User login
Repeated measures analysis of patient-reported outcomes in prostate cancer after abiraterone acetate
Background Metastatic castration-resistant prostate cancer (mCRPC) is typically associated with declining health-related quality of life (HR-QoL).
Objective To assess patient experience with abiraterone acetate (hereafter abiraterone) plus prednisone longitudinally.
Methods COU-AA-302 was a phase 3, multinational, randomized, double-blind study that enrolled asymptomatic or mildly symptomatic, chemotherapy-naïve patients with mCRPC. Patients were randomized to 1 g abiraterone daily plus 5 mg prednisone BID (n = 546) or placebo plus prednisone (n = 542) in continuous 28-day cycles. Patient-reported outcomes (PROs) were collected using the Functional Assessment of Cancer Therapy-Prostate (FACT-P) questionnaire, consisting of 4 well-being subscales (physical, social/family, emotional, functional) and a prostate cancer-specific subscale (PCS). The trial outcome index (TOI) is a composite of the physical well-being, functional well-being, and PCS scores. Least squares mean change from baseline at each cycle up to 1 year (cycle13) was compared between treatment arms using a mixed-effects model for repeated measures, which assumed that data were “missing at random.” A pattern-mixture model (PMM) with multiple imputation was performed to address the assumption that data were “missing not at random.”
Results Significant differences favoring abiraterone-prednisone were observed for FACT-P total, TOI, and PCS scores, and for all well-being subscales except social/family well-being over the first year of treatment. These results were supported by the PMM with multiple imputation.
Limitations Attrition after 1 year limited our ability to analyze the PRO data beyond that time point.
Conclusions Abiraterone-prednisone confers sustained HR-QoL benefits over the course of treatment.
Funding Janssen Research & Development
Click on the PDF icon at the top of this introduction to read the full article.
Background Metastatic castration-resistant prostate cancer (mCRPC) is typically associated with declining health-related quality of life (HR-QoL).
Objective To assess patient experience with abiraterone acetate (hereafter abiraterone) plus prednisone longitudinally.
Methods COU-AA-302 was a phase 3, multinational, randomized, double-blind study that enrolled asymptomatic or mildly symptomatic, chemotherapy-naïve patients with mCRPC. Patients were randomized to 1 g abiraterone daily plus 5 mg prednisone BID (n = 546) or placebo plus prednisone (n = 542) in continuous 28-day cycles. Patient-reported outcomes (PROs) were collected using the Functional Assessment of Cancer Therapy-Prostate (FACT-P) questionnaire, consisting of 4 well-being subscales (physical, social/family, emotional, functional) and a prostate cancer-specific subscale (PCS). The trial outcome index (TOI) is a composite of the physical well-being, functional well-being, and PCS scores. Least squares mean change from baseline at each cycle up to 1 year (cycle13) was compared between treatment arms using a mixed-effects model for repeated measures, which assumed that data were “missing at random.” A pattern-mixture model (PMM) with multiple imputation was performed to address the assumption that data were “missing not at random.”
Results Significant differences favoring abiraterone-prednisone were observed for FACT-P total, TOI, and PCS scores, and for all well-being subscales except social/family well-being over the first year of treatment. These results were supported by the PMM with multiple imputation.
Limitations Attrition after 1 year limited our ability to analyze the PRO data beyond that time point.
Conclusions Abiraterone-prednisone confers sustained HR-QoL benefits over the course of treatment.
Funding Janssen Research & Development
Click on the PDF icon at the top of this introduction to read the full article.
Background Metastatic castration-resistant prostate cancer (mCRPC) is typically associated with declining health-related quality of life (HR-QoL).
Objective To assess patient experience with abiraterone acetate (hereafter abiraterone) plus prednisone longitudinally.
Methods COU-AA-302 was a phase 3, multinational, randomized, double-blind study that enrolled asymptomatic or mildly symptomatic, chemotherapy-naïve patients with mCRPC. Patients were randomized to 1 g abiraterone daily plus 5 mg prednisone BID (n = 546) or placebo plus prednisone (n = 542) in continuous 28-day cycles. Patient-reported outcomes (PROs) were collected using the Functional Assessment of Cancer Therapy-Prostate (FACT-P) questionnaire, consisting of 4 well-being subscales (physical, social/family, emotional, functional) and a prostate cancer-specific subscale (PCS). The trial outcome index (TOI) is a composite of the physical well-being, functional well-being, and PCS scores. Least squares mean change from baseline at each cycle up to 1 year (cycle13) was compared between treatment arms using a mixed-effects model for repeated measures, which assumed that data were “missing at random.” A pattern-mixture model (PMM) with multiple imputation was performed to address the assumption that data were “missing not at random.”
Results Significant differences favoring abiraterone-prednisone were observed for FACT-P total, TOI, and PCS scores, and for all well-being subscales except social/family well-being over the first year of treatment. These results were supported by the PMM with multiple imputation.
Limitations Attrition after 1 year limited our ability to analyze the PRO data beyond that time point.
Conclusions Abiraterone-prednisone confers sustained HR-QoL benefits over the course of treatment.
Funding Janssen Research & Development
Click on the PDF icon at the top of this introduction to read the full article.
Olanzapine versus fosaprepitant for the prevention of concurrent chemotherapy radiotherapy-induced nausea and vomiting
Background Concurrent chemotherapy radiation therapy may result in significant nausea and vomiting. There have been few studies reporting effective interventions for preventing treatment-related nausea and vomiting.
Objective To compare olanzapine with fosaprepitant for the prevention of nausea and vomiting in patients receiving concurrent highly emetogenic chemotherapy (HEC) and radiotherapy for locally advanced head and neck or esophageal cancer.
Methods 120 chemotherapy and radiotherapy naïve patients with head and neck cancer who were receiving concurrent local radiation and cisplatin were randomized to receive either olanzapine or fosaprepitant in combination with palonosetron and dexamethasone for the prevention of chemotherapy- and radiation-induced nausea and vomiting. The olanzapine, palonosetron, dexamethasone (OPD) regimen was 10 mg oral olanzapine , 0.25 mg IV palonosetron, and 20 mg IV dexamethasone before chemotherapy on day 1, and 10 mg/day of oral olanzapine before chemotherapy on days 2-4. The fosaprepitant, palonosetron, dexamethasone (FPD) regimen was 150 mg IV fosaprepitant, 0.25 mg IV palonosetron, and 12 mg IV dexamethasone before chemotherapy on day 1, and 4 mg dexamethasone PO BID, before chemotherapy days 2 and 3.
Results 101 of the 120 patients were evaluable. In 51 patients who received the OPD regimen, the complete response (CR; no emesis, no rescue medication) rate was 88% for the acute period (24 h after chemotherapy), 76% for the delayed period (days 2-5), and 76% for the overall period (0-120 h). In 50 patients who received the FPD regimen, the CR was 84% acute, 74% delayed, and 74% overall (P > .01 for all periods). Patients with no nausea (0, on a scale 0-10, visual analogue scale) were: OPD: 86% acute, 71% delayed, 71% overall; FPD: 78% acute, 40% delayed, 40% overall (P > .01 for acute; P < .01 for delayed and overall) There were no grade 3 or 4 toxicities.
Conclusions CR was similar for OPD and FPD; nausea in the delayed and overall periods was signifcantly improved with OPD compared with FPD (P < .01).
Funding Reich Endowment for the Care of the Whole Patient
Click on the PDF icon at the top of this introduction to read the full article.
Background Concurrent chemotherapy radiation therapy may result in significant nausea and vomiting. There have been few studies reporting effective interventions for preventing treatment-related nausea and vomiting.
Objective To compare olanzapine with fosaprepitant for the prevention of nausea and vomiting in patients receiving concurrent highly emetogenic chemotherapy (HEC) and radiotherapy for locally advanced head and neck or esophageal cancer.
Methods 120 chemotherapy and radiotherapy naïve patients with head and neck cancer who were receiving concurrent local radiation and cisplatin were randomized to receive either olanzapine or fosaprepitant in combination with palonosetron and dexamethasone for the prevention of chemotherapy- and radiation-induced nausea and vomiting. The olanzapine, palonosetron, dexamethasone (OPD) regimen was 10 mg oral olanzapine , 0.25 mg IV palonosetron, and 20 mg IV dexamethasone before chemotherapy on day 1, and 10 mg/day of oral olanzapine before chemotherapy on days 2-4. The fosaprepitant, palonosetron, dexamethasone (FPD) regimen was 150 mg IV fosaprepitant, 0.25 mg IV palonosetron, and 12 mg IV dexamethasone before chemotherapy on day 1, and 4 mg dexamethasone PO BID, before chemotherapy days 2 and 3.
Results 101 of the 120 patients were evaluable. In 51 patients who received the OPD regimen, the complete response (CR; no emesis, no rescue medication) rate was 88% for the acute period (24 h after chemotherapy), 76% for the delayed period (days 2-5), and 76% for the overall period (0-120 h). In 50 patients who received the FPD regimen, the CR was 84% acute, 74% delayed, and 74% overall (P > .01 for all periods). Patients with no nausea (0, on a scale 0-10, visual analogue scale) were: OPD: 86% acute, 71% delayed, 71% overall; FPD: 78% acute, 40% delayed, 40% overall (P > .01 for acute; P < .01 for delayed and overall) There were no grade 3 or 4 toxicities.
Conclusions CR was similar for OPD and FPD; nausea in the delayed and overall periods was signifcantly improved with OPD compared with FPD (P < .01).
Funding Reich Endowment for the Care of the Whole Patient
Click on the PDF icon at the top of this introduction to read the full article.
Background Concurrent chemotherapy radiation therapy may result in significant nausea and vomiting. There have been few studies reporting effective interventions for preventing treatment-related nausea and vomiting.
Objective To compare olanzapine with fosaprepitant for the prevention of nausea and vomiting in patients receiving concurrent highly emetogenic chemotherapy (HEC) and radiotherapy for locally advanced head and neck or esophageal cancer.
Methods 120 chemotherapy and radiotherapy naïve patients with head and neck cancer who were receiving concurrent local radiation and cisplatin were randomized to receive either olanzapine or fosaprepitant in combination with palonosetron and dexamethasone for the prevention of chemotherapy- and radiation-induced nausea and vomiting. The olanzapine, palonosetron, dexamethasone (OPD) regimen was 10 mg oral olanzapine , 0.25 mg IV palonosetron, and 20 mg IV dexamethasone before chemotherapy on day 1, and 10 mg/day of oral olanzapine before chemotherapy on days 2-4. The fosaprepitant, palonosetron, dexamethasone (FPD) regimen was 150 mg IV fosaprepitant, 0.25 mg IV palonosetron, and 12 mg IV dexamethasone before chemotherapy on day 1, and 4 mg dexamethasone PO BID, before chemotherapy days 2 and 3.
Results 101 of the 120 patients were evaluable. In 51 patients who received the OPD regimen, the complete response (CR; no emesis, no rescue medication) rate was 88% for the acute period (24 h after chemotherapy), 76% for the delayed period (days 2-5), and 76% for the overall period (0-120 h). In 50 patients who received the FPD regimen, the CR was 84% acute, 74% delayed, and 74% overall (P > .01 for all periods). Patients with no nausea (0, on a scale 0-10, visual analogue scale) were: OPD: 86% acute, 71% delayed, 71% overall; FPD: 78% acute, 40% delayed, 40% overall (P > .01 for acute; P < .01 for delayed and overall) There were no grade 3 or 4 toxicities.
Conclusions CR was similar for OPD and FPD; nausea in the delayed and overall periods was signifcantly improved with OPD compared with FPD (P < .01).
Funding Reich Endowment for the Care of the Whole Patient
Click on the PDF icon at the top of this introduction to read the full article.
More success for immunotherapy with nivolumab approval for metastatic RCC
Change, challenge, and a farewell
OPAT at a Medical Respite Facility
Prolonged hospitalizations for complex patients with severe infections and difficult social situations are becoming very common in many institutions. Outpatient parenteral antimicrobial therapy (OPAT) is widely used[1] and has been found to be a safe, efficient, and cost‐effective way to administer intravenous (IV) antimicrobial therapy to patients, with the potential to decrease hospital length of stay (LOS) and to improve patient satisfaction.[2] Infectious disease (ID) consultation should be involved to determine appropriate candidates for OPAT as well as a suitable drug regimen and duration of therapy,[3] or if oral alternatives can be utilized.[4] OPAT patients require close laboratory monitoring and provider follow‐up for the duration of their care. The combination of ID consultation, patient selection, laboratory monitoring, and follow‐up care have been described as part of a proposed OPAT bundle in recent medical literature.[5] Appropriate patient selection is a key component as to whether or not a patient will be successful with OPAT once discharged from the hospital. Current Infectious Diseases Society of America (IDSA) guidelines recommend that patients be evaluated for stable housing and ability to perform OPAT‐specific duties prior to discharge.[3]
To our knowledge there are no published data regarding the use of OPAT at a medical respite facility for homeless patients with co‐morbid substance abuse and mental illness issues. This may be due to perceived concerns of difficulty in administering OPAT to these disadvantaged patients for multiple reasons such as unstable or no housing, inability to stay engaged in medical care, and underlying mental illness and substance abuse problems. In particular, the concern for substance abuse, specifically injection drug use (IDU), is a significant problem. The current IDSA guidelines for OPAT recommend patients who are likely to abuse a vascular access system are poor candidates for OPAT.[3]
A major barrier to successful utilization of OPAT programs is the need for stable housing so that antibiotics can be administered in a safe setting. Recommending long‐term parenteral therapy as an inpatient for all patients who are homeless or have a history of IDU can lead to prolonged hospitalizations, increased healthcare costs, and contribute to conflicts between patients and staff. Chemical dependence treatment is not available in most inpatient settings, leaving patients with addiction issues without options. Most patients would prefer, when given the choice, to be treated with OPAT outside of the inpatient setting.[6]
This study aimed to evaluate our experience with administering OPAT to homeless patients at a medical respite facility and to determine if patients could complete a successful treatment course of antibiotics for a variety of illnesses.
METHODS
Harborview Medical Center (HMC) is a 413‐bed county hospital, and serves as a major teaching hospital for the University of Washington. It is a level 1 trauma/burn center for Washington, Wyoming, Alaska, Montana, and Idaho. The hospital has 61 psychiatric beds, 29 rehabilitation beds, and 89 intensive care unit beds, with over 60,000 emergency department visits per year. Harborview also serves as a public safety‐net hospital for King County, providing $219 million in charity care in 2013.
Housed in a building adjacent to HMC is a 34‐bed medical respite program,[7] which was established in 2011 through collaboration with King County and 6 other hospitals to serve the homeless population needing medical care, similar to programs in Boston[8] and San Francisco.[9] It is staffed by a multidisciplinary team from HMC including a physician, nurse practitioners, registered nurses, medical assistants, mental health specialists, case managers, and security guards, and accepts patients from all hospitals and clinics within King County. To qualify for medical respite, patient must be homeless and require ongoing nursing needs (ie, wound care, parenteral therapy). Referred patients are screened by a nurse prior to admission. The projected daily cost at medical respite is $350 per patient.
Medical respite is a harm‐reduction model, which includes information on needle exchange programs, narcan kits and education on safer injection practices. Resources are available for patients wishing to start a rehabilitation program, including opiate replacement therapy. Patients may leave the premises during the day, but a curfew is enforced at 9 pm nightly. Patients sign a contract on admission to refrain from using their IV line for IDU and peripherally‐inserted central catheter (PICC) port is secured and monitored for manipulation. Patients who exhibit threatening behavior or who use alcohol/drugs on site are discharged from the program. Patients in need of OPAT must keep nurse visits once or twice daily depending on medication and wound care. Medications needing more frequent dosing were placed on a battery‐operated pump and changed once every 24 hours by nursing.
After obtaining approval from the University of Washington Institutional Review Board, we performed a retrospective chart review of homeless patients over 18 years old discharged from HMC who received OPAT at medical respite from January 1, 2012 to January 1, 2014. There were no exclusions for race, gender, or insurance status. Patients included in the study were respite candidates, and required prolonged parenteral antibiotic therapy. Data collection was performed using a REDCap data collection tool and REDCap grant support.[10] Demographics, diagnosis, and comorbidities, including mental illness, current IDU at time of admission, and remote IDU (last use >3 months ago) were obtained from the electronic medical record. Surgical, microbiologic, and antimicrobial therapy, including route (IV or oral), duration of therapy, and adverse events were abstracted. Primary outcome was defined as successful completion of OPAT at medical respite without nonadherence to therapy or readmission (for presumed OPAT failure). A secondary outcome was antimicrobial course completion for a specific diagnosis defined by achieving goal duration of parenteral and/or oral antibiotic therapy as deemed appropriate by an ID provider. Nonadherence is defined as missing greater than 2 doses of scheduled antibiotic, absence from respite for greater than 24 hours, evidence of line tampering, or expulsion from respite for violation of care agreement. Recurrence of infection was defined as subsequent infection at the same site, following completion of a prior antimicrobial course, at the most recent follow‐up visit.
Continuous variables are expressed as the mean standard deviation, and categorical variables are expressed as the proportion of the entire population. Categorical variables are compared using the 2 test. A 2‐sided P value of 0.05 was considered statistically significant.
RESULTS
Fifty‐one homeless patients were identified with 53 episodes of OPAT between January 1, 2012 and January 1, 2014. For ease of reporting, the number of episodes of OPAT (n = 53) was used as the denominator instead of number of patients (n = 51) for descriptive statistics. The average age was 45 10.4 years (range, 2262 years), 38 (72%) patients were male, and 39 (74%) were Caucasian. Comorbidities included 28 (53%) patients with current IDU and 9 (17%) with a remote history of IDU, 32 (60%) with hepatitis C infection, and 14 (26%) with mental illness (Table 1).
| Comorbidities | No. per Patient Episode, n = 53 (%) |
|---|---|
| |
| Hepatitis C infection | 32 (60%) |
| Current IDU | 28 (53%) |
| Psychiatric/mental illness | 14 (26%) |
| Remote IDU | 9 (17%) |
| Hypertension | 7 (13%) |
| Diabetes type 1 or type 2 | 5 (9%) |
| Rheumatologic diagnosis | 3 (6%) |
| Obesity | 2 (4%) |
| Cardiovascular disease | 2 (4%) |
| Peripheral vascular disease | 2 (4%) |
| Congestive heart failure | 2 (4%) |
| Chronic kidney disease (any stage) | 1 (2%) |
| HIV | 1 (2%) |
Forty‐six (87%) patients were evaluated by an ID physician during their admission. Diagnosis (some patients had multiple) requiring OPAT included: bacteremia in 28, osteomyelitis in 22, skin and soft tissue infection in 19, endocarditis in 15, and epidural abscess in 7 patients. Twenty‐nine patients underwent surgical intervention. The pathogens recovered were primarily gram‐positive organisms. Multidrug resistant organisms were isolated in 11 patients. The IV medications used included vancomycin, nafcillin, cefazolin, ertapenem, and daptomycin.
Forty‐six (87%) patients completed a defined course of antibiotic therapy (deemed appropriate therapy by an ID physician) for their specific infection. Thirty‐four (64%) patients were successfully treated with OPAT at medical respite. There were 19 (36%) failures, which included nonadherent patients, some of whom required urgent readmission (Table 2). There were a total of 16 readmissions, and 10 of those were considered OPAT failures, whereas the other 6 were not (patients admitted for other reasons including, surgery, and IV malfunction). Of the total readmissions, 12 of those were current or remote IDU patients. There is a trend toward a higher prevalence of current/remote IDU among those with clinical failure (15/19, 79%) compared to those with clinical success (22/34, 65%) (P = 0.2788). Overall, 27 (51%) patients were switched to oral therapy after completing an initial IV course. Oral agents used were: trimethoprim‐sulfamethoxazole, rifampin, doxycycline, fluconazole, linezolid, fluoroquinolones, and amoxicillin/clavulanic acid. The average length of OPAT was 22 days. The average daily cost of an acute‐care bed day in 2015 was $1500 at our institution. The cost savings to our institution (using $1500/day inpatient cost compared to $350 per day at medical respite) was $25,000 per episode of OPAT.
| No. of Episodes of Care, n = 53 (%) | |
|---|---|
| |
| Successfully treated at medical respite | 34 (64%) |
| Nonadherent to therapy | 19 (36%) |
| Left respite with IV line in place | 6 [2 admitted, 3 orals, 1 lost] |
| Missed IV doses and switched to orals | 5 |
| Missed IV doses and admitted | 8 admitted |
| Any hospital readmission | 16 (30%) |
| Readmissions, assumed failures | 10 (19%) |
| PICC‐lineassociated infection/bacteremia | 2 |
| SIRS with suspected line infection | 2 |
| Ongoing IDU /discharge from respite | 2 |
| Nonadherent with OPAT/altercations | 3 |
| Acute kidney injury | 1 |
| Readmissions, not counted as failures | 6 (11%) |
| PICC malfunction (leaking) | 2 [1 had further OPAT] |
| Surgery | 4 [3 had further OPAT] |
During the course of OPAT, 7 (13%) patients experienced an adverse event. Of those, we had 1 patient with drug rash, 1 with nausea, and 1 with diarrhea (not infectious). One patient developed leukopenia (white blood cells 4.0), and 2 patients developed neutropenia (absolute neutrophils 750). One patient developed significant elevation of creatinine(>1.9 upper limit of normal) and required inpatient admission. An additional 5 patients had a small elevation of creatinine that did not meet the criteria listed above and were not counted as adverse events by definition. At the study conclusion, 36 (68%) patients had no recurrence of infection at the most recent follow‐up visit at HMC; length of follow‐up ranged from 2 months to 2.5 years. One patient later died of nonOPAT‐related complications. In total, 11 (21%) patients were lost to follow‐up, 1 with a peripherally inserted central catheter line in place.
DISCUSSION
We demonstrated that 87% of homeless patients were able to complete a defined course of antibiotic therapy, and 64% were successfully treated with OPAT at medical respite. To our knowledge this is the first study evaluating this specific population in which OPAT was received at medical respite. Our rate of adverse events (some that required change in drug therapy) was similar to other OPAT studies in the published literature, ranging from 3% to 10% in 1 study,[3] and up to 11% in another.[11] Our total readmission rate of 30% was similar to what current literature suggests, ranging from 9%[11] up to 26%[12] for OPAT patients. Notably, 11% of the readmissions were not related to OPAT failure. This supports the recommendation for close scrutiny of social behaviors in the OPAT patient‐selection process; however, in certain circumstances, IDU alone may not be a reason to fully exclude someone from OPAT candidacy. Careful review of substance abuse history and evaluation of psychosocial factors, such as housing status, mental health history, and outpatient support system are needed. Furthermore, an evaluation of the patient's willingness to comply with care agreements while an inpatient and at respite, and ensuring appropriate resources for chemical dependency treatment are needed. Early consideration of oral antimicrobial options if the patient is readmitted for complications/nonadherence should be encouraged.
Our findings are consistent with results reported by Ho and colleagues, which demonstrated a success rate of 97% of IDU OPAT patients.[13] They carefully chose 29 study patients from 906 in their OPAT program over several years, giving them daily infusions under close supervision. Patients signed an agreement to refrain from accessing their IV lines for drug use. Special security seals were used on all connections and tubing to prevent line tampering. Medical respite in King County uses a similar technique, using a Tegaderm dressing to cover all valves and tubing junction sites to prevent tampering. The IV lines are inspected daily, and ID providers were contacted to discuss any patients suspicious of tampering with their lines to discuss next appropriate steps, either readmission or transition to oral antibiotics. Half of our patients were switched to oral therapy during their course, consistent with current literature.[12, 14]
Traditionally, homeless patients requiring ongoing parenteral therapy have remained inpatients for the duration of their course. Feigal and colleagues evaluated the connection between homelessness and inpatient discharge delays for placement over a 6‐month period in 2009 at an urban hospital.[15] They found homeless patients awaiting placement had an increased median LOS of 26 days, compared to housed individuals with 14 days. Homeless patients without a psychiatric disorder had a delay in discharge 60% longer compared to those with housing, with data adjusted for multiple variables. The cause for delay in discharge in homeless patients was found in those awaiting group home or nursing facility placement, in 50% of cases, whereas delay for chemical dependency program was in 17% of cases, and other local treatment center in 12% of cases.
Medical respite programs are gaining in popularity in the United States since they began in the mid‐1980s.[16] A review by Doran and colleagues found medical respite can result in cost avoidance for hospitals by limiting inpatient days and readmissions.[17] Medical respite can also help engage patients in follow‐up care and assist with housing placement. Many programs promote safe IDU practices and offer referrals for rehabilitation programs, both of which are programs that are not available in most hospitals. Medical respite may continue to be a site of OPAT expansion, as there is continued pressure to discharge nonacute patients from the hospital. Moving forward, it may be beneficial for hospitals, public health departments, and communities to support these programs, which can assist with close monitoring of homeless patients receiving OPAT.
There were several limitations in our study. This was a retrospective observational study with a small patient population comprised of a high prevalence of current and remote IDU. The single center study makes it difficult to generalize to other settings. In addition, there were no comparative data with historical controls, making it difficult to perform comparative analysis.
OPAT is effective for many patients, and it is optimal to utilize ID consultation to determine appropriate candidacy,[3, 4, 5] particularly among IDU. OPAT can be successful in a closely monitored medical respite setting for homeless patients with the help of a multidisciplinary team. Medical respite OPAT can decrease inpatient stays in patients who would otherwise require long hospitalizations, resulting in overall cost savings, and may lead to improved patient satisfaction. Future research linking other outcomes of medical respite OPAT, including substance‐dependence treatment and transition to housing, is warranted.
Acknowledgements
The authors thank the staff at the Harborview Medical Center Infectious Disease Clinic and at Edward Thomas House Medical Respite for their help in this study.
Disclosures: Presented at the oral abstract session Clinical Practice IssuesOPAT in Diverse Populations, IDWeek, October 812, 2014, Philadelphia, Pennsylvania. The authors report no conflicts of interest.
- , , , et al. Experience of infectious diseases consultants with outpatient parenteral antimicrobial therapy: results of an emerging infections network survey. Clin Infect Dis. 2006;43:1290–1295.
- , , , et al. Randomized controlled trial of intravenous antibiotic therapy for cellulitis at home compared with hospital. BMJ. 2005;330:129.
- , , , et al. Practice guidelines for outpatient parenteral antimicrobial therapy. IDSA guidelines. Clin Infect Dis. 2004;38:1651–1672.
- , , . Mandatory infectious diseases approval of outpatient parenteral antimicrobial therapy (OPAT): clinical and economic outcomes of averted cases. J Antimicrob Chemother. 2014;10:1093–1099.
- , , , . Are we ready for an outpatient parenteral antimicrobial therapy bundle? A critical appraisal of the evidence. Clin Infect Dis. 2013;57:419–424.
- , , , et al. Willingness to pay to access patient preferences for therapy in a Canadian setting. BMC Health Serv Res. 2005;5:43.
- UW Medicine. Respite program at Jefferson Terrace (Edward Thomas House). University of Washington website. Available at: http://www.uwmedicine.org/locations/respite‐program‐jefferson‐terrace. Accessed October 1, 2015.
- Boston Healthcare for the Homeless Program. Medical respite care at the Barbara McInnis House. Available at: http://www.bhchp.org/medical‐respite‐care. Accessed October 1, 2015.
- San Francisco Department of Public Health. Medical Respite and Sobering Center. Available at: https://www.sfdph.org/dph/comupg/oprograms/HUH/medrespite.asp. Accessed October 1, 2015.
- , , , , , . Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381.
- , , . Outpatient parenteral antimicrobial therapy (OPAT) in a teaching hospital‐based practice: a retrospective cohort study describing experience and evolution over 10 years. Int J Antimicro Agents. 2012;39:407–413.
- , , , et al. Prediction model for 30‐day hospital readmissions among patients discharged receiving outpatient parenteral antibiotic therapy. Clin Infect Dis. 2014;58:812–819.
- , , , . Safe and successful treatment of intravenous drug users with a peripherally inserted central catheter in an outpatient parenteral antibiotic treatment service. J Antimicrob Chemother. 2010;65:2641–2644.
- , . Outpatient parenteral antimicrobial therapy and antimicrobial stewardship: challenges and checklists. J Antimicrob Chemother. 2015;70:965–970.
- , , , , , . Homelessness and discharge delays from an urban safety net hospital. Public Health. 2014;128:1033–1035.
- , , . Medical respite care for homeless people: a growing national phenomenon. J Health Care Poor Underserved. 2009;20:36–41.
- , , , . Medical respite programs for homeless patients: a systematic review. J Health Care Poor Underserved. 2013;24:499–524.
Prolonged hospitalizations for complex patients with severe infections and difficult social situations are becoming very common in many institutions. Outpatient parenteral antimicrobial therapy (OPAT) is widely used[1] and has been found to be a safe, efficient, and cost‐effective way to administer intravenous (IV) antimicrobial therapy to patients, with the potential to decrease hospital length of stay (LOS) and to improve patient satisfaction.[2] Infectious disease (ID) consultation should be involved to determine appropriate candidates for OPAT as well as a suitable drug regimen and duration of therapy,[3] or if oral alternatives can be utilized.[4] OPAT patients require close laboratory monitoring and provider follow‐up for the duration of their care. The combination of ID consultation, patient selection, laboratory monitoring, and follow‐up care have been described as part of a proposed OPAT bundle in recent medical literature.[5] Appropriate patient selection is a key component as to whether or not a patient will be successful with OPAT once discharged from the hospital. Current Infectious Diseases Society of America (IDSA) guidelines recommend that patients be evaluated for stable housing and ability to perform OPAT‐specific duties prior to discharge.[3]
To our knowledge there are no published data regarding the use of OPAT at a medical respite facility for homeless patients with co‐morbid substance abuse and mental illness issues. This may be due to perceived concerns of difficulty in administering OPAT to these disadvantaged patients for multiple reasons such as unstable or no housing, inability to stay engaged in medical care, and underlying mental illness and substance abuse problems. In particular, the concern for substance abuse, specifically injection drug use (IDU), is a significant problem. The current IDSA guidelines for OPAT recommend patients who are likely to abuse a vascular access system are poor candidates for OPAT.[3]
A major barrier to successful utilization of OPAT programs is the need for stable housing so that antibiotics can be administered in a safe setting. Recommending long‐term parenteral therapy as an inpatient for all patients who are homeless or have a history of IDU can lead to prolonged hospitalizations, increased healthcare costs, and contribute to conflicts between patients and staff. Chemical dependence treatment is not available in most inpatient settings, leaving patients with addiction issues without options. Most patients would prefer, when given the choice, to be treated with OPAT outside of the inpatient setting.[6]
This study aimed to evaluate our experience with administering OPAT to homeless patients at a medical respite facility and to determine if patients could complete a successful treatment course of antibiotics for a variety of illnesses.
METHODS
Harborview Medical Center (HMC) is a 413‐bed county hospital, and serves as a major teaching hospital for the University of Washington. It is a level 1 trauma/burn center for Washington, Wyoming, Alaska, Montana, and Idaho. The hospital has 61 psychiatric beds, 29 rehabilitation beds, and 89 intensive care unit beds, with over 60,000 emergency department visits per year. Harborview also serves as a public safety‐net hospital for King County, providing $219 million in charity care in 2013.
Housed in a building adjacent to HMC is a 34‐bed medical respite program,[7] which was established in 2011 through collaboration with King County and 6 other hospitals to serve the homeless population needing medical care, similar to programs in Boston[8] and San Francisco.[9] It is staffed by a multidisciplinary team from HMC including a physician, nurse practitioners, registered nurses, medical assistants, mental health specialists, case managers, and security guards, and accepts patients from all hospitals and clinics within King County. To qualify for medical respite, patient must be homeless and require ongoing nursing needs (ie, wound care, parenteral therapy). Referred patients are screened by a nurse prior to admission. The projected daily cost at medical respite is $350 per patient.
Medical respite is a harm‐reduction model, which includes information on needle exchange programs, narcan kits and education on safer injection practices. Resources are available for patients wishing to start a rehabilitation program, including opiate replacement therapy. Patients may leave the premises during the day, but a curfew is enforced at 9 pm nightly. Patients sign a contract on admission to refrain from using their IV line for IDU and peripherally‐inserted central catheter (PICC) port is secured and monitored for manipulation. Patients who exhibit threatening behavior or who use alcohol/drugs on site are discharged from the program. Patients in need of OPAT must keep nurse visits once or twice daily depending on medication and wound care. Medications needing more frequent dosing were placed on a battery‐operated pump and changed once every 24 hours by nursing.
After obtaining approval from the University of Washington Institutional Review Board, we performed a retrospective chart review of homeless patients over 18 years old discharged from HMC who received OPAT at medical respite from January 1, 2012 to January 1, 2014. There were no exclusions for race, gender, or insurance status. Patients included in the study were respite candidates, and required prolonged parenteral antibiotic therapy. Data collection was performed using a REDCap data collection tool and REDCap grant support.[10] Demographics, diagnosis, and comorbidities, including mental illness, current IDU at time of admission, and remote IDU (last use >3 months ago) were obtained from the electronic medical record. Surgical, microbiologic, and antimicrobial therapy, including route (IV or oral), duration of therapy, and adverse events were abstracted. Primary outcome was defined as successful completion of OPAT at medical respite without nonadherence to therapy or readmission (for presumed OPAT failure). A secondary outcome was antimicrobial course completion for a specific diagnosis defined by achieving goal duration of parenteral and/or oral antibiotic therapy as deemed appropriate by an ID provider. Nonadherence is defined as missing greater than 2 doses of scheduled antibiotic, absence from respite for greater than 24 hours, evidence of line tampering, or expulsion from respite for violation of care agreement. Recurrence of infection was defined as subsequent infection at the same site, following completion of a prior antimicrobial course, at the most recent follow‐up visit.
Continuous variables are expressed as the mean standard deviation, and categorical variables are expressed as the proportion of the entire population. Categorical variables are compared using the 2 test. A 2‐sided P value of 0.05 was considered statistically significant.
RESULTS
Fifty‐one homeless patients were identified with 53 episodes of OPAT between January 1, 2012 and January 1, 2014. For ease of reporting, the number of episodes of OPAT (n = 53) was used as the denominator instead of number of patients (n = 51) for descriptive statistics. The average age was 45 10.4 years (range, 2262 years), 38 (72%) patients were male, and 39 (74%) were Caucasian. Comorbidities included 28 (53%) patients with current IDU and 9 (17%) with a remote history of IDU, 32 (60%) with hepatitis C infection, and 14 (26%) with mental illness (Table 1).
| Comorbidities | No. per Patient Episode, n = 53 (%) |
|---|---|
| |
| Hepatitis C infection | 32 (60%) |
| Current IDU | 28 (53%) |
| Psychiatric/mental illness | 14 (26%) |
| Remote IDU | 9 (17%) |
| Hypertension | 7 (13%) |
| Diabetes type 1 or type 2 | 5 (9%) |
| Rheumatologic diagnosis | 3 (6%) |
| Obesity | 2 (4%) |
| Cardiovascular disease | 2 (4%) |
| Peripheral vascular disease | 2 (4%) |
| Congestive heart failure | 2 (4%) |
| Chronic kidney disease (any stage) | 1 (2%) |
| HIV | 1 (2%) |
Forty‐six (87%) patients were evaluated by an ID physician during their admission. Diagnosis (some patients had multiple) requiring OPAT included: bacteremia in 28, osteomyelitis in 22, skin and soft tissue infection in 19, endocarditis in 15, and epidural abscess in 7 patients. Twenty‐nine patients underwent surgical intervention. The pathogens recovered were primarily gram‐positive organisms. Multidrug resistant organisms were isolated in 11 patients. The IV medications used included vancomycin, nafcillin, cefazolin, ertapenem, and daptomycin.
Forty‐six (87%) patients completed a defined course of antibiotic therapy (deemed appropriate therapy by an ID physician) for their specific infection. Thirty‐four (64%) patients were successfully treated with OPAT at medical respite. There were 19 (36%) failures, which included nonadherent patients, some of whom required urgent readmission (Table 2). There were a total of 16 readmissions, and 10 of those were considered OPAT failures, whereas the other 6 were not (patients admitted for other reasons including, surgery, and IV malfunction). Of the total readmissions, 12 of those were current or remote IDU patients. There is a trend toward a higher prevalence of current/remote IDU among those with clinical failure (15/19, 79%) compared to those with clinical success (22/34, 65%) (P = 0.2788). Overall, 27 (51%) patients were switched to oral therapy after completing an initial IV course. Oral agents used were: trimethoprim‐sulfamethoxazole, rifampin, doxycycline, fluconazole, linezolid, fluoroquinolones, and amoxicillin/clavulanic acid. The average length of OPAT was 22 days. The average daily cost of an acute‐care bed day in 2015 was $1500 at our institution. The cost savings to our institution (using $1500/day inpatient cost compared to $350 per day at medical respite) was $25,000 per episode of OPAT.
| No. of Episodes of Care, n = 53 (%) | |
|---|---|
| |
| Successfully treated at medical respite | 34 (64%) |
| Nonadherent to therapy | 19 (36%) |
| Left respite with IV line in place | 6 [2 admitted, 3 orals, 1 lost] |
| Missed IV doses and switched to orals | 5 |
| Missed IV doses and admitted | 8 admitted |
| Any hospital readmission | 16 (30%) |
| Readmissions, assumed failures | 10 (19%) |
| PICC‐lineassociated infection/bacteremia | 2 |
| SIRS with suspected line infection | 2 |
| Ongoing IDU /discharge from respite | 2 |
| Nonadherent with OPAT/altercations | 3 |
| Acute kidney injury | 1 |
| Readmissions, not counted as failures | 6 (11%) |
| PICC malfunction (leaking) | 2 [1 had further OPAT] |
| Surgery | 4 [3 had further OPAT] |
During the course of OPAT, 7 (13%) patients experienced an adverse event. Of those, we had 1 patient with drug rash, 1 with nausea, and 1 with diarrhea (not infectious). One patient developed leukopenia (white blood cells 4.0), and 2 patients developed neutropenia (absolute neutrophils 750). One patient developed significant elevation of creatinine(>1.9 upper limit of normal) and required inpatient admission. An additional 5 patients had a small elevation of creatinine that did not meet the criteria listed above and were not counted as adverse events by definition. At the study conclusion, 36 (68%) patients had no recurrence of infection at the most recent follow‐up visit at HMC; length of follow‐up ranged from 2 months to 2.5 years. One patient later died of nonOPAT‐related complications. In total, 11 (21%) patients were lost to follow‐up, 1 with a peripherally inserted central catheter line in place.
DISCUSSION
We demonstrated that 87% of homeless patients were able to complete a defined course of antibiotic therapy, and 64% were successfully treated with OPAT at medical respite. To our knowledge this is the first study evaluating this specific population in which OPAT was received at medical respite. Our rate of adverse events (some that required change in drug therapy) was similar to other OPAT studies in the published literature, ranging from 3% to 10% in 1 study,[3] and up to 11% in another.[11] Our total readmission rate of 30% was similar to what current literature suggests, ranging from 9%[11] up to 26%[12] for OPAT patients. Notably, 11% of the readmissions were not related to OPAT failure. This supports the recommendation for close scrutiny of social behaviors in the OPAT patient‐selection process; however, in certain circumstances, IDU alone may not be a reason to fully exclude someone from OPAT candidacy. Careful review of substance abuse history and evaluation of psychosocial factors, such as housing status, mental health history, and outpatient support system are needed. Furthermore, an evaluation of the patient's willingness to comply with care agreements while an inpatient and at respite, and ensuring appropriate resources for chemical dependency treatment are needed. Early consideration of oral antimicrobial options if the patient is readmitted for complications/nonadherence should be encouraged.
Our findings are consistent with results reported by Ho and colleagues, which demonstrated a success rate of 97% of IDU OPAT patients.[13] They carefully chose 29 study patients from 906 in their OPAT program over several years, giving them daily infusions under close supervision. Patients signed an agreement to refrain from accessing their IV lines for drug use. Special security seals were used on all connections and tubing to prevent line tampering. Medical respite in King County uses a similar technique, using a Tegaderm dressing to cover all valves and tubing junction sites to prevent tampering. The IV lines are inspected daily, and ID providers were contacted to discuss any patients suspicious of tampering with their lines to discuss next appropriate steps, either readmission or transition to oral antibiotics. Half of our patients were switched to oral therapy during their course, consistent with current literature.[12, 14]
Traditionally, homeless patients requiring ongoing parenteral therapy have remained inpatients for the duration of their course. Feigal and colleagues evaluated the connection between homelessness and inpatient discharge delays for placement over a 6‐month period in 2009 at an urban hospital.[15] They found homeless patients awaiting placement had an increased median LOS of 26 days, compared to housed individuals with 14 days. Homeless patients without a psychiatric disorder had a delay in discharge 60% longer compared to those with housing, with data adjusted for multiple variables. The cause for delay in discharge in homeless patients was found in those awaiting group home or nursing facility placement, in 50% of cases, whereas delay for chemical dependency program was in 17% of cases, and other local treatment center in 12% of cases.
Medical respite programs are gaining in popularity in the United States since they began in the mid‐1980s.[16] A review by Doran and colleagues found medical respite can result in cost avoidance for hospitals by limiting inpatient days and readmissions.[17] Medical respite can also help engage patients in follow‐up care and assist with housing placement. Many programs promote safe IDU practices and offer referrals for rehabilitation programs, both of which are programs that are not available in most hospitals. Medical respite may continue to be a site of OPAT expansion, as there is continued pressure to discharge nonacute patients from the hospital. Moving forward, it may be beneficial for hospitals, public health departments, and communities to support these programs, which can assist with close monitoring of homeless patients receiving OPAT.
There were several limitations in our study. This was a retrospective observational study with a small patient population comprised of a high prevalence of current and remote IDU. The single center study makes it difficult to generalize to other settings. In addition, there were no comparative data with historical controls, making it difficult to perform comparative analysis.
OPAT is effective for many patients, and it is optimal to utilize ID consultation to determine appropriate candidacy,[3, 4, 5] particularly among IDU. OPAT can be successful in a closely monitored medical respite setting for homeless patients with the help of a multidisciplinary team. Medical respite OPAT can decrease inpatient stays in patients who would otherwise require long hospitalizations, resulting in overall cost savings, and may lead to improved patient satisfaction. Future research linking other outcomes of medical respite OPAT, including substance‐dependence treatment and transition to housing, is warranted.
Acknowledgements
The authors thank the staff at the Harborview Medical Center Infectious Disease Clinic and at Edward Thomas House Medical Respite for their help in this study.
Disclosures: Presented at the oral abstract session Clinical Practice IssuesOPAT in Diverse Populations, IDWeek, October 812, 2014, Philadelphia, Pennsylvania. The authors report no conflicts of interest.
Prolonged hospitalizations for complex patients with severe infections and difficult social situations are becoming very common in many institutions. Outpatient parenteral antimicrobial therapy (OPAT) is widely used[1] and has been found to be a safe, efficient, and cost‐effective way to administer intravenous (IV) antimicrobial therapy to patients, with the potential to decrease hospital length of stay (LOS) and to improve patient satisfaction.[2] Infectious disease (ID) consultation should be involved to determine appropriate candidates for OPAT as well as a suitable drug regimen and duration of therapy,[3] or if oral alternatives can be utilized.[4] OPAT patients require close laboratory monitoring and provider follow‐up for the duration of their care. The combination of ID consultation, patient selection, laboratory monitoring, and follow‐up care have been described as part of a proposed OPAT bundle in recent medical literature.[5] Appropriate patient selection is a key component as to whether or not a patient will be successful with OPAT once discharged from the hospital. Current Infectious Diseases Society of America (IDSA) guidelines recommend that patients be evaluated for stable housing and ability to perform OPAT‐specific duties prior to discharge.[3]
To our knowledge there are no published data regarding the use of OPAT at a medical respite facility for homeless patients with co‐morbid substance abuse and mental illness issues. This may be due to perceived concerns of difficulty in administering OPAT to these disadvantaged patients for multiple reasons such as unstable or no housing, inability to stay engaged in medical care, and underlying mental illness and substance abuse problems. In particular, the concern for substance abuse, specifically injection drug use (IDU), is a significant problem. The current IDSA guidelines for OPAT recommend patients who are likely to abuse a vascular access system are poor candidates for OPAT.[3]
A major barrier to successful utilization of OPAT programs is the need for stable housing so that antibiotics can be administered in a safe setting. Recommending long‐term parenteral therapy as an inpatient for all patients who are homeless or have a history of IDU can lead to prolonged hospitalizations, increased healthcare costs, and contribute to conflicts between patients and staff. Chemical dependence treatment is not available in most inpatient settings, leaving patients with addiction issues without options. Most patients would prefer, when given the choice, to be treated with OPAT outside of the inpatient setting.[6]
This study aimed to evaluate our experience with administering OPAT to homeless patients at a medical respite facility and to determine if patients could complete a successful treatment course of antibiotics for a variety of illnesses.
METHODS
Harborview Medical Center (HMC) is a 413‐bed county hospital, and serves as a major teaching hospital for the University of Washington. It is a level 1 trauma/burn center for Washington, Wyoming, Alaska, Montana, and Idaho. The hospital has 61 psychiatric beds, 29 rehabilitation beds, and 89 intensive care unit beds, with over 60,000 emergency department visits per year. Harborview also serves as a public safety‐net hospital for King County, providing $219 million in charity care in 2013.
Housed in a building adjacent to HMC is a 34‐bed medical respite program,[7] which was established in 2011 through collaboration with King County and 6 other hospitals to serve the homeless population needing medical care, similar to programs in Boston[8] and San Francisco.[9] It is staffed by a multidisciplinary team from HMC including a physician, nurse practitioners, registered nurses, medical assistants, mental health specialists, case managers, and security guards, and accepts patients from all hospitals and clinics within King County. To qualify for medical respite, patient must be homeless and require ongoing nursing needs (ie, wound care, parenteral therapy). Referred patients are screened by a nurse prior to admission. The projected daily cost at medical respite is $350 per patient.
Medical respite is a harm‐reduction model, which includes information on needle exchange programs, narcan kits and education on safer injection practices. Resources are available for patients wishing to start a rehabilitation program, including opiate replacement therapy. Patients may leave the premises during the day, but a curfew is enforced at 9 pm nightly. Patients sign a contract on admission to refrain from using their IV line for IDU and peripherally‐inserted central catheter (PICC) port is secured and monitored for manipulation. Patients who exhibit threatening behavior or who use alcohol/drugs on site are discharged from the program. Patients in need of OPAT must keep nurse visits once or twice daily depending on medication and wound care. Medications needing more frequent dosing were placed on a battery‐operated pump and changed once every 24 hours by nursing.
After obtaining approval from the University of Washington Institutional Review Board, we performed a retrospective chart review of homeless patients over 18 years old discharged from HMC who received OPAT at medical respite from January 1, 2012 to January 1, 2014. There were no exclusions for race, gender, or insurance status. Patients included in the study were respite candidates, and required prolonged parenteral antibiotic therapy. Data collection was performed using a REDCap data collection tool and REDCap grant support.[10] Demographics, diagnosis, and comorbidities, including mental illness, current IDU at time of admission, and remote IDU (last use >3 months ago) were obtained from the electronic medical record. Surgical, microbiologic, and antimicrobial therapy, including route (IV or oral), duration of therapy, and adverse events were abstracted. Primary outcome was defined as successful completion of OPAT at medical respite without nonadherence to therapy or readmission (for presumed OPAT failure). A secondary outcome was antimicrobial course completion for a specific diagnosis defined by achieving goal duration of parenteral and/or oral antibiotic therapy as deemed appropriate by an ID provider. Nonadherence is defined as missing greater than 2 doses of scheduled antibiotic, absence from respite for greater than 24 hours, evidence of line tampering, or expulsion from respite for violation of care agreement. Recurrence of infection was defined as subsequent infection at the same site, following completion of a prior antimicrobial course, at the most recent follow‐up visit.
Continuous variables are expressed as the mean standard deviation, and categorical variables are expressed as the proportion of the entire population. Categorical variables are compared using the 2 test. A 2‐sided P value of 0.05 was considered statistically significant.
RESULTS
Fifty‐one homeless patients were identified with 53 episodes of OPAT between January 1, 2012 and January 1, 2014. For ease of reporting, the number of episodes of OPAT (n = 53) was used as the denominator instead of number of patients (n = 51) for descriptive statistics. The average age was 45 10.4 years (range, 2262 years), 38 (72%) patients were male, and 39 (74%) were Caucasian. Comorbidities included 28 (53%) patients with current IDU and 9 (17%) with a remote history of IDU, 32 (60%) with hepatitis C infection, and 14 (26%) with mental illness (Table 1).
| Comorbidities | No. per Patient Episode, n = 53 (%) |
|---|---|
| |
| Hepatitis C infection | 32 (60%) |
| Current IDU | 28 (53%) |
| Psychiatric/mental illness | 14 (26%) |
| Remote IDU | 9 (17%) |
| Hypertension | 7 (13%) |
| Diabetes type 1 or type 2 | 5 (9%) |
| Rheumatologic diagnosis | 3 (6%) |
| Obesity | 2 (4%) |
| Cardiovascular disease | 2 (4%) |
| Peripheral vascular disease | 2 (4%) |
| Congestive heart failure | 2 (4%) |
| Chronic kidney disease (any stage) | 1 (2%) |
| HIV | 1 (2%) |
Forty‐six (87%) patients were evaluated by an ID physician during their admission. Diagnosis (some patients had multiple) requiring OPAT included: bacteremia in 28, osteomyelitis in 22, skin and soft tissue infection in 19, endocarditis in 15, and epidural abscess in 7 patients. Twenty‐nine patients underwent surgical intervention. The pathogens recovered were primarily gram‐positive organisms. Multidrug resistant organisms were isolated in 11 patients. The IV medications used included vancomycin, nafcillin, cefazolin, ertapenem, and daptomycin.
Forty‐six (87%) patients completed a defined course of antibiotic therapy (deemed appropriate therapy by an ID physician) for their specific infection. Thirty‐four (64%) patients were successfully treated with OPAT at medical respite. There were 19 (36%) failures, which included nonadherent patients, some of whom required urgent readmission (Table 2). There were a total of 16 readmissions, and 10 of those were considered OPAT failures, whereas the other 6 were not (patients admitted for other reasons including, surgery, and IV malfunction). Of the total readmissions, 12 of those were current or remote IDU patients. There is a trend toward a higher prevalence of current/remote IDU among those with clinical failure (15/19, 79%) compared to those with clinical success (22/34, 65%) (P = 0.2788). Overall, 27 (51%) patients were switched to oral therapy after completing an initial IV course. Oral agents used were: trimethoprim‐sulfamethoxazole, rifampin, doxycycline, fluconazole, linezolid, fluoroquinolones, and amoxicillin/clavulanic acid. The average length of OPAT was 22 days. The average daily cost of an acute‐care bed day in 2015 was $1500 at our institution. The cost savings to our institution (using $1500/day inpatient cost compared to $350 per day at medical respite) was $25,000 per episode of OPAT.
| No. of Episodes of Care, n = 53 (%) | |
|---|---|
| |
| Successfully treated at medical respite | 34 (64%) |
| Nonadherent to therapy | 19 (36%) |
| Left respite with IV line in place | 6 [2 admitted, 3 orals, 1 lost] |
| Missed IV doses and switched to orals | 5 |
| Missed IV doses and admitted | 8 admitted |
| Any hospital readmission | 16 (30%) |
| Readmissions, assumed failures | 10 (19%) |
| PICC‐lineassociated infection/bacteremia | 2 |
| SIRS with suspected line infection | 2 |
| Ongoing IDU /discharge from respite | 2 |
| Nonadherent with OPAT/altercations | 3 |
| Acute kidney injury | 1 |
| Readmissions, not counted as failures | 6 (11%) |
| PICC malfunction (leaking) | 2 [1 had further OPAT] |
| Surgery | 4 [3 had further OPAT] |
During the course of OPAT, 7 (13%) patients experienced an adverse event. Of those, we had 1 patient with drug rash, 1 with nausea, and 1 with diarrhea (not infectious). One patient developed leukopenia (white blood cells 4.0), and 2 patients developed neutropenia (absolute neutrophils 750). One patient developed significant elevation of creatinine(>1.9 upper limit of normal) and required inpatient admission. An additional 5 patients had a small elevation of creatinine that did not meet the criteria listed above and were not counted as adverse events by definition. At the study conclusion, 36 (68%) patients had no recurrence of infection at the most recent follow‐up visit at HMC; length of follow‐up ranged from 2 months to 2.5 years. One patient later died of nonOPAT‐related complications. In total, 11 (21%) patients were lost to follow‐up, 1 with a peripherally inserted central catheter line in place.
DISCUSSION
We demonstrated that 87% of homeless patients were able to complete a defined course of antibiotic therapy, and 64% were successfully treated with OPAT at medical respite. To our knowledge this is the first study evaluating this specific population in which OPAT was received at medical respite. Our rate of adverse events (some that required change in drug therapy) was similar to other OPAT studies in the published literature, ranging from 3% to 10% in 1 study,[3] and up to 11% in another.[11] Our total readmission rate of 30% was similar to what current literature suggests, ranging from 9%[11] up to 26%[12] for OPAT patients. Notably, 11% of the readmissions were not related to OPAT failure. This supports the recommendation for close scrutiny of social behaviors in the OPAT patient‐selection process; however, in certain circumstances, IDU alone may not be a reason to fully exclude someone from OPAT candidacy. Careful review of substance abuse history and evaluation of psychosocial factors, such as housing status, mental health history, and outpatient support system are needed. Furthermore, an evaluation of the patient's willingness to comply with care agreements while an inpatient and at respite, and ensuring appropriate resources for chemical dependency treatment are needed. Early consideration of oral antimicrobial options if the patient is readmitted for complications/nonadherence should be encouraged.
Our findings are consistent with results reported by Ho and colleagues, which demonstrated a success rate of 97% of IDU OPAT patients.[13] They carefully chose 29 study patients from 906 in their OPAT program over several years, giving them daily infusions under close supervision. Patients signed an agreement to refrain from accessing their IV lines for drug use. Special security seals were used on all connections and tubing to prevent line tampering. Medical respite in King County uses a similar technique, using a Tegaderm dressing to cover all valves and tubing junction sites to prevent tampering. The IV lines are inspected daily, and ID providers were contacted to discuss any patients suspicious of tampering with their lines to discuss next appropriate steps, either readmission or transition to oral antibiotics. Half of our patients were switched to oral therapy during their course, consistent with current literature.[12, 14]
Traditionally, homeless patients requiring ongoing parenteral therapy have remained inpatients for the duration of their course. Feigal and colleagues evaluated the connection between homelessness and inpatient discharge delays for placement over a 6‐month period in 2009 at an urban hospital.[15] They found homeless patients awaiting placement had an increased median LOS of 26 days, compared to housed individuals with 14 days. Homeless patients without a psychiatric disorder had a delay in discharge 60% longer compared to those with housing, with data adjusted for multiple variables. The cause for delay in discharge in homeless patients was found in those awaiting group home or nursing facility placement, in 50% of cases, whereas delay for chemical dependency program was in 17% of cases, and other local treatment center in 12% of cases.
Medical respite programs are gaining in popularity in the United States since they began in the mid‐1980s.[16] A review by Doran and colleagues found medical respite can result in cost avoidance for hospitals by limiting inpatient days and readmissions.[17] Medical respite can also help engage patients in follow‐up care and assist with housing placement. Many programs promote safe IDU practices and offer referrals for rehabilitation programs, both of which are programs that are not available in most hospitals. Medical respite may continue to be a site of OPAT expansion, as there is continued pressure to discharge nonacute patients from the hospital. Moving forward, it may be beneficial for hospitals, public health departments, and communities to support these programs, which can assist with close monitoring of homeless patients receiving OPAT.
There were several limitations in our study. This was a retrospective observational study with a small patient population comprised of a high prevalence of current and remote IDU. The single center study makes it difficult to generalize to other settings. In addition, there were no comparative data with historical controls, making it difficult to perform comparative analysis.
OPAT is effective for many patients, and it is optimal to utilize ID consultation to determine appropriate candidacy,[3, 4, 5] particularly among IDU. OPAT can be successful in a closely monitored medical respite setting for homeless patients with the help of a multidisciplinary team. Medical respite OPAT can decrease inpatient stays in patients who would otherwise require long hospitalizations, resulting in overall cost savings, and may lead to improved patient satisfaction. Future research linking other outcomes of medical respite OPAT, including substance‐dependence treatment and transition to housing, is warranted.
Acknowledgements
The authors thank the staff at the Harborview Medical Center Infectious Disease Clinic and at Edward Thomas House Medical Respite for their help in this study.
Disclosures: Presented at the oral abstract session Clinical Practice IssuesOPAT in Diverse Populations, IDWeek, October 812, 2014, Philadelphia, Pennsylvania. The authors report no conflicts of interest.
- , , , et al. Experience of infectious diseases consultants with outpatient parenteral antimicrobial therapy: results of an emerging infections network survey. Clin Infect Dis. 2006;43:1290–1295.
- , , , et al. Randomized controlled trial of intravenous antibiotic therapy for cellulitis at home compared with hospital. BMJ. 2005;330:129.
- , , , et al. Practice guidelines for outpatient parenteral antimicrobial therapy. IDSA guidelines. Clin Infect Dis. 2004;38:1651–1672.
- , , . Mandatory infectious diseases approval of outpatient parenteral antimicrobial therapy (OPAT): clinical and economic outcomes of averted cases. J Antimicrob Chemother. 2014;10:1093–1099.
- , , , . Are we ready for an outpatient parenteral antimicrobial therapy bundle? A critical appraisal of the evidence. Clin Infect Dis. 2013;57:419–424.
- , , , et al. Willingness to pay to access patient preferences for therapy in a Canadian setting. BMC Health Serv Res. 2005;5:43.
- UW Medicine. Respite program at Jefferson Terrace (Edward Thomas House). University of Washington website. Available at: http://www.uwmedicine.org/locations/respite‐program‐jefferson‐terrace. Accessed October 1, 2015.
- Boston Healthcare for the Homeless Program. Medical respite care at the Barbara McInnis House. Available at: http://www.bhchp.org/medical‐respite‐care. Accessed October 1, 2015.
- San Francisco Department of Public Health. Medical Respite and Sobering Center. Available at: https://www.sfdph.org/dph/comupg/oprograms/HUH/medrespite.asp. Accessed October 1, 2015.
- , , , , , . Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381.
- , , . Outpatient parenteral antimicrobial therapy (OPAT) in a teaching hospital‐based practice: a retrospective cohort study describing experience and evolution over 10 years. Int J Antimicro Agents. 2012;39:407–413.
- , , , et al. Prediction model for 30‐day hospital readmissions among patients discharged receiving outpatient parenteral antibiotic therapy. Clin Infect Dis. 2014;58:812–819.
- , , , . Safe and successful treatment of intravenous drug users with a peripherally inserted central catheter in an outpatient parenteral antibiotic treatment service. J Antimicrob Chemother. 2010;65:2641–2644.
- , . Outpatient parenteral antimicrobial therapy and antimicrobial stewardship: challenges and checklists. J Antimicrob Chemother. 2015;70:965–970.
- , , , , , . Homelessness and discharge delays from an urban safety net hospital. Public Health. 2014;128:1033–1035.
- , , . Medical respite care for homeless people: a growing national phenomenon. J Health Care Poor Underserved. 2009;20:36–41.
- , , , . Medical respite programs for homeless patients: a systematic review. J Health Care Poor Underserved. 2013;24:499–524.
- , , , et al. Experience of infectious diseases consultants with outpatient parenteral antimicrobial therapy: results of an emerging infections network survey. Clin Infect Dis. 2006;43:1290–1295.
- , , , et al. Randomized controlled trial of intravenous antibiotic therapy for cellulitis at home compared with hospital. BMJ. 2005;330:129.
- , , , et al. Practice guidelines for outpatient parenteral antimicrobial therapy. IDSA guidelines. Clin Infect Dis. 2004;38:1651–1672.
- , , . Mandatory infectious diseases approval of outpatient parenteral antimicrobial therapy (OPAT): clinical and economic outcomes of averted cases. J Antimicrob Chemother. 2014;10:1093–1099.
- , , , . Are we ready for an outpatient parenteral antimicrobial therapy bundle? A critical appraisal of the evidence. Clin Infect Dis. 2013;57:419–424.
- , , , et al. Willingness to pay to access patient preferences for therapy in a Canadian setting. BMC Health Serv Res. 2005;5:43.
- UW Medicine. Respite program at Jefferson Terrace (Edward Thomas House). University of Washington website. Available at: http://www.uwmedicine.org/locations/respite‐program‐jefferson‐terrace. Accessed October 1, 2015.
- Boston Healthcare for the Homeless Program. Medical respite care at the Barbara McInnis House. Available at: http://www.bhchp.org/medical‐respite‐care. Accessed October 1, 2015.
- San Francisco Department of Public Health. Medical Respite and Sobering Center. Available at: https://www.sfdph.org/dph/comupg/oprograms/HUH/medrespite.asp. Accessed October 1, 2015.
- , , , , , . Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381.
- , , . Outpatient parenteral antimicrobial therapy (OPAT) in a teaching hospital‐based practice: a retrospective cohort study describing experience and evolution over 10 years. Int J Antimicro Agents. 2012;39:407–413.
- , , , et al. Prediction model for 30‐day hospital readmissions among patients discharged receiving outpatient parenteral antibiotic therapy. Clin Infect Dis. 2014;58:812–819.
- , , , . Safe and successful treatment of intravenous drug users with a peripherally inserted central catheter in an outpatient parenteral antibiotic treatment service. J Antimicrob Chemother. 2010;65:2641–2644.
- , . Outpatient parenteral antimicrobial therapy and antimicrobial stewardship: challenges and checklists. J Antimicrob Chemother. 2015;70:965–970.
- , , , , , . Homelessness and discharge delays from an urban safety net hospital. Public Health. 2014;128:1033–1035.
- , , . Medical respite care for homeless people: a growing national phenomenon. J Health Care Poor Underserved. 2009;20:36–41.
- , , , . Medical respite programs for homeless patients: a systematic review. J Health Care Poor Underserved. 2013;24:499–524.
Painful, swollen, oozing right great toe
A 68-year-old Filipino man with a history of hypertension, type 2 diabetes, and osteoarthritis presented to the emergency department with a one-week history of increasing pain, swelling, erythema, and seepage of his right great toe. The patient denied paresthesias, fever, chills, night sweats, cough, dyspnea, or any change in his diet, medications (which included lisinopril, metformin, and acetaminophen as needed), or routine. Social history was negative for alcohol use and cigarette smoking.
He previously had similar symptoms in his right fourth toe that resulted in amputation. The patient was told at the time that he had a “bone infection” and amputation was necessary.
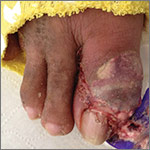
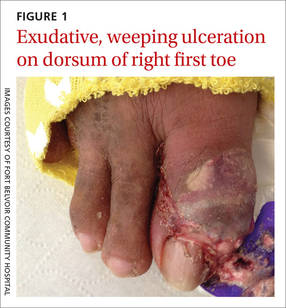
The patient was thin, alert, oriented, and in no acute distress. His vital signs and a cardiopulmonary exam were normal. The patient’s right great toe was tender to touch, with ulceration of the skin dorsally at the proximal nail fold. In addition, his toe was oozing a purulent, non-foul smelling discharge (FIGURE 1). Other pertinent findings included multiple enlarged joints on both hands with visible yellow-white subcutaneous nodules on the hands and dorsum of the forearm (FIGURE 2).
The patient’s white blood cell count was 10,800/mcL, C-reactive protein (CRP) was 18 mg/L, erythrocyte sedimentation rate (ESR) was 80 mm/hr, and uric acid was 12.5 mg/dL. His blood urea nitrogen was 52 mg/dL and creatinine was 2.5 mg/dL. A glycated hemoglobin test was 7.2%. A wound culture, aspirate from the dorsum of the toe, and x-ray were obtained.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Tophaceous gouty arthritis
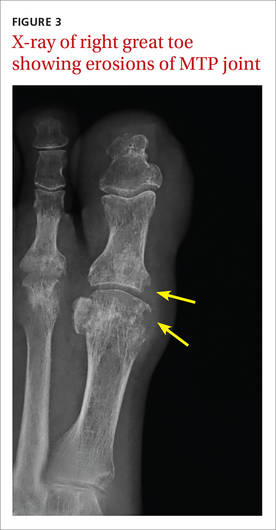
The x-ray of the right great toe showed erosions of the metatarsophalangeal (MTP) joint (FIGURE 3). Given the patient’s age, underlying diabetes, skin ulceration, and elevation of CRP and ESR, the initial concern was for septic arthritis and osteomyelitis. However, the absence of leukocytosis and hyperglycemia argued against an infectious process.
The diagnosis of tophaceous gouty arthritis was confirmed by aspiration from the tophus, which demonstrated monosodium urate (MSU) crystals on polarized light microscopy. The patient presented with acute gout on the first right toe overlaying chronic tophaceous gout, complicated by renal failure.
Four phases. Gout progresses through 4 phases: asymptomatic hyperuricemia, acute gouty arthritis, intercritical gout (intervals between acute attacks), and chronic tophaceous gout.1 Chronic tophaceous gout is characterized by tophi—collections of solid urate in connective tissues (from bone to bursa, tendons, ligaments, and entheses).2 There are often multiple tophi and they may be calcified. An acute gouty attack is marked by a relatively sudden increase in pain and swelling, and may improve spontaneously over the course of 7 to 10 days.3
Gout is the most common form of inflammatory arthritis, with a prevalence in the United States of 3.9%.4The findings of several studies suggest that the prevalence and incidence of gout have risen in recent decades, which may be attributable to a growing aging population, the rise in obesity, increasing numbers of people who have other conditions such as heart disease, kidney disease, and/or diabetes, and the use of diuretics by individuals with cardiovascular disease.5
In a meta-analysis on gouty involvement of the first MTP joint, the occurrence of acute first MTP arthritis has been reported to be an independent predictor of MSU crystal presence in patients with gout.6 The presence of first MTP arthritis and the predilection for MSU deposition in the medial and dorsal aspects of the joint suggested an association, but no causation, between the 2 disease processes. The authors concluded that the distinction between osteoarthritis and gout as the cause of the joint damage is often difficult.
The diagnosis of gout may be made clinically based on established clinical criteria. The most commonly used are the 1977 American College of Rheumatology (ACR) criteria for the classification of acute arthritis of primary gout. (See “The 12 diagnostic criteria for gout.”7) However, in 2015, the ACR/European League Against Rheumatism (EULAR) published a new set of criteria that include the signs and symptoms of chronic gout, as well.8 (The ACR-EULAR Gout Classification Criteria Calculator may be accessed at http://goutclassificationcalculator.auckland.ac.nz/.)
The 12 diagnostic criteria for gout7
1. Recurrent arthritic attack
2. Joint redness
3. Pain or swelling in the first metatarsophalangeal joint
4. Unilateral attack involving the first metatarsophalangeal joint
5. Unilateral attack involving the tarsal joint
6. Suspected tophus
7. Hyperuricemia
8. Radiographic evidence of asymmetric swelling within a joint
9. Attack of monoarticular arthritis
10. Development of maximal inflammation within one day
11. Negative culture of joint fluid for microorganisms during joint inflammation attack
12. Radiographic evidence of subcortical cyst without erosions
Differential diagnosis includes trauma, septic and reactive arthritis
The differential diagnosis of acute gouty arthritis includes trauma, pseudogout (arthritis involving calcium pyrophosphate dehydrate), septic arthritis, reactive arthritis, post-streptococcal arthritis, and Lyme disease.
Trauma with a resulting acute or stress fracture can be determined by x-ray or magnetic resonance imaging (MRI).
Pseudogout requires aspiration of fluid and examination for calcium pyrophosphate crystals under polarizing microscopy.9
Septic arthritis may present in a similar manner to other causes of acute arthritis. Therefore, arthrocentesis is needed to identify the causative infectious agent.10 Septic arthritis was considered in our patient, given his age, history of diabetes mellitus, and finding of skin ulceration over the toe. However, our patient did not have systemic symptoms, and the joint aspiration did not show the presence of bacteria.
Reactive arthritis typically presents with inflammation of the ligaments and tendons at their sites of insertion into the bone, and can affect other areas of the body, such as the genitourinary and ocular systems.11
Post-streptococcal arthritis (aka acute rheumatic fever [ARF]) and Lyme disease can also present with joint complaints. The arthritis in ARF is usually migratory and involves several joints. Fever, rash, and a history of group A streptococcal infection are also diagnostic features.12 History of travel to an endemic area or seasonal exposure would further differentiate Lyme disease from other causes of arthritis.
Deciding on a course of treatment
Initial treatment choices for acute gout include nonsteroidal anti-inflammatory drugs (NSAIDs), colchicine, and corticosteroids.13 Various NSAIDs have been studied in the treatment of acute gout, but none showed absolute superiority over others. One option is naproxen 500 mg twice daily, but the choice of NSAID is mainly based on the adverse reaction profile and the physician’s preference.
Due to frequent gastrointestinal adverse effects and the concern for toxicity and drug interactions, colchicine and corticosteroids are not typically the first-line agents for acute gout treatment. Patients with frequent recurrent gouty attacks require urate-lowering therapies, such as allopurinol or probenecid. Other indications for urate-lowering therapies include evidence of tophaceous deposits in joints and soft tissues and gouty arthropathy.
Our patient’s renal failure was likely chronic, secondary to his untreated tophaceous gouty disease. Because his creatinine clearance was between 30 and 60 mL/min per 1.73 m2, he was not treated with NSAIDs, but with colchicine 1.2 mg for his initial flare, followed with a single dose of 0.6 mg in one hour. The patient had dramatic improvement of his pain the following day.
Patient education on chronic gout was also provided. We specifically discussed the patient’s dietary habits with him, advising him to minimize his intake of seafood, animal organs, and red meat products, which are high in purines. The patient was also told that he would need to start urate-lowering therapy to prevent recurrent gouty attacks and further complications from gout.
CORRESPONDENCE
Joseph Huang, MD, Fort Belvoir Community Hospital, Family Medicine Clinic, 1st Floor Eagle Pavilion, 9300 Dewitt Loop, Fort Belvoir, VA 22060; [email protected].
1. Harris MD, Siegel LB, Alloway JA. Gout and hyperuricemia. Am Fam Physician. 1999;59:925-934.
2. Dalbeth N, Kalluru R, Aati O, et al. Tendon involvement in the feet of patients with gout: a dual-energy CT study. Ann Rheum Dis. 2013;72:1545-1548.
3. Schlesinger N, Schumacher R, Catton M, et al. Colchicine for acute gout. Cochrane Database Syst Rev. 2006;CD006190.
4. Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum. 2011;63:3136-3141.
5. Roddy E, Doherty M. Epidemiology of gout. Arthritis Res Ther. 2010;12:223.
6. Stewart S, Dalbeth N, Vandal AC, et al. The first metatarsophalangeal joint in gout: a systemic review and meta-analysis. BMC Musculoskelet Disord. 2016;17:69.
7. Wallace SL, Robinson H, Masi AT, et al. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 1977;20:895-900.
8. Neogi T, Jansen TL, Dalbeth N, et al. 2015 Gout Classification Criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheumatol. 2015;67:2557-2568.
9. Rosenthal AK. Pseudogout: Presentation, natural history, and associated conditions. In: Wortmann RL, Schumacher HR Jr, Becker MA, et al, eds. Crystal-Induced Arthropathies: Gout, Pseudogout, and Apatite-Associated Syndromes. New York: Taylor and Francis Group;2006:99.
10. Horowitz DL, Katzap E, Horowitz S, et al. Approach to septic arthritis. Am Fam Physician. 2011;84:653-660.
11. Barth WF, Segal K. Reactive arthritis (Reiter’s syndrome). Am Fam Physician. 1999;60:499-507.
12. Beaudoin A, Edison L, Introcaso CE, et al; Centers for Disease Control and Prevention (CDC). Acute rheumatic fever and rheumatic heart disease among children—American Samoa, 2011-2012. MMWR Morb Mortal Wkly Rep. 2015;64:555-558.
13. Zhang W, Doherty M, Bardin T, et al; EULAR Standing Committee for International Clinical Studies Including Therapeutics. EULAR evidence based recommendations for gout. Part II: Management. Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis. 2006;65:1312-1324.
A 68-year-old Filipino man with a history of hypertension, type 2 diabetes, and osteoarthritis presented to the emergency department with a one-week history of increasing pain, swelling, erythema, and seepage of his right great toe. The patient denied paresthesias, fever, chills, night sweats, cough, dyspnea, or any change in his diet, medications (which included lisinopril, metformin, and acetaminophen as needed), or routine. Social history was negative for alcohol use and cigarette smoking.
He previously had similar symptoms in his right fourth toe that resulted in amputation. The patient was told at the time that he had a “bone infection” and amputation was necessary.
The patient was thin, alert, oriented, and in no acute distress. His vital signs and a cardiopulmonary exam were normal. The patient’s right great toe was tender to touch, with ulceration of the skin dorsally at the proximal nail fold. In addition, his toe was oozing a purulent, non-foul smelling discharge (FIGURE 1). Other pertinent findings included multiple enlarged joints on both hands with visible yellow-white subcutaneous nodules on the hands and dorsum of the forearm (FIGURE 2).
The patient’s white blood cell count was 10,800/mcL, C-reactive protein (CRP) was 18 mg/L, erythrocyte sedimentation rate (ESR) was 80 mm/hr, and uric acid was 12.5 mg/dL. His blood urea nitrogen was 52 mg/dL and creatinine was 2.5 mg/dL. A glycated hemoglobin test was 7.2%. A wound culture, aspirate from the dorsum of the toe, and x-ray were obtained.


WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Tophaceous gouty arthritis
The x-ray of the right great toe showed erosions of the metatarsophalangeal (MTP) joint (FIGURE 3). Given the patient’s age, underlying diabetes, skin ulceration, and elevation of CRP and ESR, the initial concern was for septic arthritis and osteomyelitis. However, the absence of leukocytosis and hyperglycemia argued against an infectious process.

The diagnosis of tophaceous gouty arthritis was confirmed by aspiration from the tophus, which demonstrated monosodium urate (MSU) crystals on polarized light microscopy. The patient presented with acute gout on the first right toe overlaying chronic tophaceous gout, complicated by renal failure.
Four phases. Gout progresses through 4 phases: asymptomatic hyperuricemia, acute gouty arthritis, intercritical gout (intervals between acute attacks), and chronic tophaceous gout.1 Chronic tophaceous gout is characterized by tophi—collections of solid urate in connective tissues (from bone to bursa, tendons, ligaments, and entheses).2 There are often multiple tophi and they may be calcified. An acute gouty attack is marked by a relatively sudden increase in pain and swelling, and may improve spontaneously over the course of 7 to 10 days.3
Gout is the most common form of inflammatory arthritis, with a prevalence in the United States of 3.9%.4The findings of several studies suggest that the prevalence and incidence of gout have risen in recent decades, which may be attributable to a growing aging population, the rise in obesity, increasing numbers of people who have other conditions such as heart disease, kidney disease, and/or diabetes, and the use of diuretics by individuals with cardiovascular disease.5
In a meta-analysis on gouty involvement of the first MTP joint, the occurrence of acute first MTP arthritis has been reported to be an independent predictor of MSU crystal presence in patients with gout.6 The presence of first MTP arthritis and the predilection for MSU deposition in the medial and dorsal aspects of the joint suggested an association, but no causation, between the 2 disease processes. The authors concluded that the distinction between osteoarthritis and gout as the cause of the joint damage is often difficult.
The diagnosis of gout may be made clinically based on established clinical criteria. The most commonly used are the 1977 American College of Rheumatology (ACR) criteria for the classification of acute arthritis of primary gout. (See “The 12 diagnostic criteria for gout.”7) However, in 2015, the ACR/European League Against Rheumatism (EULAR) published a new set of criteria that include the signs and symptoms of chronic gout, as well.8 (The ACR-EULAR Gout Classification Criteria Calculator may be accessed at http://goutclassificationcalculator.auckland.ac.nz/.)
The 12 diagnostic criteria for gout7
1. Recurrent arthritic attack
2. Joint redness
3. Pain or swelling in the first metatarsophalangeal joint
4. Unilateral attack involving the first metatarsophalangeal joint
5. Unilateral attack involving the tarsal joint
6. Suspected tophus
7. Hyperuricemia
8. Radiographic evidence of asymmetric swelling within a joint
9. Attack of monoarticular arthritis
10. Development of maximal inflammation within one day
11. Negative culture of joint fluid for microorganisms during joint inflammation attack
12. Radiographic evidence of subcortical cyst without erosions
Differential diagnosis includes trauma, septic and reactive arthritis
The differential diagnosis of acute gouty arthritis includes trauma, pseudogout (arthritis involving calcium pyrophosphate dehydrate), septic arthritis, reactive arthritis, post-streptococcal arthritis, and Lyme disease.
Trauma with a resulting acute or stress fracture can be determined by x-ray or magnetic resonance imaging (MRI).
Pseudogout requires aspiration of fluid and examination for calcium pyrophosphate crystals under polarizing microscopy.9
Septic arthritis may present in a similar manner to other causes of acute arthritis. Therefore, arthrocentesis is needed to identify the causative infectious agent.10 Septic arthritis was considered in our patient, given his age, history of diabetes mellitus, and finding of skin ulceration over the toe. However, our patient did not have systemic symptoms, and the joint aspiration did not show the presence of bacteria.
Reactive arthritis typically presents with inflammation of the ligaments and tendons at their sites of insertion into the bone, and can affect other areas of the body, such as the genitourinary and ocular systems.11
Post-streptococcal arthritis (aka acute rheumatic fever [ARF]) and Lyme disease can also present with joint complaints. The arthritis in ARF is usually migratory and involves several joints. Fever, rash, and a history of group A streptococcal infection are also diagnostic features.12 History of travel to an endemic area or seasonal exposure would further differentiate Lyme disease from other causes of arthritis.
Deciding on a course of treatment
Initial treatment choices for acute gout include nonsteroidal anti-inflammatory drugs (NSAIDs), colchicine, and corticosteroids.13 Various NSAIDs have been studied in the treatment of acute gout, but none showed absolute superiority over others. One option is naproxen 500 mg twice daily, but the choice of NSAID is mainly based on the adverse reaction profile and the physician’s preference.
Due to frequent gastrointestinal adverse effects and the concern for toxicity and drug interactions, colchicine and corticosteroids are not typically the first-line agents for acute gout treatment. Patients with frequent recurrent gouty attacks require urate-lowering therapies, such as allopurinol or probenecid. Other indications for urate-lowering therapies include evidence of tophaceous deposits in joints and soft tissues and gouty arthropathy.
Our patient’s renal failure was likely chronic, secondary to his untreated tophaceous gouty disease. Because his creatinine clearance was between 30 and 60 mL/min per 1.73 m2, he was not treated with NSAIDs, but with colchicine 1.2 mg for his initial flare, followed with a single dose of 0.6 mg in one hour. The patient had dramatic improvement of his pain the following day.
Patient education on chronic gout was also provided. We specifically discussed the patient’s dietary habits with him, advising him to minimize his intake of seafood, animal organs, and red meat products, which are high in purines. The patient was also told that he would need to start urate-lowering therapy to prevent recurrent gouty attacks and further complications from gout.
CORRESPONDENCE
Joseph Huang, MD, Fort Belvoir Community Hospital, Family Medicine Clinic, 1st Floor Eagle Pavilion, 9300 Dewitt Loop, Fort Belvoir, VA 22060; [email protected].
A 68-year-old Filipino man with a history of hypertension, type 2 diabetes, and osteoarthritis presented to the emergency department with a one-week history of increasing pain, swelling, erythema, and seepage of his right great toe. The patient denied paresthesias, fever, chills, night sweats, cough, dyspnea, or any change in his diet, medications (which included lisinopril, metformin, and acetaminophen as needed), or routine. Social history was negative for alcohol use and cigarette smoking.
He previously had similar symptoms in his right fourth toe that resulted in amputation. The patient was told at the time that he had a “bone infection” and amputation was necessary.
The patient was thin, alert, oriented, and in no acute distress. His vital signs and a cardiopulmonary exam were normal. The patient’s right great toe was tender to touch, with ulceration of the skin dorsally at the proximal nail fold. In addition, his toe was oozing a purulent, non-foul smelling discharge (FIGURE 1). Other pertinent findings included multiple enlarged joints on both hands with visible yellow-white subcutaneous nodules on the hands and dorsum of the forearm (FIGURE 2).
The patient’s white blood cell count was 10,800/mcL, C-reactive protein (CRP) was 18 mg/L, erythrocyte sedimentation rate (ESR) was 80 mm/hr, and uric acid was 12.5 mg/dL. His blood urea nitrogen was 52 mg/dL and creatinine was 2.5 mg/dL. A glycated hemoglobin test was 7.2%. A wound culture, aspirate from the dorsum of the toe, and x-ray were obtained.


WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Tophaceous gouty arthritis
The x-ray of the right great toe showed erosions of the metatarsophalangeal (MTP) joint (FIGURE 3). Given the patient’s age, underlying diabetes, skin ulceration, and elevation of CRP and ESR, the initial concern was for septic arthritis and osteomyelitis. However, the absence of leukocytosis and hyperglycemia argued against an infectious process.

The diagnosis of tophaceous gouty arthritis was confirmed by aspiration from the tophus, which demonstrated monosodium urate (MSU) crystals on polarized light microscopy. The patient presented with acute gout on the first right toe overlaying chronic tophaceous gout, complicated by renal failure.
Four phases. Gout progresses through 4 phases: asymptomatic hyperuricemia, acute gouty arthritis, intercritical gout (intervals between acute attacks), and chronic tophaceous gout.1 Chronic tophaceous gout is characterized by tophi—collections of solid urate in connective tissues (from bone to bursa, tendons, ligaments, and entheses).2 There are often multiple tophi and they may be calcified. An acute gouty attack is marked by a relatively sudden increase in pain and swelling, and may improve spontaneously over the course of 7 to 10 days.3
Gout is the most common form of inflammatory arthritis, with a prevalence in the United States of 3.9%.4The findings of several studies suggest that the prevalence and incidence of gout have risen in recent decades, which may be attributable to a growing aging population, the rise in obesity, increasing numbers of people who have other conditions such as heart disease, kidney disease, and/or diabetes, and the use of diuretics by individuals with cardiovascular disease.5
In a meta-analysis on gouty involvement of the first MTP joint, the occurrence of acute first MTP arthritis has been reported to be an independent predictor of MSU crystal presence in patients with gout.6 The presence of first MTP arthritis and the predilection for MSU deposition in the medial and dorsal aspects of the joint suggested an association, but no causation, between the 2 disease processes. The authors concluded that the distinction between osteoarthritis and gout as the cause of the joint damage is often difficult.
The diagnosis of gout may be made clinically based on established clinical criteria. The most commonly used are the 1977 American College of Rheumatology (ACR) criteria for the classification of acute arthritis of primary gout. (See “The 12 diagnostic criteria for gout.”7) However, in 2015, the ACR/European League Against Rheumatism (EULAR) published a new set of criteria that include the signs and symptoms of chronic gout, as well.8 (The ACR-EULAR Gout Classification Criteria Calculator may be accessed at http://goutclassificationcalculator.auckland.ac.nz/.)
The 12 diagnostic criteria for gout7
1. Recurrent arthritic attack
2. Joint redness
3. Pain or swelling in the first metatarsophalangeal joint
4. Unilateral attack involving the first metatarsophalangeal joint
5. Unilateral attack involving the tarsal joint
6. Suspected tophus
7. Hyperuricemia
8. Radiographic evidence of asymmetric swelling within a joint
9. Attack of monoarticular arthritis
10. Development of maximal inflammation within one day
11. Negative culture of joint fluid for microorganisms during joint inflammation attack
12. Radiographic evidence of subcortical cyst without erosions
Differential diagnosis includes trauma, septic and reactive arthritis
The differential diagnosis of acute gouty arthritis includes trauma, pseudogout (arthritis involving calcium pyrophosphate dehydrate), septic arthritis, reactive arthritis, post-streptococcal arthritis, and Lyme disease.
Trauma with a resulting acute or stress fracture can be determined by x-ray or magnetic resonance imaging (MRI).
Pseudogout requires aspiration of fluid and examination for calcium pyrophosphate crystals under polarizing microscopy.9
Septic arthritis may present in a similar manner to other causes of acute arthritis. Therefore, arthrocentesis is needed to identify the causative infectious agent.10 Septic arthritis was considered in our patient, given his age, history of diabetes mellitus, and finding of skin ulceration over the toe. However, our patient did not have systemic symptoms, and the joint aspiration did not show the presence of bacteria.
Reactive arthritis typically presents with inflammation of the ligaments and tendons at their sites of insertion into the bone, and can affect other areas of the body, such as the genitourinary and ocular systems.11
Post-streptococcal arthritis (aka acute rheumatic fever [ARF]) and Lyme disease can also present with joint complaints. The arthritis in ARF is usually migratory and involves several joints. Fever, rash, and a history of group A streptococcal infection are also diagnostic features.12 History of travel to an endemic area or seasonal exposure would further differentiate Lyme disease from other causes of arthritis.
Deciding on a course of treatment
Initial treatment choices for acute gout include nonsteroidal anti-inflammatory drugs (NSAIDs), colchicine, and corticosteroids.13 Various NSAIDs have been studied in the treatment of acute gout, but none showed absolute superiority over others. One option is naproxen 500 mg twice daily, but the choice of NSAID is mainly based on the adverse reaction profile and the physician’s preference.
Due to frequent gastrointestinal adverse effects and the concern for toxicity and drug interactions, colchicine and corticosteroids are not typically the first-line agents for acute gout treatment. Patients with frequent recurrent gouty attacks require urate-lowering therapies, such as allopurinol or probenecid. Other indications for urate-lowering therapies include evidence of tophaceous deposits in joints and soft tissues and gouty arthropathy.
Our patient’s renal failure was likely chronic, secondary to his untreated tophaceous gouty disease. Because his creatinine clearance was between 30 and 60 mL/min per 1.73 m2, he was not treated with NSAIDs, but with colchicine 1.2 mg for his initial flare, followed with a single dose of 0.6 mg in one hour. The patient had dramatic improvement of his pain the following day.
Patient education on chronic gout was also provided. We specifically discussed the patient’s dietary habits with him, advising him to minimize his intake of seafood, animal organs, and red meat products, which are high in purines. The patient was also told that he would need to start urate-lowering therapy to prevent recurrent gouty attacks and further complications from gout.
CORRESPONDENCE
Joseph Huang, MD, Fort Belvoir Community Hospital, Family Medicine Clinic, 1st Floor Eagle Pavilion, 9300 Dewitt Loop, Fort Belvoir, VA 22060; [email protected].
1. Harris MD, Siegel LB, Alloway JA. Gout and hyperuricemia. Am Fam Physician. 1999;59:925-934.
2. Dalbeth N, Kalluru R, Aati O, et al. Tendon involvement in the feet of patients with gout: a dual-energy CT study. Ann Rheum Dis. 2013;72:1545-1548.
3. Schlesinger N, Schumacher R, Catton M, et al. Colchicine for acute gout. Cochrane Database Syst Rev. 2006;CD006190.
4. Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum. 2011;63:3136-3141.
5. Roddy E, Doherty M. Epidemiology of gout. Arthritis Res Ther. 2010;12:223.
6. Stewart S, Dalbeth N, Vandal AC, et al. The first metatarsophalangeal joint in gout: a systemic review and meta-analysis. BMC Musculoskelet Disord. 2016;17:69.
7. Wallace SL, Robinson H, Masi AT, et al. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 1977;20:895-900.
8. Neogi T, Jansen TL, Dalbeth N, et al. 2015 Gout Classification Criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheumatol. 2015;67:2557-2568.
9. Rosenthal AK. Pseudogout: Presentation, natural history, and associated conditions. In: Wortmann RL, Schumacher HR Jr, Becker MA, et al, eds. Crystal-Induced Arthropathies: Gout, Pseudogout, and Apatite-Associated Syndromes. New York: Taylor and Francis Group;2006:99.
10. Horowitz DL, Katzap E, Horowitz S, et al. Approach to septic arthritis. Am Fam Physician. 2011;84:653-660.
11. Barth WF, Segal K. Reactive arthritis (Reiter’s syndrome). Am Fam Physician. 1999;60:499-507.
12. Beaudoin A, Edison L, Introcaso CE, et al; Centers for Disease Control and Prevention (CDC). Acute rheumatic fever and rheumatic heart disease among children—American Samoa, 2011-2012. MMWR Morb Mortal Wkly Rep. 2015;64:555-558.
13. Zhang W, Doherty M, Bardin T, et al; EULAR Standing Committee for International Clinical Studies Including Therapeutics. EULAR evidence based recommendations for gout. Part II: Management. Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis. 2006;65:1312-1324.
1. Harris MD, Siegel LB, Alloway JA. Gout and hyperuricemia. Am Fam Physician. 1999;59:925-934.
2. Dalbeth N, Kalluru R, Aati O, et al. Tendon involvement in the feet of patients with gout: a dual-energy CT study. Ann Rheum Dis. 2013;72:1545-1548.
3. Schlesinger N, Schumacher R, Catton M, et al. Colchicine for acute gout. Cochrane Database Syst Rev. 2006;CD006190.
4. Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum. 2011;63:3136-3141.
5. Roddy E, Doherty M. Epidemiology of gout. Arthritis Res Ther. 2010;12:223.
6. Stewart S, Dalbeth N, Vandal AC, et al. The first metatarsophalangeal joint in gout: a systemic review and meta-analysis. BMC Musculoskelet Disord. 2016;17:69.
7. Wallace SL, Robinson H, Masi AT, et al. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 1977;20:895-900.
8. Neogi T, Jansen TL, Dalbeth N, et al. 2015 Gout Classification Criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheumatol. 2015;67:2557-2568.
9. Rosenthal AK. Pseudogout: Presentation, natural history, and associated conditions. In: Wortmann RL, Schumacher HR Jr, Becker MA, et al, eds. Crystal-Induced Arthropathies: Gout, Pseudogout, and Apatite-Associated Syndromes. New York: Taylor and Francis Group;2006:99.
10. Horowitz DL, Katzap E, Horowitz S, et al. Approach to septic arthritis. Am Fam Physician. 2011;84:653-660.
11. Barth WF, Segal K. Reactive arthritis (Reiter’s syndrome). Am Fam Physician. 1999;60:499-507.
12. Beaudoin A, Edison L, Introcaso CE, et al; Centers for Disease Control and Prevention (CDC). Acute rheumatic fever and rheumatic heart disease among children—American Samoa, 2011-2012. MMWR Morb Mortal Wkly Rep. 2015;64:555-558.
13. Zhang W, Doherty M, Bardin T, et al; EULAR Standing Committee for International Clinical Studies Including Therapeutics. EULAR evidence based recommendations for gout. Part II: Management. Report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis. 2006;65:1312-1324.
Do corticosteroids reduce bronchiolitis hospitalizations?
No. Corticosteroids alone don’t decrease hospital admissions or length of stay among children with bronchiolitis (strength of recommendation [SOR]: A, meta-analysis of randomized controlled trials [RCTs]).
Combining oral dexamethasone and inhaled epinephrine appears to prevent one hospital admission for every 11 patients treated (SOR: B, single large RCT).
Evidence summary
A 2013 Cochrane review of 17 RCTs with 2596 patients compared corticosteroids with placebo for treating bronchiolitis in children younger than 2 years.1 The studies used dexamethasone, prednisolone, prednisone, and budesonide delivered by oral, inhaled, intravenous (IV), or intramuscular (IM) routes, ranging between a one-day dose to a 5-day taper. Doses ranged from 0.5 to 2 mg/kg/d for oral and parenteral routes and 0.2 to 1 mg for inhalation. Outcomes were rate of admissions at Days 1 and 7 from outpatient trials and length of stay among inpatients.
Investigators found no significant difference in admission rates at Day 1 and Day 7 between children treated with corticosteroids compared with placebo (Day 1: 8 trials, 1762 patients; relative risk [RR]=0.92; 95% confidence interval [CI], 0.78-1.1; Day 7: 5 trials, 1530 patients; RR=0.86; 95% CI, 0.70-1.1). Length of hospital stay didn’t differ between children treated with corticosteroids and children who received placebo (8 trials, 633 patients; mean difference= −0.18 days; 95% CI, −0.39 to 0.04).
Corticosteroid + epinephrine can lower hospital admissions
A 2009 multicenter, double-blind RCT with 800 patients (infants 6 weeks to 12 months of age with a first episode of bronchiolitis) that was included in the 2013 Cochrane review also compared the combination of epinephrine and corticosteroid with placebo and either agent alone.2
Infants were assigned to 4 groups: oral dexamethasone alone (1 mg/kg in the emergency room [ER] on Day 1, followed by 0.6 mg/kg daily for 5 days); nebulized epinephrine alone (2 treatments of 3 mL epinephrine 1:1000 solution); combined dexamethasone and epinephrine; and placebo. The primary outcome was hospital admission as long as 7 days after being seen in the ER.
Rates of admission were similar for the dexamethasone and placebo groups (25.6% vs 26.4%, respectively; RR=0.96; 95% CI, 0.69-1.3). The epinephrine group’s rate of admission was 23.7% (RR=0.88; CI, 0.63–1.23). Only the dexamethasone-epinephrine group had a lower rate of admission compared with placebo (17% vs 26%; RR=0.65; 95% CI, 0.45-0.95). The number needed to treat with dexamethasone-epinephrine to prevent one hospital admission was 11.
Review prompts revised recommendations
Based on the Cochrane review, the American Academy of Pediatrics (AAP) revised its evidence-based clinical practice guideline in 2014 to recommend that clinicians not administer systemic corticosteroids to infants with a diagnosis of bronchiolitis in any setting (evidence quality B, strong recommendation, based on results of multiple RCTs).3 The AAP advocates additional large trials to clarify whether combination therapy (corticosteroids plus agents with α or β agonist activity) is effective.
1. Fernandes RM, Bialy LM, Vandermeer B, et al. Glucocorticoids for acute viral bronchitis in infants and children. Cochrane Database Syst Rev. 2013;(6):CD004878.
2. Plint AC, Johnson DW, Patel H, et al. Epinephrine and dexamethasone in children with bronchiolitis. N Engl J Med. 2009;360:2079-2089.
3. Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134:e1474-e1502.
No. Corticosteroids alone don’t decrease hospital admissions or length of stay among children with bronchiolitis (strength of recommendation [SOR]: A, meta-analysis of randomized controlled trials [RCTs]).
Combining oral dexamethasone and inhaled epinephrine appears to prevent one hospital admission for every 11 patients treated (SOR: B, single large RCT).
Evidence summary
A 2013 Cochrane review of 17 RCTs with 2596 patients compared corticosteroids with placebo for treating bronchiolitis in children younger than 2 years.1 The studies used dexamethasone, prednisolone, prednisone, and budesonide delivered by oral, inhaled, intravenous (IV), or intramuscular (IM) routes, ranging between a one-day dose to a 5-day taper. Doses ranged from 0.5 to 2 mg/kg/d for oral and parenteral routes and 0.2 to 1 mg for inhalation. Outcomes were rate of admissions at Days 1 and 7 from outpatient trials and length of stay among inpatients.
Investigators found no significant difference in admission rates at Day 1 and Day 7 between children treated with corticosteroids compared with placebo (Day 1: 8 trials, 1762 patients; relative risk [RR]=0.92; 95% confidence interval [CI], 0.78-1.1; Day 7: 5 trials, 1530 patients; RR=0.86; 95% CI, 0.70-1.1). Length of hospital stay didn’t differ between children treated with corticosteroids and children who received placebo (8 trials, 633 patients; mean difference= −0.18 days; 95% CI, −0.39 to 0.04).
Corticosteroid + epinephrine can lower hospital admissions
A 2009 multicenter, double-blind RCT with 800 patients (infants 6 weeks to 12 months of age with a first episode of bronchiolitis) that was included in the 2013 Cochrane review also compared the combination of epinephrine and corticosteroid with placebo and either agent alone.2
Infants were assigned to 4 groups: oral dexamethasone alone (1 mg/kg in the emergency room [ER] on Day 1, followed by 0.6 mg/kg daily for 5 days); nebulized epinephrine alone (2 treatments of 3 mL epinephrine 1:1000 solution); combined dexamethasone and epinephrine; and placebo. The primary outcome was hospital admission as long as 7 days after being seen in the ER.
Rates of admission were similar for the dexamethasone and placebo groups (25.6% vs 26.4%, respectively; RR=0.96; 95% CI, 0.69-1.3). The epinephrine group’s rate of admission was 23.7% (RR=0.88; CI, 0.63–1.23). Only the dexamethasone-epinephrine group had a lower rate of admission compared with placebo (17% vs 26%; RR=0.65; 95% CI, 0.45-0.95). The number needed to treat with dexamethasone-epinephrine to prevent one hospital admission was 11.
Review prompts revised recommendations
Based on the Cochrane review, the American Academy of Pediatrics (AAP) revised its evidence-based clinical practice guideline in 2014 to recommend that clinicians not administer systemic corticosteroids to infants with a diagnosis of bronchiolitis in any setting (evidence quality B, strong recommendation, based on results of multiple RCTs).3 The AAP advocates additional large trials to clarify whether combination therapy (corticosteroids plus agents with α or β agonist activity) is effective.
No. Corticosteroids alone don’t decrease hospital admissions or length of stay among children with bronchiolitis (strength of recommendation [SOR]: A, meta-analysis of randomized controlled trials [RCTs]).
Combining oral dexamethasone and inhaled epinephrine appears to prevent one hospital admission for every 11 patients treated (SOR: B, single large RCT).
Evidence summary
A 2013 Cochrane review of 17 RCTs with 2596 patients compared corticosteroids with placebo for treating bronchiolitis in children younger than 2 years.1 The studies used dexamethasone, prednisolone, prednisone, and budesonide delivered by oral, inhaled, intravenous (IV), or intramuscular (IM) routes, ranging between a one-day dose to a 5-day taper. Doses ranged from 0.5 to 2 mg/kg/d for oral and parenteral routes and 0.2 to 1 mg for inhalation. Outcomes were rate of admissions at Days 1 and 7 from outpatient trials and length of stay among inpatients.
Investigators found no significant difference in admission rates at Day 1 and Day 7 between children treated with corticosteroids compared with placebo (Day 1: 8 trials, 1762 patients; relative risk [RR]=0.92; 95% confidence interval [CI], 0.78-1.1; Day 7: 5 trials, 1530 patients; RR=0.86; 95% CI, 0.70-1.1). Length of hospital stay didn’t differ between children treated with corticosteroids and children who received placebo (8 trials, 633 patients; mean difference= −0.18 days; 95% CI, −0.39 to 0.04).
Corticosteroid + epinephrine can lower hospital admissions
A 2009 multicenter, double-blind RCT with 800 patients (infants 6 weeks to 12 months of age with a first episode of bronchiolitis) that was included in the 2013 Cochrane review also compared the combination of epinephrine and corticosteroid with placebo and either agent alone.2
Infants were assigned to 4 groups: oral dexamethasone alone (1 mg/kg in the emergency room [ER] on Day 1, followed by 0.6 mg/kg daily for 5 days); nebulized epinephrine alone (2 treatments of 3 mL epinephrine 1:1000 solution); combined dexamethasone and epinephrine; and placebo. The primary outcome was hospital admission as long as 7 days after being seen in the ER.
Rates of admission were similar for the dexamethasone and placebo groups (25.6% vs 26.4%, respectively; RR=0.96; 95% CI, 0.69-1.3). The epinephrine group’s rate of admission was 23.7% (RR=0.88; CI, 0.63–1.23). Only the dexamethasone-epinephrine group had a lower rate of admission compared with placebo (17% vs 26%; RR=0.65; 95% CI, 0.45-0.95). The number needed to treat with dexamethasone-epinephrine to prevent one hospital admission was 11.
Review prompts revised recommendations
Based on the Cochrane review, the American Academy of Pediatrics (AAP) revised its evidence-based clinical practice guideline in 2014 to recommend that clinicians not administer systemic corticosteroids to infants with a diagnosis of bronchiolitis in any setting (evidence quality B, strong recommendation, based on results of multiple RCTs).3 The AAP advocates additional large trials to clarify whether combination therapy (corticosteroids plus agents with α or β agonist activity) is effective.
1. Fernandes RM, Bialy LM, Vandermeer B, et al. Glucocorticoids for acute viral bronchitis in infants and children. Cochrane Database Syst Rev. 2013;(6):CD004878.
2. Plint AC, Johnson DW, Patel H, et al. Epinephrine and dexamethasone in children with bronchiolitis. N Engl J Med. 2009;360:2079-2089.
3. Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134:e1474-e1502.
1. Fernandes RM, Bialy LM, Vandermeer B, et al. Glucocorticoids for acute viral bronchitis in infants and children. Cochrane Database Syst Rev. 2013;(6):CD004878.
2. Plint AC, Johnson DW, Patel H, et al. Epinephrine and dexamethasone in children with bronchiolitis. N Engl J Med. 2009;360:2079-2089.
3. Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134:e1474-e1502.
Evidence-based answers from the Family Physicians Inquiries Network
Sublingual immunotherapy for allergy-related asthma
Immunotherapy using sublingual tablets containing house dust mite allergen extended the interval until patients developed a moderate asthma exacerbation in a manufacturer-sponsored clinical trial reported online April 26 in JAMA.
However, patients’ scores on both the Asthma Control Questionnaire and the Asthma Quality of Life Questionnaire showed no difference between active treatment and placebo. And 25%-27% of the study participants dropped out of the study, usually citing asthma exacerbations, adverse events, or “withdrawal of consent.” Further studies are needed to assess long-term efficacy and safety, said Dr. J. Christian Virchow of the department of pulmonology/intensive care medicine, University of Rostock (Germany), and his associates.
The trial, involving 834 adults with asthma related to house dust mite allergy that was not well controlled by inhaled corticosteroids and short-acting beta-agonists, was performed at 109 sites in 13 European countries during a 2-year period. These participants were randomly assigned to receive add-on daily sublingual tablets containing low-dose dust-mite extract (275 patients), high-dose extract (282 patients), or placebo (277 patients) for 7-12 months. During the final 6 months of the intervention, corticosteroids were reduced by half for 3 months and then withdrawn for 3 months.
The primary efficacy outcome (time to the first asthma exacerbation) was extended by both doses of active drug, compared with placebo, with hazard ratios of 0.69 for the lower dose and 0.66 for the higher dose, the investigators said (JAMA. 2016 Apr 26;315[16]:1715-25).
Adverse events were significantly more frequent with active treatment, affecting 39% of patients receiving the lower dose and 46% of those receiving the higher dose of active immunotherapy, compared with only 17% of patients receiving placebo. However, this study was not adequately powered to compare adverse events across groups, Dr. Virchow and his associates noted.
The most frequently reported adverse events were oral pruritus, mouth edema, and throat irritation, which developed within a median of 1-2 minutes of taking the first dose on day 1 and persisted for a median of 4-23 days. There were 32 serious adverse events, including erosive esophagitis, hepatocellular injury, arthralgia, laryngeal edema, and asthma.
This trial was limited in that treatment duration was much shorter than that for a standard course of immunotherapy, which is often 3 years. This prevents drawing conclusions regarding the sustained effect of the treatment. “Furthermore, because the ultimate aim of allergen immunotherapy is disease modification beyond the duration of treatment, a follow-up after the end of treatment would have been relevant,” the investigators said.
This study was sponsored by the Danish pharmaceutical company ALK. Dr. Virchow reported ties to 31 industry sources; his associates also reported ties to numerous industry sources.
Sublingual immunotherapy appears to be somewhat less effective than subcutaneous immunotherapy, but it offers several advantages. It doesn’t require injections, can be self-administered, doesn’t require dose escalations, and carries a much lower risk of anaphylaxis. However, in this study there were no significant differences in patients’ responses to questionnaires regarding either asthma control or quality of life.
The main disadvantage is that sublingual immunotherapy requires adherence to daily dosing, and research has consistently shown low rates of long-term adherence. In one study, 55%-82% of patients failed to complete the recommended course of sublingual immunotherapy. In another, only 44% of patients renewed their prescriptions after 1 year of treatment, only 28% did so after 2 years, and only 13% did so after 3 years.
Dr. Robert A. Wood is in the division of allergy and immunology, department of pediatrics, at Johns Hopkins University, Baltimore. He reported ties to DBV Technologies, the Immune Tolerance Network, Stallergenes, Sanofi, and UpToDate. Dr. Wood made these remarks in an editorial accompanying Dr. Virchow’s report (JAMA. 2016 Apr 26;315:1711-2).
Sublingual immunotherapy appears to be somewhat less effective than subcutaneous immunotherapy, but it offers several advantages. It doesn’t require injections, can be self-administered, doesn’t require dose escalations, and carries a much lower risk of anaphylaxis. However, in this study there were no significant differences in patients’ responses to questionnaires regarding either asthma control or quality of life.
The main disadvantage is that sublingual immunotherapy requires adherence to daily dosing, and research has consistently shown low rates of long-term adherence. In one study, 55%-82% of patients failed to complete the recommended course of sublingual immunotherapy. In another, only 44% of patients renewed their prescriptions after 1 year of treatment, only 28% did so after 2 years, and only 13% did so after 3 years.
Dr. Robert A. Wood is in the division of allergy and immunology, department of pediatrics, at Johns Hopkins University, Baltimore. He reported ties to DBV Technologies, the Immune Tolerance Network, Stallergenes, Sanofi, and UpToDate. Dr. Wood made these remarks in an editorial accompanying Dr. Virchow’s report (JAMA. 2016 Apr 26;315:1711-2).
Sublingual immunotherapy appears to be somewhat less effective than subcutaneous immunotherapy, but it offers several advantages. It doesn’t require injections, can be self-administered, doesn’t require dose escalations, and carries a much lower risk of anaphylaxis. However, in this study there were no significant differences in patients’ responses to questionnaires regarding either asthma control or quality of life.
The main disadvantage is that sublingual immunotherapy requires adherence to daily dosing, and research has consistently shown low rates of long-term adherence. In one study, 55%-82% of patients failed to complete the recommended course of sublingual immunotherapy. In another, only 44% of patients renewed their prescriptions after 1 year of treatment, only 28% did so after 2 years, and only 13% did so after 3 years.
Dr. Robert A. Wood is in the division of allergy and immunology, department of pediatrics, at Johns Hopkins University, Baltimore. He reported ties to DBV Technologies, the Immune Tolerance Network, Stallergenes, Sanofi, and UpToDate. Dr. Wood made these remarks in an editorial accompanying Dr. Virchow’s report (JAMA. 2016 Apr 26;315:1711-2).
Immunotherapy using sublingual tablets containing house dust mite allergen extended the interval until patients developed a moderate asthma exacerbation in a manufacturer-sponsored clinical trial reported online April 26 in JAMA.
However, patients’ scores on both the Asthma Control Questionnaire and the Asthma Quality of Life Questionnaire showed no difference between active treatment and placebo. And 25%-27% of the study participants dropped out of the study, usually citing asthma exacerbations, adverse events, or “withdrawal of consent.” Further studies are needed to assess long-term efficacy and safety, said Dr. J. Christian Virchow of the department of pulmonology/intensive care medicine, University of Rostock (Germany), and his associates.
The trial, involving 834 adults with asthma related to house dust mite allergy that was not well controlled by inhaled corticosteroids and short-acting beta-agonists, was performed at 109 sites in 13 European countries during a 2-year period. These participants were randomly assigned to receive add-on daily sublingual tablets containing low-dose dust-mite extract (275 patients), high-dose extract (282 patients), or placebo (277 patients) for 7-12 months. During the final 6 months of the intervention, corticosteroids were reduced by half for 3 months and then withdrawn for 3 months.
The primary efficacy outcome (time to the first asthma exacerbation) was extended by both doses of active drug, compared with placebo, with hazard ratios of 0.69 for the lower dose and 0.66 for the higher dose, the investigators said (JAMA. 2016 Apr 26;315[16]:1715-25).
Adverse events were significantly more frequent with active treatment, affecting 39% of patients receiving the lower dose and 46% of those receiving the higher dose of active immunotherapy, compared with only 17% of patients receiving placebo. However, this study was not adequately powered to compare adverse events across groups, Dr. Virchow and his associates noted.
The most frequently reported adverse events were oral pruritus, mouth edema, and throat irritation, which developed within a median of 1-2 minutes of taking the first dose on day 1 and persisted for a median of 4-23 days. There were 32 serious adverse events, including erosive esophagitis, hepatocellular injury, arthralgia, laryngeal edema, and asthma.
This trial was limited in that treatment duration was much shorter than that for a standard course of immunotherapy, which is often 3 years. This prevents drawing conclusions regarding the sustained effect of the treatment. “Furthermore, because the ultimate aim of allergen immunotherapy is disease modification beyond the duration of treatment, a follow-up after the end of treatment would have been relevant,” the investigators said.
This study was sponsored by the Danish pharmaceutical company ALK. Dr. Virchow reported ties to 31 industry sources; his associates also reported ties to numerous industry sources.
Immunotherapy using sublingual tablets containing house dust mite allergen extended the interval until patients developed a moderate asthma exacerbation in a manufacturer-sponsored clinical trial reported online April 26 in JAMA.
However, patients’ scores on both the Asthma Control Questionnaire and the Asthma Quality of Life Questionnaire showed no difference between active treatment and placebo. And 25%-27% of the study participants dropped out of the study, usually citing asthma exacerbations, adverse events, or “withdrawal of consent.” Further studies are needed to assess long-term efficacy and safety, said Dr. J. Christian Virchow of the department of pulmonology/intensive care medicine, University of Rostock (Germany), and his associates.
The trial, involving 834 adults with asthma related to house dust mite allergy that was not well controlled by inhaled corticosteroids and short-acting beta-agonists, was performed at 109 sites in 13 European countries during a 2-year period. These participants were randomly assigned to receive add-on daily sublingual tablets containing low-dose dust-mite extract (275 patients), high-dose extract (282 patients), or placebo (277 patients) for 7-12 months. During the final 6 months of the intervention, corticosteroids were reduced by half for 3 months and then withdrawn for 3 months.
The primary efficacy outcome (time to the first asthma exacerbation) was extended by both doses of active drug, compared with placebo, with hazard ratios of 0.69 for the lower dose and 0.66 for the higher dose, the investigators said (JAMA. 2016 Apr 26;315[16]:1715-25).
Adverse events were significantly more frequent with active treatment, affecting 39% of patients receiving the lower dose and 46% of those receiving the higher dose of active immunotherapy, compared with only 17% of patients receiving placebo. However, this study was not adequately powered to compare adverse events across groups, Dr. Virchow and his associates noted.
The most frequently reported adverse events were oral pruritus, mouth edema, and throat irritation, which developed within a median of 1-2 minutes of taking the first dose on day 1 and persisted for a median of 4-23 days. There were 32 serious adverse events, including erosive esophagitis, hepatocellular injury, arthralgia, laryngeal edema, and asthma.
This trial was limited in that treatment duration was much shorter than that for a standard course of immunotherapy, which is often 3 years. This prevents drawing conclusions regarding the sustained effect of the treatment. “Furthermore, because the ultimate aim of allergen immunotherapy is disease modification beyond the duration of treatment, a follow-up after the end of treatment would have been relevant,” the investigators said.
This study was sponsored by the Danish pharmaceutical company ALK. Dr. Virchow reported ties to 31 industry sources; his associates also reported ties to numerous industry sources.
FROM JAMA
Key clinical point: Sublingual tablets containing house dust mite allergen immunotherapy extended the interval until a moderate or severe asthma exacerbation.
Major finding: The primary efficacy outcome (time to the first asthma exacerbation) was extended by both doses of active drug, compared with placebo, with hazard ratios of 0.69 for the lower dose and 0.66 for the higher dose.
Data source: An industry-sponsored international randomized placebo-controlled trial involving 834 patients.
Disclosures: This study was sponsored by the Danish pharmaceutical company ALK. Dr. Virchow reported ties to 31 industry sources; his associates also reported ties to numerous industry sources.
Suture Anchor
DePuy Synthes Mitek Sports Medicine
(https://www.depuysynthes.com/hcp/mitek-sports-medicine)
Gryphon® Suture Anchor with Proknot™ Technology
Paul Favorito, MD, Wellington Orthopaedic and Sports Medicine, Cincinnati, OH
The Gryphon® suture anchor with Proknot™ technology is a doubled No. 1 Permacord® high-strength orthopedic suture with a proprietary pre-tied sliding knot. The suture construct is loaded onto a 3.0-mm Gryphonsuture anchor (Peek or Biocryl Rapide® biocomposite material) and has clinical indications for labral repair of the shoulder and hip. In a laboratory setting, Proknot technology has been tested against other high-tensile sutures and commonly tied arthroscopic knots.1 Proknot technology demonstrated higher ultimate strength, significantly less knot volume, and better reproducibility among surgeons.
Surgical pearl: I use the Gryphon Proknot suture anchor for all shoulder Bankart and superior labral anterior to posterior (SLAP) repairs. I have colleagues who also use this anchor for hip arthroscopy.
Once opened on the back table, the surgical assistant may ink the free limb of suture for easy arthroscopic identification. The anchor is placed and, in the case of hard bone frequently encountered in younger patients, a 2.5-mm drill bit may be substituted for the usual 2.4-mm. One important goal of any labral repair is to position knots away from the articular surface. The free suture limb is passed through the labrum, retrieved, and delivered through the open, pre-tied knot on the suture card.
Once the knot is released and dressed, the knot pusher is placed over the suture and the knot is advanced and preliminarily tensioned medial to the articular surface. The suture limbs are separated and one limb of the suture is removed from the knot pusher. As few as 1, or up to 3, half hitches may be placed to secure the knot, taking care to direct it away from the joint surface. The result is a strong but well-positioned knot with minimal mass securing the soft tissue.
1. Rodes SA, Favorito PJ, Piccirillo JM, Spivey JT. Performance comparison of a prettied suture knot with three conventional arthroscopic knots. Arthroscopy. 2015;31(11):2183-2190.
DePuy Synthes Mitek Sports Medicine
(https://www.depuysynthes.com/hcp/mitek-sports-medicine)
Gryphon® Suture Anchor with Proknot™ Technology
Paul Favorito, MD, Wellington Orthopaedic and Sports Medicine, Cincinnati, OH
The Gryphon® suture anchor with Proknot™ technology is a doubled No. 1 Permacord® high-strength orthopedic suture with a proprietary pre-tied sliding knot. The suture construct is loaded onto a 3.0-mm Gryphonsuture anchor (Peek or Biocryl Rapide® biocomposite material) and has clinical indications for labral repair of the shoulder and hip. In a laboratory setting, Proknot technology has been tested against other high-tensile sutures and commonly tied arthroscopic knots.1 Proknot technology demonstrated higher ultimate strength, significantly less knot volume, and better reproducibility among surgeons.
Surgical pearl: I use the Gryphon Proknot suture anchor for all shoulder Bankart and superior labral anterior to posterior (SLAP) repairs. I have colleagues who also use this anchor for hip arthroscopy.
Once opened on the back table, the surgical assistant may ink the free limb of suture for easy arthroscopic identification. The anchor is placed and, in the case of hard bone frequently encountered in younger patients, a 2.5-mm drill bit may be substituted for the usual 2.4-mm. One important goal of any labral repair is to position knots away from the articular surface. The free suture limb is passed through the labrum, retrieved, and delivered through the open, pre-tied knot on the suture card.
Once the knot is released and dressed, the knot pusher is placed over the suture and the knot is advanced and preliminarily tensioned medial to the articular surface. The suture limbs are separated and one limb of the suture is removed from the knot pusher. As few as 1, or up to 3, half hitches may be placed to secure the knot, taking care to direct it away from the joint surface. The result is a strong but well-positioned knot with minimal mass securing the soft tissue.
DePuy Synthes Mitek Sports Medicine
(https://www.depuysynthes.com/hcp/mitek-sports-medicine)
Gryphon® Suture Anchor with Proknot™ Technology
Paul Favorito, MD, Wellington Orthopaedic and Sports Medicine, Cincinnati, OH
The Gryphon® suture anchor with Proknot™ technology is a doubled No. 1 Permacord® high-strength orthopedic suture with a proprietary pre-tied sliding knot. The suture construct is loaded onto a 3.0-mm Gryphonsuture anchor (Peek or Biocryl Rapide® biocomposite material) and has clinical indications for labral repair of the shoulder and hip. In a laboratory setting, Proknot technology has been tested against other high-tensile sutures and commonly tied arthroscopic knots.1 Proknot technology demonstrated higher ultimate strength, significantly less knot volume, and better reproducibility among surgeons.
Surgical pearl: I use the Gryphon Proknot suture anchor for all shoulder Bankart and superior labral anterior to posterior (SLAP) repairs. I have colleagues who also use this anchor for hip arthroscopy.
Once opened on the back table, the surgical assistant may ink the free limb of suture for easy arthroscopic identification. The anchor is placed and, in the case of hard bone frequently encountered in younger patients, a 2.5-mm drill bit may be substituted for the usual 2.4-mm. One important goal of any labral repair is to position knots away from the articular surface. The free suture limb is passed through the labrum, retrieved, and delivered through the open, pre-tied knot on the suture card.
Once the knot is released and dressed, the knot pusher is placed over the suture and the knot is advanced and preliminarily tensioned medial to the articular surface. The suture limbs are separated and one limb of the suture is removed from the knot pusher. As few as 1, or up to 3, half hitches may be placed to secure the knot, taking care to direct it away from the joint surface. The result is a strong but well-positioned knot with minimal mass securing the soft tissue.
1. Rodes SA, Favorito PJ, Piccirillo JM, Spivey JT. Performance comparison of a prettied suture knot with three conventional arthroscopic knots. Arthroscopy. 2015;31(11):2183-2190.
1. Rodes SA, Favorito PJ, Piccirillo JM, Spivey JT. Performance comparison of a prettied suture knot with three conventional arthroscopic knots. Arthroscopy. 2015;31(11):2183-2190.
ASCR Restores Stability in Patients with Large Rotator Cuff Tears
ORLANDO, FL—Using arthroscopic superior capsule reconstruction (ASCR) to treat patients with massive rotator cuff tears can improve shoulder strength and function, according to research presented at the American Orthopedic Society for Sports Medicine’s Specialty Day.
Researchers used ASCR to treat 100 patients (average age: 66) who had irreparable rotator cuff tears that failed during previous treatment. Physical exams, x-rays, and magnetic resonance imaging were performed before surgery, at 3, 6 and 12 months following surgery, and on a yearly basis thereafter. Rates of return to work or sport were analyzed in 34 patients who were employed and 26 patients who were recreational athletes before the rotator cuff tear.
Overall, 92% of patients significantly improved their strength and shoulder function. In all, 32 patients returned fully to their previous work and 2 patients returned with reduced hours and workloads. All 26 patients who played sports prior to injury fully returned to their activities.
ORLANDO, FL—Using arthroscopic superior capsule reconstruction (ASCR) to treat patients with massive rotator cuff tears can improve shoulder strength and function, according to research presented at the American Orthopedic Society for Sports Medicine’s Specialty Day.
Researchers used ASCR to treat 100 patients (average age: 66) who had irreparable rotator cuff tears that failed during previous treatment. Physical exams, x-rays, and magnetic resonance imaging were performed before surgery, at 3, 6 and 12 months following surgery, and on a yearly basis thereafter. Rates of return to work or sport were analyzed in 34 patients who were employed and 26 patients who were recreational athletes before the rotator cuff tear.
Overall, 92% of patients significantly improved their strength and shoulder function. In all, 32 patients returned fully to their previous work and 2 patients returned with reduced hours and workloads. All 26 patients who played sports prior to injury fully returned to their activities.
ORLANDO, FL—Using arthroscopic superior capsule reconstruction (ASCR) to treat patients with massive rotator cuff tears can improve shoulder strength and function, according to research presented at the American Orthopedic Society for Sports Medicine’s Specialty Day.
Researchers used ASCR to treat 100 patients (average age: 66) who had irreparable rotator cuff tears that failed during previous treatment. Physical exams, x-rays, and magnetic resonance imaging were performed before surgery, at 3, 6 and 12 months following surgery, and on a yearly basis thereafter. Rates of return to work or sport were analyzed in 34 patients who were employed and 26 patients who were recreational athletes before the rotator cuff tear.
Overall, 92% of patients significantly improved their strength and shoulder function. In all, 32 patients returned fully to their previous work and 2 patients returned with reduced hours and workloads. All 26 patients who played sports prior to injury fully returned to their activities.