User login
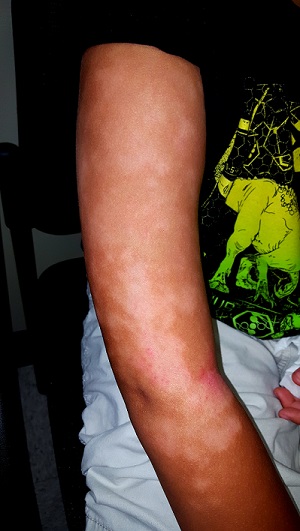
Wanna See My Not-Tan Lines?
A 16-year-old African-American girl is brought in by her mother for evaluation of skin changes affecting both arms: small, round, slightly scaly, 2- to 3-cm patches on the triceps, antecubitals, and deltoids. The changes manifested in early spring and worsened with the arrival of summer.
The condition has been previously diagnosed as vitiligo by her primary care provider and as fungal infection by an urgent care provider. Nystatin cream and clotrimazole cream have had no effect.
The patient’s history includes eczema, extensive atopy manifesting with seasonal allergies, and childhood asthma. Her siblings also had these problems.
EXAMINATION
Extensive, mottled hypopigmentation is noted on the skin of both arms, in stark contrast to the patient’s type V skin. Very little scale is seen. There are focal areas of slight erythema around the antecubital folds.
What is the diagnosis?
DISCUSSION
This phenomenon is so common in dermatology clinics that it’s a rare day when we don’t see it. This form of hypopigmentation is pityriasis alba (PA), in which areas of eczema don’t tan at all while the surrounding skin darkens with sun exposure. The lateral aspects of the arms are often affected (sparing the sun-protected medial aspects), as are the sides of the face and the posterior neck. The contrast is striking, especially on those with darker skin.
PA occurs mostly in children and young adults, becoming less frequent with age. It differs from vitiligo in that PA involves seasonal, partial pigment loss; vitiligo by contrast manifests with complete pigment loss (leaving utterly white skin) that is almost always permanent.
Treatment consists of sun protection, moisturization to prevent eczema, and use of class IV steroid creams or ointments when lesions appear. Even without treatment, PA usually clears during the winter months—when the surrounding skin loses its tan—only to recur the following spring.
TAKE-HOME LEARNING POINTS
- Pityriasis alba (PA) occurs when patches of eczema fail to tan, producing marked contrast between them and the normal surrounding skin; it is often mistaken for fungal infection.
- PA favors the antecubital, deltoid, and lateral tricep areas, as well as the lateral face.
- PA is more common in atopic individuals who are prone to eczema and appears more dramatic in those with darker skin.
- Vitiligo is a major item in the differential, but color loss with PA is only partial (rather than permanent) and almost always resolves in the winter.
- Once color is lost with PA, treatment is largely ineffective. It is then best to use preventive measures (eg, sunscreen and moisturizer), plus/minus topical steroid creams, for the eczema.
A 16-year-old African-American girl is brought in by her mother for evaluation of skin changes affecting both arms: small, round, slightly scaly, 2- to 3-cm patches on the triceps, antecubitals, and deltoids. The changes manifested in early spring and worsened with the arrival of summer.
The condition has been previously diagnosed as vitiligo by her primary care provider and as fungal infection by an urgent care provider. Nystatin cream and clotrimazole cream have had no effect.
The patient’s history includes eczema, extensive atopy manifesting with seasonal allergies, and childhood asthma. Her siblings also had these problems.
EXAMINATION
Extensive, mottled hypopigmentation is noted on the skin of both arms, in stark contrast to the patient’s type V skin. Very little scale is seen. There are focal areas of slight erythema around the antecubital folds.

What is the diagnosis?
DISCUSSION
This phenomenon is so common in dermatology clinics that it’s a rare day when we don’t see it. This form of hypopigmentation is pityriasis alba (PA), in which areas of eczema don’t tan at all while the surrounding skin darkens with sun exposure. The lateral aspects of the arms are often affected (sparing the sun-protected medial aspects), as are the sides of the face and the posterior neck. The contrast is striking, especially on those with darker skin.
PA occurs mostly in children and young adults, becoming less frequent with age. It differs from vitiligo in that PA involves seasonal, partial pigment loss; vitiligo by contrast manifests with complete pigment loss (leaving utterly white skin) that is almost always permanent.
Treatment consists of sun protection, moisturization to prevent eczema, and use of class IV steroid creams or ointments when lesions appear. Even without treatment, PA usually clears during the winter months—when the surrounding skin loses its tan—only to recur the following spring.
TAKE-HOME LEARNING POINTS
- Pityriasis alba (PA) occurs when patches of eczema fail to tan, producing marked contrast between them and the normal surrounding skin; it is often mistaken for fungal infection.
- PA favors the antecubital, deltoid, and lateral tricep areas, as well as the lateral face.
- PA is more common in atopic individuals who are prone to eczema and appears more dramatic in those with darker skin.
- Vitiligo is a major item in the differential, but color loss with PA is only partial (rather than permanent) and almost always resolves in the winter.
- Once color is lost with PA, treatment is largely ineffective. It is then best to use preventive measures (eg, sunscreen and moisturizer), plus/minus topical steroid creams, for the eczema.
A 16-year-old African-American girl is brought in by her mother for evaluation of skin changes affecting both arms: small, round, slightly scaly, 2- to 3-cm patches on the triceps, antecubitals, and deltoids. The changes manifested in early spring and worsened with the arrival of summer.
The condition has been previously diagnosed as vitiligo by her primary care provider and as fungal infection by an urgent care provider. Nystatin cream and clotrimazole cream have had no effect.
The patient’s history includes eczema, extensive atopy manifesting with seasonal allergies, and childhood asthma. Her siblings also had these problems.
EXAMINATION
Extensive, mottled hypopigmentation is noted on the skin of both arms, in stark contrast to the patient’s type V skin. Very little scale is seen. There are focal areas of slight erythema around the antecubital folds.

What is the diagnosis?
DISCUSSION
This phenomenon is so common in dermatology clinics that it’s a rare day when we don’t see it. This form of hypopigmentation is pityriasis alba (PA), in which areas of eczema don’t tan at all while the surrounding skin darkens with sun exposure. The lateral aspects of the arms are often affected (sparing the sun-protected medial aspects), as are the sides of the face and the posterior neck. The contrast is striking, especially on those with darker skin.
PA occurs mostly in children and young adults, becoming less frequent with age. It differs from vitiligo in that PA involves seasonal, partial pigment loss; vitiligo by contrast manifests with complete pigment loss (leaving utterly white skin) that is almost always permanent.
Treatment consists of sun protection, moisturization to prevent eczema, and use of class IV steroid creams or ointments when lesions appear. Even without treatment, PA usually clears during the winter months—when the surrounding skin loses its tan—only to recur the following spring.
TAKE-HOME LEARNING POINTS
- Pityriasis alba (PA) occurs when patches of eczema fail to tan, producing marked contrast between them and the normal surrounding skin; it is often mistaken for fungal infection.
- PA favors the antecubital, deltoid, and lateral tricep areas, as well as the lateral face.
- PA is more common in atopic individuals who are prone to eczema and appears more dramatic in those with darker skin.
- Vitiligo is a major item in the differential, but color loss with PA is only partial (rather than permanent) and almost always resolves in the winter.
- Once color is lost with PA, treatment is largely ineffective. It is then best to use preventive measures (eg, sunscreen and moisturizer), plus/minus topical steroid creams, for the eczema.
Novel antifungal had favorable safety, efficacy profile for onychomycosis in phase IIB study
ORLANDO – A novel orally administered antifungal showed a favorable safety and efficacy profile in the treatment of distal lateral subungual onychomycosis, in a phase IIB study presented at the annual meeting of the American Academy of Dermatology.
In the RENOVATE (Restoring Nail: An Oral VT-1161 Tablet Evaluation) study, a randomized, double-blind, placebo-controlled, dose-ranging trial, 259 adults with moderate to severe distal lateral subungual onychomycosis of the large toenail were assigned to either one of four treatment arms. They were given the antifungal, currently named VT-1161, a selective CYP51 inhibitor, at doses of 300 mg or 600 mg once weekly for 10 or 22 weeks, after receiving daily loading doses for the initial 2 weeks. The trial evaluated two dose levels of VT-1161 (300 mg and 600 mg) administered once weekly for either 10 or 22 weeks following an initial 2-week, once-daily loading dose period.
At baseline, the average involvement of the large toenail was 46%, with an average of 4.6 toenails affected. In the intent-to-treat analysis, at 48 weeks, complete cure rates in the four study drug arms ranged from 32% to 42%, compared with 0% in the placebo arm.
Amir Tavakkol, PhD, chief development officer at Viamet Pharmaceuticals, which is developing VT01161, presented the study findings during a late breaking clinical session at the meeting.
Adverse event rates and discontinuation rates were comparable to placebo through week 60, with no patients discontinuing due to any laboratory abnormalities. Nausea and muscle spasms were the most commonly reported adverse events, which Dr. Tavakkol said seemed to occur in patients given the higher doses. VT-1161 is also being studied for treatment of vulvovaginal candidiasis. In October 2016, the FDA granted the drug Qualified Infectious Disease Product and Fast Track designations for the treatment of recurrent vulvovaginal candidiasis, according to the company.
Viamet sponsored the study and Dr. Tavakkol is an employee of the company.
[email protected]
On Twitter @whitneymcknight
ORLANDO – A novel orally administered antifungal showed a favorable safety and efficacy profile in the treatment of distal lateral subungual onychomycosis, in a phase IIB study presented at the annual meeting of the American Academy of Dermatology.
In the RENOVATE (Restoring Nail: An Oral VT-1161 Tablet Evaluation) study, a randomized, double-blind, placebo-controlled, dose-ranging trial, 259 adults with moderate to severe distal lateral subungual onychomycosis of the large toenail were assigned to either one of four treatment arms. They were given the antifungal, currently named VT-1161, a selective CYP51 inhibitor, at doses of 300 mg or 600 mg once weekly for 10 or 22 weeks, after receiving daily loading doses for the initial 2 weeks. The trial evaluated two dose levels of VT-1161 (300 mg and 600 mg) administered once weekly for either 10 or 22 weeks following an initial 2-week, once-daily loading dose period.
At baseline, the average involvement of the large toenail was 46%, with an average of 4.6 toenails affected. In the intent-to-treat analysis, at 48 weeks, complete cure rates in the four study drug arms ranged from 32% to 42%, compared with 0% in the placebo arm.
Amir Tavakkol, PhD, chief development officer at Viamet Pharmaceuticals, which is developing VT01161, presented the study findings during a late breaking clinical session at the meeting.
Adverse event rates and discontinuation rates were comparable to placebo through week 60, with no patients discontinuing due to any laboratory abnormalities. Nausea and muscle spasms were the most commonly reported adverse events, which Dr. Tavakkol said seemed to occur in patients given the higher doses. VT-1161 is also being studied for treatment of vulvovaginal candidiasis. In October 2016, the FDA granted the drug Qualified Infectious Disease Product and Fast Track designations for the treatment of recurrent vulvovaginal candidiasis, according to the company.
Viamet sponsored the study and Dr. Tavakkol is an employee of the company.
[email protected]
On Twitter @whitneymcknight
ORLANDO – A novel orally administered antifungal showed a favorable safety and efficacy profile in the treatment of distal lateral subungual onychomycosis, in a phase IIB study presented at the annual meeting of the American Academy of Dermatology.
In the RENOVATE (Restoring Nail: An Oral VT-1161 Tablet Evaluation) study, a randomized, double-blind, placebo-controlled, dose-ranging trial, 259 adults with moderate to severe distal lateral subungual onychomycosis of the large toenail were assigned to either one of four treatment arms. They were given the antifungal, currently named VT-1161, a selective CYP51 inhibitor, at doses of 300 mg or 600 mg once weekly for 10 or 22 weeks, after receiving daily loading doses for the initial 2 weeks. The trial evaluated two dose levels of VT-1161 (300 mg and 600 mg) administered once weekly for either 10 or 22 weeks following an initial 2-week, once-daily loading dose period.
At baseline, the average involvement of the large toenail was 46%, with an average of 4.6 toenails affected. In the intent-to-treat analysis, at 48 weeks, complete cure rates in the four study drug arms ranged from 32% to 42%, compared with 0% in the placebo arm.
Amir Tavakkol, PhD, chief development officer at Viamet Pharmaceuticals, which is developing VT01161, presented the study findings during a late breaking clinical session at the meeting.
Adverse event rates and discontinuation rates were comparable to placebo through week 60, with no patients discontinuing due to any laboratory abnormalities. Nausea and muscle spasms were the most commonly reported adverse events, which Dr. Tavakkol said seemed to occur in patients given the higher doses. VT-1161 is also being studied for treatment of vulvovaginal candidiasis. In October 2016, the FDA granted the drug Qualified Infectious Disease Product and Fast Track designations for the treatment of recurrent vulvovaginal candidiasis, according to the company.
Viamet sponsored the study and Dr. Tavakkol is an employee of the company.
[email protected]
On Twitter @whitneymcknight
AT AAD 17
Key clinical point:
Major finding: A new selective CYP51 inhibitor, administered orally, met the primary endpoint of complete cure rates at 48 weeks.
Data source: A phase IIB, randomized, double-blind, placebo-controlled, dose-ranging study of 259 adults with moderate-to-severe distal lateral subungual onychomycosis of the large toenail.
Disclosures: Dr. Tavakkol is the chief development officer of Viamet Pharmaceuticals, the sponsor of this trial.
Match Day: Surgery positions up, other specialties grew more
Despite a record number of applicants and slots filled for first-year residents on the 2017 Match Day, growth in all surgery positions continued to be outpaced by growth in other specialties.
“While it is encouraging to see that the number of categorical surgery positions offered has increased by 101 since 2013, representing an increase of 8.6%, over the same period, the number of positions offered in emergency medicine and family medicine positions have each increased by over 300, representing increases of 17.4% and 11.5%, respectively, and the number of internal medicine positions has increased by over 900, representing an increase of 15.2%,” Patrick Bailey, MD, FACS, medical director of advocacy for the Division of Advocacy and Health Policy at the American College of Surgeons. “The fact that over 35,969 applicants submitted program choices and only 27,688 matched into a PGY-1 position is yet another indication of the need to expand the number of graduate medical education positions available,” he added.
And while more positions may be needed, the number of categorical surgery positions that went unfilled remained low, with only 5 of 1,281 slots remaining unfilled at the conclusion of Match Day 2017, according to the data released by the National Resident Match Program (NRMP).
“This result is consistent with those since 2013 during which time the number of unfilled positions has varied between two and seven positions, e.g., 99.4%-99.8% of categorical surgery positions offered were filled,” Dr. Bailey said.
Still, the ACS is advocating for further changes to the resident matching program to help build the future workforce. “The ACS believes broad reforms in the way graduate medical education is funded and administered are necessary and overdue to ensure that our nation is able to produce the physician workforce capable of meeting the needs of the U.S. population,” he said. To that end, the ACS produced a policy paper that outlined a series of steps relative to reform for which it is advocating.
Broadly, those reforms include the collection of actionable and accurate health care workforce data, maintenance of current GME funding levels with the appropriation of temporary additional funds to modernize the system, the combination of the current GME funding streams into a single stream of funds, and the consideration of a regionalized governance system.
Overall, this is the fifth straight year the NRMP has reported growth in applicants, up 1.4% from the previous year, and applicants matched, to PGY-1 positions up 3.2% from 2016.
One factor driving the increase is the “all-in” policy that required programs registering for the Match to offer all their available positions in the Match or another national matching program. The policy, which began with the 2013 Match, has resulted in significant increases for internal medicine, family medicine, and pediatrics.
Internal medicine residency programs offered 7,233 programs, accounting for about 25% of all PGY-1 positions. This was up from 7,024 programs offered last year. U.S. seniors accounted for 44.9% of the 7,101 slots filled, a rate that was slightly lower than the 46.9% of slots filled by U.S. medical school graduates in 2016.
A record-high 18,539 allopathic medical school seniors submitted program choices and 17,480 (94.3%) were matched to first-year resident programs, a rate that has been consistent for a number of years according to NRMP data.
Of the 1,279 unfilled slots, 1,177 were offered in the Match Week Supplemental Offer and Acceptance Program, the results of which will be available in May.
One trend that stood out for NRMP President and CEO Mona Signer was the decline in both U.S.- and non–U.S.-citizen international medical school graduates (IMGs) who submitted program choices.
“I was surprised that the number of U.S.-citizen and non–U.S.-citizen IMGs declined this year, but on the other hand, the good news is their match rates went up,” Ms. Signer said in an interview.
U.S.-citizen IMGs declined by 254 to 5,069, but 54.8% were matched to first-year residency positions, the highest match rate since 2004. The number of non–U.S.-citizen IMGs declined by 176 to 7,284, but 52.4% were matched to first-year positions, the highest match rate since 2005.
Ms. Signer declined to speculate what caused the decline, noting that NRMP does not collect demographic data.
Despite a record number of applicants and slots filled for first-year residents on the 2017 Match Day, growth in all surgery positions continued to be outpaced by growth in other specialties.
“While it is encouraging to see that the number of categorical surgery positions offered has increased by 101 since 2013, representing an increase of 8.6%, over the same period, the number of positions offered in emergency medicine and family medicine positions have each increased by over 300, representing increases of 17.4% and 11.5%, respectively, and the number of internal medicine positions has increased by over 900, representing an increase of 15.2%,” Patrick Bailey, MD, FACS, medical director of advocacy for the Division of Advocacy and Health Policy at the American College of Surgeons. “The fact that over 35,969 applicants submitted program choices and only 27,688 matched into a PGY-1 position is yet another indication of the need to expand the number of graduate medical education positions available,” he added.
And while more positions may be needed, the number of categorical surgery positions that went unfilled remained low, with only 5 of 1,281 slots remaining unfilled at the conclusion of Match Day 2017, according to the data released by the National Resident Match Program (NRMP).
“This result is consistent with those since 2013 during which time the number of unfilled positions has varied between two and seven positions, e.g., 99.4%-99.8% of categorical surgery positions offered were filled,” Dr. Bailey said.
Still, the ACS is advocating for further changes to the resident matching program to help build the future workforce. “The ACS believes broad reforms in the way graduate medical education is funded and administered are necessary and overdue to ensure that our nation is able to produce the physician workforce capable of meeting the needs of the U.S. population,” he said. To that end, the ACS produced a policy paper that outlined a series of steps relative to reform for which it is advocating.
Broadly, those reforms include the collection of actionable and accurate health care workforce data, maintenance of current GME funding levels with the appropriation of temporary additional funds to modernize the system, the combination of the current GME funding streams into a single stream of funds, and the consideration of a regionalized governance system.
Overall, this is the fifth straight year the NRMP has reported growth in applicants, up 1.4% from the previous year, and applicants matched, to PGY-1 positions up 3.2% from 2016.
One factor driving the increase is the “all-in” policy that required programs registering for the Match to offer all their available positions in the Match or another national matching program. The policy, which began with the 2013 Match, has resulted in significant increases for internal medicine, family medicine, and pediatrics.
Internal medicine residency programs offered 7,233 programs, accounting for about 25% of all PGY-1 positions. This was up from 7,024 programs offered last year. U.S. seniors accounted for 44.9% of the 7,101 slots filled, a rate that was slightly lower than the 46.9% of slots filled by U.S. medical school graduates in 2016.
A record-high 18,539 allopathic medical school seniors submitted program choices and 17,480 (94.3%) were matched to first-year resident programs, a rate that has been consistent for a number of years according to NRMP data.
Of the 1,279 unfilled slots, 1,177 were offered in the Match Week Supplemental Offer and Acceptance Program, the results of which will be available in May.
One trend that stood out for NRMP President and CEO Mona Signer was the decline in both U.S.- and non–U.S.-citizen international medical school graduates (IMGs) who submitted program choices.
“I was surprised that the number of U.S.-citizen and non–U.S.-citizen IMGs declined this year, but on the other hand, the good news is their match rates went up,” Ms. Signer said in an interview.
U.S.-citizen IMGs declined by 254 to 5,069, but 54.8% were matched to first-year residency positions, the highest match rate since 2004. The number of non–U.S.-citizen IMGs declined by 176 to 7,284, but 52.4% were matched to first-year positions, the highest match rate since 2005.
Ms. Signer declined to speculate what caused the decline, noting that NRMP does not collect demographic data.
Despite a record number of applicants and slots filled for first-year residents on the 2017 Match Day, growth in all surgery positions continued to be outpaced by growth in other specialties.
“While it is encouraging to see that the number of categorical surgery positions offered has increased by 101 since 2013, representing an increase of 8.6%, over the same period, the number of positions offered in emergency medicine and family medicine positions have each increased by over 300, representing increases of 17.4% and 11.5%, respectively, and the number of internal medicine positions has increased by over 900, representing an increase of 15.2%,” Patrick Bailey, MD, FACS, medical director of advocacy for the Division of Advocacy and Health Policy at the American College of Surgeons. “The fact that over 35,969 applicants submitted program choices and only 27,688 matched into a PGY-1 position is yet another indication of the need to expand the number of graduate medical education positions available,” he added.
And while more positions may be needed, the number of categorical surgery positions that went unfilled remained low, with only 5 of 1,281 slots remaining unfilled at the conclusion of Match Day 2017, according to the data released by the National Resident Match Program (NRMP).
“This result is consistent with those since 2013 during which time the number of unfilled positions has varied between two and seven positions, e.g., 99.4%-99.8% of categorical surgery positions offered were filled,” Dr. Bailey said.
Still, the ACS is advocating for further changes to the resident matching program to help build the future workforce. “The ACS believes broad reforms in the way graduate medical education is funded and administered are necessary and overdue to ensure that our nation is able to produce the physician workforce capable of meeting the needs of the U.S. population,” he said. To that end, the ACS produced a policy paper that outlined a series of steps relative to reform for which it is advocating.
Broadly, those reforms include the collection of actionable and accurate health care workforce data, maintenance of current GME funding levels with the appropriation of temporary additional funds to modernize the system, the combination of the current GME funding streams into a single stream of funds, and the consideration of a regionalized governance system.
Overall, this is the fifth straight year the NRMP has reported growth in applicants, up 1.4% from the previous year, and applicants matched, to PGY-1 positions up 3.2% from 2016.
One factor driving the increase is the “all-in” policy that required programs registering for the Match to offer all their available positions in the Match or another national matching program. The policy, which began with the 2013 Match, has resulted in significant increases for internal medicine, family medicine, and pediatrics.
Internal medicine residency programs offered 7,233 programs, accounting for about 25% of all PGY-1 positions. This was up from 7,024 programs offered last year. U.S. seniors accounted for 44.9% of the 7,101 slots filled, a rate that was slightly lower than the 46.9% of slots filled by U.S. medical school graduates in 2016.
A record-high 18,539 allopathic medical school seniors submitted program choices and 17,480 (94.3%) were matched to first-year resident programs, a rate that has been consistent for a number of years according to NRMP data.
Of the 1,279 unfilled slots, 1,177 were offered in the Match Week Supplemental Offer and Acceptance Program, the results of which will be available in May.
One trend that stood out for NRMP President and CEO Mona Signer was the decline in both U.S.- and non–U.S.-citizen international medical school graduates (IMGs) who submitted program choices.
“I was surprised that the number of U.S.-citizen and non–U.S.-citizen IMGs declined this year, but on the other hand, the good news is their match rates went up,” Ms. Signer said in an interview.
U.S.-citizen IMGs declined by 254 to 5,069, but 54.8% were matched to first-year residency positions, the highest match rate since 2004. The number of non–U.S.-citizen IMGs declined by 176 to 7,284, but 52.4% were matched to first-year positions, the highest match rate since 2005.
Ms. Signer declined to speculate what caused the decline, noting that NRMP does not collect demographic data.
No link between methylation, survival in ovarian cancer
NATIONAL HARBOR, MD. – Unlike mutation, methylation of homologous recombination DNA repair pathway genes was not linked to longer survival or platinum sensitivity in high-grade serous ovarian cancer, according to molecular and clinical analyses of 332 patients.
“Methylation may be more readily reversed than mutation, allowing more rapid development of platinum resistance and negating any impact on overall survival,” said Sarah Bernards, a second-year medical student at the University of Washington, Seattle, who presented the findings at the annual meeting of the Society of Gynecologic Oncology.
The homologous recombination (HR) DNA repair pathway is critical for repairing cross-linking and double-strand breaks, Ms. Bernards noted. Genes such as BRCA1, BRCA2, and RAD51C are important in this pathway, and inherited and sporadic mutations of these genes can lead to ovarian carcinoma. Importantly, ovarian cancers with HR gene mutations are more sensitive to platinum and radiation therapy and are associated with longer progression-free survival and overall survival compared with ovarian cancers without these mutations.
Methylation of the promoter regions of BRCA1 and RAD51C reduces expression of these genes and has been found to be associated with high-grade serous ovarian cancer, Ms. Bernards explained. One previous study found promoter methylations of BRCA1 and RAD51C in 11.5% and 3% of such tumors, respectively (Nature. 2011;474[7353]:609-15).
To further characterize HR gene methylations, Ms. Bernards and her associates used methylation-sensitive PCR to analyze ovarian carcinomas from patients who underwent primary debulking surgery and were enrolled in the University of Washington gynecologic oncology tissue bank. They also tested for damaging germline and somatic mutations in 16 HR DNA repair genes.
In all, 28% of cases had mutations in HR repair pathway genes, 7% had a BRCA1 methylation, and 3% had RAD51C methylation. BRCA1 and RAD51C methylation never overlapped with mutation (P = .001), and almost never overlapped with mutations in other HR DNA repair pathway genes, Ms. Bernards said.
Fully two-thirds of BRCA1 mutated cases were sensitive to chemotherapy, compared with 59% of BRCA1 methylated cases and 53% of wild-type BRCA1 cases (P = .03). Mutations of any HR DNA repair gene were associated with improved overall survival (hazard ratio, 0.7; 95% confidence interval, 0.5-0.9; P = .02), but methylation of RAD51C and BRCA1 was not (P = .3). Patients with HR gene mutations survived a median of 66 months after diagnosis, compared with 41 months for patients with RAD51C methylation and 43 months for patients with BRCA1 methylation.
Median age at diagnosis was 53 years for BRCA1 mutated cases (P less than .0001) and 55 years for BRCA1 methylated cases (P = .03), significantly lower than the 63-year median age for diagnosis of wild-type BRCA1 cases. High-grade histology characterized 71% of wild-type BRCA1 cases, compared with 82% of BRCA1 mutated cases (P = .001) and 91% of BRCA1 methylated cases (P = .05). Somatic TP53 mutations occurred in 67% of wild-type cases, 89% of mutated cases (P = .004), and 82% of methylated cases (P = .01).
To sum up, both methylation and mutation of BRCA1 were associated with younger age at diagnosis, high-grade histology, and TP53 mutations, but only BRCA1 mutation was tied to improved overall survival or platinum sensitivity, Ms. Bernards said.
The work was supported by a grant from the University of Washington School of Medicine Medical Student Research Training Program. Ms. Bernards conducted the work under Elizabeth Swisher, MD, a medical oncologist and head of the Swisher Lab at the University of Washington, Seattle. Ms. Bernards reported having no conflicts of interest.
NATIONAL HARBOR, MD. – Unlike mutation, methylation of homologous recombination DNA repair pathway genes was not linked to longer survival or platinum sensitivity in high-grade serous ovarian cancer, according to molecular and clinical analyses of 332 patients.
“Methylation may be more readily reversed than mutation, allowing more rapid development of platinum resistance and negating any impact on overall survival,” said Sarah Bernards, a second-year medical student at the University of Washington, Seattle, who presented the findings at the annual meeting of the Society of Gynecologic Oncology.
The homologous recombination (HR) DNA repair pathway is critical for repairing cross-linking and double-strand breaks, Ms. Bernards noted. Genes such as BRCA1, BRCA2, and RAD51C are important in this pathway, and inherited and sporadic mutations of these genes can lead to ovarian carcinoma. Importantly, ovarian cancers with HR gene mutations are more sensitive to platinum and radiation therapy and are associated with longer progression-free survival and overall survival compared with ovarian cancers without these mutations.
Methylation of the promoter regions of BRCA1 and RAD51C reduces expression of these genes and has been found to be associated with high-grade serous ovarian cancer, Ms. Bernards explained. One previous study found promoter methylations of BRCA1 and RAD51C in 11.5% and 3% of such tumors, respectively (Nature. 2011;474[7353]:609-15).
To further characterize HR gene methylations, Ms. Bernards and her associates used methylation-sensitive PCR to analyze ovarian carcinomas from patients who underwent primary debulking surgery and were enrolled in the University of Washington gynecologic oncology tissue bank. They also tested for damaging germline and somatic mutations in 16 HR DNA repair genes.
In all, 28% of cases had mutations in HR repair pathway genes, 7% had a BRCA1 methylation, and 3% had RAD51C methylation. BRCA1 and RAD51C methylation never overlapped with mutation (P = .001), and almost never overlapped with mutations in other HR DNA repair pathway genes, Ms. Bernards said.
Fully two-thirds of BRCA1 mutated cases were sensitive to chemotherapy, compared with 59% of BRCA1 methylated cases and 53% of wild-type BRCA1 cases (P = .03). Mutations of any HR DNA repair gene were associated with improved overall survival (hazard ratio, 0.7; 95% confidence interval, 0.5-0.9; P = .02), but methylation of RAD51C and BRCA1 was not (P = .3). Patients with HR gene mutations survived a median of 66 months after diagnosis, compared with 41 months for patients with RAD51C methylation and 43 months for patients with BRCA1 methylation.
Median age at diagnosis was 53 years for BRCA1 mutated cases (P less than .0001) and 55 years for BRCA1 methylated cases (P = .03), significantly lower than the 63-year median age for diagnosis of wild-type BRCA1 cases. High-grade histology characterized 71% of wild-type BRCA1 cases, compared with 82% of BRCA1 mutated cases (P = .001) and 91% of BRCA1 methylated cases (P = .05). Somatic TP53 mutations occurred in 67% of wild-type cases, 89% of mutated cases (P = .004), and 82% of methylated cases (P = .01).
To sum up, both methylation and mutation of BRCA1 were associated with younger age at diagnosis, high-grade histology, and TP53 mutations, but only BRCA1 mutation was tied to improved overall survival or platinum sensitivity, Ms. Bernards said.
The work was supported by a grant from the University of Washington School of Medicine Medical Student Research Training Program. Ms. Bernards conducted the work under Elizabeth Swisher, MD, a medical oncologist and head of the Swisher Lab at the University of Washington, Seattle. Ms. Bernards reported having no conflicts of interest.
NATIONAL HARBOR, MD. – Unlike mutation, methylation of homologous recombination DNA repair pathway genes was not linked to longer survival or platinum sensitivity in high-grade serous ovarian cancer, according to molecular and clinical analyses of 332 patients.
“Methylation may be more readily reversed than mutation, allowing more rapid development of platinum resistance and negating any impact on overall survival,” said Sarah Bernards, a second-year medical student at the University of Washington, Seattle, who presented the findings at the annual meeting of the Society of Gynecologic Oncology.
The homologous recombination (HR) DNA repair pathway is critical for repairing cross-linking and double-strand breaks, Ms. Bernards noted. Genes such as BRCA1, BRCA2, and RAD51C are important in this pathway, and inherited and sporadic mutations of these genes can lead to ovarian carcinoma. Importantly, ovarian cancers with HR gene mutations are more sensitive to platinum and radiation therapy and are associated with longer progression-free survival and overall survival compared with ovarian cancers without these mutations.
Methylation of the promoter regions of BRCA1 and RAD51C reduces expression of these genes and has been found to be associated with high-grade serous ovarian cancer, Ms. Bernards explained. One previous study found promoter methylations of BRCA1 and RAD51C in 11.5% and 3% of such tumors, respectively (Nature. 2011;474[7353]:609-15).
To further characterize HR gene methylations, Ms. Bernards and her associates used methylation-sensitive PCR to analyze ovarian carcinomas from patients who underwent primary debulking surgery and were enrolled in the University of Washington gynecologic oncology tissue bank. They also tested for damaging germline and somatic mutations in 16 HR DNA repair genes.
In all, 28% of cases had mutations in HR repair pathway genes, 7% had a BRCA1 methylation, and 3% had RAD51C methylation. BRCA1 and RAD51C methylation never overlapped with mutation (P = .001), and almost never overlapped with mutations in other HR DNA repair pathway genes, Ms. Bernards said.
Fully two-thirds of BRCA1 mutated cases were sensitive to chemotherapy, compared with 59% of BRCA1 methylated cases and 53% of wild-type BRCA1 cases (P = .03). Mutations of any HR DNA repair gene were associated with improved overall survival (hazard ratio, 0.7; 95% confidence interval, 0.5-0.9; P = .02), but methylation of RAD51C and BRCA1 was not (P = .3). Patients with HR gene mutations survived a median of 66 months after diagnosis, compared with 41 months for patients with RAD51C methylation and 43 months for patients with BRCA1 methylation.
Median age at diagnosis was 53 years for BRCA1 mutated cases (P less than .0001) and 55 years for BRCA1 methylated cases (P = .03), significantly lower than the 63-year median age for diagnosis of wild-type BRCA1 cases. High-grade histology characterized 71% of wild-type BRCA1 cases, compared with 82% of BRCA1 mutated cases (P = .001) and 91% of BRCA1 methylated cases (P = .05). Somatic TP53 mutations occurred in 67% of wild-type cases, 89% of mutated cases (P = .004), and 82% of methylated cases (P = .01).
To sum up, both methylation and mutation of BRCA1 were associated with younger age at diagnosis, high-grade histology, and TP53 mutations, but only BRCA1 mutation was tied to improved overall survival or platinum sensitivity, Ms. Bernards said.
The work was supported by a grant from the University of Washington School of Medicine Medical Student Research Training Program. Ms. Bernards conducted the work under Elizabeth Swisher, MD, a medical oncologist and head of the Swisher Lab at the University of Washington, Seattle. Ms. Bernards reported having no conflicts of interest.
AT THE ANNUAL MEETING ON WOMEN’S CANCER
Key clinical point: Unlike mutation, methylation of BRCA1 was not linked to longer survival or platinum sensitivity in high-grade serous ovarian cancer.
Major finding: Fully two-thirds of BRCA1 mutated cases were platinum-sensitive compared with 59% of BRCA1 methylated cases and 53% of wild-type BRCA1 cases (P = .03). Mutations of HR DNA repair genes were associated with improved overall survival (hazard ratio, 0.7; 95% confidence interval, 0.5-0.9; P = .02), but methylation of BRCA1 and RAD51C was not (P = .3).
Data source: Methylation-sensitive PCR and clinical assessment of 332 primary ovarian carcinomas.
Disclosures: The University of Washington School of Medicine Medical Student Research Training Program supported the work. Ms. Bernards reported having no conflicts of interest.
Foreign doctors may lose U.S. jobs after visa program suspension
While much of the drama surrounding the Trump administration’s immigration policy has centered on the so-called travel ban, changes to a specialized visa program may have a bigger impact on foreign doctors in the United States and the employers who hope to hire them.
Starting April 3, U.S. Citizenship and Immigration Services (USCIS) is temporarily suspending its expedited processing of H-1B visas, a primary route used by highly skilled foreign physicians and students to practice and train in the United States.
Under the existing “premium processing” system, foreign medical graduates – usually sponsored by a U.S. institution – pay an extra $1,200 when submitting an H-1B petition to ensure a response from USCIS within 15 days. Standard processing of H-1B applications takes 6-10 months. USCIS is terminating the expedited reviews for up to 6 months to address long-standing H-1B petitions and to reduce backlogs, according to a March announcement by the agency.
In the meantime, many foreign medical students and physicians will lose top training spots and jobs as their H-1B applications linger in the system, said Jennifer A. Minear, a Richmond, Va.–based attorney and national treasurer for the American Immigration Lawyers Association.
“As a practical matter, the percentages of physicians coming into the U.S. who are accepted into residencies or fellowships, those are the top of the top for medical graduates around the world,” Ms. Minear said in an interview. “Most of them who stay afterward wind up working in underserved areas of the United States. It really doesn’t make much sense as a policy matter to create obstacles to attracting those people to the United States that would prevent them from getting here, obtaining U.S. education, and then remaining in the U.S. and providing urgently needed care to populations that would otherwise go without.”
Changing rules, uncertain futures
The H-1B processing change has left Amr Marawan, MD, unsure if a job offer may fall through and if he will be able to work in the United States at all over the next year.
Dr. Marawan, a native of Cairo, Egypt, will finish his internal medicine residency at the University of Tennessee, Chattanooga, in June and had planned to pursue a cardiology fellowship under a continuation of his J-1 alien physician visa. After the 2016 election, he decided instead to take a position as assistant professor of internal medicine at Virginia Commonwealth University in Richmond.
Among his reasons: A J-1 visa requires foreign trainees to return to their home country for 2 years following the completion of their training. With that requirement, he said there would be a gap in his career progression and that he might face challenges returning to the United States.
However, if Dr. Marawan accepted the job at VCU and received approval to waive the 2-year home country requirement from the Virginia Department of Health and the U.S. Department of State, he could apply for a 3-year H-1B visa through the premium processing program.
To get the home country requirement waived, physicians must agree to be employed full time in H-1B status at a health care facility within a designated health professional shortage area, medically underserved area, or medically underserved population.
“The main reason I switched my plan was after the presidential election, there was a lot of talk about changes to visas, so I thought it might be better to take this step now and do the waiver and hopefully this will help me to be more secure while working in order to pursue my medical career,” Dr. Marawan said.
Like many foreign doctors, Dr. Marawan now faces a conundrum. His J-1 visa expires in June and his position at VCU is slated to start in July, but the premium processing program terminates in April. If forced to wait the typical 6-10 months for standard processing, he may lose the position.
“There’s no way we can finish the [state approval] before June,” he said. “And now if we wait and file the H-1B in June, it will take months to get approved. During that time, I cannot work.”
Immigration attorneys have been inundated with similar stories and concerns by physicians regarding how to move forward after the H-1B premium processing suspension, said Adam Cohen, a Memphis attorney. USCIS has delayed premium processing in the past, but not to this extent, he said. [polldaddy:9710548]
This change “was dropped on us with no warning and it’s left us with less than a month to get all of these H-1B [applications] together,” he said.
While foreign physicians and students are scrambling to file their H-1B petitions before April 3, there is no guarantee that the applications will be expedited, Mr. Cohen added. It’s possible USCIS will be unable to get to every application and will simply refund the premium processing fee, he said. The applications would then be subject to standard processing.
USCIS says the suspension will help to address the accumulation of older applications, but the change will only shift the backlog, according to Washington attorney Allen Orr Jr.
USCIS spokeswoman Carolyn Gwathmey said officials cannot speculate whether they will get to every application filed before April 3.
“As noted in the agency’s announcement, we will continue to premium process form I-129 H-1B petitions if the petitioner properly filed an associated form I-907 before April 3, 2017,” Ms. Gwathmey said in an interview. “We will refund the premium processing fee if the petitioner filed the form for an H-1B petition before April 3, 2017, and we did not take adjudicative action on the case within the 15-calendar-day processing period.”
Foreign medical students face rough road
Medical students applying for residencies and fellowships may also be detoured by the premium processing ban. Students who planned to train under an H-1B visa had to wait until Match Day on March 17 to file their H-1B petitions, Ms. Minear said. There is little chance they can complete all paperwork and state approvals needed in order to submit an H-1B application before April 3.
“What this really means is that physicians effectively cannot do their residencies or fellowships in H-1B status this year because they cannot file the petitions in time for a July 1 start date,” Ms. Minear said. “Effectively, what it does is force all foreign doctors who want to do residency or fellowship in the U.S. to do their training in J-1 status.”
A large number of foreign medical students already complete their training in J-1 status; however, many residency and fellowship programs agree to sponsor students in H-1B status as an attractive recruiting incentive for top talent, Ms. Minear said. Foreign doctors often prefer the latter status because they are exempt from the 2-year requirement to return home.
Foreign medical students matching to residency programs generally have the option to apply for a J-1 visa and can still train in the United States, said Matthew Shick, JD, government relations director for the Association of American Medical Colleges. He noted that the premium processing suspension will have a greater impact on faculty, scientists, and hospital staff.
However, medical students applying for J-1 visas also may experience processing delays because of President Trump’s March 6 Executive Order on immigration. A provision in that order increases uniform screening procedures for all visa classes and nationalities, while another provision suspends the Visa Interview Waiver Program. The suspension means that certain visa applicants seeking to renew a visa must be interviewed in person by a consular officer. The Hawaii federal court that blocked much of the that Executive Order did not halt the additional screening requirements or stay the Visa Interview Waiver Program rollback. Both provisions remain in effect.
“It is reasonably foreseeable, based on the portions of the Executive Order that remain in place and based on the on-the-ground reality of State Department officials and consular officer resignations and departures, that this year it will be more challenging than in prior years for an incoming Match applicant to arrive on time at their GME program on July 1, even with the J-1 path,” Ms. Harris said.
Taskforce requests carve-out
The IMG Taskforce is urging USCIS to exempt physicians from the premium processing ban. In a March 8 letter to the agency, the task force outlined examples of how IMGs benefit the country and described how application delays could harm patient care and impair U.S. medical institutions.
“The hope is that this would encourage a review and a rethink of that shift and that upon that review, H-1B cap exempt petitions would across the board be considered for continued premium processing,” Ms. Harris said in an interview. “And/or, perhaps a greater lead time than merely 4 weeks’ notice [would be granted] so that people may be able complete their obligations.”
A group of U.S. senators also has requested that USCIS reconsider the premium processing suspension as it relates to physicians. Sen. Amy Klobuchar (D-Minn.) said that the suspension will exacerbate physician shortages, particularly in rural areas.
“The [waiver] program has helped address chronic physician shortages in rural America and other underserved areas for over two decades,” Sen. Klobuchar wrote in a March 10 letter. “We understand USCIS is facing a backlog, but USCIS has addressed this problem in the past without suspending premium processing for Conrad 30 doctors. We have every faith that USCIS can address its administrative needs without sacrificing support to this successful, time-tested program.”
Ms. Gwathmey would not comment on whether USCIS would consider an exception to the suspension for physicians. As with all affected workloads, USCIS is cognizant of processing time sensitivities for IMGs who are applying to change their status to H-1B, Ms. Gwathmey said in an interview.
“USCIS will be monitoring this workload during the coming months and will evaluate any time sensitive impacts prior to the resumption of premium processing services,” she said.
Dr. Marawan meanwhile is exhausting all efforts to keep his job offer. He is considering the option of filing an H-1B application now, before his state approval comes through, in the hopes of securing premium processing, he said. However, the option comes with a catch. Foreign doctors can file an H-1B petition without a J-1 waiver, but they can’t request a change of status from J-1 to H-1B without leaving the United States unless they have the waiver. This means if Dr. Marawan’s petition is approved by USCIS, he must go back to Egypt to apply for an H-1B visa at the U.S. Embassy in Cairo.
But with increased security delays for visa applicants and reports of foreign travelers being denied entry at U.S. airports, Dr. Marawan said he is fearful.
“Once you step out [of the United States], you never know what’s going to happen,” he said. “Sometimes visas get struck. Sometimes there’s a lot of security checks. Egypt is not included in the travel ban, but it’s always hard. There’s a lot of stories of people who are rejected getting their visas for different reasons. It’s worrisome.”
[email protected]
On Twitter @legal_med
While much of the drama surrounding the Trump administration’s immigration policy has centered on the so-called travel ban, changes to a specialized visa program may have a bigger impact on foreign doctors in the United States and the employers who hope to hire them.
Starting April 3, U.S. Citizenship and Immigration Services (USCIS) is temporarily suspending its expedited processing of H-1B visas, a primary route used by highly skilled foreign physicians and students to practice and train in the United States.
Under the existing “premium processing” system, foreign medical graduates – usually sponsored by a U.S. institution – pay an extra $1,200 when submitting an H-1B petition to ensure a response from USCIS within 15 days. Standard processing of H-1B applications takes 6-10 months. USCIS is terminating the expedited reviews for up to 6 months to address long-standing H-1B petitions and to reduce backlogs, according to a March announcement by the agency.
In the meantime, many foreign medical students and physicians will lose top training spots and jobs as their H-1B applications linger in the system, said Jennifer A. Minear, a Richmond, Va.–based attorney and national treasurer for the American Immigration Lawyers Association.
“As a practical matter, the percentages of physicians coming into the U.S. who are accepted into residencies or fellowships, those are the top of the top for medical graduates around the world,” Ms. Minear said in an interview. “Most of them who stay afterward wind up working in underserved areas of the United States. It really doesn’t make much sense as a policy matter to create obstacles to attracting those people to the United States that would prevent them from getting here, obtaining U.S. education, and then remaining in the U.S. and providing urgently needed care to populations that would otherwise go without.”
Changing rules, uncertain futures
The H-1B processing change has left Amr Marawan, MD, unsure if a job offer may fall through and if he will be able to work in the United States at all over the next year.
Dr. Marawan, a native of Cairo, Egypt, will finish his internal medicine residency at the University of Tennessee, Chattanooga, in June and had planned to pursue a cardiology fellowship under a continuation of his J-1 alien physician visa. After the 2016 election, he decided instead to take a position as assistant professor of internal medicine at Virginia Commonwealth University in Richmond.
Among his reasons: A J-1 visa requires foreign trainees to return to their home country for 2 years following the completion of their training. With that requirement, he said there would be a gap in his career progression and that he might face challenges returning to the United States.
However, if Dr. Marawan accepted the job at VCU and received approval to waive the 2-year home country requirement from the Virginia Department of Health and the U.S. Department of State, he could apply for a 3-year H-1B visa through the premium processing program.
To get the home country requirement waived, physicians must agree to be employed full time in H-1B status at a health care facility within a designated health professional shortage area, medically underserved area, or medically underserved population.
“The main reason I switched my plan was after the presidential election, there was a lot of talk about changes to visas, so I thought it might be better to take this step now and do the waiver and hopefully this will help me to be more secure while working in order to pursue my medical career,” Dr. Marawan said.
Like many foreign doctors, Dr. Marawan now faces a conundrum. His J-1 visa expires in June and his position at VCU is slated to start in July, but the premium processing program terminates in April. If forced to wait the typical 6-10 months for standard processing, he may lose the position.
“There’s no way we can finish the [state approval] before June,” he said. “And now if we wait and file the H-1B in June, it will take months to get approved. During that time, I cannot work.”
Immigration attorneys have been inundated with similar stories and concerns by physicians regarding how to move forward after the H-1B premium processing suspension, said Adam Cohen, a Memphis attorney. USCIS has delayed premium processing in the past, but not to this extent, he said. [polldaddy:9710548]
This change “was dropped on us with no warning and it’s left us with less than a month to get all of these H-1B [applications] together,” he said.
While foreign physicians and students are scrambling to file their H-1B petitions before April 3, there is no guarantee that the applications will be expedited, Mr. Cohen added. It’s possible USCIS will be unable to get to every application and will simply refund the premium processing fee, he said. The applications would then be subject to standard processing.
USCIS says the suspension will help to address the accumulation of older applications, but the change will only shift the backlog, according to Washington attorney Allen Orr Jr.
USCIS spokeswoman Carolyn Gwathmey said officials cannot speculate whether they will get to every application filed before April 3.
“As noted in the agency’s announcement, we will continue to premium process form I-129 H-1B petitions if the petitioner properly filed an associated form I-907 before April 3, 2017,” Ms. Gwathmey said in an interview. “We will refund the premium processing fee if the petitioner filed the form for an H-1B petition before April 3, 2017, and we did not take adjudicative action on the case within the 15-calendar-day processing period.”
Foreign medical students face rough road
Medical students applying for residencies and fellowships may also be detoured by the premium processing ban. Students who planned to train under an H-1B visa had to wait until Match Day on March 17 to file their H-1B petitions, Ms. Minear said. There is little chance they can complete all paperwork and state approvals needed in order to submit an H-1B application before April 3.
“What this really means is that physicians effectively cannot do their residencies or fellowships in H-1B status this year because they cannot file the petitions in time for a July 1 start date,” Ms. Minear said. “Effectively, what it does is force all foreign doctors who want to do residency or fellowship in the U.S. to do their training in J-1 status.”
A large number of foreign medical students already complete their training in J-1 status; however, many residency and fellowship programs agree to sponsor students in H-1B status as an attractive recruiting incentive for top talent, Ms. Minear said. Foreign doctors often prefer the latter status because they are exempt from the 2-year requirement to return home.
Foreign medical students matching to residency programs generally have the option to apply for a J-1 visa and can still train in the United States, said Matthew Shick, JD, government relations director for the Association of American Medical Colleges. He noted that the premium processing suspension will have a greater impact on faculty, scientists, and hospital staff.
However, medical students applying for J-1 visas also may experience processing delays because of President Trump’s March 6 Executive Order on immigration. A provision in that order increases uniform screening procedures for all visa classes and nationalities, while another provision suspends the Visa Interview Waiver Program. The suspension means that certain visa applicants seeking to renew a visa must be interviewed in person by a consular officer. The Hawaii federal court that blocked much of the that Executive Order did not halt the additional screening requirements or stay the Visa Interview Waiver Program rollback. Both provisions remain in effect.
“It is reasonably foreseeable, based on the portions of the Executive Order that remain in place and based on the on-the-ground reality of State Department officials and consular officer resignations and departures, that this year it will be more challenging than in prior years for an incoming Match applicant to arrive on time at their GME program on July 1, even with the J-1 path,” Ms. Harris said.
Taskforce requests carve-out
The IMG Taskforce is urging USCIS to exempt physicians from the premium processing ban. In a March 8 letter to the agency, the task force outlined examples of how IMGs benefit the country and described how application delays could harm patient care and impair U.S. medical institutions.
“The hope is that this would encourage a review and a rethink of that shift and that upon that review, H-1B cap exempt petitions would across the board be considered for continued premium processing,” Ms. Harris said in an interview. “And/or, perhaps a greater lead time than merely 4 weeks’ notice [would be granted] so that people may be able complete their obligations.”
A group of U.S. senators also has requested that USCIS reconsider the premium processing suspension as it relates to physicians. Sen. Amy Klobuchar (D-Minn.) said that the suspension will exacerbate physician shortages, particularly in rural areas.
“The [waiver] program has helped address chronic physician shortages in rural America and other underserved areas for over two decades,” Sen. Klobuchar wrote in a March 10 letter. “We understand USCIS is facing a backlog, but USCIS has addressed this problem in the past without suspending premium processing for Conrad 30 doctors. We have every faith that USCIS can address its administrative needs without sacrificing support to this successful, time-tested program.”
Ms. Gwathmey would not comment on whether USCIS would consider an exception to the suspension for physicians. As with all affected workloads, USCIS is cognizant of processing time sensitivities for IMGs who are applying to change their status to H-1B, Ms. Gwathmey said in an interview.
“USCIS will be monitoring this workload during the coming months and will evaluate any time sensitive impacts prior to the resumption of premium processing services,” she said.
Dr. Marawan meanwhile is exhausting all efforts to keep his job offer. He is considering the option of filing an H-1B application now, before his state approval comes through, in the hopes of securing premium processing, he said. However, the option comes with a catch. Foreign doctors can file an H-1B petition without a J-1 waiver, but they can’t request a change of status from J-1 to H-1B without leaving the United States unless they have the waiver. This means if Dr. Marawan’s petition is approved by USCIS, he must go back to Egypt to apply for an H-1B visa at the U.S. Embassy in Cairo.
But with increased security delays for visa applicants and reports of foreign travelers being denied entry at U.S. airports, Dr. Marawan said he is fearful.
“Once you step out [of the United States], you never know what’s going to happen,” he said. “Sometimes visas get struck. Sometimes there’s a lot of security checks. Egypt is not included in the travel ban, but it’s always hard. There’s a lot of stories of people who are rejected getting their visas for different reasons. It’s worrisome.”
[email protected]
On Twitter @legal_med
While much of the drama surrounding the Trump administration’s immigration policy has centered on the so-called travel ban, changes to a specialized visa program may have a bigger impact on foreign doctors in the United States and the employers who hope to hire them.
Starting April 3, U.S. Citizenship and Immigration Services (USCIS) is temporarily suspending its expedited processing of H-1B visas, a primary route used by highly skilled foreign physicians and students to practice and train in the United States.
Under the existing “premium processing” system, foreign medical graduates – usually sponsored by a U.S. institution – pay an extra $1,200 when submitting an H-1B petition to ensure a response from USCIS within 15 days. Standard processing of H-1B applications takes 6-10 months. USCIS is terminating the expedited reviews for up to 6 months to address long-standing H-1B petitions and to reduce backlogs, according to a March announcement by the agency.
In the meantime, many foreign medical students and physicians will lose top training spots and jobs as their H-1B applications linger in the system, said Jennifer A. Minear, a Richmond, Va.–based attorney and national treasurer for the American Immigration Lawyers Association.
“As a practical matter, the percentages of physicians coming into the U.S. who are accepted into residencies or fellowships, those are the top of the top for medical graduates around the world,” Ms. Minear said in an interview. “Most of them who stay afterward wind up working in underserved areas of the United States. It really doesn’t make much sense as a policy matter to create obstacles to attracting those people to the United States that would prevent them from getting here, obtaining U.S. education, and then remaining in the U.S. and providing urgently needed care to populations that would otherwise go without.”
Changing rules, uncertain futures
The H-1B processing change has left Amr Marawan, MD, unsure if a job offer may fall through and if he will be able to work in the United States at all over the next year.
Dr. Marawan, a native of Cairo, Egypt, will finish his internal medicine residency at the University of Tennessee, Chattanooga, in June and had planned to pursue a cardiology fellowship under a continuation of his J-1 alien physician visa. After the 2016 election, he decided instead to take a position as assistant professor of internal medicine at Virginia Commonwealth University in Richmond.
Among his reasons: A J-1 visa requires foreign trainees to return to their home country for 2 years following the completion of their training. With that requirement, he said there would be a gap in his career progression and that he might face challenges returning to the United States.
However, if Dr. Marawan accepted the job at VCU and received approval to waive the 2-year home country requirement from the Virginia Department of Health and the U.S. Department of State, he could apply for a 3-year H-1B visa through the premium processing program.
To get the home country requirement waived, physicians must agree to be employed full time in H-1B status at a health care facility within a designated health professional shortage area, medically underserved area, or medically underserved population.
“The main reason I switched my plan was after the presidential election, there was a lot of talk about changes to visas, so I thought it might be better to take this step now and do the waiver and hopefully this will help me to be more secure while working in order to pursue my medical career,” Dr. Marawan said.
Like many foreign doctors, Dr. Marawan now faces a conundrum. His J-1 visa expires in June and his position at VCU is slated to start in July, but the premium processing program terminates in April. If forced to wait the typical 6-10 months for standard processing, he may lose the position.
“There’s no way we can finish the [state approval] before June,” he said. “And now if we wait and file the H-1B in June, it will take months to get approved. During that time, I cannot work.”
Immigration attorneys have been inundated with similar stories and concerns by physicians regarding how to move forward after the H-1B premium processing suspension, said Adam Cohen, a Memphis attorney. USCIS has delayed premium processing in the past, but not to this extent, he said. [polldaddy:9710548]
This change “was dropped on us with no warning and it’s left us with less than a month to get all of these H-1B [applications] together,” he said.
While foreign physicians and students are scrambling to file their H-1B petitions before April 3, there is no guarantee that the applications will be expedited, Mr. Cohen added. It’s possible USCIS will be unable to get to every application and will simply refund the premium processing fee, he said. The applications would then be subject to standard processing.
USCIS says the suspension will help to address the accumulation of older applications, but the change will only shift the backlog, according to Washington attorney Allen Orr Jr.
USCIS spokeswoman Carolyn Gwathmey said officials cannot speculate whether they will get to every application filed before April 3.
“As noted in the agency’s announcement, we will continue to premium process form I-129 H-1B petitions if the petitioner properly filed an associated form I-907 before April 3, 2017,” Ms. Gwathmey said in an interview. “We will refund the premium processing fee if the petitioner filed the form for an H-1B petition before April 3, 2017, and we did not take adjudicative action on the case within the 15-calendar-day processing period.”
Foreign medical students face rough road
Medical students applying for residencies and fellowships may also be detoured by the premium processing ban. Students who planned to train under an H-1B visa had to wait until Match Day on March 17 to file their H-1B petitions, Ms. Minear said. There is little chance they can complete all paperwork and state approvals needed in order to submit an H-1B application before April 3.
“What this really means is that physicians effectively cannot do their residencies or fellowships in H-1B status this year because they cannot file the petitions in time for a July 1 start date,” Ms. Minear said. “Effectively, what it does is force all foreign doctors who want to do residency or fellowship in the U.S. to do their training in J-1 status.”
A large number of foreign medical students already complete their training in J-1 status; however, many residency and fellowship programs agree to sponsor students in H-1B status as an attractive recruiting incentive for top talent, Ms. Minear said. Foreign doctors often prefer the latter status because they are exempt from the 2-year requirement to return home.
Foreign medical students matching to residency programs generally have the option to apply for a J-1 visa and can still train in the United States, said Matthew Shick, JD, government relations director for the Association of American Medical Colleges. He noted that the premium processing suspension will have a greater impact on faculty, scientists, and hospital staff.
However, medical students applying for J-1 visas also may experience processing delays because of President Trump’s March 6 Executive Order on immigration. A provision in that order increases uniform screening procedures for all visa classes and nationalities, while another provision suspends the Visa Interview Waiver Program. The suspension means that certain visa applicants seeking to renew a visa must be interviewed in person by a consular officer. The Hawaii federal court that blocked much of the that Executive Order did not halt the additional screening requirements or stay the Visa Interview Waiver Program rollback. Both provisions remain in effect.
“It is reasonably foreseeable, based on the portions of the Executive Order that remain in place and based on the on-the-ground reality of State Department officials and consular officer resignations and departures, that this year it will be more challenging than in prior years for an incoming Match applicant to arrive on time at their GME program on July 1, even with the J-1 path,” Ms. Harris said.
Taskforce requests carve-out
The IMG Taskforce is urging USCIS to exempt physicians from the premium processing ban. In a March 8 letter to the agency, the task force outlined examples of how IMGs benefit the country and described how application delays could harm patient care and impair U.S. medical institutions.
“The hope is that this would encourage a review and a rethink of that shift and that upon that review, H-1B cap exempt petitions would across the board be considered for continued premium processing,” Ms. Harris said in an interview. “And/or, perhaps a greater lead time than merely 4 weeks’ notice [would be granted] so that people may be able complete their obligations.”
A group of U.S. senators also has requested that USCIS reconsider the premium processing suspension as it relates to physicians. Sen. Amy Klobuchar (D-Minn.) said that the suspension will exacerbate physician shortages, particularly in rural areas.
“The [waiver] program has helped address chronic physician shortages in rural America and other underserved areas for over two decades,” Sen. Klobuchar wrote in a March 10 letter. “We understand USCIS is facing a backlog, but USCIS has addressed this problem in the past without suspending premium processing for Conrad 30 doctors. We have every faith that USCIS can address its administrative needs without sacrificing support to this successful, time-tested program.”
Ms. Gwathmey would not comment on whether USCIS would consider an exception to the suspension for physicians. As with all affected workloads, USCIS is cognizant of processing time sensitivities for IMGs who are applying to change their status to H-1B, Ms. Gwathmey said in an interview.
“USCIS will be monitoring this workload during the coming months and will evaluate any time sensitive impacts prior to the resumption of premium processing services,” she said.
Dr. Marawan meanwhile is exhausting all efforts to keep his job offer. He is considering the option of filing an H-1B application now, before his state approval comes through, in the hopes of securing premium processing, he said. However, the option comes with a catch. Foreign doctors can file an H-1B petition without a J-1 waiver, but they can’t request a change of status from J-1 to H-1B without leaving the United States unless they have the waiver. This means if Dr. Marawan’s petition is approved by USCIS, he must go back to Egypt to apply for an H-1B visa at the U.S. Embassy in Cairo.
But with increased security delays for visa applicants and reports of foreign travelers being denied entry at U.S. airports, Dr. Marawan said he is fearful.
“Once you step out [of the United States], you never know what’s going to happen,” he said. “Sometimes visas get struck. Sometimes there’s a lot of security checks. Egypt is not included in the travel ban, but it’s always hard. There’s a lot of stories of people who are rejected getting their visas for different reasons. It’s worrisome.”
[email protected]
On Twitter @legal_med
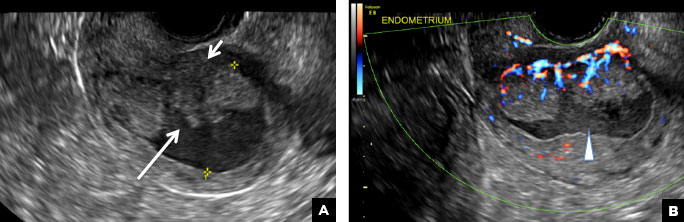
42-year-old woman with abnormal uterine bleeding
A) Endometrial polyp INCORRECT
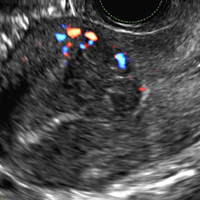
Endometrial polyps on ultrasonography appear as focal echogenic (hyperechoic) masses or as nonspecific endometrial thickening.1 Color Doppler often demonstrates a vascular stalk, which is a nonspecific finding that also can be seen in submucosal fibroids and endometrial cancer.2 On sonohysterography (SHG), endometrial polyps typically appear as well-defined echogenic/hyperechoic polypoid lesions (tissue appearance similar to that of normal endometrium) protruding into the endometrial canal but still preserving the endometrial−myometrial interface.2,3
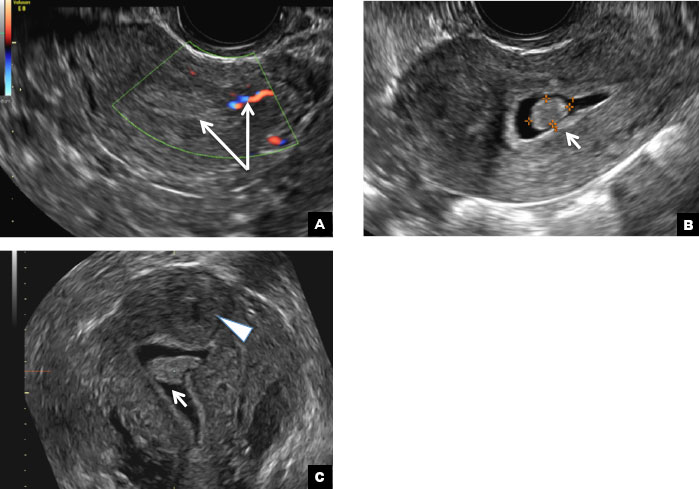
B) Submucosal fibroid CORRECT
Submucosal fibroids on ultrasonography appear as heterogeneous hypoechoic lesions distorting the endometrial cavity.1 In contrast to endometrial polyps, which involve the endometrium only, submucosal (intracavitary) fibroids originate in the myometrium, as clarified in Figures 3A & B. SHG demonstrates a broad-based mixed hypoechoic/isoechoic lesion protruding into the endometrial canal but preserving the echogenic endometrium, distinguishing myometrial from endometrial lesions. Submucosal fibroids often distort the endometrial myometrial interface and demonstrate acoustic shadowing.2,3
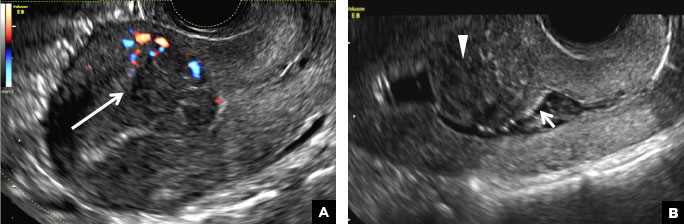
C) Endometrial carcinoma INCORRECT
Endometrial carcinoma on SHG appears as an irregular inhomogeneous lobulated vascular mass distorting the endometrial−myometrial interface.3 Additionally, irregular frond-like projections can be seen extending from the mass into the endometrial cavity, which are distended with echogenic fluid.2
D) Endometrial hyperplasia INCORRECT
On ultrasonography, endometrial hyperplasia has a nonspecific appearance, often presenting as diffuse smooth endometrial thickening.1 SHG typically demonstrates diffuse thickening of the echogenic endometrial stripe without a focal lesion and, when a focal lesion is present, can mimic a broad-based endometrial polyp.2,3

- Nalaboff KM, Pellerito JS, Ben-Levi E. Imaging the endometrium: disease and normal variants. Radiographics. 2001;21(6):1409-1424.
- Davis PC, O'Neill MJ, Yoder IC, Lee SI, Mueller PR. Sonohysterographic findings of endometrial and subendometrial conditions. Radiographics. 2002;22(4):803- 816.
- Yang T, Pandya A, Marcal L, et al. Sonohysterography: principles, technique and role in diagnosis of endometrial pathology. World J Radiol. 2013;5(3):81-87.
A) Endometrial polyp INCORRECT
Endometrial polyps on ultrasonography appear as focal echogenic (hyperechoic) masses or as nonspecific endometrial thickening.1 Color Doppler often demonstrates a vascular stalk, which is a nonspecific finding that also can be seen in submucosal fibroids and endometrial cancer.2 On sonohysterography (SHG), endometrial polyps typically appear as well-defined echogenic/hyperechoic polypoid lesions (tissue appearance similar to that of normal endometrium) protruding into the endometrial canal but still preserving the endometrial−myometrial interface.2,3

B) Submucosal fibroid CORRECT
Submucosal fibroids on ultrasonography appear as heterogeneous hypoechoic lesions distorting the endometrial cavity.1 In contrast to endometrial polyps, which involve the endometrium only, submucosal (intracavitary) fibroids originate in the myometrium, as clarified in Figures 3A & B. SHG demonstrates a broad-based mixed hypoechoic/isoechoic lesion protruding into the endometrial canal but preserving the echogenic endometrium, distinguishing myometrial from endometrial lesions. Submucosal fibroids often distort the endometrial myometrial interface and demonstrate acoustic shadowing.2,3

C) Endometrial carcinoma INCORRECT
Endometrial carcinoma on SHG appears as an irregular inhomogeneous lobulated vascular mass distorting the endometrial−myometrial interface.3 Additionally, irregular frond-like projections can be seen extending from the mass into the endometrial cavity, which are distended with echogenic fluid.2

D) Endometrial hyperplasia INCORRECT
On ultrasonography, endometrial hyperplasia has a nonspecific appearance, often presenting as diffuse smooth endometrial thickening.1 SHG typically demonstrates diffuse thickening of the echogenic endometrial stripe without a focal lesion and, when a focal lesion is present, can mimic a broad-based endometrial polyp.2,3

A) Endometrial polyp INCORRECT
Endometrial polyps on ultrasonography appear as focal echogenic (hyperechoic) masses or as nonspecific endometrial thickening.1 Color Doppler often demonstrates a vascular stalk, which is a nonspecific finding that also can be seen in submucosal fibroids and endometrial cancer.2 On sonohysterography (SHG), endometrial polyps typically appear as well-defined echogenic/hyperechoic polypoid lesions (tissue appearance similar to that of normal endometrium) protruding into the endometrial canal but still preserving the endometrial−myometrial interface.2,3

B) Submucosal fibroid CORRECT
Submucosal fibroids on ultrasonography appear as heterogeneous hypoechoic lesions distorting the endometrial cavity.1 In contrast to endometrial polyps, which involve the endometrium only, submucosal (intracavitary) fibroids originate in the myometrium, as clarified in Figures 3A & B. SHG demonstrates a broad-based mixed hypoechoic/isoechoic lesion protruding into the endometrial canal but preserving the echogenic endometrium, distinguishing myometrial from endometrial lesions. Submucosal fibroids often distort the endometrial myometrial interface and demonstrate acoustic shadowing.2,3

C) Endometrial carcinoma INCORRECT
Endometrial carcinoma on SHG appears as an irregular inhomogeneous lobulated vascular mass distorting the endometrial−myometrial interface.3 Additionally, irregular frond-like projections can be seen extending from the mass into the endometrial cavity, which are distended with echogenic fluid.2

D) Endometrial hyperplasia INCORRECT
On ultrasonography, endometrial hyperplasia has a nonspecific appearance, often presenting as diffuse smooth endometrial thickening.1 SHG typically demonstrates diffuse thickening of the echogenic endometrial stripe without a focal lesion and, when a focal lesion is present, can mimic a broad-based endometrial polyp.2,3

- Nalaboff KM, Pellerito JS, Ben-Levi E. Imaging the endometrium: disease and normal variants. Radiographics. 2001;21(6):1409-1424.
- Davis PC, O'Neill MJ, Yoder IC, Lee SI, Mueller PR. Sonohysterographic findings of endometrial and subendometrial conditions. Radiographics. 2002;22(4):803- 816.
- Yang T, Pandya A, Marcal L, et al. Sonohysterography: principles, technique and role in diagnosis of endometrial pathology. World J Radiol. 2013;5(3):81-87.
- Nalaboff KM, Pellerito JS, Ben-Levi E. Imaging the endometrium: disease and normal variants. Radiographics. 2001;21(6):1409-1424.
- Davis PC, O'Neill MJ, Yoder IC, Lee SI, Mueller PR. Sonohysterographic findings of endometrial and subendometrial conditions. Radiographics. 2002;22(4):803- 816.
- Yang T, Pandya A, Marcal L, et al. Sonohysterography: principles, technique and role in diagnosis of endometrial pathology. World J Radiol. 2013;5(3):81-87.
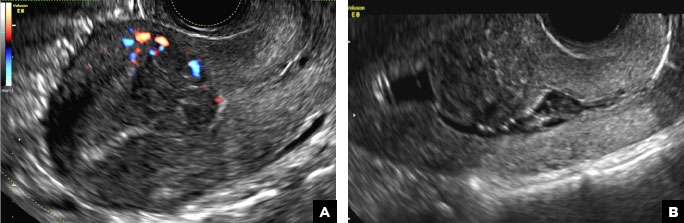
A 42-year-old woman presents to her gynecologist's office with abnormal uterine bleeding. Pelvic ultrasonography of the uterus is performed with color Doppler (A) and subsequent sonohysterogram (SHG)/saline infusion sonohysterography (SIS) (B). Figures shown above.
Pregnancy After Cervical Surgery: What Are the Risks?
Surgeries for cervical intraepithelial neoplasia (CIN) have been linked to preterm birth, low birth weight, and pregnancy loss, among other adverse effects. But the mechanisms have not been entirely explained. Are the outcomes due to the surgery, the immunologic factors, or the risk factors associated with CIN?
The greater risk of pregnancy problems derives primarily from the treatment not from characteristics of human papillomavirus or CIN, say researchers from Kaiser Permanente Northwest, Duke University, and GlaxoSmithKline. They compared 322 women who had undergone excisional and ablative cervical surgery with 4,307 women who had not and with 847 women who had undergone only diagnostic procedures.
Of the treated women, 163 had a live birth within 1 year of treatment. Women who had undergone thick excisional cervical surgical procedures ( ≥ 1.0 cm) had approximately double the risk of preterm birth and low-birth-weight infants compared with that of the other 2 groups. The risk was higher if the baby was born within 1 year of surgery. The risk of cesarean delivery rose 20% with excisional surgery. The treated women did not have a substantially higher risk of dysfunctional labor.
Related: More Evidence of HPV’s Role in Cancer
Among the women who had cervical surgery, 21% lost the pregnancy (25% of those with ablative and 19% of those with excisional surgery). By contrast, 18% of the unexposed women and 20% of the diagnostic-only group experienced pregnancy loss. The researchers say the positive association between pregnancy loss and ablative surgical treatment has not been previously reported. “These findings suggest that efforts to minimize excision thickness in cervical surgeries are prudent,” the researchers advise.
Source:
Weinmann S, Naleway A, Swamy G, et al. PLoS One. 2017;12(1):e0165276.
doi: 10.1371/journal.pone.0165276.
Surgeries for cervical intraepithelial neoplasia (CIN) have been linked to preterm birth, low birth weight, and pregnancy loss, among other adverse effects. But the mechanisms have not been entirely explained. Are the outcomes due to the surgery, the immunologic factors, or the risk factors associated with CIN?
The greater risk of pregnancy problems derives primarily from the treatment not from characteristics of human papillomavirus or CIN, say researchers from Kaiser Permanente Northwest, Duke University, and GlaxoSmithKline. They compared 322 women who had undergone excisional and ablative cervical surgery with 4,307 women who had not and with 847 women who had undergone only diagnostic procedures.
Of the treated women, 163 had a live birth within 1 year of treatment. Women who had undergone thick excisional cervical surgical procedures ( ≥ 1.0 cm) had approximately double the risk of preterm birth and low-birth-weight infants compared with that of the other 2 groups. The risk was higher if the baby was born within 1 year of surgery. The risk of cesarean delivery rose 20% with excisional surgery. The treated women did not have a substantially higher risk of dysfunctional labor.
Related: More Evidence of HPV’s Role in Cancer
Among the women who had cervical surgery, 21% lost the pregnancy (25% of those with ablative and 19% of those with excisional surgery). By contrast, 18% of the unexposed women and 20% of the diagnostic-only group experienced pregnancy loss. The researchers say the positive association between pregnancy loss and ablative surgical treatment has not been previously reported. “These findings suggest that efforts to minimize excision thickness in cervical surgeries are prudent,” the researchers advise.
Source:
Weinmann S, Naleway A, Swamy G, et al. PLoS One. 2017;12(1):e0165276.
doi: 10.1371/journal.pone.0165276.
Surgeries for cervical intraepithelial neoplasia (CIN) have been linked to preterm birth, low birth weight, and pregnancy loss, among other adverse effects. But the mechanisms have not been entirely explained. Are the outcomes due to the surgery, the immunologic factors, or the risk factors associated with CIN?
The greater risk of pregnancy problems derives primarily from the treatment not from characteristics of human papillomavirus or CIN, say researchers from Kaiser Permanente Northwest, Duke University, and GlaxoSmithKline. They compared 322 women who had undergone excisional and ablative cervical surgery with 4,307 women who had not and with 847 women who had undergone only diagnostic procedures.
Of the treated women, 163 had a live birth within 1 year of treatment. Women who had undergone thick excisional cervical surgical procedures ( ≥ 1.0 cm) had approximately double the risk of preterm birth and low-birth-weight infants compared with that of the other 2 groups. The risk was higher if the baby was born within 1 year of surgery. The risk of cesarean delivery rose 20% with excisional surgery. The treated women did not have a substantially higher risk of dysfunctional labor.
Related: More Evidence of HPV’s Role in Cancer
Among the women who had cervical surgery, 21% lost the pregnancy (25% of those with ablative and 19% of those with excisional surgery). By contrast, 18% of the unexposed women and 20% of the diagnostic-only group experienced pregnancy loss. The researchers say the positive association between pregnancy loss and ablative surgical treatment has not been previously reported. “These findings suggest that efforts to minimize excision thickness in cervical surgeries are prudent,” the researchers advise.
Source:
Weinmann S, Naleway A, Swamy G, et al. PLoS One. 2017;12(1):e0165276.
doi: 10.1371/journal.pone.0165276.
Onetime, Nondrug Treatment May Be Better for Some MS Patients
High-dose immunosuppressive therapy and autologous hematopoietic cell transplant (HDIT/HCT) has had “highly promising” results for patients with relapsing-remitting multiple sclerosis (MS), according to researchers from the HALT-MS trial. In the 5-year study, 69% of 24 participants survived without progression of disability, relapse, or new brain lesions, despite not taking MS medications.
Findings published at the 3-year mark were encouraging. The event-free survival rate was 78%. The extended findings suggest that “onetime treatment with HDIT/HCT may be substantially more effective than long-term treatment with the best available medications” for these patients, said NIAID Director Anthony Fauci, MD.
The treatment “resets” the immune system, the researchers say. First, doctors collect the patient’s blood-forming stem cells, then give the patient chemotherapy to deplete the immune system. Finally, the doctors return the patient’s stem cells to rebuild the immune system.
Adverse events were consistent with those routinely observed after HDIT/HCT. Adverse effects recorded at 4 and 5 years were not related to the transplant and were not considered severe. Three patients died, but their deaths were not related to the study treatments.
Five years later, most trial participants remained in remission and stabilized, and some showed improvements, such as recovering mobility.
High-dose immunosuppressive therapy and autologous hematopoietic cell transplant (HDIT/HCT) has had “highly promising” results for patients with relapsing-remitting multiple sclerosis (MS), according to researchers from the HALT-MS trial. In the 5-year study, 69% of 24 participants survived without progression of disability, relapse, or new brain lesions, despite not taking MS medications.
Findings published at the 3-year mark were encouraging. The event-free survival rate was 78%. The extended findings suggest that “onetime treatment with HDIT/HCT may be substantially more effective than long-term treatment with the best available medications” for these patients, said NIAID Director Anthony Fauci, MD.
The treatment “resets” the immune system, the researchers say. First, doctors collect the patient’s blood-forming stem cells, then give the patient chemotherapy to deplete the immune system. Finally, the doctors return the patient’s stem cells to rebuild the immune system.
Adverse events were consistent with those routinely observed after HDIT/HCT. Adverse effects recorded at 4 and 5 years were not related to the transplant and were not considered severe. Three patients died, but their deaths were not related to the study treatments.
Five years later, most trial participants remained in remission and stabilized, and some showed improvements, such as recovering mobility.
High-dose immunosuppressive therapy and autologous hematopoietic cell transplant (HDIT/HCT) has had “highly promising” results for patients with relapsing-remitting multiple sclerosis (MS), according to researchers from the HALT-MS trial. In the 5-year study, 69% of 24 participants survived without progression of disability, relapse, or new brain lesions, despite not taking MS medications.
Findings published at the 3-year mark were encouraging. The event-free survival rate was 78%. The extended findings suggest that “onetime treatment with HDIT/HCT may be substantially more effective than long-term treatment with the best available medications” for these patients, said NIAID Director Anthony Fauci, MD.
The treatment “resets” the immune system, the researchers say. First, doctors collect the patient’s blood-forming stem cells, then give the patient chemotherapy to deplete the immune system. Finally, the doctors return the patient’s stem cells to rebuild the immune system.
Adverse events were consistent with those routinely observed after HDIT/HCT. Adverse effects recorded at 4 and 5 years were not related to the transplant and were not considered severe. Three patients died, but their deaths were not related to the study treatments.
Five years later, most trial participants remained in remission and stabilized, and some showed improvements, such as recovering mobility.
Lungs may play key role in blood production, doc says
The lungs may play a previously unrecognized role in blood production, according to preclinical research published in Nature.
Researchers discovered large numbers of megakaryocytes in the lungs of mice and found these cells produced roughly half of the animals’ platelets.
The team also identified a pool of hematopoietic progenitors in the extravascular spaces of mouse lungs that were capable of multi-lineage bone marrow reconstitution.
“This finding definitely suggests a more sophisticated view of the lungs—that they’re not just for respiration but also a key partner in formation of crucial aspects of the blood,” said study author Mark R. Looney, MD, of the University of California - San Francisco.
“What we’ve observed here in mice strongly suggests the lung may play a key role in blood formation in humans as well.”
The researchers believe these findings could have major implications for understanding diseases in which patients suffer from thrombocytopenia.
Imaging reveals surprise
This research was made possible by a refinement of a technique known as 2-photon intravital imaging. This approach allowed the researchers to visualize the behavior of individual cells within the blood vessels of a living mouse lung.
Dr Looney and his colleagues used the technique to examine interactions between the immune system and circulating platelets in the lungs in a mouse strain engineered so that platelets emit bright green fluorescence (GFP+).
In this way, the team noticed a large population of megakaryocytes in the lung vasculature. Though megakaryocytes had been observed in the lung before, they were generally thought to live and produce platelets primarily in the bone marrow.
“When we discovered this massive population of megakaryocytes that appeared to be living in the lung, we realized we had to follow this up,” said study author Emma Lefrançais, PhD, a postdoctoral researcher in Dr Looney’s lab.
More detailed imaging sessions revealed megakaryocytes in the act of producing more than 10 million platelets per hour within the lung vasculature. This suggests that roughly half of a mouse’s total platelet production occurs in the lung, not the bone marrow, as researchers had long presumed.
Subsequent experiments also revealed a variety of previously overlooked hematopoietic progenitors outside the lung vasculature.
Transplants provide more insight
The discovery of megakaryocytes and hematopoietic progenitors in the lung raised questions about how these cells move back and forth between the lung and bone marrow. To address these questions, the researchers conducted a set of lung transplant studies.
The team transplanted lungs from wild-type mice into mice with GFP+ megakaryocytes and vice-versa. The researchers said they observed proplatelet formation from GFP+ megakaryocytes in the lung vasculature of the GFP+ mice but not in the wild-type mice.
This suggests the megakaryocytes releasing platelets in the lung circulation originate from outside the lungs, the researchers said. And subsequent experiments suggested the megakaryocytes originate in the bone marrow.
“It’s fascinating that megakaryocytes travel all the way from the bone marrow to the lungs to produce platelets,” said Guadalupe Ortiz-Muñoz, PhD, a postdoctoral researcher in Dr Looney’s lab.
“It’s possible that the lung is an ideal bioreactor for platelet production because of the mechanical force of the blood, or perhaps because of some molecular signaling we don’t yet know about.”
In another experiment, the researchers transplanted lungs with GFP+ megakaryocyte progenitors into mutant mice with low platelet counts.
The transplants successfully restored platelet levels to normal, an effect that persisted over a few months of observation—much longer than the lifespan of individual megakaryocytes or platelets.
To the researchers, this indicated that resident megakaryocyte progenitors in the transplanted lungs had become activated by the recipient mouse’s low platelet counts and had produced healthy megakaryocytes to restore proper platelet production.
Finally, the researchers tested whether lung hematopoietic progenitors were capable of multi-lineage bone marrow reconstitution.
They found that cells originating from transplanted lungs traveled to damaged bone marrow and contributed to the production of platelets and other blood cells, including neutrophils, B cells, and T cells.
The researchers said these experiments suggest the lungs play host to a variety of hematopoietic progenitors capable of reconstituting damaged bone marrow and restoring the production of many components of the blood.
“To our knowledge, this is the first description of blood progenitors resident in the lung, and it raises a lot of questions with clinical relevance for the millions of people who suffer from thrombocytopenia,” Dr Looney said.
In particular, the study suggests that researchers who have proposed treating platelet diseases with platelets produced from engineered megakaryocytes should look to the lungs as a resource for platelet production, Dr Looney noted.
The study also presents new avenues of research for stem cell biologists to explore how the bone marrow and lung collaborate to produce a healthy blood system through the mutual exchange of stem cells.
“These observations alter existing paradigms regarding blood cell formation, lung biology and disease, and transplantation,” said pulmonologist Guy A. Zimmerman, MD, who is associate chair of the Department of Internal Medicine at the University of Utah School of Medicine and was an independent reviewer of this study for Nature.
“The findings have direct clinical relevance and provide a rich group of questions for future studies of platelet genesis and megakaryocyte function in lung inflammation and other inflammatory conditions, bleeding and thrombotic disorders, and transplantation.” ![]()
The lungs may play a previously unrecognized role in blood production, according to preclinical research published in Nature.
Researchers discovered large numbers of megakaryocytes in the lungs of mice and found these cells produced roughly half of the animals’ platelets.
The team also identified a pool of hematopoietic progenitors in the extravascular spaces of mouse lungs that were capable of multi-lineage bone marrow reconstitution.
“This finding definitely suggests a more sophisticated view of the lungs—that they’re not just for respiration but also a key partner in formation of crucial aspects of the blood,” said study author Mark R. Looney, MD, of the University of California - San Francisco.
“What we’ve observed here in mice strongly suggests the lung may play a key role in blood formation in humans as well.”
The researchers believe these findings could have major implications for understanding diseases in which patients suffer from thrombocytopenia.
Imaging reveals surprise
This research was made possible by a refinement of a technique known as 2-photon intravital imaging. This approach allowed the researchers to visualize the behavior of individual cells within the blood vessels of a living mouse lung.
Dr Looney and his colleagues used the technique to examine interactions between the immune system and circulating platelets in the lungs in a mouse strain engineered so that platelets emit bright green fluorescence (GFP+).
In this way, the team noticed a large population of megakaryocytes in the lung vasculature. Though megakaryocytes had been observed in the lung before, they were generally thought to live and produce platelets primarily in the bone marrow.
“When we discovered this massive population of megakaryocytes that appeared to be living in the lung, we realized we had to follow this up,” said study author Emma Lefrançais, PhD, a postdoctoral researcher in Dr Looney’s lab.
More detailed imaging sessions revealed megakaryocytes in the act of producing more than 10 million platelets per hour within the lung vasculature. This suggests that roughly half of a mouse’s total platelet production occurs in the lung, not the bone marrow, as researchers had long presumed.
Subsequent experiments also revealed a variety of previously overlooked hematopoietic progenitors outside the lung vasculature.
Transplants provide more insight
The discovery of megakaryocytes and hematopoietic progenitors in the lung raised questions about how these cells move back and forth between the lung and bone marrow. To address these questions, the researchers conducted a set of lung transplant studies.
The team transplanted lungs from wild-type mice into mice with GFP+ megakaryocytes and vice-versa. The researchers said they observed proplatelet formation from GFP+ megakaryocytes in the lung vasculature of the GFP+ mice but not in the wild-type mice.
This suggests the megakaryocytes releasing platelets in the lung circulation originate from outside the lungs, the researchers said. And subsequent experiments suggested the megakaryocytes originate in the bone marrow.
“It’s fascinating that megakaryocytes travel all the way from the bone marrow to the lungs to produce platelets,” said Guadalupe Ortiz-Muñoz, PhD, a postdoctoral researcher in Dr Looney’s lab.
“It’s possible that the lung is an ideal bioreactor for platelet production because of the mechanical force of the blood, or perhaps because of some molecular signaling we don’t yet know about.”
In another experiment, the researchers transplanted lungs with GFP+ megakaryocyte progenitors into mutant mice with low platelet counts.
The transplants successfully restored platelet levels to normal, an effect that persisted over a few months of observation—much longer than the lifespan of individual megakaryocytes or platelets.
To the researchers, this indicated that resident megakaryocyte progenitors in the transplanted lungs had become activated by the recipient mouse’s low platelet counts and had produced healthy megakaryocytes to restore proper platelet production.
Finally, the researchers tested whether lung hematopoietic progenitors were capable of multi-lineage bone marrow reconstitution.
They found that cells originating from transplanted lungs traveled to damaged bone marrow and contributed to the production of platelets and other blood cells, including neutrophils, B cells, and T cells.
The researchers said these experiments suggest the lungs play host to a variety of hematopoietic progenitors capable of reconstituting damaged bone marrow and restoring the production of many components of the blood.
“To our knowledge, this is the first description of blood progenitors resident in the lung, and it raises a lot of questions with clinical relevance for the millions of people who suffer from thrombocytopenia,” Dr Looney said.
In particular, the study suggests that researchers who have proposed treating platelet diseases with platelets produced from engineered megakaryocytes should look to the lungs as a resource for platelet production, Dr Looney noted.
The study also presents new avenues of research for stem cell biologists to explore how the bone marrow and lung collaborate to produce a healthy blood system through the mutual exchange of stem cells.
“These observations alter existing paradigms regarding blood cell formation, lung biology and disease, and transplantation,” said pulmonologist Guy A. Zimmerman, MD, who is associate chair of the Department of Internal Medicine at the University of Utah School of Medicine and was an independent reviewer of this study for Nature.
“The findings have direct clinical relevance and provide a rich group of questions for future studies of platelet genesis and megakaryocyte function in lung inflammation and other inflammatory conditions, bleeding and thrombotic disorders, and transplantation.” ![]()
The lungs may play a previously unrecognized role in blood production, according to preclinical research published in Nature.
Researchers discovered large numbers of megakaryocytes in the lungs of mice and found these cells produced roughly half of the animals’ platelets.
The team also identified a pool of hematopoietic progenitors in the extravascular spaces of mouse lungs that were capable of multi-lineage bone marrow reconstitution.
“This finding definitely suggests a more sophisticated view of the lungs—that they’re not just for respiration but also a key partner in formation of crucial aspects of the blood,” said study author Mark R. Looney, MD, of the University of California - San Francisco.
“What we’ve observed here in mice strongly suggests the lung may play a key role in blood formation in humans as well.”
The researchers believe these findings could have major implications for understanding diseases in which patients suffer from thrombocytopenia.
Imaging reveals surprise
This research was made possible by a refinement of a technique known as 2-photon intravital imaging. This approach allowed the researchers to visualize the behavior of individual cells within the blood vessels of a living mouse lung.
Dr Looney and his colleagues used the technique to examine interactions between the immune system and circulating platelets in the lungs in a mouse strain engineered so that platelets emit bright green fluorescence (GFP+).
In this way, the team noticed a large population of megakaryocytes in the lung vasculature. Though megakaryocytes had been observed in the lung before, they were generally thought to live and produce platelets primarily in the bone marrow.
“When we discovered this massive population of megakaryocytes that appeared to be living in the lung, we realized we had to follow this up,” said study author Emma Lefrançais, PhD, a postdoctoral researcher in Dr Looney’s lab.
More detailed imaging sessions revealed megakaryocytes in the act of producing more than 10 million platelets per hour within the lung vasculature. This suggests that roughly half of a mouse’s total platelet production occurs in the lung, not the bone marrow, as researchers had long presumed.
Subsequent experiments also revealed a variety of previously overlooked hematopoietic progenitors outside the lung vasculature.
Transplants provide more insight
The discovery of megakaryocytes and hematopoietic progenitors in the lung raised questions about how these cells move back and forth between the lung and bone marrow. To address these questions, the researchers conducted a set of lung transplant studies.
The team transplanted lungs from wild-type mice into mice with GFP+ megakaryocytes and vice-versa. The researchers said they observed proplatelet formation from GFP+ megakaryocytes in the lung vasculature of the GFP+ mice but not in the wild-type mice.
This suggests the megakaryocytes releasing platelets in the lung circulation originate from outside the lungs, the researchers said. And subsequent experiments suggested the megakaryocytes originate in the bone marrow.
“It’s fascinating that megakaryocytes travel all the way from the bone marrow to the lungs to produce platelets,” said Guadalupe Ortiz-Muñoz, PhD, a postdoctoral researcher in Dr Looney’s lab.
“It’s possible that the lung is an ideal bioreactor for platelet production because of the mechanical force of the blood, or perhaps because of some molecular signaling we don’t yet know about.”
In another experiment, the researchers transplanted lungs with GFP+ megakaryocyte progenitors into mutant mice with low platelet counts.
The transplants successfully restored platelet levels to normal, an effect that persisted over a few months of observation—much longer than the lifespan of individual megakaryocytes or platelets.
To the researchers, this indicated that resident megakaryocyte progenitors in the transplanted lungs had become activated by the recipient mouse’s low platelet counts and had produced healthy megakaryocytes to restore proper platelet production.
Finally, the researchers tested whether lung hematopoietic progenitors were capable of multi-lineage bone marrow reconstitution.
They found that cells originating from transplanted lungs traveled to damaged bone marrow and contributed to the production of platelets and other blood cells, including neutrophils, B cells, and T cells.
The researchers said these experiments suggest the lungs play host to a variety of hematopoietic progenitors capable of reconstituting damaged bone marrow and restoring the production of many components of the blood.
“To our knowledge, this is the first description of blood progenitors resident in the lung, and it raises a lot of questions with clinical relevance for the millions of people who suffer from thrombocytopenia,” Dr Looney said.
In particular, the study suggests that researchers who have proposed treating platelet diseases with platelets produced from engineered megakaryocytes should look to the lungs as a resource for platelet production, Dr Looney noted.
The study also presents new avenues of research for stem cell biologists to explore how the bone marrow and lung collaborate to produce a healthy blood system through the mutual exchange of stem cells.
“These observations alter existing paradigms regarding blood cell formation, lung biology and disease, and transplantation,” said pulmonologist Guy A. Zimmerman, MD, who is associate chair of the Department of Internal Medicine at the University of Utah School of Medicine and was an independent reviewer of this study for Nature.
“The findings have direct clinical relevance and provide a rich group of questions for future studies of platelet genesis and megakaryocyte function in lung inflammation and other inflammatory conditions, bleeding and thrombotic disorders, and transplantation.” ![]()
CDT use and outcomes differ between sexes
New research has revealed sex-based differences in utilization and outcomes of catheter-directed thrombolysis (CDT) in patients with deep vein thrombosis (DVT).
The study showed that CDT use was more common in men than women, although use of the procedure increased over time for both sexes.
There were significant between-sex differences in the rates of some bleeding complications and certain in-hospital outcomes, but in-hospital mortality rates were similar between the sexes.
Riyaz Bashir, MD, of Temple University in Philadelphia, Pennsylvania, and his colleagues reported these findings in Vascular Medicine.
“The data provided some interesting findings,” Dr Bashir said. “In addition to differences in utilization, we were able to find variations in the incidence of a number of complications, including bleeding that requires blood transfusion, intracranial hemorrhage, gastrointestinal bleeding, and acute kidney injury, as well as the incidence of angioplasty, stenting, and adjunctive IVC filter placement.”
For this study, Dr Bashir and his colleagues analyzed data from the Nationwide Inpatient Sample database.
The team identified 108,243 patients age 18 or older with a primary discharge diagnosis of proximal lower extremity or caval DVT between January 2005 and December 2011. Of those patients, 4826 (4.5%) were treated with CDT.
The researchers found that women underwent CDT less often than men—4.1% and 4.9%, respectively (P<0.01).
But the rates of CDT use increased between 2005 and 2011 for both sexes—from 2.1% to 5.9% in women (P<0.01) and from 2.5% to 7.5% (P<0.01) in men.
The rate of in-hospital mortality was similar between women (1.2%) and men (1.3%, P=0.76).
Likewise, there was no significant between-sex difference in length of hospital stay or total hospital charges. The average length of stay was 7.0 ± 5.7 days for men and 7.1 ± 5.5 days for women (P=0.55).
Average total hospital charges were $88,837 ± 68,284 for men and $91,487 ± 77,129 for women (P=0.28).
Women were more likely than men to:
- Require blood transfusions—11.7% and 8.8%, respectively, (P<0.01)
- Undergo angioplasty—64.1% and 54.3%, respectively (P<0.01)
- Undergo stenting—32.2% and 19.9%, respectively (P<0.01)
- Receive an inferior vena cava filter—37.0% and 32.1%, respectively (P<0.01).
Men were more likely than women to:
- Experience acute kidney injury—9.9% and 6.5%, respectively (P<0.01)
- Have an intracranial hemorrhage (ICH)—1.2% and 0.5%, respectively (P=0.03)
- Experience gastrointestinal bleeding—2.2% and 0.9%, respectively (P<0.01).
The researchers noted that the higher rate of ICH among men overall was due to a higher incidence of ICH among men over the age of 75.
There was no significant difference between men and women when it came to procedure-related hemorrhage—1.4% and 1.2%, respectively (P=0.45).
“Future research should focus on uncovering why these sex-based differences exist,” Dr Bashir said. “The answers to those questions could help shape future treatment guidelines for patients who are suitable candidates for CDT.” ![]()
New research has revealed sex-based differences in utilization and outcomes of catheter-directed thrombolysis (CDT) in patients with deep vein thrombosis (DVT).
The study showed that CDT use was more common in men than women, although use of the procedure increased over time for both sexes.
There were significant between-sex differences in the rates of some bleeding complications and certain in-hospital outcomes, but in-hospital mortality rates were similar between the sexes.
Riyaz Bashir, MD, of Temple University in Philadelphia, Pennsylvania, and his colleagues reported these findings in Vascular Medicine.
“The data provided some interesting findings,” Dr Bashir said. “In addition to differences in utilization, we were able to find variations in the incidence of a number of complications, including bleeding that requires blood transfusion, intracranial hemorrhage, gastrointestinal bleeding, and acute kidney injury, as well as the incidence of angioplasty, stenting, and adjunctive IVC filter placement.”
For this study, Dr Bashir and his colleagues analyzed data from the Nationwide Inpatient Sample database.
The team identified 108,243 patients age 18 or older with a primary discharge diagnosis of proximal lower extremity or caval DVT between January 2005 and December 2011. Of those patients, 4826 (4.5%) were treated with CDT.
The researchers found that women underwent CDT less often than men—4.1% and 4.9%, respectively (P<0.01).
But the rates of CDT use increased between 2005 and 2011 for both sexes—from 2.1% to 5.9% in women (P<0.01) and from 2.5% to 7.5% (P<0.01) in men.
The rate of in-hospital mortality was similar between women (1.2%) and men (1.3%, P=0.76).
Likewise, there was no significant between-sex difference in length of hospital stay or total hospital charges. The average length of stay was 7.0 ± 5.7 days for men and 7.1 ± 5.5 days for women (P=0.55).
Average total hospital charges were $88,837 ± 68,284 for men and $91,487 ± 77,129 for women (P=0.28).
Women were more likely than men to:
- Require blood transfusions—11.7% and 8.8%, respectively, (P<0.01)
- Undergo angioplasty—64.1% and 54.3%, respectively (P<0.01)
- Undergo stenting—32.2% and 19.9%, respectively (P<0.01)
- Receive an inferior vena cava filter—37.0% and 32.1%, respectively (P<0.01).
Men were more likely than women to:
- Experience acute kidney injury—9.9% and 6.5%, respectively (P<0.01)
- Have an intracranial hemorrhage (ICH)—1.2% and 0.5%, respectively (P=0.03)
- Experience gastrointestinal bleeding—2.2% and 0.9%, respectively (P<0.01).
The researchers noted that the higher rate of ICH among men overall was due to a higher incidence of ICH among men over the age of 75.
There was no significant difference between men and women when it came to procedure-related hemorrhage—1.4% and 1.2%, respectively (P=0.45).
“Future research should focus on uncovering why these sex-based differences exist,” Dr Bashir said. “The answers to those questions could help shape future treatment guidelines for patients who are suitable candidates for CDT.” ![]()
New research has revealed sex-based differences in utilization and outcomes of catheter-directed thrombolysis (CDT) in patients with deep vein thrombosis (DVT).
The study showed that CDT use was more common in men than women, although use of the procedure increased over time for both sexes.
There were significant between-sex differences in the rates of some bleeding complications and certain in-hospital outcomes, but in-hospital mortality rates were similar between the sexes.
Riyaz Bashir, MD, of Temple University in Philadelphia, Pennsylvania, and his colleagues reported these findings in Vascular Medicine.
“The data provided some interesting findings,” Dr Bashir said. “In addition to differences in utilization, we were able to find variations in the incidence of a number of complications, including bleeding that requires blood transfusion, intracranial hemorrhage, gastrointestinal bleeding, and acute kidney injury, as well as the incidence of angioplasty, stenting, and adjunctive IVC filter placement.”
For this study, Dr Bashir and his colleagues analyzed data from the Nationwide Inpatient Sample database.
The team identified 108,243 patients age 18 or older with a primary discharge diagnosis of proximal lower extremity or caval DVT between January 2005 and December 2011. Of those patients, 4826 (4.5%) were treated with CDT.
The researchers found that women underwent CDT less often than men—4.1% and 4.9%, respectively (P<0.01).
But the rates of CDT use increased between 2005 and 2011 for both sexes—from 2.1% to 5.9% in women (P<0.01) and from 2.5% to 7.5% (P<0.01) in men.
The rate of in-hospital mortality was similar between women (1.2%) and men (1.3%, P=0.76).
Likewise, there was no significant between-sex difference in length of hospital stay or total hospital charges. The average length of stay was 7.0 ± 5.7 days for men and 7.1 ± 5.5 days for women (P=0.55).
Average total hospital charges were $88,837 ± 68,284 for men and $91,487 ± 77,129 for women (P=0.28).
Women were more likely than men to:
- Require blood transfusions—11.7% and 8.8%, respectively, (P<0.01)
- Undergo angioplasty—64.1% and 54.3%, respectively (P<0.01)
- Undergo stenting—32.2% and 19.9%, respectively (P<0.01)
- Receive an inferior vena cava filter—37.0% and 32.1%, respectively (P<0.01).
Men were more likely than women to:
- Experience acute kidney injury—9.9% and 6.5%, respectively (P<0.01)
- Have an intracranial hemorrhage (ICH)—1.2% and 0.5%, respectively (P=0.03)
- Experience gastrointestinal bleeding—2.2% and 0.9%, respectively (P<0.01).
The researchers noted that the higher rate of ICH among men overall was due to a higher incidence of ICH among men over the age of 75.
There was no significant difference between men and women when it came to procedure-related hemorrhage—1.4% and 1.2%, respectively (P=0.45).
“Future research should focus on uncovering why these sex-based differences exist,” Dr Bashir said. “The answers to those questions could help shape future treatment guidelines for patients who are suitable candidates for CDT.” ![]()