User login
Top Acthar prescribers reap hefty payments from drug maker
BOSTON – A new analysis finds that some of the biggest neurologist prescribers of repository corticotropin gel (Acthar) – the extraordinarily expensive multiple sclerosis (MS) relapse drug – reaped extensive payments from its manufacturer, with one taking in $130,307 in a single year.
Together, 51 neurologists accounted for 980 Medicare claims worth more than $39 million in Acthar spending in 2014, almost half of the entire estimated $83 million in Medicare spending on neurologist-prescribed Acthar that year.
“There is a small group of neurologists – less than 1% – who are prescribing Acthar at considerable cost to Medicare and may do this in part because of financial relationships with the company that sells Acthar,” said study lead author Dennis Bourdette, MD, chair and research professor of neurology at Oregon Health and Science University, Portland.
Dr. Bourdette acknowledges that the research doesn’t prove a causal relationship between payments and prescriptions. In response to questions, Acthar manufacturer Mallinckrodt Pharmaceuticals, already under fire for the $34,000-per-vial cost of the drug, questioned the study design and denied wrongdoing in a statement: “Mallinckrodt is committed to following the highest standards for integrity and compliance in all of our business practices, including our collaboration with physicians.”
Acthar, also known as H.P. Acthar, is the poster child for stunningly expensive medication. According to the Federal Trade Commission, the cost of the drug rose from $40 per vial in 2001 to more than $34,000 this year. The New York Times reports that Medicare spending on Acthar topped half a billion in 2015.
Earlier this year, Mallinckrodt ARD (formerly Questcor) and its parent company agreed to pay a $100 million fine to settle charges that it created an illegal monopoly over the drug.
Dr. Bourdette and colleagues released their findings at the annual meeting of the American Academy of Neurology.
In an interview, Dr. Bourdette said the study’s roots lie in his concern about the medication, whose transformation from an inexpensive 1950s-era medication for pulmonary sarcoidosis to high-priced MS treatment has drawn national media attention.
“I believe that Acthar is tremendously overpriced and a waste of health care money,” Dr. Bourdette said. “I wanted to find out how extensive an economic problem it was and how much money Medicare was spending on it since this data was easily accessible.”
According to the study, Medicare spent $1.3 billion from 2011 to 2015 on Acthar, with about 25% of the cost due to prescriptions from neurologists. “When I discovered that a relatively small number of neurologists was prescribing it commonly at a cost of $40-$50 million a year to Medicare, I was interested in determining why they were prescribing this expensive therapy and postulated that it might be related to financial support they were receiving from the manufacturer,” Dr. Bourdette said.
The researchers examined the Medicare Part D Public Use File to determine which neurologists prescribed Acthar frequently in 2014. They identified 51 who prescribed Acthar 10 or more times that year and were frequent prescribers of MS disease-modifying therapy, indicating they treated many people with MS.
The 51 neurologists accounted for a mean of 19 Acthar claims each in 2014 (range, 11-50) totaling a mean annual cost of $770,145 (range, $354,479-$3,623,509). Together, the neurologists accounted for total Medicare spending on Acthar of $39,277,380.
The researchers also chose a control group – 51 neurologists who prescribed glatiramer acetate (Copaxone) more than 10 times in 2014 and also prescribed a similar frequency of all MS disease-modifying therapies as the high-frequency Acthar group.
“Acthar is used episodically to treat MS relapses, and glatiramer acetate is taken chronically to prevent relapses and disability,” Dr. Bourdette said. He added that glatiramer acetate is now available in a generic, but it wasn’t in 2014.
The Acthar and Copaxone groups were nearly identical in terms of gender (about two-thirds men) and years since graduation (a mean of 26), but the Acthar prescribers were more likely to work in small practices (1-10 doctors), file more prescription claims, see more Medicare patients, and practice in the South or West. The demographic information came from CMS Physician Compare.
In terms of overall drug maker payments, neurologists in the Acthar group accepted much more (median, $54,270; range, $623-$369,847) than did the Copaxone group (median, $1,747, range, $0-$256,305, P less than .001). Payment information came from the federal Open Payments database.
As for payments directly from the manufacturers of the two drugs, the Acthar prescribers accepted a median of $5,344 (range, $0-$130,307) from its manufacturer, while the Copaxone prescribers accepted a median of $137 (range, $0-$168,373) from Teva (P = .003).
“The payments are primarily for giving lectures or serving on advisory boards,” Dr. Bourdette said. “These types of payments are commonly made by pharmaceutical companies to physicians who participate in these types of activities.”
In a statement, Mallinckrodt Pharmaceuticals contends that the study inappropriately compares Acthar, often a later-line therapy, to Copaxone, which it says is often a first-line therapy.
Dr. Bourdette responded that the researchers chose a comparison group of top prescribers of Copaxone “as a marker of neurologists who treated a significant number of patients with MS.”
Mallinckrodt also notes that “there may be unmeasured confounding factors in the matching process between the comparator physician groups. These could include differences in the patient characteristics managed by these physicians including disease severity affecting prescribing patterns.”
The researchers agree that there may be differences between the groups, Dr. Bourdette said. However, he added, “the fact remains that the two groups differed in the amount of money they received as open-source payments from pharmaceutical companies. We doubt that the severity of their case mixes should lead to one group receiving more pharmaceutical open-source payments than another.”
Finally, Mallinckrodt says there’s no proof of a causal connection between the payments and the prescriptions; the study authors agree. And, the company says, “this pattern of correlation would be expected in any scenario where a small number of prescribers are the experts in the use of a later-line drug to treat a limited subset of patients.”
Dr. Bourdette rejects this contention. Few of the high Acthar prescribers practice at academic centers, he said, and few are recognized for their MS expertise. “So to suggest that they are experts in the use of Acthar for the treatment of MS when they are not as a group recognized as being experts on the treatment of MS in general is incredible,” he said. “Why a small group of neurologists prescribe Acthar remains a mystery, but it is not because the majority are leaders in the field of MS therapeutics.”
In an interview, Eric G. Campbell, PhD, professor of medicine and director of research at the Mongan Institute Health Policy Center at Harvard Medical School, Boston, said the study findings fit in with previous research that has found that “the more money that people get, the more they use the drug.
“Any reasonable person looking at this data would assume or at least consider very strongly that there is a causal relationship here,” he said.
What should be done? “There are lots of ways to stop this,” Dr. Campbell said. “One could simply impose rules that forbid doctors who accept payments for marketing drugs from billing Medicare or private payers for the care they provide. Large provider organizations could pass rules that forbid this kind of behavior. Finally, the government could vigorously pursue stiff penalties against physicians who accept payments that are really nothing more than incentives to encourage or sustain prescribing practices.”
The study was supported by a National Multiple Sclerosis Society grant. Dr. Bourdette said that he provides consulting to Magellan Healthcare, a company that provides recommendations to insurance companies on the approval of high-cost therapies. Dr. Campbell disclosed that he serves as an expert witness on law cases related to conflicts of interest in medicine.
BOSTON – A new analysis finds that some of the biggest neurologist prescribers of repository corticotropin gel (Acthar) – the extraordinarily expensive multiple sclerosis (MS) relapse drug – reaped extensive payments from its manufacturer, with one taking in $130,307 in a single year.
Together, 51 neurologists accounted for 980 Medicare claims worth more than $39 million in Acthar spending in 2014, almost half of the entire estimated $83 million in Medicare spending on neurologist-prescribed Acthar that year.
“There is a small group of neurologists – less than 1% – who are prescribing Acthar at considerable cost to Medicare and may do this in part because of financial relationships with the company that sells Acthar,” said study lead author Dennis Bourdette, MD, chair and research professor of neurology at Oregon Health and Science University, Portland.
Dr. Bourdette acknowledges that the research doesn’t prove a causal relationship between payments and prescriptions. In response to questions, Acthar manufacturer Mallinckrodt Pharmaceuticals, already under fire for the $34,000-per-vial cost of the drug, questioned the study design and denied wrongdoing in a statement: “Mallinckrodt is committed to following the highest standards for integrity and compliance in all of our business practices, including our collaboration with physicians.”
Acthar, also known as H.P. Acthar, is the poster child for stunningly expensive medication. According to the Federal Trade Commission, the cost of the drug rose from $40 per vial in 2001 to more than $34,000 this year. The New York Times reports that Medicare spending on Acthar topped half a billion in 2015.
Earlier this year, Mallinckrodt ARD (formerly Questcor) and its parent company agreed to pay a $100 million fine to settle charges that it created an illegal monopoly over the drug.
Dr. Bourdette and colleagues released their findings at the annual meeting of the American Academy of Neurology.
In an interview, Dr. Bourdette said the study’s roots lie in his concern about the medication, whose transformation from an inexpensive 1950s-era medication for pulmonary sarcoidosis to high-priced MS treatment has drawn national media attention.
“I believe that Acthar is tremendously overpriced and a waste of health care money,” Dr. Bourdette said. “I wanted to find out how extensive an economic problem it was and how much money Medicare was spending on it since this data was easily accessible.”
According to the study, Medicare spent $1.3 billion from 2011 to 2015 on Acthar, with about 25% of the cost due to prescriptions from neurologists. “When I discovered that a relatively small number of neurologists was prescribing it commonly at a cost of $40-$50 million a year to Medicare, I was interested in determining why they were prescribing this expensive therapy and postulated that it might be related to financial support they were receiving from the manufacturer,” Dr. Bourdette said.
The researchers examined the Medicare Part D Public Use File to determine which neurologists prescribed Acthar frequently in 2014. They identified 51 who prescribed Acthar 10 or more times that year and were frequent prescribers of MS disease-modifying therapy, indicating they treated many people with MS.
The 51 neurologists accounted for a mean of 19 Acthar claims each in 2014 (range, 11-50) totaling a mean annual cost of $770,145 (range, $354,479-$3,623,509). Together, the neurologists accounted for total Medicare spending on Acthar of $39,277,380.
The researchers also chose a control group – 51 neurologists who prescribed glatiramer acetate (Copaxone) more than 10 times in 2014 and also prescribed a similar frequency of all MS disease-modifying therapies as the high-frequency Acthar group.
“Acthar is used episodically to treat MS relapses, and glatiramer acetate is taken chronically to prevent relapses and disability,” Dr. Bourdette said. He added that glatiramer acetate is now available in a generic, but it wasn’t in 2014.
The Acthar and Copaxone groups were nearly identical in terms of gender (about two-thirds men) and years since graduation (a mean of 26), but the Acthar prescribers were more likely to work in small practices (1-10 doctors), file more prescription claims, see more Medicare patients, and practice in the South or West. The demographic information came from CMS Physician Compare.
In terms of overall drug maker payments, neurologists in the Acthar group accepted much more (median, $54,270; range, $623-$369,847) than did the Copaxone group (median, $1,747, range, $0-$256,305, P less than .001). Payment information came from the federal Open Payments database.
As for payments directly from the manufacturers of the two drugs, the Acthar prescribers accepted a median of $5,344 (range, $0-$130,307) from its manufacturer, while the Copaxone prescribers accepted a median of $137 (range, $0-$168,373) from Teva (P = .003).
“The payments are primarily for giving lectures or serving on advisory boards,” Dr. Bourdette said. “These types of payments are commonly made by pharmaceutical companies to physicians who participate in these types of activities.”
In a statement, Mallinckrodt Pharmaceuticals contends that the study inappropriately compares Acthar, often a later-line therapy, to Copaxone, which it says is often a first-line therapy.
Dr. Bourdette responded that the researchers chose a comparison group of top prescribers of Copaxone “as a marker of neurologists who treated a significant number of patients with MS.”
Mallinckrodt also notes that “there may be unmeasured confounding factors in the matching process between the comparator physician groups. These could include differences in the patient characteristics managed by these physicians including disease severity affecting prescribing patterns.”
The researchers agree that there may be differences between the groups, Dr. Bourdette said. However, he added, “the fact remains that the two groups differed in the amount of money they received as open-source payments from pharmaceutical companies. We doubt that the severity of their case mixes should lead to one group receiving more pharmaceutical open-source payments than another.”
Finally, Mallinckrodt says there’s no proof of a causal connection between the payments and the prescriptions; the study authors agree. And, the company says, “this pattern of correlation would be expected in any scenario where a small number of prescribers are the experts in the use of a later-line drug to treat a limited subset of patients.”
Dr. Bourdette rejects this contention. Few of the high Acthar prescribers practice at academic centers, he said, and few are recognized for their MS expertise. “So to suggest that they are experts in the use of Acthar for the treatment of MS when they are not as a group recognized as being experts on the treatment of MS in general is incredible,” he said. “Why a small group of neurologists prescribe Acthar remains a mystery, but it is not because the majority are leaders in the field of MS therapeutics.”
In an interview, Eric G. Campbell, PhD, professor of medicine and director of research at the Mongan Institute Health Policy Center at Harvard Medical School, Boston, said the study findings fit in with previous research that has found that “the more money that people get, the more they use the drug.
“Any reasonable person looking at this data would assume or at least consider very strongly that there is a causal relationship here,” he said.
What should be done? “There are lots of ways to stop this,” Dr. Campbell said. “One could simply impose rules that forbid doctors who accept payments for marketing drugs from billing Medicare or private payers for the care they provide. Large provider organizations could pass rules that forbid this kind of behavior. Finally, the government could vigorously pursue stiff penalties against physicians who accept payments that are really nothing more than incentives to encourage or sustain prescribing practices.”
The study was supported by a National Multiple Sclerosis Society grant. Dr. Bourdette said that he provides consulting to Magellan Healthcare, a company that provides recommendations to insurance companies on the approval of high-cost therapies. Dr. Campbell disclosed that he serves as an expert witness on law cases related to conflicts of interest in medicine.
BOSTON – A new analysis finds that some of the biggest neurologist prescribers of repository corticotropin gel (Acthar) – the extraordinarily expensive multiple sclerosis (MS) relapse drug – reaped extensive payments from its manufacturer, with one taking in $130,307 in a single year.
Together, 51 neurologists accounted for 980 Medicare claims worth more than $39 million in Acthar spending in 2014, almost half of the entire estimated $83 million in Medicare spending on neurologist-prescribed Acthar that year.
“There is a small group of neurologists – less than 1% – who are prescribing Acthar at considerable cost to Medicare and may do this in part because of financial relationships with the company that sells Acthar,” said study lead author Dennis Bourdette, MD, chair and research professor of neurology at Oregon Health and Science University, Portland.
Dr. Bourdette acknowledges that the research doesn’t prove a causal relationship between payments and prescriptions. In response to questions, Acthar manufacturer Mallinckrodt Pharmaceuticals, already under fire for the $34,000-per-vial cost of the drug, questioned the study design and denied wrongdoing in a statement: “Mallinckrodt is committed to following the highest standards for integrity and compliance in all of our business practices, including our collaboration with physicians.”
Acthar, also known as H.P. Acthar, is the poster child for stunningly expensive medication. According to the Federal Trade Commission, the cost of the drug rose from $40 per vial in 2001 to more than $34,000 this year. The New York Times reports that Medicare spending on Acthar topped half a billion in 2015.
Earlier this year, Mallinckrodt ARD (formerly Questcor) and its parent company agreed to pay a $100 million fine to settle charges that it created an illegal monopoly over the drug.
Dr. Bourdette and colleagues released their findings at the annual meeting of the American Academy of Neurology.
In an interview, Dr. Bourdette said the study’s roots lie in his concern about the medication, whose transformation from an inexpensive 1950s-era medication for pulmonary sarcoidosis to high-priced MS treatment has drawn national media attention.
“I believe that Acthar is tremendously overpriced and a waste of health care money,” Dr. Bourdette said. “I wanted to find out how extensive an economic problem it was and how much money Medicare was spending on it since this data was easily accessible.”
According to the study, Medicare spent $1.3 billion from 2011 to 2015 on Acthar, with about 25% of the cost due to prescriptions from neurologists. “When I discovered that a relatively small number of neurologists was prescribing it commonly at a cost of $40-$50 million a year to Medicare, I was interested in determining why they were prescribing this expensive therapy and postulated that it might be related to financial support they were receiving from the manufacturer,” Dr. Bourdette said.
The researchers examined the Medicare Part D Public Use File to determine which neurologists prescribed Acthar frequently in 2014. They identified 51 who prescribed Acthar 10 or more times that year and were frequent prescribers of MS disease-modifying therapy, indicating they treated many people with MS.
The 51 neurologists accounted for a mean of 19 Acthar claims each in 2014 (range, 11-50) totaling a mean annual cost of $770,145 (range, $354,479-$3,623,509). Together, the neurologists accounted for total Medicare spending on Acthar of $39,277,380.
The researchers also chose a control group – 51 neurologists who prescribed glatiramer acetate (Copaxone) more than 10 times in 2014 and also prescribed a similar frequency of all MS disease-modifying therapies as the high-frequency Acthar group.
“Acthar is used episodically to treat MS relapses, and glatiramer acetate is taken chronically to prevent relapses and disability,” Dr. Bourdette said. He added that glatiramer acetate is now available in a generic, but it wasn’t in 2014.
The Acthar and Copaxone groups were nearly identical in terms of gender (about two-thirds men) and years since graduation (a mean of 26), but the Acthar prescribers were more likely to work in small practices (1-10 doctors), file more prescription claims, see more Medicare patients, and practice in the South or West. The demographic information came from CMS Physician Compare.
In terms of overall drug maker payments, neurologists in the Acthar group accepted much more (median, $54,270; range, $623-$369,847) than did the Copaxone group (median, $1,747, range, $0-$256,305, P less than .001). Payment information came from the federal Open Payments database.
As for payments directly from the manufacturers of the two drugs, the Acthar prescribers accepted a median of $5,344 (range, $0-$130,307) from its manufacturer, while the Copaxone prescribers accepted a median of $137 (range, $0-$168,373) from Teva (P = .003).
“The payments are primarily for giving lectures or serving on advisory boards,” Dr. Bourdette said. “These types of payments are commonly made by pharmaceutical companies to physicians who participate in these types of activities.”
In a statement, Mallinckrodt Pharmaceuticals contends that the study inappropriately compares Acthar, often a later-line therapy, to Copaxone, which it says is often a first-line therapy.
Dr. Bourdette responded that the researchers chose a comparison group of top prescribers of Copaxone “as a marker of neurologists who treated a significant number of patients with MS.”
Mallinckrodt also notes that “there may be unmeasured confounding factors in the matching process between the comparator physician groups. These could include differences in the patient characteristics managed by these physicians including disease severity affecting prescribing patterns.”
The researchers agree that there may be differences between the groups, Dr. Bourdette said. However, he added, “the fact remains that the two groups differed in the amount of money they received as open-source payments from pharmaceutical companies. We doubt that the severity of their case mixes should lead to one group receiving more pharmaceutical open-source payments than another.”
Finally, Mallinckrodt says there’s no proof of a causal connection between the payments and the prescriptions; the study authors agree. And, the company says, “this pattern of correlation would be expected in any scenario where a small number of prescribers are the experts in the use of a later-line drug to treat a limited subset of patients.”
Dr. Bourdette rejects this contention. Few of the high Acthar prescribers practice at academic centers, he said, and few are recognized for their MS expertise. “So to suggest that they are experts in the use of Acthar for the treatment of MS when they are not as a group recognized as being experts on the treatment of MS in general is incredible,” he said. “Why a small group of neurologists prescribe Acthar remains a mystery, but it is not because the majority are leaders in the field of MS therapeutics.”
In an interview, Eric G. Campbell, PhD, professor of medicine and director of research at the Mongan Institute Health Policy Center at Harvard Medical School, Boston, said the study findings fit in with previous research that has found that “the more money that people get, the more they use the drug.
“Any reasonable person looking at this data would assume or at least consider very strongly that there is a causal relationship here,” he said.
What should be done? “There are lots of ways to stop this,” Dr. Campbell said. “One could simply impose rules that forbid doctors who accept payments for marketing drugs from billing Medicare or private payers for the care they provide. Large provider organizations could pass rules that forbid this kind of behavior. Finally, the government could vigorously pursue stiff penalties against physicians who accept payments that are really nothing more than incentives to encourage or sustain prescribing practices.”
The study was supported by a National Multiple Sclerosis Society grant. Dr. Bourdette said that he provides consulting to Magellan Healthcare, a company that provides recommendations to insurance companies on the approval of high-cost therapies. Dr. Campbell disclosed that he serves as an expert witness on law cases related to conflicts of interest in medicine.
AT AAN 2017
Key clinical point:
Major finding: The top Acthar prescribers reaped much more in payments from drug makers overall in 2014 than did a control group of top prescribers of another MS drug (median of $54,270 vs. $1,747; P less than .001) and from the manufacturer of each drug (median of $5,344 vs. $137; P = .003).
Data source: Medicare Part D Public Use File, CMS Physician Compare, Open Payments database, 2014.
Disclosures: The work was supported by a National Multiple Sclerosis Society grant. Dr. Bourdette provides consulting to Magellan Healthcare, a company that provides recommendations to insurance companies on the approval of high-cost therapies.
Serpentine Supravenous Hyperpigmentation Following Cisplatin and Pemetrexed Chemotherapy
To the Editor:
Serpentine supravenous hyperpigmentation (SSH) is a rare phenomenon characterized by linear hyperpigmentation of the skin overlying veins secondary to intravenous antineoplastic therapy. The term was first suggested by Hrushesky1 in 1976 as an uncommon side effect of administering intravenous 5-fluorouracil (5-FU). Although 5-FU is the most frequent offending agent, cases involving treatment with actinomycin, cyclophosphamide, docetaxel, fotemustine, nitrogen mustard, nitrosoureas, taxanes, and triazinate, as well as various combinations of chemotherapeutic agents, also have been observed.2,3 We present the case of SSH following a cisplatin and pemetrexed chemotherapy regimen.
A 52-year-old man with newly diagnosed inoperable adenocarcinoma in the left upper lung lobe received 2 cycles of treatment with cisplatin 138 mg and pemetrexed 920 mg 21 days apart. The first cycle of chemotherapy was delivered intravenously through the left forearm and the second cycle through the right forearm. Each infusion was followed by a 20-cc 0.9% saline flush. The patient developed nausea, vomiting, diarrhea, and hyperpigmentation tracing the path of infusion on the right arm as well as a slight darkness on the left arm that were noted by medical staff. At that time, cisplatin was discontinued from the chemotherapeutic regimen.
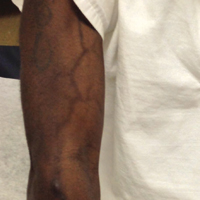
A port-a-cath was inserted into the patient’s right upper chest 4 weeks later and was used for subsequent infusions. Carboplatin 450 mg was initiated with pemetrexed thereafter. The patient was seen in the dermatology clinic 3 weeks after the insertion of the port-a-cath for evaluation of diffuse tinea versicolor of the trunk. Further examination of the arms revealed asymptomatic serpiginous hyperpigmentation overlying the superficial venous network tracing from the prior intravenous access points in the bilateral forearms to the upper arms (Figure). There was no evidence of extravasation or phlebitis prior to the hyperpigmentation. The patient was continued on pemetrexed and was subsequently lost to follow-up.
Cisplatin was the first member of the platinum-based chemotherapeutic agent class and is now one of the most potent and widely used in the treatment of solid malignancies. The cytotoxic mode of action is primarily mediated through interaction with DNA to form intrastrand cross-link adducts leading to aberrant mitosis and culminating in the activation of apoptosis. A variety of dermatologic complications have been reported with cisplatin chemotherapy including melanonychia, oral mucosal hyperpigmentation, hypersensitivity reactions, extravasation,4 Raynaud phenomenon, and flushing.5

Two cases of SSH have been reported following combination chemotherapy with cisplatin included in the regimen. A 61-year-old man with inoperable esophageal squamous cell carcinoma received cisplatin and 5-FU in addition to concurrent radiotherapy.6 After worsening renal function, cisplatin promptly was replaced with leucovorin. The patient developed SSH after the eighth infusion of 5-FU–leucovorin delivered through a peripheral catheter over a 24-hour period. The cutaneous side effect was attributed to the use of intravenous 5-FU.6 The second case involved a 48-year-old woman diagnosed with Paget disease of the breast who received adjuvant therapy with 12 courses of once-daily 5-FU and docetaxel for 5 years as well as 2 courses of vinorelbine and 1 course of cisplatin and etoposide for lung metastases.7 Serpentine supravenous hyperpigmentation lesions slowly developed over approximately 6 months. Based on the literature, the authors speculated that 5-FU and vinorelbine were most likely to be responsible. They noted, however, the inability to clarify the relationship between the onset of skin lesions and the time course of the chemotherapy.7 Although these cases do not directly implicate cisplatin as the cause of SSH, the possibility of a delayed reaction or augmentation of another drug’s effect cannot be excluded.
Pemetrexed, on the other hand, has not been associated with SSH. Several cutaneous adverse reactions have been reported, including acute generalized exanthematous pustulosis, alopecia, pityriasis lichenoides, radiation recall dermatitis, toxic epidermal necrolysis, and urticarial vasculitis.8 Three cases of pemetrexed-induced skin hyperpigmentation including the palms of the hands and soles of the feet as well as diffuse hyperpigmentation sparing only the palms and soles have been reported.8-10
Similar cases of SSH have demonstrated histopathologic findings with increased basal melanin synthesis and occasional melanophages in the papillary dermis without inflammatory changes.7,11 Although the unique serpentine pattern of hyperpigmentation is instantly recognizable, clinical differential diagnosis may include thrombophlebitis, cutis marmorata, erythema ab igne, livedo reticularis, and lichen planus.2,12
The exact mechanism of SSH has not been conclusively elucidated. Several studies postulate that direct cytotoxic damage causes loss of endothelial integrity permitting the extravasation of the agent to the overlying epidermis and interfering with melanogenesis.2,6,11 Other hypotheses include direct stimulation of melanocytes, depletion of reduced thioredoxin leading to tyrosinase stimulation, hyperthermia-related changes including reduced cytokine production and/or increased expression of melanocyte-stimulating hormone receptor, subclinical phlebitis leading to postinflammatory hyperpigmentation, or hyperpigmentation secondary to increased blood flow in certain areas and therefore increased drug deposition.12,13
Currently, there is no specific therapy recommended for SSH and the pigment may persist anywhere from a few months to more than a year after completing chemotherapy.2,7 Although discontinuing the offending agent would certainly prevent further development, due to the benign nature of the reaction, modifying therapy based on cutaneous findings alone is not recommended.12 Several authors have suggested avoiding peripheral infusions of chemotherapeutic agents known to cause SSH or have recommended using a permanent central venous catheter.6,7 Another option, which needs further investigation, is the administration of an abundant flush following chemotherapy. This technique was described in a case report of a 47-year-old man who developed persistent SSH in the right forearm following docetaxel injection.13 Copious venous washing with 1000 mL of isotonic saline solution following the second infusion in the unaffected arm prevented discoloration. The lack of subsequent reaction may support the theory that direct toxic effect on the vascular endothelium results in hyperpigmentation of the supravenous skin.13
Serpentine supravenous hyperpigmentation is an uncommon cutaneous reaction secondary to antineoplastic therapies. Given the widespread use of chemotherapeutic regimens, dermatologists should be aware of the reaction. Additional studies are warranted to better elucidate the pathogenesis and investigate how infusion techniques might aid in the prevention of skin discoloration. Although this side effect originally was described in relation to 5-FU, subsequent observations have included other chemotherapeutic agents. In light of the findings presented in this report, cisplatin and pemetrexed should be considered on the list of offending agents. Ultimately, patients should be reassured that the lesions are benign, self-limiting, and gradually resolve on their own in most cases.12
- Hrushesky WJ. Letter: serpentine supravenous fluorouracil hyperpigmentation. JAMA. 1976;236:138.
- Ghosh SK, Bandyopadhyay D, Ghoshal L, et al. Letter: docetaxel-induced supravenous serpentine dermatitis. Dermatol Online J. 2011;17:16.
- Pujol RM, Rocamora V, Lopez-Pousa A, et al. Persistent supravenous erythematous eruption: a rare local complication of intravenous 5-fluorouracil therapy. J Am Acad Dermatol. 1998;39:839-842.
- Kufe DW, Pollock RE, Weichsebaum RR, et al, eds. Holland-Frei Cancer Medicine. 6th ed. Hamilton, Ontario, Canada: BC Decker Inc; 2000.
- Mann MW, Berk DR, Popkin DL, et al. Handbook of Dermatology: A Practical Manual. Hoboken, NJ: Wiley-Blackwell; 2009.
- Chan CC, Lin SJ. Serpentine supravenous hyperpigmentation. N Engl J Med. 2010;29:363.
- Ouyang Y-H, Chu C-Y, Hu S-L. Linear hyperpigmentation of the left hand following chemotherapy. Dermatol Sinica. 2004;22:262-263.
- Piérard-Franchimont C, Quatresooz P, Reginster MA, et al. Revisiting cutaneous adverse reactions to pemetrexed. Oncol Lett. 2011;2:769-772.
- Buchinger K, Stahel R, Niggemeier V, et al. Pemetrexed-induced neutropenic enteritis and severe cutaneous hyperpigmentation in a patient with malignant pleural mesothelioma. Lung Cancer. 2013;80:347-349.
- Schallier D, Decoster L, De Greve J. Pemetrexed-induced hyperpigmentation of the skin. Anticancer Res. 2011;31:1753-1755.
- Rao R, Balachandran C. Serpentine supravenous pigmentation. a rare vasculocutaneous effect induced by systemic 5-fluoruracil. Indian J Dermatol Venereol Leprol. 2010;76:714-715.
- Geddes ER, Cohen PR. Antineoplastic agent-associated serpentine supravenous hyperpigmentation: superficial venous system hyperpigmentation following intravenous chemotherapy. South Med J. 2010;103:231-235.
- Ayodogan I, Kavak A, Parlak AH, et al. Persistent serpentine supravenous hyperpigmented eruption associated with docetaxel. J Eur Acad Dermatol Venereol. 2005;19:345-347.
To the Editor:
Serpentine supravenous hyperpigmentation (SSH) is a rare phenomenon characterized by linear hyperpigmentation of the skin overlying veins secondary to intravenous antineoplastic therapy. The term was first suggested by Hrushesky1 in 1976 as an uncommon side effect of administering intravenous 5-fluorouracil (5-FU). Although 5-FU is the most frequent offending agent, cases involving treatment with actinomycin, cyclophosphamide, docetaxel, fotemustine, nitrogen mustard, nitrosoureas, taxanes, and triazinate, as well as various combinations of chemotherapeutic agents, also have been observed.2,3 We present the case of SSH following a cisplatin and pemetrexed chemotherapy regimen.
A 52-year-old man with newly diagnosed inoperable adenocarcinoma in the left upper lung lobe received 2 cycles of treatment with cisplatin 138 mg and pemetrexed 920 mg 21 days apart. The first cycle of chemotherapy was delivered intravenously through the left forearm and the second cycle through the right forearm. Each infusion was followed by a 20-cc 0.9% saline flush. The patient developed nausea, vomiting, diarrhea, and hyperpigmentation tracing the path of infusion on the right arm as well as a slight darkness on the left arm that were noted by medical staff. At that time, cisplatin was discontinued from the chemotherapeutic regimen.
A port-a-cath was inserted into the patient’s right upper chest 4 weeks later and was used for subsequent infusions. Carboplatin 450 mg was initiated with pemetrexed thereafter. The patient was seen in the dermatology clinic 3 weeks after the insertion of the port-a-cath for evaluation of diffuse tinea versicolor of the trunk. Further examination of the arms revealed asymptomatic serpiginous hyperpigmentation overlying the superficial venous network tracing from the prior intravenous access points in the bilateral forearms to the upper arms (Figure). There was no evidence of extravasation or phlebitis prior to the hyperpigmentation. The patient was continued on pemetrexed and was subsequently lost to follow-up.
Cisplatin was the first member of the platinum-based chemotherapeutic agent class and is now one of the most potent and widely used in the treatment of solid malignancies. The cytotoxic mode of action is primarily mediated through interaction with DNA to form intrastrand cross-link adducts leading to aberrant mitosis and culminating in the activation of apoptosis. A variety of dermatologic complications have been reported with cisplatin chemotherapy including melanonychia, oral mucosal hyperpigmentation, hypersensitivity reactions, extravasation,4 Raynaud phenomenon, and flushing.5

Two cases of SSH have been reported following combination chemotherapy with cisplatin included in the regimen. A 61-year-old man with inoperable esophageal squamous cell carcinoma received cisplatin and 5-FU in addition to concurrent radiotherapy.6 After worsening renal function, cisplatin promptly was replaced with leucovorin. The patient developed SSH after the eighth infusion of 5-FU–leucovorin delivered through a peripheral catheter over a 24-hour period. The cutaneous side effect was attributed to the use of intravenous 5-FU.6 The second case involved a 48-year-old woman diagnosed with Paget disease of the breast who received adjuvant therapy with 12 courses of once-daily 5-FU and docetaxel for 5 years as well as 2 courses of vinorelbine and 1 course of cisplatin and etoposide for lung metastases.7 Serpentine supravenous hyperpigmentation lesions slowly developed over approximately 6 months. Based on the literature, the authors speculated that 5-FU and vinorelbine were most likely to be responsible. They noted, however, the inability to clarify the relationship between the onset of skin lesions and the time course of the chemotherapy.7 Although these cases do not directly implicate cisplatin as the cause of SSH, the possibility of a delayed reaction or augmentation of another drug’s effect cannot be excluded.
Pemetrexed, on the other hand, has not been associated with SSH. Several cutaneous adverse reactions have been reported, including acute generalized exanthematous pustulosis, alopecia, pityriasis lichenoides, radiation recall dermatitis, toxic epidermal necrolysis, and urticarial vasculitis.8 Three cases of pemetrexed-induced skin hyperpigmentation including the palms of the hands and soles of the feet as well as diffuse hyperpigmentation sparing only the palms and soles have been reported.8-10
Similar cases of SSH have demonstrated histopathologic findings with increased basal melanin synthesis and occasional melanophages in the papillary dermis without inflammatory changes.7,11 Although the unique serpentine pattern of hyperpigmentation is instantly recognizable, clinical differential diagnosis may include thrombophlebitis, cutis marmorata, erythema ab igne, livedo reticularis, and lichen planus.2,12
The exact mechanism of SSH has not been conclusively elucidated. Several studies postulate that direct cytotoxic damage causes loss of endothelial integrity permitting the extravasation of the agent to the overlying epidermis and interfering with melanogenesis.2,6,11 Other hypotheses include direct stimulation of melanocytes, depletion of reduced thioredoxin leading to tyrosinase stimulation, hyperthermia-related changes including reduced cytokine production and/or increased expression of melanocyte-stimulating hormone receptor, subclinical phlebitis leading to postinflammatory hyperpigmentation, or hyperpigmentation secondary to increased blood flow in certain areas and therefore increased drug deposition.12,13
Currently, there is no specific therapy recommended for SSH and the pigment may persist anywhere from a few months to more than a year after completing chemotherapy.2,7 Although discontinuing the offending agent would certainly prevent further development, due to the benign nature of the reaction, modifying therapy based on cutaneous findings alone is not recommended.12 Several authors have suggested avoiding peripheral infusions of chemotherapeutic agents known to cause SSH or have recommended using a permanent central venous catheter.6,7 Another option, which needs further investigation, is the administration of an abundant flush following chemotherapy. This technique was described in a case report of a 47-year-old man who developed persistent SSH in the right forearm following docetaxel injection.13 Copious venous washing with 1000 mL of isotonic saline solution following the second infusion in the unaffected arm prevented discoloration. The lack of subsequent reaction may support the theory that direct toxic effect on the vascular endothelium results in hyperpigmentation of the supravenous skin.13
Serpentine supravenous hyperpigmentation is an uncommon cutaneous reaction secondary to antineoplastic therapies. Given the widespread use of chemotherapeutic regimens, dermatologists should be aware of the reaction. Additional studies are warranted to better elucidate the pathogenesis and investigate how infusion techniques might aid in the prevention of skin discoloration. Although this side effect originally was described in relation to 5-FU, subsequent observations have included other chemotherapeutic agents. In light of the findings presented in this report, cisplatin and pemetrexed should be considered on the list of offending agents. Ultimately, patients should be reassured that the lesions are benign, self-limiting, and gradually resolve on their own in most cases.12
To the Editor:
Serpentine supravenous hyperpigmentation (SSH) is a rare phenomenon characterized by linear hyperpigmentation of the skin overlying veins secondary to intravenous antineoplastic therapy. The term was first suggested by Hrushesky1 in 1976 as an uncommon side effect of administering intravenous 5-fluorouracil (5-FU). Although 5-FU is the most frequent offending agent, cases involving treatment with actinomycin, cyclophosphamide, docetaxel, fotemustine, nitrogen mustard, nitrosoureas, taxanes, and triazinate, as well as various combinations of chemotherapeutic agents, also have been observed.2,3 We present the case of SSH following a cisplatin and pemetrexed chemotherapy regimen.
A 52-year-old man with newly diagnosed inoperable adenocarcinoma in the left upper lung lobe received 2 cycles of treatment with cisplatin 138 mg and pemetrexed 920 mg 21 days apart. The first cycle of chemotherapy was delivered intravenously through the left forearm and the second cycle through the right forearm. Each infusion was followed by a 20-cc 0.9% saline flush. The patient developed nausea, vomiting, diarrhea, and hyperpigmentation tracing the path of infusion on the right arm as well as a slight darkness on the left arm that were noted by medical staff. At that time, cisplatin was discontinued from the chemotherapeutic regimen.
A port-a-cath was inserted into the patient’s right upper chest 4 weeks later and was used for subsequent infusions. Carboplatin 450 mg was initiated with pemetrexed thereafter. The patient was seen in the dermatology clinic 3 weeks after the insertion of the port-a-cath for evaluation of diffuse tinea versicolor of the trunk. Further examination of the arms revealed asymptomatic serpiginous hyperpigmentation overlying the superficial venous network tracing from the prior intravenous access points in the bilateral forearms to the upper arms (Figure). There was no evidence of extravasation or phlebitis prior to the hyperpigmentation. The patient was continued on pemetrexed and was subsequently lost to follow-up.
Cisplatin was the first member of the platinum-based chemotherapeutic agent class and is now one of the most potent and widely used in the treatment of solid malignancies. The cytotoxic mode of action is primarily mediated through interaction with DNA to form intrastrand cross-link adducts leading to aberrant mitosis and culminating in the activation of apoptosis. A variety of dermatologic complications have been reported with cisplatin chemotherapy including melanonychia, oral mucosal hyperpigmentation, hypersensitivity reactions, extravasation,4 Raynaud phenomenon, and flushing.5

Two cases of SSH have been reported following combination chemotherapy with cisplatin included in the regimen. A 61-year-old man with inoperable esophageal squamous cell carcinoma received cisplatin and 5-FU in addition to concurrent radiotherapy.6 After worsening renal function, cisplatin promptly was replaced with leucovorin. The patient developed SSH after the eighth infusion of 5-FU–leucovorin delivered through a peripheral catheter over a 24-hour period. The cutaneous side effect was attributed to the use of intravenous 5-FU.6 The second case involved a 48-year-old woman diagnosed with Paget disease of the breast who received adjuvant therapy with 12 courses of once-daily 5-FU and docetaxel for 5 years as well as 2 courses of vinorelbine and 1 course of cisplatin and etoposide for lung metastases.7 Serpentine supravenous hyperpigmentation lesions slowly developed over approximately 6 months. Based on the literature, the authors speculated that 5-FU and vinorelbine were most likely to be responsible. They noted, however, the inability to clarify the relationship between the onset of skin lesions and the time course of the chemotherapy.7 Although these cases do not directly implicate cisplatin as the cause of SSH, the possibility of a delayed reaction or augmentation of another drug’s effect cannot be excluded.
Pemetrexed, on the other hand, has not been associated with SSH. Several cutaneous adverse reactions have been reported, including acute generalized exanthematous pustulosis, alopecia, pityriasis lichenoides, radiation recall dermatitis, toxic epidermal necrolysis, and urticarial vasculitis.8 Three cases of pemetrexed-induced skin hyperpigmentation including the palms of the hands and soles of the feet as well as diffuse hyperpigmentation sparing only the palms and soles have been reported.8-10
Similar cases of SSH have demonstrated histopathologic findings with increased basal melanin synthesis and occasional melanophages in the papillary dermis without inflammatory changes.7,11 Although the unique serpentine pattern of hyperpigmentation is instantly recognizable, clinical differential diagnosis may include thrombophlebitis, cutis marmorata, erythema ab igne, livedo reticularis, and lichen planus.2,12
The exact mechanism of SSH has not been conclusively elucidated. Several studies postulate that direct cytotoxic damage causes loss of endothelial integrity permitting the extravasation of the agent to the overlying epidermis and interfering with melanogenesis.2,6,11 Other hypotheses include direct stimulation of melanocytes, depletion of reduced thioredoxin leading to tyrosinase stimulation, hyperthermia-related changes including reduced cytokine production and/or increased expression of melanocyte-stimulating hormone receptor, subclinical phlebitis leading to postinflammatory hyperpigmentation, or hyperpigmentation secondary to increased blood flow in certain areas and therefore increased drug deposition.12,13
Currently, there is no specific therapy recommended for SSH and the pigment may persist anywhere from a few months to more than a year after completing chemotherapy.2,7 Although discontinuing the offending agent would certainly prevent further development, due to the benign nature of the reaction, modifying therapy based on cutaneous findings alone is not recommended.12 Several authors have suggested avoiding peripheral infusions of chemotherapeutic agents known to cause SSH or have recommended using a permanent central venous catheter.6,7 Another option, which needs further investigation, is the administration of an abundant flush following chemotherapy. This technique was described in a case report of a 47-year-old man who developed persistent SSH in the right forearm following docetaxel injection.13 Copious venous washing with 1000 mL of isotonic saline solution following the second infusion in the unaffected arm prevented discoloration. The lack of subsequent reaction may support the theory that direct toxic effect on the vascular endothelium results in hyperpigmentation of the supravenous skin.13
Serpentine supravenous hyperpigmentation is an uncommon cutaneous reaction secondary to antineoplastic therapies. Given the widespread use of chemotherapeutic regimens, dermatologists should be aware of the reaction. Additional studies are warranted to better elucidate the pathogenesis and investigate how infusion techniques might aid in the prevention of skin discoloration. Although this side effect originally was described in relation to 5-FU, subsequent observations have included other chemotherapeutic agents. In light of the findings presented in this report, cisplatin and pemetrexed should be considered on the list of offending agents. Ultimately, patients should be reassured that the lesions are benign, self-limiting, and gradually resolve on their own in most cases.12
- Hrushesky WJ. Letter: serpentine supravenous fluorouracil hyperpigmentation. JAMA. 1976;236:138.
- Ghosh SK, Bandyopadhyay D, Ghoshal L, et al. Letter: docetaxel-induced supravenous serpentine dermatitis. Dermatol Online J. 2011;17:16.
- Pujol RM, Rocamora V, Lopez-Pousa A, et al. Persistent supravenous erythematous eruption: a rare local complication of intravenous 5-fluorouracil therapy. J Am Acad Dermatol. 1998;39:839-842.
- Kufe DW, Pollock RE, Weichsebaum RR, et al, eds. Holland-Frei Cancer Medicine. 6th ed. Hamilton, Ontario, Canada: BC Decker Inc; 2000.
- Mann MW, Berk DR, Popkin DL, et al. Handbook of Dermatology: A Practical Manual. Hoboken, NJ: Wiley-Blackwell; 2009.
- Chan CC, Lin SJ. Serpentine supravenous hyperpigmentation. N Engl J Med. 2010;29:363.
- Ouyang Y-H, Chu C-Y, Hu S-L. Linear hyperpigmentation of the left hand following chemotherapy. Dermatol Sinica. 2004;22:262-263.
- Piérard-Franchimont C, Quatresooz P, Reginster MA, et al. Revisiting cutaneous adverse reactions to pemetrexed. Oncol Lett. 2011;2:769-772.
- Buchinger K, Stahel R, Niggemeier V, et al. Pemetrexed-induced neutropenic enteritis and severe cutaneous hyperpigmentation in a patient with malignant pleural mesothelioma. Lung Cancer. 2013;80:347-349.
- Schallier D, Decoster L, De Greve J. Pemetrexed-induced hyperpigmentation of the skin. Anticancer Res. 2011;31:1753-1755.
- Rao R, Balachandran C. Serpentine supravenous pigmentation. a rare vasculocutaneous effect induced by systemic 5-fluoruracil. Indian J Dermatol Venereol Leprol. 2010;76:714-715.
- Geddes ER, Cohen PR. Antineoplastic agent-associated serpentine supravenous hyperpigmentation: superficial venous system hyperpigmentation following intravenous chemotherapy. South Med J. 2010;103:231-235.
- Ayodogan I, Kavak A, Parlak AH, et al. Persistent serpentine supravenous hyperpigmented eruption associated with docetaxel. J Eur Acad Dermatol Venereol. 2005;19:345-347.
- Hrushesky WJ. Letter: serpentine supravenous fluorouracil hyperpigmentation. JAMA. 1976;236:138.
- Ghosh SK, Bandyopadhyay D, Ghoshal L, et al. Letter: docetaxel-induced supravenous serpentine dermatitis. Dermatol Online J. 2011;17:16.
- Pujol RM, Rocamora V, Lopez-Pousa A, et al. Persistent supravenous erythematous eruption: a rare local complication of intravenous 5-fluorouracil therapy. J Am Acad Dermatol. 1998;39:839-842.
- Kufe DW, Pollock RE, Weichsebaum RR, et al, eds. Holland-Frei Cancer Medicine. 6th ed. Hamilton, Ontario, Canada: BC Decker Inc; 2000.
- Mann MW, Berk DR, Popkin DL, et al. Handbook of Dermatology: A Practical Manual. Hoboken, NJ: Wiley-Blackwell; 2009.
- Chan CC, Lin SJ. Serpentine supravenous hyperpigmentation. N Engl J Med. 2010;29:363.
- Ouyang Y-H, Chu C-Y, Hu S-L. Linear hyperpigmentation of the left hand following chemotherapy. Dermatol Sinica. 2004;22:262-263.
- Piérard-Franchimont C, Quatresooz P, Reginster MA, et al. Revisiting cutaneous adverse reactions to pemetrexed. Oncol Lett. 2011;2:769-772.
- Buchinger K, Stahel R, Niggemeier V, et al. Pemetrexed-induced neutropenic enteritis and severe cutaneous hyperpigmentation in a patient with malignant pleural mesothelioma. Lung Cancer. 2013;80:347-349.
- Schallier D, Decoster L, De Greve J. Pemetrexed-induced hyperpigmentation of the skin. Anticancer Res. 2011;31:1753-1755.
- Rao R, Balachandran C. Serpentine supravenous pigmentation. a rare vasculocutaneous effect induced by systemic 5-fluoruracil. Indian J Dermatol Venereol Leprol. 2010;76:714-715.
- Geddes ER, Cohen PR. Antineoplastic agent-associated serpentine supravenous hyperpigmentation: superficial venous system hyperpigmentation following intravenous chemotherapy. South Med J. 2010;103:231-235.
- Ayodogan I, Kavak A, Parlak AH, et al. Persistent serpentine supravenous hyperpigmented eruption associated with docetaxel. J Eur Acad Dermatol Venereol. 2005;19:345-347.
Practice Points
- A variety of dermatologic complications have been reported with cisplatin chemotherapy, including serpentine supravenous hyperpigmentation (SSH); however, pemetrexed has not been associated with SSH.
- Although discontinuing the offending agent would certainly prevent further development, due to the benign nature of the reaction, modifying therapy based on cutaneous findings alone is not recommended.
Lasers may be effective for treating xanthelasma
SAN DIEGO – Laser treatments may be effective for xanthelasma palpebrarum lesions, based on a systematic review of existing studies, although the research is limited.
“The number of cases we looked at was relatively small, so you can’t come up with any definite conclusions,” said review coauthor Christopher J. Huerter, MD, head of the division of dermatology at Creighton University, Omaha. “But it’s promising since the lasers we examined all work with some efficacy, with the CO2 and Er:YAG [erbium:YAG] lasers probably having the best results.”
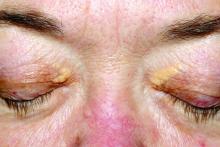
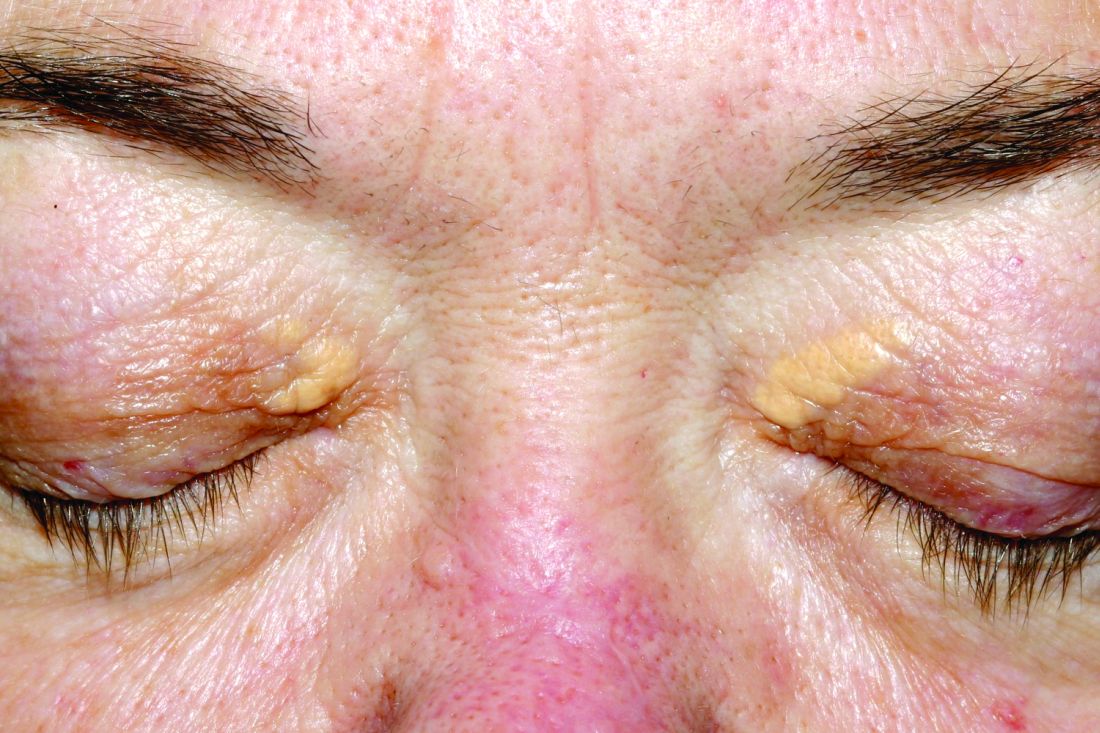
Xanthelasma lesions appear as small yellowish plaques on the eyelids. “About half the people who have it have some blood lipid abnormality,” Dr. Huerter said in an interview. “If a person has it, it’s worthwhile to do a cholesterol screen or a lipid profile.”
Treatment with trichloroacetic acid is one option, although it was more common before lasers began to be used. In addition, “surgical incision can be very effective,” he said, although the review notes that it can create undesirable scarring.
Researchers have studied laser treatment for xanthelasma for at least 30 years. Dr. Huerter and his colleagues examined 21 studies published since 1987, with the following lasers: CO2 laser (three studies), argon laser (one study), Er:YAG laser (four studies), ultrapulse CO2 laser (five studies), 1,450-nm diode laser (one study), pulsed dye laser (PDL, two studies), superpulsed or fractional CO2 laser (one study), and Q-switched neodymium:YAG laser (three studies). An additional study examined both argon and Er:YAG lasers.
The number of treated patients in the studies ranged from 1 to 50, and the number of treated lesions ranged from 1 to 76. (Patients often had more than one lesion.) “It would be nice to have bigger studies and bigger numbers,” Dr. Huerter said at the annual meeting of the American Society for Laser Medicine and Surgery.
Although the studies were limited by small cohorts, short follow-up, and lack of comparison groups, the findings did reveal signs of effectiveness: Clearance rates were 100% in CO2, argon, and PDL cases and about 85% with Er:YAG lasers. Clearance rates were lower with Nd:YAG (about 55%) and 1,450-nm diode (about 48%) lasers.
Edema was reported in all PDL cases and erythema in almost 20% of CO2 cases. Dyspigmentation was most common – at about 30% – in Er:YAG and 1,450-nm diode cases. Visible scars were reported in more than 5% of Er:YAG cases.
The review concluded that “sufficient evidence is available to suggest laser therapies to be a cosmetically excellent treatment option for xanthelasma , particularly applicable in patients who are not good candidates for surgical excision,” he said.
As for advice to dermatologists, Dr. Huerter pointed to the positive results for CO2 and Er:YAG lasers. He said PDL lasers could also be used. As for argon lasers, he noted that it’s not as likely for dermatologists to have them on hand, he said.
In regard to choosing which xanthelasma lesions to treat with laser, he said thicker ones may not be as amenable. “But if you do laser treatment and don’t get the results you want, you can always excise.”
No funding for the study was reported. Dr. Huerter reported no disclosures.
SAN DIEGO – Laser treatments may be effective for xanthelasma palpebrarum lesions, based on a systematic review of existing studies, although the research is limited.
“The number of cases we looked at was relatively small, so you can’t come up with any definite conclusions,” said review coauthor Christopher J. Huerter, MD, head of the division of dermatology at Creighton University, Omaha. “But it’s promising since the lasers we examined all work with some efficacy, with the CO2 and Er:YAG [erbium:YAG] lasers probably having the best results.”
Xanthelasma lesions appear as small yellowish plaques on the eyelids. “About half the people who have it have some blood lipid abnormality,” Dr. Huerter said in an interview. “If a person has it, it’s worthwhile to do a cholesterol screen or a lipid profile.”
Treatment with trichloroacetic acid is one option, although it was more common before lasers began to be used. In addition, “surgical incision can be very effective,” he said, although the review notes that it can create undesirable scarring.
Researchers have studied laser treatment for xanthelasma for at least 30 years. Dr. Huerter and his colleagues examined 21 studies published since 1987, with the following lasers: CO2 laser (three studies), argon laser (one study), Er:YAG laser (four studies), ultrapulse CO2 laser (five studies), 1,450-nm diode laser (one study), pulsed dye laser (PDL, two studies), superpulsed or fractional CO2 laser (one study), and Q-switched neodymium:YAG laser (three studies). An additional study examined both argon and Er:YAG lasers.
The number of treated patients in the studies ranged from 1 to 50, and the number of treated lesions ranged from 1 to 76. (Patients often had more than one lesion.) “It would be nice to have bigger studies and bigger numbers,” Dr. Huerter said at the annual meeting of the American Society for Laser Medicine and Surgery.
Although the studies were limited by small cohorts, short follow-up, and lack of comparison groups, the findings did reveal signs of effectiveness: Clearance rates were 100% in CO2, argon, and PDL cases and about 85% with Er:YAG lasers. Clearance rates were lower with Nd:YAG (about 55%) and 1,450-nm diode (about 48%) lasers.
Edema was reported in all PDL cases and erythema in almost 20% of CO2 cases. Dyspigmentation was most common – at about 30% – in Er:YAG and 1,450-nm diode cases. Visible scars were reported in more than 5% of Er:YAG cases.
The review concluded that “sufficient evidence is available to suggest laser therapies to be a cosmetically excellent treatment option for xanthelasma , particularly applicable in patients who are not good candidates for surgical excision,” he said.
As for advice to dermatologists, Dr. Huerter pointed to the positive results for CO2 and Er:YAG lasers. He said PDL lasers could also be used. As for argon lasers, he noted that it’s not as likely for dermatologists to have them on hand, he said.
In regard to choosing which xanthelasma lesions to treat with laser, he said thicker ones may not be as amenable. “But if you do laser treatment and don’t get the results you want, you can always excise.”
No funding for the study was reported. Dr. Huerter reported no disclosures.
SAN DIEGO – Laser treatments may be effective for xanthelasma palpebrarum lesions, based on a systematic review of existing studies, although the research is limited.
“The number of cases we looked at was relatively small, so you can’t come up with any definite conclusions,” said review coauthor Christopher J. Huerter, MD, head of the division of dermatology at Creighton University, Omaha. “But it’s promising since the lasers we examined all work with some efficacy, with the CO2 and Er:YAG [erbium:YAG] lasers probably having the best results.”
Xanthelasma lesions appear as small yellowish plaques on the eyelids. “About half the people who have it have some blood lipid abnormality,” Dr. Huerter said in an interview. “If a person has it, it’s worthwhile to do a cholesterol screen or a lipid profile.”
Treatment with trichloroacetic acid is one option, although it was more common before lasers began to be used. In addition, “surgical incision can be very effective,” he said, although the review notes that it can create undesirable scarring.
Researchers have studied laser treatment for xanthelasma for at least 30 years. Dr. Huerter and his colleagues examined 21 studies published since 1987, with the following lasers: CO2 laser (three studies), argon laser (one study), Er:YAG laser (four studies), ultrapulse CO2 laser (five studies), 1,450-nm diode laser (one study), pulsed dye laser (PDL, two studies), superpulsed or fractional CO2 laser (one study), and Q-switched neodymium:YAG laser (three studies). An additional study examined both argon and Er:YAG lasers.
The number of treated patients in the studies ranged from 1 to 50, and the number of treated lesions ranged from 1 to 76. (Patients often had more than one lesion.) “It would be nice to have bigger studies and bigger numbers,” Dr. Huerter said at the annual meeting of the American Society for Laser Medicine and Surgery.
Although the studies were limited by small cohorts, short follow-up, and lack of comparison groups, the findings did reveal signs of effectiveness: Clearance rates were 100% in CO2, argon, and PDL cases and about 85% with Er:YAG lasers. Clearance rates were lower with Nd:YAG (about 55%) and 1,450-nm diode (about 48%) lasers.
Edema was reported in all PDL cases and erythema in almost 20% of CO2 cases. Dyspigmentation was most common – at about 30% – in Er:YAG and 1,450-nm diode cases. Visible scars were reported in more than 5% of Er:YAG cases.
The review concluded that “sufficient evidence is available to suggest laser therapies to be a cosmetically excellent treatment option for xanthelasma , particularly applicable in patients who are not good candidates for surgical excision,” he said.
As for advice to dermatologists, Dr. Huerter pointed to the positive results for CO2 and Er:YAG lasers. He said PDL lasers could also be used. As for argon lasers, he noted that it’s not as likely for dermatologists to have them on hand, he said.
In regard to choosing which xanthelasma lesions to treat with laser, he said thicker ones may not be as amenable. “But if you do laser treatment and don’t get the results you want, you can always excise.”
No funding for the study was reported. Dr. Huerter reported no disclosures.
Key clinical point:
Major finding: Clearance rates of about 85%-100% were reported for xanthelasma treatment with CO2, argon, pulsed dye, and Er:YAG laser treatments.
Data source: A systematic review of 21 studies evaluating different laser treatments for xanthelasma.
Disclosures: No funding was reported. Dr. Huerter reported no disclosures.
Expanding Uses of Propranolol in Dermatology
Since the serendipitous discovery of expedited involution of infantile hemangiomas (IHs) with propranolol in 2008,1 current research has proliferated to discern the mechanism of action of beta-blockers in the care of IHs. Propranolol is a nonselective beta-blocker with a structure similar to catecholamines and thus competes for β-adrenergic receptors. Blocking β1-receptors is cardioselective, leading to decreased heart rate and myocardial contractility, while blocking β2-receptors leads to inhibition of smooth muscle relaxation and decreased glycogenolysis. The endothelial cells of IH express β2-adrenergic receptors; the mechanistic role of propranolol in these lesions is surmised to be due to vasoconstriction, decreased angiogenesis through inhibition of vascular endothelial growth factor, and subsequent endothelial cell apoptosis.2
After this breakthrough finding, a subsequent novel development was made when an ophthalmologist demonstrated that timolol, a topical beta-blocker, could be utilized to expedite IH involution and prevent ocular complications such as amblyopia secondary to the mass effect of the lesion. Guo and Ni3 prescribed the commercially available ophthalmologic solution of timolol maleate 0.5% for twice-daily use for 5 weeks. Remarkable reduction in the periorbital IH without rebound phenomenon was observed.3 A recent multicenter retrospective cohort of more than 700 patients with IH were treated with topical timolol with a 70% success rate, corresponding to 10% improvement from baseline; this study highlights the efficacy of timolol while confirming the safety of the medication.4
Systemic beta-blockers for IH have been used predominately for critical sites such as the nasal tip, lip, ear, perineum, and periocular area; ulcerated lesions or those that may be prone to leave a fibrofatty tissue residue after involution also have been targeted. Contraindications for use include premature infants younger than 5 weeks, infants weighing less than 2 kg, history of asthma or bronchospasm, heart rate less than 80 beats per minute, blood pressure less than 50/30 mm Hg, or hypersensitivity to the medication.5 Current guidelines for propranolol initiation vary; some dermatologists consult cardiology prior to initiation, while others perform routine vitals and an indication-driven electrocardiogram as needed based on family history of cardiac disease, maternal history of connective tissue disease, congenital heart block, or abnormal vital signs.
Given the demonstrated long-term safety of propranolol and the acceptable side-effect profile, the use of beta-blockers for IH has become increasingly mainstream. Three randomized controlled trials (RCTs) have evaluated the efficacy and minimal adverse effects of propranolol for IH. The first RCT evaluated 40 patients who received either placebo or propranolol 2 mg/kg daily (divided into 3 doses) for 6 months; IH growth stopped by week 4 in the treatment group and the largest volume difference in IH was seen at week 12.6 Léauté-Labrèze et al7 demonstrated that propranolol could be given earlier to patients and at higher doses; the treatment group included 7 patients at 3 mg/kg daily of propranolol for 15 days, followed by 15 additional days of 4 mg/kg daily of propranolol. A statistically significant (P=.004) decrease in IH volume, quantified by use of ultrasonography, was exhibited by the propranolol group.7 Lastly, the largest RCT (N=456) established the efficacy of propranolol 3 mg/kg daily for 6 months with a 60% successful treatment rate compared to 4% for patients receiving placebo.8
Given the efficacy of propranolol for IH, other investigators have experimented with nonselective beta-blockers for other dermatologic conditions. In addition to second-line use for flushing, hyperhidrosis, and adrenergic urticaria, the future of propranolol is expanding for vascular lesions in particular.9 Chow et al10 highlighted a case of progressive angiosarcoma of the scalp that responded to propranolol hydrochloride therapy at 40 mg 3 times daily with extensive regression; propranolol was given in addition to chemotherapy and radiation. The tumor was biopsied before and after propranolol therapy and exhibited a 34% decrease in the proliferative index (Ki-67).10 Interestingly, Chisholm et al11 evaluated the expression of β-adrenergic expression in 141 vascular lesions; endothelial cell expression of β2-adrenergic receptors was found positive in 100% of IHs, 67% of kaposiform hemangioendotheliomas, 41% of angiosarcomas, 50% of pyogenic granulomas, and 75% of Kaposi sarcomas, to name merely a few studied lesions.
These data have spurred physicians to further seek beta-blocker dermatologic use in specific patient populations. For example, Meseguer-Yebra et al12 employed timolol solution 0.5% twice daily for 12 weeks for 2 human immunodeficiency virus–negative patients with limited Kaposi sarcoma of the right thigh and foot; no clinical evidence of recurrence was seen at 20 months, and one of the patients had a subsequent biopsy performed with negative human herpesvirus 8 staining after therapy. In the pediatric arena, topical timolol has been used for both port-wine stains and pyogenic granulomas.13-15 Two lesions of pyogenic granulomas on the scalp of a child were treated with timolol ophthalmic solution 0.5% under occlusion for 4 weeks with resolution.15 Propranolol also has been utilized as adjunctive therapy for aggressive pediatric vascular lesions such as kaposiform hemangioendothelioma with promising results and additionally reducing the duration of therapy needed with vincristine.2
In summary, propranolol and timolol have made an indelible impression on the field of pediatric dermatology and have demonstrated a burgeoning role in the dermatologic arena. The use of nonselective beta-blockers for the management of vascular lesions can serve as adjunctive or monotherapy for certain patient populations. The relatively low adverse risk profile of propranolol makes it a versatile tool to use both systemically and topically. Although the authors of the study assessing the β2-adrenergic expression in vascular lesions admittedly stated that the positivity of the receptors does not necessarily correlate with therapeutic management, it is an interesting subject area with much potential in the future.11 This review serves to illuminate the expanding role of beta-blockers in dermatology.
- Léauté-Labrèze C, Dumas de la Roque E, Hubiche T, et al. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358:2649-2651.
- Hermans DJ, van Beynum IM, van der Vijver RJ, et al. Kaposiform hemangioendothelioma with Kasabach-Merritt syndrome: a new indication for propranolol treatment. J Pediatr Hematol Oncol. 2011;33:E171-E173.
- Guo S, Ni N. Topical treatment for capillary hemangioma of the eyelid using beta-blocker solution. Arch Ophthalmol. 2010;128:255-256.
- Püttgen K, Lucky A, Adams D, et al. Topical timolol maleate treatment of infantile hemangiomas. Pediatrics. 2016;138:3.
- Drolet BA, Frommelt PC, Chamlin SL, et al. Initiation and use of propranolol for infantile hemangioma: report of a consensus conference. Pediatrics. 2013;131:128-140.
- Hogeling M, Adams S, Wargon O. A randomized controlled trial of propranolol for infantile hemangiomas [published online July 25, 2011]. Pediatrics. 2011;128:E259-E266.
- Léauté-Labrèze C, Dumas de la Roque E, Nacka F, et al. Doubleblind randomized pilot trial evaluating the efficacy of oral propranolol on infantile haemangiomas in infants < 4 months of age. Br J Dermatol. 2013;169:181-183.
- Léauté-Labrèze C, Hoeger P, Mazereeuw-Hautier J, et al. A randomized, controlled trial of oral propranolol in infantile hemangioma. N Engl J Med. 2015;372:735-746.
- Shelley WB, Shelley ED. Adrenergic urticaria: a new form of stress induced hives. Lancet. 1985;2:1031-1033.
- Chow W, Amaya CN, Rains S, et al. Growth attenuation of cutaneous angiosarcoma with propranolol-mediated β-blockade. JAMA Dermatol. 2015;151:1226-1229.
- Chisholm KM, Chang KW, Truong MT, et al. β-adrenergic receptor expression in vascular tumors. Mod Pathol. 2012;25:1446-1451.
- Meseguer-Yebra C, Cardeñoso-Álvarez, ME, Bordel-Gómez MT, et al. Successful treatment of classic Kaposi sarcoma with topical timolol: report of two cases. Br J Dermatol. 2015;173:860-862.
- Passeron T, Maza A, Fontas E, et al. Treatment of port wine stains and pulsed dye laser and topical timolol: a multicenter randomized controlled trial. Br J Dermatol. 2014;170:1350-1353.
- Wine LL, Goff KL, Lam JM, et al. Treatment of pediatric pyogenic granulomas using β-adrenergic receptor antagonist. Pediatr Dermatol. 2014;31:203-207.
- Knöpfel N, Escudero-Góngora Mdel M, Bauzà A, et al. Timolol for the treatment of pyogenic granuloma (PG) in children. J Am Acad Dermatol. 2016;75:E105-E106.
Since the serendipitous discovery of expedited involution of infantile hemangiomas (IHs) with propranolol in 2008,1 current research has proliferated to discern the mechanism of action of beta-blockers in the care of IHs. Propranolol is a nonselective beta-blocker with a structure similar to catecholamines and thus competes for β-adrenergic receptors. Blocking β1-receptors is cardioselective, leading to decreased heart rate and myocardial contractility, while blocking β2-receptors leads to inhibition of smooth muscle relaxation and decreased glycogenolysis. The endothelial cells of IH express β2-adrenergic receptors; the mechanistic role of propranolol in these lesions is surmised to be due to vasoconstriction, decreased angiogenesis through inhibition of vascular endothelial growth factor, and subsequent endothelial cell apoptosis.2
After this breakthrough finding, a subsequent novel development was made when an ophthalmologist demonstrated that timolol, a topical beta-blocker, could be utilized to expedite IH involution and prevent ocular complications such as amblyopia secondary to the mass effect of the lesion. Guo and Ni3 prescribed the commercially available ophthalmologic solution of timolol maleate 0.5% for twice-daily use for 5 weeks. Remarkable reduction in the periorbital IH without rebound phenomenon was observed.3 A recent multicenter retrospective cohort of more than 700 patients with IH were treated with topical timolol with a 70% success rate, corresponding to 10% improvement from baseline; this study highlights the efficacy of timolol while confirming the safety of the medication.4
Systemic beta-blockers for IH have been used predominately for critical sites such as the nasal tip, lip, ear, perineum, and periocular area; ulcerated lesions or those that may be prone to leave a fibrofatty tissue residue after involution also have been targeted. Contraindications for use include premature infants younger than 5 weeks, infants weighing less than 2 kg, history of asthma or bronchospasm, heart rate less than 80 beats per minute, blood pressure less than 50/30 mm Hg, or hypersensitivity to the medication.5 Current guidelines for propranolol initiation vary; some dermatologists consult cardiology prior to initiation, while others perform routine vitals and an indication-driven electrocardiogram as needed based on family history of cardiac disease, maternal history of connective tissue disease, congenital heart block, or abnormal vital signs.
Given the demonstrated long-term safety of propranolol and the acceptable side-effect profile, the use of beta-blockers for IH has become increasingly mainstream. Three randomized controlled trials (RCTs) have evaluated the efficacy and minimal adverse effects of propranolol for IH. The first RCT evaluated 40 patients who received either placebo or propranolol 2 mg/kg daily (divided into 3 doses) for 6 months; IH growth stopped by week 4 in the treatment group and the largest volume difference in IH was seen at week 12.6 Léauté-Labrèze et al7 demonstrated that propranolol could be given earlier to patients and at higher doses; the treatment group included 7 patients at 3 mg/kg daily of propranolol for 15 days, followed by 15 additional days of 4 mg/kg daily of propranolol. A statistically significant (P=.004) decrease in IH volume, quantified by use of ultrasonography, was exhibited by the propranolol group.7 Lastly, the largest RCT (N=456) established the efficacy of propranolol 3 mg/kg daily for 6 months with a 60% successful treatment rate compared to 4% for patients receiving placebo.8
Given the efficacy of propranolol for IH, other investigators have experimented with nonselective beta-blockers for other dermatologic conditions. In addition to second-line use for flushing, hyperhidrosis, and adrenergic urticaria, the future of propranolol is expanding for vascular lesions in particular.9 Chow et al10 highlighted a case of progressive angiosarcoma of the scalp that responded to propranolol hydrochloride therapy at 40 mg 3 times daily with extensive regression; propranolol was given in addition to chemotherapy and radiation. The tumor was biopsied before and after propranolol therapy and exhibited a 34% decrease in the proliferative index (Ki-67).10 Interestingly, Chisholm et al11 evaluated the expression of β-adrenergic expression in 141 vascular lesions; endothelial cell expression of β2-adrenergic receptors was found positive in 100% of IHs, 67% of kaposiform hemangioendotheliomas, 41% of angiosarcomas, 50% of pyogenic granulomas, and 75% of Kaposi sarcomas, to name merely a few studied lesions.
These data have spurred physicians to further seek beta-blocker dermatologic use in specific patient populations. For example, Meseguer-Yebra et al12 employed timolol solution 0.5% twice daily for 12 weeks for 2 human immunodeficiency virus–negative patients with limited Kaposi sarcoma of the right thigh and foot; no clinical evidence of recurrence was seen at 20 months, and one of the patients had a subsequent biopsy performed with negative human herpesvirus 8 staining after therapy. In the pediatric arena, topical timolol has been used for both port-wine stains and pyogenic granulomas.13-15 Two lesions of pyogenic granulomas on the scalp of a child were treated with timolol ophthalmic solution 0.5% under occlusion for 4 weeks with resolution.15 Propranolol also has been utilized as adjunctive therapy for aggressive pediatric vascular lesions such as kaposiform hemangioendothelioma with promising results and additionally reducing the duration of therapy needed with vincristine.2
In summary, propranolol and timolol have made an indelible impression on the field of pediatric dermatology and have demonstrated a burgeoning role in the dermatologic arena. The use of nonselective beta-blockers for the management of vascular lesions can serve as adjunctive or monotherapy for certain patient populations. The relatively low adverse risk profile of propranolol makes it a versatile tool to use both systemically and topically. Although the authors of the study assessing the β2-adrenergic expression in vascular lesions admittedly stated that the positivity of the receptors does not necessarily correlate with therapeutic management, it is an interesting subject area with much potential in the future.11 This review serves to illuminate the expanding role of beta-blockers in dermatology.
Since the serendipitous discovery of expedited involution of infantile hemangiomas (IHs) with propranolol in 2008,1 current research has proliferated to discern the mechanism of action of beta-blockers in the care of IHs. Propranolol is a nonselective beta-blocker with a structure similar to catecholamines and thus competes for β-adrenergic receptors. Blocking β1-receptors is cardioselective, leading to decreased heart rate and myocardial contractility, while blocking β2-receptors leads to inhibition of smooth muscle relaxation and decreased glycogenolysis. The endothelial cells of IH express β2-adrenergic receptors; the mechanistic role of propranolol in these lesions is surmised to be due to vasoconstriction, decreased angiogenesis through inhibition of vascular endothelial growth factor, and subsequent endothelial cell apoptosis.2
After this breakthrough finding, a subsequent novel development was made when an ophthalmologist demonstrated that timolol, a topical beta-blocker, could be utilized to expedite IH involution and prevent ocular complications such as amblyopia secondary to the mass effect of the lesion. Guo and Ni3 prescribed the commercially available ophthalmologic solution of timolol maleate 0.5% for twice-daily use for 5 weeks. Remarkable reduction in the periorbital IH without rebound phenomenon was observed.3 A recent multicenter retrospective cohort of more than 700 patients with IH were treated with topical timolol with a 70% success rate, corresponding to 10% improvement from baseline; this study highlights the efficacy of timolol while confirming the safety of the medication.4
Systemic beta-blockers for IH have been used predominately for critical sites such as the nasal tip, lip, ear, perineum, and periocular area; ulcerated lesions or those that may be prone to leave a fibrofatty tissue residue after involution also have been targeted. Contraindications for use include premature infants younger than 5 weeks, infants weighing less than 2 kg, history of asthma or bronchospasm, heart rate less than 80 beats per minute, blood pressure less than 50/30 mm Hg, or hypersensitivity to the medication.5 Current guidelines for propranolol initiation vary; some dermatologists consult cardiology prior to initiation, while others perform routine vitals and an indication-driven electrocardiogram as needed based on family history of cardiac disease, maternal history of connective tissue disease, congenital heart block, or abnormal vital signs.
Given the demonstrated long-term safety of propranolol and the acceptable side-effect profile, the use of beta-blockers for IH has become increasingly mainstream. Three randomized controlled trials (RCTs) have evaluated the efficacy and minimal adverse effects of propranolol for IH. The first RCT evaluated 40 patients who received either placebo or propranolol 2 mg/kg daily (divided into 3 doses) for 6 months; IH growth stopped by week 4 in the treatment group and the largest volume difference in IH was seen at week 12.6 Léauté-Labrèze et al7 demonstrated that propranolol could be given earlier to patients and at higher doses; the treatment group included 7 patients at 3 mg/kg daily of propranolol for 15 days, followed by 15 additional days of 4 mg/kg daily of propranolol. A statistically significant (P=.004) decrease in IH volume, quantified by use of ultrasonography, was exhibited by the propranolol group.7 Lastly, the largest RCT (N=456) established the efficacy of propranolol 3 mg/kg daily for 6 months with a 60% successful treatment rate compared to 4% for patients receiving placebo.8
Given the efficacy of propranolol for IH, other investigators have experimented with nonselective beta-blockers for other dermatologic conditions. In addition to second-line use for flushing, hyperhidrosis, and adrenergic urticaria, the future of propranolol is expanding for vascular lesions in particular.9 Chow et al10 highlighted a case of progressive angiosarcoma of the scalp that responded to propranolol hydrochloride therapy at 40 mg 3 times daily with extensive regression; propranolol was given in addition to chemotherapy and radiation. The tumor was biopsied before and after propranolol therapy and exhibited a 34% decrease in the proliferative index (Ki-67).10 Interestingly, Chisholm et al11 evaluated the expression of β-adrenergic expression in 141 vascular lesions; endothelial cell expression of β2-adrenergic receptors was found positive in 100% of IHs, 67% of kaposiform hemangioendotheliomas, 41% of angiosarcomas, 50% of pyogenic granulomas, and 75% of Kaposi sarcomas, to name merely a few studied lesions.
These data have spurred physicians to further seek beta-blocker dermatologic use in specific patient populations. For example, Meseguer-Yebra et al12 employed timolol solution 0.5% twice daily for 12 weeks for 2 human immunodeficiency virus–negative patients with limited Kaposi sarcoma of the right thigh and foot; no clinical evidence of recurrence was seen at 20 months, and one of the patients had a subsequent biopsy performed with negative human herpesvirus 8 staining after therapy. In the pediatric arena, topical timolol has been used for both port-wine stains and pyogenic granulomas.13-15 Two lesions of pyogenic granulomas on the scalp of a child were treated with timolol ophthalmic solution 0.5% under occlusion for 4 weeks with resolution.15 Propranolol also has been utilized as adjunctive therapy for aggressive pediatric vascular lesions such as kaposiform hemangioendothelioma with promising results and additionally reducing the duration of therapy needed with vincristine.2
In summary, propranolol and timolol have made an indelible impression on the field of pediatric dermatology and have demonstrated a burgeoning role in the dermatologic arena. The use of nonselective beta-blockers for the management of vascular lesions can serve as adjunctive or monotherapy for certain patient populations. The relatively low adverse risk profile of propranolol makes it a versatile tool to use both systemically and topically. Although the authors of the study assessing the β2-adrenergic expression in vascular lesions admittedly stated that the positivity of the receptors does not necessarily correlate with therapeutic management, it is an interesting subject area with much potential in the future.11 This review serves to illuminate the expanding role of beta-blockers in dermatology.
- Léauté-Labrèze C, Dumas de la Roque E, Hubiche T, et al. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358:2649-2651.
- Hermans DJ, van Beynum IM, van der Vijver RJ, et al. Kaposiform hemangioendothelioma with Kasabach-Merritt syndrome: a new indication for propranolol treatment. J Pediatr Hematol Oncol. 2011;33:E171-E173.
- Guo S, Ni N. Topical treatment for capillary hemangioma of the eyelid using beta-blocker solution. Arch Ophthalmol. 2010;128:255-256.
- Püttgen K, Lucky A, Adams D, et al. Topical timolol maleate treatment of infantile hemangiomas. Pediatrics. 2016;138:3.
- Drolet BA, Frommelt PC, Chamlin SL, et al. Initiation and use of propranolol for infantile hemangioma: report of a consensus conference. Pediatrics. 2013;131:128-140.
- Hogeling M, Adams S, Wargon O. A randomized controlled trial of propranolol for infantile hemangiomas [published online July 25, 2011]. Pediatrics. 2011;128:E259-E266.
- Léauté-Labrèze C, Dumas de la Roque E, Nacka F, et al. Doubleblind randomized pilot trial evaluating the efficacy of oral propranolol on infantile haemangiomas in infants < 4 months of age. Br J Dermatol. 2013;169:181-183.
- Léauté-Labrèze C, Hoeger P, Mazereeuw-Hautier J, et al. A randomized, controlled trial of oral propranolol in infantile hemangioma. N Engl J Med. 2015;372:735-746.
- Shelley WB, Shelley ED. Adrenergic urticaria: a new form of stress induced hives. Lancet. 1985;2:1031-1033.
- Chow W, Amaya CN, Rains S, et al. Growth attenuation of cutaneous angiosarcoma with propranolol-mediated β-blockade. JAMA Dermatol. 2015;151:1226-1229.
- Chisholm KM, Chang KW, Truong MT, et al. β-adrenergic receptor expression in vascular tumors. Mod Pathol. 2012;25:1446-1451.
- Meseguer-Yebra C, Cardeñoso-Álvarez, ME, Bordel-Gómez MT, et al. Successful treatment of classic Kaposi sarcoma with topical timolol: report of two cases. Br J Dermatol. 2015;173:860-862.
- Passeron T, Maza A, Fontas E, et al. Treatment of port wine stains and pulsed dye laser and topical timolol: a multicenter randomized controlled trial. Br J Dermatol. 2014;170:1350-1353.
- Wine LL, Goff KL, Lam JM, et al. Treatment of pediatric pyogenic granulomas using β-adrenergic receptor antagonist. Pediatr Dermatol. 2014;31:203-207.
- Knöpfel N, Escudero-Góngora Mdel M, Bauzà A, et al. Timolol for the treatment of pyogenic granuloma (PG) in children. J Am Acad Dermatol. 2016;75:E105-E106.
- Léauté-Labrèze C, Dumas de la Roque E, Hubiche T, et al. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358:2649-2651.
- Hermans DJ, van Beynum IM, van der Vijver RJ, et al. Kaposiform hemangioendothelioma with Kasabach-Merritt syndrome: a new indication for propranolol treatment. J Pediatr Hematol Oncol. 2011;33:E171-E173.
- Guo S, Ni N. Topical treatment for capillary hemangioma of the eyelid using beta-blocker solution. Arch Ophthalmol. 2010;128:255-256.
- Püttgen K, Lucky A, Adams D, et al. Topical timolol maleate treatment of infantile hemangiomas. Pediatrics. 2016;138:3.
- Drolet BA, Frommelt PC, Chamlin SL, et al. Initiation and use of propranolol for infantile hemangioma: report of a consensus conference. Pediatrics. 2013;131:128-140.
- Hogeling M, Adams S, Wargon O. A randomized controlled trial of propranolol for infantile hemangiomas [published online July 25, 2011]. Pediatrics. 2011;128:E259-E266.
- Léauté-Labrèze C, Dumas de la Roque E, Nacka F, et al. Doubleblind randomized pilot trial evaluating the efficacy of oral propranolol on infantile haemangiomas in infants < 4 months of age. Br J Dermatol. 2013;169:181-183.
- Léauté-Labrèze C, Hoeger P, Mazereeuw-Hautier J, et al. A randomized, controlled trial of oral propranolol in infantile hemangioma. N Engl J Med. 2015;372:735-746.
- Shelley WB, Shelley ED. Adrenergic urticaria: a new form of stress induced hives. Lancet. 1985;2:1031-1033.
- Chow W, Amaya CN, Rains S, et al. Growth attenuation of cutaneous angiosarcoma with propranolol-mediated β-blockade. JAMA Dermatol. 2015;151:1226-1229.
- Chisholm KM, Chang KW, Truong MT, et al. β-adrenergic receptor expression in vascular tumors. Mod Pathol. 2012;25:1446-1451.
- Meseguer-Yebra C, Cardeñoso-Álvarez, ME, Bordel-Gómez MT, et al. Successful treatment of classic Kaposi sarcoma with topical timolol: report of two cases. Br J Dermatol. 2015;173:860-862.
- Passeron T, Maza A, Fontas E, et al. Treatment of port wine stains and pulsed dye laser and topical timolol: a multicenter randomized controlled trial. Br J Dermatol. 2014;170:1350-1353.
- Wine LL, Goff KL, Lam JM, et al. Treatment of pediatric pyogenic granulomas using β-adrenergic receptor antagonist. Pediatr Dermatol. 2014;31:203-207.
- Knöpfel N, Escudero-Góngora Mdel M, Bauzà A, et al. Timolol for the treatment of pyogenic granuloma (PG) in children. J Am Acad Dermatol. 2016;75:E105-E106.
In sickle cell disease, osteomyelitis is a tough call
MONTREAL – Osteomyelitis is an especially challenging diagnosis in children with sickle cell disease (SCD) because the bone and joint signs, elevated white cell counts, and C-reactive protein levels that are commonly used to diagnose bone infection are frequently features of SCD as well.
As a result, most patients with SCD and suspected osteomyelitis are treated without a confirmation of the diagnosis.
Among 30 patients with SCD who were followed at a single center over a decade, 29 patients had elevated ESR, but only 13 patients had leukocytosis, and only 13 had elevated CRP.
“Prior studies on sickle cell disease have shown that it is very difficult to differentiate between osteoarticular infection and bone infarction. Therefore, oftentimes, this diagnosis is very difficult to make,” Dr. Weisman said at the annual meeting of the American Society of Pediatric Hematology/Oncology.
Laboratory findings for osteomyelitis in SCD are often nonspecific, including leukocytosis, elevated CRP and ESR, and blood cultures positive for Staphylococcus aureus (the predominant pathogen in children with osteomyelitis), or, in children with hemoglobinopathies, salmonella.
In children with SCD, CRP levels can vary from normal to elevated. ESR is similarly variable, as low hematocrit values can result in higher ESR values. Additionally, sickle erythrocytes can fail to aggregate, which can lead to lower ESR values.
A decade of data
The researchers set out to get a better handle on the characteristics and outcomes of osteomyelitis in patients with SCD and to see which laboratory and imaging findings might prove most useful for diagnosing osteomyelitis in this population. They reviewed data on 59 patients who were identified with indeterminate or likely osteomyelitis over a 10-year span. Of those, 30 were diagnosed and treated for osteomyelitis, and 29 were tentatively diagnosed but not treated. The latter group likely had symptoms caused by a bone infarction or vaso-occlusive crisis, Dr. Weisman said.
Among the 30 treated patients, osteomyelitis was confirmed by bone biopsy in 3, and an organism was isolated from blood or an abscess in 6. In the other 21, osteomyelitis was presumed based on clinical, laboratory, and MRI findings.
The median patient age was 12 years (range, 8 months to 18 years), 18 were male, and all but three patients have the HbSS genotype. Of the remaining patients, two had the HbSC and one the HbSF genotypes.
Infections occurred in the lower extremities in 11 patients, in the upper extremities in 10, in the pelvis or vertebrae in 2 each, and in the scapula, clavicle, hand, rib, or mandible in 1 patient each.
Just 13 of the 30 patients (43%) had lab findings of leukocytosis (more than 15,000 cells/mm2), and an equal number had elevated CRP (greater than 10 mg/L).
In contrast, 29 patients had an ESR above 20 mm/hour, and, in three of these patients, the rate was higher than 100 mm/hour.
When the researchers compared white blood cell counts and CRP levels between the treated patients and the 29 untreated controls, they found no significant differences for either measure of inflammation. In contrast, ESR was significantly higher among treated patients (P = .03).
Looking at the receiver operating characteristic curve for ESR, they found that an ESR of more than 100 mm/hour had 100% specificity for osteomyelitis in this group of patients.
Only 6 of the 30 (20%) had bacteremia. In 9 patients, nontyphoidal salmonella was isolated from cultures of either bone biopsy (3), abscess (3), or blood (6), but no possible causative organism could be isolated in the remaining 21 patients.
All patients were treated with prolonged antibiotic therapy. Surgical drainage and/or debridement were required in 6 patients. Two patients developed chronic osteomyelitis, but infection eventually resolved in all patients.
Recommendations
Dr. Weisman recommended early consultation with infectious disease experts and orthopedists; labs studies with complete blood counts, CRP, and ESR; and imaging studies with MRI when there is clinical suspicion of osteomyelitis in patients with SCD.
When an SCD patient has indeterminate findings, a blood culture can be performed. If it is positive for salmonella and the ESR is above 100 mm/hour, the patient can then go on to treatment. If the blood culture is negative and the ESR is below 100 mm hour but the suspicion of osteomyelitis remains high, a bone biopsy can be considered, they concluded.
The study was internally funded. Dr. Weisman reported no conflicts of interest to disclose.
MONTREAL – Osteomyelitis is an especially challenging diagnosis in children with sickle cell disease (SCD) because the bone and joint signs, elevated white cell counts, and C-reactive protein levels that are commonly used to diagnose bone infection are frequently features of SCD as well.
As a result, most patients with SCD and suspected osteomyelitis are treated without a confirmation of the diagnosis.
Among 30 patients with SCD who were followed at a single center over a decade, 29 patients had elevated ESR, but only 13 patients had leukocytosis, and only 13 had elevated CRP.
“Prior studies on sickle cell disease have shown that it is very difficult to differentiate between osteoarticular infection and bone infarction. Therefore, oftentimes, this diagnosis is very difficult to make,” Dr. Weisman said at the annual meeting of the American Society of Pediatric Hematology/Oncology.
Laboratory findings for osteomyelitis in SCD are often nonspecific, including leukocytosis, elevated CRP and ESR, and blood cultures positive for Staphylococcus aureus (the predominant pathogen in children with osteomyelitis), or, in children with hemoglobinopathies, salmonella.
In children with SCD, CRP levels can vary from normal to elevated. ESR is similarly variable, as low hematocrit values can result in higher ESR values. Additionally, sickle erythrocytes can fail to aggregate, which can lead to lower ESR values.
A decade of data
The researchers set out to get a better handle on the characteristics and outcomes of osteomyelitis in patients with SCD and to see which laboratory and imaging findings might prove most useful for diagnosing osteomyelitis in this population. They reviewed data on 59 patients who were identified with indeterminate or likely osteomyelitis over a 10-year span. Of those, 30 were diagnosed and treated for osteomyelitis, and 29 were tentatively diagnosed but not treated. The latter group likely had symptoms caused by a bone infarction or vaso-occlusive crisis, Dr. Weisman said.
Among the 30 treated patients, osteomyelitis was confirmed by bone biopsy in 3, and an organism was isolated from blood or an abscess in 6. In the other 21, osteomyelitis was presumed based on clinical, laboratory, and MRI findings.
The median patient age was 12 years (range, 8 months to 18 years), 18 were male, and all but three patients have the HbSS genotype. Of the remaining patients, two had the HbSC and one the HbSF genotypes.
Infections occurred in the lower extremities in 11 patients, in the upper extremities in 10, in the pelvis or vertebrae in 2 each, and in the scapula, clavicle, hand, rib, or mandible in 1 patient each.
Just 13 of the 30 patients (43%) had lab findings of leukocytosis (more than 15,000 cells/mm2), and an equal number had elevated CRP (greater than 10 mg/L).
In contrast, 29 patients had an ESR above 20 mm/hour, and, in three of these patients, the rate was higher than 100 mm/hour.
When the researchers compared white blood cell counts and CRP levels between the treated patients and the 29 untreated controls, they found no significant differences for either measure of inflammation. In contrast, ESR was significantly higher among treated patients (P = .03).
Looking at the receiver operating characteristic curve for ESR, they found that an ESR of more than 100 mm/hour had 100% specificity for osteomyelitis in this group of patients.
Only 6 of the 30 (20%) had bacteremia. In 9 patients, nontyphoidal salmonella was isolated from cultures of either bone biopsy (3), abscess (3), or blood (6), but no possible causative organism could be isolated in the remaining 21 patients.
All patients were treated with prolonged antibiotic therapy. Surgical drainage and/or debridement were required in 6 patients. Two patients developed chronic osteomyelitis, but infection eventually resolved in all patients.
Recommendations
Dr. Weisman recommended early consultation with infectious disease experts and orthopedists; labs studies with complete blood counts, CRP, and ESR; and imaging studies with MRI when there is clinical suspicion of osteomyelitis in patients with SCD.
When an SCD patient has indeterminate findings, a blood culture can be performed. If it is positive for salmonella and the ESR is above 100 mm/hour, the patient can then go on to treatment. If the blood culture is negative and the ESR is below 100 mm hour but the suspicion of osteomyelitis remains high, a bone biopsy can be considered, they concluded.
The study was internally funded. Dr. Weisman reported no conflicts of interest to disclose.
MONTREAL – Osteomyelitis is an especially challenging diagnosis in children with sickle cell disease (SCD) because the bone and joint signs, elevated white cell counts, and C-reactive protein levels that are commonly used to diagnose bone infection are frequently features of SCD as well.
As a result, most patients with SCD and suspected osteomyelitis are treated without a confirmation of the diagnosis.
Among 30 patients with SCD who were followed at a single center over a decade, 29 patients had elevated ESR, but only 13 patients had leukocytosis, and only 13 had elevated CRP.
“Prior studies on sickle cell disease have shown that it is very difficult to differentiate between osteoarticular infection and bone infarction. Therefore, oftentimes, this diagnosis is very difficult to make,” Dr. Weisman said at the annual meeting of the American Society of Pediatric Hematology/Oncology.
Laboratory findings for osteomyelitis in SCD are often nonspecific, including leukocytosis, elevated CRP and ESR, and blood cultures positive for Staphylococcus aureus (the predominant pathogen in children with osteomyelitis), or, in children with hemoglobinopathies, salmonella.
In children with SCD, CRP levels can vary from normal to elevated. ESR is similarly variable, as low hematocrit values can result in higher ESR values. Additionally, sickle erythrocytes can fail to aggregate, which can lead to lower ESR values.
A decade of data
The researchers set out to get a better handle on the characteristics and outcomes of osteomyelitis in patients with SCD and to see which laboratory and imaging findings might prove most useful for diagnosing osteomyelitis in this population. They reviewed data on 59 patients who were identified with indeterminate or likely osteomyelitis over a 10-year span. Of those, 30 were diagnosed and treated for osteomyelitis, and 29 were tentatively diagnosed but not treated. The latter group likely had symptoms caused by a bone infarction or vaso-occlusive crisis, Dr. Weisman said.
Among the 30 treated patients, osteomyelitis was confirmed by bone biopsy in 3, and an organism was isolated from blood or an abscess in 6. In the other 21, osteomyelitis was presumed based on clinical, laboratory, and MRI findings.
The median patient age was 12 years (range, 8 months to 18 years), 18 were male, and all but three patients have the HbSS genotype. Of the remaining patients, two had the HbSC and one the HbSF genotypes.
Infections occurred in the lower extremities in 11 patients, in the upper extremities in 10, in the pelvis or vertebrae in 2 each, and in the scapula, clavicle, hand, rib, or mandible in 1 patient each.
Just 13 of the 30 patients (43%) had lab findings of leukocytosis (more than 15,000 cells/mm2), and an equal number had elevated CRP (greater than 10 mg/L).
In contrast, 29 patients had an ESR above 20 mm/hour, and, in three of these patients, the rate was higher than 100 mm/hour.
When the researchers compared white blood cell counts and CRP levels between the treated patients and the 29 untreated controls, they found no significant differences for either measure of inflammation. In contrast, ESR was significantly higher among treated patients (P = .03).
Looking at the receiver operating characteristic curve for ESR, they found that an ESR of more than 100 mm/hour had 100% specificity for osteomyelitis in this group of patients.
Only 6 of the 30 (20%) had bacteremia. In 9 patients, nontyphoidal salmonella was isolated from cultures of either bone biopsy (3), abscess (3), or blood (6), but no possible causative organism could be isolated in the remaining 21 patients.
All patients were treated with prolonged antibiotic therapy. Surgical drainage and/or debridement were required in 6 patients. Two patients developed chronic osteomyelitis, but infection eventually resolved in all patients.
Recommendations
Dr. Weisman recommended early consultation with infectious disease experts and orthopedists; labs studies with complete blood counts, CRP, and ESR; and imaging studies with MRI when there is clinical suspicion of osteomyelitis in patients with SCD.
When an SCD patient has indeterminate findings, a blood culture can be performed. If it is positive for salmonella and the ESR is above 100 mm/hour, the patient can then go on to treatment. If the blood culture is negative and the ESR is below 100 mm hour but the suspicion of osteomyelitis remains high, a bone biopsy can be considered, they concluded.
The study was internally funded. Dr. Weisman reported no conflicts of interest to disclose.
Key clinical point: ESR may be a better lab marker for osteomyelitis in sickle cell disease than either WBC or CRP.
Major finding: An ESR greater than 100 mm/hr was 100% specific for osteomyelitis in this study.
Data source: A retrospective review of data on 59 patients with sickle cell disease and suspected or probable osteomyelitis.
Disclosures: The study was internally funded. Dr. Weisman reported having no conflicts of interest to disclose.
Workshop to help hospitalists with patient flow
The “flow” of patients through the hospital can sometimes resemble a 10-lane expressway at gridlock, said Christopher Kim, MD, MBA, SFHM, associate professor of internal medicine and associate medical director of quality and safety at the University of Washington Medical Center in Seattle. That is, there’s no flow at all.
Thursday at HM17, Dr. Kim and a panel of experts will lead small workshops of audience members on how to put the “go” back in the flow. The session, “Hospitalists as Leaders in Patient Flow and Hospital Throughput,” will begin at 10 a.m.
“As hospitalists, we are at the sharp end of this problem and are often asked to be key members of project teams assembled to tackle this challenge of patient flow and capacity management,” said Dr. Kim. “We look forward to an interactive session with our audience members.”
The session will address expedited discharge, the idea of the “hospitalist quarterback,” a kind of in-house controller of patient flow; facilitators of transferring to and from outside hospitals; focused efforts on reducing length of stay; establishing short-stay units to enhance patient flow; and participating in continuous process improvement teams, Dr. Kim said.
There will be several small group workshops interspersed with presentations of background content by the workshop facilitators, who represent a wide range of geographic and care settings. Dr. Kim said they have “all experienced the challenges of patient flow and throughput at their hospitals and health systems and have taken leadership roles to address these issues at their medical centers.”
“We anticipate a highly engaging session, where audience members will be divided into different teams, and each team will be tasked to identify specific interventions and challenges in managing the patient flow and capacity management initiative,” said Dr. Berger, associate medical director for inpatient capacity at the University of Washington. “Audience members will work within their own teams, guided by the workshop facilitators, and after the teams come up with their ideas on each of the topics covered in this workshop, each team will have the opportunity to present their best ideas for sharing and feedback.”
The session comes at a time when hospitals are tapping into hospitalists’ experience.
“Many hospitals across the country are challenged with high-occupancy states, creating pressures and external challenges to serve patients’ needs,” said Dr. Parekh, associate professor of internal medicine at the University of Michigan. “Hospitalists in their daily work have great familiarity with how hospitals run and where the ‘bottlenecks’ are that [block] patient throughput in their organization. Recognizing this experience and talent for enabling patient flow, many hospitals have turned to hospitalists – and in some cases, resident trainees – to lead or contribute to the hospital’s initiatives to enhance patient throughput.”
The importance of patient flow goes beyond efficiency, Dr. Kim said. Hospital staff who work during high-occupancy days feel the brunt of the burden, and this can lead to patient safety problems.
“We will address the importance of optimal communication between providers and staff, both within hospitals and with outside hospitals to ensure patient safety and staff concerns are appropriately addressed,” he said. “This is a topic that affects everyone at the medical center, and so we believe that this topic is applicable to a broad audience. We hope the participants will be able to facilitate a dialogue with a diverse group of leaders at their hospitals.”
Hospitalists as Leaders in Patient Flow and Hospital Throughput
Thursday, 10:00–11:30 a.m.
The “flow” of patients through the hospital can sometimes resemble a 10-lane expressway at gridlock, said Christopher Kim, MD, MBA, SFHM, associate professor of internal medicine and associate medical director of quality and safety at the University of Washington Medical Center in Seattle. That is, there’s no flow at all.
Thursday at HM17, Dr. Kim and a panel of experts will lead small workshops of audience members on how to put the “go” back in the flow. The session, “Hospitalists as Leaders in Patient Flow and Hospital Throughput,” will begin at 10 a.m.
“As hospitalists, we are at the sharp end of this problem and are often asked to be key members of project teams assembled to tackle this challenge of patient flow and capacity management,” said Dr. Kim. “We look forward to an interactive session with our audience members.”
The session will address expedited discharge, the idea of the “hospitalist quarterback,” a kind of in-house controller of patient flow; facilitators of transferring to and from outside hospitals; focused efforts on reducing length of stay; establishing short-stay units to enhance patient flow; and participating in continuous process improvement teams, Dr. Kim said.
There will be several small group workshops interspersed with presentations of background content by the workshop facilitators, who represent a wide range of geographic and care settings. Dr. Kim said they have “all experienced the challenges of patient flow and throughput at their hospitals and health systems and have taken leadership roles to address these issues at their medical centers.”
“We anticipate a highly engaging session, where audience members will be divided into different teams, and each team will be tasked to identify specific interventions and challenges in managing the patient flow and capacity management initiative,” said Dr. Berger, associate medical director for inpatient capacity at the University of Washington. “Audience members will work within their own teams, guided by the workshop facilitators, and after the teams come up with their ideas on each of the topics covered in this workshop, each team will have the opportunity to present their best ideas for sharing and feedback.”
The session comes at a time when hospitals are tapping into hospitalists’ experience.
“Many hospitals across the country are challenged with high-occupancy states, creating pressures and external challenges to serve patients’ needs,” said Dr. Parekh, associate professor of internal medicine at the University of Michigan. “Hospitalists in their daily work have great familiarity with how hospitals run and where the ‘bottlenecks’ are that [block] patient throughput in their organization. Recognizing this experience and talent for enabling patient flow, many hospitals have turned to hospitalists – and in some cases, resident trainees – to lead or contribute to the hospital’s initiatives to enhance patient throughput.”
The importance of patient flow goes beyond efficiency, Dr. Kim said. Hospital staff who work during high-occupancy days feel the brunt of the burden, and this can lead to patient safety problems.
“We will address the importance of optimal communication between providers and staff, both within hospitals and with outside hospitals to ensure patient safety and staff concerns are appropriately addressed,” he said. “This is a topic that affects everyone at the medical center, and so we believe that this topic is applicable to a broad audience. We hope the participants will be able to facilitate a dialogue with a diverse group of leaders at their hospitals.”
Hospitalists as Leaders in Patient Flow and Hospital Throughput
Thursday, 10:00–11:30 a.m.
The “flow” of patients through the hospital can sometimes resemble a 10-lane expressway at gridlock, said Christopher Kim, MD, MBA, SFHM, associate professor of internal medicine and associate medical director of quality and safety at the University of Washington Medical Center in Seattle. That is, there’s no flow at all.
Thursday at HM17, Dr. Kim and a panel of experts will lead small workshops of audience members on how to put the “go” back in the flow. The session, “Hospitalists as Leaders in Patient Flow and Hospital Throughput,” will begin at 10 a.m.
“As hospitalists, we are at the sharp end of this problem and are often asked to be key members of project teams assembled to tackle this challenge of patient flow and capacity management,” said Dr. Kim. “We look forward to an interactive session with our audience members.”
The session will address expedited discharge, the idea of the “hospitalist quarterback,” a kind of in-house controller of patient flow; facilitators of transferring to and from outside hospitals; focused efforts on reducing length of stay; establishing short-stay units to enhance patient flow; and participating in continuous process improvement teams, Dr. Kim said.
There will be several small group workshops interspersed with presentations of background content by the workshop facilitators, who represent a wide range of geographic and care settings. Dr. Kim said they have “all experienced the challenges of patient flow and throughput at their hospitals and health systems and have taken leadership roles to address these issues at their medical centers.”
“We anticipate a highly engaging session, where audience members will be divided into different teams, and each team will be tasked to identify specific interventions and challenges in managing the patient flow and capacity management initiative,” said Dr. Berger, associate medical director for inpatient capacity at the University of Washington. “Audience members will work within their own teams, guided by the workshop facilitators, and after the teams come up with their ideas on each of the topics covered in this workshop, each team will have the opportunity to present their best ideas for sharing and feedback.”
The session comes at a time when hospitals are tapping into hospitalists’ experience.
“Many hospitals across the country are challenged with high-occupancy states, creating pressures and external challenges to serve patients’ needs,” said Dr. Parekh, associate professor of internal medicine at the University of Michigan. “Hospitalists in their daily work have great familiarity with how hospitals run and where the ‘bottlenecks’ are that [block] patient throughput in their organization. Recognizing this experience and talent for enabling patient flow, many hospitals have turned to hospitalists – and in some cases, resident trainees – to lead or contribute to the hospital’s initiatives to enhance patient throughput.”
The importance of patient flow goes beyond efficiency, Dr. Kim said. Hospital staff who work during high-occupancy days feel the brunt of the burden, and this can lead to patient safety problems.
“We will address the importance of optimal communication between providers and staff, both within hospitals and with outside hospitals to ensure patient safety and staff concerns are appropriately addressed,” he said. “This is a topic that affects everyone at the medical center, and so we believe that this topic is applicable to a broad audience. We hope the participants will be able to facilitate a dialogue with a diverse group of leaders at their hospitals.”
Hospitalists as Leaders in Patient Flow and Hospital Throughput
Thursday, 10:00–11:30 a.m.
Systems engineering in the hospital
Systems-engineering expert James Benneyan, PhD, doesn’t want hospitalists to look at poorly working processes in their institutions and think, “I should try to tweak this process to improve it.”
Instead, he wants them to walk out of his HM17 session at 8 a.m. Thursday – appropriately titled, “Systems Engineering in the Hospital: What Is in Your Toolkit?” – thinking like engineers, which means designing a solution, analyzing how well that process works, and then optimizing it for improvements. If that means not just tweaking a process, but redesigning it from scratch, so be it.
“Systems engineering studies the performance and how to improve the performance of complex systems, particularly sociotechnical systems,” said Dr. Benneyan, who runs the Healthcare Systems Engineering Institute at Northeastern University in Boston, which encompasses four research centers. “Health care is a perfect example … systems engineering can really help to understand and improve complex processes, whether it’s patient flow, safety, on-time discharge [or] better discharge.”
Dr. Benneyan says that systems engineering is, first and foremost, a mindset. It’s an approach to problem solving that’s different, if related, to quality improvement. Both have tremendous value, but they are based on different philosophies, tools, and work styles.
For example, many hospital operating rooms measure how many days the first procedure of the day begins on time. But instead of using that as a yardstick for quality, Dr. Benneyan said a better approach would be designing a system that can adapt to situations when the first case starts late. He compared the process to a delayed flight at an airport. An airline doesn’t back up every plane’s departure when one plane is running behind. Instead, it has systems that adapt to circumstances.
“There are methods and then there are philosophies,” Dr. Benneyan said. “I don’t think people in health care realize what my field did in the airline industry. We didn’t design things that worked and clicked properly. We designed things that … react to daily events and [everything] going on and perform pretty well.”
Dr. Benneyan says that, while health care is an incredibly complex system, other fields with similar levels of technical expertise have used systems engineering much more effectively. Manufacturing, logistics, and global distribution networks are all precise industries requiring hundreds of individual processes to ensure success.
“These are really complicated processes,” he said. “The real barrier is a cultural barrier. Health care is not the most challenging environment to work in. … I think something that people in health care have to have an appreciation for is that the process of doing this work is different from doing their other work. Systems engineering is not the same as quality improvement and can achieve fundamental breakthroughs in cases where QI has not – but also tends to take more work.”
Still, Dr. Benneyan believes his field has lessons that complement quality initiatives. To wit, health care advocates – including the Institute of Medicine, the Agency for Healthcare Research and Quality, the National Institutes of Health, and the National Science Foundation – have all pushed for greater application of systems engineering in medicine with the goal of improving how well health care does its job.
While he hopes hospitalists and other HM17 attendees at his session walk away with a newfound respect for and understanding of what systems engineering can do, he doesn’t want them to think it’s too easy.
“There’s a lack of appreciation of the process of engineering and how it’s different,” he said. “It’s a big challenge, partnering clinician with engineers. … We think differently even though we’re both scientifically trained.
“I hope hospitalists take away an appreciation for how this toolkit can be useful in their world.”
Systems Engineering in the Hospital: What Is in Your Toolkit?
Thursday, 8:00–9:30 a.m.
Systems-engineering expert James Benneyan, PhD, doesn’t want hospitalists to look at poorly working processes in their institutions and think, “I should try to tweak this process to improve it.”
Instead, he wants them to walk out of his HM17 session at 8 a.m. Thursday – appropriately titled, “Systems Engineering in the Hospital: What Is in Your Toolkit?” – thinking like engineers, which means designing a solution, analyzing how well that process works, and then optimizing it for improvements. If that means not just tweaking a process, but redesigning it from scratch, so be it.
“Systems engineering studies the performance and how to improve the performance of complex systems, particularly sociotechnical systems,” said Dr. Benneyan, who runs the Healthcare Systems Engineering Institute at Northeastern University in Boston, which encompasses four research centers. “Health care is a perfect example … systems engineering can really help to understand and improve complex processes, whether it’s patient flow, safety, on-time discharge [or] better discharge.”
Dr. Benneyan says that systems engineering is, first and foremost, a mindset. It’s an approach to problem solving that’s different, if related, to quality improvement. Both have tremendous value, but they are based on different philosophies, tools, and work styles.
For example, many hospital operating rooms measure how many days the first procedure of the day begins on time. But instead of using that as a yardstick for quality, Dr. Benneyan said a better approach would be designing a system that can adapt to situations when the first case starts late. He compared the process to a delayed flight at an airport. An airline doesn’t back up every plane’s departure when one plane is running behind. Instead, it has systems that adapt to circumstances.
“There are methods and then there are philosophies,” Dr. Benneyan said. “I don’t think people in health care realize what my field did in the airline industry. We didn’t design things that worked and clicked properly. We designed things that … react to daily events and [everything] going on and perform pretty well.”
Dr. Benneyan says that, while health care is an incredibly complex system, other fields with similar levels of technical expertise have used systems engineering much more effectively. Manufacturing, logistics, and global distribution networks are all precise industries requiring hundreds of individual processes to ensure success.
“These are really complicated processes,” he said. “The real barrier is a cultural barrier. Health care is not the most challenging environment to work in. … I think something that people in health care have to have an appreciation for is that the process of doing this work is different from doing their other work. Systems engineering is not the same as quality improvement and can achieve fundamental breakthroughs in cases where QI has not – but also tends to take more work.”
Still, Dr. Benneyan believes his field has lessons that complement quality initiatives. To wit, health care advocates – including the Institute of Medicine, the Agency for Healthcare Research and Quality, the National Institutes of Health, and the National Science Foundation – have all pushed for greater application of systems engineering in medicine with the goal of improving how well health care does its job.
While he hopes hospitalists and other HM17 attendees at his session walk away with a newfound respect for and understanding of what systems engineering can do, he doesn’t want them to think it’s too easy.
“There’s a lack of appreciation of the process of engineering and how it’s different,” he said. “It’s a big challenge, partnering clinician with engineers. … We think differently even though we’re both scientifically trained.
“I hope hospitalists take away an appreciation for how this toolkit can be useful in their world.”
Systems Engineering in the Hospital: What Is in Your Toolkit?
Thursday, 8:00–9:30 a.m.
Systems-engineering expert James Benneyan, PhD, doesn’t want hospitalists to look at poorly working processes in their institutions and think, “I should try to tweak this process to improve it.”
Instead, he wants them to walk out of his HM17 session at 8 a.m. Thursday – appropriately titled, “Systems Engineering in the Hospital: What Is in Your Toolkit?” – thinking like engineers, which means designing a solution, analyzing how well that process works, and then optimizing it for improvements. If that means not just tweaking a process, but redesigning it from scratch, so be it.
“Systems engineering studies the performance and how to improve the performance of complex systems, particularly sociotechnical systems,” said Dr. Benneyan, who runs the Healthcare Systems Engineering Institute at Northeastern University in Boston, which encompasses four research centers. “Health care is a perfect example … systems engineering can really help to understand and improve complex processes, whether it’s patient flow, safety, on-time discharge [or] better discharge.”
Dr. Benneyan says that systems engineering is, first and foremost, a mindset. It’s an approach to problem solving that’s different, if related, to quality improvement. Both have tremendous value, but they are based on different philosophies, tools, and work styles.
For example, many hospital operating rooms measure how many days the first procedure of the day begins on time. But instead of using that as a yardstick for quality, Dr. Benneyan said a better approach would be designing a system that can adapt to situations when the first case starts late. He compared the process to a delayed flight at an airport. An airline doesn’t back up every plane’s departure when one plane is running behind. Instead, it has systems that adapt to circumstances.
“There are methods and then there are philosophies,” Dr. Benneyan said. “I don’t think people in health care realize what my field did in the airline industry. We didn’t design things that worked and clicked properly. We designed things that … react to daily events and [everything] going on and perform pretty well.”
Dr. Benneyan says that, while health care is an incredibly complex system, other fields with similar levels of technical expertise have used systems engineering much more effectively. Manufacturing, logistics, and global distribution networks are all precise industries requiring hundreds of individual processes to ensure success.
“These are really complicated processes,” he said. “The real barrier is a cultural barrier. Health care is not the most challenging environment to work in. … I think something that people in health care have to have an appreciation for is that the process of doing this work is different from doing their other work. Systems engineering is not the same as quality improvement and can achieve fundamental breakthroughs in cases where QI has not – but also tends to take more work.”
Still, Dr. Benneyan believes his field has lessons that complement quality initiatives. To wit, health care advocates – including the Institute of Medicine, the Agency for Healthcare Research and Quality, the National Institutes of Health, and the National Science Foundation – have all pushed for greater application of systems engineering in medicine with the goal of improving how well health care does its job.
While he hopes hospitalists and other HM17 attendees at his session walk away with a newfound respect for and understanding of what systems engineering can do, he doesn’t want them to think it’s too easy.
“There’s a lack of appreciation of the process of engineering and how it’s different,” he said. “It’s a big challenge, partnering clinician with engineers. … We think differently even though we’re both scientifically trained.
“I hope hospitalists take away an appreciation for how this toolkit can be useful in their world.”
Systems Engineering in the Hospital: What Is in Your Toolkit?
Thursday, 8:00–9:30 a.m.
Diabetes specialist to offer disease management tips
Diabetes is a persistent presence in the hospital, and hospitalists must remain up to date on the latest in disease management.
An endocrinologist will walk the audience through four major points on caring for diabetes patients in a talk to be given Thursday at HM17. The session, “Inpatient Diabetes Management for the Hospitalist,” will begin at 7:40 a.m.
Guillermo Umpierrez, MD, CDE, FACP, FACE, professor of medicine, director of the clinical research center and section of diabetes and metabolism at Emory University, Atlanta, and section head of diabetes and endocrinology at Grady Health System, also in Atlanta, said that, “in most patients, diabetes is a comorbidity that has a serious impact on the outcome of patients with cardiovascular disease or malignancies or surgery.
“Hyperglycemia in patients with or without diabetes can be 30%-40%,” he said. “There are somewhere around 8 to 10 million hospital discharges with diabetes every year in the United States.”
Dr. Umpierrez intends to discuss the following topics in his presentation:
• Intensive insulin therapy. “There is no evidence that intensive insulin therapy aiming to normalize blood glucose [leads to] improvement in outcome and could even [worsen] outcome because of the risk of hypoglycemia. This is true for patients in intensive care and the regular floor.”
• Treatment other than insulin. Guidelines say that using insulin is the only way to manage diabetic patients in the hospital, but evidence is growing that this might not be ideal in some cases, he said.
“Recent evidence in the past 5 years has shown that maybe a one-size-fits-all approach is wrong because using insulin, especially the basal-bolus insulin regimen” – with long-lasting insulin between meals and bolus insulin at mealtime – “can be an overtreatment for some patients with multiple complications and patients with mild hyperglycemia.” In many patients, the administration of a single basal insulin dose (glargine or detemir) is sufficient to achieve reasonable glucose control. In addition, patients with blood glucose less than 180 to 200 mg/dL could benefit from the use of incretin therapy with or without insulin to “at least minimize the risk of hypoglycemia.”
• Limitations for sliding-scale insulin therapy. This approach, in which mealtime bolus insulin is based on blood-sugar level before meals and which has dominated diabetes management over the past 80 years, can bring problems, according to the latest literature, Dr. Umpierrez said.
“Now we have excessive evidence, both in the ICU and non-ICU, that the use of sliding-scale insulin therapy … is associated with higher blood glucose levels [and a] higher rate of complications compared to the use of basal insulin. So, I think that physicians are becoming more aware that sliding scale is not the only way to manage patients in the hospital.”
• Insulin at discharge. The belief that all patients need to go home with insulin might be misguided, he said. “This could be an overtreatment associated with increased risk of hypoglycemia with no benefit in outcome.”
• The use of computer-guided algorithms on insulin therapy. “Are they better than the standard insulin drip protocols that we have? Not clear,” he said. Many commercial versions and institution-generated versions have been developed, but there is uncertainty about their value, he added.
“They may reduce the risk of hypoglycemia,” Dr. Umpierrez said. “We don’t have any evidence that they are better in reducing complications in the hospital. And they can be costly. So the physician has to be aware of the cost. But, it’s an option for some institutions that have very little support from hospitalists or intensivists in their hospital to adjust insulin therapy in the rapidly changing environment in critically ill patients in the ICU.”
Inpatient Diabetes Management for the Hospitalist
Thursday, 7:40–8:15 a.m.
Diabetes is a persistent presence in the hospital, and hospitalists must remain up to date on the latest in disease management.
An endocrinologist will walk the audience through four major points on caring for diabetes patients in a talk to be given Thursday at HM17. The session, “Inpatient Diabetes Management for the Hospitalist,” will begin at 7:40 a.m.
Guillermo Umpierrez, MD, CDE, FACP, FACE, professor of medicine, director of the clinical research center and section of diabetes and metabolism at Emory University, Atlanta, and section head of diabetes and endocrinology at Grady Health System, also in Atlanta, said that, “in most patients, diabetes is a comorbidity that has a serious impact on the outcome of patients with cardiovascular disease or malignancies or surgery.
“Hyperglycemia in patients with or without diabetes can be 30%-40%,” he said. “There are somewhere around 8 to 10 million hospital discharges with diabetes every year in the United States.”
Dr. Umpierrez intends to discuss the following topics in his presentation:
• Intensive insulin therapy. “There is no evidence that intensive insulin therapy aiming to normalize blood glucose [leads to] improvement in outcome and could even [worsen] outcome because of the risk of hypoglycemia. This is true for patients in intensive care and the regular floor.”
• Treatment other than insulin. Guidelines say that using insulin is the only way to manage diabetic patients in the hospital, but evidence is growing that this might not be ideal in some cases, he said.
“Recent evidence in the past 5 years has shown that maybe a one-size-fits-all approach is wrong because using insulin, especially the basal-bolus insulin regimen” – with long-lasting insulin between meals and bolus insulin at mealtime – “can be an overtreatment for some patients with multiple complications and patients with mild hyperglycemia.” In many patients, the administration of a single basal insulin dose (glargine or detemir) is sufficient to achieve reasonable glucose control. In addition, patients with blood glucose less than 180 to 200 mg/dL could benefit from the use of incretin therapy with or without insulin to “at least minimize the risk of hypoglycemia.”
• Limitations for sliding-scale insulin therapy. This approach, in which mealtime bolus insulin is based on blood-sugar level before meals and which has dominated diabetes management over the past 80 years, can bring problems, according to the latest literature, Dr. Umpierrez said.
“Now we have excessive evidence, both in the ICU and non-ICU, that the use of sliding-scale insulin therapy … is associated with higher blood glucose levels [and a] higher rate of complications compared to the use of basal insulin. So, I think that physicians are becoming more aware that sliding scale is not the only way to manage patients in the hospital.”
• Insulin at discharge. The belief that all patients need to go home with insulin might be misguided, he said. “This could be an overtreatment associated with increased risk of hypoglycemia with no benefit in outcome.”
• The use of computer-guided algorithms on insulin therapy. “Are they better than the standard insulin drip protocols that we have? Not clear,” he said. Many commercial versions and institution-generated versions have been developed, but there is uncertainty about their value, he added.
“They may reduce the risk of hypoglycemia,” Dr. Umpierrez said. “We don’t have any evidence that they are better in reducing complications in the hospital. And they can be costly. So the physician has to be aware of the cost. But, it’s an option for some institutions that have very little support from hospitalists or intensivists in their hospital to adjust insulin therapy in the rapidly changing environment in critically ill patients in the ICU.”
Inpatient Diabetes Management for the Hospitalist
Thursday, 7:40–8:15 a.m.
Diabetes is a persistent presence in the hospital, and hospitalists must remain up to date on the latest in disease management.
An endocrinologist will walk the audience through four major points on caring for diabetes patients in a talk to be given Thursday at HM17. The session, “Inpatient Diabetes Management for the Hospitalist,” will begin at 7:40 a.m.
Guillermo Umpierrez, MD, CDE, FACP, FACE, professor of medicine, director of the clinical research center and section of diabetes and metabolism at Emory University, Atlanta, and section head of diabetes and endocrinology at Grady Health System, also in Atlanta, said that, “in most patients, diabetes is a comorbidity that has a serious impact on the outcome of patients with cardiovascular disease or malignancies or surgery.
“Hyperglycemia in patients with or without diabetes can be 30%-40%,” he said. “There are somewhere around 8 to 10 million hospital discharges with diabetes every year in the United States.”
Dr. Umpierrez intends to discuss the following topics in his presentation:
• Intensive insulin therapy. “There is no evidence that intensive insulin therapy aiming to normalize blood glucose [leads to] improvement in outcome and could even [worsen] outcome because of the risk of hypoglycemia. This is true for patients in intensive care and the regular floor.”
• Treatment other than insulin. Guidelines say that using insulin is the only way to manage diabetic patients in the hospital, but evidence is growing that this might not be ideal in some cases, he said.
“Recent evidence in the past 5 years has shown that maybe a one-size-fits-all approach is wrong because using insulin, especially the basal-bolus insulin regimen” – with long-lasting insulin between meals and bolus insulin at mealtime – “can be an overtreatment for some patients with multiple complications and patients with mild hyperglycemia.” In many patients, the administration of a single basal insulin dose (glargine or detemir) is sufficient to achieve reasonable glucose control. In addition, patients with blood glucose less than 180 to 200 mg/dL could benefit from the use of incretin therapy with or without insulin to “at least minimize the risk of hypoglycemia.”
• Limitations for sliding-scale insulin therapy. This approach, in which mealtime bolus insulin is based on blood-sugar level before meals and which has dominated diabetes management over the past 80 years, can bring problems, according to the latest literature, Dr. Umpierrez said.
“Now we have excessive evidence, both in the ICU and non-ICU, that the use of sliding-scale insulin therapy … is associated with higher blood glucose levels [and a] higher rate of complications compared to the use of basal insulin. So, I think that physicians are becoming more aware that sliding scale is not the only way to manage patients in the hospital.”
• Insulin at discharge. The belief that all patients need to go home with insulin might be misguided, he said. “This could be an overtreatment associated with increased risk of hypoglycemia with no benefit in outcome.”
• The use of computer-guided algorithms on insulin therapy. “Are they better than the standard insulin drip protocols that we have? Not clear,” he said. Many commercial versions and institution-generated versions have been developed, but there is uncertainty about their value, he added.
“They may reduce the risk of hypoglycemia,” Dr. Umpierrez said. “We don’t have any evidence that they are better in reducing complications in the hospital. And they can be costly. So the physician has to be aware of the cost. But, it’s an option for some institutions that have very little support from hospitalists or intensivists in their hospital to adjust insulin therapy in the rapidly changing environment in critically ill patients in the ICU.”
Inpatient Diabetes Management for the Hospitalist
Thursday, 7:40–8:15 a.m.
Culture change necessary to weed out health care overuse
Chris Moriates, MD, assistant dean for healthcare value and associate professor of medicine at the University of Texas at Austin Dell Medical School, drew insight from writer David Foster Wallace on Tuesday afternoon as he discussed overuse in hospital care.
He cited a story by Wallace: Two young fish are swimming along when they pass an old, wise fish who says, “Mornin’, boys. How’s the water?” One of the young fish asks the other, “What the hell is water?”
For hospitalists, the culture is their water: Even though they’re swimming in it every day, it can be an abstraction that gets little attention. But the culture is what needs to be tended to in order to turn the tide of unnecessary expenses, Dr. Moriates said in his talk, “Overcoming a Culture Overrun with Overuse.”
“How we’re addressing this problem is by making guidelines, algorithms, evidence-based medicine, Choosing Wisely lists,” he said during the well-attended session in the High-Value Care track. “It’s important that we codify our practices in these ways. But it is insufficient, because we must recognize that we are all swimming in that same water.
“If we don’t change this culture, this water, we will not make any progress.”
A 2013 survey by the Society of Academic Emergency Medicine found that 97% of physicians thought that at least some of the imaging they have personally ordered is medically unnecessary. And a 2011 Annals of Internal Medicine study found that organizational culture was a key factor in distinguishing hospitals with high and low 30-day mortality rates for patients with acute myocardial infarction.
He pointed to lessons from his previous institution, the University of California San Francisco, where they launched Caring Wisely, a program meant to support front-line clinicians who want to eradicate unnecessary costs. Part of the program involves funding clinicians up to $50,000 for their projects. One such project found that feedback to surgeons about expenses, and a small financial incentive to the department to keep costs down, was associated with reduced operating room costs.
This kind of change can be done anywhere, he said. After all, culture change, he said, can start with something as small as encouraging patient interaction by asking, “What questions do you have?” rather than “Do you have any questions?”
“I don’t want you to leave and think, ‘Well, unless I work somewhere like UCSF, where they’re going to give me $50,000 to do this, or I have an amazing boss like [Dr.] Bob Wachter [the former SHM president from UCSF who coined the term “hospitalist”], there is nothing I can do about this,” Dr. Moriates said. “It turns out that, yes, that is important for changing culture, but each of us has a personal responsibility.”
Chris Moriates, MD, assistant dean for healthcare value and associate professor of medicine at the University of Texas at Austin Dell Medical School, drew insight from writer David Foster Wallace on Tuesday afternoon as he discussed overuse in hospital care.
He cited a story by Wallace: Two young fish are swimming along when they pass an old, wise fish who says, “Mornin’, boys. How’s the water?” One of the young fish asks the other, “What the hell is water?”
For hospitalists, the culture is their water: Even though they’re swimming in it every day, it can be an abstraction that gets little attention. But the culture is what needs to be tended to in order to turn the tide of unnecessary expenses, Dr. Moriates said in his talk, “Overcoming a Culture Overrun with Overuse.”
“How we’re addressing this problem is by making guidelines, algorithms, evidence-based medicine, Choosing Wisely lists,” he said during the well-attended session in the High-Value Care track. “It’s important that we codify our practices in these ways. But it is insufficient, because we must recognize that we are all swimming in that same water.
“If we don’t change this culture, this water, we will not make any progress.”
A 2013 survey by the Society of Academic Emergency Medicine found that 97% of physicians thought that at least some of the imaging they have personally ordered is medically unnecessary. And a 2011 Annals of Internal Medicine study found that organizational culture was a key factor in distinguishing hospitals with high and low 30-day mortality rates for patients with acute myocardial infarction.
He pointed to lessons from his previous institution, the University of California San Francisco, where they launched Caring Wisely, a program meant to support front-line clinicians who want to eradicate unnecessary costs. Part of the program involves funding clinicians up to $50,000 for their projects. One such project found that feedback to surgeons about expenses, and a small financial incentive to the department to keep costs down, was associated with reduced operating room costs.
This kind of change can be done anywhere, he said. After all, culture change, he said, can start with something as small as encouraging patient interaction by asking, “What questions do you have?” rather than “Do you have any questions?”
“I don’t want you to leave and think, ‘Well, unless I work somewhere like UCSF, where they’re going to give me $50,000 to do this, or I have an amazing boss like [Dr.] Bob Wachter [the former SHM president from UCSF who coined the term “hospitalist”], there is nothing I can do about this,” Dr. Moriates said. “It turns out that, yes, that is important for changing culture, but each of us has a personal responsibility.”
Chris Moriates, MD, assistant dean for healthcare value and associate professor of medicine at the University of Texas at Austin Dell Medical School, drew insight from writer David Foster Wallace on Tuesday afternoon as he discussed overuse in hospital care.
He cited a story by Wallace: Two young fish are swimming along when they pass an old, wise fish who says, “Mornin’, boys. How’s the water?” One of the young fish asks the other, “What the hell is water?”
For hospitalists, the culture is their water: Even though they’re swimming in it every day, it can be an abstraction that gets little attention. But the culture is what needs to be tended to in order to turn the tide of unnecessary expenses, Dr. Moriates said in his talk, “Overcoming a Culture Overrun with Overuse.”
“How we’re addressing this problem is by making guidelines, algorithms, evidence-based medicine, Choosing Wisely lists,” he said during the well-attended session in the High-Value Care track. “It’s important that we codify our practices in these ways. But it is insufficient, because we must recognize that we are all swimming in that same water.
“If we don’t change this culture, this water, we will not make any progress.”
A 2013 survey by the Society of Academic Emergency Medicine found that 97% of physicians thought that at least some of the imaging they have personally ordered is medically unnecessary. And a 2011 Annals of Internal Medicine study found that organizational culture was a key factor in distinguishing hospitals with high and low 30-day mortality rates for patients with acute myocardial infarction.
He pointed to lessons from his previous institution, the University of California San Francisco, where they launched Caring Wisely, a program meant to support front-line clinicians who want to eradicate unnecessary costs. Part of the program involves funding clinicians up to $50,000 for their projects. One such project found that feedback to surgeons about expenses, and a small financial incentive to the department to keep costs down, was associated with reduced operating room costs.
This kind of change can be done anywhere, he said. After all, culture change, he said, can start with something as small as encouraging patient interaction by asking, “What questions do you have?” rather than “Do you have any questions?”
“I don’t want you to leave and think, ‘Well, unless I work somewhere like UCSF, where they’re going to give me $50,000 to do this, or I have an amazing boss like [Dr.] Bob Wachter [the former SHM president from UCSF who coined the term “hospitalist”], there is nothing I can do about this,” Dr. Moriates said. “It turns out that, yes, that is important for changing culture, but each of us has a personal responsibility.”
Cord blood/placental cell combo induces rapid immune recovery
MONTREAL – A combination of placenta-derived stem cells and umbilical cord blood was associated with early engraftment and high degrees of cord blood donor chimerism in the treatment of children with both malignant and nonmalignant hematologic conditions requiring stem cell transplantation, updated results of a pilot study show.
Among 16 children treated with the combination, the probability of neutrophil engraftment was 87.5%, and all patients who had neutrophil engraftment went on to have platelet engraftment. The probability of 12-month overall survival was 81.2%, reported Allyson Flower, MD, from Boston Children’s Health Physicians in Hawthorne, N.Y. “The probability of grade II-IV acute graft vs. host disease was 12.5%, compared with 32.5% seen with unrelated cord blood in our group’s previous studies. Cellular immune reconstitution was robust,” she said at the annual meeting of the American Society of Pediatric Hematology/Oncology.
Augmenting cord blood
Although unrelated donor cord blood transplantation expands the donor pool, is rapidly available, and is associated with decreases in both severe acute graft vs. host disease (GVHD) and chronic GVHD, compared with other stem cell sources, the technique is hampered by limited cell doses, prolonged immune reconstitution time, delays in hematopoietic recovery, and a higher incidence of graft failure.
Early studies of myeloablative conditioning followed by unrelated umbilical or placental blood transplantation showed a median of 22-24 days to neutrophil engraftment (Blood 1996 88:795-802; N Engl J Med. 1996;335:157-66), Dr. Flower noted.
More recently, a multivariate analysis of patients who underwent reduced-intensity conditioning followed by hematopoietic stem cell transplant with unrelated cord blood showed that graft failure was an independent risk factor for worse overall survival (Biol Blood Marrow Transplant. 2013 Apr;19:4;552-61).
Multiple groups have shown that adding human placenta–derived stem cells (HPDSC) to cord blood transplantation can facilitate more rapid hematopoietic engraftment by increasing the number of stem cells, increasing the proportion of hematopoietic progenitor cells, and providing additional, immature CD34+/CD45– progenitor cells.
In a single-arm, nonrandomized study, the investigators enrolled 16 patients ranging in age from 0.3 to 15.7 years with inborn errors of metabolism, marrow failure syndromes, severe immunodeficiency states, or hematologic malignancies.
Malignant conditions included B-cell precursor acute lymphoblastic leukemia (B-ALL; four patients), acute myeloid leukemia (AML; two), and T-cell ALL (one) in first complete remission, and T-cell lymphoblastic lymphoma following induction failure (one). Nonmalignant conditions included adrenoleukodystrophy (two patients), amegakaryotic thrombocytopenia (one), severe combined immunodeficiency (SCID; two), dyskeratosis congenita (one), chronic granulomatous disease (one), and severe congenital neutropenia (one).
The patients first underwent either myeloablative or reduced-intensity conditioning, followed 10 days later by infusion of unrelated cord blood and HPDSCs. Prior to HPDSC infusion, patients were medicated with diphenhydramine and hydrocortisone to prevent or reduce potential sensitivity reactions. HPDSCs were infused no sooner than 4 hours after the end of the cord blood infusion.
Patients received GVHD prophylaxis with either tacrolimus or cyclosporine, plus mycophenolate mofetil.
The combination appeared to be safe, with no cases of grade 3 or 4 toxicity secondary to HPDSC infusion.
The probability of neutrophil engraftment was 87.5%, with engraftment occurring at a median of 23 days (range 13-53). As noted before, all patients who had neutrophil engraftment had platelet engraftment, which was achieved at a median of 47 days (range, 20-98). In the group’s previous studies, median time to platelet engraftment was 53 days for patients who had undergone reduced-intensity conditioning, and 118 days for patients who had undergone myeloablation.
The probability of grade 2-4 acute GVHD within 100 days was 12.5%, and there were no cases of chronic GVHD.
Respective percentages of cord blood donor chimerism at days 30, 60, 100, and 180 were 88%, 98%, 99%, and 99%.
Immune reconstitution was strong, with normalization of mean CD3+, CD19+, and CD56+ cells occurring by day 100, CD8+ cells by day 180, and CD4+ cells by day 270.
There were three patient deaths: one from adenoviremia in a patient with B-ALL and CNS relapse, who had neutrophil engraftment at day 21; one in a patient with SCID, from adenoviremia and multiple system organ failure, who did not have engraftment before death; and one in a patient with severe congenital neutrophilia, who also did not have neutrophil engraftment.
None of the eight patients with malignant disease have experienced relapse to date, Dr. Flower noted.
The study was funded by a grant from Celgene Cellular Therapeutics. Dr. Flower reported having no conflicts of interest.
MONTREAL – A combination of placenta-derived stem cells and umbilical cord blood was associated with early engraftment and high degrees of cord blood donor chimerism in the treatment of children with both malignant and nonmalignant hematologic conditions requiring stem cell transplantation, updated results of a pilot study show.
Among 16 children treated with the combination, the probability of neutrophil engraftment was 87.5%, and all patients who had neutrophil engraftment went on to have platelet engraftment. The probability of 12-month overall survival was 81.2%, reported Allyson Flower, MD, from Boston Children’s Health Physicians in Hawthorne, N.Y. “The probability of grade II-IV acute graft vs. host disease was 12.5%, compared with 32.5% seen with unrelated cord blood in our group’s previous studies. Cellular immune reconstitution was robust,” she said at the annual meeting of the American Society of Pediatric Hematology/Oncology.
Augmenting cord blood
Although unrelated donor cord blood transplantation expands the donor pool, is rapidly available, and is associated with decreases in both severe acute graft vs. host disease (GVHD) and chronic GVHD, compared with other stem cell sources, the technique is hampered by limited cell doses, prolonged immune reconstitution time, delays in hematopoietic recovery, and a higher incidence of graft failure.
Early studies of myeloablative conditioning followed by unrelated umbilical or placental blood transplantation showed a median of 22-24 days to neutrophil engraftment (Blood 1996 88:795-802; N Engl J Med. 1996;335:157-66), Dr. Flower noted.
More recently, a multivariate analysis of patients who underwent reduced-intensity conditioning followed by hematopoietic stem cell transplant with unrelated cord blood showed that graft failure was an independent risk factor for worse overall survival (Biol Blood Marrow Transplant. 2013 Apr;19:4;552-61).
Multiple groups have shown that adding human placenta–derived stem cells (HPDSC) to cord blood transplantation can facilitate more rapid hematopoietic engraftment by increasing the number of stem cells, increasing the proportion of hematopoietic progenitor cells, and providing additional, immature CD34+/CD45– progenitor cells.
In a single-arm, nonrandomized study, the investigators enrolled 16 patients ranging in age from 0.3 to 15.7 years with inborn errors of metabolism, marrow failure syndromes, severe immunodeficiency states, or hematologic malignancies.
Malignant conditions included B-cell precursor acute lymphoblastic leukemia (B-ALL; four patients), acute myeloid leukemia (AML; two), and T-cell ALL (one) in first complete remission, and T-cell lymphoblastic lymphoma following induction failure (one). Nonmalignant conditions included adrenoleukodystrophy (two patients), amegakaryotic thrombocytopenia (one), severe combined immunodeficiency (SCID; two), dyskeratosis congenita (one), chronic granulomatous disease (one), and severe congenital neutropenia (one).
The patients first underwent either myeloablative or reduced-intensity conditioning, followed 10 days later by infusion of unrelated cord blood and HPDSCs. Prior to HPDSC infusion, patients were medicated with diphenhydramine and hydrocortisone to prevent or reduce potential sensitivity reactions. HPDSCs were infused no sooner than 4 hours after the end of the cord blood infusion.
Patients received GVHD prophylaxis with either tacrolimus or cyclosporine, plus mycophenolate mofetil.
The combination appeared to be safe, with no cases of grade 3 or 4 toxicity secondary to HPDSC infusion.
The probability of neutrophil engraftment was 87.5%, with engraftment occurring at a median of 23 days (range 13-53). As noted before, all patients who had neutrophil engraftment had platelet engraftment, which was achieved at a median of 47 days (range, 20-98). In the group’s previous studies, median time to platelet engraftment was 53 days for patients who had undergone reduced-intensity conditioning, and 118 days for patients who had undergone myeloablation.
The probability of grade 2-4 acute GVHD within 100 days was 12.5%, and there were no cases of chronic GVHD.
Respective percentages of cord blood donor chimerism at days 30, 60, 100, and 180 were 88%, 98%, 99%, and 99%.
Immune reconstitution was strong, with normalization of mean CD3+, CD19+, and CD56+ cells occurring by day 100, CD8+ cells by day 180, and CD4+ cells by day 270.
There were three patient deaths: one from adenoviremia in a patient with B-ALL and CNS relapse, who had neutrophil engraftment at day 21; one in a patient with SCID, from adenoviremia and multiple system organ failure, who did not have engraftment before death; and one in a patient with severe congenital neutrophilia, who also did not have neutrophil engraftment.
None of the eight patients with malignant disease have experienced relapse to date, Dr. Flower noted.
The study was funded by a grant from Celgene Cellular Therapeutics. Dr. Flower reported having no conflicts of interest.
MONTREAL – A combination of placenta-derived stem cells and umbilical cord blood was associated with early engraftment and high degrees of cord blood donor chimerism in the treatment of children with both malignant and nonmalignant hematologic conditions requiring stem cell transplantation, updated results of a pilot study show.
Among 16 children treated with the combination, the probability of neutrophil engraftment was 87.5%, and all patients who had neutrophil engraftment went on to have platelet engraftment. The probability of 12-month overall survival was 81.2%, reported Allyson Flower, MD, from Boston Children’s Health Physicians in Hawthorne, N.Y. “The probability of grade II-IV acute graft vs. host disease was 12.5%, compared with 32.5% seen with unrelated cord blood in our group’s previous studies. Cellular immune reconstitution was robust,” she said at the annual meeting of the American Society of Pediatric Hematology/Oncology.
Augmenting cord blood
Although unrelated donor cord blood transplantation expands the donor pool, is rapidly available, and is associated with decreases in both severe acute graft vs. host disease (GVHD) and chronic GVHD, compared with other stem cell sources, the technique is hampered by limited cell doses, prolonged immune reconstitution time, delays in hematopoietic recovery, and a higher incidence of graft failure.
Early studies of myeloablative conditioning followed by unrelated umbilical or placental blood transplantation showed a median of 22-24 days to neutrophil engraftment (Blood 1996 88:795-802; N Engl J Med. 1996;335:157-66), Dr. Flower noted.
More recently, a multivariate analysis of patients who underwent reduced-intensity conditioning followed by hematopoietic stem cell transplant with unrelated cord blood showed that graft failure was an independent risk factor for worse overall survival (Biol Blood Marrow Transplant. 2013 Apr;19:4;552-61).
Multiple groups have shown that adding human placenta–derived stem cells (HPDSC) to cord blood transplantation can facilitate more rapid hematopoietic engraftment by increasing the number of stem cells, increasing the proportion of hematopoietic progenitor cells, and providing additional, immature CD34+/CD45– progenitor cells.
In a single-arm, nonrandomized study, the investigators enrolled 16 patients ranging in age from 0.3 to 15.7 years with inborn errors of metabolism, marrow failure syndromes, severe immunodeficiency states, or hematologic malignancies.
Malignant conditions included B-cell precursor acute lymphoblastic leukemia (B-ALL; four patients), acute myeloid leukemia (AML; two), and T-cell ALL (one) in first complete remission, and T-cell lymphoblastic lymphoma following induction failure (one). Nonmalignant conditions included adrenoleukodystrophy (two patients), amegakaryotic thrombocytopenia (one), severe combined immunodeficiency (SCID; two), dyskeratosis congenita (one), chronic granulomatous disease (one), and severe congenital neutropenia (one).
The patients first underwent either myeloablative or reduced-intensity conditioning, followed 10 days later by infusion of unrelated cord blood and HPDSCs. Prior to HPDSC infusion, patients were medicated with diphenhydramine and hydrocortisone to prevent or reduce potential sensitivity reactions. HPDSCs were infused no sooner than 4 hours after the end of the cord blood infusion.
Patients received GVHD prophylaxis with either tacrolimus or cyclosporine, plus mycophenolate mofetil.
The combination appeared to be safe, with no cases of grade 3 or 4 toxicity secondary to HPDSC infusion.
The probability of neutrophil engraftment was 87.5%, with engraftment occurring at a median of 23 days (range 13-53). As noted before, all patients who had neutrophil engraftment had platelet engraftment, which was achieved at a median of 47 days (range, 20-98). In the group’s previous studies, median time to platelet engraftment was 53 days for patients who had undergone reduced-intensity conditioning, and 118 days for patients who had undergone myeloablation.
The probability of grade 2-4 acute GVHD within 100 days was 12.5%, and there were no cases of chronic GVHD.
Respective percentages of cord blood donor chimerism at days 30, 60, 100, and 180 were 88%, 98%, 99%, and 99%.
Immune reconstitution was strong, with normalization of mean CD3+, CD19+, and CD56+ cells occurring by day 100, CD8+ cells by day 180, and CD4+ cells by day 270.
There were three patient deaths: one from adenoviremia in a patient with B-ALL and CNS relapse, who had neutrophil engraftment at day 21; one in a patient with SCID, from adenoviremia and multiple system organ failure, who did not have engraftment before death; and one in a patient with severe congenital neutrophilia, who also did not have neutrophil engraftment.
None of the eight patients with malignant disease have experienced relapse to date, Dr. Flower noted.
The study was funded by a grant from Celgene Cellular Therapeutics. Dr. Flower reported having no conflicts of interest.
Key clinical point: A combination of donor cord blood and human placenta–derived stem cells induced more rapid engraftment than cord blood alone.
Major finding: The probability of 12-month overall survival was 81%.
Data source: Open-label single-arm study in 16 children with severe malignant and nonmalignant diseases requiring hematopoietic stem cell transplants.
Disclosures: The study was funded by a grant from Celgene Cellular Therapeutics. Dr. Flower reported having no conflicts of interest.