User login
Commentary: Should board exams include a technical skill assessment? A European perspective
The incidence of vascular diseases is steadily increasing because of an aging population. Vascular surgery is the only specialty that can offer all modalities of vascular therapy (endovascular, open, and conservative). It is therefore necessary to ensure implementation of all these modalities in a modern vascular surgical curricula. The creation of a vascular specialist curriculum is undoubtedly the best way to overcome further fragmentation of vascular provision and to prevent the increasingly financially-driven incentives that can mislead treatment. For obvious reasons this would be a major benefit for our patients and for our specialty.
Another reason for updating the vascular surgical curricula is the significant reduction of open aortic and peripheral vascular surgical training cases, such as abdominal aortic aneurysms and superficial femoral artery occlusions.1 Since the vast majority of these patients are now treated by endovascular means, the remaining vascular disease morphologies can technically be very demanding when requiring open vascular surgery procedures.
Nevertheless, the public and our patients quite understandably expect to be treated by well trained and competent vascular surgeons/specialists. As in all other professions, a proper assessment of all vascular competencies is therefore considered to be mandatory at the end of the training period for a vascular specialist. To this end, several proposals have been made to improve both the structure and different assessment tools including the Vascular Surgical Milestones Project,2 the Vascular Surgery In-Training Examinations (VSITE),3 the use of procedure-based assessments (PBA),4 or objective structured assessments of technical skills (OSATS).5 In addition, simulation workshops (using computer- or life-like synthetic models) play an increasing role in teaching vascular residents the ever-increasing number of different open and endovascular surgical techniques.6,7
Traditionally, the final board examination at the end of the vascular surgical training period consists of an oral assessment or a computer-based test. The obvious crucial question is whether a practical examination should be a added as a mandatory part of a vascular exit exam. This article gives an overview of the board examination of the European Board of Vascular Surgery (EBVS) at the UEMS (Union of European Medical Specialists), which adopted a technical skills assessment in 2006.
The European Vascular Surgical Examination
The UEMS was founded in 1958 as an official body of the European Union (EU). The UEMS has the remits to accredit medical meetings,8 to promote free professional movement of all doctors within Europe, and to ensure high quality of training and associated specialist standards via UEMS examinations.9,10 Currently, the UEMS represents the national medical societies of 37 member states. To date there are 42 UEMS Specialist Sections (separate and independent disciplines), UEMS Divisions (key areas within the independent disciplines, such as Interventional Radiology) and some so-called “Multidisciplinary Joint Committees” (such as Phlebology).
Since 2005, vascular surgery has been represented as an independent medical discipline within the UEMS.Politically, this was a tremendously important step that has helped many European countries to establish vascular surgery on a national level as a separate specialty. The most recent examples are Switzerland (since 2014) and Austria (since 2015).
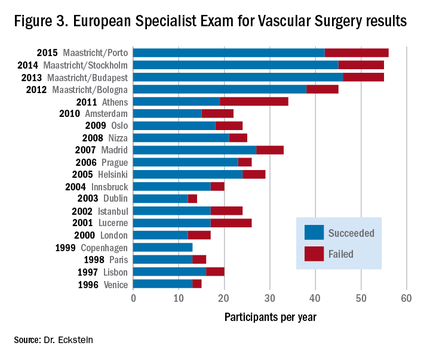
European vascular surgical examinations have been offered since 1996. The Fellowship of the European Board in Vascular Surgery (FEBVS) is voluntary in most European countries, but in some countries, such as Switzerland and the Netherlands, the European exam has now replaced the national specialist exam.12 Other countries also are in the process of accepting this European standard as a national standard, including Romania, Austria, and Sweden.
The European exam consists of a written section and a combined oral and practical exam. Candidates must be in possession of a national specialist title for surgery or vascular surgery (in countries with a monospecialty). Applications from non-EU countries also are accepted.
Applications must be made in writing, giving details of open-operative and endovascular experience. A distinction is made between assisted operations, independently performed surgery with assistance, and actual independently performed surgical procedures without specialist tutorial assistance. All candidates admitted to the examination have to pass a one-day oral and practical examination, which includes questioning on theoretical background knowledge and its practical application. This takes place mostly in the context of specific clinical case studies as well as via practical examinations on pulsatile perfused lifelike models.
The following procedures are assessed: an infrarenal aortic anastomosis, a carotid endarterectomy, and a distal bypass anastomosis.6,13,14 In the endovascular part of the examination, the applicant’s ability to introduce a guide-wire into the renal artery is assessed.15 Unlike the case in many national tests, FEBVS candidates are also presented with a specialist English-language publication (usually from the European Journal of Vascular and Endovascular Surgery). This article is then discussed with two examiners, with respect to its quality as well as its methodological content and significance. Many examination candidates fear this hurdle the most, but in fact very few participants fail this part of the test.
The European exam is designed to be unbiased and fair, with two examiners at each test station who carry out their assessments independent of each other. During the course of the examination, each candidate is interviewed by approximately 10 assessors. The assessment is validated by way of an evaluation form. The assessing auditors’ communications skills are themselves judged by observers. In the event of communication difficulties, observers are subsequently consulted.
Despite the challenging test procedures, the number of participants in the European Specialist Exam for Vascular Surgery has steadily increased in recent years. For this reason, since 2012, two examination sessions per year have been offered, one during the Annual Meeting of the European Society for Vascular Surgery (ESVS) and one at the European Vascular Course (EVC) in Maastricht. The failure rate each year fluctuates around 20%.
Benefits of being a Fellow of the European Board of Vascular Surgery (FEBVS)
There are a number of very good reasons to sit a European examination and acquire the title of Fellow of the European Board of Vascular Surgery (FEBVS). Some of them are:
Evidence of competency in job applications. Many managers know that the European exam is theoretically and practically challenging, and comprehensive. Confidence in candidates (specialists and senior physicians) who have passed the European test is therefore higher. That in turn increases the chances of getting the desired position especially when applying abroad!
Verification of open surgical and endovascular skills. Filling in the logbook16 helps to maintain a transparent open/endovascular portfolio. It is an extremely sophisticated tool to capture expertise and experience.
Commitment to the need for a European standard. The UEMS has set itself the goal of setting a European standard for medical specialists at the highest level. The European specialist exam projects this. All FEBVS support this goal via their application.
Commitment to academic knowledge-based vascular surgery. The European Vascular Surgery specialist exam covers theoretical background, knowledge of the main studies, basic academic skills, and the ability to comprehensively apply this knowledge to case studies from the entire vascular field. By obtaining this exam, all FEBVS confirm their commitment to an evidence-based approach to vascular surgery.
Commitment to competency-based vascular surgery. The European Vascular Surgery specialist exam covers a practical assessment on open vascular surgical and endovascular key competencies. By passing this part of the exam, all FEBVS give evidence that they are technically competent vascular surgeons.
Desire to belong to the best of the profession. The European specialist exam is certainly more demanding than many national board certifications. However, it offers an opportunity to belong to the European vascular surgical elite.
In conclusion, the European experience on board examinations including skills assessment shows pretty clearly that this sort of comprehensive examination is feasible. Moreover, the increasing number of applications indicates the growing attractiveness of the European certification and qualification as FEBVS. The long-term goal will be to make this examination mandatory for all EU countries – still a long way to go. By the way, since the status of FEBVS is also achievable by non-EU countries, Brexit will not prevent vascular surgeons from the United Kingdom to qualify as FEBVS in the future!
Dr. Eckstein is the Past President of the Board and Section of Vascular Surgery at the Union of European Medical Specialists (UEMS) and Past President of the German Vascular Society (DGG), and an associate editor for Vascular Specialist.
References
1. J Vasc Surg. 2014;60:945-49
2. J Vasc Surg. 2009;49:1140-6
3. Vascular surgery qualifying examination and Vsite
4. Health Technol Assess. 2011;15:i-xxi, 1-162
6. J Vasc Surg. 2013;57:1422-8
8. International Angiology. 2007;26:361-6
9. Eur J Vasc Endovasc Surg. 2009;37:109-15
10. J Vasc Surg. 2008;48:69S-75S; discussion 75S
12. Eur J Vasc Endovasc Surg. 2013;46:719-25
13. J Vasc Surg. 2013;57:1148-54
14. Brit J Surg. 2006;93:1132-8

|
Dr. Malachi Sheahan III |
Dr. Eckstein’s excellent review highlights the challenges the European Union faces in trying to standardize its certification in vascular surgery. Among European nations, the training pathways in vascular surgery are extremely varied, yet the European Economic Union calls for a medical specialist who is certified in one country to be able to practice that specialty in any EEU nation. While participation in the Fellowship of the European Board in Vascular Surgery is still mostly optional, it does provide a path toward a standard of quality that includes competence in open and endovascular procedures. In the United States, we face a similar dilemma with the advent of the integrated vascular residencies. Curricula, case volumes, and rotations still vary wildly between programs and in comparison with traditional fellowships. One solution is the Fundamentals of Vascular and Endovascular Surgery (FVEVS) project. Currently in its pilot stage, the FVEVS is designed to ensure the attainment of basic technical competencies by the mid-trainee level so the later years are focused on advanced open and endovascular training.
Dr. Malachi Sheahan III is the Associate Medical Editor for Vascular Specialist.

|
Dr. Malachi Sheahan III |
Dr. Eckstein’s excellent review highlights the challenges the European Union faces in trying to standardize its certification in vascular surgery. Among European nations, the training pathways in vascular surgery are extremely varied, yet the European Economic Union calls for a medical specialist who is certified in one country to be able to practice that specialty in any EEU nation. While participation in the Fellowship of the European Board in Vascular Surgery is still mostly optional, it does provide a path toward a standard of quality that includes competence in open and endovascular procedures. In the United States, we face a similar dilemma with the advent of the integrated vascular residencies. Curricula, case volumes, and rotations still vary wildly between programs and in comparison with traditional fellowships. One solution is the Fundamentals of Vascular and Endovascular Surgery (FVEVS) project. Currently in its pilot stage, the FVEVS is designed to ensure the attainment of basic technical competencies by the mid-trainee level so the later years are focused on advanced open and endovascular training.
Dr. Malachi Sheahan III is the Associate Medical Editor for Vascular Specialist.

|
Dr. Malachi Sheahan III |
Dr. Eckstein’s excellent review highlights the challenges the European Union faces in trying to standardize its certification in vascular surgery. Among European nations, the training pathways in vascular surgery are extremely varied, yet the European Economic Union calls for a medical specialist who is certified in one country to be able to practice that specialty in any EEU nation. While participation in the Fellowship of the European Board in Vascular Surgery is still mostly optional, it does provide a path toward a standard of quality that includes competence in open and endovascular procedures. In the United States, we face a similar dilemma with the advent of the integrated vascular residencies. Curricula, case volumes, and rotations still vary wildly between programs and in comparison with traditional fellowships. One solution is the Fundamentals of Vascular and Endovascular Surgery (FVEVS) project. Currently in its pilot stage, the FVEVS is designed to ensure the attainment of basic technical competencies by the mid-trainee level so the later years are focused on advanced open and endovascular training.
Dr. Malachi Sheahan III is the Associate Medical Editor for Vascular Specialist.
The incidence of vascular diseases is steadily increasing because of an aging population. Vascular surgery is the only specialty that can offer all modalities of vascular therapy (endovascular, open, and conservative). It is therefore necessary to ensure implementation of all these modalities in a modern vascular surgical curricula. The creation of a vascular specialist curriculum is undoubtedly the best way to overcome further fragmentation of vascular provision and to prevent the increasingly financially-driven incentives that can mislead treatment. For obvious reasons this would be a major benefit for our patients and for our specialty.
Another reason for updating the vascular surgical curricula is the significant reduction of open aortic and peripheral vascular surgical training cases, such as abdominal aortic aneurysms and superficial femoral artery occlusions.1 Since the vast majority of these patients are now treated by endovascular means, the remaining vascular disease morphologies can technically be very demanding when requiring open vascular surgery procedures.
Nevertheless, the public and our patients quite understandably expect to be treated by well trained and competent vascular surgeons/specialists. As in all other professions, a proper assessment of all vascular competencies is therefore considered to be mandatory at the end of the training period for a vascular specialist. To this end, several proposals have been made to improve both the structure and different assessment tools including the Vascular Surgical Milestones Project,2 the Vascular Surgery In-Training Examinations (VSITE),3 the use of procedure-based assessments (PBA),4 or objective structured assessments of technical skills (OSATS).5 In addition, simulation workshops (using computer- or life-like synthetic models) play an increasing role in teaching vascular residents the ever-increasing number of different open and endovascular surgical techniques.6,7
Traditionally, the final board examination at the end of the vascular surgical training period consists of an oral assessment or a computer-based test. The obvious crucial question is whether a practical examination should be a added as a mandatory part of a vascular exit exam. This article gives an overview of the board examination of the European Board of Vascular Surgery (EBVS) at the UEMS (Union of European Medical Specialists), which adopted a technical skills assessment in 2006.
The European Vascular Surgical Examination
The UEMS was founded in 1958 as an official body of the European Union (EU). The UEMS has the remits to accredit medical meetings,8 to promote free professional movement of all doctors within Europe, and to ensure high quality of training and associated specialist standards via UEMS examinations.9,10 Currently, the UEMS represents the national medical societies of 37 member states. To date there are 42 UEMS Specialist Sections (separate and independent disciplines), UEMS Divisions (key areas within the independent disciplines, such as Interventional Radiology) and some so-called “Multidisciplinary Joint Committees” (such as Phlebology).
Since 2005, vascular surgery has been represented as an independent medical discipline within the UEMS.Politically, this was a tremendously important step that has helped many European countries to establish vascular surgery on a national level as a separate specialty. The most recent examples are Switzerland (since 2014) and Austria (since 2015).

European vascular surgical examinations have been offered since 1996. The Fellowship of the European Board in Vascular Surgery (FEBVS) is voluntary in most European countries, but in some countries, such as Switzerland and the Netherlands, the European exam has now replaced the national specialist exam.12 Other countries also are in the process of accepting this European standard as a national standard, including Romania, Austria, and Sweden.
The European exam consists of a written section and a combined oral and practical exam. Candidates must be in possession of a national specialist title for surgery or vascular surgery (in countries with a monospecialty). Applications from non-EU countries also are accepted.
Applications must be made in writing, giving details of open-operative and endovascular experience. A distinction is made between assisted operations, independently performed surgery with assistance, and actual independently performed surgical procedures without specialist tutorial assistance. All candidates admitted to the examination have to pass a one-day oral and practical examination, which includes questioning on theoretical background knowledge and its practical application. This takes place mostly in the context of specific clinical case studies as well as via practical examinations on pulsatile perfused lifelike models.
The following procedures are assessed: an infrarenal aortic anastomosis, a carotid endarterectomy, and a distal bypass anastomosis.6,13,14 In the endovascular part of the examination, the applicant’s ability to introduce a guide-wire into the renal artery is assessed.15 Unlike the case in many national tests, FEBVS candidates are also presented with a specialist English-language publication (usually from the European Journal of Vascular and Endovascular Surgery). This article is then discussed with two examiners, with respect to its quality as well as its methodological content and significance. Many examination candidates fear this hurdle the most, but in fact very few participants fail this part of the test.
The European exam is designed to be unbiased and fair, with two examiners at each test station who carry out their assessments independent of each other. During the course of the examination, each candidate is interviewed by approximately 10 assessors. The assessment is validated by way of an evaluation form. The assessing auditors’ communications skills are themselves judged by observers. In the event of communication difficulties, observers are subsequently consulted.
Despite the challenging test procedures, the number of participants in the European Specialist Exam for Vascular Surgery has steadily increased in recent years. For this reason, since 2012, two examination sessions per year have been offered, one during the Annual Meeting of the European Society for Vascular Surgery (ESVS) and one at the European Vascular Course (EVC) in Maastricht. The failure rate each year fluctuates around 20%.
Benefits of being a Fellow of the European Board of Vascular Surgery (FEBVS)
There are a number of very good reasons to sit a European examination and acquire the title of Fellow of the European Board of Vascular Surgery (FEBVS). Some of them are:
Evidence of competency in job applications. Many managers know that the European exam is theoretically and practically challenging, and comprehensive. Confidence in candidates (specialists and senior physicians) who have passed the European test is therefore higher. That in turn increases the chances of getting the desired position especially when applying abroad!
Verification of open surgical and endovascular skills. Filling in the logbook16 helps to maintain a transparent open/endovascular portfolio. It is an extremely sophisticated tool to capture expertise and experience.
Commitment to the need for a European standard. The UEMS has set itself the goal of setting a European standard for medical specialists at the highest level. The European specialist exam projects this. All FEBVS support this goal via their application.
Commitment to academic knowledge-based vascular surgery. The European Vascular Surgery specialist exam covers theoretical background, knowledge of the main studies, basic academic skills, and the ability to comprehensively apply this knowledge to case studies from the entire vascular field. By obtaining this exam, all FEBVS confirm their commitment to an evidence-based approach to vascular surgery.
Commitment to competency-based vascular surgery. The European Vascular Surgery specialist exam covers a practical assessment on open vascular surgical and endovascular key competencies. By passing this part of the exam, all FEBVS give evidence that they are technically competent vascular surgeons.
Desire to belong to the best of the profession. The European specialist exam is certainly more demanding than many national board certifications. However, it offers an opportunity to belong to the European vascular surgical elite.
In conclusion, the European experience on board examinations including skills assessment shows pretty clearly that this sort of comprehensive examination is feasible. Moreover, the increasing number of applications indicates the growing attractiveness of the European certification and qualification as FEBVS. The long-term goal will be to make this examination mandatory for all EU countries – still a long way to go. By the way, since the status of FEBVS is also achievable by non-EU countries, Brexit will not prevent vascular surgeons from the United Kingdom to qualify as FEBVS in the future!
Dr. Eckstein is the Past President of the Board and Section of Vascular Surgery at the Union of European Medical Specialists (UEMS) and Past President of the German Vascular Society (DGG), and an associate editor for Vascular Specialist.
References
1. J Vasc Surg. 2014;60:945-49
2. J Vasc Surg. 2009;49:1140-6
3. Vascular surgery qualifying examination and Vsite
4. Health Technol Assess. 2011;15:i-xxi, 1-162
6. J Vasc Surg. 2013;57:1422-8
8. International Angiology. 2007;26:361-6
9. Eur J Vasc Endovasc Surg. 2009;37:109-15
10. J Vasc Surg. 2008;48:69S-75S; discussion 75S
12. Eur J Vasc Endovasc Surg. 2013;46:719-25
13. J Vasc Surg. 2013;57:1148-54
14. Brit J Surg. 2006;93:1132-8
The incidence of vascular diseases is steadily increasing because of an aging population. Vascular surgery is the only specialty that can offer all modalities of vascular therapy (endovascular, open, and conservative). It is therefore necessary to ensure implementation of all these modalities in a modern vascular surgical curricula. The creation of a vascular specialist curriculum is undoubtedly the best way to overcome further fragmentation of vascular provision and to prevent the increasingly financially-driven incentives that can mislead treatment. For obvious reasons this would be a major benefit for our patients and for our specialty.
Another reason for updating the vascular surgical curricula is the significant reduction of open aortic and peripheral vascular surgical training cases, such as abdominal aortic aneurysms and superficial femoral artery occlusions.1 Since the vast majority of these patients are now treated by endovascular means, the remaining vascular disease morphologies can technically be very demanding when requiring open vascular surgery procedures.
Nevertheless, the public and our patients quite understandably expect to be treated by well trained and competent vascular surgeons/specialists. As in all other professions, a proper assessment of all vascular competencies is therefore considered to be mandatory at the end of the training period for a vascular specialist. To this end, several proposals have been made to improve both the structure and different assessment tools including the Vascular Surgical Milestones Project,2 the Vascular Surgery In-Training Examinations (VSITE),3 the use of procedure-based assessments (PBA),4 or objective structured assessments of technical skills (OSATS).5 In addition, simulation workshops (using computer- or life-like synthetic models) play an increasing role in teaching vascular residents the ever-increasing number of different open and endovascular surgical techniques.6,7
Traditionally, the final board examination at the end of the vascular surgical training period consists of an oral assessment or a computer-based test. The obvious crucial question is whether a practical examination should be a added as a mandatory part of a vascular exit exam. This article gives an overview of the board examination of the European Board of Vascular Surgery (EBVS) at the UEMS (Union of European Medical Specialists), which adopted a technical skills assessment in 2006.
The European Vascular Surgical Examination
The UEMS was founded in 1958 as an official body of the European Union (EU). The UEMS has the remits to accredit medical meetings,8 to promote free professional movement of all doctors within Europe, and to ensure high quality of training and associated specialist standards via UEMS examinations.9,10 Currently, the UEMS represents the national medical societies of 37 member states. To date there are 42 UEMS Specialist Sections (separate and independent disciplines), UEMS Divisions (key areas within the independent disciplines, such as Interventional Radiology) and some so-called “Multidisciplinary Joint Committees” (such as Phlebology).
Since 2005, vascular surgery has been represented as an independent medical discipline within the UEMS.Politically, this was a tremendously important step that has helped many European countries to establish vascular surgery on a national level as a separate specialty. The most recent examples are Switzerland (since 2014) and Austria (since 2015).

European vascular surgical examinations have been offered since 1996. The Fellowship of the European Board in Vascular Surgery (FEBVS) is voluntary in most European countries, but in some countries, such as Switzerland and the Netherlands, the European exam has now replaced the national specialist exam.12 Other countries also are in the process of accepting this European standard as a national standard, including Romania, Austria, and Sweden.
The European exam consists of a written section and a combined oral and practical exam. Candidates must be in possession of a national specialist title for surgery or vascular surgery (in countries with a monospecialty). Applications from non-EU countries also are accepted.
Applications must be made in writing, giving details of open-operative and endovascular experience. A distinction is made between assisted operations, independently performed surgery with assistance, and actual independently performed surgical procedures without specialist tutorial assistance. All candidates admitted to the examination have to pass a one-day oral and practical examination, which includes questioning on theoretical background knowledge and its practical application. This takes place mostly in the context of specific clinical case studies as well as via practical examinations on pulsatile perfused lifelike models.
The following procedures are assessed: an infrarenal aortic anastomosis, a carotid endarterectomy, and a distal bypass anastomosis.6,13,14 In the endovascular part of the examination, the applicant’s ability to introduce a guide-wire into the renal artery is assessed.15 Unlike the case in many national tests, FEBVS candidates are also presented with a specialist English-language publication (usually from the European Journal of Vascular and Endovascular Surgery). This article is then discussed with two examiners, with respect to its quality as well as its methodological content and significance. Many examination candidates fear this hurdle the most, but in fact very few participants fail this part of the test.
The European exam is designed to be unbiased and fair, with two examiners at each test station who carry out their assessments independent of each other. During the course of the examination, each candidate is interviewed by approximately 10 assessors. The assessment is validated by way of an evaluation form. The assessing auditors’ communications skills are themselves judged by observers. In the event of communication difficulties, observers are subsequently consulted.
Despite the challenging test procedures, the number of participants in the European Specialist Exam for Vascular Surgery has steadily increased in recent years. For this reason, since 2012, two examination sessions per year have been offered, one during the Annual Meeting of the European Society for Vascular Surgery (ESVS) and one at the European Vascular Course (EVC) in Maastricht. The failure rate each year fluctuates around 20%.
Benefits of being a Fellow of the European Board of Vascular Surgery (FEBVS)
There are a number of very good reasons to sit a European examination and acquire the title of Fellow of the European Board of Vascular Surgery (FEBVS). Some of them are:
Evidence of competency in job applications. Many managers know that the European exam is theoretically and practically challenging, and comprehensive. Confidence in candidates (specialists and senior physicians) who have passed the European test is therefore higher. That in turn increases the chances of getting the desired position especially when applying abroad!
Verification of open surgical and endovascular skills. Filling in the logbook16 helps to maintain a transparent open/endovascular portfolio. It is an extremely sophisticated tool to capture expertise and experience.
Commitment to the need for a European standard. The UEMS has set itself the goal of setting a European standard for medical specialists at the highest level. The European specialist exam projects this. All FEBVS support this goal via their application.
Commitment to academic knowledge-based vascular surgery. The European Vascular Surgery specialist exam covers theoretical background, knowledge of the main studies, basic academic skills, and the ability to comprehensively apply this knowledge to case studies from the entire vascular field. By obtaining this exam, all FEBVS confirm their commitment to an evidence-based approach to vascular surgery.
Commitment to competency-based vascular surgery. The European Vascular Surgery specialist exam covers a practical assessment on open vascular surgical and endovascular key competencies. By passing this part of the exam, all FEBVS give evidence that they are technically competent vascular surgeons.
Desire to belong to the best of the profession. The European specialist exam is certainly more demanding than many national board certifications. However, it offers an opportunity to belong to the European vascular surgical elite.
In conclusion, the European experience on board examinations including skills assessment shows pretty clearly that this sort of comprehensive examination is feasible. Moreover, the increasing number of applications indicates the growing attractiveness of the European certification and qualification as FEBVS. The long-term goal will be to make this examination mandatory for all EU countries – still a long way to go. By the way, since the status of FEBVS is also achievable by non-EU countries, Brexit will not prevent vascular surgeons from the United Kingdom to qualify as FEBVS in the future!
Dr. Eckstein is the Past President of the Board and Section of Vascular Surgery at the Union of European Medical Specialists (UEMS) and Past President of the German Vascular Society (DGG), and an associate editor for Vascular Specialist.
References
1. J Vasc Surg. 2014;60:945-49
2. J Vasc Surg. 2009;49:1140-6
3. Vascular surgery qualifying examination and Vsite
4. Health Technol Assess. 2011;15:i-xxi, 1-162
6. J Vasc Surg. 2013;57:1422-8
8. International Angiology. 2007;26:361-6
9. Eur J Vasc Endovasc Surg. 2009;37:109-15
10. J Vasc Surg. 2008;48:69S-75S; discussion 75S
12. Eur J Vasc Endovasc Surg. 2013;46:719-25
13. J Vasc Surg. 2013;57:1148-54
14. Brit J Surg. 2006;93:1132-8
Current Tx for blistering disorders lacks evidence-based science
NEWPORT BEACH, CALIF. – Current treatment of autoimmune blistering diseases is not backed by evidence-based medicine and solid randomized, controlled trials, according to David T. Woodley, MD.
“These are rare diseases; there’s no consensus on the treatment of choice,” Dr. Woodley said at the annual meeting of the Pacific Dermatologic Association.
Dr. Woodley, professor of dermatology at the University of Southern California, Los Angeles, limited his discussion to pemphigus vulgaris (PV), pemphigus foliaceus (PF), and bullous pemphigoid (BP). The histologic hallmark of PV and PF is acantholysis. “Because these are intraepidermal blistering diseases, you don’t see the blister very often; maybe you’ll have a few intact loose sacklike blisters, but you mostly see erosions and crusting,” he said. “PV often only begins with mouth lesions.”
Work-up for a suspected autoimmune blistering disease includes a physical exam, histology, direct and indirect immunofluorescence, and serologic tests. The diagnosis should be based on the target autoantigen in the skin. PV and PF can appear identical on direct immunofluorescence, even though the blister cleavage plane is very high in PF and usually just above the basal keratinocytes in PV. “PF can have an intercellular keratinocyte cell surface staining pattern throughout the full epidermis or sometimes only the upper epidermal layers,” Dr. Woodley said.
At an international meeting on pemphigus in 2015, Dr. Woodley and his associates presented results from an ongoing study that is following 44 pemphigus patients at Keck Hospital of USC (a private university health care system) and 40 patients at Los Angeles County and USC Medical Center (a public safety net health care system). “The question we asked was, When the same doctors treat patients with a serious complicated disease requiring lots of details and follow-up at two very different health care systems, were there any differences in the outcomes of these patients?”
They found that the rates of clinical and immunologic remission were identical at both hospitals. However, at the county hospital, there was a higher incidence of relapses (59% vs. 30%). In addition, complications between the county and private hospitals differed in terms of hyperglycemia (38% vs. 11%, respectively), infections (79% vs. 37%), deaths (1 vs. none), medication dosage nonadherence (68% vs. 22%), and inappropriate discontinuation of one or more medications (68% vs. 15%). “Current research goals are to determine what factors cause medication compliance and noncompliance,” he said.
Dr. Woodley noted that one autoimmune blistering disease can evolve into another because of the phenomenon of epitope spreading. “The concept is that inflammation from one autoimmune blistering disease creates skin injury and reveals new neoautoantigens that get recognized by the patient’s immune system,” he said.
For example, a patient who had PF and high-titer autoantibodies to desmoglein 1 after a couple of years began to have blisters and erosions in her mouth, which is not supposed to happen in PF. Immunologic testing of this patient showed that in addition to antibodies against desmoglein 1, she began making high-titer autoantibodies against desmoglein 3 and had transformed into PV.
Bullous pemphigoid is another well-characterized autoimmune bullous disease that usually occurs in elderly patients. The blister is beneath the epidermis at the dermal-epidermal junction. These patients may have oral involvement. BP is characterized by tense subepidermal bullae on inflammatory bases. Histology reveals epidermal-dermal separation, many eosinophils and mast cells, and sometimes many polymorphonuclear leukocytes. “Many of these patients have very high IgE antibodies as well as IgG,” he said.
Features of BP include IgG and C3 deposits at the dermal-epidermal junction by direct immunofluorescence and by indirect immunofluorescence. “On salt-split human skin substrate, these antibodies bind to the epidermal roof of the separation and not to the dermal floor,” Dr. Woodley said. “You can send off the patient’s serum for ELISA [enzyme-linked immunosorbent assay] testing to detect autoantibodies against the BP230 antigen and the BP180 antigen to confirm the diagnosis. Autoantibodies to the BP180 antigen often correlate with the patient’s disease activity. The sensitivity of indirect immunofluorescence and ELISA are both above 95%.”
BP can present with urticarial plaques and pruritus and without blisters. Also, recent research has demonstrated that BP can present with just pruritus and no skin lesions. “So that’s something to keep in mind in refractory pruritus patients,” he said.
The incidence of BP seems to be increasing, from an estimated 7 cases per million in 1995 to 43 cases per million in 2008, Dr. Woodley said. “It may be that it is associated with some drugs like loop diuretics and spironolactone, but the precise reason is not known,” he said. “If your patient has dementia or Parkinson’s disease, he or she has a fourfold increased chance that they will have BP, because the BP180 and BP230 antigens are also in neuronal cells. Parkinson’s patients are known to make antibodies to the BP180 antigen, but not to the NC16A domain of the BP180 antigen. The development of BP in a Parkinson’s patient occurs when he or she begins to also make autoantibodies against the NC16A domain.”
The standard of treatment for autoimmune blistering diseases is prednisone 0.7-2 mg/kg. Nonsteroidal immunosuppressive agents are also helpful, including methotrexate, azathioprine, cyclophosphamide, and mycophenolate mofetil. Emerging evidence suggests that rituximab is the drug of choice for PV and PF, and should be the first-line therapy. Rituximab is a chimeric monoclonal antibody that targets CD20–positive B lymphocytes. “It removes cells that are ready to transform into autoantibody-producing short-lived plasma cells,” Dr. Woodley said. “The depletion lasts 6-12 months.”
Another new development in BP is the use of omalizumab, a monoclonal antibody to IgE. “Omalizumab inhibits the IgE antibody binding to the BP180,” Dr. Woodley said.
“It’s thought that the BP180 antigen is released from basal keratinocytes into the high papillary dermis. In BP patients, there are numerous mast cells at that location. IgE binds to the mast cells, which have IgE receptors on their surface, and in the presence of the BP180 antigen, forms dimers on the mast cells, and causes them to release inflammatory cytokines, some of which recruit eosinophils. This is likely why BP is such an inflammatory disorder. You cannot follow the IgE levels; you have to follow the eosinophils. The IgE is still high, but it’s nullified and inactive.”
Other biologics that have been successfully used in PV, PF, and BP include tumor necrosis factor–alpha inhibitors and rituximab (Rituxan). Common dosing for rituximab is 325 mg/m2 per week for 4 weeks. Infusion reactions are the most common side effect, he said, but other reported adverse reactions include infections, transient hypogammaglobulinemia, neutropenia, deep vein thrombosis, and pulmonary embolism. “The incidence of side effects seems to be going down, because we are now premedicating patients with antihistamines and IV hydrocortisone before giving them rituximab,” Dr. Woodley noted.
The development of progressive multifocal leukoencephalopathy has also been reported with the use of rituximab. “This is exceedingly rare with rituximab but has been described, and this is what keeps doctors who use this drug awake at night,” he said.
Elderly patients with mild BP sometimes can get by without using immunosuppressive agents to manage their disease. One option is potent topical steroids plus niacinamide 0.5-2 g after each meal as an anti-inflammatory B vitamin. “Doxycycline 100 mg b.i.d. also works to inhibit neutrophils,” he said. “Antihistamines can also be helpful, and some French dermatologists have found total body clobetasol to be useful.”
Dr. Woodley disclosed that he holds patents on human recombinant type VII collagen. He is also a consultant for Shire Pharmaceuticals and a speaker for Biofusion.
NEWPORT BEACH, CALIF. – Current treatment of autoimmune blistering diseases is not backed by evidence-based medicine and solid randomized, controlled trials, according to David T. Woodley, MD.
“These are rare diseases; there’s no consensus on the treatment of choice,” Dr. Woodley said at the annual meeting of the Pacific Dermatologic Association.
Dr. Woodley, professor of dermatology at the University of Southern California, Los Angeles, limited his discussion to pemphigus vulgaris (PV), pemphigus foliaceus (PF), and bullous pemphigoid (BP). The histologic hallmark of PV and PF is acantholysis. “Because these are intraepidermal blistering diseases, you don’t see the blister very often; maybe you’ll have a few intact loose sacklike blisters, but you mostly see erosions and crusting,” he said. “PV often only begins with mouth lesions.”
Work-up for a suspected autoimmune blistering disease includes a physical exam, histology, direct and indirect immunofluorescence, and serologic tests. The diagnosis should be based on the target autoantigen in the skin. PV and PF can appear identical on direct immunofluorescence, even though the blister cleavage plane is very high in PF and usually just above the basal keratinocytes in PV. “PF can have an intercellular keratinocyte cell surface staining pattern throughout the full epidermis or sometimes only the upper epidermal layers,” Dr. Woodley said.
At an international meeting on pemphigus in 2015, Dr. Woodley and his associates presented results from an ongoing study that is following 44 pemphigus patients at Keck Hospital of USC (a private university health care system) and 40 patients at Los Angeles County and USC Medical Center (a public safety net health care system). “The question we asked was, When the same doctors treat patients with a serious complicated disease requiring lots of details and follow-up at two very different health care systems, were there any differences in the outcomes of these patients?”
They found that the rates of clinical and immunologic remission were identical at both hospitals. However, at the county hospital, there was a higher incidence of relapses (59% vs. 30%). In addition, complications between the county and private hospitals differed in terms of hyperglycemia (38% vs. 11%, respectively), infections (79% vs. 37%), deaths (1 vs. none), medication dosage nonadherence (68% vs. 22%), and inappropriate discontinuation of one or more medications (68% vs. 15%). “Current research goals are to determine what factors cause medication compliance and noncompliance,” he said.
Dr. Woodley noted that one autoimmune blistering disease can evolve into another because of the phenomenon of epitope spreading. “The concept is that inflammation from one autoimmune blistering disease creates skin injury and reveals new neoautoantigens that get recognized by the patient’s immune system,” he said.
For example, a patient who had PF and high-titer autoantibodies to desmoglein 1 after a couple of years began to have blisters and erosions in her mouth, which is not supposed to happen in PF. Immunologic testing of this patient showed that in addition to antibodies against desmoglein 1, she began making high-titer autoantibodies against desmoglein 3 and had transformed into PV.
Bullous pemphigoid is another well-characterized autoimmune bullous disease that usually occurs in elderly patients. The blister is beneath the epidermis at the dermal-epidermal junction. These patients may have oral involvement. BP is characterized by tense subepidermal bullae on inflammatory bases. Histology reveals epidermal-dermal separation, many eosinophils and mast cells, and sometimes many polymorphonuclear leukocytes. “Many of these patients have very high IgE antibodies as well as IgG,” he said.
Features of BP include IgG and C3 deposits at the dermal-epidermal junction by direct immunofluorescence and by indirect immunofluorescence. “On salt-split human skin substrate, these antibodies bind to the epidermal roof of the separation and not to the dermal floor,” Dr. Woodley said. “You can send off the patient’s serum for ELISA [enzyme-linked immunosorbent assay] testing to detect autoantibodies against the BP230 antigen and the BP180 antigen to confirm the diagnosis. Autoantibodies to the BP180 antigen often correlate with the patient’s disease activity. The sensitivity of indirect immunofluorescence and ELISA are both above 95%.”
BP can present with urticarial plaques and pruritus and without blisters. Also, recent research has demonstrated that BP can present with just pruritus and no skin lesions. “So that’s something to keep in mind in refractory pruritus patients,” he said.
The incidence of BP seems to be increasing, from an estimated 7 cases per million in 1995 to 43 cases per million in 2008, Dr. Woodley said. “It may be that it is associated with some drugs like loop diuretics and spironolactone, but the precise reason is not known,” he said. “If your patient has dementia or Parkinson’s disease, he or she has a fourfold increased chance that they will have BP, because the BP180 and BP230 antigens are also in neuronal cells. Parkinson’s patients are known to make antibodies to the BP180 antigen, but not to the NC16A domain of the BP180 antigen. The development of BP in a Parkinson’s patient occurs when he or she begins to also make autoantibodies against the NC16A domain.”
The standard of treatment for autoimmune blistering diseases is prednisone 0.7-2 mg/kg. Nonsteroidal immunosuppressive agents are also helpful, including methotrexate, azathioprine, cyclophosphamide, and mycophenolate mofetil. Emerging evidence suggests that rituximab is the drug of choice for PV and PF, and should be the first-line therapy. Rituximab is a chimeric monoclonal antibody that targets CD20–positive B lymphocytes. “It removes cells that are ready to transform into autoantibody-producing short-lived plasma cells,” Dr. Woodley said. “The depletion lasts 6-12 months.”
Another new development in BP is the use of omalizumab, a monoclonal antibody to IgE. “Omalizumab inhibits the IgE antibody binding to the BP180,” Dr. Woodley said.
“It’s thought that the BP180 antigen is released from basal keratinocytes into the high papillary dermis. In BP patients, there are numerous mast cells at that location. IgE binds to the mast cells, which have IgE receptors on their surface, and in the presence of the BP180 antigen, forms dimers on the mast cells, and causes them to release inflammatory cytokines, some of which recruit eosinophils. This is likely why BP is such an inflammatory disorder. You cannot follow the IgE levels; you have to follow the eosinophils. The IgE is still high, but it’s nullified and inactive.”
Other biologics that have been successfully used in PV, PF, and BP include tumor necrosis factor–alpha inhibitors and rituximab (Rituxan). Common dosing for rituximab is 325 mg/m2 per week for 4 weeks. Infusion reactions are the most common side effect, he said, but other reported adverse reactions include infections, transient hypogammaglobulinemia, neutropenia, deep vein thrombosis, and pulmonary embolism. “The incidence of side effects seems to be going down, because we are now premedicating patients with antihistamines and IV hydrocortisone before giving them rituximab,” Dr. Woodley noted.
The development of progressive multifocal leukoencephalopathy has also been reported with the use of rituximab. “This is exceedingly rare with rituximab but has been described, and this is what keeps doctors who use this drug awake at night,” he said.
Elderly patients with mild BP sometimes can get by without using immunosuppressive agents to manage their disease. One option is potent topical steroids plus niacinamide 0.5-2 g after each meal as an anti-inflammatory B vitamin. “Doxycycline 100 mg b.i.d. also works to inhibit neutrophils,” he said. “Antihistamines can also be helpful, and some French dermatologists have found total body clobetasol to be useful.”
Dr. Woodley disclosed that he holds patents on human recombinant type VII collagen. He is also a consultant for Shire Pharmaceuticals and a speaker for Biofusion.
NEWPORT BEACH, CALIF. – Current treatment of autoimmune blistering diseases is not backed by evidence-based medicine and solid randomized, controlled trials, according to David T. Woodley, MD.
“These are rare diseases; there’s no consensus on the treatment of choice,” Dr. Woodley said at the annual meeting of the Pacific Dermatologic Association.
Dr. Woodley, professor of dermatology at the University of Southern California, Los Angeles, limited his discussion to pemphigus vulgaris (PV), pemphigus foliaceus (PF), and bullous pemphigoid (BP). The histologic hallmark of PV and PF is acantholysis. “Because these are intraepidermal blistering diseases, you don’t see the blister very often; maybe you’ll have a few intact loose sacklike blisters, but you mostly see erosions and crusting,” he said. “PV often only begins with mouth lesions.”
Work-up for a suspected autoimmune blistering disease includes a physical exam, histology, direct and indirect immunofluorescence, and serologic tests. The diagnosis should be based on the target autoantigen in the skin. PV and PF can appear identical on direct immunofluorescence, even though the blister cleavage plane is very high in PF and usually just above the basal keratinocytes in PV. “PF can have an intercellular keratinocyte cell surface staining pattern throughout the full epidermis or sometimes only the upper epidermal layers,” Dr. Woodley said.
At an international meeting on pemphigus in 2015, Dr. Woodley and his associates presented results from an ongoing study that is following 44 pemphigus patients at Keck Hospital of USC (a private university health care system) and 40 patients at Los Angeles County and USC Medical Center (a public safety net health care system). “The question we asked was, When the same doctors treat patients with a serious complicated disease requiring lots of details and follow-up at two very different health care systems, were there any differences in the outcomes of these patients?”
They found that the rates of clinical and immunologic remission were identical at both hospitals. However, at the county hospital, there was a higher incidence of relapses (59% vs. 30%). In addition, complications between the county and private hospitals differed in terms of hyperglycemia (38% vs. 11%, respectively), infections (79% vs. 37%), deaths (1 vs. none), medication dosage nonadherence (68% vs. 22%), and inappropriate discontinuation of one or more medications (68% vs. 15%). “Current research goals are to determine what factors cause medication compliance and noncompliance,” he said.
Dr. Woodley noted that one autoimmune blistering disease can evolve into another because of the phenomenon of epitope spreading. “The concept is that inflammation from one autoimmune blistering disease creates skin injury and reveals new neoautoantigens that get recognized by the patient’s immune system,” he said.
For example, a patient who had PF and high-titer autoantibodies to desmoglein 1 after a couple of years began to have blisters and erosions in her mouth, which is not supposed to happen in PF. Immunologic testing of this patient showed that in addition to antibodies against desmoglein 1, she began making high-titer autoantibodies against desmoglein 3 and had transformed into PV.
Bullous pemphigoid is another well-characterized autoimmune bullous disease that usually occurs in elderly patients. The blister is beneath the epidermis at the dermal-epidermal junction. These patients may have oral involvement. BP is characterized by tense subepidermal bullae on inflammatory bases. Histology reveals epidermal-dermal separation, many eosinophils and mast cells, and sometimes many polymorphonuclear leukocytes. “Many of these patients have very high IgE antibodies as well as IgG,” he said.
Features of BP include IgG and C3 deposits at the dermal-epidermal junction by direct immunofluorescence and by indirect immunofluorescence. “On salt-split human skin substrate, these antibodies bind to the epidermal roof of the separation and not to the dermal floor,” Dr. Woodley said. “You can send off the patient’s serum for ELISA [enzyme-linked immunosorbent assay] testing to detect autoantibodies against the BP230 antigen and the BP180 antigen to confirm the diagnosis. Autoantibodies to the BP180 antigen often correlate with the patient’s disease activity. The sensitivity of indirect immunofluorescence and ELISA are both above 95%.”
BP can present with urticarial plaques and pruritus and without blisters. Also, recent research has demonstrated that BP can present with just pruritus and no skin lesions. “So that’s something to keep in mind in refractory pruritus patients,” he said.
The incidence of BP seems to be increasing, from an estimated 7 cases per million in 1995 to 43 cases per million in 2008, Dr. Woodley said. “It may be that it is associated with some drugs like loop diuretics and spironolactone, but the precise reason is not known,” he said. “If your patient has dementia or Parkinson’s disease, he or she has a fourfold increased chance that they will have BP, because the BP180 and BP230 antigens are also in neuronal cells. Parkinson’s patients are known to make antibodies to the BP180 antigen, but not to the NC16A domain of the BP180 antigen. The development of BP in a Parkinson’s patient occurs when he or she begins to also make autoantibodies against the NC16A domain.”
The standard of treatment for autoimmune blistering diseases is prednisone 0.7-2 mg/kg. Nonsteroidal immunosuppressive agents are also helpful, including methotrexate, azathioprine, cyclophosphamide, and mycophenolate mofetil. Emerging evidence suggests that rituximab is the drug of choice for PV and PF, and should be the first-line therapy. Rituximab is a chimeric monoclonal antibody that targets CD20–positive B lymphocytes. “It removes cells that are ready to transform into autoantibody-producing short-lived plasma cells,” Dr. Woodley said. “The depletion lasts 6-12 months.”
Another new development in BP is the use of omalizumab, a monoclonal antibody to IgE. “Omalizumab inhibits the IgE antibody binding to the BP180,” Dr. Woodley said.
“It’s thought that the BP180 antigen is released from basal keratinocytes into the high papillary dermis. In BP patients, there are numerous mast cells at that location. IgE binds to the mast cells, which have IgE receptors on their surface, and in the presence of the BP180 antigen, forms dimers on the mast cells, and causes them to release inflammatory cytokines, some of which recruit eosinophils. This is likely why BP is such an inflammatory disorder. You cannot follow the IgE levels; you have to follow the eosinophils. The IgE is still high, but it’s nullified and inactive.”
Other biologics that have been successfully used in PV, PF, and BP include tumor necrosis factor–alpha inhibitors and rituximab (Rituxan). Common dosing for rituximab is 325 mg/m2 per week for 4 weeks. Infusion reactions are the most common side effect, he said, but other reported adverse reactions include infections, transient hypogammaglobulinemia, neutropenia, deep vein thrombosis, and pulmonary embolism. “The incidence of side effects seems to be going down, because we are now premedicating patients with antihistamines and IV hydrocortisone before giving them rituximab,” Dr. Woodley noted.
The development of progressive multifocal leukoencephalopathy has also been reported with the use of rituximab. “This is exceedingly rare with rituximab but has been described, and this is what keeps doctors who use this drug awake at night,” he said.
Elderly patients with mild BP sometimes can get by without using immunosuppressive agents to manage their disease. One option is potent topical steroids plus niacinamide 0.5-2 g after each meal as an anti-inflammatory B vitamin. “Doxycycline 100 mg b.i.d. also works to inhibit neutrophils,” he said. “Antihistamines can also be helpful, and some French dermatologists have found total body clobetasol to be useful.”
Dr. Woodley disclosed that he holds patents on human recombinant type VII collagen. He is also a consultant for Shire Pharmaceuticals and a speaker for Biofusion.
EXPERT ANALYSIS AT PDA 2016
Gluten-free adherence triples while celiac disease prevalence remains stable
The number of people adhering to a gluten-free diet more than tripled between 2009 and 2014, despite the fact that the prevalence of celiac disease has remained largely stable over the same period, according to data from the National Health and Nutrition Examination Survey.
Hyun-seok Kim, MD, MPH, and colleagues from Rutgers New Jersey Medical School noted that there is a popular trend of people choosing gluten-free diets, which exceeds the numbers that would be solely attributable to an increasing prevalence of celiac disease.
In a report published online Sept. 6 in JAMA Internal Medicine, the researchers noted that of 22,278 persons aged 6 years or older for whom data were available on celiac disease status and gluten-free diet status, 106 (0.69%) had a diagnosis of celiac disease, and 213 (1.08%) followed a gluten-free diet but did not have celiac disease.
At a U.S. population level, this would correspond to an estimated 1.76 million individuals with celiac disease, and 2.7 million individuals without celiac disease who follow a gluten-free diet.
The prevalence of celiac disease ranged from 0.70% during 2009-2010, to 0.77% during 2011-2012, and 0.58% during 2013-2014 (JAMA Intern Med. 2016 Sept 6. doi: 10.1001/jamainternmed.2016.5254).
In contrast, the prevalence of a gluten-free diet without celiac disease increased from 0.52% during 2009-2010 to 0.99% during 2011-2012 and 1.69% during 2013-2014, although the increase was even greater among non-Hispanic whites.
“The two trends may be related because gluten consumption has been identified as a risk factor of celiac disease, such that steady or even decreasing gluten consumption may be contributing to a plateau in celiac disease,” they reported.
The authors suggested that there were a number of reasons why individuals without celiac disease might choose to follow a gluten-free diet. “The public perception is that gluten-free diets are healthier and may provide benefits to nonspecific gastrointestinal symptoms,” they wrote, pointing out that gluten-free products are now also more widely available in supermarkets and online.
“There is also an increasing number of individuals with self-diagnosed gluten sensitivity but not the typical enteropathic or serologic features of celiac disease who have improved gastrointestinal health after avoidance of gluten-containing products.”
They stressed that the numbers of individuals in the survey with celiac disease or adhering to a gluten-free diet were relatively small, and that a diagnosis of celiac disease was not confirmed by intestinal biopsy, relying instead on serological tests and prior diagnosis by a health professional.
No conflicts of interest were declared.
Part of what may be driving this gluten-free diet trend is simply a belief, fueled by marketing and media, that these foods are healthier. However, surveys suggest that many individuals who adhere to a gluten-free diet believe that the exclusion of gluten has resulted in subjective health benefits from weight loss to reduced symptoms of inflammation and gastrointestinal distress.
Because a gluten-free diet may have negative social, financial, and health repercussions, it is important for clinicians to understand whether, in most cases, it is the elimination of the protein gluten that is responsible for symptom improvement or whether following a gluten-free diet is simply a marker of other dietary choices that are creating positive effects.
Although the choice to be gluten free may be driven in part by marketing and a misperception that gluten free is healthier, it is important that this choice not be dismissed as an unfounded trend except for those with celiac disease and wheat allergy.
Dr. Daphne Miller is from the department of family and community medicine at the University of California, San Francisco. The comments are taken from an editorial (JAMA Intern Med. 2016 Sept 6. doi: 10.1001/jamainternmed.2016.5271). No conflicts of interest were declared.
Part of what may be driving this gluten-free diet trend is simply a belief, fueled by marketing and media, that these foods are healthier. However, surveys suggest that many individuals who adhere to a gluten-free diet believe that the exclusion of gluten has resulted in subjective health benefits from weight loss to reduced symptoms of inflammation and gastrointestinal distress.
Because a gluten-free diet may have negative social, financial, and health repercussions, it is important for clinicians to understand whether, in most cases, it is the elimination of the protein gluten that is responsible for symptom improvement or whether following a gluten-free diet is simply a marker of other dietary choices that are creating positive effects.
Although the choice to be gluten free may be driven in part by marketing and a misperception that gluten free is healthier, it is important that this choice not be dismissed as an unfounded trend except for those with celiac disease and wheat allergy.
Dr. Daphne Miller is from the department of family and community medicine at the University of California, San Francisco. The comments are taken from an editorial (JAMA Intern Med. 2016 Sept 6. doi: 10.1001/jamainternmed.2016.5271). No conflicts of interest were declared.
Part of what may be driving this gluten-free diet trend is simply a belief, fueled by marketing and media, that these foods are healthier. However, surveys suggest that many individuals who adhere to a gluten-free diet believe that the exclusion of gluten has resulted in subjective health benefits from weight loss to reduced symptoms of inflammation and gastrointestinal distress.
Because a gluten-free diet may have negative social, financial, and health repercussions, it is important for clinicians to understand whether, in most cases, it is the elimination of the protein gluten that is responsible for symptom improvement or whether following a gluten-free diet is simply a marker of other dietary choices that are creating positive effects.
Although the choice to be gluten free may be driven in part by marketing and a misperception that gluten free is healthier, it is important that this choice not be dismissed as an unfounded trend except for those with celiac disease and wheat allergy.
Dr. Daphne Miller is from the department of family and community medicine at the University of California, San Francisco. The comments are taken from an editorial (JAMA Intern Med. 2016 Sept 6. doi: 10.1001/jamainternmed.2016.5271). No conflicts of interest were declared.
The number of people adhering to a gluten-free diet more than tripled between 2009 and 2014, despite the fact that the prevalence of celiac disease has remained largely stable over the same period, according to data from the National Health and Nutrition Examination Survey.
Hyun-seok Kim, MD, MPH, and colleagues from Rutgers New Jersey Medical School noted that there is a popular trend of people choosing gluten-free diets, which exceeds the numbers that would be solely attributable to an increasing prevalence of celiac disease.
In a report published online Sept. 6 in JAMA Internal Medicine, the researchers noted that of 22,278 persons aged 6 years or older for whom data were available on celiac disease status and gluten-free diet status, 106 (0.69%) had a diagnosis of celiac disease, and 213 (1.08%) followed a gluten-free diet but did not have celiac disease.
At a U.S. population level, this would correspond to an estimated 1.76 million individuals with celiac disease, and 2.7 million individuals without celiac disease who follow a gluten-free diet.
The prevalence of celiac disease ranged from 0.70% during 2009-2010, to 0.77% during 2011-2012, and 0.58% during 2013-2014 (JAMA Intern Med. 2016 Sept 6. doi: 10.1001/jamainternmed.2016.5254).
In contrast, the prevalence of a gluten-free diet without celiac disease increased from 0.52% during 2009-2010 to 0.99% during 2011-2012 and 1.69% during 2013-2014, although the increase was even greater among non-Hispanic whites.
“The two trends may be related because gluten consumption has been identified as a risk factor of celiac disease, such that steady or even decreasing gluten consumption may be contributing to a plateau in celiac disease,” they reported.
The authors suggested that there were a number of reasons why individuals without celiac disease might choose to follow a gluten-free diet. “The public perception is that gluten-free diets are healthier and may provide benefits to nonspecific gastrointestinal symptoms,” they wrote, pointing out that gluten-free products are now also more widely available in supermarkets and online.
“There is also an increasing number of individuals with self-diagnosed gluten sensitivity but not the typical enteropathic or serologic features of celiac disease who have improved gastrointestinal health after avoidance of gluten-containing products.”
They stressed that the numbers of individuals in the survey with celiac disease or adhering to a gluten-free diet were relatively small, and that a diagnosis of celiac disease was not confirmed by intestinal biopsy, relying instead on serological tests and prior diagnosis by a health professional.
No conflicts of interest were declared.
The number of people adhering to a gluten-free diet more than tripled between 2009 and 2014, despite the fact that the prevalence of celiac disease has remained largely stable over the same period, according to data from the National Health and Nutrition Examination Survey.
Hyun-seok Kim, MD, MPH, and colleagues from Rutgers New Jersey Medical School noted that there is a popular trend of people choosing gluten-free diets, which exceeds the numbers that would be solely attributable to an increasing prevalence of celiac disease.
In a report published online Sept. 6 in JAMA Internal Medicine, the researchers noted that of 22,278 persons aged 6 years or older for whom data were available on celiac disease status and gluten-free diet status, 106 (0.69%) had a diagnosis of celiac disease, and 213 (1.08%) followed a gluten-free diet but did not have celiac disease.
At a U.S. population level, this would correspond to an estimated 1.76 million individuals with celiac disease, and 2.7 million individuals without celiac disease who follow a gluten-free diet.
The prevalence of celiac disease ranged from 0.70% during 2009-2010, to 0.77% during 2011-2012, and 0.58% during 2013-2014 (JAMA Intern Med. 2016 Sept 6. doi: 10.1001/jamainternmed.2016.5254).
In contrast, the prevalence of a gluten-free diet without celiac disease increased from 0.52% during 2009-2010 to 0.99% during 2011-2012 and 1.69% during 2013-2014, although the increase was even greater among non-Hispanic whites.
“The two trends may be related because gluten consumption has been identified as a risk factor of celiac disease, such that steady or even decreasing gluten consumption may be contributing to a plateau in celiac disease,” they reported.
The authors suggested that there were a number of reasons why individuals without celiac disease might choose to follow a gluten-free diet. “The public perception is that gluten-free diets are healthier and may provide benefits to nonspecific gastrointestinal symptoms,” they wrote, pointing out that gluten-free products are now also more widely available in supermarkets and online.
“There is also an increasing number of individuals with self-diagnosed gluten sensitivity but not the typical enteropathic or serologic features of celiac disease who have improved gastrointestinal health after avoidance of gluten-containing products.”
They stressed that the numbers of individuals in the survey with celiac disease or adhering to a gluten-free diet were relatively small, and that a diagnosis of celiac disease was not confirmed by intestinal biopsy, relying instead on serological tests and prior diagnosis by a health professional.
No conflicts of interest were declared.
FROM JAMA INTERNAL MEDICINE
Key clinical point: The number of people adhering to a gluten-free diet more than tripled between 2009 and 2014, while the prevalence of celiac disease has remained largely stable over the same period.
Major finding: The prevalence of celiac disease ranged from 0.70% during 2009-2010 to 0.58% in 2013-2014, while the prevalence of a gluten-free diet without celiac disease increased from 0.52% in 2009-2010 to 1.69% during 2013-2014.
Data source: Analysis of data from 22,278 participants in the National Health and Nutrition Examination Survey.
Disclosures: No conflicts of interest were disclosed.
Teledermatology making inroads
NEWPORT BEACH, CALIF. – As the health care delivery landscape continues to evolve, expect the use of telemedicine to expand in dermatology.
“We have a robust evidence base that this is a valid way to deliver health care that can significantly improve patient access and offer comparable diagnostic and therapeutic outcomes, compared with in-person dermatology,” Ivy A. Lee, MD, said at the annual meeting of the Pacific Dermatologic Association. “This is an efficient way to improve access to our small and mighty workforce.”
Many factors are driving teledermatology’s rise in popularity, including patient access issues. “These are getting more prominent and more prevalent, not only in the uninsured and the underinsured, but also in our insured population,” said Dr. Lee, a Pasadena, Calif.–based dermatologist who also chairs the American Academy of Dermatology’s Telemedicine Task Force. “In my private practice, it’s about a 6- to 8-week wait for a new patient appointment, and this is in an affluent part of Los Angeles.”
Then there’s the increasing expectations of today’s health care consumers, who “want a better medical experience,” she said. “They don’t want to spend all their day in our waiting room, and they want to be able to get in touch with their physicians and not play phone tag with the front office staff. There’s also omnipresent technology. We have devices in our office, in our home, and in our hand – in every aspect of our lives. That’s also affecting our experience and expectations of medicine and health.”
Current forms of telemedicine range from “store and forward” (which refers to the submission of clinical images and history to a remote provider who reviews the material and sends a recommendation at a different time), “live interactive” (which uses synchronous video technology similar to FaceTime or Skype where you’re interacting with the patient or with the patient’s provider at the same time, even though you are separated by space), and “hybrid” (a combination of the two forms).
Dr. Lee said that store and forward currently is the predominant form of teledermatology “because of the convenience and ease of use. We’re a lot more efficient with that modality. We’ve used it predominantly for care delivery in a consultative practice model (provider interacts with another provider for consultation or triage of a patient). But, we also see an increasing interest and practice of direct-to-patient or direct-to-consumer care where providers interact with patients. This trend is predominantly led by telemedicine companies as a response to increasing patient demand for convenience, but also we see this practice model being adopted by larger health care systems and private practices.”
In the past, she continued, most telemedicine technology has consisted of stand-alone software platforms. The new focus is integration, asking software platforms to be more interoperable with EHRs. “Also, some EHRs are offering telemedicine capabilities,” she said. Recent interest in value-based medicine, especially since the passage of the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA), also plays a role in the growing adoption of telemedicine. “The focus on value and data collection is increasingly imposed upon us by multiple sources,” Dr. Lee said.
Reimbursement remains a barrier for wide adoption of teledermatology. Current models include volume-based (fee-for-service) and value-based models such as capitation, bundled payments, and pay for performance. Advances in the volume-based realm include increasing adoption of parity laws for private insurers (29 states hold such laws, which call for telemedicine services to be covered to the same extent and in a similar manner as in-person services).
In this era of higher-deductible insurance plans and narrowing networks, patients are also paying for their own care out of pocket or by using flexible savings accounts, to the tune of $30-$200 per teledermatology encounter. “We also have seen some progress with government payers in the fee-for-service models: Medicare, and less so with Medicaid,” she said. Telemedicine payments from Medicare have increased, from about $5 million in 2011 to about $18 million in 2015, but it currently reimburses live video with geographic restrictions and reimburses for store-and-forward technologies only in Alaska and Hawaii.
Medicaid “lags behind other payers,” Dr. Lee said. “Few states reimburse for store and forward, and reimbursement depends on distance requirements, eligible patient populations and health care providers, authorized technologies, and patient consent. Currently, reimbursement varies significantly across the country; it varies by state and, within each state, by payer. With the passage of MACRA, we have to start thinking about how we will practice and measure the value-based care we deliver and whether we as dermatologists will implement an alternative payment model or a merit-based incentive system. There are a lot of legislative changes in terms of getting properly paid for these services.”
On the legislative front, the Federation of State Medical Boards Interstate Medical Licensure Compact should help promote the adoption of teledermatology. This is proposed legislation to provide expedited and streamlined processes for physicians to obtain a multistate license to provide care. It’s been enacted by 17 states and proposed by 9 states and will go into effect in 2017.
“For teledermatology, this is an exciting time full of changes in practice, utilization, reimbursement, regulation, and research,” Dr. Lee concluded. “We see telemedicine increasingly integrated into mainstream medicine and health maintenance, and the outlook for dermatology is very positive.”
She reported having no relevant financial disclosures.
NEWPORT BEACH, CALIF. – As the health care delivery landscape continues to evolve, expect the use of telemedicine to expand in dermatology.
“We have a robust evidence base that this is a valid way to deliver health care that can significantly improve patient access and offer comparable diagnostic and therapeutic outcomes, compared with in-person dermatology,” Ivy A. Lee, MD, said at the annual meeting of the Pacific Dermatologic Association. “This is an efficient way to improve access to our small and mighty workforce.”
Many factors are driving teledermatology’s rise in popularity, including patient access issues. “These are getting more prominent and more prevalent, not only in the uninsured and the underinsured, but also in our insured population,” said Dr. Lee, a Pasadena, Calif.–based dermatologist who also chairs the American Academy of Dermatology’s Telemedicine Task Force. “In my private practice, it’s about a 6- to 8-week wait for a new patient appointment, and this is in an affluent part of Los Angeles.”
Then there’s the increasing expectations of today’s health care consumers, who “want a better medical experience,” she said. “They don’t want to spend all their day in our waiting room, and they want to be able to get in touch with their physicians and not play phone tag with the front office staff. There’s also omnipresent technology. We have devices in our office, in our home, and in our hand – in every aspect of our lives. That’s also affecting our experience and expectations of medicine and health.”
Current forms of telemedicine range from “store and forward” (which refers to the submission of clinical images and history to a remote provider who reviews the material and sends a recommendation at a different time), “live interactive” (which uses synchronous video technology similar to FaceTime or Skype where you’re interacting with the patient or with the patient’s provider at the same time, even though you are separated by space), and “hybrid” (a combination of the two forms).
Dr. Lee said that store and forward currently is the predominant form of teledermatology “because of the convenience and ease of use. We’re a lot more efficient with that modality. We’ve used it predominantly for care delivery in a consultative practice model (provider interacts with another provider for consultation or triage of a patient). But, we also see an increasing interest and practice of direct-to-patient or direct-to-consumer care where providers interact with patients. This trend is predominantly led by telemedicine companies as a response to increasing patient demand for convenience, but also we see this practice model being adopted by larger health care systems and private practices.”
In the past, she continued, most telemedicine technology has consisted of stand-alone software platforms. The new focus is integration, asking software platforms to be more interoperable with EHRs. “Also, some EHRs are offering telemedicine capabilities,” she said. Recent interest in value-based medicine, especially since the passage of the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA), also plays a role in the growing adoption of telemedicine. “The focus on value and data collection is increasingly imposed upon us by multiple sources,” Dr. Lee said.
Reimbursement remains a barrier for wide adoption of teledermatology. Current models include volume-based (fee-for-service) and value-based models such as capitation, bundled payments, and pay for performance. Advances in the volume-based realm include increasing adoption of parity laws for private insurers (29 states hold such laws, which call for telemedicine services to be covered to the same extent and in a similar manner as in-person services).
In this era of higher-deductible insurance plans and narrowing networks, patients are also paying for their own care out of pocket or by using flexible savings accounts, to the tune of $30-$200 per teledermatology encounter. “We also have seen some progress with government payers in the fee-for-service models: Medicare, and less so with Medicaid,” she said. Telemedicine payments from Medicare have increased, from about $5 million in 2011 to about $18 million in 2015, but it currently reimburses live video with geographic restrictions and reimburses for store-and-forward technologies only in Alaska and Hawaii.
Medicaid “lags behind other payers,” Dr. Lee said. “Few states reimburse for store and forward, and reimbursement depends on distance requirements, eligible patient populations and health care providers, authorized technologies, and patient consent. Currently, reimbursement varies significantly across the country; it varies by state and, within each state, by payer. With the passage of MACRA, we have to start thinking about how we will practice and measure the value-based care we deliver and whether we as dermatologists will implement an alternative payment model or a merit-based incentive system. There are a lot of legislative changes in terms of getting properly paid for these services.”
On the legislative front, the Federation of State Medical Boards Interstate Medical Licensure Compact should help promote the adoption of teledermatology. This is proposed legislation to provide expedited and streamlined processes for physicians to obtain a multistate license to provide care. It’s been enacted by 17 states and proposed by 9 states and will go into effect in 2017.
“For teledermatology, this is an exciting time full of changes in practice, utilization, reimbursement, regulation, and research,” Dr. Lee concluded. “We see telemedicine increasingly integrated into mainstream medicine and health maintenance, and the outlook for dermatology is very positive.”
She reported having no relevant financial disclosures.
NEWPORT BEACH, CALIF. – As the health care delivery landscape continues to evolve, expect the use of telemedicine to expand in dermatology.
“We have a robust evidence base that this is a valid way to deliver health care that can significantly improve patient access and offer comparable diagnostic and therapeutic outcomes, compared with in-person dermatology,” Ivy A. Lee, MD, said at the annual meeting of the Pacific Dermatologic Association. “This is an efficient way to improve access to our small and mighty workforce.”
Many factors are driving teledermatology’s rise in popularity, including patient access issues. “These are getting more prominent and more prevalent, not only in the uninsured and the underinsured, but also in our insured population,” said Dr. Lee, a Pasadena, Calif.–based dermatologist who also chairs the American Academy of Dermatology’s Telemedicine Task Force. “In my private practice, it’s about a 6- to 8-week wait for a new patient appointment, and this is in an affluent part of Los Angeles.”
Then there’s the increasing expectations of today’s health care consumers, who “want a better medical experience,” she said. “They don’t want to spend all their day in our waiting room, and they want to be able to get in touch with their physicians and not play phone tag with the front office staff. There’s also omnipresent technology. We have devices in our office, in our home, and in our hand – in every aspect of our lives. That’s also affecting our experience and expectations of medicine and health.”
Current forms of telemedicine range from “store and forward” (which refers to the submission of clinical images and history to a remote provider who reviews the material and sends a recommendation at a different time), “live interactive” (which uses synchronous video technology similar to FaceTime or Skype where you’re interacting with the patient or with the patient’s provider at the same time, even though you are separated by space), and “hybrid” (a combination of the two forms).
Dr. Lee said that store and forward currently is the predominant form of teledermatology “because of the convenience and ease of use. We’re a lot more efficient with that modality. We’ve used it predominantly for care delivery in a consultative practice model (provider interacts with another provider for consultation or triage of a patient). But, we also see an increasing interest and practice of direct-to-patient or direct-to-consumer care where providers interact with patients. This trend is predominantly led by telemedicine companies as a response to increasing patient demand for convenience, but also we see this practice model being adopted by larger health care systems and private practices.”
In the past, she continued, most telemedicine technology has consisted of stand-alone software platforms. The new focus is integration, asking software platforms to be more interoperable with EHRs. “Also, some EHRs are offering telemedicine capabilities,” she said. Recent interest in value-based medicine, especially since the passage of the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA), also plays a role in the growing adoption of telemedicine. “The focus on value and data collection is increasingly imposed upon us by multiple sources,” Dr. Lee said.
Reimbursement remains a barrier for wide adoption of teledermatology. Current models include volume-based (fee-for-service) and value-based models such as capitation, bundled payments, and pay for performance. Advances in the volume-based realm include increasing adoption of parity laws for private insurers (29 states hold such laws, which call for telemedicine services to be covered to the same extent and in a similar manner as in-person services).
In this era of higher-deductible insurance plans and narrowing networks, patients are also paying for their own care out of pocket or by using flexible savings accounts, to the tune of $30-$200 per teledermatology encounter. “We also have seen some progress with government payers in the fee-for-service models: Medicare, and less so with Medicaid,” she said. Telemedicine payments from Medicare have increased, from about $5 million in 2011 to about $18 million in 2015, but it currently reimburses live video with geographic restrictions and reimburses for store-and-forward technologies only in Alaska and Hawaii.
Medicaid “lags behind other payers,” Dr. Lee said. “Few states reimburse for store and forward, and reimbursement depends on distance requirements, eligible patient populations and health care providers, authorized technologies, and patient consent. Currently, reimbursement varies significantly across the country; it varies by state and, within each state, by payer. With the passage of MACRA, we have to start thinking about how we will practice and measure the value-based care we deliver and whether we as dermatologists will implement an alternative payment model or a merit-based incentive system. There are a lot of legislative changes in terms of getting properly paid for these services.”
On the legislative front, the Federation of State Medical Boards Interstate Medical Licensure Compact should help promote the adoption of teledermatology. This is proposed legislation to provide expedited and streamlined processes for physicians to obtain a multistate license to provide care. It’s been enacted by 17 states and proposed by 9 states and will go into effect in 2017.
“For teledermatology, this is an exciting time full of changes in practice, utilization, reimbursement, regulation, and research,” Dr. Lee concluded. “We see telemedicine increasingly integrated into mainstream medicine and health maintenance, and the outlook for dermatology is very positive.”
She reported having no relevant financial disclosures.
EXPERT ANALYSIS AT PDA 2016
Make Your Nominations for SHM Designations Now
- Awards of Excellence: www.hospitalmedicine.org/awards
- Board of Directors: www.hospitalmedicine.org/boardelection
- Committee nominations: www.hospitalmedicine.org/committee
- Masters of Hospital Medicine: www.hospitalmedicine.org/masters
- Awards of Excellence: www.hospitalmedicine.org/awards
- Board of Directors: www.hospitalmedicine.org/boardelection
- Committee nominations: www.hospitalmedicine.org/committee
- Masters of Hospital Medicine: www.hospitalmedicine.org/masters
- Awards of Excellence: www.hospitalmedicine.org/awards
- Board of Directors: www.hospitalmedicine.org/boardelection
- Committee nominations: www.hospitalmedicine.org/committee
- Masters of Hospital Medicine: www.hospitalmedicine.org/masters
Earn Fellow in Hospital Medicine Designation
If you applied for early decision on or before September 15, you’ll hear back on or before October 28, 2016. The regular decision application will remain open through November 30, with a decision on or before December 31, 2016. Apply now and learn how you can join other hospitalists who have earned this exclusive designation and recognition at www.hospitalmedicine.org/fellows.
If you applied for early decision on or before September 15, you’ll hear back on or before October 28, 2016. The regular decision application will remain open through November 30, with a decision on or before December 31, 2016. Apply now and learn how you can join other hospitalists who have earned this exclusive designation and recognition at www.hospitalmedicine.org/fellows.
If you applied for early decision on or before September 15, you’ll hear back on or before October 28, 2016. The regular decision application will remain open through November 30, with a decision on or before December 31, 2016. Apply now and learn how you can join other hospitalists who have earned this exclusive designation and recognition at www.hospitalmedicine.org/fellows.
Authors Needed for SHM Clinical Quick Talks
Busy clinical services and multiple demands on hospitalists’ time make it difficult to prepare brief talks to give to residents and students. The SHM Education Committee has created SHM Clinical Quick Talks, a bank of short prepared lectures on the SHM website. SHM Clinical Quick Talks are designed to be given in fewer than 10 minutes and are intended for use during teaching rounds, for a brief sit-down, or whenever time allows.
SHM is looking for additional authors for this series of micro-lectures. Read more and learn how to submit at connect.hospitalmedicine.org/clinicalquicktalks.
Busy clinical services and multiple demands on hospitalists’ time make it difficult to prepare brief talks to give to residents and students. The SHM Education Committee has created SHM Clinical Quick Talks, a bank of short prepared lectures on the SHM website. SHM Clinical Quick Talks are designed to be given in fewer than 10 minutes and are intended for use during teaching rounds, for a brief sit-down, or whenever time allows.
SHM is looking for additional authors for this series of micro-lectures. Read more and learn how to submit at connect.hospitalmedicine.org/clinicalquicktalks.
Busy clinical services and multiple demands on hospitalists’ time make it difficult to prepare brief talks to give to residents and students. The SHM Education Committee has created SHM Clinical Quick Talks, a bank of short prepared lectures on the SHM website. SHM Clinical Quick Talks are designed to be given in fewer than 10 minutes and are intended for use during teaching rounds, for a brief sit-down, or whenever time allows.
SHM is looking for additional authors for this series of micro-lectures. Read more and learn how to submit at connect.hospitalmedicine.org/clinicalquicktalks.
Parents support school-based HPV vaccination
Approximately 86% of middle school students were vaccinated against HPV in a school setting in a review of data from a service project involving more than 8,000 students.
“School-located vaccination programs (SLVPs) provide access to vaccination for those adolescents whose parents cannot miss work or other daytime commitments or those who have multiple after-school commitments,” wrote Dr. Amy Middleman of the University of Oklahoma Health Science Center, Oklahoma City, and her colleagues. Data from previous studies suggest reluctance on the part of parents to allow their children to receive the human papillomavirus vaccine (HPV) in a school setting, but such data may not reflect parents’ opinions when a school-based program is actually available, the researchers noted.
The researchers tested SLVPs at eight middle schools including 8,333 students; 80% were Hispanic, 17% were black, 1% were white, and the remainder of students’ ethnicities were not identified (Human Vaccines & Immunotherapeutics. 2016 Aug. doi: 10.1080/21645515.2016.1208326).
School-based vaccinations were scheduled for three times: September/October 2012, March/April 2013, and September/October 2013; the findings reflect the first two visits.
The SLVPs included the following vaccines: HPV, influenza, Tdap, meningococcal conjugate vaccine (MCV4), hepatitis A, varicella, and MMR. A total of 1,674 vaccines were administered in the fall of 2012, and 532 were administered in the spring of 2013. Overall, 449 of 524 (86%) students in the fall program and 161 of 188 (86%) in the spring program received the HPV vaccine.
The study was limited by several factors including the descriptive nature of the service project and the inability to obtain the vaccination status of all enrolled students. In addition, the schools were located in lower income areas, which might limit the generalizability of the findings, the researchers noted. However, the results suggest that “SLVPs may be more successful not only when they include all vaccines, but also when conducted in the fall prior to the onset of preparation for high-stakes state testing,” they said.
The study was supported by the Society for Adolescent Health through a grant from Merck. One study coauthor previously received salary support from Merck.
Approximately 86% of middle school students were vaccinated against HPV in a school setting in a review of data from a service project involving more than 8,000 students.
“School-located vaccination programs (SLVPs) provide access to vaccination for those adolescents whose parents cannot miss work or other daytime commitments or those who have multiple after-school commitments,” wrote Dr. Amy Middleman of the University of Oklahoma Health Science Center, Oklahoma City, and her colleagues. Data from previous studies suggest reluctance on the part of parents to allow their children to receive the human papillomavirus vaccine (HPV) in a school setting, but such data may not reflect parents’ opinions when a school-based program is actually available, the researchers noted.
The researchers tested SLVPs at eight middle schools including 8,333 students; 80% were Hispanic, 17% were black, 1% were white, and the remainder of students’ ethnicities were not identified (Human Vaccines & Immunotherapeutics. 2016 Aug. doi: 10.1080/21645515.2016.1208326).
School-based vaccinations were scheduled for three times: September/October 2012, March/April 2013, and September/October 2013; the findings reflect the first two visits.
The SLVPs included the following vaccines: HPV, influenza, Tdap, meningococcal conjugate vaccine (MCV4), hepatitis A, varicella, and MMR. A total of 1,674 vaccines were administered in the fall of 2012, and 532 were administered in the spring of 2013. Overall, 449 of 524 (86%) students in the fall program and 161 of 188 (86%) in the spring program received the HPV vaccine.
The study was limited by several factors including the descriptive nature of the service project and the inability to obtain the vaccination status of all enrolled students. In addition, the schools were located in lower income areas, which might limit the generalizability of the findings, the researchers noted. However, the results suggest that “SLVPs may be more successful not only when they include all vaccines, but also when conducted in the fall prior to the onset of preparation for high-stakes state testing,” they said.
The study was supported by the Society for Adolescent Health through a grant from Merck. One study coauthor previously received salary support from Merck.
Approximately 86% of middle school students were vaccinated against HPV in a school setting in a review of data from a service project involving more than 8,000 students.
“School-located vaccination programs (SLVPs) provide access to vaccination for those adolescents whose parents cannot miss work or other daytime commitments or those who have multiple after-school commitments,” wrote Dr. Amy Middleman of the University of Oklahoma Health Science Center, Oklahoma City, and her colleagues. Data from previous studies suggest reluctance on the part of parents to allow their children to receive the human papillomavirus vaccine (HPV) in a school setting, but such data may not reflect parents’ opinions when a school-based program is actually available, the researchers noted.
The researchers tested SLVPs at eight middle schools including 8,333 students; 80% were Hispanic, 17% were black, 1% were white, and the remainder of students’ ethnicities were not identified (Human Vaccines & Immunotherapeutics. 2016 Aug. doi: 10.1080/21645515.2016.1208326).
School-based vaccinations were scheduled for three times: September/October 2012, March/April 2013, and September/October 2013; the findings reflect the first two visits.
The SLVPs included the following vaccines: HPV, influenza, Tdap, meningococcal conjugate vaccine (MCV4), hepatitis A, varicella, and MMR. A total of 1,674 vaccines were administered in the fall of 2012, and 532 were administered in the spring of 2013. Overall, 449 of 524 (86%) students in the fall program and 161 of 188 (86%) in the spring program received the HPV vaccine.
The study was limited by several factors including the descriptive nature of the service project and the inability to obtain the vaccination status of all enrolled students. In addition, the schools were located in lower income areas, which might limit the generalizability of the findings, the researchers noted. However, the results suggest that “SLVPs may be more successful not only when they include all vaccines, but also when conducted in the fall prior to the onset of preparation for high-stakes state testing,” they said.
The study was supported by the Society for Adolescent Health through a grant from Merck. One study coauthor previously received salary support from Merck.
FROM HUMAN VACCINES & IMMUNOTHERAPEUTICS
Key clinical point: Parents already supportive of school-based vaccination are likely to support school-based HPV vaccination as well.
Major finding: Approximately 86% of adolescents participating in a school-based program received an HPV vaccine.
Data source: A descriptive study based on a service project that included 8,333 middle school students and parents.
Disclosures: The study was supported by the Society for Adolescent Health through a grant from Merck. One study coauthor previously received salary support from Merck.
Data-based Recommendations for Dialysis
Q) I work in a cardiology practice. Recently, a patient on dialysis mentioned that her nephrology practitioner recommended either home therapy or nocturnal dialysis. Why would someone recommend these, and what are the differences between home, nocturnal, and regular daytime dialysis?
Patients usually require dialysis when 90% or more of their renal function is lost.5 This can happen acutely or result from a chronic process. Dialysis performs many of the functions of a kidney, such as removing waste and fluid buildup that damaged kidneys cannot. It also helps maintain electrolyte balance.
There are several forms of hemodialysis including home, incenter, and nocturnal; the most frequently used is in-center hemodialysis.5 Patients on in-center hemodialysis visit the center three times a week, and their treatments last from three to five hours; the nationwide average is four hours. These patients have very restricted schedules and must maintain their appointments with limited flexibility. Food, drinks, and nonmedical personnel may not be allowed in the treatment area. Between treatments, patients must follow a diet that restricts fluid, sodium, and potassium intake.
Home dialysis has become a popular alternative, since it may be done in a location and at a time that is convenient for the patient. With more flexibility, many patients are able to continue working and feel like they have a more “normal” life. Types of home dialysis include home hemodialysis (HHD) or peritoneal dialysis (PD). A relative or friend may need to assist the patient during HHD, which is undergone more frequently (between five and seven days per week) and for a shorter duration of time than in-center dialysis. PD is done every day, either at night or multiple times throughout the day. Although no partner is needed for PD, a medical provider is available by phone to address any concerns that may arise during treatment.
Nocturnal hemodialysis is similar to daytime in-center hemodialysis, but it occurs while the patient is asleep. The treatment duration is longer (an average of eight hours per treatment). The slower blood flow allows for gentler dialysis. Patients who undergo nocturnal hemodialysis have higher survival and lower hospitalization rates, with better phosphorus control and blood pressure.6 This is attributed to the slower removal of excess fluid and more effective clearance of toxins.
So, why is your patient being encouraged to consider home or nocturnal dialysis? Studies have shown that for the cardiac patient, slower, gentler dialysis is preferable.7 The clinician who recommended it has the patient’s best interest in mind. —TAH
Tricia A. Howard, MHS, PA-C, DFAAPA
PA Program, South University, Savannah, Georgia
5. Gilbert S, Weiner DE. National Kidney Foundation Primer on Kidney Diseases. 6th ed. Philadelphia, PA: Elsevier; 2014.
6. Lacson E, Wang W, Lester K, et al. Outcomes associated with in-center nocturnal hemodialysis from a large multicenter program. Clin J Am Soc Nephrol. 2010;5(2):220-226.
7. Lin J, Berns JS. Is hemodialysis bad for the heart? Semin Dial. 2012;25(1):86-87.
Q) I work in a cardiology practice. Recently, a patient on dialysis mentioned that her nephrology practitioner recommended either home therapy or nocturnal dialysis. Why would someone recommend these, and what are the differences between home, nocturnal, and regular daytime dialysis?
Patients usually require dialysis when 90% or more of their renal function is lost.5 This can happen acutely or result from a chronic process. Dialysis performs many of the functions of a kidney, such as removing waste and fluid buildup that damaged kidneys cannot. It also helps maintain electrolyte balance.
There are several forms of hemodialysis including home, incenter, and nocturnal; the most frequently used is in-center hemodialysis.5 Patients on in-center hemodialysis visit the center three times a week, and their treatments last from three to five hours; the nationwide average is four hours. These patients have very restricted schedules and must maintain their appointments with limited flexibility. Food, drinks, and nonmedical personnel may not be allowed in the treatment area. Between treatments, patients must follow a diet that restricts fluid, sodium, and potassium intake.
Home dialysis has become a popular alternative, since it may be done in a location and at a time that is convenient for the patient. With more flexibility, many patients are able to continue working and feel like they have a more “normal” life. Types of home dialysis include home hemodialysis (HHD) or peritoneal dialysis (PD). A relative or friend may need to assist the patient during HHD, which is undergone more frequently (between five and seven days per week) and for a shorter duration of time than in-center dialysis. PD is done every day, either at night or multiple times throughout the day. Although no partner is needed for PD, a medical provider is available by phone to address any concerns that may arise during treatment.
Nocturnal hemodialysis is similar to daytime in-center hemodialysis, but it occurs while the patient is asleep. The treatment duration is longer (an average of eight hours per treatment). The slower blood flow allows for gentler dialysis. Patients who undergo nocturnal hemodialysis have higher survival and lower hospitalization rates, with better phosphorus control and blood pressure.6 This is attributed to the slower removal of excess fluid and more effective clearance of toxins.
So, why is your patient being encouraged to consider home or nocturnal dialysis? Studies have shown that for the cardiac patient, slower, gentler dialysis is preferable.7 The clinician who recommended it has the patient’s best interest in mind. —TAH
Tricia A. Howard, MHS, PA-C, DFAAPA
PA Program, South University, Savannah, Georgia
Q) I work in a cardiology practice. Recently, a patient on dialysis mentioned that her nephrology practitioner recommended either home therapy or nocturnal dialysis. Why would someone recommend these, and what are the differences between home, nocturnal, and regular daytime dialysis?
Patients usually require dialysis when 90% or more of their renal function is lost.5 This can happen acutely or result from a chronic process. Dialysis performs many of the functions of a kidney, such as removing waste and fluid buildup that damaged kidneys cannot. It also helps maintain electrolyte balance.
There are several forms of hemodialysis including home, incenter, and nocturnal; the most frequently used is in-center hemodialysis.5 Patients on in-center hemodialysis visit the center three times a week, and their treatments last from three to five hours; the nationwide average is four hours. These patients have very restricted schedules and must maintain their appointments with limited flexibility. Food, drinks, and nonmedical personnel may not be allowed in the treatment area. Between treatments, patients must follow a diet that restricts fluid, sodium, and potassium intake.
Home dialysis has become a popular alternative, since it may be done in a location and at a time that is convenient for the patient. With more flexibility, many patients are able to continue working and feel like they have a more “normal” life. Types of home dialysis include home hemodialysis (HHD) or peritoneal dialysis (PD). A relative or friend may need to assist the patient during HHD, which is undergone more frequently (between five and seven days per week) and for a shorter duration of time than in-center dialysis. PD is done every day, either at night or multiple times throughout the day. Although no partner is needed for PD, a medical provider is available by phone to address any concerns that may arise during treatment.
Nocturnal hemodialysis is similar to daytime in-center hemodialysis, but it occurs while the patient is asleep. The treatment duration is longer (an average of eight hours per treatment). The slower blood flow allows for gentler dialysis. Patients who undergo nocturnal hemodialysis have higher survival and lower hospitalization rates, with better phosphorus control and blood pressure.6 This is attributed to the slower removal of excess fluid and more effective clearance of toxins.
So, why is your patient being encouraged to consider home or nocturnal dialysis? Studies have shown that for the cardiac patient, slower, gentler dialysis is preferable.7 The clinician who recommended it has the patient’s best interest in mind. —TAH
Tricia A. Howard, MHS, PA-C, DFAAPA
PA Program, South University, Savannah, Georgia
5. Gilbert S, Weiner DE. National Kidney Foundation Primer on Kidney Diseases. 6th ed. Philadelphia, PA: Elsevier; 2014.
6. Lacson E, Wang W, Lester K, et al. Outcomes associated with in-center nocturnal hemodialysis from a large multicenter program. Clin J Am Soc Nephrol. 2010;5(2):220-226.
7. Lin J, Berns JS. Is hemodialysis bad for the heart? Semin Dial. 2012;25(1):86-87.
5. Gilbert S, Weiner DE. National Kidney Foundation Primer on Kidney Diseases. 6th ed. Philadelphia, PA: Elsevier; 2014.
6. Lacson E, Wang W, Lester K, et al. Outcomes associated with in-center nocturnal hemodialysis from a large multicenter program. Clin J Am Soc Nephrol. 2010;5(2):220-226.
7. Lin J, Berns JS. Is hemodialysis bad for the heart? Semin Dial. 2012;25(1):86-87.
ASH launches sickle cell coalition
To improve the lives of individuals with sickle cell disease, the American Society of Hematology has launched the Sickle Cell Disease Coalition (SCDC). Creation of the coalition with 20 other organizations coincides with the release of “State of Sickle Cell Disease: 2016” – a report detailing the status of access to care for patients with sickle cell disease, gaps in the training and education of health care professionals, areas of interest for research and clinical trials, and the global state of the disease. All four of these areas need improvement, ASH officials said in a statement.
“Not only are individuals with [sickle cell disease] burdened by the pain and disability that comes with a chronic condition, but they also have very few accessible treatment options due to our fragmented health care system,” Dr. Charles S. Abrams, president of ASH, said in the statement. Sickle cell disease affects approximately 100,000 Americans and remains a growing global health concern, according to the statement.
The coalition’s goals include development of evidence-based treatment guidelines to improve quality of care, education of clinicians to increase the number of health care providers available to treat sickle cell disease, investment in research and clinical trials to improve existing treatments and develop new ones, and expansion of early intervention programs to help ease the global burden of sickle cell disease.
For more information regarding the coalition, click here.
To improve the lives of individuals with sickle cell disease, the American Society of Hematology has launched the Sickle Cell Disease Coalition (SCDC). Creation of the coalition with 20 other organizations coincides with the release of “State of Sickle Cell Disease: 2016” – a report detailing the status of access to care for patients with sickle cell disease, gaps in the training and education of health care professionals, areas of interest for research and clinical trials, and the global state of the disease. All four of these areas need improvement, ASH officials said in a statement.
“Not only are individuals with [sickle cell disease] burdened by the pain and disability that comes with a chronic condition, but they also have very few accessible treatment options due to our fragmented health care system,” Dr. Charles S. Abrams, president of ASH, said in the statement. Sickle cell disease affects approximately 100,000 Americans and remains a growing global health concern, according to the statement.
The coalition’s goals include development of evidence-based treatment guidelines to improve quality of care, education of clinicians to increase the number of health care providers available to treat sickle cell disease, investment in research and clinical trials to improve existing treatments and develop new ones, and expansion of early intervention programs to help ease the global burden of sickle cell disease.
For more information regarding the coalition, click here.
To improve the lives of individuals with sickle cell disease, the American Society of Hematology has launched the Sickle Cell Disease Coalition (SCDC). Creation of the coalition with 20 other organizations coincides with the release of “State of Sickle Cell Disease: 2016” – a report detailing the status of access to care for patients with sickle cell disease, gaps in the training and education of health care professionals, areas of interest for research and clinical trials, and the global state of the disease. All four of these areas need improvement, ASH officials said in a statement.
“Not only are individuals with [sickle cell disease] burdened by the pain and disability that comes with a chronic condition, but they also have very few accessible treatment options due to our fragmented health care system,” Dr. Charles S. Abrams, president of ASH, said in the statement. Sickle cell disease affects approximately 100,000 Americans and remains a growing global health concern, according to the statement.
The coalition’s goals include development of evidence-based treatment guidelines to improve quality of care, education of clinicians to increase the number of health care providers available to treat sickle cell disease, investment in research and clinical trials to improve existing treatments and develop new ones, and expansion of early intervention programs to help ease the global burden of sickle cell disease.
For more information regarding the coalition, click here.