User login
FDA approves neratinib for extended adjuvant treatment of HER2+ breast cancer
The Food and Drug Administration has approved neratinib, an oral tyrosine kinase inhibitor, for the extended adjuvant treatment of patients with early-stage, HER2-positive breast cancer who have previously been treated with trastuzumab.
Approval was based on improved invasive disease-free survival (iDFS) in the phase 3 ExteNET trial of 2,840 women with early-stage HER2-positive breast cancer who were within 2 years of completing adjuvant trastuzumab. Patients were randomized to receive either neratinib or placebo daily for 1 year. After 2 years of follow-up, iDFS was 94.2% in patients treated with neratinib, compared with 91.9% in those receiving placebo (hazard ratio, 0.66; 95% confidence interval, 0.49, 0.90; P = .008), according to the FDA statement.
The most common adverse reactions to neratinib in ExteNET were diarrhea, nausea, abdominal pain, fatigue, vomiting, rash, stomatitis, decreased appetite, muscle spasms, dyspepsia, AST or ALT increase, nail disorder, dry skin, abdominal distention, weight loss, and urinary tract infection. Diarrhea was observed in 16.8% of neratinib-treated patients.
The recommended dose of of neratinib is 240 mg (six 40 mg tablets) given orally once daily with food, continuously, for 1 year. Patients should be given antidiarrheal prophylaxis for the first 56 days of treatment with neratinib and as needed thereafter to help manage diarrhea, the FDA said.
The Food and Drug Administration has approved neratinib, an oral tyrosine kinase inhibitor, for the extended adjuvant treatment of patients with early-stage, HER2-positive breast cancer who have previously been treated with trastuzumab.
Approval was based on improved invasive disease-free survival (iDFS) in the phase 3 ExteNET trial of 2,840 women with early-stage HER2-positive breast cancer who were within 2 years of completing adjuvant trastuzumab. Patients were randomized to receive either neratinib or placebo daily for 1 year. After 2 years of follow-up, iDFS was 94.2% in patients treated with neratinib, compared with 91.9% in those receiving placebo (hazard ratio, 0.66; 95% confidence interval, 0.49, 0.90; P = .008), according to the FDA statement.
The most common adverse reactions to neratinib in ExteNET were diarrhea, nausea, abdominal pain, fatigue, vomiting, rash, stomatitis, decreased appetite, muscle spasms, dyspepsia, AST or ALT increase, nail disorder, dry skin, abdominal distention, weight loss, and urinary tract infection. Diarrhea was observed in 16.8% of neratinib-treated patients.
The recommended dose of of neratinib is 240 mg (six 40 mg tablets) given orally once daily with food, continuously, for 1 year. Patients should be given antidiarrheal prophylaxis for the first 56 days of treatment with neratinib and as needed thereafter to help manage diarrhea, the FDA said.
The Food and Drug Administration has approved neratinib, an oral tyrosine kinase inhibitor, for the extended adjuvant treatment of patients with early-stage, HER2-positive breast cancer who have previously been treated with trastuzumab.
Approval was based on improved invasive disease-free survival (iDFS) in the phase 3 ExteNET trial of 2,840 women with early-stage HER2-positive breast cancer who were within 2 years of completing adjuvant trastuzumab. Patients were randomized to receive either neratinib or placebo daily for 1 year. After 2 years of follow-up, iDFS was 94.2% in patients treated with neratinib, compared with 91.9% in those receiving placebo (hazard ratio, 0.66; 95% confidence interval, 0.49, 0.90; P = .008), according to the FDA statement.
The most common adverse reactions to neratinib in ExteNET were diarrhea, nausea, abdominal pain, fatigue, vomiting, rash, stomatitis, decreased appetite, muscle spasms, dyspepsia, AST or ALT increase, nail disorder, dry skin, abdominal distention, weight loss, and urinary tract infection. Diarrhea was observed in 16.8% of neratinib-treated patients.
The recommended dose of of neratinib is 240 mg (six 40 mg tablets) given orally once daily with food, continuously, for 1 year. Patients should be given antidiarrheal prophylaxis for the first 56 days of treatment with neratinib and as needed thereafter to help manage diarrhea, the FDA said.
Small brain infarcts’ cognitive impact equals that of large infarcts
LONDON – Small infarct-like brain lesions have long been ignored in both research and clinical settings, but an ongoing analysis of an observational cohort shows that they can be just as cognitively damaging as large infarcts. Having both is a serious one-two punch to thinking and memory.
At less than 3 mm, infarct-like lesions (ILL) may be tiny, but, over 20 years, they exerted exactly the same deleterious effect on cognition as lesions 3 mm or larger, Beverly Gwen Windham, MD, said at the Alzheimer’s Association International Conference.
When neuroradiologists began to look at brain infarcts several decades ago, they used then state-of-the-art 1.5 Tesla MRI. As infarct description and classification evolved, anything measuring smaller than 3 mm was classified as an infarct-like lesion and anything 3 mm or larger as a large infarct. There was some concern that readers would confuse the ILLs with perivascular spaces and flag these normal voids as pathological changes, Dr. Windham said. As a result, research studies have always excluded them. Since they’re usually associated with silent events, without any clinical signs or symptoms, they’ve been clinically disregarded as well, adding to the perception that they have little long-term impact.
However, in 2015, Dr. Windham and her team proved this perception incorrect, at least when it came to stroke and stroke mortality. Using the large Atherosclerosis Risk in Communities (ARIC) Study cohort, they showed that ILLs alone tripled the risk of both a stroke and stroke mortality. Patients who had both ILLs and large infarcts were nine times more likely to have a stroke and seven times more likely to die from a stroke than were patients who had no lesions.
Dr. Windham used the same ARIC cohort in the 20-year cognition study. Begun in 1987, ARIC enrolled 15,800 middle-aged adults who have been followed regularly with physical and neurocognitive testing. Its goals are largely to investigate the natural history and multiple risk factors of cardiovascular and cerebrovascular disease. In 1993, subjects who had not experienced a stroke also had a brain MRI to add more detail to the study. The investigators also have performed cognitive assessments of a large number of participants five times since 1993 on measures of delayed word recall, digit symbol substitution, and word fluency. The outcome was the change in the composite Z-score over time.
The cognition study comprised 1,881 who had brain MRI and the full five cognitive assessments over a 20-year period. The participants were stratified as having no infarcts (1,611), only ILLs (50) or large infarcts (185), or both lesions (35).
At baseline, these subjects had a mean age of 63 years, 17% had diabetes, and 48% had hypertension. About one-third were positive for an ApoE e4 allele. The mean white matter intensity score was 1.4.
“In general, everyone in the cohort had some decline in cognitive function as they got older,” Dr. Windham said. But, a striking observation was that those with ILLs only and those with large lesions only had virtually identical decline slopes over the 20-year follow-up. The change from baseline in global Z-score was 0.18 standard deviations for the ILL-only group and 0.24 standard deviations for the large infarct group. For those with both lesions, the change from baseline was 0.62.
“At the end of 20 years, those with no lesion burden declined 1.3 standard deviations from baseline, those with only ILLs declined 1.5 standard deviations, and those with large infarcts, 1.6 standard deviations,” Dr. Windham said. “But, subjects who had both lesions declined 2.5 standard deviations from baseline. This is equivalent to adding 27 years of aging. The effect of having both was nearly four times greater than [that of] having only large lesions, which, up until now, have been the only ones read on MRI in either clinical practice or in research. Overall, our findings confirm that the relationship of ILLs to cognition is very similar that of large infarcts.
“The presence of midlife ILLs appears to amplify the effect of large infarcts on cognition, and we hypothesize that this process may represent vascular disease at midlife. We may also be able to identify people at high risk of cognitive decline or even dementia at midlife. I also think that we need to be rethinking how we read MRIs. Stopping at the 3-mm threshold may be too conservative. We should be looking at other studies on the consequences of these small lesions,” she said.
Dr. Windham had no financial disclosures.
[email protected]
On Twitter @alz_gal
Virtually every time researchers look for an effect of brain vascular health on cognition, they find it. Not only do these data reveal a surprisingly large effect of a previously ignored type of brain lesion on cognition, they also highlight that poor management of vascular risk factors in midlife may lead to dementia decades later. This suggests we need more research to understand the long-term impact of these small lesions on brain health and the development of Alzheimer’s disease.
Keith Fargo, PhD, is the Alzheimer’s Association’s director of scientific programs and outreach.
Virtually every time researchers look for an effect of brain vascular health on cognition, they find it. Not only do these data reveal a surprisingly large effect of a previously ignored type of brain lesion on cognition, they also highlight that poor management of vascular risk factors in midlife may lead to dementia decades later. This suggests we need more research to understand the long-term impact of these small lesions on brain health and the development of Alzheimer’s disease.
Keith Fargo, PhD, is the Alzheimer’s Association’s director of scientific programs and outreach.
Virtually every time researchers look for an effect of brain vascular health on cognition, they find it. Not only do these data reveal a surprisingly large effect of a previously ignored type of brain lesion on cognition, they also highlight that poor management of vascular risk factors in midlife may lead to dementia decades later. This suggests we need more research to understand the long-term impact of these small lesions on brain health and the development of Alzheimer’s disease.
Keith Fargo, PhD, is the Alzheimer’s Association’s director of scientific programs and outreach.
LONDON – Small infarct-like brain lesions have long been ignored in both research and clinical settings, but an ongoing analysis of an observational cohort shows that they can be just as cognitively damaging as large infarcts. Having both is a serious one-two punch to thinking and memory.
At less than 3 mm, infarct-like lesions (ILL) may be tiny, but, over 20 years, they exerted exactly the same deleterious effect on cognition as lesions 3 mm or larger, Beverly Gwen Windham, MD, said at the Alzheimer’s Association International Conference.
When neuroradiologists began to look at brain infarcts several decades ago, they used then state-of-the-art 1.5 Tesla MRI. As infarct description and classification evolved, anything measuring smaller than 3 mm was classified as an infarct-like lesion and anything 3 mm or larger as a large infarct. There was some concern that readers would confuse the ILLs with perivascular spaces and flag these normal voids as pathological changes, Dr. Windham said. As a result, research studies have always excluded them. Since they’re usually associated with silent events, without any clinical signs or symptoms, they’ve been clinically disregarded as well, adding to the perception that they have little long-term impact.
However, in 2015, Dr. Windham and her team proved this perception incorrect, at least when it came to stroke and stroke mortality. Using the large Atherosclerosis Risk in Communities (ARIC) Study cohort, they showed that ILLs alone tripled the risk of both a stroke and stroke mortality. Patients who had both ILLs and large infarcts were nine times more likely to have a stroke and seven times more likely to die from a stroke than were patients who had no lesions.
Dr. Windham used the same ARIC cohort in the 20-year cognition study. Begun in 1987, ARIC enrolled 15,800 middle-aged adults who have been followed regularly with physical and neurocognitive testing. Its goals are largely to investigate the natural history and multiple risk factors of cardiovascular and cerebrovascular disease. In 1993, subjects who had not experienced a stroke also had a brain MRI to add more detail to the study. The investigators also have performed cognitive assessments of a large number of participants five times since 1993 on measures of delayed word recall, digit symbol substitution, and word fluency. The outcome was the change in the composite Z-score over time.
The cognition study comprised 1,881 who had brain MRI and the full five cognitive assessments over a 20-year period. The participants were stratified as having no infarcts (1,611), only ILLs (50) or large infarcts (185), or both lesions (35).
At baseline, these subjects had a mean age of 63 years, 17% had diabetes, and 48% had hypertension. About one-third were positive for an ApoE e4 allele. The mean white matter intensity score was 1.4.
“In general, everyone in the cohort had some decline in cognitive function as they got older,” Dr. Windham said. But, a striking observation was that those with ILLs only and those with large lesions only had virtually identical decline slopes over the 20-year follow-up. The change from baseline in global Z-score was 0.18 standard deviations for the ILL-only group and 0.24 standard deviations for the large infarct group. For those with both lesions, the change from baseline was 0.62.
“At the end of 20 years, those with no lesion burden declined 1.3 standard deviations from baseline, those with only ILLs declined 1.5 standard deviations, and those with large infarcts, 1.6 standard deviations,” Dr. Windham said. “But, subjects who had both lesions declined 2.5 standard deviations from baseline. This is equivalent to adding 27 years of aging. The effect of having both was nearly four times greater than [that of] having only large lesions, which, up until now, have been the only ones read on MRI in either clinical practice or in research. Overall, our findings confirm that the relationship of ILLs to cognition is very similar that of large infarcts.
“The presence of midlife ILLs appears to amplify the effect of large infarcts on cognition, and we hypothesize that this process may represent vascular disease at midlife. We may also be able to identify people at high risk of cognitive decline or even dementia at midlife. I also think that we need to be rethinking how we read MRIs. Stopping at the 3-mm threshold may be too conservative. We should be looking at other studies on the consequences of these small lesions,” she said.
Dr. Windham had no financial disclosures.
[email protected]
On Twitter @alz_gal
LONDON – Small infarct-like brain lesions have long been ignored in both research and clinical settings, but an ongoing analysis of an observational cohort shows that they can be just as cognitively damaging as large infarcts. Having both is a serious one-two punch to thinking and memory.
At less than 3 mm, infarct-like lesions (ILL) may be tiny, but, over 20 years, they exerted exactly the same deleterious effect on cognition as lesions 3 mm or larger, Beverly Gwen Windham, MD, said at the Alzheimer’s Association International Conference.
When neuroradiologists began to look at brain infarcts several decades ago, they used then state-of-the-art 1.5 Tesla MRI. As infarct description and classification evolved, anything measuring smaller than 3 mm was classified as an infarct-like lesion and anything 3 mm or larger as a large infarct. There was some concern that readers would confuse the ILLs with perivascular spaces and flag these normal voids as pathological changes, Dr. Windham said. As a result, research studies have always excluded them. Since they’re usually associated with silent events, without any clinical signs or symptoms, they’ve been clinically disregarded as well, adding to the perception that they have little long-term impact.
However, in 2015, Dr. Windham and her team proved this perception incorrect, at least when it came to stroke and stroke mortality. Using the large Atherosclerosis Risk in Communities (ARIC) Study cohort, they showed that ILLs alone tripled the risk of both a stroke and stroke mortality. Patients who had both ILLs and large infarcts were nine times more likely to have a stroke and seven times more likely to die from a stroke than were patients who had no lesions.
Dr. Windham used the same ARIC cohort in the 20-year cognition study. Begun in 1987, ARIC enrolled 15,800 middle-aged adults who have been followed regularly with physical and neurocognitive testing. Its goals are largely to investigate the natural history and multiple risk factors of cardiovascular and cerebrovascular disease. In 1993, subjects who had not experienced a stroke also had a brain MRI to add more detail to the study. The investigators also have performed cognitive assessments of a large number of participants five times since 1993 on measures of delayed word recall, digit symbol substitution, and word fluency. The outcome was the change in the composite Z-score over time.
The cognition study comprised 1,881 who had brain MRI and the full five cognitive assessments over a 20-year period. The participants were stratified as having no infarcts (1,611), only ILLs (50) or large infarcts (185), or both lesions (35).
At baseline, these subjects had a mean age of 63 years, 17% had diabetes, and 48% had hypertension. About one-third were positive for an ApoE e4 allele. The mean white matter intensity score was 1.4.
“In general, everyone in the cohort had some decline in cognitive function as they got older,” Dr. Windham said. But, a striking observation was that those with ILLs only and those with large lesions only had virtually identical decline slopes over the 20-year follow-up. The change from baseline in global Z-score was 0.18 standard deviations for the ILL-only group and 0.24 standard deviations for the large infarct group. For those with both lesions, the change from baseline was 0.62.
“At the end of 20 years, those with no lesion burden declined 1.3 standard deviations from baseline, those with only ILLs declined 1.5 standard deviations, and those with large infarcts, 1.6 standard deviations,” Dr. Windham said. “But, subjects who had both lesions declined 2.5 standard deviations from baseline. This is equivalent to adding 27 years of aging. The effect of having both was nearly four times greater than [that of] having only large lesions, which, up until now, have been the only ones read on MRI in either clinical practice or in research. Overall, our findings confirm that the relationship of ILLs to cognition is very similar that of large infarcts.
“The presence of midlife ILLs appears to amplify the effect of large infarcts on cognition, and we hypothesize that this process may represent vascular disease at midlife. We may also be able to identify people at high risk of cognitive decline or even dementia at midlife. I also think that we need to be rethinking how we read MRIs. Stopping at the 3-mm threshold may be too conservative. We should be looking at other studies on the consequences of these small lesions,” she said.
Dr. Windham had no financial disclosures.
[email protected]
On Twitter @alz_gal
AT AAIC 2017
Key clinical point:
Major finding: A composite cognitive measure showed a decline of 1.5 standard deviations from baseline in patients with only infarct-like lesions, 1.6 standard deviations in those with large infarcts, and 2.5 standard deviations in subjects who had both lesions.
Data source: The ARIC substudy comprised 1,881 subjects followed for 20 years.
Disclosures: Dr. Windham had no financial disclosures.
High Value Care curriculum reduced echocardiogram ordering
Study Title
The impact of a High Value Care curriculum on rate of repeat of trans-thoracic echocardiogram ordering among medical residents
Background
There is little data to confirm the impact of a High Value Care curriculum on echocardiogram ordering practices in a residency training program. We sought to evaluate the rate of performance of repeat transthoracic echocardiograms (TTE) before and after implementation of a High Vale Care curriculum.
Methods
A High Value Care curriculum was developed for the medical residents at Griffin Hospital, a community hospital, in 2015. The curriculum included a series of lectures aimed at promoting cost-conscious care while maintaining high quality. It also involved house staff in different quality improvement (QI) projects aimed at promoting high value care.
A group of residents decided to work on an initiative to reduce repeat echocardiograms. Repeat echocardiograms were defined as those performed within 6 months of a previous echocardiogram on the same patient. Only results in our EHR reflecting in-patient echocardiograms were utilized.
We retrospectively examined the rates of repeat echocardiograms performed in a 6 month period in 2014 before the High Vale Care curriculum was initiated. We assessed data from a 5 month period in 2016 to determine the rate of repeat electrocardiograms ordered at our institution.
Results
A total of 1,709 echocardiograms were reviewed in both time periods. Of these, 275 were considered repeat. At baseline, or before the implementation of a High Value Care curriculum, we examined 908 echocardiograms that were ordered, of which 21% were repeats.
After the implementation of a High Vale Care curriculum, 801 echocardiograms were ordered. Only 11% of these were repeats. This corresponds to a 52% reduction in the rate of repeated ordering of echocardiograms.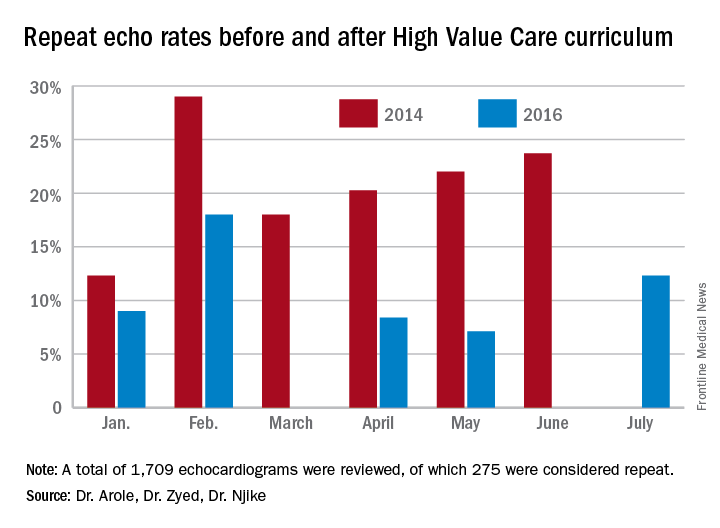
Discussion
The significant improvement in the rate of repeat echocardiograms was noted without any initiative directed specifically at echocardiogram ordering practices. During the planning phases of the proposed QI initiative to reduce repeat echocardiograms, house staff noted that their colleagues were already being more selective in their echocardiogram ordering practices because of the impact of the-cost conscious care lectures they had attended, as well as hospital-wide attention on the first resident-driven QI initiative that was aimed at reducing repetitive daily labs.
As part of the reducing repetitive labs QI, house staff had to provide clear rationale for why they were ordering daily labs. The received regular updates about their lab ordering practices and direct feedback if they consistently did not provide clear rationale for ordering daily labs.
Conclusion
Our study clearly showed a greater than 50% reduction in the ordering of repeat echocardiograms because of a High Value Care curriculum in our residency training program.
The improvement was brought on by increased awareness by house staff regarding provision of high quality care while being cognizant of the costs involved. The reduction in repeat echocardiograms resulted in more efficient use of a limited hospital resource.
Dr. Arole is chief of hospital medicine at Griffin Hospital, Derby, Conn. Dr. Zyed is in the department of internal medicine at Griffin Hospital. Dr. Njike is with the Yale University Prevention Research Center at Griffin Hospital.
Study Title
The impact of a High Value Care curriculum on rate of repeat of trans-thoracic echocardiogram ordering among medical residents
Background
There is little data to confirm the impact of a High Value Care curriculum on echocardiogram ordering practices in a residency training program. We sought to evaluate the rate of performance of repeat transthoracic echocardiograms (TTE) before and after implementation of a High Vale Care curriculum.
Methods
A High Value Care curriculum was developed for the medical residents at Griffin Hospital, a community hospital, in 2015. The curriculum included a series of lectures aimed at promoting cost-conscious care while maintaining high quality. It also involved house staff in different quality improvement (QI) projects aimed at promoting high value care.
A group of residents decided to work on an initiative to reduce repeat echocardiograms. Repeat echocardiograms were defined as those performed within 6 months of a previous echocardiogram on the same patient. Only results in our EHR reflecting in-patient echocardiograms were utilized.
We retrospectively examined the rates of repeat echocardiograms performed in a 6 month period in 2014 before the High Vale Care curriculum was initiated. We assessed data from a 5 month period in 2016 to determine the rate of repeat electrocardiograms ordered at our institution.
Results
A total of 1,709 echocardiograms were reviewed in both time periods. Of these, 275 were considered repeat. At baseline, or before the implementation of a High Value Care curriculum, we examined 908 echocardiograms that were ordered, of which 21% were repeats.
After the implementation of a High Vale Care curriculum, 801 echocardiograms were ordered. Only 11% of these were repeats. This corresponds to a 52% reduction in the rate of repeated ordering of echocardiograms.
Discussion
The significant improvement in the rate of repeat echocardiograms was noted without any initiative directed specifically at echocardiogram ordering practices. During the planning phases of the proposed QI initiative to reduce repeat echocardiograms, house staff noted that their colleagues were already being more selective in their echocardiogram ordering practices because of the impact of the-cost conscious care lectures they had attended, as well as hospital-wide attention on the first resident-driven QI initiative that was aimed at reducing repetitive daily labs.
As part of the reducing repetitive labs QI, house staff had to provide clear rationale for why they were ordering daily labs. The received regular updates about their lab ordering practices and direct feedback if they consistently did not provide clear rationale for ordering daily labs.
Conclusion
Our study clearly showed a greater than 50% reduction in the ordering of repeat echocardiograms because of a High Value Care curriculum in our residency training program.
The improvement was brought on by increased awareness by house staff regarding provision of high quality care while being cognizant of the costs involved. The reduction in repeat echocardiograms resulted in more efficient use of a limited hospital resource.
Dr. Arole is chief of hospital medicine at Griffin Hospital, Derby, Conn. Dr. Zyed is in the department of internal medicine at Griffin Hospital. Dr. Njike is with the Yale University Prevention Research Center at Griffin Hospital.
Study Title
The impact of a High Value Care curriculum on rate of repeat of trans-thoracic echocardiogram ordering among medical residents
Background
There is little data to confirm the impact of a High Value Care curriculum on echocardiogram ordering practices in a residency training program. We sought to evaluate the rate of performance of repeat transthoracic echocardiograms (TTE) before and after implementation of a High Vale Care curriculum.
Methods
A High Value Care curriculum was developed for the medical residents at Griffin Hospital, a community hospital, in 2015. The curriculum included a series of lectures aimed at promoting cost-conscious care while maintaining high quality. It also involved house staff in different quality improvement (QI) projects aimed at promoting high value care.
A group of residents decided to work on an initiative to reduce repeat echocardiograms. Repeat echocardiograms were defined as those performed within 6 months of a previous echocardiogram on the same patient. Only results in our EHR reflecting in-patient echocardiograms were utilized.
We retrospectively examined the rates of repeat echocardiograms performed in a 6 month period in 2014 before the High Vale Care curriculum was initiated. We assessed data from a 5 month period in 2016 to determine the rate of repeat electrocardiograms ordered at our institution.
Results
A total of 1,709 echocardiograms were reviewed in both time periods. Of these, 275 were considered repeat. At baseline, or before the implementation of a High Value Care curriculum, we examined 908 echocardiograms that were ordered, of which 21% were repeats.
After the implementation of a High Vale Care curriculum, 801 echocardiograms were ordered. Only 11% of these were repeats. This corresponds to a 52% reduction in the rate of repeated ordering of echocardiograms.
Discussion
The significant improvement in the rate of repeat echocardiograms was noted without any initiative directed specifically at echocardiogram ordering practices. During the planning phases of the proposed QI initiative to reduce repeat echocardiograms, house staff noted that their colleagues were already being more selective in their echocardiogram ordering practices because of the impact of the-cost conscious care lectures they had attended, as well as hospital-wide attention on the first resident-driven QI initiative that was aimed at reducing repetitive daily labs.
As part of the reducing repetitive labs QI, house staff had to provide clear rationale for why they were ordering daily labs. The received regular updates about their lab ordering practices and direct feedback if they consistently did not provide clear rationale for ordering daily labs.
Conclusion
Our study clearly showed a greater than 50% reduction in the ordering of repeat echocardiograms because of a High Value Care curriculum in our residency training program.
The improvement was brought on by increased awareness by house staff regarding provision of high quality care while being cognizant of the costs involved. The reduction in repeat echocardiograms resulted in more efficient use of a limited hospital resource.
Dr. Arole is chief of hospital medicine at Griffin Hospital, Derby, Conn. Dr. Zyed is in the department of internal medicine at Griffin Hospital. Dr. Njike is with the Yale University Prevention Research Center at Griffin Hospital.
Immune signature shows good prognostic performance in early-stage NSCLC
A new tumor immune-related gene signature may help take the guesswork out of prognostication in patients with early-stage non–small cell lung cancer (NSCLC), according to a retrospective cohort study.
“Various components of the immune system have been shown to be a determining factor during cancer initiation and progression,” note the investigators, who were led by Bailiang Li, PhD, of Stanford (Calif.) University. “Recent immunotherapies targeting specific immune checkpoints such as programmed death 1 or programmed death ligand 1 have demonstrated a remarkable, durable response in NSCLC. Certain histopathologic patterns, such as intratumoral infiltration by cytotoxic lymphocytes, have also been associated with better prognoses in several cancer types, including NSCLC.”
For the study, the investigators developed and validated an immune-related gene signature using frozen tumors from 2,414 patients with stage I or II nonsquamous NSCLC from 19 public cohorts who underwent resection with negative margins and did not receive any neoadjuvant or adjuvant therapy.
The new signature contained 25 gene pairs consisting of 40 unique immune-related genes, Dr. Li and associates report (JAMA Oncol. 2017 Jul 6. doi: 10.1001/jamaoncol.2017.1609).
Processes such as chemotaxis were enriched among the included genes.
The signature significantly stratified patients into groups that have high and low risks of death during follow-up, both across and within subsets with stage I, IA, IB, or II disease. Relative to counterparts falling into the signature-defined low-risk group, those falling into the signature-defined high-risk group had roughly twice the risk of death after adjustment for clinical and pathologic characteristics, with a hazard ratio range of 1.72 (P less than .001) to 2.36 (P less than .001).
Accuracy of the immune signature exceeded that of two commercialized gene signatures for estimating survival in similar validation cohorts (mean concordance index [C-index], 0.64 vs 0.53 and 0.61).
Moreover, the combination of the immune signature with clinical factors outperformed the signature alone (mean C-index, 0.70 vs 0.63) and another commercialized clinical-molecular combination signature (mean C-index, 0.68 vs 0.65).
“The proposed immune-related gene pair–based signature is a promising prognostic biomarker in nonsquamous NSCLC, including early-stage disease,” concluded the investigators. “Prospective studies are needed to further validate its analytical accuracy for estimating prognoses and to test its clinical utility in individualized management of nonsquamous NSCLC.”
M. Patricia Rivera, MD, FCCP, comments: As lung cancer screening implementation increases, it is expected that the prevalence of early-stage non–small cell lung cancer (NSCLC) will increase.
Currently, adjuvant therapy is reserved for patients with tumors greater than 4 cm or those with N1 disease. Having reliable biomarkers to identify patients at a high risk for recurrence after surgical resection is a significant clinical advantage in order to guide adjuvant therapy. The clinical-immune signature described in this study is an exciting and promising biomarker for estimating overall survival in NSCLC.
M. Patricia Rivera, MD, FCCP, comments: As lung cancer screening implementation increases, it is expected that the prevalence of early-stage non–small cell lung cancer (NSCLC) will increase.
Currently, adjuvant therapy is reserved for patients with tumors greater than 4 cm or those with N1 disease. Having reliable biomarkers to identify patients at a high risk for recurrence after surgical resection is a significant clinical advantage in order to guide adjuvant therapy. The clinical-immune signature described in this study is an exciting and promising biomarker for estimating overall survival in NSCLC.
M. Patricia Rivera, MD, FCCP, comments: As lung cancer screening implementation increases, it is expected that the prevalence of early-stage non–small cell lung cancer (NSCLC) will increase.
Currently, adjuvant therapy is reserved for patients with tumors greater than 4 cm or those with N1 disease. Having reliable biomarkers to identify patients at a high risk for recurrence after surgical resection is a significant clinical advantage in order to guide adjuvant therapy. The clinical-immune signature described in this study is an exciting and promising biomarker for estimating overall survival in NSCLC.
A new tumor immune-related gene signature may help take the guesswork out of prognostication in patients with early-stage non–small cell lung cancer (NSCLC), according to a retrospective cohort study.
“Various components of the immune system have been shown to be a determining factor during cancer initiation and progression,” note the investigators, who were led by Bailiang Li, PhD, of Stanford (Calif.) University. “Recent immunotherapies targeting specific immune checkpoints such as programmed death 1 or programmed death ligand 1 have demonstrated a remarkable, durable response in NSCLC. Certain histopathologic patterns, such as intratumoral infiltration by cytotoxic lymphocytes, have also been associated with better prognoses in several cancer types, including NSCLC.”
For the study, the investigators developed and validated an immune-related gene signature using frozen tumors from 2,414 patients with stage I or II nonsquamous NSCLC from 19 public cohorts who underwent resection with negative margins and did not receive any neoadjuvant or adjuvant therapy.
The new signature contained 25 gene pairs consisting of 40 unique immune-related genes, Dr. Li and associates report (JAMA Oncol. 2017 Jul 6. doi: 10.1001/jamaoncol.2017.1609).
Processes such as chemotaxis were enriched among the included genes.
The signature significantly stratified patients into groups that have high and low risks of death during follow-up, both across and within subsets with stage I, IA, IB, or II disease. Relative to counterparts falling into the signature-defined low-risk group, those falling into the signature-defined high-risk group had roughly twice the risk of death after adjustment for clinical and pathologic characteristics, with a hazard ratio range of 1.72 (P less than .001) to 2.36 (P less than .001).
Accuracy of the immune signature exceeded that of two commercialized gene signatures for estimating survival in similar validation cohorts (mean concordance index [C-index], 0.64 vs 0.53 and 0.61).
Moreover, the combination of the immune signature with clinical factors outperformed the signature alone (mean C-index, 0.70 vs 0.63) and another commercialized clinical-molecular combination signature (mean C-index, 0.68 vs 0.65).
“The proposed immune-related gene pair–based signature is a promising prognostic biomarker in nonsquamous NSCLC, including early-stage disease,” concluded the investigators. “Prospective studies are needed to further validate its analytical accuracy for estimating prognoses and to test its clinical utility in individualized management of nonsquamous NSCLC.”
A new tumor immune-related gene signature may help take the guesswork out of prognostication in patients with early-stage non–small cell lung cancer (NSCLC), according to a retrospective cohort study.
“Various components of the immune system have been shown to be a determining factor during cancer initiation and progression,” note the investigators, who were led by Bailiang Li, PhD, of Stanford (Calif.) University. “Recent immunotherapies targeting specific immune checkpoints such as programmed death 1 or programmed death ligand 1 have demonstrated a remarkable, durable response in NSCLC. Certain histopathologic patterns, such as intratumoral infiltration by cytotoxic lymphocytes, have also been associated with better prognoses in several cancer types, including NSCLC.”
For the study, the investigators developed and validated an immune-related gene signature using frozen tumors from 2,414 patients with stage I or II nonsquamous NSCLC from 19 public cohorts who underwent resection with negative margins and did not receive any neoadjuvant or adjuvant therapy.
The new signature contained 25 gene pairs consisting of 40 unique immune-related genes, Dr. Li and associates report (JAMA Oncol. 2017 Jul 6. doi: 10.1001/jamaoncol.2017.1609).
Processes such as chemotaxis were enriched among the included genes.
The signature significantly stratified patients into groups that have high and low risks of death during follow-up, both across and within subsets with stage I, IA, IB, or II disease. Relative to counterparts falling into the signature-defined low-risk group, those falling into the signature-defined high-risk group had roughly twice the risk of death after adjustment for clinical and pathologic characteristics, with a hazard ratio range of 1.72 (P less than .001) to 2.36 (P less than .001).
Accuracy of the immune signature exceeded that of two commercialized gene signatures for estimating survival in similar validation cohorts (mean concordance index [C-index], 0.64 vs 0.53 and 0.61).
Moreover, the combination of the immune signature with clinical factors outperformed the signature alone (mean C-index, 0.70 vs 0.63) and another commercialized clinical-molecular combination signature (mean C-index, 0.68 vs 0.65).
“The proposed immune-related gene pair–based signature is a promising prognostic biomarker in nonsquamous NSCLC, including early-stage disease,” concluded the investigators. “Prospective studies are needed to further validate its analytical accuracy for estimating prognoses and to test its clinical utility in individualized management of nonsquamous NSCLC.”
FROM JAMA ONCOLOGY
Key clinical point:
Major finding: Compared with peers in the signature-defined low-risk group, patients in the signature-defined high-risk group had roughly twice the adjusted risk of death (hazard ratio range, 1.72-2.36).
Data source: A retrospective cohort study using frozen tumors from 2,414 patients with stage I or II nonsquamous NSCLC who underwent complete resection and did not receive adjuvant or neoadjuvant therapy.
Disclosures: The investigators reported that they had no relevant disclosures. The study was supported in part by the National Institutes of Health.
Time for dermatologists in nine states to start submitting CPT code 99024
The Centers for Medicare & Medicaid Services survey period is upon us, and it’s time for dermatologists in nine test states to act.
In my April column, I discussed the CMS survey, which is intended to gather data on when follow-up visits for surgical procedures take place. Reporting started July 1st and will continue for several months, at least, possibly for a year.
All of you in these nine test states recently received a two-page letter from CMS telling you that, if you are in a practice with fewer than 10 dermatologists, you don’t have to report. That’s correct – you don’t have to – but is not reporting in the best interest of dermatology? I contend it is not; you can and must report!
Simply put, you need to generate and append code 99024 to your claims whenever possible. The 99024 code is a “no charge” code that informs CMS you did some follow-up work, either in person or on the phone.
That’s right, generate a 99024 after every visit when you or your staff do not bill for an evaluation and management code – and whenever you, or your physician assistant, nurse practitioner, nurse, medical assistant, or receptionist even speak to a patient on the phone. Yes, phone contacts count for a 99024.
So, when you or a member of your staff call back biopsy or lab results after a procedure, or call to schedule or change a postop appointment, speak to a relative, give instructions to the visiting nurse, or provide reassurance after a procedure, you or your staff member should generate a very brief note in the chart, plug in the working diagnosis, put that 99024 in there, and make sure the billing company posts it. Some of your billing systems may require that a physician finalize the receptionist note or that you charge a penny to get the software to cooperate, but you should still put in 99024.
(And I tell you what, I am personally good to cover all the 1-cent charges that get generated and you don’t want to write off. Just have the patients send the bill to good ole “Hotsteel” here in Cincinnati!)
Some of you may say, “Hey, a skin biopsy is a 0-day global, so why report a follow-up? Here’s why. How often do you do a skin biopsy using a shave code, or without freezing an actinic keratosis? Reporting the 99024 when you call back with the biopsy results correctly documents the actinic keratosis and shave-embedded follow-up visit, so you should do it.
When you see that patient back to remove her sutures after an excision, submit the 99024.
When you see him to inject a hypertrophic scar from an electrodessication, submit the 99024.
I know we see our patients at follow-up visits and communicate with them by phone – sometimes for years after a procedure, at no charge. I hope to see hundreds of thousands of 99024 codes generated from small groups and solo dermatologists. You need to make sure these services are acknowledged and that dermatologists get credit when credit is due. The future of our specialty depends on your doing so.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
The Centers for Medicare & Medicaid Services survey period is upon us, and it’s time for dermatologists in nine test states to act.
In my April column, I discussed the CMS survey, which is intended to gather data on when follow-up visits for surgical procedures take place. Reporting started July 1st and will continue for several months, at least, possibly for a year.
All of you in these nine test states recently received a two-page letter from CMS telling you that, if you are in a practice with fewer than 10 dermatologists, you don’t have to report. That’s correct – you don’t have to – but is not reporting in the best interest of dermatology? I contend it is not; you can and must report!
Simply put, you need to generate and append code 99024 to your claims whenever possible. The 99024 code is a “no charge” code that informs CMS you did some follow-up work, either in person or on the phone.
That’s right, generate a 99024 after every visit when you or your staff do not bill for an evaluation and management code – and whenever you, or your physician assistant, nurse practitioner, nurse, medical assistant, or receptionist even speak to a patient on the phone. Yes, phone contacts count for a 99024.
So, when you or a member of your staff call back biopsy or lab results after a procedure, or call to schedule or change a postop appointment, speak to a relative, give instructions to the visiting nurse, or provide reassurance after a procedure, you or your staff member should generate a very brief note in the chart, plug in the working diagnosis, put that 99024 in there, and make sure the billing company posts it. Some of your billing systems may require that a physician finalize the receptionist note or that you charge a penny to get the software to cooperate, but you should still put in 99024.
(And I tell you what, I am personally good to cover all the 1-cent charges that get generated and you don’t want to write off. Just have the patients send the bill to good ole “Hotsteel” here in Cincinnati!)
Some of you may say, “Hey, a skin biopsy is a 0-day global, so why report a follow-up? Here’s why. How often do you do a skin biopsy using a shave code, or without freezing an actinic keratosis? Reporting the 99024 when you call back with the biopsy results correctly documents the actinic keratosis and shave-embedded follow-up visit, so you should do it.
When you see that patient back to remove her sutures after an excision, submit the 99024.
When you see him to inject a hypertrophic scar from an electrodessication, submit the 99024.
I know we see our patients at follow-up visits and communicate with them by phone – sometimes for years after a procedure, at no charge. I hope to see hundreds of thousands of 99024 codes generated from small groups and solo dermatologists. You need to make sure these services are acknowledged and that dermatologists get credit when credit is due. The future of our specialty depends on your doing so.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
The Centers for Medicare & Medicaid Services survey period is upon us, and it’s time for dermatologists in nine test states to act.
In my April column, I discussed the CMS survey, which is intended to gather data on when follow-up visits for surgical procedures take place. Reporting started July 1st and will continue for several months, at least, possibly for a year.
All of you in these nine test states recently received a two-page letter from CMS telling you that, if you are in a practice with fewer than 10 dermatologists, you don’t have to report. That’s correct – you don’t have to – but is not reporting in the best interest of dermatology? I contend it is not; you can and must report!
Simply put, you need to generate and append code 99024 to your claims whenever possible. The 99024 code is a “no charge” code that informs CMS you did some follow-up work, either in person or on the phone.
That’s right, generate a 99024 after every visit when you or your staff do not bill for an evaluation and management code – and whenever you, or your physician assistant, nurse practitioner, nurse, medical assistant, or receptionist even speak to a patient on the phone. Yes, phone contacts count for a 99024.
So, when you or a member of your staff call back biopsy or lab results after a procedure, or call to schedule or change a postop appointment, speak to a relative, give instructions to the visiting nurse, or provide reassurance after a procedure, you or your staff member should generate a very brief note in the chart, plug in the working diagnosis, put that 99024 in there, and make sure the billing company posts it. Some of your billing systems may require that a physician finalize the receptionist note or that you charge a penny to get the software to cooperate, but you should still put in 99024.
(And I tell you what, I am personally good to cover all the 1-cent charges that get generated and you don’t want to write off. Just have the patients send the bill to good ole “Hotsteel” here in Cincinnati!)
Some of you may say, “Hey, a skin biopsy is a 0-day global, so why report a follow-up? Here’s why. How often do you do a skin biopsy using a shave code, or without freezing an actinic keratosis? Reporting the 99024 when you call back with the biopsy results correctly documents the actinic keratosis and shave-embedded follow-up visit, so you should do it.
When you see that patient back to remove her sutures after an excision, submit the 99024.
When you see him to inject a hypertrophic scar from an electrodessication, submit the 99024.
I know we see our patients at follow-up visits and communicate with them by phone – sometimes for years after a procedure, at no charge. I hope to see hundreds of thousands of 99024 codes generated from small groups and solo dermatologists. You need to make sure these services are acknowledged and that dermatologists get credit when credit is due. The future of our specialty depends on your doing so.
Dr. Coldiron is in private practice but maintains a clinical assistant professorship at the University of Cincinnati. He cares for patients, teaches medical students and residents, and has several active clinical research projects. Dr. Coldiron is the author of more than 80 scientific letters, papers, and several book chapters, and he speaks frequently on a variety of topics. He is a past president of the American Academy of Dermatology. Write to him at [email protected].
Sick of Your Job—or Sick Because of Your Job?
Does your work make you sick? If it does, do you have paid sick leave? A NIOSH study found that aspects of anoccupation influences people’s health in a multitude of ways, including work conditions, how the work is organized, job-related tasks, long work hours, and work-life balance. “Work is an important determinant of health,” the researchers conclude.
NIOSH researchers analyzed data from 10,767 adults in many occupations who participated in the 2010 National Health Interview Survey. People employed in business operations jobs, such as marketing and human resources, were 85% more likely to rate their health as fair or poor. Workers with no paid sick leave were 35% more likely to report fair or poor health. Workers who were worried about becoming unemployed were 43% more likely to report fair or poor health. Those who reported difficulty combining work and family were 23% more likely. Those who reported being bullied at work were 82% more likely.
“We believe this is the first study to show an association between business operations jobs and poor health,” said Sara Luckhaupt, MD, NIOSH medical officer and lead author of the study. “Knowing which aspects of a person’s job can lead to poor health can help public health and employee wellness professionals develop—ideally with worker input—tailored workplace interventions to advance worker well-being.”
Does your work make you sick? If it does, do you have paid sick leave? A NIOSH study found that aspects of anoccupation influences people’s health in a multitude of ways, including work conditions, how the work is organized, job-related tasks, long work hours, and work-life balance. “Work is an important determinant of health,” the researchers conclude.
NIOSH researchers analyzed data from 10,767 adults in many occupations who participated in the 2010 National Health Interview Survey. People employed in business operations jobs, such as marketing and human resources, were 85% more likely to rate their health as fair or poor. Workers with no paid sick leave were 35% more likely to report fair or poor health. Workers who were worried about becoming unemployed were 43% more likely to report fair or poor health. Those who reported difficulty combining work and family were 23% more likely. Those who reported being bullied at work were 82% more likely.
“We believe this is the first study to show an association between business operations jobs and poor health,” said Sara Luckhaupt, MD, NIOSH medical officer and lead author of the study. “Knowing which aspects of a person’s job can lead to poor health can help public health and employee wellness professionals develop—ideally with worker input—tailored workplace interventions to advance worker well-being.”
Does your work make you sick? If it does, do you have paid sick leave? A NIOSH study found that aspects of anoccupation influences people’s health in a multitude of ways, including work conditions, how the work is organized, job-related tasks, long work hours, and work-life balance. “Work is an important determinant of health,” the researchers conclude.
NIOSH researchers analyzed data from 10,767 adults in many occupations who participated in the 2010 National Health Interview Survey. People employed in business operations jobs, such as marketing and human resources, were 85% more likely to rate their health as fair or poor. Workers with no paid sick leave were 35% more likely to report fair or poor health. Workers who were worried about becoming unemployed were 43% more likely to report fair or poor health. Those who reported difficulty combining work and family were 23% more likely. Those who reported being bullied at work were 82% more likely.
“We believe this is the first study to show an association between business operations jobs and poor health,” said Sara Luckhaupt, MD, NIOSH medical officer and lead author of the study. “Knowing which aspects of a person’s job can lead to poor health can help public health and employee wellness professionals develop—ideally with worker input—tailored workplace interventions to advance worker well-being.”
A New ‘Triplet’ Treatment for Multiple Myeloma
Carfilzomib, a selective second-generation proteasome inhibitor, has performed well in clinical trials. So because other “triplets”—combinations of alkylator, proteasome inhibitor, and steroid—had shown “encouraging” response rates, researchers from the Center for Cancer and Blood Disorders in Bethesda, Maryland, and others, conducted a multicenter study to evaluate the safety and tolerability of twice-weekly carfilzomib combined with cyclophosphamide and dexamethasone (KCyd) for patients newly diagnosed with multiple myeloma (MM).
The researchers tested 3 doses of carfilzomib: 36 mg/m2, 45 mg/m2, and 56 mg/m2. Of the 22 enrolled patients, 16 were treated with the maximum dose.
Fourteen patients completed all 8 cycles of treatment; 10 in the maximum-dose group completed all 8. At 56 mg/m2, the overall response rate was 87.5%. Among the 14 patients whose disease responded to therapy, the median time to response was 1 month.
Five patients discontinued treatment because of adverse effects (AEs), but the researchers found no dose-limiting toxicities at any of the dose levels. The most common AEs were nausea, diarrhea, and anemia.
The researchers concluded that based on previous research, KCyd with 36 mg/m2 is safe and effective in patients aged ≥ 65 years with newly diagnosed MM. However, their findings suggest that twice-weekly carfilzomib 56 mg/m2 in combination with cyclophosphamide and dexamethasone also is effective with “manageable toxicity.”
Source:
Boccia RV, Bessudo A, Agajanian R, et al. Clin Lymphoma Myeloma Leuk. In press.
doi: 10.1016/j.clml.2017.05.009.
Carfilzomib, a selective second-generation proteasome inhibitor, has performed well in clinical trials. So because other “triplets”—combinations of alkylator, proteasome inhibitor, and steroid—had shown “encouraging” response rates, researchers from the Center for Cancer and Blood Disorders in Bethesda, Maryland, and others, conducted a multicenter study to evaluate the safety and tolerability of twice-weekly carfilzomib combined with cyclophosphamide and dexamethasone (KCyd) for patients newly diagnosed with multiple myeloma (MM).
The researchers tested 3 doses of carfilzomib: 36 mg/m2, 45 mg/m2, and 56 mg/m2. Of the 22 enrolled patients, 16 were treated with the maximum dose.
Fourteen patients completed all 8 cycles of treatment; 10 in the maximum-dose group completed all 8. At 56 mg/m2, the overall response rate was 87.5%. Among the 14 patients whose disease responded to therapy, the median time to response was 1 month.
Five patients discontinued treatment because of adverse effects (AEs), but the researchers found no dose-limiting toxicities at any of the dose levels. The most common AEs were nausea, diarrhea, and anemia.
The researchers concluded that based on previous research, KCyd with 36 mg/m2 is safe and effective in patients aged ≥ 65 years with newly diagnosed MM. However, their findings suggest that twice-weekly carfilzomib 56 mg/m2 in combination with cyclophosphamide and dexamethasone also is effective with “manageable toxicity.”
Source:
Boccia RV, Bessudo A, Agajanian R, et al. Clin Lymphoma Myeloma Leuk. In press.
doi: 10.1016/j.clml.2017.05.009.
Carfilzomib, a selective second-generation proteasome inhibitor, has performed well in clinical trials. So because other “triplets”—combinations of alkylator, proteasome inhibitor, and steroid—had shown “encouraging” response rates, researchers from the Center for Cancer and Blood Disorders in Bethesda, Maryland, and others, conducted a multicenter study to evaluate the safety and tolerability of twice-weekly carfilzomib combined with cyclophosphamide and dexamethasone (KCyd) for patients newly diagnosed with multiple myeloma (MM).
The researchers tested 3 doses of carfilzomib: 36 mg/m2, 45 mg/m2, and 56 mg/m2. Of the 22 enrolled patients, 16 were treated with the maximum dose.
Fourteen patients completed all 8 cycles of treatment; 10 in the maximum-dose group completed all 8. At 56 mg/m2, the overall response rate was 87.5%. Among the 14 patients whose disease responded to therapy, the median time to response was 1 month.
Five patients discontinued treatment because of adverse effects (AEs), but the researchers found no dose-limiting toxicities at any of the dose levels. The most common AEs were nausea, diarrhea, and anemia.
The researchers concluded that based on previous research, KCyd with 36 mg/m2 is safe and effective in patients aged ≥ 65 years with newly diagnosed MM. However, their findings suggest that twice-weekly carfilzomib 56 mg/m2 in combination with cyclophosphamide and dexamethasone also is effective with “manageable toxicity.”
Source:
Boccia RV, Bessudo A, Agajanian R, et al. Clin Lymphoma Myeloma Leuk. In press.
doi: 10.1016/j.clml.2017.05.009.
RNAi therapeutic reduces ABR in hemophilia A and B
BERLIN—Researchers have reported positive results from an ongoing phase 2 trial of fitusiran in patients with hemophilia A or B, with or without inhibitors.
Once-monthly treatment with fitusiran reduced the median annualized bleeding rate (ABR) from 20 to 1 in all patients. In patients with inhibitors, the median ABR fell from 38 to 0.
Most adverse events (AEs) were mild or moderate in severity, with the most common AEs being alanine aminotransferase (ALT) increases and injection-site reactions.
These results were presented* at the International Society on Thrombosis and Haemostasis (ISTH) 2017 Congress.
The research was sponsored by Alnylam Pharmaceuticals, Inc., the company developing fitusiran in collaboration with Sanofi Genzyme.
Fitusiran is an RNAi therapeutic targeting antithrombin for the treatment of patients with hemophilia A and B. Fitusiran is designed to lower levels of antithrombin and promote sufficient thrombin generation upon activation of the clotting cascade to restore hemostasis and prevent bleeding.
John Pasi, PhD, of Barts and the London School of Medicine and Dentistry in London, UK, and his colleagues tested fitusiran in 33 patients, ages 19 to 61, with hemophilia A (n=27) or hemophilia B (n=6), with inhibitors (n=14) or without (n=19).
Fitusiran was administered as a monthly, subcutaneous, fixed dose of 50 mg (n=13) or 80 mg (n=20).
Patients were treated for up to 20 months, with a median of 11 months on study. Five patients discontinued treatment—4 due to withdrawn consent and 1 due to an AE.
Safety
The incidence of AEs was 70%. Most were mild or moderate in severity and unrelated to fitusiran.
Non-laboratory AEs included injection site reactions (18%, n=6), abdominal pain (9%, n=3), diarrhea (9%, n=3), and headache (9%, n=3).
Eleven patients had asymptomatic ALT increases greater than 3 times the upper limit of normal, without concurrent elevations in bilirubin greater than 2 times the upper limit of normal. All of these patients were hepatitis C antibody-positive.
At last follow-up, all ALT elevations were resolved (n=10) or resolving (n=1).
There were serious AEs in 6 patients, and 2 of these events were considered possibly related to fitusiran. One event was seizure with confusion in a patient with a prior history of seizure disorder.
The other serious AE was an asymptomatic ALT elevation in a patient with chronic hepatitis C virus infection. This patient discontinued treatment due to the event.
There were no thromboembolic events, no laboratory evidence for pathological clot formation, and no instances of anti-drug antibody formation.
Efficacy
Fitusiran resulted in approximately 80% lowering of antithrombin, with corresponding increases in thrombin generation.
In all patients, fitusiran reduced the median ABR from 20 (interquartile range [IQR]: 4-36) to 1 (IQR: 0-3).
In patients with inhibitors, fitusiran reduced the median ABR from 38 (IQR: 20-48) to 0 (IQR: 0-3).
Forty-eight percent of all patients (n=16) remained bleed-free during the observation period, and 67% (n=22) did not experience any spontaneous bleeds.
All breakthrough bleeds were successfully managed with replacement factor (recombinant factor VIII or recombinant factor IX) or bypassing agents (recombinant factor VIIa or activated prothrombin complex concentrate).
Based on these results, Sanofi and Alnylam initiated the ATLAS phase 3 program for fitusiran in patients with hemophilia A and B, with or without inhibitors. ![]()
*Data in the abstract differ from data presented at the meeting.
BERLIN—Researchers have reported positive results from an ongoing phase 2 trial of fitusiran in patients with hemophilia A or B, with or without inhibitors.
Once-monthly treatment with fitusiran reduced the median annualized bleeding rate (ABR) from 20 to 1 in all patients. In patients with inhibitors, the median ABR fell from 38 to 0.
Most adverse events (AEs) were mild or moderate in severity, with the most common AEs being alanine aminotransferase (ALT) increases and injection-site reactions.
These results were presented* at the International Society on Thrombosis and Haemostasis (ISTH) 2017 Congress.
The research was sponsored by Alnylam Pharmaceuticals, Inc., the company developing fitusiran in collaboration with Sanofi Genzyme.
Fitusiran is an RNAi therapeutic targeting antithrombin for the treatment of patients with hemophilia A and B. Fitusiran is designed to lower levels of antithrombin and promote sufficient thrombin generation upon activation of the clotting cascade to restore hemostasis and prevent bleeding.
John Pasi, PhD, of Barts and the London School of Medicine and Dentistry in London, UK, and his colleagues tested fitusiran in 33 patients, ages 19 to 61, with hemophilia A (n=27) or hemophilia B (n=6), with inhibitors (n=14) or without (n=19).
Fitusiran was administered as a monthly, subcutaneous, fixed dose of 50 mg (n=13) or 80 mg (n=20).
Patients were treated for up to 20 months, with a median of 11 months on study. Five patients discontinued treatment—4 due to withdrawn consent and 1 due to an AE.
Safety
The incidence of AEs was 70%. Most were mild or moderate in severity and unrelated to fitusiran.
Non-laboratory AEs included injection site reactions (18%, n=6), abdominal pain (9%, n=3), diarrhea (9%, n=3), and headache (9%, n=3).
Eleven patients had asymptomatic ALT increases greater than 3 times the upper limit of normal, without concurrent elevations in bilirubin greater than 2 times the upper limit of normal. All of these patients were hepatitis C antibody-positive.
At last follow-up, all ALT elevations were resolved (n=10) or resolving (n=1).
There were serious AEs in 6 patients, and 2 of these events were considered possibly related to fitusiran. One event was seizure with confusion in a patient with a prior history of seizure disorder.
The other serious AE was an asymptomatic ALT elevation in a patient with chronic hepatitis C virus infection. This patient discontinued treatment due to the event.
There were no thromboembolic events, no laboratory evidence for pathological clot formation, and no instances of anti-drug antibody formation.
Efficacy
Fitusiran resulted in approximately 80% lowering of antithrombin, with corresponding increases in thrombin generation.
In all patients, fitusiran reduced the median ABR from 20 (interquartile range [IQR]: 4-36) to 1 (IQR: 0-3).
In patients with inhibitors, fitusiran reduced the median ABR from 38 (IQR: 20-48) to 0 (IQR: 0-3).
Forty-eight percent of all patients (n=16) remained bleed-free during the observation period, and 67% (n=22) did not experience any spontaneous bleeds.
All breakthrough bleeds were successfully managed with replacement factor (recombinant factor VIII or recombinant factor IX) or bypassing agents (recombinant factor VIIa or activated prothrombin complex concentrate).
Based on these results, Sanofi and Alnylam initiated the ATLAS phase 3 program for fitusiran in patients with hemophilia A and B, with or without inhibitors. ![]()
*Data in the abstract differ from data presented at the meeting.
BERLIN—Researchers have reported positive results from an ongoing phase 2 trial of fitusiran in patients with hemophilia A or B, with or without inhibitors.
Once-monthly treatment with fitusiran reduced the median annualized bleeding rate (ABR) from 20 to 1 in all patients. In patients with inhibitors, the median ABR fell from 38 to 0.
Most adverse events (AEs) were mild or moderate in severity, with the most common AEs being alanine aminotransferase (ALT) increases and injection-site reactions.
These results were presented* at the International Society on Thrombosis and Haemostasis (ISTH) 2017 Congress.
The research was sponsored by Alnylam Pharmaceuticals, Inc., the company developing fitusiran in collaboration with Sanofi Genzyme.
Fitusiran is an RNAi therapeutic targeting antithrombin for the treatment of patients with hemophilia A and B. Fitusiran is designed to lower levels of antithrombin and promote sufficient thrombin generation upon activation of the clotting cascade to restore hemostasis and prevent bleeding.
John Pasi, PhD, of Barts and the London School of Medicine and Dentistry in London, UK, and his colleagues tested fitusiran in 33 patients, ages 19 to 61, with hemophilia A (n=27) or hemophilia B (n=6), with inhibitors (n=14) or without (n=19).
Fitusiran was administered as a monthly, subcutaneous, fixed dose of 50 mg (n=13) or 80 mg (n=20).
Patients were treated for up to 20 months, with a median of 11 months on study. Five patients discontinued treatment—4 due to withdrawn consent and 1 due to an AE.
Safety
The incidence of AEs was 70%. Most were mild or moderate in severity and unrelated to fitusiran.
Non-laboratory AEs included injection site reactions (18%, n=6), abdominal pain (9%, n=3), diarrhea (9%, n=3), and headache (9%, n=3).
Eleven patients had asymptomatic ALT increases greater than 3 times the upper limit of normal, without concurrent elevations in bilirubin greater than 2 times the upper limit of normal. All of these patients were hepatitis C antibody-positive.
At last follow-up, all ALT elevations were resolved (n=10) or resolving (n=1).
There were serious AEs in 6 patients, and 2 of these events were considered possibly related to fitusiran. One event was seizure with confusion in a patient with a prior history of seizure disorder.
The other serious AE was an asymptomatic ALT elevation in a patient with chronic hepatitis C virus infection. This patient discontinued treatment due to the event.
There were no thromboembolic events, no laboratory evidence for pathological clot formation, and no instances of anti-drug antibody formation.
Efficacy
Fitusiran resulted in approximately 80% lowering of antithrombin, with corresponding increases in thrombin generation.
In all patients, fitusiran reduced the median ABR from 20 (interquartile range [IQR]: 4-36) to 1 (IQR: 0-3).
In patients with inhibitors, fitusiran reduced the median ABR from 38 (IQR: 20-48) to 0 (IQR: 0-3).
Forty-eight percent of all patients (n=16) remained bleed-free during the observation period, and 67% (n=22) did not experience any spontaneous bleeds.
All breakthrough bleeds were successfully managed with replacement factor (recombinant factor VIII or recombinant factor IX) or bypassing agents (recombinant factor VIIa or activated prothrombin complex concentrate).
Based on these results, Sanofi and Alnylam initiated the ATLAS phase 3 program for fitusiran in patients with hemophilia A and B, with or without inhibitors. ![]()
*Data in the abstract differ from data presented at the meeting.
GAD-M produces high ORR in treatment-naïve ENKTL
LUGANO, SWITZERLAND—A 4-drug regimen has demonstrated efficacy in a phase 2 trial of patients with treatment-naïve extranodal natural killer/T-cell lymphoma (ENKTL).
Treatment with gemcitabine, PEG-asparaginase, dexamethasone, and methotrexate (GAD-M) produced a 94% overall response rate (ORR) and an 83% complete response (CR) rate in this trial.
Responses and survival rates were higher in patients with stage I/II disease, who also received radiotherapy, than in patients with stage III/IV disease.
Grade 1/2 toxicities were frequent, but there were few grade 3/4 non-hematologic toxicities. One patient died of treatment-related toxicity.
Zhiming Li, of Sun Yet-sen University Cancer Center in Guangzhou, China, presented these results at the 14th International Conference on Malignant Lymphoma (ICML).
Patients and treatment
The trial enrolled 41 patients with treatment-naïve ENKTL, and 36 of them were evaluable.
The patients’ median age was 45 (range, 18-75), and 30.6% were female. Most patients (86.1%) had stage I/II disease, 13.9% had an ECOG performance status of 2 or greater, and 41.7% had an IPI score of 2 or greater.
The GAD-M regimen consisted of:
- Gemcitabine given at 1000 mg/m2 via intravenous drip on days 1 and 8
- PEG-asparaginase given at 2500 U/m2 intramuscularly on day 1
- Dexamethasone given at 20 mg via intravenous drip on days 1 to 3
- Methotrexate given at 3000 mg/m2 via continuous, 12-hour infusion on day 1.
The regimen was repeated every 3 weeks.
For patients with stage I/II disease, 2 to 4 cycles of the GAD-M regimen was followed by extensive involved-field radiotherapy and an additional 2 to 4 cycles. For patients with stage III/IV disease, GAD-M was repeated for 6 cycles.
Response and survival
The ORR was 94.4%, both after 2 cycles of GAD-M and after 6 cycles. The CR rate was 50% after 2 cycles and 83.3% after 6 cycles.
In patients with stage I/II disease, the ORR was 100% after 2 cycles and 6 cycles. The CR rate was 54.8% after 2 cycles and 90.3% after 6 cycles.
In patients with stage III/IV disease, the ORR was 60% after 2 cycles and 6 cycles. The CR rate was 20% after 2 cycles and 40% after 6 cycles.
At median follow-up of 23.3 months, the estimated 3-year progression-free survival (PFS) was 72.1%, and the overall survival (OS) was 76.3%.
For patients with stage I/II disease, the PFS was 77.3%, and the OS was 79.3%. For patients with stage III/IV disease, the PFS was 40%, and the OS was 60%.
Safety
Hematologic adverse events (AEs) included anemia (97.2% total, 52.8% grade 3/4), leukocytopenia (94.4%, 27.8% grade 3/4), neutropenia (88.9%, 5.6% grade 3), and thrombocytopenia (47.2%, 13.9% grade 3/4).
Non-hematologic AEs included hypoalbuminemia (100%, 5.6% grade 3), increased transaminases (88.9%, 5.6% grade 3), hyperbilirubinemia (52.8%, 11.1% grade 3), decreased fibrinogen (19.4%, 11.1% grade 4), vomiting (13.9%, 2.8% grade 5), increased creatinine (8.3%, 2.8% grade 3), and abdominal pain (5.6% grade 1).
The grade 5 treatment-related AE occurred in a 61-year-old man. He died of electrolyte disorders caused by severe vomiting. ![]()
LUGANO, SWITZERLAND—A 4-drug regimen has demonstrated efficacy in a phase 2 trial of patients with treatment-naïve extranodal natural killer/T-cell lymphoma (ENKTL).
Treatment with gemcitabine, PEG-asparaginase, dexamethasone, and methotrexate (GAD-M) produced a 94% overall response rate (ORR) and an 83% complete response (CR) rate in this trial.
Responses and survival rates were higher in patients with stage I/II disease, who also received radiotherapy, than in patients with stage III/IV disease.
Grade 1/2 toxicities were frequent, but there were few grade 3/4 non-hematologic toxicities. One patient died of treatment-related toxicity.
Zhiming Li, of Sun Yet-sen University Cancer Center in Guangzhou, China, presented these results at the 14th International Conference on Malignant Lymphoma (ICML).
Patients and treatment
The trial enrolled 41 patients with treatment-naïve ENKTL, and 36 of them were evaluable.
The patients’ median age was 45 (range, 18-75), and 30.6% were female. Most patients (86.1%) had stage I/II disease, 13.9% had an ECOG performance status of 2 or greater, and 41.7% had an IPI score of 2 or greater.
The GAD-M regimen consisted of:
- Gemcitabine given at 1000 mg/m2 via intravenous drip on days 1 and 8
- PEG-asparaginase given at 2500 U/m2 intramuscularly on day 1
- Dexamethasone given at 20 mg via intravenous drip on days 1 to 3
- Methotrexate given at 3000 mg/m2 via continuous, 12-hour infusion on day 1.
The regimen was repeated every 3 weeks.
For patients with stage I/II disease, 2 to 4 cycles of the GAD-M regimen was followed by extensive involved-field radiotherapy and an additional 2 to 4 cycles. For patients with stage III/IV disease, GAD-M was repeated for 6 cycles.
Response and survival
The ORR was 94.4%, both after 2 cycles of GAD-M and after 6 cycles. The CR rate was 50% after 2 cycles and 83.3% after 6 cycles.
In patients with stage I/II disease, the ORR was 100% after 2 cycles and 6 cycles. The CR rate was 54.8% after 2 cycles and 90.3% after 6 cycles.
In patients with stage III/IV disease, the ORR was 60% after 2 cycles and 6 cycles. The CR rate was 20% after 2 cycles and 40% after 6 cycles.
At median follow-up of 23.3 months, the estimated 3-year progression-free survival (PFS) was 72.1%, and the overall survival (OS) was 76.3%.
For patients with stage I/II disease, the PFS was 77.3%, and the OS was 79.3%. For patients with stage III/IV disease, the PFS was 40%, and the OS was 60%.
Safety
Hematologic adverse events (AEs) included anemia (97.2% total, 52.8% grade 3/4), leukocytopenia (94.4%, 27.8% grade 3/4), neutropenia (88.9%, 5.6% grade 3), and thrombocytopenia (47.2%, 13.9% grade 3/4).
Non-hematologic AEs included hypoalbuminemia (100%, 5.6% grade 3), increased transaminases (88.9%, 5.6% grade 3), hyperbilirubinemia (52.8%, 11.1% grade 3), decreased fibrinogen (19.4%, 11.1% grade 4), vomiting (13.9%, 2.8% grade 5), increased creatinine (8.3%, 2.8% grade 3), and abdominal pain (5.6% grade 1).
The grade 5 treatment-related AE occurred in a 61-year-old man. He died of electrolyte disorders caused by severe vomiting. ![]()
LUGANO, SWITZERLAND—A 4-drug regimen has demonstrated efficacy in a phase 2 trial of patients with treatment-naïve extranodal natural killer/T-cell lymphoma (ENKTL).
Treatment with gemcitabine, PEG-asparaginase, dexamethasone, and methotrexate (GAD-M) produced a 94% overall response rate (ORR) and an 83% complete response (CR) rate in this trial.
Responses and survival rates were higher in patients with stage I/II disease, who also received radiotherapy, than in patients with stage III/IV disease.
Grade 1/2 toxicities were frequent, but there were few grade 3/4 non-hematologic toxicities. One patient died of treatment-related toxicity.
Zhiming Li, of Sun Yet-sen University Cancer Center in Guangzhou, China, presented these results at the 14th International Conference on Malignant Lymphoma (ICML).
Patients and treatment
The trial enrolled 41 patients with treatment-naïve ENKTL, and 36 of them were evaluable.
The patients’ median age was 45 (range, 18-75), and 30.6% were female. Most patients (86.1%) had stage I/II disease, 13.9% had an ECOG performance status of 2 or greater, and 41.7% had an IPI score of 2 or greater.
The GAD-M regimen consisted of:
- Gemcitabine given at 1000 mg/m2 via intravenous drip on days 1 and 8
- PEG-asparaginase given at 2500 U/m2 intramuscularly on day 1
- Dexamethasone given at 20 mg via intravenous drip on days 1 to 3
- Methotrexate given at 3000 mg/m2 via continuous, 12-hour infusion on day 1.
The regimen was repeated every 3 weeks.
For patients with stage I/II disease, 2 to 4 cycles of the GAD-M regimen was followed by extensive involved-field radiotherapy and an additional 2 to 4 cycles. For patients with stage III/IV disease, GAD-M was repeated for 6 cycles.
Response and survival
The ORR was 94.4%, both after 2 cycles of GAD-M and after 6 cycles. The CR rate was 50% after 2 cycles and 83.3% after 6 cycles.
In patients with stage I/II disease, the ORR was 100% after 2 cycles and 6 cycles. The CR rate was 54.8% after 2 cycles and 90.3% after 6 cycles.
In patients with stage III/IV disease, the ORR was 60% after 2 cycles and 6 cycles. The CR rate was 20% after 2 cycles and 40% after 6 cycles.
At median follow-up of 23.3 months, the estimated 3-year progression-free survival (PFS) was 72.1%, and the overall survival (OS) was 76.3%.
For patients with stage I/II disease, the PFS was 77.3%, and the OS was 79.3%. For patients with stage III/IV disease, the PFS was 40%, and the OS was 60%.
Safety
Hematologic adverse events (AEs) included anemia (97.2% total, 52.8% grade 3/4), leukocytopenia (94.4%, 27.8% grade 3/4), neutropenia (88.9%, 5.6% grade 3), and thrombocytopenia (47.2%, 13.9% grade 3/4).
Non-hematologic AEs included hypoalbuminemia (100%, 5.6% grade 3), increased transaminases (88.9%, 5.6% grade 3), hyperbilirubinemia (52.8%, 11.1% grade 3), decreased fibrinogen (19.4%, 11.1% grade 4), vomiting (13.9%, 2.8% grade 5), increased creatinine (8.3%, 2.8% grade 3), and abdominal pain (5.6% grade 1).
The grade 5 treatment-related AE occurred in a 61-year-old man. He died of electrolyte disorders caused by severe vomiting. ![]()
Mutations linked to Fanconi anemia
New research suggests that mutations in the RFWD3 gene cause Fanconi anemia (FA).
Investigators detected mutations in the RFWD3 gene in a child with FA and confirmed the relationship between the mutations and the disorder via functional studies in cell and animal models.
The team described this research in The Journal of Clinical Oncology.
Previously, there was knowledge of 21 genes involved in FA.
“The discovery of new genes is essential not only for genetic diagnosis and advice, but also for the development of new therapies,” said study author Jordi Surrallés, PhD, of the Hospital de la Santa Creu i Sant Pau and Universitat Autonoma de Barcelona in Spain.
“The RFWD3 protein is one of the few deficient proteins in patients with Fanconi anemia in which we can see a clear enzymatic activity (ubiquitin ligase), which opens the door to massive drug screenings. In this sense, my group has already worked on several screenings of thousands of therapeutic molecules with the aim of repositioning a drug for this disease.” ![]()
New research suggests that mutations in the RFWD3 gene cause Fanconi anemia (FA).
Investigators detected mutations in the RFWD3 gene in a child with FA and confirmed the relationship between the mutations and the disorder via functional studies in cell and animal models.
The team described this research in The Journal of Clinical Oncology.
Previously, there was knowledge of 21 genes involved in FA.
“The discovery of new genes is essential not only for genetic diagnosis and advice, but also for the development of new therapies,” said study author Jordi Surrallés, PhD, of the Hospital de la Santa Creu i Sant Pau and Universitat Autonoma de Barcelona in Spain.
“The RFWD3 protein is one of the few deficient proteins in patients with Fanconi anemia in which we can see a clear enzymatic activity (ubiquitin ligase), which opens the door to massive drug screenings. In this sense, my group has already worked on several screenings of thousands of therapeutic molecules with the aim of repositioning a drug for this disease.” ![]()
New research suggests that mutations in the RFWD3 gene cause Fanconi anemia (FA).
Investigators detected mutations in the RFWD3 gene in a child with FA and confirmed the relationship between the mutations and the disorder via functional studies in cell and animal models.
The team described this research in The Journal of Clinical Oncology.
Previously, there was knowledge of 21 genes involved in FA.
“The discovery of new genes is essential not only for genetic diagnosis and advice, but also for the development of new therapies,” said study author Jordi Surrallés, PhD, of the Hospital de la Santa Creu i Sant Pau and Universitat Autonoma de Barcelona in Spain.
“The RFWD3 protein is one of the few deficient proteins in patients with Fanconi anemia in which we can see a clear enzymatic activity (ubiquitin ligase), which opens the door to massive drug screenings. In this sense, my group has already worked on several screenings of thousands of therapeutic molecules with the aim of repositioning a drug for this disease.” ![]()