
The approval was based on a global randomized phase 3 study comparing the product with plasma-based therapies in 46 patients with congenital TTP. Patients in the trial were randomized to receive 6 months of treatment with either intravenous Adzynma — given once every other week as prophylactic enzyme replacement therapy or once daily as on-demand enzyme replacement therapy — or plasma-based therapies. The patients then crossed over to the other treatment for 6 months.
Interim findings from the study showed that Adzynma reduced the incidence of thrombocytopenia — the most common symptom of congenital TTP — by 60% compared with plasma-based therapy (rate ratio, 0.40). No patients experienced an acute TTP event during Adzynma prophylaxis, Takeda said.
Significantly more patients receiving plasma-based therapies experienced treatment-emergent adverse events compared with those receiving the biologic.
The most common side effects associated with the biologic were headache (31.3%), diarrhea (16.7%), migraine (14.6%), abdominal pain (12.5%), nausea (12.5%), upper respiratory tract infection (12.5%), dizziness (10.4%), and vomiting (10.4%). No treatment-related adverse events, including allergic reactions, were observed during administration.
“The FDA remains deeply committed in our efforts to help facilitate the development and approval of safe and effective therapies for patients with rare diseases,” Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, stated. The “approval reflects important progress in the development of much-needed treatment options for patients affected by this life-threatening disorder.”
A version of this article first appeared on Medscape.com.
Congenital TTP affects fewer than 1,000 people in the United States and is caused by a mutation in the ADAMTS13 gene, which makes an enzyme that regulates blood clotting. Patients with the congenital TTP typically receive prophylactic plasma-based therapy to replenish the ADAMTS13 enzyme and reduce the risk for clotting and bleeding. The condition, however, can be fatal if left untreated.
The new agent is a purified recombinant form of the ADAMTS13 enzyme that works by replacing low levels of the deficient enzyme in patients with congenital TTP. Adzynma is given prophylactically to reduce the risk for disease symptoms and on demand when a patient is experiencing an acute event, according to the FDA approval announcement.
The approval was based on a global randomized phase 3 study comparing the product with plasma-based therapies in 46 patients with congenital TTP. Patients in the trial were randomized to receive 6 months of treatment with either intravenous Adzynma — given once every other week as prophylactic enzyme replacement therapy or once daily as on-demand enzyme replacement therapy — or plasma-based therapies. The patients then crossed over to the other treatment for 6 months.
Interim findings from the study showed that Adzynma reduced the incidence of thrombocytopenia — the most common symptom of congenital TTP — by 60% compared with plasma-based therapy (rate ratio, 0.40). No patients experienced an acute TTP event during Adzynma prophylaxis, Takeda said.
Significantly more patients receiving plasma-based therapies experienced treatment-emergent adverse events compared with those receiving the biologic.
The most common side effects associated with the biologic were headache (31.3%), diarrhea (16.7%), migraine (14.6%), abdominal pain (12.5%), nausea (12.5%), upper respiratory tract infection (12.5%), dizziness (10.4%), and vomiting (10.4%). No treatment-related adverse events, including allergic reactions, were observed during administration.
“The FDA remains deeply committed in our efforts to help facilitate the development and approval of safe and effective therapies for patients with rare diseases,” Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, stated. The “approval reflects important progress in the development of much-needed treatment options for patients affected by this life-threatening disorder.”
A version of this article first appeared on Medscape.com.
Highlights in Metastatic Breast Cancer From ESMO 2023
Developments in metastatic breast cancer (MBC) were reported at the European Society for Medical Oncology (ESMO) 2023 Congress and are discussed by Dr Ann Partridge of Dana-Farber Cancer Institute.
To begin, Dr Partridge highlights a late-breaking abstract showing that use of the antibody-drug conjugate (ADC) datopotamab deruxtecan in hormone receptor–positive (HR+)/human epidermal growth factor receptor 2–negative (HER2-) MBC results in improved progression-free survival (PFS) compared with chemotherapy.
Next, Dr Partridge turns to two studies on another ADC, trastuzumab deruxtecan (T-DXd), in MBC. The first study showed positive PFS and overall survival results among patients with either estrogen receptor–positive (ER+)/HER2-low or triple-negative/HER2-low breast cancer. The second T-DXd study examined the ADC's impact on brain metastases in patients with HER2+ disease and reported favorable results.
She then highlights promising phase 2 results for a novel agent called OP-1250, or palazestrant, studied in patients with ER+/HER2- MBC.
Finally, Dr Partridge points to a study of a supportive-care program called MOATT, designed for patients on oral MBC therapy, which aims to improve home management. Compared with local standard of care, patients in the program show higher rates of persistence in therapy management and, importantly, concomitant improvements in PFS.
--
Ann H. Partridge, MD, Professor of Medicine, Harvard Medical School; Vice Chair of Clinical Oncology, Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, Massachusetts
Ann H. Partridge, MD, MPH, has disclosed no relevant financial relationships.
Developments in metastatic breast cancer (MBC) were reported at the European Society for Medical Oncology (ESMO) 2023 Congress and are discussed by Dr Ann Partridge of Dana-Farber Cancer Institute.
To begin, Dr Partridge highlights a late-breaking abstract showing that use of the antibody-drug conjugate (ADC) datopotamab deruxtecan in hormone receptor–positive (HR+)/human epidermal growth factor receptor 2–negative (HER2-) MBC results in improved progression-free survival (PFS) compared with chemotherapy.
Next, Dr Partridge turns to two studies on another ADC, trastuzumab deruxtecan (T-DXd), in MBC. The first study showed positive PFS and overall survival results among patients with either estrogen receptor–positive (ER+)/HER2-low or triple-negative/HER2-low breast cancer. The second T-DXd study examined the ADC's impact on brain metastases in patients with HER2+ disease and reported favorable results.
She then highlights promising phase 2 results for a novel agent called OP-1250, or palazestrant, studied in patients with ER+/HER2- MBC.
Finally, Dr Partridge points to a study of a supportive-care program called MOATT, designed for patients on oral MBC therapy, which aims to improve home management. Compared with local standard of care, patients in the program show higher rates of persistence in therapy management and, importantly, concomitant improvements in PFS.
--
Ann H. Partridge, MD, Professor of Medicine, Harvard Medical School; Vice Chair of Clinical Oncology, Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, Massachusetts
Ann H. Partridge, MD, MPH, has disclosed no relevant financial relationships.
Developments in metastatic breast cancer (MBC) were reported at the European Society for Medical Oncology (ESMO) 2023 Congress and are discussed by Dr Ann Partridge of Dana-Farber Cancer Institute.
To begin, Dr Partridge highlights a late-breaking abstract showing that use of the antibody-drug conjugate (ADC) datopotamab deruxtecan in hormone receptor–positive (HR+)/human epidermal growth factor receptor 2–negative (HER2-) MBC results in improved progression-free survival (PFS) compared with chemotherapy.
Next, Dr Partridge turns to two studies on another ADC, trastuzumab deruxtecan (T-DXd), in MBC. The first study showed positive PFS and overall survival results among patients with either estrogen receptor–positive (ER+)/HER2-low or triple-negative/HER2-low breast cancer. The second T-DXd study examined the ADC's impact on brain metastases in patients with HER2+ disease and reported favorable results.
She then highlights promising phase 2 results for a novel agent called OP-1250, or palazestrant, studied in patients with ER+/HER2- MBC.
Finally, Dr Partridge points to a study of a supportive-care program called MOATT, designed for patients on oral MBC therapy, which aims to improve home management. Compared with local standard of care, patients in the program show higher rates of persistence in therapy management and, importantly, concomitant improvements in PFS.
--
Ann H. Partridge, MD, Professor of Medicine, Harvard Medical School; Vice Chair of Clinical Oncology, Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, Massachusetts
Ann H. Partridge, MD, MPH, has disclosed no relevant financial relationships.