User login
Emergency Ultrasound: Tips and Tricks for Imaging Digits
Clinicians familiar with point-of-care (POC) ultrasound know that structures such as the hands and feet require the use of the linear high-frequency transducer to obtain quality images. In reality, however, employing the standard technique (ie, applying gel to the probe surface and scanning the structure) can be challenging due to the uneven surfaces of the fingers and toes; therefore, obtaining good contact with the transducer is harder than it may seem at first glance. Additionally, since these structures are superficial, they are usually seen on the top half of the ultrasound display, while the focal zone of most ultrasound machines is located in the middle of the display and is nonadjustable.
We describe two simple adjuncts to POC ultrasound that can assist in visualizing digital structures with greater ease and improved image resolution: the water bath1,2 and standoff pad techniques.
Water Bath Technique
In the water bath technique, one fills a small basin with lukewarm water to a depth point where the extremity being studied (ie, hand or foot) is mostly—but not completely—submerged in the water bath. After the extremity is submerged, the high-frequency probe is then placed into the water bath (Figure 1). When employing this technique, the transducer does not need to make contact with the patient’s skin. Since the water acts as an excellent conduction medium for sound waves, no ultrasound gel is required. For a video demonstrating the use of the water bath technique to evaluate the distal tip of the finger, see below.
Standoff Pad
Another technique that enhances POC imaging of the digits involves a standoff pad. A variety of commercially available standoff pads can be used for this technique. Alternatively, the clinician can easily create a standoff pad using supplies that are readily available in the ED. One such method is to fill a latex glove with water, tie off the filled glove, and place it on top of the extremity to be imaged (Figure 2). The water in the glove will facilitate sound-wave transmission.
Pathology
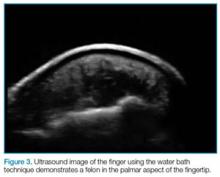
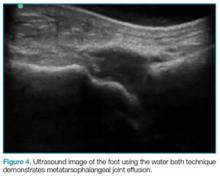
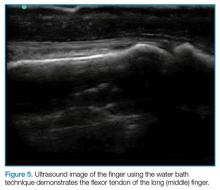
The water bath and standoff pad techniques can facilitate visualization of several pathologies, including felons (Figure 3), flexor tenosynovitis, phalangeal and metacarpal/metatarsal fractures, and interphalangeal, metacarpophalangeal, and metatarsophalangeal joint effusions (Figure 4). In addition, these techniques also assist in visualizing digit tendons to evaluate for tears in these structures (Figure 5).
Summary
Point-of-care ultrasound imaging to evaluate superficial body parts such as hands or feet can be challenging due to the irregular shape and uneven surface of these structures. The employment of adjuncts such as the water bath or standoff pad techniques can mitigate these challenges, facilitating the acquisition of high-resolution images and providing easier identification of pathology.
1. Krishnamurthy R, Yoo JH, Thapa M, Callahan MJ. Water-bath method for sonographic evaluation of superficial structures of the extremities in children. Pediatr Radiol. 2013;4(Suppl 1):S41-S47. DOI:10.1007/s00247-012-2592-y.
2. Jeong HY, Krishnamurthy R. 1012: Water-bath method for sonographic evaluation of superficial structures of the extremities. Ultrasound Med Biol. 2009;35(8):S101-S102. doi:10.1016/j.ultrasmedbio.2009.06.394.
Clinicians familiar with point-of-care (POC) ultrasound know that structures such as the hands and feet require the use of the linear high-frequency transducer to obtain quality images. In reality, however, employing the standard technique (ie, applying gel to the probe surface and scanning the structure) can be challenging due to the uneven surfaces of the fingers and toes; therefore, obtaining good contact with the transducer is harder than it may seem at first glance. Additionally, since these structures are superficial, they are usually seen on the top half of the ultrasound display, while the focal zone of most ultrasound machines is located in the middle of the display and is nonadjustable.
We describe two simple adjuncts to POC ultrasound that can assist in visualizing digital structures with greater ease and improved image resolution: the water bath1,2 and standoff pad techniques.
Water Bath Technique
In the water bath technique, one fills a small basin with lukewarm water to a depth point where the extremity being studied (ie, hand or foot) is mostly—but not completely—submerged in the water bath. After the extremity is submerged, the high-frequency probe is then placed into the water bath (Figure 1). When employing this technique, the transducer does not need to make contact with the patient’s skin. Since the water acts as an excellent conduction medium for sound waves, no ultrasound gel is required. For a video demonstrating the use of the water bath technique to evaluate the distal tip of the finger, see below.
Standoff Pad
Another technique that enhances POC imaging of the digits involves a standoff pad. A variety of commercially available standoff pads can be used for this technique. Alternatively, the clinician can easily create a standoff pad using supplies that are readily available in the ED. One such method is to fill a latex glove with water, tie off the filled glove, and place it on top of the extremity to be imaged (Figure 2). The water in the glove will facilitate sound-wave transmission.
Pathology
The water bath and standoff pad techniques can facilitate visualization of several pathologies, including felons (Figure 3), flexor tenosynovitis, phalangeal and metacarpal/metatarsal fractures, and interphalangeal, metacarpophalangeal, and metatarsophalangeal joint effusions (Figure 4). In addition, these techniques also assist in visualizing digit tendons to evaluate for tears in these structures (Figure 5).
Summary
Point-of-care ultrasound imaging to evaluate superficial body parts such as hands or feet can be challenging due to the irregular shape and uneven surface of these structures. The employment of adjuncts such as the water bath or standoff pad techniques can mitigate these challenges, facilitating the acquisition of high-resolution images and providing easier identification of pathology.
Clinicians familiar with point-of-care (POC) ultrasound know that structures such as the hands and feet require the use of the linear high-frequency transducer to obtain quality images. In reality, however, employing the standard technique (ie, applying gel to the probe surface and scanning the structure) can be challenging due to the uneven surfaces of the fingers and toes; therefore, obtaining good contact with the transducer is harder than it may seem at first glance. Additionally, since these structures are superficial, they are usually seen on the top half of the ultrasound display, while the focal zone of most ultrasound machines is located in the middle of the display and is nonadjustable.
We describe two simple adjuncts to POC ultrasound that can assist in visualizing digital structures with greater ease and improved image resolution: the water bath1,2 and standoff pad techniques.
Water Bath Technique
In the water bath technique, one fills a small basin with lukewarm water to a depth point where the extremity being studied (ie, hand or foot) is mostly—but not completely—submerged in the water bath. After the extremity is submerged, the high-frequency probe is then placed into the water bath (Figure 1). When employing this technique, the transducer does not need to make contact with the patient’s skin. Since the water acts as an excellent conduction medium for sound waves, no ultrasound gel is required. For a video demonstrating the use of the water bath technique to evaluate the distal tip of the finger, see below.
Standoff Pad
Another technique that enhances POC imaging of the digits involves a standoff pad. A variety of commercially available standoff pads can be used for this technique. Alternatively, the clinician can easily create a standoff pad using supplies that are readily available in the ED. One such method is to fill a latex glove with water, tie off the filled glove, and place it on top of the extremity to be imaged (Figure 2). The water in the glove will facilitate sound-wave transmission.
Pathology
The water bath and standoff pad techniques can facilitate visualization of several pathologies, including felons (Figure 3), flexor tenosynovitis, phalangeal and metacarpal/metatarsal fractures, and interphalangeal, metacarpophalangeal, and metatarsophalangeal joint effusions (Figure 4). In addition, these techniques also assist in visualizing digit tendons to evaluate for tears in these structures (Figure 5).
Summary
Point-of-care ultrasound imaging to evaluate superficial body parts such as hands or feet can be challenging due to the irregular shape and uneven surface of these structures. The employment of adjuncts such as the water bath or standoff pad techniques can mitigate these challenges, facilitating the acquisition of high-resolution images and providing easier identification of pathology.
1. Krishnamurthy R, Yoo JH, Thapa M, Callahan MJ. Water-bath method for sonographic evaluation of superficial structures of the extremities in children. Pediatr Radiol. 2013;4(Suppl 1):S41-S47. DOI:10.1007/s00247-012-2592-y.
2. Jeong HY, Krishnamurthy R. 1012: Water-bath method for sonographic evaluation of superficial structures of the extremities. Ultrasound Med Biol. 2009;35(8):S101-S102. doi:10.1016/j.ultrasmedbio.2009.06.394.
1. Krishnamurthy R, Yoo JH, Thapa M, Callahan MJ. Water-bath method for sonographic evaluation of superficial structures of the extremities in children. Pediatr Radiol. 2013;4(Suppl 1):S41-S47. DOI:10.1007/s00247-012-2592-y.
2. Jeong HY, Krishnamurthy R. 1012: Water-bath method for sonographic evaluation of superficial structures of the extremities. Ultrasound Med Biol. 2009;35(8):S101-S102. doi:10.1016/j.ultrasmedbio.2009.06.394.
No more popping, swatting, slapping, and spanking
During a recent primary care rotation in northeast Philadelphia, I was privileged to witness a community experience on a daily basis. Each morning I took the elevated subway to the end of the line out of the city, and transferred to a bus to get to the office. The 24 bus at 8:30 a.m. has the same faces every day, making their way to work at various stops along the route. There was also a mother of a particularly cute set of twin boys. Every day she also got on the “El” and transferred to the bus with me, in order to get her boys to the day care she also went to as a child, where she told me she trusted her kids so much it was worth the daily trip.
On my last day of the rotation, enjoying the familiar scene again of people saying good morning to each other on the 24, everyone’s pleasant morning was suddenly interrupted. The twins were being particularly annoying to their mother that day, and she began disciplining them. The entire bus witnessed this: a mother “popping” her boys on the arms repeatedly while yelling loudly, “No hitting! You don’t hit each other and you don’t hit mommy!”
As a pediatrician, this was hard to watch. “Popping” is a common practice here in Philadelphia, and it involves a quick but loud slap that leaves no mark and I assume only stings a second or two, and thus is not too physically harmful. I chose not to speak up as they are not my patients, and it was not my place to be confrontational at that moment. But the thought that went through my head immediately was, “How can this caring and well-intentioned mother expect her sons to learn the lesson to not hit while she is doing exactly that?”
Get online and you’ll see plenty of bloggers arguing the topic of popping, swatting, slapping, and spanking. People say, “My generation was spanked and we turned out fine!” or “It toughens kids up and teaches them discipline.” But the main problem here is the mixed message. The old adage, “Do as I say and not as I do,” simply does not work in childhood. The young developing brain of a child can’t make that distinction, and learning by example from their most loved ones on this planet – their parents – is the single most influential factor in their education.
Just because something is common does not make it right. A few short decades ago seat belts were not commonly worn, and we all know of their benefits now. Currently in America, obesity is becoming the normal body shape for adults and children alike, and every physician is trying their best to combat it. Popping, swatting, slapping, and spanking, in this pediatric resident’s opinion, is far too common, and if explained to parents why the practice is counter-intuitive and ineffectual, I do hope it can be a thing of the past some day, too.
Dr. Beardmore is a pediatric resident at Albert Einstein Medical Center and St. Christopher’s Hospital for Children, Philadelphia. Email him at [email protected].
During a recent primary care rotation in northeast Philadelphia, I was privileged to witness a community experience on a daily basis. Each morning I took the elevated subway to the end of the line out of the city, and transferred to a bus to get to the office. The 24 bus at 8:30 a.m. has the same faces every day, making their way to work at various stops along the route. There was also a mother of a particularly cute set of twin boys. Every day she also got on the “El” and transferred to the bus with me, in order to get her boys to the day care she also went to as a child, where she told me she trusted her kids so much it was worth the daily trip.
On my last day of the rotation, enjoying the familiar scene again of people saying good morning to each other on the 24, everyone’s pleasant morning was suddenly interrupted. The twins were being particularly annoying to their mother that day, and she began disciplining them. The entire bus witnessed this: a mother “popping” her boys on the arms repeatedly while yelling loudly, “No hitting! You don’t hit each other and you don’t hit mommy!”
As a pediatrician, this was hard to watch. “Popping” is a common practice here in Philadelphia, and it involves a quick but loud slap that leaves no mark and I assume only stings a second or two, and thus is not too physically harmful. I chose not to speak up as they are not my patients, and it was not my place to be confrontational at that moment. But the thought that went through my head immediately was, “How can this caring and well-intentioned mother expect her sons to learn the lesson to not hit while she is doing exactly that?”
Get online and you’ll see plenty of bloggers arguing the topic of popping, swatting, slapping, and spanking. People say, “My generation was spanked and we turned out fine!” or “It toughens kids up and teaches them discipline.” But the main problem here is the mixed message. The old adage, “Do as I say and not as I do,” simply does not work in childhood. The young developing brain of a child can’t make that distinction, and learning by example from their most loved ones on this planet – their parents – is the single most influential factor in their education.
Just because something is common does not make it right. A few short decades ago seat belts were not commonly worn, and we all know of their benefits now. Currently in America, obesity is becoming the normal body shape for adults and children alike, and every physician is trying their best to combat it. Popping, swatting, slapping, and spanking, in this pediatric resident’s opinion, is far too common, and if explained to parents why the practice is counter-intuitive and ineffectual, I do hope it can be a thing of the past some day, too.
Dr. Beardmore is a pediatric resident at Albert Einstein Medical Center and St. Christopher’s Hospital for Children, Philadelphia. Email him at [email protected].
During a recent primary care rotation in northeast Philadelphia, I was privileged to witness a community experience on a daily basis. Each morning I took the elevated subway to the end of the line out of the city, and transferred to a bus to get to the office. The 24 bus at 8:30 a.m. has the same faces every day, making their way to work at various stops along the route. There was also a mother of a particularly cute set of twin boys. Every day she also got on the “El” and transferred to the bus with me, in order to get her boys to the day care she also went to as a child, where she told me she trusted her kids so much it was worth the daily trip.
On my last day of the rotation, enjoying the familiar scene again of people saying good morning to each other on the 24, everyone’s pleasant morning was suddenly interrupted. The twins were being particularly annoying to their mother that day, and she began disciplining them. The entire bus witnessed this: a mother “popping” her boys on the arms repeatedly while yelling loudly, “No hitting! You don’t hit each other and you don’t hit mommy!”
As a pediatrician, this was hard to watch. “Popping” is a common practice here in Philadelphia, and it involves a quick but loud slap that leaves no mark and I assume only stings a second or two, and thus is not too physically harmful. I chose not to speak up as they are not my patients, and it was not my place to be confrontational at that moment. But the thought that went through my head immediately was, “How can this caring and well-intentioned mother expect her sons to learn the lesson to not hit while she is doing exactly that?”
Get online and you’ll see plenty of bloggers arguing the topic of popping, swatting, slapping, and spanking. People say, “My generation was spanked and we turned out fine!” or “It toughens kids up and teaches them discipline.” But the main problem here is the mixed message. The old adage, “Do as I say and not as I do,” simply does not work in childhood. The young developing brain of a child can’t make that distinction, and learning by example from their most loved ones on this planet – their parents – is the single most influential factor in their education.
Just because something is common does not make it right. A few short decades ago seat belts were not commonly worn, and we all know of their benefits now. Currently in America, obesity is becoming the normal body shape for adults and children alike, and every physician is trying their best to combat it. Popping, swatting, slapping, and spanking, in this pediatric resident’s opinion, is far too common, and if explained to parents why the practice is counter-intuitive and ineffectual, I do hope it can be a thing of the past some day, too.
Dr. Beardmore is a pediatric resident at Albert Einstein Medical Center and St. Christopher’s Hospital for Children, Philadelphia. Email him at [email protected].
Drug combo shows promise for non–clear cell renal cell carcinoma
The combination of an mTOR complex 1 inhibitor (everolimus) plus a VEGF inhibitor (bevacizumab) showed promise against advanced non–clear cell renal cell carcinoma characterized by papillary features in a small manufacturer-sponsored phase II trial, according to a report published online Sept. 6 in the Journal of Clinical Oncology.
Non–clear cell renal cell carcinomas (ncRCCs) are a diverse mixture of heterogeneous malignancies and include papillary, chromophobe, medullary, collecting duct, and a variety of unclassified tumor types. Researchers performed a single-center trial to assess the effectiveness of combined everolimus plus bevacizumab in 35 treatment-naive patients who presented with advanced disease representing all of these histologic types. The unclassified subgroup (23 patients) included several tumors with prominent papillary architectural features that did not fulfill other criteria for papillary RCC, said Martin H. Voss, MD, of Memorial Sloan Kettering Cancer Center, New York, and his associates.
A total of 18 patients (53%) were alive and free of disease progression at 6 months, and 10 (29%) were alive and progression free at 12 months. Two patients still were receiving study treatment at the time of publication, after 20.2 and 30.4 months of therapy, respectively.
“Objective responses were observed in a sizable proportion of subjects with significant papillary (7 of 18) or chromophobe (2 of 5) tumor components but rarely in patients with unclassified RCC without papillary features (1 of 9) or those with medullary RCC (0 of 2),” Dr. Voss and his associates reported. Among patients with unclassified RCC, the 14 whose cancer had a major papillary component showed an objective response rate of 43%, a median progression-free survival of 12.9 months, and a median overall survival of 28.2 months. In contrast, the nine patients whose cancer did not have a major papillary component showed an objective response rate of 11%, a median progression-free survival of 1.9 months, and a median overall survival of 9.3 months, the investigators said (J Clin Oncol. 2016 Sept 6. doi: 10.1200/JCO.2016.67.9084).
Treatment was generally well tolerated, even though there were frequent low-grade toxicities. High-grade toxicities known to be associated with mTOR complex 1 inhibitors or VEGF inhibitors included hyperglycemia (11%), hypertriglyceridemia (14%), lymphopenia (20%), hypertension (29%), and proteinuria (18%). There were two patient deaths from gastrointestinal hemorrhage, one of which was considered possibly related to bevacizumab.
Archived tissue samples were available for genetic analysis for some patients. Acquired mutations in the ARID1A gene were noted in 5 of 14 tumors with major papillary components, and all 5 of those patients achieved more than 6 months of progression-free survival with the combination therapy. In contrast, no ARID1A mutations were detected in any of the patients who had shorter progression-free survival, and none were detected in any of the tumors that did not have papillary components. This suggests that ARID1A “merits further study for its functional role in papillary RCC variants and as a candidate biomarker for future study of everolimus plus bevacizumab,” Dr. Voss and his associates said.
Novartis supported the study. Dr. Voss reported ties to Novartis, Calithera Biosciences, Natera, GlaxoSmithKline, Exelixis, Pfizer, Bristol-Myers Squibb, Genentech, and Takeda; his associates reported ties to numerous industry sources.
The signal of activity Voss et al. found in the subgroup of non–clear cell RCCs with papillary features is compelling, but studies with larger sample sizes are necessary. We need multicenter randomized trials that specifically focus on particular histologic subtypes and provide detailed molecular characterization.
Fortunately, another recent phase II study also found that pairing an mTOR inhibitor with a VEGF inhibitor (everolimus plus lenvatinib) improved the response rate, progression-free survival, and overall survival in clear cell RCC. The ensuing regulatory approval of this combination paves the way for further studies of similar regimens for tumors with papillary features.
Sumanta K. Pal, MD, is in the department of medical oncology at City of Hope Comprehensive Cancer Center, Duarte, Calif. Financial disclosures for Dr. Pal and his associates are available at www.jco.org. Dr. Pal and his associates made these remarks in an editorial accompanying Dr. Voss’s report (J Clin Oncol. 2016 Sept 6. doi: 10.1200/JCO.2016.69.3572).
The signal of activity Voss et al. found in the subgroup of non–clear cell RCCs with papillary features is compelling, but studies with larger sample sizes are necessary. We need multicenter randomized trials that specifically focus on particular histologic subtypes and provide detailed molecular characterization.
Fortunately, another recent phase II study also found that pairing an mTOR inhibitor with a VEGF inhibitor (everolimus plus lenvatinib) improved the response rate, progression-free survival, and overall survival in clear cell RCC. The ensuing regulatory approval of this combination paves the way for further studies of similar regimens for tumors with papillary features.
Sumanta K. Pal, MD, is in the department of medical oncology at City of Hope Comprehensive Cancer Center, Duarte, Calif. Financial disclosures for Dr. Pal and his associates are available at www.jco.org. Dr. Pal and his associates made these remarks in an editorial accompanying Dr. Voss’s report (J Clin Oncol. 2016 Sept 6. doi: 10.1200/JCO.2016.69.3572).
The signal of activity Voss et al. found in the subgroup of non–clear cell RCCs with papillary features is compelling, but studies with larger sample sizes are necessary. We need multicenter randomized trials that specifically focus on particular histologic subtypes and provide detailed molecular characterization.
Fortunately, another recent phase II study also found that pairing an mTOR inhibitor with a VEGF inhibitor (everolimus plus lenvatinib) improved the response rate, progression-free survival, and overall survival in clear cell RCC. The ensuing regulatory approval of this combination paves the way for further studies of similar regimens for tumors with papillary features.
Sumanta K. Pal, MD, is in the department of medical oncology at City of Hope Comprehensive Cancer Center, Duarte, Calif. Financial disclosures for Dr. Pal and his associates are available at www.jco.org. Dr. Pal and his associates made these remarks in an editorial accompanying Dr. Voss’s report (J Clin Oncol. 2016 Sept 6. doi: 10.1200/JCO.2016.69.3572).
The combination of an mTOR complex 1 inhibitor (everolimus) plus a VEGF inhibitor (bevacizumab) showed promise against advanced non–clear cell renal cell carcinoma characterized by papillary features in a small manufacturer-sponsored phase II trial, according to a report published online Sept. 6 in the Journal of Clinical Oncology.
Non–clear cell renal cell carcinomas (ncRCCs) are a diverse mixture of heterogeneous malignancies and include papillary, chromophobe, medullary, collecting duct, and a variety of unclassified tumor types. Researchers performed a single-center trial to assess the effectiveness of combined everolimus plus bevacizumab in 35 treatment-naive patients who presented with advanced disease representing all of these histologic types. The unclassified subgroup (23 patients) included several tumors with prominent papillary architectural features that did not fulfill other criteria for papillary RCC, said Martin H. Voss, MD, of Memorial Sloan Kettering Cancer Center, New York, and his associates.
A total of 18 patients (53%) were alive and free of disease progression at 6 months, and 10 (29%) were alive and progression free at 12 months. Two patients still were receiving study treatment at the time of publication, after 20.2 and 30.4 months of therapy, respectively.
“Objective responses were observed in a sizable proportion of subjects with significant papillary (7 of 18) or chromophobe (2 of 5) tumor components but rarely in patients with unclassified RCC without papillary features (1 of 9) or those with medullary RCC (0 of 2),” Dr. Voss and his associates reported. Among patients with unclassified RCC, the 14 whose cancer had a major papillary component showed an objective response rate of 43%, a median progression-free survival of 12.9 months, and a median overall survival of 28.2 months. In contrast, the nine patients whose cancer did not have a major papillary component showed an objective response rate of 11%, a median progression-free survival of 1.9 months, and a median overall survival of 9.3 months, the investigators said (J Clin Oncol. 2016 Sept 6. doi: 10.1200/JCO.2016.67.9084).
Treatment was generally well tolerated, even though there were frequent low-grade toxicities. High-grade toxicities known to be associated with mTOR complex 1 inhibitors or VEGF inhibitors included hyperglycemia (11%), hypertriglyceridemia (14%), lymphopenia (20%), hypertension (29%), and proteinuria (18%). There were two patient deaths from gastrointestinal hemorrhage, one of which was considered possibly related to bevacizumab.
Archived tissue samples were available for genetic analysis for some patients. Acquired mutations in the ARID1A gene were noted in 5 of 14 tumors with major papillary components, and all 5 of those patients achieved more than 6 months of progression-free survival with the combination therapy. In contrast, no ARID1A mutations were detected in any of the patients who had shorter progression-free survival, and none were detected in any of the tumors that did not have papillary components. This suggests that ARID1A “merits further study for its functional role in papillary RCC variants and as a candidate biomarker for future study of everolimus plus bevacizumab,” Dr. Voss and his associates said.
Novartis supported the study. Dr. Voss reported ties to Novartis, Calithera Biosciences, Natera, GlaxoSmithKline, Exelixis, Pfizer, Bristol-Myers Squibb, Genentech, and Takeda; his associates reported ties to numerous industry sources.
The combination of an mTOR complex 1 inhibitor (everolimus) plus a VEGF inhibitor (bevacizumab) showed promise against advanced non–clear cell renal cell carcinoma characterized by papillary features in a small manufacturer-sponsored phase II trial, according to a report published online Sept. 6 in the Journal of Clinical Oncology.
Non–clear cell renal cell carcinomas (ncRCCs) are a diverse mixture of heterogeneous malignancies and include papillary, chromophobe, medullary, collecting duct, and a variety of unclassified tumor types. Researchers performed a single-center trial to assess the effectiveness of combined everolimus plus bevacizumab in 35 treatment-naive patients who presented with advanced disease representing all of these histologic types. The unclassified subgroup (23 patients) included several tumors with prominent papillary architectural features that did not fulfill other criteria for papillary RCC, said Martin H. Voss, MD, of Memorial Sloan Kettering Cancer Center, New York, and his associates.
A total of 18 patients (53%) were alive and free of disease progression at 6 months, and 10 (29%) were alive and progression free at 12 months. Two patients still were receiving study treatment at the time of publication, after 20.2 and 30.4 months of therapy, respectively.
“Objective responses were observed in a sizable proportion of subjects with significant papillary (7 of 18) or chromophobe (2 of 5) tumor components but rarely in patients with unclassified RCC without papillary features (1 of 9) or those with medullary RCC (0 of 2),” Dr. Voss and his associates reported. Among patients with unclassified RCC, the 14 whose cancer had a major papillary component showed an objective response rate of 43%, a median progression-free survival of 12.9 months, and a median overall survival of 28.2 months. In contrast, the nine patients whose cancer did not have a major papillary component showed an objective response rate of 11%, a median progression-free survival of 1.9 months, and a median overall survival of 9.3 months, the investigators said (J Clin Oncol. 2016 Sept 6. doi: 10.1200/JCO.2016.67.9084).
Treatment was generally well tolerated, even though there were frequent low-grade toxicities. High-grade toxicities known to be associated with mTOR complex 1 inhibitors or VEGF inhibitors included hyperglycemia (11%), hypertriglyceridemia (14%), lymphopenia (20%), hypertension (29%), and proteinuria (18%). There were two patient deaths from gastrointestinal hemorrhage, one of which was considered possibly related to bevacizumab.
Archived tissue samples were available for genetic analysis for some patients. Acquired mutations in the ARID1A gene were noted in 5 of 14 tumors with major papillary components, and all 5 of those patients achieved more than 6 months of progression-free survival with the combination therapy. In contrast, no ARID1A mutations were detected in any of the patients who had shorter progression-free survival, and none were detected in any of the tumors that did not have papillary components. This suggests that ARID1A “merits further study for its functional role in papillary RCC variants and as a candidate biomarker for future study of everolimus plus bevacizumab,” Dr. Voss and his associates said.
Novartis supported the study. Dr. Voss reported ties to Novartis, Calithera Biosciences, Natera, GlaxoSmithKline, Exelixis, Pfizer, Bristol-Myers Squibb, Genentech, and Takeda; his associates reported ties to numerous industry sources.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: The combination of everolimus plus bevacizumab showed promise against advanced non–clear cell renal cell carcinoma characterized by papillary features.
Major finding: Among patients with unclassified RCC, the 14 whose cancer had a major papillary component showed an objective response rate of 43%, a median progression-free survival of 12.9 months, and a median overall survival of 28.2 months.
Data source: A small prospective single-center phase II trial involving 35 adults with treatment-naive advanced non–clear cell RCC.
Disclosures: Novartis supported the study. Dr. Voss reported ties to Novartis, Calithera Biosciences, Natera, GlaxoSmithKline, Exelixis, Pfizer, Bristol-Myers Squibb, Genentech, and Takeda; his associates reported ties to numerous industry sources.
Better survival with primary surgery in stage IIIC ovarian ca
Although the use of neoadjuvant chemotherapy for treatment of women with advanced ovarian cancer has grown significantly in recent years, a new study shows that it is associated with worse overall survival for women with stage IIIC disease, compared with primary cytoreductive surgery.
Among 594 women with advanced ovarian cancer treated at one of six major comprehensive cancer centers, median overall survival (OS) for women with stage IIIC cancers treated with neoadjuvant chemotherapy (NACT) was 33 months, compared with 43 months for women treated with primary cytoreductive surgery (PCS), reported Larissa A. Meyer, MD, of the University of Texas M.D. Anderson Cancer Center in Houston, and her colleagues.
There were no significant survival differences between chemotherapy and surgery for women with stage IV disease, however, and for these patients neoadjuvant chemotherapy was associated with fewer morbidities, and may be a better therapeutic option, the investigators reported.
“Although additional biases may persist despite propensity-score matching, our results suggest that in carefully selected patients with stage IIIC disease, PCS is associated with a survival advantage, with overall low rates of surgical morbidity. In contrast, for patients with stage IV disease, our results confirm that NACT is noninferior to PCS for survival, with fewer ICU admissions and rehospitalizations, which suggests that NACT may be preferable for patients with stage IV ovarian cancer,” they wrote in the Journal of Clinical Oncology (2016. doi: 10.1200/JCO.2016.68.1239).
The increase in the use of NACT in women with advanced ovarian cancer in the United States was spurred by two randomized clinical trials, the investigators noted. The first, published in 2010 showed that survival was similar for women with stage IIIC or IV ovarian cancer treated with either neoadjuvant chemotherapy followed by interval debulking surgery or with primary surgery followed by chemotherapy. The second study, published in 2015, found that “in women with stage III or IV ovarian cancer, survival with primary chemotherapy is noninferior to primary surgery. In this study population, the researchers stated that “giving primary chemotherapy before surgery is an acceptable standard of care for women with advanced ovarian cancer.”
To see what effect these trials had on clinical practice and outcomes in the United States, the authors conducted an observational study of patients treated at six National Cancer Institute–designated cancer centers, looking at NACT use in 1,538 women diagnosed with ovarian cancer from 2003 through 2012, and at OS, morbidity, and postoperative residual disease in a propensity score–matched sample of 594 patients.
They found that for women with stage IIIC disease, NACT use increased from 16% during the period 2003-2010, to 34% during 2011-2012. For women with stage IV disease, NACT use grew from 41% to 62% during the respective time periods (P for trend for both comparisons = .001).
As noted before, median overall survival among women with stage IIIC disease in the propensity score–matched sample was significantly shorter for those treated with primary NACT vs. PCS.
For women with stage IV disease, however, there was no significant difference in OS between those treated with NACT (median 31 months) vs. those treated with PCS (median 36 months, hazard ratio 1.16, not significant).
Women with stages IIIC and IV disease who received NACT were less likely to have one or more centimeters of residual disease postoperatively and were less likely to have an ICU admission or rehospitalization (P for all comparisons = .04). However, overall survival was lower among women with stage IIIC disease who had only microscopic residual disease or residual disease measuring 1 cm or less (HR, 1.49; P = .04).
“Future studies should prospectively consider the efficacy of NACT by extent of residual disease in unselected patients,” the authors recommended.
The study was supported by grants from the National Cancer Institute and Cancer Prevention and Research Institute of Texas. Dr. Meyer and multiple coauthors disclosed honoraria, research funding, and/or advising/consulting with various pharmaceutical companies.
Although the use of neoadjuvant chemotherapy for treatment of women with advanced ovarian cancer has grown significantly in recent years, a new study shows that it is associated with worse overall survival for women with stage IIIC disease, compared with primary cytoreductive surgery.
Among 594 women with advanced ovarian cancer treated at one of six major comprehensive cancer centers, median overall survival (OS) for women with stage IIIC cancers treated with neoadjuvant chemotherapy (NACT) was 33 months, compared with 43 months for women treated with primary cytoreductive surgery (PCS), reported Larissa A. Meyer, MD, of the University of Texas M.D. Anderson Cancer Center in Houston, and her colleagues.
There were no significant survival differences between chemotherapy and surgery for women with stage IV disease, however, and for these patients neoadjuvant chemotherapy was associated with fewer morbidities, and may be a better therapeutic option, the investigators reported.
“Although additional biases may persist despite propensity-score matching, our results suggest that in carefully selected patients with stage IIIC disease, PCS is associated with a survival advantage, with overall low rates of surgical morbidity. In contrast, for patients with stage IV disease, our results confirm that NACT is noninferior to PCS for survival, with fewer ICU admissions and rehospitalizations, which suggests that NACT may be preferable for patients with stage IV ovarian cancer,” they wrote in the Journal of Clinical Oncology (2016. doi: 10.1200/JCO.2016.68.1239).
The increase in the use of NACT in women with advanced ovarian cancer in the United States was spurred by two randomized clinical trials, the investigators noted. The first, published in 2010 showed that survival was similar for women with stage IIIC or IV ovarian cancer treated with either neoadjuvant chemotherapy followed by interval debulking surgery or with primary surgery followed by chemotherapy. The second study, published in 2015, found that “in women with stage III or IV ovarian cancer, survival with primary chemotherapy is noninferior to primary surgery. In this study population, the researchers stated that “giving primary chemotherapy before surgery is an acceptable standard of care for women with advanced ovarian cancer.”
To see what effect these trials had on clinical practice and outcomes in the United States, the authors conducted an observational study of patients treated at six National Cancer Institute–designated cancer centers, looking at NACT use in 1,538 women diagnosed with ovarian cancer from 2003 through 2012, and at OS, morbidity, and postoperative residual disease in a propensity score–matched sample of 594 patients.
They found that for women with stage IIIC disease, NACT use increased from 16% during the period 2003-2010, to 34% during 2011-2012. For women with stage IV disease, NACT use grew from 41% to 62% during the respective time periods (P for trend for both comparisons = .001).
As noted before, median overall survival among women with stage IIIC disease in the propensity score–matched sample was significantly shorter for those treated with primary NACT vs. PCS.
For women with stage IV disease, however, there was no significant difference in OS between those treated with NACT (median 31 months) vs. those treated with PCS (median 36 months, hazard ratio 1.16, not significant).
Women with stages IIIC and IV disease who received NACT were less likely to have one or more centimeters of residual disease postoperatively and were less likely to have an ICU admission or rehospitalization (P for all comparisons = .04). However, overall survival was lower among women with stage IIIC disease who had only microscopic residual disease or residual disease measuring 1 cm or less (HR, 1.49; P = .04).
“Future studies should prospectively consider the efficacy of NACT by extent of residual disease in unselected patients,” the authors recommended.
The study was supported by grants from the National Cancer Institute and Cancer Prevention and Research Institute of Texas. Dr. Meyer and multiple coauthors disclosed honoraria, research funding, and/or advising/consulting with various pharmaceutical companies.
Although the use of neoadjuvant chemotherapy for treatment of women with advanced ovarian cancer has grown significantly in recent years, a new study shows that it is associated with worse overall survival for women with stage IIIC disease, compared with primary cytoreductive surgery.
Among 594 women with advanced ovarian cancer treated at one of six major comprehensive cancer centers, median overall survival (OS) for women with stage IIIC cancers treated with neoadjuvant chemotherapy (NACT) was 33 months, compared with 43 months for women treated with primary cytoreductive surgery (PCS), reported Larissa A. Meyer, MD, of the University of Texas M.D. Anderson Cancer Center in Houston, and her colleagues.
There were no significant survival differences between chemotherapy and surgery for women with stage IV disease, however, and for these patients neoadjuvant chemotherapy was associated with fewer morbidities, and may be a better therapeutic option, the investigators reported.
“Although additional biases may persist despite propensity-score matching, our results suggest that in carefully selected patients with stage IIIC disease, PCS is associated with a survival advantage, with overall low rates of surgical morbidity. In contrast, for patients with stage IV disease, our results confirm that NACT is noninferior to PCS for survival, with fewer ICU admissions and rehospitalizations, which suggests that NACT may be preferable for patients with stage IV ovarian cancer,” they wrote in the Journal of Clinical Oncology (2016. doi: 10.1200/JCO.2016.68.1239).
The increase in the use of NACT in women with advanced ovarian cancer in the United States was spurred by two randomized clinical trials, the investigators noted. The first, published in 2010 showed that survival was similar for women with stage IIIC or IV ovarian cancer treated with either neoadjuvant chemotherapy followed by interval debulking surgery or with primary surgery followed by chemotherapy. The second study, published in 2015, found that “in women with stage III or IV ovarian cancer, survival with primary chemotherapy is noninferior to primary surgery. In this study population, the researchers stated that “giving primary chemotherapy before surgery is an acceptable standard of care for women with advanced ovarian cancer.”
To see what effect these trials had on clinical practice and outcomes in the United States, the authors conducted an observational study of patients treated at six National Cancer Institute–designated cancer centers, looking at NACT use in 1,538 women diagnosed with ovarian cancer from 2003 through 2012, and at OS, morbidity, and postoperative residual disease in a propensity score–matched sample of 594 patients.
They found that for women with stage IIIC disease, NACT use increased from 16% during the period 2003-2010, to 34% during 2011-2012. For women with stage IV disease, NACT use grew from 41% to 62% during the respective time periods (P for trend for both comparisons = .001).
As noted before, median overall survival among women with stage IIIC disease in the propensity score–matched sample was significantly shorter for those treated with primary NACT vs. PCS.
For women with stage IV disease, however, there was no significant difference in OS between those treated with NACT (median 31 months) vs. those treated with PCS (median 36 months, hazard ratio 1.16, not significant).
Women with stages IIIC and IV disease who received NACT were less likely to have one or more centimeters of residual disease postoperatively and were less likely to have an ICU admission or rehospitalization (P for all comparisons = .04). However, overall survival was lower among women with stage IIIC disease who had only microscopic residual disease or residual disease measuring 1 cm or less (HR, 1.49; P = .04).
“Future studies should prospectively consider the efficacy of NACT by extent of residual disease in unselected patients,” the authors recommended.
The study was supported by grants from the National Cancer Institute and Cancer Prevention and Research Institute of Texas. Dr. Meyer and multiple coauthors disclosed honoraria, research funding, and/or advising/consulting with various pharmaceutical companies.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Neoadjuvant chemotherapy was associated with lower overall survival of stage IIIC but not stage IV ovarian cancer.
Major finding: Median OS was 33 months with neoadjuvant chemotherapy vs. 43 months with primary cytoreductive surgery.
Data source: Observational study of 1,538 patients with ovarian cancer, and propensity score–matched sample of 594 patients for clinical outcomes.
Disclosures: The study was supported by grants from the National Cancer Institute and Cancer Prevention and Research Institute of Texas. Larissa A. Meyer and multiple coauthors disclosed honoraria, research funding, and/or advising/consulting with various pharmaceutical companies.
High free T4 levels linked to sudden cardiac death
Higher levels of free thyroxine are associated with an increased risk of sudden cardiac death, even in euthyroid adults, according to a report published online Sept. 6 in Circulation.
Thyroid dysfunction, even in the subclinical range, is known to correlate with increased cardiovascular disease, but until now a possible link between free thyroxine levels and sudden cardiac death (SCD) has never been explored in the general population. Any factors that could improve prediction of SCD in the general population would be helpful because almost half of these cases are the first indication that the patient had heart disease, said Layal Chaker, MD, of the Rotterdam Thyroid Center and the departments of internal medicine and epidemiology, Erasmus University, Rotterdam, and her associates.
They assessed SCD among 10,318 participants in the Rotterdam Study, a prospective population-based cohort study examining endocrine, cardiovascular, neurologic, ophthalmologic, and psychiatric diseases in middle-aged and older adults in the Netherlands. Men and women aged 45-106 years who had thyroid testing at baseline were followed for a median of 9.2 years (range, 4-21 years) for the development of SCD. There were 261 cases of SCD, and 231 of these occurred in euthyroid participants.
Higher levels of free thyroxine (T4) were associated with an increased risk of SCD, with a hazard ratio of 1.87 for every 1 ng/dL increase in free T4. When the analysis was confined to the 231 euthyroid participants, this association was even stronger, with an HR of 2.26, the investigators said (Circulation 2016 Sept 6. doi: 10.1161/CirculationAHA.115.020789).
The findings were similar in several sensitivity analyses, including one that excluded participants who had an unwitnessed SCD. In addition, adjustment of the data to account for the presence or absence of diabetes, as well as exclusion of patients who had heart failure, did not alter the risk estimates significantly. The results also were consistent across all age groups and both sexes, Dr. Chaker and her associates said.
The exact mechanism for the association between free thyroxine and SCD is not yet known but appears to be independent of traditional cardiovascular risk factors. “Bigger sample size and more detailed data are needed to determine whether these associations share the same or have distinct pathways,” they added.
The Netherlands Organisation for Health Research and Development and Erasmus Medical Center supported the study. Dr. Chaker and her associates reported having no relevant financial disclosures.
Higher levels of free thyroxine are associated with an increased risk of sudden cardiac death, even in euthyroid adults, according to a report published online Sept. 6 in Circulation.
Thyroid dysfunction, even in the subclinical range, is known to correlate with increased cardiovascular disease, but until now a possible link between free thyroxine levels and sudden cardiac death (SCD) has never been explored in the general population. Any factors that could improve prediction of SCD in the general population would be helpful because almost half of these cases are the first indication that the patient had heart disease, said Layal Chaker, MD, of the Rotterdam Thyroid Center and the departments of internal medicine and epidemiology, Erasmus University, Rotterdam, and her associates.
They assessed SCD among 10,318 participants in the Rotterdam Study, a prospective population-based cohort study examining endocrine, cardiovascular, neurologic, ophthalmologic, and psychiatric diseases in middle-aged and older adults in the Netherlands. Men and women aged 45-106 years who had thyroid testing at baseline were followed for a median of 9.2 years (range, 4-21 years) for the development of SCD. There were 261 cases of SCD, and 231 of these occurred in euthyroid participants.
Higher levels of free thyroxine (T4) were associated with an increased risk of SCD, with a hazard ratio of 1.87 for every 1 ng/dL increase in free T4. When the analysis was confined to the 231 euthyroid participants, this association was even stronger, with an HR of 2.26, the investigators said (Circulation 2016 Sept 6. doi: 10.1161/CirculationAHA.115.020789).
The findings were similar in several sensitivity analyses, including one that excluded participants who had an unwitnessed SCD. In addition, adjustment of the data to account for the presence or absence of diabetes, as well as exclusion of patients who had heart failure, did not alter the risk estimates significantly. The results also were consistent across all age groups and both sexes, Dr. Chaker and her associates said.
The exact mechanism for the association between free thyroxine and SCD is not yet known but appears to be independent of traditional cardiovascular risk factors. “Bigger sample size and more detailed data are needed to determine whether these associations share the same or have distinct pathways,” they added.
The Netherlands Organisation for Health Research and Development and Erasmus Medical Center supported the study. Dr. Chaker and her associates reported having no relevant financial disclosures.
Higher levels of free thyroxine are associated with an increased risk of sudden cardiac death, even in euthyroid adults, according to a report published online Sept. 6 in Circulation.
Thyroid dysfunction, even in the subclinical range, is known to correlate with increased cardiovascular disease, but until now a possible link between free thyroxine levels and sudden cardiac death (SCD) has never been explored in the general population. Any factors that could improve prediction of SCD in the general population would be helpful because almost half of these cases are the first indication that the patient had heart disease, said Layal Chaker, MD, of the Rotterdam Thyroid Center and the departments of internal medicine and epidemiology, Erasmus University, Rotterdam, and her associates.
They assessed SCD among 10,318 participants in the Rotterdam Study, a prospective population-based cohort study examining endocrine, cardiovascular, neurologic, ophthalmologic, and psychiatric diseases in middle-aged and older adults in the Netherlands. Men and women aged 45-106 years who had thyroid testing at baseline were followed for a median of 9.2 years (range, 4-21 years) for the development of SCD. There were 261 cases of SCD, and 231 of these occurred in euthyroid participants.
Higher levels of free thyroxine (T4) were associated with an increased risk of SCD, with a hazard ratio of 1.87 for every 1 ng/dL increase in free T4. When the analysis was confined to the 231 euthyroid participants, this association was even stronger, with an HR of 2.26, the investigators said (Circulation 2016 Sept 6. doi: 10.1161/CirculationAHA.115.020789).
The findings were similar in several sensitivity analyses, including one that excluded participants who had an unwitnessed SCD. In addition, adjustment of the data to account for the presence or absence of diabetes, as well as exclusion of patients who had heart failure, did not alter the risk estimates significantly. The results also were consistent across all age groups and both sexes, Dr. Chaker and her associates said.
The exact mechanism for the association between free thyroxine and SCD is not yet known but appears to be independent of traditional cardiovascular risk factors. “Bigger sample size and more detailed data are needed to determine whether these associations share the same or have distinct pathways,” they added.
The Netherlands Organisation for Health Research and Development and Erasmus Medical Center supported the study. Dr. Chaker and her associates reported having no relevant financial disclosures.
FROM CIRCULATION
Key clinical point: High levels of free thyroxine are associated with an increased risk of sudden cardiac death, even in euthyroid adults.
Major finding: Higher levels of free thyroxine (T4) were associated with an increased risk of SCD, with a hazard ratio of 1.87 for every 1 ng/dL increase in free T4.
Data source: A prospective population-based cohort study involving 10,318 older adults in the Netherlands followed for a median of 9 years.
Disclosures: The Netherlands Organisation for Health Research and Development and Erasmus Medical Center supported the study. Dr. Chaker and her associates reported having no relevant financial disclosures.
Dengue Fever: Two Unexpected Findings
Dengue fever is the most commonly transmitted arboviral disease in the world, affecting an estimated 2.5 billion people who live in areas endemic to the virus. This exposure yields an annual incidence of 100 million cases of dengue, which translates into 250,000 cases of hemorrhagic fever. With an expanding geographic distribution and increasing number of epidemics, the World Health Organization (WHO) has classified dengue as a major public health concern.1 Enhanced globalization and changing climate patterns have resulted in a dramatic increase in the incidence of dengue in both North and Central America. Aggregate North and Central American data from 2010 to the present revealed over 1.7 million cases of dengue, nearly 80,000 of which were severe, and 747 deaths.2 Based on these statistics, dengue fever should be considered in the differential diagnosis of febrile ED patients in the developed world who had a history of recent travel. We present two cases that highlight the complexity of diagnosis and novel complications associated with dengue fever.
Case Reports
Case 1
A 24-year-old man presented to the ED with a 4-day history of intermittent fever of up to 102.02°F, which was accompanied by chills, myalgia, and rigors. The patient stated that he had visited Vietnam, Thailand, Indonesia, and Malaysia 8 days prior to presentation, and had experienced mosquito bites daily throughout his travels. He further noted that his symptoms had improved on day 3 of his illness, but acutely worsened on day 4, which prompted him to visit the ED. The patient’s primary complaint was a severe retro-orbital headache, fever, and one episode of epistaxis.
On physical examination, the patient had conjunctivitis and hepatosplenomegaly, but otherwise appeared well. His laboratory evaluation was significant for leukopenia (white blood cell [WBC] count, 2.40 x 109/L), thrombocytopenia (platelet count, 123 x 109/L), and a positive mononuclear spot test. Both dengue immunoglobulin G (IgG) and immunoglobulin M (IgM) tests sent from the ED were negative. Based on the patient’s thrombocytopenia and epistaxis, as well as concerns that the patient was entering into the critical phase of dengue fever, he was admitted to the inpatient hospital for observation.
The patient’s course improved during his stay with symptomatic treatment and blood-count monitoring, and he was discharged home on hospital day 3. He followed up at our hospital travel clinic the day after discharge; a repeat dengue IgM test taken during this visit came back positive.
Case 2
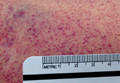
A 51-year-old man presented to the ED with a 3-day history of intermittent fever and diffuse myalgia. He reported chills, night sweats, and the feeling of abdominal fullness. He denied nausea, vomiting, or changes in the character of his stool. He had no known sick contacts, but reported he had traveled from the Philippines 3 days prior to presentation and that his symptoms had developed en route to the United States. The patient also denied any known tick, mosquito, or animal exposures. He said he had treated his symptoms with acetaminophen and nonsteroidal anti-inflammatory drugs. Prior to his arrival at the ED, he had twice presented to a walk-in clinic earlier that day. Repeated laboratory testing at the ED showed a decrease in WBC count from 42.0 x 109/L to 31.0 x 109/L, as well as a declining platelet count from 123 x 109/L to 87 x 109/L. On physical examination, the patient was ill-appearing, diaphoretic, and had a temperature of 100.6°F. His vital signs were otherwise within normal limits.
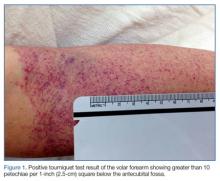
With the exception of a mild diffuse petechial rash on the patient’s thighs bilaterally, the physical examination was unrevealing. A tourniquet test (TT) to assess capillary fragility was performed at bedside, and yielded a positive result (Figure 1). Work-up further demonstrated a declining WBC of 2.70 x 109/L and declining platelet count of 65 x 109/L.
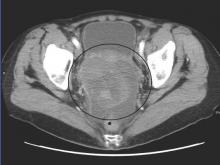
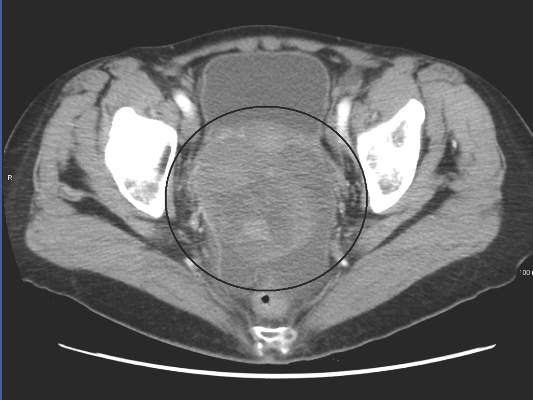
A polymerase chain reaction (PCR) test confirmed a diagnosis of dengue, with a positive dengue type-4 (DEN-4) serotype detection. Supportive care was initiated, and the patient was admitted to the inpatient hospital for continued treatment. He was discharged home on hospital day 5; however, he returned to the ED later that day with increasing headache and left flank pain. Work-up included axial and coronal computed tomography scans of the abdomen and pelvis, which revealed hematuria and a left upper pole renal infarction surrounded by mild perinephric fat stranding (Figure 2a and 2b) with maintenance of left renal artery/vein patency.
The patient was admitted to an inpatient floor, where symptomatic management was employed. He underwent unrevealing bubble echocardiography and lower extremity Doppler ultrasound imaging, and anticoagulation therapy was initiated per a consultation with hematology services. The patient was discharged home in improved, stable condition on hospital day 8.
Discussion
Dengue virus is a single-stranded, nonsegmented RNA virus in the Flaviviridae family. Four major subtypes exist: DEN-1, DEN-2, DEN-3, and DEN-4. Lifelong serotype-specific immunity is conferred following infection. The virus is transmitted by the female Aedes aegypti mosquito, which is found worldwide but has a predilection for tropical and subtropical regions. The Aedes aegypti mosquito remains an effective vector secondary to its diurnal feeding habit and nearly imperceptible bite.1,3
The viral incubation period for dengue is typically 3 to 7 days4; therefore, dengue is highly unlikely in patients whose symptoms begin more than 2 weeks after departure from an endemic area. Replication primarily occurs in the regional lymph nodes and disseminates through the lymphatic system and bloodstream.1
The 1997 WHO guidelines previously classified dengue into three categories: undifferentiated fever, dengue fever, and dengue hemorrhagic fever (which was further classified by four severity grades, with grades III and IV defined as dengue shock syndrome). However, changes in epidemiology of the disease and reports of difficulty applying the criteria in the clinical setting led to reclassification of dengue on a continuum from dengue to severe dengue in the WHO’s updated 2009 guidelines.4
Signs and Symptoms
The ramifications of dengue infection can range from asymptomatic (typically in young, immunocompetent patients) to lethal. Key symptoms of dengue fever include nausea, vomiting, fever, respiratory symptoms, morbilliform or maculopapular rash, and headache or retro-orbital pain. In addition, arthralgia (hence the colloquial name for dengue of “breakbone fever”), myalgia, and conjunctivitis may exist.3,4 Fever usually lasts 5 to 7 days and can be biphasic, with a return of symptoms after the initial resolution as seen in case report 1.4 Severe dengue is characterized by capillary leakage, hemorrhage, or end-organ damage.3-5 The most common bleeding sites are the skin, nose, and gums.
Diagnosis
Bedside evaluation for dengue can be performed with the TT—one of the WHO’s case definitions for dengue.6 This is accomplished by placing a manual blood pressure (BP) cuff on the arm and inflating it to halfway between systolic and diastolic BP for 5 minutes. The test is positive for dengue if more than 10 petechiae appear per 1-inch (2.5-cm) square below the antecubital fossa.7 Of note, the test has poor sensitivity (51.6%, 95% confidence interval [CI], 33-69), but good specificity (82.4%, 95% CI, 76-87).7,8 A positive TT combined with leukopenia increases the sensitivity to 93.9%, [95% CI, 89-96].7 While not specific to dengue infection, in the right clinical scenario, the TT is a simple bedside test to help confirm the diagnosis and is extremely useful in resource-limited settings.
During the initial days of illness, the virus may be detected by PCR, as viremia and fever usually correlate. Once defervescence occurs, IgM and then IgG antibodies become detectable. When using these antibody tests to evaluate for dengue, clinicians should be aware of cross-reactivity with other flavivirus infections, such as yellow fever or Japanese encephalitis (including immunological cross-reactivity).1 New diagnostic modalities include enzyme immunoassays that can detect dengue viral RNA within 24 to 48 hours, and viral antigen-detection kits, which can yield results in less than 1 hour.4
Aside from advanced laboratory testing, worsening thrombocytopenia in light of a rising hematocrit can be highly suggestive of dengue. Leukopenia with lymphopenia and mild elevation of hepatic enzymes (typically 2 to 5 times the upper limits of the normal reference range) are also often seen in active infections.1 The occurrence of these signs in conjunction with a rapid reduction in the platelets often signals transition to the critical phase of plasma leakage.1,4
Treatment
Treatment of dengue consists of supportive care and transfusion when necessary. The WHO recommends strict observation of patients with suspected dengue who have warning signs of severe disease (eg, abdominal pain, persistent vomiting, mucosal bleeding, lethargy, hepatomegaly, rapid increase in hematocrit with concomitant drop in platelet count). Inpatient treatment centers on judicious fluid management, trending blood count parameters, and monitoring for signs of plasma leakage and hemorrhage. Fluid resuscitation is titrated to optimize central and peripheral circulation and end-organ perfusion. Blood-product administration should be reserved for suspected or severe bleeding.4
While dengue fever was the final diagnosis in both of our case presentations, these cases also highlight key diagnostic and treatment dilemmas associated with dengue. The patient in the first case report demonstrated the characteristic biphasic fever seen with dengue—resolution of symptoms on day 3, but then return of fever and symptoms on day 4. Often the dengue-specific antibodies are not formed until after the resolution of fever. This patient represents a classic example of dengue as the serologic studies sent on day 4 of the patient’s illness were negative but then turned positive on day 7, illustrating the need for high clinical suspicion and underscoring the importance of initiating treatment despite laboratory confirmation.
Further, regarding the patient in the second case, though proteinuria, hematuria, acute renal failure, and glomerulonephritis are previously described renal complications of dengue,9 a thorough literature search yielded no prior published accounts of renal infarction. Given the patient’s previous healthy status and the lack of other hypothesis as to the mechanism of injury, we suspect this patient’s renal infarction was due to the transient hypercoagulability characteristic of dengue and responsible for other clinical manifestations of the disease.
Conclusion
In addition to more prevalent illnesses such as malaria, acute traveler’s diarrhea, and respiratory tract infections, dengue fever should be included in the differential diagnosis when evaluating a febrile patient who has a history of recent travel to countries where dengue is endemic. A high clinical suspicion, combined with a thorough history and physical examination, is essential to making the diagnosis.
Both of our case reports demonstrate some of the diagnostic limitations in the acute setting, and the breadth of clinical complications that can occur in this complex disease. With the increasing prevalence of dengue fever in North and Central America, it is likely that patients with the disease will present to EDs in the United States. Early diagnosis and awareness of potential complications can lead to timely initiation of life-saving supportive care.
1. Wilder-Smith A, Schwartz E. Dengue in travelers. N Engl J Med. 2005;353(9):924-932. dpo:10.1056/NEJMra041927
2. Pan American Health Organization, World Health Organization. Number of reported cases of dengue and severe dengue (SD), Region of the Americas (by country and subregion). Washington, DC: Pan American Health Organization. http://www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=&gid=35610&lang=es. Updated August 5, 2016. Accessed August 17, 2016.
3. Whitehorn J, Farrar J. Dengue. Clin Med (Lond). 2011;11(5):483-487.
4. World Health Organization. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control. New Edition. Geneva, Switzerland: World Health Organization; 2009. http://www.who.int/tdr/publications/documents/dengue-diagnosis.pdf. Accessed August 17, 2016.
5. Halstead SB. Dengue. Lancet. 2007;370(9599):1644-1652. doi:10.1016/S0140-6736(07)61687-0.
6. Centers for Disease Control and Prevention. Dengue Clinical Case Management E-learning. http://www.cdc.gov/dengue/training/cme/ccm/page73112.html; http://www.cdc.gov/dengue/training/cme/ccm/Tourniquet%20Test_F.pdf. Accessed August 17, 2016.
7. Gregory CJ, Lorenzi OD, Colón L, et al. Utility of the tourniquet test and the white blood cell count to differentiate dengue among acute febrile illnesses in the emergency room. PLoS Negl Trop Dis. 2011;5(12):e1400.
8. Mayxay M, Phetsouvanh R, Moore CE, et al. Predictive diagnostic value of the tourniquet test for the diagnosis of dengue infection in adults. Trop Med Int Health. 2011;16(1):127-133.
9. Lizarraga KJ, Nayer A. Dengue-associated kidney disease. J Nephropathol. 2014;3(2):57-62.
Dengue fever is the most commonly transmitted arboviral disease in the world, affecting an estimated 2.5 billion people who live in areas endemic to the virus. This exposure yields an annual incidence of 100 million cases of dengue, which translates into 250,000 cases of hemorrhagic fever. With an expanding geographic distribution and increasing number of epidemics, the World Health Organization (WHO) has classified dengue as a major public health concern.1 Enhanced globalization and changing climate patterns have resulted in a dramatic increase in the incidence of dengue in both North and Central America. Aggregate North and Central American data from 2010 to the present revealed over 1.7 million cases of dengue, nearly 80,000 of which were severe, and 747 deaths.2 Based on these statistics, dengue fever should be considered in the differential diagnosis of febrile ED patients in the developed world who had a history of recent travel. We present two cases that highlight the complexity of diagnosis and novel complications associated with dengue fever.
Case Reports
Case 1
A 24-year-old man presented to the ED with a 4-day history of intermittent fever of up to 102.02°F, which was accompanied by chills, myalgia, and rigors. The patient stated that he had visited Vietnam, Thailand, Indonesia, and Malaysia 8 days prior to presentation, and had experienced mosquito bites daily throughout his travels. He further noted that his symptoms had improved on day 3 of his illness, but acutely worsened on day 4, which prompted him to visit the ED. The patient’s primary complaint was a severe retro-orbital headache, fever, and one episode of epistaxis.
On physical examination, the patient had conjunctivitis and hepatosplenomegaly, but otherwise appeared well. His laboratory evaluation was significant for leukopenia (white blood cell [WBC] count, 2.40 x 109/L), thrombocytopenia (platelet count, 123 x 109/L), and a positive mononuclear spot test. Both dengue immunoglobulin G (IgG) and immunoglobulin M (IgM) tests sent from the ED were negative. Based on the patient’s thrombocytopenia and epistaxis, as well as concerns that the patient was entering into the critical phase of dengue fever, he was admitted to the inpatient hospital for observation.
The patient’s course improved during his stay with symptomatic treatment and blood-count monitoring, and he was discharged home on hospital day 3. He followed up at our hospital travel clinic the day after discharge; a repeat dengue IgM test taken during this visit came back positive.
Case 2
A 51-year-old man presented to the ED with a 3-day history of intermittent fever and diffuse myalgia. He reported chills, night sweats, and the feeling of abdominal fullness. He denied nausea, vomiting, or changes in the character of his stool. He had no known sick contacts, but reported he had traveled from the Philippines 3 days prior to presentation and that his symptoms had developed en route to the United States. The patient also denied any known tick, mosquito, or animal exposures. He said he had treated his symptoms with acetaminophen and nonsteroidal anti-inflammatory drugs. Prior to his arrival at the ED, he had twice presented to a walk-in clinic earlier that day. Repeated laboratory testing at the ED showed a decrease in WBC count from 42.0 x 109/L to 31.0 x 109/L, as well as a declining platelet count from 123 x 109/L to 87 x 109/L. On physical examination, the patient was ill-appearing, diaphoretic, and had a temperature of 100.6°F. His vital signs were otherwise within normal limits.
With the exception of a mild diffuse petechial rash on the patient’s thighs bilaterally, the physical examination was unrevealing. A tourniquet test (TT) to assess capillary fragility was performed at bedside, and yielded a positive result (Figure 1). Work-up further demonstrated a declining WBC of 2.70 x 109/L and declining platelet count of 65 x 109/L.
A polymerase chain reaction (PCR) test confirmed a diagnosis of dengue, with a positive dengue type-4 (DEN-4) serotype detection. Supportive care was initiated, and the patient was admitted to the inpatient hospital for continued treatment. He was discharged home on hospital day 5; however, he returned to the ED later that day with increasing headache and left flank pain. Work-up included axial and coronal computed tomography scans of the abdomen and pelvis, which revealed hematuria and a left upper pole renal infarction surrounded by mild perinephric fat stranding (Figure 2a and 2b) with maintenance of left renal artery/vein patency.
The patient was admitted to an inpatient floor, where symptomatic management was employed. He underwent unrevealing bubble echocardiography and lower extremity Doppler ultrasound imaging, and anticoagulation therapy was initiated per a consultation with hematology services. The patient was discharged home in improved, stable condition on hospital day 8.
Discussion
Dengue virus is a single-stranded, nonsegmented RNA virus in the Flaviviridae family. Four major subtypes exist: DEN-1, DEN-2, DEN-3, and DEN-4. Lifelong serotype-specific immunity is conferred following infection. The virus is transmitted by the female Aedes aegypti mosquito, which is found worldwide but has a predilection for tropical and subtropical regions. The Aedes aegypti mosquito remains an effective vector secondary to its diurnal feeding habit and nearly imperceptible bite.1,3
The viral incubation period for dengue is typically 3 to 7 days4; therefore, dengue is highly unlikely in patients whose symptoms begin more than 2 weeks after departure from an endemic area. Replication primarily occurs in the regional lymph nodes and disseminates through the lymphatic system and bloodstream.1
The 1997 WHO guidelines previously classified dengue into three categories: undifferentiated fever, dengue fever, and dengue hemorrhagic fever (which was further classified by four severity grades, with grades III and IV defined as dengue shock syndrome). However, changes in epidemiology of the disease and reports of difficulty applying the criteria in the clinical setting led to reclassification of dengue on a continuum from dengue to severe dengue in the WHO’s updated 2009 guidelines.4
Signs and Symptoms
The ramifications of dengue infection can range from asymptomatic (typically in young, immunocompetent patients) to lethal. Key symptoms of dengue fever include nausea, vomiting, fever, respiratory symptoms, morbilliform or maculopapular rash, and headache or retro-orbital pain. In addition, arthralgia (hence the colloquial name for dengue of “breakbone fever”), myalgia, and conjunctivitis may exist.3,4 Fever usually lasts 5 to 7 days and can be biphasic, with a return of symptoms after the initial resolution as seen in case report 1.4 Severe dengue is characterized by capillary leakage, hemorrhage, or end-organ damage.3-5 The most common bleeding sites are the skin, nose, and gums.
Diagnosis
Bedside evaluation for dengue can be performed with the TT—one of the WHO’s case definitions for dengue.6 This is accomplished by placing a manual blood pressure (BP) cuff on the arm and inflating it to halfway between systolic and diastolic BP for 5 minutes. The test is positive for dengue if more than 10 petechiae appear per 1-inch (2.5-cm) square below the antecubital fossa.7 Of note, the test has poor sensitivity (51.6%, 95% confidence interval [CI], 33-69), but good specificity (82.4%, 95% CI, 76-87).7,8 A positive TT combined with leukopenia increases the sensitivity to 93.9%, [95% CI, 89-96].7 While not specific to dengue infection, in the right clinical scenario, the TT is a simple bedside test to help confirm the diagnosis and is extremely useful in resource-limited settings.
During the initial days of illness, the virus may be detected by PCR, as viremia and fever usually correlate. Once defervescence occurs, IgM and then IgG antibodies become detectable. When using these antibody tests to evaluate for dengue, clinicians should be aware of cross-reactivity with other flavivirus infections, such as yellow fever or Japanese encephalitis (including immunological cross-reactivity).1 New diagnostic modalities include enzyme immunoassays that can detect dengue viral RNA within 24 to 48 hours, and viral antigen-detection kits, which can yield results in less than 1 hour.4
Aside from advanced laboratory testing, worsening thrombocytopenia in light of a rising hematocrit can be highly suggestive of dengue. Leukopenia with lymphopenia and mild elevation of hepatic enzymes (typically 2 to 5 times the upper limits of the normal reference range) are also often seen in active infections.1 The occurrence of these signs in conjunction with a rapid reduction in the platelets often signals transition to the critical phase of plasma leakage.1,4
Treatment
Treatment of dengue consists of supportive care and transfusion when necessary. The WHO recommends strict observation of patients with suspected dengue who have warning signs of severe disease (eg, abdominal pain, persistent vomiting, mucosal bleeding, lethargy, hepatomegaly, rapid increase in hematocrit with concomitant drop in platelet count). Inpatient treatment centers on judicious fluid management, trending blood count parameters, and monitoring for signs of plasma leakage and hemorrhage. Fluid resuscitation is titrated to optimize central and peripheral circulation and end-organ perfusion. Blood-product administration should be reserved for suspected or severe bleeding.4
While dengue fever was the final diagnosis in both of our case presentations, these cases also highlight key diagnostic and treatment dilemmas associated with dengue. The patient in the first case report demonstrated the characteristic biphasic fever seen with dengue—resolution of symptoms on day 3, but then return of fever and symptoms on day 4. Often the dengue-specific antibodies are not formed until after the resolution of fever. This patient represents a classic example of dengue as the serologic studies sent on day 4 of the patient’s illness were negative but then turned positive on day 7, illustrating the need for high clinical suspicion and underscoring the importance of initiating treatment despite laboratory confirmation.
Further, regarding the patient in the second case, though proteinuria, hematuria, acute renal failure, and glomerulonephritis are previously described renal complications of dengue,9 a thorough literature search yielded no prior published accounts of renal infarction. Given the patient’s previous healthy status and the lack of other hypothesis as to the mechanism of injury, we suspect this patient’s renal infarction was due to the transient hypercoagulability characteristic of dengue and responsible for other clinical manifestations of the disease.
Conclusion
In addition to more prevalent illnesses such as malaria, acute traveler’s diarrhea, and respiratory tract infections, dengue fever should be included in the differential diagnosis when evaluating a febrile patient who has a history of recent travel to countries where dengue is endemic. A high clinical suspicion, combined with a thorough history and physical examination, is essential to making the diagnosis.
Both of our case reports demonstrate some of the diagnostic limitations in the acute setting, and the breadth of clinical complications that can occur in this complex disease. With the increasing prevalence of dengue fever in North and Central America, it is likely that patients with the disease will present to EDs in the United States. Early diagnosis and awareness of potential complications can lead to timely initiation of life-saving supportive care.
Dengue fever is the most commonly transmitted arboviral disease in the world, affecting an estimated 2.5 billion people who live in areas endemic to the virus. This exposure yields an annual incidence of 100 million cases of dengue, which translates into 250,000 cases of hemorrhagic fever. With an expanding geographic distribution and increasing number of epidemics, the World Health Organization (WHO) has classified dengue as a major public health concern.1 Enhanced globalization and changing climate patterns have resulted in a dramatic increase in the incidence of dengue in both North and Central America. Aggregate North and Central American data from 2010 to the present revealed over 1.7 million cases of dengue, nearly 80,000 of which were severe, and 747 deaths.2 Based on these statistics, dengue fever should be considered in the differential diagnosis of febrile ED patients in the developed world who had a history of recent travel. We present two cases that highlight the complexity of diagnosis and novel complications associated with dengue fever.
Case Reports
Case 1
A 24-year-old man presented to the ED with a 4-day history of intermittent fever of up to 102.02°F, which was accompanied by chills, myalgia, and rigors. The patient stated that he had visited Vietnam, Thailand, Indonesia, and Malaysia 8 days prior to presentation, and had experienced mosquito bites daily throughout his travels. He further noted that his symptoms had improved on day 3 of his illness, but acutely worsened on day 4, which prompted him to visit the ED. The patient’s primary complaint was a severe retro-orbital headache, fever, and one episode of epistaxis.
On physical examination, the patient had conjunctivitis and hepatosplenomegaly, but otherwise appeared well. His laboratory evaluation was significant for leukopenia (white blood cell [WBC] count, 2.40 x 109/L), thrombocytopenia (platelet count, 123 x 109/L), and a positive mononuclear spot test. Both dengue immunoglobulin G (IgG) and immunoglobulin M (IgM) tests sent from the ED were negative. Based on the patient’s thrombocytopenia and epistaxis, as well as concerns that the patient was entering into the critical phase of dengue fever, he was admitted to the inpatient hospital for observation.
The patient’s course improved during his stay with symptomatic treatment and blood-count monitoring, and he was discharged home on hospital day 3. He followed up at our hospital travel clinic the day after discharge; a repeat dengue IgM test taken during this visit came back positive.
Case 2
A 51-year-old man presented to the ED with a 3-day history of intermittent fever and diffuse myalgia. He reported chills, night sweats, and the feeling of abdominal fullness. He denied nausea, vomiting, or changes in the character of his stool. He had no known sick contacts, but reported he had traveled from the Philippines 3 days prior to presentation and that his symptoms had developed en route to the United States. The patient also denied any known tick, mosquito, or animal exposures. He said he had treated his symptoms with acetaminophen and nonsteroidal anti-inflammatory drugs. Prior to his arrival at the ED, he had twice presented to a walk-in clinic earlier that day. Repeated laboratory testing at the ED showed a decrease in WBC count from 42.0 x 109/L to 31.0 x 109/L, as well as a declining platelet count from 123 x 109/L to 87 x 109/L. On physical examination, the patient was ill-appearing, diaphoretic, and had a temperature of 100.6°F. His vital signs were otherwise within normal limits.
With the exception of a mild diffuse petechial rash on the patient’s thighs bilaterally, the physical examination was unrevealing. A tourniquet test (TT) to assess capillary fragility was performed at bedside, and yielded a positive result (Figure 1). Work-up further demonstrated a declining WBC of 2.70 x 109/L and declining platelet count of 65 x 109/L.
A polymerase chain reaction (PCR) test confirmed a diagnosis of dengue, with a positive dengue type-4 (DEN-4) serotype detection. Supportive care was initiated, and the patient was admitted to the inpatient hospital for continued treatment. He was discharged home on hospital day 5; however, he returned to the ED later that day with increasing headache and left flank pain. Work-up included axial and coronal computed tomography scans of the abdomen and pelvis, which revealed hematuria and a left upper pole renal infarction surrounded by mild perinephric fat stranding (Figure 2a and 2b) with maintenance of left renal artery/vein patency.
The patient was admitted to an inpatient floor, where symptomatic management was employed. He underwent unrevealing bubble echocardiography and lower extremity Doppler ultrasound imaging, and anticoagulation therapy was initiated per a consultation with hematology services. The patient was discharged home in improved, stable condition on hospital day 8.
Discussion
Dengue virus is a single-stranded, nonsegmented RNA virus in the Flaviviridae family. Four major subtypes exist: DEN-1, DEN-2, DEN-3, and DEN-4. Lifelong serotype-specific immunity is conferred following infection. The virus is transmitted by the female Aedes aegypti mosquito, which is found worldwide but has a predilection for tropical and subtropical regions. The Aedes aegypti mosquito remains an effective vector secondary to its diurnal feeding habit and nearly imperceptible bite.1,3
The viral incubation period for dengue is typically 3 to 7 days4; therefore, dengue is highly unlikely in patients whose symptoms begin more than 2 weeks after departure from an endemic area. Replication primarily occurs in the regional lymph nodes and disseminates through the lymphatic system and bloodstream.1
The 1997 WHO guidelines previously classified dengue into three categories: undifferentiated fever, dengue fever, and dengue hemorrhagic fever (which was further classified by four severity grades, with grades III and IV defined as dengue shock syndrome). However, changes in epidemiology of the disease and reports of difficulty applying the criteria in the clinical setting led to reclassification of dengue on a continuum from dengue to severe dengue in the WHO’s updated 2009 guidelines.4
Signs and Symptoms
The ramifications of dengue infection can range from asymptomatic (typically in young, immunocompetent patients) to lethal. Key symptoms of dengue fever include nausea, vomiting, fever, respiratory symptoms, morbilliform or maculopapular rash, and headache or retro-orbital pain. In addition, arthralgia (hence the colloquial name for dengue of “breakbone fever”), myalgia, and conjunctivitis may exist.3,4 Fever usually lasts 5 to 7 days and can be biphasic, with a return of symptoms after the initial resolution as seen in case report 1.4 Severe dengue is characterized by capillary leakage, hemorrhage, or end-organ damage.3-5 The most common bleeding sites are the skin, nose, and gums.
Diagnosis
Bedside evaluation for dengue can be performed with the TT—one of the WHO’s case definitions for dengue.6 This is accomplished by placing a manual blood pressure (BP) cuff on the arm and inflating it to halfway between systolic and diastolic BP for 5 minutes. The test is positive for dengue if more than 10 petechiae appear per 1-inch (2.5-cm) square below the antecubital fossa.7 Of note, the test has poor sensitivity (51.6%, 95% confidence interval [CI], 33-69), but good specificity (82.4%, 95% CI, 76-87).7,8 A positive TT combined with leukopenia increases the sensitivity to 93.9%, [95% CI, 89-96].7 While not specific to dengue infection, in the right clinical scenario, the TT is a simple bedside test to help confirm the diagnosis and is extremely useful in resource-limited settings.
During the initial days of illness, the virus may be detected by PCR, as viremia and fever usually correlate. Once defervescence occurs, IgM and then IgG antibodies become detectable. When using these antibody tests to evaluate for dengue, clinicians should be aware of cross-reactivity with other flavivirus infections, such as yellow fever or Japanese encephalitis (including immunological cross-reactivity).1 New diagnostic modalities include enzyme immunoassays that can detect dengue viral RNA within 24 to 48 hours, and viral antigen-detection kits, which can yield results in less than 1 hour.4
Aside from advanced laboratory testing, worsening thrombocytopenia in light of a rising hematocrit can be highly suggestive of dengue. Leukopenia with lymphopenia and mild elevation of hepatic enzymes (typically 2 to 5 times the upper limits of the normal reference range) are also often seen in active infections.1 The occurrence of these signs in conjunction with a rapid reduction in the platelets often signals transition to the critical phase of plasma leakage.1,4
Treatment
Treatment of dengue consists of supportive care and transfusion when necessary. The WHO recommends strict observation of patients with suspected dengue who have warning signs of severe disease (eg, abdominal pain, persistent vomiting, mucosal bleeding, lethargy, hepatomegaly, rapid increase in hematocrit with concomitant drop in platelet count). Inpatient treatment centers on judicious fluid management, trending blood count parameters, and monitoring for signs of plasma leakage and hemorrhage. Fluid resuscitation is titrated to optimize central and peripheral circulation and end-organ perfusion. Blood-product administration should be reserved for suspected or severe bleeding.4
While dengue fever was the final diagnosis in both of our case presentations, these cases also highlight key diagnostic and treatment dilemmas associated with dengue. The patient in the first case report demonstrated the characteristic biphasic fever seen with dengue—resolution of symptoms on day 3, but then return of fever and symptoms on day 4. Often the dengue-specific antibodies are not formed until after the resolution of fever. This patient represents a classic example of dengue as the serologic studies sent on day 4 of the patient’s illness were negative but then turned positive on day 7, illustrating the need for high clinical suspicion and underscoring the importance of initiating treatment despite laboratory confirmation.
Further, regarding the patient in the second case, though proteinuria, hematuria, acute renal failure, and glomerulonephritis are previously described renal complications of dengue,9 a thorough literature search yielded no prior published accounts of renal infarction. Given the patient’s previous healthy status and the lack of other hypothesis as to the mechanism of injury, we suspect this patient’s renal infarction was due to the transient hypercoagulability characteristic of dengue and responsible for other clinical manifestations of the disease.
Conclusion
In addition to more prevalent illnesses such as malaria, acute traveler’s diarrhea, and respiratory tract infections, dengue fever should be included in the differential diagnosis when evaluating a febrile patient who has a history of recent travel to countries where dengue is endemic. A high clinical suspicion, combined with a thorough history and physical examination, is essential to making the diagnosis.
Both of our case reports demonstrate some of the diagnostic limitations in the acute setting, and the breadth of clinical complications that can occur in this complex disease. With the increasing prevalence of dengue fever in North and Central America, it is likely that patients with the disease will present to EDs in the United States. Early diagnosis and awareness of potential complications can lead to timely initiation of life-saving supportive care.
1. Wilder-Smith A, Schwartz E. Dengue in travelers. N Engl J Med. 2005;353(9):924-932. dpo:10.1056/NEJMra041927
2. Pan American Health Organization, World Health Organization. Number of reported cases of dengue and severe dengue (SD), Region of the Americas (by country and subregion). Washington, DC: Pan American Health Organization. http://www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=&gid=35610&lang=es. Updated August 5, 2016. Accessed August 17, 2016.
3. Whitehorn J, Farrar J. Dengue. Clin Med (Lond). 2011;11(5):483-487.
4. World Health Organization. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control. New Edition. Geneva, Switzerland: World Health Organization; 2009. http://www.who.int/tdr/publications/documents/dengue-diagnosis.pdf. Accessed August 17, 2016.
5. Halstead SB. Dengue. Lancet. 2007;370(9599):1644-1652. doi:10.1016/S0140-6736(07)61687-0.
6. Centers for Disease Control and Prevention. Dengue Clinical Case Management E-learning. http://www.cdc.gov/dengue/training/cme/ccm/page73112.html; http://www.cdc.gov/dengue/training/cme/ccm/Tourniquet%20Test_F.pdf. Accessed August 17, 2016.
7. Gregory CJ, Lorenzi OD, Colón L, et al. Utility of the tourniquet test and the white blood cell count to differentiate dengue among acute febrile illnesses in the emergency room. PLoS Negl Trop Dis. 2011;5(12):e1400.
8. Mayxay M, Phetsouvanh R, Moore CE, et al. Predictive diagnostic value of the tourniquet test for the diagnosis of dengue infection in adults. Trop Med Int Health. 2011;16(1):127-133.
9. Lizarraga KJ, Nayer A. Dengue-associated kidney disease. J Nephropathol. 2014;3(2):57-62.
1. Wilder-Smith A, Schwartz E. Dengue in travelers. N Engl J Med. 2005;353(9):924-932. dpo:10.1056/NEJMra041927
2. Pan American Health Organization, World Health Organization. Number of reported cases of dengue and severe dengue (SD), Region of the Americas (by country and subregion). Washington, DC: Pan American Health Organization. http://www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=&gid=35610&lang=es. Updated August 5, 2016. Accessed August 17, 2016.
3. Whitehorn J, Farrar J. Dengue. Clin Med (Lond). 2011;11(5):483-487.
4. World Health Organization. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control. New Edition. Geneva, Switzerland: World Health Organization; 2009. http://www.who.int/tdr/publications/documents/dengue-diagnosis.pdf. Accessed August 17, 2016.
5. Halstead SB. Dengue. Lancet. 2007;370(9599):1644-1652. doi:10.1016/S0140-6736(07)61687-0.
6. Centers for Disease Control and Prevention. Dengue Clinical Case Management E-learning. http://www.cdc.gov/dengue/training/cme/ccm/page73112.html; http://www.cdc.gov/dengue/training/cme/ccm/Tourniquet%20Test_F.pdf. Accessed August 17, 2016.
7. Gregory CJ, Lorenzi OD, Colón L, et al. Utility of the tourniquet test and the white blood cell count to differentiate dengue among acute febrile illnesses in the emergency room. PLoS Negl Trop Dis. 2011;5(12):e1400.
8. Mayxay M, Phetsouvanh R, Moore CE, et al. Predictive diagnostic value of the tourniquet test for the diagnosis of dengue infection in adults. Trop Med Int Health. 2011;16(1):127-133.
9. Lizarraga KJ, Nayer A. Dengue-associated kidney disease. J Nephropathol. 2014;3(2):57-62.
Patient Safety in the Emergency Department
Patient safety has received increased attention since the late 1990s. In 1999, The Institute of Medicine published “To Err is Human: Building a Safer Health System,”1 followed by “Crossing the Quality Chasm: A New Health System for the 21st Century”2 in 2001 to document patient-safety issues and recommend improvements in medical care to reduce errors. These reports and other patient-safety studies, however, likely underestimate the extent of medical errors and preventable harm. After these reports appeared, many specialties began to seriously evaluate their own safety issues.
Among the specialties, emergency medicine (EM) identified several problem areas and attempted to determine the epidemiology of errors. One study of 62 urban EDs found that at least 7% of patients who presented for myocardial infarctions (MIs), asthma exacerbations, or joint dislocations requiring reduction with procedural sedation experienced an actual or near-miss adverse event.3 Another study showed that up to 12% of all return visits to the ED within 7 days were related to adverse events.4
The ED setting itself undoubtedly contributes significantly to the risk of harm. This article illustrates and discusses ED patient-safety issues, and offers some recommendations for improvement in care and prevention of harm.
The ED Setting
The ED is unlike any other area of the hospital or health-care setting. Patients seek care for both primary care and urgent care complaints at any time of the day or night, on any day of the week, when no other source of care is available. Emergency physicians (EPs) are required to care for multiple patients of different ages while prioritizing care of the critically ill who have MI, stroke, sepsis, respiratory distress, or multisystem trauma. For many ED patients, diagnosis and treatment can be complex.
The ED setting is fast-paced and requires quick thinking, a broad depth of knowledge about many medical conditions, and a broad range of skills to perform emergent and life-saving procedures. Often, patients are presenting to a hospital ED for the first time, with incomplete medical records. They may not know their medical conditions or medications, or be in a position to communicate this information. Any of these situations alone can lead to an adverse event; in combination, they can significantly increase the risk for harm. In addition, ED overcrowding due to limited availability of inpatient hospital beds may consume resources and staffing needed to care for active ED patients and new patients coming through the door.
Safety factors in the ED can be categorized as those related to patients, providers, or the environment/systems (Table 1).5-7 When a large academic urban ED studied its errors, two-thirds were attributed to systems issues.5
Culture of Safety
Developing and maintaining a “culture of safety” is a commitment to minimize adverse events when performing high-risk jobs that can result in harm.8 This concept originated in other industries such as the airline and nuclear energy industries. Organizations and companies are considered high-reliability organizations (HROs) when they are dedicated to preventing harm at all staff levels—from the frontline to the corporate level. These HROs promote the reporting of errors and “near misses” without fear of blame or loss of employment.8 In the ED, a culture of safety encourages teamwork, event reporting, communication openness, transparency with feedback and learning from errors, and administrator collaboration for safety.9
In EDs with a strong safety culture, near misses are more likely to be intercepted to reduce patient harm.3 Teamwork training improves communication and reduces errors.10 One such program, Team Strategies and Tools to Enhance Performance and Patient Safety (TeamSTEPPS), was developed by a joint effort of the US Department of Defense and Agency for Healthcare Research and Quality to promote interprofessional communication between all providers in the hospital. This program provides many tools, including one to obtain attention in difficult situations and one to escalate concerns to focus on an important safety issue.11 One ED’s experience with TeamSTEPPS led it to identify specific steps to ensure continued success after the initial start. To maintain the high level of teamwork and successful communication, this ED recognized a need for continued champions at all staff levels and all new staff members were required to go through the training.12
Another important aspect of a strong safety culture is creating an environment that promotes reporting of adverse events and near misses. The culture should allow a person involved in an adverse event to feel comfortable reporting such events. In one study of 522 “unintended events” at 10 EDs in the Netherlands, nurses reported 85% of events, and resident physicians reported 13% of events. Approximately 83% of reports were filed by a person involved in the event.13 This study highlights EDs that foster a “no blame” environment, where staff members feel comfortable admitting mistakes, and there is no fear of punishment or concern for job loss. When administration supports such reporting, the true safety problems in the ED are identified and can be targeted for improvement.
Medication Safety
Case Scenario 1
A 65-year-old woman presented to the ED with atrial fibrillation with a rapid ventricular rate of 165 beats/minute. Her heart rate was controlled with intravenous (IV) diltiazem, and a heparin infusion was ordered based on her estimated weight of 150 lb. As the pharmacist prepared the infusion, she rechecked the patient’s weight and discovered that the heparin order had been placed using pounds instead of kilograms. The pharmacist discussed the order with the physician, and the order was changed to avoid a double-dosing error.
Discussion
Many medications are required to treat critical illnesses and complex medical conditions; such polypharmacy is further complicated by the sheer volume of patients seen in the ED. The wide range of medications used in the ED and the different doses appropriate for age, gender, and body weight can lead to patient harm when the prescriber is confused. In addition, many medications can be administered via multiple routes, including IV, intramuscular, subcutaneous, or oral. In situations where a critically ill patient is close to death, verbal orders are often used and then followed by computer orders when the physician is able to leave the bedside. Clinicians may be simultaneously treating multiple patients with similar conditions or with similar names. In addition, due to the acuity of patient complaints, “high-alert” medications are often used in the ED,14 such as paralytics, opioids, anticoagulants, antithrombotics, insulins, sedatives, and vasopressors.15 Considering all of these factors, it is not surprising that up to 60% of ED patients experienced medication errors in one study.16 Fortunately, most of these errors do not result in immediate patient harm, but have the potential to lead to harm.17
The addition of a pharmacist to the ED 24 hours a day, 7 days a week can greatly improve medication safety. Emergency department pharmacists are available for immediate bedside consultation or discussion of a medication order, and can intercept prescribing errors in the ordering system before they are administered and before they result in patient harm.18 In general, medication errors are 13.5 times less likely to occur when a pharmacist is on duty in the ED.19 Pharmacists can recommend appropriate antibiotic dosing,20 as well as aid in the timely administration of medications for such emergent conditions and procedures as stroke, MI, trauma, and rapid-sequence intubation. In our ED, the pharmacists also ensure that look-alike/sound-alike (LASA) medications are not confused. Importantly, in overcrowded EDs, the pharmacist reviews medication orders for all inpatients boarding in the ED and ensures that the nurses obtain the appropriate medications from the automated dispensing cabinets. In some instances, neither the EP nor the ED nurses may be familiar with proper doses and scheduling of medications typically used only in the inpatient service.
Pharmacists can prevent errors with formulation confusion, LASA confusion, weight-based dose errors, and dosing frequency errors. They also can ensure that the most up-to-date evidence is used to support a medication ordered, ensuring best practices and adherence to hospital policies. Table 214 summarizes additional information on best practices for medication safety in the ED.
Discharge Process
Case Scenario 2
A 55-year-old man on warfarin presented to the ED with cough, dyspnea, and fever. His chest X-ray revealed right lower lobe pneumonia. He was prescribed levofloxacin and discharged home. His discharge instructions included a discussion of pneumonia, fever control, and the importance of taking his antibiotic appropriately, but he was not told to have his international normalized ratio (INR) checked regularly while taking levofloxacin. When the patient returned to the ED 5 days later because of rectal bleeding, his INR was elevated to 6 (normal range in a patient taking warfarin is 2.0-3.0).
Discussion
When patients who do not require admission to the hospital are discharged home, they need instructions to ensure that they fully understand the nature of their problem and what they need to do to get better. For the provider, the discharge process must include three tasks: communicating crucial information (diagnosis and return precautions), verifying the patient’s comprehension of the information presented, and addressing and correcting specific concerns and misunderstandings.21 The encounter must be standardized but also be flexible enough to ensure patient understanding across a wide range of health care literacy and cultural backgrounds.21 Patients frequently are not given appropriate verbal and written instructions, and if they do not understand their diagnosis, they may not follow up when necessary; may not realize that they need to take specific medications; or may not take their newly prescribed medications as intended.
In an evaluation of written discharge instructions, only 76% included a diagnosis or an explanation of the patient’s symptoms, and only 34% provided instructions on when and how to return.22 Another study of the discharge process showed that the average verbal discharge exchange lasted only 76 seconds and that 65% of instructions were not complete. Patients were often not given a diagnosis, an explanation of their prescriptions, or proper return precautions.23 Deficits in the discharge process places patients at risk for medical and medication errors.
The discharge exchange must provide information on the diagnosis, what was done in the ED, and what needs to happen next. This must be done both verbally and in writing, in the patient’s native language, and at his or her health-literacy level. There should be time for the patients and those accompanying them and who are also responsible for their health to ask questions to ensure that everyone understands what has taken place and what must be done after leaving the ED. Patients should be given information on all prescription and over-the-counter medications they are instructed to take, as well as any changes to their previously prescribed medications.
Patients should be told specifically with whom to follow up and within what time frame. If possible, the exact time and location of a follow-up appointment should be provided. For patients with lower health literacy and less understanding of the health-care system, a process should be in place to help them navigate and ensure they get to necessary appointments.21
Handoffs and Transitions of Care
Case Scenario 3
A 70-year-old man with hypertension and hyperlipidemia had an episode of chest pain and was evaluated in the ED for possible myocardial ischemia. His initial electrocardiogram was interpreted as nonischemic and his troponin level was below detection 30 minutes after the episode. As the initial provider was leaving the ED, he endorsed the patient to the oncoming EP, with instructions to follow up on the chest X-ray interpretation. The initial provider, however, did not tell the oncoming EP to check the results of a repeat troponin determination. The patient was discharged home after the second troponin test had been sent to the laboratory, but before the results had been checked.
Discussion
Emergency department patients still under evaluation or in the process of being admitted to the inpatient hospital are “handed off” to the next shift of providers. Handoffs, or transitions of care, place patients at high risk for adverse events or bad outcomes. Important information can be lost whenever care is transferred to another provider. For example, there can be a lack of communication about pending tests that require follow-up, the need for further testing, or contingency planning for any problems that may arise. Loss of information and lack of follow-up can lead to diagnostic error and improper disposition.
According to the Joint Commission and a 2006 National Patient Safety Goal, handoffs should be standardized.24 The four stages for safe ED-provider-to-ED-provider handoffs are pre-turnover, arrival of new provider, meeting of providers, and post-turnover.25 During pre-turnover, the initial provider should review what has happened in the patient’s care and the next steps needed to finalize patient disposition. The arrival of the new provider signals the start of a new shift. During the meeting with the new provider, important information should be verbally transmitted to the oncoming provider.25 This meeting needs to be standardized to include a patient summary, tasks and tests to follow up, and contingency planning. Many tools can aid in transitions of care, including verbal mnemonics, tools to integrate with the medical record, and tools to develop a complete process for transition of care. Post-turnover is completed by the oncoming provider as he or she finishes any tasks related to the patient’s care to ensure the treatment plan is completed.25
There are many ways to improve the safety of handoffs. First, the number of handoffs should be limited. Having more patients dispositioned by the provider who initiated their care reduces the risk of an adverse event. This can be accomplished by having overlapping shifts to allow out-going providers time to complete care for their patients. During handoffs, interruptions and distractions should be limited to give the off-going provider appropriate time to present a succinct but complete overview of the patient’s care and communicate all outstanding tasks as “to-do” or “action lists,” with contingency planning for any changes in the patient’s status, test results, etc. There should be time for the oncoming provider to ask questions to ensure he or she is clear about the next steps.25 At the end of the transition, there should be some signal that the patient’s care is passed on to the oncoming provider and the outgoing physician should leave the patient-care area to finish documentation.
Many ED patients will need transition from “ED patient” to “admitted patient”—ie, admission to the hospital and transfer of care to an inpatient service provider. Studies on transitions of care from the ED to an inpatient medical service have found multiple barriers to a seamless transition of care. These include communication failures; information technology failures; inability of inpatient providers to review vital signs, laboratory values, and medications given; a change of the inpatient team to whom the patient was assigned; and patient transfers to areas remote from the ED and/or inpatient floors, such as to a dialysis unit. In one survey, 29% of respondents reported that a patient of theirs had experienced an adverse event or near misses due to a poor handoff between the ED and medical service.26 Just as there needs to be a standardized process for ED-provider-to-ED-provider handoffs, there also should be a standardized process for ED-to-inpatient or -outpatient service provider handoffs. There should be verbal and possibly written transmission of vital information, with patient summaries, “to-do” lists of follow-ups, situational knowledge with contingency planning, and time for questions (Table 3).25,26 The Joint Commission’s Transitions of Care Portal (https://www.jointcommission.org
/toc.aspx) offers tools to help facilities formalize this process.
Health Information Technology
Case Scenario 4
An EM intern was instructed to order a dose of morphine for a patient with a fractured hip. The intern used electronic ordering. Afterward, the nurse caring for the patient asked the attending EP if she really wanted to order patient-controlled morphine analgesia for the patient. Upon reviewing the order, the attending discovered the intern had selected the first morphine on the drop-down list instead of scrolling down to find the range of individual doses available.
Discussion
The use of electronic health records (EHRs) and health information technology (HIT) systems has both improved patient care and introduced new errors. Physician handwriting may no longer be a problem, but some hospitals use several types of EHRs simultaneously, with different systems for inpatients, outpatients, and EDs. In these settings, there may not be a seamless system to allow for review of inpatient, outpatient, and ED records. Additional concerns include communication failure, misidentification of patient orders, poor data display, and “alert fatigue.”27 Communication failures include the lack of bedside or face-to-face discussion among care providers. Physicians may enter orders at a computer away from the nursing station and never directly inform the nurse about the plan for the patient.
Incorrect patient orders are usually self-explanatory. Other errors include choosing the wrong LASA medication from a drop-down list or ordering imaging studies for the wrong side of the patient’s body. Poor data display may not alert providers of two or more patients with the same last name or allow vital signs to be displayed in a meaningful way. Other data-display problems include the inability to distinguish abnormal results from normal results because the system uses the same display color for both. Conversely, alert fatigue occurs when too many warning messages appear while providers are trying to enter orders for patient care. These warnings can range from important messages such as allergy identification or severe drug interactions to noncritical alerts about the cost of a test.
Recommendations to improve patient safety with the use of EHRs or HIT systems involve having a frontline staff champion to identify areas for performance improvement and having a review process to identify and examine safety issues with these technologies. A multidisciplinary group, including frontline staff, can usually develop effective solutions to these safety issues.27
Conclusion
The ED is a high-risk setting for errors because it features high-acuity patients, patients of widely divergent ages, the frequent need to use high-alert medications, the need to simultaneously care for multiple patients, many interruptions and distractions, and the lack of an established relationship with patients. This environment can lead to communication failures in handoffs and transitions of care, medication errors, and poor follow-up due to poor discharge processes. Additional difficulties arise when HIT systems, such as EHRs, are not set up to ensure the success of frontline staff caring for ill patients. The ED can become a much safer place by establishing strategies such as those outlined in this article to reduce error in all of these areas.
1. Institute of Medicine. To Err is Human: building a Safer Health System. LT Kohn, JM Corrigan, MS Donaldson, eds. Washington, DC: National Academy Press, 1999.
2. Institute of Medicine. Crossing the Quality Chasm: a New Health System for the 21st Century. Washington, DC: National Academy Press, 2001.
3. Camargo CA Jr, Tsai CL, Sullivan AF, et al. Safety climate and medical errors in 62 US emergency departments. Ann Emerg Med. 2012;60(5):555-563.e20.
4. Calder L, Pozgay A, Riff S, et al. Adverse events in patients with return emergency department visits. BMJ Qual Saf. 2015;24(2):142-148.
5. Jepson ZK, Darling CE, Kotkowski KA, et al. Emergency department patient safety incident characterization: an observational analysis of the findings of a standardized peer review process. BMC Emerg Med. 2014:14:20.
6. Ramlakhan S, Qayyum H, Burke D, Brown R. The safety of emergency medicine. Emerg Med J. 2016;33(4):293-299.
7. Sklar DP, Crandall C. What do we know about emergency department safety? Perspectives on Safety. Patient Safety Network. https://psnet.ahrq.gov/perspectives/perspective/88/what-do-we-know-about-emergency-department-safety. Published June 2010. Accessed June 30, 2016.
8. Patient Safey Network. Safety culture. https://psnet.ahrq.gov/primers/primer/5/safety-culture. Updated July 2016. Accessed July 1, 2016.
9. Verbeek-VanNoord I, Wagner C, VanDyck C, Twisk JW, DeBruijne MC. Is culture associated with patient safety in the emergency department? A study of staff perspectives. Int J Qual Health Care. 2014;26(1):64-70.
10. Morey JC, Simon R, Jay GD, et al. Error reduction and performance improvement in the emergency department through formal teamwork training: evaluation results of the MedTeams project. Health Serv Res. 2002;37(6):1553-1581.
11. Agency for Healthcare Research and Quality. About TeamSTEPPS.http://www.ahrq.gov/teamstepps/about-teamstepps/index.html. Accessed July 1, 2016.
12. Turner P. Implementation of TeamSTEPPS in the emergency department. Crit Care Nursing Q. 2012;35(3):208-212.
13. Smits M, Groenewegen PP, Timmermans TRM, van der Wal G, Wagner C. The nature and causes of unintended events reported at ten emergency departments. BMC Emerg Med. 2009;9:16.
14. Croskerry P, Shapiro M, Campbell S, et al. Profiles in patient safety: medication errors in the emergency department. Acad Emerg Med. 2004;11(3):289-299.
15. Institute for Safe Medicine Practices. ISMP List of High-Alert Medications in Acute Care Settings. http://www.ismp.org/Tools/highalertmedications.pdf. Updated 2014. Accessed July 15, 2016.
16. Patanwala AE, Warholak TL, Sanders AB, Erstad BL. A prospective observational study of medication errors in a tertiary care emergency department. Ann Emerg Med. 2010;55(6):522-526.
17. Patanwala AE, Hays DP, Sanders AB, Erstad BL. Severity and probability of harm of medication errors intercepted by an emergency department pharmacist. Int J Pharm Pract. 2011;19(5):358-362.
18. Patanwala AE, Sanders AB, Thomas MC, et al. A prospective, multicenter study of pharmacist activities resulting in medication error interception in the emergency department. Ann Emerg Med. 2012;59(5):369-373.
19. Ernst AA, Weiss SJ, Sullivan A 4th, et al. On-site pharmacists in the ED improve medical errors. Am J Emerg Med. 2012;30(5):717-725.
20. Dewitt KM, Weiss SJ, Rankin S, Ernst A, Sarangarm P. Impact of an emergency medicine pharmacist on antibiotic dosing adjustment. Am J Emerg Med. 2016;34(6):980-984.
21. Samuels-Kalow ME, Stack AM, Porter SC. Effective discharge communication in the emergency department. Ann Emerg Med. 2012;60(2):152-159.
22. Vashi A, Rhodes KV. “Sign right here and you’re good to go”: a content analysis of audiotaped emergency department discharge instructions. Ann Emerg Med. 2011;57(4):315-322.e1.
23. Rhodes KV, Vieth T, He T, et al. Resuscitating the physician-patient relationship: emergency department communication in an academic medical center. Ann Emerg Med. 2004;44(3):262-267.
24. The Joint Commission. 2016 National Patient Safety Goals. http://www.jointcommission.org/PatientSafety/NationalPatientSafetyGoals/06_npsg_cah.htm. Accessed June 24, 2016.
25. Cheung DS, Kelly JJ, Beach C, et al. Improving handoffs in the emergency department. Ann Emerg Med. 2010;55(2):171-180.
26. Horowitz LI, Meredith T, Schuur JD, Shah NR, Kulkarni RG, Jeng GY. Dropping the baton: a qualitative analysis of failures during the transition from emergency department to inpatient care. Ann Emerg Med. 2009;53(6):701-710.e4.
27. Farley HL, Baumlin KM, Hamedani AG, et al. Quality and safety implications of emergency department information systems. Ann Emerg Med. 2013;62(4):399-407.
Patient safety has received increased attention since the late 1990s. In 1999, The Institute of Medicine published “To Err is Human: Building a Safer Health System,”1 followed by “Crossing the Quality Chasm: A New Health System for the 21st Century”2 in 2001 to document patient-safety issues and recommend improvements in medical care to reduce errors. These reports and other patient-safety studies, however, likely underestimate the extent of medical errors and preventable harm. After these reports appeared, many specialties began to seriously evaluate their own safety issues.
Among the specialties, emergency medicine (EM) identified several problem areas and attempted to determine the epidemiology of errors. One study of 62 urban EDs found that at least 7% of patients who presented for myocardial infarctions (MIs), asthma exacerbations, or joint dislocations requiring reduction with procedural sedation experienced an actual or near-miss adverse event.3 Another study showed that up to 12% of all return visits to the ED within 7 days were related to adverse events.4
The ED setting itself undoubtedly contributes significantly to the risk of harm. This article illustrates and discusses ED patient-safety issues, and offers some recommendations for improvement in care and prevention of harm.
The ED Setting
The ED is unlike any other area of the hospital or health-care setting. Patients seek care for both primary care and urgent care complaints at any time of the day or night, on any day of the week, when no other source of care is available. Emergency physicians (EPs) are required to care for multiple patients of different ages while prioritizing care of the critically ill who have MI, stroke, sepsis, respiratory distress, or multisystem trauma. For many ED patients, diagnosis and treatment can be complex.
The ED setting is fast-paced and requires quick thinking, a broad depth of knowledge about many medical conditions, and a broad range of skills to perform emergent and life-saving procedures. Often, patients are presenting to a hospital ED for the first time, with incomplete medical records. They may not know their medical conditions or medications, or be in a position to communicate this information. Any of these situations alone can lead to an adverse event; in combination, they can significantly increase the risk for harm. In addition, ED overcrowding due to limited availability of inpatient hospital beds may consume resources and staffing needed to care for active ED patients and new patients coming through the door.
Safety factors in the ED can be categorized as those related to patients, providers, or the environment/systems (Table 1).5-7 When a large academic urban ED studied its errors, two-thirds were attributed to systems issues.5
Culture of Safety
Developing and maintaining a “culture of safety” is a commitment to minimize adverse events when performing high-risk jobs that can result in harm.8 This concept originated in other industries such as the airline and nuclear energy industries. Organizations and companies are considered high-reliability organizations (HROs) when they are dedicated to preventing harm at all staff levels—from the frontline to the corporate level. These HROs promote the reporting of errors and “near misses” without fear of blame or loss of employment.8 In the ED, a culture of safety encourages teamwork, event reporting, communication openness, transparency with feedback and learning from errors, and administrator collaboration for safety.9
In EDs with a strong safety culture, near misses are more likely to be intercepted to reduce patient harm.3 Teamwork training improves communication and reduces errors.10 One such program, Team Strategies and Tools to Enhance Performance and Patient Safety (TeamSTEPPS), was developed by a joint effort of the US Department of Defense and Agency for Healthcare Research and Quality to promote interprofessional communication between all providers in the hospital. This program provides many tools, including one to obtain attention in difficult situations and one to escalate concerns to focus on an important safety issue.11 One ED’s experience with TeamSTEPPS led it to identify specific steps to ensure continued success after the initial start. To maintain the high level of teamwork and successful communication, this ED recognized a need for continued champions at all staff levels and all new staff members were required to go through the training.12
Another important aspect of a strong safety culture is creating an environment that promotes reporting of adverse events and near misses. The culture should allow a person involved in an adverse event to feel comfortable reporting such events. In one study of 522 “unintended events” at 10 EDs in the Netherlands, nurses reported 85% of events, and resident physicians reported 13% of events. Approximately 83% of reports were filed by a person involved in the event.13 This study highlights EDs that foster a “no blame” environment, where staff members feel comfortable admitting mistakes, and there is no fear of punishment or concern for job loss. When administration supports such reporting, the true safety problems in the ED are identified and can be targeted for improvement.
Medication Safety
Case Scenario 1
A 65-year-old woman presented to the ED with atrial fibrillation with a rapid ventricular rate of 165 beats/minute. Her heart rate was controlled with intravenous (IV) diltiazem, and a heparin infusion was ordered based on her estimated weight of 150 lb. As the pharmacist prepared the infusion, she rechecked the patient’s weight and discovered that the heparin order had been placed using pounds instead of kilograms. The pharmacist discussed the order with the physician, and the order was changed to avoid a double-dosing error.
Discussion
Many medications are required to treat critical illnesses and complex medical conditions; such polypharmacy is further complicated by the sheer volume of patients seen in the ED. The wide range of medications used in the ED and the different doses appropriate for age, gender, and body weight can lead to patient harm when the prescriber is confused. In addition, many medications can be administered via multiple routes, including IV, intramuscular, subcutaneous, or oral. In situations where a critically ill patient is close to death, verbal orders are often used and then followed by computer orders when the physician is able to leave the bedside. Clinicians may be simultaneously treating multiple patients with similar conditions or with similar names. In addition, due to the acuity of patient complaints, “high-alert” medications are often used in the ED,14 such as paralytics, opioids, anticoagulants, antithrombotics, insulins, sedatives, and vasopressors.15 Considering all of these factors, it is not surprising that up to 60% of ED patients experienced medication errors in one study.16 Fortunately, most of these errors do not result in immediate patient harm, but have the potential to lead to harm.17
The addition of a pharmacist to the ED 24 hours a day, 7 days a week can greatly improve medication safety. Emergency department pharmacists are available for immediate bedside consultation or discussion of a medication order, and can intercept prescribing errors in the ordering system before they are administered and before they result in patient harm.18 In general, medication errors are 13.5 times less likely to occur when a pharmacist is on duty in the ED.19 Pharmacists can recommend appropriate antibiotic dosing,20 as well as aid in the timely administration of medications for such emergent conditions and procedures as stroke, MI, trauma, and rapid-sequence intubation. In our ED, the pharmacists also ensure that look-alike/sound-alike (LASA) medications are not confused. Importantly, in overcrowded EDs, the pharmacist reviews medication orders for all inpatients boarding in the ED and ensures that the nurses obtain the appropriate medications from the automated dispensing cabinets. In some instances, neither the EP nor the ED nurses may be familiar with proper doses and scheduling of medications typically used only in the inpatient service.
Pharmacists can prevent errors with formulation confusion, LASA confusion, weight-based dose errors, and dosing frequency errors. They also can ensure that the most up-to-date evidence is used to support a medication ordered, ensuring best practices and adherence to hospital policies. Table 214 summarizes additional information on best practices for medication safety in the ED.
Discharge Process
Case Scenario 2
A 55-year-old man on warfarin presented to the ED with cough, dyspnea, and fever. His chest X-ray revealed right lower lobe pneumonia. He was prescribed levofloxacin and discharged home. His discharge instructions included a discussion of pneumonia, fever control, and the importance of taking his antibiotic appropriately, but he was not told to have his international normalized ratio (INR) checked regularly while taking levofloxacin. When the patient returned to the ED 5 days later because of rectal bleeding, his INR was elevated to 6 (normal range in a patient taking warfarin is 2.0-3.0).
Discussion
When patients who do not require admission to the hospital are discharged home, they need instructions to ensure that they fully understand the nature of their problem and what they need to do to get better. For the provider, the discharge process must include three tasks: communicating crucial information (diagnosis and return precautions), verifying the patient’s comprehension of the information presented, and addressing and correcting specific concerns and misunderstandings.21 The encounter must be standardized but also be flexible enough to ensure patient understanding across a wide range of health care literacy and cultural backgrounds.21 Patients frequently are not given appropriate verbal and written instructions, and if they do not understand their diagnosis, they may not follow up when necessary; may not realize that they need to take specific medications; or may not take their newly prescribed medications as intended.
In an evaluation of written discharge instructions, only 76% included a diagnosis or an explanation of the patient’s symptoms, and only 34% provided instructions on when and how to return.22 Another study of the discharge process showed that the average verbal discharge exchange lasted only 76 seconds and that 65% of instructions were not complete. Patients were often not given a diagnosis, an explanation of their prescriptions, or proper return precautions.23 Deficits in the discharge process places patients at risk for medical and medication errors.
The discharge exchange must provide information on the diagnosis, what was done in the ED, and what needs to happen next. This must be done both verbally and in writing, in the patient’s native language, and at his or her health-literacy level. There should be time for the patients and those accompanying them and who are also responsible for their health to ask questions to ensure that everyone understands what has taken place and what must be done after leaving the ED. Patients should be given information on all prescription and over-the-counter medications they are instructed to take, as well as any changes to their previously prescribed medications.
Patients should be told specifically with whom to follow up and within what time frame. If possible, the exact time and location of a follow-up appointment should be provided. For patients with lower health literacy and less understanding of the health-care system, a process should be in place to help them navigate and ensure they get to necessary appointments.21
Handoffs and Transitions of Care
Case Scenario 3
A 70-year-old man with hypertension and hyperlipidemia had an episode of chest pain and was evaluated in the ED for possible myocardial ischemia. His initial electrocardiogram was interpreted as nonischemic and his troponin level was below detection 30 minutes after the episode. As the initial provider was leaving the ED, he endorsed the patient to the oncoming EP, with instructions to follow up on the chest X-ray interpretation. The initial provider, however, did not tell the oncoming EP to check the results of a repeat troponin determination. The patient was discharged home after the second troponin test had been sent to the laboratory, but before the results had been checked.
Discussion
Emergency department patients still under evaluation or in the process of being admitted to the inpatient hospital are “handed off” to the next shift of providers. Handoffs, or transitions of care, place patients at high risk for adverse events or bad outcomes. Important information can be lost whenever care is transferred to another provider. For example, there can be a lack of communication about pending tests that require follow-up, the need for further testing, or contingency planning for any problems that may arise. Loss of information and lack of follow-up can lead to diagnostic error and improper disposition.
According to the Joint Commission and a 2006 National Patient Safety Goal, handoffs should be standardized.24 The four stages for safe ED-provider-to-ED-provider handoffs are pre-turnover, arrival of new provider, meeting of providers, and post-turnover.25 During pre-turnover, the initial provider should review what has happened in the patient’s care and the next steps needed to finalize patient disposition. The arrival of the new provider signals the start of a new shift. During the meeting with the new provider, important information should be verbally transmitted to the oncoming provider.25 This meeting needs to be standardized to include a patient summary, tasks and tests to follow up, and contingency planning. Many tools can aid in transitions of care, including verbal mnemonics, tools to integrate with the medical record, and tools to develop a complete process for transition of care. Post-turnover is completed by the oncoming provider as he or she finishes any tasks related to the patient’s care to ensure the treatment plan is completed.25
There are many ways to improve the safety of handoffs. First, the number of handoffs should be limited. Having more patients dispositioned by the provider who initiated their care reduces the risk of an adverse event. This can be accomplished by having overlapping shifts to allow out-going providers time to complete care for their patients. During handoffs, interruptions and distractions should be limited to give the off-going provider appropriate time to present a succinct but complete overview of the patient’s care and communicate all outstanding tasks as “to-do” or “action lists,” with contingency planning for any changes in the patient’s status, test results, etc. There should be time for the oncoming provider to ask questions to ensure he or she is clear about the next steps.25 At the end of the transition, there should be some signal that the patient’s care is passed on to the oncoming provider and the outgoing physician should leave the patient-care area to finish documentation.
Many ED patients will need transition from “ED patient” to “admitted patient”—ie, admission to the hospital and transfer of care to an inpatient service provider. Studies on transitions of care from the ED to an inpatient medical service have found multiple barriers to a seamless transition of care. These include communication failures; information technology failures; inability of inpatient providers to review vital signs, laboratory values, and medications given; a change of the inpatient team to whom the patient was assigned; and patient transfers to areas remote from the ED and/or inpatient floors, such as to a dialysis unit. In one survey, 29% of respondents reported that a patient of theirs had experienced an adverse event or near misses due to a poor handoff between the ED and medical service.26 Just as there needs to be a standardized process for ED-provider-to-ED-provider handoffs, there also should be a standardized process for ED-to-inpatient or -outpatient service provider handoffs. There should be verbal and possibly written transmission of vital information, with patient summaries, “to-do” lists of follow-ups, situational knowledge with contingency planning, and time for questions (Table 3).25,26 The Joint Commission’s Transitions of Care Portal (https://www.jointcommission.org
/toc.aspx) offers tools to help facilities formalize this process.
Health Information Technology
Case Scenario 4
An EM intern was instructed to order a dose of morphine for a patient with a fractured hip. The intern used electronic ordering. Afterward, the nurse caring for the patient asked the attending EP if she really wanted to order patient-controlled morphine analgesia for the patient. Upon reviewing the order, the attending discovered the intern had selected the first morphine on the drop-down list instead of scrolling down to find the range of individual doses available.
Discussion
The use of electronic health records (EHRs) and health information technology (HIT) systems has both improved patient care and introduced new errors. Physician handwriting may no longer be a problem, but some hospitals use several types of EHRs simultaneously, with different systems for inpatients, outpatients, and EDs. In these settings, there may not be a seamless system to allow for review of inpatient, outpatient, and ED records. Additional concerns include communication failure, misidentification of patient orders, poor data display, and “alert fatigue.”27 Communication failures include the lack of bedside or face-to-face discussion among care providers. Physicians may enter orders at a computer away from the nursing station and never directly inform the nurse about the plan for the patient.
Incorrect patient orders are usually self-explanatory. Other errors include choosing the wrong LASA medication from a drop-down list or ordering imaging studies for the wrong side of the patient’s body. Poor data display may not alert providers of two or more patients with the same last name or allow vital signs to be displayed in a meaningful way. Other data-display problems include the inability to distinguish abnormal results from normal results because the system uses the same display color for both. Conversely, alert fatigue occurs when too many warning messages appear while providers are trying to enter orders for patient care. These warnings can range from important messages such as allergy identification or severe drug interactions to noncritical alerts about the cost of a test.
Recommendations to improve patient safety with the use of EHRs or HIT systems involve having a frontline staff champion to identify areas for performance improvement and having a review process to identify and examine safety issues with these technologies. A multidisciplinary group, including frontline staff, can usually develop effective solutions to these safety issues.27
Conclusion
The ED is a high-risk setting for errors because it features high-acuity patients, patients of widely divergent ages, the frequent need to use high-alert medications, the need to simultaneously care for multiple patients, many interruptions and distractions, and the lack of an established relationship with patients. This environment can lead to communication failures in handoffs and transitions of care, medication errors, and poor follow-up due to poor discharge processes. Additional difficulties arise when HIT systems, such as EHRs, are not set up to ensure the success of frontline staff caring for ill patients. The ED can become a much safer place by establishing strategies such as those outlined in this article to reduce error in all of these areas.
Patient safety has received increased attention since the late 1990s. In 1999, The Institute of Medicine published “To Err is Human: Building a Safer Health System,”1 followed by “Crossing the Quality Chasm: A New Health System for the 21st Century”2 in 2001 to document patient-safety issues and recommend improvements in medical care to reduce errors. These reports and other patient-safety studies, however, likely underestimate the extent of medical errors and preventable harm. After these reports appeared, many specialties began to seriously evaluate their own safety issues.
Among the specialties, emergency medicine (EM) identified several problem areas and attempted to determine the epidemiology of errors. One study of 62 urban EDs found that at least 7% of patients who presented for myocardial infarctions (MIs), asthma exacerbations, or joint dislocations requiring reduction with procedural sedation experienced an actual or near-miss adverse event.3 Another study showed that up to 12% of all return visits to the ED within 7 days were related to adverse events.4
The ED setting itself undoubtedly contributes significantly to the risk of harm. This article illustrates and discusses ED patient-safety issues, and offers some recommendations for improvement in care and prevention of harm.
The ED Setting
The ED is unlike any other area of the hospital or health-care setting. Patients seek care for both primary care and urgent care complaints at any time of the day or night, on any day of the week, when no other source of care is available. Emergency physicians (EPs) are required to care for multiple patients of different ages while prioritizing care of the critically ill who have MI, stroke, sepsis, respiratory distress, or multisystem trauma. For many ED patients, diagnosis and treatment can be complex.
The ED setting is fast-paced and requires quick thinking, a broad depth of knowledge about many medical conditions, and a broad range of skills to perform emergent and life-saving procedures. Often, patients are presenting to a hospital ED for the first time, with incomplete medical records. They may not know their medical conditions or medications, or be in a position to communicate this information. Any of these situations alone can lead to an adverse event; in combination, they can significantly increase the risk for harm. In addition, ED overcrowding due to limited availability of inpatient hospital beds may consume resources and staffing needed to care for active ED patients and new patients coming through the door.
Safety factors in the ED can be categorized as those related to patients, providers, or the environment/systems (Table 1).5-7 When a large academic urban ED studied its errors, two-thirds were attributed to systems issues.5
Culture of Safety
Developing and maintaining a “culture of safety” is a commitment to minimize adverse events when performing high-risk jobs that can result in harm.8 This concept originated in other industries such as the airline and nuclear energy industries. Organizations and companies are considered high-reliability organizations (HROs) when they are dedicated to preventing harm at all staff levels—from the frontline to the corporate level. These HROs promote the reporting of errors and “near misses” without fear of blame or loss of employment.8 In the ED, a culture of safety encourages teamwork, event reporting, communication openness, transparency with feedback and learning from errors, and administrator collaboration for safety.9
In EDs with a strong safety culture, near misses are more likely to be intercepted to reduce patient harm.3 Teamwork training improves communication and reduces errors.10 One such program, Team Strategies and Tools to Enhance Performance and Patient Safety (TeamSTEPPS), was developed by a joint effort of the US Department of Defense and Agency for Healthcare Research and Quality to promote interprofessional communication between all providers in the hospital. This program provides many tools, including one to obtain attention in difficult situations and one to escalate concerns to focus on an important safety issue.11 One ED’s experience with TeamSTEPPS led it to identify specific steps to ensure continued success after the initial start. To maintain the high level of teamwork and successful communication, this ED recognized a need for continued champions at all staff levels and all new staff members were required to go through the training.12
Another important aspect of a strong safety culture is creating an environment that promotes reporting of adverse events and near misses. The culture should allow a person involved in an adverse event to feel comfortable reporting such events. In one study of 522 “unintended events” at 10 EDs in the Netherlands, nurses reported 85% of events, and resident physicians reported 13% of events. Approximately 83% of reports were filed by a person involved in the event.13 This study highlights EDs that foster a “no blame” environment, where staff members feel comfortable admitting mistakes, and there is no fear of punishment or concern for job loss. When administration supports such reporting, the true safety problems in the ED are identified and can be targeted for improvement.
Medication Safety
Case Scenario 1
A 65-year-old woman presented to the ED with atrial fibrillation with a rapid ventricular rate of 165 beats/minute. Her heart rate was controlled with intravenous (IV) diltiazem, and a heparin infusion was ordered based on her estimated weight of 150 lb. As the pharmacist prepared the infusion, she rechecked the patient’s weight and discovered that the heparin order had been placed using pounds instead of kilograms. The pharmacist discussed the order with the physician, and the order was changed to avoid a double-dosing error.
Discussion
Many medications are required to treat critical illnesses and complex medical conditions; such polypharmacy is further complicated by the sheer volume of patients seen in the ED. The wide range of medications used in the ED and the different doses appropriate for age, gender, and body weight can lead to patient harm when the prescriber is confused. In addition, many medications can be administered via multiple routes, including IV, intramuscular, subcutaneous, or oral. In situations where a critically ill patient is close to death, verbal orders are often used and then followed by computer orders when the physician is able to leave the bedside. Clinicians may be simultaneously treating multiple patients with similar conditions or with similar names. In addition, due to the acuity of patient complaints, “high-alert” medications are often used in the ED,14 such as paralytics, opioids, anticoagulants, antithrombotics, insulins, sedatives, and vasopressors.15 Considering all of these factors, it is not surprising that up to 60% of ED patients experienced medication errors in one study.16 Fortunately, most of these errors do not result in immediate patient harm, but have the potential to lead to harm.17
The addition of a pharmacist to the ED 24 hours a day, 7 days a week can greatly improve medication safety. Emergency department pharmacists are available for immediate bedside consultation or discussion of a medication order, and can intercept prescribing errors in the ordering system before they are administered and before they result in patient harm.18 In general, medication errors are 13.5 times less likely to occur when a pharmacist is on duty in the ED.19 Pharmacists can recommend appropriate antibiotic dosing,20 as well as aid in the timely administration of medications for such emergent conditions and procedures as stroke, MI, trauma, and rapid-sequence intubation. In our ED, the pharmacists also ensure that look-alike/sound-alike (LASA) medications are not confused. Importantly, in overcrowded EDs, the pharmacist reviews medication orders for all inpatients boarding in the ED and ensures that the nurses obtain the appropriate medications from the automated dispensing cabinets. In some instances, neither the EP nor the ED nurses may be familiar with proper doses and scheduling of medications typically used only in the inpatient service.
Pharmacists can prevent errors with formulation confusion, LASA confusion, weight-based dose errors, and dosing frequency errors. They also can ensure that the most up-to-date evidence is used to support a medication ordered, ensuring best practices and adherence to hospital policies. Table 214 summarizes additional information on best practices for medication safety in the ED.
Discharge Process
Case Scenario 2
A 55-year-old man on warfarin presented to the ED with cough, dyspnea, and fever. His chest X-ray revealed right lower lobe pneumonia. He was prescribed levofloxacin and discharged home. His discharge instructions included a discussion of pneumonia, fever control, and the importance of taking his antibiotic appropriately, but he was not told to have his international normalized ratio (INR) checked regularly while taking levofloxacin. When the patient returned to the ED 5 days later because of rectal bleeding, his INR was elevated to 6 (normal range in a patient taking warfarin is 2.0-3.0).
Discussion
When patients who do not require admission to the hospital are discharged home, they need instructions to ensure that they fully understand the nature of their problem and what they need to do to get better. For the provider, the discharge process must include three tasks: communicating crucial information (diagnosis and return precautions), verifying the patient’s comprehension of the information presented, and addressing and correcting specific concerns and misunderstandings.21 The encounter must be standardized but also be flexible enough to ensure patient understanding across a wide range of health care literacy and cultural backgrounds.21 Patients frequently are not given appropriate verbal and written instructions, and if they do not understand their diagnosis, they may not follow up when necessary; may not realize that they need to take specific medications; or may not take their newly prescribed medications as intended.
In an evaluation of written discharge instructions, only 76% included a diagnosis or an explanation of the patient’s symptoms, and only 34% provided instructions on when and how to return.22 Another study of the discharge process showed that the average verbal discharge exchange lasted only 76 seconds and that 65% of instructions were not complete. Patients were often not given a diagnosis, an explanation of their prescriptions, or proper return precautions.23 Deficits in the discharge process places patients at risk for medical and medication errors.
The discharge exchange must provide information on the diagnosis, what was done in the ED, and what needs to happen next. This must be done both verbally and in writing, in the patient’s native language, and at his or her health-literacy level. There should be time for the patients and those accompanying them and who are also responsible for their health to ask questions to ensure that everyone understands what has taken place and what must be done after leaving the ED. Patients should be given information on all prescription and over-the-counter medications they are instructed to take, as well as any changes to their previously prescribed medications.
Patients should be told specifically with whom to follow up and within what time frame. If possible, the exact time and location of a follow-up appointment should be provided. For patients with lower health literacy and less understanding of the health-care system, a process should be in place to help them navigate and ensure they get to necessary appointments.21
Handoffs and Transitions of Care
Case Scenario 3
A 70-year-old man with hypertension and hyperlipidemia had an episode of chest pain and was evaluated in the ED for possible myocardial ischemia. His initial electrocardiogram was interpreted as nonischemic and his troponin level was below detection 30 minutes after the episode. As the initial provider was leaving the ED, he endorsed the patient to the oncoming EP, with instructions to follow up on the chest X-ray interpretation. The initial provider, however, did not tell the oncoming EP to check the results of a repeat troponin determination. The patient was discharged home after the second troponin test had been sent to the laboratory, but before the results had been checked.
Discussion
Emergency department patients still under evaluation or in the process of being admitted to the inpatient hospital are “handed off” to the next shift of providers. Handoffs, or transitions of care, place patients at high risk for adverse events or bad outcomes. Important information can be lost whenever care is transferred to another provider. For example, there can be a lack of communication about pending tests that require follow-up, the need for further testing, or contingency planning for any problems that may arise. Loss of information and lack of follow-up can lead to diagnostic error and improper disposition.
According to the Joint Commission and a 2006 National Patient Safety Goal, handoffs should be standardized.24 The four stages for safe ED-provider-to-ED-provider handoffs are pre-turnover, arrival of new provider, meeting of providers, and post-turnover.25 During pre-turnover, the initial provider should review what has happened in the patient’s care and the next steps needed to finalize patient disposition. The arrival of the new provider signals the start of a new shift. During the meeting with the new provider, important information should be verbally transmitted to the oncoming provider.25 This meeting needs to be standardized to include a patient summary, tasks and tests to follow up, and contingency planning. Many tools can aid in transitions of care, including verbal mnemonics, tools to integrate with the medical record, and tools to develop a complete process for transition of care. Post-turnover is completed by the oncoming provider as he or she finishes any tasks related to the patient’s care to ensure the treatment plan is completed.25
There are many ways to improve the safety of handoffs. First, the number of handoffs should be limited. Having more patients dispositioned by the provider who initiated their care reduces the risk of an adverse event. This can be accomplished by having overlapping shifts to allow out-going providers time to complete care for their patients. During handoffs, interruptions and distractions should be limited to give the off-going provider appropriate time to present a succinct but complete overview of the patient’s care and communicate all outstanding tasks as “to-do” or “action lists,” with contingency planning for any changes in the patient’s status, test results, etc. There should be time for the oncoming provider to ask questions to ensure he or she is clear about the next steps.25 At the end of the transition, there should be some signal that the patient’s care is passed on to the oncoming provider and the outgoing physician should leave the patient-care area to finish documentation.
Many ED patients will need transition from “ED patient” to “admitted patient”—ie, admission to the hospital and transfer of care to an inpatient service provider. Studies on transitions of care from the ED to an inpatient medical service have found multiple barriers to a seamless transition of care. These include communication failures; information technology failures; inability of inpatient providers to review vital signs, laboratory values, and medications given; a change of the inpatient team to whom the patient was assigned; and patient transfers to areas remote from the ED and/or inpatient floors, such as to a dialysis unit. In one survey, 29% of respondents reported that a patient of theirs had experienced an adverse event or near misses due to a poor handoff between the ED and medical service.26 Just as there needs to be a standardized process for ED-provider-to-ED-provider handoffs, there also should be a standardized process for ED-to-inpatient or -outpatient service provider handoffs. There should be verbal and possibly written transmission of vital information, with patient summaries, “to-do” lists of follow-ups, situational knowledge with contingency planning, and time for questions (Table 3).25,26 The Joint Commission’s Transitions of Care Portal (https://www.jointcommission.org
/toc.aspx) offers tools to help facilities formalize this process.
Health Information Technology
Case Scenario 4
An EM intern was instructed to order a dose of morphine for a patient with a fractured hip. The intern used electronic ordering. Afterward, the nurse caring for the patient asked the attending EP if she really wanted to order patient-controlled morphine analgesia for the patient. Upon reviewing the order, the attending discovered the intern had selected the first morphine on the drop-down list instead of scrolling down to find the range of individual doses available.
Discussion
The use of electronic health records (EHRs) and health information technology (HIT) systems has both improved patient care and introduced new errors. Physician handwriting may no longer be a problem, but some hospitals use several types of EHRs simultaneously, with different systems for inpatients, outpatients, and EDs. In these settings, there may not be a seamless system to allow for review of inpatient, outpatient, and ED records. Additional concerns include communication failure, misidentification of patient orders, poor data display, and “alert fatigue.”27 Communication failures include the lack of bedside or face-to-face discussion among care providers. Physicians may enter orders at a computer away from the nursing station and never directly inform the nurse about the plan for the patient.
Incorrect patient orders are usually self-explanatory. Other errors include choosing the wrong LASA medication from a drop-down list or ordering imaging studies for the wrong side of the patient’s body. Poor data display may not alert providers of two or more patients with the same last name or allow vital signs to be displayed in a meaningful way. Other data-display problems include the inability to distinguish abnormal results from normal results because the system uses the same display color for both. Conversely, alert fatigue occurs when too many warning messages appear while providers are trying to enter orders for patient care. These warnings can range from important messages such as allergy identification or severe drug interactions to noncritical alerts about the cost of a test.
Recommendations to improve patient safety with the use of EHRs or HIT systems involve having a frontline staff champion to identify areas for performance improvement and having a review process to identify and examine safety issues with these technologies. A multidisciplinary group, including frontline staff, can usually develop effective solutions to these safety issues.27
Conclusion
The ED is a high-risk setting for errors because it features high-acuity patients, patients of widely divergent ages, the frequent need to use high-alert medications, the need to simultaneously care for multiple patients, many interruptions and distractions, and the lack of an established relationship with patients. This environment can lead to communication failures in handoffs and transitions of care, medication errors, and poor follow-up due to poor discharge processes. Additional difficulties arise when HIT systems, such as EHRs, are not set up to ensure the success of frontline staff caring for ill patients. The ED can become a much safer place by establishing strategies such as those outlined in this article to reduce error in all of these areas.
1. Institute of Medicine. To Err is Human: building a Safer Health System. LT Kohn, JM Corrigan, MS Donaldson, eds. Washington, DC: National Academy Press, 1999.
2. Institute of Medicine. Crossing the Quality Chasm: a New Health System for the 21st Century. Washington, DC: National Academy Press, 2001.
3. Camargo CA Jr, Tsai CL, Sullivan AF, et al. Safety climate and medical errors in 62 US emergency departments. Ann Emerg Med. 2012;60(5):555-563.e20.
4. Calder L, Pozgay A, Riff S, et al. Adverse events in patients with return emergency department visits. BMJ Qual Saf. 2015;24(2):142-148.
5. Jepson ZK, Darling CE, Kotkowski KA, et al. Emergency department patient safety incident characterization: an observational analysis of the findings of a standardized peer review process. BMC Emerg Med. 2014:14:20.
6. Ramlakhan S, Qayyum H, Burke D, Brown R. The safety of emergency medicine. Emerg Med J. 2016;33(4):293-299.
7. Sklar DP, Crandall C. What do we know about emergency department safety? Perspectives on Safety. Patient Safety Network. https://psnet.ahrq.gov/perspectives/perspective/88/what-do-we-know-about-emergency-department-safety. Published June 2010. Accessed June 30, 2016.
8. Patient Safey Network. Safety culture. https://psnet.ahrq.gov/primers/primer/5/safety-culture. Updated July 2016. Accessed July 1, 2016.
9. Verbeek-VanNoord I, Wagner C, VanDyck C, Twisk JW, DeBruijne MC. Is culture associated with patient safety in the emergency department? A study of staff perspectives. Int J Qual Health Care. 2014;26(1):64-70.
10. Morey JC, Simon R, Jay GD, et al. Error reduction and performance improvement in the emergency department through formal teamwork training: evaluation results of the MedTeams project. Health Serv Res. 2002;37(6):1553-1581.
11. Agency for Healthcare Research and Quality. About TeamSTEPPS.http://www.ahrq.gov/teamstepps/about-teamstepps/index.html. Accessed July 1, 2016.
12. Turner P. Implementation of TeamSTEPPS in the emergency department. Crit Care Nursing Q. 2012;35(3):208-212.
13. Smits M, Groenewegen PP, Timmermans TRM, van der Wal G, Wagner C. The nature and causes of unintended events reported at ten emergency departments. BMC Emerg Med. 2009;9:16.
14. Croskerry P, Shapiro M, Campbell S, et al. Profiles in patient safety: medication errors in the emergency department. Acad Emerg Med. 2004;11(3):289-299.
15. Institute for Safe Medicine Practices. ISMP List of High-Alert Medications in Acute Care Settings. http://www.ismp.org/Tools/highalertmedications.pdf. Updated 2014. Accessed July 15, 2016.
16. Patanwala AE, Warholak TL, Sanders AB, Erstad BL. A prospective observational study of medication errors in a tertiary care emergency department. Ann Emerg Med. 2010;55(6):522-526.
17. Patanwala AE, Hays DP, Sanders AB, Erstad BL. Severity and probability of harm of medication errors intercepted by an emergency department pharmacist. Int J Pharm Pract. 2011;19(5):358-362.
18. Patanwala AE, Sanders AB, Thomas MC, et al. A prospective, multicenter study of pharmacist activities resulting in medication error interception in the emergency department. Ann Emerg Med. 2012;59(5):369-373.
19. Ernst AA, Weiss SJ, Sullivan A 4th, et al. On-site pharmacists in the ED improve medical errors. Am J Emerg Med. 2012;30(5):717-725.
20. Dewitt KM, Weiss SJ, Rankin S, Ernst A, Sarangarm P. Impact of an emergency medicine pharmacist on antibiotic dosing adjustment. Am J Emerg Med. 2016;34(6):980-984.
21. Samuels-Kalow ME, Stack AM, Porter SC. Effective discharge communication in the emergency department. Ann Emerg Med. 2012;60(2):152-159.
22. Vashi A, Rhodes KV. “Sign right here and you’re good to go”: a content analysis of audiotaped emergency department discharge instructions. Ann Emerg Med. 2011;57(4):315-322.e1.
23. Rhodes KV, Vieth T, He T, et al. Resuscitating the physician-patient relationship: emergency department communication in an academic medical center. Ann Emerg Med. 2004;44(3):262-267.
24. The Joint Commission. 2016 National Patient Safety Goals. http://www.jointcommission.org/PatientSafety/NationalPatientSafetyGoals/06_npsg_cah.htm. Accessed June 24, 2016.
25. Cheung DS, Kelly JJ, Beach C, et al. Improving handoffs in the emergency department. Ann Emerg Med. 2010;55(2):171-180.
26. Horowitz LI, Meredith T, Schuur JD, Shah NR, Kulkarni RG, Jeng GY. Dropping the baton: a qualitative analysis of failures during the transition from emergency department to inpatient care. Ann Emerg Med. 2009;53(6):701-710.e4.
27. Farley HL, Baumlin KM, Hamedani AG, et al. Quality and safety implications of emergency department information systems. Ann Emerg Med. 2013;62(4):399-407.
1. Institute of Medicine. To Err is Human: building a Safer Health System. LT Kohn, JM Corrigan, MS Donaldson, eds. Washington, DC: National Academy Press, 1999.
2. Institute of Medicine. Crossing the Quality Chasm: a New Health System for the 21st Century. Washington, DC: National Academy Press, 2001.
3. Camargo CA Jr, Tsai CL, Sullivan AF, et al. Safety climate and medical errors in 62 US emergency departments. Ann Emerg Med. 2012;60(5):555-563.e20.
4. Calder L, Pozgay A, Riff S, et al. Adverse events in patients with return emergency department visits. BMJ Qual Saf. 2015;24(2):142-148.
5. Jepson ZK, Darling CE, Kotkowski KA, et al. Emergency department patient safety incident characterization: an observational analysis of the findings of a standardized peer review process. BMC Emerg Med. 2014:14:20.
6. Ramlakhan S, Qayyum H, Burke D, Brown R. The safety of emergency medicine. Emerg Med J. 2016;33(4):293-299.
7. Sklar DP, Crandall C. What do we know about emergency department safety? Perspectives on Safety. Patient Safety Network. https://psnet.ahrq.gov/perspectives/perspective/88/what-do-we-know-about-emergency-department-safety. Published June 2010. Accessed June 30, 2016.
8. Patient Safey Network. Safety culture. https://psnet.ahrq.gov/primers/primer/5/safety-culture. Updated July 2016. Accessed July 1, 2016.
9. Verbeek-VanNoord I, Wagner C, VanDyck C, Twisk JW, DeBruijne MC. Is culture associated with patient safety in the emergency department? A study of staff perspectives. Int J Qual Health Care. 2014;26(1):64-70.
10. Morey JC, Simon R, Jay GD, et al. Error reduction and performance improvement in the emergency department through formal teamwork training: evaluation results of the MedTeams project. Health Serv Res. 2002;37(6):1553-1581.
11. Agency for Healthcare Research and Quality. About TeamSTEPPS.http://www.ahrq.gov/teamstepps/about-teamstepps/index.html. Accessed July 1, 2016.
12. Turner P. Implementation of TeamSTEPPS in the emergency department. Crit Care Nursing Q. 2012;35(3):208-212.
13. Smits M, Groenewegen PP, Timmermans TRM, van der Wal G, Wagner C. The nature and causes of unintended events reported at ten emergency departments. BMC Emerg Med. 2009;9:16.
14. Croskerry P, Shapiro M, Campbell S, et al. Profiles in patient safety: medication errors in the emergency department. Acad Emerg Med. 2004;11(3):289-299.
15. Institute for Safe Medicine Practices. ISMP List of High-Alert Medications in Acute Care Settings. http://www.ismp.org/Tools/highalertmedications.pdf. Updated 2014. Accessed July 15, 2016.
16. Patanwala AE, Warholak TL, Sanders AB, Erstad BL. A prospective observational study of medication errors in a tertiary care emergency department. Ann Emerg Med. 2010;55(6):522-526.
17. Patanwala AE, Hays DP, Sanders AB, Erstad BL. Severity and probability of harm of medication errors intercepted by an emergency department pharmacist. Int J Pharm Pract. 2011;19(5):358-362.
18. Patanwala AE, Sanders AB, Thomas MC, et al. A prospective, multicenter study of pharmacist activities resulting in medication error interception in the emergency department. Ann Emerg Med. 2012;59(5):369-373.
19. Ernst AA, Weiss SJ, Sullivan A 4th, et al. On-site pharmacists in the ED improve medical errors. Am J Emerg Med. 2012;30(5):717-725.
20. Dewitt KM, Weiss SJ, Rankin S, Ernst A, Sarangarm P. Impact of an emergency medicine pharmacist on antibiotic dosing adjustment. Am J Emerg Med. 2016;34(6):980-984.
21. Samuels-Kalow ME, Stack AM, Porter SC. Effective discharge communication in the emergency department. Ann Emerg Med. 2012;60(2):152-159.
22. Vashi A, Rhodes KV. “Sign right here and you’re good to go”: a content analysis of audiotaped emergency department discharge instructions. Ann Emerg Med. 2011;57(4):315-322.e1.
23. Rhodes KV, Vieth T, He T, et al. Resuscitating the physician-patient relationship: emergency department communication in an academic medical center. Ann Emerg Med. 2004;44(3):262-267.
24. The Joint Commission. 2016 National Patient Safety Goals. http://www.jointcommission.org/PatientSafety/NationalPatientSafetyGoals/06_npsg_cah.htm. Accessed June 24, 2016.
25. Cheung DS, Kelly JJ, Beach C, et al. Improving handoffs in the emergency department. Ann Emerg Med. 2010;55(2):171-180.
26. Horowitz LI, Meredith T, Schuur JD, Shah NR, Kulkarni RG, Jeng GY. Dropping the baton: a qualitative analysis of failures during the transition from emergency department to inpatient care. Ann Emerg Med. 2009;53(6):701-710.e4.
27. Farley HL, Baumlin KM, Hamedani AG, et al. Quality and safety implications of emergency department information systems. Ann Emerg Med. 2013;62(4):399-407.
Cosmetic Corner: Dermatologists Weigh in on Nail Care Products
To improve patient care and outcomes, leading dermatologists offered their recommendations on nail care products. Consideration must be given to:
- Aquaphor Healing Ointment
Beiersdorf, Inc
Recommended by Gary Goldenberg, MD, New York, New York
- Biotin Oral Supplements
Manufacturers vary
“Biotin is a helpful supplement for brittle nails. It may take 6 months to see improvement in the nails.”—Shari Lipner, MD, PhD, New York, New York
Recommended by Gary Goldenberg, MD, New York, New York
- Deep Comfort Hand and Cuticle Cream
Clinique
“It has good hydration for cuticles with sodium hyaluronate and squalene. It also is fragrance free.”—Anthony M. Rossi, MD, New York, New York
- Genadur
Medimetriks Pharmaceuticals, Inc
Recommended by Gary Goldenberg, MD, New York, New York
- Lanolin-Rich Nail Conditioner
Elon
“It’s great for moisturizing and nail hardening.”—Marta Rendon, MD, Boca Raton, Florida
- Nail Renewal System
Dr. Dana
“Developed by dermatologist Dr. Dana Stern, the system combines glycolic acid to improve discoloration and ridging, along with hydrating and strengthening botanicals to improve the look, feel, and overall health of the nails.”— Joshua Zeichner, MD, New York, New York
Cutis invites readers to send us their recommendations. Acne scar treatments, self-tanners, and cleansing devices will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on nail care products. Consideration must be given to:
- Aquaphor Healing Ointment
Beiersdorf, Inc
Recommended by Gary Goldenberg, MD, New York, New York
- Biotin Oral Supplements
Manufacturers vary
“Biotin is a helpful supplement for brittle nails. It may take 6 months to see improvement in the nails.”—Shari Lipner, MD, PhD, New York, New York
Recommended by Gary Goldenberg, MD, New York, New York
- Deep Comfort Hand and Cuticle Cream
Clinique
“It has good hydration for cuticles with sodium hyaluronate and squalene. It also is fragrance free.”—Anthony M. Rossi, MD, New York, New York
- Genadur
Medimetriks Pharmaceuticals, Inc
Recommended by Gary Goldenberg, MD, New York, New York
- Lanolin-Rich Nail Conditioner
Elon
“It’s great for moisturizing and nail hardening.”—Marta Rendon, MD, Boca Raton, Florida
- Nail Renewal System
Dr. Dana
“Developed by dermatologist Dr. Dana Stern, the system combines glycolic acid to improve discoloration and ridging, along with hydrating and strengthening botanicals to improve the look, feel, and overall health of the nails.”— Joshua Zeichner, MD, New York, New York
Cutis invites readers to send us their recommendations. Acne scar treatments, self-tanners, and cleansing devices will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on nail care products. Consideration must be given to:
- Aquaphor Healing Ointment
Beiersdorf, Inc
Recommended by Gary Goldenberg, MD, New York, New York
- Biotin Oral Supplements
Manufacturers vary
“Biotin is a helpful supplement for brittle nails. It may take 6 months to see improvement in the nails.”—Shari Lipner, MD, PhD, New York, New York
Recommended by Gary Goldenberg, MD, New York, New York
- Deep Comfort Hand and Cuticle Cream
Clinique
“It has good hydration for cuticles with sodium hyaluronate and squalene. It also is fragrance free.”—Anthony M. Rossi, MD, New York, New York
- Genadur
Medimetriks Pharmaceuticals, Inc
Recommended by Gary Goldenberg, MD, New York, New York
- Lanolin-Rich Nail Conditioner
Elon
“It’s great for moisturizing and nail hardening.”—Marta Rendon, MD, Boca Raton, Florida
- Nail Renewal System
Dr. Dana
“Developed by dermatologist Dr. Dana Stern, the system combines glycolic acid to improve discoloration and ridging, along with hydrating and strengthening botanicals to improve the look, feel, and overall health of the nails.”— Joshua Zeichner, MD, New York, New York
Cutis invites readers to send us their recommendations. Acne scar treatments, self-tanners, and cleansing devices will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
First EDition: News for and about the practice of emergency medicine
Black Patients Are Less Likely to Receive Opioids for Back Pain, Abdominal Pain, But Not for “Definitive” Pain
BY JEFF BAUER
FROM PLOS ONE
Black patients who present to the ED with back pain or abdominal pain are significantly less likely to be treated with or prescribed an opioid than are white patients who report similar pain, according to an analysis of data on national ED visits. However, there were no differences in opioid use among black patients and white patients for more objective pain conditions, such as kidney stones or long bone fractures.
Researchers evaluated National Hospital Ambulatory Medical Care Survey data that included descriptions of ED visits made by adults ages 18 to 65 years from 2007 to 2011. They looked specifically at pain-related visits, and defined the reason for each visit as being a “nondefinitive condition” (toothache, back pain, or abdominal pain) or a “definitive condition” (long bone fractures or kidney stones). These nondefinitive conditions have been associated with drug-seeking behavior.
The subjects were categorized as non-Hispanic white, non-Hispanic black, Hispanic, or non-Hispanic other. Pain was rated on a scale from 0 (no pain) to 10 (severe). Researchers also measured whether the patients received an opioid while they were in the ED, were discharged with a prescription for an opioid, or both.
During the study period, there were approximately 36.5 million ED visits for abdominal pain, 14.3 visits for back pain, 6.9 million visits for toothache, 3.4 million visits for kidney stones, and 2.1 million visits for long bone fractures. For each of these conditions, most visits were associated with severe pain.
After adjusting for pain severity, non-Hispanic black patients with abdominal pain or back pain were significantly less likely than their white counterparts to be administered an opioid while in the ED or to be discharged with a prescription for an opioid. However, there was no significant difference between these groups in opioid administration or prescription for patients with long bone fractures, kidney stones, or toothache. Researchers suggested that although toothache was considered a nondefinitive condition in this study, physicians might have been able to verify dental disease during examination of the mouth, thus limiting the subjectivity in their decision to prescribe an opioid.
1. Singhal A, Tien YY, Hsia RY. Racial-ethnic disparities in opioid prescriptions at emergency department visits for conditions commonly associated with prescription drug abuse. PLoS One. 2016;11(8):e0159224. doi: 10.1371/journal.pone.0159224.
FDA Updates Warning Label for Systemic Fluoroquinolones
BY DEEPAK CHITNIS
FRONTLINE MEDICAL NEWS
The Food and Drug Administration (FDA) has amended the boxed warning on labels for fluoroquinolone antibiotics, taken either orally or by injection, to reflect recent findings of the drugs’ potential adverse events.
“These medicines are associated with disabling and potentially permanent side effects of the tendons, muscles, joints, nerves, and central nervous system that can occur together in the same patient,” the FDA stated in its Safety Announcement.
As a result, health care providers should reserve systemic fluoroquinolones for patients who have no other treatment options for any of the following conditions: acute bacterial sinusitis (ABS), acute bacterial exacerbation of chronic bronchitis (ABECB), and uncomplicated urinary tract infections (UTIs). The FDA also said that for some serious bacterial infections, the benefits of fluoroquinolones outweigh the risks, and it is appropriate for them to remain available as a therapeutic option.
Patients taking fluoroquinolones must also be vigilant and let their provider know immediately if they begin suffering from any new pain in their joints, tendons, or muscles. Additionally, if patients begin feeling any numbness in their arms and legs, a prickling or “pins and needles” sensation, or confusion and hallucinations, they should contact their health care provider right away so that they may be switched to a nonfluoroquinolone antibacterial drug for the remainder of their treatment regimen.
Avelox (Moxifloxacin); Cipro, both standard and extended release (ciprofloxacin); Factive (gemifloxacin); Levaquin (levofloxacin); and ofloxacin are the fluoroquinolones currently approved by the FDA for systemic use.
Additional adverse effects for patients taking fluoroquinolones could include tendinitis, tendon rupture, and joint swelling. Central nervous system afflictions could include depression and thoughts of suicide. Fluoroquinolones could also bring about skin rashes, sunburn, arrhythmia, and diarrhea, and could aggravate myasthenia gravis in patients who suffer from it. Warnings regarding these conditions are already included on the drugs’ existing boxed warning.
“In addition to updating information in the Boxed Warning, we are also including information about these safety issues in the Warnings and Precautions section of the label,” the FDA stated. “The Indications and Usage section contains new limitation-of-use statements to reserve fluoroquinolones for patients who do not have other available treatment options for ABS, ABECB, and uncomplicated UTIs.”
The FDA added that it will continue to monitor and assess safety issues associated with fluoroquinolones and will issue any further updates if necessary.
1. US Food and Drug Administration. FDA Drug Safety Communication: FDA updates warnings for oral and injectable fluoroquinolone antibiotics due to disabling side effects. http://www.fda.gov/Drugs/DrugSafety/ucm511530.htm. Published July 26, 2016 Accessed August 26, 2016.
Skin Rash in a Recent Traveler? Think Dengue Fever
BY SHARON WORCESTER
FRONTLINE MEDICAL NEWS
Maintain clinical suspicion for dengue fever among individuals with recent travel to endemic areas who present with a rash and other signs and symptoms of infection, an expert advised at the American Academy of Dermatology summer meeting.
Dengue fever accounts for nearly 10% of skin rashes among individuals returning from endemic areas, and related illness can range from mild to fatal, said Jose Dario Martinez, MD, chief of the Internal Medicine Clinic at University Hospital J.E. Gonzalez, UANL Monterrey, Mexico.
“This is the most prevalent arthropod-borne virus in the world at this time, and it is a resurgent disease in some countries, like Mexico, Brazil, and Colombia,” he noted.
Worldwide, more than 2.5 billion people are at risk of dengue infection, and between 50 million and 100 million cases occur each year, while about 250,000 to 500,000 cases of dengue hemorrhagic fever (DHF) occur each year, and about 25,000 related deaths occur.
In 2005, there was a dengue outbreak in Texas, where 25 cases occurred; in southern Florida, an outbreak of 90 cases was reported in 2009 and 2010. More recently, in 2015, there was an outbreak of 107 cases of locally acquired dengue on the Big Island, Hawaii. But in Mexico, 18,000 new cases occurred in 2015, Dr Martinez said.
Of the RNA virus serotypes 1 to 4, type 1 (DEN-1) is the most common, and DEN-2 and DEN-3 are the most severe, but up to 40% of cases are asymptomatic, he noted, adding that the virus has an incubation period of 2 to 8 days. When symptoms occur, they are representative of acute febrile illness, and may include headache, high fever, myalgia, arthralgia, retro-orbital pain, and fatigue. A faint, itchy, macular rash commonly occurs at 2 to 6 days into the illness. According to the World Health Organization (WHO), a probable dengue fever case includes acute febrile illness and at least two of the following: headache, retro-orbital pain, myalgia, arthralgia, rash, hemorrhagic manifestations, leukopenia, or supportive serology.
“Sometimes the nose bleeds, the gums bleed, and there is bruising in the patient,” Dr Martinez said. “Most important are retro-orbital pain and hemorrhagic manifestations, but also supportive serology.”
About 1% of patients progress to DHF or dengue shock syndrome (DSS) during the critical phase (days 4-7) of illness. This is most likely in those with serotypes 2 and 3, but can occur with all serotypes. Warning signs of such severe disease include abdominal pain or tenderness, persistent vomiting, pleural effusion or ascites, and of particular importance—mucosal bleeding, Dr Martinez said.
By the WHO definition, a diagnosis of DHF requires the presence of fever for at least 2 to 7 days, hemorrhagic tendencies, thrombocytopenia, and evidence and signs of plasma leakage; DSS requires these, as well as evidence of circulatory failure, such as rapid and weak pulse, narrow pulse pressure, hypotension, and shock.
It is important to maintain clinical suspicion for dengue fever, particularly in anyone who has traveled to an endemic area in the 2 weeks before presentation. Serologic tests are important to detect anti-dengue antibodies. Immunoglobulin G is important because its presence could suggest recurrent infection and thus the potential for severe disease, Dr Martinez said. Polymerase chain reaction can be used for detection in the first 4 to 5 days of infection, and the nonstructural glycoprotein 1 rapid test can be positive on the first day, he noted.
The differential diagnosis for dengue fever is broad, and can include chikungunya fever, malaria, leptospirosis, meningococcemia, drug eruption, and Zika fever.
Management of dengue fever includes bed rest, liquids, and mosquito net isolation to prevent reinfection, as more severe disease can occur after reinfection. Acetaminophen can be used for pain relief; aspirin should be avoided due to risk of bleeding, Dr Martinez said. Hospitalization and supportive care are required for those with DHF or DSS. Intensive care unit admission may be required.
Of note, a vaccine against dengue fever has shown promise in phase III trials. The vaccine has been approved in Mexico and Brazil, but not yet in the United States.
For more on dengue fever, see the case report “Dengue Fever: Two Unexpected Findings” on page 408.
Pertussis Often Goes Undiagnosed, Especially in Adults
BY ABIGAIL CRUZ
FRONTLINE MEDICAL NEWS
A majority of pertussis cases in the United States may go undetected in people younger than age 50 years, particularly in adults, results of a retrospective database cohort study suggest.
“The incidence of pertussis in adolescents and adults is very difficult to quantify,” wrote Chi-Chang Chen, MD, of IMS Health, Plymouth Meeting, Pennsylvania, and associates. Symptoms may be misdiagnosed as other respiratory illnesses; infected individuals may not seek treatment; and pertussis may not be considered as a possible diagnosis in adults, they noted.
To project the possible range of pertussis incidence in this population, investigators used three different models to analyze information from private insurance and laboratory databases as well as data from the Centers for Disease Control and Prevention for a 6-year period. The first method, which used medical claims for International Classification of Diseases (ICD-9) diagnosed pertussis, found an annual incidence rate of 9 per 100,000 population. The second used a proxy pertussis model that was based on symptoms that could indicate undiagnosed pertussis, showing an incidence rate of 21 per 100,000. The third method used pathogen data to estimate the fraction of cough illness statistically attributable to pertussis, resulting in an incidence rate of 649 per 100,000 population, which is 58 to 93 times higher than the ICD-9 estimated incidence.
These estimates “highlight the need for improved preventive measures—such as increased vaccination—against pertussis,” the investigators said, noting that immunization recommendations for additional age groups and research involving strategies to reduce waning immunity after vaccination should be considered.
1. Chen CC, Balderston McGuiness C, Krishnarajah G, et al. Estimated incidence of pertussis in people aged <50 years in the United States. Hum Vaccin Immunother. 2016;31:1-10. [Epub ahead of print]
Black Patients Are Less Likely to Receive Opioids for Back Pain, Abdominal Pain, But Not for “Definitive” Pain
BY JEFF BAUER
FROM PLOS ONE
Black patients who present to the ED with back pain or abdominal pain are significantly less likely to be treated with or prescribed an opioid than are white patients who report similar pain, according to an analysis of data on national ED visits. However, there were no differences in opioid use among black patients and white patients for more objective pain conditions, such as kidney stones or long bone fractures.
Researchers evaluated National Hospital Ambulatory Medical Care Survey data that included descriptions of ED visits made by adults ages 18 to 65 years from 2007 to 2011. They looked specifically at pain-related visits, and defined the reason for each visit as being a “nondefinitive condition” (toothache, back pain, or abdominal pain) or a “definitive condition” (long bone fractures or kidney stones). These nondefinitive conditions have been associated with drug-seeking behavior.
The subjects were categorized as non-Hispanic white, non-Hispanic black, Hispanic, or non-Hispanic other. Pain was rated on a scale from 0 (no pain) to 10 (severe). Researchers also measured whether the patients received an opioid while they were in the ED, were discharged with a prescription for an opioid, or both.
During the study period, there were approximately 36.5 million ED visits for abdominal pain, 14.3 visits for back pain, 6.9 million visits for toothache, 3.4 million visits for kidney stones, and 2.1 million visits for long bone fractures. For each of these conditions, most visits were associated with severe pain.
After adjusting for pain severity, non-Hispanic black patients with abdominal pain or back pain were significantly less likely than their white counterparts to be administered an opioid while in the ED or to be discharged with a prescription for an opioid. However, there was no significant difference between these groups in opioid administration or prescription for patients with long bone fractures, kidney stones, or toothache. Researchers suggested that although toothache was considered a nondefinitive condition in this study, physicians might have been able to verify dental disease during examination of the mouth, thus limiting the subjectivity in their decision to prescribe an opioid.
1. Singhal A, Tien YY, Hsia RY. Racial-ethnic disparities in opioid prescriptions at emergency department visits for conditions commonly associated with prescription drug abuse. PLoS One. 2016;11(8):e0159224. doi: 10.1371/journal.pone.0159224.
FDA Updates Warning Label for Systemic Fluoroquinolones
BY DEEPAK CHITNIS
FRONTLINE MEDICAL NEWS
The Food and Drug Administration (FDA) has amended the boxed warning on labels for fluoroquinolone antibiotics, taken either orally or by injection, to reflect recent findings of the drugs’ potential adverse events.
“These medicines are associated with disabling and potentially permanent side effects of the tendons, muscles, joints, nerves, and central nervous system that can occur together in the same patient,” the FDA stated in its Safety Announcement.
As a result, health care providers should reserve systemic fluoroquinolones for patients who have no other treatment options for any of the following conditions: acute bacterial sinusitis (ABS), acute bacterial exacerbation of chronic bronchitis (ABECB), and uncomplicated urinary tract infections (UTIs). The FDA also said that for some serious bacterial infections, the benefits of fluoroquinolones outweigh the risks, and it is appropriate for them to remain available as a therapeutic option.
Patients taking fluoroquinolones must also be vigilant and let their provider know immediately if they begin suffering from any new pain in their joints, tendons, or muscles. Additionally, if patients begin feeling any numbness in their arms and legs, a prickling or “pins and needles” sensation, or confusion and hallucinations, they should contact their health care provider right away so that they may be switched to a nonfluoroquinolone antibacterial drug for the remainder of their treatment regimen.
Avelox (Moxifloxacin); Cipro, both standard and extended release (ciprofloxacin); Factive (gemifloxacin); Levaquin (levofloxacin); and ofloxacin are the fluoroquinolones currently approved by the FDA for systemic use.
Additional adverse effects for patients taking fluoroquinolones could include tendinitis, tendon rupture, and joint swelling. Central nervous system afflictions could include depression and thoughts of suicide. Fluoroquinolones could also bring about skin rashes, sunburn, arrhythmia, and diarrhea, and could aggravate myasthenia gravis in patients who suffer from it. Warnings regarding these conditions are already included on the drugs’ existing boxed warning.
“In addition to updating information in the Boxed Warning, we are also including information about these safety issues in the Warnings and Precautions section of the label,” the FDA stated. “The Indications and Usage section contains new limitation-of-use statements to reserve fluoroquinolones for patients who do not have other available treatment options for ABS, ABECB, and uncomplicated UTIs.”
The FDA added that it will continue to monitor and assess safety issues associated with fluoroquinolones and will issue any further updates if necessary.
1. US Food and Drug Administration. FDA Drug Safety Communication: FDA updates warnings for oral and injectable fluoroquinolone antibiotics due to disabling side effects. http://www.fda.gov/Drugs/DrugSafety/ucm511530.htm. Published July 26, 2016 Accessed August 26, 2016.
Skin Rash in a Recent Traveler? Think Dengue Fever
BY SHARON WORCESTER
FRONTLINE MEDICAL NEWS
Maintain clinical suspicion for dengue fever among individuals with recent travel to endemic areas who present with a rash and other signs and symptoms of infection, an expert advised at the American Academy of Dermatology summer meeting.
Dengue fever accounts for nearly 10% of skin rashes among individuals returning from endemic areas, and related illness can range from mild to fatal, said Jose Dario Martinez, MD, chief of the Internal Medicine Clinic at University Hospital J.E. Gonzalez, UANL Monterrey, Mexico.
“This is the most prevalent arthropod-borne virus in the world at this time, and it is a resurgent disease in some countries, like Mexico, Brazil, and Colombia,” he noted.
Worldwide, more than 2.5 billion people are at risk of dengue infection, and between 50 million and 100 million cases occur each year, while about 250,000 to 500,000 cases of dengue hemorrhagic fever (DHF) occur each year, and about 25,000 related deaths occur.
In 2005, there was a dengue outbreak in Texas, where 25 cases occurred; in southern Florida, an outbreak of 90 cases was reported in 2009 and 2010. More recently, in 2015, there was an outbreak of 107 cases of locally acquired dengue on the Big Island, Hawaii. But in Mexico, 18,000 new cases occurred in 2015, Dr Martinez said.
Of the RNA virus serotypes 1 to 4, type 1 (DEN-1) is the most common, and DEN-2 and DEN-3 are the most severe, but up to 40% of cases are asymptomatic, he noted, adding that the virus has an incubation period of 2 to 8 days. When symptoms occur, they are representative of acute febrile illness, and may include headache, high fever, myalgia, arthralgia, retro-orbital pain, and fatigue. A faint, itchy, macular rash commonly occurs at 2 to 6 days into the illness. According to the World Health Organization (WHO), a probable dengue fever case includes acute febrile illness and at least two of the following: headache, retro-orbital pain, myalgia, arthralgia, rash, hemorrhagic manifestations, leukopenia, or supportive serology.
“Sometimes the nose bleeds, the gums bleed, and there is bruising in the patient,” Dr Martinez said. “Most important are retro-orbital pain and hemorrhagic manifestations, but also supportive serology.”
About 1% of patients progress to DHF or dengue shock syndrome (DSS) during the critical phase (days 4-7) of illness. This is most likely in those with serotypes 2 and 3, but can occur with all serotypes. Warning signs of such severe disease include abdominal pain or tenderness, persistent vomiting, pleural effusion or ascites, and of particular importance—mucosal bleeding, Dr Martinez said.
By the WHO definition, a diagnosis of DHF requires the presence of fever for at least 2 to 7 days, hemorrhagic tendencies, thrombocytopenia, and evidence and signs of plasma leakage; DSS requires these, as well as evidence of circulatory failure, such as rapid and weak pulse, narrow pulse pressure, hypotension, and shock.
It is important to maintain clinical suspicion for dengue fever, particularly in anyone who has traveled to an endemic area in the 2 weeks before presentation. Serologic tests are important to detect anti-dengue antibodies. Immunoglobulin G is important because its presence could suggest recurrent infection and thus the potential for severe disease, Dr Martinez said. Polymerase chain reaction can be used for detection in the first 4 to 5 days of infection, and the nonstructural glycoprotein 1 rapid test can be positive on the first day, he noted.
The differential diagnosis for dengue fever is broad, and can include chikungunya fever, malaria, leptospirosis, meningococcemia, drug eruption, and Zika fever.
Management of dengue fever includes bed rest, liquids, and mosquito net isolation to prevent reinfection, as more severe disease can occur after reinfection. Acetaminophen can be used for pain relief; aspirin should be avoided due to risk of bleeding, Dr Martinez said. Hospitalization and supportive care are required for those with DHF or DSS. Intensive care unit admission may be required.
Of note, a vaccine against dengue fever has shown promise in phase III trials. The vaccine has been approved in Mexico and Brazil, but not yet in the United States.
For more on dengue fever, see the case report “Dengue Fever: Two Unexpected Findings” on page 408.
Pertussis Often Goes Undiagnosed, Especially in Adults
BY ABIGAIL CRUZ
FRONTLINE MEDICAL NEWS
A majority of pertussis cases in the United States may go undetected in people younger than age 50 years, particularly in adults, results of a retrospective database cohort study suggest.
“The incidence of pertussis in adolescents and adults is very difficult to quantify,” wrote Chi-Chang Chen, MD, of IMS Health, Plymouth Meeting, Pennsylvania, and associates. Symptoms may be misdiagnosed as other respiratory illnesses; infected individuals may not seek treatment; and pertussis may not be considered as a possible diagnosis in adults, they noted.
To project the possible range of pertussis incidence in this population, investigators used three different models to analyze information from private insurance and laboratory databases as well as data from the Centers for Disease Control and Prevention for a 6-year period. The first method, which used medical claims for International Classification of Diseases (ICD-9) diagnosed pertussis, found an annual incidence rate of 9 per 100,000 population. The second used a proxy pertussis model that was based on symptoms that could indicate undiagnosed pertussis, showing an incidence rate of 21 per 100,000. The third method used pathogen data to estimate the fraction of cough illness statistically attributable to pertussis, resulting in an incidence rate of 649 per 100,000 population, which is 58 to 93 times higher than the ICD-9 estimated incidence.
These estimates “highlight the need for improved preventive measures—such as increased vaccination—against pertussis,” the investigators said, noting that immunization recommendations for additional age groups and research involving strategies to reduce waning immunity after vaccination should be considered.
1. Chen CC, Balderston McGuiness C, Krishnarajah G, et al. Estimated incidence of pertussis in people aged <50 years in the United States. Hum Vaccin Immunother. 2016;31:1-10. [Epub ahead of print]
Black Patients Are Less Likely to Receive Opioids for Back Pain, Abdominal Pain, But Not for “Definitive” Pain
BY JEFF BAUER
FROM PLOS ONE
Black patients who present to the ED with back pain or abdominal pain are significantly less likely to be treated with or prescribed an opioid than are white patients who report similar pain, according to an analysis of data on national ED visits. However, there were no differences in opioid use among black patients and white patients for more objective pain conditions, such as kidney stones or long bone fractures.
Researchers evaluated National Hospital Ambulatory Medical Care Survey data that included descriptions of ED visits made by adults ages 18 to 65 years from 2007 to 2011. They looked specifically at pain-related visits, and defined the reason for each visit as being a “nondefinitive condition” (toothache, back pain, or abdominal pain) or a “definitive condition” (long bone fractures or kidney stones). These nondefinitive conditions have been associated with drug-seeking behavior.
The subjects were categorized as non-Hispanic white, non-Hispanic black, Hispanic, or non-Hispanic other. Pain was rated on a scale from 0 (no pain) to 10 (severe). Researchers also measured whether the patients received an opioid while they were in the ED, were discharged with a prescription for an opioid, or both.
During the study period, there were approximately 36.5 million ED visits for abdominal pain, 14.3 visits for back pain, 6.9 million visits for toothache, 3.4 million visits for kidney stones, and 2.1 million visits for long bone fractures. For each of these conditions, most visits were associated with severe pain.
After adjusting for pain severity, non-Hispanic black patients with abdominal pain or back pain were significantly less likely than their white counterparts to be administered an opioid while in the ED or to be discharged with a prescription for an opioid. However, there was no significant difference between these groups in opioid administration or prescription for patients with long bone fractures, kidney stones, or toothache. Researchers suggested that although toothache was considered a nondefinitive condition in this study, physicians might have been able to verify dental disease during examination of the mouth, thus limiting the subjectivity in their decision to prescribe an opioid.
1. Singhal A, Tien YY, Hsia RY. Racial-ethnic disparities in opioid prescriptions at emergency department visits for conditions commonly associated with prescription drug abuse. PLoS One. 2016;11(8):e0159224. doi: 10.1371/journal.pone.0159224.
FDA Updates Warning Label for Systemic Fluoroquinolones
BY DEEPAK CHITNIS
FRONTLINE MEDICAL NEWS
The Food and Drug Administration (FDA) has amended the boxed warning on labels for fluoroquinolone antibiotics, taken either orally or by injection, to reflect recent findings of the drugs’ potential adverse events.
“These medicines are associated with disabling and potentially permanent side effects of the tendons, muscles, joints, nerves, and central nervous system that can occur together in the same patient,” the FDA stated in its Safety Announcement.
As a result, health care providers should reserve systemic fluoroquinolones for patients who have no other treatment options for any of the following conditions: acute bacterial sinusitis (ABS), acute bacterial exacerbation of chronic bronchitis (ABECB), and uncomplicated urinary tract infections (UTIs). The FDA also said that for some serious bacterial infections, the benefits of fluoroquinolones outweigh the risks, and it is appropriate for them to remain available as a therapeutic option.
Patients taking fluoroquinolones must also be vigilant and let their provider know immediately if they begin suffering from any new pain in their joints, tendons, or muscles. Additionally, if patients begin feeling any numbness in their arms and legs, a prickling or “pins and needles” sensation, or confusion and hallucinations, they should contact their health care provider right away so that they may be switched to a nonfluoroquinolone antibacterial drug for the remainder of their treatment regimen.
Avelox (Moxifloxacin); Cipro, both standard and extended release (ciprofloxacin); Factive (gemifloxacin); Levaquin (levofloxacin); and ofloxacin are the fluoroquinolones currently approved by the FDA for systemic use.
Additional adverse effects for patients taking fluoroquinolones could include tendinitis, tendon rupture, and joint swelling. Central nervous system afflictions could include depression and thoughts of suicide. Fluoroquinolones could also bring about skin rashes, sunburn, arrhythmia, and diarrhea, and could aggravate myasthenia gravis in patients who suffer from it. Warnings regarding these conditions are already included on the drugs’ existing boxed warning.
“In addition to updating information in the Boxed Warning, we are also including information about these safety issues in the Warnings and Precautions section of the label,” the FDA stated. “The Indications and Usage section contains new limitation-of-use statements to reserve fluoroquinolones for patients who do not have other available treatment options for ABS, ABECB, and uncomplicated UTIs.”
The FDA added that it will continue to monitor and assess safety issues associated with fluoroquinolones and will issue any further updates if necessary.
1. US Food and Drug Administration. FDA Drug Safety Communication: FDA updates warnings for oral and injectable fluoroquinolone antibiotics due to disabling side effects. http://www.fda.gov/Drugs/DrugSafety/ucm511530.htm. Published July 26, 2016 Accessed August 26, 2016.
Skin Rash in a Recent Traveler? Think Dengue Fever
BY SHARON WORCESTER
FRONTLINE MEDICAL NEWS
Maintain clinical suspicion for dengue fever among individuals with recent travel to endemic areas who present with a rash and other signs and symptoms of infection, an expert advised at the American Academy of Dermatology summer meeting.
Dengue fever accounts for nearly 10% of skin rashes among individuals returning from endemic areas, and related illness can range from mild to fatal, said Jose Dario Martinez, MD, chief of the Internal Medicine Clinic at University Hospital J.E. Gonzalez, UANL Monterrey, Mexico.
“This is the most prevalent arthropod-borne virus in the world at this time, and it is a resurgent disease in some countries, like Mexico, Brazil, and Colombia,” he noted.
Worldwide, more than 2.5 billion people are at risk of dengue infection, and between 50 million and 100 million cases occur each year, while about 250,000 to 500,000 cases of dengue hemorrhagic fever (DHF) occur each year, and about 25,000 related deaths occur.
In 2005, there was a dengue outbreak in Texas, where 25 cases occurred; in southern Florida, an outbreak of 90 cases was reported in 2009 and 2010. More recently, in 2015, there was an outbreak of 107 cases of locally acquired dengue on the Big Island, Hawaii. But in Mexico, 18,000 new cases occurred in 2015, Dr Martinez said.
Of the RNA virus serotypes 1 to 4, type 1 (DEN-1) is the most common, and DEN-2 and DEN-3 are the most severe, but up to 40% of cases are asymptomatic, he noted, adding that the virus has an incubation period of 2 to 8 days. When symptoms occur, they are representative of acute febrile illness, and may include headache, high fever, myalgia, arthralgia, retro-orbital pain, and fatigue. A faint, itchy, macular rash commonly occurs at 2 to 6 days into the illness. According to the World Health Organization (WHO), a probable dengue fever case includes acute febrile illness and at least two of the following: headache, retro-orbital pain, myalgia, arthralgia, rash, hemorrhagic manifestations, leukopenia, or supportive serology.
“Sometimes the nose bleeds, the gums bleed, and there is bruising in the patient,” Dr Martinez said. “Most important are retro-orbital pain and hemorrhagic manifestations, but also supportive serology.”
About 1% of patients progress to DHF or dengue shock syndrome (DSS) during the critical phase (days 4-7) of illness. This is most likely in those with serotypes 2 and 3, but can occur with all serotypes. Warning signs of such severe disease include abdominal pain or tenderness, persistent vomiting, pleural effusion or ascites, and of particular importance—mucosal bleeding, Dr Martinez said.
By the WHO definition, a diagnosis of DHF requires the presence of fever for at least 2 to 7 days, hemorrhagic tendencies, thrombocytopenia, and evidence and signs of plasma leakage; DSS requires these, as well as evidence of circulatory failure, such as rapid and weak pulse, narrow pulse pressure, hypotension, and shock.
It is important to maintain clinical suspicion for dengue fever, particularly in anyone who has traveled to an endemic area in the 2 weeks before presentation. Serologic tests are important to detect anti-dengue antibodies. Immunoglobulin G is important because its presence could suggest recurrent infection and thus the potential for severe disease, Dr Martinez said. Polymerase chain reaction can be used for detection in the first 4 to 5 days of infection, and the nonstructural glycoprotein 1 rapid test can be positive on the first day, he noted.
The differential diagnosis for dengue fever is broad, and can include chikungunya fever, malaria, leptospirosis, meningococcemia, drug eruption, and Zika fever.
Management of dengue fever includes bed rest, liquids, and mosquito net isolation to prevent reinfection, as more severe disease can occur after reinfection. Acetaminophen can be used for pain relief; aspirin should be avoided due to risk of bleeding, Dr Martinez said. Hospitalization and supportive care are required for those with DHF or DSS. Intensive care unit admission may be required.
Of note, a vaccine against dengue fever has shown promise in phase III trials. The vaccine has been approved in Mexico and Brazil, but not yet in the United States.
For more on dengue fever, see the case report “Dengue Fever: Two Unexpected Findings” on page 408.
Pertussis Often Goes Undiagnosed, Especially in Adults
BY ABIGAIL CRUZ
FRONTLINE MEDICAL NEWS
A majority of pertussis cases in the United States may go undetected in people younger than age 50 years, particularly in adults, results of a retrospective database cohort study suggest.
“The incidence of pertussis in adolescents and adults is very difficult to quantify,” wrote Chi-Chang Chen, MD, of IMS Health, Plymouth Meeting, Pennsylvania, and associates. Symptoms may be misdiagnosed as other respiratory illnesses; infected individuals may not seek treatment; and pertussis may not be considered as a possible diagnosis in adults, they noted.
To project the possible range of pertussis incidence in this population, investigators used three different models to analyze information from private insurance and laboratory databases as well as data from the Centers for Disease Control and Prevention for a 6-year period. The first method, which used medical claims for International Classification of Diseases (ICD-9) diagnosed pertussis, found an annual incidence rate of 9 per 100,000 population. The second used a proxy pertussis model that was based on symptoms that could indicate undiagnosed pertussis, showing an incidence rate of 21 per 100,000. The third method used pathogen data to estimate the fraction of cough illness statistically attributable to pertussis, resulting in an incidence rate of 649 per 100,000 population, which is 58 to 93 times higher than the ICD-9 estimated incidence.
These estimates “highlight the need for improved preventive measures—such as increased vaccination—against pertussis,” the investigators said, noting that immunization recommendations for additional age groups and research involving strategies to reduce waning immunity after vaccination should be considered.
1. Chen CC, Balderston McGuiness C, Krishnarajah G, et al. Estimated incidence of pertussis in people aged <50 years in the United States. Hum Vaccin Immunother. 2016;31:1-10. [Epub ahead of print]
The ED Is a Safer Place…and Can Be Safer Still
Improving medication accuracy, transitions of care, health information technology, and other ED patient-safety strategies are offered in this month’s Emergency Medicine cover article, “Patient Safety in the Emergency Department,” by emergency physician (EP)/toxicologist Brenna M. Farmer, MD, a colleague for many years.
As Dr Farmer notes in her introduction, patient safety—in the ED and elsewhere—has received a great deal of attention since the publication of the two landmark Institute of Medicine (IOM) studies in 1999 and 2001 that documented an enormous number of medical errors and recommended improvements in medical care. More than a decade and a half after their publication, is there any evidence that these reports have led to a reduction in the number of serious adverse effects and deaths due to medical errors?
Although most EPs believe that ED safety measures have reduced the overall number of errors, there is a scarcity of published data demonstrating a direct cause-and-effect relationship in reducing the number of adverse events and deaths. A recent analysis of National Hospital Ambulatory Medical Care Survey data by EPs Kanzaria, Probst, and Hsia (Health Aff [Millwood]. 2016;35[7]:1303-1308) found that ED death rates dropped by nearly 50% between 1997 and 2011. Most of this reporting period includes the years following the IOM reports before the implementation of the Affordable Care Act measures. One might reasonably assume that the decrease in ED death rates since 1997 is at least partly due to the safety measures described by Dr Farmer. However, Kanzaria et al hypothesize that the reduction is probably due to palliative and prehospital care efforts which “shift the locus of deaths,” to recent advances in emergency critical care, and to public health successes in smoking cessation, motor vehicle safety, etc. Conspicuously absent from their list of possible measures responsible for the reduction in ED death rates are ED safety measures.
If Kanzaria et al are correct in attributing the reduction in ED deaths to measures taken by others to decrease the number of dying patients brought to EDs, then it may be reasonable to look for the benefit of eliminating serious ED errors to a decrease in death rates after patients leave the ED for inpatient services. Though inpatient death-rate data is available only since 2005, Kanzaria et al report no significant change in the inpatient death rate between 2005 and 2011. It is possible, however, that the improvements in ED critical care hypothesized by the authors to be partly responsible for reducing ED death rates enable sicker patients to survive longer and ultimately succumb to their serious illnesses as inpatients. If so, this could offset any evident reduction in inpatient mortality from the avoidance of serious errors in the ED.
In any case, Dr Farmer does present direct evidence that safety measures are effective in reducing morbidity, and probably mortality. For example, in one study cited, medication errors were 13.5 times less likely to occur when an ED pharmacist was present, and clearly, avoiding doubling the doses of potent cardiac medications or sedative hypnotics, avoiding dangerous drug interactions, and choosing the correct type, dose, and time of administration of antibiotics and all meds must be responsible for reducing morbidity and ultimately mortality. It is also worth recalling that with respect to patient safety, emergency medicine is undoubtedly the safest medical specialty ever created, pioneering from its inception 24/7 bedside attending presence and mandatory recertifications, years to decades before other specialties adopted these practices. Thanks to these efforts, EDs are much safer than they had been previously, and by implementing the measures described by Dr Farmer will be safer still.
Improving medication accuracy, transitions of care, health information technology, and other ED patient-safety strategies are offered in this month’s Emergency Medicine cover article, “Patient Safety in the Emergency Department,” by emergency physician (EP)/toxicologist Brenna M. Farmer, MD, a colleague for many years.
As Dr Farmer notes in her introduction, patient safety—in the ED and elsewhere—has received a great deal of attention since the publication of the two landmark Institute of Medicine (IOM) studies in 1999 and 2001 that documented an enormous number of medical errors and recommended improvements in medical care. More than a decade and a half after their publication, is there any evidence that these reports have led to a reduction in the number of serious adverse effects and deaths due to medical errors?
Although most EPs believe that ED safety measures have reduced the overall number of errors, there is a scarcity of published data demonstrating a direct cause-and-effect relationship in reducing the number of adverse events and deaths. A recent analysis of National Hospital Ambulatory Medical Care Survey data by EPs Kanzaria, Probst, and Hsia (Health Aff [Millwood]. 2016;35[7]:1303-1308) found that ED death rates dropped by nearly 50% between 1997 and 2011. Most of this reporting period includes the years following the IOM reports before the implementation of the Affordable Care Act measures. One might reasonably assume that the decrease in ED death rates since 1997 is at least partly due to the safety measures described by Dr Farmer. However, Kanzaria et al hypothesize that the reduction is probably due to palliative and prehospital care efforts which “shift the locus of deaths,” to recent advances in emergency critical care, and to public health successes in smoking cessation, motor vehicle safety, etc. Conspicuously absent from their list of possible measures responsible for the reduction in ED death rates are ED safety measures.
If Kanzaria et al are correct in attributing the reduction in ED deaths to measures taken by others to decrease the number of dying patients brought to EDs, then it may be reasonable to look for the benefit of eliminating serious ED errors to a decrease in death rates after patients leave the ED for inpatient services. Though inpatient death-rate data is available only since 2005, Kanzaria et al report no significant change in the inpatient death rate between 2005 and 2011. It is possible, however, that the improvements in ED critical care hypothesized by the authors to be partly responsible for reducing ED death rates enable sicker patients to survive longer and ultimately succumb to their serious illnesses as inpatients. If so, this could offset any evident reduction in inpatient mortality from the avoidance of serious errors in the ED.
In any case, Dr Farmer does present direct evidence that safety measures are effective in reducing morbidity, and probably mortality. For example, in one study cited, medication errors were 13.5 times less likely to occur when an ED pharmacist was present, and clearly, avoiding doubling the doses of potent cardiac medications or sedative hypnotics, avoiding dangerous drug interactions, and choosing the correct type, dose, and time of administration of antibiotics and all meds must be responsible for reducing morbidity and ultimately mortality. It is also worth recalling that with respect to patient safety, emergency medicine is undoubtedly the safest medical specialty ever created, pioneering from its inception 24/7 bedside attending presence and mandatory recertifications, years to decades before other specialties adopted these practices. Thanks to these efforts, EDs are much safer than they had been previously, and by implementing the measures described by Dr Farmer will be safer still.
Improving medication accuracy, transitions of care, health information technology, and other ED patient-safety strategies are offered in this month’s Emergency Medicine cover article, “Patient Safety in the Emergency Department,” by emergency physician (EP)/toxicologist Brenna M. Farmer, MD, a colleague for many years.
As Dr Farmer notes in her introduction, patient safety—in the ED and elsewhere—has received a great deal of attention since the publication of the two landmark Institute of Medicine (IOM) studies in 1999 and 2001 that documented an enormous number of medical errors and recommended improvements in medical care. More than a decade and a half after their publication, is there any evidence that these reports have led to a reduction in the number of serious adverse effects and deaths due to medical errors?
Although most EPs believe that ED safety measures have reduced the overall number of errors, there is a scarcity of published data demonstrating a direct cause-and-effect relationship in reducing the number of adverse events and deaths. A recent analysis of National Hospital Ambulatory Medical Care Survey data by EPs Kanzaria, Probst, and Hsia (Health Aff [Millwood]. 2016;35[7]:1303-1308) found that ED death rates dropped by nearly 50% between 1997 and 2011. Most of this reporting period includes the years following the IOM reports before the implementation of the Affordable Care Act measures. One might reasonably assume that the decrease in ED death rates since 1997 is at least partly due to the safety measures described by Dr Farmer. However, Kanzaria et al hypothesize that the reduction is probably due to palliative and prehospital care efforts which “shift the locus of deaths,” to recent advances in emergency critical care, and to public health successes in smoking cessation, motor vehicle safety, etc. Conspicuously absent from their list of possible measures responsible for the reduction in ED death rates are ED safety measures.
If Kanzaria et al are correct in attributing the reduction in ED deaths to measures taken by others to decrease the number of dying patients brought to EDs, then it may be reasonable to look for the benefit of eliminating serious ED errors to a decrease in death rates after patients leave the ED for inpatient services. Though inpatient death-rate data is available only since 2005, Kanzaria et al report no significant change in the inpatient death rate between 2005 and 2011. It is possible, however, that the improvements in ED critical care hypothesized by the authors to be partly responsible for reducing ED death rates enable sicker patients to survive longer and ultimately succumb to their serious illnesses as inpatients. If so, this could offset any evident reduction in inpatient mortality from the avoidance of serious errors in the ED.
In any case, Dr Farmer does present direct evidence that safety measures are effective in reducing morbidity, and probably mortality. For example, in one study cited, medication errors were 13.5 times less likely to occur when an ED pharmacist was present, and clearly, avoiding doubling the doses of potent cardiac medications or sedative hypnotics, avoiding dangerous drug interactions, and choosing the correct type, dose, and time of administration of antibiotics and all meds must be responsible for reducing morbidity and ultimately mortality. It is also worth recalling that with respect to patient safety, emergency medicine is undoubtedly the safest medical specialty ever created, pioneering from its inception 24/7 bedside attending presence and mandatory recertifications, years to decades before other specialties adopted these practices. Thanks to these efforts, EDs are much safer than they had been previously, and by implementing the measures described by Dr Farmer will be safer still.