User login
Residual symptoms of schizophrenia: What are realistic treatment goals?
The course of chronic psychiatric conditions, such as schizophrenia, differs from chronic medical conditions, such as diabetes. Some patients with chronic psychiatric conditions achieve remission and become symptom-free, while others continue to have lingering signs of disease for life.
Residual symptoms of schizophrenia are not fully defined in the literature, which poses a challenge because they are central in the overall treatment of schizophrenia spectrum disorders.1 During this phase of schizophrenia, patients continue to have symptoms after psychosis has subsided. These patients might continue to have negative symptoms such as social and emotional withdrawal and low energy. Although frank psychotic behavior has disappeared, the patient might continue to hold strange beliefs. Pharmacotherapy is the primary treatment option for psychiatric conditions, but the psychosocial aspect may have greater importance when treating residual symptoms and patients with chronic psychiatric illness.2
A naturalistic study in Germany evaluated the occurrence and characteristics of residual symptoms in patients with schizophrenia.3 The authors used a Positive and Negative Syndrome Scale symptom severity score >1 for those purposes, which is possibly a stringent criterion to define residual symptoms. This multicenter study enrolled 399 individuals age 18 to 65 with a DSM-IV-TR diagnosis of schizophrenia, schizophreniform disorder, delusional disorder, or schizoaffective disorder.3 Of the 236 patients achieving remission at discharge, 94% had at least 1 residual symptom and 69% had at least 4 residual symptoms. Therefore, residual symptoms were highly prevalent in remitted patients. The most frequent residual symptoms were:
- blunted affect
- conceptual disorganization
- passive or apathetic social withdrawal
- emotional withdrawal
- lack of judgment and insight
- poor attention
- somatic concern
- difficulty with abstract thinking
- anxiety
- poor rapport.3
Of note, positive symptoms, such as delusions and hallucinatory behavior, were found in remitted patients at discharge (17% and 10%, respectively). The study concluded that the severity of residual symptoms was associated with relapse risk and had an overall negative impact on the outcome of patients with schizophrenia.3 The study noted that residual symptoms may be greater in number or volume than negative symptoms and questioned the origins of residual symptoms because most were present at baseline in more than two-third of patients.
Patients with residual symptoms of schizophrenia usually are older and therefore present specific management challenges for clinicians. Changes associated with aging, such as medical problems, cognitive deficits, and lack of social support, could create new care needs for this patient population. Although the biopsychosocial model used to treat chronic psychiatric conditions, especially schizophrenia, is preferred, older schizophrenia patients with residual symptoms often need more psychosocial interventions compared with young adults with schizophrenia.
Managing residual symptoms in schizophrenia
Few studies are devoted to pharmacological treatment of older adults with schizophrenia, likely because pharmacotherapy for older patients with schizophrenia can be challenging. Evidence-based treatment is based primarily on findings from younger patients who survived into later life. Clinicians often use the adage of geriatric psychiatry, “start low, go slow,” because older patients are susceptible to adverse effects associated with psychiatric medications, including cardiovascular, metabolic, anticholinergic, and extrapyramidal effects, orthostasis, sedation, falls, and neuroleptic malignant syndrome.
Older patients with schizophrenia are at an increased risk for extrapyramidal symptoms (EPS) and anticholinergic adverse effects, perhaps because of degeneration of dopaminergic and cholinergic neurons.4 Lowering the anticholinergic load by discontinuing or reducing the dosage of medications with anticholinergic properties, when possible, is a key principle when treating these patients. This tactic could help improve cognition and quality of life by decreasing the risk of other anticholinergic adverse effects, including delirium, constipation, urinary retention, and blurred vision.
Patients treated with typical antipsychotics are nearly twice as likely to develop tardive dyskinesia compared with those receiving atypical antipsychotics.5 Sedation, orthostatic hypotension, and anticholinergic effects can cause cognitive clouding, worsen cognitive impairment, and increase the risk of falls, especially in older patients.6 Clozapine and olanzapine have the strongest association with clinically significant weight gain and treatment-induced type 2 diabetes mellitus.7
The appropriate starting dosage of antipsychotics in older patients with schizophrenia is one-fourth of the starting adult dosage. Total daily maintenance dosages may be one-third to one-half of the adult dosage.6 Consensus guidelines for dosing atypical antipsychotics for older patients with schizophrenia are as shown in Table 1.8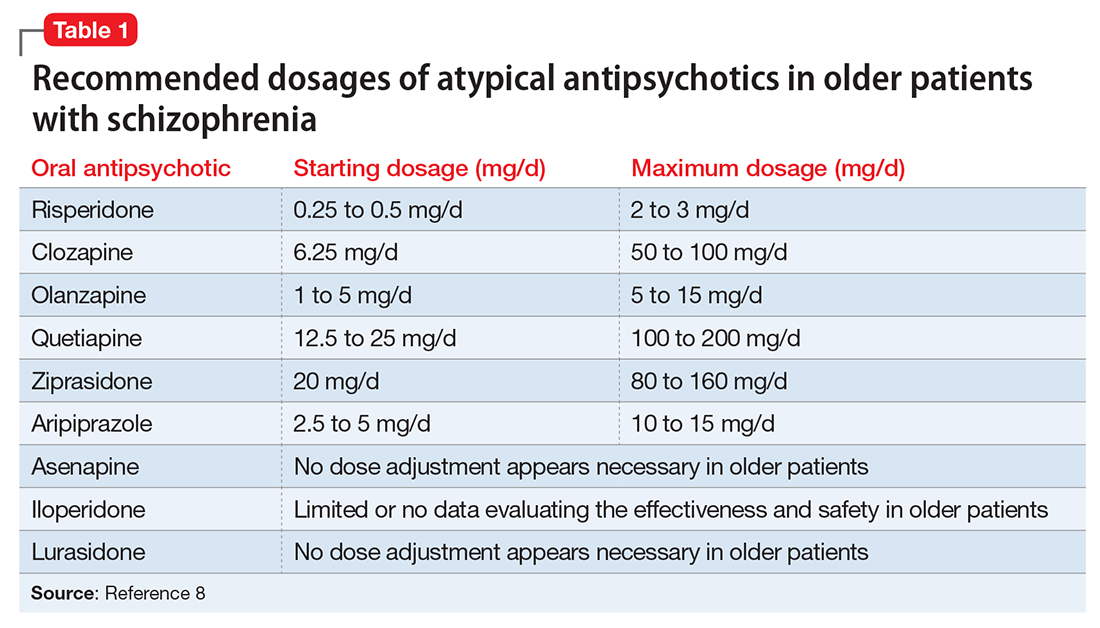
To ensure safety, patients should be regularly monitored with a complete blood count, comprehensive metabolic panel, lipid panel, hemoglobin A1C, electrocardiogram, orthostatic vital signs, Abnormal Involuntary Movement Scale, and weight check.7,9
When negative symptoms remain after a patient has achieved remission, it is important to evaluate whether the symptoms are related to adverse effects of medication (eg, parkinsonism syndrome), untreated depressive symptoms, or persistent positive symptoms, such as paranoia. Management of these symptoms consists of treating the cause, for example, using antipsychotics for primary positive symptoms, antidepressants for depression, anxiolytics for anxiety, and anti-parkinsonian agents or antipsychotic dosage reduction for EPS.
It is important to differentiate between negative symptoms of schizophrenia and depression in these patients. Negative symptoms of schizophrenia include affective flattening, alogia, avolition, and anhedonia. In depression, patients could have depressed mood, cognitive problems, sleep disturbances, and loss of appetite. Also, long-term symptoms are more consistent with negative symptomatology.
Keep in mind the potential for pharmacokinetic drug–drug interaction when using a combination of selective serotonin reuptake inhibitors (SSRIs), such as fluoxetine, paroxetine, and fluvoxamine (to treat negative/depressive symptoms), because all are significant inhibitors of cytochrome P450 enzymes and increase antipsychotic plasma level. The Expert Treatment Guidelines for Patients with Schizophrenia recommends SSRIs, followed by venlafaxine then bupropion to treat depressive symptoms after optimizing second-generation antipsychotics.9
Another point to consider when treating residual symptoms in patients with schizophrenia is to not discontinue antipsychotic medications. Relapse rates for these patients can occur up to 5 times higher than for those who continue treatment.10 A way to address this problem could be the use of depot antipsychotic medications, but there are no set recommendations for the use of long-acting injectable antipsychotics in older patients. These medications should be used with caution and at lowest effective dosages to offset potential adverse effects.
With the introduction of typical and atypical antipsychotics, the use of electroconvulsive therapy in older patients with schizophrenia has declined. In a 2009 meta-analysis of studies that included patients with refractory schizophrenia and repetitive transcranial magnetic stimulation (rTMS), results revealed a mixed effect size for controlled and uncontrolled studies. The authors stated the need for further controlled trials, assessing the efficacy of rTMS on negative and positive symptoms of schizophrenia.11
Psychotherapy and psychosocial interventions
Patients with schizophrenia who have persistent psychotic symptoms while receiving adequate pharmacotherapy should be offered adjunctive cognitive, behaviorally oriented psychotherapy to reduce symptom severity. Cognitive-behavioral therapy (CBT) has been shown to help reduce relapse rates, reduce psychotic symptoms, and improve patients’ mental state.12 Amotivation and lack of insight can be particularly troublesome, which CBT can help address.12 Psychoeducation can:
- empower patients to understand their illness
- help them cope with their disease
- be aware of symptom relapse
- seek help sooner rather than later.
Also, counseling and supportive therapy are recommended by the American Psychiatric Association guidelines. Providers should involve family and loved ones in this discussion, so that they can help collaborate with care and provide a supportive and non-judgmental environment.
Older patients with residual symptoms of schizophrenia are less likely to have completed their education, pursued a career, or developed long-lasting relationships. Family members who were their support system earlier in life, such as parents, often are unable to provide care for them by the time patients with schizophrenia become older. These patients also are less likely to get married or have children, meaning that they are more likely to live alone. The advent of the interdisciplinary team, integration of several therapeutic modalities, the provision of case managers, and assertive community treatment (ACT) teams has provided help with social support, relapses, and hospitalizations, for older patients with schizophrenia.13 Key elements of ACT include:
- a multidisciplinary team, including a medication prescriber
- a shared caseload among team members
- direct service provision by team members
- frequent patient contact
- low patient to staff ratios
- outreach to patients in the community.
Medical care
Patients with schizophrenia are at higher risk for several comorbid medical conditions, such as diabetes, coronary artery disease, and digestive and liver disorders, compared with individuals without schizophrenia. This risk is associated with numerous factors, including sedentary lifestyle, high rates of lifetime cigarette use (70% to 80% of schizophrenia outpatients age <67 smoke), poor self-management skills, frequent homelessness, and unhealthy diet.
Although substantial attention is devoted to the psychiatric and behavioral management of patients with schizophrenia, many barriers impede the detection and treatment of their medical conditions. Patients with schizophrenia could experience delays in diagnosing a medical disorder, leading to more acute comorbidities at the time of diagnosis and premature mortality. Studies have confirmed that cardiovascular diseases are the leading cause of premature death among psychiatric patients in the United States.14 Key risk factors include smoking, obesity, hypertension, dyslipidemia, diabetes, and lack of physical activity, all of which are more common among patients with schizophrenia compared with the general population.15 In addition, antipsychotics are associated with adverse metabolic effects.16
What are realistic treatment goals to manage residual symptoms in schizophrenia?
We believe that because remission in schizophrenia has been defined consensually, the bar for treatment expectations is set higher than it was 20 years ago. There can be patient-, family-, and system-related variables affecting the feasibility of treating residual symptoms. Providers who treat patients with schizophrenia should consider the following treatment goals:
- Prevent relapse and acute psychiatric hospitalization
- Use evidence-based strategies to minimize or monitor adverse effects
- Monitor compliance and consider use of depot antipsychotics combined with patients’ preference
- Facilitate ongoing safety assessment, including suicide risk
- Monitor negative and cognitive symptoms in addition to positive symptoms, using evidence-based management
- Encourage collaboration of care with family, caretakers, and other members of the treatment team
- Empower patients by providing psychoeducation and social skills training and assisting in their vocational rehabilitation
- Educate the patient and family about healthy lifestyle interventions and medical comorbidities common with schizophrenia
- Perform baseline screening and follow-up for early detection and treatment of medical comorbidities in patients with schizophrenia
- Improve functional status and quality of life.
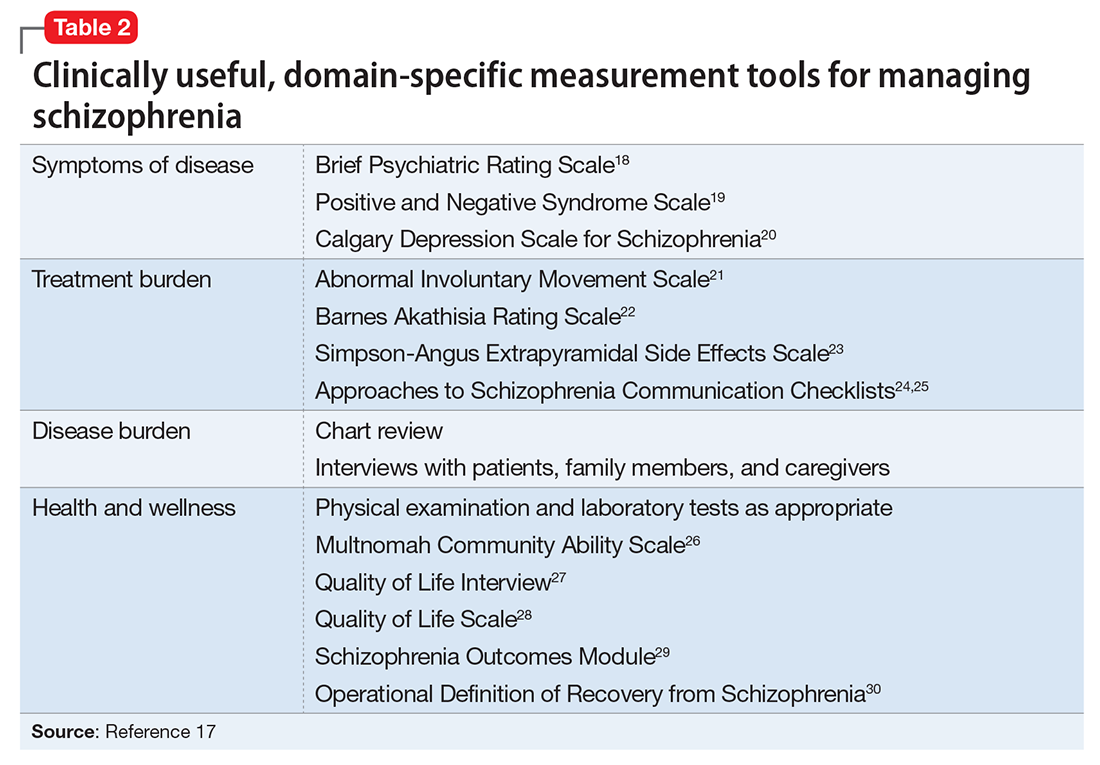
In addition to meeting these treatment goals, a measurement-based method can be implemented to monitor improvement and status of the independent treatment domains. A collection of rating instruments can be found in Table 2.17-30
The clinical presentation of patients with residual symptoms of schizophrenia differs from that of other patients with schizophrenia. Our understanding of residual symptoms in schizophrenia has come a long way in the last decade; however, we are still far from pinning the complex nature of these symptoms, let alone their management. Given the risk of morbidity and disability, there clearly is a need for further investigation and investment of time and resources to support developing novel pharmacological treatment options to manage residual symptoms in patients with schizophrenia.
Because patients with residual symptoms of schizophrenia usually are older, psychiatrists should be responsible for implementing necessary screening assessments and should closely collaborate with primary care practitioners and other specialists, and when necessary, treat comorbid medical conditions. The importance of educating patients, their families, and the treatment team cannot be overlooked. Further, psychiatric treatment facilities should offer and promote healthy lifestyle interventions.
1. Kaiser S, Lyne J, Agartz I, et al. Individual negative symptoms and domains - relevance for assessment, pathomechanisms and treatment [published online July 21, 2016]. Schizophr Res. doi:10.1016/j.schres.2016.07.013.
2. Taylor M, Chaudhry I, Cross M, et al. Towards consensus in the long-term management of relapse prevention in schizophrenia. Hum Psychopharmacol. 2005;20(3):175-181.
3. Schennach R, Riedel M, Obermeier M, et al. What are residual symptoms in schizophrenia spectrum disorder? Clinical description and 1-year persistence within a naturalistic trial. Eur Arch Psychiatry Clin Neurosci. 2015;265(2):107-116.
4. Caligiuri MP, Jeste DV, Lacro JP. Antipsychotic-induced movement disorders in the elderly: epidemiology and treatment recommendations. Drugs Aging. 2000;17(5):363-384.
5. Dolder CR, Jeste DV. Incidence of tardive dyskinesia with typical versus atypical antipsychotics in very high risk patients. Biol Psychiatry. 2003;53(12):1142-1145.
6. Sable JA, Jeste DV. Antipsychotic treatment for late-life schizophrenia. Curr Psychiatry Rep. 2002;4(4):299-306.
7. Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs. 2005;19(suppl 1):1-93.
8. Khan AY, Redden W, Ovais M, et al. Current concepts in the diagnosis and treatment of schizophrenia in later life. Current Geriatric Reports. 2015;4(4):290-300.
9. Alexopoulos GS, Streim J, Carpenter D, et al; Expert Consensus Panel for Using Antipsychotic Drugs in Older Patients. Using antipsychotic agents in older patients. J Clin Psychiatry. 2004;65(suppl 2):5-99; discussion 100-102; quiz 103-104.
10. Robinson D, Woerner MG, Alvir JM, et al. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry. 1999;56(3):241-247.
11. Freitas C, Fregni F, Pascual-Leone A. Meta-analysis of the effects of repetitive transcranial magnetic stimulation (rTMS) on negative and positive symptoms in schizophrenia. Schizophr Res. 2009;108(1-3):11-24.
12. Rector NA, Beck AT. Cognitive behavioral therapy for schizophrenia: an empirical review. J Nerv Ment Dis. 2012;200(10):832-839.
13. Stobbe J, Mulder NC, Roosenschoon BJ, et al. Assertive community treatment for elderly people with severe mental illness. BMC Psychiatry. 2010;10:84.
14. Hennekens CH, Hennekens AR, Hollar D, et al. Schizophrenia and increased risks of cardiovascular disease. Am Heart J. 2005;150(6):1115-1121.
15. Bushe CJ, Taylor M, Haukka J. Mortality in schizophrenia: a measurable clinical point. J Psychopharmacol. 2010;24(suppl 4):17-25.
16. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia, and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
17. Nasrallah HA, Targum SD, Tandon R, et al. Defining and measuring clinical effectiveness in the treatment of schizophrenia. Psychiatr Serv. 2005;56(3):273-282.
18. Overall JE, Gorham DR. The Brief Psychiatric Rating Scale (BPRS): recent developments in ascertainment and scaling. Psychopharmacol Bull. 1988;24:97-99.
19. Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276.
20. Addington D, Addington J, Schissel B. A depression rating scale for schizophrenics. Schizophr Res. 1990;3(4):247-251.
21. Guy W. ECDEU Assessment manual for psychopharmacology revised, 1976. Rockville, MD: US Department of Health, Education, and Welfare; Public Health Service; Alcohol, Drug Abuse, and Mental Health Administration; National Institute of Mental Health Psychopharmacology Research Branch, Division of Extramural Research Programs; 1976.
22. Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154:672-676.
23. Simpson GM, Angus JWS. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand. 1970;45(212):11-19.
24. Dott SG, Weiden P, Hopwood P, et al. An innovative approach to clinical communication in schizophrenia: the Approaches to Schizophrenia Communication checklists. CNS Spectr. 2001;6(4):333-338.
25. Dott SG, Knesevich J, Miller A, et al. Using the ASC program: a training guide. J Psychiatr Pract. 2001;7(1):64-68.
26. Barker S, Barron N, McFarland BH, et al. Multnomah Community Ability Scale: user’s manual. Portland, OR: Western Mental Health Research Center, Oregon Health Sciences University; 1994.
27. Lehman AF. A quality of life interview for the chronically mentally ill. Eval Program Plann. 1988;11(1):51-62.
28. Heinrichs DW, Hanlon TE, Carpenter WT Jr. The Quality of Life Scale: an instrument for rating the schizophrenic deficit syndrome. Schizophr Bull. 1984;10(3):388-398.
29. Becker M, Diamond R, Sainfort F. A new patient focused index for measuring quality of life in persons with severe and persistent mental illness. Qual Life Res. 1993;2(4):239-251.
30. Liberman RP, Kopelowicz A, Ventura J, et al. Operational criteria and factors related to recovery from schizophrenia. Int Rev Psychiatry. 2009;14(4):256-272.
The course of chronic psychiatric conditions, such as schizophrenia, differs from chronic medical conditions, such as diabetes. Some patients with chronic psychiatric conditions achieve remission and become symptom-free, while others continue to have lingering signs of disease for life.
Residual symptoms of schizophrenia are not fully defined in the literature, which poses a challenge because they are central in the overall treatment of schizophrenia spectrum disorders.1 During this phase of schizophrenia, patients continue to have symptoms after psychosis has subsided. These patients might continue to have negative symptoms such as social and emotional withdrawal and low energy. Although frank psychotic behavior has disappeared, the patient might continue to hold strange beliefs. Pharmacotherapy is the primary treatment option for psychiatric conditions, but the psychosocial aspect may have greater importance when treating residual symptoms and patients with chronic psychiatric illness.2
A naturalistic study in Germany evaluated the occurrence and characteristics of residual symptoms in patients with schizophrenia.3 The authors used a Positive and Negative Syndrome Scale symptom severity score >1 for those purposes, which is possibly a stringent criterion to define residual symptoms. This multicenter study enrolled 399 individuals age 18 to 65 with a DSM-IV-TR diagnosis of schizophrenia, schizophreniform disorder, delusional disorder, or schizoaffective disorder.3 Of the 236 patients achieving remission at discharge, 94% had at least 1 residual symptom and 69% had at least 4 residual symptoms. Therefore, residual symptoms were highly prevalent in remitted patients. The most frequent residual symptoms were:
- blunted affect
- conceptual disorganization
- passive or apathetic social withdrawal
- emotional withdrawal
- lack of judgment and insight
- poor attention
- somatic concern
- difficulty with abstract thinking
- anxiety
- poor rapport.3
Of note, positive symptoms, such as delusions and hallucinatory behavior, were found in remitted patients at discharge (17% and 10%, respectively). The study concluded that the severity of residual symptoms was associated with relapse risk and had an overall negative impact on the outcome of patients with schizophrenia.3 The study noted that residual symptoms may be greater in number or volume than negative symptoms and questioned the origins of residual symptoms because most were present at baseline in more than two-third of patients.
Patients with residual symptoms of schizophrenia usually are older and therefore present specific management challenges for clinicians. Changes associated with aging, such as medical problems, cognitive deficits, and lack of social support, could create new care needs for this patient population. Although the biopsychosocial model used to treat chronic psychiatric conditions, especially schizophrenia, is preferred, older schizophrenia patients with residual symptoms often need more psychosocial interventions compared with young adults with schizophrenia.
Managing residual symptoms in schizophrenia
Few studies are devoted to pharmacological treatment of older adults with schizophrenia, likely because pharmacotherapy for older patients with schizophrenia can be challenging. Evidence-based treatment is based primarily on findings from younger patients who survived into later life. Clinicians often use the adage of geriatric psychiatry, “start low, go slow,” because older patients are susceptible to adverse effects associated with psychiatric medications, including cardiovascular, metabolic, anticholinergic, and extrapyramidal effects, orthostasis, sedation, falls, and neuroleptic malignant syndrome.
Older patients with schizophrenia are at an increased risk for extrapyramidal symptoms (EPS) and anticholinergic adverse effects, perhaps because of degeneration of dopaminergic and cholinergic neurons.4 Lowering the anticholinergic load by discontinuing or reducing the dosage of medications with anticholinergic properties, when possible, is a key principle when treating these patients. This tactic could help improve cognition and quality of life by decreasing the risk of other anticholinergic adverse effects, including delirium, constipation, urinary retention, and blurred vision.
Patients treated with typical antipsychotics are nearly twice as likely to develop tardive dyskinesia compared with those receiving atypical antipsychotics.5 Sedation, orthostatic hypotension, and anticholinergic effects can cause cognitive clouding, worsen cognitive impairment, and increase the risk of falls, especially in older patients.6 Clozapine and olanzapine have the strongest association with clinically significant weight gain and treatment-induced type 2 diabetes mellitus.7
The appropriate starting dosage of antipsychotics in older patients with schizophrenia is one-fourth of the starting adult dosage. Total daily maintenance dosages may be one-third to one-half of the adult dosage.6 Consensus guidelines for dosing atypical antipsychotics for older patients with schizophrenia are as shown in Table 1.8
To ensure safety, patients should be regularly monitored with a complete blood count, comprehensive metabolic panel, lipid panel, hemoglobin A1C, electrocardiogram, orthostatic vital signs, Abnormal Involuntary Movement Scale, and weight check.7,9
When negative symptoms remain after a patient has achieved remission, it is important to evaluate whether the symptoms are related to adverse effects of medication (eg, parkinsonism syndrome), untreated depressive symptoms, or persistent positive symptoms, such as paranoia. Management of these symptoms consists of treating the cause, for example, using antipsychotics for primary positive symptoms, antidepressants for depression, anxiolytics for anxiety, and anti-parkinsonian agents or antipsychotic dosage reduction for EPS.
It is important to differentiate between negative symptoms of schizophrenia and depression in these patients. Negative symptoms of schizophrenia include affective flattening, alogia, avolition, and anhedonia. In depression, patients could have depressed mood, cognitive problems, sleep disturbances, and loss of appetite. Also, long-term symptoms are more consistent with negative symptomatology.
Keep in mind the potential for pharmacokinetic drug–drug interaction when using a combination of selective serotonin reuptake inhibitors (SSRIs), such as fluoxetine, paroxetine, and fluvoxamine (to treat negative/depressive symptoms), because all are significant inhibitors of cytochrome P450 enzymes and increase antipsychotic plasma level. The Expert Treatment Guidelines for Patients with Schizophrenia recommends SSRIs, followed by venlafaxine then bupropion to treat depressive symptoms after optimizing second-generation antipsychotics.9
Another point to consider when treating residual symptoms in patients with schizophrenia is to not discontinue antipsychotic medications. Relapse rates for these patients can occur up to 5 times higher than for those who continue treatment.10 A way to address this problem could be the use of depot antipsychotic medications, but there are no set recommendations for the use of long-acting injectable antipsychotics in older patients. These medications should be used with caution and at lowest effective dosages to offset potential adverse effects.
With the introduction of typical and atypical antipsychotics, the use of electroconvulsive therapy in older patients with schizophrenia has declined. In a 2009 meta-analysis of studies that included patients with refractory schizophrenia and repetitive transcranial magnetic stimulation (rTMS), results revealed a mixed effect size for controlled and uncontrolled studies. The authors stated the need for further controlled trials, assessing the efficacy of rTMS on negative and positive symptoms of schizophrenia.11
Psychotherapy and psychosocial interventions
Patients with schizophrenia who have persistent psychotic symptoms while receiving adequate pharmacotherapy should be offered adjunctive cognitive, behaviorally oriented psychotherapy to reduce symptom severity. Cognitive-behavioral therapy (CBT) has been shown to help reduce relapse rates, reduce psychotic symptoms, and improve patients’ mental state.12 Amotivation and lack of insight can be particularly troublesome, which CBT can help address.12 Psychoeducation can:
- empower patients to understand their illness
- help them cope with their disease
- be aware of symptom relapse
- seek help sooner rather than later.
Also, counseling and supportive therapy are recommended by the American Psychiatric Association guidelines. Providers should involve family and loved ones in this discussion, so that they can help collaborate with care and provide a supportive and non-judgmental environment.
Older patients with residual symptoms of schizophrenia are less likely to have completed their education, pursued a career, or developed long-lasting relationships. Family members who were their support system earlier in life, such as parents, often are unable to provide care for them by the time patients with schizophrenia become older. These patients also are less likely to get married or have children, meaning that they are more likely to live alone. The advent of the interdisciplinary team, integration of several therapeutic modalities, the provision of case managers, and assertive community treatment (ACT) teams has provided help with social support, relapses, and hospitalizations, for older patients with schizophrenia.13 Key elements of ACT include:
- a multidisciplinary team, including a medication prescriber
- a shared caseload among team members
- direct service provision by team members
- frequent patient contact
- low patient to staff ratios
- outreach to patients in the community.
Medical care
Patients with schizophrenia are at higher risk for several comorbid medical conditions, such as diabetes, coronary artery disease, and digestive and liver disorders, compared with individuals without schizophrenia. This risk is associated with numerous factors, including sedentary lifestyle, high rates of lifetime cigarette use (70% to 80% of schizophrenia outpatients age <67 smoke), poor self-management skills, frequent homelessness, and unhealthy diet.
Although substantial attention is devoted to the psychiatric and behavioral management of patients with schizophrenia, many barriers impede the detection and treatment of their medical conditions. Patients with schizophrenia could experience delays in diagnosing a medical disorder, leading to more acute comorbidities at the time of diagnosis and premature mortality. Studies have confirmed that cardiovascular diseases are the leading cause of premature death among psychiatric patients in the United States.14 Key risk factors include smoking, obesity, hypertension, dyslipidemia, diabetes, and lack of physical activity, all of which are more common among patients with schizophrenia compared with the general population.15 In addition, antipsychotics are associated with adverse metabolic effects.16
What are realistic treatment goals to manage residual symptoms in schizophrenia?
We believe that because remission in schizophrenia has been defined consensually, the bar for treatment expectations is set higher than it was 20 years ago. There can be patient-, family-, and system-related variables affecting the feasibility of treating residual symptoms. Providers who treat patients with schizophrenia should consider the following treatment goals:
- Prevent relapse and acute psychiatric hospitalization
- Use evidence-based strategies to minimize or monitor adverse effects
- Monitor compliance and consider use of depot antipsychotics combined with patients’ preference
- Facilitate ongoing safety assessment, including suicide risk
- Monitor negative and cognitive symptoms in addition to positive symptoms, using evidence-based management
- Encourage collaboration of care with family, caretakers, and other members of the treatment team
- Empower patients by providing psychoeducation and social skills training and assisting in their vocational rehabilitation
- Educate the patient and family about healthy lifestyle interventions and medical comorbidities common with schizophrenia
- Perform baseline screening and follow-up for early detection and treatment of medical comorbidities in patients with schizophrenia
- Improve functional status and quality of life.
In addition to meeting these treatment goals, a measurement-based method can be implemented to monitor improvement and status of the independent treatment domains. A collection of rating instruments can be found in Table 2.17-30

The clinical presentation of patients with residual symptoms of schizophrenia differs from that of other patients with schizophrenia. Our understanding of residual symptoms in schizophrenia has come a long way in the last decade; however, we are still far from pinning the complex nature of these symptoms, let alone their management. Given the risk of morbidity and disability, there clearly is a need for further investigation and investment of time and resources to support developing novel pharmacological treatment options to manage residual symptoms in patients with schizophrenia.
Because patients with residual symptoms of schizophrenia usually are older, psychiatrists should be responsible for implementing necessary screening assessments and should closely collaborate with primary care practitioners and other specialists, and when necessary, treat comorbid medical conditions. The importance of educating patients, their families, and the treatment team cannot be overlooked. Further, psychiatric treatment facilities should offer and promote healthy lifestyle interventions.
The course of chronic psychiatric conditions, such as schizophrenia, differs from chronic medical conditions, such as diabetes. Some patients with chronic psychiatric conditions achieve remission and become symptom-free, while others continue to have lingering signs of disease for life.
Residual symptoms of schizophrenia are not fully defined in the literature, which poses a challenge because they are central in the overall treatment of schizophrenia spectrum disorders.1 During this phase of schizophrenia, patients continue to have symptoms after psychosis has subsided. These patients might continue to have negative symptoms such as social and emotional withdrawal and low energy. Although frank psychotic behavior has disappeared, the patient might continue to hold strange beliefs. Pharmacotherapy is the primary treatment option for psychiatric conditions, but the psychosocial aspect may have greater importance when treating residual symptoms and patients with chronic psychiatric illness.2
A naturalistic study in Germany evaluated the occurrence and characteristics of residual symptoms in patients with schizophrenia.3 The authors used a Positive and Negative Syndrome Scale symptom severity score >1 for those purposes, which is possibly a stringent criterion to define residual symptoms. This multicenter study enrolled 399 individuals age 18 to 65 with a DSM-IV-TR diagnosis of schizophrenia, schizophreniform disorder, delusional disorder, or schizoaffective disorder.3 Of the 236 patients achieving remission at discharge, 94% had at least 1 residual symptom and 69% had at least 4 residual symptoms. Therefore, residual symptoms were highly prevalent in remitted patients. The most frequent residual symptoms were:
- blunted affect
- conceptual disorganization
- passive or apathetic social withdrawal
- emotional withdrawal
- lack of judgment and insight
- poor attention
- somatic concern
- difficulty with abstract thinking
- anxiety
- poor rapport.3
Of note, positive symptoms, such as delusions and hallucinatory behavior, were found in remitted patients at discharge (17% and 10%, respectively). The study concluded that the severity of residual symptoms was associated with relapse risk and had an overall negative impact on the outcome of patients with schizophrenia.3 The study noted that residual symptoms may be greater in number or volume than negative symptoms and questioned the origins of residual symptoms because most were present at baseline in more than two-third of patients.
Patients with residual symptoms of schizophrenia usually are older and therefore present specific management challenges for clinicians. Changes associated with aging, such as medical problems, cognitive deficits, and lack of social support, could create new care needs for this patient population. Although the biopsychosocial model used to treat chronic psychiatric conditions, especially schizophrenia, is preferred, older schizophrenia patients with residual symptoms often need more psychosocial interventions compared with young adults with schizophrenia.
Managing residual symptoms in schizophrenia
Few studies are devoted to pharmacological treatment of older adults with schizophrenia, likely because pharmacotherapy for older patients with schizophrenia can be challenging. Evidence-based treatment is based primarily on findings from younger patients who survived into later life. Clinicians often use the adage of geriatric psychiatry, “start low, go slow,” because older patients are susceptible to adverse effects associated with psychiatric medications, including cardiovascular, metabolic, anticholinergic, and extrapyramidal effects, orthostasis, sedation, falls, and neuroleptic malignant syndrome.
Older patients with schizophrenia are at an increased risk for extrapyramidal symptoms (EPS) and anticholinergic adverse effects, perhaps because of degeneration of dopaminergic and cholinergic neurons.4 Lowering the anticholinergic load by discontinuing or reducing the dosage of medications with anticholinergic properties, when possible, is a key principle when treating these patients. This tactic could help improve cognition and quality of life by decreasing the risk of other anticholinergic adverse effects, including delirium, constipation, urinary retention, and blurred vision.
Patients treated with typical antipsychotics are nearly twice as likely to develop tardive dyskinesia compared with those receiving atypical antipsychotics.5 Sedation, orthostatic hypotension, and anticholinergic effects can cause cognitive clouding, worsen cognitive impairment, and increase the risk of falls, especially in older patients.6 Clozapine and olanzapine have the strongest association with clinically significant weight gain and treatment-induced type 2 diabetes mellitus.7
The appropriate starting dosage of antipsychotics in older patients with schizophrenia is one-fourth of the starting adult dosage. Total daily maintenance dosages may be one-third to one-half of the adult dosage.6 Consensus guidelines for dosing atypical antipsychotics for older patients with schizophrenia are as shown in Table 1.8
To ensure safety, patients should be regularly monitored with a complete blood count, comprehensive metabolic panel, lipid panel, hemoglobin A1C, electrocardiogram, orthostatic vital signs, Abnormal Involuntary Movement Scale, and weight check.7,9
When negative symptoms remain after a patient has achieved remission, it is important to evaluate whether the symptoms are related to adverse effects of medication (eg, parkinsonism syndrome), untreated depressive symptoms, or persistent positive symptoms, such as paranoia. Management of these symptoms consists of treating the cause, for example, using antipsychotics for primary positive symptoms, antidepressants for depression, anxiolytics for anxiety, and anti-parkinsonian agents or antipsychotic dosage reduction for EPS.
It is important to differentiate between negative symptoms of schizophrenia and depression in these patients. Negative symptoms of schizophrenia include affective flattening, alogia, avolition, and anhedonia. In depression, patients could have depressed mood, cognitive problems, sleep disturbances, and loss of appetite. Also, long-term symptoms are more consistent with negative symptomatology.
Keep in mind the potential for pharmacokinetic drug–drug interaction when using a combination of selective serotonin reuptake inhibitors (SSRIs), such as fluoxetine, paroxetine, and fluvoxamine (to treat negative/depressive symptoms), because all are significant inhibitors of cytochrome P450 enzymes and increase antipsychotic plasma level. The Expert Treatment Guidelines for Patients with Schizophrenia recommends SSRIs, followed by venlafaxine then bupropion to treat depressive symptoms after optimizing second-generation antipsychotics.9
Another point to consider when treating residual symptoms in patients with schizophrenia is to not discontinue antipsychotic medications. Relapse rates for these patients can occur up to 5 times higher than for those who continue treatment.10 A way to address this problem could be the use of depot antipsychotic medications, but there are no set recommendations for the use of long-acting injectable antipsychotics in older patients. These medications should be used with caution and at lowest effective dosages to offset potential adverse effects.
With the introduction of typical and atypical antipsychotics, the use of electroconvulsive therapy in older patients with schizophrenia has declined. In a 2009 meta-analysis of studies that included patients with refractory schizophrenia and repetitive transcranial magnetic stimulation (rTMS), results revealed a mixed effect size for controlled and uncontrolled studies. The authors stated the need for further controlled trials, assessing the efficacy of rTMS on negative and positive symptoms of schizophrenia.11
Psychotherapy and psychosocial interventions
Patients with schizophrenia who have persistent psychotic symptoms while receiving adequate pharmacotherapy should be offered adjunctive cognitive, behaviorally oriented psychotherapy to reduce symptom severity. Cognitive-behavioral therapy (CBT) has been shown to help reduce relapse rates, reduce psychotic symptoms, and improve patients’ mental state.12 Amotivation and lack of insight can be particularly troublesome, which CBT can help address.12 Psychoeducation can:
- empower patients to understand their illness
- help them cope with their disease
- be aware of symptom relapse
- seek help sooner rather than later.
Also, counseling and supportive therapy are recommended by the American Psychiatric Association guidelines. Providers should involve family and loved ones in this discussion, so that they can help collaborate with care and provide a supportive and non-judgmental environment.
Older patients with residual symptoms of schizophrenia are less likely to have completed their education, pursued a career, or developed long-lasting relationships. Family members who were their support system earlier in life, such as parents, often are unable to provide care for them by the time patients with schizophrenia become older. These patients also are less likely to get married or have children, meaning that they are more likely to live alone. The advent of the interdisciplinary team, integration of several therapeutic modalities, the provision of case managers, and assertive community treatment (ACT) teams has provided help with social support, relapses, and hospitalizations, for older patients with schizophrenia.13 Key elements of ACT include:
- a multidisciplinary team, including a medication prescriber
- a shared caseload among team members
- direct service provision by team members
- frequent patient contact
- low patient to staff ratios
- outreach to patients in the community.
Medical care
Patients with schizophrenia are at higher risk for several comorbid medical conditions, such as diabetes, coronary artery disease, and digestive and liver disorders, compared with individuals without schizophrenia. This risk is associated with numerous factors, including sedentary lifestyle, high rates of lifetime cigarette use (70% to 80% of schizophrenia outpatients age <67 smoke), poor self-management skills, frequent homelessness, and unhealthy diet.
Although substantial attention is devoted to the psychiatric and behavioral management of patients with schizophrenia, many barriers impede the detection and treatment of their medical conditions. Patients with schizophrenia could experience delays in diagnosing a medical disorder, leading to more acute comorbidities at the time of diagnosis and premature mortality. Studies have confirmed that cardiovascular diseases are the leading cause of premature death among psychiatric patients in the United States.14 Key risk factors include smoking, obesity, hypertension, dyslipidemia, diabetes, and lack of physical activity, all of which are more common among patients with schizophrenia compared with the general population.15 In addition, antipsychotics are associated with adverse metabolic effects.16
What are realistic treatment goals to manage residual symptoms in schizophrenia?
We believe that because remission in schizophrenia has been defined consensually, the bar for treatment expectations is set higher than it was 20 years ago. There can be patient-, family-, and system-related variables affecting the feasibility of treating residual symptoms. Providers who treat patients with schizophrenia should consider the following treatment goals:
- Prevent relapse and acute psychiatric hospitalization
- Use evidence-based strategies to minimize or monitor adverse effects
- Monitor compliance and consider use of depot antipsychotics combined with patients’ preference
- Facilitate ongoing safety assessment, including suicide risk
- Monitor negative and cognitive symptoms in addition to positive symptoms, using evidence-based management
- Encourage collaboration of care with family, caretakers, and other members of the treatment team
- Empower patients by providing psychoeducation and social skills training and assisting in their vocational rehabilitation
- Educate the patient and family about healthy lifestyle interventions and medical comorbidities common with schizophrenia
- Perform baseline screening and follow-up for early detection and treatment of medical comorbidities in patients with schizophrenia
- Improve functional status and quality of life.
In addition to meeting these treatment goals, a measurement-based method can be implemented to monitor improvement and status of the independent treatment domains. A collection of rating instruments can be found in Table 2.17-30

The clinical presentation of patients with residual symptoms of schizophrenia differs from that of other patients with schizophrenia. Our understanding of residual symptoms in schizophrenia has come a long way in the last decade; however, we are still far from pinning the complex nature of these symptoms, let alone their management. Given the risk of morbidity and disability, there clearly is a need for further investigation and investment of time and resources to support developing novel pharmacological treatment options to manage residual symptoms in patients with schizophrenia.
Because patients with residual symptoms of schizophrenia usually are older, psychiatrists should be responsible for implementing necessary screening assessments and should closely collaborate with primary care practitioners and other specialists, and when necessary, treat comorbid medical conditions. The importance of educating patients, their families, and the treatment team cannot be overlooked. Further, psychiatric treatment facilities should offer and promote healthy lifestyle interventions.
1. Kaiser S, Lyne J, Agartz I, et al. Individual negative symptoms and domains - relevance for assessment, pathomechanisms and treatment [published online July 21, 2016]. Schizophr Res. doi:10.1016/j.schres.2016.07.013.
2. Taylor M, Chaudhry I, Cross M, et al. Towards consensus in the long-term management of relapse prevention in schizophrenia. Hum Psychopharmacol. 2005;20(3):175-181.
3. Schennach R, Riedel M, Obermeier M, et al. What are residual symptoms in schizophrenia spectrum disorder? Clinical description and 1-year persistence within a naturalistic trial. Eur Arch Psychiatry Clin Neurosci. 2015;265(2):107-116.
4. Caligiuri MP, Jeste DV, Lacro JP. Antipsychotic-induced movement disorders in the elderly: epidemiology and treatment recommendations. Drugs Aging. 2000;17(5):363-384.
5. Dolder CR, Jeste DV. Incidence of tardive dyskinesia with typical versus atypical antipsychotics in very high risk patients. Biol Psychiatry. 2003;53(12):1142-1145.
6. Sable JA, Jeste DV. Antipsychotic treatment for late-life schizophrenia. Curr Psychiatry Rep. 2002;4(4):299-306.
7. Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs. 2005;19(suppl 1):1-93.
8. Khan AY, Redden W, Ovais M, et al. Current concepts in the diagnosis and treatment of schizophrenia in later life. Current Geriatric Reports. 2015;4(4):290-300.
9. Alexopoulos GS, Streim J, Carpenter D, et al; Expert Consensus Panel for Using Antipsychotic Drugs in Older Patients. Using antipsychotic agents in older patients. J Clin Psychiatry. 2004;65(suppl 2):5-99; discussion 100-102; quiz 103-104.
10. Robinson D, Woerner MG, Alvir JM, et al. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry. 1999;56(3):241-247.
11. Freitas C, Fregni F, Pascual-Leone A. Meta-analysis of the effects of repetitive transcranial magnetic stimulation (rTMS) on negative and positive symptoms in schizophrenia. Schizophr Res. 2009;108(1-3):11-24.
12. Rector NA, Beck AT. Cognitive behavioral therapy for schizophrenia: an empirical review. J Nerv Ment Dis. 2012;200(10):832-839.
13. Stobbe J, Mulder NC, Roosenschoon BJ, et al. Assertive community treatment for elderly people with severe mental illness. BMC Psychiatry. 2010;10:84.
14. Hennekens CH, Hennekens AR, Hollar D, et al. Schizophrenia and increased risks of cardiovascular disease. Am Heart J. 2005;150(6):1115-1121.
15. Bushe CJ, Taylor M, Haukka J. Mortality in schizophrenia: a measurable clinical point. J Psychopharmacol. 2010;24(suppl 4):17-25.
16. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia, and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
17. Nasrallah HA, Targum SD, Tandon R, et al. Defining and measuring clinical effectiveness in the treatment of schizophrenia. Psychiatr Serv. 2005;56(3):273-282.
18. Overall JE, Gorham DR. The Brief Psychiatric Rating Scale (BPRS): recent developments in ascertainment and scaling. Psychopharmacol Bull. 1988;24:97-99.
19. Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276.
20. Addington D, Addington J, Schissel B. A depression rating scale for schizophrenics. Schizophr Res. 1990;3(4):247-251.
21. Guy W. ECDEU Assessment manual for psychopharmacology revised, 1976. Rockville, MD: US Department of Health, Education, and Welfare; Public Health Service; Alcohol, Drug Abuse, and Mental Health Administration; National Institute of Mental Health Psychopharmacology Research Branch, Division of Extramural Research Programs; 1976.
22. Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154:672-676.
23. Simpson GM, Angus JWS. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand. 1970;45(212):11-19.
24. Dott SG, Weiden P, Hopwood P, et al. An innovative approach to clinical communication in schizophrenia: the Approaches to Schizophrenia Communication checklists. CNS Spectr. 2001;6(4):333-338.
25. Dott SG, Knesevich J, Miller A, et al. Using the ASC program: a training guide. J Psychiatr Pract. 2001;7(1):64-68.
26. Barker S, Barron N, McFarland BH, et al. Multnomah Community Ability Scale: user’s manual. Portland, OR: Western Mental Health Research Center, Oregon Health Sciences University; 1994.
27. Lehman AF. A quality of life interview for the chronically mentally ill. Eval Program Plann. 1988;11(1):51-62.
28. Heinrichs DW, Hanlon TE, Carpenter WT Jr. The Quality of Life Scale: an instrument for rating the schizophrenic deficit syndrome. Schizophr Bull. 1984;10(3):388-398.
29. Becker M, Diamond R, Sainfort F. A new patient focused index for measuring quality of life in persons with severe and persistent mental illness. Qual Life Res. 1993;2(4):239-251.
30. Liberman RP, Kopelowicz A, Ventura J, et al. Operational criteria and factors related to recovery from schizophrenia. Int Rev Psychiatry. 2009;14(4):256-272.
1. Kaiser S, Lyne J, Agartz I, et al. Individual negative symptoms and domains - relevance for assessment, pathomechanisms and treatment [published online July 21, 2016]. Schizophr Res. doi:10.1016/j.schres.2016.07.013.
2. Taylor M, Chaudhry I, Cross M, et al. Towards consensus in the long-term management of relapse prevention in schizophrenia. Hum Psychopharmacol. 2005;20(3):175-181.
3. Schennach R, Riedel M, Obermeier M, et al. What are residual symptoms in schizophrenia spectrum disorder? Clinical description and 1-year persistence within a naturalistic trial. Eur Arch Psychiatry Clin Neurosci. 2015;265(2):107-116.
4. Caligiuri MP, Jeste DV, Lacro JP. Antipsychotic-induced movement disorders in the elderly: epidemiology and treatment recommendations. Drugs Aging. 2000;17(5):363-384.
5. Dolder CR, Jeste DV. Incidence of tardive dyskinesia with typical versus atypical antipsychotics in very high risk patients. Biol Psychiatry. 2003;53(12):1142-1145.
6. Sable JA, Jeste DV. Antipsychotic treatment for late-life schizophrenia. Curr Psychiatry Rep. 2002;4(4):299-306.
7. Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs. 2005;19(suppl 1):1-93.
8. Khan AY, Redden W, Ovais M, et al. Current concepts in the diagnosis and treatment of schizophrenia in later life. Current Geriatric Reports. 2015;4(4):290-300.
9. Alexopoulos GS, Streim J, Carpenter D, et al; Expert Consensus Panel for Using Antipsychotic Drugs in Older Patients. Using antipsychotic agents in older patients. J Clin Psychiatry. 2004;65(suppl 2):5-99; discussion 100-102; quiz 103-104.
10. Robinson D, Woerner MG, Alvir JM, et al. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry. 1999;56(3):241-247.
11. Freitas C, Fregni F, Pascual-Leone A. Meta-analysis of the effects of repetitive transcranial magnetic stimulation (rTMS) on negative and positive symptoms in schizophrenia. Schizophr Res. 2009;108(1-3):11-24.
12. Rector NA, Beck AT. Cognitive behavioral therapy for schizophrenia: an empirical review. J Nerv Ment Dis. 2012;200(10):832-839.
13. Stobbe J, Mulder NC, Roosenschoon BJ, et al. Assertive community treatment for elderly people with severe mental illness. BMC Psychiatry. 2010;10:84.
14. Hennekens CH, Hennekens AR, Hollar D, et al. Schizophrenia and increased risks of cardiovascular disease. Am Heart J. 2005;150(6):1115-1121.
15. Bushe CJ, Taylor M, Haukka J. Mortality in schizophrenia: a measurable clinical point. J Psychopharmacol. 2010;24(suppl 4):17-25.
16. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia, and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
17. Nasrallah HA, Targum SD, Tandon R, et al. Defining and measuring clinical effectiveness in the treatment of schizophrenia. Psychiatr Serv. 2005;56(3):273-282.
18. Overall JE, Gorham DR. The Brief Psychiatric Rating Scale (BPRS): recent developments in ascertainment and scaling. Psychopharmacol Bull. 1988;24:97-99.
19. Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276.
20. Addington D, Addington J, Schissel B. A depression rating scale for schizophrenics. Schizophr Res. 1990;3(4):247-251.
21. Guy W. ECDEU Assessment manual for psychopharmacology revised, 1976. Rockville, MD: US Department of Health, Education, and Welfare; Public Health Service; Alcohol, Drug Abuse, and Mental Health Administration; National Institute of Mental Health Psychopharmacology Research Branch, Division of Extramural Research Programs; 1976.
22. Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154:672-676.
23. Simpson GM, Angus JWS. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand. 1970;45(212):11-19.
24. Dott SG, Weiden P, Hopwood P, et al. An innovative approach to clinical communication in schizophrenia: the Approaches to Schizophrenia Communication checklists. CNS Spectr. 2001;6(4):333-338.
25. Dott SG, Knesevich J, Miller A, et al. Using the ASC program: a training guide. J Psychiatr Pract. 2001;7(1):64-68.
26. Barker S, Barron N, McFarland BH, et al. Multnomah Community Ability Scale: user’s manual. Portland, OR: Western Mental Health Research Center, Oregon Health Sciences University; 1994.
27. Lehman AF. A quality of life interview for the chronically mentally ill. Eval Program Plann. 1988;11(1):51-62.
28. Heinrichs DW, Hanlon TE, Carpenter WT Jr. The Quality of Life Scale: an instrument for rating the schizophrenic deficit syndrome. Schizophr Bull. 1984;10(3):388-398.
29. Becker M, Diamond R, Sainfort F. A new patient focused index for measuring quality of life in persons with severe and persistent mental illness. Qual Life Res. 1993;2(4):239-251.
30. Liberman RP, Kopelowicz A, Ventura J, et al. Operational criteria and factors related to recovery from schizophrenia. Int Rev Psychiatry. 2009;14(4):256-272.
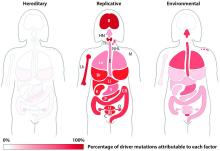
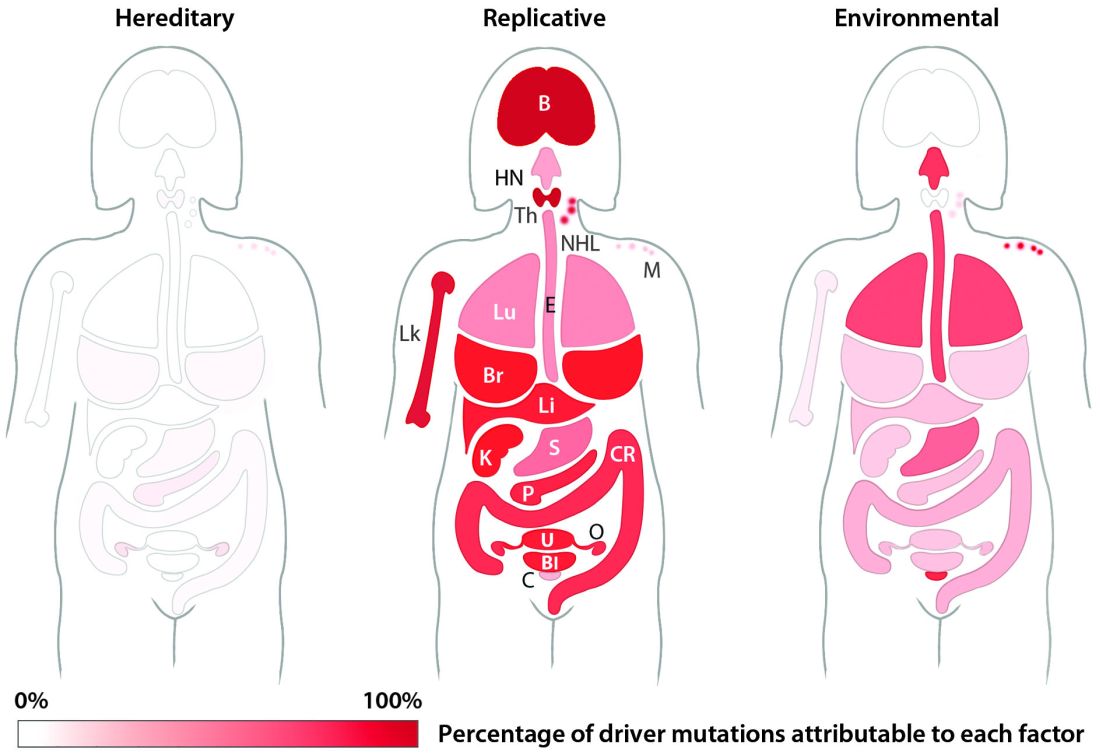
Most blood cancer mutations due to DNA replication errors
A new study supports the idea that most cancer-driving mutations are a result of DNA replication errors, not heredity or lifestyle/environmental factors.
For all 32 cancer types studied, researchers found that 66% of driver mutations resulted from DNA replication errors, 29% could be attributed to lifestyle or environmental factors, and the remaining 5% were inherited.
In hematologic malignancies, the percentage of mutations caused by DNA replication errors was even higher—70% in Hodgkin lymphoma, 85% in leukemias, 96% in non-Hodgkin lymphomas, and 99% in myeloma.
Cristian Tomasetti, PhD, of Johns Hopkins University School of Medicine in Baltimore, Maryland, and his colleagues reported these findings in Science.
“It is well-known that we must avoid environmental factors such as smoking to decrease our risk of getting cancer, but it is not as well-known that each time a normal cell divides and copies its DNA to produce 2 new cells, it makes multiple mistakes,” Dr Tomasetti said.
“These copying mistakes are a potent source of cancer mutations that, historically, have been scientifically undervalued, and this new work provides the first estimate of the fraction of mutations caused by these mistakes.”
In 2015, Dr Tomasetti and his colleagues reported that DNA replication errors could explain why certain cancers occur more often than others in the US.
The current study builds upon that research but includes additional cancers and encompasses an international population.
The researchers first studied the relationship between the number of normal stem cell divisions and the risk of 17 cancer types in 69 countries representing 4.8 billion people, or more than half of the world’s population.
The team said they observed a strong correlation between cancer incidence and normal stem cell divisions in all countries, regardless of their environment.
Next, the researchers set out to determine the percentage of driver mutations caused by DNA replication errors in 32 cancer types. The team developed a mathematical model using DNA sequencing data from The Cancer Genome Atlas and epidemiologic data from the Cancer Research UK database.
According to the researchers, it generally takes 2 or more critical mutations for cancer to occur. In an individual, these mutations can be due to random DNA replication errors, the environment, or inherited genes.
Knowing this, the researchers used their mathematical model to show, for example, that when critical mutations in leukemia are added together, 85.2% of them are due to random DNA replication errors, 14.3% to environmental factors, and 0.5% to heredity.
In Hodgkin lymphoma, 69.5% are due to DNA replication errors, 30% to environmental factors, and 0.5% to heredity. In non-Hodgkin lymphoma, 95.6% are due to random DNA replication errors, 3.9% to environmental factors, and 0.5% to heredity.
In myeloma, 99.3% are due to DNA replication errors, 0.2% to environmental factors, and 0.5% to heredity.
Dr Tomasetti said these random DNA replication errors will only get more important as aging populations continue to grow, prolonging the opportunity for cells to make more and more errors.
“We need to continue to encourage people to avoid environmental agents and lifestyles that increase their risk of developing cancer mutations,” said study author Bert Vogelstein, MD, of The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University.
“However, many people will still develop cancers due to these random DNA copying errors, and better methods to detect all cancers earlier, while they are still curable, are urgently needed.” ![]()
A new study supports the idea that most cancer-driving mutations are a result of DNA replication errors, not heredity or lifestyle/environmental factors.
For all 32 cancer types studied, researchers found that 66% of driver mutations resulted from DNA replication errors, 29% could be attributed to lifestyle or environmental factors, and the remaining 5% were inherited.
In hematologic malignancies, the percentage of mutations caused by DNA replication errors was even higher—70% in Hodgkin lymphoma, 85% in leukemias, 96% in non-Hodgkin lymphomas, and 99% in myeloma.
Cristian Tomasetti, PhD, of Johns Hopkins University School of Medicine in Baltimore, Maryland, and his colleagues reported these findings in Science.
“It is well-known that we must avoid environmental factors such as smoking to decrease our risk of getting cancer, but it is not as well-known that each time a normal cell divides and copies its DNA to produce 2 new cells, it makes multiple mistakes,” Dr Tomasetti said.
“These copying mistakes are a potent source of cancer mutations that, historically, have been scientifically undervalued, and this new work provides the first estimate of the fraction of mutations caused by these mistakes.”
In 2015, Dr Tomasetti and his colleagues reported that DNA replication errors could explain why certain cancers occur more often than others in the US.
The current study builds upon that research but includes additional cancers and encompasses an international population.
The researchers first studied the relationship between the number of normal stem cell divisions and the risk of 17 cancer types in 69 countries representing 4.8 billion people, or more than half of the world’s population.
The team said they observed a strong correlation between cancer incidence and normal stem cell divisions in all countries, regardless of their environment.
Next, the researchers set out to determine the percentage of driver mutations caused by DNA replication errors in 32 cancer types. The team developed a mathematical model using DNA sequencing data from The Cancer Genome Atlas and epidemiologic data from the Cancer Research UK database.
According to the researchers, it generally takes 2 or more critical mutations for cancer to occur. In an individual, these mutations can be due to random DNA replication errors, the environment, or inherited genes.
Knowing this, the researchers used their mathematical model to show, for example, that when critical mutations in leukemia are added together, 85.2% of them are due to random DNA replication errors, 14.3% to environmental factors, and 0.5% to heredity.
In Hodgkin lymphoma, 69.5% are due to DNA replication errors, 30% to environmental factors, and 0.5% to heredity. In non-Hodgkin lymphoma, 95.6% are due to random DNA replication errors, 3.9% to environmental factors, and 0.5% to heredity.
In myeloma, 99.3% are due to DNA replication errors, 0.2% to environmental factors, and 0.5% to heredity.
Dr Tomasetti said these random DNA replication errors will only get more important as aging populations continue to grow, prolonging the opportunity for cells to make more and more errors.
“We need to continue to encourage people to avoid environmental agents and lifestyles that increase their risk of developing cancer mutations,” said study author Bert Vogelstein, MD, of The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University.
“However, many people will still develop cancers due to these random DNA copying errors, and better methods to detect all cancers earlier, while they are still curable, are urgently needed.” ![]()
A new study supports the idea that most cancer-driving mutations are a result of DNA replication errors, not heredity or lifestyle/environmental factors.
For all 32 cancer types studied, researchers found that 66% of driver mutations resulted from DNA replication errors, 29% could be attributed to lifestyle or environmental factors, and the remaining 5% were inherited.
In hematologic malignancies, the percentage of mutations caused by DNA replication errors was even higher—70% in Hodgkin lymphoma, 85% in leukemias, 96% in non-Hodgkin lymphomas, and 99% in myeloma.
Cristian Tomasetti, PhD, of Johns Hopkins University School of Medicine in Baltimore, Maryland, and his colleagues reported these findings in Science.
“It is well-known that we must avoid environmental factors such as smoking to decrease our risk of getting cancer, but it is not as well-known that each time a normal cell divides and copies its DNA to produce 2 new cells, it makes multiple mistakes,” Dr Tomasetti said.
“These copying mistakes are a potent source of cancer mutations that, historically, have been scientifically undervalued, and this new work provides the first estimate of the fraction of mutations caused by these mistakes.”
In 2015, Dr Tomasetti and his colleagues reported that DNA replication errors could explain why certain cancers occur more often than others in the US.
The current study builds upon that research but includes additional cancers and encompasses an international population.
The researchers first studied the relationship between the number of normal stem cell divisions and the risk of 17 cancer types in 69 countries representing 4.8 billion people, or more than half of the world’s population.
The team said they observed a strong correlation between cancer incidence and normal stem cell divisions in all countries, regardless of their environment.
Next, the researchers set out to determine the percentage of driver mutations caused by DNA replication errors in 32 cancer types. The team developed a mathematical model using DNA sequencing data from The Cancer Genome Atlas and epidemiologic data from the Cancer Research UK database.
According to the researchers, it generally takes 2 or more critical mutations for cancer to occur. In an individual, these mutations can be due to random DNA replication errors, the environment, or inherited genes.
Knowing this, the researchers used their mathematical model to show, for example, that when critical mutations in leukemia are added together, 85.2% of them are due to random DNA replication errors, 14.3% to environmental factors, and 0.5% to heredity.
In Hodgkin lymphoma, 69.5% are due to DNA replication errors, 30% to environmental factors, and 0.5% to heredity. In non-Hodgkin lymphoma, 95.6% are due to random DNA replication errors, 3.9% to environmental factors, and 0.5% to heredity.
In myeloma, 99.3% are due to DNA replication errors, 0.2% to environmental factors, and 0.5% to heredity.
Dr Tomasetti said these random DNA replication errors will only get more important as aging populations continue to grow, prolonging the opportunity for cells to make more and more errors.
“We need to continue to encourage people to avoid environmental agents and lifestyles that increase their risk of developing cancer mutations,” said study author Bert Vogelstein, MD, of The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University.
“However, many people will still develop cancers due to these random DNA copying errors, and better methods to detect all cancers earlier, while they are still curable, are urgently needed.” ![]()
Preterm births more common in cancer survivors
Women diagnosed with cancer during their childbearing years have an increased risk of preterm births, according to research published in JAMA Oncology.
The study showed that cancer survivors were more likely than women who never had cancer to give birth prematurely, have underweight babies, and undergo cesarean section deliveries.
The researchers said women diagnosed with cancer during pregnancy may be delivering early in order to start their cancer treatment, but that does not fully explain these findings.
The team also detected an increased risk of preterm delivery in women who had already received cancer treatment.
“We found that women were more likely to deliver preterm if they’ve been treated for cancer overall, with greater risks for women who had chemotherapy,” said study author Hazel B. Nichols, PhD, of University of North Carolina Lineberger Comprehensive Cancer Center in Chapel Hill.
“While we believe these findings are something women should be aware of, we still have a lot of work to do to understand why this risk is becoming apparent and whether or not the children who are born preterm to these women go on to develop any health concerns.”
Dr Nichols and her colleagues analyzed data on 2598 births to female adolescent and young adult cancer survivors (ages 15 to 39) and 12,990 births to women without a cancer diagnosis.
Among cancer survivors, there was a significantly increased prevalence of preterm birth (prevalence ratio [PR]=1.52), low birth weight (PR=1.59), and cesarean delivery (PR=1.08), compared to women without a cancer diagnosis.
Timing of diagnosis and cancer type
When the researchers broke the data down by cancer diagnosis, they found a higher risk of preterm birth and low birth weight for women with lymphoma as well as breast and gynecologic cancers.
The PR for preterm birth was 1.59 for Hodgkin lymphoma, 1.98 for breast cancer, 2.11 for non-Hodgkin lymphoma, and 2.58 for gynecologic cancer. The PR for low birth weight was 1.59 for breast cancer, 2.41 for non-Hodgkin lymphoma, and 2.74 for gynecologic cancer.
The researchers found an increased risk of adverse birth outcomes among women who were diagnosed with cancer while pregnant and before pregnancy.
Among women diagnosed while pregnant, the PR was 2.97 for preterm birth, 2.82 for low birth weight, 1.21 for cesarean delivery, and 1.90 for low Apgar score. Among women diagnosed before pregnancy, the PR was 1.23 for preterm birth and 1.36 for low birth weight.
Role of treatment
Compared to women without a cancer diagnosis, cancer survivors who received chemotherapy but no radiation were more likely to have preterm births (PR=2.11), infants with low birth weight (PR=2.36), and cesarean deliveries (PR=1.16).
There was no significant increase in adverse birth outcomes among cancer survivors who received radiation but not chemotherapy.
Among the cancer survivors, women who received chemotherapy without radiation were more likely to have preterm births (PR=2.12), infants with low birth weight (PR=2.13), and infants who were small for their gestational age (PR=1.43) when compared to women treated with surgery only.
Dr Nichols said the role of treatment is an area of possible future research.
“We’d like to get better information about the types of chemotherapy women receive,” she said. “Chemotherapy is a very broad category, and the agents have very different effects on the body. In the future, we’d like to get more detailed information on the types of drugs that were involved in treatment.” ![]()
Women diagnosed with cancer during their childbearing years have an increased risk of preterm births, according to research published in JAMA Oncology.
The study showed that cancer survivors were more likely than women who never had cancer to give birth prematurely, have underweight babies, and undergo cesarean section deliveries.
The researchers said women diagnosed with cancer during pregnancy may be delivering early in order to start their cancer treatment, but that does not fully explain these findings.
The team also detected an increased risk of preterm delivery in women who had already received cancer treatment.
“We found that women were more likely to deliver preterm if they’ve been treated for cancer overall, with greater risks for women who had chemotherapy,” said study author Hazel B. Nichols, PhD, of University of North Carolina Lineberger Comprehensive Cancer Center in Chapel Hill.
“While we believe these findings are something women should be aware of, we still have a lot of work to do to understand why this risk is becoming apparent and whether or not the children who are born preterm to these women go on to develop any health concerns.”
Dr Nichols and her colleagues analyzed data on 2598 births to female adolescent and young adult cancer survivors (ages 15 to 39) and 12,990 births to women without a cancer diagnosis.
Among cancer survivors, there was a significantly increased prevalence of preterm birth (prevalence ratio [PR]=1.52), low birth weight (PR=1.59), and cesarean delivery (PR=1.08), compared to women without a cancer diagnosis.
Timing of diagnosis and cancer type
When the researchers broke the data down by cancer diagnosis, they found a higher risk of preterm birth and low birth weight for women with lymphoma as well as breast and gynecologic cancers.
The PR for preterm birth was 1.59 for Hodgkin lymphoma, 1.98 for breast cancer, 2.11 for non-Hodgkin lymphoma, and 2.58 for gynecologic cancer. The PR for low birth weight was 1.59 for breast cancer, 2.41 for non-Hodgkin lymphoma, and 2.74 for gynecologic cancer.
The researchers found an increased risk of adverse birth outcomes among women who were diagnosed with cancer while pregnant and before pregnancy.
Among women diagnosed while pregnant, the PR was 2.97 for preterm birth, 2.82 for low birth weight, 1.21 for cesarean delivery, and 1.90 for low Apgar score. Among women diagnosed before pregnancy, the PR was 1.23 for preterm birth and 1.36 for low birth weight.
Role of treatment
Compared to women without a cancer diagnosis, cancer survivors who received chemotherapy but no radiation were more likely to have preterm births (PR=2.11), infants with low birth weight (PR=2.36), and cesarean deliveries (PR=1.16).
There was no significant increase in adverse birth outcomes among cancer survivors who received radiation but not chemotherapy.
Among the cancer survivors, women who received chemotherapy without radiation were more likely to have preterm births (PR=2.12), infants with low birth weight (PR=2.13), and infants who were small for their gestational age (PR=1.43) when compared to women treated with surgery only.
Dr Nichols said the role of treatment is an area of possible future research.
“We’d like to get better information about the types of chemotherapy women receive,” she said. “Chemotherapy is a very broad category, and the agents have very different effects on the body. In the future, we’d like to get more detailed information on the types of drugs that were involved in treatment.” ![]()
Women diagnosed with cancer during their childbearing years have an increased risk of preterm births, according to research published in JAMA Oncology.
The study showed that cancer survivors were more likely than women who never had cancer to give birth prematurely, have underweight babies, and undergo cesarean section deliveries.
The researchers said women diagnosed with cancer during pregnancy may be delivering early in order to start their cancer treatment, but that does not fully explain these findings.
The team also detected an increased risk of preterm delivery in women who had already received cancer treatment.
“We found that women were more likely to deliver preterm if they’ve been treated for cancer overall, with greater risks for women who had chemotherapy,” said study author Hazel B. Nichols, PhD, of University of North Carolina Lineberger Comprehensive Cancer Center in Chapel Hill.
“While we believe these findings are something women should be aware of, we still have a lot of work to do to understand why this risk is becoming apparent and whether or not the children who are born preterm to these women go on to develop any health concerns.”
Dr Nichols and her colleagues analyzed data on 2598 births to female adolescent and young adult cancer survivors (ages 15 to 39) and 12,990 births to women without a cancer diagnosis.
Among cancer survivors, there was a significantly increased prevalence of preterm birth (prevalence ratio [PR]=1.52), low birth weight (PR=1.59), and cesarean delivery (PR=1.08), compared to women without a cancer diagnosis.
Timing of diagnosis and cancer type
When the researchers broke the data down by cancer diagnosis, they found a higher risk of preterm birth and low birth weight for women with lymphoma as well as breast and gynecologic cancers.
The PR for preterm birth was 1.59 for Hodgkin lymphoma, 1.98 for breast cancer, 2.11 for non-Hodgkin lymphoma, and 2.58 for gynecologic cancer. The PR for low birth weight was 1.59 for breast cancer, 2.41 for non-Hodgkin lymphoma, and 2.74 for gynecologic cancer.
The researchers found an increased risk of adverse birth outcomes among women who were diagnosed with cancer while pregnant and before pregnancy.
Among women diagnosed while pregnant, the PR was 2.97 for preterm birth, 2.82 for low birth weight, 1.21 for cesarean delivery, and 1.90 for low Apgar score. Among women diagnosed before pregnancy, the PR was 1.23 for preterm birth and 1.36 for low birth weight.
Role of treatment
Compared to women without a cancer diagnosis, cancer survivors who received chemotherapy but no radiation were more likely to have preterm births (PR=2.11), infants with low birth weight (PR=2.36), and cesarean deliveries (PR=1.16).
There was no significant increase in adverse birth outcomes among cancer survivors who received radiation but not chemotherapy.
Among the cancer survivors, women who received chemotherapy without radiation were more likely to have preterm births (PR=2.12), infants with low birth weight (PR=2.13), and infants who were small for their gestational age (PR=1.43) when compared to women treated with surgery only.
Dr Nichols said the role of treatment is an area of possible future research.
“We’d like to get better information about the types of chemotherapy women receive,” she said. “Chemotherapy is a very broad category, and the agents have very different effects on the body. In the future, we’d like to get more detailed information on the types of drugs that were involved in treatment.” ![]()
ASCO reports progress, challenges in cancer care
The US cancer care delivery system is undergoing changes to better meet the needs of cancer patients, but persistent hurdles threaten to slow progress, according to the American Society of Clinical Oncology (ASCO).
ASCO’s “The State of Cancer Care in America, 2017” report describes areas of progress, including new approaches for cancer diagnosis and treatment, improved data sharing to drive innovation, and an increased focus on value-based healthcare.
However, the report also suggests that access and affordability challenges, along with increased practice burdens, continue to pose barriers to high-value, high-quality cancer care.
The report was published in the Journal of Oncology Practice.
Challenges
The report notes that the US population is growing rapidly, changing demographically, and living longer. And all of these factors contribute to a record number of cancer cases/survivors.
It has been estimated that the number of cancer survivors in the US will grow from 15.5 million to 20.3 million by 2026.
Unfortunately, the report says, cancer care is unaffordable for many patients, even those with health insurance.
And significant health disparities persist that are independent of insurance status. Socioeconomic status, geography, and race/ethnicity all impact patient health outcomes.
The report also suggests that oncology practices are facing increased administrative burdens that divert time and resources from their patients.
Progress
Despite the aforementioned challenges, the report paints an optimistic vision about the future of cancer care and highlights activity in the past year aimed at improving care.
For instance, the Food and Drug Administration approved 5 new anticancer therapies, expanded the use of 13, and approved several diagnostic tests in 2016.
In addition, overall cancer incidence and mortality rates were lower in 2016 than in previous decades.
“Since 1991, we’ve been able to save 2.1 million lives because of significant advances in prevention, diagnosis, and treatment—something unimaginable even a decade ago,” said ASCO President Daniel F. Hayes, MD.
“But there’s still more work to be done to ensure that every patient with cancer, no matter who they are or where they live, has access to high-quality, high-value cancer care.”
The report includes a list of recommendations that, ASCO believes, could help bring the US closer to achieving that goal. ![]()
The US cancer care delivery system is undergoing changes to better meet the needs of cancer patients, but persistent hurdles threaten to slow progress, according to the American Society of Clinical Oncology (ASCO).
ASCO’s “The State of Cancer Care in America, 2017” report describes areas of progress, including new approaches for cancer diagnosis and treatment, improved data sharing to drive innovation, and an increased focus on value-based healthcare.
However, the report also suggests that access and affordability challenges, along with increased practice burdens, continue to pose barriers to high-value, high-quality cancer care.
The report was published in the Journal of Oncology Practice.
Challenges
The report notes that the US population is growing rapidly, changing demographically, and living longer. And all of these factors contribute to a record number of cancer cases/survivors.
It has been estimated that the number of cancer survivors in the US will grow from 15.5 million to 20.3 million by 2026.
Unfortunately, the report says, cancer care is unaffordable for many patients, even those with health insurance.
And significant health disparities persist that are independent of insurance status. Socioeconomic status, geography, and race/ethnicity all impact patient health outcomes.
The report also suggests that oncology practices are facing increased administrative burdens that divert time and resources from their patients.
Progress
Despite the aforementioned challenges, the report paints an optimistic vision about the future of cancer care and highlights activity in the past year aimed at improving care.
For instance, the Food and Drug Administration approved 5 new anticancer therapies, expanded the use of 13, and approved several diagnostic tests in 2016.
In addition, overall cancer incidence and mortality rates were lower in 2016 than in previous decades.
“Since 1991, we’ve been able to save 2.1 million lives because of significant advances in prevention, diagnosis, and treatment—something unimaginable even a decade ago,” said ASCO President Daniel F. Hayes, MD.
“But there’s still more work to be done to ensure that every patient with cancer, no matter who they are or where they live, has access to high-quality, high-value cancer care.”
The report includes a list of recommendations that, ASCO believes, could help bring the US closer to achieving that goal. ![]()
The US cancer care delivery system is undergoing changes to better meet the needs of cancer patients, but persistent hurdles threaten to slow progress, according to the American Society of Clinical Oncology (ASCO).
ASCO’s “The State of Cancer Care in America, 2017” report describes areas of progress, including new approaches for cancer diagnosis and treatment, improved data sharing to drive innovation, and an increased focus on value-based healthcare.
However, the report also suggests that access and affordability challenges, along with increased practice burdens, continue to pose barriers to high-value, high-quality cancer care.
The report was published in the Journal of Oncology Practice.
Challenges
The report notes that the US population is growing rapidly, changing demographically, and living longer. And all of these factors contribute to a record number of cancer cases/survivors.
It has been estimated that the number of cancer survivors in the US will grow from 15.5 million to 20.3 million by 2026.
Unfortunately, the report says, cancer care is unaffordable for many patients, even those with health insurance.
And significant health disparities persist that are independent of insurance status. Socioeconomic status, geography, and race/ethnicity all impact patient health outcomes.
The report also suggests that oncology practices are facing increased administrative burdens that divert time and resources from their patients.
Progress
Despite the aforementioned challenges, the report paints an optimistic vision about the future of cancer care and highlights activity in the past year aimed at improving care.
For instance, the Food and Drug Administration approved 5 new anticancer therapies, expanded the use of 13, and approved several diagnostic tests in 2016.
In addition, overall cancer incidence and mortality rates were lower in 2016 than in previous decades.
“Since 1991, we’ve been able to save 2.1 million lives because of significant advances in prevention, diagnosis, and treatment—something unimaginable even a decade ago,” said ASCO President Daniel F. Hayes, MD.
“But there’s still more work to be done to ensure that every patient with cancer, no matter who they are or where they live, has access to high-quality, high-value cancer care.”
The report includes a list of recommendations that, ASCO believes, could help bring the US closer to achieving that goal. ![]()
New Onset Type 1 Diabetes: "Fear Not - Easing Your Concerns"
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
EMA recommends orphan designation for cord blood product
The European Medicines Agency’s (EMA’s) Committee for Orphan Medicinal Products has recommended that NiCord® receive orphan designation as a treatment for patients who require a hematopoietic stem cell transplant.
NiCord is a stand-alone graft derived from a single umbilical cord blood unit that has been expanded in culture and enriched with stem and progenitor cells.
NiCord already has orphan designation in the European Union as a treatment for patients with acute myeloid leukemia.
Orphan designation provides regulatory and financial incentives for companies to develop and market therapies that treat life-threatening or chronically debilitating conditions affecting no more than 5 in 10,000 people in the European Union, and where no satisfactory treatment is available.
Orphan designation provides a 10-year period of marketing exclusivity if the drug receives regulatory approval. The designation also provides incentives for companies seeking protocol assistance from the EMA during the product development phase and direct access to the centralized authorization procedure.
The EMA adopts an opinion on the granting of orphan drug designation, and that opinion is submitted to the European Commission for a final decision. The European Commission typically makes a decision within 30 days.
NiCord also has orphan designation and breakthrough designation from the US Food and Drug Administration for the treatment of hematologic malignancies.
NiCord research
Data from the pilot study of NiCord suggested the therapy can provide a clinically meaningful improvement in time to neutrophil engraftment over traditional cord blood transplant.
And research presented at EBMT 2016 showed that patients who received NiCord had fewer infections, shorter hospital stays, quicker platelet engraftment, and improved non-relapse mortality when compared to patients who received a traditional cord blood transplant.
NiCord is currently being studied in a phase 3 registration study as a graft for patients with hematologic malignancies who do not have a rapidly available, fully matched donor.
Gamida Cell, the company developing NiCord, announced last month that the first patient in the study had been transplanted. ![]()
The European Medicines Agency’s (EMA’s) Committee for Orphan Medicinal Products has recommended that NiCord® receive orphan designation as a treatment for patients who require a hematopoietic stem cell transplant.
NiCord is a stand-alone graft derived from a single umbilical cord blood unit that has been expanded in culture and enriched with stem and progenitor cells.
NiCord already has orphan designation in the European Union as a treatment for patients with acute myeloid leukemia.
Orphan designation provides regulatory and financial incentives for companies to develop and market therapies that treat life-threatening or chronically debilitating conditions affecting no more than 5 in 10,000 people in the European Union, and where no satisfactory treatment is available.
Orphan designation provides a 10-year period of marketing exclusivity if the drug receives regulatory approval. The designation also provides incentives for companies seeking protocol assistance from the EMA during the product development phase and direct access to the centralized authorization procedure.
The EMA adopts an opinion on the granting of orphan drug designation, and that opinion is submitted to the European Commission for a final decision. The European Commission typically makes a decision within 30 days.
NiCord also has orphan designation and breakthrough designation from the US Food and Drug Administration for the treatment of hematologic malignancies.
NiCord research
Data from the pilot study of NiCord suggested the therapy can provide a clinically meaningful improvement in time to neutrophil engraftment over traditional cord blood transplant.
And research presented at EBMT 2016 showed that patients who received NiCord had fewer infections, shorter hospital stays, quicker platelet engraftment, and improved non-relapse mortality when compared to patients who received a traditional cord blood transplant.
NiCord is currently being studied in a phase 3 registration study as a graft for patients with hematologic malignancies who do not have a rapidly available, fully matched donor.
Gamida Cell, the company developing NiCord, announced last month that the first patient in the study had been transplanted. ![]()
The European Medicines Agency’s (EMA’s) Committee for Orphan Medicinal Products has recommended that NiCord® receive orphan designation as a treatment for patients who require a hematopoietic stem cell transplant.
NiCord is a stand-alone graft derived from a single umbilical cord blood unit that has been expanded in culture and enriched with stem and progenitor cells.
NiCord already has orphan designation in the European Union as a treatment for patients with acute myeloid leukemia.
Orphan designation provides regulatory and financial incentives for companies to develop and market therapies that treat life-threatening or chronically debilitating conditions affecting no more than 5 in 10,000 people in the European Union, and where no satisfactory treatment is available.
Orphan designation provides a 10-year period of marketing exclusivity if the drug receives regulatory approval. The designation also provides incentives for companies seeking protocol assistance from the EMA during the product development phase and direct access to the centralized authorization procedure.
The EMA adopts an opinion on the granting of orphan drug designation, and that opinion is submitted to the European Commission for a final decision. The European Commission typically makes a decision within 30 days.
NiCord also has orphan designation and breakthrough designation from the US Food and Drug Administration for the treatment of hematologic malignancies.
NiCord research
Data from the pilot study of NiCord suggested the therapy can provide a clinically meaningful improvement in time to neutrophil engraftment over traditional cord blood transplant.
And research presented at EBMT 2016 showed that patients who received NiCord had fewer infections, shorter hospital stays, quicker platelet engraftment, and improved non-relapse mortality when compared to patients who received a traditional cord blood transplant.
NiCord is currently being studied in a phase 3 registration study as a graft for patients with hematologic malignancies who do not have a rapidly available, fully matched donor.
Gamida Cell, the company developing NiCord, announced last month that the first patient in the study had been transplanted. ![]()
Sneak Peek: Journal of Hospital Medicine
BACKGROUND: Readmissions after hospitalization for pneumonia are common, but the few risk-prediction models have poor to modest predictive ability. Data routinely collected in the EHR may improve prediction.
DESIGN: Observational cohort study using backward-stepwise selection and cross validation.
SUBJECTS: Consecutive pneumonia hospitalizations from six diverse hospitals in north Texas from 2009 to 2010.
MEASURES: All-cause, nonelective, 30-day readmissions, ascertained from 75 regional hospitals.
RESULTS: Of 1,463 patients, 13.6% were readmitted. The first-day, pneumonia-specific model included sociodemographic factors, prior hospitalizations, thrombocytosis, and a modified pneumonia severity index. The full-stay model included disposition status, vital sign instabilities on discharge, and an updated pneumonia severity index calculated using values from the day of discharge as additional predictors. The full-stay, pneumonia-specific model outperformed the first-day model (C-statistic, 0.731 vs. 0.695; P = .02; net reclassification index = 0.08). Compared with a validated multicondition readmission model, the Centers for Medicare & Medicaid Services pneumonia model, and two commonly used pneumonia severity of illness scores, the full-stay pneumonia-specific model had better discrimination (C-statistic, 0.604-0.681; P less than 0.01 for all comparisons), predicted a broader range of risk, and better reclassified individuals by their true risk (net reclassification index range, 0.09-0.18).
CONCLUSIONS: EHR data collected from the entire hospitalization can accurately predict readmission risk among patients hospitalized for pneumonia. This approach outperforms a first-day, pneumonia-specific model, the Centers for Medicare & Medicaid Services pneumonia model, and two commonly used pneumonia severity of illness scores.
Also In JHM This Month
Evaluating automated rules for rapid response system alarm triggers in medical and surgical patients
AUTHORS: Santiago Romero-Brufau, MD; Bruce W. Morlan, MS; Matthew Johnson, MPH; Joel Hickman; Lisa L. Kirkland, MD; James M. Naessens, ScD; Jeanne Huddleston, MD, FACP, FHM
Prognosticating with the Hospital-Patient One-year Mortality Risk score using information abstracted from the medical record
AUTHORS: Genevieve Casey, MD, and Carl van Walraven, MD, FRCPC, MSc
Automating venous thromboembolism risk calculation using electronic health record data upon hospital admission: The Automated Padua Prediction Score
AUTHORS: Pierre Elias, MD; Raman Khanna, MD; Adams Dudley, MD, MBA; Jason Davies, MD, PhD; Ronald Jacolbia, MSN; Kara McArthur, BA; Andrew D. Auerbach, MD, MPH, SFHM
The value of ultrasound in cellulitis to rule out deep venous thrombosis
AUTHORS: Hyung J. Cho, MD, and Andrew S. Dunn, MD, SFHM
Hospital medicine and perioperative care: A framework for high quality, high value collaborative care
AUTHORS: Rachel E. Thompson, MD, MPH, SFHM; Kurt Pfeifer, MD, FHM; Paul Grant, MD, SFHM; Cornelia Taylor, MD; Barbara Slawski, MD, FACP, MS, SFHM; Christopher Whinney, MD, FACP, FHM; Laurence Wellikson, MD, MHM; Amir K. Jaffer, MD, MBA, SFHM
BACKGROUND: Readmissions after hospitalization for pneumonia are common, but the few risk-prediction models have poor to modest predictive ability. Data routinely collected in the EHR may improve prediction.
DESIGN: Observational cohort study using backward-stepwise selection and cross validation.
SUBJECTS: Consecutive pneumonia hospitalizations from six diverse hospitals in north Texas from 2009 to 2010.
MEASURES: All-cause, nonelective, 30-day readmissions, ascertained from 75 regional hospitals.
RESULTS: Of 1,463 patients, 13.6% were readmitted. The first-day, pneumonia-specific model included sociodemographic factors, prior hospitalizations, thrombocytosis, and a modified pneumonia severity index. The full-stay model included disposition status, vital sign instabilities on discharge, and an updated pneumonia severity index calculated using values from the day of discharge as additional predictors. The full-stay, pneumonia-specific model outperformed the first-day model (C-statistic, 0.731 vs. 0.695; P = .02; net reclassification index = 0.08). Compared with a validated multicondition readmission model, the Centers for Medicare & Medicaid Services pneumonia model, and two commonly used pneumonia severity of illness scores, the full-stay pneumonia-specific model had better discrimination (C-statistic, 0.604-0.681; P less than 0.01 for all comparisons), predicted a broader range of risk, and better reclassified individuals by their true risk (net reclassification index range, 0.09-0.18).
CONCLUSIONS: EHR data collected from the entire hospitalization can accurately predict readmission risk among patients hospitalized for pneumonia. This approach outperforms a first-day, pneumonia-specific model, the Centers for Medicare & Medicaid Services pneumonia model, and two commonly used pneumonia severity of illness scores.
Also In JHM This Month
Evaluating automated rules for rapid response system alarm triggers in medical and surgical patients
AUTHORS: Santiago Romero-Brufau, MD; Bruce W. Morlan, MS; Matthew Johnson, MPH; Joel Hickman; Lisa L. Kirkland, MD; James M. Naessens, ScD; Jeanne Huddleston, MD, FACP, FHM
Prognosticating with the Hospital-Patient One-year Mortality Risk score using information abstracted from the medical record
AUTHORS: Genevieve Casey, MD, and Carl van Walraven, MD, FRCPC, MSc
Automating venous thromboembolism risk calculation using electronic health record data upon hospital admission: The Automated Padua Prediction Score
AUTHORS: Pierre Elias, MD; Raman Khanna, MD; Adams Dudley, MD, MBA; Jason Davies, MD, PhD; Ronald Jacolbia, MSN; Kara McArthur, BA; Andrew D. Auerbach, MD, MPH, SFHM
The value of ultrasound in cellulitis to rule out deep venous thrombosis
AUTHORS: Hyung J. Cho, MD, and Andrew S. Dunn, MD, SFHM
Hospital medicine and perioperative care: A framework for high quality, high value collaborative care
AUTHORS: Rachel E. Thompson, MD, MPH, SFHM; Kurt Pfeifer, MD, FHM; Paul Grant, MD, SFHM; Cornelia Taylor, MD; Barbara Slawski, MD, FACP, MS, SFHM; Christopher Whinney, MD, FACP, FHM; Laurence Wellikson, MD, MHM; Amir K. Jaffer, MD, MBA, SFHM
BACKGROUND: Readmissions after hospitalization for pneumonia are common, but the few risk-prediction models have poor to modest predictive ability. Data routinely collected in the EHR may improve prediction.
DESIGN: Observational cohort study using backward-stepwise selection and cross validation.
SUBJECTS: Consecutive pneumonia hospitalizations from six diverse hospitals in north Texas from 2009 to 2010.
MEASURES: All-cause, nonelective, 30-day readmissions, ascertained from 75 regional hospitals.
RESULTS: Of 1,463 patients, 13.6% were readmitted. The first-day, pneumonia-specific model included sociodemographic factors, prior hospitalizations, thrombocytosis, and a modified pneumonia severity index. The full-stay model included disposition status, vital sign instabilities on discharge, and an updated pneumonia severity index calculated using values from the day of discharge as additional predictors. The full-stay, pneumonia-specific model outperformed the first-day model (C-statistic, 0.731 vs. 0.695; P = .02; net reclassification index = 0.08). Compared with a validated multicondition readmission model, the Centers for Medicare & Medicaid Services pneumonia model, and two commonly used pneumonia severity of illness scores, the full-stay pneumonia-specific model had better discrimination (C-statistic, 0.604-0.681; P less than 0.01 for all comparisons), predicted a broader range of risk, and better reclassified individuals by their true risk (net reclassification index range, 0.09-0.18).
CONCLUSIONS: EHR data collected from the entire hospitalization can accurately predict readmission risk among patients hospitalized for pneumonia. This approach outperforms a first-day, pneumonia-specific model, the Centers for Medicare & Medicaid Services pneumonia model, and two commonly used pneumonia severity of illness scores.
Also In JHM This Month
Evaluating automated rules for rapid response system alarm triggers in medical and surgical patients
AUTHORS: Santiago Romero-Brufau, MD; Bruce W. Morlan, MS; Matthew Johnson, MPH; Joel Hickman; Lisa L. Kirkland, MD; James M. Naessens, ScD; Jeanne Huddleston, MD, FACP, FHM
Prognosticating with the Hospital-Patient One-year Mortality Risk score using information abstracted from the medical record
AUTHORS: Genevieve Casey, MD, and Carl van Walraven, MD, FRCPC, MSc
Automating venous thromboembolism risk calculation using electronic health record data upon hospital admission: The Automated Padua Prediction Score
AUTHORS: Pierre Elias, MD; Raman Khanna, MD; Adams Dudley, MD, MBA; Jason Davies, MD, PhD; Ronald Jacolbia, MSN; Kara McArthur, BA; Andrew D. Auerbach, MD, MPH, SFHM
The value of ultrasound in cellulitis to rule out deep venous thrombosis
AUTHORS: Hyung J. Cho, MD, and Andrew S. Dunn, MD, SFHM
Hospital medicine and perioperative care: A framework for high quality, high value collaborative care
AUTHORS: Rachel E. Thompson, MD, MPH, SFHM; Kurt Pfeifer, MD, FHM; Paul Grant, MD, SFHM; Cornelia Taylor, MD; Barbara Slawski, MD, FACP, MS, SFHM; Christopher Whinney, MD, FACP, FHM; Laurence Wellikson, MD, MHM; Amir K. Jaffer, MD, MBA, SFHM
Dabigatran crushes warfarin for AF ablation
Washington – Uninterrupted dabigatran for periprocedural anticoagulation in patients undergoing catheter ablation for atrial fibrillation proved far superior to uninterrupted warfarin – the current standard – in the randomized multicenter RE-CIRCUIT trial, Hugh Calkins, MD, reported at the annual meeting of the American College of Cardiology.
The primary study endpoint – the incidence of major bleeding events from the time of the first femoral puncture at the procedure’s start through the subsequent 8 weeks – occurred in 1.6% of the dabigatran (Pradaxa) group and 6.9% of the warfarin group, for an absolute 5.9% reduction in risk and a 77% relative risk reduction favoring the novel anticoagulant.
“This trial will definitely affect my own practice, and I think it will quickly affect the practices of electrophysiologists around the world,” declared Dr. Calkins, professor of cardiology and medicine and director of the clinical electrophysiology laboratory and the arrhythmia service at Johns Hopkins University, Baltimore.
RE-CIRCUIT (Randomized Evaluation of Dabigatran Etexilate Compared to Warfarin in Pulmonary Vein Ablation: Assessment of an Uninterrupted Periprocedural Anticoagulation Strategy) was a multicenter, prospective, international trial conducted in 635 atrial fibrillation (AF) patients who underwent catheter ablation at 104 sites. The trial was of necessity open label because of the need for frequent adjustments of warfarin dosing; however, outcome assessment was carried out by a blinded panel of six cardiologists and three neurologists.
Standard practice among AF ablationists is to continue oral anticoagulation periprocedurally because prior studies have convincingly shown that periprocedural interruption of warfarin in an effort to reduce bleeding results in a sharply increased risk of periprocedural stroke. So participants in RE-CIRCUIT were randomized to 4-8 weeks of uninterrupted anticoagulation with either dabigatran at 150 mg b.i.d. or warfarin with a target international normalized ratio (INR) of 2.0-3.0 prior to the ablation procedure, during it, and for 8 weeks afterward, at which time an individualized decision was made as to whether to stop or continue the drug.
Major bleeding events were defined by the International Society on Thrombosis and Hemostatis criteria. Most of these bleeds occurred within the first day or two after the procedure. Pericardial tamponades and groin hematomas were significantly less common with dabigatran.
The incidence of minor bleeding events was similar, at around 18% in the two treatment arms. No strokes or systemic embolisms occurred in the study. One patient on warfarin experienced a transient ischemic attack.
Dr. Calkins elaborated on why RE-CIRCUIT will change clinical practice: “A stroke is a terrible thing during an AF procedure and cardiac tamponade is the most common cause of death from the procedure. And now we have high-quality data showing that if you perform this procedure on uninterrupted dabigatran, the risk of stroke and other systemic embolic events is extremely low, and the rate of major bleeding was 77% less.
“Plus, the logistics of warfarin are a pain,” he continued. “If the patient presents on the day of ablation with an INR that’s too high, the procedure is canceled, and if they present with an INR that’s too low and the procedure is carried out, it’s done so with an increased stroke risk.”
Dr. Calkins said he suspects the sharp reduction in major bleeding events during and after AF catheter ablation is a class effect shared by the other NOACs. Studies with those agents are ongoing. But for now, the unique availability of an immediate reversal agent in the form of idarucizumab (Praxbind) for dabigatran in the event of uncontrolled major bleeding is a source of reassurance for operators and patients alike. The antidote was never required in RE-CIRCUIT, though, the cardiologist noted.
Discussant William G. Stevenson, MD, called the trial “informative and helpful.”
“Something we’ve all been struggling with was that some concern was earlier raised that dabigatran might be associated with more thromboembolic events in this scenario. This study clearly refutes that concern,” observed Dr. Stevenson, director of the clinical cardiac electrophysiology program at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School, both in Boston.
Dr. Stevenson wondered whether the outcome differences between the two study groups could be explained by differences in operator techniques and tools. That’s highly unlikely, Dr. Calkins replied. Randomization was done patient by patient, not center by center.
Then why the big difference in major bleeding complications? Dr. Stevenson asked.
“It may be that, if you poke a hole when a patient is on a more forgiving anticoagulant like dabigatran, the bleeding doesn’t persist and turn into a tamponade, whereas if you poke a hole on warfarin it turns into a bigger problem,” Dr. Calkins responded. “When you think about it, warfarin really impacts the whole coagulation cascade through factors VII, IX, and X, so multiple coagulation factors are rendered impotent, whereas dabigatran is a direct thrombin inhibitor, so you’re selectively knocking out just one component of the coagulation cascade. It provides more leeway in preventing a small hole from turning into a big effusion,” he said.
The RE-CIRCUIT trial was funded by Boehringer Ingelheim. Dr. Calkins reported receiving lecture fees from that company and from Medtronic, and serving as a consultant to Medtronic, Abbott Medical, and AtriCure.
Simultaneously with Dr. Calkins’ presentation at ACC 17, the RE-CIRCUIT study was published online (N Engl J Med. 2017 Mar 19. doi: 10.1056/NEJMoa1701005).
Washington – Uninterrupted dabigatran for periprocedural anticoagulation in patients undergoing catheter ablation for atrial fibrillation proved far superior to uninterrupted warfarin – the current standard – in the randomized multicenter RE-CIRCUIT trial, Hugh Calkins, MD, reported at the annual meeting of the American College of Cardiology.
The primary study endpoint – the incidence of major bleeding events from the time of the first femoral puncture at the procedure’s start through the subsequent 8 weeks – occurred in 1.6% of the dabigatran (Pradaxa) group and 6.9% of the warfarin group, for an absolute 5.9% reduction in risk and a 77% relative risk reduction favoring the novel anticoagulant.
“This trial will definitely affect my own practice, and I think it will quickly affect the practices of electrophysiologists around the world,” declared Dr. Calkins, professor of cardiology and medicine and director of the clinical electrophysiology laboratory and the arrhythmia service at Johns Hopkins University, Baltimore.
RE-CIRCUIT (Randomized Evaluation of Dabigatran Etexilate Compared to Warfarin in Pulmonary Vein Ablation: Assessment of an Uninterrupted Periprocedural Anticoagulation Strategy) was a multicenter, prospective, international trial conducted in 635 atrial fibrillation (AF) patients who underwent catheter ablation at 104 sites. The trial was of necessity open label because of the need for frequent adjustments of warfarin dosing; however, outcome assessment was carried out by a blinded panel of six cardiologists and three neurologists.
Standard practice among AF ablationists is to continue oral anticoagulation periprocedurally because prior studies have convincingly shown that periprocedural interruption of warfarin in an effort to reduce bleeding results in a sharply increased risk of periprocedural stroke. So participants in RE-CIRCUIT were randomized to 4-8 weeks of uninterrupted anticoagulation with either dabigatran at 150 mg b.i.d. or warfarin with a target international normalized ratio (INR) of 2.0-3.0 prior to the ablation procedure, during it, and for 8 weeks afterward, at which time an individualized decision was made as to whether to stop or continue the drug.
Major bleeding events were defined by the International Society on Thrombosis and Hemostatis criteria. Most of these bleeds occurred within the first day or two after the procedure. Pericardial tamponades and groin hematomas were significantly less common with dabigatran.
The incidence of minor bleeding events was similar, at around 18% in the two treatment arms. No strokes or systemic embolisms occurred in the study. One patient on warfarin experienced a transient ischemic attack.
Dr. Calkins elaborated on why RE-CIRCUIT will change clinical practice: “A stroke is a terrible thing during an AF procedure and cardiac tamponade is the most common cause of death from the procedure. And now we have high-quality data showing that if you perform this procedure on uninterrupted dabigatran, the risk of stroke and other systemic embolic events is extremely low, and the rate of major bleeding was 77% less.
“Plus, the logistics of warfarin are a pain,” he continued. “If the patient presents on the day of ablation with an INR that’s too high, the procedure is canceled, and if they present with an INR that’s too low and the procedure is carried out, it’s done so with an increased stroke risk.”
Dr. Calkins said he suspects the sharp reduction in major bleeding events during and after AF catheter ablation is a class effect shared by the other NOACs. Studies with those agents are ongoing. But for now, the unique availability of an immediate reversal agent in the form of idarucizumab (Praxbind) for dabigatran in the event of uncontrolled major bleeding is a source of reassurance for operators and patients alike. The antidote was never required in RE-CIRCUIT, though, the cardiologist noted.
Discussant William G. Stevenson, MD, called the trial “informative and helpful.”
“Something we’ve all been struggling with was that some concern was earlier raised that dabigatran might be associated with more thromboembolic events in this scenario. This study clearly refutes that concern,” observed Dr. Stevenson, director of the clinical cardiac electrophysiology program at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School, both in Boston.
Dr. Stevenson wondered whether the outcome differences between the two study groups could be explained by differences in operator techniques and tools. That’s highly unlikely, Dr. Calkins replied. Randomization was done patient by patient, not center by center.
Then why the big difference in major bleeding complications? Dr. Stevenson asked.
“It may be that, if you poke a hole when a patient is on a more forgiving anticoagulant like dabigatran, the bleeding doesn’t persist and turn into a tamponade, whereas if you poke a hole on warfarin it turns into a bigger problem,” Dr. Calkins responded. “When you think about it, warfarin really impacts the whole coagulation cascade through factors VII, IX, and X, so multiple coagulation factors are rendered impotent, whereas dabigatran is a direct thrombin inhibitor, so you’re selectively knocking out just one component of the coagulation cascade. It provides more leeway in preventing a small hole from turning into a big effusion,” he said.
The RE-CIRCUIT trial was funded by Boehringer Ingelheim. Dr. Calkins reported receiving lecture fees from that company and from Medtronic, and serving as a consultant to Medtronic, Abbott Medical, and AtriCure.
Simultaneously with Dr. Calkins’ presentation at ACC 17, the RE-CIRCUIT study was published online (N Engl J Med. 2017 Mar 19. doi: 10.1056/NEJMoa1701005).
Washington – Uninterrupted dabigatran for periprocedural anticoagulation in patients undergoing catheter ablation for atrial fibrillation proved far superior to uninterrupted warfarin – the current standard – in the randomized multicenter RE-CIRCUIT trial, Hugh Calkins, MD, reported at the annual meeting of the American College of Cardiology.
The primary study endpoint – the incidence of major bleeding events from the time of the first femoral puncture at the procedure’s start through the subsequent 8 weeks – occurred in 1.6% of the dabigatran (Pradaxa) group and 6.9% of the warfarin group, for an absolute 5.9% reduction in risk and a 77% relative risk reduction favoring the novel anticoagulant.
“This trial will definitely affect my own practice, and I think it will quickly affect the practices of electrophysiologists around the world,” declared Dr. Calkins, professor of cardiology and medicine and director of the clinical electrophysiology laboratory and the arrhythmia service at Johns Hopkins University, Baltimore.
RE-CIRCUIT (Randomized Evaluation of Dabigatran Etexilate Compared to Warfarin in Pulmonary Vein Ablation: Assessment of an Uninterrupted Periprocedural Anticoagulation Strategy) was a multicenter, prospective, international trial conducted in 635 atrial fibrillation (AF) patients who underwent catheter ablation at 104 sites. The trial was of necessity open label because of the need for frequent adjustments of warfarin dosing; however, outcome assessment was carried out by a blinded panel of six cardiologists and three neurologists.
Standard practice among AF ablationists is to continue oral anticoagulation periprocedurally because prior studies have convincingly shown that periprocedural interruption of warfarin in an effort to reduce bleeding results in a sharply increased risk of periprocedural stroke. So participants in RE-CIRCUIT were randomized to 4-8 weeks of uninterrupted anticoagulation with either dabigatran at 150 mg b.i.d. or warfarin with a target international normalized ratio (INR) of 2.0-3.0 prior to the ablation procedure, during it, and for 8 weeks afterward, at which time an individualized decision was made as to whether to stop or continue the drug.
Major bleeding events were defined by the International Society on Thrombosis and Hemostatis criteria. Most of these bleeds occurred within the first day or two after the procedure. Pericardial tamponades and groin hematomas were significantly less common with dabigatran.
The incidence of minor bleeding events was similar, at around 18% in the two treatment arms. No strokes or systemic embolisms occurred in the study. One patient on warfarin experienced a transient ischemic attack.
Dr. Calkins elaborated on why RE-CIRCUIT will change clinical practice: “A stroke is a terrible thing during an AF procedure and cardiac tamponade is the most common cause of death from the procedure. And now we have high-quality data showing that if you perform this procedure on uninterrupted dabigatran, the risk of stroke and other systemic embolic events is extremely low, and the rate of major bleeding was 77% less.
“Plus, the logistics of warfarin are a pain,” he continued. “If the patient presents on the day of ablation with an INR that’s too high, the procedure is canceled, and if they present with an INR that’s too low and the procedure is carried out, it’s done so with an increased stroke risk.”
Dr. Calkins said he suspects the sharp reduction in major bleeding events during and after AF catheter ablation is a class effect shared by the other NOACs. Studies with those agents are ongoing. But for now, the unique availability of an immediate reversal agent in the form of idarucizumab (Praxbind) for dabigatran in the event of uncontrolled major bleeding is a source of reassurance for operators and patients alike. The antidote was never required in RE-CIRCUIT, though, the cardiologist noted.
Discussant William G. Stevenson, MD, called the trial “informative and helpful.”
“Something we’ve all been struggling with was that some concern was earlier raised that dabigatran might be associated with more thromboembolic events in this scenario. This study clearly refutes that concern,” observed Dr. Stevenson, director of the clinical cardiac electrophysiology program at Brigham and Women’s Hospital and professor of medicine at Harvard Medical School, both in Boston.
Dr. Stevenson wondered whether the outcome differences between the two study groups could be explained by differences in operator techniques and tools. That’s highly unlikely, Dr. Calkins replied. Randomization was done patient by patient, not center by center.
Then why the big difference in major bleeding complications? Dr. Stevenson asked.
“It may be that, if you poke a hole when a patient is on a more forgiving anticoagulant like dabigatran, the bleeding doesn’t persist and turn into a tamponade, whereas if you poke a hole on warfarin it turns into a bigger problem,” Dr. Calkins responded. “When you think about it, warfarin really impacts the whole coagulation cascade through factors VII, IX, and X, so multiple coagulation factors are rendered impotent, whereas dabigatran is a direct thrombin inhibitor, so you’re selectively knocking out just one component of the coagulation cascade. It provides more leeway in preventing a small hole from turning into a big effusion,” he said.
The RE-CIRCUIT trial was funded by Boehringer Ingelheim. Dr. Calkins reported receiving lecture fees from that company and from Medtronic, and serving as a consultant to Medtronic, Abbott Medical, and AtriCure.
Simultaneously with Dr. Calkins’ presentation at ACC 17, the RE-CIRCUIT study was published online (N Engl J Med. 2017 Mar 19. doi: 10.1056/NEJMoa1701005).
At ACC 17
Key clinical point:
Major finding: The incidence of major bleeding events in conjunction with catheter ablation of atrial fibrillation was reduced by 77% in patients on periprocedural dabigatran compared with those on warfarin.
Data source: A randomized multicenter prospective international trial of 635 patients who underwent catheter ablation for atrial fibrillation supported by uninterrupted oral anticoagulation.
Disclosures: The RE-CIRCUIT trial was funded by Boehringer Ingelheim. Dr. Calkins reported receiving lecture fees from that company and from Medtronic, and serving as a consultant to Medtronic, Abbott Medical, and AtriCure.
Unavoidable, random DNA replication errors are the most common cancer drivers
Up to two-thirds of the mutations that drive human cancers may be due to DNA replication errors in normally dividing stem cells, not by inherited or environmentally induced mutations, according to a mathematical modeling study.
The proportion of replication error-driven mutations varied widely among 17 cancers analyzed, but the overall attributable risk of these errors was remarkably consistent among 69 countries included in the study, said Cristian Tomasetti, PhD, a coauthor of the paper and a biostatistician at Johns Hopkins University, Baltimore.
The findings should be a game-changer in the cancer field, Dr. Tomasetti said during a press briefing sponsored by the American Association for the Advancement of Science. Research dogma has long held that most cancers are related to lifestyle and environmental exposure, with a few primarily due to genetic factors.
“We have now determined that there is a third factor, and that it causes most of the mutations that drive cancer,” Dr. Tomasetti said. “We cannot ignore it and pretend it doesn’t exist. This is a complete paradigm shift in how we think of cancer and what causes it.”
The finding that 66% of cancer-driving mutations are based on unavoidable replication errors doesn’t challenge well-established epidemiology, said Dr. Tomasetti and his coauthor, Bert Vogelstein, MD. Rather, it fits perfectly with several key understandings of cancer: that about 40% of cases are preventable, that rapidly dividing tissues are more prone to develop cancers, and that cancer incidence rises exponentially as humans age.
“If we have as our starting point the assumption that 42% of cancers are preventable, we are completely consistent with that,” in finding that about 60% of cancers are unavoidable, Dr. Tomasetti said. “Those two numbers go perfectly together.”
The study also found that replication-error mutations (R) were most likely to drive cancers in tissues with rapid turnover, such as colorectal tissue. This makes intuitive sense, given that basal mutation rates hover at about three errors per cell replication cycle regardless of tissue type.
“The basal mutation rate in all cells is pretty even,” said Dr. Vogelstein, the Clayton Professor of Oncology and Pathology at John Hopkins University, Baltimore. “The difference is the number of stem cells. The more cells, the more divisions, and the more mistakes.”
R-mutations also contribute to age-related cancer incidence. As a person ages, more cell divisions accumulate, thus increasing the risk of a cancer-driving R-error. But these mutations also occur in children, who have rapid cell division in all their tissues. In fact, the colleagues suspect that R-errors are the main drivers of almost all pediatric cancers.
The new study bolsters the duo’s controversial 2015 work.
The theory sparked controversy among scholars and researchers. They challenged it on a number of technical fronts, from stem cell counts and division rates to charges that it didn’t adequately assess the interaction between R-mutations and environmental risks.
Some commentators, perceiving nihilism in the paper, expressed concern that clinicians and patients would get the idea that cancer prevention strategies were useless, since most cancers were simply a case of “bad luck.”
A pervading theme of these counter arguments was one familiar to any researcher: Correlation does not equal causation. The new study was an attempt to expand upon and strengthen the original findings, Dr. Tomasetti said.
“There are well-known environmental risk variations across the world, and there was a question of how our findings might change if we did this analysis in a different country. This paper is also the very first time that someone has ever looked at the proportions of mutations in each cancer type and assigned them to these factors.”
The new study employed a similar mathematical model, but comprised data from 423 cancer registries in 69 countries. The researchers examined the relationship between the lifetime risk of 17 cancers (including breast and prostate, which were not included in the 2015 study) and lifetime stem cell divisions for each tissue. The median correlation coefficient was 0.80; 89% of the countries examined had a correlation of greater than 0.70. This was “remarkably similar” to the correlation determined in the 2015 U.S.-only study.
The team’s next step was to determine what fraction of cancer-driving mutations arose from R-errors, from environmental factors (E), and from hereditary factors (H). They examined these proportions in 32 different cancers in which environmental, lifestyle, and genetic factors have been thoroughly studied. Overall, 29% of the driver mutations were due to environment, 5% to heredity, and 66% to R-errors.
The proportions of these drivers did vary widely between the cancer types, the team noted. For example, lung and esophageal cancers and melanoma were primarily driven by environmental factors (more than 60% each). However, they wrote, “even in lung adenocarcinomas, R contributes a third of the total mutations, with tobacco smoke [including secondhand smoke], diet, radiation, and occupational exposures contributing the remainder. In cancers that are less strongly associated with environmental factors, such as those of the pancreas, brain, bone, or prostate, the majority of the mutations are attributable to R.”
During the press briefing, Dr. Tomasetti and Dr. Vogelstein stressed that most of the inevitable R-errors don’t precipitate cancer – and that even if they do increase risk, that risk may not ever trip the disease process.
“Most of the time these replicative mutations do no harm,” Dr Vogelstein said. “They occur in junk DNA genes, or in areas that are unimportant with respect to cancer. That’s the good luck. Occasionally, they occur in a cancer driver gene, and that is bad luck.”
But even a dose of bad luck isn’t enough to cause cancer. Most cancers require multiple hits to develop – which makes primary prevention strategies more important than ever, Dr. Tomasetti said.
“In the case of lung cancer, for instance, three or more mutations are needed. We showed that these mutations are caused by a combination of environment and R-errors. In theory, then, all of these cancers are preventable because if we can prevent even one of the environmentally caused mutations, then that patient won’t develop cancer.”
However, he said, some cancers do appear to be entirely driven by E-errors and, thus, appear entirely unavoidable. This is an extremely difficult area for clinicians and patients to navigate, said Dr. Vogelstein, a former pediatrician.
“We hope that understanding this will offer some comfort to the literally millions of patients who develop cancer despite having lead a near-perfect life,” in terms of managing risk factors. “Cancer develops in people who haven’t smoked, who avoided the sun and wore sunscreen, who eat perfectly healthy diets and exercise regularly. This is a particularly important concept for parents of children who have cancer, who think ‘I either transmitted a bad gene or unknowingly exposed my child to an environmental agent that caused their cancer.’ They need to understand that these cancers would have occurred no matter what they did.”
Dr. Tomasetti had no disclosures. Dr. Vogelstein is on the scientific advisory boards of Morphotek, Exelixis GP, and Sysmex Inostics, and is a founder of PapGene and Personal Genome Diagnostics.
[email protected]
On Twitter @Alz_gal
Up to two-thirds of the mutations that drive human cancers may be due to DNA replication errors in normally dividing stem cells, not by inherited or environmentally induced mutations, according to a mathematical modeling study.
The proportion of replication error-driven mutations varied widely among 17 cancers analyzed, but the overall attributable risk of these errors was remarkably consistent among 69 countries included in the study, said Cristian Tomasetti, PhD, a coauthor of the paper and a biostatistician at Johns Hopkins University, Baltimore.
The findings should be a game-changer in the cancer field, Dr. Tomasetti said during a press briefing sponsored by the American Association for the Advancement of Science. Research dogma has long held that most cancers are related to lifestyle and environmental exposure, with a few primarily due to genetic factors.
“We have now determined that there is a third factor, and that it causes most of the mutations that drive cancer,” Dr. Tomasetti said. “We cannot ignore it and pretend it doesn’t exist. This is a complete paradigm shift in how we think of cancer and what causes it.”
The finding that 66% of cancer-driving mutations are based on unavoidable replication errors doesn’t challenge well-established epidemiology, said Dr. Tomasetti and his coauthor, Bert Vogelstein, MD. Rather, it fits perfectly with several key understandings of cancer: that about 40% of cases are preventable, that rapidly dividing tissues are more prone to develop cancers, and that cancer incidence rises exponentially as humans age.
“If we have as our starting point the assumption that 42% of cancers are preventable, we are completely consistent with that,” in finding that about 60% of cancers are unavoidable, Dr. Tomasetti said. “Those two numbers go perfectly together.”
The study also found that replication-error mutations (R) were most likely to drive cancers in tissues with rapid turnover, such as colorectal tissue. This makes intuitive sense, given that basal mutation rates hover at about three errors per cell replication cycle regardless of tissue type.
“The basal mutation rate in all cells is pretty even,” said Dr. Vogelstein, the Clayton Professor of Oncology and Pathology at John Hopkins University, Baltimore. “The difference is the number of stem cells. The more cells, the more divisions, and the more mistakes.”
R-mutations also contribute to age-related cancer incidence. As a person ages, more cell divisions accumulate, thus increasing the risk of a cancer-driving R-error. But these mutations also occur in children, who have rapid cell division in all their tissues. In fact, the colleagues suspect that R-errors are the main drivers of almost all pediatric cancers.
The new study bolsters the duo’s controversial 2015 work.
The theory sparked controversy among scholars and researchers. They challenged it on a number of technical fronts, from stem cell counts and division rates to charges that it didn’t adequately assess the interaction between R-mutations and environmental risks.
Some commentators, perceiving nihilism in the paper, expressed concern that clinicians and patients would get the idea that cancer prevention strategies were useless, since most cancers were simply a case of “bad luck.”
A pervading theme of these counter arguments was one familiar to any researcher: Correlation does not equal causation. The new study was an attempt to expand upon and strengthen the original findings, Dr. Tomasetti said.
“There are well-known environmental risk variations across the world, and there was a question of how our findings might change if we did this analysis in a different country. This paper is also the very first time that someone has ever looked at the proportions of mutations in each cancer type and assigned them to these factors.”
The new study employed a similar mathematical model, but comprised data from 423 cancer registries in 69 countries. The researchers examined the relationship between the lifetime risk of 17 cancers (including breast and prostate, which were not included in the 2015 study) and lifetime stem cell divisions for each tissue. The median correlation coefficient was 0.80; 89% of the countries examined had a correlation of greater than 0.70. This was “remarkably similar” to the correlation determined in the 2015 U.S.-only study.
The team’s next step was to determine what fraction of cancer-driving mutations arose from R-errors, from environmental factors (E), and from hereditary factors (H). They examined these proportions in 32 different cancers in which environmental, lifestyle, and genetic factors have been thoroughly studied. Overall, 29% of the driver mutations were due to environment, 5% to heredity, and 66% to R-errors.
The proportions of these drivers did vary widely between the cancer types, the team noted. For example, lung and esophageal cancers and melanoma were primarily driven by environmental factors (more than 60% each). However, they wrote, “even in lung adenocarcinomas, R contributes a third of the total mutations, with tobacco smoke [including secondhand smoke], diet, radiation, and occupational exposures contributing the remainder. In cancers that are less strongly associated with environmental factors, such as those of the pancreas, brain, bone, or prostate, the majority of the mutations are attributable to R.”
During the press briefing, Dr. Tomasetti and Dr. Vogelstein stressed that most of the inevitable R-errors don’t precipitate cancer – and that even if they do increase risk, that risk may not ever trip the disease process.
“Most of the time these replicative mutations do no harm,” Dr Vogelstein said. “They occur in junk DNA genes, or in areas that are unimportant with respect to cancer. That’s the good luck. Occasionally, they occur in a cancer driver gene, and that is bad luck.”
But even a dose of bad luck isn’t enough to cause cancer. Most cancers require multiple hits to develop – which makes primary prevention strategies more important than ever, Dr. Tomasetti said.
“In the case of lung cancer, for instance, three or more mutations are needed. We showed that these mutations are caused by a combination of environment and R-errors. In theory, then, all of these cancers are preventable because if we can prevent even one of the environmentally caused mutations, then that patient won’t develop cancer.”
However, he said, some cancers do appear to be entirely driven by E-errors and, thus, appear entirely unavoidable. This is an extremely difficult area for clinicians and patients to navigate, said Dr. Vogelstein, a former pediatrician.
“We hope that understanding this will offer some comfort to the literally millions of patients who develop cancer despite having lead a near-perfect life,” in terms of managing risk factors. “Cancer develops in people who haven’t smoked, who avoided the sun and wore sunscreen, who eat perfectly healthy diets and exercise regularly. This is a particularly important concept for parents of children who have cancer, who think ‘I either transmitted a bad gene or unknowingly exposed my child to an environmental agent that caused their cancer.’ They need to understand that these cancers would have occurred no matter what they did.”
Dr. Tomasetti had no disclosures. Dr. Vogelstein is on the scientific advisory boards of Morphotek, Exelixis GP, and Sysmex Inostics, and is a founder of PapGene and Personal Genome Diagnostics.
[email protected]
On Twitter @Alz_gal
Up to two-thirds of the mutations that drive human cancers may be due to DNA replication errors in normally dividing stem cells, not by inherited or environmentally induced mutations, according to a mathematical modeling study.
The proportion of replication error-driven mutations varied widely among 17 cancers analyzed, but the overall attributable risk of these errors was remarkably consistent among 69 countries included in the study, said Cristian Tomasetti, PhD, a coauthor of the paper and a biostatistician at Johns Hopkins University, Baltimore.
The findings should be a game-changer in the cancer field, Dr. Tomasetti said during a press briefing sponsored by the American Association for the Advancement of Science. Research dogma has long held that most cancers are related to lifestyle and environmental exposure, with a few primarily due to genetic factors.
“We have now determined that there is a third factor, and that it causes most of the mutations that drive cancer,” Dr. Tomasetti said. “We cannot ignore it and pretend it doesn’t exist. This is a complete paradigm shift in how we think of cancer and what causes it.”
The finding that 66% of cancer-driving mutations are based on unavoidable replication errors doesn’t challenge well-established epidemiology, said Dr. Tomasetti and his coauthor, Bert Vogelstein, MD. Rather, it fits perfectly with several key understandings of cancer: that about 40% of cases are preventable, that rapidly dividing tissues are more prone to develop cancers, and that cancer incidence rises exponentially as humans age.
“If we have as our starting point the assumption that 42% of cancers are preventable, we are completely consistent with that,” in finding that about 60% of cancers are unavoidable, Dr. Tomasetti said. “Those two numbers go perfectly together.”
The study also found that replication-error mutations (R) were most likely to drive cancers in tissues with rapid turnover, such as colorectal tissue. This makes intuitive sense, given that basal mutation rates hover at about three errors per cell replication cycle regardless of tissue type.
“The basal mutation rate in all cells is pretty even,” said Dr. Vogelstein, the Clayton Professor of Oncology and Pathology at John Hopkins University, Baltimore. “The difference is the number of stem cells. The more cells, the more divisions, and the more mistakes.”
R-mutations also contribute to age-related cancer incidence. As a person ages, more cell divisions accumulate, thus increasing the risk of a cancer-driving R-error. But these mutations also occur in children, who have rapid cell division in all their tissues. In fact, the colleagues suspect that R-errors are the main drivers of almost all pediatric cancers.
The new study bolsters the duo’s controversial 2015 work.
The theory sparked controversy among scholars and researchers. They challenged it on a number of technical fronts, from stem cell counts and division rates to charges that it didn’t adequately assess the interaction between R-mutations and environmental risks.
Some commentators, perceiving nihilism in the paper, expressed concern that clinicians and patients would get the idea that cancer prevention strategies were useless, since most cancers were simply a case of “bad luck.”
A pervading theme of these counter arguments was one familiar to any researcher: Correlation does not equal causation. The new study was an attempt to expand upon and strengthen the original findings, Dr. Tomasetti said.
“There are well-known environmental risk variations across the world, and there was a question of how our findings might change if we did this analysis in a different country. This paper is also the very first time that someone has ever looked at the proportions of mutations in each cancer type and assigned them to these factors.”
The new study employed a similar mathematical model, but comprised data from 423 cancer registries in 69 countries. The researchers examined the relationship between the lifetime risk of 17 cancers (including breast and prostate, which were not included in the 2015 study) and lifetime stem cell divisions for each tissue. The median correlation coefficient was 0.80; 89% of the countries examined had a correlation of greater than 0.70. This was “remarkably similar” to the correlation determined in the 2015 U.S.-only study.
The team’s next step was to determine what fraction of cancer-driving mutations arose from R-errors, from environmental factors (E), and from hereditary factors (H). They examined these proportions in 32 different cancers in which environmental, lifestyle, and genetic factors have been thoroughly studied. Overall, 29% of the driver mutations were due to environment, 5% to heredity, and 66% to R-errors.
The proportions of these drivers did vary widely between the cancer types, the team noted. For example, lung and esophageal cancers and melanoma were primarily driven by environmental factors (more than 60% each). However, they wrote, “even in lung adenocarcinomas, R contributes a third of the total mutations, with tobacco smoke [including secondhand smoke], diet, radiation, and occupational exposures contributing the remainder. In cancers that are less strongly associated with environmental factors, such as those of the pancreas, brain, bone, or prostate, the majority of the mutations are attributable to R.”
During the press briefing, Dr. Tomasetti and Dr. Vogelstein stressed that most of the inevitable R-errors don’t precipitate cancer – and that even if they do increase risk, that risk may not ever trip the disease process.
“Most of the time these replicative mutations do no harm,” Dr Vogelstein said. “They occur in junk DNA genes, or in areas that are unimportant with respect to cancer. That’s the good luck. Occasionally, they occur in a cancer driver gene, and that is bad luck.”
But even a dose of bad luck isn’t enough to cause cancer. Most cancers require multiple hits to develop – which makes primary prevention strategies more important than ever, Dr. Tomasetti said.
“In the case of lung cancer, for instance, three or more mutations are needed. We showed that these mutations are caused by a combination of environment and R-errors. In theory, then, all of these cancers are preventable because if we can prevent even one of the environmentally caused mutations, then that patient won’t develop cancer.”
However, he said, some cancers do appear to be entirely driven by E-errors and, thus, appear entirely unavoidable. This is an extremely difficult area for clinicians and patients to navigate, said Dr. Vogelstein, a former pediatrician.
“We hope that understanding this will offer some comfort to the literally millions of patients who develop cancer despite having lead a near-perfect life,” in terms of managing risk factors. “Cancer develops in people who haven’t smoked, who avoided the sun and wore sunscreen, who eat perfectly healthy diets and exercise regularly. This is a particularly important concept for parents of children who have cancer, who think ‘I either transmitted a bad gene or unknowingly exposed my child to an environmental agent that caused their cancer.’ They need to understand that these cancers would have occurred no matter what they did.”
Dr. Tomasetti had no disclosures. Dr. Vogelstein is on the scientific advisory boards of Morphotek, Exelixis GP, and Sysmex Inostics, and is a founder of PapGene and Personal Genome Diagnostics.
[email protected]
On Twitter @Alz_gal
Key clinical point:
Major finding: Two-thirds (66%) of cancer drivers are replication errors, 29% are environmentally induced, and 5% are hereditary.
Data source: The researchers examined cancer mutation drivers in two cohorts that spanned 69 countries.
Disclosures: Dr. Tomasetti had no disclosures. Dr. Vogelstein is on the scientific advisory boards of Morphotek, Exelixis GP, and Sysmex Inostics, and is a founder of PapGene and Personal Genome Diagnostics.
American Samoa could be a model for responding to Zika outbreaks
Syndromic and enhanced surveillance employed during a Zika outbreak in American Samoa last year is credited for bringing a quick end to ongoing transmission and could be used to predict when ongoing transmission in similarly small populations will end.
“Establishing a timeline for discontinuing screening of asymptomatic pregnant women allows ASDoH [the American Samoa Department of Health] to allocate resources appropriately toward early interventions for children and families affected by Zika virus,” W. Thane Hancock, MD, of the CDC’s Office of Public Health Preparedness and Response, and his colleagues wrote in the Morbidity and Mortality Weekly Report (2017 Mar 24;66[11]:299-301).
Laboratory-confirmed Zika cases first cropped up in American Samoa in January 2016, prompting the implementation of protocols to reduce the mosquito population, along with syndromic surveillance based on electronic health records to determine which patients may be at higher risk. Routine testing of all asymptomatic pregnant woman, along with individuals with one or more symptoms of Zika virus, also became standard procedure. Real-time, reverse transcription–polymerase chain reaction (rRT-PCR) testing was used to confirm suspected Zika virus cases.
“Early in the response, collection and testing of specimens from pregnant women was prioritized over the collection from symptomatic nonpregnant patients because of limited testing and shipping capacity,” the researchers wrote.
By May 2016, suspected cases had dropped from six per week during January and February, to just one per week, with the last laboratory-confirmed case occurring on June 19, 2016. In August, the ASDoH proposed ending routine screening of asymptomatic pregnant women by Oct. 15, 2016. Transmission data from American Samoa and the CDC were used to determine the Oct. 15 date as an estimate for when active mosquito-borne transmission would end.
To confirm that local transmission was no longer ongoing, enhanced surveillance was implemented from Aug. 31 to Oct. 15, involving free testing at local clinics. Over the course of 45 days, there were a total of 32 patients suspected of having a Zika virus infection.
Two of these subjects’ specimens were not sufficient in sample size to be tested, but the other 30 underwent rRT-PCR testing within 30 days of symptom onset, and all the results were negative. Additionally, 277 asymptomatic pregnant women were tested and 86 (31%) tested anti-Zika IgM–positive or equivocal. All 86 specimens later tested negative by rRT-PCR.
Given the possibility that Zika virus can persist in semen for 6 months, officials recommended permissive testing of asymptomatic pregnant women who conceive through April 15, 2017 “as a conservative approach.”
“The surveillance processes outlined and the timeline established for American Samoa might have implications for jurisdictions where small populations and similar population immunity following widespread Zika virus exposure can facilitate interruption of transmission,” the researchers wrote.
Syndromic and enhanced surveillance employed during a Zika outbreak in American Samoa last year is credited for bringing a quick end to ongoing transmission and could be used to predict when ongoing transmission in similarly small populations will end.
“Establishing a timeline for discontinuing screening of asymptomatic pregnant women allows ASDoH [the American Samoa Department of Health] to allocate resources appropriately toward early interventions for children and families affected by Zika virus,” W. Thane Hancock, MD, of the CDC’s Office of Public Health Preparedness and Response, and his colleagues wrote in the Morbidity and Mortality Weekly Report (2017 Mar 24;66[11]:299-301).
Laboratory-confirmed Zika cases first cropped up in American Samoa in January 2016, prompting the implementation of protocols to reduce the mosquito population, along with syndromic surveillance based on electronic health records to determine which patients may be at higher risk. Routine testing of all asymptomatic pregnant woman, along with individuals with one or more symptoms of Zika virus, also became standard procedure. Real-time, reverse transcription–polymerase chain reaction (rRT-PCR) testing was used to confirm suspected Zika virus cases.
“Early in the response, collection and testing of specimens from pregnant women was prioritized over the collection from symptomatic nonpregnant patients because of limited testing and shipping capacity,” the researchers wrote.
By May 2016, suspected cases had dropped from six per week during January and February, to just one per week, with the last laboratory-confirmed case occurring on June 19, 2016. In August, the ASDoH proposed ending routine screening of asymptomatic pregnant women by Oct. 15, 2016. Transmission data from American Samoa and the CDC were used to determine the Oct. 15 date as an estimate for when active mosquito-borne transmission would end.
To confirm that local transmission was no longer ongoing, enhanced surveillance was implemented from Aug. 31 to Oct. 15, involving free testing at local clinics. Over the course of 45 days, there were a total of 32 patients suspected of having a Zika virus infection.
Two of these subjects’ specimens were not sufficient in sample size to be tested, but the other 30 underwent rRT-PCR testing within 30 days of symptom onset, and all the results were negative. Additionally, 277 asymptomatic pregnant women were tested and 86 (31%) tested anti-Zika IgM–positive or equivocal. All 86 specimens later tested negative by rRT-PCR.
Given the possibility that Zika virus can persist in semen for 6 months, officials recommended permissive testing of asymptomatic pregnant women who conceive through April 15, 2017 “as a conservative approach.”
“The surveillance processes outlined and the timeline established for American Samoa might have implications for jurisdictions where small populations and similar population immunity following widespread Zika virus exposure can facilitate interruption of transmission,” the researchers wrote.
Syndromic and enhanced surveillance employed during a Zika outbreak in American Samoa last year is credited for bringing a quick end to ongoing transmission and could be used to predict when ongoing transmission in similarly small populations will end.
“Establishing a timeline for discontinuing screening of asymptomatic pregnant women allows ASDoH [the American Samoa Department of Health] to allocate resources appropriately toward early interventions for children and families affected by Zika virus,” W. Thane Hancock, MD, of the CDC’s Office of Public Health Preparedness and Response, and his colleagues wrote in the Morbidity and Mortality Weekly Report (2017 Mar 24;66[11]:299-301).
Laboratory-confirmed Zika cases first cropped up in American Samoa in January 2016, prompting the implementation of protocols to reduce the mosquito population, along with syndromic surveillance based on electronic health records to determine which patients may be at higher risk. Routine testing of all asymptomatic pregnant woman, along with individuals with one or more symptoms of Zika virus, also became standard procedure. Real-time, reverse transcription–polymerase chain reaction (rRT-PCR) testing was used to confirm suspected Zika virus cases.
“Early in the response, collection and testing of specimens from pregnant women was prioritized over the collection from symptomatic nonpregnant patients because of limited testing and shipping capacity,” the researchers wrote.
By May 2016, suspected cases had dropped from six per week during January and February, to just one per week, with the last laboratory-confirmed case occurring on June 19, 2016. In August, the ASDoH proposed ending routine screening of asymptomatic pregnant women by Oct. 15, 2016. Transmission data from American Samoa and the CDC were used to determine the Oct. 15 date as an estimate for when active mosquito-borne transmission would end.
To confirm that local transmission was no longer ongoing, enhanced surveillance was implemented from Aug. 31 to Oct. 15, involving free testing at local clinics. Over the course of 45 days, there were a total of 32 patients suspected of having a Zika virus infection.
Two of these subjects’ specimens were not sufficient in sample size to be tested, but the other 30 underwent rRT-PCR testing within 30 days of symptom onset, and all the results were negative. Additionally, 277 asymptomatic pregnant women were tested and 86 (31%) tested anti-Zika IgM–positive or equivocal. All 86 specimens later tested negative by rRT-PCR.
Given the possibility that Zika virus can persist in semen for 6 months, officials recommended permissive testing of asymptomatic pregnant women who conceive through April 15, 2017 “as a conservative approach.”
“The surveillance processes outlined and the timeline established for American Samoa might have implications for jurisdictions where small populations and similar population immunity following widespread Zika virus exposure can facilitate interruption of transmission,” the researchers wrote.
FROM MMWR