User login
Cherokee Nation Sues 6 Pharmacies Over Opioid Prescribing
The Cherokee Nation has filed a lawsuit against 6 companies, accusing them of “turning a blind eye” to opioids being illegally prescribed to Cherokee adults and children. The lawsuit is the first of its kind filed in the U.S. “As we fight this epidemic in our hospitals, our schools, and our Cherokee homes, we will also use our legal system to make sure the companies, who put profits over people while our society is crippled by this epidemic, are held responsible for their actions,” said Cherokee Nation Principal Chief Bill John Baker.
According to DEA statistics, about 845 mg of opioids were distributed in the 14 counties of the Cherokee Nation in 2015—or between 360 and 720 opioid pills per every prescription. In The Washington Post, one of the lawyers for the Cherokee Nation said the “flood of opioids into Oklahoma has torn apart families and cost the Cherokees hundreds of millions of dollars.” Over the past 3 years, 2,684 opioid-related deaths have been reported in the state, according to Oklahoma Attorney General Mike Hunter.
The lawsuit names McKesson Corporation, Cardinal Health, Inc., and AmerisourceBergen (which together control roughly 85% of prescription drug distribution in the U.S.) as well as CVS, Walgreens, and Walmart. The companies “enabled prescription opioids to fall into illicit distribution channels, failed to alert regulators of extreme volume, and incentivized sales of these drugs with financial bonuses,” said Cherokee Nation Attorney General Todd Hembree. He said the drug distributors and pharmacies knew or should have known that the amount of drugs they were sending and dispensing were suspicious. He charged that the corporations’ profit seeking has unleashed “a plague.”
“Tribal nations have survived disease, removal from our homelands, termination, and other adversities and still we prospered,” said Chief Baker. “However, I fear the opioid epidemic is emerging as the next great challenge of our modern era.”
The full petition is available at www.cherokeecourts.org.
The Cherokee Nation has filed a lawsuit against 6 companies, accusing them of “turning a blind eye” to opioids being illegally prescribed to Cherokee adults and children. The lawsuit is the first of its kind filed in the U.S. “As we fight this epidemic in our hospitals, our schools, and our Cherokee homes, we will also use our legal system to make sure the companies, who put profits over people while our society is crippled by this epidemic, are held responsible for their actions,” said Cherokee Nation Principal Chief Bill John Baker.
According to DEA statistics, about 845 mg of opioids were distributed in the 14 counties of the Cherokee Nation in 2015—or between 360 and 720 opioid pills per every prescription. In The Washington Post, one of the lawyers for the Cherokee Nation said the “flood of opioids into Oklahoma has torn apart families and cost the Cherokees hundreds of millions of dollars.” Over the past 3 years, 2,684 opioid-related deaths have been reported in the state, according to Oklahoma Attorney General Mike Hunter.
The lawsuit names McKesson Corporation, Cardinal Health, Inc., and AmerisourceBergen (which together control roughly 85% of prescription drug distribution in the U.S.) as well as CVS, Walgreens, and Walmart. The companies “enabled prescription opioids to fall into illicit distribution channels, failed to alert regulators of extreme volume, and incentivized sales of these drugs with financial bonuses,” said Cherokee Nation Attorney General Todd Hembree. He said the drug distributors and pharmacies knew or should have known that the amount of drugs they were sending and dispensing were suspicious. He charged that the corporations’ profit seeking has unleashed “a plague.”
“Tribal nations have survived disease, removal from our homelands, termination, and other adversities and still we prospered,” said Chief Baker. “However, I fear the opioid epidemic is emerging as the next great challenge of our modern era.”
The full petition is available at www.cherokeecourts.org.
The Cherokee Nation has filed a lawsuit against 6 companies, accusing them of “turning a blind eye” to opioids being illegally prescribed to Cherokee adults and children. The lawsuit is the first of its kind filed in the U.S. “As we fight this epidemic in our hospitals, our schools, and our Cherokee homes, we will also use our legal system to make sure the companies, who put profits over people while our society is crippled by this epidemic, are held responsible for their actions,” said Cherokee Nation Principal Chief Bill John Baker.
According to DEA statistics, about 845 mg of opioids were distributed in the 14 counties of the Cherokee Nation in 2015—or between 360 and 720 opioid pills per every prescription. In The Washington Post, one of the lawyers for the Cherokee Nation said the “flood of opioids into Oklahoma has torn apart families and cost the Cherokees hundreds of millions of dollars.” Over the past 3 years, 2,684 opioid-related deaths have been reported in the state, according to Oklahoma Attorney General Mike Hunter.
The lawsuit names McKesson Corporation, Cardinal Health, Inc., and AmerisourceBergen (which together control roughly 85% of prescription drug distribution in the U.S.) as well as CVS, Walgreens, and Walmart. The companies “enabled prescription opioids to fall into illicit distribution channels, failed to alert regulators of extreme volume, and incentivized sales of these drugs with financial bonuses,” said Cherokee Nation Attorney General Todd Hembree. He said the drug distributors and pharmacies knew or should have known that the amount of drugs they were sending and dispensing were suspicious. He charged that the corporations’ profit seeking has unleashed “a plague.”
“Tribal nations have survived disease, removal from our homelands, termination, and other adversities and still we prospered,” said Chief Baker. “However, I fear the opioid epidemic is emerging as the next great challenge of our modern era.”
The full petition is available at www.cherokeecourts.org.
Rituximab Stretches Survival in Follicular Lymphoma
Follicular lymphoma (FL), which accounts for about 20% to 30% of all non-Hodgkin lymphomas, is an incurable disease. Although people with FL are living longer, the median overall survival (OS) had been about 10 years—until recently. According to researchers from the Spanish Lymphoma Oncology Group, OS may be changing. In their long-term study of 1,074 patients with newly diagnosed FL, median OS was more than 20 years.
Related: Six Open Clinical Trials That Are Expanding Our Understanding of Immunotherapies
The difference, the researchers say, may be chemoimmunotherapy, specifically, the anti-CD20 monoclonal antibody rituximab. Patients were enrolled between 1980 and 2013 and followed for a median of 55 months. The researchers note that rituximab was added to the treatment options in 2003. When the researchers analyzed the patients who were still alive 10 years beyond the diagnosis, they found that 118 of 166 were free of evident clinical disease.
The prognostic factors the researchers enumerate are similar to those of other studies. The variables significantly associated with survival at 10 years were stage great than II, aged < 60 years, low Follicular Lymphoma International Prognostic Index, normal β2 microglobulin, no B symptoms on diagnosis, Performance Status 0 to 1, and treatment with anthracyclines and rituximab. But their data, the researchers add, support the conclusion that the initial combined treatment with rituximab and anthracyclines “could be considered key factors.” They note that randomized studies and meta-analyses have repeatedly made improvements in the survival of patients with FL.
Related: New Treatments Offer Hope in Diffuse Large B-Cell Lymphoma
“We believe that the weight of the introduction of [rituximab] in a young population, associated with chemotherapy,” the researchers say, “has given these high rates of survival in an unselected population.” They conclude, “in the [rituximab] era, the strategy of ‘wait and watch’ remains valid for patients with favorable prognostic factors and low-grade tumors.”
Source:
Provencio M, Sabín P, Gomez-Codina J, et al. PLoS One. 2017;12(5):e0177204.
doi: 10.1371/journal.pone.0177204
Follicular lymphoma (FL), which accounts for about 20% to 30% of all non-Hodgkin lymphomas, is an incurable disease. Although people with FL are living longer, the median overall survival (OS) had been about 10 years—until recently. According to researchers from the Spanish Lymphoma Oncology Group, OS may be changing. In their long-term study of 1,074 patients with newly diagnosed FL, median OS was more than 20 years.
Related: Six Open Clinical Trials That Are Expanding Our Understanding of Immunotherapies
The difference, the researchers say, may be chemoimmunotherapy, specifically, the anti-CD20 monoclonal antibody rituximab. Patients were enrolled between 1980 and 2013 and followed for a median of 55 months. The researchers note that rituximab was added to the treatment options in 2003. When the researchers analyzed the patients who were still alive 10 years beyond the diagnosis, they found that 118 of 166 were free of evident clinical disease.
The prognostic factors the researchers enumerate are similar to those of other studies. The variables significantly associated with survival at 10 years were stage great than II, aged < 60 years, low Follicular Lymphoma International Prognostic Index, normal β2 microglobulin, no B symptoms on diagnosis, Performance Status 0 to 1, and treatment with anthracyclines and rituximab. But their data, the researchers add, support the conclusion that the initial combined treatment with rituximab and anthracyclines “could be considered key factors.” They note that randomized studies and meta-analyses have repeatedly made improvements in the survival of patients with FL.
Related: New Treatments Offer Hope in Diffuse Large B-Cell Lymphoma
“We believe that the weight of the introduction of [rituximab] in a young population, associated with chemotherapy,” the researchers say, “has given these high rates of survival in an unselected population.” They conclude, “in the [rituximab] era, the strategy of ‘wait and watch’ remains valid for patients with favorable prognostic factors and low-grade tumors.”
Source:
Provencio M, Sabín P, Gomez-Codina J, et al. PLoS One. 2017;12(5):e0177204.
doi: 10.1371/journal.pone.0177204
Follicular lymphoma (FL), which accounts for about 20% to 30% of all non-Hodgkin lymphomas, is an incurable disease. Although people with FL are living longer, the median overall survival (OS) had been about 10 years—until recently. According to researchers from the Spanish Lymphoma Oncology Group, OS may be changing. In their long-term study of 1,074 patients with newly diagnosed FL, median OS was more than 20 years.
Related: Six Open Clinical Trials That Are Expanding Our Understanding of Immunotherapies
The difference, the researchers say, may be chemoimmunotherapy, specifically, the anti-CD20 monoclonal antibody rituximab. Patients were enrolled between 1980 and 2013 and followed for a median of 55 months. The researchers note that rituximab was added to the treatment options in 2003. When the researchers analyzed the patients who were still alive 10 years beyond the diagnosis, they found that 118 of 166 were free of evident clinical disease.
The prognostic factors the researchers enumerate are similar to those of other studies. The variables significantly associated with survival at 10 years were stage great than II, aged < 60 years, low Follicular Lymphoma International Prognostic Index, normal β2 microglobulin, no B symptoms on diagnosis, Performance Status 0 to 1, and treatment with anthracyclines and rituximab. But their data, the researchers add, support the conclusion that the initial combined treatment with rituximab and anthracyclines “could be considered key factors.” They note that randomized studies and meta-analyses have repeatedly made improvements in the survival of patients with FL.
Related: New Treatments Offer Hope in Diffuse Large B-Cell Lymphoma
“We believe that the weight of the introduction of [rituximab] in a young population, associated with chemotherapy,” the researchers say, “has given these high rates of survival in an unselected population.” They conclude, “in the [rituximab] era, the strategy of ‘wait and watch’ remains valid for patients with favorable prognostic factors and low-grade tumors.”
Source:
Provencio M, Sabín P, Gomez-Codina J, et al. PLoS One. 2017;12(5):e0177204.
doi: 10.1371/journal.pone.0177204
Call for Cardiovascular Disease Papers
Federal Practitioner is inviting VA, DoD, and PHS health care providers and researchers to contribute to a special issue that will be published in November 2017 and will examine cardiovascular disease treatments, such as heart failure, atrial fibrillation, anticoagulation treatments, and other related conditions.
Interested authors should submit a brief 2 to 3 sentence abstract to [email protected] by July 7, 2017. Federal Practitioner welcomes case studies, literature reviews, original research, program profiles, guest editorials, and other evidence-based articles. The updated and complete submission guidelines, including details about the style and format, can be found here:
http://www.mdedge.com/fedprac/page/submission-guidelines
Federal Practitioner uses Editorial Manager, a web-based manuscript submission and review system. All manuscripts must be submitted through this system.
All manuscripts submitted to Federal Practitioner for both special and regular issues will be subject to peer review. Peer reviews are conducted in a double-blind fashion, and the reviewers are asked to comment on the manuscript’s importance, accuracy, relevance, clarity, timeliness, balance, and reference citation. Final decisions on all submitted manuscripts are made by the Editor-in-Chief (or, in the event of a potential conflict of interest, a designated surrogate from the journal’s Editorial Advisory Association).
Federal Practitioner is inviting VA, DoD, and PHS health care providers and researchers to contribute to a special issue that will be published in November 2017 and will examine cardiovascular disease treatments, such as heart failure, atrial fibrillation, anticoagulation treatments, and other related conditions.
Interested authors should submit a brief 2 to 3 sentence abstract to [email protected] by July 7, 2017. Federal Practitioner welcomes case studies, literature reviews, original research, program profiles, guest editorials, and other evidence-based articles. The updated and complete submission guidelines, including details about the style and format, can be found here:
http://www.mdedge.com/fedprac/page/submission-guidelines
Federal Practitioner uses Editorial Manager, a web-based manuscript submission and review system. All manuscripts must be submitted through this system.
All manuscripts submitted to Federal Practitioner for both special and regular issues will be subject to peer review. Peer reviews are conducted in a double-blind fashion, and the reviewers are asked to comment on the manuscript’s importance, accuracy, relevance, clarity, timeliness, balance, and reference citation. Final decisions on all submitted manuscripts are made by the Editor-in-Chief (or, in the event of a potential conflict of interest, a designated surrogate from the journal’s Editorial Advisory Association).
Federal Practitioner is inviting VA, DoD, and PHS health care providers and researchers to contribute to a special issue that will be published in November 2017 and will examine cardiovascular disease treatments, such as heart failure, atrial fibrillation, anticoagulation treatments, and other related conditions.
Interested authors should submit a brief 2 to 3 sentence abstract to [email protected] by July 7, 2017. Federal Practitioner welcomes case studies, literature reviews, original research, program profiles, guest editorials, and other evidence-based articles. The updated and complete submission guidelines, including details about the style and format, can be found here:
http://www.mdedge.com/fedprac/page/submission-guidelines
Federal Practitioner uses Editorial Manager, a web-based manuscript submission and review system. All manuscripts must be submitted through this system.
All manuscripts submitted to Federal Practitioner for both special and regular issues will be subject to peer review. Peer reviews are conducted in a double-blind fashion, and the reviewers are asked to comment on the manuscript’s importance, accuracy, relevance, clarity, timeliness, balance, and reference citation. Final decisions on all submitted manuscripts are made by the Editor-in-Chief (or, in the event of a potential conflict of interest, a designated surrogate from the journal’s Editorial Advisory Association).
New type of CAR for multiple myeloma
CHICAGO—A new type of chimeric antigen receptor (CAR) T cell, one that is specific for the B-cell maturation antigen (BCMA), has produced durable remissions in patients with multiple myeloma (MM), according to research reported at the 2017 ASCO Annual Meeting (abstract LBA3001).
BCMA is a cell surface antigen universally expressed on malignant plasma cells. It plays a role in the progression of MM and is turning out to be a highly selective antigen to target in novel treatments for MM.
This trial of LCAR-B38M is one of the first clinical trials of CAR T cells to target BCMA.
“[W]hat makes our CAR T different from other CAR T all over the world is we are truly a bispecific CAR T modality,” Frank (Xiaohu) Fan, MD, PhD, explained in a media briefing, “especially our antigen-binding units compared to single domain antibodies.” Dr Fan is CSO of Nanjing Legend Biotech in China, the developer of LCAR-B38M.
“We believe targeting BCMA alone should be enough to get a good efficacy,” he said.
To date the objective response rate is 100%.
The investigators treated 35 relapsed/refractory MM patients thus far with LCAR-B38M. Patients received the modified CAR T cells in 3 doses, on days 0, 2, and 6.
The investigators reported on 19 patients who they followed for more than 4 months, a criterion established by the International Myeloma Working Group for full efficacy evaluation.
Efficacy
Of the 19 patients, 14 (74%) achieved a stringent complete response (sCR), 4 (21%) a very good partial response (VGPR), and 1 (5%) a PR.
One patient who achieved a VGPR relapsed due to an extramedullary lesion.
Investigators observed no evidence of relapse among patients who achieved sCR.
Five patients have been followed for more than a year and all have maintained sCR.
Safety
Safety is a major issue with CAR T-cell therapies, with a frequent and major adverse event being cytokine release syndrome (CRS).
Of the 35 patients treated, 6 experienced no CRS, 17 had grade 1, 10 had grade 2, and 2 had grade 3 CRS. No patient experienced grade 4 CRS or any grade 5 event.
Because LCAR-B38M demonstrates “outstanding” efficacy with a “great” safety profile, the investigators believe this technology raises the hope of cure for MM patients.
A clinical trial of LCAR-B38M is planned in the United States. ![]()
CHICAGO—A new type of chimeric antigen receptor (CAR) T cell, one that is specific for the B-cell maturation antigen (BCMA), has produced durable remissions in patients with multiple myeloma (MM), according to research reported at the 2017 ASCO Annual Meeting (abstract LBA3001).
BCMA is a cell surface antigen universally expressed on malignant plasma cells. It plays a role in the progression of MM and is turning out to be a highly selective antigen to target in novel treatments for MM.
This trial of LCAR-B38M is one of the first clinical trials of CAR T cells to target BCMA.
“[W]hat makes our CAR T different from other CAR T all over the world is we are truly a bispecific CAR T modality,” Frank (Xiaohu) Fan, MD, PhD, explained in a media briefing, “especially our antigen-binding units compared to single domain antibodies.” Dr Fan is CSO of Nanjing Legend Biotech in China, the developer of LCAR-B38M.
“We believe targeting BCMA alone should be enough to get a good efficacy,” he said.
To date the objective response rate is 100%.
The investigators treated 35 relapsed/refractory MM patients thus far with LCAR-B38M. Patients received the modified CAR T cells in 3 doses, on days 0, 2, and 6.
The investigators reported on 19 patients who they followed for more than 4 months, a criterion established by the International Myeloma Working Group for full efficacy evaluation.
Efficacy
Of the 19 patients, 14 (74%) achieved a stringent complete response (sCR), 4 (21%) a very good partial response (VGPR), and 1 (5%) a PR.
One patient who achieved a VGPR relapsed due to an extramedullary lesion.
Investigators observed no evidence of relapse among patients who achieved sCR.
Five patients have been followed for more than a year and all have maintained sCR.
Safety
Safety is a major issue with CAR T-cell therapies, with a frequent and major adverse event being cytokine release syndrome (CRS).
Of the 35 patients treated, 6 experienced no CRS, 17 had grade 1, 10 had grade 2, and 2 had grade 3 CRS. No patient experienced grade 4 CRS or any grade 5 event.
Because LCAR-B38M demonstrates “outstanding” efficacy with a “great” safety profile, the investigators believe this technology raises the hope of cure for MM patients.
A clinical trial of LCAR-B38M is planned in the United States. ![]()
CHICAGO—A new type of chimeric antigen receptor (CAR) T cell, one that is specific for the B-cell maturation antigen (BCMA), has produced durable remissions in patients with multiple myeloma (MM), according to research reported at the 2017 ASCO Annual Meeting (abstract LBA3001).
BCMA is a cell surface antigen universally expressed on malignant plasma cells. It plays a role in the progression of MM and is turning out to be a highly selective antigen to target in novel treatments for MM.
This trial of LCAR-B38M is one of the first clinical trials of CAR T cells to target BCMA.
“[W]hat makes our CAR T different from other CAR T all over the world is we are truly a bispecific CAR T modality,” Frank (Xiaohu) Fan, MD, PhD, explained in a media briefing, “especially our antigen-binding units compared to single domain antibodies.” Dr Fan is CSO of Nanjing Legend Biotech in China, the developer of LCAR-B38M.
“We believe targeting BCMA alone should be enough to get a good efficacy,” he said.
To date the objective response rate is 100%.
The investigators treated 35 relapsed/refractory MM patients thus far with LCAR-B38M. Patients received the modified CAR T cells in 3 doses, on days 0, 2, and 6.
The investigators reported on 19 patients who they followed for more than 4 months, a criterion established by the International Myeloma Working Group for full efficacy evaluation.
Efficacy
Of the 19 patients, 14 (74%) achieved a stringent complete response (sCR), 4 (21%) a very good partial response (VGPR), and 1 (5%) a PR.
One patient who achieved a VGPR relapsed due to an extramedullary lesion.
Investigators observed no evidence of relapse among patients who achieved sCR.
Five patients have been followed for more than a year and all have maintained sCR.
Safety
Safety is a major issue with CAR T-cell therapies, with a frequent and major adverse event being cytokine release syndrome (CRS).
Of the 35 patients treated, 6 experienced no CRS, 17 had grade 1, 10 had grade 2, and 2 had grade 3 CRS. No patient experienced grade 4 CRS or any grade 5 event.
Because LCAR-B38M demonstrates “outstanding” efficacy with a “great” safety profile, the investigators believe this technology raises the hope of cure for MM patients.
A clinical trial of LCAR-B38M is planned in the United States. ![]()
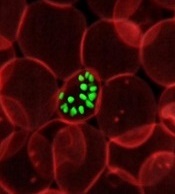
Malaria infection can lead to chronic bone loss
Malaria infection caused by Plasmodium falciparum, even after a one-time illness, can cause long-term bone loss, researchers report.
Parasite byproducts remain in the bone marrow and induce MyD88-dependent inflammatory responses in osteoclast and osteoblast precursors, resulting in bone resorption.
Researchers found that treatment with the vitamin D3 analog alfacalcidol can prevent bone loss related to malaria, at least in animals.
They suggest that bone therapies combined with anti-malarial drugs may prevent bone loss in infected individuals.
Cevayir Coban, MD, from Osaka University in Japan, and colleagues reported their findings in Science Immunology.
"Although chronic inflammatory conditions are known to facilitate bone disorders, our study—for the first time—shows that malaria can do the same thing,” Dr Coban said.
“One may think that the infection has been completely cured by anti-malarial treatment, and be feeling fully recovered,” she said. “However, sustained long-term accumulation of parasite by-products leave the bone in a state of chronic inflammation, leading to long-term bone loss. This is particularly worrisome in the young of age, where it may cause growth problems and osteoporotic, fragile bones."
To study the effect of Plasmodium infection on bone, the team used two mouse models, Plasmodium yoelii nonlethal (PyNL) and Plasmodium chabaudi chabaudi (Pcc).
PyNL infection invades reticulocytes and resembles Plasmodium vivax infection in humans. Pcc infection more closely resembles Plasmodium falciparum infection in humans. The investigators used Pcc infection to evaluate the effect of low-level chronic infection on bone homeostasis.
They infected 6-week-old adult mice with these parasite species and assessed changes in bone during the acute, convalescent, and chronic phases of infection.
Both parasites caused significantly reduced trabecular bone volume and increased trabecular bone spacing in infected mice. This continued for over 60 days after parasite clearance.
Michelle Lee, a PhD candidate and the first author of the study explains, "We found that Plasmodium products continuously accumulate in the bone marrow niche, which turns the bone noticeably black in color, and results in it being ‘eaten-up’ by bone resorbing cells known as osteoclasts, eventually disrupting bone homeostasis".
The research team also investigated the impact of malaria infection on bone growth at a young age by infecting 3-week-old mice with PyNL. These mice had significantly shorter femurs and lower trabecular bone volume compared with uninfected litter mates 3 weeks after infection, when the mice were 6 weeks old.
A key finding of the study, investigators report, is that although Plasmodium infection resolves systemically, a state of chronic inflammation evolves in the bone marrow that may be associated with continued accumulation of Plasmodium byproducts—proteins, hemozoin, and nucleic acids—in the bone marrow. These byproducts are capable of modulating immune responses.
The team also evaluated whether vitamin D supplementation would alleviate bone loss caused by Plasmodium. They treated mice with alfacalcidol, which is used to treat osteoporosis. Treatment with lower doses of oral alfacalcidol was sufficient to prevent malaria-induced bone loss, while higher doses enhanced bone growth despite infection.
This work was supported by the Grants-in-Aid for Scientific Research, the Japan Agency for Medical Research and Development, the Uehara Memorial and Yamada Science, the Grant-in-Aid for Young Scientists, and a Japanese Government Scholarship (Ministry of Education, Culture, Sports, Science and Technology). ![]()
Malaria infection caused by Plasmodium falciparum, even after a one-time illness, can cause long-term bone loss, researchers report.
Parasite byproducts remain in the bone marrow and induce MyD88-dependent inflammatory responses in osteoclast and osteoblast precursors, resulting in bone resorption.
Researchers found that treatment with the vitamin D3 analog alfacalcidol can prevent bone loss related to malaria, at least in animals.
They suggest that bone therapies combined with anti-malarial drugs may prevent bone loss in infected individuals.
Cevayir Coban, MD, from Osaka University in Japan, and colleagues reported their findings in Science Immunology.
"Although chronic inflammatory conditions are known to facilitate bone disorders, our study—for the first time—shows that malaria can do the same thing,” Dr Coban said.
“One may think that the infection has been completely cured by anti-malarial treatment, and be feeling fully recovered,” she said. “However, sustained long-term accumulation of parasite by-products leave the bone in a state of chronic inflammation, leading to long-term bone loss. This is particularly worrisome in the young of age, where it may cause growth problems and osteoporotic, fragile bones."
To study the effect of Plasmodium infection on bone, the team used two mouse models, Plasmodium yoelii nonlethal (PyNL) and Plasmodium chabaudi chabaudi (Pcc).
PyNL infection invades reticulocytes and resembles Plasmodium vivax infection in humans. Pcc infection more closely resembles Plasmodium falciparum infection in humans. The investigators used Pcc infection to evaluate the effect of low-level chronic infection on bone homeostasis.
They infected 6-week-old adult mice with these parasite species and assessed changes in bone during the acute, convalescent, and chronic phases of infection.
Both parasites caused significantly reduced trabecular bone volume and increased trabecular bone spacing in infected mice. This continued for over 60 days after parasite clearance.
Michelle Lee, a PhD candidate and the first author of the study explains, "We found that Plasmodium products continuously accumulate in the bone marrow niche, which turns the bone noticeably black in color, and results in it being ‘eaten-up’ by bone resorbing cells known as osteoclasts, eventually disrupting bone homeostasis".
The research team also investigated the impact of malaria infection on bone growth at a young age by infecting 3-week-old mice with PyNL. These mice had significantly shorter femurs and lower trabecular bone volume compared with uninfected litter mates 3 weeks after infection, when the mice were 6 weeks old.
A key finding of the study, investigators report, is that although Plasmodium infection resolves systemically, a state of chronic inflammation evolves in the bone marrow that may be associated with continued accumulation of Plasmodium byproducts—proteins, hemozoin, and nucleic acids—in the bone marrow. These byproducts are capable of modulating immune responses.
The team also evaluated whether vitamin D supplementation would alleviate bone loss caused by Plasmodium. They treated mice with alfacalcidol, which is used to treat osteoporosis. Treatment with lower doses of oral alfacalcidol was sufficient to prevent malaria-induced bone loss, while higher doses enhanced bone growth despite infection.
This work was supported by the Grants-in-Aid for Scientific Research, the Japan Agency for Medical Research and Development, the Uehara Memorial and Yamada Science, the Grant-in-Aid for Young Scientists, and a Japanese Government Scholarship (Ministry of Education, Culture, Sports, Science and Technology). ![]()
Malaria infection caused by Plasmodium falciparum, even after a one-time illness, can cause long-term bone loss, researchers report.
Parasite byproducts remain in the bone marrow and induce MyD88-dependent inflammatory responses in osteoclast and osteoblast precursors, resulting in bone resorption.
Researchers found that treatment with the vitamin D3 analog alfacalcidol can prevent bone loss related to malaria, at least in animals.
They suggest that bone therapies combined with anti-malarial drugs may prevent bone loss in infected individuals.
Cevayir Coban, MD, from Osaka University in Japan, and colleagues reported their findings in Science Immunology.
"Although chronic inflammatory conditions are known to facilitate bone disorders, our study—for the first time—shows that malaria can do the same thing,” Dr Coban said.
“One may think that the infection has been completely cured by anti-malarial treatment, and be feeling fully recovered,” she said. “However, sustained long-term accumulation of parasite by-products leave the bone in a state of chronic inflammation, leading to long-term bone loss. This is particularly worrisome in the young of age, where it may cause growth problems and osteoporotic, fragile bones."
To study the effect of Plasmodium infection on bone, the team used two mouse models, Plasmodium yoelii nonlethal (PyNL) and Plasmodium chabaudi chabaudi (Pcc).
PyNL infection invades reticulocytes and resembles Plasmodium vivax infection in humans. Pcc infection more closely resembles Plasmodium falciparum infection in humans. The investigators used Pcc infection to evaluate the effect of low-level chronic infection on bone homeostasis.
They infected 6-week-old adult mice with these parasite species and assessed changes in bone during the acute, convalescent, and chronic phases of infection.
Both parasites caused significantly reduced trabecular bone volume and increased trabecular bone spacing in infected mice. This continued for over 60 days after parasite clearance.
Michelle Lee, a PhD candidate and the first author of the study explains, "We found that Plasmodium products continuously accumulate in the bone marrow niche, which turns the bone noticeably black in color, and results in it being ‘eaten-up’ by bone resorbing cells known as osteoclasts, eventually disrupting bone homeostasis".
The research team also investigated the impact of malaria infection on bone growth at a young age by infecting 3-week-old mice with PyNL. These mice had significantly shorter femurs and lower trabecular bone volume compared with uninfected litter mates 3 weeks after infection, when the mice were 6 weeks old.
A key finding of the study, investigators report, is that although Plasmodium infection resolves systemically, a state of chronic inflammation evolves in the bone marrow that may be associated with continued accumulation of Plasmodium byproducts—proteins, hemozoin, and nucleic acids—in the bone marrow. These byproducts are capable of modulating immune responses.
The team also evaluated whether vitamin D supplementation would alleviate bone loss caused by Plasmodium. They treated mice with alfacalcidol, which is used to treat osteoporosis. Treatment with lower doses of oral alfacalcidol was sufficient to prevent malaria-induced bone loss, while higher doses enhanced bone growth despite infection.
This work was supported by the Grants-in-Aid for Scientific Research, the Japan Agency for Medical Research and Development, the Uehara Memorial and Yamada Science, the Grant-in-Aid for Young Scientists, and a Japanese Government Scholarship (Ministry of Education, Culture, Sports, Science and Technology). ![]()
Consider Melatonin for Migraine Prevention

A 32-year-old woman comes to your office for help with her recurrent migraines, which she’s had since her early 20s. She is otherwise healthy and active. She is frustrated by the frequency of her migraines and the resulting debilitation. She has tried prophylactic medications in the past but stopped taking them because of the adverse effects. What do you recommend for treatment?
Daily preventive medication can be helpful for patients whose chronic migraines have a significant impact on their lives. Many have a goal of reducing headache frequency, severity, and/or disability, while avoiding acute medication escalation.2 An estimated 38% of patients with migraine are appropriate candidates for prophylactic therapy, but only 3% to 13% are taking preventive medications.3
Evidence-based guidelines from the American Academy of Neurology and the American Headache Society state that antiepileptic drugs (divalproex sodium, sodium valproate, topiramate) and many ß-blockers (metoprolol, propranolol, timolol) are effective and should be recommended for migraine prevention.2 Medications such as antidepressants (amitriptyline, venlafaxine) and other ß-blockers (atenolol, nadolol) are probably effective and can be considered.2 However, adverse effects—including somnolence—are listed as “frequent” with amitriptyline and “occasional to frequent” with topiramate.4
Researchers have investigated melatonin before. But a 2010 double-blind, crossover RCT of 46 patients with two to seven migraine attacks per month found no significant difference in reduction of headache frequency between extended-release melatonin (2 mg taken 1 h before bed) and placebo over an eight-week period.5
STUDY SUMMARY
More than 50% reduction in headache frequency
This RCT, conducted in Brazil, compared the effectiveness of melatonin to amitriptyline and placebo for migraine prevention in 196 adults (ages 18 to 65) with chronic migraine.1 Eligible patients had a history of at least three migraine attacks or four migraine headache days per month. Patients were randomized to take identical-appearing melatonin (3 mg), amitriptyline (25 mg), or placebo nightly. The investigators appear to have concealed allocation adequately and used double-blinding.
The primary outcome was the number of headache days per month, compared to baseline. Secondary endpoints included reduction in migraine intensity, duration, number of analgesics used, and percentage of patients with more than 50% reduction in migraine headache days.
Compared to placebo, headache days per month were reduced in both the melatonin group (6.2 d vs 4.6 d, respectively; mean difference [MD], –1.6) and the amitriptyline group (6.2 d vs 5 d, respectively; MD, –1.2) at 12 weeks, based on intention-to-treat analysis. Mean headache intensity (0-10 pain scale) was also lower at 12 weeks in the melatonin group (4.8 vs 3.6; MD, –1.2) and in the amitriptyline group (4.8 vs 3.5; MD, –1.3), compared to placebo.
Headache duration (hours/month) at 12 weeks was reduced in both groups (MD, –4.4 h for amitriptyline and –4.8 h for melatonin), as was the number of analgesics used (MD for amitriptyline and for melatonin, –1) when compared to placebo. There was no significant difference between the melatonin and amitriptyline groups for these outcomes.
Patients taking melatonin were more likely to have more than 50% improvement in headache frequency compared to those taking amitriptyline (54% vs 39%; number needed to treat [NNT], 7). Melatonin worked much better than placebo (54% vs 20%; NNT, 3).
Adverse events were reported more often in the amitriptyline group than in the melatonin group (46 vs 16), with daytime sleepiness being the most frequent complaint (41% of patients in the amitriptyline group vs 18% of the melatonin group; number needed to harm [NNH], 5). There was no significant difference in adverse events between melatonin and placebo (16 vs 17). Melatonin resulted in weight loss (mean, –0.14 kg), whereas those taking amitriptyline gained weight (+0.97 kg).
WHAT’S NEW
Effective alternative with minimal adverse effects
Melatonin is an accessible and affordable option for prevention of migraine. The 3-mg dosing reduces headache frequency—measured by both the number of migraine headache days per month and the percentage of patients with a more than 50% reduction in headache events—as well as headache intensity, with minimal adverse effects.
CAVEATS
Product consistency, missing study data
This trial used 3-mg dosing, so it is not clear if other doses are also effective. In addition, melatonin’s OTC status means there could be a lack of consistency in quality/actual doses between brands.
Furthermore, in this trial, neither the amitriptyline nor the melatonin dose was titrated according to patient response or adverse effects, as it might be in clinical practice. As a result, we are not sure of the actual lowest effective dose or if greater effect (with continued minimal adverse effects) could be achieved with higher doses.
Lastly, 69% to 75% of patients in the treatment groups completed the 16-week trial, and the researchers reported using three different analytic techniques to estimate missing data. (For example, the primary endpoint analysis included data for 90.8% of randomized patients [178 of 196], and the authors treated all missing data as nonheadache days.) It is unclear how the missing data would affect the outcome—although in this type of analysis, it would tend toward a null effect.
CHALLENGES TO IMPLEMENTATION
Challenges are negligible
There are really no challenges to implementing this practice changer; melatonin is readily available and is affordable.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2017;66[5]:320-322).
1. Gonçalves AL, Martini Ferreira A, Ribeiro RT, et al. Randomised clinical trial comparing melatonin 3 mg, amitriptyline 25 mg and placebo for migraine prevention. J Neurol Neurosurg Psychiatry. 2016;87:1127-1132.
2. Silberstein SD, Holland S, Freitag F, et al. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337-1345.
3. Lipton RB, Bigal ME, Diamond M, et al; The American Migraine Prevalence and Prevention Advisory Group. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343-349.
4. Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;55:754-762.
5. Alstadhaug KB, Odeh F, Salvesen R, et al. Prophylaxis of migraine with melatonin: a randomized controlled trial. Neurology. 2010;75:1527-1532.

A 32-year-old woman comes to your office for help with her recurrent migraines, which she’s had since her early 20s. She is otherwise healthy and active. She is frustrated by the frequency of her migraines and the resulting debilitation. She has tried prophylactic medications in the past but stopped taking them because of the adverse effects. What do you recommend for treatment?
Daily preventive medication can be helpful for patients whose chronic migraines have a significant impact on their lives. Many have a goal of reducing headache frequency, severity, and/or disability, while avoiding acute medication escalation.2 An estimated 38% of patients with migraine are appropriate candidates for prophylactic therapy, but only 3% to 13% are taking preventive medications.3
Evidence-based guidelines from the American Academy of Neurology and the American Headache Society state that antiepileptic drugs (divalproex sodium, sodium valproate, topiramate) and many ß-blockers (metoprolol, propranolol, timolol) are effective and should be recommended for migraine prevention.2 Medications such as antidepressants (amitriptyline, venlafaxine) and other ß-blockers (atenolol, nadolol) are probably effective and can be considered.2 However, adverse effects—including somnolence—are listed as “frequent” with amitriptyline and “occasional to frequent” with topiramate.4
Researchers have investigated melatonin before. But a 2010 double-blind, crossover RCT of 46 patients with two to seven migraine attacks per month found no significant difference in reduction of headache frequency between extended-release melatonin (2 mg taken 1 h before bed) and placebo over an eight-week period.5
STUDY SUMMARY
More than 50% reduction in headache frequency
This RCT, conducted in Brazil, compared the effectiveness of melatonin to amitriptyline and placebo for migraine prevention in 196 adults (ages 18 to 65) with chronic migraine.1 Eligible patients had a history of at least three migraine attacks or four migraine headache days per month. Patients were randomized to take identical-appearing melatonin (3 mg), amitriptyline (25 mg), or placebo nightly. The investigators appear to have concealed allocation adequately and used double-blinding.
The primary outcome was the number of headache days per month, compared to baseline. Secondary endpoints included reduction in migraine intensity, duration, number of analgesics used, and percentage of patients with more than 50% reduction in migraine headache days.
Compared to placebo, headache days per month were reduced in both the melatonin group (6.2 d vs 4.6 d, respectively; mean difference [MD], –1.6) and the amitriptyline group (6.2 d vs 5 d, respectively; MD, –1.2) at 12 weeks, based on intention-to-treat analysis. Mean headache intensity (0-10 pain scale) was also lower at 12 weeks in the melatonin group (4.8 vs 3.6; MD, –1.2) and in the amitriptyline group (4.8 vs 3.5; MD, –1.3), compared to placebo.
Headache duration (hours/month) at 12 weeks was reduced in both groups (MD, –4.4 h for amitriptyline and –4.8 h for melatonin), as was the number of analgesics used (MD for amitriptyline and for melatonin, –1) when compared to placebo. There was no significant difference between the melatonin and amitriptyline groups for these outcomes.
Patients taking melatonin were more likely to have more than 50% improvement in headache frequency compared to those taking amitriptyline (54% vs 39%; number needed to treat [NNT], 7). Melatonin worked much better than placebo (54% vs 20%; NNT, 3).
Adverse events were reported more often in the amitriptyline group than in the melatonin group (46 vs 16), with daytime sleepiness being the most frequent complaint (41% of patients in the amitriptyline group vs 18% of the melatonin group; number needed to harm [NNH], 5). There was no significant difference in adverse events between melatonin and placebo (16 vs 17). Melatonin resulted in weight loss (mean, –0.14 kg), whereas those taking amitriptyline gained weight (+0.97 kg).
WHAT’S NEW
Effective alternative with minimal adverse effects
Melatonin is an accessible and affordable option for prevention of migraine. The 3-mg dosing reduces headache frequency—measured by both the number of migraine headache days per month and the percentage of patients with a more than 50% reduction in headache events—as well as headache intensity, with minimal adverse effects.
CAVEATS
Product consistency, missing study data
This trial used 3-mg dosing, so it is not clear if other doses are also effective. In addition, melatonin’s OTC status means there could be a lack of consistency in quality/actual doses between brands.
Furthermore, in this trial, neither the amitriptyline nor the melatonin dose was titrated according to patient response or adverse effects, as it might be in clinical practice. As a result, we are not sure of the actual lowest effective dose or if greater effect (with continued minimal adverse effects) could be achieved with higher doses.
Lastly, 69% to 75% of patients in the treatment groups completed the 16-week trial, and the researchers reported using three different analytic techniques to estimate missing data. (For example, the primary endpoint analysis included data for 90.8% of randomized patients [178 of 196], and the authors treated all missing data as nonheadache days.) It is unclear how the missing data would affect the outcome—although in this type of analysis, it would tend toward a null effect.
CHALLENGES TO IMPLEMENTATION
Challenges are negligible
There are really no challenges to implementing this practice changer; melatonin is readily available and is affordable.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2017;66[5]:320-322).

A 32-year-old woman comes to your office for help with her recurrent migraines, which she’s had since her early 20s. She is otherwise healthy and active. She is frustrated by the frequency of her migraines and the resulting debilitation. She has tried prophylactic medications in the past but stopped taking them because of the adverse effects. What do you recommend for treatment?
Daily preventive medication can be helpful for patients whose chronic migraines have a significant impact on their lives. Many have a goal of reducing headache frequency, severity, and/or disability, while avoiding acute medication escalation.2 An estimated 38% of patients with migraine are appropriate candidates for prophylactic therapy, but only 3% to 13% are taking preventive medications.3
Evidence-based guidelines from the American Academy of Neurology and the American Headache Society state that antiepileptic drugs (divalproex sodium, sodium valproate, topiramate) and many ß-blockers (metoprolol, propranolol, timolol) are effective and should be recommended for migraine prevention.2 Medications such as antidepressants (amitriptyline, venlafaxine) and other ß-blockers (atenolol, nadolol) are probably effective and can be considered.2 However, adverse effects—including somnolence—are listed as “frequent” with amitriptyline and “occasional to frequent” with topiramate.4
Researchers have investigated melatonin before. But a 2010 double-blind, crossover RCT of 46 patients with two to seven migraine attacks per month found no significant difference in reduction of headache frequency between extended-release melatonin (2 mg taken 1 h before bed) and placebo over an eight-week period.5
STUDY SUMMARY
More than 50% reduction in headache frequency
This RCT, conducted in Brazil, compared the effectiveness of melatonin to amitriptyline and placebo for migraine prevention in 196 adults (ages 18 to 65) with chronic migraine.1 Eligible patients had a history of at least three migraine attacks or four migraine headache days per month. Patients were randomized to take identical-appearing melatonin (3 mg), amitriptyline (25 mg), or placebo nightly. The investigators appear to have concealed allocation adequately and used double-blinding.
The primary outcome was the number of headache days per month, compared to baseline. Secondary endpoints included reduction in migraine intensity, duration, number of analgesics used, and percentage of patients with more than 50% reduction in migraine headache days.
Compared to placebo, headache days per month were reduced in both the melatonin group (6.2 d vs 4.6 d, respectively; mean difference [MD], –1.6) and the amitriptyline group (6.2 d vs 5 d, respectively; MD, –1.2) at 12 weeks, based on intention-to-treat analysis. Mean headache intensity (0-10 pain scale) was also lower at 12 weeks in the melatonin group (4.8 vs 3.6; MD, –1.2) and in the amitriptyline group (4.8 vs 3.5; MD, –1.3), compared to placebo.
Headache duration (hours/month) at 12 weeks was reduced in both groups (MD, –4.4 h for amitriptyline and –4.8 h for melatonin), as was the number of analgesics used (MD for amitriptyline and for melatonin, –1) when compared to placebo. There was no significant difference between the melatonin and amitriptyline groups for these outcomes.
Patients taking melatonin were more likely to have more than 50% improvement in headache frequency compared to those taking amitriptyline (54% vs 39%; number needed to treat [NNT], 7). Melatonin worked much better than placebo (54% vs 20%; NNT, 3).
Adverse events were reported more often in the amitriptyline group than in the melatonin group (46 vs 16), with daytime sleepiness being the most frequent complaint (41% of patients in the amitriptyline group vs 18% of the melatonin group; number needed to harm [NNH], 5). There was no significant difference in adverse events between melatonin and placebo (16 vs 17). Melatonin resulted in weight loss (mean, –0.14 kg), whereas those taking amitriptyline gained weight (+0.97 kg).
WHAT’S NEW
Effective alternative with minimal adverse effects
Melatonin is an accessible and affordable option for prevention of migraine. The 3-mg dosing reduces headache frequency—measured by both the number of migraine headache days per month and the percentage of patients with a more than 50% reduction in headache events—as well as headache intensity, with minimal adverse effects.
CAVEATS
Product consistency, missing study data
This trial used 3-mg dosing, so it is not clear if other doses are also effective. In addition, melatonin’s OTC status means there could be a lack of consistency in quality/actual doses between brands.
Furthermore, in this trial, neither the amitriptyline nor the melatonin dose was titrated according to patient response or adverse effects, as it might be in clinical practice. As a result, we are not sure of the actual lowest effective dose or if greater effect (with continued minimal adverse effects) could be achieved with higher doses.
Lastly, 69% to 75% of patients in the treatment groups completed the 16-week trial, and the researchers reported using three different analytic techniques to estimate missing data. (For example, the primary endpoint analysis included data for 90.8% of randomized patients [178 of 196], and the authors treated all missing data as nonheadache days.) It is unclear how the missing data would affect the outcome—although in this type of analysis, it would tend toward a null effect.
CHALLENGES TO IMPLEMENTATION
Challenges are negligible
There are really no challenges to implementing this practice changer; melatonin is readily available and is affordable.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2017;66[5]:320-322).
1. Gonçalves AL, Martini Ferreira A, Ribeiro RT, et al. Randomised clinical trial comparing melatonin 3 mg, amitriptyline 25 mg and placebo for migraine prevention. J Neurol Neurosurg Psychiatry. 2016;87:1127-1132.
2. Silberstein SD, Holland S, Freitag F, et al. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337-1345.
3. Lipton RB, Bigal ME, Diamond M, et al; The American Migraine Prevalence and Prevention Advisory Group. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343-349.
4. Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;55:754-762.
5. Alstadhaug KB, Odeh F, Salvesen R, et al. Prophylaxis of migraine with melatonin: a randomized controlled trial. Neurology. 2010;75:1527-1532.
1. Gonçalves AL, Martini Ferreira A, Ribeiro RT, et al. Randomised clinical trial comparing melatonin 3 mg, amitriptyline 25 mg and placebo for migraine prevention. J Neurol Neurosurg Psychiatry. 2016;87:1127-1132.
2. Silberstein SD, Holland S, Freitag F, et al. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337-1345.
3. Lipton RB, Bigal ME, Diamond M, et al; The American Migraine Prevalence and Prevention Advisory Group. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343-349.
4. Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;55:754-762.
5. Alstadhaug KB, Odeh F, Salvesen R, et al. Prophylaxis of migraine with melatonin: a randomized controlled trial. Neurology. 2010;75:1527-1532.
Ulipristal acetate: A new option for endometriosis pain?
VANCOUVER – Not too long ago, clinicians at McMaster University in Hamilton, Ont., noticed that ulipristal acetate seemed to reduce pelvic pain in women who were taking it to shrink fibroids and reduce bleeding and other symptoms.
Fibroids aren’t usually painful, however, so they had a hunch it was helping with pain from other causes and decided to take a closer look.
All of the women at McMaster started out with moderate to severe pelvic pain, but ,while on the drug, nine (50%) said they had less pain, and seven (39%) said they had no pain at all. Most of the women reduced or stopped other pain medications.
Ten of the women had surgery, generally for fibroid control, and nine turned out to have endometriosis, adenomyosis, or both. The women were 25-49 years old and took the drug per Canadian fibroid labeling – 5 mg daily for 3 months.
“I was surprised by how many women actually had an improvement in their pain and how many went down to zero,” said lead investigator Sarah Scattolon, MD, a minimally invasive gynecologic surgery fellow at McMaster. Almost all of the women “had used multiple treatments in the past that didn’t work, including opioids. That makes a placebo effect less likely.”
The need for better endometriosis treatments was a frequent topic at the World Congress on Endometriosis, where Dr. Scattolon presented the study findings. Gonadotropin-releasing hormone agonists, progestins, and other options can help, but they don’t work for everyone, and some women can’t tolerate the side effects.
The idea that ulipristal acetate and other selective progesterone receptor modulators might be useful additions to the armamentarium has been around for a while, but there’s not much research. Dr. Scattolon said her next step is a prospective study among women with pelvic pain and, ultimately, a randomized trial. A small prospective study is already underway at Northwestern University, Chicago, for pelvic pain associated with endometriosis.
It’s unclear how the drug helps pelvic pain. It suppresses ovulation, which might help women with pain related to menstruation. “It presumably works in a different way” than other anovulatory options, Dr. Scattolon said.
Hot flashes and headaches are the most common adverse reactions, but Allergan’s Canadian Fibristal monograph states that side effects are mild or moderate and do not often lead to discontinuation.
Allergan wasn’t involved in the study, but Dr. Scattolon was scheduled to give her first paid talk for the company.
VANCOUVER – Not too long ago, clinicians at McMaster University in Hamilton, Ont., noticed that ulipristal acetate seemed to reduce pelvic pain in women who were taking it to shrink fibroids and reduce bleeding and other symptoms.
Fibroids aren’t usually painful, however, so they had a hunch it was helping with pain from other causes and decided to take a closer look.
All of the women at McMaster started out with moderate to severe pelvic pain, but ,while on the drug, nine (50%) said they had less pain, and seven (39%) said they had no pain at all. Most of the women reduced or stopped other pain medications.
Ten of the women had surgery, generally for fibroid control, and nine turned out to have endometriosis, adenomyosis, or both. The women were 25-49 years old and took the drug per Canadian fibroid labeling – 5 mg daily for 3 months.
“I was surprised by how many women actually had an improvement in their pain and how many went down to zero,” said lead investigator Sarah Scattolon, MD, a minimally invasive gynecologic surgery fellow at McMaster. Almost all of the women “had used multiple treatments in the past that didn’t work, including opioids. That makes a placebo effect less likely.”
The need for better endometriosis treatments was a frequent topic at the World Congress on Endometriosis, where Dr. Scattolon presented the study findings. Gonadotropin-releasing hormone agonists, progestins, and other options can help, but they don’t work for everyone, and some women can’t tolerate the side effects.
The idea that ulipristal acetate and other selective progesterone receptor modulators might be useful additions to the armamentarium has been around for a while, but there’s not much research. Dr. Scattolon said her next step is a prospective study among women with pelvic pain and, ultimately, a randomized trial. A small prospective study is already underway at Northwestern University, Chicago, for pelvic pain associated with endometriosis.
It’s unclear how the drug helps pelvic pain. It suppresses ovulation, which might help women with pain related to menstruation. “It presumably works in a different way” than other anovulatory options, Dr. Scattolon said.
Hot flashes and headaches are the most common adverse reactions, but Allergan’s Canadian Fibristal monograph states that side effects are mild or moderate and do not often lead to discontinuation.
Allergan wasn’t involved in the study, but Dr. Scattolon was scheduled to give her first paid talk for the company.
VANCOUVER – Not too long ago, clinicians at McMaster University in Hamilton, Ont., noticed that ulipristal acetate seemed to reduce pelvic pain in women who were taking it to shrink fibroids and reduce bleeding and other symptoms.
Fibroids aren’t usually painful, however, so they had a hunch it was helping with pain from other causes and decided to take a closer look.
All of the women at McMaster started out with moderate to severe pelvic pain, but ,while on the drug, nine (50%) said they had less pain, and seven (39%) said they had no pain at all. Most of the women reduced or stopped other pain medications.
Ten of the women had surgery, generally for fibroid control, and nine turned out to have endometriosis, adenomyosis, or both. The women were 25-49 years old and took the drug per Canadian fibroid labeling – 5 mg daily for 3 months.
“I was surprised by how many women actually had an improvement in their pain and how many went down to zero,” said lead investigator Sarah Scattolon, MD, a minimally invasive gynecologic surgery fellow at McMaster. Almost all of the women “had used multiple treatments in the past that didn’t work, including opioids. That makes a placebo effect less likely.”
The need for better endometriosis treatments was a frequent topic at the World Congress on Endometriosis, where Dr. Scattolon presented the study findings. Gonadotropin-releasing hormone agonists, progestins, and other options can help, but they don’t work for everyone, and some women can’t tolerate the side effects.
The idea that ulipristal acetate and other selective progesterone receptor modulators might be useful additions to the armamentarium has been around for a while, but there’s not much research. Dr. Scattolon said her next step is a prospective study among women with pelvic pain and, ultimately, a randomized trial. A small prospective study is already underway at Northwestern University, Chicago, for pelvic pain associated with endometriosis.
It’s unclear how the drug helps pelvic pain. It suppresses ovulation, which might help women with pain related to menstruation. “It presumably works in a different way” than other anovulatory options, Dr. Scattolon said.
Hot flashes and headaches are the most common adverse reactions, but Allergan’s Canadian Fibristal monograph states that side effects are mild or moderate and do not often lead to discontinuation.
Allergan wasn’t involved in the study, but Dr. Scattolon was scheduled to give her first paid talk for the company.
AT WCE 2017
Key clinical point:
Major finding: Of 18 women with moderate-to-severe pelvic pain, 9 (50%) noted less pain while on ulipristal acetate for fibroids, and 7 (39%) said they had no pain at all.
Data source: A chart review of 18 women.
Disclosures: Allergan, the drug’s maker, wasn’t involved in the study, but the lead investigator was scheduled to give her first paid talk for the company.
New chest x-ray assessment reflects ARDS severity
WASHINGTON – A new way to semiquantitatively score chest x-rays that takes into account lung density and consolidation may be a useful adjunct to current methods for assessing severity of acute respiratory distress syndrome.
The score, know as the Radiographic Assessment of Lung Edema (RALE) score, showed good correlations with lung edema, the severity of acute respiratory distress syndrome (ARDS), and response to fluid management resulting in reduced pulmonary edema, Melissa A. Warren, MD, said at an international conference of the American Thoracic Society.
The RALE score that Dr. Warren and her associates devised rates a patient’s chest x-ray for two parameters: consolidation, which is based on the extent of alveolar opacity in each of the four lung quadrants (left upper, left lower, right upper, and right lower), and a density score that is based on the density of alveolar opacity in each quadrant.
The consolidation score for each quadrant is rated on a 0-4 scale with 0 corresponding to no opacity, 1 for 1%-24% opacity, 2 for 25%-49% opacity, 3 for 50%-75% opacity, and 4 for more than 75%. The density score is rated on a scale of 1-3 with 1 for hazy opacity, 2 for moderate opacity, and 3 for dense opacity. The score for each quadrant is obtained by multiplying the extent score by the density score. A patient’s total RALE score sums the scores from all four quadrants.
The researchers ran three tests of the clinical relevance of this scoring system. First, they used it to score chest x-rays of 72 preprocurement lungs donated for transplant but unable to be used for that purpose, and compared the scores with the extent of lung edema measured by the actual weight of each explanted lung. This showed high correlation between the scores and the amount of edema, Dr. Warren reported.
Next they assessed the RALE score as a marker of ARDS by retrospectively calculating the scores of 174 patients with baseline chest x-rays enrolled in the Fluids and Catheters Treatment Trial (FACTT) (N Engl J Med. 2006;354[24]:2564-75). This analysis showed that patients with the highest RALE scores had significantly worse survival during 90-day follow-up, compared with the patients with the lowest scores.
Finally, the researchers assessed how the RALE score changed in response to either the liberal or conservative fluid management approaches tested in FACTT. This showed that at baseline the average RALE scores were similar among 92 patients randomized to the liberal fluid management treatment arm and 82 patients assigned to the conservative fluid management arm. But after 3 days of treatment, patients in the conservative arm showed a roughly one third reduction in their average RALE score, while patients in the liberal fluid arm showed virtually no change in their score.
“A conservative fluid management strategy favorably impacted the RALE score, reflecting a decrease in pulmonary edema,” Dr. Warren concluded.
[email protected]
On Twitter @mitchelzoler
Eric Gartman, MD, FCCP, comments: The results obtained from the use of this scoring system could be important in the prognostication of patients with ARDS, although it is unclear how the score would be used to alter clinical decision making. Further, issues may arise in its implementation given the somewhat subjective nature of the scoring (e.g., hazy vs. moderate vs. dense opacity), changing factors in the ICU that may affect the lung density on x-ray (e.g., different levels of positive end-expiratory pressure), and the variable quality of chest x-rays in the ICU.
Eric Gartman, MD, FCCP, comments: The results obtained from the use of this scoring system could be important in the prognostication of patients with ARDS, although it is unclear how the score would be used to alter clinical decision making. Further, issues may arise in its implementation given the somewhat subjective nature of the scoring (e.g., hazy vs. moderate vs. dense opacity), changing factors in the ICU that may affect the lung density on x-ray (e.g., different levels of positive end-expiratory pressure), and the variable quality of chest x-rays in the ICU.
Eric Gartman, MD, FCCP, comments: The results obtained from the use of this scoring system could be important in the prognostication of patients with ARDS, although it is unclear how the score would be used to alter clinical decision making. Further, issues may arise in its implementation given the somewhat subjective nature of the scoring (e.g., hazy vs. moderate vs. dense opacity), changing factors in the ICU that may affect the lung density on x-ray (e.g., different levels of positive end-expiratory pressure), and the variable quality of chest x-rays in the ICU.
WASHINGTON – A new way to semiquantitatively score chest x-rays that takes into account lung density and consolidation may be a useful adjunct to current methods for assessing severity of acute respiratory distress syndrome.
The score, know as the Radiographic Assessment of Lung Edema (RALE) score, showed good correlations with lung edema, the severity of acute respiratory distress syndrome (ARDS), and response to fluid management resulting in reduced pulmonary edema, Melissa A. Warren, MD, said at an international conference of the American Thoracic Society.
The RALE score that Dr. Warren and her associates devised rates a patient’s chest x-ray for two parameters: consolidation, which is based on the extent of alveolar opacity in each of the four lung quadrants (left upper, left lower, right upper, and right lower), and a density score that is based on the density of alveolar opacity in each quadrant.
The consolidation score for each quadrant is rated on a 0-4 scale with 0 corresponding to no opacity, 1 for 1%-24% opacity, 2 for 25%-49% opacity, 3 for 50%-75% opacity, and 4 for more than 75%. The density score is rated on a scale of 1-3 with 1 for hazy opacity, 2 for moderate opacity, and 3 for dense opacity. The score for each quadrant is obtained by multiplying the extent score by the density score. A patient’s total RALE score sums the scores from all four quadrants.
The researchers ran three tests of the clinical relevance of this scoring system. First, they used it to score chest x-rays of 72 preprocurement lungs donated for transplant but unable to be used for that purpose, and compared the scores with the extent of lung edema measured by the actual weight of each explanted lung. This showed high correlation between the scores and the amount of edema, Dr. Warren reported.
Next they assessed the RALE score as a marker of ARDS by retrospectively calculating the scores of 174 patients with baseline chest x-rays enrolled in the Fluids and Catheters Treatment Trial (FACTT) (N Engl J Med. 2006;354[24]:2564-75). This analysis showed that patients with the highest RALE scores had significantly worse survival during 90-day follow-up, compared with the patients with the lowest scores.
Finally, the researchers assessed how the RALE score changed in response to either the liberal or conservative fluid management approaches tested in FACTT. This showed that at baseline the average RALE scores were similar among 92 patients randomized to the liberal fluid management treatment arm and 82 patients assigned to the conservative fluid management arm. But after 3 days of treatment, patients in the conservative arm showed a roughly one third reduction in their average RALE score, while patients in the liberal fluid arm showed virtually no change in their score.
“A conservative fluid management strategy favorably impacted the RALE score, reflecting a decrease in pulmonary edema,” Dr. Warren concluded.
[email protected]
On Twitter @mitchelzoler
WASHINGTON – A new way to semiquantitatively score chest x-rays that takes into account lung density and consolidation may be a useful adjunct to current methods for assessing severity of acute respiratory distress syndrome.
The score, know as the Radiographic Assessment of Lung Edema (RALE) score, showed good correlations with lung edema, the severity of acute respiratory distress syndrome (ARDS), and response to fluid management resulting in reduced pulmonary edema, Melissa A. Warren, MD, said at an international conference of the American Thoracic Society.
The RALE score that Dr. Warren and her associates devised rates a patient’s chest x-ray for two parameters: consolidation, which is based on the extent of alveolar opacity in each of the four lung quadrants (left upper, left lower, right upper, and right lower), and a density score that is based on the density of alveolar opacity in each quadrant.
The consolidation score for each quadrant is rated on a 0-4 scale with 0 corresponding to no opacity, 1 for 1%-24% opacity, 2 for 25%-49% opacity, 3 for 50%-75% opacity, and 4 for more than 75%. The density score is rated on a scale of 1-3 with 1 for hazy opacity, 2 for moderate opacity, and 3 for dense opacity. The score for each quadrant is obtained by multiplying the extent score by the density score. A patient’s total RALE score sums the scores from all four quadrants.
The researchers ran three tests of the clinical relevance of this scoring system. First, they used it to score chest x-rays of 72 preprocurement lungs donated for transplant but unable to be used for that purpose, and compared the scores with the extent of lung edema measured by the actual weight of each explanted lung. This showed high correlation between the scores and the amount of edema, Dr. Warren reported.
Next they assessed the RALE score as a marker of ARDS by retrospectively calculating the scores of 174 patients with baseline chest x-rays enrolled in the Fluids and Catheters Treatment Trial (FACTT) (N Engl J Med. 2006;354[24]:2564-75). This analysis showed that patients with the highest RALE scores had significantly worse survival during 90-day follow-up, compared with the patients with the lowest scores.
Finally, the researchers assessed how the RALE score changed in response to either the liberal or conservative fluid management approaches tested in FACTT. This showed that at baseline the average RALE scores were similar among 92 patients randomized to the liberal fluid management treatment arm and 82 patients assigned to the conservative fluid management arm. But after 3 days of treatment, patients in the conservative arm showed a roughly one third reduction in their average RALE score, while patients in the liberal fluid arm showed virtually no change in their score.
“A conservative fluid management strategy favorably impacted the RALE score, reflecting a decrease in pulmonary edema,” Dr. Warren concluded.
[email protected]
On Twitter @mitchelzoler
AT ATS 2017
Key clinical point:
Major finding: The RALE score correlated with lung edema and ARDS severity, and changed in response to conservative fluid management.
Data source: A subgroup of 174 patients with ARDS enrolled in the FACTT trial, and also 72 donor lungs not available for transplantation.
Disclosures: Dr. Warren had no disclosures.
A prescription for heart failure success: Change the name
PARIS – Does heart failure’s name doom any progress against the disease?
That was the provocative premise advanced by Lynne Warner Stevenson, MD, who suggested that efforts to prevent, diagnose, and treat the disease would go better if it could only jettison that unfortunate word “failure,” its hard-wired albatross.
Dr. Stevenson offered several potentially superior alternatives, including cardiac insufficiency, heart dysfunction, and her favorite, cardiomyopathy.
“Is heart failure still the best diagnosis” for the entire spectrum of disease that most patients progress through ,including the many patients in earlier stages of the disease who do not have a truly failing heart? “Perhaps cardiomyopathy is the condition and heart failure is the transition,” she proposed.
To Dr. Stevenson, it’s more than just semantics.
“Words are hugely powerful,” she explained in an interview following her talk. “I think patients do not want to be seen as having heart failure. They don’t want to think of themselves as having heart failure. I think it can make them delay getting care, and it makes them ignore the disease. I worry about that a lot. I also worry that patients don’t provide support to each other that they could. Patients tend to hide that they have heart failure. We need to come up with a term that does not make patients ashamed of their disease.”
Part of the problem, Dr. Stevenson said, is that the name heart failure can be very misleading depending on the stage of the disease that patients have. Patients with stage B (presymptomatic) disease and those with mild stage C disease “don’t see themselves as having heart failure,” as having a heart that has failed them. “We need to be able to convince these patients that they have a disease that we need to treat carefully and aggressively.”
Additionally, labeling tens of millions of people as having stage A heart failure, which is presymptomatic and occurs before the heart shows any sign of damage or dysfunction, is also counterproductive, maintained Dr. Stevenson, professor of medicine at Harvard Medical School and director of the Cardiomyopathy and Heart Failure Program at Brigham and Women’s Hospital in Boston.
“So many people are at risk of developing heart failure,” she noted, including patients with hypertension, diabetes, or coronary artery disease. To label them all as already also having heart failure at that stage “tends to make them ignore the disease that we are trying to get them to pay attention to. Telling patients they have the disease that we are trying to prevent doesn’t help.”
Calling the whole range of the disease heart failure also confuses patients and others. “Patients ask me, ‘How can I have heart failure without any symptoms?’ ‘My ejection fraction improved to almost normal; do I still have heart failure?’ and ‘I don’t understand how my heart muscle is strong but my heart is failing,’ ” she said
For Dr. Stevenson, perhaps the biggest problem is the stigma of failure and the way that word ties a huge weight to the disease that prompts patients and caregivers alike to relegate it to a hidden and neglected place.
“It’s failure. Who is proud to have heart failure? Where are the marches for heart failure? Where are the celebrity champions for heart failure? We have celebrities who are happy to admit that they have Parkinson’s disease, ALS [amyotrophic lateral sclerosis], drug addiction, and even erectile dysfunction, but no one wants to say they have heart failure. We can’t get any traction behind heart failure. It doesn’t sound very inspiring,” an issue that even percolates down to dissuading clinicians from pursuing a career in heart failure care. Young people do not aspire to go into failure, she said.
“We need to call it something else.”
[email protected]
On Twitter @mitchelzoler
PARIS – Does heart failure’s name doom any progress against the disease?
That was the provocative premise advanced by Lynne Warner Stevenson, MD, who suggested that efforts to prevent, diagnose, and treat the disease would go better if it could only jettison that unfortunate word “failure,” its hard-wired albatross.
Dr. Stevenson offered several potentially superior alternatives, including cardiac insufficiency, heart dysfunction, and her favorite, cardiomyopathy.
“Is heart failure still the best diagnosis” for the entire spectrum of disease that most patients progress through ,including the many patients in earlier stages of the disease who do not have a truly failing heart? “Perhaps cardiomyopathy is the condition and heart failure is the transition,” she proposed.
To Dr. Stevenson, it’s more than just semantics.
“Words are hugely powerful,” she explained in an interview following her talk. “I think patients do not want to be seen as having heart failure. They don’t want to think of themselves as having heart failure. I think it can make them delay getting care, and it makes them ignore the disease. I worry about that a lot. I also worry that patients don’t provide support to each other that they could. Patients tend to hide that they have heart failure. We need to come up with a term that does not make patients ashamed of their disease.”
Part of the problem, Dr. Stevenson said, is that the name heart failure can be very misleading depending on the stage of the disease that patients have. Patients with stage B (presymptomatic) disease and those with mild stage C disease “don’t see themselves as having heart failure,” as having a heart that has failed them. “We need to be able to convince these patients that they have a disease that we need to treat carefully and aggressively.”
Additionally, labeling tens of millions of people as having stage A heart failure, which is presymptomatic and occurs before the heart shows any sign of damage or dysfunction, is also counterproductive, maintained Dr. Stevenson, professor of medicine at Harvard Medical School and director of the Cardiomyopathy and Heart Failure Program at Brigham and Women’s Hospital in Boston.
“So many people are at risk of developing heart failure,” she noted, including patients with hypertension, diabetes, or coronary artery disease. To label them all as already also having heart failure at that stage “tends to make them ignore the disease that we are trying to get them to pay attention to. Telling patients they have the disease that we are trying to prevent doesn’t help.”
Calling the whole range of the disease heart failure also confuses patients and others. “Patients ask me, ‘How can I have heart failure without any symptoms?’ ‘My ejection fraction improved to almost normal; do I still have heart failure?’ and ‘I don’t understand how my heart muscle is strong but my heart is failing,’ ” she said
For Dr. Stevenson, perhaps the biggest problem is the stigma of failure and the way that word ties a huge weight to the disease that prompts patients and caregivers alike to relegate it to a hidden and neglected place.
“It’s failure. Who is proud to have heart failure? Where are the marches for heart failure? Where are the celebrity champions for heart failure? We have celebrities who are happy to admit that they have Parkinson’s disease, ALS [amyotrophic lateral sclerosis], drug addiction, and even erectile dysfunction, but no one wants to say they have heart failure. We can’t get any traction behind heart failure. It doesn’t sound very inspiring,” an issue that even percolates down to dissuading clinicians from pursuing a career in heart failure care. Young people do not aspire to go into failure, she said.
“We need to call it something else.”
[email protected]
On Twitter @mitchelzoler
PARIS – Does heart failure’s name doom any progress against the disease?
That was the provocative premise advanced by Lynne Warner Stevenson, MD, who suggested that efforts to prevent, diagnose, and treat the disease would go better if it could only jettison that unfortunate word “failure,” its hard-wired albatross.
Dr. Stevenson offered several potentially superior alternatives, including cardiac insufficiency, heart dysfunction, and her favorite, cardiomyopathy.
“Is heart failure still the best diagnosis” for the entire spectrum of disease that most patients progress through ,including the many patients in earlier stages of the disease who do not have a truly failing heart? “Perhaps cardiomyopathy is the condition and heart failure is the transition,” she proposed.
To Dr. Stevenson, it’s more than just semantics.
“Words are hugely powerful,” she explained in an interview following her talk. “I think patients do not want to be seen as having heart failure. They don’t want to think of themselves as having heart failure. I think it can make them delay getting care, and it makes them ignore the disease. I worry about that a lot. I also worry that patients don’t provide support to each other that they could. Patients tend to hide that they have heart failure. We need to come up with a term that does not make patients ashamed of their disease.”
Part of the problem, Dr. Stevenson said, is that the name heart failure can be very misleading depending on the stage of the disease that patients have. Patients with stage B (presymptomatic) disease and those with mild stage C disease “don’t see themselves as having heart failure,” as having a heart that has failed them. “We need to be able to convince these patients that they have a disease that we need to treat carefully and aggressively.”
Additionally, labeling tens of millions of people as having stage A heart failure, which is presymptomatic and occurs before the heart shows any sign of damage or dysfunction, is also counterproductive, maintained Dr. Stevenson, professor of medicine at Harvard Medical School and director of the Cardiomyopathy and Heart Failure Program at Brigham and Women’s Hospital in Boston.
“So many people are at risk of developing heart failure,” she noted, including patients with hypertension, diabetes, or coronary artery disease. To label them all as already also having heart failure at that stage “tends to make them ignore the disease that we are trying to get them to pay attention to. Telling patients they have the disease that we are trying to prevent doesn’t help.”
Calling the whole range of the disease heart failure also confuses patients and others. “Patients ask me, ‘How can I have heart failure without any symptoms?’ ‘My ejection fraction improved to almost normal; do I still have heart failure?’ and ‘I don’t understand how my heart muscle is strong but my heart is failing,’ ” she said
For Dr. Stevenson, perhaps the biggest problem is the stigma of failure and the way that word ties a huge weight to the disease that prompts patients and caregivers alike to relegate it to a hidden and neglected place.
“It’s failure. Who is proud to have heart failure? Where are the marches for heart failure? Where are the celebrity champions for heart failure? We have celebrities who are happy to admit that they have Parkinson’s disease, ALS [amyotrophic lateral sclerosis], drug addiction, and even erectile dysfunction, but no one wants to say they have heart failure. We can’t get any traction behind heart failure. It doesn’t sound very inspiring,” an issue that even percolates down to dissuading clinicians from pursuing a career in heart failure care. Young people do not aspire to go into failure, she said.
“We need to call it something else.”
[email protected]
On Twitter @mitchelzoler
EXPERT ANALYSIS FROM HEART FAILURE 2017
Short Takes
Community-based palliative care reduces emergency department visits
By Bryan J. Huang, MD, FHM
Retrospective cohort study showed that patients receiving community-based palliative care were less likely to seek ED care. The reduction was greater for older patients and for patients living in areas of higher socioeconomic status.
Time to intubation after cardiac arrest: Earlier may not be better
By Sarah Horman, MD
In a retrospective, observational, cohort study of 86,628 adults with in-hospital cardiac arrest, intubation during the first 15 minutes was associated with decreased survival, compared with no intubation.
Reference: Andersen, LW, Granfeldt, A, Callaway, CW, et al. Association between Tracheal intubation during adult in-hospital cardiac arrest and survival. JAMA. 2017;317(5):494-506.
DNR orders often not transferred to ED from outside care facilities
By Leslie M. Martin, MD
Prospective chart review of patients presenting from extended care facilities to an urban trauma center found hospital staff did a poor job of noting do not resuscitate preferences, and extended care facilities were inconsistent in providing their patients’ DNR forms.
Reference: McQuown CM, Frey JA, Amireh A, Chaudhary A. Transfer of do not resuscitate orders to the emergency department from extended care facilities. Am J Emerg Med. Published on 4 Feb 2017. doi: 10.1016/j.ajem.2017.02.007.
A quasi-experimental, before-after trial examining the impact of an emergency department mechanical ventilator protocol on clinical outcomes and lung-protective ventilation in acute respiratory distress syndrome
By William James Frederick III, MD, PhD
A single center, quasi-experimental, before-after trial shows a lung-protective mechanical ventilation protocol for emergency department and intensive care patients with Acute Respiratory Distress Syndrome reduced mortality and increased ventilator-free days.
Reference: Fuller BM, Ferguson IT, Mohr NM, et al. A Quasi-Experimental, Before-After Trial Examining the Impact of an Emergency Department Mechanical Ventilator Protocol on Clinical Outcomes and Lung-Protective Ventilation in Acute Respiratory Distress Syndrome. Crit Care Med. 2017;45(4);645-52.
Community-based palliative care reduces emergency department visits
By Bryan J. Huang, MD, FHM
Retrospective cohort study showed that patients receiving community-based palliative care were less likely to seek ED care. The reduction was greater for older patients and for patients living in areas of higher socioeconomic status.
Time to intubation after cardiac arrest: Earlier may not be better
By Sarah Horman, MD
In a retrospective, observational, cohort study of 86,628 adults with in-hospital cardiac arrest, intubation during the first 15 minutes was associated with decreased survival, compared with no intubation.
Reference: Andersen, LW, Granfeldt, A, Callaway, CW, et al. Association between Tracheal intubation during adult in-hospital cardiac arrest and survival. JAMA. 2017;317(5):494-506.
DNR orders often not transferred to ED from outside care facilities
By Leslie M. Martin, MD
Prospective chart review of patients presenting from extended care facilities to an urban trauma center found hospital staff did a poor job of noting do not resuscitate preferences, and extended care facilities were inconsistent in providing their patients’ DNR forms.
Reference: McQuown CM, Frey JA, Amireh A, Chaudhary A. Transfer of do not resuscitate orders to the emergency department from extended care facilities. Am J Emerg Med. Published on 4 Feb 2017. doi: 10.1016/j.ajem.2017.02.007.
A quasi-experimental, before-after trial examining the impact of an emergency department mechanical ventilator protocol on clinical outcomes and lung-protective ventilation in acute respiratory distress syndrome
By William James Frederick III, MD, PhD
A single center, quasi-experimental, before-after trial shows a lung-protective mechanical ventilation protocol for emergency department and intensive care patients with Acute Respiratory Distress Syndrome reduced mortality and increased ventilator-free days.
Reference: Fuller BM, Ferguson IT, Mohr NM, et al. A Quasi-Experimental, Before-After Trial Examining the Impact of an Emergency Department Mechanical Ventilator Protocol on Clinical Outcomes and Lung-Protective Ventilation in Acute Respiratory Distress Syndrome. Crit Care Med. 2017;45(4);645-52.
Community-based palliative care reduces emergency department visits
By Bryan J. Huang, MD, FHM
Retrospective cohort study showed that patients receiving community-based palliative care were less likely to seek ED care. The reduction was greater for older patients and for patients living in areas of higher socioeconomic status.
Time to intubation after cardiac arrest: Earlier may not be better
By Sarah Horman, MD
In a retrospective, observational, cohort study of 86,628 adults with in-hospital cardiac arrest, intubation during the first 15 minutes was associated with decreased survival, compared with no intubation.
Reference: Andersen, LW, Granfeldt, A, Callaway, CW, et al. Association between Tracheal intubation during adult in-hospital cardiac arrest and survival. JAMA. 2017;317(5):494-506.
DNR orders often not transferred to ED from outside care facilities
By Leslie M. Martin, MD
Prospective chart review of patients presenting from extended care facilities to an urban trauma center found hospital staff did a poor job of noting do not resuscitate preferences, and extended care facilities were inconsistent in providing their patients’ DNR forms.
Reference: McQuown CM, Frey JA, Amireh A, Chaudhary A. Transfer of do not resuscitate orders to the emergency department from extended care facilities. Am J Emerg Med. Published on 4 Feb 2017. doi: 10.1016/j.ajem.2017.02.007.
A quasi-experimental, before-after trial examining the impact of an emergency department mechanical ventilator protocol on clinical outcomes and lung-protective ventilation in acute respiratory distress syndrome
By William James Frederick III, MD, PhD
A single center, quasi-experimental, before-after trial shows a lung-protective mechanical ventilation protocol for emergency department and intensive care patients with Acute Respiratory Distress Syndrome reduced mortality and increased ventilator-free days.
Reference: Fuller BM, Ferguson IT, Mohr NM, et al. A Quasi-Experimental, Before-After Trial Examining the Impact of an Emergency Department Mechanical Ventilator Protocol on Clinical Outcomes and Lung-Protective Ventilation in Acute Respiratory Distress Syndrome. Crit Care Med. 2017;45(4);645-52.