User login
New resource designed to help prevent CINV
The Hematology/Oncology Pharmacy Association (HOPA) has announced the release of a toolkit intended to help prevent chemotherapy-induced nausea and vomiting (CINV) in cancer patients.
The Time to Talk CINV™ toolkit was designed to facilitate dialogue between patients and their healthcare teams to ensure no patient is needlessly suffering from CINV.
The tools in the kit, which are targeted to both patients and healthcare providers, were created with guidance from patients, caregivers, pharmacists, and nurses.
The entire toolkit is available for free download at TimeToTalkCINV.com.
The toolkit is part of the Time to Talk CINV™ campaign, which is a collaboration between HOPA, Eisai Inc., and Helsinn Therapeutics (US), Inc. (funded by Eisai and Helsinn Therapeutics).
The campaign began in response to results from a survey of cancer patients.
“Our research revealed a vast majority of patients on chemotherapy who experience nausea and vomiting expect to have this side effect, and 95% of these patients said, at some point, chemo-induced nausea and vomiting had an impact on their daily lives,” said Sarah Peters, PharmD, president of HOPA.
“In response to these findings, as well as the finding that healthcare team members are looking for better communication tools, the Time to Talk CINV toolkit was created to facilitate efficient and effective conversations about nausea and vomiting from chemotherapy to ensure that each patient is receiving the right information and effective management approaches.”
The Time to Talk CINV toolkit includes the following resources:
- A list of myths and truths about CINV intended to eliminate common misperceptions
- A checklist of questions patients can ask healthcare providers to better understand CINV
- A chemotherapy side effect tracker, which enables patients to track their experience and report back to their healthcare team
- A communication checklist for the healthcare team outlining best practices for communicating with patients to prevent CINV.
Each tool can be printed or filled out digitally.
Additional information about CINV, including videos and infographics, can be found on the Time to Talk CINV website. ![]()
The Hematology/Oncology Pharmacy Association (HOPA) has announced the release of a toolkit intended to help prevent chemotherapy-induced nausea and vomiting (CINV) in cancer patients.
The Time to Talk CINV™ toolkit was designed to facilitate dialogue between patients and their healthcare teams to ensure no patient is needlessly suffering from CINV.
The tools in the kit, which are targeted to both patients and healthcare providers, were created with guidance from patients, caregivers, pharmacists, and nurses.
The entire toolkit is available for free download at TimeToTalkCINV.com.
The toolkit is part of the Time to Talk CINV™ campaign, which is a collaboration between HOPA, Eisai Inc., and Helsinn Therapeutics (US), Inc. (funded by Eisai and Helsinn Therapeutics).
The campaign began in response to results from a survey of cancer patients.
“Our research revealed a vast majority of patients on chemotherapy who experience nausea and vomiting expect to have this side effect, and 95% of these patients said, at some point, chemo-induced nausea and vomiting had an impact on their daily lives,” said Sarah Peters, PharmD, president of HOPA.
“In response to these findings, as well as the finding that healthcare team members are looking for better communication tools, the Time to Talk CINV toolkit was created to facilitate efficient and effective conversations about nausea and vomiting from chemotherapy to ensure that each patient is receiving the right information and effective management approaches.”
The Time to Talk CINV toolkit includes the following resources:
- A list of myths and truths about CINV intended to eliminate common misperceptions
- A checklist of questions patients can ask healthcare providers to better understand CINV
- A chemotherapy side effect tracker, which enables patients to track their experience and report back to their healthcare team
- A communication checklist for the healthcare team outlining best practices for communicating with patients to prevent CINV.
Each tool can be printed or filled out digitally.
Additional information about CINV, including videos and infographics, can be found on the Time to Talk CINV website. ![]()
The Hematology/Oncology Pharmacy Association (HOPA) has announced the release of a toolkit intended to help prevent chemotherapy-induced nausea and vomiting (CINV) in cancer patients.
The Time to Talk CINV™ toolkit was designed to facilitate dialogue between patients and their healthcare teams to ensure no patient is needlessly suffering from CINV.
The tools in the kit, which are targeted to both patients and healthcare providers, were created with guidance from patients, caregivers, pharmacists, and nurses.
The entire toolkit is available for free download at TimeToTalkCINV.com.
The toolkit is part of the Time to Talk CINV™ campaign, which is a collaboration between HOPA, Eisai Inc., and Helsinn Therapeutics (US), Inc. (funded by Eisai and Helsinn Therapeutics).
The campaign began in response to results from a survey of cancer patients.
“Our research revealed a vast majority of patients on chemotherapy who experience nausea and vomiting expect to have this side effect, and 95% of these patients said, at some point, chemo-induced nausea and vomiting had an impact on their daily lives,” said Sarah Peters, PharmD, president of HOPA.
“In response to these findings, as well as the finding that healthcare team members are looking for better communication tools, the Time to Talk CINV toolkit was created to facilitate efficient and effective conversations about nausea and vomiting from chemotherapy to ensure that each patient is receiving the right information and effective management approaches.”
The Time to Talk CINV toolkit includes the following resources:
- A list of myths and truths about CINV intended to eliminate common misperceptions
- A checklist of questions patients can ask healthcare providers to better understand CINV
- A chemotherapy side effect tracker, which enables patients to track their experience and report back to their healthcare team
- A communication checklist for the healthcare team outlining best practices for communicating with patients to prevent CINV.
Each tool can be printed or filled out digitally.
Additional information about CINV, including videos and infographics, can be found on the Time to Talk CINV website. ![]()
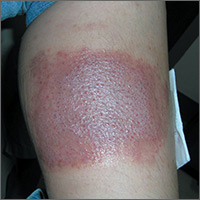
Large leg rash
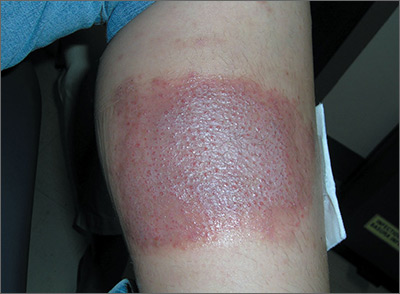
The family physician (FP) asked some more questions about the antibiotic ointment and discovered that it was a triple antibiotic ointment (which typically contains neomycin, bacitracin, and polymyxin). The patient acknowledged that after applying the ointment to the bug bite, she’d used a bandage that was rectangular in shape—just like the patch of erythematous skin with papules.
The FP deduced that this was a case of contact dermatitis and the patient was reacting to at least one of the topical antibiotics in the triple antibiotic preparation. The most likely offending topical antibiotic was neomycin, as it’s a common contact allergen. However, bacitracin is also a common contact allergen.
The FP told the patient to stop using the triple antibiotic ointment and to avoid using any preparations that contain neomycin, bacitracin—or both—in the future. The FP explained that most minor skin injuries, including bug bites and minor scrapes, do not require topical antibiotics.
The FP prescribed 0.1% triamcinolone cream to be applied twice daily to treat the erythema and itching caused by the contact dermatitis. At a follow-up visit one month later, the only skin problem that persisted was some postinflammatory hyperpigmentation. The FP explained that this would likely fade over time.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R. Contact dermatitis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:591-596.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

The family physician (FP) asked some more questions about the antibiotic ointment and discovered that it was a triple antibiotic ointment (which typically contains neomycin, bacitracin, and polymyxin). The patient acknowledged that after applying the ointment to the bug bite, she’d used a bandage that was rectangular in shape—just like the patch of erythematous skin with papules.
The FP deduced that this was a case of contact dermatitis and the patient was reacting to at least one of the topical antibiotics in the triple antibiotic preparation. The most likely offending topical antibiotic was neomycin, as it’s a common contact allergen. However, bacitracin is also a common contact allergen.
The FP told the patient to stop using the triple antibiotic ointment and to avoid using any preparations that contain neomycin, bacitracin—or both—in the future. The FP explained that most minor skin injuries, including bug bites and minor scrapes, do not require topical antibiotics.
The FP prescribed 0.1% triamcinolone cream to be applied twice daily to treat the erythema and itching caused by the contact dermatitis. At a follow-up visit one month later, the only skin problem that persisted was some postinflammatory hyperpigmentation. The FP explained that this would likely fade over time.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R. Contact dermatitis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:591-596.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

The family physician (FP) asked some more questions about the antibiotic ointment and discovered that it was a triple antibiotic ointment (which typically contains neomycin, bacitracin, and polymyxin). The patient acknowledged that after applying the ointment to the bug bite, she’d used a bandage that was rectangular in shape—just like the patch of erythematous skin with papules.
The FP deduced that this was a case of contact dermatitis and the patient was reacting to at least one of the topical antibiotics in the triple antibiotic preparation. The most likely offending topical antibiotic was neomycin, as it’s a common contact allergen. However, bacitracin is also a common contact allergen.
The FP told the patient to stop using the triple antibiotic ointment and to avoid using any preparations that contain neomycin, bacitracin—or both—in the future. The FP explained that most minor skin injuries, including bug bites and minor scrapes, do not require topical antibiotics.
The FP prescribed 0.1% triamcinolone cream to be applied twice daily to treat the erythema and itching caused by the contact dermatitis. At a follow-up visit one month later, the only skin problem that persisted was some postinflammatory hyperpigmentation. The FP explained that this would likely fade over time.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R. Contact dermatitis. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:591-596.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
Perioperative pharmacological thromboprophylaxis in patients with cancer: a systematic review and meta-analysis
Clinical Question: What are the benefits and harms of perioperative pharmacological thromboprophylaxis in cancer patients undergoing surgery?
Background: Both cancer and surgery increase the risk of venous thromboembolism (VTE). In postsurgical patients with cancer, the benefits and harms of anticoagulation remain unknown.
Synopsis: Thirty-nine trials were deemed eligible for inclusion in the meta-analysis. Twenty-five of these were prospective and 14 were retrospective. The overall incidence of deep venous thrombosis (DVT) and pulmonary embolism was 0.9% (across 20 studies) and 0.3% (across 19 studies), respectively. Pharmacologic prophylaxis overall reduced DVT incidence (0.5% vs. 1.2%; relative risk, 0.51; P = .03). Subgroup analysis demonstrated this was significant for abdominal/pelvic surgeries and with low molecular weight heparin. Six studies compared duration of standard prophylaxis (10 days) with extended prophylaxis (4 weeks), with a lower VTE rate in the extended group. Bleeding events were noted in 13 studies and pharmacologic prophylaxis significantly increased bleeding risk (2.7% vs. 8%; RR, 2.51; P less than .0001).
Bottom Line: Perioperative pharmacologic prophylaxis reduces DVT risk in patients with cancer, with greatest risk reduction seen in patients undergoing abdominal/pelvic surgeries. This comes at the cost of increased bleeding complications.
Citations: Guo Q, Huang B, Zhao J, et al. Perioperative pharmacological thromboprophylaxis in patients with cancer: a systematic review and meta-analysis. Ann Surg. 2016 Nov. doi: 10.1097/SLA.0000000000002074.
Dr. Patil is a clinical instructor, Division of Hospital Medicine, University of Colorado School of Medicine, Aurora.
Clinical Question: What are the benefits and harms of perioperative pharmacological thromboprophylaxis in cancer patients undergoing surgery?
Background: Both cancer and surgery increase the risk of venous thromboembolism (VTE). In postsurgical patients with cancer, the benefits and harms of anticoagulation remain unknown.
Synopsis: Thirty-nine trials were deemed eligible for inclusion in the meta-analysis. Twenty-five of these were prospective and 14 were retrospective. The overall incidence of deep venous thrombosis (DVT) and pulmonary embolism was 0.9% (across 20 studies) and 0.3% (across 19 studies), respectively. Pharmacologic prophylaxis overall reduced DVT incidence (0.5% vs. 1.2%; relative risk, 0.51; P = .03). Subgroup analysis demonstrated this was significant for abdominal/pelvic surgeries and with low molecular weight heparin. Six studies compared duration of standard prophylaxis (10 days) with extended prophylaxis (4 weeks), with a lower VTE rate in the extended group. Bleeding events were noted in 13 studies and pharmacologic prophylaxis significantly increased bleeding risk (2.7% vs. 8%; RR, 2.51; P less than .0001).
Bottom Line: Perioperative pharmacologic prophylaxis reduces DVT risk in patients with cancer, with greatest risk reduction seen in patients undergoing abdominal/pelvic surgeries. This comes at the cost of increased bleeding complications.
Citations: Guo Q, Huang B, Zhao J, et al. Perioperative pharmacological thromboprophylaxis in patients with cancer: a systematic review and meta-analysis. Ann Surg. 2016 Nov. doi: 10.1097/SLA.0000000000002074.
Dr. Patil is a clinical instructor, Division of Hospital Medicine, University of Colorado School of Medicine, Aurora.
Clinical Question: What are the benefits and harms of perioperative pharmacological thromboprophylaxis in cancer patients undergoing surgery?
Background: Both cancer and surgery increase the risk of venous thromboembolism (VTE). In postsurgical patients with cancer, the benefits and harms of anticoagulation remain unknown.
Synopsis: Thirty-nine trials were deemed eligible for inclusion in the meta-analysis. Twenty-five of these were prospective and 14 were retrospective. The overall incidence of deep venous thrombosis (DVT) and pulmonary embolism was 0.9% (across 20 studies) and 0.3% (across 19 studies), respectively. Pharmacologic prophylaxis overall reduced DVT incidence (0.5% vs. 1.2%; relative risk, 0.51; P = .03). Subgroup analysis demonstrated this was significant for abdominal/pelvic surgeries and with low molecular weight heparin. Six studies compared duration of standard prophylaxis (10 days) with extended prophylaxis (4 weeks), with a lower VTE rate in the extended group. Bleeding events were noted in 13 studies and pharmacologic prophylaxis significantly increased bleeding risk (2.7% vs. 8%; RR, 2.51; P less than .0001).
Bottom Line: Perioperative pharmacologic prophylaxis reduces DVT risk in patients with cancer, with greatest risk reduction seen in patients undergoing abdominal/pelvic surgeries. This comes at the cost of increased bleeding complications.
Citations: Guo Q, Huang B, Zhao J, et al. Perioperative pharmacological thromboprophylaxis in patients with cancer: a systematic review and meta-analysis. Ann Surg. 2016 Nov. doi: 10.1097/SLA.0000000000002074.
Dr. Patil is a clinical instructor, Division of Hospital Medicine, University of Colorado School of Medicine, Aurora.
Readmission rates after passage of the hospital readmissions reduction program
Clinical Question: Did hospitals receiving the highest penalties for readmissions have accelerated improvement in this metric after passage of Medicare Hospital Readmissions Reduction Program (HRRP)?
Background: Medicare passed the HRRP to incentivize reductions in readmission rates. The impact of penalties on relative hospital improvement rates remains unknown.
Setting: Query of national Medicare Provider Analysis and Review files.
Synopsis: 2,868 hospitals were identified as candidates for analysis and were stratified into four risk groups based on penalty size under HRRP: highest-performing, average-performing, low-performing, and lowest-performing. The primary outcomes were hospital-specific, 30-day, all-cause risk-standardized readmission rates (RSRRs) for patients discharged with acute MI, HF, or pneumonia. The investigators separated data into a pre-law period and post-law period. They fitted a logistic regression model to pre-law RSRRs and developed a piecewise linear model on post-law RSRRs with pre-law data as the dependent variable. All hospital groups had reductions in RSRRs, with the lowest quartile demonstrating greatest improvement.
Bottom Line: HRRP has resulted in reductions in RSRRs with greatest improvement in hospitals with lowest pre-law performance.
Citations: Wasfy JH, Zigler CM, Choirat C, et al. Readmission rates after passage of the hospital readmissions reduction program: a pre-post analysis. Ann Intern Med. 2017 Mar;166(5):324-31.
Dr. Patil is a clinical instructor, Division of Hospital Medicine, University of Colorado School of Medicine, Aurora.
Clinical Question: Did hospitals receiving the highest penalties for readmissions have accelerated improvement in this metric after passage of Medicare Hospital Readmissions Reduction Program (HRRP)?
Background: Medicare passed the HRRP to incentivize reductions in readmission rates. The impact of penalties on relative hospital improvement rates remains unknown.
Setting: Query of national Medicare Provider Analysis and Review files.
Synopsis: 2,868 hospitals were identified as candidates for analysis and were stratified into four risk groups based on penalty size under HRRP: highest-performing, average-performing, low-performing, and lowest-performing. The primary outcomes were hospital-specific, 30-day, all-cause risk-standardized readmission rates (RSRRs) for patients discharged with acute MI, HF, or pneumonia. The investigators separated data into a pre-law period and post-law period. They fitted a logistic regression model to pre-law RSRRs and developed a piecewise linear model on post-law RSRRs with pre-law data as the dependent variable. All hospital groups had reductions in RSRRs, with the lowest quartile demonstrating greatest improvement.
Bottom Line: HRRP has resulted in reductions in RSRRs with greatest improvement in hospitals with lowest pre-law performance.
Citations: Wasfy JH, Zigler CM, Choirat C, et al. Readmission rates after passage of the hospital readmissions reduction program: a pre-post analysis. Ann Intern Med. 2017 Mar;166(5):324-31.
Dr. Patil is a clinical instructor, Division of Hospital Medicine, University of Colorado School of Medicine, Aurora.
Clinical Question: Did hospitals receiving the highest penalties for readmissions have accelerated improvement in this metric after passage of Medicare Hospital Readmissions Reduction Program (HRRP)?
Background: Medicare passed the HRRP to incentivize reductions in readmission rates. The impact of penalties on relative hospital improvement rates remains unknown.
Setting: Query of national Medicare Provider Analysis and Review files.
Synopsis: 2,868 hospitals were identified as candidates for analysis and were stratified into four risk groups based on penalty size under HRRP: highest-performing, average-performing, low-performing, and lowest-performing. The primary outcomes were hospital-specific, 30-day, all-cause risk-standardized readmission rates (RSRRs) for patients discharged with acute MI, HF, or pneumonia. The investigators separated data into a pre-law period and post-law period. They fitted a logistic regression model to pre-law RSRRs and developed a piecewise linear model on post-law RSRRs with pre-law data as the dependent variable. All hospital groups had reductions in RSRRs, with the lowest quartile demonstrating greatest improvement.
Bottom Line: HRRP has resulted in reductions in RSRRs with greatest improvement in hospitals with lowest pre-law performance.
Citations: Wasfy JH, Zigler CM, Choirat C, et al. Readmission rates after passage of the hospital readmissions reduction program: a pre-post analysis. Ann Intern Med. 2017 Mar;166(5):324-31.
Dr. Patil is a clinical instructor, Division of Hospital Medicine, University of Colorado School of Medicine, Aurora.
Point of prostate cancer diagnosis experiences and needs of black men: the Florida CaPCaS study
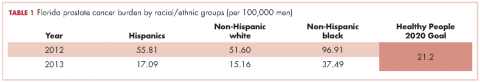
As of 2016, Florida ranks second among all states in the United States in estimated new cases of prostate cancer and second in estimated deaths from prostate cancer.1 Disparities in diagnosis, mortality rates, and access to cancer care also continue to be a major problem in Florida, especially for black men. For example, black men were the only racial/ethnic group that did not meet the Healthy People (HP) 2010 objective to reduce the prostate cancer death rate to 28.2 per 100,000 men and that has not met the HP 2020 objective to reduce the prostate cancer death rate to 21.2 per 100,000 men (Table 1). Based on the 2013 prostate cancer mortality rates for Florida,2 the death rate for black men is almost twice the HP 2020 goal (37.49 per 100,000).
A diagnosis of prostate cancer is a life changing event for a man. In particular, there is limited research on the experiences and coping mechanisms of black men at diagnosis. This limited body of research indicates that black men’s reactions to their initial diagnoses varied, from being shocked when notified of their initial diagnosis of prostate cancer,3 to perceiving that they had received a “death sentence”.4 In regard to having to make decisions about their treatment options, some black men indicated that the information about treatment that they received from physicians decreased their anxiety,5 whereas others noted that they had not been given adequate information by a physician to make a decision.6 Patients have also reported that they felt as though they were not knowledgeable enough to ask questions concerning treatment options and preferred for the physician to make the treatment choice for them.6 Decisional regret is now a common observation among men who are not involved in making decisions about their treatment.3
According to the American Cancer Society, about 30,000 black men were diagnosed with prostate cancer in 2016.7 It is important to understand these men’s needs and help them cope effectively as they navigate the survivorship continuum. In line with our research program’s goal of ensuring quality cancer care for black men, the primary objective of this study was to explore the experiences and needs of black men at the point of prostate cancer diagnosis (PPCD). Specifically, we developed an interpretative framework for black men’s experiences at the PPCD, focusing on United States or native-born black men (NBBM) and Caribbean-born black men (CBBM). African-born black men were not included in this study because of the low sample size for that ethnicity. This study is part of a large-scale study that focuses on developing a model of prostate cancer care and survivorship (CaPCaS model) using grounded theory to study black, ethnically diverse prostate cancer survivors.
Methods
The study aims to close the prostate health disparity gap for black men in Florida through community engaged research in partnership with survivors of prostate cancer and their advocates. The current study was a prospective, grounded theory study that involved one-on-one, in-depth interviews with 31 prostate cancer patients about their care and survivorship experiences. Specifically, 17 NBBM and 14 CBBM were enrolled in the project. Appropriate human subjects review and approval were obtained from the University of Florida, the Florida Department of Health, and the Department of Defense.
Research design
This is a qualitative research study. Based on the principles of community engaged research and using a rigorous qualitative research methodology, we recruited NBBM and CBBM with a personal history of prostate cancer. Guided by open-ended questions developed by the team, one-on-one in-depth interviews were conducted with each participant in their home or at a convenient location in the community. Our primary focus was on the participants’ care and survivorship experiences, with primary focus on their prostate cancer diagnosis. Qualitative research was our methodology of choice because little is known about the PPCD experiences of black men.8 With qualitative research, we were able to get our participants to “relive” their experiences in the presence of a culturally competent, well-trained interviewer and elicit the information about their care and survivorship experiences based on their interpretation. In addition, we were able to capture the dynamic processes associated with their experiences, documenting sequential patterns and change through both verbal and nonverbal communications, because the participants were interviewed twice.
Research population and recruitment
The study setting was Florida. The inclusion criteria were: black men, personal history of prostate cancer, ability to complete two separate interviews with each one expected to last 2-3 hours, and flexibility to meet interviewers at a convenient community site for the interviews. Participants were identified through the Florida Cancer Data System (FCDS)9 database. At the time of the study, the most recent FCDS database was for 2010. The FCDS has collected the number of new cancer cancers diagnosed in the state of Florida annually since 1981. It is a comprehensive incidence-only registry and does not extract data on patients with a death certificate. All investigators are bounded by the confidential pledge required for the use of the FCDS data.
We used the Florida Department of Health’s (DoH’s) Bureau of Epidemiology standard procedure for the FCDS9 to recruit participants. Our recruitment strategies included: initial patient contact by written correspondence; second mailing that included a telephone opt-out card after 3 weeks for nonrespondents (the telephone opt-out card explained to the patient that if no response was received, the study investigator would attempt a telephone call to introduce the study); and a telephone call by a study staff to introduce the study for nonrespondents. As per the Florida DoH standard procedure, we did not disclose on the cover of the study mailings that the patient was being contacted for a study specific to cancer. Efforts to recruit a patient stopped immediately if a patient indicated that he did not wish to participate. All of the study staff making participant contact were extensively trained to provide a clear and accurate description of cancer registration in Florida. In addition, to assist the study staff in providing clear and accurate responses, responses to frequently asked questions were made available to the study staff. During the participant recruitment phase, anyone who seemed to be upset when contacted was reported immediately (within 24 hours) to the DoH cancer epidemiologist. In addition, the name of anyone who stated that he did not wish to be contacted again was given to the DoH so that the person would not be re-contacted.
Prescreening of participants for eligibility
All eligible participants who agreed to participate in the study comprised the pool of potential study participants. For those who agreed to participate, the following information was obtained by telephone interview using REDCap software:10 name and contact information, country of birth, age, marital status, and education level. The demographic information facilitated a purposeful systematic selection of black men of diverse age groups (younger than 50 years or older than 50 years), marital status (single, including divorced or separated, or married/in a relationship), and educational level (college degree or not college educated). An incentive of a $5 gift card was provided to all the men who participated in the screening phase. Using systematic sampling to ensure demographically diverse participants, 40 participants (20 NBBM, 20 CBBM) were selected from the initial pool of participants to participate in the study.
Data collection
The data collection was conducted by a trained Community Health Worker (CHW) using semi-structured interview process. The interview guide was constructed by the research team and the study community advisory board members to ensure language appropriateness, understanding and cultural sensitivity. For this study, the interview questions focused on participants’ background information and diagnosis history, including: participants’ personal story of diagnosis, feelings, emotions, reactions, regrets and level of personal/family/physician involvement in diagnosis. For the CBBM, we also obtained information on the age at which they immigrated to the United States. The CHW interviewer was trained to question participants and encourage them to elaborate on areas of importance to their experience.
A total time of about 5-6 hours was scheduled for the data collection per participant, which is sufficient for gathering in-depth perspectives. We scheduled two interviews lasting not more than 3 hours at a time so as not to create burden for study participants. Participants had the choice to have the interviews completed in a single session or spread out over 2 days. The interviews were audio-recorded to provide ease of transcription and back-up of data. At the end of the interviews, participants were compensated for participating in the study.
Data management and analyses
The study dataset included interview transcripts and field notes of the CHW interviewer describing his insights about the interviews. The data analyses included preparing and verifying the narrative data, coding data, and developing an interpretative framework for black men’s experiences at the PPCD. Interviews were transcribed verbatim by a professional transcription service that has policies in place for protected health information. Each transcript was then verified for accuracy by the CHW interviewer. The interview transcripts were imported directly into NVivo 11, a computer-assisted data analysis software that allows coding and modeling of complex narrative data. The data coding was conducted by our interdisciplinary team of clinicians, behavioral scientists, and social scientists. It is important to note that the NVivo 11 software was not used to analyze the data per se. However, it provided a sophisticated and systematic way to manage the following tasks for the analyses: organizing large quantities of narrative data, coding text, retrieving text by codes, querying the data, comparing sets of data interpretation between NBBM and CBBM; and developing analytic models. The study team members coded the data in weekly team meetings. The coding consisted of reading the data and identifying major themes, then assigning labels to and defining emerging categories.
Two levels of coding were used. The first, open coding, refers to an approach to data with no preconceived ideas about what will be found; and the second, focused or axial coding, refers to reviewing data for the purpose of more richly coding on a particular theme.11 We used dimensional analysis to ensure that each emerging concept was carefully defined. The study team went back and forth between the data and the emerging analytic framework, using constant comparison of new data with already coded data and new categories with previously analyzed text.12
To ensure trustworthiness and credibility,13 the study team maintained an audit trail that documented how and when analytic decisions were made. In addition, peer debriefing was conducted to ensure credibility, including the presentation of findings to the study community advisory board members as part of the community engaged research approach.
Results
Description of participants
The FCDS provided a database of 1,813 participants identified as black men diagnosed with prostate cancer in 2010. Because the FCDS does not extract data on patients with a death certificate, we found out during the pre-screening phase that a few of the men were deceased. In addition, there were a significant number of incorrect addresses. We obtained a total of 212 completed responses by phone during the prescreening phase. The majority of the participants were aged 60-69 years (48.2%), had a high school diploma only (26.1%), and were currently married (65.3%). Relative to ethnicity, 67% of participants classified themselves NBBM, 24% as CBBM, 3.5% as black men born in Africa, and 5.5% as Other/Don’t know/Refused. For the CBBM, the most common countries of birth were Jamaica, Haiti, and Guyana, respectively.
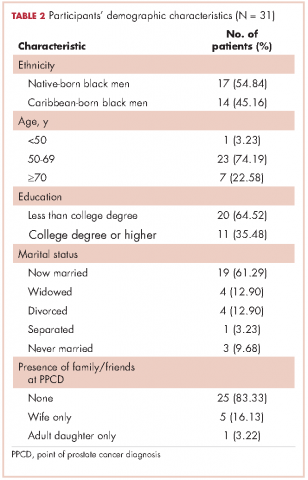
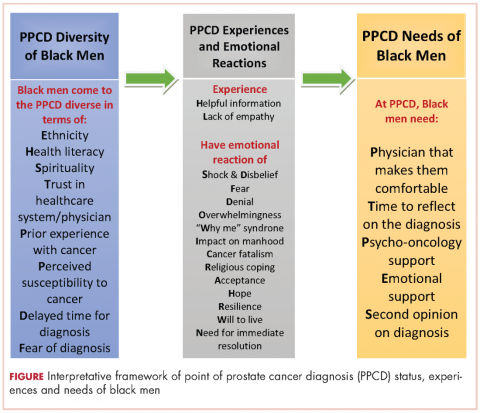
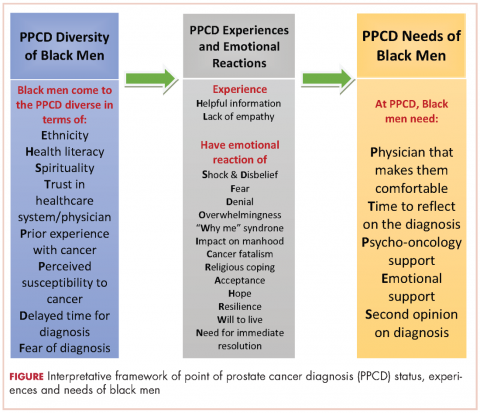
In all, 40 participants (20 NBBM and 20 CBBM) were selected from the 212 participants to participate in the study. Selection was conducted systematically to ensure representation in terms of age, marital status, education, and geographical location. Data saturation was achieved with 17 NBBM and 14 CBBM, after which we ended data collection (Table 2). Data saturation is the standard for deciding that we are not finding anything different from the interviews first coded and last coded. Although we were specifically looking for differences between the two groups (NBBM and CBBM), no between-group differences emerged. Each man’s experience was unique to him with some common themes emerging described hereinafter (Figure).
Moderating factors and experiences at PPCD
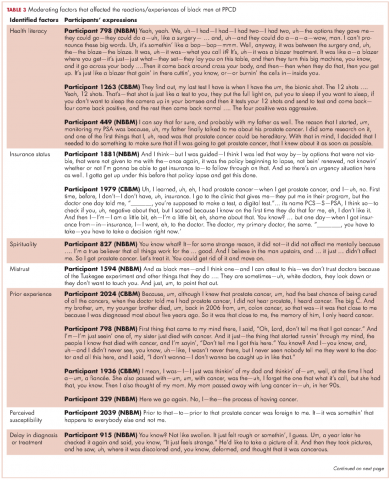
Some of the moderating factors that the study participants identified as affecting their reactions to the PPCD included health literacy, insurance status, spirituality, mistrust, prior experience with cancer, perceived susceptibility to cancer, and delay in diagnosis (Table 3). Health literacy, defined as personal, cognitive, and social skills that determine the ability of individuals to gain access to, understand, and use information to promote and maintain good health, was one of the moderating factors found in this study.13 Some of the black men came to the PPCD with a low level of health literacy, which had an impact on their understanding of the treatment options. For example, in the interview, participant 798 (NBBM) was confused about what tests had been done and was not able to accurately describe the treatments offered to him. Participant 1263 (CBBM) struggled to express the purpose and procedures associated with diagnostic biopsy. However, there were participants with a high level of health literacy (eg, participant 449 [NBBM]), who decided to research the disease.
Another factor to consider is the insurance status of participants at the PPCD. The majority of the participants had good insurance coverage, but some were affected by poor insurance coverage. Participant 1881(NBBM) made his treatment decision primarily on the basis of the pending lapse of his insurance coverage rather than the best clinical option for him. Participant 1979 (CBBM) described both his confusion on the screening tests and the impact of not having insurance coverage. Upon obtaining insurance coverage, he sought treatment for his prostate cancer with an urgency that he did not experience when he was first diagnosed when uninsured.
The spirituality of black men was another moderating factor at the PPCD. Participant 827 (NBBM) noted that he was unaffected when he received his diagnosis because he was a true believer. Some of the black men also came to the PPCD with lack of trust in the physician and/or the health care system and perceived a sense of contempt from the physician. Participant 1594 (NBBM) described mistrust based on the history of medical exploitation of black men as well as a perception of current discriminatory practices.
Another important PPCD status to note for black men is prior experience with cancer, including prior personal cancer history and/or prior cancer history of a family member. Participant 2024 (CBBM) described the meaning of cancer to him, while participant 798 (NBBM) echoed the despair of the cancer diagnosis based on experience with other cancers in the family. Sometimes there were multiple cancers in the family or even among the significant others of the participant, as was the case with Participant 1936 (CBBM).
Of greatest concern were men who delayed their diagnosis or treatment, perhaps resulting in their cancer being at a more advanced stage when they eventually did return for care. Finally, some of the men came to the PPCD appointment with a low expectation of receiving a diagnosis of prostate cancer, whereas others came to the PPCD fearful of the results of their testing.
In describing their experiences, participants expressed both positive and negative experiences: on the positive side, they found the information provided by the physician to be helpful; but on the negative side, the sterile or medically focused encounter was perceived as a lack empathy on the part of the physician.
Cognitive, emotional, and behavioral coping experiences
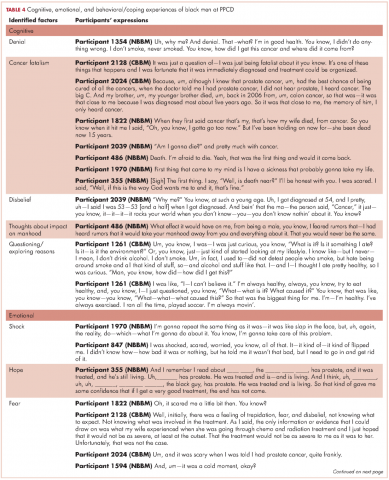
As expected, there were ranges of emotions, including shock, disbelief and denial (Table 4). Some of the men questioned why this (the cancer) was happening to them when they had done “nothing” to deserve it. Doing nothing in this case meant that they had lived a healthy lifestyle with no obvious apparent cause to have the cancer. Fear and cancer fatalism were experienced by a significant number of the men, with their thoughts immediately turning to death and dying. This was especially the case for men who had lost a loved one to cancer. Conversely, some of the men wanted immediate resolution, focusing instead on ways to beat the cancer and with a strong will to live.
Reliance on faith was a big part of coping at the PPCD. Some of the men drew strength from their faith to get them through their cancer journey. Others found a way to accept the diagnosis – one participant accepted the diagnosis and the fact that this could mean dying (after living a good life), whereas another participant accepted the diagnosis with the hope that he would find a cure. Hope was more realistic with the knowledge that other men had survived prostate cancer.
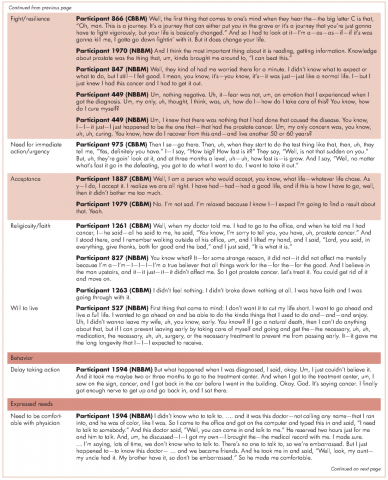
Reflecting back on their experiences, the men also identified clear needs at the PPCD. One of the needs they identified was having a physician they were comfortable with to discuss their diagnosis. Another need was for a second opinion. Participant 1594 (NBBM) advised that it was important for black men to take control by requesting a second opinion. Participant 2039 (NBBM) described a feeling of navigating blindly and trying to find answers that would be helpful to him in his cancer journey. However, his experience with a second opinion was not helpful because the second physician was at the same clinic as his primary physician. His recommendation was to get a second opinion at a different clinic or center. Another important need was emotional support at the PPCD. Participant 2024 (CBBM) made a strong case for emotional support, especially for men who are not accompanied during diagnosis. In addition, Participant 2024’s (CBBM) reflections underscore the fact that the PPCD may not be an ideal place or time to discuss treatment options. With the range of emotions that the men go through at the PPCD, it is difficult to comprehend any follow-up discussions after hearing the words “you have prostate cancer.” Participant 2024 (CBBM) also strongly expressed that men need time to deal with the diagnosis at the PPCD.
Discussion
The primary goal of this study was to develop an interpretative framework of black men’s experiences at the PPCD. The Figure provides a pictorial summary of the framework. Study results indicated that black men come to the PPCD with different emotions and different experiences. Although the majority of the men were NBBM, there is a significantly increasing number of foreign-born black men receiving a diagnosis of prostate cancer in the United States. Given that black men carry a disproportionate burden of the disease, with a significantly higher incidence compared with any other racial group, it is important that tailored services are provided to black men at the PPCD.
We also found that black men came to the PPCD diverse in terms of their ethnicity, health literacy, spirituality, trust in health care system/physician, prior experience with cancer, perceived susceptibility to cancer, delayed time for diagnosis, and fear of diagnosis. Of importance for physicians is that the black race is not homogeneous. There is a significant number of foreign-born blacks at the PPCD, and they often have different cultural beliefs and values compared with NBBM. In addition, some of the foreign-born black men may not have English proficiency and may need a medical interpreter during the PPCD consultation. In addition, a patient’s pre-existing lack of trust in the health care system may have a negative impact on the PPCD consultation. It is thus important that the physician takes the time to instill trust and make the men comfortable during the PPCD consultation.
For some of the men who had fear of a prostate cancer diagnosis and/or prior experience with cancer, cancer fatalism was experienced at the PPCD. Cancer fatalism, defined as an individual’s belief that death is bound to happen when diagnosed with cancer, has been documented as a major barrier to cancer detection and control.15 For example, fatalistic perspectives have been reported to affect cervical cancer,16 breast cancer,17,18 colorectal cancer,19 and prostate cancer20,21 among blacks. It is thus important to effectively address fatalistic beliefs when a man is diagnosed with prostate cancer.
Other emotions at the PPCD that may affect effective treatment decision making also need to be addressed immediately. For example, the emotions of fear, denial, and feeling overwhelmed are potential barriers to timely treatment decision making. Psycho-oncology interventions to appropriately deal with these emotions at PPCD or right after the diagnosis may be crucial for the men. In particular, a group-based psychosocial intervention focusing on: provision of education about treatment options for prostate cancer and their acute and late effects; negotiating treatment and treatment side effects; enhancing communication with treatment providers; managing distress; and engaging positive family- and community-based social support to optimize emotional, behavioral, social, and physical outcomes in black men with prostate cancer.
In addition to having physicians make them comfortable at PPCD, the PPCD needs expressed by participants included having time to come to terms with the diagnosis and receiving psycho-oncology/emotional support. Anyone who has just received a diagnosis of cancer cannot be expected to immediately continue to function as he did before the PPCD. This is especially difficult for men who are alone at the PPCD. Nevertheless, it is expected that they will listen attentively and understand subsequent consultation by the physician, then leave the consultation room almost immediately, and be able drive home or back to work right after the diagnosis. There seems to be a support gap that needs to be closed at the PPCD. Providing the men with immediate support to cope with the diagnosis may make a significant difference in effective treatment choices and eliminating treatment decisional regrets.
Methodological rigor was established through purposeful sampling, extended time with participants, standardized procedures for data collection, management and analysis, multidisciplinary interpretation, and validation of results with the community advisory board. Because the research participants were purposefully selected from a statewide database of black men diagnosed with CaP, generalizability of findings to the two target groups of NBBM and CBBM can be assumed, with the caveat that men with different experiences may have chosen not to respond to recruitment efforts or refused participation. Black men who were not sufficiently fluent in English to be interviewed were also excluded and are not represented in these findings. Black men of other nativity (including African-born black men) and residing outside of Florida were also not represented.
In conclusion, the PPCD interpretative framework developed in this study, describes the status of black men at the PPCD, their experiences during the PPCD, and their needs at the PPCD. The framework provides information that can be used by physicians to prepare for their PPCD consultation with black men as well as develop a support system for black men at the PPCD.
Acknowledgments
The authors thank the men who participated in the CaPCaS study. They also thank the CaPCaS project community advisory board chairs (Mr Jim West, Dr Angela Adams, and Prince Oladapo Odedina) and all the CaPCaS project community advisory board members for their effort throughout the project. Finally, they rxecognize the effort of additional CaPCaS scientific team, especially the primary interviewer, Mr Kenneth Stokes. Weekly meeting support for this study was provided by the University of Florida MiCaRT Center, which is funded by the NIH-National Cancer Institute Award # 1P20CA192990-02. REDCaP software was supported by the UF Clinical and Translational Science Institute, which is funded in part by the NIH Clinical and Translational Science Award program (grants UL1TR001427, KL2TR001429 and TL1TR001428).
1. American Cancer Society. Cancer facts & figures 2016. http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2016/. Published 2016. Accessed January 10, 2017.
2. Florida Cancer Data System. Florida Statewide Population-Based Cancer Registry. https://fcds.med.miami.edu/scripts/fcdspubrates/production/doSelection.aspx?election=map. Processed February 16, 2016.
3. Sinfield P, Baker R, Camosso-Stefinovic J, et al. Men’s and carers’ experiences of care for prostate cancer: a narrative literature review. Health Expect. 2009;12:301-312.
4. Maliski SL, Connor SE, Williams L, Litwin MS. Faith among low-income, African American/ black men treated for prostate cancer. Cancer Nurs. 2010;33(6):470-478.
5. Jones RA, Wenzel J, Hinton I, et el. Exploring cancer support needs for older African-American men with prostate cancer. Support Care Cancer. 2011;19(9):1411-1419.
6. Sinfield P, Baker R, Agarwal S, Tarrant C. Patient-centred care: what are the experiences of prostate cancer patients and their partners? Patient Educ Couns. 2008;73(1):91-96.
7. American Cancer Society. Cancer facts & figures for African Americans 2016-2018. http://www.cancer.org/research/cancerfactsstatistics/cancer-facts-figures-for-african-americans. Published 2016. Accessed January 10, 2017.
8. Patton MQ. Qualitative research & evaluation methods. 4th ed. Thousand Oaks, CA: Sage Publications; 2001.
9. Florida Department of Health, Bureau of Epidemiology. Procedure guide for studies that utilize patient identifiable data from the Florida Cancer Data System. http://www.fcds.med.miami.edu/downloads/datarequest/Procedure%20Guide_Revised%20
October%202007.pdf. Accessed July 24, 2010.
10. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381.
11. Strauss A. Qualitative analysis for social scientists. New York, NY: Cambridge University Press; 1987.
12. Glaser BG, Strauss AL. The discovery of grounded theory: strategies for qualitative research. Chicago, IL: Aldine; 1967.
13. Miles MB, Huberman AM. Qualitative data analysis: an expanded sourcebook. 2nd ed. Thousand Oaks, CA: Sage Publications; 1994.
14. Nutbeam, D. Health literacy as a public health goal: a challenge for contemporary health education and communication strategies into the 21st century. Health Promot Int. 2000;15(3):259-267.
15. Powe BD, Finnie R. Cancer fatalism: the state of the science. Cancer Nurs. 2003;26:454-467.
16. Powe BD. Fatalism among elderly African Americans: effects on colorectal cancer screening. Cancer Nurs. 1995;18:285-392.
17. Powe BD. Cancer fatalism among elderly Caucasians and African Americans. Oncol Nurs Forum. 1995;22(9):1355-1359.
18. Thoresen CE. Spirituality, health, and science: the coming revival? In: Roth RS, Kurpius SR, eds. The emerging role of counseling psychology in health care. New York, NY: WW Norton; 1998.
19. Carver CS, Scheier MF, Weintraub JK. Assessing coping strategies: a theoretically based approach. J Pers Soc Psychol. 1989;56:267-283.
21. Odedina FT, Yu D, Akinremi TO, Reams RR, Freedman ML, Kumar N. Prostate cancer cognitive-behavioral factors in a West African population. J Immigr Minor Health. 2009;11(4):258-267.
22. Odedina FT, Scrivens JJ Jr, Larose-Pierre M, et al. Modifiable prostate cancer risk reduction and early detection behaviors in black men. Am J Health Behav. 2011;35(4):470-484.
As of 2016, Florida ranks second among all states in the United States in estimated new cases of prostate cancer and second in estimated deaths from prostate cancer.1 Disparities in diagnosis, mortality rates, and access to cancer care also continue to be a major problem in Florida, especially for black men. For example, black men were the only racial/ethnic group that did not meet the Healthy People (HP) 2010 objective to reduce the prostate cancer death rate to 28.2 per 100,000 men and that has not met the HP 2020 objective to reduce the prostate cancer death rate to 21.2 per 100,000 men (Table 1). Based on the 2013 prostate cancer mortality rates for Florida,2 the death rate for black men is almost twice the HP 2020 goal (37.49 per 100,000).
A diagnosis of prostate cancer is a life changing event for a man. In particular, there is limited research on the experiences and coping mechanisms of black men at diagnosis. This limited body of research indicates that black men’s reactions to their initial diagnoses varied, from being shocked when notified of their initial diagnosis of prostate cancer,3 to perceiving that they had received a “death sentence”.4 In regard to having to make decisions about their treatment options, some black men indicated that the information about treatment that they received from physicians decreased their anxiety,5 whereas others noted that they had not been given adequate information by a physician to make a decision.6 Patients have also reported that they felt as though they were not knowledgeable enough to ask questions concerning treatment options and preferred for the physician to make the treatment choice for them.6 Decisional regret is now a common observation among men who are not involved in making decisions about their treatment.3
According to the American Cancer Society, about 30,000 black men were diagnosed with prostate cancer in 2016.7 It is important to understand these men’s needs and help them cope effectively as they navigate the survivorship continuum. In line with our research program’s goal of ensuring quality cancer care for black men, the primary objective of this study was to explore the experiences and needs of black men at the point of prostate cancer diagnosis (PPCD). Specifically, we developed an interpretative framework for black men’s experiences at the PPCD, focusing on United States or native-born black men (NBBM) and Caribbean-born black men (CBBM). African-born black men were not included in this study because of the low sample size for that ethnicity. This study is part of a large-scale study that focuses on developing a model of prostate cancer care and survivorship (CaPCaS model) using grounded theory to study black, ethnically diverse prostate cancer survivors.
Methods
The study aims to close the prostate health disparity gap for black men in Florida through community engaged research in partnership with survivors of prostate cancer and their advocates. The current study was a prospective, grounded theory study that involved one-on-one, in-depth interviews with 31 prostate cancer patients about their care and survivorship experiences. Specifically, 17 NBBM and 14 CBBM were enrolled in the project. Appropriate human subjects review and approval were obtained from the University of Florida, the Florida Department of Health, and the Department of Defense.
Research design
This is a qualitative research study. Based on the principles of community engaged research and using a rigorous qualitative research methodology, we recruited NBBM and CBBM with a personal history of prostate cancer. Guided by open-ended questions developed by the team, one-on-one in-depth interviews were conducted with each participant in their home or at a convenient location in the community. Our primary focus was on the participants’ care and survivorship experiences, with primary focus on their prostate cancer diagnosis. Qualitative research was our methodology of choice because little is known about the PPCD experiences of black men.8 With qualitative research, we were able to get our participants to “relive” their experiences in the presence of a culturally competent, well-trained interviewer and elicit the information about their care and survivorship experiences based on their interpretation. In addition, we were able to capture the dynamic processes associated with their experiences, documenting sequential patterns and change through both verbal and nonverbal communications, because the participants were interviewed twice.
Research population and recruitment
The study setting was Florida. The inclusion criteria were: black men, personal history of prostate cancer, ability to complete two separate interviews with each one expected to last 2-3 hours, and flexibility to meet interviewers at a convenient community site for the interviews. Participants were identified through the Florida Cancer Data System (FCDS)9 database. At the time of the study, the most recent FCDS database was for 2010. The FCDS has collected the number of new cancer cancers diagnosed in the state of Florida annually since 1981. It is a comprehensive incidence-only registry and does not extract data on patients with a death certificate. All investigators are bounded by the confidential pledge required for the use of the FCDS data.
We used the Florida Department of Health’s (DoH’s) Bureau of Epidemiology standard procedure for the FCDS9 to recruit participants. Our recruitment strategies included: initial patient contact by written correspondence; second mailing that included a telephone opt-out card after 3 weeks for nonrespondents (the telephone opt-out card explained to the patient that if no response was received, the study investigator would attempt a telephone call to introduce the study); and a telephone call by a study staff to introduce the study for nonrespondents. As per the Florida DoH standard procedure, we did not disclose on the cover of the study mailings that the patient was being contacted for a study specific to cancer. Efforts to recruit a patient stopped immediately if a patient indicated that he did not wish to participate. All of the study staff making participant contact were extensively trained to provide a clear and accurate description of cancer registration in Florida. In addition, to assist the study staff in providing clear and accurate responses, responses to frequently asked questions were made available to the study staff. During the participant recruitment phase, anyone who seemed to be upset when contacted was reported immediately (within 24 hours) to the DoH cancer epidemiologist. In addition, the name of anyone who stated that he did not wish to be contacted again was given to the DoH so that the person would not be re-contacted.
Prescreening of participants for eligibility
All eligible participants who agreed to participate in the study comprised the pool of potential study participants. For those who agreed to participate, the following information was obtained by telephone interview using REDCap software:10 name and contact information, country of birth, age, marital status, and education level. The demographic information facilitated a purposeful systematic selection of black men of diverse age groups (younger than 50 years or older than 50 years), marital status (single, including divorced or separated, or married/in a relationship), and educational level (college degree or not college educated). An incentive of a $5 gift card was provided to all the men who participated in the screening phase. Using systematic sampling to ensure demographically diverse participants, 40 participants (20 NBBM, 20 CBBM) were selected from the initial pool of participants to participate in the study.
Data collection
The data collection was conducted by a trained Community Health Worker (CHW) using semi-structured interview process. The interview guide was constructed by the research team and the study community advisory board members to ensure language appropriateness, understanding and cultural sensitivity. For this study, the interview questions focused on participants’ background information and diagnosis history, including: participants’ personal story of diagnosis, feelings, emotions, reactions, regrets and level of personal/family/physician involvement in diagnosis. For the CBBM, we also obtained information on the age at which they immigrated to the United States. The CHW interviewer was trained to question participants and encourage them to elaborate on areas of importance to their experience.
A total time of about 5-6 hours was scheduled for the data collection per participant, which is sufficient for gathering in-depth perspectives. We scheduled two interviews lasting not more than 3 hours at a time so as not to create burden for study participants. Participants had the choice to have the interviews completed in a single session or spread out over 2 days. The interviews were audio-recorded to provide ease of transcription and back-up of data. At the end of the interviews, participants were compensated for participating in the study.
Data management and analyses
The study dataset included interview transcripts and field notes of the CHW interviewer describing his insights about the interviews. The data analyses included preparing and verifying the narrative data, coding data, and developing an interpretative framework for black men’s experiences at the PPCD. Interviews were transcribed verbatim by a professional transcription service that has policies in place for protected health information. Each transcript was then verified for accuracy by the CHW interviewer. The interview transcripts were imported directly into NVivo 11, a computer-assisted data analysis software that allows coding and modeling of complex narrative data. The data coding was conducted by our interdisciplinary team of clinicians, behavioral scientists, and social scientists. It is important to note that the NVivo 11 software was not used to analyze the data per se. However, it provided a sophisticated and systematic way to manage the following tasks for the analyses: organizing large quantities of narrative data, coding text, retrieving text by codes, querying the data, comparing sets of data interpretation between NBBM and CBBM; and developing analytic models. The study team members coded the data in weekly team meetings. The coding consisted of reading the data and identifying major themes, then assigning labels to and defining emerging categories.
Two levels of coding were used. The first, open coding, refers to an approach to data with no preconceived ideas about what will be found; and the second, focused or axial coding, refers to reviewing data for the purpose of more richly coding on a particular theme.11 We used dimensional analysis to ensure that each emerging concept was carefully defined. The study team went back and forth between the data and the emerging analytic framework, using constant comparison of new data with already coded data and new categories with previously analyzed text.12
To ensure trustworthiness and credibility,13 the study team maintained an audit trail that documented how and when analytic decisions were made. In addition, peer debriefing was conducted to ensure credibility, including the presentation of findings to the study community advisory board members as part of the community engaged research approach.
Results
Description of participants
The FCDS provided a database of 1,813 participants identified as black men diagnosed with prostate cancer in 2010. Because the FCDS does not extract data on patients with a death certificate, we found out during the pre-screening phase that a few of the men were deceased. In addition, there were a significant number of incorrect addresses. We obtained a total of 212 completed responses by phone during the prescreening phase. The majority of the participants were aged 60-69 years (48.2%), had a high school diploma only (26.1%), and were currently married (65.3%). Relative to ethnicity, 67% of participants classified themselves NBBM, 24% as CBBM, 3.5% as black men born in Africa, and 5.5% as Other/Don’t know/Refused. For the CBBM, the most common countries of birth were Jamaica, Haiti, and Guyana, respectively.
In all, 40 participants (20 NBBM and 20 CBBM) were selected from the 212 participants to participate in the study. Selection was conducted systematically to ensure representation in terms of age, marital status, education, and geographical location. Data saturation was achieved with 17 NBBM and 14 CBBM, after which we ended data collection (Table 2). Data saturation is the standard for deciding that we are not finding anything different from the interviews first coded and last coded. Although we were specifically looking for differences between the two groups (NBBM and CBBM), no between-group differences emerged. Each man’s experience was unique to him with some common themes emerging described hereinafter (Figure).
Moderating factors and experiences at PPCD
Some of the moderating factors that the study participants identified as affecting their reactions to the PPCD included health literacy, insurance status, spirituality, mistrust, prior experience with cancer, perceived susceptibility to cancer, and delay in diagnosis (Table 3). Health literacy, defined as personal, cognitive, and social skills that determine the ability of individuals to gain access to, understand, and use information to promote and maintain good health, was one of the moderating factors found in this study.13 Some of the black men came to the PPCD with a low level of health literacy, which had an impact on their understanding of the treatment options. For example, in the interview, participant 798 (NBBM) was confused about what tests had been done and was not able to accurately describe the treatments offered to him. Participant 1263 (CBBM) struggled to express the purpose and procedures associated with diagnostic biopsy. However, there were participants with a high level of health literacy (eg, participant 449 [NBBM]), who decided to research the disease.
Another factor to consider is the insurance status of participants at the PPCD. The majority of the participants had good insurance coverage, but some were affected by poor insurance coverage. Participant 1881(NBBM) made his treatment decision primarily on the basis of the pending lapse of his insurance coverage rather than the best clinical option for him. Participant 1979 (CBBM) described both his confusion on the screening tests and the impact of not having insurance coverage. Upon obtaining insurance coverage, he sought treatment for his prostate cancer with an urgency that he did not experience when he was first diagnosed when uninsured.
The spirituality of black men was another moderating factor at the PPCD. Participant 827 (NBBM) noted that he was unaffected when he received his diagnosis because he was a true believer. Some of the black men also came to the PPCD with lack of trust in the physician and/or the health care system and perceived a sense of contempt from the physician. Participant 1594 (NBBM) described mistrust based on the history of medical exploitation of black men as well as a perception of current discriminatory practices.
Another important PPCD status to note for black men is prior experience with cancer, including prior personal cancer history and/or prior cancer history of a family member. Participant 2024 (CBBM) described the meaning of cancer to him, while participant 798 (NBBM) echoed the despair of the cancer diagnosis based on experience with other cancers in the family. Sometimes there were multiple cancers in the family or even among the significant others of the participant, as was the case with Participant 1936 (CBBM).
Of greatest concern were men who delayed their diagnosis or treatment, perhaps resulting in their cancer being at a more advanced stage when they eventually did return for care. Finally, some of the men came to the PPCD appointment with a low expectation of receiving a diagnosis of prostate cancer, whereas others came to the PPCD fearful of the results of their testing.
In describing their experiences, participants expressed both positive and negative experiences: on the positive side, they found the information provided by the physician to be helpful; but on the negative side, the sterile or medically focused encounter was perceived as a lack empathy on the part of the physician.
Cognitive, emotional, and behavioral coping experiences
As expected, there were ranges of emotions, including shock, disbelief and denial (Table 4). Some of the men questioned why this (the cancer) was happening to them when they had done “nothing” to deserve it. Doing nothing in this case meant that they had lived a healthy lifestyle with no obvious apparent cause to have the cancer. Fear and cancer fatalism were experienced by a significant number of the men, with their thoughts immediately turning to death and dying. This was especially the case for men who had lost a loved one to cancer. Conversely, some of the men wanted immediate resolution, focusing instead on ways to beat the cancer and with a strong will to live.
Reliance on faith was a big part of coping at the PPCD. Some of the men drew strength from their faith to get them through their cancer journey. Others found a way to accept the diagnosis – one participant accepted the diagnosis and the fact that this could mean dying (after living a good life), whereas another participant accepted the diagnosis with the hope that he would find a cure. Hope was more realistic with the knowledge that other men had survived prostate cancer.
Reflecting back on their experiences, the men also identified clear needs at the PPCD. One of the needs they identified was having a physician they were comfortable with to discuss their diagnosis. Another need was for a second opinion. Participant 1594 (NBBM) advised that it was important for black men to take control by requesting a second opinion. Participant 2039 (NBBM) described a feeling of navigating blindly and trying to find answers that would be helpful to him in his cancer journey. However, his experience with a second opinion was not helpful because the second physician was at the same clinic as his primary physician. His recommendation was to get a second opinion at a different clinic or center. Another important need was emotional support at the PPCD. Participant 2024 (CBBM) made a strong case for emotional support, especially for men who are not accompanied during diagnosis. In addition, Participant 2024’s (CBBM) reflections underscore the fact that the PPCD may not be an ideal place or time to discuss treatment options. With the range of emotions that the men go through at the PPCD, it is difficult to comprehend any follow-up discussions after hearing the words “you have prostate cancer.” Participant 2024 (CBBM) also strongly expressed that men need time to deal with the diagnosis at the PPCD.
Discussion
The primary goal of this study was to develop an interpretative framework of black men’s experiences at the PPCD. The Figure provides a pictorial summary of the framework. Study results indicated that black men come to the PPCD with different emotions and different experiences. Although the majority of the men were NBBM, there is a significantly increasing number of foreign-born black men receiving a diagnosis of prostate cancer in the United States. Given that black men carry a disproportionate burden of the disease, with a significantly higher incidence compared with any other racial group, it is important that tailored services are provided to black men at the PPCD.
We also found that black men came to the PPCD diverse in terms of their ethnicity, health literacy, spirituality, trust in health care system/physician, prior experience with cancer, perceived susceptibility to cancer, delayed time for diagnosis, and fear of diagnosis. Of importance for physicians is that the black race is not homogeneous. There is a significant number of foreign-born blacks at the PPCD, and they often have different cultural beliefs and values compared with NBBM. In addition, some of the foreign-born black men may not have English proficiency and may need a medical interpreter during the PPCD consultation. In addition, a patient’s pre-existing lack of trust in the health care system may have a negative impact on the PPCD consultation. It is thus important that the physician takes the time to instill trust and make the men comfortable during the PPCD consultation.
For some of the men who had fear of a prostate cancer diagnosis and/or prior experience with cancer, cancer fatalism was experienced at the PPCD. Cancer fatalism, defined as an individual’s belief that death is bound to happen when diagnosed with cancer, has been documented as a major barrier to cancer detection and control.15 For example, fatalistic perspectives have been reported to affect cervical cancer,16 breast cancer,17,18 colorectal cancer,19 and prostate cancer20,21 among blacks. It is thus important to effectively address fatalistic beliefs when a man is diagnosed with prostate cancer.
Other emotions at the PPCD that may affect effective treatment decision making also need to be addressed immediately. For example, the emotions of fear, denial, and feeling overwhelmed are potential barriers to timely treatment decision making. Psycho-oncology interventions to appropriately deal with these emotions at PPCD or right after the diagnosis may be crucial for the men. In particular, a group-based psychosocial intervention focusing on: provision of education about treatment options for prostate cancer and their acute and late effects; negotiating treatment and treatment side effects; enhancing communication with treatment providers; managing distress; and engaging positive family- and community-based social support to optimize emotional, behavioral, social, and physical outcomes in black men with prostate cancer.
In addition to having physicians make them comfortable at PPCD, the PPCD needs expressed by participants included having time to come to terms with the diagnosis and receiving psycho-oncology/emotional support. Anyone who has just received a diagnosis of cancer cannot be expected to immediately continue to function as he did before the PPCD. This is especially difficult for men who are alone at the PPCD. Nevertheless, it is expected that they will listen attentively and understand subsequent consultation by the physician, then leave the consultation room almost immediately, and be able drive home or back to work right after the diagnosis. There seems to be a support gap that needs to be closed at the PPCD. Providing the men with immediate support to cope with the diagnosis may make a significant difference in effective treatment choices and eliminating treatment decisional regrets.
Methodological rigor was established through purposeful sampling, extended time with participants, standardized procedures for data collection, management and analysis, multidisciplinary interpretation, and validation of results with the community advisory board. Because the research participants were purposefully selected from a statewide database of black men diagnosed with CaP, generalizability of findings to the two target groups of NBBM and CBBM can be assumed, with the caveat that men with different experiences may have chosen not to respond to recruitment efforts or refused participation. Black men who were not sufficiently fluent in English to be interviewed were also excluded and are not represented in these findings. Black men of other nativity (including African-born black men) and residing outside of Florida were also not represented.
In conclusion, the PPCD interpretative framework developed in this study, describes the status of black men at the PPCD, their experiences during the PPCD, and their needs at the PPCD. The framework provides information that can be used by physicians to prepare for their PPCD consultation with black men as well as develop a support system for black men at the PPCD.
Acknowledgments
The authors thank the men who participated in the CaPCaS study. They also thank the CaPCaS project community advisory board chairs (Mr Jim West, Dr Angela Adams, and Prince Oladapo Odedina) and all the CaPCaS project community advisory board members for their effort throughout the project. Finally, they rxecognize the effort of additional CaPCaS scientific team, especially the primary interviewer, Mr Kenneth Stokes. Weekly meeting support for this study was provided by the University of Florida MiCaRT Center, which is funded by the NIH-National Cancer Institute Award # 1P20CA192990-02. REDCaP software was supported by the UF Clinical and Translational Science Institute, which is funded in part by the NIH Clinical and Translational Science Award program (grants UL1TR001427, KL2TR001429 and TL1TR001428).
As of 2016, Florida ranks second among all states in the United States in estimated new cases of prostate cancer and second in estimated deaths from prostate cancer.1 Disparities in diagnosis, mortality rates, and access to cancer care also continue to be a major problem in Florida, especially for black men. For example, black men were the only racial/ethnic group that did not meet the Healthy People (HP) 2010 objective to reduce the prostate cancer death rate to 28.2 per 100,000 men and that has not met the HP 2020 objective to reduce the prostate cancer death rate to 21.2 per 100,000 men (Table 1). Based on the 2013 prostate cancer mortality rates for Florida,2 the death rate for black men is almost twice the HP 2020 goal (37.49 per 100,000).
A diagnosis of prostate cancer is a life changing event for a man. In particular, there is limited research on the experiences and coping mechanisms of black men at diagnosis. This limited body of research indicates that black men’s reactions to their initial diagnoses varied, from being shocked when notified of their initial diagnosis of prostate cancer,3 to perceiving that they had received a “death sentence”.4 In regard to having to make decisions about their treatment options, some black men indicated that the information about treatment that they received from physicians decreased their anxiety,5 whereas others noted that they had not been given adequate information by a physician to make a decision.6 Patients have also reported that they felt as though they were not knowledgeable enough to ask questions concerning treatment options and preferred for the physician to make the treatment choice for them.6 Decisional regret is now a common observation among men who are not involved in making decisions about their treatment.3
According to the American Cancer Society, about 30,000 black men were diagnosed with prostate cancer in 2016.7 It is important to understand these men’s needs and help them cope effectively as they navigate the survivorship continuum. In line with our research program’s goal of ensuring quality cancer care for black men, the primary objective of this study was to explore the experiences and needs of black men at the point of prostate cancer diagnosis (PPCD). Specifically, we developed an interpretative framework for black men’s experiences at the PPCD, focusing on United States or native-born black men (NBBM) and Caribbean-born black men (CBBM). African-born black men were not included in this study because of the low sample size for that ethnicity. This study is part of a large-scale study that focuses on developing a model of prostate cancer care and survivorship (CaPCaS model) using grounded theory to study black, ethnically diverse prostate cancer survivors.
Methods
The study aims to close the prostate health disparity gap for black men in Florida through community engaged research in partnership with survivors of prostate cancer and their advocates. The current study was a prospective, grounded theory study that involved one-on-one, in-depth interviews with 31 prostate cancer patients about their care and survivorship experiences. Specifically, 17 NBBM and 14 CBBM were enrolled in the project. Appropriate human subjects review and approval were obtained from the University of Florida, the Florida Department of Health, and the Department of Defense.
Research design
This is a qualitative research study. Based on the principles of community engaged research and using a rigorous qualitative research methodology, we recruited NBBM and CBBM with a personal history of prostate cancer. Guided by open-ended questions developed by the team, one-on-one in-depth interviews were conducted with each participant in their home or at a convenient location in the community. Our primary focus was on the participants’ care and survivorship experiences, with primary focus on their prostate cancer diagnosis. Qualitative research was our methodology of choice because little is known about the PPCD experiences of black men.8 With qualitative research, we were able to get our participants to “relive” their experiences in the presence of a culturally competent, well-trained interviewer and elicit the information about their care and survivorship experiences based on their interpretation. In addition, we were able to capture the dynamic processes associated with their experiences, documenting sequential patterns and change through both verbal and nonverbal communications, because the participants were interviewed twice.
Research population and recruitment
The study setting was Florida. The inclusion criteria were: black men, personal history of prostate cancer, ability to complete two separate interviews with each one expected to last 2-3 hours, and flexibility to meet interviewers at a convenient community site for the interviews. Participants were identified through the Florida Cancer Data System (FCDS)9 database. At the time of the study, the most recent FCDS database was for 2010. The FCDS has collected the number of new cancer cancers diagnosed in the state of Florida annually since 1981. It is a comprehensive incidence-only registry and does not extract data on patients with a death certificate. All investigators are bounded by the confidential pledge required for the use of the FCDS data.
We used the Florida Department of Health’s (DoH’s) Bureau of Epidemiology standard procedure for the FCDS9 to recruit participants. Our recruitment strategies included: initial patient contact by written correspondence; second mailing that included a telephone opt-out card after 3 weeks for nonrespondents (the telephone opt-out card explained to the patient that if no response was received, the study investigator would attempt a telephone call to introduce the study); and a telephone call by a study staff to introduce the study for nonrespondents. As per the Florida DoH standard procedure, we did not disclose on the cover of the study mailings that the patient was being contacted for a study specific to cancer. Efforts to recruit a patient stopped immediately if a patient indicated that he did not wish to participate. All of the study staff making participant contact were extensively trained to provide a clear and accurate description of cancer registration in Florida. In addition, to assist the study staff in providing clear and accurate responses, responses to frequently asked questions were made available to the study staff. During the participant recruitment phase, anyone who seemed to be upset when contacted was reported immediately (within 24 hours) to the DoH cancer epidemiologist. In addition, the name of anyone who stated that he did not wish to be contacted again was given to the DoH so that the person would not be re-contacted.
Prescreening of participants for eligibility
All eligible participants who agreed to participate in the study comprised the pool of potential study participants. For those who agreed to participate, the following information was obtained by telephone interview using REDCap software:10 name and contact information, country of birth, age, marital status, and education level. The demographic information facilitated a purposeful systematic selection of black men of diverse age groups (younger than 50 years or older than 50 years), marital status (single, including divorced or separated, or married/in a relationship), and educational level (college degree or not college educated). An incentive of a $5 gift card was provided to all the men who participated in the screening phase. Using systematic sampling to ensure demographically diverse participants, 40 participants (20 NBBM, 20 CBBM) were selected from the initial pool of participants to participate in the study.
Data collection
The data collection was conducted by a trained Community Health Worker (CHW) using semi-structured interview process. The interview guide was constructed by the research team and the study community advisory board members to ensure language appropriateness, understanding and cultural sensitivity. For this study, the interview questions focused on participants’ background information and diagnosis history, including: participants’ personal story of diagnosis, feelings, emotions, reactions, regrets and level of personal/family/physician involvement in diagnosis. For the CBBM, we also obtained information on the age at which they immigrated to the United States. The CHW interviewer was trained to question participants and encourage them to elaborate on areas of importance to their experience.
A total time of about 5-6 hours was scheduled for the data collection per participant, which is sufficient for gathering in-depth perspectives. We scheduled two interviews lasting not more than 3 hours at a time so as not to create burden for study participants. Participants had the choice to have the interviews completed in a single session or spread out over 2 days. The interviews were audio-recorded to provide ease of transcription and back-up of data. At the end of the interviews, participants were compensated for participating in the study.
Data management and analyses
The study dataset included interview transcripts and field notes of the CHW interviewer describing his insights about the interviews. The data analyses included preparing and verifying the narrative data, coding data, and developing an interpretative framework for black men’s experiences at the PPCD. Interviews were transcribed verbatim by a professional transcription service that has policies in place for protected health information. Each transcript was then verified for accuracy by the CHW interviewer. The interview transcripts were imported directly into NVivo 11, a computer-assisted data analysis software that allows coding and modeling of complex narrative data. The data coding was conducted by our interdisciplinary team of clinicians, behavioral scientists, and social scientists. It is important to note that the NVivo 11 software was not used to analyze the data per se. However, it provided a sophisticated and systematic way to manage the following tasks for the analyses: organizing large quantities of narrative data, coding text, retrieving text by codes, querying the data, comparing sets of data interpretation between NBBM and CBBM; and developing analytic models. The study team members coded the data in weekly team meetings. The coding consisted of reading the data and identifying major themes, then assigning labels to and defining emerging categories.
Two levels of coding were used. The first, open coding, refers to an approach to data with no preconceived ideas about what will be found; and the second, focused or axial coding, refers to reviewing data for the purpose of more richly coding on a particular theme.11 We used dimensional analysis to ensure that each emerging concept was carefully defined. The study team went back and forth between the data and the emerging analytic framework, using constant comparison of new data with already coded data and new categories with previously analyzed text.12
To ensure trustworthiness and credibility,13 the study team maintained an audit trail that documented how and when analytic decisions were made. In addition, peer debriefing was conducted to ensure credibility, including the presentation of findings to the study community advisory board members as part of the community engaged research approach.
Results
Description of participants
The FCDS provided a database of 1,813 participants identified as black men diagnosed with prostate cancer in 2010. Because the FCDS does not extract data on patients with a death certificate, we found out during the pre-screening phase that a few of the men were deceased. In addition, there were a significant number of incorrect addresses. We obtained a total of 212 completed responses by phone during the prescreening phase. The majority of the participants were aged 60-69 years (48.2%), had a high school diploma only (26.1%), and were currently married (65.3%). Relative to ethnicity, 67% of participants classified themselves NBBM, 24% as CBBM, 3.5% as black men born in Africa, and 5.5% as Other/Don’t know/Refused. For the CBBM, the most common countries of birth were Jamaica, Haiti, and Guyana, respectively.
In all, 40 participants (20 NBBM and 20 CBBM) were selected from the 212 participants to participate in the study. Selection was conducted systematically to ensure representation in terms of age, marital status, education, and geographical location. Data saturation was achieved with 17 NBBM and 14 CBBM, after which we ended data collection (Table 2). Data saturation is the standard for deciding that we are not finding anything different from the interviews first coded and last coded. Although we were specifically looking for differences between the two groups (NBBM and CBBM), no between-group differences emerged. Each man’s experience was unique to him with some common themes emerging described hereinafter (Figure).
Moderating factors and experiences at PPCD
Some of the moderating factors that the study participants identified as affecting their reactions to the PPCD included health literacy, insurance status, spirituality, mistrust, prior experience with cancer, perceived susceptibility to cancer, and delay in diagnosis (Table 3). Health literacy, defined as personal, cognitive, and social skills that determine the ability of individuals to gain access to, understand, and use information to promote and maintain good health, was one of the moderating factors found in this study.13 Some of the black men came to the PPCD with a low level of health literacy, which had an impact on their understanding of the treatment options. For example, in the interview, participant 798 (NBBM) was confused about what tests had been done and was not able to accurately describe the treatments offered to him. Participant 1263 (CBBM) struggled to express the purpose and procedures associated with diagnostic biopsy. However, there were participants with a high level of health literacy (eg, participant 449 [NBBM]), who decided to research the disease.
Another factor to consider is the insurance status of participants at the PPCD. The majority of the participants had good insurance coverage, but some were affected by poor insurance coverage. Participant 1881(NBBM) made his treatment decision primarily on the basis of the pending lapse of his insurance coverage rather than the best clinical option for him. Participant 1979 (CBBM) described both his confusion on the screening tests and the impact of not having insurance coverage. Upon obtaining insurance coverage, he sought treatment for his prostate cancer with an urgency that he did not experience when he was first diagnosed when uninsured.
The spirituality of black men was another moderating factor at the PPCD. Participant 827 (NBBM) noted that he was unaffected when he received his diagnosis because he was a true believer. Some of the black men also came to the PPCD with lack of trust in the physician and/or the health care system and perceived a sense of contempt from the physician. Participant 1594 (NBBM) described mistrust based on the history of medical exploitation of black men as well as a perception of current discriminatory practices.
Another important PPCD status to note for black men is prior experience with cancer, including prior personal cancer history and/or prior cancer history of a family member. Participant 2024 (CBBM) described the meaning of cancer to him, while participant 798 (NBBM) echoed the despair of the cancer diagnosis based on experience with other cancers in the family. Sometimes there were multiple cancers in the family or even among the significant others of the participant, as was the case with Participant 1936 (CBBM).
Of greatest concern were men who delayed their diagnosis or treatment, perhaps resulting in their cancer being at a more advanced stage when they eventually did return for care. Finally, some of the men came to the PPCD appointment with a low expectation of receiving a diagnosis of prostate cancer, whereas others came to the PPCD fearful of the results of their testing.
In describing their experiences, participants expressed both positive and negative experiences: on the positive side, they found the information provided by the physician to be helpful; but on the negative side, the sterile or medically focused encounter was perceived as a lack empathy on the part of the physician.
Cognitive, emotional, and behavioral coping experiences
As expected, there were ranges of emotions, including shock, disbelief and denial (Table 4). Some of the men questioned why this (the cancer) was happening to them when they had done “nothing” to deserve it. Doing nothing in this case meant that they had lived a healthy lifestyle with no obvious apparent cause to have the cancer. Fear and cancer fatalism were experienced by a significant number of the men, with their thoughts immediately turning to death and dying. This was especially the case for men who had lost a loved one to cancer. Conversely, some of the men wanted immediate resolution, focusing instead on ways to beat the cancer and with a strong will to live.
Reliance on faith was a big part of coping at the PPCD. Some of the men drew strength from their faith to get them through their cancer journey. Others found a way to accept the diagnosis – one participant accepted the diagnosis and the fact that this could mean dying (after living a good life), whereas another participant accepted the diagnosis with the hope that he would find a cure. Hope was more realistic with the knowledge that other men had survived prostate cancer.
Reflecting back on their experiences, the men also identified clear needs at the PPCD. One of the needs they identified was having a physician they were comfortable with to discuss their diagnosis. Another need was for a second opinion. Participant 1594 (NBBM) advised that it was important for black men to take control by requesting a second opinion. Participant 2039 (NBBM) described a feeling of navigating blindly and trying to find answers that would be helpful to him in his cancer journey. However, his experience with a second opinion was not helpful because the second physician was at the same clinic as his primary physician. His recommendation was to get a second opinion at a different clinic or center. Another important need was emotional support at the PPCD. Participant 2024 (CBBM) made a strong case for emotional support, especially for men who are not accompanied during diagnosis. In addition, Participant 2024’s (CBBM) reflections underscore the fact that the PPCD may not be an ideal place or time to discuss treatment options. With the range of emotions that the men go through at the PPCD, it is difficult to comprehend any follow-up discussions after hearing the words “you have prostate cancer.” Participant 2024 (CBBM) also strongly expressed that men need time to deal with the diagnosis at the PPCD.
Discussion
The primary goal of this study was to develop an interpretative framework of black men’s experiences at the PPCD. The Figure provides a pictorial summary of the framework. Study results indicated that black men come to the PPCD with different emotions and different experiences. Although the majority of the men were NBBM, there is a significantly increasing number of foreign-born black men receiving a diagnosis of prostate cancer in the United States. Given that black men carry a disproportionate burden of the disease, with a significantly higher incidence compared with any other racial group, it is important that tailored services are provided to black men at the PPCD.
We also found that black men came to the PPCD diverse in terms of their ethnicity, health literacy, spirituality, trust in health care system/physician, prior experience with cancer, perceived susceptibility to cancer, delayed time for diagnosis, and fear of diagnosis. Of importance for physicians is that the black race is not homogeneous. There is a significant number of foreign-born blacks at the PPCD, and they often have different cultural beliefs and values compared with NBBM. In addition, some of the foreign-born black men may not have English proficiency and may need a medical interpreter during the PPCD consultation. In addition, a patient’s pre-existing lack of trust in the health care system may have a negative impact on the PPCD consultation. It is thus important that the physician takes the time to instill trust and make the men comfortable during the PPCD consultation.
For some of the men who had fear of a prostate cancer diagnosis and/or prior experience with cancer, cancer fatalism was experienced at the PPCD. Cancer fatalism, defined as an individual’s belief that death is bound to happen when diagnosed with cancer, has been documented as a major barrier to cancer detection and control.15 For example, fatalistic perspectives have been reported to affect cervical cancer,16 breast cancer,17,18 colorectal cancer,19 and prostate cancer20,21 among blacks. It is thus important to effectively address fatalistic beliefs when a man is diagnosed with prostate cancer.
Other emotions at the PPCD that may affect effective treatment decision making also need to be addressed immediately. For example, the emotions of fear, denial, and feeling overwhelmed are potential barriers to timely treatment decision making. Psycho-oncology interventions to appropriately deal with these emotions at PPCD or right after the diagnosis may be crucial for the men. In particular, a group-based psychosocial intervention focusing on: provision of education about treatment options for prostate cancer and their acute and late effects; negotiating treatment and treatment side effects; enhancing communication with treatment providers; managing distress; and engaging positive family- and community-based social support to optimize emotional, behavioral, social, and physical outcomes in black men with prostate cancer.
In addition to having physicians make them comfortable at PPCD, the PPCD needs expressed by participants included having time to come to terms with the diagnosis and receiving psycho-oncology/emotional support. Anyone who has just received a diagnosis of cancer cannot be expected to immediately continue to function as he did before the PPCD. This is especially difficult for men who are alone at the PPCD. Nevertheless, it is expected that they will listen attentively and understand subsequent consultation by the physician, then leave the consultation room almost immediately, and be able drive home or back to work right after the diagnosis. There seems to be a support gap that needs to be closed at the PPCD. Providing the men with immediate support to cope with the diagnosis may make a significant difference in effective treatment choices and eliminating treatment decisional regrets.
Methodological rigor was established through purposeful sampling, extended time with participants, standardized procedures for data collection, management and analysis, multidisciplinary interpretation, and validation of results with the community advisory board. Because the research participants were purposefully selected from a statewide database of black men diagnosed with CaP, generalizability of findings to the two target groups of NBBM and CBBM can be assumed, with the caveat that men with different experiences may have chosen not to respond to recruitment efforts or refused participation. Black men who were not sufficiently fluent in English to be interviewed were also excluded and are not represented in these findings. Black men of other nativity (including African-born black men) and residing outside of Florida were also not represented.
In conclusion, the PPCD interpretative framework developed in this study, describes the status of black men at the PPCD, their experiences during the PPCD, and their needs at the PPCD. The framework provides information that can be used by physicians to prepare for their PPCD consultation with black men as well as develop a support system for black men at the PPCD.
Acknowledgments
The authors thank the men who participated in the CaPCaS study. They also thank the CaPCaS project community advisory board chairs (Mr Jim West, Dr Angela Adams, and Prince Oladapo Odedina) and all the CaPCaS project community advisory board members for their effort throughout the project. Finally, they rxecognize the effort of additional CaPCaS scientific team, especially the primary interviewer, Mr Kenneth Stokes. Weekly meeting support for this study was provided by the University of Florida MiCaRT Center, which is funded by the NIH-National Cancer Institute Award # 1P20CA192990-02. REDCaP software was supported by the UF Clinical and Translational Science Institute, which is funded in part by the NIH Clinical and Translational Science Award program (grants UL1TR001427, KL2TR001429 and TL1TR001428).
1. American Cancer Society. Cancer facts & figures 2016. http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2016/. Published 2016. Accessed January 10, 2017.
2. Florida Cancer Data System. Florida Statewide Population-Based Cancer Registry. https://fcds.med.miami.edu/scripts/fcdspubrates/production/doSelection.aspx?election=map. Processed February 16, 2016.
3. Sinfield P, Baker R, Camosso-Stefinovic J, et al. Men’s and carers’ experiences of care for prostate cancer: a narrative literature review. Health Expect. 2009;12:301-312.
4. Maliski SL, Connor SE, Williams L, Litwin MS. Faith among low-income, African American/ black men treated for prostate cancer. Cancer Nurs. 2010;33(6):470-478.
5. Jones RA, Wenzel J, Hinton I, et el. Exploring cancer support needs for older African-American men with prostate cancer. Support Care Cancer. 2011;19(9):1411-1419.
6. Sinfield P, Baker R, Agarwal S, Tarrant C. Patient-centred care: what are the experiences of prostate cancer patients and their partners? Patient Educ Couns. 2008;73(1):91-96.
7. American Cancer Society. Cancer facts & figures for African Americans 2016-2018. http://www.cancer.org/research/cancerfactsstatistics/cancer-facts-figures-for-african-americans. Published 2016. Accessed January 10, 2017.
8. Patton MQ. Qualitative research & evaluation methods. 4th ed. Thousand Oaks, CA: Sage Publications; 2001.
9. Florida Department of Health, Bureau of Epidemiology. Procedure guide for studies that utilize patient identifiable data from the Florida Cancer Data System. http://www.fcds.med.miami.edu/downloads/datarequest/Procedure%20Guide_Revised%20
October%202007.pdf. Accessed July 24, 2010.
10. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381.
11. Strauss A. Qualitative analysis for social scientists. New York, NY: Cambridge University Press; 1987.
12. Glaser BG, Strauss AL. The discovery of grounded theory: strategies for qualitative research. Chicago, IL: Aldine; 1967.
13. Miles MB, Huberman AM. Qualitative data analysis: an expanded sourcebook. 2nd ed. Thousand Oaks, CA: Sage Publications; 1994.
14. Nutbeam, D. Health literacy as a public health goal: a challenge for contemporary health education and communication strategies into the 21st century. Health Promot Int. 2000;15(3):259-267.
15. Powe BD, Finnie R. Cancer fatalism: the state of the science. Cancer Nurs. 2003;26:454-467.
16. Powe BD. Fatalism among elderly African Americans: effects on colorectal cancer screening. Cancer Nurs. 1995;18:285-392.
17. Powe BD. Cancer fatalism among elderly Caucasians and African Americans. Oncol Nurs Forum. 1995;22(9):1355-1359.
18. Thoresen CE. Spirituality, health, and science: the coming revival? In: Roth RS, Kurpius SR, eds. The emerging role of counseling psychology in health care. New York, NY: WW Norton; 1998.
19. Carver CS, Scheier MF, Weintraub JK. Assessing coping strategies: a theoretically based approach. J Pers Soc Psychol. 1989;56:267-283.
21. Odedina FT, Yu D, Akinremi TO, Reams RR, Freedman ML, Kumar N. Prostate cancer cognitive-behavioral factors in a West African population. J Immigr Minor Health. 2009;11(4):258-267.
22. Odedina FT, Scrivens JJ Jr, Larose-Pierre M, et al. Modifiable prostate cancer risk reduction and early detection behaviors in black men. Am J Health Behav. 2011;35(4):470-484.
1. American Cancer Society. Cancer facts & figures 2016. http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2016/. Published 2016. Accessed January 10, 2017.
2. Florida Cancer Data System. Florida Statewide Population-Based Cancer Registry. https://fcds.med.miami.edu/scripts/fcdspubrates/production/doSelection.aspx?election=map. Processed February 16, 2016.
3. Sinfield P, Baker R, Camosso-Stefinovic J, et al. Men’s and carers’ experiences of care for prostate cancer: a narrative literature review. Health Expect. 2009;12:301-312.
4. Maliski SL, Connor SE, Williams L, Litwin MS. Faith among low-income, African American/ black men treated for prostate cancer. Cancer Nurs. 2010;33(6):470-478.
5. Jones RA, Wenzel J, Hinton I, et el. Exploring cancer support needs for older African-American men with prostate cancer. Support Care Cancer. 2011;19(9):1411-1419.
6. Sinfield P, Baker R, Agarwal S, Tarrant C. Patient-centred care: what are the experiences of prostate cancer patients and their partners? Patient Educ Couns. 2008;73(1):91-96.
7. American Cancer Society. Cancer facts & figures for African Americans 2016-2018. http://www.cancer.org/research/cancerfactsstatistics/cancer-facts-figures-for-african-americans. Published 2016. Accessed January 10, 2017.
8. Patton MQ. Qualitative research & evaluation methods. 4th ed. Thousand Oaks, CA: Sage Publications; 2001.
9. Florida Department of Health, Bureau of Epidemiology. Procedure guide for studies that utilize patient identifiable data from the Florida Cancer Data System. http://www.fcds.med.miami.edu/downloads/datarequest/Procedure%20Guide_Revised%20
October%202007.pdf. Accessed July 24, 2010.
10. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381.
11. Strauss A. Qualitative analysis for social scientists. New York, NY: Cambridge University Press; 1987.
12. Glaser BG, Strauss AL. The discovery of grounded theory: strategies for qualitative research. Chicago, IL: Aldine; 1967.
13. Miles MB, Huberman AM. Qualitative data analysis: an expanded sourcebook. 2nd ed. Thousand Oaks, CA: Sage Publications; 1994.
14. Nutbeam, D. Health literacy as a public health goal: a challenge for contemporary health education and communication strategies into the 21st century. Health Promot Int. 2000;15(3):259-267.
15. Powe BD, Finnie R. Cancer fatalism: the state of the science. Cancer Nurs. 2003;26:454-467.
16. Powe BD. Fatalism among elderly African Americans: effects on colorectal cancer screening. Cancer Nurs. 1995;18:285-392.
17. Powe BD. Cancer fatalism among elderly Caucasians and African Americans. Oncol Nurs Forum. 1995;22(9):1355-1359.
18. Thoresen CE. Spirituality, health, and science: the coming revival? In: Roth RS, Kurpius SR, eds. The emerging role of counseling psychology in health care. New York, NY: WW Norton; 1998.
19. Carver CS, Scheier MF, Weintraub JK. Assessing coping strategies: a theoretically based approach. J Pers Soc Psychol. 1989;56:267-283.
21. Odedina FT, Yu D, Akinremi TO, Reams RR, Freedman ML, Kumar N. Prostate cancer cognitive-behavioral factors in a West African population. J Immigr Minor Health. 2009;11(4):258-267.
22. Odedina FT, Scrivens JJ Jr, Larose-Pierre M, et al. Modifiable prostate cancer risk reduction and early detection behaviors in black men. Am J Health Behav. 2011;35(4):470-484.
The power of interaction – Supporting language and play development
Engaging caregivers in the management and treatment of early childhood developmental challenges is a critical component of effective intervention.1 Family-centered care helps to promote positive outcomes with early intervention (across developmental domains), and there’s increasing evidence that parent-training programs can be effective in promoting skill generalization and targeting core impairments in toddlers with autism.2
Furthermore, a 2014 randomized controlled trial revealed that individual Early Social Interaction (ESI) with home coaching using the SCERTS (Social Communication, Emotional Regulation, and Transactional Support) curriculum was associated with improvement of a range of child outcomes, compared with group ESI. The authors commented on the importance of individualized parent coaching in natural environments as a way to improve social components of communication and receptive language for toddlers with autism.3
For many parents and at-home caregivers, however, engaging in home-based and parent-delivered interventions can be overwhelming and anxiety-provoking, as well as complicated by other barriers (competing responsibilities, cultural beliefs, and so on). Additionally, these interventions can themselves be a source of stress for some families.
Case
Jake is a 3-year-old boy with a history of global developmental delays, who presents with particular struggles: relating his expressive communication, ability to engage peers in an age-appropriate manner, and capacity to self-regulate when frustrated. He and his family participated in an comprehensive autism diagnostic assessment. In reviewing the history and presentation, considerable challenges in the two core symptom domains that characterize an autism spectrum disorder were noted. A diagnosis of autism was provided, and treatment recommendations were discussed. “What can I do at home to help Jake learn?” his mother asked, noting that, with one-on-one attention, he does seem to demonstrate increased responsiveness, less use of echolalic language, and improved eye contact.
Discussion
To complement the autism services that Jake would likely qualify for through an Early Education program, in-home interaction and play to ensure skill development was discussed at length with his mother, who readily acknowledged her own care-giving struggles that, in part, are informed by her own mental health troubles.
We openly explored Jake’s mother’s perceived challenges in engaging with her son at home and developed initial recommendations for interaction that didn’t risk overwhelming her. We impressed upon Jake’s mother that, regardless of a child’s developmental profile, toddlers use play to learn and she can be Jake’s “favorite toy.” After all, “play is really the work of childhood,” as Fred Rogers said.
With all children, back-and-forth interactions serve as the foundation for future development. Using scaffolding techniques, parent support is a primary driver of “how children develop cognitive, language, social-emotional, and higher-level thinking skills.”4 In particular, the quality of parental interaction can influence language development, and, when considering children with autism, there are several recommendations for what parents can do to help build social, play, and communication skills.5 The Hanen Program is a great resource for providers and parents to learn more about parent engagement in early learning, the power of building communication through everyday experiences and attention to responsiveness, and the use of a child’s strengths to help make family interactions more meaningful and enjoyable. Additionally, the 2012 book “An Early Start for Your Child with Autism: Using Everyday Activities to Help Kids Connect, Communicate, and Learn” by Sally Rogers, PhD, et al. is an easy-to-read text for parents and caregivers for learning effective and practical strategies for engaging their child with autism.
With Jake and his mother, our team offered the following in-home recommendations:
- Try to keep interaction fun. Be enthusiastic when encouraging Jake’s attempts to communicate.
- Teach Jake song-gesture games. Encourage him to produce routine, predictable gestures to keep the song going (in imitation of mom). Using songs with vowel emphasis is encouraged (for example: Farmer in the Dell with “E I E I OOOOO”).
- Encourage Jake to produce responsive gestures in play and daily routines not involving songs, such as open arms to receive a ball, reaching to mom when about to be tickled, or having his arms up to have his shirt taken off.
- Capitalize on Jake’s natural desires and personal preferences. Activate a wind-up toy, let it deactivate, and then hand it to Jake.
- Initiate a familiar social game with Jake until he expresses pleasure. Then stop the game and wait for him to initiate continuance.
- Adapt the environment so that Jake will need to frequently request objects of assistance to make choices (place favorite toys in clear containers which may be difficult to open so that he must request help).
Clinical pearl
The United States Department of Education recognizes the importance of family engagement in a child’s early years. Their 2015 policy statement notes that “families are their children’s first and most important teachers, advocates, and nurturers. As such, strong family engagement is central – not supplemental – to the success of early childhood systems and programs that promote children’s healthy development, learning, and wellness.”
By recognizing this principle, primary care providers are in a position to talk with parents about how much youth learn through play and regular interaction. This especially holds true for children with autism. Developing in-home strategies to facilitate active engagement, even strategies that may not be a formal component of a home-based intervention program, are instrumental in fostering positive family- and child-based outcomes and wellness.
Dr. Dickerson, a child and adolescent psychiatrist, is assistant professor of psychiatry at the University of Vermont, Burlington, where he is director of the autism diagnostic clinic. Email him at [email protected].
References
1. Annu Rev Clin Psychol. 2010;6:447-68.
2. J Autism Dev Disord. 2010 Sep;40(9):1045-56.
3. Pediatrics. 2014 Dec;134(6):1084-93.
4. JAMA Pediatr. 2016 Feb;170(2):112-3.
5. Child Dev. 2012 Sep-Oct;83(5):1762-74.
Engaging caregivers in the management and treatment of early childhood developmental challenges is a critical component of effective intervention.1 Family-centered care helps to promote positive outcomes with early intervention (across developmental domains), and there’s increasing evidence that parent-training programs can be effective in promoting skill generalization and targeting core impairments in toddlers with autism.2
Furthermore, a 2014 randomized controlled trial revealed that individual Early Social Interaction (ESI) with home coaching using the SCERTS (Social Communication, Emotional Regulation, and Transactional Support) curriculum was associated with improvement of a range of child outcomes, compared with group ESI. The authors commented on the importance of individualized parent coaching in natural environments as a way to improve social components of communication and receptive language for toddlers with autism.3
For many parents and at-home caregivers, however, engaging in home-based and parent-delivered interventions can be overwhelming and anxiety-provoking, as well as complicated by other barriers (competing responsibilities, cultural beliefs, and so on). Additionally, these interventions can themselves be a source of stress for some families.
Case
Jake is a 3-year-old boy with a history of global developmental delays, who presents with particular struggles: relating his expressive communication, ability to engage peers in an age-appropriate manner, and capacity to self-regulate when frustrated. He and his family participated in an comprehensive autism diagnostic assessment. In reviewing the history and presentation, considerable challenges in the two core symptom domains that characterize an autism spectrum disorder were noted. A diagnosis of autism was provided, and treatment recommendations were discussed. “What can I do at home to help Jake learn?” his mother asked, noting that, with one-on-one attention, he does seem to demonstrate increased responsiveness, less use of echolalic language, and improved eye contact.
Discussion
To complement the autism services that Jake would likely qualify for through an Early Education program, in-home interaction and play to ensure skill development was discussed at length with his mother, who readily acknowledged her own care-giving struggles that, in part, are informed by her own mental health troubles.
We openly explored Jake’s mother’s perceived challenges in engaging with her son at home and developed initial recommendations for interaction that didn’t risk overwhelming her. We impressed upon Jake’s mother that, regardless of a child’s developmental profile, toddlers use play to learn and she can be Jake’s “favorite toy.” After all, “play is really the work of childhood,” as Fred Rogers said.
With all children, back-and-forth interactions serve as the foundation for future development. Using scaffolding techniques, parent support is a primary driver of “how children develop cognitive, language, social-emotional, and higher-level thinking skills.”4 In particular, the quality of parental interaction can influence language development, and, when considering children with autism, there are several recommendations for what parents can do to help build social, play, and communication skills.5 The Hanen Program is a great resource for providers and parents to learn more about parent engagement in early learning, the power of building communication through everyday experiences and attention to responsiveness, and the use of a child’s strengths to help make family interactions more meaningful and enjoyable. Additionally, the 2012 book “An Early Start for Your Child with Autism: Using Everyday Activities to Help Kids Connect, Communicate, and Learn” by Sally Rogers, PhD, et al. is an easy-to-read text for parents and caregivers for learning effective and practical strategies for engaging their child with autism.
With Jake and his mother, our team offered the following in-home recommendations:
- Try to keep interaction fun. Be enthusiastic when encouraging Jake’s attempts to communicate.
- Teach Jake song-gesture games. Encourage him to produce routine, predictable gestures to keep the song going (in imitation of mom). Using songs with vowel emphasis is encouraged (for example: Farmer in the Dell with “E I E I OOOOO”).
- Encourage Jake to produce responsive gestures in play and daily routines not involving songs, such as open arms to receive a ball, reaching to mom when about to be tickled, or having his arms up to have his shirt taken off.
- Capitalize on Jake’s natural desires and personal preferences. Activate a wind-up toy, let it deactivate, and then hand it to Jake.
- Initiate a familiar social game with Jake until he expresses pleasure. Then stop the game and wait for him to initiate continuance.
- Adapt the environment so that Jake will need to frequently request objects of assistance to make choices (place favorite toys in clear containers which may be difficult to open so that he must request help).
Clinical pearl
The United States Department of Education recognizes the importance of family engagement in a child’s early years. Their 2015 policy statement notes that “families are their children’s first and most important teachers, advocates, and nurturers. As such, strong family engagement is central – not supplemental – to the success of early childhood systems and programs that promote children’s healthy development, learning, and wellness.”
By recognizing this principle, primary care providers are in a position to talk with parents about how much youth learn through play and regular interaction. This especially holds true for children with autism. Developing in-home strategies to facilitate active engagement, even strategies that may not be a formal component of a home-based intervention program, are instrumental in fostering positive family- and child-based outcomes and wellness.
Dr. Dickerson, a child and adolescent psychiatrist, is assistant professor of psychiatry at the University of Vermont, Burlington, where he is director of the autism diagnostic clinic. Email him at [email protected].
References
1. Annu Rev Clin Psychol. 2010;6:447-68.
2. J Autism Dev Disord. 2010 Sep;40(9):1045-56.
3. Pediatrics. 2014 Dec;134(6):1084-93.
4. JAMA Pediatr. 2016 Feb;170(2):112-3.
5. Child Dev. 2012 Sep-Oct;83(5):1762-74.
Engaging caregivers in the management and treatment of early childhood developmental challenges is a critical component of effective intervention.1 Family-centered care helps to promote positive outcomes with early intervention (across developmental domains), and there’s increasing evidence that parent-training programs can be effective in promoting skill generalization and targeting core impairments in toddlers with autism.2
Furthermore, a 2014 randomized controlled trial revealed that individual Early Social Interaction (ESI) with home coaching using the SCERTS (Social Communication, Emotional Regulation, and Transactional Support) curriculum was associated with improvement of a range of child outcomes, compared with group ESI. The authors commented on the importance of individualized parent coaching in natural environments as a way to improve social components of communication and receptive language for toddlers with autism.3
For many parents and at-home caregivers, however, engaging in home-based and parent-delivered interventions can be overwhelming and anxiety-provoking, as well as complicated by other barriers (competing responsibilities, cultural beliefs, and so on). Additionally, these interventions can themselves be a source of stress for some families.
Case
Jake is a 3-year-old boy with a history of global developmental delays, who presents with particular struggles: relating his expressive communication, ability to engage peers in an age-appropriate manner, and capacity to self-regulate when frustrated. He and his family participated in an comprehensive autism diagnostic assessment. In reviewing the history and presentation, considerable challenges in the two core symptom domains that characterize an autism spectrum disorder were noted. A diagnosis of autism was provided, and treatment recommendations were discussed. “What can I do at home to help Jake learn?” his mother asked, noting that, with one-on-one attention, he does seem to demonstrate increased responsiveness, less use of echolalic language, and improved eye contact.
Discussion
To complement the autism services that Jake would likely qualify for through an Early Education program, in-home interaction and play to ensure skill development was discussed at length with his mother, who readily acknowledged her own care-giving struggles that, in part, are informed by her own mental health troubles.
We openly explored Jake’s mother’s perceived challenges in engaging with her son at home and developed initial recommendations for interaction that didn’t risk overwhelming her. We impressed upon Jake’s mother that, regardless of a child’s developmental profile, toddlers use play to learn and she can be Jake’s “favorite toy.” After all, “play is really the work of childhood,” as Fred Rogers said.
With all children, back-and-forth interactions serve as the foundation for future development. Using scaffolding techniques, parent support is a primary driver of “how children develop cognitive, language, social-emotional, and higher-level thinking skills.”4 In particular, the quality of parental interaction can influence language development, and, when considering children with autism, there are several recommendations for what parents can do to help build social, play, and communication skills.5 The Hanen Program is a great resource for providers and parents to learn more about parent engagement in early learning, the power of building communication through everyday experiences and attention to responsiveness, and the use of a child’s strengths to help make family interactions more meaningful and enjoyable. Additionally, the 2012 book “An Early Start for Your Child with Autism: Using Everyday Activities to Help Kids Connect, Communicate, and Learn” by Sally Rogers, PhD, et al. is an easy-to-read text for parents and caregivers for learning effective and practical strategies for engaging their child with autism.
With Jake and his mother, our team offered the following in-home recommendations:
- Try to keep interaction fun. Be enthusiastic when encouraging Jake’s attempts to communicate.
- Teach Jake song-gesture games. Encourage him to produce routine, predictable gestures to keep the song going (in imitation of mom). Using songs with vowel emphasis is encouraged (for example: Farmer in the Dell with “E I E I OOOOO”).
- Encourage Jake to produce responsive gestures in play and daily routines not involving songs, such as open arms to receive a ball, reaching to mom when about to be tickled, or having his arms up to have his shirt taken off.
- Capitalize on Jake’s natural desires and personal preferences. Activate a wind-up toy, let it deactivate, and then hand it to Jake.
- Initiate a familiar social game with Jake until he expresses pleasure. Then stop the game and wait for him to initiate continuance.
- Adapt the environment so that Jake will need to frequently request objects of assistance to make choices (place favorite toys in clear containers which may be difficult to open so that he must request help).
Clinical pearl
The United States Department of Education recognizes the importance of family engagement in a child’s early years. Their 2015 policy statement notes that “families are their children’s first and most important teachers, advocates, and nurturers. As such, strong family engagement is central – not supplemental – to the success of early childhood systems and programs that promote children’s healthy development, learning, and wellness.”
By recognizing this principle, primary care providers are in a position to talk with parents about how much youth learn through play and regular interaction. This especially holds true for children with autism. Developing in-home strategies to facilitate active engagement, even strategies that may not be a formal component of a home-based intervention program, are instrumental in fostering positive family- and child-based outcomes and wellness.
Dr. Dickerson, a child and adolescent psychiatrist, is assistant professor of psychiatry at the University of Vermont, Burlington, where he is director of the autism diagnostic clinic. Email him at [email protected].
References
1. Annu Rev Clin Psychol. 2010;6:447-68.
2. J Autism Dev Disord. 2010 Sep;40(9):1045-56.
3. Pediatrics. 2014 Dec;134(6):1084-93.
4. JAMA Pediatr. 2016 Feb;170(2):112-3.
5. Child Dev. 2012 Sep-Oct;83(5):1762-74.
Childhood adversities tied to depressive symptoms in older homeless adults
Childhood adversities, which are known contributors to psychiatric morbidity in adults, continue to be disruptive later in life, a cross-sectional study of 350 older homeless adults shows.
“Clinicians should collect information about childhood adversities among this high-risk population to inform risk assessment and treatment recommendations,” wrote Chuan-Mei Lee, MD, and her associates (Am J Geriatr Psychiatry. 2017 Feb;25[2]:107-17).
At the start of the study, Dr. Lee and her associates recruited 350 adults who met the participation criteria from several places frequented by homeless individuals in Oakland, Calif., including shelters and encampments.
The median age of the participants was 58.1 years, 77.1% were men, and 79.7% were African American. All of the participants were English speakers and were homeless as defined by the Homeless Emergency Assistance and Rapid Transition to Housing Act (Psychiatr Ser. 2009;60[4]:465-72). Many of the participants (43.4%) had experienced their first homeless episode at age 50 years or later, reported Dr. Lee of the department of psychiatry at the University of California, San Francisco, and her associates.
The older homeless adults were accessed clinically and asked whether a health care provider had ever told them that they had any of several conditions, including hypertension, coronary artery disease, diabetes, stroke, cancer, and HIV/AIDS. The investigators used the Modified Mini-Mental State examination to assess the participants’ cognitive statuses.
The participants were asked about seven categories of adverse events that they might have experienced before age 18. Specifically, they were asked if they had experienced physical neglect, verbal abuse, physical abuse, sexual abuse, the death of either parent, incarceration of a parent for 1 month or more, or placement in the child welfare system. Each person received a childhood adversity score ranging from 0 to 7.
“Because of the low prevalence of scores of 5 or greater, we grouped study participants with four or more childhood adversities together,” Dr. Lee and her associates wrote.
Depressive symptoms were assessed using the Center for Epidemiologic Studies Depression Scale (CESD). A CESD score equal to or greater than 22 was used to define moderate to severe depressive symptoms.
Participants who completed the enrollment procedures received a $5 gift card, and those who completed the enrollment interview received a $20 gift card.
Of the 350 total participants, 251 reported a history of childhood adversity, and 99 reported no such history. Verbal abuse was the most commonly reported childhood adversity (49.3%), followed by physical abuse (33.3%) and parental death (21.4%).
Overall, the investigators found that more than one-third of the participants (38.3%) received CESD scores high enough to place them in the moderate to severe depressive symptoms category. Furthermore, they found a dose-response relationship between the number of adverse experiences and higher odds of moderate to severe depressive symptoms.
Participants with “exposure to one childhood adversity had a twofold increase in odds of reporting moderate to severe depressive symptoms (adjusted odds ratio, 2.0; 95% confidence interval, 1.1-3.7), whereas those with exposure to four or more childhood adversities had a sixfold increase (AOR, 6.0; 95% CI, 2.4-15.4), compared with those with no adverse events,” the investigators noted.
Similar dose-response relationships were found between the number of adverse childhood events and the number of lifetime suicide attempts.
Dr. Lee and her associates said their findings have implications for clinical practice. The Substance Abuse and Mental Health Services Administration recommends that clinicians screen all patients for physical and sexual trauma but not for parental loss, they said.
“Our findings suggest that mental health and primary care providers should consider screening older homeless adults for all childhood adversities,” they wrote. “This may enhance suicide risk assessment by identifying those with multiple adversities, who are at highest risk.”
The investigators cited several limitations. For example, estimates of childhood adversity might have been underreported. Also, the study’s cross-sectional design made it difficult to establish causation between childhood adversity and psychiatric morbidity.
Nevertheless, “psychiatrists working with low-income, older populations should screen for homelessness,” they wrote. “The high prevalence of psychiatric morbidity in this medically complex population presents challenges to the mental health workforce [amid] a shortage of geriatric psychiatrists.”
Dr. Lee reported no conflicts of interest. The principal investigator, Margot Kushel, MD, reported serving on the leadership board of EveryOne Home, a group that seeks to bring an end to homelessness in Alameda County, Calif. The National Institute on Aging, the National Institute of Mental Health, and SAMHSA provided funding support.
[email protected]
On Twitter @ginalhenderson
Childhood adversities, which are known contributors to psychiatric morbidity in adults, continue to be disruptive later in life, a cross-sectional study of 350 older homeless adults shows.
“Clinicians should collect information about childhood adversities among this high-risk population to inform risk assessment and treatment recommendations,” wrote Chuan-Mei Lee, MD, and her associates (Am J Geriatr Psychiatry. 2017 Feb;25[2]:107-17).
At the start of the study, Dr. Lee and her associates recruited 350 adults who met the participation criteria from several places frequented by homeless individuals in Oakland, Calif., including shelters and encampments.
The median age of the participants was 58.1 years, 77.1% were men, and 79.7% were African American. All of the participants were English speakers and were homeless as defined by the Homeless Emergency Assistance and Rapid Transition to Housing Act (Psychiatr Ser. 2009;60[4]:465-72). Many of the participants (43.4%) had experienced their first homeless episode at age 50 years or later, reported Dr. Lee of the department of psychiatry at the University of California, San Francisco, and her associates.
The older homeless adults were accessed clinically and asked whether a health care provider had ever told them that they had any of several conditions, including hypertension, coronary artery disease, diabetes, stroke, cancer, and HIV/AIDS. The investigators used the Modified Mini-Mental State examination to assess the participants’ cognitive statuses.
The participants were asked about seven categories of adverse events that they might have experienced before age 18. Specifically, they were asked if they had experienced physical neglect, verbal abuse, physical abuse, sexual abuse, the death of either parent, incarceration of a parent for 1 month or more, or placement in the child welfare system. Each person received a childhood adversity score ranging from 0 to 7.
“Because of the low prevalence of scores of 5 or greater, we grouped study participants with four or more childhood adversities together,” Dr. Lee and her associates wrote.
Depressive symptoms were assessed using the Center for Epidemiologic Studies Depression Scale (CESD). A CESD score equal to or greater than 22 was used to define moderate to severe depressive symptoms.
Participants who completed the enrollment procedures received a $5 gift card, and those who completed the enrollment interview received a $20 gift card.
Of the 350 total participants, 251 reported a history of childhood adversity, and 99 reported no such history. Verbal abuse was the most commonly reported childhood adversity (49.3%), followed by physical abuse (33.3%) and parental death (21.4%).
Overall, the investigators found that more than one-third of the participants (38.3%) received CESD scores high enough to place them in the moderate to severe depressive symptoms category. Furthermore, they found a dose-response relationship between the number of adverse experiences and higher odds of moderate to severe depressive symptoms.
Participants with “exposure to one childhood adversity had a twofold increase in odds of reporting moderate to severe depressive symptoms (adjusted odds ratio, 2.0; 95% confidence interval, 1.1-3.7), whereas those with exposure to four or more childhood adversities had a sixfold increase (AOR, 6.0; 95% CI, 2.4-15.4), compared with those with no adverse events,” the investigators noted.
Similar dose-response relationships were found between the number of adverse childhood events and the number of lifetime suicide attempts.
Dr. Lee and her associates said their findings have implications for clinical practice. The Substance Abuse and Mental Health Services Administration recommends that clinicians screen all patients for physical and sexual trauma but not for parental loss, they said.
“Our findings suggest that mental health and primary care providers should consider screening older homeless adults for all childhood adversities,” they wrote. “This may enhance suicide risk assessment by identifying those with multiple adversities, who are at highest risk.”
The investigators cited several limitations. For example, estimates of childhood adversity might have been underreported. Also, the study’s cross-sectional design made it difficult to establish causation between childhood adversity and psychiatric morbidity.
Nevertheless, “psychiatrists working with low-income, older populations should screen for homelessness,” they wrote. “The high prevalence of psychiatric morbidity in this medically complex population presents challenges to the mental health workforce [amid] a shortage of geriatric psychiatrists.”
Dr. Lee reported no conflicts of interest. The principal investigator, Margot Kushel, MD, reported serving on the leadership board of EveryOne Home, a group that seeks to bring an end to homelessness in Alameda County, Calif. The National Institute on Aging, the National Institute of Mental Health, and SAMHSA provided funding support.
[email protected]
On Twitter @ginalhenderson
Childhood adversities, which are known contributors to psychiatric morbidity in adults, continue to be disruptive later in life, a cross-sectional study of 350 older homeless adults shows.
“Clinicians should collect information about childhood adversities among this high-risk population to inform risk assessment and treatment recommendations,” wrote Chuan-Mei Lee, MD, and her associates (Am J Geriatr Psychiatry. 2017 Feb;25[2]:107-17).
At the start of the study, Dr. Lee and her associates recruited 350 adults who met the participation criteria from several places frequented by homeless individuals in Oakland, Calif., including shelters and encampments.
The median age of the participants was 58.1 years, 77.1% were men, and 79.7% were African American. All of the participants were English speakers and were homeless as defined by the Homeless Emergency Assistance and Rapid Transition to Housing Act (Psychiatr Ser. 2009;60[4]:465-72). Many of the participants (43.4%) had experienced their first homeless episode at age 50 years or later, reported Dr. Lee of the department of psychiatry at the University of California, San Francisco, and her associates.
The older homeless adults were accessed clinically and asked whether a health care provider had ever told them that they had any of several conditions, including hypertension, coronary artery disease, diabetes, stroke, cancer, and HIV/AIDS. The investigators used the Modified Mini-Mental State examination to assess the participants’ cognitive statuses.
The participants were asked about seven categories of adverse events that they might have experienced before age 18. Specifically, they were asked if they had experienced physical neglect, verbal abuse, physical abuse, sexual abuse, the death of either parent, incarceration of a parent for 1 month or more, or placement in the child welfare system. Each person received a childhood adversity score ranging from 0 to 7.
“Because of the low prevalence of scores of 5 or greater, we grouped study participants with four or more childhood adversities together,” Dr. Lee and her associates wrote.
Depressive symptoms were assessed using the Center for Epidemiologic Studies Depression Scale (CESD). A CESD score equal to or greater than 22 was used to define moderate to severe depressive symptoms.
Participants who completed the enrollment procedures received a $5 gift card, and those who completed the enrollment interview received a $20 gift card.
Of the 350 total participants, 251 reported a history of childhood adversity, and 99 reported no such history. Verbal abuse was the most commonly reported childhood adversity (49.3%), followed by physical abuse (33.3%) and parental death (21.4%).
Overall, the investigators found that more than one-third of the participants (38.3%) received CESD scores high enough to place them in the moderate to severe depressive symptoms category. Furthermore, they found a dose-response relationship between the number of adverse experiences and higher odds of moderate to severe depressive symptoms.
Participants with “exposure to one childhood adversity had a twofold increase in odds of reporting moderate to severe depressive symptoms (adjusted odds ratio, 2.0; 95% confidence interval, 1.1-3.7), whereas those with exposure to four or more childhood adversities had a sixfold increase (AOR, 6.0; 95% CI, 2.4-15.4), compared with those with no adverse events,” the investigators noted.
Similar dose-response relationships were found between the number of adverse childhood events and the number of lifetime suicide attempts.
Dr. Lee and her associates said their findings have implications for clinical practice. The Substance Abuse and Mental Health Services Administration recommends that clinicians screen all patients for physical and sexual trauma but not for parental loss, they said.
“Our findings suggest that mental health and primary care providers should consider screening older homeless adults for all childhood adversities,” they wrote. “This may enhance suicide risk assessment by identifying those with multiple adversities, who are at highest risk.”
The investigators cited several limitations. For example, estimates of childhood adversity might have been underreported. Also, the study’s cross-sectional design made it difficult to establish causation between childhood adversity and psychiatric morbidity.
Nevertheless, “psychiatrists working with low-income, older populations should screen for homelessness,” they wrote. “The high prevalence of psychiatric morbidity in this medically complex population presents challenges to the mental health workforce [amid] a shortage of geriatric psychiatrists.”
Dr. Lee reported no conflicts of interest. The principal investigator, Margot Kushel, MD, reported serving on the leadership board of EveryOne Home, a group that seeks to bring an end to homelessness in Alameda County, Calif. The National Institute on Aging, the National Institute of Mental Health, and SAMHSA provided funding support.
[email protected]
On Twitter @ginalhenderson
FROM THE AMERICAN JOURNAL OF GERIATRIC PSYCHIATRY
Experts warn against readmissions as sole quality measure in ovarian cancer
NATIONAL HARBOR, MD. – For patients with ovarian cancer, focusing solely on hospital readmission rates as a quality measure might worsen long-term outcomes while unfairly penalizing the best hospitals, suggest the results of two analyses of the National Cancer Database presented at the annual meeting of the Society of Gynecologic Oncology.
Hospitals with the highest ovarian cancer caseloads had the best rates of overall survival and postsurgical mortality, but also had the highest rates of readmission within 30 days after postsurgical discharge, reported Shitanshu Uppal, MBBS, of the obstetrics and gynecology department at the University of Michigan in Ann Arbor.
A separate analysis linked neoadjuvant chemotherapy with significantly fewer readmissions, but also with significantly worse overall survival compared with primary debulking surgery, reported Emma L. Barber, MD, of the University of North Carolina at Chapel Hill.
“Policies that prioritize decreased readmission rates inherently encourage neoadjuvant chemotherapy over primary debulking surgery,” Dr. Barber stressed. She recommended that policies and incentive programs incorporate both short- and long-term outcomes for patients with ovarian cancer.
Thirty-day hospital readmissions cost Medicare $17.4 billion in 2004 alone, noted Dr. Uppal. Research shows that almost one in seven patients is readmitted to the hospital within 30 days of discharge after major surgery, and that high-volume hospitals with low surgical mortality rates have lower surgical readmission rates than other hospitals do (N Engl J Med. 2013;369:1134-42).
Accordingly, the Centers for Medicare and Medicaid Services limits reimbursements if hospitals exceed 30-day readmission thresholds after surgeries for certain conditions, including acute myocardial infarction, heart failure, pneumonia, chronic obstructive pulmonary disease, coronary artery disease, and total hip and total knee arthroplasty. For now, these procedures do not include primary or interval debulking, but hospitals do monitor readmission rates, in general, as part of the Hospital Readmission Reduction Program, Dr. Barber noted.
Studies of ovarian cancer have shown postsurgical readmission rates of 10%-20%, Dr. Uppal said. To evaluate readmissions as a quality metric in this setting, he and his associates identified patients diagnosed with stage III or stage IV high-grade serous carcinoma between 2004 and 2013 who underwent cytoreductive surgery as primary treatment. More than 44,000 patients meeting these criteria were treated at hospitals that handled anywhere from under 10 to more than 30 cases annually.
The overall rate of unplanned readmissions was 6.2% (95% confidence interval, 5.2%-6.4%), and patients resembled each other clinically and demographically, irrespective of hospital caseload, Dr. Uppal said. After adjustment for other risk factors for readmission, including higher-stage disease, hospitals that treated more than 30 cases of ovarian cancer per year had significantly higher 30-day readmission rates than did the lowest-volume hospitals – nearly 10%, compared with about 7.5% (adjusted odd ratio, 1.25; 95% CI, 1.1-1.5; P less than .05). However, high-volume hospitals had the lowest 30-day and 90-day mortality rates (P less than .05), the highest rate of 5-year overall survival (adjusted hazard ratio, 0.83; 95% CI, 0.78-0.88; P less than .05), and a significantly higher frequency of adherence to NCCN guidelines compared with low-volume hospitals.
“Not all readmissions reflect a failed discharge,” Dr. Uppal emphasized. “Certain readmissions may be appropriate and necessary, and some surgeries merit appropriate aggression and result in a high readmission rate.”
The study by Dr. Barber included data from nearly 27,000 patients who underwent chemotherapy and surgery for stage IIIC epithelial ovarian cancer at Commission on Cancer–accredited institutions between 2006 and 2012. The overall 30-day readmission rate was 11%, and 57% of readmissions were unplanned. About 16% of patients received neoadjuvant chemotherapy followed by interval debulking surgery, and the rest underwent primary debulking surgery, Dr. Barber said.
Rates of 30-day readmissions were 6.5% after neoadjuvant chemotherapy with interval debulking, compared with 12% after primary debulking. Neoadjuvant chemotherapy was associated with a 48% drop in the risk of readmission and with a 37% decrease in the chances of unplanned readmission after the researchers controlled for age, race, insurance status, comorbidities, and histology, Dr. Barber said. However, primary debulking significantly increased the chances of survival at next follow-up (HR, 1.36; 95% CI, 1.29-1.42; P less than .001). Median overall survival was 47 months with primary debulking surgery and 37 months with neoadjuvant chemotherapy (P less than .001).
These findings show how broad health policies that are not designed for specific patient subgroups can create unique, unintended, and undesirable incentives and consequences, Dr. Barber said. Unfairly penalizing high-volume hospitals by concentrating solely on readmissions, rather than taking a more holistic view of cancer care, could inadvertently encourage the use of less aggressive debulking surgeries and increase the use of neoadjuvant therapies in cases where primary debulking is more appropriate, Dr. Uppal added.
Dr. Barber and Dr. Uppal acknowledged no external funding sources and reported having no conflicts of interest.
NATIONAL HARBOR, MD. – For patients with ovarian cancer, focusing solely on hospital readmission rates as a quality measure might worsen long-term outcomes while unfairly penalizing the best hospitals, suggest the results of two analyses of the National Cancer Database presented at the annual meeting of the Society of Gynecologic Oncology.
Hospitals with the highest ovarian cancer caseloads had the best rates of overall survival and postsurgical mortality, but also had the highest rates of readmission within 30 days after postsurgical discharge, reported Shitanshu Uppal, MBBS, of the obstetrics and gynecology department at the University of Michigan in Ann Arbor.
A separate analysis linked neoadjuvant chemotherapy with significantly fewer readmissions, but also with significantly worse overall survival compared with primary debulking surgery, reported Emma L. Barber, MD, of the University of North Carolina at Chapel Hill.
“Policies that prioritize decreased readmission rates inherently encourage neoadjuvant chemotherapy over primary debulking surgery,” Dr. Barber stressed. She recommended that policies and incentive programs incorporate both short- and long-term outcomes for patients with ovarian cancer.
Thirty-day hospital readmissions cost Medicare $17.4 billion in 2004 alone, noted Dr. Uppal. Research shows that almost one in seven patients is readmitted to the hospital within 30 days of discharge after major surgery, and that high-volume hospitals with low surgical mortality rates have lower surgical readmission rates than other hospitals do (N Engl J Med. 2013;369:1134-42).
Accordingly, the Centers for Medicare and Medicaid Services limits reimbursements if hospitals exceed 30-day readmission thresholds after surgeries for certain conditions, including acute myocardial infarction, heart failure, pneumonia, chronic obstructive pulmonary disease, coronary artery disease, and total hip and total knee arthroplasty. For now, these procedures do not include primary or interval debulking, but hospitals do monitor readmission rates, in general, as part of the Hospital Readmission Reduction Program, Dr. Barber noted.
Studies of ovarian cancer have shown postsurgical readmission rates of 10%-20%, Dr. Uppal said. To evaluate readmissions as a quality metric in this setting, he and his associates identified patients diagnosed with stage III or stage IV high-grade serous carcinoma between 2004 and 2013 who underwent cytoreductive surgery as primary treatment. More than 44,000 patients meeting these criteria were treated at hospitals that handled anywhere from under 10 to more than 30 cases annually.
The overall rate of unplanned readmissions was 6.2% (95% confidence interval, 5.2%-6.4%), and patients resembled each other clinically and demographically, irrespective of hospital caseload, Dr. Uppal said. After adjustment for other risk factors for readmission, including higher-stage disease, hospitals that treated more than 30 cases of ovarian cancer per year had significantly higher 30-day readmission rates than did the lowest-volume hospitals – nearly 10%, compared with about 7.5% (adjusted odd ratio, 1.25; 95% CI, 1.1-1.5; P less than .05). However, high-volume hospitals had the lowest 30-day and 90-day mortality rates (P less than .05), the highest rate of 5-year overall survival (adjusted hazard ratio, 0.83; 95% CI, 0.78-0.88; P less than .05), and a significantly higher frequency of adherence to NCCN guidelines compared with low-volume hospitals.
“Not all readmissions reflect a failed discharge,” Dr. Uppal emphasized. “Certain readmissions may be appropriate and necessary, and some surgeries merit appropriate aggression and result in a high readmission rate.”
The study by Dr. Barber included data from nearly 27,000 patients who underwent chemotherapy and surgery for stage IIIC epithelial ovarian cancer at Commission on Cancer–accredited institutions between 2006 and 2012. The overall 30-day readmission rate was 11%, and 57% of readmissions were unplanned. About 16% of patients received neoadjuvant chemotherapy followed by interval debulking surgery, and the rest underwent primary debulking surgery, Dr. Barber said.
Rates of 30-day readmissions were 6.5% after neoadjuvant chemotherapy with interval debulking, compared with 12% after primary debulking. Neoadjuvant chemotherapy was associated with a 48% drop in the risk of readmission and with a 37% decrease in the chances of unplanned readmission after the researchers controlled for age, race, insurance status, comorbidities, and histology, Dr. Barber said. However, primary debulking significantly increased the chances of survival at next follow-up (HR, 1.36; 95% CI, 1.29-1.42; P less than .001). Median overall survival was 47 months with primary debulking surgery and 37 months with neoadjuvant chemotherapy (P less than .001).
These findings show how broad health policies that are not designed for specific patient subgroups can create unique, unintended, and undesirable incentives and consequences, Dr. Barber said. Unfairly penalizing high-volume hospitals by concentrating solely on readmissions, rather than taking a more holistic view of cancer care, could inadvertently encourage the use of less aggressive debulking surgeries and increase the use of neoadjuvant therapies in cases where primary debulking is more appropriate, Dr. Uppal added.
Dr. Barber and Dr. Uppal acknowledged no external funding sources and reported having no conflicts of interest.
NATIONAL HARBOR, MD. – For patients with ovarian cancer, focusing solely on hospital readmission rates as a quality measure might worsen long-term outcomes while unfairly penalizing the best hospitals, suggest the results of two analyses of the National Cancer Database presented at the annual meeting of the Society of Gynecologic Oncology.
Hospitals with the highest ovarian cancer caseloads had the best rates of overall survival and postsurgical mortality, but also had the highest rates of readmission within 30 days after postsurgical discharge, reported Shitanshu Uppal, MBBS, of the obstetrics and gynecology department at the University of Michigan in Ann Arbor.
A separate analysis linked neoadjuvant chemotherapy with significantly fewer readmissions, but also with significantly worse overall survival compared with primary debulking surgery, reported Emma L. Barber, MD, of the University of North Carolina at Chapel Hill.
“Policies that prioritize decreased readmission rates inherently encourage neoadjuvant chemotherapy over primary debulking surgery,” Dr. Barber stressed. She recommended that policies and incentive programs incorporate both short- and long-term outcomes for patients with ovarian cancer.
Thirty-day hospital readmissions cost Medicare $17.4 billion in 2004 alone, noted Dr. Uppal. Research shows that almost one in seven patients is readmitted to the hospital within 30 days of discharge after major surgery, and that high-volume hospitals with low surgical mortality rates have lower surgical readmission rates than other hospitals do (N Engl J Med. 2013;369:1134-42).
Accordingly, the Centers for Medicare and Medicaid Services limits reimbursements if hospitals exceed 30-day readmission thresholds after surgeries for certain conditions, including acute myocardial infarction, heart failure, pneumonia, chronic obstructive pulmonary disease, coronary artery disease, and total hip and total knee arthroplasty. For now, these procedures do not include primary or interval debulking, but hospitals do monitor readmission rates, in general, as part of the Hospital Readmission Reduction Program, Dr. Barber noted.
Studies of ovarian cancer have shown postsurgical readmission rates of 10%-20%, Dr. Uppal said. To evaluate readmissions as a quality metric in this setting, he and his associates identified patients diagnosed with stage III or stage IV high-grade serous carcinoma between 2004 and 2013 who underwent cytoreductive surgery as primary treatment. More than 44,000 patients meeting these criteria were treated at hospitals that handled anywhere from under 10 to more than 30 cases annually.
The overall rate of unplanned readmissions was 6.2% (95% confidence interval, 5.2%-6.4%), and patients resembled each other clinically and demographically, irrespective of hospital caseload, Dr. Uppal said. After adjustment for other risk factors for readmission, including higher-stage disease, hospitals that treated more than 30 cases of ovarian cancer per year had significantly higher 30-day readmission rates than did the lowest-volume hospitals – nearly 10%, compared with about 7.5% (adjusted odd ratio, 1.25; 95% CI, 1.1-1.5; P less than .05). However, high-volume hospitals had the lowest 30-day and 90-day mortality rates (P less than .05), the highest rate of 5-year overall survival (adjusted hazard ratio, 0.83; 95% CI, 0.78-0.88; P less than .05), and a significantly higher frequency of adherence to NCCN guidelines compared with low-volume hospitals.
“Not all readmissions reflect a failed discharge,” Dr. Uppal emphasized. “Certain readmissions may be appropriate and necessary, and some surgeries merit appropriate aggression and result in a high readmission rate.”
The study by Dr. Barber included data from nearly 27,000 patients who underwent chemotherapy and surgery for stage IIIC epithelial ovarian cancer at Commission on Cancer–accredited institutions between 2006 and 2012. The overall 30-day readmission rate was 11%, and 57% of readmissions were unplanned. About 16% of patients received neoadjuvant chemotherapy followed by interval debulking surgery, and the rest underwent primary debulking surgery, Dr. Barber said.
Rates of 30-day readmissions were 6.5% after neoadjuvant chemotherapy with interval debulking, compared with 12% after primary debulking. Neoadjuvant chemotherapy was associated with a 48% drop in the risk of readmission and with a 37% decrease in the chances of unplanned readmission after the researchers controlled for age, race, insurance status, comorbidities, and histology, Dr. Barber said. However, primary debulking significantly increased the chances of survival at next follow-up (HR, 1.36; 95% CI, 1.29-1.42; P less than .001). Median overall survival was 47 months with primary debulking surgery and 37 months with neoadjuvant chemotherapy (P less than .001).
These findings show how broad health policies that are not designed for specific patient subgroups can create unique, unintended, and undesirable incentives and consequences, Dr. Barber said. Unfairly penalizing high-volume hospitals by concentrating solely on readmissions, rather than taking a more holistic view of cancer care, could inadvertently encourage the use of less aggressive debulking surgeries and increase the use of neoadjuvant therapies in cases where primary debulking is more appropriate, Dr. Uppal added.
Dr. Barber and Dr. Uppal acknowledged no external funding sources and reported having no conflicts of interest.
AT THE ANNUAL MEETING ON WOMEN’S CANCER
Key clinical point: For patients with ovarian cancer, focusing solely on postsurgical hospital readmissions as a quality measure could worsen outcomes and might penalize the best hospitals.
Major finding: Hospitals that treated the most cases of ovarian cancer annually had significantly better rates of overall survival and postsurgical mortality, as well as the highest 30-day readmission rates (P less than .05). A separate analysis linked neoadjuvant chemotherapy with significantly fewer readmissions, but also with significantly worse overall survival compared with primary debulking surgery.
Data source: Two large retrospective analyses of the National Cancer Database.
Disclosures: Dr. Barber and Dr. Uppal acknowledged no external funding sources and reported having no conflicts of interest.
HM17: Plenaries – Conway and DeSalvo
The first two plenary addresses at HM17 are focused on policy at a time when the dynamically evolving U.S. health care delivery system may seem daunting, opaque, and labyrinthine.
Some might view the health care landscape as hopelessly confusing. Yet both of the keynote speakers use the same word for what they hope to leave their listeners with: optimism.
“Though it feels uncertain in the headlines, the reality is that the health care world feels pretty united in that we need to continue the progress we’ve made on moving away from the fee-for-service model and to let people practice medicine the way they want – to work better as teams and focus on patients and outcomes,” said Karen DeSalvo, MD, MPH, MSc, former acting assistant secretary for health in the U.S. Department of Health and Human Services (HHS) and former national coordinator for health information technology.
“I would view it as an opportunity as well,” said Dr. Conway, who still moonlights as a pediatric academic hospitalist on weekends in greater Washington, D.C. “I think the pieces are coming together. Everything from data, to new payment models, to the MACRA Medicare Physician payment legislation, really suggests a time of positive change.”
Dr. DeSalvo, a former political appointee, joined HHS as the national coordinator for health information technology in 2014 and soon thereafter assumed the acting assistant secretary role. Dr. Conway has attained one of the country’s highest-ranking public health care jobs since joining CMS in 2011. He retained the top post at CMS while President Donald Trump’s nominee to lead the agency, Seema Verma, awaited a confirmation hearing before the U.S. Senate. Dr. Conway’s prior title was principal deputy administrator and CMS chief medical officer.
“I don’t want people to lose sight of the fact that there’s this entire care system that everybody’s working and innovating in every day, trying to find more efficient, effective ways to get better outcomes,” she said. “Hospitalists, quite frankly, have been leading that for their entire existence. They really understand in great granular detail what it takes.”
Dr. DeSalvo believes that the progress of the past 5 years has established a path that must be followed. The public sector move away from fee-for-service has combined with emerging technology platforms to create a new age where physicians and insurers can judge, in real time, how well care is working.
“We’re now in a feedback loop where we can say – ‘When we’ve built a care system like this or when we pay this way, we are actually seeing improved outcomes’ – and change doesn’t take as long,” Dr. DeSalvo said.
Dr. Conway, whose working title for his speech is “Health care System Transformation,” said hospitalists should be encouraged by how well the field has already adapted to the proliferation of accountable care organizations (ACOs), value-based purchasing (VBP), alternative payment models (APM), and the Medicare Access and CHIP Reauthorization Act (MACRA) of 2015. He noted that, as innovations lead to better and more coordinated patient care, hospitalists, patients, and hospitals would all benefit.
“I want to leave people with the idea that value-based payment innovation and delivery system reform will continue to be critical aspects of improving our health system,” he said. “I also want hospitalists to continue to stay engaged with these new payment models, help lead them, and provide better patient care as a part of them.”
The first two plenary addresses at HM17 are focused on policy at a time when the dynamically evolving U.S. health care delivery system may seem daunting, opaque, and labyrinthine.
Some might view the health care landscape as hopelessly confusing. Yet both of the keynote speakers use the same word for what they hope to leave their listeners with: optimism.
“Though it feels uncertain in the headlines, the reality is that the health care world feels pretty united in that we need to continue the progress we’ve made on moving away from the fee-for-service model and to let people practice medicine the way they want – to work better as teams and focus on patients and outcomes,” said Karen DeSalvo, MD, MPH, MSc, former acting assistant secretary for health in the U.S. Department of Health and Human Services (HHS) and former national coordinator for health information technology.
“I would view it as an opportunity as well,” said Dr. Conway, who still moonlights as a pediatric academic hospitalist on weekends in greater Washington, D.C. “I think the pieces are coming together. Everything from data, to new payment models, to the MACRA Medicare Physician payment legislation, really suggests a time of positive change.”
Dr. DeSalvo, a former political appointee, joined HHS as the national coordinator for health information technology in 2014 and soon thereafter assumed the acting assistant secretary role. Dr. Conway has attained one of the country’s highest-ranking public health care jobs since joining CMS in 2011. He retained the top post at CMS while President Donald Trump’s nominee to lead the agency, Seema Verma, awaited a confirmation hearing before the U.S. Senate. Dr. Conway’s prior title was principal deputy administrator and CMS chief medical officer.
“I don’t want people to lose sight of the fact that there’s this entire care system that everybody’s working and innovating in every day, trying to find more efficient, effective ways to get better outcomes,” she said. “Hospitalists, quite frankly, have been leading that for their entire existence. They really understand in great granular detail what it takes.”
Dr. DeSalvo believes that the progress of the past 5 years has established a path that must be followed. The public sector move away from fee-for-service has combined with emerging technology platforms to create a new age where physicians and insurers can judge, in real time, how well care is working.
“We’re now in a feedback loop where we can say – ‘When we’ve built a care system like this or when we pay this way, we are actually seeing improved outcomes’ – and change doesn’t take as long,” Dr. DeSalvo said.
Dr. Conway, whose working title for his speech is “Health care System Transformation,” said hospitalists should be encouraged by how well the field has already adapted to the proliferation of accountable care organizations (ACOs), value-based purchasing (VBP), alternative payment models (APM), and the Medicare Access and CHIP Reauthorization Act (MACRA) of 2015. He noted that, as innovations lead to better and more coordinated patient care, hospitalists, patients, and hospitals would all benefit.
“I want to leave people with the idea that value-based payment innovation and delivery system reform will continue to be critical aspects of improving our health system,” he said. “I also want hospitalists to continue to stay engaged with these new payment models, help lead them, and provide better patient care as a part of them.”
The first two plenary addresses at HM17 are focused on policy at a time when the dynamically evolving U.S. health care delivery system may seem daunting, opaque, and labyrinthine.
Some might view the health care landscape as hopelessly confusing. Yet both of the keynote speakers use the same word for what they hope to leave their listeners with: optimism.
“Though it feels uncertain in the headlines, the reality is that the health care world feels pretty united in that we need to continue the progress we’ve made on moving away from the fee-for-service model and to let people practice medicine the way they want – to work better as teams and focus on patients and outcomes,” said Karen DeSalvo, MD, MPH, MSc, former acting assistant secretary for health in the U.S. Department of Health and Human Services (HHS) and former national coordinator for health information technology.
“I would view it as an opportunity as well,” said Dr. Conway, who still moonlights as a pediatric academic hospitalist on weekends in greater Washington, D.C. “I think the pieces are coming together. Everything from data, to new payment models, to the MACRA Medicare Physician payment legislation, really suggests a time of positive change.”
Dr. DeSalvo, a former political appointee, joined HHS as the national coordinator for health information technology in 2014 and soon thereafter assumed the acting assistant secretary role. Dr. Conway has attained one of the country’s highest-ranking public health care jobs since joining CMS in 2011. He retained the top post at CMS while President Donald Trump’s nominee to lead the agency, Seema Verma, awaited a confirmation hearing before the U.S. Senate. Dr. Conway’s prior title was principal deputy administrator and CMS chief medical officer.
“I don’t want people to lose sight of the fact that there’s this entire care system that everybody’s working and innovating in every day, trying to find more efficient, effective ways to get better outcomes,” she said. “Hospitalists, quite frankly, have been leading that for their entire existence. They really understand in great granular detail what it takes.”
Dr. DeSalvo believes that the progress of the past 5 years has established a path that must be followed. The public sector move away from fee-for-service has combined with emerging technology platforms to create a new age where physicians and insurers can judge, in real time, how well care is working.
“We’re now in a feedback loop where we can say – ‘When we’ve built a care system like this or when we pay this way, we are actually seeing improved outcomes’ – and change doesn’t take as long,” Dr. DeSalvo said.
Dr. Conway, whose working title for his speech is “Health care System Transformation,” said hospitalists should be encouraged by how well the field has already adapted to the proliferation of accountable care organizations (ACOs), value-based purchasing (VBP), alternative payment models (APM), and the Medicare Access and CHIP Reauthorization Act (MACRA) of 2015. He noted that, as innovations lead to better and more coordinated patient care, hospitalists, patients, and hospitals would all benefit.
“I want to leave people with the idea that value-based payment innovation and delivery system reform will continue to be critical aspects of improving our health system,” he said. “I also want hospitalists to continue to stay engaged with these new payment models, help lead them, and provide better patient care as a part of them.”
New self-persuasion app to promote HPV vaccine appears effective
A new tablet application using a self-persuasion method has been successful in convincing parents in underserved communities to have their children vaccinated for human papillomavirus (HPV), according to a study.
Of 45 participating parents, 27 of the 33 (82%) parents whose adolescents were not vaccinated reported that they decided to get their children vaccinated after completing the application. The remaining 12 already had an HPV-vaccinated adolescent. Children of participating parents were aged 11-17 years (Patient Educ Couns. 2016. doi: 10.1016/j.pec.2016.11.014).
Of the 45 parents, 31 (69%) were Hispanic and 29 (64%) held a high school education or less, according to the study.
To test the effectiveness of self-persuasion, researchers developed a tablet application which started with a 5 minute video on HPV and vaccine efficacy, and then asked parents to complete two tasks: answer questions about the HPV vaccine that prompt thought on its benefits and come up with personal reasons for why having their child vaccinated is important.
Parents then participated in a 45-60 minute interview with one of six research assistants, who prompted participants to address four research points: Did they like the application? Which questions generated interest in the HPV vaccine without raising concerns? Were they able to communicate reasons for vaccination? Were they convinced to have their children vaccinated?
After watching the video, 18 (55%) of the 33 parents whose children were not vaccinated changed their minds, and, after participating in the self-persuasion questionnaire, an additional 9 parents decided to vaccinate their children, according to the study. Five parents remained undecided, and one decided against HPV vaccination.
Overall, participants reported that the application questions were helpful in their decision to vaccinate their children, with question ratings ranging from 4.33 to 4.98 on a scale of 1 to 5.
Mr. Baldwin and his colleagues said that, while the initial test showed promise, further research must be done on actual vaccine behavior, as these tests only studied verbally reported decisions by parents.
One of the limitations to the study was that the research was primarily conducted at a research facility. While some was conducted in local clinics, Mr. Baldwin and his colleagues consider that further studies should be conducted in more public areas. All but one of the participants were female, which may make it hard to generalize about the effects of this application with male parents.
The researchers reported no relevant financial disclosures.
[email protected]
On Twitter @EAZTweets
The media has not been kind when it comes to HPV vaccinations. When most parents are approached with the option to have their children vaccinated, they are hesitant because of what they have heard from television, news articles, or online mom chat rooms about the dangerous side effects of the vaccine. When a mother hears about other mothers’ daughters fainting or being subjected to pain, she usually wonders if it is really worth the risk.
The same goes for parents who expect their daughters to practice celibacy until marriage. After all, why expose a child to negative side effects when she won’t be having sex anyway? The problem with this thinking, however, is that, often, it is not based in reality. When I approach families about HPV vaccine, or any other vaccine for that matter, I make sure to present studies that explain the lack of evidence on negative vaccine side effects, as well as information pertaining to how common HPV is.
However, simply handing out a fact sheet is not enough. Parents who are convinced that their children do not need to be vaccinated will not be persuaded through statistics but through personal anecdotes. I like to tell mothers to ask their girlfriends about being vaccinated, to not only see how common HPV is but to break the stigma as well. I explain to parents who do not want their child having sex now that, at some point, they might want their child to grow their family, and, when that time comes, don’t they want their child to be safe? Even if it is just one time, shouldn’t their child be protected? From that point on, the language of conversation has changed, and the channels of communication are more open.
As pediatricians, we are the medical advocates for our patients and their families, not just someone to prescribe medication for them. Establish that role through knowledge and conversation, and persuasion will soon follow.
Francine Pearce, MD, is a pediatrician in Frankfort, Ill. She writes the Pediatric News teen column, Pearce-ings .
The media has not been kind when it comes to HPV vaccinations. When most parents are approached with the option to have their children vaccinated, they are hesitant because of what they have heard from television, news articles, or online mom chat rooms about the dangerous side effects of the vaccine. When a mother hears about other mothers’ daughters fainting or being subjected to pain, she usually wonders if it is really worth the risk.
The same goes for parents who expect their daughters to practice celibacy until marriage. After all, why expose a child to negative side effects when she won’t be having sex anyway? The problem with this thinking, however, is that, often, it is not based in reality. When I approach families about HPV vaccine, or any other vaccine for that matter, I make sure to present studies that explain the lack of evidence on negative vaccine side effects, as well as information pertaining to how common HPV is.
However, simply handing out a fact sheet is not enough. Parents who are convinced that their children do not need to be vaccinated will not be persuaded through statistics but through personal anecdotes. I like to tell mothers to ask their girlfriends about being vaccinated, to not only see how common HPV is but to break the stigma as well. I explain to parents who do not want their child having sex now that, at some point, they might want their child to grow their family, and, when that time comes, don’t they want their child to be safe? Even if it is just one time, shouldn’t their child be protected? From that point on, the language of conversation has changed, and the channels of communication are more open.
As pediatricians, we are the medical advocates for our patients and their families, not just someone to prescribe medication for them. Establish that role through knowledge and conversation, and persuasion will soon follow.
Francine Pearce, MD, is a pediatrician in Frankfort, Ill. She writes the Pediatric News teen column, Pearce-ings .
The media has not been kind when it comes to HPV vaccinations. When most parents are approached with the option to have their children vaccinated, they are hesitant because of what they have heard from television, news articles, or online mom chat rooms about the dangerous side effects of the vaccine. When a mother hears about other mothers’ daughters fainting or being subjected to pain, she usually wonders if it is really worth the risk.
The same goes for parents who expect their daughters to practice celibacy until marriage. After all, why expose a child to negative side effects when she won’t be having sex anyway? The problem with this thinking, however, is that, often, it is not based in reality. When I approach families about HPV vaccine, or any other vaccine for that matter, I make sure to present studies that explain the lack of evidence on negative vaccine side effects, as well as information pertaining to how common HPV is.
However, simply handing out a fact sheet is not enough. Parents who are convinced that their children do not need to be vaccinated will not be persuaded through statistics but through personal anecdotes. I like to tell mothers to ask their girlfriends about being vaccinated, to not only see how common HPV is but to break the stigma as well. I explain to parents who do not want their child having sex now that, at some point, they might want their child to grow their family, and, when that time comes, don’t they want their child to be safe? Even if it is just one time, shouldn’t their child be protected? From that point on, the language of conversation has changed, and the channels of communication are more open.
As pediatricians, we are the medical advocates for our patients and their families, not just someone to prescribe medication for them. Establish that role through knowledge and conversation, and persuasion will soon follow.
Francine Pearce, MD, is a pediatrician in Frankfort, Ill. She writes the Pediatric News teen column, Pearce-ings .
A new tablet application using a self-persuasion method has been successful in convincing parents in underserved communities to have their children vaccinated for human papillomavirus (HPV), according to a study.
Of 45 participating parents, 27 of the 33 (82%) parents whose adolescents were not vaccinated reported that they decided to get their children vaccinated after completing the application. The remaining 12 already had an HPV-vaccinated adolescent. Children of participating parents were aged 11-17 years (Patient Educ Couns. 2016. doi: 10.1016/j.pec.2016.11.014).
Of the 45 parents, 31 (69%) were Hispanic and 29 (64%) held a high school education or less, according to the study.
To test the effectiveness of self-persuasion, researchers developed a tablet application which started with a 5 minute video on HPV and vaccine efficacy, and then asked parents to complete two tasks: answer questions about the HPV vaccine that prompt thought on its benefits and come up with personal reasons for why having their child vaccinated is important.
Parents then participated in a 45-60 minute interview with one of six research assistants, who prompted participants to address four research points: Did they like the application? Which questions generated interest in the HPV vaccine without raising concerns? Were they able to communicate reasons for vaccination? Were they convinced to have their children vaccinated?
After watching the video, 18 (55%) of the 33 parents whose children were not vaccinated changed their minds, and, after participating in the self-persuasion questionnaire, an additional 9 parents decided to vaccinate their children, according to the study. Five parents remained undecided, and one decided against HPV vaccination.
Overall, participants reported that the application questions were helpful in their decision to vaccinate their children, with question ratings ranging from 4.33 to 4.98 on a scale of 1 to 5.
Mr. Baldwin and his colleagues said that, while the initial test showed promise, further research must be done on actual vaccine behavior, as these tests only studied verbally reported decisions by parents.
One of the limitations to the study was that the research was primarily conducted at a research facility. While some was conducted in local clinics, Mr. Baldwin and his colleagues consider that further studies should be conducted in more public areas. All but one of the participants were female, which may make it hard to generalize about the effects of this application with male parents.
The researchers reported no relevant financial disclosures.
[email protected]
On Twitter @EAZTweets
A new tablet application using a self-persuasion method has been successful in convincing parents in underserved communities to have their children vaccinated for human papillomavirus (HPV), according to a study.
Of 45 participating parents, 27 of the 33 (82%) parents whose adolescents were not vaccinated reported that they decided to get their children vaccinated after completing the application. The remaining 12 already had an HPV-vaccinated adolescent. Children of participating parents were aged 11-17 years (Patient Educ Couns. 2016. doi: 10.1016/j.pec.2016.11.014).
Of the 45 parents, 31 (69%) were Hispanic and 29 (64%) held a high school education or less, according to the study.
To test the effectiveness of self-persuasion, researchers developed a tablet application which started with a 5 minute video on HPV and vaccine efficacy, and then asked parents to complete two tasks: answer questions about the HPV vaccine that prompt thought on its benefits and come up with personal reasons for why having their child vaccinated is important.
Parents then participated in a 45-60 minute interview with one of six research assistants, who prompted participants to address four research points: Did they like the application? Which questions generated interest in the HPV vaccine without raising concerns? Were they able to communicate reasons for vaccination? Were they convinced to have their children vaccinated?
After watching the video, 18 (55%) of the 33 parents whose children were not vaccinated changed their minds, and, after participating in the self-persuasion questionnaire, an additional 9 parents decided to vaccinate their children, according to the study. Five parents remained undecided, and one decided against HPV vaccination.
Overall, participants reported that the application questions were helpful in their decision to vaccinate their children, with question ratings ranging from 4.33 to 4.98 on a scale of 1 to 5.
Mr. Baldwin and his colleagues said that, while the initial test showed promise, further research must be done on actual vaccine behavior, as these tests only studied verbally reported decisions by parents.
One of the limitations to the study was that the research was primarily conducted at a research facility. While some was conducted in local clinics, Mr. Baldwin and his colleagues consider that further studies should be conducted in more public areas. All but one of the participants were female, which may make it hard to generalize about the effects of this application with male parents.
The researchers reported no relevant financial disclosures.
[email protected]
On Twitter @EAZTweets
FROM PATIENT EDUCATION & COUNSELING
Key clinical point:
Major finding: Of the 33 parents whose children were not vaccinated, 27 (818%) decided to have their children vaccinated after using the application.
Data source: A study of 45 parents in low-income communities, evaluated through self-reporting questionnaires and in-person interviews conducted by research assistants.
Disclosures: Researchers reported no relevant financial disclosures.