User login
IV tigecycline scores as alternative C. difficile treatment
AMSTERDAM – Intravenous tigecycline was significantly more effective than standard therapy at curing refractory Clostridium difficile infections, according to a case-control study presented at the European Society of Clinical Microbiology and Infectious Diseases annual congress.
Tigecycline effected a 76% clinical cure rate, compared with 53% for the combination regimen of intravenous metronidazole and oral vancomycin, Dr. Baltin Gergely Szabo reported. And despite the fact that those who took tigecycline had more clinically severe disease, no colectomies were required in that group, while two patients in the standard treatment arm did need the procedure.
However, tigecycline didn’t significantly improve relapse rates or mortality, noted Dr. Szabo of the St. Stephan and St. Ladislaus Hospital-Clinic, Budapest, Hungary.
He presented the results of a matched case-control study of 90 patients with severe C. difficile infections, who were treated with either of the protocols. Patients who took tigecycline were more likely to have a recurrent infection (38% vs. 29%). Thus, they were also more likely to have previously been treated with metronidazole (38% vs. 24%) and vancomycin (24% vs. 7%). Prior tigecycline use was very rare in both groups (2% vs. 0%).
Those who took tigecycline were significantly younger as well (72 vs. 78 years), and more often men (56% vs. 30%). They were more likely to be hypertensive, have chronic obstructive pulmonary disease, have cancers, be immunosuppressed, and be chronic users of corticosteroids.
However, the Charlson comorbidity index was similar between the tigecycline and standard therapy groups (4.6 vs. 5). They were also matched for ATLAS scores (mean 7.8 in each group).
Significantly more patients taking tigecycline had acquired their infections during hospitalization (64% vs. 30%). They also had a longer duration of symptoms (17 vs. 10 days).
Imaging showed more severe disease in the tigecycline group with significantly more colonic distension, mural thickening, and ascites. Tigecycline patients had also undergone significantly more colonoscopies and blood cultures.
Tigecycline was given in the hospital for 7-10 days, with a 100-mg loading dose and subsequent 50-mg daily doses. The main duration of therapy was 10 days, but that varied widely, from 2 to 22 days. It was given only as first-line treatment to 15% of patients; the rest received tigecycline as an alternative treatment, often after the combination of metronidazole/vancomycin had failed. No adverse drug reactions occurred in the group.
Clinical cure was achieved in 76% of the tigecycline group and 53% of the standard protocol group – a significant difference. The drug was associated with a decreased rate of complicated disease course (29% vs. 53%) and significantly fewer colectomies (0 vs. 2).
Rates of toxic megacolon were equal (7% each group); ileus was more frequent in the tigecycline group (11% vs. 9%), but this difference was not statistically significant.
However, tigecycline had no impact on either in-hospital or 90-day relapse, or on in-hospital mortality (15 vs. 16 deaths). At 90 days, fewer patients taking the drug had died (17 vs. 21), but that difference was not statistically significant (P = 0.52).
A multivariate analysis identified several characteristics associated with a beneficial response to tigecycline:
• Male sex.
• Being immunosuppressed.
• Chronic steroid treatment.
• Malignancy.
• Longer duration of symptoms.
• Prior C. difficile infections.
• Nosocomial onset.
• Signs of severe infection on imaging.
Dr. Szabo said these characteristics can be used to create a profile of patients who might be good candidates for the drug.
He had no relevant financial declarations.
On Twitter @Alz_Gal
AMSTERDAM – Intravenous tigecycline was significantly more effective than standard therapy at curing refractory Clostridium difficile infections, according to a case-control study presented at the European Society of Clinical Microbiology and Infectious Diseases annual congress.
Tigecycline effected a 76% clinical cure rate, compared with 53% for the combination regimen of intravenous metronidazole and oral vancomycin, Dr. Baltin Gergely Szabo reported. And despite the fact that those who took tigecycline had more clinically severe disease, no colectomies were required in that group, while two patients in the standard treatment arm did need the procedure.
However, tigecycline didn’t significantly improve relapse rates or mortality, noted Dr. Szabo of the St. Stephan and St. Ladislaus Hospital-Clinic, Budapest, Hungary.
He presented the results of a matched case-control study of 90 patients with severe C. difficile infections, who were treated with either of the protocols. Patients who took tigecycline were more likely to have a recurrent infection (38% vs. 29%). Thus, they were also more likely to have previously been treated with metronidazole (38% vs. 24%) and vancomycin (24% vs. 7%). Prior tigecycline use was very rare in both groups (2% vs. 0%).
Those who took tigecycline were significantly younger as well (72 vs. 78 years), and more often men (56% vs. 30%). They were more likely to be hypertensive, have chronic obstructive pulmonary disease, have cancers, be immunosuppressed, and be chronic users of corticosteroids.
However, the Charlson comorbidity index was similar between the tigecycline and standard therapy groups (4.6 vs. 5). They were also matched for ATLAS scores (mean 7.8 in each group).
Significantly more patients taking tigecycline had acquired their infections during hospitalization (64% vs. 30%). They also had a longer duration of symptoms (17 vs. 10 days).
Imaging showed more severe disease in the tigecycline group with significantly more colonic distension, mural thickening, and ascites. Tigecycline patients had also undergone significantly more colonoscopies and blood cultures.
Tigecycline was given in the hospital for 7-10 days, with a 100-mg loading dose and subsequent 50-mg daily doses. The main duration of therapy was 10 days, but that varied widely, from 2 to 22 days. It was given only as first-line treatment to 15% of patients; the rest received tigecycline as an alternative treatment, often after the combination of metronidazole/vancomycin had failed. No adverse drug reactions occurred in the group.
Clinical cure was achieved in 76% of the tigecycline group and 53% of the standard protocol group – a significant difference. The drug was associated with a decreased rate of complicated disease course (29% vs. 53%) and significantly fewer colectomies (0 vs. 2).
Rates of toxic megacolon were equal (7% each group); ileus was more frequent in the tigecycline group (11% vs. 9%), but this difference was not statistically significant.
However, tigecycline had no impact on either in-hospital or 90-day relapse, or on in-hospital mortality (15 vs. 16 deaths). At 90 days, fewer patients taking the drug had died (17 vs. 21), but that difference was not statistically significant (P = 0.52).
A multivariate analysis identified several characteristics associated with a beneficial response to tigecycline:
• Male sex.
• Being immunosuppressed.
• Chronic steroid treatment.
• Malignancy.
• Longer duration of symptoms.
• Prior C. difficile infections.
• Nosocomial onset.
• Signs of severe infection on imaging.
Dr. Szabo said these characteristics can be used to create a profile of patients who might be good candidates for the drug.
He had no relevant financial declarations.
On Twitter @Alz_Gal
AMSTERDAM – Intravenous tigecycline was significantly more effective than standard therapy at curing refractory Clostridium difficile infections, according to a case-control study presented at the European Society of Clinical Microbiology and Infectious Diseases annual congress.
Tigecycline effected a 76% clinical cure rate, compared with 53% for the combination regimen of intravenous metronidazole and oral vancomycin, Dr. Baltin Gergely Szabo reported. And despite the fact that those who took tigecycline had more clinically severe disease, no colectomies were required in that group, while two patients in the standard treatment arm did need the procedure.
However, tigecycline didn’t significantly improve relapse rates or mortality, noted Dr. Szabo of the St. Stephan and St. Ladislaus Hospital-Clinic, Budapest, Hungary.
He presented the results of a matched case-control study of 90 patients with severe C. difficile infections, who were treated with either of the protocols. Patients who took tigecycline were more likely to have a recurrent infection (38% vs. 29%). Thus, they were also more likely to have previously been treated with metronidazole (38% vs. 24%) and vancomycin (24% vs. 7%). Prior tigecycline use was very rare in both groups (2% vs. 0%).
Those who took tigecycline were significantly younger as well (72 vs. 78 years), and more often men (56% vs. 30%). They were more likely to be hypertensive, have chronic obstructive pulmonary disease, have cancers, be immunosuppressed, and be chronic users of corticosteroids.
However, the Charlson comorbidity index was similar between the tigecycline and standard therapy groups (4.6 vs. 5). They were also matched for ATLAS scores (mean 7.8 in each group).
Significantly more patients taking tigecycline had acquired their infections during hospitalization (64% vs. 30%). They also had a longer duration of symptoms (17 vs. 10 days).
Imaging showed more severe disease in the tigecycline group with significantly more colonic distension, mural thickening, and ascites. Tigecycline patients had also undergone significantly more colonoscopies and blood cultures.
Tigecycline was given in the hospital for 7-10 days, with a 100-mg loading dose and subsequent 50-mg daily doses. The main duration of therapy was 10 days, but that varied widely, from 2 to 22 days. It was given only as first-line treatment to 15% of patients; the rest received tigecycline as an alternative treatment, often after the combination of metronidazole/vancomycin had failed. No adverse drug reactions occurred in the group.
Clinical cure was achieved in 76% of the tigecycline group and 53% of the standard protocol group – a significant difference. The drug was associated with a decreased rate of complicated disease course (29% vs. 53%) and significantly fewer colectomies (0 vs. 2).
Rates of toxic megacolon were equal (7% each group); ileus was more frequent in the tigecycline group (11% vs. 9%), but this difference was not statistically significant.
However, tigecycline had no impact on either in-hospital or 90-day relapse, or on in-hospital mortality (15 vs. 16 deaths). At 90 days, fewer patients taking the drug had died (17 vs. 21), but that difference was not statistically significant (P = 0.52).
A multivariate analysis identified several characteristics associated with a beneficial response to tigecycline:
• Male sex.
• Being immunosuppressed.
• Chronic steroid treatment.
• Malignancy.
• Longer duration of symptoms.
• Prior C. difficile infections.
• Nosocomial onset.
• Signs of severe infection on imaging.
Dr. Szabo said these characteristics can be used to create a profile of patients who might be good candidates for the drug.
He had no relevant financial declarations.
On Twitter @Alz_Gal
AT ACCMID 2016
Key clinical point: Tigecycline was an effective therapy for patients with severe C. difficile infections.
Major finding: The drug effected a clinical cure in 76% of patients, compared with a 53% cure rate in those taking metronidazole and vancomycin.
Data source: A retrospective case-control study involving 90 patients.
Disclosures: Dr. Szabo had no relevant financial disclosures.
Sexually Transmitted Diseases
Review the PDF of the fact sheet on sexually transmitted diseases with board-relevant, easy-to-review material. This month's fact sheet offers a comprehensive review of the etiology, clinical findings, and management of common STDs.
Practice Questions
1. A 44-year-old woman presents with fever, lymphadenopathy, and headaches. She has noticed a rash on her palms and soles that is not itchy. What is the diagnosis?
a. chancroid
b. gonorrhea
c. granuloma inguinale
d. lymphogranuloma venereum
e. secondary syphilis
2. A 37-year-old man presents with dysuria and purulent discharge. What is the appropriate test for diagnosis?
a. dark field microscopy
b. Giemsa staining
c. McCoy culture
d. porphyrin test (hemin [X factor]) culture
e. Thayer-Martin medium
3. Which disease in the neonate is preventable with silver nitrate drops?
a. chancroid
b. gonorrhea
c. granuloma inguinale
d. lymphogranuloma venereum
e. syphilis
4. A 22-year-old pregnant woman develops a painless indurated ulcer on the vagina. What is the treatment of choice?
a. azithromycin
b. ceftriaxone
c. doxycycline
d. penicillin G
e. TMP-SMX
5. What sexually transmitted disease facilitates the transmission of HIV?
a. chancroid
b. gonorrhea
c. lymphogranuloma venereum
d. syphilis
e. all of the above
Answers to practice questions provided on next page
Practice Question Answers
1. A 44-year-old woman presents with fever, lymphadenopathy, and headaches. She has noticed a rash on her palms and soles that is not itchy. What is the diagnosis?
a. chancroid
b. gonorrhea
c. granuloma inguinale
d. lymphogranuloma venereum
e. secondary syphilis
2. A 37-year-old man presents with dysuria and purulent discharge. What is the appropriate test for diagnosis?
a. dark field microscopy
b. Giemsa staining
c. McCoy culture
d. porphyrin test (hemin [X factor]) culture
e. Thayer-Martin medium
3. Which disease in the neonate is preventable with silver nitrate drops?
a. chancroid
b. gonorrhea
c. granuloma inguinale
d. lymphogranuloma venereum
e. syphilis
4. A 22-year-old pregnant woman develops a painless indurated ulcer on the vagina. What is the treatment of choice?
a. azithromycin
b. ceftriaxone
c. doxycycline
d. penicillin G
e. TMP-SMX
5. What sexually transmitted disease facilitates the transmission of HIV?
a. chancroid
b. gonorrhea
c. lymphogranuloma venereum
d. syphilis
e. all of the above
Review the PDF of the fact sheet on sexually transmitted diseases with board-relevant, easy-to-review material. This month's fact sheet offers a comprehensive review of the etiology, clinical findings, and management of common STDs.
Practice Questions
1. A 44-year-old woman presents with fever, lymphadenopathy, and headaches. She has noticed a rash on her palms and soles that is not itchy. What is the diagnosis?
a. chancroid
b. gonorrhea
c. granuloma inguinale
d. lymphogranuloma venereum
e. secondary syphilis
2. A 37-year-old man presents with dysuria and purulent discharge. What is the appropriate test for diagnosis?
a. dark field microscopy
b. Giemsa staining
c. McCoy culture
d. porphyrin test (hemin [X factor]) culture
e. Thayer-Martin medium
3. Which disease in the neonate is preventable with silver nitrate drops?
a. chancroid
b. gonorrhea
c. granuloma inguinale
d. lymphogranuloma venereum
e. syphilis
4. A 22-year-old pregnant woman develops a painless indurated ulcer on the vagina. What is the treatment of choice?
a. azithromycin
b. ceftriaxone
c. doxycycline
d. penicillin G
e. TMP-SMX
5. What sexually transmitted disease facilitates the transmission of HIV?
a. chancroid
b. gonorrhea
c. lymphogranuloma venereum
d. syphilis
e. all of the above
Answers to practice questions provided on next page
Practice Question Answers
1. A 44-year-old woman presents with fever, lymphadenopathy, and headaches. She has noticed a rash on her palms and soles that is not itchy. What is the diagnosis?
a. chancroid
b. gonorrhea
c. granuloma inguinale
d. lymphogranuloma venereum
e. secondary syphilis
2. A 37-year-old man presents with dysuria and purulent discharge. What is the appropriate test for diagnosis?
a. dark field microscopy
b. Giemsa staining
c. McCoy culture
d. porphyrin test (hemin [X factor]) culture
e. Thayer-Martin medium
3. Which disease in the neonate is preventable with silver nitrate drops?
a. chancroid
b. gonorrhea
c. granuloma inguinale
d. lymphogranuloma venereum
e. syphilis
4. A 22-year-old pregnant woman develops a painless indurated ulcer on the vagina. What is the treatment of choice?
a. azithromycin
b. ceftriaxone
c. doxycycline
d. penicillin G
e. TMP-SMX
5. What sexually transmitted disease facilitates the transmission of HIV?
a. chancroid
b. gonorrhea
c. lymphogranuloma venereum
d. syphilis
e. all of the above
Review the PDF of the fact sheet on sexually transmitted diseases with board-relevant, easy-to-review material. This month's fact sheet offers a comprehensive review of the etiology, clinical findings, and management of common STDs.
Practice Questions
1. A 44-year-old woman presents with fever, lymphadenopathy, and headaches. She has noticed a rash on her palms and soles that is not itchy. What is the diagnosis?
a. chancroid
b. gonorrhea
c. granuloma inguinale
d. lymphogranuloma venereum
e. secondary syphilis
2. A 37-year-old man presents with dysuria and purulent discharge. What is the appropriate test for diagnosis?
a. dark field microscopy
b. Giemsa staining
c. McCoy culture
d. porphyrin test (hemin [X factor]) culture
e. Thayer-Martin medium
3. Which disease in the neonate is preventable with silver nitrate drops?
a. chancroid
b. gonorrhea
c. granuloma inguinale
d. lymphogranuloma venereum
e. syphilis
4. A 22-year-old pregnant woman develops a painless indurated ulcer on the vagina. What is the treatment of choice?
a. azithromycin
b. ceftriaxone
c. doxycycline
d. penicillin G
e. TMP-SMX
5. What sexually transmitted disease facilitates the transmission of HIV?
a. chancroid
b. gonorrhea
c. lymphogranuloma venereum
d. syphilis
e. all of the above
Answers to practice questions provided on next page
Practice Question Answers
1. A 44-year-old woman presents with fever, lymphadenopathy, and headaches. She has noticed a rash on her palms and soles that is not itchy. What is the diagnosis?
a. chancroid
b. gonorrhea
c. granuloma inguinale
d. lymphogranuloma venereum
e. secondary syphilis
2. A 37-year-old man presents with dysuria and purulent discharge. What is the appropriate test for diagnosis?
a. dark field microscopy
b. Giemsa staining
c. McCoy culture
d. porphyrin test (hemin [X factor]) culture
e. Thayer-Martin medium
3. Which disease in the neonate is preventable with silver nitrate drops?
a. chancroid
b. gonorrhea
c. granuloma inguinale
d. lymphogranuloma venereum
e. syphilis
4. A 22-year-old pregnant woman develops a painless indurated ulcer on the vagina. What is the treatment of choice?
a. azithromycin
b. ceftriaxone
c. doxycycline
d. penicillin G
e. TMP-SMX
5. What sexually transmitted disease facilitates the transmission of HIV?
a. chancroid
b. gonorrhea
c. lymphogranuloma venereum
d. syphilis
e. all of the above
$30 million NIA Consortium Explores Links Between Vascular Health and Alzheimer Disease
BETHESDA, MD. – Cerebral vascular dysfunction exerts a significant negative influence on cognition, doubling the risk of dementia in old age and speeding the rate of cognitive decline.
These findings have been confirmed in a number of studies, and their advancement in both research and clinical arenas continues. But studies of the vascular conditions that affect cognition remain largely observational. Intervention trials are few and limited in scope. The dearth of animal models that express compromised cerebral vascular function has made conducting basic studies feel like wheels spinning in the mud.
According to investigators who discussed the problem at the recent Alzheimer’s Disease–Related Dementias 2016 Summit, sponsored by the National Institutes of Health, the situation calls for a targeted push to better understand vascular complications and their impact on cognition and the development of dementias – and a new, 5-year NIH research program aims to do just that.
M²OVE–AD: Molecular Mechanisms of the Vascular Etiology of Alzheimer’s Disease, is a $30 million initiative that brings together more than a dozen research teams. Investigators will employ new molecular profiling technologies and big data analytics to understand how vascular dysfunction influences the development of Alzheimer’s. The teams will collaborate on five different projects, each exploring a different facet of these complex processes, according to Dr. Suzana Petanceska, program director of the neuroscience division at the National Institute on Aging, Bethesda, Md., who shared her thoughts after the meeting.
“The central goal of the consortium is to generate a deeper understanding of the molecular mechanisms linking vascular risk factors, cerebrovascular disease, and Alzheimer’s, and to generate a new big-data resource that will aid the discovery of therapeutic targets for disease treatment and prevention and molecular signatures that can be used as biomarkers for disease risk,” Dr. Petanceska said in an interview.
Following a new trend of sharing Alzheimer’s research data across public and academic domains, data generated by this program will be made rapidly available to the greater research community. “Making these complex biological data sets available and usable by researchers other than the data generators is key to accelerating the pace at which the research community can generate new knowledge and replicate new findings. The M²OVE–AD initiative builds on the open-science approach established by the Accelerating Medicines Partnership – AD Programand Alzheimer’s Disease Neuroimaging Initiative (ADNI). By coordinating the experimental and analytical approaches the research teams will maximize the usability of the data generated on these projects.”
Five complementary projects comprise the consortium:
Integrative Translational Discovery of Vascular Risk Factors in Aging and Dementia
Researchers at the Mayo Clinics in Minnesota and Florida will collaborate with those at the Icahn Institute for Genomics and Multiscale Biology, New York, to explore how molecular networks influence vascular risk in normal aging, as well as in Alzheimer’s and other dementias.
The project’s goal is to understand how gender and the Alzheimer’s disease risk factor gene ApoE4 influence the molecular processes that lead to Alzheimer’s-related cerebral amyloid angiopathy (CAA).
CAA appears to be a key player in the progression of Alzheimer’s disease. The health of small vessels of the brain is important not only in age-related cognitive decline, but also in amyloid clearance. When amyloid collects in these vessels, it may cause a potentially self-sustaining loop of vascular injury and impaired amyloid clearance, which causes more intravascular amyloid deposition, more CAA, and increasing amyloid pathology.
The team intends to use genetic and expression profiling data from human brain and bloods samples, as well as existing molecular, clinical, and pathologic data in hopes of discovering therapeutic targets. The dynamic interaction between gender, apoE4 and aging and its impact on various AD pathologic and clinical traits will be explored in an array of existing and new animal models.
“Integrating the analysis of multidimensional human data with studies in animal models will accelerate the speed with which the findings can be translated to new interventions for treatment and prevention,” Dr. Petanceska said.
Type 2 diabetes mellitus and prediabetes metabolic abnormalities affect one-third of U.S. adults and the majority of persons aged 60 years and older. Diabetes is associated with a higher risk of the clinical manifestations of AD, including dementia and mild cognitive impairment. Hispanics in the United States have higher rates of diabetes, putting them at greater risk for developing Alzheimer’s. Investigators at Columbia University and SUNY Downstate Medical Center, both in New York, will examine the complex relationship between diabetes, cerebrovascular disease, and Alzheimer’s in a cohort of 200 middle-aged Hispanic participants, with either normal glucose metabolism, prediabetes, or type 2 diabetes; the subjects will be followed for 5 years with whole-brain magnetic resonance imaging and a variety of cognitive measures. The brain imaging will track AD-like functional and pathologic changes and vascular lesions.
In addition, the team will carry out molecular profiling of plasma samples collected in the same participants to identify metabolic and protein signatures that may predict clinical, pathologic, and physiologic outcomes related to Alzheimer’s and cerebrovascular disease.
In a companion study using mouse models with diabetes and Alzheimer’s pathology traits, the researchers will examine how the interaction between diabetes and Alzheimer’s pathology affects the structure and function of neural circuits important to learning and memory.
“This project is addressing two critical knowledge gaps,” Dr. Petanceska said. “The first is understanding the mechanisms by which dysregulated glucose metabolism impacts the onset and progression of pathologic changes in the course of the preclinical phase of Alzheimer’s disease; the second is understanding the molecular determinants of AD risk in Hispanics, a population with higher prevalence of diabetes and at greater risk for AD.”
The Role of Renin-Angiotensin-Endothelial Pathway in Alzheimer’s Disease
Researchers at Emory University, Atlanta, will focus on understanding the molecular mechanisms by which vascular dysfunction associated with high blood pressure affects the onset and progression of Alzheimer’s.
The research cohort comprises 160 subjects from the Emory Cardiovascular Biobank and Predictive Health Study– 80 with normal cognition and 80 with mild cognitive impairment – who will be followed for 2 years. Molecular data (genomic, epigenetic and metabolomic) combined with clinical data on the same subjects collected over 2 years, will be used to build a molecular network model of the interaction between vascular dysfunction and various Alzheimer’s disease traits.
Parallel studies in a rat model that uniquely exhibits human-like AD neuropathology will help uncover the temporal relationship between vascular dysfunction and AD and examine the potential of the molecular regulators of vascular function, such as the renin-angiotensin system, as therapeutic targets for AD. The goal is to characterize this pathway as a therapeutic target.
Metabolic Signatures Underlying Vascular Risk Factors for Alzheimer’s-Type Dementias
Teams at Duke University, Durham, N.C., and the University of Pennsylvania, Philadelphia, will carry out extensive profiling of plasma samples from 900 ADNI participants and from participants in the Duke University MURDOCK Memory and Cognitive Health Study in search for lipid metabolites that are associated with cardiovascular disease and cognitive change. These lipidomic profiles will be integrated with the vast array of clinical and other molecular data available for these participants to identify molecular signatures that may be used to differentiate among various risk-factor types of AD.
In addition, in a subset of subjects, the team will compare the lipidomic profiles between plasma and cerebrospinal fluid; this will enable the team to test hypotheses about the role of systemic vascular and metabolic factors on cognitive aging and AD.
Cerebral Amyloid Angiopathy and Mechanisms of Brain Amyloid Accumulation
Investigators at Massachusetts General Hospital, Boston, will investigate the molecular underpinnings of CAA and its impact on Alzheimer’s disease. Employing a mouse model and human subjects with CAA, the study will explore this cycle of progressive amyloid deposition and brain injury. The team’s approach combines noninvasive detection and analysis of human CAA, real-time measurement of vascular structure and physiology in living transgenic mouse models, and molecular analysis of gene expression in brain microvessels. Ultimately, the team hopes to identify candidate therapies with which could block it.
“This highly multidisciplinary investigation into how the vascular effects of amyloid at the molecular, single-blood vessel, and whole-brain levels influence the clinical disease promises to deliver new, well-characterized therapeutic targets for disease prevention,” Dr. Petanceska said.
She predicted that the wide-ranging projects of the M²OVE–AD consortium will bring invaluable understanding to an enormously important, but still unexplored, aspect of Alzheimer’s pathology.
“We hope that this large-scale team science effort will generate an in-depth understanding of how vascular and metabolic factors contribute to neurodegenerative changes that result in cognitive decline and dementia and that the data and knowledge generated by this program will be the basis for developing effective interventions for disease treatment and prevention.”
BETHESDA, MD. – Cerebral vascular dysfunction exerts a significant negative influence on cognition, doubling the risk of dementia in old age and speeding the rate of cognitive decline.
These findings have been confirmed in a number of studies, and their advancement in both research and clinical arenas continues. But studies of the vascular conditions that affect cognition remain largely observational. Intervention trials are few and limited in scope. The dearth of animal models that express compromised cerebral vascular function has made conducting basic studies feel like wheels spinning in the mud.
According to investigators who discussed the problem at the recent Alzheimer’s Disease–Related Dementias 2016 Summit, sponsored by the National Institutes of Health, the situation calls for a targeted push to better understand vascular complications and their impact on cognition and the development of dementias – and a new, 5-year NIH research program aims to do just that.
M²OVE–AD: Molecular Mechanisms of the Vascular Etiology of Alzheimer’s Disease, is a $30 million initiative that brings together more than a dozen research teams. Investigators will employ new molecular profiling technologies and big data analytics to understand how vascular dysfunction influences the development of Alzheimer’s. The teams will collaborate on five different projects, each exploring a different facet of these complex processes, according to Dr. Suzana Petanceska, program director of the neuroscience division at the National Institute on Aging, Bethesda, Md., who shared her thoughts after the meeting.
“The central goal of the consortium is to generate a deeper understanding of the molecular mechanisms linking vascular risk factors, cerebrovascular disease, and Alzheimer’s, and to generate a new big-data resource that will aid the discovery of therapeutic targets for disease treatment and prevention and molecular signatures that can be used as biomarkers for disease risk,” Dr. Petanceska said in an interview.
Following a new trend of sharing Alzheimer’s research data across public and academic domains, data generated by this program will be made rapidly available to the greater research community. “Making these complex biological data sets available and usable by researchers other than the data generators is key to accelerating the pace at which the research community can generate new knowledge and replicate new findings. The M²OVE–AD initiative builds on the open-science approach established by the Accelerating Medicines Partnership – AD Programand Alzheimer’s Disease Neuroimaging Initiative (ADNI). By coordinating the experimental and analytical approaches the research teams will maximize the usability of the data generated on these projects.”
Five complementary projects comprise the consortium:
Integrative Translational Discovery of Vascular Risk Factors in Aging and Dementia
Researchers at the Mayo Clinics in Minnesota and Florida will collaborate with those at the Icahn Institute for Genomics and Multiscale Biology, New York, to explore how molecular networks influence vascular risk in normal aging, as well as in Alzheimer’s and other dementias.
The project’s goal is to understand how gender and the Alzheimer’s disease risk factor gene ApoE4 influence the molecular processes that lead to Alzheimer’s-related cerebral amyloid angiopathy (CAA).
CAA appears to be a key player in the progression of Alzheimer’s disease. The health of small vessels of the brain is important not only in age-related cognitive decline, but also in amyloid clearance. When amyloid collects in these vessels, it may cause a potentially self-sustaining loop of vascular injury and impaired amyloid clearance, which causes more intravascular amyloid deposition, more CAA, and increasing amyloid pathology.
The team intends to use genetic and expression profiling data from human brain and bloods samples, as well as existing molecular, clinical, and pathologic data in hopes of discovering therapeutic targets. The dynamic interaction between gender, apoE4 and aging and its impact on various AD pathologic and clinical traits will be explored in an array of existing and new animal models.
“Integrating the analysis of multidimensional human data with studies in animal models will accelerate the speed with which the findings can be translated to new interventions for treatment and prevention,” Dr. Petanceska said.
Type 2 diabetes mellitus and prediabetes metabolic abnormalities affect one-third of U.S. adults and the majority of persons aged 60 years and older. Diabetes is associated with a higher risk of the clinical manifestations of AD, including dementia and mild cognitive impairment. Hispanics in the United States have higher rates of diabetes, putting them at greater risk for developing Alzheimer’s. Investigators at Columbia University and SUNY Downstate Medical Center, both in New York, will examine the complex relationship between diabetes, cerebrovascular disease, and Alzheimer’s in a cohort of 200 middle-aged Hispanic participants, with either normal glucose metabolism, prediabetes, or type 2 diabetes; the subjects will be followed for 5 years with whole-brain magnetic resonance imaging and a variety of cognitive measures. The brain imaging will track AD-like functional and pathologic changes and vascular lesions.
In addition, the team will carry out molecular profiling of plasma samples collected in the same participants to identify metabolic and protein signatures that may predict clinical, pathologic, and physiologic outcomes related to Alzheimer’s and cerebrovascular disease.
In a companion study using mouse models with diabetes and Alzheimer’s pathology traits, the researchers will examine how the interaction between diabetes and Alzheimer’s pathology affects the structure and function of neural circuits important to learning and memory.
“This project is addressing two critical knowledge gaps,” Dr. Petanceska said. “The first is understanding the mechanisms by which dysregulated glucose metabolism impacts the onset and progression of pathologic changes in the course of the preclinical phase of Alzheimer’s disease; the second is understanding the molecular determinants of AD risk in Hispanics, a population with higher prevalence of diabetes and at greater risk for AD.”
The Role of Renin-Angiotensin-Endothelial Pathway in Alzheimer’s Disease
Researchers at Emory University, Atlanta, will focus on understanding the molecular mechanisms by which vascular dysfunction associated with high blood pressure affects the onset and progression of Alzheimer’s.
The research cohort comprises 160 subjects from the Emory Cardiovascular Biobank and Predictive Health Study– 80 with normal cognition and 80 with mild cognitive impairment – who will be followed for 2 years. Molecular data (genomic, epigenetic and metabolomic) combined with clinical data on the same subjects collected over 2 years, will be used to build a molecular network model of the interaction between vascular dysfunction and various Alzheimer’s disease traits.
Parallel studies in a rat model that uniquely exhibits human-like AD neuropathology will help uncover the temporal relationship between vascular dysfunction and AD and examine the potential of the molecular regulators of vascular function, such as the renin-angiotensin system, as therapeutic targets for AD. The goal is to characterize this pathway as a therapeutic target.
Metabolic Signatures Underlying Vascular Risk Factors for Alzheimer’s-Type Dementias
Teams at Duke University, Durham, N.C., and the University of Pennsylvania, Philadelphia, will carry out extensive profiling of plasma samples from 900 ADNI participants and from participants in the Duke University MURDOCK Memory and Cognitive Health Study in search for lipid metabolites that are associated with cardiovascular disease and cognitive change. These lipidomic profiles will be integrated with the vast array of clinical and other molecular data available for these participants to identify molecular signatures that may be used to differentiate among various risk-factor types of AD.
In addition, in a subset of subjects, the team will compare the lipidomic profiles between plasma and cerebrospinal fluid; this will enable the team to test hypotheses about the role of systemic vascular and metabolic factors on cognitive aging and AD.
Cerebral Amyloid Angiopathy and Mechanisms of Brain Amyloid Accumulation
Investigators at Massachusetts General Hospital, Boston, will investigate the molecular underpinnings of CAA and its impact on Alzheimer’s disease. Employing a mouse model and human subjects with CAA, the study will explore this cycle of progressive amyloid deposition and brain injury. The team’s approach combines noninvasive detection and analysis of human CAA, real-time measurement of vascular structure and physiology in living transgenic mouse models, and molecular analysis of gene expression in brain microvessels. Ultimately, the team hopes to identify candidate therapies with which could block it.
“This highly multidisciplinary investigation into how the vascular effects of amyloid at the molecular, single-blood vessel, and whole-brain levels influence the clinical disease promises to deliver new, well-characterized therapeutic targets for disease prevention,” Dr. Petanceska said.
She predicted that the wide-ranging projects of the M²OVE–AD consortium will bring invaluable understanding to an enormously important, but still unexplored, aspect of Alzheimer’s pathology.
“We hope that this large-scale team science effort will generate an in-depth understanding of how vascular and metabolic factors contribute to neurodegenerative changes that result in cognitive decline and dementia and that the data and knowledge generated by this program will be the basis for developing effective interventions for disease treatment and prevention.”
BETHESDA, MD. – Cerebral vascular dysfunction exerts a significant negative influence on cognition, doubling the risk of dementia in old age and speeding the rate of cognitive decline.
These findings have been confirmed in a number of studies, and their advancement in both research and clinical arenas continues. But studies of the vascular conditions that affect cognition remain largely observational. Intervention trials are few and limited in scope. The dearth of animal models that express compromised cerebral vascular function has made conducting basic studies feel like wheels spinning in the mud.
According to investigators who discussed the problem at the recent Alzheimer’s Disease–Related Dementias 2016 Summit, sponsored by the National Institutes of Health, the situation calls for a targeted push to better understand vascular complications and their impact on cognition and the development of dementias – and a new, 5-year NIH research program aims to do just that.
M²OVE–AD: Molecular Mechanisms of the Vascular Etiology of Alzheimer’s Disease, is a $30 million initiative that brings together more than a dozen research teams. Investigators will employ new molecular profiling technologies and big data analytics to understand how vascular dysfunction influences the development of Alzheimer’s. The teams will collaborate on five different projects, each exploring a different facet of these complex processes, according to Dr. Suzana Petanceska, program director of the neuroscience division at the National Institute on Aging, Bethesda, Md., who shared her thoughts after the meeting.
“The central goal of the consortium is to generate a deeper understanding of the molecular mechanisms linking vascular risk factors, cerebrovascular disease, and Alzheimer’s, and to generate a new big-data resource that will aid the discovery of therapeutic targets for disease treatment and prevention and molecular signatures that can be used as biomarkers for disease risk,” Dr. Petanceska said in an interview.
Following a new trend of sharing Alzheimer’s research data across public and academic domains, data generated by this program will be made rapidly available to the greater research community. “Making these complex biological data sets available and usable by researchers other than the data generators is key to accelerating the pace at which the research community can generate new knowledge and replicate new findings. The M²OVE–AD initiative builds on the open-science approach established by the Accelerating Medicines Partnership – AD Programand Alzheimer’s Disease Neuroimaging Initiative (ADNI). By coordinating the experimental and analytical approaches the research teams will maximize the usability of the data generated on these projects.”
Five complementary projects comprise the consortium:
Integrative Translational Discovery of Vascular Risk Factors in Aging and Dementia
Researchers at the Mayo Clinics in Minnesota and Florida will collaborate with those at the Icahn Institute for Genomics and Multiscale Biology, New York, to explore how molecular networks influence vascular risk in normal aging, as well as in Alzheimer’s and other dementias.
The project’s goal is to understand how gender and the Alzheimer’s disease risk factor gene ApoE4 influence the molecular processes that lead to Alzheimer’s-related cerebral amyloid angiopathy (CAA).
CAA appears to be a key player in the progression of Alzheimer’s disease. The health of small vessels of the brain is important not only in age-related cognitive decline, but also in amyloid clearance. When amyloid collects in these vessels, it may cause a potentially self-sustaining loop of vascular injury and impaired amyloid clearance, which causes more intravascular amyloid deposition, more CAA, and increasing amyloid pathology.
The team intends to use genetic and expression profiling data from human brain and bloods samples, as well as existing molecular, clinical, and pathologic data in hopes of discovering therapeutic targets. The dynamic interaction between gender, apoE4 and aging and its impact on various AD pathologic and clinical traits will be explored in an array of existing and new animal models.
“Integrating the analysis of multidimensional human data with studies in animal models will accelerate the speed with which the findings can be translated to new interventions for treatment and prevention,” Dr. Petanceska said.
Type 2 diabetes mellitus and prediabetes metabolic abnormalities affect one-third of U.S. adults and the majority of persons aged 60 years and older. Diabetes is associated with a higher risk of the clinical manifestations of AD, including dementia and mild cognitive impairment. Hispanics in the United States have higher rates of diabetes, putting them at greater risk for developing Alzheimer’s. Investigators at Columbia University and SUNY Downstate Medical Center, both in New York, will examine the complex relationship between diabetes, cerebrovascular disease, and Alzheimer’s in a cohort of 200 middle-aged Hispanic participants, with either normal glucose metabolism, prediabetes, or type 2 diabetes; the subjects will be followed for 5 years with whole-brain magnetic resonance imaging and a variety of cognitive measures. The brain imaging will track AD-like functional and pathologic changes and vascular lesions.
In addition, the team will carry out molecular profiling of plasma samples collected in the same participants to identify metabolic and protein signatures that may predict clinical, pathologic, and physiologic outcomes related to Alzheimer’s and cerebrovascular disease.
In a companion study using mouse models with diabetes and Alzheimer’s pathology traits, the researchers will examine how the interaction between diabetes and Alzheimer’s pathology affects the structure and function of neural circuits important to learning and memory.
“This project is addressing two critical knowledge gaps,” Dr. Petanceska said. “The first is understanding the mechanisms by which dysregulated glucose metabolism impacts the onset and progression of pathologic changes in the course of the preclinical phase of Alzheimer’s disease; the second is understanding the molecular determinants of AD risk in Hispanics, a population with higher prevalence of diabetes and at greater risk for AD.”
The Role of Renin-Angiotensin-Endothelial Pathway in Alzheimer’s Disease
Researchers at Emory University, Atlanta, will focus on understanding the molecular mechanisms by which vascular dysfunction associated with high blood pressure affects the onset and progression of Alzheimer’s.
The research cohort comprises 160 subjects from the Emory Cardiovascular Biobank and Predictive Health Study– 80 with normal cognition and 80 with mild cognitive impairment – who will be followed for 2 years. Molecular data (genomic, epigenetic and metabolomic) combined with clinical data on the same subjects collected over 2 years, will be used to build a molecular network model of the interaction between vascular dysfunction and various Alzheimer’s disease traits.
Parallel studies in a rat model that uniquely exhibits human-like AD neuropathology will help uncover the temporal relationship between vascular dysfunction and AD and examine the potential of the molecular regulators of vascular function, such as the renin-angiotensin system, as therapeutic targets for AD. The goal is to characterize this pathway as a therapeutic target.
Metabolic Signatures Underlying Vascular Risk Factors for Alzheimer’s-Type Dementias
Teams at Duke University, Durham, N.C., and the University of Pennsylvania, Philadelphia, will carry out extensive profiling of plasma samples from 900 ADNI participants and from participants in the Duke University MURDOCK Memory and Cognitive Health Study in search for lipid metabolites that are associated with cardiovascular disease and cognitive change. These lipidomic profiles will be integrated with the vast array of clinical and other molecular data available for these participants to identify molecular signatures that may be used to differentiate among various risk-factor types of AD.
In addition, in a subset of subjects, the team will compare the lipidomic profiles between plasma and cerebrospinal fluid; this will enable the team to test hypotheses about the role of systemic vascular and metabolic factors on cognitive aging and AD.
Cerebral Amyloid Angiopathy and Mechanisms of Brain Amyloid Accumulation
Investigators at Massachusetts General Hospital, Boston, will investigate the molecular underpinnings of CAA and its impact on Alzheimer’s disease. Employing a mouse model and human subjects with CAA, the study will explore this cycle of progressive amyloid deposition and brain injury. The team’s approach combines noninvasive detection and analysis of human CAA, real-time measurement of vascular structure and physiology in living transgenic mouse models, and molecular analysis of gene expression in brain microvessels. Ultimately, the team hopes to identify candidate therapies with which could block it.
“This highly multidisciplinary investigation into how the vascular effects of amyloid at the molecular, single-blood vessel, and whole-brain levels influence the clinical disease promises to deliver new, well-characterized therapeutic targets for disease prevention,” Dr. Petanceska said.
She predicted that the wide-ranging projects of the M²OVE–AD consortium will bring invaluable understanding to an enormously important, but still unexplored, aspect of Alzheimer’s pathology.
“We hope that this large-scale team science effort will generate an in-depth understanding of how vascular and metabolic factors contribute to neurodegenerative changes that result in cognitive decline and dementia and that the data and knowledge generated by this program will be the basis for developing effective interventions for disease treatment and prevention.”
AT ADRD 2016 SUMMIT
$30 million NIA consortium explores links between vascular health and Alzheimer’s disease
BETHESDA, MD. – Cerebral vascular dysfunction exerts a significant negative influence on cognition, doubling the risk of dementia in old age and speeding the rate of cognitive decline.
These findings have been confirmed in a number of studies, and their advancement in both research and clinical arenas continues. But studies of the vascular conditions that affect cognition remain largely observational. Intervention trials are few and limited in scope. The dearth of animal models that express compromised cerebral vascular function has made conducting basic studies feel like wheels spinning in the mud.
According to investigators who discussed the problem at the recent Alzheimer’s Disease–Related Dementias 2016 Summit, sponsored by the National Institutes of Health, the situation calls for a targeted push to better understand vascular complications and their impact on cognition and the development of dementias – and a new, 5-year NIH research program aims to do just that.
M²OVE–AD: Molecular Mechanisms of the Vascular Etiology of Alzheimer’s Disease, is a $30 million initiative that brings together more than a dozen research teams. Investigators will employ new molecular profiling technologies and big data analytics to understand how vascular dysfunction influences the development of Alzheimer’s. The teams will collaborate on five different projects, each exploring a different facet of these complex processes, according to Dr. Suzana Petanceska, program director of the neuroscience division at the National Institute on Aging, Bethesda, Md., who shared her thoughts after the meeting.
“The central goal of the consortium is to generate a deeper understanding of the molecular mechanisms linking vascular risk factors, cerebrovascular disease, and Alzheimer’s, and to generate a new big-data resource that will aid the discovery of therapeutic targets for disease treatment and prevention and molecular signatures that can be used as biomarkers for disease risk,” Dr. Petanceska said in an interview.
Following a new trend of sharing Alzheimer’s research data across public and academic domains, data generated by this program will be made rapidly available to the greater research community. “Making these complex biological data sets available and usable by researchers other than the data generators is key to accelerating the pace at which the research community can generate new knowledge and replicate new findings. The M²OVE–AD initiative builds on the open-science approach established by the Accelerating Medicines Partnership – AD Programand Alzheimer’s Disease Neuroimaging Initiative (ADNI). By coordinating the experimental and analytical approaches the research teams will maximize the usability of the data generated on these projects.”
Five complementary projects comprise the consortium:
Integrative Translational Discovery of Vascular Risk Factors in Aging and Dementia
Researchers at the Mayo Clinics in Minnesota and Florida will collaborate with those at the Icahn Institute for Genomics and Multiscale Biology, New York, to explore how molecular networks influence vascular risk in normal aging, as well as in Alzheimer’s and other dementias.
The project’s goal is to understand how gender and the Alzheimer’s disease risk factor gene ApoE4 influence the molecular processes that lead to Alzheimer’s-related cerebral amyloid angiopathy (CAA).
CAA appears to be a key player in the progression of Alzheimer’s disease. The health of small vessels of the brain is important not only in age-related cognitive decline, but also in amyloid clearance. When amyloid collects in these vessels, it may cause a potentially self-sustaining loop of vascular injury and impaired amyloid clearance, which causes more intravascular amyloid deposition, more CAA, and increasing amyloid pathology.
The team intends to use genetic and expression profiling data from human brain and bloods samples, as well as existing molecular, clinical, and pathologic data in hopes of discovering therapeutic targets. The dynamic interaction between gender, apoE4 and aging and its impact on various AD pathologic and clinical traits will be explored in an array of existing and new animal models.
“Integrating the analysis of multidimensional human data with studies in animal models will accelerate the speed with which the findings can be translated to new interventions for treatment and prevention,” Dr. Petanceska said.
Type 2 diabetes mellitus and prediabetes metabolic abnormalities affect one-third of U.S. adults and the majority of persons aged 60 years and older. Diabetes is associated with a higher risk of the clinical manifestations of AD, including dementia and mild cognitive impairment. Hispanics in the United States have higher rates of diabetes, putting them at greater risk for developing Alzheimer’s. Investigators at Columbia University and SUNY Downstate Medical Center, both in New York, will examine the complex relationship between diabetes, cerebrovascular disease, and Alzheimer’s in a cohort of 200 middle-aged Hispanic participants, with either normal glucose metabolism, prediabetes, or type 2 diabetes; the subjects will be followed for 5 years with whole-brain magnetic resonance imaging and a variety of cognitive measures. The brain imaging will track AD-like functional and pathologic changes and vascular lesions.
In addition, the team will carry out molecular profiling of plasma samples collected in the same participants to identify metabolic and protein signatures that may predict clinical, pathologic, and physiologic outcomes related to Alzheimer’s and cerebrovascular disease.
In a companion study using mouse models with diabetes and Alzheimer’s pathology traits, the researchers will examine how the interaction between diabetes and Alzheimer’s pathology affects the structure and function of neural circuits important to learning and memory.
“This project is addressing two critical knowledge gaps,” Dr. Petanceska said. “The first is understanding the mechanisms by which dysregulated glucose metabolism impacts the onset and progression of pathologic changes in the course of the preclinical phase of Alzheimer’s disease; the second is understanding the molecular determinants of AD risk in Hispanics, a population with higher prevalence of diabetes and at greater risk for AD.”
The Role of Renin-Angiotensin-Endothelial Pathway in Alzheimer’s Disease
Researchers at Emory University, Atlanta, will focus on understanding the molecular mechanisms by which vascular dysfunction associated with high blood pressure affects the onset and progression of Alzheimer’s.
The research cohort comprises 160 subjects from the Emory Cardiovascular Biobank and Predictive Health Study– 80 with normal cognition and 80 with mild cognitive impairment – who will be followed for 2 years. Molecular data (genomic, epigenetic and metabolomic) combined with clinical data on the same subjects collected over 2 years, will be used to build a molecular network model of the interaction between vascular dysfunction and various Alzheimer’s disease traits.
Parallel studies in a rat model that uniquely exhibits human-like AD neuropathology will help uncover the temporal relationship between vascular dysfunction and AD and examine the potential of the molecular regulators of vascular function, such as the renin-angiotensin system, as therapeutic targets for AD. The goal is to characterize this pathway as a therapeutic target.
Metabolic Signatures Underlying Vascular Risk Factors for Alzheimer’s-Type Dementias
Teams at Duke University, Durham, N.C., and the University of Pennsylvania, Philadelphia, will carry out extensive profiling of plasma samples from 900 ADNI participants and from participants in the Duke University MURDOCK Memory and Cognitive Health Study in search for lipid metabolites that are associated with cardiovascular disease and cognitive change. These lipidomic profiles will be integrated with the vast array of clinical and other molecular data available for these participants to identify molecular signatures that may be used to differentiate among various risk-factor types of AD.
In addition, in a subset of subjects, the team will compare the lipidomic profiles between plasma and cerebrospinal fluid; this will enable the team to test hypotheses about the role of systemic vascular and metabolic factors on cognitive aging and AD.
Cerebral Amyloid Angiopathy and Mechanisms of Brain Amyloid Accumulation
Investigators at Massachusetts General Hospital, Boston, will investigate the molecular underpinnings of CAA and its impact on Alzheimer’s disease. Employing a mouse model and human subjects with CAA, the study will explore this cycle of progressive amyloid deposition and brain injury. The team’s approach combines noninvasive detection and analysis of human CAA, real-time measurement of vascular structure and physiology in living transgenic mouse models, and molecular analysis of gene expression in brain microvessels. Ultimately, the team hopes to identify candidate therapies with which could block it.
“This highly multidisciplinary investigation into how the vascular effects of amyloid at the molecular, single-blood vessel, and whole-brain levels influence the clinical disease promises to deliver new, well-characterized therapeutic targets for disease prevention,” Dr. Petanceska said.
She predicted that the wide-ranging projects of the M²OVE–AD consortium will bring invaluable understanding to an enormously important, but still unexplored, aspect of Alzheimer’s pathology.
“We hope that this large-scale team science effort will generate an in-depth understanding of how vascular and metabolic factors contribute to neurodegenerative changes that result in cognitive decline and dementia and that the data and knowledge generated by this program will be the basis for developing effective interventions for disease treatment and prevention.”
On Twitter @Alz_Gal
BETHESDA, MD. – Cerebral vascular dysfunction exerts a significant negative influence on cognition, doubling the risk of dementia in old age and speeding the rate of cognitive decline.
These findings have been confirmed in a number of studies, and their advancement in both research and clinical arenas continues. But studies of the vascular conditions that affect cognition remain largely observational. Intervention trials are few and limited in scope. The dearth of animal models that express compromised cerebral vascular function has made conducting basic studies feel like wheels spinning in the mud.
According to investigators who discussed the problem at the recent Alzheimer’s Disease–Related Dementias 2016 Summit, sponsored by the National Institutes of Health, the situation calls for a targeted push to better understand vascular complications and their impact on cognition and the development of dementias – and a new, 5-year NIH research program aims to do just that.
M²OVE–AD: Molecular Mechanisms of the Vascular Etiology of Alzheimer’s Disease, is a $30 million initiative that brings together more than a dozen research teams. Investigators will employ new molecular profiling technologies and big data analytics to understand how vascular dysfunction influences the development of Alzheimer’s. The teams will collaborate on five different projects, each exploring a different facet of these complex processes, according to Dr. Suzana Petanceska, program director of the neuroscience division at the National Institute on Aging, Bethesda, Md., who shared her thoughts after the meeting.
“The central goal of the consortium is to generate a deeper understanding of the molecular mechanisms linking vascular risk factors, cerebrovascular disease, and Alzheimer’s, and to generate a new big-data resource that will aid the discovery of therapeutic targets for disease treatment and prevention and molecular signatures that can be used as biomarkers for disease risk,” Dr. Petanceska said in an interview.
Following a new trend of sharing Alzheimer’s research data across public and academic domains, data generated by this program will be made rapidly available to the greater research community. “Making these complex biological data sets available and usable by researchers other than the data generators is key to accelerating the pace at which the research community can generate new knowledge and replicate new findings. The M²OVE–AD initiative builds on the open-science approach established by the Accelerating Medicines Partnership – AD Programand Alzheimer’s Disease Neuroimaging Initiative (ADNI). By coordinating the experimental and analytical approaches the research teams will maximize the usability of the data generated on these projects.”
Five complementary projects comprise the consortium:
Integrative Translational Discovery of Vascular Risk Factors in Aging and Dementia
Researchers at the Mayo Clinics in Minnesota and Florida will collaborate with those at the Icahn Institute for Genomics and Multiscale Biology, New York, to explore how molecular networks influence vascular risk in normal aging, as well as in Alzheimer’s and other dementias.
The project’s goal is to understand how gender and the Alzheimer’s disease risk factor gene ApoE4 influence the molecular processes that lead to Alzheimer’s-related cerebral amyloid angiopathy (CAA).
CAA appears to be a key player in the progression of Alzheimer’s disease. The health of small vessels of the brain is important not only in age-related cognitive decline, but also in amyloid clearance. When amyloid collects in these vessels, it may cause a potentially self-sustaining loop of vascular injury and impaired amyloid clearance, which causes more intravascular amyloid deposition, more CAA, and increasing amyloid pathology.
The team intends to use genetic and expression profiling data from human brain and bloods samples, as well as existing molecular, clinical, and pathologic data in hopes of discovering therapeutic targets. The dynamic interaction between gender, apoE4 and aging and its impact on various AD pathologic and clinical traits will be explored in an array of existing and new animal models.
“Integrating the analysis of multidimensional human data with studies in animal models will accelerate the speed with which the findings can be translated to new interventions for treatment and prevention,” Dr. Petanceska said.
Type 2 diabetes mellitus and prediabetes metabolic abnormalities affect one-third of U.S. adults and the majority of persons aged 60 years and older. Diabetes is associated with a higher risk of the clinical manifestations of AD, including dementia and mild cognitive impairment. Hispanics in the United States have higher rates of diabetes, putting them at greater risk for developing Alzheimer’s. Investigators at Columbia University and SUNY Downstate Medical Center, both in New York, will examine the complex relationship between diabetes, cerebrovascular disease, and Alzheimer’s in a cohort of 200 middle-aged Hispanic participants, with either normal glucose metabolism, prediabetes, or type 2 diabetes; the subjects will be followed for 5 years with whole-brain magnetic resonance imaging and a variety of cognitive measures. The brain imaging will track AD-like functional and pathologic changes and vascular lesions.
In addition, the team will carry out molecular profiling of plasma samples collected in the same participants to identify metabolic and protein signatures that may predict clinical, pathologic, and physiologic outcomes related to Alzheimer’s and cerebrovascular disease.
In a companion study using mouse models with diabetes and Alzheimer’s pathology traits, the researchers will examine how the interaction between diabetes and Alzheimer’s pathology affects the structure and function of neural circuits important to learning and memory.
“This project is addressing two critical knowledge gaps,” Dr. Petanceska said. “The first is understanding the mechanisms by which dysregulated glucose metabolism impacts the onset and progression of pathologic changes in the course of the preclinical phase of Alzheimer’s disease; the second is understanding the molecular determinants of AD risk in Hispanics, a population with higher prevalence of diabetes and at greater risk for AD.”
The Role of Renin-Angiotensin-Endothelial Pathway in Alzheimer’s Disease
Researchers at Emory University, Atlanta, will focus on understanding the molecular mechanisms by which vascular dysfunction associated with high blood pressure affects the onset and progression of Alzheimer’s.
The research cohort comprises 160 subjects from the Emory Cardiovascular Biobank and Predictive Health Study– 80 with normal cognition and 80 with mild cognitive impairment – who will be followed for 2 years. Molecular data (genomic, epigenetic and metabolomic) combined with clinical data on the same subjects collected over 2 years, will be used to build a molecular network model of the interaction between vascular dysfunction and various Alzheimer’s disease traits.
Parallel studies in a rat model that uniquely exhibits human-like AD neuropathology will help uncover the temporal relationship between vascular dysfunction and AD and examine the potential of the molecular regulators of vascular function, such as the renin-angiotensin system, as therapeutic targets for AD. The goal is to characterize this pathway as a therapeutic target.
Metabolic Signatures Underlying Vascular Risk Factors for Alzheimer’s-Type Dementias
Teams at Duke University, Durham, N.C., and the University of Pennsylvania, Philadelphia, will carry out extensive profiling of plasma samples from 900 ADNI participants and from participants in the Duke University MURDOCK Memory and Cognitive Health Study in search for lipid metabolites that are associated with cardiovascular disease and cognitive change. These lipidomic profiles will be integrated with the vast array of clinical and other molecular data available for these participants to identify molecular signatures that may be used to differentiate among various risk-factor types of AD.
In addition, in a subset of subjects, the team will compare the lipidomic profiles between plasma and cerebrospinal fluid; this will enable the team to test hypotheses about the role of systemic vascular and metabolic factors on cognitive aging and AD.
Cerebral Amyloid Angiopathy and Mechanisms of Brain Amyloid Accumulation
Investigators at Massachusetts General Hospital, Boston, will investigate the molecular underpinnings of CAA and its impact on Alzheimer’s disease. Employing a mouse model and human subjects with CAA, the study will explore this cycle of progressive amyloid deposition and brain injury. The team’s approach combines noninvasive detection and analysis of human CAA, real-time measurement of vascular structure and physiology in living transgenic mouse models, and molecular analysis of gene expression in brain microvessels. Ultimately, the team hopes to identify candidate therapies with which could block it.
“This highly multidisciplinary investigation into how the vascular effects of amyloid at the molecular, single-blood vessel, and whole-brain levels influence the clinical disease promises to deliver new, well-characterized therapeutic targets for disease prevention,” Dr. Petanceska said.
She predicted that the wide-ranging projects of the M²OVE–AD consortium will bring invaluable understanding to an enormously important, but still unexplored, aspect of Alzheimer’s pathology.
“We hope that this large-scale team science effort will generate an in-depth understanding of how vascular and metabolic factors contribute to neurodegenerative changes that result in cognitive decline and dementia and that the data and knowledge generated by this program will be the basis for developing effective interventions for disease treatment and prevention.”
On Twitter @Alz_Gal
BETHESDA, MD. – Cerebral vascular dysfunction exerts a significant negative influence on cognition, doubling the risk of dementia in old age and speeding the rate of cognitive decline.
These findings have been confirmed in a number of studies, and their advancement in both research and clinical arenas continues. But studies of the vascular conditions that affect cognition remain largely observational. Intervention trials are few and limited in scope. The dearth of animal models that express compromised cerebral vascular function has made conducting basic studies feel like wheels spinning in the mud.
According to investigators who discussed the problem at the recent Alzheimer’s Disease–Related Dementias 2016 Summit, sponsored by the National Institutes of Health, the situation calls for a targeted push to better understand vascular complications and their impact on cognition and the development of dementias – and a new, 5-year NIH research program aims to do just that.
M²OVE–AD: Molecular Mechanisms of the Vascular Etiology of Alzheimer’s Disease, is a $30 million initiative that brings together more than a dozen research teams. Investigators will employ new molecular profiling technologies and big data analytics to understand how vascular dysfunction influences the development of Alzheimer’s. The teams will collaborate on five different projects, each exploring a different facet of these complex processes, according to Dr. Suzana Petanceska, program director of the neuroscience division at the National Institute on Aging, Bethesda, Md., who shared her thoughts after the meeting.
“The central goal of the consortium is to generate a deeper understanding of the molecular mechanisms linking vascular risk factors, cerebrovascular disease, and Alzheimer’s, and to generate a new big-data resource that will aid the discovery of therapeutic targets for disease treatment and prevention and molecular signatures that can be used as biomarkers for disease risk,” Dr. Petanceska said in an interview.
Following a new trend of sharing Alzheimer’s research data across public and academic domains, data generated by this program will be made rapidly available to the greater research community. “Making these complex biological data sets available and usable by researchers other than the data generators is key to accelerating the pace at which the research community can generate new knowledge and replicate new findings. The M²OVE–AD initiative builds on the open-science approach established by the Accelerating Medicines Partnership – AD Programand Alzheimer’s Disease Neuroimaging Initiative (ADNI). By coordinating the experimental and analytical approaches the research teams will maximize the usability of the data generated on these projects.”
Five complementary projects comprise the consortium:
Integrative Translational Discovery of Vascular Risk Factors in Aging and Dementia
Researchers at the Mayo Clinics in Minnesota and Florida will collaborate with those at the Icahn Institute for Genomics and Multiscale Biology, New York, to explore how molecular networks influence vascular risk in normal aging, as well as in Alzheimer’s and other dementias.
The project’s goal is to understand how gender and the Alzheimer’s disease risk factor gene ApoE4 influence the molecular processes that lead to Alzheimer’s-related cerebral amyloid angiopathy (CAA).
CAA appears to be a key player in the progression of Alzheimer’s disease. The health of small vessels of the brain is important not only in age-related cognitive decline, but also in amyloid clearance. When amyloid collects in these vessels, it may cause a potentially self-sustaining loop of vascular injury and impaired amyloid clearance, which causes more intravascular amyloid deposition, more CAA, and increasing amyloid pathology.
The team intends to use genetic and expression profiling data from human brain and bloods samples, as well as existing molecular, clinical, and pathologic data in hopes of discovering therapeutic targets. The dynamic interaction between gender, apoE4 and aging and its impact on various AD pathologic and clinical traits will be explored in an array of existing and new animal models.
“Integrating the analysis of multidimensional human data with studies in animal models will accelerate the speed with which the findings can be translated to new interventions for treatment and prevention,” Dr. Petanceska said.
Type 2 diabetes mellitus and prediabetes metabolic abnormalities affect one-third of U.S. adults and the majority of persons aged 60 years and older. Diabetes is associated with a higher risk of the clinical manifestations of AD, including dementia and mild cognitive impairment. Hispanics in the United States have higher rates of diabetes, putting them at greater risk for developing Alzheimer’s. Investigators at Columbia University and SUNY Downstate Medical Center, both in New York, will examine the complex relationship between diabetes, cerebrovascular disease, and Alzheimer’s in a cohort of 200 middle-aged Hispanic participants, with either normal glucose metabolism, prediabetes, or type 2 diabetes; the subjects will be followed for 5 years with whole-brain magnetic resonance imaging and a variety of cognitive measures. The brain imaging will track AD-like functional and pathologic changes and vascular lesions.
In addition, the team will carry out molecular profiling of plasma samples collected in the same participants to identify metabolic and protein signatures that may predict clinical, pathologic, and physiologic outcomes related to Alzheimer’s and cerebrovascular disease.
In a companion study using mouse models with diabetes and Alzheimer’s pathology traits, the researchers will examine how the interaction between diabetes and Alzheimer’s pathology affects the structure and function of neural circuits important to learning and memory.
“This project is addressing two critical knowledge gaps,” Dr. Petanceska said. “The first is understanding the mechanisms by which dysregulated glucose metabolism impacts the onset and progression of pathologic changes in the course of the preclinical phase of Alzheimer’s disease; the second is understanding the molecular determinants of AD risk in Hispanics, a population with higher prevalence of diabetes and at greater risk for AD.”
The Role of Renin-Angiotensin-Endothelial Pathway in Alzheimer’s Disease
Researchers at Emory University, Atlanta, will focus on understanding the molecular mechanisms by which vascular dysfunction associated with high blood pressure affects the onset and progression of Alzheimer’s.
The research cohort comprises 160 subjects from the Emory Cardiovascular Biobank and Predictive Health Study– 80 with normal cognition and 80 with mild cognitive impairment – who will be followed for 2 years. Molecular data (genomic, epigenetic and metabolomic) combined with clinical data on the same subjects collected over 2 years, will be used to build a molecular network model of the interaction between vascular dysfunction and various Alzheimer’s disease traits.
Parallel studies in a rat model that uniquely exhibits human-like AD neuropathology will help uncover the temporal relationship between vascular dysfunction and AD and examine the potential of the molecular regulators of vascular function, such as the renin-angiotensin system, as therapeutic targets for AD. The goal is to characterize this pathway as a therapeutic target.
Metabolic Signatures Underlying Vascular Risk Factors for Alzheimer’s-Type Dementias
Teams at Duke University, Durham, N.C., and the University of Pennsylvania, Philadelphia, will carry out extensive profiling of plasma samples from 900 ADNI participants and from participants in the Duke University MURDOCK Memory and Cognitive Health Study in search for lipid metabolites that are associated with cardiovascular disease and cognitive change. These lipidomic profiles will be integrated with the vast array of clinical and other molecular data available for these participants to identify molecular signatures that may be used to differentiate among various risk-factor types of AD.
In addition, in a subset of subjects, the team will compare the lipidomic profiles between plasma and cerebrospinal fluid; this will enable the team to test hypotheses about the role of systemic vascular and metabolic factors on cognitive aging and AD.
Cerebral Amyloid Angiopathy and Mechanisms of Brain Amyloid Accumulation
Investigators at Massachusetts General Hospital, Boston, will investigate the molecular underpinnings of CAA and its impact on Alzheimer’s disease. Employing a mouse model and human subjects with CAA, the study will explore this cycle of progressive amyloid deposition and brain injury. The team’s approach combines noninvasive detection and analysis of human CAA, real-time measurement of vascular structure and physiology in living transgenic mouse models, and molecular analysis of gene expression in brain microvessels. Ultimately, the team hopes to identify candidate therapies with which could block it.
“This highly multidisciplinary investigation into how the vascular effects of amyloid at the molecular, single-blood vessel, and whole-brain levels influence the clinical disease promises to deliver new, well-characterized therapeutic targets for disease prevention,” Dr. Petanceska said.
She predicted that the wide-ranging projects of the M²OVE–AD consortium will bring invaluable understanding to an enormously important, but still unexplored, aspect of Alzheimer’s pathology.
“We hope that this large-scale team science effort will generate an in-depth understanding of how vascular and metabolic factors contribute to neurodegenerative changes that result in cognitive decline and dementia and that the data and knowledge generated by this program will be the basis for developing effective interventions for disease treatment and prevention.”
On Twitter @Alz_Gal
AT ADRD 2016 SUMMIT
Premenopausal age linked to lower sexual function after gynecologic cancer surgery
INDIAN WELLS, CALIF. – Premenopausal age was associated with a greater temporary decline in sexual desire 1 month after undergoing surgery for suspected gynecologic malignancies, results from an ancillary analysis showed.
“Sexual health is an important dimension of quality of life for women with gynecologic cancer,” Dr. C. Emi Bretschneider, lead study author, said at the annual scientific meeting of the Society of Gynecologic Surgeons. “Limited data exists on the impact of surgery for treatment of gynecologic cancer on patient-reported sexual desire and interest.”
In an effort to evaluate the impact on sexual function in women undergoing surgery for presumed or known gynecologic malignancies, the researchers performed an ancillary analysis of a cohort study analyzing quality-of-life and operative outcomes in 185 women who underwent gynecologic oncology procedures at the University of North Carolina, Chapel Hill, between October 2013 and October 2014.
Study participants completed the Patient-Reported Outcomes Measurement Information System Sexual Function and Satisfaction Questionnaire (PROMIS-SFQ) at baseline and at 1, 3, and 6 months postoperatively. The questionnaire evaluates four subdomains of sexual function: global satisfaction with sex life, interest in sexual activity, lubrication, and vaginal discomfort. The researchers used student t-test and linear regression to compare mean score changes between cancer types, surgical route, menopausal status, and postoperative complications, said Dr. Bretschneider of the university’s department of obstetrics and gynecology.
Of the 281 patients initially enrolled, 185 (66%) completed the PROMIS-SFQ at baseline and at 1 month postoperatively, forming the primary cohort from which the researchers performed the analysis. Of these 185 patients, 170 (92%) completed the PROMIS-SFQ at 3 months and 174 (94%) completed the survey at 6 months postoperatively.
The average age of patients at baseline was 56 years: most (77%) were white, mean body mass index was 32.9 kg/m2, 62% were partnered, and 63% underwent minimally invasive procedures. Following surgery, 131 of the patients (71%) were diagnosed with a malignancy, most commonly uterine cancer (84%), followed by ovarian (23%), cervical (17%), and vulvar cancer (3%).
Dr. Bretschneider reported that the mean baseline sexual interest score among all study participants was 44.8. At 1 month postoperatively, the mean scores decreased a mean of 3.8 points from baseline to 41. By 3 and 6 months postoperatively, the mean sexual interest scores increased from baseline by 1.9 and 2.7 points, respectively, to 46.7 and 47.5.
Women younger than age 55 years had a greater decrease in sexual interest between baseline and 1 month postoperatively, compared with their counterparts aged 55 and older (a mean of –5.5 vs. –2.3 points, respectively; P = .02).
On multivariate analysis adjusted for cancer diagnosis, minimally invasive surgery, and cancer site, women younger than age 55 continued to have a greater decrease in sexual interest between baseline and 1 month postoperatively, compared with their counterparts aged 55 and older (a mean of –4.59 points). Additionally, women who had cancer had a greater drop in sexual desire, compared with those with benign disease (a mean of –5.6 points).
“This study offers new information on the impact of surgery on sexual function for women with gynecologic cancer,” Dr. Bretschneider said at the meeting, which was jointly sponsored by the American College of Surgeons. “The study was further strengthened by its prospective design and well-characterized, large cohort of women.” Weaknesses, she continued, include its generalizability, “which may be limited, as the study cohort was recruited from a single academic institution. Also, the small sample size for some cancer sites reduced our ability to sense cancer site as a causal agent for sexual dysfunction.”
Dr. Bretschneider reported having no financial disclosures.
INDIAN WELLS, CALIF. – Premenopausal age was associated with a greater temporary decline in sexual desire 1 month after undergoing surgery for suspected gynecologic malignancies, results from an ancillary analysis showed.
“Sexual health is an important dimension of quality of life for women with gynecologic cancer,” Dr. C. Emi Bretschneider, lead study author, said at the annual scientific meeting of the Society of Gynecologic Surgeons. “Limited data exists on the impact of surgery for treatment of gynecologic cancer on patient-reported sexual desire and interest.”
In an effort to evaluate the impact on sexual function in women undergoing surgery for presumed or known gynecologic malignancies, the researchers performed an ancillary analysis of a cohort study analyzing quality-of-life and operative outcomes in 185 women who underwent gynecologic oncology procedures at the University of North Carolina, Chapel Hill, between October 2013 and October 2014.
Study participants completed the Patient-Reported Outcomes Measurement Information System Sexual Function and Satisfaction Questionnaire (PROMIS-SFQ) at baseline and at 1, 3, and 6 months postoperatively. The questionnaire evaluates four subdomains of sexual function: global satisfaction with sex life, interest in sexual activity, lubrication, and vaginal discomfort. The researchers used student t-test and linear regression to compare mean score changes between cancer types, surgical route, menopausal status, and postoperative complications, said Dr. Bretschneider of the university’s department of obstetrics and gynecology.
Of the 281 patients initially enrolled, 185 (66%) completed the PROMIS-SFQ at baseline and at 1 month postoperatively, forming the primary cohort from which the researchers performed the analysis. Of these 185 patients, 170 (92%) completed the PROMIS-SFQ at 3 months and 174 (94%) completed the survey at 6 months postoperatively.
The average age of patients at baseline was 56 years: most (77%) were white, mean body mass index was 32.9 kg/m2, 62% were partnered, and 63% underwent minimally invasive procedures. Following surgery, 131 of the patients (71%) were diagnosed with a malignancy, most commonly uterine cancer (84%), followed by ovarian (23%), cervical (17%), and vulvar cancer (3%).
Dr. Bretschneider reported that the mean baseline sexual interest score among all study participants was 44.8. At 1 month postoperatively, the mean scores decreased a mean of 3.8 points from baseline to 41. By 3 and 6 months postoperatively, the mean sexual interest scores increased from baseline by 1.9 and 2.7 points, respectively, to 46.7 and 47.5.
Women younger than age 55 years had a greater decrease in sexual interest between baseline and 1 month postoperatively, compared with their counterparts aged 55 and older (a mean of –5.5 vs. –2.3 points, respectively; P = .02).
On multivariate analysis adjusted for cancer diagnosis, minimally invasive surgery, and cancer site, women younger than age 55 continued to have a greater decrease in sexual interest between baseline and 1 month postoperatively, compared with their counterparts aged 55 and older (a mean of –4.59 points). Additionally, women who had cancer had a greater drop in sexual desire, compared with those with benign disease (a mean of –5.6 points).
“This study offers new information on the impact of surgery on sexual function for women with gynecologic cancer,” Dr. Bretschneider said at the meeting, which was jointly sponsored by the American College of Surgeons. “The study was further strengthened by its prospective design and well-characterized, large cohort of women.” Weaknesses, she continued, include its generalizability, “which may be limited, as the study cohort was recruited from a single academic institution. Also, the small sample size for some cancer sites reduced our ability to sense cancer site as a causal agent for sexual dysfunction.”
Dr. Bretschneider reported having no financial disclosures.
INDIAN WELLS, CALIF. – Premenopausal age was associated with a greater temporary decline in sexual desire 1 month after undergoing surgery for suspected gynecologic malignancies, results from an ancillary analysis showed.
“Sexual health is an important dimension of quality of life for women with gynecologic cancer,” Dr. C. Emi Bretschneider, lead study author, said at the annual scientific meeting of the Society of Gynecologic Surgeons. “Limited data exists on the impact of surgery for treatment of gynecologic cancer on patient-reported sexual desire and interest.”
In an effort to evaluate the impact on sexual function in women undergoing surgery for presumed or known gynecologic malignancies, the researchers performed an ancillary analysis of a cohort study analyzing quality-of-life and operative outcomes in 185 women who underwent gynecologic oncology procedures at the University of North Carolina, Chapel Hill, between October 2013 and October 2014.
Study participants completed the Patient-Reported Outcomes Measurement Information System Sexual Function and Satisfaction Questionnaire (PROMIS-SFQ) at baseline and at 1, 3, and 6 months postoperatively. The questionnaire evaluates four subdomains of sexual function: global satisfaction with sex life, interest in sexual activity, lubrication, and vaginal discomfort. The researchers used student t-test and linear regression to compare mean score changes between cancer types, surgical route, menopausal status, and postoperative complications, said Dr. Bretschneider of the university’s department of obstetrics and gynecology.
Of the 281 patients initially enrolled, 185 (66%) completed the PROMIS-SFQ at baseline and at 1 month postoperatively, forming the primary cohort from which the researchers performed the analysis. Of these 185 patients, 170 (92%) completed the PROMIS-SFQ at 3 months and 174 (94%) completed the survey at 6 months postoperatively.
The average age of patients at baseline was 56 years: most (77%) were white, mean body mass index was 32.9 kg/m2, 62% were partnered, and 63% underwent minimally invasive procedures. Following surgery, 131 of the patients (71%) were diagnosed with a malignancy, most commonly uterine cancer (84%), followed by ovarian (23%), cervical (17%), and vulvar cancer (3%).
Dr. Bretschneider reported that the mean baseline sexual interest score among all study participants was 44.8. At 1 month postoperatively, the mean scores decreased a mean of 3.8 points from baseline to 41. By 3 and 6 months postoperatively, the mean sexual interest scores increased from baseline by 1.9 and 2.7 points, respectively, to 46.7 and 47.5.
Women younger than age 55 years had a greater decrease in sexual interest between baseline and 1 month postoperatively, compared with their counterparts aged 55 and older (a mean of –5.5 vs. –2.3 points, respectively; P = .02).
On multivariate analysis adjusted for cancer diagnosis, minimally invasive surgery, and cancer site, women younger than age 55 continued to have a greater decrease in sexual interest between baseline and 1 month postoperatively, compared with their counterparts aged 55 and older (a mean of –4.59 points). Additionally, women who had cancer had a greater drop in sexual desire, compared with those with benign disease (a mean of –5.6 points).
“This study offers new information on the impact of surgery on sexual function for women with gynecologic cancer,” Dr. Bretschneider said at the meeting, which was jointly sponsored by the American College of Surgeons. “The study was further strengthened by its prospective design and well-characterized, large cohort of women.” Weaknesses, she continued, include its generalizability, “which may be limited, as the study cohort was recruited from a single academic institution. Also, the small sample size for some cancer sites reduced our ability to sense cancer site as a causal agent for sexual dysfunction.”
Dr. Bretschneider reported having no financial disclosures.
AT SGS 2016
Key clinical point: Premenopausal age was associated with a greater temporary decline in sexual function following gynecologic oncology procedures.
Major finding: Women younger than age 55 years had a greater decrease in sexual interest between baseline and 1 month postoperatively, compared with their counterparts aged 55 and older (a mean of –5.5 vs. –2.3 points on the PROMIS-SFQ, respectively; P = .02).
Data source: An ancillary analysis of a cohort study analyzing quality-of-life and operative outcomes in 185 women who underwent gynecologic oncology procedures between October 2013 and October 2014.
Disclosures: Dr. Bretschneider reported having no financial disclosures.
Which nonhormonal treatments are effective for hot flashes?
Selective serotonin reuptake inhibitors (SSRIs [fluoxetine, sertraline, paroxetine]) and the selective norepinephrine reuptake inhibitor (SNRI) venlafaxine, as well as clonidine and gabapentin, reduce hot flashes by about 25% (approximately one per day) in women with and without a history of breast cancer. No studies compare medications against each other to determine a single best option (strength of recommendation [SOR]: A, systematic reviews and meta-analyses of randomized controlled trials [RCTs]). In comparison, estrogen reduces the frequency of hot flashes by about 75%, or 2.5 to 3 per day.
The phytoestrogens (soy isoflavones, red clover extract, black cohosh), vitamin E, and nonpharmacologic measures (relaxation therapy, exercise, acupuncture, homeopathy, magnet therapy) lack evidence of effectiveness (SOR: A, meta-analyses of RCTs, many of which were low quality).
EVIDENCE SUMMARY
A systematic review of 6 RCTs that evaluated SSRIs and SNRIs (fluoxetine, sertraline, paroxetine, venlafaxine) found them all to be effective for reducing hot flash frequency and symptom scores in women with previous breast cancer1 (TABLE1,2).
A 2006 meta-analysis combined the results of 7 RCTs (each evaluating a single SSRI [fluoxetine, paroxetine] or SNRI [venlafaxine]) and found that as a group, they reduced mean hot flash frequency (–1.13 hot flashes/d; 95% confidence interval [CI], –1.70 to –0.57) in women with and without breast cancer.2 No trial compared medications head to head, and the populations differed among studies, so that investigators couldn’t determine a single best agent.
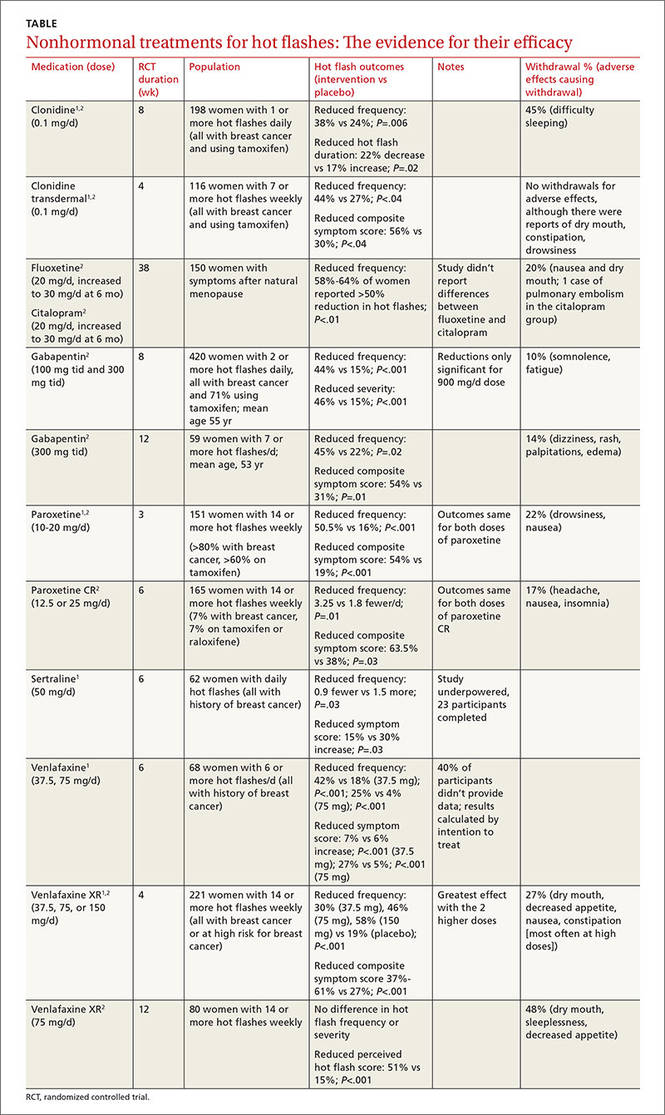
Clonidine and gabapentin decrease hot flash frequency
The 2006 meta-analysis also included 10 RCTs (743 patients) that studied clonidine in women with and without a history of breast cancer, and 2 RCTs (479 patients) that evaluated gabapentin in women with breast cancer.2 Both drugs reduced mean hot flash frequency (clonidine: –0.95 hot flashes/d, 95% CI, –1.44 to –0.47 at 4 weeks and –1.63 hot flashes/d, 95% CI, –2.76 to –0.05 at 8 weeks; gabapentin: –2.05 hot flashes/d; 95% CI, −2.80 to –1.30).
Phytoestrogens: The jury is still out
A meta-analysis of 43 RCTs (4364 patients) evaluated phytoestrogens that included dietary soy, soy extracts, red clover extracts, genistein extracts, and other types of phytoestrogens.3 The data from the only 5 RCTs (300 patients) that could be combined showed no effect from red clover extract on hot flash frequency. However, another 4 individual trials that couldn’t be combined each found that extracts with high levels of the phytoestrogen genistein (>30 mg/d) did reduce frequency. Investigators reported that many of the trials were small and had a high risk of bias.
A meta-analysis of 16 RCTs (2027 patients) that assessed black cohosh found that it didn’t reduce hot flash frequency (3 RCTs, 393 patients) or symptom severity scores (4 RCTs, 357 patients).4 Investigators reported high heterogeneity and recommended further research.
Nonpharmacologic therapies and vitamin E don’t help
Systematic reviews found that relaxation therapy (4 RCTs, 281 patients), exercise (3 RCTs, 454 patients), and acupuncture (8 RCTs, 414 patients) didn’t reduce hot flashes.5-7 In another review, vitamin E (1 RCT, 105 patients), homeopathy (2 RCTs, 124 patients), and magnetic devices (1 RCT, 11 patients) also produced no benefit.1
1. Rada G, Capurro D, Pantoja T, et al. Non-hormonal interventions for hot flushes in women with a history of breast cancer. Cochrane Database Syst Rev. 2010;(9):CD004923.
2. Nelson HD, Vesco KK, Haney E, et al. Nonhormonal therapies of menopausal hot flashes: systematic review and meta-analysis. JAMA. 2006;295:2057-2071.
3. Lethaby A, Marjoribanks J, Kronenberg F, et al. Phytoestrogens for menopausal vasomotor symptoms. Cochrane Database Syst Rev. 2013;(12):CD001395.
4. Leach MJ, Moore V. Black cohosh (Cimicifuga spp.) for menopausal symptoms. Cochrane Database Syst Rev. 2012;(9):CD007244.
5. Saensak S, Vutyavanich T, Somboonporn W, et al. Relaxation for perimenopausal and postmenopausal symptoms. Cochrane Database Syst Rev. 2014;(7):CD008582.
6. Daley A, Stokes-Lampard H, Thomas A, et al. Exercise for vasomotor menopausal symptoms. Cochrane Database Syst Rev. 2014:(11):CD006108.
7. Dodin S, Blanchet C, Marc I, et al. Acupuncture for menopausal hot flashes. Cochrane Database Syst Rev. 2013;(7):CD007410.
Selective serotonin reuptake inhibitors (SSRIs [fluoxetine, sertraline, paroxetine]) and the selective norepinephrine reuptake inhibitor (SNRI) venlafaxine, as well as clonidine and gabapentin, reduce hot flashes by about 25% (approximately one per day) in women with and without a history of breast cancer. No studies compare medications against each other to determine a single best option (strength of recommendation [SOR]: A, systematic reviews and meta-analyses of randomized controlled trials [RCTs]). In comparison, estrogen reduces the frequency of hot flashes by about 75%, or 2.5 to 3 per day.
The phytoestrogens (soy isoflavones, red clover extract, black cohosh), vitamin E, and nonpharmacologic measures (relaxation therapy, exercise, acupuncture, homeopathy, magnet therapy) lack evidence of effectiveness (SOR: A, meta-analyses of RCTs, many of which were low quality).
EVIDENCE SUMMARY
A systematic review of 6 RCTs that evaluated SSRIs and SNRIs (fluoxetine, sertraline, paroxetine, venlafaxine) found them all to be effective for reducing hot flash frequency and symptom scores in women with previous breast cancer1 (TABLE1,2).
A 2006 meta-analysis combined the results of 7 RCTs (each evaluating a single SSRI [fluoxetine, paroxetine] or SNRI [venlafaxine]) and found that as a group, they reduced mean hot flash frequency (–1.13 hot flashes/d; 95% confidence interval [CI], –1.70 to –0.57) in women with and without breast cancer.2 No trial compared medications head to head, and the populations differed among studies, so that investigators couldn’t determine a single best agent.

Clonidine and gabapentin decrease hot flash frequency
The 2006 meta-analysis also included 10 RCTs (743 patients) that studied clonidine in women with and without a history of breast cancer, and 2 RCTs (479 patients) that evaluated gabapentin in women with breast cancer.2 Both drugs reduced mean hot flash frequency (clonidine: –0.95 hot flashes/d, 95% CI, –1.44 to –0.47 at 4 weeks and –1.63 hot flashes/d, 95% CI, –2.76 to –0.05 at 8 weeks; gabapentin: –2.05 hot flashes/d; 95% CI, −2.80 to –1.30).
Phytoestrogens: The jury is still out
A meta-analysis of 43 RCTs (4364 patients) evaluated phytoestrogens that included dietary soy, soy extracts, red clover extracts, genistein extracts, and other types of phytoestrogens.3 The data from the only 5 RCTs (300 patients) that could be combined showed no effect from red clover extract on hot flash frequency. However, another 4 individual trials that couldn’t be combined each found that extracts with high levels of the phytoestrogen genistein (>30 mg/d) did reduce frequency. Investigators reported that many of the trials were small and had a high risk of bias.
A meta-analysis of 16 RCTs (2027 patients) that assessed black cohosh found that it didn’t reduce hot flash frequency (3 RCTs, 393 patients) or symptom severity scores (4 RCTs, 357 patients).4 Investigators reported high heterogeneity and recommended further research.
Nonpharmacologic therapies and vitamin E don’t help
Systematic reviews found that relaxation therapy (4 RCTs, 281 patients), exercise (3 RCTs, 454 patients), and acupuncture (8 RCTs, 414 patients) didn’t reduce hot flashes.5-7 In another review, vitamin E (1 RCT, 105 patients), homeopathy (2 RCTs, 124 patients), and magnetic devices (1 RCT, 11 patients) also produced no benefit.1
Selective serotonin reuptake inhibitors (SSRIs [fluoxetine, sertraline, paroxetine]) and the selective norepinephrine reuptake inhibitor (SNRI) venlafaxine, as well as clonidine and gabapentin, reduce hot flashes by about 25% (approximately one per day) in women with and without a history of breast cancer. No studies compare medications against each other to determine a single best option (strength of recommendation [SOR]: A, systematic reviews and meta-analyses of randomized controlled trials [RCTs]). In comparison, estrogen reduces the frequency of hot flashes by about 75%, or 2.5 to 3 per day.
The phytoestrogens (soy isoflavones, red clover extract, black cohosh), vitamin E, and nonpharmacologic measures (relaxation therapy, exercise, acupuncture, homeopathy, magnet therapy) lack evidence of effectiveness (SOR: A, meta-analyses of RCTs, many of which were low quality).
EVIDENCE SUMMARY
A systematic review of 6 RCTs that evaluated SSRIs and SNRIs (fluoxetine, sertraline, paroxetine, venlafaxine) found them all to be effective for reducing hot flash frequency and symptom scores in women with previous breast cancer1 (TABLE1,2).
A 2006 meta-analysis combined the results of 7 RCTs (each evaluating a single SSRI [fluoxetine, paroxetine] or SNRI [venlafaxine]) and found that as a group, they reduced mean hot flash frequency (–1.13 hot flashes/d; 95% confidence interval [CI], –1.70 to –0.57) in women with and without breast cancer.2 No trial compared medications head to head, and the populations differed among studies, so that investigators couldn’t determine a single best agent.

Clonidine and gabapentin decrease hot flash frequency
The 2006 meta-analysis also included 10 RCTs (743 patients) that studied clonidine in women with and without a history of breast cancer, and 2 RCTs (479 patients) that evaluated gabapentin in women with breast cancer.2 Both drugs reduced mean hot flash frequency (clonidine: –0.95 hot flashes/d, 95% CI, –1.44 to –0.47 at 4 weeks and –1.63 hot flashes/d, 95% CI, –2.76 to –0.05 at 8 weeks; gabapentin: –2.05 hot flashes/d; 95% CI, −2.80 to –1.30).
Phytoestrogens: The jury is still out
A meta-analysis of 43 RCTs (4364 patients) evaluated phytoestrogens that included dietary soy, soy extracts, red clover extracts, genistein extracts, and other types of phytoestrogens.3 The data from the only 5 RCTs (300 patients) that could be combined showed no effect from red clover extract on hot flash frequency. However, another 4 individual trials that couldn’t be combined each found that extracts with high levels of the phytoestrogen genistein (>30 mg/d) did reduce frequency. Investigators reported that many of the trials were small and had a high risk of bias.
A meta-analysis of 16 RCTs (2027 patients) that assessed black cohosh found that it didn’t reduce hot flash frequency (3 RCTs, 393 patients) or symptom severity scores (4 RCTs, 357 patients).4 Investigators reported high heterogeneity and recommended further research.
Nonpharmacologic therapies and vitamin E don’t help
Systematic reviews found that relaxation therapy (4 RCTs, 281 patients), exercise (3 RCTs, 454 patients), and acupuncture (8 RCTs, 414 patients) didn’t reduce hot flashes.5-7 In another review, vitamin E (1 RCT, 105 patients), homeopathy (2 RCTs, 124 patients), and magnetic devices (1 RCT, 11 patients) also produced no benefit.1
1. Rada G, Capurro D, Pantoja T, et al. Non-hormonal interventions for hot flushes in women with a history of breast cancer. Cochrane Database Syst Rev. 2010;(9):CD004923.
2. Nelson HD, Vesco KK, Haney E, et al. Nonhormonal therapies of menopausal hot flashes: systematic review and meta-analysis. JAMA. 2006;295:2057-2071.
3. Lethaby A, Marjoribanks J, Kronenberg F, et al. Phytoestrogens for menopausal vasomotor symptoms. Cochrane Database Syst Rev. 2013;(12):CD001395.
4. Leach MJ, Moore V. Black cohosh (Cimicifuga spp.) for menopausal symptoms. Cochrane Database Syst Rev. 2012;(9):CD007244.
5. Saensak S, Vutyavanich T, Somboonporn W, et al. Relaxation for perimenopausal and postmenopausal symptoms. Cochrane Database Syst Rev. 2014;(7):CD008582.
6. Daley A, Stokes-Lampard H, Thomas A, et al. Exercise for vasomotor menopausal symptoms. Cochrane Database Syst Rev. 2014:(11):CD006108.
7. Dodin S, Blanchet C, Marc I, et al. Acupuncture for menopausal hot flashes. Cochrane Database Syst Rev. 2013;(7):CD007410.
1. Rada G, Capurro D, Pantoja T, et al. Non-hormonal interventions for hot flushes in women with a history of breast cancer. Cochrane Database Syst Rev. 2010;(9):CD004923.
2. Nelson HD, Vesco KK, Haney E, et al. Nonhormonal therapies of menopausal hot flashes: systematic review and meta-analysis. JAMA. 2006;295:2057-2071.
3. Lethaby A, Marjoribanks J, Kronenberg F, et al. Phytoestrogens for menopausal vasomotor symptoms. Cochrane Database Syst Rev. 2013;(12):CD001395.
4. Leach MJ, Moore V. Black cohosh (Cimicifuga spp.) for menopausal symptoms. Cochrane Database Syst Rev. 2012;(9):CD007244.
5. Saensak S, Vutyavanich T, Somboonporn W, et al. Relaxation for perimenopausal and postmenopausal symptoms. Cochrane Database Syst Rev. 2014;(7):CD008582.
6. Daley A, Stokes-Lampard H, Thomas A, et al. Exercise for vasomotor menopausal symptoms. Cochrane Database Syst Rev. 2014:(11):CD006108.
7. Dodin S, Blanchet C, Marc I, et al. Acupuncture for menopausal hot flashes. Cochrane Database Syst Rev. 2013;(7):CD007410.
Evidence-based answers from the Family Physicians Inquiries Network
Hepatitis B test identifies active carriers
A one-time test combining hepatitis B virus (HBV) surface antigen and HBV DNA measurement accurately distinguished inactive carriers from active cases of chronic HBV, based on data from 1,529 patients published online in the journal Hepatology.
Correct identification of active chronic HBV patients vs. inactive cases “is a crucial step in identifying patients in need of less stringent follow-up, as well as patients who may benefit from additional follow-up and earlier initiation of antiviral therapy,” wrote Dr. Jessica Liu of the Genomics Research Center, Academia Sinica, in Taipei, Taiwan, and her colleagues (Hepatology. 2016. doi: 10.1002/hep.28552).
The investigators reviewed data from patients in the REVEAL-HBV cohort, which included individuals aged 30-65 years with chronic HBV who were recruited between 1991 and 1992. All patients were free of liver cirrhosis and were seronegative for anti–hepatitis C virus and hepatitis B surface antigen (HBsAg) at baseline, and were characterized as inactive carriers or active cases based on their serologic profiles at 18 months’ follow-up. Blood was collected at study entry and every 6-12 months.
At 18 months’ follow-up, the combined test distinguished inactive carriers from active cases with a sensitivity of 71% and a specificity of 85%. The positive and negative predictive values were 83% and 74%, respectively, and the diagnostic accuracy was 78%. In addition, the one-time combination measurement predicted cirrhosis, hepatocelluar carcinoma, and HBsAg seroclearance with areas under the receiver operating characteristic curves of 0.72, 0.79, and 0.78, respectively. The combination test also yielded information about the predictability of inactive carrier status, showing that inactive carriers had a significantly lower risk of cirrhosis and hepatocelluar carcinoma; adjusted hazard ratios were 0.43 and 0.13, respectively.
“To our knowledge, this is the first study to externally validate the usage of a one-time measurement of serum HBsAg less than 1,000 IU/mL and HBV DNA less than 2,000 IU/mL,” the researchers wrote.
The study was limited by a relatively short follow-up period, but the results suggest that “this single-point strategy may provide new and complementary information useful for simplifying or tailoring management of patients with chronic hepatitis B infection,” they said.
The study was supported by the Taiwan Department of Health, Bristol-Myers Squibb, Roche Diagnostics, and Academia Sinica. One of the coauthors is employed by Roche Diagnostics.
A one-time test combining hepatitis B virus (HBV) surface antigen and HBV DNA measurement accurately distinguished inactive carriers from active cases of chronic HBV, based on data from 1,529 patients published online in the journal Hepatology.
Correct identification of active chronic HBV patients vs. inactive cases “is a crucial step in identifying patients in need of less stringent follow-up, as well as patients who may benefit from additional follow-up and earlier initiation of antiviral therapy,” wrote Dr. Jessica Liu of the Genomics Research Center, Academia Sinica, in Taipei, Taiwan, and her colleagues (Hepatology. 2016. doi: 10.1002/hep.28552).
The investigators reviewed data from patients in the REVEAL-HBV cohort, which included individuals aged 30-65 years with chronic HBV who were recruited between 1991 and 1992. All patients were free of liver cirrhosis and were seronegative for anti–hepatitis C virus and hepatitis B surface antigen (HBsAg) at baseline, and were characterized as inactive carriers or active cases based on their serologic profiles at 18 months’ follow-up. Blood was collected at study entry and every 6-12 months.
At 18 months’ follow-up, the combined test distinguished inactive carriers from active cases with a sensitivity of 71% and a specificity of 85%. The positive and negative predictive values were 83% and 74%, respectively, and the diagnostic accuracy was 78%. In addition, the one-time combination measurement predicted cirrhosis, hepatocelluar carcinoma, and HBsAg seroclearance with areas under the receiver operating characteristic curves of 0.72, 0.79, and 0.78, respectively. The combination test also yielded information about the predictability of inactive carrier status, showing that inactive carriers had a significantly lower risk of cirrhosis and hepatocelluar carcinoma; adjusted hazard ratios were 0.43 and 0.13, respectively.
“To our knowledge, this is the first study to externally validate the usage of a one-time measurement of serum HBsAg less than 1,000 IU/mL and HBV DNA less than 2,000 IU/mL,” the researchers wrote.
The study was limited by a relatively short follow-up period, but the results suggest that “this single-point strategy may provide new and complementary information useful for simplifying or tailoring management of patients with chronic hepatitis B infection,” they said.
The study was supported by the Taiwan Department of Health, Bristol-Myers Squibb, Roche Diagnostics, and Academia Sinica. One of the coauthors is employed by Roche Diagnostics.
A one-time test combining hepatitis B virus (HBV) surface antigen and HBV DNA measurement accurately distinguished inactive carriers from active cases of chronic HBV, based on data from 1,529 patients published online in the journal Hepatology.
Correct identification of active chronic HBV patients vs. inactive cases “is a crucial step in identifying patients in need of less stringent follow-up, as well as patients who may benefit from additional follow-up and earlier initiation of antiviral therapy,” wrote Dr. Jessica Liu of the Genomics Research Center, Academia Sinica, in Taipei, Taiwan, and her colleagues (Hepatology. 2016. doi: 10.1002/hep.28552).
The investigators reviewed data from patients in the REVEAL-HBV cohort, which included individuals aged 30-65 years with chronic HBV who were recruited between 1991 and 1992. All patients were free of liver cirrhosis and were seronegative for anti–hepatitis C virus and hepatitis B surface antigen (HBsAg) at baseline, and were characterized as inactive carriers or active cases based on their serologic profiles at 18 months’ follow-up. Blood was collected at study entry and every 6-12 months.
At 18 months’ follow-up, the combined test distinguished inactive carriers from active cases with a sensitivity of 71% and a specificity of 85%. The positive and negative predictive values were 83% and 74%, respectively, and the diagnostic accuracy was 78%. In addition, the one-time combination measurement predicted cirrhosis, hepatocelluar carcinoma, and HBsAg seroclearance with areas under the receiver operating characteristic curves of 0.72, 0.79, and 0.78, respectively. The combination test also yielded information about the predictability of inactive carrier status, showing that inactive carriers had a significantly lower risk of cirrhosis and hepatocelluar carcinoma; adjusted hazard ratios were 0.43 and 0.13, respectively.
“To our knowledge, this is the first study to externally validate the usage of a one-time measurement of serum HBsAg less than 1,000 IU/mL and HBV DNA less than 2,000 IU/mL,” the researchers wrote.
The study was limited by a relatively short follow-up period, but the results suggest that “this single-point strategy may provide new and complementary information useful for simplifying or tailoring management of patients with chronic hepatitis B infection,” they said.
The study was supported by the Taiwan Department of Health, Bristol-Myers Squibb, Roche Diagnostics, and Academia Sinica. One of the coauthors is employed by Roche Diagnostics.
FROM HEPATOLOGY
Key clinical point: The data confirm the predictability of a one-time test for chronic hepatitis B progression that combines hepatitis B surface antigen and hepatitis B DNA measurement.
Major finding: The one-time combined test distinguished inactive carriers from active cases with a sensitivity of 71% and specificity of 85%.
Data source: A study of 1,529 adults aged 30-65 years with chronic hepatitis B.
Disclosures: The study was supported by the Taiwan Department of Health, Bristol-Myers Squibb, Roche Diagnostics, and Academia Sinica. One of the coauthors is employed by Roche Diagnostics.
Painful lesions on hand
This patient was given a diagnosis of herpetic whitlow, or herpes simplex virus (HSV) infection of the hand. Herpetic whitlow classically has a bimodal age distribution and may be seen in children younger than 10 years of age or in adults between 20 and 30 years of age. In children, it is caused almost exclusively by HSV-1, whereas in adults it can be caused by HSV-1 or HSV-2.
HSV infection of the hand classically occurs as a result of autoinoculation following herpetic gingivostomatitis. After inoculation, the virus has an incubation period of 2 to 20 days before vesicles appear. The appearance of the lesions is associated with intense throbbing pain. Fever and systemic symptoms are rare.
Patients with herpetic whitlow will develop a single vesicle or cluster of vesicles on a single digit a few days after their skin has been irritated or exposed to minor trauma. Vesicles are typically clear in color and have an erythematous base. However, they are often superinfected with bacteria and may exhibit signs of impetiginization. The most common location of the vesicles is on the terminal phalanx of the thumb, index, or middle finger.
The diagnosis of herpetic whitlow is clinical. If the diagnosis is unclear, tests include a viral culture, serum antibody titers, a Tzanck smear, lesion specimen antigen testing, or histopathologic examinations.
The natural history of untreated, uncomplicated herpetic whitlow is complete clearance within 2 to 3 weeks. Rare complications include systemic viremia, ocular infection, nail dystrophy, nail loss, scarring, and localized hyperesthesia or hypoesthesia.
Oral acyclovir (2 g/day in 3 doses for 10 days) taken during the prodromal stage of recurrent HSV-2 herpetic whitlow has been shown to reduce the duration of symptoms from 10.1 to 3.7 days. Prophylactic use of oral acyclovir (200 mg, 4 times daily for up to 2 years) has been shown to be effective in suppressing recurrent HSV infection of nongenital skin.
The patient described here was given a 10-day course of acyclovir 400 mg orally 3 times daily and cefadroxil 500 mg orally twice daily (for superimposed bacterial infection). The lesions had completely disappeared upon follow-up 2 weeks later.
Adapted from: Ishak RS, Abbas O. Recurrent vesicular eruption on the right hand. J Fam Pract. 2014;63:33-35.
This patient was given a diagnosis of herpetic whitlow, or herpes simplex virus (HSV) infection of the hand. Herpetic whitlow classically has a bimodal age distribution and may be seen in children younger than 10 years of age or in adults between 20 and 30 years of age. In children, it is caused almost exclusively by HSV-1, whereas in adults it can be caused by HSV-1 or HSV-2.
HSV infection of the hand classically occurs as a result of autoinoculation following herpetic gingivostomatitis. After inoculation, the virus has an incubation period of 2 to 20 days before vesicles appear. The appearance of the lesions is associated with intense throbbing pain. Fever and systemic symptoms are rare.
Patients with herpetic whitlow will develop a single vesicle or cluster of vesicles on a single digit a few days after their skin has been irritated or exposed to minor trauma. Vesicles are typically clear in color and have an erythematous base. However, they are often superinfected with bacteria and may exhibit signs of impetiginization. The most common location of the vesicles is on the terminal phalanx of the thumb, index, or middle finger.
The diagnosis of herpetic whitlow is clinical. If the diagnosis is unclear, tests include a viral culture, serum antibody titers, a Tzanck smear, lesion specimen antigen testing, or histopathologic examinations.
The natural history of untreated, uncomplicated herpetic whitlow is complete clearance within 2 to 3 weeks. Rare complications include systemic viremia, ocular infection, nail dystrophy, nail loss, scarring, and localized hyperesthesia or hypoesthesia.
Oral acyclovir (2 g/day in 3 doses for 10 days) taken during the prodromal stage of recurrent HSV-2 herpetic whitlow has been shown to reduce the duration of symptoms from 10.1 to 3.7 days. Prophylactic use of oral acyclovir (200 mg, 4 times daily for up to 2 years) has been shown to be effective in suppressing recurrent HSV infection of nongenital skin.
The patient described here was given a 10-day course of acyclovir 400 mg orally 3 times daily and cefadroxil 500 mg orally twice daily (for superimposed bacterial infection). The lesions had completely disappeared upon follow-up 2 weeks later.
Adapted from: Ishak RS, Abbas O. Recurrent vesicular eruption on the right hand. J Fam Pract. 2014;63:33-35.
This patient was given a diagnosis of herpetic whitlow, or herpes simplex virus (HSV) infection of the hand. Herpetic whitlow classically has a bimodal age distribution and may be seen in children younger than 10 years of age or in adults between 20 and 30 years of age. In children, it is caused almost exclusively by HSV-1, whereas in adults it can be caused by HSV-1 or HSV-2.
HSV infection of the hand classically occurs as a result of autoinoculation following herpetic gingivostomatitis. After inoculation, the virus has an incubation period of 2 to 20 days before vesicles appear. The appearance of the lesions is associated with intense throbbing pain. Fever and systemic symptoms are rare.
Patients with herpetic whitlow will develop a single vesicle or cluster of vesicles on a single digit a few days after their skin has been irritated or exposed to minor trauma. Vesicles are typically clear in color and have an erythematous base. However, they are often superinfected with bacteria and may exhibit signs of impetiginization. The most common location of the vesicles is on the terminal phalanx of the thumb, index, or middle finger.
The diagnosis of herpetic whitlow is clinical. If the diagnosis is unclear, tests include a viral culture, serum antibody titers, a Tzanck smear, lesion specimen antigen testing, or histopathologic examinations.
The natural history of untreated, uncomplicated herpetic whitlow is complete clearance within 2 to 3 weeks. Rare complications include systemic viremia, ocular infection, nail dystrophy, nail loss, scarring, and localized hyperesthesia or hypoesthesia.
Oral acyclovir (2 g/day in 3 doses for 10 days) taken during the prodromal stage of recurrent HSV-2 herpetic whitlow has been shown to reduce the duration of symptoms from 10.1 to 3.7 days. Prophylactic use of oral acyclovir (200 mg, 4 times daily for up to 2 years) has been shown to be effective in suppressing recurrent HSV infection of nongenital skin.
The patient described here was given a 10-day course of acyclovir 400 mg orally 3 times daily and cefadroxil 500 mg orally twice daily (for superimposed bacterial infection). The lesions had completely disappeared upon follow-up 2 weeks later.
Adapted from: Ishak RS, Abbas O. Recurrent vesicular eruption on the right hand. J Fam Pract. 2014;63:33-35.
ACOSOG Z0011: Good long-term results with SLND without ALND
CHICAGO – Sentinel lymph node dissection without axillary lymph node dissection offers excellent regional control in select patients with early metastatic breast cancer who are treated using breast-conserving therapy and adjuvant systemic therapy, according to 10-year results from the American College of Surgeons Oncology Group (ACOSOG) Z0011 Randomized Trial. ACOSOG is now part of Alliance for Clinical Trials in Oncology.
The findings confirm the previously reported 5-year outcomes, which demonstrated no significant difference in locoregional recurrence for patients with positive sentinel nodes who were randomized to undergo axillary lymph node dissection (ALND) or no further axillary treatment, Dr. Armando E. Giuliano of Cedars-Sinai Medical Center, Los Angeles, reported at the annual meeting of the American Surgical Association.
“In fact, the [5-year] results were highly significant showing noninferiority of sentinel lymph node dissection,” he said.
At a median follow-up of 9.25 years, there still was no statistically significant difference between 446 sentinel lymph node dissection (SLND)–only patients and 445 completion ALND patients with respect to the rate of locoregional recurrence, Dr. Giuliano said.
“The 10-year locoregional recurrence incidence after axillary lymph node dissection is 6.2%, compared to 5.3% after sentinel lymph node dissection alone,” he said, noting that most recurrences were seen in the first 5 years.
Of the ALND patients, 27% had additional positive nodes removed beyond the sentinel nodes.
“Therefore, about 27% of patients who underwent sentinel node dissection alone had residual disease remaining in the axilla undissected. Despite this high possibility of residual disease, very few regional recurrences were seen in either arm,” he noted.
Local recurrences occurred in 19 (5.6%) of patients in the ALND group and 12 (3.8%) in the SLND group, and regional recurrence was seen in 2 (0.5%) patients in the ALND group and 5 (1.5%) in the SLND group. The differences were not statistically significant.
Only hormone receptor status, Bloom-Richardson score, and tumor size were associated with locoregional recurrence. Omission of radiation increased local but not regional recurrence, but numbers were too few to draw further conclusions, he said.
“We can conclude, however, that sentinel lymph node dissection provides excellent locoregional control comparable to completion axillary lymph node dissection in these selected patients,” he said.
ACOSOG Z0011 subjects were patients with hematoxylin-eosin (H&E)–detected sentinel lymph node metastases undergoing breast-conserving therapy. The groups randomized to undergo ALND or to receive no further axillary treatment were similar with respect to age, Bloom-Richardson score, estrogen-receptor status, adjuvant systemic therapy, histology, and tumor size.
Nearly all patients had adjuvant systemic therapy (96% and 97% in the ALND and SLND groups, respectively), and about 60% in each group received chemotherapy.
Dr. Giuliano concluded that, despite the potential for residual axillary disease after SLND, SLND without ALND offers excellent regional control for selected patients with early metastatic breast cancer treated with breast-conserving therapy and adjuvant systemic therapy.
“Axillary lymph node dissection is not necessary for patients with early metastatic breast cancer and should be abandoned,” he said.
Dr. Giuliano had no disclosures.
The complete manuscript of this presentation is anticipated to be published in the Annals of Surgery pending editorial review
CHICAGO – Sentinel lymph node dissection without axillary lymph node dissection offers excellent regional control in select patients with early metastatic breast cancer who are treated using breast-conserving therapy and adjuvant systemic therapy, according to 10-year results from the American College of Surgeons Oncology Group (ACOSOG) Z0011 Randomized Trial. ACOSOG is now part of Alliance for Clinical Trials in Oncology.
The findings confirm the previously reported 5-year outcomes, which demonstrated no significant difference in locoregional recurrence for patients with positive sentinel nodes who were randomized to undergo axillary lymph node dissection (ALND) or no further axillary treatment, Dr. Armando E. Giuliano of Cedars-Sinai Medical Center, Los Angeles, reported at the annual meeting of the American Surgical Association.
“In fact, the [5-year] results were highly significant showing noninferiority of sentinel lymph node dissection,” he said.
At a median follow-up of 9.25 years, there still was no statistically significant difference between 446 sentinel lymph node dissection (SLND)–only patients and 445 completion ALND patients with respect to the rate of locoregional recurrence, Dr. Giuliano said.
“The 10-year locoregional recurrence incidence after axillary lymph node dissection is 6.2%, compared to 5.3% after sentinel lymph node dissection alone,” he said, noting that most recurrences were seen in the first 5 years.
Of the ALND patients, 27% had additional positive nodes removed beyond the sentinel nodes.
“Therefore, about 27% of patients who underwent sentinel node dissection alone had residual disease remaining in the axilla undissected. Despite this high possibility of residual disease, very few regional recurrences were seen in either arm,” he noted.
Local recurrences occurred in 19 (5.6%) of patients in the ALND group and 12 (3.8%) in the SLND group, and regional recurrence was seen in 2 (0.5%) patients in the ALND group and 5 (1.5%) in the SLND group. The differences were not statistically significant.
Only hormone receptor status, Bloom-Richardson score, and tumor size were associated with locoregional recurrence. Omission of radiation increased local but not regional recurrence, but numbers were too few to draw further conclusions, he said.
“We can conclude, however, that sentinel lymph node dissection provides excellent locoregional control comparable to completion axillary lymph node dissection in these selected patients,” he said.
ACOSOG Z0011 subjects were patients with hematoxylin-eosin (H&E)–detected sentinel lymph node metastases undergoing breast-conserving therapy. The groups randomized to undergo ALND or to receive no further axillary treatment were similar with respect to age, Bloom-Richardson score, estrogen-receptor status, adjuvant systemic therapy, histology, and tumor size.
Nearly all patients had adjuvant systemic therapy (96% and 97% in the ALND and SLND groups, respectively), and about 60% in each group received chemotherapy.
Dr. Giuliano concluded that, despite the potential for residual axillary disease after SLND, SLND without ALND offers excellent regional control for selected patients with early metastatic breast cancer treated with breast-conserving therapy and adjuvant systemic therapy.
“Axillary lymph node dissection is not necessary for patients with early metastatic breast cancer and should be abandoned,” he said.
Dr. Giuliano had no disclosures.
The complete manuscript of this presentation is anticipated to be published in the Annals of Surgery pending editorial review
CHICAGO – Sentinel lymph node dissection without axillary lymph node dissection offers excellent regional control in select patients with early metastatic breast cancer who are treated using breast-conserving therapy and adjuvant systemic therapy, according to 10-year results from the American College of Surgeons Oncology Group (ACOSOG) Z0011 Randomized Trial. ACOSOG is now part of Alliance for Clinical Trials in Oncology.
The findings confirm the previously reported 5-year outcomes, which demonstrated no significant difference in locoregional recurrence for patients with positive sentinel nodes who were randomized to undergo axillary lymph node dissection (ALND) or no further axillary treatment, Dr. Armando E. Giuliano of Cedars-Sinai Medical Center, Los Angeles, reported at the annual meeting of the American Surgical Association.
“In fact, the [5-year] results were highly significant showing noninferiority of sentinel lymph node dissection,” he said.
At a median follow-up of 9.25 years, there still was no statistically significant difference between 446 sentinel lymph node dissection (SLND)–only patients and 445 completion ALND patients with respect to the rate of locoregional recurrence, Dr. Giuliano said.
“The 10-year locoregional recurrence incidence after axillary lymph node dissection is 6.2%, compared to 5.3% after sentinel lymph node dissection alone,” he said, noting that most recurrences were seen in the first 5 years.
Of the ALND patients, 27% had additional positive nodes removed beyond the sentinel nodes.
“Therefore, about 27% of patients who underwent sentinel node dissection alone had residual disease remaining in the axilla undissected. Despite this high possibility of residual disease, very few regional recurrences were seen in either arm,” he noted.
Local recurrences occurred in 19 (5.6%) of patients in the ALND group and 12 (3.8%) in the SLND group, and regional recurrence was seen in 2 (0.5%) patients in the ALND group and 5 (1.5%) in the SLND group. The differences were not statistically significant.
Only hormone receptor status, Bloom-Richardson score, and tumor size were associated with locoregional recurrence. Omission of radiation increased local but not regional recurrence, but numbers were too few to draw further conclusions, he said.
“We can conclude, however, that sentinel lymph node dissection provides excellent locoregional control comparable to completion axillary lymph node dissection in these selected patients,” he said.
ACOSOG Z0011 subjects were patients with hematoxylin-eosin (H&E)–detected sentinel lymph node metastases undergoing breast-conserving therapy. The groups randomized to undergo ALND or to receive no further axillary treatment were similar with respect to age, Bloom-Richardson score, estrogen-receptor status, adjuvant systemic therapy, histology, and tumor size.
Nearly all patients had adjuvant systemic therapy (96% and 97% in the ALND and SLND groups, respectively), and about 60% in each group received chemotherapy.
Dr. Giuliano concluded that, despite the potential for residual axillary disease after SLND, SLND without ALND offers excellent regional control for selected patients with early metastatic breast cancer treated with breast-conserving therapy and adjuvant systemic therapy.
“Axillary lymph node dissection is not necessary for patients with early metastatic breast cancer and should be abandoned,” he said.
Dr. Giuliano had no disclosures.
The complete manuscript of this presentation is anticipated to be published in the Annals of Surgery pending editorial review
AT THE ASA ANNUAL MEETING
Key clinical point: Sentinel lymph node dissection without axillary lymph node dissection offers excellent long-term regional control in select patients with early metastatic breast cancer who are treated using breast-conserving therapy and adjuvant systemic therapy.
Major finding: The 10-year locoregional recurrence after axillary lymph node dissection was 6.2%, compared with 5.3% after SLND alone.
Data source: The American College of Surgeons Oncology Group (ACOSOG) Z0011 Randomized Trial involving 891 patients.
Disclosures: Dr. Giuliano had no disclosures.
Perioperative bundle implementation reduced SSIs after hysterectomy
INDIAN WELLS, CALIF. – Implementation of a gynecologic perioperative infection prevention bundle for patients undergoing hysterectomy in a large academic hospital led to a 53% decrease in surgical site infections (SSIs) and a 50% drop in deep and organ space infections, a retrospective study found.
“There are approximately 600,000 hysterectomies performed each year in the United States, and the infection rate is widely reported as 1%-4%,” Dr. Sarah E. Andiman said at the annual scientific meeting of the Society of Gynecologic Surgeons. “SSIs lead to increased morbidity, negative patient experiences, prolonged hospital stays, additional procedures, and increased costs. The exact costs of SSIs related to hysterectomy are not known. However, the Centers for Medicare & Medicaid Services has required public reporting of SSIs after hysterectomy since 2013.”
An interdisciplinary team at Yale–New Haven Hospital designed a perioperative gynecology-specific bundle aimed at reducing the SSI rate in hysterectomies. Dr. Andiman of the department of obstetrics, gynecology, and reproductive sciences at Yale University, New Haven, Conn., and her associates examined the efficacy of the infection prevention bundle. The primary outcome was SSI rate change, while the secondary outcome was hospital cost of admission for the initial care episode.
The bundle consists of a preoperative phase that includes chlorhexidine wipes, patient-controlled warming, and a standard antibiotic regimen consisting of 2 g of cefazolin within 1 hour of incision and 500 mg of metronidazole administered when there is a potential for bowel involvement.
The intraoperative phase of the bundle includes a standardized method of vaginal preparation with chlorhexidine and an abdominal prep with ChloraPrep. “Staff and trainees underwent training with an educational video that is available over our intranet,” Dr. Andiman said. “Also included was antibiotic redosing at 3 hours and intraoperative maintenance of temperature above 36° C.” The postoperative phase includes maintenance of a surgical dressing for 24-48 hours.
The researchers collected data prospectively according to institutional guidelines for tracking SSIs using definitions from the Centers for Disease Control and Prevention. All cases of SSIs were reviewed by a committee. In instances where the protocol was not followed, direct feedback was given to appropriate team members within 2 weeks.
The preintervention period was defined as the beginning of data collection through full bundle implementation, which was April 2013 through November 2014. The postbundle implementation period was December 2014 through June 2015. The analysis was limited to total abdominal, total laparoscopic, robotic-assisted total laparoscopic, and laparoscopic-assisted vaginal hysterectomies. Transvaginal and obstetric hysterectomies were excluded from the study, leaving a total of 1,763 procedures for inclusion.
Between the prebundle and postbundle period, the researchers observed a 53% decrease in SSIs and a 50% decrease in deep and organ space infections (P = .04). The difference was primarily driven by the decrease in the infection rate for total abdominal hysterectomies, Dr. Andiman said at the meeting, which was jointly sponsored by the American College of Surgeons.
The researchers also found that the cost of hospital admissions decreased 17.6% between the prebundle and postbundle period, from $7,452 per case to $6,142 per case (P = .002).
Dr. Andiman acknowledged certain limitations of the analysis, including the staggered implementation of the bundle components. “However, in the next stage of our study, we will be looking at comprehensive compliance data to examine this further,” she said. “Finally, we currently only have cost data for the cost of the hospital admission for the index surgery. We are also analyzing cost data for patients who were readmitted up to 30 days postoperatively to assess how this factors into overall costs.”
In an interview, Dr. Linda Fan, a gynecologic surgeon at Yale and the senior study author, said that a perioperative care bundle “by itself is not enough” to decrease SSI rates following hysterectomy.
“Education of staff is really important in terms of the uptake of these sorts of interventions,” she said. “As we move forward and everyone is looking at value, we have to teach people how to implement the different elements of the bundle.”
The researchers reported having no relevant financial disclosures.
INDIAN WELLS, CALIF. – Implementation of a gynecologic perioperative infection prevention bundle for patients undergoing hysterectomy in a large academic hospital led to a 53% decrease in surgical site infections (SSIs) and a 50% drop in deep and organ space infections, a retrospective study found.
“There are approximately 600,000 hysterectomies performed each year in the United States, and the infection rate is widely reported as 1%-4%,” Dr. Sarah E. Andiman said at the annual scientific meeting of the Society of Gynecologic Surgeons. “SSIs lead to increased morbidity, negative patient experiences, prolonged hospital stays, additional procedures, and increased costs. The exact costs of SSIs related to hysterectomy are not known. However, the Centers for Medicare & Medicaid Services has required public reporting of SSIs after hysterectomy since 2013.”
An interdisciplinary team at Yale–New Haven Hospital designed a perioperative gynecology-specific bundle aimed at reducing the SSI rate in hysterectomies. Dr. Andiman of the department of obstetrics, gynecology, and reproductive sciences at Yale University, New Haven, Conn., and her associates examined the efficacy of the infection prevention bundle. The primary outcome was SSI rate change, while the secondary outcome was hospital cost of admission for the initial care episode.
The bundle consists of a preoperative phase that includes chlorhexidine wipes, patient-controlled warming, and a standard antibiotic regimen consisting of 2 g of cefazolin within 1 hour of incision and 500 mg of metronidazole administered when there is a potential for bowel involvement.
The intraoperative phase of the bundle includes a standardized method of vaginal preparation with chlorhexidine and an abdominal prep with ChloraPrep. “Staff and trainees underwent training with an educational video that is available over our intranet,” Dr. Andiman said. “Also included was antibiotic redosing at 3 hours and intraoperative maintenance of temperature above 36° C.” The postoperative phase includes maintenance of a surgical dressing for 24-48 hours.
The researchers collected data prospectively according to institutional guidelines for tracking SSIs using definitions from the Centers for Disease Control and Prevention. All cases of SSIs were reviewed by a committee. In instances where the protocol was not followed, direct feedback was given to appropriate team members within 2 weeks.
The preintervention period was defined as the beginning of data collection through full bundle implementation, which was April 2013 through November 2014. The postbundle implementation period was December 2014 through June 2015. The analysis was limited to total abdominal, total laparoscopic, robotic-assisted total laparoscopic, and laparoscopic-assisted vaginal hysterectomies. Transvaginal and obstetric hysterectomies were excluded from the study, leaving a total of 1,763 procedures for inclusion.
Between the prebundle and postbundle period, the researchers observed a 53% decrease in SSIs and a 50% decrease in deep and organ space infections (P = .04). The difference was primarily driven by the decrease in the infection rate for total abdominal hysterectomies, Dr. Andiman said at the meeting, which was jointly sponsored by the American College of Surgeons.
The researchers also found that the cost of hospital admissions decreased 17.6% between the prebundle and postbundle period, from $7,452 per case to $6,142 per case (P = .002).
Dr. Andiman acknowledged certain limitations of the analysis, including the staggered implementation of the bundle components. “However, in the next stage of our study, we will be looking at comprehensive compliance data to examine this further,” she said. “Finally, we currently only have cost data for the cost of the hospital admission for the index surgery. We are also analyzing cost data for patients who were readmitted up to 30 days postoperatively to assess how this factors into overall costs.”
In an interview, Dr. Linda Fan, a gynecologic surgeon at Yale and the senior study author, said that a perioperative care bundle “by itself is not enough” to decrease SSI rates following hysterectomy.
“Education of staff is really important in terms of the uptake of these sorts of interventions,” she said. “As we move forward and everyone is looking at value, we have to teach people how to implement the different elements of the bundle.”
The researchers reported having no relevant financial disclosures.
INDIAN WELLS, CALIF. – Implementation of a gynecologic perioperative infection prevention bundle for patients undergoing hysterectomy in a large academic hospital led to a 53% decrease in surgical site infections (SSIs) and a 50% drop in deep and organ space infections, a retrospective study found.
“There are approximately 600,000 hysterectomies performed each year in the United States, and the infection rate is widely reported as 1%-4%,” Dr. Sarah E. Andiman said at the annual scientific meeting of the Society of Gynecologic Surgeons. “SSIs lead to increased morbidity, negative patient experiences, prolonged hospital stays, additional procedures, and increased costs. The exact costs of SSIs related to hysterectomy are not known. However, the Centers for Medicare & Medicaid Services has required public reporting of SSIs after hysterectomy since 2013.”
An interdisciplinary team at Yale–New Haven Hospital designed a perioperative gynecology-specific bundle aimed at reducing the SSI rate in hysterectomies. Dr. Andiman of the department of obstetrics, gynecology, and reproductive sciences at Yale University, New Haven, Conn., and her associates examined the efficacy of the infection prevention bundle. The primary outcome was SSI rate change, while the secondary outcome was hospital cost of admission for the initial care episode.
The bundle consists of a preoperative phase that includes chlorhexidine wipes, patient-controlled warming, and a standard antibiotic regimen consisting of 2 g of cefazolin within 1 hour of incision and 500 mg of metronidazole administered when there is a potential for bowel involvement.
The intraoperative phase of the bundle includes a standardized method of vaginal preparation with chlorhexidine and an abdominal prep with ChloraPrep. “Staff and trainees underwent training with an educational video that is available over our intranet,” Dr. Andiman said. “Also included was antibiotic redosing at 3 hours and intraoperative maintenance of temperature above 36° C.” The postoperative phase includes maintenance of a surgical dressing for 24-48 hours.
The researchers collected data prospectively according to institutional guidelines for tracking SSIs using definitions from the Centers for Disease Control and Prevention. All cases of SSIs were reviewed by a committee. In instances where the protocol was not followed, direct feedback was given to appropriate team members within 2 weeks.
The preintervention period was defined as the beginning of data collection through full bundle implementation, which was April 2013 through November 2014. The postbundle implementation period was December 2014 through June 2015. The analysis was limited to total abdominal, total laparoscopic, robotic-assisted total laparoscopic, and laparoscopic-assisted vaginal hysterectomies. Transvaginal and obstetric hysterectomies were excluded from the study, leaving a total of 1,763 procedures for inclusion.
Between the prebundle and postbundle period, the researchers observed a 53% decrease in SSIs and a 50% decrease in deep and organ space infections (P = .04). The difference was primarily driven by the decrease in the infection rate for total abdominal hysterectomies, Dr. Andiman said at the meeting, which was jointly sponsored by the American College of Surgeons.
The researchers also found that the cost of hospital admissions decreased 17.6% between the prebundle and postbundle period, from $7,452 per case to $6,142 per case (P = .002).
Dr. Andiman acknowledged certain limitations of the analysis, including the staggered implementation of the bundle components. “However, in the next stage of our study, we will be looking at comprehensive compliance data to examine this further,” she said. “Finally, we currently only have cost data for the cost of the hospital admission for the index surgery. We are also analyzing cost data for patients who were readmitted up to 30 days postoperatively to assess how this factors into overall costs.”
In an interview, Dr. Linda Fan, a gynecologic surgeon at Yale and the senior study author, said that a perioperative care bundle “by itself is not enough” to decrease SSI rates following hysterectomy.
“Education of staff is really important in terms of the uptake of these sorts of interventions,” she said. “As we move forward and everyone is looking at value, we have to teach people how to implement the different elements of the bundle.”
The researchers reported having no relevant financial disclosures.
AT SGS 2016
Key clinical point: Implementation of a gynecologic perioperative bundle helped reduce surgical site infections following hysterectomy.
Major finding: Between the prebundle and postbundle period, the researchers observed a 53% decrease in surgical site infections and a 50% decrease in deep and organ space infections (P = .04).
Data source: A retrospective cohort study of 1,763 hysterectomies performed before and after implementation of a gynecologic perioperative bundle designed to prevent surgical site infections.
Disclosures: The researchers reported having no relevant financial disclosures.