User login
Anticoagulant therapy not contraindicated in brain metastases
Venous thromboembolism (VTE) is common in cancer patients with brain metastases but therapeutic anticoagulation does not increase the risk for intracranial hemorrhage, according to new data.
In a matched, retrospective cohort study of 293 cancer patients with brain metastases (104 treated with therapeutic enoxaparin and 189 controls), there were no differences at 1 year between the two groups for measurable (19% vs 21%; Gray test, P = .97; hazard ratio, 1.02; 90% confidence interval [CI], 0.66-1.59), significant (21% vs 22%; P = .87), and total (44% vs 37%; P = .13) intracranial hemorrhages.
“Reassuringly, the cumulative incidence of intracranial hemorrhage was not significantly different in those patients who received therapeutic enoxaparin compared with controls for all outcomes including measurable, total, and significant intracranial hemorrhages,” wrote Dr. Jessica Donato of Harvard Medical School, Boston, and colleagues (Blood 2015 Jul 23 doi:10.1182/blood-2015-02-626788).
The only covariate that was predictive of hemorrhage in this study was the combined group of renal cell carcinoma and melanoma, as the risk for intracranial hemorrhage was fourfold higher (adjusted hazard ratio, 3.98; 90% CI, 2.41-6.57; P less than .001) in those subgroups.
In an accompanying commentary in the same issue of Blood, Dr. Lisa Baumann Kreuziger of Medical College of Wisconsin, Milwaukee, wrote that although the study was well designed, “its retrospective nature creates inherent limitations.”
As an example, besides tumor type, the multivariable analysis did not identify other clinical factors that could guide clinicians when assessing the risk of intracranial hemorrhage, she wrote (Blood 2015 Jul 23. doi: 10.1182/blood-2015-06-648089]).
But in spite of the limitations, the study “further supports the statement from the 2014 American Society of Clinical Oncology Guidelines that brain metastases are not a contraindication to treatment of VTE with low-molecular-weight heparin,” she concluded.
Venous thromboembolism (VTE) is common in cancer patients with brain metastases but therapeutic anticoagulation does not increase the risk for intracranial hemorrhage, according to new data.
In a matched, retrospective cohort study of 293 cancer patients with brain metastases (104 treated with therapeutic enoxaparin and 189 controls), there were no differences at 1 year between the two groups for measurable (19% vs 21%; Gray test, P = .97; hazard ratio, 1.02; 90% confidence interval [CI], 0.66-1.59), significant (21% vs 22%; P = .87), and total (44% vs 37%; P = .13) intracranial hemorrhages.
“Reassuringly, the cumulative incidence of intracranial hemorrhage was not significantly different in those patients who received therapeutic enoxaparin compared with controls for all outcomes including measurable, total, and significant intracranial hemorrhages,” wrote Dr. Jessica Donato of Harvard Medical School, Boston, and colleagues (Blood 2015 Jul 23 doi:10.1182/blood-2015-02-626788).
The only covariate that was predictive of hemorrhage in this study was the combined group of renal cell carcinoma and melanoma, as the risk for intracranial hemorrhage was fourfold higher (adjusted hazard ratio, 3.98; 90% CI, 2.41-6.57; P less than .001) in those subgroups.
In an accompanying commentary in the same issue of Blood, Dr. Lisa Baumann Kreuziger of Medical College of Wisconsin, Milwaukee, wrote that although the study was well designed, “its retrospective nature creates inherent limitations.”
As an example, besides tumor type, the multivariable analysis did not identify other clinical factors that could guide clinicians when assessing the risk of intracranial hemorrhage, she wrote (Blood 2015 Jul 23. doi: 10.1182/blood-2015-06-648089]).
But in spite of the limitations, the study “further supports the statement from the 2014 American Society of Clinical Oncology Guidelines that brain metastases are not a contraindication to treatment of VTE with low-molecular-weight heparin,” she concluded.
Venous thromboembolism (VTE) is common in cancer patients with brain metastases but therapeutic anticoagulation does not increase the risk for intracranial hemorrhage, according to new data.
In a matched, retrospective cohort study of 293 cancer patients with brain metastases (104 treated with therapeutic enoxaparin and 189 controls), there were no differences at 1 year between the two groups for measurable (19% vs 21%; Gray test, P = .97; hazard ratio, 1.02; 90% confidence interval [CI], 0.66-1.59), significant (21% vs 22%; P = .87), and total (44% vs 37%; P = .13) intracranial hemorrhages.
“Reassuringly, the cumulative incidence of intracranial hemorrhage was not significantly different in those patients who received therapeutic enoxaparin compared with controls for all outcomes including measurable, total, and significant intracranial hemorrhages,” wrote Dr. Jessica Donato of Harvard Medical School, Boston, and colleagues (Blood 2015 Jul 23 doi:10.1182/blood-2015-02-626788).
The only covariate that was predictive of hemorrhage in this study was the combined group of renal cell carcinoma and melanoma, as the risk for intracranial hemorrhage was fourfold higher (adjusted hazard ratio, 3.98; 90% CI, 2.41-6.57; P less than .001) in those subgroups.
In an accompanying commentary in the same issue of Blood, Dr. Lisa Baumann Kreuziger of Medical College of Wisconsin, Milwaukee, wrote that although the study was well designed, “its retrospective nature creates inherent limitations.”
As an example, besides tumor type, the multivariable analysis did not identify other clinical factors that could guide clinicians when assessing the risk of intracranial hemorrhage, she wrote (Blood 2015 Jul 23. doi: 10.1182/blood-2015-06-648089]).
But in spite of the limitations, the study “further supports the statement from the 2014 American Society of Clinical Oncology Guidelines that brain metastases are not a contraindication to treatment of VTE with low-molecular-weight heparin,” she concluded.
FROM BLOOD
Key clinical point: Therapeutic anticoagulation does not increase the risk for intracranial hemorrhage in patients with brain metastases.
Major finding: There were no differences in the cumulative incidence of intracranial hemorrhage at 1 year in the enoxaparin and control cohorts for total (44% vs 37%; P = .13) intracranial hemorrhages.
Data source: A matched, retrospective cohort study of 293 cancer patients with brain metastases.
Disclosures: The Harvard Catalyst/The Harvard Clinical and Translational Science Center and a Dana-Farber/Harvard Cancer Center Core Grant supported the study. Dr. Zwicker received prior research funding from Sanofi, serves on advisory committees for Portola Pharmaceuticals and Merck, and receives consulting fees from Parexel. The remaining authors declared no financial conflicts.
Plasma tau level chronically elevated in TBI
Peripheral plasma levels of the CNS protein tau are chronically elevated after traumatic brain injury and appear to correlate with the severity of postconcussive symptoms, according to a report published Aug. 3 in JAMA Neurology.
If these findings are confirmed, this will be the first biomarker that is sensitive and specific to persistent traumatic brain injury–related symptoms. The results also suggest that “months to years after the primary brain injury, there may be a continuation of secondary injuries with residual axonal degeneration and blood-brain barrier disruptions in this population that may contribute to the maintenance of postconcussive disorder symptoms and affect symptom severity,” wrote Anlys Olivera, Ph.D., of the National Institute of Nursing Research, Bethesda, Md., and her associates.
Tau is a protein that stabilizes the structure of the axonal cytoskeleton. It is elevated in the cerebrospinal fluid and the peripheral blood (albeit in extremely low concentrations) of patients with severe traumatic brain injury (TBI), professional boxers, and athletes who sustain concussions. The extremely low levels of tau in the peripheral blood have been very difficult to measure until the recent development of an ultrahigh-sensitivity immunoassay technology. Using this innovation, the researchers were able to examine for the first time the associations between plasma tau levels and the frequency and severity of deployment-related TBIs.
Over a 2-year period, Dr. Olivera and her associates assessed tau levels in 70 members of the military who self-reported one or more TBIs and 28 control subjects without TBI who were matched for age, sex, race, time since deployment, and number of deployments. Almost all of those in the TBI group had been injured at least 18 months previously. The most common sources of TBI were blows to the head, exposure to blasts, vehicular crashes, and sports-related concussions.
Total tau was significantly increased in the TBI group (mean level, 1.13 pg/mL), compared with the control group (0.63 pg/mL). Total tau also increased with increasing severity of the initial brain injury, with increasing numbers of TBIs, and increasing severity of present-day postconcussive symptoms. These associations, moreover, were independent of symptoms of posttraumatic brain disorder (PTSD) and depression, which were prevalent in the TBI group, the investigators said (JAMA Neurol. 2015 Aug 3 doi: 10.1001/jamaneurol.2015.1383).
Tau is not only a marker of brain injury; it also can contribute to secondary injury processes such as inflammation, which makes it a potential target for therapy. If the findings of this study are confirmed and extended to demonstrate a direct mechanistic relationship between TBI and tau aggregation, treatments such as the direct delivery of proteasomes “would be invaluable, considering the dearth of treatments for TBIs and chronic [postconcussive disorder] symptoms,” Dr. Olivera and her associates said.
Among the limitations cited by the investigators are lack of neuroimaging and neuropsychological data.
This study was supported by the National Institutes of Health’s National Institute of Nursing Research and the Center for Neuroscience and Regenerative Medicine, which is a collaborative program between the Department of Defense and the NIH. Dr. Olivera reported having no relevant financial disclosures. One of her associates reported ties to Quanterix, developer of the ultrahigh-sensitivity Simoa technology used in this study, which allows measurement of extremely low levels of tau and other CNS-derived biomarkers in the plasma or serum.
Researchers and funding agencies have been hoping that the recent development of ultrahigh-sensitivity technology to measure the extremely low levels of CNS-derived biomarkers in the blood would be a game changer for minor TBI, and it appears that our hopes are beginning to be borne out.
The usefulness of plasma tau as a marker of brain pathology in TBI, however, was relatively weak in this study, and it remains to be seen whether this marker will prove helpful in clinical practice. The between-group differences in mean plasma tau levels were small in this study, and the levels in individual samples overlapped substantially between affected patients and controls.
Dr. Elaine R. Peskind is with the Veterans Affairs Northwest Network Mental Illness Research, Education, and Clinical Center and the department of psychiatry and behavioral sciences at the University of Washington, both in Seattle. She reported having no relevant financial conflicts of interest. Dr. Peskind and her associates made these remarks in an editorial accompanying Dr. Olivera’s report (JAMA Neurol. 2015 Aug 3 doi: 10.1001/jamaneurol.2015.1789).
Researchers and funding agencies have been hoping that the recent development of ultrahigh-sensitivity technology to measure the extremely low levels of CNS-derived biomarkers in the blood would be a game changer for minor TBI, and it appears that our hopes are beginning to be borne out.
The usefulness of plasma tau as a marker of brain pathology in TBI, however, was relatively weak in this study, and it remains to be seen whether this marker will prove helpful in clinical practice. The between-group differences in mean plasma tau levels were small in this study, and the levels in individual samples overlapped substantially between affected patients and controls.
Dr. Elaine R. Peskind is with the Veterans Affairs Northwest Network Mental Illness Research, Education, and Clinical Center and the department of psychiatry and behavioral sciences at the University of Washington, both in Seattle. She reported having no relevant financial conflicts of interest. Dr. Peskind and her associates made these remarks in an editorial accompanying Dr. Olivera’s report (JAMA Neurol. 2015 Aug 3 doi: 10.1001/jamaneurol.2015.1789).
Researchers and funding agencies have been hoping that the recent development of ultrahigh-sensitivity technology to measure the extremely low levels of CNS-derived biomarkers in the blood would be a game changer for minor TBI, and it appears that our hopes are beginning to be borne out.
The usefulness of plasma tau as a marker of brain pathology in TBI, however, was relatively weak in this study, and it remains to be seen whether this marker will prove helpful in clinical practice. The between-group differences in mean plasma tau levels were small in this study, and the levels in individual samples overlapped substantially between affected patients and controls.
Dr. Elaine R. Peskind is with the Veterans Affairs Northwest Network Mental Illness Research, Education, and Clinical Center and the department of psychiatry and behavioral sciences at the University of Washington, both in Seattle. She reported having no relevant financial conflicts of interest. Dr. Peskind and her associates made these remarks in an editorial accompanying Dr. Olivera’s report (JAMA Neurol. 2015 Aug 3 doi: 10.1001/jamaneurol.2015.1789).
Peripheral plasma levels of the CNS protein tau are chronically elevated after traumatic brain injury and appear to correlate with the severity of postconcussive symptoms, according to a report published Aug. 3 in JAMA Neurology.
If these findings are confirmed, this will be the first biomarker that is sensitive and specific to persistent traumatic brain injury–related symptoms. The results also suggest that “months to years after the primary brain injury, there may be a continuation of secondary injuries with residual axonal degeneration and blood-brain barrier disruptions in this population that may contribute to the maintenance of postconcussive disorder symptoms and affect symptom severity,” wrote Anlys Olivera, Ph.D., of the National Institute of Nursing Research, Bethesda, Md., and her associates.
Tau is a protein that stabilizes the structure of the axonal cytoskeleton. It is elevated in the cerebrospinal fluid and the peripheral blood (albeit in extremely low concentrations) of patients with severe traumatic brain injury (TBI), professional boxers, and athletes who sustain concussions. The extremely low levels of tau in the peripheral blood have been very difficult to measure until the recent development of an ultrahigh-sensitivity immunoassay technology. Using this innovation, the researchers were able to examine for the first time the associations between plasma tau levels and the frequency and severity of deployment-related TBIs.
Over a 2-year period, Dr. Olivera and her associates assessed tau levels in 70 members of the military who self-reported one or more TBIs and 28 control subjects without TBI who were matched for age, sex, race, time since deployment, and number of deployments. Almost all of those in the TBI group had been injured at least 18 months previously. The most common sources of TBI were blows to the head, exposure to blasts, vehicular crashes, and sports-related concussions.
Total tau was significantly increased in the TBI group (mean level, 1.13 pg/mL), compared with the control group (0.63 pg/mL). Total tau also increased with increasing severity of the initial brain injury, with increasing numbers of TBIs, and increasing severity of present-day postconcussive symptoms. These associations, moreover, were independent of symptoms of posttraumatic brain disorder (PTSD) and depression, which were prevalent in the TBI group, the investigators said (JAMA Neurol. 2015 Aug 3 doi: 10.1001/jamaneurol.2015.1383).
Tau is not only a marker of brain injury; it also can contribute to secondary injury processes such as inflammation, which makes it a potential target for therapy. If the findings of this study are confirmed and extended to demonstrate a direct mechanistic relationship between TBI and tau aggregation, treatments such as the direct delivery of proteasomes “would be invaluable, considering the dearth of treatments for TBIs and chronic [postconcussive disorder] symptoms,” Dr. Olivera and her associates said.
Among the limitations cited by the investigators are lack of neuroimaging and neuropsychological data.
This study was supported by the National Institutes of Health’s National Institute of Nursing Research and the Center for Neuroscience and Regenerative Medicine, which is a collaborative program between the Department of Defense and the NIH. Dr. Olivera reported having no relevant financial disclosures. One of her associates reported ties to Quanterix, developer of the ultrahigh-sensitivity Simoa technology used in this study, which allows measurement of extremely low levels of tau and other CNS-derived biomarkers in the plasma or serum.
Peripheral plasma levels of the CNS protein tau are chronically elevated after traumatic brain injury and appear to correlate with the severity of postconcussive symptoms, according to a report published Aug. 3 in JAMA Neurology.
If these findings are confirmed, this will be the first biomarker that is sensitive and specific to persistent traumatic brain injury–related symptoms. The results also suggest that “months to years after the primary brain injury, there may be a continuation of secondary injuries with residual axonal degeneration and blood-brain barrier disruptions in this population that may contribute to the maintenance of postconcussive disorder symptoms and affect symptom severity,” wrote Anlys Olivera, Ph.D., of the National Institute of Nursing Research, Bethesda, Md., and her associates.
Tau is a protein that stabilizes the structure of the axonal cytoskeleton. It is elevated in the cerebrospinal fluid and the peripheral blood (albeit in extremely low concentrations) of patients with severe traumatic brain injury (TBI), professional boxers, and athletes who sustain concussions. The extremely low levels of tau in the peripheral blood have been very difficult to measure until the recent development of an ultrahigh-sensitivity immunoassay technology. Using this innovation, the researchers were able to examine for the first time the associations between plasma tau levels and the frequency and severity of deployment-related TBIs.
Over a 2-year period, Dr. Olivera and her associates assessed tau levels in 70 members of the military who self-reported one or more TBIs and 28 control subjects without TBI who were matched for age, sex, race, time since deployment, and number of deployments. Almost all of those in the TBI group had been injured at least 18 months previously. The most common sources of TBI were blows to the head, exposure to blasts, vehicular crashes, and sports-related concussions.
Total tau was significantly increased in the TBI group (mean level, 1.13 pg/mL), compared with the control group (0.63 pg/mL). Total tau also increased with increasing severity of the initial brain injury, with increasing numbers of TBIs, and increasing severity of present-day postconcussive symptoms. These associations, moreover, were independent of symptoms of posttraumatic brain disorder (PTSD) and depression, which were prevalent in the TBI group, the investigators said (JAMA Neurol. 2015 Aug 3 doi: 10.1001/jamaneurol.2015.1383).
Tau is not only a marker of brain injury; it also can contribute to secondary injury processes such as inflammation, which makes it a potential target for therapy. If the findings of this study are confirmed and extended to demonstrate a direct mechanistic relationship between TBI and tau aggregation, treatments such as the direct delivery of proteasomes “would be invaluable, considering the dearth of treatments for TBIs and chronic [postconcussive disorder] symptoms,” Dr. Olivera and her associates said.
Among the limitations cited by the investigators are lack of neuroimaging and neuropsychological data.
This study was supported by the National Institutes of Health’s National Institute of Nursing Research and the Center for Neuroscience and Regenerative Medicine, which is a collaborative program between the Department of Defense and the NIH. Dr. Olivera reported having no relevant financial disclosures. One of her associates reported ties to Quanterix, developer of the ultrahigh-sensitivity Simoa technology used in this study, which allows measurement of extremely low levels of tau and other CNS-derived biomarkers in the plasma or serum.
FROM JAMA NEUROLOGY
Key clinical point: Peripheral plasma levels of the CNS protein tau are chronically elevated after traumatic brain injury and correlate with the severity of postconcussive symptoms.
Major finding: Total tau was significantly increased in the TBI group (mean level, 1.13 pg/mL), compared with the control group (0.63 pg/mL).
Data source: An observational case-control study assessing plasma tau levels in 70 military personnel with a history of TBI and 28 control subjects.
Disclosures: This study was supported by the National Institutes of Health’s National Institute of Nursing Research and the Center for Neuroscience and Regenerative Medicine, which is a collaborative program between the Department of Defense and the NIH. Dr. Olivera reported having no relevant financial disclosures. One of her associates reported ties to Quanterix, developer of the ultrahigh-sensitivity Simoa technology used in this study, which allows measurement of extremely low levels of tau and other CNS-derived biomarkers in the plasma or serum.
Direct oral anticoagulants may be inappropriate for frail elderly
Caution must be used when prescribing direct oral anticoagulants to older patients, especially those in a frail condition, according to an opinion piece by Anne-Laure Sennesael and her associates.
In the case study, an 86-year-old woman weighing 55 kg was admitted to an emergency department with persistent epistaxis. The patient was taking 20 mg of rivaroxaban and 80 mg of aspirin daily. There was a history of peripheral arterial disease, and the patient had received a bioprosthetic heart implant 4 years prior. Creatinine clearance was 21 mL/min, hemoglobin was 9.4 g/dL, and prothrombin time Quick value was 30%.
Rivaroxaban was discontinued, and the patient was switched to a new anticoagulant, acenocoumarol. Aspirin also was discontinued, and the patient was discharged, reported Ms. Sennesael, of the Université catholique de Louvain in Brussels.
Use of direct oral anticoagulants (DOACs) are increasing rapidly, the authors write, mostly because doctors and patients view them as more convenient to use. However, in some patients, vitamin K antagonists (VKAs) remain the best course of treatment. In this case, the patient was receiving too much rivaroxaban in light of her low creatinine level. In addition, aspirin plus a DOAC is not appropriate, as it increases the risk of major bleeding significantly.
“A conservative strategy may be more valuable,” Ms. Sennesael and her associates wrote. “This kind of ‘less is more’ approach includes avoiding prescribing DOACs rather than VKAs without clear, compelling, evidence-based reasons; regularly reassessing renal function; monitoring for adverse effects; and reappraising aspirin prescription.”
Read the full study at JAMA Internal Medicine (doi:10.1001/jamainternmed.2015.3589).
Caution must be used when prescribing direct oral anticoagulants to older patients, especially those in a frail condition, according to an opinion piece by Anne-Laure Sennesael and her associates.
In the case study, an 86-year-old woman weighing 55 kg was admitted to an emergency department with persistent epistaxis. The patient was taking 20 mg of rivaroxaban and 80 mg of aspirin daily. There was a history of peripheral arterial disease, and the patient had received a bioprosthetic heart implant 4 years prior. Creatinine clearance was 21 mL/min, hemoglobin was 9.4 g/dL, and prothrombin time Quick value was 30%.
Rivaroxaban was discontinued, and the patient was switched to a new anticoagulant, acenocoumarol. Aspirin also was discontinued, and the patient was discharged, reported Ms. Sennesael, of the Université catholique de Louvain in Brussels.
Use of direct oral anticoagulants (DOACs) are increasing rapidly, the authors write, mostly because doctors and patients view them as more convenient to use. However, in some patients, vitamin K antagonists (VKAs) remain the best course of treatment. In this case, the patient was receiving too much rivaroxaban in light of her low creatinine level. In addition, aspirin plus a DOAC is not appropriate, as it increases the risk of major bleeding significantly.
“A conservative strategy may be more valuable,” Ms. Sennesael and her associates wrote. “This kind of ‘less is more’ approach includes avoiding prescribing DOACs rather than VKAs without clear, compelling, evidence-based reasons; regularly reassessing renal function; monitoring for adverse effects; and reappraising aspirin prescription.”
Read the full study at JAMA Internal Medicine (doi:10.1001/jamainternmed.2015.3589).
Caution must be used when prescribing direct oral anticoagulants to older patients, especially those in a frail condition, according to an opinion piece by Anne-Laure Sennesael and her associates.
In the case study, an 86-year-old woman weighing 55 kg was admitted to an emergency department with persistent epistaxis. The patient was taking 20 mg of rivaroxaban and 80 mg of aspirin daily. There was a history of peripheral arterial disease, and the patient had received a bioprosthetic heart implant 4 years prior. Creatinine clearance was 21 mL/min, hemoglobin was 9.4 g/dL, and prothrombin time Quick value was 30%.
Rivaroxaban was discontinued, and the patient was switched to a new anticoagulant, acenocoumarol. Aspirin also was discontinued, and the patient was discharged, reported Ms. Sennesael, of the Université catholique de Louvain in Brussels.
Use of direct oral anticoagulants (DOACs) are increasing rapidly, the authors write, mostly because doctors and patients view them as more convenient to use. However, in some patients, vitamin K antagonists (VKAs) remain the best course of treatment. In this case, the patient was receiving too much rivaroxaban in light of her low creatinine level. In addition, aspirin plus a DOAC is not appropriate, as it increases the risk of major bleeding significantly.
“A conservative strategy may be more valuable,” Ms. Sennesael and her associates wrote. “This kind of ‘less is more’ approach includes avoiding prescribing DOACs rather than VKAs without clear, compelling, evidence-based reasons; regularly reassessing renal function; monitoring for adverse effects; and reappraising aspirin prescription.”
Read the full study at JAMA Internal Medicine (doi:10.1001/jamainternmed.2015.3589).
SVS: AAA surveillance comes at an emotional cost
CHICAGO – For some patients, surveillance of low-risk abdominal aortic aneurysms is so stressful that early repair might be a better option.
Until now, though, it’s been hard to know who those patients are. There hasn’t been a way to quantify the impact of abdominal aortic aneurysm (AAA) surveillance on quality of life.
Dr. Bjoern Suckow, a vascular surgeon at Dartmouth-Hitchcock Medical Center in Lebanon, N.H., and his colleagues at the University of Massachusetts and elsewhere are working to fix that problem. “I do believe that there is a certain subset of patients who we know are” at low risk for rupture “who are so consumed by fear and anxiety during surveillance that the impact on quality of life might make us want to repair them slightly sooner. I hope this will help us weed out who that subgroup might be,” Dr. Suckow said at a meeting hosted by the Society for Vascular Surgery.
With the help of patient and physician focus groups and interviews, the team developed AAA-specific quality of life (QOL) surveys and administered them to 351 patients under surveillance for aneurysms below about 5.5 cm, and 657 who had undergone mostly endovascular AAA repair at six United States institutions.
The surveys included nine questions to assess concerns about rupture, surgery, costs, and death. The responses were averaged to give an emotional impact score (EIS) ranging from 0 to 100, with higher scores indicating worse emotional QOL. The survey also included 10 questions to assess changes in heavy lifting, strenuous activity, travel habits, and other behaviors. Those results were averaged to give a behavioral change score (BCS) that also ranged from 0 to 100, with higher scores indicating greater negative impact.
A significant portion of the surveillance patients thought it was “very likely” their aneurysm would rupture within a year; their EIS was 45 and BCS 30; patients who thought rupture was unlikely had an EIS of 12 and BCS of 13 (P less than .001). Overall, patients under surveillance had worse emotional impact sores than did those who had undergone repair.
“We routinely counsel patients with small aneurysms that the rupture risk is low” – less than 5% – “and outweighed by the higher risk of repair. We were surprised that even though we feel we do a great job counseling and educating our patients, some of them do not understand or retain what we mean.” Eventually, surveys could be used in the clinic to identify patients with “less understanding, so [we can] spend more time with them,” Dr. Suckow said.
In general, “the range of impact on QOL by AAA surveillance is broad. For most patients, the impact is minimal, but for some, especially those with a greater perceived rupture risk, it is severe. Overall, surveillance has a persistent negative impact on QOL, particularly emotional QOL. This impact appears to diminish following either open or endovascular repair,” he said.
The respondents were about 76 years old, on average. Most were white men, and about half were high school graduates.
Dr. Suckow has no relevant financial conflicts. The work was funded by the National Institutes of Health and career development awards from the Society for Vascular Surgery and the American College of Surgeons.
The diagnosis of a small aortic aneurysm, whether by screening or as an incidental finding, causes anxiety in our patients. The risk of rupture of small AAA has been demonstrated to be low – less than 1% per year below 5.0 cm in males (Health Technol. Assess. 2013;41:1-108) . Therefore, appropriate counseling and surveillance intervals should optimize the management of AAA patients. This study highlights the adverse effects of a diagnosis of small AAA on a proportion of our patients, despite appropriate explanation. Frequently patients know someone who died of AAA rupture and many do not understand the risk when it is explained in routine consultations. Perhaps we should all ensure that a member of our team contacts patients with small AAA post review and perform a short Quality of Life questionnaire by phone so that we can identify those who are suffering a negative impact on their QOL. We could then intensify our counseling and reassurance for this cohort of patients. This study should make us all reflect on whether our surveillance programs need to be modified, to ensure that our patients are not adversely affected by a diagnosis of small AAA.
Dr. Robert Fitridge is professor of vascular surgery, University of Adelaide, Australia, and associate medical editor of Vascular Specialist.
The diagnosis of a small aortic aneurysm, whether by screening or as an incidental finding, causes anxiety in our patients. The risk of rupture of small AAA has been demonstrated to be low – less than 1% per year below 5.0 cm in males (Health Technol. Assess. 2013;41:1-108) . Therefore, appropriate counseling and surveillance intervals should optimize the management of AAA patients. This study highlights the adverse effects of a diagnosis of small AAA on a proportion of our patients, despite appropriate explanation. Frequently patients know someone who died of AAA rupture and many do not understand the risk when it is explained in routine consultations. Perhaps we should all ensure that a member of our team contacts patients with small AAA post review and perform a short Quality of Life questionnaire by phone so that we can identify those who are suffering a negative impact on their QOL. We could then intensify our counseling and reassurance for this cohort of patients. This study should make us all reflect on whether our surveillance programs need to be modified, to ensure that our patients are not adversely affected by a diagnosis of small AAA.
Dr. Robert Fitridge is professor of vascular surgery, University of Adelaide, Australia, and associate medical editor of Vascular Specialist.
The diagnosis of a small aortic aneurysm, whether by screening or as an incidental finding, causes anxiety in our patients. The risk of rupture of small AAA has been demonstrated to be low – less than 1% per year below 5.0 cm in males (Health Technol. Assess. 2013;41:1-108) . Therefore, appropriate counseling and surveillance intervals should optimize the management of AAA patients. This study highlights the adverse effects of a diagnosis of small AAA on a proportion of our patients, despite appropriate explanation. Frequently patients know someone who died of AAA rupture and many do not understand the risk when it is explained in routine consultations. Perhaps we should all ensure that a member of our team contacts patients with small AAA post review and perform a short Quality of Life questionnaire by phone so that we can identify those who are suffering a negative impact on their QOL. We could then intensify our counseling and reassurance for this cohort of patients. This study should make us all reflect on whether our surveillance programs need to be modified, to ensure that our patients are not adversely affected by a diagnosis of small AAA.
Dr. Robert Fitridge is professor of vascular surgery, University of Adelaide, Australia, and associate medical editor of Vascular Specialist.
CHICAGO – For some patients, surveillance of low-risk abdominal aortic aneurysms is so stressful that early repair might be a better option.
Until now, though, it’s been hard to know who those patients are. There hasn’t been a way to quantify the impact of abdominal aortic aneurysm (AAA) surveillance on quality of life.
Dr. Bjoern Suckow, a vascular surgeon at Dartmouth-Hitchcock Medical Center in Lebanon, N.H., and his colleagues at the University of Massachusetts and elsewhere are working to fix that problem. “I do believe that there is a certain subset of patients who we know are” at low risk for rupture “who are so consumed by fear and anxiety during surveillance that the impact on quality of life might make us want to repair them slightly sooner. I hope this will help us weed out who that subgroup might be,” Dr. Suckow said at a meeting hosted by the Society for Vascular Surgery.
With the help of patient and physician focus groups and interviews, the team developed AAA-specific quality of life (QOL) surveys and administered them to 351 patients under surveillance for aneurysms below about 5.5 cm, and 657 who had undergone mostly endovascular AAA repair at six United States institutions.
The surveys included nine questions to assess concerns about rupture, surgery, costs, and death. The responses were averaged to give an emotional impact score (EIS) ranging from 0 to 100, with higher scores indicating worse emotional QOL. The survey also included 10 questions to assess changes in heavy lifting, strenuous activity, travel habits, and other behaviors. Those results were averaged to give a behavioral change score (BCS) that also ranged from 0 to 100, with higher scores indicating greater negative impact.
A significant portion of the surveillance patients thought it was “very likely” their aneurysm would rupture within a year; their EIS was 45 and BCS 30; patients who thought rupture was unlikely had an EIS of 12 and BCS of 13 (P less than .001). Overall, patients under surveillance had worse emotional impact sores than did those who had undergone repair.
“We routinely counsel patients with small aneurysms that the rupture risk is low” – less than 5% – “and outweighed by the higher risk of repair. We were surprised that even though we feel we do a great job counseling and educating our patients, some of them do not understand or retain what we mean.” Eventually, surveys could be used in the clinic to identify patients with “less understanding, so [we can] spend more time with them,” Dr. Suckow said.
In general, “the range of impact on QOL by AAA surveillance is broad. For most patients, the impact is minimal, but for some, especially those with a greater perceived rupture risk, it is severe. Overall, surveillance has a persistent negative impact on QOL, particularly emotional QOL. This impact appears to diminish following either open or endovascular repair,” he said.
The respondents were about 76 years old, on average. Most were white men, and about half were high school graduates.
Dr. Suckow has no relevant financial conflicts. The work was funded by the National Institutes of Health and career development awards from the Society for Vascular Surgery and the American College of Surgeons.
CHICAGO – For some patients, surveillance of low-risk abdominal aortic aneurysms is so stressful that early repair might be a better option.
Until now, though, it’s been hard to know who those patients are. There hasn’t been a way to quantify the impact of abdominal aortic aneurysm (AAA) surveillance on quality of life.
Dr. Bjoern Suckow, a vascular surgeon at Dartmouth-Hitchcock Medical Center in Lebanon, N.H., and his colleagues at the University of Massachusetts and elsewhere are working to fix that problem. “I do believe that there is a certain subset of patients who we know are” at low risk for rupture “who are so consumed by fear and anxiety during surveillance that the impact on quality of life might make us want to repair them slightly sooner. I hope this will help us weed out who that subgroup might be,” Dr. Suckow said at a meeting hosted by the Society for Vascular Surgery.
With the help of patient and physician focus groups and interviews, the team developed AAA-specific quality of life (QOL) surveys and administered them to 351 patients under surveillance for aneurysms below about 5.5 cm, and 657 who had undergone mostly endovascular AAA repair at six United States institutions.
The surveys included nine questions to assess concerns about rupture, surgery, costs, and death. The responses were averaged to give an emotional impact score (EIS) ranging from 0 to 100, with higher scores indicating worse emotional QOL. The survey also included 10 questions to assess changes in heavy lifting, strenuous activity, travel habits, and other behaviors. Those results were averaged to give a behavioral change score (BCS) that also ranged from 0 to 100, with higher scores indicating greater negative impact.
A significant portion of the surveillance patients thought it was “very likely” their aneurysm would rupture within a year; their EIS was 45 and BCS 30; patients who thought rupture was unlikely had an EIS of 12 and BCS of 13 (P less than .001). Overall, patients under surveillance had worse emotional impact sores than did those who had undergone repair.
“We routinely counsel patients with small aneurysms that the rupture risk is low” – less than 5% – “and outweighed by the higher risk of repair. We were surprised that even though we feel we do a great job counseling and educating our patients, some of them do not understand or retain what we mean.” Eventually, surveys could be used in the clinic to identify patients with “less understanding, so [we can] spend more time with them,” Dr. Suckow said.
In general, “the range of impact on QOL by AAA surveillance is broad. For most patients, the impact is minimal, but for some, especially those with a greater perceived rupture risk, it is severe. Overall, surveillance has a persistent negative impact on QOL, particularly emotional QOL. This impact appears to diminish following either open or endovascular repair,” he said.
The respondents were about 76 years old, on average. Most were white men, and about half were high school graduates.
Dr. Suckow has no relevant financial conflicts. The work was funded by the National Institutes of Health and career development awards from the Society for Vascular Surgery and the American College of Surgeons.
AT The 2015 Vascular Annual Meeting
Key clinical point: Check with your AAA surveillance patients to make sure they know their rupture risk is low.
Major finding: Surveillance patients who thought it was “very likely” their aneurysm would rupture within a year had an emotional impact score of 45. Patients who thought rupture was unlikely had a sore of 12 (P less than .001).
Data source: Surveys of 1,008 AAA patients at six U.S. medical centers.
Disclosures: There was no outside funding for the work, and the lead investigator has no relevant disclosures.
Outpatient venography can be performed safely
Venoplasties and stenting carried out in an office-based setting have the same therapeutic results and carry no greater risk as the same procedure done in an inpatient setting, researchers reported.
Dr. Arkady Ganelin and researchers from the Total Vascular Center in Brooklyn, N.Y. evaluated 245 patients who had undergone venography for the correction of suspected iliac vein stenosis at their office-based center. Overall, 90 women and 47 men underwent unilateral intervention and 23 women and 14 men underwent bilateral intervention.
There was a low incidence of complications such as thrombosis (2%), a figure that was similar to an inpatient setting, the researchers reported (J Vasc Surg: Venous and Lym Dis. 2015 doi: 10.1016/j.jvsv.2015.03.007).
One patient had a retroperitoneal hematoma, which occurred more than 30 days after the procedure. The average pain score was 2 out of 10 on the Likert scale.
“Our initial experience with conducting office-based procedures that were formerly only inpatient procedures has demonstrated that an office-based procedure can be safely performed with minimal complications,” the study authors wrote.
The financial burden of U.S. health care has been continuously increasing and the shift of endovascular procedures from the hospital to an office-based setting is the natural next step, they said.
If the results are sustained over the long term, office-based iliac venography and stent placement may replace the need of performing these procedures in the hospital, they concluded.
This conclusion, however, poses the question of which option would be chosen by a patient, they added.
The researchers reported having no financial disclosures.
There are more than 500 office-based labs. Complicated endovascular procedures are performed in this setting with results comparable or better than hospital-based procedures with extremely high patient satisfaction. Experience with complicated venous procedures in the office has been limited because there is no reimbursement for use of intravascular ultrasound (IVUS) in the office. This may change in January as the Centers for Medicare & Medicaid Services may start reimbursing the use of IVUS in office. IVUS is an important element in endovascular management of venous obstruction.
Researchers from Brooklyn, N.Y., performed 285 venous angioplasties and stent placements in an office setting. There was a 2% incidence of thrombosis that occurred in patients with a previous history of deep venous thrombosis. This subset of patients would naturally be at a higher risk for thrombosis. There was one bleeding complication after 30 days, which was successfully managed by nonoperative means. The complication rate was comparable to the procedures done in the hospital setting. One would expect similar complication rates when the same operator is doing the procedure at two different sites. However, the indications for these procedures are not well defined in the literature and there are very few studies showing long-term results. Accordingly, there is a real need for a prospective randomized study to determine the indications and efficacy of these procedures.
Dr. Krishna Jain is clinical associate professor of surgery, Western Michigan University School of Medicine, Kalamazoo. He is an associate medical editor of Vascular Specialist.
There are more than 500 office-based labs. Complicated endovascular procedures are performed in this setting with results comparable or better than hospital-based procedures with extremely high patient satisfaction. Experience with complicated venous procedures in the office has been limited because there is no reimbursement for use of intravascular ultrasound (IVUS) in the office. This may change in January as the Centers for Medicare & Medicaid Services may start reimbursing the use of IVUS in office. IVUS is an important element in endovascular management of venous obstruction.
Researchers from Brooklyn, N.Y., performed 285 venous angioplasties and stent placements in an office setting. There was a 2% incidence of thrombosis that occurred in patients with a previous history of deep venous thrombosis. This subset of patients would naturally be at a higher risk for thrombosis. There was one bleeding complication after 30 days, which was successfully managed by nonoperative means. The complication rate was comparable to the procedures done in the hospital setting. One would expect similar complication rates when the same operator is doing the procedure at two different sites. However, the indications for these procedures are not well defined in the literature and there are very few studies showing long-term results. Accordingly, there is a real need for a prospective randomized study to determine the indications and efficacy of these procedures.
Dr. Krishna Jain is clinical associate professor of surgery, Western Michigan University School of Medicine, Kalamazoo. He is an associate medical editor of Vascular Specialist.
There are more than 500 office-based labs. Complicated endovascular procedures are performed in this setting with results comparable or better than hospital-based procedures with extremely high patient satisfaction. Experience with complicated venous procedures in the office has been limited because there is no reimbursement for use of intravascular ultrasound (IVUS) in the office. This may change in January as the Centers for Medicare & Medicaid Services may start reimbursing the use of IVUS in office. IVUS is an important element in endovascular management of venous obstruction.
Researchers from Brooklyn, N.Y., performed 285 venous angioplasties and stent placements in an office setting. There was a 2% incidence of thrombosis that occurred in patients with a previous history of deep venous thrombosis. This subset of patients would naturally be at a higher risk for thrombosis. There was one bleeding complication after 30 days, which was successfully managed by nonoperative means. The complication rate was comparable to the procedures done in the hospital setting. One would expect similar complication rates when the same operator is doing the procedure at two different sites. However, the indications for these procedures are not well defined in the literature and there are very few studies showing long-term results. Accordingly, there is a real need for a prospective randomized study to determine the indications and efficacy of these procedures.
Dr. Krishna Jain is clinical associate professor of surgery, Western Michigan University School of Medicine, Kalamazoo. He is an associate medical editor of Vascular Specialist.
Venoplasties and stenting carried out in an office-based setting have the same therapeutic results and carry no greater risk as the same procedure done in an inpatient setting, researchers reported.
Dr. Arkady Ganelin and researchers from the Total Vascular Center in Brooklyn, N.Y. evaluated 245 patients who had undergone venography for the correction of suspected iliac vein stenosis at their office-based center. Overall, 90 women and 47 men underwent unilateral intervention and 23 women and 14 men underwent bilateral intervention.
There was a low incidence of complications such as thrombosis (2%), a figure that was similar to an inpatient setting, the researchers reported (J Vasc Surg: Venous and Lym Dis. 2015 doi: 10.1016/j.jvsv.2015.03.007).
One patient had a retroperitoneal hematoma, which occurred more than 30 days after the procedure. The average pain score was 2 out of 10 on the Likert scale.
“Our initial experience with conducting office-based procedures that were formerly only inpatient procedures has demonstrated that an office-based procedure can be safely performed with minimal complications,” the study authors wrote.
The financial burden of U.S. health care has been continuously increasing and the shift of endovascular procedures from the hospital to an office-based setting is the natural next step, they said.
If the results are sustained over the long term, office-based iliac venography and stent placement may replace the need of performing these procedures in the hospital, they concluded.
This conclusion, however, poses the question of which option would be chosen by a patient, they added.
The researchers reported having no financial disclosures.
Venoplasties and stenting carried out in an office-based setting have the same therapeutic results and carry no greater risk as the same procedure done in an inpatient setting, researchers reported.
Dr. Arkady Ganelin and researchers from the Total Vascular Center in Brooklyn, N.Y. evaluated 245 patients who had undergone venography for the correction of suspected iliac vein stenosis at their office-based center. Overall, 90 women and 47 men underwent unilateral intervention and 23 women and 14 men underwent bilateral intervention.
There was a low incidence of complications such as thrombosis (2%), a figure that was similar to an inpatient setting, the researchers reported (J Vasc Surg: Venous and Lym Dis. 2015 doi: 10.1016/j.jvsv.2015.03.007).
One patient had a retroperitoneal hematoma, which occurred more than 30 days after the procedure. The average pain score was 2 out of 10 on the Likert scale.
“Our initial experience with conducting office-based procedures that were formerly only inpatient procedures has demonstrated that an office-based procedure can be safely performed with minimal complications,” the study authors wrote.
The financial burden of U.S. health care has been continuously increasing and the shift of endovascular procedures from the hospital to an office-based setting is the natural next step, they said.
If the results are sustained over the long term, office-based iliac venography and stent placement may replace the need of performing these procedures in the hospital, they concluded.
This conclusion, however, poses the question of which option would be chosen by a patient, they added.
The researchers reported having no financial disclosures.
FROM THE JOURNAL OF VASCULAR SURGERY: VENOUS AND LYMPHATIC DISORDERS
Key clinical point: Office-based iliac venography and stent placement may replace the need to perform these procedures in the hospital.
Major finding: Outpatient venography had the same therapeutic results and carried no greater risk as the same procedure done in an inpatient setting.
Data source: 245 patients who had undergone venography for the correction of suspected iliac vein stenosis in an office-based setting.
Disclosures: The researchers reported having no financial disclosures.
Order of Food During a Meal May Influence Blood Sugar
(Reuters Health) - Overweight and obese people with type 2 diabetes may feel better after a meal if they start it off with vegetables or proteins and end with the carbs, suggests a new study of 11 people.
Finishing the broccoli and chicken before tucking into bread and fruit juice was tied to a lower rise in blood sugar levels over the next two hours, compared to eating the same foods in the opposite order, researchers report in Diabetes Care.
"When we saw the result, we were really encouraged that this is something that could potentially benefit people," said Dr.Louis Aronne, the study's senior author from Weill Cornell Medical College in New York.
Approximately 29 million Americans - about 9 percent of the U.S. population - have diabetes, according to the Centers for Disease Control and Prevention. About 30 percent of those people are undiagnosed.
Type 2 is the most common form of diabetes and is often linked to obesity. In type 2 diabetes, the body's cells are resistant to the hormone insulin, or the body doesn't make enough of it. Insulin helps the body's cells use glucose in the blood for fuel.
Drinking whey protein shakes before meals has been linked to lower blood sugar levels after eating, but little was known about the influence of foods, and the order in which they're consumed, on blood sugar levels following a meal, the researchers write.
Blood sugar normally rises after eating, but for people with diabetes it can spike dangerously. Diabetics are often told to avoid foods high on the glycemic index - a measure of how rapidly a food gets converted to glucose in the blood - like white breads and sugary drinks.
The new research suggests that people may benefit from timing their consumption of carbohydrates during a meal instead of simply avoiding certain foods.
The researchers recruited 11 people with type 2 diabetes who were all overweight or obese. They were also taking a drug called metformin, which helps to control blood sugar.
The participants all fasted for 12 hours overnight before consuming a 628 calorie meal with protein, carbohydrates and fat.
One week, they consumed the carbohydrates (ciabatta bread and orange juice) first. Then they ate skinless grilled chicken, a small salad and buttered steamed broccoli 15 minutes later.
The participants ate the same meal a week later, but the order of the foods was reversed, with the salad and broccoli first, then the chicken, then the carbs.
The researchers also took blood samples before the meals and 30, 60 and 120 minutes afterward.
When the participants ate vegetables and proteins first, their blood sugar levels were about 29 percent lower 30 minutes after starting the meal, compared to when they ate the carbs first. At 60 and 120 minutes after participants began eating, blood sugar levels were 37 percent and 17 percent lower, respectively, compared to when the carbs came first.
"It's possible what this is doing is delaying or tempering how fast the carbohydrates get absorbed," said Dr. Sethu Reddy, chief of the Adult Diabetes Section at the Joslin Diabetes Center in Boston.
"I think certainly it's an interesting study that says eating a good salad before your meal may help with glucose absorption," said Reddy, who was not involved with the new study.
The new study may not be the full story, Reddy told Reuters Health. For example, he said, it will be important to see what happens beyond two hours, and what's happening to the carbohydrates.
The researchers also say more studies with longer follow-up times are needed.
"We're doing the next study," Aronne told Reuters Health. "We're doing a longer study and we're looking at some of the other key hormones."
As of now, he said, the theory is that the absorption of the carbohydrates is somehow slowed down by eating vegetables, which are low on the glycemic index.
"This shows that the highly desired foods can be a part of a diet if we sneak them in there," Aronne said.
(Reuters Health) - Overweight and obese people with type 2 diabetes may feel better after a meal if they start it off with vegetables or proteins and end with the carbs, suggests a new study of 11 people.
Finishing the broccoli and chicken before tucking into bread and fruit juice was tied to a lower rise in blood sugar levels over the next two hours, compared to eating the same foods in the opposite order, researchers report in Diabetes Care.
"When we saw the result, we were really encouraged that this is something that could potentially benefit people," said Dr.Louis Aronne, the study's senior author from Weill Cornell Medical College in New York.
Approximately 29 million Americans - about 9 percent of the U.S. population - have diabetes, according to the Centers for Disease Control and Prevention. About 30 percent of those people are undiagnosed.
Type 2 is the most common form of diabetes and is often linked to obesity. In type 2 diabetes, the body's cells are resistant to the hormone insulin, or the body doesn't make enough of it. Insulin helps the body's cells use glucose in the blood for fuel.
Drinking whey protein shakes before meals has been linked to lower blood sugar levels after eating, but little was known about the influence of foods, and the order in which they're consumed, on blood sugar levels following a meal, the researchers write.
Blood sugar normally rises after eating, but for people with diabetes it can spike dangerously. Diabetics are often told to avoid foods high on the glycemic index - a measure of how rapidly a food gets converted to glucose in the blood - like white breads and sugary drinks.
The new research suggests that people may benefit from timing their consumption of carbohydrates during a meal instead of simply avoiding certain foods.
The researchers recruited 11 people with type 2 diabetes who were all overweight or obese. They were also taking a drug called metformin, which helps to control blood sugar.
The participants all fasted for 12 hours overnight before consuming a 628 calorie meal with protein, carbohydrates and fat.
One week, they consumed the carbohydrates (ciabatta bread and orange juice) first. Then they ate skinless grilled chicken, a small salad and buttered steamed broccoli 15 minutes later.
The participants ate the same meal a week later, but the order of the foods was reversed, with the salad and broccoli first, then the chicken, then the carbs.
The researchers also took blood samples before the meals and 30, 60 and 120 minutes afterward.
When the participants ate vegetables and proteins first, their blood sugar levels were about 29 percent lower 30 minutes after starting the meal, compared to when they ate the carbs first. At 60 and 120 minutes after participants began eating, blood sugar levels were 37 percent and 17 percent lower, respectively, compared to when the carbs came first.
"It's possible what this is doing is delaying or tempering how fast the carbohydrates get absorbed," said Dr. Sethu Reddy, chief of the Adult Diabetes Section at the Joslin Diabetes Center in Boston.
"I think certainly it's an interesting study that says eating a good salad before your meal may help with glucose absorption," said Reddy, who was not involved with the new study.
The new study may not be the full story, Reddy told Reuters Health. For example, he said, it will be important to see what happens beyond two hours, and what's happening to the carbohydrates.
The researchers also say more studies with longer follow-up times are needed.
"We're doing the next study," Aronne told Reuters Health. "We're doing a longer study and we're looking at some of the other key hormones."
As of now, he said, the theory is that the absorption of the carbohydrates is somehow slowed down by eating vegetables, which are low on the glycemic index.
"This shows that the highly desired foods can be a part of a diet if we sneak them in there," Aronne said.
(Reuters Health) - Overweight and obese people with type 2 diabetes may feel better after a meal if they start it off with vegetables or proteins and end with the carbs, suggests a new study of 11 people.
Finishing the broccoli and chicken before tucking into bread and fruit juice was tied to a lower rise in blood sugar levels over the next two hours, compared to eating the same foods in the opposite order, researchers report in Diabetes Care.
"When we saw the result, we were really encouraged that this is something that could potentially benefit people," said Dr.Louis Aronne, the study's senior author from Weill Cornell Medical College in New York.
Approximately 29 million Americans - about 9 percent of the U.S. population - have diabetes, according to the Centers for Disease Control and Prevention. About 30 percent of those people are undiagnosed.
Type 2 is the most common form of diabetes and is often linked to obesity. In type 2 diabetes, the body's cells are resistant to the hormone insulin, or the body doesn't make enough of it. Insulin helps the body's cells use glucose in the blood for fuel.
Drinking whey protein shakes before meals has been linked to lower blood sugar levels after eating, but little was known about the influence of foods, and the order in which they're consumed, on blood sugar levels following a meal, the researchers write.
Blood sugar normally rises after eating, but for people with diabetes it can spike dangerously. Diabetics are often told to avoid foods high on the glycemic index - a measure of how rapidly a food gets converted to glucose in the blood - like white breads and sugary drinks.
The new research suggests that people may benefit from timing their consumption of carbohydrates during a meal instead of simply avoiding certain foods.
The researchers recruited 11 people with type 2 diabetes who were all overweight or obese. They were also taking a drug called metformin, which helps to control blood sugar.
The participants all fasted for 12 hours overnight before consuming a 628 calorie meal with protein, carbohydrates and fat.
One week, they consumed the carbohydrates (ciabatta bread and orange juice) first. Then they ate skinless grilled chicken, a small salad and buttered steamed broccoli 15 minutes later.
The participants ate the same meal a week later, but the order of the foods was reversed, with the salad and broccoli first, then the chicken, then the carbs.
The researchers also took blood samples before the meals and 30, 60 and 120 minutes afterward.
When the participants ate vegetables and proteins first, their blood sugar levels were about 29 percent lower 30 minutes after starting the meal, compared to when they ate the carbs first. At 60 and 120 minutes after participants began eating, blood sugar levels were 37 percent and 17 percent lower, respectively, compared to when the carbs came first.
"It's possible what this is doing is delaying or tempering how fast the carbohydrates get absorbed," said Dr. Sethu Reddy, chief of the Adult Diabetes Section at the Joslin Diabetes Center in Boston.
"I think certainly it's an interesting study that says eating a good salad before your meal may help with glucose absorption," said Reddy, who was not involved with the new study.
The new study may not be the full story, Reddy told Reuters Health. For example, he said, it will be important to see what happens beyond two hours, and what's happening to the carbohydrates.
The researchers also say more studies with longer follow-up times are needed.
"We're doing the next study," Aronne told Reuters Health. "We're doing a longer study and we're looking at some of the other key hormones."
As of now, he said, the theory is that the absorption of the carbohydrates is somehow slowed down by eating vegetables, which are low on the glycemic index.
"This shows that the highly desired foods can be a part of a diet if we sneak them in there," Aronne said.
Girl, 10, Asks Tough Questions About Skin Problem
A 10-year-old girl is seen in dermatology for evaluation of dry skin. She reports few if any symptoms but expresses frustration at her inability to curb the problem; she’s tried several different moisturizers to no avail.
Additional history-taking reveals that she’s had patches of dry skin since age 4; these have appeared and disappeared on her arms, legs, and neck. None has ever been problematic enough to require medical attention.
But recently, a dry patch manifested on the patient’s forearm that her primary care provider diagnosed as fungal infection. Unfortunately, the prescribed antifungal creams (terbinafine and clotrimazole) had no positive impact on the situation.
The patient and her mother deny any family history of skin disease.
EXAMINATION
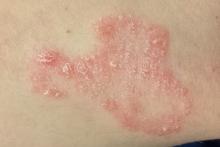
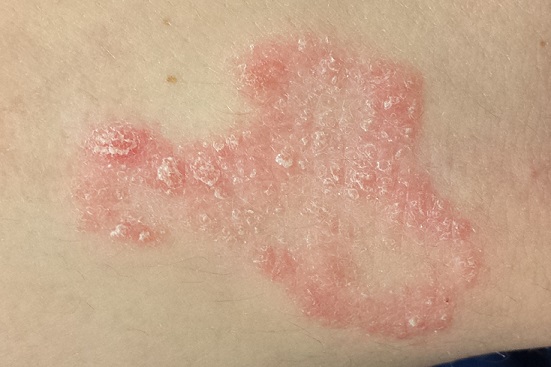
The scaly, annular plaque on the patient’s extensor left forearm is distinctly salmon-pink, with a tenacious white scale. Elsewhere, there are scaly areas in both post-auricular sulci. There are no significant changes to the skin on the patient’s knees, elbows, or scalp. Several tiny pits are observed on her fingernails.
What is the diagnosis?
DISCUSSION
It would be difficult to imagine a more clear-cut case of psoriasis: not only manifesting with a classic plaque on the left extensor forearm but also with corroboratory stigmata behind the ears and classic fingernail pits. Then why, you might ask, was the diagnosis missed?
Psoriasis has a classic look: annular borders, salmon-pink color, and thick, tenacious scale. Distribution matters, since the condition tends to manifest in specific areas, particularly the extensor portions of extremities. It’s helpful to know that psoriasis affects almost 3% of the white population in the United States, meaning that you will see it with considerable frequency. It would also help if you knew the diagnosis can be corroborated by identification of other, lesser known features.
But if you’re unaware of these facts, you won’t look for these things—and if you don’t look specifically for them, you won’t see them. Then, to add insult to injury, when you consult your bag of “diseases that present with annular borders and scaly surfaces,” you’ll find one item in your differential: fungal infection. When antifungal medications don’t work, you’ll find yourself stuck, because you simply don’t have any other diagnoses to consider.
I’ve known internists who have practiced for more than 25 years and still make this diagnostic mistake. So it’s not a matter of lack of intelligence. They simply do not invest the time to expand their knowledge of dermatologic conditions.
My job was to break the news to the patient and her mother about the diagnosis and, perhaps more importantly, her prognosis. The only good news is that the patient is living in the golden age of psoriasis treatment; if her condition flares, and even if she eventually develops psoriatic arthritis (as do almost 25% of psoriasis patients), we have terrific treatment for it.
I prescribed topical fluocinonide 0.05% ointment (for bid application) to address her plaque and scheduled a follow-up visit for one month later. Before she left, though, I had to address her most pressing question: “Why did my doctor say this was a fungal infection?” Good question indeed.
TAKE-HOME LEARNING POINTS
• Psoriasis is quite commonly seen in primary care, since it affects almost 3% of the white population.
• Psoriasis is the quintessential papulosquamous disorder, manifesting with salmon-pink scaly patches on extensor extremities, behind ears, and/or in scalp.
• The diagnosis may be corroborated by identification of pits in the fingernails (25% of cases).
• Confirmation of the diagnosis can be made by punch biopsy, which usually shows characteristic changes.
• The overarching learning point: Your differential for “round and scaly” needs more than one item in it.
A 10-year-old girl is seen in dermatology for evaluation of dry skin. She reports few if any symptoms but expresses frustration at her inability to curb the problem; she’s tried several different moisturizers to no avail.
Additional history-taking reveals that she’s had patches of dry skin since age 4; these have appeared and disappeared on her arms, legs, and neck. None has ever been problematic enough to require medical attention.
But recently, a dry patch manifested on the patient’s forearm that her primary care provider diagnosed as fungal infection. Unfortunately, the prescribed antifungal creams (terbinafine and clotrimazole) had no positive impact on the situation.
The patient and her mother deny any family history of skin disease.
EXAMINATION
The scaly, annular plaque on the patient’s extensor left forearm is distinctly salmon-pink, with a tenacious white scale. Elsewhere, there are scaly areas in both post-auricular sulci. There are no significant changes to the skin on the patient’s knees, elbows, or scalp. Several tiny pits are observed on her fingernails.
What is the diagnosis?
DISCUSSION
It would be difficult to imagine a more clear-cut case of psoriasis: not only manifesting with a classic plaque on the left extensor forearm but also with corroboratory stigmata behind the ears and classic fingernail pits. Then why, you might ask, was the diagnosis missed?
Psoriasis has a classic look: annular borders, salmon-pink color, and thick, tenacious scale. Distribution matters, since the condition tends to manifest in specific areas, particularly the extensor portions of extremities. It’s helpful to know that psoriasis affects almost 3% of the white population in the United States, meaning that you will see it with considerable frequency. It would also help if you knew the diagnosis can be corroborated by identification of other, lesser known features.
But if you’re unaware of these facts, you won’t look for these things—and if you don’t look specifically for them, you won’t see them. Then, to add insult to injury, when you consult your bag of “diseases that present with annular borders and scaly surfaces,” you’ll find one item in your differential: fungal infection. When antifungal medications don’t work, you’ll find yourself stuck, because you simply don’t have any other diagnoses to consider.
I’ve known internists who have practiced for more than 25 years and still make this diagnostic mistake. So it’s not a matter of lack of intelligence. They simply do not invest the time to expand their knowledge of dermatologic conditions.
My job was to break the news to the patient and her mother about the diagnosis and, perhaps more importantly, her prognosis. The only good news is that the patient is living in the golden age of psoriasis treatment; if her condition flares, and even if she eventually develops psoriatic arthritis (as do almost 25% of psoriasis patients), we have terrific treatment for it.
I prescribed topical fluocinonide 0.05% ointment (for bid application) to address her plaque and scheduled a follow-up visit for one month later. Before she left, though, I had to address her most pressing question: “Why did my doctor say this was a fungal infection?” Good question indeed.
TAKE-HOME LEARNING POINTS
• Psoriasis is quite commonly seen in primary care, since it affects almost 3% of the white population.
• Psoriasis is the quintessential papulosquamous disorder, manifesting with salmon-pink scaly patches on extensor extremities, behind ears, and/or in scalp.
• The diagnosis may be corroborated by identification of pits in the fingernails (25% of cases).
• Confirmation of the diagnosis can be made by punch biopsy, which usually shows characteristic changes.
• The overarching learning point: Your differential for “round and scaly” needs more than one item in it.
A 10-year-old girl is seen in dermatology for evaluation of dry skin. She reports few if any symptoms but expresses frustration at her inability to curb the problem; she’s tried several different moisturizers to no avail.
Additional history-taking reveals that she’s had patches of dry skin since age 4; these have appeared and disappeared on her arms, legs, and neck. None has ever been problematic enough to require medical attention.
But recently, a dry patch manifested on the patient’s forearm that her primary care provider diagnosed as fungal infection. Unfortunately, the prescribed antifungal creams (terbinafine and clotrimazole) had no positive impact on the situation.
The patient and her mother deny any family history of skin disease.
EXAMINATION
The scaly, annular plaque on the patient’s extensor left forearm is distinctly salmon-pink, with a tenacious white scale. Elsewhere, there are scaly areas in both post-auricular sulci. There are no significant changes to the skin on the patient’s knees, elbows, or scalp. Several tiny pits are observed on her fingernails.
What is the diagnosis?
DISCUSSION
It would be difficult to imagine a more clear-cut case of psoriasis: not only manifesting with a classic plaque on the left extensor forearm but also with corroboratory stigmata behind the ears and classic fingernail pits. Then why, you might ask, was the diagnosis missed?
Psoriasis has a classic look: annular borders, salmon-pink color, and thick, tenacious scale. Distribution matters, since the condition tends to manifest in specific areas, particularly the extensor portions of extremities. It’s helpful to know that psoriasis affects almost 3% of the white population in the United States, meaning that you will see it with considerable frequency. It would also help if you knew the diagnosis can be corroborated by identification of other, lesser known features.
But if you’re unaware of these facts, you won’t look for these things—and if you don’t look specifically for them, you won’t see them. Then, to add insult to injury, when you consult your bag of “diseases that present with annular borders and scaly surfaces,” you’ll find one item in your differential: fungal infection. When antifungal medications don’t work, you’ll find yourself stuck, because you simply don’t have any other diagnoses to consider.
I’ve known internists who have practiced for more than 25 years and still make this diagnostic mistake. So it’s not a matter of lack of intelligence. They simply do not invest the time to expand their knowledge of dermatologic conditions.
My job was to break the news to the patient and her mother about the diagnosis and, perhaps more importantly, her prognosis. The only good news is that the patient is living in the golden age of psoriasis treatment; if her condition flares, and even if she eventually develops psoriatic arthritis (as do almost 25% of psoriasis patients), we have terrific treatment for it.
I prescribed topical fluocinonide 0.05% ointment (for bid application) to address her plaque and scheduled a follow-up visit for one month later. Before she left, though, I had to address her most pressing question: “Why did my doctor say this was a fungal infection?” Good question indeed.
TAKE-HOME LEARNING POINTS
• Psoriasis is quite commonly seen in primary care, since it affects almost 3% of the white population.
• Psoriasis is the quintessential papulosquamous disorder, manifesting with salmon-pink scaly patches on extensor extremities, behind ears, and/or in scalp.
• The diagnosis may be corroborated by identification of pits in the fingernails (25% of cases).
• Confirmation of the diagnosis can be made by punch biopsy, which usually shows characteristic changes.
• The overarching learning point: Your differential for “round and scaly” needs more than one item in it.
How a molecule turns B cells into macrophages

pseudopodia to engulf particles
The transcription factor C/EBPα reprograms B cells into macrophages by “short-circuiting” the cells so they re-express genes reserved for embryonic development, according to research published in Stem Cell Reports.
Over the past 28 years, researchers have shown that a number of specialized cell types can be forcibly converted into other cell types, but the science of how this change takes place is still emerging.
“For a long time, it was unclear whether forcing cell fate decisions by expressing transcription factors in the wrong cell type could teach us something about what happens normally during physiological differentiation,” said Thomas Graf, PhD, of the Center for Genomic Regulation in Barcelona, Spain.
“What we have now found is that the two processes are actually surprisingly similar.”
The researchers found that B-cell transdifferentiation takes place when C/EBPα binds to two regions of DNA that act as gene expression enhancers. One of these regions is normally active in immune cells, and the other is only turned on when macrophage precursors are ready to differentiate.
This indicates that the convergence of these two enhancer pathways can cause the B cell to act like a macrophage precursor, thus triggering the unnatural transdifferentiation.
“This has taught us a great deal about how a transcription factor can activate a new gene expression program (in our case, that of macrophages) but has left us in the dark about the other part of the equation; namely, how the factor silences the B-cell program, something that must happen if transdifferentiation is to work,” Dr Graf said. “This is one of the questions we are focusing on now.”
Dr Graf is interested in this pathway because C/EBPα-induced, B cell-to-macrophage transdifferentiation can convert both human B-cell lymphoma and leukemia cells into functional, non-cancerous macrophages.
He believes that induced transdifferentiation could become therapeutically relevant if a drug could be found that can replace the transcription factor. And understanding the mechanisms of the process could help labs that use this transdifferentiation approach to generate cells for regenerative purposes. ![]()

pseudopodia to engulf particles
The transcription factor C/EBPα reprograms B cells into macrophages by “short-circuiting” the cells so they re-express genes reserved for embryonic development, according to research published in Stem Cell Reports.
Over the past 28 years, researchers have shown that a number of specialized cell types can be forcibly converted into other cell types, but the science of how this change takes place is still emerging.
“For a long time, it was unclear whether forcing cell fate decisions by expressing transcription factors in the wrong cell type could teach us something about what happens normally during physiological differentiation,” said Thomas Graf, PhD, of the Center for Genomic Regulation in Barcelona, Spain.
“What we have now found is that the two processes are actually surprisingly similar.”
The researchers found that B-cell transdifferentiation takes place when C/EBPα binds to two regions of DNA that act as gene expression enhancers. One of these regions is normally active in immune cells, and the other is only turned on when macrophage precursors are ready to differentiate.
This indicates that the convergence of these two enhancer pathways can cause the B cell to act like a macrophage precursor, thus triggering the unnatural transdifferentiation.
“This has taught us a great deal about how a transcription factor can activate a new gene expression program (in our case, that of macrophages) but has left us in the dark about the other part of the equation; namely, how the factor silences the B-cell program, something that must happen if transdifferentiation is to work,” Dr Graf said. “This is one of the questions we are focusing on now.”
Dr Graf is interested in this pathway because C/EBPα-induced, B cell-to-macrophage transdifferentiation can convert both human B-cell lymphoma and leukemia cells into functional, non-cancerous macrophages.
He believes that induced transdifferentiation could become therapeutically relevant if a drug could be found that can replace the transcription factor. And understanding the mechanisms of the process could help labs that use this transdifferentiation approach to generate cells for regenerative purposes. ![]()

pseudopodia to engulf particles
The transcription factor C/EBPα reprograms B cells into macrophages by “short-circuiting” the cells so they re-express genes reserved for embryonic development, according to research published in Stem Cell Reports.
Over the past 28 years, researchers have shown that a number of specialized cell types can be forcibly converted into other cell types, but the science of how this change takes place is still emerging.
“For a long time, it was unclear whether forcing cell fate decisions by expressing transcription factors in the wrong cell type could teach us something about what happens normally during physiological differentiation,” said Thomas Graf, PhD, of the Center for Genomic Regulation in Barcelona, Spain.
“What we have now found is that the two processes are actually surprisingly similar.”
The researchers found that B-cell transdifferentiation takes place when C/EBPα binds to two regions of DNA that act as gene expression enhancers. One of these regions is normally active in immune cells, and the other is only turned on when macrophage precursors are ready to differentiate.
This indicates that the convergence of these two enhancer pathways can cause the B cell to act like a macrophage precursor, thus triggering the unnatural transdifferentiation.
“This has taught us a great deal about how a transcription factor can activate a new gene expression program (in our case, that of macrophages) but has left us in the dark about the other part of the equation; namely, how the factor silences the B-cell program, something that must happen if transdifferentiation is to work,” Dr Graf said. “This is one of the questions we are focusing on now.”
Dr Graf is interested in this pathway because C/EBPα-induced, B cell-to-macrophage transdifferentiation can convert both human B-cell lymphoma and leukemia cells into functional, non-cancerous macrophages.
He believes that induced transdifferentiation could become therapeutically relevant if a drug could be found that can replace the transcription factor. And understanding the mechanisms of the process could help labs that use this transdifferentiation approach to generate cells for regenerative purposes. ![]()
Microfluidics can improve epigenomic analysis, team says
his student, Zhenning Cao.
Dr Lu is holding the chip
used in the study.
Photo from Virginia Tech
A new technique enables epigenomic analysis using fewer cells than other methods involving chromatin immunoprecipitation and deep sequencing (ChIP-seq), according to researchers.
The technique, microfluidic oscillatory washing-based ChIP-seq (MOWChIP-seq), allows for genome-wide analysis of histone modifications using as few as 100
cells.
Using MOWChIP-seq, researchers uncovered new information regarding early hematopoiesis.
The team described this work in Nature Methods.
They used multilayer soft lithography to create a poly (dimethylsiloxane) device with a microfluidic chamber for ChIP. The researchers flowed magnetic beads coated with a ChIP antibody into the chamber, which formed a packed bed.
They then flowed sonicated chromatin fragments through the chamber, which were adsorbed onto the bead surface. The team said the gaps between the immunoprecipitation beads are smaller than 2 μm and facilitate rapid, high-efficiency adsorption of target chromatin fragments under the small diffusion length.
The researchers washed the beads by oscillatory washing in two different buffers to remove nonspecifically adsorbed chromatin fragments. Then, they flowed the beads out of the chamber and collected them for off-chip processing.
“The use of a packed bed of beads for ChIP allowed us to collect the chromatin fragments with a very high efficiency,” said study author Chang Lu, PhD, of Virginia Tech in Blacksburg.
“At the same time, effective washing for removing undesired molecules and debris guarantees the purity of the collected molecules. These two factors constitute a successful strategy for epigenomic analysis with extremely high sensitivity.”
In addition, the entire MOWChIP-seq process takes about 90 minutes.
To test MOWChIP-seq, Dr Lu and his colleagues used the technique to study the epigenomes of hematopoietic stem and progenitor cells (HSPCs) isolated from the fetal liver of a mouse.
“Little is known about the dynamics of the epigenome during embryonic hematopoiesis, largely due to the difficulty in isolating sufficient quantities of these cells from developing embryos,” said study author Kai Tan, PhD, of the University of Iowa in Iowa City. “This technology is the perfect tool for tackling this problem.”
MOWChIP-seq revealed new enhancers and super enhancers in the HSPCs.
By comparing all of the enhancers they identified to an enhancer catalog covering 16 blood cell types, the researchers found that 2561 (58%) of the enhancers they found were unique to fetal liver HSPCs. They said this suggests enhancer activity is highly dynamic during early hematopoiesis.
Now, the researchers plan to use MOWChIP-seq to study other epigenomic changes involved in inflammation and cancer.
“Our technology paves the way for studies of epigenomes with extremely low numbers of cells from animals and from patients,” Dr Lu said.
Virginia Tech Intellectual Properties has filed a utility patent on MOWChIP-seq on behalf of Dr Lu. ![]()

his student, Zhenning Cao.
Dr Lu is holding the chip
used in the study.
Photo from Virginia Tech
A new technique enables epigenomic analysis using fewer cells than other methods involving chromatin immunoprecipitation and deep sequencing (ChIP-seq), according to researchers.
The technique, microfluidic oscillatory washing-based ChIP-seq (MOWChIP-seq), allows for genome-wide analysis of histone modifications using as few as 100
cells.
Using MOWChIP-seq, researchers uncovered new information regarding early hematopoiesis.
The team described this work in Nature Methods.
They used multilayer soft lithography to create a poly (dimethylsiloxane) device with a microfluidic chamber for ChIP. The researchers flowed magnetic beads coated with a ChIP antibody into the chamber, which formed a packed bed.
They then flowed sonicated chromatin fragments through the chamber, which were adsorbed onto the bead surface. The team said the gaps between the immunoprecipitation beads are smaller than 2 μm and facilitate rapid, high-efficiency adsorption of target chromatin fragments under the small diffusion length.
The researchers washed the beads by oscillatory washing in two different buffers to remove nonspecifically adsorbed chromatin fragments. Then, they flowed the beads out of the chamber and collected them for off-chip processing.
“The use of a packed bed of beads for ChIP allowed us to collect the chromatin fragments with a very high efficiency,” said study author Chang Lu, PhD, of Virginia Tech in Blacksburg.
“At the same time, effective washing for removing undesired molecules and debris guarantees the purity of the collected molecules. These two factors constitute a successful strategy for epigenomic analysis with extremely high sensitivity.”
In addition, the entire MOWChIP-seq process takes about 90 minutes.
To test MOWChIP-seq, Dr Lu and his colleagues used the technique to study the epigenomes of hematopoietic stem and progenitor cells (HSPCs) isolated from the fetal liver of a mouse.
“Little is known about the dynamics of the epigenome during embryonic hematopoiesis, largely due to the difficulty in isolating sufficient quantities of these cells from developing embryos,” said study author Kai Tan, PhD, of the University of Iowa in Iowa City. “This technology is the perfect tool for tackling this problem.”
MOWChIP-seq revealed new enhancers and super enhancers in the HSPCs.
By comparing all of the enhancers they identified to an enhancer catalog covering 16 blood cell types, the researchers found that 2561 (58%) of the enhancers they found were unique to fetal liver HSPCs. They said this suggests enhancer activity is highly dynamic during early hematopoiesis.
Now, the researchers plan to use MOWChIP-seq to study other epigenomic changes involved in inflammation and cancer.
“Our technology paves the way for studies of epigenomes with extremely low numbers of cells from animals and from patients,” Dr Lu said.
Virginia Tech Intellectual Properties has filed a utility patent on MOWChIP-seq on behalf of Dr Lu. ![]()

his student, Zhenning Cao.
Dr Lu is holding the chip
used in the study.
Photo from Virginia Tech
A new technique enables epigenomic analysis using fewer cells than other methods involving chromatin immunoprecipitation and deep sequencing (ChIP-seq), according to researchers.
The technique, microfluidic oscillatory washing-based ChIP-seq (MOWChIP-seq), allows for genome-wide analysis of histone modifications using as few as 100
cells.
Using MOWChIP-seq, researchers uncovered new information regarding early hematopoiesis.
The team described this work in Nature Methods.
They used multilayer soft lithography to create a poly (dimethylsiloxane) device with a microfluidic chamber for ChIP. The researchers flowed magnetic beads coated with a ChIP antibody into the chamber, which formed a packed bed.
They then flowed sonicated chromatin fragments through the chamber, which were adsorbed onto the bead surface. The team said the gaps between the immunoprecipitation beads are smaller than 2 μm and facilitate rapid, high-efficiency adsorption of target chromatin fragments under the small diffusion length.
The researchers washed the beads by oscillatory washing in two different buffers to remove nonspecifically adsorbed chromatin fragments. Then, they flowed the beads out of the chamber and collected them for off-chip processing.
“The use of a packed bed of beads for ChIP allowed us to collect the chromatin fragments with a very high efficiency,” said study author Chang Lu, PhD, of Virginia Tech in Blacksburg.
“At the same time, effective washing for removing undesired molecules and debris guarantees the purity of the collected molecules. These two factors constitute a successful strategy for epigenomic analysis with extremely high sensitivity.”
In addition, the entire MOWChIP-seq process takes about 90 minutes.
To test MOWChIP-seq, Dr Lu and his colleagues used the technique to study the epigenomes of hematopoietic stem and progenitor cells (HSPCs) isolated from the fetal liver of a mouse.
“Little is known about the dynamics of the epigenome during embryonic hematopoiesis, largely due to the difficulty in isolating sufficient quantities of these cells from developing embryos,” said study author Kai Tan, PhD, of the University of Iowa in Iowa City. “This technology is the perfect tool for tackling this problem.”
MOWChIP-seq revealed new enhancers and super enhancers in the HSPCs.
By comparing all of the enhancers they identified to an enhancer catalog covering 16 blood cell types, the researchers found that 2561 (58%) of the enhancers they found were unique to fetal liver HSPCs. They said this suggests enhancer activity is highly dynamic during early hematopoiesis.
Now, the researchers plan to use MOWChIP-seq to study other epigenomic changes involved in inflammation and cancer.
“Our technology paves the way for studies of epigenomes with extremely low numbers of cells from animals and from patients,” Dr Lu said.
Virginia Tech Intellectual Properties has filed a utility patent on MOWChIP-seq on behalf of Dr Lu. ![]()
Newfound mechanism could be used to fight cancers

apoptosis in cancer cells
Researchers say they have identified a new mechanism by which the tumor suppressor protein p53 triggers apoptosis, and they believe this process could be harnessed to kill cancer cells.
The team discovered how p53 acts in the cytoplasm to trigger cell death by binding to and activating the BAX protein.
The process involves a shape change in one of p53’s amino acids that serves as the “switch” to activate BAX and trigger the apoptotic pathway.
The team also identified the enzyme in the cytoplasm that promotes the change that controls the “switch.”
Richard Kriwacki, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee, and his colleagues described these findings in Molecular Cell.
Like up to half of all proteins, p53 includes both structured and disordered regions. A disordered region is a segment that does not adopt a single shape but
remains flexible and constantly switches between different shapes until
it encounters a partner.
Dr Kriwacki and his colleagues showed that both structured and disordered regions of p53 play a role in BAX activation in the cytoplasm.
The process starts when a structured region of p53 known as the DNA-binding domain binds to BAX. That sets the stage for the unstructured region of p53 to form a second bond, which activates BAX and triggers apoptosis.
“There were no previous reports of this disordered region of p53 binding to BAX, so the finding that this region was the key to BAX activation was a total surprise,” Dr Kriwacki said.
The disordered p53 segment included the amino acid proline, which can change between two shapes, particularly in the presence of the enzyme Pin1.
Using NMR spectroscopy, the researchers showed that the proline shape change promotes p53 binding and activation of BAX.
“These results expand our understanding of the different ways p53 modulates cell behavior,” Dr Kriwacki said. “The findings also raise the possibility of killing tumor cells using small molecules to trigger BAX-dependent apoptosis.” ![]()

apoptosis in cancer cells
Researchers say they have identified a new mechanism by which the tumor suppressor protein p53 triggers apoptosis, and they believe this process could be harnessed to kill cancer cells.
The team discovered how p53 acts in the cytoplasm to trigger cell death by binding to and activating the BAX protein.
The process involves a shape change in one of p53’s amino acids that serves as the “switch” to activate BAX and trigger the apoptotic pathway.
The team also identified the enzyme in the cytoplasm that promotes the change that controls the “switch.”
Richard Kriwacki, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee, and his colleagues described these findings in Molecular Cell.
Like up to half of all proteins, p53 includes both structured and disordered regions. A disordered region is a segment that does not adopt a single shape but
remains flexible and constantly switches between different shapes until
it encounters a partner.
Dr Kriwacki and his colleagues showed that both structured and disordered regions of p53 play a role in BAX activation in the cytoplasm.
The process starts when a structured region of p53 known as the DNA-binding domain binds to BAX. That sets the stage for the unstructured region of p53 to form a second bond, which activates BAX and triggers apoptosis.
“There were no previous reports of this disordered region of p53 binding to BAX, so the finding that this region was the key to BAX activation was a total surprise,” Dr Kriwacki said.
The disordered p53 segment included the amino acid proline, which can change between two shapes, particularly in the presence of the enzyme Pin1.
Using NMR spectroscopy, the researchers showed that the proline shape change promotes p53 binding and activation of BAX.
“These results expand our understanding of the different ways p53 modulates cell behavior,” Dr Kriwacki said. “The findings also raise the possibility of killing tumor cells using small molecules to trigger BAX-dependent apoptosis.” ![]()

apoptosis in cancer cells
Researchers say they have identified a new mechanism by which the tumor suppressor protein p53 triggers apoptosis, and they believe this process could be harnessed to kill cancer cells.
The team discovered how p53 acts in the cytoplasm to trigger cell death by binding to and activating the BAX protein.
The process involves a shape change in one of p53’s amino acids that serves as the “switch” to activate BAX and trigger the apoptotic pathway.
The team also identified the enzyme in the cytoplasm that promotes the change that controls the “switch.”
Richard Kriwacki, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee, and his colleagues described these findings in Molecular Cell.
Like up to half of all proteins, p53 includes both structured and disordered regions. A disordered region is a segment that does not adopt a single shape but
remains flexible and constantly switches between different shapes until
it encounters a partner.
Dr Kriwacki and his colleagues showed that both structured and disordered regions of p53 play a role in BAX activation in the cytoplasm.
The process starts when a structured region of p53 known as the DNA-binding domain binds to BAX. That sets the stage for the unstructured region of p53 to form a second bond, which activates BAX and triggers apoptosis.
“There were no previous reports of this disordered region of p53 binding to BAX, so the finding that this region was the key to BAX activation was a total surprise,” Dr Kriwacki said.
The disordered p53 segment included the amino acid proline, which can change between two shapes, particularly in the presence of the enzyme Pin1.
Using NMR spectroscopy, the researchers showed that the proline shape change promotes p53 binding and activation of BAX.
“These results expand our understanding of the different ways p53 modulates cell behavior,” Dr Kriwacki said. “The findings also raise the possibility of killing tumor cells using small molecules to trigger BAX-dependent apoptosis.” ![]()