User login
Women and Heart Disease
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Sex-discordant transfusions don’t increase death risk

Photo by Elise Amendola
There is no association between sex-discordant blood transfusions and the risk of death after cardiac surgery, according to research published in Circulation.
Two previous studies suggested that patients who received red blood cells (RBCs) from a donor of the opposite sex had an increased risk of death after cardiac surgery.
However, the current study showed no significant difference between same-sex and opposite-sex donor-recipient pairs.
The researchers said the reason for the difference between the new and older studies is that, in the new study, the team “carefully adjusted” for the number of transfusions performed and allowed for the effect of RBC transfusions on mortality to differ between men and women.
“The consequences of the findings from [the earlier studies], if proved true, would have been immense and necessitated radical changes to how blood transfusions are managed around the world,” said Martin Holzmann, MD, PhD, of Karolinska Institutet in Stockholm, Sweden.
“Our results clearly show that there is no real connection between sex-discordant blood transfusions and the risk of death.”
Therefore, Dr Holzmann and his colleagues believe there is no need to consider donor sex when allocating RBC units for transfusion.
To come to this conclusion, the researchers analyzed data on 45,090 patients who underwent cardiac surgery and received at least 1 RBC transfusion.
All patients were adults who had undergone isolated coronary artery bypass grafting, isolated valve repair/replacement surgery, or a combination of these procedures between 1997 and 2012.
The researchers estimated the relative hazard of death in relation to exposure to sex-discordant transfusions, adjusting their analyses for potential confounding factors, such as patient sex, age, blood group, and number of transfusions.
Results
The researchers found that women were more likely to receive sex-discordant transfusions than same-sex transfusions—45.3% and 19.8%, respectively. And patients who received sex-discordant transfusions tended to receive more transfusions—a mean of 4.2 vs 2.0 for same-sex transfusions.
However, there were no other significant differences between the sex-discordant and same-sex groups.
The researchers noted that, during the 30-day follow-up period, there were more deaths among patients who received sex-discordant transfusions than those who did not—1701 (4.9%) and 205 (1.9%), respectively.
However, when the team adjusted for potential confounding factors, the relative risk of death was similar for patients who received at least 1 unit of sex-discordant blood and those who did not. The hazard ratio was 0.97 at 30 days of follow-up, 0.97 at the 2-year mark, and 0.98 at 10 years of follow-up.
The risk of death did increase as the number of sex-discordant units transfused increased. However, the increase was not statistically significant. ![]()

Photo by Elise Amendola
There is no association between sex-discordant blood transfusions and the risk of death after cardiac surgery, according to research published in Circulation.
Two previous studies suggested that patients who received red blood cells (RBCs) from a donor of the opposite sex had an increased risk of death after cardiac surgery.
However, the current study showed no significant difference between same-sex and opposite-sex donor-recipient pairs.
The researchers said the reason for the difference between the new and older studies is that, in the new study, the team “carefully adjusted” for the number of transfusions performed and allowed for the effect of RBC transfusions on mortality to differ between men and women.
“The consequences of the findings from [the earlier studies], if proved true, would have been immense and necessitated radical changes to how blood transfusions are managed around the world,” said Martin Holzmann, MD, PhD, of Karolinska Institutet in Stockholm, Sweden.
“Our results clearly show that there is no real connection between sex-discordant blood transfusions and the risk of death.”
Therefore, Dr Holzmann and his colleagues believe there is no need to consider donor sex when allocating RBC units for transfusion.
To come to this conclusion, the researchers analyzed data on 45,090 patients who underwent cardiac surgery and received at least 1 RBC transfusion.
All patients were adults who had undergone isolated coronary artery bypass grafting, isolated valve repair/replacement surgery, or a combination of these procedures between 1997 and 2012.
The researchers estimated the relative hazard of death in relation to exposure to sex-discordant transfusions, adjusting their analyses for potential confounding factors, such as patient sex, age, blood group, and number of transfusions.
Results
The researchers found that women were more likely to receive sex-discordant transfusions than same-sex transfusions—45.3% and 19.8%, respectively. And patients who received sex-discordant transfusions tended to receive more transfusions—a mean of 4.2 vs 2.0 for same-sex transfusions.
However, there were no other significant differences between the sex-discordant and same-sex groups.
The researchers noted that, during the 30-day follow-up period, there were more deaths among patients who received sex-discordant transfusions than those who did not—1701 (4.9%) and 205 (1.9%), respectively.
However, when the team adjusted for potential confounding factors, the relative risk of death was similar for patients who received at least 1 unit of sex-discordant blood and those who did not. The hazard ratio was 0.97 at 30 days of follow-up, 0.97 at the 2-year mark, and 0.98 at 10 years of follow-up.
The risk of death did increase as the number of sex-discordant units transfused increased. However, the increase was not statistically significant. ![]()

Photo by Elise Amendola
There is no association between sex-discordant blood transfusions and the risk of death after cardiac surgery, according to research published in Circulation.
Two previous studies suggested that patients who received red blood cells (RBCs) from a donor of the opposite sex had an increased risk of death after cardiac surgery.
However, the current study showed no significant difference between same-sex and opposite-sex donor-recipient pairs.
The researchers said the reason for the difference between the new and older studies is that, in the new study, the team “carefully adjusted” for the number of transfusions performed and allowed for the effect of RBC transfusions on mortality to differ between men and women.
“The consequences of the findings from [the earlier studies], if proved true, would have been immense and necessitated radical changes to how blood transfusions are managed around the world,” said Martin Holzmann, MD, PhD, of Karolinska Institutet in Stockholm, Sweden.
“Our results clearly show that there is no real connection between sex-discordant blood transfusions and the risk of death.”
Therefore, Dr Holzmann and his colleagues believe there is no need to consider donor sex when allocating RBC units for transfusion.
To come to this conclusion, the researchers analyzed data on 45,090 patients who underwent cardiac surgery and received at least 1 RBC transfusion.
All patients were adults who had undergone isolated coronary artery bypass grafting, isolated valve repair/replacement surgery, or a combination of these procedures between 1997 and 2012.
The researchers estimated the relative hazard of death in relation to exposure to sex-discordant transfusions, adjusting their analyses for potential confounding factors, such as patient sex, age, blood group, and number of transfusions.
Results
The researchers found that women were more likely to receive sex-discordant transfusions than same-sex transfusions—45.3% and 19.8%, respectively. And patients who received sex-discordant transfusions tended to receive more transfusions—a mean of 4.2 vs 2.0 for same-sex transfusions.
However, there were no other significant differences between the sex-discordant and same-sex groups.
The researchers noted that, during the 30-day follow-up period, there were more deaths among patients who received sex-discordant transfusions than those who did not—1701 (4.9%) and 205 (1.9%), respectively.
However, when the team adjusted for potential confounding factors, the relative risk of death was similar for patients who received at least 1 unit of sex-discordant blood and those who did not. The hazard ratio was 0.97 at 30 days of follow-up, 0.97 at the 2-year mark, and 0.98 at 10 years of follow-up.
The risk of death did increase as the number of sex-discordant units transfused increased. However, the increase was not statistically significant. ![]()
EC grants drug orphan designation for PNH

The European Commission (EC) has granted orphan drug designation to RA101495 for the treatment of paroxysmal nocturnal hemoglobinuria (PNH).
RA101495 is a synthetic macrocyclic peptide inhibitor of complement component C5.
Ra Pharmaceuticals is developing RA101495 as a self-administered, subcutaneous injection for the treatment of PNH, refractory generalized myasthenia gravis, and lupus nephritis.
RA101495 binds complement C5 with subnanomolar affinity and allosterically inhibits its cleavage into C5a and C5b upon activation of the classical, alternative, or lectin pathways.
RA101495 also directly binds to C5b, disrupting the interaction between C5b and C6 and preventing assembly of the membrane attack complex.
According to Ra Pharmaceuticals, repeat dosing of RA101495 in vivo has demonstrated “sustained and predictable” inhibition of complement activity with an “excellent” safety profile.
The company also said phase 1 data have suggested that RA101495 is potent inhibitor of C5-mediated hemolysis with a favorable safety profile.
Preclinical research involving RA101495 was presented at the 2015 ASH Annual Meeting, and phase 1 data were presented at the 21st Congress of the European Hematology Association earlier this year.
RA101495’s orphan designation
The EC grants orphan designation to therapies intended to treat life-threatening or chronically debilitating conditions affecting no more than 5 in 10,000 people in the European Union, and where no satisfactory treatment is available.
In situations where there is already an approved standard of care—such as with PNH, where the monoclonal antibody eculizumab (Soliris) is currently available—the EC requires companies developing a potential orphan drug to provide evidence that the drug is expected to provide significant benefits over the standard of care.
In the case of RA101495, the decision to grant orphan designation was based on the potential for improved patient convenience with subcutaneous self-administration, as well as the potential to treat patients who do not respond to eculizumab.
Orphan designation provides the company developing a drug with regulatory and financial incentives, including protocol assistance, 10 years of market exclusivity once the drug is approved, and, in some cases, reductions in fees. ![]()

The European Commission (EC) has granted orphan drug designation to RA101495 for the treatment of paroxysmal nocturnal hemoglobinuria (PNH).
RA101495 is a synthetic macrocyclic peptide inhibitor of complement component C5.
Ra Pharmaceuticals is developing RA101495 as a self-administered, subcutaneous injection for the treatment of PNH, refractory generalized myasthenia gravis, and lupus nephritis.
RA101495 binds complement C5 with subnanomolar affinity and allosterically inhibits its cleavage into C5a and C5b upon activation of the classical, alternative, or lectin pathways.
RA101495 also directly binds to C5b, disrupting the interaction between C5b and C6 and preventing assembly of the membrane attack complex.
According to Ra Pharmaceuticals, repeat dosing of RA101495 in vivo has demonstrated “sustained and predictable” inhibition of complement activity with an “excellent” safety profile.
The company also said phase 1 data have suggested that RA101495 is potent inhibitor of C5-mediated hemolysis with a favorable safety profile.
Preclinical research involving RA101495 was presented at the 2015 ASH Annual Meeting, and phase 1 data were presented at the 21st Congress of the European Hematology Association earlier this year.
RA101495’s orphan designation
The EC grants orphan designation to therapies intended to treat life-threatening or chronically debilitating conditions affecting no more than 5 in 10,000 people in the European Union, and where no satisfactory treatment is available.
In situations where there is already an approved standard of care—such as with PNH, where the monoclonal antibody eculizumab (Soliris) is currently available—the EC requires companies developing a potential orphan drug to provide evidence that the drug is expected to provide significant benefits over the standard of care.
In the case of RA101495, the decision to grant orphan designation was based on the potential for improved patient convenience with subcutaneous self-administration, as well as the potential to treat patients who do not respond to eculizumab.
Orphan designation provides the company developing a drug with regulatory and financial incentives, including protocol assistance, 10 years of market exclusivity once the drug is approved, and, in some cases, reductions in fees. ![]()

The European Commission (EC) has granted orphan drug designation to RA101495 for the treatment of paroxysmal nocturnal hemoglobinuria (PNH).
RA101495 is a synthetic macrocyclic peptide inhibitor of complement component C5.
Ra Pharmaceuticals is developing RA101495 as a self-administered, subcutaneous injection for the treatment of PNH, refractory generalized myasthenia gravis, and lupus nephritis.
RA101495 binds complement C5 with subnanomolar affinity and allosterically inhibits its cleavage into C5a and C5b upon activation of the classical, alternative, or lectin pathways.
RA101495 also directly binds to C5b, disrupting the interaction between C5b and C6 and preventing assembly of the membrane attack complex.
According to Ra Pharmaceuticals, repeat dosing of RA101495 in vivo has demonstrated “sustained and predictable” inhibition of complement activity with an “excellent” safety profile.
The company also said phase 1 data have suggested that RA101495 is potent inhibitor of C5-mediated hemolysis with a favorable safety profile.
Preclinical research involving RA101495 was presented at the 2015 ASH Annual Meeting, and phase 1 data were presented at the 21st Congress of the European Hematology Association earlier this year.
RA101495’s orphan designation
The EC grants orphan designation to therapies intended to treat life-threatening or chronically debilitating conditions affecting no more than 5 in 10,000 people in the European Union, and where no satisfactory treatment is available.
In situations where there is already an approved standard of care—such as with PNH, where the monoclonal antibody eculizumab (Soliris) is currently available—the EC requires companies developing a potential orphan drug to provide evidence that the drug is expected to provide significant benefits over the standard of care.
In the case of RA101495, the decision to grant orphan designation was based on the potential for improved patient convenience with subcutaneous self-administration, as well as the potential to treat patients who do not respond to eculizumab.
Orphan designation provides the company developing a drug with regulatory and financial incentives, including protocol assistance, 10 years of market exclusivity once the drug is approved, and, in some cases, reductions in fees. ![]()
Robotic Technology Produces More Conservative Tibial Resection Than Conventional Techniques in UKA
Unicompartmental knee arthroplasty (UKA) is considered a less invasive approach for the treatment of unicompartmental knee arthritis when compared with total knee arthroplasty (TKA), with optimal preservation of kinematics.1 Despite excellent functional outcomes, conversion to TKA may be necessary if the UKA fails, or in patients with progressive knee arthritis. Some studies have found UKA conversion to TKA to be comparable with primary TKA,2,3 whereas others have found that conversion often requires bone graft, augments, and stemmed components and has increased complications and inferior results compared to primary TKA.4-7 While some studies report that <10% of UKA conversions to TKA require augments,2 others have found that as many as 76% require augments.4-8
Schwarzkopf and colleagues9 recently demonstrated that UKA conversion to TKA is comparable with primary TKA when a conservative tibial resection is performed during the index procedure. However, they reported increased complexity when greater tibial resection was performed and thicker polyethylene inserts were used at the time of the index UKA. The odds ratio of needing an augment or stem during the conversion to TKA was 26.8 (95% confidence interval, 3.71-194) when an aggressive tibial resection was performed during the UKA.9 Tibial resection thickness may thus be predictive of anticipated complexity of UKA revision to TKA and may aid in preoperative planning.
Robotic assistance has been shown to enhance the accuracy of bone preparation, implant component alignment, and soft tissue balance in UKA.10-15 It has yet to be determined whether this improved accuracy translates to improved clinical performance or longevity of the UKA implant. However, the enhanced accuracy of robotic technology may result in more conservative tibial resection when compared to conventional UKA and may be advantageous if conversion to TKA becomes necessary.
The purpose of this study was to compare the distribution of polyethylene insert sizes implanted during conventional and robotic-assisted UKA. We hypothesized that robotic assistance would demonstrate more conservative tibial resection compared to conventional methods of bone preparation.
Methods
We retrospectively compared the distribution of polyethylene insert sizes implanted during consecutive conventional and robotic-assisted UKA procedures. Several manufacturers were queried to provide a listing of the polyethylene insert sizes utilized, ranging from 8 mm to 14 mm. The analysis included 8421 robotic-assisted UKA cases and 27,989 conventional UKA cases. Data were provided by Zimmer Biomet and Smith & Nephew regarding conventional cases, as well as Blue Belt Technologies (now part of Smith & Nephew) and MAKO Surgical (now part of Stryker) regarding robotic-assisted cases. (Dr. Lonner has an ongoing relationship as a consultant with Blue Belt Technologies, whose data was utilized in this study.) Using tibial insert thickness as a surrogate measure of the extent of tibial resection, an insert size of ≥10 mm was defined as aggressive while <10 mm was considered conservative. This cutoff was established based on its corresponding resection level with primary TKA and the anticipated need for augments. Statistical analysis was performed using a Mann-Whitney-Wilcoxon test. Significance was set at P < .05.
Results
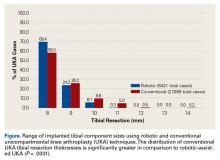
Tibial resection thickness was found to be most commonly conservative in nature, with sizes 8-mm and 9-mm polyethylene inserts utilized in the majority of both robotic-assisted and conventional UKA cases. However, statistically more 8-mm and 9-mm polyethylene inserts were used in the robotic group (93.6%) than in the conventional group (84.5%) (P < .0001; Figure). Aggressive tibial resection, requiring tibial inserts ≥10 mm, was performed in 6.4% of robotic-assisted cases and 15.5% of conventional cases.
Discussion
Robotic assistance enhances the accuracy of bone preparation, implant component alignment, and soft tissue balance in UKA.10-15 It has yet to be determined whether this improved accuracy translates to improved clinical performance or longevity of the UKA implant. However, we demonstrate that the enhanced accuracy of robotic technology results in more conservative tibial resection when compared to conventional techniques with a potential benefit suggested in the literature upon conversion to TKA.
The findings of this study have important implications for patients undergoing conversion of UKA to TKA, potentially optimizing the ease of revision and clinical outcomes. The outcomes of UKA conversion to TKA are often considered inferior to those of primary TKA, compromised by bone loss, need for augmentation, and challenges of restoring the joint line and rotation.9,16-22 Barrett and Scott18 reported only 66% of patients had good or excellent results at an average of 4.6 years of follow-up after UKA conversion to TKA. Over 50% required stemmed implants and bone graft or bone cement augmentation to address osseous insufficiency. The authors suggested that the primary determinant of the complexity of the conversion to TKA was the surgical technique used in the index procedure. They concluded that UKA conversion to TKA can be as successful as a primary TKA and primary TKA implants can be used without bone augmentation or stems during the revision procedure if minimal tibial bone is resected at the time of the index UKA.18 Schwarzkopf and colleagues9 supported this conclusion when they found that aggressive tibial resection during UKA resulted in the need for bone graft, stem, wedge, or augment in 70% of cases when converted to TKA. Similarly, Khan and colleagues23 found that 26% of patients required bone grafting and 26% required some form of augmentation, and Springer and colleagues3 reported that 68% required a graft, augment, or stem.3,22 Using data from the New Zealand Joint Registry, Pearse and colleagues5 reported that revision TKA components were necessary in 28% of patients and concluded that converting a UKA to TKA gives a less reliable result than primary TKA, and with functional results that are not significantly better than a revision from a TKA.
Conservative tibial resection during UKA minimizes the complexity and concerns of bone loss upon conversion to TKA. Schwarzkopf and colleagues9 found 96.6% of patients with conservative tibial resection received a primary TKA implant, without augments or stems. Furthermore, patients with a primary TKA implant showed improved tibial survivorship, with revision as an end point, compared with patients who received a TKA implant that required stems and augments or bone graft for support.9 Also emphasizing the importance of minimal tibial resection, O’Donnell and colleagues8 compared a cohort of patients undergoing conversion of a minimal resection resurfacing onlay-type UKA to TKA with a cohort of patients undergoing primary TKA. They found that 40% of patients required bone grafting for contained defects, 3.6% required metal augments, and 1.8% required stems.8 There was no significant difference between the groups in terms of range of motion, functional outcome, or radiologic outcomes. The authors concluded that revision of minimal resection resurfacing implants to TKA is associated with similar results to primary TKA and is superior to revision of UKA with greater bone loss. Prior studies have shown that one of the advantages of robotic-assisted UKA is the accuracy and precision of bone resection. The present study supports this premise by showing that tibial resection is significantly more conservative using robotic-assisted techniques when using tibial component thickness as a surrogate for extent of bone resection. While our study did not address implant durability or the impact of conservative resection on conversion to TKA, studies referenced above suggest that the conservative nature of bone preparation would have a relevant impact on the revision of the implant to TKA.
Our study is a retrospective case series that reports tibial component thickness as a surrogate for volume of tibial resection during UKA. While the implication is that more conservative tibial resection may optimize durability and ease of conversion to TKA, future study will be needed to compare robotic-assisted and conventional cases of UKA upon conversion to TKA in order to ascertain whether the more conventional resections of robotic-assisted UKA in fact lead to revision that is comparable with primary TKA in terms of bone loss at the time of revision, components utilized, the need for bone graft, augments, or stems, and clinical outcomes. Given the method of data collection in this study, we could not control for clinical deformity, selection bias, surgeon experience, or medial vs lateral knee compartments. These potential confounders represent weaknesses of this study.
In conclusion, conversion of UKA to TKA may be associated with significant osseous insufficiency, which may compromise patient outcomes in comparison to primary TKA. Studies have shown that UKA conversion to TKA is comparable to primary TKA when minimal tibial resection is performed during the UKA, and the need for augmentation, grafting or stems is increased with more aggressive tibial resection. This study has shown that when robotic assistance is utilized, tibial resection is more precise, less variable, and more conservative compared to conventional techniques.
Am J Orthop. 2016;45(7):E465-E468. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Patil S, Colwell CW Jr, Ezzet KA, D’Lima DD. Can normal knee kinematics be restored with unicompartmental knee replacement? J Bone Joint Surg Am. 2005;87(2):332-338.
2. Johnson S, Jones P, Newman JH. The survivorship and results of total knee replacements converted from unicompartmental knee replacements. Knee. 2007;14(2):154-157.
3. Springer BD, Scott RD, Thornhill TS. Conversion of failed unicompartmental knee arthroplasty to TKA. Clin Orthop Relat Res. 2006;446:214-220.
4. Järvenpää J, Kettunen J, Miettinen H, Kröger H. The clinical outcome of revision knee replacement after unicompartmental knee arthroplasty versus primary total knee arthroplasty: 8-17 years follow-up study of 49 patients. Int Orthop. 2010;34(5):649-653.
5. Pearse AJ, Hooper GJ, Rothwell AG, Frampton C. Osteotomy and unicompartmental knee arthroplasty converted to total knee arthroplasty: data from the New Zealand Joint Registry. J Arthroplasty. 2012;27(10):1827-1831.
6. Rancourt MF, Kemp KA, Plamondon SM, Kim PR, Dervin GF. Unicompartmental knee arthroplasties revised to total knee arthroplasties compared with primary total knee arthroplasties. J Arthroplasty. 2012;27(8 Suppl):106-110.
7. Sierra RJ, Kassel CA, Wetters NG, Berend KR, Della Valle CJ, Lombardi AV. Revision of unicompartmental arthroplasty to total knee arthroplasty: not always a slam dunk! J Arthroplasty. 2013;28(8 Suppl):128-132.
8. O’Donnell TM, Abouazza O, Neil MJ. Revision of minimal resection resurfacing unicondylar knee arthroplasty to total knee arthroplasty: results compared with primary total knee arthroplasty. J Arthroplasty. 2013;28(1):33-39.
9. Schwarzkopf R, Mikhael B, Li L, Josephs L, Scott RD. Effect of initial tibial resection thickness on outcomes of revision UKA. Orthopedics. 2013;36(4):e409-e414.
10. Conditt MA, Roche MW. Minimally invasive robotic-arm-guided unicompartmental knee arthroplasty. J Bone Joint Surg Am. 2009;91 Suppl 1:63-68.
11. Dunbar NJ, Roche MW, Park BH, Branch SH, Conditt MA, Banks SA. Accuracy of dynamic tactile-guided unicompartmental knee arthroplasty. J Arthroplasty. 2012;27(5):803-808.e1.
12. Karia M, Masjedi M, Andrews B, Jaffry Z, Cobb J. Robotic assistance enables inexperienced surgeons to perform unicompartmental knee arthroplasties on dry bone models with accuracy superior to conventional methods. Adv Orthop. 2013;2013:481039.
13. Lonner JH, John TK, Conditt MA. Robotic arm-assisted UKA improves tibial component alignment: a pilot study. Clin Orthop Relat Res. 2010;468(1):141-146.
14. Lonner JH, Smith JR, Picard F, Hamlin B, Rowe PJ, Riches PE. High degree of accuracy of a novel image-free handheld robot for unicondylar knee arthroplasty in a cadaveric study. Clin Orthop Relat Res. 2015;473(1):206-212.
15. Smith JR, Picard F, Rowe PJ, Deakin A, Riches PE. The accuracy of a robotically-controlled freehand sculpting tool for unicondylar knee arthroplasty. Bone Joint J. 2013;95-B(suppl 28):68.
16. Chakrabarty G, Newman JH, Ackroyd CE. Revision of unicompartmental arthroplasty of the knee. Clinical and technical considerations. J Arthroplasty. 1998;13(2):191-196.
17. Levine WN, Ozuna RM, Scott RD, Thornhill TS. Conversion of failed modern unicompartmental arthroplasty to total knee arthroplasty. J Arthroplasty. 1996;11(7):797-801.
18. Barrett WP, Scott RD. Revision of failed unicondylar unicompartmental knee arthroplasty. J Bone Joint Surg Am. 1987;69(9):1328-1335.
19. Padgett DE, Stern SH, Insall JN. Revision total knee arthroplasty for failed unicompartmental replacement. J Bone Joint Surg Am. 1991;73(2):186-190.
20. Aleto TJ, Berend ME, Ritter MA, Faris PM, Meneghini RM. Early failure of unicompartmental knee arthroplasty leading to revision. J Arthroplasty. 2008;23(2):159-163.
21. McAuley JP, Engh GA, Ammeen DJ. Revision of failed unicompartmental knee arthroplasty. Clin Orthop Relat Res. 2001;(392):279-282.22. Böhm I, Landsiedl F. Revision surgery after failed unicompartmental knee arthroplasty: a study of 35 cases. J Arthroplasty. 2000;15(8):982-989.
23. Khan Z, Nawaz SZ, Kahane S, Ester C, Chatterji U. Conversion of unicompartmental knee arthroplasty to total knee arthroplasty: the challenges and need for augments. Acta Orthop Belg. 2013;79(6):699-705.
Unicompartmental knee arthroplasty (UKA) is considered a less invasive approach for the treatment of unicompartmental knee arthritis when compared with total knee arthroplasty (TKA), with optimal preservation of kinematics.1 Despite excellent functional outcomes, conversion to TKA may be necessary if the UKA fails, or in patients with progressive knee arthritis. Some studies have found UKA conversion to TKA to be comparable with primary TKA,2,3 whereas others have found that conversion often requires bone graft, augments, and stemmed components and has increased complications and inferior results compared to primary TKA.4-7 While some studies report that <10% of UKA conversions to TKA require augments,2 others have found that as many as 76% require augments.4-8
Schwarzkopf and colleagues9 recently demonstrated that UKA conversion to TKA is comparable with primary TKA when a conservative tibial resection is performed during the index procedure. However, they reported increased complexity when greater tibial resection was performed and thicker polyethylene inserts were used at the time of the index UKA. The odds ratio of needing an augment or stem during the conversion to TKA was 26.8 (95% confidence interval, 3.71-194) when an aggressive tibial resection was performed during the UKA.9 Tibial resection thickness may thus be predictive of anticipated complexity of UKA revision to TKA and may aid in preoperative planning.
Robotic assistance has been shown to enhance the accuracy of bone preparation, implant component alignment, and soft tissue balance in UKA.10-15 It has yet to be determined whether this improved accuracy translates to improved clinical performance or longevity of the UKA implant. However, the enhanced accuracy of robotic technology may result in more conservative tibial resection when compared to conventional UKA and may be advantageous if conversion to TKA becomes necessary.
The purpose of this study was to compare the distribution of polyethylene insert sizes implanted during conventional and robotic-assisted UKA. We hypothesized that robotic assistance would demonstrate more conservative tibial resection compared to conventional methods of bone preparation.
Methods
We retrospectively compared the distribution of polyethylene insert sizes implanted during consecutive conventional and robotic-assisted UKA procedures. Several manufacturers were queried to provide a listing of the polyethylene insert sizes utilized, ranging from 8 mm to 14 mm. The analysis included 8421 robotic-assisted UKA cases and 27,989 conventional UKA cases. Data were provided by Zimmer Biomet and Smith & Nephew regarding conventional cases, as well as Blue Belt Technologies (now part of Smith & Nephew) and MAKO Surgical (now part of Stryker) regarding robotic-assisted cases. (Dr. Lonner has an ongoing relationship as a consultant with Blue Belt Technologies, whose data was utilized in this study.) Using tibial insert thickness as a surrogate measure of the extent of tibial resection, an insert size of ≥10 mm was defined as aggressive while <10 mm was considered conservative. This cutoff was established based on its corresponding resection level with primary TKA and the anticipated need for augments. Statistical analysis was performed using a Mann-Whitney-Wilcoxon test. Significance was set at P < .05.
Results
Tibial resection thickness was found to be most commonly conservative in nature, with sizes 8-mm and 9-mm polyethylene inserts utilized in the majority of both robotic-assisted and conventional UKA cases. However, statistically more 8-mm and 9-mm polyethylene inserts were used in the robotic group (93.6%) than in the conventional group (84.5%) (P < .0001; Figure). Aggressive tibial resection, requiring tibial inserts ≥10 mm, was performed in 6.4% of robotic-assisted cases and 15.5% of conventional cases.
Discussion
Robotic assistance enhances the accuracy of bone preparation, implant component alignment, and soft tissue balance in UKA.10-15 It has yet to be determined whether this improved accuracy translates to improved clinical performance or longevity of the UKA implant. However, we demonstrate that the enhanced accuracy of robotic technology results in more conservative tibial resection when compared to conventional techniques with a potential benefit suggested in the literature upon conversion to TKA.
The findings of this study have important implications for patients undergoing conversion of UKA to TKA, potentially optimizing the ease of revision and clinical outcomes. The outcomes of UKA conversion to TKA are often considered inferior to those of primary TKA, compromised by bone loss, need for augmentation, and challenges of restoring the joint line and rotation.9,16-22 Barrett and Scott18 reported only 66% of patients had good or excellent results at an average of 4.6 years of follow-up after UKA conversion to TKA. Over 50% required stemmed implants and bone graft or bone cement augmentation to address osseous insufficiency. The authors suggested that the primary determinant of the complexity of the conversion to TKA was the surgical technique used in the index procedure. They concluded that UKA conversion to TKA can be as successful as a primary TKA and primary TKA implants can be used without bone augmentation or stems during the revision procedure if minimal tibial bone is resected at the time of the index UKA.18 Schwarzkopf and colleagues9 supported this conclusion when they found that aggressive tibial resection during UKA resulted in the need for bone graft, stem, wedge, or augment in 70% of cases when converted to TKA. Similarly, Khan and colleagues23 found that 26% of patients required bone grafting and 26% required some form of augmentation, and Springer and colleagues3 reported that 68% required a graft, augment, or stem.3,22 Using data from the New Zealand Joint Registry, Pearse and colleagues5 reported that revision TKA components were necessary in 28% of patients and concluded that converting a UKA to TKA gives a less reliable result than primary TKA, and with functional results that are not significantly better than a revision from a TKA.
Conservative tibial resection during UKA minimizes the complexity and concerns of bone loss upon conversion to TKA. Schwarzkopf and colleagues9 found 96.6% of patients with conservative tibial resection received a primary TKA implant, without augments or stems. Furthermore, patients with a primary TKA implant showed improved tibial survivorship, with revision as an end point, compared with patients who received a TKA implant that required stems and augments or bone graft for support.9 Also emphasizing the importance of minimal tibial resection, O’Donnell and colleagues8 compared a cohort of patients undergoing conversion of a minimal resection resurfacing onlay-type UKA to TKA with a cohort of patients undergoing primary TKA. They found that 40% of patients required bone grafting for contained defects, 3.6% required metal augments, and 1.8% required stems.8 There was no significant difference between the groups in terms of range of motion, functional outcome, or radiologic outcomes. The authors concluded that revision of minimal resection resurfacing implants to TKA is associated with similar results to primary TKA and is superior to revision of UKA with greater bone loss. Prior studies have shown that one of the advantages of robotic-assisted UKA is the accuracy and precision of bone resection. The present study supports this premise by showing that tibial resection is significantly more conservative using robotic-assisted techniques when using tibial component thickness as a surrogate for extent of bone resection. While our study did not address implant durability or the impact of conservative resection on conversion to TKA, studies referenced above suggest that the conservative nature of bone preparation would have a relevant impact on the revision of the implant to TKA.
Our study is a retrospective case series that reports tibial component thickness as a surrogate for volume of tibial resection during UKA. While the implication is that more conservative tibial resection may optimize durability and ease of conversion to TKA, future study will be needed to compare robotic-assisted and conventional cases of UKA upon conversion to TKA in order to ascertain whether the more conventional resections of robotic-assisted UKA in fact lead to revision that is comparable with primary TKA in terms of bone loss at the time of revision, components utilized, the need for bone graft, augments, or stems, and clinical outcomes. Given the method of data collection in this study, we could not control for clinical deformity, selection bias, surgeon experience, or medial vs lateral knee compartments. These potential confounders represent weaknesses of this study.
In conclusion, conversion of UKA to TKA may be associated with significant osseous insufficiency, which may compromise patient outcomes in comparison to primary TKA. Studies have shown that UKA conversion to TKA is comparable to primary TKA when minimal tibial resection is performed during the UKA, and the need for augmentation, grafting or stems is increased with more aggressive tibial resection. This study has shown that when robotic assistance is utilized, tibial resection is more precise, less variable, and more conservative compared to conventional techniques.
Am J Orthop. 2016;45(7):E465-E468. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Unicompartmental knee arthroplasty (UKA) is considered a less invasive approach for the treatment of unicompartmental knee arthritis when compared with total knee arthroplasty (TKA), with optimal preservation of kinematics.1 Despite excellent functional outcomes, conversion to TKA may be necessary if the UKA fails, or in patients with progressive knee arthritis. Some studies have found UKA conversion to TKA to be comparable with primary TKA,2,3 whereas others have found that conversion often requires bone graft, augments, and stemmed components and has increased complications and inferior results compared to primary TKA.4-7 While some studies report that <10% of UKA conversions to TKA require augments,2 others have found that as many as 76% require augments.4-8
Schwarzkopf and colleagues9 recently demonstrated that UKA conversion to TKA is comparable with primary TKA when a conservative tibial resection is performed during the index procedure. However, they reported increased complexity when greater tibial resection was performed and thicker polyethylene inserts were used at the time of the index UKA. The odds ratio of needing an augment or stem during the conversion to TKA was 26.8 (95% confidence interval, 3.71-194) when an aggressive tibial resection was performed during the UKA.9 Tibial resection thickness may thus be predictive of anticipated complexity of UKA revision to TKA and may aid in preoperative planning.
Robotic assistance has been shown to enhance the accuracy of bone preparation, implant component alignment, and soft tissue balance in UKA.10-15 It has yet to be determined whether this improved accuracy translates to improved clinical performance or longevity of the UKA implant. However, the enhanced accuracy of robotic technology may result in more conservative tibial resection when compared to conventional UKA and may be advantageous if conversion to TKA becomes necessary.
The purpose of this study was to compare the distribution of polyethylene insert sizes implanted during conventional and robotic-assisted UKA. We hypothesized that robotic assistance would demonstrate more conservative tibial resection compared to conventional methods of bone preparation.
Methods
We retrospectively compared the distribution of polyethylene insert sizes implanted during consecutive conventional and robotic-assisted UKA procedures. Several manufacturers were queried to provide a listing of the polyethylene insert sizes utilized, ranging from 8 mm to 14 mm. The analysis included 8421 robotic-assisted UKA cases and 27,989 conventional UKA cases. Data were provided by Zimmer Biomet and Smith & Nephew regarding conventional cases, as well as Blue Belt Technologies (now part of Smith & Nephew) and MAKO Surgical (now part of Stryker) regarding robotic-assisted cases. (Dr. Lonner has an ongoing relationship as a consultant with Blue Belt Technologies, whose data was utilized in this study.) Using tibial insert thickness as a surrogate measure of the extent of tibial resection, an insert size of ≥10 mm was defined as aggressive while <10 mm was considered conservative. This cutoff was established based on its corresponding resection level with primary TKA and the anticipated need for augments. Statistical analysis was performed using a Mann-Whitney-Wilcoxon test. Significance was set at P < .05.
Results
Tibial resection thickness was found to be most commonly conservative in nature, with sizes 8-mm and 9-mm polyethylene inserts utilized in the majority of both robotic-assisted and conventional UKA cases. However, statistically more 8-mm and 9-mm polyethylene inserts were used in the robotic group (93.6%) than in the conventional group (84.5%) (P < .0001; Figure). Aggressive tibial resection, requiring tibial inserts ≥10 mm, was performed in 6.4% of robotic-assisted cases and 15.5% of conventional cases.
Discussion
Robotic assistance enhances the accuracy of bone preparation, implant component alignment, and soft tissue balance in UKA.10-15 It has yet to be determined whether this improved accuracy translates to improved clinical performance or longevity of the UKA implant. However, we demonstrate that the enhanced accuracy of robotic technology results in more conservative tibial resection when compared to conventional techniques with a potential benefit suggested in the literature upon conversion to TKA.
The findings of this study have important implications for patients undergoing conversion of UKA to TKA, potentially optimizing the ease of revision and clinical outcomes. The outcomes of UKA conversion to TKA are often considered inferior to those of primary TKA, compromised by bone loss, need for augmentation, and challenges of restoring the joint line and rotation.9,16-22 Barrett and Scott18 reported only 66% of patients had good or excellent results at an average of 4.6 years of follow-up after UKA conversion to TKA. Over 50% required stemmed implants and bone graft or bone cement augmentation to address osseous insufficiency. The authors suggested that the primary determinant of the complexity of the conversion to TKA was the surgical technique used in the index procedure. They concluded that UKA conversion to TKA can be as successful as a primary TKA and primary TKA implants can be used without bone augmentation or stems during the revision procedure if minimal tibial bone is resected at the time of the index UKA.18 Schwarzkopf and colleagues9 supported this conclusion when they found that aggressive tibial resection during UKA resulted in the need for bone graft, stem, wedge, or augment in 70% of cases when converted to TKA. Similarly, Khan and colleagues23 found that 26% of patients required bone grafting and 26% required some form of augmentation, and Springer and colleagues3 reported that 68% required a graft, augment, or stem.3,22 Using data from the New Zealand Joint Registry, Pearse and colleagues5 reported that revision TKA components were necessary in 28% of patients and concluded that converting a UKA to TKA gives a less reliable result than primary TKA, and with functional results that are not significantly better than a revision from a TKA.
Conservative tibial resection during UKA minimizes the complexity and concerns of bone loss upon conversion to TKA. Schwarzkopf and colleagues9 found 96.6% of patients with conservative tibial resection received a primary TKA implant, without augments or stems. Furthermore, patients with a primary TKA implant showed improved tibial survivorship, with revision as an end point, compared with patients who received a TKA implant that required stems and augments or bone graft for support.9 Also emphasizing the importance of minimal tibial resection, O’Donnell and colleagues8 compared a cohort of patients undergoing conversion of a minimal resection resurfacing onlay-type UKA to TKA with a cohort of patients undergoing primary TKA. They found that 40% of patients required bone grafting for contained defects, 3.6% required metal augments, and 1.8% required stems.8 There was no significant difference between the groups in terms of range of motion, functional outcome, or radiologic outcomes. The authors concluded that revision of minimal resection resurfacing implants to TKA is associated with similar results to primary TKA and is superior to revision of UKA with greater bone loss. Prior studies have shown that one of the advantages of robotic-assisted UKA is the accuracy and precision of bone resection. The present study supports this premise by showing that tibial resection is significantly more conservative using robotic-assisted techniques when using tibial component thickness as a surrogate for extent of bone resection. While our study did not address implant durability or the impact of conservative resection on conversion to TKA, studies referenced above suggest that the conservative nature of bone preparation would have a relevant impact on the revision of the implant to TKA.
Our study is a retrospective case series that reports tibial component thickness as a surrogate for volume of tibial resection during UKA. While the implication is that more conservative tibial resection may optimize durability and ease of conversion to TKA, future study will be needed to compare robotic-assisted and conventional cases of UKA upon conversion to TKA in order to ascertain whether the more conventional resections of robotic-assisted UKA in fact lead to revision that is comparable with primary TKA in terms of bone loss at the time of revision, components utilized, the need for bone graft, augments, or stems, and clinical outcomes. Given the method of data collection in this study, we could not control for clinical deformity, selection bias, surgeon experience, or medial vs lateral knee compartments. These potential confounders represent weaknesses of this study.
In conclusion, conversion of UKA to TKA may be associated with significant osseous insufficiency, which may compromise patient outcomes in comparison to primary TKA. Studies have shown that UKA conversion to TKA is comparable to primary TKA when minimal tibial resection is performed during the UKA, and the need for augmentation, grafting or stems is increased with more aggressive tibial resection. This study has shown that when robotic assistance is utilized, tibial resection is more precise, less variable, and more conservative compared to conventional techniques.
Am J Orthop. 2016;45(7):E465-E468. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Patil S, Colwell CW Jr, Ezzet KA, D’Lima DD. Can normal knee kinematics be restored with unicompartmental knee replacement? J Bone Joint Surg Am. 2005;87(2):332-338.
2. Johnson S, Jones P, Newman JH. The survivorship and results of total knee replacements converted from unicompartmental knee replacements. Knee. 2007;14(2):154-157.
3. Springer BD, Scott RD, Thornhill TS. Conversion of failed unicompartmental knee arthroplasty to TKA. Clin Orthop Relat Res. 2006;446:214-220.
4. Järvenpää J, Kettunen J, Miettinen H, Kröger H. The clinical outcome of revision knee replacement after unicompartmental knee arthroplasty versus primary total knee arthroplasty: 8-17 years follow-up study of 49 patients. Int Orthop. 2010;34(5):649-653.
5. Pearse AJ, Hooper GJ, Rothwell AG, Frampton C. Osteotomy and unicompartmental knee arthroplasty converted to total knee arthroplasty: data from the New Zealand Joint Registry. J Arthroplasty. 2012;27(10):1827-1831.
6. Rancourt MF, Kemp KA, Plamondon SM, Kim PR, Dervin GF. Unicompartmental knee arthroplasties revised to total knee arthroplasties compared with primary total knee arthroplasties. J Arthroplasty. 2012;27(8 Suppl):106-110.
7. Sierra RJ, Kassel CA, Wetters NG, Berend KR, Della Valle CJ, Lombardi AV. Revision of unicompartmental arthroplasty to total knee arthroplasty: not always a slam dunk! J Arthroplasty. 2013;28(8 Suppl):128-132.
8. O’Donnell TM, Abouazza O, Neil MJ. Revision of minimal resection resurfacing unicondylar knee arthroplasty to total knee arthroplasty: results compared with primary total knee arthroplasty. J Arthroplasty. 2013;28(1):33-39.
9. Schwarzkopf R, Mikhael B, Li L, Josephs L, Scott RD. Effect of initial tibial resection thickness on outcomes of revision UKA. Orthopedics. 2013;36(4):e409-e414.
10. Conditt MA, Roche MW. Minimally invasive robotic-arm-guided unicompartmental knee arthroplasty. J Bone Joint Surg Am. 2009;91 Suppl 1:63-68.
11. Dunbar NJ, Roche MW, Park BH, Branch SH, Conditt MA, Banks SA. Accuracy of dynamic tactile-guided unicompartmental knee arthroplasty. J Arthroplasty. 2012;27(5):803-808.e1.
12. Karia M, Masjedi M, Andrews B, Jaffry Z, Cobb J. Robotic assistance enables inexperienced surgeons to perform unicompartmental knee arthroplasties on dry bone models with accuracy superior to conventional methods. Adv Orthop. 2013;2013:481039.
13. Lonner JH, John TK, Conditt MA. Robotic arm-assisted UKA improves tibial component alignment: a pilot study. Clin Orthop Relat Res. 2010;468(1):141-146.
14. Lonner JH, Smith JR, Picard F, Hamlin B, Rowe PJ, Riches PE. High degree of accuracy of a novel image-free handheld robot for unicondylar knee arthroplasty in a cadaveric study. Clin Orthop Relat Res. 2015;473(1):206-212.
15. Smith JR, Picard F, Rowe PJ, Deakin A, Riches PE. The accuracy of a robotically-controlled freehand sculpting tool for unicondylar knee arthroplasty. Bone Joint J. 2013;95-B(suppl 28):68.
16. Chakrabarty G, Newman JH, Ackroyd CE. Revision of unicompartmental arthroplasty of the knee. Clinical and technical considerations. J Arthroplasty. 1998;13(2):191-196.
17. Levine WN, Ozuna RM, Scott RD, Thornhill TS. Conversion of failed modern unicompartmental arthroplasty to total knee arthroplasty. J Arthroplasty. 1996;11(7):797-801.
18. Barrett WP, Scott RD. Revision of failed unicondylar unicompartmental knee arthroplasty. J Bone Joint Surg Am. 1987;69(9):1328-1335.
19. Padgett DE, Stern SH, Insall JN. Revision total knee arthroplasty for failed unicompartmental replacement. J Bone Joint Surg Am. 1991;73(2):186-190.
20. Aleto TJ, Berend ME, Ritter MA, Faris PM, Meneghini RM. Early failure of unicompartmental knee arthroplasty leading to revision. J Arthroplasty. 2008;23(2):159-163.
21. McAuley JP, Engh GA, Ammeen DJ. Revision of failed unicompartmental knee arthroplasty. Clin Orthop Relat Res. 2001;(392):279-282.22. Böhm I, Landsiedl F. Revision surgery after failed unicompartmental knee arthroplasty: a study of 35 cases. J Arthroplasty. 2000;15(8):982-989.
23. Khan Z, Nawaz SZ, Kahane S, Ester C, Chatterji U. Conversion of unicompartmental knee arthroplasty to total knee arthroplasty: the challenges and need for augments. Acta Orthop Belg. 2013;79(6):699-705.
1. Patil S, Colwell CW Jr, Ezzet KA, D’Lima DD. Can normal knee kinematics be restored with unicompartmental knee replacement? J Bone Joint Surg Am. 2005;87(2):332-338.
2. Johnson S, Jones P, Newman JH. The survivorship and results of total knee replacements converted from unicompartmental knee replacements. Knee. 2007;14(2):154-157.
3. Springer BD, Scott RD, Thornhill TS. Conversion of failed unicompartmental knee arthroplasty to TKA. Clin Orthop Relat Res. 2006;446:214-220.
4. Järvenpää J, Kettunen J, Miettinen H, Kröger H. The clinical outcome of revision knee replacement after unicompartmental knee arthroplasty versus primary total knee arthroplasty: 8-17 years follow-up study of 49 patients. Int Orthop. 2010;34(5):649-653.
5. Pearse AJ, Hooper GJ, Rothwell AG, Frampton C. Osteotomy and unicompartmental knee arthroplasty converted to total knee arthroplasty: data from the New Zealand Joint Registry. J Arthroplasty. 2012;27(10):1827-1831.
6. Rancourt MF, Kemp KA, Plamondon SM, Kim PR, Dervin GF. Unicompartmental knee arthroplasties revised to total knee arthroplasties compared with primary total knee arthroplasties. J Arthroplasty. 2012;27(8 Suppl):106-110.
7. Sierra RJ, Kassel CA, Wetters NG, Berend KR, Della Valle CJ, Lombardi AV. Revision of unicompartmental arthroplasty to total knee arthroplasty: not always a slam dunk! J Arthroplasty. 2013;28(8 Suppl):128-132.
8. O’Donnell TM, Abouazza O, Neil MJ. Revision of minimal resection resurfacing unicondylar knee arthroplasty to total knee arthroplasty: results compared with primary total knee arthroplasty. J Arthroplasty. 2013;28(1):33-39.
9. Schwarzkopf R, Mikhael B, Li L, Josephs L, Scott RD. Effect of initial tibial resection thickness on outcomes of revision UKA. Orthopedics. 2013;36(4):e409-e414.
10. Conditt MA, Roche MW. Minimally invasive robotic-arm-guided unicompartmental knee arthroplasty. J Bone Joint Surg Am. 2009;91 Suppl 1:63-68.
11. Dunbar NJ, Roche MW, Park BH, Branch SH, Conditt MA, Banks SA. Accuracy of dynamic tactile-guided unicompartmental knee arthroplasty. J Arthroplasty. 2012;27(5):803-808.e1.
12. Karia M, Masjedi M, Andrews B, Jaffry Z, Cobb J. Robotic assistance enables inexperienced surgeons to perform unicompartmental knee arthroplasties on dry bone models with accuracy superior to conventional methods. Adv Orthop. 2013;2013:481039.
13. Lonner JH, John TK, Conditt MA. Robotic arm-assisted UKA improves tibial component alignment: a pilot study. Clin Orthop Relat Res. 2010;468(1):141-146.
14. Lonner JH, Smith JR, Picard F, Hamlin B, Rowe PJ, Riches PE. High degree of accuracy of a novel image-free handheld robot for unicondylar knee arthroplasty in a cadaveric study. Clin Orthop Relat Res. 2015;473(1):206-212.
15. Smith JR, Picard F, Rowe PJ, Deakin A, Riches PE. The accuracy of a robotically-controlled freehand sculpting tool for unicondylar knee arthroplasty. Bone Joint J. 2013;95-B(suppl 28):68.
16. Chakrabarty G, Newman JH, Ackroyd CE. Revision of unicompartmental arthroplasty of the knee. Clinical and technical considerations. J Arthroplasty. 1998;13(2):191-196.
17. Levine WN, Ozuna RM, Scott RD, Thornhill TS. Conversion of failed modern unicompartmental arthroplasty to total knee arthroplasty. J Arthroplasty. 1996;11(7):797-801.
18. Barrett WP, Scott RD. Revision of failed unicondylar unicompartmental knee arthroplasty. J Bone Joint Surg Am. 1987;69(9):1328-1335.
19. Padgett DE, Stern SH, Insall JN. Revision total knee arthroplasty for failed unicompartmental replacement. J Bone Joint Surg Am. 1991;73(2):186-190.
20. Aleto TJ, Berend ME, Ritter MA, Faris PM, Meneghini RM. Early failure of unicompartmental knee arthroplasty leading to revision. J Arthroplasty. 2008;23(2):159-163.
21. McAuley JP, Engh GA, Ammeen DJ. Revision of failed unicompartmental knee arthroplasty. Clin Orthop Relat Res. 2001;(392):279-282.22. Böhm I, Landsiedl F. Revision surgery after failed unicompartmental knee arthroplasty: a study of 35 cases. J Arthroplasty. 2000;15(8):982-989.
23. Khan Z, Nawaz SZ, Kahane S, Ester C, Chatterji U. Conversion of unicompartmental knee arthroplasty to total knee arthroplasty: the challenges and need for augments. Acta Orthop Belg. 2013;79(6):699-705.
Hospitalist Tracy Gulling-Leftwich, DO, Spends Her Free Time Caring for Rescue Animals
Tracy Gulling-Leftwich, DO, remembers Chewy very well. He was a 70-pound English bulldog she was caring for last year on behalf of the Rescue Ohio English Bulldogs, an English bulldog rescue group.
She soon learned that Chewy was anemic and suffered from bone cancer of the jaw. Ironically, considering his name, he could barely chew, so Dr. Gulling-Leftwich and her husband, Samuel Leftwich, pureed his food, spoon-fed the animal, and administered around-the-clock pain medications for roughly two weeks. But his pain grew too intense, and Chewy had to be euthanized.
For many people, that would end their experience with an animal organization. People typically compare the heartbreaking experience to losing a beloved family member or friend. But as an animal lover and hospitalist at the Cleveland Clinic, Dr. Gulling-Leftwich has no intentions of looking the other way whenever an animal—or human—is in need. Ever since she was in college, she has been rescuing lab rats and dogs, trying to keep them happy, healthy, and loved throughout their relatively short lives.
Underground Railroad
Dr. Gulling-Leftwich graduated from the Lake Erie College of Osteopathic Medicine in Erie, Penn., in 2007. The following year, she pursued an osteopathic rotating internship at the University of Connecticut. While attending the same university from 2008 to 2010, she completed a traditional, categorical, allopathic medicine residency.
After completing her medical education, she held several positions. She worked as a teaching hospitalist at the Hartford Hospital for one year, served as a primary-care physician for the next three years at The Hospital of Central Connecticut, and then joined the Cleveland Clinic as a hospitalist in 2014.
Her involvement in animal rescue began many years earlier while attending undergraduate school at Westminster College in New Wilmington, Penn. She tells the story how one student at the college kidnapped a rat from the school’s neuroscience lab just before Christmas break.
Since the student’s mother would not allow her to bring a rat home over the six-week holiday, Dr. Gulling-Leftwich babysat him until she returned. However, the student intended on releasing him into the wild. Fearing the worst, that the rat could not fend for itself since it had been caged and fed for many months, Dr. Gulling-Leftwich convinced the student to relinquish custody of the rat to her.
That’s how it all began. Dr. Gulling-Leftwich named the rat Templeton. She suspects he died of a pituitary tumor four years later; still, that’s a long life for a rat. Most live just two years. Just shows what a little love can do.
Since then, she has rescued approximately 21 rats from Kentucky and Connecticut. Years ago, she says, there were multiple Yahoo chat groups of people involved in an underground railroad of sorts for rescued lab rats. People would often drive the rats to different cities, even across state borders, so these rats could enjoy a permanent home.
While she has never broken into a research lab, her opinion is torn on animal research. She believes it is not necessary for consumer products, such as makeup, but can see its value in other fields of science like the development of new medications.
“What I can hope for is that we work toward finding a way of not requiring animals for research in the future,” she says.
Full House
After getting married in 2013, Dr. Gulling-Leftwich told her husband she wanted a dog. But because of their hectic schedules, no one would be home to care for the animal, so the couple waited another two years to adopt a rescue animal.
In 2015, they had purchased a house in Cleveland when they adopted Boomer, a pug and beagle designer breed, as their family pet.
“I had really wanted an English bulldog. They’re just cute, their face is squishy,” she says, adding she had been monitoring English bulldog rescue websites. “I won’t buy a puppy. I will only get a dog that needs a home.”
In September that year, the rescue organization emailed a desperate plea to its followers. Can anyone rescue an English bulldog named Chewy? Dr. Gulling-Leftwich immediately filled out the paperwork and adopted him. But Chewy only stayed with them for two weeks before he was euthanized. She brought him to the vet after he attacked Boomer.
“Chewy wasn’t being a jerk,” she says. “His attacking behavior had to do with his pain and discomfort. He had blood everywhere around his mouth. We had a hard time letting him go.”
One month later, another English bulldog named Olive joined their family. She’s roughly two years old and weighs only 30 pounds mainly because of her disease: congenital cardiomyopathy. They plan to care for Olive until she dies.
She says Olive takes six pills a day for her condition and occasionally receives nitroglycerin when she overexerts herself and passes out.
Meanwhile, Dr. Gulling-Leftwich and her husband care for one rat named Harvey and a cat called Lily in addition to the two dogs. Boomer doesn’t like Olive. Olive doesn’t like the cat. And both dogs and the cat pay no attention to the rat.
“My husband says rescuing animals and taking care of people is one of my more endearing qualities,” she says. “Then he follows it up with, ‘No, you can’t have that bunny that needs a home.’”
She believes caring for these animals balances her work in hospital medicine. While hospital patients often are in pain, act grouchy, and appear unappreciative, she says her four-legged family members are always excited to see her and routinely demonstrate unconditional love.
“You definitely have to be open-minded because you never know what you’ll be walking into when you rescue an animal,” she says, adding that rescue groups tend to pay for vet bills and medicine. “You have to be prepared for what potentially could be the worst.”
Carol Patton is a freelance writer in Las Vegas.
Tracy Gulling-Leftwich, DO, remembers Chewy very well. He was a 70-pound English bulldog she was caring for last year on behalf of the Rescue Ohio English Bulldogs, an English bulldog rescue group.
She soon learned that Chewy was anemic and suffered from bone cancer of the jaw. Ironically, considering his name, he could barely chew, so Dr. Gulling-Leftwich and her husband, Samuel Leftwich, pureed his food, spoon-fed the animal, and administered around-the-clock pain medications for roughly two weeks. But his pain grew too intense, and Chewy had to be euthanized.
For many people, that would end their experience with an animal organization. People typically compare the heartbreaking experience to losing a beloved family member or friend. But as an animal lover and hospitalist at the Cleveland Clinic, Dr. Gulling-Leftwich has no intentions of looking the other way whenever an animal—or human—is in need. Ever since she was in college, she has been rescuing lab rats and dogs, trying to keep them happy, healthy, and loved throughout their relatively short lives.
Underground Railroad
Dr. Gulling-Leftwich graduated from the Lake Erie College of Osteopathic Medicine in Erie, Penn., in 2007. The following year, she pursued an osteopathic rotating internship at the University of Connecticut. While attending the same university from 2008 to 2010, she completed a traditional, categorical, allopathic medicine residency.
After completing her medical education, she held several positions. She worked as a teaching hospitalist at the Hartford Hospital for one year, served as a primary-care physician for the next three years at The Hospital of Central Connecticut, and then joined the Cleveland Clinic as a hospitalist in 2014.
Her involvement in animal rescue began many years earlier while attending undergraduate school at Westminster College in New Wilmington, Penn. She tells the story how one student at the college kidnapped a rat from the school’s neuroscience lab just before Christmas break.
Since the student’s mother would not allow her to bring a rat home over the six-week holiday, Dr. Gulling-Leftwich babysat him until she returned. However, the student intended on releasing him into the wild. Fearing the worst, that the rat could not fend for itself since it had been caged and fed for many months, Dr. Gulling-Leftwich convinced the student to relinquish custody of the rat to her.
That’s how it all began. Dr. Gulling-Leftwich named the rat Templeton. She suspects he died of a pituitary tumor four years later; still, that’s a long life for a rat. Most live just two years. Just shows what a little love can do.
Since then, she has rescued approximately 21 rats from Kentucky and Connecticut. Years ago, she says, there were multiple Yahoo chat groups of people involved in an underground railroad of sorts for rescued lab rats. People would often drive the rats to different cities, even across state borders, so these rats could enjoy a permanent home.
While she has never broken into a research lab, her opinion is torn on animal research. She believes it is not necessary for consumer products, such as makeup, but can see its value in other fields of science like the development of new medications.
“What I can hope for is that we work toward finding a way of not requiring animals for research in the future,” she says.
Full House
After getting married in 2013, Dr. Gulling-Leftwich told her husband she wanted a dog. But because of their hectic schedules, no one would be home to care for the animal, so the couple waited another two years to adopt a rescue animal.
In 2015, they had purchased a house in Cleveland when they adopted Boomer, a pug and beagle designer breed, as their family pet.
“I had really wanted an English bulldog. They’re just cute, their face is squishy,” she says, adding she had been monitoring English bulldog rescue websites. “I won’t buy a puppy. I will only get a dog that needs a home.”
In September that year, the rescue organization emailed a desperate plea to its followers. Can anyone rescue an English bulldog named Chewy? Dr. Gulling-Leftwich immediately filled out the paperwork and adopted him. But Chewy only stayed with them for two weeks before he was euthanized. She brought him to the vet after he attacked Boomer.
“Chewy wasn’t being a jerk,” she says. “His attacking behavior had to do with his pain and discomfort. He had blood everywhere around his mouth. We had a hard time letting him go.”
One month later, another English bulldog named Olive joined their family. She’s roughly two years old and weighs only 30 pounds mainly because of her disease: congenital cardiomyopathy. They plan to care for Olive until she dies.
She says Olive takes six pills a day for her condition and occasionally receives nitroglycerin when she overexerts herself and passes out.
Meanwhile, Dr. Gulling-Leftwich and her husband care for one rat named Harvey and a cat called Lily in addition to the two dogs. Boomer doesn’t like Olive. Olive doesn’t like the cat. And both dogs and the cat pay no attention to the rat.
“My husband says rescuing animals and taking care of people is one of my more endearing qualities,” she says. “Then he follows it up with, ‘No, you can’t have that bunny that needs a home.’”
She believes caring for these animals balances her work in hospital medicine. While hospital patients often are in pain, act grouchy, and appear unappreciative, she says her four-legged family members are always excited to see her and routinely demonstrate unconditional love.
“You definitely have to be open-minded because you never know what you’ll be walking into when you rescue an animal,” she says, adding that rescue groups tend to pay for vet bills and medicine. “You have to be prepared for what potentially could be the worst.”
Carol Patton is a freelance writer in Las Vegas.
Tracy Gulling-Leftwich, DO, remembers Chewy very well. He was a 70-pound English bulldog she was caring for last year on behalf of the Rescue Ohio English Bulldogs, an English bulldog rescue group.
She soon learned that Chewy was anemic and suffered from bone cancer of the jaw. Ironically, considering his name, he could barely chew, so Dr. Gulling-Leftwich and her husband, Samuel Leftwich, pureed his food, spoon-fed the animal, and administered around-the-clock pain medications for roughly two weeks. But his pain grew too intense, and Chewy had to be euthanized.
For many people, that would end their experience with an animal organization. People typically compare the heartbreaking experience to losing a beloved family member or friend. But as an animal lover and hospitalist at the Cleveland Clinic, Dr. Gulling-Leftwich has no intentions of looking the other way whenever an animal—or human—is in need. Ever since she was in college, she has been rescuing lab rats and dogs, trying to keep them happy, healthy, and loved throughout their relatively short lives.
Underground Railroad
Dr. Gulling-Leftwich graduated from the Lake Erie College of Osteopathic Medicine in Erie, Penn., in 2007. The following year, she pursued an osteopathic rotating internship at the University of Connecticut. While attending the same university from 2008 to 2010, she completed a traditional, categorical, allopathic medicine residency.
After completing her medical education, she held several positions. She worked as a teaching hospitalist at the Hartford Hospital for one year, served as a primary-care physician for the next three years at The Hospital of Central Connecticut, and then joined the Cleveland Clinic as a hospitalist in 2014.
Her involvement in animal rescue began many years earlier while attending undergraduate school at Westminster College in New Wilmington, Penn. She tells the story how one student at the college kidnapped a rat from the school’s neuroscience lab just before Christmas break.
Since the student’s mother would not allow her to bring a rat home over the six-week holiday, Dr. Gulling-Leftwich babysat him until she returned. However, the student intended on releasing him into the wild. Fearing the worst, that the rat could not fend for itself since it had been caged and fed for many months, Dr. Gulling-Leftwich convinced the student to relinquish custody of the rat to her.
That’s how it all began. Dr. Gulling-Leftwich named the rat Templeton. She suspects he died of a pituitary tumor four years later; still, that’s a long life for a rat. Most live just two years. Just shows what a little love can do.
Since then, she has rescued approximately 21 rats from Kentucky and Connecticut. Years ago, she says, there were multiple Yahoo chat groups of people involved in an underground railroad of sorts for rescued lab rats. People would often drive the rats to different cities, even across state borders, so these rats could enjoy a permanent home.
While she has never broken into a research lab, her opinion is torn on animal research. She believes it is not necessary for consumer products, such as makeup, but can see its value in other fields of science like the development of new medications.
“What I can hope for is that we work toward finding a way of not requiring animals for research in the future,” she says.
Full House
After getting married in 2013, Dr. Gulling-Leftwich told her husband she wanted a dog. But because of their hectic schedules, no one would be home to care for the animal, so the couple waited another two years to adopt a rescue animal.
In 2015, they had purchased a house in Cleveland when they adopted Boomer, a pug and beagle designer breed, as their family pet.
“I had really wanted an English bulldog. They’re just cute, their face is squishy,” she says, adding she had been monitoring English bulldog rescue websites. “I won’t buy a puppy. I will only get a dog that needs a home.”
In September that year, the rescue organization emailed a desperate plea to its followers. Can anyone rescue an English bulldog named Chewy? Dr. Gulling-Leftwich immediately filled out the paperwork and adopted him. But Chewy only stayed with them for two weeks before he was euthanized. She brought him to the vet after he attacked Boomer.
“Chewy wasn’t being a jerk,” she says. “His attacking behavior had to do with his pain and discomfort. He had blood everywhere around his mouth. We had a hard time letting him go.”
One month later, another English bulldog named Olive joined their family. She’s roughly two years old and weighs only 30 pounds mainly because of her disease: congenital cardiomyopathy. They plan to care for Olive until she dies.
She says Olive takes six pills a day for her condition and occasionally receives nitroglycerin when she overexerts herself and passes out.
Meanwhile, Dr. Gulling-Leftwich and her husband care for one rat named Harvey and a cat called Lily in addition to the two dogs. Boomer doesn’t like Olive. Olive doesn’t like the cat. And both dogs and the cat pay no attention to the rat.
“My husband says rescuing animals and taking care of people is one of my more endearing qualities,” she says. “Then he follows it up with, ‘No, you can’t have that bunny that needs a home.’”
She believes caring for these animals balances her work in hospital medicine. While hospital patients often are in pain, act grouchy, and appear unappreciative, she says her four-legged family members are always excited to see her and routinely demonstrate unconditional love.
“You definitely have to be open-minded because you never know what you’ll be walking into when you rescue an animal,” she says, adding that rescue groups tend to pay for vet bills and medicine. “You have to be prepared for what potentially could be the worst.”
Carol Patton is a freelance writer in Las Vegas.
CAR T-cell trial placed on hold again
Once again, the phase 2 ROCKET trial has been placed on clinical hold due to patient deaths.
In this trial, researchers are testing the chimeric antigen receptor (CAR) T-cell therapy JCAR015 in adults with relapsed or refractory B-cell acute lymphoblastic leukemia.
Juno Therapeutics, Inc. voluntarily put the trial on hold after 2 more patients suffered cerebral edema and died.
A total of 5 patients have died of cerebral edema in this trial.
Juno has notified the US Food and Drug Administration (FDA) of the latest clinical hold on the ROCKET trial and is working with the agency and the company’s data and safety monitoring board to determine next steps.
The ROCKET trial was previously placed on clinical hold in July, after 3 patients died of cerebral edema. The FDA lifted the hold less than a week later, allowing the trial to continue with a revised protocol.
Juno had theorized the deaths were likely a result of adding fludarabine to the conditioning regimen.
Patients enrolled in ROCKET initially received conditioning with cyclophosphamide alone, but researchers later decided to add fludarabine in the hopes of increasing efficacy. Previous trials of 2 other CAR T-cell therapies, JCAR014 and JCAR017, had suggested that adding fludarabine to conditioning could increase efficacy.
However, in the ROCKET trial, the addition of fludarabine was associated with an increase in the incidence of severe neurotoxicity and the 3 deaths from cerebral edema.
Juno said that, although other factors may have contributed to the deaths, fludarabine was the most likely culprit. So the company revised the trial protocol, and the FDA allowed ROCKET to continue with a conditioning regimen consisting of cyclophosphamide alone.
Since that time, 12 patients have been treated on the ROCKET trial. Two patients who were treated the week of November 14 developed cerebral edema and died on November 22 and 23, respectively.
In a conference call, Juno’s Chief Medical Officer Mark Gilbert, MD, said the etiology of cerebral edema is multi-factorial, and Juno will need more time to draw even preliminary conclusions about what factors contributed to the cases of cerebral edema in ROCKET.
Right now, the company is assessing data from the cases and the trial and is evaluating its options regarding the JCAR015 program.
Juno’s President and CEO Hans Bishop said the options for JCAR015 going forward include continuing the ROCKET trial with a modified protocol, beginning a new study of JCAR015, and terminating the JCAR015 development program.
Bishop said the company expects to provide an update on the status of ROCKET and JCAR015 in the next few weeks.
Juno’s other trials and plans for its other CD19-directed CAR T-cell product candidates are not affected by the issues with ROCKET and JCAR015.
ROCKET is not the first trial of JCAR015 to be placed on hold. The phase 1 trial of the therapy was placed on clinical hold in 2014, after 2 patients died of cytokine release syndrome.
That hold was lifted following changes to enrollment criteria and dosing. Results from this trial were presented at ASCO 2015 and ASCO 2016.
Once again, the phase 2 ROCKET trial has been placed on clinical hold due to patient deaths.
In this trial, researchers are testing the chimeric antigen receptor (CAR) T-cell therapy JCAR015 in adults with relapsed or refractory B-cell acute lymphoblastic leukemia.
Juno Therapeutics, Inc. voluntarily put the trial on hold after 2 more patients suffered cerebral edema and died.
A total of 5 patients have died of cerebral edema in this trial.
Juno has notified the US Food and Drug Administration (FDA) of the latest clinical hold on the ROCKET trial and is working with the agency and the company’s data and safety monitoring board to determine next steps.
The ROCKET trial was previously placed on clinical hold in July, after 3 patients died of cerebral edema. The FDA lifted the hold less than a week later, allowing the trial to continue with a revised protocol.
Juno had theorized the deaths were likely a result of adding fludarabine to the conditioning regimen.
Patients enrolled in ROCKET initially received conditioning with cyclophosphamide alone, but researchers later decided to add fludarabine in the hopes of increasing efficacy. Previous trials of 2 other CAR T-cell therapies, JCAR014 and JCAR017, had suggested that adding fludarabine to conditioning could increase efficacy.
However, in the ROCKET trial, the addition of fludarabine was associated with an increase in the incidence of severe neurotoxicity and the 3 deaths from cerebral edema.
Juno said that, although other factors may have contributed to the deaths, fludarabine was the most likely culprit. So the company revised the trial protocol, and the FDA allowed ROCKET to continue with a conditioning regimen consisting of cyclophosphamide alone.
Since that time, 12 patients have been treated on the ROCKET trial. Two patients who were treated the week of November 14 developed cerebral edema and died on November 22 and 23, respectively.
In a conference call, Juno’s Chief Medical Officer Mark Gilbert, MD, said the etiology of cerebral edema is multi-factorial, and Juno will need more time to draw even preliminary conclusions about what factors contributed to the cases of cerebral edema in ROCKET.
Right now, the company is assessing data from the cases and the trial and is evaluating its options regarding the JCAR015 program.
Juno’s President and CEO Hans Bishop said the options for JCAR015 going forward include continuing the ROCKET trial with a modified protocol, beginning a new study of JCAR015, and terminating the JCAR015 development program.
Bishop said the company expects to provide an update on the status of ROCKET and JCAR015 in the next few weeks.
Juno’s other trials and plans for its other CD19-directed CAR T-cell product candidates are not affected by the issues with ROCKET and JCAR015.
ROCKET is not the first trial of JCAR015 to be placed on hold. The phase 1 trial of the therapy was placed on clinical hold in 2014, after 2 patients died of cytokine release syndrome.
That hold was lifted following changes to enrollment criteria and dosing. Results from this trial were presented at ASCO 2015 and ASCO 2016.
Once again, the phase 2 ROCKET trial has been placed on clinical hold due to patient deaths.
In this trial, researchers are testing the chimeric antigen receptor (CAR) T-cell therapy JCAR015 in adults with relapsed or refractory B-cell acute lymphoblastic leukemia.
Juno Therapeutics, Inc. voluntarily put the trial on hold after 2 more patients suffered cerebral edema and died.
A total of 5 patients have died of cerebral edema in this trial.
Juno has notified the US Food and Drug Administration (FDA) of the latest clinical hold on the ROCKET trial and is working with the agency and the company’s data and safety monitoring board to determine next steps.
The ROCKET trial was previously placed on clinical hold in July, after 3 patients died of cerebral edema. The FDA lifted the hold less than a week later, allowing the trial to continue with a revised protocol.
Juno had theorized the deaths were likely a result of adding fludarabine to the conditioning regimen.
Patients enrolled in ROCKET initially received conditioning with cyclophosphamide alone, but researchers later decided to add fludarabine in the hopes of increasing efficacy. Previous trials of 2 other CAR T-cell therapies, JCAR014 and JCAR017, had suggested that adding fludarabine to conditioning could increase efficacy.
However, in the ROCKET trial, the addition of fludarabine was associated with an increase in the incidence of severe neurotoxicity and the 3 deaths from cerebral edema.
Juno said that, although other factors may have contributed to the deaths, fludarabine was the most likely culprit. So the company revised the trial protocol, and the FDA allowed ROCKET to continue with a conditioning regimen consisting of cyclophosphamide alone.
Since that time, 12 patients have been treated on the ROCKET trial. Two patients who were treated the week of November 14 developed cerebral edema and died on November 22 and 23, respectively.
In a conference call, Juno’s Chief Medical Officer Mark Gilbert, MD, said the etiology of cerebral edema is multi-factorial, and Juno will need more time to draw even preliminary conclusions about what factors contributed to the cases of cerebral edema in ROCKET.
Right now, the company is assessing data from the cases and the trial and is evaluating its options regarding the JCAR015 program.
Juno’s President and CEO Hans Bishop said the options for JCAR015 going forward include continuing the ROCKET trial with a modified protocol, beginning a new study of JCAR015, and terminating the JCAR015 development program.
Bishop said the company expects to provide an update on the status of ROCKET and JCAR015 in the next few weeks.
Juno’s other trials and plans for its other CD19-directed CAR T-cell product candidates are not affected by the issues with ROCKET and JCAR015.
ROCKET is not the first trial of JCAR015 to be placed on hold. The phase 1 trial of the therapy was placed on clinical hold in 2014, after 2 patients died of cytokine release syndrome.
That hold was lifted following changes to enrollment criteria and dosing. Results from this trial were presented at ASCO 2015 and ASCO 2016.
EC grants drug orphan status for AML, sarcoma

The European Commission (EC) has granted orphan drug designation to crenolanib for the treatment of acute myeloid leukemia (AML) and soft tissue sarcoma.
Crenolanib is a benzimidazole type I kinase inhibitor that selectively inhibits signaling of wild-type and mutant isoforms of FLT3 and PDGFRα/β.
The drug is under investigation as a treatment for multiple cancers. It is being developed by Arog Pharmaceuticals, Inc.
Results from a phase 2 trial of crenolanib in relapsed/refractory, FLT3+ AML were presented at the 2016 ASCO Annual Meeting.
About orphan designation
The EC grants orphan designation to therapies intended to treat life-threatening or chronically debilitating conditions affecting no more than 5 in 10,000 people in the European Union, and where no satisfactory treatment is available.
Orphan designation provides companies developing such drugs with regulatory and financial incentives, including protocol assistance, 10 years of market exclusivity once the drug is approved, and, in some cases, reductions in fees. ![]()

The European Commission (EC) has granted orphan drug designation to crenolanib for the treatment of acute myeloid leukemia (AML) and soft tissue sarcoma.
Crenolanib is a benzimidazole type I kinase inhibitor that selectively inhibits signaling of wild-type and mutant isoforms of FLT3 and PDGFRα/β.
The drug is under investigation as a treatment for multiple cancers. It is being developed by Arog Pharmaceuticals, Inc.
Results from a phase 2 trial of crenolanib in relapsed/refractory, FLT3+ AML were presented at the 2016 ASCO Annual Meeting.
About orphan designation
The EC grants orphan designation to therapies intended to treat life-threatening or chronically debilitating conditions affecting no more than 5 in 10,000 people in the European Union, and where no satisfactory treatment is available.
Orphan designation provides companies developing such drugs with regulatory and financial incentives, including protocol assistance, 10 years of market exclusivity once the drug is approved, and, in some cases, reductions in fees. ![]()

The European Commission (EC) has granted orphan drug designation to crenolanib for the treatment of acute myeloid leukemia (AML) and soft tissue sarcoma.
Crenolanib is a benzimidazole type I kinase inhibitor that selectively inhibits signaling of wild-type and mutant isoforms of FLT3 and PDGFRα/β.
The drug is under investigation as a treatment for multiple cancers. It is being developed by Arog Pharmaceuticals, Inc.
Results from a phase 2 trial of crenolanib in relapsed/refractory, FLT3+ AML were presented at the 2016 ASCO Annual Meeting.
About orphan designation
The EC grants orphan designation to therapies intended to treat life-threatening or chronically debilitating conditions affecting no more than 5 in 10,000 people in the European Union, and where no satisfactory treatment is available.
Orphan designation provides companies developing such drugs with regulatory and financial incentives, including protocol assistance, 10 years of market exclusivity once the drug is approved, and, in some cases, reductions in fees. ![]()
Implementation and Evaluation of an APRN-Led Opioid Monitoring Clinic
Chronic pain, defined as pain lasting longer than 3 to 6 months in duration, affects about 100 million Americans.1 The use of opioids in the management of chronic nonmalignant pain is common in primary care. The U.S., with only 5% of the global population, nevertheless is the world’s leading opioid consumer.2 For example, it is estimated that the U.S. consumes 56% of the global supply of morphine, 99% of hydrocodone, and 83% of oxycodone; this consumption is a growing problem in the use of chronic opioid therapy in managing chronic nonmalignant pain.2,3 The high rates of use of opioids continues, despite a lack of solid evidence on the long-term effectiveness of opioids for managing chronic nonmalignant pain and on the associated risks of opioid addiction, abuse, and misuse.3,4 Among veterans, the prevalence of opioid abuse and misuse has been reported to be about 30%, a nearly 7-fold occurrence compared with that in the general population.5,6
Due to the pervasiveness of opioid abuse and misuse among veterans, a project was initiated to develop, implement, and evaluate an Opioid Monitoring Clinic (OMC) as a clinical referral system within the primary care service of the VA Southern Nevada Healthcare System (VASNHS) in North Las Vegas. A health care provider (HCP) needs assessment was conducted at the facility, resulting in recommendations to improve adherence to evidence-based clinical practice guidelines in opioid management and regular monitoring of veterans on chronic opioid therapy for the identification of opioid abuse and misuse. Based on the results, an advanced practice registered nurse (APRN) in consultation with the chief of primary care at VASNHS and teamlet support (a registered nurse, licensed practical nurse, and medical support assistant) started the OMC. The OMC was developed consistent with the 2010 VA/DoD clinical practice guidelines for managing opioid therapy for chronic pain.7
After 6 months of OMC operation, the project also was evaluated for efficacy. First, a retrospective chart review of participants was conducted to identify the use of opioid pain agreements, prescription drug monitoring programs (PDMP) for controlled substance use review, and urine drug screens (UDSs). The chart review also included the average daily morphine equivalent dose (MED) for patients and OMC retention rates. Second, an online survey of primary care providers (PCPs) assessed their adherence to evidence-based guidelines in opioid management and satisfaction of the OMC services.
Background
In 1997, the average sales and distribution of opioids in the U.S. was 96 mg MED per individual, which increased to 710 mg per individual in 2010.8,9 The MED is a standardized daily dose measure for all opioids.10 At VASNHS North Las Vegas, there were 5,881 patients on opioid therapy in 2013 with about 13% of patients on opioid therapy using about 100 mg MED/d. The potential for abuse and misuse was great. Almost 30% of patients on chronic opioid therapy for chronic nonmalignant pain abuse their opioid prescriptions.5,7 Subsequently, opioid analgesics were responsible for nearly 60% of overdose deaths in 2010.11
In 2010, there were about 12 million people in the U.S. who abused or misused prescription opioids, using them for nonmedical reasons; annually, the prevalence rate of Americans who abuse and misuse opioids is about 2 million people.12 The 5-year prevalence rate of opioid abuse among veterans is at least 3%.6 This results in health care expenditures with an average excess medical cost of $20,546 per year for patients who misuse opioids compared with those who do not.13 The economic burden among veterans is even higher. Baser and colleagues reported that the annual economic cost for veterans who abused their opioid prescriptions was nearly $29,000.6
Unfortunately, injudicious opioid prescribing by HCPs is often cited as a contributor to the growing problem of opioid abuse and misuse in the U.S.14 Health care provider education on the proper and judicious use of opioids and adherence to clinical practice guidelines in the management of chronic pain is a crucial factor in reducing the complications of chron
Method
This project evaluated the APRN-led OMC with both quantitative and qualitative data from patients and PCPs. The OMC was implemented at VASNHS North Las Vegas, which serves almost 60,000 veterans. Patients referred to the OMC who were eligible for admission were veterans aged ≥ 18 years, who had chronic nonmalignant pain for at least 3 months, were receiving chronic opioid therapy, and were considered high risk for abuse or misuse of opioids. Patients were considered high risk if they had documented aberrant behaviors, such as multiple early refill requests, history of lost medications, drug screens not showing prescribed opioid(s), positive drug screens for controlled substance not prescribed, nonadherence with plan of care, or a history of substance abuse, including alcohol, cocaine, heroin, and marijuana. Veterans found to be suicidal or homicidal were excluded from the OMC and instead were referred to appropriate specialty care for further evaluation. A total of 61 veteran participants were successfully recruited.
Primary care physicians, physician assistants, and APRNs working at VASNHS on a full-time and part-time basis were eligible for participation as PCPs in this project. Thirty of 42 eligible PCPs participated and responded to the secure online provider survey (71% response rate).
Risks and benefits were discussed, and a written informed consent was obtained for each participant. There were no apparent risks or adverse effects encountered during project implementation and evaluation. Prior to data collection, permission for the study was obtained from the VA facility. An institutional review board application through the University of Nevada, Las Vegas’ Office of Research Integrity-Human Subjects was also submitted and approved through an expedited review.
Results
Table 1 details patient data, including demographics on the veteran participants. The av
Review of PDMPs revealed that 20% of the participants received controlled substances from other HCPs. Consequently, all OMC patients completed and had reinforced OPAs that served 2 purposes. First, the content in the agreement provided patient education regarding the potential hazards of opioid use. Second, the content provided specific expectations from the patient if opioid therapy is continued, including opioid risk reduction strategies (ie, random UDSs and pill counts, PDMP for controlled substance utilization review), and the consequences of lack of adherence to opioid management.
The majority of patients remained as patients and continued to be monitored after 6 months at the OMC (Table 2). However, only 64% were retained in the OMC, in large part because 36% had their opioids discontinued due to discovery of active illicit substance use, opioi
The mean MED/d among OMC participants was 54 mg/d before admission, which decreased gradually to 22 mg/d after 6 months of OMC care; this represented a 59% MED reduction (Table 3). Using the exact single-tailed Wilcoxon signed rank
Primary Care Providers
Based on the survey of PCPs, 40% of respondents referred at least 1 patient to the OMC. The majority (90%) acknowledged following the VA/DoD guidelines for opioid management, with 80% using an OPA more than usual since the implementation of the OMC, and 54% routinely accessing a PDMP for controlled substance utilization review. Moreover, 93% of PCPs routinely ordered a UDS when indicated.
For 3 months prior to opening the OMC in 2013, 1,606 UDSs were ordered at VASNHS. The UDS number steadily increased, and 6 months after opening the OMC, UDSs increased to 2,293 from months 4 to 6, representing a 30% increase. This was an expected outcome from the OMC—more PCPs now were following the VA/DoD clinical practice guidelines regarding the monitoring of patients on chronic opioid therapy.
A survey assessed the level of satisfaction among PCPs who had referred their patients to the OMC. Although only 11 PCPs responded to this question, a large majority found the OMC to have a positive effect on primary care services; many noted receiving fewer complaints regarding pain medications and fewer walk-ins. The majority recognized the advantage of the OMC in facilitating more PCP time for managing other medical problems beyond opioid use, which tends to be challenging and time consuming. Overall, 100% were satisfied with the OMC service.
Finally, 82% of PCPs reported that the OMC referral process did not need improvement. Two PCPs (18%) left positive feedback, reporting that the OMC was “simple, easy and accessible” and that the referral process “so far, it is great.” Another PCP noted, “I want patients who are suicidal but still need pain control with narcotics to be addressed.”
Discussion
The OMC has shown great promise in identifying abuse and misuse of opioids through evidence-based guidelines and risk-mitigation strategies. In the past, VA clinics specifically focused on opioid renewal have been implemented. In 2002, the Philadelphia VAMC opened an opioid renewal clinic (ORC) to assist PCPs in the management of patients with chronic pain on chronic opioid therapy.15 The Philadelphia VAMC ORC was operated collaboratively by PCPs and Pharmacy Service. They reported that 51% of their patients initially had documented aberrant behaviors, and 45% of these patients resolved their aberrant behaviors through intensive opioid monitoring using random UDSs.14 Thirteen percent of their patients were found to have an opioid addiction disorder and eventually were referred to addiction treatment; and 4% were weaned off opioids due to consistently negative UDSs.14
In the same manner, the OMC has effectively identified patients who abused and misused their opioids and consequently referred these patients for pain interventional management or to the VASNHS alcohol and drug treatment program as appropriate, which falls under the VASNHS Mental Health Care line, a service that is vital for veterans who are suicidal and homicidal. The importance of mental health care cannot be understated, as many patients with chronic pain also experience mental health challenges.
The Malcom Randall VAMC in Gainesville, Florida, structured a nurse-led, multidisciplinary ORC in 2003.16 A retrospective review of their program showed that 33% of patients had a positive UDS for marijuana, cocaine, or alcohol. The ORC had increased patient involvement in substance abuse treatment, resulting in some patients taking lower opioid dosages than before.
The New York Harbor VAMC reduced opioid cost by effectively switching veterans on expensive long-acting opioids, such as oxycodone and fentanyl, to less expensive alternatives, such a long-acting morphine.17 A secondary purpose of the New York initiative was to reduce the potential for inappropriate use of expensive long-acting opioids. Accordingly, the initiative reduced the number of expensive long-acting and potentially inappropriate opioids from 165 to 69 prescriptions in less than 6 months (November 2007 through March 2008). Similarly, after 6 months of operation, the OMC significantly reduced opioid prescription from 54 mg to 22 MED/d. This reduction represents a significant pharmacy cost savings. The combination of the discontinuation of opioids for patients found to be abusing and misusing opioids coupled with the decrease in pill burden resulting from changes from short-acting to long-acting opioids also resulted in significant savings for VA facilities.
The impact of the OMC on PCP adherence to opioid management guidelines as well as PCP satisfaction with the OMC services was significant. Similarly, Wiedemer and colleagues found significant PCP satisfaction with the ORC.15 The ability to spend more time with patients on other medical problems while allowing the OMC to focus on opioid and pain management was found to be beneficial. Buy-in among PCPs coupled with their concerns for chronic opioid therapy for high-risk patients facilitated the success of the OMC. The commitment of the VASNHS leadership to the OMC and their support for the APRN in leading this initiative were important facilitators in the success of the OMC.
Limitations
Long-term evaluation of the OMC with a larger sample is needed to fully evaluate its impact on decreasing opioid misuse and abuse. This project was limited by a small sample size, although the results are promising. Pharmacy costs, emergency department visits, as well as patient satisfaction, physical and emotional function, and pain levels are outcomes that need to be considered over the long term. Incorporating mental health counseling, cognitive behavior therapy, self-management programs, and group educational sessions have the potential to be important OMC services. The continued success and cost-effectiveness of the OMC can be a potentially significant model for this type of service that can be applied to clinics outside the VA system.
Implications
Possible implications to practice settings that are considering an OMC or ORC include the chance that patients will want to be discharged from such a clinic and return to the PCP for opioid management. Collaborative relationships and communication between PCPs and OMC providers are important to facilitate adherence and consistency with pain care. Collaboration and effective communication can be facilitated by electronic recording and reporting. For example, the VA Computerized Patient Record System can alert PCPs to patient discharges from the OMC along with OMC provider recommendations for patient care. Another challenge for the OMC would be a lack of referrals for patients who are at high risk for opioid abuse or misuse. These challenges can be mitigated by providing in-services, educational flyers, and advertisement promotions regarding OMC services. With the high prevalence of opioid abuse and misuse as well as the subsequent exorbitant health-related costs and deaths associated with opioids, OMCs and ORCs are viable options for improving opioid management in the treatment of patients with nonmalignant chronic pain.
Conclusion
The OMC effectively reduced the MED of patients referred to the clinic by 59%. The significant reduction in the opioid dose of patients referred to the OMC resulted from the implementation of evidence-based strategies that were used to identify abuse of prescription opioids, the use of illicit substances that can cause opioid-related complications, and the discovery of doctor shopping, coupled with gradual dose reductions for patients when appropriate. Provider satisfaction and increased use of evidence-based guidelines in opioid management and risk mitigation strategies, such as OPAs, PDMP databases, and UDS were evident. These results suggest that an OMC can be an effective program to help identify abuse and misuse of prescription opioids among high-risk patients and can improve patient safety and provider satisfaction.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the VA Southern Nevada Healthcare System.
1. Institute of Medicine (IOM). Relieving pain in America: a blueprint for transforming prevention, care, education, and research. http://iom.nationalacademies.org/~/media/Files/Report%20Files/2011/Relieving-Pain-in-America-A-Blueprint-for-Transforming-Prevention-Care-Education-Research/Pain%20Research%202011%20Report%20Brief.pdf. Published June 2011. Accessed August 25, 2016.
2. International Narcotics Control Board. Report of the International Narcotics Control Board on the availability of internationally controlled drugs. https://www.incb.org/documents/Publications/AnnualReports/AR2010/Supplement-AR10_availability_English.pdf. Published January 2011. Accessed August 25, 2016.
3. Manchikanti L, Helm S II, Fellows B, et al. Opioid epidemic in the United States. Pain Physician. 2012;15(3)(suppl):ES9-ES38.
4. Krebs EE, Ramsey DC, Miloshoff JM, Bair MJ. Primary care monitoring of long-term opioid therapy among veterans with chronic pain. Pain Med. 2011;12(5):740-746.
5. Von Korff M, Kolodny A, Deyo RA, Chou R. Long-term opioid therapy reconsidered. Ann Intern Med. 2011;155(5):325-328.
6. Baser O, Xie L, Mardekian J, Schaaf D, Wang L, Joshi AV. Prevalence of diagnosed opioid abuse and its economic burden in the Veterans’ Health Administration. Pain Pract. 2014;14(5):437-445.
7. Department of Veterans Affairs, Department of Defense. VA/DoD Clinical Practice Guideline for Management of Opioid Therapy for Chronic Pain. Guideline summary. http://www.va.gov/painmanagement/docs/cpg_opioidtherapy_summary.pdf. Published May 2010. Accessed August 25, 2016.
8. Centers for Disease Control and Prevention (CDC). CDC grand rounds: prescription drug overdoses—a U.S. epidemic. MMWR Morb Mortal Wkly Rep. 2012;61(1):10-13.
9. Hansen H, Noe CE, Racz GB. The evolving role of opioid treatment in chronic pain management. In: Hansen H, Racz GB, Noe CE, eds. Pain and Treatment. http://www.intechopen.com/books/pain-and-treatment/the-evolving-role-of-opioid-treatment-in-chronic-pain-management. Published July 10, 2014. Accessed October 5, 2016.
10. McAuley D. Opioids—equianalgesic dosages. http://www.globalrph.com/narcotic.htm. Updated August 5, 2016. Accessed October 5, 2016.
11. U.S. Department of Health and Human Services. Addressing prescription drug abuse in the United States: current activities and future opportunities. https://www.cdc.gov/drugoverdose/pdf/hhs_prescription_drug_abuse_report_09.2013.pdf. Accessed August 25, 2016.
12. Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration, U.S. Department of Health and Human Services, RTI International. Results from the 2010 National survey on drug use and health: summary of national findings. http://archive.samhsa.gov/data/NSDUH/2k10NSDUH/2k10Results.htm. Published September 2011. Accessed October 5, 2016.
13. Birnbaum HG, White AG, Schiller M, Waldman T, Cleveland JM, Roland CL. Societal costs of prescription opioid abuse, dependence, and misuse in the United States. Pain Med. 2011;12(4):657-667.
14. Federation of State Medical Boards. Model policy on the use of opioid analgesics in the treatment of chronic pain. http://www.fsmb.org/Media/Default/PDF/FSMB/Advocacy/pain_policy_july2013.pdf.Published July 2013. Accessed October 5, 2016.
15. Wiedemer NL, Harden PS, Arndt IO, Gallagher RM. The opioid renewal clinic: a primary care, managed approach to opioid therapy in chronic pain patients at risk for substance abuse. Pain Med. 2007;8(7):573-584.
16. Sampson JM, Havens S, Marsh B, Murrhee R. Managing chronic, nonmalignant pain in patients with a substance use disorder. Fed Pract. 2005;22(11):10, 16, 18, 25-26, 29.
17. Kharlamb V. (2008). VISN Opioid cost avoidance plan. New York Harbor VA, New York. Unpublished raw data.
Chronic pain, defined as pain lasting longer than 3 to 6 months in duration, affects about 100 million Americans.1 The use of opioids in the management of chronic nonmalignant pain is common in primary care. The U.S., with only 5% of the global population, nevertheless is the world’s leading opioid consumer.2 For example, it is estimated that the U.S. consumes 56% of the global supply of morphine, 99% of hydrocodone, and 83% of oxycodone; this consumption is a growing problem in the use of chronic opioid therapy in managing chronic nonmalignant pain.2,3 The high rates of use of opioids continues, despite a lack of solid evidence on the long-term effectiveness of opioids for managing chronic nonmalignant pain and on the associated risks of opioid addiction, abuse, and misuse.3,4 Among veterans, the prevalence of opioid abuse and misuse has been reported to be about 30%, a nearly 7-fold occurrence compared with that in the general population.5,6
Due to the pervasiveness of opioid abuse and misuse among veterans, a project was initiated to develop, implement, and evaluate an Opioid Monitoring Clinic (OMC) as a clinical referral system within the primary care service of the VA Southern Nevada Healthcare System (VASNHS) in North Las Vegas. A health care provider (HCP) needs assessment was conducted at the facility, resulting in recommendations to improve adherence to evidence-based clinical practice guidelines in opioid management and regular monitoring of veterans on chronic opioid therapy for the identification of opioid abuse and misuse. Based on the results, an advanced practice registered nurse (APRN) in consultation with the chief of primary care at VASNHS and teamlet support (a registered nurse, licensed practical nurse, and medical support assistant) started the OMC. The OMC was developed consistent with the 2010 VA/DoD clinical practice guidelines for managing opioid therapy for chronic pain.7
After 6 months of OMC operation, the project also was evaluated for efficacy. First, a retrospective chart review of participants was conducted to identify the use of opioid pain agreements, prescription drug monitoring programs (PDMP) for controlled substance use review, and urine drug screens (UDSs). The chart review also included the average daily morphine equivalent dose (MED) for patients and OMC retention rates. Second, an online survey of primary care providers (PCPs) assessed their adherence to evidence-based guidelines in opioid management and satisfaction of the OMC services.
Background
In 1997, the average sales and distribution of opioids in the U.S. was 96 mg MED per individual, which increased to 710 mg per individual in 2010.8,9 The MED is a standardized daily dose measure for all opioids.10 At VASNHS North Las Vegas, there were 5,881 patients on opioid therapy in 2013 with about 13% of patients on opioid therapy using about 100 mg MED/d. The potential for abuse and misuse was great. Almost 30% of patients on chronic opioid therapy for chronic nonmalignant pain abuse their opioid prescriptions.5,7 Subsequently, opioid analgesics were responsible for nearly 60% of overdose deaths in 2010.11
In 2010, there were about 12 million people in the U.S. who abused or misused prescription opioids, using them for nonmedical reasons; annually, the prevalence rate of Americans who abuse and misuse opioids is about 2 million people.12 The 5-year prevalence rate of opioid abuse among veterans is at least 3%.6 This results in health care expenditures with an average excess medical cost of $20,546 per year for patients who misuse opioids compared with those who do not.13 The economic burden among veterans is even higher. Baser and colleagues reported that the annual economic cost for veterans who abused their opioid prescriptions was nearly $29,000.6
Unfortunately, injudicious opioid prescribing by HCPs is often cited as a contributor to the growing problem of opioid abuse and misuse in the U.S.14 Health care provider education on the proper and judicious use of opioids and adherence to clinical practice guidelines in the management of chronic pain is a crucial factor in reducing the complications of chron
Method
This project evaluated the APRN-led OMC with both quantitative and qualitative data from patients and PCPs. The OMC was implemented at VASNHS North Las Vegas, which serves almost 60,000 veterans. Patients referred to the OMC who were eligible for admission were veterans aged ≥ 18 years, who had chronic nonmalignant pain for at least 3 months, were receiving chronic opioid therapy, and were considered high risk for abuse or misuse of opioids. Patients were considered high risk if they had documented aberrant behaviors, such as multiple early refill requests, history of lost medications, drug screens not showing prescribed opioid(s), positive drug screens for controlled substance not prescribed, nonadherence with plan of care, or a history of substance abuse, including alcohol, cocaine, heroin, and marijuana. Veterans found to be suicidal or homicidal were excluded from the OMC and instead were referred to appropriate specialty care for further evaluation. A total of 61 veteran participants were successfully recruited.
Primary care physicians, physician assistants, and APRNs working at VASNHS on a full-time and part-time basis were eligible for participation as PCPs in this project. Thirty of 42 eligible PCPs participated and responded to the secure online provider survey (71% response rate).
Risks and benefits were discussed, and a written informed consent was obtained for each participant. There were no apparent risks or adverse effects encountered during project implementation and evaluation. Prior to data collection, permission for the study was obtained from the VA facility. An institutional review board application through the University of Nevada, Las Vegas’ Office of Research Integrity-Human Subjects was also submitted and approved through an expedited review.
Results
Table 1 details patient data, including demographics on the veteran participants. The av
Review of PDMPs revealed that 20% of the participants received controlled substances from other HCPs. Consequently, all OMC patients completed and had reinforced OPAs that served 2 purposes. First, the content in the agreement provided patient education regarding the potential hazards of opioid use. Second, the content provided specific expectations from the patient if opioid therapy is continued, including opioid risk reduction strategies (ie, random UDSs and pill counts, PDMP for controlled substance utilization review), and the consequences of lack of adherence to opioid management.
The majority of patients remained as patients and continued to be monitored after 6 months at the OMC (Table 2). However, only 64% were retained in the OMC, in large part because 36% had their opioids discontinued due to discovery of active illicit substance use, opioi
The mean MED/d among OMC participants was 54 mg/d before admission, which decreased gradually to 22 mg/d after 6 months of OMC care; this represented a 59% MED reduction (Table 3). Using the exact single-tailed Wilcoxon signed rank
Primary Care Providers
Based on the survey of PCPs, 40% of respondents referred at least 1 patient to the OMC. The majority (90%) acknowledged following the VA/DoD guidelines for opioid management, with 80% using an OPA more than usual since the implementation of the OMC, and 54% routinely accessing a PDMP for controlled substance utilization review. Moreover, 93% of PCPs routinely ordered a UDS when indicated.
For 3 months prior to opening the OMC in 2013, 1,606 UDSs were ordered at VASNHS. The UDS number steadily increased, and 6 months after opening the OMC, UDSs increased to 2,293 from months 4 to 6, representing a 30% increase. This was an expected outcome from the OMC—more PCPs now were following the VA/DoD clinical practice guidelines regarding the monitoring of patients on chronic opioid therapy.
A survey assessed the level of satisfaction among PCPs who had referred their patients to the OMC. Although only 11 PCPs responded to this question, a large majority found the OMC to have a positive effect on primary care services; many noted receiving fewer complaints regarding pain medications and fewer walk-ins. The majority recognized the advantage of the OMC in facilitating more PCP time for managing other medical problems beyond opioid use, which tends to be challenging and time consuming. Overall, 100% were satisfied with the OMC service.
Finally, 82% of PCPs reported that the OMC referral process did not need improvement. Two PCPs (18%) left positive feedback, reporting that the OMC was “simple, easy and accessible” and that the referral process “so far, it is great.” Another PCP noted, “I want patients who are suicidal but still need pain control with narcotics to be addressed.”
Discussion
The OMC has shown great promise in identifying abuse and misuse of opioids through evidence-based guidelines and risk-mitigation strategies. In the past, VA clinics specifically focused on opioid renewal have been implemented. In 2002, the Philadelphia VAMC opened an opioid renewal clinic (ORC) to assist PCPs in the management of patients with chronic pain on chronic opioid therapy.15 The Philadelphia VAMC ORC was operated collaboratively by PCPs and Pharmacy Service. They reported that 51% of their patients initially had documented aberrant behaviors, and 45% of these patients resolved their aberrant behaviors through intensive opioid monitoring using random UDSs.14 Thirteen percent of their patients were found to have an opioid addiction disorder and eventually were referred to addiction treatment; and 4% were weaned off opioids due to consistently negative UDSs.14
In the same manner, the OMC has effectively identified patients who abused and misused their opioids and consequently referred these patients for pain interventional management or to the VASNHS alcohol and drug treatment program as appropriate, which falls under the VASNHS Mental Health Care line, a service that is vital for veterans who are suicidal and homicidal. The importance of mental health care cannot be understated, as many patients with chronic pain also experience mental health challenges.
The Malcom Randall VAMC in Gainesville, Florida, structured a nurse-led, multidisciplinary ORC in 2003.16 A retrospective review of their program showed that 33% of patients had a positive UDS for marijuana, cocaine, or alcohol. The ORC had increased patient involvement in substance abuse treatment, resulting in some patients taking lower opioid dosages than before.
The New York Harbor VAMC reduced opioid cost by effectively switching veterans on expensive long-acting opioids, such as oxycodone and fentanyl, to less expensive alternatives, such a long-acting morphine.17 A secondary purpose of the New York initiative was to reduce the potential for inappropriate use of expensive long-acting opioids. Accordingly, the initiative reduced the number of expensive long-acting and potentially inappropriate opioids from 165 to 69 prescriptions in less than 6 months (November 2007 through March 2008). Similarly, after 6 months of operation, the OMC significantly reduced opioid prescription from 54 mg to 22 MED/d. This reduction represents a significant pharmacy cost savings. The combination of the discontinuation of opioids for patients found to be abusing and misusing opioids coupled with the decrease in pill burden resulting from changes from short-acting to long-acting opioids also resulted in significant savings for VA facilities.
The impact of the OMC on PCP adherence to opioid management guidelines as well as PCP satisfaction with the OMC services was significant. Similarly, Wiedemer and colleagues found significant PCP satisfaction with the ORC.15 The ability to spend more time with patients on other medical problems while allowing the OMC to focus on opioid and pain management was found to be beneficial. Buy-in among PCPs coupled with their concerns for chronic opioid therapy for high-risk patients facilitated the success of the OMC. The commitment of the VASNHS leadership to the OMC and their support for the APRN in leading this initiative were important facilitators in the success of the OMC.
Limitations
Long-term evaluation of the OMC with a larger sample is needed to fully evaluate its impact on decreasing opioid misuse and abuse. This project was limited by a small sample size, although the results are promising. Pharmacy costs, emergency department visits, as well as patient satisfaction, physical and emotional function, and pain levels are outcomes that need to be considered over the long term. Incorporating mental health counseling, cognitive behavior therapy, self-management programs, and group educational sessions have the potential to be important OMC services. The continued success and cost-effectiveness of the OMC can be a potentially significant model for this type of service that can be applied to clinics outside the VA system.
Implications
Possible implications to practice settings that are considering an OMC or ORC include the chance that patients will want to be discharged from such a clinic and return to the PCP for opioid management. Collaborative relationships and communication between PCPs and OMC providers are important to facilitate adherence and consistency with pain care. Collaboration and effective communication can be facilitated by electronic recording and reporting. For example, the VA Computerized Patient Record System can alert PCPs to patient discharges from the OMC along with OMC provider recommendations for patient care. Another challenge for the OMC would be a lack of referrals for patients who are at high risk for opioid abuse or misuse. These challenges can be mitigated by providing in-services, educational flyers, and advertisement promotions regarding OMC services. With the high prevalence of opioid abuse and misuse as well as the subsequent exorbitant health-related costs and deaths associated with opioids, OMCs and ORCs are viable options for improving opioid management in the treatment of patients with nonmalignant chronic pain.
Conclusion
The OMC effectively reduced the MED of patients referred to the clinic by 59%. The significant reduction in the opioid dose of patients referred to the OMC resulted from the implementation of evidence-based strategies that were used to identify abuse of prescription opioids, the use of illicit substances that can cause opioid-related complications, and the discovery of doctor shopping, coupled with gradual dose reductions for patients when appropriate. Provider satisfaction and increased use of evidence-based guidelines in opioid management and risk mitigation strategies, such as OPAs, PDMP databases, and UDS were evident. These results suggest that an OMC can be an effective program to help identify abuse and misuse of prescription opioids among high-risk patients and can improve patient safety and provider satisfaction.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the VA Southern Nevada Healthcare System.
Chronic pain, defined as pain lasting longer than 3 to 6 months in duration, affects about 100 million Americans.1 The use of opioids in the management of chronic nonmalignant pain is common in primary care. The U.S., with only 5% of the global population, nevertheless is the world’s leading opioid consumer.2 For example, it is estimated that the U.S. consumes 56% of the global supply of morphine, 99% of hydrocodone, and 83% of oxycodone; this consumption is a growing problem in the use of chronic opioid therapy in managing chronic nonmalignant pain.2,3 The high rates of use of opioids continues, despite a lack of solid evidence on the long-term effectiveness of opioids for managing chronic nonmalignant pain and on the associated risks of opioid addiction, abuse, and misuse.3,4 Among veterans, the prevalence of opioid abuse and misuse has been reported to be about 30%, a nearly 7-fold occurrence compared with that in the general population.5,6
Due to the pervasiveness of opioid abuse and misuse among veterans, a project was initiated to develop, implement, and evaluate an Opioid Monitoring Clinic (OMC) as a clinical referral system within the primary care service of the VA Southern Nevada Healthcare System (VASNHS) in North Las Vegas. A health care provider (HCP) needs assessment was conducted at the facility, resulting in recommendations to improve adherence to evidence-based clinical practice guidelines in opioid management and regular monitoring of veterans on chronic opioid therapy for the identification of opioid abuse and misuse. Based on the results, an advanced practice registered nurse (APRN) in consultation with the chief of primary care at VASNHS and teamlet support (a registered nurse, licensed practical nurse, and medical support assistant) started the OMC. The OMC was developed consistent with the 2010 VA/DoD clinical practice guidelines for managing opioid therapy for chronic pain.7
After 6 months of OMC operation, the project also was evaluated for efficacy. First, a retrospective chart review of participants was conducted to identify the use of opioid pain agreements, prescription drug monitoring programs (PDMP) for controlled substance use review, and urine drug screens (UDSs). The chart review also included the average daily morphine equivalent dose (MED) for patients and OMC retention rates. Second, an online survey of primary care providers (PCPs) assessed their adherence to evidence-based guidelines in opioid management and satisfaction of the OMC services.
Background
In 1997, the average sales and distribution of opioids in the U.S. was 96 mg MED per individual, which increased to 710 mg per individual in 2010.8,9 The MED is a standardized daily dose measure for all opioids.10 At VASNHS North Las Vegas, there were 5,881 patients on opioid therapy in 2013 with about 13% of patients on opioid therapy using about 100 mg MED/d. The potential for abuse and misuse was great. Almost 30% of patients on chronic opioid therapy for chronic nonmalignant pain abuse their opioid prescriptions.5,7 Subsequently, opioid analgesics were responsible for nearly 60% of overdose deaths in 2010.11
In 2010, there were about 12 million people in the U.S. who abused or misused prescription opioids, using them for nonmedical reasons; annually, the prevalence rate of Americans who abuse and misuse opioids is about 2 million people.12 The 5-year prevalence rate of opioid abuse among veterans is at least 3%.6 This results in health care expenditures with an average excess medical cost of $20,546 per year for patients who misuse opioids compared with those who do not.13 The economic burden among veterans is even higher. Baser and colleagues reported that the annual economic cost for veterans who abused their opioid prescriptions was nearly $29,000.6
Unfortunately, injudicious opioid prescribing by HCPs is often cited as a contributor to the growing problem of opioid abuse and misuse in the U.S.14 Health care provider education on the proper and judicious use of opioids and adherence to clinical practice guidelines in the management of chronic pain is a crucial factor in reducing the complications of chron
Method
This project evaluated the APRN-led OMC with both quantitative and qualitative data from patients and PCPs. The OMC was implemented at VASNHS North Las Vegas, which serves almost 60,000 veterans. Patients referred to the OMC who were eligible for admission were veterans aged ≥ 18 years, who had chronic nonmalignant pain for at least 3 months, were receiving chronic opioid therapy, and were considered high risk for abuse or misuse of opioids. Patients were considered high risk if they had documented aberrant behaviors, such as multiple early refill requests, history of lost medications, drug screens not showing prescribed opioid(s), positive drug screens for controlled substance not prescribed, nonadherence with plan of care, or a history of substance abuse, including alcohol, cocaine, heroin, and marijuana. Veterans found to be suicidal or homicidal were excluded from the OMC and instead were referred to appropriate specialty care for further evaluation. A total of 61 veteran participants were successfully recruited.
Primary care physicians, physician assistants, and APRNs working at VASNHS on a full-time and part-time basis were eligible for participation as PCPs in this project. Thirty of 42 eligible PCPs participated and responded to the secure online provider survey (71% response rate).
Risks and benefits were discussed, and a written informed consent was obtained for each participant. There were no apparent risks or adverse effects encountered during project implementation and evaluation. Prior to data collection, permission for the study was obtained from the VA facility. An institutional review board application through the University of Nevada, Las Vegas’ Office of Research Integrity-Human Subjects was also submitted and approved through an expedited review.
Results
Table 1 details patient data, including demographics on the veteran participants. The av
Review of PDMPs revealed that 20% of the participants received controlled substances from other HCPs. Consequently, all OMC patients completed and had reinforced OPAs that served 2 purposes. First, the content in the agreement provided patient education regarding the potential hazards of opioid use. Second, the content provided specific expectations from the patient if opioid therapy is continued, including opioid risk reduction strategies (ie, random UDSs and pill counts, PDMP for controlled substance utilization review), and the consequences of lack of adherence to opioid management.
The majority of patients remained as patients and continued to be monitored after 6 months at the OMC (Table 2). However, only 64% were retained in the OMC, in large part because 36% had their opioids discontinued due to discovery of active illicit substance use, opioi
The mean MED/d among OMC participants was 54 mg/d before admission, which decreased gradually to 22 mg/d after 6 months of OMC care; this represented a 59% MED reduction (Table 3). Using the exact single-tailed Wilcoxon signed rank
Primary Care Providers
Based on the survey of PCPs, 40% of respondents referred at least 1 patient to the OMC. The majority (90%) acknowledged following the VA/DoD guidelines for opioid management, with 80% using an OPA more than usual since the implementation of the OMC, and 54% routinely accessing a PDMP for controlled substance utilization review. Moreover, 93% of PCPs routinely ordered a UDS when indicated.
For 3 months prior to opening the OMC in 2013, 1,606 UDSs were ordered at VASNHS. The UDS number steadily increased, and 6 months after opening the OMC, UDSs increased to 2,293 from months 4 to 6, representing a 30% increase. This was an expected outcome from the OMC—more PCPs now were following the VA/DoD clinical practice guidelines regarding the monitoring of patients on chronic opioid therapy.
A survey assessed the level of satisfaction among PCPs who had referred their patients to the OMC. Although only 11 PCPs responded to this question, a large majority found the OMC to have a positive effect on primary care services; many noted receiving fewer complaints regarding pain medications and fewer walk-ins. The majority recognized the advantage of the OMC in facilitating more PCP time for managing other medical problems beyond opioid use, which tends to be challenging and time consuming. Overall, 100% were satisfied with the OMC service.
Finally, 82% of PCPs reported that the OMC referral process did not need improvement. Two PCPs (18%) left positive feedback, reporting that the OMC was “simple, easy and accessible” and that the referral process “so far, it is great.” Another PCP noted, “I want patients who are suicidal but still need pain control with narcotics to be addressed.”
Discussion
The OMC has shown great promise in identifying abuse and misuse of opioids through evidence-based guidelines and risk-mitigation strategies. In the past, VA clinics specifically focused on opioid renewal have been implemented. In 2002, the Philadelphia VAMC opened an opioid renewal clinic (ORC) to assist PCPs in the management of patients with chronic pain on chronic opioid therapy.15 The Philadelphia VAMC ORC was operated collaboratively by PCPs and Pharmacy Service. They reported that 51% of their patients initially had documented aberrant behaviors, and 45% of these patients resolved their aberrant behaviors through intensive opioid monitoring using random UDSs.14 Thirteen percent of their patients were found to have an opioid addiction disorder and eventually were referred to addiction treatment; and 4% were weaned off opioids due to consistently negative UDSs.14
In the same manner, the OMC has effectively identified patients who abused and misused their opioids and consequently referred these patients for pain interventional management or to the VASNHS alcohol and drug treatment program as appropriate, which falls under the VASNHS Mental Health Care line, a service that is vital for veterans who are suicidal and homicidal. The importance of mental health care cannot be understated, as many patients with chronic pain also experience mental health challenges.
The Malcom Randall VAMC in Gainesville, Florida, structured a nurse-led, multidisciplinary ORC in 2003.16 A retrospective review of their program showed that 33% of patients had a positive UDS for marijuana, cocaine, or alcohol. The ORC had increased patient involvement in substance abuse treatment, resulting in some patients taking lower opioid dosages than before.
The New York Harbor VAMC reduced opioid cost by effectively switching veterans on expensive long-acting opioids, such as oxycodone and fentanyl, to less expensive alternatives, such a long-acting morphine.17 A secondary purpose of the New York initiative was to reduce the potential for inappropriate use of expensive long-acting opioids. Accordingly, the initiative reduced the number of expensive long-acting and potentially inappropriate opioids from 165 to 69 prescriptions in less than 6 months (November 2007 through March 2008). Similarly, after 6 months of operation, the OMC significantly reduced opioid prescription from 54 mg to 22 MED/d. This reduction represents a significant pharmacy cost savings. The combination of the discontinuation of opioids for patients found to be abusing and misusing opioids coupled with the decrease in pill burden resulting from changes from short-acting to long-acting opioids also resulted in significant savings for VA facilities.
The impact of the OMC on PCP adherence to opioid management guidelines as well as PCP satisfaction with the OMC services was significant. Similarly, Wiedemer and colleagues found significant PCP satisfaction with the ORC.15 The ability to spend more time with patients on other medical problems while allowing the OMC to focus on opioid and pain management was found to be beneficial. Buy-in among PCPs coupled with their concerns for chronic opioid therapy for high-risk patients facilitated the success of the OMC. The commitment of the VASNHS leadership to the OMC and their support for the APRN in leading this initiative were important facilitators in the success of the OMC.
Limitations
Long-term evaluation of the OMC with a larger sample is needed to fully evaluate its impact on decreasing opioid misuse and abuse. This project was limited by a small sample size, although the results are promising. Pharmacy costs, emergency department visits, as well as patient satisfaction, physical and emotional function, and pain levels are outcomes that need to be considered over the long term. Incorporating mental health counseling, cognitive behavior therapy, self-management programs, and group educational sessions have the potential to be important OMC services. The continued success and cost-effectiveness of the OMC can be a potentially significant model for this type of service that can be applied to clinics outside the VA system.
Implications
Possible implications to practice settings that are considering an OMC or ORC include the chance that patients will want to be discharged from such a clinic and return to the PCP for opioid management. Collaborative relationships and communication between PCPs and OMC providers are important to facilitate adherence and consistency with pain care. Collaboration and effective communication can be facilitated by electronic recording and reporting. For example, the VA Computerized Patient Record System can alert PCPs to patient discharges from the OMC along with OMC provider recommendations for patient care. Another challenge for the OMC would be a lack of referrals for patients who are at high risk for opioid abuse or misuse. These challenges can be mitigated by providing in-services, educational flyers, and advertisement promotions regarding OMC services. With the high prevalence of opioid abuse and misuse as well as the subsequent exorbitant health-related costs and deaths associated with opioids, OMCs and ORCs are viable options for improving opioid management in the treatment of patients with nonmalignant chronic pain.
Conclusion
The OMC effectively reduced the MED of patients referred to the clinic by 59%. The significant reduction in the opioid dose of patients referred to the OMC resulted from the implementation of evidence-based strategies that were used to identify abuse of prescription opioids, the use of illicit substances that can cause opioid-related complications, and the discovery of doctor shopping, coupled with gradual dose reductions for patients when appropriate. Provider satisfaction and increased use of evidence-based guidelines in opioid management and risk mitigation strategies, such as OPAs, PDMP databases, and UDS were evident. These results suggest that an OMC can be an effective program to help identify abuse and misuse of prescription opioids among high-risk patients and can improve patient safety and provider satisfaction.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the VA Southern Nevada Healthcare System.
1. Institute of Medicine (IOM). Relieving pain in America: a blueprint for transforming prevention, care, education, and research. http://iom.nationalacademies.org/~/media/Files/Report%20Files/2011/Relieving-Pain-in-America-A-Blueprint-for-Transforming-Prevention-Care-Education-Research/Pain%20Research%202011%20Report%20Brief.pdf. Published June 2011. Accessed August 25, 2016.
2. International Narcotics Control Board. Report of the International Narcotics Control Board on the availability of internationally controlled drugs. https://www.incb.org/documents/Publications/AnnualReports/AR2010/Supplement-AR10_availability_English.pdf. Published January 2011. Accessed August 25, 2016.
3. Manchikanti L, Helm S II, Fellows B, et al. Opioid epidemic in the United States. Pain Physician. 2012;15(3)(suppl):ES9-ES38.
4. Krebs EE, Ramsey DC, Miloshoff JM, Bair MJ. Primary care monitoring of long-term opioid therapy among veterans with chronic pain. Pain Med. 2011;12(5):740-746.
5. Von Korff M, Kolodny A, Deyo RA, Chou R. Long-term opioid therapy reconsidered. Ann Intern Med. 2011;155(5):325-328.
6. Baser O, Xie L, Mardekian J, Schaaf D, Wang L, Joshi AV. Prevalence of diagnosed opioid abuse and its economic burden in the Veterans’ Health Administration. Pain Pract. 2014;14(5):437-445.
7. Department of Veterans Affairs, Department of Defense. VA/DoD Clinical Practice Guideline for Management of Opioid Therapy for Chronic Pain. Guideline summary. http://www.va.gov/painmanagement/docs/cpg_opioidtherapy_summary.pdf. Published May 2010. Accessed August 25, 2016.
8. Centers for Disease Control and Prevention (CDC). CDC grand rounds: prescription drug overdoses—a U.S. epidemic. MMWR Morb Mortal Wkly Rep. 2012;61(1):10-13.
9. Hansen H, Noe CE, Racz GB. The evolving role of opioid treatment in chronic pain management. In: Hansen H, Racz GB, Noe CE, eds. Pain and Treatment. http://www.intechopen.com/books/pain-and-treatment/the-evolving-role-of-opioid-treatment-in-chronic-pain-management. Published July 10, 2014. Accessed October 5, 2016.
10. McAuley D. Opioids—equianalgesic dosages. http://www.globalrph.com/narcotic.htm. Updated August 5, 2016. Accessed October 5, 2016.
11. U.S. Department of Health and Human Services. Addressing prescription drug abuse in the United States: current activities and future opportunities. https://www.cdc.gov/drugoverdose/pdf/hhs_prescription_drug_abuse_report_09.2013.pdf. Accessed August 25, 2016.
12. Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration, U.S. Department of Health and Human Services, RTI International. Results from the 2010 National survey on drug use and health: summary of national findings. http://archive.samhsa.gov/data/NSDUH/2k10NSDUH/2k10Results.htm. Published September 2011. Accessed October 5, 2016.
13. Birnbaum HG, White AG, Schiller M, Waldman T, Cleveland JM, Roland CL. Societal costs of prescription opioid abuse, dependence, and misuse in the United States. Pain Med. 2011;12(4):657-667.
14. Federation of State Medical Boards. Model policy on the use of opioid analgesics in the treatment of chronic pain. http://www.fsmb.org/Media/Default/PDF/FSMB/Advocacy/pain_policy_july2013.pdf.Published July 2013. Accessed October 5, 2016.
15. Wiedemer NL, Harden PS, Arndt IO, Gallagher RM. The opioid renewal clinic: a primary care, managed approach to opioid therapy in chronic pain patients at risk for substance abuse. Pain Med. 2007;8(7):573-584.
16. Sampson JM, Havens S, Marsh B, Murrhee R. Managing chronic, nonmalignant pain in patients with a substance use disorder. Fed Pract. 2005;22(11):10, 16, 18, 25-26, 29.
17. Kharlamb V. (2008). VISN Opioid cost avoidance plan. New York Harbor VA, New York. Unpublished raw data.
1. Institute of Medicine (IOM). Relieving pain in America: a blueprint for transforming prevention, care, education, and research. http://iom.nationalacademies.org/~/media/Files/Report%20Files/2011/Relieving-Pain-in-America-A-Blueprint-for-Transforming-Prevention-Care-Education-Research/Pain%20Research%202011%20Report%20Brief.pdf. Published June 2011. Accessed August 25, 2016.
2. International Narcotics Control Board. Report of the International Narcotics Control Board on the availability of internationally controlled drugs. https://www.incb.org/documents/Publications/AnnualReports/AR2010/Supplement-AR10_availability_English.pdf. Published January 2011. Accessed August 25, 2016.
3. Manchikanti L, Helm S II, Fellows B, et al. Opioid epidemic in the United States. Pain Physician. 2012;15(3)(suppl):ES9-ES38.
4. Krebs EE, Ramsey DC, Miloshoff JM, Bair MJ. Primary care monitoring of long-term opioid therapy among veterans with chronic pain. Pain Med. 2011;12(5):740-746.
5. Von Korff M, Kolodny A, Deyo RA, Chou R. Long-term opioid therapy reconsidered. Ann Intern Med. 2011;155(5):325-328.
6. Baser O, Xie L, Mardekian J, Schaaf D, Wang L, Joshi AV. Prevalence of diagnosed opioid abuse and its economic burden in the Veterans’ Health Administration. Pain Pract. 2014;14(5):437-445.
7. Department of Veterans Affairs, Department of Defense. VA/DoD Clinical Practice Guideline for Management of Opioid Therapy for Chronic Pain. Guideline summary. http://www.va.gov/painmanagement/docs/cpg_opioidtherapy_summary.pdf. Published May 2010. Accessed August 25, 2016.
8. Centers for Disease Control and Prevention (CDC). CDC grand rounds: prescription drug overdoses—a U.S. epidemic. MMWR Morb Mortal Wkly Rep. 2012;61(1):10-13.
9. Hansen H, Noe CE, Racz GB. The evolving role of opioid treatment in chronic pain management. In: Hansen H, Racz GB, Noe CE, eds. Pain and Treatment. http://www.intechopen.com/books/pain-and-treatment/the-evolving-role-of-opioid-treatment-in-chronic-pain-management. Published July 10, 2014. Accessed October 5, 2016.
10. McAuley D. Opioids—equianalgesic dosages. http://www.globalrph.com/narcotic.htm. Updated August 5, 2016. Accessed October 5, 2016.
11. U.S. Department of Health and Human Services. Addressing prescription drug abuse in the United States: current activities and future opportunities. https://www.cdc.gov/drugoverdose/pdf/hhs_prescription_drug_abuse_report_09.2013.pdf. Accessed August 25, 2016.
12. Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration, U.S. Department of Health and Human Services, RTI International. Results from the 2010 National survey on drug use and health: summary of national findings. http://archive.samhsa.gov/data/NSDUH/2k10NSDUH/2k10Results.htm. Published September 2011. Accessed October 5, 2016.
13. Birnbaum HG, White AG, Schiller M, Waldman T, Cleveland JM, Roland CL. Societal costs of prescription opioid abuse, dependence, and misuse in the United States. Pain Med. 2011;12(4):657-667.
14. Federation of State Medical Boards. Model policy on the use of opioid analgesics in the treatment of chronic pain. http://www.fsmb.org/Media/Default/PDF/FSMB/Advocacy/pain_policy_july2013.pdf.Published July 2013. Accessed October 5, 2016.
15. Wiedemer NL, Harden PS, Arndt IO, Gallagher RM. The opioid renewal clinic: a primary care, managed approach to opioid therapy in chronic pain patients at risk for substance abuse. Pain Med. 2007;8(7):573-584.
16. Sampson JM, Havens S, Marsh B, Murrhee R. Managing chronic, nonmalignant pain in patients with a substance use disorder. Fed Pract. 2005;22(11):10, 16, 18, 25-26, 29.
17. Kharlamb V. (2008). VISN Opioid cost avoidance plan. New York Harbor VA, New York. Unpublished raw data.
Decitabine elicits favorable response in high-risk AML/MDS
Single-agent therapy with decitabine elicited favorable responses in patients with acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS) who had cytogenetic abnormalities associated with an unfavorable risk profile, a study showed.
Even though the responses were not long lasting, the overall survival rates were similar to those of AML patients who had an intermediate-risk cytogenetic profile and who had received the same treatment regimen.
Adult AML patients with karyotypes associated with unfavorable risk and older patients with AML (aged 60 or older) generally have poor outcomes, with a median survival in the range of 1 year. Those with AML and TP53 mutations tend to be older (median age, 61-67 years), and nearly all patients have karyotypes associated with unfavorable risk. These patients have particularly dismal outcomes, with median survival in the range of 4-6 months, if they receive cytotoxic chemotherapy.
In their study, John S Welch, MD, of Washington University, St Louis, and his colleagues evaluated somatic mutations and their relationships to clinical responses in 84 adult patients with AML or MDS who received treatment with decitabine as monotherapy.
Decitabine was administered at a dose of 20 mg/m2 of body surface area per day for 10 consecutive days in monthly cycles. An extension cohort that included 32 additional patients also were treated with decitabine but in different protocols.
Of the entire cohort of 116 patients, 15 patients (13%) achieved a complete remission, and 38 patients had bone marrow blast clearance with less than 5% blasts (complete remission with incomplete count recovery or morphologic complete remission). The overall response rate was 46%. In addition, 9 patients (8%), achieved a partial response, stable disease was observed in 23 patients (20%), and progressive disease was seen in 19 patients (16%).
Clinical responses were strongly correlated with karyotypes associated with unfavorable risk and the presence of TP53 mutations. Bone marrow blast clearance (complete remission, complete remission with incomplete count recovery, or morphologic complete remission) occurred in 29 of 43 patients with karyotypes associated with unfavorable risk (67%), compared with 24 of 71 patients with karyotypes associated with intermediate or favorable risk (34%). The same pattern was observed in all patients with TP53 mutations (100%) versus 32 of 78 patients with wildtype TP53 mutations (41%).
“Additional studies will be required to determine whether these differences in survival are truly due to improved responses associated with decitabine or whether conventional chemotherapy with an anthracycline and cytarabine actually decreases the rate of survival among patients with unfavorable-risk cytogenetic profiles,” wrote Dr. Welch and his colleagues.
The study was supported by the Specialized Program of Research Excellence in AML of the National Cancer Institute and the Genomics of AML Program Project. Dr. Welch had no disclosures, and several of his coauthors reported relationships with industry.
The current study raises the important question of the definition of a response. Complete remission conventionally entails bone marrow with less than 5% blasts and normalization of blood counts; absent these, remission is considered incomplete. After various confounding factors are taken into account, a complete response with cytotoxic therapy is associated with longer remissions and longer survival than is complete remission with incomplete count recovery.
The authors of this paper considered a response to be blast clearance to less than 5%, but complete remission was seen in only 4 of the 21 patients with TP53 mutations who fulfilled this criterion. The mutant allele burden was also similar in patients who had a response, regardless of blood count recovery.
In contrast, measurable residual disease is considerably more frequent in patients with complete remission with incomplete count recovery than in patients with complete remission who have received cytotoxic therapy, indicating that more data are needed on subsequent clinical outcomes according to whether clearance of blasts is accompanied by count recovery in patients with AML and TP53 mutations who have received decitabine.
AML “targeted-therapy” trials typically involve one drug, and this policy is called into question by the diverse molecular architecture (and brief remissions) observed in this trial. The trial by Welch et al. points to inevitable, rational replacement of large trials in which homogeneous therapy is administered for a heterogeneous disease by smaller, subgroup-specific trials.
The article also suggests questions that are likely to complicate this future.
Dr. Elihu Estey is with the division of hematology, University of Washington Medical Center, and the clinical research division, Fred Hutchinson Cancer Research Center, Seattle. He had no disclosures. These remarks were taken from an editorial accompanying Dr. Welch’s paper (N Engl J Med. 2016;375:2023-36).
The current study raises the important question of the definition of a response. Complete remission conventionally entails bone marrow with less than 5% blasts and normalization of blood counts; absent these, remission is considered incomplete. After various confounding factors are taken into account, a complete response with cytotoxic therapy is associated with longer remissions and longer survival than is complete remission with incomplete count recovery.
The authors of this paper considered a response to be blast clearance to less than 5%, but complete remission was seen in only 4 of the 21 patients with TP53 mutations who fulfilled this criterion. The mutant allele burden was also similar in patients who had a response, regardless of blood count recovery.
In contrast, measurable residual disease is considerably more frequent in patients with complete remission with incomplete count recovery than in patients with complete remission who have received cytotoxic therapy, indicating that more data are needed on subsequent clinical outcomes according to whether clearance of blasts is accompanied by count recovery in patients with AML and TP53 mutations who have received decitabine.
AML “targeted-therapy” trials typically involve one drug, and this policy is called into question by the diverse molecular architecture (and brief remissions) observed in this trial. The trial by Welch et al. points to inevitable, rational replacement of large trials in which homogeneous therapy is administered for a heterogeneous disease by smaller, subgroup-specific trials.
The article also suggests questions that are likely to complicate this future.
Dr. Elihu Estey is with the division of hematology, University of Washington Medical Center, and the clinical research division, Fred Hutchinson Cancer Research Center, Seattle. He had no disclosures. These remarks were taken from an editorial accompanying Dr. Welch’s paper (N Engl J Med. 2016;375:2023-36).
The current study raises the important question of the definition of a response. Complete remission conventionally entails bone marrow with less than 5% blasts and normalization of blood counts; absent these, remission is considered incomplete. After various confounding factors are taken into account, a complete response with cytotoxic therapy is associated with longer remissions and longer survival than is complete remission with incomplete count recovery.
The authors of this paper considered a response to be blast clearance to less than 5%, but complete remission was seen in only 4 of the 21 patients with TP53 mutations who fulfilled this criterion. The mutant allele burden was also similar in patients who had a response, regardless of blood count recovery.
In contrast, measurable residual disease is considerably more frequent in patients with complete remission with incomplete count recovery than in patients with complete remission who have received cytotoxic therapy, indicating that more data are needed on subsequent clinical outcomes according to whether clearance of blasts is accompanied by count recovery in patients with AML and TP53 mutations who have received decitabine.
AML “targeted-therapy” trials typically involve one drug, and this policy is called into question by the diverse molecular architecture (and brief remissions) observed in this trial. The trial by Welch et al. points to inevitable, rational replacement of large trials in which homogeneous therapy is administered for a heterogeneous disease by smaller, subgroup-specific trials.
The article also suggests questions that are likely to complicate this future.
Dr. Elihu Estey is with the division of hematology, University of Washington Medical Center, and the clinical research division, Fred Hutchinson Cancer Research Center, Seattle. He had no disclosures. These remarks were taken from an editorial accompanying Dr. Welch’s paper (N Engl J Med. 2016;375:2023-36).
Single-agent therapy with decitabine elicited favorable responses in patients with acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS) who had cytogenetic abnormalities associated with an unfavorable risk profile, a study showed.
Even though the responses were not long lasting, the overall survival rates were similar to those of AML patients who had an intermediate-risk cytogenetic profile and who had received the same treatment regimen.
Adult AML patients with karyotypes associated with unfavorable risk and older patients with AML (aged 60 or older) generally have poor outcomes, with a median survival in the range of 1 year. Those with AML and TP53 mutations tend to be older (median age, 61-67 years), and nearly all patients have karyotypes associated with unfavorable risk. These patients have particularly dismal outcomes, with median survival in the range of 4-6 months, if they receive cytotoxic chemotherapy.
In their study, John S Welch, MD, of Washington University, St Louis, and his colleagues evaluated somatic mutations and their relationships to clinical responses in 84 adult patients with AML or MDS who received treatment with decitabine as monotherapy.
Decitabine was administered at a dose of 20 mg/m2 of body surface area per day for 10 consecutive days in monthly cycles. An extension cohort that included 32 additional patients also were treated with decitabine but in different protocols.
Of the entire cohort of 116 patients, 15 patients (13%) achieved a complete remission, and 38 patients had bone marrow blast clearance with less than 5% blasts (complete remission with incomplete count recovery or morphologic complete remission). The overall response rate was 46%. In addition, 9 patients (8%), achieved a partial response, stable disease was observed in 23 patients (20%), and progressive disease was seen in 19 patients (16%).
Clinical responses were strongly correlated with karyotypes associated with unfavorable risk and the presence of TP53 mutations. Bone marrow blast clearance (complete remission, complete remission with incomplete count recovery, or morphologic complete remission) occurred in 29 of 43 patients with karyotypes associated with unfavorable risk (67%), compared with 24 of 71 patients with karyotypes associated with intermediate or favorable risk (34%). The same pattern was observed in all patients with TP53 mutations (100%) versus 32 of 78 patients with wildtype TP53 mutations (41%).
“Additional studies will be required to determine whether these differences in survival are truly due to improved responses associated with decitabine or whether conventional chemotherapy with an anthracycline and cytarabine actually decreases the rate of survival among patients with unfavorable-risk cytogenetic profiles,” wrote Dr. Welch and his colleagues.
The study was supported by the Specialized Program of Research Excellence in AML of the National Cancer Institute and the Genomics of AML Program Project. Dr. Welch had no disclosures, and several of his coauthors reported relationships with industry.
Single-agent therapy with decitabine elicited favorable responses in patients with acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS) who had cytogenetic abnormalities associated with an unfavorable risk profile, a study showed.
Even though the responses were not long lasting, the overall survival rates were similar to those of AML patients who had an intermediate-risk cytogenetic profile and who had received the same treatment regimen.
Adult AML patients with karyotypes associated with unfavorable risk and older patients with AML (aged 60 or older) generally have poor outcomes, with a median survival in the range of 1 year. Those with AML and TP53 mutations tend to be older (median age, 61-67 years), and nearly all patients have karyotypes associated with unfavorable risk. These patients have particularly dismal outcomes, with median survival in the range of 4-6 months, if they receive cytotoxic chemotherapy.
In their study, John S Welch, MD, of Washington University, St Louis, and his colleagues evaluated somatic mutations and their relationships to clinical responses in 84 adult patients with AML or MDS who received treatment with decitabine as monotherapy.
Decitabine was administered at a dose of 20 mg/m2 of body surface area per day for 10 consecutive days in monthly cycles. An extension cohort that included 32 additional patients also were treated with decitabine but in different protocols.
Of the entire cohort of 116 patients, 15 patients (13%) achieved a complete remission, and 38 patients had bone marrow blast clearance with less than 5% blasts (complete remission with incomplete count recovery or morphologic complete remission). The overall response rate was 46%. In addition, 9 patients (8%), achieved a partial response, stable disease was observed in 23 patients (20%), and progressive disease was seen in 19 patients (16%).
Clinical responses were strongly correlated with karyotypes associated with unfavorable risk and the presence of TP53 mutations. Bone marrow blast clearance (complete remission, complete remission with incomplete count recovery, or morphologic complete remission) occurred in 29 of 43 patients with karyotypes associated with unfavorable risk (67%), compared with 24 of 71 patients with karyotypes associated with intermediate or favorable risk (34%). The same pattern was observed in all patients with TP53 mutations (100%) versus 32 of 78 patients with wildtype TP53 mutations (41%).
“Additional studies will be required to determine whether these differences in survival are truly due to improved responses associated with decitabine or whether conventional chemotherapy with an anthracycline and cytarabine actually decreases the rate of survival among patients with unfavorable-risk cytogenetic profiles,” wrote Dr. Welch and his colleagues.
The study was supported by the Specialized Program of Research Excellence in AML of the National Cancer Institute and the Genomics of AML Program Project. Dr. Welch had no disclosures, and several of his coauthors reported relationships with industry.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: Of 116 patients, 53 (46%) experienced bone marrow blast clearance (less than 5% blasts), and response rates were higher among those with an unfavorable cytogenetic risk profile.
Data source: A prospective single-center clinical trial that evaluated single-agent decitabine in 84 adult patients with AML or MDS, with an extension cohort.
Disclosures: The study was supported by the Specialized Program of Research Excellence in AML of the National Cancer Institute and the Genomics of AML Program Project. Dr. Welch had no disclosures, and several of his coauthors reported relationships with industry.
What are the modern cardiovascular risk indicators and how should ObGyns be using them in their practice?
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
- Visit NAMS
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
- Visit NAMS
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
- Visit NAMS