User login
Study identifies cognitive impairment in elderly urogynecologic patients
INDIAN WELLS, CALIF. – A rapid screening tool found that about 5% of urogynecologic patients aged 65-74 years showed signs of cognitive impairment, with that figure rising to more than 30% for patients age 85 and older, according to the results of a single-center study.
“As our gynecologic patients continue to age, it’s increasingly important that we continue to identify and manage the risk factors for cognitive decline that occur in the ambulatory and the perioperative care settings,” Dr. Elisa R. Trowbridge, lead study author, said at the annual scientific meeting of the Society of Gynecologic Surgeons. “However, data are lacking to describe the prevalence of cognitive impairment in this very specific population.”
In 2013, the Centers for Disease Control and Prevention estimated that one in eight patients older than 60 years of age deal with memory loss and confusion. However, fewer than 20% of these patients report this to their health care providers, said Dr. Trowbridge, division director of the University of Virginia Women’s Center for Continence and Pelvic Surgery in Charlottesville.
“For this reason the aim of our study was to evaluate the prevalence of cognitive impairment in a urogynecologic ambulatory population, and to evaluate the feasibility of using a standardized, validated screening questionnaire in the tertiary care setting,” she said.
The researchers invited 371 English-speaking patients aged 65 and older to participate and used two cognitive screening tools: the Mini-Cog and the AD8 (8-item Interview to Differentiate Aging and Dementia). They also used the Geriatric Depression Scale, as there is an association between depression and cognition in the elderly.
“Advantages of the Mini-Cog are that it’s administered in less than 3 minutes, it requires no special equipment, and it is not influenced by level of education, or any language variations,” Dr. Trowbridge said.
Of the 371 patients, 39 were excluded due to pre-existing cognitive impairment/dementia, active psychotic disorders, acute/unstable medical conditions, neurologic injury/disorders, alcohol/drug abuse, severe visual/hearing impairment, and illiteracy. An additional 37 patients declined to participate because they “were frustrated that we had asked to evaluate their memory,” she said. This left a total of 295 patients with a mean age of 75 years. Most (97%) were Caucasian, 62% were married, and each had an average of four major medical conditions, including hypertension, hyperlipidemia, and depression. The researchers stratified patients into three age groups: 65-74, 75-84, and 85 and older.
Cognitive impairment as measured by the Mini-Cog was identified in 5.3% of patients aged 65-74 years, 13.7% of those aged 75-84 years, and 31% of those aged 85 and older. The difference in impairment between those aged 65-74 years and those aged 75 years and older reached significance, with a P value of less than .001.
Cognitive impairment as measured by the AD8 found that all three age groups perceived themselves to have early cognitive changes: 25.9% of patients aged 65-74 years, 31.9% of those aged 75-84 years, and 40% of those aged 85 and older. There were no significant between-group differences in these results (P = .4). The most commonly identified areas of impairment were problems with thinking and memory (62%), judgment (52%), and trouble learning new tools or gadgets (44%).
Dr. Trowbridge also reported that 6.4% of the study population screened positive for depression on the Geriatric Depression Scale, with no significant differences between the age groups.
“In our study population, cognitive impairment as measured by a validated questionnaire is prevalent among women greater than 75 years of age,” she said at the meeting, which was jointly sponsored by the American College of Surgeons. “The Mini-Cog is a feasible screening tool for routine use in clinical practice that can be integrated easily into the urogynecologic evaluation. However, remember these are screening tools that effectively screen for previously unrecognized impairment, but a definitive diagnosis requires additional evaluation.”
Dr. Trowbridge reported having no financial disclosures.
INDIAN WELLS, CALIF. – A rapid screening tool found that about 5% of urogynecologic patients aged 65-74 years showed signs of cognitive impairment, with that figure rising to more than 30% for patients age 85 and older, according to the results of a single-center study.
“As our gynecologic patients continue to age, it’s increasingly important that we continue to identify and manage the risk factors for cognitive decline that occur in the ambulatory and the perioperative care settings,” Dr. Elisa R. Trowbridge, lead study author, said at the annual scientific meeting of the Society of Gynecologic Surgeons. “However, data are lacking to describe the prevalence of cognitive impairment in this very specific population.”
In 2013, the Centers for Disease Control and Prevention estimated that one in eight patients older than 60 years of age deal with memory loss and confusion. However, fewer than 20% of these patients report this to their health care providers, said Dr. Trowbridge, division director of the University of Virginia Women’s Center for Continence and Pelvic Surgery in Charlottesville.
“For this reason the aim of our study was to evaluate the prevalence of cognitive impairment in a urogynecologic ambulatory population, and to evaluate the feasibility of using a standardized, validated screening questionnaire in the tertiary care setting,” she said.
The researchers invited 371 English-speaking patients aged 65 and older to participate and used two cognitive screening tools: the Mini-Cog and the AD8 (8-item Interview to Differentiate Aging and Dementia). They also used the Geriatric Depression Scale, as there is an association between depression and cognition in the elderly.
“Advantages of the Mini-Cog are that it’s administered in less than 3 minutes, it requires no special equipment, and it is not influenced by level of education, or any language variations,” Dr. Trowbridge said.
Of the 371 patients, 39 were excluded due to pre-existing cognitive impairment/dementia, active psychotic disorders, acute/unstable medical conditions, neurologic injury/disorders, alcohol/drug abuse, severe visual/hearing impairment, and illiteracy. An additional 37 patients declined to participate because they “were frustrated that we had asked to evaluate their memory,” she said. This left a total of 295 patients with a mean age of 75 years. Most (97%) were Caucasian, 62% were married, and each had an average of four major medical conditions, including hypertension, hyperlipidemia, and depression. The researchers stratified patients into three age groups: 65-74, 75-84, and 85 and older.
Cognitive impairment as measured by the Mini-Cog was identified in 5.3% of patients aged 65-74 years, 13.7% of those aged 75-84 years, and 31% of those aged 85 and older. The difference in impairment between those aged 65-74 years and those aged 75 years and older reached significance, with a P value of less than .001.
Cognitive impairment as measured by the AD8 found that all three age groups perceived themselves to have early cognitive changes: 25.9% of patients aged 65-74 years, 31.9% of those aged 75-84 years, and 40% of those aged 85 and older. There were no significant between-group differences in these results (P = .4). The most commonly identified areas of impairment were problems with thinking and memory (62%), judgment (52%), and trouble learning new tools or gadgets (44%).
Dr. Trowbridge also reported that 6.4% of the study population screened positive for depression on the Geriatric Depression Scale, with no significant differences between the age groups.
“In our study population, cognitive impairment as measured by a validated questionnaire is prevalent among women greater than 75 years of age,” she said at the meeting, which was jointly sponsored by the American College of Surgeons. “The Mini-Cog is a feasible screening tool for routine use in clinical practice that can be integrated easily into the urogynecologic evaluation. However, remember these are screening tools that effectively screen for previously unrecognized impairment, but a definitive diagnosis requires additional evaluation.”
Dr. Trowbridge reported having no financial disclosures.
INDIAN WELLS, CALIF. – A rapid screening tool found that about 5% of urogynecologic patients aged 65-74 years showed signs of cognitive impairment, with that figure rising to more than 30% for patients age 85 and older, according to the results of a single-center study.
“As our gynecologic patients continue to age, it’s increasingly important that we continue to identify and manage the risk factors for cognitive decline that occur in the ambulatory and the perioperative care settings,” Dr. Elisa R. Trowbridge, lead study author, said at the annual scientific meeting of the Society of Gynecologic Surgeons. “However, data are lacking to describe the prevalence of cognitive impairment in this very specific population.”
In 2013, the Centers for Disease Control and Prevention estimated that one in eight patients older than 60 years of age deal with memory loss and confusion. However, fewer than 20% of these patients report this to their health care providers, said Dr. Trowbridge, division director of the University of Virginia Women’s Center for Continence and Pelvic Surgery in Charlottesville.
“For this reason the aim of our study was to evaluate the prevalence of cognitive impairment in a urogynecologic ambulatory population, and to evaluate the feasibility of using a standardized, validated screening questionnaire in the tertiary care setting,” she said.
The researchers invited 371 English-speaking patients aged 65 and older to participate and used two cognitive screening tools: the Mini-Cog and the AD8 (8-item Interview to Differentiate Aging and Dementia). They also used the Geriatric Depression Scale, as there is an association between depression and cognition in the elderly.
“Advantages of the Mini-Cog are that it’s administered in less than 3 minutes, it requires no special equipment, and it is not influenced by level of education, or any language variations,” Dr. Trowbridge said.
Of the 371 patients, 39 were excluded due to pre-existing cognitive impairment/dementia, active psychotic disorders, acute/unstable medical conditions, neurologic injury/disorders, alcohol/drug abuse, severe visual/hearing impairment, and illiteracy. An additional 37 patients declined to participate because they “were frustrated that we had asked to evaluate their memory,” she said. This left a total of 295 patients with a mean age of 75 years. Most (97%) were Caucasian, 62% were married, and each had an average of four major medical conditions, including hypertension, hyperlipidemia, and depression. The researchers stratified patients into three age groups: 65-74, 75-84, and 85 and older.
Cognitive impairment as measured by the Mini-Cog was identified in 5.3% of patients aged 65-74 years, 13.7% of those aged 75-84 years, and 31% of those aged 85 and older. The difference in impairment between those aged 65-74 years and those aged 75 years and older reached significance, with a P value of less than .001.
Cognitive impairment as measured by the AD8 found that all three age groups perceived themselves to have early cognitive changes: 25.9% of patients aged 65-74 years, 31.9% of those aged 75-84 years, and 40% of those aged 85 and older. There were no significant between-group differences in these results (P = .4). The most commonly identified areas of impairment were problems with thinking and memory (62%), judgment (52%), and trouble learning new tools or gadgets (44%).
Dr. Trowbridge also reported that 6.4% of the study population screened positive for depression on the Geriatric Depression Scale, with no significant differences between the age groups.
“In our study population, cognitive impairment as measured by a validated questionnaire is prevalent among women greater than 75 years of age,” she said at the meeting, which was jointly sponsored by the American College of Surgeons. “The Mini-Cog is a feasible screening tool for routine use in clinical practice that can be integrated easily into the urogynecologic evaluation. However, remember these are screening tools that effectively screen for previously unrecognized impairment, but a definitive diagnosis requires additional evaluation.”
Dr. Trowbridge reported having no financial disclosures.
AT SGS 2016
Key clinical point: The Mini-Cog is a feasible screening tool for routine use in clinical practice that can be integrated easily into the urogynecologic evaluation.
Major finding: Cognitive impairment as measured by the Mini-Cog was identified in 5.3% of patients aged 65-74 years, 13.7% of those aged 75-84 years, and 31% of those aged 85 and older.
Data source: A single-center study of 295 urogynecologic patients aged 65 and older.
Disclosures: Dr. Trowbridge reported having no financial disclosures.
Bortezomib-based regimen + transplant increased progression-free survival in primary plasma cell leukemia
In a prospective study of 40 patients with primary plasma cell leukemia, upfront autotransplantation followed by allotransplant for younger patients and by consolidation/maintenance for older patients was associated with a median overall survival of 36.3 months and a median progression-free survival of 15.1 months.
Patients with this aggressive form of multiple myeloma received a regimen that combined standard chemotherapy, a proteasome inhibitor, high-dose melphalan followed by autologous stem cell transplantation, and allogeneic transplantation or immunomodulatory drugs, reported Dr. Bruno Royer of University Hospital in Amiens, France, and his associates.
Induction therapy consisted of four 21-day cycles: Cycles 1 and 3 included subcutaneous bortezomib, intravenous pegylated doxorubicin, and oral dexamethasone; cycles 2 and 4 included subcutaneous bortezomib, oral cyclophosphamide, and oral dexamethasone. Of 39 patients – one patient died 24 hours after study inclusion – 35 completed the four cycles. The overall response rate to induction was 69%: 10% of patients had a complete response and 26% had a very good partial response. Of 27 responding patients, 25 underwent high-dose melphalan followed by autologous stem cell transplantation.
The high response rates allowed 16 patients who were younger than 66 years and had an HLA-matched donor to then receive high-dose melphalan followed by autologous stem cell transplantation followed by consolidation with either an reduced-intensity conditioning allograft or a second high-dose melphalan followed by autologous stem cell transplantation and subsequent maintenance with lenalidomide, bortezomib, and dexamethasone for 1 year, the researchers said (J Clin Oncol. 2016 Apr 25. doi: 10.1200/JCO.2015.63.1929).
A total of 20% of patients had a complete response to the entire treatment protocol, 13% had a stringent complete response, 26% had a very good partial response, 5% had stable disease, and 5% had progressive disease. Thirteen patients died of progressive disease and four died of infections, including three that occurred during induction or after allograft.
This is only the second prospective trial in patients with primary plasma cell leukemia, an aggressive form of multiple myeloma that accounts for 2%-4% of cases, the researchers said. Future prospective trials should seek to optimize induction with newer combinations, such as carfilzomib, lenalidomide, dexamethasone, or monoclonal anti-CD38 antibodies. Also, optimizing the stem cell conditioning procedure and the postallograft immunomodulation may further benefit younger patients.
Dr. Royer reported receiving honoraria from Amgen and having served as a consultant or advisor for Octapharma Plasma. Fifteen coinvestigators also reported financial relationships with a number of pharmaceutical companies.
The study by Dr. Royer and associates is the first prospective trial to confirm that bortezomib-based regimens combined with a transplantation program may be effective and feasible in a significant proportion of patients with primary plasma cell leukemia. Response to induction therapy, however, was not remarkable; thus, although both cyclophosphamide and doxorubicin have demonstrated efficacy in primary plasma cell leukemia, the introduction of lenalidomide and/or incorporation of newer agents such as pomalidomide, carfilzomib, or daratumumab could hopefully optimize the induction phase and increase the rate and quality of response in future studies.
Hopefully, sequential phases of induction therapy, multiple transplantations (if applicable), further consolidation, and maintenance should ensure rapid disease control and reduction of early deaths from initial complications, a contrasting of clonal evolution that may induce drug resistance, and activity on residual disease by decreasing the risk of relapse. Feasibility of these approaches, however, may be limited, especially for older and frail patients who are unable to tolerate intensive induction or prolonged treatments. Personalized therapies with acceptable toxicities should be considered for these patients.
Dr. Pellegrino Musto is at Referral Cancer Center of Basilicata, Rionero in Vulture, Italy. He reported receiving honoraria from Celgene, Janssen-Cilag, Novartis, Sanofi, and Bristol-Myers Squibb. These comments are from an editorial (J Clin Oncol. 2016 Apr 25. doi: 10.1200/JCO.2016.66.6115) that accompanied the published study.
The study by Dr. Royer and associates is the first prospective trial to confirm that bortezomib-based regimens combined with a transplantation program may be effective and feasible in a significant proportion of patients with primary plasma cell leukemia. Response to induction therapy, however, was not remarkable; thus, although both cyclophosphamide and doxorubicin have demonstrated efficacy in primary plasma cell leukemia, the introduction of lenalidomide and/or incorporation of newer agents such as pomalidomide, carfilzomib, or daratumumab could hopefully optimize the induction phase and increase the rate and quality of response in future studies.
Hopefully, sequential phases of induction therapy, multiple transplantations (if applicable), further consolidation, and maintenance should ensure rapid disease control and reduction of early deaths from initial complications, a contrasting of clonal evolution that may induce drug resistance, and activity on residual disease by decreasing the risk of relapse. Feasibility of these approaches, however, may be limited, especially for older and frail patients who are unable to tolerate intensive induction or prolonged treatments. Personalized therapies with acceptable toxicities should be considered for these patients.
Dr. Pellegrino Musto is at Referral Cancer Center of Basilicata, Rionero in Vulture, Italy. He reported receiving honoraria from Celgene, Janssen-Cilag, Novartis, Sanofi, and Bristol-Myers Squibb. These comments are from an editorial (J Clin Oncol. 2016 Apr 25. doi: 10.1200/JCO.2016.66.6115) that accompanied the published study.
The study by Dr. Royer and associates is the first prospective trial to confirm that bortezomib-based regimens combined with a transplantation program may be effective and feasible in a significant proportion of patients with primary plasma cell leukemia. Response to induction therapy, however, was not remarkable; thus, although both cyclophosphamide and doxorubicin have demonstrated efficacy in primary plasma cell leukemia, the introduction of lenalidomide and/or incorporation of newer agents such as pomalidomide, carfilzomib, or daratumumab could hopefully optimize the induction phase and increase the rate and quality of response in future studies.
Hopefully, sequential phases of induction therapy, multiple transplantations (if applicable), further consolidation, and maintenance should ensure rapid disease control and reduction of early deaths from initial complications, a contrasting of clonal evolution that may induce drug resistance, and activity on residual disease by decreasing the risk of relapse. Feasibility of these approaches, however, may be limited, especially for older and frail patients who are unable to tolerate intensive induction or prolonged treatments. Personalized therapies with acceptable toxicities should be considered for these patients.
Dr. Pellegrino Musto is at Referral Cancer Center of Basilicata, Rionero in Vulture, Italy. He reported receiving honoraria from Celgene, Janssen-Cilag, Novartis, Sanofi, and Bristol-Myers Squibb. These comments are from an editorial (J Clin Oncol. 2016 Apr 25. doi: 10.1200/JCO.2016.66.6115) that accompanied the published study.
In a prospective study of 40 patients with primary plasma cell leukemia, upfront autotransplantation followed by allotransplant for younger patients and by consolidation/maintenance for older patients was associated with a median overall survival of 36.3 months and a median progression-free survival of 15.1 months.
Patients with this aggressive form of multiple myeloma received a regimen that combined standard chemotherapy, a proteasome inhibitor, high-dose melphalan followed by autologous stem cell transplantation, and allogeneic transplantation or immunomodulatory drugs, reported Dr. Bruno Royer of University Hospital in Amiens, France, and his associates.
Induction therapy consisted of four 21-day cycles: Cycles 1 and 3 included subcutaneous bortezomib, intravenous pegylated doxorubicin, and oral dexamethasone; cycles 2 and 4 included subcutaneous bortezomib, oral cyclophosphamide, and oral dexamethasone. Of 39 patients – one patient died 24 hours after study inclusion – 35 completed the four cycles. The overall response rate to induction was 69%: 10% of patients had a complete response and 26% had a very good partial response. Of 27 responding patients, 25 underwent high-dose melphalan followed by autologous stem cell transplantation.
The high response rates allowed 16 patients who were younger than 66 years and had an HLA-matched donor to then receive high-dose melphalan followed by autologous stem cell transplantation followed by consolidation with either an reduced-intensity conditioning allograft or a second high-dose melphalan followed by autologous stem cell transplantation and subsequent maintenance with lenalidomide, bortezomib, and dexamethasone for 1 year, the researchers said (J Clin Oncol. 2016 Apr 25. doi: 10.1200/JCO.2015.63.1929).
A total of 20% of patients had a complete response to the entire treatment protocol, 13% had a stringent complete response, 26% had a very good partial response, 5% had stable disease, and 5% had progressive disease. Thirteen patients died of progressive disease and four died of infections, including three that occurred during induction or after allograft.
This is only the second prospective trial in patients with primary plasma cell leukemia, an aggressive form of multiple myeloma that accounts for 2%-4% of cases, the researchers said. Future prospective trials should seek to optimize induction with newer combinations, such as carfilzomib, lenalidomide, dexamethasone, or monoclonal anti-CD38 antibodies. Also, optimizing the stem cell conditioning procedure and the postallograft immunomodulation may further benefit younger patients.
Dr. Royer reported receiving honoraria from Amgen and having served as a consultant or advisor for Octapharma Plasma. Fifteen coinvestigators also reported financial relationships with a number of pharmaceutical companies.
In a prospective study of 40 patients with primary plasma cell leukemia, upfront autotransplantation followed by allotransplant for younger patients and by consolidation/maintenance for older patients was associated with a median overall survival of 36.3 months and a median progression-free survival of 15.1 months.
Patients with this aggressive form of multiple myeloma received a regimen that combined standard chemotherapy, a proteasome inhibitor, high-dose melphalan followed by autologous stem cell transplantation, and allogeneic transplantation or immunomodulatory drugs, reported Dr. Bruno Royer of University Hospital in Amiens, France, and his associates.
Induction therapy consisted of four 21-day cycles: Cycles 1 and 3 included subcutaneous bortezomib, intravenous pegylated doxorubicin, and oral dexamethasone; cycles 2 and 4 included subcutaneous bortezomib, oral cyclophosphamide, and oral dexamethasone. Of 39 patients – one patient died 24 hours after study inclusion – 35 completed the four cycles. The overall response rate to induction was 69%: 10% of patients had a complete response and 26% had a very good partial response. Of 27 responding patients, 25 underwent high-dose melphalan followed by autologous stem cell transplantation.
The high response rates allowed 16 patients who were younger than 66 years and had an HLA-matched donor to then receive high-dose melphalan followed by autologous stem cell transplantation followed by consolidation with either an reduced-intensity conditioning allograft or a second high-dose melphalan followed by autologous stem cell transplantation and subsequent maintenance with lenalidomide, bortezomib, and dexamethasone for 1 year, the researchers said (J Clin Oncol. 2016 Apr 25. doi: 10.1200/JCO.2015.63.1929).
A total of 20% of patients had a complete response to the entire treatment protocol, 13% had a stringent complete response, 26% had a very good partial response, 5% had stable disease, and 5% had progressive disease. Thirteen patients died of progressive disease and four died of infections, including three that occurred during induction or after allograft.
This is only the second prospective trial in patients with primary plasma cell leukemia, an aggressive form of multiple myeloma that accounts for 2%-4% of cases, the researchers said. Future prospective trials should seek to optimize induction with newer combinations, such as carfilzomib, lenalidomide, dexamethasone, or monoclonal anti-CD38 antibodies. Also, optimizing the stem cell conditioning procedure and the postallograft immunomodulation may further benefit younger patients.
Dr. Royer reported receiving honoraria from Amgen and having served as a consultant or advisor for Octapharma Plasma. Fifteen coinvestigators also reported financial relationships with a number of pharmaceutical companies.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Progression-free survival was improved in patients who had primary plasma cell leukemia and underwent four cycles of induction; high-dose melphalan followed by autologous stem cell transplantation; consolidation with either a reduced-intensity conditioning allograft or a second high-dose melphalan followed by autologous stem cell transplantation; and subsequent maintenance with lenalidomide, bortezomib, and dexamethasone for 1 year.
Major finding: Median overall survival was 36.3 months and median progression-free survival was 15.1 months.
Data source: A prospective phase II study of 40 adults with newly diagnosed primary plasma cell leukemia.
Disclosures: Dr. Royer reported receiving honoraria from Amgen and having served as a consultant or advisor for Octapharma Plasma. Fifteen coinvestigators also reported financial relationships with various drug companies.
Engaging Your Patients in Decision-Making Processes Yields Better Outcomes
Editor’s note: “Everything We Say and Do” is an informational series developed by SHM’s Patient Experience Committee to provide readers with thoughtful and actionable communication tactics that have great potential to positively impact patients’ experience of care. Each column will focus on how the contributor applies one of the “Key Communication” areas in practice.
View a chart outlining key communication tactics
What I Say and Do
I counsel and deliver the diagnosis or give recommendations through a dialogue, instead of a monologue, using active listening.
Why I Do It
The monologue, or lecture, is among the least effective ways to instill behavior change. Research studies have demonstrated that, after a monologue, only around 20% to 60% of medical information is remembered by the end of a visit. Out of what is remembered, less than 50% is accurate. Furthermore, 47% of Americans have health literacy levels below the intermediate range, defined as the ability to determine when to take a medication with food from reading the label.
Lecturing the patient without first understanding what the patient knows and finds important, and understanding the barriers to plan implementation, runs the risk of decreased comprehension, a lack of understanding, or a lack of personal relevance—all leading to decreased adherence. Doing the opposite, by involving the patient in decision making, inspires change that comes from within in the context of the patient’s own needs. This approach is more enduring, emphasizes self-accountability, and ultimately leads to better outcomes.
How I Do It
I open up a dialogue using the Cleveland Clinic’s ARIA approach as adapted from the REDE model of healthcare communication.1
- First, assess: What does the patient know about diagnosis and treatment? How much and what type of education does the patient desire/need? What are the patient’s treatment preferences and health literacy?
- Second, reflect on what the patient just said. Validate meaning and emotion.
- Third, inform the patient within the context of the patient’s perspectives and preferences. Speak slowly and provide small chunks of information at a time. Use understandable language and visual aids. (This will increase recall by 60%.)
- Finally, assess the patient’s understanding and emotional reaction to information provided.
- Repeat the cycle to introduce other chunks of information.
Dr. Velez is director of faculty development in the Center for Excellence in Healthcare Communication at the Cleveland Clinic.
Reference
- Windover A, Boissy A, Rice T, Gilligan T, Velez V, Merlino J. The REDE model of healthcare communication: optimizing relationship as a therapeutic agent. J Patient Exp. 2014;1(1):8-13.
Editor’s note: “Everything We Say and Do” is an informational series developed by SHM’s Patient Experience Committee to provide readers with thoughtful and actionable communication tactics that have great potential to positively impact patients’ experience of care. Each column will focus on how the contributor applies one of the “Key Communication” areas in practice.
View a chart outlining key communication tactics
What I Say and Do
I counsel and deliver the diagnosis or give recommendations through a dialogue, instead of a monologue, using active listening.
Why I Do It
The monologue, or lecture, is among the least effective ways to instill behavior change. Research studies have demonstrated that, after a monologue, only around 20% to 60% of medical information is remembered by the end of a visit. Out of what is remembered, less than 50% is accurate. Furthermore, 47% of Americans have health literacy levels below the intermediate range, defined as the ability to determine when to take a medication with food from reading the label.
Lecturing the patient without first understanding what the patient knows and finds important, and understanding the barriers to plan implementation, runs the risk of decreased comprehension, a lack of understanding, or a lack of personal relevance—all leading to decreased adherence. Doing the opposite, by involving the patient in decision making, inspires change that comes from within in the context of the patient’s own needs. This approach is more enduring, emphasizes self-accountability, and ultimately leads to better outcomes.
How I Do It
I open up a dialogue using the Cleveland Clinic’s ARIA approach as adapted from the REDE model of healthcare communication.1
- First, assess: What does the patient know about diagnosis and treatment? How much and what type of education does the patient desire/need? What are the patient’s treatment preferences and health literacy?
- Second, reflect on what the patient just said. Validate meaning and emotion.
- Third, inform the patient within the context of the patient’s perspectives and preferences. Speak slowly and provide small chunks of information at a time. Use understandable language and visual aids. (This will increase recall by 60%.)
- Finally, assess the patient’s understanding and emotional reaction to information provided.
- Repeat the cycle to introduce other chunks of information.
Dr. Velez is director of faculty development in the Center for Excellence in Healthcare Communication at the Cleveland Clinic.
Reference
- Windover A, Boissy A, Rice T, Gilligan T, Velez V, Merlino J. The REDE model of healthcare communication: optimizing relationship as a therapeutic agent. J Patient Exp. 2014;1(1):8-13.
Editor’s note: “Everything We Say and Do” is an informational series developed by SHM’s Patient Experience Committee to provide readers with thoughtful and actionable communication tactics that have great potential to positively impact patients’ experience of care. Each column will focus on how the contributor applies one of the “Key Communication” areas in practice.
View a chart outlining key communication tactics
What I Say and Do
I counsel and deliver the diagnosis or give recommendations through a dialogue, instead of a monologue, using active listening.
Why I Do It
The monologue, or lecture, is among the least effective ways to instill behavior change. Research studies have demonstrated that, after a monologue, only around 20% to 60% of medical information is remembered by the end of a visit. Out of what is remembered, less than 50% is accurate. Furthermore, 47% of Americans have health literacy levels below the intermediate range, defined as the ability to determine when to take a medication with food from reading the label.
Lecturing the patient without first understanding what the patient knows and finds important, and understanding the barriers to plan implementation, runs the risk of decreased comprehension, a lack of understanding, or a lack of personal relevance—all leading to decreased adherence. Doing the opposite, by involving the patient in decision making, inspires change that comes from within in the context of the patient’s own needs. This approach is more enduring, emphasizes self-accountability, and ultimately leads to better outcomes.
How I Do It
I open up a dialogue using the Cleveland Clinic’s ARIA approach as adapted from the REDE model of healthcare communication.1
- First, assess: What does the patient know about diagnosis and treatment? How much and what type of education does the patient desire/need? What are the patient’s treatment preferences and health literacy?
- Second, reflect on what the patient just said. Validate meaning and emotion.
- Third, inform the patient within the context of the patient’s perspectives and preferences. Speak slowly and provide small chunks of information at a time. Use understandable language and visual aids. (This will increase recall by 60%.)
- Finally, assess the patient’s understanding and emotional reaction to information provided.
- Repeat the cycle to introduce other chunks of information.
Dr. Velez is director of faculty development in the Center for Excellence in Healthcare Communication at the Cleveland Clinic.
Reference
- Windover A, Boissy A, Rice T, Gilligan T, Velez V, Merlino J. The REDE model of healthcare communication: optimizing relationship as a therapeutic agent. J Patient Exp. 2014;1(1):8-13.
Inflammation has negative effects on HSCs

in the bone marrow
Preclinical research suggests chronic inflammation leads to an imbalanced blood system, which may have an impact on hematopoietic stem cell (HSC) transplant.
The study showed that chronic exposure to an inflammatory “emergency” signal, interleukin-1 (IL-1), has a negative effect on HSCs—restricting differentiation, impairing self-renewal capacity, and priming HSCs to fail massive replicative challenges such as transplantation.
However, these effects proved to be fully reversible.
Eric M. Pietras, PhD, of the University of Colorado Anschutz Medical Campus in Aurora, and his colleagues recounted these findings in Nature Cell Biology.
While HSCs are usually dormant in the bone marrow, Dr Pietras said he and his colleagues showed that, “these cells are also exquisitely sensitive to changes in their environment and react accordingly.”
The team showed that HSCs are sensitive to the amount of IL-1 they encounter. Chronic IL-1 exposure prompts accelerated cell division and pushes HSCs toward myeloid differentiation through activation of the NF-κB pathway and engagement of a PU.1-dependent myeloid gene program.
So HSCs that are overexposed to IL-1 lose their ability to differentiate into lymphoid and erythroid cells.
“[The HSCs are] receiving a signal telling them they need to keep building myeloid cells, and, as a result, they don’t make the other blood cells you need,” Dr Pietras explained.
“You can end up with too few red blood cells, reducing the body’s ability to deliver oxygen to cells. Or we see decreased production of new lymphoid cells, leaving the system potentially immunodeficient. These are all common features of chronically inflamed, and even aged, blood systems.”
Chronic IL-1 exposure also led to decreased self-renewal activity and regenerative potential in HSCs in response to transplantation in mice. Dr Pietras and his colleagues believe these findings may translate to HSC transplant in humans.
“Our results show that not only should we be looking for markers of blood system compatibility [in HSC donors], but we may also want to explore whether a potential donor’s [HSCs] have been exposed to inflammation and may not be as effective at rebuilding the patient’s blood system,” Dr Pietras said.
“Likewise, the presence of inflammation in the individual receiving the [HSC transplant] could also be an important factor in how well the stem cells regenerate a new blood system once they are transplanted.”
Fortunately, Dr Pietras and his colleagues found the damaging effects of chronic IL-1 exposure could be reversed upon IL-1 withdrawal.
To test the durability of IL-1’s effects, the researchers treated mice with IL-1 for 20 days and then stopped for several weeks to see if the HSCs recovered.
“Our data suggest that it is possible to turn back the clock and reverse the effects of chronic inflammation on [HSCs], perhaps using therapies already available in the clinic to block inflammatory signals such as IL-1,” Dr Pietras said.
“Of course, we don’t yet know, on a human scale, how long it takes a stem cell to ‘remember’ these insults. It may be that, after a longer period of exposure to IL-1, these changes become more fixed.” ![]()

in the bone marrow
Preclinical research suggests chronic inflammation leads to an imbalanced blood system, which may have an impact on hematopoietic stem cell (HSC) transplant.
The study showed that chronic exposure to an inflammatory “emergency” signal, interleukin-1 (IL-1), has a negative effect on HSCs—restricting differentiation, impairing self-renewal capacity, and priming HSCs to fail massive replicative challenges such as transplantation.
However, these effects proved to be fully reversible.
Eric M. Pietras, PhD, of the University of Colorado Anschutz Medical Campus in Aurora, and his colleagues recounted these findings in Nature Cell Biology.
While HSCs are usually dormant in the bone marrow, Dr Pietras said he and his colleagues showed that, “these cells are also exquisitely sensitive to changes in their environment and react accordingly.”
The team showed that HSCs are sensitive to the amount of IL-1 they encounter. Chronic IL-1 exposure prompts accelerated cell division and pushes HSCs toward myeloid differentiation through activation of the NF-κB pathway and engagement of a PU.1-dependent myeloid gene program.
So HSCs that are overexposed to IL-1 lose their ability to differentiate into lymphoid and erythroid cells.
“[The HSCs are] receiving a signal telling them they need to keep building myeloid cells, and, as a result, they don’t make the other blood cells you need,” Dr Pietras explained.
“You can end up with too few red blood cells, reducing the body’s ability to deliver oxygen to cells. Or we see decreased production of new lymphoid cells, leaving the system potentially immunodeficient. These are all common features of chronically inflamed, and even aged, blood systems.”
Chronic IL-1 exposure also led to decreased self-renewal activity and regenerative potential in HSCs in response to transplantation in mice. Dr Pietras and his colleagues believe these findings may translate to HSC transplant in humans.
“Our results show that not only should we be looking for markers of blood system compatibility [in HSC donors], but we may also want to explore whether a potential donor’s [HSCs] have been exposed to inflammation and may not be as effective at rebuilding the patient’s blood system,” Dr Pietras said.
“Likewise, the presence of inflammation in the individual receiving the [HSC transplant] could also be an important factor in how well the stem cells regenerate a new blood system once they are transplanted.”
Fortunately, Dr Pietras and his colleagues found the damaging effects of chronic IL-1 exposure could be reversed upon IL-1 withdrawal.
To test the durability of IL-1’s effects, the researchers treated mice with IL-1 for 20 days and then stopped for several weeks to see if the HSCs recovered.
“Our data suggest that it is possible to turn back the clock and reverse the effects of chronic inflammation on [HSCs], perhaps using therapies already available in the clinic to block inflammatory signals such as IL-1,” Dr Pietras said.
“Of course, we don’t yet know, on a human scale, how long it takes a stem cell to ‘remember’ these insults. It may be that, after a longer period of exposure to IL-1, these changes become more fixed.” ![]()

in the bone marrow
Preclinical research suggests chronic inflammation leads to an imbalanced blood system, which may have an impact on hematopoietic stem cell (HSC) transplant.
The study showed that chronic exposure to an inflammatory “emergency” signal, interleukin-1 (IL-1), has a negative effect on HSCs—restricting differentiation, impairing self-renewal capacity, and priming HSCs to fail massive replicative challenges such as transplantation.
However, these effects proved to be fully reversible.
Eric M. Pietras, PhD, of the University of Colorado Anschutz Medical Campus in Aurora, and his colleagues recounted these findings in Nature Cell Biology.
While HSCs are usually dormant in the bone marrow, Dr Pietras said he and his colleagues showed that, “these cells are also exquisitely sensitive to changes in their environment and react accordingly.”
The team showed that HSCs are sensitive to the amount of IL-1 they encounter. Chronic IL-1 exposure prompts accelerated cell division and pushes HSCs toward myeloid differentiation through activation of the NF-κB pathway and engagement of a PU.1-dependent myeloid gene program.
So HSCs that are overexposed to IL-1 lose their ability to differentiate into lymphoid and erythroid cells.
“[The HSCs are] receiving a signal telling them they need to keep building myeloid cells, and, as a result, they don’t make the other blood cells you need,” Dr Pietras explained.
“You can end up with too few red blood cells, reducing the body’s ability to deliver oxygen to cells. Or we see decreased production of new lymphoid cells, leaving the system potentially immunodeficient. These are all common features of chronically inflamed, and even aged, blood systems.”
Chronic IL-1 exposure also led to decreased self-renewal activity and regenerative potential in HSCs in response to transplantation in mice. Dr Pietras and his colleagues believe these findings may translate to HSC transplant in humans.
“Our results show that not only should we be looking for markers of blood system compatibility [in HSC donors], but we may also want to explore whether a potential donor’s [HSCs] have been exposed to inflammation and may not be as effective at rebuilding the patient’s blood system,” Dr Pietras said.
“Likewise, the presence of inflammation in the individual receiving the [HSC transplant] could also be an important factor in how well the stem cells regenerate a new blood system once they are transplanted.”
Fortunately, Dr Pietras and his colleagues found the damaging effects of chronic IL-1 exposure could be reversed upon IL-1 withdrawal.
To test the durability of IL-1’s effects, the researchers treated mice with IL-1 for 20 days and then stopped for several weeks to see if the HSCs recovered.
“Our data suggest that it is possible to turn back the clock and reverse the effects of chronic inflammation on [HSCs], perhaps using therapies already available in the clinic to block inflammatory signals such as IL-1,” Dr Pietras said.
“Of course, we don’t yet know, on a human scale, how long it takes a stem cell to ‘remember’ these insults. It may be that, after a longer period of exposure to IL-1, these changes become more fixed.” ![]()
CDC, OSHA issue guidance to protect workers from Zika virus
Photo by William Weinert
The US Centers for Disease Control and Prevention (CDC) and the Occupational Safety and Health Administration (OSHA) have issued an interim guidance for protecting workers from occupational exposure to the Zika virus.
The guidance is for healthcare and laboratory workers, outdoor workers, mosquito control workers, and business travelers.
It includes recommendations to help protect these workers from mosquito bites and exposure to an infected person’s blood or other body fluids.
The CDC noted that, although Zika virus is primarily spread by infected mosquitoes, exposure to an infected person’s blood or other body fluids may also result in transmission.
So healthcare workers who may be exposed to contaminated blood or other potentially infectious materials from people infected with Zika virus may require additional protection.
Recommendations for healthcare and laboratory workers
Employers and workers in healthcare settings and laboratories should follow standard infection control and biosafety practices (including universal precautions) as appropriate to prevent or minimize the risk of Zika virus transmission.
Standard precautions include, but are not limited to, hand hygiene and the use of personal protective equipment (PPE) to avoid direct contact with blood and other potentially infectious materials, including laboratory specimens/samples. PPE may include gloves, gowns, masks, and eye protection.
Hand hygiene consists of washing with soap and water or using alcohol-based hand rubs containing at least 60% alcohol. Soap and water are best for hands that are visibly soiled. Perform hand hygiene before and after any contact with a patient, after any contact with potentially infectious material, and before putting on and upon removing PPE, including gloves.
Laboratories should ensure that their facilities and practices meet the appropriate Biosafety Level for the type of work being conducted (including the specific biologic agents—in this case, Zika virus) in the laboratory.
Employers should ensure that workers follow workplace standard operating procedures (eg, workplace exposure control plans) and use the engineering controls and work practices available in the workplace to prevent exposure to blood or other potentially infectious materials.
Employers should ensure workers do not bend, recap, or remove contaminated needles or other contaminated sharps. Properly dispose of these items in closable, puncture-resistant, leak-proof, and labeled or color-coded containers. Workers should use sharps with engineered sharps injury protection to avoid sharps-related injuries.
Additional details and recommendations for business travelers, outdoor workers, and mosquito control workers are available in the full guidance document.
The CDC said it will continue to update this guidance based on accumulating evidence. For updates, visit www.cdc.gov/zika. ![]()

Photo by William Weinert
The US Centers for Disease Control and Prevention (CDC) and the Occupational Safety and Health Administration (OSHA) have issued an interim guidance for protecting workers from occupational exposure to the Zika virus.
The guidance is for healthcare and laboratory workers, outdoor workers, mosquito control workers, and business travelers.
It includes recommendations to help protect these workers from mosquito bites and exposure to an infected person’s blood or other body fluids.
The CDC noted that, although Zika virus is primarily spread by infected mosquitoes, exposure to an infected person’s blood or other body fluids may also result in transmission.
So healthcare workers who may be exposed to contaminated blood or other potentially infectious materials from people infected with Zika virus may require additional protection.
Recommendations for healthcare and laboratory workers
Employers and workers in healthcare settings and laboratories should follow standard infection control and biosafety practices (including universal precautions) as appropriate to prevent or minimize the risk of Zika virus transmission.
Standard precautions include, but are not limited to, hand hygiene and the use of personal protective equipment (PPE) to avoid direct contact with blood and other potentially infectious materials, including laboratory specimens/samples. PPE may include gloves, gowns, masks, and eye protection.
Hand hygiene consists of washing with soap and water or using alcohol-based hand rubs containing at least 60% alcohol. Soap and water are best for hands that are visibly soiled. Perform hand hygiene before and after any contact with a patient, after any contact with potentially infectious material, and before putting on and upon removing PPE, including gloves.
Laboratories should ensure that their facilities and practices meet the appropriate Biosafety Level for the type of work being conducted (including the specific biologic agents—in this case, Zika virus) in the laboratory.
Employers should ensure that workers follow workplace standard operating procedures (eg, workplace exposure control plans) and use the engineering controls and work practices available in the workplace to prevent exposure to blood or other potentially infectious materials.
Employers should ensure workers do not bend, recap, or remove contaminated needles or other contaminated sharps. Properly dispose of these items in closable, puncture-resistant, leak-proof, and labeled or color-coded containers. Workers should use sharps with engineered sharps injury protection to avoid sharps-related injuries.
Additional details and recommendations for business travelers, outdoor workers, and mosquito control workers are available in the full guidance document.
The CDC said it will continue to update this guidance based on accumulating evidence. For updates, visit www.cdc.gov/zika. ![]()

Photo by William Weinert
The US Centers for Disease Control and Prevention (CDC) and the Occupational Safety and Health Administration (OSHA) have issued an interim guidance for protecting workers from occupational exposure to the Zika virus.
The guidance is for healthcare and laboratory workers, outdoor workers, mosquito control workers, and business travelers.
It includes recommendations to help protect these workers from mosquito bites and exposure to an infected person’s blood or other body fluids.
The CDC noted that, although Zika virus is primarily spread by infected mosquitoes, exposure to an infected person’s blood or other body fluids may also result in transmission.
So healthcare workers who may be exposed to contaminated blood or other potentially infectious materials from people infected with Zika virus may require additional protection.
Recommendations for healthcare and laboratory workers
Employers and workers in healthcare settings and laboratories should follow standard infection control and biosafety practices (including universal precautions) as appropriate to prevent or minimize the risk of Zika virus transmission.
Standard precautions include, but are not limited to, hand hygiene and the use of personal protective equipment (PPE) to avoid direct contact with blood and other potentially infectious materials, including laboratory specimens/samples. PPE may include gloves, gowns, masks, and eye protection.
Hand hygiene consists of washing with soap and water or using alcohol-based hand rubs containing at least 60% alcohol. Soap and water are best for hands that are visibly soiled. Perform hand hygiene before and after any contact with a patient, after any contact with potentially infectious material, and before putting on and upon removing PPE, including gloves.
Laboratories should ensure that their facilities and practices meet the appropriate Biosafety Level for the type of work being conducted (including the specific biologic agents—in this case, Zika virus) in the laboratory.
Employers should ensure that workers follow workplace standard operating procedures (eg, workplace exposure control plans) and use the engineering controls and work practices available in the workplace to prevent exposure to blood or other potentially infectious materials.
Employers should ensure workers do not bend, recap, or remove contaminated needles or other contaminated sharps. Properly dispose of these items in closable, puncture-resistant, leak-proof, and labeled or color-coded containers. Workers should use sharps with engineered sharps injury protection to avoid sharps-related injuries.
Additional details and recommendations for business travelers, outdoor workers, and mosquito control workers are available in the full guidance document.
The CDC said it will continue to update this guidance based on accumulating evidence. For updates, visit www.cdc.gov/zika. ![]()
Combo may be active in refractory MM

Photo courtesy of the CDC
The combination of vorinostat and bortezomib, with or without dexamethasone, can be active in patients with multiple myeloma (MM) that is refractory to novel treatments, according to researchers.
In a phase 2 trial, the combination produced an overall response rate of 11% among MM patients who were refractory to bortezomib and were either refractory to or could not receive treatment with an immunomodulatory agent (IMiD).
About 92% of patients had drug-related adverse events (AEs), and 20% had serious drug-related AEs.
These results were published in Clinical Lymphoma, Myeloma & Leukemia. The study was funded by Merck & Co., Inc., which markets vorinostat as Zolinza.
This trial (VANTAGE 095) enrolled 143 MM patients from 41 centers in 12 countries. The patients had a median age of 63 (range, 37-81) and had received a median of 4 prior lines of therapy (range, 2-17).
All 143 patients were considered refractory to previous bortezomib, which was defined as less than 25% response on therapy or progression during/less than 60 days after the completion of bortezomib therapy.
All but 1 patient had been exposed to 1 or more IMiDs (99.3%). Roughly 87% of patients exposed to IMiDs were considered to have disease refractory to at least 1 IMiD and 40% to at least 2 different IMiDs. Three percent of patients were considered ineligible for further IMiD-based therapy because of previous toxicities.
For this study, patients received 21-day cycles of bortezomib (1.3 mg/m2 intravenously; days 1, 4, 8, and 11) plus oral vorinostat at 400 mg/d on days 1 to 14.
If a patient had no change as the best response after 4 cycles of treatment or progressive disease after 2 cycles of treatment, oral dexamethasone at 20 mg on the day of and day after each dose of bortezomib could be added to the treatment regimen.
Patients were treated until disease progression, unacceptable toxicities, or withdrawal from the study.
One hundred and forty-two patients were evaluable for safety and efficacy. Fifty-seven of these patients received dexamethasone per protocol.
The overall response rate (partial response or better), as assessed by an independent adjudication committee, was 11.3%.
All 16 responses were partial responses. The median time to response was 44 days (range, 22-71), and the median duration of response was 211 days (range, 64-550 days).
Eleven patients (7.7%) had a minimal response, and 87 (61.3%) had stable disease.
The median progression-free survival was 3.13 months, and the median time to progression was 3.47 months. The median overall survival was 11.2 months, with a 2-year survival rate of 32%.
All 142 patients had at least 1 AE, and 131 (92.3%) had drug-related AEs. Most AEs were grade 3 (28.9%) or 4 (50.7%). Serious AEs occurred in 64.8% of patients, and serious drug-related AEs occurred in 20.4%.
Twenty-seven patients (19%) discontinued treatment due to an AE. And 24 patients (16.9%) died from an AE, although 18 of these deaths were attributable to progression of underlying MM.
The most common AEs were thrombocytopenia (69.7%), nausea (57.0%), diarrhea (53.5%), anemia (52.1%), and fatigue (48.6%). ![]()

Photo courtesy of the CDC
The combination of vorinostat and bortezomib, with or without dexamethasone, can be active in patients with multiple myeloma (MM) that is refractory to novel treatments, according to researchers.
In a phase 2 trial, the combination produced an overall response rate of 11% among MM patients who were refractory to bortezomib and were either refractory to or could not receive treatment with an immunomodulatory agent (IMiD).
About 92% of patients had drug-related adverse events (AEs), and 20% had serious drug-related AEs.
These results were published in Clinical Lymphoma, Myeloma & Leukemia. The study was funded by Merck & Co., Inc., which markets vorinostat as Zolinza.
This trial (VANTAGE 095) enrolled 143 MM patients from 41 centers in 12 countries. The patients had a median age of 63 (range, 37-81) and had received a median of 4 prior lines of therapy (range, 2-17).
All 143 patients were considered refractory to previous bortezomib, which was defined as less than 25% response on therapy or progression during/less than 60 days after the completion of bortezomib therapy.
All but 1 patient had been exposed to 1 or more IMiDs (99.3%). Roughly 87% of patients exposed to IMiDs were considered to have disease refractory to at least 1 IMiD and 40% to at least 2 different IMiDs. Three percent of patients were considered ineligible for further IMiD-based therapy because of previous toxicities.
For this study, patients received 21-day cycles of bortezomib (1.3 mg/m2 intravenously; days 1, 4, 8, and 11) plus oral vorinostat at 400 mg/d on days 1 to 14.
If a patient had no change as the best response after 4 cycles of treatment or progressive disease after 2 cycles of treatment, oral dexamethasone at 20 mg on the day of and day after each dose of bortezomib could be added to the treatment regimen.
Patients were treated until disease progression, unacceptable toxicities, or withdrawal from the study.
One hundred and forty-two patients were evaluable for safety and efficacy. Fifty-seven of these patients received dexamethasone per protocol.
The overall response rate (partial response or better), as assessed by an independent adjudication committee, was 11.3%.
All 16 responses were partial responses. The median time to response was 44 days (range, 22-71), and the median duration of response was 211 days (range, 64-550 days).
Eleven patients (7.7%) had a minimal response, and 87 (61.3%) had stable disease.
The median progression-free survival was 3.13 months, and the median time to progression was 3.47 months. The median overall survival was 11.2 months, with a 2-year survival rate of 32%.
All 142 patients had at least 1 AE, and 131 (92.3%) had drug-related AEs. Most AEs were grade 3 (28.9%) or 4 (50.7%). Serious AEs occurred in 64.8% of patients, and serious drug-related AEs occurred in 20.4%.
Twenty-seven patients (19%) discontinued treatment due to an AE. And 24 patients (16.9%) died from an AE, although 18 of these deaths were attributable to progression of underlying MM.
The most common AEs were thrombocytopenia (69.7%), nausea (57.0%), diarrhea (53.5%), anemia (52.1%), and fatigue (48.6%). ![]()

Photo courtesy of the CDC
The combination of vorinostat and bortezomib, with or without dexamethasone, can be active in patients with multiple myeloma (MM) that is refractory to novel treatments, according to researchers.
In a phase 2 trial, the combination produced an overall response rate of 11% among MM patients who were refractory to bortezomib and were either refractory to or could not receive treatment with an immunomodulatory agent (IMiD).
About 92% of patients had drug-related adverse events (AEs), and 20% had serious drug-related AEs.
These results were published in Clinical Lymphoma, Myeloma & Leukemia. The study was funded by Merck & Co., Inc., which markets vorinostat as Zolinza.
This trial (VANTAGE 095) enrolled 143 MM patients from 41 centers in 12 countries. The patients had a median age of 63 (range, 37-81) and had received a median of 4 prior lines of therapy (range, 2-17).
All 143 patients were considered refractory to previous bortezomib, which was defined as less than 25% response on therapy or progression during/less than 60 days after the completion of bortezomib therapy.
All but 1 patient had been exposed to 1 or more IMiDs (99.3%). Roughly 87% of patients exposed to IMiDs were considered to have disease refractory to at least 1 IMiD and 40% to at least 2 different IMiDs. Three percent of patients were considered ineligible for further IMiD-based therapy because of previous toxicities.
For this study, patients received 21-day cycles of bortezomib (1.3 mg/m2 intravenously; days 1, 4, 8, and 11) plus oral vorinostat at 400 mg/d on days 1 to 14.
If a patient had no change as the best response after 4 cycles of treatment or progressive disease after 2 cycles of treatment, oral dexamethasone at 20 mg on the day of and day after each dose of bortezomib could be added to the treatment regimen.
Patients were treated until disease progression, unacceptable toxicities, or withdrawal from the study.
One hundred and forty-two patients were evaluable for safety and efficacy. Fifty-seven of these patients received dexamethasone per protocol.
The overall response rate (partial response or better), as assessed by an independent adjudication committee, was 11.3%.
All 16 responses were partial responses. The median time to response was 44 days (range, 22-71), and the median duration of response was 211 days (range, 64-550 days).
Eleven patients (7.7%) had a minimal response, and 87 (61.3%) had stable disease.
The median progression-free survival was 3.13 months, and the median time to progression was 3.47 months. The median overall survival was 11.2 months, with a 2-year survival rate of 32%.
All 142 patients had at least 1 AE, and 131 (92.3%) had drug-related AEs. Most AEs were grade 3 (28.9%) or 4 (50.7%). Serious AEs occurred in 64.8% of patients, and serious drug-related AEs occurred in 20.4%.
Twenty-seven patients (19%) discontinued treatment due to an AE. And 24 patients (16.9%) died from an AE, although 18 of these deaths were attributable to progression of underlying MM.
The most common AEs were thrombocytopenia (69.7%), nausea (57.0%), diarrhea (53.5%), anemia (52.1%), and fatigue (48.6%). ![]()
Factors may increase risk of asparaginase-induced pancreatitis in ALL

(left) and Chengcheng Liu
Photo courtesy of St. Jude
Children’s Research Hospital
and Peter Barta
Researchers have identified several factors that may increase the risk of asparaginase-induced pancreatitis in patients with acute lymphoblastic leukemia (ALL).
The team found that 16 variants in the CPA2 gene—and 1 rare variant in particular—were associated with a higher risk of asparaginase-induced pancreatitis.
Patients also had a higher risk if they had genetically defined Native American ancestry, were older, and received higher doses of asparaginase.
The researchers reported these findings in the Journal of Clinical Oncology.
“In this study, we identified several independent risk factors for asparaginase-induced pancreatitis and also gained insight into the mechanism responsible for this serious treatment complication,” said study author Mary Relling, PharmD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
“Understanding the risk factors for acute pancreatitis is important because, in patients who can tolerate the drug, asparaginase reduces the likelihood that ALL patients will relapse.”
The research included 5398 ALL patients (ages 0 to 30) who were treated in clinical trials organized by St. Jude or the Children’s Oncology Group. In all, 188 patients developed pancreatitis at least once during ALL therapy.
To search for risk factors associated with asparaginase-induced pancreatitis, the researchers checked patient DNA for more than 920,000 gene variants.
The team also sequenced 283 genes, including genes associated with ALL risk and treatment outcome and genes linked to an elevated risk of pancreatitis in patients with different health problems.
The results revealed a rare nonsense variant in CPA2 (rs199695765) that yields a truncated version of the pancreatic enzyme proCPA2. The researchers said this variant was “highly associated” with pancreatitis, with a hazard ratio (HR) of 587 (P=9.0×10−9).
Two study participants each carried 1 copy of the variant, and both patients developed severe pancreatitis within weeks of receiving their first dose of asparaginase.
“That suggests patients with this rare variant cannot tolerate the drug long enough to benefit from treatment,” Dr Relling said. “For these patients, ALL treatment regimens that do not depend on asparaginase may be preferable.”
The researchers estimated that about 9 in 100,000 individuals carry the suspected high-risk CPA2 variant.
The team also found an excess of additional CPA2 variants in patients who developed pancreatitis compared to those who did not (P=0.001).
In all, the researchers identified 380 variants in CPA2. Sixteen of them were significantly associated (P<0.05) with pancreatitis, and 54% (13/24) of patients who carried at least 1 of these variants developed pancreatitis.
The researchers also found links between clinical factors and asparaginase-induced pancreatitis. A multivariate analysis suggested the following were associated with pancreatitis:
- Older age (HR=1.1 per year; P<0.001)
- Genetically defined Native American ancestry (HR=1.2 for every 10% increase in Native American ancestry; P<0.001)
- High-dose (≥240,000 U/m2) asparaginase regimens (HR=3.2; P<0.001).


(left) and Chengcheng Liu
Photo courtesy of St. Jude
Children’s Research Hospital
and Peter Barta
Researchers have identified several factors that may increase the risk of asparaginase-induced pancreatitis in patients with acute lymphoblastic leukemia (ALL).
The team found that 16 variants in the CPA2 gene—and 1 rare variant in particular—were associated with a higher risk of asparaginase-induced pancreatitis.
Patients also had a higher risk if they had genetically defined Native American ancestry, were older, and received higher doses of asparaginase.
The researchers reported these findings in the Journal of Clinical Oncology.
“In this study, we identified several independent risk factors for asparaginase-induced pancreatitis and also gained insight into the mechanism responsible for this serious treatment complication,” said study author Mary Relling, PharmD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
“Understanding the risk factors for acute pancreatitis is important because, in patients who can tolerate the drug, asparaginase reduces the likelihood that ALL patients will relapse.”
The research included 5398 ALL patients (ages 0 to 30) who were treated in clinical trials organized by St. Jude or the Children’s Oncology Group. In all, 188 patients developed pancreatitis at least once during ALL therapy.
To search for risk factors associated with asparaginase-induced pancreatitis, the researchers checked patient DNA for more than 920,000 gene variants.
The team also sequenced 283 genes, including genes associated with ALL risk and treatment outcome and genes linked to an elevated risk of pancreatitis in patients with different health problems.
The results revealed a rare nonsense variant in CPA2 (rs199695765) that yields a truncated version of the pancreatic enzyme proCPA2. The researchers said this variant was “highly associated” with pancreatitis, with a hazard ratio (HR) of 587 (P=9.0×10−9).
Two study participants each carried 1 copy of the variant, and both patients developed severe pancreatitis within weeks of receiving their first dose of asparaginase.
“That suggests patients with this rare variant cannot tolerate the drug long enough to benefit from treatment,” Dr Relling said. “For these patients, ALL treatment regimens that do not depend on asparaginase may be preferable.”
The researchers estimated that about 9 in 100,000 individuals carry the suspected high-risk CPA2 variant.
The team also found an excess of additional CPA2 variants in patients who developed pancreatitis compared to those who did not (P=0.001).
In all, the researchers identified 380 variants in CPA2. Sixteen of them were significantly associated (P<0.05) with pancreatitis, and 54% (13/24) of patients who carried at least 1 of these variants developed pancreatitis.
The researchers also found links between clinical factors and asparaginase-induced pancreatitis. A multivariate analysis suggested the following were associated with pancreatitis:
- Older age (HR=1.1 per year; P<0.001)
- Genetically defined Native American ancestry (HR=1.2 for every 10% increase in Native American ancestry; P<0.001)
- High-dose (≥240,000 U/m2) asparaginase regimens (HR=3.2; P<0.001).


(left) and Chengcheng Liu
Photo courtesy of St. Jude
Children’s Research Hospital
and Peter Barta
Researchers have identified several factors that may increase the risk of asparaginase-induced pancreatitis in patients with acute lymphoblastic leukemia (ALL).
The team found that 16 variants in the CPA2 gene—and 1 rare variant in particular—were associated with a higher risk of asparaginase-induced pancreatitis.
Patients also had a higher risk if they had genetically defined Native American ancestry, were older, and received higher doses of asparaginase.
The researchers reported these findings in the Journal of Clinical Oncology.
“In this study, we identified several independent risk factors for asparaginase-induced pancreatitis and also gained insight into the mechanism responsible for this serious treatment complication,” said study author Mary Relling, PharmD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
“Understanding the risk factors for acute pancreatitis is important because, in patients who can tolerate the drug, asparaginase reduces the likelihood that ALL patients will relapse.”
The research included 5398 ALL patients (ages 0 to 30) who were treated in clinical trials organized by St. Jude or the Children’s Oncology Group. In all, 188 patients developed pancreatitis at least once during ALL therapy.
To search for risk factors associated with asparaginase-induced pancreatitis, the researchers checked patient DNA for more than 920,000 gene variants.
The team also sequenced 283 genes, including genes associated with ALL risk and treatment outcome and genes linked to an elevated risk of pancreatitis in patients with different health problems.
The results revealed a rare nonsense variant in CPA2 (rs199695765) that yields a truncated version of the pancreatic enzyme proCPA2. The researchers said this variant was “highly associated” with pancreatitis, with a hazard ratio (HR) of 587 (P=9.0×10−9).
Two study participants each carried 1 copy of the variant, and both patients developed severe pancreatitis within weeks of receiving their first dose of asparaginase.
“That suggests patients with this rare variant cannot tolerate the drug long enough to benefit from treatment,” Dr Relling said. “For these patients, ALL treatment regimens that do not depend on asparaginase may be preferable.”
The researchers estimated that about 9 in 100,000 individuals carry the suspected high-risk CPA2 variant.
The team also found an excess of additional CPA2 variants in patients who developed pancreatitis compared to those who did not (P=0.001).
In all, the researchers identified 380 variants in CPA2. Sixteen of them were significantly associated (P<0.05) with pancreatitis, and 54% (13/24) of patients who carried at least 1 of these variants developed pancreatitis.
The researchers also found links between clinical factors and asparaginase-induced pancreatitis. A multivariate analysis suggested the following were associated with pancreatitis:
- Older age (HR=1.1 per year; P<0.001)
- Genetically defined Native American ancestry (HR=1.2 for every 10% increase in Native American ancestry; P<0.001)
- High-dose (≥240,000 U/m2) asparaginase regimens (HR=3.2; P<0.001).

“Go low” or say “No” to aggressive systolic BP goals?
Consider treating non-diabetic patients age ≥50 years to a systolic blood pressure (SBP) target <120 mm Hg as compared to <140 mm Hg when the benefits—lower rates of fatal and nonfatal cardiovascular (CV) events and death from any cause—are likely to outweigh the risks from possible additional medication.1
Strength of recommendation
B: Based on a single, good-quality randomized controlled trial (RCT).
Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
Illustrative Case
A 55-year-old man with hypertension and stage 3 chronic kidney disease (CKD) comes in to your office for routine care. His blood pressure is 135/85 mm Hg, and he is presently taking lisinopril 40 mg daily. Should you increase his antihypertensive regimen?
Hypertension is common and leads to significant morbidity and mortality, but pharmacologic treatment reduces incidence of stroke by 35% to 40%, myocardial infarction (MI) by 15% to 25%, and heart failure by up to 64%.2-4 Specific blood pressure targets for defined populations continue to be studied.
In patients with diabetes, the ACCORD (Action to Control Cardiovascular Risk in Diabetes) trial found that more intensive BP targets did not reduce the rate of major CV events, but the study may have been underpowered.5 The members of The Eighth Joint National Committee recommended treating patients over age 60 years to BP goals <150/90 mm Hg.6 This was based on evidence from 6 randomized controlled trials (RCTs),7-12 but there remains debate—even among the members of the Committee—as to appropriate BP goals in patients of any age without CV disease who have BP measurements of 140-159/90-99 mm Hg.13
Study Summary
Treating to SBP <120 mm Hg lowers mortality
The Systolic Blood Pressure Intervention Trial (SPRINT) was a multicenter RCT designed to determine if treating to lower SBP targets in non-diabetic patients at high risk for CV events improves outcomes as compared to standard care. Patients were at least 50 years of age with SBP of 130 to 180 mm Hg and were at increased CV risk as defined by clinical or subclinical CV disease other than stroke, CKD with glomerular filtration rate (GFR) 20 to 60 mL/min/1.73 m2, 10-year risk of CV disease >15% on Framingham risk score, or age ≥75 years of age. Patients with diabetes; prior stroke; polycystic kidney disease; significant proteinuria within the past 6 months; symptomatic heart failure within the past 6 months; or left ventricular ejection fraction <35% were excluded.1
Patients (N=9361) were randomly assigned to an SBP target <120 mm Hg in the intensive group or <140 mm Hg in the standard treatment group, in an open-label design. Allocation was concealed. The study protocol encouraged, but did not require, the use of thiazide-type diuretics, loop diuretics (for those with advanced renal disease), angiotensin-converting enzyme inhibitors or angiotensin receptor blocker agents, calcium channel blockers, and beta-blockers. Clinicians could add other agents as needed. All major classes of antihypertensives were used.
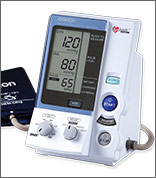
Medication dosing adjustments were based on the average of 3 BP measurements taken with an automated measurement system (Omron Healthcare, Model 907) with the patient seated after 5 minutes of quiet rest. Target SBP in the standard therapy group was 135 to 139 mm Hg. Medication dosages were lowered if SBP was <130 mm Hg at a single visit or <135 mm Hg at 2 consecutive visits.1
The primary composite outcome included the first occurrence of MI, acute coronary syndrome, stroke, heart failure, or death from CV causes. Secondary outcomes were the individual components of the primary composite outcome, death from any cause, and the composite of the primary outcome or death from any cause.1
Study halted early. The study was stopped early due to significantly lower rates of the primary outcome in the intensive therapy group vs the standard therapy group (1.65% per year vs 2.19% per year, respectively, hazard ratio [HR] with intensive treatment=0.75; 95% confidence interval [CI], 0.64-0.89; P<.001). The resulting median follow-up time was 3.26 years.1 This corresponds to a 25% lower relative risk of the primary outcome, with a decrease in event rates from 6.8% to 5.2% over the trial period. All-cause mortality was also lower in the intensive therapy group: 3.4% vs 4.5% (HR=0.73; 95% CI, 0.60-0.90; P=.003).
The number needed to treat (NNT) over 3.26 years to prevent a primary outcome event, death from any cause, and death from CV causes was 61, 90, and 172, respectively. Serious adverse events occurred more frequently in the intensive therapy group than in the standard therapy group (38.3% vs 37.1%; HR=1.04; P=.25) with a number needed to harm (NNH) of 46 over the study period.1 (When looking at serious adverse events identified as likely associated with the intervention, rates were 4.7% vs 2.5%, respectively [P<.001].) Hypotension, syncope, electrolyte abnormalities, and acute kidney injury/acute renal failure reached statistical significance. The incidence of bradycardia and injurious falls was higher in the intensive treatment group, but did not reach statistical significance. In the subgroup of patients ≥75 years of age, 48% in each study group experienced a serious adverse event.1
Throughout the study, mean SBP was 121.5 mm Hg in the intensive therapy group and 134.6 mm Hg in the standard treatment group. This required an average of one additional BP medication in the intensive therapy group (2.8 vs 1.8, respectively).1
What’s New
Lower SBP produces mortality benefits in those under, and over, age 75
This trial builds on a body of evidence that shows the advantages of lowering SBP to <150 mm Hg7,11,12 by demonstrating benefits, including lower all-cause mortality, for lower SBP targets in non-diabetic patients at high risk of CV disease. The SPRINT trial also showed that the benefits of intensive therapy remained true in a subgroup of patients ≥75 years of age.
The incidence of the primary outcome in the cohort ≥75 years of age receiving intensive therapy was 7.7% vs 10.9% for those receiving standard therapy (HR=0.67; 95% CI, 0.51-0.86; NNT=31). All-cause mortality was also lower in the intensive therapy group than in the standard therapy group among patients ≥75 years of age: 5.5% vs 8.04% (HR=0.68; 95% CI, 0.50-0.92; NNT=38).1
Caveats
Many do not benefit from—or are harmed by—increased medication
The absolute risk reduction for the primary outcome is 1.6%, meaning 98.4% of patients receiving more intensive treatment will not benefit. In a group of 1000 patients, an estimated 16 patients will benefit, 22 patients will be seriously harmed, and 962 patients will experience neither benefit nor harm.14 The difference between how BP was measured in this trial (an average of 3 readings after the patient had rested for 5 minutes) and that which occurs typically in clinical practice could potentially lead to overtreatment in practice.
Also, reducing antihypertensive therapies when the SBP was about 130 to 135 mm Hg in the standard therapy group likely exaggerated the difference in outcomes between the intensive and standard therapy groups, and is neither routine nor recommended in clinical practice.6 Finally, the trial specifically studied non-diabetic patients at high risk of CV disease ≥50 years of age, limiting generalizability to other populations.
Challenges to implementation
Who will benefit/who can achieve intensive SBP goals?
Identifying patients most likely to benefit from more intensive BP targets remains challenging. The SPRINT trial showed a mortality benefit, but at a cost of increased morbidity.1,14 In particular, caution should be exercised in the subgroup of patients ≥75 years. Despite a lower NNT than the rest of the study population, serious adverse events happened more frequently. Also, this particular cohort of volunteers may not be representative of those ≥75 years of age in the general population.
Additionally, achieving intensive SBP goals can be challenging. In the SPRINT trial, only half of the intensive target group achieved an SBP <120 mm Hg.1 And in a 2011-12 National Health and Nutrition Examination Survey, only 52% of patients in the general population achieved a BP target <140/90 mm Hg.15 Lower morbidity and mortality should remain the ultimate goals to the management of hypertension, requiring physicians to carefully assess an individual patient’s likelihood of benefit vs harm.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
2. Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560-2572.
3. Neal B, MacMahon S, Chapman N. Effects of ACE inhibitors, calcium antagonists, and other blood-pressure-lowering drugs: results of prospectively designed overviews of randomised trials. Lancet. 2000;356:1955-1964.
4. Psaty BM, Smith NL, Siscovick DS, et al. Health outcomes associated with antihypertensive therapies used as first-line agents. A systematic review and meta-analysis. JAMA. 1997;277:739-745.
5. Margolis KL, O’Connor PJ, Morgan TM, et al. Outcomes of combined cardiovascular risk factor management strategies in type 2 diabetes: the ACCORD randomized trial. Diabetes Care. 2014;37:1721-1728.
6. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520.
7. Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887-1898.
8. Verdecchia P, Staessen JA, Angeli F, et al. Usual versus tight control of systolic blood pressure in non-diabetic patients with hypertension (Cardio-Sis): an open-label randomised trial. Lancet. 2009;374:525-533.
9. JATOS Study Group. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens Res. 2008;31:2115-2127.
10. Ogihara T, Saruta T, Rakugi H, et al. Target blood pressure for treatment of isolated systolic hypertension in the elderly: valsartan in elderly isolated systolic hypertension study. Hypertension. 2010;56:196-202.
11. Staessen JA, Fagard R, Thijs L, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet. 1997;350:757-764.
12. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group. JAMA. 1991;265:3255-3264.
13. Cundiff DK, Gueyffier F, Wright JM. Guidelines for managing high blood pressure. JAMA. 2014; 312:294.
14. Ortiz E, James PA. Let’s not SPRINT to judgment about new blood pressure goals. Ann Intern Med. 2016.
15. Nwankwo T, Yoon SS, Burt V, et al. Hypertension among adults in the United States: National Health and Nutrition Examination Survey, 2011-2012. NCHS Data Brief. 2013;1-8.
Consider treating non-diabetic patients age ≥50 years to a systolic blood pressure (SBP) target <120 mm Hg as compared to <140 mm Hg when the benefits—lower rates of fatal and nonfatal cardiovascular (CV) events and death from any cause—are likely to outweigh the risks from possible additional medication.1
Strength of recommendation
B: Based on a single, good-quality randomized controlled trial (RCT).
Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
Illustrative Case
A 55-year-old man with hypertension and stage 3 chronic kidney disease (CKD) comes in to your office for routine care. His blood pressure is 135/85 mm Hg, and he is presently taking lisinopril 40 mg daily. Should you increase his antihypertensive regimen?
Hypertension is common and leads to significant morbidity and mortality, but pharmacologic treatment reduces incidence of stroke by 35% to 40%, myocardial infarction (MI) by 15% to 25%, and heart failure by up to 64%.2-4 Specific blood pressure targets for defined populations continue to be studied.
In patients with diabetes, the ACCORD (Action to Control Cardiovascular Risk in Diabetes) trial found that more intensive BP targets did not reduce the rate of major CV events, but the study may have been underpowered.5 The members of The Eighth Joint National Committee recommended treating patients over age 60 years to BP goals <150/90 mm Hg.6 This was based on evidence from 6 randomized controlled trials (RCTs),7-12 but there remains debate—even among the members of the Committee—as to appropriate BP goals in patients of any age without CV disease who have BP measurements of 140-159/90-99 mm Hg.13
Study Summary
Treating to SBP <120 mm Hg lowers mortality
The Systolic Blood Pressure Intervention Trial (SPRINT) was a multicenter RCT designed to determine if treating to lower SBP targets in non-diabetic patients at high risk for CV events improves outcomes as compared to standard care. Patients were at least 50 years of age with SBP of 130 to 180 mm Hg and were at increased CV risk as defined by clinical or subclinical CV disease other than stroke, CKD with glomerular filtration rate (GFR) 20 to 60 mL/min/1.73 m2, 10-year risk of CV disease >15% on Framingham risk score, or age ≥75 years of age. Patients with diabetes; prior stroke; polycystic kidney disease; significant proteinuria within the past 6 months; symptomatic heart failure within the past 6 months; or left ventricular ejection fraction <35% were excluded.1
Patients (N=9361) were randomly assigned to an SBP target <120 mm Hg in the intensive group or <140 mm Hg in the standard treatment group, in an open-label design. Allocation was concealed. The study protocol encouraged, but did not require, the use of thiazide-type diuretics, loop diuretics (for those with advanced renal disease), angiotensin-converting enzyme inhibitors or angiotensin receptor blocker agents, calcium channel blockers, and beta-blockers. Clinicians could add other agents as needed. All major classes of antihypertensives were used.
Medication dosing adjustments were based on the average of 3 BP measurements taken with an automated measurement system (Omron Healthcare, Model 907) with the patient seated after 5 minutes of quiet rest. Target SBP in the standard therapy group was 135 to 139 mm Hg. Medication dosages were lowered if SBP was <130 mm Hg at a single visit or <135 mm Hg at 2 consecutive visits.1
The primary composite outcome included the first occurrence of MI, acute coronary syndrome, stroke, heart failure, or death from CV causes. Secondary outcomes were the individual components of the primary composite outcome, death from any cause, and the composite of the primary outcome or death from any cause.1
Study halted early. The study was stopped early due to significantly lower rates of the primary outcome in the intensive therapy group vs the standard therapy group (1.65% per year vs 2.19% per year, respectively, hazard ratio [HR] with intensive treatment=0.75; 95% confidence interval [CI], 0.64-0.89; P<.001). The resulting median follow-up time was 3.26 years.1 This corresponds to a 25% lower relative risk of the primary outcome, with a decrease in event rates from 6.8% to 5.2% over the trial period. All-cause mortality was also lower in the intensive therapy group: 3.4% vs 4.5% (HR=0.73; 95% CI, 0.60-0.90; P=.003).
The number needed to treat (NNT) over 3.26 years to prevent a primary outcome event, death from any cause, and death from CV causes was 61, 90, and 172, respectively. Serious adverse events occurred more frequently in the intensive therapy group than in the standard therapy group (38.3% vs 37.1%; HR=1.04; P=.25) with a number needed to harm (NNH) of 46 over the study period.1 (When looking at serious adverse events identified as likely associated with the intervention, rates were 4.7% vs 2.5%, respectively [P<.001].) Hypotension, syncope, electrolyte abnormalities, and acute kidney injury/acute renal failure reached statistical significance. The incidence of bradycardia and injurious falls was higher in the intensive treatment group, but did not reach statistical significance. In the subgroup of patients ≥75 years of age, 48% in each study group experienced a serious adverse event.1
Throughout the study, mean SBP was 121.5 mm Hg in the intensive therapy group and 134.6 mm Hg in the standard treatment group. This required an average of one additional BP medication in the intensive therapy group (2.8 vs 1.8, respectively).1
What’s New
Lower SBP produces mortality benefits in those under, and over, age 75
This trial builds on a body of evidence that shows the advantages of lowering SBP to <150 mm Hg7,11,12 by demonstrating benefits, including lower all-cause mortality, for lower SBP targets in non-diabetic patients at high risk of CV disease. The SPRINT trial also showed that the benefits of intensive therapy remained true in a subgroup of patients ≥75 years of age.
The incidence of the primary outcome in the cohort ≥75 years of age receiving intensive therapy was 7.7% vs 10.9% for those receiving standard therapy (HR=0.67; 95% CI, 0.51-0.86; NNT=31). All-cause mortality was also lower in the intensive therapy group than in the standard therapy group among patients ≥75 years of age: 5.5% vs 8.04% (HR=0.68; 95% CI, 0.50-0.92; NNT=38).1
Caveats
Many do not benefit from—or are harmed by—increased medication
The absolute risk reduction for the primary outcome is 1.6%, meaning 98.4% of patients receiving more intensive treatment will not benefit. In a group of 1000 patients, an estimated 16 patients will benefit, 22 patients will be seriously harmed, and 962 patients will experience neither benefit nor harm.14 The difference between how BP was measured in this trial (an average of 3 readings after the patient had rested for 5 minutes) and that which occurs typically in clinical practice could potentially lead to overtreatment in practice.
Also, reducing antihypertensive therapies when the SBP was about 130 to 135 mm Hg in the standard therapy group likely exaggerated the difference in outcomes between the intensive and standard therapy groups, and is neither routine nor recommended in clinical practice.6 Finally, the trial specifically studied non-diabetic patients at high risk of CV disease ≥50 years of age, limiting generalizability to other populations.
Challenges to implementation
Who will benefit/who can achieve intensive SBP goals?
Identifying patients most likely to benefit from more intensive BP targets remains challenging. The SPRINT trial showed a mortality benefit, but at a cost of increased morbidity.1,14 In particular, caution should be exercised in the subgroup of patients ≥75 years. Despite a lower NNT than the rest of the study population, serious adverse events happened more frequently. Also, this particular cohort of volunteers may not be representative of those ≥75 years of age in the general population.
Additionally, achieving intensive SBP goals can be challenging. In the SPRINT trial, only half of the intensive target group achieved an SBP <120 mm Hg.1 And in a 2011-12 National Health and Nutrition Examination Survey, only 52% of patients in the general population achieved a BP target <140/90 mm Hg.15 Lower morbidity and mortality should remain the ultimate goals to the management of hypertension, requiring physicians to carefully assess an individual patient’s likelihood of benefit vs harm.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Consider treating non-diabetic patients age ≥50 years to a systolic blood pressure (SBP) target <120 mm Hg as compared to <140 mm Hg when the benefits—lower rates of fatal and nonfatal cardiovascular (CV) events and death from any cause—are likely to outweigh the risks from possible additional medication.1
Strength of recommendation
B: Based on a single, good-quality randomized controlled trial (RCT).
Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
Illustrative Case
A 55-year-old man with hypertension and stage 3 chronic kidney disease (CKD) comes in to your office for routine care. His blood pressure is 135/85 mm Hg, and he is presently taking lisinopril 40 mg daily. Should you increase his antihypertensive regimen?
Hypertension is common and leads to significant morbidity and mortality, but pharmacologic treatment reduces incidence of stroke by 35% to 40%, myocardial infarction (MI) by 15% to 25%, and heart failure by up to 64%.2-4 Specific blood pressure targets for defined populations continue to be studied.
In patients with diabetes, the ACCORD (Action to Control Cardiovascular Risk in Diabetes) trial found that more intensive BP targets did not reduce the rate of major CV events, but the study may have been underpowered.5 The members of The Eighth Joint National Committee recommended treating patients over age 60 years to BP goals <150/90 mm Hg.6 This was based on evidence from 6 randomized controlled trials (RCTs),7-12 but there remains debate—even among the members of the Committee—as to appropriate BP goals in patients of any age without CV disease who have BP measurements of 140-159/90-99 mm Hg.13
Study Summary
Treating to SBP <120 mm Hg lowers mortality
The Systolic Blood Pressure Intervention Trial (SPRINT) was a multicenter RCT designed to determine if treating to lower SBP targets in non-diabetic patients at high risk for CV events improves outcomes as compared to standard care. Patients were at least 50 years of age with SBP of 130 to 180 mm Hg and were at increased CV risk as defined by clinical or subclinical CV disease other than stroke, CKD with glomerular filtration rate (GFR) 20 to 60 mL/min/1.73 m2, 10-year risk of CV disease >15% on Framingham risk score, or age ≥75 years of age. Patients with diabetes; prior stroke; polycystic kidney disease; significant proteinuria within the past 6 months; symptomatic heart failure within the past 6 months; or left ventricular ejection fraction <35% were excluded.1
Patients (N=9361) were randomly assigned to an SBP target <120 mm Hg in the intensive group or <140 mm Hg in the standard treatment group, in an open-label design. Allocation was concealed. The study protocol encouraged, but did not require, the use of thiazide-type diuretics, loop diuretics (for those with advanced renal disease), angiotensin-converting enzyme inhibitors or angiotensin receptor blocker agents, calcium channel blockers, and beta-blockers. Clinicians could add other agents as needed. All major classes of antihypertensives were used.
Medication dosing adjustments were based on the average of 3 BP measurements taken with an automated measurement system (Omron Healthcare, Model 907) with the patient seated after 5 minutes of quiet rest. Target SBP in the standard therapy group was 135 to 139 mm Hg. Medication dosages were lowered if SBP was <130 mm Hg at a single visit or <135 mm Hg at 2 consecutive visits.1
The primary composite outcome included the first occurrence of MI, acute coronary syndrome, stroke, heart failure, or death from CV causes. Secondary outcomes were the individual components of the primary composite outcome, death from any cause, and the composite of the primary outcome or death from any cause.1
Study halted early. The study was stopped early due to significantly lower rates of the primary outcome in the intensive therapy group vs the standard therapy group (1.65% per year vs 2.19% per year, respectively, hazard ratio [HR] with intensive treatment=0.75; 95% confidence interval [CI], 0.64-0.89; P<.001). The resulting median follow-up time was 3.26 years.1 This corresponds to a 25% lower relative risk of the primary outcome, with a decrease in event rates from 6.8% to 5.2% over the trial period. All-cause mortality was also lower in the intensive therapy group: 3.4% vs 4.5% (HR=0.73; 95% CI, 0.60-0.90; P=.003).
The number needed to treat (NNT) over 3.26 years to prevent a primary outcome event, death from any cause, and death from CV causes was 61, 90, and 172, respectively. Serious adverse events occurred more frequently in the intensive therapy group than in the standard therapy group (38.3% vs 37.1%; HR=1.04; P=.25) with a number needed to harm (NNH) of 46 over the study period.1 (When looking at serious adverse events identified as likely associated with the intervention, rates were 4.7% vs 2.5%, respectively [P<.001].) Hypotension, syncope, electrolyte abnormalities, and acute kidney injury/acute renal failure reached statistical significance. The incidence of bradycardia and injurious falls was higher in the intensive treatment group, but did not reach statistical significance. In the subgroup of patients ≥75 years of age, 48% in each study group experienced a serious adverse event.1
Throughout the study, mean SBP was 121.5 mm Hg in the intensive therapy group and 134.6 mm Hg in the standard treatment group. This required an average of one additional BP medication in the intensive therapy group (2.8 vs 1.8, respectively).1
What’s New
Lower SBP produces mortality benefits in those under, and over, age 75
This trial builds on a body of evidence that shows the advantages of lowering SBP to <150 mm Hg7,11,12 by demonstrating benefits, including lower all-cause mortality, for lower SBP targets in non-diabetic patients at high risk of CV disease. The SPRINT trial also showed that the benefits of intensive therapy remained true in a subgroup of patients ≥75 years of age.
The incidence of the primary outcome in the cohort ≥75 years of age receiving intensive therapy was 7.7% vs 10.9% for those receiving standard therapy (HR=0.67; 95% CI, 0.51-0.86; NNT=31). All-cause mortality was also lower in the intensive therapy group than in the standard therapy group among patients ≥75 years of age: 5.5% vs 8.04% (HR=0.68; 95% CI, 0.50-0.92; NNT=38).1
Caveats
Many do not benefit from—or are harmed by—increased medication
The absolute risk reduction for the primary outcome is 1.6%, meaning 98.4% of patients receiving more intensive treatment will not benefit. In a group of 1000 patients, an estimated 16 patients will benefit, 22 patients will be seriously harmed, and 962 patients will experience neither benefit nor harm.14 The difference between how BP was measured in this trial (an average of 3 readings after the patient had rested for 5 minutes) and that which occurs typically in clinical practice could potentially lead to overtreatment in practice.
Also, reducing antihypertensive therapies when the SBP was about 130 to 135 mm Hg in the standard therapy group likely exaggerated the difference in outcomes between the intensive and standard therapy groups, and is neither routine nor recommended in clinical practice.6 Finally, the trial specifically studied non-diabetic patients at high risk of CV disease ≥50 years of age, limiting generalizability to other populations.
Challenges to implementation
Who will benefit/who can achieve intensive SBP goals?
Identifying patients most likely to benefit from more intensive BP targets remains challenging. The SPRINT trial showed a mortality benefit, but at a cost of increased morbidity.1,14 In particular, caution should be exercised in the subgroup of patients ≥75 years. Despite a lower NNT than the rest of the study population, serious adverse events happened more frequently. Also, this particular cohort of volunteers may not be representative of those ≥75 years of age in the general population.
Additionally, achieving intensive SBP goals can be challenging. In the SPRINT trial, only half of the intensive target group achieved an SBP <120 mm Hg.1 And in a 2011-12 National Health and Nutrition Examination Survey, only 52% of patients in the general population achieved a BP target <140/90 mm Hg.15 Lower morbidity and mortality should remain the ultimate goals to the management of hypertension, requiring physicians to carefully assess an individual patient’s likelihood of benefit vs harm.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
2. Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560-2572.
3. Neal B, MacMahon S, Chapman N. Effects of ACE inhibitors, calcium antagonists, and other blood-pressure-lowering drugs: results of prospectively designed overviews of randomised trials. Lancet. 2000;356:1955-1964.
4. Psaty BM, Smith NL, Siscovick DS, et al. Health outcomes associated with antihypertensive therapies used as first-line agents. A systematic review and meta-analysis. JAMA. 1997;277:739-745.
5. Margolis KL, O’Connor PJ, Morgan TM, et al. Outcomes of combined cardiovascular risk factor management strategies in type 2 diabetes: the ACCORD randomized trial. Diabetes Care. 2014;37:1721-1728.
6. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520.
7. Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887-1898.
8. Verdecchia P, Staessen JA, Angeli F, et al. Usual versus tight control of systolic blood pressure in non-diabetic patients with hypertension (Cardio-Sis): an open-label randomised trial. Lancet. 2009;374:525-533.
9. JATOS Study Group. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens Res. 2008;31:2115-2127.
10. Ogihara T, Saruta T, Rakugi H, et al. Target blood pressure for treatment of isolated systolic hypertension in the elderly: valsartan in elderly isolated systolic hypertension study. Hypertension. 2010;56:196-202.
11. Staessen JA, Fagard R, Thijs L, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet. 1997;350:757-764.
12. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group. JAMA. 1991;265:3255-3264.
13. Cundiff DK, Gueyffier F, Wright JM. Guidelines for managing high blood pressure. JAMA. 2014; 312:294.
14. Ortiz E, James PA. Let’s not SPRINT to judgment about new blood pressure goals. Ann Intern Med. 2016.
15. Nwankwo T, Yoon SS, Burt V, et al. Hypertension among adults in the United States: National Health and Nutrition Examination Survey, 2011-2012. NCHS Data Brief. 2013;1-8.
1. Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
2. Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560-2572.
3. Neal B, MacMahon S, Chapman N. Effects of ACE inhibitors, calcium antagonists, and other blood-pressure-lowering drugs: results of prospectively designed overviews of randomised trials. Lancet. 2000;356:1955-1964.
4. Psaty BM, Smith NL, Siscovick DS, et al. Health outcomes associated with antihypertensive therapies used as first-line agents. A systematic review and meta-analysis. JAMA. 1997;277:739-745.
5. Margolis KL, O’Connor PJ, Morgan TM, et al. Outcomes of combined cardiovascular risk factor management strategies in type 2 diabetes: the ACCORD randomized trial. Diabetes Care. 2014;37:1721-1728.
6. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520.
7. Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887-1898.
8. Verdecchia P, Staessen JA, Angeli F, et al. Usual versus tight control of systolic blood pressure in non-diabetic patients with hypertension (Cardio-Sis): an open-label randomised trial. Lancet. 2009;374:525-533.
9. JATOS Study Group. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens Res. 2008;31:2115-2127.
10. Ogihara T, Saruta T, Rakugi H, et al. Target blood pressure for treatment of isolated systolic hypertension in the elderly: valsartan in elderly isolated systolic hypertension study. Hypertension. 2010;56:196-202.
11. Staessen JA, Fagard R, Thijs L, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet. 1997;350:757-764.
12. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group. JAMA. 1991;265:3255-3264.
13. Cundiff DK, Gueyffier F, Wright JM. Guidelines for managing high blood pressure. JAMA. 2014; 312:294.
14. Ortiz E, James PA. Let’s not SPRINT to judgment about new blood pressure goals. Ann Intern Med. 2016.
15. Nwankwo T, Yoon SS, Burt V, et al. Hypertension among adults in the United States: National Health and Nutrition Examination Survey, 2011-2012. NCHS Data Brief. 2013;1-8.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.
8 USPSTF recommendations FPs need to know about
The US Preventive Services Task Force made 8 recommendations in 2015 that family physicians should implement in their practices (TABLE 11). The conditions addressed are high blood pressure, abnormal blood glucose, breast cancer, depression, and tobacco use. The Task Force also issued 13 “I” statements (TABLE 21) reflecting insufficient evidence to recommend for or against a particular intervention—once again underscoring the inadequate evidence base for many commonly-accepted practices aimed at prevention. Four such interventions were targeted toward children.
High blood pressure: Verify before starting treatment
The Task Force continues to give strong backing to the practice of screening for high blood pressure (HBP) and treating those with HBP to prevent cardiovascular and renal disease. The new recommendation, however, recognizes there is significant over-diagnosis of this condition and advises that, before starting treatment, HBP found with office measurement be confirmed with either ambulatory blood pressure monitoring or home blood pressure monitoring. This topic was covered in more depth in a recent Practice Alert.2
Since cardiovascular disease is the leading cause of death in the United States and much of this mortality is preventable, the Task Force also has recommendations in place for screening and treatment of other risks for cardiovascular disease, including obesity, hyperlipidemia, elevated blood glucose (discussed below), and tobacco use.1
Blood glucose: Focus is now on overweight/obese individuals
The Task Force’s new recommendation for diabetes screening differs from the one made in 2008, which recommended screening for type 2 diabetes (T2DM) only in adults with hypertension. The Task Force now recommends screening for abnormal blood glucose in all obese and overweight adults between the ages of 40 and 70. The Task Force analysis is detailed3 and will be the subject of the next Practice Alert, with only the highlights described here.
The recommendation is limited to overweight and obese adults because they are most likely to have abnormal blood glucose and to benefit from interventions. Screening can be done by measuring fasting blood glucose levels, performing a glucose tolerance test, or measuring glycated hemoglobin levels. The optimal screening frequency is unknown but suggested to be every 3 years. Refer patients with abnormal screen results to an intensive behavioral counseling program that promotes healthy eating and physical activity. Those with T2DM should also receive these services and consider pharmacotherapy.
Breast cancer: Mammography advice is age dependent
The Task Force breast cancer screening recommendations, first proposed in 2015 and finalized in early 2016, essentially reaffirm those made in 2009. Women ages 50 through 74 should be screened with mammography every 2 years, and individuals younger than age 50 should make a decision to receive screening—or not—based on the known benefits and risks of mammography at their age and their personal risks and preferences.
Insufficient evidence exists to make recommendations regarding mammography for women ages 75 and up, the use of digital breast tomosynthesis as a primary screening tool, and the use of any modality to augment screening in women with dense breasts who have normal mammogram results. Details of these recommendations were described in a Practice Alert last year.4
Depression: Use screening tools designed for specific patients
The 2015 updates on screening for depression essentially reconfirm the Task Force’s previous findings and recommendations on this topic. Screening for depression is recommended for all adults, including pregnant and postpartum women,5 and adolescents starting at age 12.6 Once again, the evidence is insufficient to make a recommendation on screening for depression in children younger than age 12.
Both recommendations emphasize the importance of follow-up steps after screening to ensure accurate diagnosis, adequate treatment, and appropriate follow-up. Treatment for adults and adolescents can include pharmacotherapy, cognitive-behavioral therapy, and/or psychosocial counseling. However, pharmacotherapy is not recommended for pregnant and breastfeeding women because of potential harms to the fetus and newborn.
The Task Force deems a number of screening tools acceptable. For adolescents, it suggests the Patient Health Questionnaire for Adolescents and the primary care version of the Beck Depression Inventory.6 For adults, the Task Force suggests the Patient Health Questionnaire, the Hospital Anxiety and Depression Scales, the Geriatric Depression Scale for older adults, and the Edinburgh Postnatal Depression Scale for postpartum and pregnant women.5
There is no known optimal frequency of screening or evidence on the value of repeated screening. The Task Force suggests one initial screen with repeated screening based on individual characteristics.
Tobacco use: Ask every adult patient about it
Preventing the harms from tobacco use is one of the most important and productive primary care interventions. The Task Force has affirmed its previous recommendation to ask all adults about tobacco use, encourage those that use tobacco to quit, and to offer behavioral and pharmacologic interventions to assist with quitting.7 The new recommendations emphasize the importance of smoking cessation during pregnancy; however, because of concern about the unknown potential harms from pharmacologic interventions, they advise only behavioral therapy to assist pregnant women to quit smoking.
The Task Force also examined the potential of electronic nicotine delivery systems for smoking cessation and concluded the evidence is insufficient to make a recommendation. It also concluded that the availability of other proven methods of smoking cessation make them the preferred alternatives.
Services with insufficient evidence
TABLE 21 lists the interventions that the Task Force studied this past year and found insufficient evidence to support a recommendation for or against. For adults, these “I” recommendations include screening for visual acuity disorders in older adults, screening for thyroid disorders, screening for iron deficiency anemia during pregnancy, and routinely providing iron supplementation during pregnancy.
The persistent inadequate evidence for the effectiveness of preventive services in infants and children was highlighted by the results of last year’s examination of 4 screening tests, all recommended by the American Academy of Pediatrics, but given an “I” recommendation by the Task Force. These included screening for autism spectrum disorder (ASD) in young children (18-30 months), iron deficiency anemia in children ages 6 to 24 months, depression in those ages 11 and younger, and speech and language delay and disorders in children ages 5 or younger. (Ages noted are from the Task Force.)
The Task Force is careful to emphasize that the statement about ASD screening refers to infants and children who appear normal and for whom no concerns of ASD have been raised by their parents. Screening all young children for this disorder is problematic, according to the Task Force, because of possible over-diagnosis and unclear benefits of early intervention.8
1. US Preventive Services Task Force. Published recommendations. Available at: http://www.uspreventiveservicestaskforce.org/BrowseRec/Index/browse-recommendations. Accessed March 18, 2016.
2. Campos-Outcalt D. USPSTF urges extra step before treating hypertension. J Fam Pract. 2016;65:41-44.
3. US Preventive Services Task Force. Abnormal blood glucose and diabetes type 2: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/screening-for-abnormal-blood-glucose-and-type-2-diabetes. Accessed March 18, 2016.
4. Campos-Outcalt D. Breast cancer screening: the latest from the USPSTF. J Fam Pract. 2015;64:407-410.
5. US Preventive Services Task Force. Depression in adults: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/depression-in-adults-screening1. Accessed March 18, 2016.
6. US Preventive Services Task Force. Depression in children and adolescents: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/depression-in-children-and-adolescents-screening1. Accessed March 18, 2016.
7. US Preventive Services Task Force. Tobacco smoking cessation in adults, including pregnant women: Behavioral and pharmacotherapy interventions. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions1. Accessed April 7, 2016.
8. US Preventive Services Task Force. Autism spectrum disorder in young children: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/autism-spectrum-disorder-in-young-children-screening. Accessed March 18, 2016.
The US Preventive Services Task Force made 8 recommendations in 2015 that family physicians should implement in their practices (TABLE 11). The conditions addressed are high blood pressure, abnormal blood glucose, breast cancer, depression, and tobacco use. The Task Force also issued 13 “I” statements (TABLE 21) reflecting insufficient evidence to recommend for or against a particular intervention—once again underscoring the inadequate evidence base for many commonly-accepted practices aimed at prevention. Four such interventions were targeted toward children.
High blood pressure: Verify before starting treatment
The Task Force continues to give strong backing to the practice of screening for high blood pressure (HBP) and treating those with HBP to prevent cardiovascular and renal disease. The new recommendation, however, recognizes there is significant over-diagnosis of this condition and advises that, before starting treatment, HBP found with office measurement be confirmed with either ambulatory blood pressure monitoring or home blood pressure monitoring. This topic was covered in more depth in a recent Practice Alert.2
Since cardiovascular disease is the leading cause of death in the United States and much of this mortality is preventable, the Task Force also has recommendations in place for screening and treatment of other risks for cardiovascular disease, including obesity, hyperlipidemia, elevated blood glucose (discussed below), and tobacco use.1
Blood glucose: Focus is now on overweight/obese individuals
The Task Force’s new recommendation for diabetes screening differs from the one made in 2008, which recommended screening for type 2 diabetes (T2DM) only in adults with hypertension. The Task Force now recommends screening for abnormal blood glucose in all obese and overweight adults between the ages of 40 and 70. The Task Force analysis is detailed3 and will be the subject of the next Practice Alert, with only the highlights described here.
The recommendation is limited to overweight and obese adults because they are most likely to have abnormal blood glucose and to benefit from interventions. Screening can be done by measuring fasting blood glucose levels, performing a glucose tolerance test, or measuring glycated hemoglobin levels. The optimal screening frequency is unknown but suggested to be every 3 years. Refer patients with abnormal screen results to an intensive behavioral counseling program that promotes healthy eating and physical activity. Those with T2DM should also receive these services and consider pharmacotherapy.
Breast cancer: Mammography advice is age dependent
The Task Force breast cancer screening recommendations, first proposed in 2015 and finalized in early 2016, essentially reaffirm those made in 2009. Women ages 50 through 74 should be screened with mammography every 2 years, and individuals younger than age 50 should make a decision to receive screening—or not—based on the known benefits and risks of mammography at their age and their personal risks and preferences.
Insufficient evidence exists to make recommendations regarding mammography for women ages 75 and up, the use of digital breast tomosynthesis as a primary screening tool, and the use of any modality to augment screening in women with dense breasts who have normal mammogram results. Details of these recommendations were described in a Practice Alert last year.4
Depression: Use screening tools designed for specific patients
The 2015 updates on screening for depression essentially reconfirm the Task Force’s previous findings and recommendations on this topic. Screening for depression is recommended for all adults, including pregnant and postpartum women,5 and adolescents starting at age 12.6 Once again, the evidence is insufficient to make a recommendation on screening for depression in children younger than age 12.
Both recommendations emphasize the importance of follow-up steps after screening to ensure accurate diagnosis, adequate treatment, and appropriate follow-up. Treatment for adults and adolescents can include pharmacotherapy, cognitive-behavioral therapy, and/or psychosocial counseling. However, pharmacotherapy is not recommended for pregnant and breastfeeding women because of potential harms to the fetus and newborn.
The Task Force deems a number of screening tools acceptable. For adolescents, it suggests the Patient Health Questionnaire for Adolescents and the primary care version of the Beck Depression Inventory.6 For adults, the Task Force suggests the Patient Health Questionnaire, the Hospital Anxiety and Depression Scales, the Geriatric Depression Scale for older adults, and the Edinburgh Postnatal Depression Scale for postpartum and pregnant women.5
There is no known optimal frequency of screening or evidence on the value of repeated screening. The Task Force suggests one initial screen with repeated screening based on individual characteristics.
Tobacco use: Ask every adult patient about it
Preventing the harms from tobacco use is one of the most important and productive primary care interventions. The Task Force has affirmed its previous recommendation to ask all adults about tobacco use, encourage those that use tobacco to quit, and to offer behavioral and pharmacologic interventions to assist with quitting.7 The new recommendations emphasize the importance of smoking cessation during pregnancy; however, because of concern about the unknown potential harms from pharmacologic interventions, they advise only behavioral therapy to assist pregnant women to quit smoking.
The Task Force also examined the potential of electronic nicotine delivery systems for smoking cessation and concluded the evidence is insufficient to make a recommendation. It also concluded that the availability of other proven methods of smoking cessation make them the preferred alternatives.
Services with insufficient evidence
TABLE 21 lists the interventions that the Task Force studied this past year and found insufficient evidence to support a recommendation for or against. For adults, these “I” recommendations include screening for visual acuity disorders in older adults, screening for thyroid disorders, screening for iron deficiency anemia during pregnancy, and routinely providing iron supplementation during pregnancy.
The persistent inadequate evidence for the effectiveness of preventive services in infants and children was highlighted by the results of last year’s examination of 4 screening tests, all recommended by the American Academy of Pediatrics, but given an “I” recommendation by the Task Force. These included screening for autism spectrum disorder (ASD) in young children (18-30 months), iron deficiency anemia in children ages 6 to 24 months, depression in those ages 11 and younger, and speech and language delay and disorders in children ages 5 or younger. (Ages noted are from the Task Force.)
The Task Force is careful to emphasize that the statement about ASD screening refers to infants and children who appear normal and for whom no concerns of ASD have been raised by their parents. Screening all young children for this disorder is problematic, according to the Task Force, because of possible over-diagnosis and unclear benefits of early intervention.8
The US Preventive Services Task Force made 8 recommendations in 2015 that family physicians should implement in their practices (TABLE 11). The conditions addressed are high blood pressure, abnormal blood glucose, breast cancer, depression, and tobacco use. The Task Force also issued 13 “I” statements (TABLE 21) reflecting insufficient evidence to recommend for or against a particular intervention—once again underscoring the inadequate evidence base for many commonly-accepted practices aimed at prevention. Four such interventions were targeted toward children.
High blood pressure: Verify before starting treatment
The Task Force continues to give strong backing to the practice of screening for high blood pressure (HBP) and treating those with HBP to prevent cardiovascular and renal disease. The new recommendation, however, recognizes there is significant over-diagnosis of this condition and advises that, before starting treatment, HBP found with office measurement be confirmed with either ambulatory blood pressure monitoring or home blood pressure monitoring. This topic was covered in more depth in a recent Practice Alert.2
Since cardiovascular disease is the leading cause of death in the United States and much of this mortality is preventable, the Task Force also has recommendations in place for screening and treatment of other risks for cardiovascular disease, including obesity, hyperlipidemia, elevated blood glucose (discussed below), and tobacco use.1
Blood glucose: Focus is now on overweight/obese individuals
The Task Force’s new recommendation for diabetes screening differs from the one made in 2008, which recommended screening for type 2 diabetes (T2DM) only in adults with hypertension. The Task Force now recommends screening for abnormal blood glucose in all obese and overweight adults between the ages of 40 and 70. The Task Force analysis is detailed3 and will be the subject of the next Practice Alert, with only the highlights described here.
The recommendation is limited to overweight and obese adults because they are most likely to have abnormal blood glucose and to benefit from interventions. Screening can be done by measuring fasting blood glucose levels, performing a glucose tolerance test, or measuring glycated hemoglobin levels. The optimal screening frequency is unknown but suggested to be every 3 years. Refer patients with abnormal screen results to an intensive behavioral counseling program that promotes healthy eating and physical activity. Those with T2DM should also receive these services and consider pharmacotherapy.
Breast cancer: Mammography advice is age dependent
The Task Force breast cancer screening recommendations, first proposed in 2015 and finalized in early 2016, essentially reaffirm those made in 2009. Women ages 50 through 74 should be screened with mammography every 2 years, and individuals younger than age 50 should make a decision to receive screening—or not—based on the known benefits and risks of mammography at their age and their personal risks and preferences.
Insufficient evidence exists to make recommendations regarding mammography for women ages 75 and up, the use of digital breast tomosynthesis as a primary screening tool, and the use of any modality to augment screening in women with dense breasts who have normal mammogram results. Details of these recommendations were described in a Practice Alert last year.4
Depression: Use screening tools designed for specific patients
The 2015 updates on screening for depression essentially reconfirm the Task Force’s previous findings and recommendations on this topic. Screening for depression is recommended for all adults, including pregnant and postpartum women,5 and adolescents starting at age 12.6 Once again, the evidence is insufficient to make a recommendation on screening for depression in children younger than age 12.
Both recommendations emphasize the importance of follow-up steps after screening to ensure accurate diagnosis, adequate treatment, and appropriate follow-up. Treatment for adults and adolescents can include pharmacotherapy, cognitive-behavioral therapy, and/or psychosocial counseling. However, pharmacotherapy is not recommended for pregnant and breastfeeding women because of potential harms to the fetus and newborn.
The Task Force deems a number of screening tools acceptable. For adolescents, it suggests the Patient Health Questionnaire for Adolescents and the primary care version of the Beck Depression Inventory.6 For adults, the Task Force suggests the Patient Health Questionnaire, the Hospital Anxiety and Depression Scales, the Geriatric Depression Scale for older adults, and the Edinburgh Postnatal Depression Scale for postpartum and pregnant women.5
There is no known optimal frequency of screening or evidence on the value of repeated screening. The Task Force suggests one initial screen with repeated screening based on individual characteristics.
Tobacco use: Ask every adult patient about it
Preventing the harms from tobacco use is one of the most important and productive primary care interventions. The Task Force has affirmed its previous recommendation to ask all adults about tobacco use, encourage those that use tobacco to quit, and to offer behavioral and pharmacologic interventions to assist with quitting.7 The new recommendations emphasize the importance of smoking cessation during pregnancy; however, because of concern about the unknown potential harms from pharmacologic interventions, they advise only behavioral therapy to assist pregnant women to quit smoking.
The Task Force also examined the potential of electronic nicotine delivery systems for smoking cessation and concluded the evidence is insufficient to make a recommendation. It also concluded that the availability of other proven methods of smoking cessation make them the preferred alternatives.
Services with insufficient evidence
TABLE 21 lists the interventions that the Task Force studied this past year and found insufficient evidence to support a recommendation for or against. For adults, these “I” recommendations include screening for visual acuity disorders in older adults, screening for thyroid disorders, screening for iron deficiency anemia during pregnancy, and routinely providing iron supplementation during pregnancy.
The persistent inadequate evidence for the effectiveness of preventive services in infants and children was highlighted by the results of last year’s examination of 4 screening tests, all recommended by the American Academy of Pediatrics, but given an “I” recommendation by the Task Force. These included screening for autism spectrum disorder (ASD) in young children (18-30 months), iron deficiency anemia in children ages 6 to 24 months, depression in those ages 11 and younger, and speech and language delay and disorders in children ages 5 or younger. (Ages noted are from the Task Force.)
The Task Force is careful to emphasize that the statement about ASD screening refers to infants and children who appear normal and for whom no concerns of ASD have been raised by their parents. Screening all young children for this disorder is problematic, according to the Task Force, because of possible over-diagnosis and unclear benefits of early intervention.8
1. US Preventive Services Task Force. Published recommendations. Available at: http://www.uspreventiveservicestaskforce.org/BrowseRec/Index/browse-recommendations. Accessed March 18, 2016.
2. Campos-Outcalt D. USPSTF urges extra step before treating hypertension. J Fam Pract. 2016;65:41-44.
3. US Preventive Services Task Force. Abnormal blood glucose and diabetes type 2: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/screening-for-abnormal-blood-glucose-and-type-2-diabetes. Accessed March 18, 2016.
4. Campos-Outcalt D. Breast cancer screening: the latest from the USPSTF. J Fam Pract. 2015;64:407-410.
5. US Preventive Services Task Force. Depression in adults: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/depression-in-adults-screening1. Accessed March 18, 2016.
6. US Preventive Services Task Force. Depression in children and adolescents: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/depression-in-children-and-adolescents-screening1. Accessed March 18, 2016.
7. US Preventive Services Task Force. Tobacco smoking cessation in adults, including pregnant women: Behavioral and pharmacotherapy interventions. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions1. Accessed April 7, 2016.
8. US Preventive Services Task Force. Autism spectrum disorder in young children: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/autism-spectrum-disorder-in-young-children-screening. Accessed March 18, 2016.
1. US Preventive Services Task Force. Published recommendations. Available at: http://www.uspreventiveservicestaskforce.org/BrowseRec/Index/browse-recommendations. Accessed March 18, 2016.
2. Campos-Outcalt D. USPSTF urges extra step before treating hypertension. J Fam Pract. 2016;65:41-44.
3. US Preventive Services Task Force. Abnormal blood glucose and diabetes type 2: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/screening-for-abnormal-blood-glucose-and-type-2-diabetes. Accessed March 18, 2016.
4. Campos-Outcalt D. Breast cancer screening: the latest from the USPSTF. J Fam Pract. 2015;64:407-410.
5. US Preventive Services Task Force. Depression in adults: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/depression-in-adults-screening1. Accessed March 18, 2016.
6. US Preventive Services Task Force. Depression in children and adolescents: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/depression-in-children-and-adolescents-screening1. Accessed March 18, 2016.
7. US Preventive Services Task Force. Tobacco smoking cessation in adults, including pregnant women: Behavioral and pharmacotherapy interventions. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions1. Accessed April 7, 2016.
8. US Preventive Services Task Force. Autism spectrum disorder in young children: screening. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/autism-spectrum-disorder-in-young-children-screening. Accessed March 18, 2016.
From The Journal of Family Practice | 2016;65(5):338-341.
No rise in serious HF seen in patients taking saxagliptin or sitagliptin
Neither saxagliptin nor sitagliptin, the two oral DPP-4 inhibitors most commonly used as antihyperglycemic medications, raised the risk of hospitalization for heart failure in a large population-based cohort study that analyzed data from a Food and Drug Administration surveillance program.
The report was published online April 25 in Annals of Internal Medicine.
The cardiovascular safety of DPP-4 inhibitors is controversial: Several postmarketing studies have produced conflicting results, particularly with regard to HF risk. “Patients with diabetes have a higher HF risk than those without, so any antihyperglycemic agent that modifies the risk warrants further examination,” said Sengwee Toh, Sc.D., a pharmacoepidemiologist in the department of population medicine, Harvard Medical School and Harvard Pilgrim Health Institute, Boston, and his associates.
They compared rates of HF among demographically and geographically diverse patients who initiated antidiabetic medications during a 7-year period in routine clinical settings. The study population included 78,553 adults who initiated saxagliptin and 298,124 who initiated sitagliptin, who were compared with patients who initiated pioglitazone, second-generation sulfonylureas, or long-acting insulins. Mean follow-up was 7-9 months.
There was no evidence of an increased risk of hospitalization for HF among new users of saxagliptin or sitagliptin. The hazard ratios for developing HF were 0.83 for saxagliptin vs. sitagliptin, 0.63 for saxagliptin vs. pioglitazone, 0.69 for saxagliptin vs. sulfonylureas, and 0.61 for saxagliptin vs. insulin. Similarly, the hazard ratios for developing HF were 0.74 for sitagliptin vs. pioglitazone, 0.86 for sitagliptin vs. sulfonylureas, and 0.71 for sitagliptin vs. insulin.
These results were consistent across sensitivity analyses and subgroup analyses that categorized patients by whether or not they had preexisting cardiovascular disease and whether or not they had a history of prior HF, the investigators said (Ann Intern Med. 2016 April 25. doi:10.7326/M15-2568).
However, this was an observational study with a relatively short follow-up. “Well-designed randomized trials with hospitalization for HF as the main endpoint or observational studies that address the limitations of our study will help provide more definitive evidence on the topic,” Dr. Toh and his associates said.
This study was supported by the FDA. Dr. Toh reported having no relevant financial disclosures; one of his associates reported receiving personal fees from Novartis unrelated to this work.
The findings of Toh et al. allay concerns about a saxagliptin- or sitagliptin-associated risk for heart failure. This risk was similar between the two agents and either comparable to or lower than that in all other comparator groups.
Beyond reassuring clinicians, this study illustrates the value of large, longitudinal databases built from clinical and administrative data, to complement the findings of clinical trials. These investigators were able to draw their conclusions from rich demographic, diagnostic, prescription, and utilization data based in routine real-world practice.
Joseph V. Selby, M.D., is at the Patient-Centered Outcomes Research Institute, Washington. He reported having no relevant financial disclosures. Dr. Selby made these remarks in an editorial accompanying Dr. Toh’s report (Ann. Intern. Med. 2016 April 25. doi:10.7326/M16-0869).
The findings of Toh et al. allay concerns about a saxagliptin- or sitagliptin-associated risk for heart failure. This risk was similar between the two agents and either comparable to or lower than that in all other comparator groups.
Beyond reassuring clinicians, this study illustrates the value of large, longitudinal databases built from clinical and administrative data, to complement the findings of clinical trials. These investigators were able to draw their conclusions from rich demographic, diagnostic, prescription, and utilization data based in routine real-world practice.
Joseph V. Selby, M.D., is at the Patient-Centered Outcomes Research Institute, Washington. He reported having no relevant financial disclosures. Dr. Selby made these remarks in an editorial accompanying Dr. Toh’s report (Ann. Intern. Med. 2016 April 25. doi:10.7326/M16-0869).
The findings of Toh et al. allay concerns about a saxagliptin- or sitagliptin-associated risk for heart failure. This risk was similar between the two agents and either comparable to or lower than that in all other comparator groups.
Beyond reassuring clinicians, this study illustrates the value of large, longitudinal databases built from clinical and administrative data, to complement the findings of clinical trials. These investigators were able to draw their conclusions from rich demographic, diagnostic, prescription, and utilization data based in routine real-world practice.
Joseph V. Selby, M.D., is at the Patient-Centered Outcomes Research Institute, Washington. He reported having no relevant financial disclosures. Dr. Selby made these remarks in an editorial accompanying Dr. Toh’s report (Ann. Intern. Med. 2016 April 25. doi:10.7326/M16-0869).
Neither saxagliptin nor sitagliptin, the two oral DPP-4 inhibitors most commonly used as antihyperglycemic medications, raised the risk of hospitalization for heart failure in a large population-based cohort study that analyzed data from a Food and Drug Administration surveillance program.
The report was published online April 25 in Annals of Internal Medicine.
The cardiovascular safety of DPP-4 inhibitors is controversial: Several postmarketing studies have produced conflicting results, particularly with regard to HF risk. “Patients with diabetes have a higher HF risk than those without, so any antihyperglycemic agent that modifies the risk warrants further examination,” said Sengwee Toh, Sc.D., a pharmacoepidemiologist in the department of population medicine, Harvard Medical School and Harvard Pilgrim Health Institute, Boston, and his associates.
They compared rates of HF among demographically and geographically diverse patients who initiated antidiabetic medications during a 7-year period in routine clinical settings. The study population included 78,553 adults who initiated saxagliptin and 298,124 who initiated sitagliptin, who were compared with patients who initiated pioglitazone, second-generation sulfonylureas, or long-acting insulins. Mean follow-up was 7-9 months.
There was no evidence of an increased risk of hospitalization for HF among new users of saxagliptin or sitagliptin. The hazard ratios for developing HF were 0.83 for saxagliptin vs. sitagliptin, 0.63 for saxagliptin vs. pioglitazone, 0.69 for saxagliptin vs. sulfonylureas, and 0.61 for saxagliptin vs. insulin. Similarly, the hazard ratios for developing HF were 0.74 for sitagliptin vs. pioglitazone, 0.86 for sitagliptin vs. sulfonylureas, and 0.71 for sitagliptin vs. insulin.
These results were consistent across sensitivity analyses and subgroup analyses that categorized patients by whether or not they had preexisting cardiovascular disease and whether or not they had a history of prior HF, the investigators said (Ann Intern Med. 2016 April 25. doi:10.7326/M15-2568).
However, this was an observational study with a relatively short follow-up. “Well-designed randomized trials with hospitalization for HF as the main endpoint or observational studies that address the limitations of our study will help provide more definitive evidence on the topic,” Dr. Toh and his associates said.
This study was supported by the FDA. Dr. Toh reported having no relevant financial disclosures; one of his associates reported receiving personal fees from Novartis unrelated to this work.
Neither saxagliptin nor sitagliptin, the two oral DPP-4 inhibitors most commonly used as antihyperglycemic medications, raised the risk of hospitalization for heart failure in a large population-based cohort study that analyzed data from a Food and Drug Administration surveillance program.
The report was published online April 25 in Annals of Internal Medicine.
The cardiovascular safety of DPP-4 inhibitors is controversial: Several postmarketing studies have produced conflicting results, particularly with regard to HF risk. “Patients with diabetes have a higher HF risk than those without, so any antihyperglycemic agent that modifies the risk warrants further examination,” said Sengwee Toh, Sc.D., a pharmacoepidemiologist in the department of population medicine, Harvard Medical School and Harvard Pilgrim Health Institute, Boston, and his associates.
They compared rates of HF among demographically and geographically diverse patients who initiated antidiabetic medications during a 7-year period in routine clinical settings. The study population included 78,553 adults who initiated saxagliptin and 298,124 who initiated sitagliptin, who were compared with patients who initiated pioglitazone, second-generation sulfonylureas, or long-acting insulins. Mean follow-up was 7-9 months.
There was no evidence of an increased risk of hospitalization for HF among new users of saxagliptin or sitagliptin. The hazard ratios for developing HF were 0.83 for saxagliptin vs. sitagliptin, 0.63 for saxagliptin vs. pioglitazone, 0.69 for saxagliptin vs. sulfonylureas, and 0.61 for saxagliptin vs. insulin. Similarly, the hazard ratios for developing HF were 0.74 for sitagliptin vs. pioglitazone, 0.86 for sitagliptin vs. sulfonylureas, and 0.71 for sitagliptin vs. insulin.
These results were consistent across sensitivity analyses and subgroup analyses that categorized patients by whether or not they had preexisting cardiovascular disease and whether or not they had a history of prior HF, the investigators said (Ann Intern Med. 2016 April 25. doi:10.7326/M15-2568).
However, this was an observational study with a relatively short follow-up. “Well-designed randomized trials with hospitalization for HF as the main endpoint or observational studies that address the limitations of our study will help provide more definitive evidence on the topic,” Dr. Toh and his associates said.
This study was supported by the FDA. Dr. Toh reported having no relevant financial disclosures; one of his associates reported receiving personal fees from Novartis unrelated to this work.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point: No increase in the risk of hospitalization for heart failure was found in a large cohort study that used an FDA surveillance program.
Major finding: The hazard ratios for developing HF were 0.83 for saxagliptin vs. sitagliptin, 0.63 for saxagliptin vs. pioglitazone, 0.69 for saxagliptin vs. sulfonylureas, and 0.61 for saxagliptin vs. insulin.
Data source: A population-based retrospective cohort study involving 78,553 new users of saxagliptin and 298,124 of sitagliptin during a 7-year period.
Disclosures: This study was supported by the FDA. Dr. Toh reported having no relevant financial disclosures; one of his associates reported receiving personal fees from Novartis unrelated to this work.