User login
Recognizing granulosa cell ovarian tumors
Granulosa cell tumors arise from ovarian sex cords and make up an estimated 1% of all ovarian cancer cases but comprise more than 70% of all sex cord stromal tumors.
Granulosa cell tumors (GCTs) can be divided into adult and juvenile types. Adult GCTs are much more common, representing 95% of all GCTs. Women diagnosed with adult GCTs are typically younger as compared with those with epithelial ovarian cancer. The average age of diagnosis for adult GCTs is 50 years, and for women with juvenile GCTs, the average age at diagnosis is 20 years.
Granulosa cell tumors have been shown to be more common in nonwhite women, those with a high body mass index, and a family history of breast or ovarian cancer.1
Adult GCTs can be associated with Peutz-Jeghers and Potter syndromes. Juvenile GCTs are exceedingly rare but can also be associated with mesodermal dysplastic syndromes characterized by the presence of enchondromatosis and hemangioma formation, such as Ollier disease or Maffucci syndrome.
Adult granulosa cell tumors are large, hormonally active tumors; typically secreting estrogen and associated with symptoms of hyperestrogenism. In one study, 55% of women with GCTs were reported to have hyperestrogenic findings such as breast tenderness, virulism, abnormal or postmenopausal bleeding, and hyperplasia, and those with juvenile GCTs may present with precocious puberty.2,3
In pregnancy, hormonal symptoms are temporized, thus the most common presentation is acute rupture. Initial evaluation of women with adult GCTs will reveal a palpable unilateral pelvic mass typically larger than 10cm. Juvenile and adult GCTs are unilateral in 95% of cases.4
In women presenting with a large adnexal mass, the appropriate initial clinical evaluation includes radiographic and laboratory studies. Endovaginal ultrasound typically reveals a large adnexal mass with heterogeneous solid and cystic components, areas of hemorrhage or necrosis and increased vascularity on Doppler. Juvenile GCTs have a more distinct appearance of solid growth with focal areas of follicular formation.
Laboratory findings suggestive of GCT include elevated inhibin-A, inhibin-B, anti-Mullerian hormone (AMH), and CA-125. Inhibin-B is the most commonly used tumor marker for the clinical monitoring of adult GCTs, but AMH may be the most specific.5 Lastly, an endometrial biopsy should be considered in all patients with abnormal uterine bleeding and in all postmenopausal women with an adnexal mass and an endometrial stripe greater than 5mm.6
Surgical staging for adult GCTs is the standard of care. For women who do not desire fertility, this includes total hysterectomy, bilateral salpingo-oophorectomy and removal of all gross disease. Comprehensive nodal dissection is not indicated except when necessary for complete cytoreduction. In contrast to epithelial ovarian cancer, approximately 80% of women with adult GCTs are diagnosed with stage I disease. For stage IA disease, treatment with surgery alone is sufficient, yet in women with stage II-IV disease or with tumors that are ruptured intraoperatively, platinum-based chemotherapy is recommended. The most common regimen is bleomycin, etoposide, and cisplatin, though there is increasing experience with an outpatient regimen of paclitaxel and carboplatin.
The gross appearance of both adult and juvenile GCTs are of a large, tan-yellow tumor with cystic, solid, and hemorrhagic components. Microscopically, juvenile GCTs are more distinct than that of adult GCTs. Whereas adult GCTs comprise diffuse cords or trabeculae and small follicles termed Call-Exner bodies of rounded cells with scant cytoplasm and pale “coffee-bean” nuclei, juvenile GCTs have nuclei that are rounded, hyperchromatic with moderate to abundant eosinophilic or vacuolated cytoplasm.
The prognosis of GCTs is largely dependent on the stage at diagnosis and presence of residual disease after debulking. Negative prognostic factors for recurrence include tumor size, rupture, atypia and increased mitotic activity.
There are distinct clinical, radiographic, and laboratory characteristics that may raise the suspicion of the practicing gynecologist for a GCT. In such cases, expedient referral for surgical exploration to a gynecologic oncologist is warranted. If the tumor is encountered inadvertently, intraoperative consultation from a gynecologic oncologist should be requested. If a gynecologic oncologist is not available, it is paramount to optimize surgical exposure to clearly document any abnormal pelvic or intra-abdominal findings, take care to prevent surgical spillage, and preserve fertility if indicated.
If referred appropriately and completely resected, the 5-year overall survival of stage IA disease can be upward of 90%. Recurrences are stage dependent with an average time to recurrence of just under 5 years. When recurrences occur, they tend to happen in the pelvis. All women with a history of GCT will require surveillance and monitoring.
References
1. Gynecol Oncol. 2005 May;97(2):519-23.
2. “Rare and Uncommon Gynecological Cancers: A Clinical Guide” (Heidelberg: Springer, 2011): Reed N.
3. Obstet Gynecol. 1980 Feb;55(2):231-8.
4. “Principles and Practice of Gynecologic Oncology” (Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins, 2013): Barakat R.
5. Indian J Surg Oncol. 2013 Mar;4(1):37-47.
6. “Uncommon Gynecologic Cancers” (Indianapolis: Wiley-Blackwell, 2014): Del Carmen M.
Dr. Gehrig is professor and director of gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Castellano is a resident physician in the obstetrics and gynecology program at the university. They reported having no relevant financial disclosures. To comment, email them at [email protected].
Granulosa cell tumors arise from ovarian sex cords and make up an estimated 1% of all ovarian cancer cases but comprise more than 70% of all sex cord stromal tumors.
Granulosa cell tumors (GCTs) can be divided into adult and juvenile types. Adult GCTs are much more common, representing 95% of all GCTs. Women diagnosed with adult GCTs are typically younger as compared with those with epithelial ovarian cancer. The average age of diagnosis for adult GCTs is 50 years, and for women with juvenile GCTs, the average age at diagnosis is 20 years.
Granulosa cell tumors have been shown to be more common in nonwhite women, those with a high body mass index, and a family history of breast or ovarian cancer.1
Adult GCTs can be associated with Peutz-Jeghers and Potter syndromes. Juvenile GCTs are exceedingly rare but can also be associated with mesodermal dysplastic syndromes characterized by the presence of enchondromatosis and hemangioma formation, such as Ollier disease or Maffucci syndrome.
Adult granulosa cell tumors are large, hormonally active tumors; typically secreting estrogen and associated with symptoms of hyperestrogenism. In one study, 55% of women with GCTs were reported to have hyperestrogenic findings such as breast tenderness, virulism, abnormal or postmenopausal bleeding, and hyperplasia, and those with juvenile GCTs may present with precocious puberty.2,3
In pregnancy, hormonal symptoms are temporized, thus the most common presentation is acute rupture. Initial evaluation of women with adult GCTs will reveal a palpable unilateral pelvic mass typically larger than 10cm. Juvenile and adult GCTs are unilateral in 95% of cases.4
In women presenting with a large adnexal mass, the appropriate initial clinical evaluation includes radiographic and laboratory studies. Endovaginal ultrasound typically reveals a large adnexal mass with heterogeneous solid and cystic components, areas of hemorrhage or necrosis and increased vascularity on Doppler. Juvenile GCTs have a more distinct appearance of solid growth with focal areas of follicular formation.
Laboratory findings suggestive of GCT include elevated inhibin-A, inhibin-B, anti-Mullerian hormone (AMH), and CA-125. Inhibin-B is the most commonly used tumor marker for the clinical monitoring of adult GCTs, but AMH may be the most specific.5 Lastly, an endometrial biopsy should be considered in all patients with abnormal uterine bleeding and in all postmenopausal women with an adnexal mass and an endometrial stripe greater than 5mm.6
Surgical staging for adult GCTs is the standard of care. For women who do not desire fertility, this includes total hysterectomy, bilateral salpingo-oophorectomy and removal of all gross disease. Comprehensive nodal dissection is not indicated except when necessary for complete cytoreduction. In contrast to epithelial ovarian cancer, approximately 80% of women with adult GCTs are diagnosed with stage I disease. For stage IA disease, treatment with surgery alone is sufficient, yet in women with stage II-IV disease or with tumors that are ruptured intraoperatively, platinum-based chemotherapy is recommended. The most common regimen is bleomycin, etoposide, and cisplatin, though there is increasing experience with an outpatient regimen of paclitaxel and carboplatin.
The gross appearance of both adult and juvenile GCTs are of a large, tan-yellow tumor with cystic, solid, and hemorrhagic components. Microscopically, juvenile GCTs are more distinct than that of adult GCTs. Whereas adult GCTs comprise diffuse cords or trabeculae and small follicles termed Call-Exner bodies of rounded cells with scant cytoplasm and pale “coffee-bean” nuclei, juvenile GCTs have nuclei that are rounded, hyperchromatic with moderate to abundant eosinophilic or vacuolated cytoplasm.
The prognosis of GCTs is largely dependent on the stage at diagnosis and presence of residual disease after debulking. Negative prognostic factors for recurrence include tumor size, rupture, atypia and increased mitotic activity.
There are distinct clinical, radiographic, and laboratory characteristics that may raise the suspicion of the practicing gynecologist for a GCT. In such cases, expedient referral for surgical exploration to a gynecologic oncologist is warranted. If the tumor is encountered inadvertently, intraoperative consultation from a gynecologic oncologist should be requested. If a gynecologic oncologist is not available, it is paramount to optimize surgical exposure to clearly document any abnormal pelvic or intra-abdominal findings, take care to prevent surgical spillage, and preserve fertility if indicated.
If referred appropriately and completely resected, the 5-year overall survival of stage IA disease can be upward of 90%. Recurrences are stage dependent with an average time to recurrence of just under 5 years. When recurrences occur, they tend to happen in the pelvis. All women with a history of GCT will require surveillance and monitoring.
References
1. Gynecol Oncol. 2005 May;97(2):519-23.
2. “Rare and Uncommon Gynecological Cancers: A Clinical Guide” (Heidelberg: Springer, 2011): Reed N.
3. Obstet Gynecol. 1980 Feb;55(2):231-8.
4. “Principles and Practice of Gynecologic Oncology” (Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins, 2013): Barakat R.
5. Indian J Surg Oncol. 2013 Mar;4(1):37-47.
6. “Uncommon Gynecologic Cancers” (Indianapolis: Wiley-Blackwell, 2014): Del Carmen M.
Dr. Gehrig is professor and director of gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Castellano is a resident physician in the obstetrics and gynecology program at the university. They reported having no relevant financial disclosures. To comment, email them at [email protected].
Granulosa cell tumors arise from ovarian sex cords and make up an estimated 1% of all ovarian cancer cases but comprise more than 70% of all sex cord stromal tumors.
Granulosa cell tumors (GCTs) can be divided into adult and juvenile types. Adult GCTs are much more common, representing 95% of all GCTs. Women diagnosed with adult GCTs are typically younger as compared with those with epithelial ovarian cancer. The average age of diagnosis for adult GCTs is 50 years, and for women with juvenile GCTs, the average age at diagnosis is 20 years.
Granulosa cell tumors have been shown to be more common in nonwhite women, those with a high body mass index, and a family history of breast or ovarian cancer.1
Adult GCTs can be associated with Peutz-Jeghers and Potter syndromes. Juvenile GCTs are exceedingly rare but can also be associated with mesodermal dysplastic syndromes characterized by the presence of enchondromatosis and hemangioma formation, such as Ollier disease or Maffucci syndrome.
Adult granulosa cell tumors are large, hormonally active tumors; typically secreting estrogen and associated with symptoms of hyperestrogenism. In one study, 55% of women with GCTs were reported to have hyperestrogenic findings such as breast tenderness, virulism, abnormal or postmenopausal bleeding, and hyperplasia, and those with juvenile GCTs may present with precocious puberty.2,3
In pregnancy, hormonal symptoms are temporized, thus the most common presentation is acute rupture. Initial evaluation of women with adult GCTs will reveal a palpable unilateral pelvic mass typically larger than 10cm. Juvenile and adult GCTs are unilateral in 95% of cases.4
In women presenting with a large adnexal mass, the appropriate initial clinical evaluation includes radiographic and laboratory studies. Endovaginal ultrasound typically reveals a large adnexal mass with heterogeneous solid and cystic components, areas of hemorrhage or necrosis and increased vascularity on Doppler. Juvenile GCTs have a more distinct appearance of solid growth with focal areas of follicular formation.
Laboratory findings suggestive of GCT include elevated inhibin-A, inhibin-B, anti-Mullerian hormone (AMH), and CA-125. Inhibin-B is the most commonly used tumor marker for the clinical monitoring of adult GCTs, but AMH may be the most specific.5 Lastly, an endometrial biopsy should be considered in all patients with abnormal uterine bleeding and in all postmenopausal women with an adnexal mass and an endometrial stripe greater than 5mm.6
Surgical staging for adult GCTs is the standard of care. For women who do not desire fertility, this includes total hysterectomy, bilateral salpingo-oophorectomy and removal of all gross disease. Comprehensive nodal dissection is not indicated except when necessary for complete cytoreduction. In contrast to epithelial ovarian cancer, approximately 80% of women with adult GCTs are diagnosed with stage I disease. For stage IA disease, treatment with surgery alone is sufficient, yet in women with stage II-IV disease or with tumors that are ruptured intraoperatively, platinum-based chemotherapy is recommended. The most common regimen is bleomycin, etoposide, and cisplatin, though there is increasing experience with an outpatient regimen of paclitaxel and carboplatin.
The gross appearance of both adult and juvenile GCTs are of a large, tan-yellow tumor with cystic, solid, and hemorrhagic components. Microscopically, juvenile GCTs are more distinct than that of adult GCTs. Whereas adult GCTs comprise diffuse cords or trabeculae and small follicles termed Call-Exner bodies of rounded cells with scant cytoplasm and pale “coffee-bean” nuclei, juvenile GCTs have nuclei that are rounded, hyperchromatic with moderate to abundant eosinophilic or vacuolated cytoplasm.
The prognosis of GCTs is largely dependent on the stage at diagnosis and presence of residual disease after debulking. Negative prognostic factors for recurrence include tumor size, rupture, atypia and increased mitotic activity.
There are distinct clinical, radiographic, and laboratory characteristics that may raise the suspicion of the practicing gynecologist for a GCT. In such cases, expedient referral for surgical exploration to a gynecologic oncologist is warranted. If the tumor is encountered inadvertently, intraoperative consultation from a gynecologic oncologist should be requested. If a gynecologic oncologist is not available, it is paramount to optimize surgical exposure to clearly document any abnormal pelvic or intra-abdominal findings, take care to prevent surgical spillage, and preserve fertility if indicated.
If referred appropriately and completely resected, the 5-year overall survival of stage IA disease can be upward of 90%. Recurrences are stage dependent with an average time to recurrence of just under 5 years. When recurrences occur, they tend to happen in the pelvis. All women with a history of GCT will require surveillance and monitoring.
References
1. Gynecol Oncol. 2005 May;97(2):519-23.
2. “Rare and Uncommon Gynecological Cancers: A Clinical Guide” (Heidelberg: Springer, 2011): Reed N.
3. Obstet Gynecol. 1980 Feb;55(2):231-8.
4. “Principles and Practice of Gynecologic Oncology” (Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins, 2013): Barakat R.
5. Indian J Surg Oncol. 2013 Mar;4(1):37-47.
6. “Uncommon Gynecologic Cancers” (Indianapolis: Wiley-Blackwell, 2014): Del Carmen M.
Dr. Gehrig is professor and director of gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Castellano is a resident physician in the obstetrics and gynecology program at the university. They reported having no relevant financial disclosures. To comment, email them at [email protected].
Blood pressure targets
To the Editor: In their review,1 Thomas et al noted that the benefits of intensive blood pressure lowering seen in the SPRINT study2 were not observed in the Action to Control Cardiovascular Risk in Diabetes-Blood pressure (ACCORD BP) trial3 or in the Secondary Prevention of Small Subcortical Strokes (SPS3) trial.4 In addition to the reasons discussed in their review, the discrepancy may be due to the surprisingly low rate of statin use in the patients enrolled in SPRINT. Even though 61% of the patients in SPRINT had a 10-year Framingham risk score greater than 15%, only 44% of the patients were on statin therapy. In comparison, rates of statin use in ACCORD BP and SPS3 were 65% and 83%, respectively.
A possible interaction between statin use and intensive blood pressure lowering is consistent with previous data on angiotensin-converting enzyme (ACE) inhibitor use in high-risk populations.
The Heart Outcomes Prevention Evaluation (HOPE) trial,5 in which only 29% of patients received lipid-lowering therapy, found that ACE inhibitor use was associated with a significant reduction in a composite cardiovascular outcome, whereas the Prevention of Events With Angiotensin-Converting Enzyme Inhibitor Therapy (PEACE) trial,6 in which 70% of patients were on lipid-lowering therapy, did not show a benefit for ACE inhibitor therapy. In addition, there are many drug interactions between statins and calcium channel blockers, potentially limiting options for simultaneous aggressive treatment of lipid levels and blood pressure.
In summary, aggressive use of statins may confer sufficient cardiovascular protection when aggressive antihypertensive therapy provides little or no incremental benefit. Hopefully, further analyses of these trials will shed light on this important question.
- Thomas G, Nally JV, Pohl MA. Interpreting SPRINT: how low should you go? Cleve Clin J Med 2016; 83:187–195.
- SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373:2103–2116.
- ACCORD Study Group; Cushma WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362:1575–1585.
- SPS3 Study Group; Benavente OR, Coffey CS, Conwit R, et al. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet 2013; 382:507–515.
- The Heart Outcomes Prevention Evaluation Study Investigators. Effects of an angiotensin-converting enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med 2000; 342:145–153.
- The PEACE Trial Investigators. Angiotensin-converting–enzyme inhibition in stable coronary artery disease. N Engl J Med 2004; 351:2058–2068.
To the Editor: In their review,1 Thomas et al noted that the benefits of intensive blood pressure lowering seen in the SPRINT study2 were not observed in the Action to Control Cardiovascular Risk in Diabetes-Blood pressure (ACCORD BP) trial3 or in the Secondary Prevention of Small Subcortical Strokes (SPS3) trial.4 In addition to the reasons discussed in their review, the discrepancy may be due to the surprisingly low rate of statin use in the patients enrolled in SPRINT. Even though 61% of the patients in SPRINT had a 10-year Framingham risk score greater than 15%, only 44% of the patients were on statin therapy. In comparison, rates of statin use in ACCORD BP and SPS3 were 65% and 83%, respectively.
A possible interaction between statin use and intensive blood pressure lowering is consistent with previous data on angiotensin-converting enzyme (ACE) inhibitor use in high-risk populations.
The Heart Outcomes Prevention Evaluation (HOPE) trial,5 in which only 29% of patients received lipid-lowering therapy, found that ACE inhibitor use was associated with a significant reduction in a composite cardiovascular outcome, whereas the Prevention of Events With Angiotensin-Converting Enzyme Inhibitor Therapy (PEACE) trial,6 in which 70% of patients were on lipid-lowering therapy, did not show a benefit for ACE inhibitor therapy. In addition, there are many drug interactions between statins and calcium channel blockers, potentially limiting options for simultaneous aggressive treatment of lipid levels and blood pressure.
In summary, aggressive use of statins may confer sufficient cardiovascular protection when aggressive antihypertensive therapy provides little or no incremental benefit. Hopefully, further analyses of these trials will shed light on this important question.
To the Editor: In their review,1 Thomas et al noted that the benefits of intensive blood pressure lowering seen in the SPRINT study2 were not observed in the Action to Control Cardiovascular Risk in Diabetes-Blood pressure (ACCORD BP) trial3 or in the Secondary Prevention of Small Subcortical Strokes (SPS3) trial.4 In addition to the reasons discussed in their review, the discrepancy may be due to the surprisingly low rate of statin use in the patients enrolled in SPRINT. Even though 61% of the patients in SPRINT had a 10-year Framingham risk score greater than 15%, only 44% of the patients were on statin therapy. In comparison, rates of statin use in ACCORD BP and SPS3 were 65% and 83%, respectively.
A possible interaction between statin use and intensive blood pressure lowering is consistent with previous data on angiotensin-converting enzyme (ACE) inhibitor use in high-risk populations.
The Heart Outcomes Prevention Evaluation (HOPE) trial,5 in which only 29% of patients received lipid-lowering therapy, found that ACE inhibitor use was associated with a significant reduction in a composite cardiovascular outcome, whereas the Prevention of Events With Angiotensin-Converting Enzyme Inhibitor Therapy (PEACE) trial,6 in which 70% of patients were on lipid-lowering therapy, did not show a benefit for ACE inhibitor therapy. In addition, there are many drug interactions between statins and calcium channel blockers, potentially limiting options for simultaneous aggressive treatment of lipid levels and blood pressure.
In summary, aggressive use of statins may confer sufficient cardiovascular protection when aggressive antihypertensive therapy provides little or no incremental benefit. Hopefully, further analyses of these trials will shed light on this important question.
- Thomas G, Nally JV, Pohl MA. Interpreting SPRINT: how low should you go? Cleve Clin J Med 2016; 83:187–195.
- SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373:2103–2116.
- ACCORD Study Group; Cushma WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362:1575–1585.
- SPS3 Study Group; Benavente OR, Coffey CS, Conwit R, et al. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet 2013; 382:507–515.
- The Heart Outcomes Prevention Evaluation Study Investigators. Effects of an angiotensin-converting enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med 2000; 342:145–153.
- The PEACE Trial Investigators. Angiotensin-converting–enzyme inhibition in stable coronary artery disease. N Engl J Med 2004; 351:2058–2068.
- Thomas G, Nally JV, Pohl MA. Interpreting SPRINT: how low should you go? Cleve Clin J Med 2016; 83:187–195.
- SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373:2103–2116.
- ACCORD Study Group; Cushma WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362:1575–1585.
- SPS3 Study Group; Benavente OR, Coffey CS, Conwit R, et al. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet 2013; 382:507–515.
- The Heart Outcomes Prevention Evaluation Study Investigators. Effects of an angiotensin-converting enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med 2000; 342:145–153.
- The PEACE Trial Investigators. Angiotensin-converting–enzyme inhibition in stable coronary artery disease. N Engl J Med 2004; 351:2058–2068.
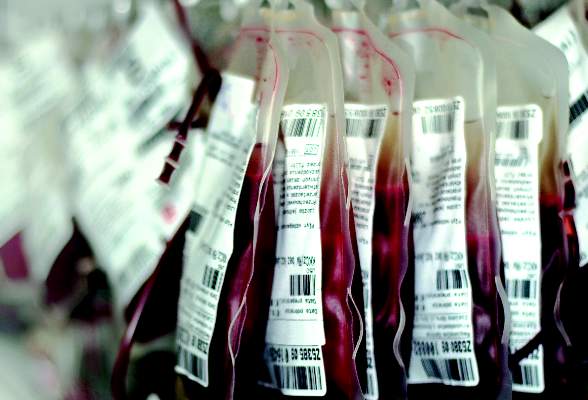
No evidence to show Alzheimer’s or Parkinson’s transmission via blood transfusion
A retrospective study of nearly 1.5 million patients over the course of 44 years showed no evidence to support the claim that neurodegenerative disorders can be transmitted between individuals through blood transfusions.
“Given that the neurodegenerative diseases on the dementia spectrum are relatively common, the misfolded proteins in affected persons have a wide tissue distribution, and the diseases may have a protracted prediagnostic course, potential transmission through transfusion could have important public health implications,” explained study authors led by Gustaf Edgren, MD, PhD, of the Karolinska Institute in Stockholm.
The retrospective cohort study analyzed data on 1,465,845 patients listed in nationwide transfusion registers, all of whom had undergone transfusions between 1968 and 2012 in Sweden, or during 1981-2012 in Denmark. The investigators looked for transfusions in which the donor had a diagnosis – either before or after the transfusion – of Alzheimer’s disease, Parkinson’s disease, or amyotrophic lateral sclerosis, and compared them with transfusions involving two healthy control groups. Creutzfeldt-Jakob disease was not included in the analysis (Ann Intern Med. 2016 Jun 28. doi: 10.7326/M15-2421).
All transfusion recipients were followed from 180 days after the transfusion date in order to minimize the risk of including a patient who had a neurodegenerative disorder that was present but not registered at the time of the transfusion. Follow-ups lasted until either the first diagnosis of a neurodegenerative disorder, death, emigration, or the end of the trial period on Dec. 31, 2012.
If a donor had received a diagnosis of a neurodegenerative disorder within 20 years of the transfusion, all recipients from said donor were considered to be exposed. “The maximum latency of 20 years was used as a compromise between allowing a long preclinical phase – during which donors may still be infectious – and avoiding classifying clearly healthy donors as potentially infectious,” the authors wrote.
Only 42,254 (2.9%) of subjects were deemed to be exposed to a neurodegenerative disorder based on their blood transfusion donor. However, there was no evidence of any increased risk of dementia of any type from exposure to blood from affected donors in any of the recipients over the course of the follow-up period (hazard ratio, 1.04; 95% confidence interval, 0.99-1.09), and similar results were obtained individually for Alzheimer’s disease and Parkinson’s disease. As a control measure, the investigators also tested for hepatitis C transmission before and after transfusions, and were “as expected, readily able to detect transmission of viral hepatitis,” despite finding no traces of neurodegenerative conditions.
“Despite accumulating scientific support for horizontal transmissibility of a range of disorders in the neurodegenerative spectrum through various methods of inoculation in animal models and of variant Creutzfeldt-Jakob disease from human and animal model data, this study provides evidence against blood transfusion as an important route of transmission of neurodegenerative diseases between humans,” the authors concluded.
The authors also added that “given that the study was based on the entire computerized blood banking history in two countries with several decades of follow-up, more comprehensive data are unlikely to be available in the foreseeable future.”
Funding for the study was provided by the Swedish Research Council, the Swedish Heart-Lung Foundation, the Swedish Society for Medical Research, and the Danish Council for Independent Research. One coauthor reported receiving a grant from the Danish Council for Independent Research, and another reported receiving grants from the Swedish Research Council and the Swedish Heart-Lung Foundation during the conduct of the study. No other authors reported any relevant financial disclosures.
A retrospective study of nearly 1.5 million patients over the course of 44 years showed no evidence to support the claim that neurodegenerative disorders can be transmitted between individuals through blood transfusions.
“Given that the neurodegenerative diseases on the dementia spectrum are relatively common, the misfolded proteins in affected persons have a wide tissue distribution, and the diseases may have a protracted prediagnostic course, potential transmission through transfusion could have important public health implications,” explained study authors led by Gustaf Edgren, MD, PhD, of the Karolinska Institute in Stockholm.
The retrospective cohort study analyzed data on 1,465,845 patients listed in nationwide transfusion registers, all of whom had undergone transfusions between 1968 and 2012 in Sweden, or during 1981-2012 in Denmark. The investigators looked for transfusions in which the donor had a diagnosis – either before or after the transfusion – of Alzheimer’s disease, Parkinson’s disease, or amyotrophic lateral sclerosis, and compared them with transfusions involving two healthy control groups. Creutzfeldt-Jakob disease was not included in the analysis (Ann Intern Med. 2016 Jun 28. doi: 10.7326/M15-2421).
All transfusion recipients were followed from 180 days after the transfusion date in order to minimize the risk of including a patient who had a neurodegenerative disorder that was present but not registered at the time of the transfusion. Follow-ups lasted until either the first diagnosis of a neurodegenerative disorder, death, emigration, or the end of the trial period on Dec. 31, 2012.
If a donor had received a diagnosis of a neurodegenerative disorder within 20 years of the transfusion, all recipients from said donor were considered to be exposed. “The maximum latency of 20 years was used as a compromise between allowing a long preclinical phase – during which donors may still be infectious – and avoiding classifying clearly healthy donors as potentially infectious,” the authors wrote.
Only 42,254 (2.9%) of subjects were deemed to be exposed to a neurodegenerative disorder based on their blood transfusion donor. However, there was no evidence of any increased risk of dementia of any type from exposure to blood from affected donors in any of the recipients over the course of the follow-up period (hazard ratio, 1.04; 95% confidence interval, 0.99-1.09), and similar results were obtained individually for Alzheimer’s disease and Parkinson’s disease. As a control measure, the investigators also tested for hepatitis C transmission before and after transfusions, and were “as expected, readily able to detect transmission of viral hepatitis,” despite finding no traces of neurodegenerative conditions.
“Despite accumulating scientific support for horizontal transmissibility of a range of disorders in the neurodegenerative spectrum through various methods of inoculation in animal models and of variant Creutzfeldt-Jakob disease from human and animal model data, this study provides evidence against blood transfusion as an important route of transmission of neurodegenerative diseases between humans,” the authors concluded.
The authors also added that “given that the study was based on the entire computerized blood banking history in two countries with several decades of follow-up, more comprehensive data are unlikely to be available in the foreseeable future.”
Funding for the study was provided by the Swedish Research Council, the Swedish Heart-Lung Foundation, the Swedish Society for Medical Research, and the Danish Council for Independent Research. One coauthor reported receiving a grant from the Danish Council for Independent Research, and another reported receiving grants from the Swedish Research Council and the Swedish Heart-Lung Foundation during the conduct of the study. No other authors reported any relevant financial disclosures.
A retrospective study of nearly 1.5 million patients over the course of 44 years showed no evidence to support the claim that neurodegenerative disorders can be transmitted between individuals through blood transfusions.
“Given that the neurodegenerative diseases on the dementia spectrum are relatively common, the misfolded proteins in affected persons have a wide tissue distribution, and the diseases may have a protracted prediagnostic course, potential transmission through transfusion could have important public health implications,” explained study authors led by Gustaf Edgren, MD, PhD, of the Karolinska Institute in Stockholm.
The retrospective cohort study analyzed data on 1,465,845 patients listed in nationwide transfusion registers, all of whom had undergone transfusions between 1968 and 2012 in Sweden, or during 1981-2012 in Denmark. The investigators looked for transfusions in which the donor had a diagnosis – either before or after the transfusion – of Alzheimer’s disease, Parkinson’s disease, or amyotrophic lateral sclerosis, and compared them with transfusions involving two healthy control groups. Creutzfeldt-Jakob disease was not included in the analysis (Ann Intern Med. 2016 Jun 28. doi: 10.7326/M15-2421).
All transfusion recipients were followed from 180 days after the transfusion date in order to minimize the risk of including a patient who had a neurodegenerative disorder that was present but not registered at the time of the transfusion. Follow-ups lasted until either the first diagnosis of a neurodegenerative disorder, death, emigration, or the end of the trial period on Dec. 31, 2012.
If a donor had received a diagnosis of a neurodegenerative disorder within 20 years of the transfusion, all recipients from said donor were considered to be exposed. “The maximum latency of 20 years was used as a compromise between allowing a long preclinical phase – during which donors may still be infectious – and avoiding classifying clearly healthy donors as potentially infectious,” the authors wrote.
Only 42,254 (2.9%) of subjects were deemed to be exposed to a neurodegenerative disorder based on their blood transfusion donor. However, there was no evidence of any increased risk of dementia of any type from exposure to blood from affected donors in any of the recipients over the course of the follow-up period (hazard ratio, 1.04; 95% confidence interval, 0.99-1.09), and similar results were obtained individually for Alzheimer’s disease and Parkinson’s disease. As a control measure, the investigators also tested for hepatitis C transmission before and after transfusions, and were “as expected, readily able to detect transmission of viral hepatitis,” despite finding no traces of neurodegenerative conditions.
“Despite accumulating scientific support for horizontal transmissibility of a range of disorders in the neurodegenerative spectrum through various methods of inoculation in animal models and of variant Creutzfeldt-Jakob disease from human and animal model data, this study provides evidence against blood transfusion as an important route of transmission of neurodegenerative diseases between humans,” the authors concluded.
The authors also added that “given that the study was based on the entire computerized blood banking history in two countries with several decades of follow-up, more comprehensive data are unlikely to be available in the foreseeable future.”
Funding for the study was provided by the Swedish Research Council, the Swedish Heart-Lung Foundation, the Swedish Society for Medical Research, and the Danish Council for Independent Research. One coauthor reported receiving a grant from the Danish Council for Independent Research, and another reported receiving grants from the Swedish Research Council and the Swedish Heart-Lung Foundation during the conduct of the study. No other authors reported any relevant financial disclosures.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point: There are no data to support the notion that human transmission of Alzheimer’s or Parkinson’s diseases can occur via blood transfusions.
Major finding: There was no evidence of any increased risk of dementia of any type from exposure to blood from affected donors in any of the recipients over the course of the follow-up period (HR, 1.04; 95% CI, 0.99-1.09).
Data source: Retrospective case-control study of 1,465,845 transfusion patients in Sweden and Denmark during 1968-2012.
Disclosures: The study was funded by the Swedish Research Council, the Swedish Heart-Lung Foundation, the Swedish Society for Medical Research, and the Danish Council for Independent Research. Two coauthors disclosed receiving grants from the funding institutions.
Metformin Continues to Be First-Line Therapy for Type 2 Diabetes
Clinical question: Which medications are most safe and effective at managing type 2 diabetes?
Background: Patients and practitioners need an updated review of the evidence to select the optimal medication for type 2 diabetes management.
Study design: Systematic review.
Synopsis: The authors reviewed 179 trials and 25 observational studies. When comparing metformin to sulfonylureas, metformin was associated with less cardiovascular mortality.
However, when trying to make comparisons based on all-cause mortality or microvascular complications, the evidence is limited. Improvements in hemoglobin A1c levels are similar when comparing different monotherapy options, and low blood sugar was most common with sulfonylureas. The short duration of many trials limits the ability to provide better data on long-term outcomes.
Bottom line: Metformin remains the first-line agent for type 2 diabetes management.
Citation: Maruthur NM, Tseng E, Hutfless S, et al. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: a systemic review and meta-analysis. Ann Intern Med. 2016;164(1):740-751.
Short Take
Patients Discharge Readiness May Not Be Adequately Assessed and/or Addressed During Hospitalization
Prospective observational study found unresolved barriers to discharge were common in at least 90% of patients. Patients frequently cited issues including unresolved pain, lack of understanding around discharge plans, and ability to provide self-care.
Citation: Harrison JD, Greysen RS, Jacolbia R, Nguyen A, Auerbach AD. Not ready, not set…discharge: patient-reported barriers to discharge readiness at an academic medical center [published online ahead of print April 15, 2016]. J Hosp Med. doi:10.1002/jhm.2591.
Clinical question: Which medications are most safe and effective at managing type 2 diabetes?
Background: Patients and practitioners need an updated review of the evidence to select the optimal medication for type 2 diabetes management.
Study design: Systematic review.
Synopsis: The authors reviewed 179 trials and 25 observational studies. When comparing metformin to sulfonylureas, metformin was associated with less cardiovascular mortality.
However, when trying to make comparisons based on all-cause mortality or microvascular complications, the evidence is limited. Improvements in hemoglobin A1c levels are similar when comparing different monotherapy options, and low blood sugar was most common with sulfonylureas. The short duration of many trials limits the ability to provide better data on long-term outcomes.
Bottom line: Metformin remains the first-line agent for type 2 diabetes management.
Citation: Maruthur NM, Tseng E, Hutfless S, et al. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: a systemic review and meta-analysis. Ann Intern Med. 2016;164(1):740-751.
Short Take
Patients Discharge Readiness May Not Be Adequately Assessed and/or Addressed During Hospitalization
Prospective observational study found unresolved barriers to discharge were common in at least 90% of patients. Patients frequently cited issues including unresolved pain, lack of understanding around discharge plans, and ability to provide self-care.
Citation: Harrison JD, Greysen RS, Jacolbia R, Nguyen A, Auerbach AD. Not ready, not set…discharge: patient-reported barriers to discharge readiness at an academic medical center [published online ahead of print April 15, 2016]. J Hosp Med. doi:10.1002/jhm.2591.
Clinical question: Which medications are most safe and effective at managing type 2 diabetes?
Background: Patients and practitioners need an updated review of the evidence to select the optimal medication for type 2 diabetes management.
Study design: Systematic review.
Synopsis: The authors reviewed 179 trials and 25 observational studies. When comparing metformin to sulfonylureas, metformin was associated with less cardiovascular mortality.
However, when trying to make comparisons based on all-cause mortality or microvascular complications, the evidence is limited. Improvements in hemoglobin A1c levels are similar when comparing different monotherapy options, and low blood sugar was most common with sulfonylureas. The short duration of many trials limits the ability to provide better data on long-term outcomes.
Bottom line: Metformin remains the first-line agent for type 2 diabetes management.
Citation: Maruthur NM, Tseng E, Hutfless S, et al. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: a systemic review and meta-analysis. Ann Intern Med. 2016;164(1):740-751.
Short Take
Patients Discharge Readiness May Not Be Adequately Assessed and/or Addressed During Hospitalization
Prospective observational study found unresolved barriers to discharge were common in at least 90% of patients. Patients frequently cited issues including unresolved pain, lack of understanding around discharge plans, and ability to provide self-care.
Citation: Harrison JD, Greysen RS, Jacolbia R, Nguyen A, Auerbach AD. Not ready, not set…discharge: patient-reported barriers to discharge readiness at an academic medical center [published online ahead of print April 15, 2016]. J Hosp Med. doi:10.1002/jhm.2591.
Reevaluating Cardiovascular Risk after TIA
Clinical question: What is the prognosis of patients who have a TIA or minor stroke?
Background: Prior studies had estimated the risk in the three months following a TIA or minor stroke of having a stroke or acute coronary syndrome (ACS) as 12% to 20%, but this may not reflect the risk of modern patients receiving the current standards of care.
Study design: Prospective observational registry of patients with recent TIA or minor stroke.
Setting: International, including 21 countries.
Synopsis: Adults with recent TIA or minor stroke were included in this multi-center, international registry, and one-year outcomes were reported. At one year, the Kaplan-Meier estimated event rate for the combined outcome of stroke, ACS, or death from cardiovascular causes was 6.2%. The risk of the cardiovascular events was found to be lower than previously reported, suggesting an improvement in outcomes with current interventions. Elevated ABCD2 score, infarction seen on brain imaging, and large-artery atherosclerosis were each associated with higher risk.
Bottom line: Elevated ABCD2 score, brain imaging findings, and large-artery atherosclerosis suggest increased risk for recurrent stroke.
Citation: Amarenco P, Lavallée PC, Labreuche J, et al. One-year risk of stroke after transient ischemic attack or minor stroke. N Engl J Med. 2016;374(16):1533-1542.
Clinical question: What is the prognosis of patients who have a TIA or minor stroke?
Background: Prior studies had estimated the risk in the three months following a TIA or minor stroke of having a stroke or acute coronary syndrome (ACS) as 12% to 20%, but this may not reflect the risk of modern patients receiving the current standards of care.
Study design: Prospective observational registry of patients with recent TIA or minor stroke.
Setting: International, including 21 countries.
Synopsis: Adults with recent TIA or minor stroke were included in this multi-center, international registry, and one-year outcomes were reported. At one year, the Kaplan-Meier estimated event rate for the combined outcome of stroke, ACS, or death from cardiovascular causes was 6.2%. The risk of the cardiovascular events was found to be lower than previously reported, suggesting an improvement in outcomes with current interventions. Elevated ABCD2 score, infarction seen on brain imaging, and large-artery atherosclerosis were each associated with higher risk.
Bottom line: Elevated ABCD2 score, brain imaging findings, and large-artery atherosclerosis suggest increased risk for recurrent stroke.
Citation: Amarenco P, Lavallée PC, Labreuche J, et al. One-year risk of stroke after transient ischemic attack or minor stroke. N Engl J Med. 2016;374(16):1533-1542.
Clinical question: What is the prognosis of patients who have a TIA or minor stroke?
Background: Prior studies had estimated the risk in the three months following a TIA or minor stroke of having a stroke or acute coronary syndrome (ACS) as 12% to 20%, but this may not reflect the risk of modern patients receiving the current standards of care.
Study design: Prospective observational registry of patients with recent TIA or minor stroke.
Setting: International, including 21 countries.
Synopsis: Adults with recent TIA or minor stroke were included in this multi-center, international registry, and one-year outcomes were reported. At one year, the Kaplan-Meier estimated event rate for the combined outcome of stroke, ACS, or death from cardiovascular causes was 6.2%. The risk of the cardiovascular events was found to be lower than previously reported, suggesting an improvement in outcomes with current interventions. Elevated ABCD2 score, infarction seen on brain imaging, and large-artery atherosclerosis were each associated with higher risk.
Bottom line: Elevated ABCD2 score, brain imaging findings, and large-artery atherosclerosis suggest increased risk for recurrent stroke.
Citation: Amarenco P, Lavallée PC, Labreuche J, et al. One-year risk of stroke after transient ischemic attack or minor stroke. N Engl J Med. 2016;374(16):1533-1542.
Old drug could treat CMV

Image by Ed Uthman
Research published in PLOS Pathogens suggests that emetine, a drug once used to treat amebiasis and induce vomiting in cases of poisoning, may also halt replication of cytomegalovirus (CMV).
Although there are drugs available to treat CMV, they can cause serious toxicity.
Furthermore, resistant strains of CMV can emerge, creating “a desperate need for other ways to control this virus,” according to Ravit Boger, MD, of the Johns Hopkins University School of Medicine in Baltimore, Maryland.
Dr Boger and her colleagues decided to search for drugs to treat CMV by screening 1280 pharmacologically active compounds already approved by the US Food and Drug Administration.
The researchers found a “hit” with emetine, a drug that was used in the past to treat amebiasis before other drugs took its place decades ago.
Results showed that emetine can inhibit CMV at much lower doses than those used for amebiasis, and less frequent doses might be effective for CMV inhibition as well.
In vitro and in vivo tests showed that very low doses of emetine significantly reduced viral replication—75 nm in vitro and 0.1 mg/kg in mice.
Furthermore, with a half-life of 35 hours, the drug exerted its effects over a sustained period, effectively inhibiting virus replication at 14 days after 3 doses.
Additional investigation revealed that emetine’s action against CMV was due to its effects on proteins that control the cell cycle.
Dr Boger cautioned that there is a long way to go before emetine or similar agents can be considered for the treatment of CMV.
“But if further research affirms its potential value, emetine might eventually be used in patients who don’t respond to approved anti-CMV drugs, alone or in combination with these,” she said.
Dr Boger and her colleagues also believe that gaining a better understanding of emetine’s activity in cells could lead to the discovery of new drugs that take advantage of the same or similar pathways. ![]()

Image by Ed Uthman
Research published in PLOS Pathogens suggests that emetine, a drug once used to treat amebiasis and induce vomiting in cases of poisoning, may also halt replication of cytomegalovirus (CMV).
Although there are drugs available to treat CMV, they can cause serious toxicity.
Furthermore, resistant strains of CMV can emerge, creating “a desperate need for other ways to control this virus,” according to Ravit Boger, MD, of the Johns Hopkins University School of Medicine in Baltimore, Maryland.
Dr Boger and her colleagues decided to search for drugs to treat CMV by screening 1280 pharmacologically active compounds already approved by the US Food and Drug Administration.
The researchers found a “hit” with emetine, a drug that was used in the past to treat amebiasis before other drugs took its place decades ago.
Results showed that emetine can inhibit CMV at much lower doses than those used for amebiasis, and less frequent doses might be effective for CMV inhibition as well.
In vitro and in vivo tests showed that very low doses of emetine significantly reduced viral replication—75 nm in vitro and 0.1 mg/kg in mice.
Furthermore, with a half-life of 35 hours, the drug exerted its effects over a sustained period, effectively inhibiting virus replication at 14 days after 3 doses.
Additional investigation revealed that emetine’s action against CMV was due to its effects on proteins that control the cell cycle.
Dr Boger cautioned that there is a long way to go before emetine or similar agents can be considered for the treatment of CMV.
“But if further research affirms its potential value, emetine might eventually be used in patients who don’t respond to approved anti-CMV drugs, alone or in combination with these,” she said.
Dr Boger and her colleagues also believe that gaining a better understanding of emetine’s activity in cells could lead to the discovery of new drugs that take advantage of the same or similar pathways. ![]()

Image by Ed Uthman
Research published in PLOS Pathogens suggests that emetine, a drug once used to treat amebiasis and induce vomiting in cases of poisoning, may also halt replication of cytomegalovirus (CMV).
Although there are drugs available to treat CMV, they can cause serious toxicity.
Furthermore, resistant strains of CMV can emerge, creating “a desperate need for other ways to control this virus,” according to Ravit Boger, MD, of the Johns Hopkins University School of Medicine in Baltimore, Maryland.
Dr Boger and her colleagues decided to search for drugs to treat CMV by screening 1280 pharmacologically active compounds already approved by the US Food and Drug Administration.
The researchers found a “hit” with emetine, a drug that was used in the past to treat amebiasis before other drugs took its place decades ago.
Results showed that emetine can inhibit CMV at much lower doses than those used for amebiasis, and less frequent doses might be effective for CMV inhibition as well.
In vitro and in vivo tests showed that very low doses of emetine significantly reduced viral replication—75 nm in vitro and 0.1 mg/kg in mice.
Furthermore, with a half-life of 35 hours, the drug exerted its effects over a sustained period, effectively inhibiting virus replication at 14 days after 3 doses.
Additional investigation revealed that emetine’s action against CMV was due to its effects on proteins that control the cell cycle.
Dr Boger cautioned that there is a long way to go before emetine or similar agents can be considered for the treatment of CMV.
“But if further research affirms its potential value, emetine might eventually be used in patients who don’t respond to approved anti-CMV drugs, alone or in combination with these,” she said.
Dr Boger and her colleagues also believe that gaining a better understanding of emetine’s activity in cells could lead to the discovery of new drugs that take advantage of the same or similar pathways. ![]()
Computer model shows how spleen filters blood

Researchers have created a computer model that shows how tiny slits in the spleen prevent old, diseased, or misshapen red blood cells from re-entering the bloodstream.
The team says the model can be used to study the spleen’s role in controlling diseases that affect the size and structure of red blood cells—such as sickle cell anemia, thalassemia, and malaria—and to develop new diagnostics and therapeutics for these diseases.
The researchers described the model in PNAS.
Previous studies have shown that part of the spleen’s filtration process relies on having red blood cells squeeze through tiny slits between the endothelial cells that line the spleen’s blood vessels. These “interendothelial slits” are no larger than 1.2 µm tall, 4 µm wide, and 1.9 µm deep.
More rigid and misshapen blood cells might not be able to pass through these narrow passages. And this process cannot be observed in vivo because of the minute size of the slits.
In order to “see” how the interendothelial slits regulate red blood cell circulation, the researchers created a computer simulation based on dissipative particle dynamics, a modeling method.
Their model allowed the team to determine the range of cell sizes and shapes that could fit through the slits. The range closely mirrored the range of sizes and shapes for healthy red blood cells, indicating that only healthy cells should be able to pass through the slits.
“The computational and analytical models from this work, along with a variety of experimental observations, point to a more detailed picture of how the physiology of the human spleen likely influences several key geometrical characteristics of red blood cells,” said study author Subra Suresh, ScD, of Carnegie Mellon University in Pittsburgh, Pennsylvania.
“They also offer better understanding of how the circulatory bottleneck for the red blood cell in the spleen could affect a variety of acute and chronic disease states arising from hereditary disorders, human cancers, and infectious diseases, with implications for therapeutic interventions and drug efficacy assays.”
In addition to giving researchers a better picture of how the spleen functions, the findings provide new insights into drug treatments.
A class of drugs currently in development for treating malaria alters the shape of red blood cells infected with malaria, theoretically preventing them from passing through interendothelial slits. One such drug, spiroindoline KAE609, is in clinical trials.
The researchers’ results might also explain why artemisinin-based antimalarial drugs, which stiffen healthy and malaria-infected red blood cells, can lead to severe anemia. ![]()

Researchers have created a computer model that shows how tiny slits in the spleen prevent old, diseased, or misshapen red blood cells from re-entering the bloodstream.
The team says the model can be used to study the spleen’s role in controlling diseases that affect the size and structure of red blood cells—such as sickle cell anemia, thalassemia, and malaria—and to develop new diagnostics and therapeutics for these diseases.
The researchers described the model in PNAS.
Previous studies have shown that part of the spleen’s filtration process relies on having red blood cells squeeze through tiny slits between the endothelial cells that line the spleen’s blood vessels. These “interendothelial slits” are no larger than 1.2 µm tall, 4 µm wide, and 1.9 µm deep.
More rigid and misshapen blood cells might not be able to pass through these narrow passages. And this process cannot be observed in vivo because of the minute size of the slits.
In order to “see” how the interendothelial slits regulate red blood cell circulation, the researchers created a computer simulation based on dissipative particle dynamics, a modeling method.
Their model allowed the team to determine the range of cell sizes and shapes that could fit through the slits. The range closely mirrored the range of sizes and shapes for healthy red blood cells, indicating that only healthy cells should be able to pass through the slits.
“The computational and analytical models from this work, along with a variety of experimental observations, point to a more detailed picture of how the physiology of the human spleen likely influences several key geometrical characteristics of red blood cells,” said study author Subra Suresh, ScD, of Carnegie Mellon University in Pittsburgh, Pennsylvania.
“They also offer better understanding of how the circulatory bottleneck for the red blood cell in the spleen could affect a variety of acute and chronic disease states arising from hereditary disorders, human cancers, and infectious diseases, with implications for therapeutic interventions and drug efficacy assays.”
In addition to giving researchers a better picture of how the spleen functions, the findings provide new insights into drug treatments.
A class of drugs currently in development for treating malaria alters the shape of red blood cells infected with malaria, theoretically preventing them from passing through interendothelial slits. One such drug, spiroindoline KAE609, is in clinical trials.
The researchers’ results might also explain why artemisinin-based antimalarial drugs, which stiffen healthy and malaria-infected red blood cells, can lead to severe anemia. ![]()

Researchers have created a computer model that shows how tiny slits in the spleen prevent old, diseased, or misshapen red blood cells from re-entering the bloodstream.
The team says the model can be used to study the spleen’s role in controlling diseases that affect the size and structure of red blood cells—such as sickle cell anemia, thalassemia, and malaria—and to develop new diagnostics and therapeutics for these diseases.
The researchers described the model in PNAS.
Previous studies have shown that part of the spleen’s filtration process relies on having red blood cells squeeze through tiny slits between the endothelial cells that line the spleen’s blood vessels. These “interendothelial slits” are no larger than 1.2 µm tall, 4 µm wide, and 1.9 µm deep.
More rigid and misshapen blood cells might not be able to pass through these narrow passages. And this process cannot be observed in vivo because of the minute size of the slits.
In order to “see” how the interendothelial slits regulate red blood cell circulation, the researchers created a computer simulation based on dissipative particle dynamics, a modeling method.
Their model allowed the team to determine the range of cell sizes and shapes that could fit through the slits. The range closely mirrored the range of sizes and shapes for healthy red blood cells, indicating that only healthy cells should be able to pass through the slits.
“The computational and analytical models from this work, along with a variety of experimental observations, point to a more detailed picture of how the physiology of the human spleen likely influences several key geometrical characteristics of red blood cells,” said study author Subra Suresh, ScD, of Carnegie Mellon University in Pittsburgh, Pennsylvania.
“They also offer better understanding of how the circulatory bottleneck for the red blood cell in the spleen could affect a variety of acute and chronic disease states arising from hereditary disorders, human cancers, and infectious diseases, with implications for therapeutic interventions and drug efficacy assays.”
In addition to giving researchers a better picture of how the spleen functions, the findings provide new insights into drug treatments.
A class of drugs currently in development for treating malaria alters the shape of red blood cells infected with malaria, theoretically preventing them from passing through interendothelial slits. One such drug, spiroindoline KAE609, is in clinical trials.
The researchers’ results might also explain why artemisinin-based antimalarial drugs, which stiffen healthy and malaria-infected red blood cells, can lead to severe anemia. ![]()
New type of CAR T cells can produce responses in NHL

Image courtesy of NIAID
Results of a phase 1 study suggest that chimeric antigen receptor T cells specific for the κ light chain (κ.CAR T cells) can produce responses in patients with relapsed or refractory B-cell malignancies, largely without side effects.
The therapy induced complete and partial responses in some patients with non-Hodgkin lymphoma (NHL), and it allowed other patients with chronic lymphocytic leukemia (CLL) or multiple myeloma (MM) to maintain stable disease.
There was 1 adverse event considered possibly related to the treatment.
The researchers reported these results in The Journal of Clinical Investigation.
The κ.CAR T cells were designed to recognize κ-restricted cells and spare normal B cells expressing the nontargeted λ light chain.
“We reasoned that targeting the light chain expressed by malignant B cells should efficiently kill tumor cells while sparing normal B cells expressing the other type of light chain,” said study author Carlos Ramos, MD, of Baylor College of Medicine in Houston, Texas.
He and his colleagues tested the κ.CAR T cells in 16 patients with relapsed or refractory κ+ NHL (n=7), CLL (n=2), or MM (n=7).
The team isolated T cells from these patients and modified the cells so they could target the κ light chain on the surface of malignant B cells. The modified T cells were infused back into the patients, and each patient was monitored for disease progression and side effects.
Eleven patients stopped receiving other treatments at least 4 weeks prior to T-cell infusion. Six patients without lymphopenia received cyclophosphamide at 12.5 mg/kg 4 days before κ.CAR T-cell infusion (0.2×108 to 2×108 κ.CAR T cells/m2).
“We found the treatment to be feasible and safe at all the dose levels studied,” Dr Ramos said.
One MM patient had grade 3 lymphopenia that was deemed possibly related to treatment, but none of the other adverse events were thought to result from the κ.CAR T cells.
Two patients with NHL achieved a complete response to treatment, 1 lasting more than 32 months and the other lasting 6 weeks. A third NHL patient had a partial response lasting 3 months, and a CLL patient had stable disease lasting 6 weeks.
Five of the 7 MM patients had stable disease, 3 lasting 6 weeks, 1 lasting 17 months, and 1 lasting 24 months.
“Our approach, although we are still optimizing it, offers a new possibility for patients in whom other treatments have not been successful,” Dr Ramos concluded. ![]()

Image courtesy of NIAID
Results of a phase 1 study suggest that chimeric antigen receptor T cells specific for the κ light chain (κ.CAR T cells) can produce responses in patients with relapsed or refractory B-cell malignancies, largely without side effects.
The therapy induced complete and partial responses in some patients with non-Hodgkin lymphoma (NHL), and it allowed other patients with chronic lymphocytic leukemia (CLL) or multiple myeloma (MM) to maintain stable disease.
There was 1 adverse event considered possibly related to the treatment.
The researchers reported these results in The Journal of Clinical Investigation.
The κ.CAR T cells were designed to recognize κ-restricted cells and spare normal B cells expressing the nontargeted λ light chain.
“We reasoned that targeting the light chain expressed by malignant B cells should efficiently kill tumor cells while sparing normal B cells expressing the other type of light chain,” said study author Carlos Ramos, MD, of Baylor College of Medicine in Houston, Texas.
He and his colleagues tested the κ.CAR T cells in 16 patients with relapsed or refractory κ+ NHL (n=7), CLL (n=2), or MM (n=7).
The team isolated T cells from these patients and modified the cells so they could target the κ light chain on the surface of malignant B cells. The modified T cells were infused back into the patients, and each patient was monitored for disease progression and side effects.
Eleven patients stopped receiving other treatments at least 4 weeks prior to T-cell infusion. Six patients without lymphopenia received cyclophosphamide at 12.5 mg/kg 4 days before κ.CAR T-cell infusion (0.2×108 to 2×108 κ.CAR T cells/m2).
“We found the treatment to be feasible and safe at all the dose levels studied,” Dr Ramos said.
One MM patient had grade 3 lymphopenia that was deemed possibly related to treatment, but none of the other adverse events were thought to result from the κ.CAR T cells.
Two patients with NHL achieved a complete response to treatment, 1 lasting more than 32 months and the other lasting 6 weeks. A third NHL patient had a partial response lasting 3 months, and a CLL patient had stable disease lasting 6 weeks.
Five of the 7 MM patients had stable disease, 3 lasting 6 weeks, 1 lasting 17 months, and 1 lasting 24 months.
“Our approach, although we are still optimizing it, offers a new possibility for patients in whom other treatments have not been successful,” Dr Ramos concluded. ![]()

Image courtesy of NIAID
Results of a phase 1 study suggest that chimeric antigen receptor T cells specific for the κ light chain (κ.CAR T cells) can produce responses in patients with relapsed or refractory B-cell malignancies, largely without side effects.
The therapy induced complete and partial responses in some patients with non-Hodgkin lymphoma (NHL), and it allowed other patients with chronic lymphocytic leukemia (CLL) or multiple myeloma (MM) to maintain stable disease.
There was 1 adverse event considered possibly related to the treatment.
The researchers reported these results in The Journal of Clinical Investigation.
The κ.CAR T cells were designed to recognize κ-restricted cells and spare normal B cells expressing the nontargeted λ light chain.
“We reasoned that targeting the light chain expressed by malignant B cells should efficiently kill tumor cells while sparing normal B cells expressing the other type of light chain,” said study author Carlos Ramos, MD, of Baylor College of Medicine in Houston, Texas.
He and his colleagues tested the κ.CAR T cells in 16 patients with relapsed or refractory κ+ NHL (n=7), CLL (n=2), or MM (n=7).
The team isolated T cells from these patients and modified the cells so they could target the κ light chain on the surface of malignant B cells. The modified T cells were infused back into the patients, and each patient was monitored for disease progression and side effects.
Eleven patients stopped receiving other treatments at least 4 weeks prior to T-cell infusion. Six patients without lymphopenia received cyclophosphamide at 12.5 mg/kg 4 days before κ.CAR T-cell infusion (0.2×108 to 2×108 κ.CAR T cells/m2).
“We found the treatment to be feasible and safe at all the dose levels studied,” Dr Ramos said.
One MM patient had grade 3 lymphopenia that was deemed possibly related to treatment, but none of the other adverse events were thought to result from the κ.CAR T cells.
Two patients with NHL achieved a complete response to treatment, 1 lasting more than 32 months and the other lasting 6 weeks. A third NHL patient had a partial response lasting 3 months, and a CLL patient had stable disease lasting 6 weeks.
Five of the 7 MM patients had stable disease, 3 lasting 6 weeks, 1 lasting 17 months, and 1 lasting 24 months.
“Our approach, although we are still optimizing it, offers a new possibility for patients in whom other treatments have not been successful,” Dr Ramos concluded. ![]()
Health Canada approves mAb for MM

Photo courtesy of Janssen
Health Canada has granted conditional approval, or a Notice of Compliance with Conditions (NOC/c), for daratumumab (Darzalex), a monoclonal antibody (mAb) targeting CD38.
The mAb is now approved to treat patients with multiple myeloma (MM) who have received at least 3 prior lines of therapy, including a proteasome inhibitor (PI) and an immunomodulatory drug (IMiD), or MM patients who are refractory to both a PI and an IMiD.
An NOC/c is authorization to market a drug with the condition that the sponsor—in this case, Janssen Inc.—undertake additional studies to verify a clinical benefit.
The NOC/c policy is designed to provide access to:
- Drugs that can treat serious, life-threatening, or severely debilitating diseases
- Drugs that can treat conditions for which no drug is currently marketed in Canada
- Drugs that provide a significant increase in efficacy or significant decrease in risk when compared to existing drugs marketed in Canada.
Studies of daratumumab
The NOC/c for daratumumab was based on a review of data from the phase 2 SIRIUS study, the phase 1/2 GEN501 study, and additional supportive studies.
The GEN501 study enrolled 102 patients with relapsed MM or relapsed MM that was refractory to 2 or more prior lines of therapy. The patients received daratumumab at a range of doses and on a number of different schedules.
The results suggested daratumumab is most effective at a dose of 16 mg/kg. At this dose, the overall response rate was 36%. Most adverse events in this study were grade 1 or 2, although serious events did occur.
The SIRIUS study enrolled 124 MM patients who had received 3 or more prior lines of therapy. They received daratumumab at different doses and on different schedules, but 106 patients received the drug at 16 mg/kg.
Twenty-nine percent of the 106 patients responded to treatment, and the median duration of response was 7 months. Thirty percent of patients experienced serious adverse events.
Findings from a combined efficacy analysis of the GEN501 and SIRIUS trials demonstrated that, after a mean follow-up of 14.8 months, the estimated median overall survival for patients who received single-agent daratumumab at 16 mg/kg was 20 months.
Five phase 3 clinical studies with daratumumab in MM patients—in relapsed and frontline settings—are ongoing. Additional studies are ongoing or planned to assess the mAb’s potential in other malignant and pre-malignant diseases in which CD38 is expressed.
Janssen has exclusive worldwide rights to the development, manufacturing, and commercialization of daratumumab for all potential indications. The company licensed daratumumab from Genmab A/S in August 2012. ![]()

Photo courtesy of Janssen
Health Canada has granted conditional approval, or a Notice of Compliance with Conditions (NOC/c), for daratumumab (Darzalex), a monoclonal antibody (mAb) targeting CD38.
The mAb is now approved to treat patients with multiple myeloma (MM) who have received at least 3 prior lines of therapy, including a proteasome inhibitor (PI) and an immunomodulatory drug (IMiD), or MM patients who are refractory to both a PI and an IMiD.
An NOC/c is authorization to market a drug with the condition that the sponsor—in this case, Janssen Inc.—undertake additional studies to verify a clinical benefit.
The NOC/c policy is designed to provide access to:
- Drugs that can treat serious, life-threatening, or severely debilitating diseases
- Drugs that can treat conditions for which no drug is currently marketed in Canada
- Drugs that provide a significant increase in efficacy or significant decrease in risk when compared to existing drugs marketed in Canada.
Studies of daratumumab
The NOC/c for daratumumab was based on a review of data from the phase 2 SIRIUS study, the phase 1/2 GEN501 study, and additional supportive studies.
The GEN501 study enrolled 102 patients with relapsed MM or relapsed MM that was refractory to 2 or more prior lines of therapy. The patients received daratumumab at a range of doses and on a number of different schedules.
The results suggested daratumumab is most effective at a dose of 16 mg/kg. At this dose, the overall response rate was 36%. Most adverse events in this study were grade 1 or 2, although serious events did occur.
The SIRIUS study enrolled 124 MM patients who had received 3 or more prior lines of therapy. They received daratumumab at different doses and on different schedules, but 106 patients received the drug at 16 mg/kg.
Twenty-nine percent of the 106 patients responded to treatment, and the median duration of response was 7 months. Thirty percent of patients experienced serious adverse events.
Findings from a combined efficacy analysis of the GEN501 and SIRIUS trials demonstrated that, after a mean follow-up of 14.8 months, the estimated median overall survival for patients who received single-agent daratumumab at 16 mg/kg was 20 months.
Five phase 3 clinical studies with daratumumab in MM patients—in relapsed and frontline settings—are ongoing. Additional studies are ongoing or planned to assess the mAb’s potential in other malignant and pre-malignant diseases in which CD38 is expressed.
Janssen has exclusive worldwide rights to the development, manufacturing, and commercialization of daratumumab for all potential indications. The company licensed daratumumab from Genmab A/S in August 2012. ![]()

Photo courtesy of Janssen
Health Canada has granted conditional approval, or a Notice of Compliance with Conditions (NOC/c), for daratumumab (Darzalex), a monoclonal antibody (mAb) targeting CD38.
The mAb is now approved to treat patients with multiple myeloma (MM) who have received at least 3 prior lines of therapy, including a proteasome inhibitor (PI) and an immunomodulatory drug (IMiD), or MM patients who are refractory to both a PI and an IMiD.
An NOC/c is authorization to market a drug with the condition that the sponsor—in this case, Janssen Inc.—undertake additional studies to verify a clinical benefit.
The NOC/c policy is designed to provide access to:
- Drugs that can treat serious, life-threatening, or severely debilitating diseases
- Drugs that can treat conditions for which no drug is currently marketed in Canada
- Drugs that provide a significant increase in efficacy or significant decrease in risk when compared to existing drugs marketed in Canada.
Studies of daratumumab
The NOC/c for daratumumab was based on a review of data from the phase 2 SIRIUS study, the phase 1/2 GEN501 study, and additional supportive studies.
The GEN501 study enrolled 102 patients with relapsed MM or relapsed MM that was refractory to 2 or more prior lines of therapy. The patients received daratumumab at a range of doses and on a number of different schedules.
The results suggested daratumumab is most effective at a dose of 16 mg/kg. At this dose, the overall response rate was 36%. Most adverse events in this study were grade 1 or 2, although serious events did occur.
The SIRIUS study enrolled 124 MM patients who had received 3 or more prior lines of therapy. They received daratumumab at different doses and on different schedules, but 106 patients received the drug at 16 mg/kg.
Twenty-nine percent of the 106 patients responded to treatment, and the median duration of response was 7 months. Thirty percent of patients experienced serious adverse events.
Findings from a combined efficacy analysis of the GEN501 and SIRIUS trials demonstrated that, after a mean follow-up of 14.8 months, the estimated median overall survival for patients who received single-agent daratumumab at 16 mg/kg was 20 months.
Five phase 3 clinical studies with daratumumab in MM patients—in relapsed and frontline settings—are ongoing. Additional studies are ongoing or planned to assess the mAb’s potential in other malignant and pre-malignant diseases in which CD38 is expressed.
Janssen has exclusive worldwide rights to the development, manufacturing, and commercialization of daratumumab for all potential indications. The company licensed daratumumab from Genmab A/S in August 2012. ![]()
July 2016 Digital Edition
Click here to access the July 2016 Digital Edition.
Table of Contents
- What is the VA? The Largest Educator of Health Care Professionals in the U.S.
- The Relationship Between Sustained Gripping and the Development of Carpal Tunnel Syndrome
- Efficacy and Safety Outcomes for Patients Taking Warfarin Who Were Switched From Face-to-Face to Telephone Anticoagulation Clinic
- The Most Expensive Drug in the World: To Continue or Discontinue, That Is the Question
- The Unique Value of Externships to Nursing Education and Health Care Organizations
- The Cost of Oncology Drugs: A Pharmacy Perspective, Part 2
- Diabetic Peripheral Neuropathy: The Learning Curve
- Let’s Dance: A Holistic Approach to Treating Veterans With Posttraumatic Stress Disorder

Click here to access the July 2016 Digital Edition.
Table of Contents
- What is the VA? The Largest Educator of Health Care Professionals in the U.S.
- The Relationship Between Sustained Gripping and the Development of Carpal Tunnel Syndrome
- Efficacy and Safety Outcomes for Patients Taking Warfarin Who Were Switched From Face-to-Face to Telephone Anticoagulation Clinic
- The Most Expensive Drug in the World: To Continue or Discontinue, That Is the Question
- The Unique Value of Externships to Nursing Education and Health Care Organizations
- The Cost of Oncology Drugs: A Pharmacy Perspective, Part 2
- Diabetic Peripheral Neuropathy: The Learning Curve
- Let’s Dance: A Holistic Approach to Treating Veterans With Posttraumatic Stress Disorder

Click here to access the July 2016 Digital Edition.
Table of Contents
- What is the VA? The Largest Educator of Health Care Professionals in the U.S.
- The Relationship Between Sustained Gripping and the Development of Carpal Tunnel Syndrome
- Efficacy and Safety Outcomes for Patients Taking Warfarin Who Were Switched From Face-to-Face to Telephone Anticoagulation Clinic
- The Most Expensive Drug in the World: To Continue or Discontinue, That Is the Question
- The Unique Value of Externships to Nursing Education and Health Care Organizations
- The Cost of Oncology Drugs: A Pharmacy Perspective, Part 2
- Diabetic Peripheral Neuropathy: The Learning Curve
- Let’s Dance: A Holistic Approach to Treating Veterans With Posttraumatic Stress Disorder