User login
Mobilization in Severe Sepsis
Severe sepsis, defined as an infection leading to systemic inflammatory response and acute organ dysfunction, is a significant cause of morbidity and mortality.[1, 2, 3] Although it has been a condition classically attributed to patients in the intensive care unit (ICU), accumulating data suggest that a substantial proportion of patients with severe sepsis are managed by hospitalists and floor teams in non‐ICU, general ward settings.[1, 4, 5] Although the incidence of severe sepsis continues to rise both in the United States and other developed nations,[2, 6, 7] advances in early recognition, management, and care of this condition have resulted in improved rates of survival.[8] The resultant increase in a severe sepsis survivor population[6] make the long‐term sequelae of this condition an important public health problem.[9]
In both the ICU and on general wards, severe sepsis survivors suffer from decreased functional status, worsened quality of life, increased cognitive dysfunction, and sarcopenia.[4, 6, 10, 11, 12, 13, 14] Not surprisingly, many such patients are discharged to long‐term care facilities for physical rehabilitation,[15] with escalating utilization of resources[16] and cost.[17, 18] Inexpensive interventions that improve outcomes following sepsis would thus be welcomed.
It is well known that physical therapy (PT) and early mobilization are beneficial in mitigating functional decline in a number of conditions.[19, 20, 21, 22] PT can improve outcomes in several ways: prevention of bed rest deconditioning, mitigation of mechanisms that lead to sarcopenia, increased pulmonary and tissue aerobic capacity, and improved sense of well‐being. Indeed, among the population cared for in ICU settings, early mobility and PT lead to more ventilator‐free days, better functional status at discharge, shorter duration of delirium, and even a potentially reduced risk of central line‐associated bloodstream infection (CLABSI).[23, 24] However, whether initiating early PT can improve outcomes in patients with severe sepsis treated by either intensivists or hospitalists/floor teams outside the ICU is unknown.
Therefore, to better understand this phenomenon, we systematically reviewed and integrated the literature regarding early mobilization and PT for severe sepsis outside the ICU. To be more inclusive, a secondary review including populations with any infectious etiology and severe sepsis treated within the ICU was also conducted. Our review begins by providing an overview of the pathophysiology behind functional decline in severe sepsis, along with existing evidence on early mobilization efficacy in other patient populations. We then proceed with a review of the extant literature on the aforementioned topic. We conclude with an evaluation of the current evidence on the subject, along with assertions regarding future research in the area.
PATHOPHYSIOLOGY OF DISABILITY FOLLOWING HOSPITALIZATION FOR SEVERE SEPSIS
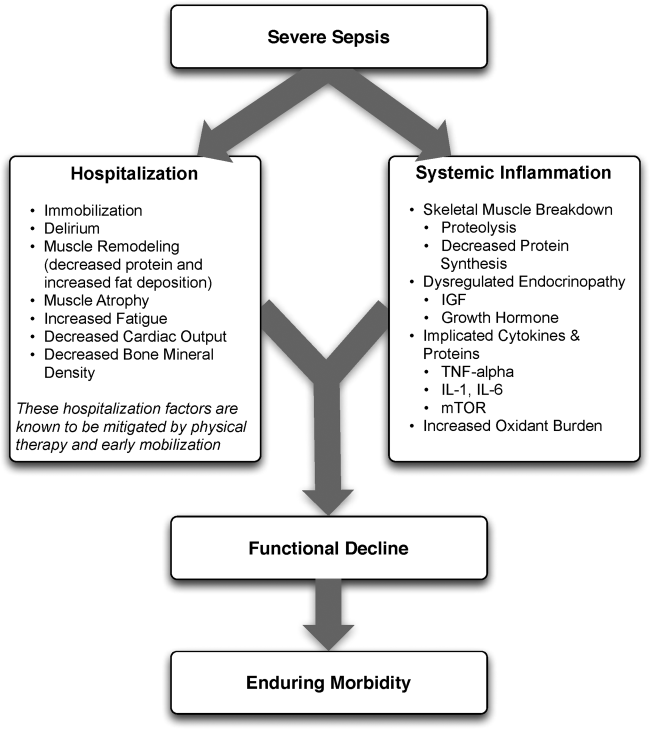
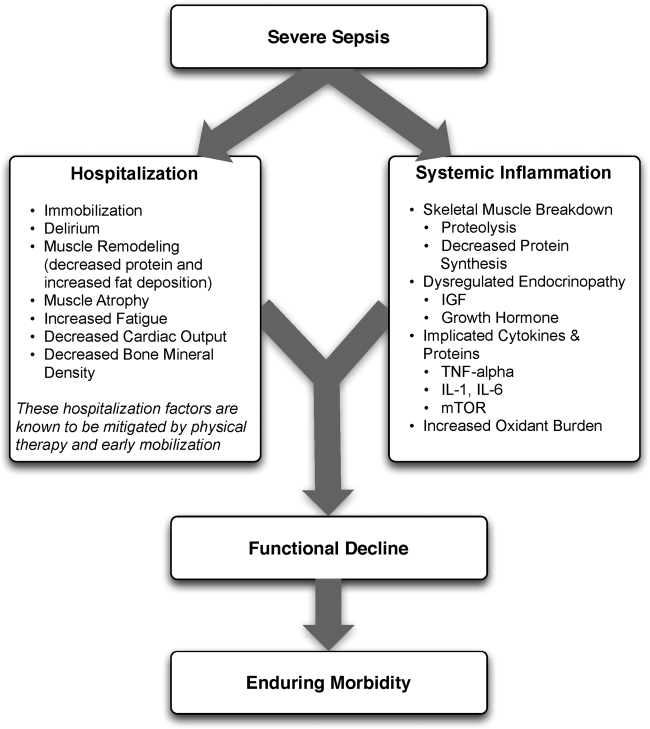
The pathophysiology behind functional decline in patients hospitalized with severe sepsis is multifactorial (Figure 1). During hospitalization, it is well known that patients suffer from restricted mobility,[25] and that this impediment is linked to poor functional outcomes.[26] Described as far back as Hippocrates,[27] more recent studies have elucidated how prolonged bed rest leads to a multitude of physiological changes that promote deconditioning.[28] Specifically, skeletal muscle atrophy and decreased protein synthesis, independent of ongoing disease processes and acute illness, have been demonstrated in both animal and human models of prolonged inactivity.[29, 30] Additionally, bed rest leading to insensible fluid losses, a decline in stroke volume and effective cardiac output, bone loss, and decreased insulin sensitivity has been reported.[28, 31] There is little doubt that the aforementioned issues pertain to severe sepsis patients outside the ICU. In fact, nearly all of the acute mechanisms driving Creditor's hazards of hospitalization are noted among patients with severe sepsis.[32]
Furthermore, several factors preceding hospitalization may increase risk of disability. For example, Covinsky et al. described a number of risk factors, such as comorbid conditions, cognitive impairment, and various psychosocial aspects such as depression and limited social support, as being associated with increased risk of functional decline.[33] Thus, both in‐hospital and prehospital factors likely combine within an individual patient's context to determine risk of physical decline.
On this backdrop and the inherent immobilization associated with hospitalization, sepsis and inflammation catalyze physiologic changes that further propagate deconditioning.[7] Implicated pathways and proteins for this process include the mammalian target of rapamycin, human growth hormone, insulin‐like growth factors, interleukin‐1, and tumor necrosis factor‐. Through several metabolic alterations, sepsis independently promotes skeletal muscle breakdown and impairs skeletal muscle synthesis.[34, 35, 36] Inflammation associated with sepsis also increases oxidant burden, further leading to muscle dysfunction and dysregulation.[7, 31, 37, 38]
EFFECTS OF PHYSICAL THERAPY AND MOBILIZATION ON CLINICAL OUTCOMES
In patients with nonsepsis conditions who are at risk for functional decline, the effectiveness of physical therapy has been studied in multiple settings with positive outcomes. For example, in hospitalized elderly patients with general deconditioning, PT‐based interventions have demonstrated reductions in length of hospital stay.[39] Additionally, exercise in healthy subjects who have been subjected to bed rest has been shown to attenuate physiological changes, and maintain plasma and red cell volume and work capacity.[40] Adequate safety and improved outcomes have also been demonstrated in the general population of critically ill patients who receive early PT and mobilization. Improved functional capacity at discharge, decreases in duration of delirium, increased ventilator‐free days, decreased risk for CLABSI, and a better general sense of well‐being following these interventions have been widely reported in the literature.[14, 19, 23, 24, 41, 42, 43, 44, 45] Interestingly, critically ill patients may have a dose‐ and time‐dependent response to PT; that is, high intensity and early onset mobility‐based interventions are often associated with more ventilator‐free time and improved functional outcomes, resulting in shorter ICU and hospital length of stay.[42, 46, 47, 48]
Moderate intensity exercise has also been shown to improve 6‐minute walking distance in patients convalescing from coronary artery bypass grafting surgery.[49] Furthermore, in the postoperative setting, patients suffering traumatic hip fractures are known to benefit from physical and occupational therapies with shorter time to ambulation and improved locomotion in the recovery period.[21, 50, 51] Among patients with stroke, PT and gait training has led to improvements in speed, gait, independence during walking, activities of daily living, and extended activities of daily living.[52, 53, 54] A recent meta‐analysis also suggested that extra PT compared to regular treatment in patients with acute and subacute conditions such as stroke and postoperative states improved mobility and quality of life, while reducing length of hospital stay.[22]
Although this evidence suggests potential benefits for PT and mobilization, it is important to note that the effect of these treatments in dissimilar populations is unknown and may not necessarily be positive. For example, a recent study examining PT and its impact on patients with hip osteoarthritis showed no clinical benefit.[55] Mobilizing patients in severe illness may be associated with important risks, including falls, worsening of their clinical status, or moral discouragement in the setting of limited capacity. Therefore, understanding which elements of mobilization efforts create the greatest impact in the context of delivery of the intervention is critical to assessing the risk, benefit, and efficacy of PT‐based interventions.
EARLY PHYSICAL THERAPY FOR SEVERE SEPSIS OUTSIDE THE ICU: LITERATURE REVIEW
Given the functional decline associated with severe sepsis and the evidence of PT efficacy in other populations, we reviewed the current literature for studies evaluating physical therapy in severe sepsis patients outside the ICU. With the assistance of medical reference librarians, we searched MEDLINE via PubMed (1950present), EMBASE (1946present), Cochrane CENTRAL Register of Controlled Trials, and the Cochrane Database of Reviews of Effectiveness (1960present via Ovid). The search was last updated in June 2014.
We searched for studies that (1) involved human patients 18 years of age, (2) included patients with a primary diagnosis of sepsis or severe sepsis being treated outside the ICU, (3) featured a primary intervention that included PT or an early mobilization‐based initiative, and (4) reported a primary clinical or functional outcome of interest. Early was defined based on the included studies' definition. To be fully inclusive, we also conducted a secondary review with inclusion criteria expanded to studies of either any infectious pathology or severe sepsis patient in the ICU that employed PT interventions.
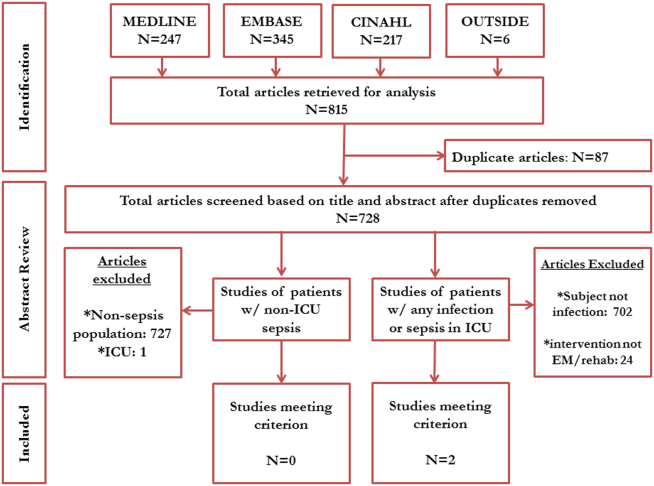
Our electronic search retrieved 815 records (Figure 2). Despite this approach, no publications met our primary inclusion criteria as we found no study that implemented a mobility intervention directed toward patients with sepsis treated outside the ICU. Our expanded secondary review included patients with any infectious pathology or those with severe sepsis in the ICU treated with PT; in this review, 2 studies met eligibility criteria.[56] In a 2003 cluster‐randomized trial, Mundy and colleagues randomized patients admitted with pneumonia to receive early PT or usual care. The outcomes of interest were hospital length of stay, mortality, number of chest radiographs, emergency department visits, and readmissions at 30 and 90 days after hospital admission. Although the study has important limitations (including patient‐level difference between trial arms, subjective definition of early mobilization), the authors found a significant decrease in length of stay among patients with pneumonia who received early PT compared to controls (5.8 vs 6.9 days, absolute difference 1.1 days, 95% confidence interval: 02.2 days). The study also reported a substantial decrease in adjusted mean hospital charges for the early mobilization group versus the usual care group ($10,159 per patient vs. $12,868 per patient, P=0.05). In the second study, Sossdorf et al. retrospectively evaluated a cohort of 999 patients with severe sepsis and septic shock and assessed whether onset and frequency of PT‐based interventions was associated with clinical benefit. After multivariate analysis, the authors reported a small mortality benefit associated with the relative number of PT interventions (hazard ratio: 0.982, P0.001).[45]
EXPLAINING THE VOID
Our integrative review of the current literature reveals a gap in our understanding of the role of early mobilization in severe sepsis both within and beyond the ICU. Given the promise of PT‐based interventions and the toll of severe sepsis, one must ask: why may this be so?
First, the understanding that severe sepsis leads to significant, long‐term consequences for survivors has only been identified recently. Thus, it is possible that the burden and consequences related to this condition have not been fully recognized in clinical settings, leading to a paucity of research and interventions. Although the association between sepsis and mortality has been known since the 1990s,[57] long‐term complications and enduring morbidity of this disease continue to be realized. Indeed, many studies delineating the longer‐term effects of sepsis have been only recently published.[6, 10, 11, 12, 13]
Second, it is likely that many clinicians ascribe to the viewpoint that severe sepsis is an ICU‐only condition, a myth that has been discounted by multiple studies.[1, 4, 5] Although our study shows a paucity of evidence in both ICU and nonICU‐based severe sepsis, almost half of severe sepsis occurs outside the ICU, carrying with it many of the same clinical implications. Additionally, increased morbidity, mortality, and resource utilization are known to be true in all patients with severe sepsis, irrespective of where they receive treatment in the hospital.[4, 5, 6] Recent evidence has also shown that severe sepsis treated on the floor may be clinically, epidemiologically, and even prognostically unique from its ICU counterpart.[5, 58, 59] Therefore, it appears that research domains with tailored interventions to both ICU and non‐ICU severe sepsis patients are important areas of inquiry for clinicians. Such research may serve the purpose of assessing impact of early mobilization and unmasking any treatment heterogeneity that may exist when dealing with severe sepsis. Though trials of PT in ICU‐based severe sepsis are underway,[60] it is prudent that these also extend beyond the ICU‐setting.
Third, variability in early mobility practices and billing documentation for severe sepsis patients may exist, adding barriers to performing high‐quality research on this topic. In fact, administrative billing records for PT may offer insufficient granularity about services provided or therapies administered, particularly in the ICU where variability in early mobilization practices have been shown despite common employment of physiotherapists.[61]
Finally, many hospitalists may believe that patients with severe sepsis are simply too sick for early mobilization or PT, possibly limiting their participation in clinical or research‐based interventions. This perception has been well described in ICU populations, where it has been well studied and shown to be false.[41, 42, 43] Nevertheless, if severe sepsis patients are viewed as relatively sick hospitalized patients, it is plausible that resistance against early mobilization interventions may exist.[62] Understanding these biases and being mindful of such barriers when conducting studies in this area would be important.
CONCLUSION AND FUTURE DIRECTIONS
The cost burdens of severe sepsis are substantial. Elixhauser et al. suggest that it is currently the single most expensive cause of acute hospitalization in the United States.[63] Importantly, a large proportion of patients with severe sepsis receive care from hospitalists and/or floor teams on the general wards. Our integrative review has demonstrated a knowledge gap when it comes to rigorous assessments of PT and mobilization treatments in patients with severe sepsis within and beyond the ICU. Existing evidence provides a strong rationale for why functional decline occurs in patients with severe sepsis. A reasonable argument for PT‐based interventions to mitigate functional decline in this subset exists, but rigorous evaluation of such interventions is necessary. Physical and mobilization‐based treatments are routinely available and efficacious in several other settings and populations. It could be rapidly deployed and potentially improve outcomes in those with severe sepsis. Research would be welcomed to establish optimal dosing, efficacy, and cost effectiveness of PT and early mobilization for severe sepsis, particularly in patients treated on the general wards by hospitalists and floor teams.
How may such a research agenda be launched? A balanced multipronged approach is necessary. First, large‐scale epidemiological data to understand variation in practice are needed. Focused studies carried out by community and academic hospitalists on septic patients treated outside the ICU are the call of the hour. These data, in turn, can help create registries that assess for risk factors, quality of treatment, and long‐term outcomes among survivors of this condition. Second, evaluation and improvement of the coding and precision of physical and occupational therapy billing records is necessary so that their added value can be assessed and tracked using administrative data. Third, targeted prospective studies and clinical trials to directly evaluate the effect of PT in well‐defined patient populations with sepsis outside the ICU are needed. In this arena, hospitalist expertise and trained physical therapists will be crucial. The focus of this work should be directed toward both short‐term and long‐term functional outcomes, as well as mortality and morbidity assessments. Fourth, these patient‐centered efforts should loop back and inform the foundational biology of severe sepsis, thus illuminating patient‐centered end points, from biomarker analysis to physiometric measurements in basic and translational research.
In conclusion, this review sheds light on the fact that interventions that may mitigate the functional and cognitive decline in survivors of severe sepsis appear underdeveloped. Although the precise benefit of such interventions remains unclear, the low‐cost, widespread availability and generalizability of PT‐based interventions make it a worthy candidate for future research. As the numbers of survivors of sepsis expand, an unmet public health need for interventions to improve the long‐term outcomes of this population exists. Hospitalists and intensivists caring for severe sepsis patients must rise to meet this need. Together, we can help improve the lives of patients afflicted with severe sepsis, wherever they may receive care in the hospital.
Acknowledgements
The authors acknowledge the efforts of medical research librarians Andy Hickner, MSI, and Marissa Conte, MSI, on this project.
Disclosures
This work was supported by the National Institutes of HealthK08, HL091249 (T.J.I.) and VA HSR&D IIR‐11109 (T.J.I.). The views expressed here are the authors' own and do not necessarily represent the views of the US government or the Department of Veterans' Affairs. The authors report no conflicts of interest.
- , . Epidemiology of sepsis: an update. Crit Care Med. 2001;29:S109–S116.
- , , , et al. Nationwide trends of severe sepsis in the 21st century (2000–2007). Chest. 2011;140:1223–1231.
- , , , et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554.
- , , , et al. Functional outcomes of general medical patients with severe sepsis. BMC Infect Dis. 2013;13:588.
- , , , et al. The epidemiology of acute organ system dysfunction from severe sepsis outside of the intensive care unit. J Hosp Med. 2013;8:243–247.
- , , , et al. Population burden of long‐term survivorship after severe sepsis in older Americans. J Am Geriatr Soc. 2012;60:1070–1077.
- , , , et al. Systemic inflammatory response syndrome increases immobility‐induced neuromuscular weakness. Crit Care Med. 2008;36:910–916.
- , , , et al. Early goal‐directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377.
- . The lingering consequences of sepsis: a hidden public health disaster? JAMA. 2010;304:1833–1834.
- , , , et al. Long‐term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787–1794.
- , , , et al. Spurious inferences about long‐term outcomes: the case of severe sepsis and geriatric conditions. Am J Respir Crit Care Med. 2012;185:835–841.
- , , , et al. Long‐term outcome and quality‐adjusted life years after severe sepsis. Crit Care Med. 2009;37:1268–1274.
- , , , et al. Long‐term mortality and quality of life in sepsis: a systematic review. Crit Care Med. 2010;38:1276–1283.
- , , , et al. Improving post‐intensive care unit neuropsychiatric outcomes: understanding cognitive effects of physical activity. Am J Respir Crit Care Med. 2012;186:1220–1228.
- , , , et al. Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med. 2012;40:754–761.
- , , , et al. Long‐term acute care hospital utilization after critical illness. JAMA. 2010;303:2253–2259.
- , , , et al. Long‐term survival and healthcare utilization outcomes attributable to sepsis and pneumonia. BMC Health Serv Res. 2012;12:432.
- , , , et al. Long‐term mortality and medical care charges in patients with severe sepsis. Crit Care Med. 2003;31:2316–2323.
- , , , et al. Early exercise in critically ill patients enhances short‐term functional recovery. Crit Care Med. 2009;37:2499–2505.
- , , , et al. Exercise‐based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2011;(7):CD001800.
- , , , et al. What is the role of timing in the surgical and rehabilitative care of community‐dwelling older persons with acute hip fracture? Arch Intern Med. 1997;157:513–520.
- , , . Extra physical therapy reduces patient length of stay and improves functional outcomes and quality of life in people with acute or subacute conditions: a systematic review. Arch Phys Med Rehabilil. 2011;92:1490–1500.
- , , . Reduction of intensive care unit length of stay: the case of early mobilization. Health Care Manag (Frederick). 2014;33:128–135.
- , , , et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373:1874–1882.
- , , , et al. Hospitalization, restricted activity, and the development of disability among older persons. JAMA. 2004;292:2115–2124.
- , , , et al. Low mobility during hospitalization and functional decline in older adults. J Am Geriatr Soc. 2011;59:266–273.
- , . The Medical Works of Hippocrates. Oxford, United Kingdom: Blackwell; 1950.
- , , . An overview of the issues: physiological effects of bed rest and restricted physical activity. Med Sci Sports Exerc. 1997;29:187–190.
- , , , et al. Prolonged bed rest decreases skeletal muscle and whole body protein synthesis. Am J Physiol. 1996;270:E627–E633.
- , . Metabolic consequences of muscle disuse atrophy. J Nutr. 2005;135:1824S–1828S.
- . Inactivity and inflammation in the critically ill patient. Crit Care Clin. 2007;23:21–34.
- . Hazards of hospitalization of the elderly. Ann Intern Med. 1993;118:219–223.
- , , . Hospitalization‐associated disability: “She was probably able to ambulate, but I'm not sure”. JAMA. 2011;306:1782–1793.
- , , , et al. A sustained rat model for studying the long‐lasting catabolic state of sepsis. Infect Immun. 1999;67:1079–1085.
- . Regulation of skeletal muscle protein turnover during sepsis. Curr Opin Clin Nutr. Metab Care. 1998;1:217–224.
- , , . Regulation of muscle protein synthesis during sepsis and inflammation. Am J Physiol Endocrinol Metab. 2007;293:E453–E459.
- , . From muscle disuse to myopathy in COPD: potential contribution of oxidative stress. Eur Respir J. 2005;26:703–719.
- , , . Oxidative stress and gene expression in sepsis. Br J Anaesth. 2003;90:221–232.
- , , , et al. Early ambulation and length of stay in older adults hospitalized for acute illness. Arch Intern Med. 2010;170:1942–1943.
- . Intensive exercise training during bed rest attenuates deconditioning. Med Sci Sports Exerc. 1997;29:207–215.
- , , , et al. Early activity is feasible and safe in respiratory failure patients. Crit Care Med. 2007;35:139–145.
- , , , et al. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med. 2008;36:2238–2243.
- . Clinical trials of early mobilization of critically ill patients. Crit Care Med. 2009;37:S442–S447.
- . Mobilizing patients in the intensive care unit: improving neuromuscular weakness and physical function. JAMA. 2008;300:1685–1690.
- , , , et al. Potential effect of physiotherapeutic treatment on mortality rate in patients with severe sepsis and septic shock: a retrospective cohort analysis. J Crit Care. 2013;28:954–958.
- , , , et al. Effects of physical training on functional status in patients with prolonged mechanical ventilation. Phys Ther. 2006;86:1271–1281.
- , , , et al. Impact of whole‐body rehabilitation in patients receiving chronic mechanical ventilation. Crit Care Med. 2005;33:2259–2265.
- . Rehabilitation of patients admitted to a respiratory intensive care unit. Arch Phys Med Rehabil. 1998;79:849–854.
- , , , et al. Supervised moderate intensity exercise improves distance walked at hospital discharge following coronary artery bypass graft surgery—a randomised controlled trial. Heart Lung Circ. 2008;17:129–138.
- , , , et al. Systematic review of hip fracture rehabilitation practices in the elderly. Arch Phys Med Rehabil. 2009;90:246–262.
- , , , et al. Physical therapy and mobility 2 and 6 months after hip fracture. J Am Geriatr Soc. 2004;52:1114–1120.
- , , , et al. Physical fitness training for stroke patients. Cochrane Database Syst Rev. 2011;(11):CD003316.
- , , , et al. Effects of augmented exercise therapy on outcome of gait and gait‐related activities in the first 6 months after stroke: a meta‐analysis. Stroke. 2011;42:3311–3315.
- , , , et al. Effects of augmented exercise therapy time after stroke: a meta‐analysis. Stroke. 2004;35:2529–2539.
- , , , et al. Effect of physical therapy on pain and function in patients with hip osteoarthritis: a randomized clinical trial. JAMA. 2014;311:1987–1997.
- , , , et al. Early mobilization of patients hospitalized with community‐acquired pneumonia. Chest. 2003;124:883–889.
- , , , et al. Magnitude and duration of the effect of sepsis on survival. Department of Veterans Affairs Systemic Sepsis Cooperative Studies Group. JAMA. 1997;277:1058–1063.
- , , , et al. Epidemiology of sepsis in Victoria, Australia. Crit Care Med. 2005;33:71–80.
- , , , et al. Sepsis incidence and outcome: contrasting the intensive care unit with the hospital ward. Crit Care Med. 2007;35:1284–1289.
- , , . Early rehabilitation in sepsis: a prospective randomised controlled trial investigating functional and physiological outcomes The i‐PERFORM Trial (Protocol Article). BMC Anesthesiol. 2011;11:21.
- , , , et al. TEAM: a prospective multi‐centre cohort study of early activity and mobilisation in ICU. In: American Thoracic Society 2013 International Conference; May 17–22, 2013; Philadelphia, PA. Am J Respir Crit Care Med. 2013;187:A3625.
- , , , et al. Improving long‐term outcomes after discharge from intensive care unit: report from a stakeholders' conference. Crit Care Med. 2012;40:502–509.
- , , . Septicemia in U.S. hospitals, 2009: statistical brief #122. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville, MD; 2006.
Severe sepsis, defined as an infection leading to systemic inflammatory response and acute organ dysfunction, is a significant cause of morbidity and mortality.[1, 2, 3] Although it has been a condition classically attributed to patients in the intensive care unit (ICU), accumulating data suggest that a substantial proportion of patients with severe sepsis are managed by hospitalists and floor teams in non‐ICU, general ward settings.[1, 4, 5] Although the incidence of severe sepsis continues to rise both in the United States and other developed nations,[2, 6, 7] advances in early recognition, management, and care of this condition have resulted in improved rates of survival.[8] The resultant increase in a severe sepsis survivor population[6] make the long‐term sequelae of this condition an important public health problem.[9]
In both the ICU and on general wards, severe sepsis survivors suffer from decreased functional status, worsened quality of life, increased cognitive dysfunction, and sarcopenia.[4, 6, 10, 11, 12, 13, 14] Not surprisingly, many such patients are discharged to long‐term care facilities for physical rehabilitation,[15] with escalating utilization of resources[16] and cost.[17, 18] Inexpensive interventions that improve outcomes following sepsis would thus be welcomed.
It is well known that physical therapy (PT) and early mobilization are beneficial in mitigating functional decline in a number of conditions.[19, 20, 21, 22] PT can improve outcomes in several ways: prevention of bed rest deconditioning, mitigation of mechanisms that lead to sarcopenia, increased pulmonary and tissue aerobic capacity, and improved sense of well‐being. Indeed, among the population cared for in ICU settings, early mobility and PT lead to more ventilator‐free days, better functional status at discharge, shorter duration of delirium, and even a potentially reduced risk of central line‐associated bloodstream infection (CLABSI).[23, 24] However, whether initiating early PT can improve outcomes in patients with severe sepsis treated by either intensivists or hospitalists/floor teams outside the ICU is unknown.
Therefore, to better understand this phenomenon, we systematically reviewed and integrated the literature regarding early mobilization and PT for severe sepsis outside the ICU. To be more inclusive, a secondary review including populations with any infectious etiology and severe sepsis treated within the ICU was also conducted. Our review begins by providing an overview of the pathophysiology behind functional decline in severe sepsis, along with existing evidence on early mobilization efficacy in other patient populations. We then proceed with a review of the extant literature on the aforementioned topic. We conclude with an evaluation of the current evidence on the subject, along with assertions regarding future research in the area.
PATHOPHYSIOLOGY OF DISABILITY FOLLOWING HOSPITALIZATION FOR SEVERE SEPSIS
The pathophysiology behind functional decline in patients hospitalized with severe sepsis is multifactorial (Figure 1). During hospitalization, it is well known that patients suffer from restricted mobility,[25] and that this impediment is linked to poor functional outcomes.[26] Described as far back as Hippocrates,[27] more recent studies have elucidated how prolonged bed rest leads to a multitude of physiological changes that promote deconditioning.[28] Specifically, skeletal muscle atrophy and decreased protein synthesis, independent of ongoing disease processes and acute illness, have been demonstrated in both animal and human models of prolonged inactivity.[29, 30] Additionally, bed rest leading to insensible fluid losses, a decline in stroke volume and effective cardiac output, bone loss, and decreased insulin sensitivity has been reported.[28, 31] There is little doubt that the aforementioned issues pertain to severe sepsis patients outside the ICU. In fact, nearly all of the acute mechanisms driving Creditor's hazards of hospitalization are noted among patients with severe sepsis.[32]

Furthermore, several factors preceding hospitalization may increase risk of disability. For example, Covinsky et al. described a number of risk factors, such as comorbid conditions, cognitive impairment, and various psychosocial aspects such as depression and limited social support, as being associated with increased risk of functional decline.[33] Thus, both in‐hospital and prehospital factors likely combine within an individual patient's context to determine risk of physical decline.
On this backdrop and the inherent immobilization associated with hospitalization, sepsis and inflammation catalyze physiologic changes that further propagate deconditioning.[7] Implicated pathways and proteins for this process include the mammalian target of rapamycin, human growth hormone, insulin‐like growth factors, interleukin‐1, and tumor necrosis factor‐. Through several metabolic alterations, sepsis independently promotes skeletal muscle breakdown and impairs skeletal muscle synthesis.[34, 35, 36] Inflammation associated with sepsis also increases oxidant burden, further leading to muscle dysfunction and dysregulation.[7, 31, 37, 38]
EFFECTS OF PHYSICAL THERAPY AND MOBILIZATION ON CLINICAL OUTCOMES
In patients with nonsepsis conditions who are at risk for functional decline, the effectiveness of physical therapy has been studied in multiple settings with positive outcomes. For example, in hospitalized elderly patients with general deconditioning, PT‐based interventions have demonstrated reductions in length of hospital stay.[39] Additionally, exercise in healthy subjects who have been subjected to bed rest has been shown to attenuate physiological changes, and maintain plasma and red cell volume and work capacity.[40] Adequate safety and improved outcomes have also been demonstrated in the general population of critically ill patients who receive early PT and mobilization. Improved functional capacity at discharge, decreases in duration of delirium, increased ventilator‐free days, decreased risk for CLABSI, and a better general sense of well‐being following these interventions have been widely reported in the literature.[14, 19, 23, 24, 41, 42, 43, 44, 45] Interestingly, critically ill patients may have a dose‐ and time‐dependent response to PT; that is, high intensity and early onset mobility‐based interventions are often associated with more ventilator‐free time and improved functional outcomes, resulting in shorter ICU and hospital length of stay.[42, 46, 47, 48]
Moderate intensity exercise has also been shown to improve 6‐minute walking distance in patients convalescing from coronary artery bypass grafting surgery.[49] Furthermore, in the postoperative setting, patients suffering traumatic hip fractures are known to benefit from physical and occupational therapies with shorter time to ambulation and improved locomotion in the recovery period.[21, 50, 51] Among patients with stroke, PT and gait training has led to improvements in speed, gait, independence during walking, activities of daily living, and extended activities of daily living.[52, 53, 54] A recent meta‐analysis also suggested that extra PT compared to regular treatment in patients with acute and subacute conditions such as stroke and postoperative states improved mobility and quality of life, while reducing length of hospital stay.[22]
Although this evidence suggests potential benefits for PT and mobilization, it is important to note that the effect of these treatments in dissimilar populations is unknown and may not necessarily be positive. For example, a recent study examining PT and its impact on patients with hip osteoarthritis showed no clinical benefit.[55] Mobilizing patients in severe illness may be associated with important risks, including falls, worsening of their clinical status, or moral discouragement in the setting of limited capacity. Therefore, understanding which elements of mobilization efforts create the greatest impact in the context of delivery of the intervention is critical to assessing the risk, benefit, and efficacy of PT‐based interventions.
EARLY PHYSICAL THERAPY FOR SEVERE SEPSIS OUTSIDE THE ICU: LITERATURE REVIEW
Given the functional decline associated with severe sepsis and the evidence of PT efficacy in other populations, we reviewed the current literature for studies evaluating physical therapy in severe sepsis patients outside the ICU. With the assistance of medical reference librarians, we searched MEDLINE via PubMed (1950present), EMBASE (1946present), Cochrane CENTRAL Register of Controlled Trials, and the Cochrane Database of Reviews of Effectiveness (1960present via Ovid). The search was last updated in June 2014.
We searched for studies that (1) involved human patients 18 years of age, (2) included patients with a primary diagnosis of sepsis or severe sepsis being treated outside the ICU, (3) featured a primary intervention that included PT or an early mobilization‐based initiative, and (4) reported a primary clinical or functional outcome of interest. Early was defined based on the included studies' definition. To be fully inclusive, we also conducted a secondary review with inclusion criteria expanded to studies of either any infectious pathology or severe sepsis patient in the ICU that employed PT interventions.
Our electronic search retrieved 815 records (Figure 2). Despite this approach, no publications met our primary inclusion criteria as we found no study that implemented a mobility intervention directed toward patients with sepsis treated outside the ICU. Our expanded secondary review included patients with any infectious pathology or those with severe sepsis in the ICU treated with PT; in this review, 2 studies met eligibility criteria.[56] In a 2003 cluster‐randomized trial, Mundy and colleagues randomized patients admitted with pneumonia to receive early PT or usual care. The outcomes of interest were hospital length of stay, mortality, number of chest radiographs, emergency department visits, and readmissions at 30 and 90 days after hospital admission. Although the study has important limitations (including patient‐level difference between trial arms, subjective definition of early mobilization), the authors found a significant decrease in length of stay among patients with pneumonia who received early PT compared to controls (5.8 vs 6.9 days, absolute difference 1.1 days, 95% confidence interval: 02.2 days). The study also reported a substantial decrease in adjusted mean hospital charges for the early mobilization group versus the usual care group ($10,159 per patient vs. $12,868 per patient, P=0.05). In the second study, Sossdorf et al. retrospectively evaluated a cohort of 999 patients with severe sepsis and septic shock and assessed whether onset and frequency of PT‐based interventions was associated with clinical benefit. After multivariate analysis, the authors reported a small mortality benefit associated with the relative number of PT interventions (hazard ratio: 0.982, P0.001).[45]

EXPLAINING THE VOID
Our integrative review of the current literature reveals a gap in our understanding of the role of early mobilization in severe sepsis both within and beyond the ICU. Given the promise of PT‐based interventions and the toll of severe sepsis, one must ask: why may this be so?
First, the understanding that severe sepsis leads to significant, long‐term consequences for survivors has only been identified recently. Thus, it is possible that the burden and consequences related to this condition have not been fully recognized in clinical settings, leading to a paucity of research and interventions. Although the association between sepsis and mortality has been known since the 1990s,[57] long‐term complications and enduring morbidity of this disease continue to be realized. Indeed, many studies delineating the longer‐term effects of sepsis have been only recently published.[6, 10, 11, 12, 13]
Second, it is likely that many clinicians ascribe to the viewpoint that severe sepsis is an ICU‐only condition, a myth that has been discounted by multiple studies.[1, 4, 5] Although our study shows a paucity of evidence in both ICU and nonICU‐based severe sepsis, almost half of severe sepsis occurs outside the ICU, carrying with it many of the same clinical implications. Additionally, increased morbidity, mortality, and resource utilization are known to be true in all patients with severe sepsis, irrespective of where they receive treatment in the hospital.[4, 5, 6] Recent evidence has also shown that severe sepsis treated on the floor may be clinically, epidemiologically, and even prognostically unique from its ICU counterpart.[5, 58, 59] Therefore, it appears that research domains with tailored interventions to both ICU and non‐ICU severe sepsis patients are important areas of inquiry for clinicians. Such research may serve the purpose of assessing impact of early mobilization and unmasking any treatment heterogeneity that may exist when dealing with severe sepsis. Though trials of PT in ICU‐based severe sepsis are underway,[60] it is prudent that these also extend beyond the ICU‐setting.
Third, variability in early mobility practices and billing documentation for severe sepsis patients may exist, adding barriers to performing high‐quality research on this topic. In fact, administrative billing records for PT may offer insufficient granularity about services provided or therapies administered, particularly in the ICU where variability in early mobilization practices have been shown despite common employment of physiotherapists.[61]
Finally, many hospitalists may believe that patients with severe sepsis are simply too sick for early mobilization or PT, possibly limiting their participation in clinical or research‐based interventions. This perception has been well described in ICU populations, where it has been well studied and shown to be false.[41, 42, 43] Nevertheless, if severe sepsis patients are viewed as relatively sick hospitalized patients, it is plausible that resistance against early mobilization interventions may exist.[62] Understanding these biases and being mindful of such barriers when conducting studies in this area would be important.
CONCLUSION AND FUTURE DIRECTIONS
The cost burdens of severe sepsis are substantial. Elixhauser et al. suggest that it is currently the single most expensive cause of acute hospitalization in the United States.[63] Importantly, a large proportion of patients with severe sepsis receive care from hospitalists and/or floor teams on the general wards. Our integrative review has demonstrated a knowledge gap when it comes to rigorous assessments of PT and mobilization treatments in patients with severe sepsis within and beyond the ICU. Existing evidence provides a strong rationale for why functional decline occurs in patients with severe sepsis. A reasonable argument for PT‐based interventions to mitigate functional decline in this subset exists, but rigorous evaluation of such interventions is necessary. Physical and mobilization‐based treatments are routinely available and efficacious in several other settings and populations. It could be rapidly deployed and potentially improve outcomes in those with severe sepsis. Research would be welcomed to establish optimal dosing, efficacy, and cost effectiveness of PT and early mobilization for severe sepsis, particularly in patients treated on the general wards by hospitalists and floor teams.
How may such a research agenda be launched? A balanced multipronged approach is necessary. First, large‐scale epidemiological data to understand variation in practice are needed. Focused studies carried out by community and academic hospitalists on septic patients treated outside the ICU are the call of the hour. These data, in turn, can help create registries that assess for risk factors, quality of treatment, and long‐term outcomes among survivors of this condition. Second, evaluation and improvement of the coding and precision of physical and occupational therapy billing records is necessary so that their added value can be assessed and tracked using administrative data. Third, targeted prospective studies and clinical trials to directly evaluate the effect of PT in well‐defined patient populations with sepsis outside the ICU are needed. In this arena, hospitalist expertise and trained physical therapists will be crucial. The focus of this work should be directed toward both short‐term and long‐term functional outcomes, as well as mortality and morbidity assessments. Fourth, these patient‐centered efforts should loop back and inform the foundational biology of severe sepsis, thus illuminating patient‐centered end points, from biomarker analysis to physiometric measurements in basic and translational research.
In conclusion, this review sheds light on the fact that interventions that may mitigate the functional and cognitive decline in survivors of severe sepsis appear underdeveloped. Although the precise benefit of such interventions remains unclear, the low‐cost, widespread availability and generalizability of PT‐based interventions make it a worthy candidate for future research. As the numbers of survivors of sepsis expand, an unmet public health need for interventions to improve the long‐term outcomes of this population exists. Hospitalists and intensivists caring for severe sepsis patients must rise to meet this need. Together, we can help improve the lives of patients afflicted with severe sepsis, wherever they may receive care in the hospital.
Acknowledgements
The authors acknowledge the efforts of medical research librarians Andy Hickner, MSI, and Marissa Conte, MSI, on this project.
Disclosures
This work was supported by the National Institutes of HealthK08, HL091249 (T.J.I.) and VA HSR&D IIR‐11109 (T.J.I.). The views expressed here are the authors' own and do not necessarily represent the views of the US government or the Department of Veterans' Affairs. The authors report no conflicts of interest.
Severe sepsis, defined as an infection leading to systemic inflammatory response and acute organ dysfunction, is a significant cause of morbidity and mortality.[1, 2, 3] Although it has been a condition classically attributed to patients in the intensive care unit (ICU), accumulating data suggest that a substantial proportion of patients with severe sepsis are managed by hospitalists and floor teams in non‐ICU, general ward settings.[1, 4, 5] Although the incidence of severe sepsis continues to rise both in the United States and other developed nations,[2, 6, 7] advances in early recognition, management, and care of this condition have resulted in improved rates of survival.[8] The resultant increase in a severe sepsis survivor population[6] make the long‐term sequelae of this condition an important public health problem.[9]
In both the ICU and on general wards, severe sepsis survivors suffer from decreased functional status, worsened quality of life, increased cognitive dysfunction, and sarcopenia.[4, 6, 10, 11, 12, 13, 14] Not surprisingly, many such patients are discharged to long‐term care facilities for physical rehabilitation,[15] with escalating utilization of resources[16] and cost.[17, 18] Inexpensive interventions that improve outcomes following sepsis would thus be welcomed.
It is well known that physical therapy (PT) and early mobilization are beneficial in mitigating functional decline in a number of conditions.[19, 20, 21, 22] PT can improve outcomes in several ways: prevention of bed rest deconditioning, mitigation of mechanisms that lead to sarcopenia, increased pulmonary and tissue aerobic capacity, and improved sense of well‐being. Indeed, among the population cared for in ICU settings, early mobility and PT lead to more ventilator‐free days, better functional status at discharge, shorter duration of delirium, and even a potentially reduced risk of central line‐associated bloodstream infection (CLABSI).[23, 24] However, whether initiating early PT can improve outcomes in patients with severe sepsis treated by either intensivists or hospitalists/floor teams outside the ICU is unknown.
Therefore, to better understand this phenomenon, we systematically reviewed and integrated the literature regarding early mobilization and PT for severe sepsis outside the ICU. To be more inclusive, a secondary review including populations with any infectious etiology and severe sepsis treated within the ICU was also conducted. Our review begins by providing an overview of the pathophysiology behind functional decline in severe sepsis, along with existing evidence on early mobilization efficacy in other patient populations. We then proceed with a review of the extant literature on the aforementioned topic. We conclude with an evaluation of the current evidence on the subject, along with assertions regarding future research in the area.
PATHOPHYSIOLOGY OF DISABILITY FOLLOWING HOSPITALIZATION FOR SEVERE SEPSIS
The pathophysiology behind functional decline in patients hospitalized with severe sepsis is multifactorial (Figure 1). During hospitalization, it is well known that patients suffer from restricted mobility,[25] and that this impediment is linked to poor functional outcomes.[26] Described as far back as Hippocrates,[27] more recent studies have elucidated how prolonged bed rest leads to a multitude of physiological changes that promote deconditioning.[28] Specifically, skeletal muscle atrophy and decreased protein synthesis, independent of ongoing disease processes and acute illness, have been demonstrated in both animal and human models of prolonged inactivity.[29, 30] Additionally, bed rest leading to insensible fluid losses, a decline in stroke volume and effective cardiac output, bone loss, and decreased insulin sensitivity has been reported.[28, 31] There is little doubt that the aforementioned issues pertain to severe sepsis patients outside the ICU. In fact, nearly all of the acute mechanisms driving Creditor's hazards of hospitalization are noted among patients with severe sepsis.[32]

Furthermore, several factors preceding hospitalization may increase risk of disability. For example, Covinsky et al. described a number of risk factors, such as comorbid conditions, cognitive impairment, and various psychosocial aspects such as depression and limited social support, as being associated with increased risk of functional decline.[33] Thus, both in‐hospital and prehospital factors likely combine within an individual patient's context to determine risk of physical decline.
On this backdrop and the inherent immobilization associated with hospitalization, sepsis and inflammation catalyze physiologic changes that further propagate deconditioning.[7] Implicated pathways and proteins for this process include the mammalian target of rapamycin, human growth hormone, insulin‐like growth factors, interleukin‐1, and tumor necrosis factor‐. Through several metabolic alterations, sepsis independently promotes skeletal muscle breakdown and impairs skeletal muscle synthesis.[34, 35, 36] Inflammation associated with sepsis also increases oxidant burden, further leading to muscle dysfunction and dysregulation.[7, 31, 37, 38]
EFFECTS OF PHYSICAL THERAPY AND MOBILIZATION ON CLINICAL OUTCOMES
In patients with nonsepsis conditions who are at risk for functional decline, the effectiveness of physical therapy has been studied in multiple settings with positive outcomes. For example, in hospitalized elderly patients with general deconditioning, PT‐based interventions have demonstrated reductions in length of hospital stay.[39] Additionally, exercise in healthy subjects who have been subjected to bed rest has been shown to attenuate physiological changes, and maintain plasma and red cell volume and work capacity.[40] Adequate safety and improved outcomes have also been demonstrated in the general population of critically ill patients who receive early PT and mobilization. Improved functional capacity at discharge, decreases in duration of delirium, increased ventilator‐free days, decreased risk for CLABSI, and a better general sense of well‐being following these interventions have been widely reported in the literature.[14, 19, 23, 24, 41, 42, 43, 44, 45] Interestingly, critically ill patients may have a dose‐ and time‐dependent response to PT; that is, high intensity and early onset mobility‐based interventions are often associated with more ventilator‐free time and improved functional outcomes, resulting in shorter ICU and hospital length of stay.[42, 46, 47, 48]
Moderate intensity exercise has also been shown to improve 6‐minute walking distance in patients convalescing from coronary artery bypass grafting surgery.[49] Furthermore, in the postoperative setting, patients suffering traumatic hip fractures are known to benefit from physical and occupational therapies with shorter time to ambulation and improved locomotion in the recovery period.[21, 50, 51] Among patients with stroke, PT and gait training has led to improvements in speed, gait, independence during walking, activities of daily living, and extended activities of daily living.[52, 53, 54] A recent meta‐analysis also suggested that extra PT compared to regular treatment in patients with acute and subacute conditions such as stroke and postoperative states improved mobility and quality of life, while reducing length of hospital stay.[22]
Although this evidence suggests potential benefits for PT and mobilization, it is important to note that the effect of these treatments in dissimilar populations is unknown and may not necessarily be positive. For example, a recent study examining PT and its impact on patients with hip osteoarthritis showed no clinical benefit.[55] Mobilizing patients in severe illness may be associated with important risks, including falls, worsening of their clinical status, or moral discouragement in the setting of limited capacity. Therefore, understanding which elements of mobilization efforts create the greatest impact in the context of delivery of the intervention is critical to assessing the risk, benefit, and efficacy of PT‐based interventions.
EARLY PHYSICAL THERAPY FOR SEVERE SEPSIS OUTSIDE THE ICU: LITERATURE REVIEW
Given the functional decline associated with severe sepsis and the evidence of PT efficacy in other populations, we reviewed the current literature for studies evaluating physical therapy in severe sepsis patients outside the ICU. With the assistance of medical reference librarians, we searched MEDLINE via PubMed (1950present), EMBASE (1946present), Cochrane CENTRAL Register of Controlled Trials, and the Cochrane Database of Reviews of Effectiveness (1960present via Ovid). The search was last updated in June 2014.
We searched for studies that (1) involved human patients 18 years of age, (2) included patients with a primary diagnosis of sepsis or severe sepsis being treated outside the ICU, (3) featured a primary intervention that included PT or an early mobilization‐based initiative, and (4) reported a primary clinical or functional outcome of interest. Early was defined based on the included studies' definition. To be fully inclusive, we also conducted a secondary review with inclusion criteria expanded to studies of either any infectious pathology or severe sepsis patient in the ICU that employed PT interventions.
Our electronic search retrieved 815 records (Figure 2). Despite this approach, no publications met our primary inclusion criteria as we found no study that implemented a mobility intervention directed toward patients with sepsis treated outside the ICU. Our expanded secondary review included patients with any infectious pathology or those with severe sepsis in the ICU treated with PT; in this review, 2 studies met eligibility criteria.[56] In a 2003 cluster‐randomized trial, Mundy and colleagues randomized patients admitted with pneumonia to receive early PT or usual care. The outcomes of interest were hospital length of stay, mortality, number of chest radiographs, emergency department visits, and readmissions at 30 and 90 days after hospital admission. Although the study has important limitations (including patient‐level difference between trial arms, subjective definition of early mobilization), the authors found a significant decrease in length of stay among patients with pneumonia who received early PT compared to controls (5.8 vs 6.9 days, absolute difference 1.1 days, 95% confidence interval: 02.2 days). The study also reported a substantial decrease in adjusted mean hospital charges for the early mobilization group versus the usual care group ($10,159 per patient vs. $12,868 per patient, P=0.05). In the second study, Sossdorf et al. retrospectively evaluated a cohort of 999 patients with severe sepsis and septic shock and assessed whether onset and frequency of PT‐based interventions was associated with clinical benefit. After multivariate analysis, the authors reported a small mortality benefit associated with the relative number of PT interventions (hazard ratio: 0.982, P0.001).[45]

EXPLAINING THE VOID
Our integrative review of the current literature reveals a gap in our understanding of the role of early mobilization in severe sepsis both within and beyond the ICU. Given the promise of PT‐based interventions and the toll of severe sepsis, one must ask: why may this be so?
First, the understanding that severe sepsis leads to significant, long‐term consequences for survivors has only been identified recently. Thus, it is possible that the burden and consequences related to this condition have not been fully recognized in clinical settings, leading to a paucity of research and interventions. Although the association between sepsis and mortality has been known since the 1990s,[57] long‐term complications and enduring morbidity of this disease continue to be realized. Indeed, many studies delineating the longer‐term effects of sepsis have been only recently published.[6, 10, 11, 12, 13]
Second, it is likely that many clinicians ascribe to the viewpoint that severe sepsis is an ICU‐only condition, a myth that has been discounted by multiple studies.[1, 4, 5] Although our study shows a paucity of evidence in both ICU and nonICU‐based severe sepsis, almost half of severe sepsis occurs outside the ICU, carrying with it many of the same clinical implications. Additionally, increased morbidity, mortality, and resource utilization are known to be true in all patients with severe sepsis, irrespective of where they receive treatment in the hospital.[4, 5, 6] Recent evidence has also shown that severe sepsis treated on the floor may be clinically, epidemiologically, and even prognostically unique from its ICU counterpart.[5, 58, 59] Therefore, it appears that research domains with tailored interventions to both ICU and non‐ICU severe sepsis patients are important areas of inquiry for clinicians. Such research may serve the purpose of assessing impact of early mobilization and unmasking any treatment heterogeneity that may exist when dealing with severe sepsis. Though trials of PT in ICU‐based severe sepsis are underway,[60] it is prudent that these also extend beyond the ICU‐setting.
Third, variability in early mobility practices and billing documentation for severe sepsis patients may exist, adding barriers to performing high‐quality research on this topic. In fact, administrative billing records for PT may offer insufficient granularity about services provided or therapies administered, particularly in the ICU where variability in early mobilization practices have been shown despite common employment of physiotherapists.[61]
Finally, many hospitalists may believe that patients with severe sepsis are simply too sick for early mobilization or PT, possibly limiting their participation in clinical or research‐based interventions. This perception has been well described in ICU populations, where it has been well studied and shown to be false.[41, 42, 43] Nevertheless, if severe sepsis patients are viewed as relatively sick hospitalized patients, it is plausible that resistance against early mobilization interventions may exist.[62] Understanding these biases and being mindful of such barriers when conducting studies in this area would be important.
CONCLUSION AND FUTURE DIRECTIONS
The cost burdens of severe sepsis are substantial. Elixhauser et al. suggest that it is currently the single most expensive cause of acute hospitalization in the United States.[63] Importantly, a large proportion of patients with severe sepsis receive care from hospitalists and/or floor teams on the general wards. Our integrative review has demonstrated a knowledge gap when it comes to rigorous assessments of PT and mobilization treatments in patients with severe sepsis within and beyond the ICU. Existing evidence provides a strong rationale for why functional decline occurs in patients with severe sepsis. A reasonable argument for PT‐based interventions to mitigate functional decline in this subset exists, but rigorous evaluation of such interventions is necessary. Physical and mobilization‐based treatments are routinely available and efficacious in several other settings and populations. It could be rapidly deployed and potentially improve outcomes in those with severe sepsis. Research would be welcomed to establish optimal dosing, efficacy, and cost effectiveness of PT and early mobilization for severe sepsis, particularly in patients treated on the general wards by hospitalists and floor teams.
How may such a research agenda be launched? A balanced multipronged approach is necessary. First, large‐scale epidemiological data to understand variation in practice are needed. Focused studies carried out by community and academic hospitalists on septic patients treated outside the ICU are the call of the hour. These data, in turn, can help create registries that assess for risk factors, quality of treatment, and long‐term outcomes among survivors of this condition. Second, evaluation and improvement of the coding and precision of physical and occupational therapy billing records is necessary so that their added value can be assessed and tracked using administrative data. Third, targeted prospective studies and clinical trials to directly evaluate the effect of PT in well‐defined patient populations with sepsis outside the ICU are needed. In this arena, hospitalist expertise and trained physical therapists will be crucial. The focus of this work should be directed toward both short‐term and long‐term functional outcomes, as well as mortality and morbidity assessments. Fourth, these patient‐centered efforts should loop back and inform the foundational biology of severe sepsis, thus illuminating patient‐centered end points, from biomarker analysis to physiometric measurements in basic and translational research.
In conclusion, this review sheds light on the fact that interventions that may mitigate the functional and cognitive decline in survivors of severe sepsis appear underdeveloped. Although the precise benefit of such interventions remains unclear, the low‐cost, widespread availability and generalizability of PT‐based interventions make it a worthy candidate for future research. As the numbers of survivors of sepsis expand, an unmet public health need for interventions to improve the long‐term outcomes of this population exists. Hospitalists and intensivists caring for severe sepsis patients must rise to meet this need. Together, we can help improve the lives of patients afflicted with severe sepsis, wherever they may receive care in the hospital.
Acknowledgements
The authors acknowledge the efforts of medical research librarians Andy Hickner, MSI, and Marissa Conte, MSI, on this project.
Disclosures
This work was supported by the National Institutes of HealthK08, HL091249 (T.J.I.) and VA HSR&D IIR‐11109 (T.J.I.). The views expressed here are the authors' own and do not necessarily represent the views of the US government or the Department of Veterans' Affairs. The authors report no conflicts of interest.
- , . Epidemiology of sepsis: an update. Crit Care Med. 2001;29:S109–S116.
- , , , et al. Nationwide trends of severe sepsis in the 21st century (2000–2007). Chest. 2011;140:1223–1231.
- , , , et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554.
- , , , et al. Functional outcomes of general medical patients with severe sepsis. BMC Infect Dis. 2013;13:588.
- , , , et al. The epidemiology of acute organ system dysfunction from severe sepsis outside of the intensive care unit. J Hosp Med. 2013;8:243–247.
- , , , et al. Population burden of long‐term survivorship after severe sepsis in older Americans. J Am Geriatr Soc. 2012;60:1070–1077.
- , , , et al. Systemic inflammatory response syndrome increases immobility‐induced neuromuscular weakness. Crit Care Med. 2008;36:910–916.
- , , , et al. Early goal‐directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377.
- . The lingering consequences of sepsis: a hidden public health disaster? JAMA. 2010;304:1833–1834.
- , , , et al. Long‐term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787–1794.
- , , , et al. Spurious inferences about long‐term outcomes: the case of severe sepsis and geriatric conditions. Am J Respir Crit Care Med. 2012;185:835–841.
- , , , et al. Long‐term outcome and quality‐adjusted life years after severe sepsis. Crit Care Med. 2009;37:1268–1274.
- , , , et al. Long‐term mortality and quality of life in sepsis: a systematic review. Crit Care Med. 2010;38:1276–1283.
- , , , et al. Improving post‐intensive care unit neuropsychiatric outcomes: understanding cognitive effects of physical activity. Am J Respir Crit Care Med. 2012;186:1220–1228.
- , , , et al. Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med. 2012;40:754–761.
- , , , et al. Long‐term acute care hospital utilization after critical illness. JAMA. 2010;303:2253–2259.
- , , , et al. Long‐term survival and healthcare utilization outcomes attributable to sepsis and pneumonia. BMC Health Serv Res. 2012;12:432.
- , , , et al. Long‐term mortality and medical care charges in patients with severe sepsis. Crit Care Med. 2003;31:2316–2323.
- , , , et al. Early exercise in critically ill patients enhances short‐term functional recovery. Crit Care Med. 2009;37:2499–2505.
- , , , et al. Exercise‐based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2011;(7):CD001800.
- , , , et al. What is the role of timing in the surgical and rehabilitative care of community‐dwelling older persons with acute hip fracture? Arch Intern Med. 1997;157:513–520.
- , , . Extra physical therapy reduces patient length of stay and improves functional outcomes and quality of life in people with acute or subacute conditions: a systematic review. Arch Phys Med Rehabilil. 2011;92:1490–1500.
- , , . Reduction of intensive care unit length of stay: the case of early mobilization. Health Care Manag (Frederick). 2014;33:128–135.
- , , , et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373:1874–1882.
- , , , et al. Hospitalization, restricted activity, and the development of disability among older persons. JAMA. 2004;292:2115–2124.
- , , , et al. Low mobility during hospitalization and functional decline in older adults. J Am Geriatr Soc. 2011;59:266–273.
- , . The Medical Works of Hippocrates. Oxford, United Kingdom: Blackwell; 1950.
- , , . An overview of the issues: physiological effects of bed rest and restricted physical activity. Med Sci Sports Exerc. 1997;29:187–190.
- , , , et al. Prolonged bed rest decreases skeletal muscle and whole body protein synthesis. Am J Physiol. 1996;270:E627–E633.
- , . Metabolic consequences of muscle disuse atrophy. J Nutr. 2005;135:1824S–1828S.
- . Inactivity and inflammation in the critically ill patient. Crit Care Clin. 2007;23:21–34.
- . Hazards of hospitalization of the elderly. Ann Intern Med. 1993;118:219–223.
- , , . Hospitalization‐associated disability: “She was probably able to ambulate, but I'm not sure”. JAMA. 2011;306:1782–1793.
- , , , et al. A sustained rat model for studying the long‐lasting catabolic state of sepsis. Infect Immun. 1999;67:1079–1085.
- . Regulation of skeletal muscle protein turnover during sepsis. Curr Opin Clin Nutr. Metab Care. 1998;1:217–224.
- , , . Regulation of muscle protein synthesis during sepsis and inflammation. Am J Physiol Endocrinol Metab. 2007;293:E453–E459.
- , . From muscle disuse to myopathy in COPD: potential contribution of oxidative stress. Eur Respir J. 2005;26:703–719.
- , , . Oxidative stress and gene expression in sepsis. Br J Anaesth. 2003;90:221–232.
- , , , et al. Early ambulation and length of stay in older adults hospitalized for acute illness. Arch Intern Med. 2010;170:1942–1943.
- . Intensive exercise training during bed rest attenuates deconditioning. Med Sci Sports Exerc. 1997;29:207–215.
- , , , et al. Early activity is feasible and safe in respiratory failure patients. Crit Care Med. 2007;35:139–145.
- , , , et al. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med. 2008;36:2238–2243.
- . Clinical trials of early mobilization of critically ill patients. Crit Care Med. 2009;37:S442–S447.
- . Mobilizing patients in the intensive care unit: improving neuromuscular weakness and physical function. JAMA. 2008;300:1685–1690.
- , , , et al. Potential effect of physiotherapeutic treatment on mortality rate in patients with severe sepsis and septic shock: a retrospective cohort analysis. J Crit Care. 2013;28:954–958.
- , , , et al. Effects of physical training on functional status in patients with prolonged mechanical ventilation. Phys Ther. 2006;86:1271–1281.
- , , , et al. Impact of whole‐body rehabilitation in patients receiving chronic mechanical ventilation. Crit Care Med. 2005;33:2259–2265.
- . Rehabilitation of patients admitted to a respiratory intensive care unit. Arch Phys Med Rehabil. 1998;79:849–854.
- , , , et al. Supervised moderate intensity exercise improves distance walked at hospital discharge following coronary artery bypass graft surgery—a randomised controlled trial. Heart Lung Circ. 2008;17:129–138.
- , , , et al. Systematic review of hip fracture rehabilitation practices in the elderly. Arch Phys Med Rehabil. 2009;90:246–262.
- , , , et al. Physical therapy and mobility 2 and 6 months after hip fracture. J Am Geriatr Soc. 2004;52:1114–1120.
- , , , et al. Physical fitness training for stroke patients. Cochrane Database Syst Rev. 2011;(11):CD003316.
- , , , et al. Effects of augmented exercise therapy on outcome of gait and gait‐related activities in the first 6 months after stroke: a meta‐analysis. Stroke. 2011;42:3311–3315.
- , , , et al. Effects of augmented exercise therapy time after stroke: a meta‐analysis. Stroke. 2004;35:2529–2539.
- , , , et al. Effect of physical therapy on pain and function in patients with hip osteoarthritis: a randomized clinical trial. JAMA. 2014;311:1987–1997.
- , , , et al. Early mobilization of patients hospitalized with community‐acquired pneumonia. Chest. 2003;124:883–889.
- , , , et al. Magnitude and duration of the effect of sepsis on survival. Department of Veterans Affairs Systemic Sepsis Cooperative Studies Group. JAMA. 1997;277:1058–1063.
- , , , et al. Epidemiology of sepsis in Victoria, Australia. Crit Care Med. 2005;33:71–80.
- , , , et al. Sepsis incidence and outcome: contrasting the intensive care unit with the hospital ward. Crit Care Med. 2007;35:1284–1289.
- , , . Early rehabilitation in sepsis: a prospective randomised controlled trial investigating functional and physiological outcomes The i‐PERFORM Trial (Protocol Article). BMC Anesthesiol. 2011;11:21.
- , , , et al. TEAM: a prospective multi‐centre cohort study of early activity and mobilisation in ICU. In: American Thoracic Society 2013 International Conference; May 17–22, 2013; Philadelphia, PA. Am J Respir Crit Care Med. 2013;187:A3625.
- , , , et al. Improving long‐term outcomes after discharge from intensive care unit: report from a stakeholders' conference. Crit Care Med. 2012;40:502–509.
- , , . Septicemia in U.S. hospitals, 2009: statistical brief #122. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville, MD; 2006.
- , . Epidemiology of sepsis: an update. Crit Care Med. 2001;29:S109–S116.
- , , , et al. Nationwide trends of severe sepsis in the 21st century (2000–2007). Chest. 2011;140:1223–1231.
- , , , et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554.
- , , , et al. Functional outcomes of general medical patients with severe sepsis. BMC Infect Dis. 2013;13:588.
- , , , et al. The epidemiology of acute organ system dysfunction from severe sepsis outside of the intensive care unit. J Hosp Med. 2013;8:243–247.
- , , , et al. Population burden of long‐term survivorship after severe sepsis in older Americans. J Am Geriatr Soc. 2012;60:1070–1077.
- , , , et al. Systemic inflammatory response syndrome increases immobility‐induced neuromuscular weakness. Crit Care Med. 2008;36:910–916.
- , , , et al. Early goal‐directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377.
- . The lingering consequences of sepsis: a hidden public health disaster? JAMA. 2010;304:1833–1834.
- , , , et al. Long‐term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787–1794.
- , , , et al. Spurious inferences about long‐term outcomes: the case of severe sepsis and geriatric conditions. Am J Respir Crit Care Med. 2012;185:835–841.
- , , , et al. Long‐term outcome and quality‐adjusted life years after severe sepsis. Crit Care Med. 2009;37:1268–1274.
- , , , et al. Long‐term mortality and quality of life in sepsis: a systematic review. Crit Care Med. 2010;38:1276–1283.
- , , , et al. Improving post‐intensive care unit neuropsychiatric outcomes: understanding cognitive effects of physical activity. Am J Respir Crit Care Med. 2012;186:1220–1228.
- , , , et al. Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med. 2012;40:754–761.
- , , , et al. Long‐term acute care hospital utilization after critical illness. JAMA. 2010;303:2253–2259.
- , , , et al. Long‐term survival and healthcare utilization outcomes attributable to sepsis and pneumonia. BMC Health Serv Res. 2012;12:432.
- , , , et al. Long‐term mortality and medical care charges in patients with severe sepsis. Crit Care Med. 2003;31:2316–2323.
- , , , et al. Early exercise in critically ill patients enhances short‐term functional recovery. Crit Care Med. 2009;37:2499–2505.
- , , , et al. Exercise‐based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2011;(7):CD001800.
- , , , et al. What is the role of timing in the surgical and rehabilitative care of community‐dwelling older persons with acute hip fracture? Arch Intern Med. 1997;157:513–520.
- , , . Extra physical therapy reduces patient length of stay and improves functional outcomes and quality of life in people with acute or subacute conditions: a systematic review. Arch Phys Med Rehabilil. 2011;92:1490–1500.
- , , . Reduction of intensive care unit length of stay: the case of early mobilization. Health Care Manag (Frederick). 2014;33:128–135.
- , , , et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373:1874–1882.
- , , , et al. Hospitalization, restricted activity, and the development of disability among older persons. JAMA. 2004;292:2115–2124.
- , , , et al. Low mobility during hospitalization and functional decline in older adults. J Am Geriatr Soc. 2011;59:266–273.
- , . The Medical Works of Hippocrates. Oxford, United Kingdom: Blackwell; 1950.
- , , . An overview of the issues: physiological effects of bed rest and restricted physical activity. Med Sci Sports Exerc. 1997;29:187–190.
- , , , et al. Prolonged bed rest decreases skeletal muscle and whole body protein synthesis. Am J Physiol. 1996;270:E627–E633.
- , . Metabolic consequences of muscle disuse atrophy. J Nutr. 2005;135:1824S–1828S.
- . Inactivity and inflammation in the critically ill patient. Crit Care Clin. 2007;23:21–34.
- . Hazards of hospitalization of the elderly. Ann Intern Med. 1993;118:219–223.
- , , . Hospitalization‐associated disability: “She was probably able to ambulate, but I'm not sure”. JAMA. 2011;306:1782–1793.
- , , , et al. A sustained rat model for studying the long‐lasting catabolic state of sepsis. Infect Immun. 1999;67:1079–1085.
- . Regulation of skeletal muscle protein turnover during sepsis. Curr Opin Clin Nutr. Metab Care. 1998;1:217–224.
- , , . Regulation of muscle protein synthesis during sepsis and inflammation. Am J Physiol Endocrinol Metab. 2007;293:E453–E459.
- , . From muscle disuse to myopathy in COPD: potential contribution of oxidative stress. Eur Respir J. 2005;26:703–719.
- , , . Oxidative stress and gene expression in sepsis. Br J Anaesth. 2003;90:221–232.
- , , , et al. Early ambulation and length of stay in older adults hospitalized for acute illness. Arch Intern Med. 2010;170:1942–1943.
- . Intensive exercise training during bed rest attenuates deconditioning. Med Sci Sports Exerc. 1997;29:207–215.
- , , , et al. Early activity is feasible and safe in respiratory failure patients. Crit Care Med. 2007;35:139–145.
- , , , et al. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med. 2008;36:2238–2243.
- . Clinical trials of early mobilization of critically ill patients. Crit Care Med. 2009;37:S442–S447.
- . Mobilizing patients in the intensive care unit: improving neuromuscular weakness and physical function. JAMA. 2008;300:1685–1690.
- , , , et al. Potential effect of physiotherapeutic treatment on mortality rate in patients with severe sepsis and septic shock: a retrospective cohort analysis. J Crit Care. 2013;28:954–958.
- , , , et al. Effects of physical training on functional status in patients with prolonged mechanical ventilation. Phys Ther. 2006;86:1271–1281.
- , , , et al. Impact of whole‐body rehabilitation in patients receiving chronic mechanical ventilation. Crit Care Med. 2005;33:2259–2265.
- . Rehabilitation of patients admitted to a respiratory intensive care unit. Arch Phys Med Rehabil. 1998;79:849–854.
- , , , et al. Supervised moderate intensity exercise improves distance walked at hospital discharge following coronary artery bypass graft surgery—a randomised controlled trial. Heart Lung Circ. 2008;17:129–138.
- , , , et al. Systematic review of hip fracture rehabilitation practices in the elderly. Arch Phys Med Rehabil. 2009;90:246–262.
- , , , et al. Physical therapy and mobility 2 and 6 months after hip fracture. J Am Geriatr Soc. 2004;52:1114–1120.
- , , , et al. Physical fitness training for stroke patients. Cochrane Database Syst Rev. 2011;(11):CD003316.
- , , , et al. Effects of augmented exercise therapy on outcome of gait and gait‐related activities in the first 6 months after stroke: a meta‐analysis. Stroke. 2011;42:3311–3315.
- , , , et al. Effects of augmented exercise therapy time after stroke: a meta‐analysis. Stroke. 2004;35:2529–2539.
- , , , et al. Effect of physical therapy on pain and function in patients with hip osteoarthritis: a randomized clinical trial. JAMA. 2014;311:1987–1997.
- , , , et al. Early mobilization of patients hospitalized with community‐acquired pneumonia. Chest. 2003;124:883–889.
- , , , et al. Magnitude and duration of the effect of sepsis on survival. Department of Veterans Affairs Systemic Sepsis Cooperative Studies Group. JAMA. 1997;277:1058–1063.
- , , , et al. Epidemiology of sepsis in Victoria, Australia. Crit Care Med. 2005;33:71–80.
- , , , et al. Sepsis incidence and outcome: contrasting the intensive care unit with the hospital ward. Crit Care Med. 2007;35:1284–1289.
- , , . Early rehabilitation in sepsis: a prospective randomised controlled trial investigating functional and physiological outcomes The i‐PERFORM Trial (Protocol Article). BMC Anesthesiol. 2011;11:21.
- , , , et al. TEAM: a prospective multi‐centre cohort study of early activity and mobilisation in ICU. In: American Thoracic Society 2013 International Conference; May 17–22, 2013; Philadelphia, PA. Am J Respir Crit Care Med. 2013;187:A3625.
- , , , et al. Improving long‐term outcomes after discharge from intensive care unit: report from a stakeholders' conference. Crit Care Med. 2012;40:502–509.
- , , . Septicemia in U.S. hospitals, 2009: statistical brief #122. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville, MD; 2006.
Understanding U.S. Cardiologists’ Perspectives on Non-Valvular Atrial Fibrillation (NVAF)
A supplement to Cardiology News. This supplement was sponsored by Daiichi Sankyo Inc.
Faculty:
Hugh Calkins, MD, FHRS, Immediate Past President of The Heart Rhythm Society; Director of Cardiac Arrhythmia Service, Johns Hopkins Hospital; and Professor of Medicine at Johns Hopkins University School of Medicine, Baltimore, MD
Disclosures:
Daiichi Sankyo has provided financial support to the Heart Rhythm Society for conducting this survey. Dr. Calkins consults on behalf of Daiichi Sankyo.
A supplement to Cardiology News. This supplement was sponsored by Daiichi Sankyo Inc.
Faculty:
Hugh Calkins, MD, FHRS, Immediate Past President of The Heart Rhythm Society; Director of Cardiac Arrhythmia Service, Johns Hopkins Hospital; and Professor of Medicine at Johns Hopkins University School of Medicine, Baltimore, MD
Disclosures:
Daiichi Sankyo has provided financial support to the Heart Rhythm Society for conducting this survey. Dr. Calkins consults on behalf of Daiichi Sankyo.
A supplement to Cardiology News. This supplement was sponsored by Daiichi Sankyo Inc.
Faculty:
Hugh Calkins, MD, FHRS, Immediate Past President of The Heart Rhythm Society; Director of Cardiac Arrhythmia Service, Johns Hopkins Hospital; and Professor of Medicine at Johns Hopkins University School of Medicine, Baltimore, MD
Disclosures:
Daiichi Sankyo has provided financial support to the Heart Rhythm Society for conducting this survey. Dr. Calkins consults on behalf of Daiichi Sankyo.
Laser treatments for men
In our final segment on male dermatology, we will be focusing on laser treatments in men. There has been a steady increase in cosmetic procedures in men over the last decade, and laser procedures tend to be some of the most popular. In general, laser treatments provide faster results than topical or oral treatments and offer subtle aesthetic improvements with little to no downtime depending on the procedure. These factors appeal to male patients, who generally are generally less risk tolerant than women, and want masculinizing treatments with little downtime and natural results.
• Hair growth. Men tend to have highly pigmented, thicker hair in contrast to women, and often seek laser hair removal for excess body hair. Common sites include the back, upper arms, posterior hairline, lower beardline, and chest. Similar precautions apply to both men and women, such as proper cooling of the skin and avoidance of tanned skin. However, laser settings for male patients may need to be adjusted given the thicker, darkly pigmented hairs and often lower pain threshold. In addition, proper counseling of men is necessary with laser hair removal, because men often need more treatments than women and may need a topical anesthetic for highly sensitive areas.
• Body contouring. Men tend to deposit fat in hard-to-lose areas, such as the central abdomen and flanks. The expanding array of noninvasive devices using cold temperatures to freeze the fat, or ultrasound and radiofrequency devices to heat and thereby tighten the subcutaneous tissue have made body contouring one of the fastest growing cosmetic markets for men. Men are great candidates for these procedures given the fast results, minor discomfort, and noninvasive nature. Although many men have visceral abdominal fat that does not respond to these treatments, areas often treated with great long-term results include the upper and lower abdomen, flanks, arms, chest, and back.
• Rosacea. Men have a higher density of facial blood vessels than women, and they often seek treatment for telangiectasias and overall facial erythema. For noninflammatory erythematotelangiectatic rosacea, vascular laser treatments are the most effective treatments. Pulsed dye laser is often the best laser to target both large and small facial blood vessels and flushing erythema. Intense pulsed light (IPL) lasers are often a more popular choice for men because they involve less downtime and can treat brown spots as well. However, IPL must be used with caution in skin of color and tanned skin because of the risks of scarring and hyperpigmentation. Men may need more treatments and higher energy settings than women. Men also prefer minimal downtime and thus more frequent nonpurpuric settings are often preferred with any vascular laser. In addition, with IPL, men should be warned of the possibility of the laser temporarily stunting hair growth or causing hair to grow in patchy temporarily when using the device in the beard or mustache area.
• Laser resurfacing. Laser skin resurfacing can be performed for acne scars, rhytids, age sports, sun spots, melasma, and overall skin laxity. Options include ablative and nonablative skin resurfacing. The choice of procedure depends on the type of problem being treated, skin type, and downtime. Ablative CO2, erbium:YAG, and fractional ablative lasers provide the best results for deep rhytids, acne scars, surgical scars, and skin laxity. However, men often shy away from these procedures given the pain, postprocedure care necessary, and downtime. Nonablative lasers may be a better choice for men, particularly for fine rhytids, melasma, and sun spots. With multiple treatments, they also may be used for scars and skin laxity. Postprocedure skincare and downtime are the critical factors for men when choosing resurfacing procedures, and detailed review of the care, complexity, and side effects are essential in the care of male patients.
Dr. Talakoub and Dr. Wesley are co-contributors to a monthly Aesthetic Dermatology column in Skin & Allergy News. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub.
In our final segment on male dermatology, we will be focusing on laser treatments in men. There has been a steady increase in cosmetic procedures in men over the last decade, and laser procedures tend to be some of the most popular. In general, laser treatments provide faster results than topical or oral treatments and offer subtle aesthetic improvements with little to no downtime depending on the procedure. These factors appeal to male patients, who generally are generally less risk tolerant than women, and want masculinizing treatments with little downtime and natural results.
• Hair growth. Men tend to have highly pigmented, thicker hair in contrast to women, and often seek laser hair removal for excess body hair. Common sites include the back, upper arms, posterior hairline, lower beardline, and chest. Similar precautions apply to both men and women, such as proper cooling of the skin and avoidance of tanned skin. However, laser settings for male patients may need to be adjusted given the thicker, darkly pigmented hairs and often lower pain threshold. In addition, proper counseling of men is necessary with laser hair removal, because men often need more treatments than women and may need a topical anesthetic for highly sensitive areas.
• Body contouring. Men tend to deposit fat in hard-to-lose areas, such as the central abdomen and flanks. The expanding array of noninvasive devices using cold temperatures to freeze the fat, or ultrasound and radiofrequency devices to heat and thereby tighten the subcutaneous tissue have made body contouring one of the fastest growing cosmetic markets for men. Men are great candidates for these procedures given the fast results, minor discomfort, and noninvasive nature. Although many men have visceral abdominal fat that does not respond to these treatments, areas often treated with great long-term results include the upper and lower abdomen, flanks, arms, chest, and back.
• Rosacea. Men have a higher density of facial blood vessels than women, and they often seek treatment for telangiectasias and overall facial erythema. For noninflammatory erythematotelangiectatic rosacea, vascular laser treatments are the most effective treatments. Pulsed dye laser is often the best laser to target both large and small facial blood vessels and flushing erythema. Intense pulsed light (IPL) lasers are often a more popular choice for men because they involve less downtime and can treat brown spots as well. However, IPL must be used with caution in skin of color and tanned skin because of the risks of scarring and hyperpigmentation. Men may need more treatments and higher energy settings than women. Men also prefer minimal downtime and thus more frequent nonpurpuric settings are often preferred with any vascular laser. In addition, with IPL, men should be warned of the possibility of the laser temporarily stunting hair growth or causing hair to grow in patchy temporarily when using the device in the beard or mustache area.
• Laser resurfacing. Laser skin resurfacing can be performed for acne scars, rhytids, age sports, sun spots, melasma, and overall skin laxity. Options include ablative and nonablative skin resurfacing. The choice of procedure depends on the type of problem being treated, skin type, and downtime. Ablative CO2, erbium:YAG, and fractional ablative lasers provide the best results for deep rhytids, acne scars, surgical scars, and skin laxity. However, men often shy away from these procedures given the pain, postprocedure care necessary, and downtime. Nonablative lasers may be a better choice for men, particularly for fine rhytids, melasma, and sun spots. With multiple treatments, they also may be used for scars and skin laxity. Postprocedure skincare and downtime are the critical factors for men when choosing resurfacing procedures, and detailed review of the care, complexity, and side effects are essential in the care of male patients.
Dr. Talakoub and Dr. Wesley are co-contributors to a monthly Aesthetic Dermatology column in Skin & Allergy News. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub.
In our final segment on male dermatology, we will be focusing on laser treatments in men. There has been a steady increase in cosmetic procedures in men over the last decade, and laser procedures tend to be some of the most popular. In general, laser treatments provide faster results than topical or oral treatments and offer subtle aesthetic improvements with little to no downtime depending on the procedure. These factors appeal to male patients, who generally are generally less risk tolerant than women, and want masculinizing treatments with little downtime and natural results.
• Hair growth. Men tend to have highly pigmented, thicker hair in contrast to women, and often seek laser hair removal for excess body hair. Common sites include the back, upper arms, posterior hairline, lower beardline, and chest. Similar precautions apply to both men and women, such as proper cooling of the skin and avoidance of tanned skin. However, laser settings for male patients may need to be adjusted given the thicker, darkly pigmented hairs and often lower pain threshold. In addition, proper counseling of men is necessary with laser hair removal, because men often need more treatments than women and may need a topical anesthetic for highly sensitive areas.
• Body contouring. Men tend to deposit fat in hard-to-lose areas, such as the central abdomen and flanks. The expanding array of noninvasive devices using cold temperatures to freeze the fat, or ultrasound and radiofrequency devices to heat and thereby tighten the subcutaneous tissue have made body contouring one of the fastest growing cosmetic markets for men. Men are great candidates for these procedures given the fast results, minor discomfort, and noninvasive nature. Although many men have visceral abdominal fat that does not respond to these treatments, areas often treated with great long-term results include the upper and lower abdomen, flanks, arms, chest, and back.
• Rosacea. Men have a higher density of facial blood vessels than women, and they often seek treatment for telangiectasias and overall facial erythema. For noninflammatory erythematotelangiectatic rosacea, vascular laser treatments are the most effective treatments. Pulsed dye laser is often the best laser to target both large and small facial blood vessels and flushing erythema. Intense pulsed light (IPL) lasers are often a more popular choice for men because they involve less downtime and can treat brown spots as well. However, IPL must be used with caution in skin of color and tanned skin because of the risks of scarring and hyperpigmentation. Men may need more treatments and higher energy settings than women. Men also prefer minimal downtime and thus more frequent nonpurpuric settings are often preferred with any vascular laser. In addition, with IPL, men should be warned of the possibility of the laser temporarily stunting hair growth or causing hair to grow in patchy temporarily when using the device in the beard or mustache area.
• Laser resurfacing. Laser skin resurfacing can be performed for acne scars, rhytids, age sports, sun spots, melasma, and overall skin laxity. Options include ablative and nonablative skin resurfacing. The choice of procedure depends on the type of problem being treated, skin type, and downtime. Ablative CO2, erbium:YAG, and fractional ablative lasers provide the best results for deep rhytids, acne scars, surgical scars, and skin laxity. However, men often shy away from these procedures given the pain, postprocedure care necessary, and downtime. Nonablative lasers may be a better choice for men, particularly for fine rhytids, melasma, and sun spots. With multiple treatments, they also may be used for scars and skin laxity. Postprocedure skincare and downtime are the critical factors for men when choosing resurfacing procedures, and detailed review of the care, complexity, and side effects are essential in the care of male patients.
Dr. Talakoub and Dr. Wesley are co-contributors to a monthly Aesthetic Dermatology column in Skin & Allergy News. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub.
Digital resource provides tips on diabetes management, treatment
More than a dozen federal agencies have banded together to create the Guiding Principles for the Care of People With or at Risk for Diabetes, a digital resource center aimed at providing clinically relevant information on diabetes management to clinicians and health care professionals.
The tool, created by the National Diabetes Education Program, is based on areas of general agreement among existing diabetes management protocols, can better inform primary care providers and health care teams on how to deliver quality care to adults with or at risk of diabetes.
The guidelines are not intended as a definitive resource for diabetes management and do not provide information on specific clinical management of diabetes.
Supporters of the guidelines include the American Diabetes Association, the Academy of Nutrition and Dietetics, the American Association of Nurse Practitioners, the Office of Minority Health, and the Agency for Healthcare Research and Quality.
The resource guide has information about the following topics:
• Identify undiagnosed diabetes and prediabetes.
• Manage prediabetes.
• Provide self-management education and support.
• Provide individualized nutrition therapy.
• Encourage regular physical activity.
• Control blood glucose.
• Reduce cardiovascular disease risk.
• Detect and monitor microvascular complications.
• Consider special populations.
• Provide patient-centered care.
To learn more about the Guiding Principles, check out the website here.
More than a dozen federal agencies have banded together to create the Guiding Principles for the Care of People With or at Risk for Diabetes, a digital resource center aimed at providing clinically relevant information on diabetes management to clinicians and health care professionals.
The tool, created by the National Diabetes Education Program, is based on areas of general agreement among existing diabetes management protocols, can better inform primary care providers and health care teams on how to deliver quality care to adults with or at risk of diabetes.
The guidelines are not intended as a definitive resource for diabetes management and do not provide information on specific clinical management of diabetes.
Supporters of the guidelines include the American Diabetes Association, the Academy of Nutrition and Dietetics, the American Association of Nurse Practitioners, the Office of Minority Health, and the Agency for Healthcare Research and Quality.
The resource guide has information about the following topics:
• Identify undiagnosed diabetes and prediabetes.
• Manage prediabetes.
• Provide self-management education and support.
• Provide individualized nutrition therapy.
• Encourage regular physical activity.
• Control blood glucose.
• Reduce cardiovascular disease risk.
• Detect and monitor microvascular complications.
• Consider special populations.
• Provide patient-centered care.
To learn more about the Guiding Principles, check out the website here.
More than a dozen federal agencies have banded together to create the Guiding Principles for the Care of People With or at Risk for Diabetes, a digital resource center aimed at providing clinically relevant information on diabetes management to clinicians and health care professionals.
The tool, created by the National Diabetes Education Program, is based on areas of general agreement among existing diabetes management protocols, can better inform primary care providers and health care teams on how to deliver quality care to adults with or at risk of diabetes.
The guidelines are not intended as a definitive resource for diabetes management and do not provide information on specific clinical management of diabetes.
Supporters of the guidelines include the American Diabetes Association, the Academy of Nutrition and Dietetics, the American Association of Nurse Practitioners, the Office of Minority Health, and the Agency for Healthcare Research and Quality.
The resource guide has information about the following topics:
• Identify undiagnosed diabetes and prediabetes.
• Manage prediabetes.
• Provide self-management education and support.
• Provide individualized nutrition therapy.
• Encourage regular physical activity.
• Control blood glucose.
• Reduce cardiovascular disease risk.
• Detect and monitor microvascular complications.
• Consider special populations.
• Provide patient-centered care.
To learn more about the Guiding Principles, check out the website here.
PODCAST: Program restores competency of defendants
CHICAGO– A competency restoration program in the District of Columbia is helping defendants with mental illness become fit to stand trial, while saving the district money. The program could serve as a model for other jurisdictions that want to improve the competency of unfit defendants, Dr. Nicole R. Johnson, director of outpatient forensic service at the district’s Department of Behavioral Health said in an interview.
The District of Columbia’s Outpatient Competency Restoration Program (OCRP) started in 2009 and receives court-ordered referrals for individuals to participate in the program. The program works to restore defendants’ competency through question and answer sessions, games, and educational lessons, among other methods, Dr. Johnson reported at the American Academy of Psychiatry and the Law meeting. Some participants have mental illness and about a third have cognitive or neurologic limitations, she said. The majority of those referred receive mental health services from separate district agencies. If such needs are being not being met, OCRP administrators refer them to a clinic for treatment.
Of 170 individuals enrolled in the OCRP from 2009 to 2013, 54 were deemed competent to stand trial after completion, said Dr. Johnson, who oversees the program.
In an interview at the meeting, Dr. Johnson discussed how much money the program has saved the district and whether other jurisdictions can feasibly start similar programs.
On Twitter @legal_med
CHICAGO– A competency restoration program in the District of Columbia is helping defendants with mental illness become fit to stand trial, while saving the district money. The program could serve as a model for other jurisdictions that want to improve the competency of unfit defendants, Dr. Nicole R. Johnson, director of outpatient forensic service at the district’s Department of Behavioral Health said in an interview.
The District of Columbia’s Outpatient Competency Restoration Program (OCRP) started in 2009 and receives court-ordered referrals for individuals to participate in the program. The program works to restore defendants’ competency through question and answer sessions, games, and educational lessons, among other methods, Dr. Johnson reported at the American Academy of Psychiatry and the Law meeting. Some participants have mental illness and about a third have cognitive or neurologic limitations, she said. The majority of those referred receive mental health services from separate district agencies. If such needs are being not being met, OCRP administrators refer them to a clinic for treatment.
Of 170 individuals enrolled in the OCRP from 2009 to 2013, 54 were deemed competent to stand trial after completion, said Dr. Johnson, who oversees the program.
In an interview at the meeting, Dr. Johnson discussed how much money the program has saved the district and whether other jurisdictions can feasibly start similar programs.
On Twitter @legal_med
CHICAGO– A competency restoration program in the District of Columbia is helping defendants with mental illness become fit to stand trial, while saving the district money. The program could serve as a model for other jurisdictions that want to improve the competency of unfit defendants, Dr. Nicole R. Johnson, director of outpatient forensic service at the district’s Department of Behavioral Health said in an interview.
The District of Columbia’s Outpatient Competency Restoration Program (OCRP) started in 2009 and receives court-ordered referrals for individuals to participate in the program. The program works to restore defendants’ competency through question and answer sessions, games, and educational lessons, among other methods, Dr. Johnson reported at the American Academy of Psychiatry and the Law meeting. Some participants have mental illness and about a third have cognitive or neurologic limitations, she said. The majority of those referred receive mental health services from separate district agencies. If such needs are being not being met, OCRP administrators refer them to a clinic for treatment.
Of 170 individuals enrolled in the OCRP from 2009 to 2013, 54 were deemed competent to stand trial after completion, said Dr. Johnson, who oversees the program.
In an interview at the meeting, Dr. Johnson discussed how much money the program has saved the district and whether other jurisdictions can feasibly start similar programs.
On Twitter @legal_med
AT THE ANNUAL MEETING OF THE AMERICAN ACADEMY OF PSYCHIATRY AND THE LAW
Blood sample storage may hinder leukemia research

Credit: Graham Colm
Storing blood samples at room temperature can induce changes that may cloud research findings, according to a group of investigators.
The team initially found that blood samples from leukemia patients had high levels of malformed RNA, a discovery they believed could explain leukemia’s origins.
But additional research showed this abnormality was a result of storing blood samples at room temperature for hours, or even days, prior to processing.
Heidi Dvinge, PhD, of the Fred Hutchinson Cancer Research Center in Seattle, and her colleagues reported these findings in PNAS.
The investigators had searched databases to collect genomic information on leukemia patients and healthy control subjects. In all but one of the datasets they analyzed, the team found high levels of abnormal RNA in leukemia cells.
Another finding was that samples from pediatric leukemia patients had the highest levels of abnormal RNA. And this led the researchers to speculate about the cause.
They realized that pediatric leukemias are rare, so blood samples can be difficult to obtain. Therefore, the samples in the databases were collected at facilities throughout the world and shipped to where they were needed. So the samples could be stored at room temperature for days at a time.
To confirm that this practice can affect blood samples, the investigators conducted an experiment. They collected samples from 4 healthy subjects (2 men and 2 women) and looked for differences between samples that were processed immediately and samples that sat in the lab at room temperature for up to 48 hours.
Sure enough, the team observed changes in the stored samples, even if they were only stored for 4 hours. Stored samples exhibited biased activation of biological pathways and upregulation of pseudogenes, antisense RNAs, and unannotated coding isoforms.
Storage affected a number of genes that play roles in biological pathways relevant to leukemia, including cytokine production, NF-κB signaling, chromatin modification, and RNA splicing. Additionally, storage inhibited RNA surveillance, leading to the genome-wide expression of normally degraded RNAs.
The researchers said these findings coincide with the database findings, as they observed inhibited RNA surveillance in all but one of the datasets they analyzed.
The samples from that dataset were processed immediately after collection. And samples from healthy subjects were processed immediately, which explains why those samples were normal as well.
The team also noted that they did not observe inhibited RNA surveillance in lymphoma or solid tumor datasets.
These results suggest previous research utilizing these types of databases may contain errors, and future work making use of these databases could be affected as well.
Fortunately, the investigators found that putting blood samples on ice can prevent the negative effects they observed. The group also identified biomarkers that indicate prolonged storage, so researchers can look for those biomarkers if chilling blood samples is not an option.
In addition to describing these findings in PNAS, Dr Dvinge and her colleagues are planning to present their work at the upcoming ASH Annual Meeting. ![]()

Credit: Graham Colm
Storing blood samples at room temperature can induce changes that may cloud research findings, according to a group of investigators.
The team initially found that blood samples from leukemia patients had high levels of malformed RNA, a discovery they believed could explain leukemia’s origins.
But additional research showed this abnormality was a result of storing blood samples at room temperature for hours, or even days, prior to processing.
Heidi Dvinge, PhD, of the Fred Hutchinson Cancer Research Center in Seattle, and her colleagues reported these findings in PNAS.
The investigators had searched databases to collect genomic information on leukemia patients and healthy control subjects. In all but one of the datasets they analyzed, the team found high levels of abnormal RNA in leukemia cells.
Another finding was that samples from pediatric leukemia patients had the highest levels of abnormal RNA. And this led the researchers to speculate about the cause.
They realized that pediatric leukemias are rare, so blood samples can be difficult to obtain. Therefore, the samples in the databases were collected at facilities throughout the world and shipped to where they were needed. So the samples could be stored at room temperature for days at a time.
To confirm that this practice can affect blood samples, the investigators conducted an experiment. They collected samples from 4 healthy subjects (2 men and 2 women) and looked for differences between samples that were processed immediately and samples that sat in the lab at room temperature for up to 48 hours.
Sure enough, the team observed changes in the stored samples, even if they were only stored for 4 hours. Stored samples exhibited biased activation of biological pathways and upregulation of pseudogenes, antisense RNAs, and unannotated coding isoforms.
Storage affected a number of genes that play roles in biological pathways relevant to leukemia, including cytokine production, NF-κB signaling, chromatin modification, and RNA splicing. Additionally, storage inhibited RNA surveillance, leading to the genome-wide expression of normally degraded RNAs.
The researchers said these findings coincide with the database findings, as they observed inhibited RNA surveillance in all but one of the datasets they analyzed.
The samples from that dataset were processed immediately after collection. And samples from healthy subjects were processed immediately, which explains why those samples were normal as well.
The team also noted that they did not observe inhibited RNA surveillance in lymphoma or solid tumor datasets.
These results suggest previous research utilizing these types of databases may contain errors, and future work making use of these databases could be affected as well.
Fortunately, the investigators found that putting blood samples on ice can prevent the negative effects they observed. The group also identified biomarkers that indicate prolonged storage, so researchers can look for those biomarkers if chilling blood samples is not an option.
In addition to describing these findings in PNAS, Dr Dvinge and her colleagues are planning to present their work at the upcoming ASH Annual Meeting. ![]()

Credit: Graham Colm
Storing blood samples at room temperature can induce changes that may cloud research findings, according to a group of investigators.
The team initially found that blood samples from leukemia patients had high levels of malformed RNA, a discovery they believed could explain leukemia’s origins.
But additional research showed this abnormality was a result of storing blood samples at room temperature for hours, or even days, prior to processing.
Heidi Dvinge, PhD, of the Fred Hutchinson Cancer Research Center in Seattle, and her colleagues reported these findings in PNAS.
The investigators had searched databases to collect genomic information on leukemia patients and healthy control subjects. In all but one of the datasets they analyzed, the team found high levels of abnormal RNA in leukemia cells.
Another finding was that samples from pediatric leukemia patients had the highest levels of abnormal RNA. And this led the researchers to speculate about the cause.
They realized that pediatric leukemias are rare, so blood samples can be difficult to obtain. Therefore, the samples in the databases were collected at facilities throughout the world and shipped to where they were needed. So the samples could be stored at room temperature for days at a time.
To confirm that this practice can affect blood samples, the investigators conducted an experiment. They collected samples from 4 healthy subjects (2 men and 2 women) and looked for differences between samples that were processed immediately and samples that sat in the lab at room temperature for up to 48 hours.
Sure enough, the team observed changes in the stored samples, even if they were only stored for 4 hours. Stored samples exhibited biased activation of biological pathways and upregulation of pseudogenes, antisense RNAs, and unannotated coding isoforms.
Storage affected a number of genes that play roles in biological pathways relevant to leukemia, including cytokine production, NF-κB signaling, chromatin modification, and RNA splicing. Additionally, storage inhibited RNA surveillance, leading to the genome-wide expression of normally degraded RNAs.
The researchers said these findings coincide with the database findings, as they observed inhibited RNA surveillance in all but one of the datasets they analyzed.
The samples from that dataset were processed immediately after collection. And samples from healthy subjects were processed immediately, which explains why those samples were normal as well.
The team also noted that they did not observe inhibited RNA surveillance in lymphoma or solid tumor datasets.
These results suggest previous research utilizing these types of databases may contain errors, and future work making use of these databases could be affected as well.
Fortunately, the investigators found that putting blood samples on ice can prevent the negative effects they observed. The group also identified biomarkers that indicate prolonged storage, so researchers can look for those biomarkers if chilling blood samples is not an option.
In addition to describing these findings in PNAS, Dr Dvinge and her colleagues are planning to present their work at the upcoming ASH Annual Meeting. ![]()
Sharing research with public prompts more citations

Credit: Rhoda Baer
Academic researchers who talk to the press and use social media are more likely than their less communicative peers to have their work cited, a new study suggests.
The research revealed a connection between h-index—a measure of the quality of a researcher’s work and influence—and whether the scientists interact with reporters and get mentioned on Twitter.
The results appear in Journalism & Mass Communications Quarterly.
“I’ve been in science communication for a while now, and I am really seeing a change—especially among the younger scientists—in their willingness to share their work,” said study author Dominique Brossard, PhD, of the University of Wisconsin-Madison.
Attention from reporters is good news for h-index, Dr Brossard noted. But couple that with attention on Twitter, and you see a more pronounced spike in reputation.
“If you talk to reporters and you tweet about your research, your work is more likely to be cited than people who do one or the other,” she said.
That sort of activity hasn’t always been encouraged, Dr Brossard pointed out. Any distraction from a researcher’s work can draw criticism as a waste of a precious resource. But Dr Brossard hopes a new understanding of the relationship between research and communicating with the public can change that.
“What this shows us is that sharing your science with the public is not hurting the science by stealing time,” she said. “If the goal is to encourage people, ultimately, to be productive scientists, and if directors of labs are discouraging people from engaging in this activity, they’re actually hurting the science itself. Because people who do this are cited more often in scientific journals, [and] they’re making science accessible to broader audiences at the same time.”
Social media use is rising in other professional circles as well, according to Michael Xenos, PhD, also of the University of Wisconsin-Madison.
“As in other areas, such as politics for example, social media was once met with skepticism but is increasingly part of the culture,” he said. “Just like it became the norm there, our research shows it may one day become the norm in science.”
Even if you flip the connection between social media attention and h-index on its head, it’s still worth taking to heart, according to the researchers.
“The counter argument is that it may be just the other way around—that it may just be the big names that get mentions,” said study author Dietram A. Scheufele, PhD, also of the University of Wisconsin-Madison.
“But then, the lesson should be that the most successful people in your field are also the ones that are good at getting outside the ivory tower. That should be something to emulate.” ![]()

Credit: Rhoda Baer
Academic researchers who talk to the press and use social media are more likely than their less communicative peers to have their work cited, a new study suggests.
The research revealed a connection between h-index—a measure of the quality of a researcher’s work and influence—and whether the scientists interact with reporters and get mentioned on Twitter.
The results appear in Journalism & Mass Communications Quarterly.
“I’ve been in science communication for a while now, and I am really seeing a change—especially among the younger scientists—in their willingness to share their work,” said study author Dominique Brossard, PhD, of the University of Wisconsin-Madison.
Attention from reporters is good news for h-index, Dr Brossard noted. But couple that with attention on Twitter, and you see a more pronounced spike in reputation.
“If you talk to reporters and you tweet about your research, your work is more likely to be cited than people who do one or the other,” she said.
That sort of activity hasn’t always been encouraged, Dr Brossard pointed out. Any distraction from a researcher’s work can draw criticism as a waste of a precious resource. But Dr Brossard hopes a new understanding of the relationship between research and communicating with the public can change that.
“What this shows us is that sharing your science with the public is not hurting the science by stealing time,” she said. “If the goal is to encourage people, ultimately, to be productive scientists, and if directors of labs are discouraging people from engaging in this activity, they’re actually hurting the science itself. Because people who do this are cited more often in scientific journals, [and] they’re making science accessible to broader audiences at the same time.”
Social media use is rising in other professional circles as well, according to Michael Xenos, PhD, also of the University of Wisconsin-Madison.
“As in other areas, such as politics for example, social media was once met with skepticism but is increasingly part of the culture,” he said. “Just like it became the norm there, our research shows it may one day become the norm in science.”
Even if you flip the connection between social media attention and h-index on its head, it’s still worth taking to heart, according to the researchers.
“The counter argument is that it may be just the other way around—that it may just be the big names that get mentions,” said study author Dietram A. Scheufele, PhD, also of the University of Wisconsin-Madison.
“But then, the lesson should be that the most successful people in your field are also the ones that are good at getting outside the ivory tower. That should be something to emulate.” ![]()

Credit: Rhoda Baer
Academic researchers who talk to the press and use social media are more likely than their less communicative peers to have their work cited, a new study suggests.
The research revealed a connection between h-index—a measure of the quality of a researcher’s work and influence—and whether the scientists interact with reporters and get mentioned on Twitter.
The results appear in Journalism & Mass Communications Quarterly.
“I’ve been in science communication for a while now, and I am really seeing a change—especially among the younger scientists—in their willingness to share their work,” said study author Dominique Brossard, PhD, of the University of Wisconsin-Madison.
Attention from reporters is good news for h-index, Dr Brossard noted. But couple that with attention on Twitter, and you see a more pronounced spike in reputation.
“If you talk to reporters and you tweet about your research, your work is more likely to be cited than people who do one or the other,” she said.
That sort of activity hasn’t always been encouraged, Dr Brossard pointed out. Any distraction from a researcher’s work can draw criticism as a waste of a precious resource. But Dr Brossard hopes a new understanding of the relationship between research and communicating with the public can change that.
“What this shows us is that sharing your science with the public is not hurting the science by stealing time,” she said. “If the goal is to encourage people, ultimately, to be productive scientists, and if directors of labs are discouraging people from engaging in this activity, they’re actually hurting the science itself. Because people who do this are cited more often in scientific journals, [and] they’re making science accessible to broader audiences at the same time.”
Social media use is rising in other professional circles as well, according to Michael Xenos, PhD, also of the University of Wisconsin-Madison.
“As in other areas, such as politics for example, social media was once met with skepticism but is increasingly part of the culture,” he said. “Just like it became the norm there, our research shows it may one day become the norm in science.”
Even if you flip the connection between social media attention and h-index on its head, it’s still worth taking to heart, according to the researchers.
“The counter argument is that it may be just the other way around—that it may just be the big names that get mentions,” said study author Dietram A. Scheufele, PhD, also of the University of Wisconsin-Madison.
“But then, the lesson should be that the most successful people in your field are also the ones that are good at getting outside the ivory tower. That should be something to emulate.” ![]()
Hospice cuts cost and use of care for cancer patients

Credit: CDC
Patients with advanced cancer receive less aggressive care and have lower healthcare costs during their last year of life if they use hospice care, according to research published in JAMA.
Patients who entered hospice had significantly lower rates of hospitalization, intensive care unit admissions, and invasive procedures, compared to patients who did not enter hospice.
Furthermore, patients who chose hospice were about 5 times less likely to die in hospitals and nursing homes.
“Our study shows very clearly that hospice matters,” said Ziad Obermeyer, MD, of Brigham and Women’s Hospital in Boston.
“Hospice and non-hospice patients had very similar patterns of healthcare utilization right up until the week of hospice enrollment. Then, the care started to look very different. Patients who didn’t enroll in hospice ended up with far more aggressive care in their last year of life, most of it related to acute complications like infections and organ failure, and not directly related to their cancer diagnosis.”
To conduct this study, Dr Obermeyer and his colleagues used data from Medicare beneficiaries with poor-prognosis cancers, including hematologic malignancies. The study included a nationally representative 20% sample of Medicare fee-for-service beneficiaries who died in 2011.
Among 86,851 patients, 51,924 (60%) entered hospice before death. Matching patients based on various criteria produced hospice and non-hospice groups, each with 18,165 patients. The median hospice duration was 11 days.
The researchers found that non-hospice patients had significantly greater healthcare utilization, largely for acute conditions not directly related to cancer.
This included rates of hospitalization (65% vs 42%), intensive care unit admissions (36% vs 15%), invasive procedures (51% vs 27%), and death in a hospital or nursing facility (74% vs 14%).
The costs of care for hospice and non-hospice patients were not significantly different before hospice care began. But they diverged sharply thereafter, contributing to a significant difference in total costs of $8697 over the last year of life—$71,517 for non-hospice patients and $62,819 for hospice patients.
“These findings highlight the importance of honest discussions between doctors and patients about our patients’ goals of their care at the end of life, relating to treatment decisions and quality of life,” Dr Obermeyer said. “This is of particular importance now, in light of the ongoing policy discussions around reimbursing providers for advance-care planning.” ![]()

Credit: CDC
Patients with advanced cancer receive less aggressive care and have lower healthcare costs during their last year of life if they use hospice care, according to research published in JAMA.
Patients who entered hospice had significantly lower rates of hospitalization, intensive care unit admissions, and invasive procedures, compared to patients who did not enter hospice.
Furthermore, patients who chose hospice were about 5 times less likely to die in hospitals and nursing homes.
“Our study shows very clearly that hospice matters,” said Ziad Obermeyer, MD, of Brigham and Women’s Hospital in Boston.
“Hospice and non-hospice patients had very similar patterns of healthcare utilization right up until the week of hospice enrollment. Then, the care started to look very different. Patients who didn’t enroll in hospice ended up with far more aggressive care in their last year of life, most of it related to acute complications like infections and organ failure, and not directly related to their cancer diagnosis.”
To conduct this study, Dr Obermeyer and his colleagues used data from Medicare beneficiaries with poor-prognosis cancers, including hematologic malignancies. The study included a nationally representative 20% sample of Medicare fee-for-service beneficiaries who died in 2011.
Among 86,851 patients, 51,924 (60%) entered hospice before death. Matching patients based on various criteria produced hospice and non-hospice groups, each with 18,165 patients. The median hospice duration was 11 days.
The researchers found that non-hospice patients had significantly greater healthcare utilization, largely for acute conditions not directly related to cancer.
This included rates of hospitalization (65% vs 42%), intensive care unit admissions (36% vs 15%), invasive procedures (51% vs 27%), and death in a hospital or nursing facility (74% vs 14%).
The costs of care for hospice and non-hospice patients were not significantly different before hospice care began. But they diverged sharply thereafter, contributing to a significant difference in total costs of $8697 over the last year of life—$71,517 for non-hospice patients and $62,819 for hospice patients.
“These findings highlight the importance of honest discussions between doctors and patients about our patients’ goals of their care at the end of life, relating to treatment decisions and quality of life,” Dr Obermeyer said. “This is of particular importance now, in light of the ongoing policy discussions around reimbursing providers for advance-care planning.” ![]()

Credit: CDC
Patients with advanced cancer receive less aggressive care and have lower healthcare costs during their last year of life if they use hospice care, according to research published in JAMA.
Patients who entered hospice had significantly lower rates of hospitalization, intensive care unit admissions, and invasive procedures, compared to patients who did not enter hospice.
Furthermore, patients who chose hospice were about 5 times less likely to die in hospitals and nursing homes.
“Our study shows very clearly that hospice matters,” said Ziad Obermeyer, MD, of Brigham and Women’s Hospital in Boston.
“Hospice and non-hospice patients had very similar patterns of healthcare utilization right up until the week of hospice enrollment. Then, the care started to look very different. Patients who didn’t enroll in hospice ended up with far more aggressive care in their last year of life, most of it related to acute complications like infections and organ failure, and not directly related to their cancer diagnosis.”
To conduct this study, Dr Obermeyer and his colleagues used data from Medicare beneficiaries with poor-prognosis cancers, including hematologic malignancies. The study included a nationally representative 20% sample of Medicare fee-for-service beneficiaries who died in 2011.
Among 86,851 patients, 51,924 (60%) entered hospice before death. Matching patients based on various criteria produced hospice and non-hospice groups, each with 18,165 patients. The median hospice duration was 11 days.
The researchers found that non-hospice patients had significantly greater healthcare utilization, largely for acute conditions not directly related to cancer.
This included rates of hospitalization (65% vs 42%), intensive care unit admissions (36% vs 15%), invasive procedures (51% vs 27%), and death in a hospital or nursing facility (74% vs 14%).
The costs of care for hospice and non-hospice patients were not significantly different before hospice care began. But they diverged sharply thereafter, contributing to a significant difference in total costs of $8697 over the last year of life—$71,517 for non-hospice patients and $62,819 for hospice patients.
“These findings highlight the importance of honest discussions between doctors and patients about our patients’ goals of their care at the end of life, relating to treatment decisions and quality of life,” Dr Obermeyer said. “This is of particular importance now, in light of the ongoing policy discussions around reimbursing providers for advance-care planning.” ![]()
NICE supports use of catheter-positioning device

The UK’s National Institute for Health and Care Excellence (NICE) has opened consultation on a draft guidance about a device designed to help healthcare professionals correctly place peripherally inserted central catheters
(PICCs).
The draft guidance supports using the Sherlock 3CG Tip Confirmation System for placing PICCs.
The standard procedure for placing PICCs is blind insertion, followed by a chest X-ray to check the catheter’s position.
In some cases, fluoroscopy is used instead of standard X-ray to assist with positioning the PICC when placing it proves difficult.
The Sherlock system, on the other hand, uses magnetic and electrocardiographic real-time tracking of a PICC to enable the person placing the catheter to detect and correct any error in how the tip is positioned.
The device’s manufacturer, C.R. Bard, says the Sherlock System eliminates the need for the patient to have an X-ray, thus preventing delays in treatment or monitoring.
“Using the technology also increases staff and patient confidence of the accuracy of the procedure during catheter insertion,” said Professor Carole Longson, director of the NICE centre for health technology evaluation.
The cost of the Sherlock 3CG TCS is stated in the manufacturer’s submission as £9990 (excluding value-added tax). The cost of consumables associated with each insertion is £189.91. Maintenance costs associated with the technology are £595 per year.
Across the whole population in which PICCs are placed, the cost of using the Sherlock system is similar to blind insertion followed by X-ray, but it can save up to £106 per patient in specific clinical situations.
In intensive care, where PICCs are more likely to be placed incorrectly using blind insertion, the savings from using the Sherlock system and a confirmatory X-ray are estimated at around £41 per patient, compared with blind insertion and standard X-ray. (In intensive care settings, staff members sometimes initially use Sherlock with confirmatory X-ray while they are becoming accustomed to the system.)
For more information on the system, see the draft guidance consultation. It is open for public comment until December 9. {HT_DN}

The UK’s National Institute for Health and Care Excellence (NICE) has opened consultation on a draft guidance about a device designed to help healthcare professionals correctly place peripherally inserted central catheters
(PICCs).
The draft guidance supports using the Sherlock 3CG Tip Confirmation System for placing PICCs.
The standard procedure for placing PICCs is blind insertion, followed by a chest X-ray to check the catheter’s position.
In some cases, fluoroscopy is used instead of standard X-ray to assist with positioning the PICC when placing it proves difficult.
The Sherlock system, on the other hand, uses magnetic and electrocardiographic real-time tracking of a PICC to enable the person placing the catheter to detect and correct any error in how the tip is positioned.
The device’s manufacturer, C.R. Bard, says the Sherlock System eliminates the need for the patient to have an X-ray, thus preventing delays in treatment or monitoring.
“Using the technology also increases staff and patient confidence of the accuracy of the procedure during catheter insertion,” said Professor Carole Longson, director of the NICE centre for health technology evaluation.
The cost of the Sherlock 3CG TCS is stated in the manufacturer’s submission as £9990 (excluding value-added tax). The cost of consumables associated with each insertion is £189.91. Maintenance costs associated with the technology are £595 per year.
Across the whole population in which PICCs are placed, the cost of using the Sherlock system is similar to blind insertion followed by X-ray, but it can save up to £106 per patient in specific clinical situations.
In intensive care, where PICCs are more likely to be placed incorrectly using blind insertion, the savings from using the Sherlock system and a confirmatory X-ray are estimated at around £41 per patient, compared with blind insertion and standard X-ray. (In intensive care settings, staff members sometimes initially use Sherlock with confirmatory X-ray while they are becoming accustomed to the system.)
For more information on the system, see the draft guidance consultation. It is open for public comment until December 9. {HT_DN}

The UK’s National Institute for Health and Care Excellence (NICE) has opened consultation on a draft guidance about a device designed to help healthcare professionals correctly place peripherally inserted central catheters
(PICCs).
The draft guidance supports using the Sherlock 3CG Tip Confirmation System for placing PICCs.
The standard procedure for placing PICCs is blind insertion, followed by a chest X-ray to check the catheter’s position.
In some cases, fluoroscopy is used instead of standard X-ray to assist with positioning the PICC when placing it proves difficult.
The Sherlock system, on the other hand, uses magnetic and electrocardiographic real-time tracking of a PICC to enable the person placing the catheter to detect and correct any error in how the tip is positioned.
The device’s manufacturer, C.R. Bard, says the Sherlock System eliminates the need for the patient to have an X-ray, thus preventing delays in treatment or monitoring.
“Using the technology also increases staff and patient confidence of the accuracy of the procedure during catheter insertion,” said Professor Carole Longson, director of the NICE centre for health technology evaluation.
The cost of the Sherlock 3CG TCS is stated in the manufacturer’s submission as £9990 (excluding value-added tax). The cost of consumables associated with each insertion is £189.91. Maintenance costs associated with the technology are £595 per year.
Across the whole population in which PICCs are placed, the cost of using the Sherlock system is similar to blind insertion followed by X-ray, but it can save up to £106 per patient in specific clinical situations.
In intensive care, where PICCs are more likely to be placed incorrectly using blind insertion, the savings from using the Sherlock system and a confirmatory X-ray are estimated at around £41 per patient, compared with blind insertion and standard X-ray. (In intensive care settings, staff members sometimes initially use Sherlock with confirmatory X-ray while they are becoming accustomed to the system.)
For more information on the system, see the draft guidance consultation. It is open for public comment until December 9. {HT_DN}
Preop risk assessment, prophylaxis for VTE
The majority of women with gynecologic cancer will undergo surgery for their disease. Deep vein thrombosis and pulmonary embolism, or venous thromboembolic events are common, serious complications. The rate of pulmonary embolism in women with gynecologic malignancy may be as high as 6.8%, with the case fatality rate being 11%-12%. Hence, one key strategy to lower the rate of fatal pulmonary embolism depends on proper prophylaxis for deep vein thrombosis prevention.
Factors associated with the development of venous thromboembolic events (VTE) include prior VTE, malignancy, older age, African American race, prolonged operative time, and prior radiation therapy (Obstet. Gynecol. 1987;69:146-50). The risk of pulmonary embolism (PE) in women undergoing gynecologic surgery is quadrupled in the presence of malignancy (Obstet. Gynecol. 2006;107:666-71) and these patients are twice as likely to die from a VTE, compared with matched controls (Gynecol. Oncol. 2007;106:439-45). In addition, cancer patients are typically older and have longer and more complex surgeries. Furthermore, the presence of a pelvic mass further contributes to venous stasis (Obstet. Gynecol. 2012;119:155-67).
Other risk factors associated with the development of VTE include hormone replacement therapy, oral contraceptives, use of tamoxifen, and inherited thrombophilias. The most common is factor V Leiden deficiency, affecting up to 20% of patients with VTE. Affected heterozygotes have a 3- to 8-fold increased risk of VTE, whereas homozygotes have a 50- to 80-fold increased risk (Blood 1995;85:1504-8).
Depending on additional risk factors, both the American College of Obstetricians and Gynecologists (ACOG) Practice Bulletin and guidelines published by the American College of Chest Physicians (ACCP) place women with gynecologic cancers into "high" or "highest" risk categories (Obstet. Gynecol. 2007;110:429-40; Chest 2012;141:e227S-77S).
Currently, thromboprophylaxis regimens include mechanical and pharmacologic methods. Mechanical devices include graduated compression stockings and intermittent pneumatic compression, which reduce venous stasis and may promote endogenous fibrinolysis. Pharmacologic prophylaxis includes unfractionated heparin (UFH) and low-molecular weight heparin (LMWH). Prospective controlled trials have shown that UFH reduces VTE in patients with gynecologic cancer. Trials comparing LMWH with UFH have demonstrated equivalent efficacy and similar bleeding complications. The recommended prophylactic dose for LMWH is 40 mg subcutaneous injection daily. However, this dose may need to be adjusted in morbidly obese patients (body mass index greater than 40 kg/m2) as well as in women with abnormal renal clearance. UFH should be administered as a dose of 5,000 units subcutaneously three times daily. Intermittent pneumatic compression also has been shown to reduce the incidence of VTE in this patient population.
A combined regimen of pharmacologic and mechanical prophylaxis may improve efficacy, especially in the highest-risk patients, such as women with gynecologic cancer. Although limited data exist to support this approach in gynecology patients, studies from other surgical disciplines suggest benefit from a combined regimen. With regards to addressing the timing of initiation, a large retrospective trial of patients undergoing hysterectomy for benign indications concluded that postoperative rather than preoperative administration of UFH or LMWH may reduce the risk of bleeding complications without apparent risk of increased VTE (Acta. Obstet. Gynecol. Scand. 2008;87:1039-47).
In summary, the majority of gynecologic oncology patients are considered to be at the highest risk for developing VTE. For this group of women, double prophylaxis with either UFH or LMWH, and a mechanical method (intermittent pneumatic compression) are recommended in the perioperative setting. In addition, ACCP further recommends that these patients receive extended postoperative prophylaxis with LMWH for 4 weeks. Further evidence is needed to determine acceptable timing for initiation of therapy in order to find a balance between adequate thromboprophylaxis and bleeding complications.
Dr. Roque is a fellow in the gynecologic oncology program at the University of North Carolina at Chapel Hill. Dr. Clarke-Pearson is the chair and the Robert A. Ross Distinguished Professor of Obstetrics and Gynecology and a professor in the division of gynecologic oncology at the university. Dr. Roque and Dr. Clarke-Pearson said they had no relevant disclosures. Scan this QR code or go to obgynnews.com to view similar columns.
The majority of women with gynecologic cancer will undergo surgery for their disease. Deep vein thrombosis and pulmonary embolism, or venous thromboembolic events are common, serious complications. The rate of pulmonary embolism in women with gynecologic malignancy may be as high as 6.8%, with the case fatality rate being 11%-12%. Hence, one key strategy to lower the rate of fatal pulmonary embolism depends on proper prophylaxis for deep vein thrombosis prevention.
Factors associated with the development of venous thromboembolic events (VTE) include prior VTE, malignancy, older age, African American race, prolonged operative time, and prior radiation therapy (Obstet. Gynecol. 1987;69:146-50). The risk of pulmonary embolism (PE) in women undergoing gynecologic surgery is quadrupled in the presence of malignancy (Obstet. Gynecol. 2006;107:666-71) and these patients are twice as likely to die from a VTE, compared with matched controls (Gynecol. Oncol. 2007;106:439-45). In addition, cancer patients are typically older and have longer and more complex surgeries. Furthermore, the presence of a pelvic mass further contributes to venous stasis (Obstet. Gynecol. 2012;119:155-67).
Other risk factors associated with the development of VTE include hormone replacement therapy, oral contraceptives, use of tamoxifen, and inherited thrombophilias. The most common is factor V Leiden deficiency, affecting up to 20% of patients with VTE. Affected heterozygotes have a 3- to 8-fold increased risk of VTE, whereas homozygotes have a 50- to 80-fold increased risk (Blood 1995;85:1504-8).
Depending on additional risk factors, both the American College of Obstetricians and Gynecologists (ACOG) Practice Bulletin and guidelines published by the American College of Chest Physicians (ACCP) place women with gynecologic cancers into "high" or "highest" risk categories (Obstet. Gynecol. 2007;110:429-40; Chest 2012;141:e227S-77S).
Currently, thromboprophylaxis regimens include mechanical and pharmacologic methods. Mechanical devices include graduated compression stockings and intermittent pneumatic compression, which reduce venous stasis and may promote endogenous fibrinolysis. Pharmacologic prophylaxis includes unfractionated heparin (UFH) and low-molecular weight heparin (LMWH). Prospective controlled trials have shown that UFH reduces VTE in patients with gynecologic cancer. Trials comparing LMWH with UFH have demonstrated equivalent efficacy and similar bleeding complications. The recommended prophylactic dose for LMWH is 40 mg subcutaneous injection daily. However, this dose may need to be adjusted in morbidly obese patients (body mass index greater than 40 kg/m2) as well as in women with abnormal renal clearance. UFH should be administered as a dose of 5,000 units subcutaneously three times daily. Intermittent pneumatic compression also has been shown to reduce the incidence of VTE in this patient population.
A combined regimen of pharmacologic and mechanical prophylaxis may improve efficacy, especially in the highest-risk patients, such as women with gynecologic cancer. Although limited data exist to support this approach in gynecology patients, studies from other surgical disciplines suggest benefit from a combined regimen. With regards to addressing the timing of initiation, a large retrospective trial of patients undergoing hysterectomy for benign indications concluded that postoperative rather than preoperative administration of UFH or LMWH may reduce the risk of bleeding complications without apparent risk of increased VTE (Acta. Obstet. Gynecol. Scand. 2008;87:1039-47).
In summary, the majority of gynecologic oncology patients are considered to be at the highest risk for developing VTE. For this group of women, double prophylaxis with either UFH or LMWH, and a mechanical method (intermittent pneumatic compression) are recommended in the perioperative setting. In addition, ACCP further recommends that these patients receive extended postoperative prophylaxis with LMWH for 4 weeks. Further evidence is needed to determine acceptable timing for initiation of therapy in order to find a balance between adequate thromboprophylaxis and bleeding complications.
Dr. Roque is a fellow in the gynecologic oncology program at the University of North Carolina at Chapel Hill. Dr. Clarke-Pearson is the chair and the Robert A. Ross Distinguished Professor of Obstetrics and Gynecology and a professor in the division of gynecologic oncology at the university. Dr. Roque and Dr. Clarke-Pearson said they had no relevant disclosures. Scan this QR code or go to obgynnews.com to view similar columns.
The majority of women with gynecologic cancer will undergo surgery for their disease. Deep vein thrombosis and pulmonary embolism, or venous thromboembolic events are common, serious complications. The rate of pulmonary embolism in women with gynecologic malignancy may be as high as 6.8%, with the case fatality rate being 11%-12%. Hence, one key strategy to lower the rate of fatal pulmonary embolism depends on proper prophylaxis for deep vein thrombosis prevention.
Factors associated with the development of venous thromboembolic events (VTE) include prior VTE, malignancy, older age, African American race, prolonged operative time, and prior radiation therapy (Obstet. Gynecol. 1987;69:146-50). The risk of pulmonary embolism (PE) in women undergoing gynecologic surgery is quadrupled in the presence of malignancy (Obstet. Gynecol. 2006;107:666-71) and these patients are twice as likely to die from a VTE, compared with matched controls (Gynecol. Oncol. 2007;106:439-45). In addition, cancer patients are typically older and have longer and more complex surgeries. Furthermore, the presence of a pelvic mass further contributes to venous stasis (Obstet. Gynecol. 2012;119:155-67).
Other risk factors associated with the development of VTE include hormone replacement therapy, oral contraceptives, use of tamoxifen, and inherited thrombophilias. The most common is factor V Leiden deficiency, affecting up to 20% of patients with VTE. Affected heterozygotes have a 3- to 8-fold increased risk of VTE, whereas homozygotes have a 50- to 80-fold increased risk (Blood 1995;85:1504-8).
Depending on additional risk factors, both the American College of Obstetricians and Gynecologists (ACOG) Practice Bulletin and guidelines published by the American College of Chest Physicians (ACCP) place women with gynecologic cancers into "high" or "highest" risk categories (Obstet. Gynecol. 2007;110:429-40; Chest 2012;141:e227S-77S).
Currently, thromboprophylaxis regimens include mechanical and pharmacologic methods. Mechanical devices include graduated compression stockings and intermittent pneumatic compression, which reduce venous stasis and may promote endogenous fibrinolysis. Pharmacologic prophylaxis includes unfractionated heparin (UFH) and low-molecular weight heparin (LMWH). Prospective controlled trials have shown that UFH reduces VTE in patients with gynecologic cancer. Trials comparing LMWH with UFH have demonstrated equivalent efficacy and similar bleeding complications. The recommended prophylactic dose for LMWH is 40 mg subcutaneous injection daily. However, this dose may need to be adjusted in morbidly obese patients (body mass index greater than 40 kg/m2) as well as in women with abnormal renal clearance. UFH should be administered as a dose of 5,000 units subcutaneously three times daily. Intermittent pneumatic compression also has been shown to reduce the incidence of VTE in this patient population.
A combined regimen of pharmacologic and mechanical prophylaxis may improve efficacy, especially in the highest-risk patients, such as women with gynecologic cancer. Although limited data exist to support this approach in gynecology patients, studies from other surgical disciplines suggest benefit from a combined regimen. With regards to addressing the timing of initiation, a large retrospective trial of patients undergoing hysterectomy for benign indications concluded that postoperative rather than preoperative administration of UFH or LMWH may reduce the risk of bleeding complications without apparent risk of increased VTE (Acta. Obstet. Gynecol. Scand. 2008;87:1039-47).
In summary, the majority of gynecologic oncology patients are considered to be at the highest risk for developing VTE. For this group of women, double prophylaxis with either UFH or LMWH, and a mechanical method (intermittent pneumatic compression) are recommended in the perioperative setting. In addition, ACCP further recommends that these patients receive extended postoperative prophylaxis with LMWH for 4 weeks. Further evidence is needed to determine acceptable timing for initiation of therapy in order to find a balance between adequate thromboprophylaxis and bleeding complications.
Dr. Roque is a fellow in the gynecologic oncology program at the University of North Carolina at Chapel Hill. Dr. Clarke-Pearson is the chair and the Robert A. Ross Distinguished Professor of Obstetrics and Gynecology and a professor in the division of gynecologic oncology at the university. Dr. Roque and Dr. Clarke-Pearson said they had no relevant disclosures. Scan this QR code or go to obgynnews.com to view similar columns.