User login
Experts to review ‘hot topics’ in pediatric hospital medicine research
Pediatric hospital medicine (PHM) is a fast-moving field, so having the best information is part of being a good doctor. But try going through all the relevant journals every month and pulling out the relevant findings. It’s a tall task.
Never fear! Akshata Hopkins, MD, an academic hospitalist at Johns Hopkins All Children’s Hospital in St. Petersburg, Fla., and Amit Singh, MD, of Stanford (Calif.) Children’s Health, have done the work for you. They reviewed every issue from 18 relevant journals over the last year and chose studies that are “hot topics” and involve important, evolving clinical questions that any physician caring for hospitalized children should know about.
As pediatric hospitalists, “we’re looking at articles from a pediatric hospital medicine standpoint, but the way that we chose the articles was based on topics that are prevalent to not only academic centers but community centers – and so it’s more broad,” Dr. Hopkins said. “The topics themselves are not necessarily new, but there are nuances to management [for which] every year there is new data that’s coming out. So what we’ve done is digest it for them.”
[[{"fid":"195832","view_mode":"medstat_image_flush_right","attributes":{"alt":"Dr. Akshata Hopkins","height":"220","width":"146","class":"media-element file-medstat-image-flush-right","data-delta":"1"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Dr. Akshata Hopkins","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"1":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Dr. Akshata Hopkins","field_file_image_credit[und][0][value]":""}}}]]Their session at 5:30 p.m. today, “Pediatric Update: Top Articles in Pediatric Hospital Medicine 2016,” will touch on both clinical and systems issues, often with a case used as a way to introduce topics, followed by a review of findings from a recent article. Dr. Hopkins said that questions will be answered throughout the session. Topics will include the management of young febrile infants, nasogastric feeding in bronchiolitis, prediction of severe pneumonia outcomes in children, and a review of quality measures that include patient experience and antibiotic stewardship.
“With the rise of more PCR testing and discussions of Choosing Wisely and high-value care, there’s more testing available,” Dr. Hopkins said. “But what is that going to cost versus what are the benefits that it brings? Are these tests valuable and in what way? And that’s kind of a hot topic. It depends on the age of the child and actually the results of [testing] are a little surprising.”
Dr. Singh said he hopes the session appeals to hospitalists in a wide array of care settings. “You want to make sure you are covering the breadth and scope of practice we might find ourselves in, whether it is in an adult hospital as the only hospital-based pediatrician covering ED consults, a NICU, a delivery room, and a small pediatric ward, or whether it is a pediatric hospitalist leading a team of medical students and residents in a large, free-standing, university-affiliated, children’s hospital,” he said.
Pediatric Update: Top Articles in Pediatric Hospital Medicine 2016
Wednesday, 5:30–6:20 p.m.
Pediatric hospital medicine (PHM) is a fast-moving field, so having the best information is part of being a good doctor. But try going through all the relevant journals every month and pulling out the relevant findings. It’s a tall task.
Never fear! Akshata Hopkins, MD, an academic hospitalist at Johns Hopkins All Children’s Hospital in St. Petersburg, Fla., and Amit Singh, MD, of Stanford (Calif.) Children’s Health, have done the work for you. They reviewed every issue from 18 relevant journals over the last year and chose studies that are “hot topics” and involve important, evolving clinical questions that any physician caring for hospitalized children should know about.
As pediatric hospitalists, “we’re looking at articles from a pediatric hospital medicine standpoint, but the way that we chose the articles was based on topics that are prevalent to not only academic centers but community centers – and so it’s more broad,” Dr. Hopkins said. “The topics themselves are not necessarily new, but there are nuances to management [for which] every year there is new data that’s coming out. So what we’ve done is digest it for them.”
[[{"fid":"195832","view_mode":"medstat_image_flush_right","attributes":{"alt":"Dr. Akshata Hopkins","height":"220","width":"146","class":"media-element file-medstat-image-flush-right","data-delta":"1"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Dr. Akshata Hopkins","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"1":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Dr. Akshata Hopkins","field_file_image_credit[und][0][value]":""}}}]]Their session at 5:30 p.m. today, “Pediatric Update: Top Articles in Pediatric Hospital Medicine 2016,” will touch on both clinical and systems issues, often with a case used as a way to introduce topics, followed by a review of findings from a recent article. Dr. Hopkins said that questions will be answered throughout the session. Topics will include the management of young febrile infants, nasogastric feeding in bronchiolitis, prediction of severe pneumonia outcomes in children, and a review of quality measures that include patient experience and antibiotic stewardship.
“With the rise of more PCR testing and discussions of Choosing Wisely and high-value care, there’s more testing available,” Dr. Hopkins said. “But what is that going to cost versus what are the benefits that it brings? Are these tests valuable and in what way? And that’s kind of a hot topic. It depends on the age of the child and actually the results of [testing] are a little surprising.”
Dr. Singh said he hopes the session appeals to hospitalists in a wide array of care settings. “You want to make sure you are covering the breadth and scope of practice we might find ourselves in, whether it is in an adult hospital as the only hospital-based pediatrician covering ED consults, a NICU, a delivery room, and a small pediatric ward, or whether it is a pediatric hospitalist leading a team of medical students and residents in a large, free-standing, university-affiliated, children’s hospital,” he said.
Pediatric Update: Top Articles in Pediatric Hospital Medicine 2016
Wednesday, 5:30–6:20 p.m.
Pediatric hospital medicine (PHM) is a fast-moving field, so having the best information is part of being a good doctor. But try going through all the relevant journals every month and pulling out the relevant findings. It’s a tall task.
Never fear! Akshata Hopkins, MD, an academic hospitalist at Johns Hopkins All Children’s Hospital in St. Petersburg, Fla., and Amit Singh, MD, of Stanford (Calif.) Children’s Health, have done the work for you. They reviewed every issue from 18 relevant journals over the last year and chose studies that are “hot topics” and involve important, evolving clinical questions that any physician caring for hospitalized children should know about.
As pediatric hospitalists, “we’re looking at articles from a pediatric hospital medicine standpoint, but the way that we chose the articles was based on topics that are prevalent to not only academic centers but community centers – and so it’s more broad,” Dr. Hopkins said. “The topics themselves are not necessarily new, but there are nuances to management [for which] every year there is new data that’s coming out. So what we’ve done is digest it for them.”
[[{"fid":"195832","view_mode":"medstat_image_flush_right","attributes":{"alt":"Dr. Akshata Hopkins","height":"220","width":"146","class":"media-element file-medstat-image-flush-right","data-delta":"1"},"fields":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Dr. Akshata Hopkins","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"1":{"format":"medstat_image_flush_right","field_file_image_caption[und][0][value]":"Dr. Akshata Hopkins","field_file_image_credit[und][0][value]":""}}}]]Their session at 5:30 p.m. today, “Pediatric Update: Top Articles in Pediatric Hospital Medicine 2016,” will touch on both clinical and systems issues, often with a case used as a way to introduce topics, followed by a review of findings from a recent article. Dr. Hopkins said that questions will be answered throughout the session. Topics will include the management of young febrile infants, nasogastric feeding in bronchiolitis, prediction of severe pneumonia outcomes in children, and a review of quality measures that include patient experience and antibiotic stewardship.
“With the rise of more PCR testing and discussions of Choosing Wisely and high-value care, there’s more testing available,” Dr. Hopkins said. “But what is that going to cost versus what are the benefits that it brings? Are these tests valuable and in what way? And that’s kind of a hot topic. It depends on the age of the child and actually the results of [testing] are a little surprising.”
Dr. Singh said he hopes the session appeals to hospitalists in a wide array of care settings. “You want to make sure you are covering the breadth and scope of practice we might find ourselves in, whether it is in an adult hospital as the only hospital-based pediatrician covering ED consults, a NICU, a delivery room, and a small pediatric ward, or whether it is a pediatric hospitalist leading a team of medical students and residents in a large, free-standing, university-affiliated, children’s hospital,” he said.
Pediatric Update: Top Articles in Pediatric Hospital Medicine 2016
Wednesday, 5:30–6:20 p.m.
Telehospitalists can expand capability, capacity
The cost of health care is on the lips of everyone, from the thousands of HM17 attendees to congressional leaders to President Donald Trump. Yet, one long-promoted answer – telemedicine practiced by telehospitalists – is not as widely adopted as its proponents say it should be. After Wednesday’s session, “Foundations of a Hospital Medicine Telemedicine Program,” which begins at 4:15 p.m., at least a few more physicians will see it as an option.
“The timing is there,” said copresenter Talbot “Mac” McCormick, MD, president and chief executive officer of Eagle Telemedicine of Atlanta. “Telemedicine has come of age.”
[[{"fid":"194482","view_mode":"medstat_image_flush_left","attributes":{"alt":"Dr. Talbot \\\u0022Mac\u0022 McCormick, Eagle telemedicine, Atlanta","height":"220","width":"157","class":"media-element file-medstat-image-flush-left","data-delta":"1"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Dr. Talbot \u0022Mac\u0022 McCormick","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"1":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Dr. Talbot \u0022Mac\u0022 McCormick","field_file_image_credit[und][0][value]":""}}}]]Copresenter Shannon Carpenter, BS, MBA, vice president at Charlotte, N.C.–based Carolinas Healthcare System, said telemedicine is a “relevancy issue.”
“We are hearing so much about a need for an alternative care model, [and] the virtual-care model is incredibly relevant in today’s environment,” Ms. Carpenter said. Adding to telemedicine’s basic advantage is its ability to help alleviate staffing issues.
“Telemedicine and telehospitalists can support and fill out gaps [and] can expand capability and capacity,” Dr. McCormick added. “Then, the other part is economy of scale. You can share a telehospitalist amongst a couple of small hospitals ... an advantage over each one trying to do it themselves.”
For example, individual institutions might not be able to keep a hospitalist busy 12 hours a night, but a nocturnist is still a requirement.
“When they need somebody, they sure need them,” Dr. McCormick said. “So, you get an economy of scale that several small hospitals effectively can share one physician because the space continuum – not necessarily the time continuum – but the space continuum and the geographic continuum is different engaging telemedicine versus people physically on the ground.”
Ms. Carpenter said that one of the obstacles to telemedicine is simply getting physicians to change habits.
“The biggest hurdles that we experienced were with buy-in for the care-delivery model,” she said. “Surprisingly, it’s not from the patients’ perspectives. It’s from either from the physicians who should be providing the service and/or from the staff in the hospital who aren’t used to the technology or the method of care delivery. To avoid this, it’s just like anything else: over-communication, education, and an ability to explain why and how care will be delivered.”
In addition to the difficulty of changing the culture, integration failures are another potential pitfall, according to Dr. McCormick.
“I think that communication gets to part of that,” he said. “I think [telemedicine should be viewed] ... not [as] a segmented silo of a hospitalist team – to be functional and to work well, it has to be integrated with the team so that it’s just a seamless part of the care team of the doctors, the nurses, the nurse practitioners.”
Both presenters agree that, as value-based payments and alternative payment models proliferate in the coming years, telemedicine will only grow as hospitals and hospitalist group leaders look for cost efficiencies. It will also be broader than just nocturnist services.
Ms. Carpenter said future uses could include expansion to ambulatory clinics for transitioning patients from acute care back to their medical home environment or to telehospitalists supporting paramedics on home visits.
“The use of tools and technology like this can allow care to bridge across these multiple geographic locations of care and do it in a way that provides continuity, economy of scale, consistent and high quality care,” Dr. McCormick said.
Foundations of a Hospital Medicine Telemedicine Program
Wednesday, 4:15–5:20 p.m.
Available via HM17 On Demand
The cost of health care is on the lips of everyone, from the thousands of HM17 attendees to congressional leaders to President Donald Trump. Yet, one long-promoted answer – telemedicine practiced by telehospitalists – is not as widely adopted as its proponents say it should be. After Wednesday’s session, “Foundations of a Hospital Medicine Telemedicine Program,” which begins at 4:15 p.m., at least a few more physicians will see it as an option.
“The timing is there,” said copresenter Talbot “Mac” McCormick, MD, president and chief executive officer of Eagle Telemedicine of Atlanta. “Telemedicine has come of age.”
[[{"fid":"194482","view_mode":"medstat_image_flush_left","attributes":{"alt":"Dr. Talbot \\\u0022Mac\u0022 McCormick, Eagle telemedicine, Atlanta","height":"220","width":"157","class":"media-element file-medstat-image-flush-left","data-delta":"1"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Dr. Talbot \u0022Mac\u0022 McCormick","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"1":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Dr. Talbot \u0022Mac\u0022 McCormick","field_file_image_credit[und][0][value]":""}}}]]Copresenter Shannon Carpenter, BS, MBA, vice president at Charlotte, N.C.–based Carolinas Healthcare System, said telemedicine is a “relevancy issue.”
“We are hearing so much about a need for an alternative care model, [and] the virtual-care model is incredibly relevant in today’s environment,” Ms. Carpenter said. Adding to telemedicine’s basic advantage is its ability to help alleviate staffing issues.
“Telemedicine and telehospitalists can support and fill out gaps [and] can expand capability and capacity,” Dr. McCormick added. “Then, the other part is economy of scale. You can share a telehospitalist amongst a couple of small hospitals ... an advantage over each one trying to do it themselves.”
For example, individual institutions might not be able to keep a hospitalist busy 12 hours a night, but a nocturnist is still a requirement.
“When they need somebody, they sure need them,” Dr. McCormick said. “So, you get an economy of scale that several small hospitals effectively can share one physician because the space continuum – not necessarily the time continuum – but the space continuum and the geographic continuum is different engaging telemedicine versus people physically on the ground.”
Ms. Carpenter said that one of the obstacles to telemedicine is simply getting physicians to change habits.
“The biggest hurdles that we experienced were with buy-in for the care-delivery model,” she said. “Surprisingly, it’s not from the patients’ perspectives. It’s from either from the physicians who should be providing the service and/or from the staff in the hospital who aren’t used to the technology or the method of care delivery. To avoid this, it’s just like anything else: over-communication, education, and an ability to explain why and how care will be delivered.”
In addition to the difficulty of changing the culture, integration failures are another potential pitfall, according to Dr. McCormick.
“I think that communication gets to part of that,” he said. “I think [telemedicine should be viewed] ... not [as] a segmented silo of a hospitalist team – to be functional and to work well, it has to be integrated with the team so that it’s just a seamless part of the care team of the doctors, the nurses, the nurse practitioners.”
Both presenters agree that, as value-based payments and alternative payment models proliferate in the coming years, telemedicine will only grow as hospitals and hospitalist group leaders look for cost efficiencies. It will also be broader than just nocturnist services.
Ms. Carpenter said future uses could include expansion to ambulatory clinics for transitioning patients from acute care back to their medical home environment or to telehospitalists supporting paramedics on home visits.
“The use of tools and technology like this can allow care to bridge across these multiple geographic locations of care and do it in a way that provides continuity, economy of scale, consistent and high quality care,” Dr. McCormick said.
Foundations of a Hospital Medicine Telemedicine Program
Wednesday, 4:15–5:20 p.m.
Available via HM17 On Demand
The cost of health care is on the lips of everyone, from the thousands of HM17 attendees to congressional leaders to President Donald Trump. Yet, one long-promoted answer – telemedicine practiced by telehospitalists – is not as widely adopted as its proponents say it should be. After Wednesday’s session, “Foundations of a Hospital Medicine Telemedicine Program,” which begins at 4:15 p.m., at least a few more physicians will see it as an option.
“The timing is there,” said copresenter Talbot “Mac” McCormick, MD, president and chief executive officer of Eagle Telemedicine of Atlanta. “Telemedicine has come of age.”
[[{"fid":"194482","view_mode":"medstat_image_flush_left","attributes":{"alt":"Dr. Talbot \\\u0022Mac\u0022 McCormick, Eagle telemedicine, Atlanta","height":"220","width":"157","class":"media-element file-medstat-image-flush-left","data-delta":"1"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Dr. Talbot \u0022Mac\u0022 McCormick","field_file_image_credit[und][0][value]":"","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"1":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Dr. Talbot \u0022Mac\u0022 McCormick","field_file_image_credit[und][0][value]":""}}}]]Copresenter Shannon Carpenter, BS, MBA, vice president at Charlotte, N.C.–based Carolinas Healthcare System, said telemedicine is a “relevancy issue.”
“We are hearing so much about a need for an alternative care model, [and] the virtual-care model is incredibly relevant in today’s environment,” Ms. Carpenter said. Adding to telemedicine’s basic advantage is its ability to help alleviate staffing issues.
“Telemedicine and telehospitalists can support and fill out gaps [and] can expand capability and capacity,” Dr. McCormick added. “Then, the other part is economy of scale. You can share a telehospitalist amongst a couple of small hospitals ... an advantage over each one trying to do it themselves.”
For example, individual institutions might not be able to keep a hospitalist busy 12 hours a night, but a nocturnist is still a requirement.
“When they need somebody, they sure need them,” Dr. McCormick said. “So, you get an economy of scale that several small hospitals effectively can share one physician because the space continuum – not necessarily the time continuum – but the space continuum and the geographic continuum is different engaging telemedicine versus people physically on the ground.”
Ms. Carpenter said that one of the obstacles to telemedicine is simply getting physicians to change habits.
“The biggest hurdles that we experienced were with buy-in for the care-delivery model,” she said. “Surprisingly, it’s not from the patients’ perspectives. It’s from either from the physicians who should be providing the service and/or from the staff in the hospital who aren’t used to the technology or the method of care delivery. To avoid this, it’s just like anything else: over-communication, education, and an ability to explain why and how care will be delivered.”
In addition to the difficulty of changing the culture, integration failures are another potential pitfall, according to Dr. McCormick.
“I think that communication gets to part of that,” he said. “I think [telemedicine should be viewed] ... not [as] a segmented silo of a hospitalist team – to be functional and to work well, it has to be integrated with the team so that it’s just a seamless part of the care team of the doctors, the nurses, the nurse practitioners.”
Both presenters agree that, as value-based payments and alternative payment models proliferate in the coming years, telemedicine will only grow as hospitals and hospitalist group leaders look for cost efficiencies. It will also be broader than just nocturnist services.
Ms. Carpenter said future uses could include expansion to ambulatory clinics for transitioning patients from acute care back to their medical home environment or to telehospitalists supporting paramedics on home visits.
“The use of tools and technology like this can allow care to bridge across these multiple geographic locations of care and do it in a way that provides continuity, economy of scale, consistent and high quality care,” Dr. McCormick said.
Foundations of a Hospital Medicine Telemedicine Program
Wednesday, 4:15–5:20 p.m.
Available via HM17 On Demand
Welcome to Annual Meeting Day 2
Day 1 has already raised the bar for the SHM Annual Meeting. Day 2, Wednesday, will set a whole new standard with courses, speakers, content, and perspective.
Leading off the day will be the presentation of the best of Research and Innovations. This year, we had hundreds of submissions, and the kickoff of Day 2 will showcase the very best of the best! Following immediately, we will recognize the winners of the SHM Awards of Excellence.
Then, off to the main meeting!
This year, we anticipated that some sessions would be so hot that we would have to hold them twice to meet the demand. These sessions are labeled with their own track and include my own personal favorite – and ironically named – series at SHM, “Things We Do for No Reason.” So if you missed this or any of the other talks – heart failure, pulmonary embolism, infectious diseases, delirium, and syncope – here’s your second chance!
But wait – there’s more! This year, for the first time, we have Maintenance of Certification credit available for attendees of the MOC-Clinical Updates sessions and the Rapid Fire Sessions. So, go right ahead and get the MOC credits while you catch up on the latest evidence.
Every year, hospitalists, residents, and students from all over submit hundreds of insightful clinical vignettes posters. Lunch and learn in the Exhibit Hall and peruse the great cases while the judges debate over the absolute best. Afterward is the can’t- miss feature at every Annual Meeting: the Update in Hospital Medicine – this year being delivered by a pair of hospitalist leaders from the heartland, Rachel Thompson, MD, MPH, SFHM and Chad Miller, MD, FHM. Come for Rachel and Chad’s interpretation of the most important and relevant recent literature in adult hospital medicine.
Resident or medical student? You’re in good company at HM17. We have more trainees here than ever before. At 5:30 p.m., we’re holding a special session for you: A skills workshop on “Mastering the Job Interview.” We don’t learn these things in medical or residency – learn them at HM17!
A few other key sessions close out Day 2: Ron Greeno, MD, FCCP, MHM, and Nasim Afsar, MD, SFHM, present on the role hospitalists can (and must) play in the rollout and management of Alternative Payment Models. Then, there’s the mysteriously titled “Myths, Misunderstandings, Medicare & Money: PA/NP and Physician Teams in Hospital Medicine.”
Finally, wind down and see what’s new in the pediatrics world with the Pediatric Hospital Medicine Update with Akshata Hopkins, MD, and Amit Singh, MD.
If yesterday set the tone and tomorrow is the wrap up, Day 2 – today – is the middle act of HM17 and is sure to be educational, provocative, exciting, and an exceptional learning experience. Be sure to take time to walk through the exhibit hall. Please also stop by the SHM Booth to meet the hardworking SHM staff who have made this meeting a great success and introduce yourself to members of the Board who will be present in the booth during the course of the meeting!
Dr. Harte is outgoing president of SHM and president of Cleveland Clinic Akron General and Southern Region.
Day 1 has already raised the bar for the SHM Annual Meeting. Day 2, Wednesday, will set a whole new standard with courses, speakers, content, and perspective.
Leading off the day will be the presentation of the best of Research and Innovations. This year, we had hundreds of submissions, and the kickoff of Day 2 will showcase the very best of the best! Following immediately, we will recognize the winners of the SHM Awards of Excellence.
Then, off to the main meeting!
This year, we anticipated that some sessions would be so hot that we would have to hold them twice to meet the demand. These sessions are labeled with their own track and include my own personal favorite – and ironically named – series at SHM, “Things We Do for No Reason.” So if you missed this or any of the other talks – heart failure, pulmonary embolism, infectious diseases, delirium, and syncope – here’s your second chance!
But wait – there’s more! This year, for the first time, we have Maintenance of Certification credit available for attendees of the MOC-Clinical Updates sessions and the Rapid Fire Sessions. So, go right ahead and get the MOC credits while you catch up on the latest evidence.
Every year, hospitalists, residents, and students from all over submit hundreds of insightful clinical vignettes posters. Lunch and learn in the Exhibit Hall and peruse the great cases while the judges debate over the absolute best. Afterward is the can’t- miss feature at every Annual Meeting: the Update in Hospital Medicine – this year being delivered by a pair of hospitalist leaders from the heartland, Rachel Thompson, MD, MPH, SFHM and Chad Miller, MD, FHM. Come for Rachel and Chad’s interpretation of the most important and relevant recent literature in adult hospital medicine.
Resident or medical student? You’re in good company at HM17. We have more trainees here than ever before. At 5:30 p.m., we’re holding a special session for you: A skills workshop on “Mastering the Job Interview.” We don’t learn these things in medical or residency – learn them at HM17!
A few other key sessions close out Day 2: Ron Greeno, MD, FCCP, MHM, and Nasim Afsar, MD, SFHM, present on the role hospitalists can (and must) play in the rollout and management of Alternative Payment Models. Then, there’s the mysteriously titled “Myths, Misunderstandings, Medicare & Money: PA/NP and Physician Teams in Hospital Medicine.”
Finally, wind down and see what’s new in the pediatrics world with the Pediatric Hospital Medicine Update with Akshata Hopkins, MD, and Amit Singh, MD.
If yesterday set the tone and tomorrow is the wrap up, Day 2 – today – is the middle act of HM17 and is sure to be educational, provocative, exciting, and an exceptional learning experience. Be sure to take time to walk through the exhibit hall. Please also stop by the SHM Booth to meet the hardworking SHM staff who have made this meeting a great success and introduce yourself to members of the Board who will be present in the booth during the course of the meeting!
Dr. Harte is outgoing president of SHM and president of Cleveland Clinic Akron General and Southern Region.
Day 1 has already raised the bar for the SHM Annual Meeting. Day 2, Wednesday, will set a whole new standard with courses, speakers, content, and perspective.
Leading off the day will be the presentation of the best of Research and Innovations. This year, we had hundreds of submissions, and the kickoff of Day 2 will showcase the very best of the best! Following immediately, we will recognize the winners of the SHM Awards of Excellence.
Then, off to the main meeting!
This year, we anticipated that some sessions would be so hot that we would have to hold them twice to meet the demand. These sessions are labeled with their own track and include my own personal favorite – and ironically named – series at SHM, “Things We Do for No Reason.” So if you missed this or any of the other talks – heart failure, pulmonary embolism, infectious diseases, delirium, and syncope – here’s your second chance!
But wait – there’s more! This year, for the first time, we have Maintenance of Certification credit available for attendees of the MOC-Clinical Updates sessions and the Rapid Fire Sessions. So, go right ahead and get the MOC credits while you catch up on the latest evidence.
Every year, hospitalists, residents, and students from all over submit hundreds of insightful clinical vignettes posters. Lunch and learn in the Exhibit Hall and peruse the great cases while the judges debate over the absolute best. Afterward is the can’t- miss feature at every Annual Meeting: the Update in Hospital Medicine – this year being delivered by a pair of hospitalist leaders from the heartland, Rachel Thompson, MD, MPH, SFHM and Chad Miller, MD, FHM. Come for Rachel and Chad’s interpretation of the most important and relevant recent literature in adult hospital medicine.
Resident or medical student? You’re in good company at HM17. We have more trainees here than ever before. At 5:30 p.m., we’re holding a special session for you: A skills workshop on “Mastering the Job Interview.” We don’t learn these things in medical or residency – learn them at HM17!
A few other key sessions close out Day 2: Ron Greeno, MD, FCCP, MHM, and Nasim Afsar, MD, SFHM, present on the role hospitalists can (and must) play in the rollout and management of Alternative Payment Models. Then, there’s the mysteriously titled “Myths, Misunderstandings, Medicare & Money: PA/NP and Physician Teams in Hospital Medicine.”
Finally, wind down and see what’s new in the pediatrics world with the Pediatric Hospital Medicine Update with Akshata Hopkins, MD, and Amit Singh, MD.
If yesterday set the tone and tomorrow is the wrap up, Day 2 – today – is the middle act of HM17 and is sure to be educational, provocative, exciting, and an exceptional learning experience. Be sure to take time to walk through the exhibit hall. Please also stop by the SHM Booth to meet the hardworking SHM staff who have made this meeting a great success and introduce yourself to members of the Board who will be present in the booth during the course of the meeting!
Dr. Harte is outgoing president of SHM and president of Cleveland Clinic Akron General and Southern Region.
Accountability and Whistleblowers in VA Spotlight
In an ongoing effort to improve oversight and to protect potential whistleblowers at the VA, President Trump has signed a new executive order creating the Office of Accountability and Whistleblower Protection. The executive order establishes an office that will report directly to VA Secretary David. J. Shulkin, MD. “Accountability is an important issue to us at VA and something that we’re focusing on to make sure that we have employees who work and are committed to the mission of serving our veterans,” Dr. Shulkin explained at an April 26 press conference. “When we find employees that have deviated from those values, we want to make sure that we can move them outside the VA and not have them working at VA.”
The new office is not the first effort at the VA to protect whistleblowers or to expedite the removal of employees. In 2014 the Office of Accountability and Review was established to increase central office scrutiny of senior-level executives at local and regional VA facilities. In contrast Dr. Shulkin noted, “This is a broader office that will be taking a look at all of our employees.” The current VA Whistleblower Office, created just last year will be incorporated into the new office, according to Dr. Shulkin.
Related: VA Secretary Shulkin Calls for New Powers to Fire VA Employees
Not everyone greeted the announcement with praise. “This rush to fire feds faster, first at VA, but with attempts to spread it across government, comes with a serious risk,” argued Washington Post columnist Joe Davidson. “Yes, due process rights can be slow and cumbersome. They protect, however, not just employees, but more importantly, also the public from a politicized system that favors citizens of one political party over another. Reforms must respect civil service protections. They should be acknowledged by government leaders and not be ignored as they were at the signing.”
Officials at the Project on Government Oversight expressed concern that whistleblowers should have an independent channel to report their concerns. Like the current whistleblower office, the new structure “may do far more damage than good,” the organization reported “It is incredibly important that whistleblowers have the ability to go to an independent office to report wrongdoing, since an internal office could be pressured to act in the VA’s interest by covering up problems and silencing whistleblowers.”
Related: VA Launches Investigation into Cincinnati Facility Mismanagement
Dr. Shulkin insisted that the office did not negate the need for the new legislation that he has called for that would speed the process of firing problem employees. Nor will the new office replace the hot line set up by the White House for veteran complaints about VA service. “These are all 3 efforts that are important for us to identify issues that are preventing us from doing the very best job that we can,” he explained. “We’re keeping our employees and our executives accountable to the values, to be able to work at the VA. We are soliciting input from veterans who feel that they have issues that they want to share with us, and that’s what the hotline will be doing.”
While the focus on the effort is on employees malfeasance, Dr. Shulkin cautioned that the VA was still concerned about employee morale and protecting whistleblowers from retaliation. “Our employees have to feel safe, when they see something, to tell us about it,” he explained. “The message is clear that we will not tolerate whistleblower retaliation in the [VA]. And we will take actions if we do determine that retaliation has been imposed upon an employee who has come forth with an issue.”
Related: Deputy Secretary of Veterans Affairs Gibson Defends VA Discipline Guidelines
Dr. Shulkin also announced a new task force that would tackle fraud, waste, and abuse, “to make sure that we are aggressively investigating any issues that might lead to the waste of taxpayer dollars.”
In an ongoing effort to improve oversight and to protect potential whistleblowers at the VA, President Trump has signed a new executive order creating the Office of Accountability and Whistleblower Protection. The executive order establishes an office that will report directly to VA Secretary David. J. Shulkin, MD. “Accountability is an important issue to us at VA and something that we’re focusing on to make sure that we have employees who work and are committed to the mission of serving our veterans,” Dr. Shulkin explained at an April 26 press conference. “When we find employees that have deviated from those values, we want to make sure that we can move them outside the VA and not have them working at VA.”
The new office is not the first effort at the VA to protect whistleblowers or to expedite the removal of employees. In 2014 the Office of Accountability and Review was established to increase central office scrutiny of senior-level executives at local and regional VA facilities. In contrast Dr. Shulkin noted, “This is a broader office that will be taking a look at all of our employees.” The current VA Whistleblower Office, created just last year will be incorporated into the new office, according to Dr. Shulkin.
Related: VA Secretary Shulkin Calls for New Powers to Fire VA Employees
Not everyone greeted the announcement with praise. “This rush to fire feds faster, first at VA, but with attempts to spread it across government, comes with a serious risk,” argued Washington Post columnist Joe Davidson. “Yes, due process rights can be slow and cumbersome. They protect, however, not just employees, but more importantly, also the public from a politicized system that favors citizens of one political party over another. Reforms must respect civil service protections. They should be acknowledged by government leaders and not be ignored as they were at the signing.”
Officials at the Project on Government Oversight expressed concern that whistleblowers should have an independent channel to report their concerns. Like the current whistleblower office, the new structure “may do far more damage than good,” the organization reported “It is incredibly important that whistleblowers have the ability to go to an independent office to report wrongdoing, since an internal office could be pressured to act in the VA’s interest by covering up problems and silencing whistleblowers.”
Related: VA Launches Investigation into Cincinnati Facility Mismanagement
Dr. Shulkin insisted that the office did not negate the need for the new legislation that he has called for that would speed the process of firing problem employees. Nor will the new office replace the hot line set up by the White House for veteran complaints about VA service. “These are all 3 efforts that are important for us to identify issues that are preventing us from doing the very best job that we can,” he explained. “We’re keeping our employees and our executives accountable to the values, to be able to work at the VA. We are soliciting input from veterans who feel that they have issues that they want to share with us, and that’s what the hotline will be doing.”
While the focus on the effort is on employees malfeasance, Dr. Shulkin cautioned that the VA was still concerned about employee morale and protecting whistleblowers from retaliation. “Our employees have to feel safe, when they see something, to tell us about it,” he explained. “The message is clear that we will not tolerate whistleblower retaliation in the [VA]. And we will take actions if we do determine that retaliation has been imposed upon an employee who has come forth with an issue.”
Related: Deputy Secretary of Veterans Affairs Gibson Defends VA Discipline Guidelines
Dr. Shulkin also announced a new task force that would tackle fraud, waste, and abuse, “to make sure that we are aggressively investigating any issues that might lead to the waste of taxpayer dollars.”
In an ongoing effort to improve oversight and to protect potential whistleblowers at the VA, President Trump has signed a new executive order creating the Office of Accountability and Whistleblower Protection. The executive order establishes an office that will report directly to VA Secretary David. J. Shulkin, MD. “Accountability is an important issue to us at VA and something that we’re focusing on to make sure that we have employees who work and are committed to the mission of serving our veterans,” Dr. Shulkin explained at an April 26 press conference. “When we find employees that have deviated from those values, we want to make sure that we can move them outside the VA and not have them working at VA.”
The new office is not the first effort at the VA to protect whistleblowers or to expedite the removal of employees. In 2014 the Office of Accountability and Review was established to increase central office scrutiny of senior-level executives at local and regional VA facilities. In contrast Dr. Shulkin noted, “This is a broader office that will be taking a look at all of our employees.” The current VA Whistleblower Office, created just last year will be incorporated into the new office, according to Dr. Shulkin.
Related: VA Secretary Shulkin Calls for New Powers to Fire VA Employees
Not everyone greeted the announcement with praise. “This rush to fire feds faster, first at VA, but with attempts to spread it across government, comes with a serious risk,” argued Washington Post columnist Joe Davidson. “Yes, due process rights can be slow and cumbersome. They protect, however, not just employees, but more importantly, also the public from a politicized system that favors citizens of one political party over another. Reforms must respect civil service protections. They should be acknowledged by government leaders and not be ignored as they were at the signing.”
Officials at the Project on Government Oversight expressed concern that whistleblowers should have an independent channel to report their concerns. Like the current whistleblower office, the new structure “may do far more damage than good,” the organization reported “It is incredibly important that whistleblowers have the ability to go to an independent office to report wrongdoing, since an internal office could be pressured to act in the VA’s interest by covering up problems and silencing whistleblowers.”
Related: VA Launches Investigation into Cincinnati Facility Mismanagement
Dr. Shulkin insisted that the office did not negate the need for the new legislation that he has called for that would speed the process of firing problem employees. Nor will the new office replace the hot line set up by the White House for veteran complaints about VA service. “These are all 3 efforts that are important for us to identify issues that are preventing us from doing the very best job that we can,” he explained. “We’re keeping our employees and our executives accountable to the values, to be able to work at the VA. We are soliciting input from veterans who feel that they have issues that they want to share with us, and that’s what the hotline will be doing.”
While the focus on the effort is on employees malfeasance, Dr. Shulkin cautioned that the VA was still concerned about employee morale and protecting whistleblowers from retaliation. “Our employees have to feel safe, when they see something, to tell us about it,” he explained. “The message is clear that we will not tolerate whistleblower retaliation in the [VA]. And we will take actions if we do determine that retaliation has been imposed upon an employee who has come forth with an issue.”
Related: Deputy Secretary of Veterans Affairs Gibson Defends VA Discipline Guidelines
Dr. Shulkin also announced a new task force that would tackle fraud, waste, and abuse, “to make sure that we are aggressively investigating any issues that might lead to the waste of taxpayer dollars.”
Up the Creek Without a Provider
ANSWER
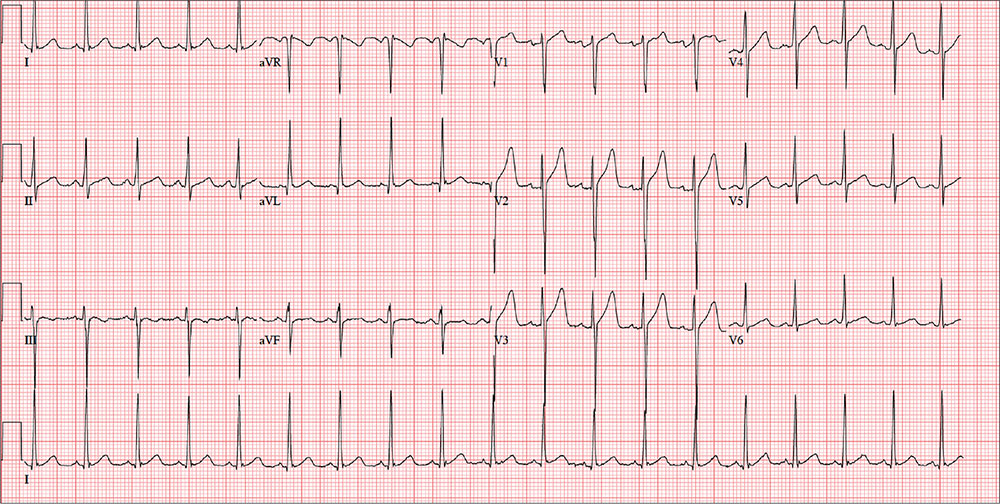
The ECG is remarkable for sinus tachycardia and left ventricular hypertrophy. Equal numbers of P and QRS complexes with a consistent PR interval indicate sinus tachycardia. High voltages in the limb leads (R in lead I and S in lead III ≥ 25 mm) or precordial leads (S in lead V1 and R in lead V5 or V6 ≥ 35 mm) constitute left ventricular hypertrophy.
ANSWER
The ECG is remarkable for sinus tachycardia and left ventricular hypertrophy. Equal numbers of P and QRS complexes with a consistent PR interval indicate sinus tachycardia. High voltages in the limb leads (R in lead I and S in lead III ≥ 25 mm) or precordial leads (S in lead V1 and R in lead V5 or V6 ≥ 35 mm) constitute left ventricular hypertrophy.
ANSWER
The ECG is remarkable for sinus tachycardia and left ventricular hypertrophy. Equal numbers of P and QRS complexes with a consistent PR interval indicate sinus tachycardia. High voltages in the limb leads (R in lead I and S in lead III ≥ 25 mm) or precordial leads (S in lead V1 and R in lead V5 or V6 ≥ 35 mm) constitute left ventricular hypertrophy.
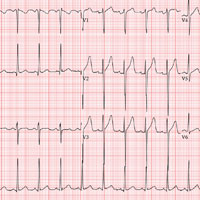
A 40-year-old man presents to establish care with you as his primary care provider; he was recently forced to make this change because his prior network stopped accepting his insurance policy. He works as an electrical engineer and is a competitive rower. He began rowing at age 14; he was on his university’s team and now rows in a competitive, age-matched league.
He has never had any health-related issues, apart from sprained ankles and a fractured right clavicle in childhood. He exercises daily at an exclusive men’s club. He has no history of hypertension, diabetes, hyperlipidemia, chest pain or discomfort, shortness of breath, or exertional dyspnea. He has no surgical history.
The patient is single and
He is not taking any medications and has no known drug allergies. The review of systems is remarkable only for recent rhinitis, which is resolving.
Vital signs include a blood pressure of 124/60 mm Hg; pulse, 110 beats/min; respiratory rate, 14 breaths/min-1; and temperature, 98.4°F. His weight is 194 lb and his height, 75 in.
The physical exam reveals a mildly anxious male in no acute distress. When asked if he is nervous, he says yes, because he’s “used to having someone else examine him.” His HEENT exam is normal, as is his thyroid exam. He has no jugular venous distention. The lungs are clear bilaterally. His heart rate is 110 beats/min and regular with no murmurs, rubs, or gallops. The abdomen is soft and nontender, with no palpable organomegaly. Peripheral pulses are strong and equal bilaterally, and there is no peripheral edema. The neurologic exam is grossly intact.
Routine blood tests are performed, and an HIV titer, ECG, and chest x-ray are ordered. The ECG reveals a heart rate of 112 beats/min; PR interval, 132 ms; QRS duration, 76 ms; QT/QTc interval, 326/444 ms; P axis, 59°; R axis, –8°; T axis, 26°. What is your interpretation of this ECG?
Hospitalists can do better at end-of-life care, expert says
As a 99-year-old friend neared the end of her life, she offered a lesson for the health care world, said Deborah Korenstein, MD, chief of general internal medicine and director of clinical effectiveness at Memorial Sloan Kettering Cancer Center, N.Y., in the Tuesday session “Finding High Value Inpatient Care at the End of Life.”
The woman, nicknamed “Mitch,” had bluntly made her preference clear, Dr. Korenstein said: “She wanted to live independently as long as she could, and then, she wanted to be dead.”
But when a pathology report showed urothelial cancer, that preference didn’t stop an oncology urologist from suggesting that Mitch enter a clinical trial on an unproven therapy. Worse, Mitch initially said “yes” to this idea, seemingly because she thought that’s what she was expected to say.
It was only when Dr. Korenstein spoke with her that she changed her mind, entered inpatient hospice care, and died peacefully.
“I think it’s a cautionary tale about when a patient is crystal clear about their wishes,” she said. “The wave of the medical system kind of pushes them along in a particular direction that may go against their wishes.”
Dr. Korenstein said U.S. health care system does fairly well in some areas – for instance, research shows that about 60% of people die in their preferred location, whether at home or somewhere else. But it does not do so well in others – a 2013 Journal of General Internal Medicine study found that, during 2002-2008, Medicare beneficiaries typically spent $39,000 out of pocket on their medical care, and in 25% of cases, what they spent exceeded the total value of their assets.
As far as individual preferences, these tend to correlate poorly with the care that people actually get, Dr. Korenstein said. Patients often don’t express their wishes, doctors are poor judges of what matters to individual people, and care is largely driven by physician preferences and by the care setting involved, she said.
Given those problems, she said, “we cannot possibly be providing high-value individualized care.”
Hospitalists are well positioned to help patients’ preferences align with care, she added. Sometimes, a sustained relationship with a patient, while generally a positive thing, might lead a provider to become invested in their care in “ways that are not always rational.” So a hospitalist can have a helpful vantage point.
As a 99-year-old friend neared the end of her life, she offered a lesson for the health care world, said Deborah Korenstein, MD, chief of general internal medicine and director of clinical effectiveness at Memorial Sloan Kettering Cancer Center, N.Y., in the Tuesday session “Finding High Value Inpatient Care at the End of Life.”
The woman, nicknamed “Mitch,” had bluntly made her preference clear, Dr. Korenstein said: “She wanted to live independently as long as she could, and then, she wanted to be dead.”
But when a pathology report showed urothelial cancer, that preference didn’t stop an oncology urologist from suggesting that Mitch enter a clinical trial on an unproven therapy. Worse, Mitch initially said “yes” to this idea, seemingly because she thought that’s what she was expected to say.
It was only when Dr. Korenstein spoke with her that she changed her mind, entered inpatient hospice care, and died peacefully.
“I think it’s a cautionary tale about when a patient is crystal clear about their wishes,” she said. “The wave of the medical system kind of pushes them along in a particular direction that may go against their wishes.”
Dr. Korenstein said U.S. health care system does fairly well in some areas – for instance, research shows that about 60% of people die in their preferred location, whether at home or somewhere else. But it does not do so well in others – a 2013 Journal of General Internal Medicine study found that, during 2002-2008, Medicare beneficiaries typically spent $39,000 out of pocket on their medical care, and in 25% of cases, what they spent exceeded the total value of their assets.
As far as individual preferences, these tend to correlate poorly with the care that people actually get, Dr. Korenstein said. Patients often don’t express their wishes, doctors are poor judges of what matters to individual people, and care is largely driven by physician preferences and by the care setting involved, she said.
Given those problems, she said, “we cannot possibly be providing high-value individualized care.”
Hospitalists are well positioned to help patients’ preferences align with care, she added. Sometimes, a sustained relationship with a patient, while generally a positive thing, might lead a provider to become invested in their care in “ways that are not always rational.” So a hospitalist can have a helpful vantage point.
As a 99-year-old friend neared the end of her life, she offered a lesson for the health care world, said Deborah Korenstein, MD, chief of general internal medicine and director of clinical effectiveness at Memorial Sloan Kettering Cancer Center, N.Y., in the Tuesday session “Finding High Value Inpatient Care at the End of Life.”
The woman, nicknamed “Mitch,” had bluntly made her preference clear, Dr. Korenstein said: “She wanted to live independently as long as she could, and then, she wanted to be dead.”
But when a pathology report showed urothelial cancer, that preference didn’t stop an oncology urologist from suggesting that Mitch enter a clinical trial on an unproven therapy. Worse, Mitch initially said “yes” to this idea, seemingly because she thought that’s what she was expected to say.
It was only when Dr. Korenstein spoke with her that she changed her mind, entered inpatient hospice care, and died peacefully.
“I think it’s a cautionary tale about when a patient is crystal clear about their wishes,” she said. “The wave of the medical system kind of pushes them along in a particular direction that may go against their wishes.”
Dr. Korenstein said U.S. health care system does fairly well in some areas – for instance, research shows that about 60% of people die in their preferred location, whether at home or somewhere else. But it does not do so well in others – a 2013 Journal of General Internal Medicine study found that, during 2002-2008, Medicare beneficiaries typically spent $39,000 out of pocket on their medical care, and in 25% of cases, what they spent exceeded the total value of their assets.
As far as individual preferences, these tend to correlate poorly with the care that people actually get, Dr. Korenstein said. Patients often don’t express their wishes, doctors are poor judges of what matters to individual people, and care is largely driven by physician preferences and by the care setting involved, she said.
Given those problems, she said, “we cannot possibly be providing high-value individualized care.”
Hospitalists are well positioned to help patients’ preferences align with care, she added. Sometimes, a sustained relationship with a patient, while generally a positive thing, might lead a provider to become invested in their care in “ways that are not always rational.” So a hospitalist can have a helpful vantage point.
VIDEO: Policy-focused SHM president thinks hospitalists can impact global, systems change
An original member of the Society of Hospital Medicine, new SHM Board President Ron Greeno, MD, MHM, is excited about helping to guide hospitalists into a new era of health system transformation.
The former chair of SHM’s Public Policy Committee, Dr. Greeno believes payment reforms like MACRA will have a “huge impact” on both hospitalists and the hospitals/health systems they work in. He expects hospital medicine, as a field, is well positioned for such changes and can play a vital role in systems change at the global level.
“In order to impact those things, hospitalists have to be ready to help change systems,” he said after his plenary address Tuesday at HM17.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
An original member of the Society of Hospital Medicine, new SHM Board President Ron Greeno, MD, MHM, is excited about helping to guide hospitalists into a new era of health system transformation.
The former chair of SHM’s Public Policy Committee, Dr. Greeno believes payment reforms like MACRA will have a “huge impact” on both hospitalists and the hospitals/health systems they work in. He expects hospital medicine, as a field, is well positioned for such changes and can play a vital role in systems change at the global level.
“In order to impact those things, hospitalists have to be ready to help change systems,” he said after his plenary address Tuesday at HM17.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
An original member of the Society of Hospital Medicine, new SHM Board President Ron Greeno, MD, MHM, is excited about helping to guide hospitalists into a new era of health system transformation.
The former chair of SHM’s Public Policy Committee, Dr. Greeno believes payment reforms like MACRA will have a “huge impact” on both hospitalists and the hospitals/health systems they work in. He expects hospital medicine, as a field, is well positioned for such changes and can play a vital role in systems change at the global level.
“In order to impact those things, hospitalists have to be ready to help change systems,” he said after his plenary address Tuesday at HM17.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Adalimumab strikes out for hand osteoarthritis
LAS VEGAS – Adalimumab proved no better than placebo for the treatment of erosive hand osteoarthritis in the double-blind, placebo-controlled, randomized HUMOR trial, throwing cold water on hopes that a disease-modifying treatment for osteoarthritis might at long last have been found.
“This randomized controlled trial demonstrated that subcutaneous adalimumab at 40 mg every other week was no different from placebo for alleviation of pain, synovitis, or bone marrow lesions in patients with erosive hand osteoarthritis presenting with MRI-detected synovitis. This suggests that pain and inflammation are not responsive to TNF [tumor necrosis factor] inhibition in this patient population,” Dawn Aitken, PhD, declared at the World Congress on Osteoarthritis.
The primary endpoint was change in the visual analog pain score over the course of 12 weeks of therapy. Scores dropped by an average of 3.0 points from a mean baseline of 63.6 with adalimumab and by 0.7 points with placebo. That between-group difference wasn’t statistically significant, nor were those modest reductions clinically meaningful. By convention, a clinically meaningful treatment result requires at least a 15-point improvement on the self-assessed pain scale, noted Dr. Aitken of the University of Tasmania in Hobart, Australia.
It made no difference which treatment arm came first.
“There was absolutely no placebo effect,” she said.
The study proved negative for all prespecified secondary endpoints, too. These included change in the Australian/Canadian Hand OA Index pain, function, and stiffness subscales, as well as change in bone marrow lesions and synovitis. A mere 12% of patients showed improvement in synovitis scores with adalimumab, as did 10% on placebo. Five percent of the adalimumab group and 7% on placebo showed improvement in bone marrow lesion scores.
The proinflammatory cytokine TNF-alpha has been shown to play a key role in OA development and progression. However, several prior studies failed to show a benefit for anti-TNF therapy in terms of reducing pain in hand OA patients, although in one of those negative studies, a post hoc analysis found that adalimumab halted erosive progression in the subset of interphalangeal joints with palpable soft tissue swelling at baseline (Ann Rheum Dis. 2012 Jun;71[6]:891-8).
However, there was a positive signal: In a subgroup analysis confined to the patients with inflammatory joints, the use of etanercept was associated with less structural damage of those joints over time as assessed using the quantitative Ghent University Scoring System, or GUSS, according to Dr. Kloppenburg, professor of rheumatology at Leiden (the Netherlands) University.
The emphatically negative results of the HUMOR trial were greeted with dismay by several disappointed audience members. They raised questions: Might a study featuring more than 12 weeks of treatment have brought positive results? How about a larger study? Why no placebo effect, given that patients were receiving injections? Is this area of investigation of TNF-inhibitor therapy now a dead end? Or could adalimumab and etanercept have differential efficacy in hand OA, even though they are both TNF inhibitors?
Dr. Aitken had an answer for every question.
“I think our study was big enough based on our power calculations to detect a difference of 15 mm on a visual analog scale, which is a clinically important difference. And that’s what we should be chasing in OA, that big effect. Patients are not going to be interested in a treatment that improves their pain by 5 mm,” she said.
As for the possibility that 12 weeks of adalimumab might have been too short to see a treatment effect, Dr. Aitken noted that anti-TNF therapy in rheumatoid arthritis brings a rapid response.
“Ideally, if you did have a disease-modifying drug for osteoarthritis you would want it to have a relatively quick response for pain; if patients aren’t seeing an effect after 3 months they might be less interested in taking it,” she continued.
Also, studies with a crossover design, like HUMOR, are known to have a much smaller placebo effect because patients know that at some point they’re certain to receive the active agent, Dr. Aitken observed.
As for the possibility that etanercept might be effective for erosive hand OA, while adalimumab is not, one physician commented, “That’s really scraping the bottom of the barrel.”
The HUMOR trial was sponsored by AbbVie. Dr. Aitken reported having no financial conflicts.
LAS VEGAS – Adalimumab proved no better than placebo for the treatment of erosive hand osteoarthritis in the double-blind, placebo-controlled, randomized HUMOR trial, throwing cold water on hopes that a disease-modifying treatment for osteoarthritis might at long last have been found.
“This randomized controlled trial demonstrated that subcutaneous adalimumab at 40 mg every other week was no different from placebo for alleviation of pain, synovitis, or bone marrow lesions in patients with erosive hand osteoarthritis presenting with MRI-detected synovitis. This suggests that pain and inflammation are not responsive to TNF [tumor necrosis factor] inhibition in this patient population,” Dawn Aitken, PhD, declared at the World Congress on Osteoarthritis.
The primary endpoint was change in the visual analog pain score over the course of 12 weeks of therapy. Scores dropped by an average of 3.0 points from a mean baseline of 63.6 with adalimumab and by 0.7 points with placebo. That between-group difference wasn’t statistically significant, nor were those modest reductions clinically meaningful. By convention, a clinically meaningful treatment result requires at least a 15-point improvement on the self-assessed pain scale, noted Dr. Aitken of the University of Tasmania in Hobart, Australia.
It made no difference which treatment arm came first.
“There was absolutely no placebo effect,” she said.
The study proved negative for all prespecified secondary endpoints, too. These included change in the Australian/Canadian Hand OA Index pain, function, and stiffness subscales, as well as change in bone marrow lesions and synovitis. A mere 12% of patients showed improvement in synovitis scores with adalimumab, as did 10% on placebo. Five percent of the adalimumab group and 7% on placebo showed improvement in bone marrow lesion scores.
The proinflammatory cytokine TNF-alpha has been shown to play a key role in OA development and progression. However, several prior studies failed to show a benefit for anti-TNF therapy in terms of reducing pain in hand OA patients, although in one of those negative studies, a post hoc analysis found that adalimumab halted erosive progression in the subset of interphalangeal joints with palpable soft tissue swelling at baseline (Ann Rheum Dis. 2012 Jun;71[6]:891-8).
However, there was a positive signal: In a subgroup analysis confined to the patients with inflammatory joints, the use of etanercept was associated with less structural damage of those joints over time as assessed using the quantitative Ghent University Scoring System, or GUSS, according to Dr. Kloppenburg, professor of rheumatology at Leiden (the Netherlands) University.
The emphatically negative results of the HUMOR trial were greeted with dismay by several disappointed audience members. They raised questions: Might a study featuring more than 12 weeks of treatment have brought positive results? How about a larger study? Why no placebo effect, given that patients were receiving injections? Is this area of investigation of TNF-inhibitor therapy now a dead end? Or could adalimumab and etanercept have differential efficacy in hand OA, even though they are both TNF inhibitors?
Dr. Aitken had an answer for every question.
“I think our study was big enough based on our power calculations to detect a difference of 15 mm on a visual analog scale, which is a clinically important difference. And that’s what we should be chasing in OA, that big effect. Patients are not going to be interested in a treatment that improves their pain by 5 mm,” she said.
As for the possibility that 12 weeks of adalimumab might have been too short to see a treatment effect, Dr. Aitken noted that anti-TNF therapy in rheumatoid arthritis brings a rapid response.
“Ideally, if you did have a disease-modifying drug for osteoarthritis you would want it to have a relatively quick response for pain; if patients aren’t seeing an effect after 3 months they might be less interested in taking it,” she continued.
Also, studies with a crossover design, like HUMOR, are known to have a much smaller placebo effect because patients know that at some point they’re certain to receive the active agent, Dr. Aitken observed.
As for the possibility that etanercept might be effective for erosive hand OA, while adalimumab is not, one physician commented, “That’s really scraping the bottom of the barrel.”
The HUMOR trial was sponsored by AbbVie. Dr. Aitken reported having no financial conflicts.
LAS VEGAS – Adalimumab proved no better than placebo for the treatment of erosive hand osteoarthritis in the double-blind, placebo-controlled, randomized HUMOR trial, throwing cold water on hopes that a disease-modifying treatment for osteoarthritis might at long last have been found.
“This randomized controlled trial demonstrated that subcutaneous adalimumab at 40 mg every other week was no different from placebo for alleviation of pain, synovitis, or bone marrow lesions in patients with erosive hand osteoarthritis presenting with MRI-detected synovitis. This suggests that pain and inflammation are not responsive to TNF [tumor necrosis factor] inhibition in this patient population,” Dawn Aitken, PhD, declared at the World Congress on Osteoarthritis.
The primary endpoint was change in the visual analog pain score over the course of 12 weeks of therapy. Scores dropped by an average of 3.0 points from a mean baseline of 63.6 with adalimumab and by 0.7 points with placebo. That between-group difference wasn’t statistically significant, nor were those modest reductions clinically meaningful. By convention, a clinically meaningful treatment result requires at least a 15-point improvement on the self-assessed pain scale, noted Dr. Aitken of the University of Tasmania in Hobart, Australia.
It made no difference which treatment arm came first.
“There was absolutely no placebo effect,” she said.
The study proved negative for all prespecified secondary endpoints, too. These included change in the Australian/Canadian Hand OA Index pain, function, and stiffness subscales, as well as change in bone marrow lesions and synovitis. A mere 12% of patients showed improvement in synovitis scores with adalimumab, as did 10% on placebo. Five percent of the adalimumab group and 7% on placebo showed improvement in bone marrow lesion scores.
The proinflammatory cytokine TNF-alpha has been shown to play a key role in OA development and progression. However, several prior studies failed to show a benefit for anti-TNF therapy in terms of reducing pain in hand OA patients, although in one of those negative studies, a post hoc analysis found that adalimumab halted erosive progression in the subset of interphalangeal joints with palpable soft tissue swelling at baseline (Ann Rheum Dis. 2012 Jun;71[6]:891-8).
However, there was a positive signal: In a subgroup analysis confined to the patients with inflammatory joints, the use of etanercept was associated with less structural damage of those joints over time as assessed using the quantitative Ghent University Scoring System, or GUSS, according to Dr. Kloppenburg, professor of rheumatology at Leiden (the Netherlands) University.
The emphatically negative results of the HUMOR trial were greeted with dismay by several disappointed audience members. They raised questions: Might a study featuring more than 12 weeks of treatment have brought positive results? How about a larger study? Why no placebo effect, given that patients were receiving injections? Is this area of investigation of TNF-inhibitor therapy now a dead end? Or could adalimumab and etanercept have differential efficacy in hand OA, even though they are both TNF inhibitors?
Dr. Aitken had an answer for every question.
“I think our study was big enough based on our power calculations to detect a difference of 15 mm on a visual analog scale, which is a clinically important difference. And that’s what we should be chasing in OA, that big effect. Patients are not going to be interested in a treatment that improves their pain by 5 mm,” she said.
As for the possibility that 12 weeks of adalimumab might have been too short to see a treatment effect, Dr. Aitken noted that anti-TNF therapy in rheumatoid arthritis brings a rapid response.
“Ideally, if you did have a disease-modifying drug for osteoarthritis you would want it to have a relatively quick response for pain; if patients aren’t seeing an effect after 3 months they might be less interested in taking it,” she continued.
Also, studies with a crossover design, like HUMOR, are known to have a much smaller placebo effect because patients know that at some point they’re certain to receive the active agent, Dr. Aitken observed.
As for the possibility that etanercept might be effective for erosive hand OA, while adalimumab is not, one physician commented, “That’s really scraping the bottom of the barrel.”
The HUMOR trial was sponsored by AbbVie. Dr. Aitken reported having no financial conflicts.
FROM OARSI 2017
Key clinical point:
Major finding: Scores dropped by an average of 3.0 points from a mean baseline of 63.6 with adalimumab and by 0.7 points with placebo on the primary endpoint of change in the visual analog pain score over the course of 12 weeks.
Data source: The HUMOR trial was a double-blind, placebo-controlled, randomized, crossover trial in which 43 participants with erosive hand osteoarthritis received 12 weeks of treatment with adalimumab and 12 weeks of placebo.
Disclosures: The study was sponsored by AbbVie. The presenter reported having no financial conflicts.
DeSalvo: HM needs holistic approach to health care
LAS VEGAS – To deliver her message of inclusion Tuesday morning, former acting assistant secretary for health in the U.S. Department of Health and Human Services (HHS) Karen DeSalvo, MD, MPH, MSc, could think of “no finer group” than those assembled before her at HM17.
The thousands of hospitalists gathered to hear her keynote address, “Rethinking Health: The Vital Role of Hospitals and the Hospitalist,” listened as she talked about including more than just the best medical care in HM’s scope of practice. The job must evolve to include a focus on such social issues as economic stability, neighborhood and physical environment, education, and access to healthy options for food.
In other words, Dr. DeSalvo wondered aloud, what good is treating a grandmother’s heart failure over and over if she’s always going to return to the hospital because her home, her neighborhood, or her finances mean she is unable to prevent recurring health issues? [[{"fid":"195561","view_mode":"medstat_image_flush_left","attributes":{"alt":"Dr. Brian Harte conducts an interveiw with Dr. Karen DeSalvo duing the opening plenary Tuesday at HM17.","height":"147","width":"220","class":"media-element file-medstat-image-flush-left","data-delta":"1"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Dr. Brian Harte conducts an interview with Dr. Karen DeSalvo during the opening plenary Tuesday at HM17.","field_file_image_credit[und][0][value]":"Darnell Scott","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"1":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Dr. Brian Harte conducts an interview with Dr. Karen DeSalvo during the opening plenary Tuesday at HM17.","field_file_image_credit[und][0][value]":"Darnell Scott"}}}]]
Hospitalists “have been at the center of change, not only in building a new field and showing us that medicine doesn’t have to always be the way it always was,” she said. “You have been at the forefront of seeing that we’re getting better value out of our health care system and, though that work must continue, you must also begin to broaden our thinking and understand that the drivers of health are much more than [just] health care. There are social determinants, social factors.”
Dr. DeSalvo, an internist by training, understands that dealing with social issues may seem like a role for others, but she said that the implications of those factors directly impact hospitalists and their institutions via issues such as readmissions.
“These things … don’t just matter conceptually,” she said. “They [have] direct relationships with mortality and morbidity and cost. They are literally affecting people’s lives in this country every day. When we begin to adjust them, to impact them, you can see that it also affects the health care system.”
On the front lines, Dr. DeSalvo said that hospitalists and others can work to take advantage of their hospital’s existing tools to link their patients to available resources, partner with local public health offices, and push to make their hospitals “anchor institutions to build community capacity to address these social determinants.”
Dr. DeSalvo also praised HM as a field that has already embraced value-based payment (VBP) models. She said that ability to anticipate and adapt to health care’s changing needs positions the field well as the Medicare Access and CHIP Reauthorization Act (MACRA) moves health care from fee-for-service to payment models that seek to manage risk and penalize mistakes.
LAS VEGAS – To deliver her message of inclusion Tuesday morning, former acting assistant secretary for health in the U.S. Department of Health and Human Services (HHS) Karen DeSalvo, MD, MPH, MSc, could think of “no finer group” than those assembled before her at HM17.
The thousands of hospitalists gathered to hear her keynote address, “Rethinking Health: The Vital Role of Hospitals and the Hospitalist,” listened as she talked about including more than just the best medical care in HM’s scope of practice. The job must evolve to include a focus on such social issues as economic stability, neighborhood and physical environment, education, and access to healthy options for food.
In other words, Dr. DeSalvo wondered aloud, what good is treating a grandmother’s heart failure over and over if she’s always going to return to the hospital because her home, her neighborhood, or her finances mean she is unable to prevent recurring health issues? [[{"fid":"195561","view_mode":"medstat_image_flush_left","attributes":{"alt":"Dr. Brian Harte conducts an interveiw with Dr. Karen DeSalvo duing the opening plenary Tuesday at HM17.","height":"147","width":"220","class":"media-element file-medstat-image-flush-left","data-delta":"1"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Dr. Brian Harte conducts an interview with Dr. Karen DeSalvo during the opening plenary Tuesday at HM17.","field_file_image_credit[und][0][value]":"Darnell Scott","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"1":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Dr. Brian Harte conducts an interview with Dr. Karen DeSalvo during the opening plenary Tuesday at HM17.","field_file_image_credit[und][0][value]":"Darnell Scott"}}}]]
Hospitalists “have been at the center of change, not only in building a new field and showing us that medicine doesn’t have to always be the way it always was,” she said. “You have been at the forefront of seeing that we’re getting better value out of our health care system and, though that work must continue, you must also begin to broaden our thinking and understand that the drivers of health are much more than [just] health care. There are social determinants, social factors.”
Dr. DeSalvo, an internist by training, understands that dealing with social issues may seem like a role for others, but she said that the implications of those factors directly impact hospitalists and their institutions via issues such as readmissions.
“These things … don’t just matter conceptually,” she said. “They [have] direct relationships with mortality and morbidity and cost. They are literally affecting people’s lives in this country every day. When we begin to adjust them, to impact them, you can see that it also affects the health care system.”
On the front lines, Dr. DeSalvo said that hospitalists and others can work to take advantage of their hospital’s existing tools to link their patients to available resources, partner with local public health offices, and push to make their hospitals “anchor institutions to build community capacity to address these social determinants.”
Dr. DeSalvo also praised HM as a field that has already embraced value-based payment (VBP) models. She said that ability to anticipate and adapt to health care’s changing needs positions the field well as the Medicare Access and CHIP Reauthorization Act (MACRA) moves health care from fee-for-service to payment models that seek to manage risk and penalize mistakes.
LAS VEGAS – To deliver her message of inclusion Tuesday morning, former acting assistant secretary for health in the U.S. Department of Health and Human Services (HHS) Karen DeSalvo, MD, MPH, MSc, could think of “no finer group” than those assembled before her at HM17.
The thousands of hospitalists gathered to hear her keynote address, “Rethinking Health: The Vital Role of Hospitals and the Hospitalist,” listened as she talked about including more than just the best medical care in HM’s scope of practice. The job must evolve to include a focus on such social issues as economic stability, neighborhood and physical environment, education, and access to healthy options for food.
In other words, Dr. DeSalvo wondered aloud, what good is treating a grandmother’s heart failure over and over if she’s always going to return to the hospital because her home, her neighborhood, or her finances mean she is unable to prevent recurring health issues? [[{"fid":"195561","view_mode":"medstat_image_flush_left","attributes":{"alt":"Dr. Brian Harte conducts an interveiw with Dr. Karen DeSalvo duing the opening plenary Tuesday at HM17.","height":"147","width":"220","class":"media-element file-medstat-image-flush-left","data-delta":"1"},"fields":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Dr. Brian Harte conducts an interview with Dr. Karen DeSalvo during the opening plenary Tuesday at HM17.","field_file_image_credit[und][0][value]":"Darnell Scott","field_file_image_caption[und][0][format]":"plain_text","field_file_image_credit[und][0][format]":"plain_text"},"type":"media","field_deltas":{"1":{"format":"medstat_image_flush_left","field_file_image_caption[und][0][value]":"Dr. Brian Harte conducts an interview with Dr. Karen DeSalvo during the opening plenary Tuesday at HM17.","field_file_image_credit[und][0][value]":"Darnell Scott"}}}]]
Hospitalists “have been at the center of change, not only in building a new field and showing us that medicine doesn’t have to always be the way it always was,” she said. “You have been at the forefront of seeing that we’re getting better value out of our health care system and, though that work must continue, you must also begin to broaden our thinking and understand that the drivers of health are much more than [just] health care. There are social determinants, social factors.”
Dr. DeSalvo, an internist by training, understands that dealing with social issues may seem like a role for others, but she said that the implications of those factors directly impact hospitalists and their institutions via issues such as readmissions.
“These things … don’t just matter conceptually,” she said. “They [have] direct relationships with mortality and morbidity and cost. They are literally affecting people’s lives in this country every day. When we begin to adjust them, to impact them, you can see that it also affects the health care system.”
On the front lines, Dr. DeSalvo said that hospitalists and others can work to take advantage of their hospital’s existing tools to link their patients to available resources, partner with local public health offices, and push to make their hospitals “anchor institutions to build community capacity to address these social determinants.”
Dr. DeSalvo also praised HM as a field that has already embraced value-based payment (VBP) models. She said that ability to anticipate and adapt to health care’s changing needs positions the field well as the Medicare Access and CHIP Reauthorization Act (MACRA) moves health care from fee-for-service to payment models that seek to manage risk and penalize mistakes.
Rapid-fire session troubleshoots mechanical ventilation
Troubleshooting problems with mechanical ventilation starts with assessing how much control one has over specific variables, according to an expert at HM17.
“You want to be in charge of everything when you’re dealing with a ventilator, but you have to acknowledge that you only get to be in charge of some stuff,” said Peter Clardy, MD, an assistant professor of medicine at Harvard University in Cambridge, Mass., and its affiliate, Mount Auburn Hospital. He made his remarks during a rapid-fire science session at HM17.
Since successful algorithms for acute mechanical ventilation require control over many independent variables, knowing what is most stable and going from there can allow the physician to develop a workable plan of action, according to Dr. Clardy.
“It’s really good to be explicit about what is dependent and what is independent,” he said. Independent variables might be those specific to the ventilator, but will always include the positive end-expiratory pressure and the fraction of inspired oxygen. Other independent variables will depend on the mode of ventilation – either fully assisted, partially assisted, or noninvasive.
“If you’re in charge of volume, you have to worry about pressure,” he noted. “If you’re in charge of pressure you have to worry about volume.”
Dependent variables also can vary by mode of ventilation. Once the independent and dependent variables are mapped, it is easier to glean more information about the respiratory mechanics of the situation and the physiologic processes, such as the metabolic cost of breathing and whether it can be reduced, what can be done to prevent ventilator-induced lung injury, and how gas exchange can be supported.
Understanding the independent/dependent variable ratio can also help provide valuable clinical information, such as whether reversing hypoxemia and/or hypercarbia is necessary, or if there are signs of respiratory distress or dyspnea. Other clinical indications might include whether there is a need to prevent or reverse atelectasis, or reduce ventilatory muscle fatigue. Additionally, it will be easier to know whether sedation is possible, or if a neuromuscular blockade should be used. Such information can help determine whether to protect the airway.
“Respiratory distress in a patient who is already ventilated is quite common, so having a routinized way to assess these patients and their stability can help you think about what your moves are right there while you’re in the room,” Dr. Clardy explained. “All of that can be incredibly helpful.”
Dr. Clardy had no relevant financial disclosures.
Troubleshooting problems with mechanical ventilation starts with assessing how much control one has over specific variables, according to an expert at HM17.
“You want to be in charge of everything when you’re dealing with a ventilator, but you have to acknowledge that you only get to be in charge of some stuff,” said Peter Clardy, MD, an assistant professor of medicine at Harvard University in Cambridge, Mass., and its affiliate, Mount Auburn Hospital. He made his remarks during a rapid-fire science session at HM17.
Since successful algorithms for acute mechanical ventilation require control over many independent variables, knowing what is most stable and going from there can allow the physician to develop a workable plan of action, according to Dr. Clardy.
“It’s really good to be explicit about what is dependent and what is independent,” he said. Independent variables might be those specific to the ventilator, but will always include the positive end-expiratory pressure and the fraction of inspired oxygen. Other independent variables will depend on the mode of ventilation – either fully assisted, partially assisted, or noninvasive.
“If you’re in charge of volume, you have to worry about pressure,” he noted. “If you’re in charge of pressure you have to worry about volume.”
Dependent variables also can vary by mode of ventilation. Once the independent and dependent variables are mapped, it is easier to glean more information about the respiratory mechanics of the situation and the physiologic processes, such as the metabolic cost of breathing and whether it can be reduced, what can be done to prevent ventilator-induced lung injury, and how gas exchange can be supported.
Understanding the independent/dependent variable ratio can also help provide valuable clinical information, such as whether reversing hypoxemia and/or hypercarbia is necessary, or if there are signs of respiratory distress or dyspnea. Other clinical indications might include whether there is a need to prevent or reverse atelectasis, or reduce ventilatory muscle fatigue. Additionally, it will be easier to know whether sedation is possible, or if a neuromuscular blockade should be used. Such information can help determine whether to protect the airway.
“Respiratory distress in a patient who is already ventilated is quite common, so having a routinized way to assess these patients and their stability can help you think about what your moves are right there while you’re in the room,” Dr. Clardy explained. “All of that can be incredibly helpful.”
Dr. Clardy had no relevant financial disclosures.
Troubleshooting problems with mechanical ventilation starts with assessing how much control one has over specific variables, according to an expert at HM17.
“You want to be in charge of everything when you’re dealing with a ventilator, but you have to acknowledge that you only get to be in charge of some stuff,” said Peter Clardy, MD, an assistant professor of medicine at Harvard University in Cambridge, Mass., and its affiliate, Mount Auburn Hospital. He made his remarks during a rapid-fire science session at HM17.
Since successful algorithms for acute mechanical ventilation require control over many independent variables, knowing what is most stable and going from there can allow the physician to develop a workable plan of action, according to Dr. Clardy.
“It’s really good to be explicit about what is dependent and what is independent,” he said. Independent variables might be those specific to the ventilator, but will always include the positive end-expiratory pressure and the fraction of inspired oxygen. Other independent variables will depend on the mode of ventilation – either fully assisted, partially assisted, or noninvasive.
“If you’re in charge of volume, you have to worry about pressure,” he noted. “If you’re in charge of pressure you have to worry about volume.”
Dependent variables also can vary by mode of ventilation. Once the independent and dependent variables are mapped, it is easier to glean more information about the respiratory mechanics of the situation and the physiologic processes, such as the metabolic cost of breathing and whether it can be reduced, what can be done to prevent ventilator-induced lung injury, and how gas exchange can be supported.
Understanding the independent/dependent variable ratio can also help provide valuable clinical information, such as whether reversing hypoxemia and/or hypercarbia is necessary, or if there are signs of respiratory distress or dyspnea. Other clinical indications might include whether there is a need to prevent or reverse atelectasis, or reduce ventilatory muscle fatigue. Additionally, it will be easier to know whether sedation is possible, or if a neuromuscular blockade should be used. Such information can help determine whether to protect the airway.
“Respiratory distress in a patient who is already ventilated is quite common, so having a routinized way to assess these patients and their stability can help you think about what your moves are right there while you’re in the room,” Dr. Clardy explained. “All of that can be incredibly helpful.”
Dr. Clardy had no relevant financial disclosures.