User login
USPSTF opposes screening for obstructive sleep apnea in draft recommendation
The U.S. Preventive Services Task Force has issued a draft recommendation opposing screening for obstructive sleep apnea (OSA) in adults who are asymptomatic for the breathing disorder.
The USPSTF’s opposition is based on its determination that there is insufficient evidence to assess the balance of benefits and harms of screening for OSA in asymptomatic adults in primary care settings, giving the service an “I” grade. The recommendation and a draft evidence review are available for public comment until July 11 at 8:00 p.m. EST.
The draft recommendation is the first that the USPSTF has ever made about sleep apnea, according to the draft evidence review. The recommendation “applies to asymptomatic adults (aged 18 years and older) and adults with unrecognized symptoms of OSA.” It does not apply to children, adolescents, pregnant women, persons presenting with symptoms of or concerns about OSA, those who are being referred for evaluation or treatment of suspected OSA, and those who have acute conditions that could trigger the onset of OSA.
“Reported estimates of OSA prevalence vary due to differing definitions of OSA, sampling bias, and year of study publication. A 2013 systematic review reported an estimated prevalence of 2%-14% based on four community-based studies, while two U.S.-based studies conducted in the 1990s reported an estimated prevalence of 10% for mild OSA and 3.8%-6.5% for moderate or severe OSA,” according to the recommendation.
The USPSTF was unable to find adequate evidence on the direct harms of screening for OSA or the benefits of screening for OSA in asymptomatic populations, including their magnitude.
Most primary care clinicians do not routinely screen for OSA, according to the recommendation. While the Epworth Sleepiness Scale, STOP Questionnaire, STOPBang Questionnaire, Berlin Questionnaire, and Wisconsin Sleep Questionnaire are potential screening tests for OSA, none of these questionnaires has been validated in a primary care setting.
“There is uncertainty about the clinical utility of all potential screening tools,” and the USPSTF found no studies that prospectively evaluated screening questionnaires or clinical prediction tools to report calibration or clinical utility for improving health outcomes,” the draft evidence review said.
The USPSTF also found no studies evaluating the effect of screening for OSA on health outcomes or that directly evaluated benefits or harms of screening for OSA.
The recommendation calls for further research on the health outcomes of screening for OSA in asymptomatic persons and the role of sleepiness in determining health outcomes. The following are needed:
• The identification of valid and reliable clinical prediction tools that could accurately determine which asymptomatic persons (or persons with unrecognized symptoms) would benefit from further evaluation and testing for OSA.
• Studies that evaluate the effect of OSA treatments or interventions on health outcomes that are adequately powered and have an appropriate length of follow-up.
• Studies that evaluate whether improvement in the apnea-hypopnea index leads to improvement in health outcomes.
• More data on the natural history of mild sleep apnea.
The final evidence review will be used to inform the final USPSTF recommendation statement.

|
| Dr. David Schulman, FCCP |
Dr. David A. Schulman, FCCP, comments: The draft statement from the Preventative Services Task Force recommending against screening asymptomatic patients with standardized OSA questionnaires warrants a careful read. Many sleepy patients may not complain of their fatigue unless specifically asked, choosing to attribute their symptoms to inactivity, age, weight or a lack of exercise instead of a potential underlying sleep disorder.
Assessment of patients’ sleep habits and patterns by primary care physicians remains a critical component of preventative health to improve identification of the twenty-plus million Americans with sleep disordered breathing.

|
| Dr. David Schulman, FCCP |
Dr. David A. Schulman, FCCP, comments: The draft statement from the Preventative Services Task Force recommending against screening asymptomatic patients with standardized OSA questionnaires warrants a careful read. Many sleepy patients may not complain of their fatigue unless specifically asked, choosing to attribute their symptoms to inactivity, age, weight or a lack of exercise instead of a potential underlying sleep disorder.
Assessment of patients’ sleep habits and patterns by primary care physicians remains a critical component of preventative health to improve identification of the twenty-plus million Americans with sleep disordered breathing.

|
| Dr. David Schulman, FCCP |
Dr. David A. Schulman, FCCP, comments: The draft statement from the Preventative Services Task Force recommending against screening asymptomatic patients with standardized OSA questionnaires warrants a careful read. Many sleepy patients may not complain of their fatigue unless specifically asked, choosing to attribute their symptoms to inactivity, age, weight or a lack of exercise instead of a potential underlying sleep disorder.
Assessment of patients’ sleep habits and patterns by primary care physicians remains a critical component of preventative health to improve identification of the twenty-plus million Americans with sleep disordered breathing.
The U.S. Preventive Services Task Force has issued a draft recommendation opposing screening for obstructive sleep apnea (OSA) in adults who are asymptomatic for the breathing disorder.
The USPSTF’s opposition is based on its determination that there is insufficient evidence to assess the balance of benefits and harms of screening for OSA in asymptomatic adults in primary care settings, giving the service an “I” grade. The recommendation and a draft evidence review are available for public comment until July 11 at 8:00 p.m. EST.
The draft recommendation is the first that the USPSTF has ever made about sleep apnea, according to the draft evidence review. The recommendation “applies to asymptomatic adults (aged 18 years and older) and adults with unrecognized symptoms of OSA.” It does not apply to children, adolescents, pregnant women, persons presenting with symptoms of or concerns about OSA, those who are being referred for evaluation or treatment of suspected OSA, and those who have acute conditions that could trigger the onset of OSA.
“Reported estimates of OSA prevalence vary due to differing definitions of OSA, sampling bias, and year of study publication. A 2013 systematic review reported an estimated prevalence of 2%-14% based on four community-based studies, while two U.S.-based studies conducted in the 1990s reported an estimated prevalence of 10% for mild OSA and 3.8%-6.5% for moderate or severe OSA,” according to the recommendation.
The USPSTF was unable to find adequate evidence on the direct harms of screening for OSA or the benefits of screening for OSA in asymptomatic populations, including their magnitude.
Most primary care clinicians do not routinely screen for OSA, according to the recommendation. While the Epworth Sleepiness Scale, STOP Questionnaire, STOPBang Questionnaire, Berlin Questionnaire, and Wisconsin Sleep Questionnaire are potential screening tests for OSA, none of these questionnaires has been validated in a primary care setting.
“There is uncertainty about the clinical utility of all potential screening tools,” and the USPSTF found no studies that prospectively evaluated screening questionnaires or clinical prediction tools to report calibration or clinical utility for improving health outcomes,” the draft evidence review said.
The USPSTF also found no studies evaluating the effect of screening for OSA on health outcomes or that directly evaluated benefits or harms of screening for OSA.
The recommendation calls for further research on the health outcomes of screening for OSA in asymptomatic persons and the role of sleepiness in determining health outcomes. The following are needed:
• The identification of valid and reliable clinical prediction tools that could accurately determine which asymptomatic persons (or persons with unrecognized symptoms) would benefit from further evaluation and testing for OSA.
• Studies that evaluate the effect of OSA treatments or interventions on health outcomes that are adequately powered and have an appropriate length of follow-up.
• Studies that evaluate whether improvement in the apnea-hypopnea index leads to improvement in health outcomes.
• More data on the natural history of mild sleep apnea.
The final evidence review will be used to inform the final USPSTF recommendation statement.
The U.S. Preventive Services Task Force has issued a draft recommendation opposing screening for obstructive sleep apnea (OSA) in adults who are asymptomatic for the breathing disorder.
The USPSTF’s opposition is based on its determination that there is insufficient evidence to assess the balance of benefits and harms of screening for OSA in asymptomatic adults in primary care settings, giving the service an “I” grade. The recommendation and a draft evidence review are available for public comment until July 11 at 8:00 p.m. EST.
The draft recommendation is the first that the USPSTF has ever made about sleep apnea, according to the draft evidence review. The recommendation “applies to asymptomatic adults (aged 18 years and older) and adults with unrecognized symptoms of OSA.” It does not apply to children, adolescents, pregnant women, persons presenting with symptoms of or concerns about OSA, those who are being referred for evaluation or treatment of suspected OSA, and those who have acute conditions that could trigger the onset of OSA.
“Reported estimates of OSA prevalence vary due to differing definitions of OSA, sampling bias, and year of study publication. A 2013 systematic review reported an estimated prevalence of 2%-14% based on four community-based studies, while two U.S.-based studies conducted in the 1990s reported an estimated prevalence of 10% for mild OSA and 3.8%-6.5% for moderate or severe OSA,” according to the recommendation.
The USPSTF was unable to find adequate evidence on the direct harms of screening for OSA or the benefits of screening for OSA in asymptomatic populations, including their magnitude.
Most primary care clinicians do not routinely screen for OSA, according to the recommendation. While the Epworth Sleepiness Scale, STOP Questionnaire, STOPBang Questionnaire, Berlin Questionnaire, and Wisconsin Sleep Questionnaire are potential screening tests for OSA, none of these questionnaires has been validated in a primary care setting.
“There is uncertainty about the clinical utility of all potential screening tools,” and the USPSTF found no studies that prospectively evaluated screening questionnaires or clinical prediction tools to report calibration or clinical utility for improving health outcomes,” the draft evidence review said.
The USPSTF also found no studies evaluating the effect of screening for OSA on health outcomes or that directly evaluated benefits or harms of screening for OSA.
The recommendation calls for further research on the health outcomes of screening for OSA in asymptomatic persons and the role of sleepiness in determining health outcomes. The following are needed:
• The identification of valid and reliable clinical prediction tools that could accurately determine which asymptomatic persons (or persons with unrecognized symptoms) would benefit from further evaluation and testing for OSA.
• Studies that evaluate the effect of OSA treatments or interventions on health outcomes that are adequately powered and have an appropriate length of follow-up.
• Studies that evaluate whether improvement in the apnea-hypopnea index leads to improvement in health outcomes.
• More data on the natural history of mild sleep apnea.
The final evidence review will be used to inform the final USPSTF recommendation statement.
Study links mismatch repair defects to lower PFS in endometrial cancer
Endometrioid endometrial tumors with DNA mismatch repair (MMR) defects were associated with lower progression-free survival than were tumors that lacked these epigenetic defects, according to a large prospective study published online June 20 in the Journal of Clinical Oncology.
“The overall effect was less than would be expected given the strong association [between MMR defects and] higher grade (66% grade 2 or 3), higher stage (22% stage III or IV), and frequent lymphovascular space invasion (33%),” Dr. Scott McMeekin of the University of Oklahoma Health Sciences Center, Oklahoma City, and his associates wrote. Perhaps MMR-deficient tumors “are eliciting an antitumor immune response, as has been described for POLE ultramutated tumors,” they suggested.
Endometrioid endometrial tumors comprise about 80% of uterine cancers, and about 20%-40% of endometrioid endometrial cancer (EEC) tumors show loss of MMR, but “the relationship between MMR defects and outcomes in patients with endometrial cancer has not been fully established,” the researchers wrote.
Their study looked for links between MMR classes, clinicopathologic features, and outcomes, including response to adjuvant therapy. Understanding these links “will be critical to the design and implementation of trials for treating advanced-stage and recurrent EEC, including biologic therapies such as immune checkpoint blockade,” they wrote (J Clin Oncol. 2016. doi: 10.1200/JCO.2016.67.8722).
The study included 1,043 women with primary EEC prospectively recruited between 2003 and 2007 by the NRG/Gynecologic Oncology Group.
Tumor slides and clinical reports for a total of 1,024 EECs were centrally reviewed, and the tumors were tested for microsatellite instability (MSI), MLH1 methylation, and MMR protein expression. Each tumor was classified as being either MMR normal, having an epigenetic defect, having a probable mutation (that is, an MMR defect not attributable to MLH1 methylation), or as being MSI-low.
Progression-free survival was significantly lower among women whose tumors had epigenetic defects, compared with women whose tumors were MMR normal (hazard ratio, 1.37; 95% confidence interval, 1.00-1.86; P less than .05).
However, trends in disease-specific survival were similar, regardless of MMR type, the researchers wrote. Importantly, an exploratory analysis revealed a possible link between MMR status and response to adjuvant therapy. Specifically, women classified as having probable MMR mutations who received adjuvant therapy had substantially better progression-free survival, with a P-value that trended toward significant (HR, 0.24; 95% CI, 0.05-1.16; P = .07).
“No such effect was seen in the epigenetic defect group, and the differences in outcomes for patients with the two different classes of MMR defect are highly suggestive,” the researchers wrote. “Despite the large size of our study, our power to detect differences in survival is limited by the modest number of cases whose tumors were classified as probable mutation (99, 10% of cohort) and further by the fact that only 26 subjects received adjuvant therapy.”
Since many cancer centers regularly test tumor specimens for MMR defects and MLH1 methylation, “it should be possible to rapidly undertake retrospective studies to validate the findings we report here,” the researchers noted.
Dr. McMeekin reported having no financial disclosures. His coauthors reported ties to several companies, including Repros Therapeutics, Ethicon, Vitatex, Genentech, Janssen Oncology, Tesaro, AstraZeneca, and OvaGene Oncology.
Endometrioid endometrial tumors with DNA mismatch repair (MMR) defects were associated with lower progression-free survival than were tumors that lacked these epigenetic defects, according to a large prospective study published online June 20 in the Journal of Clinical Oncology.
“The overall effect was less than would be expected given the strong association [between MMR defects and] higher grade (66% grade 2 or 3), higher stage (22% stage III or IV), and frequent lymphovascular space invasion (33%),” Dr. Scott McMeekin of the University of Oklahoma Health Sciences Center, Oklahoma City, and his associates wrote. Perhaps MMR-deficient tumors “are eliciting an antitumor immune response, as has been described for POLE ultramutated tumors,” they suggested.
Endometrioid endometrial tumors comprise about 80% of uterine cancers, and about 20%-40% of endometrioid endometrial cancer (EEC) tumors show loss of MMR, but “the relationship between MMR defects and outcomes in patients with endometrial cancer has not been fully established,” the researchers wrote.
Their study looked for links between MMR classes, clinicopathologic features, and outcomes, including response to adjuvant therapy. Understanding these links “will be critical to the design and implementation of trials for treating advanced-stage and recurrent EEC, including biologic therapies such as immune checkpoint blockade,” they wrote (J Clin Oncol. 2016. doi: 10.1200/JCO.2016.67.8722).
The study included 1,043 women with primary EEC prospectively recruited between 2003 and 2007 by the NRG/Gynecologic Oncology Group.
Tumor slides and clinical reports for a total of 1,024 EECs were centrally reviewed, and the tumors were tested for microsatellite instability (MSI), MLH1 methylation, and MMR protein expression. Each tumor was classified as being either MMR normal, having an epigenetic defect, having a probable mutation (that is, an MMR defect not attributable to MLH1 methylation), or as being MSI-low.
Progression-free survival was significantly lower among women whose tumors had epigenetic defects, compared with women whose tumors were MMR normal (hazard ratio, 1.37; 95% confidence interval, 1.00-1.86; P less than .05).
However, trends in disease-specific survival were similar, regardless of MMR type, the researchers wrote. Importantly, an exploratory analysis revealed a possible link between MMR status and response to adjuvant therapy. Specifically, women classified as having probable MMR mutations who received adjuvant therapy had substantially better progression-free survival, with a P-value that trended toward significant (HR, 0.24; 95% CI, 0.05-1.16; P = .07).
“No such effect was seen in the epigenetic defect group, and the differences in outcomes for patients with the two different classes of MMR defect are highly suggestive,” the researchers wrote. “Despite the large size of our study, our power to detect differences in survival is limited by the modest number of cases whose tumors were classified as probable mutation (99, 10% of cohort) and further by the fact that only 26 subjects received adjuvant therapy.”
Since many cancer centers regularly test tumor specimens for MMR defects and MLH1 methylation, “it should be possible to rapidly undertake retrospective studies to validate the findings we report here,” the researchers noted.
Dr. McMeekin reported having no financial disclosures. His coauthors reported ties to several companies, including Repros Therapeutics, Ethicon, Vitatex, Genentech, Janssen Oncology, Tesaro, AstraZeneca, and OvaGene Oncology.
Endometrioid endometrial tumors with DNA mismatch repair (MMR) defects were associated with lower progression-free survival than were tumors that lacked these epigenetic defects, according to a large prospective study published online June 20 in the Journal of Clinical Oncology.
“The overall effect was less than would be expected given the strong association [between MMR defects and] higher grade (66% grade 2 or 3), higher stage (22% stage III or IV), and frequent lymphovascular space invasion (33%),” Dr. Scott McMeekin of the University of Oklahoma Health Sciences Center, Oklahoma City, and his associates wrote. Perhaps MMR-deficient tumors “are eliciting an antitumor immune response, as has been described for POLE ultramutated tumors,” they suggested.
Endometrioid endometrial tumors comprise about 80% of uterine cancers, and about 20%-40% of endometrioid endometrial cancer (EEC) tumors show loss of MMR, but “the relationship between MMR defects and outcomes in patients with endometrial cancer has not been fully established,” the researchers wrote.
Their study looked for links between MMR classes, clinicopathologic features, and outcomes, including response to adjuvant therapy. Understanding these links “will be critical to the design and implementation of trials for treating advanced-stage and recurrent EEC, including biologic therapies such as immune checkpoint blockade,” they wrote (J Clin Oncol. 2016. doi: 10.1200/JCO.2016.67.8722).
The study included 1,043 women with primary EEC prospectively recruited between 2003 and 2007 by the NRG/Gynecologic Oncology Group.
Tumor slides and clinical reports for a total of 1,024 EECs were centrally reviewed, and the tumors were tested for microsatellite instability (MSI), MLH1 methylation, and MMR protein expression. Each tumor was classified as being either MMR normal, having an epigenetic defect, having a probable mutation (that is, an MMR defect not attributable to MLH1 methylation), or as being MSI-low.
Progression-free survival was significantly lower among women whose tumors had epigenetic defects, compared with women whose tumors were MMR normal (hazard ratio, 1.37; 95% confidence interval, 1.00-1.86; P less than .05).
However, trends in disease-specific survival were similar, regardless of MMR type, the researchers wrote. Importantly, an exploratory analysis revealed a possible link between MMR status and response to adjuvant therapy. Specifically, women classified as having probable MMR mutations who received adjuvant therapy had substantially better progression-free survival, with a P-value that trended toward significant (HR, 0.24; 95% CI, 0.05-1.16; P = .07).
“No such effect was seen in the epigenetic defect group, and the differences in outcomes for patients with the two different classes of MMR defect are highly suggestive,” the researchers wrote. “Despite the large size of our study, our power to detect differences in survival is limited by the modest number of cases whose tumors were classified as probable mutation (99, 10% of cohort) and further by the fact that only 26 subjects received adjuvant therapy.”
Since many cancer centers regularly test tumor specimens for MMR defects and MLH1 methylation, “it should be possible to rapidly undertake retrospective studies to validate the findings we report here,” the researchers noted.
Dr. McMeekin reported having no financial disclosures. His coauthors reported ties to several companies, including Repros Therapeutics, Ethicon, Vitatex, Genentech, Janssen Oncology, Tesaro, AstraZeneca, and OvaGene Oncology.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: DNA mismatch repair defects have prognostic importance in endometrioid endometrial cancers.
Major finding: Progression-free survival was significantly lower among women whose tumors had epigenetic defects, compared with women whose tumors were MMR normal (HR, 1.37; 95% confidence interval, 1.00-1.86; P less than .05).
Data source: A prospective study of 1,024 endometrioid endometrial tumors, with central review of tumor slides and clinical data.
Disclosures: Dr. McMeekin reported having no financial disclosures. His coauthors reported ties to several companies, including Repros Therapeutics, Ethicon, Vitatex, Genentech, Janssen Oncology, Tesaro, AstraZeneca, and OvaGene Oncology.
Study confirms role of oral contraceptives in preventing ovarian cancer
Every 5-year increase in duration of oral contraceptive use was associated with about a 13% decrease in the risk of invasive ovarian cancers, and this protective effect persisted for all histologic subtypes except mucinous tumors, according to results of a meta-analysis.
“Oral contraceptives continue to be an important preventive factor for most types of ovarian cancer. Few other risk factors for ovarian cancer are modifiable, and those that are, such as smoking and obesity, did not show clear associations with serous carcinomas, the most common and fatal subtype,” Dr. Nicolas Wentzensen of the National Cancer Institute and his associates wrote June 20 in the Journal of Clinical Oncology.
Ovarian cancer is heterogeneous and its etiology poorly understood. Power limitations have prevented individual studies from parsing risk factors for distinct ovarian cancer histotypes, the researchers added. To solve this issue, they examined links between 14 different hormonal, reproductive, and lifestyle characteristics and histologic subtypes of tumors in the Ovarian Cancer Cohort Consortium.
Their analysis included 5,584 invasive ovarian cancers from more than 1.3 million women enrolled in 21 prospective studies (J Clin Oncol. 2016 Jun. doi: 10.1200/JCO.2016.66.8178).
Patients who reported having ever used oral contraceptives were about 16% less likely to develop any type of invasive epithelial ovarian cancer, compared with those who had never used oral contraceptives (relative risk, 0.84; 95% confidence interval, 0.79-0.89). Associations were similar for high-grade serous, endometrioid, and clear cell histotypes, although OC use did not appear to protect against mucinous tumors, the researchers wrote.
Some of the strongest associations were for endometrioid and clear cell carcinomas, they noted. For example, the risk of endometrioid carcinoma fell by about 22% for every birth (RR per birth, 0.78; 95% CI, 0.74-0.83), and the risk of clear cell carcinoma fell by about 32% per birth (RR, 0.68; 95% CI, 0.61-0.76).
“Likewise, age at menopause, endometriosis, and tubal ligation were associated only with clear cell and endometrioid tumors,” the researchers wrote.
Smoking was tied to a higher risk of mucinous tumors (RR per 20 pack-years, 1.20; 95% CI, 1.04-1.39) but was associated with a lower risk of clear cell tumors (RR, 0.68; 95% CI, 0.53-0.89).
“The substantial heterogeneity of individual risk factor associations across ovarian cancer subtypes [suggests] that subtypes are indeed different diseases, and underscores the importance of evaluating risk factors and biomarkers by ovarian cancer subtypes,” the researchers concluded. “Due to weaker associations observed for high-grade serous carcinomas, prediction of the clinically most important subtype may perform worse than for other types, which underscores the importance of finding better risk factors for serous carcinomas.”
The consortium plans to search for more tumor predictors by analyzing circulating biomarkers and genetic data.
Dr. Wentzensen reported having no financial disclosures. Other researchers reported financial relationships with GlaxoSmithKline and Cepheid.
Every 5-year increase in duration of oral contraceptive use was associated with about a 13% decrease in the risk of invasive ovarian cancers, and this protective effect persisted for all histologic subtypes except mucinous tumors, according to results of a meta-analysis.
“Oral contraceptives continue to be an important preventive factor for most types of ovarian cancer. Few other risk factors for ovarian cancer are modifiable, and those that are, such as smoking and obesity, did not show clear associations with serous carcinomas, the most common and fatal subtype,” Dr. Nicolas Wentzensen of the National Cancer Institute and his associates wrote June 20 in the Journal of Clinical Oncology.
Ovarian cancer is heterogeneous and its etiology poorly understood. Power limitations have prevented individual studies from parsing risk factors for distinct ovarian cancer histotypes, the researchers added. To solve this issue, they examined links between 14 different hormonal, reproductive, and lifestyle characteristics and histologic subtypes of tumors in the Ovarian Cancer Cohort Consortium.
Their analysis included 5,584 invasive ovarian cancers from more than 1.3 million women enrolled in 21 prospective studies (J Clin Oncol. 2016 Jun. doi: 10.1200/JCO.2016.66.8178).
Patients who reported having ever used oral contraceptives were about 16% less likely to develop any type of invasive epithelial ovarian cancer, compared with those who had never used oral contraceptives (relative risk, 0.84; 95% confidence interval, 0.79-0.89). Associations were similar for high-grade serous, endometrioid, and clear cell histotypes, although OC use did not appear to protect against mucinous tumors, the researchers wrote.
Some of the strongest associations were for endometrioid and clear cell carcinomas, they noted. For example, the risk of endometrioid carcinoma fell by about 22% for every birth (RR per birth, 0.78; 95% CI, 0.74-0.83), and the risk of clear cell carcinoma fell by about 32% per birth (RR, 0.68; 95% CI, 0.61-0.76).
“Likewise, age at menopause, endometriosis, and tubal ligation were associated only with clear cell and endometrioid tumors,” the researchers wrote.
Smoking was tied to a higher risk of mucinous tumors (RR per 20 pack-years, 1.20; 95% CI, 1.04-1.39) but was associated with a lower risk of clear cell tumors (RR, 0.68; 95% CI, 0.53-0.89).
“The substantial heterogeneity of individual risk factor associations across ovarian cancer subtypes [suggests] that subtypes are indeed different diseases, and underscores the importance of evaluating risk factors and biomarkers by ovarian cancer subtypes,” the researchers concluded. “Due to weaker associations observed for high-grade serous carcinomas, prediction of the clinically most important subtype may perform worse than for other types, which underscores the importance of finding better risk factors for serous carcinomas.”
The consortium plans to search for more tumor predictors by analyzing circulating biomarkers and genetic data.
Dr. Wentzensen reported having no financial disclosures. Other researchers reported financial relationships with GlaxoSmithKline and Cepheid.
Every 5-year increase in duration of oral contraceptive use was associated with about a 13% decrease in the risk of invasive ovarian cancers, and this protective effect persisted for all histologic subtypes except mucinous tumors, according to results of a meta-analysis.
“Oral contraceptives continue to be an important preventive factor for most types of ovarian cancer. Few other risk factors for ovarian cancer are modifiable, and those that are, such as smoking and obesity, did not show clear associations with serous carcinomas, the most common and fatal subtype,” Dr. Nicolas Wentzensen of the National Cancer Institute and his associates wrote June 20 in the Journal of Clinical Oncology.
Ovarian cancer is heterogeneous and its etiology poorly understood. Power limitations have prevented individual studies from parsing risk factors for distinct ovarian cancer histotypes, the researchers added. To solve this issue, they examined links between 14 different hormonal, reproductive, and lifestyle characteristics and histologic subtypes of tumors in the Ovarian Cancer Cohort Consortium.
Their analysis included 5,584 invasive ovarian cancers from more than 1.3 million women enrolled in 21 prospective studies (J Clin Oncol. 2016 Jun. doi: 10.1200/JCO.2016.66.8178).
Patients who reported having ever used oral contraceptives were about 16% less likely to develop any type of invasive epithelial ovarian cancer, compared with those who had never used oral contraceptives (relative risk, 0.84; 95% confidence interval, 0.79-0.89). Associations were similar for high-grade serous, endometrioid, and clear cell histotypes, although OC use did not appear to protect against mucinous tumors, the researchers wrote.
Some of the strongest associations were for endometrioid and clear cell carcinomas, they noted. For example, the risk of endometrioid carcinoma fell by about 22% for every birth (RR per birth, 0.78; 95% CI, 0.74-0.83), and the risk of clear cell carcinoma fell by about 32% per birth (RR, 0.68; 95% CI, 0.61-0.76).
“Likewise, age at menopause, endometriosis, and tubal ligation were associated only with clear cell and endometrioid tumors,” the researchers wrote.
Smoking was tied to a higher risk of mucinous tumors (RR per 20 pack-years, 1.20; 95% CI, 1.04-1.39) but was associated with a lower risk of clear cell tumors (RR, 0.68; 95% CI, 0.53-0.89).
“The substantial heterogeneity of individual risk factor associations across ovarian cancer subtypes [suggests] that subtypes are indeed different diseases, and underscores the importance of evaluating risk factors and biomarkers by ovarian cancer subtypes,” the researchers concluded. “Due to weaker associations observed for high-grade serous carcinomas, prediction of the clinically most important subtype may perform worse than for other types, which underscores the importance of finding better risk factors for serous carcinomas.”
The consortium plans to search for more tumor predictors by analyzing circulating biomarkers and genetic data.
Dr. Wentzensen reported having no financial disclosures. Other researchers reported financial relationships with GlaxoSmithKline and Cepheid.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Oral contraceptive (OC) use was inversely associated with the risk of several types of ovarian cancer.
Major finding: Use of OCs was associated with a 16% decrease in the risk of any type of ovarian cancer (relative risk, 0.84; 95% confidence interval, 0.79-0.89).
Data source: A meta-analysis of 21 prospective studies that included 5,584 invasive ovarian cancer cases from more than 1.3 million women.
Disclosures: Dr. Wentzensen reported having no financial disclosures. Other researchers reported financial relationships with GlaxoSmithKline and Cepheid.
Continuous Rd again beat MPT for transplant-ineligible multiple myeloma
Individuals with newly diagnosed multiple myeloma who were ineligible for stem cell transplantation were 31% less likely to die or progress on continuous lenalidomide plus low-dose dexamethasone (Rd continuous) than were those on melphalan, prednisone, and thalidomide (MPT), according to an extended follow-up of patients from the FIRST trial.
In addition, Rd continuous was associated with a statistically significant 20% decrease in risk of death or disease progression, compared with MPT among patients older than 75 years, reported Dr. Cyrille Hulin of Bordeaux (France) Hospital University Center and associates. The finding “establishes continuous treatment with Rd until disease progression as a new standard of care for patients with newly diagnosed multiple myeloma who are ineligible for stem cell transplantation, regardless of age. With proper monitoring and dose adjustment, Rd continuous is an effective and tolerable treatment option for even the most elderly patients,” they wrote online June 20 in the Journal of Clinical Oncology.
FIRST (Frontline Investigation of Revlimid Plus Dexamethasone Versus Standard Thalidomide) was an international, randomized, phase III open-label study of patients with untreated symptomatic multiple myeloma enrolled between 2008 and 2011. Patients were randomly assigned to Rd continuous, 72 weeks/18 cycles of Rd (Rd18), or MPT. The primary endpoint was progression-free survival (PFS). In the original analysis, Rd continuous led to a 28% lower risk of progression or death, compared with MPT, for a statistically significant hazard ratio (HR) of 0.72. The current study involved longer follow-up, with an updated data cutoff that was 3 years after the end of recruitment, the researchers said (J Clin Oncol. 2016 Jun 20. doi: 10.1200/JCO.2016.66.7295).
The cohort included 1,623 patients, of whom 567 (35%) were older than 75 years. The intention-to-treat populations included 535 Rd continuous patients, 541 Rd18 patients, and 547 MPT patients. Survivors were followed for a median of 45.5 months. As in the earlier analysis of FIRST data, the PFS was longer with Rd continuous than with MPT. For the overall intention-to-treat group, the median PFS was 26 months with Rd continuous, and 21.9 months with MPT (HR, 0.69; 95% confidence interval, 0.59-0.80). Among patients aged 75 years and younger, the median PFS was 28.1 months and 22.4 months, respectively (HR, 0.64; 95% CI, 0.53-0.77). Among patients over age 75 years, the median PFS was 20.3 months and 19.8 months, respectively (HR, 0.80, 95% CI, 0.62-1.03). In addition, the 4-year PFS “was more than doubled with Rd continuous versus MPT, regardless of age,” the investigators said. In contrast, Rd18 and MPT led to a similar median PFS, regardless of age.
As in the prior FIRST analysis, MPT was more often linked to grade 3 and 4 neutropenia (40% and 47% of older and younger patients, respectively, versus about 28% of Rd continuous patients), while Rd more often led to grade 3 and 4 infections (about 30% of Rd continuous patients, about 22% of Rd18 patients, and 16%-20% of MPT patients).
A total of 40% of younger Rd patients remained at their starting lenalidomide dose at 72 weeks, while only 16% of MPT patients stayed at their starting thalidomide dose. Similarly, 30% of older Rd continuous patients remained on their starting dose at 72 weeks, compared with 19% of older MPT patients.
Older age was associated with International Staging System stage III disease, renal impairment, and more comorbidities, but not with high-risk cytogenetics (that is, del[17p] and t[4;14]). “Although chronologic age is not necessarily an indicator of frailty, FIRST trial results did show greater PFS and OS [overall survival] benefits with Rd continuous versus MPT therapy, regardless of age,” the researchers commented.
The study was funded by Intergroupe Francophone du Myélome and Celgene. Dr. Hulin disclosed honoraria from Celgene, Amgen, Bristol-Myers Squibb, and Novartis.
Individuals with newly diagnosed multiple myeloma who were ineligible for stem cell transplantation were 31% less likely to die or progress on continuous lenalidomide plus low-dose dexamethasone (Rd continuous) than were those on melphalan, prednisone, and thalidomide (MPT), according to an extended follow-up of patients from the FIRST trial.
In addition, Rd continuous was associated with a statistically significant 20% decrease in risk of death or disease progression, compared with MPT among patients older than 75 years, reported Dr. Cyrille Hulin of Bordeaux (France) Hospital University Center and associates. The finding “establishes continuous treatment with Rd until disease progression as a new standard of care for patients with newly diagnosed multiple myeloma who are ineligible for stem cell transplantation, regardless of age. With proper monitoring and dose adjustment, Rd continuous is an effective and tolerable treatment option for even the most elderly patients,” they wrote online June 20 in the Journal of Clinical Oncology.
FIRST (Frontline Investigation of Revlimid Plus Dexamethasone Versus Standard Thalidomide) was an international, randomized, phase III open-label study of patients with untreated symptomatic multiple myeloma enrolled between 2008 and 2011. Patients were randomly assigned to Rd continuous, 72 weeks/18 cycles of Rd (Rd18), or MPT. The primary endpoint was progression-free survival (PFS). In the original analysis, Rd continuous led to a 28% lower risk of progression or death, compared with MPT, for a statistically significant hazard ratio (HR) of 0.72. The current study involved longer follow-up, with an updated data cutoff that was 3 years after the end of recruitment, the researchers said (J Clin Oncol. 2016 Jun 20. doi: 10.1200/JCO.2016.66.7295).
The cohort included 1,623 patients, of whom 567 (35%) were older than 75 years. The intention-to-treat populations included 535 Rd continuous patients, 541 Rd18 patients, and 547 MPT patients. Survivors were followed for a median of 45.5 months. As in the earlier analysis of FIRST data, the PFS was longer with Rd continuous than with MPT. For the overall intention-to-treat group, the median PFS was 26 months with Rd continuous, and 21.9 months with MPT (HR, 0.69; 95% confidence interval, 0.59-0.80). Among patients aged 75 years and younger, the median PFS was 28.1 months and 22.4 months, respectively (HR, 0.64; 95% CI, 0.53-0.77). Among patients over age 75 years, the median PFS was 20.3 months and 19.8 months, respectively (HR, 0.80, 95% CI, 0.62-1.03). In addition, the 4-year PFS “was more than doubled with Rd continuous versus MPT, regardless of age,” the investigators said. In contrast, Rd18 and MPT led to a similar median PFS, regardless of age.
As in the prior FIRST analysis, MPT was more often linked to grade 3 and 4 neutropenia (40% and 47% of older and younger patients, respectively, versus about 28% of Rd continuous patients), while Rd more often led to grade 3 and 4 infections (about 30% of Rd continuous patients, about 22% of Rd18 patients, and 16%-20% of MPT patients).
A total of 40% of younger Rd patients remained at their starting lenalidomide dose at 72 weeks, while only 16% of MPT patients stayed at their starting thalidomide dose. Similarly, 30% of older Rd continuous patients remained on their starting dose at 72 weeks, compared with 19% of older MPT patients.
Older age was associated with International Staging System stage III disease, renal impairment, and more comorbidities, but not with high-risk cytogenetics (that is, del[17p] and t[4;14]). “Although chronologic age is not necessarily an indicator of frailty, FIRST trial results did show greater PFS and OS [overall survival] benefits with Rd continuous versus MPT therapy, regardless of age,” the researchers commented.
The study was funded by Intergroupe Francophone du Myélome and Celgene. Dr. Hulin disclosed honoraria from Celgene, Amgen, Bristol-Myers Squibb, and Novartis.
Individuals with newly diagnosed multiple myeloma who were ineligible for stem cell transplantation were 31% less likely to die or progress on continuous lenalidomide plus low-dose dexamethasone (Rd continuous) than were those on melphalan, prednisone, and thalidomide (MPT), according to an extended follow-up of patients from the FIRST trial.
In addition, Rd continuous was associated with a statistically significant 20% decrease in risk of death or disease progression, compared with MPT among patients older than 75 years, reported Dr. Cyrille Hulin of Bordeaux (France) Hospital University Center and associates. The finding “establishes continuous treatment with Rd until disease progression as a new standard of care for patients with newly diagnosed multiple myeloma who are ineligible for stem cell transplantation, regardless of age. With proper monitoring and dose adjustment, Rd continuous is an effective and tolerable treatment option for even the most elderly patients,” they wrote online June 20 in the Journal of Clinical Oncology.
FIRST (Frontline Investigation of Revlimid Plus Dexamethasone Versus Standard Thalidomide) was an international, randomized, phase III open-label study of patients with untreated symptomatic multiple myeloma enrolled between 2008 and 2011. Patients were randomly assigned to Rd continuous, 72 weeks/18 cycles of Rd (Rd18), or MPT. The primary endpoint was progression-free survival (PFS). In the original analysis, Rd continuous led to a 28% lower risk of progression or death, compared with MPT, for a statistically significant hazard ratio (HR) of 0.72. The current study involved longer follow-up, with an updated data cutoff that was 3 years after the end of recruitment, the researchers said (J Clin Oncol. 2016 Jun 20. doi: 10.1200/JCO.2016.66.7295).
The cohort included 1,623 patients, of whom 567 (35%) were older than 75 years. The intention-to-treat populations included 535 Rd continuous patients, 541 Rd18 patients, and 547 MPT patients. Survivors were followed for a median of 45.5 months. As in the earlier analysis of FIRST data, the PFS was longer with Rd continuous than with MPT. For the overall intention-to-treat group, the median PFS was 26 months with Rd continuous, and 21.9 months with MPT (HR, 0.69; 95% confidence interval, 0.59-0.80). Among patients aged 75 years and younger, the median PFS was 28.1 months and 22.4 months, respectively (HR, 0.64; 95% CI, 0.53-0.77). Among patients over age 75 years, the median PFS was 20.3 months and 19.8 months, respectively (HR, 0.80, 95% CI, 0.62-1.03). In addition, the 4-year PFS “was more than doubled with Rd continuous versus MPT, regardless of age,” the investigators said. In contrast, Rd18 and MPT led to a similar median PFS, regardless of age.
As in the prior FIRST analysis, MPT was more often linked to grade 3 and 4 neutropenia (40% and 47% of older and younger patients, respectively, versus about 28% of Rd continuous patients), while Rd more often led to grade 3 and 4 infections (about 30% of Rd continuous patients, about 22% of Rd18 patients, and 16%-20% of MPT patients).
A total of 40% of younger Rd patients remained at their starting lenalidomide dose at 72 weeks, while only 16% of MPT patients stayed at their starting thalidomide dose. Similarly, 30% of older Rd continuous patients remained on their starting dose at 72 weeks, compared with 19% of older MPT patients.
Older age was associated with International Staging System stage III disease, renal impairment, and more comorbidities, but not with high-risk cytogenetics (that is, del[17p] and t[4;14]). “Although chronologic age is not necessarily an indicator of frailty, FIRST trial results did show greater PFS and OS [overall survival] benefits with Rd continuous versus MPT therapy, regardless of age,” the researchers commented.
The study was funded by Intergroupe Francophone du Myélome and Celgene. Dr. Hulin disclosed honoraria from Celgene, Amgen, Bristol-Myers Squibb, and Novartis.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: For patients with newly diagnosed multiple myeloma who were ineligible for transplant, including older patients, continuous lenalidomide plus low-dose dexamethasone (Rd continuous) was associated with significantly longer progression-free survival than was treatment with melphalan, prednisone, and thalidomide (MPT).
Major finding: Continuous Rd was associated with a 31% lower risk of death or progression in the overall intention-to-treat analysis, a 36% decrease among patients aged 75 years or younger, and a 20% decrease among patients older than 75 years.
Data source: An updated analysis of 1,623 patients from the FIRST trial.
Disclosures: The study was funded by Intergroupe Francophone du Myélome and Celgene. Dr. Hulin disclosed honoraria from Celgene, Amgen, Bristol-Myers Squibb, and Novartis.
In-person, telephone genetic counseling yield similar outcomes
Genetic counseling by telephone was noninferior to in-person counseling among women at increased risk of hereditary breast and/or ovarian cancer (HBOC) for all psychosocial, decision-making, and quality-of-life measures, investigators found.
In addition, genetic testing was more common among women who received in-person counseling and women who lived in rural settings.
“This trial provides important evidence that telephone genetic counseling for HBOC is noninferior to in-person counseling and can be delivered as safely as in-person counseling without an adverse effect on long-term psychological, quality-of-life, and decision-making outcomes,” according to Anita Kinney, Ph.D., of the University of New Mexico, Albuquerque, and her associates (J Clin Oncol. 2016 Jun. doi: 10.1200/JCO.2015.65.9557).
Investigators used the Utah Population Database and Utah Cancer Registry to identify breast and ovarian cancer survivors and their at-risk female relatives with deleterious BRCA 1/2 mutations. Of the 988 women who met study requirements, 495 were randomly assigned to receive in-person genetic counseling, and 493 women were assigned to receive genetic counseling via telephone. All patients received counseling by a certified cancer genetic counselor according to standardized national protocols. Importantly, there were no significant differences in age, race/ethnicity, marital status, education level, rural vs. urban residence, income, employment status or health care coverage between the two study arms.
At the 1-year follow-up, there was no significant difference between patients receiving in-person or telephone counseling for all psychosocial and informed decision-making outcomes which included anxiety (average brief symptom inventory scores, 2.37 vs. 2.74), cancer-specific distress (average impact of event scores, 10.06 vs. 11.19), quality-of-life for physical health (average short form health survey scores, 50.54 vs. 49.75), quality-of-life for mental health (50.51 vs. 50.74), decisional conflict (average decisional conflict score, 26.88 vs. 26.76), decisional regret (average decision regret score, 21.38 vs. 21.07), and perceived personal control (average questionnaire scores, 1.53 vs. 1.52).
Genetic testing was more common among women who received in-person counseling (37.3% vs. 27.9%; 95% confidence interval comparing difference in testing uptake, 2.2%-16.8%). Interestingly, testing was higher for rural, compared with urban residents, for both telephone and in-person counseling.
This study received funding from the National Institutes of Health, the Huntsman Cancer Foundation, the University of New Mexico Comprehensive Cancer Center, and the University of Utah. Twelve of the investigators had no disclosures to report. Two investigators reported serving in advisory roles or receiving honoraria from Myriad Genetics or In Vitae.
On Twitter @jessnicolecraig
Genetic counseling by telephone was noninferior to in-person counseling among women at increased risk of hereditary breast and/or ovarian cancer (HBOC) for all psychosocial, decision-making, and quality-of-life measures, investigators found.
In addition, genetic testing was more common among women who received in-person counseling and women who lived in rural settings.
“This trial provides important evidence that telephone genetic counseling for HBOC is noninferior to in-person counseling and can be delivered as safely as in-person counseling without an adverse effect on long-term psychological, quality-of-life, and decision-making outcomes,” according to Anita Kinney, Ph.D., of the University of New Mexico, Albuquerque, and her associates (J Clin Oncol. 2016 Jun. doi: 10.1200/JCO.2015.65.9557).
Investigators used the Utah Population Database and Utah Cancer Registry to identify breast and ovarian cancer survivors and their at-risk female relatives with deleterious BRCA 1/2 mutations. Of the 988 women who met study requirements, 495 were randomly assigned to receive in-person genetic counseling, and 493 women were assigned to receive genetic counseling via telephone. All patients received counseling by a certified cancer genetic counselor according to standardized national protocols. Importantly, there were no significant differences in age, race/ethnicity, marital status, education level, rural vs. urban residence, income, employment status or health care coverage between the two study arms.
At the 1-year follow-up, there was no significant difference between patients receiving in-person or telephone counseling for all psychosocial and informed decision-making outcomes which included anxiety (average brief symptom inventory scores, 2.37 vs. 2.74), cancer-specific distress (average impact of event scores, 10.06 vs. 11.19), quality-of-life for physical health (average short form health survey scores, 50.54 vs. 49.75), quality-of-life for mental health (50.51 vs. 50.74), decisional conflict (average decisional conflict score, 26.88 vs. 26.76), decisional regret (average decision regret score, 21.38 vs. 21.07), and perceived personal control (average questionnaire scores, 1.53 vs. 1.52).
Genetic testing was more common among women who received in-person counseling (37.3% vs. 27.9%; 95% confidence interval comparing difference in testing uptake, 2.2%-16.8%). Interestingly, testing was higher for rural, compared with urban residents, for both telephone and in-person counseling.
This study received funding from the National Institutes of Health, the Huntsman Cancer Foundation, the University of New Mexico Comprehensive Cancer Center, and the University of Utah. Twelve of the investigators had no disclosures to report. Two investigators reported serving in advisory roles or receiving honoraria from Myriad Genetics or In Vitae.
On Twitter @jessnicolecraig
Genetic counseling by telephone was noninferior to in-person counseling among women at increased risk of hereditary breast and/or ovarian cancer (HBOC) for all psychosocial, decision-making, and quality-of-life measures, investigators found.
In addition, genetic testing was more common among women who received in-person counseling and women who lived in rural settings.
“This trial provides important evidence that telephone genetic counseling for HBOC is noninferior to in-person counseling and can be delivered as safely as in-person counseling without an adverse effect on long-term psychological, quality-of-life, and decision-making outcomes,” according to Anita Kinney, Ph.D., of the University of New Mexico, Albuquerque, and her associates (J Clin Oncol. 2016 Jun. doi: 10.1200/JCO.2015.65.9557).
Investigators used the Utah Population Database and Utah Cancer Registry to identify breast and ovarian cancer survivors and their at-risk female relatives with deleterious BRCA 1/2 mutations. Of the 988 women who met study requirements, 495 were randomly assigned to receive in-person genetic counseling, and 493 women were assigned to receive genetic counseling via telephone. All patients received counseling by a certified cancer genetic counselor according to standardized national protocols. Importantly, there were no significant differences in age, race/ethnicity, marital status, education level, rural vs. urban residence, income, employment status or health care coverage between the two study arms.
At the 1-year follow-up, there was no significant difference between patients receiving in-person or telephone counseling for all psychosocial and informed decision-making outcomes which included anxiety (average brief symptom inventory scores, 2.37 vs. 2.74), cancer-specific distress (average impact of event scores, 10.06 vs. 11.19), quality-of-life for physical health (average short form health survey scores, 50.54 vs. 49.75), quality-of-life for mental health (50.51 vs. 50.74), decisional conflict (average decisional conflict score, 26.88 vs. 26.76), decisional regret (average decision regret score, 21.38 vs. 21.07), and perceived personal control (average questionnaire scores, 1.53 vs. 1.52).
Genetic testing was more common among women who received in-person counseling (37.3% vs. 27.9%; 95% confidence interval comparing difference in testing uptake, 2.2%-16.8%). Interestingly, testing was higher for rural, compared with urban residents, for both telephone and in-person counseling.
This study received funding from the National Institutes of Health, the Huntsman Cancer Foundation, the University of New Mexico Comprehensive Cancer Center, and the University of Utah. Twelve of the investigators had no disclosures to report. Two investigators reported serving in advisory roles or receiving honoraria from Myriad Genetics or In Vitae.
On Twitter @jessnicolecraig
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Telephone genetic counseling was noninferior to in-person counseling for women at increased risk of hereditary breast and/or ovarian cancer.
Major finding: Anxiety, cancer-specific distress, quality-of-life for physical and mental health, decisional conflict, decisional regret, and perceived personal control measures were not significantly different between the two study arms.
Data source: A randomized noninferiority trial of 988 women at increased risk of hereditary breast and/or ovarian cancer.
Disclosures: This study received funding from the National Institutes of Health, the Huntsman Cancer Foundation, the University of New Mexico Comprehensive Cancer Center, and the University of Utah. Twelve of the investigators had no disclosures to report. Two investigators reported serving in advisory roles or receiving honoraria from Myriad Genetics or In Vitae.
Lasers for Darker Skin Types
Alternative therapies can augment treatment in resistant depression
SCOTTSDALE, ARIZ. – Simple, effective, nonpharmacologic therapies can significantly augment pharmacotherapy for patients with treatment-resistant depression, according to Dr. Mark Hyman Rapaport.
More complex complementary approaches are being explored and may hold promise as part of the future of precision medicine, but well-tested strategies can still help many patients, he said.
Speaking at a session focused on treatment-resistant depression at a meeting of the American Society of Clinical Psychopharmacology, Dr. Rapaport, chairman of the department of psychiatry and behavioral sciences and Reunette W. Harris Professor at Emory University, Atlanta, began with the basics.
Citing a recent meta-analysis examining the way in which exercise works as a treatment for depression, Dr. Rapaport said, “looking at the world’s literature in a very thoughtful way, [the meta-analysis] did demonstrate a significant effect for aerobic exercise in decreasing signs and symptoms of depression.” The meta-analysis, he said, found that exercise provided the equivalent of a 5.07-point improvement on the Hamilton Rating Scale for Depression (HAM-D) (P = .0007). “I think it is something we should encourage our patients to use,” he said.
Similarly, though the treatments “have fallen out of favor,” Dr. Rapaport said “meta-analyses clearly demonstrate” a significant effect of bright white light (BL) therapy in seasonal affective disorder, both as monotherapy and in combination with antidepressants. The light should be dosed at 5000 lux or more for 1 hour daily.
Further, said Dr. Rapaport, BL as monotherapy or used in combination with antidepressants, or with sleep deprivation can be moderately effective in treating major depressive disorder. “It’s something to consider. We do not use it; we should use it,” Dr. Rapaport said. For patients with bipolar disorder, BL therapy can effectively augment medication use as well.
Focused, intensive treatment with light and sleep adjustments can be effective in both bipolar depression and treatment-resistant depression, Dr. Rapaport said. This approach, termed “adjunctive triple chronotherapy,” begins with total sleep deprivation for a period of 33-36 hours. Then, the patient receives BL therapy at 5,000 lux for at least 1 hour for 3 consecutive nights. Finally, patients are asked to advance their sleep phase for 3 days so that they sleep from 6 p.m. to 1 a.m. the 1st night, 8 p.m. to 3 a.m. the 2nd night, and 10 p.m. to 5 a.m. the 3rd night.
The triple chronotherapy approach, said Dr. Rapaport, results in a “rapid and sustained response, both in unipolar and bipolar depression, in the studies that have been done to date.”
Massage can effectively improve mood for individuals with depression as well. In a study of HIV-positive individuals with major depressive disorder and on stable medication, those who received weekly massage had a 33% decrease in HAM-D scores, compared with a 12% increase for those receiving light touch, and a 9% decrease for those on a wait list who received neither touch nor massage (P less than .05). The response rate of 40% for those receiving massage also was significantly higher than the 6.3% for those receiving light touch and the 14% for those on the wait list (P less than .05).
Recently completed unpublished work by Dr. Rapaport and his colleagues involving patients with generalized anxiety disorder also showed significantly greater improvement on a self-rating scale for anxiety for those receiving twice-weekly Swedish massage therapy, compared with those receiving light touch.
“Our patients – their symptoms aren’t just what we see on the monitors – they deal with quality of life, they deal with functioning,” said Dr. Rapaport, “so there are many things that we as clinicians need to look at,” he said at the meeting. “The key is this: There’s a slowly increasing but limited data set for [treatment-resistant depression]. We need to find the right treatments for the right subjects. “
The ASCP meeting was formerly known as the New Clinical Drug Evaluation Unit meeting. Dr. Rapaport reported no relevant financial conflicts.
On Twitter @karioakes
SCOTTSDALE, ARIZ. – Simple, effective, nonpharmacologic therapies can significantly augment pharmacotherapy for patients with treatment-resistant depression, according to Dr. Mark Hyman Rapaport.
More complex complementary approaches are being explored and may hold promise as part of the future of precision medicine, but well-tested strategies can still help many patients, he said.
Speaking at a session focused on treatment-resistant depression at a meeting of the American Society of Clinical Psychopharmacology, Dr. Rapaport, chairman of the department of psychiatry and behavioral sciences and Reunette W. Harris Professor at Emory University, Atlanta, began with the basics.
Citing a recent meta-analysis examining the way in which exercise works as a treatment for depression, Dr. Rapaport said, “looking at the world’s literature in a very thoughtful way, [the meta-analysis] did demonstrate a significant effect for aerobic exercise in decreasing signs and symptoms of depression.” The meta-analysis, he said, found that exercise provided the equivalent of a 5.07-point improvement on the Hamilton Rating Scale for Depression (HAM-D) (P = .0007). “I think it is something we should encourage our patients to use,” he said.
Similarly, though the treatments “have fallen out of favor,” Dr. Rapaport said “meta-analyses clearly demonstrate” a significant effect of bright white light (BL) therapy in seasonal affective disorder, both as monotherapy and in combination with antidepressants. The light should be dosed at 5000 lux or more for 1 hour daily.
Further, said Dr. Rapaport, BL as monotherapy or used in combination with antidepressants, or with sleep deprivation can be moderately effective in treating major depressive disorder. “It’s something to consider. We do not use it; we should use it,” Dr. Rapaport said. For patients with bipolar disorder, BL therapy can effectively augment medication use as well.
Focused, intensive treatment with light and sleep adjustments can be effective in both bipolar depression and treatment-resistant depression, Dr. Rapaport said. This approach, termed “adjunctive triple chronotherapy,” begins with total sleep deprivation for a period of 33-36 hours. Then, the patient receives BL therapy at 5,000 lux for at least 1 hour for 3 consecutive nights. Finally, patients are asked to advance their sleep phase for 3 days so that they sleep from 6 p.m. to 1 a.m. the 1st night, 8 p.m. to 3 a.m. the 2nd night, and 10 p.m. to 5 a.m. the 3rd night.
The triple chronotherapy approach, said Dr. Rapaport, results in a “rapid and sustained response, both in unipolar and bipolar depression, in the studies that have been done to date.”
Massage can effectively improve mood for individuals with depression as well. In a study of HIV-positive individuals with major depressive disorder and on stable medication, those who received weekly massage had a 33% decrease in HAM-D scores, compared with a 12% increase for those receiving light touch, and a 9% decrease for those on a wait list who received neither touch nor massage (P less than .05). The response rate of 40% for those receiving massage also was significantly higher than the 6.3% for those receiving light touch and the 14% for those on the wait list (P less than .05).
Recently completed unpublished work by Dr. Rapaport and his colleagues involving patients with generalized anxiety disorder also showed significantly greater improvement on a self-rating scale for anxiety for those receiving twice-weekly Swedish massage therapy, compared with those receiving light touch.
“Our patients – their symptoms aren’t just what we see on the monitors – they deal with quality of life, they deal with functioning,” said Dr. Rapaport, “so there are many things that we as clinicians need to look at,” he said at the meeting. “The key is this: There’s a slowly increasing but limited data set for [treatment-resistant depression]. We need to find the right treatments for the right subjects. “
The ASCP meeting was formerly known as the New Clinical Drug Evaluation Unit meeting. Dr. Rapaport reported no relevant financial conflicts.
On Twitter @karioakes
SCOTTSDALE, ARIZ. – Simple, effective, nonpharmacologic therapies can significantly augment pharmacotherapy for patients with treatment-resistant depression, according to Dr. Mark Hyman Rapaport.
More complex complementary approaches are being explored and may hold promise as part of the future of precision medicine, but well-tested strategies can still help many patients, he said.
Speaking at a session focused on treatment-resistant depression at a meeting of the American Society of Clinical Psychopharmacology, Dr. Rapaport, chairman of the department of psychiatry and behavioral sciences and Reunette W. Harris Professor at Emory University, Atlanta, began with the basics.
Citing a recent meta-analysis examining the way in which exercise works as a treatment for depression, Dr. Rapaport said, “looking at the world’s literature in a very thoughtful way, [the meta-analysis] did demonstrate a significant effect for aerobic exercise in decreasing signs and symptoms of depression.” The meta-analysis, he said, found that exercise provided the equivalent of a 5.07-point improvement on the Hamilton Rating Scale for Depression (HAM-D) (P = .0007). “I think it is something we should encourage our patients to use,” he said.
Similarly, though the treatments “have fallen out of favor,” Dr. Rapaport said “meta-analyses clearly demonstrate” a significant effect of bright white light (BL) therapy in seasonal affective disorder, both as monotherapy and in combination with antidepressants. The light should be dosed at 5000 lux or more for 1 hour daily.
Further, said Dr. Rapaport, BL as monotherapy or used in combination with antidepressants, or with sleep deprivation can be moderately effective in treating major depressive disorder. “It’s something to consider. We do not use it; we should use it,” Dr. Rapaport said. For patients with bipolar disorder, BL therapy can effectively augment medication use as well.
Focused, intensive treatment with light and sleep adjustments can be effective in both bipolar depression and treatment-resistant depression, Dr. Rapaport said. This approach, termed “adjunctive triple chronotherapy,” begins with total sleep deprivation for a period of 33-36 hours. Then, the patient receives BL therapy at 5,000 lux for at least 1 hour for 3 consecutive nights. Finally, patients are asked to advance their sleep phase for 3 days so that they sleep from 6 p.m. to 1 a.m. the 1st night, 8 p.m. to 3 a.m. the 2nd night, and 10 p.m. to 5 a.m. the 3rd night.
The triple chronotherapy approach, said Dr. Rapaport, results in a “rapid and sustained response, both in unipolar and bipolar depression, in the studies that have been done to date.”
Massage can effectively improve mood for individuals with depression as well. In a study of HIV-positive individuals with major depressive disorder and on stable medication, those who received weekly massage had a 33% decrease in HAM-D scores, compared with a 12% increase for those receiving light touch, and a 9% decrease for those on a wait list who received neither touch nor massage (P less than .05). The response rate of 40% for those receiving massage also was significantly higher than the 6.3% for those receiving light touch and the 14% for those on the wait list (P less than .05).
Recently completed unpublished work by Dr. Rapaport and his colleagues involving patients with generalized anxiety disorder also showed significantly greater improvement on a self-rating scale for anxiety for those receiving twice-weekly Swedish massage therapy, compared with those receiving light touch.
“Our patients – their symptoms aren’t just what we see on the monitors – they deal with quality of life, they deal with functioning,” said Dr. Rapaport, “so there are many things that we as clinicians need to look at,” he said at the meeting. “The key is this: There’s a slowly increasing but limited data set for [treatment-resistant depression]. We need to find the right treatments for the right subjects. “
The ASCP meeting was formerly known as the New Clinical Drug Evaluation Unit meeting. Dr. Rapaport reported no relevant financial conflicts.
On Twitter @karioakes
EXPERT ANALYSIS FROM THE ASCP ANNUAL MEETING
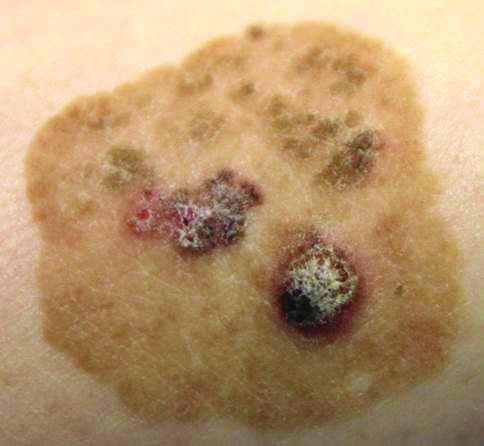
Brown Papules and a Plaque on the Calf
The Diagnosis: Irritated Seborrheic Keratosis
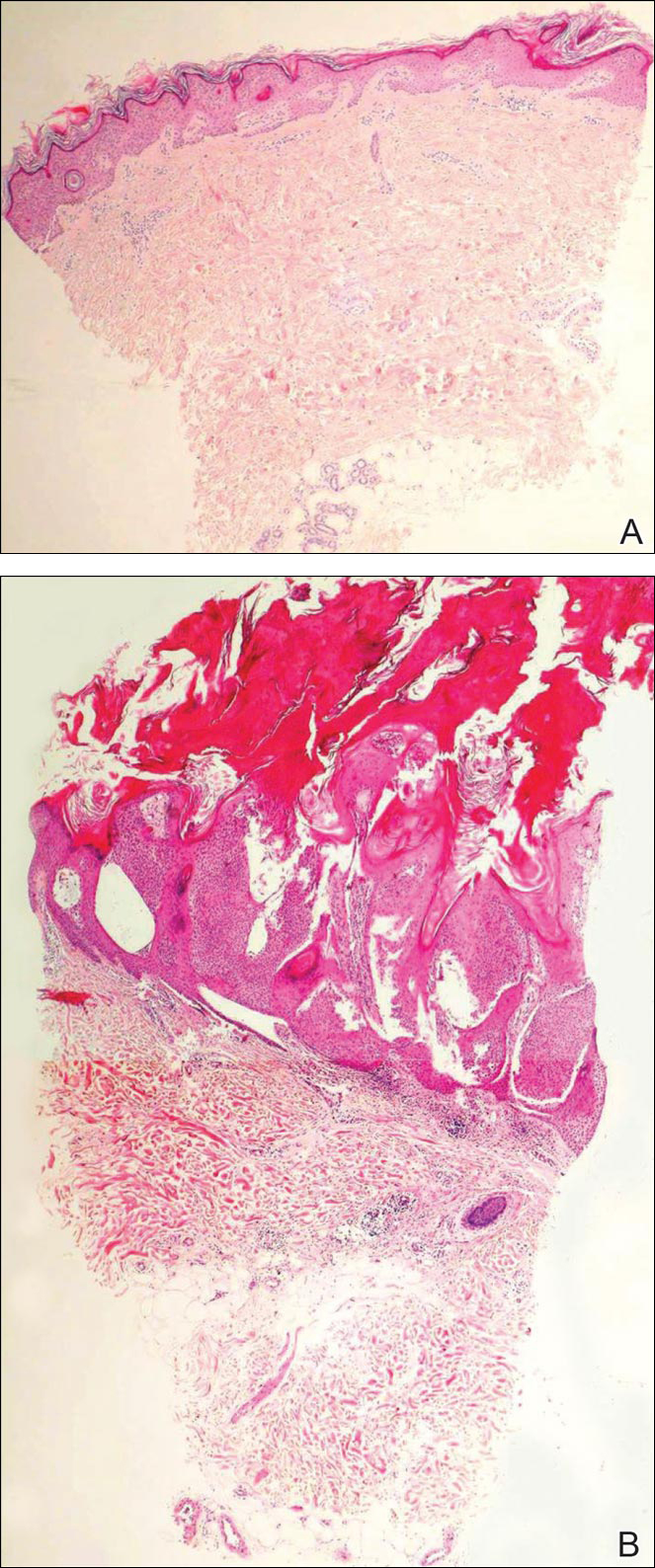
Biopsies of one of the protruding papules and the underlying plaque were performed. The specimen from the papule showed hyperkeratosis, acanthosis, papillomatosis, and a flattened dermoepidermal junction with demarcated horizontal margin, which demonstrated apparent upward growth of the epidermis. Moderate lymphocytic infiltration in the upper dermis also was observed (Figure, A). The histologic findings of the plaque showed acanthosis, several pseudohorn cysts, hyperpigmentation of the basal layer, and a horizontal demarcation of the dermoepidermal junction (Figure, B).
Seborrheic keratosis is the most common benign epidermal tumor of the skin with variable appearance.1 It usually begins with well-circumscribed, dull, flat, tan or brown patches that then grow into waxy verrucous papules.1 There are many clinicopathologic variants of SK such as common SK, stucco keratosis, and dermatosis papulosa nigra in clinical variation, as well as acanthotic, hyperkeratotic, clonal, reticulated, irritated, and melanoacanthoma subtypes based on histological variation.2,3
Seborrheic keratosis is a tumor of keratinocytic origin. Although genetics, sun exposure,4 and human papillomavirus infection5 are thought to be causative factors, the precise etiology of SK is unknown.1
The histology of SK shows monotonous basaloid tumor cells without atypia. It generally is comprised of focal acanthosis and papillomatosis with a sharp flat base. Intraepithelial horn pseudocysts are notable features of SK and increased melanin often is seen.2,6
Irritated SK is a histologic variant of SK that has been mechanically or chemically irritated or is involved in immunologic responses. Histologically, the dermis underlying an SK lesion filled with a dense lymphocytic infiltration is characteristic.1,2
For symptomatic or cosmetically undesirable lesions, complete removal of the lesion is the preferred treatment. Cryotherapy, electrodesiccation followed by curettage, curettage followed by desiccation, laser ablation, and surgical excision are effective treatments.1
- Valencia DT, Nicholas RS, Ken KL, et al. Benign epithelial tumors, hamartomas, and hyperplasias. In: Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill Professional; 2012:1319-1336.
- Kirkharn N. Tumors and cysts of the epidermis. In: Elder DE, Elenitsas R, Johnson BL Jr, eds. Lever’s Histopathology of the Skin. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:791-850.
- Rajesh G, Thappa DM, Jaisankar TJ, et al. Spectrum of seborrheic keratoses in South Indians: a clinical and dermoscopic study. Indian J Dermatol Venereol Leprol. 2011;77:483-488.
- Yeatman JM, Kilkenny M, Marks R. The prevalence of seborrhoeic keratoses in an Australian population: does exposure to sunlight play a part in their frequency? Br J Dermatol. 1997;137:411-414.
- Li YH, Chen G, Dong XP, et al. Detection of epidermodysplasia verruciformis-associated human papillomavirus DNA in nongenital seborrhoeic keratosis. Br J Dermatol. 2004;151:1060-1065.
- Brinster NK, Liu V, Diwan AH, et al. Dermatopathology. Philadelphia, PA: Saunders/Elsevier; 2011.
The Diagnosis: Irritated Seborrheic Keratosis
Biopsies of one of the protruding papules and the underlying plaque were performed. The specimen from the papule showed hyperkeratosis, acanthosis, papillomatosis, and a flattened dermoepidermal junction with demarcated horizontal margin, which demonstrated apparent upward growth of the epidermis. Moderate lymphocytic infiltration in the upper dermis also was observed (Figure, A). The histologic findings of the plaque showed acanthosis, several pseudohorn cysts, hyperpigmentation of the basal layer, and a horizontal demarcation of the dermoepidermal junction (Figure, B).

Seborrheic keratosis is the most common benign epidermal tumor of the skin with variable appearance.1 It usually begins with well-circumscribed, dull, flat, tan or brown patches that then grow into waxy verrucous papules.1 There are many clinicopathologic variants of SK such as common SK, stucco keratosis, and dermatosis papulosa nigra in clinical variation, as well as acanthotic, hyperkeratotic, clonal, reticulated, irritated, and melanoacanthoma subtypes based on histological variation.2,3
Seborrheic keratosis is a tumor of keratinocytic origin. Although genetics, sun exposure,4 and human papillomavirus infection5 are thought to be causative factors, the precise etiology of SK is unknown.1
The histology of SK shows monotonous basaloid tumor cells without atypia. It generally is comprised of focal acanthosis and papillomatosis with a sharp flat base. Intraepithelial horn pseudocysts are notable features of SK and increased melanin often is seen.2,6
Irritated SK is a histologic variant of SK that has been mechanically or chemically irritated or is involved in immunologic responses. Histologically, the dermis underlying an SK lesion filled with a dense lymphocytic infiltration is characteristic.1,2
For symptomatic or cosmetically undesirable lesions, complete removal of the lesion is the preferred treatment. Cryotherapy, electrodesiccation followed by curettage, curettage followed by desiccation, laser ablation, and surgical excision are effective treatments.1
The Diagnosis: Irritated Seborrheic Keratosis
Biopsies of one of the protruding papules and the underlying plaque were performed. The specimen from the papule showed hyperkeratosis, acanthosis, papillomatosis, and a flattened dermoepidermal junction with demarcated horizontal margin, which demonstrated apparent upward growth of the epidermis. Moderate lymphocytic infiltration in the upper dermis also was observed (Figure, A). The histologic findings of the plaque showed acanthosis, several pseudohorn cysts, hyperpigmentation of the basal layer, and a horizontal demarcation of the dermoepidermal junction (Figure, B).

Seborrheic keratosis is the most common benign epidermal tumor of the skin with variable appearance.1 It usually begins with well-circumscribed, dull, flat, tan or brown patches that then grow into waxy verrucous papules.1 There are many clinicopathologic variants of SK such as common SK, stucco keratosis, and dermatosis papulosa nigra in clinical variation, as well as acanthotic, hyperkeratotic, clonal, reticulated, irritated, and melanoacanthoma subtypes based on histological variation.2,3
Seborrheic keratosis is a tumor of keratinocytic origin. Although genetics, sun exposure,4 and human papillomavirus infection5 are thought to be causative factors, the precise etiology of SK is unknown.1
The histology of SK shows monotonous basaloid tumor cells without atypia. It generally is comprised of focal acanthosis and papillomatosis with a sharp flat base. Intraepithelial horn pseudocysts are notable features of SK and increased melanin often is seen.2,6
Irritated SK is a histologic variant of SK that has been mechanically or chemically irritated or is involved in immunologic responses. Histologically, the dermis underlying an SK lesion filled with a dense lymphocytic infiltration is characteristic.1,2
For symptomatic or cosmetically undesirable lesions, complete removal of the lesion is the preferred treatment. Cryotherapy, electrodesiccation followed by curettage, curettage followed by desiccation, laser ablation, and surgical excision are effective treatments.1
- Valencia DT, Nicholas RS, Ken KL, et al. Benign epithelial tumors, hamartomas, and hyperplasias. In: Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill Professional; 2012:1319-1336.
- Kirkharn N. Tumors and cysts of the epidermis. In: Elder DE, Elenitsas R, Johnson BL Jr, eds. Lever’s Histopathology of the Skin. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:791-850.
- Rajesh G, Thappa DM, Jaisankar TJ, et al. Spectrum of seborrheic keratoses in South Indians: a clinical and dermoscopic study. Indian J Dermatol Venereol Leprol. 2011;77:483-488.
- Yeatman JM, Kilkenny M, Marks R. The prevalence of seborrhoeic keratoses in an Australian population: does exposure to sunlight play a part in their frequency? Br J Dermatol. 1997;137:411-414.
- Li YH, Chen G, Dong XP, et al. Detection of epidermodysplasia verruciformis-associated human papillomavirus DNA in nongenital seborrhoeic keratosis. Br J Dermatol. 2004;151:1060-1065.
- Brinster NK, Liu V, Diwan AH, et al. Dermatopathology. Philadelphia, PA: Saunders/Elsevier; 2011.
- Valencia DT, Nicholas RS, Ken KL, et al. Benign epithelial tumors, hamartomas, and hyperplasias. In: Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill Professional; 2012:1319-1336.
- Kirkharn N. Tumors and cysts of the epidermis. In: Elder DE, Elenitsas R, Johnson BL Jr, eds. Lever’s Histopathology of the Skin. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:791-850.
- Rajesh G, Thappa DM, Jaisankar TJ, et al. Spectrum of seborrheic keratoses in South Indians: a clinical and dermoscopic study. Indian J Dermatol Venereol Leprol. 2011;77:483-488.
- Yeatman JM, Kilkenny M, Marks R. The prevalence of seborrhoeic keratoses in an Australian population: does exposure to sunlight play a part in their frequency? Br J Dermatol. 1997;137:411-414.
- Li YH, Chen G, Dong XP, et al. Detection of epidermodysplasia verruciformis-associated human papillomavirus DNA in nongenital seborrhoeic keratosis. Br J Dermatol. 2004;151:1060-1065.
- Brinster NK, Liu V, Diwan AH, et al. Dermatopathology. Philadelphia, PA: Saunders/Elsevier; 2011.
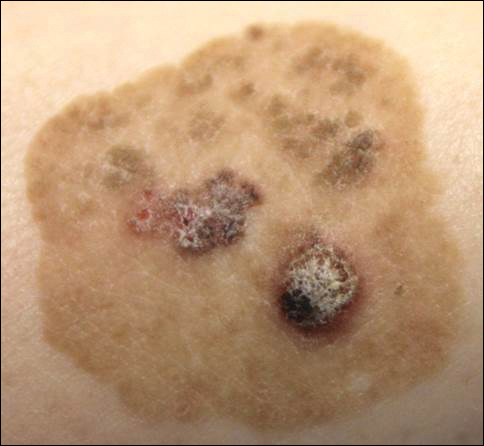
Phentermine-topiramate tops competition for long-term weight loss
SAN DIEGO – Phentermine-topiramate was the most effective long-term weight loss drug, based on findings from a network meta-analysis from the University of California, San Diego.
The investigators pooled data from 28 studies of phentermine-topiramate (Qsymia) and four other drugs: orlistat (Xenical, Alli), lorcaserin (Belviq), naltrexone-bupropion (Contrave), and liraglutide (Victoza, Saxenda). The most commonly prescribed weight loss drug in the United States – generic phentermine – was not included because it’s not indicated for long-term use.
“It’s good to have a problem of plenty,” said Dr. Siddharth Singh, but it also makes it hard to figure out which drug to prescribe, especially given the lack of head-to-head studies. He said he hopes the results will help. However, the 30%-45% attrition rate across the studies means that the results have to be interpreted cautiously, said Dr. Singh, who is in the division of gastroenterology in the department of internal medicine, UCSD.
Most of the studies were company-funded phase III trials of weight loss drugs vs. placebo; the trials all were at least 1 year long and included more than 29,000 overweight or obese adults. The analysis was limited to patients on the maximum recommended dose of each drug.
All the drugs were more effective than placebo, but phentermine-topiramate was the most effective, with about 75% of patients losing at least 5% of their weight at 1 year and more than half losing at least 10%, with an average weight loss above placebo of 9 kg. Phentermine-topiramate patients were 10 times more likely than patients on placebo to hit the 5% mark at 1 year; liraglutide patients were 5.5 times more likely to do so; naltrexone-bupropion patients were 4 times more likely; lorcaserin patients, 3.1 times more likely; and orlistat patients, 2.7 times more likely to hit the 5% mark. The spread was similar for weight loss of 10% or more at 1 year vs. placebo.
Phentermine-topiramate’s tolerability profile was acceptable, with 10% of patients quitting the drug by 1 year because of adverse events. The best tolerated was lorcaserin, with a discontinuation rate of 6%, and the least tolerated was liraglutide, with a 13% discontinuation rate. Patients were more likely to quit active drug than placebo in all the studies.
Of course, there are other considerations, especially comorbidities; liraglutide might be the best choice in diabetics, for instance, and patients with psychiatric issues might want to avoid naltrexone-bupropion and lorcaserin, Dr. Singh said.
Subjects were a median of 46 years old, and 74% were women. The median body mass index was 36 kg/m2. All the patients had lifestyle and nutrition counseling along with their study medications.
The investigators next plan to compare the drugs’ safety and efficacy in real world settings.
There was no industry funding for the work, and Dr. Singh had no relevant financial disclosures.

|
Dr. John Morton |
The findings do not correlate with the way weight loss drugs are prescribed. The drug that’s being prescribed the most – outside of generic phentermine – is Contrave (naltrexone-bupropion), but the one that seemed to have the most effect was Qsymia (phentermine-topiramate), which is not being prescribed as often. Qsymia is probably underprescribed, perhaps as a result of its high price tag. Cost is a big factor for these drugs.
Dr. John Morton is chief of bariatric and minimally invasive surgery at Stanford (Calif.) University. He moderated Dr. Singh’s presentation, and had no disclosures.

|
Dr. John Morton |
The findings do not correlate with the way weight loss drugs are prescribed. The drug that’s being prescribed the most – outside of generic phentermine – is Contrave (naltrexone-bupropion), but the one that seemed to have the most effect was Qsymia (phentermine-topiramate), which is not being prescribed as often. Qsymia is probably underprescribed, perhaps as a result of its high price tag. Cost is a big factor for these drugs.
Dr. John Morton is chief of bariatric and minimally invasive surgery at Stanford (Calif.) University. He moderated Dr. Singh’s presentation, and had no disclosures.

|
Dr. John Morton |
The findings do not correlate with the way weight loss drugs are prescribed. The drug that’s being prescribed the most – outside of generic phentermine – is Contrave (naltrexone-bupropion), but the one that seemed to have the most effect was Qsymia (phentermine-topiramate), which is not being prescribed as often. Qsymia is probably underprescribed, perhaps as a result of its high price tag. Cost is a big factor for these drugs.
Dr. John Morton is chief of bariatric and minimally invasive surgery at Stanford (Calif.) University. He moderated Dr. Singh’s presentation, and had no disclosures.
SAN DIEGO – Phentermine-topiramate was the most effective long-term weight loss drug, based on findings from a network meta-analysis from the University of California, San Diego.
The investigators pooled data from 28 studies of phentermine-topiramate (Qsymia) and four other drugs: orlistat (Xenical, Alli), lorcaserin (Belviq), naltrexone-bupropion (Contrave), and liraglutide (Victoza, Saxenda). The most commonly prescribed weight loss drug in the United States – generic phentermine – was not included because it’s not indicated for long-term use.
“It’s good to have a problem of plenty,” said Dr. Siddharth Singh, but it also makes it hard to figure out which drug to prescribe, especially given the lack of head-to-head studies. He said he hopes the results will help. However, the 30%-45% attrition rate across the studies means that the results have to be interpreted cautiously, said Dr. Singh, who is in the division of gastroenterology in the department of internal medicine, UCSD.
Most of the studies were company-funded phase III trials of weight loss drugs vs. placebo; the trials all were at least 1 year long and included more than 29,000 overweight or obese adults. The analysis was limited to patients on the maximum recommended dose of each drug.
All the drugs were more effective than placebo, but phentermine-topiramate was the most effective, with about 75% of patients losing at least 5% of their weight at 1 year and more than half losing at least 10%, with an average weight loss above placebo of 9 kg. Phentermine-topiramate patients were 10 times more likely than patients on placebo to hit the 5% mark at 1 year; liraglutide patients were 5.5 times more likely to do so; naltrexone-bupropion patients were 4 times more likely; lorcaserin patients, 3.1 times more likely; and orlistat patients, 2.7 times more likely to hit the 5% mark. The spread was similar for weight loss of 10% or more at 1 year vs. placebo.
Phentermine-topiramate’s tolerability profile was acceptable, with 10% of patients quitting the drug by 1 year because of adverse events. The best tolerated was lorcaserin, with a discontinuation rate of 6%, and the least tolerated was liraglutide, with a 13% discontinuation rate. Patients were more likely to quit active drug than placebo in all the studies.
Of course, there are other considerations, especially comorbidities; liraglutide might be the best choice in diabetics, for instance, and patients with psychiatric issues might want to avoid naltrexone-bupropion and lorcaserin, Dr. Singh said.
Subjects were a median of 46 years old, and 74% were women. The median body mass index was 36 kg/m2. All the patients had lifestyle and nutrition counseling along with their study medications.
The investigators next plan to compare the drugs’ safety and efficacy in real world settings.
There was no industry funding for the work, and Dr. Singh had no relevant financial disclosures.
SAN DIEGO – Phentermine-topiramate was the most effective long-term weight loss drug, based on findings from a network meta-analysis from the University of California, San Diego.
The investigators pooled data from 28 studies of phentermine-topiramate (Qsymia) and four other drugs: orlistat (Xenical, Alli), lorcaserin (Belviq), naltrexone-bupropion (Contrave), and liraglutide (Victoza, Saxenda). The most commonly prescribed weight loss drug in the United States – generic phentermine – was not included because it’s not indicated for long-term use.
“It’s good to have a problem of plenty,” said Dr. Siddharth Singh, but it also makes it hard to figure out which drug to prescribe, especially given the lack of head-to-head studies. He said he hopes the results will help. However, the 30%-45% attrition rate across the studies means that the results have to be interpreted cautiously, said Dr. Singh, who is in the division of gastroenterology in the department of internal medicine, UCSD.
Most of the studies were company-funded phase III trials of weight loss drugs vs. placebo; the trials all were at least 1 year long and included more than 29,000 overweight or obese adults. The analysis was limited to patients on the maximum recommended dose of each drug.
All the drugs were more effective than placebo, but phentermine-topiramate was the most effective, with about 75% of patients losing at least 5% of their weight at 1 year and more than half losing at least 10%, with an average weight loss above placebo of 9 kg. Phentermine-topiramate patients were 10 times more likely than patients on placebo to hit the 5% mark at 1 year; liraglutide patients were 5.5 times more likely to do so; naltrexone-bupropion patients were 4 times more likely; lorcaserin patients, 3.1 times more likely; and orlistat patients, 2.7 times more likely to hit the 5% mark. The spread was similar for weight loss of 10% or more at 1 year vs. placebo.
Phentermine-topiramate’s tolerability profile was acceptable, with 10% of patients quitting the drug by 1 year because of adverse events. The best tolerated was lorcaserin, with a discontinuation rate of 6%, and the least tolerated was liraglutide, with a 13% discontinuation rate. Patients were more likely to quit active drug than placebo in all the studies.
Of course, there are other considerations, especially comorbidities; liraglutide might be the best choice in diabetics, for instance, and patients with psychiatric issues might want to avoid naltrexone-bupropion and lorcaserin, Dr. Singh said.
Subjects were a median of 46 years old, and 74% were women. The median body mass index was 36 kg/m2. All the patients had lifestyle and nutrition counseling along with their study medications.
The investigators next plan to compare the drugs’ safety and efficacy in real world settings.
There was no industry funding for the work, and Dr. Singh had no relevant financial disclosures.
AT DDW® 2016
Key clinical point: Phentermine-topiramate was the most effective long-term weight loss drug in a network meta-analysis from the University of California, San Diego.
Major finding: All the drugs were better than placebo, but phentermine-topiramate was the most effective, with 75% of patients losing at least 5% of their weight at 1 year and more than half losing at least 10%.
Data source: Twenty-eight clinical trials involving more than 29,000 overweight or obese adults
Disclosures: There was no industry funding, and the presenter didn’t have any relevant financial disclosures.
VIDEO: RA patients on subcutaneous methotrexate avoid biologics
LONDON – Subcutaneous methotrexate monotherapy may be more effective at helping recently diagnosed patients with rheumatoid arthritis avoid biologic therapy, compared with similar patients on oral methotrexate, based on an analysis of data collected from 483 Canadian patients in routine care and enrolled in a national registry.
“This is a signal for improved efficacy with subcutaneous methotrexate, compared with oral methotrexate,” said Dr. Stephanie Gottheil, who reported these results at the European Congress of Rheumatology.
“In general, as long as patients with rheumatoid arthritis are under good control without a biologic drug, that is preferable” to initiating biologic treatment, said Dr. Gottheil, a researcher at Western University in London, Ont. Delaying the start of biologic treatment saves money, avoids the increased risk of infection that comes with biologic treatment, and defers a patient’s immune response to a biologic drug that can eventually compromise the biologic’s efficacy, she said in an interview.
“These data did not come from a randomized trial and so are by no means conclusive, but this is a signal that supports other data that subcutaneous methotrexate potentially puts patients into remission faster, and we know that earlier remission predicts more sustained remission,” she said.
“The biggest barrier to subcutaneous administration of methotrexate is patient preference to not inject themselves, but results from some studies have also shown that subcutaneous methotrexate is better tolerated,” compared with oral dosing, she added.
The study used data collected in the Canadian Early Arthritis Cohort (CATCH), which enrolls patients at several centers throughout Canada diagnosed with rheumatoid arthritis for less than 12 months. Dr. Gottheil and her associates particularly focused on 1,189 early RA patients with moderate to severe disease activity enrolled in CATCH during 2007-2012 who received methotrexate and had never previously received a biologic drug. The study’s primary endpoint was time to first treatment with a biologic during 3 years of follow-up after entry into the registry.
The patients’ average age at enrollment was 56 years, more than two-thirds were women, and their average methotrexate dosage was 20 mg/week. The cohort included 483 patients on methotrexate monotherapy – with virtually equal numbers on oral methotrexate and subcutaneous methotrexate – and 706 on a regimen that combined methotrexate with one or more additional (nonbiologic) drugs at baseline. The patients in each of the methotrexate monotherapy subgroups, those on oral or subcutaneous therapy, were very similar in their demographic and clinical profiles.
The analysis showed no statistically significant difference in time to first biologic use between the patients on a combination regimen and those on oral methotrexate monotherapy.
But when the researchers compared the time to first biologic among those on subcutaneous methotrexate monotherapy with those on oral methotrexate monotherapy, the subcutaneous patients showed a statistically significant, 47% reduced rate of starting any biologic drug during follow-up in an analysis that controlled for age, sex, education, comorbidities, disease duration, baseline disease activity, baseline corticosteroid use, joint erosions at baseline, and score on the health-assessment questionnaire at baseline, Dr. Gottheil reported.
The analysis also revealed three other variables that significantly linked with a slower progression to biologic treatment: older age, no use of corticosteroid treatment at baseline, and lower disease activity at baseline.
The CATCH registry research program is sponsored by AbbVie, Amgen, Bristol-Myers Squibb, Hoffmann-La Roche, Janssen, Pfizer, and UCB. Dr. Gottheil had no relevant disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
LONDON – Subcutaneous methotrexate monotherapy may be more effective at helping recently diagnosed patients with rheumatoid arthritis avoid biologic therapy, compared with similar patients on oral methotrexate, based on an analysis of data collected from 483 Canadian patients in routine care and enrolled in a national registry.
“This is a signal for improved efficacy with subcutaneous methotrexate, compared with oral methotrexate,” said Dr. Stephanie Gottheil, who reported these results at the European Congress of Rheumatology.
“In general, as long as patients with rheumatoid arthritis are under good control without a biologic drug, that is preferable” to initiating biologic treatment, said Dr. Gottheil, a researcher at Western University in London, Ont. Delaying the start of biologic treatment saves money, avoids the increased risk of infection that comes with biologic treatment, and defers a patient’s immune response to a biologic drug that can eventually compromise the biologic’s efficacy, she said in an interview.
“These data did not come from a randomized trial and so are by no means conclusive, but this is a signal that supports other data that subcutaneous methotrexate potentially puts patients into remission faster, and we know that earlier remission predicts more sustained remission,” she said.
“The biggest barrier to subcutaneous administration of methotrexate is patient preference to not inject themselves, but results from some studies have also shown that subcutaneous methotrexate is better tolerated,” compared with oral dosing, she added.
The study used data collected in the Canadian Early Arthritis Cohort (CATCH), which enrolls patients at several centers throughout Canada diagnosed with rheumatoid arthritis for less than 12 months. Dr. Gottheil and her associates particularly focused on 1,189 early RA patients with moderate to severe disease activity enrolled in CATCH during 2007-2012 who received methotrexate and had never previously received a biologic drug. The study’s primary endpoint was time to first treatment with a biologic during 3 years of follow-up after entry into the registry.
The patients’ average age at enrollment was 56 years, more than two-thirds were women, and their average methotrexate dosage was 20 mg/week. The cohort included 483 patients on methotrexate monotherapy – with virtually equal numbers on oral methotrexate and subcutaneous methotrexate – and 706 on a regimen that combined methotrexate with one or more additional (nonbiologic) drugs at baseline. The patients in each of the methotrexate monotherapy subgroups, those on oral or subcutaneous therapy, were very similar in their demographic and clinical profiles.
The analysis showed no statistically significant difference in time to first biologic use between the patients on a combination regimen and those on oral methotrexate monotherapy.
But when the researchers compared the time to first biologic among those on subcutaneous methotrexate monotherapy with those on oral methotrexate monotherapy, the subcutaneous patients showed a statistically significant, 47% reduced rate of starting any biologic drug during follow-up in an analysis that controlled for age, sex, education, comorbidities, disease duration, baseline disease activity, baseline corticosteroid use, joint erosions at baseline, and score on the health-assessment questionnaire at baseline, Dr. Gottheil reported.
The analysis also revealed three other variables that significantly linked with a slower progression to biologic treatment: older age, no use of corticosteroid treatment at baseline, and lower disease activity at baseline.
The CATCH registry research program is sponsored by AbbVie, Amgen, Bristol-Myers Squibb, Hoffmann-La Roche, Janssen, Pfizer, and UCB. Dr. Gottheil had no relevant disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
LONDON – Subcutaneous methotrexate monotherapy may be more effective at helping recently diagnosed patients with rheumatoid arthritis avoid biologic therapy, compared with similar patients on oral methotrexate, based on an analysis of data collected from 483 Canadian patients in routine care and enrolled in a national registry.
“This is a signal for improved efficacy with subcutaneous methotrexate, compared with oral methotrexate,” said Dr. Stephanie Gottheil, who reported these results at the European Congress of Rheumatology.
“In general, as long as patients with rheumatoid arthritis are under good control without a biologic drug, that is preferable” to initiating biologic treatment, said Dr. Gottheil, a researcher at Western University in London, Ont. Delaying the start of biologic treatment saves money, avoids the increased risk of infection that comes with biologic treatment, and defers a patient’s immune response to a biologic drug that can eventually compromise the biologic’s efficacy, she said in an interview.
“These data did not come from a randomized trial and so are by no means conclusive, but this is a signal that supports other data that subcutaneous methotrexate potentially puts patients into remission faster, and we know that earlier remission predicts more sustained remission,” she said.
“The biggest barrier to subcutaneous administration of methotrexate is patient preference to not inject themselves, but results from some studies have also shown that subcutaneous methotrexate is better tolerated,” compared with oral dosing, she added.
The study used data collected in the Canadian Early Arthritis Cohort (CATCH), which enrolls patients at several centers throughout Canada diagnosed with rheumatoid arthritis for less than 12 months. Dr. Gottheil and her associates particularly focused on 1,189 early RA patients with moderate to severe disease activity enrolled in CATCH during 2007-2012 who received methotrexate and had never previously received a biologic drug. The study’s primary endpoint was time to first treatment with a biologic during 3 years of follow-up after entry into the registry.
The patients’ average age at enrollment was 56 years, more than two-thirds were women, and their average methotrexate dosage was 20 mg/week. The cohort included 483 patients on methotrexate monotherapy – with virtually equal numbers on oral methotrexate and subcutaneous methotrexate – and 706 on a regimen that combined methotrexate with one or more additional (nonbiologic) drugs at baseline. The patients in each of the methotrexate monotherapy subgroups, those on oral or subcutaneous therapy, were very similar in their demographic and clinical profiles.
The analysis showed no statistically significant difference in time to first biologic use between the patients on a combination regimen and those on oral methotrexate monotherapy.
But when the researchers compared the time to first biologic among those on subcutaneous methotrexate monotherapy with those on oral methotrexate monotherapy, the subcutaneous patients showed a statistically significant, 47% reduced rate of starting any biologic drug during follow-up in an analysis that controlled for age, sex, education, comorbidities, disease duration, baseline disease activity, baseline corticosteroid use, joint erosions at baseline, and score on the health-assessment questionnaire at baseline, Dr. Gottheil reported.
The analysis also revealed three other variables that significantly linked with a slower progression to biologic treatment: older age, no use of corticosteroid treatment at baseline, and lower disease activity at baseline.
The CATCH registry research program is sponsored by AbbVie, Amgen, Bristol-Myers Squibb, Hoffmann-La Roche, Janssen, Pfizer, and UCB. Dr. Gottheil had no relevant disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
AT THE EULAR 2016 CONGRESS
Key clinical point: Among patients with recently diagnosed, moderate to severe rheumatoid arthritis, subcutaneous methotrexate monotherapy postponed the need for biologic therapy longer than did oral methotrexate monotherapy.
Major finding: Progression to biologic therapy was 47% less common among RA patients on subcutaneous methotrexate monotherapy, compared with oral methotrexate.
Data source: Three-year follow-up of 483 Canadian patients with recently diagnosed rheumatoid arthritis enrolled in CATCH, a national, real-world registry.
Disclosures: The CATCH registry research program is sponsored by AbbVie, Amgen, Bristol-Myers Squibb, Hoffmann-La Roche, Janssen, Pfizer, and UCB. Dr. Gottheil had no relevant disclosures.