User login
Low Back Pain: Evidence-based Diagnosis and Treatment
CE/CME No: CR-1605
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Identify "red flag" items in the history and physical exam that make low back pain (LBP) "complicated."
• Stratify patients into three categories: simple back pain, complicated back pain, and back pain with sciatica.
• Discuss when appropriate additional testing/imaging is needed based on LBP categories.
• Discuss patient perceptions and costs associated with imaging and LBP.
• Describe basic treatment options for noncomplicated acute LBP.
FACULTY
Mike Roscoe is the PA Program Director at the University of Evansville, Indiana. Alyssa Nishihira is in her final year of the PA program at Butler University, Indianapolis; after graduation, she will be practicing at Advanced Neurosurgery in Reno, Nevada.
The authors have no financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of May 2016.
Article begins on next page >>
Low back pain (LBP) is one of the most common reasons for an office visit, but most cases—at least 95%—have a benign underlying cause. Evaluation of LBP patients in the primary care setting, therefore, must focus on identifying “red flags” in the history and physical exam that suggest a significant underlying process requiring further work-up, including imaging. This evidence-based approach helps control costs and prevents the detrimental effects of unnecessary testing.
Low back pain (LBP) plagues many Americans and is a common reason for office visits in the United States. In 2010, back symptoms were the principal reason for 1.3% of office visits in the US.1 Recent data suggest that 75% to 85% of all Americans will experience an episode of LBP at least once in their lifetime.2 It is the leading cause of years lived with disability in the US3 and is a common reason for work disability. From a health care system standpoint, LBP imposes a considerable burden, accounting for more than $85 billion annually in direct costs.2
The etiology of LBP can be related to several anatomic and physiologic changes. Potential origins of LBP include, but are not limited to, pathology of the vertebrospinal ligaments, musculature, facet joints, fascia, vertebra and vertebral disks, and the extensive neurovascular components of the lumbar region. Although the potential causes of LBP are many, the majority of patients presenting with acute LBP usually improve with minimal clinical intervention within the first month. This is true even for patients who report limitations in daily activities and those with severe, acute cases of LBP.
A single standard of care for patients presenting with LBP has not been established. The wide array of choices for diagnosis and treatment of LBP is one factor that hinders the development of a standard diagnostic protocol. The challenge to clinicians when diagnosing LBP is to differentiate the patients with benign, self-limiting LBP (simple), who comprise the vast majority of LBP patients, from the 1% to 5% with a serious underlying pathology (complicated).4
Continue for stratification of low back pain >>
STRATIFICATION OF LOW BACK PAIN
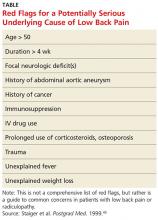
Koes and colleagues analyzed 13 different national guidelines and two international guidelines for the management of LBP.5 They found that the guidelines consistently recommend focusing the history and physical exam (HPE) on identifying features suggestive of underlying serious pathology, or “red flags,” and excluding specific diseases.5 They also found that none of the guidelines recommends the routine use of imaging in patients without suspected serious pathology.5 The American College of Radiology simplified this approach to patients with LBP by creating a list of red flags to look for during the HPE.3 The presence of red flags indicates a case of complicated LBP, and patients who present with them should undergo additional diagnostic studies to screen for serious underlying conditions (see the Table).
The HPE should ultimately separate patients into three categories to determine the need for imaging (and course of treatment): (1) simple acute back pain, (2) complicated back pain with red flag (ie, a potential underlying systemic disease), and (3) LBP with neurologic deficits potentially requiring surgery.5
Simple acute low back pain
Up to 85% of patients presenting with LBP may never receive a definitive diagnosis due to lack of specific symptoms and ambiguous imaging results.6 Clinicians can assume that LBP in these patients is due to a mechanical cause, by far the most common cause of LBP.7 It is therefore more useful to rule out serious or potentially fatal causes of LBP (complicated LBP) rather than rule in a cause for patients presenting with LBP.
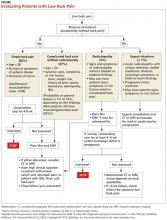
It is generally accepted among practitioners that a thorough HPE alone is sufficient for evaluating most patients presenting with acute LBP lasting less than four weeks.5 Patients presenting without red flags should be assured that improvement of acute LBP is typical, and that no diagnostic intervention is needed unless they do not improve as expected per patient or provider (eg, in terms of activities of daily living or work restrictions). The Figure depicts an appropriate approach to diagnosis and treatment in patients presenting with LBP.8 Clinicians should also offer patient education for self-care and discuss noninvasive treatment options, including pharmacologic and nonpharmacologic therapy.9
Low back pain with red flags (complicated)
Patient history is more useful than the physical exam in screening for spinal malignancies. In one particular combination (age > 50, history of cancer, unexplained weight loss, and failure to improve with conservative therapy), red flag symptoms are 100% sensitive for detecting malignancy.10 However, malignant neoplasms of the spine make up less than 1% of the diagnoses of patients presenting with LBP in primary care.4 Additionally, Deyo and Diehl reviewed five studies of a large series of consecutive spine films with large sample sizes and found the incidence of tumors, infections, and inflammatory spondyloarthropathies together were present in less than 2%.11 This low prevalence underscores the challenge of diagnosing serious pathology of the spine in the primary care setting.
Patients with complicated back pain presenting with red flags should always be examined for an underlying systemic disease. There is one red flag that, seen in isolation, meaningfully increases the likelihood of cancer: a previous history of cancer.4 Otherwise, inflammatory markers (eg, erythrocyte sedimentation rate) can be used to determine the need for advanced imaging (see the Figure).10
Low back pain with neurologic findings (sciatica)
Screening (HPE) for neurologic damage is difficult because traditional findings of neurologic injury (paresis or muscle weakness, impaired reflexes, sensory deficits, and decreased range of motion) all have low sensitivity with higher specificity.12 For this reason, these tests are of limited value as screening tools during the HPE. Specific exams, such as the straight leg raise and crossed straight leg tests, are also of limited value, especially in the primary care setting, because of inconsistent sensitivity and specificity.
This is the primary reason that the HPE in patients with LBP who have neurologic findings must include evaluation for urgent findings (see the Figure). If any red flags are present, advanced imaging is immediately warranted. Otherwise, inflammatory markers and plain radiography may be obtained, and advanced imaging may be considered if the plain radiography and/or inflammatory markers are abnormal.
There is also an approach that advocates the use of advanced imaging in patients with significant functional disability due to their LBP. Two questionnaires, the Oswestry Low Back Pain Disability Index and the Roland-Morris Disability Questionnaire, evaluate subjective data to determine a patient’s functional disability due to LBP.The validity of both tests has been confirmed.13
Continue for diagnostic imaging >>
DIAGNOSTIC IMAGING
The majority of patients presenting with LBP without concerning symptoms can be assumed to have nonspecific mechanical back pain. These patients do not need radiography unless the pain has not improved after four to six weeks of conservative care, because plain radiographs often detect findings (degenerative joint disease, bone spurs, spondylosis) that are unrelated to symptoms.9 Advanced imaging is generally recommended only for LBP patients with red flags due to the potentially critical nature of these cases.5 Patients with LBP presenting with any of these factors require further testing, even if the duration of their pain is less than four weeks.
If a patient’s LBP persists beyond four weeks, the clinician must decide which diagnostic test to order. General medical knowledge suggests that MRI is superior to plain radiography because it shows soft tissue and can detect more concerning abnormalities, such as infections, cancer, and metastatic tumors. CT is better for showing bony abnormalities, but these rarely correlate with a patient’s LBP, and CT subjects patients to levels of radiation that can increase cancer risks.14 Plain radiography in this cohort (LBP > 4 wk) is not generally recommended as it cannot show intervertebral discs or evaluate the degree of spinal stenosis as accurately as MRI. Additionally, these lumbar radiographs expose patients to more than 35 times the radiation delivered in a single chest radiograph.15
COSTS AND PATIENT OUTCOMES
The estimated cost of unnecessary imaging for LBP is $300 million per year.16 There is evidence of a strong association between advanced lumbar spine imaging and increased rates of surgery and significantly higher total medical expenditures.17,18 One study examined patients with nonspecific LBP who either received MRI within 30 days post-onset (defined as “early MRI”) or did not receive MRI. Early-MRI patients had significantly higher total medical expenses ($12,948, P < .0001) than the no-MRI group.17 The early-MRI group also had significantly longer periods of disability and were less likely to go off disability than the no-MRI group (P < .0001).
Cost-effectiveness studies of plain radiographs, dating back to 1982, have yielded similar findings. Liang et al suggested that if radiography was done routinely at the initial visit in patients with acute LBP but no red flags, the cost would be more than $2,000 (in 1982 dollars) to avert one day of pain.19 A more recent study examined patients with acute LBP who received MRI, with one group blinded (both patients and physicians) to their MRI results for six months while the other group received their results within 48 hours.20 All patients underwent a physical exam by a study coordinator, and treatment was assigned prior to imaging. At six weeks and one year, there was no significant difference in treatment assignments or self-reported surveys between groups, indicating that the MRI results had no significant influence on patient outcomes.
Despite the large increase in the use of advanced diagnostic imaging aimed at improving patient care and outcomes, there is a lack of data showing any correlative or causative connection between the two. Given this lack of evidence, and the potentially detrimental radiation exposure and increased costs to patients, clinicians should follow evidence-based guidelines when considering diagnostic imaging in patients presenting with LBP.
Continue for patient perception >>
PATIENT PERCEPTION
Patient satisfaction plays a very important role in health care and may correlate with compliance and other outcomes. One study showed that while radiography in patients with LBP was not associated with improved clinical outcomes, it did increase patients’ satisfaction with the care they received.21 A study that grouped patients requiring imaging for LBP into rapid MRI and plain film radiography cohorts found that patients who received rapid MRI were more assured by their results than were patients in the radiography group (74% vs 58%, P = .002).22 Both groups showed significant clinical improvement in the first three months, but there was no difference between groups at either the three- or 12-month mark. In both groups, reassurance was positively correlated with patient satisfaction (Pearson correlation coefficients, 0.55-0.59, P < .001).
Patients may be reassured by imaging, even when it is unnecessary. Effectively explaining symptomatology during the HPE to patients with LBP should be of high priority to clinicians. A study found that when patients with mechanical LBP did not receive an adequate explanation of the problem, they were less satisfied with their visit and wanted more diagnostic tests.11 Another study found that when low-risk patients were randomly assigned to a control group and received an educational intervention only, they reported equal satisfaction with their care and had clinical outcomes equal to those of the treatment group that received a plain radiograph.11
Given the costs, radiation risks, and other negative aspects of unnecessary imaging, additional diagnostic tests may not be in a patient’s best interest. A careful physical exam should be performed, with the clinician providing ongoing commentary to reassure patients that the clinician is neither dismissing the patient’s symptoms nor inappropriately avoiding further tests.
Often, medical providers order imaging with the intention to reassure patients with the results and thus ultimately increase the patient’s sense of well-being. However, the opposite effect may occur, with patients actually developing a decreased sense of wellness with no alteration of outcomes. A study evaluated general health (GH) scores (based on results from several screening questionnaires that assessed the patient’s current physical and mental health state) in patients receiving MRI results.20 The patients were divided into those who received results (within 48 hours), and those who did not unless it was critical to patient management (blinded group). At six weeks, the blinded group’s GH score was significantly higher than the early-informed group’s GH score. This suggests that receiving MRI results may negatively influence patients’ perception of their general health.20
The same meta-analysis that reviewed patient outcomes also evaluated mental health and quality-of-life scores of LBP patients who received either MRI, CT, or radiography.23 There was no short-term (< 3 mo) or long-term (6-12 mo) difference between patients who received radiography versus advanced imaging. This indicates that using imaging of any kind in patients with LBP but without indications of serious underlying conditions does not improve clinical outcomes and is negatively correlated with quality-of-life measures at short- and long-term intervals.23
Continue for treatment >>
TREATMENT
The prognosis of simple acute mechanical LBP is excellent. Although back pain is a leading reason for visiting health care providers, many affected individuals never seek medical care and apparently improve on their own. In a random telephone survey of North Carolina residents, only 39% of persons with LBP sought medical care.24 Therefore, patients who do seek treatment should be given reassurance, and therapies should be tailored to the individual in the least invasive and most cost-effective manner. Many treatment options are available for LBP, but often strong evidence of benefit is lacking.
Pharmacologic therapy
Anti-inflammatories. It can be assumed that when a patient comes to the practitioner for evaluation of LBP, there is an expectation that some type of medication will be recommended or prescribed for pain relief. Unless there is a contraindication, NSAIDs are often first-line therapy, and they are effective for short-term symptom relief when compared with placebo.25 A mild pain medication, such as acetaminophen, is also a common treatment. The 2007 joint practice guideline from the American Pain Society (APS) and the American College of Physicians (ACP) recommends acetaminophen or NSAIDs as first-line therapy for acute LBP.3 Neither agent—NSAIDs or acetaminophen—has shown superiority, and combining the two has shown no additional benefits.26 Caution must be used, however, as NSAIDs have a risk for gastrointestinal toxicity and nephrotoxicity, and acetaminophen has a dose- and patient-dependent risk for hepatotoxicity.
Muscle relaxants. Muscle relaxants are another pharmacologic treatment option for LBP. Most pain reduction from this class of medication occurs in the first one to two weeks of therapy, although benefit may continue for up to four weeks.27 There is also evidence that a combination of an NSAID and a muscle relaxer has added benefits.27 These medications are centrally acting, so sedation and dizziness are common; all medications in this class have these adverse effects to some degree. Carisoprodol has as its first metabolite meprobamate, which is a tranquilizer used to treat anxiety disorders; it has a potential for abuse and should be used with caution in certain populations.
Opioids. Opioids are commonly prescribed to patients with LBP, though there are limited data regarding efficacy. One trial compared an NSAID alone versus an NSAID plus oxycodone/acetaminophen and found no significant difference in pain or disability after seven days.28 In addition, the adverse effects of opioids, which include sedation, constipation, nausea, and confusion, may be amplified in the elderly population; therefore, opioids should be prescribed with caution in these patients. If prescribed to treat acute LBP, opioids should be used in short, scheduled dosing regimens since NSAIDs or acetaminophen suffice for most patients.
Corticosteroids. Oral glucocorticoids are sometimes given to patients with acute LBP, and they likely are used more frequently in patients with radicular symptoms. However, the APS/ACP 2007 joint guidelines recommend against use of systemic glucocorticoids for acute LBP due to lack of proven benefit.3 Epidural steroid injections are not generally beneficial for isolated acute LBP, but there is evidence that they are helpful with persistent radicular pain.29 Zarghooni and colleagues found significant reductions in pain and use of pain medication after single-shot epidural injections.29
Other pharmacologic therapies, acupuncture, sclerotherapy, and other methods are used to treat back pain, but these are typically reserved for chronic, not acute, LBP.
Nonpharmacologic therapy
Physical therapy. Physical therapy is a commonly prescribed treatment for LBP. Systematic literature reviews indicate that for patients with acute LBP (< 6 wk), there is no difference in the effectiveness of exercise therapy compared to no treatment and care provided by a general practitioner or to manipulations.30 For patients with subacute (6-12 wk) and chronic (≥ 12 wk) LBP, exercise therapy is effective compared to no treatment.30 There is debate, however, over which exercise activities should be used. Research supports strength/resistance and coordination/stabilization exercises.
Most therapists recommend the McKenzie method or spine stabilization exercises.31 The McKenzie method is used for LBP with sciatica; the patient moves through exercises within the prone position and focuses on extension of the spine. Spine stabilization is an active form of exercise based on a “neutral spine” position and helps strengthen muscles to maintain this position (core stabilization). The McKenzie method, when added to first-line care for LBP, does not produce significant improvements in pain or other clinical outcomes, although it may reduce health care utilization.32 Spine stabilization exercises have been shown to decrease pain, disability, and risk for recurrence after a first episode of back pain.33 The apparent success of physical therapy is attributed to compliance with directed home exercise programs, which have been shown to reduce the rate of recurrence, decrease episodes of acute LBP, and decrease the need for health services.34
Spinal traction. Traction or nonsurgical spinal decompression has emerged as a treatment for LBP. Unfortunately, there are little data to support its use as a treatment for acute LBP. Only a few randomized trials showed benefit, and these were small studies with a high risk for bias. A Cochrane review published in 2013 looked at 32 studies involving 2,762 patients with acute, subacute, and chronic LBP.35 The review did not find any evidence that traction alone or in combination with other therapy was any better than placebo treatment.35
Spinal manipulation. Spinal manipulation may be more effective than placebo treatment in reducing pain when the pain has been present for less than six weeks, but it is not more effective in reducing disability.36 There is little or no high-level evidence about spinal manipulation for acute LBP. However, there is some evidence of cost-effectiveness when using spinal manipulation in subacute to chronic pain.37 Chiropractic techniques are considered safe (when performed by a trained provider), but a systematic review found that these techniques provide no clinically relevant improvement in pain or disability when compared to other treatments.38
Bed rest. Bed rest has not been shown to improve outcomes, and in fact patients who had bed rest had less favorable outcomes than those who stayed active.39 Bed rest is less effective at reducing pain and improving function when compared to staying active.39
Continue for recommended management >>
Recommended management
A patient who presents with nonspecific acute LBP should have a thorough HPE to evaluate for the presence of red flags. If no concerning findings are present, the initial visit should focus on patient education based on the following items: (1) good prognosis with little intervention, (2) staying active and avoiding bed rest as much as possible, and (3) avoiding pain-causing movements when possible. The second step is to initiate a trial of an NSAID or acetaminophen and consider a muscle relaxant based on pain severity. Avoid opioid therapy if possible, but use conservative dosing if required for severe pain. Patients should be advised to return in two to four weeks if they do not experience significant improvement. At this time, the clinician may consider referring the patient for physical therapy, changing NSAIDs, ordering inflammatory markers, and/or referring to a specialist.
CONCLUSION
Although no single diagnostic protocol for LBP exists, the clinician must be able to distinguish simple from complex types. A thorough HPE is useful for categorizing the patient’s pain, with diagnostic imaging reserved for those patients with severe or progressive neurologic deficits, suspicion of serious underlying conditions, or LBP lasting more than four weeks without improvement. MRI, if available, is generally preferred over CT because it does not use ionizing radiation and provides better visualization of soft tissue, vertebral marrow, and the spinal cord. Symptomatology should be explained to patients with LBP during the HPE, with ongoing commentary to increase patient satisfaction and compliance. About two-thirds of patients with LBP do not seek evaluation from a health care provider; therefore, those who do seek treatment should be reassured, and therapies tailored to the individual in the least invasive and most cost-effective manner possible.
1. CDC. National Ambulatory Medical Care Survey: 2010 Summary Tables. Table 9. www.cdc.gov/nchs/data/ahcd/namcs_summary/2010_namcs_web_tables.pdf. Accessed March 29, 2016.
2. Davies C, Nitz AJ, Mattacola CG, et al. Practice patterns when treating patients with low back pain: a survey of physical therapists. Physiother Theor Pract. 2014;30(6):399-408.
3. American College of Radiology. ACR Appropriateness Criteria. Low back pain. 2015. www.acr.org/~/media/ACR/Documents/AppCriteria/Diagnostic/LowBackPain.pdf. Accessed March 10, 2016.
4. Henschke N, Maher CG, Ostelo RW, et al. Red flags to screen for malignancy in patients with low back pain. Cochrane Database Syst Rev. 2013;2:CD008686.
5. Koes BW, Tulder M, Lin CW, et al. An updated overview of clinical guidelines for the management of non-specific low back pain in primary care. Eur Spine J. 2010;19(12):2075-2094.
6. Deyo RA, Rainville J, Kent DL. What can the history and physical examination tell us about low back pain? JAMA. 1992;268(6):760-765.
7. Jarvik JG. Diagnostic evaluation of low back pain with emphasis on imaging. Ann Intern Med. 2002;137:586-597.
8. Diagnostic testing for low back pain. In: Post TW (ed), UpToDate, Waltham, MA. www.uptodate.com. Accessed March 16, 2016.
9. Chou R, Qaseem A, Snow V, et al; Clinical Efficacy Assessment Subcommittee of the American College of Physicians; American College of Physicians; American Pain Society Low Back Pain Guidelines Panel. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007;147(7):478-491.
10. Deyo RA, Diehl AK. Cancer as a cause of back pain: frequency, clinical presentation, and diagnostic strategies. J Gen Intern Med. 1988;3(3):230-238.
11. Deyo RA, Diehl AK. Patient satisfaction with medical care for low-back pain. Spine. 1986;11(1):28-30.
12. Pradeep S, Rainville J, Katz JN, et al. The accuracy of the physical examination for the diagnosis of midlumbar and low lumbar nerve root impingement. Spine. 2011;36(1):63-73.
13. Leclaire R, Blier F, Fortin L, Proulx R. A cross-sectional study comparing the Oswestry and Roland-Morris Functional Disability Scales in two populations of patients with low back pain of different levels of severity. Spine. 1997;22(1):68-71
14. FDA. Radiation emitting products. www.fda.gov/Radiation-EmittingProducts/RadiationEmittingProductsandProcedures/MedicalImaging/MedicalX-Rays/ucm115317.htm. Accessed March 29, 2016.
15. Simpson AK, Whang PG, Jonisch A, et al. The radiation exposure associated with cervical and lumbar spine radiographs. J Spinal Disord Tech. 2008;21(6):409-412.
16. Srinivas S, Deyo R, Berger Z. Application of “less is more” to lower back pain. Arch Intern Med. 2012;172(13):1016-1020.
17. Webster BS, Bauer AZ, Choi Y, et al. Iatrogenic consequences of early magnetic resonance imaging in acute, work-related, disabling back pain. Spine. 2013;38(22):1939-1946.
18. Webster BS, Bauer AZ, Choi Y, et al. The cascade of medical services and associated longitudinal costs due to nonadherent magnetic resonance imaging for low back pain. Spine. 2014;39(17):1433-1440.
19. Liang M, Komaroff AL. Roentgenograms in primary care patients with acute low back pain: a cost-effectiveness analysis. Arch Intern Med. 1982;142(6):1108-1112.
20. Ash LM, Modic MT, Obuchowski NA, et al. Effects of diagnostic information, per se, on patient outcomes in acute radiculopathy and low back pain. AJNR Am J Neuroradiol. 2008;29(6):1098-1103.
21. Kendrick D, Fielding K, Bentley E, et al. Radiography of the lumbar spine in primary care patients with low back pain: randomized controlled trial. BMJ. 2001;322(7283):400-405.
22. Jarvik JG, Hollingworth W, Martin B, et al. Rapid magnetic resonance imaging vs radiographs for patients with low back pain. JAMA. 2003;289(21):2810-2818.
23. Chou R, Fu R, Carrino JA, Deyo RA. Imaging strategies for low-back pain: systematic review and meta-analysis. Lancet. 2009;373(9662):463-472.
24. Carey TS, Evans AT, Hadler NM, et al. Acute severe low back pain: a population-based study of prevalence and care-seeking. Spine. 1996;21(3):339-344.
25. Roelofs PD, Deyo RA, Koes BW, et al. Nonsteroidal anti-inflammatory drugs for low back pain. Spine. 2008;33(16):1766-1774.
26. Hancock MJ, Maher CG, Latimer J, et al. Assessment of diclofenac or spinal manipulative therapy, or both, in addition to recommended first-line treatment for acute low back pain: a randomized controlled trial. Lancet. 2007;370(10):1638-1643.
27. Van Tulder MW, Touray T, Furlan AD, et al. Muscle relaxants for non-specific low-back pain. Cochrane Database Syst Rev. 2003;(4):CD004252.
28. Friedman BW, Dym AA, Davitt M, et al. Naproxen with cyclobenzaprine, oxycodone/acetaminophen, or placebo for treating acute low back pain: a randomized clinical trial. JAMA. 2015;314(15):1572-1580.
29. Zarghooni K, Rashidi A, Siewe, J, et al. Single-shot epidural injections in the management of radicular pain. Orthop Rev (Pavia). 2015;7(4):5985.
30. Smidt N, deVet HC, Bouter LM, et al. Effectiveness of exercise therapy: A best-evidence summary of systematic reviews. Aust J Physiother. 2005;51(2):71-85.
31. Casazza BA. Diagnosis and treatment of acute low back pain. Am Fam Physician. 2012;85(4):343-350.
32. Machado LA, Maher CG, Herbert RD, et al. The effectiveness of the McKenzie method in addition to first-line care for acute low back pain: a randomized controlled trial. BMC Med. 2010;8(10):1-10.
33. Cho I, Jeon C, Lee S, et al. Effects of lumbar stabilization exercise on functional disability and lumbar lordosis angle in patients with chronic low back pain. J Phys Ther Sci. 2015;27(6):1983-1985.
34. Choi BK, Verbeek JH, Tam WW, Jiang JY. Exercises for prevention of recurrences of low-back pain (review). Cochrane Database Syst Rev. 2010;(1):CD006555.
35. Wegner I, Widyahening IS, van Tulder MW, et al. Traction for low-back pain with or without sciatica (review). Cochrane Database Syst Rev. 2013;(8):CD003010.
36. Hoiriis KT, Pfleger B, McDuffie FC, et al. A randomized clinical trial comparing chiropractic adjustments to muscle relaxants for subacute low back pain. J Manipulative Physiol Ther. 2004;27(6):388-398.
37. Lin CC, Haas M, Maher CG, et al. Cost-effectiveness of guideline-endorsed treatments for low back pain: a systematic review. Eur Spine J. 2011;20:1024-1038.
38. Walker BF, French SD, Grant W, Green S. A Cochrane Review of combined chiropractic interventions for low-back pain. Spine. 2011;36(3): 230-242.
39. Dahm KT, Brurberg KG, Jamtvedt G, Hagen KB. Advice to rest in bed versus advice to stay active for acute low-back pain and sciatica. Cochrane Database Syst Rev. 2010;(6):CD007612.
40. Staiger T, Paauw D, Deyo A, Jarvik JG. Imaging studies for acute low back pain. When and when not to order them. Postgrad Med. 1999;105(4):161-162,165-166,171-172.
CE/CME No: CR-1605
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Identify "red flag" items in the history and physical exam that make low back pain (LBP) "complicated."
• Stratify patients into three categories: simple back pain, complicated back pain, and back pain with sciatica.
• Discuss when appropriate additional testing/imaging is needed based on LBP categories.
• Discuss patient perceptions and costs associated with imaging and LBP.
• Describe basic treatment options for noncomplicated acute LBP.
FACULTY
Mike Roscoe is the PA Program Director at the University of Evansville, Indiana. Alyssa Nishihira is in her final year of the PA program at Butler University, Indianapolis; after graduation, she will be practicing at Advanced Neurosurgery in Reno, Nevada.
The authors have no financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of May 2016.
Article begins on next page >>
Low back pain (LBP) is one of the most common reasons for an office visit, but most cases—at least 95%—have a benign underlying cause. Evaluation of LBP patients in the primary care setting, therefore, must focus on identifying “red flags” in the history and physical exam that suggest a significant underlying process requiring further work-up, including imaging. This evidence-based approach helps control costs and prevents the detrimental effects of unnecessary testing.
Low back pain (LBP) plagues many Americans and is a common reason for office visits in the United States. In 2010, back symptoms were the principal reason for 1.3% of office visits in the US.1 Recent data suggest that 75% to 85% of all Americans will experience an episode of LBP at least once in their lifetime.2 It is the leading cause of years lived with disability in the US3 and is a common reason for work disability. From a health care system standpoint, LBP imposes a considerable burden, accounting for more than $85 billion annually in direct costs.2
The etiology of LBP can be related to several anatomic and physiologic changes. Potential origins of LBP include, but are not limited to, pathology of the vertebrospinal ligaments, musculature, facet joints, fascia, vertebra and vertebral disks, and the extensive neurovascular components of the lumbar region. Although the potential causes of LBP are many, the majority of patients presenting with acute LBP usually improve with minimal clinical intervention within the first month. This is true even for patients who report limitations in daily activities and those with severe, acute cases of LBP.
A single standard of care for patients presenting with LBP has not been established. The wide array of choices for diagnosis and treatment of LBP is one factor that hinders the development of a standard diagnostic protocol. The challenge to clinicians when diagnosing LBP is to differentiate the patients with benign, self-limiting LBP (simple), who comprise the vast majority of LBP patients, from the 1% to 5% with a serious underlying pathology (complicated).4
Continue for stratification of low back pain >>
STRATIFICATION OF LOW BACK PAIN
Koes and colleagues analyzed 13 different national guidelines and two international guidelines for the management of LBP.5 They found that the guidelines consistently recommend focusing the history and physical exam (HPE) on identifying features suggestive of underlying serious pathology, or “red flags,” and excluding specific diseases.5 They also found that none of the guidelines recommends the routine use of imaging in patients without suspected serious pathology.5 The American College of Radiology simplified this approach to patients with LBP by creating a list of red flags to look for during the HPE.3 The presence of red flags indicates a case of complicated LBP, and patients who present with them should undergo additional diagnostic studies to screen for serious underlying conditions (see the Table).
The HPE should ultimately separate patients into three categories to determine the need for imaging (and course of treatment): (1) simple acute back pain, (2) complicated back pain with red flag (ie, a potential underlying systemic disease), and (3) LBP with neurologic deficits potentially requiring surgery.5
Simple acute low back pain
Up to 85% of patients presenting with LBP may never receive a definitive diagnosis due to lack of specific symptoms and ambiguous imaging results.6 Clinicians can assume that LBP in these patients is due to a mechanical cause, by far the most common cause of LBP.7 It is therefore more useful to rule out serious or potentially fatal causes of LBP (complicated LBP) rather than rule in a cause for patients presenting with LBP.
It is generally accepted among practitioners that a thorough HPE alone is sufficient for evaluating most patients presenting with acute LBP lasting less than four weeks.5 Patients presenting without red flags should be assured that improvement of acute LBP is typical, and that no diagnostic intervention is needed unless they do not improve as expected per patient or provider (eg, in terms of activities of daily living or work restrictions). The Figure depicts an appropriate approach to diagnosis and treatment in patients presenting with LBP.8 Clinicians should also offer patient education for self-care and discuss noninvasive treatment options, including pharmacologic and nonpharmacologic therapy.9
Low back pain with red flags (complicated)
Patient history is more useful than the physical exam in screening for spinal malignancies. In one particular combination (age > 50, history of cancer, unexplained weight loss, and failure to improve with conservative therapy), red flag symptoms are 100% sensitive for detecting malignancy.10 However, malignant neoplasms of the spine make up less than 1% of the diagnoses of patients presenting with LBP in primary care.4 Additionally, Deyo and Diehl reviewed five studies of a large series of consecutive spine films with large sample sizes and found the incidence of tumors, infections, and inflammatory spondyloarthropathies together were present in less than 2%.11 This low prevalence underscores the challenge of diagnosing serious pathology of the spine in the primary care setting.
Patients with complicated back pain presenting with red flags should always be examined for an underlying systemic disease. There is one red flag that, seen in isolation, meaningfully increases the likelihood of cancer: a previous history of cancer.4 Otherwise, inflammatory markers (eg, erythrocyte sedimentation rate) can be used to determine the need for advanced imaging (see the Figure).10
Low back pain with neurologic findings (sciatica)
Screening (HPE) for neurologic damage is difficult because traditional findings of neurologic injury (paresis or muscle weakness, impaired reflexes, sensory deficits, and decreased range of motion) all have low sensitivity with higher specificity.12 For this reason, these tests are of limited value as screening tools during the HPE. Specific exams, such as the straight leg raise and crossed straight leg tests, are also of limited value, especially in the primary care setting, because of inconsistent sensitivity and specificity.
This is the primary reason that the HPE in patients with LBP who have neurologic findings must include evaluation for urgent findings (see the Figure). If any red flags are present, advanced imaging is immediately warranted. Otherwise, inflammatory markers and plain radiography may be obtained, and advanced imaging may be considered if the plain radiography and/or inflammatory markers are abnormal.
There is also an approach that advocates the use of advanced imaging in patients with significant functional disability due to their LBP. Two questionnaires, the Oswestry Low Back Pain Disability Index and the Roland-Morris Disability Questionnaire, evaluate subjective data to determine a patient’s functional disability due to LBP.The validity of both tests has been confirmed.13
Continue for diagnostic imaging >>
DIAGNOSTIC IMAGING
The majority of patients presenting with LBP without concerning symptoms can be assumed to have nonspecific mechanical back pain. These patients do not need radiography unless the pain has not improved after four to six weeks of conservative care, because plain radiographs often detect findings (degenerative joint disease, bone spurs, spondylosis) that are unrelated to symptoms.9 Advanced imaging is generally recommended only for LBP patients with red flags due to the potentially critical nature of these cases.5 Patients with LBP presenting with any of these factors require further testing, even if the duration of their pain is less than four weeks.
If a patient’s LBP persists beyond four weeks, the clinician must decide which diagnostic test to order. General medical knowledge suggests that MRI is superior to plain radiography because it shows soft tissue and can detect more concerning abnormalities, such as infections, cancer, and metastatic tumors. CT is better for showing bony abnormalities, but these rarely correlate with a patient’s LBP, and CT subjects patients to levels of radiation that can increase cancer risks.14 Plain radiography in this cohort (LBP > 4 wk) is not generally recommended as it cannot show intervertebral discs or evaluate the degree of spinal stenosis as accurately as MRI. Additionally, these lumbar radiographs expose patients to more than 35 times the radiation delivered in a single chest radiograph.15
COSTS AND PATIENT OUTCOMES
The estimated cost of unnecessary imaging for LBP is $300 million per year.16 There is evidence of a strong association between advanced lumbar spine imaging and increased rates of surgery and significantly higher total medical expenditures.17,18 One study examined patients with nonspecific LBP who either received MRI within 30 days post-onset (defined as “early MRI”) or did not receive MRI. Early-MRI patients had significantly higher total medical expenses ($12,948, P < .0001) than the no-MRI group.17 The early-MRI group also had significantly longer periods of disability and were less likely to go off disability than the no-MRI group (P < .0001).
Cost-effectiveness studies of plain radiographs, dating back to 1982, have yielded similar findings. Liang et al suggested that if radiography was done routinely at the initial visit in patients with acute LBP but no red flags, the cost would be more than $2,000 (in 1982 dollars) to avert one day of pain.19 A more recent study examined patients with acute LBP who received MRI, with one group blinded (both patients and physicians) to their MRI results for six months while the other group received their results within 48 hours.20 All patients underwent a physical exam by a study coordinator, and treatment was assigned prior to imaging. At six weeks and one year, there was no significant difference in treatment assignments or self-reported surveys between groups, indicating that the MRI results had no significant influence on patient outcomes.
Despite the large increase in the use of advanced diagnostic imaging aimed at improving patient care and outcomes, there is a lack of data showing any correlative or causative connection between the two. Given this lack of evidence, and the potentially detrimental radiation exposure and increased costs to patients, clinicians should follow evidence-based guidelines when considering diagnostic imaging in patients presenting with LBP.
Continue for patient perception >>
PATIENT PERCEPTION
Patient satisfaction plays a very important role in health care and may correlate with compliance and other outcomes. One study showed that while radiography in patients with LBP was not associated with improved clinical outcomes, it did increase patients’ satisfaction with the care they received.21 A study that grouped patients requiring imaging for LBP into rapid MRI and plain film radiography cohorts found that patients who received rapid MRI were more assured by their results than were patients in the radiography group (74% vs 58%, P = .002).22 Both groups showed significant clinical improvement in the first three months, but there was no difference between groups at either the three- or 12-month mark. In both groups, reassurance was positively correlated with patient satisfaction (Pearson correlation coefficients, 0.55-0.59, P < .001).
Patients may be reassured by imaging, even when it is unnecessary. Effectively explaining symptomatology during the HPE to patients with LBP should be of high priority to clinicians. A study found that when patients with mechanical LBP did not receive an adequate explanation of the problem, they were less satisfied with their visit and wanted more diagnostic tests.11 Another study found that when low-risk patients were randomly assigned to a control group and received an educational intervention only, they reported equal satisfaction with their care and had clinical outcomes equal to those of the treatment group that received a plain radiograph.11
Given the costs, radiation risks, and other negative aspects of unnecessary imaging, additional diagnostic tests may not be in a patient’s best interest. A careful physical exam should be performed, with the clinician providing ongoing commentary to reassure patients that the clinician is neither dismissing the patient’s symptoms nor inappropriately avoiding further tests.
Often, medical providers order imaging with the intention to reassure patients with the results and thus ultimately increase the patient’s sense of well-being. However, the opposite effect may occur, with patients actually developing a decreased sense of wellness with no alteration of outcomes. A study evaluated general health (GH) scores (based on results from several screening questionnaires that assessed the patient’s current physical and mental health state) in patients receiving MRI results.20 The patients were divided into those who received results (within 48 hours), and those who did not unless it was critical to patient management (blinded group). At six weeks, the blinded group’s GH score was significantly higher than the early-informed group’s GH score. This suggests that receiving MRI results may negatively influence patients’ perception of their general health.20
The same meta-analysis that reviewed patient outcomes also evaluated mental health and quality-of-life scores of LBP patients who received either MRI, CT, or radiography.23 There was no short-term (< 3 mo) or long-term (6-12 mo) difference between patients who received radiography versus advanced imaging. This indicates that using imaging of any kind in patients with LBP but without indications of serious underlying conditions does not improve clinical outcomes and is negatively correlated with quality-of-life measures at short- and long-term intervals.23
Continue for treatment >>
TREATMENT
The prognosis of simple acute mechanical LBP is excellent. Although back pain is a leading reason for visiting health care providers, many affected individuals never seek medical care and apparently improve on their own. In a random telephone survey of North Carolina residents, only 39% of persons with LBP sought medical care.24 Therefore, patients who do seek treatment should be given reassurance, and therapies should be tailored to the individual in the least invasive and most cost-effective manner. Many treatment options are available for LBP, but often strong evidence of benefit is lacking.
Pharmacologic therapy
Anti-inflammatories. It can be assumed that when a patient comes to the practitioner for evaluation of LBP, there is an expectation that some type of medication will be recommended or prescribed for pain relief. Unless there is a contraindication, NSAIDs are often first-line therapy, and they are effective for short-term symptom relief when compared with placebo.25 A mild pain medication, such as acetaminophen, is also a common treatment. The 2007 joint practice guideline from the American Pain Society (APS) and the American College of Physicians (ACP) recommends acetaminophen or NSAIDs as first-line therapy for acute LBP.3 Neither agent—NSAIDs or acetaminophen—has shown superiority, and combining the two has shown no additional benefits.26 Caution must be used, however, as NSAIDs have a risk for gastrointestinal toxicity and nephrotoxicity, and acetaminophen has a dose- and patient-dependent risk for hepatotoxicity.
Muscle relaxants. Muscle relaxants are another pharmacologic treatment option for LBP. Most pain reduction from this class of medication occurs in the first one to two weeks of therapy, although benefit may continue for up to four weeks.27 There is also evidence that a combination of an NSAID and a muscle relaxer has added benefits.27 These medications are centrally acting, so sedation and dizziness are common; all medications in this class have these adverse effects to some degree. Carisoprodol has as its first metabolite meprobamate, which is a tranquilizer used to treat anxiety disorders; it has a potential for abuse and should be used with caution in certain populations.
Opioids. Opioids are commonly prescribed to patients with LBP, though there are limited data regarding efficacy. One trial compared an NSAID alone versus an NSAID plus oxycodone/acetaminophen and found no significant difference in pain or disability after seven days.28 In addition, the adverse effects of opioids, which include sedation, constipation, nausea, and confusion, may be amplified in the elderly population; therefore, opioids should be prescribed with caution in these patients. If prescribed to treat acute LBP, opioids should be used in short, scheduled dosing regimens since NSAIDs or acetaminophen suffice for most patients.
Corticosteroids. Oral glucocorticoids are sometimes given to patients with acute LBP, and they likely are used more frequently in patients with radicular symptoms. However, the APS/ACP 2007 joint guidelines recommend against use of systemic glucocorticoids for acute LBP due to lack of proven benefit.3 Epidural steroid injections are not generally beneficial for isolated acute LBP, but there is evidence that they are helpful with persistent radicular pain.29 Zarghooni and colleagues found significant reductions in pain and use of pain medication after single-shot epidural injections.29
Other pharmacologic therapies, acupuncture, sclerotherapy, and other methods are used to treat back pain, but these are typically reserved for chronic, not acute, LBP.
Nonpharmacologic therapy
Physical therapy. Physical therapy is a commonly prescribed treatment for LBP. Systematic literature reviews indicate that for patients with acute LBP (< 6 wk), there is no difference in the effectiveness of exercise therapy compared to no treatment and care provided by a general practitioner or to manipulations.30 For patients with subacute (6-12 wk) and chronic (≥ 12 wk) LBP, exercise therapy is effective compared to no treatment.30 There is debate, however, over which exercise activities should be used. Research supports strength/resistance and coordination/stabilization exercises.
Most therapists recommend the McKenzie method or spine stabilization exercises.31 The McKenzie method is used for LBP with sciatica; the patient moves through exercises within the prone position and focuses on extension of the spine. Spine stabilization is an active form of exercise based on a “neutral spine” position and helps strengthen muscles to maintain this position (core stabilization). The McKenzie method, when added to first-line care for LBP, does not produce significant improvements in pain or other clinical outcomes, although it may reduce health care utilization.32 Spine stabilization exercises have been shown to decrease pain, disability, and risk for recurrence after a first episode of back pain.33 The apparent success of physical therapy is attributed to compliance with directed home exercise programs, which have been shown to reduce the rate of recurrence, decrease episodes of acute LBP, and decrease the need for health services.34
Spinal traction. Traction or nonsurgical spinal decompression has emerged as a treatment for LBP. Unfortunately, there are little data to support its use as a treatment for acute LBP. Only a few randomized trials showed benefit, and these were small studies with a high risk for bias. A Cochrane review published in 2013 looked at 32 studies involving 2,762 patients with acute, subacute, and chronic LBP.35 The review did not find any evidence that traction alone or in combination with other therapy was any better than placebo treatment.35
Spinal manipulation. Spinal manipulation may be more effective than placebo treatment in reducing pain when the pain has been present for less than six weeks, but it is not more effective in reducing disability.36 There is little or no high-level evidence about spinal manipulation for acute LBP. However, there is some evidence of cost-effectiveness when using spinal manipulation in subacute to chronic pain.37 Chiropractic techniques are considered safe (when performed by a trained provider), but a systematic review found that these techniques provide no clinically relevant improvement in pain or disability when compared to other treatments.38
Bed rest. Bed rest has not been shown to improve outcomes, and in fact patients who had bed rest had less favorable outcomes than those who stayed active.39 Bed rest is less effective at reducing pain and improving function when compared to staying active.39
Continue for recommended management >>
Recommended management
A patient who presents with nonspecific acute LBP should have a thorough HPE to evaluate for the presence of red flags. If no concerning findings are present, the initial visit should focus on patient education based on the following items: (1) good prognosis with little intervention, (2) staying active and avoiding bed rest as much as possible, and (3) avoiding pain-causing movements when possible. The second step is to initiate a trial of an NSAID or acetaminophen and consider a muscle relaxant based on pain severity. Avoid opioid therapy if possible, but use conservative dosing if required for severe pain. Patients should be advised to return in two to four weeks if they do not experience significant improvement. At this time, the clinician may consider referring the patient for physical therapy, changing NSAIDs, ordering inflammatory markers, and/or referring to a specialist.
CONCLUSION
Although no single diagnostic protocol for LBP exists, the clinician must be able to distinguish simple from complex types. A thorough HPE is useful for categorizing the patient’s pain, with diagnostic imaging reserved for those patients with severe or progressive neurologic deficits, suspicion of serious underlying conditions, or LBP lasting more than four weeks without improvement. MRI, if available, is generally preferred over CT because it does not use ionizing radiation and provides better visualization of soft tissue, vertebral marrow, and the spinal cord. Symptomatology should be explained to patients with LBP during the HPE, with ongoing commentary to increase patient satisfaction and compliance. About two-thirds of patients with LBP do not seek evaluation from a health care provider; therefore, those who do seek treatment should be reassured, and therapies tailored to the individual in the least invasive and most cost-effective manner possible.
CE/CME No: CR-1605
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Identify "red flag" items in the history and physical exam that make low back pain (LBP) "complicated."
• Stratify patients into three categories: simple back pain, complicated back pain, and back pain with sciatica.
• Discuss when appropriate additional testing/imaging is needed based on LBP categories.
• Discuss patient perceptions and costs associated with imaging and LBP.
• Describe basic treatment options for noncomplicated acute LBP.
FACULTY
Mike Roscoe is the PA Program Director at the University of Evansville, Indiana. Alyssa Nishihira is in her final year of the PA program at Butler University, Indianapolis; after graduation, she will be practicing at Advanced Neurosurgery in Reno, Nevada.
The authors have no financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of May 2016.
Article begins on next page >>
Low back pain (LBP) is one of the most common reasons for an office visit, but most cases—at least 95%—have a benign underlying cause. Evaluation of LBP patients in the primary care setting, therefore, must focus on identifying “red flags” in the history and physical exam that suggest a significant underlying process requiring further work-up, including imaging. This evidence-based approach helps control costs and prevents the detrimental effects of unnecessary testing.
Low back pain (LBP) plagues many Americans and is a common reason for office visits in the United States. In 2010, back symptoms were the principal reason for 1.3% of office visits in the US.1 Recent data suggest that 75% to 85% of all Americans will experience an episode of LBP at least once in their lifetime.2 It is the leading cause of years lived with disability in the US3 and is a common reason for work disability. From a health care system standpoint, LBP imposes a considerable burden, accounting for more than $85 billion annually in direct costs.2
The etiology of LBP can be related to several anatomic and physiologic changes. Potential origins of LBP include, but are not limited to, pathology of the vertebrospinal ligaments, musculature, facet joints, fascia, vertebra and vertebral disks, and the extensive neurovascular components of the lumbar region. Although the potential causes of LBP are many, the majority of patients presenting with acute LBP usually improve with minimal clinical intervention within the first month. This is true even for patients who report limitations in daily activities and those with severe, acute cases of LBP.
A single standard of care for patients presenting with LBP has not been established. The wide array of choices for diagnosis and treatment of LBP is one factor that hinders the development of a standard diagnostic protocol. The challenge to clinicians when diagnosing LBP is to differentiate the patients with benign, self-limiting LBP (simple), who comprise the vast majority of LBP patients, from the 1% to 5% with a serious underlying pathology (complicated).4
Continue for stratification of low back pain >>
STRATIFICATION OF LOW BACK PAIN
Koes and colleagues analyzed 13 different national guidelines and two international guidelines for the management of LBP.5 They found that the guidelines consistently recommend focusing the history and physical exam (HPE) on identifying features suggestive of underlying serious pathology, or “red flags,” and excluding specific diseases.5 They also found that none of the guidelines recommends the routine use of imaging in patients without suspected serious pathology.5 The American College of Radiology simplified this approach to patients with LBP by creating a list of red flags to look for during the HPE.3 The presence of red flags indicates a case of complicated LBP, and patients who present with them should undergo additional diagnostic studies to screen for serious underlying conditions (see the Table).
The HPE should ultimately separate patients into three categories to determine the need for imaging (and course of treatment): (1) simple acute back pain, (2) complicated back pain with red flag (ie, a potential underlying systemic disease), and (3) LBP with neurologic deficits potentially requiring surgery.5
Simple acute low back pain
Up to 85% of patients presenting with LBP may never receive a definitive diagnosis due to lack of specific symptoms and ambiguous imaging results.6 Clinicians can assume that LBP in these patients is due to a mechanical cause, by far the most common cause of LBP.7 It is therefore more useful to rule out serious or potentially fatal causes of LBP (complicated LBP) rather than rule in a cause for patients presenting with LBP.
It is generally accepted among practitioners that a thorough HPE alone is sufficient for evaluating most patients presenting with acute LBP lasting less than four weeks.5 Patients presenting without red flags should be assured that improvement of acute LBP is typical, and that no diagnostic intervention is needed unless they do not improve as expected per patient or provider (eg, in terms of activities of daily living or work restrictions). The Figure depicts an appropriate approach to diagnosis and treatment in patients presenting with LBP.8 Clinicians should also offer patient education for self-care and discuss noninvasive treatment options, including pharmacologic and nonpharmacologic therapy.9
Low back pain with red flags (complicated)
Patient history is more useful than the physical exam in screening for spinal malignancies. In one particular combination (age > 50, history of cancer, unexplained weight loss, and failure to improve with conservative therapy), red flag symptoms are 100% sensitive for detecting malignancy.10 However, malignant neoplasms of the spine make up less than 1% of the diagnoses of patients presenting with LBP in primary care.4 Additionally, Deyo and Diehl reviewed five studies of a large series of consecutive spine films with large sample sizes and found the incidence of tumors, infections, and inflammatory spondyloarthropathies together were present in less than 2%.11 This low prevalence underscores the challenge of diagnosing serious pathology of the spine in the primary care setting.
Patients with complicated back pain presenting with red flags should always be examined for an underlying systemic disease. There is one red flag that, seen in isolation, meaningfully increases the likelihood of cancer: a previous history of cancer.4 Otherwise, inflammatory markers (eg, erythrocyte sedimentation rate) can be used to determine the need for advanced imaging (see the Figure).10
Low back pain with neurologic findings (sciatica)
Screening (HPE) for neurologic damage is difficult because traditional findings of neurologic injury (paresis or muscle weakness, impaired reflexes, sensory deficits, and decreased range of motion) all have low sensitivity with higher specificity.12 For this reason, these tests are of limited value as screening tools during the HPE. Specific exams, such as the straight leg raise and crossed straight leg tests, are also of limited value, especially in the primary care setting, because of inconsistent sensitivity and specificity.
This is the primary reason that the HPE in patients with LBP who have neurologic findings must include evaluation for urgent findings (see the Figure). If any red flags are present, advanced imaging is immediately warranted. Otherwise, inflammatory markers and plain radiography may be obtained, and advanced imaging may be considered if the plain radiography and/or inflammatory markers are abnormal.
There is also an approach that advocates the use of advanced imaging in patients with significant functional disability due to their LBP. Two questionnaires, the Oswestry Low Back Pain Disability Index and the Roland-Morris Disability Questionnaire, evaluate subjective data to determine a patient’s functional disability due to LBP.The validity of both tests has been confirmed.13
Continue for diagnostic imaging >>
DIAGNOSTIC IMAGING
The majority of patients presenting with LBP without concerning symptoms can be assumed to have nonspecific mechanical back pain. These patients do not need radiography unless the pain has not improved after four to six weeks of conservative care, because plain radiographs often detect findings (degenerative joint disease, bone spurs, spondylosis) that are unrelated to symptoms.9 Advanced imaging is generally recommended only for LBP patients with red flags due to the potentially critical nature of these cases.5 Patients with LBP presenting with any of these factors require further testing, even if the duration of their pain is less than four weeks.
If a patient’s LBP persists beyond four weeks, the clinician must decide which diagnostic test to order. General medical knowledge suggests that MRI is superior to plain radiography because it shows soft tissue and can detect more concerning abnormalities, such as infections, cancer, and metastatic tumors. CT is better for showing bony abnormalities, but these rarely correlate with a patient’s LBP, and CT subjects patients to levels of radiation that can increase cancer risks.14 Plain radiography in this cohort (LBP > 4 wk) is not generally recommended as it cannot show intervertebral discs or evaluate the degree of spinal stenosis as accurately as MRI. Additionally, these lumbar radiographs expose patients to more than 35 times the radiation delivered in a single chest radiograph.15
COSTS AND PATIENT OUTCOMES
The estimated cost of unnecessary imaging for LBP is $300 million per year.16 There is evidence of a strong association between advanced lumbar spine imaging and increased rates of surgery and significantly higher total medical expenditures.17,18 One study examined patients with nonspecific LBP who either received MRI within 30 days post-onset (defined as “early MRI”) or did not receive MRI. Early-MRI patients had significantly higher total medical expenses ($12,948, P < .0001) than the no-MRI group.17 The early-MRI group also had significantly longer periods of disability and were less likely to go off disability than the no-MRI group (P < .0001).
Cost-effectiveness studies of plain radiographs, dating back to 1982, have yielded similar findings. Liang et al suggested that if radiography was done routinely at the initial visit in patients with acute LBP but no red flags, the cost would be more than $2,000 (in 1982 dollars) to avert one day of pain.19 A more recent study examined patients with acute LBP who received MRI, with one group blinded (both patients and physicians) to their MRI results for six months while the other group received their results within 48 hours.20 All patients underwent a physical exam by a study coordinator, and treatment was assigned prior to imaging. At six weeks and one year, there was no significant difference in treatment assignments or self-reported surveys between groups, indicating that the MRI results had no significant influence on patient outcomes.
Despite the large increase in the use of advanced diagnostic imaging aimed at improving patient care and outcomes, there is a lack of data showing any correlative or causative connection between the two. Given this lack of evidence, and the potentially detrimental radiation exposure and increased costs to patients, clinicians should follow evidence-based guidelines when considering diagnostic imaging in patients presenting with LBP.
Continue for patient perception >>
PATIENT PERCEPTION
Patient satisfaction plays a very important role in health care and may correlate with compliance and other outcomes. One study showed that while radiography in patients with LBP was not associated with improved clinical outcomes, it did increase patients’ satisfaction with the care they received.21 A study that grouped patients requiring imaging for LBP into rapid MRI and plain film radiography cohorts found that patients who received rapid MRI were more assured by their results than were patients in the radiography group (74% vs 58%, P = .002).22 Both groups showed significant clinical improvement in the first three months, but there was no difference between groups at either the three- or 12-month mark. In both groups, reassurance was positively correlated with patient satisfaction (Pearson correlation coefficients, 0.55-0.59, P < .001).
Patients may be reassured by imaging, even when it is unnecessary. Effectively explaining symptomatology during the HPE to patients with LBP should be of high priority to clinicians. A study found that when patients with mechanical LBP did not receive an adequate explanation of the problem, they were less satisfied with their visit and wanted more diagnostic tests.11 Another study found that when low-risk patients were randomly assigned to a control group and received an educational intervention only, they reported equal satisfaction with their care and had clinical outcomes equal to those of the treatment group that received a plain radiograph.11
Given the costs, radiation risks, and other negative aspects of unnecessary imaging, additional diagnostic tests may not be in a patient’s best interest. A careful physical exam should be performed, with the clinician providing ongoing commentary to reassure patients that the clinician is neither dismissing the patient’s symptoms nor inappropriately avoiding further tests.
Often, medical providers order imaging with the intention to reassure patients with the results and thus ultimately increase the patient’s sense of well-being. However, the opposite effect may occur, with patients actually developing a decreased sense of wellness with no alteration of outcomes. A study evaluated general health (GH) scores (based on results from several screening questionnaires that assessed the patient’s current physical and mental health state) in patients receiving MRI results.20 The patients were divided into those who received results (within 48 hours), and those who did not unless it was critical to patient management (blinded group). At six weeks, the blinded group’s GH score was significantly higher than the early-informed group’s GH score. This suggests that receiving MRI results may negatively influence patients’ perception of their general health.20
The same meta-analysis that reviewed patient outcomes also evaluated mental health and quality-of-life scores of LBP patients who received either MRI, CT, or radiography.23 There was no short-term (< 3 mo) or long-term (6-12 mo) difference between patients who received radiography versus advanced imaging. This indicates that using imaging of any kind in patients with LBP but without indications of serious underlying conditions does not improve clinical outcomes and is negatively correlated with quality-of-life measures at short- and long-term intervals.23
Continue for treatment >>
TREATMENT
The prognosis of simple acute mechanical LBP is excellent. Although back pain is a leading reason for visiting health care providers, many affected individuals never seek medical care and apparently improve on their own. In a random telephone survey of North Carolina residents, only 39% of persons with LBP sought medical care.24 Therefore, patients who do seek treatment should be given reassurance, and therapies should be tailored to the individual in the least invasive and most cost-effective manner. Many treatment options are available for LBP, but often strong evidence of benefit is lacking.
Pharmacologic therapy
Anti-inflammatories. It can be assumed that when a patient comes to the practitioner for evaluation of LBP, there is an expectation that some type of medication will be recommended or prescribed for pain relief. Unless there is a contraindication, NSAIDs are often first-line therapy, and they are effective for short-term symptom relief when compared with placebo.25 A mild pain medication, such as acetaminophen, is also a common treatment. The 2007 joint practice guideline from the American Pain Society (APS) and the American College of Physicians (ACP) recommends acetaminophen or NSAIDs as first-line therapy for acute LBP.3 Neither agent—NSAIDs or acetaminophen—has shown superiority, and combining the two has shown no additional benefits.26 Caution must be used, however, as NSAIDs have a risk for gastrointestinal toxicity and nephrotoxicity, and acetaminophen has a dose- and patient-dependent risk for hepatotoxicity.
Muscle relaxants. Muscle relaxants are another pharmacologic treatment option for LBP. Most pain reduction from this class of medication occurs in the first one to two weeks of therapy, although benefit may continue for up to four weeks.27 There is also evidence that a combination of an NSAID and a muscle relaxer has added benefits.27 These medications are centrally acting, so sedation and dizziness are common; all medications in this class have these adverse effects to some degree. Carisoprodol has as its first metabolite meprobamate, which is a tranquilizer used to treat anxiety disorders; it has a potential for abuse and should be used with caution in certain populations.
Opioids. Opioids are commonly prescribed to patients with LBP, though there are limited data regarding efficacy. One trial compared an NSAID alone versus an NSAID plus oxycodone/acetaminophen and found no significant difference in pain or disability after seven days.28 In addition, the adverse effects of opioids, which include sedation, constipation, nausea, and confusion, may be amplified in the elderly population; therefore, opioids should be prescribed with caution in these patients. If prescribed to treat acute LBP, opioids should be used in short, scheduled dosing regimens since NSAIDs or acetaminophen suffice for most patients.
Corticosteroids. Oral glucocorticoids are sometimes given to patients with acute LBP, and they likely are used more frequently in patients with radicular symptoms. However, the APS/ACP 2007 joint guidelines recommend against use of systemic glucocorticoids for acute LBP due to lack of proven benefit.3 Epidural steroid injections are not generally beneficial for isolated acute LBP, but there is evidence that they are helpful with persistent radicular pain.29 Zarghooni and colleagues found significant reductions in pain and use of pain medication after single-shot epidural injections.29
Other pharmacologic therapies, acupuncture, sclerotherapy, and other methods are used to treat back pain, but these are typically reserved for chronic, not acute, LBP.
Nonpharmacologic therapy
Physical therapy. Physical therapy is a commonly prescribed treatment for LBP. Systematic literature reviews indicate that for patients with acute LBP (< 6 wk), there is no difference in the effectiveness of exercise therapy compared to no treatment and care provided by a general practitioner or to manipulations.30 For patients with subacute (6-12 wk) and chronic (≥ 12 wk) LBP, exercise therapy is effective compared to no treatment.30 There is debate, however, over which exercise activities should be used. Research supports strength/resistance and coordination/stabilization exercises.
Most therapists recommend the McKenzie method or spine stabilization exercises.31 The McKenzie method is used for LBP with sciatica; the patient moves through exercises within the prone position and focuses on extension of the spine. Spine stabilization is an active form of exercise based on a “neutral spine” position and helps strengthen muscles to maintain this position (core stabilization). The McKenzie method, when added to first-line care for LBP, does not produce significant improvements in pain or other clinical outcomes, although it may reduce health care utilization.32 Spine stabilization exercises have been shown to decrease pain, disability, and risk for recurrence after a first episode of back pain.33 The apparent success of physical therapy is attributed to compliance with directed home exercise programs, which have been shown to reduce the rate of recurrence, decrease episodes of acute LBP, and decrease the need for health services.34
Spinal traction. Traction or nonsurgical spinal decompression has emerged as a treatment for LBP. Unfortunately, there are little data to support its use as a treatment for acute LBP. Only a few randomized trials showed benefit, and these were small studies with a high risk for bias. A Cochrane review published in 2013 looked at 32 studies involving 2,762 patients with acute, subacute, and chronic LBP.35 The review did not find any evidence that traction alone or in combination with other therapy was any better than placebo treatment.35
Spinal manipulation. Spinal manipulation may be more effective than placebo treatment in reducing pain when the pain has been present for less than six weeks, but it is not more effective in reducing disability.36 There is little or no high-level evidence about spinal manipulation for acute LBP. However, there is some evidence of cost-effectiveness when using spinal manipulation in subacute to chronic pain.37 Chiropractic techniques are considered safe (when performed by a trained provider), but a systematic review found that these techniques provide no clinically relevant improvement in pain or disability when compared to other treatments.38
Bed rest. Bed rest has not been shown to improve outcomes, and in fact patients who had bed rest had less favorable outcomes than those who stayed active.39 Bed rest is less effective at reducing pain and improving function when compared to staying active.39
Continue for recommended management >>
Recommended management
A patient who presents with nonspecific acute LBP should have a thorough HPE to evaluate for the presence of red flags. If no concerning findings are present, the initial visit should focus on patient education based on the following items: (1) good prognosis with little intervention, (2) staying active and avoiding bed rest as much as possible, and (3) avoiding pain-causing movements when possible. The second step is to initiate a trial of an NSAID or acetaminophen and consider a muscle relaxant based on pain severity. Avoid opioid therapy if possible, but use conservative dosing if required for severe pain. Patients should be advised to return in two to four weeks if they do not experience significant improvement. At this time, the clinician may consider referring the patient for physical therapy, changing NSAIDs, ordering inflammatory markers, and/or referring to a specialist.
CONCLUSION
Although no single diagnostic protocol for LBP exists, the clinician must be able to distinguish simple from complex types. A thorough HPE is useful for categorizing the patient’s pain, with diagnostic imaging reserved for those patients with severe or progressive neurologic deficits, suspicion of serious underlying conditions, or LBP lasting more than four weeks without improvement. MRI, if available, is generally preferred over CT because it does not use ionizing radiation and provides better visualization of soft tissue, vertebral marrow, and the spinal cord. Symptomatology should be explained to patients with LBP during the HPE, with ongoing commentary to increase patient satisfaction and compliance. About two-thirds of patients with LBP do not seek evaluation from a health care provider; therefore, those who do seek treatment should be reassured, and therapies tailored to the individual in the least invasive and most cost-effective manner possible.
1. CDC. National Ambulatory Medical Care Survey: 2010 Summary Tables. Table 9. www.cdc.gov/nchs/data/ahcd/namcs_summary/2010_namcs_web_tables.pdf. Accessed March 29, 2016.
2. Davies C, Nitz AJ, Mattacola CG, et al. Practice patterns when treating patients with low back pain: a survey of physical therapists. Physiother Theor Pract. 2014;30(6):399-408.
3. American College of Radiology. ACR Appropriateness Criteria. Low back pain. 2015. www.acr.org/~/media/ACR/Documents/AppCriteria/Diagnostic/LowBackPain.pdf. Accessed March 10, 2016.
4. Henschke N, Maher CG, Ostelo RW, et al. Red flags to screen for malignancy in patients with low back pain. Cochrane Database Syst Rev. 2013;2:CD008686.
5. Koes BW, Tulder M, Lin CW, et al. An updated overview of clinical guidelines for the management of non-specific low back pain in primary care. Eur Spine J. 2010;19(12):2075-2094.
6. Deyo RA, Rainville J, Kent DL. What can the history and physical examination tell us about low back pain? JAMA. 1992;268(6):760-765.
7. Jarvik JG. Diagnostic evaluation of low back pain with emphasis on imaging. Ann Intern Med. 2002;137:586-597.
8. Diagnostic testing for low back pain. In: Post TW (ed), UpToDate, Waltham, MA. www.uptodate.com. Accessed March 16, 2016.
9. Chou R, Qaseem A, Snow V, et al; Clinical Efficacy Assessment Subcommittee of the American College of Physicians; American College of Physicians; American Pain Society Low Back Pain Guidelines Panel. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007;147(7):478-491.
10. Deyo RA, Diehl AK. Cancer as a cause of back pain: frequency, clinical presentation, and diagnostic strategies. J Gen Intern Med. 1988;3(3):230-238.
11. Deyo RA, Diehl AK. Patient satisfaction with medical care for low-back pain. Spine. 1986;11(1):28-30.
12. Pradeep S, Rainville J, Katz JN, et al. The accuracy of the physical examination for the diagnosis of midlumbar and low lumbar nerve root impingement. Spine. 2011;36(1):63-73.
13. Leclaire R, Blier F, Fortin L, Proulx R. A cross-sectional study comparing the Oswestry and Roland-Morris Functional Disability Scales in two populations of patients with low back pain of different levels of severity. Spine. 1997;22(1):68-71
14. FDA. Radiation emitting products. www.fda.gov/Radiation-EmittingProducts/RadiationEmittingProductsandProcedures/MedicalImaging/MedicalX-Rays/ucm115317.htm. Accessed March 29, 2016.
15. Simpson AK, Whang PG, Jonisch A, et al. The radiation exposure associated with cervical and lumbar spine radiographs. J Spinal Disord Tech. 2008;21(6):409-412.
16. Srinivas S, Deyo R, Berger Z. Application of “less is more” to lower back pain. Arch Intern Med. 2012;172(13):1016-1020.
17. Webster BS, Bauer AZ, Choi Y, et al. Iatrogenic consequences of early magnetic resonance imaging in acute, work-related, disabling back pain. Spine. 2013;38(22):1939-1946.
18. Webster BS, Bauer AZ, Choi Y, et al. The cascade of medical services and associated longitudinal costs due to nonadherent magnetic resonance imaging for low back pain. Spine. 2014;39(17):1433-1440.
19. Liang M, Komaroff AL. Roentgenograms in primary care patients with acute low back pain: a cost-effectiveness analysis. Arch Intern Med. 1982;142(6):1108-1112.
20. Ash LM, Modic MT, Obuchowski NA, et al. Effects of diagnostic information, per se, on patient outcomes in acute radiculopathy and low back pain. AJNR Am J Neuroradiol. 2008;29(6):1098-1103.
21. Kendrick D, Fielding K, Bentley E, et al. Radiography of the lumbar spine in primary care patients with low back pain: randomized controlled trial. BMJ. 2001;322(7283):400-405.
22. Jarvik JG, Hollingworth W, Martin B, et al. Rapid magnetic resonance imaging vs radiographs for patients with low back pain. JAMA. 2003;289(21):2810-2818.
23. Chou R, Fu R, Carrino JA, Deyo RA. Imaging strategies for low-back pain: systematic review and meta-analysis. Lancet. 2009;373(9662):463-472.
24. Carey TS, Evans AT, Hadler NM, et al. Acute severe low back pain: a population-based study of prevalence and care-seeking. Spine. 1996;21(3):339-344.
25. Roelofs PD, Deyo RA, Koes BW, et al. Nonsteroidal anti-inflammatory drugs for low back pain. Spine. 2008;33(16):1766-1774.
26. Hancock MJ, Maher CG, Latimer J, et al. Assessment of diclofenac or spinal manipulative therapy, or both, in addition to recommended first-line treatment for acute low back pain: a randomized controlled trial. Lancet. 2007;370(10):1638-1643.
27. Van Tulder MW, Touray T, Furlan AD, et al. Muscle relaxants for non-specific low-back pain. Cochrane Database Syst Rev. 2003;(4):CD004252.
28. Friedman BW, Dym AA, Davitt M, et al. Naproxen with cyclobenzaprine, oxycodone/acetaminophen, or placebo for treating acute low back pain: a randomized clinical trial. JAMA. 2015;314(15):1572-1580.
29. Zarghooni K, Rashidi A, Siewe, J, et al. Single-shot epidural injections in the management of radicular pain. Orthop Rev (Pavia). 2015;7(4):5985.
30. Smidt N, deVet HC, Bouter LM, et al. Effectiveness of exercise therapy: A best-evidence summary of systematic reviews. Aust J Physiother. 2005;51(2):71-85.
31. Casazza BA. Diagnosis and treatment of acute low back pain. Am Fam Physician. 2012;85(4):343-350.
32. Machado LA, Maher CG, Herbert RD, et al. The effectiveness of the McKenzie method in addition to first-line care for acute low back pain: a randomized controlled trial. BMC Med. 2010;8(10):1-10.
33. Cho I, Jeon C, Lee S, et al. Effects of lumbar stabilization exercise on functional disability and lumbar lordosis angle in patients with chronic low back pain. J Phys Ther Sci. 2015;27(6):1983-1985.
34. Choi BK, Verbeek JH, Tam WW, Jiang JY. Exercises for prevention of recurrences of low-back pain (review). Cochrane Database Syst Rev. 2010;(1):CD006555.
35. Wegner I, Widyahening IS, van Tulder MW, et al. Traction for low-back pain with or without sciatica (review). Cochrane Database Syst Rev. 2013;(8):CD003010.
36. Hoiriis KT, Pfleger B, McDuffie FC, et al. A randomized clinical trial comparing chiropractic adjustments to muscle relaxants for subacute low back pain. J Manipulative Physiol Ther. 2004;27(6):388-398.
37. Lin CC, Haas M, Maher CG, et al. Cost-effectiveness of guideline-endorsed treatments for low back pain: a systematic review. Eur Spine J. 2011;20:1024-1038.
38. Walker BF, French SD, Grant W, Green S. A Cochrane Review of combined chiropractic interventions for low-back pain. Spine. 2011;36(3): 230-242.
39. Dahm KT, Brurberg KG, Jamtvedt G, Hagen KB. Advice to rest in bed versus advice to stay active for acute low-back pain and sciatica. Cochrane Database Syst Rev. 2010;(6):CD007612.
40. Staiger T, Paauw D, Deyo A, Jarvik JG. Imaging studies for acute low back pain. When and when not to order them. Postgrad Med. 1999;105(4):161-162,165-166,171-172.
1. CDC. National Ambulatory Medical Care Survey: 2010 Summary Tables. Table 9. www.cdc.gov/nchs/data/ahcd/namcs_summary/2010_namcs_web_tables.pdf. Accessed March 29, 2016.
2. Davies C, Nitz AJ, Mattacola CG, et al. Practice patterns when treating patients with low back pain: a survey of physical therapists. Physiother Theor Pract. 2014;30(6):399-408.
3. American College of Radiology. ACR Appropriateness Criteria. Low back pain. 2015. www.acr.org/~/media/ACR/Documents/AppCriteria/Diagnostic/LowBackPain.pdf. Accessed March 10, 2016.
4. Henschke N, Maher CG, Ostelo RW, et al. Red flags to screen for malignancy in patients with low back pain. Cochrane Database Syst Rev. 2013;2:CD008686.
5. Koes BW, Tulder M, Lin CW, et al. An updated overview of clinical guidelines for the management of non-specific low back pain in primary care. Eur Spine J. 2010;19(12):2075-2094.
6. Deyo RA, Rainville J, Kent DL. What can the history and physical examination tell us about low back pain? JAMA. 1992;268(6):760-765.
7. Jarvik JG. Diagnostic evaluation of low back pain with emphasis on imaging. Ann Intern Med. 2002;137:586-597.
8. Diagnostic testing for low back pain. In: Post TW (ed), UpToDate, Waltham, MA. www.uptodate.com. Accessed March 16, 2016.
9. Chou R, Qaseem A, Snow V, et al; Clinical Efficacy Assessment Subcommittee of the American College of Physicians; American College of Physicians; American Pain Society Low Back Pain Guidelines Panel. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007;147(7):478-491.
10. Deyo RA, Diehl AK. Cancer as a cause of back pain: frequency, clinical presentation, and diagnostic strategies. J Gen Intern Med. 1988;3(3):230-238.
11. Deyo RA, Diehl AK. Patient satisfaction with medical care for low-back pain. Spine. 1986;11(1):28-30.
12. Pradeep S, Rainville J, Katz JN, et al. The accuracy of the physical examination for the diagnosis of midlumbar and low lumbar nerve root impingement. Spine. 2011;36(1):63-73.
13. Leclaire R, Blier F, Fortin L, Proulx R. A cross-sectional study comparing the Oswestry and Roland-Morris Functional Disability Scales in two populations of patients with low back pain of different levels of severity. Spine. 1997;22(1):68-71
14. FDA. Radiation emitting products. www.fda.gov/Radiation-EmittingProducts/RadiationEmittingProductsandProcedures/MedicalImaging/MedicalX-Rays/ucm115317.htm. Accessed March 29, 2016.
15. Simpson AK, Whang PG, Jonisch A, et al. The radiation exposure associated with cervical and lumbar spine radiographs. J Spinal Disord Tech. 2008;21(6):409-412.
16. Srinivas S, Deyo R, Berger Z. Application of “less is more” to lower back pain. Arch Intern Med. 2012;172(13):1016-1020.
17. Webster BS, Bauer AZ, Choi Y, et al. Iatrogenic consequences of early magnetic resonance imaging in acute, work-related, disabling back pain. Spine. 2013;38(22):1939-1946.
18. Webster BS, Bauer AZ, Choi Y, et al. The cascade of medical services and associated longitudinal costs due to nonadherent magnetic resonance imaging for low back pain. Spine. 2014;39(17):1433-1440.
19. Liang M, Komaroff AL. Roentgenograms in primary care patients with acute low back pain: a cost-effectiveness analysis. Arch Intern Med. 1982;142(6):1108-1112.
20. Ash LM, Modic MT, Obuchowski NA, et al. Effects of diagnostic information, per se, on patient outcomes in acute radiculopathy and low back pain. AJNR Am J Neuroradiol. 2008;29(6):1098-1103.
21. Kendrick D, Fielding K, Bentley E, et al. Radiography of the lumbar spine in primary care patients with low back pain: randomized controlled trial. BMJ. 2001;322(7283):400-405.
22. Jarvik JG, Hollingworth W, Martin B, et al. Rapid magnetic resonance imaging vs radiographs for patients with low back pain. JAMA. 2003;289(21):2810-2818.
23. Chou R, Fu R, Carrino JA, Deyo RA. Imaging strategies for low-back pain: systematic review and meta-analysis. Lancet. 2009;373(9662):463-472.
24. Carey TS, Evans AT, Hadler NM, et al. Acute severe low back pain: a population-based study of prevalence and care-seeking. Spine. 1996;21(3):339-344.
25. Roelofs PD, Deyo RA, Koes BW, et al. Nonsteroidal anti-inflammatory drugs for low back pain. Spine. 2008;33(16):1766-1774.
26. Hancock MJ, Maher CG, Latimer J, et al. Assessment of diclofenac or spinal manipulative therapy, or both, in addition to recommended first-line treatment for acute low back pain: a randomized controlled trial. Lancet. 2007;370(10):1638-1643.
27. Van Tulder MW, Touray T, Furlan AD, et al. Muscle relaxants for non-specific low-back pain. Cochrane Database Syst Rev. 2003;(4):CD004252.
28. Friedman BW, Dym AA, Davitt M, et al. Naproxen with cyclobenzaprine, oxycodone/acetaminophen, or placebo for treating acute low back pain: a randomized clinical trial. JAMA. 2015;314(15):1572-1580.
29. Zarghooni K, Rashidi A, Siewe, J, et al. Single-shot epidural injections in the management of radicular pain. Orthop Rev (Pavia). 2015;7(4):5985.
30. Smidt N, deVet HC, Bouter LM, et al. Effectiveness of exercise therapy: A best-evidence summary of systematic reviews. Aust J Physiother. 2005;51(2):71-85.
31. Casazza BA. Diagnosis and treatment of acute low back pain. Am Fam Physician. 2012;85(4):343-350.
32. Machado LA, Maher CG, Herbert RD, et al. The effectiveness of the McKenzie method in addition to first-line care for acute low back pain: a randomized controlled trial. BMC Med. 2010;8(10):1-10.
33. Cho I, Jeon C, Lee S, et al. Effects of lumbar stabilization exercise on functional disability and lumbar lordosis angle in patients with chronic low back pain. J Phys Ther Sci. 2015;27(6):1983-1985.
34. Choi BK, Verbeek JH, Tam WW, Jiang JY. Exercises for prevention of recurrences of low-back pain (review). Cochrane Database Syst Rev. 2010;(1):CD006555.
35. Wegner I, Widyahening IS, van Tulder MW, et al. Traction for low-back pain with or without sciatica (review). Cochrane Database Syst Rev. 2013;(8):CD003010.
36. Hoiriis KT, Pfleger B, McDuffie FC, et al. A randomized clinical trial comparing chiropractic adjustments to muscle relaxants for subacute low back pain. J Manipulative Physiol Ther. 2004;27(6):388-398.
37. Lin CC, Haas M, Maher CG, et al. Cost-effectiveness of guideline-endorsed treatments for low back pain: a systematic review. Eur Spine J. 2011;20:1024-1038.
38. Walker BF, French SD, Grant W, Green S. A Cochrane Review of combined chiropractic interventions for low-back pain. Spine. 2011;36(3): 230-242.
39. Dahm KT, Brurberg KG, Jamtvedt G, Hagen KB. Advice to rest in bed versus advice to stay active for acute low-back pain and sciatica. Cochrane Database Syst Rev. 2010;(6):CD007612.
40. Staiger T, Paauw D, Deyo A, Jarvik JG. Imaging studies for acute low back pain. When and when not to order them. Postgrad Med. 1999;105(4):161-162,165-166,171-172.
Elderly woman with sharp shoulder pain
A 78-year-old woman sought care at our emergency department for sudden-onset right shoulder pain that had begun 5 days earlier. She said the pain was sharp and that it radiated to the scapula, right arm, and chest. She said that nonsteroidal anti-inflammatory drugs provided pain relief.
The patient denied any recent trauma or heavy lifting, and was not experiencing extremity weakness, numbness, or tingling. She reported no fever, chills, cough, or night sweats, but said she’d lost 10 pounds over the previous month. The patient was a former smoker whose medical history included diabetes mellitus, hypertension, and hyperlipidemia. Two years ago, she was treated for recurrent right-sided pleural effusions with pleurocentesis, which was negative for cytology and acid-fast bacilli.
Auscultation revealed crackles in the right lower lung field with decreased breath sounds. The patient had full range of motion in her right shoulder and experienced minimal pain on flexion. She had no swelling, erythema, or tenderness in her right upper extremity and there was no sign of lymphadenopathy. Her laboratory data were noncontributory. A chest radiograph was obtained (FIGURE 1).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Malignant pleural mesothelioma
The chest radiograph revealed pleural-based masses extending along the convexity of the right chest wall. (Note the diminished volume of the right lung in FIGURE 1.) A coronal enhanced computed tomography (CT) scan (FIGURE 2) showed a large heterogeneous mass extending through the chest wall into the right axillary soft tissue and encasing the right brachial plexus (yellow arrows). Transdiaphragmatic extension was evident as an irregular mass at the hepatic dome (red arrow). The constellation of findings prompted the radiologist to suspect pleural malignancy.
An aggressive cancer
MPM is a highly aggressive neoplasm of the pleura with rising incidence around the globe.1,2 The annual incidence of mesothelioma in the United States is approximately 3000 cases per year, with the majority linked to asbestos exposure. The latency period is long, typically ranging from 35 to 40 years.1 Our patient, however, did not have a history of asbestos exposure. She was a housewife who had no exposure to building construction or demolition.
Nonspecific complaints. Clinical findings of MPM are frequently nonspecific and may masquerade as innocuous shoulder pain, as illustrated in this case. Patients may also present with dyspnea, nonpleuritic chest pain, and incidental pleural effusions. On examination, unilateral dullness on percussion at the lung base, palpable chest wall masses, and scoliosis toward the sides of the lesion may be present.3
Differential Dx of shoulder pain includes rotator cuff disorders, tears
The differential diagnosis of shoulder pain includes rotator cuff disorders, acromioclavicular osteoarthritis, and cervical radiculopathy from degenerative spondylosis.
Rotator cuff tendinopathy and tears usually present with pain on overhead activity, positional test muscle weakness, and evidence of impingement. Acromioclavicular osteoarthritis usually causes acromioclavicular joint tenderness, which can be relieved with diagnostic intra-articular anesthetic injections. Pain from cervical spondylosis is usually accompanied by numbness and weakness in the arms and hands and muscle spasms in the neck. Also, the symptoms are usually aggravated by downward compression of the head (Spurling’s test).4,5
Chest radiographs are useful, but CT scans are more sensitive
Often, MPM is initially suspected because of unilateral pleural nodularity or thickening with a large, unilateral pleural effusion on a chest radiograph.6 Pleural plaques may also be seen. As the tumor grows, encasing the lung and invading the fissures, it leads to volume loss of the affected side, which can also be identified radiographically.1
CT is a more sensitive way to detect pleural and pulmonary parenchymal involvement, as well as invasion of adjacent thoracic structures, including the chest wall, pericardium, diaphragm, and the mediastinal lymph nodes.1
When mesothelioma is suspected because of clinical or radiologic data, experts recommend that cytologic findings from thoracentesis be followed by tissue confirmation from thoracoscopy or CT biopsy.2
Chemotherapy, Yes, but there are many Tx unknowns
The best approach to treatment of MPM remains controversial due to the rarity of the disease and the scarcity of randomized prospective trials. Surgical resection is most often performed when the disease is confined to the pleural space. An extrapleural pneumonectomy is usually performed for stage I disease, when the tumor is limited to one hemithorax, invading the pleura and involving the lung, endothoracic fascia, diaphragm, or pericardium.7
Unfortunately, mesothelioma is highly radioresistant; patients often endure severe toxicity due to large radiation fields. Chemotherapy, either as single agents or in combination, can be administered systemically or directly into the pleural space. Combination chemotherapy using cisplatin and pemetrexed is currently the standard of care, based upon a phase III trial that demonstrated prolonged overall survival with the combination compared to treatment with cisplatin alone (12.1 months vs 9.3 months).8
Other agents used to treat MPM. Five other chemotherapy agents are also used in the treatment of MPM. Used individually, the maximum response rates to these agents are as follows: methotrexate (37%), mitomycin (21%), doxorubicin (16%), cyclophosphamide (13%), and carboplatin (11%).7
Our patient. The rest of our patient’s hospital course was uncomplicated. She was not a surgical candidate because she had such extensive tumor involvement. She was discharged with a referral to an outpatient oncology clinic. Despite 2 cycles of carboplatin and pemetrexed, and palliative radiation therapy to the right upper thoracic mass, the disease progressed with worsening right upper extremity pain and neurologic deficits.
CORRESPONDENCE
Mark Guelfguat, DO, Jacobi Medical Center, Albert Einstein College of Medicine, 1400 S Pelham Parkway, Building 1, Room 4N15, Bronx, NY 10461; [email protected].
1. Miller BH, Rosado-de-Christenson ML, Mason AC, et al. From the archives of the AFIP. Malignant pleural mesothelioma: radiologic-pathologic correlation. Radiographics. 1996;16:613-644.
2. Scherpereel A, Astoul P, Baas P, et al; European Respiratory Society/European Society of Thoracic Surgeons Task Force. Guidelines of the European Respiratory Society and the European Society of Thoracic Surgeons for the management of malignant pleural mesothelioma. Eur Respir J. 2010;35:479-495.
3. Antman KH. Natural history and epidemiology of malignant mesothelioma. Chest. 1993;103:373S-376S.
4. Burbank KM, Stevenson JH, Czarnecki GR, et al. Chronic shoulder pain: part I. Evaluation and diagnosis. Am Fam Physician. 2008;77:453-460.
5. Anekstein Y, Blecher R, Smorgick Y, et al. What is the best way to apply the Spurling test for cervical radiculopathy? Clin Orthop Relat Res. 2012;470:2566-2572.
6. British Thoracic Society Standards of Care Committee. Statement on malignant mesothelioma in the United Kingdom. Thorax. 2001;56:250-265.
7. Aisner J. Current approach to malignant mesothelioma of the pleura. Chest. 1995;107:332S-344S.
8. Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21:2636-2644.
A 78-year-old woman sought care at our emergency department for sudden-onset right shoulder pain that had begun 5 days earlier. She said the pain was sharp and that it radiated to the scapula, right arm, and chest. She said that nonsteroidal anti-inflammatory drugs provided pain relief.
The patient denied any recent trauma or heavy lifting, and was not experiencing extremity weakness, numbness, or tingling. She reported no fever, chills, cough, or night sweats, but said she’d lost 10 pounds over the previous month. The patient was a former smoker whose medical history included diabetes mellitus, hypertension, and hyperlipidemia. Two years ago, she was treated for recurrent right-sided pleural effusions with pleurocentesis, which was negative for cytology and acid-fast bacilli.
Auscultation revealed crackles in the right lower lung field with decreased breath sounds. The patient had full range of motion in her right shoulder and experienced minimal pain on flexion. She had no swelling, erythema, or tenderness in her right upper extremity and there was no sign of lymphadenopathy. Her laboratory data were noncontributory. A chest radiograph was obtained (FIGURE 1).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Malignant pleural mesothelioma
The chest radiograph revealed pleural-based masses extending along the convexity of the right chest wall. (Note the diminished volume of the right lung in FIGURE 1.) A coronal enhanced computed tomography (CT) scan (FIGURE 2) showed a large heterogeneous mass extending through the chest wall into the right axillary soft tissue and encasing the right brachial plexus (yellow arrows). Transdiaphragmatic extension was evident as an irregular mass at the hepatic dome (red arrow). The constellation of findings prompted the radiologist to suspect pleural malignancy.
An aggressive cancer
MPM is a highly aggressive neoplasm of the pleura with rising incidence around the globe.1,2 The annual incidence of mesothelioma in the United States is approximately 3000 cases per year, with the majority linked to asbestos exposure. The latency period is long, typically ranging from 35 to 40 years.1 Our patient, however, did not have a history of asbestos exposure. She was a housewife who had no exposure to building construction or demolition.
Nonspecific complaints. Clinical findings of MPM are frequently nonspecific and may masquerade as innocuous shoulder pain, as illustrated in this case. Patients may also present with dyspnea, nonpleuritic chest pain, and incidental pleural effusions. On examination, unilateral dullness on percussion at the lung base, palpable chest wall masses, and scoliosis toward the sides of the lesion may be present.3
Differential Dx of shoulder pain includes rotator cuff disorders, tears
The differential diagnosis of shoulder pain includes rotator cuff disorders, acromioclavicular osteoarthritis, and cervical radiculopathy from degenerative spondylosis.
Rotator cuff tendinopathy and tears usually present with pain on overhead activity, positional test muscle weakness, and evidence of impingement. Acromioclavicular osteoarthritis usually causes acromioclavicular joint tenderness, which can be relieved with diagnostic intra-articular anesthetic injections. Pain from cervical spondylosis is usually accompanied by numbness and weakness in the arms and hands and muscle spasms in the neck. Also, the symptoms are usually aggravated by downward compression of the head (Spurling’s test).4,5
Chest radiographs are useful, but CT scans are more sensitive
Often, MPM is initially suspected because of unilateral pleural nodularity or thickening with a large, unilateral pleural effusion on a chest radiograph.6 Pleural plaques may also be seen. As the tumor grows, encasing the lung and invading the fissures, it leads to volume loss of the affected side, which can also be identified radiographically.1
CT is a more sensitive way to detect pleural and pulmonary parenchymal involvement, as well as invasion of adjacent thoracic structures, including the chest wall, pericardium, diaphragm, and the mediastinal lymph nodes.1
When mesothelioma is suspected because of clinical or radiologic data, experts recommend that cytologic findings from thoracentesis be followed by tissue confirmation from thoracoscopy or CT biopsy.2
Chemotherapy, Yes, but there are many Tx unknowns
The best approach to treatment of MPM remains controversial due to the rarity of the disease and the scarcity of randomized prospective trials. Surgical resection is most often performed when the disease is confined to the pleural space. An extrapleural pneumonectomy is usually performed for stage I disease, when the tumor is limited to one hemithorax, invading the pleura and involving the lung, endothoracic fascia, diaphragm, or pericardium.7
Unfortunately, mesothelioma is highly radioresistant; patients often endure severe toxicity due to large radiation fields. Chemotherapy, either as single agents or in combination, can be administered systemically or directly into the pleural space. Combination chemotherapy using cisplatin and pemetrexed is currently the standard of care, based upon a phase III trial that demonstrated prolonged overall survival with the combination compared to treatment with cisplatin alone (12.1 months vs 9.3 months).8
Other agents used to treat MPM. Five other chemotherapy agents are also used in the treatment of MPM. Used individually, the maximum response rates to these agents are as follows: methotrexate (37%), mitomycin (21%), doxorubicin (16%), cyclophosphamide (13%), and carboplatin (11%).7
Our patient. The rest of our patient’s hospital course was uncomplicated. She was not a surgical candidate because she had such extensive tumor involvement. She was discharged with a referral to an outpatient oncology clinic. Despite 2 cycles of carboplatin and pemetrexed, and palliative radiation therapy to the right upper thoracic mass, the disease progressed with worsening right upper extremity pain and neurologic deficits.
CORRESPONDENCE
Mark Guelfguat, DO, Jacobi Medical Center, Albert Einstein College of Medicine, 1400 S Pelham Parkway, Building 1, Room 4N15, Bronx, NY 10461; [email protected].
A 78-year-old woman sought care at our emergency department for sudden-onset right shoulder pain that had begun 5 days earlier. She said the pain was sharp and that it radiated to the scapula, right arm, and chest. She said that nonsteroidal anti-inflammatory drugs provided pain relief.
The patient denied any recent trauma or heavy lifting, and was not experiencing extremity weakness, numbness, or tingling. She reported no fever, chills, cough, or night sweats, but said she’d lost 10 pounds over the previous month. The patient was a former smoker whose medical history included diabetes mellitus, hypertension, and hyperlipidemia. Two years ago, she was treated for recurrent right-sided pleural effusions with pleurocentesis, which was negative for cytology and acid-fast bacilli.
Auscultation revealed crackles in the right lower lung field with decreased breath sounds. The patient had full range of motion in her right shoulder and experienced minimal pain on flexion. She had no swelling, erythema, or tenderness in her right upper extremity and there was no sign of lymphadenopathy. Her laboratory data were noncontributory. A chest radiograph was obtained (FIGURE 1).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Malignant pleural mesothelioma
The chest radiograph revealed pleural-based masses extending along the convexity of the right chest wall. (Note the diminished volume of the right lung in FIGURE 1.) A coronal enhanced computed tomography (CT) scan (FIGURE 2) showed a large heterogeneous mass extending through the chest wall into the right axillary soft tissue and encasing the right brachial plexus (yellow arrows). Transdiaphragmatic extension was evident as an irregular mass at the hepatic dome (red arrow). The constellation of findings prompted the radiologist to suspect pleural malignancy.
An aggressive cancer
MPM is a highly aggressive neoplasm of the pleura with rising incidence around the globe.1,2 The annual incidence of mesothelioma in the United States is approximately 3000 cases per year, with the majority linked to asbestos exposure. The latency period is long, typically ranging from 35 to 40 years.1 Our patient, however, did not have a history of asbestos exposure. She was a housewife who had no exposure to building construction or demolition.
Nonspecific complaints. Clinical findings of MPM are frequently nonspecific and may masquerade as innocuous shoulder pain, as illustrated in this case. Patients may also present with dyspnea, nonpleuritic chest pain, and incidental pleural effusions. On examination, unilateral dullness on percussion at the lung base, palpable chest wall masses, and scoliosis toward the sides of the lesion may be present.3
Differential Dx of shoulder pain includes rotator cuff disorders, tears
The differential diagnosis of shoulder pain includes rotator cuff disorders, acromioclavicular osteoarthritis, and cervical radiculopathy from degenerative spondylosis.
Rotator cuff tendinopathy and tears usually present with pain on overhead activity, positional test muscle weakness, and evidence of impingement. Acromioclavicular osteoarthritis usually causes acromioclavicular joint tenderness, which can be relieved with diagnostic intra-articular anesthetic injections. Pain from cervical spondylosis is usually accompanied by numbness and weakness in the arms and hands and muscle spasms in the neck. Also, the symptoms are usually aggravated by downward compression of the head (Spurling’s test).4,5
Chest radiographs are useful, but CT scans are more sensitive
Often, MPM is initially suspected because of unilateral pleural nodularity or thickening with a large, unilateral pleural effusion on a chest radiograph.6 Pleural plaques may also be seen. As the tumor grows, encasing the lung and invading the fissures, it leads to volume loss of the affected side, which can also be identified radiographically.1
CT is a more sensitive way to detect pleural and pulmonary parenchymal involvement, as well as invasion of adjacent thoracic structures, including the chest wall, pericardium, diaphragm, and the mediastinal lymph nodes.1
When mesothelioma is suspected because of clinical or radiologic data, experts recommend that cytologic findings from thoracentesis be followed by tissue confirmation from thoracoscopy or CT biopsy.2
Chemotherapy, Yes, but there are many Tx unknowns
The best approach to treatment of MPM remains controversial due to the rarity of the disease and the scarcity of randomized prospective trials. Surgical resection is most often performed when the disease is confined to the pleural space. An extrapleural pneumonectomy is usually performed for stage I disease, when the tumor is limited to one hemithorax, invading the pleura and involving the lung, endothoracic fascia, diaphragm, or pericardium.7
Unfortunately, mesothelioma is highly radioresistant; patients often endure severe toxicity due to large radiation fields. Chemotherapy, either as single agents or in combination, can be administered systemically or directly into the pleural space. Combination chemotherapy using cisplatin and pemetrexed is currently the standard of care, based upon a phase III trial that demonstrated prolonged overall survival with the combination compared to treatment with cisplatin alone (12.1 months vs 9.3 months).8
Other agents used to treat MPM. Five other chemotherapy agents are also used in the treatment of MPM. Used individually, the maximum response rates to these agents are as follows: methotrexate (37%), mitomycin (21%), doxorubicin (16%), cyclophosphamide (13%), and carboplatin (11%).7
Our patient. The rest of our patient’s hospital course was uncomplicated. She was not a surgical candidate because she had such extensive tumor involvement. She was discharged with a referral to an outpatient oncology clinic. Despite 2 cycles of carboplatin and pemetrexed, and palliative radiation therapy to the right upper thoracic mass, the disease progressed with worsening right upper extremity pain and neurologic deficits.
CORRESPONDENCE
Mark Guelfguat, DO, Jacobi Medical Center, Albert Einstein College of Medicine, 1400 S Pelham Parkway, Building 1, Room 4N15, Bronx, NY 10461; [email protected].
1. Miller BH, Rosado-de-Christenson ML, Mason AC, et al. From the archives of the AFIP. Malignant pleural mesothelioma: radiologic-pathologic correlation. Radiographics. 1996;16:613-644.
2. Scherpereel A, Astoul P, Baas P, et al; European Respiratory Society/European Society of Thoracic Surgeons Task Force. Guidelines of the European Respiratory Society and the European Society of Thoracic Surgeons for the management of malignant pleural mesothelioma. Eur Respir J. 2010;35:479-495.
3. Antman KH. Natural history and epidemiology of malignant mesothelioma. Chest. 1993;103:373S-376S.
4. Burbank KM, Stevenson JH, Czarnecki GR, et al. Chronic shoulder pain: part I. Evaluation and diagnosis. Am Fam Physician. 2008;77:453-460.
5. Anekstein Y, Blecher R, Smorgick Y, et al. What is the best way to apply the Spurling test for cervical radiculopathy? Clin Orthop Relat Res. 2012;470:2566-2572.
6. British Thoracic Society Standards of Care Committee. Statement on malignant mesothelioma in the United Kingdom. Thorax. 2001;56:250-265.
7. Aisner J. Current approach to malignant mesothelioma of the pleura. Chest. 1995;107:332S-344S.
8. Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21:2636-2644.
1. Miller BH, Rosado-de-Christenson ML, Mason AC, et al. From the archives of the AFIP. Malignant pleural mesothelioma: radiologic-pathologic correlation. Radiographics. 1996;16:613-644.
2. Scherpereel A, Astoul P, Baas P, et al; European Respiratory Society/European Society of Thoracic Surgeons Task Force. Guidelines of the European Respiratory Society and the European Society of Thoracic Surgeons for the management of malignant pleural mesothelioma. Eur Respir J. 2010;35:479-495.
3. Antman KH. Natural history and epidemiology of malignant mesothelioma. Chest. 1993;103:373S-376S.
4. Burbank KM, Stevenson JH, Czarnecki GR, et al. Chronic shoulder pain: part I. Evaluation and diagnosis. Am Fam Physician. 2008;77:453-460.
5. Anekstein Y, Blecher R, Smorgick Y, et al. What is the best way to apply the Spurling test for cervical radiculopathy? Clin Orthop Relat Res. 2012;470:2566-2572.
6. British Thoracic Society Standards of Care Committee. Statement on malignant mesothelioma in the United Kingdom. Thorax. 2001;56:250-265.
7. Aisner J. Current approach to malignant mesothelioma of the pleura. Chest. 1995;107:332S-344S.
8. Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21:2636-2644.
VIDEO: Adding ixazomib to len-dex boosts progression-free survival in multiple myeloma
Adding ixazomib to lenalidomide and dexamethasone was associated with longer progression-free survival and limited additional toxic effects in patients with multiple myeloma, based on the published phase 3 results of the TOURMALINE trial.
The double-blind, placebo-controlled trial included 722 patients who had relapsed, refractory, or relapsed and refractory multiple myeloma and were randomly assigned to receive the oral proteasome inhibitor plus lenalidomide-dexamethasone or placebo plus lenalidomide-dexamethasone (len-dex), according to Dr. Philippe Moreau of University Hospital Hôtel
Dieu, Nantes, France, and his colleagues in the TOURMALINE-MM1 Study Group.
At a median follow-up of nearly 14.7 months, median progression-free survival was 20.6 months in the ixazomib plus len-dex group and 14.7 months in the placebo plus len-dex group, a significant difference for ixazomib with a 0.74 hazard ratio for disease progression or death (P = .01). The benefit was noted for all prespecified patient subgroups, including patients with high-risk cytogenetic abnormalities. The overall rates of response were 78% in the ixazomib plus len-dex group and 72% in the placebo plus len-dex group, and the corresponding rates of complete response plus very good partial response were 48% and 39%, respectively. At a median follow-up of approximately 23 months, the median duration of response was 20.5 months for ixazomib plus len-dex and 15 months for len-dex alone, the researchers reported (N Engl J Med. 2016;374:1621-34. doi: 10.1056/NEJMoa1516282).
The rates of serious adverse events were 47% in the ixazomib plus len-dex group and 49% in the placebo plus len-dex group; the rates of death during the study period were 4% and 6%, respectively.
The results of the trial also were presented at the annual meeting of the American Society of Hematology, where Dr. Shaji Kumar, one the study investigators, discussed the implications of the TOURMALINE results in a video interview.
The study was sponsored by Millennium Pharmaceuticals, the makers of ixazomib (Ninlaro). Dr. Moreau reports receiving fees for serving on advisory boards for Millennium Pharmaceuticals and several other drug companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @maryjodales
Adding ixazomib to lenalidomide and dexamethasone was associated with longer progression-free survival and limited additional toxic effects in patients with multiple myeloma, based on the published phase 3 results of the TOURMALINE trial.
The double-blind, placebo-controlled trial included 722 patients who had relapsed, refractory, or relapsed and refractory multiple myeloma and were randomly assigned to receive the oral proteasome inhibitor plus lenalidomide-dexamethasone or placebo plus lenalidomide-dexamethasone (len-dex), according to Dr. Philippe Moreau of University Hospital Hôtel
Dieu, Nantes, France, and his colleagues in the TOURMALINE-MM1 Study Group.
At a median follow-up of nearly 14.7 months, median progression-free survival was 20.6 months in the ixazomib plus len-dex group and 14.7 months in the placebo plus len-dex group, a significant difference for ixazomib with a 0.74 hazard ratio for disease progression or death (P = .01). The benefit was noted for all prespecified patient subgroups, including patients with high-risk cytogenetic abnormalities. The overall rates of response were 78% in the ixazomib plus len-dex group and 72% in the placebo plus len-dex group, and the corresponding rates of complete response plus very good partial response were 48% and 39%, respectively. At a median follow-up of approximately 23 months, the median duration of response was 20.5 months for ixazomib plus len-dex and 15 months for len-dex alone, the researchers reported (N Engl J Med. 2016;374:1621-34. doi: 10.1056/NEJMoa1516282).
The rates of serious adverse events were 47% in the ixazomib plus len-dex group and 49% in the placebo plus len-dex group; the rates of death during the study period were 4% and 6%, respectively.
The results of the trial also were presented at the annual meeting of the American Society of Hematology, where Dr. Shaji Kumar, one the study investigators, discussed the implications of the TOURMALINE results in a video interview.
The study was sponsored by Millennium Pharmaceuticals, the makers of ixazomib (Ninlaro). Dr. Moreau reports receiving fees for serving on advisory boards for Millennium Pharmaceuticals and several other drug companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @maryjodales
Adding ixazomib to lenalidomide and dexamethasone was associated with longer progression-free survival and limited additional toxic effects in patients with multiple myeloma, based on the published phase 3 results of the TOURMALINE trial.
The double-blind, placebo-controlled trial included 722 patients who had relapsed, refractory, or relapsed and refractory multiple myeloma and were randomly assigned to receive the oral proteasome inhibitor plus lenalidomide-dexamethasone or placebo plus lenalidomide-dexamethasone (len-dex), according to Dr. Philippe Moreau of University Hospital Hôtel
Dieu, Nantes, France, and his colleagues in the TOURMALINE-MM1 Study Group.
At a median follow-up of nearly 14.7 months, median progression-free survival was 20.6 months in the ixazomib plus len-dex group and 14.7 months in the placebo plus len-dex group, a significant difference for ixazomib with a 0.74 hazard ratio for disease progression or death (P = .01). The benefit was noted for all prespecified patient subgroups, including patients with high-risk cytogenetic abnormalities. The overall rates of response were 78% in the ixazomib plus len-dex group and 72% in the placebo plus len-dex group, and the corresponding rates of complete response plus very good partial response were 48% and 39%, respectively. At a median follow-up of approximately 23 months, the median duration of response was 20.5 months for ixazomib plus len-dex and 15 months for len-dex alone, the researchers reported (N Engl J Med. 2016;374:1621-34. doi: 10.1056/NEJMoa1516282).
The rates of serious adverse events were 47% in the ixazomib plus len-dex group and 49% in the placebo plus len-dex group; the rates of death during the study period were 4% and 6%, respectively.
The results of the trial also were presented at the annual meeting of the American Society of Hematology, where Dr. Shaji Kumar, one the study investigators, discussed the implications of the TOURMALINE results in a video interview.
The study was sponsored by Millennium Pharmaceuticals, the makers of ixazomib (Ninlaro). Dr. Moreau reports receiving fees for serving on advisory boards for Millennium Pharmaceuticals and several other drug companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @maryjodales
FROM NEJM
Key clinical point: Adding ixazomib to lenalidomide and dexamethasone was associated with a longer progression-free survival and limited additional toxic effects in patients with multiple myeloma.
Major finding: At a median follow-up of nearly 14.7 months, median progression-free survival was 20.6 months in the ixazomib plus len-dex group and 14.7 months in the placebo plus len-dex group.
Data source: Phase III results on 722 patients in the TOURMALINE trial.
Disclosures: The study was sponsored by Millennium Pharmaceuticals, the makers of ixazomib (Ninlaro). Dr. Moreau reports receiving fees for serving on advisory boards for Millennium Pharmaceuticals and several other drug companies.
IV tigecycline scores as alternative C. difficile treatment
AMSTERDAM – Intravenous tigecycline was significantly more effective than standard therapy at curing refractory Clostridium difficile infections, according to a case-control study presented at the European Society of Clinical Microbiology and Infectious Diseases annual congress.
Tigecycline effected a 76% clinical cure rate, compared with 53% for the combination regimen of intravenous metronidazole and oral vancomycin, Dr. Baltin Gergely Szabo reported. And despite the fact that those who took tigecycline had more clinically severe disease, no colectomies were required in that group, while two patients in the standard treatment arm did need the procedure.
However, tigecycline didn’t significantly improve relapse rates or mortality, noted Dr. Szabo of the St. Stephan and St. Ladislaus Hospital-Clinic, Budapest, Hungary.
He presented the results of a matched case-control study of 90 patients with severe C. difficile infections, who were treated with either of the protocols. Patients who took tigecycline were more likely to have a recurrent infection (38% vs. 29%). Thus, they were also more likely to have previously been treated with metronidazole (38% vs. 24%) and vancomycin (24% vs. 7%). Prior tigecycline use was very rare in both groups (2% vs. 0%).
Those who took tigecycline were significantly younger as well (72 vs. 78 years), and more often men (56% vs. 30%). They were more likely to be hypertensive, have chronic obstructive pulmonary disease, have cancers, be immunosuppressed, and be chronic users of corticosteroids.
However, the Charlson comorbidity index was similar between the tigecycline and standard therapy groups (4.6 vs. 5). They were also matched for ATLAS scores (mean 7.8 in each group).
Significantly more patients taking tigecycline had acquired their infections during hospitalization (64% vs. 30%). They also had a longer duration of symptoms (17 vs. 10 days).
Imaging showed more severe disease in the tigecycline group with significantly more colonic distension, mural thickening, and ascites. Tigecycline patients had also undergone significantly more colonoscopies and blood cultures.
Tigecycline was given in the hospital for 7-10 days, with a 100-mg loading dose and subsequent 50-mg daily doses. The main duration of therapy was 10 days, but that varied widely, from 2 to 22 days. It was given only as first-line treatment to 15% of patients; the rest received tigecycline as an alternative treatment, often after the combination of metronidazole/vancomycin had failed. No adverse drug reactions occurred in the group.
Clinical cure was achieved in 76% of the tigecycline group and 53% of the standard protocol group – a significant difference. The drug was associated with a decreased rate of complicated disease course (29% vs. 53%) and significantly fewer colectomies (0 vs. 2).
Rates of toxic megacolon were equal (7% each group); ileus was more frequent in the tigecycline group (11% vs. 9%), but this difference was not statistically significant.
However, tigecycline had no impact on either in-hospital or 90-day relapse, or on in-hospital mortality (15 vs. 16 deaths). At 90 days, fewer patients taking the drug had died (17 vs. 21), but that difference was not statistically significant (P = 0.52).
A multivariate analysis identified several characteristics associated with a beneficial response to tigecycline:
• Male sex.
• Being immunosuppressed.
• Chronic steroid treatment.
• Malignancy.
• Longer duration of symptoms.
• Prior C. difficile infections.
• Nosocomial onset.
• Signs of severe infection on imaging.
Dr. Szabo said these characteristics can be used to create a profile of patients who might be good candidates for the drug.
He had no relevant financial declarations.
On Twitter @Alz_Gal
AMSTERDAM – Intravenous tigecycline was significantly more effective than standard therapy at curing refractory Clostridium difficile infections, according to a case-control study presented at the European Society of Clinical Microbiology and Infectious Diseases annual congress.
Tigecycline effected a 76% clinical cure rate, compared with 53% for the combination regimen of intravenous metronidazole and oral vancomycin, Dr. Baltin Gergely Szabo reported. And despite the fact that those who took tigecycline had more clinically severe disease, no colectomies were required in that group, while two patients in the standard treatment arm did need the procedure.
However, tigecycline didn’t significantly improve relapse rates or mortality, noted Dr. Szabo of the St. Stephan and St. Ladislaus Hospital-Clinic, Budapest, Hungary.
He presented the results of a matched case-control study of 90 patients with severe C. difficile infections, who were treated with either of the protocols. Patients who took tigecycline were more likely to have a recurrent infection (38% vs. 29%). Thus, they were also more likely to have previously been treated with metronidazole (38% vs. 24%) and vancomycin (24% vs. 7%). Prior tigecycline use was very rare in both groups (2% vs. 0%).
Those who took tigecycline were significantly younger as well (72 vs. 78 years), and more often men (56% vs. 30%). They were more likely to be hypertensive, have chronic obstructive pulmonary disease, have cancers, be immunosuppressed, and be chronic users of corticosteroids.
However, the Charlson comorbidity index was similar between the tigecycline and standard therapy groups (4.6 vs. 5). They were also matched for ATLAS scores (mean 7.8 in each group).
Significantly more patients taking tigecycline had acquired their infections during hospitalization (64% vs. 30%). They also had a longer duration of symptoms (17 vs. 10 days).
Imaging showed more severe disease in the tigecycline group with significantly more colonic distension, mural thickening, and ascites. Tigecycline patients had also undergone significantly more colonoscopies and blood cultures.
Tigecycline was given in the hospital for 7-10 days, with a 100-mg loading dose and subsequent 50-mg daily doses. The main duration of therapy was 10 days, but that varied widely, from 2 to 22 days. It was given only as first-line treatment to 15% of patients; the rest received tigecycline as an alternative treatment, often after the combination of metronidazole/vancomycin had failed. No adverse drug reactions occurred in the group.
Clinical cure was achieved in 76% of the tigecycline group and 53% of the standard protocol group – a significant difference. The drug was associated with a decreased rate of complicated disease course (29% vs. 53%) and significantly fewer colectomies (0 vs. 2).
Rates of toxic megacolon were equal (7% each group); ileus was more frequent in the tigecycline group (11% vs. 9%), but this difference was not statistically significant.
However, tigecycline had no impact on either in-hospital or 90-day relapse, or on in-hospital mortality (15 vs. 16 deaths). At 90 days, fewer patients taking the drug had died (17 vs. 21), but that difference was not statistically significant (P = 0.52).
A multivariate analysis identified several characteristics associated with a beneficial response to tigecycline:
• Male sex.
• Being immunosuppressed.
• Chronic steroid treatment.
• Malignancy.
• Longer duration of symptoms.
• Prior C. difficile infections.
• Nosocomial onset.
• Signs of severe infection on imaging.
Dr. Szabo said these characteristics can be used to create a profile of patients who might be good candidates for the drug.
He had no relevant financial declarations.
On Twitter @Alz_Gal
AMSTERDAM – Intravenous tigecycline was significantly more effective than standard therapy at curing refractory Clostridium difficile infections, according to a case-control study presented at the European Society of Clinical Microbiology and Infectious Diseases annual congress.
Tigecycline effected a 76% clinical cure rate, compared with 53% for the combination regimen of intravenous metronidazole and oral vancomycin, Dr. Baltin Gergely Szabo reported. And despite the fact that those who took tigecycline had more clinically severe disease, no colectomies were required in that group, while two patients in the standard treatment arm did need the procedure.
However, tigecycline didn’t significantly improve relapse rates or mortality, noted Dr. Szabo of the St. Stephan and St. Ladislaus Hospital-Clinic, Budapest, Hungary.
He presented the results of a matched case-control study of 90 patients with severe C. difficile infections, who were treated with either of the protocols. Patients who took tigecycline were more likely to have a recurrent infection (38% vs. 29%). Thus, they were also more likely to have previously been treated with metronidazole (38% vs. 24%) and vancomycin (24% vs. 7%). Prior tigecycline use was very rare in both groups (2% vs. 0%).
Those who took tigecycline were significantly younger as well (72 vs. 78 years), and more often men (56% vs. 30%). They were more likely to be hypertensive, have chronic obstructive pulmonary disease, have cancers, be immunosuppressed, and be chronic users of corticosteroids.
However, the Charlson comorbidity index was similar between the tigecycline and standard therapy groups (4.6 vs. 5). They were also matched for ATLAS scores (mean 7.8 in each group).
Significantly more patients taking tigecycline had acquired their infections during hospitalization (64% vs. 30%). They also had a longer duration of symptoms (17 vs. 10 days).
Imaging showed more severe disease in the tigecycline group with significantly more colonic distension, mural thickening, and ascites. Tigecycline patients had also undergone significantly more colonoscopies and blood cultures.
Tigecycline was given in the hospital for 7-10 days, with a 100-mg loading dose and subsequent 50-mg daily doses. The main duration of therapy was 10 days, but that varied widely, from 2 to 22 days. It was given only as first-line treatment to 15% of patients; the rest received tigecycline as an alternative treatment, often after the combination of metronidazole/vancomycin had failed. No adverse drug reactions occurred in the group.
Clinical cure was achieved in 76% of the tigecycline group and 53% of the standard protocol group – a significant difference. The drug was associated with a decreased rate of complicated disease course (29% vs. 53%) and significantly fewer colectomies (0 vs. 2).
Rates of toxic megacolon were equal (7% each group); ileus was more frequent in the tigecycline group (11% vs. 9%), but this difference was not statistically significant.
However, tigecycline had no impact on either in-hospital or 90-day relapse, or on in-hospital mortality (15 vs. 16 deaths). At 90 days, fewer patients taking the drug had died (17 vs. 21), but that difference was not statistically significant (P = 0.52).
A multivariate analysis identified several characteristics associated with a beneficial response to tigecycline:
• Male sex.
• Being immunosuppressed.
• Chronic steroid treatment.
• Malignancy.
• Longer duration of symptoms.
• Prior C. difficile infections.
• Nosocomial onset.
• Signs of severe infection on imaging.
Dr. Szabo said these characteristics can be used to create a profile of patients who might be good candidates for the drug.
He had no relevant financial declarations.
On Twitter @Alz_Gal
AT ACCMID 2016
Key clinical point: Tigecycline was an effective therapy for patients with severe C. difficile infections.
Major finding: The drug effected a clinical cure in 76% of patients, compared with a 53% cure rate in those taking metronidazole and vancomycin.
Data source: A retrospective case-control study involving 90 patients.
Disclosures: Dr. Szabo had no relevant financial disclosures.
Sexually Transmitted Diseases
Review the PDF of the fact sheet on sexually transmitted diseases with board-relevant, easy-to-review material. This month's fact sheet offers a comprehensive review of the etiology, clinical findings, and management of common STDs.
Practice Questions
1. A 44-year-old woman presents with fever, lymphadenopathy, and headaches. She has noticed a rash on her palms and soles that is not itchy. What is the diagnosis?
a. chancroid
b. gonorrhea
c. granuloma inguinale
d. lymphogranuloma venereum
e. secondary syphilis
2. A 37-year-old man presents with dysuria and purulent discharge. What is the appropriate test for diagnosis?
a. dark field microscopy
b. Giemsa staining
c. McCoy culture
d. porphyrin test (hemin [X factor]) culture
e. Thayer-Martin medium
3. Which disease in the neonate is preventable with silver nitrate drops?
a. chancroid
b. gonorrhea
c. granuloma inguinale
d. lymphogranuloma venereum
e. syphilis
4. A 22-year-old pregnant woman develops a painless indurated ulcer on the vagina. What is the treatment of choice?
a. azithromycin
b. ceftriaxone
c. doxycycline
d. penicillin G
e. TMP-SMX
5. What sexually transmitted disease facilitates the transmission of HIV?
a. chancroid
b. gonorrhea
c. lymphogranuloma venereum
d. syphilis
e. all of the above
Answers to practice questions provided on next page
Practice Question Answers
1. A 44-year-old woman presents with fever, lymphadenopathy, and headaches. She has noticed a rash on her palms and soles that is not itchy. What is the diagnosis?
a. chancroid
b. gonorrhea
c. granuloma inguinale
d. lymphogranuloma venereum
e. secondary syphilis
2. A 37-year-old man presents with dysuria and purulent discharge. What is the appropriate test for diagnosis?
a. dark field microscopy
b. Giemsa staining
c. McCoy culture
d. porphyrin test (hemin [X factor]) culture
e. Thayer-Martin medium
3. Which disease in the neonate is preventable with silver nitrate drops?
a. chancroid
b. gonorrhea
c. granuloma inguinale
d. lymphogranuloma venereum
e. syphilis
4. A 22-year-old pregnant woman develops a painless indurated ulcer on the vagina. What is the treatment of choice?
a. azithromycin
b. ceftriaxone
c. doxycycline
d. penicillin G
e. TMP-SMX
5. What sexually transmitted disease facilitates the transmission of HIV?
a. chancroid
b. gonorrhea
c. lymphogranuloma venereum
d. syphilis
e. all of the above
Review the PDF of the fact sheet on sexually transmitted diseases with board-relevant, easy-to-review material. This month's fact sheet offers a comprehensive review of the etiology, clinical findings, and management of common STDs.
Practice Questions
1. A 44-year-old woman presents with fever, lymphadenopathy, and headaches. She has noticed a rash on her palms and soles that is not itchy. What is the diagnosis?
a. chancroid
b. gonorrhea
c. granuloma inguinale
d. lymphogranuloma venereum
e. secondary syphilis
2. A 37-year-old man presents with dysuria and purulent discharge. What is the appropriate test for diagnosis?
a. dark field microscopy
b. Giemsa staining
c. McCoy culture
d. porphyrin test (hemin [X factor]) culture
e. Thayer-Martin medium
3. Which disease in the neonate is preventable with silver nitrate drops?
a. chancroid
b. gonorrhea
c. granuloma inguinale
d. lymphogranuloma venereum
e. syphilis
4. A 22-year-old pregnant woman develops a painless indurated ulcer on the vagina. What is the treatment of choice?
a. azithromycin
b. ceftriaxone
c. doxycycline
d. penicillin G
e. TMP-SMX
5. What sexually transmitted disease facilitates the transmission of HIV?
a. chancroid
b. gonorrhea
c. lymphogranuloma venereum
d. syphilis
e. all of the above
Answers to practice questions provided on next page
Practice Question Answers
1. A 44-year-old woman presents with fever, lymphadenopathy, and headaches. She has noticed a rash on her palms and soles that is not itchy. What is the diagnosis?
a. chancroid
b. gonorrhea
c. granuloma inguinale
d. lymphogranuloma venereum
e. secondary syphilis
2. A 37-year-old man presents with dysuria and purulent discharge. What is the appropriate test for diagnosis?
a. dark field microscopy
b. Giemsa staining
c. McCoy culture
d. porphyrin test (hemin [X factor]) culture
e. Thayer-Martin medium
3. Which disease in the neonate is preventable with silver nitrate drops?
a. chancroid
b. gonorrhea
c. granuloma inguinale
d. lymphogranuloma venereum
e. syphilis
4. A 22-year-old pregnant woman develops a painless indurated ulcer on the vagina. What is the treatment of choice?
a. azithromycin
b. ceftriaxone
c. doxycycline
d. penicillin G
e. TMP-SMX
5. What sexually transmitted disease facilitates the transmission of HIV?
a. chancroid
b. gonorrhea
c. lymphogranuloma venereum
d. syphilis
e. all of the above
Review the PDF of the fact sheet on sexually transmitted diseases with board-relevant, easy-to-review material. This month's fact sheet offers a comprehensive review of the etiology, clinical findings, and management of common STDs.
Practice Questions
1. A 44-year-old woman presents with fever, lymphadenopathy, and headaches. She has noticed a rash on her palms and soles that is not itchy. What is the diagnosis?
a. chancroid
b. gonorrhea
c. granuloma inguinale
d. lymphogranuloma venereum
e. secondary syphilis
2. A 37-year-old man presents with dysuria and purulent discharge. What is the appropriate test for diagnosis?
a. dark field microscopy
b. Giemsa staining
c. McCoy culture
d. porphyrin test (hemin [X factor]) culture
e. Thayer-Martin medium
3. Which disease in the neonate is preventable with silver nitrate drops?
a. chancroid
b. gonorrhea
c. granuloma inguinale
d. lymphogranuloma venereum
e. syphilis
4. A 22-year-old pregnant woman develops a painless indurated ulcer on the vagina. What is the treatment of choice?
a. azithromycin
b. ceftriaxone
c. doxycycline
d. penicillin G
e. TMP-SMX
5. What sexually transmitted disease facilitates the transmission of HIV?
a. chancroid
b. gonorrhea
c. lymphogranuloma venereum
d. syphilis
e. all of the above
Answers to practice questions provided on next page
Practice Question Answers
1. A 44-year-old woman presents with fever, lymphadenopathy, and headaches. She has noticed a rash on her palms and soles that is not itchy. What is the diagnosis?
a. chancroid
b. gonorrhea
c. granuloma inguinale
d. lymphogranuloma venereum
e. secondary syphilis
2. A 37-year-old man presents with dysuria and purulent discharge. What is the appropriate test for diagnosis?
a. dark field microscopy
b. Giemsa staining
c. McCoy culture
d. porphyrin test (hemin [X factor]) culture
e. Thayer-Martin medium
3. Which disease in the neonate is preventable with silver nitrate drops?
a. chancroid
b. gonorrhea
c. granuloma inguinale
d. lymphogranuloma venereum
e. syphilis
4. A 22-year-old pregnant woman develops a painless indurated ulcer on the vagina. What is the treatment of choice?
a. azithromycin
b. ceftriaxone
c. doxycycline
d. penicillin G
e. TMP-SMX
5. What sexually transmitted disease facilitates the transmission of HIV?
a. chancroid
b. gonorrhea
c. lymphogranuloma venereum
d. syphilis
e. all of the above
$30 million NIA Consortium Explores Links Between Vascular Health and Alzheimer Disease
BETHESDA, MD. – Cerebral vascular dysfunction exerts a significant negative influence on cognition, doubling the risk of dementia in old age and speeding the rate of cognitive decline.
These findings have been confirmed in a number of studies, and their advancement in both research and clinical arenas continues. But studies of the vascular conditions that affect cognition remain largely observational. Intervention trials are few and limited in scope. The dearth of animal models that express compromised cerebral vascular function has made conducting basic studies feel like wheels spinning in the mud.
According to investigators who discussed the problem at the recent Alzheimer’s Disease–Related Dementias 2016 Summit, sponsored by the National Institutes of Health, the situation calls for a targeted push to better understand vascular complications and their impact on cognition and the development of dementias – and a new, 5-year NIH research program aims to do just that.
M²OVE–AD: Molecular Mechanisms of the Vascular Etiology of Alzheimer’s Disease, is a $30 million initiative that brings together more than a dozen research teams. Investigators will employ new molecular profiling technologies and big data analytics to understand how vascular dysfunction influences the development of Alzheimer’s. The teams will collaborate on five different projects, each exploring a different facet of these complex processes, according to Dr. Suzana Petanceska, program director of the neuroscience division at the National Institute on Aging, Bethesda, Md., who shared her thoughts after the meeting.
“The central goal of the consortium is to generate a deeper understanding of the molecular mechanisms linking vascular risk factors, cerebrovascular disease, and Alzheimer’s, and to generate a new big-data resource that will aid the discovery of therapeutic targets for disease treatment and prevention and molecular signatures that can be used as biomarkers for disease risk,” Dr. Petanceska said in an interview.
Following a new trend of sharing Alzheimer’s research data across public and academic domains, data generated by this program will be made rapidly available to the greater research community. “Making these complex biological data sets available and usable by researchers other than the data generators is key to accelerating the pace at which the research community can generate new knowledge and replicate new findings. The M²OVE–AD initiative builds on the open-science approach established by the Accelerating Medicines Partnership – AD Programand Alzheimer’s Disease Neuroimaging Initiative (ADNI). By coordinating the experimental and analytical approaches the research teams will maximize the usability of the data generated on these projects.”
Five complementary projects comprise the consortium:
Integrative Translational Discovery of Vascular Risk Factors in Aging and Dementia
Researchers at the Mayo Clinics in Minnesota and Florida will collaborate with those at the Icahn Institute for Genomics and Multiscale Biology, New York, to explore how molecular networks influence vascular risk in normal aging, as well as in Alzheimer’s and other dementias.
The project’s goal is to understand how gender and the Alzheimer’s disease risk factor gene ApoE4 influence the molecular processes that lead to Alzheimer’s-related cerebral amyloid angiopathy (CAA).
CAA appears to be a key player in the progression of Alzheimer’s disease. The health of small vessels of the brain is important not only in age-related cognitive decline, but also in amyloid clearance. When amyloid collects in these vessels, it may cause a potentially self-sustaining loop of vascular injury and impaired amyloid clearance, which causes more intravascular amyloid deposition, more CAA, and increasing amyloid pathology.
The team intends to use genetic and expression profiling data from human brain and bloods samples, as well as existing molecular, clinical, and pathologic data in hopes of discovering therapeutic targets. The dynamic interaction between gender, apoE4 and aging and its impact on various AD pathologic and clinical traits will be explored in an array of existing and new animal models.
“Integrating the analysis of multidimensional human data with studies in animal models will accelerate the speed with which the findings can be translated to new interventions for treatment and prevention,” Dr. Petanceska said.
Type 2 diabetes mellitus and prediabetes metabolic abnormalities affect one-third of U.S. adults and the majority of persons aged 60 years and older. Diabetes is associated with a higher risk of the clinical manifestations of AD, including dementia and mild cognitive impairment. Hispanics in the United States have higher rates of diabetes, putting them at greater risk for developing Alzheimer’s. Investigators at Columbia University and SUNY Downstate Medical Center, both in New York, will examine the complex relationship between diabetes, cerebrovascular disease, and Alzheimer’s in a cohort of 200 middle-aged Hispanic participants, with either normal glucose metabolism, prediabetes, or type 2 diabetes; the subjects will be followed for 5 years with whole-brain magnetic resonance imaging and a variety of cognitive measures. The brain imaging will track AD-like functional and pathologic changes and vascular lesions.
In addition, the team will carry out molecular profiling of plasma samples collected in the same participants to identify metabolic and protein signatures that may predict clinical, pathologic, and physiologic outcomes related to Alzheimer’s and cerebrovascular disease.
In a companion study using mouse models with diabetes and Alzheimer’s pathology traits, the researchers will examine how the interaction between diabetes and Alzheimer’s pathology affects the structure and function of neural circuits important to learning and memory.
“This project is addressing two critical knowledge gaps,” Dr. Petanceska said. “The first is understanding the mechanisms by which dysregulated glucose metabolism impacts the onset and progression of pathologic changes in the course of the preclinical phase of Alzheimer’s disease; the second is understanding the molecular determinants of AD risk in Hispanics, a population with higher prevalence of diabetes and at greater risk for AD.”
The Role of Renin-Angiotensin-Endothelial Pathway in Alzheimer’s Disease
Researchers at Emory University, Atlanta, will focus on understanding the molecular mechanisms by which vascular dysfunction associated with high blood pressure affects the onset and progression of Alzheimer’s.
The research cohort comprises 160 subjects from the Emory Cardiovascular Biobank and Predictive Health Study– 80 with normal cognition and 80 with mild cognitive impairment – who will be followed for 2 years. Molecular data (genomic, epigenetic and metabolomic) combined with clinical data on the same subjects collected over 2 years, will be used to build a molecular network model of the interaction between vascular dysfunction and various Alzheimer’s disease traits.
Parallel studies in a rat model that uniquely exhibits human-like AD neuropathology will help uncover the temporal relationship between vascular dysfunction and AD and examine the potential of the molecular regulators of vascular function, such as the renin-angiotensin system, as therapeutic targets for AD. The goal is to characterize this pathway as a therapeutic target.
Metabolic Signatures Underlying Vascular Risk Factors for Alzheimer’s-Type Dementias
Teams at Duke University, Durham, N.C., and the University of Pennsylvania, Philadelphia, will carry out extensive profiling of plasma samples from 900 ADNI participants and from participants in the Duke University MURDOCK Memory and Cognitive Health Study in search for lipid metabolites that are associated with cardiovascular disease and cognitive change. These lipidomic profiles will be integrated with the vast array of clinical and other molecular data available for these participants to identify molecular signatures that may be used to differentiate among various risk-factor types of AD.
In addition, in a subset of subjects, the team will compare the lipidomic profiles between plasma and cerebrospinal fluid; this will enable the team to test hypotheses about the role of systemic vascular and metabolic factors on cognitive aging and AD.
Cerebral Amyloid Angiopathy and Mechanisms of Brain Amyloid Accumulation
Investigators at Massachusetts General Hospital, Boston, will investigate the molecular underpinnings of CAA and its impact on Alzheimer’s disease. Employing a mouse model and human subjects with CAA, the study will explore this cycle of progressive amyloid deposition and brain injury. The team’s approach combines noninvasive detection and analysis of human CAA, real-time measurement of vascular structure and physiology in living transgenic mouse models, and molecular analysis of gene expression in brain microvessels. Ultimately, the team hopes to identify candidate therapies with which could block it.
“This highly multidisciplinary investigation into how the vascular effects of amyloid at the molecular, single-blood vessel, and whole-brain levels influence the clinical disease promises to deliver new, well-characterized therapeutic targets for disease prevention,” Dr. Petanceska said.
She predicted that the wide-ranging projects of the M²OVE–AD consortium will bring invaluable understanding to an enormously important, but still unexplored, aspect of Alzheimer’s pathology.
“We hope that this large-scale team science effort will generate an in-depth understanding of how vascular and metabolic factors contribute to neurodegenerative changes that result in cognitive decline and dementia and that the data and knowledge generated by this program will be the basis for developing effective interventions for disease treatment and prevention.”
BETHESDA, MD. – Cerebral vascular dysfunction exerts a significant negative influence on cognition, doubling the risk of dementia in old age and speeding the rate of cognitive decline.
These findings have been confirmed in a number of studies, and their advancement in both research and clinical arenas continues. But studies of the vascular conditions that affect cognition remain largely observational. Intervention trials are few and limited in scope. The dearth of animal models that express compromised cerebral vascular function has made conducting basic studies feel like wheels spinning in the mud.
According to investigators who discussed the problem at the recent Alzheimer’s Disease–Related Dementias 2016 Summit, sponsored by the National Institutes of Health, the situation calls for a targeted push to better understand vascular complications and their impact on cognition and the development of dementias – and a new, 5-year NIH research program aims to do just that.
M²OVE–AD: Molecular Mechanisms of the Vascular Etiology of Alzheimer’s Disease, is a $30 million initiative that brings together more than a dozen research teams. Investigators will employ new molecular profiling technologies and big data analytics to understand how vascular dysfunction influences the development of Alzheimer’s. The teams will collaborate on five different projects, each exploring a different facet of these complex processes, according to Dr. Suzana Petanceska, program director of the neuroscience division at the National Institute on Aging, Bethesda, Md., who shared her thoughts after the meeting.
“The central goal of the consortium is to generate a deeper understanding of the molecular mechanisms linking vascular risk factors, cerebrovascular disease, and Alzheimer’s, and to generate a new big-data resource that will aid the discovery of therapeutic targets for disease treatment and prevention and molecular signatures that can be used as biomarkers for disease risk,” Dr. Petanceska said in an interview.
Following a new trend of sharing Alzheimer’s research data across public and academic domains, data generated by this program will be made rapidly available to the greater research community. “Making these complex biological data sets available and usable by researchers other than the data generators is key to accelerating the pace at which the research community can generate new knowledge and replicate new findings. The M²OVE–AD initiative builds on the open-science approach established by the Accelerating Medicines Partnership – AD Programand Alzheimer’s Disease Neuroimaging Initiative (ADNI). By coordinating the experimental and analytical approaches the research teams will maximize the usability of the data generated on these projects.”
Five complementary projects comprise the consortium:
Integrative Translational Discovery of Vascular Risk Factors in Aging and Dementia
Researchers at the Mayo Clinics in Minnesota and Florida will collaborate with those at the Icahn Institute for Genomics and Multiscale Biology, New York, to explore how molecular networks influence vascular risk in normal aging, as well as in Alzheimer’s and other dementias.
The project’s goal is to understand how gender and the Alzheimer’s disease risk factor gene ApoE4 influence the molecular processes that lead to Alzheimer’s-related cerebral amyloid angiopathy (CAA).
CAA appears to be a key player in the progression of Alzheimer’s disease. The health of small vessels of the brain is important not only in age-related cognitive decline, but also in amyloid clearance. When amyloid collects in these vessels, it may cause a potentially self-sustaining loop of vascular injury and impaired amyloid clearance, which causes more intravascular amyloid deposition, more CAA, and increasing amyloid pathology.
The team intends to use genetic and expression profiling data from human brain and bloods samples, as well as existing molecular, clinical, and pathologic data in hopes of discovering therapeutic targets. The dynamic interaction between gender, apoE4 and aging and its impact on various AD pathologic and clinical traits will be explored in an array of existing and new animal models.
“Integrating the analysis of multidimensional human data with studies in animal models will accelerate the speed with which the findings can be translated to new interventions for treatment and prevention,” Dr. Petanceska said.
Type 2 diabetes mellitus and prediabetes metabolic abnormalities affect one-third of U.S. adults and the majority of persons aged 60 years and older. Diabetes is associated with a higher risk of the clinical manifestations of AD, including dementia and mild cognitive impairment. Hispanics in the United States have higher rates of diabetes, putting them at greater risk for developing Alzheimer’s. Investigators at Columbia University and SUNY Downstate Medical Center, both in New York, will examine the complex relationship between diabetes, cerebrovascular disease, and Alzheimer’s in a cohort of 200 middle-aged Hispanic participants, with either normal glucose metabolism, prediabetes, or type 2 diabetes; the subjects will be followed for 5 years with whole-brain magnetic resonance imaging and a variety of cognitive measures. The brain imaging will track AD-like functional and pathologic changes and vascular lesions.
In addition, the team will carry out molecular profiling of plasma samples collected in the same participants to identify metabolic and protein signatures that may predict clinical, pathologic, and physiologic outcomes related to Alzheimer’s and cerebrovascular disease.
In a companion study using mouse models with diabetes and Alzheimer’s pathology traits, the researchers will examine how the interaction between diabetes and Alzheimer’s pathology affects the structure and function of neural circuits important to learning and memory.
“This project is addressing two critical knowledge gaps,” Dr. Petanceska said. “The first is understanding the mechanisms by which dysregulated glucose metabolism impacts the onset and progression of pathologic changes in the course of the preclinical phase of Alzheimer’s disease; the second is understanding the molecular determinants of AD risk in Hispanics, a population with higher prevalence of diabetes and at greater risk for AD.”
The Role of Renin-Angiotensin-Endothelial Pathway in Alzheimer’s Disease
Researchers at Emory University, Atlanta, will focus on understanding the molecular mechanisms by which vascular dysfunction associated with high blood pressure affects the onset and progression of Alzheimer’s.
The research cohort comprises 160 subjects from the Emory Cardiovascular Biobank and Predictive Health Study– 80 with normal cognition and 80 with mild cognitive impairment – who will be followed for 2 years. Molecular data (genomic, epigenetic and metabolomic) combined with clinical data on the same subjects collected over 2 years, will be used to build a molecular network model of the interaction between vascular dysfunction and various Alzheimer’s disease traits.
Parallel studies in a rat model that uniquely exhibits human-like AD neuropathology will help uncover the temporal relationship between vascular dysfunction and AD and examine the potential of the molecular regulators of vascular function, such as the renin-angiotensin system, as therapeutic targets for AD. The goal is to characterize this pathway as a therapeutic target.
Metabolic Signatures Underlying Vascular Risk Factors for Alzheimer’s-Type Dementias
Teams at Duke University, Durham, N.C., and the University of Pennsylvania, Philadelphia, will carry out extensive profiling of plasma samples from 900 ADNI participants and from participants in the Duke University MURDOCK Memory and Cognitive Health Study in search for lipid metabolites that are associated with cardiovascular disease and cognitive change. These lipidomic profiles will be integrated with the vast array of clinical and other molecular data available for these participants to identify molecular signatures that may be used to differentiate among various risk-factor types of AD.
In addition, in a subset of subjects, the team will compare the lipidomic profiles between plasma and cerebrospinal fluid; this will enable the team to test hypotheses about the role of systemic vascular and metabolic factors on cognitive aging and AD.
Cerebral Amyloid Angiopathy and Mechanisms of Brain Amyloid Accumulation
Investigators at Massachusetts General Hospital, Boston, will investigate the molecular underpinnings of CAA and its impact on Alzheimer’s disease. Employing a mouse model and human subjects with CAA, the study will explore this cycle of progressive amyloid deposition and brain injury. The team’s approach combines noninvasive detection and analysis of human CAA, real-time measurement of vascular structure and physiology in living transgenic mouse models, and molecular analysis of gene expression in brain microvessels. Ultimately, the team hopes to identify candidate therapies with which could block it.
“This highly multidisciplinary investigation into how the vascular effects of amyloid at the molecular, single-blood vessel, and whole-brain levels influence the clinical disease promises to deliver new, well-characterized therapeutic targets for disease prevention,” Dr. Petanceska said.
She predicted that the wide-ranging projects of the M²OVE–AD consortium will bring invaluable understanding to an enormously important, but still unexplored, aspect of Alzheimer’s pathology.
“We hope that this large-scale team science effort will generate an in-depth understanding of how vascular and metabolic factors contribute to neurodegenerative changes that result in cognitive decline and dementia and that the data and knowledge generated by this program will be the basis for developing effective interventions for disease treatment and prevention.”
BETHESDA, MD. – Cerebral vascular dysfunction exerts a significant negative influence on cognition, doubling the risk of dementia in old age and speeding the rate of cognitive decline.
These findings have been confirmed in a number of studies, and their advancement in both research and clinical arenas continues. But studies of the vascular conditions that affect cognition remain largely observational. Intervention trials are few and limited in scope. The dearth of animal models that express compromised cerebral vascular function has made conducting basic studies feel like wheels spinning in the mud.
According to investigators who discussed the problem at the recent Alzheimer’s Disease–Related Dementias 2016 Summit, sponsored by the National Institutes of Health, the situation calls for a targeted push to better understand vascular complications and their impact on cognition and the development of dementias – and a new, 5-year NIH research program aims to do just that.
M²OVE–AD: Molecular Mechanisms of the Vascular Etiology of Alzheimer’s Disease, is a $30 million initiative that brings together more than a dozen research teams. Investigators will employ new molecular profiling technologies and big data analytics to understand how vascular dysfunction influences the development of Alzheimer’s. The teams will collaborate on five different projects, each exploring a different facet of these complex processes, according to Dr. Suzana Petanceska, program director of the neuroscience division at the National Institute on Aging, Bethesda, Md., who shared her thoughts after the meeting.
“The central goal of the consortium is to generate a deeper understanding of the molecular mechanisms linking vascular risk factors, cerebrovascular disease, and Alzheimer’s, and to generate a new big-data resource that will aid the discovery of therapeutic targets for disease treatment and prevention and molecular signatures that can be used as biomarkers for disease risk,” Dr. Petanceska said in an interview.
Following a new trend of sharing Alzheimer’s research data across public and academic domains, data generated by this program will be made rapidly available to the greater research community. “Making these complex biological data sets available and usable by researchers other than the data generators is key to accelerating the pace at which the research community can generate new knowledge and replicate new findings. The M²OVE–AD initiative builds on the open-science approach established by the Accelerating Medicines Partnership – AD Programand Alzheimer’s Disease Neuroimaging Initiative (ADNI). By coordinating the experimental and analytical approaches the research teams will maximize the usability of the data generated on these projects.”
Five complementary projects comprise the consortium:
Integrative Translational Discovery of Vascular Risk Factors in Aging and Dementia
Researchers at the Mayo Clinics in Minnesota and Florida will collaborate with those at the Icahn Institute for Genomics and Multiscale Biology, New York, to explore how molecular networks influence vascular risk in normal aging, as well as in Alzheimer’s and other dementias.
The project’s goal is to understand how gender and the Alzheimer’s disease risk factor gene ApoE4 influence the molecular processes that lead to Alzheimer’s-related cerebral amyloid angiopathy (CAA).
CAA appears to be a key player in the progression of Alzheimer’s disease. The health of small vessels of the brain is important not only in age-related cognitive decline, but also in amyloid clearance. When amyloid collects in these vessels, it may cause a potentially self-sustaining loop of vascular injury and impaired amyloid clearance, which causes more intravascular amyloid deposition, more CAA, and increasing amyloid pathology.
The team intends to use genetic and expression profiling data from human brain and bloods samples, as well as existing molecular, clinical, and pathologic data in hopes of discovering therapeutic targets. The dynamic interaction between gender, apoE4 and aging and its impact on various AD pathologic and clinical traits will be explored in an array of existing and new animal models.
“Integrating the analysis of multidimensional human data with studies in animal models will accelerate the speed with which the findings can be translated to new interventions for treatment and prevention,” Dr. Petanceska said.
Type 2 diabetes mellitus and prediabetes metabolic abnormalities affect one-third of U.S. adults and the majority of persons aged 60 years and older. Diabetes is associated with a higher risk of the clinical manifestations of AD, including dementia and mild cognitive impairment. Hispanics in the United States have higher rates of diabetes, putting them at greater risk for developing Alzheimer’s. Investigators at Columbia University and SUNY Downstate Medical Center, both in New York, will examine the complex relationship between diabetes, cerebrovascular disease, and Alzheimer’s in a cohort of 200 middle-aged Hispanic participants, with either normal glucose metabolism, prediabetes, or type 2 diabetes; the subjects will be followed for 5 years with whole-brain magnetic resonance imaging and a variety of cognitive measures. The brain imaging will track AD-like functional and pathologic changes and vascular lesions.
In addition, the team will carry out molecular profiling of plasma samples collected in the same participants to identify metabolic and protein signatures that may predict clinical, pathologic, and physiologic outcomes related to Alzheimer’s and cerebrovascular disease.
In a companion study using mouse models with diabetes and Alzheimer’s pathology traits, the researchers will examine how the interaction between diabetes and Alzheimer’s pathology affects the structure and function of neural circuits important to learning and memory.
“This project is addressing two critical knowledge gaps,” Dr. Petanceska said. “The first is understanding the mechanisms by which dysregulated glucose metabolism impacts the onset and progression of pathologic changes in the course of the preclinical phase of Alzheimer’s disease; the second is understanding the molecular determinants of AD risk in Hispanics, a population with higher prevalence of diabetes and at greater risk for AD.”
The Role of Renin-Angiotensin-Endothelial Pathway in Alzheimer’s Disease
Researchers at Emory University, Atlanta, will focus on understanding the molecular mechanisms by which vascular dysfunction associated with high blood pressure affects the onset and progression of Alzheimer’s.
The research cohort comprises 160 subjects from the Emory Cardiovascular Biobank and Predictive Health Study– 80 with normal cognition and 80 with mild cognitive impairment – who will be followed for 2 years. Molecular data (genomic, epigenetic and metabolomic) combined with clinical data on the same subjects collected over 2 years, will be used to build a molecular network model of the interaction between vascular dysfunction and various Alzheimer’s disease traits.
Parallel studies in a rat model that uniquely exhibits human-like AD neuropathology will help uncover the temporal relationship between vascular dysfunction and AD and examine the potential of the molecular regulators of vascular function, such as the renin-angiotensin system, as therapeutic targets for AD. The goal is to characterize this pathway as a therapeutic target.
Metabolic Signatures Underlying Vascular Risk Factors for Alzheimer’s-Type Dementias
Teams at Duke University, Durham, N.C., and the University of Pennsylvania, Philadelphia, will carry out extensive profiling of plasma samples from 900 ADNI participants and from participants in the Duke University MURDOCK Memory and Cognitive Health Study in search for lipid metabolites that are associated with cardiovascular disease and cognitive change. These lipidomic profiles will be integrated with the vast array of clinical and other molecular data available for these participants to identify molecular signatures that may be used to differentiate among various risk-factor types of AD.
In addition, in a subset of subjects, the team will compare the lipidomic profiles between plasma and cerebrospinal fluid; this will enable the team to test hypotheses about the role of systemic vascular and metabolic factors on cognitive aging and AD.
Cerebral Amyloid Angiopathy and Mechanisms of Brain Amyloid Accumulation
Investigators at Massachusetts General Hospital, Boston, will investigate the molecular underpinnings of CAA and its impact on Alzheimer’s disease. Employing a mouse model and human subjects with CAA, the study will explore this cycle of progressive amyloid deposition and brain injury. The team’s approach combines noninvasive detection and analysis of human CAA, real-time measurement of vascular structure and physiology in living transgenic mouse models, and molecular analysis of gene expression in brain microvessels. Ultimately, the team hopes to identify candidate therapies with which could block it.
“This highly multidisciplinary investigation into how the vascular effects of amyloid at the molecular, single-blood vessel, and whole-brain levels influence the clinical disease promises to deliver new, well-characterized therapeutic targets for disease prevention,” Dr. Petanceska said.
She predicted that the wide-ranging projects of the M²OVE–AD consortium will bring invaluable understanding to an enormously important, but still unexplored, aspect of Alzheimer’s pathology.
“We hope that this large-scale team science effort will generate an in-depth understanding of how vascular and metabolic factors contribute to neurodegenerative changes that result in cognitive decline and dementia and that the data and knowledge generated by this program will be the basis for developing effective interventions for disease treatment and prevention.”
AT ADRD 2016 SUMMIT
$30 million NIA consortium explores links between vascular health and Alzheimer’s disease
BETHESDA, MD. – Cerebral vascular dysfunction exerts a significant negative influence on cognition, doubling the risk of dementia in old age and speeding the rate of cognitive decline.
These findings have been confirmed in a number of studies, and their advancement in both research and clinical arenas continues. But studies of the vascular conditions that affect cognition remain largely observational. Intervention trials are few and limited in scope. The dearth of animal models that express compromised cerebral vascular function has made conducting basic studies feel like wheels spinning in the mud.
According to investigators who discussed the problem at the recent Alzheimer’s Disease–Related Dementias 2016 Summit, sponsored by the National Institutes of Health, the situation calls for a targeted push to better understand vascular complications and their impact on cognition and the development of dementias – and a new, 5-year NIH research program aims to do just that.
M²OVE–AD: Molecular Mechanisms of the Vascular Etiology of Alzheimer’s Disease, is a $30 million initiative that brings together more than a dozen research teams. Investigators will employ new molecular profiling technologies and big data analytics to understand how vascular dysfunction influences the development of Alzheimer’s. The teams will collaborate on five different projects, each exploring a different facet of these complex processes, according to Dr. Suzana Petanceska, program director of the neuroscience division at the National Institute on Aging, Bethesda, Md., who shared her thoughts after the meeting.
“The central goal of the consortium is to generate a deeper understanding of the molecular mechanisms linking vascular risk factors, cerebrovascular disease, and Alzheimer’s, and to generate a new big-data resource that will aid the discovery of therapeutic targets for disease treatment and prevention and molecular signatures that can be used as biomarkers for disease risk,” Dr. Petanceska said in an interview.
Following a new trend of sharing Alzheimer’s research data across public and academic domains, data generated by this program will be made rapidly available to the greater research community. “Making these complex biological data sets available and usable by researchers other than the data generators is key to accelerating the pace at which the research community can generate new knowledge and replicate new findings. The M²OVE–AD initiative builds on the open-science approach established by the Accelerating Medicines Partnership – AD Programand Alzheimer’s Disease Neuroimaging Initiative (ADNI). By coordinating the experimental and analytical approaches the research teams will maximize the usability of the data generated on these projects.”
Five complementary projects comprise the consortium:
Integrative Translational Discovery of Vascular Risk Factors in Aging and Dementia
Researchers at the Mayo Clinics in Minnesota and Florida will collaborate with those at the Icahn Institute for Genomics and Multiscale Biology, New York, to explore how molecular networks influence vascular risk in normal aging, as well as in Alzheimer’s and other dementias.
The project’s goal is to understand how gender and the Alzheimer’s disease risk factor gene ApoE4 influence the molecular processes that lead to Alzheimer’s-related cerebral amyloid angiopathy (CAA).
CAA appears to be a key player in the progression of Alzheimer’s disease. The health of small vessels of the brain is important not only in age-related cognitive decline, but also in amyloid clearance. When amyloid collects in these vessels, it may cause a potentially self-sustaining loop of vascular injury and impaired amyloid clearance, which causes more intravascular amyloid deposition, more CAA, and increasing amyloid pathology.
The team intends to use genetic and expression profiling data from human brain and bloods samples, as well as existing molecular, clinical, and pathologic data in hopes of discovering therapeutic targets. The dynamic interaction between gender, apoE4 and aging and its impact on various AD pathologic and clinical traits will be explored in an array of existing and new animal models.
“Integrating the analysis of multidimensional human data with studies in animal models will accelerate the speed with which the findings can be translated to new interventions for treatment and prevention,” Dr. Petanceska said.
Type 2 diabetes mellitus and prediabetes metabolic abnormalities affect one-third of U.S. adults and the majority of persons aged 60 years and older. Diabetes is associated with a higher risk of the clinical manifestations of AD, including dementia and mild cognitive impairment. Hispanics in the United States have higher rates of diabetes, putting them at greater risk for developing Alzheimer’s. Investigators at Columbia University and SUNY Downstate Medical Center, both in New York, will examine the complex relationship between diabetes, cerebrovascular disease, and Alzheimer’s in a cohort of 200 middle-aged Hispanic participants, with either normal glucose metabolism, prediabetes, or type 2 diabetes; the subjects will be followed for 5 years with whole-brain magnetic resonance imaging and a variety of cognitive measures. The brain imaging will track AD-like functional and pathologic changes and vascular lesions.
In addition, the team will carry out molecular profiling of plasma samples collected in the same participants to identify metabolic and protein signatures that may predict clinical, pathologic, and physiologic outcomes related to Alzheimer’s and cerebrovascular disease.
In a companion study using mouse models with diabetes and Alzheimer’s pathology traits, the researchers will examine how the interaction between diabetes and Alzheimer’s pathology affects the structure and function of neural circuits important to learning and memory.
“This project is addressing two critical knowledge gaps,” Dr. Petanceska said. “The first is understanding the mechanisms by which dysregulated glucose metabolism impacts the onset and progression of pathologic changes in the course of the preclinical phase of Alzheimer’s disease; the second is understanding the molecular determinants of AD risk in Hispanics, a population with higher prevalence of diabetes and at greater risk for AD.”
The Role of Renin-Angiotensin-Endothelial Pathway in Alzheimer’s Disease
Researchers at Emory University, Atlanta, will focus on understanding the molecular mechanisms by which vascular dysfunction associated with high blood pressure affects the onset and progression of Alzheimer’s.
The research cohort comprises 160 subjects from the Emory Cardiovascular Biobank and Predictive Health Study– 80 with normal cognition and 80 with mild cognitive impairment – who will be followed for 2 years. Molecular data (genomic, epigenetic and metabolomic) combined with clinical data on the same subjects collected over 2 years, will be used to build a molecular network model of the interaction between vascular dysfunction and various Alzheimer’s disease traits.
Parallel studies in a rat model that uniquely exhibits human-like AD neuropathology will help uncover the temporal relationship between vascular dysfunction and AD and examine the potential of the molecular regulators of vascular function, such as the renin-angiotensin system, as therapeutic targets for AD. The goal is to characterize this pathway as a therapeutic target.
Metabolic Signatures Underlying Vascular Risk Factors for Alzheimer’s-Type Dementias
Teams at Duke University, Durham, N.C., and the University of Pennsylvania, Philadelphia, will carry out extensive profiling of plasma samples from 900 ADNI participants and from participants in the Duke University MURDOCK Memory and Cognitive Health Study in search for lipid metabolites that are associated with cardiovascular disease and cognitive change. These lipidomic profiles will be integrated with the vast array of clinical and other molecular data available for these participants to identify molecular signatures that may be used to differentiate among various risk-factor types of AD.
In addition, in a subset of subjects, the team will compare the lipidomic profiles between plasma and cerebrospinal fluid; this will enable the team to test hypotheses about the role of systemic vascular and metabolic factors on cognitive aging and AD.
Cerebral Amyloid Angiopathy and Mechanisms of Brain Amyloid Accumulation
Investigators at Massachusetts General Hospital, Boston, will investigate the molecular underpinnings of CAA and its impact on Alzheimer’s disease. Employing a mouse model and human subjects with CAA, the study will explore this cycle of progressive amyloid deposition and brain injury. The team’s approach combines noninvasive detection and analysis of human CAA, real-time measurement of vascular structure and physiology in living transgenic mouse models, and molecular analysis of gene expression in brain microvessels. Ultimately, the team hopes to identify candidate therapies with which could block it.
“This highly multidisciplinary investigation into how the vascular effects of amyloid at the molecular, single-blood vessel, and whole-brain levels influence the clinical disease promises to deliver new, well-characterized therapeutic targets for disease prevention,” Dr. Petanceska said.
She predicted that the wide-ranging projects of the M²OVE–AD consortium will bring invaluable understanding to an enormously important, but still unexplored, aspect of Alzheimer’s pathology.
“We hope that this large-scale team science effort will generate an in-depth understanding of how vascular and metabolic factors contribute to neurodegenerative changes that result in cognitive decline and dementia and that the data and knowledge generated by this program will be the basis for developing effective interventions for disease treatment and prevention.”
On Twitter @Alz_Gal
BETHESDA, MD. – Cerebral vascular dysfunction exerts a significant negative influence on cognition, doubling the risk of dementia in old age and speeding the rate of cognitive decline.
These findings have been confirmed in a number of studies, and their advancement in both research and clinical arenas continues. But studies of the vascular conditions that affect cognition remain largely observational. Intervention trials are few and limited in scope. The dearth of animal models that express compromised cerebral vascular function has made conducting basic studies feel like wheels spinning in the mud.
According to investigators who discussed the problem at the recent Alzheimer’s Disease–Related Dementias 2016 Summit, sponsored by the National Institutes of Health, the situation calls for a targeted push to better understand vascular complications and their impact on cognition and the development of dementias – and a new, 5-year NIH research program aims to do just that.
M²OVE–AD: Molecular Mechanisms of the Vascular Etiology of Alzheimer’s Disease, is a $30 million initiative that brings together more than a dozen research teams. Investigators will employ new molecular profiling technologies and big data analytics to understand how vascular dysfunction influences the development of Alzheimer’s. The teams will collaborate on five different projects, each exploring a different facet of these complex processes, according to Dr. Suzana Petanceska, program director of the neuroscience division at the National Institute on Aging, Bethesda, Md., who shared her thoughts after the meeting.
“The central goal of the consortium is to generate a deeper understanding of the molecular mechanisms linking vascular risk factors, cerebrovascular disease, and Alzheimer’s, and to generate a new big-data resource that will aid the discovery of therapeutic targets for disease treatment and prevention and molecular signatures that can be used as biomarkers for disease risk,” Dr. Petanceska said in an interview.
Following a new trend of sharing Alzheimer’s research data across public and academic domains, data generated by this program will be made rapidly available to the greater research community. “Making these complex biological data sets available and usable by researchers other than the data generators is key to accelerating the pace at which the research community can generate new knowledge and replicate new findings. The M²OVE–AD initiative builds on the open-science approach established by the Accelerating Medicines Partnership – AD Programand Alzheimer’s Disease Neuroimaging Initiative (ADNI). By coordinating the experimental and analytical approaches the research teams will maximize the usability of the data generated on these projects.”
Five complementary projects comprise the consortium:
Integrative Translational Discovery of Vascular Risk Factors in Aging and Dementia
Researchers at the Mayo Clinics in Minnesota and Florida will collaborate with those at the Icahn Institute for Genomics and Multiscale Biology, New York, to explore how molecular networks influence vascular risk in normal aging, as well as in Alzheimer’s and other dementias.
The project’s goal is to understand how gender and the Alzheimer’s disease risk factor gene ApoE4 influence the molecular processes that lead to Alzheimer’s-related cerebral amyloid angiopathy (CAA).
CAA appears to be a key player in the progression of Alzheimer’s disease. The health of small vessels of the brain is important not only in age-related cognitive decline, but also in amyloid clearance. When amyloid collects in these vessels, it may cause a potentially self-sustaining loop of vascular injury and impaired amyloid clearance, which causes more intravascular amyloid deposition, more CAA, and increasing amyloid pathology.
The team intends to use genetic and expression profiling data from human brain and bloods samples, as well as existing molecular, clinical, and pathologic data in hopes of discovering therapeutic targets. The dynamic interaction between gender, apoE4 and aging and its impact on various AD pathologic and clinical traits will be explored in an array of existing and new animal models.
“Integrating the analysis of multidimensional human data with studies in animal models will accelerate the speed with which the findings can be translated to new interventions for treatment and prevention,” Dr. Petanceska said.
Type 2 diabetes mellitus and prediabetes metabolic abnormalities affect one-third of U.S. adults and the majority of persons aged 60 years and older. Diabetes is associated with a higher risk of the clinical manifestations of AD, including dementia and mild cognitive impairment. Hispanics in the United States have higher rates of diabetes, putting them at greater risk for developing Alzheimer’s. Investigators at Columbia University and SUNY Downstate Medical Center, both in New York, will examine the complex relationship between diabetes, cerebrovascular disease, and Alzheimer’s in a cohort of 200 middle-aged Hispanic participants, with either normal glucose metabolism, prediabetes, or type 2 diabetes; the subjects will be followed for 5 years with whole-brain magnetic resonance imaging and a variety of cognitive measures. The brain imaging will track AD-like functional and pathologic changes and vascular lesions.
In addition, the team will carry out molecular profiling of plasma samples collected in the same participants to identify metabolic and protein signatures that may predict clinical, pathologic, and physiologic outcomes related to Alzheimer’s and cerebrovascular disease.
In a companion study using mouse models with diabetes and Alzheimer’s pathology traits, the researchers will examine how the interaction between diabetes and Alzheimer’s pathology affects the structure and function of neural circuits important to learning and memory.
“This project is addressing two critical knowledge gaps,” Dr. Petanceska said. “The first is understanding the mechanisms by which dysregulated glucose metabolism impacts the onset and progression of pathologic changes in the course of the preclinical phase of Alzheimer’s disease; the second is understanding the molecular determinants of AD risk in Hispanics, a population with higher prevalence of diabetes and at greater risk for AD.”
The Role of Renin-Angiotensin-Endothelial Pathway in Alzheimer’s Disease
Researchers at Emory University, Atlanta, will focus on understanding the molecular mechanisms by which vascular dysfunction associated with high blood pressure affects the onset and progression of Alzheimer’s.
The research cohort comprises 160 subjects from the Emory Cardiovascular Biobank and Predictive Health Study– 80 with normal cognition and 80 with mild cognitive impairment – who will be followed for 2 years. Molecular data (genomic, epigenetic and metabolomic) combined with clinical data on the same subjects collected over 2 years, will be used to build a molecular network model of the interaction between vascular dysfunction and various Alzheimer’s disease traits.
Parallel studies in a rat model that uniquely exhibits human-like AD neuropathology will help uncover the temporal relationship between vascular dysfunction and AD and examine the potential of the molecular regulators of vascular function, such as the renin-angiotensin system, as therapeutic targets for AD. The goal is to characterize this pathway as a therapeutic target.
Metabolic Signatures Underlying Vascular Risk Factors for Alzheimer’s-Type Dementias
Teams at Duke University, Durham, N.C., and the University of Pennsylvania, Philadelphia, will carry out extensive profiling of plasma samples from 900 ADNI participants and from participants in the Duke University MURDOCK Memory and Cognitive Health Study in search for lipid metabolites that are associated with cardiovascular disease and cognitive change. These lipidomic profiles will be integrated with the vast array of clinical and other molecular data available for these participants to identify molecular signatures that may be used to differentiate among various risk-factor types of AD.
In addition, in a subset of subjects, the team will compare the lipidomic profiles between plasma and cerebrospinal fluid; this will enable the team to test hypotheses about the role of systemic vascular and metabolic factors on cognitive aging and AD.
Cerebral Amyloid Angiopathy and Mechanisms of Brain Amyloid Accumulation
Investigators at Massachusetts General Hospital, Boston, will investigate the molecular underpinnings of CAA and its impact on Alzheimer’s disease. Employing a mouse model and human subjects with CAA, the study will explore this cycle of progressive amyloid deposition and brain injury. The team’s approach combines noninvasive detection and analysis of human CAA, real-time measurement of vascular structure and physiology in living transgenic mouse models, and molecular analysis of gene expression in brain microvessels. Ultimately, the team hopes to identify candidate therapies with which could block it.
“This highly multidisciplinary investigation into how the vascular effects of amyloid at the molecular, single-blood vessel, and whole-brain levels influence the clinical disease promises to deliver new, well-characterized therapeutic targets for disease prevention,” Dr. Petanceska said.
She predicted that the wide-ranging projects of the M²OVE–AD consortium will bring invaluable understanding to an enormously important, but still unexplored, aspect of Alzheimer’s pathology.
“We hope that this large-scale team science effort will generate an in-depth understanding of how vascular and metabolic factors contribute to neurodegenerative changes that result in cognitive decline and dementia and that the data and knowledge generated by this program will be the basis for developing effective interventions for disease treatment and prevention.”
On Twitter @Alz_Gal
BETHESDA, MD. – Cerebral vascular dysfunction exerts a significant negative influence on cognition, doubling the risk of dementia in old age and speeding the rate of cognitive decline.
These findings have been confirmed in a number of studies, and their advancement in both research and clinical arenas continues. But studies of the vascular conditions that affect cognition remain largely observational. Intervention trials are few and limited in scope. The dearth of animal models that express compromised cerebral vascular function has made conducting basic studies feel like wheels spinning in the mud.
According to investigators who discussed the problem at the recent Alzheimer’s Disease–Related Dementias 2016 Summit, sponsored by the National Institutes of Health, the situation calls for a targeted push to better understand vascular complications and their impact on cognition and the development of dementias – and a new, 5-year NIH research program aims to do just that.
M²OVE–AD: Molecular Mechanisms of the Vascular Etiology of Alzheimer’s Disease, is a $30 million initiative that brings together more than a dozen research teams. Investigators will employ new molecular profiling technologies and big data analytics to understand how vascular dysfunction influences the development of Alzheimer’s. The teams will collaborate on five different projects, each exploring a different facet of these complex processes, according to Dr. Suzana Petanceska, program director of the neuroscience division at the National Institute on Aging, Bethesda, Md., who shared her thoughts after the meeting.
“The central goal of the consortium is to generate a deeper understanding of the molecular mechanisms linking vascular risk factors, cerebrovascular disease, and Alzheimer’s, and to generate a new big-data resource that will aid the discovery of therapeutic targets for disease treatment and prevention and molecular signatures that can be used as biomarkers for disease risk,” Dr. Petanceska said in an interview.
Following a new trend of sharing Alzheimer’s research data across public and academic domains, data generated by this program will be made rapidly available to the greater research community. “Making these complex biological data sets available and usable by researchers other than the data generators is key to accelerating the pace at which the research community can generate new knowledge and replicate new findings. The M²OVE–AD initiative builds on the open-science approach established by the Accelerating Medicines Partnership – AD Programand Alzheimer’s Disease Neuroimaging Initiative (ADNI). By coordinating the experimental and analytical approaches the research teams will maximize the usability of the data generated on these projects.”
Five complementary projects comprise the consortium:
Integrative Translational Discovery of Vascular Risk Factors in Aging and Dementia
Researchers at the Mayo Clinics in Minnesota and Florida will collaborate with those at the Icahn Institute for Genomics and Multiscale Biology, New York, to explore how molecular networks influence vascular risk in normal aging, as well as in Alzheimer’s and other dementias.
The project’s goal is to understand how gender and the Alzheimer’s disease risk factor gene ApoE4 influence the molecular processes that lead to Alzheimer’s-related cerebral amyloid angiopathy (CAA).
CAA appears to be a key player in the progression of Alzheimer’s disease. The health of small vessels of the brain is important not only in age-related cognitive decline, but also in amyloid clearance. When amyloid collects in these vessels, it may cause a potentially self-sustaining loop of vascular injury and impaired amyloid clearance, which causes more intravascular amyloid deposition, more CAA, and increasing amyloid pathology.
The team intends to use genetic and expression profiling data from human brain and bloods samples, as well as existing molecular, clinical, and pathologic data in hopes of discovering therapeutic targets. The dynamic interaction between gender, apoE4 and aging and its impact on various AD pathologic and clinical traits will be explored in an array of existing and new animal models.
“Integrating the analysis of multidimensional human data with studies in animal models will accelerate the speed with which the findings can be translated to new interventions for treatment and prevention,” Dr. Petanceska said.
Type 2 diabetes mellitus and prediabetes metabolic abnormalities affect one-third of U.S. adults and the majority of persons aged 60 years and older. Diabetes is associated with a higher risk of the clinical manifestations of AD, including dementia and mild cognitive impairment. Hispanics in the United States have higher rates of diabetes, putting them at greater risk for developing Alzheimer’s. Investigators at Columbia University and SUNY Downstate Medical Center, both in New York, will examine the complex relationship between diabetes, cerebrovascular disease, and Alzheimer’s in a cohort of 200 middle-aged Hispanic participants, with either normal glucose metabolism, prediabetes, or type 2 diabetes; the subjects will be followed for 5 years with whole-brain magnetic resonance imaging and a variety of cognitive measures. The brain imaging will track AD-like functional and pathologic changes and vascular lesions.
In addition, the team will carry out molecular profiling of plasma samples collected in the same participants to identify metabolic and protein signatures that may predict clinical, pathologic, and physiologic outcomes related to Alzheimer’s and cerebrovascular disease.
In a companion study using mouse models with diabetes and Alzheimer’s pathology traits, the researchers will examine how the interaction between diabetes and Alzheimer’s pathology affects the structure and function of neural circuits important to learning and memory.
“This project is addressing two critical knowledge gaps,” Dr. Petanceska said. “The first is understanding the mechanisms by which dysregulated glucose metabolism impacts the onset and progression of pathologic changes in the course of the preclinical phase of Alzheimer’s disease; the second is understanding the molecular determinants of AD risk in Hispanics, a population with higher prevalence of diabetes and at greater risk for AD.”
The Role of Renin-Angiotensin-Endothelial Pathway in Alzheimer’s Disease
Researchers at Emory University, Atlanta, will focus on understanding the molecular mechanisms by which vascular dysfunction associated with high blood pressure affects the onset and progression of Alzheimer’s.
The research cohort comprises 160 subjects from the Emory Cardiovascular Biobank and Predictive Health Study– 80 with normal cognition and 80 with mild cognitive impairment – who will be followed for 2 years. Molecular data (genomic, epigenetic and metabolomic) combined with clinical data on the same subjects collected over 2 years, will be used to build a molecular network model of the interaction between vascular dysfunction and various Alzheimer’s disease traits.
Parallel studies in a rat model that uniquely exhibits human-like AD neuropathology will help uncover the temporal relationship between vascular dysfunction and AD and examine the potential of the molecular regulators of vascular function, such as the renin-angiotensin system, as therapeutic targets for AD. The goal is to characterize this pathway as a therapeutic target.
Metabolic Signatures Underlying Vascular Risk Factors for Alzheimer’s-Type Dementias
Teams at Duke University, Durham, N.C., and the University of Pennsylvania, Philadelphia, will carry out extensive profiling of plasma samples from 900 ADNI participants and from participants in the Duke University MURDOCK Memory and Cognitive Health Study in search for lipid metabolites that are associated with cardiovascular disease and cognitive change. These lipidomic profiles will be integrated with the vast array of clinical and other molecular data available for these participants to identify molecular signatures that may be used to differentiate among various risk-factor types of AD.
In addition, in a subset of subjects, the team will compare the lipidomic profiles between plasma and cerebrospinal fluid; this will enable the team to test hypotheses about the role of systemic vascular and metabolic factors on cognitive aging and AD.
Cerebral Amyloid Angiopathy and Mechanisms of Brain Amyloid Accumulation
Investigators at Massachusetts General Hospital, Boston, will investigate the molecular underpinnings of CAA and its impact on Alzheimer’s disease. Employing a mouse model and human subjects with CAA, the study will explore this cycle of progressive amyloid deposition and brain injury. The team’s approach combines noninvasive detection and analysis of human CAA, real-time measurement of vascular structure and physiology in living transgenic mouse models, and molecular analysis of gene expression in brain microvessels. Ultimately, the team hopes to identify candidate therapies with which could block it.
“This highly multidisciplinary investigation into how the vascular effects of amyloid at the molecular, single-blood vessel, and whole-brain levels influence the clinical disease promises to deliver new, well-characterized therapeutic targets for disease prevention,” Dr. Petanceska said.
She predicted that the wide-ranging projects of the M²OVE–AD consortium will bring invaluable understanding to an enormously important, but still unexplored, aspect of Alzheimer’s pathology.
“We hope that this large-scale team science effort will generate an in-depth understanding of how vascular and metabolic factors contribute to neurodegenerative changes that result in cognitive decline and dementia and that the data and knowledge generated by this program will be the basis for developing effective interventions for disease treatment and prevention.”
On Twitter @Alz_Gal
AT ADRD 2016 SUMMIT
Premenopausal age linked to lower sexual function after gynecologic cancer surgery
INDIAN WELLS, CALIF. – Premenopausal age was associated with a greater temporary decline in sexual desire 1 month after undergoing surgery for suspected gynecologic malignancies, results from an ancillary analysis showed.
“Sexual health is an important dimension of quality of life for women with gynecologic cancer,” Dr. C. Emi Bretschneider, lead study author, said at the annual scientific meeting of the Society of Gynecologic Surgeons. “Limited data exists on the impact of surgery for treatment of gynecologic cancer on patient-reported sexual desire and interest.”
In an effort to evaluate the impact on sexual function in women undergoing surgery for presumed or known gynecologic malignancies, the researchers performed an ancillary analysis of a cohort study analyzing quality-of-life and operative outcomes in 185 women who underwent gynecologic oncology procedures at the University of North Carolina, Chapel Hill, between October 2013 and October 2014.
Study participants completed the Patient-Reported Outcomes Measurement Information System Sexual Function and Satisfaction Questionnaire (PROMIS-SFQ) at baseline and at 1, 3, and 6 months postoperatively. The questionnaire evaluates four subdomains of sexual function: global satisfaction with sex life, interest in sexual activity, lubrication, and vaginal discomfort. The researchers used student t-test and linear regression to compare mean score changes between cancer types, surgical route, menopausal status, and postoperative complications, said Dr. Bretschneider of the university’s department of obstetrics and gynecology.
Of the 281 patients initially enrolled, 185 (66%) completed the PROMIS-SFQ at baseline and at 1 month postoperatively, forming the primary cohort from which the researchers performed the analysis. Of these 185 patients, 170 (92%) completed the PROMIS-SFQ at 3 months and 174 (94%) completed the survey at 6 months postoperatively.
The average age of patients at baseline was 56 years: most (77%) were white, mean body mass index was 32.9 kg/m2, 62% were partnered, and 63% underwent minimally invasive procedures. Following surgery, 131 of the patients (71%) were diagnosed with a malignancy, most commonly uterine cancer (84%), followed by ovarian (23%), cervical (17%), and vulvar cancer (3%).
Dr. Bretschneider reported that the mean baseline sexual interest score among all study participants was 44.8. At 1 month postoperatively, the mean scores decreased a mean of 3.8 points from baseline to 41. By 3 and 6 months postoperatively, the mean sexual interest scores increased from baseline by 1.9 and 2.7 points, respectively, to 46.7 and 47.5.
Women younger than age 55 years had a greater decrease in sexual interest between baseline and 1 month postoperatively, compared with their counterparts aged 55 and older (a mean of –5.5 vs. –2.3 points, respectively; P = .02).
On multivariate analysis adjusted for cancer diagnosis, minimally invasive surgery, and cancer site, women younger than age 55 continued to have a greater decrease in sexual interest between baseline and 1 month postoperatively, compared with their counterparts aged 55 and older (a mean of –4.59 points). Additionally, women who had cancer had a greater drop in sexual desire, compared with those with benign disease (a mean of –5.6 points).
“This study offers new information on the impact of surgery on sexual function for women with gynecologic cancer,” Dr. Bretschneider said at the meeting, which was jointly sponsored by the American College of Surgeons. “The study was further strengthened by its prospective design and well-characterized, large cohort of women.” Weaknesses, she continued, include its generalizability, “which may be limited, as the study cohort was recruited from a single academic institution. Also, the small sample size for some cancer sites reduced our ability to sense cancer site as a causal agent for sexual dysfunction.”
Dr. Bretschneider reported having no financial disclosures.
INDIAN WELLS, CALIF. – Premenopausal age was associated with a greater temporary decline in sexual desire 1 month after undergoing surgery for suspected gynecologic malignancies, results from an ancillary analysis showed.
“Sexual health is an important dimension of quality of life for women with gynecologic cancer,” Dr. C. Emi Bretschneider, lead study author, said at the annual scientific meeting of the Society of Gynecologic Surgeons. “Limited data exists on the impact of surgery for treatment of gynecologic cancer on patient-reported sexual desire and interest.”
In an effort to evaluate the impact on sexual function in women undergoing surgery for presumed or known gynecologic malignancies, the researchers performed an ancillary analysis of a cohort study analyzing quality-of-life and operative outcomes in 185 women who underwent gynecologic oncology procedures at the University of North Carolina, Chapel Hill, between October 2013 and October 2014.
Study participants completed the Patient-Reported Outcomes Measurement Information System Sexual Function and Satisfaction Questionnaire (PROMIS-SFQ) at baseline and at 1, 3, and 6 months postoperatively. The questionnaire evaluates four subdomains of sexual function: global satisfaction with sex life, interest in sexual activity, lubrication, and vaginal discomfort. The researchers used student t-test and linear regression to compare mean score changes between cancer types, surgical route, menopausal status, and postoperative complications, said Dr. Bretschneider of the university’s department of obstetrics and gynecology.
Of the 281 patients initially enrolled, 185 (66%) completed the PROMIS-SFQ at baseline and at 1 month postoperatively, forming the primary cohort from which the researchers performed the analysis. Of these 185 patients, 170 (92%) completed the PROMIS-SFQ at 3 months and 174 (94%) completed the survey at 6 months postoperatively.
The average age of patients at baseline was 56 years: most (77%) were white, mean body mass index was 32.9 kg/m2, 62% were partnered, and 63% underwent minimally invasive procedures. Following surgery, 131 of the patients (71%) were diagnosed with a malignancy, most commonly uterine cancer (84%), followed by ovarian (23%), cervical (17%), and vulvar cancer (3%).
Dr. Bretschneider reported that the mean baseline sexual interest score among all study participants was 44.8. At 1 month postoperatively, the mean scores decreased a mean of 3.8 points from baseline to 41. By 3 and 6 months postoperatively, the mean sexual interest scores increased from baseline by 1.9 and 2.7 points, respectively, to 46.7 and 47.5.
Women younger than age 55 years had a greater decrease in sexual interest between baseline and 1 month postoperatively, compared with their counterparts aged 55 and older (a mean of –5.5 vs. –2.3 points, respectively; P = .02).
On multivariate analysis adjusted for cancer diagnosis, minimally invasive surgery, and cancer site, women younger than age 55 continued to have a greater decrease in sexual interest between baseline and 1 month postoperatively, compared with their counterparts aged 55 and older (a mean of –4.59 points). Additionally, women who had cancer had a greater drop in sexual desire, compared with those with benign disease (a mean of –5.6 points).
“This study offers new information on the impact of surgery on sexual function for women with gynecologic cancer,” Dr. Bretschneider said at the meeting, which was jointly sponsored by the American College of Surgeons. “The study was further strengthened by its prospective design and well-characterized, large cohort of women.” Weaknesses, she continued, include its generalizability, “which may be limited, as the study cohort was recruited from a single academic institution. Also, the small sample size for some cancer sites reduced our ability to sense cancer site as a causal agent for sexual dysfunction.”
Dr. Bretschneider reported having no financial disclosures.
INDIAN WELLS, CALIF. – Premenopausal age was associated with a greater temporary decline in sexual desire 1 month after undergoing surgery for suspected gynecologic malignancies, results from an ancillary analysis showed.
“Sexual health is an important dimension of quality of life for women with gynecologic cancer,” Dr. C. Emi Bretschneider, lead study author, said at the annual scientific meeting of the Society of Gynecologic Surgeons. “Limited data exists on the impact of surgery for treatment of gynecologic cancer on patient-reported sexual desire and interest.”
In an effort to evaluate the impact on sexual function in women undergoing surgery for presumed or known gynecologic malignancies, the researchers performed an ancillary analysis of a cohort study analyzing quality-of-life and operative outcomes in 185 women who underwent gynecologic oncology procedures at the University of North Carolina, Chapel Hill, between October 2013 and October 2014.
Study participants completed the Patient-Reported Outcomes Measurement Information System Sexual Function and Satisfaction Questionnaire (PROMIS-SFQ) at baseline and at 1, 3, and 6 months postoperatively. The questionnaire evaluates four subdomains of sexual function: global satisfaction with sex life, interest in sexual activity, lubrication, and vaginal discomfort. The researchers used student t-test and linear regression to compare mean score changes between cancer types, surgical route, menopausal status, and postoperative complications, said Dr. Bretschneider of the university’s department of obstetrics and gynecology.
Of the 281 patients initially enrolled, 185 (66%) completed the PROMIS-SFQ at baseline and at 1 month postoperatively, forming the primary cohort from which the researchers performed the analysis. Of these 185 patients, 170 (92%) completed the PROMIS-SFQ at 3 months and 174 (94%) completed the survey at 6 months postoperatively.
The average age of patients at baseline was 56 years: most (77%) were white, mean body mass index was 32.9 kg/m2, 62% were partnered, and 63% underwent minimally invasive procedures. Following surgery, 131 of the patients (71%) were diagnosed with a malignancy, most commonly uterine cancer (84%), followed by ovarian (23%), cervical (17%), and vulvar cancer (3%).
Dr. Bretschneider reported that the mean baseline sexual interest score among all study participants was 44.8. At 1 month postoperatively, the mean scores decreased a mean of 3.8 points from baseline to 41. By 3 and 6 months postoperatively, the mean sexual interest scores increased from baseline by 1.9 and 2.7 points, respectively, to 46.7 and 47.5.
Women younger than age 55 years had a greater decrease in sexual interest between baseline and 1 month postoperatively, compared with their counterparts aged 55 and older (a mean of –5.5 vs. –2.3 points, respectively; P = .02).
On multivariate analysis adjusted for cancer diagnosis, minimally invasive surgery, and cancer site, women younger than age 55 continued to have a greater decrease in sexual interest between baseline and 1 month postoperatively, compared with their counterparts aged 55 and older (a mean of –4.59 points). Additionally, women who had cancer had a greater drop in sexual desire, compared with those with benign disease (a mean of –5.6 points).
“This study offers new information on the impact of surgery on sexual function for women with gynecologic cancer,” Dr. Bretschneider said at the meeting, which was jointly sponsored by the American College of Surgeons. “The study was further strengthened by its prospective design and well-characterized, large cohort of women.” Weaknesses, she continued, include its generalizability, “which may be limited, as the study cohort was recruited from a single academic institution. Also, the small sample size for some cancer sites reduced our ability to sense cancer site as a causal agent for sexual dysfunction.”
Dr. Bretschneider reported having no financial disclosures.
AT SGS 2016
Key clinical point: Premenopausal age was associated with a greater temporary decline in sexual function following gynecologic oncology procedures.
Major finding: Women younger than age 55 years had a greater decrease in sexual interest between baseline and 1 month postoperatively, compared with their counterparts aged 55 and older (a mean of –5.5 vs. –2.3 points on the PROMIS-SFQ, respectively; P = .02).
Data source: An ancillary analysis of a cohort study analyzing quality-of-life and operative outcomes in 185 women who underwent gynecologic oncology procedures between October 2013 and October 2014.
Disclosures: Dr. Bretschneider reported having no financial disclosures.
Which nonhormonal treatments are effective for hot flashes?
Selective serotonin reuptake inhibitors (SSRIs [fluoxetine, sertraline, paroxetine]) and the selective norepinephrine reuptake inhibitor (SNRI) venlafaxine, as well as clonidine and gabapentin, reduce hot flashes by about 25% (approximately one per day) in women with and without a history of breast cancer. No studies compare medications against each other to determine a single best option (strength of recommendation [SOR]: A, systematic reviews and meta-analyses of randomized controlled trials [RCTs]). In comparison, estrogen reduces the frequency of hot flashes by about 75%, or 2.5 to 3 per day.
The phytoestrogens (soy isoflavones, red clover extract, black cohosh), vitamin E, and nonpharmacologic measures (relaxation therapy, exercise, acupuncture, homeopathy, magnet therapy) lack evidence of effectiveness (SOR: A, meta-analyses of RCTs, many of which were low quality).
EVIDENCE SUMMARY
A systematic review of 6 RCTs that evaluated SSRIs and SNRIs (fluoxetine, sertraline, paroxetine, venlafaxine) found them all to be effective for reducing hot flash frequency and symptom scores in women with previous breast cancer1 (TABLE1,2).
A 2006 meta-analysis combined the results of 7 RCTs (each evaluating a single SSRI [fluoxetine, paroxetine] or SNRI [venlafaxine]) and found that as a group, they reduced mean hot flash frequency (–1.13 hot flashes/d; 95% confidence interval [CI], –1.70 to –0.57) in women with and without breast cancer.2 No trial compared medications head to head, and the populations differed among studies, so that investigators couldn’t determine a single best agent.
Clonidine and gabapentin decrease hot flash frequency
The 2006 meta-analysis also included 10 RCTs (743 patients) that studied clonidine in women with and without a history of breast cancer, and 2 RCTs (479 patients) that evaluated gabapentin in women with breast cancer.2 Both drugs reduced mean hot flash frequency (clonidine: –0.95 hot flashes/d, 95% CI, –1.44 to –0.47 at 4 weeks and –1.63 hot flashes/d, 95% CI, –2.76 to –0.05 at 8 weeks; gabapentin: –2.05 hot flashes/d; 95% CI, −2.80 to –1.30).
Phytoestrogens: The jury is still out
A meta-analysis of 43 RCTs (4364 patients) evaluated phytoestrogens that included dietary soy, soy extracts, red clover extracts, genistein extracts, and other types of phytoestrogens.3 The data from the only 5 RCTs (300 patients) that could be combined showed no effect from red clover extract on hot flash frequency. However, another 4 individual trials that couldn’t be combined each found that extracts with high levels of the phytoestrogen genistein (>30 mg/d) did reduce frequency. Investigators reported that many of the trials were small and had a high risk of bias.
A meta-analysis of 16 RCTs (2027 patients) that assessed black cohosh found that it didn’t reduce hot flash frequency (3 RCTs, 393 patients) or symptom severity scores (4 RCTs, 357 patients).4 Investigators reported high heterogeneity and recommended further research.
Nonpharmacologic therapies and vitamin E don’t help
Systematic reviews found that relaxation therapy (4 RCTs, 281 patients), exercise (3 RCTs, 454 patients), and acupuncture (8 RCTs, 414 patients) didn’t reduce hot flashes.5-7 In another review, vitamin E (1 RCT, 105 patients), homeopathy (2 RCTs, 124 patients), and magnetic devices (1 RCT, 11 patients) also produced no benefit.1
1. Rada G, Capurro D, Pantoja T, et al. Non-hormonal interventions for hot flushes in women with a history of breast cancer. Cochrane Database Syst Rev. 2010;(9):CD004923.
2. Nelson HD, Vesco KK, Haney E, et al. Nonhormonal therapies of menopausal hot flashes: systematic review and meta-analysis. JAMA. 2006;295:2057-2071.
3. Lethaby A, Marjoribanks J, Kronenberg F, et al. Phytoestrogens for menopausal vasomotor symptoms. Cochrane Database Syst Rev. 2013;(12):CD001395.
4. Leach MJ, Moore V. Black cohosh (Cimicifuga spp.) for menopausal symptoms. Cochrane Database Syst Rev. 2012;(9):CD007244.
5. Saensak S, Vutyavanich T, Somboonporn W, et al. Relaxation for perimenopausal and postmenopausal symptoms. Cochrane Database Syst Rev. 2014;(7):CD008582.
6. Daley A, Stokes-Lampard H, Thomas A, et al. Exercise for vasomotor menopausal symptoms. Cochrane Database Syst Rev. 2014:(11):CD006108.
7. Dodin S, Blanchet C, Marc I, et al. Acupuncture for menopausal hot flashes. Cochrane Database Syst Rev. 2013;(7):CD007410.
Selective serotonin reuptake inhibitors (SSRIs [fluoxetine, sertraline, paroxetine]) and the selective norepinephrine reuptake inhibitor (SNRI) venlafaxine, as well as clonidine and gabapentin, reduce hot flashes by about 25% (approximately one per day) in women with and without a history of breast cancer. No studies compare medications against each other to determine a single best option (strength of recommendation [SOR]: A, systematic reviews and meta-analyses of randomized controlled trials [RCTs]). In comparison, estrogen reduces the frequency of hot flashes by about 75%, or 2.5 to 3 per day.
The phytoestrogens (soy isoflavones, red clover extract, black cohosh), vitamin E, and nonpharmacologic measures (relaxation therapy, exercise, acupuncture, homeopathy, magnet therapy) lack evidence of effectiveness (SOR: A, meta-analyses of RCTs, many of which were low quality).
EVIDENCE SUMMARY
A systematic review of 6 RCTs that evaluated SSRIs and SNRIs (fluoxetine, sertraline, paroxetine, venlafaxine) found them all to be effective for reducing hot flash frequency and symptom scores in women with previous breast cancer1 (TABLE1,2).
A 2006 meta-analysis combined the results of 7 RCTs (each evaluating a single SSRI [fluoxetine, paroxetine] or SNRI [venlafaxine]) and found that as a group, they reduced mean hot flash frequency (–1.13 hot flashes/d; 95% confidence interval [CI], –1.70 to –0.57) in women with and without breast cancer.2 No trial compared medications head to head, and the populations differed among studies, so that investigators couldn’t determine a single best agent.

Clonidine and gabapentin decrease hot flash frequency
The 2006 meta-analysis also included 10 RCTs (743 patients) that studied clonidine in women with and without a history of breast cancer, and 2 RCTs (479 patients) that evaluated gabapentin in women with breast cancer.2 Both drugs reduced mean hot flash frequency (clonidine: –0.95 hot flashes/d, 95% CI, –1.44 to –0.47 at 4 weeks and –1.63 hot flashes/d, 95% CI, –2.76 to –0.05 at 8 weeks; gabapentin: –2.05 hot flashes/d; 95% CI, −2.80 to –1.30).
Phytoestrogens: The jury is still out
A meta-analysis of 43 RCTs (4364 patients) evaluated phytoestrogens that included dietary soy, soy extracts, red clover extracts, genistein extracts, and other types of phytoestrogens.3 The data from the only 5 RCTs (300 patients) that could be combined showed no effect from red clover extract on hot flash frequency. However, another 4 individual trials that couldn’t be combined each found that extracts with high levels of the phytoestrogen genistein (>30 mg/d) did reduce frequency. Investigators reported that many of the trials were small and had a high risk of bias.
A meta-analysis of 16 RCTs (2027 patients) that assessed black cohosh found that it didn’t reduce hot flash frequency (3 RCTs, 393 patients) or symptom severity scores (4 RCTs, 357 patients).4 Investigators reported high heterogeneity and recommended further research.
Nonpharmacologic therapies and vitamin E don’t help
Systematic reviews found that relaxation therapy (4 RCTs, 281 patients), exercise (3 RCTs, 454 patients), and acupuncture (8 RCTs, 414 patients) didn’t reduce hot flashes.5-7 In another review, vitamin E (1 RCT, 105 patients), homeopathy (2 RCTs, 124 patients), and magnetic devices (1 RCT, 11 patients) also produced no benefit.1
Selective serotonin reuptake inhibitors (SSRIs [fluoxetine, sertraline, paroxetine]) and the selective norepinephrine reuptake inhibitor (SNRI) venlafaxine, as well as clonidine and gabapentin, reduce hot flashes by about 25% (approximately one per day) in women with and without a history of breast cancer. No studies compare medications against each other to determine a single best option (strength of recommendation [SOR]: A, systematic reviews and meta-analyses of randomized controlled trials [RCTs]). In comparison, estrogen reduces the frequency of hot flashes by about 75%, or 2.5 to 3 per day.
The phytoestrogens (soy isoflavones, red clover extract, black cohosh), vitamin E, and nonpharmacologic measures (relaxation therapy, exercise, acupuncture, homeopathy, magnet therapy) lack evidence of effectiveness (SOR: A, meta-analyses of RCTs, many of which were low quality).
EVIDENCE SUMMARY
A systematic review of 6 RCTs that evaluated SSRIs and SNRIs (fluoxetine, sertraline, paroxetine, venlafaxine) found them all to be effective for reducing hot flash frequency and symptom scores in women with previous breast cancer1 (TABLE1,2).
A 2006 meta-analysis combined the results of 7 RCTs (each evaluating a single SSRI [fluoxetine, paroxetine] or SNRI [venlafaxine]) and found that as a group, they reduced mean hot flash frequency (–1.13 hot flashes/d; 95% confidence interval [CI], –1.70 to –0.57) in women with and without breast cancer.2 No trial compared medications head to head, and the populations differed among studies, so that investigators couldn’t determine a single best agent.

Clonidine and gabapentin decrease hot flash frequency
The 2006 meta-analysis also included 10 RCTs (743 patients) that studied clonidine in women with and without a history of breast cancer, and 2 RCTs (479 patients) that evaluated gabapentin in women with breast cancer.2 Both drugs reduced mean hot flash frequency (clonidine: –0.95 hot flashes/d, 95% CI, –1.44 to –0.47 at 4 weeks and –1.63 hot flashes/d, 95% CI, –2.76 to –0.05 at 8 weeks; gabapentin: –2.05 hot flashes/d; 95% CI, −2.80 to –1.30).
Phytoestrogens: The jury is still out
A meta-analysis of 43 RCTs (4364 patients) evaluated phytoestrogens that included dietary soy, soy extracts, red clover extracts, genistein extracts, and other types of phytoestrogens.3 The data from the only 5 RCTs (300 patients) that could be combined showed no effect from red clover extract on hot flash frequency. However, another 4 individual trials that couldn’t be combined each found that extracts with high levels of the phytoestrogen genistein (>30 mg/d) did reduce frequency. Investigators reported that many of the trials were small and had a high risk of bias.
A meta-analysis of 16 RCTs (2027 patients) that assessed black cohosh found that it didn’t reduce hot flash frequency (3 RCTs, 393 patients) or symptom severity scores (4 RCTs, 357 patients).4 Investigators reported high heterogeneity and recommended further research.
Nonpharmacologic therapies and vitamin E don’t help
Systematic reviews found that relaxation therapy (4 RCTs, 281 patients), exercise (3 RCTs, 454 patients), and acupuncture (8 RCTs, 414 patients) didn’t reduce hot flashes.5-7 In another review, vitamin E (1 RCT, 105 patients), homeopathy (2 RCTs, 124 patients), and magnetic devices (1 RCT, 11 patients) also produced no benefit.1
1. Rada G, Capurro D, Pantoja T, et al. Non-hormonal interventions for hot flushes in women with a history of breast cancer. Cochrane Database Syst Rev. 2010;(9):CD004923.
2. Nelson HD, Vesco KK, Haney E, et al. Nonhormonal therapies of menopausal hot flashes: systematic review and meta-analysis. JAMA. 2006;295:2057-2071.
3. Lethaby A, Marjoribanks J, Kronenberg F, et al. Phytoestrogens for menopausal vasomotor symptoms. Cochrane Database Syst Rev. 2013;(12):CD001395.
4. Leach MJ, Moore V. Black cohosh (Cimicifuga spp.) for menopausal symptoms. Cochrane Database Syst Rev. 2012;(9):CD007244.
5. Saensak S, Vutyavanich T, Somboonporn W, et al. Relaxation for perimenopausal and postmenopausal symptoms. Cochrane Database Syst Rev. 2014;(7):CD008582.
6. Daley A, Stokes-Lampard H, Thomas A, et al. Exercise for vasomotor menopausal symptoms. Cochrane Database Syst Rev. 2014:(11):CD006108.
7. Dodin S, Blanchet C, Marc I, et al. Acupuncture for menopausal hot flashes. Cochrane Database Syst Rev. 2013;(7):CD007410.
1. Rada G, Capurro D, Pantoja T, et al. Non-hormonal interventions for hot flushes in women with a history of breast cancer. Cochrane Database Syst Rev. 2010;(9):CD004923.
2. Nelson HD, Vesco KK, Haney E, et al. Nonhormonal therapies of menopausal hot flashes: systematic review and meta-analysis. JAMA. 2006;295:2057-2071.
3. Lethaby A, Marjoribanks J, Kronenberg F, et al. Phytoestrogens for menopausal vasomotor symptoms. Cochrane Database Syst Rev. 2013;(12):CD001395.
4. Leach MJ, Moore V. Black cohosh (Cimicifuga spp.) for menopausal symptoms. Cochrane Database Syst Rev. 2012;(9):CD007244.
5. Saensak S, Vutyavanich T, Somboonporn W, et al. Relaxation for perimenopausal and postmenopausal symptoms. Cochrane Database Syst Rev. 2014;(7):CD008582.
6. Daley A, Stokes-Lampard H, Thomas A, et al. Exercise for vasomotor menopausal symptoms. Cochrane Database Syst Rev. 2014:(11):CD006108.
7. Dodin S, Blanchet C, Marc I, et al. Acupuncture for menopausal hot flashes. Cochrane Database Syst Rev. 2013;(7):CD007410.
Evidence-based answers from the Family Physicians Inquiries Network
Hepatitis B test identifies active carriers
A one-time test combining hepatitis B virus (HBV) surface antigen and HBV DNA measurement accurately distinguished inactive carriers from active cases of chronic HBV, based on data from 1,529 patients published online in the journal Hepatology.
Correct identification of active chronic HBV patients vs. inactive cases “is a crucial step in identifying patients in need of less stringent follow-up, as well as patients who may benefit from additional follow-up and earlier initiation of antiviral therapy,” wrote Dr. Jessica Liu of the Genomics Research Center, Academia Sinica, in Taipei, Taiwan, and her colleagues (Hepatology. 2016. doi: 10.1002/hep.28552).
The investigators reviewed data from patients in the REVEAL-HBV cohort, which included individuals aged 30-65 years with chronic HBV who were recruited between 1991 and 1992. All patients were free of liver cirrhosis and were seronegative for anti–hepatitis C virus and hepatitis B surface antigen (HBsAg) at baseline, and were characterized as inactive carriers or active cases based on their serologic profiles at 18 months’ follow-up. Blood was collected at study entry and every 6-12 months.
At 18 months’ follow-up, the combined test distinguished inactive carriers from active cases with a sensitivity of 71% and a specificity of 85%. The positive and negative predictive values were 83% and 74%, respectively, and the diagnostic accuracy was 78%. In addition, the one-time combination measurement predicted cirrhosis, hepatocelluar carcinoma, and HBsAg seroclearance with areas under the receiver operating characteristic curves of 0.72, 0.79, and 0.78, respectively. The combination test also yielded information about the predictability of inactive carrier status, showing that inactive carriers had a significantly lower risk of cirrhosis and hepatocelluar carcinoma; adjusted hazard ratios were 0.43 and 0.13, respectively.
“To our knowledge, this is the first study to externally validate the usage of a one-time measurement of serum HBsAg less than 1,000 IU/mL and HBV DNA less than 2,000 IU/mL,” the researchers wrote.
The study was limited by a relatively short follow-up period, but the results suggest that “this single-point strategy may provide new and complementary information useful for simplifying or tailoring management of patients with chronic hepatitis B infection,” they said.
The study was supported by the Taiwan Department of Health, Bristol-Myers Squibb, Roche Diagnostics, and Academia Sinica. One of the coauthors is employed by Roche Diagnostics.
A one-time test combining hepatitis B virus (HBV) surface antigen and HBV DNA measurement accurately distinguished inactive carriers from active cases of chronic HBV, based on data from 1,529 patients published online in the journal Hepatology.
Correct identification of active chronic HBV patients vs. inactive cases “is a crucial step in identifying patients in need of less stringent follow-up, as well as patients who may benefit from additional follow-up and earlier initiation of antiviral therapy,” wrote Dr. Jessica Liu of the Genomics Research Center, Academia Sinica, in Taipei, Taiwan, and her colleagues (Hepatology. 2016. doi: 10.1002/hep.28552).
The investigators reviewed data from patients in the REVEAL-HBV cohort, which included individuals aged 30-65 years with chronic HBV who were recruited between 1991 and 1992. All patients were free of liver cirrhosis and were seronegative for anti–hepatitis C virus and hepatitis B surface antigen (HBsAg) at baseline, and were characterized as inactive carriers or active cases based on their serologic profiles at 18 months’ follow-up. Blood was collected at study entry and every 6-12 months.
At 18 months’ follow-up, the combined test distinguished inactive carriers from active cases with a sensitivity of 71% and a specificity of 85%. The positive and negative predictive values were 83% and 74%, respectively, and the diagnostic accuracy was 78%. In addition, the one-time combination measurement predicted cirrhosis, hepatocelluar carcinoma, and HBsAg seroclearance with areas under the receiver operating characteristic curves of 0.72, 0.79, and 0.78, respectively. The combination test also yielded information about the predictability of inactive carrier status, showing that inactive carriers had a significantly lower risk of cirrhosis and hepatocelluar carcinoma; adjusted hazard ratios were 0.43 and 0.13, respectively.
“To our knowledge, this is the first study to externally validate the usage of a one-time measurement of serum HBsAg less than 1,000 IU/mL and HBV DNA less than 2,000 IU/mL,” the researchers wrote.
The study was limited by a relatively short follow-up period, but the results suggest that “this single-point strategy may provide new and complementary information useful for simplifying or tailoring management of patients with chronic hepatitis B infection,” they said.
The study was supported by the Taiwan Department of Health, Bristol-Myers Squibb, Roche Diagnostics, and Academia Sinica. One of the coauthors is employed by Roche Diagnostics.
A one-time test combining hepatitis B virus (HBV) surface antigen and HBV DNA measurement accurately distinguished inactive carriers from active cases of chronic HBV, based on data from 1,529 patients published online in the journal Hepatology.
Correct identification of active chronic HBV patients vs. inactive cases “is a crucial step in identifying patients in need of less stringent follow-up, as well as patients who may benefit from additional follow-up and earlier initiation of antiviral therapy,” wrote Dr. Jessica Liu of the Genomics Research Center, Academia Sinica, in Taipei, Taiwan, and her colleagues (Hepatology. 2016. doi: 10.1002/hep.28552).
The investigators reviewed data from patients in the REVEAL-HBV cohort, which included individuals aged 30-65 years with chronic HBV who were recruited between 1991 and 1992. All patients were free of liver cirrhosis and were seronegative for anti–hepatitis C virus and hepatitis B surface antigen (HBsAg) at baseline, and were characterized as inactive carriers or active cases based on their serologic profiles at 18 months’ follow-up. Blood was collected at study entry and every 6-12 months.
At 18 months’ follow-up, the combined test distinguished inactive carriers from active cases with a sensitivity of 71% and a specificity of 85%. The positive and negative predictive values were 83% and 74%, respectively, and the diagnostic accuracy was 78%. In addition, the one-time combination measurement predicted cirrhosis, hepatocelluar carcinoma, and HBsAg seroclearance with areas under the receiver operating characteristic curves of 0.72, 0.79, and 0.78, respectively. The combination test also yielded information about the predictability of inactive carrier status, showing that inactive carriers had a significantly lower risk of cirrhosis and hepatocelluar carcinoma; adjusted hazard ratios were 0.43 and 0.13, respectively.
“To our knowledge, this is the first study to externally validate the usage of a one-time measurement of serum HBsAg less than 1,000 IU/mL and HBV DNA less than 2,000 IU/mL,” the researchers wrote.
The study was limited by a relatively short follow-up period, but the results suggest that “this single-point strategy may provide new and complementary information useful for simplifying or tailoring management of patients with chronic hepatitis B infection,” they said.
The study was supported by the Taiwan Department of Health, Bristol-Myers Squibb, Roche Diagnostics, and Academia Sinica. One of the coauthors is employed by Roche Diagnostics.
FROM HEPATOLOGY
Key clinical point: The data confirm the predictability of a one-time test for chronic hepatitis B progression that combines hepatitis B surface antigen and hepatitis B DNA measurement.
Major finding: The one-time combined test distinguished inactive carriers from active cases with a sensitivity of 71% and specificity of 85%.
Data source: A study of 1,529 adults aged 30-65 years with chronic hepatitis B.
Disclosures: The study was supported by the Taiwan Department of Health, Bristol-Myers Squibb, Roche Diagnostics, and Academia Sinica. One of the coauthors is employed by Roche Diagnostics.