User login
Pediatric Dermatology Consult - July 2016
By Ellen S. Haddock, MBA, and Lawrence F. Eichenfield, MD
Molluscum
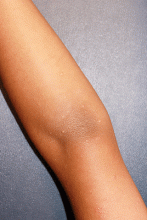
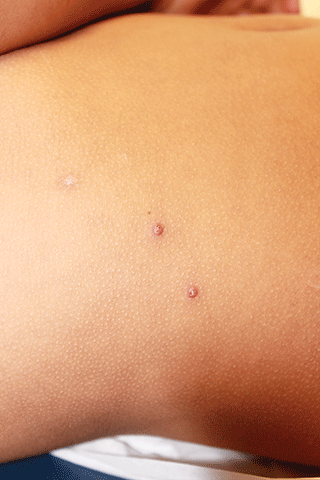
Molluscum typically presents as smooth, flesh-colored, flat-topped papules 2-8 mm in diameter with central whitish area, which is composed of the causative molluscum pox virus. While central depressions called umbilications are common, they may not be present in early molluscum lesions.1,2 Lesions most often occur on the trunk and arms, but can occur anywhere.3-5 Individuals usually have multiple lesions, which may be clustered especially in areas of skin-to-skin contact.5,6 Molluscum lesions can be itchy.
Molluscum is a benign viral skin infection caused by the molluscum contagiosum virus, which is a member of the poxvirus family. Molluscum infections are common, affecting 5%-11% of children.5,7 Molluscum most often affects children younger than 8-years-old,5 with an average age of 5.8 years.8 The infection is spread through skin-to-skin contact with other individuals and by autoinoculation, which means that the infection can be spread from one area of an individual's skin to another when he or she scratches a lesion and then touches another area. It also can be spread by contact with fomites like towels and sponges.1 An association between public swimming pool use and molluscum infection has been reported, but this may have more to do with shared towels and equipment like kick-boards than transmission through the water itself.9 Molluscum sometimes is spread through contact sports like wrestling6 and between children sharing a bath.9 In adults, in whom molluscum is much less common because of acquired immunity,10 molluscum may be sexually transmitted or associated with HIV; however, this is rarely the case in children.1
Children with atopic dermatitis have increased risk of molluscum infection in part because breaks in their skin and pruritus facilitate autoinoculation through scratching.11 Not uncommonly, molluscum lesions become inflamed, with tenderness, erythema, and crust. In a study by Berger et al., 22% of patients had inflamed molluscum lesions.3 The appearance of inflamed molluscum lesions may raise concern about bacterial infection, but more often, the inflammation is a sign that the immune system is reacting to the viral infection and has almost "won the battle."10 After inflamed molluscum lesions develop, the total number of molluscum lesions typically declines,3 and some consider inflamed molluscum lesions to be a "beginning of the end sign," indicating that the infection may soon resolve.10 If the child is afebrile, lesions are itchy and painless, skin culture is negative, and there is no lymphangitis or spreading erythema, the inflammation is more likely a sign of impending resolution than bacterial secondary infection, and the urge to prescribe antibiotics should be resisted.10
As seen in this case, some patients with molluscum (5%) develop a diffuse, monomorphous, papular, or papulovesicular eruption that is an id reaction.3 This may appear to be eczema-like, lichenoid in appearance, or mimicking Gianotti-Crosti syndrome (papular acrodermatitis of childhood).3 It typically affects the arms and legs, is bilateral, and may be pruritic. The id reaction may occur in conjunction with inflamed molluscum, as is true in this case. The diffuse eruption can sometimes be mistaken for a sudden increase in the number of molluscum lesions, but the papular dermatitis lesions do not have the flat-topped dome-shape nor white centers.3 On average, the id reaction lasts about 6 weeks, after which both it and the primary molluscum lesions typically resolve.3 Although not seen in this case, more than a third of molluscum patients develop a pruritic, erythematous, eczematous area around molluscum lesions, termed molluscum dermatitis or eczema molluscatum, which may be more prominent than the molluscum itself.3 The eczematous patch typically surrounds molluscum lesions but also may occur at distant sites.12 This reaction is especially common in patients with atopic dermatitis, 51% of whom develop it.3 It is considered a hypersensitivity reaction and may be asymptomatic or minimally pruritic.12 Molluscum dermatitis suggests that the immune system has taken notice of the infection and is fighting it13; however, it does not necessarily indicate impending resolution.3
Differential diagnosis
The differential diagnosis for molluscum includes herpes simplex, warts, and milia.14 Like molluscum, herpes simplex lesions can have central umbilication, but the lesions are vesicular rather than solid. Warts typically have a rough, jagged surface in contrast to the smooth surface of molluscum lesions. Milia tend to be smaller and not flat topped. They are more common in infants and adults than in children and primarily affect the face.
Inflamed molluscum lesions and molluscum dermatitis can be mistaken for atopic dermatitis, and molluscum-associated id reactions may exacerbate atopic dermatitis. Inflamed molluscum and molluscum with id reaction could be confused with scabies, which may become crusted and also may be accompanied by id reaction. Presence of serpiginous linear burrows would suggest scabies rather than molluscum, and the diagnosis of scabies can be confirmed by scraping a burrow and looking for a mite or its feces under a microscope.
Prognosis and treatment
Molluscum infections typically resolve spontaneously in months to years (average duration, 13 months),14 so treatment may not be required. The goal of treatment is to accelerate the resolution of the infection, but some studies have found that common treatments may not shorten the time to resolution.11 However, if there is substantial pruritus, lesions are cosmetically undesirable, or a child has atopic dermatitis and is at increased risk for autoinoculation, treatment may be warranted.15 Furthermore, molluscum lesions can scar, so prevention of autoinoculation may help minimize scarring.16
Few high-quality studies of the efficacy of molluscum treatments exist, and a 2009 Cochrane review found insufficient evidence to recommend any therapy for molluscum. The most common treatment used by pediatric dermatologists is cantharidin,17 and this treatment also is available to primary care practitioners. This option is preferred over other destructive methods such as curettage or liquid nitrogen cryotherapy because it is not painful or traumatic and is well tolerated by pediatric patients.8 Parent and physician satisfaction with the therapy is high; 78%-95% of parents would use cantharidin treatment again for molluscum recurrence.4,8,18 Originally extracted from the blister beetle but now synthesized commercially,19 cantharidin causes vesiculation at the dermoepidermal junction6 by destroying intercellular connections.4 Vesiculation of the skin causes extrusion of the molluscum body, which facilitates resolution of the lesion.19 The cantharidin formulation is applied directly to molluscum lesions with the wooden end of a cotton-tipped applicator.4 Patients may be directed to wash it off after 4-6 hours. Blistering is an expected, desired outcome. A minority of patients may experience mild temporary pain (7%), more significant blistering (2.5%), burning (less than 1%), pruritus (less than 1%), or irritation (less than 1%).4 There is a risk of scarring and pigmentary changes, but these risks also exist for untreated lesions.19 Cantharidin treatment is repeated approximately every 4 weeks, and 90% of cases resolve after an average of 2.1 treatments.18 Topical retinoids can be used in an attempt to trigger an irritant response by the immune system, and they are the preferred therapy for facial lesions, but they are inconsistently effective.4 Randomized controlled trials found that imiquimod, a previously popular treatment is not effective,20 and the evidence for cimetidine is contradictory.21,22 Molluscum dermatitis and id reaction can be treated with medium strength topical steroids.
References
- Viral diseases of the skin, in "Hurwitz Clinical Pediatric Dermatology," 4 ed. (New York: Elsevier, 2011, pp. 348-69). .
- Molluscum, in "Red Book Report of the Committee on Infectious Diseases," 2015.
Ms. Haddock is a medical student at the University of California, San Diego, and a research associate at Rady Children's Hospital-San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children's Hospital-San Diego and professor of dermatology and pediatrics at the University of California, San Diego. Dr. Eichenfield and Ms. Haddock state they have no relevant financial disclosures.
By Ellen S. Haddock, MBA, and Lawrence F. Eichenfield, MD
Molluscum
Molluscum typically presents as smooth, flesh-colored, flat-topped papules 2-8 mm in diameter with central whitish area, which is composed of the causative molluscum pox virus. While central depressions called umbilications are common, they may not be present in early molluscum lesions.1,2 Lesions most often occur on the trunk and arms, but can occur anywhere.3-5 Individuals usually have multiple lesions, which may be clustered especially in areas of skin-to-skin contact.5,6 Molluscum lesions can be itchy.
Molluscum is a benign viral skin infection caused by the molluscum contagiosum virus, which is a member of the poxvirus family. Molluscum infections are common, affecting 5%-11% of children.5,7 Molluscum most often affects children younger than 8-years-old,5 with an average age of 5.8 years.8 The infection is spread through skin-to-skin contact with other individuals and by autoinoculation, which means that the infection can be spread from one area of an individual's skin to another when he or she scratches a lesion and then touches another area. It also can be spread by contact with fomites like towels and sponges.1 An association between public swimming pool use and molluscum infection has been reported, but this may have more to do with shared towels and equipment like kick-boards than transmission through the water itself.9 Molluscum sometimes is spread through contact sports like wrestling6 and between children sharing a bath.9 In adults, in whom molluscum is much less common because of acquired immunity,10 molluscum may be sexually transmitted or associated with HIV; however, this is rarely the case in children.1
Children with atopic dermatitis have increased risk of molluscum infection in part because breaks in their skin and pruritus facilitate autoinoculation through scratching.11 Not uncommonly, molluscum lesions become inflamed, with tenderness, erythema, and crust. In a study by Berger et al., 22% of patients had inflamed molluscum lesions.3 The appearance of inflamed molluscum lesions may raise concern about bacterial infection, but more often, the inflammation is a sign that the immune system is reacting to the viral infection and has almost "won the battle."10 After inflamed molluscum lesions develop, the total number of molluscum lesions typically declines,3 and some consider inflamed molluscum lesions to be a "beginning of the end sign," indicating that the infection may soon resolve.10 If the child is afebrile, lesions are itchy and painless, skin culture is negative, and there is no lymphangitis or spreading erythema, the inflammation is more likely a sign of impending resolution than bacterial secondary infection, and the urge to prescribe antibiotics should be resisted.10
As seen in this case, some patients with molluscum (5%) develop a diffuse, monomorphous, papular, or papulovesicular eruption that is an id reaction.3 This may appear to be eczema-like, lichenoid in appearance, or mimicking Gianotti-Crosti syndrome (papular acrodermatitis of childhood).3 It typically affects the arms and legs, is bilateral, and may be pruritic. The id reaction may occur in conjunction with inflamed molluscum, as is true in this case. The diffuse eruption can sometimes be mistaken for a sudden increase in the number of molluscum lesions, but the papular dermatitis lesions do not have the flat-topped dome-shape nor white centers.3 On average, the id reaction lasts about 6 weeks, after which both it and the primary molluscum lesions typically resolve.3 Although not seen in this case, more than a third of molluscum patients develop a pruritic, erythematous, eczematous area around molluscum lesions, termed molluscum dermatitis or eczema molluscatum, which may be more prominent than the molluscum itself.3 The eczematous patch typically surrounds molluscum lesions but also may occur at distant sites.12 This reaction is especially common in patients with atopic dermatitis, 51% of whom develop it.3 It is considered a hypersensitivity reaction and may be asymptomatic or minimally pruritic.12 Molluscum dermatitis suggests that the immune system has taken notice of the infection and is fighting it13; however, it does not necessarily indicate impending resolution.3
Differential diagnosis
The differential diagnosis for molluscum includes herpes simplex, warts, and milia.14 Like molluscum, herpes simplex lesions can have central umbilication, but the lesions are vesicular rather than solid. Warts typically have a rough, jagged surface in contrast to the smooth surface of molluscum lesions. Milia tend to be smaller and not flat topped. They are more common in infants and adults than in children and primarily affect the face.
Inflamed molluscum lesions and molluscum dermatitis can be mistaken for atopic dermatitis, and molluscum-associated id reactions may exacerbate atopic dermatitis. Inflamed molluscum and molluscum with id reaction could be confused with scabies, which may become crusted and also may be accompanied by id reaction. Presence of serpiginous linear burrows would suggest scabies rather than molluscum, and the diagnosis of scabies can be confirmed by scraping a burrow and looking for a mite or its feces under a microscope.
Prognosis and treatment
Molluscum infections typically resolve spontaneously in months to years (average duration, 13 months),14 so treatment may not be required. The goal of treatment is to accelerate the resolution of the infection, but some studies have found that common treatments may not shorten the time to resolution.11 However, if there is substantial pruritus, lesions are cosmetically undesirable, or a child has atopic dermatitis and is at increased risk for autoinoculation, treatment may be warranted.15 Furthermore, molluscum lesions can scar, so prevention of autoinoculation may help minimize scarring.16
Few high-quality studies of the efficacy of molluscum treatments exist, and a 2009 Cochrane review found insufficient evidence to recommend any therapy for molluscum. The most common treatment used by pediatric dermatologists is cantharidin,17 and this treatment also is available to primary care practitioners. This option is preferred over other destructive methods such as curettage or liquid nitrogen cryotherapy because it is not painful or traumatic and is well tolerated by pediatric patients.8 Parent and physician satisfaction with the therapy is high; 78%-95% of parents would use cantharidin treatment again for molluscum recurrence.4,8,18 Originally extracted from the blister beetle but now synthesized commercially,19 cantharidin causes vesiculation at the dermoepidermal junction6 by destroying intercellular connections.4 Vesiculation of the skin causes extrusion of the molluscum body, which facilitates resolution of the lesion.19 The cantharidin formulation is applied directly to molluscum lesions with the wooden end of a cotton-tipped applicator.4 Patients may be directed to wash it off after 4-6 hours. Blistering is an expected, desired outcome. A minority of patients may experience mild temporary pain (7%), more significant blistering (2.5%), burning (less than 1%), pruritus (less than 1%), or irritation (less than 1%).4 There is a risk of scarring and pigmentary changes, but these risks also exist for untreated lesions.19 Cantharidin treatment is repeated approximately every 4 weeks, and 90% of cases resolve after an average of 2.1 treatments.18 Topical retinoids can be used in an attempt to trigger an irritant response by the immune system, and they are the preferred therapy for facial lesions, but they are inconsistently effective.4 Randomized controlled trials found that imiquimod, a previously popular treatment is not effective,20 and the evidence for cimetidine is contradictory.21,22 Molluscum dermatitis and id reaction can be treated with medium strength topical steroids.
References
- Viral diseases of the skin, in "Hurwitz Clinical Pediatric Dermatology," 4 ed. (New York: Elsevier, 2011, pp. 348-69). .
- Molluscum, in "Red Book Report of the Committee on Infectious Diseases," 2015.
Ms. Haddock is a medical student at the University of California, San Diego, and a research associate at Rady Children's Hospital-San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children's Hospital-San Diego and professor of dermatology and pediatrics at the University of California, San Diego. Dr. Eichenfield and Ms. Haddock state they have no relevant financial disclosures.
By Ellen S. Haddock, MBA, and Lawrence F. Eichenfield, MD
Molluscum
Molluscum typically presents as smooth, flesh-colored, flat-topped papules 2-8 mm in diameter with central whitish area, which is composed of the causative molluscum pox virus. While central depressions called umbilications are common, they may not be present in early molluscum lesions.1,2 Lesions most often occur on the trunk and arms, but can occur anywhere.3-5 Individuals usually have multiple lesions, which may be clustered especially in areas of skin-to-skin contact.5,6 Molluscum lesions can be itchy.
Molluscum is a benign viral skin infection caused by the molluscum contagiosum virus, which is a member of the poxvirus family. Molluscum infections are common, affecting 5%-11% of children.5,7 Molluscum most often affects children younger than 8-years-old,5 with an average age of 5.8 years.8 The infection is spread through skin-to-skin contact with other individuals and by autoinoculation, which means that the infection can be spread from one area of an individual's skin to another when he or she scratches a lesion and then touches another area. It also can be spread by contact with fomites like towels and sponges.1 An association between public swimming pool use and molluscum infection has been reported, but this may have more to do with shared towels and equipment like kick-boards than transmission through the water itself.9 Molluscum sometimes is spread through contact sports like wrestling6 and between children sharing a bath.9 In adults, in whom molluscum is much less common because of acquired immunity,10 molluscum may be sexually transmitted or associated with HIV; however, this is rarely the case in children.1
Children with atopic dermatitis have increased risk of molluscum infection in part because breaks in their skin and pruritus facilitate autoinoculation through scratching.11 Not uncommonly, molluscum lesions become inflamed, with tenderness, erythema, and crust. In a study by Berger et al., 22% of patients had inflamed molluscum lesions.3 The appearance of inflamed molluscum lesions may raise concern about bacterial infection, but more often, the inflammation is a sign that the immune system is reacting to the viral infection and has almost "won the battle."10 After inflamed molluscum lesions develop, the total number of molluscum lesions typically declines,3 and some consider inflamed molluscum lesions to be a "beginning of the end sign," indicating that the infection may soon resolve.10 If the child is afebrile, lesions are itchy and painless, skin culture is negative, and there is no lymphangitis or spreading erythema, the inflammation is more likely a sign of impending resolution than bacterial secondary infection, and the urge to prescribe antibiotics should be resisted.10
As seen in this case, some patients with molluscum (5%) develop a diffuse, monomorphous, papular, or papulovesicular eruption that is an id reaction.3 This may appear to be eczema-like, lichenoid in appearance, or mimicking Gianotti-Crosti syndrome (papular acrodermatitis of childhood).3 It typically affects the arms and legs, is bilateral, and may be pruritic. The id reaction may occur in conjunction with inflamed molluscum, as is true in this case. The diffuse eruption can sometimes be mistaken for a sudden increase in the number of molluscum lesions, but the papular dermatitis lesions do not have the flat-topped dome-shape nor white centers.3 On average, the id reaction lasts about 6 weeks, after which both it and the primary molluscum lesions typically resolve.3 Although not seen in this case, more than a third of molluscum patients develop a pruritic, erythematous, eczematous area around molluscum lesions, termed molluscum dermatitis or eczema molluscatum, which may be more prominent than the molluscum itself.3 The eczematous patch typically surrounds molluscum lesions but also may occur at distant sites.12 This reaction is especially common in patients with atopic dermatitis, 51% of whom develop it.3 It is considered a hypersensitivity reaction and may be asymptomatic or minimally pruritic.12 Molluscum dermatitis suggests that the immune system has taken notice of the infection and is fighting it13; however, it does not necessarily indicate impending resolution.3
Differential diagnosis
The differential diagnosis for molluscum includes herpes simplex, warts, and milia.14 Like molluscum, herpes simplex lesions can have central umbilication, but the lesions are vesicular rather than solid. Warts typically have a rough, jagged surface in contrast to the smooth surface of molluscum lesions. Milia tend to be smaller and not flat topped. They are more common in infants and adults than in children and primarily affect the face.
Inflamed molluscum lesions and molluscum dermatitis can be mistaken for atopic dermatitis, and molluscum-associated id reactions may exacerbate atopic dermatitis. Inflamed molluscum and molluscum with id reaction could be confused with scabies, which may become crusted and also may be accompanied by id reaction. Presence of serpiginous linear burrows would suggest scabies rather than molluscum, and the diagnosis of scabies can be confirmed by scraping a burrow and looking for a mite or its feces under a microscope.
Prognosis and treatment
Molluscum infections typically resolve spontaneously in months to years (average duration, 13 months),14 so treatment may not be required. The goal of treatment is to accelerate the resolution of the infection, but some studies have found that common treatments may not shorten the time to resolution.11 However, if there is substantial pruritus, lesions are cosmetically undesirable, or a child has atopic dermatitis and is at increased risk for autoinoculation, treatment may be warranted.15 Furthermore, molluscum lesions can scar, so prevention of autoinoculation may help minimize scarring.16
Few high-quality studies of the efficacy of molluscum treatments exist, and a 2009 Cochrane review found insufficient evidence to recommend any therapy for molluscum. The most common treatment used by pediatric dermatologists is cantharidin,17 and this treatment also is available to primary care practitioners. This option is preferred over other destructive methods such as curettage or liquid nitrogen cryotherapy because it is not painful or traumatic and is well tolerated by pediatric patients.8 Parent and physician satisfaction with the therapy is high; 78%-95% of parents would use cantharidin treatment again for molluscum recurrence.4,8,18 Originally extracted from the blister beetle but now synthesized commercially,19 cantharidin causes vesiculation at the dermoepidermal junction6 by destroying intercellular connections.4 Vesiculation of the skin causes extrusion of the molluscum body, which facilitates resolution of the lesion.19 The cantharidin formulation is applied directly to molluscum lesions with the wooden end of a cotton-tipped applicator.4 Patients may be directed to wash it off after 4-6 hours. Blistering is an expected, desired outcome. A minority of patients may experience mild temporary pain (7%), more significant blistering (2.5%), burning (less than 1%), pruritus (less than 1%), or irritation (less than 1%).4 There is a risk of scarring and pigmentary changes, but these risks also exist for untreated lesions.19 Cantharidin treatment is repeated approximately every 4 weeks, and 90% of cases resolve after an average of 2.1 treatments.18 Topical retinoids can be used in an attempt to trigger an irritant response by the immune system, and they are the preferred therapy for facial lesions, but they are inconsistently effective.4 Randomized controlled trials found that imiquimod, a previously popular treatment is not effective,20 and the evidence for cimetidine is contradictory.21,22 Molluscum dermatitis and id reaction can be treated with medium strength topical steroids.
References
- Viral diseases of the skin, in "Hurwitz Clinical Pediatric Dermatology," 4 ed. (New York: Elsevier, 2011, pp. 348-69). .
- Molluscum, in "Red Book Report of the Committee on Infectious Diseases," 2015.
Ms. Haddock is a medical student at the University of California, San Diego, and a research associate at Rady Children's Hospital-San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children's Hospital-San Diego and professor of dermatology and pediatrics at the University of California, San Diego. Dr. Eichenfield and Ms. Haddock state they have no relevant financial disclosures.
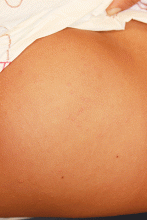
An otherwise healthy 9-year-old girl presented for evaluation of multiple small, skin-colored bumps on her belly, arms, knees, and buttocks. She first noticed a few bumps on her belly 4 months ago. Some of the original bumps have resolved, leaving only two of the originals remaining, but a few weeks ago she developed many additional itchy bumps on her arms, knees, and buttocks. On physical exam, she has two erythematous, flat-topped papules on her abdomen with white centers. (See photo.) A hypopigmented macule also is present on the abdomen. On her legs, arms, and buttocks she has multiple skin-colored to pink papules without white centers.
Maximize depression treatment efforts with measurement-based care
WASHINGTON – Challenging patients with depression to stay engaged in their recovery while rigorously monitoring and measuring their treatment response can mean the difference between remission or resistance, according to an expert.
“We all wish for better treatments, but we really don’t have any coming available to us in the near future, so our task is to do as well as we can with what we have,” Michael E. Thase, MD, professor of psychiatry at the University of Pennsylvania, Philadelphia, said at Summit in Neurology & Psychiatry.
“We know far too well that there is a large gap between what we might be able to accomplish and what actually happens in clinical practice,” Dr. Thase said.
To close that gap, start with evaluating what can be done to combat patient nonadherence. A 2007 study indicated that just under 20% of patients adhere to antidepressant medication guidelines (Prim Care Companion J Clin Psychiatry. 2007;9[2]:91-9.), and according to Dr. Thase, less than 10% of patients being seen for depression in primary care actually fill their prescriptions.
“Don’t delude yourself that these patients drop out of treatment because they no longer need it. They drop out mostly because they have gotten disgusted, disappointed, or discouraged,” said Dr. Thase, attributing the high rate of nonadherence to the nature of depression itself.
“Depression is a state of pessimism, low confidence, and of not really feeling capable.”
Helping patients overcome their reluctance to follow treatment guidelines begins with accurately assessing their symptom severity. Patients who rate 4 or less on the Patient Health Questionnaire–9 (PHQ-9), which is based on the DSM-IV, are considered in remission or not to have severe depression. Similarly, patients who score 5 or less on the Quick Inventory of Depressive Symptomatology (QIDS) are not considered in the danger zone.
The severity ratings included in the DSM-5 can help clinicians pinpoint the level of depression a patient is experiencing, as can a therapeutic relationship with the patient and the family, Dr. Thase said at the summit, held by Global Academy for Medical Education.
He urged clinicians to monitor adherence routinely by asking patients whether or not they have filled or refilled their prescriptions. It is helpful to ask patients about missing doses and whether they are feeling any improvement, he said.
“It’s a very simple process: You keep track of the patients. Chase after them if they don’t follow through. If you’re not keeping track, the patients think [what they do] doesn’t matter,” Dr. Thase said.
A 2010 study of chronic depression treatment in primary care practices showed that compared with regular care, aggressive monitoring of patient adherence and outcomes in 728 adults with depression resulted in better remission rates across 18 months of treatment. At 6 months 43.4% of patients who had been contacted regularly by nursing and social worker staff were in remission, compared with 33.3% of the 78 who had received regular care (P = .11). At 12 months, the results were 52% vs. 33.9% (P = .012), and at 18 months remission was reported in 49.2% vs. 27.3% (P = .004) (Ann Fam Med. 2010 Sep;8[5]:387-96).
By being alert to side effects, using a rating scale to measure symptom reduction, and not staying “locked” into the idea that all treatments require 6-8 weeks before patients see any improvement, clinicians can boost chances for adherence – and thus for remission – since patients who don’t improve in the first 2 weeks have just a 15% chance for improvement, Dr. Thase said. “You need 6-8 weeks to get to the maximum titration. That old 8-week rule applies mostly to patients who are showing improvement by week 2.”
If by week 2, the patient is not tolerating the medication’s side effects and has no symptom reduction, then move on to the next medication, Dr. Thase said, emphasizing the importance of seeing patients at least twice monthly. “If we’re only seeing a patient once a month, we’ve already fallen behind in the up-titration part, and we’ve missed the opportunity for early intervention if the patient is having side effects.”
Algorithms for choosing depression treatments should be a “fact of life,” he said. Start with the safest, easiest to tolerate, and least expensive of the available antidepressants, such as escitalopram and fluoxetine before working through the second- and third-line therapies and adjunctive therapies, all of which have greater risk profiles as you move through them. This algorithmic approach has been shown to result in patients scoring at least 10 points higher on a depression rating scale (JAMA Psychiatry. 2004;61:[7]669-80), which Dr. Thase said is equivalent to about a 30% higher chance of remission. “Don’t go over and over with the simple treatments that aren’t [evoking] a response. Move briskly on to the more complex treatments until patients respond.”
Treatment algorithms can include psychotherapies, either in combination with pharmacotherapy or as monotherapy. He encouraged clinicians to refer to the American Psychiatric Association (APA) practice guideline for depression for more details. The absence of depressive symptoms alone is not indicative of remission, according to the guideline, which Dr. Thase coauthored. The presence of positive emotions and resilience, along with a sense of control over emotions and hope for the future, indicates remission.
If after applying those methods a patient remains depressed and has been negatively screened for bipolar disorder, the use of tricyclics or monoamine oxidase inhibitors (MAOIs) may be appropriate. “MAOIs account for less than 1 in every 1,000 prescriptions for antidepressants, yet for people who don’t respond to modern antidepressants, they still can carry a 30%-40% response rate. So, if you don’t prescribe them yourself, please get access to someone who does,” Dr. Thase said.
The tenets of measurement-based care are essentially the lessons learned from the landmark Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial, which sought to address how pharmacologic care for depression could be delivered based on adequate dosing, attenuation of symptoms, fewer side effects, and other factors (Am J Psychiatry. 2006;163:1905-17).
Now,10 years later, measurement-based treatment is still finding its way into practice, Dr. Thase said in an interview. “But, there’s no reason to be unduly pessimistic. Ten years ago, depression screening was in the same position, and now it is both considered to be the standard of care and is widely done.”
Dr. Thase reported having extensive industry relationships, noting that he has been involved in the development of nearly every drug for the treatment of mood disorders. Global Academy and this news organization are owned by the same company.
On Twitter @whitneymcknight
Ten years ago, the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study highlighted the importance of close monitoring of patients with depression. This is best done with a team-based approach; it’s best suited to handle issues of compliance, side effects, efficacy, and close follow-up. The team may consist of a psychotherapist, nurse, and, possibly, a pharmacist – in addition to the internist, Nitin S. Damle, MD, said in an interview.

|
| Courtesy American College of Physicians Dr. Nitin S. Damle |
Integrating behavioral health into primary care practices involves structure, which includes office visits – even every 2 weeks until stable – follow-up phone calls by the nurse to assess adherence and problems with medication, and a pharmacist to track refill rates and side effects, and to recommend changes to medication due to lack of efficacy or side effects, he said.
Treatment algorithms are an effective means to find the most effective and safest medication, and screening with PHQ-9 and even QIDS has become more common in the primary care office. Still, the adaption of measurement-based care has been slow, partly because of the absence of adequate funding for an integrated primary care team-based approach to mental illness. Now there is no mechanism to cover the costs of personnel and infrastructure to provide for the level of monitoring and treatment that measurement-based care requires, according to Dr. Damle.
Health plans need to review the evidence, such as that from the STAR*D and other studies, and create funding mechanisms so that their members stay healthy and avoid complications of mental illness.
Dr. Damle is president of the American College of Physicians and clinical associate professor of medicine at Brown University, Providence, R.I. He has no relevant disclosures.
Ten years ago, the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study highlighted the importance of close monitoring of patients with depression. This is best done with a team-based approach; it’s best suited to handle issues of compliance, side effects, efficacy, and close follow-up. The team may consist of a psychotherapist, nurse, and, possibly, a pharmacist – in addition to the internist, Nitin S. Damle, MD, said in an interview.

|
| Courtesy American College of Physicians Dr. Nitin S. Damle |
Integrating behavioral health into primary care practices involves structure, which includes office visits – even every 2 weeks until stable – follow-up phone calls by the nurse to assess adherence and problems with medication, and a pharmacist to track refill rates and side effects, and to recommend changes to medication due to lack of efficacy or side effects, he said.
Treatment algorithms are an effective means to find the most effective and safest medication, and screening with PHQ-9 and even QIDS has become more common in the primary care office. Still, the adaption of measurement-based care has been slow, partly because of the absence of adequate funding for an integrated primary care team-based approach to mental illness. Now there is no mechanism to cover the costs of personnel and infrastructure to provide for the level of monitoring and treatment that measurement-based care requires, according to Dr. Damle.
Health plans need to review the evidence, such as that from the STAR*D and other studies, and create funding mechanisms so that their members stay healthy and avoid complications of mental illness.
Dr. Damle is president of the American College of Physicians and clinical associate professor of medicine at Brown University, Providence, R.I. He has no relevant disclosures.
Ten years ago, the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study highlighted the importance of close monitoring of patients with depression. This is best done with a team-based approach; it’s best suited to handle issues of compliance, side effects, efficacy, and close follow-up. The team may consist of a psychotherapist, nurse, and, possibly, a pharmacist – in addition to the internist, Nitin S. Damle, MD, said in an interview.

|
| Courtesy American College of Physicians Dr. Nitin S. Damle |
Integrating behavioral health into primary care practices involves structure, which includes office visits – even every 2 weeks until stable – follow-up phone calls by the nurse to assess adherence and problems with medication, and a pharmacist to track refill rates and side effects, and to recommend changes to medication due to lack of efficacy or side effects, he said.
Treatment algorithms are an effective means to find the most effective and safest medication, and screening with PHQ-9 and even QIDS has become more common in the primary care office. Still, the adaption of measurement-based care has been slow, partly because of the absence of adequate funding for an integrated primary care team-based approach to mental illness. Now there is no mechanism to cover the costs of personnel and infrastructure to provide for the level of monitoring and treatment that measurement-based care requires, according to Dr. Damle.
Health plans need to review the evidence, such as that from the STAR*D and other studies, and create funding mechanisms so that their members stay healthy and avoid complications of mental illness.
Dr. Damle is president of the American College of Physicians and clinical associate professor of medicine at Brown University, Providence, R.I. He has no relevant disclosures.
WASHINGTON – Challenging patients with depression to stay engaged in their recovery while rigorously monitoring and measuring their treatment response can mean the difference between remission or resistance, according to an expert.
“We all wish for better treatments, but we really don’t have any coming available to us in the near future, so our task is to do as well as we can with what we have,” Michael E. Thase, MD, professor of psychiatry at the University of Pennsylvania, Philadelphia, said at Summit in Neurology & Psychiatry.
“We know far too well that there is a large gap between what we might be able to accomplish and what actually happens in clinical practice,” Dr. Thase said.
To close that gap, start with evaluating what can be done to combat patient nonadherence. A 2007 study indicated that just under 20% of patients adhere to antidepressant medication guidelines (Prim Care Companion J Clin Psychiatry. 2007;9[2]:91-9.), and according to Dr. Thase, less than 10% of patients being seen for depression in primary care actually fill their prescriptions.
“Don’t delude yourself that these patients drop out of treatment because they no longer need it. They drop out mostly because they have gotten disgusted, disappointed, or discouraged,” said Dr. Thase, attributing the high rate of nonadherence to the nature of depression itself.
“Depression is a state of pessimism, low confidence, and of not really feeling capable.”
Helping patients overcome their reluctance to follow treatment guidelines begins with accurately assessing their symptom severity. Patients who rate 4 or less on the Patient Health Questionnaire–9 (PHQ-9), which is based on the DSM-IV, are considered in remission or not to have severe depression. Similarly, patients who score 5 or less on the Quick Inventory of Depressive Symptomatology (QIDS) are not considered in the danger zone.
The severity ratings included in the DSM-5 can help clinicians pinpoint the level of depression a patient is experiencing, as can a therapeutic relationship with the patient and the family, Dr. Thase said at the summit, held by Global Academy for Medical Education.
He urged clinicians to monitor adherence routinely by asking patients whether or not they have filled or refilled their prescriptions. It is helpful to ask patients about missing doses and whether they are feeling any improvement, he said.
“It’s a very simple process: You keep track of the patients. Chase after them if they don’t follow through. If you’re not keeping track, the patients think [what they do] doesn’t matter,” Dr. Thase said.
A 2010 study of chronic depression treatment in primary care practices showed that compared with regular care, aggressive monitoring of patient adherence and outcomes in 728 adults with depression resulted in better remission rates across 18 months of treatment. At 6 months 43.4% of patients who had been contacted regularly by nursing and social worker staff were in remission, compared with 33.3% of the 78 who had received regular care (P = .11). At 12 months, the results were 52% vs. 33.9% (P = .012), and at 18 months remission was reported in 49.2% vs. 27.3% (P = .004) (Ann Fam Med. 2010 Sep;8[5]:387-96).
By being alert to side effects, using a rating scale to measure symptom reduction, and not staying “locked” into the idea that all treatments require 6-8 weeks before patients see any improvement, clinicians can boost chances for adherence – and thus for remission – since patients who don’t improve in the first 2 weeks have just a 15% chance for improvement, Dr. Thase said. “You need 6-8 weeks to get to the maximum titration. That old 8-week rule applies mostly to patients who are showing improvement by week 2.”
If by week 2, the patient is not tolerating the medication’s side effects and has no symptom reduction, then move on to the next medication, Dr. Thase said, emphasizing the importance of seeing patients at least twice monthly. “If we’re only seeing a patient once a month, we’ve already fallen behind in the up-titration part, and we’ve missed the opportunity for early intervention if the patient is having side effects.”
Algorithms for choosing depression treatments should be a “fact of life,” he said. Start with the safest, easiest to tolerate, and least expensive of the available antidepressants, such as escitalopram and fluoxetine before working through the second- and third-line therapies and adjunctive therapies, all of which have greater risk profiles as you move through them. This algorithmic approach has been shown to result in patients scoring at least 10 points higher on a depression rating scale (JAMA Psychiatry. 2004;61:[7]669-80), which Dr. Thase said is equivalent to about a 30% higher chance of remission. “Don’t go over and over with the simple treatments that aren’t [evoking] a response. Move briskly on to the more complex treatments until patients respond.”
Treatment algorithms can include psychotherapies, either in combination with pharmacotherapy or as monotherapy. He encouraged clinicians to refer to the American Psychiatric Association (APA) practice guideline for depression for more details. The absence of depressive symptoms alone is not indicative of remission, according to the guideline, which Dr. Thase coauthored. The presence of positive emotions and resilience, along with a sense of control over emotions and hope for the future, indicates remission.
If after applying those methods a patient remains depressed and has been negatively screened for bipolar disorder, the use of tricyclics or monoamine oxidase inhibitors (MAOIs) may be appropriate. “MAOIs account for less than 1 in every 1,000 prescriptions for antidepressants, yet for people who don’t respond to modern antidepressants, they still can carry a 30%-40% response rate. So, if you don’t prescribe them yourself, please get access to someone who does,” Dr. Thase said.
The tenets of measurement-based care are essentially the lessons learned from the landmark Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial, which sought to address how pharmacologic care for depression could be delivered based on adequate dosing, attenuation of symptoms, fewer side effects, and other factors (Am J Psychiatry. 2006;163:1905-17).
Now,10 years later, measurement-based treatment is still finding its way into practice, Dr. Thase said in an interview. “But, there’s no reason to be unduly pessimistic. Ten years ago, depression screening was in the same position, and now it is both considered to be the standard of care and is widely done.”
Dr. Thase reported having extensive industry relationships, noting that he has been involved in the development of nearly every drug for the treatment of mood disorders. Global Academy and this news organization are owned by the same company.
On Twitter @whitneymcknight
WASHINGTON – Challenging patients with depression to stay engaged in their recovery while rigorously monitoring and measuring their treatment response can mean the difference between remission or resistance, according to an expert.
“We all wish for better treatments, but we really don’t have any coming available to us in the near future, so our task is to do as well as we can with what we have,” Michael E. Thase, MD, professor of psychiatry at the University of Pennsylvania, Philadelphia, said at Summit in Neurology & Psychiatry.
“We know far too well that there is a large gap between what we might be able to accomplish and what actually happens in clinical practice,” Dr. Thase said.
To close that gap, start with evaluating what can be done to combat patient nonadherence. A 2007 study indicated that just under 20% of patients adhere to antidepressant medication guidelines (Prim Care Companion J Clin Psychiatry. 2007;9[2]:91-9.), and according to Dr. Thase, less than 10% of patients being seen for depression in primary care actually fill their prescriptions.
“Don’t delude yourself that these patients drop out of treatment because they no longer need it. They drop out mostly because they have gotten disgusted, disappointed, or discouraged,” said Dr. Thase, attributing the high rate of nonadherence to the nature of depression itself.
“Depression is a state of pessimism, low confidence, and of not really feeling capable.”
Helping patients overcome their reluctance to follow treatment guidelines begins with accurately assessing their symptom severity. Patients who rate 4 or less on the Patient Health Questionnaire–9 (PHQ-9), which is based on the DSM-IV, are considered in remission or not to have severe depression. Similarly, patients who score 5 or less on the Quick Inventory of Depressive Symptomatology (QIDS) are not considered in the danger zone.
The severity ratings included in the DSM-5 can help clinicians pinpoint the level of depression a patient is experiencing, as can a therapeutic relationship with the patient and the family, Dr. Thase said at the summit, held by Global Academy for Medical Education.
He urged clinicians to monitor adherence routinely by asking patients whether or not they have filled or refilled their prescriptions. It is helpful to ask patients about missing doses and whether they are feeling any improvement, he said.
“It’s a very simple process: You keep track of the patients. Chase after them if they don’t follow through. If you’re not keeping track, the patients think [what they do] doesn’t matter,” Dr. Thase said.
A 2010 study of chronic depression treatment in primary care practices showed that compared with regular care, aggressive monitoring of patient adherence and outcomes in 728 adults with depression resulted in better remission rates across 18 months of treatment. At 6 months 43.4% of patients who had been contacted regularly by nursing and social worker staff were in remission, compared with 33.3% of the 78 who had received regular care (P = .11). At 12 months, the results were 52% vs. 33.9% (P = .012), and at 18 months remission was reported in 49.2% vs. 27.3% (P = .004) (Ann Fam Med. 2010 Sep;8[5]:387-96).
By being alert to side effects, using a rating scale to measure symptom reduction, and not staying “locked” into the idea that all treatments require 6-8 weeks before patients see any improvement, clinicians can boost chances for adherence – and thus for remission – since patients who don’t improve in the first 2 weeks have just a 15% chance for improvement, Dr. Thase said. “You need 6-8 weeks to get to the maximum titration. That old 8-week rule applies mostly to patients who are showing improvement by week 2.”
If by week 2, the patient is not tolerating the medication’s side effects and has no symptom reduction, then move on to the next medication, Dr. Thase said, emphasizing the importance of seeing patients at least twice monthly. “If we’re only seeing a patient once a month, we’ve already fallen behind in the up-titration part, and we’ve missed the opportunity for early intervention if the patient is having side effects.”
Algorithms for choosing depression treatments should be a “fact of life,” he said. Start with the safest, easiest to tolerate, and least expensive of the available antidepressants, such as escitalopram and fluoxetine before working through the second- and third-line therapies and adjunctive therapies, all of which have greater risk profiles as you move through them. This algorithmic approach has been shown to result in patients scoring at least 10 points higher on a depression rating scale (JAMA Psychiatry. 2004;61:[7]669-80), which Dr. Thase said is equivalent to about a 30% higher chance of remission. “Don’t go over and over with the simple treatments that aren’t [evoking] a response. Move briskly on to the more complex treatments until patients respond.”
Treatment algorithms can include psychotherapies, either in combination with pharmacotherapy or as monotherapy. He encouraged clinicians to refer to the American Psychiatric Association (APA) practice guideline for depression for more details. The absence of depressive symptoms alone is not indicative of remission, according to the guideline, which Dr. Thase coauthored. The presence of positive emotions and resilience, along with a sense of control over emotions and hope for the future, indicates remission.
If after applying those methods a patient remains depressed and has been negatively screened for bipolar disorder, the use of tricyclics or monoamine oxidase inhibitors (MAOIs) may be appropriate. “MAOIs account for less than 1 in every 1,000 prescriptions for antidepressants, yet for people who don’t respond to modern antidepressants, they still can carry a 30%-40% response rate. So, if you don’t prescribe them yourself, please get access to someone who does,” Dr. Thase said.
The tenets of measurement-based care are essentially the lessons learned from the landmark Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial, which sought to address how pharmacologic care for depression could be delivered based on adequate dosing, attenuation of symptoms, fewer side effects, and other factors (Am J Psychiatry. 2006;163:1905-17).
Now,10 years later, measurement-based treatment is still finding its way into practice, Dr. Thase said in an interview. “But, there’s no reason to be unduly pessimistic. Ten years ago, depression screening was in the same position, and now it is both considered to be the standard of care and is widely done.”
Dr. Thase reported having extensive industry relationships, noting that he has been involved in the development of nearly every drug for the treatment of mood disorders. Global Academy and this news organization are owned by the same company.
On Twitter @whitneymcknight
EXPERT ANALYSIS FROM SUMMIT OF NEUROLOGY & PSYCHIATRY
Zika Understanding Unfolds
Inundating our popular and academic media circles is information regarding the Zika virus. A recent article by Farahnik et al in the Journal of the American Academy of Dermatology (2016;74:1286-1287) briefly outlines what is known about Zika infection thus far and its dermatologic manifestations. Pairing this article with Centers for Disease Control and Prevention guidelines on the topic, we are presented with an evolving introduction to this new entity. Here’s what we know:
- It is a single-stranded RNA arbovirus in the Flavivirus family transmitted by the bite of Aedes mosquitoes, with cases reported so far in Africa, Asia, and the Americas (particularly southern coastal and island destinations).
- It also is transmitted via transfusion of blood, sexual contact, and mother to fetus.
- There is theoretical risk for fetal microcephaly, intracranial calcifications, and other brain and eye abnormalities.
- Only 1 in 5 affected patients show any systemic manifestations of infection, including self-limited flulike symptoms and nonspecific exanthema, typically sparing acral sites and occurring within 1 to 2 weeks of virus exposure.
- Testing is recommended for pregnant women with possible Zika exposure (ie, travel to an area with active transmission of Zika virus, unprotected sex with a male with this travel history).
- Diagnosis can be made through state health departments, employing real-time reverse transcriptase–polymerase chain reaction (rRT-PCR) or enzyme-linked immunosorbent assay the week after symptom onset using serum, or rRT-PCR 2 weeks after symptom onset using urine. Further antibody testing can be done if a false-negative is suspected, but false-positives also are possible if a patient was exposed to or vaccinated against other flaviviruses (eg, dengue virus, West Nile virus, yellow fever virus)
- Testing is inaccurate if ordered within 7 days or more than 12 weeks following presumed exposure.
- If positive or inconclusive testing arises, serial fetal ultrasonography should be considered; if testing is negative, then a single fetal ultrasound is recommended to detect Zika abnormalities.
- Test results are automatically reported to respective state health departments.
- There is no treatment of this infection aside from supportive care.
What’s the issue?
As with any new outbreak, the applicability to the general population and true risks remain to be seen. Each of our clinics recalls the stark changes in patient intake and screening questions with infections as ubiquitous as methicillin-resistant Staphylococcus aureus to much rarer exposures such as Ebola virus, each with progressive understanding of risk groups, disease manifestations, and eradication and prevention measures.
By mid-June 2016, 30 hits on PubMed addressing Zika had already been cited just within the month, outlining various aspects of the infection, and many specialties, particularly neurology, obstetrics, primary care, infectious disease, and dermatology, are weighing in. Unfortunately, the majority of cases of primary Zika infection do not manifest with skin or systemic symptoms, and even cases that do are nonspecific, exanthematous, and flulike.
Vague as it may be so far, it is nonetheless imperative that clinicians be familiar with what is concretely known about Zika virus and acquaint ourselves with the travel distribution and restrictions, disease risk factors, known sequelae, testing availability and limitations, and reporting guidelines. From personal experience, as I traveled to Belize earlier this year during my first trimester of pregnancy before the travel restrictions were outlined, even obstetricians are not wholly familiar with the manner in which to order testing and the appropriate window to do so. I have been asymptomatic, my blood was drawn in a period of time that exceeded the interval for accurate results (as outlined above) and was therefore inappropriately recommended/ordered, and now serial fetal ultrasonography is being implemented every few weeks.
With lack of ubiquitous knowledge about the infection, clinicians are not universally certain of the appropriate next steps when a patient presents with Zika risk factors, and therefore anxiety remains high for pregnant patients and their contacts. The Centers for Disease Control and Prevention website is the official home base, and we should review it and await their further evolving specific recommendations as more cases unfortunately accumulate.
Have you encountered any patients this year with exposure to or symptoms of Zika infection, and what, if anything, have you outlined for them?
Inundating our popular and academic media circles is information regarding the Zika virus. A recent article by Farahnik et al in the Journal of the American Academy of Dermatology (2016;74:1286-1287) briefly outlines what is known about Zika infection thus far and its dermatologic manifestations. Pairing this article with Centers for Disease Control and Prevention guidelines on the topic, we are presented with an evolving introduction to this new entity. Here’s what we know:
- It is a single-stranded RNA arbovirus in the Flavivirus family transmitted by the bite of Aedes mosquitoes, with cases reported so far in Africa, Asia, and the Americas (particularly southern coastal and island destinations).
- It also is transmitted via transfusion of blood, sexual contact, and mother to fetus.
- There is theoretical risk for fetal microcephaly, intracranial calcifications, and other brain and eye abnormalities.
- Only 1 in 5 affected patients show any systemic manifestations of infection, including self-limited flulike symptoms and nonspecific exanthema, typically sparing acral sites and occurring within 1 to 2 weeks of virus exposure.
- Testing is recommended for pregnant women with possible Zika exposure (ie, travel to an area with active transmission of Zika virus, unprotected sex with a male with this travel history).
- Diagnosis can be made through state health departments, employing real-time reverse transcriptase–polymerase chain reaction (rRT-PCR) or enzyme-linked immunosorbent assay the week after symptom onset using serum, or rRT-PCR 2 weeks after symptom onset using urine. Further antibody testing can be done if a false-negative is suspected, but false-positives also are possible if a patient was exposed to or vaccinated against other flaviviruses (eg, dengue virus, West Nile virus, yellow fever virus)
- Testing is inaccurate if ordered within 7 days or more than 12 weeks following presumed exposure.
- If positive or inconclusive testing arises, serial fetal ultrasonography should be considered; if testing is negative, then a single fetal ultrasound is recommended to detect Zika abnormalities.
- Test results are automatically reported to respective state health departments.
- There is no treatment of this infection aside from supportive care.
What’s the issue?
As with any new outbreak, the applicability to the general population and true risks remain to be seen. Each of our clinics recalls the stark changes in patient intake and screening questions with infections as ubiquitous as methicillin-resistant Staphylococcus aureus to much rarer exposures such as Ebola virus, each with progressive understanding of risk groups, disease manifestations, and eradication and prevention measures.
By mid-June 2016, 30 hits on PubMed addressing Zika had already been cited just within the month, outlining various aspects of the infection, and many specialties, particularly neurology, obstetrics, primary care, infectious disease, and dermatology, are weighing in. Unfortunately, the majority of cases of primary Zika infection do not manifest with skin or systemic symptoms, and even cases that do are nonspecific, exanthematous, and flulike.
Vague as it may be so far, it is nonetheless imperative that clinicians be familiar with what is concretely known about Zika virus and acquaint ourselves with the travel distribution and restrictions, disease risk factors, known sequelae, testing availability and limitations, and reporting guidelines. From personal experience, as I traveled to Belize earlier this year during my first trimester of pregnancy before the travel restrictions were outlined, even obstetricians are not wholly familiar with the manner in which to order testing and the appropriate window to do so. I have been asymptomatic, my blood was drawn in a period of time that exceeded the interval for accurate results (as outlined above) and was therefore inappropriately recommended/ordered, and now serial fetal ultrasonography is being implemented every few weeks.
With lack of ubiquitous knowledge about the infection, clinicians are not universally certain of the appropriate next steps when a patient presents with Zika risk factors, and therefore anxiety remains high for pregnant patients and their contacts. The Centers for Disease Control and Prevention website is the official home base, and we should review it and await their further evolving specific recommendations as more cases unfortunately accumulate.
Have you encountered any patients this year with exposure to or symptoms of Zika infection, and what, if anything, have you outlined for them?
Inundating our popular and academic media circles is information regarding the Zika virus. A recent article by Farahnik et al in the Journal of the American Academy of Dermatology (2016;74:1286-1287) briefly outlines what is known about Zika infection thus far and its dermatologic manifestations. Pairing this article with Centers for Disease Control and Prevention guidelines on the topic, we are presented with an evolving introduction to this new entity. Here’s what we know:
- It is a single-stranded RNA arbovirus in the Flavivirus family transmitted by the bite of Aedes mosquitoes, with cases reported so far in Africa, Asia, and the Americas (particularly southern coastal and island destinations).
- It also is transmitted via transfusion of blood, sexual contact, and mother to fetus.
- There is theoretical risk for fetal microcephaly, intracranial calcifications, and other brain and eye abnormalities.
- Only 1 in 5 affected patients show any systemic manifestations of infection, including self-limited flulike symptoms and nonspecific exanthema, typically sparing acral sites and occurring within 1 to 2 weeks of virus exposure.
- Testing is recommended for pregnant women with possible Zika exposure (ie, travel to an area with active transmission of Zika virus, unprotected sex with a male with this travel history).
- Diagnosis can be made through state health departments, employing real-time reverse transcriptase–polymerase chain reaction (rRT-PCR) or enzyme-linked immunosorbent assay the week after symptom onset using serum, or rRT-PCR 2 weeks after symptom onset using urine. Further antibody testing can be done if a false-negative is suspected, but false-positives also are possible if a patient was exposed to or vaccinated against other flaviviruses (eg, dengue virus, West Nile virus, yellow fever virus)
- Testing is inaccurate if ordered within 7 days or more than 12 weeks following presumed exposure.
- If positive or inconclusive testing arises, serial fetal ultrasonography should be considered; if testing is negative, then a single fetal ultrasound is recommended to detect Zika abnormalities.
- Test results are automatically reported to respective state health departments.
- There is no treatment of this infection aside from supportive care.
What’s the issue?
As with any new outbreak, the applicability to the general population and true risks remain to be seen. Each of our clinics recalls the stark changes in patient intake and screening questions with infections as ubiquitous as methicillin-resistant Staphylococcus aureus to much rarer exposures such as Ebola virus, each with progressive understanding of risk groups, disease manifestations, and eradication and prevention measures.
By mid-June 2016, 30 hits on PubMed addressing Zika had already been cited just within the month, outlining various aspects of the infection, and many specialties, particularly neurology, obstetrics, primary care, infectious disease, and dermatology, are weighing in. Unfortunately, the majority of cases of primary Zika infection do not manifest with skin or systemic symptoms, and even cases that do are nonspecific, exanthematous, and flulike.
Vague as it may be so far, it is nonetheless imperative that clinicians be familiar with what is concretely known about Zika virus and acquaint ourselves with the travel distribution and restrictions, disease risk factors, known sequelae, testing availability and limitations, and reporting guidelines. From personal experience, as I traveled to Belize earlier this year during my first trimester of pregnancy before the travel restrictions were outlined, even obstetricians are not wholly familiar with the manner in which to order testing and the appropriate window to do so. I have been asymptomatic, my blood was drawn in a period of time that exceeded the interval for accurate results (as outlined above) and was therefore inappropriately recommended/ordered, and now serial fetal ultrasonography is being implemented every few weeks.
With lack of ubiquitous knowledge about the infection, clinicians are not universally certain of the appropriate next steps when a patient presents with Zika risk factors, and therefore anxiety remains high for pregnant patients and their contacts. The Centers for Disease Control and Prevention website is the official home base, and we should review it and await their further evolving specific recommendations as more cases unfortunately accumulate.
Have you encountered any patients this year with exposure to or symptoms of Zika infection, and what, if anything, have you outlined for them?
Stop using rectal misoprostol for the treatment of postpartum hemorrhage caused by uterine atony
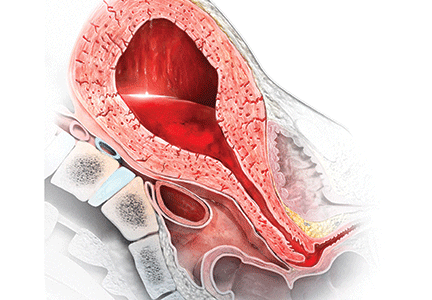
Most authorities recommend that, following delivery, all women should receive a uterotonic medication to reduce the risk of postpartum hemorrhage (PPH).1 In the United States, the preferred uterotonic for this preventive effort is oxytocin—a low-cost, highly effective agent that typically is administered as an intravenous (IV) infusion or intramuscular (IM) injection. Unfortunately, even with the universal administration of oxytocin in the third stage of labor, PPH occurs in about 3% of vaginal deliveries.
A key decision in treating a PPH due to uterine atony is treatment with an optimal uterotonic. The options include:
- additional oxytocin
- carboprost tromethamine(Hemabate)
- methylergonovine (Methergine)
- misoprostol.
Many obstetricians choose rectal misoprostol alone or in combination with oxytocin as the preferred treatment of PPH. However, evidence from clinical trials and pharmacokinetic studies suggest that rectal misoprostol is not an optimal choice if parenteral uterotonics are available. Here I pre-sent this evidence and urge you to stop the practice of using rectal misoprostol in efforts to manage PPH.
RCTs do not support the use of rectal misoprostolRandomized clinical trials (RCTs) have not demonstrated that misoprostol is superior to oxytocin for the treatment of PPH caused by uterine atony.2 For example, Blum and colleagues studied 31,055 women who received oxytocin (by IV or IM route) at vaginal delivery and observed that 809 (3%) developed a PPH.3 The women who developed PPH were randomly assigned to treatment with misoprostol 800 µg sublingual or oxytocin 40 U in 1,000 mL as an IV infusion over 15 minutes.
Both oxytocin and misoprostol had similar efficacy for controlling bleeding within 20 minutes (90% and 89%, respectively). Fewer women had blood loss of 1,000 mL or greater when treated with oxytocin compared with misoprostol (1% vs 3%, respectively; P = .062). In addition, oxytocin was associated with fewer temperature elevations of 38°C (100.4°F) or above (15% vs 22% for misoprostol, P = .007) and fewer temperature elevations of 40°C (104°F) or above (0.2% vs 1.2% for misoprostol, P = .11).
In another trial, women with a vaginal delivery who were not treated with a uterotonic in the third stage were monitored for the development of a PPH.4 PPH did develop in 1,422 women, who were then randomly assigned to receive oxytocin (10 U IV or IM) plus a placebo tablet or oxytocin plus misoprostol (600 µg sublingual).
Comparing oxytocin alone versus oxytocin plus misoprostol, there was no difference in blood loss of 500 mL or greater after treatment initiation (14% vs 14%). However, 90 minutes following treatment, temperature elevations occurred much more often in the women who received oxytocin plus misoprostol compared with the women who received oxytocin alone (temperature ≥38°C: 58% vs 19%; temperature ≥40°C: 6.8% vs 0.4%).
Bottom line: If you have access to oxytocin, there is no advantage to using misoprostol to treat a PPH due to uterine atony.5
Rectal misoprostol does not achieve optimal circulating concentrations of the drugMisoprostol tablets are formulated for oral administration, not rectal administration. The studies in the TABLE show that rectal administration of misoprostol results in lower circulating concentration of the medication compared to oral, buccal, or vaginal administration.6−8 After rectal administration it takes about 60 minutes to reach the peak circulating concentration of misoprostol.6,7 By contrast, parenteral oxytocin, methylergonovine, and carboprost tromethamine reach peak serum concentration much more quickly after administration.

In a study of misoprostol stimulation of uterine contractility as measured by an intrauterine pressure catheter, buccal administration resulted in higher peak uterine tone than rectal administration (49 vs 31 mm Hg).8 In addition, time to onset of uterine contractility was 41 minutes and 103 minutes, respectively, for buccal and rectal administration.
These studies show that rectal misoprostol is associated with lower serum concentrations, longer time to onset of uterine contraction, and less contractility than buccal administration. The one advantage of rectal administration is that it has a longer duration of action than the oral, buccal, or sublingual routes. In pharmacokinetic comparisons of buccal versus sublingual administration of misoprostol, the sublingual route results in greater peak concentration, which may cause more adverse effects.9,10
Misoprostol is a useful uterotonic if parenteral agents are not available
Worldwide, approximately one maternal death occurs every 7 minutes. Postpartum hemorrhage (PPH) is a common cause of maternal death. Oxytocin, methylergonovine, and carboprost tromethamine should be stored in a refrigerated environment to ensure the stability and bioavailability of the drug. In settings in which reliable refrigeration is not available, misoprostol, a medication that is heat-stable, is often used to prevent and treat PPH.
One approach to preventing PPH is to provide 600 µg of misoprostol to women delivering at home without a skilled birth attendant that they can self- administer after the delivery.1,2 Another approach is to recommend that skilled birth attendants administer misoprostol following the delivery.3
Although I am recommending that we not use rectal misoprostol to treat PPH in the United States, it is clear that misoprostol plays an important role in preventing PPH in countries where parenteral uterotonics are not available. If a clinician in the United States was involved in a home birth complicated by PPH due to uterine atony, and if misoprostol was the only available uterotonic, it would be wise to administer it promptly.
References
- Rajbhandari S, Hodgins S, Sanghvi H, McPherson R, Pradhan YV, Baqui AH; Misoprostol Study Group. Expanding uterotonic protection following childbirth through community-based distribution of misoprostol: operations research study in Nepal. Int J Gynaecol Obstet. 2010;108(3):282–288.
- Sanghvi H, Ansari N, Prata NJ, Gibson H, Ehsan AT, Smith JM. Prevention of postpartum hemorrhage at home birth in Afghanistan. Int J Gynaecol Obstet. 2010;108(3):276–281.
- Prata N, Mbaruku G, Campbell M, Potts M, Vahidnia F. Controlling postpartum hemorrhage after home births in Tanzania. Int J Gynaecol Obstet. 2005;90(1):51–55.
Prioritize oxytocin, methergine, and carboprost tromethamineWhen treating PPH, administration of oxytocin, methylergonovine, or carboprost tromethamine rapidly provides therapeutic concentration of medication. For oxytocin, 40 U in 1 L, administered at a rate sufficient to control atony, or 10 U IM injection are often effective in controlling bleeding due to atony. Carboprost tromethamine 0.25 mg administered intramuscularly every 15 minutes up to 8 doses provides an excellent second-line agent. Carboprost tromethamine is contraindicated for women with asthma.
Methylergonovine 0.2 mg administered intramuscularly only can be given every 2 to 4 hours. Consequently, because time is of the essence in managing a severe PPH, it is unusual to be able to administer more than one dose of the agent during the course of treatment. Methylergonovine is contraindicated for women with hypertension.
There is scant evidence that misoprostol is more effective than oxytocin, and misoprostol clearly causes a higher rate of elevated temperature than any of the parenteral uterotonic agents. In your practice stop using rectal misoprostol for the treatment of PPH caused by uterine atony, and prioritize the use of parenteral uterotonics.

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Westhoff G, Cotter AM, Tolosa JE. Prophylactic oxytocin for the third stage of labour to prevent postpartum hemorrhage. Cochrane Database Syst Rev. 2013;(10):CD001808.
- Gibbons KJ, Albright CM, Rouse DJ. Postpartum hemorrhage in the developed world: whither misoprostol? Am J Obstet Gynecol. 2013;208(3):181−183.
- Blum J, Winikoff B, Raghavan S, et al. Treatment of postpartum hemorrhage with sublingual misoprostol versus oxytocin in women receiving prophylactic oxytocin: a double-blind, randomised, non-inferiority trial. Lancet. 2010;375(9710):217−223.
- Widmer M, Blum J, Hofmeyr GJ, et al. Misoprostolas an adjunct to standard uterotonics for treatment of post-partum haemorrhage: a multicentre, double-blind randomised trial. Lancet. 2010;375(9728):1808−1813.
- Weeks A. The prevention and treatment of postpartum hemorrhage: what do we know and where do we go to next? BJOG. 2015;122(2):202−210.
- Khan RU, El-Refaey H. Pharmacokinetics and adverse-effect profile of rectally administered misoprostol in the third stage of labor. Obstet Gynecol. 2003;101(5 pt 1):968−974.
- Khan RU, El-Refaey H, Sharma S, Sooranna D, Stafford M. Oral, rectal and vaginal pharmacokinetics of misoprostol. Obstet Gynecol. 2004;103(5 pt 1):866−870.
- Meckstroth KR, Whitaker AK, Bertisch S, Goldberg AB, Darney PD. Misoprostol administered by epithelial routes: drug absorption and uterine response. Obstet Gynecol. 2006;108(3 pt 1):582−590.
- Schaff EA, DiCenzo R, Fielding SL. Comparison of misoprostol plasma concentrations following buccal and sublingual administration. Contraception. 2005;71(1):22−25.
- Frye LJ, Byrne ME, Winikoff B. A crossover pharmacokinetic study of misoprostol by the oral, sublingual and buccal routes [published online ahead of print April 22, 2016]. Eur J Contracept Reprod Health Care. doi:10.3109/13625187.2016.1168799.

Most authorities recommend that, following delivery, all women should receive a uterotonic medication to reduce the risk of postpartum hemorrhage (PPH).1 In the United States, the preferred uterotonic for this preventive effort is oxytocin—a low-cost, highly effective agent that typically is administered as an intravenous (IV) infusion or intramuscular (IM) injection. Unfortunately, even with the universal administration of oxytocin in the third stage of labor, PPH occurs in about 3% of vaginal deliveries.
A key decision in treating a PPH due to uterine atony is treatment with an optimal uterotonic. The options include:
- additional oxytocin
- carboprost tromethamine(Hemabate)
- methylergonovine (Methergine)
- misoprostol.
Many obstetricians choose rectal misoprostol alone or in combination with oxytocin as the preferred treatment of PPH. However, evidence from clinical trials and pharmacokinetic studies suggest that rectal misoprostol is not an optimal choice if parenteral uterotonics are available. Here I pre-sent this evidence and urge you to stop the practice of using rectal misoprostol in efforts to manage PPH.
RCTs do not support the use of rectal misoprostolRandomized clinical trials (RCTs) have not demonstrated that misoprostol is superior to oxytocin for the treatment of PPH caused by uterine atony.2 For example, Blum and colleagues studied 31,055 women who received oxytocin (by IV or IM route) at vaginal delivery and observed that 809 (3%) developed a PPH.3 The women who developed PPH were randomly assigned to treatment with misoprostol 800 µg sublingual or oxytocin 40 U in 1,000 mL as an IV infusion over 15 minutes.
Both oxytocin and misoprostol had similar efficacy for controlling bleeding within 20 minutes (90% and 89%, respectively). Fewer women had blood loss of 1,000 mL or greater when treated with oxytocin compared with misoprostol (1% vs 3%, respectively; P = .062). In addition, oxytocin was associated with fewer temperature elevations of 38°C (100.4°F) or above (15% vs 22% for misoprostol, P = .007) and fewer temperature elevations of 40°C (104°F) or above (0.2% vs 1.2% for misoprostol, P = .11).
In another trial, women with a vaginal delivery who were not treated with a uterotonic in the third stage were monitored for the development of a PPH.4 PPH did develop in 1,422 women, who were then randomly assigned to receive oxytocin (10 U IV or IM) plus a placebo tablet or oxytocin plus misoprostol (600 µg sublingual).
Comparing oxytocin alone versus oxytocin plus misoprostol, there was no difference in blood loss of 500 mL or greater after treatment initiation (14% vs 14%). However, 90 minutes following treatment, temperature elevations occurred much more often in the women who received oxytocin plus misoprostol compared with the women who received oxytocin alone (temperature ≥38°C: 58% vs 19%; temperature ≥40°C: 6.8% vs 0.4%).
Bottom line: If you have access to oxytocin, there is no advantage to using misoprostol to treat a PPH due to uterine atony.5
Rectal misoprostol does not achieve optimal circulating concentrations of the drugMisoprostol tablets are formulated for oral administration, not rectal administration. The studies in the TABLE show that rectal administration of misoprostol results in lower circulating concentration of the medication compared to oral, buccal, or vaginal administration.6−8 After rectal administration it takes about 60 minutes to reach the peak circulating concentration of misoprostol.6,7 By contrast, parenteral oxytocin, methylergonovine, and carboprost tromethamine reach peak serum concentration much more quickly after administration.

In a study of misoprostol stimulation of uterine contractility as measured by an intrauterine pressure catheter, buccal administration resulted in higher peak uterine tone than rectal administration (49 vs 31 mm Hg).8 In addition, time to onset of uterine contractility was 41 minutes and 103 minutes, respectively, for buccal and rectal administration.
These studies show that rectal misoprostol is associated with lower serum concentrations, longer time to onset of uterine contraction, and less contractility than buccal administration. The one advantage of rectal administration is that it has a longer duration of action than the oral, buccal, or sublingual routes. In pharmacokinetic comparisons of buccal versus sublingual administration of misoprostol, the sublingual route results in greater peak concentration, which may cause more adverse effects.9,10
Misoprostol is a useful uterotonic if parenteral agents are not available
Worldwide, approximately one maternal death occurs every 7 minutes. Postpartum hemorrhage (PPH) is a common cause of maternal death. Oxytocin, methylergonovine, and carboprost tromethamine should be stored in a refrigerated environment to ensure the stability and bioavailability of the drug. In settings in which reliable refrigeration is not available, misoprostol, a medication that is heat-stable, is often used to prevent and treat PPH.
One approach to preventing PPH is to provide 600 µg of misoprostol to women delivering at home without a skilled birth attendant that they can self- administer after the delivery.1,2 Another approach is to recommend that skilled birth attendants administer misoprostol following the delivery.3
Although I am recommending that we not use rectal misoprostol to treat PPH in the United States, it is clear that misoprostol plays an important role in preventing PPH in countries where parenteral uterotonics are not available. If a clinician in the United States was involved in a home birth complicated by PPH due to uterine atony, and if misoprostol was the only available uterotonic, it would be wise to administer it promptly.
References
- Rajbhandari S, Hodgins S, Sanghvi H, McPherson R, Pradhan YV, Baqui AH; Misoprostol Study Group. Expanding uterotonic protection following childbirth through community-based distribution of misoprostol: operations research study in Nepal. Int J Gynaecol Obstet. 2010;108(3):282–288.
- Sanghvi H, Ansari N, Prata NJ, Gibson H, Ehsan AT, Smith JM. Prevention of postpartum hemorrhage at home birth in Afghanistan. Int J Gynaecol Obstet. 2010;108(3):276–281.
- Prata N, Mbaruku G, Campbell M, Potts M, Vahidnia F. Controlling postpartum hemorrhage after home births in Tanzania. Int J Gynaecol Obstet. 2005;90(1):51–55.
Prioritize oxytocin, methergine, and carboprost tromethamineWhen treating PPH, administration of oxytocin, methylergonovine, or carboprost tromethamine rapidly provides therapeutic concentration of medication. For oxytocin, 40 U in 1 L, administered at a rate sufficient to control atony, or 10 U IM injection are often effective in controlling bleeding due to atony. Carboprost tromethamine 0.25 mg administered intramuscularly every 15 minutes up to 8 doses provides an excellent second-line agent. Carboprost tromethamine is contraindicated for women with asthma.
Methylergonovine 0.2 mg administered intramuscularly only can be given every 2 to 4 hours. Consequently, because time is of the essence in managing a severe PPH, it is unusual to be able to administer more than one dose of the agent during the course of treatment. Methylergonovine is contraindicated for women with hypertension.
There is scant evidence that misoprostol is more effective than oxytocin, and misoprostol clearly causes a higher rate of elevated temperature than any of the parenteral uterotonic agents. In your practice stop using rectal misoprostol for the treatment of PPH caused by uterine atony, and prioritize the use of parenteral uterotonics.

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.

Most authorities recommend that, following delivery, all women should receive a uterotonic medication to reduce the risk of postpartum hemorrhage (PPH).1 In the United States, the preferred uterotonic for this preventive effort is oxytocin—a low-cost, highly effective agent that typically is administered as an intravenous (IV) infusion or intramuscular (IM) injection. Unfortunately, even with the universal administration of oxytocin in the third stage of labor, PPH occurs in about 3% of vaginal deliveries.
A key decision in treating a PPH due to uterine atony is treatment with an optimal uterotonic. The options include:
- additional oxytocin
- carboprost tromethamine(Hemabate)
- methylergonovine (Methergine)
- misoprostol.
Many obstetricians choose rectal misoprostol alone or in combination with oxytocin as the preferred treatment of PPH. However, evidence from clinical trials and pharmacokinetic studies suggest that rectal misoprostol is not an optimal choice if parenteral uterotonics are available. Here I pre-sent this evidence and urge you to stop the practice of using rectal misoprostol in efforts to manage PPH.
RCTs do not support the use of rectal misoprostolRandomized clinical trials (RCTs) have not demonstrated that misoprostol is superior to oxytocin for the treatment of PPH caused by uterine atony.2 For example, Blum and colleagues studied 31,055 women who received oxytocin (by IV or IM route) at vaginal delivery and observed that 809 (3%) developed a PPH.3 The women who developed PPH were randomly assigned to treatment with misoprostol 800 µg sublingual or oxytocin 40 U in 1,000 mL as an IV infusion over 15 minutes.
Both oxytocin and misoprostol had similar efficacy for controlling bleeding within 20 minutes (90% and 89%, respectively). Fewer women had blood loss of 1,000 mL or greater when treated with oxytocin compared with misoprostol (1% vs 3%, respectively; P = .062). In addition, oxytocin was associated with fewer temperature elevations of 38°C (100.4°F) or above (15% vs 22% for misoprostol, P = .007) and fewer temperature elevations of 40°C (104°F) or above (0.2% vs 1.2% for misoprostol, P = .11).
In another trial, women with a vaginal delivery who were not treated with a uterotonic in the third stage were monitored for the development of a PPH.4 PPH did develop in 1,422 women, who were then randomly assigned to receive oxytocin (10 U IV or IM) plus a placebo tablet or oxytocin plus misoprostol (600 µg sublingual).
Comparing oxytocin alone versus oxytocin plus misoprostol, there was no difference in blood loss of 500 mL or greater after treatment initiation (14% vs 14%). However, 90 minutes following treatment, temperature elevations occurred much more often in the women who received oxytocin plus misoprostol compared with the women who received oxytocin alone (temperature ≥38°C: 58% vs 19%; temperature ≥40°C: 6.8% vs 0.4%).
Bottom line: If you have access to oxytocin, there is no advantage to using misoprostol to treat a PPH due to uterine atony.5
Rectal misoprostol does not achieve optimal circulating concentrations of the drugMisoprostol tablets are formulated for oral administration, not rectal administration. The studies in the TABLE show that rectal administration of misoprostol results in lower circulating concentration of the medication compared to oral, buccal, or vaginal administration.6−8 After rectal administration it takes about 60 minutes to reach the peak circulating concentration of misoprostol.6,7 By contrast, parenteral oxytocin, methylergonovine, and carboprost tromethamine reach peak serum concentration much more quickly after administration.

In a study of misoprostol stimulation of uterine contractility as measured by an intrauterine pressure catheter, buccal administration resulted in higher peak uterine tone than rectal administration (49 vs 31 mm Hg).8 In addition, time to onset of uterine contractility was 41 minutes and 103 minutes, respectively, for buccal and rectal administration.
These studies show that rectal misoprostol is associated with lower serum concentrations, longer time to onset of uterine contraction, and less contractility than buccal administration. The one advantage of rectal administration is that it has a longer duration of action than the oral, buccal, or sublingual routes. In pharmacokinetic comparisons of buccal versus sublingual administration of misoprostol, the sublingual route results in greater peak concentration, which may cause more adverse effects.9,10
Misoprostol is a useful uterotonic if parenteral agents are not available
Worldwide, approximately one maternal death occurs every 7 minutes. Postpartum hemorrhage (PPH) is a common cause of maternal death. Oxytocin, methylergonovine, and carboprost tromethamine should be stored in a refrigerated environment to ensure the stability and bioavailability of the drug. In settings in which reliable refrigeration is not available, misoprostol, a medication that is heat-stable, is often used to prevent and treat PPH.
One approach to preventing PPH is to provide 600 µg of misoprostol to women delivering at home without a skilled birth attendant that they can self- administer after the delivery.1,2 Another approach is to recommend that skilled birth attendants administer misoprostol following the delivery.3
Although I am recommending that we not use rectal misoprostol to treat PPH in the United States, it is clear that misoprostol plays an important role in preventing PPH in countries where parenteral uterotonics are not available. If a clinician in the United States was involved in a home birth complicated by PPH due to uterine atony, and if misoprostol was the only available uterotonic, it would be wise to administer it promptly.
References
- Rajbhandari S, Hodgins S, Sanghvi H, McPherson R, Pradhan YV, Baqui AH; Misoprostol Study Group. Expanding uterotonic protection following childbirth through community-based distribution of misoprostol: operations research study in Nepal. Int J Gynaecol Obstet. 2010;108(3):282–288.
- Sanghvi H, Ansari N, Prata NJ, Gibson H, Ehsan AT, Smith JM. Prevention of postpartum hemorrhage at home birth in Afghanistan. Int J Gynaecol Obstet. 2010;108(3):276–281.
- Prata N, Mbaruku G, Campbell M, Potts M, Vahidnia F. Controlling postpartum hemorrhage after home births in Tanzania. Int J Gynaecol Obstet. 2005;90(1):51–55.
Prioritize oxytocin, methergine, and carboprost tromethamineWhen treating PPH, administration of oxytocin, methylergonovine, or carboprost tromethamine rapidly provides therapeutic concentration of medication. For oxytocin, 40 U in 1 L, administered at a rate sufficient to control atony, or 10 U IM injection are often effective in controlling bleeding due to atony. Carboprost tromethamine 0.25 mg administered intramuscularly every 15 minutes up to 8 doses provides an excellent second-line agent. Carboprost tromethamine is contraindicated for women with asthma.
Methylergonovine 0.2 mg administered intramuscularly only can be given every 2 to 4 hours. Consequently, because time is of the essence in managing a severe PPH, it is unusual to be able to administer more than one dose of the agent during the course of treatment. Methylergonovine is contraindicated for women with hypertension.
There is scant evidence that misoprostol is more effective than oxytocin, and misoprostol clearly causes a higher rate of elevated temperature than any of the parenteral uterotonic agents. In your practice stop using rectal misoprostol for the treatment of PPH caused by uterine atony, and prioritize the use of parenteral uterotonics.

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Westhoff G, Cotter AM, Tolosa JE. Prophylactic oxytocin for the third stage of labour to prevent postpartum hemorrhage. Cochrane Database Syst Rev. 2013;(10):CD001808.
- Gibbons KJ, Albright CM, Rouse DJ. Postpartum hemorrhage in the developed world: whither misoprostol? Am J Obstet Gynecol. 2013;208(3):181−183.
- Blum J, Winikoff B, Raghavan S, et al. Treatment of postpartum hemorrhage with sublingual misoprostol versus oxytocin in women receiving prophylactic oxytocin: a double-blind, randomised, non-inferiority trial. Lancet. 2010;375(9710):217−223.
- Widmer M, Blum J, Hofmeyr GJ, et al. Misoprostolas an adjunct to standard uterotonics for treatment of post-partum haemorrhage: a multicentre, double-blind randomised trial. Lancet. 2010;375(9728):1808−1813.
- Weeks A. The prevention and treatment of postpartum hemorrhage: what do we know and where do we go to next? BJOG. 2015;122(2):202−210.
- Khan RU, El-Refaey H. Pharmacokinetics and adverse-effect profile of rectally administered misoprostol in the third stage of labor. Obstet Gynecol. 2003;101(5 pt 1):968−974.
- Khan RU, El-Refaey H, Sharma S, Sooranna D, Stafford M. Oral, rectal and vaginal pharmacokinetics of misoprostol. Obstet Gynecol. 2004;103(5 pt 1):866−870.
- Meckstroth KR, Whitaker AK, Bertisch S, Goldberg AB, Darney PD. Misoprostol administered by epithelial routes: drug absorption and uterine response. Obstet Gynecol. 2006;108(3 pt 1):582−590.
- Schaff EA, DiCenzo R, Fielding SL. Comparison of misoprostol plasma concentrations following buccal and sublingual administration. Contraception. 2005;71(1):22−25.
- Frye LJ, Byrne ME, Winikoff B. A crossover pharmacokinetic study of misoprostol by the oral, sublingual and buccal routes [published online ahead of print April 22, 2016]. Eur J Contracept Reprod Health Care. doi:10.3109/13625187.2016.1168799.
- Westhoff G, Cotter AM, Tolosa JE. Prophylactic oxytocin for the third stage of labour to prevent postpartum hemorrhage. Cochrane Database Syst Rev. 2013;(10):CD001808.
- Gibbons KJ, Albright CM, Rouse DJ. Postpartum hemorrhage in the developed world: whither misoprostol? Am J Obstet Gynecol. 2013;208(3):181−183.
- Blum J, Winikoff B, Raghavan S, et al. Treatment of postpartum hemorrhage with sublingual misoprostol versus oxytocin in women receiving prophylactic oxytocin: a double-blind, randomised, non-inferiority trial. Lancet. 2010;375(9710):217−223.
- Widmer M, Blum J, Hofmeyr GJ, et al. Misoprostolas an adjunct to standard uterotonics for treatment of post-partum haemorrhage: a multicentre, double-blind randomised trial. Lancet. 2010;375(9728):1808−1813.
- Weeks A. The prevention and treatment of postpartum hemorrhage: what do we know and where do we go to next? BJOG. 2015;122(2):202−210.
- Khan RU, El-Refaey H. Pharmacokinetics and adverse-effect profile of rectally administered misoprostol in the third stage of labor. Obstet Gynecol. 2003;101(5 pt 1):968−974.
- Khan RU, El-Refaey H, Sharma S, Sooranna D, Stafford M. Oral, rectal and vaginal pharmacokinetics of misoprostol. Obstet Gynecol. 2004;103(5 pt 1):866−870.
- Meckstroth KR, Whitaker AK, Bertisch S, Goldberg AB, Darney PD. Misoprostol administered by epithelial routes: drug absorption and uterine response. Obstet Gynecol. 2006;108(3 pt 1):582−590.
- Schaff EA, DiCenzo R, Fielding SL. Comparison of misoprostol plasma concentrations following buccal and sublingual administration. Contraception. 2005;71(1):22−25.
- Frye LJ, Byrne ME, Winikoff B. A crossover pharmacokinetic study of misoprostol by the oral, sublingual and buccal routes [published online ahead of print April 22, 2016]. Eur J Contracept Reprod Health Care. doi:10.3109/13625187.2016.1168799.
2016 Update on menopause
In this Update, I discuss important new study results regarding the cardiovascular safety of hormone therapy (HT) in early menopausal women. In addition, I review survey data that reveal a huge number of US women are using compounded HT preparations, which have unproven efficacy and safety.
Earlier initiation is better: ELITE trial provides strong support for the estrogen timing hypothesis
Hodis HN, Mack WJ, Henderson VW, et al; for the ELITE Research Group. Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med. 2016;374(13):1221-1231.
Keaney JF, Solomon G. Postmenopausal hormone therapy and atherosclerosis--time is of the essence [editorial]. N Engl J Med. 2016;374(13):1279-1280.
A substantial amount of published data, including from the Women's Health Initiative (WHI), supports the timing hypothesis, which proposes that HT slows the progression of atherosclerosis among recently menopausal women but has a neutral or adverse effect among women who are a decade or more past menopause onset.1 To directly test this hypothesis, Hodis and colleagues randomly assigned healthy postmenopausal women (<6 years or ≥10 years past menopause) without cardiovascular disease (CVD) to oral estradiol 1 mg or placebo. Women with a uterus also were randomly assigned to receive either vaginal progesterone gel or placebo gel. The primary outcome was the rate of change in carotid artery intima-media thickness (CIMT), which was assessed at baseline and each 6 months of the study. (An earlier report had noted that baseline CIMT correlated well with CVD risk factors.2) Coronary artery atherosclerosis, a secondary outcome, was assessed at study completion using computed tomography (CT).
Details of the study
Among the 643 participants in the Early versus Late Intervention Trial with Estradiol (ELITE), the median years since menopause and the median age at enrollment were 3.5 and 55.4, respectively, in the early postmenopause group and 14.3 and 63.6, respectively, in the late postmenopause group.
Among the younger women, after a median of 5 years of study medications, the estradiol group had less progression of CIMT than the placebo group (P = .008). By contrast, in the older group, rates of CIMT progression were similar in the HT and placebo groups (P = .29). The relationship between estrogen and CIMT progression differed significantly between the younger and older groups (P = .007). Use of progesterone did not change these trends. Coronary artery CT parameters did not differ significantly between the placebo and HT groups in the age group or in the time-since-menopause group.
What this evidence means for practice
In an editorial accompanying the published results of the ELITE trial, Keaney and Solomon concluded that, although estrogen had a favorable effect on atherosclerosis in early menopause, it would be premature to recommend HT for prevention of cardiovascular events. I agree with them, but I also would like to note that the use of HT for the treatment of menopausal symptoms has plummeted since the initial WHI findings in 2002, with infrequent HT use even among symptomatic women in early menopause.3 (And I refer you to the special inset featuring JoAnn E. Manson, MD, DrPH) The takeaway message is that this important new clinical trial provides additional reassurance regarding the cardiovascular safety of HT when initiated by recently menopausal women to treat bothersome vasomotor symptoms. This message represents welcome news for women with bothersome menopausal symptoms considering use of HT.
A word about the vaginal progesterone gel used in the ELITE trial in relation to clinical practice: Given the need for vaginal placement of progesterone gel, potential messiness, and high cost, few clinicians may prescribe this formulation, and few women probably would choose to use it. As an alternative, micronized progesterone 100-mg capsules are less expensive and well accepted by most patients. These capsules are formulated with peanut oil. Because they may cause women to feel drowsy, the capsules should be taken at bedtime. In women with an intact uterus who are taking oral estradiol 1-mg tablets, one appropriate progestogen regimen for endometrial suppression is a 100-mg micronized progesterone capsule each night, continuously.
WHI, ELITE and the timing hypothesis:
New evidence on HT in early menopause is reassuring
Q&A with JoAnn E. Manson, MD, DrPH
In this interview, Dr. JoAnn Manson discusses the reassuring results of recent hormone therapy (HT) trials in early versus later postmenopausal women, examines these outcomes in the context of the Women's Health Initiative (WHI) trial and ELITE trial, and debunks an enduring common misconception about the WHI.
Q You have said for several years that there has been a misconception about the WHI trial. What is that misconception, and what has been its impact on clinicians, women, and the use of HT?
A The WHI HT trial has been largely misunderstood. It was designed to address the balance of benefits and risks of long-term HT for the prevention of chronic disease in postmenopausal women across a broad range of ages (average age 63).1,2 It was not intended to evaluate the clinical role of HT for managing menopausal symptoms in young and early menopausal women.3 Overall, the WHI study findings have been inappropriately extrapolated to women in their 40s and early 50s who report distressing hot flashes, night sweats, and other menopausal symptoms, and they are often used as a reason to deny therapy when in fact many of these women would be appropriate candidates for HT.
There is increasing evidence that younger women in early menopause who are taking HT have a lower risk of adverse outcomes and lower absolute risks of disease than older women.2,3 In younger, early menopausal women with bothersome hot flashes, night sweats, or other menopausal symptoms and who have no contraindications to HT, the benefits of treatment are likely to outweigh the risks, and these patients derive quality-of-life benefits from treatment.
Q How do the results of the recent ELITE (Early versus Late Intervention Trial with Estradiol) trial build on cardiovascular safety, in particular, of HT and when HT is optimally initiated?
A The ELITE trial directly tested the "timing hypothesis" and the role of HT in slowing the progression of atherosclerosis in early menopause (defined as within 6 years of menopause onset) compared with the effect in women in later menopause (defined as at least 10 yearspast menopause).4 The investigators used carotid artery intima-media thickness (CIMT) as a surrogate end point. In this trial, 643 women were randomly assigned according to whether they were in early or later menopause to receive either placebo or estradiol 1 mg daily; women with a uterus also received progesterone 45 mg as a 4% vaginal gel or matching placebo gel. The median duration of intervention was 5 years.
The ELITE study results provide support for the "critical window hypothesis" in that the estradiol-treated younger women closer to onset of menopause had slowing of atherosclerosis compared with the placebo group, while the older women more distant from menopause did not have slowing of atherosclerosis with estradiol.
The ELITE trial was not large enough, however, to assess clinical end points--rates of heart attack, stroke, or other cardiovascular events. So it remains unclear whether the findings for the surrogate end point of CIMT would translate into a reduced risk of clinical events in the younger women. Nevertheless, ELITE does provide more reassurance about the use of HT in early menopause and supports the possibility that the overall results of the WHI among women enrolled at an average age of 63 years may not apply directly to younger women in early menopause.
Q What impact on clinical practice do you anticipate as a result of the ELITE trial results?
A The findings provide further support for the timing hypothesis and offer additional reassurance regarding the safety of HT in early menopause for management of menopausal symptoms. However, the trial does not provide conclusive evidence to support recommendations to use HT for the express purpose of preventing cardiovascular disease (CVD), even if HT is started in early menopause. Using a surrogate end point for atherosclerosis (CIMT) is not the same as looking at clinical events. There are many biologic pathways for heart attacks, strokes, and other cardiovascular events. In addition to atherosclerosis, for example, there is thrombosis, clotting, thrombo-occlusion within a blood vessel, and plaque rupture. Again, we do not know whether the CIMT-based results would translate directly into a reduction in clinical heart attacks and stroke.
The main takeaway point from the ELITE trial results is further reassurance for use of HT for management of menopausal symptoms in early menopause, but not for long-term chronic disease prevention at any age.
Q Another recent study, published in the Journal of Clinical Endocrinology and Metabolism, addresses HT and the timing hypothesis but in this instance relating to glucose tolerance.5 What did these study authors find?
A This study by Pereira and colleagues is very interesting and suggests that the window of opportunity for initiating estrogen therapy may apply not only to coronary events but also to glucose tolerance, insulin sensitivity, and diabetes risk.5
The authors investigated the effects of short-term high-dose transdermal estradiol on the insulin-mediated glucose disposal rate (GDR), which is a measure of insulin-stimulated glucose uptake. Participants in this randomized, crossover, placebo-controlled study included 22 women who were in early menopause (6 years or less since final menses) and 24 women who were in later menopause (10 years or longer since final menses). All of the women were naïve to hormone therapy, and baseline GDR did not differ between groups. After 1 week of treatment with transdermal estradiol (a high dose of 150 μg) or placebo, the participants' GDR was measured via a hyperinsulinemic-euglycemic clamp.
The investigators found that in the younger women, estradiol had a favorable effect on insulin sensitivity and GDR, whereas in the older women, there was no evidence of a favorable effect and, in fact, there was a signal for risk and more adverse findings in this group.
Several studies in the WHI also looked at glucose tolerance and at the risk of being diagnosed with diabetes. While the results of the WHI estrogen-alone trial revealed a reduction in diabetes and favorable effects across age groups, in the WHI estrogen-plus-progestin trial we did see a signal that the results for diabetes may have been more favorable in the younger than in the older women, somewhat consistent with the findings of Pereira and colleagues.2,5
Overall this issue requires more research, but the Pereira study provides further support for the possibility that estrogen's metabolic effects may vary by age and time since menopause, and there is evidence that the estrogen receptors may be more functional and more sensitive in early rather than later menopause. These findings are very interesting and consistent with the overall hypothesis about the importance of age and time since menopause in relation to estrogen action. Again, they offer further support for use of HT for managing bothersome menopausal symptoms in early menopause, but they should not be interpreted as endorsing the use of HT to prevent either diabetes or CVD, due to the potential for other risks.
Q Where would you like to see future research conducted regarding the timing hypothesis?
A I would like to see more research on the role of oral versus transdermal estrogen in relation to insulin sensitivity, diabetes risk, and CVD risk, and more research on the role of estrogen dose, different types of progestogens, and the benefits and risks of novel formulations, including selective estrogen receptor modulators and tissue selective estrogen complexes.
Dr. Manson is Professor of Medicine and the Michael and Lee Bell Professor of Women's Health at Harvard Medical School and Chief of the Division of Preventive Medicine at Brigham and Women's Hospital, Boston, Massachusetts. She is a past President of the North American Menopause Society (NAMS) and a NAMS Certified Menopause Practitioner.
The author reports no financial relationships relevant to this article.
References
- Rossouw JE, Anderson GL, Prentice RL, et al; Writing Group for the Women's Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288(3):321-333.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women's Health Initiative randomized trials. JAMA. 2013(13);310:1353-1368.
- Manson JE, Kaunitz AM. Menopause management-- getting clinical care back on track. N Engl J Med. 2016;374(19):803-806.
- Hodis HN, Mack WJ, Henderson VW, et al; ELITE Research Group. Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med. 2016;374(13):1221-1231.
- Pereira RI, Casey BA, Swibas TA, Erickson CB, Wolfe P, Van Pelt RE. Timing of estradiol treatment after menopause may determine benefit or harm to insulin action. J Clin Endocrinol Metab. 2015;100(12):4456-4462.
FDA-approved HT is preferable to compounded HT formulations
Pinkerton JV, Santoro N. Compounded bioidentical hormone therapy: identifying use trends and knowledge gaps among US women. Menopause. 2015;22(9):926-936.
Pinkerton JV, Constantine GD. Compounded non-FDA-approved menopausal hormone therapy prescriptions have increased: results of a pharmacy survey. Menopause. 2016;23(4):359-367.
Gass ML, Stuenkel CA, Utian WH, LaCroix A, Liu JH, Shifren JL.; North American Menopause Society (NAMS) Advisory Panel consisting of representatives of NAMS Board of Trustees and other experts in women's health. Use of compounded hormone therapy in the United States: report of The North American Menopause Society Survey. Menopause. 2015;22(12):1276-1284.
Consider how you would manage this clinical scenario: During a well-woman visit, your 54-year-old patient mentions that, after seeing an advertisement on television, she visited a clinic that sells compounded hormones. There, she underwent some testing and received an estrogen-testosterone implant and a progesterone cream that she applies to her skin each night to treat her menopausal symptoms. Now what?
The use of HT for menopausal symptoms declined considerably following the 2002 publication of the initial findings from the WHI, and its use remains low.4 Symptomatic menopausal women often find that their physicians are reluctant to consider prescribing treatment for menopausal symptoms because of safety concerns regarding HT use. Further, confusion about HT safety has opened the door to the increasing use of compounded bioidentical HT formulations, which are not approved by the US Food and Drug Administration (FDA).3 Since the publication of my 2015 Update on menopause (OBG Manag. 2015;27(6):37−40,42−43), several reports have addressed the use of "custom compounded" bioidentical menopausal HT in US women.
Millions use compounded HT for menopausal symptoms
A recent study by Pinkerton and Santoro that analyzed data from 2 national surveys suggested that as many as 2.5 million US women currently use non−FDA-approved custom-compounded HT. The authors also found that more than three-quarters of women using compounded HT are unaware that these medications, which include oral, topical, injectable, and implantable (pellet) formulations, are not FDA approved. In a study by Pinkerton and Constantine, total annual sales of compounded HT were estimated at approximately $1.5 billion. The dramatic growth in the use of compounded HT appears to have stemmed from celebrity endorsements, aggressive and unregulated marketing, and beliefs about the safety of "natural" hormones.5
Spurious laboratory testing. Women seeking care from physicians and clinics that provide compounded HT are often advised to undergo saliva and serum testing to determine hormone levels. Many women are unaware, however, that saliva testing does not correlate with serum levels of hormones. Further, in contrast with conditions such as thyroid disease and diabetes, routine laboratory testing is neither indicated nor helpful in the management of menopausal symptoms.6 Of note, insurance companies often do not reimburse for the cost of saliva hormone testing or for non-FDA-approved hormones.5
Inadequate endometrial protection. Topical progesterone cream, which is not absorbed in sufficient quantities to generate therapeutic effects, is often prescribed by practitioners who sell bioidentical compounded hormones to their patients.7 According to a report by the North American Menopause Society, several cases of endometrial cancer have been reported among women using compounded HT. These cases may reflect use of systemic estrogen without adequate progesterone protection, as could occur when topical progesterone cream is prescribed to women with an intact uterus using systemic estrogen therapy.
What this evidence means for practice
Clinicians should be alert to the growing prevalence of use of compounded HT and should educate themselves and their patients about the differences between non−FDA-approved HT and FDA-approved HT. Further, women interested in using "natural," "bioidentical," or "custom compounded" HT should be aware that FDA-approved estradiol (oral, transdermal, and vaginal) and progesterone (oral and vaginal) formulations are available.
Because the FDA does not test custom compounded hormones for efficacy or safety and the standardization and purity of these products are uncertain, the American College of Obstetricians and Gynecologists has stated that FDA-approved HT is preferred for management of menopausal symptoms.8 Similarly, the North American Menopause Society does not recommend the use of compounded HT for treatment of menopausal symptoms unless a patient is allergic to ingredients contained in FDA-approved HT formulations.9
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368.
- Hodis HN, Mack WJ, Shoupe D, et al. Methods and baseline cardiovascular data from the Early versus Late Intervention Trial with Estradiol testing the menopausal hormone timing hypothesis. Menopause. 2015;22(4):391–401.
- Manson JE, Kaunitz AM. Menopause management—getting clinical care back on track. N Engl J Med. 2016;374(9):803–806.
- Rossouw JE, Anderson GL, Prentice RL, et al; Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333.
- Kaunitz AM, Kaunitz JD. Compounded bioidentical hormone therapy: time for a reality check? Menopause. 2015;22(9):919–920.
- Kaunitz AM, Manson JE. Management of menopausal symptoms. Obstet Gynecol. 2015;126(4):859–876.
- Benster B, Carey A, Wadsworth F, Vashisht A, Domoney C, Studd J. A double-blind placebo-controlled study to evaluate the effect of progestelle progesterone cream on postmenopausal women. Menopause Int. 2009;15(2):63–69.
- American College of Obstetricians Gynecologists. ACOG Practice Bulletin No. 141: management of menopausal symptoms. Obstet Gynecol. 2014;123(1):202–216.
- North American Menopause Society. The 2012 hormone therapy position statement of The North American Menopause Society. Menopause. 2012;19(3):257–271.
In this Update, I discuss important new study results regarding the cardiovascular safety of hormone therapy (HT) in early menopausal women. In addition, I review survey data that reveal a huge number of US women are using compounded HT preparations, which have unproven efficacy and safety.
Earlier initiation is better: ELITE trial provides strong support for the estrogen timing hypothesis
Hodis HN, Mack WJ, Henderson VW, et al; for the ELITE Research Group. Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med. 2016;374(13):1221-1231.
Keaney JF, Solomon G. Postmenopausal hormone therapy and atherosclerosis--time is of the essence [editorial]. N Engl J Med. 2016;374(13):1279-1280.
A substantial amount of published data, including from the Women's Health Initiative (WHI), supports the timing hypothesis, which proposes that HT slows the progression of atherosclerosis among recently menopausal women but has a neutral or adverse effect among women who are a decade or more past menopause onset.1 To directly test this hypothesis, Hodis and colleagues randomly assigned healthy postmenopausal women (<6 years or ≥10 years past menopause) without cardiovascular disease (CVD) to oral estradiol 1 mg or placebo. Women with a uterus also were randomly assigned to receive either vaginal progesterone gel or placebo gel. The primary outcome was the rate of change in carotid artery intima-media thickness (CIMT), which was assessed at baseline and each 6 months of the study. (An earlier report had noted that baseline CIMT correlated well with CVD risk factors.2) Coronary artery atherosclerosis, a secondary outcome, was assessed at study completion using computed tomography (CT).
Details of the study
Among the 643 participants in the Early versus Late Intervention Trial with Estradiol (ELITE), the median years since menopause and the median age at enrollment were 3.5 and 55.4, respectively, in the early postmenopause group and 14.3 and 63.6, respectively, in the late postmenopause group.
Among the younger women, after a median of 5 years of study medications, the estradiol group had less progression of CIMT than the placebo group (P = .008). By contrast, in the older group, rates of CIMT progression were similar in the HT and placebo groups (P = .29). The relationship between estrogen and CIMT progression differed significantly between the younger and older groups (P = .007). Use of progesterone did not change these trends. Coronary artery CT parameters did not differ significantly between the placebo and HT groups in the age group or in the time-since-menopause group.
What this evidence means for practice
In an editorial accompanying the published results of the ELITE trial, Keaney and Solomon concluded that, although estrogen had a favorable effect on atherosclerosis in early menopause, it would be premature to recommend HT for prevention of cardiovascular events. I agree with them, but I also would like to note that the use of HT for the treatment of menopausal symptoms has plummeted since the initial WHI findings in 2002, with infrequent HT use even among symptomatic women in early menopause.3 (And I refer you to the special inset featuring JoAnn E. Manson, MD, DrPH) The takeaway message is that this important new clinical trial provides additional reassurance regarding the cardiovascular safety of HT when initiated by recently menopausal women to treat bothersome vasomotor symptoms. This message represents welcome news for women with bothersome menopausal symptoms considering use of HT.
A word about the vaginal progesterone gel used in the ELITE trial in relation to clinical practice: Given the need for vaginal placement of progesterone gel, potential messiness, and high cost, few clinicians may prescribe this formulation, and few women probably would choose to use it. As an alternative, micronized progesterone 100-mg capsules are less expensive and well accepted by most patients. These capsules are formulated with peanut oil. Because they may cause women to feel drowsy, the capsules should be taken at bedtime. In women with an intact uterus who are taking oral estradiol 1-mg tablets, one appropriate progestogen regimen for endometrial suppression is a 100-mg micronized progesterone capsule each night, continuously.
WHI, ELITE and the timing hypothesis:
New evidence on HT in early menopause is reassuring
Q&A with JoAnn E. Manson, MD, DrPH
In this interview, Dr. JoAnn Manson discusses the reassuring results of recent hormone therapy (HT) trials in early versus later postmenopausal women, examines these outcomes in the context of the Women's Health Initiative (WHI) trial and ELITE trial, and debunks an enduring common misconception about the WHI.
Q You have said for several years that there has been a misconception about the WHI trial. What is that misconception, and what has been its impact on clinicians, women, and the use of HT?
A The WHI HT trial has been largely misunderstood. It was designed to address the balance of benefits and risks of long-term HT for the prevention of chronic disease in postmenopausal women across a broad range of ages (average age 63).1,2 It was not intended to evaluate the clinical role of HT for managing menopausal symptoms in young and early menopausal women.3 Overall, the WHI study findings have been inappropriately extrapolated to women in their 40s and early 50s who report distressing hot flashes, night sweats, and other menopausal symptoms, and they are often used as a reason to deny therapy when in fact many of these women would be appropriate candidates for HT.
There is increasing evidence that younger women in early menopause who are taking HT have a lower risk of adverse outcomes and lower absolute risks of disease than older women.2,3 In younger, early menopausal women with bothersome hot flashes, night sweats, or other menopausal symptoms and who have no contraindications to HT, the benefits of treatment are likely to outweigh the risks, and these patients derive quality-of-life benefits from treatment.
Q How do the results of the recent ELITE (Early versus Late Intervention Trial with Estradiol) trial build on cardiovascular safety, in particular, of HT and when HT is optimally initiated?
A The ELITE trial directly tested the "timing hypothesis" and the role of HT in slowing the progression of atherosclerosis in early menopause (defined as within 6 years of menopause onset) compared with the effect in women in later menopause (defined as at least 10 yearspast menopause).4 The investigators used carotid artery intima-media thickness (CIMT) as a surrogate end point. In this trial, 643 women were randomly assigned according to whether they were in early or later menopause to receive either placebo or estradiol 1 mg daily; women with a uterus also received progesterone 45 mg as a 4% vaginal gel or matching placebo gel. The median duration of intervention was 5 years.
The ELITE study results provide support for the "critical window hypothesis" in that the estradiol-treated younger women closer to onset of menopause had slowing of atherosclerosis compared with the placebo group, while the older women more distant from menopause did not have slowing of atherosclerosis with estradiol.
The ELITE trial was not large enough, however, to assess clinical end points--rates of heart attack, stroke, or other cardiovascular events. So it remains unclear whether the findings for the surrogate end point of CIMT would translate into a reduced risk of clinical events in the younger women. Nevertheless, ELITE does provide more reassurance about the use of HT in early menopause and supports the possibility that the overall results of the WHI among women enrolled at an average age of 63 years may not apply directly to younger women in early menopause.
Q What impact on clinical practice do you anticipate as a result of the ELITE trial results?
A The findings provide further support for the timing hypothesis and offer additional reassurance regarding the safety of HT in early menopause for management of menopausal symptoms. However, the trial does not provide conclusive evidence to support recommendations to use HT for the express purpose of preventing cardiovascular disease (CVD), even if HT is started in early menopause. Using a surrogate end point for atherosclerosis (CIMT) is not the same as looking at clinical events. There are many biologic pathways for heart attacks, strokes, and other cardiovascular events. In addition to atherosclerosis, for example, there is thrombosis, clotting, thrombo-occlusion within a blood vessel, and plaque rupture. Again, we do not know whether the CIMT-based results would translate directly into a reduction in clinical heart attacks and stroke.
The main takeaway point from the ELITE trial results is further reassurance for use of HT for management of menopausal symptoms in early menopause, but not for long-term chronic disease prevention at any age.
Q Another recent study, published in the Journal of Clinical Endocrinology and Metabolism, addresses HT and the timing hypothesis but in this instance relating to glucose tolerance.5 What did these study authors find?
A This study by Pereira and colleagues is very interesting and suggests that the window of opportunity for initiating estrogen therapy may apply not only to coronary events but also to glucose tolerance, insulin sensitivity, and diabetes risk.5
The authors investigated the effects of short-term high-dose transdermal estradiol on the insulin-mediated glucose disposal rate (GDR), which is a measure of insulin-stimulated glucose uptake. Participants in this randomized, crossover, placebo-controlled study included 22 women who were in early menopause (6 years or less since final menses) and 24 women who were in later menopause (10 years or longer since final menses). All of the women were naïve to hormone therapy, and baseline GDR did not differ between groups. After 1 week of treatment with transdermal estradiol (a high dose of 150 μg) or placebo, the participants' GDR was measured via a hyperinsulinemic-euglycemic clamp.
The investigators found that in the younger women, estradiol had a favorable effect on insulin sensitivity and GDR, whereas in the older women, there was no evidence of a favorable effect and, in fact, there was a signal for risk and more adverse findings in this group.
Several studies in the WHI also looked at glucose tolerance and at the risk of being diagnosed with diabetes. While the results of the WHI estrogen-alone trial revealed a reduction in diabetes and favorable effects across age groups, in the WHI estrogen-plus-progestin trial we did see a signal that the results for diabetes may have been more favorable in the younger than in the older women, somewhat consistent with the findings of Pereira and colleagues.2,5
Overall this issue requires more research, but the Pereira study provides further support for the possibility that estrogen's metabolic effects may vary by age and time since menopause, and there is evidence that the estrogen receptors may be more functional and more sensitive in early rather than later menopause. These findings are very interesting and consistent with the overall hypothesis about the importance of age and time since menopause in relation to estrogen action. Again, they offer further support for use of HT for managing bothersome menopausal symptoms in early menopause, but they should not be interpreted as endorsing the use of HT to prevent either diabetes or CVD, due to the potential for other risks.
Q Where would you like to see future research conducted regarding the timing hypothesis?
A I would like to see more research on the role of oral versus transdermal estrogen in relation to insulin sensitivity, diabetes risk, and CVD risk, and more research on the role of estrogen dose, different types of progestogens, and the benefits and risks of novel formulations, including selective estrogen receptor modulators and tissue selective estrogen complexes.
Dr. Manson is Professor of Medicine and the Michael and Lee Bell Professor of Women's Health at Harvard Medical School and Chief of the Division of Preventive Medicine at Brigham and Women's Hospital, Boston, Massachusetts. She is a past President of the North American Menopause Society (NAMS) and a NAMS Certified Menopause Practitioner.
The author reports no financial relationships relevant to this article.
References
- Rossouw JE, Anderson GL, Prentice RL, et al; Writing Group for the Women's Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288(3):321-333.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women's Health Initiative randomized trials. JAMA. 2013(13);310:1353-1368.
- Manson JE, Kaunitz AM. Menopause management-- getting clinical care back on track. N Engl J Med. 2016;374(19):803-806.
- Hodis HN, Mack WJ, Henderson VW, et al; ELITE Research Group. Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med. 2016;374(13):1221-1231.
- Pereira RI, Casey BA, Swibas TA, Erickson CB, Wolfe P, Van Pelt RE. Timing of estradiol treatment after menopause may determine benefit or harm to insulin action. J Clin Endocrinol Metab. 2015;100(12):4456-4462.
FDA-approved HT is preferable to compounded HT formulations
Pinkerton JV, Santoro N. Compounded bioidentical hormone therapy: identifying use trends and knowledge gaps among US women. Menopause. 2015;22(9):926-936.
Pinkerton JV, Constantine GD. Compounded non-FDA-approved menopausal hormone therapy prescriptions have increased: results of a pharmacy survey. Menopause. 2016;23(4):359-367.
Gass ML, Stuenkel CA, Utian WH, LaCroix A, Liu JH, Shifren JL.; North American Menopause Society (NAMS) Advisory Panel consisting of representatives of NAMS Board of Trustees and other experts in women's health. Use of compounded hormone therapy in the United States: report of The North American Menopause Society Survey. Menopause. 2015;22(12):1276-1284.
Consider how you would manage this clinical scenario: During a well-woman visit, your 54-year-old patient mentions that, after seeing an advertisement on television, she visited a clinic that sells compounded hormones. There, she underwent some testing and received an estrogen-testosterone implant and a progesterone cream that she applies to her skin each night to treat her menopausal symptoms. Now what?
The use of HT for menopausal symptoms declined considerably following the 2002 publication of the initial findings from the WHI, and its use remains low.4 Symptomatic menopausal women often find that their physicians are reluctant to consider prescribing treatment for menopausal symptoms because of safety concerns regarding HT use. Further, confusion about HT safety has opened the door to the increasing use of compounded bioidentical HT formulations, which are not approved by the US Food and Drug Administration (FDA).3 Since the publication of my 2015 Update on menopause (OBG Manag. 2015;27(6):37−40,42−43), several reports have addressed the use of "custom compounded" bioidentical menopausal HT in US women.
Millions use compounded HT for menopausal symptoms
A recent study by Pinkerton and Santoro that analyzed data from 2 national surveys suggested that as many as 2.5 million US women currently use non−FDA-approved custom-compounded HT. The authors also found that more than three-quarters of women using compounded HT are unaware that these medications, which include oral, topical, injectable, and implantable (pellet) formulations, are not FDA approved. In a study by Pinkerton and Constantine, total annual sales of compounded HT were estimated at approximately $1.5 billion. The dramatic growth in the use of compounded HT appears to have stemmed from celebrity endorsements, aggressive and unregulated marketing, and beliefs about the safety of "natural" hormones.5
Spurious laboratory testing. Women seeking care from physicians and clinics that provide compounded HT are often advised to undergo saliva and serum testing to determine hormone levels. Many women are unaware, however, that saliva testing does not correlate with serum levels of hormones. Further, in contrast with conditions such as thyroid disease and diabetes, routine laboratory testing is neither indicated nor helpful in the management of menopausal symptoms.6 Of note, insurance companies often do not reimburse for the cost of saliva hormone testing or for non-FDA-approved hormones.5
Inadequate endometrial protection. Topical progesterone cream, which is not absorbed in sufficient quantities to generate therapeutic effects, is often prescribed by practitioners who sell bioidentical compounded hormones to their patients.7 According to a report by the North American Menopause Society, several cases of endometrial cancer have been reported among women using compounded HT. These cases may reflect use of systemic estrogen without adequate progesterone protection, as could occur when topical progesterone cream is prescribed to women with an intact uterus using systemic estrogen therapy.
What this evidence means for practice
Clinicians should be alert to the growing prevalence of use of compounded HT and should educate themselves and their patients about the differences between non−FDA-approved HT and FDA-approved HT. Further, women interested in using "natural," "bioidentical," or "custom compounded" HT should be aware that FDA-approved estradiol (oral, transdermal, and vaginal) and progesterone (oral and vaginal) formulations are available.
Because the FDA does not test custom compounded hormones for efficacy or safety and the standardization and purity of these products are uncertain, the American College of Obstetricians and Gynecologists has stated that FDA-approved HT is preferred for management of menopausal symptoms.8 Similarly, the North American Menopause Society does not recommend the use of compounded HT for treatment of menopausal symptoms unless a patient is allergic to ingredients contained in FDA-approved HT formulations.9
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
In this Update, I discuss important new study results regarding the cardiovascular safety of hormone therapy (HT) in early menopausal women. In addition, I review survey data that reveal a huge number of US women are using compounded HT preparations, which have unproven efficacy and safety.
Earlier initiation is better: ELITE trial provides strong support for the estrogen timing hypothesis
Hodis HN, Mack WJ, Henderson VW, et al; for the ELITE Research Group. Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med. 2016;374(13):1221-1231.
Keaney JF, Solomon G. Postmenopausal hormone therapy and atherosclerosis--time is of the essence [editorial]. N Engl J Med. 2016;374(13):1279-1280.
A substantial amount of published data, including from the Women's Health Initiative (WHI), supports the timing hypothesis, which proposes that HT slows the progression of atherosclerosis among recently menopausal women but has a neutral or adverse effect among women who are a decade or more past menopause onset.1 To directly test this hypothesis, Hodis and colleagues randomly assigned healthy postmenopausal women (<6 years or ≥10 years past menopause) without cardiovascular disease (CVD) to oral estradiol 1 mg or placebo. Women with a uterus also were randomly assigned to receive either vaginal progesterone gel or placebo gel. The primary outcome was the rate of change in carotid artery intima-media thickness (CIMT), which was assessed at baseline and each 6 months of the study. (An earlier report had noted that baseline CIMT correlated well with CVD risk factors.2) Coronary artery atherosclerosis, a secondary outcome, was assessed at study completion using computed tomography (CT).
Details of the study
Among the 643 participants in the Early versus Late Intervention Trial with Estradiol (ELITE), the median years since menopause and the median age at enrollment were 3.5 and 55.4, respectively, in the early postmenopause group and 14.3 and 63.6, respectively, in the late postmenopause group.
Among the younger women, after a median of 5 years of study medications, the estradiol group had less progression of CIMT than the placebo group (P = .008). By contrast, in the older group, rates of CIMT progression were similar in the HT and placebo groups (P = .29). The relationship between estrogen and CIMT progression differed significantly between the younger and older groups (P = .007). Use of progesterone did not change these trends. Coronary artery CT parameters did not differ significantly between the placebo and HT groups in the age group or in the time-since-menopause group.
What this evidence means for practice
In an editorial accompanying the published results of the ELITE trial, Keaney and Solomon concluded that, although estrogen had a favorable effect on atherosclerosis in early menopause, it would be premature to recommend HT for prevention of cardiovascular events. I agree with them, but I also would like to note that the use of HT for the treatment of menopausal symptoms has plummeted since the initial WHI findings in 2002, with infrequent HT use even among symptomatic women in early menopause.3 (And I refer you to the special inset featuring JoAnn E. Manson, MD, DrPH) The takeaway message is that this important new clinical trial provides additional reassurance regarding the cardiovascular safety of HT when initiated by recently menopausal women to treat bothersome vasomotor symptoms. This message represents welcome news for women with bothersome menopausal symptoms considering use of HT.
A word about the vaginal progesterone gel used in the ELITE trial in relation to clinical practice: Given the need for vaginal placement of progesterone gel, potential messiness, and high cost, few clinicians may prescribe this formulation, and few women probably would choose to use it. As an alternative, micronized progesterone 100-mg capsules are less expensive and well accepted by most patients. These capsules are formulated with peanut oil. Because they may cause women to feel drowsy, the capsules should be taken at bedtime. In women with an intact uterus who are taking oral estradiol 1-mg tablets, one appropriate progestogen regimen for endometrial suppression is a 100-mg micronized progesterone capsule each night, continuously.
WHI, ELITE and the timing hypothesis:
New evidence on HT in early menopause is reassuring
Q&A with JoAnn E. Manson, MD, DrPH
In this interview, Dr. JoAnn Manson discusses the reassuring results of recent hormone therapy (HT) trials in early versus later postmenopausal women, examines these outcomes in the context of the Women's Health Initiative (WHI) trial and ELITE trial, and debunks an enduring common misconception about the WHI.
Q You have said for several years that there has been a misconception about the WHI trial. What is that misconception, and what has been its impact on clinicians, women, and the use of HT?
A The WHI HT trial has been largely misunderstood. It was designed to address the balance of benefits and risks of long-term HT for the prevention of chronic disease in postmenopausal women across a broad range of ages (average age 63).1,2 It was not intended to evaluate the clinical role of HT for managing menopausal symptoms in young and early menopausal women.3 Overall, the WHI study findings have been inappropriately extrapolated to women in their 40s and early 50s who report distressing hot flashes, night sweats, and other menopausal symptoms, and they are often used as a reason to deny therapy when in fact many of these women would be appropriate candidates for HT.
There is increasing evidence that younger women in early menopause who are taking HT have a lower risk of adverse outcomes and lower absolute risks of disease than older women.2,3 In younger, early menopausal women with bothersome hot flashes, night sweats, or other menopausal symptoms and who have no contraindications to HT, the benefits of treatment are likely to outweigh the risks, and these patients derive quality-of-life benefits from treatment.
Q How do the results of the recent ELITE (Early versus Late Intervention Trial with Estradiol) trial build on cardiovascular safety, in particular, of HT and when HT is optimally initiated?
A The ELITE trial directly tested the "timing hypothesis" and the role of HT in slowing the progression of atherosclerosis in early menopause (defined as within 6 years of menopause onset) compared with the effect in women in later menopause (defined as at least 10 yearspast menopause).4 The investigators used carotid artery intima-media thickness (CIMT) as a surrogate end point. In this trial, 643 women were randomly assigned according to whether they were in early or later menopause to receive either placebo or estradiol 1 mg daily; women with a uterus also received progesterone 45 mg as a 4% vaginal gel or matching placebo gel. The median duration of intervention was 5 years.
The ELITE study results provide support for the "critical window hypothesis" in that the estradiol-treated younger women closer to onset of menopause had slowing of atherosclerosis compared with the placebo group, while the older women more distant from menopause did not have slowing of atherosclerosis with estradiol.
The ELITE trial was not large enough, however, to assess clinical end points--rates of heart attack, stroke, or other cardiovascular events. So it remains unclear whether the findings for the surrogate end point of CIMT would translate into a reduced risk of clinical events in the younger women. Nevertheless, ELITE does provide more reassurance about the use of HT in early menopause and supports the possibility that the overall results of the WHI among women enrolled at an average age of 63 years may not apply directly to younger women in early menopause.
Q What impact on clinical practice do you anticipate as a result of the ELITE trial results?
A The findings provide further support for the timing hypothesis and offer additional reassurance regarding the safety of HT in early menopause for management of menopausal symptoms. However, the trial does not provide conclusive evidence to support recommendations to use HT for the express purpose of preventing cardiovascular disease (CVD), even if HT is started in early menopause. Using a surrogate end point for atherosclerosis (CIMT) is not the same as looking at clinical events. There are many biologic pathways for heart attacks, strokes, and other cardiovascular events. In addition to atherosclerosis, for example, there is thrombosis, clotting, thrombo-occlusion within a blood vessel, and plaque rupture. Again, we do not know whether the CIMT-based results would translate directly into a reduction in clinical heart attacks and stroke.
The main takeaway point from the ELITE trial results is further reassurance for use of HT for management of menopausal symptoms in early menopause, but not for long-term chronic disease prevention at any age.
Q Another recent study, published in the Journal of Clinical Endocrinology and Metabolism, addresses HT and the timing hypothesis but in this instance relating to glucose tolerance.5 What did these study authors find?
A This study by Pereira and colleagues is very interesting and suggests that the window of opportunity for initiating estrogen therapy may apply not only to coronary events but also to glucose tolerance, insulin sensitivity, and diabetes risk.5
The authors investigated the effects of short-term high-dose transdermal estradiol on the insulin-mediated glucose disposal rate (GDR), which is a measure of insulin-stimulated glucose uptake. Participants in this randomized, crossover, placebo-controlled study included 22 women who were in early menopause (6 years or less since final menses) and 24 women who were in later menopause (10 years or longer since final menses). All of the women were naïve to hormone therapy, and baseline GDR did not differ between groups. After 1 week of treatment with transdermal estradiol (a high dose of 150 μg) or placebo, the participants' GDR was measured via a hyperinsulinemic-euglycemic clamp.
The investigators found that in the younger women, estradiol had a favorable effect on insulin sensitivity and GDR, whereas in the older women, there was no evidence of a favorable effect and, in fact, there was a signal for risk and more adverse findings in this group.
Several studies in the WHI also looked at glucose tolerance and at the risk of being diagnosed with diabetes. While the results of the WHI estrogen-alone trial revealed a reduction in diabetes and favorable effects across age groups, in the WHI estrogen-plus-progestin trial we did see a signal that the results for diabetes may have been more favorable in the younger than in the older women, somewhat consistent with the findings of Pereira and colleagues.2,5
Overall this issue requires more research, but the Pereira study provides further support for the possibility that estrogen's metabolic effects may vary by age and time since menopause, and there is evidence that the estrogen receptors may be more functional and more sensitive in early rather than later menopause. These findings are very interesting and consistent with the overall hypothesis about the importance of age and time since menopause in relation to estrogen action. Again, they offer further support for use of HT for managing bothersome menopausal symptoms in early menopause, but they should not be interpreted as endorsing the use of HT to prevent either diabetes or CVD, due to the potential for other risks.
Q Where would you like to see future research conducted regarding the timing hypothesis?
A I would like to see more research on the role of oral versus transdermal estrogen in relation to insulin sensitivity, diabetes risk, and CVD risk, and more research on the role of estrogen dose, different types of progestogens, and the benefits and risks of novel formulations, including selective estrogen receptor modulators and tissue selective estrogen complexes.
Dr. Manson is Professor of Medicine and the Michael and Lee Bell Professor of Women's Health at Harvard Medical School and Chief of the Division of Preventive Medicine at Brigham and Women's Hospital, Boston, Massachusetts. She is a past President of the North American Menopause Society (NAMS) and a NAMS Certified Menopause Practitioner.
The author reports no financial relationships relevant to this article.
References
- Rossouw JE, Anderson GL, Prentice RL, et al; Writing Group for the Women's Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288(3):321-333.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women's Health Initiative randomized trials. JAMA. 2013(13);310:1353-1368.
- Manson JE, Kaunitz AM. Menopause management-- getting clinical care back on track. N Engl J Med. 2016;374(19):803-806.
- Hodis HN, Mack WJ, Henderson VW, et al; ELITE Research Group. Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med. 2016;374(13):1221-1231.
- Pereira RI, Casey BA, Swibas TA, Erickson CB, Wolfe P, Van Pelt RE. Timing of estradiol treatment after menopause may determine benefit or harm to insulin action. J Clin Endocrinol Metab. 2015;100(12):4456-4462.
FDA-approved HT is preferable to compounded HT formulations
Pinkerton JV, Santoro N. Compounded bioidentical hormone therapy: identifying use trends and knowledge gaps among US women. Menopause. 2015;22(9):926-936.
Pinkerton JV, Constantine GD. Compounded non-FDA-approved menopausal hormone therapy prescriptions have increased: results of a pharmacy survey. Menopause. 2016;23(4):359-367.
Gass ML, Stuenkel CA, Utian WH, LaCroix A, Liu JH, Shifren JL.; North American Menopause Society (NAMS) Advisory Panel consisting of representatives of NAMS Board of Trustees and other experts in women's health. Use of compounded hormone therapy in the United States: report of The North American Menopause Society Survey. Menopause. 2015;22(12):1276-1284.
Consider how you would manage this clinical scenario: During a well-woman visit, your 54-year-old patient mentions that, after seeing an advertisement on television, she visited a clinic that sells compounded hormones. There, she underwent some testing and received an estrogen-testosterone implant and a progesterone cream that she applies to her skin each night to treat her menopausal symptoms. Now what?
The use of HT for menopausal symptoms declined considerably following the 2002 publication of the initial findings from the WHI, and its use remains low.4 Symptomatic menopausal women often find that their physicians are reluctant to consider prescribing treatment for menopausal symptoms because of safety concerns regarding HT use. Further, confusion about HT safety has opened the door to the increasing use of compounded bioidentical HT formulations, which are not approved by the US Food and Drug Administration (FDA).3 Since the publication of my 2015 Update on menopause (OBG Manag. 2015;27(6):37−40,42−43), several reports have addressed the use of "custom compounded" bioidentical menopausal HT in US women.
Millions use compounded HT for menopausal symptoms
A recent study by Pinkerton and Santoro that analyzed data from 2 national surveys suggested that as many as 2.5 million US women currently use non−FDA-approved custom-compounded HT. The authors also found that more than three-quarters of women using compounded HT are unaware that these medications, which include oral, topical, injectable, and implantable (pellet) formulations, are not FDA approved. In a study by Pinkerton and Constantine, total annual sales of compounded HT were estimated at approximately $1.5 billion. The dramatic growth in the use of compounded HT appears to have stemmed from celebrity endorsements, aggressive and unregulated marketing, and beliefs about the safety of "natural" hormones.5
Spurious laboratory testing. Women seeking care from physicians and clinics that provide compounded HT are often advised to undergo saliva and serum testing to determine hormone levels. Many women are unaware, however, that saliva testing does not correlate with serum levels of hormones. Further, in contrast with conditions such as thyroid disease and diabetes, routine laboratory testing is neither indicated nor helpful in the management of menopausal symptoms.6 Of note, insurance companies often do not reimburse for the cost of saliva hormone testing or for non-FDA-approved hormones.5
Inadequate endometrial protection. Topical progesterone cream, which is not absorbed in sufficient quantities to generate therapeutic effects, is often prescribed by practitioners who sell bioidentical compounded hormones to their patients.7 According to a report by the North American Menopause Society, several cases of endometrial cancer have been reported among women using compounded HT. These cases may reflect use of systemic estrogen without adequate progesterone protection, as could occur when topical progesterone cream is prescribed to women with an intact uterus using systemic estrogen therapy.
What this evidence means for practice
Clinicians should be alert to the growing prevalence of use of compounded HT and should educate themselves and their patients about the differences between non−FDA-approved HT and FDA-approved HT. Further, women interested in using "natural," "bioidentical," or "custom compounded" HT should be aware that FDA-approved estradiol (oral, transdermal, and vaginal) and progesterone (oral and vaginal) formulations are available.
Because the FDA does not test custom compounded hormones for efficacy or safety and the standardization and purity of these products are uncertain, the American College of Obstetricians and Gynecologists has stated that FDA-approved HT is preferred for management of menopausal symptoms.8 Similarly, the North American Menopause Society does not recommend the use of compounded HT for treatment of menopausal symptoms unless a patient is allergic to ingredients contained in FDA-approved HT formulations.9
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368.
- Hodis HN, Mack WJ, Shoupe D, et al. Methods and baseline cardiovascular data from the Early versus Late Intervention Trial with Estradiol testing the menopausal hormone timing hypothesis. Menopause. 2015;22(4):391–401.
- Manson JE, Kaunitz AM. Menopause management—getting clinical care back on track. N Engl J Med. 2016;374(9):803–806.
- Rossouw JE, Anderson GL, Prentice RL, et al; Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333.
- Kaunitz AM, Kaunitz JD. Compounded bioidentical hormone therapy: time for a reality check? Menopause. 2015;22(9):919–920.
- Kaunitz AM, Manson JE. Management of menopausal symptoms. Obstet Gynecol. 2015;126(4):859–876.
- Benster B, Carey A, Wadsworth F, Vashisht A, Domoney C, Studd J. A double-blind placebo-controlled study to evaluate the effect of progestelle progesterone cream on postmenopausal women. Menopause Int. 2009;15(2):63–69.
- American College of Obstetricians Gynecologists. ACOG Practice Bulletin No. 141: management of menopausal symptoms. Obstet Gynecol. 2014;123(1):202–216.
- North American Menopause Society. The 2012 hormone therapy position statement of The North American Menopause Society. Menopause. 2012;19(3):257–271.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310(13):1353–1368.
- Hodis HN, Mack WJ, Shoupe D, et al. Methods and baseline cardiovascular data from the Early versus Late Intervention Trial with Estradiol testing the menopausal hormone timing hypothesis. Menopause. 2015;22(4):391–401.
- Manson JE, Kaunitz AM. Menopause management—getting clinical care back on track. N Engl J Med. 2016;374(9):803–806.
- Rossouw JE, Anderson GL, Prentice RL, et al; Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333.
- Kaunitz AM, Kaunitz JD. Compounded bioidentical hormone therapy: time for a reality check? Menopause. 2015;22(9):919–920.
- Kaunitz AM, Manson JE. Management of menopausal symptoms. Obstet Gynecol. 2015;126(4):859–876.
- Benster B, Carey A, Wadsworth F, Vashisht A, Domoney C, Studd J. A double-blind placebo-controlled study to evaluate the effect of progestelle progesterone cream on postmenopausal women. Menopause Int. 2009;15(2):63–69.
- American College of Obstetricians Gynecologists. ACOG Practice Bulletin No. 141: management of menopausal symptoms. Obstet Gynecol. 2014;123(1):202–216.
- North American Menopause Society. The 2012 hormone therapy position statement of The North American Menopause Society. Menopause. 2012;19(3):257–271.
In this article
• JoAnn E. Manson discusses new data on HT benefits vs risks
• Use of compounded hormones growing
How do you break the ice with patients to ask about their sexual health?
CASE Patient may benefit from treatment for dyspareuniaA 54-year-old woman has been in your care for more than 15 years. Three years ago, at her well-woman examination, she was not yet having symptoms of menopause. Now, during her current examination, she reports hot flashes, which she says are not bothersome. In passing, she also says, “I don’t want to take hormone therapy,” but then is not overly conversational or responsive to your questions. She does mention having had 3 urinary tract infections over the past 8 months. On physical examination, you note mildly atrophied vaginal tissue.
Your patient does not bring up any sexual concerns, and so far you have not directly asked about sexual health. However, the time remaining in this visit is limited, and your patient, whose daughter is sitting in the waiting area, seems anxious to finish and leave. Still, you want to broach the subject of your patient’s sexual health. What are your best options?
We learned a lot about women’s perceptions regarding their sexual health in the 2008 Prevalence of Female Sexual Problems Associated with Distress and Determinants of Treatment Seeking study (PRESIDE). Approximately 43% of 31,581 questionnaire respondents reported dysfunction in sexual desire, arousal, or orgasm.1 Results also showed that 11.5% of the respondents with any of these types of female sexual dysfunction (FSD) were distressed about it. For clinicians, knowing who these women are is key in recognizing and treating FSD.
Important to the opening case, in PRESIDE, Shifren and colleagues found that women in their midlife years (aged 45 to 64) had the highest rate of any distressing sexual problem: 14.8%. Younger women (aged 18 to 44 years) had a rate of 10.8%; older women (aged 65 years or older) had a rate of 8.9%.1
The most prevalent FSD was hypoactive sexual desire disorder,1 which in 2013 was renamed sexual interest and arousal disorder in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition.2 As with any distressing FSD, reports of being distressed about low sexual desire were highest for midlife women (12.3%) relative to younger (8.9%) and older (7.4%) women.1
Unfortunately, decreased desire can have a ripple effect that goes well beyond a patient’s sexual health. A less-than-satisfying sex life can have a significant negative impact on self-image, possibly leading to depression or overall mood instability, which in turn can put undue strain on personal relationships.1,3 A patient’s entire quality of life can be affected negatively.
With so much at stake, it is important for physicians to take a more active role in addressing the sexual health of their patients. Emphasizing wellness can help reduce the stigma of sexual dysfunction, break the silence, and open up patient–physician communication.4 There is also much to be gained by helping patients realize that having positive and respectful relationships is protective for health, including sexual health.4 Likewise, patients benefit from acknowledging that sexual health is an element of overall health and contributes to it.4
Toward these ends, more discussion with patients is needed. According to a 2008 national study, although 63% of US ObGyns surveyed indicated that they routinely asked their patients about sexual activity, only 40% asked about sexual problems, and only 29% asked patients if their sex lives were satisfying.5
Without communication, information is missed, and clinicians easily can overlook their patients’ sexual dysfunction and need for intervention. For midlife women, who are disproportionately affected by dysfunction relative to younger and older women, and for whom the rate of menopausal symptoms increases over the transition years, the results of going undiagnosed and untreated can be especially troubling. As reported in one study, for example, the rate of bothersome vulvovaginal atrophy, which can be a source of sexual dysfunction, increased from less than 5% at premenopause to almost 50% at 3 years postmenopause.6 What is standing in our way, however, and how can we overcome the hurdles to an open-door approach and meaningful conversation?
Obstacles to taking a sexual historyInitiating a sexual history can be like opening Pandora’s box. How do clinicians deal with the problems that come out? Some clinicians worry about embarrassing a patient with the first few questions about sexual health. Male gynecologists may feel awkward asking a patient about sex—particularly an older, midlife patient. The problem with not starting the conversation is that the midlife patient is often the one in the most distress, and the one most in need of treatment. Only by having the sexual health discussion can clinicians identify any issues and begin to address them.
Icebreakers to jump-start the conversation
Asking open-ended questions works best. Here are some options for starting a conversation with a midlife patient:
- say, “Many women around menopause develop sexual problems. Have you noticed any changes?”
- say, “It is part of my routine to ask about sexual health. Tell me if you have any concerns.”
- add a brief sexual symptom checklist (FIGURE 1) to the patient history or intake form. The checklist shown here starts by asking if the patient is satisfied, yes or no, with her sexual function. If yes, the satisfied patient (and the clinician) can proceed to the next section on the form. If no, the dissatisfied patient can answer additional questions about problems related to sexual desire, arousal, orgasm, and dyspareunia.
Such tools as checklists are often needed to bridge the wide communication gap between patients and physicians. Of the 255 women who reported experiencing dyspareunia in the Revealing Vaginal Effects at Midlife (REVEAL) study, almost half (44%) indicated that they had not spoken with their health care clinician about it.7 Another 44% had spoken about the problem but on their own initiative. In only 10% of cases had a physician started the conversation.
Clinicians can and should do better. Many of us have known our patients for years—given them their annual examinations, delivered their babies, performed their surgeries, become familiar with their bodies and intimate medical histories. We are uniquely qualified to start conversations on sexual health. A clinician who examines tissues and sees a decrease in vaginal caliber and pallor must say something. In some cases, the vagina is dry, but the patient has not been having lubrication problems. In other cases, a more serious condition might be involved. The important thing is to open up a conversation and talk about treatments.
CASE Continued
As today’s office visit wraps up and your patient begins moving for the door, you say, “Your hot flashes aren’t bothering you, but some women start experiencing certain sexual problems around this time in life. Have you noticed any issues?”
“Well, I have been having more burning during intercourse,” your patient responds.
On hearing this, you say, “That’s very important, Mrs. X, and I am glad you told me about it. I would like to discuss your concern a bit more, so let’s make another appointment to do just that.”
At the next visit, as part of the discussion, you give your patient a 15-minute sexual status examination.
Sexual status examination
Performing this examination helps clinicians see patterns in both sexual behavior and sexual health, which in turn can make it easier to recognize any dysfunction that might subsequently develop. The key to this process is establishing trust with the patient and having her feel comfortable with the discussion.
The patient remains fully clothed during this 15-minute session, which takes place with guarantees of nonjudgmental listening, confidentiality, privacy, and no interruptions. With the topic of sex being so personal, it should be emphasized that she is simply giving the clinician information, as she does on other health-related matters.
Establish her sexual status. Begin by asking the patient to describe her most recent or typical sexual encounter, including details such as day, time, location, type of activity, thoughts and feelings, and responses.
Potential issues can become apparent immediately. A patient may not have had a sexual encounter recently, or ever. Another may want sex, or more sex, but sees obstacles or lack of opportunity. Each of these is an issue to be explored, if the patient allows.
A patient can be sexually active in a number of ways, as the definition varies among population groups (race and age) and individuals. Sex is not only intercourse or oral sex—it is also kissing, touching, and hugging. Some people have an expansive view of what it is to be sexually active. When the patient mentions an encounter, ask what day, what time, where (at home, in a hotel room, at the office), and what type of activity (foreplay, oral sex, manual stimulation, intercourse, and position). Following up, ask what the patient was thinking or feeling about the encounter. For example, were there distracting thoughts or feelings of guilt? How did the patient and her partner respond during the encounter?
Assess for sexual dysfunction. After assessing the patient’s sexual status, turn to dysfunction. Arousal, pain, orgasm, and satisfaction are 4 areas of interest. Did the patient have difficulty becoming aroused? Was there a problem with lubrication? Did she have an orgasm? Was sex painful? How did she feel in terms of overall satisfaction?
In general, patients are comfortable speaking about sexual function and health. Having this talk can help identify a pattern, which can be discussed further during another visit. Such a follow-up would not take long—a level 3 visit should suffice.
Differential diagnosis. Consider the effects of current medications.8,9 The psychiatric illnesses and general health factors that may affect sexual function should be considered as well (FIGURE 2).10–22
When is it important to refer?
There are many reasons to refer a patient to another physician, including:
- a recommended treatment is not working
- abuse is suspected
- the patient shows symptoms of depression, anxiety, or another psychiatric condition
- a chronic, generalized (vs situational) disorder may be involved
- physical pain issues must be addressed
- you simply do not feel comfortable with a particular problem or patient.
Given the range of potential issues associated with sexual function, it is important to be able to provide the patient with expert assistance from a multidisciplinary team of specialists. This team can include psychologists, psychiatrists, counselors, sex educators, and, for pain issues, pelvic floor specialists and pelvic floor physical therapists. These colleagues are thoroughly familiar with the kinds of issues that can arise, and can offer alternative and adjunctive therapies.
Referrals also can be made for the latest nonpharmacologic and FDA-approved pharmacologic treatment options. Specialists tend to be familiar with these options, some of which are available only recently.
It is important to ask patients about sexual function and, if necessary, give them access to the best treatment options.
CASE Resolved
During the sexual status examination, your patient describes her most recent sexual encounter with her husband. She is frustrated with her lack of sexual response and describes a dry, tearing sensation during intercourse. You recommend first-line treatment with vaginal lubricants, preferably iso-osmolar aqueous− or silicone/dimethicone−based lubricants during intercourse. You also can discuss topical estrogen therapy via estradiol cream, conjugated equine estrogen cream, estradiol tablets in the vagina, or the estrogen ring. She is reassured that topical estrogen use will not pose significant risk for cancer, stroke, heart disease, or blood clot and that progesterone treatment is not necessary.
For patients who are particularly concerned about vaginal estrogen use, 2 or 3 times weekly use of a vaginal moisturizer could be an alternative for genitourinary symptoms and dyspareunia.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Shifren JL, Monz BU, Russo PA, Segreti A, Johannes CB. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112(5):970−978.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). Washington, DC: American Psychiatric Association; 2013.
- Leiblum SR, Koochaki PE, Rodenberg CA, Barton IP, Rosen RC. Hypoactive sexual desire disorder in postmenopausal women: US results from the Women’s International Study of Health and Sexuality (WISHeS). Menopause. 2006;13(1):46−56.
- Satcher D, Hook EW 3rd, Coleman E. Sexual health in America: improving patient care and public health. JAMA. 2015;314(8):765−766.
- Sobecki JN, Curlin FA, Rasinski KA, Lindau ST. What we don’t talk about when we don’t talk about sex: results of a national survey of U.S. obstetrician/gynecologists. J Sex Med. 2012;9(5):1285−1294.
- Dennerstein L, Dudley EC, Hopper JL, Guthrie JR, Burger HG. A prospective population-based study of menopausal symptoms. Obstet Gynecol. 2000;96(3):351−358.
- Shifren JL, Johannes CB, Monz BU, Russo PA, Bennett L, Rosen R. Help-seeking behavior of women with self-reported distressing sexual problems. J Womens Health. 2009;18(4):461−468.
- Basson R, Schultz WW. Sexual sequelae of general medical disorders. Lancet. 2007;369(9559):409−424.
- Kingsberg SA, Janata JW. Female sexual disorders: assessment, diagnosis, and treatment. Urol Clin North Am. 2007;34(4):497−506, v−vi.
- Casper RC, Redmond DE Jr, Katz MM, Schaffer CB, Davis JM, Koslow SH. Somatic symptoms in primary affective disorder. Presence and relationship to the classification of depression. Arch Gen Psychiatry. 1985;42(11):1098−1104.
- van Lankveld JJ, Grotjohann Y. Psychiatric comorbidity in heterosexual couples with sexual dysfunction assessed with the Composite International Diagnostic Interview. Arch Sex Behav. 2000;29(5):479−498.
- Shifren JL, Monz BU, Russo PA, Segreti A, Johannes CB. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112(5):970−978.
- Friedman S, Harrison G. Sexual histories, attitudes, and behavior of schizophrenic and “normal” women. Arch Sex Behav. 1984;13(6):555−567.
- Okeahialam BN, Obeka NC. Sexual dysfunction in female hypertensives. J Natl Med Assoc. 2006;98(4):638−640.
- Rees PM, Fowler CJ, Maas CP. Sexual function in men and women with neurological disorders. Lancet. 2007;369(9560):512−525.
- Bhasin S, Enzlin P, Coviello A, Basson R. Sexual dysfunction in men and women with endocrine disorders. Lancet. 2007;369(9561):597−611.
- Aslan G, KöseoTimesğlu H, Sadik O, Gimen S, Cihan A, Esen A. Sexual function in women with urinary incontinence. Int J Impot Res. 2005;17(3):248−251.
- Smith EM, Ritchie JM, Galask R, Pugh EE, Jia J, Ricks-McGillan J. Case–control study of vulvar vestibulitis risk associated with genital infections. Infect Dis Obstet Gynecol. 2002;10(4):193−202.
- Baksu B, Davas I, Agar E, Akyol A, Varolan A. The effect of mode of delivery on postpartum sexual functioning in primiparous women. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(4):401−406.
- Abdel-Nasser AM, Ali EI. Determinants of sexual disability and dissatisfaction in female patients with rheumatoid arthritis. Clin Rheumatol. 2006;25(6):822−830.
- Sampogna F, Gisondi P, Tabolli S, Abeni D; IDI Multipurpose Psoriasis Research on Vital Experiences investigators. Impairment of sexual life in patients with psoriasis. Dermatology. 2007;214(2):144−150.
- Mathias C, Cardeal Mendes CM, Pondé de Sena E, et al. An open-label, fixed-dose study of bupropion effect on sexual function scores in women treated for breast cancer. Ann Oncol. 2006;17(12):1792−1796.
CASE Patient may benefit from treatment for dyspareuniaA 54-year-old woman has been in your care for more than 15 years. Three years ago, at her well-woman examination, she was not yet having symptoms of menopause. Now, during her current examination, she reports hot flashes, which she says are not bothersome. In passing, she also says, “I don’t want to take hormone therapy,” but then is not overly conversational or responsive to your questions. She does mention having had 3 urinary tract infections over the past 8 months. On physical examination, you note mildly atrophied vaginal tissue.
Your patient does not bring up any sexual concerns, and so far you have not directly asked about sexual health. However, the time remaining in this visit is limited, and your patient, whose daughter is sitting in the waiting area, seems anxious to finish and leave. Still, you want to broach the subject of your patient’s sexual health. What are your best options?
We learned a lot about women’s perceptions regarding their sexual health in the 2008 Prevalence of Female Sexual Problems Associated with Distress and Determinants of Treatment Seeking study (PRESIDE). Approximately 43% of 31,581 questionnaire respondents reported dysfunction in sexual desire, arousal, or orgasm.1 Results also showed that 11.5% of the respondents with any of these types of female sexual dysfunction (FSD) were distressed about it. For clinicians, knowing who these women are is key in recognizing and treating FSD.
Important to the opening case, in PRESIDE, Shifren and colleagues found that women in their midlife years (aged 45 to 64) had the highest rate of any distressing sexual problem: 14.8%. Younger women (aged 18 to 44 years) had a rate of 10.8%; older women (aged 65 years or older) had a rate of 8.9%.1
The most prevalent FSD was hypoactive sexual desire disorder,1 which in 2013 was renamed sexual interest and arousal disorder in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition.2 As with any distressing FSD, reports of being distressed about low sexual desire were highest for midlife women (12.3%) relative to younger (8.9%) and older (7.4%) women.1
Unfortunately, decreased desire can have a ripple effect that goes well beyond a patient’s sexual health. A less-than-satisfying sex life can have a significant negative impact on self-image, possibly leading to depression or overall mood instability, which in turn can put undue strain on personal relationships.1,3 A patient’s entire quality of life can be affected negatively.
With so much at stake, it is important for physicians to take a more active role in addressing the sexual health of their patients. Emphasizing wellness can help reduce the stigma of sexual dysfunction, break the silence, and open up patient–physician communication.4 There is also much to be gained by helping patients realize that having positive and respectful relationships is protective for health, including sexual health.4 Likewise, patients benefit from acknowledging that sexual health is an element of overall health and contributes to it.4
Toward these ends, more discussion with patients is needed. According to a 2008 national study, although 63% of US ObGyns surveyed indicated that they routinely asked their patients about sexual activity, only 40% asked about sexual problems, and only 29% asked patients if their sex lives were satisfying.5
Without communication, information is missed, and clinicians easily can overlook their patients’ sexual dysfunction and need for intervention. For midlife women, who are disproportionately affected by dysfunction relative to younger and older women, and for whom the rate of menopausal symptoms increases over the transition years, the results of going undiagnosed and untreated can be especially troubling. As reported in one study, for example, the rate of bothersome vulvovaginal atrophy, which can be a source of sexual dysfunction, increased from less than 5% at premenopause to almost 50% at 3 years postmenopause.6 What is standing in our way, however, and how can we overcome the hurdles to an open-door approach and meaningful conversation?
Obstacles to taking a sexual historyInitiating a sexual history can be like opening Pandora’s box. How do clinicians deal with the problems that come out? Some clinicians worry about embarrassing a patient with the first few questions about sexual health. Male gynecologists may feel awkward asking a patient about sex—particularly an older, midlife patient. The problem with not starting the conversation is that the midlife patient is often the one in the most distress, and the one most in need of treatment. Only by having the sexual health discussion can clinicians identify any issues and begin to address them.
Icebreakers to jump-start the conversation
Asking open-ended questions works best. Here are some options for starting a conversation with a midlife patient:
- say, “Many women around menopause develop sexual problems. Have you noticed any changes?”
- say, “It is part of my routine to ask about sexual health. Tell me if you have any concerns.”
- add a brief sexual symptom checklist (FIGURE 1) to the patient history or intake form. The checklist shown here starts by asking if the patient is satisfied, yes or no, with her sexual function. If yes, the satisfied patient (and the clinician) can proceed to the next section on the form. If no, the dissatisfied patient can answer additional questions about problems related to sexual desire, arousal, orgasm, and dyspareunia.
Such tools as checklists are often needed to bridge the wide communication gap between patients and physicians. Of the 255 women who reported experiencing dyspareunia in the Revealing Vaginal Effects at Midlife (REVEAL) study, almost half (44%) indicated that they had not spoken with their health care clinician about it.7 Another 44% had spoken about the problem but on their own initiative. In only 10% of cases had a physician started the conversation.
Clinicians can and should do better. Many of us have known our patients for years—given them their annual examinations, delivered their babies, performed their surgeries, become familiar with their bodies and intimate medical histories. We are uniquely qualified to start conversations on sexual health. A clinician who examines tissues and sees a decrease in vaginal caliber and pallor must say something. In some cases, the vagina is dry, but the patient has not been having lubrication problems. In other cases, a more serious condition might be involved. The important thing is to open up a conversation and talk about treatments.
CASE Continued
As today’s office visit wraps up and your patient begins moving for the door, you say, “Your hot flashes aren’t bothering you, but some women start experiencing certain sexual problems around this time in life. Have you noticed any issues?”
“Well, I have been having more burning during intercourse,” your patient responds.
On hearing this, you say, “That’s very important, Mrs. X, and I am glad you told me about it. I would like to discuss your concern a bit more, so let’s make another appointment to do just that.”
At the next visit, as part of the discussion, you give your patient a 15-minute sexual status examination.
Sexual status examination
Performing this examination helps clinicians see patterns in both sexual behavior and sexual health, which in turn can make it easier to recognize any dysfunction that might subsequently develop. The key to this process is establishing trust with the patient and having her feel comfortable with the discussion.
The patient remains fully clothed during this 15-minute session, which takes place with guarantees of nonjudgmental listening, confidentiality, privacy, and no interruptions. With the topic of sex being so personal, it should be emphasized that she is simply giving the clinician information, as she does on other health-related matters.
Establish her sexual status. Begin by asking the patient to describe her most recent or typical sexual encounter, including details such as day, time, location, type of activity, thoughts and feelings, and responses.
Potential issues can become apparent immediately. A patient may not have had a sexual encounter recently, or ever. Another may want sex, or more sex, but sees obstacles or lack of opportunity. Each of these is an issue to be explored, if the patient allows.
A patient can be sexually active in a number of ways, as the definition varies among population groups (race and age) and individuals. Sex is not only intercourse or oral sex—it is also kissing, touching, and hugging. Some people have an expansive view of what it is to be sexually active. When the patient mentions an encounter, ask what day, what time, where (at home, in a hotel room, at the office), and what type of activity (foreplay, oral sex, manual stimulation, intercourse, and position). Following up, ask what the patient was thinking or feeling about the encounter. For example, were there distracting thoughts or feelings of guilt? How did the patient and her partner respond during the encounter?
Assess for sexual dysfunction. After assessing the patient’s sexual status, turn to dysfunction. Arousal, pain, orgasm, and satisfaction are 4 areas of interest. Did the patient have difficulty becoming aroused? Was there a problem with lubrication? Did she have an orgasm? Was sex painful? How did she feel in terms of overall satisfaction?
In general, patients are comfortable speaking about sexual function and health. Having this talk can help identify a pattern, which can be discussed further during another visit. Such a follow-up would not take long—a level 3 visit should suffice.
Differential diagnosis. Consider the effects of current medications.8,9 The psychiatric illnesses and general health factors that may affect sexual function should be considered as well (FIGURE 2).10–22
When is it important to refer?
There are many reasons to refer a patient to another physician, including:
- a recommended treatment is not working
- abuse is suspected
- the patient shows symptoms of depression, anxiety, or another psychiatric condition
- a chronic, generalized (vs situational) disorder may be involved
- physical pain issues must be addressed
- you simply do not feel comfortable with a particular problem or patient.
Given the range of potential issues associated with sexual function, it is important to be able to provide the patient with expert assistance from a multidisciplinary team of specialists. This team can include psychologists, psychiatrists, counselors, sex educators, and, for pain issues, pelvic floor specialists and pelvic floor physical therapists. These colleagues are thoroughly familiar with the kinds of issues that can arise, and can offer alternative and adjunctive therapies.
Referrals also can be made for the latest nonpharmacologic and FDA-approved pharmacologic treatment options. Specialists tend to be familiar with these options, some of which are available only recently.
It is important to ask patients about sexual function and, if necessary, give them access to the best treatment options.
CASE Resolved
During the sexual status examination, your patient describes her most recent sexual encounter with her husband. She is frustrated with her lack of sexual response and describes a dry, tearing sensation during intercourse. You recommend first-line treatment with vaginal lubricants, preferably iso-osmolar aqueous− or silicone/dimethicone−based lubricants during intercourse. You also can discuss topical estrogen therapy via estradiol cream, conjugated equine estrogen cream, estradiol tablets in the vagina, or the estrogen ring. She is reassured that topical estrogen use will not pose significant risk for cancer, stroke, heart disease, or blood clot and that progesterone treatment is not necessary.
For patients who are particularly concerned about vaginal estrogen use, 2 or 3 times weekly use of a vaginal moisturizer could be an alternative for genitourinary symptoms and dyspareunia.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
CASE Patient may benefit from treatment for dyspareuniaA 54-year-old woman has been in your care for more than 15 years. Three years ago, at her well-woman examination, she was not yet having symptoms of menopause. Now, during her current examination, she reports hot flashes, which she says are not bothersome. In passing, she also says, “I don’t want to take hormone therapy,” but then is not overly conversational or responsive to your questions. She does mention having had 3 urinary tract infections over the past 8 months. On physical examination, you note mildly atrophied vaginal tissue.
Your patient does not bring up any sexual concerns, and so far you have not directly asked about sexual health. However, the time remaining in this visit is limited, and your patient, whose daughter is sitting in the waiting area, seems anxious to finish and leave. Still, you want to broach the subject of your patient’s sexual health. What are your best options?
We learned a lot about women’s perceptions regarding their sexual health in the 2008 Prevalence of Female Sexual Problems Associated with Distress and Determinants of Treatment Seeking study (PRESIDE). Approximately 43% of 31,581 questionnaire respondents reported dysfunction in sexual desire, arousal, or orgasm.1 Results also showed that 11.5% of the respondents with any of these types of female sexual dysfunction (FSD) were distressed about it. For clinicians, knowing who these women are is key in recognizing and treating FSD.
Important to the opening case, in PRESIDE, Shifren and colleagues found that women in their midlife years (aged 45 to 64) had the highest rate of any distressing sexual problem: 14.8%. Younger women (aged 18 to 44 years) had a rate of 10.8%; older women (aged 65 years or older) had a rate of 8.9%.1
The most prevalent FSD was hypoactive sexual desire disorder,1 which in 2013 was renamed sexual interest and arousal disorder in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition.2 As with any distressing FSD, reports of being distressed about low sexual desire were highest for midlife women (12.3%) relative to younger (8.9%) and older (7.4%) women.1
Unfortunately, decreased desire can have a ripple effect that goes well beyond a patient’s sexual health. A less-than-satisfying sex life can have a significant negative impact on self-image, possibly leading to depression or overall mood instability, which in turn can put undue strain on personal relationships.1,3 A patient’s entire quality of life can be affected negatively.
With so much at stake, it is important for physicians to take a more active role in addressing the sexual health of their patients. Emphasizing wellness can help reduce the stigma of sexual dysfunction, break the silence, and open up patient–physician communication.4 There is also much to be gained by helping patients realize that having positive and respectful relationships is protective for health, including sexual health.4 Likewise, patients benefit from acknowledging that sexual health is an element of overall health and contributes to it.4
Toward these ends, more discussion with patients is needed. According to a 2008 national study, although 63% of US ObGyns surveyed indicated that they routinely asked their patients about sexual activity, only 40% asked about sexual problems, and only 29% asked patients if their sex lives were satisfying.5
Without communication, information is missed, and clinicians easily can overlook their patients’ sexual dysfunction and need for intervention. For midlife women, who are disproportionately affected by dysfunction relative to younger and older women, and for whom the rate of menopausal symptoms increases over the transition years, the results of going undiagnosed and untreated can be especially troubling. As reported in one study, for example, the rate of bothersome vulvovaginal atrophy, which can be a source of sexual dysfunction, increased from less than 5% at premenopause to almost 50% at 3 years postmenopause.6 What is standing in our way, however, and how can we overcome the hurdles to an open-door approach and meaningful conversation?
Obstacles to taking a sexual historyInitiating a sexual history can be like opening Pandora’s box. How do clinicians deal with the problems that come out? Some clinicians worry about embarrassing a patient with the first few questions about sexual health. Male gynecologists may feel awkward asking a patient about sex—particularly an older, midlife patient. The problem with not starting the conversation is that the midlife patient is often the one in the most distress, and the one most in need of treatment. Only by having the sexual health discussion can clinicians identify any issues and begin to address them.
Icebreakers to jump-start the conversation
Asking open-ended questions works best. Here are some options for starting a conversation with a midlife patient:
- say, “Many women around menopause develop sexual problems. Have you noticed any changes?”
- say, “It is part of my routine to ask about sexual health. Tell me if you have any concerns.”
- add a brief sexual symptom checklist (FIGURE 1) to the patient history or intake form. The checklist shown here starts by asking if the patient is satisfied, yes or no, with her sexual function. If yes, the satisfied patient (and the clinician) can proceed to the next section on the form. If no, the dissatisfied patient can answer additional questions about problems related to sexual desire, arousal, orgasm, and dyspareunia.
Such tools as checklists are often needed to bridge the wide communication gap between patients and physicians. Of the 255 women who reported experiencing dyspareunia in the Revealing Vaginal Effects at Midlife (REVEAL) study, almost half (44%) indicated that they had not spoken with their health care clinician about it.7 Another 44% had spoken about the problem but on their own initiative. In only 10% of cases had a physician started the conversation.
Clinicians can and should do better. Many of us have known our patients for years—given them their annual examinations, delivered their babies, performed their surgeries, become familiar with their bodies and intimate medical histories. We are uniquely qualified to start conversations on sexual health. A clinician who examines tissues and sees a decrease in vaginal caliber and pallor must say something. In some cases, the vagina is dry, but the patient has not been having lubrication problems. In other cases, a more serious condition might be involved. The important thing is to open up a conversation and talk about treatments.
CASE Continued
As today’s office visit wraps up and your patient begins moving for the door, you say, “Your hot flashes aren’t bothering you, but some women start experiencing certain sexual problems around this time in life. Have you noticed any issues?”
“Well, I have been having more burning during intercourse,” your patient responds.
On hearing this, you say, “That’s very important, Mrs. X, and I am glad you told me about it. I would like to discuss your concern a bit more, so let’s make another appointment to do just that.”
At the next visit, as part of the discussion, you give your patient a 15-minute sexual status examination.
Sexual status examination
Performing this examination helps clinicians see patterns in both sexual behavior and sexual health, which in turn can make it easier to recognize any dysfunction that might subsequently develop. The key to this process is establishing trust with the patient and having her feel comfortable with the discussion.
The patient remains fully clothed during this 15-minute session, which takes place with guarantees of nonjudgmental listening, confidentiality, privacy, and no interruptions. With the topic of sex being so personal, it should be emphasized that she is simply giving the clinician information, as she does on other health-related matters.
Establish her sexual status. Begin by asking the patient to describe her most recent or typical sexual encounter, including details such as day, time, location, type of activity, thoughts and feelings, and responses.
Potential issues can become apparent immediately. A patient may not have had a sexual encounter recently, or ever. Another may want sex, or more sex, but sees obstacles or lack of opportunity. Each of these is an issue to be explored, if the patient allows.
A patient can be sexually active in a number of ways, as the definition varies among population groups (race and age) and individuals. Sex is not only intercourse or oral sex—it is also kissing, touching, and hugging. Some people have an expansive view of what it is to be sexually active. When the patient mentions an encounter, ask what day, what time, where (at home, in a hotel room, at the office), and what type of activity (foreplay, oral sex, manual stimulation, intercourse, and position). Following up, ask what the patient was thinking or feeling about the encounter. For example, were there distracting thoughts or feelings of guilt? How did the patient and her partner respond during the encounter?
Assess for sexual dysfunction. After assessing the patient’s sexual status, turn to dysfunction. Arousal, pain, orgasm, and satisfaction are 4 areas of interest. Did the patient have difficulty becoming aroused? Was there a problem with lubrication? Did she have an orgasm? Was sex painful? How did she feel in terms of overall satisfaction?
In general, patients are comfortable speaking about sexual function and health. Having this talk can help identify a pattern, which can be discussed further during another visit. Such a follow-up would not take long—a level 3 visit should suffice.
Differential diagnosis. Consider the effects of current medications.8,9 The psychiatric illnesses and general health factors that may affect sexual function should be considered as well (FIGURE 2).10–22
When is it important to refer?
There are many reasons to refer a patient to another physician, including:
- a recommended treatment is not working
- abuse is suspected
- the patient shows symptoms of depression, anxiety, or another psychiatric condition
- a chronic, generalized (vs situational) disorder may be involved
- physical pain issues must be addressed
- you simply do not feel comfortable with a particular problem or patient.
Given the range of potential issues associated with sexual function, it is important to be able to provide the patient with expert assistance from a multidisciplinary team of specialists. This team can include psychologists, psychiatrists, counselors, sex educators, and, for pain issues, pelvic floor specialists and pelvic floor physical therapists. These colleagues are thoroughly familiar with the kinds of issues that can arise, and can offer alternative and adjunctive therapies.
Referrals also can be made for the latest nonpharmacologic and FDA-approved pharmacologic treatment options. Specialists tend to be familiar with these options, some of which are available only recently.
It is important to ask patients about sexual function and, if necessary, give them access to the best treatment options.
CASE Resolved
During the sexual status examination, your patient describes her most recent sexual encounter with her husband. She is frustrated with her lack of sexual response and describes a dry, tearing sensation during intercourse. You recommend first-line treatment with vaginal lubricants, preferably iso-osmolar aqueous− or silicone/dimethicone−based lubricants during intercourse. You also can discuss topical estrogen therapy via estradiol cream, conjugated equine estrogen cream, estradiol tablets in the vagina, or the estrogen ring. She is reassured that topical estrogen use will not pose significant risk for cancer, stroke, heart disease, or blood clot and that progesterone treatment is not necessary.
For patients who are particularly concerned about vaginal estrogen use, 2 or 3 times weekly use of a vaginal moisturizer could be an alternative for genitourinary symptoms and dyspareunia.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Shifren JL, Monz BU, Russo PA, Segreti A, Johannes CB. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112(5):970−978.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). Washington, DC: American Psychiatric Association; 2013.
- Leiblum SR, Koochaki PE, Rodenberg CA, Barton IP, Rosen RC. Hypoactive sexual desire disorder in postmenopausal women: US results from the Women’s International Study of Health and Sexuality (WISHeS). Menopause. 2006;13(1):46−56.
- Satcher D, Hook EW 3rd, Coleman E. Sexual health in America: improving patient care and public health. JAMA. 2015;314(8):765−766.
- Sobecki JN, Curlin FA, Rasinski KA, Lindau ST. What we don’t talk about when we don’t talk about sex: results of a national survey of U.S. obstetrician/gynecologists. J Sex Med. 2012;9(5):1285−1294.
- Dennerstein L, Dudley EC, Hopper JL, Guthrie JR, Burger HG. A prospective population-based study of menopausal symptoms. Obstet Gynecol. 2000;96(3):351−358.
- Shifren JL, Johannes CB, Monz BU, Russo PA, Bennett L, Rosen R. Help-seeking behavior of women with self-reported distressing sexual problems. J Womens Health. 2009;18(4):461−468.
- Basson R, Schultz WW. Sexual sequelae of general medical disorders. Lancet. 2007;369(9559):409−424.
- Kingsberg SA, Janata JW. Female sexual disorders: assessment, diagnosis, and treatment. Urol Clin North Am. 2007;34(4):497−506, v−vi.
- Casper RC, Redmond DE Jr, Katz MM, Schaffer CB, Davis JM, Koslow SH. Somatic symptoms in primary affective disorder. Presence and relationship to the classification of depression. Arch Gen Psychiatry. 1985;42(11):1098−1104.
- van Lankveld JJ, Grotjohann Y. Psychiatric comorbidity in heterosexual couples with sexual dysfunction assessed with the Composite International Diagnostic Interview. Arch Sex Behav. 2000;29(5):479−498.
- Shifren JL, Monz BU, Russo PA, Segreti A, Johannes CB. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112(5):970−978.
- Friedman S, Harrison G. Sexual histories, attitudes, and behavior of schizophrenic and “normal” women. Arch Sex Behav. 1984;13(6):555−567.
- Okeahialam BN, Obeka NC. Sexual dysfunction in female hypertensives. J Natl Med Assoc. 2006;98(4):638−640.
- Rees PM, Fowler CJ, Maas CP. Sexual function in men and women with neurological disorders. Lancet. 2007;369(9560):512−525.
- Bhasin S, Enzlin P, Coviello A, Basson R. Sexual dysfunction in men and women with endocrine disorders. Lancet. 2007;369(9561):597−611.
- Aslan G, KöseoTimesğlu H, Sadik O, Gimen S, Cihan A, Esen A. Sexual function in women with urinary incontinence. Int J Impot Res. 2005;17(3):248−251.
- Smith EM, Ritchie JM, Galask R, Pugh EE, Jia J, Ricks-McGillan J. Case–control study of vulvar vestibulitis risk associated with genital infections. Infect Dis Obstet Gynecol. 2002;10(4):193−202.
- Baksu B, Davas I, Agar E, Akyol A, Varolan A. The effect of mode of delivery on postpartum sexual functioning in primiparous women. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(4):401−406.
- Abdel-Nasser AM, Ali EI. Determinants of sexual disability and dissatisfaction in female patients with rheumatoid arthritis. Clin Rheumatol. 2006;25(6):822−830.
- Sampogna F, Gisondi P, Tabolli S, Abeni D; IDI Multipurpose Psoriasis Research on Vital Experiences investigators. Impairment of sexual life in patients with psoriasis. Dermatology. 2007;214(2):144−150.
- Mathias C, Cardeal Mendes CM, Pondé de Sena E, et al. An open-label, fixed-dose study of bupropion effect on sexual function scores in women treated for breast cancer. Ann Oncol. 2006;17(12):1792−1796.
- Shifren JL, Monz BU, Russo PA, Segreti A, Johannes CB. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112(5):970−978.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). Washington, DC: American Psychiatric Association; 2013.
- Leiblum SR, Koochaki PE, Rodenberg CA, Barton IP, Rosen RC. Hypoactive sexual desire disorder in postmenopausal women: US results from the Women’s International Study of Health and Sexuality (WISHeS). Menopause. 2006;13(1):46−56.
- Satcher D, Hook EW 3rd, Coleman E. Sexual health in America: improving patient care and public health. JAMA. 2015;314(8):765−766.
- Sobecki JN, Curlin FA, Rasinski KA, Lindau ST. What we don’t talk about when we don’t talk about sex: results of a national survey of U.S. obstetrician/gynecologists. J Sex Med. 2012;9(5):1285−1294.
- Dennerstein L, Dudley EC, Hopper JL, Guthrie JR, Burger HG. A prospective population-based study of menopausal symptoms. Obstet Gynecol. 2000;96(3):351−358.
- Shifren JL, Johannes CB, Monz BU, Russo PA, Bennett L, Rosen R. Help-seeking behavior of women with self-reported distressing sexual problems. J Womens Health. 2009;18(4):461−468.
- Basson R, Schultz WW. Sexual sequelae of general medical disorders. Lancet. 2007;369(9559):409−424.
- Kingsberg SA, Janata JW. Female sexual disorders: assessment, diagnosis, and treatment. Urol Clin North Am. 2007;34(4):497−506, v−vi.
- Casper RC, Redmond DE Jr, Katz MM, Schaffer CB, Davis JM, Koslow SH. Somatic symptoms in primary affective disorder. Presence and relationship to the classification of depression. Arch Gen Psychiatry. 1985;42(11):1098−1104.
- van Lankveld JJ, Grotjohann Y. Psychiatric comorbidity in heterosexual couples with sexual dysfunction assessed with the Composite International Diagnostic Interview. Arch Sex Behav. 2000;29(5):479−498.
- Shifren JL, Monz BU, Russo PA, Segreti A, Johannes CB. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112(5):970−978.
- Friedman S, Harrison G. Sexual histories, attitudes, and behavior of schizophrenic and “normal” women. Arch Sex Behav. 1984;13(6):555−567.
- Okeahialam BN, Obeka NC. Sexual dysfunction in female hypertensives. J Natl Med Assoc. 2006;98(4):638−640.
- Rees PM, Fowler CJ, Maas CP. Sexual function in men and women with neurological disorders. Lancet. 2007;369(9560):512−525.
- Bhasin S, Enzlin P, Coviello A, Basson R. Sexual dysfunction in men and women with endocrine disorders. Lancet. 2007;369(9561):597−611.
- Aslan G, KöseoTimesğlu H, Sadik O, Gimen S, Cihan A, Esen A. Sexual function in women with urinary incontinence. Int J Impot Res. 2005;17(3):248−251.
- Smith EM, Ritchie JM, Galask R, Pugh EE, Jia J, Ricks-McGillan J. Case–control study of vulvar vestibulitis risk associated with genital infections. Infect Dis Obstet Gynecol. 2002;10(4):193−202.
- Baksu B, Davas I, Agar E, Akyol A, Varolan A. The effect of mode of delivery on postpartum sexual functioning in primiparous women. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(4):401−406.
- Abdel-Nasser AM, Ali EI. Determinants of sexual disability and dissatisfaction in female patients with rheumatoid arthritis. Clin Rheumatol. 2006;25(6):822−830.
- Sampogna F, Gisondi P, Tabolli S, Abeni D; IDI Multipurpose Psoriasis Research on Vital Experiences investigators. Impairment of sexual life in patients with psoriasis. Dermatology. 2007;214(2):144−150.
- Mathias C, Cardeal Mendes CM, Pondé de Sena E, et al. An open-label, fixed-dose study of bupropion effect on sexual function scores in women treated for breast cancer. Ann Oncol. 2006;17(12):1792−1796.
In this Article
- Conversation icebreakers
- The sexual status examination
- When to refer
Multisite NIH-sponsored research can now use single IRB
In an effort to streamline multisite clinical research, the National Institutes of Health announced a new policy related to the use of institutional review boards (IRBs).
The new policy sets “the expectation that multisite studies conducting the same protocol use a single IRB to carry out the ethical review of the proposed research,” NIH Director Francis S. Collins, MD, said in a June 21 statement. The policy goes into effect May 25, 2017, and applies only to domestic research.
Currently, for most multisite studies, the IRB at each site conducts an independent review of protocol and consent documents, which Dr. Collins said “adds time, but generally does not meaningfully enhance protections for the participants. This new NIH policy seeks to end duplicative reviews that slow down the start of the research.”
Michael Pichichero, MD, director of the research institute, Rochester (N.Y.) General Hospital, called the change “a good policy. Allowing a single IRB to review and not go through multiple reviews will help get clinical trials going faster,” he said in an interview. “The policies and principles of IRB review are the same for all U.S. Food and Drug Administration–approved IRBs, so the concern that inappropriate approval might be given is highly unlikely.”
In an effort to streamline multisite clinical research, the National Institutes of Health announced a new policy related to the use of institutional review boards (IRBs).
The new policy sets “the expectation that multisite studies conducting the same protocol use a single IRB to carry out the ethical review of the proposed research,” NIH Director Francis S. Collins, MD, said in a June 21 statement. The policy goes into effect May 25, 2017, and applies only to domestic research.
Currently, for most multisite studies, the IRB at each site conducts an independent review of protocol and consent documents, which Dr. Collins said “adds time, but generally does not meaningfully enhance protections for the participants. This new NIH policy seeks to end duplicative reviews that slow down the start of the research.”
Michael Pichichero, MD, director of the research institute, Rochester (N.Y.) General Hospital, called the change “a good policy. Allowing a single IRB to review and not go through multiple reviews will help get clinical trials going faster,” he said in an interview. “The policies and principles of IRB review are the same for all U.S. Food and Drug Administration–approved IRBs, so the concern that inappropriate approval might be given is highly unlikely.”
In an effort to streamline multisite clinical research, the National Institutes of Health announced a new policy related to the use of institutional review boards (IRBs).
The new policy sets “the expectation that multisite studies conducting the same protocol use a single IRB to carry out the ethical review of the proposed research,” NIH Director Francis S. Collins, MD, said in a June 21 statement. The policy goes into effect May 25, 2017, and applies only to domestic research.
Currently, for most multisite studies, the IRB at each site conducts an independent review of protocol and consent documents, which Dr. Collins said “adds time, but generally does not meaningfully enhance protections for the participants. This new NIH policy seeks to end duplicative reviews that slow down the start of the research.”
Michael Pichichero, MD, director of the research institute, Rochester (N.Y.) General Hospital, called the change “a good policy. Allowing a single IRB to review and not go through multiple reviews will help get clinical trials going faster,” he said in an interview. “The policies and principles of IRB review are the same for all U.S. Food and Drug Administration–approved IRBs, so the concern that inappropriate approval might be given is highly unlikely.”
Laparoscopic salpingectomy and cornual resection repurposed
I am pleased to introduce this month’s video, from the Division of Minimally Invasive Gynecologic Surgery (MIGS) at Penn State. Dr. Michelle Pacis addresses an increasingly important topic to MIGS surgeons: removal of previously hysteroscopically placed tubal occlusion devices. These devices offer a permanent alterative to laparoscopic sterilization. If women require their removal, however, whether desired or because of complications, gynecologic surgeons must be familiar with the steps involved.
The following video was produced in order to demonstrate a reproducible technique for laparoscopic removal of a device for women requesting this procedure. Key objectives of the video include:
- review of the techniques available to remove hysteroscopic tubal occlusion devices, as well as the contraindications and advantages/disadvantages of these approaches.
- discuss recommended imaging and materials required for the technique described and tips for their use.
- demonstrate salpingectomy and repair technique.
I hope that you find this month’s video helpful to your surgical practice.

Share your thoughts on this video! Send your Letter to the Editor to [email protected]
I am pleased to introduce this month’s video, from the Division of Minimally Invasive Gynecologic Surgery (MIGS) at Penn State. Dr. Michelle Pacis addresses an increasingly important topic to MIGS surgeons: removal of previously hysteroscopically placed tubal occlusion devices. These devices offer a permanent alterative to laparoscopic sterilization. If women require their removal, however, whether desired or because of complications, gynecologic surgeons must be familiar with the steps involved.
The following video was produced in order to demonstrate a reproducible technique for laparoscopic removal of a device for women requesting this procedure. Key objectives of the video include:
- review of the techniques available to remove hysteroscopic tubal occlusion devices, as well as the contraindications and advantages/disadvantages of these approaches.
- discuss recommended imaging and materials required for the technique described and tips for their use.
- demonstrate salpingectomy and repair technique.
I hope that you find this month’s video helpful to your surgical practice.

Share your thoughts on this video! Send your Letter to the Editor to [email protected]
I am pleased to introduce this month’s video, from the Division of Minimally Invasive Gynecologic Surgery (MIGS) at Penn State. Dr. Michelle Pacis addresses an increasingly important topic to MIGS surgeons: removal of previously hysteroscopically placed tubal occlusion devices. These devices offer a permanent alterative to laparoscopic sterilization. If women require their removal, however, whether desired or because of complications, gynecologic surgeons must be familiar with the steps involved.
The following video was produced in order to demonstrate a reproducible technique for laparoscopic removal of a device for women requesting this procedure. Key objectives of the video include:
- review of the techniques available to remove hysteroscopic tubal occlusion devices, as well as the contraindications and advantages/disadvantages of these approaches.
- discuss recommended imaging and materials required for the technique described and tips for their use.
- demonstrate salpingectomy and repair technique.
I hope that you find this month’s video helpful to your surgical practice.

Share your thoughts on this video! Send your Letter to the Editor to [email protected]
Team documentation—the good, the surprising
The clerical work involved in managing the electronic health record (EHR) is clearly not at the top of the skill set for physicians, yet many office-based clinicians find themselves bogged down in this work with no easy way out.
However, practices that are adopting team-based care—where each team member works at the top of his or her skill set—are finding a solution in the form of scribing or team documentation. This approach can ease that burden and perhaps even help to curb physician burnout in the process. But many questions still surround this approach, notably: What do we know about the quality of this documentation?
A recent study published by Misra-Hebert and colleagues reported on this issue.1 It provides some insight—and reason for optimism, especially because the study authors found that outpatient notes from trained staff stack up quite well when compared to those of physicians. Having worked myself with this approach to documentation, I can attest to its benefits, as well.
Team documented notes compare well
There are 2 different ways that physicians can get help with documentation. One involves the use of trained scribes, who come from a variety of backgrounds and are charged with writing down, or scribing, what the physician says. The other involves training staff, usually certified medical assistants (CMAs) or licensed practical nurses (LPNs), to take on a wide variety of additional duties including refill management, care gap closure, and most of the duties concerning the EHR—including documentation.
Misra-Hebert and colleagues studied the second approach in a retrospective chart review of ambulatory progress notes written before and after 8 practice sites transitioned to using medical assistants as scribes. Comparing notes relating to diabetes encounters and same-day appointments, the study authors found important evidence that using staff in this way does not adversely affect—and may even enhance—documentation previously done entirely by physicians. For diabetes encounters, scribed notes were rated higher in overall quality, as well as more up to date, thorough, useful, and comprehensible, than unscribed notes.1
Scribing versus team documentation: terminology can be important
A person who is serving in a medical scribe role is a "personal assistant to the physician; performing documentation in the [electronic health record], gathering information for the patient's visit, and partnering with the physician to deliver the pinnacle of efficient patient care," according to Scribe America, the largest US company that employs scribes, providing their services to hospitals, emergency departments, and outpatient care and urgent care facilities, etc.1
Scribes versus team-based care
Scribe America mainly employs medical students, offering students a way to become exposed to physicians, mentors, and medical care. In fact, they note on their website that "a background in medical scribing is quickly becoming the standard for premedical experience, and is suggested by medical school acceptance committees across the country."2
Bellin Health, and many practices transitioning to team-based care, do not use medical students as scribes but rather "train up" employed certified medical assistants and licensed practical nurses to perform what they call "team documentation." This is the model described by Misra-Hebert and colleagues.3 The advantage is that these licensed health care workers can perform many other aspects of patient care, such as agenda setting and basic health coaching, and can perform additional work in the electronic health record, such as order entry and pending refills.
References
- What is a medical scribe? ScribeAmerica website. http://www.scribeamerica.com/what_is_medical_scribe.html. Accessed June 20, 2016. .
- Why be a medical scribe? ScribeAmerica website. http://www.scribeamerica.com/why_be_a_medical_scribe.html. Accessed June 20, 2016.
- Misra-Hebert AD, Amah L, Rabovsky A, et al. Medical scribes: how do their notes stack up? J Fam Pract. 2016;65(3):155−159.
In my experience
This change in the way we approach EHRs involves commitment, as I have seen first hand. There needs to be significant training to make this work and there needs to be more staff, since physicians require 2 of these valuable team members to function effectively. (At least that has been our experience.)
How has the physician’s role changed? Prior to team-based care, I would try to enter information into the EHR in the room while seeing the patient. After the visit, I would go to the computer at my station and use voice recognition software to add information. Now, the CMA/LPN does the initial documentation and other EHR work, while I am able to focus on the patient without the distraction of the computer. When I leave the room, the CMA/LPN stays with the patient, arranging necessary tests or consults, scheduling future labs and appointments, and reviewing the chart, all before seeing the next patient.
We are in the process of implementing team-based care throughout our 32-location health care system and have found that using CMAs and LPNs to assist with documentation is a “win” for everyone. And since the problems with burnout and all of the other reasons for making this transformation to team-based care applies to all office-based specialties, we plan to have our entire system adopt this model.
An ObGyn group was the first specialty group to pilot this model in our system. In fact, the American College of Obstetricians and Gynecologists’ interprofessional Task Force of Collaborative Practice published in March 2016 a strong recommendation that all practices across all specialties adopt team-based care.2 Among the aims of this care are that it should “respond to emerging demands and reduce undue burdens on health care providers.”
In order for this transformation to teambased care to be successful and sustainable at Bellin Health, we realized that we had to achieve 3 wins:
A win for the patient. Patients immediately notice that their physicians are now able to focus on them during the office visit, since the physicians no longer have to tend to the demands of the computer. In addition, since the CMAs/LPNs are with patients during the entire visit, the patients bond with them and feel the extra support from this relationship.
A win for the care team. Physician satisfaction has never been higher. Charts are usually closed at the end of each half-day. There is no need to take work home at night. CMAs/LPNs feel empowered and meaningfully involved in patient care. Their increase in satisfaction mirrors that of the physicians.
A win for the system. Not only are quality measures improving, but access improves since this team support increases efficiency. We are able to see more patients per day and are billing at a higher level of service, since there is more time to attend to more of the patient’s needs, thanks to the additional team support.
Team documentation can help bring the joy back
There is much talk about putting joy back into the practice of medicine. But the benchmark of any change needs to be whether it helps our patients. I believe that team documentation does. Happier, less burned-out physicians are able to better focus on patients during their visit. As one patient recently said to me at the end of a visit, “I feel like I’ve got my doctor back.” That is something that patients, and doctors alike, can feel good about.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Misra-Hebert AD, Amah L, Rabovsky A, et al. Medical scribes: how do their notes stack up? J Fam Pract. 2016;65(3):155−159.
- Jennings J, Nielson P, Buck, ML, et al. Executive summary: Collaboration in Practice: Implementing Team-Based Care: Report of the American College of Obstetricians and Gynecologists’ Task Force on Collaborative Practice. Obstet Gynecol. 2016;127(3):612−627.
The clerical work involved in managing the electronic health record (EHR) is clearly not at the top of the skill set for physicians, yet many office-based clinicians find themselves bogged down in this work with no easy way out.
However, practices that are adopting team-based care—where each team member works at the top of his or her skill set—are finding a solution in the form of scribing or team documentation. This approach can ease that burden and perhaps even help to curb physician burnout in the process. But many questions still surround this approach, notably: What do we know about the quality of this documentation?
A recent study published by Misra-Hebert and colleagues reported on this issue.1 It provides some insight—and reason for optimism, especially because the study authors found that outpatient notes from trained staff stack up quite well when compared to those of physicians. Having worked myself with this approach to documentation, I can attest to its benefits, as well.
Team documented notes compare well
There are 2 different ways that physicians can get help with documentation. One involves the use of trained scribes, who come from a variety of backgrounds and are charged with writing down, or scribing, what the physician says. The other involves training staff, usually certified medical assistants (CMAs) or licensed practical nurses (LPNs), to take on a wide variety of additional duties including refill management, care gap closure, and most of the duties concerning the EHR—including documentation.
Misra-Hebert and colleagues studied the second approach in a retrospective chart review of ambulatory progress notes written before and after 8 practice sites transitioned to using medical assistants as scribes. Comparing notes relating to diabetes encounters and same-day appointments, the study authors found important evidence that using staff in this way does not adversely affect—and may even enhance—documentation previously done entirely by physicians. For diabetes encounters, scribed notes were rated higher in overall quality, as well as more up to date, thorough, useful, and comprehensible, than unscribed notes.1
Scribing versus team documentation: terminology can be important
A person who is serving in a medical scribe role is a "personal assistant to the physician; performing documentation in the [electronic health record], gathering information for the patient's visit, and partnering with the physician to deliver the pinnacle of efficient patient care," according to Scribe America, the largest US company that employs scribes, providing their services to hospitals, emergency departments, and outpatient care and urgent care facilities, etc.1
Scribes versus team-based care
Scribe America mainly employs medical students, offering students a way to become exposed to physicians, mentors, and medical care. In fact, they note on their website that "a background in medical scribing is quickly becoming the standard for premedical experience, and is suggested by medical school acceptance committees across the country."2
Bellin Health, and many practices transitioning to team-based care, do not use medical students as scribes but rather "train up" employed certified medical assistants and licensed practical nurses to perform what they call "team documentation." This is the model described by Misra-Hebert and colleagues.3 The advantage is that these licensed health care workers can perform many other aspects of patient care, such as agenda setting and basic health coaching, and can perform additional work in the electronic health record, such as order entry and pending refills.
References
- What is a medical scribe? ScribeAmerica website. http://www.scribeamerica.com/what_is_medical_scribe.html. Accessed June 20, 2016. .
- Why be a medical scribe? ScribeAmerica website. http://www.scribeamerica.com/why_be_a_medical_scribe.html. Accessed June 20, 2016.
- Misra-Hebert AD, Amah L, Rabovsky A, et al. Medical scribes: how do their notes stack up? J Fam Pract. 2016;65(3):155−159.
In my experience
This change in the way we approach EHRs involves commitment, as I have seen first hand. There needs to be significant training to make this work and there needs to be more staff, since physicians require 2 of these valuable team members to function effectively. (At least that has been our experience.)
How has the physician’s role changed? Prior to team-based care, I would try to enter information into the EHR in the room while seeing the patient. After the visit, I would go to the computer at my station and use voice recognition software to add information. Now, the CMA/LPN does the initial documentation and other EHR work, while I am able to focus on the patient without the distraction of the computer. When I leave the room, the CMA/LPN stays with the patient, arranging necessary tests or consults, scheduling future labs and appointments, and reviewing the chart, all before seeing the next patient.
We are in the process of implementing team-based care throughout our 32-location health care system and have found that using CMAs and LPNs to assist with documentation is a “win” for everyone. And since the problems with burnout and all of the other reasons for making this transformation to team-based care applies to all office-based specialties, we plan to have our entire system adopt this model.
An ObGyn group was the first specialty group to pilot this model in our system. In fact, the American College of Obstetricians and Gynecologists’ interprofessional Task Force of Collaborative Practice published in March 2016 a strong recommendation that all practices across all specialties adopt team-based care.2 Among the aims of this care are that it should “respond to emerging demands and reduce undue burdens on health care providers.”
In order for this transformation to teambased care to be successful and sustainable at Bellin Health, we realized that we had to achieve 3 wins:
A win for the patient. Patients immediately notice that their physicians are now able to focus on them during the office visit, since the physicians no longer have to tend to the demands of the computer. In addition, since the CMAs/LPNs are with patients during the entire visit, the patients bond with them and feel the extra support from this relationship.
A win for the care team. Physician satisfaction has never been higher. Charts are usually closed at the end of each half-day. There is no need to take work home at night. CMAs/LPNs feel empowered and meaningfully involved in patient care. Their increase in satisfaction mirrors that of the physicians.
A win for the system. Not only are quality measures improving, but access improves since this team support increases efficiency. We are able to see more patients per day and are billing at a higher level of service, since there is more time to attend to more of the patient’s needs, thanks to the additional team support.
Team documentation can help bring the joy back
There is much talk about putting joy back into the practice of medicine. But the benchmark of any change needs to be whether it helps our patients. I believe that team documentation does. Happier, less burned-out physicians are able to better focus on patients during their visit. As one patient recently said to me at the end of a visit, “I feel like I’ve got my doctor back.” That is something that patients, and doctors alike, can feel good about.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The clerical work involved in managing the electronic health record (EHR) is clearly not at the top of the skill set for physicians, yet many office-based clinicians find themselves bogged down in this work with no easy way out.
However, practices that are adopting team-based care—where each team member works at the top of his or her skill set—are finding a solution in the form of scribing or team documentation. This approach can ease that burden and perhaps even help to curb physician burnout in the process. But many questions still surround this approach, notably: What do we know about the quality of this documentation?
A recent study published by Misra-Hebert and colleagues reported on this issue.1 It provides some insight—and reason for optimism, especially because the study authors found that outpatient notes from trained staff stack up quite well when compared to those of physicians. Having worked myself with this approach to documentation, I can attest to its benefits, as well.
Team documented notes compare well
There are 2 different ways that physicians can get help with documentation. One involves the use of trained scribes, who come from a variety of backgrounds and are charged with writing down, or scribing, what the physician says. The other involves training staff, usually certified medical assistants (CMAs) or licensed practical nurses (LPNs), to take on a wide variety of additional duties including refill management, care gap closure, and most of the duties concerning the EHR—including documentation.
Misra-Hebert and colleagues studied the second approach in a retrospective chart review of ambulatory progress notes written before and after 8 practice sites transitioned to using medical assistants as scribes. Comparing notes relating to diabetes encounters and same-day appointments, the study authors found important evidence that using staff in this way does not adversely affect—and may even enhance—documentation previously done entirely by physicians. For diabetes encounters, scribed notes were rated higher in overall quality, as well as more up to date, thorough, useful, and comprehensible, than unscribed notes.1
Scribing versus team documentation: terminology can be important
A person who is serving in a medical scribe role is a "personal assistant to the physician; performing documentation in the [electronic health record], gathering information for the patient's visit, and partnering with the physician to deliver the pinnacle of efficient patient care," according to Scribe America, the largest US company that employs scribes, providing their services to hospitals, emergency departments, and outpatient care and urgent care facilities, etc.1
Scribes versus team-based care
Scribe America mainly employs medical students, offering students a way to become exposed to physicians, mentors, and medical care. In fact, they note on their website that "a background in medical scribing is quickly becoming the standard for premedical experience, and is suggested by medical school acceptance committees across the country."2
Bellin Health, and many practices transitioning to team-based care, do not use medical students as scribes but rather "train up" employed certified medical assistants and licensed practical nurses to perform what they call "team documentation." This is the model described by Misra-Hebert and colleagues.3 The advantage is that these licensed health care workers can perform many other aspects of patient care, such as agenda setting and basic health coaching, and can perform additional work in the electronic health record, such as order entry and pending refills.
References
- What is a medical scribe? ScribeAmerica website. http://www.scribeamerica.com/what_is_medical_scribe.html. Accessed June 20, 2016. .
- Why be a medical scribe? ScribeAmerica website. http://www.scribeamerica.com/why_be_a_medical_scribe.html. Accessed June 20, 2016.
- Misra-Hebert AD, Amah L, Rabovsky A, et al. Medical scribes: how do their notes stack up? J Fam Pract. 2016;65(3):155−159.
In my experience
This change in the way we approach EHRs involves commitment, as I have seen first hand. There needs to be significant training to make this work and there needs to be more staff, since physicians require 2 of these valuable team members to function effectively. (At least that has been our experience.)
How has the physician’s role changed? Prior to team-based care, I would try to enter information into the EHR in the room while seeing the patient. After the visit, I would go to the computer at my station and use voice recognition software to add information. Now, the CMA/LPN does the initial documentation and other EHR work, while I am able to focus on the patient without the distraction of the computer. When I leave the room, the CMA/LPN stays with the patient, arranging necessary tests or consults, scheduling future labs and appointments, and reviewing the chart, all before seeing the next patient.
We are in the process of implementing team-based care throughout our 32-location health care system and have found that using CMAs and LPNs to assist with documentation is a “win” for everyone. And since the problems with burnout and all of the other reasons for making this transformation to team-based care applies to all office-based specialties, we plan to have our entire system adopt this model.
An ObGyn group was the first specialty group to pilot this model in our system. In fact, the American College of Obstetricians and Gynecologists’ interprofessional Task Force of Collaborative Practice published in March 2016 a strong recommendation that all practices across all specialties adopt team-based care.2 Among the aims of this care are that it should “respond to emerging demands and reduce undue burdens on health care providers.”
In order for this transformation to teambased care to be successful and sustainable at Bellin Health, we realized that we had to achieve 3 wins:
A win for the patient. Patients immediately notice that their physicians are now able to focus on them during the office visit, since the physicians no longer have to tend to the demands of the computer. In addition, since the CMAs/LPNs are with patients during the entire visit, the patients bond with them and feel the extra support from this relationship.
A win for the care team. Physician satisfaction has never been higher. Charts are usually closed at the end of each half-day. There is no need to take work home at night. CMAs/LPNs feel empowered and meaningfully involved in patient care. Their increase in satisfaction mirrors that of the physicians.
A win for the system. Not only are quality measures improving, but access improves since this team support increases efficiency. We are able to see more patients per day and are billing at a higher level of service, since there is more time to attend to more of the patient’s needs, thanks to the additional team support.
Team documentation can help bring the joy back
There is much talk about putting joy back into the practice of medicine. But the benchmark of any change needs to be whether it helps our patients. I believe that team documentation does. Happier, less burned-out physicians are able to better focus on patients during their visit. As one patient recently said to me at the end of a visit, “I feel like I’ve got my doctor back.” That is something that patients, and doctors alike, can feel good about.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Misra-Hebert AD, Amah L, Rabovsky A, et al. Medical scribes: how do their notes stack up? J Fam Pract. 2016;65(3):155−159.
- Jennings J, Nielson P, Buck, ML, et al. Executive summary: Collaboration in Practice: Implementing Team-Based Care: Report of the American College of Obstetricians and Gynecologists’ Task Force on Collaborative Practice. Obstet Gynecol. 2016;127(3):612−627.
- Misra-Hebert AD, Amah L, Rabovsky A, et al. Medical scribes: how do their notes stack up? J Fam Pract. 2016;65(3):155−159.
- Jennings J, Nielson P, Buck, ML, et al. Executive summary: Collaboration in Practice: Implementing Team-Based Care: Report of the American College of Obstetricians and Gynecologists’ Task Force on Collaborative Practice. Obstet Gynecol. 2016;127(3):612−627.
In this Article
- Scribing vs team documentation
- How has the physician’s role changed?
Public speaking fundamentals. Preparation: Tips that lead to a solid, engaging presentation
Public speaking is one of the best ways to market and promote your skills as a physician. It is an ethical way of communicating and showcasing your areas of interest and expertise to professional or lay audiences. Most physicians and health care professionals take pride in their ability to communicate. After all, that is how we take a history, discuss our findings with patients, and educate individuals on restoring or maintaining their health. Public speaking, though, for the most part is a learned skill. Except for presentations to faculty at bedside or at grand rounds, we have received little training in public speaking.
Few of us are naturally comfortable in front of a live audience or a TV or video camera. But with a little practice and diligent preparation, we can become good or even excellent, confident public speakers. This article—the first in a series of 3—provides you with preparatory tips and techniques to enhance your public speaking skills.
First, know your audience
Whether you are presenting to a group of 20 or 200, you can do certain things in advance to ensure that your presentation achieves the desired response. Most important: Know your audience. Don’t assume the audience is like you. To connect with them, you need to understand why your topic is important to them. What do they expect to learn from the presentation? Each attendee will be asking, “What’s in it for me?”
To keep listeners interested and engaged, you also must know their level of knowledge about the topic. If you are speaking to a group of residents about pelvic organ prolapse, you would use different language and content than if you were speaking to practicing primary care doctors; and these elements would be different again if you were speaking to a group of practicing urogynecologists. It’s insulting to recite basic information to highly knowledgeable physicians, or to present sophisticated technical content and complicated slides to novice physicians or lay people.
When presenting in a foreign country, learn how the culture of the audience differs from yours. How do they dress? What style of humor do they favor? How do they typically communicate? What gestures are appropriate or inappropriate? Are there religious influences to consider?
Practical steps. Before the meeting or event, speak to the organizer or meeting planner and find out the audience’s level of knowledge on the topic. Ask about audience expectations as well as demographics (such as age and background). If you are speaking at an industry event, research the event’s website and familiarize yourself with the mission of the event and who are the typical attendees. If you are presenting to a corporation, learn as much as you can about it by visiting its website, reading news reports, and reviewing associated blogs.
In addition to knowing the needs of the audience, ask the meeting planner about the goals and objectives for the program to make certain you can deliver on the requests.
Know your talk stem to stern
Review your slide material thoroughly. Understand each slide in the presentation and be comfortable with its content.
Avoid reading from slides. Reciting content that viewers can read for themselves breeds boredom and makes them lose interest. Further, when you are looking at the slides, you are not making eye contact with the audience and risk losing their attention. Good speakers are so comfortable with their slides that they can discuss each one without having to look at it.
Rehearse. The best way to achieve the foregoing is to rehearse. Your audience will be able to tell if you took the slide deck directly from a CD and loaded it into a computer and are giving the talk for the first time. You’ll need to know how long the program is to last and how long you are to speak. We suggest you practice with a timer to be certain you do not exceed the allotted time. Rehearse your talk aloud several times with all the props and audiovisual equipment you plan to use. This practice will help to curb filler words such as “ah” and “um.” It is also helpful to practice slide transitions, pauses, and even your breathing.
Prepare for the unexpected, too. Dinner meetings, for instance, may not start on time due to office or hospital delays for attending physicians, possibly resulting in a need to shorten your presentation.
Ask about the meeting agenda. If a meal is to be served, will you be speaking beforehand? This is the least favorable time slot, as you are holding people hostage before they can eat. Our preference is to speak after the appetizer is served and the orders have been taken by the wait staff. This way, attendees are not starving and they have something to drink. You can assure the waiters they won’t be disturbing you, and you can ask them to avoid walking in front of the projector. Ideally, you should end your presentation before dessert arrives and use the remaining time to field questions.
We suggest that you prepare a handout to be distributed at the end of the program, not before. You want your audience focused on you and your slides as you are speaking. Tell the audience you will be providing a handout of your presentation, which will minimize note-taking during your talk.
Your speech opening
The first and last 30 seconds of any speech probably have the most impact.1 Give extra thought, time, and effort to your opening and closing remarks. Do not open with “Good evening, it is a pleasure to be here tonight.” That wastes precious seconds.
While opening a speech with a joke or funny story is the conventional wisdom, ask yourself1:
- Is my selection appropriate to the occasion and for this audience?
- Is it in good taste?
- Does it relate to me (my service) or to the event or the group? Does it support my topic or its key points?
A humorous story or inspirational vignette that relates to your topic or audience can grab the audience’s attention. If you feel that demands more presentation skill than you possess at the outset of your public-speaking career, give the audience what you know and what they most want to hear. You know the questions that you have heard most at cocktail receptions or professional society meetings. So, put the answers to those questions in your speech.
For example. A scientist working with a major corporation was preparing a speech for a lay audience. Since most of the audience did not know what scientists do, he offered the following analogy: “Being a scientist is like doing a jigsaw puzzle in a snowstorm at night...you don’t have all the pieces...and you don’t have the picture on the front of the box to work from.” You can say more with less.1
Your closing
The closing is an important aspect of your speech. Summarize the key elements to your presentation. If you are going to take questions, a good approach is to say, “Before my closing remarks, are there any questions?” Following the questions, finish with a takeaway message that ties into your theme.1
Prepare an autobiographical introduction
We suggest that you write your own introduction and e-mail it to the person who will be introducing you. Let them know it is a suggestion that they are welcome to modify. We have found that most emcees or meeting planners are delighted to have the introduction and will use it just as you have written it. Also bring hard copy with you; many emcees will have forgotten to download what you sent. The figure shows an example of the introduction that one of us (NHB) uses, and you are welcome to modify it for your own use.
Ask about and confirm audiovisual support
Ask the meeting planner ahead of time if they will be providing the computer, projector, and screen. And if, for instance, they will provide a projector but not a computer, make sure the computer you will bring is compatible with their projector. Also, you will probably not require a microphone for a small group, but if you are speaking in a loud restaurant, a portable microphone-speaker system may be helpful.
Arrive early at the program venue to make sure the computers, projector, screen placement, and seating arrangement are all in order. Nothing can sidetrack a speaker (even a seasoned one) like a problem with the computer or equipment setup—for example, your flash drive requiring a USB port cannot connect to the sponsor’s computer, or your program created on a Mac does not run on the sponsor’s PC.
Show time: Getting ready
Another benefit in arriving early, besides being able to check on the equipment, is the chance to greet the audience members as they enter. It is easier to speak to a group of friends than strangers. And if you can remember their names, you can call on them and ask their opinion or how they might manage a patient who has the condition you are discussing. You also could suggest to the meeting planner that name tags for attendees would be helpful.
Warming up. A public speaker, like an athlete, needs to warm up physically before the event. If the facility has an anteroom available, use it for the following exercises suggested by public speaking coach Patricia Fripp1:
- Stand on one leg and shake the other (remove high heels first). When you place your raised foot back on the floor, it will feel lighter than the other one. Repeat the exercise using the other leg. Imagine your energy going down through the floor and up out of your head. While this sounds quite comical, it is not. It is a practical technique used by actors.
- Shake your hands vigorously. Hold them above your head, bending your wrists and elbows, then return your arms to your sides. This will make your hand movements more natural.
- Warm up your facial muscles by chewing in a highly exaggerated way. Do shoulder and neck rolls. Imagine you are at eye level with a clock. As you look at 12 o’clock, pull as much of your face up to the 12 as you can; move your eyes to 3 and repeat, then down to 6, and finally over to 9.
Not only do these exercises warm you up but they also relax you. The exaggerated movements help your movements to flow more naturally.1
This is just the start
Thorough preparation is key to making a solid presentation. But other factors are important too. Your goal is for the audience to take action or to implement suggestions from your presentation. In part 2 of this series, we will share tips on elements of the presentation itself that will encourage audience engagement and message retention. We will discuss how to make your message “stick” and how to make a dynamic, effective presentation that holds your audience’s attention for your entire talk.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Reference
- Fripp P. Add credibility to your business reputation through public speaking. Patricia Fripp website. http://www.fripp.com/add-credibility-to-your-business-reputation-through-public-speaking/. Accessed June 15, 2016.
Public speaking is one of the best ways to market and promote your skills as a physician. It is an ethical way of communicating and showcasing your areas of interest and expertise to professional or lay audiences. Most physicians and health care professionals take pride in their ability to communicate. After all, that is how we take a history, discuss our findings with patients, and educate individuals on restoring or maintaining their health. Public speaking, though, for the most part is a learned skill. Except for presentations to faculty at bedside or at grand rounds, we have received little training in public speaking.
Few of us are naturally comfortable in front of a live audience or a TV or video camera. But with a little practice and diligent preparation, we can become good or even excellent, confident public speakers. This article—the first in a series of 3—provides you with preparatory tips and techniques to enhance your public speaking skills.
First, know your audience
Whether you are presenting to a group of 20 or 200, you can do certain things in advance to ensure that your presentation achieves the desired response. Most important: Know your audience. Don’t assume the audience is like you. To connect with them, you need to understand why your topic is important to them. What do they expect to learn from the presentation? Each attendee will be asking, “What’s in it for me?”
To keep listeners interested and engaged, you also must know their level of knowledge about the topic. If you are speaking to a group of residents about pelvic organ prolapse, you would use different language and content than if you were speaking to practicing primary care doctors; and these elements would be different again if you were speaking to a group of practicing urogynecologists. It’s insulting to recite basic information to highly knowledgeable physicians, or to present sophisticated technical content and complicated slides to novice physicians or lay people.
When presenting in a foreign country, learn how the culture of the audience differs from yours. How do they dress? What style of humor do they favor? How do they typically communicate? What gestures are appropriate or inappropriate? Are there religious influences to consider?
Practical steps. Before the meeting or event, speak to the organizer or meeting planner and find out the audience’s level of knowledge on the topic. Ask about audience expectations as well as demographics (such as age and background). If you are speaking at an industry event, research the event’s website and familiarize yourself with the mission of the event and who are the typical attendees. If you are presenting to a corporation, learn as much as you can about it by visiting its website, reading news reports, and reviewing associated blogs.
In addition to knowing the needs of the audience, ask the meeting planner about the goals and objectives for the program to make certain you can deliver on the requests.
Know your talk stem to stern
Review your slide material thoroughly. Understand each slide in the presentation and be comfortable with its content.
Avoid reading from slides. Reciting content that viewers can read for themselves breeds boredom and makes them lose interest. Further, when you are looking at the slides, you are not making eye contact with the audience and risk losing their attention. Good speakers are so comfortable with their slides that they can discuss each one without having to look at it.
Rehearse. The best way to achieve the foregoing is to rehearse. Your audience will be able to tell if you took the slide deck directly from a CD and loaded it into a computer and are giving the talk for the first time. You’ll need to know how long the program is to last and how long you are to speak. We suggest you practice with a timer to be certain you do not exceed the allotted time. Rehearse your talk aloud several times with all the props and audiovisual equipment you plan to use. This practice will help to curb filler words such as “ah” and “um.” It is also helpful to practice slide transitions, pauses, and even your breathing.
Prepare for the unexpected, too. Dinner meetings, for instance, may not start on time due to office or hospital delays for attending physicians, possibly resulting in a need to shorten your presentation.
Ask about the meeting agenda. If a meal is to be served, will you be speaking beforehand? This is the least favorable time slot, as you are holding people hostage before they can eat. Our preference is to speak after the appetizer is served and the orders have been taken by the wait staff. This way, attendees are not starving and they have something to drink. You can assure the waiters they won’t be disturbing you, and you can ask them to avoid walking in front of the projector. Ideally, you should end your presentation before dessert arrives and use the remaining time to field questions.
We suggest that you prepare a handout to be distributed at the end of the program, not before. You want your audience focused on you and your slides as you are speaking. Tell the audience you will be providing a handout of your presentation, which will minimize note-taking during your talk.
Your speech opening
The first and last 30 seconds of any speech probably have the most impact.1 Give extra thought, time, and effort to your opening and closing remarks. Do not open with “Good evening, it is a pleasure to be here tonight.” That wastes precious seconds.
While opening a speech with a joke or funny story is the conventional wisdom, ask yourself1:
- Is my selection appropriate to the occasion and for this audience?
- Is it in good taste?
- Does it relate to me (my service) or to the event or the group? Does it support my topic or its key points?
A humorous story or inspirational vignette that relates to your topic or audience can grab the audience’s attention. If you feel that demands more presentation skill than you possess at the outset of your public-speaking career, give the audience what you know and what they most want to hear. You know the questions that you have heard most at cocktail receptions or professional society meetings. So, put the answers to those questions in your speech.
For example. A scientist working with a major corporation was preparing a speech for a lay audience. Since most of the audience did not know what scientists do, he offered the following analogy: “Being a scientist is like doing a jigsaw puzzle in a snowstorm at night...you don’t have all the pieces...and you don’t have the picture on the front of the box to work from.” You can say more with less.1
Your closing
The closing is an important aspect of your speech. Summarize the key elements to your presentation. If you are going to take questions, a good approach is to say, “Before my closing remarks, are there any questions?” Following the questions, finish with a takeaway message that ties into your theme.1
Prepare an autobiographical introduction
We suggest that you write your own introduction and e-mail it to the person who will be introducing you. Let them know it is a suggestion that they are welcome to modify. We have found that most emcees or meeting planners are delighted to have the introduction and will use it just as you have written it. Also bring hard copy with you; many emcees will have forgotten to download what you sent. The figure shows an example of the introduction that one of us (NHB) uses, and you are welcome to modify it for your own use.
Ask about and confirm audiovisual support
Ask the meeting planner ahead of time if they will be providing the computer, projector, and screen. And if, for instance, they will provide a projector but not a computer, make sure the computer you will bring is compatible with their projector. Also, you will probably not require a microphone for a small group, but if you are speaking in a loud restaurant, a portable microphone-speaker system may be helpful.
Arrive early at the program venue to make sure the computers, projector, screen placement, and seating arrangement are all in order. Nothing can sidetrack a speaker (even a seasoned one) like a problem with the computer or equipment setup—for example, your flash drive requiring a USB port cannot connect to the sponsor’s computer, or your program created on a Mac does not run on the sponsor’s PC.
Show time: Getting ready
Another benefit in arriving early, besides being able to check on the equipment, is the chance to greet the audience members as they enter. It is easier to speak to a group of friends than strangers. And if you can remember their names, you can call on them and ask their opinion or how they might manage a patient who has the condition you are discussing. You also could suggest to the meeting planner that name tags for attendees would be helpful.
Warming up. A public speaker, like an athlete, needs to warm up physically before the event. If the facility has an anteroom available, use it for the following exercises suggested by public speaking coach Patricia Fripp1:
- Stand on one leg and shake the other (remove high heels first). When you place your raised foot back on the floor, it will feel lighter than the other one. Repeat the exercise using the other leg. Imagine your energy going down through the floor and up out of your head. While this sounds quite comical, it is not. It is a practical technique used by actors.
- Shake your hands vigorously. Hold them above your head, bending your wrists and elbows, then return your arms to your sides. This will make your hand movements more natural.
- Warm up your facial muscles by chewing in a highly exaggerated way. Do shoulder and neck rolls. Imagine you are at eye level with a clock. As you look at 12 o’clock, pull as much of your face up to the 12 as you can; move your eyes to 3 and repeat, then down to 6, and finally over to 9.
Not only do these exercises warm you up but they also relax you. The exaggerated movements help your movements to flow more naturally.1
This is just the start
Thorough preparation is key to making a solid presentation. But other factors are important too. Your goal is for the audience to take action or to implement suggestions from your presentation. In part 2 of this series, we will share tips on elements of the presentation itself that will encourage audience engagement and message retention. We will discuss how to make your message “stick” and how to make a dynamic, effective presentation that holds your audience’s attention for your entire talk.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Public speaking is one of the best ways to market and promote your skills as a physician. It is an ethical way of communicating and showcasing your areas of interest and expertise to professional or lay audiences. Most physicians and health care professionals take pride in their ability to communicate. After all, that is how we take a history, discuss our findings with patients, and educate individuals on restoring or maintaining their health. Public speaking, though, for the most part is a learned skill. Except for presentations to faculty at bedside or at grand rounds, we have received little training in public speaking.
Few of us are naturally comfortable in front of a live audience or a TV or video camera. But with a little practice and diligent preparation, we can become good or even excellent, confident public speakers. This article—the first in a series of 3—provides you with preparatory tips and techniques to enhance your public speaking skills.
First, know your audience
Whether you are presenting to a group of 20 or 200, you can do certain things in advance to ensure that your presentation achieves the desired response. Most important: Know your audience. Don’t assume the audience is like you. To connect with them, you need to understand why your topic is important to them. What do they expect to learn from the presentation? Each attendee will be asking, “What’s in it for me?”
To keep listeners interested and engaged, you also must know their level of knowledge about the topic. If you are speaking to a group of residents about pelvic organ prolapse, you would use different language and content than if you were speaking to practicing primary care doctors; and these elements would be different again if you were speaking to a group of practicing urogynecologists. It’s insulting to recite basic information to highly knowledgeable physicians, or to present sophisticated technical content and complicated slides to novice physicians or lay people.
When presenting in a foreign country, learn how the culture of the audience differs from yours. How do they dress? What style of humor do they favor? How do they typically communicate? What gestures are appropriate or inappropriate? Are there religious influences to consider?
Practical steps. Before the meeting or event, speak to the organizer or meeting planner and find out the audience’s level of knowledge on the topic. Ask about audience expectations as well as demographics (such as age and background). If you are speaking at an industry event, research the event’s website and familiarize yourself with the mission of the event and who are the typical attendees. If you are presenting to a corporation, learn as much as you can about it by visiting its website, reading news reports, and reviewing associated blogs.
In addition to knowing the needs of the audience, ask the meeting planner about the goals and objectives for the program to make certain you can deliver on the requests.
Know your talk stem to stern
Review your slide material thoroughly. Understand each slide in the presentation and be comfortable with its content.
Avoid reading from slides. Reciting content that viewers can read for themselves breeds boredom and makes them lose interest. Further, when you are looking at the slides, you are not making eye contact with the audience and risk losing their attention. Good speakers are so comfortable with their slides that they can discuss each one without having to look at it.
Rehearse. The best way to achieve the foregoing is to rehearse. Your audience will be able to tell if you took the slide deck directly from a CD and loaded it into a computer and are giving the talk for the first time. You’ll need to know how long the program is to last and how long you are to speak. We suggest you practice with a timer to be certain you do not exceed the allotted time. Rehearse your talk aloud several times with all the props and audiovisual equipment you plan to use. This practice will help to curb filler words such as “ah” and “um.” It is also helpful to practice slide transitions, pauses, and even your breathing.
Prepare for the unexpected, too. Dinner meetings, for instance, may not start on time due to office or hospital delays for attending physicians, possibly resulting in a need to shorten your presentation.
Ask about the meeting agenda. If a meal is to be served, will you be speaking beforehand? This is the least favorable time slot, as you are holding people hostage before they can eat. Our preference is to speak after the appetizer is served and the orders have been taken by the wait staff. This way, attendees are not starving and they have something to drink. You can assure the waiters they won’t be disturbing you, and you can ask them to avoid walking in front of the projector. Ideally, you should end your presentation before dessert arrives and use the remaining time to field questions.
We suggest that you prepare a handout to be distributed at the end of the program, not before. You want your audience focused on you and your slides as you are speaking. Tell the audience you will be providing a handout of your presentation, which will minimize note-taking during your talk.
Your speech opening
The first and last 30 seconds of any speech probably have the most impact.1 Give extra thought, time, and effort to your opening and closing remarks. Do not open with “Good evening, it is a pleasure to be here tonight.” That wastes precious seconds.
While opening a speech with a joke or funny story is the conventional wisdom, ask yourself1:
- Is my selection appropriate to the occasion and for this audience?
- Is it in good taste?
- Does it relate to me (my service) or to the event or the group? Does it support my topic or its key points?
A humorous story or inspirational vignette that relates to your topic or audience can grab the audience’s attention. If you feel that demands more presentation skill than you possess at the outset of your public-speaking career, give the audience what you know and what they most want to hear. You know the questions that you have heard most at cocktail receptions or professional society meetings. So, put the answers to those questions in your speech.
For example. A scientist working with a major corporation was preparing a speech for a lay audience. Since most of the audience did not know what scientists do, he offered the following analogy: “Being a scientist is like doing a jigsaw puzzle in a snowstorm at night...you don’t have all the pieces...and you don’t have the picture on the front of the box to work from.” You can say more with less.1
Your closing
The closing is an important aspect of your speech. Summarize the key elements to your presentation. If you are going to take questions, a good approach is to say, “Before my closing remarks, are there any questions?” Following the questions, finish with a takeaway message that ties into your theme.1
Prepare an autobiographical introduction
We suggest that you write your own introduction and e-mail it to the person who will be introducing you. Let them know it is a suggestion that they are welcome to modify. We have found that most emcees or meeting planners are delighted to have the introduction and will use it just as you have written it. Also bring hard copy with you; many emcees will have forgotten to download what you sent. The figure shows an example of the introduction that one of us (NHB) uses, and you are welcome to modify it for your own use.
Ask about and confirm audiovisual support
Ask the meeting planner ahead of time if they will be providing the computer, projector, and screen. And if, for instance, they will provide a projector but not a computer, make sure the computer you will bring is compatible with their projector. Also, you will probably not require a microphone for a small group, but if you are speaking in a loud restaurant, a portable microphone-speaker system may be helpful.
Arrive early at the program venue to make sure the computers, projector, screen placement, and seating arrangement are all in order. Nothing can sidetrack a speaker (even a seasoned one) like a problem with the computer or equipment setup—for example, your flash drive requiring a USB port cannot connect to the sponsor’s computer, or your program created on a Mac does not run on the sponsor’s PC.
Show time: Getting ready
Another benefit in arriving early, besides being able to check on the equipment, is the chance to greet the audience members as they enter. It is easier to speak to a group of friends than strangers. And if you can remember their names, you can call on them and ask their opinion or how they might manage a patient who has the condition you are discussing. You also could suggest to the meeting planner that name tags for attendees would be helpful.
Warming up. A public speaker, like an athlete, needs to warm up physically before the event. If the facility has an anteroom available, use it for the following exercises suggested by public speaking coach Patricia Fripp1:
- Stand on one leg and shake the other (remove high heels first). When you place your raised foot back on the floor, it will feel lighter than the other one. Repeat the exercise using the other leg. Imagine your energy going down through the floor and up out of your head. While this sounds quite comical, it is not. It is a practical technique used by actors.
- Shake your hands vigorously. Hold them above your head, bending your wrists and elbows, then return your arms to your sides. This will make your hand movements more natural.
- Warm up your facial muscles by chewing in a highly exaggerated way. Do shoulder and neck rolls. Imagine you are at eye level with a clock. As you look at 12 o’clock, pull as much of your face up to the 12 as you can; move your eyes to 3 and repeat, then down to 6, and finally over to 9.
Not only do these exercises warm you up but they also relax you. The exaggerated movements help your movements to flow more naturally.1
This is just the start
Thorough preparation is key to making a solid presentation. But other factors are important too. Your goal is for the audience to take action or to implement suggestions from your presentation. In part 2 of this series, we will share tips on elements of the presentation itself that will encourage audience engagement and message retention. We will discuss how to make your message “stick” and how to make a dynamic, effective presentation that holds your audience’s attention for your entire talk.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Reference
- Fripp P. Add credibility to your business reputation through public speaking. Patricia Fripp website. http://www.fripp.com/add-credibility-to-your-business-reputation-through-public-speaking/. Accessed June 15, 2016.
Reference
- Fripp P. Add credibility to your business reputation through public speaking. Patricia Fripp website. http://www.fripp.com/add-credibility-to-your-business-reputation-through-public-speaking/. Accessed June 15, 2016.
In this Article
- Preparing a presentation
- Your speech opening
- AV equipment and support