User login
Checklists Improve Outcomes, Require Care-team Buy-in
Dr. Ramiro Jervis and Dr. Umesh Gidwani urge hospitalists to experiment with checklists during the 7th annual Hospital Medicine Symposium in New York City.
Dr. Ramiro Jervis and Dr. Umesh Gidwani urge hospitalists to experiment with checklists during the 7th annual Hospital Medicine Symposium in New York City.
Dr. Ramiro Jervis and Dr. Umesh Gidwani urge hospitalists to experiment with checklists during the 7th annual Hospital Medicine Symposium in New York City.
LISTEN NOW: Dr. Kendall Rogers, MD, SFHM, Encourages Hospitalists to Work as Part of Quality Teams to Achieve Glycemic Control
As SHM's glycemic control lead mentor and a hospitalist at the University of New Mexico in Albuquerque, Kendall Rogers, MD, CPE, FACP, SFHM, offers advice to hospitalists when working as part of a quality team in achieving glycemic control.
As SHM's glycemic control lead mentor and a hospitalist at the University of New Mexico in Albuquerque, Kendall Rogers, MD, CPE, FACP, SFHM, offers advice to hospitalists when working as part of a quality team in achieving glycemic control.
As SHM's glycemic control lead mentor and a hospitalist at the University of New Mexico in Albuquerque, Kendall Rogers, MD, CPE, FACP, SFHM, offers advice to hospitalists when working as part of a quality team in achieving glycemic control.
LISTEN NOW: Kristen Kulasa, MD, Explains How Hospitalists Can Work with Nutritionists and Dieticians
Kristen Kulasa, MD, assistant clinical professor of medicine and director of Inpatient Glycemic Control, Division of Endocrinology, Diabetes, and Metabolism at the University of California in San Diego, provides tips on how hospitalists can work with nutritionists and dieticians for the betterment of diabetic patients. As a mentor for SHM's care coordination program on inpatient diabetes, Dr. Kulasa offers hospitalists advice in treating diabetic patients. She points to SHM’s website, which has a lot of resources to help hospitalists feel comfortable with insulin dosing.
Kristen Kulasa, MD, assistant clinical professor of medicine and director of Inpatient Glycemic Control, Division of Endocrinology, Diabetes, and Metabolism at the University of California in San Diego, provides tips on how hospitalists can work with nutritionists and dieticians for the betterment of diabetic patients. As a mentor for SHM's care coordination program on inpatient diabetes, Dr. Kulasa offers hospitalists advice in treating diabetic patients. She points to SHM’s website, which has a lot of resources to help hospitalists feel comfortable with insulin dosing.
Kristen Kulasa, MD, assistant clinical professor of medicine and director of Inpatient Glycemic Control, Division of Endocrinology, Diabetes, and Metabolism at the University of California in San Diego, provides tips on how hospitalists can work with nutritionists and dieticians for the betterment of diabetic patients. As a mentor for SHM's care coordination program on inpatient diabetes, Dr. Kulasa offers hospitalists advice in treating diabetic patients. She points to SHM’s website, which has a lot of resources to help hospitalists feel comfortable with insulin dosing.
LISTEN NOW: Dr. Carolyn Zelop, MD, Discusses Cardiovascular Emergencies in Pregnant Women
Listen now to excerpts of our interview with Dr. Zelop, a board certified maternal-fetal medicine specialist and director of perinatal ultrasound and research at Valley Hospital in Ridgewood, N.J.
Listen now to excerpts of our interview with Dr. Zelop, a board certified maternal-fetal medicine specialist and director of perinatal ultrasound and research at Valley Hospital in Ridgewood, N.J.
Listen now to excerpts of our interview with Dr. Zelop, a board certified maternal-fetal medicine specialist and director of perinatal ultrasound and research at Valley Hospital in Ridgewood, N.J.
Product News: 11 2014
Effaclar Dermatological Acne System
La Roche-Posay Laboratoire Dermatologique introduces the Effaclar Dermatological Acne System, an over-the-counter system to cleanse, tone, and treat using 3 products: Effaclar Medicated Gel Cleanser, Effaclar Clarifying Solution, and Effaclar Duo. The system is designed to reduce acne with little to no drying or irritation. The Effaclar Dermatological Acne System targets excess sebum, hyperkeratinization, and the main triggers of acne. It can be purchased at select physicians’ offices and pharmacies as well as online. For more information, visit www.laroche-posay.us.
Hydrate Moisturizers
Obagi Medical Products, Inc, introduces 2 moisturizers that provide long-lasting hydration: Hydrate Facial Moisturizer and Hydrate Luxe. Hydrate Facial Moisturizer provides all-day moisture protection for every skin type with immediate and lasting effects on skin barrier function. Hydrate Luxe is engineered with biomimetic peptides to work overnight. It saturates skin with 8-hour moisture protection, and also promotes skin radiance. Both products are dispensed in dermatology, plastic surgery, and other aesthetic physicians’ practices. For more information, visit www.obagi.com.
Stretch Mark Crème
Natural skin care company derma e reveals Stretch Mark Crème to visibly diminish the look of stretch marks, as well as improve texture, color, and overall appearance. Stretch Mark Crème contains argan oil, cocoa butter, coconut oil, and shea butter to intensely condition skin to help increase elasticity and resiliency. It also contains vitamin E to promote self-healing and hyaluronic acid to attract and bind moisture to the skin. For more information, visit www.dermae.com.
Taclonex
LEO Pharma Inc announces a new pediatric indication for Taclonex (calcipotriene 0.005%–betamethasone dipropionate 0.064%) Topical Suspension. Taclonex is a first-line, once-daily combination product indicated for treatment of both scalp and body psoriasis in adults 18 years and older for up to 8 weeks. It is now also indicated for the treatment of plaque psoriasis of the scalp in patients aged 12 to 17 years for the same period. For more information, visit www.taclonex.com.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at [email protected]
Effaclar Dermatological Acne System
La Roche-Posay Laboratoire Dermatologique introduces the Effaclar Dermatological Acne System, an over-the-counter system to cleanse, tone, and treat using 3 products: Effaclar Medicated Gel Cleanser, Effaclar Clarifying Solution, and Effaclar Duo. The system is designed to reduce acne with little to no drying or irritation. The Effaclar Dermatological Acne System targets excess sebum, hyperkeratinization, and the main triggers of acne. It can be purchased at select physicians’ offices and pharmacies as well as online. For more information, visit www.laroche-posay.us.
Hydrate Moisturizers
Obagi Medical Products, Inc, introduces 2 moisturizers that provide long-lasting hydration: Hydrate Facial Moisturizer and Hydrate Luxe. Hydrate Facial Moisturizer provides all-day moisture protection for every skin type with immediate and lasting effects on skin barrier function. Hydrate Luxe is engineered with biomimetic peptides to work overnight. It saturates skin with 8-hour moisture protection, and also promotes skin radiance. Both products are dispensed in dermatology, plastic surgery, and other aesthetic physicians’ practices. For more information, visit www.obagi.com.
Stretch Mark Crème
Natural skin care company derma e reveals Stretch Mark Crème to visibly diminish the look of stretch marks, as well as improve texture, color, and overall appearance. Stretch Mark Crème contains argan oil, cocoa butter, coconut oil, and shea butter to intensely condition skin to help increase elasticity and resiliency. It also contains vitamin E to promote self-healing and hyaluronic acid to attract and bind moisture to the skin. For more information, visit www.dermae.com.
Taclonex
LEO Pharma Inc announces a new pediatric indication for Taclonex (calcipotriene 0.005%–betamethasone dipropionate 0.064%) Topical Suspension. Taclonex is a first-line, once-daily combination product indicated for treatment of both scalp and body psoriasis in adults 18 years and older for up to 8 weeks. It is now also indicated for the treatment of plaque psoriasis of the scalp in patients aged 12 to 17 years for the same period. For more information, visit www.taclonex.com.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at [email protected]
Effaclar Dermatological Acne System
La Roche-Posay Laboratoire Dermatologique introduces the Effaclar Dermatological Acne System, an over-the-counter system to cleanse, tone, and treat using 3 products: Effaclar Medicated Gel Cleanser, Effaclar Clarifying Solution, and Effaclar Duo. The system is designed to reduce acne with little to no drying or irritation. The Effaclar Dermatological Acne System targets excess sebum, hyperkeratinization, and the main triggers of acne. It can be purchased at select physicians’ offices and pharmacies as well as online. For more information, visit www.laroche-posay.us.
Hydrate Moisturizers
Obagi Medical Products, Inc, introduces 2 moisturizers that provide long-lasting hydration: Hydrate Facial Moisturizer and Hydrate Luxe. Hydrate Facial Moisturizer provides all-day moisture protection for every skin type with immediate and lasting effects on skin barrier function. Hydrate Luxe is engineered with biomimetic peptides to work overnight. It saturates skin with 8-hour moisture protection, and also promotes skin radiance. Both products are dispensed in dermatology, plastic surgery, and other aesthetic physicians’ practices. For more information, visit www.obagi.com.
Stretch Mark Crème
Natural skin care company derma e reveals Stretch Mark Crème to visibly diminish the look of stretch marks, as well as improve texture, color, and overall appearance. Stretch Mark Crème contains argan oil, cocoa butter, coconut oil, and shea butter to intensely condition skin to help increase elasticity and resiliency. It also contains vitamin E to promote self-healing and hyaluronic acid to attract and bind moisture to the skin. For more information, visit www.dermae.com.
Taclonex
LEO Pharma Inc announces a new pediatric indication for Taclonex (calcipotriene 0.005%–betamethasone dipropionate 0.064%) Topical Suspension. Taclonex is a first-line, once-daily combination product indicated for treatment of both scalp and body psoriasis in adults 18 years and older for up to 8 weeks. It is now also indicated for the treatment of plaque psoriasis of the scalp in patients aged 12 to 17 years for the same period. For more information, visit www.taclonex.com.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at [email protected]
Insulin Rules in the Hospital
Although new medications to manage and treat hyperglycemia and diabetes continuously appear on the market, national guidelines and position statements consistently refer to insulin as the treatment of choice in the inpatient hospital setting.
“When patients are admitted to the hospital, our standard is to switch from the outpatient regimen [wide variety of medications] to the inpatient regimen—insulin,” says Paul M. Szumita, PharmD, BCPS, clinical pharmacy practice manager director at Brigham and Women’s Hospital in Boston.
For critically ill patients in ICUs or during the peri-operative period, intravenous infusion of insulin is preferred. Most general medicine and surgery patients are managed with subcutaneous insulin.
“Using a basal bolus regimen starting at a total daily dose of 0.3-0.5 unit/kg is sufficient for most patients,” says Guillermo Umpierrez, MD, CDE, FCAE, FACP, professor of medicine at Emory University in Atlanta, Ga., and a member of the board of directors for the American Diabetes Association; however, for most general medicine and surgical patients who have low oral intake or are NPO, a recent trial reported that the administration of basal insulin alone plus correction doses with rapid-acting insulin analogs before meals is as good as a basal bolus regimen. A regimen should be tweaked throughout the inpatient’s stay with an aim to reach the goal of minimal or no hypoglycemia.1
Planning for a discharge regimen should start early in the hospital stay, Dr. Szumita says, and should be based on several factors:
- The patient’s Hb1c;
- The prior regimen and how it was performing;
- The patient’s wishes; and
- Collaboration with outpatient providers.
At discharge, it is critical that patients be clear about what medications they should be on post-discharge and that they follow-up with outpatient providers in a timely manner. TH
Karen Appold is a freelance writer in Pennsylvania.
Reference
- Umpierrez GE, Smiley D, Hermayer K, et al. Randomized study comparing a basal-bolus with a basal plus correction insulin regimen for the hospital management of medical and surgical patients with type 2 diabetes: basal plus trial. Diabetes Care. 2013;36(8):2169-2174.
Although new medications to manage and treat hyperglycemia and diabetes continuously appear on the market, national guidelines and position statements consistently refer to insulin as the treatment of choice in the inpatient hospital setting.
“When patients are admitted to the hospital, our standard is to switch from the outpatient regimen [wide variety of medications] to the inpatient regimen—insulin,” says Paul M. Szumita, PharmD, BCPS, clinical pharmacy practice manager director at Brigham and Women’s Hospital in Boston.
For critically ill patients in ICUs or during the peri-operative period, intravenous infusion of insulin is preferred. Most general medicine and surgery patients are managed with subcutaneous insulin.
“Using a basal bolus regimen starting at a total daily dose of 0.3-0.5 unit/kg is sufficient for most patients,” says Guillermo Umpierrez, MD, CDE, FCAE, FACP, professor of medicine at Emory University in Atlanta, Ga., and a member of the board of directors for the American Diabetes Association; however, for most general medicine and surgical patients who have low oral intake or are NPO, a recent trial reported that the administration of basal insulin alone plus correction doses with rapid-acting insulin analogs before meals is as good as a basal bolus regimen. A regimen should be tweaked throughout the inpatient’s stay with an aim to reach the goal of minimal or no hypoglycemia.1
Planning for a discharge regimen should start early in the hospital stay, Dr. Szumita says, and should be based on several factors:
- The patient’s Hb1c;
- The prior regimen and how it was performing;
- The patient’s wishes; and
- Collaboration with outpatient providers.
At discharge, it is critical that patients be clear about what medications they should be on post-discharge and that they follow-up with outpatient providers in a timely manner. TH
Karen Appold is a freelance writer in Pennsylvania.
Reference
- Umpierrez GE, Smiley D, Hermayer K, et al. Randomized study comparing a basal-bolus with a basal plus correction insulin regimen for the hospital management of medical and surgical patients with type 2 diabetes: basal plus trial. Diabetes Care. 2013;36(8):2169-2174.
Although new medications to manage and treat hyperglycemia and diabetes continuously appear on the market, national guidelines and position statements consistently refer to insulin as the treatment of choice in the inpatient hospital setting.
“When patients are admitted to the hospital, our standard is to switch from the outpatient regimen [wide variety of medications] to the inpatient regimen—insulin,” says Paul M. Szumita, PharmD, BCPS, clinical pharmacy practice manager director at Brigham and Women’s Hospital in Boston.
For critically ill patients in ICUs or during the peri-operative period, intravenous infusion of insulin is preferred. Most general medicine and surgery patients are managed with subcutaneous insulin.
“Using a basal bolus regimen starting at a total daily dose of 0.3-0.5 unit/kg is sufficient for most patients,” says Guillermo Umpierrez, MD, CDE, FCAE, FACP, professor of medicine at Emory University in Atlanta, Ga., and a member of the board of directors for the American Diabetes Association; however, for most general medicine and surgical patients who have low oral intake or are NPO, a recent trial reported that the administration of basal insulin alone plus correction doses with rapid-acting insulin analogs before meals is as good as a basal bolus regimen. A regimen should be tweaked throughout the inpatient’s stay with an aim to reach the goal of minimal or no hypoglycemia.1
Planning for a discharge regimen should start early in the hospital stay, Dr. Szumita says, and should be based on several factors:
- The patient’s Hb1c;
- The prior regimen and how it was performing;
- The patient’s wishes; and
- Collaboration with outpatient providers.
At discharge, it is critical that patients be clear about what medications they should be on post-discharge and that they follow-up with outpatient providers in a timely manner. TH
Karen Appold is a freelance writer in Pennsylvania.
Reference
- Umpierrez GE, Smiley D, Hermayer K, et al. Randomized study comparing a basal-bolus with a basal plus correction insulin regimen for the hospital management of medical and surgical patients with type 2 diabetes: basal plus trial. Diabetes Care. 2013;36(8):2169-2174.
The Increasing Presence of Pregnant Patients in Hospital Medicine
Twenty years ago, pregnant women rarely appeared in the hospital for reasons other than delivery. Two trends responsible for that shift are advanced maternal age and rising rates of obesity, defined as a body mass index of >30.
The birth rate for women ages 35-44 has continued to rise, and that has brought new challenges to treating pregnancy, many of which result in hospital visits.1
OB/GYN hospitalist Robert Olson, MD, SFHM, has witnessed the winds of change firsthand. “Older patients are more likely to have medical conditions such as hypertension and diabetes, as well as the unusual medical problems such as status post heart attack, status post heart transplant, status post chemotherapy for cancer, as well as being on medications for chronic disease,” says Dr. Olson, who practices in Bellingham, Wash., and is the founding president of the Society of OB/GYN Hospitalists.
According to the National Health and Nutrition Examination Survey, more than one third of U.S. women are obese and more than half of all pregnant women are overweight or obese and therefore prone to complications that send them to the hospital, including gestational diabetes, hypertension, and preeclampsia.3
As an inpatient, obese pregnant women present their own challenges, including increased risk of thromboembolism. When treating this type of patient, remember pneumatic compression devices are recommended if the patient will be immobile for any length of time.4
Click here to listen to Dr. Carolyn Zelop discuss cardiovascular emergencies in pregnant patients.
Clinicians might also have significant difficulty intubating the overweight mother-to-be. Whether for cesarean section, other surgical procedures, or an acute medical crisis, physicians must approach intubation with caution as a result of excessive adipose tissue, obscured landmarks, difficulty positioning, and edema, as well as progesterone-induced relaxation of the sphincter between the esophagus and stomach.5 It is vital to make use of your most experienced staff when intubating this special needs patient. TH
Maybelle Cowan-Lincoln is a freelance writer in New Jersey.
References
- Martin JA, Hamilton BE, Ventura SJ, et al. National Vital Statistics Reports: Volume 62, Number 1. June 28, 2013. Available at: http://www.cdc.gov/nchs/data/nvsr/nvsr62/nvsr62_01.pdf. Accessed October 6, 2014.
- Olson, Robert. Founding president, Society of OB/GYN Hospitalists; OB/GYN hospitalist, PeaceHealth St. Joseph Medical Center, Bellingham, Wash. E-mail interview. November 13, 2013.
- Leddy MA, Power ML, Schulkin J. The impact of maternal obesity on maternal and fetal health. Rev Obstet Gynecol. 2008;1(4):170-178.
- ACOG committee opinion number 549. Obstet Gynecol. 2013;121(1):213-217.
- Zelop, Carolyn M. Director, perinatal ultrasound and research, Valley Hospital, Ridgewood, N.J. Telephone interview. October 30, 2013.
Twenty years ago, pregnant women rarely appeared in the hospital for reasons other than delivery. Two trends responsible for that shift are advanced maternal age and rising rates of obesity, defined as a body mass index of >30.
The birth rate for women ages 35-44 has continued to rise, and that has brought new challenges to treating pregnancy, many of which result in hospital visits.1
OB/GYN hospitalist Robert Olson, MD, SFHM, has witnessed the winds of change firsthand. “Older patients are more likely to have medical conditions such as hypertension and diabetes, as well as the unusual medical problems such as status post heart attack, status post heart transplant, status post chemotherapy for cancer, as well as being on medications for chronic disease,” says Dr. Olson, who practices in Bellingham, Wash., and is the founding president of the Society of OB/GYN Hospitalists.
According to the National Health and Nutrition Examination Survey, more than one third of U.S. women are obese and more than half of all pregnant women are overweight or obese and therefore prone to complications that send them to the hospital, including gestational diabetes, hypertension, and preeclampsia.3
As an inpatient, obese pregnant women present their own challenges, including increased risk of thromboembolism. When treating this type of patient, remember pneumatic compression devices are recommended if the patient will be immobile for any length of time.4
Click here to listen to Dr. Carolyn Zelop discuss cardiovascular emergencies in pregnant patients.
Clinicians might also have significant difficulty intubating the overweight mother-to-be. Whether for cesarean section, other surgical procedures, or an acute medical crisis, physicians must approach intubation with caution as a result of excessive adipose tissue, obscured landmarks, difficulty positioning, and edema, as well as progesterone-induced relaxation of the sphincter between the esophagus and stomach.5 It is vital to make use of your most experienced staff when intubating this special needs patient. TH
Maybelle Cowan-Lincoln is a freelance writer in New Jersey.
References
- Martin JA, Hamilton BE, Ventura SJ, et al. National Vital Statistics Reports: Volume 62, Number 1. June 28, 2013. Available at: http://www.cdc.gov/nchs/data/nvsr/nvsr62/nvsr62_01.pdf. Accessed October 6, 2014.
- Olson, Robert. Founding president, Society of OB/GYN Hospitalists; OB/GYN hospitalist, PeaceHealth St. Joseph Medical Center, Bellingham, Wash. E-mail interview. November 13, 2013.
- Leddy MA, Power ML, Schulkin J. The impact of maternal obesity on maternal and fetal health. Rev Obstet Gynecol. 2008;1(4):170-178.
- ACOG committee opinion number 549. Obstet Gynecol. 2013;121(1):213-217.
- Zelop, Carolyn M. Director, perinatal ultrasound and research, Valley Hospital, Ridgewood, N.J. Telephone interview. October 30, 2013.
Twenty years ago, pregnant women rarely appeared in the hospital for reasons other than delivery. Two trends responsible for that shift are advanced maternal age and rising rates of obesity, defined as a body mass index of >30.
The birth rate for women ages 35-44 has continued to rise, and that has brought new challenges to treating pregnancy, many of which result in hospital visits.1
OB/GYN hospitalist Robert Olson, MD, SFHM, has witnessed the winds of change firsthand. “Older patients are more likely to have medical conditions such as hypertension and diabetes, as well as the unusual medical problems such as status post heart attack, status post heart transplant, status post chemotherapy for cancer, as well as being on medications for chronic disease,” says Dr. Olson, who practices in Bellingham, Wash., and is the founding president of the Society of OB/GYN Hospitalists.
According to the National Health and Nutrition Examination Survey, more than one third of U.S. women are obese and more than half of all pregnant women are overweight or obese and therefore prone to complications that send them to the hospital, including gestational diabetes, hypertension, and preeclampsia.3
As an inpatient, obese pregnant women present their own challenges, including increased risk of thromboembolism. When treating this type of patient, remember pneumatic compression devices are recommended if the patient will be immobile for any length of time.4
Click here to listen to Dr. Carolyn Zelop discuss cardiovascular emergencies in pregnant patients.
Clinicians might also have significant difficulty intubating the overweight mother-to-be. Whether for cesarean section, other surgical procedures, or an acute medical crisis, physicians must approach intubation with caution as a result of excessive adipose tissue, obscured landmarks, difficulty positioning, and edema, as well as progesterone-induced relaxation of the sphincter between the esophagus and stomach.5 It is vital to make use of your most experienced staff when intubating this special needs patient. TH
Maybelle Cowan-Lincoln is a freelance writer in New Jersey.
References
- Martin JA, Hamilton BE, Ventura SJ, et al. National Vital Statistics Reports: Volume 62, Number 1. June 28, 2013. Available at: http://www.cdc.gov/nchs/data/nvsr/nvsr62/nvsr62_01.pdf. Accessed October 6, 2014.
- Olson, Robert. Founding president, Society of OB/GYN Hospitalists; OB/GYN hospitalist, PeaceHealth St. Joseph Medical Center, Bellingham, Wash. E-mail interview. November 13, 2013.
- Leddy MA, Power ML, Schulkin J. The impact of maternal obesity on maternal and fetal health. Rev Obstet Gynecol. 2008;1(4):170-178.
- ACOG committee opinion number 549. Obstet Gynecol. 2013;121(1):213-217.
- Zelop, Carolyn M. Director, perinatal ultrasound and research, Valley Hospital, Ridgewood, N.J. Telephone interview. October 30, 2013.
No survival benefit of RAI seen in early-stage thyroid cancer
CORONADO, CALIF. – In a large cohort of patients with differentiated thyroid cancer, the use of radioactive iodine was associated with improved disease-specific survival in those with advanced disease but not in those with papillary thyroid microcarcinoma.
“Everything in medicine is a risk-benefit balance,” lead author Dr. Ryan K. Orosco said in an interview in advance of the annual meeting of the American Thyroid Association, where the work was presented. “Any two patients that receive radioactive iodine (RAI) for differentiated thyroid cancer are likely to have different survival benefit from that therapy. This study provides a quantitative comparison of the impact of RAI in various patient subgroups.”
In one of the largest studies of its kind, Dr. Orosco of the division of head and neck surgery at the University of California, San Diego, and his associates identified 85,740 patients with differentiated thyroid carcinoma from the Surveillance, Epidemiology, and End Results database from 1973 through 2009. They used multivariate analyses to explore the association between RAI and cancer-specific survival in 149 population subgroups, controlling for age, decade of diagnosis, race, gender, tumor type, nodal involvement, metastasis stage, and RAI therapy.
More than three-quarters of the patients (78%) were female, 68% were white, their mean age at diagnosis was 46 years, and the median follow-up time was 85 months. The researchers found that nearly half of patients (43%) received RAI. By American Joint Committee on Cancer stage, RAI was used in 55% of stage I patients, 41% of stage II patients, 94% of stage III patients, and 85% of stage IV patients. In addition, 42% of patients with T1a disease and 88% of those with T4 disease received RAI.
Use of RAI was positively associated with survival in the overall cohort (hazard ratio 1.3; P = .002), while statistically significant HRs for RAI were observed in 49 population subgroups. In patients with metastatic disease, use of RAI was associated with a decreased risk for disease-specific mortality (HR range of 2.28-3.82). Protective effects of RAI were also observed in patients with regional metastases (HR 1.4-1.9), those with T3-positive tumors (HR 1.36-1.39), those with T4 tumors (HR 1.85), and in those with stage IV disease (HR 1.47-1.73).
Dr. Orosco and his associates observed a negative effect of RAI in patients with macropapillary carcinoma. Specifically, those with T1a disease had an increased likelihood of thyroid cancer–specific mortality (HR .13; P less than .001), while similar associations were seen in multiple subgroups of patients with T1a disease (HR 0.04-0.25). No statistically significant effects of RAI were observed in patients with T1b or T2 tumors.
“RAI appears to offer the best survival impact in patients with advanced differentiated thyroid carcinoma,” Dr. Orosco said. “Its use in early-stage patients should be carefully considered.”
In their abstract, the researchers noted that the findings “might help clinicians personalize RAI therapy to specific differentiated thyroid cancer populations – offering treatment in patients most likely to benefit, and sparing others unnecessary costs and potential side effects.”
Dr. Orosco acknowledged certain limitations of the study, including the fact that the SEER database does not contain details about each patient’s surgery, the dose of RAI used, other comorbidities, or data on cancer recurrence. “This study does not attempt to explore the reasons behind the apparent survival disadvantage seen in patients with T1a disease,” he said. “We don’t know exactly why early-stage patients have an increased risk of disease-specific mortality when RAI is used. Additional work is needed to explore this further.”
Dr. Orosco reported having no financial disclosures.
On Twitter @dougbrunk
CORONADO, CALIF. – In a large cohort of patients with differentiated thyroid cancer, the use of radioactive iodine was associated with improved disease-specific survival in those with advanced disease but not in those with papillary thyroid microcarcinoma.
“Everything in medicine is a risk-benefit balance,” lead author Dr. Ryan K. Orosco said in an interview in advance of the annual meeting of the American Thyroid Association, where the work was presented. “Any two patients that receive radioactive iodine (RAI) for differentiated thyroid cancer are likely to have different survival benefit from that therapy. This study provides a quantitative comparison of the impact of RAI in various patient subgroups.”
In one of the largest studies of its kind, Dr. Orosco of the division of head and neck surgery at the University of California, San Diego, and his associates identified 85,740 patients with differentiated thyroid carcinoma from the Surveillance, Epidemiology, and End Results database from 1973 through 2009. They used multivariate analyses to explore the association between RAI and cancer-specific survival in 149 population subgroups, controlling for age, decade of diagnosis, race, gender, tumor type, nodal involvement, metastasis stage, and RAI therapy.
More than three-quarters of the patients (78%) were female, 68% were white, their mean age at diagnosis was 46 years, and the median follow-up time was 85 months. The researchers found that nearly half of patients (43%) received RAI. By American Joint Committee on Cancer stage, RAI was used in 55% of stage I patients, 41% of stage II patients, 94% of stage III patients, and 85% of stage IV patients. In addition, 42% of patients with T1a disease and 88% of those with T4 disease received RAI.
Use of RAI was positively associated with survival in the overall cohort (hazard ratio 1.3; P = .002), while statistically significant HRs for RAI were observed in 49 population subgroups. In patients with metastatic disease, use of RAI was associated with a decreased risk for disease-specific mortality (HR range of 2.28-3.82). Protective effects of RAI were also observed in patients with regional metastases (HR 1.4-1.9), those with T3-positive tumors (HR 1.36-1.39), those with T4 tumors (HR 1.85), and in those with stage IV disease (HR 1.47-1.73).
Dr. Orosco and his associates observed a negative effect of RAI in patients with macropapillary carcinoma. Specifically, those with T1a disease had an increased likelihood of thyroid cancer–specific mortality (HR .13; P less than .001), while similar associations were seen in multiple subgroups of patients with T1a disease (HR 0.04-0.25). No statistically significant effects of RAI were observed in patients with T1b or T2 tumors.
“RAI appears to offer the best survival impact in patients with advanced differentiated thyroid carcinoma,” Dr. Orosco said. “Its use in early-stage patients should be carefully considered.”
In their abstract, the researchers noted that the findings “might help clinicians personalize RAI therapy to specific differentiated thyroid cancer populations – offering treatment in patients most likely to benefit, and sparing others unnecessary costs and potential side effects.”
Dr. Orosco acknowledged certain limitations of the study, including the fact that the SEER database does not contain details about each patient’s surgery, the dose of RAI used, other comorbidities, or data on cancer recurrence. “This study does not attempt to explore the reasons behind the apparent survival disadvantage seen in patients with T1a disease,” he said. “We don’t know exactly why early-stage patients have an increased risk of disease-specific mortality when RAI is used. Additional work is needed to explore this further.”
Dr. Orosco reported having no financial disclosures.
On Twitter @dougbrunk
CORONADO, CALIF. – In a large cohort of patients with differentiated thyroid cancer, the use of radioactive iodine was associated with improved disease-specific survival in those with advanced disease but not in those with papillary thyroid microcarcinoma.
“Everything in medicine is a risk-benefit balance,” lead author Dr. Ryan K. Orosco said in an interview in advance of the annual meeting of the American Thyroid Association, where the work was presented. “Any two patients that receive radioactive iodine (RAI) for differentiated thyroid cancer are likely to have different survival benefit from that therapy. This study provides a quantitative comparison of the impact of RAI in various patient subgroups.”
In one of the largest studies of its kind, Dr. Orosco of the division of head and neck surgery at the University of California, San Diego, and his associates identified 85,740 patients with differentiated thyroid carcinoma from the Surveillance, Epidemiology, and End Results database from 1973 through 2009. They used multivariate analyses to explore the association between RAI and cancer-specific survival in 149 population subgroups, controlling for age, decade of diagnosis, race, gender, tumor type, nodal involvement, metastasis stage, and RAI therapy.
More than three-quarters of the patients (78%) were female, 68% were white, their mean age at diagnosis was 46 years, and the median follow-up time was 85 months. The researchers found that nearly half of patients (43%) received RAI. By American Joint Committee on Cancer stage, RAI was used in 55% of stage I patients, 41% of stage II patients, 94% of stage III patients, and 85% of stage IV patients. In addition, 42% of patients with T1a disease and 88% of those with T4 disease received RAI.
Use of RAI was positively associated with survival in the overall cohort (hazard ratio 1.3; P = .002), while statistically significant HRs for RAI were observed in 49 population subgroups. In patients with metastatic disease, use of RAI was associated with a decreased risk for disease-specific mortality (HR range of 2.28-3.82). Protective effects of RAI were also observed in patients with regional metastases (HR 1.4-1.9), those with T3-positive tumors (HR 1.36-1.39), those with T4 tumors (HR 1.85), and in those with stage IV disease (HR 1.47-1.73).
Dr. Orosco and his associates observed a negative effect of RAI in patients with macropapillary carcinoma. Specifically, those with T1a disease had an increased likelihood of thyroid cancer–specific mortality (HR .13; P less than .001), while similar associations were seen in multiple subgroups of patients with T1a disease (HR 0.04-0.25). No statistically significant effects of RAI were observed in patients with T1b or T2 tumors.
“RAI appears to offer the best survival impact in patients with advanced differentiated thyroid carcinoma,” Dr. Orosco said. “Its use in early-stage patients should be carefully considered.”
In their abstract, the researchers noted that the findings “might help clinicians personalize RAI therapy to specific differentiated thyroid cancer populations – offering treatment in patients most likely to benefit, and sparing others unnecessary costs and potential side effects.”
Dr. Orosco acknowledged certain limitations of the study, including the fact that the SEER database does not contain details about each patient’s surgery, the dose of RAI used, other comorbidities, or data on cancer recurrence. “This study does not attempt to explore the reasons behind the apparent survival disadvantage seen in patients with T1a disease,” he said. “We don’t know exactly why early-stage patients have an increased risk of disease-specific mortality when RAI is used. Additional work is needed to explore this further.”
Dr. Orosco reported having no financial disclosures.
On Twitter @dougbrunk
AT THE ATA ANNUAL MEETING
Key clinical point: Radioactive iodine appears to offer the best survival impact in patients with advanced differentiated thyroid carcinoma.
Major finding: In patients with metastatic disease, use of RAI was associated with a decreased risk for disease-specific mortality (HR range of 2.28-3.82). However, those with T1a disease had an increased likelihood of thyroid cancer-specific mortality (HR .13; P less than .001), while similar associations were seen in multiple subgroups of patients with T1a disease (HR .04-.25).
Data source: An analysis of 85,740 patients with differentiated thyroid carcinoma from the Surveillance, Epidemiology, and End Results database from 1973 through 2009.
Disclosures: Dr. Orosco reported having no financial disclosures.
Sulfur Spring Dermatitis
Sulfur spring dermatitis is characterized by multiple punched-out erosions and pits. In prior case reports, patients often presented with painful swollen lesions that developed within 24 hours of bathing in hot sulfur springs.1 Because spa therapy and thermal spring baths are common in modern society, dermatologists should be aware of sulfur spring dermatitis as a potential adverse effect.
Case Report
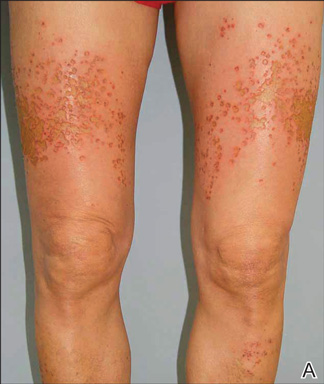
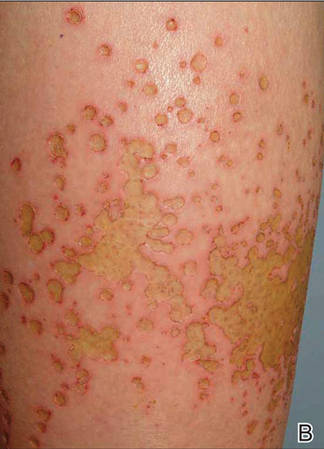
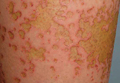
A healthy 65-year-old man presented with painful skin lesions on the legs that developed after bathing for 25 minutes in a hot sulfur spring 1 day prior. The patient had no history of dermatologic disease. He reported a 10-year history of bathing in a hot sulfur spring for 20 minutes every 3 days in the winter. This time, he bathed 5 minutes longer than usual. No skin condition was noted prior to bathing, but he reported feeling a tickling sensation and scratching the legs while he was immersed in the water. One hour after bathing, he noted confluent, punched-out, round ulcers with peripheral erythema on the thighs and shins (Figure 1).
|
|
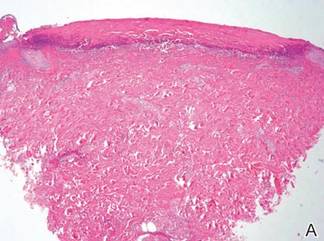
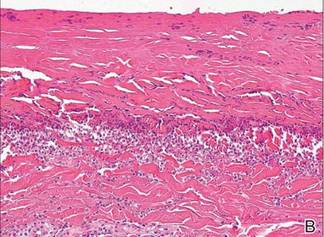
A skin biopsy revealed sharply demarcated, homogeneous coagulation necrosis of the epidermis. Many neutrophils were present under the necrosis (Figure 2). Periodic acid–Schiff and acid-fast stains were negative for infectious organisms, and a skin tissue culture yielded negative results. Intensive wound care was started with nitrofurazone ointment 0.2%. The ulcers healed gradually in the following months with scar formation and hyperpigmentation.
Comment
Thermal sulfur baths are a form of balneotherapy promoted in many cultures for improvement of skin conditions; however, certain uncommon skin problems may occur after bathing in hot sulfur springs.2 In particular, sulfur spring dermatitis is a potential adverse effect.
Thermal sulfur water is known to exert anti-inflammatory, keratoplastic, and antipruriginous effects. As a result, it often is used in many cultures as an alternative treatment of various skin conditions.2-4 Moreover, thermal sulfur baths are popular in northeastern Asian countries for their effects on mental health.5 Hot springs in northern Taiwan, which contain large amounts of hydrogen sulfide, sulfate, and sulfur differ from other thermal springs in that they are rather acidic in nature and release geothermal energy from volcanic activity.6 In addition to hot sulfur springs, there are neutral salt and CO2 springs in Taiwan.5 However, spring dermatitis has only been associated with bathing in hot sulfur springs due to high concentrations of hydrogen sulfide that break down keratin and cause dissolution of the stratum corneum.7
The incidence of sulfur spring dermatitis is unknown. Although the largest known case series reported 44 cases occurring within a decade in Taiwan,1 it is rarely seen in our daily practice. Previously reported cases of sulfur spring dermatitis noted clinical findings of swelling of the affected area followed by punched-out erosions with surrounding erythema. Most lesions gradually healed with dry brownish crusts. A patch test with sulfur spring water and sulfur compounds showed negative results; therefore, the mechanism is unlikely to be allergic reaction.1 The clinical differential diagnosis includes factitious ulcers as well as viral and fungal infections. A tissue culture should be performed to exclude infectious conditions.
This characteristic skin disease does not present in all individuals after bathing in hot sulfur springs. Lesions may present anywhere on the body with a predilection for skin folds, including the penis and scrotum. Preexisting skin conditions such as pruritus and xerosis are considered to be contributing factors. The possible etiology of sulfur spring dermatitis may be acid irritation from the unstable amount of soluble sulfur in the water, which is enhanced by the heat.1 In our patient, no prior skin disease was noted, but he scratched the skin on the thighs while bathing, which may have contributed to the development of lesions in this area rather than in the skin folds.
The skin biopsy specimen demonstrated epidermal coagulation necrosis, mild superficial dermal damage, and preservation of the pilosebaceous appendages. The ulcers were painful during healing and resolved with scarring and hyperpigmentation. The histopathologic findings and clinical course in our patient were similar to cases of superficial second-degree burns.8 It is possible that the keratoplastic effect of sulfur at high concentrations along with thermal water caused the skin condition.
Conclusion
Individuals who engage in thermal sulfur baths should be aware of potential adverse effects such as sulfur spring dermatitis, especially those with preexisting skin disorders.
1. Sun CC, Sue MS. Sulfur spring dermatitis. Contact Dermatitis. 1995;32:31-34.
2. Matz H, Orion E, Wolf R. Balneotherapy in dermatology. Dermatol Ther. 2003;16:132-140.
3. Leslie KS, Millington GW, Levell NJ. Sulphur and skin: from Satan to Saddam! J Cosmet Dermatol. 2004;3:94-98.
4. Millikan LE. Unapproved treatments or indications in dermatology: physical therapy including balneotherapy. Clin Dermatol. 2000;18:125-129.
5. Nirei H, Furuno K, Kusuda T. Medical geology in Japan. In: Selinus O, Finkelman RB, Centeno JA, eds. Medical Geology: A Regional Synthesis. New York, NY: Springer; 2010:329-354.
6. Liu CM, Song SR, Chen YL, et al. Characteristics and origins of hot springs in the Tatun Volcano Group in northern Taiwan. Terr Atmos Ocean Sci. 2011;22:475-489.
7. Lin AN, Reimer RJ, Carter DM. Sulfur revisited. J Am Acad Dermatol. 1988;18:553-558.
8. Weedon D. Reaction to physical agents. In: Weedon D. Weedon’s Skin Pathology. 3rd ed. London, England: Churchill Livingstone, Elsevier Health; 2010:525-540.
Sulfur spring dermatitis is characterized by multiple punched-out erosions and pits. In prior case reports, patients often presented with painful swollen lesions that developed within 24 hours of bathing in hot sulfur springs.1 Because spa therapy and thermal spring baths are common in modern society, dermatologists should be aware of sulfur spring dermatitis as a potential adverse effect.
Case Report
A healthy 65-year-old man presented with painful skin lesions on the legs that developed after bathing for 25 minutes in a hot sulfur spring 1 day prior. The patient had no history of dermatologic disease. He reported a 10-year history of bathing in a hot sulfur spring for 20 minutes every 3 days in the winter. This time, he bathed 5 minutes longer than usual. No skin condition was noted prior to bathing, but he reported feeling a tickling sensation and scratching the legs while he was immersed in the water. One hour after bathing, he noted confluent, punched-out, round ulcers with peripheral erythema on the thighs and shins (Figure 1).
|
|
A skin biopsy revealed sharply demarcated, homogeneous coagulation necrosis of the epidermis. Many neutrophils were present under the necrosis (Figure 2). Periodic acid–Schiff and acid-fast stains were negative for infectious organisms, and a skin tissue culture yielded negative results. Intensive wound care was started with nitrofurazone ointment 0.2%. The ulcers healed gradually in the following months with scar formation and hyperpigmentation.
Comment
Thermal sulfur baths are a form of balneotherapy promoted in many cultures for improvement of skin conditions; however, certain uncommon skin problems may occur after bathing in hot sulfur springs.2 In particular, sulfur spring dermatitis is a potential adverse effect.
Thermal sulfur water is known to exert anti-inflammatory, keratoplastic, and antipruriginous effects. As a result, it often is used in many cultures as an alternative treatment of various skin conditions.2-4 Moreover, thermal sulfur baths are popular in northeastern Asian countries for their effects on mental health.5 Hot springs in northern Taiwan, which contain large amounts of hydrogen sulfide, sulfate, and sulfur differ from other thermal springs in that they are rather acidic in nature and release geothermal energy from volcanic activity.6 In addition to hot sulfur springs, there are neutral salt and CO2 springs in Taiwan.5 However, spring dermatitis has only been associated with bathing in hot sulfur springs due to high concentrations of hydrogen sulfide that break down keratin and cause dissolution of the stratum corneum.7
The incidence of sulfur spring dermatitis is unknown. Although the largest known case series reported 44 cases occurring within a decade in Taiwan,1 it is rarely seen in our daily practice. Previously reported cases of sulfur spring dermatitis noted clinical findings of swelling of the affected area followed by punched-out erosions with surrounding erythema. Most lesions gradually healed with dry brownish crusts. A patch test with sulfur spring water and sulfur compounds showed negative results; therefore, the mechanism is unlikely to be allergic reaction.1 The clinical differential diagnosis includes factitious ulcers as well as viral and fungal infections. A tissue culture should be performed to exclude infectious conditions.
This characteristic skin disease does not present in all individuals after bathing in hot sulfur springs. Lesions may present anywhere on the body with a predilection for skin folds, including the penis and scrotum. Preexisting skin conditions such as pruritus and xerosis are considered to be contributing factors. The possible etiology of sulfur spring dermatitis may be acid irritation from the unstable amount of soluble sulfur in the water, which is enhanced by the heat.1 In our patient, no prior skin disease was noted, but he scratched the skin on the thighs while bathing, which may have contributed to the development of lesions in this area rather than in the skin folds.
The skin biopsy specimen demonstrated epidermal coagulation necrosis, mild superficial dermal damage, and preservation of the pilosebaceous appendages. The ulcers were painful during healing and resolved with scarring and hyperpigmentation. The histopathologic findings and clinical course in our patient were similar to cases of superficial second-degree burns.8 It is possible that the keratoplastic effect of sulfur at high concentrations along with thermal water caused the skin condition.
Conclusion
Individuals who engage in thermal sulfur baths should be aware of potential adverse effects such as sulfur spring dermatitis, especially those with preexisting skin disorders.
Sulfur spring dermatitis is characterized by multiple punched-out erosions and pits. In prior case reports, patients often presented with painful swollen lesions that developed within 24 hours of bathing in hot sulfur springs.1 Because spa therapy and thermal spring baths are common in modern society, dermatologists should be aware of sulfur spring dermatitis as a potential adverse effect.
Case Report
A healthy 65-year-old man presented with painful skin lesions on the legs that developed after bathing for 25 minutes in a hot sulfur spring 1 day prior. The patient had no history of dermatologic disease. He reported a 10-year history of bathing in a hot sulfur spring for 20 minutes every 3 days in the winter. This time, he bathed 5 minutes longer than usual. No skin condition was noted prior to bathing, but he reported feeling a tickling sensation and scratching the legs while he was immersed in the water. One hour after bathing, he noted confluent, punched-out, round ulcers with peripheral erythema on the thighs and shins (Figure 1).
|
|
A skin biopsy revealed sharply demarcated, homogeneous coagulation necrosis of the epidermis. Many neutrophils were present under the necrosis (Figure 2). Periodic acid–Schiff and acid-fast stains were negative for infectious organisms, and a skin tissue culture yielded negative results. Intensive wound care was started with nitrofurazone ointment 0.2%. The ulcers healed gradually in the following months with scar formation and hyperpigmentation.
Comment
Thermal sulfur baths are a form of balneotherapy promoted in many cultures for improvement of skin conditions; however, certain uncommon skin problems may occur after bathing in hot sulfur springs.2 In particular, sulfur spring dermatitis is a potential adverse effect.
Thermal sulfur water is known to exert anti-inflammatory, keratoplastic, and antipruriginous effects. As a result, it often is used in many cultures as an alternative treatment of various skin conditions.2-4 Moreover, thermal sulfur baths are popular in northeastern Asian countries for their effects on mental health.5 Hot springs in northern Taiwan, which contain large amounts of hydrogen sulfide, sulfate, and sulfur differ from other thermal springs in that they are rather acidic in nature and release geothermal energy from volcanic activity.6 In addition to hot sulfur springs, there are neutral salt and CO2 springs in Taiwan.5 However, spring dermatitis has only been associated with bathing in hot sulfur springs due to high concentrations of hydrogen sulfide that break down keratin and cause dissolution of the stratum corneum.7
The incidence of sulfur spring dermatitis is unknown. Although the largest known case series reported 44 cases occurring within a decade in Taiwan,1 it is rarely seen in our daily practice. Previously reported cases of sulfur spring dermatitis noted clinical findings of swelling of the affected area followed by punched-out erosions with surrounding erythema. Most lesions gradually healed with dry brownish crusts. A patch test with sulfur spring water and sulfur compounds showed negative results; therefore, the mechanism is unlikely to be allergic reaction.1 The clinical differential diagnosis includes factitious ulcers as well as viral and fungal infections. A tissue culture should be performed to exclude infectious conditions.
This characteristic skin disease does not present in all individuals after bathing in hot sulfur springs. Lesions may present anywhere on the body with a predilection for skin folds, including the penis and scrotum. Preexisting skin conditions such as pruritus and xerosis are considered to be contributing factors. The possible etiology of sulfur spring dermatitis may be acid irritation from the unstable amount of soluble sulfur in the water, which is enhanced by the heat.1 In our patient, no prior skin disease was noted, but he scratched the skin on the thighs while bathing, which may have contributed to the development of lesions in this area rather than in the skin folds.
The skin biopsy specimen demonstrated epidermal coagulation necrosis, mild superficial dermal damage, and preservation of the pilosebaceous appendages. The ulcers were painful during healing and resolved with scarring and hyperpigmentation. The histopathologic findings and clinical course in our patient were similar to cases of superficial second-degree burns.8 It is possible that the keratoplastic effect of sulfur at high concentrations along with thermal water caused the skin condition.
Conclusion
Individuals who engage in thermal sulfur baths should be aware of potential adverse effects such as sulfur spring dermatitis, especially those with preexisting skin disorders.
1. Sun CC, Sue MS. Sulfur spring dermatitis. Contact Dermatitis. 1995;32:31-34.
2. Matz H, Orion E, Wolf R. Balneotherapy in dermatology. Dermatol Ther. 2003;16:132-140.
3. Leslie KS, Millington GW, Levell NJ. Sulphur and skin: from Satan to Saddam! J Cosmet Dermatol. 2004;3:94-98.
4. Millikan LE. Unapproved treatments or indications in dermatology: physical therapy including balneotherapy. Clin Dermatol. 2000;18:125-129.
5. Nirei H, Furuno K, Kusuda T. Medical geology in Japan. In: Selinus O, Finkelman RB, Centeno JA, eds. Medical Geology: A Regional Synthesis. New York, NY: Springer; 2010:329-354.
6. Liu CM, Song SR, Chen YL, et al. Characteristics and origins of hot springs in the Tatun Volcano Group in northern Taiwan. Terr Atmos Ocean Sci. 2011;22:475-489.
7. Lin AN, Reimer RJ, Carter DM. Sulfur revisited. J Am Acad Dermatol. 1988;18:553-558.
8. Weedon D. Reaction to physical agents. In: Weedon D. Weedon’s Skin Pathology. 3rd ed. London, England: Churchill Livingstone, Elsevier Health; 2010:525-540.
1. Sun CC, Sue MS. Sulfur spring dermatitis. Contact Dermatitis. 1995;32:31-34.
2. Matz H, Orion E, Wolf R. Balneotherapy in dermatology. Dermatol Ther. 2003;16:132-140.
3. Leslie KS, Millington GW, Levell NJ. Sulphur and skin: from Satan to Saddam! J Cosmet Dermatol. 2004;3:94-98.
4. Millikan LE. Unapproved treatments or indications in dermatology: physical therapy including balneotherapy. Clin Dermatol. 2000;18:125-129.
5. Nirei H, Furuno K, Kusuda T. Medical geology in Japan. In: Selinus O, Finkelman RB, Centeno JA, eds. Medical Geology: A Regional Synthesis. New York, NY: Springer; 2010:329-354.
6. Liu CM, Song SR, Chen YL, et al. Characteristics and origins of hot springs in the Tatun Volcano Group in northern Taiwan. Terr Atmos Ocean Sci. 2011;22:475-489.
7. Lin AN, Reimer RJ, Carter DM. Sulfur revisited. J Am Acad Dermatol. 1988;18:553-558.
8. Weedon D. Reaction to physical agents. In: Weedon D. Weedon’s Skin Pathology. 3rd ed. London, England: Churchill Livingstone, Elsevier Health; 2010:525-540.
Practice Points
- The clinical findings of sulfur spring dermatitis are similar to those of a superficial second-degree burn.
- Careful evaluation of the patient’s clinical history and recognition of characteristic findings are important for correct diagnosis.
- Patients with preexisting skin disorders who engage in thermal sulfur baths should be aware of the potential adverse effect of sulfur spring dermatitis.
Five Reasons You Should Attend Hospital Medicine 2013 in Washington, D.C.
Hospital Medicine 2013 offers expert speakers, 90 educational offerings, and networking with the best and brightest hospital medicine has to offer.
Hospital Medicine 2013 offers expert speakers, 90 educational offerings, and networking with the best and brightest hospital medicine has to offer.
Hospital Medicine 2013 offers expert speakers, 90 educational offerings, and networking with the best and brightest hospital medicine has to offer.