User login
Preoperative evaluation: A time-saving algorithm
PRACTICE RECOMMENDATIONS
› Recommend that patients quit smoking 8 weeks before surgery; keep in mind, though, that quitting closer to the date of surgery does not increase the risk of complications. A
› Use the online American College of Surgeons/National Surgical Quality Improvement Program surgical risk calculator to estimate a patient’s surgical risk. C
› Send a patient directly to surgery if he or she has an estimated cardiac risk <1% or <2 risk factors of the Revised Cardiac Risk Index. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
About 27 million Americans undergo surgery every year1 and before doing so, they turn to you—their primary care physician—or their cardiologist for a preoperative evaluation. Of course, the goal of this evaluation is to determine an individual patient’s risk and compare it to procedural averages in an effort to identify opportunities for risk mitigation. But the preoperative evaluation is also an opportunity to make recommendations regarding perioperative management of medications. And certainly we want to conduct these evaluations in a way that is both expeditious and in keeping with the latest guidelines.
Current guidelines for preoperative evaluations are less complicated than they used to be and focus on cardiac and pulmonary risk stratification. While a risk calculator remains your primary tool, elements such as smoking cessation and identifying sleep apnea are important parts of the preop equation. In the review that follows, we present a simple algorithm (FIGURE 12-6) that we developed that can be completed in a single visit.

Cardiac assessment: A risk calculator is the primary tool

Cardiac risk estimation is perhaps the most important element in determining a patient’s overall surgical risk. But before you begin, you'll need to determine whether the preoperative evaluation of the patient is best handled by you or a specialist. Current guidelines recommend preoperative evaluation by a specialist when a patient has certain conditions, such as moderate or greater valvular stenosis/regurgitation, a cardiac implantable electronic device, pulmonary hypertension, congenital heart disease, or severe systemic disease.2
If these conditions are not present (and an immediate referral is not required), you can turn your attention to the cardiac assessment. The first step is to determine which cardiac risk calculator you’d like to use. In its comprehensive guideline on perioperative cardiovascular evaluation, the American College of Cardiology/American Heart Association (ACC/AHA) recommends the use of one of the 2 calculators described below.2
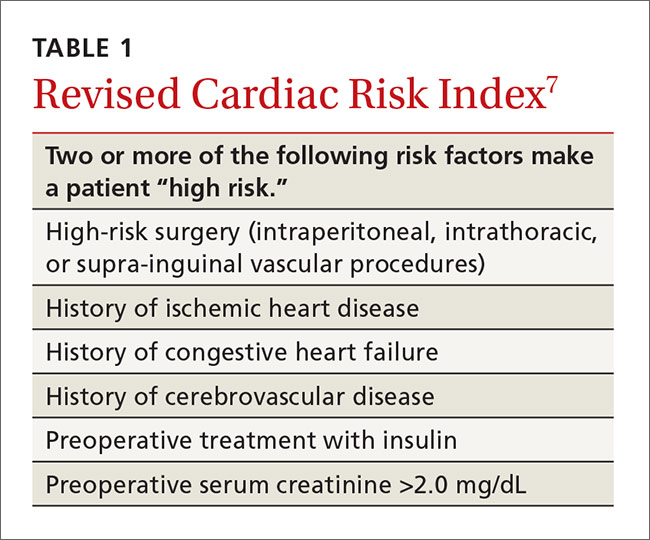
Revised Cardiac Risk Index. The most well-known cardiac risk calculator is the 6-element Revised Cardiac Risk Index (RCRI) (TABLE 1).7 Published in 1999, the RCRI was derived from a cohort of 2800 patients, verified in 1400 patients, and has been validated in numerous studies.2 Each element increases the odds of a cardiac complication by a factor of 2 to 3, and more than one positive response indicates the patient is at high risk for complications.7
The ACS NSQIP risk calculator has been criticized because it has not been validated in a group separate from the initial patient population used in its development.2 Another criticism is the inclusion of the American Society of Anesthesiologists' (ASA) classification of the overall health of the patient, a simple yet subjective and unreliable method of patient characterization.2
Choosing a calculator. The ACS NSQIP calculator may be more useful for primary care physicians because it provides individualized risks for numerous complications and is easy to use. The output page can be printed as documentation of the preoperative evaluation, and is useful for counseling patients about reconsideration of surgery or risk-reduction strategies. The RCRI is also simple to use, but considers only cardiac risk. Although the RCRI has been validated in numerous studies, the ACS NSQIP was derived from a more substantial 1.4 million patients.
Mapping out next steps based on risk score
The next step in the preoperative evaluation process is to calculate your patient’s risk score and determine whether it is low or high. If the risk is determined to be low—either an RCRI score <2 or an ACS NSQIP cardiac complication risk <1%—the patient can be referred to surgery without further evaluation.2
If the calculator suggests higher risk, the patient’s functional status should be assessed. If the patient has a functional status of >4 metabolic equivalents (METs), then the patient can be recommended for surgery without further evaluation.2 Examples of activities that are greater than 4 METs are yard work such as raking leaves, weeding, or pushing a power mower; sexual relations; climbing a flight of stairs; walking up a hill; and participating in moderate recreational activities like golf, bowling, dancing, doubles tennis, or throwing a baseball or football.9
Patient can’t perform >4 METs? If the patient does not have a functional capacity of >4 METs, further risk stratification should be considered if the results would change management.2 Prior guidelines recommended either perioperative beta-blockers to mitigate risk or coronary interventions, but both are controversial due to lack of proven benefit.
Perioperative beta-blocker use. A recommendation to consider starting beta-blockers at least one day prior to surgery remains in the 2014 ACC/AHA guidelines for patients with 3 or more RCRI risk factors.2 But a group of studies supporting beta-blocker use has been discredited due to serious flaws and fabricated data. At the same time, a large study arguing against perioperative beta-blockers has been criticized for starting high doses of beta-blockers on the day of surgery.2,10,11 In the end, mortality benefit from perioperative beta-blockers is uncertain, and the suggested reduction in cardiac events is partially offset by an increased risk of stroke.2
Stress testing is of questionable value. A patient with high cardiac risk (as evaluated with a calculator) may need to forego the surgical procedure or undergo a modified procedure. Alternatively, he or she may need to be referred to a cardiologist for consultation and possible pharmacologic nuclear stress testing. Although a normal stress test has a high negative predictive value, an abnormal test often leads to percutaneous coronary intervention or bypass surgery, and neither has been shown to reduce cardiac surgical risk.2 Percutaneous coronary interventions require a period of dual antiplatelet therapy, delaying surgery for unproven benefit.2
EKGs and echocardiograms are of limited use. An anesthesia group or surgical center will often require an electrocardiogram (EKG) as part of a preoperative evaluation, but preoperative evaluation by EKG or echocardiogram is controversial due to unproven benefits and potential risks. The 2014 ACC/AHA guidelines recommend against a 12-lead EKG for patients with low cardiac risk using the RCRI or ACS NSQIP or those who are having a low-risk procedure, such as endoscopy or cataract surgery.2 The United States Preventive Health Services Task Force also recommends against screening low-risk patients and says that screening EKGs and stress testing in asymptomatic medium- to high-risk patients is of undetermined value.12 They noted no evidence of benefit from resting or exercise EKG, with harm from a 1.7% complication rate of angiography, which is performed after up to 2.9% of exercise EKG testing.12
There are no recommendations for preoperative echocardiogram in the asymptomatic patient. Only unexplained dyspnea or other clinical signs of heart failure require an echocardiogram. For patients with known heart failure that is clinically stable, the ACC/AHA guidelines suggest that an echocardiogram should be performed within the year prior to surgery, although this is based on expert opinion.2
Because of the controversy over both coronary interventions and perioperative beta-blocker therapy, consider cardiology referral for a patient with poor functional activity level who does not meet low-risk criteria. While stress testing is acceptable, it may not lead to improved patient outcomes.
Optimize preventive care
Begin by ensuring that blood pressure (BP) and cholesterol are managed according to ACC/AHA guidelines. Then consider whether to start preoperative medications. You'll also want to screen for sleep apnea and discuss smoking status and cessation, if appropriate.
Initiate medications preoperatively?
In addition to having value as long-term primary prevention, there is some evidence that statins help prevent cardiac events during surgery. A randomized trial of over 200 vascular surgery patients showed that starting statins an average of 30 days prior to surgery significantly reduced cardiac complications.13 Another systematic review demonstrated that preoperative statins significantly reduce acute kidney injury from surgery.14
Unlike statins, aspirin started prior to surgery does not confer benefit. Aspirin was shown to significantly increase bleeding risk without improving cardiac outcomes in a large trial.15
Screen for sleep apnea
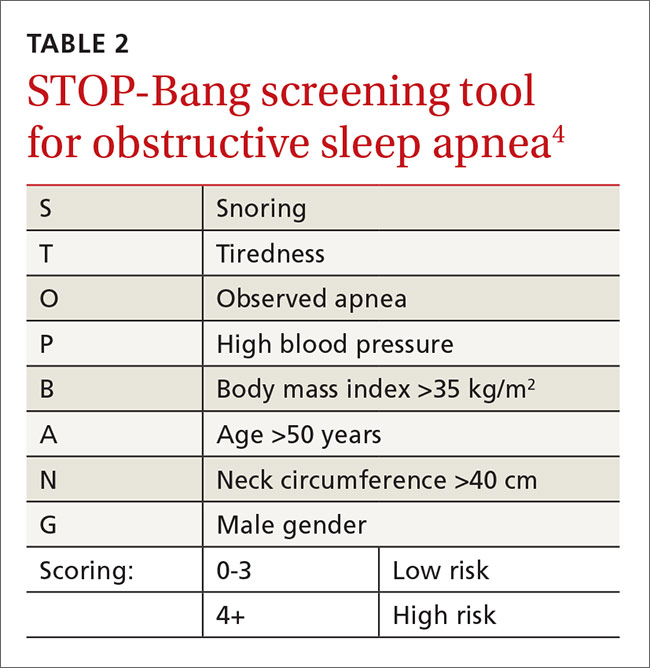
Sleep apnea increases the rate of respiratory failure between 2 and 3 times and the rate of cardiac complications about 1.5 times.16 This is a potentially correctable risk factor. The European Society of Anaesthesiology recommends clinical screening for obstructive sleep apnea using the STOP-Bang screening tool5 (TABLE 24). For its part, the ASA combines screening questions from the STOP-Bang screening questionnaire with a review of the medical record and physical exam, because the STOP-Bang questionnaire alone has an insufficient negative predictive value, ranging from 30% and 82%.6 Obstructive sleep apnea is not addressed on published pulmonary and cardiac risk tools.2,3,7,8
Is the patient a smoker?
Smoking is a reversible risk factor for pulmonary, cardiac, and infectious complications, as well as overall mortality. Perioperative smoking cessation counseling has been complicated by concerns that stopping smoking within 8 weeks of surgery might worsen postoperative outcomes. A study that looked at intraoperative sputum retrieved via tracheal suction showed that patients who had stopped smoking for 2 months or more prior to surgery had the same amount of sputum as non-smokers, while those that quit smoking <2 months before surgery were more likely to have increased intraoperative sputum volume.18 This study did not demonstrate a difference in postoperative pulmonary complications, likely because it included patients receiving minor surgeries only. But based on this study, a cessation period of 2 months was often recommended.
A recent systematic review showed that smoking cessation shortly before surgery does not increase risk.19 In fact, although the review did not show a statistically significant reduction in postoperative complications among recent quitters as compared with continued smokers, there was a trend toward a reduction in overall complications with only a slight increase in pulmonary complications.19
Address potential pulmonary complications
In addition to screening for issues that could lead to cardiac complications, it’s important to address the potential for pulmonary complications. Postoperative pulmonary complications are at least as common as cardiac complications, and include all possible respiratory related outcomes of surgery, from pneumonia to respiratory failure.
The seminal study on postoperative pulmonary complications is a systematic review published in 2006, which showed that the most important risk factors were surgery type, advanced age, ASA classification of overall health ≥II, and congestive heart failure.3 Chronic lung diseases and cigarette use were less predictive of pulmonary issues. All of these factors are included in the ACS NSQIP risk calculator.
Perioperative medication management
One aspect of the preoperative evaluation that should not be overlooked is a thorough medication reconciliation. Primary care providers can support the operative team by recommending medication adjustments prior to surgery. Several classes of medications have specific perioperative recommendations, which are summarized here.
Hypertension medications
- Beta-blockers. A patient who regularly takes a beta-blocker should continue the medication on the day of surgery and restart after surgery.2,20
- Calcium channel blockers. Calcium channel blockers can be continued through the day of surgery.2,20
- Renin-angiotensin system antagonists. Given the increased risk of hypotension following anesthesia induction, have patients refrain from taking angiotensin-converting enzyme inhibitors and angiotensin receptor blocker medications for at least 10 hours prior to surgery.20
- Diuretics. Diuretics can be given on the day of surgery, although they increase the risk of hypovolemia and electrolyte disturbances.20
Diabetes medications
- Insulin. For patients with type I diabetes, recommend basal insulin of 0.2 to 0.3 units/kg/day of long-acting insulin.21 If the patient is using an insulin pump, basal rate should be continued. For patients with type 2 diabetes, the simplest method is to use one-half the normal long-acting insulin dose on the morning of surgery.22
- Metformin. Discontinue metformin 24 hours prior to surgery because of the risk for lactic acidosis.21,22 While the risk of lactic acidosis from metformin is low, mortality rates as high as 50% have been documented after lactic acidosis occurred with similar medications.22
- Sulfonylureas. Sulfonylureas should be held on the day of surgery due to the risk of hypoglycemia and a possible increased risk of ischemia.21,22
- Thiazolidinediones, dipeptidyl peptidase-4 inhibitors, and glucagon-like peptide-1 agonists. All should be held on the day of surgery.21
Anticoagulant medications
- Vitamin K antagonists (warfarin). Discontinue warfarin 5 days prior to the procedure. The half-life is approximately 40 hours, requiring at least 5 days for the anticoagulant effect to be eliminated from the body.23 Use of bridging therapy with regular- or low-molecular weight heparin remains controversial due to increased surgical bleeding risk without evidence of a decrease in cardiovascular events.24 The patient’s risks of stroke and venous thromboembolism should be taken into account when deciding whether to use bridging therapy or not.
- Factor Xa inhibitor. Management of factor Xa inhibitors (rivaroxaban, apixaban) depends on the bleeding risk of the surgery and the patient’s renal function.24,25 For instance, a patient undergoing cataract surgery (low risk) needs a shorter cessation time than a patient undergoing hip arthroplasty (high risk). Discontinuation times are listed in TABLE 3.24
- Direct thrombin inhibitor. Management of direct thrombin inhibitors (dabigatran) is also dependent on surgical bleeding risk and renal function (TABLE 3).23,24
- Aspirin, clopidogrel, ticlopidine, prasugrel. All should be stopped 7 to 10 days prior to surgery to allow new platelet growth. Low-dose aspirin for secondary prevention of cardiovascular disease or primary prevention in a high-risk patient can be continued through surgery.23

Other

- Corticosteroids. Recent evidence suggests that stress-dose steroids are not needed to prevent adrenal insufficiency in patients taking corticosteroids chronically.26 These patients should continue maintenance therapy at regular dosing.26,27 Stress dosing of corticosteroids is only required when a patient has signs of adrenal insufficiency.26
- Statins. Statin medications should be continued on the day of surgery.2
- Nonsteroidal anti-inflammatory drugs. NSAIDs should be stopped 5 days prior to surgery to reverse antiplatelet effects.23
CORRESPONDENCE
CDR Michael J. Arnold, Naval Hospital, 2080 Child Street, Jacksonville, FL 32214; [email protected].
ACKNOWLEDGEMENT
The authors thank CDR Kristian Sanchack and LCDR Dustin Smith for their assistance with this manuscript.
1. Wier LM, Steiner CA, Owens PL. Surgeries in hospital-owned outpatient facilities, 2012. HCUP Statistical Brief #188. February 2015. Available at: https://www.hcup-us.ahrq.gov/reports/statbriefs/sb188-Surgeries-Hospital-Outpatient-Facilities-2012.jsp. Accessed September 15, 2016.
2. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. J Am Coll Cardiol. 2014;64:e77-e137.
3. Smetana GW, Lawrence VA, Cornell JE. Preoperative pulmonary risk stratification for noncardiothoracic surgery: systematic review for the American College of Physicians. Ann Intern Med. 2006;144:581-595.
4. Chung F, Abdullah HR, Liao P. STOP-Bang questionnaire: a prac tical approach to screen for obstructive sleep apnea. Chest. 2016;149:631-638.
5. De Hert S, Imberger G, Carlisle J, et al. Preoperative evaluation of the adult patient undergoing non-cardiac surgery: guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol. 2011;28:684-722.
6. American Society of Anesthesiologists Task Force on Perioperative Management of Patients with Obstructive Sleep Apnea. Practice guidelines for the perioperative management of patients with obstructive sleep apnea; an updated report by the American Society of Anesthesiologists Task Force on Perioperative Management of Patients with Obstructive Sleep Apnea. Anesthesiology. 2014;120:268-286.
7. Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100:1043-1049.
8. Bilimoria KY, Liu Y, Paruch JL, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aide and informed consent tool for patients and surgeons. J Am Coll Surg. 2013;217:833-842.
9. Hlatky MA, Boineau RE, Higginbotham MB, et al. A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index). Am J Cardiol. 1989;64:651-654.
10. Bouri S, Shun-Shin MJ, Cole GD, et al. Meta-analysis of secure randomized controlled trials of B-blockade to prevent perioperative death in non-cardiac surgery. Heart. 2014;100:456-464.
11. Mounsey A, Roque JM, Egan M. Why you shouldn’t start beta-blockers before surgery. J Fam Pract. 2014;63:E15-E16.
12. Chou R, Arora B, Dana T, et al. Screening asymptomatic adults with resting or exercise electrocardiography: a review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;155:375-385.
13. Durazzo AES, Machado FS, Ikeoka DT, et al. Reduction in cardiovascular events after vascular surgery with atorvastatin: a randomized trial. J Vasc Surg. 2004;39:967-975.
14. Pan SY, Wu VC, Huang TM, et al. Effect of preoperative statin therapy on postoperative acute kidney injury in patients undergoing major surgery: systemic review and meta-analysis. Nephrology. 2014;19:750-763.
15. Devereux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med. 2014;370:1494-1503.
16. Adesanya AO, Lee W, Greilich NB, et al. Perioperative management of obstructive sleep apnea. Chest. 2010;138:1489-1498.
17. Chung F, Nagappa M, Singh M, et al. CPAP in the perioperative setting: evidence of support. Chest. 2016;149:586-597.
18. Yamashita S, Yamaguchi H, Sakaguchi M, et al. Effect of smoking on intraoperative sputum and postoperative pulmonary complication in minor surgical patients. Respir Med. 2004;98:760-766.
19. Myers K, Hajek P, Hinds
20. Lonjaret L, Lairez O, Minville V, et al. Optimal perioperative management of arterial blood pressure. Integr Blood Press Control. 2014;7:49-59.
21. Sudhakaran S, Surani SR. Guidelines for perioperative management of the diabetic patient. Surg Res Pract. 2015;2015:284063.
22. Duncan AE. Hyperglycemia and perioperative glucose management. Curr Pharm Des. 2012;18:6195-6203.
23. Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e326S-e350S.
24. Faraoni D, Levy JH, Albaladejo P, et al. Updates in the perioperative and emergency management of non-vitamin K antagonist oral anticoagulants. Crit Care. 2015;19:203.
25. Shamoun F, Obeid H, Ramakrishna H. Novel anticoagulants in atrial fibrillation: monitoring, reversal and perioperative management. Biomed Res Int. 2015;2015:424031.
26. Kelly KN, Domajnko B. Perioperative stress-dose steroids. Clin Colon Rectal Surg. 2013;26:163-167.
27. Scanzello CR, Nestor BJ. Perioperative management of medications used in the treatment of rheumatoid arthritis. HSSJ. 2006;2:141-147.
PRACTICE RECOMMENDATIONS
› Recommend that patients quit smoking 8 weeks before surgery; keep in mind, though, that quitting closer to the date of surgery does not increase the risk of complications. A
› Use the online American College of Surgeons/National Surgical Quality Improvement Program surgical risk calculator to estimate a patient’s surgical risk. C
› Send a patient directly to surgery if he or she has an estimated cardiac risk <1% or <2 risk factors of the Revised Cardiac Risk Index. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
About 27 million Americans undergo surgery every year1 and before doing so, they turn to you—their primary care physician—or their cardiologist for a preoperative evaluation. Of course, the goal of this evaluation is to determine an individual patient’s risk and compare it to procedural averages in an effort to identify opportunities for risk mitigation. But the preoperative evaluation is also an opportunity to make recommendations regarding perioperative management of medications. And certainly we want to conduct these evaluations in a way that is both expeditious and in keeping with the latest guidelines.
Current guidelines for preoperative evaluations are less complicated than they used to be and focus on cardiac and pulmonary risk stratification. While a risk calculator remains your primary tool, elements such as smoking cessation and identifying sleep apnea are important parts of the preop equation. In the review that follows, we present a simple algorithm (FIGURE 12-6) that we developed that can be completed in a single visit.

Cardiac assessment: A risk calculator is the primary tool

Cardiac risk estimation is perhaps the most important element in determining a patient’s overall surgical risk. But before you begin, you'll need to determine whether the preoperative evaluation of the patient is best handled by you or a specialist. Current guidelines recommend preoperative evaluation by a specialist when a patient has certain conditions, such as moderate or greater valvular stenosis/regurgitation, a cardiac implantable electronic device, pulmonary hypertension, congenital heart disease, or severe systemic disease.2
If these conditions are not present (and an immediate referral is not required), you can turn your attention to the cardiac assessment. The first step is to determine which cardiac risk calculator you’d like to use. In its comprehensive guideline on perioperative cardiovascular evaluation, the American College of Cardiology/American Heart Association (ACC/AHA) recommends the use of one of the 2 calculators described below.2
Revised Cardiac Risk Index. The most well-known cardiac risk calculator is the 6-element Revised Cardiac Risk Index (RCRI) (TABLE 1).7 Published in 1999, the RCRI was derived from a cohort of 2800 patients, verified in 1400 patients, and has been validated in numerous studies.2 Each element increases the odds of a cardiac complication by a factor of 2 to 3, and more than one positive response indicates the patient is at high risk for complications.7
The ACS NSQIP risk calculator has been criticized because it has not been validated in a group separate from the initial patient population used in its development.2 Another criticism is the inclusion of the American Society of Anesthesiologists' (ASA) classification of the overall health of the patient, a simple yet subjective and unreliable method of patient characterization.2
Choosing a calculator. The ACS NSQIP calculator may be more useful for primary care physicians because it provides individualized risks for numerous complications and is easy to use. The output page can be printed as documentation of the preoperative evaluation, and is useful for counseling patients about reconsideration of surgery or risk-reduction strategies. The RCRI is also simple to use, but considers only cardiac risk. Although the RCRI has been validated in numerous studies, the ACS NSQIP was derived from a more substantial 1.4 million patients.

Mapping out next steps based on risk score
The next step in the preoperative evaluation process is to calculate your patient’s risk score and determine whether it is low or high. If the risk is determined to be low—either an RCRI score <2 or an ACS NSQIP cardiac complication risk <1%—the patient can be referred to surgery without further evaluation.2
If the calculator suggests higher risk, the patient’s functional status should be assessed. If the patient has a functional status of >4 metabolic equivalents (METs), then the patient can be recommended for surgery without further evaluation.2 Examples of activities that are greater than 4 METs are yard work such as raking leaves, weeding, or pushing a power mower; sexual relations; climbing a flight of stairs; walking up a hill; and participating in moderate recreational activities like golf, bowling, dancing, doubles tennis, or throwing a baseball or football.9
Patient can’t perform >4 METs? If the patient does not have a functional capacity of >4 METs, further risk stratification should be considered if the results would change management.2 Prior guidelines recommended either perioperative beta-blockers to mitigate risk or coronary interventions, but both are controversial due to lack of proven benefit.
Perioperative beta-blocker use. A recommendation to consider starting beta-blockers at least one day prior to surgery remains in the 2014 ACC/AHA guidelines for patients with 3 or more RCRI risk factors.2 But a group of studies supporting beta-blocker use has been discredited due to serious flaws and fabricated data. At the same time, a large study arguing against perioperative beta-blockers has been criticized for starting high doses of beta-blockers on the day of surgery.2,10,11 In the end, mortality benefit from perioperative beta-blockers is uncertain, and the suggested reduction in cardiac events is partially offset by an increased risk of stroke.2
Stress testing is of questionable value. A patient with high cardiac risk (as evaluated with a calculator) may need to forego the surgical procedure or undergo a modified procedure. Alternatively, he or she may need to be referred to a cardiologist for consultation and possible pharmacologic nuclear stress testing. Although a normal stress test has a high negative predictive value, an abnormal test often leads to percutaneous coronary intervention or bypass surgery, and neither has been shown to reduce cardiac surgical risk.2 Percutaneous coronary interventions require a period of dual antiplatelet therapy, delaying surgery for unproven benefit.2
EKGs and echocardiograms are of limited use. An anesthesia group or surgical center will often require an electrocardiogram (EKG) as part of a preoperative evaluation, but preoperative evaluation by EKG or echocardiogram is controversial due to unproven benefits and potential risks. The 2014 ACC/AHA guidelines recommend against a 12-lead EKG for patients with low cardiac risk using the RCRI or ACS NSQIP or those who are having a low-risk procedure, such as endoscopy or cataract surgery.2 The United States Preventive Health Services Task Force also recommends against screening low-risk patients and says that screening EKGs and stress testing in asymptomatic medium- to high-risk patients is of undetermined value.12 They noted no evidence of benefit from resting or exercise EKG, with harm from a 1.7% complication rate of angiography, which is performed after up to 2.9% of exercise EKG testing.12
There are no recommendations for preoperative echocardiogram in the asymptomatic patient. Only unexplained dyspnea or other clinical signs of heart failure require an echocardiogram. For patients with known heart failure that is clinically stable, the ACC/AHA guidelines suggest that an echocardiogram should be performed within the year prior to surgery, although this is based on expert opinion.2
Because of the controversy over both coronary interventions and perioperative beta-blocker therapy, consider cardiology referral for a patient with poor functional activity level who does not meet low-risk criteria. While stress testing is acceptable, it may not lead to improved patient outcomes.
Optimize preventive care
Begin by ensuring that blood pressure (BP) and cholesterol are managed according to ACC/AHA guidelines. Then consider whether to start preoperative medications. You'll also want to screen for sleep apnea and discuss smoking status and cessation, if appropriate.
Initiate medications preoperatively?
In addition to having value as long-term primary prevention, there is some evidence that statins help prevent cardiac events during surgery. A randomized trial of over 200 vascular surgery patients showed that starting statins an average of 30 days prior to surgery significantly reduced cardiac complications.13 Another systematic review demonstrated that preoperative statins significantly reduce acute kidney injury from surgery.14
Unlike statins, aspirin started prior to surgery does not confer benefit. Aspirin was shown to significantly increase bleeding risk without improving cardiac outcomes in a large trial.15
Screen for sleep apnea
Sleep apnea increases the rate of respiratory failure between 2 and 3 times and the rate of cardiac complications about 1.5 times.16 This is a potentially correctable risk factor. The European Society of Anaesthesiology recommends clinical screening for obstructive sleep apnea using the STOP-Bang screening tool5 (TABLE 24). For its part, the ASA combines screening questions from the STOP-Bang screening questionnaire with a review of the medical record and physical exam, because the STOP-Bang questionnaire alone has an insufficient negative predictive value, ranging from 30% and 82%.6 Obstructive sleep apnea is not addressed on published pulmonary and cardiac risk tools.2,3,7,8

Is the patient a smoker?
Smoking is a reversible risk factor for pulmonary, cardiac, and infectious complications, as well as overall mortality. Perioperative smoking cessation counseling has been complicated by concerns that stopping smoking within 8 weeks of surgery might worsen postoperative outcomes. A study that looked at intraoperative sputum retrieved via tracheal suction showed that patients who had stopped smoking for 2 months or more prior to surgery had the same amount of sputum as non-smokers, while those that quit smoking <2 months before surgery were more likely to have increased intraoperative sputum volume.18 This study did not demonstrate a difference in postoperative pulmonary complications, likely because it included patients receiving minor surgeries only. But based on this study, a cessation period of 2 months was often recommended.
A recent systematic review showed that smoking cessation shortly before surgery does not increase risk.19 In fact, although the review did not show a statistically significant reduction in postoperative complications among recent quitters as compared with continued smokers, there was a trend toward a reduction in overall complications with only a slight increase in pulmonary complications.19
Address potential pulmonary complications
In addition to screening for issues that could lead to cardiac complications, it’s important to address the potential for pulmonary complications. Postoperative pulmonary complications are at least as common as cardiac complications, and include all possible respiratory related outcomes of surgery, from pneumonia to respiratory failure.
The seminal study on postoperative pulmonary complications is a systematic review published in 2006, which showed that the most important risk factors were surgery type, advanced age, ASA classification of overall health ≥II, and congestive heart failure.3 Chronic lung diseases and cigarette use were less predictive of pulmonary issues. All of these factors are included in the ACS NSQIP risk calculator.
Perioperative medication management
One aspect of the preoperative evaluation that should not be overlooked is a thorough medication reconciliation. Primary care providers can support the operative team by recommending medication adjustments prior to surgery. Several classes of medications have specific perioperative recommendations, which are summarized here.
Hypertension medications
- Beta-blockers. A patient who regularly takes a beta-blocker should continue the medication on the day of surgery and restart after surgery.2,20
- Calcium channel blockers. Calcium channel blockers can be continued through the day of surgery.2,20
- Renin-angiotensin system antagonists. Given the increased risk of hypotension following anesthesia induction, have patients refrain from taking angiotensin-converting enzyme inhibitors and angiotensin receptor blocker medications for at least 10 hours prior to surgery.20
- Diuretics. Diuretics can be given on the day of surgery, although they increase the risk of hypovolemia and electrolyte disturbances.20
Diabetes medications
- Insulin. For patients with type I diabetes, recommend basal insulin of 0.2 to 0.3 units/kg/day of long-acting insulin.21 If the patient is using an insulin pump, basal rate should be continued. For patients with type 2 diabetes, the simplest method is to use one-half the normal long-acting insulin dose on the morning of surgery.22
- Metformin. Discontinue metformin 24 hours prior to surgery because of the risk for lactic acidosis.21,22 While the risk of lactic acidosis from metformin is low, mortality rates as high as 50% have been documented after lactic acidosis occurred with similar medications.22
- Sulfonylureas. Sulfonylureas should be held on the day of surgery due to the risk of hypoglycemia and a possible increased risk of ischemia.21,22
- Thiazolidinediones, dipeptidyl peptidase-4 inhibitors, and glucagon-like peptide-1 agonists. All should be held on the day of surgery.21
Anticoagulant medications
- Vitamin K antagonists (warfarin). Discontinue warfarin 5 days prior to the procedure. The half-life is approximately 40 hours, requiring at least 5 days for the anticoagulant effect to be eliminated from the body.23 Use of bridging therapy with regular- or low-molecular weight heparin remains controversial due to increased surgical bleeding risk without evidence of a decrease in cardiovascular events.24 The patient’s risks of stroke and venous thromboembolism should be taken into account when deciding whether to use bridging therapy or not.
- Factor Xa inhibitor. Management of factor Xa inhibitors (rivaroxaban, apixaban) depends on the bleeding risk of the surgery and the patient’s renal function.24,25 For instance, a patient undergoing cataract surgery (low risk) needs a shorter cessation time than a patient undergoing hip arthroplasty (high risk). Discontinuation times are listed in TABLE 3.24
- Direct thrombin inhibitor. Management of direct thrombin inhibitors (dabigatran) is also dependent on surgical bleeding risk and renal function (TABLE 3).23,24
- Aspirin, clopidogrel, ticlopidine, prasugrel. All should be stopped 7 to 10 days prior to surgery to allow new platelet growth. Low-dose aspirin for secondary prevention of cardiovascular disease or primary prevention in a high-risk patient can be continued through surgery.23

Other

- Corticosteroids. Recent evidence suggests that stress-dose steroids are not needed to prevent adrenal insufficiency in patients taking corticosteroids chronically.26 These patients should continue maintenance therapy at regular dosing.26,27 Stress dosing of corticosteroids is only required when a patient has signs of adrenal insufficiency.26
- Statins. Statin medications should be continued on the day of surgery.2
- Nonsteroidal anti-inflammatory drugs. NSAIDs should be stopped 5 days prior to surgery to reverse antiplatelet effects.23
CORRESPONDENCE
CDR Michael J. Arnold, Naval Hospital, 2080 Child Street, Jacksonville, FL 32214; [email protected].
ACKNOWLEDGEMENT
The authors thank CDR Kristian Sanchack and LCDR Dustin Smith for their assistance with this manuscript.
PRACTICE RECOMMENDATIONS
› Recommend that patients quit smoking 8 weeks before surgery; keep in mind, though, that quitting closer to the date of surgery does not increase the risk of complications. A
› Use the online American College of Surgeons/National Surgical Quality Improvement Program surgical risk calculator to estimate a patient’s surgical risk. C
› Send a patient directly to surgery if he or she has an estimated cardiac risk <1% or <2 risk factors of the Revised Cardiac Risk Index. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
About 27 million Americans undergo surgery every year1 and before doing so, they turn to you—their primary care physician—or their cardiologist for a preoperative evaluation. Of course, the goal of this evaluation is to determine an individual patient’s risk and compare it to procedural averages in an effort to identify opportunities for risk mitigation. But the preoperative evaluation is also an opportunity to make recommendations regarding perioperative management of medications. And certainly we want to conduct these evaluations in a way that is both expeditious and in keeping with the latest guidelines.
Current guidelines for preoperative evaluations are less complicated than they used to be and focus on cardiac and pulmonary risk stratification. While a risk calculator remains your primary tool, elements such as smoking cessation and identifying sleep apnea are important parts of the preop equation. In the review that follows, we present a simple algorithm (FIGURE 12-6) that we developed that can be completed in a single visit.

Cardiac assessment: A risk calculator is the primary tool

Cardiac risk estimation is perhaps the most important element in determining a patient’s overall surgical risk. But before you begin, you'll need to determine whether the preoperative evaluation of the patient is best handled by you or a specialist. Current guidelines recommend preoperative evaluation by a specialist when a patient has certain conditions, such as moderate or greater valvular stenosis/regurgitation, a cardiac implantable electronic device, pulmonary hypertension, congenital heart disease, or severe systemic disease.2
If these conditions are not present (and an immediate referral is not required), you can turn your attention to the cardiac assessment. The first step is to determine which cardiac risk calculator you’d like to use. In its comprehensive guideline on perioperative cardiovascular evaluation, the American College of Cardiology/American Heart Association (ACC/AHA) recommends the use of one of the 2 calculators described below.2
Revised Cardiac Risk Index. The most well-known cardiac risk calculator is the 6-element Revised Cardiac Risk Index (RCRI) (TABLE 1).7 Published in 1999, the RCRI was derived from a cohort of 2800 patients, verified in 1400 patients, and has been validated in numerous studies.2 Each element increases the odds of a cardiac complication by a factor of 2 to 3, and more than one positive response indicates the patient is at high risk for complications.7
The ACS NSQIP risk calculator has been criticized because it has not been validated in a group separate from the initial patient population used in its development.2 Another criticism is the inclusion of the American Society of Anesthesiologists' (ASA) classification of the overall health of the patient, a simple yet subjective and unreliable method of patient characterization.2
Choosing a calculator. The ACS NSQIP calculator may be more useful for primary care physicians because it provides individualized risks for numerous complications and is easy to use. The output page can be printed as documentation of the preoperative evaluation, and is useful for counseling patients about reconsideration of surgery or risk-reduction strategies. The RCRI is also simple to use, but considers only cardiac risk. Although the RCRI has been validated in numerous studies, the ACS NSQIP was derived from a more substantial 1.4 million patients.

Mapping out next steps based on risk score
The next step in the preoperative evaluation process is to calculate your patient’s risk score and determine whether it is low or high. If the risk is determined to be low—either an RCRI score <2 or an ACS NSQIP cardiac complication risk <1%—the patient can be referred to surgery without further evaluation.2
If the calculator suggests higher risk, the patient’s functional status should be assessed. If the patient has a functional status of >4 metabolic equivalents (METs), then the patient can be recommended for surgery without further evaluation.2 Examples of activities that are greater than 4 METs are yard work such as raking leaves, weeding, or pushing a power mower; sexual relations; climbing a flight of stairs; walking up a hill; and participating in moderate recreational activities like golf, bowling, dancing, doubles tennis, or throwing a baseball or football.9
Patient can’t perform >4 METs? If the patient does not have a functional capacity of >4 METs, further risk stratification should be considered if the results would change management.2 Prior guidelines recommended either perioperative beta-blockers to mitigate risk or coronary interventions, but both are controversial due to lack of proven benefit.
Perioperative beta-blocker use. A recommendation to consider starting beta-blockers at least one day prior to surgery remains in the 2014 ACC/AHA guidelines for patients with 3 or more RCRI risk factors.2 But a group of studies supporting beta-blocker use has been discredited due to serious flaws and fabricated data. At the same time, a large study arguing against perioperative beta-blockers has been criticized for starting high doses of beta-blockers on the day of surgery.2,10,11 In the end, mortality benefit from perioperative beta-blockers is uncertain, and the suggested reduction in cardiac events is partially offset by an increased risk of stroke.2
Stress testing is of questionable value. A patient with high cardiac risk (as evaluated with a calculator) may need to forego the surgical procedure or undergo a modified procedure. Alternatively, he or she may need to be referred to a cardiologist for consultation and possible pharmacologic nuclear stress testing. Although a normal stress test has a high negative predictive value, an abnormal test often leads to percutaneous coronary intervention or bypass surgery, and neither has been shown to reduce cardiac surgical risk.2 Percutaneous coronary interventions require a period of dual antiplatelet therapy, delaying surgery for unproven benefit.2
EKGs and echocardiograms are of limited use. An anesthesia group or surgical center will often require an electrocardiogram (EKG) as part of a preoperative evaluation, but preoperative evaluation by EKG or echocardiogram is controversial due to unproven benefits and potential risks. The 2014 ACC/AHA guidelines recommend against a 12-lead EKG for patients with low cardiac risk using the RCRI or ACS NSQIP or those who are having a low-risk procedure, such as endoscopy or cataract surgery.2 The United States Preventive Health Services Task Force also recommends against screening low-risk patients and says that screening EKGs and stress testing in asymptomatic medium- to high-risk patients is of undetermined value.12 They noted no evidence of benefit from resting or exercise EKG, with harm from a 1.7% complication rate of angiography, which is performed after up to 2.9% of exercise EKG testing.12
There are no recommendations for preoperative echocardiogram in the asymptomatic patient. Only unexplained dyspnea or other clinical signs of heart failure require an echocardiogram. For patients with known heart failure that is clinically stable, the ACC/AHA guidelines suggest that an echocardiogram should be performed within the year prior to surgery, although this is based on expert opinion.2
Because of the controversy over both coronary interventions and perioperative beta-blocker therapy, consider cardiology referral for a patient with poor functional activity level who does not meet low-risk criteria. While stress testing is acceptable, it may not lead to improved patient outcomes.
Optimize preventive care
Begin by ensuring that blood pressure (BP) and cholesterol are managed according to ACC/AHA guidelines. Then consider whether to start preoperative medications. You'll also want to screen for sleep apnea and discuss smoking status and cessation, if appropriate.
Initiate medications preoperatively?
In addition to having value as long-term primary prevention, there is some evidence that statins help prevent cardiac events during surgery. A randomized trial of over 200 vascular surgery patients showed that starting statins an average of 30 days prior to surgery significantly reduced cardiac complications.13 Another systematic review demonstrated that preoperative statins significantly reduce acute kidney injury from surgery.14
Unlike statins, aspirin started prior to surgery does not confer benefit. Aspirin was shown to significantly increase bleeding risk without improving cardiac outcomes in a large trial.15
Screen for sleep apnea
Sleep apnea increases the rate of respiratory failure between 2 and 3 times and the rate of cardiac complications about 1.5 times.16 This is a potentially correctable risk factor. The European Society of Anaesthesiology recommends clinical screening for obstructive sleep apnea using the STOP-Bang screening tool5 (TABLE 24). For its part, the ASA combines screening questions from the STOP-Bang screening questionnaire with a review of the medical record and physical exam, because the STOP-Bang questionnaire alone has an insufficient negative predictive value, ranging from 30% and 82%.6 Obstructive sleep apnea is not addressed on published pulmonary and cardiac risk tools.2,3,7,8

Is the patient a smoker?
Smoking is a reversible risk factor for pulmonary, cardiac, and infectious complications, as well as overall mortality. Perioperative smoking cessation counseling has been complicated by concerns that stopping smoking within 8 weeks of surgery might worsen postoperative outcomes. A study that looked at intraoperative sputum retrieved via tracheal suction showed that patients who had stopped smoking for 2 months or more prior to surgery had the same amount of sputum as non-smokers, while those that quit smoking <2 months before surgery were more likely to have increased intraoperative sputum volume.18 This study did not demonstrate a difference in postoperative pulmonary complications, likely because it included patients receiving minor surgeries only. But based on this study, a cessation period of 2 months was often recommended.
A recent systematic review showed that smoking cessation shortly before surgery does not increase risk.19 In fact, although the review did not show a statistically significant reduction in postoperative complications among recent quitters as compared with continued smokers, there was a trend toward a reduction in overall complications with only a slight increase in pulmonary complications.19
Address potential pulmonary complications
In addition to screening for issues that could lead to cardiac complications, it’s important to address the potential for pulmonary complications. Postoperative pulmonary complications are at least as common as cardiac complications, and include all possible respiratory related outcomes of surgery, from pneumonia to respiratory failure.
The seminal study on postoperative pulmonary complications is a systematic review published in 2006, which showed that the most important risk factors were surgery type, advanced age, ASA classification of overall health ≥II, and congestive heart failure.3 Chronic lung diseases and cigarette use were less predictive of pulmonary issues. All of these factors are included in the ACS NSQIP risk calculator.
Perioperative medication management
One aspect of the preoperative evaluation that should not be overlooked is a thorough medication reconciliation. Primary care providers can support the operative team by recommending medication adjustments prior to surgery. Several classes of medications have specific perioperative recommendations, which are summarized here.
Hypertension medications
- Beta-blockers. A patient who regularly takes a beta-blocker should continue the medication on the day of surgery and restart after surgery.2,20
- Calcium channel blockers. Calcium channel blockers can be continued through the day of surgery.2,20
- Renin-angiotensin system antagonists. Given the increased risk of hypotension following anesthesia induction, have patients refrain from taking angiotensin-converting enzyme inhibitors and angiotensin receptor blocker medications for at least 10 hours prior to surgery.20
- Diuretics. Diuretics can be given on the day of surgery, although they increase the risk of hypovolemia and electrolyte disturbances.20
Diabetes medications
- Insulin. For patients with type I diabetes, recommend basal insulin of 0.2 to 0.3 units/kg/day of long-acting insulin.21 If the patient is using an insulin pump, basal rate should be continued. For patients with type 2 diabetes, the simplest method is to use one-half the normal long-acting insulin dose on the morning of surgery.22
- Metformin. Discontinue metformin 24 hours prior to surgery because of the risk for lactic acidosis.21,22 While the risk of lactic acidosis from metformin is low, mortality rates as high as 50% have been documented after lactic acidosis occurred with similar medications.22
- Sulfonylureas. Sulfonylureas should be held on the day of surgery due to the risk of hypoglycemia and a possible increased risk of ischemia.21,22
- Thiazolidinediones, dipeptidyl peptidase-4 inhibitors, and glucagon-like peptide-1 agonists. All should be held on the day of surgery.21
Anticoagulant medications
- Vitamin K antagonists (warfarin). Discontinue warfarin 5 days prior to the procedure. The half-life is approximately 40 hours, requiring at least 5 days for the anticoagulant effect to be eliminated from the body.23 Use of bridging therapy with regular- or low-molecular weight heparin remains controversial due to increased surgical bleeding risk without evidence of a decrease in cardiovascular events.24 The patient’s risks of stroke and venous thromboembolism should be taken into account when deciding whether to use bridging therapy or not.
- Factor Xa inhibitor. Management of factor Xa inhibitors (rivaroxaban, apixaban) depends on the bleeding risk of the surgery and the patient’s renal function.24,25 For instance, a patient undergoing cataract surgery (low risk) needs a shorter cessation time than a patient undergoing hip arthroplasty (high risk). Discontinuation times are listed in TABLE 3.24
- Direct thrombin inhibitor. Management of direct thrombin inhibitors (dabigatran) is also dependent on surgical bleeding risk and renal function (TABLE 3).23,24
- Aspirin, clopidogrel, ticlopidine, prasugrel. All should be stopped 7 to 10 days prior to surgery to allow new platelet growth. Low-dose aspirin for secondary prevention of cardiovascular disease or primary prevention in a high-risk patient can be continued through surgery.23

Other

- Corticosteroids. Recent evidence suggests that stress-dose steroids are not needed to prevent adrenal insufficiency in patients taking corticosteroids chronically.26 These patients should continue maintenance therapy at regular dosing.26,27 Stress dosing of corticosteroids is only required when a patient has signs of adrenal insufficiency.26
- Statins. Statin medications should be continued on the day of surgery.2
- Nonsteroidal anti-inflammatory drugs. NSAIDs should be stopped 5 days prior to surgery to reverse antiplatelet effects.23
CORRESPONDENCE
CDR Michael J. Arnold, Naval Hospital, 2080 Child Street, Jacksonville, FL 32214; [email protected].
ACKNOWLEDGEMENT
The authors thank CDR Kristian Sanchack and LCDR Dustin Smith for their assistance with this manuscript.
1. Wier LM, Steiner CA, Owens PL. Surgeries in hospital-owned outpatient facilities, 2012. HCUP Statistical Brief #188. February 2015. Available at: https://www.hcup-us.ahrq.gov/reports/statbriefs/sb188-Surgeries-Hospital-Outpatient-Facilities-2012.jsp. Accessed September 15, 2016.
2. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. J Am Coll Cardiol. 2014;64:e77-e137.
3. Smetana GW, Lawrence VA, Cornell JE. Preoperative pulmonary risk stratification for noncardiothoracic surgery: systematic review for the American College of Physicians. Ann Intern Med. 2006;144:581-595.
4. Chung F, Abdullah HR, Liao P. STOP-Bang questionnaire: a prac tical approach to screen for obstructive sleep apnea. Chest. 2016;149:631-638.
5. De Hert S, Imberger G, Carlisle J, et al. Preoperative evaluation of the adult patient undergoing non-cardiac surgery: guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol. 2011;28:684-722.
6. American Society of Anesthesiologists Task Force on Perioperative Management of Patients with Obstructive Sleep Apnea. Practice guidelines for the perioperative management of patients with obstructive sleep apnea; an updated report by the American Society of Anesthesiologists Task Force on Perioperative Management of Patients with Obstructive Sleep Apnea. Anesthesiology. 2014;120:268-286.
7. Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100:1043-1049.
8. Bilimoria KY, Liu Y, Paruch JL, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aide and informed consent tool for patients and surgeons. J Am Coll Surg. 2013;217:833-842.
9. Hlatky MA, Boineau RE, Higginbotham MB, et al. A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index). Am J Cardiol. 1989;64:651-654.
10. Bouri S, Shun-Shin MJ, Cole GD, et al. Meta-analysis of secure randomized controlled trials of B-blockade to prevent perioperative death in non-cardiac surgery. Heart. 2014;100:456-464.
11. Mounsey A, Roque JM, Egan M. Why you shouldn’t start beta-blockers before surgery. J Fam Pract. 2014;63:E15-E16.
12. Chou R, Arora B, Dana T, et al. Screening asymptomatic adults with resting or exercise electrocardiography: a review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;155:375-385.
13. Durazzo AES, Machado FS, Ikeoka DT, et al. Reduction in cardiovascular events after vascular surgery with atorvastatin: a randomized trial. J Vasc Surg. 2004;39:967-975.
14. Pan SY, Wu VC, Huang TM, et al. Effect of preoperative statin therapy on postoperative acute kidney injury in patients undergoing major surgery: systemic review and meta-analysis. Nephrology. 2014;19:750-763.
15. Devereux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med. 2014;370:1494-1503.
16. Adesanya AO, Lee W, Greilich NB, et al. Perioperative management of obstructive sleep apnea. Chest. 2010;138:1489-1498.
17. Chung F, Nagappa M, Singh M, et al. CPAP in the perioperative setting: evidence of support. Chest. 2016;149:586-597.
18. Yamashita S, Yamaguchi H, Sakaguchi M, et al. Effect of smoking on intraoperative sputum and postoperative pulmonary complication in minor surgical patients. Respir Med. 2004;98:760-766.
19. Myers K, Hajek P, Hinds
20. Lonjaret L, Lairez O, Minville V, et al. Optimal perioperative management of arterial blood pressure. Integr Blood Press Control. 2014;7:49-59.
21. Sudhakaran S, Surani SR. Guidelines for perioperative management of the diabetic patient. Surg Res Pract. 2015;2015:284063.
22. Duncan AE. Hyperglycemia and perioperative glucose management. Curr Pharm Des. 2012;18:6195-6203.
23. Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e326S-e350S.
24. Faraoni D, Levy JH, Albaladejo P, et al. Updates in the perioperative and emergency management of non-vitamin K antagonist oral anticoagulants. Crit Care. 2015;19:203.
25. Shamoun F, Obeid H, Ramakrishna H. Novel anticoagulants in atrial fibrillation: monitoring, reversal and perioperative management. Biomed Res Int. 2015;2015:424031.
26. Kelly KN, Domajnko B. Perioperative stress-dose steroids. Clin Colon Rectal Surg. 2013;26:163-167.
27. Scanzello CR, Nestor BJ. Perioperative management of medications used in the treatment of rheumatoid arthritis. HSSJ. 2006;2:141-147.
1. Wier LM, Steiner CA, Owens PL. Surgeries in hospital-owned outpatient facilities, 2012. HCUP Statistical Brief #188. February 2015. Available at: https://www.hcup-us.ahrq.gov/reports/statbriefs/sb188-Surgeries-Hospital-Outpatient-Facilities-2012.jsp. Accessed September 15, 2016.
2. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. J Am Coll Cardiol. 2014;64:e77-e137.
3. Smetana GW, Lawrence VA, Cornell JE. Preoperative pulmonary risk stratification for noncardiothoracic surgery: systematic review for the American College of Physicians. Ann Intern Med. 2006;144:581-595.
4. Chung F, Abdullah HR, Liao P. STOP-Bang questionnaire: a prac tical approach to screen for obstructive sleep apnea. Chest. 2016;149:631-638.
5. De Hert S, Imberger G, Carlisle J, et al. Preoperative evaluation of the adult patient undergoing non-cardiac surgery: guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol. 2011;28:684-722.
6. American Society of Anesthesiologists Task Force on Perioperative Management of Patients with Obstructive Sleep Apnea. Practice guidelines for the perioperative management of patients with obstructive sleep apnea; an updated report by the American Society of Anesthesiologists Task Force on Perioperative Management of Patients with Obstructive Sleep Apnea. Anesthesiology. 2014;120:268-286.
7. Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100:1043-1049.
8. Bilimoria KY, Liu Y, Paruch JL, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aide and informed consent tool for patients and surgeons. J Am Coll Surg. 2013;217:833-842.
9. Hlatky MA, Boineau RE, Higginbotham MB, et al. A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index). Am J Cardiol. 1989;64:651-654.
10. Bouri S, Shun-Shin MJ, Cole GD, et al. Meta-analysis of secure randomized controlled trials of B-blockade to prevent perioperative death in non-cardiac surgery. Heart. 2014;100:456-464.
11. Mounsey A, Roque JM, Egan M. Why you shouldn’t start beta-blockers before surgery. J Fam Pract. 2014;63:E15-E16.
12. Chou R, Arora B, Dana T, et al. Screening asymptomatic adults with resting or exercise electrocardiography: a review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;155:375-385.
13. Durazzo AES, Machado FS, Ikeoka DT, et al. Reduction in cardiovascular events after vascular surgery with atorvastatin: a randomized trial. J Vasc Surg. 2004;39:967-975.
14. Pan SY, Wu VC, Huang TM, et al. Effect of preoperative statin therapy on postoperative acute kidney injury in patients undergoing major surgery: systemic review and meta-analysis. Nephrology. 2014;19:750-763.
15. Devereux PJ, Mrkobrada M, Sessler DI, et al. Aspirin in patients undergoing noncardiac surgery. N Engl J Med. 2014;370:1494-1503.
16. Adesanya AO, Lee W, Greilich NB, et al. Perioperative management of obstructive sleep apnea. Chest. 2010;138:1489-1498.
17. Chung F, Nagappa M, Singh M, et al. CPAP in the perioperative setting: evidence of support. Chest. 2016;149:586-597.
18. Yamashita S, Yamaguchi H, Sakaguchi M, et al. Effect of smoking on intraoperative sputum and postoperative pulmonary complication in minor surgical patients. Respir Med. 2004;98:760-766.
19. Myers K, Hajek P, Hinds
20. Lonjaret L, Lairez O, Minville V, et al. Optimal perioperative management of arterial blood pressure. Integr Blood Press Control. 2014;7:49-59.
21. Sudhakaran S, Surani SR. Guidelines for perioperative management of the diabetic patient. Surg Res Pract. 2015;2015:284063.
22. Duncan AE. Hyperglycemia and perioperative glucose management. Curr Pharm Des. 2012;18:6195-6203.
23. Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e326S-e350S.
24. Faraoni D, Levy JH, Albaladejo P, et al. Updates in the perioperative and emergency management of non-vitamin K antagonist oral anticoagulants. Crit Care. 2015;19:203.
25. Shamoun F, Obeid H, Ramakrishna H. Novel anticoagulants in atrial fibrillation: monitoring, reversal and perioperative management. Biomed Res Int. 2015;2015:424031.
26. Kelly KN, Domajnko B. Perioperative stress-dose steroids. Clin Colon Rectal Surg. 2013;26:163-167.
27. Scanzello CR, Nestor BJ. Perioperative management of medications used in the treatment of rheumatoid arthritis. HSSJ. 2006;2:141-147.
It’s Not Too Late to Register for the NORD Summit
Food and Drug Administration (FDA) Commissioner Robert Califf, MD, will deliver a keynote address on the opening morning of the NORD Rare Diseases and Orphan Products Breakthrough Summit to take place Oct. 17 and 18 in Arlington Virginia. Approximately one-third of the novel new drugs approved by FDA in recent years have been “orphan” drugs for rare diseases.
Dr. Califf will be joined on the program agenda by more than 25 additional FDA speakers, including Janet Woodcock, MD, Director of FDA’s Center for Drug Evaluation and Research, and Peter Marks, MD, PhD, Director of FDA’s Center for Biologics Evaluation and Research.
On Day Two of the conference, Kate Rawson, Senior Editor at Prevision Policy, will provide the morning keynote with a look ahead at possible implications of the national election for the rare disease community. The NORD Summit attracts medical professionals, patient advocates, and others to examine issues related to rare disease research, diagnosis, treatment, and patient access to care. The conference is open to all. Click here to view the agenda.
Food and Drug Administration (FDA) Commissioner Robert Califf, MD, will deliver a keynote address on the opening morning of the NORD Rare Diseases and Orphan Products Breakthrough Summit to take place Oct. 17 and 18 in Arlington Virginia. Approximately one-third of the novel new drugs approved by FDA in recent years have been “orphan” drugs for rare diseases.
Dr. Califf will be joined on the program agenda by more than 25 additional FDA speakers, including Janet Woodcock, MD, Director of FDA’s Center for Drug Evaluation and Research, and Peter Marks, MD, PhD, Director of FDA’s Center for Biologics Evaluation and Research.
On Day Two of the conference, Kate Rawson, Senior Editor at Prevision Policy, will provide the morning keynote with a look ahead at possible implications of the national election for the rare disease community. The NORD Summit attracts medical professionals, patient advocates, and others to examine issues related to rare disease research, diagnosis, treatment, and patient access to care. The conference is open to all. Click here to view the agenda.
Food and Drug Administration (FDA) Commissioner Robert Califf, MD, will deliver a keynote address on the opening morning of the NORD Rare Diseases and Orphan Products Breakthrough Summit to take place Oct. 17 and 18 in Arlington Virginia. Approximately one-third of the novel new drugs approved by FDA in recent years have been “orphan” drugs for rare diseases.
Dr. Califf will be joined on the program agenda by more than 25 additional FDA speakers, including Janet Woodcock, MD, Director of FDA’s Center for Drug Evaluation and Research, and Peter Marks, MD, PhD, Director of FDA’s Center for Biologics Evaluation and Research.
On Day Two of the conference, Kate Rawson, Senior Editor at Prevision Policy, will provide the morning keynote with a look ahead at possible implications of the national election for the rare disease community. The NORD Summit attracts medical professionals, patient advocates, and others to examine issues related to rare disease research, diagnosis, treatment, and patient access to care. The conference is open to all. Click here to view the agenda.
Skin findings associated with nutritional deficiencies
Although vitamin and mineral deficiencies are relatively uncommon in the United States and other developed countries, physicians must be alert to them, particularly in specific populations such as infants, pregnant women, alcoholics, vegetarians, people of lower socioeconomic status, and patients on dialysis, on certain medications, or with a history of malabsorption or gastrointestinal surgery. The skin is commonly affected by nutritional deficiencies and can provide important diagnostic clues.
This article reviews the consequences of deficiencies of zinc and vitamins A, B2, B3, B6, and C, emphasizing dermatologic findings.
ZINC DEFICIENCY
Case: A colon cancer patient on total parenteral nutrition
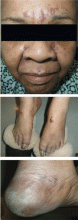
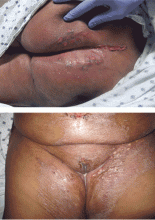
A 65-year-old woman who had been on total parenteral nutrition for 4 months after undergoing surgical debulking for metastatic colon cancer was admitted for evaluation of a rash on her face and extremities and failure to thrive. The rash had started 10 days earlier as small red papules and vesicles on the forehead and progressed to cover the forehead and lips. She had been prescribed prednisone 20 mg daily, but the condition had not improved.
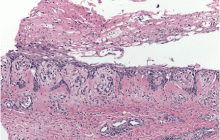
Physical examination revealed numerous violaceous papules, plaques, and vesicles on her face, legs, and feet (Figure 1). The vesicles were tender to touch and some were crusted. Biopsy of a lesion on her leg revealed psoriasiform dermatitis with prominent epidermal pallor and necrosis (Figure 2), suggestive of a nutritional deficiency.
Blood testing revealed low levels of alkaline phosphatase and zinc. She was started on zinc supplementation (3 mg/kg/day), and her cutaneous lesions improved within a month, confirming the diagnosis of zinc deficiency.
Zinc is an essential trace element
Zinc is an essential trace element required for function of many metalloproteases and transcription factors involved in reproduction, immunology, and wound repair. Additionally, its antioxidant properties help prevent ultraviolet radiation damage.1
The recommended dietary allowance (RDA) for zinc is 11 mg/day for men and 8 mg/day for women, with higher amounts for pregnant and lactating women.1 The human body does not store zinc, and meat and eggs are the most important dietary sources.1
The normal plasma zinc level is 70 to 250 µL/dL, and hypozincemia can be diagnosed with a blood test. For the test to be accurate, zinc-free tubes should be used, anticoagulants should be avoided, the blood should not come into contact with rubber stoppers, and blood should be drawn in the morning due to diurnal variation in zinc levels. Additionally, zinc levels may be transiently low secondary to infection. Thus, the clinical picture, along with zinc levels, histopathology, and clinical response to zinc supplementation are necessary for the diagnosis of zinc deficiency.2
Since zinc is required for the activity of alkaline phosphatase (a metalloenzyme), serum levels of alkaline phosphatase correlate with zinc levels and can be used as a serologic marker for zinc levels.3
Zinc deficiency is a worldwide problem, with a higher prevalence in developing countries. It can result from either inadequate diet or impaired absorption, which can be acquired or inherited.
Clinical forms of zinc deficiency
Acrodermatitis enteropathica, an inherited form of zinc deficiency, is due to a mutation in the SLC39A4 gene encoding a zinc uptake protein.4 Patients typically present during infancy a few weeks after being weaned from breast milk. Clinical presentations include diarrhea, periorificial (eg, around the mouth) and acral dermatitis, and alopecia, although only 20% of patients have all these findings at presentation.5 Occasionally, diaper rash, photosensitivity, nail dystrophy, angular stomatitis, conjunctivitis, blepharitis, and growth retardation are observed. Serum levels of zinc and alkaline phosphatase are low.5 Clinical and serologic markers improve within 2 to 3 weeks with oral zinc supplementation (2–3 mg/kg/day).
Acquired forms of zinc deficiency are linked to poor socioeconomic status, diet, infections, renal failure, pancreatic insufficiency, cystic fibrosis, and malabsorption syndromes.1,6,7 Cutaneous findings in acquired cases of zinc deficiency are similar to those seen in acrodermatitis enteropathica. Periorificial lesions are a hallmark of this condition, and angular cheilitis is an early manifestation. Eczematous annular plaques typically develop in areas subjected to repeated friction and pressure and may evolve into vesicles, pustules, and bullae.2 On biopsy study, lesions are characterized by cytoplasmic pallor, vacuolization, and necrosis of keratinocytes, which are common findings in nutritional deficiencies.8 Dystrophic nails, structural hair changes, and diminished growth of both hair and nails have been reported.2
Cutaneous lesions due to hypozincemia respond quickly to zinc supplementation (1–3 mg/kg/day), usually without permanent damage.2 However, areas of hypo- and hyperpigmentation may persist.
VITAMIN C DEFICIENCY
Case: A lung transplant recipient on peritoneal dialysis
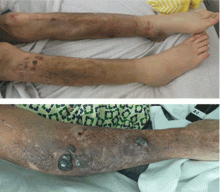
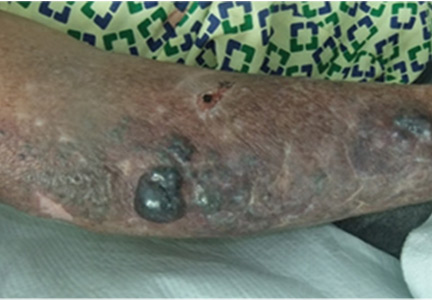
A 59-year-old bilateral lung transplant patient with a history of chronic kidney disease on peritoneal dialysis for the past 2 years was admitted for peritonitis. He had developed tender violaceous papules and nodules coalescing into large plaques on his arms and perifollicular purpuric macules on both legs 3 days before admission (Figure 3). The lesions were painful to the touch, and some bled at times. Tender gums, bilateral edema, and corkscrew hair were also noted (corkscrew hair is shown in another patient in Figure 4).
Biopsy of a lesion on the forearm was consistent with lymphangiectasia secondary to edema. Staining for bacteria and fungi was negative.
Serologic investigation revealed low vitamin C serum levels (7 µmol/L, reference range 23–114 µmol/L). Supplementation with 1 g/day of vitamin C was started and resulted in gradual improvement of the purpura. The patient died 4 months later of complications of comorbidities.
An important antioxidant
Vitamin C, or ascorbic acid, is an important antioxidant involved in the synthesis of tyrosine, tryptophan, and folic acid and in the hydroxylation of glycine and proline, a required step in the formation of collagen.9 Humans cannot synthesize vitamin C and must acquire it in the diet.9 Plants are the most important dietary sources.9 Although vitamin C is generally not toxic and its metabolites are renally cleared, diarrhea and other gastrointestinal disturbances can occur if large amounts are ingested.10
Vitamin C deficiency is rare in developed countries and is linked to malnutrition. Risk factors include alcoholism, severe psychiatric illness, anorexia, and low socioeconomic status. Moreover, multiple conditions including stress, viral illness, smoking, fever, and use of antibiotics lead to diminished vitamin C bioavailability.9 Patients on dialysis are at increased risk of vitamin C deficiency since it is lost during the process.11
The RDA for vitamin C is 90 mg for men and 75 mg for women, with higher requirements during pregnancy and lactation.12 This is much higher than the amount needed to prevent scurvy, 10 mg/day.13
Scurvy is the classic manifestation
The classic manifestations of vitamin C deficiency are scurvy and Barlow disease, also known as infantile scurvy.
Early manifestations of vitamin C deficiency such as fatigue, mood changes, and depression appear after 1 to 3 months of inadequate intake.13 Other manifestations are anemia, bone pain, hemorrhage into joints, abnormal vision, and possibly osteoporosis.
Cutaneous findings are a hallmark of scurvy. Follicular hyperkeratosis with fragmented corkscrew hair and perifollicular hemorrhages on posterior thighs, forearms, and abdomen are pathognomonic findings that occur early in the disease.13 The cutaneous hemorrhages can become palpable, particularly in the lower limbs. Diffuse petechiae are a later finding along with ecchymosis, particularly in pressure sites such as the buttocks.13 “Woody edema” of the legs with ecchymosis, pain, and limited motion can also arise.14 Nail findings including koilonychia and splinter hemorrhages are common.13,14
Vitamin C deficiency results in poor wound healing with consequent ulcer formation due to impaired collagen synthesis. Hair abnormalities including corkscrew and swan-neck hairs are common in scurvy due to vitamin C’s role in disulfide bond formation, which is necessary for hair synthesis.13
Scurvy also affects the oral cavity: gums typically appear red, swollen, and shiny earlier in the disease and can become black and necrotic later.13 Loosening and loss of teeth is also common.13
Scurvy responds quickly to vitamin C supplementation. Patients with scurvy should receive 1 to 2 g of vitamin C daily for 2 to 3 days, 500 mg daily for the next week, and 100 mg daily for the next 1 to 3 months.15 Fatigue, pain, and confusion usually improve in the first 24 hours of treatment, cutaneous manifestations respond in 2 weeks, and hair within 1 month. Complete recovery is expected within 3 months on vitamin C supplementation.15
VITAMIN A DEFICIENCY
Case: A girl with short-bowel syndrome on total parenteral nutrition
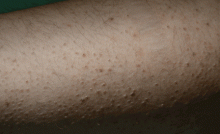
A 14-year-old girl who had been on total parenteral nutrition for the past 3 years due to short-bowel syndrome was admitted for evaluation for a second small-bowel transplant. She complained of dry skin and dry eyes. She was found to have rough, toad-like skin with prominent brown perifollicular hyperkeratotic papules on buttocks and extremities (Figure 5). Additionally, corkscrew hairs were noted. Physical examination was consistent with phrynoderma.
Blood work revealed low levels of vitamin A (8 µg/dL, reference range 20–120 µg/dL) and vitamin C (20 µmol/L, reference range 23–114 µmol/L). After bowel transplant, her vitamin A levels normalized within 2 weeks and her skin improved without vitamin A supplementation.
Essential for protein synthesis
Vitamin A is a group of fat-soluble isoprenoids that includes retinol, retinoic acid, and beta-carotene. It is stored in hepatic stellate cells, which can release it in circulation for distribution to peripheral organs when needed.16
Vitamin A is essential for protein synthesis in the eye and is a crucial component of phototransduction.17 It is also an important modulator of the immune system, as it enhances cytotoxicity and proliferation of T cells while suppressing B-cell proliferation.18 Additionally, vitamin A plays an important role in the skin, where it promotes cell mitosis and increases epithelial thickness, the number of Langerhans cells, and glycosaminoglycan synthesis.19–21
Deficiency associated with malabsorption, liver disease, small-bowel surgery
Vitamin A deficiency is rare in developed countries overall, but it is associated with malabsorption, liver disease, and small-bowel surgery.22 Indeed, 4 years after undergoing bariatric surgery, 69% of patients in one series had deficiencies in vitamin A and other fat-soluble vitamins.23 The typical manifestations are nyctalopia (night blindness) and xerophthalmia (inability to produce tears).
Phrynoderma, or “toad skin,” is a cutaneous manifestation of vitamin A deficiency. The association between phrynoderma and vitamin A deficiency was established in 1933 when prisoners in Africa with nyctalopia, xerophthalmia, and phrynoderma showed improvement in all three conditions when treated with cod oil, which is rich in vitamin A.24
Phrynoderma is characterized by dry, hyperkeratotic papules with central intrafollicular plugs projecting from hair follicles.25 The lesions are typically symmetrically distributed on the face, the skull, and the extensor surfaces of the shoulders, buttocks, and extremities, but they can extend to the entire body in severe cases.25 They typically get better with improved nutrition.
Evidence is mounting to suggest phrynoderma is a cutaneous manifestation of diverse nutritional deficiencies, not just vitamin A. For example, some children with phrynoderma have normal levels of vitamin A,26 and a trial showed that patients with phrynoderma benefited from intramuscular injections of either vitamin A or vitamin B complex, particularly when also treated with topical keratolytics.27 Thus, patients who present with the typical lesions of phrynoderma should be screened for nutritional deficiencies beyond vitamin A.
VITAMIN B6 DEFICIENCY
Case: A woman with sepsis
A 62-year-old woman with a 4-year history of unspecified dermatitis, intertriginous rashes, and skin ulcerations with polymicrobial infections was admitted for sepsis. She reported that her rash had worsened over the previous 2 weeks. Physical examination revealed generalized xerosis, an inflamed bright red tongue with atrophy of distal papillae, and red painful erosions in intertriginous areas (Figure 6).
Blood testing revealed low levels of vitamin B2 (< 5.0 nmol/L, reference range 6.2–39 nmol/L) and vitamin B6 (3.1 nmol/L, reference range 20–125 nmol/L). She was started on supplementation with vitamin B6 50 mg/day and vitamin B2 200 mg/day, and her dermatitis and ulcers improved.
Pyridoxine and its derivatives
Pyridoxine and its derivatives are collectively known as vitamin B6. Vitamin B6 can be stored throughout the body, particularly in muscle and the liver, whereas its oxidized version is excreted mostly in the urine.28,29 Vitamin B6 serves as a cofactor to more than 140 enzymes, it is required for tryptophan metabolism and synthesis of nicotinic acid, and it is a cofactor for alanine aminotransferase and aspartate aminotransferase.28,29
Vitamin B6 deficiency is rare in the general population. The median daily intake is 2 mg/day for men and 1.5 mg/day for women, whereas the RDA for adults is 1.3 mg/day. No signs of vitamin B6 deficiency have been noted at intakes greater than 0.5 mg/day in clinical studies.28
However, chronic alcoholism poses a high risk of this deficiency because it decreases the intake of vitamin B6 and decreases the ability of the liver to store it. Additionally, patients with eclampsia or preeclampsia or who are on dialysis have higher vitamin B6 requirements.28 Certain medications are also associated with a low vitamin B6 level, in particular the antituberculosis medication isoniazid, penicillamine, and hydralazine.28
Although clinical manifestations of vitamin B6 deficiency are rare, subclinical deficiency may be common, particularly in the elderly,28 as up to 23% of people ages 65 to 75 and 40% of those older than 85 have vitamin B6 deficiency.30,31
Features of vitamin B6 deficiency
Vitamin B6 deficiency is associated with anemia (hypochromic, microcytic, iron-refractory), impaired immune function, seizures, peripheral neuropathy, and glossitis. Experimentally induced deficiency of vitamin B6 results in periorificial dermatitis within 3 weeks.32 Intriguingly, multiple studies have shown an inverse correlation between B6 levels and diverse cancers, including colorectal, pancreatic, and lung cancer.28
Given its role in the synthesis of nicotinic acid, vitamin B6 deficiency results in abnormal levels of B3. Thus, vitamin B6 deficiency may result in a pellagra-like presentation (reviewed in detail below in the discussion of vitamin B3 deficiency). In this case, giving vitamin B3 does not result in significant improvement, and this failure helps to establish the diagnosis of vitamin B6 deficiency.32 It is believed that pellagrous lesions in vitamin B6 deficiency are due to decreased synthesis of proline from ornithine, as suggested by decreased levels of the enzyme ornithine aminotransferase in patients with low vitamin B6.33 Other cutaneous manifestations of vitamin B6 deficiency include eczema and seborrheic dermatitis.33
Vitamin B6 can be measured in blood and urine. Although these levels only reflect recent intake, plasma values lower than 20 nmol/L are indicative of vitamin B6 deficiency.34 Therapeutic oral supplementation of vitamin B6 is the treatment of choice. Vitamin B6 treatment is safe, but exposure to high levels of vitamin B6 may result in photosensitivity and dermatitis.35
Vitamin B2 (riboflavin) deficiency
Riboflavin, or vitamin B2, is a water-soluble vitamin involved in diverse reduction-oxidation reactions. Its active forms—flavin adenine dinucleotide and flavin mononucleotide—act as electron carriers in the respiratory electron transfer chain, and the former is necessary for the oxidation of fatty acids.36 The human body does not store riboflavin, and excess intake is excreted in the urine.36
Milk, dairy products, and meat are the major dietary sources of vitamin B2. Additionally, some colonic bacteria synthesize it and provide an additional source.36 Patients whose diets are low in dairy and meat products, in particular vegetarians, alcoholics, and the elderly, are at risk of this deficiency. Other populations at risk are pregnant women, lactating women, premature infants, infants exposed to phototherapy for hyperbilirubinemia, and infants of mothers with low vitamin B2 levels.36,37
The RDA for vitamin B2 is 1.3 mg/day for men and 1.1 mg/day per women, with higher requirements for pregnant and lactating women. Fortunately, the median intake of riboflavin from diet in the United States is 2 mg/day for men and 1.5 mg/day for women.38
Features of vitamin B2 deficiency
Features of vitamin B2 deficiency include angular stomatitis, glossitis, cheilosis, nasolabial dermatitis, and rarely corneal vascularization.39,40 Dermatitic lesions around the scrotum and labia are common and are in many cases the initial manifestation of vitamin B2 deficiency.39,40 Riboflavin deficiency during development results in muscular, skeletal, and gastrointestinal abnormalities. In adults, riboflavin deficiency is associated with anemia, decreased iron absorption, neurodegeneration, and peripheral neuropathy.36
Vitamin B2 deficiency usually coexists with other deficiencies, and riboflavin is involved in the metabolism of other B vitamins including B3, B6, B9 (folate), and B12. Thus, the clinical presentation of vitamin B2 deficiency is similar to that of vitamin B3 and B6 deficiency (reviewed above and below) and has been described as pellagra sine pellagra (pellagra without pellagra). Moreover, correction of riboflavin deficiency results in increased levels of vitamin B3 and B6.36
Vitamin B2 levels can be measured in the urine and blood.37 Oral supplementation is safe (up to 60 mg/day) and is the treatment of choice.36,38 Clearance of lesions within 3 to 5 days of riboflavin supplementation confirms the diagnosis.40
Vitamin B3 (niacin) deficiency
Niacin, or vitamin B3, is a water-soluble vitamin abundant in meat, eggs, and legumes. It is an essential cofactor for coenzyme I and coenzyme II; therefore, it plays a crucial role in ATP synthesis, glycolysis, and metabolism of fatty acids and amino acids.41,42
Most niacin is acquired in the diet, but humans can synthesize it from tryptophan in the presence of vitamin B6 and thiamine.42 Thus, a deficiency in tryptophan, vitamin B6, or thiamine can also lead to low niacin, and an excess of dietary leucine can interfere with niacin synthesis and result in deficiency.42
The RDA for niacin is 6 to 20 mg/day, based on sex and age, with higher requirements for pregnant and lactating women.38
Pellagra, the clinical manifestation
Pellagra is the clinical manifestation of niacin deficiency, although it is thought that lack of tryptophan, vitamin B6, or thiamine may also be required for clinical symptoms to appear.41
Sporadic cases of pellagra occur in homeless people, alcoholics, drug abusers, people with anorexia, and food faddists.41,42 Symptoms typically develop after about 50 days of a niacin-free diet.41 Pellagra may also develop due to impaired absorption or metabolism, particularly in patients with prolonged diarrhea, colitis, ileitis, hepatic cirrhosis, or Hartnup disease.42–45 Certain medications, eg, isoniazid, 5-fluorouracil, azathioprine, and 6-mercaptopurine, interfere with niacin synthesis and may induce pellagra in susceptible patients.42
The clinical course of pellagra is often described by the four “Ds”: dermatitis, dementia, diarrhea, and, when not corrected, death. Early symptoms of insufficient vitamin B3 are weakness, fatigue, loss of appetite, depression, and mood changes.42
The cutaneous manifestations of pellagra are impressive and include photosensitive eruptions, perineal lesions, and thickened and pigmented skin.41 Biopsy of affected and unaffected skin in pellagra patients shows abnormal keratinization.
Photosensitivity is an initial manifestation of pellagra.46 It is believed that vitamin B3 deficiency results in a lack of urocanic acid, a compound that protects against ultraviolet B damage and accumulation of kynurenic acid, a known phototoxic agent.47
The initial stage of acute pellagra can resemble a sunburn on the face, neck, and dorsal extremities47 that becomes darker with time instead of fading.46 Sharply demarcated hyperpigmented areas on the arms and legs are known as the “glove” and “boot” of pellagra.46 Nearly all patients have involvement of the dorsum of the hand.42 The Casal necklace may be present, a characteristic eruption observed in up to 76% of patients on the front of the neck in the region of C3-C4.48
As the disease progresses, lesions harden and become brittle—hence, the name pellagra, which means “rough skin.” Perineal lesions are also common, along with fissures and ulcerations. Additionally, about a third of pellagra patients have involvement of the lips, tongue, and oral mucosa.42 Notably, patients with drug-induced or Hartnup-related pellagra do not develop genital, perineal, oral, or hyperkeratotic lesions.46
Although untreated pellagra can lead to death in 5 years,42 the disease responds dramatically to oral nicotinamide (250–500 mg/day), which is preferred over niacin due to the latter’s vasomotor effects.41 Therapy also includes caloric supplementation, other B vitamins, zinc, and magnesium.42
NUTRITIONAL DEFICIENCIES TEND TO COEXIST
The clinical scenarios presented here emphasize how different nutritional deficiencies can manifest with overlapping features. But nutritional deficiencies, particularly those associated with underlying conditions, tend to coexist rather than occur in isolation.
Although associated with significant morbidity, nutritional deficiencies can be easily addressed, particularly when promptly identified. Careful evaluation of the history and clinical and serologic findings is necessary to correctly diagnose and address these conditions.
- Gupta M, Mahajan VK, Mehta KS, Chauhan PS. Zinc therapy in dermatology: a review. Dermatol Res Pract 2014; 2014:709152.
- Kumar P, Lal NR, Mondal AK, Mondal A, Gharami RC, Maiti A. Zinc and skin: a brief summary. Dermatol Online J 2012; 18:1.
- Kiliç I, Ozalp I, Coskun T, et al. The effect of zinc-supplemented bread consumption on school children with asymptomatic zinc deficiency. J Pediatr Gastroenterol Nutr 1998; 26:167–171.
- Küry S, Dréno B, Bézieau S, et al. Identification of SLC39A4, a gene involved in acrodermatitis enteropathica. Nat Genet 2002; 31:239–240.
- Maverakis E, Fung MA, Lynch PJ, et al. Acrodermatitis enteropathica and an overview of zinc metabolism. J Am Acad Dermatol 2007; 56:116–124.
- Younoszai HD. Clinical zinc deficiency in total parenteral nutrition: zinc supplementation. JPEN J Parenter Enteral Nutr 1983; 7:72–74.
- Muñiz AE, Bartle S, Foster R. Edema, anemia, hypoproteinemia, and acrodermatitis enteropathica: an uncommon initial presentation of cystic fibrosis. Pediatr Emerg Care 2004; 20:112–114.
- Corbo MD, Lam J. Zinc deficiency and its management in the pediatric population: a literature review and proposed etiologic classification. J Am Acad Dermatol 2013; 69:616–624.e1.
- Chambial S, Dwivedi S, Shukla KK, John PJ, Sharma P. Vitamin C in disease prevention and cure: an overview. Indian J Clin Biochem 2013; 28:314–328.
- Johnston CS. Biomarkers for establishing a tolerable upper intake level for vitamin C. Nutr Rev 1999; 57:71–77.
- Raimann JG, Levin NW, Craig RG, Sirover W, Kotanko P, Handelman G. Is vitamin C intake too low in dialysis patients? Semin Dial 2013; 26:1–5.
- Institute of Medicine (US) Panel on Dietary Antioxidants and Related Compounds. Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids. Washington, DC: National Academies Press (US); 2000. www.ncbi.nlm.nih.gov/books/NBK225483/. Accessed September 12, 2016.
- Hirschmann JV, Raugi GJ. Adult scurvy. J Am Acad Dermatol 1999; 41:895–906.
- Barthelemy H, Chouvet B, Cambazard F. Skin and mucosal manifestations in vitamin deficiency. J Am Acad Dermatol 1986; 15:1263–1274.
- Léger D. Scurvy: reemergence of nutritional deficiencies. Can Fam Physician 2008; 54:1403–1406.
- Senoo H, Yoshikawa K, Morii M, Miura M, Imai K, Mezaki Y. Hepatic stellate cell (vitamin A-storing cell) and its relative—past, present and future. Cell Biol Int 2010; 34:1247–1272.
- Saari JC. Vitamin A metabolism in rod and cone visual cycles. Annu Rev Nutr 2012; 32:125–145.
- Ross AC. Vitamin A and retinoic acid in T cell–related immunity. Am J Clin Nutr 2012; 96:1166S–1172S.
- King IA, Tabiowo A. The effect of all-trans-retinoic acid on the synthesis of epidermal cell-surface-associated carbohydrates. Biochem J 1981; 194:341–351.
- Kafi R, Kwak HS, Schumacher WE, et al. Improvement of naturally aged skin with vitamin A (retinol). Arch Dermatol 2007; 143:606–612.
- Schiltz JR, Lanigan J, Nabial W, Petty B, Birnbaum JE. Retinoic acid induces cyclic changes in epidermal thickness and dermal collagen and glycosaminoglycan biosynthesis rates. J Invest Dermatol 1986; 87:663–667.
- Ocón J, Cabrejas C, Altemir J, Moros M. Phrynoderma: a rare dermatologic complication of bariatric surgery. JPEN J Parenter Enteral Nutr 2012; 36:361–364.
- Slater GH, Ren CJ, Siegel N, et al. Serum fat-soluble vitamin deficiency and abnormal calcium metabolism after malabsorptive bariatric surgery. J Gastrointest Surg 2004; 8:48–55.
- Nicholls L. Phrynoderma: a condition due to vitamin deficiency. Indian Med Gaz 1933; 68:681–687.
- Ragunatha S, Kumar VJ, Murugesh SB. A clinical study of 125 patients with phrynoderma. Indian J Dermatol 2011; 56:389–392.
- Nakjang Y, Yuttanavivat T. Phrynoderma: a review of 105 cases. J Dermatol 1988; 15:531–534.
- S R, Kumar V J, S B M, M R, G N, Kapoor M. Therapeutic response of vitamin A, vitamin B complex, essential fatty acids (EFA) and vitamin E in the treatment of phrynoderma: a randomized controlled study. J Clin Diagn Res 2014; 8:116–118.
- Spinneker A, Sola R, Lemmen V, Castillo MJ, Pietrzik K, González-Gross M. Vitamin B6 status, deficiency and its consequences—an overview. Nutr Hosp 2007; 22:7–24.
- Lang F, editor. Encyclopedia of Molecular Mechanisms of Disease. Heidelberg, Germany: Springer Berlin Heidelberg; 2009:2217–2218. http://link.springer.com/referenceworkentry/10.1007/978-3-540-29676-8_1853. Accessed September 6, 2016.
- Herrmann W, Knapp JP. Hyperhomocysteinemia: a new risk factor for degenerative diseases. Clin Lab 2002; 48:471–481.
- Haller J, Löwik MR, Ferry M, Ferro-Luzzi A. Nutritional status: blood vitamins A, E, B6, B12, folic acid and carotene. Euronut SENECA investigators. Eur J Clin Nutr 1991; 45(suppl 3):63–82.
- Barthelemy H, Chouvet B, Cambazard F. Skin and mucosal manifestations in vitamin deficiency. J Am Acad Dermatol 1986; 15:1263–1274.
- Inubushi T, Takasawa T, Tuboi Y, Watanabe N, Aki K, Katunuma N. Changes of glucose metabolism and skin-collagen neogenesis in vitamin B6 deficiency. Biofactors 2005; 23:59–67.
- Lui A, Lumeng L, Aronoff GR, Li TK. Relationship between body store of vitamin B6 and plasma pyridoxal-P clearance: metabolic balance studies in humans. J Lab Clin Med 1985; 106:491–497.
- Bajaj AK, Rastogi S, Misra A, Misra K, Bajaj S. Occupational and systemic contact dermatitis with photosensitivity due to vitamin B6. Contact Dermatitis 2001; 44:184.
- Powers HJ. Riboflavin (vitamin B-2) and health. Am J Clin Nutr 2003; 77:1352–1360.
- Graham JM, Peerson JM, Haskell MJ, Shrestha RK, Brown KH, Allen LH. Erythrocyte riboflavin for the detection of riboflavin deficiency in pregnant Nepali women. Clin Chem 2005; 51:2162–2165.
- Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, DC: National Academies Press (US); 1998. www.ncbi.nlm.nih.gov/books/NBK114310/. Accessed September 6, 2016.
- Ryan AS, Goldsmith LA. Nutrition and the skin. Clin Dermatol 1996; 14:389–406.
- Roe DA. Riboflavin deficiency: mucocutaneous signs of acute and chronic deficiency. Semin Dermatol 1991; 10:293–295.
- Karthikeyan K, Thappa DM. Pellagra and skin. Int J Dermatol 2002; 41:476–481.
- Hegyi J, Schwartz RA, Hegyi V. Pellagra: dermatitis, dementia, and diarrhea. Int J Dermatol 2004; 43:1–5.
- Armstrong JR. Pellagra associated with Crohn’s disease. Lancet 1952; 2:1253–1254.
- Oakley A, Wallace J. Hartnup disease presenting in an adult. Clin Exp Dermatol 1994; 19:407–408.
- Lu JY, Yu CL, Wu MZ. Pellagra in an immunocompetent patient with cytomegalovirus colitis. Am J Gastroenterol 2001; 96:932–934.
- Wan P, Moat S, Anstey A. Pellagra: a review with emphasis on photosensitivity. Br J Dermatol 2011; 164:1188–1200.
- Hendricks WM. Pellagra and pellagralike dermatoses: etiology, differential diagnosis, dermatopathology, and treatment. Semin Dermatol 1991; 10:282–292.
- Malfait P, Moren A, Dillon JC, et al. An outbreak of pellagra related to changes in dietary niacin among Mozambican refugees in Malawi. Int J Epidemiol 1993; 22:504–511.
Although vitamin and mineral deficiencies are relatively uncommon in the United States and other developed countries, physicians must be alert to them, particularly in specific populations such as infants, pregnant women, alcoholics, vegetarians, people of lower socioeconomic status, and patients on dialysis, on certain medications, or with a history of malabsorption or gastrointestinal surgery. The skin is commonly affected by nutritional deficiencies and can provide important diagnostic clues.
This article reviews the consequences of deficiencies of zinc and vitamins A, B2, B3, B6, and C, emphasizing dermatologic findings.
ZINC DEFICIENCY
Case: A colon cancer patient on total parenteral nutrition
A 65-year-old woman who had been on total parenteral nutrition for 4 months after undergoing surgical debulking for metastatic colon cancer was admitted for evaluation of a rash on her face and extremities and failure to thrive. The rash had started 10 days earlier as small red papules and vesicles on the forehead and progressed to cover the forehead and lips. She had been prescribed prednisone 20 mg daily, but the condition had not improved.
Physical examination revealed numerous violaceous papules, plaques, and vesicles on her face, legs, and feet (Figure 1). The vesicles were tender to touch and some were crusted. Biopsy of a lesion on her leg revealed psoriasiform dermatitis with prominent epidermal pallor and necrosis (Figure 2), suggestive of a nutritional deficiency.
Blood testing revealed low levels of alkaline phosphatase and zinc. She was started on zinc supplementation (3 mg/kg/day), and her cutaneous lesions improved within a month, confirming the diagnosis of zinc deficiency.
Zinc is an essential trace element
Zinc is an essential trace element required for function of many metalloproteases and transcription factors involved in reproduction, immunology, and wound repair. Additionally, its antioxidant properties help prevent ultraviolet radiation damage.1
The recommended dietary allowance (RDA) for zinc is 11 mg/day for men and 8 mg/day for women, with higher amounts for pregnant and lactating women.1 The human body does not store zinc, and meat and eggs are the most important dietary sources.1
The normal plasma zinc level is 70 to 250 µL/dL, and hypozincemia can be diagnosed with a blood test. For the test to be accurate, zinc-free tubes should be used, anticoagulants should be avoided, the blood should not come into contact with rubber stoppers, and blood should be drawn in the morning due to diurnal variation in zinc levels. Additionally, zinc levels may be transiently low secondary to infection. Thus, the clinical picture, along with zinc levels, histopathology, and clinical response to zinc supplementation are necessary for the diagnosis of zinc deficiency.2
Since zinc is required for the activity of alkaline phosphatase (a metalloenzyme), serum levels of alkaline phosphatase correlate with zinc levels and can be used as a serologic marker for zinc levels.3
Zinc deficiency is a worldwide problem, with a higher prevalence in developing countries. It can result from either inadequate diet or impaired absorption, which can be acquired or inherited.
Clinical forms of zinc deficiency
Acrodermatitis enteropathica, an inherited form of zinc deficiency, is due to a mutation in the SLC39A4 gene encoding a zinc uptake protein.4 Patients typically present during infancy a few weeks after being weaned from breast milk. Clinical presentations include diarrhea, periorificial (eg, around the mouth) and acral dermatitis, and alopecia, although only 20% of patients have all these findings at presentation.5 Occasionally, diaper rash, photosensitivity, nail dystrophy, angular stomatitis, conjunctivitis, blepharitis, and growth retardation are observed. Serum levels of zinc and alkaline phosphatase are low.5 Clinical and serologic markers improve within 2 to 3 weeks with oral zinc supplementation (2–3 mg/kg/day).
Acquired forms of zinc deficiency are linked to poor socioeconomic status, diet, infections, renal failure, pancreatic insufficiency, cystic fibrosis, and malabsorption syndromes.1,6,7 Cutaneous findings in acquired cases of zinc deficiency are similar to those seen in acrodermatitis enteropathica. Periorificial lesions are a hallmark of this condition, and angular cheilitis is an early manifestation. Eczematous annular plaques typically develop in areas subjected to repeated friction and pressure and may evolve into vesicles, pustules, and bullae.2 On biopsy study, lesions are characterized by cytoplasmic pallor, vacuolization, and necrosis of keratinocytes, which are common findings in nutritional deficiencies.8 Dystrophic nails, structural hair changes, and diminished growth of both hair and nails have been reported.2
Cutaneous lesions due to hypozincemia respond quickly to zinc supplementation (1–3 mg/kg/day), usually without permanent damage.2 However, areas of hypo- and hyperpigmentation may persist.
VITAMIN C DEFICIENCY
Case: A lung transplant recipient on peritoneal dialysis
A 59-year-old bilateral lung transplant patient with a history of chronic kidney disease on peritoneal dialysis for the past 2 years was admitted for peritonitis. He had developed tender violaceous papules and nodules coalescing into large plaques on his arms and perifollicular purpuric macules on both legs 3 days before admission (Figure 3). The lesions were painful to the touch, and some bled at times. Tender gums, bilateral edema, and corkscrew hair were also noted (corkscrew hair is shown in another patient in Figure 4).
Biopsy of a lesion on the forearm was consistent with lymphangiectasia secondary to edema. Staining for bacteria and fungi was negative.
Serologic investigation revealed low vitamin C serum levels (7 µmol/L, reference range 23–114 µmol/L). Supplementation with 1 g/day of vitamin C was started and resulted in gradual improvement of the purpura. The patient died 4 months later of complications of comorbidities.
An important antioxidant
Vitamin C, or ascorbic acid, is an important antioxidant involved in the synthesis of tyrosine, tryptophan, and folic acid and in the hydroxylation of glycine and proline, a required step in the formation of collagen.9 Humans cannot synthesize vitamin C and must acquire it in the diet.9 Plants are the most important dietary sources.9 Although vitamin C is generally not toxic and its metabolites are renally cleared, diarrhea and other gastrointestinal disturbances can occur if large amounts are ingested.10
Vitamin C deficiency is rare in developed countries and is linked to malnutrition. Risk factors include alcoholism, severe psychiatric illness, anorexia, and low socioeconomic status. Moreover, multiple conditions including stress, viral illness, smoking, fever, and use of antibiotics lead to diminished vitamin C bioavailability.9 Patients on dialysis are at increased risk of vitamin C deficiency since it is lost during the process.11
The RDA for vitamin C is 90 mg for men and 75 mg for women, with higher requirements during pregnancy and lactation.12 This is much higher than the amount needed to prevent scurvy, 10 mg/day.13
Scurvy is the classic manifestation
The classic manifestations of vitamin C deficiency are scurvy and Barlow disease, also known as infantile scurvy.
Early manifestations of vitamin C deficiency such as fatigue, mood changes, and depression appear after 1 to 3 months of inadequate intake.13 Other manifestations are anemia, bone pain, hemorrhage into joints, abnormal vision, and possibly osteoporosis.
Cutaneous findings are a hallmark of scurvy. Follicular hyperkeratosis with fragmented corkscrew hair and perifollicular hemorrhages on posterior thighs, forearms, and abdomen are pathognomonic findings that occur early in the disease.13 The cutaneous hemorrhages can become palpable, particularly in the lower limbs. Diffuse petechiae are a later finding along with ecchymosis, particularly in pressure sites such as the buttocks.13 “Woody edema” of the legs with ecchymosis, pain, and limited motion can also arise.14 Nail findings including koilonychia and splinter hemorrhages are common.13,14
Vitamin C deficiency results in poor wound healing with consequent ulcer formation due to impaired collagen synthesis. Hair abnormalities including corkscrew and swan-neck hairs are common in scurvy due to vitamin C’s role in disulfide bond formation, which is necessary for hair synthesis.13
Scurvy also affects the oral cavity: gums typically appear red, swollen, and shiny earlier in the disease and can become black and necrotic later.13 Loosening and loss of teeth is also common.13
Scurvy responds quickly to vitamin C supplementation. Patients with scurvy should receive 1 to 2 g of vitamin C daily for 2 to 3 days, 500 mg daily for the next week, and 100 mg daily for the next 1 to 3 months.15 Fatigue, pain, and confusion usually improve in the first 24 hours of treatment, cutaneous manifestations respond in 2 weeks, and hair within 1 month. Complete recovery is expected within 3 months on vitamin C supplementation.15
VITAMIN A DEFICIENCY
Case: A girl with short-bowel syndrome on total parenteral nutrition
A 14-year-old girl who had been on total parenteral nutrition for the past 3 years due to short-bowel syndrome was admitted for evaluation for a second small-bowel transplant. She complained of dry skin and dry eyes. She was found to have rough, toad-like skin with prominent brown perifollicular hyperkeratotic papules on buttocks and extremities (Figure 5). Additionally, corkscrew hairs were noted. Physical examination was consistent with phrynoderma.
Blood work revealed low levels of vitamin A (8 µg/dL, reference range 20–120 µg/dL) and vitamin C (20 µmol/L, reference range 23–114 µmol/L). After bowel transplant, her vitamin A levels normalized within 2 weeks and her skin improved without vitamin A supplementation.
Essential for protein synthesis
Vitamin A is a group of fat-soluble isoprenoids that includes retinol, retinoic acid, and beta-carotene. It is stored in hepatic stellate cells, which can release it in circulation for distribution to peripheral organs when needed.16
Vitamin A is essential for protein synthesis in the eye and is a crucial component of phototransduction.17 It is also an important modulator of the immune system, as it enhances cytotoxicity and proliferation of T cells while suppressing B-cell proliferation.18 Additionally, vitamin A plays an important role in the skin, where it promotes cell mitosis and increases epithelial thickness, the number of Langerhans cells, and glycosaminoglycan synthesis.19–21
Deficiency associated with malabsorption, liver disease, small-bowel surgery
Vitamin A deficiency is rare in developed countries overall, but it is associated with malabsorption, liver disease, and small-bowel surgery.22 Indeed, 4 years after undergoing bariatric surgery, 69% of patients in one series had deficiencies in vitamin A and other fat-soluble vitamins.23 The typical manifestations are nyctalopia (night blindness) and xerophthalmia (inability to produce tears).
Phrynoderma, or “toad skin,” is a cutaneous manifestation of vitamin A deficiency. The association between phrynoderma and vitamin A deficiency was established in 1933 when prisoners in Africa with nyctalopia, xerophthalmia, and phrynoderma showed improvement in all three conditions when treated with cod oil, which is rich in vitamin A.24
Phrynoderma is characterized by dry, hyperkeratotic papules with central intrafollicular plugs projecting from hair follicles.25 The lesions are typically symmetrically distributed on the face, the skull, and the extensor surfaces of the shoulders, buttocks, and extremities, but they can extend to the entire body in severe cases.25 They typically get better with improved nutrition.
Evidence is mounting to suggest phrynoderma is a cutaneous manifestation of diverse nutritional deficiencies, not just vitamin A. For example, some children with phrynoderma have normal levels of vitamin A,26 and a trial showed that patients with phrynoderma benefited from intramuscular injections of either vitamin A or vitamin B complex, particularly when also treated with topical keratolytics.27 Thus, patients who present with the typical lesions of phrynoderma should be screened for nutritional deficiencies beyond vitamin A.
VITAMIN B6 DEFICIENCY
Case: A woman with sepsis
A 62-year-old woman with a 4-year history of unspecified dermatitis, intertriginous rashes, and skin ulcerations with polymicrobial infections was admitted for sepsis. She reported that her rash had worsened over the previous 2 weeks. Physical examination revealed generalized xerosis, an inflamed bright red tongue with atrophy of distal papillae, and red painful erosions in intertriginous areas (Figure 6).
Blood testing revealed low levels of vitamin B2 (< 5.0 nmol/L, reference range 6.2–39 nmol/L) and vitamin B6 (3.1 nmol/L, reference range 20–125 nmol/L). She was started on supplementation with vitamin B6 50 mg/day and vitamin B2 200 mg/day, and her dermatitis and ulcers improved.
Pyridoxine and its derivatives
Pyridoxine and its derivatives are collectively known as vitamin B6. Vitamin B6 can be stored throughout the body, particularly in muscle and the liver, whereas its oxidized version is excreted mostly in the urine.28,29 Vitamin B6 serves as a cofactor to more than 140 enzymes, it is required for tryptophan metabolism and synthesis of nicotinic acid, and it is a cofactor for alanine aminotransferase and aspartate aminotransferase.28,29
Vitamin B6 deficiency is rare in the general population. The median daily intake is 2 mg/day for men and 1.5 mg/day for women, whereas the RDA for adults is 1.3 mg/day. No signs of vitamin B6 deficiency have been noted at intakes greater than 0.5 mg/day in clinical studies.28
However, chronic alcoholism poses a high risk of this deficiency because it decreases the intake of vitamin B6 and decreases the ability of the liver to store it. Additionally, patients with eclampsia or preeclampsia or who are on dialysis have higher vitamin B6 requirements.28 Certain medications are also associated with a low vitamin B6 level, in particular the antituberculosis medication isoniazid, penicillamine, and hydralazine.28
Although clinical manifestations of vitamin B6 deficiency are rare, subclinical deficiency may be common, particularly in the elderly,28 as up to 23% of people ages 65 to 75 and 40% of those older than 85 have vitamin B6 deficiency.30,31
Features of vitamin B6 deficiency
Vitamin B6 deficiency is associated with anemia (hypochromic, microcytic, iron-refractory), impaired immune function, seizures, peripheral neuropathy, and glossitis. Experimentally induced deficiency of vitamin B6 results in periorificial dermatitis within 3 weeks.32 Intriguingly, multiple studies have shown an inverse correlation between B6 levels and diverse cancers, including colorectal, pancreatic, and lung cancer.28
Given its role in the synthesis of nicotinic acid, vitamin B6 deficiency results in abnormal levels of B3. Thus, vitamin B6 deficiency may result in a pellagra-like presentation (reviewed in detail below in the discussion of vitamin B3 deficiency). In this case, giving vitamin B3 does not result in significant improvement, and this failure helps to establish the diagnosis of vitamin B6 deficiency.32 It is believed that pellagrous lesions in vitamin B6 deficiency are due to decreased synthesis of proline from ornithine, as suggested by decreased levels of the enzyme ornithine aminotransferase in patients with low vitamin B6.33 Other cutaneous manifestations of vitamin B6 deficiency include eczema and seborrheic dermatitis.33
Vitamin B6 can be measured in blood and urine. Although these levels only reflect recent intake, plasma values lower than 20 nmol/L are indicative of vitamin B6 deficiency.34 Therapeutic oral supplementation of vitamin B6 is the treatment of choice. Vitamin B6 treatment is safe, but exposure to high levels of vitamin B6 may result in photosensitivity and dermatitis.35
Vitamin B2 (riboflavin) deficiency
Riboflavin, or vitamin B2, is a water-soluble vitamin involved in diverse reduction-oxidation reactions. Its active forms—flavin adenine dinucleotide and flavin mononucleotide—act as electron carriers in the respiratory electron transfer chain, and the former is necessary for the oxidation of fatty acids.36 The human body does not store riboflavin, and excess intake is excreted in the urine.36
Milk, dairy products, and meat are the major dietary sources of vitamin B2. Additionally, some colonic bacteria synthesize it and provide an additional source.36 Patients whose diets are low in dairy and meat products, in particular vegetarians, alcoholics, and the elderly, are at risk of this deficiency. Other populations at risk are pregnant women, lactating women, premature infants, infants exposed to phototherapy for hyperbilirubinemia, and infants of mothers with low vitamin B2 levels.36,37
The RDA for vitamin B2 is 1.3 mg/day for men and 1.1 mg/day per women, with higher requirements for pregnant and lactating women. Fortunately, the median intake of riboflavin from diet in the United States is 2 mg/day for men and 1.5 mg/day for women.38
Features of vitamin B2 deficiency
Features of vitamin B2 deficiency include angular stomatitis, glossitis, cheilosis, nasolabial dermatitis, and rarely corneal vascularization.39,40 Dermatitic lesions around the scrotum and labia are common and are in many cases the initial manifestation of vitamin B2 deficiency.39,40 Riboflavin deficiency during development results in muscular, skeletal, and gastrointestinal abnormalities. In adults, riboflavin deficiency is associated with anemia, decreased iron absorption, neurodegeneration, and peripheral neuropathy.36
Vitamin B2 deficiency usually coexists with other deficiencies, and riboflavin is involved in the metabolism of other B vitamins including B3, B6, B9 (folate), and B12. Thus, the clinical presentation of vitamin B2 deficiency is similar to that of vitamin B3 and B6 deficiency (reviewed above and below) and has been described as pellagra sine pellagra (pellagra without pellagra). Moreover, correction of riboflavin deficiency results in increased levels of vitamin B3 and B6.36
Vitamin B2 levels can be measured in the urine and blood.37 Oral supplementation is safe (up to 60 mg/day) and is the treatment of choice.36,38 Clearance of lesions within 3 to 5 days of riboflavin supplementation confirms the diagnosis.40
Vitamin B3 (niacin) deficiency
Niacin, or vitamin B3, is a water-soluble vitamin abundant in meat, eggs, and legumes. It is an essential cofactor for coenzyme I and coenzyme II; therefore, it plays a crucial role in ATP synthesis, glycolysis, and metabolism of fatty acids and amino acids.41,42
Most niacin is acquired in the diet, but humans can synthesize it from tryptophan in the presence of vitamin B6 and thiamine.42 Thus, a deficiency in tryptophan, vitamin B6, or thiamine can also lead to low niacin, and an excess of dietary leucine can interfere with niacin synthesis and result in deficiency.42
The RDA for niacin is 6 to 20 mg/day, based on sex and age, with higher requirements for pregnant and lactating women.38
Pellagra, the clinical manifestation
Pellagra is the clinical manifestation of niacin deficiency, although it is thought that lack of tryptophan, vitamin B6, or thiamine may also be required for clinical symptoms to appear.41
Sporadic cases of pellagra occur in homeless people, alcoholics, drug abusers, people with anorexia, and food faddists.41,42 Symptoms typically develop after about 50 days of a niacin-free diet.41 Pellagra may also develop due to impaired absorption or metabolism, particularly in patients with prolonged diarrhea, colitis, ileitis, hepatic cirrhosis, or Hartnup disease.42–45 Certain medications, eg, isoniazid, 5-fluorouracil, azathioprine, and 6-mercaptopurine, interfere with niacin synthesis and may induce pellagra in susceptible patients.42
The clinical course of pellagra is often described by the four “Ds”: dermatitis, dementia, diarrhea, and, when not corrected, death. Early symptoms of insufficient vitamin B3 are weakness, fatigue, loss of appetite, depression, and mood changes.42
The cutaneous manifestations of pellagra are impressive and include photosensitive eruptions, perineal lesions, and thickened and pigmented skin.41 Biopsy of affected and unaffected skin in pellagra patients shows abnormal keratinization.
Photosensitivity is an initial manifestation of pellagra.46 It is believed that vitamin B3 deficiency results in a lack of urocanic acid, a compound that protects against ultraviolet B damage and accumulation of kynurenic acid, a known phototoxic agent.47
The initial stage of acute pellagra can resemble a sunburn on the face, neck, and dorsal extremities47 that becomes darker with time instead of fading.46 Sharply demarcated hyperpigmented areas on the arms and legs are known as the “glove” and “boot” of pellagra.46 Nearly all patients have involvement of the dorsum of the hand.42 The Casal necklace may be present, a characteristic eruption observed in up to 76% of patients on the front of the neck in the region of C3-C4.48
As the disease progresses, lesions harden and become brittle—hence, the name pellagra, which means “rough skin.” Perineal lesions are also common, along with fissures and ulcerations. Additionally, about a third of pellagra patients have involvement of the lips, tongue, and oral mucosa.42 Notably, patients with drug-induced or Hartnup-related pellagra do not develop genital, perineal, oral, or hyperkeratotic lesions.46
Although untreated pellagra can lead to death in 5 years,42 the disease responds dramatically to oral nicotinamide (250–500 mg/day), which is preferred over niacin due to the latter’s vasomotor effects.41 Therapy also includes caloric supplementation, other B vitamins, zinc, and magnesium.42
NUTRITIONAL DEFICIENCIES TEND TO COEXIST
The clinical scenarios presented here emphasize how different nutritional deficiencies can manifest with overlapping features. But nutritional deficiencies, particularly those associated with underlying conditions, tend to coexist rather than occur in isolation.
Although associated with significant morbidity, nutritional deficiencies can be easily addressed, particularly when promptly identified. Careful evaluation of the history and clinical and serologic findings is necessary to correctly diagnose and address these conditions.
Although vitamin and mineral deficiencies are relatively uncommon in the United States and other developed countries, physicians must be alert to them, particularly in specific populations such as infants, pregnant women, alcoholics, vegetarians, people of lower socioeconomic status, and patients on dialysis, on certain medications, or with a history of malabsorption or gastrointestinal surgery. The skin is commonly affected by nutritional deficiencies and can provide important diagnostic clues.
This article reviews the consequences of deficiencies of zinc and vitamins A, B2, B3, B6, and C, emphasizing dermatologic findings.
ZINC DEFICIENCY
Case: A colon cancer patient on total parenteral nutrition
A 65-year-old woman who had been on total parenteral nutrition for 4 months after undergoing surgical debulking for metastatic colon cancer was admitted for evaluation of a rash on her face and extremities and failure to thrive. The rash had started 10 days earlier as small red papules and vesicles on the forehead and progressed to cover the forehead and lips. She had been prescribed prednisone 20 mg daily, but the condition had not improved.
Physical examination revealed numerous violaceous papules, plaques, and vesicles on her face, legs, and feet (Figure 1). The vesicles were tender to touch and some were crusted. Biopsy of a lesion on her leg revealed psoriasiform dermatitis with prominent epidermal pallor and necrosis (Figure 2), suggestive of a nutritional deficiency.
Blood testing revealed low levels of alkaline phosphatase and zinc. She was started on zinc supplementation (3 mg/kg/day), and her cutaneous lesions improved within a month, confirming the diagnosis of zinc deficiency.
Zinc is an essential trace element
Zinc is an essential trace element required for function of many metalloproteases and transcription factors involved in reproduction, immunology, and wound repair. Additionally, its antioxidant properties help prevent ultraviolet radiation damage.1
The recommended dietary allowance (RDA) for zinc is 11 mg/day for men and 8 mg/day for women, with higher amounts for pregnant and lactating women.1 The human body does not store zinc, and meat and eggs are the most important dietary sources.1
The normal plasma zinc level is 70 to 250 µL/dL, and hypozincemia can be diagnosed with a blood test. For the test to be accurate, zinc-free tubes should be used, anticoagulants should be avoided, the blood should not come into contact with rubber stoppers, and blood should be drawn in the morning due to diurnal variation in zinc levels. Additionally, zinc levels may be transiently low secondary to infection. Thus, the clinical picture, along with zinc levels, histopathology, and clinical response to zinc supplementation are necessary for the diagnosis of zinc deficiency.2
Since zinc is required for the activity of alkaline phosphatase (a metalloenzyme), serum levels of alkaline phosphatase correlate with zinc levels and can be used as a serologic marker for zinc levels.3
Zinc deficiency is a worldwide problem, with a higher prevalence in developing countries. It can result from either inadequate diet or impaired absorption, which can be acquired or inherited.
Clinical forms of zinc deficiency
Acrodermatitis enteropathica, an inherited form of zinc deficiency, is due to a mutation in the SLC39A4 gene encoding a zinc uptake protein.4 Patients typically present during infancy a few weeks after being weaned from breast milk. Clinical presentations include diarrhea, periorificial (eg, around the mouth) and acral dermatitis, and alopecia, although only 20% of patients have all these findings at presentation.5 Occasionally, diaper rash, photosensitivity, nail dystrophy, angular stomatitis, conjunctivitis, blepharitis, and growth retardation are observed. Serum levels of zinc and alkaline phosphatase are low.5 Clinical and serologic markers improve within 2 to 3 weeks with oral zinc supplementation (2–3 mg/kg/day).
Acquired forms of zinc deficiency are linked to poor socioeconomic status, diet, infections, renal failure, pancreatic insufficiency, cystic fibrosis, and malabsorption syndromes.1,6,7 Cutaneous findings in acquired cases of zinc deficiency are similar to those seen in acrodermatitis enteropathica. Periorificial lesions are a hallmark of this condition, and angular cheilitis is an early manifestation. Eczematous annular plaques typically develop in areas subjected to repeated friction and pressure and may evolve into vesicles, pustules, and bullae.2 On biopsy study, lesions are characterized by cytoplasmic pallor, vacuolization, and necrosis of keratinocytes, which are common findings in nutritional deficiencies.8 Dystrophic nails, structural hair changes, and diminished growth of both hair and nails have been reported.2
Cutaneous lesions due to hypozincemia respond quickly to zinc supplementation (1–3 mg/kg/day), usually without permanent damage.2 However, areas of hypo- and hyperpigmentation may persist.
VITAMIN C DEFICIENCY
Case: A lung transplant recipient on peritoneal dialysis
A 59-year-old bilateral lung transplant patient with a history of chronic kidney disease on peritoneal dialysis for the past 2 years was admitted for peritonitis. He had developed tender violaceous papules and nodules coalescing into large plaques on his arms and perifollicular purpuric macules on both legs 3 days before admission (Figure 3). The lesions were painful to the touch, and some bled at times. Tender gums, bilateral edema, and corkscrew hair were also noted (corkscrew hair is shown in another patient in Figure 4).
Biopsy of a lesion on the forearm was consistent with lymphangiectasia secondary to edema. Staining for bacteria and fungi was negative.
Serologic investigation revealed low vitamin C serum levels (7 µmol/L, reference range 23–114 µmol/L). Supplementation with 1 g/day of vitamin C was started and resulted in gradual improvement of the purpura. The patient died 4 months later of complications of comorbidities.
An important antioxidant
Vitamin C, or ascorbic acid, is an important antioxidant involved in the synthesis of tyrosine, tryptophan, and folic acid and in the hydroxylation of glycine and proline, a required step in the formation of collagen.9 Humans cannot synthesize vitamin C and must acquire it in the diet.9 Plants are the most important dietary sources.9 Although vitamin C is generally not toxic and its metabolites are renally cleared, diarrhea and other gastrointestinal disturbances can occur if large amounts are ingested.10
Vitamin C deficiency is rare in developed countries and is linked to malnutrition. Risk factors include alcoholism, severe psychiatric illness, anorexia, and low socioeconomic status. Moreover, multiple conditions including stress, viral illness, smoking, fever, and use of antibiotics lead to diminished vitamin C bioavailability.9 Patients on dialysis are at increased risk of vitamin C deficiency since it is lost during the process.11
The RDA for vitamin C is 90 mg for men and 75 mg for women, with higher requirements during pregnancy and lactation.12 This is much higher than the amount needed to prevent scurvy, 10 mg/day.13
Scurvy is the classic manifestation
The classic manifestations of vitamin C deficiency are scurvy and Barlow disease, also known as infantile scurvy.
Early manifestations of vitamin C deficiency such as fatigue, mood changes, and depression appear after 1 to 3 months of inadequate intake.13 Other manifestations are anemia, bone pain, hemorrhage into joints, abnormal vision, and possibly osteoporosis.
Cutaneous findings are a hallmark of scurvy. Follicular hyperkeratosis with fragmented corkscrew hair and perifollicular hemorrhages on posterior thighs, forearms, and abdomen are pathognomonic findings that occur early in the disease.13 The cutaneous hemorrhages can become palpable, particularly in the lower limbs. Diffuse petechiae are a later finding along with ecchymosis, particularly in pressure sites such as the buttocks.13 “Woody edema” of the legs with ecchymosis, pain, and limited motion can also arise.14 Nail findings including koilonychia and splinter hemorrhages are common.13,14
Vitamin C deficiency results in poor wound healing with consequent ulcer formation due to impaired collagen synthesis. Hair abnormalities including corkscrew and swan-neck hairs are common in scurvy due to vitamin C’s role in disulfide bond formation, which is necessary for hair synthesis.13
Scurvy also affects the oral cavity: gums typically appear red, swollen, and shiny earlier in the disease and can become black and necrotic later.13 Loosening and loss of teeth is also common.13
Scurvy responds quickly to vitamin C supplementation. Patients with scurvy should receive 1 to 2 g of vitamin C daily for 2 to 3 days, 500 mg daily for the next week, and 100 mg daily for the next 1 to 3 months.15 Fatigue, pain, and confusion usually improve in the first 24 hours of treatment, cutaneous manifestations respond in 2 weeks, and hair within 1 month. Complete recovery is expected within 3 months on vitamin C supplementation.15
VITAMIN A DEFICIENCY
Case: A girl with short-bowel syndrome on total parenteral nutrition
A 14-year-old girl who had been on total parenteral nutrition for the past 3 years due to short-bowel syndrome was admitted for evaluation for a second small-bowel transplant. She complained of dry skin and dry eyes. She was found to have rough, toad-like skin with prominent brown perifollicular hyperkeratotic papules on buttocks and extremities (Figure 5). Additionally, corkscrew hairs were noted. Physical examination was consistent with phrynoderma.
Blood work revealed low levels of vitamin A (8 µg/dL, reference range 20–120 µg/dL) and vitamin C (20 µmol/L, reference range 23–114 µmol/L). After bowel transplant, her vitamin A levels normalized within 2 weeks and her skin improved without vitamin A supplementation.
Essential for protein synthesis
Vitamin A is a group of fat-soluble isoprenoids that includes retinol, retinoic acid, and beta-carotene. It is stored in hepatic stellate cells, which can release it in circulation for distribution to peripheral organs when needed.16
Vitamin A is essential for protein synthesis in the eye and is a crucial component of phototransduction.17 It is also an important modulator of the immune system, as it enhances cytotoxicity and proliferation of T cells while suppressing B-cell proliferation.18 Additionally, vitamin A plays an important role in the skin, where it promotes cell mitosis and increases epithelial thickness, the number of Langerhans cells, and glycosaminoglycan synthesis.19–21
Deficiency associated with malabsorption, liver disease, small-bowel surgery
Vitamin A deficiency is rare in developed countries overall, but it is associated with malabsorption, liver disease, and small-bowel surgery.22 Indeed, 4 years after undergoing bariatric surgery, 69% of patients in one series had deficiencies in vitamin A and other fat-soluble vitamins.23 The typical manifestations are nyctalopia (night blindness) and xerophthalmia (inability to produce tears).
Phrynoderma, or “toad skin,” is a cutaneous manifestation of vitamin A deficiency. The association between phrynoderma and vitamin A deficiency was established in 1933 when prisoners in Africa with nyctalopia, xerophthalmia, and phrynoderma showed improvement in all three conditions when treated with cod oil, which is rich in vitamin A.24
Phrynoderma is characterized by dry, hyperkeratotic papules with central intrafollicular plugs projecting from hair follicles.25 The lesions are typically symmetrically distributed on the face, the skull, and the extensor surfaces of the shoulders, buttocks, and extremities, but they can extend to the entire body in severe cases.25 They typically get better with improved nutrition.
Evidence is mounting to suggest phrynoderma is a cutaneous manifestation of diverse nutritional deficiencies, not just vitamin A. For example, some children with phrynoderma have normal levels of vitamin A,26 and a trial showed that patients with phrynoderma benefited from intramuscular injections of either vitamin A or vitamin B complex, particularly when also treated with topical keratolytics.27 Thus, patients who present with the typical lesions of phrynoderma should be screened for nutritional deficiencies beyond vitamin A.
VITAMIN B6 DEFICIENCY
Case: A woman with sepsis
A 62-year-old woman with a 4-year history of unspecified dermatitis, intertriginous rashes, and skin ulcerations with polymicrobial infections was admitted for sepsis. She reported that her rash had worsened over the previous 2 weeks. Physical examination revealed generalized xerosis, an inflamed bright red tongue with atrophy of distal papillae, and red painful erosions in intertriginous areas (Figure 6).
Blood testing revealed low levels of vitamin B2 (< 5.0 nmol/L, reference range 6.2–39 nmol/L) and vitamin B6 (3.1 nmol/L, reference range 20–125 nmol/L). She was started on supplementation with vitamin B6 50 mg/day and vitamin B2 200 mg/day, and her dermatitis and ulcers improved.
Pyridoxine and its derivatives
Pyridoxine and its derivatives are collectively known as vitamin B6. Vitamin B6 can be stored throughout the body, particularly in muscle and the liver, whereas its oxidized version is excreted mostly in the urine.28,29 Vitamin B6 serves as a cofactor to more than 140 enzymes, it is required for tryptophan metabolism and synthesis of nicotinic acid, and it is a cofactor for alanine aminotransferase and aspartate aminotransferase.28,29
Vitamin B6 deficiency is rare in the general population. The median daily intake is 2 mg/day for men and 1.5 mg/day for women, whereas the RDA for adults is 1.3 mg/day. No signs of vitamin B6 deficiency have been noted at intakes greater than 0.5 mg/day in clinical studies.28
However, chronic alcoholism poses a high risk of this deficiency because it decreases the intake of vitamin B6 and decreases the ability of the liver to store it. Additionally, patients with eclampsia or preeclampsia or who are on dialysis have higher vitamin B6 requirements.28 Certain medications are also associated with a low vitamin B6 level, in particular the antituberculosis medication isoniazid, penicillamine, and hydralazine.28
Although clinical manifestations of vitamin B6 deficiency are rare, subclinical deficiency may be common, particularly in the elderly,28 as up to 23% of people ages 65 to 75 and 40% of those older than 85 have vitamin B6 deficiency.30,31
Features of vitamin B6 deficiency
Vitamin B6 deficiency is associated with anemia (hypochromic, microcytic, iron-refractory), impaired immune function, seizures, peripheral neuropathy, and glossitis. Experimentally induced deficiency of vitamin B6 results in periorificial dermatitis within 3 weeks.32 Intriguingly, multiple studies have shown an inverse correlation between B6 levels and diverse cancers, including colorectal, pancreatic, and lung cancer.28
Given its role in the synthesis of nicotinic acid, vitamin B6 deficiency results in abnormal levels of B3. Thus, vitamin B6 deficiency may result in a pellagra-like presentation (reviewed in detail below in the discussion of vitamin B3 deficiency). In this case, giving vitamin B3 does not result in significant improvement, and this failure helps to establish the diagnosis of vitamin B6 deficiency.32 It is believed that pellagrous lesions in vitamin B6 deficiency are due to decreased synthesis of proline from ornithine, as suggested by decreased levels of the enzyme ornithine aminotransferase in patients with low vitamin B6.33 Other cutaneous manifestations of vitamin B6 deficiency include eczema and seborrheic dermatitis.33
Vitamin B6 can be measured in blood and urine. Although these levels only reflect recent intake, plasma values lower than 20 nmol/L are indicative of vitamin B6 deficiency.34 Therapeutic oral supplementation of vitamin B6 is the treatment of choice. Vitamin B6 treatment is safe, but exposure to high levels of vitamin B6 may result in photosensitivity and dermatitis.35
Vitamin B2 (riboflavin) deficiency
Riboflavin, or vitamin B2, is a water-soluble vitamin involved in diverse reduction-oxidation reactions. Its active forms—flavin adenine dinucleotide and flavin mononucleotide—act as electron carriers in the respiratory electron transfer chain, and the former is necessary for the oxidation of fatty acids.36 The human body does not store riboflavin, and excess intake is excreted in the urine.36
Milk, dairy products, and meat are the major dietary sources of vitamin B2. Additionally, some colonic bacteria synthesize it and provide an additional source.36 Patients whose diets are low in dairy and meat products, in particular vegetarians, alcoholics, and the elderly, are at risk of this deficiency. Other populations at risk are pregnant women, lactating women, premature infants, infants exposed to phototherapy for hyperbilirubinemia, and infants of mothers with low vitamin B2 levels.36,37
The RDA for vitamin B2 is 1.3 mg/day for men and 1.1 mg/day per women, with higher requirements for pregnant and lactating women. Fortunately, the median intake of riboflavin from diet in the United States is 2 mg/day for men and 1.5 mg/day for women.38
Features of vitamin B2 deficiency
Features of vitamin B2 deficiency include angular stomatitis, glossitis, cheilosis, nasolabial dermatitis, and rarely corneal vascularization.39,40 Dermatitic lesions around the scrotum and labia are common and are in many cases the initial manifestation of vitamin B2 deficiency.39,40 Riboflavin deficiency during development results in muscular, skeletal, and gastrointestinal abnormalities. In adults, riboflavin deficiency is associated with anemia, decreased iron absorption, neurodegeneration, and peripheral neuropathy.36
Vitamin B2 deficiency usually coexists with other deficiencies, and riboflavin is involved in the metabolism of other B vitamins including B3, B6, B9 (folate), and B12. Thus, the clinical presentation of vitamin B2 deficiency is similar to that of vitamin B3 and B6 deficiency (reviewed above and below) and has been described as pellagra sine pellagra (pellagra without pellagra). Moreover, correction of riboflavin deficiency results in increased levels of vitamin B3 and B6.36
Vitamin B2 levels can be measured in the urine and blood.37 Oral supplementation is safe (up to 60 mg/day) and is the treatment of choice.36,38 Clearance of lesions within 3 to 5 days of riboflavin supplementation confirms the diagnosis.40
Vitamin B3 (niacin) deficiency
Niacin, or vitamin B3, is a water-soluble vitamin abundant in meat, eggs, and legumes. It is an essential cofactor for coenzyme I and coenzyme II; therefore, it plays a crucial role in ATP synthesis, glycolysis, and metabolism of fatty acids and amino acids.41,42
Most niacin is acquired in the diet, but humans can synthesize it from tryptophan in the presence of vitamin B6 and thiamine.42 Thus, a deficiency in tryptophan, vitamin B6, or thiamine can also lead to low niacin, and an excess of dietary leucine can interfere with niacin synthesis and result in deficiency.42
The RDA for niacin is 6 to 20 mg/day, based on sex and age, with higher requirements for pregnant and lactating women.38
Pellagra, the clinical manifestation
Pellagra is the clinical manifestation of niacin deficiency, although it is thought that lack of tryptophan, vitamin B6, or thiamine may also be required for clinical symptoms to appear.41
Sporadic cases of pellagra occur in homeless people, alcoholics, drug abusers, people with anorexia, and food faddists.41,42 Symptoms typically develop after about 50 days of a niacin-free diet.41 Pellagra may also develop due to impaired absorption or metabolism, particularly in patients with prolonged diarrhea, colitis, ileitis, hepatic cirrhosis, or Hartnup disease.42–45 Certain medications, eg, isoniazid, 5-fluorouracil, azathioprine, and 6-mercaptopurine, interfere with niacin synthesis and may induce pellagra in susceptible patients.42
The clinical course of pellagra is often described by the four “Ds”: dermatitis, dementia, diarrhea, and, when not corrected, death. Early symptoms of insufficient vitamin B3 are weakness, fatigue, loss of appetite, depression, and mood changes.42
The cutaneous manifestations of pellagra are impressive and include photosensitive eruptions, perineal lesions, and thickened and pigmented skin.41 Biopsy of affected and unaffected skin in pellagra patients shows abnormal keratinization.
Photosensitivity is an initial manifestation of pellagra.46 It is believed that vitamin B3 deficiency results in a lack of urocanic acid, a compound that protects against ultraviolet B damage and accumulation of kynurenic acid, a known phototoxic agent.47
The initial stage of acute pellagra can resemble a sunburn on the face, neck, and dorsal extremities47 that becomes darker with time instead of fading.46 Sharply demarcated hyperpigmented areas on the arms and legs are known as the “glove” and “boot” of pellagra.46 Nearly all patients have involvement of the dorsum of the hand.42 The Casal necklace may be present, a characteristic eruption observed in up to 76% of patients on the front of the neck in the region of C3-C4.48
As the disease progresses, lesions harden and become brittle—hence, the name pellagra, which means “rough skin.” Perineal lesions are also common, along with fissures and ulcerations. Additionally, about a third of pellagra patients have involvement of the lips, tongue, and oral mucosa.42 Notably, patients with drug-induced or Hartnup-related pellagra do not develop genital, perineal, oral, or hyperkeratotic lesions.46
Although untreated pellagra can lead to death in 5 years,42 the disease responds dramatically to oral nicotinamide (250–500 mg/day), which is preferred over niacin due to the latter’s vasomotor effects.41 Therapy also includes caloric supplementation, other B vitamins, zinc, and magnesium.42
NUTRITIONAL DEFICIENCIES TEND TO COEXIST
The clinical scenarios presented here emphasize how different nutritional deficiencies can manifest with overlapping features. But nutritional deficiencies, particularly those associated with underlying conditions, tend to coexist rather than occur in isolation.
Although associated with significant morbidity, nutritional deficiencies can be easily addressed, particularly when promptly identified. Careful evaluation of the history and clinical and serologic findings is necessary to correctly diagnose and address these conditions.
- Gupta M, Mahajan VK, Mehta KS, Chauhan PS. Zinc therapy in dermatology: a review. Dermatol Res Pract 2014; 2014:709152.
- Kumar P, Lal NR, Mondal AK, Mondal A, Gharami RC, Maiti A. Zinc and skin: a brief summary. Dermatol Online J 2012; 18:1.
- Kiliç I, Ozalp I, Coskun T, et al. The effect of zinc-supplemented bread consumption on school children with asymptomatic zinc deficiency. J Pediatr Gastroenterol Nutr 1998; 26:167–171.
- Küry S, Dréno B, Bézieau S, et al. Identification of SLC39A4, a gene involved in acrodermatitis enteropathica. Nat Genet 2002; 31:239–240.
- Maverakis E, Fung MA, Lynch PJ, et al. Acrodermatitis enteropathica and an overview of zinc metabolism. J Am Acad Dermatol 2007; 56:116–124.
- Younoszai HD. Clinical zinc deficiency in total parenteral nutrition: zinc supplementation. JPEN J Parenter Enteral Nutr 1983; 7:72–74.
- Muñiz AE, Bartle S, Foster R. Edema, anemia, hypoproteinemia, and acrodermatitis enteropathica: an uncommon initial presentation of cystic fibrosis. Pediatr Emerg Care 2004; 20:112–114.
- Corbo MD, Lam J. Zinc deficiency and its management in the pediatric population: a literature review and proposed etiologic classification. J Am Acad Dermatol 2013; 69:616–624.e1.
- Chambial S, Dwivedi S, Shukla KK, John PJ, Sharma P. Vitamin C in disease prevention and cure: an overview. Indian J Clin Biochem 2013; 28:314–328.
- Johnston CS. Biomarkers for establishing a tolerable upper intake level for vitamin C. Nutr Rev 1999; 57:71–77.
- Raimann JG, Levin NW, Craig RG, Sirover W, Kotanko P, Handelman G. Is vitamin C intake too low in dialysis patients? Semin Dial 2013; 26:1–5.
- Institute of Medicine (US) Panel on Dietary Antioxidants and Related Compounds. Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids. Washington, DC: National Academies Press (US); 2000. www.ncbi.nlm.nih.gov/books/NBK225483/. Accessed September 12, 2016.
- Hirschmann JV, Raugi GJ. Adult scurvy. J Am Acad Dermatol 1999; 41:895–906.
- Barthelemy H, Chouvet B, Cambazard F. Skin and mucosal manifestations in vitamin deficiency. J Am Acad Dermatol 1986; 15:1263–1274.
- Léger D. Scurvy: reemergence of nutritional deficiencies. Can Fam Physician 2008; 54:1403–1406.
- Senoo H, Yoshikawa K, Morii M, Miura M, Imai K, Mezaki Y. Hepatic stellate cell (vitamin A-storing cell) and its relative—past, present and future. Cell Biol Int 2010; 34:1247–1272.
- Saari JC. Vitamin A metabolism in rod and cone visual cycles. Annu Rev Nutr 2012; 32:125–145.
- Ross AC. Vitamin A and retinoic acid in T cell–related immunity. Am J Clin Nutr 2012; 96:1166S–1172S.
- King IA, Tabiowo A. The effect of all-trans-retinoic acid on the synthesis of epidermal cell-surface-associated carbohydrates. Biochem J 1981; 194:341–351.
- Kafi R, Kwak HS, Schumacher WE, et al. Improvement of naturally aged skin with vitamin A (retinol). Arch Dermatol 2007; 143:606–612.
- Schiltz JR, Lanigan J, Nabial W, Petty B, Birnbaum JE. Retinoic acid induces cyclic changes in epidermal thickness and dermal collagen and glycosaminoglycan biosynthesis rates. J Invest Dermatol 1986; 87:663–667.
- Ocón J, Cabrejas C, Altemir J, Moros M. Phrynoderma: a rare dermatologic complication of bariatric surgery. JPEN J Parenter Enteral Nutr 2012; 36:361–364.
- Slater GH, Ren CJ, Siegel N, et al. Serum fat-soluble vitamin deficiency and abnormal calcium metabolism after malabsorptive bariatric surgery. J Gastrointest Surg 2004; 8:48–55.
- Nicholls L. Phrynoderma: a condition due to vitamin deficiency. Indian Med Gaz 1933; 68:681–687.
- Ragunatha S, Kumar VJ, Murugesh SB. A clinical study of 125 patients with phrynoderma. Indian J Dermatol 2011; 56:389–392.
- Nakjang Y, Yuttanavivat T. Phrynoderma: a review of 105 cases. J Dermatol 1988; 15:531–534.
- S R, Kumar V J, S B M, M R, G N, Kapoor M. Therapeutic response of vitamin A, vitamin B complex, essential fatty acids (EFA) and vitamin E in the treatment of phrynoderma: a randomized controlled study. J Clin Diagn Res 2014; 8:116–118.
- Spinneker A, Sola R, Lemmen V, Castillo MJ, Pietrzik K, González-Gross M. Vitamin B6 status, deficiency and its consequences—an overview. Nutr Hosp 2007; 22:7–24.
- Lang F, editor. Encyclopedia of Molecular Mechanisms of Disease. Heidelberg, Germany: Springer Berlin Heidelberg; 2009:2217–2218. http://link.springer.com/referenceworkentry/10.1007/978-3-540-29676-8_1853. Accessed September 6, 2016.
- Herrmann W, Knapp JP. Hyperhomocysteinemia: a new risk factor for degenerative diseases. Clin Lab 2002; 48:471–481.
- Haller J, Löwik MR, Ferry M, Ferro-Luzzi A. Nutritional status: blood vitamins A, E, B6, B12, folic acid and carotene. Euronut SENECA investigators. Eur J Clin Nutr 1991; 45(suppl 3):63–82.
- Barthelemy H, Chouvet B, Cambazard F. Skin and mucosal manifestations in vitamin deficiency. J Am Acad Dermatol 1986; 15:1263–1274.
- Inubushi T, Takasawa T, Tuboi Y, Watanabe N, Aki K, Katunuma N. Changes of glucose metabolism and skin-collagen neogenesis in vitamin B6 deficiency. Biofactors 2005; 23:59–67.
- Lui A, Lumeng L, Aronoff GR, Li TK. Relationship between body store of vitamin B6 and plasma pyridoxal-P clearance: metabolic balance studies in humans. J Lab Clin Med 1985; 106:491–497.
- Bajaj AK, Rastogi S, Misra A, Misra K, Bajaj S. Occupational and systemic contact dermatitis with photosensitivity due to vitamin B6. Contact Dermatitis 2001; 44:184.
- Powers HJ. Riboflavin (vitamin B-2) and health. Am J Clin Nutr 2003; 77:1352–1360.
- Graham JM, Peerson JM, Haskell MJ, Shrestha RK, Brown KH, Allen LH. Erythrocyte riboflavin for the detection of riboflavin deficiency in pregnant Nepali women. Clin Chem 2005; 51:2162–2165.
- Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, DC: National Academies Press (US); 1998. www.ncbi.nlm.nih.gov/books/NBK114310/. Accessed September 6, 2016.
- Ryan AS, Goldsmith LA. Nutrition and the skin. Clin Dermatol 1996; 14:389–406.
- Roe DA. Riboflavin deficiency: mucocutaneous signs of acute and chronic deficiency. Semin Dermatol 1991; 10:293–295.
- Karthikeyan K, Thappa DM. Pellagra and skin. Int J Dermatol 2002; 41:476–481.
- Hegyi J, Schwartz RA, Hegyi V. Pellagra: dermatitis, dementia, and diarrhea. Int J Dermatol 2004; 43:1–5.
- Armstrong JR. Pellagra associated with Crohn’s disease. Lancet 1952; 2:1253–1254.
- Oakley A, Wallace J. Hartnup disease presenting in an adult. Clin Exp Dermatol 1994; 19:407–408.
- Lu JY, Yu CL, Wu MZ. Pellagra in an immunocompetent patient with cytomegalovirus colitis. Am J Gastroenterol 2001; 96:932–934.
- Wan P, Moat S, Anstey A. Pellagra: a review with emphasis on photosensitivity. Br J Dermatol 2011; 164:1188–1200.
- Hendricks WM. Pellagra and pellagralike dermatoses: etiology, differential diagnosis, dermatopathology, and treatment. Semin Dermatol 1991; 10:282–292.
- Malfait P, Moren A, Dillon JC, et al. An outbreak of pellagra related to changes in dietary niacin among Mozambican refugees in Malawi. Int J Epidemiol 1993; 22:504–511.
- Gupta M, Mahajan VK, Mehta KS, Chauhan PS. Zinc therapy in dermatology: a review. Dermatol Res Pract 2014; 2014:709152.
- Kumar P, Lal NR, Mondal AK, Mondal A, Gharami RC, Maiti A. Zinc and skin: a brief summary. Dermatol Online J 2012; 18:1.
- Kiliç I, Ozalp I, Coskun T, et al. The effect of zinc-supplemented bread consumption on school children with asymptomatic zinc deficiency. J Pediatr Gastroenterol Nutr 1998; 26:167–171.
- Küry S, Dréno B, Bézieau S, et al. Identification of SLC39A4, a gene involved in acrodermatitis enteropathica. Nat Genet 2002; 31:239–240.
- Maverakis E, Fung MA, Lynch PJ, et al. Acrodermatitis enteropathica and an overview of zinc metabolism. J Am Acad Dermatol 2007; 56:116–124.
- Younoszai HD. Clinical zinc deficiency in total parenteral nutrition: zinc supplementation. JPEN J Parenter Enteral Nutr 1983; 7:72–74.
- Muñiz AE, Bartle S, Foster R. Edema, anemia, hypoproteinemia, and acrodermatitis enteropathica: an uncommon initial presentation of cystic fibrosis. Pediatr Emerg Care 2004; 20:112–114.
- Corbo MD, Lam J. Zinc deficiency and its management in the pediatric population: a literature review and proposed etiologic classification. J Am Acad Dermatol 2013; 69:616–624.e1.
- Chambial S, Dwivedi S, Shukla KK, John PJ, Sharma P. Vitamin C in disease prevention and cure: an overview. Indian J Clin Biochem 2013; 28:314–328.
- Johnston CS. Biomarkers for establishing a tolerable upper intake level for vitamin C. Nutr Rev 1999; 57:71–77.
- Raimann JG, Levin NW, Craig RG, Sirover W, Kotanko P, Handelman G. Is vitamin C intake too low in dialysis patients? Semin Dial 2013; 26:1–5.
- Institute of Medicine (US) Panel on Dietary Antioxidants and Related Compounds. Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids. Washington, DC: National Academies Press (US); 2000. www.ncbi.nlm.nih.gov/books/NBK225483/. Accessed September 12, 2016.
- Hirschmann JV, Raugi GJ. Adult scurvy. J Am Acad Dermatol 1999; 41:895–906.
- Barthelemy H, Chouvet B, Cambazard F. Skin and mucosal manifestations in vitamin deficiency. J Am Acad Dermatol 1986; 15:1263–1274.
- Léger D. Scurvy: reemergence of nutritional deficiencies. Can Fam Physician 2008; 54:1403–1406.
- Senoo H, Yoshikawa K, Morii M, Miura M, Imai K, Mezaki Y. Hepatic stellate cell (vitamin A-storing cell) and its relative—past, present and future. Cell Biol Int 2010; 34:1247–1272.
- Saari JC. Vitamin A metabolism in rod and cone visual cycles. Annu Rev Nutr 2012; 32:125–145.
- Ross AC. Vitamin A and retinoic acid in T cell–related immunity. Am J Clin Nutr 2012; 96:1166S–1172S.
- King IA, Tabiowo A. The effect of all-trans-retinoic acid on the synthesis of epidermal cell-surface-associated carbohydrates. Biochem J 1981; 194:341–351.
- Kafi R, Kwak HS, Schumacher WE, et al. Improvement of naturally aged skin with vitamin A (retinol). Arch Dermatol 2007; 143:606–612.
- Schiltz JR, Lanigan J, Nabial W, Petty B, Birnbaum JE. Retinoic acid induces cyclic changes in epidermal thickness and dermal collagen and glycosaminoglycan biosynthesis rates. J Invest Dermatol 1986; 87:663–667.
- Ocón J, Cabrejas C, Altemir J, Moros M. Phrynoderma: a rare dermatologic complication of bariatric surgery. JPEN J Parenter Enteral Nutr 2012; 36:361–364.
- Slater GH, Ren CJ, Siegel N, et al. Serum fat-soluble vitamin deficiency and abnormal calcium metabolism after malabsorptive bariatric surgery. J Gastrointest Surg 2004; 8:48–55.
- Nicholls L. Phrynoderma: a condition due to vitamin deficiency. Indian Med Gaz 1933; 68:681–687.
- Ragunatha S, Kumar VJ, Murugesh SB. A clinical study of 125 patients with phrynoderma. Indian J Dermatol 2011; 56:389–392.
- Nakjang Y, Yuttanavivat T. Phrynoderma: a review of 105 cases. J Dermatol 1988; 15:531–534.
- S R, Kumar V J, S B M, M R, G N, Kapoor M. Therapeutic response of vitamin A, vitamin B complex, essential fatty acids (EFA) and vitamin E in the treatment of phrynoderma: a randomized controlled study. J Clin Diagn Res 2014; 8:116–118.
- Spinneker A, Sola R, Lemmen V, Castillo MJ, Pietrzik K, González-Gross M. Vitamin B6 status, deficiency and its consequences—an overview. Nutr Hosp 2007; 22:7–24.
- Lang F, editor. Encyclopedia of Molecular Mechanisms of Disease. Heidelberg, Germany: Springer Berlin Heidelberg; 2009:2217–2218. http://link.springer.com/referenceworkentry/10.1007/978-3-540-29676-8_1853. Accessed September 6, 2016.
- Herrmann W, Knapp JP. Hyperhomocysteinemia: a new risk factor for degenerative diseases. Clin Lab 2002; 48:471–481.
- Haller J, Löwik MR, Ferry M, Ferro-Luzzi A. Nutritional status: blood vitamins A, E, B6, B12, folic acid and carotene. Euronut SENECA investigators. Eur J Clin Nutr 1991; 45(suppl 3):63–82.
- Barthelemy H, Chouvet B, Cambazard F. Skin and mucosal manifestations in vitamin deficiency. J Am Acad Dermatol 1986; 15:1263–1274.
- Inubushi T, Takasawa T, Tuboi Y, Watanabe N, Aki K, Katunuma N. Changes of glucose metabolism and skin-collagen neogenesis in vitamin B6 deficiency. Biofactors 2005; 23:59–67.
- Lui A, Lumeng L, Aronoff GR, Li TK. Relationship between body store of vitamin B6 and plasma pyridoxal-P clearance: metabolic balance studies in humans. J Lab Clin Med 1985; 106:491–497.
- Bajaj AK, Rastogi S, Misra A, Misra K, Bajaj S. Occupational and systemic contact dermatitis with photosensitivity due to vitamin B6. Contact Dermatitis 2001; 44:184.
- Powers HJ. Riboflavin (vitamin B-2) and health. Am J Clin Nutr 2003; 77:1352–1360.
- Graham JM, Peerson JM, Haskell MJ, Shrestha RK, Brown KH, Allen LH. Erythrocyte riboflavin for the detection of riboflavin deficiency in pregnant Nepali women. Clin Chem 2005; 51:2162–2165.
- Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, DC: National Academies Press (US); 1998. www.ncbi.nlm.nih.gov/books/NBK114310/. Accessed September 6, 2016.
- Ryan AS, Goldsmith LA. Nutrition and the skin. Clin Dermatol 1996; 14:389–406.
- Roe DA. Riboflavin deficiency: mucocutaneous signs of acute and chronic deficiency. Semin Dermatol 1991; 10:293–295.
- Karthikeyan K, Thappa DM. Pellagra and skin. Int J Dermatol 2002; 41:476–481.
- Hegyi J, Schwartz RA, Hegyi V. Pellagra: dermatitis, dementia, and diarrhea. Int J Dermatol 2004; 43:1–5.
- Armstrong JR. Pellagra associated with Crohn’s disease. Lancet 1952; 2:1253–1254.
- Oakley A, Wallace J. Hartnup disease presenting in an adult. Clin Exp Dermatol 1994; 19:407–408.
- Lu JY, Yu CL, Wu MZ. Pellagra in an immunocompetent patient with cytomegalovirus colitis. Am J Gastroenterol 2001; 96:932–934.
- Wan P, Moat S, Anstey A. Pellagra: a review with emphasis on photosensitivity. Br J Dermatol 2011; 164:1188–1200.
- Hendricks WM. Pellagra and pellagralike dermatoses: etiology, differential diagnosis, dermatopathology, and treatment. Semin Dermatol 1991; 10:282–292.
- Malfait P, Moren A, Dillon JC, et al. An outbreak of pellagra related to changes in dietary niacin among Mozambican refugees in Malawi. Int J Epidemiol 1993; 22:504–511.
KEY POINTS
- Although nutritional deficiencies are relatively uncommon in the general population, certain groups have a higher risk, including infants, pregnant women, alcoholics, vegetarians, persons of poor socioeconomic status, and patients on dialysis, on certain medications, or with a history of malabsorption or gastrointestinal surgery.
- Often, patients present with more than one deficiency.
- Zinc deficiency can result from either inadequate diet or impaired absorption, which can be acquired or inherited.
- The classic manifestations of vitamin C deficiency are scurvy and Barlow disease, also known as infantile scurvy.
- Manifestations of vitamin A deficiency include night-blindness, dry eyes, and phrynoderma (“toad skin”).
- The B-complex vitamins are linked. Vitamin B2 (riboflavin) deficiency usually coexists with other deficiencies, and riboflavin is involved in the metabolism of other B vitamins including B3, B6, B9 (folate), and B12.
Correction: Anemia of chronic kidney disease
The article “Anemia of chronic kidney disease: Treat it, but not too aggressively” by Drs. Georges Nakhoul and James F. Simon (Cleve Clin J Med 2016; 83:613–624) contained a typographical error. In Table 2, the target ferritin level in chronic kidney disease is given as greater than 100 ng/dL, and for end-stage renal disease 200 to 1,200 ng/dL. Ferritin levels are measured in ng/mL, not ng/dL.
The article “Anemia of chronic kidney disease: Treat it, but not too aggressively” by Drs. Georges Nakhoul and James F. Simon (Cleve Clin J Med 2016; 83:613–624) contained a typographical error. In Table 2, the target ferritin level in chronic kidney disease is given as greater than 100 ng/dL, and for end-stage renal disease 200 to 1,200 ng/dL. Ferritin levels are measured in ng/mL, not ng/dL.
The article “Anemia of chronic kidney disease: Treat it, but not too aggressively” by Drs. Georges Nakhoul and James F. Simon (Cleve Clin J Med 2016; 83:613–624) contained a typographical error. In Table 2, the target ferritin level in chronic kidney disease is given as greater than 100 ng/dL, and for end-stage renal disease 200 to 1,200 ng/dL. Ferritin levels are measured in ng/mL, not ng/dL.
In reply: Obstructive sleep apnea
In Reply: We thank Dr. Keller for his thorough reading of our article.1
Regarding the predictive value of neck circumference for obstructive sleep apnea, (OSA), neck circumference is one of many tools to screen for OSA. If neck circumference greater than 38 cm is applied without other predictors (such as the presence of snoring, daytime sleepiness, or elevated body mass index), it provides only a 58% sensitivity and 79% specificity.2 It is less an issue of inches vs collar size vs centimeters than of combining circumference with other parameters (as in the STOP-BANG questionnaire) before proceeding with a sleep study. The senior author of our article (G.R.) uses 38 cm.
With respect to home vs sleep lab monitoring, the question was beyond the scope of the paper and outside our expertise, as we are both general internists. The home venue recommendations in this instance were taken directly from the American Academy of Sleep Medicine.3 We would rely on consultation with a sleep specialist before ordering home monitoring to determine the potential success of non-CPAP interventions for OSA.
As for Parkinson disease as an exception to OSA and hypertension, we wrote in the paper, “Untreated OSA is associated with a number of conditions.”1 Yes, resistant hypertension is prominent in today’s epidemic of obesity, diabetes, and OSA, but not everyone with coronary artery disease, atrial fibrillation, or heart failure—as in persons with Parkinson disease—has hypertension. The associated conditions in our paper are more typical of a general medical practice, but we agree that Parkinson disease is associated with OSA. Patients with hypertension and OSA are more prevalent because the clinical risk factors for OSA and hypertension are common to both conditions.4
In adults, apnea is considered present when the airflow drops by 90% or more from the pre-event baseline. Hypopnea in adults is present when the airflow drops by 30% or more of the pre-event baseline for 10 or more seconds in association with either 3% or greater arterial oxygen desaturation or an electroencephalographic arousal.5 Studies have shown that episodes of hypopnea with 2% oxygen desaturation are associated with an increased prevalence of metabolic impairment.6 A higher degree of desaturation, ie, more than 4%, was associated with increased prevalence of self-reported cardiovascular disease.7 But the significance of episodes of hypopnea without arterial desaturation is not well known to us and was beyond the scope of our article.
Our article was primarily focused on screening for OSA in ambulatory clinical practice and was not intended as a comprehensive review of screening in different settings of patient care. As to the importance of recognizing OSA in patients undergoing elective surgery under general anesthesia, we agree that screening is important to reduce the risk of postoperative adverse respiratory events in patients with a high pretest probability of OSA. In a recent study by Seet et al,8 patients with high STOP-BANG questionnaire scores (≥ 3) had higher rates of intraoperative and early postoperative adverse events than those with low scores (< 3). The risk of adverse events correlated with higher scores, and patients with a STOP-BANG score of 5 or more had a five times greater risk of unexpected intraoperative and early postoperative adverse events, whereas those with a STOP-BANG score of 3 or more had a one in four chance of an adverse event. We recommend polysomnography for patients with a STOP-BANG score of 5 or more before elective surgery.
- Manne MB, Rutecki G. Obstructive sleep apnea: who should be tested, and how? Cleve Clin J Med 2016; 83:25–27.
- Cizza G, de Jonge L, Piaggi P, et al. Neck circumference is a predictor of metabolic syndrome and obstructive sleep apnea in short-sleeping obese men and women. Met Syndr Relat Disord 2014; 12:231–241.
- Collop NA, Anderson WM, Boehlecke B, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. J Clin Sleep Med 2007; 3:737–747.
- Min HJ, Cho Y, Kim C, et al. Clinical features of obstructive sleep apnea that determine its high prevalence in resistant hypertension. Yonsei Med J 2015; 56:1258–1265.
- Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. J Clin Sleep Med 2012; 8:597–619.
- Stamatakis K, Sanders MH, Caffo B, et al. Fasting glycemia in sleep disordered breathing: lowering the threshold on oxyhemoglobin desaturation. Sleep 2008; 31:1018–1024.
- Punjabi NM, Newman AB, Young TB, Resnick HE, Sanders MH. Sleep-disordered breathing and cardiovascular disease: an outcome-based definition of hypopneas. Am J Respir Crit Care Med 2008; 177:1150–1155.
- Seet E, Chua M, Liaw CM. High STOP-BANG questionnaire scores predict intraoperative and early postoperative adverse events. Singapore Med J 2015; 56:212–216.
In Reply: We thank Dr. Keller for his thorough reading of our article.1
Regarding the predictive value of neck circumference for obstructive sleep apnea, (OSA), neck circumference is one of many tools to screen for OSA. If neck circumference greater than 38 cm is applied without other predictors (such as the presence of snoring, daytime sleepiness, or elevated body mass index), it provides only a 58% sensitivity and 79% specificity.2 It is less an issue of inches vs collar size vs centimeters than of combining circumference with other parameters (as in the STOP-BANG questionnaire) before proceeding with a sleep study. The senior author of our article (G.R.) uses 38 cm.
With respect to home vs sleep lab monitoring, the question was beyond the scope of the paper and outside our expertise, as we are both general internists. The home venue recommendations in this instance were taken directly from the American Academy of Sleep Medicine.3 We would rely on consultation with a sleep specialist before ordering home monitoring to determine the potential success of non-CPAP interventions for OSA.
As for Parkinson disease as an exception to OSA and hypertension, we wrote in the paper, “Untreated OSA is associated with a number of conditions.”1 Yes, resistant hypertension is prominent in today’s epidemic of obesity, diabetes, and OSA, but not everyone with coronary artery disease, atrial fibrillation, or heart failure—as in persons with Parkinson disease—has hypertension. The associated conditions in our paper are more typical of a general medical practice, but we agree that Parkinson disease is associated with OSA. Patients with hypertension and OSA are more prevalent because the clinical risk factors for OSA and hypertension are common to both conditions.4
In adults, apnea is considered present when the airflow drops by 90% or more from the pre-event baseline. Hypopnea in adults is present when the airflow drops by 30% or more of the pre-event baseline for 10 or more seconds in association with either 3% or greater arterial oxygen desaturation or an electroencephalographic arousal.5 Studies have shown that episodes of hypopnea with 2% oxygen desaturation are associated with an increased prevalence of metabolic impairment.6 A higher degree of desaturation, ie, more than 4%, was associated with increased prevalence of self-reported cardiovascular disease.7 But the significance of episodes of hypopnea without arterial desaturation is not well known to us and was beyond the scope of our article.
Our article was primarily focused on screening for OSA in ambulatory clinical practice and was not intended as a comprehensive review of screening in different settings of patient care. As to the importance of recognizing OSA in patients undergoing elective surgery under general anesthesia, we agree that screening is important to reduce the risk of postoperative adverse respiratory events in patients with a high pretest probability of OSA. In a recent study by Seet et al,8 patients with high STOP-BANG questionnaire scores (≥ 3) had higher rates of intraoperative and early postoperative adverse events than those with low scores (< 3). The risk of adverse events correlated with higher scores, and patients with a STOP-BANG score of 5 or more had a five times greater risk of unexpected intraoperative and early postoperative adverse events, whereas those with a STOP-BANG score of 3 or more had a one in four chance of an adverse event. We recommend polysomnography for patients with a STOP-BANG score of 5 or more before elective surgery.
In Reply: We thank Dr. Keller for his thorough reading of our article.1
Regarding the predictive value of neck circumference for obstructive sleep apnea, (OSA), neck circumference is one of many tools to screen for OSA. If neck circumference greater than 38 cm is applied without other predictors (such as the presence of snoring, daytime sleepiness, or elevated body mass index), it provides only a 58% sensitivity and 79% specificity.2 It is less an issue of inches vs collar size vs centimeters than of combining circumference with other parameters (as in the STOP-BANG questionnaire) before proceeding with a sleep study. The senior author of our article (G.R.) uses 38 cm.
With respect to home vs sleep lab monitoring, the question was beyond the scope of the paper and outside our expertise, as we are both general internists. The home venue recommendations in this instance were taken directly from the American Academy of Sleep Medicine.3 We would rely on consultation with a sleep specialist before ordering home monitoring to determine the potential success of non-CPAP interventions for OSA.
As for Parkinson disease as an exception to OSA and hypertension, we wrote in the paper, “Untreated OSA is associated with a number of conditions.”1 Yes, resistant hypertension is prominent in today’s epidemic of obesity, diabetes, and OSA, but not everyone with coronary artery disease, atrial fibrillation, or heart failure—as in persons with Parkinson disease—has hypertension. The associated conditions in our paper are more typical of a general medical practice, but we agree that Parkinson disease is associated with OSA. Patients with hypertension and OSA are more prevalent because the clinical risk factors for OSA and hypertension are common to both conditions.4
In adults, apnea is considered present when the airflow drops by 90% or more from the pre-event baseline. Hypopnea in adults is present when the airflow drops by 30% or more of the pre-event baseline for 10 or more seconds in association with either 3% or greater arterial oxygen desaturation or an electroencephalographic arousal.5 Studies have shown that episodes of hypopnea with 2% oxygen desaturation are associated with an increased prevalence of metabolic impairment.6 A higher degree of desaturation, ie, more than 4%, was associated with increased prevalence of self-reported cardiovascular disease.7 But the significance of episodes of hypopnea without arterial desaturation is not well known to us and was beyond the scope of our article.
Our article was primarily focused on screening for OSA in ambulatory clinical practice and was not intended as a comprehensive review of screening in different settings of patient care. As to the importance of recognizing OSA in patients undergoing elective surgery under general anesthesia, we agree that screening is important to reduce the risk of postoperative adverse respiratory events in patients with a high pretest probability of OSA. In a recent study by Seet et al,8 patients with high STOP-BANG questionnaire scores (≥ 3) had higher rates of intraoperative and early postoperative adverse events than those with low scores (< 3). The risk of adverse events correlated with higher scores, and patients with a STOP-BANG score of 5 or more had a five times greater risk of unexpected intraoperative and early postoperative adverse events, whereas those with a STOP-BANG score of 3 or more had a one in four chance of an adverse event. We recommend polysomnography for patients with a STOP-BANG score of 5 or more before elective surgery.
- Manne MB, Rutecki G. Obstructive sleep apnea: who should be tested, and how? Cleve Clin J Med 2016; 83:25–27.
- Cizza G, de Jonge L, Piaggi P, et al. Neck circumference is a predictor of metabolic syndrome and obstructive sleep apnea in short-sleeping obese men and women. Met Syndr Relat Disord 2014; 12:231–241.
- Collop NA, Anderson WM, Boehlecke B, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. J Clin Sleep Med 2007; 3:737–747.
- Min HJ, Cho Y, Kim C, et al. Clinical features of obstructive sleep apnea that determine its high prevalence in resistant hypertension. Yonsei Med J 2015; 56:1258–1265.
- Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. J Clin Sleep Med 2012; 8:597–619.
- Stamatakis K, Sanders MH, Caffo B, et al. Fasting glycemia in sleep disordered breathing: lowering the threshold on oxyhemoglobin desaturation. Sleep 2008; 31:1018–1024.
- Punjabi NM, Newman AB, Young TB, Resnick HE, Sanders MH. Sleep-disordered breathing and cardiovascular disease: an outcome-based definition of hypopneas. Am J Respir Crit Care Med 2008; 177:1150–1155.
- Seet E, Chua M, Liaw CM. High STOP-BANG questionnaire scores predict intraoperative and early postoperative adverse events. Singapore Med J 2015; 56:212–216.
- Manne MB, Rutecki G. Obstructive sleep apnea: who should be tested, and how? Cleve Clin J Med 2016; 83:25–27.
- Cizza G, de Jonge L, Piaggi P, et al. Neck circumference is a predictor of metabolic syndrome and obstructive sleep apnea in short-sleeping obese men and women. Met Syndr Relat Disord 2014; 12:231–241.
- Collop NA, Anderson WM, Boehlecke B, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. J Clin Sleep Med 2007; 3:737–747.
- Min HJ, Cho Y, Kim C, et al. Clinical features of obstructive sleep apnea that determine its high prevalence in resistant hypertension. Yonsei Med J 2015; 56:1258–1265.
- Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. J Clin Sleep Med 2012; 8:597–619.
- Stamatakis K, Sanders MH, Caffo B, et al. Fasting glycemia in sleep disordered breathing: lowering the threshold on oxyhemoglobin desaturation. Sleep 2008; 31:1018–1024.
- Punjabi NM, Newman AB, Young TB, Resnick HE, Sanders MH. Sleep-disordered breathing and cardiovascular disease: an outcome-based definition of hypopneas. Am J Respir Crit Care Med 2008; 177:1150–1155.
- Seet E, Chua M, Liaw CM. High STOP-BANG questionnaire scores predict intraoperative and early postoperative adverse events. Singapore Med J 2015; 56:212–216.
Evolution of heart failure management: Miles to go
The woods are lovely, dark and deep,
But I have promises to keep,
And miles to go before I sleep,
And miles to go before I sleep.
—Robert Frost, “Stopping by Woods on a Snowy Evening”1
Frost's words are simple yet elegant. They can be interpreted many ways. I see the allegory of life as a journey in this poem. The passage, like the woods, is beautiful, but there is a long, long way to go.
And so it is with the treatment of heart failure. There is beauty in our understanding of the syndrome’s physiologic complexities and natural history, and of effective treatments uncovered. Still, we’ve a monstrous climb ahead to get to the summit of this clinical challenge in order to start a real descent.
THE PAST, PRESENT, AND FUTURE OF HEART FAILURE THERAPY
Okwuosa et al,2 in this issue of the Journal, have capably summarized the ABCs of treating heart failure with reduced ejection fraction (also called systolic heart failure), approaching the subject from a perspective on past, present, and future therapies. They summarize heart failure interventions with a guideline-based philosophy, pointing out that these care paths are supposed to be evidence-based. They observe that in the 1960s the standard of care was digitalis, diuretics (furosemide first became available in 1967), and rest. That was about all we had for this problem.
There are now many drugs, devices, and operations that help patients with heart failure. But they never really cure the disease or, more aptly, the syndrome—and therapies are supposed to cure. This limitation of present therapies is important, given the disturbing epidemiology of heart failure, its economic cost, and the suffering of patients. That burden is well detailed.
In addition to curing, the overarching goals of treatment generally are to ameliorate distressing symptoms and to prevent comorbidities. In heart failure with reduced ejection fraction, we want to prevent premature death, stroke, myocardial infarction, congestive states, hospitalization, renal insufficiency, renal failure, cachexia, inanition, feebleness, and respiratory distress, among others.
The ABC mnemonic of Okwuosa et al will help caregivers remember the basics. It is important, however, to put algorithms into proper perspective and to look toward the future.
PROBLEMS WITH EVIDENCE-BASED MEDICINE
Several problems with our current heart failure treatments are rooted in how we perform clinical trials, arguably the premier method of determining truth in clinical practice and the foundation of evidence-based medicine.3,4
Do the trials represent real-world practice?
Were the clinical trials that led to regulatory approval and professional society endorsement of the therapies that we prescribe in our offices done in the same sorts of patients as those in our waiting rooms asking for help? Perhaps, for the most part, they have been. And thus, Okwuosa et al have crafted a work relevant to all of us and every patient.
But I believe there are major gaps in the types of participants enrolled in trials, eg, underrepresentation of certain racial and ethnic groups, not to mention the relative paucity of women. The very elderly (a rapidly growing population) have largely been ignored as well, and participants with significant renal insufficiency, anemia, and diabetes mellitus seem far fewer than what we deal with in a busy clinic.
In addition, Okwuosa et al focus only on patients with reduced left ventricular ejection fraction, a group that makes up only about half of the heart failure crowd.
What about quality of life and other important outcomes?
Clinical trials in heart failure with reduced ejection fraction have generally focused on major clinical end points (primarily, but not exclusively, mortality), to the exclusion of quality of life. Though sometimes included in trials, quality-of-life metrics generally get relegated to second-class seats or ‘tween-deck steerage. Perhaps that is because measuring quality of life can be time-consuming and difficult.
Yet, in the words of sociologist William Bruce Cameron, not everything that counts can be counted, and not everything that can be counted counts. That goes for quality of life.
Lies, damned lies, and P values
Quandaries in data management and analysis include what to do about trial dropouts, study power, precision of statistical analysis, intention-to-treat principles, and choice of the P value that defines significance (or not) for any end point observation. Of course, there are myriad sophisticated mathematical and statistical reasons to justify why we don’t simply count on-treatment participants or allow imputation of results when patients or results drop out, forcing us to worship at the altar of P < .05.
A review of the P value concept5 recently appeared with an accompanying editorial by Kyriacou6 that concluded that “the automatic application of dichotomized hypothesis testing based on prearranged levels of statistical significance should be substituted with a more complex process using effect estimates, confidence intervals, and even P values, thereby permitting scientists, statisticians, and clinicians to use their own inferential capabilities to assign scientific significance.”6
How many great treatments have we tossed out because of rigid reliance on old-fashioned approaches to determining therapeutic evidence? Many treatments studied have had great results in a minority of patients in clinical trials but did not have a major positive (or negative) impact on the overall cohort (with lack of primary end point statistical significance). And what to do when the primary end point is a neutral or negative one but secondary end points are positive? Why not focus more attention on those patients benefiting from an intervention despite the overall results of any trial?
Dilemmas of trials
Other issues are that clinical trials cost too much, and that recruitment and follow-up take too long. Intercurrent therapies (and guidelines) can emerge that jeopardize the trial itself or make observations untimely. The dilemma of stacking therapies one on top of another, often making patient compliance impossible, is another problem with clinical trials. Yet this is how we get to the ABCs.
A NEW WAY TO DO TRIALS
The information provided by Okwuosa et al is useful and encouraging, but too many gaps exist in our heart failure therapies to permit us to celebrate with exuberance. Too many patients still suffer, too many die too young, and the costs are still too great.
Perhaps the future of therapeutic development should embrace different and better ways to demonstrate real value (relying on the equation of value equals outcomes meaningful to patients, divided by cost) of therapies, including the old, the new, the trashed and the underdeveloped. More creative data analysis to reexamine the current tools on the shelf and the ones tried but discarded is essential.
A position paper from the Cardiovascular Round Table of the European Society of Cardiology concluded that “a coordinated effort involving academia, regulators, industry and payors will help to foster better and more effective conduct of clinical cardiovascular trials, supporting earlier availability of innovative therapies and better management of cardiovascular diseases.”7
Lauer and D’Agostino,8 also in an editorial, argued for innovative methods of doing clinical trials and discovering truth about therapies that are applicable to the future of developing treatments for heart failure with reduced ejection fraction. They noted that “the randomized registry trial represents a disruptive technology” and wondered if it will be “given serious consideration as a way to resolve the recognized limitations of current clinical-trial design.”8
Indeed, conducting megatrials with existing megadatabases using a registry format could help. Registries emerging from early adaptive trial design efforts, particularly when Bayesian analysis theory is applied, might help inform clinical experience faster and more efficiently. Bayesian analysis is a statistical approach that attempts to estimate parameters of an underlying distribution of events in an ongoing fashion based on the observed distribution. A clinical trial of stem cell therapies could, at the end of the trial, be turned into a multicenter registry that would continue to inform us about the more real-world application of newer treatment approaches.
Though the therapeutic cupboard for heart failure is certainly not bare, as Okwuosa et al point out, it is wanting. Let’s look for new therapeutic ABCs differently. We should be attacking the real challenge—curing the disease processes that cause the syndrome. Yes, there are miles to go before we sleep.
- Frost R. Stopping by Woods on a Snowy Evening. In: New Hampshire. New York, Henry Holt, 1923.
- Okwuoso IS, Ojeifo O, Nwabueze C, et al. The ABCs of managing systolic heart failure: the past, present and future. Cleve Clin J Med 2016; 83:753–765.
- Samman Tahhan A, Vaduganathan M, Kelkar A, et al. Trends in heart failure clinical trials from 2001–2012. J Card Fail 2016; 22:171–179.
- Cohn JN. Trials and tribulations. J Card Fail 2016; 22:180–181.
- Chavalarias D, Wallach JD, Li AH, Ioannidis JP. Evolution of reporting P values in the biomedical literature, 1990–2015. JAMA 2016; 315:1141–1148.
- Kyriacou DN. The enduring evolution of the P value. JAMA 2016; 315:1113–1115.
- Jackson N, Atar D, Borentain M, et al. Improving clinical trials for cardiovascular diseases: a position paper from the Cardiovascular Round Table of the European Society of Cardiology. Eur Heart J 2016; 37:747–754.
- Lauer MS, D’Agostino RB Sr. The randomized registry trial—the next disruptive technology in clinical research? N Engl J Med 2013; 369:1579–1581.
The woods are lovely, dark and deep,
But I have promises to keep,
And miles to go before I sleep,
And miles to go before I sleep.
—Robert Frost, “Stopping by Woods on a Snowy Evening”1
Frost's words are simple yet elegant. They can be interpreted many ways. I see the allegory of life as a journey in this poem. The passage, like the woods, is beautiful, but there is a long, long way to go.
And so it is with the treatment of heart failure. There is beauty in our understanding of the syndrome’s physiologic complexities and natural history, and of effective treatments uncovered. Still, we’ve a monstrous climb ahead to get to the summit of this clinical challenge in order to start a real descent.
THE PAST, PRESENT, AND FUTURE OF HEART FAILURE THERAPY
Okwuosa et al,2 in this issue of the Journal, have capably summarized the ABCs of treating heart failure with reduced ejection fraction (also called systolic heart failure), approaching the subject from a perspective on past, present, and future therapies. They summarize heart failure interventions with a guideline-based philosophy, pointing out that these care paths are supposed to be evidence-based. They observe that in the 1960s the standard of care was digitalis, diuretics (furosemide first became available in 1967), and rest. That was about all we had for this problem.
There are now many drugs, devices, and operations that help patients with heart failure. But they never really cure the disease or, more aptly, the syndrome—and therapies are supposed to cure. This limitation of present therapies is important, given the disturbing epidemiology of heart failure, its economic cost, and the suffering of patients. That burden is well detailed.
In addition to curing, the overarching goals of treatment generally are to ameliorate distressing symptoms and to prevent comorbidities. In heart failure with reduced ejection fraction, we want to prevent premature death, stroke, myocardial infarction, congestive states, hospitalization, renal insufficiency, renal failure, cachexia, inanition, feebleness, and respiratory distress, among others.
The ABC mnemonic of Okwuosa et al will help caregivers remember the basics. It is important, however, to put algorithms into proper perspective and to look toward the future.
PROBLEMS WITH EVIDENCE-BASED MEDICINE
Several problems with our current heart failure treatments are rooted in how we perform clinical trials, arguably the premier method of determining truth in clinical practice and the foundation of evidence-based medicine.3,4
Do the trials represent real-world practice?
Were the clinical trials that led to regulatory approval and professional society endorsement of the therapies that we prescribe in our offices done in the same sorts of patients as those in our waiting rooms asking for help? Perhaps, for the most part, they have been. And thus, Okwuosa et al have crafted a work relevant to all of us and every patient.
But I believe there are major gaps in the types of participants enrolled in trials, eg, underrepresentation of certain racial and ethnic groups, not to mention the relative paucity of women. The very elderly (a rapidly growing population) have largely been ignored as well, and participants with significant renal insufficiency, anemia, and diabetes mellitus seem far fewer than what we deal with in a busy clinic.
In addition, Okwuosa et al focus only on patients with reduced left ventricular ejection fraction, a group that makes up only about half of the heart failure crowd.
What about quality of life and other important outcomes?
Clinical trials in heart failure with reduced ejection fraction have generally focused on major clinical end points (primarily, but not exclusively, mortality), to the exclusion of quality of life. Though sometimes included in trials, quality-of-life metrics generally get relegated to second-class seats or ‘tween-deck steerage. Perhaps that is because measuring quality of life can be time-consuming and difficult.
Yet, in the words of sociologist William Bruce Cameron, not everything that counts can be counted, and not everything that can be counted counts. That goes for quality of life.
Lies, damned lies, and P values
Quandaries in data management and analysis include what to do about trial dropouts, study power, precision of statistical analysis, intention-to-treat principles, and choice of the P value that defines significance (or not) for any end point observation. Of course, there are myriad sophisticated mathematical and statistical reasons to justify why we don’t simply count on-treatment participants or allow imputation of results when patients or results drop out, forcing us to worship at the altar of P < .05.
A review of the P value concept5 recently appeared with an accompanying editorial by Kyriacou6 that concluded that “the automatic application of dichotomized hypothesis testing based on prearranged levels of statistical significance should be substituted with a more complex process using effect estimates, confidence intervals, and even P values, thereby permitting scientists, statisticians, and clinicians to use their own inferential capabilities to assign scientific significance.”6
How many great treatments have we tossed out because of rigid reliance on old-fashioned approaches to determining therapeutic evidence? Many treatments studied have had great results in a minority of patients in clinical trials but did not have a major positive (or negative) impact on the overall cohort (with lack of primary end point statistical significance). And what to do when the primary end point is a neutral or negative one but secondary end points are positive? Why not focus more attention on those patients benefiting from an intervention despite the overall results of any trial?
Dilemmas of trials
Other issues are that clinical trials cost too much, and that recruitment and follow-up take too long. Intercurrent therapies (and guidelines) can emerge that jeopardize the trial itself or make observations untimely. The dilemma of stacking therapies one on top of another, often making patient compliance impossible, is another problem with clinical trials. Yet this is how we get to the ABCs.
A NEW WAY TO DO TRIALS
The information provided by Okwuosa et al is useful and encouraging, but too many gaps exist in our heart failure therapies to permit us to celebrate with exuberance. Too many patients still suffer, too many die too young, and the costs are still too great.
Perhaps the future of therapeutic development should embrace different and better ways to demonstrate real value (relying on the equation of value equals outcomes meaningful to patients, divided by cost) of therapies, including the old, the new, the trashed and the underdeveloped. More creative data analysis to reexamine the current tools on the shelf and the ones tried but discarded is essential.
A position paper from the Cardiovascular Round Table of the European Society of Cardiology concluded that “a coordinated effort involving academia, regulators, industry and payors will help to foster better and more effective conduct of clinical cardiovascular trials, supporting earlier availability of innovative therapies and better management of cardiovascular diseases.”7
Lauer and D’Agostino,8 also in an editorial, argued for innovative methods of doing clinical trials and discovering truth about therapies that are applicable to the future of developing treatments for heart failure with reduced ejection fraction. They noted that “the randomized registry trial represents a disruptive technology” and wondered if it will be “given serious consideration as a way to resolve the recognized limitations of current clinical-trial design.”8
Indeed, conducting megatrials with existing megadatabases using a registry format could help. Registries emerging from early adaptive trial design efforts, particularly when Bayesian analysis theory is applied, might help inform clinical experience faster and more efficiently. Bayesian analysis is a statistical approach that attempts to estimate parameters of an underlying distribution of events in an ongoing fashion based on the observed distribution. A clinical trial of stem cell therapies could, at the end of the trial, be turned into a multicenter registry that would continue to inform us about the more real-world application of newer treatment approaches.
Though the therapeutic cupboard for heart failure is certainly not bare, as Okwuosa et al point out, it is wanting. Let’s look for new therapeutic ABCs differently. We should be attacking the real challenge—curing the disease processes that cause the syndrome. Yes, there are miles to go before we sleep.
The woods are lovely, dark and deep,
But I have promises to keep,
And miles to go before I sleep,
And miles to go before I sleep.
—Robert Frost, “Stopping by Woods on a Snowy Evening”1
Frost's words are simple yet elegant. They can be interpreted many ways. I see the allegory of life as a journey in this poem. The passage, like the woods, is beautiful, but there is a long, long way to go.
And so it is with the treatment of heart failure. There is beauty in our understanding of the syndrome’s physiologic complexities and natural history, and of effective treatments uncovered. Still, we’ve a monstrous climb ahead to get to the summit of this clinical challenge in order to start a real descent.
THE PAST, PRESENT, AND FUTURE OF HEART FAILURE THERAPY
Okwuosa et al,2 in this issue of the Journal, have capably summarized the ABCs of treating heart failure with reduced ejection fraction (also called systolic heart failure), approaching the subject from a perspective on past, present, and future therapies. They summarize heart failure interventions with a guideline-based philosophy, pointing out that these care paths are supposed to be evidence-based. They observe that in the 1960s the standard of care was digitalis, diuretics (furosemide first became available in 1967), and rest. That was about all we had for this problem.
There are now many drugs, devices, and operations that help patients with heart failure. But they never really cure the disease or, more aptly, the syndrome—and therapies are supposed to cure. This limitation of present therapies is important, given the disturbing epidemiology of heart failure, its economic cost, and the suffering of patients. That burden is well detailed.
In addition to curing, the overarching goals of treatment generally are to ameliorate distressing symptoms and to prevent comorbidities. In heart failure with reduced ejection fraction, we want to prevent premature death, stroke, myocardial infarction, congestive states, hospitalization, renal insufficiency, renal failure, cachexia, inanition, feebleness, and respiratory distress, among others.
The ABC mnemonic of Okwuosa et al will help caregivers remember the basics. It is important, however, to put algorithms into proper perspective and to look toward the future.
PROBLEMS WITH EVIDENCE-BASED MEDICINE
Several problems with our current heart failure treatments are rooted in how we perform clinical trials, arguably the premier method of determining truth in clinical practice and the foundation of evidence-based medicine.3,4
Do the trials represent real-world practice?
Were the clinical trials that led to regulatory approval and professional society endorsement of the therapies that we prescribe in our offices done in the same sorts of patients as those in our waiting rooms asking for help? Perhaps, for the most part, they have been. And thus, Okwuosa et al have crafted a work relevant to all of us and every patient.
But I believe there are major gaps in the types of participants enrolled in trials, eg, underrepresentation of certain racial and ethnic groups, not to mention the relative paucity of women. The very elderly (a rapidly growing population) have largely been ignored as well, and participants with significant renal insufficiency, anemia, and diabetes mellitus seem far fewer than what we deal with in a busy clinic.
In addition, Okwuosa et al focus only on patients with reduced left ventricular ejection fraction, a group that makes up only about half of the heart failure crowd.
What about quality of life and other important outcomes?
Clinical trials in heart failure with reduced ejection fraction have generally focused on major clinical end points (primarily, but not exclusively, mortality), to the exclusion of quality of life. Though sometimes included in trials, quality-of-life metrics generally get relegated to second-class seats or ‘tween-deck steerage. Perhaps that is because measuring quality of life can be time-consuming and difficult.
Yet, in the words of sociologist William Bruce Cameron, not everything that counts can be counted, and not everything that can be counted counts. That goes for quality of life.
Lies, damned lies, and P values
Quandaries in data management and analysis include what to do about trial dropouts, study power, precision of statistical analysis, intention-to-treat principles, and choice of the P value that defines significance (or not) for any end point observation. Of course, there are myriad sophisticated mathematical and statistical reasons to justify why we don’t simply count on-treatment participants or allow imputation of results when patients or results drop out, forcing us to worship at the altar of P < .05.
A review of the P value concept5 recently appeared with an accompanying editorial by Kyriacou6 that concluded that “the automatic application of dichotomized hypothesis testing based on prearranged levels of statistical significance should be substituted with a more complex process using effect estimates, confidence intervals, and even P values, thereby permitting scientists, statisticians, and clinicians to use their own inferential capabilities to assign scientific significance.”6
How many great treatments have we tossed out because of rigid reliance on old-fashioned approaches to determining therapeutic evidence? Many treatments studied have had great results in a minority of patients in clinical trials but did not have a major positive (or negative) impact on the overall cohort (with lack of primary end point statistical significance). And what to do when the primary end point is a neutral or negative one but secondary end points are positive? Why not focus more attention on those patients benefiting from an intervention despite the overall results of any trial?
Dilemmas of trials
Other issues are that clinical trials cost too much, and that recruitment and follow-up take too long. Intercurrent therapies (and guidelines) can emerge that jeopardize the trial itself or make observations untimely. The dilemma of stacking therapies one on top of another, often making patient compliance impossible, is another problem with clinical trials. Yet this is how we get to the ABCs.
A NEW WAY TO DO TRIALS
The information provided by Okwuosa et al is useful and encouraging, but too many gaps exist in our heart failure therapies to permit us to celebrate with exuberance. Too many patients still suffer, too many die too young, and the costs are still too great.
Perhaps the future of therapeutic development should embrace different and better ways to demonstrate real value (relying on the equation of value equals outcomes meaningful to patients, divided by cost) of therapies, including the old, the new, the trashed and the underdeveloped. More creative data analysis to reexamine the current tools on the shelf and the ones tried but discarded is essential.
A position paper from the Cardiovascular Round Table of the European Society of Cardiology concluded that “a coordinated effort involving academia, regulators, industry and payors will help to foster better and more effective conduct of clinical cardiovascular trials, supporting earlier availability of innovative therapies and better management of cardiovascular diseases.”7
Lauer and D’Agostino,8 also in an editorial, argued for innovative methods of doing clinical trials and discovering truth about therapies that are applicable to the future of developing treatments for heart failure with reduced ejection fraction. They noted that “the randomized registry trial represents a disruptive technology” and wondered if it will be “given serious consideration as a way to resolve the recognized limitations of current clinical-trial design.”8
Indeed, conducting megatrials with existing megadatabases using a registry format could help. Registries emerging from early adaptive trial design efforts, particularly when Bayesian analysis theory is applied, might help inform clinical experience faster and more efficiently. Bayesian analysis is a statistical approach that attempts to estimate parameters of an underlying distribution of events in an ongoing fashion based on the observed distribution. A clinical trial of stem cell therapies could, at the end of the trial, be turned into a multicenter registry that would continue to inform us about the more real-world application of newer treatment approaches.
Though the therapeutic cupboard for heart failure is certainly not bare, as Okwuosa et al point out, it is wanting. Let’s look for new therapeutic ABCs differently. We should be attacking the real challenge—curing the disease processes that cause the syndrome. Yes, there are miles to go before we sleep.
- Frost R. Stopping by Woods on a Snowy Evening. In: New Hampshire. New York, Henry Holt, 1923.
- Okwuoso IS, Ojeifo O, Nwabueze C, et al. The ABCs of managing systolic heart failure: the past, present and future. Cleve Clin J Med 2016; 83:753–765.
- Samman Tahhan A, Vaduganathan M, Kelkar A, et al. Trends in heart failure clinical trials from 2001–2012. J Card Fail 2016; 22:171–179.
- Cohn JN. Trials and tribulations. J Card Fail 2016; 22:180–181.
- Chavalarias D, Wallach JD, Li AH, Ioannidis JP. Evolution of reporting P values in the biomedical literature, 1990–2015. JAMA 2016; 315:1141–1148.
- Kyriacou DN. The enduring evolution of the P value. JAMA 2016; 315:1113–1115.
- Jackson N, Atar D, Borentain M, et al. Improving clinical trials for cardiovascular diseases: a position paper from the Cardiovascular Round Table of the European Society of Cardiology. Eur Heart J 2016; 37:747–754.
- Lauer MS, D’Agostino RB Sr. The randomized registry trial—the next disruptive technology in clinical research? N Engl J Med 2013; 369:1579–1581.
- Frost R. Stopping by Woods on a Snowy Evening. In: New Hampshire. New York, Henry Holt, 1923.
- Okwuoso IS, Ojeifo O, Nwabueze C, et al. The ABCs of managing systolic heart failure: the past, present and future. Cleve Clin J Med 2016; 83:753–765.
- Samman Tahhan A, Vaduganathan M, Kelkar A, et al. Trends in heart failure clinical trials from 2001–2012. J Card Fail 2016; 22:171–179.
- Cohn JN. Trials and tribulations. J Card Fail 2016; 22:180–181.
- Chavalarias D, Wallach JD, Li AH, Ioannidis JP. Evolution of reporting P values in the biomedical literature, 1990–2015. JAMA 2016; 315:1141–1148.
- Kyriacou DN. The enduring evolution of the P value. JAMA 2016; 315:1113–1115.
- Jackson N, Atar D, Borentain M, et al. Improving clinical trials for cardiovascular diseases: a position paper from the Cardiovascular Round Table of the European Society of Cardiology. Eur Heart J 2016; 37:747–754.
- Lauer MS, D’Agostino RB Sr. The randomized registry trial—the next disruptive technology in clinical research? N Engl J Med 2013; 369:1579–1581.
Nonatherosclerotic limb ischemia: Prompt evaluation and diagnosis
Timely diagnosis of limb ischemia is critical to limb health and limb salvage. The cause in most cases is related to atherosclerosis, and patients with limb ischemia are usually older and have risk factors for atherosclerosis, such as smoking, diabetes, hypertension, hyperlipidemia, and coronary artery disease. When younger patients develop limb ischemia, the diagnosis is often delayed since the index of suspicion is quite low in the absence of the usual risk factors.
Here, we discuss several nonatherosclerotic causes of limb ischemia: popliteal artery entrapment syndrome, popliteal artery aneurysm, cystic adventitial disease, persistent sciatic artery, phlegmasia cerulea dolens, Buerger disease, Takayasu arteritis, arterial thoracic outlet syndrome, and external iliac endofibrosis (Table 1). Our goal is to help clinicians make a timely diagnosis and ultimately save the patient’s limb.
POPLITEAL ARTERY ENTRAPMENT SYNDROME
Popliteal artery entrapment syndrome occurs when the popliteal artery becomes compressed in the popliteal fossa, particularly during exercise.1,2 The underlying problem may be that the popliteal artery has an aberrant course lateral to the medial head of the gastrocnemius muscle, or the medial head of the gastrocnemius may have an abnormal insertion, or there may be fibrous bands in the popliteal fossa, or a combination of these (Figure 1).1–3 Functional popliteal artery entrapment syndrome occurs when there is compression of the artery without an anatomic cause.1–3
The classic clinical presentation is a young athletic patient with calf or foot claudication (crampy pain with exercise, relieved with rest), but other symptoms can include coldness, paresthesias, and numbness. Pain at rest and tissue loss are rare on presentation but may develop if the diagnosis and treatment are delayed.3
Continued compression and microtrauma to the artery may lead to an intramural hematoma, thrombus formation, aneurysmal degeneration, dissection, or even acute thrombosis.2 If the diagnosis is delayed, the patient’s condition may progress from intermittent arterial compression with plantar flexion to complete arterial thrombosis and critical limb ischemia, putting the patient at risk of limb loss.
Diagnosing popliteal artery entrapment syndrome
The diagnostic workup includes a detailed history with a focus on the cause of pain (usually exercise), a comprehensive physical examination that includes looking for wounds, and a thorough pulse examination.
The workup should start with noninvasive imaging such as duplex arterial ultrasonography with and without provocative measures (plantar flexion), the ankle-brachial index with and without provocative measures, and exercise treadmill testing with ankle-brachial index measurement.1,2 Plantar flexion may be necessary to elicit arterial compression that is usually absent at rest.
Magnetic resonance imaging (MRI) and computed tomography (CT) of the lower extremity are useful to identify an arterial abnormality and aberrant muscle anatomy1,3; MRI is currently the gold standard for delineating the muscles of the popliteal fossa.4 If these studies do not shed light on the diagnosis, arterial angiography with and without provocative maneuvers is useful in identifying compression of the popliteal artery.1–3
Treating popliteal artery entrapment syndrome
Treatment depends on the level of arterial injury.
For patients with symptoms but no evidence of arterial injury, the most common procedure offered is popliteal fossa decompression.1–3 This involves surgical release of the medial head of the gastrocnemius muscle and other muscles compressing the popliteal artery.
For patients with evidence of arterial injury such as stenosis, dissection, or aneurysm, bypass grafting may be required.
For patients who present with acute limb ischemia, both surgical thrombectomy with possible bypass and intraarterial lysis have been described.1,2,5
POPLITEAL ARTERY ANEURYSM
Popliteal artery aneurysm (Figure 2) is the most common type of aneurysm of the peripheral arteries of the lower extremity and is present in about 1% of men over age 65. Fifty percent are bilateral, and 50% are associated with an abdominal aortic aneurysm.6,7 While up to 80% patients with this type of aneurysm have no symptoms at the time of diagnosis, symptoms develop at a rate of 14% per year, with acute limb ischemia occurring in up to one-third of cases.6,7
When popliteal artery aneurysm progresses to acute limb ischemia, the consequences are often deleterious, as the tibial arteries distal to the popliteal artery are often occluded, limiting treatment options.
Popliteal artery aneurysm is defined as a local dilation of the artery of 2 cm or greater or an increase in the diameter to 1.5 times normal.6
Acute thrombosis of the aneurysm with limb ischemia is the most common presenting symptom and occurs in 50% of symptomatic cases of popliteal artery aneurysm.7 Almost 25% of patients present with intermittent claudication secondary to thrombosis, partial thrombosis with distal embolization, or combined aneurysmal and atherosclerotic disease. Compression of the popliteal vein by the popliteal artery aneurysm can cause leg swelling with or without deep vein thrombosis in up to 5% of patients.6 Rupture is very rare, with a rate of 2% to 4%.6,7
Diagnosing popliteal artery aneurysm
The diagnosis can be made with arterial duplex ultrasonography, which is also useful for follow-up surveillance.6–8 In the acute setting, computed tomographic angiography (CTA) or magnetic resonance angiography (MRA) is useful not only to identify the popliteal aneurysm, but also to define the distal tibial outflow vessels.6,7
Treating popliteal artery aneurysm
Management of an acutely thrombosed popliteal artery aneurysm starts with systemic anticoagulation with intravenous heparin, followed initially by arterial angiography and lysis.8–11 This approach has been shown to be safe and effective even in the absence of arterial runoff distal to the thrombosed popliteal aneurysm. Conversion to open thrombectomy and bypass can be done if initial lytic therapy fails, if the patient develops complications of lytic therapy, or if the patient needs emergency revascularization because of motor and neurologic deficits in the affected extremity.8,10,11
How to manage the asymptomatic patient depends on the size of the aneurysm. Most studies recommend 2 cm or larger as the criterion for repair,6–8,12 while others suggest treating even smaller aneurysms if thrombus is detected.9 Preoperative imaging before elective treatment of an asymptomatic popliteal artery aneurysm includes either CTA or MRA,8,10 which allows the surgeon to visualize the full extent of the aneurysm to best plan the surgical approach. Diagnostic angiography can help determine the most suitable bypass target and can better characterize tibial outflow.
Asymptomatic popliteal artery aneurysm has traditionally been treated with surgical bypass with exclusion of the aneurysm,6–8,12 but more recently, endovascular approaches using self-expanding stent grafts have been described. Further study is needed to determine the long-term efficacy of the endovascular approach.8,10
CYSTIC ADVENTITIAL DISEASE
Cystic adventitial disease is a rare condition in which a blood vessel is narrowed due to mucin-containing cysts in the adventitia. More than 80% of cases occur in the popliteal artery, but it has been described in other peripheral arteries and veins.13,14 It is more common in men than in women and typically occurs in the 4th or 5th decade of life. Most patients present with the sudden onset of calf claudication without the usual risk factors for peripheral vascular disease.13
Diagnosing cystic adventitial disease
Noninvasive arterial or venous duplex ultrasonography can be a good screening tool, as the cysts appear hypoechoic, but results are operator-dependent. CTA and MRA are the imaging tests of choice, as they can detect the cystic lesions and define vessel anatomy for intervention. Diagnostic angiography does not show the cysts themselves but instead reveals a classic “hourglass” and “scimitar” pattern of arterial narrowing that suggests the underlying pathology.13,14
Treating cystic adventitial disease
Usual treatment is complete cyst resection and vessel reconstruction by surgical bypass. Other therapies include open surgical cyst evacuation and removal of the cyst wall, open surgical cyst aspiration, aspiration guided by ultrasonography or CT, and percutaneous angioplasty. However, these nonsurgical treatments have not been shown to be as effective and long-lasting as cyst excision and bypass.13,14
PERSISTENT SCIATIC ARTERY
Persistent sciatic artery is a rare developmental abnormality.15–17 Normally, as the femoral artery develops in the embryo, the sciatic artery involutes to form the inferior gluteal artery. But if the femoral system fails to mature, the sciatic artery, which is adjacent to the sciatic nerve posteriorly as it goes through the sciatic foramen, persists and functions as the major artery supplying the lower extremity, continuing to the posterior thigh and joining the popliteal artery (Figure 3).15,17
Persistent sciatic artery has an incidence of 2.5 to 4 per 10,000 per year15 and is bilateral in almost half of cases.16 Up to 40% of patients have no symptoms, but symptoms may develop by age 40 to 50. Because of repeated trauma to the vessel as it passes through the sciatic foramen,18 the persistent sciatic artery typically sustains accelerated atherosclerotic changes that make it susceptible to aneurysm formation,15 and up to 46% of patients present with aneurysmal degeneration.17
Classically, patients present with lower extremity ischemia from atherosclerotic changes in the persistent sciatic artery or aneurysmal degeneration and thromboembolism.15 Rarely, these aneurysms rupture.15,17 Other signs and symptoms include a pulsatile mass in the buttock, lower extremity numbness, motor weakness, and radicular pain along the sciatic nerve distribution from nerve compression.15–17
Physical findings vary but are distinguished by the lack of femoral pulses in the presence of pedal pulses. A pulsatile buttock mass with evidence of lower extremity nerve compression or limb ischemia or both is pathognomonic of a persistent sciatic artery aneurysm.16,18
Diagnosing persistent sciatic artery
Diagnostic angiography is the gold standard imaging test,15,19 although CTA is starting to replace it.16,18
Treating persistent sciatic artery
Persistent sciatic artery that is asymptomatic and is found incidentally does not require repair; however, it should be followed with duplex ultrasonography to look for evidence of aneurysm degeneration. Degeneration requires repair in most cases.15,16,18,19 When the persistent sciatic artery is the only blood supply to the distal extremity, open aneurysm excision and bypass is the treatment of choice.15,16,19 If collateral flow is adequate, endovascular coil embolization is an option.15 Endovascular stent graft placement has also been described.16,19
PHLEGMASIA CERULEA DOLENS
Phlegmasia cerulea dolens is a rare syndrome caused by extensive acute thrombosis of the ileofemoral vein.20–23 It is defined as total or near-total occlusion of the venous outflow of an extremity, causing massive swelling and congestion that impedes arterial inflow.20,22
Phlegmasia cerulea dolens is associated with four cardinal signs: edema, violaceous discoloration, pain, and severe venous outflow obstruction (Figure 4).22 Patients present with sudden onset of lower extremity pain, swelling, cyanosis, and arterial ischemia with or without loss of distal pulses.20,22
This syndrome can progress to gangrene and massive fluid sequestration leading to shock and death.21–23 From 25% to 40% of patients die, and of those who survive, 20% to 50% require amputation of the limb.20,23
Risk factors include malignancy, immobility, heart failure, heparin-induced thrombocytopenia, antiphospholipid syndrome, pregnancy, venous catheterization (eg, to insert an inferior vena cava filter), and surgery.20–22
Diagnosing phlegmasia cerulea dolens
The diagnosis is made on clinical suspicion with evidence of iliofemoral deep vein thrombosis. Most experts suggest venous duplex ultrasonography to identify the deep vein thrombosis,23 although CT or MR venography can be used to better delineate the proximal extent of the thrombus.20,23
Treating phlegmasia cerulea dolens
Initial management is aggressive fluid resuscitation, elevation of the affected limb, strict bed rest, and anticoagulation with intravenous heparin.20,23 Interventions are aimed at urgently restoring venous outflow to prevent progression to venous gangrene and limb loss.
Although conservative therapy can succeed by itself,23 if the condition does not improve or has already progressed to an advanced stage, the two mainstays of treatment are open venous thrombectomy and endovascular treatment.21–23 Endovascular treatment includes catheter-directed thrombolytic therapy (with or without percutaneous mechanical or pharmacomechanical thrombectomy) and stenting.20,23 The success rate for endovascular therapy can be as high as 90% with near-complete resolution of thrombosis.20 A disadvantage is that, compared with open surgical thrombectomy, more time is needed to achieve venous outflow.20,22
If endovascular therapy is ineffective, if lytic therapy is contraindicated, or if the disease has progressed to gangrene, open surgical thrombectomy with possible fasciotomy is the preferred option.20,21,23 Open surgery has the advantage of restoring venous outflow faster, but disadvantages include the inability to open the smaller veins of the extremity, blood loss, and risks associated with general anesthesia.20–22
BUERGER DISEASE
Buerger disease (thromboangiitis obliterans) is a nonatherosclerotic segmental inflammatory disease involving the small and medium-sized vessels of the arms and legs.24–27 It is differentiated from other vasculitides by its marked male predominance, its close association with smoking, the rarity of systemic signs and symptoms, and the absence of elevated inflammatory markers.26
The rate of major amputation is reported to be 11% at 5 years and 23% at 20 years.24
The classic patient is a young male smoker with symptoms of arterial disease before age 45.24,26 Patients can present with migratory thrombophlebitis or signs of arterial insufficiency in the upper or lower extremities. Two or more limbs are commonly involved. Arterial insufficiency can range from claudication and exertional discomfort of the extremity to ischemic pain at rest leading to ulceration of the distal fingers and toes. Physical findings are similar to those seen in peripheral vascular disease and arterial insufficiency, with decreased arterial brachial index, cool extremities, and wounds.
Diagnosing Buerger disease
- The Shionoya diagnostic criteria for Buerger disease are the following five clinical features24,27:
- History of smoking
- Onset before age 50
- Infrapopliteal arterial occlusive disease
- Upper-limb involvement or phlebitis migrans
- Absence of atherosclerotic risk factors other than heavy smoking.
Various other major and minor criteria have been described to make the diagnosis as well.24
There is no specific laboratory test to confirm the diagnosis of Buerger disease. A full panel of laboratory tests should be sent to rule out other causes of arterial insufficiency and vasculitides; these tests should include C-reactive protein, rheumatoid factor, erythrocyte sedimentation rate, antinuclear antibodies, antiphospholipid antibodies, anti-Scl-70 antibodies, anticentromere antibodies, complement level measurement, and hypercoagulability workup.
Imaging studies include arterial duplex ultrasonography with ankle-brachial indices and segmental pressures and CTA or MRA.26 Angiography can show a “corkscrew” pattern of occlusive disease and collateral formation, which is highly associated with Buerger disease.24
Treating Buerger disease
The only treatment shown to reduce the risk of amputation is complete abstention from tobacco and nicotine (smoking, secondhand smoke, and nicotine patches and gum).24,26
Symptoms of claudication can be managed with aspirin, clopidogrel, vasodilators, pentoxifylline, and cilostazol.26
Surgical bypass is rarely an option, as Buerger disease typically affects the distal blood vessels, thus precluding bypass, and the 5-year patency rate is only 49%.26 Other treatments including arterial thrombolysis, sympathectomy, stem cell injection, spinal cord stimulators, omental grafting, and immunomodulation have been described, but there are only limited data to offer guidance in choosing the appropriate one.24
TAKAYASU ARTERITIS
Takayasu arteritis is a form of vasculitis involving the aorta and its main branches (Figure 5).28 Although seen around the world, it has a higher incidence in young Asian women. Patients can present with systemic symptoms such as fever, fatigue, vague pain, and cardinal signs of limb ischemia associated with Takayasu arteritis, such as weak or absent pulses, differences between the arms in pulses and blood pressures, unobtainable blood pressure measurement in one or both arms, limb fatigability, and pain.28
Diagnosing Takayasu arteritis
Multiple diagnostic criteria have been proposed to define Takayasu arteritis.28 CTA, MRA, and positron emission tomography have replaced invasive angiography as the diagnostic imaging tests of choice.29
Treating Takayasu arteritis
Takayasu arteritis has an acute and chronic course. Interventions are typically reserved for severe cases, with indications that include uncontrollable hypertension from renal artery stenosis, severe coronary or cerebrovascular disease, severe aortic regurgitation or coarctation, stenotic or occlusive lesions resulting in critical limb ischemia, and aneurysm at risk of rupture.28–30
THORACIC OUTLET SYNDROME
Thoracic outlet syndrome is compression of the brachial plexus, subclavian vein, or subclavian artery as it exits the thoracic outlet through an area known as the scalene triangle, which is bordered by the anterior scalene, first rib, and clavicle.31 Presenting symptoms depend on the structure compressed.
By far the most common presentation32 is neurogenic thoracic outlet syndrome, accounting for more than 90% of cases, followed by venous thoracic outlet syndrome. Arterial thoracic outlet syndrome is the least frequent at less than 1%, but carries the greatest morbidity with potential for limb loss.31–33
The subclavian artery exits the thoracic outlet between the anterior and middle scalene muscles, and then travels over the first rib and underneath the clavicle.31 Repeated trauma from compression of the artery results in intimal injury leading to compression, stenosis, occlusion, or aneurysm formation.31,32
Symptoms of arterial thoracic outlet syndrome can start out as effort fatigue of the upper extremity secondary to compression. These symptoms are usually vague and difficult to define,31 as these patients typically are young and do not have atherosclerotic risk factors that would prompt suspicion of a vascular cause.
The most common presentation of arterial thoracic outlet syndrome is upper extremity embolization from a partially thrombosed aneurysm or area of stenosis with ischemia.32 Symptoms can range from ischemia of the fingers due to microembolization to acute limb ischemia due to complete thrombosis of the subclavian artery.31,32 Arterial thoracic outlet syndrome is most commonly associated with a bony abnormality (ie, cervical rib or anomalous first rib),31–33 and on physical examination the bony abnormality may be palpated in the supraclavicular fossa.31
Other physical findings include a bruit over the subclavian artery, a blood pressure difference of 20 mm Hg or more between the affected and unaffected arms, loss of brachial, radial, or ulnar pulses with arm abduction, and loss of the radial pulse with the head rotated to the affected side as the patient takes a deep breath (the Adson maneuver).31 While postural changes in the pulse examination hint at arterial thoracic outlet syndrome, extremity pulses may be reduced or even absent in up to 60% of normal patients.32
Diagnosing thoracic outlet syndrome
The workup should start with noninvasive imaging with pulse volume recording and wrist and finger systolic pressures, followed by arterial duplex ultrasonography.
Chest radiography may be able to identify bony abnormalities, and MRA or CTA with the patient in two positions—ie, arms down at the sides, and arms held above the head—can help identify arterial compression from bony or muscular structures in the thoracic outlet. Upper extremity angiography provides high-resolution imaging of the digital arteries and can help identify a subclavian artery aneurysm, which may be a subtle finding.31
It is important to have objective evidence of arterial or venous mechanical obstruction before deciding to remove the first rib.
Treating thoracic outlet syndrome
Treatment is determined by the severity and acuity of symptoms. If the patient presents with acute limb ischemia, prompt treatment with either open surgery or endovascular treatment is required.31,32,34 Once the acute phase has resolved or if the patient presents with chronic disease, open surgical repair is needed to remove the compression of the artery. If an arterial abnormality is identified (aneurysm or significant stenosis), an arterial reconstruction with bypass may be required.31
The standard treatment for thoracic outlet syndrome is resection of the first rib (and removal of the cervical rib if present).31,34 This can be by a transaxillary approach unless arterial reconstruction is needed, in which case a supraclavicular approach is used.31,34 When a patient without symptoms is found to have evidence of arterial compression, most experts would recommend resection of the first rib if there is evidence of an arterial abnormality, or follow-up with duplex imaging for patients with only subtle findings.31
EXTERNAL ILIAC ENDOFIBROSIS
External iliac endofibrosis is a rare cause of intermittent claudication, typically in high-performance athletes, resulting from thickening of the intima in the external iliac artery causing luminal narrowing and resultant ischemia.35–37 The estimated incidence is as high as 20% in elite competitive cyclists, and the condition has been described in other sports as well.37
External iliac endofibrosis typically presents as unilateral leg pain or cramping at near-maximal exercise with an associated feeling of swelling and numbness on the affected side.35,37 It is bilateral in up to 15% of cases.35 While claudication of the thigh is the predominant presenting symptom, dissection and thrombosis of the external iliac artery have been described, presenting with acute limb ischemia in up to 4% of patients.35,36
The condition has been attributed to factors such as physical position, psoas hypertrophy, tethering of the external iliac artery to the psoas muscle, kinking and tortuosity of the vessel, and high-flow states secondary to increased cardiac output and adaptive systolic hypertension.36,37
Diagnosing external iliac endofibrosis
The diagnosis is difficult, as symptoms typically manifest only during maximal exercise. Delays of 12 to 41 months between the onset of symptoms and diagnosis have been reported.37 Physical findings are nonspecific, and pulses and ankle-brachial indices are typically normal at rest. A careful history with a focus on location and duration of symptoms and a high index of suspicion have been shown to increase the sensitivity of diagnosis.36
Noninvasive vascular imaging with arterial duplex ultrasonography with physiologic studies (the ankle-brachial index) at rest and at maximal exertion should be obtained first.35,37 If findings on ultrasonography are positive, CTA or MRA can be used to identify a suspected stenosis.
Diagnostic angiography is still the gold standard for imaging, as real-time images of the artery with different leg positions can be obtained and pressure gradients can be measured with or without the use of a vasodilator to determine the hemodynamic significance of a lesion.35–37
Treating external iliac endofibrosis
Treatment should initially be conservative. Recreational athletes should consider changing to a sport that does not require hip flexion, and cyclists should be advised to reduce the amount of time spent cycling and to raise the handlebars or bring the saddle position forward to minimize hip flexion.37
Definitive treatment is open surgical repair. Surgical options include arterial release of the tethered artery, endofibrosectomy and vessel shortening, endofibrosectomy and patch angioplasty, and interposition bypass grafting.35–37
- Sinha S, Houghton J, Holt PJ, Thompson MM, Loftus IM, Hinchliffe RJ. Popliteal entrapment syndrome. J Vasc Surg 2012; 55:252–262.e30.
- Gokkus K, Sagtas E, Bakalim T, Taskaya E, Aydin AT. Popliteal entrapment syndrome. A systematic review of the literature and case presentation. Muscles Ligaments Tendons J 2014; 4:141–148.
- Pillai J. A current interpretation of popliteal vascular entrapment. J Vasc Surg 2008; 48(suppl 6):61S–65S.
- Liu Y, Sun Y, He X, et al. Imaging diagnosis and surgical treatment of popliteal artery entrapment syndrome: a single-center experience. Ann Vasc Surg 2014; 28:330–337.
- Kim SY, Min SK, Ahn S, Min SI, Ha J, Kim SJ. Long-term outcomes after revascularization for advanced popliteal artery entrapment syndrome with segmental arterial occlusion. J Vasc Surg 2012; 55:90–97.
- Galland RB. Popliteal aneurysms: from John Hunter to the 21st century. Ann R Coll Surg Engl 2007; 89:466–471.
- Dawson J, Fitridge R. Update on aneurysm disease: current insights and controversies: peripheral aneurysms: when to intervene—is rupture really a danger? Prog Cardiovasc Dis 2013; 56:26–35.
- Stone PA, Jagannath P, Thompson SN, et al. Evolving treatment of popliteal artery aneurysms. J Vasc Surg 2013; 57:1306–1310.
- Eslami MH, Rybin D, Doros G, Farber A. Open repair of asymptomatic popliteal artery aneurysm is associated with better outcomes than endovascular repair. J Vasc Surg 2015; 61:663–669.
- Serrano Hernando FJ, Martínez López I, Hernández Mateo MM, et al. Comparison of popliteal artery aneurysm therapies. J Vasc Surg 2015; 61:655–661.
- Marty B, Wicky S, Ris HB, et al. Success of thrombolysis as a predictor of outcome in acute thrombosis of popliteal aneurysms. J Vasc Surg 2002; 35:487–493.
- Hall HA, Minc S, Babrowski T. Peripheral artery aneurysm. Surg Clin North Am 2013; 93:911–923.
- Veraldi GF, Scudo G, Scorsone L, Mezzetto L, Castellani RL. Cystic adventitial disease of the popliteal artery: report of two cases and review of the literature. G Chir 2014; 35:229–234.
- Desy NM, Spinner RJ. The etiology and management of cystic adventitial disease. J Vasc Surg 2014; 60:235–245.e1–e11.
- Patel MV, Patel NH, Schneider JR, Kim S, Verta MJ. Persistent sciatic artery presenting with limb ischemia. J Vasc Surg 2013; 57:225–229.
- Kesri G, Mangtani J, Kumar G, Dangayach KK. Persistent sciatic artery aneurysm with lower limb ischemia. Case Rep Vasc Med 2014; 2014:183969.
- Nuño-Escobar C, Pérez-Durán MA, Ramos-López R, et al. Persistent sciatic artery aneurysm. Ann Vasc Surg 2013; 27:1182.e13–e16.
- Vaz C, Machado R, Rego D, Matos A, Almeida R. Hybrid approach in a case of persistent sciatic artery aneurysm. Ann Vasc Surg 2014; 28:1313.e5–e7.
- Abularrage CJ, Crawford RS, Patel VI, Conrad MF. Diagnostic strategies for the persistent sciatic artery. Vasc Endovascular Surg 2009; 43:485–489.
- Suwanabol PA, Tefera G, Schwarze ML. Syndromes associated with the deep veins: phlegmasia cerulea dolens, May-Thurner syndrome, and nutcracker syndrome. Perspect Vasc Surg Endovasc Ther 2010; 22:223–230.
- Vysetti S, Shinde S, Chaudhry S, Subramoney K. Phlegmasia cerulea dolens—a rare, life-threatening condition. ScientificWorldJournal 2009; 9:1105–1106.
- Mumoli N, Invernizzi C, Luschi R, Carmignani G, Camaiti A, Cei M. Phlegmasia cerulea dolens. Circulation 2012; 125:1056–1057.
- Chinsakchai K, Ten Duis K, Moll FL, de Borst GJ. Trends in management of phlegmasia cerulea dolens. Vasc Endovascular Surg 2011; 45:5–14.
- Dargon PT, Landry GJ. Buerger’s disease. Ann Vasc Surg 2012; 26:871–880.
- Faizer R, Forbes TL. Buerger’s disease. J Vasc Surg 2007; 46:812.
- Vijayakumar A, Tiwari R, Kumar Prabhuswamy V. Thromboangiitis obliterans (Buerger’s disease)—current practices. Int J Inflam 2013; 2013:156905.
- Ohta T, Ishibashi H, Sugimoto I, et al. The clinical course of Buerger’s disease. Ann Vasc Dis 2008; 1:85–90.
- de Souza AWS, de Carvalho JF. Diagnostic and classification criteria of Takayasu arteritis. J Autoimmun 2014; 48–49:79–83.
- Perera AH, Mason JC, Wolfe JH. Takayasu arteritis: criteria for surgical intervention should not be ignored. Int J Vasc Med 2013; 2013:618910.
- Keser G, Direskeneli H, Aksu K. Management of Takayasu arteritis: a systematic review. Rheumatology (Oxford) 2014; 53:793–801.
- Sanders RJ, Annest SJ. Thoracic outlet and pectoralis minor syndromes. Semin Vasc Surg 2014; 27:86–117.
- Criado E, Berguer R, Greenfield L. The spectrum of arterial compression at the thoracic outlet. J Vasc Surg 2010; 52:406–411.
- Povlsen B, Hansson T, Povlsen SD. Treatment for thoracic outlet syndrome. Cochrane Database Syst Rev 2014; 11:CD007218.
- Orlando MS, Likes KC, Mirza S, et al. A decade of excellent outcomes after surgical intervention in 538 patients with thoracic outlet syndrome. J Am Coll Surg 2015; 220:934–939.
- Bucci F, Ottaviani N, Plagnol P. Acute thrombosis of external iliac artery secondary to endofibrosis. Ann Vasc Surg 2011; 25:698.e5–e7.
- Willson TD, Revesz E, Podbielski FJ, Blecha MJ. External iliac artery dissection secondary to endofibrosis in a cyclist. J Vasc Surg 2010; 52:219–221.
- Peach G, Schep G, Palfreeman R, Beard JD, Thompson MM, Hinchliffe RJ. Endofibrosis and kinking of the Iliac arteries in athletes: a systematic review. Eur J Vasc Endovasc Surg 2012; 43:208–217.
Timely diagnosis of limb ischemia is critical to limb health and limb salvage. The cause in most cases is related to atherosclerosis, and patients with limb ischemia are usually older and have risk factors for atherosclerosis, such as smoking, diabetes, hypertension, hyperlipidemia, and coronary artery disease. When younger patients develop limb ischemia, the diagnosis is often delayed since the index of suspicion is quite low in the absence of the usual risk factors.
Here, we discuss several nonatherosclerotic causes of limb ischemia: popliteal artery entrapment syndrome, popliteal artery aneurysm, cystic adventitial disease, persistent sciatic artery, phlegmasia cerulea dolens, Buerger disease, Takayasu arteritis, arterial thoracic outlet syndrome, and external iliac endofibrosis (Table 1). Our goal is to help clinicians make a timely diagnosis and ultimately save the patient’s limb.
POPLITEAL ARTERY ENTRAPMENT SYNDROME
Popliteal artery entrapment syndrome occurs when the popliteal artery becomes compressed in the popliteal fossa, particularly during exercise.1,2 The underlying problem may be that the popliteal artery has an aberrant course lateral to the medial head of the gastrocnemius muscle, or the medial head of the gastrocnemius may have an abnormal insertion, or there may be fibrous bands in the popliteal fossa, or a combination of these (Figure 1).1–3 Functional popliteal artery entrapment syndrome occurs when there is compression of the artery without an anatomic cause.1–3
The classic clinical presentation is a young athletic patient with calf or foot claudication (crampy pain with exercise, relieved with rest), but other symptoms can include coldness, paresthesias, and numbness. Pain at rest and tissue loss are rare on presentation but may develop if the diagnosis and treatment are delayed.3
Continued compression and microtrauma to the artery may lead to an intramural hematoma, thrombus formation, aneurysmal degeneration, dissection, or even acute thrombosis.2 If the diagnosis is delayed, the patient’s condition may progress from intermittent arterial compression with plantar flexion to complete arterial thrombosis and critical limb ischemia, putting the patient at risk of limb loss.
Diagnosing popliteal artery entrapment syndrome
The diagnostic workup includes a detailed history with a focus on the cause of pain (usually exercise), a comprehensive physical examination that includes looking for wounds, and a thorough pulse examination.
The workup should start with noninvasive imaging such as duplex arterial ultrasonography with and without provocative measures (plantar flexion), the ankle-brachial index with and without provocative measures, and exercise treadmill testing with ankle-brachial index measurement.1,2 Plantar flexion may be necessary to elicit arterial compression that is usually absent at rest.
Magnetic resonance imaging (MRI) and computed tomography (CT) of the lower extremity are useful to identify an arterial abnormality and aberrant muscle anatomy1,3; MRI is currently the gold standard for delineating the muscles of the popliteal fossa.4 If these studies do not shed light on the diagnosis, arterial angiography with and without provocative maneuvers is useful in identifying compression of the popliteal artery.1–3
Treating popliteal artery entrapment syndrome
Treatment depends on the level of arterial injury.
For patients with symptoms but no evidence of arterial injury, the most common procedure offered is popliteal fossa decompression.1–3 This involves surgical release of the medial head of the gastrocnemius muscle and other muscles compressing the popliteal artery.
For patients with evidence of arterial injury such as stenosis, dissection, or aneurysm, bypass grafting may be required.
For patients who present with acute limb ischemia, both surgical thrombectomy with possible bypass and intraarterial lysis have been described.1,2,5
POPLITEAL ARTERY ANEURYSM
Popliteal artery aneurysm (Figure 2) is the most common type of aneurysm of the peripheral arteries of the lower extremity and is present in about 1% of men over age 65. Fifty percent are bilateral, and 50% are associated with an abdominal aortic aneurysm.6,7 While up to 80% patients with this type of aneurysm have no symptoms at the time of diagnosis, symptoms develop at a rate of 14% per year, with acute limb ischemia occurring in up to one-third of cases.6,7
When popliteal artery aneurysm progresses to acute limb ischemia, the consequences are often deleterious, as the tibial arteries distal to the popliteal artery are often occluded, limiting treatment options.
Popliteal artery aneurysm is defined as a local dilation of the artery of 2 cm or greater or an increase in the diameter to 1.5 times normal.6
Acute thrombosis of the aneurysm with limb ischemia is the most common presenting symptom and occurs in 50% of symptomatic cases of popliteal artery aneurysm.7 Almost 25% of patients present with intermittent claudication secondary to thrombosis, partial thrombosis with distal embolization, or combined aneurysmal and atherosclerotic disease. Compression of the popliteal vein by the popliteal artery aneurysm can cause leg swelling with or without deep vein thrombosis in up to 5% of patients.6 Rupture is very rare, with a rate of 2% to 4%.6,7
Diagnosing popliteal artery aneurysm
The diagnosis can be made with arterial duplex ultrasonography, which is also useful for follow-up surveillance.6–8 In the acute setting, computed tomographic angiography (CTA) or magnetic resonance angiography (MRA) is useful not only to identify the popliteal aneurysm, but also to define the distal tibial outflow vessels.6,7
Treating popliteal artery aneurysm
Management of an acutely thrombosed popliteal artery aneurysm starts with systemic anticoagulation with intravenous heparin, followed initially by arterial angiography and lysis.8–11 This approach has been shown to be safe and effective even in the absence of arterial runoff distal to the thrombosed popliteal aneurysm. Conversion to open thrombectomy and bypass can be done if initial lytic therapy fails, if the patient develops complications of lytic therapy, or if the patient needs emergency revascularization because of motor and neurologic deficits in the affected extremity.8,10,11
How to manage the asymptomatic patient depends on the size of the aneurysm. Most studies recommend 2 cm or larger as the criterion for repair,6–8,12 while others suggest treating even smaller aneurysms if thrombus is detected.9 Preoperative imaging before elective treatment of an asymptomatic popliteal artery aneurysm includes either CTA or MRA,8,10 which allows the surgeon to visualize the full extent of the aneurysm to best plan the surgical approach. Diagnostic angiography can help determine the most suitable bypass target and can better characterize tibial outflow.
Asymptomatic popliteal artery aneurysm has traditionally been treated with surgical bypass with exclusion of the aneurysm,6–8,12 but more recently, endovascular approaches using self-expanding stent grafts have been described. Further study is needed to determine the long-term efficacy of the endovascular approach.8,10
CYSTIC ADVENTITIAL DISEASE
Cystic adventitial disease is a rare condition in which a blood vessel is narrowed due to mucin-containing cysts in the adventitia. More than 80% of cases occur in the popliteal artery, but it has been described in other peripheral arteries and veins.13,14 It is more common in men than in women and typically occurs in the 4th or 5th decade of life. Most patients present with the sudden onset of calf claudication without the usual risk factors for peripheral vascular disease.13
Diagnosing cystic adventitial disease
Noninvasive arterial or venous duplex ultrasonography can be a good screening tool, as the cysts appear hypoechoic, but results are operator-dependent. CTA and MRA are the imaging tests of choice, as they can detect the cystic lesions and define vessel anatomy for intervention. Diagnostic angiography does not show the cysts themselves but instead reveals a classic “hourglass” and “scimitar” pattern of arterial narrowing that suggests the underlying pathology.13,14
Treating cystic adventitial disease
Usual treatment is complete cyst resection and vessel reconstruction by surgical bypass. Other therapies include open surgical cyst evacuation and removal of the cyst wall, open surgical cyst aspiration, aspiration guided by ultrasonography or CT, and percutaneous angioplasty. However, these nonsurgical treatments have not been shown to be as effective and long-lasting as cyst excision and bypass.13,14
PERSISTENT SCIATIC ARTERY
Persistent sciatic artery is a rare developmental abnormality.15–17 Normally, as the femoral artery develops in the embryo, the sciatic artery involutes to form the inferior gluteal artery. But if the femoral system fails to mature, the sciatic artery, which is adjacent to the sciatic nerve posteriorly as it goes through the sciatic foramen, persists and functions as the major artery supplying the lower extremity, continuing to the posterior thigh and joining the popliteal artery (Figure 3).15,17
Persistent sciatic artery has an incidence of 2.5 to 4 per 10,000 per year15 and is bilateral in almost half of cases.16 Up to 40% of patients have no symptoms, but symptoms may develop by age 40 to 50. Because of repeated trauma to the vessel as it passes through the sciatic foramen,18 the persistent sciatic artery typically sustains accelerated atherosclerotic changes that make it susceptible to aneurysm formation,15 and up to 46% of patients present with aneurysmal degeneration.17
Classically, patients present with lower extremity ischemia from atherosclerotic changes in the persistent sciatic artery or aneurysmal degeneration and thromboembolism.15 Rarely, these aneurysms rupture.15,17 Other signs and symptoms include a pulsatile mass in the buttock, lower extremity numbness, motor weakness, and radicular pain along the sciatic nerve distribution from nerve compression.15–17
Physical findings vary but are distinguished by the lack of femoral pulses in the presence of pedal pulses. A pulsatile buttock mass with evidence of lower extremity nerve compression or limb ischemia or both is pathognomonic of a persistent sciatic artery aneurysm.16,18
Diagnosing persistent sciatic artery
Diagnostic angiography is the gold standard imaging test,15,19 although CTA is starting to replace it.16,18
Treating persistent sciatic artery
Persistent sciatic artery that is asymptomatic and is found incidentally does not require repair; however, it should be followed with duplex ultrasonography to look for evidence of aneurysm degeneration. Degeneration requires repair in most cases.15,16,18,19 When the persistent sciatic artery is the only blood supply to the distal extremity, open aneurysm excision and bypass is the treatment of choice.15,16,19 If collateral flow is adequate, endovascular coil embolization is an option.15 Endovascular stent graft placement has also been described.16,19
PHLEGMASIA CERULEA DOLENS
Phlegmasia cerulea dolens is a rare syndrome caused by extensive acute thrombosis of the ileofemoral vein.20–23 It is defined as total or near-total occlusion of the venous outflow of an extremity, causing massive swelling and congestion that impedes arterial inflow.20,22
Phlegmasia cerulea dolens is associated with four cardinal signs: edema, violaceous discoloration, pain, and severe venous outflow obstruction (Figure 4).22 Patients present with sudden onset of lower extremity pain, swelling, cyanosis, and arterial ischemia with or without loss of distal pulses.20,22
This syndrome can progress to gangrene and massive fluid sequestration leading to shock and death.21–23 From 25% to 40% of patients die, and of those who survive, 20% to 50% require amputation of the limb.20,23
Risk factors include malignancy, immobility, heart failure, heparin-induced thrombocytopenia, antiphospholipid syndrome, pregnancy, venous catheterization (eg, to insert an inferior vena cava filter), and surgery.20–22
Diagnosing phlegmasia cerulea dolens
The diagnosis is made on clinical suspicion with evidence of iliofemoral deep vein thrombosis. Most experts suggest venous duplex ultrasonography to identify the deep vein thrombosis,23 although CT or MR venography can be used to better delineate the proximal extent of the thrombus.20,23
Treating phlegmasia cerulea dolens
Initial management is aggressive fluid resuscitation, elevation of the affected limb, strict bed rest, and anticoagulation with intravenous heparin.20,23 Interventions are aimed at urgently restoring venous outflow to prevent progression to venous gangrene and limb loss.
Although conservative therapy can succeed by itself,23 if the condition does not improve or has already progressed to an advanced stage, the two mainstays of treatment are open venous thrombectomy and endovascular treatment.21–23 Endovascular treatment includes catheter-directed thrombolytic therapy (with or without percutaneous mechanical or pharmacomechanical thrombectomy) and stenting.20,23 The success rate for endovascular therapy can be as high as 90% with near-complete resolution of thrombosis.20 A disadvantage is that, compared with open surgical thrombectomy, more time is needed to achieve venous outflow.20,22
If endovascular therapy is ineffective, if lytic therapy is contraindicated, or if the disease has progressed to gangrene, open surgical thrombectomy with possible fasciotomy is the preferred option.20,21,23 Open surgery has the advantage of restoring venous outflow faster, but disadvantages include the inability to open the smaller veins of the extremity, blood loss, and risks associated with general anesthesia.20–22
BUERGER DISEASE
Buerger disease (thromboangiitis obliterans) is a nonatherosclerotic segmental inflammatory disease involving the small and medium-sized vessels of the arms and legs.24–27 It is differentiated from other vasculitides by its marked male predominance, its close association with smoking, the rarity of systemic signs and symptoms, and the absence of elevated inflammatory markers.26
The rate of major amputation is reported to be 11% at 5 years and 23% at 20 years.24
The classic patient is a young male smoker with symptoms of arterial disease before age 45.24,26 Patients can present with migratory thrombophlebitis or signs of arterial insufficiency in the upper or lower extremities. Two or more limbs are commonly involved. Arterial insufficiency can range from claudication and exertional discomfort of the extremity to ischemic pain at rest leading to ulceration of the distal fingers and toes. Physical findings are similar to those seen in peripheral vascular disease and arterial insufficiency, with decreased arterial brachial index, cool extremities, and wounds.
Diagnosing Buerger disease
- The Shionoya diagnostic criteria for Buerger disease are the following five clinical features24,27:
- History of smoking
- Onset before age 50
- Infrapopliteal arterial occlusive disease
- Upper-limb involvement or phlebitis migrans
- Absence of atherosclerotic risk factors other than heavy smoking.
Various other major and minor criteria have been described to make the diagnosis as well.24
There is no specific laboratory test to confirm the diagnosis of Buerger disease. A full panel of laboratory tests should be sent to rule out other causes of arterial insufficiency and vasculitides; these tests should include C-reactive protein, rheumatoid factor, erythrocyte sedimentation rate, antinuclear antibodies, antiphospholipid antibodies, anti-Scl-70 antibodies, anticentromere antibodies, complement level measurement, and hypercoagulability workup.
Imaging studies include arterial duplex ultrasonography with ankle-brachial indices and segmental pressures and CTA or MRA.26 Angiography can show a “corkscrew” pattern of occlusive disease and collateral formation, which is highly associated with Buerger disease.24
Treating Buerger disease
The only treatment shown to reduce the risk of amputation is complete abstention from tobacco and nicotine (smoking, secondhand smoke, and nicotine patches and gum).24,26
Symptoms of claudication can be managed with aspirin, clopidogrel, vasodilators, pentoxifylline, and cilostazol.26
Surgical bypass is rarely an option, as Buerger disease typically affects the distal blood vessels, thus precluding bypass, and the 5-year patency rate is only 49%.26 Other treatments including arterial thrombolysis, sympathectomy, stem cell injection, spinal cord stimulators, omental grafting, and immunomodulation have been described, but there are only limited data to offer guidance in choosing the appropriate one.24
TAKAYASU ARTERITIS
Takayasu arteritis is a form of vasculitis involving the aorta and its main branches (Figure 5).28 Although seen around the world, it has a higher incidence in young Asian women. Patients can present with systemic symptoms such as fever, fatigue, vague pain, and cardinal signs of limb ischemia associated with Takayasu arteritis, such as weak or absent pulses, differences between the arms in pulses and blood pressures, unobtainable blood pressure measurement in one or both arms, limb fatigability, and pain.28
Diagnosing Takayasu arteritis
Multiple diagnostic criteria have been proposed to define Takayasu arteritis.28 CTA, MRA, and positron emission tomography have replaced invasive angiography as the diagnostic imaging tests of choice.29
Treating Takayasu arteritis
Takayasu arteritis has an acute and chronic course. Interventions are typically reserved for severe cases, with indications that include uncontrollable hypertension from renal artery stenosis, severe coronary or cerebrovascular disease, severe aortic regurgitation or coarctation, stenotic or occlusive lesions resulting in critical limb ischemia, and aneurysm at risk of rupture.28–30
THORACIC OUTLET SYNDROME
Thoracic outlet syndrome is compression of the brachial plexus, subclavian vein, or subclavian artery as it exits the thoracic outlet through an area known as the scalene triangle, which is bordered by the anterior scalene, first rib, and clavicle.31 Presenting symptoms depend on the structure compressed.
By far the most common presentation32 is neurogenic thoracic outlet syndrome, accounting for more than 90% of cases, followed by venous thoracic outlet syndrome. Arterial thoracic outlet syndrome is the least frequent at less than 1%, but carries the greatest morbidity with potential for limb loss.31–33
The subclavian artery exits the thoracic outlet between the anterior and middle scalene muscles, and then travels over the first rib and underneath the clavicle.31 Repeated trauma from compression of the artery results in intimal injury leading to compression, stenosis, occlusion, or aneurysm formation.31,32
Symptoms of arterial thoracic outlet syndrome can start out as effort fatigue of the upper extremity secondary to compression. These symptoms are usually vague and difficult to define,31 as these patients typically are young and do not have atherosclerotic risk factors that would prompt suspicion of a vascular cause.
The most common presentation of arterial thoracic outlet syndrome is upper extremity embolization from a partially thrombosed aneurysm or area of stenosis with ischemia.32 Symptoms can range from ischemia of the fingers due to microembolization to acute limb ischemia due to complete thrombosis of the subclavian artery.31,32 Arterial thoracic outlet syndrome is most commonly associated with a bony abnormality (ie, cervical rib or anomalous first rib),31–33 and on physical examination the bony abnormality may be palpated in the supraclavicular fossa.31
Other physical findings include a bruit over the subclavian artery, a blood pressure difference of 20 mm Hg or more between the affected and unaffected arms, loss of brachial, radial, or ulnar pulses with arm abduction, and loss of the radial pulse with the head rotated to the affected side as the patient takes a deep breath (the Adson maneuver).31 While postural changes in the pulse examination hint at arterial thoracic outlet syndrome, extremity pulses may be reduced or even absent in up to 60% of normal patients.32
Diagnosing thoracic outlet syndrome
The workup should start with noninvasive imaging with pulse volume recording and wrist and finger systolic pressures, followed by arterial duplex ultrasonography.
Chest radiography may be able to identify bony abnormalities, and MRA or CTA with the patient in two positions—ie, arms down at the sides, and arms held above the head—can help identify arterial compression from bony or muscular structures in the thoracic outlet. Upper extremity angiography provides high-resolution imaging of the digital arteries and can help identify a subclavian artery aneurysm, which may be a subtle finding.31
It is important to have objective evidence of arterial or venous mechanical obstruction before deciding to remove the first rib.
Treating thoracic outlet syndrome
Treatment is determined by the severity and acuity of symptoms. If the patient presents with acute limb ischemia, prompt treatment with either open surgery or endovascular treatment is required.31,32,34 Once the acute phase has resolved or if the patient presents with chronic disease, open surgical repair is needed to remove the compression of the artery. If an arterial abnormality is identified (aneurysm or significant stenosis), an arterial reconstruction with bypass may be required.31
The standard treatment for thoracic outlet syndrome is resection of the first rib (and removal of the cervical rib if present).31,34 This can be by a transaxillary approach unless arterial reconstruction is needed, in which case a supraclavicular approach is used.31,34 When a patient without symptoms is found to have evidence of arterial compression, most experts would recommend resection of the first rib if there is evidence of an arterial abnormality, or follow-up with duplex imaging for patients with only subtle findings.31
EXTERNAL ILIAC ENDOFIBROSIS
External iliac endofibrosis is a rare cause of intermittent claudication, typically in high-performance athletes, resulting from thickening of the intima in the external iliac artery causing luminal narrowing and resultant ischemia.35–37 The estimated incidence is as high as 20% in elite competitive cyclists, and the condition has been described in other sports as well.37
External iliac endofibrosis typically presents as unilateral leg pain or cramping at near-maximal exercise with an associated feeling of swelling and numbness on the affected side.35,37 It is bilateral in up to 15% of cases.35 While claudication of the thigh is the predominant presenting symptom, dissection and thrombosis of the external iliac artery have been described, presenting with acute limb ischemia in up to 4% of patients.35,36
The condition has been attributed to factors such as physical position, psoas hypertrophy, tethering of the external iliac artery to the psoas muscle, kinking and tortuosity of the vessel, and high-flow states secondary to increased cardiac output and adaptive systolic hypertension.36,37
Diagnosing external iliac endofibrosis
The diagnosis is difficult, as symptoms typically manifest only during maximal exercise. Delays of 12 to 41 months between the onset of symptoms and diagnosis have been reported.37 Physical findings are nonspecific, and pulses and ankle-brachial indices are typically normal at rest. A careful history with a focus on location and duration of symptoms and a high index of suspicion have been shown to increase the sensitivity of diagnosis.36
Noninvasive vascular imaging with arterial duplex ultrasonography with physiologic studies (the ankle-brachial index) at rest and at maximal exertion should be obtained first.35,37 If findings on ultrasonography are positive, CTA or MRA can be used to identify a suspected stenosis.
Diagnostic angiography is still the gold standard for imaging, as real-time images of the artery with different leg positions can be obtained and pressure gradients can be measured with or without the use of a vasodilator to determine the hemodynamic significance of a lesion.35–37
Treating external iliac endofibrosis
Treatment should initially be conservative. Recreational athletes should consider changing to a sport that does not require hip flexion, and cyclists should be advised to reduce the amount of time spent cycling and to raise the handlebars or bring the saddle position forward to minimize hip flexion.37
Definitive treatment is open surgical repair. Surgical options include arterial release of the tethered artery, endofibrosectomy and vessel shortening, endofibrosectomy and patch angioplasty, and interposition bypass grafting.35–37
Timely diagnosis of limb ischemia is critical to limb health and limb salvage. The cause in most cases is related to atherosclerosis, and patients with limb ischemia are usually older and have risk factors for atherosclerosis, such as smoking, diabetes, hypertension, hyperlipidemia, and coronary artery disease. When younger patients develop limb ischemia, the diagnosis is often delayed since the index of suspicion is quite low in the absence of the usual risk factors.
Here, we discuss several nonatherosclerotic causes of limb ischemia: popliteal artery entrapment syndrome, popliteal artery aneurysm, cystic adventitial disease, persistent sciatic artery, phlegmasia cerulea dolens, Buerger disease, Takayasu arteritis, arterial thoracic outlet syndrome, and external iliac endofibrosis (Table 1). Our goal is to help clinicians make a timely diagnosis and ultimately save the patient’s limb.
POPLITEAL ARTERY ENTRAPMENT SYNDROME
Popliteal artery entrapment syndrome occurs when the popliteal artery becomes compressed in the popliteal fossa, particularly during exercise.1,2 The underlying problem may be that the popliteal artery has an aberrant course lateral to the medial head of the gastrocnemius muscle, or the medial head of the gastrocnemius may have an abnormal insertion, or there may be fibrous bands in the popliteal fossa, or a combination of these (Figure 1).1–3 Functional popliteal artery entrapment syndrome occurs when there is compression of the artery without an anatomic cause.1–3
The classic clinical presentation is a young athletic patient with calf or foot claudication (crampy pain with exercise, relieved with rest), but other symptoms can include coldness, paresthesias, and numbness. Pain at rest and tissue loss are rare on presentation but may develop if the diagnosis and treatment are delayed.3
Continued compression and microtrauma to the artery may lead to an intramural hematoma, thrombus formation, aneurysmal degeneration, dissection, or even acute thrombosis.2 If the diagnosis is delayed, the patient’s condition may progress from intermittent arterial compression with plantar flexion to complete arterial thrombosis and critical limb ischemia, putting the patient at risk of limb loss.
Diagnosing popliteal artery entrapment syndrome
The diagnostic workup includes a detailed history with a focus on the cause of pain (usually exercise), a comprehensive physical examination that includes looking for wounds, and a thorough pulse examination.
The workup should start with noninvasive imaging such as duplex arterial ultrasonography with and without provocative measures (plantar flexion), the ankle-brachial index with and without provocative measures, and exercise treadmill testing with ankle-brachial index measurement.1,2 Plantar flexion may be necessary to elicit arterial compression that is usually absent at rest.
Magnetic resonance imaging (MRI) and computed tomography (CT) of the lower extremity are useful to identify an arterial abnormality and aberrant muscle anatomy1,3; MRI is currently the gold standard for delineating the muscles of the popliteal fossa.4 If these studies do not shed light on the diagnosis, arterial angiography with and without provocative maneuvers is useful in identifying compression of the popliteal artery.1–3
Treating popliteal artery entrapment syndrome
Treatment depends on the level of arterial injury.
For patients with symptoms but no evidence of arterial injury, the most common procedure offered is popliteal fossa decompression.1–3 This involves surgical release of the medial head of the gastrocnemius muscle and other muscles compressing the popliteal artery.
For patients with evidence of arterial injury such as stenosis, dissection, or aneurysm, bypass grafting may be required.
For patients who present with acute limb ischemia, both surgical thrombectomy with possible bypass and intraarterial lysis have been described.1,2,5
POPLITEAL ARTERY ANEURYSM
Popliteal artery aneurysm (Figure 2) is the most common type of aneurysm of the peripheral arteries of the lower extremity and is present in about 1% of men over age 65. Fifty percent are bilateral, and 50% are associated with an abdominal aortic aneurysm.6,7 While up to 80% patients with this type of aneurysm have no symptoms at the time of diagnosis, symptoms develop at a rate of 14% per year, with acute limb ischemia occurring in up to one-third of cases.6,7
When popliteal artery aneurysm progresses to acute limb ischemia, the consequences are often deleterious, as the tibial arteries distal to the popliteal artery are often occluded, limiting treatment options.
Popliteal artery aneurysm is defined as a local dilation of the artery of 2 cm or greater or an increase in the diameter to 1.5 times normal.6
Acute thrombosis of the aneurysm with limb ischemia is the most common presenting symptom and occurs in 50% of symptomatic cases of popliteal artery aneurysm.7 Almost 25% of patients present with intermittent claudication secondary to thrombosis, partial thrombosis with distal embolization, or combined aneurysmal and atherosclerotic disease. Compression of the popliteal vein by the popliteal artery aneurysm can cause leg swelling with or without deep vein thrombosis in up to 5% of patients.6 Rupture is very rare, with a rate of 2% to 4%.6,7
Diagnosing popliteal artery aneurysm
The diagnosis can be made with arterial duplex ultrasonography, which is also useful for follow-up surveillance.6–8 In the acute setting, computed tomographic angiography (CTA) or magnetic resonance angiography (MRA) is useful not only to identify the popliteal aneurysm, but also to define the distal tibial outflow vessels.6,7
Treating popliteal artery aneurysm
Management of an acutely thrombosed popliteal artery aneurysm starts with systemic anticoagulation with intravenous heparin, followed initially by arterial angiography and lysis.8–11 This approach has been shown to be safe and effective even in the absence of arterial runoff distal to the thrombosed popliteal aneurysm. Conversion to open thrombectomy and bypass can be done if initial lytic therapy fails, if the patient develops complications of lytic therapy, or if the patient needs emergency revascularization because of motor and neurologic deficits in the affected extremity.8,10,11
How to manage the asymptomatic patient depends on the size of the aneurysm. Most studies recommend 2 cm or larger as the criterion for repair,6–8,12 while others suggest treating even smaller aneurysms if thrombus is detected.9 Preoperative imaging before elective treatment of an asymptomatic popliteal artery aneurysm includes either CTA or MRA,8,10 which allows the surgeon to visualize the full extent of the aneurysm to best plan the surgical approach. Diagnostic angiography can help determine the most suitable bypass target and can better characterize tibial outflow.
Asymptomatic popliteal artery aneurysm has traditionally been treated with surgical bypass with exclusion of the aneurysm,6–8,12 but more recently, endovascular approaches using self-expanding stent grafts have been described. Further study is needed to determine the long-term efficacy of the endovascular approach.8,10
CYSTIC ADVENTITIAL DISEASE
Cystic adventitial disease is a rare condition in which a blood vessel is narrowed due to mucin-containing cysts in the adventitia. More than 80% of cases occur in the popliteal artery, but it has been described in other peripheral arteries and veins.13,14 It is more common in men than in women and typically occurs in the 4th or 5th decade of life. Most patients present with the sudden onset of calf claudication without the usual risk factors for peripheral vascular disease.13
Diagnosing cystic adventitial disease
Noninvasive arterial or venous duplex ultrasonography can be a good screening tool, as the cysts appear hypoechoic, but results are operator-dependent. CTA and MRA are the imaging tests of choice, as they can detect the cystic lesions and define vessel anatomy for intervention. Diagnostic angiography does not show the cysts themselves but instead reveals a classic “hourglass” and “scimitar” pattern of arterial narrowing that suggests the underlying pathology.13,14
Treating cystic adventitial disease
Usual treatment is complete cyst resection and vessel reconstruction by surgical bypass. Other therapies include open surgical cyst evacuation and removal of the cyst wall, open surgical cyst aspiration, aspiration guided by ultrasonography or CT, and percutaneous angioplasty. However, these nonsurgical treatments have not been shown to be as effective and long-lasting as cyst excision and bypass.13,14
PERSISTENT SCIATIC ARTERY
Persistent sciatic artery is a rare developmental abnormality.15–17 Normally, as the femoral artery develops in the embryo, the sciatic artery involutes to form the inferior gluteal artery. But if the femoral system fails to mature, the sciatic artery, which is adjacent to the sciatic nerve posteriorly as it goes through the sciatic foramen, persists and functions as the major artery supplying the lower extremity, continuing to the posterior thigh and joining the popliteal artery (Figure 3).15,17
Persistent sciatic artery has an incidence of 2.5 to 4 per 10,000 per year15 and is bilateral in almost half of cases.16 Up to 40% of patients have no symptoms, but symptoms may develop by age 40 to 50. Because of repeated trauma to the vessel as it passes through the sciatic foramen,18 the persistent sciatic artery typically sustains accelerated atherosclerotic changes that make it susceptible to aneurysm formation,15 and up to 46% of patients present with aneurysmal degeneration.17
Classically, patients present with lower extremity ischemia from atherosclerotic changes in the persistent sciatic artery or aneurysmal degeneration and thromboembolism.15 Rarely, these aneurysms rupture.15,17 Other signs and symptoms include a pulsatile mass in the buttock, lower extremity numbness, motor weakness, and radicular pain along the sciatic nerve distribution from nerve compression.15–17
Physical findings vary but are distinguished by the lack of femoral pulses in the presence of pedal pulses. A pulsatile buttock mass with evidence of lower extremity nerve compression or limb ischemia or both is pathognomonic of a persistent sciatic artery aneurysm.16,18
Diagnosing persistent sciatic artery
Diagnostic angiography is the gold standard imaging test,15,19 although CTA is starting to replace it.16,18
Treating persistent sciatic artery
Persistent sciatic artery that is asymptomatic and is found incidentally does not require repair; however, it should be followed with duplex ultrasonography to look for evidence of aneurysm degeneration. Degeneration requires repair in most cases.15,16,18,19 When the persistent sciatic artery is the only blood supply to the distal extremity, open aneurysm excision and bypass is the treatment of choice.15,16,19 If collateral flow is adequate, endovascular coil embolization is an option.15 Endovascular stent graft placement has also been described.16,19
PHLEGMASIA CERULEA DOLENS
Phlegmasia cerulea dolens is a rare syndrome caused by extensive acute thrombosis of the ileofemoral vein.20–23 It is defined as total or near-total occlusion of the venous outflow of an extremity, causing massive swelling and congestion that impedes arterial inflow.20,22
Phlegmasia cerulea dolens is associated with four cardinal signs: edema, violaceous discoloration, pain, and severe venous outflow obstruction (Figure 4).22 Patients present with sudden onset of lower extremity pain, swelling, cyanosis, and arterial ischemia with or without loss of distal pulses.20,22
This syndrome can progress to gangrene and massive fluid sequestration leading to shock and death.21–23 From 25% to 40% of patients die, and of those who survive, 20% to 50% require amputation of the limb.20,23
Risk factors include malignancy, immobility, heart failure, heparin-induced thrombocytopenia, antiphospholipid syndrome, pregnancy, venous catheterization (eg, to insert an inferior vena cava filter), and surgery.20–22
Diagnosing phlegmasia cerulea dolens
The diagnosis is made on clinical suspicion with evidence of iliofemoral deep vein thrombosis. Most experts suggest venous duplex ultrasonography to identify the deep vein thrombosis,23 although CT or MR venography can be used to better delineate the proximal extent of the thrombus.20,23
Treating phlegmasia cerulea dolens
Initial management is aggressive fluid resuscitation, elevation of the affected limb, strict bed rest, and anticoagulation with intravenous heparin.20,23 Interventions are aimed at urgently restoring venous outflow to prevent progression to venous gangrene and limb loss.
Although conservative therapy can succeed by itself,23 if the condition does not improve or has already progressed to an advanced stage, the two mainstays of treatment are open venous thrombectomy and endovascular treatment.21–23 Endovascular treatment includes catheter-directed thrombolytic therapy (with or without percutaneous mechanical or pharmacomechanical thrombectomy) and stenting.20,23 The success rate for endovascular therapy can be as high as 90% with near-complete resolution of thrombosis.20 A disadvantage is that, compared with open surgical thrombectomy, more time is needed to achieve venous outflow.20,22
If endovascular therapy is ineffective, if lytic therapy is contraindicated, or if the disease has progressed to gangrene, open surgical thrombectomy with possible fasciotomy is the preferred option.20,21,23 Open surgery has the advantage of restoring venous outflow faster, but disadvantages include the inability to open the smaller veins of the extremity, blood loss, and risks associated with general anesthesia.20–22
BUERGER DISEASE
Buerger disease (thromboangiitis obliterans) is a nonatherosclerotic segmental inflammatory disease involving the small and medium-sized vessels of the arms and legs.24–27 It is differentiated from other vasculitides by its marked male predominance, its close association with smoking, the rarity of systemic signs and symptoms, and the absence of elevated inflammatory markers.26
The rate of major amputation is reported to be 11% at 5 years and 23% at 20 years.24
The classic patient is a young male smoker with symptoms of arterial disease before age 45.24,26 Patients can present with migratory thrombophlebitis or signs of arterial insufficiency in the upper or lower extremities. Two or more limbs are commonly involved. Arterial insufficiency can range from claudication and exertional discomfort of the extremity to ischemic pain at rest leading to ulceration of the distal fingers and toes. Physical findings are similar to those seen in peripheral vascular disease and arterial insufficiency, with decreased arterial brachial index, cool extremities, and wounds.
Diagnosing Buerger disease
- The Shionoya diagnostic criteria for Buerger disease are the following five clinical features24,27:
- History of smoking
- Onset before age 50
- Infrapopliteal arterial occlusive disease
- Upper-limb involvement or phlebitis migrans
- Absence of atherosclerotic risk factors other than heavy smoking.
Various other major and minor criteria have been described to make the diagnosis as well.24
There is no specific laboratory test to confirm the diagnosis of Buerger disease. A full panel of laboratory tests should be sent to rule out other causes of arterial insufficiency and vasculitides; these tests should include C-reactive protein, rheumatoid factor, erythrocyte sedimentation rate, antinuclear antibodies, antiphospholipid antibodies, anti-Scl-70 antibodies, anticentromere antibodies, complement level measurement, and hypercoagulability workup.
Imaging studies include arterial duplex ultrasonography with ankle-brachial indices and segmental pressures and CTA or MRA.26 Angiography can show a “corkscrew” pattern of occlusive disease and collateral formation, which is highly associated with Buerger disease.24
Treating Buerger disease
The only treatment shown to reduce the risk of amputation is complete abstention from tobacco and nicotine (smoking, secondhand smoke, and nicotine patches and gum).24,26
Symptoms of claudication can be managed with aspirin, clopidogrel, vasodilators, pentoxifylline, and cilostazol.26
Surgical bypass is rarely an option, as Buerger disease typically affects the distal blood vessels, thus precluding bypass, and the 5-year patency rate is only 49%.26 Other treatments including arterial thrombolysis, sympathectomy, stem cell injection, spinal cord stimulators, omental grafting, and immunomodulation have been described, but there are only limited data to offer guidance in choosing the appropriate one.24
TAKAYASU ARTERITIS
Takayasu arteritis is a form of vasculitis involving the aorta and its main branches (Figure 5).28 Although seen around the world, it has a higher incidence in young Asian women. Patients can present with systemic symptoms such as fever, fatigue, vague pain, and cardinal signs of limb ischemia associated with Takayasu arteritis, such as weak or absent pulses, differences between the arms in pulses and blood pressures, unobtainable blood pressure measurement in one or both arms, limb fatigability, and pain.28
Diagnosing Takayasu arteritis
Multiple diagnostic criteria have been proposed to define Takayasu arteritis.28 CTA, MRA, and positron emission tomography have replaced invasive angiography as the diagnostic imaging tests of choice.29
Treating Takayasu arteritis
Takayasu arteritis has an acute and chronic course. Interventions are typically reserved for severe cases, with indications that include uncontrollable hypertension from renal artery stenosis, severe coronary or cerebrovascular disease, severe aortic regurgitation or coarctation, stenotic or occlusive lesions resulting in critical limb ischemia, and aneurysm at risk of rupture.28–30
THORACIC OUTLET SYNDROME
Thoracic outlet syndrome is compression of the brachial plexus, subclavian vein, or subclavian artery as it exits the thoracic outlet through an area known as the scalene triangle, which is bordered by the anterior scalene, first rib, and clavicle.31 Presenting symptoms depend on the structure compressed.
By far the most common presentation32 is neurogenic thoracic outlet syndrome, accounting for more than 90% of cases, followed by venous thoracic outlet syndrome. Arterial thoracic outlet syndrome is the least frequent at less than 1%, but carries the greatest morbidity with potential for limb loss.31–33
The subclavian artery exits the thoracic outlet between the anterior and middle scalene muscles, and then travels over the first rib and underneath the clavicle.31 Repeated trauma from compression of the artery results in intimal injury leading to compression, stenosis, occlusion, or aneurysm formation.31,32
Symptoms of arterial thoracic outlet syndrome can start out as effort fatigue of the upper extremity secondary to compression. These symptoms are usually vague and difficult to define,31 as these patients typically are young and do not have atherosclerotic risk factors that would prompt suspicion of a vascular cause.
The most common presentation of arterial thoracic outlet syndrome is upper extremity embolization from a partially thrombosed aneurysm or area of stenosis with ischemia.32 Symptoms can range from ischemia of the fingers due to microembolization to acute limb ischemia due to complete thrombosis of the subclavian artery.31,32 Arterial thoracic outlet syndrome is most commonly associated with a bony abnormality (ie, cervical rib or anomalous first rib),31–33 and on physical examination the bony abnormality may be palpated in the supraclavicular fossa.31
Other physical findings include a bruit over the subclavian artery, a blood pressure difference of 20 mm Hg or more between the affected and unaffected arms, loss of brachial, radial, or ulnar pulses with arm abduction, and loss of the radial pulse with the head rotated to the affected side as the patient takes a deep breath (the Adson maneuver).31 While postural changes in the pulse examination hint at arterial thoracic outlet syndrome, extremity pulses may be reduced or even absent in up to 60% of normal patients.32
Diagnosing thoracic outlet syndrome
The workup should start with noninvasive imaging with pulse volume recording and wrist and finger systolic pressures, followed by arterial duplex ultrasonography.
Chest radiography may be able to identify bony abnormalities, and MRA or CTA with the patient in two positions—ie, arms down at the sides, and arms held above the head—can help identify arterial compression from bony or muscular structures in the thoracic outlet. Upper extremity angiography provides high-resolution imaging of the digital arteries and can help identify a subclavian artery aneurysm, which may be a subtle finding.31
It is important to have objective evidence of arterial or venous mechanical obstruction before deciding to remove the first rib.
Treating thoracic outlet syndrome
Treatment is determined by the severity and acuity of symptoms. If the patient presents with acute limb ischemia, prompt treatment with either open surgery or endovascular treatment is required.31,32,34 Once the acute phase has resolved or if the patient presents with chronic disease, open surgical repair is needed to remove the compression of the artery. If an arterial abnormality is identified (aneurysm or significant stenosis), an arterial reconstruction with bypass may be required.31
The standard treatment for thoracic outlet syndrome is resection of the first rib (and removal of the cervical rib if present).31,34 This can be by a transaxillary approach unless arterial reconstruction is needed, in which case a supraclavicular approach is used.31,34 When a patient without symptoms is found to have evidence of arterial compression, most experts would recommend resection of the first rib if there is evidence of an arterial abnormality, or follow-up with duplex imaging for patients with only subtle findings.31
EXTERNAL ILIAC ENDOFIBROSIS
External iliac endofibrosis is a rare cause of intermittent claudication, typically in high-performance athletes, resulting from thickening of the intima in the external iliac artery causing luminal narrowing and resultant ischemia.35–37 The estimated incidence is as high as 20% in elite competitive cyclists, and the condition has been described in other sports as well.37
External iliac endofibrosis typically presents as unilateral leg pain or cramping at near-maximal exercise with an associated feeling of swelling and numbness on the affected side.35,37 It is bilateral in up to 15% of cases.35 While claudication of the thigh is the predominant presenting symptom, dissection and thrombosis of the external iliac artery have been described, presenting with acute limb ischemia in up to 4% of patients.35,36
The condition has been attributed to factors such as physical position, psoas hypertrophy, tethering of the external iliac artery to the psoas muscle, kinking and tortuosity of the vessel, and high-flow states secondary to increased cardiac output and adaptive systolic hypertension.36,37
Diagnosing external iliac endofibrosis
The diagnosis is difficult, as symptoms typically manifest only during maximal exercise. Delays of 12 to 41 months between the onset of symptoms and diagnosis have been reported.37 Physical findings are nonspecific, and pulses and ankle-brachial indices are typically normal at rest. A careful history with a focus on location and duration of symptoms and a high index of suspicion have been shown to increase the sensitivity of diagnosis.36
Noninvasive vascular imaging with arterial duplex ultrasonography with physiologic studies (the ankle-brachial index) at rest and at maximal exertion should be obtained first.35,37 If findings on ultrasonography are positive, CTA or MRA can be used to identify a suspected stenosis.
Diagnostic angiography is still the gold standard for imaging, as real-time images of the artery with different leg positions can be obtained and pressure gradients can be measured with or without the use of a vasodilator to determine the hemodynamic significance of a lesion.35–37
Treating external iliac endofibrosis
Treatment should initially be conservative. Recreational athletes should consider changing to a sport that does not require hip flexion, and cyclists should be advised to reduce the amount of time spent cycling and to raise the handlebars or bring the saddle position forward to minimize hip flexion.37
Definitive treatment is open surgical repair. Surgical options include arterial release of the tethered artery, endofibrosectomy and vessel shortening, endofibrosectomy and patch angioplasty, and interposition bypass grafting.35–37
- Sinha S, Houghton J, Holt PJ, Thompson MM, Loftus IM, Hinchliffe RJ. Popliteal entrapment syndrome. J Vasc Surg 2012; 55:252–262.e30.
- Gokkus K, Sagtas E, Bakalim T, Taskaya E, Aydin AT. Popliteal entrapment syndrome. A systematic review of the literature and case presentation. Muscles Ligaments Tendons J 2014; 4:141–148.
- Pillai J. A current interpretation of popliteal vascular entrapment. J Vasc Surg 2008; 48(suppl 6):61S–65S.
- Liu Y, Sun Y, He X, et al. Imaging diagnosis and surgical treatment of popliteal artery entrapment syndrome: a single-center experience. Ann Vasc Surg 2014; 28:330–337.
- Kim SY, Min SK, Ahn S, Min SI, Ha J, Kim SJ. Long-term outcomes after revascularization for advanced popliteal artery entrapment syndrome with segmental arterial occlusion. J Vasc Surg 2012; 55:90–97.
- Galland RB. Popliteal aneurysms: from John Hunter to the 21st century. Ann R Coll Surg Engl 2007; 89:466–471.
- Dawson J, Fitridge R. Update on aneurysm disease: current insights and controversies: peripheral aneurysms: when to intervene—is rupture really a danger? Prog Cardiovasc Dis 2013; 56:26–35.
- Stone PA, Jagannath P, Thompson SN, et al. Evolving treatment of popliteal artery aneurysms. J Vasc Surg 2013; 57:1306–1310.
- Eslami MH, Rybin D, Doros G, Farber A. Open repair of asymptomatic popliteal artery aneurysm is associated with better outcomes than endovascular repair. J Vasc Surg 2015; 61:663–669.
- Serrano Hernando FJ, Martínez López I, Hernández Mateo MM, et al. Comparison of popliteal artery aneurysm therapies. J Vasc Surg 2015; 61:655–661.
- Marty B, Wicky S, Ris HB, et al. Success of thrombolysis as a predictor of outcome in acute thrombosis of popliteal aneurysms. J Vasc Surg 2002; 35:487–493.
- Hall HA, Minc S, Babrowski T. Peripheral artery aneurysm. Surg Clin North Am 2013; 93:911–923.
- Veraldi GF, Scudo G, Scorsone L, Mezzetto L, Castellani RL. Cystic adventitial disease of the popliteal artery: report of two cases and review of the literature. G Chir 2014; 35:229–234.
- Desy NM, Spinner RJ. The etiology and management of cystic adventitial disease. J Vasc Surg 2014; 60:235–245.e1–e11.
- Patel MV, Patel NH, Schneider JR, Kim S, Verta MJ. Persistent sciatic artery presenting with limb ischemia. J Vasc Surg 2013; 57:225–229.
- Kesri G, Mangtani J, Kumar G, Dangayach KK. Persistent sciatic artery aneurysm with lower limb ischemia. Case Rep Vasc Med 2014; 2014:183969.
- Nuño-Escobar C, Pérez-Durán MA, Ramos-López R, et al. Persistent sciatic artery aneurysm. Ann Vasc Surg 2013; 27:1182.e13–e16.
- Vaz C, Machado R, Rego D, Matos A, Almeida R. Hybrid approach in a case of persistent sciatic artery aneurysm. Ann Vasc Surg 2014; 28:1313.e5–e7.
- Abularrage CJ, Crawford RS, Patel VI, Conrad MF. Diagnostic strategies for the persistent sciatic artery. Vasc Endovascular Surg 2009; 43:485–489.
- Suwanabol PA, Tefera G, Schwarze ML. Syndromes associated with the deep veins: phlegmasia cerulea dolens, May-Thurner syndrome, and nutcracker syndrome. Perspect Vasc Surg Endovasc Ther 2010; 22:223–230.
- Vysetti S, Shinde S, Chaudhry S, Subramoney K. Phlegmasia cerulea dolens—a rare, life-threatening condition. ScientificWorldJournal 2009; 9:1105–1106.
- Mumoli N, Invernizzi C, Luschi R, Carmignani G, Camaiti A, Cei M. Phlegmasia cerulea dolens. Circulation 2012; 125:1056–1057.
- Chinsakchai K, Ten Duis K, Moll FL, de Borst GJ. Trends in management of phlegmasia cerulea dolens. Vasc Endovascular Surg 2011; 45:5–14.
- Dargon PT, Landry GJ. Buerger’s disease. Ann Vasc Surg 2012; 26:871–880.
- Faizer R, Forbes TL. Buerger’s disease. J Vasc Surg 2007; 46:812.
- Vijayakumar A, Tiwari R, Kumar Prabhuswamy V. Thromboangiitis obliterans (Buerger’s disease)—current practices. Int J Inflam 2013; 2013:156905.
- Ohta T, Ishibashi H, Sugimoto I, et al. The clinical course of Buerger’s disease. Ann Vasc Dis 2008; 1:85–90.
- de Souza AWS, de Carvalho JF. Diagnostic and classification criteria of Takayasu arteritis. J Autoimmun 2014; 48–49:79–83.
- Perera AH, Mason JC, Wolfe JH. Takayasu arteritis: criteria for surgical intervention should not be ignored. Int J Vasc Med 2013; 2013:618910.
- Keser G, Direskeneli H, Aksu K. Management of Takayasu arteritis: a systematic review. Rheumatology (Oxford) 2014; 53:793–801.
- Sanders RJ, Annest SJ. Thoracic outlet and pectoralis minor syndromes. Semin Vasc Surg 2014; 27:86–117.
- Criado E, Berguer R, Greenfield L. The spectrum of arterial compression at the thoracic outlet. J Vasc Surg 2010; 52:406–411.
- Povlsen B, Hansson T, Povlsen SD. Treatment for thoracic outlet syndrome. Cochrane Database Syst Rev 2014; 11:CD007218.
- Orlando MS, Likes KC, Mirza S, et al. A decade of excellent outcomes after surgical intervention in 538 patients with thoracic outlet syndrome. J Am Coll Surg 2015; 220:934–939.
- Bucci F, Ottaviani N, Plagnol P. Acute thrombosis of external iliac artery secondary to endofibrosis. Ann Vasc Surg 2011; 25:698.e5–e7.
- Willson TD, Revesz E, Podbielski FJ, Blecha MJ. External iliac artery dissection secondary to endofibrosis in a cyclist. J Vasc Surg 2010; 52:219–221.
- Peach G, Schep G, Palfreeman R, Beard JD, Thompson MM, Hinchliffe RJ. Endofibrosis and kinking of the Iliac arteries in athletes: a systematic review. Eur J Vasc Endovasc Surg 2012; 43:208–217.
- Sinha S, Houghton J, Holt PJ, Thompson MM, Loftus IM, Hinchliffe RJ. Popliteal entrapment syndrome. J Vasc Surg 2012; 55:252–262.e30.
- Gokkus K, Sagtas E, Bakalim T, Taskaya E, Aydin AT. Popliteal entrapment syndrome. A systematic review of the literature and case presentation. Muscles Ligaments Tendons J 2014; 4:141–148.
- Pillai J. A current interpretation of popliteal vascular entrapment. J Vasc Surg 2008; 48(suppl 6):61S–65S.
- Liu Y, Sun Y, He X, et al. Imaging diagnosis and surgical treatment of popliteal artery entrapment syndrome: a single-center experience. Ann Vasc Surg 2014; 28:330–337.
- Kim SY, Min SK, Ahn S, Min SI, Ha J, Kim SJ. Long-term outcomes after revascularization for advanced popliteal artery entrapment syndrome with segmental arterial occlusion. J Vasc Surg 2012; 55:90–97.
- Galland RB. Popliteal aneurysms: from John Hunter to the 21st century. Ann R Coll Surg Engl 2007; 89:466–471.
- Dawson J, Fitridge R. Update on aneurysm disease: current insights and controversies: peripheral aneurysms: when to intervene—is rupture really a danger? Prog Cardiovasc Dis 2013; 56:26–35.
- Stone PA, Jagannath P, Thompson SN, et al. Evolving treatment of popliteal artery aneurysms. J Vasc Surg 2013; 57:1306–1310.
- Eslami MH, Rybin D, Doros G, Farber A. Open repair of asymptomatic popliteal artery aneurysm is associated with better outcomes than endovascular repair. J Vasc Surg 2015; 61:663–669.
- Serrano Hernando FJ, Martínez López I, Hernández Mateo MM, et al. Comparison of popliteal artery aneurysm therapies. J Vasc Surg 2015; 61:655–661.
- Marty B, Wicky S, Ris HB, et al. Success of thrombolysis as a predictor of outcome in acute thrombosis of popliteal aneurysms. J Vasc Surg 2002; 35:487–493.
- Hall HA, Minc S, Babrowski T. Peripheral artery aneurysm. Surg Clin North Am 2013; 93:911–923.
- Veraldi GF, Scudo G, Scorsone L, Mezzetto L, Castellani RL. Cystic adventitial disease of the popliteal artery: report of two cases and review of the literature. G Chir 2014; 35:229–234.
- Desy NM, Spinner RJ. The etiology and management of cystic adventitial disease. J Vasc Surg 2014; 60:235–245.e1–e11.
- Patel MV, Patel NH, Schneider JR, Kim S, Verta MJ. Persistent sciatic artery presenting with limb ischemia. J Vasc Surg 2013; 57:225–229.
- Kesri G, Mangtani J, Kumar G, Dangayach KK. Persistent sciatic artery aneurysm with lower limb ischemia. Case Rep Vasc Med 2014; 2014:183969.
- Nuño-Escobar C, Pérez-Durán MA, Ramos-López R, et al. Persistent sciatic artery aneurysm. Ann Vasc Surg 2013; 27:1182.e13–e16.
- Vaz C, Machado R, Rego D, Matos A, Almeida R. Hybrid approach in a case of persistent sciatic artery aneurysm. Ann Vasc Surg 2014; 28:1313.e5–e7.
- Abularrage CJ, Crawford RS, Patel VI, Conrad MF. Diagnostic strategies for the persistent sciatic artery. Vasc Endovascular Surg 2009; 43:485–489.
- Suwanabol PA, Tefera G, Schwarze ML. Syndromes associated with the deep veins: phlegmasia cerulea dolens, May-Thurner syndrome, and nutcracker syndrome. Perspect Vasc Surg Endovasc Ther 2010; 22:223–230.
- Vysetti S, Shinde S, Chaudhry S, Subramoney K. Phlegmasia cerulea dolens—a rare, life-threatening condition. ScientificWorldJournal 2009; 9:1105–1106.
- Mumoli N, Invernizzi C, Luschi R, Carmignani G, Camaiti A, Cei M. Phlegmasia cerulea dolens. Circulation 2012; 125:1056–1057.
- Chinsakchai K, Ten Duis K, Moll FL, de Borst GJ. Trends in management of phlegmasia cerulea dolens. Vasc Endovascular Surg 2011; 45:5–14.
- Dargon PT, Landry GJ. Buerger’s disease. Ann Vasc Surg 2012; 26:871–880.
- Faizer R, Forbes TL. Buerger’s disease. J Vasc Surg 2007; 46:812.
- Vijayakumar A, Tiwari R, Kumar Prabhuswamy V. Thromboangiitis obliterans (Buerger’s disease)—current practices. Int J Inflam 2013; 2013:156905.
- Ohta T, Ishibashi H, Sugimoto I, et al. The clinical course of Buerger’s disease. Ann Vasc Dis 2008; 1:85–90.
- de Souza AWS, de Carvalho JF. Diagnostic and classification criteria of Takayasu arteritis. J Autoimmun 2014; 48–49:79–83.
- Perera AH, Mason JC, Wolfe JH. Takayasu arteritis: criteria for surgical intervention should not be ignored. Int J Vasc Med 2013; 2013:618910.
- Keser G, Direskeneli H, Aksu K. Management of Takayasu arteritis: a systematic review. Rheumatology (Oxford) 2014; 53:793–801.
- Sanders RJ, Annest SJ. Thoracic outlet and pectoralis minor syndromes. Semin Vasc Surg 2014; 27:86–117.
- Criado E, Berguer R, Greenfield L. The spectrum of arterial compression at the thoracic outlet. J Vasc Surg 2010; 52:406–411.
- Povlsen B, Hansson T, Povlsen SD. Treatment for thoracic outlet syndrome. Cochrane Database Syst Rev 2014; 11:CD007218.
- Orlando MS, Likes KC, Mirza S, et al. A decade of excellent outcomes after surgical intervention in 538 patients with thoracic outlet syndrome. J Am Coll Surg 2015; 220:934–939.
- Bucci F, Ottaviani N, Plagnol P. Acute thrombosis of external iliac artery secondary to endofibrosis. Ann Vasc Surg 2011; 25:698.e5–e7.
- Willson TD, Revesz E, Podbielski FJ, Blecha MJ. External iliac artery dissection secondary to endofibrosis in a cyclist. J Vasc Surg 2010; 52:219–221.
- Peach G, Schep G, Palfreeman R, Beard JD, Thompson MM, Hinchliffe RJ. Endofibrosis and kinking of the Iliac arteries in athletes: a systematic review. Eur J Vasc Endovasc Surg 2012; 43:208–217.
KEY POINTS
- A high index of suspicion should be maintained to recognize symptoms consistent with limb ischemia in a younger patient in the absence of the usual atherosclerosis risk factors.
- A workup for most conditions includes noninvasive vascular ultrasonography to detect and quantify limb ischemia.
- Prompt referral for surgical or endovascular treatment is necessary for optimal limb salvage.
October 2016: Click for Credit
Here are 5 articles in the October issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Autism Follow-up Screening by PCPs Yields High Accuracy
To take the posttest, go to: http://bit.ly/2bTLhFS
Expires August 19, 2017
VITALS
Key clinical point:
Primary care providers can conduct the M-CHAT/F following a positive M-CHAT screening for autism spectrum disorders.
Major finding:
Primary care providers and trained interviewers agreed 86.6% of the time on the screening results of the M-CHAT/F for ASDs.
Data source:
A cohort study of 5,071 children, mean age 23 months, screened with the M-CHAT, and a subsequent 197 children screened with the M-CHAT/F in 22 Maryland primary care practices.
Disclosures:
The National Institutes of Mental Health funded the research. Dr. Sturner is director of Total Child Health (TCH), a for-profit subsidiary of the Center for Promotion of Child Development through Primary Care, which conducted the study. Barbara Howard, MD, is president of TCH. Tanya Morrel, PhD, is an employee of and stockholder in TCH, and Paul Bergmann has consulted for the company. The remaining authors had no relevant disclosures.
2. Gallstone Disease Boosts Heart Risk
To take the posttest, go to: http://bit.ly/2c7TP7D
Expires August 18, 2017
VITALS
Key clinical point:
Gallstone disease is associated with an increased risk for coronary heart disease; preventing the former can help mitigate chances of developing the latter.
Major finding:
A meta-analysis revealed a 23% increased chance of CHD in gallstone disease patients.
Data source:
A meta-analysis of seven studies involving 842,553 subjects, and a prospective cohort study of 269,142 participants in three separate studies that took place from 1980 to 2011.
Disclosures:
Funding provided by NIH, Boston Obesity Nutrition Research Center, and United States-Israel Binational Science Foundation. The authors had no relevant financial disclosures.
3. New HER2-testing Guidelines Result in More Women Eligible for Directed Treatment
To take the posttest, go to: http://bit.ly/2cd9llO
Expires July 25, 2017
VITALS
Key clinical point:
New IHC and FISH pathology guidelines categorize more breast cancers as "equivocal" regarding HER2 positivity and ultimately lead to identifying more of them as HER2 positive.
Major finding:
By using 2013 guidelines, 358 additional tumors were interpreted as positive, compared with the 2007 guidelines and 298 additional tumors were considered positive, compared with the FDA criteria.
Data source:
A cohort study involving 2,851 breast cancer samples analyzed according to three different pathology guidelines during a 1-year period.
Disclosures:
This study was supported by the Mayo Clinic. Dr. Shah reported having no relevant financial disclosures; his associates reported ties to Merck, Hospira, Ariad Pharmaceuticals, Abbott Molecular, and Genome Diagnostics.
4. Extreme Alcohol Use Worsens HIV Disease
To take the posttest, go to: http://bit.ly/2coIzG3
Expires August 14, 2017
VITALS
Key clinical point:
A pattern of heavy alcohol use over time in HIV-infected patients was associated with accelerated HIV disease progression.
Major finding:
Long-term heavy alcohol use by middle-aged, HIV-infected military veterans was associated with a 1.83-fold increased likelihood of also being in the highest-risk group for accelerated progression of HIV disease.
Data source:
This study included 3,539 U.S. military veterans receiving care for HIV infection at eight VA centers. The impact of their long-term pattern of alcohol use on HIV disease progression was assessed over an 8-year period by annual assessments using validated instruments.
Disclosures:
The presenter reported having no financial conflicts of interest regarding the study, funded by the National Institute on Alcohol Abuse and Alcoholism and the National Institute of Allergy and Infectious Diseases.
5. Weight Loss Boosts TNFis' Psoriatic Arthritis Efficacy
To take the posttest, go to: http://bit.ly/2chD4M1
Expires July 23, 2017
Here are 5 articles in the October issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Autism Follow-up Screening by PCPs Yields High Accuracy
To take the posttest, go to: http://bit.ly/2bTLhFS
Expires August 19, 2017
VITALS
Key clinical point:
Primary care providers can conduct the M-CHAT/F following a positive M-CHAT screening for autism spectrum disorders.
Major finding:
Primary care providers and trained interviewers agreed 86.6% of the time on the screening results of the M-CHAT/F for ASDs.
Data source:
A cohort study of 5,071 children, mean age 23 months, screened with the M-CHAT, and a subsequent 197 children screened with the M-CHAT/F in 22 Maryland primary care practices.
Disclosures:
The National Institutes of Mental Health funded the research. Dr. Sturner is director of Total Child Health (TCH), a for-profit subsidiary of the Center for Promotion of Child Development through Primary Care, which conducted the study. Barbara Howard, MD, is president of TCH. Tanya Morrel, PhD, is an employee of and stockholder in TCH, and Paul Bergmann has consulted for the company. The remaining authors had no relevant disclosures.
2. Gallstone Disease Boosts Heart Risk
To take the posttest, go to: http://bit.ly/2c7TP7D
Expires August 18, 2017
VITALS
Key clinical point:
Gallstone disease is associated with an increased risk for coronary heart disease; preventing the former can help mitigate chances of developing the latter.
Major finding:
A meta-analysis revealed a 23% increased chance of CHD in gallstone disease patients.
Data source:
A meta-analysis of seven studies involving 842,553 subjects, and a prospective cohort study of 269,142 participants in three separate studies that took place from 1980 to 2011.
Disclosures:
Funding provided by NIH, Boston Obesity Nutrition Research Center, and United States-Israel Binational Science Foundation. The authors had no relevant financial disclosures.
3. New HER2-testing Guidelines Result in More Women Eligible for Directed Treatment
To take the posttest, go to: http://bit.ly/2cd9llO
Expires July 25, 2017
VITALS
Key clinical point:
New IHC and FISH pathology guidelines categorize more breast cancers as "equivocal" regarding HER2 positivity and ultimately lead to identifying more of them as HER2 positive.
Major finding:
By using 2013 guidelines, 358 additional tumors were interpreted as positive, compared with the 2007 guidelines and 298 additional tumors were considered positive, compared with the FDA criteria.
Data source:
A cohort study involving 2,851 breast cancer samples analyzed according to three different pathology guidelines during a 1-year period.
Disclosures:
This study was supported by the Mayo Clinic. Dr. Shah reported having no relevant financial disclosures; his associates reported ties to Merck, Hospira, Ariad Pharmaceuticals, Abbott Molecular, and Genome Diagnostics.
4. Extreme Alcohol Use Worsens HIV Disease
To take the posttest, go to: http://bit.ly/2coIzG3
Expires August 14, 2017
VITALS
Key clinical point:
A pattern of heavy alcohol use over time in HIV-infected patients was associated with accelerated HIV disease progression.
Major finding:
Long-term heavy alcohol use by middle-aged, HIV-infected military veterans was associated with a 1.83-fold increased likelihood of also being in the highest-risk group for accelerated progression of HIV disease.
Data source:
This study included 3,539 U.S. military veterans receiving care for HIV infection at eight VA centers. The impact of their long-term pattern of alcohol use on HIV disease progression was assessed over an 8-year period by annual assessments using validated instruments.
Disclosures:
The presenter reported having no financial conflicts of interest regarding the study, funded by the National Institute on Alcohol Abuse and Alcoholism and the National Institute of Allergy and Infectious Diseases.
5. Weight Loss Boosts TNFis' Psoriatic Arthritis Efficacy
To take the posttest, go to: http://bit.ly/2chD4M1
Expires July 23, 2017
Here are 5 articles in the October issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Autism Follow-up Screening by PCPs Yields High Accuracy
To take the posttest, go to: http://bit.ly/2bTLhFS
Expires August 19, 2017
VITALS
Key clinical point:
Primary care providers can conduct the M-CHAT/F following a positive M-CHAT screening for autism spectrum disorders.
Major finding:
Primary care providers and trained interviewers agreed 86.6% of the time on the screening results of the M-CHAT/F for ASDs.
Data source:
A cohort study of 5,071 children, mean age 23 months, screened with the M-CHAT, and a subsequent 197 children screened with the M-CHAT/F in 22 Maryland primary care practices.
Disclosures:
The National Institutes of Mental Health funded the research. Dr. Sturner is director of Total Child Health (TCH), a for-profit subsidiary of the Center for Promotion of Child Development through Primary Care, which conducted the study. Barbara Howard, MD, is president of TCH. Tanya Morrel, PhD, is an employee of and stockholder in TCH, and Paul Bergmann has consulted for the company. The remaining authors had no relevant disclosures.
2. Gallstone Disease Boosts Heart Risk
To take the posttest, go to: http://bit.ly/2c7TP7D
Expires August 18, 2017
VITALS
Key clinical point:
Gallstone disease is associated with an increased risk for coronary heart disease; preventing the former can help mitigate chances of developing the latter.
Major finding:
A meta-analysis revealed a 23% increased chance of CHD in gallstone disease patients.
Data source:
A meta-analysis of seven studies involving 842,553 subjects, and a prospective cohort study of 269,142 participants in three separate studies that took place from 1980 to 2011.
Disclosures:
Funding provided by NIH, Boston Obesity Nutrition Research Center, and United States-Israel Binational Science Foundation. The authors had no relevant financial disclosures.
3. New HER2-testing Guidelines Result in More Women Eligible for Directed Treatment
To take the posttest, go to: http://bit.ly/2cd9llO
Expires July 25, 2017
VITALS
Key clinical point:
New IHC and FISH pathology guidelines categorize more breast cancers as "equivocal" regarding HER2 positivity and ultimately lead to identifying more of them as HER2 positive.
Major finding:
By using 2013 guidelines, 358 additional tumors were interpreted as positive, compared with the 2007 guidelines and 298 additional tumors were considered positive, compared with the FDA criteria.
Data source:
A cohort study involving 2,851 breast cancer samples analyzed according to three different pathology guidelines during a 1-year period.
Disclosures:
This study was supported by the Mayo Clinic. Dr. Shah reported having no relevant financial disclosures; his associates reported ties to Merck, Hospira, Ariad Pharmaceuticals, Abbott Molecular, and Genome Diagnostics.
4. Extreme Alcohol Use Worsens HIV Disease
To take the posttest, go to: http://bit.ly/2coIzG3
Expires August 14, 2017
VITALS
Key clinical point:
A pattern of heavy alcohol use over time in HIV-infected patients was associated with accelerated HIV disease progression.
Major finding:
Long-term heavy alcohol use by middle-aged, HIV-infected military veterans was associated with a 1.83-fold increased likelihood of also being in the highest-risk group for accelerated progression of HIV disease.
Data source:
This study included 3,539 U.S. military veterans receiving care for HIV infection at eight VA centers. The impact of their long-term pattern of alcohol use on HIV disease progression was assessed over an 8-year period by annual assessments using validated instruments.
Disclosures:
The presenter reported having no financial conflicts of interest regarding the study, funded by the National Institute on Alcohol Abuse and Alcoholism and the National Institute of Allergy and Infectious Diseases.
5. Weight Loss Boosts TNFis' Psoriatic Arthritis Efficacy
To take the posttest, go to: http://bit.ly/2chD4M1
Expires July 23, 2017
Hypoactive Sexual Desire Disorder (HSDD): A Primer for Clinicians
- Be comfortable taking a sexual history from their patients.
- Understand the prevalence of HSDD.
- Understand how to diagnose HSDD and distinguish it from other sexual complaints.
- Understand data from trials involving pharmacologic agents both approved and not approved in attempts to treat HSDD.
- Employ multidisciplinary communication strategies to improve quality of life in patients with PDP
This CME supplement entitles the reader to 1 free CME credit.
Click here to read supplement.
After reading the supplement, click here to access the CME posttest
- Be comfortable taking a sexual history from their patients.
- Understand the prevalence of HSDD.
- Understand how to diagnose HSDD and distinguish it from other sexual complaints.
- Understand data from trials involving pharmacologic agents both approved and not approved in attempts to treat HSDD.
- Employ multidisciplinary communication strategies to improve quality of life in patients with PDP
This CME supplement entitles the reader to 1 free CME credit.
Click here to read supplement.
After reading the supplement, click here to access the CME posttest
- Be comfortable taking a sexual history from their patients.
- Understand the prevalence of HSDD.
- Understand how to diagnose HSDD and distinguish it from other sexual complaints.
- Understand data from trials involving pharmacologic agents both approved and not approved in attempts to treat HSDD.
- Employ multidisciplinary communication strategies to improve quality of life in patients with PDP
This CME supplement entitles the reader to 1 free CME credit.
Click here to read supplement.
After reading the supplement, click here to access the CME posttest
The Utility and Interpretation of Ambulatory Glucose Profiles
Achieving the glycemic target—a key goal of diabetes management—remains elusive despite pharmacological and technological advances in insulin delivery and glucose monitoring
FACULTY
Davida F. Kruger, MSN, APN-BC, BC-ADM
Certified Nurse Practitioner
Henry Ford Medical Group
Division of Endocrinology, Diabetes, Bone, and Mineral
Disease
Detroit, Michigan
DISCLOSURES
Davida Kruger discloses that she is on the advisory boards for Abbott; Eli Lilly and Company; Janssen Pharmaceuticals, Inc.; Novo Nordisk; and sanofi-aventis U.S. LLC; and on the speakers’ bureaus for Animas Corporation, AstraZeneca; Boehringer Ingelheim/Eli Lilly and Company; Dexcom, Inc.; Janssen Pharmaceuticals, Inc.; Novo Nordisk; and Valeritas, Inc. She owns stock in Dexcom, Inc.
Stephen Brunton, MD, discloses that he is on the advisory boards and speakers’ bureaus for AstraZeneca; Boehringer Ingelheim; Becton, Dickinson and Company; Eli Lilly and Company; Janssen Pharmaceuticals, Inc.; and Novo Nordisk. He is also on the advisory board for Abbott Diabetes.
Click here to read the supplement.
To receive CME credit, please read the article and on completion, go to www.pcmg-us.org/agpCME to complete the online evaluation and receive your certificate of completion.
Achieving the glycemic target—a key goal of diabetes management—remains elusive despite pharmacological and technological advances in insulin delivery and glucose monitoring
FACULTY
Davida F. Kruger, MSN, APN-BC, BC-ADM
Certified Nurse Practitioner
Henry Ford Medical Group
Division of Endocrinology, Diabetes, Bone, and Mineral
Disease
Detroit, Michigan
DISCLOSURES
Davida Kruger discloses that she is on the advisory boards for Abbott; Eli Lilly and Company; Janssen Pharmaceuticals, Inc.; Novo Nordisk; and sanofi-aventis U.S. LLC; and on the speakers’ bureaus for Animas Corporation, AstraZeneca; Boehringer Ingelheim/Eli Lilly and Company; Dexcom, Inc.; Janssen Pharmaceuticals, Inc.; Novo Nordisk; and Valeritas, Inc. She owns stock in Dexcom, Inc.
Stephen Brunton, MD, discloses that he is on the advisory boards and speakers’ bureaus for AstraZeneca; Boehringer Ingelheim; Becton, Dickinson and Company; Eli Lilly and Company; Janssen Pharmaceuticals, Inc.; and Novo Nordisk. He is also on the advisory board for Abbott Diabetes.
Click here to read the supplement.
To receive CME credit, please read the article and on completion, go to www.pcmg-us.org/agpCME to complete the online evaluation and receive your certificate of completion.
Achieving the glycemic target—a key goal of diabetes management—remains elusive despite pharmacological and technological advances in insulin delivery and glucose monitoring
FACULTY
Davida F. Kruger, MSN, APN-BC, BC-ADM
Certified Nurse Practitioner
Henry Ford Medical Group
Division of Endocrinology, Diabetes, Bone, and Mineral
Disease
Detroit, Michigan
DISCLOSURES
Davida Kruger discloses that she is on the advisory boards for Abbott; Eli Lilly and Company; Janssen Pharmaceuticals, Inc.; Novo Nordisk; and sanofi-aventis U.S. LLC; and on the speakers’ bureaus for Animas Corporation, AstraZeneca; Boehringer Ingelheim/Eli Lilly and Company; Dexcom, Inc.; Janssen Pharmaceuticals, Inc.; Novo Nordisk; and Valeritas, Inc. She owns stock in Dexcom, Inc.
Stephen Brunton, MD, discloses that he is on the advisory boards and speakers’ bureaus for AstraZeneca; Boehringer Ingelheim; Becton, Dickinson and Company; Eli Lilly and Company; Janssen Pharmaceuticals, Inc.; and Novo Nordisk. He is also on the advisory board for Abbott Diabetes.
Click here to read the supplement.
To receive CME credit, please read the article and on completion, go to www.pcmg-us.org/agpCME to complete the online evaluation and receive your certificate of completion.