User login
In critically ill patients, dalteparin is more cost-effective for VTE prevention
The low molecular weight heparin dalteparin and unfractionated heparin are associated with similar rates of thrombosis and major bleeding, but dalteparin is associated with lower rates of pulmonary embolus and heparin-induced thrombocytopenia, based on results from a prospective randomized study.
Given for prevention of venous thromboembolism, median hospital costs per patient were $39,508 for dalteparin users and $40,805 for unfractionated heparin users. Dalteparin remained the least costly strategy until its acquisition costs rose from $8 per dose to $179, as reported online 1 November in the Journal of the American Medical Association [doi:10.1001/jama.2014.15101].
The economic analysis—conducted alongside the multi-centre, randomized PROTECT trial in 2344 critically-ill medical-surgical patients— showed no matter how low the acquisition cost of unfractionated heparin, there was no threshold that favored that form of prophylaxis, according to data also presented at the Critical Care Canada Forum.
“From a health care payer perspective, VTE prophylaxis with the LMWH [low molecular weight heparin] dalteparin in critically ill medical-surgical patients was more effective and had similar or lower costs than the use of UFH [unfractionated heparin],” wrote Dr. Robert A. Fowler, from the Sunnybrook Health Sciences Centre, University of Toronto, and colleagues.The E-PROTECT study was funded by the Heart and Stroke Foundation (Ontario, Canada), the University of Toronto, and the Canadian Intensive Care Foundation. PROTECT was funded by the Canadian Institutes of Health Research, the Heart and Stroke Foundation (Canada), and the Australian and New Zealand College of Anesthetists Research Foundation. Some authors reported fees, support, and consultancies from the pharmaceutical industry.
The low molecular weight heparin dalteparin and unfractionated heparin are associated with similar rates of thrombosis and major bleeding, but dalteparin is associated with lower rates of pulmonary embolus and heparin-induced thrombocytopenia, based on results from a prospective randomized study.
Given for prevention of venous thromboembolism, median hospital costs per patient were $39,508 for dalteparin users and $40,805 for unfractionated heparin users. Dalteparin remained the least costly strategy until its acquisition costs rose from $8 per dose to $179, as reported online 1 November in the Journal of the American Medical Association [doi:10.1001/jama.2014.15101].
The economic analysis—conducted alongside the multi-centre, randomized PROTECT trial in 2344 critically-ill medical-surgical patients— showed no matter how low the acquisition cost of unfractionated heparin, there was no threshold that favored that form of prophylaxis, according to data also presented at the Critical Care Canada Forum.
“From a health care payer perspective, VTE prophylaxis with the LMWH [low molecular weight heparin] dalteparin in critically ill medical-surgical patients was more effective and had similar or lower costs than the use of UFH [unfractionated heparin],” wrote Dr. Robert A. Fowler, from the Sunnybrook Health Sciences Centre, University of Toronto, and colleagues.The E-PROTECT study was funded by the Heart and Stroke Foundation (Ontario, Canada), the University of Toronto, and the Canadian Intensive Care Foundation. PROTECT was funded by the Canadian Institutes of Health Research, the Heart and Stroke Foundation (Canada), and the Australian and New Zealand College of Anesthetists Research Foundation. Some authors reported fees, support, and consultancies from the pharmaceutical industry.
The low molecular weight heparin dalteparin and unfractionated heparin are associated with similar rates of thrombosis and major bleeding, but dalteparin is associated with lower rates of pulmonary embolus and heparin-induced thrombocytopenia, based on results from a prospective randomized study.
Given for prevention of venous thromboembolism, median hospital costs per patient were $39,508 for dalteparin users and $40,805 for unfractionated heparin users. Dalteparin remained the least costly strategy until its acquisition costs rose from $8 per dose to $179, as reported online 1 November in the Journal of the American Medical Association [doi:10.1001/jama.2014.15101].
The economic analysis—conducted alongside the multi-centre, randomized PROTECT trial in 2344 critically-ill medical-surgical patients— showed no matter how low the acquisition cost of unfractionated heparin, there was no threshold that favored that form of prophylaxis, according to data also presented at the Critical Care Canada Forum.
“From a health care payer perspective, VTE prophylaxis with the LMWH [low molecular weight heparin] dalteparin in critically ill medical-surgical patients was more effective and had similar or lower costs than the use of UFH [unfractionated heparin],” wrote Dr. Robert A. Fowler, from the Sunnybrook Health Sciences Centre, University of Toronto, and colleagues.The E-PROTECT study was funded by the Heart and Stroke Foundation (Ontario, Canada), the University of Toronto, and the Canadian Intensive Care Foundation. PROTECT was funded by the Canadian Institutes of Health Research, the Heart and Stroke Foundation (Canada), and the Australian and New Zealand College of Anesthetists Research Foundation. Some authors reported fees, support, and consultancies from the pharmaceutical industry.
FROM JAMA
Key clinical point: Dalteparin is more cost-effective than unfractionated heparin in the prevention of venous thromboembolism.
Major finding: Dalteparin is as effective as unfractionated heparin in reducing thrombosis, for the same cost, but with less pulmonary embolus and heparin-induced thrombocytopenia.
Data source: Economic analysis of a prospective randomized controlled trial of low molecular weight heparin dalteparin versus unfractionated heparin in 2344 critically-ill medical-surgical patients
Disclosures: The E-PROTECT study was funded by the Heart and Stroke Foundation (Ontario, Canada), the University of Toronto, and the Canadian Intensive Care Foundation. PROTECT was funded by the Canadian Institutes of Health Research, the Heart and Stroke Foundation (Canada), and the Australian and New Zealand College of Anesthetists Research Foundation. Some authors reported fees, support, and consultancies to the pharmaceutical industry.
How to avoid diagnostic errors
As a generalist specialty, family medicine faces more diagnostic challenges than any other specialty because we see so many undifferentiated problems. However, only 2 family physicians attended this meeting: I was one, because of my research interests in proper use of lab testing, and John Ely, MD, from the University of Iowa, was the other. He has been researching diagnostic errors for most of his career. Dr. Ely has been testing an idea borrowed from aviation: using a diagnostic checklist. He developed a packet of note cards that lists the top 10 to 20 diagnoses for complaints commonly seen in family medicine, such as headache and abdominal pain. Before the patient leaves the exam room, he pulls out the appropriate checklist and goes through it out loud, just like a pilot before takeoff. He says for most patients, this process is pretty quick and it reassures both them and him that he has not missed an important diagnosis. (You can download Dr. Ely’s checklists from http://www.improvediagnosis.org/resource/resmgr/docs/diffdx.doc.)
How are the rest of us avoiding diagnostic errors? Some day IBM’s Watson or another diagnostic software program embedded in the electronic health record will guide us to the right diagnosis. In the meantime, I have developed a list of 7 low-tech ways to arrive at the correct diagnosis (and to rapidly correct a diagnostic error, should one occur):
2. Find out what dreaded diagnosis the patient believes he or she has so you can rule it in or out.
3. Don’t forget the pertinent past history. It makes a big difference if this is the patient’s first bad headache or the latest in a string of them.
4. Don’t skip the physical exam; even a negative exam, if documented properly, may keep you out of court.
5. Negotiate the diagnosis and treatment plan with the patient. This often brings out new information and new concerns.
6. Follow up, follow up, follow up, and do so in a timely manner.
7. Quickly reconsider your diagnosis and/or get a consultation if things are not going as expected.
As a generalist specialty, family medicine faces more diagnostic challenges than any other specialty because we see so many undifferentiated problems. However, only 2 family physicians attended this meeting: I was one, because of my research interests in proper use of lab testing, and John Ely, MD, from the University of Iowa, was the other. He has been researching diagnostic errors for most of his career. Dr. Ely has been testing an idea borrowed from aviation: using a diagnostic checklist. He developed a packet of note cards that lists the top 10 to 20 diagnoses for complaints commonly seen in family medicine, such as headache and abdominal pain. Before the patient leaves the exam room, he pulls out the appropriate checklist and goes through it out loud, just like a pilot before takeoff. He says for most patients, this process is pretty quick and it reassures both them and him that he has not missed an important diagnosis. (You can download Dr. Ely’s checklists from http://www.improvediagnosis.org/resource/resmgr/docs/diffdx.doc.)
How are the rest of us avoiding diagnostic errors? Some day IBM’s Watson or another diagnostic software program embedded in the electronic health record will guide us to the right diagnosis. In the meantime, I have developed a list of 7 low-tech ways to arrive at the correct diagnosis (and to rapidly correct a diagnostic error, should one occur):
2. Find out what dreaded diagnosis the patient believes he or she has so you can rule it in or out.
3. Don’t forget the pertinent past history. It makes a big difference if this is the patient’s first bad headache or the latest in a string of them.
4. Don’t skip the physical exam; even a negative exam, if documented properly, may keep you out of court.
5. Negotiate the diagnosis and treatment plan with the patient. This often brings out new information and new concerns.
6. Follow up, follow up, follow up, and do so in a timely manner.
7. Quickly reconsider your diagnosis and/or get a consultation if things are not going as expected.
As a generalist specialty, family medicine faces more diagnostic challenges than any other specialty because we see so many undifferentiated problems. However, only 2 family physicians attended this meeting: I was one, because of my research interests in proper use of lab testing, and John Ely, MD, from the University of Iowa, was the other. He has been researching diagnostic errors for most of his career. Dr. Ely has been testing an idea borrowed from aviation: using a diagnostic checklist. He developed a packet of note cards that lists the top 10 to 20 diagnoses for complaints commonly seen in family medicine, such as headache and abdominal pain. Before the patient leaves the exam room, he pulls out the appropriate checklist and goes through it out loud, just like a pilot before takeoff. He says for most patients, this process is pretty quick and it reassures both them and him that he has not missed an important diagnosis. (You can download Dr. Ely’s checklists from http://www.improvediagnosis.org/resource/resmgr/docs/diffdx.doc.)
How are the rest of us avoiding diagnostic errors? Some day IBM’s Watson or another diagnostic software program embedded in the electronic health record will guide us to the right diagnosis. In the meantime, I have developed a list of 7 low-tech ways to arrive at the correct diagnosis (and to rapidly correct a diagnostic error, should one occur):
2. Find out what dreaded diagnosis the patient believes he or she has so you can rule it in or out.
3. Don’t forget the pertinent past history. It makes a big difference if this is the patient’s first bad headache or the latest in a string of them.
4. Don’t skip the physical exam; even a negative exam, if documented properly, may keep you out of court.
5. Negotiate the diagnosis and treatment plan with the patient. This often brings out new information and new concerns.
6. Follow up, follow up, follow up, and do so in a timely manner.
7. Quickly reconsider your diagnosis and/or get a consultation if things are not going as expected.
4 pregnant women with an unusual finding at delivery
THE CASES
CASE 1 › A 32-year-old G2P1 with an uncomplicated prenatal course presented for induction at 41 weeks and 2 days of gestation. Fetal heart tracing showed no abnormalities. A compound presentation and a prolonged second stage of labor made vacuum assistance necessary. The infant had both a true umbilical cord knot (TUCK) (FIGURE 1A) and double nuchal cord.
CASE 2 › A 46-year-old G3P0 at 38 weeks of gestation by in vitro fertilization underwent an uncomplicated primary low transverse cesarean (C-section) delivery of dichorionic/diamniotic twins. The C-section had been necessary because baby A had been in the breech position. Fetal heart tracing showed no abnormalities. Baby A had a velamentous cord insertion, and baby B had a succenturiate lobe and a TUCK.
CASE 3 › A 23-year-old G2P1 with an uncomplicated prenatal course chose to have a repeat C-section and delivered at 41 weeks in active labor. Fetal heart monitoring showed no abnormalities. Umbilical artery pH and venous pH were normal. A TUCK was noted at time of delivery.
CASE 4 › A 30-year-old G1P0 with an uncomplicated prenatal course presented in active labor at 40 weeks and 4 days of gestation. At 7 cm cervical dilation, monitoring showed repeated deep variable fetal heart rate decelerations. The patient underwent an uncomplicated primary C-section. Umbilical artery pH and venous pH were normal. A TUCK (FIGURE 1B) and double nuchal cord were found at time of delivery.
DISCUSSION
TUCKs are thought to occur when a fetus passes through a loop in the umbilical cord. They occur in <2% of term deliveries.1,2 TUCKs differ from false knots. False knots are exaggerated loops of cord vasculature.
Risk factors that have been independently associated with TUCK include advanced maternal age (AMA; >35 years), multiparity, diabetes mellitus, gestational diabetes, polyhydramnios, and previous spontaneous abortion.1-3 In one study, 72% of women with a TUCK were multiparous.3 Hershkovitz et al2 suggested that laxity of uterine and abdominal musculature in multiparous patients may contribute to increased room for TUCK formation.
The adjusted odds ratio of having a TUCK is 2.53 in women with diabetes mellitus.3 Hyperglycemia can contribute to increased fetal movements, thereby increasing the risk of TUCK development.2 Polyhydramnios is often found in patients with diabetes mellitus and gestational diabetes.3 The incidence is higher in monoamniotic twins.4
Being a male and having a longer umbilical cord may also increase the risk of TUCK. On average, male infants have longer cords than females, which may predispose them to TUCKs.3 Räisänen et al3 found that the mean cord length in TUCK infants was 16.9 cm longer than in infants without a TUCK.
Of our 4 patients, one was of AMA, 2 were multiparous, and 3 of the 4 infants who developed TUCK were male.
TUCK is usually diagnosed at delivery
Most cases of TUCK are found incidentally at the time of delivery. Antenatal diagnosis is difficult, because loops of cord lying together are easily mistaken for knots on ultrasound.5 Sepulveda et al6 evaluated the use of 3D power Doppler in 8 cases of suspected TUCK; only 63% were confirmed at delivery. Some researchers have found improved detection of TUCK with color Doppler and 4D ultrasound, which have demonstrated a “hanging noose sign” (a transverse section of umbilical cord surrounded by a loop of cord) as well as views of the cord under pressure.7-10
Outcomes associated with TUCK vary greatly. Neonates affected by TUCK have a 4% to 10% increased risk of stillbirth, usually attributed to knot tightening.2,4,11,12
In addition, there is an increased incidence of fetal heart rate abnormalities during labor.1,3,12,13
There is no increase in the incidence of assisted vaginal or C-section delivery.12 And as for whether TUCK affects an infant’s size or weight, one study found TUCK infants had a 3.2-fold higher risk of measuring small for gestational age, potentially due to chronic umbilical cord compromise; however, mean birth weight between study and control groups did not differ significantly.3
Outcomes for our patients and their infants. All 4 cases had good outcomes (TABLE). The umbilical cord knot produced no detectable fetal compromise in cases 1 through 3. In Case 4, electronic fetal monitoring showed repeated variable fetal heart rate decelerations, presumably associated with cord compression.
THE TAKEAWAY
Pregnant women who may be at risk for experiencing a TUCK include those who are older than age 35, multiparous, carrying a boy, or have diabetes mellitus, gestational diabetes, or polyhydramnios. While it is good to be aware of these risk factors, there are no recommended changes in management based on risk or ultrasound findings unless there is additional concern for fetal compromise.
Antenatal diagnosis of TUCK is challenging, but Doppler ultrasound may be able to identify the condition. Most cases of TUCK are noted on delivery, and outcomes are generally positive, although infants in whom the TUCK tightens may have an increased risk of heart rate abnormalities or stillbirth.
1. Joura EA, Zeisler H, Sator MO. Epidemiology and clinical value of true umbilical cord knots [in German]. Wien Klin Wochenschr. 1998;110:232-235.
2. Hershkovitz R, Silberstein T, Sheiner E, et al. Risk factors associated with true knots of the umbilical cord. Eur J Obstet Gynecol Reprod Biol. 2001;98:36-39.
3. Räisänen S, Georgiadis L, Harju M, et al. True umbilical cord knot and obstetric outcome. Int J Gynaecol Obstet. 2013;122: 18-21.
4. Maher JT, Conti JA. A comparison of umbilical cord blood gas values between newborns with and without true knots. Obstet Gynecol. 1996;88:863-866.
5. Clerici G, Koutras I, Luzietti R, et al. Multiple true umbilical knots: a silent risk for intrauterine growth restriction with anomalous hemodynamic pattern. Fetal Diagn Ther. 2007;22:440-443.
6. Sepulveda W, Shennan AH, Bower S, et al. True knot of the umbilical cord: a difficult prenatal ultrasonographic diagnosis. Ultrasound Obstet Gynecol. 1995;5:106-108.
7. Hasbun J, Alcalde JL, Sepulveda W. Three-dimensional power Doppler sonography in the prenatal diagnosis of a true knot of the umbilical cord: value and limitations. J Ultrasound Med. 2007;26:1215-1220.
8. Rodriguez N, Angarita AM, Casasbuenas A, et al. Three-dimensional high-definition flow imaging in prenatal diagnosis of a true umbilical cord knot. Ultrasound Obstet Gynecol. 2012;39:245-246.
9. Scioscia M, Fornalè M, Bruni F, et al. Four-dimensional and Doppler sonography in the diagnosis and surveillance of a true cord knot. J Clin Ultrasound. 2011;39: 157-159.
10. Sherer DM, Dalloul M, Zigalo A, et al. Power Doppler and 3-dimensional sonographic diagnosis of multiple separate true knots of the umbilical cord. J Ultrasound Med. 2005;24: 1321-1323.
11. Sørnes T. Umbilical cord knots. Acta Obstet Gynecol Scand. 2000;79:157-159.
12. Airas U, Heinonen S. Clinical significance of true umbilical knots: a population-based analysis. Am J Perinatol. 2002;19:127-132.
13. Szczepanik ME, Wittich AC. True knot of the umbilical cord: a report of 13 cases. Mil Med. 2007;172:892-894.
THE CASES
CASE 1 › A 32-year-old G2P1 with an uncomplicated prenatal course presented for induction at 41 weeks and 2 days of gestation. Fetal heart tracing showed no abnormalities. A compound presentation and a prolonged second stage of labor made vacuum assistance necessary. The infant had both a true umbilical cord knot (TUCK) (FIGURE 1A) and double nuchal cord.
CASE 2 › A 46-year-old G3P0 at 38 weeks of gestation by in vitro fertilization underwent an uncomplicated primary low transverse cesarean (C-section) delivery of dichorionic/diamniotic twins. The C-section had been necessary because baby A had been in the breech position. Fetal heart tracing showed no abnormalities. Baby A had a velamentous cord insertion, and baby B had a succenturiate lobe and a TUCK.
CASE 3 › A 23-year-old G2P1 with an uncomplicated prenatal course chose to have a repeat C-section and delivered at 41 weeks in active labor. Fetal heart monitoring showed no abnormalities. Umbilical artery pH and venous pH were normal. A TUCK was noted at time of delivery.
CASE 4 › A 30-year-old G1P0 with an uncomplicated prenatal course presented in active labor at 40 weeks and 4 days of gestation. At 7 cm cervical dilation, monitoring showed repeated deep variable fetal heart rate decelerations. The patient underwent an uncomplicated primary C-section. Umbilical artery pH and venous pH were normal. A TUCK (FIGURE 1B) and double nuchal cord were found at time of delivery.
DISCUSSION
TUCKs are thought to occur when a fetus passes through a loop in the umbilical cord. They occur in <2% of term deliveries.1,2 TUCKs differ from false knots. False knots are exaggerated loops of cord vasculature.
Risk factors that have been independently associated with TUCK include advanced maternal age (AMA; >35 years), multiparity, diabetes mellitus, gestational diabetes, polyhydramnios, and previous spontaneous abortion.1-3 In one study, 72% of women with a TUCK were multiparous.3 Hershkovitz et al2 suggested that laxity of uterine and abdominal musculature in multiparous patients may contribute to increased room for TUCK formation.
The adjusted odds ratio of having a TUCK is 2.53 in women with diabetes mellitus.3 Hyperglycemia can contribute to increased fetal movements, thereby increasing the risk of TUCK development.2 Polyhydramnios is often found in patients with diabetes mellitus and gestational diabetes.3 The incidence is higher in monoamniotic twins.4
Being a male and having a longer umbilical cord may also increase the risk of TUCK. On average, male infants have longer cords than females, which may predispose them to TUCKs.3 Räisänen et al3 found that the mean cord length in TUCK infants was 16.9 cm longer than in infants without a TUCK.
Of our 4 patients, one was of AMA, 2 were multiparous, and 3 of the 4 infants who developed TUCK were male.
TUCK is usually diagnosed at delivery
Most cases of TUCK are found incidentally at the time of delivery. Antenatal diagnosis is difficult, because loops of cord lying together are easily mistaken for knots on ultrasound.5 Sepulveda et al6 evaluated the use of 3D power Doppler in 8 cases of suspected TUCK; only 63% were confirmed at delivery. Some researchers have found improved detection of TUCK with color Doppler and 4D ultrasound, which have demonstrated a “hanging noose sign” (a transverse section of umbilical cord surrounded by a loop of cord) as well as views of the cord under pressure.7-10
Outcomes associated with TUCK vary greatly. Neonates affected by TUCK have a 4% to 10% increased risk of stillbirth, usually attributed to knot tightening.2,4,11,12
In addition, there is an increased incidence of fetal heart rate abnormalities during labor.1,3,12,13
There is no increase in the incidence of assisted vaginal or C-section delivery.12 And as for whether TUCK affects an infant’s size or weight, one study found TUCK infants had a 3.2-fold higher risk of measuring small for gestational age, potentially due to chronic umbilical cord compromise; however, mean birth weight between study and control groups did not differ significantly.3
Outcomes for our patients and their infants. All 4 cases had good outcomes (TABLE). The umbilical cord knot produced no detectable fetal compromise in cases 1 through 3. In Case 4, electronic fetal monitoring showed repeated variable fetal heart rate decelerations, presumably associated with cord compression.
THE TAKEAWAY
Pregnant women who may be at risk for experiencing a TUCK include those who are older than age 35, multiparous, carrying a boy, or have diabetes mellitus, gestational diabetes, or polyhydramnios. While it is good to be aware of these risk factors, there are no recommended changes in management based on risk or ultrasound findings unless there is additional concern for fetal compromise.
Antenatal diagnosis of TUCK is challenging, but Doppler ultrasound may be able to identify the condition. Most cases of TUCK are noted on delivery, and outcomes are generally positive, although infants in whom the TUCK tightens may have an increased risk of heart rate abnormalities or stillbirth.
THE CASES
CASE 1 › A 32-year-old G2P1 with an uncomplicated prenatal course presented for induction at 41 weeks and 2 days of gestation. Fetal heart tracing showed no abnormalities. A compound presentation and a prolonged second stage of labor made vacuum assistance necessary. The infant had both a true umbilical cord knot (TUCK) (FIGURE 1A) and double nuchal cord.
CASE 2 › A 46-year-old G3P0 at 38 weeks of gestation by in vitro fertilization underwent an uncomplicated primary low transverse cesarean (C-section) delivery of dichorionic/diamniotic twins. The C-section had been necessary because baby A had been in the breech position. Fetal heart tracing showed no abnormalities. Baby A had a velamentous cord insertion, and baby B had a succenturiate lobe and a TUCK.
CASE 3 › A 23-year-old G2P1 with an uncomplicated prenatal course chose to have a repeat C-section and delivered at 41 weeks in active labor. Fetal heart monitoring showed no abnormalities. Umbilical artery pH and venous pH were normal. A TUCK was noted at time of delivery.
CASE 4 › A 30-year-old G1P0 with an uncomplicated prenatal course presented in active labor at 40 weeks and 4 days of gestation. At 7 cm cervical dilation, monitoring showed repeated deep variable fetal heart rate decelerations. The patient underwent an uncomplicated primary C-section. Umbilical artery pH and venous pH were normal. A TUCK (FIGURE 1B) and double nuchal cord were found at time of delivery.
DISCUSSION
TUCKs are thought to occur when a fetus passes through a loop in the umbilical cord. They occur in <2% of term deliveries.1,2 TUCKs differ from false knots. False knots are exaggerated loops of cord vasculature.
Risk factors that have been independently associated with TUCK include advanced maternal age (AMA; >35 years), multiparity, diabetes mellitus, gestational diabetes, polyhydramnios, and previous spontaneous abortion.1-3 In one study, 72% of women with a TUCK were multiparous.3 Hershkovitz et al2 suggested that laxity of uterine and abdominal musculature in multiparous patients may contribute to increased room for TUCK formation.
The adjusted odds ratio of having a TUCK is 2.53 in women with diabetes mellitus.3 Hyperglycemia can contribute to increased fetal movements, thereby increasing the risk of TUCK development.2 Polyhydramnios is often found in patients with diabetes mellitus and gestational diabetes.3 The incidence is higher in monoamniotic twins.4
Being a male and having a longer umbilical cord may also increase the risk of TUCK. On average, male infants have longer cords than females, which may predispose them to TUCKs.3 Räisänen et al3 found that the mean cord length in TUCK infants was 16.9 cm longer than in infants without a TUCK.
Of our 4 patients, one was of AMA, 2 were multiparous, and 3 of the 4 infants who developed TUCK were male.
TUCK is usually diagnosed at delivery
Most cases of TUCK are found incidentally at the time of delivery. Antenatal diagnosis is difficult, because loops of cord lying together are easily mistaken for knots on ultrasound.5 Sepulveda et al6 evaluated the use of 3D power Doppler in 8 cases of suspected TUCK; only 63% were confirmed at delivery. Some researchers have found improved detection of TUCK with color Doppler and 4D ultrasound, which have demonstrated a “hanging noose sign” (a transverse section of umbilical cord surrounded by a loop of cord) as well as views of the cord under pressure.7-10
Outcomes associated with TUCK vary greatly. Neonates affected by TUCK have a 4% to 10% increased risk of stillbirth, usually attributed to knot tightening.2,4,11,12
In addition, there is an increased incidence of fetal heart rate abnormalities during labor.1,3,12,13
There is no increase in the incidence of assisted vaginal or C-section delivery.12 And as for whether TUCK affects an infant’s size or weight, one study found TUCK infants had a 3.2-fold higher risk of measuring small for gestational age, potentially due to chronic umbilical cord compromise; however, mean birth weight between study and control groups did not differ significantly.3
Outcomes for our patients and their infants. All 4 cases had good outcomes (TABLE). The umbilical cord knot produced no detectable fetal compromise in cases 1 through 3. In Case 4, electronic fetal monitoring showed repeated variable fetal heart rate decelerations, presumably associated with cord compression.
THE TAKEAWAY
Pregnant women who may be at risk for experiencing a TUCK include those who are older than age 35, multiparous, carrying a boy, or have diabetes mellitus, gestational diabetes, or polyhydramnios. While it is good to be aware of these risk factors, there are no recommended changes in management based on risk or ultrasound findings unless there is additional concern for fetal compromise.
Antenatal diagnosis of TUCK is challenging, but Doppler ultrasound may be able to identify the condition. Most cases of TUCK are noted on delivery, and outcomes are generally positive, although infants in whom the TUCK tightens may have an increased risk of heart rate abnormalities or stillbirth.
1. Joura EA, Zeisler H, Sator MO. Epidemiology and clinical value of true umbilical cord knots [in German]. Wien Klin Wochenschr. 1998;110:232-235.
2. Hershkovitz R, Silberstein T, Sheiner E, et al. Risk factors associated with true knots of the umbilical cord. Eur J Obstet Gynecol Reprod Biol. 2001;98:36-39.
3. Räisänen S, Georgiadis L, Harju M, et al. True umbilical cord knot and obstetric outcome. Int J Gynaecol Obstet. 2013;122: 18-21.
4. Maher JT, Conti JA. A comparison of umbilical cord blood gas values between newborns with and without true knots. Obstet Gynecol. 1996;88:863-866.
5. Clerici G, Koutras I, Luzietti R, et al. Multiple true umbilical knots: a silent risk for intrauterine growth restriction with anomalous hemodynamic pattern. Fetal Diagn Ther. 2007;22:440-443.
6. Sepulveda W, Shennan AH, Bower S, et al. True knot of the umbilical cord: a difficult prenatal ultrasonographic diagnosis. Ultrasound Obstet Gynecol. 1995;5:106-108.
7. Hasbun J, Alcalde JL, Sepulveda W. Three-dimensional power Doppler sonography in the prenatal diagnosis of a true knot of the umbilical cord: value and limitations. J Ultrasound Med. 2007;26:1215-1220.
8. Rodriguez N, Angarita AM, Casasbuenas A, et al. Three-dimensional high-definition flow imaging in prenatal diagnosis of a true umbilical cord knot. Ultrasound Obstet Gynecol. 2012;39:245-246.
9. Scioscia M, Fornalè M, Bruni F, et al. Four-dimensional and Doppler sonography in the diagnosis and surveillance of a true cord knot. J Clin Ultrasound. 2011;39: 157-159.
10. Sherer DM, Dalloul M, Zigalo A, et al. Power Doppler and 3-dimensional sonographic diagnosis of multiple separate true knots of the umbilical cord. J Ultrasound Med. 2005;24: 1321-1323.
11. Sørnes T. Umbilical cord knots. Acta Obstet Gynecol Scand. 2000;79:157-159.
12. Airas U, Heinonen S. Clinical significance of true umbilical knots: a population-based analysis. Am J Perinatol. 2002;19:127-132.
13. Szczepanik ME, Wittich AC. True knot of the umbilical cord: a report of 13 cases. Mil Med. 2007;172:892-894.
1. Joura EA, Zeisler H, Sator MO. Epidemiology and clinical value of true umbilical cord knots [in German]. Wien Klin Wochenschr. 1998;110:232-235.
2. Hershkovitz R, Silberstein T, Sheiner E, et al. Risk factors associated with true knots of the umbilical cord. Eur J Obstet Gynecol Reprod Biol. 2001;98:36-39.
3. Räisänen S, Georgiadis L, Harju M, et al. True umbilical cord knot and obstetric outcome. Int J Gynaecol Obstet. 2013;122: 18-21.
4. Maher JT, Conti JA. A comparison of umbilical cord blood gas values between newborns with and without true knots. Obstet Gynecol. 1996;88:863-866.
5. Clerici G, Koutras I, Luzietti R, et al. Multiple true umbilical knots: a silent risk for intrauterine growth restriction with anomalous hemodynamic pattern. Fetal Diagn Ther. 2007;22:440-443.
6. Sepulveda W, Shennan AH, Bower S, et al. True knot of the umbilical cord: a difficult prenatal ultrasonographic diagnosis. Ultrasound Obstet Gynecol. 1995;5:106-108.
7. Hasbun J, Alcalde JL, Sepulveda W. Three-dimensional power Doppler sonography in the prenatal diagnosis of a true knot of the umbilical cord: value and limitations. J Ultrasound Med. 2007;26:1215-1220.
8. Rodriguez N, Angarita AM, Casasbuenas A, et al. Three-dimensional high-definition flow imaging in prenatal diagnosis of a true umbilical cord knot. Ultrasound Obstet Gynecol. 2012;39:245-246.
9. Scioscia M, Fornalè M, Bruni F, et al. Four-dimensional and Doppler sonography in the diagnosis and surveillance of a true cord knot. J Clin Ultrasound. 2011;39: 157-159.
10. Sherer DM, Dalloul M, Zigalo A, et al. Power Doppler and 3-dimensional sonographic diagnosis of multiple separate true knots of the umbilical cord. J Ultrasound Med. 2005;24: 1321-1323.
11. Sørnes T. Umbilical cord knots. Acta Obstet Gynecol Scand. 2000;79:157-159.
12. Airas U, Heinonen S. Clinical significance of true umbilical knots: a population-based analysis. Am J Perinatol. 2002;19:127-132.
13. Szczepanik ME, Wittich AC. True knot of the umbilical cord: a report of 13 cases. Mil Med. 2007;172:892-894.
Radiating low back pain • history of urinary symptoms • past surgery for scoliosis • Dx?
THE CASE
A 23-year-old immunocompetent woman was referred to our spinal clinic with a 6-month history of low back pain that radiated to her right flank, buttock, and groin. She’d had intermittent urinary problems, including mild dysuria and frequency, and had been treated with antibiotics for a presumed urinary tract infection on 3 previous occasions, but her pain gradually increased and eventually became constant.
The patient had no history of fever, malaise, or weight loss. She denied consuming unpasteurized milk or undercooked poultry, and hadn’t recently experienced diarrhea or vomiting.
Eight years earlier, she had undergone anterior fusion of her spine for idiopathic scoliosis. At that time, she was at Risser grade 1, and her Cobb angle was 50°; metallic instrumentation was implanted at T10 to L3 to prevent progression of the scoliosis. Her recovery had been uneventful.
During examination, her temperature, pulse, respiratory rate, blood pressure, and nervous system were all normal. Her hips appeared normal, as well, and a straight leg raise was negative bilaterally. The patient had mild midline lumbar tenderness. Spinal range of movement revealed decreased flexion and mild pain.
X-rays (FIGURE 1) showed no changes in the previous metalwork in her spine. There was decreased disk height at the L3/4 level, but no significant bony erosion or soft-tissue shadows. Laboratory testing revealed a C-reactive protein (CRP) level of 240 mg/dL (normal, <1 mg/dL) and her erythrocyte sedimentation rate (ESR) was 102 mm/h—more than 5 times higher than it should have been.1 The patient had a normal peripheral white cell count (WCC). Midstream urine cultures were negative.
The patient was admitted to the hospital for further work-up. Magnetic resonance imaging (MRI) of the lumbar spine showed gross abnormality at the L3-L4 disk level with erosion of the end plates, fluid in the disk space, marked enhancing edema, and mild surrounding soft-tissue edematous changes, but no evidence of any epidural abscess (FIGURE 2). The patient had a fluoroscopy-guided needle biopsy of the disk on the same day and received intravenous (IV) ceftriaxone 2 g twice a day. Blood and urine cultures were negative.
THE DIAGNOSIS
We suspected our patient had spondylodiscitis, an infection of the spine that includes spondylitis (inflammation of the vertebrae) and discitis (inflammation of the vertebral disk space). After 48 hours, the biopsy sample grew Salmonella typhimurium and confirmed the diagnosis. The organism was sensitive to ceftriaxone and ciprofloxacin; parenteral ceftriaxone was continued and the patient wore a thoracolumbar brace for immobilization. For 3 days, her inflammatory marker levels were checked daily, then every other day for the rest of that first week, and then 2 more times in the following week.
DISCUSSION
Thoracic and lumbar vertebrae are the most common sites of spondylodiscitis.2 Spondylodiscitis accounts for 3% to 5% of pyogenic osteomyelitis in patients in developed countries.3 The incidence of pyogenic spondylodiscitis may be rising due to an increase in the number of elderly and immunocompromised patients, as well as a rise in invasive medical procedures.4-6
If left untreated, spondylodiscitis can spread longitudinally (involving the adjacent levels), posteriorly (causing bacterial meningitis, abscess formation, and cord compromise), or anteriorly (causing paravertebral abscess). Untreated spondylodiscitis can also send distant infective emboli and cause endocarditis7-9 or mycotic abdominal aneurysm.10
Historically, mortality in patients with vertebral osteomyelitis has been as high as 25%.11 The combination of earlier diagnosis, antibiotics, and surgical debridement and stabilization has decreased mortality to less than 15%.12-14
Risk factors for spondylodiscitis include male sex, IV drug abuse, diabetes, morbid obesity, having had a genitourinary or spinal procedure, and being immunocompromised (eg, from alcohol abuse, malignancy, organ transplantation, chemotherapy, or corticosteroid use).12,15,16
Gram-positive organisms cause most spine infections in both adults and children, with 40% to 90% caused by Staphylococcus aureus.17 Gram-negative organisms (Escherichia coli, Pseudomonas, and Proteus), which can also cause spondylodiscitis, typically occur after genitourinary infections or procedures. IV drug abusers are prone to Pseudomonas infections.18 Anaerobic infections may be seen in patients with diabetes or after penetrating trauma.15 Salmonella species can cause spondylodiscitis, especially in patients with sickle cell disease from an intestinal source.19
Mycobacterium tuberculosis is the most common nonpyogenic infecting agent that also can cause spondylodiscitis. Infection caused by tuberculosis (TB) has had a recent resurgence with resistant strains, especially in patients with human immunodeficiency virus.15 Although less than 10% of patients with TB have skeletal involvement, 50% of the skeletal involvement occurs in the spine.15
The clinical presentation of spondylodiscitis depends on the location of the infection, the virulence of the organism, and the immune status of the patient. Discitis can present as pain in the back, hip, abdomen (especially in children20) and, occasionally, with meningeal involvement.11 Patients with discitis often have a normal temperature.15,21 In patients with discitis, the patient’s WCC will be normal, but the ESR is almost always elevated.15,22 Suspect spondylodiscitis in patients who present with persistent or increasing pain 3 to 4 weeks after back surgery. For such patients, measure inflammatory markers and order imaging of the spine.
X-ray findings for patients with spondylodiscitis will include osteolysis and end plate erosions (early) and narrowing and collapse of the disk space (late). (In TB, relative preservation of the disk spaces is seen, with significant vertebral destruction.)
MRI is the modality of choice for diagnosis and assessment of suspected spondylodiscitis because it can provide imaging of the soft tissue, neural elements, and bony changes with a high sensitivity and specificity.23 Once infection is suspected, the diagnosis should be confirmed by fluoroscopic- or computed tomography-guided biopsy before starting antibiotic treatment.
Long-term antibiotics are required to prevent recurrence
IV antibiotics are the mainstay of treatment for spondylodiscitis;24 the specific drug used will depend upon the organism identified. Patients typically receive 2 to 6 weeks of IV therapy. Then, once the patient improves and inflammatory markers return to normal levels, the patient receives a course of oral antibiotics for 2 to 6 more weeks. Grados et al19 found recurrence rates of 10% to 15% for patients who were treated 4 to 8 weeks compared to 3.9% in those treated for 12 weeks or longer; therefore, a total duration of 12 weeks is commonly chosen.25-28
To minimize the risk of spondylolisthesis, kyphosis, and fractures of the infected bone, patients are advised to rest and the spine is often immobilized with a spinal brace. Surgery may be needed if antibiotics are not effective, or for patients who develop complications such as fluid collection, neurologic deficits, or deformity.
Our patient’s pain improved after 2 weeks and she became more comfortable wearing the thoracolumbar brace. Her CRP and ESR also improved and there was no radiologic evidence of fluid collection. The patient was discharged with a peripherally inserted central catheter in place and received IV ceftriaxone for 6 more weeks at home. This was followed by 4 weeks of oral ciprofloxacin 750 mg twice daily, thereby completing a 12-week course of antibiotics.
Our patient’s response to treatment was monitored clinically and the inflammatory markers were checked weekly after discharge until the end of treatment and at 6 and 12 months after start of treatment. At 12 months, our patient’s CRP was <1 mg/dL and ESR was 22 mm/h. One year later, our patient remained asymptomatic with normal inflammatory marker levels and no evidence of recurrence.
THE TAKEAWAY
Spondylodiscitis is an important differential diagnosis of lower back, flank, groin, and buttock pain. It’s important to be aware of this diagnosis, especially in patients who have risk factors such as IV drug abuse, diabetes, and morbid obesity. Although previous spinal surgery is a risk factor, spondylodiscitis should be considered in patients with persistent back pain even if they haven’t had spinal surgery. It can be present even when there is no tenderness over the spinous process or any fever.
Checking inflammatory markers is a reasonable next step if a patient’s pain does not resolve after at least 4 weeks. If levels of inflammatory markers such as CRP and ESR are elevated and symptoms continue, MRI can confirm or rule out the presence of spondylodiscitis. Treatments include orthotic support, antibiotics, and surgical intervention when complications arise.
1. Miller A, Green M, Robinson D. Simple rule for calculating normal erythrocyte sedimentation rate. Br Med J. 1983;286:266.
2. Calhoun JH, Manring MM. Adult osteomyelitis. Infect Dis Clin North Am. 2005;19:765-786.
3. Sobottke R, Seifert H, Fätkenheuer G, et al. Current diagnosis and treatment of spondylodiscitis. Dtsch Arztebl Int. 2008;105:181-187.
4. Beronius M, Bergman B, Andersson R. Vertebral osteomyelitis in Göteborg, Sweden: a retrospective study of patients during 1990-95. Scand J Infect Dis. 2001;33:527-532.
5. Digby JM, Kersley JB. Pyogenic non-tuberculous spinal infection: an analysis of thirty cases. J Bone Joint Surg Br. 1979;61: 47-55.
6. Gouliouris T, Aliyu SH, Brown NM. Spondylodiscitis: update on diagnosis and management. J Antimicrob Chemother. 2010;65 suppl 3:iii11-iii24.
7. Aoki K, Watanabe M, Ohzeki H. Successful surgical treatment of tricuspid valve endocarditis associated with vertebral osteomyelitis. Ann Thorac Cardiovasc Surg. 2010;16:207-209.
8. Gonzalez-Juanatey C, Testa-Fernandez A, Gonzalez-Gay MA. Septic discitis as initial manifestation of streptococcus bovis endocarditis. Int J Cardiol. 2006;108:128-129.
9. Morelli S, Carmenini E, Caporossi AP, et al. Spondylodiscitis and infective endocarditis: case studies and review of the literature. Spine (Phila Pa 1976). 2001;26:499-500.
10. Learch TJ, Sakamoto B, Ling AC, et al. Salmonella spondylodiscitis associated with a mycotic abdominal aortic aneurysm and paravertebral abscess. Emerg Radiol. 2009;16:147-150.
11. Guri JP. Pyogenic osteomyelitis of the spine. J Bone Joint Surg Am. 1946;28:29-39.
12. Carragee EJ. Pyogenic vertebral osteomyelitis. J Bone Joint Surg Am. 1997;79:874-880.
13. Garcia A Jr, Grantham SA. Hematogenous pyogenic vertebral osteomyelitis. J Bone Joint Surg Am. 1960;42-A:429-436.
14. Eismont FJ, Bohlman HH, Soni PL, et al. Pyogenic and fungal vertebral osteomyelitis with paralysis. J Bone Joint Surg Am. 1983;65:19-29.
15. Tay BK, Deckey J, Hu SS. Spinal infections. J Am Acad Orthop Surg. 2002;10:188-197.
16. Krogsgaard MR, Wagn P, Bengtsson J. Epidemiology of acute vertebral osteomyelitis in Denmark: 137 cases in Denmark 1978-1982, compared to cases reported to the National Patient Register 1991-1993. Acta Orthop Scand. 1998;69:513-517.
17. Francis X. Infections of spine. In: Canale ST, Beaty JH, eds. Campbell’s Operative Orthopaedics. 11th ed. New York, NY: Mosby; 2007:2241.
18. Roca RP, Yoshikawa TT. Primary skeletal infections in heroin users: a clinical characterization, diagnosis and therapy. Clin Orthop Relat Res. 1979;(144):238-248.
19. Grados F, Lescure FX, Senneville E, et al. Suggestions for managing pyogenic (non-tuberculous) discitis in adults. Joint Bone Spine. 2007;74:133-139.
20. Cheyne G, Runau F, Lloyd DM. Right upper quadrant pain and raised alkaline phosphatase is not always a hepatobiliary problem. Ann R Coll Surg Engl. 2014;96:118E-120E.
21. Varma R, Lander P, Assaf A. Imaging of pyogenic infectious spondylodiskitis. Radiol Clin North Am. 2001;39: 203-213.
22. Lehovsky J. Pyogenic vertebral osteomyelitis/disc infection. Baillieres Best Pract Res Clin Rheumatol. 1999;13:59-75.
23. Modic MT, Feiglin DH, Piraino DW, et al. Vertebral osteomyelitis: assessment using MR. Radiology. 1985;157:157-166.
24. Amritanand R, Venkatesh K, Sundararaj GD. Salmonella spondylodiscitis in the immunocompetent: our experience with eleven patients. Spine (Phila Pa 1976). 2010;35:E1317-E1321.
25. Govender S. Spinal infections. J Bone Joint Surg Br. 2005;87:1454-1458.
26. Lam KS, Webb JK. Discitis. Hosp Med. 2004;65:280-286.
27. Gasbarrini AL, Bertoldi E, Mazzetti M, et al. Clinical features, diagnostic and therapeutic approaches to haematogenous vertebral osteomyelitis. Eur Rev Med Pharmacol Sci. 2005;9: 53-66.
28. Cottle L, Riordan T. Infectious spondylodiscitis. J Infect. 2008;56:401-412.
THE CASE
A 23-year-old immunocompetent woman was referred to our spinal clinic with a 6-month history of low back pain that radiated to her right flank, buttock, and groin. She’d had intermittent urinary problems, including mild dysuria and frequency, and had been treated with antibiotics for a presumed urinary tract infection on 3 previous occasions, but her pain gradually increased and eventually became constant.
The patient had no history of fever, malaise, or weight loss. She denied consuming unpasteurized milk or undercooked poultry, and hadn’t recently experienced diarrhea or vomiting.
Eight years earlier, she had undergone anterior fusion of her spine for idiopathic scoliosis. At that time, she was at Risser grade 1, and her Cobb angle was 50°; metallic instrumentation was implanted at T10 to L3 to prevent progression of the scoliosis. Her recovery had been uneventful.
During examination, her temperature, pulse, respiratory rate, blood pressure, and nervous system were all normal. Her hips appeared normal, as well, and a straight leg raise was negative bilaterally. The patient had mild midline lumbar tenderness. Spinal range of movement revealed decreased flexion and mild pain.
X-rays (FIGURE 1) showed no changes in the previous metalwork in her spine. There was decreased disk height at the L3/4 level, but no significant bony erosion or soft-tissue shadows. Laboratory testing revealed a C-reactive protein (CRP) level of 240 mg/dL (normal, <1 mg/dL) and her erythrocyte sedimentation rate (ESR) was 102 mm/h—more than 5 times higher than it should have been.1 The patient had a normal peripheral white cell count (WCC). Midstream urine cultures were negative.
The patient was admitted to the hospital for further work-up. Magnetic resonance imaging (MRI) of the lumbar spine showed gross abnormality at the L3-L4 disk level with erosion of the end plates, fluid in the disk space, marked enhancing edema, and mild surrounding soft-tissue edematous changes, but no evidence of any epidural abscess (FIGURE 2). The patient had a fluoroscopy-guided needle biopsy of the disk on the same day and received intravenous (IV) ceftriaxone 2 g twice a day. Blood and urine cultures were negative.
THE DIAGNOSIS
We suspected our patient had spondylodiscitis, an infection of the spine that includes spondylitis (inflammation of the vertebrae) and discitis (inflammation of the vertebral disk space). After 48 hours, the biopsy sample grew Salmonella typhimurium and confirmed the diagnosis. The organism was sensitive to ceftriaxone and ciprofloxacin; parenteral ceftriaxone was continued and the patient wore a thoracolumbar brace for immobilization. For 3 days, her inflammatory marker levels were checked daily, then every other day for the rest of that first week, and then 2 more times in the following week.
DISCUSSION
Thoracic and lumbar vertebrae are the most common sites of spondylodiscitis.2 Spondylodiscitis accounts for 3% to 5% of pyogenic osteomyelitis in patients in developed countries.3 The incidence of pyogenic spondylodiscitis may be rising due to an increase in the number of elderly and immunocompromised patients, as well as a rise in invasive medical procedures.4-6
If left untreated, spondylodiscitis can spread longitudinally (involving the adjacent levels), posteriorly (causing bacterial meningitis, abscess formation, and cord compromise), or anteriorly (causing paravertebral abscess). Untreated spondylodiscitis can also send distant infective emboli and cause endocarditis7-9 or mycotic abdominal aneurysm.10
Historically, mortality in patients with vertebral osteomyelitis has been as high as 25%.11 The combination of earlier diagnosis, antibiotics, and surgical debridement and stabilization has decreased mortality to less than 15%.12-14
Risk factors for spondylodiscitis include male sex, IV drug abuse, diabetes, morbid obesity, having had a genitourinary or spinal procedure, and being immunocompromised (eg, from alcohol abuse, malignancy, organ transplantation, chemotherapy, or corticosteroid use).12,15,16
Gram-positive organisms cause most spine infections in both adults and children, with 40% to 90% caused by Staphylococcus aureus.17 Gram-negative organisms (Escherichia coli, Pseudomonas, and Proteus), which can also cause spondylodiscitis, typically occur after genitourinary infections or procedures. IV drug abusers are prone to Pseudomonas infections.18 Anaerobic infections may be seen in patients with diabetes or after penetrating trauma.15 Salmonella species can cause spondylodiscitis, especially in patients with sickle cell disease from an intestinal source.19
Mycobacterium tuberculosis is the most common nonpyogenic infecting agent that also can cause spondylodiscitis. Infection caused by tuberculosis (TB) has had a recent resurgence with resistant strains, especially in patients with human immunodeficiency virus.15 Although less than 10% of patients with TB have skeletal involvement, 50% of the skeletal involvement occurs in the spine.15
The clinical presentation of spondylodiscitis depends on the location of the infection, the virulence of the organism, and the immune status of the patient. Discitis can present as pain in the back, hip, abdomen (especially in children20) and, occasionally, with meningeal involvement.11 Patients with discitis often have a normal temperature.15,21 In patients with discitis, the patient’s WCC will be normal, but the ESR is almost always elevated.15,22 Suspect spondylodiscitis in patients who present with persistent or increasing pain 3 to 4 weeks after back surgery. For such patients, measure inflammatory markers and order imaging of the spine.
X-ray findings for patients with spondylodiscitis will include osteolysis and end plate erosions (early) and narrowing and collapse of the disk space (late). (In TB, relative preservation of the disk spaces is seen, with significant vertebral destruction.)
MRI is the modality of choice for diagnosis and assessment of suspected spondylodiscitis because it can provide imaging of the soft tissue, neural elements, and bony changes with a high sensitivity and specificity.23 Once infection is suspected, the diagnosis should be confirmed by fluoroscopic- or computed tomography-guided biopsy before starting antibiotic treatment.
Long-term antibiotics are required to prevent recurrence
IV antibiotics are the mainstay of treatment for spondylodiscitis;24 the specific drug used will depend upon the organism identified. Patients typically receive 2 to 6 weeks of IV therapy. Then, once the patient improves and inflammatory markers return to normal levels, the patient receives a course of oral antibiotics for 2 to 6 more weeks. Grados et al19 found recurrence rates of 10% to 15% for patients who were treated 4 to 8 weeks compared to 3.9% in those treated for 12 weeks or longer; therefore, a total duration of 12 weeks is commonly chosen.25-28
To minimize the risk of spondylolisthesis, kyphosis, and fractures of the infected bone, patients are advised to rest and the spine is often immobilized with a spinal brace. Surgery may be needed if antibiotics are not effective, or for patients who develop complications such as fluid collection, neurologic deficits, or deformity.
Our patient’s pain improved after 2 weeks and she became more comfortable wearing the thoracolumbar brace. Her CRP and ESR also improved and there was no radiologic evidence of fluid collection. The patient was discharged with a peripherally inserted central catheter in place and received IV ceftriaxone for 6 more weeks at home. This was followed by 4 weeks of oral ciprofloxacin 750 mg twice daily, thereby completing a 12-week course of antibiotics.
Our patient’s response to treatment was monitored clinically and the inflammatory markers were checked weekly after discharge until the end of treatment and at 6 and 12 months after start of treatment. At 12 months, our patient’s CRP was <1 mg/dL and ESR was 22 mm/h. One year later, our patient remained asymptomatic with normal inflammatory marker levels and no evidence of recurrence.
THE TAKEAWAY
Spondylodiscitis is an important differential diagnosis of lower back, flank, groin, and buttock pain. It’s important to be aware of this diagnosis, especially in patients who have risk factors such as IV drug abuse, diabetes, and morbid obesity. Although previous spinal surgery is a risk factor, spondylodiscitis should be considered in patients with persistent back pain even if they haven’t had spinal surgery. It can be present even when there is no tenderness over the spinous process or any fever.
Checking inflammatory markers is a reasonable next step if a patient’s pain does not resolve after at least 4 weeks. If levels of inflammatory markers such as CRP and ESR are elevated and symptoms continue, MRI can confirm or rule out the presence of spondylodiscitis. Treatments include orthotic support, antibiotics, and surgical intervention when complications arise.
THE CASE
A 23-year-old immunocompetent woman was referred to our spinal clinic with a 6-month history of low back pain that radiated to her right flank, buttock, and groin. She’d had intermittent urinary problems, including mild dysuria and frequency, and had been treated with antibiotics for a presumed urinary tract infection on 3 previous occasions, but her pain gradually increased and eventually became constant.
The patient had no history of fever, malaise, or weight loss. She denied consuming unpasteurized milk or undercooked poultry, and hadn’t recently experienced diarrhea or vomiting.
Eight years earlier, she had undergone anterior fusion of her spine for idiopathic scoliosis. At that time, she was at Risser grade 1, and her Cobb angle was 50°; metallic instrumentation was implanted at T10 to L3 to prevent progression of the scoliosis. Her recovery had been uneventful.
During examination, her temperature, pulse, respiratory rate, blood pressure, and nervous system were all normal. Her hips appeared normal, as well, and a straight leg raise was negative bilaterally. The patient had mild midline lumbar tenderness. Spinal range of movement revealed decreased flexion and mild pain.
X-rays (FIGURE 1) showed no changes in the previous metalwork in her spine. There was decreased disk height at the L3/4 level, but no significant bony erosion or soft-tissue shadows. Laboratory testing revealed a C-reactive protein (CRP) level of 240 mg/dL (normal, <1 mg/dL) and her erythrocyte sedimentation rate (ESR) was 102 mm/h—more than 5 times higher than it should have been.1 The patient had a normal peripheral white cell count (WCC). Midstream urine cultures were negative.
The patient was admitted to the hospital for further work-up. Magnetic resonance imaging (MRI) of the lumbar spine showed gross abnormality at the L3-L4 disk level with erosion of the end plates, fluid in the disk space, marked enhancing edema, and mild surrounding soft-tissue edematous changes, but no evidence of any epidural abscess (FIGURE 2). The patient had a fluoroscopy-guided needle biopsy of the disk on the same day and received intravenous (IV) ceftriaxone 2 g twice a day. Blood and urine cultures were negative.
THE DIAGNOSIS
We suspected our patient had spondylodiscitis, an infection of the spine that includes spondylitis (inflammation of the vertebrae) and discitis (inflammation of the vertebral disk space). After 48 hours, the biopsy sample grew Salmonella typhimurium and confirmed the diagnosis. The organism was sensitive to ceftriaxone and ciprofloxacin; parenteral ceftriaxone was continued and the patient wore a thoracolumbar brace for immobilization. For 3 days, her inflammatory marker levels were checked daily, then every other day for the rest of that first week, and then 2 more times in the following week.
DISCUSSION
Thoracic and lumbar vertebrae are the most common sites of spondylodiscitis.2 Spondylodiscitis accounts for 3% to 5% of pyogenic osteomyelitis in patients in developed countries.3 The incidence of pyogenic spondylodiscitis may be rising due to an increase in the number of elderly and immunocompromised patients, as well as a rise in invasive medical procedures.4-6
If left untreated, spondylodiscitis can spread longitudinally (involving the adjacent levels), posteriorly (causing bacterial meningitis, abscess formation, and cord compromise), or anteriorly (causing paravertebral abscess). Untreated spondylodiscitis can also send distant infective emboli and cause endocarditis7-9 or mycotic abdominal aneurysm.10
Historically, mortality in patients with vertebral osteomyelitis has been as high as 25%.11 The combination of earlier diagnosis, antibiotics, and surgical debridement and stabilization has decreased mortality to less than 15%.12-14
Risk factors for spondylodiscitis include male sex, IV drug abuse, diabetes, morbid obesity, having had a genitourinary or spinal procedure, and being immunocompromised (eg, from alcohol abuse, malignancy, organ transplantation, chemotherapy, or corticosteroid use).12,15,16
Gram-positive organisms cause most spine infections in both adults and children, with 40% to 90% caused by Staphylococcus aureus.17 Gram-negative organisms (Escherichia coli, Pseudomonas, and Proteus), which can also cause spondylodiscitis, typically occur after genitourinary infections or procedures. IV drug abusers are prone to Pseudomonas infections.18 Anaerobic infections may be seen in patients with diabetes or after penetrating trauma.15 Salmonella species can cause spondylodiscitis, especially in patients with sickle cell disease from an intestinal source.19
Mycobacterium tuberculosis is the most common nonpyogenic infecting agent that also can cause spondylodiscitis. Infection caused by tuberculosis (TB) has had a recent resurgence with resistant strains, especially in patients with human immunodeficiency virus.15 Although less than 10% of patients with TB have skeletal involvement, 50% of the skeletal involvement occurs in the spine.15
The clinical presentation of spondylodiscitis depends on the location of the infection, the virulence of the organism, and the immune status of the patient. Discitis can present as pain in the back, hip, abdomen (especially in children20) and, occasionally, with meningeal involvement.11 Patients with discitis often have a normal temperature.15,21 In patients with discitis, the patient’s WCC will be normal, but the ESR is almost always elevated.15,22 Suspect spondylodiscitis in patients who present with persistent or increasing pain 3 to 4 weeks after back surgery. For such patients, measure inflammatory markers and order imaging of the spine.
X-ray findings for patients with spondylodiscitis will include osteolysis and end plate erosions (early) and narrowing and collapse of the disk space (late). (In TB, relative preservation of the disk spaces is seen, with significant vertebral destruction.)
MRI is the modality of choice for diagnosis and assessment of suspected spondylodiscitis because it can provide imaging of the soft tissue, neural elements, and bony changes with a high sensitivity and specificity.23 Once infection is suspected, the diagnosis should be confirmed by fluoroscopic- or computed tomography-guided biopsy before starting antibiotic treatment.
Long-term antibiotics are required to prevent recurrence
IV antibiotics are the mainstay of treatment for spondylodiscitis;24 the specific drug used will depend upon the organism identified. Patients typically receive 2 to 6 weeks of IV therapy. Then, once the patient improves and inflammatory markers return to normal levels, the patient receives a course of oral antibiotics for 2 to 6 more weeks. Grados et al19 found recurrence rates of 10% to 15% for patients who were treated 4 to 8 weeks compared to 3.9% in those treated for 12 weeks or longer; therefore, a total duration of 12 weeks is commonly chosen.25-28
To minimize the risk of spondylolisthesis, kyphosis, and fractures of the infected bone, patients are advised to rest and the spine is often immobilized with a spinal brace. Surgery may be needed if antibiotics are not effective, or for patients who develop complications such as fluid collection, neurologic deficits, or deformity.
Our patient’s pain improved after 2 weeks and she became more comfortable wearing the thoracolumbar brace. Her CRP and ESR also improved and there was no radiologic evidence of fluid collection. The patient was discharged with a peripherally inserted central catheter in place and received IV ceftriaxone for 6 more weeks at home. This was followed by 4 weeks of oral ciprofloxacin 750 mg twice daily, thereby completing a 12-week course of antibiotics.
Our patient’s response to treatment was monitored clinically and the inflammatory markers were checked weekly after discharge until the end of treatment and at 6 and 12 months after start of treatment. At 12 months, our patient’s CRP was <1 mg/dL and ESR was 22 mm/h. One year later, our patient remained asymptomatic with normal inflammatory marker levels and no evidence of recurrence.
THE TAKEAWAY
Spondylodiscitis is an important differential diagnosis of lower back, flank, groin, and buttock pain. It’s important to be aware of this diagnosis, especially in patients who have risk factors such as IV drug abuse, diabetes, and morbid obesity. Although previous spinal surgery is a risk factor, spondylodiscitis should be considered in patients with persistent back pain even if they haven’t had spinal surgery. It can be present even when there is no tenderness over the spinous process or any fever.
Checking inflammatory markers is a reasonable next step if a patient’s pain does not resolve after at least 4 weeks. If levels of inflammatory markers such as CRP and ESR are elevated and symptoms continue, MRI can confirm or rule out the presence of spondylodiscitis. Treatments include orthotic support, antibiotics, and surgical intervention when complications arise.
1. Miller A, Green M, Robinson D. Simple rule for calculating normal erythrocyte sedimentation rate. Br Med J. 1983;286:266.
2. Calhoun JH, Manring MM. Adult osteomyelitis. Infect Dis Clin North Am. 2005;19:765-786.
3. Sobottke R, Seifert H, Fätkenheuer G, et al. Current diagnosis and treatment of spondylodiscitis. Dtsch Arztebl Int. 2008;105:181-187.
4. Beronius M, Bergman B, Andersson R. Vertebral osteomyelitis in Göteborg, Sweden: a retrospective study of patients during 1990-95. Scand J Infect Dis. 2001;33:527-532.
5. Digby JM, Kersley JB. Pyogenic non-tuberculous spinal infection: an analysis of thirty cases. J Bone Joint Surg Br. 1979;61: 47-55.
6. Gouliouris T, Aliyu SH, Brown NM. Spondylodiscitis: update on diagnosis and management. J Antimicrob Chemother. 2010;65 suppl 3:iii11-iii24.
7. Aoki K, Watanabe M, Ohzeki H. Successful surgical treatment of tricuspid valve endocarditis associated with vertebral osteomyelitis. Ann Thorac Cardiovasc Surg. 2010;16:207-209.
8. Gonzalez-Juanatey C, Testa-Fernandez A, Gonzalez-Gay MA. Septic discitis as initial manifestation of streptococcus bovis endocarditis. Int J Cardiol. 2006;108:128-129.
9. Morelli S, Carmenini E, Caporossi AP, et al. Spondylodiscitis and infective endocarditis: case studies and review of the literature. Spine (Phila Pa 1976). 2001;26:499-500.
10. Learch TJ, Sakamoto B, Ling AC, et al. Salmonella spondylodiscitis associated with a mycotic abdominal aortic aneurysm and paravertebral abscess. Emerg Radiol. 2009;16:147-150.
11. Guri JP. Pyogenic osteomyelitis of the spine. J Bone Joint Surg Am. 1946;28:29-39.
12. Carragee EJ. Pyogenic vertebral osteomyelitis. J Bone Joint Surg Am. 1997;79:874-880.
13. Garcia A Jr, Grantham SA. Hematogenous pyogenic vertebral osteomyelitis. J Bone Joint Surg Am. 1960;42-A:429-436.
14. Eismont FJ, Bohlman HH, Soni PL, et al. Pyogenic and fungal vertebral osteomyelitis with paralysis. J Bone Joint Surg Am. 1983;65:19-29.
15. Tay BK, Deckey J, Hu SS. Spinal infections. J Am Acad Orthop Surg. 2002;10:188-197.
16. Krogsgaard MR, Wagn P, Bengtsson J. Epidemiology of acute vertebral osteomyelitis in Denmark: 137 cases in Denmark 1978-1982, compared to cases reported to the National Patient Register 1991-1993. Acta Orthop Scand. 1998;69:513-517.
17. Francis X. Infections of spine. In: Canale ST, Beaty JH, eds. Campbell’s Operative Orthopaedics. 11th ed. New York, NY: Mosby; 2007:2241.
18. Roca RP, Yoshikawa TT. Primary skeletal infections in heroin users: a clinical characterization, diagnosis and therapy. Clin Orthop Relat Res. 1979;(144):238-248.
19. Grados F, Lescure FX, Senneville E, et al. Suggestions for managing pyogenic (non-tuberculous) discitis in adults. Joint Bone Spine. 2007;74:133-139.
20. Cheyne G, Runau F, Lloyd DM. Right upper quadrant pain and raised alkaline phosphatase is not always a hepatobiliary problem. Ann R Coll Surg Engl. 2014;96:118E-120E.
21. Varma R, Lander P, Assaf A. Imaging of pyogenic infectious spondylodiskitis. Radiol Clin North Am. 2001;39: 203-213.
22. Lehovsky J. Pyogenic vertebral osteomyelitis/disc infection. Baillieres Best Pract Res Clin Rheumatol. 1999;13:59-75.
23. Modic MT, Feiglin DH, Piraino DW, et al. Vertebral osteomyelitis: assessment using MR. Radiology. 1985;157:157-166.
24. Amritanand R, Venkatesh K, Sundararaj GD. Salmonella spondylodiscitis in the immunocompetent: our experience with eleven patients. Spine (Phila Pa 1976). 2010;35:E1317-E1321.
25. Govender S. Spinal infections. J Bone Joint Surg Br. 2005;87:1454-1458.
26. Lam KS, Webb JK. Discitis. Hosp Med. 2004;65:280-286.
27. Gasbarrini AL, Bertoldi E, Mazzetti M, et al. Clinical features, diagnostic and therapeutic approaches to haematogenous vertebral osteomyelitis. Eur Rev Med Pharmacol Sci. 2005;9: 53-66.
28. Cottle L, Riordan T. Infectious spondylodiscitis. J Infect. 2008;56:401-412.
1. Miller A, Green M, Robinson D. Simple rule for calculating normal erythrocyte sedimentation rate. Br Med J. 1983;286:266.
2. Calhoun JH, Manring MM. Adult osteomyelitis. Infect Dis Clin North Am. 2005;19:765-786.
3. Sobottke R, Seifert H, Fätkenheuer G, et al. Current diagnosis and treatment of spondylodiscitis. Dtsch Arztebl Int. 2008;105:181-187.
4. Beronius M, Bergman B, Andersson R. Vertebral osteomyelitis in Göteborg, Sweden: a retrospective study of patients during 1990-95. Scand J Infect Dis. 2001;33:527-532.
5. Digby JM, Kersley JB. Pyogenic non-tuberculous spinal infection: an analysis of thirty cases. J Bone Joint Surg Br. 1979;61: 47-55.
6. Gouliouris T, Aliyu SH, Brown NM. Spondylodiscitis: update on diagnosis and management. J Antimicrob Chemother. 2010;65 suppl 3:iii11-iii24.
7. Aoki K, Watanabe M, Ohzeki H. Successful surgical treatment of tricuspid valve endocarditis associated with vertebral osteomyelitis. Ann Thorac Cardiovasc Surg. 2010;16:207-209.
8. Gonzalez-Juanatey C, Testa-Fernandez A, Gonzalez-Gay MA. Septic discitis as initial manifestation of streptococcus bovis endocarditis. Int J Cardiol. 2006;108:128-129.
9. Morelli S, Carmenini E, Caporossi AP, et al. Spondylodiscitis and infective endocarditis: case studies and review of the literature. Spine (Phila Pa 1976). 2001;26:499-500.
10. Learch TJ, Sakamoto B, Ling AC, et al. Salmonella spondylodiscitis associated with a mycotic abdominal aortic aneurysm and paravertebral abscess. Emerg Radiol. 2009;16:147-150.
11. Guri JP. Pyogenic osteomyelitis of the spine. J Bone Joint Surg Am. 1946;28:29-39.
12. Carragee EJ. Pyogenic vertebral osteomyelitis. J Bone Joint Surg Am. 1997;79:874-880.
13. Garcia A Jr, Grantham SA. Hematogenous pyogenic vertebral osteomyelitis. J Bone Joint Surg Am. 1960;42-A:429-436.
14. Eismont FJ, Bohlman HH, Soni PL, et al. Pyogenic and fungal vertebral osteomyelitis with paralysis. J Bone Joint Surg Am. 1983;65:19-29.
15. Tay BK, Deckey J, Hu SS. Spinal infections. J Am Acad Orthop Surg. 2002;10:188-197.
16. Krogsgaard MR, Wagn P, Bengtsson J. Epidemiology of acute vertebral osteomyelitis in Denmark: 137 cases in Denmark 1978-1982, compared to cases reported to the National Patient Register 1991-1993. Acta Orthop Scand. 1998;69:513-517.
17. Francis X. Infections of spine. In: Canale ST, Beaty JH, eds. Campbell’s Operative Orthopaedics. 11th ed. New York, NY: Mosby; 2007:2241.
18. Roca RP, Yoshikawa TT. Primary skeletal infections in heroin users: a clinical characterization, diagnosis and therapy. Clin Orthop Relat Res. 1979;(144):238-248.
19. Grados F, Lescure FX, Senneville E, et al. Suggestions for managing pyogenic (non-tuberculous) discitis in adults. Joint Bone Spine. 2007;74:133-139.
20. Cheyne G, Runau F, Lloyd DM. Right upper quadrant pain and raised alkaline phosphatase is not always a hepatobiliary problem. Ann R Coll Surg Engl. 2014;96:118E-120E.
21. Varma R, Lander P, Assaf A. Imaging of pyogenic infectious spondylodiskitis. Radiol Clin North Am. 2001;39: 203-213.
22. Lehovsky J. Pyogenic vertebral osteomyelitis/disc infection. Baillieres Best Pract Res Clin Rheumatol. 1999;13:59-75.
23. Modic MT, Feiglin DH, Piraino DW, et al. Vertebral osteomyelitis: assessment using MR. Radiology. 1985;157:157-166.
24. Amritanand R, Venkatesh K, Sundararaj GD. Salmonella spondylodiscitis in the immunocompetent: our experience with eleven patients. Spine (Phila Pa 1976). 2010;35:E1317-E1321.
25. Govender S. Spinal infections. J Bone Joint Surg Br. 2005;87:1454-1458.
26. Lam KS, Webb JK. Discitis. Hosp Med. 2004;65:280-286.
27. Gasbarrini AL, Bertoldi E, Mazzetti M, et al. Clinical features, diagnostic and therapeutic approaches to haematogenous vertebral osteomyelitis. Eur Rev Med Pharmacol Sci. 2005;9: 53-66.
28. Cottle L, Riordan T. Infectious spondylodiscitis. J Infect. 2008;56:401-412.
Suspect myopathy? Take this approach to the work-up
› Categorize patients with muscle complaints into suspected myositic, intrinsic, or toxic myopathy to help guide subsequent work-up. C
› Look for diffusely painful, swollen, or boggy-feeling muscles—as well as weakness and pain with exertion—in patients you suspect may have viral myopathy. C
› Consider electromyography and muscle biopsy for patients you suspect may have dermatomyositis. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Marie C, a 75-year-old Asian woman, reports weakness in her legs and arms with unsteadiness when walking. She has a vague but persistent ache in her large muscles. Her symptoms have developed slowly over the past 3 months. She denies recent signs or symptoms of infection or other illness. Her medical history includes hypertension, hyperlipidemia, osteopenia, and obesity. Ms. C takes lisinopril 10 mg/d and atorvastatin, which was recently increased from 10 to 20 mg/d.
What would your next steps be in caring for this patient?
Patients who experience muscle-related symptoms such as pain, fatigue, or weakness often seek help from their family physician (FP). The list of possible causes of these complaints can be lengthy and vary greatly, from nonmyopathic conditions such as fibromyalgia to worrisome forms of myopathy such as inclusion body myositis or polymyositis. This article will help you to quickly identify which patients with muscle-related complaints should be evaluated for myopathy and what your work-up should include.
Myopathy or not?
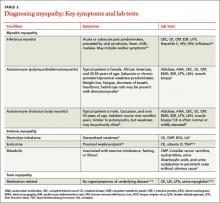
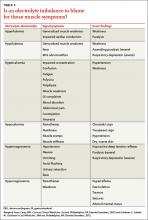
Distinguishing between myopathy and nonmyopathic muscle pain or weakness is the first step in evaluating patients with muscle-related complaints. Many conditions share muscle-related symptoms, but actual muscle damage is not always present (eg, fibromyalgia, chronic pain, and chronic fatigue syndromes).1 While there is some overlap in presentation between patients with myopathy and nonmyopathic conditions, there are important differences in symptoms, physical exam findings, and lab test results (TABLE 11-4). Notably, in myopathic disease, patients’ symptoms are usually progressive, vital signs are abnormal, and weakness is common, whereas patients with nonmyopathic disease typically have remitting and relapsing symptoms, normal vital signs, and no weakness.
Myopathy itself is divided into 3 categories—myositic, intrinsic, and toxic—which reflect the condition, or medication, that brought on the muscle damage (TABLE 22,4-15). Placing patients into one of these categories based on their risk factors, history, and physical exam findings can help to focus the diagnostic work-up on areas most likely to provide useful information.
Myositic myopathy can be caused by infection or autoimmunity
Myositic myopathies result in inflammatory destruction of muscle tissue. Patients with myositic myopathy often exhibit fever, malaise, weight loss, and general fatigue. Though weakness and pain are common, both can be variable or even absent in myositic myopathy.2,5 Myositic myopathy can be caused by infectious agents or can develop from an autoimmune disease.
Infectious myositic myopathy is one of the more common types of myopathy that FPs will encounter.2 Viruses such as influenza, parainfluenza, coxsackievirus, human immunodeficiency virus, cytomegalovirus, echovirus, adenovirus, Epstein-Barr, and hepatitis C are common causes.2,4,16 Bacterial and fungal myositides are relatively rare. Both most often occur as the result of penetrating trauma or immunocompromise, and are generally not subtle.2 Parasitic myopathy can occur from the invasion of skeletal muscle by trichinella after ingesting undercooked, infected meat.2 Although previously a more common problem, currently only 10 to 20 cases of trichinellosis are reported in the United States each year.17 Due to their rarity, bacterial, fungal, and parasitic myositides are not reviewed here.
Patients with a viral myositis often report prodromal symptoms such as fever, upper respiratory illness, or gastrointestinal distress one to 2 weeks before the onset of muscle complaints. Muscle pain is usually multifocal, involving larger, bilateral muscle groups, and may be associated with swelling.
Patients with viral myositis may exhibit diffusely painful, swollen, or boggy-feeling muscles as well as weakness and pain with exertion. Other signs of viral infection such as rash, fever, upper respiratory symptoms, or meningeal signs may be present. Severe signs include arrhythmia or respiratory failure due to cardiac muscle or diaphragm involvement, or signs of renal failure due to precipitation of myoglobin in the renal system (ie, rhabdomyolysis).2 If the infection affects the heart, patients may develop palpitations, pleuritic chest pain, or shortness of breath.2
Diagnosis of viral myositis relies heavily on clinical suspicion in patients with a fitting history and physical exam findings. Helpful lab tests include a complete blood count (CBC), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), creatine kinase (CK), and liver function tests (LFTs), all of which can be abnormal in viral myositis. Viral polymerase chain reaction, culture, or antigen testing may be helpful in severe or confusing cases, but in most cases such testing is unnecessary. Muscle biopsy is not recommended except in persistent cases, where definitive identification of the causative agent might alter treatment or when nonviral infection is suspected.2
Autoimmune myositic myopathy. Unlike infectious myopathies, autoimmune myopathies are usually chronic, subtle, and relatively rare. The 3 most common autoimmune myopathies—polymyositis, dermatomyositis, and inclusion body myositis—have a combined prevalence of approximately 10:100,000.6 Although these types of myopathies are uncommon, FPs will likely be the first to evaluate a patient with one of them.
Patients with an autoimmune myopathy typically complain of weakness and mild to moderate muscle pain, although pain may be absent. Compared to infectious myopathies, autoimmune myopathies usually exhibit a more indolent course. Patients with advanced disease may report fever, weight loss, shortness of breath from cardiomyopathy, heartburn from a weakened lower esophageal sphincter, and/or a rash.5
Physical examination may reveal symmetric, proximal muscle weakness. Atrophy is typically not seen until late in the disease. Skin exam usually is normal in patients with inclusion body myositis and polymyositis. The typical rash of dermatomyositis is a heliotrope (blue-purple) discoloration on the upper eyelids and a raised, violaceous, scaly eruption on the knuckles (Gottron’s papules).
Laboratory tests that can be helpful include CK, lactate dehydrogenase (LDH), aldolase, and LFTs (reflecting muscle injury, not liver involvement). For polymyositis and dermatomyositis, CK is the most sensitive lab test and often exhibits the highest elevation above normal.6 Conversely, CK is often normal or only mildly elevated in inclusion body myositis. Up to 80% of patients with autoimmune myopathy will have antinuclear antibodies.3,5 ESR and CRP levels are also often elevated.
Both electromyography (EMG) and muscle biopsy may be required to diagnose autoimmune myopathy, but these are typically done under the direction of a rheumatologist after an FP’s initial work-up is inconclusive.
Intrinsic myopathy: Suspect electrolyte problems, other causes
Intrinsic myopathy occurs in patients with electrolyte disorders, diseases of the endocrine system, or underlying metabolic dysfunction.
Electrolyte disorders. Muscle-related symptoms are unlikely to be the chief complaint of patients with severe electrolyte imbalance. However, a patient with mild to moderate electrolyte problems may develop muscle fatigue, weakness, or pain. TABLE 3 reviews other signs and symptoms of electrolyte abnormalities that may be helpful in establishing a diagnosis in a patient with muscle complaints.
Ordering a complete metabolic panel (CMP), CK, and urinalysis (UA) can help rule out electrolyte disorders. If electrolyte disorders are detected, an electrocardiogram is useful to evaluate for cardiac dysfunction. Once an electrolyte disorder is identified, investigate its underlying cause. Correcting the electrolyte disorder should help improve symptoms of myopathy.
Endocrine myopathy can be associated with hypothyroidism, hyperthyroidism, parathyroid disease, vitamin D deficiency, or Cushing syndrome.8-10,18,19 Although less common than some other causes, identifying endocrine myopathy is crucial because correcting the underlying disease will often improve multiple aspects of the patient’s health.
The presentation of endocrine myopathy may be subtle. Patients with hypothyroidism may experience muscle pain or weakness, fatigue, cold sensitivity, constipation, and dry skin.20 Muscle-related symptoms may be the only sign of endocrine myopathy in a patient who would otherwise be considered to have subclinical hypothyroidism.8,18 Hyperthyroidism can present with weight loss, heat intolerance, frequent bowel movements, tachycardia, and muscle weakness.21
Patients with parathyroid disease— especially patients with chronic renal failure—may report proximal muscle weakness, often in the lower extremities.19 Complaints of muscle weakness or pain can occur with severe vitamin D deficiency.10 Patients with Cushing syndrome often experience proximal weakness and weight gain.9
Patients with a personal or family history of endocrine disorders, previous thyroid surgery, or those taking medications that can impair thyroid function, such as lithium, amiodarone, or interferon, are at risk for endocrine myopathy.18-20 Suspect hyperparathyroidism in patients with chronic kidney disease who complain of weakness.
Vitamin D deficiency is relatively common, with at minimum 20% of elderly adults estimated to be deficient.10 Patients at risk for Cushing disease are most likely receiving pharmacologic doses of glucocorticoids, which can increase their risk of myopathy, or to have ectopic adrenocorticotropic hormone secretion.
Metabolic myopathy results from a lack of sufficient energy production in the muscle. The 3 main groups of metabolic myopathy are impaired muscle glycogenoses, disorders of fatty acid oxidation, and mitochondrial myopathies.7
Because metabolic myopathy can occur at any age, a thorough history and physical is crucial for diagnosis. Proximal weakness in metabolic myopathy is often associated with exercise intolerance, stressful illness, or fasting. Patients often present with dynamic abnormalities such as fatigue, muscle cramping, and even rhabdomyolysis during exertion.7
When evaluating patients you suspect may have metabolic myopathy, a physical exam may reveal muscle contractures, muscle swelling, or proximal muscle weakness. Patients with certain types of fatty acid oxidation disorders or mitochondrial disorders may also exhibit cardiomyopathy, neuropathy, retinopathy, ataxia, hearing loss, or other systemic manifestations.7
Basic labs for investigating suspected metabolic myopathy include serum electrolytes, glucose, LFTs, CK (which may or may not be elevated), lactate, ammonia, and UA for myoglobinuria. More advanced labs, such as serum total carnitine and acylcarnitine as well as urinary levels of dicarboxylic acids and acylglycines, may be needed if a metabolic disorder is strongly suspected.7 Muscle biopsy, EMG, and genetic testing can also prove helpful in diagnosis. Definitive diagnosis and treatment of metabolic myopathy usually requires a multidisciplinary team of providers, including subspecialty referral.
Toxic myopathy
Toxic myopathy refers to muscle damage caused by an exogenous chemical agent, most often a drug. The mechanism of toxicity is not always clear and may result from the activation of inflammatory responses similar to autoimmune myopathy.22 Toxic myopathies may result from several commonly used medications; cholesterol-lowering medications are a common culprit.13-15,23-25 Drug-induced myopathies vary in frequency and severity. For instance, in patients taking statins, the rate of myalgias is 6%, while the incidence of rhabdomyolysis is estimated to be 4 per 100,000, and is found most often in patients taking concomitant fibrates.23
Drug-induced toxic myopathy differs from previously discussed myopathies in that symptoms are usually more insidious, findings on exam are more often mixed muscular and neurologic, and lab abnormalities are usually more subtle.11,12 Symptoms of myopathy typically occur weeks or months after initiating a drug and usually improve or resolve within weeks after discontinuing the offending agent. Knowing the patient’s medication list and which medications cause certain patterns of myopathy symptoms can help guide the differential diagnosis (TABLE 411-15,22-25).
Risk factors for most medication-related myopathies are polypharmacy, renal or liver disease, and age over 50 years13-15,23-25 The physical exam for patients with drug- or toxin-related myopathy will most often reveal relatively minor abnormalities such as muscle tenderness and mild weakness, except for the most severe or advanced cases. Most patients will not have physical signs that suggest an underlying illness. CK levels and LFTs should be obtained. Basic chemistry and UA may also be helpful in patients with risk factors for renal disease.
CASE › Ms. C has been taking a statin for more than 10 years, and the dose was recently increased. You are aware that statin-related muscle injury can develop even after years of use, and suspect the statin may be causing her myopathy. You order a CK test, which is mildly elevated. You recommend discontinuing the statin. After 8 weeks off her statin, Ms. C’s Symptoms do not improve. Given her lack of systemic complaints, myositic myopathy from an infectious or rheumatologic cause seems unlikely. You begin to consider an intrinsic cause of myopathy, and order the following tests: a CMP, UA, thyroid-stimulating hormone, repeat CK, and vitamin D level. This testing reveals a vitamin D deficiency at 17 ng/ml (normal range: 30-74 ng/ml). You recommend vitamin D, 50,000 IU per week for 8 weeks. At follow-up, Ms. C's vitamin D level is 40. She says she feels better and her muscle complaints have resolved.
CORRESPONDENCE
Brent W. Smith, MD, Travis Air Force Base Family Medicine Residency, 101 Bodin Circle, Travis Air Force Base, CA 94535; [email protected]
1. Huynh CN, Yanni LM, Morgan LA. Fibromyalgia: diagnosis and management for the primary healthcare provider. J Womens Health. 2008;8:1379-1387.
2. Crum-Cianflone NF. Bacterial, fungal, parasitic, and viral myositis. Clin Microbiol Rev. 2008;21:473-494.
3. Reichlin M, Arnett FC Jr. Multiplicity of antibodies in myositis sera. Arthritis Rheum. 1984;27:1150-1156.
4. Yoshino M, Suzuki S, Adachi K, et al. High incidence of acute myositis with type A influenza virus infection in the elderly. Intern Med. 2000;39:431-432.
5. Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362:971-982.
6. Wilson FC, Ytterberg SR, St Sauver JL, et al. Epidemiology of sporadic inclusion body myositis and polymyositis in Olmsted County, Minnesota. J Rheumatol. 2008;35:445-447.
7. Smith EC, El-Gharbawy A, Koeberl DD. Metabolic myopathies: clinical features and diagnostic approach. Rheum Dis Clin N Am. 2011:37:201-217.
8. Reuters V, Teixeira Pde F, Vigário PS, et al. Functional capacity and muscular abnormalities in subclinical hypothyroidism. Am J Med Sci. 2009;338:259-263.
9. Nieman LK, Biller BM, Findling JW, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2008;93:1526-1540.
10. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911-1930.
11. Antons KA, Williams CD, Baker SK, et al. Clinical perspectives of statin-induced rhabdomyolysis. Am J Med. 2006;119:400-409.
12. Phillips PS, Haas RH, Bannykh S, et al; Scripps Mercy Clinical Research Center. Statin-associated myopathy with normal creatine kinase levels. Ann Intern Med. 2002;137:581-585.
13. Pereira RM, Freire de Carvalho J. Glucocorticoid-induced myopathy. Joint Bone Spine. 2011;78:41-44.
14. Posada C, García-Cruz A, García-Doval I, et al. Chloroquine-induced myopathy. Lupus. 2011;20:773-774.
15. Uri DS, Biavis M. Colchicine neuromyopathy. J Clin Rheumatol. 1996;2:163-166.
16. Mannix R, Tan ML, Wright R, et al. Acute pediatric rhabdomyolysis: causes and rates of renal failure. Pediatrics. 2006;118:2119-2125.
17. Pozio E. World distribution of Trichinella spp. infections in animals and humans. Vet Parasitol. 2007;149:3-21.
18. Rodolico C, Toscano A, Benvenga S, et al. Myopathy as the persistently isolated symptomatology of primary autoimmune hypothyroidism. Thyroid.1998;8:1033-1038.
19. AACE/AAES Task Force on Primary Hyperparathyroidism. The American Association of Clinical Endocrinologists and The American Association of Endocrine Surgeons position statement on the diagnosis and management of primary hyperparathyroidism. Endocr Pract. 2005;11:49-54.
20. Garber JR, Cobin RH, Gharib H, et al; American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocrine Pract. 2012;18:988-1028.
21. Bahn Chair RS, Burch HB, Cooper DS, et al; American Thyroid Association; American Association of Clinical Endocrinologists. Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Thyroid. 2011;21:593-646.
22. Mammen AL, Amato AA. Statin myopathy: a review of recent progress. Curr Opin Rheumatol. 2010;22:644-650.
23. Buettner C, Davis RB, Leveille SG, et al. Prevalence of musculoskeletal pain and statin use. J Gen Intern Med. 2008;23: 1182-1186.
24. Marot A, Morelle J, Chouinard VA, et al. Concomitant use of simvastatin and amiodarone resulting in severe rhabdomyolysis: a case report and review of the literature. Acta Clin Belg. 2011;66:134-136.
25. Peters BS, Winer J, Landon DN, et al. Mitochondrial myopathy associated with chronic zidovudine therapy in AIDS. Q J Med. 1993;86:5-15.
› Categorize patients with muscle complaints into suspected myositic, intrinsic, or toxic myopathy to help guide subsequent work-up. C
› Look for diffusely painful, swollen, or boggy-feeling muscles—as well as weakness and pain with exertion—in patients you suspect may have viral myopathy. C
› Consider electromyography and muscle biopsy for patients you suspect may have dermatomyositis. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Marie C, a 75-year-old Asian woman, reports weakness in her legs and arms with unsteadiness when walking. She has a vague but persistent ache in her large muscles. Her symptoms have developed slowly over the past 3 months. She denies recent signs or symptoms of infection or other illness. Her medical history includes hypertension, hyperlipidemia, osteopenia, and obesity. Ms. C takes lisinopril 10 mg/d and atorvastatin, which was recently increased from 10 to 20 mg/d.
What would your next steps be in caring for this patient?
Patients who experience muscle-related symptoms such as pain, fatigue, or weakness often seek help from their family physician (FP). The list of possible causes of these complaints can be lengthy and vary greatly, from nonmyopathic conditions such as fibromyalgia to worrisome forms of myopathy such as inclusion body myositis or polymyositis. This article will help you to quickly identify which patients with muscle-related complaints should be evaluated for myopathy and what your work-up should include.
Myopathy or not?
Distinguishing between myopathy and nonmyopathic muscle pain or weakness is the first step in evaluating patients with muscle-related complaints. Many conditions share muscle-related symptoms, but actual muscle damage is not always present (eg, fibromyalgia, chronic pain, and chronic fatigue syndromes).1 While there is some overlap in presentation between patients with myopathy and nonmyopathic conditions, there are important differences in symptoms, physical exam findings, and lab test results (TABLE 11-4). Notably, in myopathic disease, patients’ symptoms are usually progressive, vital signs are abnormal, and weakness is common, whereas patients with nonmyopathic disease typically have remitting and relapsing symptoms, normal vital signs, and no weakness.
Myopathy itself is divided into 3 categories—myositic, intrinsic, and toxic—which reflect the condition, or medication, that brought on the muscle damage (TABLE 22,4-15). Placing patients into one of these categories based on their risk factors, history, and physical exam findings can help to focus the diagnostic work-up on areas most likely to provide useful information.
Myositic myopathy can be caused by infection or autoimmunity
Myositic myopathies result in inflammatory destruction of muscle tissue. Patients with myositic myopathy often exhibit fever, malaise, weight loss, and general fatigue. Though weakness and pain are common, both can be variable or even absent in myositic myopathy.2,5 Myositic myopathy can be caused by infectious agents or can develop from an autoimmune disease.
Infectious myositic myopathy is one of the more common types of myopathy that FPs will encounter.2 Viruses such as influenza, parainfluenza, coxsackievirus, human immunodeficiency virus, cytomegalovirus, echovirus, adenovirus, Epstein-Barr, and hepatitis C are common causes.2,4,16 Bacterial and fungal myositides are relatively rare. Both most often occur as the result of penetrating trauma or immunocompromise, and are generally not subtle.2 Parasitic myopathy can occur from the invasion of skeletal muscle by trichinella after ingesting undercooked, infected meat.2 Although previously a more common problem, currently only 10 to 20 cases of trichinellosis are reported in the United States each year.17 Due to their rarity, bacterial, fungal, and parasitic myositides are not reviewed here.
Patients with a viral myositis often report prodromal symptoms such as fever, upper respiratory illness, or gastrointestinal distress one to 2 weeks before the onset of muscle complaints. Muscle pain is usually multifocal, involving larger, bilateral muscle groups, and may be associated with swelling.
Patients with viral myositis may exhibit diffusely painful, swollen, or boggy-feeling muscles as well as weakness and pain with exertion. Other signs of viral infection such as rash, fever, upper respiratory symptoms, or meningeal signs may be present. Severe signs include arrhythmia or respiratory failure due to cardiac muscle or diaphragm involvement, or signs of renal failure due to precipitation of myoglobin in the renal system (ie, rhabdomyolysis).2 If the infection affects the heart, patients may develop palpitations, pleuritic chest pain, or shortness of breath.2
Diagnosis of viral myositis relies heavily on clinical suspicion in patients with a fitting history and physical exam findings. Helpful lab tests include a complete blood count (CBC), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), creatine kinase (CK), and liver function tests (LFTs), all of which can be abnormal in viral myositis. Viral polymerase chain reaction, culture, or antigen testing may be helpful in severe or confusing cases, but in most cases such testing is unnecessary. Muscle biopsy is not recommended except in persistent cases, where definitive identification of the causative agent might alter treatment or when nonviral infection is suspected.2
Autoimmune myositic myopathy. Unlike infectious myopathies, autoimmune myopathies are usually chronic, subtle, and relatively rare. The 3 most common autoimmune myopathies—polymyositis, dermatomyositis, and inclusion body myositis—have a combined prevalence of approximately 10:100,000.6 Although these types of myopathies are uncommon, FPs will likely be the first to evaluate a patient with one of them.
Patients with an autoimmune myopathy typically complain of weakness and mild to moderate muscle pain, although pain may be absent. Compared to infectious myopathies, autoimmune myopathies usually exhibit a more indolent course. Patients with advanced disease may report fever, weight loss, shortness of breath from cardiomyopathy, heartburn from a weakened lower esophageal sphincter, and/or a rash.5
Physical examination may reveal symmetric, proximal muscle weakness. Atrophy is typically not seen until late in the disease. Skin exam usually is normal in patients with inclusion body myositis and polymyositis. The typical rash of dermatomyositis is a heliotrope (blue-purple) discoloration on the upper eyelids and a raised, violaceous, scaly eruption on the knuckles (Gottron’s papules).
Laboratory tests that can be helpful include CK, lactate dehydrogenase (LDH), aldolase, and LFTs (reflecting muscle injury, not liver involvement). For polymyositis and dermatomyositis, CK is the most sensitive lab test and often exhibits the highest elevation above normal.6 Conversely, CK is often normal or only mildly elevated in inclusion body myositis. Up to 80% of patients with autoimmune myopathy will have antinuclear antibodies.3,5 ESR and CRP levels are also often elevated.
Both electromyography (EMG) and muscle biopsy may be required to diagnose autoimmune myopathy, but these are typically done under the direction of a rheumatologist after an FP’s initial work-up is inconclusive.
Intrinsic myopathy: Suspect electrolyte problems, other causes
Intrinsic myopathy occurs in patients with electrolyte disorders, diseases of the endocrine system, or underlying metabolic dysfunction.
Electrolyte disorders. Muscle-related symptoms are unlikely to be the chief complaint of patients with severe electrolyte imbalance. However, a patient with mild to moderate electrolyte problems may develop muscle fatigue, weakness, or pain. TABLE 3 reviews other signs and symptoms of electrolyte abnormalities that may be helpful in establishing a diagnosis in a patient with muscle complaints.
Ordering a complete metabolic panel (CMP), CK, and urinalysis (UA) can help rule out electrolyte disorders. If electrolyte disorders are detected, an electrocardiogram is useful to evaluate for cardiac dysfunction. Once an electrolyte disorder is identified, investigate its underlying cause. Correcting the electrolyte disorder should help improve symptoms of myopathy.
Endocrine myopathy can be associated with hypothyroidism, hyperthyroidism, parathyroid disease, vitamin D deficiency, or Cushing syndrome.8-10,18,19 Although less common than some other causes, identifying endocrine myopathy is crucial because correcting the underlying disease will often improve multiple aspects of the patient’s health.
The presentation of endocrine myopathy may be subtle. Patients with hypothyroidism may experience muscle pain or weakness, fatigue, cold sensitivity, constipation, and dry skin.20 Muscle-related symptoms may be the only sign of endocrine myopathy in a patient who would otherwise be considered to have subclinical hypothyroidism.8,18 Hyperthyroidism can present with weight loss, heat intolerance, frequent bowel movements, tachycardia, and muscle weakness.21
Patients with parathyroid disease— especially patients with chronic renal failure—may report proximal muscle weakness, often in the lower extremities.19 Complaints of muscle weakness or pain can occur with severe vitamin D deficiency.10 Patients with Cushing syndrome often experience proximal weakness and weight gain.9
Patients with a personal or family history of endocrine disorders, previous thyroid surgery, or those taking medications that can impair thyroid function, such as lithium, amiodarone, or interferon, are at risk for endocrine myopathy.18-20 Suspect hyperparathyroidism in patients with chronic kidney disease who complain of weakness.
Vitamin D deficiency is relatively common, with at minimum 20% of elderly adults estimated to be deficient.10 Patients at risk for Cushing disease are most likely receiving pharmacologic doses of glucocorticoids, which can increase their risk of myopathy, or to have ectopic adrenocorticotropic hormone secretion.
Metabolic myopathy results from a lack of sufficient energy production in the muscle. The 3 main groups of metabolic myopathy are impaired muscle glycogenoses, disorders of fatty acid oxidation, and mitochondrial myopathies.7
Because metabolic myopathy can occur at any age, a thorough history and physical is crucial for diagnosis. Proximal weakness in metabolic myopathy is often associated with exercise intolerance, stressful illness, or fasting. Patients often present with dynamic abnormalities such as fatigue, muscle cramping, and even rhabdomyolysis during exertion.7
When evaluating patients you suspect may have metabolic myopathy, a physical exam may reveal muscle contractures, muscle swelling, or proximal muscle weakness. Patients with certain types of fatty acid oxidation disorders or mitochondrial disorders may also exhibit cardiomyopathy, neuropathy, retinopathy, ataxia, hearing loss, or other systemic manifestations.7
Basic labs for investigating suspected metabolic myopathy include serum electrolytes, glucose, LFTs, CK (which may or may not be elevated), lactate, ammonia, and UA for myoglobinuria. More advanced labs, such as serum total carnitine and acylcarnitine as well as urinary levels of dicarboxylic acids and acylglycines, may be needed if a metabolic disorder is strongly suspected.7 Muscle biopsy, EMG, and genetic testing can also prove helpful in diagnosis. Definitive diagnosis and treatment of metabolic myopathy usually requires a multidisciplinary team of providers, including subspecialty referral.
Toxic myopathy
Toxic myopathy refers to muscle damage caused by an exogenous chemical agent, most often a drug. The mechanism of toxicity is not always clear and may result from the activation of inflammatory responses similar to autoimmune myopathy.22 Toxic myopathies may result from several commonly used medications; cholesterol-lowering medications are a common culprit.13-15,23-25 Drug-induced myopathies vary in frequency and severity. For instance, in patients taking statins, the rate of myalgias is 6%, while the incidence of rhabdomyolysis is estimated to be 4 per 100,000, and is found most often in patients taking concomitant fibrates.23
Drug-induced toxic myopathy differs from previously discussed myopathies in that symptoms are usually more insidious, findings on exam are more often mixed muscular and neurologic, and lab abnormalities are usually more subtle.11,12 Symptoms of myopathy typically occur weeks or months after initiating a drug and usually improve or resolve within weeks after discontinuing the offending agent. Knowing the patient’s medication list and which medications cause certain patterns of myopathy symptoms can help guide the differential diagnosis (TABLE 411-15,22-25).
Risk factors for most medication-related myopathies are polypharmacy, renal or liver disease, and age over 50 years13-15,23-25 The physical exam for patients with drug- or toxin-related myopathy will most often reveal relatively minor abnormalities such as muscle tenderness and mild weakness, except for the most severe or advanced cases. Most patients will not have physical signs that suggest an underlying illness. CK levels and LFTs should be obtained. Basic chemistry and UA may also be helpful in patients with risk factors for renal disease.
CASE › Ms. C has been taking a statin for more than 10 years, and the dose was recently increased. You are aware that statin-related muscle injury can develop even after years of use, and suspect the statin may be causing her myopathy. You order a CK test, which is mildly elevated. You recommend discontinuing the statin. After 8 weeks off her statin, Ms. C’s Symptoms do not improve. Given her lack of systemic complaints, myositic myopathy from an infectious or rheumatologic cause seems unlikely. You begin to consider an intrinsic cause of myopathy, and order the following tests: a CMP, UA, thyroid-stimulating hormone, repeat CK, and vitamin D level. This testing reveals a vitamin D deficiency at 17 ng/ml (normal range: 30-74 ng/ml). You recommend vitamin D, 50,000 IU per week for 8 weeks. At follow-up, Ms. C's vitamin D level is 40. She says she feels better and her muscle complaints have resolved.
CORRESPONDENCE
Brent W. Smith, MD, Travis Air Force Base Family Medicine Residency, 101 Bodin Circle, Travis Air Force Base, CA 94535; [email protected]
› Categorize patients with muscle complaints into suspected myositic, intrinsic, or toxic myopathy to help guide subsequent work-up. C
› Look for diffusely painful, swollen, or boggy-feeling muscles—as well as weakness and pain with exertion—in patients you suspect may have viral myopathy. C
› Consider electromyography and muscle biopsy for patients you suspect may have dermatomyositis. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Marie C, a 75-year-old Asian woman, reports weakness in her legs and arms with unsteadiness when walking. She has a vague but persistent ache in her large muscles. Her symptoms have developed slowly over the past 3 months. She denies recent signs or symptoms of infection or other illness. Her medical history includes hypertension, hyperlipidemia, osteopenia, and obesity. Ms. C takes lisinopril 10 mg/d and atorvastatin, which was recently increased from 10 to 20 mg/d.
What would your next steps be in caring for this patient?
Patients who experience muscle-related symptoms such as pain, fatigue, or weakness often seek help from their family physician (FP). The list of possible causes of these complaints can be lengthy and vary greatly, from nonmyopathic conditions such as fibromyalgia to worrisome forms of myopathy such as inclusion body myositis or polymyositis. This article will help you to quickly identify which patients with muscle-related complaints should be evaluated for myopathy and what your work-up should include.
Myopathy or not?
Distinguishing between myopathy and nonmyopathic muscle pain or weakness is the first step in evaluating patients with muscle-related complaints. Many conditions share muscle-related symptoms, but actual muscle damage is not always present (eg, fibromyalgia, chronic pain, and chronic fatigue syndromes).1 While there is some overlap in presentation between patients with myopathy and nonmyopathic conditions, there are important differences in symptoms, physical exam findings, and lab test results (TABLE 11-4). Notably, in myopathic disease, patients’ symptoms are usually progressive, vital signs are abnormal, and weakness is common, whereas patients with nonmyopathic disease typically have remitting and relapsing symptoms, normal vital signs, and no weakness.
Myopathy itself is divided into 3 categories—myositic, intrinsic, and toxic—which reflect the condition, or medication, that brought on the muscle damage (TABLE 22,4-15). Placing patients into one of these categories based on their risk factors, history, and physical exam findings can help to focus the diagnostic work-up on areas most likely to provide useful information.
Myositic myopathy can be caused by infection or autoimmunity
Myositic myopathies result in inflammatory destruction of muscle tissue. Patients with myositic myopathy often exhibit fever, malaise, weight loss, and general fatigue. Though weakness and pain are common, both can be variable or even absent in myositic myopathy.2,5 Myositic myopathy can be caused by infectious agents or can develop from an autoimmune disease.
Infectious myositic myopathy is one of the more common types of myopathy that FPs will encounter.2 Viruses such as influenza, parainfluenza, coxsackievirus, human immunodeficiency virus, cytomegalovirus, echovirus, adenovirus, Epstein-Barr, and hepatitis C are common causes.2,4,16 Bacterial and fungal myositides are relatively rare. Both most often occur as the result of penetrating trauma or immunocompromise, and are generally not subtle.2 Parasitic myopathy can occur from the invasion of skeletal muscle by trichinella after ingesting undercooked, infected meat.2 Although previously a more common problem, currently only 10 to 20 cases of trichinellosis are reported in the United States each year.17 Due to their rarity, bacterial, fungal, and parasitic myositides are not reviewed here.
Patients with a viral myositis often report prodromal symptoms such as fever, upper respiratory illness, or gastrointestinal distress one to 2 weeks before the onset of muscle complaints. Muscle pain is usually multifocal, involving larger, bilateral muscle groups, and may be associated with swelling.
Patients with viral myositis may exhibit diffusely painful, swollen, or boggy-feeling muscles as well as weakness and pain with exertion. Other signs of viral infection such as rash, fever, upper respiratory symptoms, or meningeal signs may be present. Severe signs include arrhythmia or respiratory failure due to cardiac muscle or diaphragm involvement, or signs of renal failure due to precipitation of myoglobin in the renal system (ie, rhabdomyolysis).2 If the infection affects the heart, patients may develop palpitations, pleuritic chest pain, or shortness of breath.2
Diagnosis of viral myositis relies heavily on clinical suspicion in patients with a fitting history and physical exam findings. Helpful lab tests include a complete blood count (CBC), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), creatine kinase (CK), and liver function tests (LFTs), all of which can be abnormal in viral myositis. Viral polymerase chain reaction, culture, or antigen testing may be helpful in severe or confusing cases, but in most cases such testing is unnecessary. Muscle biopsy is not recommended except in persistent cases, where definitive identification of the causative agent might alter treatment or when nonviral infection is suspected.2
Autoimmune myositic myopathy. Unlike infectious myopathies, autoimmune myopathies are usually chronic, subtle, and relatively rare. The 3 most common autoimmune myopathies—polymyositis, dermatomyositis, and inclusion body myositis—have a combined prevalence of approximately 10:100,000.6 Although these types of myopathies are uncommon, FPs will likely be the first to evaluate a patient with one of them.
Patients with an autoimmune myopathy typically complain of weakness and mild to moderate muscle pain, although pain may be absent. Compared to infectious myopathies, autoimmune myopathies usually exhibit a more indolent course. Patients with advanced disease may report fever, weight loss, shortness of breath from cardiomyopathy, heartburn from a weakened lower esophageal sphincter, and/or a rash.5
Physical examination may reveal symmetric, proximal muscle weakness. Atrophy is typically not seen until late in the disease. Skin exam usually is normal in patients with inclusion body myositis and polymyositis. The typical rash of dermatomyositis is a heliotrope (blue-purple) discoloration on the upper eyelids and a raised, violaceous, scaly eruption on the knuckles (Gottron’s papules).
Laboratory tests that can be helpful include CK, lactate dehydrogenase (LDH), aldolase, and LFTs (reflecting muscle injury, not liver involvement). For polymyositis and dermatomyositis, CK is the most sensitive lab test and often exhibits the highest elevation above normal.6 Conversely, CK is often normal or only mildly elevated in inclusion body myositis. Up to 80% of patients with autoimmune myopathy will have antinuclear antibodies.3,5 ESR and CRP levels are also often elevated.
Both electromyography (EMG) and muscle biopsy may be required to diagnose autoimmune myopathy, but these are typically done under the direction of a rheumatologist after an FP’s initial work-up is inconclusive.
Intrinsic myopathy: Suspect electrolyte problems, other causes
Intrinsic myopathy occurs in patients with electrolyte disorders, diseases of the endocrine system, or underlying metabolic dysfunction.
Electrolyte disorders. Muscle-related symptoms are unlikely to be the chief complaint of patients with severe electrolyte imbalance. However, a patient with mild to moderate electrolyte problems may develop muscle fatigue, weakness, or pain. TABLE 3 reviews other signs and symptoms of electrolyte abnormalities that may be helpful in establishing a diagnosis in a patient with muscle complaints.
Ordering a complete metabolic panel (CMP), CK, and urinalysis (UA) can help rule out electrolyte disorders. If electrolyte disorders are detected, an electrocardiogram is useful to evaluate for cardiac dysfunction. Once an electrolyte disorder is identified, investigate its underlying cause. Correcting the electrolyte disorder should help improve symptoms of myopathy.
Endocrine myopathy can be associated with hypothyroidism, hyperthyroidism, parathyroid disease, vitamin D deficiency, or Cushing syndrome.8-10,18,19 Although less common than some other causes, identifying endocrine myopathy is crucial because correcting the underlying disease will often improve multiple aspects of the patient’s health.
The presentation of endocrine myopathy may be subtle. Patients with hypothyroidism may experience muscle pain or weakness, fatigue, cold sensitivity, constipation, and dry skin.20 Muscle-related symptoms may be the only sign of endocrine myopathy in a patient who would otherwise be considered to have subclinical hypothyroidism.8,18 Hyperthyroidism can present with weight loss, heat intolerance, frequent bowel movements, tachycardia, and muscle weakness.21
Patients with parathyroid disease— especially patients with chronic renal failure—may report proximal muscle weakness, often in the lower extremities.19 Complaints of muscle weakness or pain can occur with severe vitamin D deficiency.10 Patients with Cushing syndrome often experience proximal weakness and weight gain.9
Patients with a personal or family history of endocrine disorders, previous thyroid surgery, or those taking medications that can impair thyroid function, such as lithium, amiodarone, or interferon, are at risk for endocrine myopathy.18-20 Suspect hyperparathyroidism in patients with chronic kidney disease who complain of weakness.
Vitamin D deficiency is relatively common, with at minimum 20% of elderly adults estimated to be deficient.10 Patients at risk for Cushing disease are most likely receiving pharmacologic doses of glucocorticoids, which can increase their risk of myopathy, or to have ectopic adrenocorticotropic hormone secretion.
Metabolic myopathy results from a lack of sufficient energy production in the muscle. The 3 main groups of metabolic myopathy are impaired muscle glycogenoses, disorders of fatty acid oxidation, and mitochondrial myopathies.7
Because metabolic myopathy can occur at any age, a thorough history and physical is crucial for diagnosis. Proximal weakness in metabolic myopathy is often associated with exercise intolerance, stressful illness, or fasting. Patients often present with dynamic abnormalities such as fatigue, muscle cramping, and even rhabdomyolysis during exertion.7
When evaluating patients you suspect may have metabolic myopathy, a physical exam may reveal muscle contractures, muscle swelling, or proximal muscle weakness. Patients with certain types of fatty acid oxidation disorders or mitochondrial disorders may also exhibit cardiomyopathy, neuropathy, retinopathy, ataxia, hearing loss, or other systemic manifestations.7
Basic labs for investigating suspected metabolic myopathy include serum electrolytes, glucose, LFTs, CK (which may or may not be elevated), lactate, ammonia, and UA for myoglobinuria. More advanced labs, such as serum total carnitine and acylcarnitine as well as urinary levels of dicarboxylic acids and acylglycines, may be needed if a metabolic disorder is strongly suspected.7 Muscle biopsy, EMG, and genetic testing can also prove helpful in diagnosis. Definitive diagnosis and treatment of metabolic myopathy usually requires a multidisciplinary team of providers, including subspecialty referral.
Toxic myopathy
Toxic myopathy refers to muscle damage caused by an exogenous chemical agent, most often a drug. The mechanism of toxicity is not always clear and may result from the activation of inflammatory responses similar to autoimmune myopathy.22 Toxic myopathies may result from several commonly used medications; cholesterol-lowering medications are a common culprit.13-15,23-25 Drug-induced myopathies vary in frequency and severity. For instance, in patients taking statins, the rate of myalgias is 6%, while the incidence of rhabdomyolysis is estimated to be 4 per 100,000, and is found most often in patients taking concomitant fibrates.23
Drug-induced toxic myopathy differs from previously discussed myopathies in that symptoms are usually more insidious, findings on exam are more often mixed muscular and neurologic, and lab abnormalities are usually more subtle.11,12 Symptoms of myopathy typically occur weeks or months after initiating a drug and usually improve or resolve within weeks after discontinuing the offending agent. Knowing the patient’s medication list and which medications cause certain patterns of myopathy symptoms can help guide the differential diagnosis (TABLE 411-15,22-25).
Risk factors for most medication-related myopathies are polypharmacy, renal or liver disease, and age over 50 years13-15,23-25 The physical exam for patients with drug- or toxin-related myopathy will most often reveal relatively minor abnormalities such as muscle tenderness and mild weakness, except for the most severe or advanced cases. Most patients will not have physical signs that suggest an underlying illness. CK levels and LFTs should be obtained. Basic chemistry and UA may also be helpful in patients with risk factors for renal disease.
CASE › Ms. C has been taking a statin for more than 10 years, and the dose was recently increased. You are aware that statin-related muscle injury can develop even after years of use, and suspect the statin may be causing her myopathy. You order a CK test, which is mildly elevated. You recommend discontinuing the statin. After 8 weeks off her statin, Ms. C’s Symptoms do not improve. Given her lack of systemic complaints, myositic myopathy from an infectious or rheumatologic cause seems unlikely. You begin to consider an intrinsic cause of myopathy, and order the following tests: a CMP, UA, thyroid-stimulating hormone, repeat CK, and vitamin D level. This testing reveals a vitamin D deficiency at 17 ng/ml (normal range: 30-74 ng/ml). You recommend vitamin D, 50,000 IU per week for 8 weeks. At follow-up, Ms. C's vitamin D level is 40. She says she feels better and her muscle complaints have resolved.
CORRESPONDENCE
Brent W. Smith, MD, Travis Air Force Base Family Medicine Residency, 101 Bodin Circle, Travis Air Force Base, CA 94535; [email protected]
1. Huynh CN, Yanni LM, Morgan LA. Fibromyalgia: diagnosis and management for the primary healthcare provider. J Womens Health. 2008;8:1379-1387.
2. Crum-Cianflone NF. Bacterial, fungal, parasitic, and viral myositis. Clin Microbiol Rev. 2008;21:473-494.
3. Reichlin M, Arnett FC Jr. Multiplicity of antibodies in myositis sera. Arthritis Rheum. 1984;27:1150-1156.
4. Yoshino M, Suzuki S, Adachi K, et al. High incidence of acute myositis with type A influenza virus infection in the elderly. Intern Med. 2000;39:431-432.
5. Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362:971-982.
6. Wilson FC, Ytterberg SR, St Sauver JL, et al. Epidemiology of sporadic inclusion body myositis and polymyositis in Olmsted County, Minnesota. J Rheumatol. 2008;35:445-447.
7. Smith EC, El-Gharbawy A, Koeberl DD. Metabolic myopathies: clinical features and diagnostic approach. Rheum Dis Clin N Am. 2011:37:201-217.
8. Reuters V, Teixeira Pde F, Vigário PS, et al. Functional capacity and muscular abnormalities in subclinical hypothyroidism. Am J Med Sci. 2009;338:259-263.
9. Nieman LK, Biller BM, Findling JW, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2008;93:1526-1540.
10. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911-1930.
11. Antons KA, Williams CD, Baker SK, et al. Clinical perspectives of statin-induced rhabdomyolysis. Am J Med. 2006;119:400-409.
12. Phillips PS, Haas RH, Bannykh S, et al; Scripps Mercy Clinical Research Center. Statin-associated myopathy with normal creatine kinase levels. Ann Intern Med. 2002;137:581-585.
13. Pereira RM, Freire de Carvalho J. Glucocorticoid-induced myopathy. Joint Bone Spine. 2011;78:41-44.
14. Posada C, García-Cruz A, García-Doval I, et al. Chloroquine-induced myopathy. Lupus. 2011;20:773-774.
15. Uri DS, Biavis M. Colchicine neuromyopathy. J Clin Rheumatol. 1996;2:163-166.
16. Mannix R, Tan ML, Wright R, et al. Acute pediatric rhabdomyolysis: causes and rates of renal failure. Pediatrics. 2006;118:2119-2125.
17. Pozio E. World distribution of Trichinella spp. infections in animals and humans. Vet Parasitol. 2007;149:3-21.
18. Rodolico C, Toscano A, Benvenga S, et al. Myopathy as the persistently isolated symptomatology of primary autoimmune hypothyroidism. Thyroid.1998;8:1033-1038.
19. AACE/AAES Task Force on Primary Hyperparathyroidism. The American Association of Clinical Endocrinologists and The American Association of Endocrine Surgeons position statement on the diagnosis and management of primary hyperparathyroidism. Endocr Pract. 2005;11:49-54.
20. Garber JR, Cobin RH, Gharib H, et al; American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocrine Pract. 2012;18:988-1028.
21. Bahn Chair RS, Burch HB, Cooper DS, et al; American Thyroid Association; American Association of Clinical Endocrinologists. Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Thyroid. 2011;21:593-646.
22. Mammen AL, Amato AA. Statin myopathy: a review of recent progress. Curr Opin Rheumatol. 2010;22:644-650.
23. Buettner C, Davis RB, Leveille SG, et al. Prevalence of musculoskeletal pain and statin use. J Gen Intern Med. 2008;23: 1182-1186.
24. Marot A, Morelle J, Chouinard VA, et al. Concomitant use of simvastatin and amiodarone resulting in severe rhabdomyolysis: a case report and review of the literature. Acta Clin Belg. 2011;66:134-136.
25. Peters BS, Winer J, Landon DN, et al. Mitochondrial myopathy associated with chronic zidovudine therapy in AIDS. Q J Med. 1993;86:5-15.
1. Huynh CN, Yanni LM, Morgan LA. Fibromyalgia: diagnosis and management for the primary healthcare provider. J Womens Health. 2008;8:1379-1387.
2. Crum-Cianflone NF. Bacterial, fungal, parasitic, and viral myositis. Clin Microbiol Rev. 2008;21:473-494.
3. Reichlin M, Arnett FC Jr. Multiplicity of antibodies in myositis sera. Arthritis Rheum. 1984;27:1150-1156.
4. Yoshino M, Suzuki S, Adachi K, et al. High incidence of acute myositis with type A influenza virus infection in the elderly. Intern Med. 2000;39:431-432.
5. Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362:971-982.
6. Wilson FC, Ytterberg SR, St Sauver JL, et al. Epidemiology of sporadic inclusion body myositis and polymyositis in Olmsted County, Minnesota. J Rheumatol. 2008;35:445-447.
7. Smith EC, El-Gharbawy A, Koeberl DD. Metabolic myopathies: clinical features and diagnostic approach. Rheum Dis Clin N Am. 2011:37:201-217.
8. Reuters V, Teixeira Pde F, Vigário PS, et al. Functional capacity and muscular abnormalities in subclinical hypothyroidism. Am J Med Sci. 2009;338:259-263.
9. Nieman LK, Biller BM, Findling JW, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2008;93:1526-1540.
10. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911-1930.
11. Antons KA, Williams CD, Baker SK, et al. Clinical perspectives of statin-induced rhabdomyolysis. Am J Med. 2006;119:400-409.
12. Phillips PS, Haas RH, Bannykh S, et al; Scripps Mercy Clinical Research Center. Statin-associated myopathy with normal creatine kinase levels. Ann Intern Med. 2002;137:581-585.
13. Pereira RM, Freire de Carvalho J. Glucocorticoid-induced myopathy. Joint Bone Spine. 2011;78:41-44.
14. Posada C, García-Cruz A, García-Doval I, et al. Chloroquine-induced myopathy. Lupus. 2011;20:773-774.
15. Uri DS, Biavis M. Colchicine neuromyopathy. J Clin Rheumatol. 1996;2:163-166.
16. Mannix R, Tan ML, Wright R, et al. Acute pediatric rhabdomyolysis: causes and rates of renal failure. Pediatrics. 2006;118:2119-2125.
17. Pozio E. World distribution of Trichinella spp. infections in animals and humans. Vet Parasitol. 2007;149:3-21.
18. Rodolico C, Toscano A, Benvenga S, et al. Myopathy as the persistently isolated symptomatology of primary autoimmune hypothyroidism. Thyroid.1998;8:1033-1038.
19. AACE/AAES Task Force on Primary Hyperparathyroidism. The American Association of Clinical Endocrinologists and The American Association of Endocrine Surgeons position statement on the diagnosis and management of primary hyperparathyroidism. Endocr Pract. 2005;11:49-54.
20. Garber JR, Cobin RH, Gharib H, et al; American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocrine Pract. 2012;18:988-1028.
21. Bahn Chair RS, Burch HB, Cooper DS, et al; American Thyroid Association; American Association of Clinical Endocrinologists. Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Thyroid. 2011;21:593-646.
22. Mammen AL, Amato AA. Statin myopathy: a review of recent progress. Curr Opin Rheumatol. 2010;22:644-650.
23. Buettner C, Davis RB, Leveille SG, et al. Prevalence of musculoskeletal pain and statin use. J Gen Intern Med. 2008;23: 1182-1186.
24. Marot A, Morelle J, Chouinard VA, et al. Concomitant use of simvastatin and amiodarone resulting in severe rhabdomyolysis: a case report and review of the literature. Acta Clin Belg. 2011;66:134-136.
25. Peters BS, Winer J, Landon DN, et al. Mitochondrial myopathy associated with chronic zidovudine therapy in AIDS. Q J Med. 1993;86:5-15.
Premature infant has CP: $14.5M verdict
Premature infant has CP: $14.5M verdict
After learning that, 14 years earlier, a 36-year-old woman had undergone an emergency cesarean delivery at 32 weeks’ gestation, her health-care providers planned a cesarean delivery for the new pregnancy. The woman was admitted to the hospital in preterm labor. Three days later, she was discharged, but readmitted twice more over a 2-week period. At each admission, preterm labor was halted using medication and bed rest.
The patient’s water broke and she was admitted to the hospital at 25 weeks’ gestation, about a week after the previous admission. Shortly after admission, the patient asked about a cesarean delivery, but no action was taken. When her ObGyn arrived at the hospital 5 hours later, the patient asked for a cesarean delivery; the ObGyn said he wanted to wait to see how her labor was progressing. After 3 hours, the fetus showed signs of distress, and an emergency cesarean delivery was undertaken. The infant experienced a massive brain hemorrhage, resulting in cerebral palsy (CP). The child has cognitive delays, visual impairment, and additional problems; he will require lifelong care.
PARENTS’ CLAIM The ObGyn and hospital were negligent in discharging the woman from admission for preterm labor. Cesarean delivery should have been performed much earlier due to nonreassuring fetal heart tones. Severe variable decelerations caused cerebral blood flow fluctuations that led to the hemorrhage.
DEFENDANTS’ DEFENSE The child’s prematurity and a severe placental infection led to the injuries. Nothing would have changed the outcome.
VERDICT A $14.5 million Ohio verdict was returned, including $1.5 million for the mother.
_______________
Costs returned afterverdict for the defense
A 65-year-old woman underwent a hysterectomy for treatment of uterine cancer performed by a gynecologic oncologist. Postoperatively, the patient developed an infection. A small-bowel injury was surgically repaired. The patient was hospitalized for 4 months for treatment of sepsis.
PARENTS’ CLAIM The physician was negligent for injuring the patient’s bowel and then failing to identify and repair the injury during surgery.
PHYSICIAN’S DEFENSE There was no negligence. The patient had significant adhesions from prior surgeries. The physician noted minor serosal tears of the bowel, several of which were repaired during surgery. He checked the length of the bowel for tears/perforations several times during the procedure, but found none. The patient had areas of weakness in her bowel, one of which broke down after surgery. The perforation was repaired in a timely manner.
VERDICT A Michigan defense verdict was returned. The physician was awarded $14,535 in costs.
_______________
Colon injury after cystectomy
A 21-year-old woman underwent laparoscopic ovarian cystectomy, performed by her gynecologist, and was discharged the next day. Eight days later, the patient went to the emergency department (ED) with pelvic pain. Testing revealed a perforated colon with peritonitis. She underwent repair by laparotomy, including bowel resection and colostomy, which was reversed several months later. She has not regained regular bowel function, cannot digest food that has not been finely sliced, and constantly uses laxatives.
PARENTS’ CLAIM The colon injury occurred during cystectomy because the gynecologist was negligent in failing to maintain proper anatomical landmarks. The injury should have been recognized at the time of surgery by injecting saline solution into the colon. She had not been informed of the risk of colon injury.
DEFENDANTS’ DEFENSE Colon injury is a known complication of cystectomy. The injury could have occurred after surgery due to a minor nick of the colon that was undetectable during surgery. Proper informed consent was acquired.
VERDICT A $340,000 New York settlement was reached.
_______________
Mother hemorrhages, dies after delivery: $1M settlement
A 19-year-old woman presented at full term to a community hospital. After several hours of labor, an emergency cesarean delivery was performed due to arrested descent.
Fifteen minutes after delivery, the mother exhibited moderate bleeding with decreasing blood pressure and tachycardia. The post-anesthesia care unit nurse assessed the patient’s uterus as “boggy,” and alerted the ObGyn, who immediately reacted by expressing clots from the uterus. He noted that the fundus was firm. He ordered intravenous (IV) oxytocin, but the patient continued to hemorrhage. Fifteen minutes later, the patient’s vital signs worsened. The ObGyn ordered blood products, uterotonics, and an additional IV line for fluid resuscitation. He began to massage the fundus and expressed clots.
When the patient did not stabilize, she was returned to the OR. After attempting to stop the bleeding with O’Leary stitches, the ObGyn performed a hysterectomy. Six hours after surgery, and after transfusion of a total of 12 units of blood, the woman coded multiple times. She died 14 hours after delivery. Cause of death was disseminated intravascular coagulopathy caused by an atonic uterus.
ESTATE’S CLAIM The ObGyn failed to recognize the extent of the postpartum hemorrhage and should have acted more aggressively with resuscitation. He should have returned her to the OR earlier. The ObGyn was negligent in waiting 45 minutes for cross-matched blood rather than using universal donor O-negative blood that was readily available.
PHYSICIAN’S DEFENSE The ObGyn denied negligence and maintained that he had acted properly. He returned the patient to the OR within 90 minutes of first learning of the hemorrhage.
VERDICT A $1 million Virginia settlement was reached.
_______________
Infant born with broken arms, collarbone, facial bones
A 23-year-old woman had gestational diabetes. She is 5’9” tall and weighed 300 lb while pregnant. She went to the hospital in labor.
During delivery, shoulder dystocia was encountered. The ObGyn performed a variety of techniques, including the McRobert’s maneuver. Forceps were eventually used for delivery.
Both of the newborn’s arms were broken, and she had a broken collarbone and facial fractures. The mother also suffered significant vaginal lacerations and required an episiotomy. She continues to complain of bladder and bowel problems.
PARENTS’ CLAIM A vaginal delivery should not have been attempted due to the mother’s gestational diabetes and the risk of having a macrosomic baby. A cesarean delivery should have been performed. The ObGyn did not use the proper techniques when delivering the child after shoulder dystocia was encountered.
PHYSICIAN’S DEFENSE The ObGyn denied negligence. He claimed that the baby recovered well from her injuries. The mother underwent surgery and now has excellent bladder and bowel control.
VERDICT A confidential Louisiana settlement was reached with the hospital before trial. A defense verdict was returned for the ObGyn.
_______________
Protein found in urine at 39 weeks’ gestation: mother and child die
At 39 weeks' gestation, a woman saw her ObGyn for a prenatal visit. During the examination, the ObGyn found high levels of protein in the woman’s urine, an accumulation of fluid in her ankles, and the highest blood pressure (BP) reading of the woman’s pregnancy. However, because the BP reading was lower than that required to diagnose preeclampsia, the ObGyn sent the patient home and scheduled the next prenatal visit for the following week. The woman and her unborn child died 5 days later.
ESTATE’S CLAIM The ObGyn was negligent in failing to order a urine study and more closely monitor the mother’s symptoms when signs of preeclampsia were evident at 39 weeks’ gestation. Delivery of the child would have resolved the problem and saved both lives.
PHYSICIAN’S DEFENSE The case was settled during the trial.
VERDICT A $3 million Illinois settlement was reached.
_______________
Baby dies from group B strep
A 16-year-old woman planned delivery at a local hospital. Her ObGyn’s practice regularly sends the hospital its patients’ prenatal records, starting at 25 weeks’ gestation. At 33 weeks, the ObGyn took a vaginal culture to test for group B Streptococcus (GBS) bacteria. The laboratory reported positive GBS results to a computer in the ObGyn’s office, but the results were not entered into the patient’s chart.
The mother went to the ED in labor a week later; she was evaluated and discharged. Several days later, she returned to the ED, but was again discharged. She returned the next day, now in gestational week 36. An on-call ObGyn admitted her. A labor and delivery nurse claimed that the ObGyn’s office reported that the mother was GBS negative, so the nurse placed a negative sign in the prenatal record in the chart. When the patient’s ObGyn arrived at the hospital, he noticed the negative sign in the chart.
At birth, the baby’s Apgar scores were 7 at 1 minute and 7 at 5 minutes. She appeared limp and was grunting. A pediatrician diagnosed transient respiratory problems related to prematurity. The baby continued to deteriorate; antibiotics were ordered 7 hours after birth. After the child was transported to another facility, she died. The cause of death was GBS sepsis and pneumonia.
PARENTS’ CLAIM The ObGyn was negligent in failing to properly and timely note the positive GBS test result in the mother’s chart. The ObGyn’s office staff was negligent in miscommunicating the GBS status to the nurse.
DEFENDANTS’ DEFENSE The ObGyn usually noted laboratory results at the next prenatal visit, but the mother gave birth before that occurred. The on-call ObGyn failed to give antibiotics when the mother presented in preterm labor with unknown GBS status. The hospital did not have a protocol that required the on-call ObGyn to prescribe prophylactic antibiotics in this context. The nurse was negligent for failing to verify the oral telephone report of GBS-negative status with a written or faxed laboratory report.
The ObGyn surmised that the infection had occurred in utero, not during birth; antibiotics would not have changed the outcome.
VERDICT The parents settled with the hospital for a confidential amount. An Arizona defense verdict was returned for the ObGyn.
_______________
Child has quadraparetic CP after oxytocin-augmented delivery
A pregnant woman was hospitalized for 23-hour observation with blood work and obstetric ultrasonography. The admitting nurse noted that the patient was having mild contractions and that fetal heart tones were 130 bpm with moderate variability. The mother’s cervix was dilated to 2.5 cm, 70% effaced, at –1 station, with intact and bulging membranes and normal maternal vital signs. The ObGyn ordered intravenous ampicillin and sent the mother to labor and delivery. He prescribed oxytocin (6 mU/min), but, after its initiation, oxytocin was discontinued for almost 2 hours. When the mother had five contractions in 10 minutes, oxytocin was restarted at 8 mU/min. The oxytocin dosage was later increased to 10 mU/min, and then to 12 mU/min.
When shoulder dystocia was encountered, various maneuvers were performed. The baby was delivered using vacuum extraction. The newborn was immediately sent to the neonatal intensive care unit (NICU) with a suspected humerus fracture and poor respiration. Mechanical ventilation and treatment for hypoperfusion were initiated. She had persistently low Apgar scores, intracranial hemorrhaging, seizures, severe metabolic acidosis, and hypoxic ischemic encephalopathy. She has quadraparetic cerebral palsy with related disabilities.
PARENTS’ CLAIM The ObGyn and hospital were negligent in the treatment of the mother during labor and delivery, causing the child to be born with serious injuries.
DEFENDANTS’ DEFENSE The case was settled during the trial.
VERDICT A $4,250,000 Texas settlement was reached, including $75,000 for the parents, and the remainder placed into a trust for the child.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Premature infant has CP: $14.5M verdict
After learning that, 14 years earlier, a 36-year-old woman had undergone an emergency cesarean delivery at 32 weeks’ gestation, her health-care providers planned a cesarean delivery for the new pregnancy. The woman was admitted to the hospital in preterm labor. Three days later, she was discharged, but readmitted twice more over a 2-week period. At each admission, preterm labor was halted using medication and bed rest.
The patient’s water broke and she was admitted to the hospital at 25 weeks’ gestation, about a week after the previous admission. Shortly after admission, the patient asked about a cesarean delivery, but no action was taken. When her ObGyn arrived at the hospital 5 hours later, the patient asked for a cesarean delivery; the ObGyn said he wanted to wait to see how her labor was progressing. After 3 hours, the fetus showed signs of distress, and an emergency cesarean delivery was undertaken. The infant experienced a massive brain hemorrhage, resulting in cerebral palsy (CP). The child has cognitive delays, visual impairment, and additional problems; he will require lifelong care.
PARENTS’ CLAIM The ObGyn and hospital were negligent in discharging the woman from admission for preterm labor. Cesarean delivery should have been performed much earlier due to nonreassuring fetal heart tones. Severe variable decelerations caused cerebral blood flow fluctuations that led to the hemorrhage.
DEFENDANTS’ DEFENSE The child’s prematurity and a severe placental infection led to the injuries. Nothing would have changed the outcome.
VERDICT A $14.5 million Ohio verdict was returned, including $1.5 million for the mother.
_______________
Costs returned afterverdict for the defense
A 65-year-old woman underwent a hysterectomy for treatment of uterine cancer performed by a gynecologic oncologist. Postoperatively, the patient developed an infection. A small-bowel injury was surgically repaired. The patient was hospitalized for 4 months for treatment of sepsis.
PARENTS’ CLAIM The physician was negligent for injuring the patient’s bowel and then failing to identify and repair the injury during surgery.
PHYSICIAN’S DEFENSE There was no negligence. The patient had significant adhesions from prior surgeries. The physician noted minor serosal tears of the bowel, several of which were repaired during surgery. He checked the length of the bowel for tears/perforations several times during the procedure, but found none. The patient had areas of weakness in her bowel, one of which broke down after surgery. The perforation was repaired in a timely manner.
VERDICT A Michigan defense verdict was returned. The physician was awarded $14,535 in costs.
_______________
Colon injury after cystectomy
A 21-year-old woman underwent laparoscopic ovarian cystectomy, performed by her gynecologist, and was discharged the next day. Eight days later, the patient went to the emergency department (ED) with pelvic pain. Testing revealed a perforated colon with peritonitis. She underwent repair by laparotomy, including bowel resection and colostomy, which was reversed several months later. She has not regained regular bowel function, cannot digest food that has not been finely sliced, and constantly uses laxatives.
PARENTS’ CLAIM The colon injury occurred during cystectomy because the gynecologist was negligent in failing to maintain proper anatomical landmarks. The injury should have been recognized at the time of surgery by injecting saline solution into the colon. She had not been informed of the risk of colon injury.
DEFENDANTS’ DEFENSE Colon injury is a known complication of cystectomy. The injury could have occurred after surgery due to a minor nick of the colon that was undetectable during surgery. Proper informed consent was acquired.
VERDICT A $340,000 New York settlement was reached.
_______________
Mother hemorrhages, dies after delivery: $1M settlement
A 19-year-old woman presented at full term to a community hospital. After several hours of labor, an emergency cesarean delivery was performed due to arrested descent.
Fifteen minutes after delivery, the mother exhibited moderate bleeding with decreasing blood pressure and tachycardia. The post-anesthesia care unit nurse assessed the patient’s uterus as “boggy,” and alerted the ObGyn, who immediately reacted by expressing clots from the uterus. He noted that the fundus was firm. He ordered intravenous (IV) oxytocin, but the patient continued to hemorrhage. Fifteen minutes later, the patient’s vital signs worsened. The ObGyn ordered blood products, uterotonics, and an additional IV line for fluid resuscitation. He began to massage the fundus and expressed clots.
When the patient did not stabilize, she was returned to the OR. After attempting to stop the bleeding with O’Leary stitches, the ObGyn performed a hysterectomy. Six hours after surgery, and after transfusion of a total of 12 units of blood, the woman coded multiple times. She died 14 hours after delivery. Cause of death was disseminated intravascular coagulopathy caused by an atonic uterus.
ESTATE’S CLAIM The ObGyn failed to recognize the extent of the postpartum hemorrhage and should have acted more aggressively with resuscitation. He should have returned her to the OR earlier. The ObGyn was negligent in waiting 45 minutes for cross-matched blood rather than using universal donor O-negative blood that was readily available.
PHYSICIAN’S DEFENSE The ObGyn denied negligence and maintained that he had acted properly. He returned the patient to the OR within 90 minutes of first learning of the hemorrhage.
VERDICT A $1 million Virginia settlement was reached.
_______________
Infant born with broken arms, collarbone, facial bones
A 23-year-old woman had gestational diabetes. She is 5’9” tall and weighed 300 lb while pregnant. She went to the hospital in labor.
During delivery, shoulder dystocia was encountered. The ObGyn performed a variety of techniques, including the McRobert’s maneuver. Forceps were eventually used for delivery.
Both of the newborn’s arms were broken, and she had a broken collarbone and facial fractures. The mother also suffered significant vaginal lacerations and required an episiotomy. She continues to complain of bladder and bowel problems.
PARENTS’ CLAIM A vaginal delivery should not have been attempted due to the mother’s gestational diabetes and the risk of having a macrosomic baby. A cesarean delivery should have been performed. The ObGyn did not use the proper techniques when delivering the child after shoulder dystocia was encountered.
PHYSICIAN’S DEFENSE The ObGyn denied negligence. He claimed that the baby recovered well from her injuries. The mother underwent surgery and now has excellent bladder and bowel control.
VERDICT A confidential Louisiana settlement was reached with the hospital before trial. A defense verdict was returned for the ObGyn.
_______________
Protein found in urine at 39 weeks’ gestation: mother and child die
At 39 weeks' gestation, a woman saw her ObGyn for a prenatal visit. During the examination, the ObGyn found high levels of protein in the woman’s urine, an accumulation of fluid in her ankles, and the highest blood pressure (BP) reading of the woman’s pregnancy. However, because the BP reading was lower than that required to diagnose preeclampsia, the ObGyn sent the patient home and scheduled the next prenatal visit for the following week. The woman and her unborn child died 5 days later.
ESTATE’S CLAIM The ObGyn was negligent in failing to order a urine study and more closely monitor the mother’s symptoms when signs of preeclampsia were evident at 39 weeks’ gestation. Delivery of the child would have resolved the problem and saved both lives.
PHYSICIAN’S DEFENSE The case was settled during the trial.
VERDICT A $3 million Illinois settlement was reached.
_______________
Baby dies from group B strep
A 16-year-old woman planned delivery at a local hospital. Her ObGyn’s practice regularly sends the hospital its patients’ prenatal records, starting at 25 weeks’ gestation. At 33 weeks, the ObGyn took a vaginal culture to test for group B Streptococcus (GBS) bacteria. The laboratory reported positive GBS results to a computer in the ObGyn’s office, but the results were not entered into the patient’s chart.
The mother went to the ED in labor a week later; she was evaluated and discharged. Several days later, she returned to the ED, but was again discharged. She returned the next day, now in gestational week 36. An on-call ObGyn admitted her. A labor and delivery nurse claimed that the ObGyn’s office reported that the mother was GBS negative, so the nurse placed a negative sign in the prenatal record in the chart. When the patient’s ObGyn arrived at the hospital, he noticed the negative sign in the chart.
At birth, the baby’s Apgar scores were 7 at 1 minute and 7 at 5 minutes. She appeared limp and was grunting. A pediatrician diagnosed transient respiratory problems related to prematurity. The baby continued to deteriorate; antibiotics were ordered 7 hours after birth. After the child was transported to another facility, she died. The cause of death was GBS sepsis and pneumonia.
PARENTS’ CLAIM The ObGyn was negligent in failing to properly and timely note the positive GBS test result in the mother’s chart. The ObGyn’s office staff was negligent in miscommunicating the GBS status to the nurse.
DEFENDANTS’ DEFENSE The ObGyn usually noted laboratory results at the next prenatal visit, but the mother gave birth before that occurred. The on-call ObGyn failed to give antibiotics when the mother presented in preterm labor with unknown GBS status. The hospital did not have a protocol that required the on-call ObGyn to prescribe prophylactic antibiotics in this context. The nurse was negligent for failing to verify the oral telephone report of GBS-negative status with a written or faxed laboratory report.
The ObGyn surmised that the infection had occurred in utero, not during birth; antibiotics would not have changed the outcome.
VERDICT The parents settled with the hospital for a confidential amount. An Arizona defense verdict was returned for the ObGyn.
_______________
Child has quadraparetic CP after oxytocin-augmented delivery
A pregnant woman was hospitalized for 23-hour observation with blood work and obstetric ultrasonography. The admitting nurse noted that the patient was having mild contractions and that fetal heart tones were 130 bpm with moderate variability. The mother’s cervix was dilated to 2.5 cm, 70% effaced, at –1 station, with intact and bulging membranes and normal maternal vital signs. The ObGyn ordered intravenous ampicillin and sent the mother to labor and delivery. He prescribed oxytocin (6 mU/min), but, after its initiation, oxytocin was discontinued for almost 2 hours. When the mother had five contractions in 10 minutes, oxytocin was restarted at 8 mU/min. The oxytocin dosage was later increased to 10 mU/min, and then to 12 mU/min.
When shoulder dystocia was encountered, various maneuvers were performed. The baby was delivered using vacuum extraction. The newborn was immediately sent to the neonatal intensive care unit (NICU) with a suspected humerus fracture and poor respiration. Mechanical ventilation and treatment for hypoperfusion were initiated. She had persistently low Apgar scores, intracranial hemorrhaging, seizures, severe metabolic acidosis, and hypoxic ischemic encephalopathy. She has quadraparetic cerebral palsy with related disabilities.
PARENTS’ CLAIM The ObGyn and hospital were negligent in the treatment of the mother during labor and delivery, causing the child to be born with serious injuries.
DEFENDANTS’ DEFENSE The case was settled during the trial.
VERDICT A $4,250,000 Texas settlement was reached, including $75,000 for the parents, and the remainder placed into a trust for the child.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Premature infant has CP: $14.5M verdict
After learning that, 14 years earlier, a 36-year-old woman had undergone an emergency cesarean delivery at 32 weeks’ gestation, her health-care providers planned a cesarean delivery for the new pregnancy. The woman was admitted to the hospital in preterm labor. Three days later, she was discharged, but readmitted twice more over a 2-week period. At each admission, preterm labor was halted using medication and bed rest.
The patient’s water broke and she was admitted to the hospital at 25 weeks’ gestation, about a week after the previous admission. Shortly after admission, the patient asked about a cesarean delivery, but no action was taken. When her ObGyn arrived at the hospital 5 hours later, the patient asked for a cesarean delivery; the ObGyn said he wanted to wait to see how her labor was progressing. After 3 hours, the fetus showed signs of distress, and an emergency cesarean delivery was undertaken. The infant experienced a massive brain hemorrhage, resulting in cerebral palsy (CP). The child has cognitive delays, visual impairment, and additional problems; he will require lifelong care.
PARENTS’ CLAIM The ObGyn and hospital were negligent in discharging the woman from admission for preterm labor. Cesarean delivery should have been performed much earlier due to nonreassuring fetal heart tones. Severe variable decelerations caused cerebral blood flow fluctuations that led to the hemorrhage.
DEFENDANTS’ DEFENSE The child’s prematurity and a severe placental infection led to the injuries. Nothing would have changed the outcome.
VERDICT A $14.5 million Ohio verdict was returned, including $1.5 million for the mother.
_______________
Costs returned afterverdict for the defense
A 65-year-old woman underwent a hysterectomy for treatment of uterine cancer performed by a gynecologic oncologist. Postoperatively, the patient developed an infection. A small-bowel injury was surgically repaired. The patient was hospitalized for 4 months for treatment of sepsis.
PARENTS’ CLAIM The physician was negligent for injuring the patient’s bowel and then failing to identify and repair the injury during surgery.
PHYSICIAN’S DEFENSE There was no negligence. The patient had significant adhesions from prior surgeries. The physician noted minor serosal tears of the bowel, several of which were repaired during surgery. He checked the length of the bowel for tears/perforations several times during the procedure, but found none. The patient had areas of weakness in her bowel, one of which broke down after surgery. The perforation was repaired in a timely manner.
VERDICT A Michigan defense verdict was returned. The physician was awarded $14,535 in costs.
_______________
Colon injury after cystectomy
A 21-year-old woman underwent laparoscopic ovarian cystectomy, performed by her gynecologist, and was discharged the next day. Eight days later, the patient went to the emergency department (ED) with pelvic pain. Testing revealed a perforated colon with peritonitis. She underwent repair by laparotomy, including bowel resection and colostomy, which was reversed several months later. She has not regained regular bowel function, cannot digest food that has not been finely sliced, and constantly uses laxatives.
PARENTS’ CLAIM The colon injury occurred during cystectomy because the gynecologist was negligent in failing to maintain proper anatomical landmarks. The injury should have been recognized at the time of surgery by injecting saline solution into the colon. She had not been informed of the risk of colon injury.
DEFENDANTS’ DEFENSE Colon injury is a known complication of cystectomy. The injury could have occurred after surgery due to a minor nick of the colon that was undetectable during surgery. Proper informed consent was acquired.
VERDICT A $340,000 New York settlement was reached.
_______________
Mother hemorrhages, dies after delivery: $1M settlement
A 19-year-old woman presented at full term to a community hospital. After several hours of labor, an emergency cesarean delivery was performed due to arrested descent.
Fifteen minutes after delivery, the mother exhibited moderate bleeding with decreasing blood pressure and tachycardia. The post-anesthesia care unit nurse assessed the patient’s uterus as “boggy,” and alerted the ObGyn, who immediately reacted by expressing clots from the uterus. He noted that the fundus was firm. He ordered intravenous (IV) oxytocin, but the patient continued to hemorrhage. Fifteen minutes later, the patient’s vital signs worsened. The ObGyn ordered blood products, uterotonics, and an additional IV line for fluid resuscitation. He began to massage the fundus and expressed clots.
When the patient did not stabilize, she was returned to the OR. After attempting to stop the bleeding with O’Leary stitches, the ObGyn performed a hysterectomy. Six hours after surgery, and after transfusion of a total of 12 units of blood, the woman coded multiple times. She died 14 hours after delivery. Cause of death was disseminated intravascular coagulopathy caused by an atonic uterus.
ESTATE’S CLAIM The ObGyn failed to recognize the extent of the postpartum hemorrhage and should have acted more aggressively with resuscitation. He should have returned her to the OR earlier. The ObGyn was negligent in waiting 45 minutes for cross-matched blood rather than using universal donor O-negative blood that was readily available.
PHYSICIAN’S DEFENSE The ObGyn denied negligence and maintained that he had acted properly. He returned the patient to the OR within 90 minutes of first learning of the hemorrhage.
VERDICT A $1 million Virginia settlement was reached.
_______________
Infant born with broken arms, collarbone, facial bones
A 23-year-old woman had gestational diabetes. She is 5’9” tall and weighed 300 lb while pregnant. She went to the hospital in labor.
During delivery, shoulder dystocia was encountered. The ObGyn performed a variety of techniques, including the McRobert’s maneuver. Forceps were eventually used for delivery.
Both of the newborn’s arms were broken, and she had a broken collarbone and facial fractures. The mother also suffered significant vaginal lacerations and required an episiotomy. She continues to complain of bladder and bowel problems.
PARENTS’ CLAIM A vaginal delivery should not have been attempted due to the mother’s gestational diabetes and the risk of having a macrosomic baby. A cesarean delivery should have been performed. The ObGyn did not use the proper techniques when delivering the child after shoulder dystocia was encountered.
PHYSICIAN’S DEFENSE The ObGyn denied negligence. He claimed that the baby recovered well from her injuries. The mother underwent surgery and now has excellent bladder and bowel control.
VERDICT A confidential Louisiana settlement was reached with the hospital before trial. A defense verdict was returned for the ObGyn.
_______________
Protein found in urine at 39 weeks’ gestation: mother and child die
At 39 weeks' gestation, a woman saw her ObGyn for a prenatal visit. During the examination, the ObGyn found high levels of protein in the woman’s urine, an accumulation of fluid in her ankles, and the highest blood pressure (BP) reading of the woman’s pregnancy. However, because the BP reading was lower than that required to diagnose preeclampsia, the ObGyn sent the patient home and scheduled the next prenatal visit for the following week. The woman and her unborn child died 5 days later.
ESTATE’S CLAIM The ObGyn was negligent in failing to order a urine study and more closely monitor the mother’s symptoms when signs of preeclampsia were evident at 39 weeks’ gestation. Delivery of the child would have resolved the problem and saved both lives.
PHYSICIAN’S DEFENSE The case was settled during the trial.
VERDICT A $3 million Illinois settlement was reached.
_______________
Baby dies from group B strep
A 16-year-old woman planned delivery at a local hospital. Her ObGyn’s practice regularly sends the hospital its patients’ prenatal records, starting at 25 weeks’ gestation. At 33 weeks, the ObGyn took a vaginal culture to test for group B Streptococcus (GBS) bacteria. The laboratory reported positive GBS results to a computer in the ObGyn’s office, but the results were not entered into the patient’s chart.
The mother went to the ED in labor a week later; she was evaluated and discharged. Several days later, she returned to the ED, but was again discharged. She returned the next day, now in gestational week 36. An on-call ObGyn admitted her. A labor and delivery nurse claimed that the ObGyn’s office reported that the mother was GBS negative, so the nurse placed a negative sign in the prenatal record in the chart. When the patient’s ObGyn arrived at the hospital, he noticed the negative sign in the chart.
At birth, the baby’s Apgar scores were 7 at 1 minute and 7 at 5 minutes. She appeared limp and was grunting. A pediatrician diagnosed transient respiratory problems related to prematurity. The baby continued to deteriorate; antibiotics were ordered 7 hours after birth. After the child was transported to another facility, she died. The cause of death was GBS sepsis and pneumonia.
PARENTS’ CLAIM The ObGyn was negligent in failing to properly and timely note the positive GBS test result in the mother’s chart. The ObGyn’s office staff was negligent in miscommunicating the GBS status to the nurse.
DEFENDANTS’ DEFENSE The ObGyn usually noted laboratory results at the next prenatal visit, but the mother gave birth before that occurred. The on-call ObGyn failed to give antibiotics when the mother presented in preterm labor with unknown GBS status. The hospital did not have a protocol that required the on-call ObGyn to prescribe prophylactic antibiotics in this context. The nurse was negligent for failing to verify the oral telephone report of GBS-negative status with a written or faxed laboratory report.
The ObGyn surmised that the infection had occurred in utero, not during birth; antibiotics would not have changed the outcome.
VERDICT The parents settled with the hospital for a confidential amount. An Arizona defense verdict was returned for the ObGyn.
_______________
Child has quadraparetic CP after oxytocin-augmented delivery
A pregnant woman was hospitalized for 23-hour observation with blood work and obstetric ultrasonography. The admitting nurse noted that the patient was having mild contractions and that fetal heart tones were 130 bpm with moderate variability. The mother’s cervix was dilated to 2.5 cm, 70% effaced, at –1 station, with intact and bulging membranes and normal maternal vital signs. The ObGyn ordered intravenous ampicillin and sent the mother to labor and delivery. He prescribed oxytocin (6 mU/min), but, after its initiation, oxytocin was discontinued for almost 2 hours. When the mother had five contractions in 10 minutes, oxytocin was restarted at 8 mU/min. The oxytocin dosage was later increased to 10 mU/min, and then to 12 mU/min.
When shoulder dystocia was encountered, various maneuvers were performed. The baby was delivered using vacuum extraction. The newborn was immediately sent to the neonatal intensive care unit (NICU) with a suspected humerus fracture and poor respiration. Mechanical ventilation and treatment for hypoperfusion were initiated. She had persistently low Apgar scores, intracranial hemorrhaging, seizures, severe metabolic acidosis, and hypoxic ischemic encephalopathy. She has quadraparetic cerebral palsy with related disabilities.
PARENTS’ CLAIM The ObGyn and hospital were negligent in the treatment of the mother during labor and delivery, causing the child to be born with serious injuries.
DEFENDANTS’ DEFENSE The case was settled during the trial.
VERDICT A $4,250,000 Texas settlement was reached, including $75,000 for the parents, and the remainder placed into a trust for the child.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
EMA grants product orphan status for AML
The European Medicines Agency (EMA) has granted orphan status to Atir, a product consisting of T-cell-depleted donor immune cells, for the treatment of acute myeloid leukemia (AML).
The EMA and the US Food and Drug Administration previously granted Atir orphan status for the prevention of acute graft-vs-host-disease (GVHD) following hematopoietic stem cell transplant (HSCT).
The EMA’s orphan designation provides incentives to support drug development. This includes fee reductions and a 10-year period of market exclusivity in the European Union after product approval.
About Atir
Atir consists of donor immune cells from which the alloreactive T-cells that would otherwise attack the patient’s body have been selectively eliminated.
The product is produced using a molecule known as TH9402 to selectively remove those T cells from the donor graft, while preserving other immune cells. To activate patient-reactive T cells, the graft is mixed (ex vivo) with patient cells.
Then, TH9402 is added. As this phototoxic compound selectively accumulates in activated T cells, the cells can be eliminated by exposing the cell mixture to light of a specific wavelength. The resulting Atir product can be frozen and stored and is infused into the patient in a scheduled procedure.
Trial data
Researchers said Atir proved safe and effective in a phase 1/2 study in which high-risk leukemia patients with very poor prognosis were treated with escalating doses of Atir after a haploidentical HSCT.
The overall survival of 19 patients who received an optimal dose of Atir was 78% at 1 year and 67% at 5 years, rates that compare favorably to outcomes of HSCTs from fully matched donors. The data also suggest that immune cells responsible for the graft-vs-leukemia effect are retained in Atir.
Five-year follow-up data show that none of the 19 patients developed acute grade 3/4 GVHD, compared to an incidence of 30% in matched unrelated HSCTs. In the 9 patients who received an optimal dose of Atir, there was no transplant-related mortality.
Researchers are currently testing Atir in a phase 2 study of patients with AML, acute lymphoblastic leukemia, and myelodysplastic syndrome, to corroborate and extend the safety and efficacy results from the phase 1/2 study. Data from this trial are expected in the second half of 2014.
Atir is under development by Kiadis Pharma. For more information, visit the company’s website. ![]()
The European Medicines Agency (EMA) has granted orphan status to Atir, a product consisting of T-cell-depleted donor immune cells, for the treatment of acute myeloid leukemia (AML).
The EMA and the US Food and Drug Administration previously granted Atir orphan status for the prevention of acute graft-vs-host-disease (GVHD) following hematopoietic stem cell transplant (HSCT).
The EMA’s orphan designation provides incentives to support drug development. This includes fee reductions and a 10-year period of market exclusivity in the European Union after product approval.
About Atir
Atir consists of donor immune cells from which the alloreactive T-cells that would otherwise attack the patient’s body have been selectively eliminated.
The product is produced using a molecule known as TH9402 to selectively remove those T cells from the donor graft, while preserving other immune cells. To activate patient-reactive T cells, the graft is mixed (ex vivo) with patient cells.
Then, TH9402 is added. As this phototoxic compound selectively accumulates in activated T cells, the cells can be eliminated by exposing the cell mixture to light of a specific wavelength. The resulting Atir product can be frozen and stored and is infused into the patient in a scheduled procedure.
Trial data
Researchers said Atir proved safe and effective in a phase 1/2 study in which high-risk leukemia patients with very poor prognosis were treated with escalating doses of Atir after a haploidentical HSCT.
The overall survival of 19 patients who received an optimal dose of Atir was 78% at 1 year and 67% at 5 years, rates that compare favorably to outcomes of HSCTs from fully matched donors. The data also suggest that immune cells responsible for the graft-vs-leukemia effect are retained in Atir.
Five-year follow-up data show that none of the 19 patients developed acute grade 3/4 GVHD, compared to an incidence of 30% in matched unrelated HSCTs. In the 9 patients who received an optimal dose of Atir, there was no transplant-related mortality.
Researchers are currently testing Atir in a phase 2 study of patients with AML, acute lymphoblastic leukemia, and myelodysplastic syndrome, to corroborate and extend the safety and efficacy results from the phase 1/2 study. Data from this trial are expected in the second half of 2014.
Atir is under development by Kiadis Pharma. For more information, visit the company’s website. ![]()
The European Medicines Agency (EMA) has granted orphan status to Atir, a product consisting of T-cell-depleted donor immune cells, for the treatment of acute myeloid leukemia (AML).
The EMA and the US Food and Drug Administration previously granted Atir orphan status for the prevention of acute graft-vs-host-disease (GVHD) following hematopoietic stem cell transplant (HSCT).
The EMA’s orphan designation provides incentives to support drug development. This includes fee reductions and a 10-year period of market exclusivity in the European Union after product approval.
About Atir
Atir consists of donor immune cells from which the alloreactive T-cells that would otherwise attack the patient’s body have been selectively eliminated.
The product is produced using a molecule known as TH9402 to selectively remove those T cells from the donor graft, while preserving other immune cells. To activate patient-reactive T cells, the graft is mixed (ex vivo) with patient cells.
Then, TH9402 is added. As this phototoxic compound selectively accumulates in activated T cells, the cells can be eliminated by exposing the cell mixture to light of a specific wavelength. The resulting Atir product can be frozen and stored and is infused into the patient in a scheduled procedure.
Trial data
Researchers said Atir proved safe and effective in a phase 1/2 study in which high-risk leukemia patients with very poor prognosis were treated with escalating doses of Atir after a haploidentical HSCT.
The overall survival of 19 patients who received an optimal dose of Atir was 78% at 1 year and 67% at 5 years, rates that compare favorably to outcomes of HSCTs from fully matched donors. The data also suggest that immune cells responsible for the graft-vs-leukemia effect are retained in Atir.
Five-year follow-up data show that none of the 19 patients developed acute grade 3/4 GVHD, compared to an incidence of 30% in matched unrelated HSCTs. In the 9 patients who received an optimal dose of Atir, there was no transplant-related mortality.
Researchers are currently testing Atir in a phase 2 study of patients with AML, acute lymphoblastic leukemia, and myelodysplastic syndrome, to corroborate and extend the safety and efficacy results from the phase 1/2 study. Data from this trial are expected in the second half of 2014.
Atir is under development by Kiadis Pharma. For more information, visit the company’s website. ![]()
Topical steroid might improve mucosal integrity in eosinophilic esophagitis
Topical steroid therapy improved some indicators of mucosal integrity in patients with eosinophilic esophagitis, but proton pump inhibitor therapy did not, according to two studies reported in the November issue of Clinical Gastroenterology and Hepatology.
The first study found that topical fluticasone therapy at a dose of 880 mcg twice daily for 2 months helped correct esophageal spongiosis, or dilated intercellular space, in patients with eosinophilic esophagitis (EoE). Spongiosis scores for treated patients were significantly lower than for untreated patients (0.4 vs. 1.3; P = .016), said Dr. David Katzka at the Mayo Clinic in Rochester, Minn. and his associates (Clin. Gastroenterol. Hepatol. 2014 [doi:10.1016/j.cgh.2014.02.039]).
In the study, histologic analyses also showed that improved spongiosis scores in treated patients correlated with increased density of two tight junction proteins, filaggrin (P = .001) and zonula occludens-3 (P = .016), said the investigators. These proteins might help regulate antigenic penetration of the esophageal mucosa and also could permit migration of white blood cells, they said. “Loss of tight junction regulators and dilation of intercellular spaces appear to be involved in the pathophysiology of EoE and could be targets for treatment,” the researchers concluded. But they also noted that their study did not examine the same patients before and after steroid therapy and did not look at desmosomes, intercellular junctions that past research has suggested might be affected in EoE.
For the second study, Dr. Bram van Rhijn and his associates at the Academic Medical Center in the Netherlands compared endoscopies of 16 patients with dysphagia and suspected (unconfirmed) EoE with 11 controls, both at baseline and after 8 weeks of high-dose esomeprazole treatment. Esophageal mucosal integrity was “severely impaired” in patients with confirmed EoE and in those with proton pump inhibitor–responsive eosinophilia (PPRE), the researchers said (Clin. Gasteroenterol. Hepatol. 2014 [doi:10.1016/j.cgh.2014.02.037]).
In both forms of disease, molecules as large as 40,000 daltons were able to pass through the compromised esophageal mucosa, Dr. Bram van Rhijn and his associates reported. “This size is similar to the size of most plant and animal food allergens to which EoE patients are sensitized,” they added. Esophageal permeability might increase the rate of immune exposure to allergens, thereby mediating EoE and PPRE, they said.
On mucosal functional tests, both EoE and PPRE were associated with reduced transepithelial electrical resistance and lower electrical tissue impedance, most notably in patients with EoE (P less than .001 for both, compared with controls), the investigators reported. Proton pump inhibitor treatment partially reversed these changes in patients with PPRE but showed no effect for patients with EoE, they said. This finding suggests that acid reflux might play a role in PPRE, but not in EOE, they concluded.
Dr. Katzka and his associates disclosed no funding sources and reported having no conflicts of interest. Dr. Rhijn and his associates were supported by the Netherlands Organization for Scientific Research. Two of Dr. Rhijn’s coauthors reported financial relationships with AstraZeneca, Endostim, Medical Measurement Systems, Shire, and GlaxoSmithKline.
In the past year, the topic of mucosal integrity in eosinophilic esophagitis has garnered growing attention. Epithelial permeability defects have been described in the pathogenesis of GI disorders, including inflammatory bowel disease and celiac sprue, as well as allergic disorders such as atopic dermatitis. In EoE, both experimental as well as clinical studies have shown an eosinophil-predominant inflammatory response to specific antigens, particularly common food allergens. Increased permeability may predispose genetically susceptible individuals to swallowed allergen penetration through the esophageal epithelium. Beneath the epithelial barrier, antigens have access to antigen presenting cells, including dendritic cells, leading to both allergic sensitization and perpetuation of the TH-2 chronic inflammatory response.

|
| Dr. Ikuo Hirano |
The article by Dr. Katzka and his colleagues supports the concept of epithelial barrier defects in EoE through the demonstration of reduced immunohistochemical expression of filaggrin, zonula occludens-3, and claudin-1, important tight junction proteins. Expression was increased in EoE patients treated with topical steroids. Similarly, the study by Dr. van Rhijn and his associates identified impaired mucosal integrity in EoE by a variety of techniques that included electron microscopic demonstration of dilated intercellular spaces, electrical tissue impedance as an in vivo biomarker, and in vitro transepithelial molecular flux in an Ussing chamber. Furthermore, they found that proton pump inhibitor therapy partially restored mucosal permeability defects to a greater degree in patients with PPI-responsive esophageal eosinophilia, compared with patients with EoE. These two studies substantiate studies from the Cincinnati group that previously identified reduced mRNA expression of filaggrin in esophageal mucosal biopsies as well as reduced expression of the intercellular adhesion molecule, desmoglein 1.
In spite of these novel data, the exact role of altered esophageal epithelial permeability in the pathogenesis of EoE is yet unclear. The reversibility of the defect with medical therapy argues against defective cell junction proteins as an intrinsic abnormality. Furthermore, the location of antigen presentation in EoE may occur through other routes such as the small intestine, nasal epithelium, or skin. In the meantime, these studies provide an important advance in our understanding of EoE and open the door to novel therapeutic approaches.
Dr. Ikuo Hirano, AGAF, is professor of medicine at Northwestern University, Chicago. He reported no conflicts of interest.
In the past year, the topic of mucosal integrity in eosinophilic esophagitis has garnered growing attention. Epithelial permeability defects have been described in the pathogenesis of GI disorders, including inflammatory bowel disease and celiac sprue, as well as allergic disorders such as atopic dermatitis. In EoE, both experimental as well as clinical studies have shown an eosinophil-predominant inflammatory response to specific antigens, particularly common food allergens. Increased permeability may predispose genetically susceptible individuals to swallowed allergen penetration through the esophageal epithelium. Beneath the epithelial barrier, antigens have access to antigen presenting cells, including dendritic cells, leading to both allergic sensitization and perpetuation of the TH-2 chronic inflammatory response.

|
| Dr. Ikuo Hirano |
The article by Dr. Katzka and his colleagues supports the concept of epithelial barrier defects in EoE through the demonstration of reduced immunohistochemical expression of filaggrin, zonula occludens-3, and claudin-1, important tight junction proteins. Expression was increased in EoE patients treated with topical steroids. Similarly, the study by Dr. van Rhijn and his associates identified impaired mucosal integrity in EoE by a variety of techniques that included electron microscopic demonstration of dilated intercellular spaces, electrical tissue impedance as an in vivo biomarker, and in vitro transepithelial molecular flux in an Ussing chamber. Furthermore, they found that proton pump inhibitor therapy partially restored mucosal permeability defects to a greater degree in patients with PPI-responsive esophageal eosinophilia, compared with patients with EoE. These two studies substantiate studies from the Cincinnati group that previously identified reduced mRNA expression of filaggrin in esophageal mucosal biopsies as well as reduced expression of the intercellular adhesion molecule, desmoglein 1.
In spite of these novel data, the exact role of altered esophageal epithelial permeability in the pathogenesis of EoE is yet unclear. The reversibility of the defect with medical therapy argues against defective cell junction proteins as an intrinsic abnormality. Furthermore, the location of antigen presentation in EoE may occur through other routes such as the small intestine, nasal epithelium, or skin. In the meantime, these studies provide an important advance in our understanding of EoE and open the door to novel therapeutic approaches.
Dr. Ikuo Hirano, AGAF, is professor of medicine at Northwestern University, Chicago. He reported no conflicts of interest.
In the past year, the topic of mucosal integrity in eosinophilic esophagitis has garnered growing attention. Epithelial permeability defects have been described in the pathogenesis of GI disorders, including inflammatory bowel disease and celiac sprue, as well as allergic disorders such as atopic dermatitis. In EoE, both experimental as well as clinical studies have shown an eosinophil-predominant inflammatory response to specific antigens, particularly common food allergens. Increased permeability may predispose genetically susceptible individuals to swallowed allergen penetration through the esophageal epithelium. Beneath the epithelial barrier, antigens have access to antigen presenting cells, including dendritic cells, leading to both allergic sensitization and perpetuation of the TH-2 chronic inflammatory response.

|
| Dr. Ikuo Hirano |
The article by Dr. Katzka and his colleagues supports the concept of epithelial barrier defects in EoE through the demonstration of reduced immunohistochemical expression of filaggrin, zonula occludens-3, and claudin-1, important tight junction proteins. Expression was increased in EoE patients treated with topical steroids. Similarly, the study by Dr. van Rhijn and his associates identified impaired mucosal integrity in EoE by a variety of techniques that included electron microscopic demonstration of dilated intercellular spaces, electrical tissue impedance as an in vivo biomarker, and in vitro transepithelial molecular flux in an Ussing chamber. Furthermore, they found that proton pump inhibitor therapy partially restored mucosal permeability defects to a greater degree in patients with PPI-responsive esophageal eosinophilia, compared with patients with EoE. These two studies substantiate studies from the Cincinnati group that previously identified reduced mRNA expression of filaggrin in esophageal mucosal biopsies as well as reduced expression of the intercellular adhesion molecule, desmoglein 1.
In spite of these novel data, the exact role of altered esophageal epithelial permeability in the pathogenesis of EoE is yet unclear. The reversibility of the defect with medical therapy argues against defective cell junction proteins as an intrinsic abnormality. Furthermore, the location of antigen presentation in EoE may occur through other routes such as the small intestine, nasal epithelium, or skin. In the meantime, these studies provide an important advance in our understanding of EoE and open the door to novel therapeutic approaches.
Dr. Ikuo Hirano, AGAF, is professor of medicine at Northwestern University, Chicago. He reported no conflicts of interest.
Topical steroid therapy improved some indicators of mucosal integrity in patients with eosinophilic esophagitis, but proton pump inhibitor therapy did not, according to two studies reported in the November issue of Clinical Gastroenterology and Hepatology.
The first study found that topical fluticasone therapy at a dose of 880 mcg twice daily for 2 months helped correct esophageal spongiosis, or dilated intercellular space, in patients with eosinophilic esophagitis (EoE). Spongiosis scores for treated patients were significantly lower than for untreated patients (0.4 vs. 1.3; P = .016), said Dr. David Katzka at the Mayo Clinic in Rochester, Minn. and his associates (Clin. Gastroenterol. Hepatol. 2014 [doi:10.1016/j.cgh.2014.02.039]).
In the study, histologic analyses also showed that improved spongiosis scores in treated patients correlated with increased density of two tight junction proteins, filaggrin (P = .001) and zonula occludens-3 (P = .016), said the investigators. These proteins might help regulate antigenic penetration of the esophageal mucosa and also could permit migration of white blood cells, they said. “Loss of tight junction regulators and dilation of intercellular spaces appear to be involved in the pathophysiology of EoE and could be targets for treatment,” the researchers concluded. But they also noted that their study did not examine the same patients before and after steroid therapy and did not look at desmosomes, intercellular junctions that past research has suggested might be affected in EoE.
For the second study, Dr. Bram van Rhijn and his associates at the Academic Medical Center in the Netherlands compared endoscopies of 16 patients with dysphagia and suspected (unconfirmed) EoE with 11 controls, both at baseline and after 8 weeks of high-dose esomeprazole treatment. Esophageal mucosal integrity was “severely impaired” in patients with confirmed EoE and in those with proton pump inhibitor–responsive eosinophilia (PPRE), the researchers said (Clin. Gasteroenterol. Hepatol. 2014 [doi:10.1016/j.cgh.2014.02.037]).
In both forms of disease, molecules as large as 40,000 daltons were able to pass through the compromised esophageal mucosa, Dr. Bram van Rhijn and his associates reported. “This size is similar to the size of most plant and animal food allergens to which EoE patients are sensitized,” they added. Esophageal permeability might increase the rate of immune exposure to allergens, thereby mediating EoE and PPRE, they said.
On mucosal functional tests, both EoE and PPRE were associated with reduced transepithelial electrical resistance and lower electrical tissue impedance, most notably in patients with EoE (P less than .001 for both, compared with controls), the investigators reported. Proton pump inhibitor treatment partially reversed these changes in patients with PPRE but showed no effect for patients with EoE, they said. This finding suggests that acid reflux might play a role in PPRE, but not in EOE, they concluded.
Dr. Katzka and his associates disclosed no funding sources and reported having no conflicts of interest. Dr. Rhijn and his associates were supported by the Netherlands Organization for Scientific Research. Two of Dr. Rhijn’s coauthors reported financial relationships with AstraZeneca, Endostim, Medical Measurement Systems, Shire, and GlaxoSmithKline.
Topical steroid therapy improved some indicators of mucosal integrity in patients with eosinophilic esophagitis, but proton pump inhibitor therapy did not, according to two studies reported in the November issue of Clinical Gastroenterology and Hepatology.
The first study found that topical fluticasone therapy at a dose of 880 mcg twice daily for 2 months helped correct esophageal spongiosis, or dilated intercellular space, in patients with eosinophilic esophagitis (EoE). Spongiosis scores for treated patients were significantly lower than for untreated patients (0.4 vs. 1.3; P = .016), said Dr. David Katzka at the Mayo Clinic in Rochester, Minn. and his associates (Clin. Gastroenterol. Hepatol. 2014 [doi:10.1016/j.cgh.2014.02.039]).
In the study, histologic analyses also showed that improved spongiosis scores in treated patients correlated with increased density of two tight junction proteins, filaggrin (P = .001) and zonula occludens-3 (P = .016), said the investigators. These proteins might help regulate antigenic penetration of the esophageal mucosa and also could permit migration of white blood cells, they said. “Loss of tight junction regulators and dilation of intercellular spaces appear to be involved in the pathophysiology of EoE and could be targets for treatment,” the researchers concluded. But they also noted that their study did not examine the same patients before and after steroid therapy and did not look at desmosomes, intercellular junctions that past research has suggested might be affected in EoE.
For the second study, Dr. Bram van Rhijn and his associates at the Academic Medical Center in the Netherlands compared endoscopies of 16 patients with dysphagia and suspected (unconfirmed) EoE with 11 controls, both at baseline and after 8 weeks of high-dose esomeprazole treatment. Esophageal mucosal integrity was “severely impaired” in patients with confirmed EoE and in those with proton pump inhibitor–responsive eosinophilia (PPRE), the researchers said (Clin. Gasteroenterol. Hepatol. 2014 [doi:10.1016/j.cgh.2014.02.037]).
In both forms of disease, molecules as large as 40,000 daltons were able to pass through the compromised esophageal mucosa, Dr. Bram van Rhijn and his associates reported. “This size is similar to the size of most plant and animal food allergens to which EoE patients are sensitized,” they added. Esophageal permeability might increase the rate of immune exposure to allergens, thereby mediating EoE and PPRE, they said.
On mucosal functional tests, both EoE and PPRE were associated with reduced transepithelial electrical resistance and lower electrical tissue impedance, most notably in patients with EoE (P less than .001 for both, compared with controls), the investigators reported. Proton pump inhibitor treatment partially reversed these changes in patients with PPRE but showed no effect for patients with EoE, they said. This finding suggests that acid reflux might play a role in PPRE, but not in EOE, they concluded.
Dr. Katzka and his associates disclosed no funding sources and reported having no conflicts of interest. Dr. Rhijn and his associates were supported by the Netherlands Organization for Scientific Research. Two of Dr. Rhijn’s coauthors reported financial relationships with AstraZeneca, Endostim, Medical Measurement Systems, Shire, and GlaxoSmithKline.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Topical steroids seemed to improve mucosal integrity in patients with eosinophilic esophagitis, but proton pump inhibitor therapy did not.
Major finding: Mean spongiosis score was significantly lower among treated vs. untreated patients (0.4 vs. 1.3; P = .016).
Data source: Immunohistochemistry, histology, endoscopy, and mucosal functional analyses of 57 subjects in two separate studies.
Disclosures: Dr. Katzka and associates disclosed no funding sources and reported having no conflicts of interest. Dr. Rhijn and associates were supported by the Netherlands Organization for Scientific Research. Two of Dr. Rhijn’s coauthors reported financial relationships with AstraZeneca, Endostim, Medical Measurement Systems, Shire, and GlaxoSmithKline.
Stage III Non–Small Cell Lung Cancer
Series Editor: Arthur T. Skarin, MD, FACP, FCCP
Each year approximately 228,000 Americans will be diagnosed with lung cancer, and 159,000 will die of this disease. An estimated 85% of lung cancer cases are non–small cell lung cancer (NSCLC), more than 50% of NSCLC is comprised of adenocarcinoma, the median age at diagnosis is 71 years, and 25% of patients with this diagnosis present with stage III disease. In 2010 the seventh edition of the American Joint Committee on Cancer (AJCC) TNM staging system for lung cancer was released, and several changes were made which affect the patient population designated as having stage III disease.
To read the full article in PDF:
Series Editor: Arthur T. Skarin, MD, FACP, FCCP
Each year approximately 228,000 Americans will be diagnosed with lung cancer, and 159,000 will die of this disease. An estimated 85% of lung cancer cases are non–small cell lung cancer (NSCLC), more than 50% of NSCLC is comprised of adenocarcinoma, the median age at diagnosis is 71 years, and 25% of patients with this diagnosis present with stage III disease. In 2010 the seventh edition of the American Joint Committee on Cancer (AJCC) TNM staging system for lung cancer was released, and several changes were made which affect the patient population designated as having stage III disease.
To read the full article in PDF:
Series Editor: Arthur T. Skarin, MD, FACP, FCCP
Each year approximately 228,000 Americans will be diagnosed with lung cancer, and 159,000 will die of this disease. An estimated 85% of lung cancer cases are non–small cell lung cancer (NSCLC), more than 50% of NSCLC is comprised of adenocarcinoma, the median age at diagnosis is 71 years, and 25% of patients with this diagnosis present with stage III disease. In 2010 the seventh edition of the American Joint Committee on Cancer (AJCC) TNM staging system for lung cancer was released, and several changes were made which affect the patient population designated as having stage III disease.
To read the full article in PDF:
CAPO Aspiration Pneumonia
Pneumonia is a common clinical syndrome with well‐described epidemiology and microbiology. Aspiration pneumonia comprises 5% to 15% of patients with pneumonia acquired outside of the hospital,[1] but is less well characterized despite being a major syndrome of pneumonia in the elderly.[2, 3] Difficulties in studying aspiration pneumonia include the lack of a sensitive and specific marker for aspiration as well as the potential overlap between aspiration pneumonia and other forms of pneumonia.[4, 5, 6] Additionally, clinicians have difficulty distinguishing between aspiration pneumonia, which develops after the aspiration of oropharyngeal contents, and aspiration pneumonitis, wherein inhalation of gastric contents causes inflammation without the subsequent development of bacterial infection.[7, 8] Central to the study of aspiration pneumonia is whether it should exist as its own entity, or if aspiration is really a designation used for pneumonia in an older patient with greater comorbidities. The ability to clearly understand how a clinician diagnoses aspiration pneumonia, and whether that method has face validity with expert definitions may allow for improved future research, improved generalizability of current or past research, and possibly better clinical care.
Several validated mortality prediction models exist for community‐acquired pneumonia (CAP) using a variety of clinical predictors, but their performance in patients with aspiration pneumonia is less well characterized. Most studies validating pneumonia severity scoring systems excluded aspiration pneumonia from their study population.[9, 10, 11] Severity scoring systems for CAP may not accurately predict disease severity in patients with aspiration pneumonia. The CURB‐65[9] (confusion, uremia, respiratory rate, blood pressure, age 65 years) and the eCURB[12] scoring systems are poor predictors of mortality in patients with aspiration pneumonia, perhaps because they do not account for patient comorbidities.[13] The pneumonia severity index (PSI)[10] might predict mortality better than CURB‐65 in the aspiration population due to the inclusion of comorbidities.
Previous studies have demonstrated that patients with aspiration pneumonia are older and have greater disease severity and more comorbidities.[13, 14, 15] These single‐center studies also demonstrated greater mortality, more frequent admission to an intensive care unit (ICU), and longer hospital lengths of stay in patients with aspiration pneumonia. These studies identified aspiration pneumonia by the presence of a risk factor for aspiration[15] or by physician billing codes.[13] In practice, however, the bedside clinician diagnoses a patient as having aspiration pneumonia, but the logic is likely vague and inconsistent. Despite the potential for variability with individual judgment, an aggregate estimation from independent judgments may perform better than individual judgments.[16] Because there is no gold standard for defining aspiration pneumonia, all previous research has been limited to definitions created by investigators. This multicenter study seeks to determine what clinical characteristics lead physicians to diagnose a patient as having aspiration pneumonia, and whether or not the clinician‐derived diagnosis is distinct and clinically useful.
Our objectives were to: (1) identify covariates associated with bedside clinicians diagnosing a pneumonia patient as having aspiration pneumonia; (2) compare aspiration pneumonia and nonaspiration pneumonia in regard to disease severity, patient demographics, comorbidities, and clinical outcomes; and (3) measure the performance of the PSI in aspiration pneumonia versus nonaspiration pneumonia.
PATIENTS AND METHODS
Study Design and Setting
We performed a secondary analysis of the Community‐Acquired Pneumonia Organization (CAPO) database, which contains retrospectively collected data from 71 hospitals in 16 countries between June 2001 and December 2012. In each participating center, primary investigators selected nonconsecutive, adult hospitalized patients diagnosed with CAP. To decrease systematic selection biases, the selection of patients with CAP for enrollment in the trial was based on the date of hospital admission. Each investigator completed a case report form that was transferred via the internet to the CAPO study center at the University of Louisville (Louisville, KY). A sample of the data collection form is available at the study website (
Inclusion and Exclusion Criteria
Patients 18 years of age and satisfying criteria for CAP were included in this study. A diagnosis of CAP required a new pulmonary infiltrate at time of hospitalization, and at least 1 of the following: new or increased cough; leukocytosis; leukopenia, or left shift pattern on white blood cell count; and temperature >37.8C or <35.6 C. We excluded patients with pneumonia attributed to mycobacterial or fungal infection, and patients infected with human immunodeficiency virus, as we believed these types of pneumonia differ fundamentally from typical CAP.
Patient Variables
Patient variables included presence of aspiration pneumonia, laboratory data, comorbidities, and measures of disease severity, including the PSI. The clinician made a clinical diagnosis of the presence or absence of aspiration for each patient by marking a box on the case report form. Outcomes included in‐hospital mortality, hospital length of stay up to 14 days, and time to clinical stability up to 8 days. All variables were obtained directly from the case report form. In accordance with previously published definitions, we defined clinical stability as the day the following criteria were all met: improved clinical signs (improved cough and shortness of breath), lack of fever for >8 hours, improving leukocytosis (decreased at least 10% from the previous day), and tolerating oral intake.[17, 18]
Statistical Analysis
Baseline characteristics of patients with aspiration and nonaspiration CAP were compared using 2 or Fisher exact tests for categorical variables and the Mann‐Whitney U test for continuous variables.
To determine which patient variables were important in the physician diagnosis of aspiration pneumonia, we performed logistic regression with initial covariates comprising the demographic, comorbidity, and disease severity measurements listed in Table 1. We included interactions between cerebrovascular disease and age, nursing home status, and confusion to improve model fit. We centered all variables (including binary indicators) according to the method outlined by Kraemer and Blasey to improve interpretation of the main effects.[19]
| Aspiration Pneumonia, N=451 | Nonaspiration Pneumonia, N=4,734 | P Value | |
|---|---|---|---|
|
|||
| Demographics | |||
| Age, y | 79 (6587) | 69 (5380) | <0.001 |
| % Male | 59% | 60% | 0.58 |
| Nursing home residence | 25% | 5% | <0.001 |
| Recent (30 days) antibiotic use | 21% | 16% | 0.017 |
| Comorbidities | |||
| Cerebrovascular disease | 35% | 14% | <0.001 |
| Chronic obstructive pulmonary disease | 25% | 27% | 0.62 |
| Congestive heart failure | 23% | 19% | 0.027 |
| Diabetes | 18% | 18% | 0.85 |
| Cancer | 12% | 10% | 0.12 |
| Renal disease | 10% | 11% | 0.53 |
| Liver disease | 6% | 5% | 0.29 |
| Disease severity | |||
| Pneumonia severity index | 123 (99153) | 92 (68117) | <0.001 |
| Confusion | 49% | 12% | <0.001 |
| PaO2 <60 mm Hg | 43% | 33% | <0.001 |
| BUN >30 g/dL | 42% | 23% | <0.001 |
| Multilobar pneumonia | 34% | 28% | 0.003 |
| Pleural effusion | 25% | 21% | 0.07 |
| Respiratory rate >30 breaths/minute | 21% | 20% | 0.95 |
| pH <7.35 | 13% | 5% | <0.001 |
| Hematocrit <30% | 11% | 6% | 0.001 |
| Temperature >37.8C or <35.6C | 9% | 7% | 0.30 |
| Systolic blood pressure <90 mm Hg | 8% | 9% | 0.003 |
| Sodium <130 mEq/L | 8% | 6% | 0.08 |
| Heart rate >125 beats/minute | 8% | 5% | 0.71 |
| Glucose >250 mg/dL | 6% | 7% | 0.06 |
| Cavitary lesion | 0% | 0% | 0.67 |
| Clinical outcomes | |||
| In‐hospital mortality | 23% | 9% | <0.001 |
| Intensive care unit admission | 19% | 13% | 0.002 |
| Hospital length of stay, d | 9 (515) | 7 (412) | <0.001 |
| Time to clinical stability, d | 8 (48) | 4 (38) | <0.001 |
To determine if aspiration pneumonia had worse clinical outcomes compared to nonaspiration pneumonia, multiple methods were used. To compare the differences between the 2 groups with respect to time to clinical stability and length of hospital stay, we constructed Kaplan‐Meier survival curves and Cox proportional hazards regression models. The log‐rank test was used to determine statistical differences between the Kaplan‐Meier survival curves. To compare the impact of aspiration on mortality in patients with CAP, we conducted a propensity scorematched analysis. We chose propensity score matching over traditional logistic regression to balance variables among groups and to avoid the potential for overfit and multicollinearity. We considered a variable balanced after matching if its standardized difference was <10. All variables in the propensity scorematched analysis were balanced.
Although our dataset contained minimal missing data, we imputed any missing values to maintain the full study population in the creation of the propensity score. Missing data were imputed using the aregImpute function of the hmisc package of R (The R Foundation for Statistical Computing, Vienna, Austria).[20, 21] We built the propensity score model using a variable selection algorithm described by Bursac et al.[22] Our model included variables for region (United States/Canada, Europe, Asia/Africa or Latin America) and the variables listed in Table 1, with the exception of the PSI and the 4 clinical outcomes. Given that previous analyses accounting for clustering by physician did not substantially affect our results,[23] our model did not include physician‐level variables and did not account for the clustering effects of physicians. Using the propensity scores generated from this model, we matched a case of aspiration CAP with a case of nonaspiration CAP.[24] We then constructed a general linear model using the matched dataset to obtain the magnitude of effect of aspiration on mortality.
We used receiver operating characteristic curves to define the diagnostic accuracy of the pneumonia severity index for the prediction of mortality among patients with aspiration pneumonia and those with nonaspiration pneumonia. SAS version 9.3 (SAS Institute, Cary, NC) and R version 2.15.3 (The R Foundation for Statistical Computing) were used for all analyses. P values of 0.05 were considered statistically significant in all analyses.
RESULTS
Our initial query, after exclusion criteria, yielded a study population of 5185 patients (Figure 1). We compared 451 patients diagnosed with aspiration pneumonia to 4734 with CAP (Figure 1). Patient characteristics are summarized in Table 1. Patients with aspiration pneumonia were older, more likely to live in a nursing home, had greater disease severity, and were more likely to be admitted to an ICU. Patients with aspiration pneumonia had longer adjusted hospital lengths of stay and took more days to achieve clinical stability than patients with nonaspiration pneumonia (Figure 2). After adjusting for all variables in Table 1, the Cox proportional hazards models demonstrated that aspiration pneumonia was associated with ongoing hospitalization (hazard ratio [HR] for discharge: 0.77, 95% confidence interval [CI]: 0.65‐0.91, P=0.002) and clinical instability (HR for attaining clinical stability: 0.72, 95% CI: 0.61‐0.84, P<0.001). Patients with aspiration pneumonia presented with greater disease severity than those with nonaspiration pneumonia. Although there was no difference between groups in regard to temperature, respiratory rate, hyponatremia, or presence of pleural effusions or cavitary lesions, all other measured indices of disease severity were worse in patients with aspiration pneumonia. Patients with aspiration pneumonia were more likely to have cerebrovascular disease than those with nonaspiration pneumonia. Aspiration pneumonia patients also had increased prevalence of congestive heart failure. There was no appreciable difference between groups among other measured comorbidities.
The patient characteristics most associated with a physician diagnosis of aspiration pneumonia, identified using logistic regression, were confusion, residence in nursing home, and presence of cerebrovascular disease (odds ratio [OR]: of 4.4, 2.9, and 2.3, respectively), whereas renal disease was associated with decreased physician diagnosis of aspiration pneumonia over nonaspiration pneumonia (OR: 0.58) (Table 2).
| Covariate | Odds Ratio | 95% Confidence Intervals | P Value |
|---|---|---|---|
|
|||
| Demographics | |||
| Age, y | 1.00 | 0.991.01 | 0.948 |
| Male | 1.20 | 0.941.54 | 0.148 |
| Nursing home residence | 2.93 | 2.134.00 | <0.001 |
| Comorbidities | |||
| Cerebrovascular disease | 2.26 | 1.533.32 | <0.001 |
| Renal disease | 0.58 | 0.390.85 | 0.006 |
| Disease severity | |||
| Confusion | 4.41 | 3.405.72 | <0.001 |
| Hematocrit <30% | 1.59 | 1.062.33 | 0.020 |
| pH <7.35 | 1.67 | 1.102.47 | 0.013 |
| Temperature >37.8C or <35.6C | 1.60 | 1.072.35 | 0.019 |
| Multilobar pneumonia | 1.29 | 1.001.65 | 0.047 |
| Interaction terms | |||
| Age * cerebrovascular disease | 0.98 | 0.960.99 | 0.011 |
| Nursing home * cerebrovascular disease | 0.51 | 0.270.96 | 0.037 |
| Confusion * cerebrovascular disease | 0.70 | 0.421.17 | 0.175 |
Observed in‐patient mortality of aspiration pneumonia was 23%. This mortality was considerably higher than a mean PSI score of 123 would predict (class IV risk group, with expected 30‐day mortality of 8%9%[25]). The PSI score's ability to predict inpatient mortality in patients with aspiration pneumonia was moderate, with an area under the curve (AUC) of 0.71. This was similar to its performance in patients with nonaspiration pneumonia (AUC of 0.75) (Figure 3). These values are lower than the AUC of 0.81 for the PSI in predicting mortality derived from a meta‐analysis of 31 other studies.[26]
Our regression model after propensity score matching demonstrated that aspiration pneumonia independently confers a 2.3‐fold increased odds for inpatient mortality (95% CI: 1.56‐3.45, P<0.001).
DISCUSSION
Pneumonia patients with confusion, nursing home residence, or cerebrovascular disease are more likely to be diagnosed with aspiration pneumonia by clinicians. Although this is unsurprising, it is notable that these patients are more than twice as likely to die in the inpatient setting, even after accounting for age, comorbidities, and disease severity. These findings are similar to three previously published studies comparing aspiration and nonaspiration pneumonia at single institutions, albeit using different aspiration pneumonia definitions.[13, 14, 15] This study is the first large, multicenter, multinational study to demonstrate these findings.
Central to the interpretation of our results is the method of diagnosing aspiration versus nonaspiration. A bottom‐up method that relies on a clinician to check a box for aspiration may appear poorly reproducible. Because there is no diagnostic gold standard, clinicians may use different criteria to diagnose aspiration, creating potential for idiosyncratic noise. The strength of the wisdom of the crowd method used in this study is that an aggregate estimation from independent judgments may reduce the noise from individual judgments.[16] Although clinicians may vary in why they diagnose a particular patient as having aspiration pneumonia, it appears that the overwhelming reason for diagnosing a patient as having aspiration pneumonia is the presence of confusion, followed by previous nursing home residence or cerebrovascular disease. This finding has some face validity when compared with studies using an investigator definition, as altered mental status, chronic debility, and cerebrovascular disease are either prominent features of the definition of aspiration pneumonia[8] or frequently observed in patients with aspiration pneumonia.[13, 15] The distribution of cerebrovascular disease among our study's aspiration and nonaspiration pneumonia patients was similar to studies that used formal criteria in their definitions.[13, 15] Although nursing home residence was more likely in aspiration pneumonia patients, the majority of aspiration pneumonia patients were residing in the community, suggesting that aspiration is not simply a surrogate for healthcare‐associated pneumonia. Although patients with aspiration pneumonia are typically older than their nonaspiration counterparts, it appears that age is not a key determinant in the diagnosis of aspiration. With aspiration pneumonia, confusion, nursing home residence, and the presence of cerebrovascular disease are the greatest contributors in the clinical diagnosis, more than age.
Our data demonstrate that aspiration pneumonia confers increased odds for mortality, even after adjustment for age, disease severity, and comorbidities. These data suggest that aspiration pneumonia is a distinct entity from nonaspiration pneumonia, and that this disease is worse than nonaspiration CAP. If aspiration pneumonia is distinct from nonaspiration pneumonia, some unrecognized host factor other than age, disease severity, or the captured comorbidities decreases survival in aspiration pneumonia patients. However, it is also possible that aspiration pneumonia is merely a clinical designation for one end of the pneumonia spectrum, and we and others have failed to completely account for all measures of disease severity or all measures of comorbidities. Examples of unmeasured comorbidities would include presence of oropharyngeal dysphagia, which is not assessed in the database but could have a significant effect on clinical diagnosis. Unmeasured covariates can include measures beyond that of disease severity or comorbidity, such as the presence of a do not resuscitate (DNR) order, which could have a significant confounding effect on the observed association. A previous, single‐center study demonstrated that increased 30‐day mortality in aspiration pneumonia was mostly attributable to greater disease severity and comorbidities, although aspiration pneumonia independently conferred greater risk for adverse long‐term outcomes.[15] We propose that aspiration pneumonia represents a clinically distinct entity from nonaspiration pneumonia. Patients with chronic aspiration are often chronically malnourished and may have different oral flora than patients without chronic aspiration.[27, 28] Chronic aspiration has been associated with granulomatous reaction, organizing pneumonia, diffuse alveolar damage, and chronic bronchiolitis.[29] Chronic aspiration may elicit changes in the host physiology, and may render the host more susceptible to the development of secondary bacterial infection with morbid consequences.
The ability of the PSI to predict inpatient mortality was moderate (AUC only 0.7), with no significant additional discrimination between the aspiration and nonaspiration pneumonia groups. Although the PSI had moderate ability to predict inpatient mortality, the observed mortality was considerably higher than predicted. It is possible that the PSI incompletely captures clinically relevant comorbidities (eg, malnutrition). Further study to improve mortality prediction of aspiration pneumonia patients could employ sensitivity analysis to determine optimal thresholds and weighting of the PSI components.
Patients with aspiration pneumonia had longer hospital lengths of stay and took longer to achieve clinical stability than their nonaspiration counterparts. Time to clinical stability has been associated with increased posthospitalization mortality and is associated with time to switch from intravenous to oral antibiotics.[17] Although some component of hospital length‐of‐stay is subject to local practice patterns, time to clinical stability has explicit criteria for clinical improvement and failure, and therefore is less likely to be affected by local practice patterns.
We noted a relatively high (16%21%) incidence of prior antibiotic use among patients in this database. Analysis of antibiotic prescription patterns was limited, given the several different countries from which the database draws its cases. Although we used accepted criteria to define CAP cases, it is possible that this population may have a higher rate of resistant or uncommon pathogens than other studies of CAP that have populations with lower incidence of prior antibiotic use. Although not assessed, we suspect a significant component of the prior antibiotic use represented outpatient pneumonia treatment during the few days prior to visiting the hospital.
This study has several limitations, of which the most important may be that we used clinical determination for defining presence of aspiration pneumonia. This method is susceptible to the subjective perceptions of the treating clinician. We did not account for the effect of individual physicians in our model, although we did adjust for regional differences. The retrospective identification of patients allows for the possibility of selection bias, and therefore we have not attempted to make inferences regarding the relative incidence of pneumonia, nor did we adjust for temporal trends in diagnosis. The ratio of aspiration pneumonia patients to nonaspiration pneumonia patients may not necessarily reflect that observed in reality. Microbiologic and antibiotic data were unavailable for analysis. This study cannot inform on nonhospitalized patients with aspiration pneumonia, as only hospitalized patients were enrolled. The database identified cases of pneumonia, so it is possible for a patient to enter into the database more than once. Detection of mortality was limited to the inpatient setting rather than a set interval of 30 days. Inpatient mortality depends on length‐of‐stay patterns that may bias the mortality endpoint.[30] Also not assessed was the presence of a DNR order. It is possible that an older patient with greater comorbidities and disease severity may have care intentionally limited or withdrawn early by the family or clinicians.
Strengths of the study include its size and its multicenter, multinational population. The CAPO database is a large and well‐described population of patients with CAP.[17, 31] These attributes, as well as the clinician‐determined diagnosis, increase the generalizability of the study compared to a single‐center, single‐country study that employs investigator‐defined criteria.
CONCLUSION
Pneumonia patients with confusion, who are nursing home residence, and have cerebrovascular disease are more likely to be diagnosed with aspiration pneumonia by clinicians. Our clinician‐diagnosed cohort appears similar to those derived using an investigator definition. Patients with aspiration pneumonia are older, and have greater disease severity and more comorbidities than patients with nonaspiration pneumonia. They have greater mortality than their PSI score class would predict. Even after accounting for age, disease severity, and comorbidities, the presence of aspiration pneumonia independently conferred a greater than 2‐fold increase in inpatient mortality. These findings together suggest that aspiration pneumonia should be considered a distinct entity from typical pneumonia, and that additional research should be done in this field.
ACKNOWLEDGMENTS
Disclosures: M.J.L. contributed to the study design, data analysis, statistical analysis, and writing of the manuscript. P.P. contributed to the study design and revision of the manuscript for important intellectual content. T.W. and E.W. contributed to the study design, statistical analysis, and revision of the manuscript for important intellectual content. J.A.R. and N.C.D. contributed to the study design and revision of the manuscript for important intellectual content. All authors read and approved the final manuscript. M.L. takes responsibility for the integrity of the work as a whole, from inception to published article. This investigation was partly supported with funding from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health (grant 8UL1TR000105 [formerly UL1RR025764]). The authors report no conflicts of interest.
- , , , et al. Severe community‐acquired pneumonia. Epidemiology and prognostic factors. Am Rev Respir Dis. 1991;144(2):312–318.
- , , . Risk factors for pneumonia in the elderly. Am J Med. 1994;96(4):313–320.
- , . Aspiration pneumonia and dysphagia in the elderly. Chest. 2003;124(1):328–336.
- , , . Pneumonia versus aspiration pneumonitis in nursing home residents: diagnosis and management. J Am Geriatr Soc. 2003;51(1):17–23.
- . Aspiration pneumonia: mixing apples with oranges and tangerines. Crit Care Med. 2004;32(5):1236; author reply 1236–1237.
- , , , , . Epidemiology and impact of aspiration pneumonia in patients undergoing surgery in Maryland, 1999–2000. Crit Care Med. 2003;31(7):1930–1937.
- . Aspiration syndromes: aspiration pneumonia and pneumonitis. Hosp Pract (Minneap). 2010;38(1):35–42.
- . Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344(9):665–671.
- , , , et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58(5):377–382.
- , , , et al. Comparison of a disease‐specific and a generic severity of illness measure for patients with community‐acquired pneumonia. J Gen Intern Med. 1995;10(7):359–368.
- , , , et al. Development and validation of a clinical prediction rule for severe community‐acquired pneumonia. Am J Respir Crit Care Med. 2006;174(11):1249–1256.
- , , , et al. CURB‐65 pneumonia severity assessment adapted for electronic decision support. Chest. 2011;140(1):156–163.
- , , , . Mortality, morbidity, and disease severity of patients with aspiration pneumonia. J Hosp Med. 2013;8(2):83–90.
- , , , , . Pneumonia Severity Index (PSI), CURB‐65, and mortality in hospitalized elderly patients with aspiration pneumonia [in German]. Z Gerontol Geriatr. 2011;44(4):229–234.
- , , , , . Risk factors for aspiration in community‐acquired pneumonia: analysis of a hospitalized UK cohort. Am J Med. 2013;126(11):995–1001.
- , , , . The wisdom of the crowd in combinatorial problems. Cogn Sci. 2012;36(3):452–470.
- , , , et al. Association between time to clinical stability and outcomes after discharge in hospitalized patients with community‐acquired pneumonia. Chest. 2011;140(2):482–488.
- . Clinical stability and switch therapy in hospitalised patients with community‐acquired pneumonia: are we there yet? Eur Respir J. 2013;41(1):5–6.
- , . Centring in regression analyses: a strategy to prevent errors in statistical inference. Int J Methods Psychiatr Res. 2004;13(3):141–151.
- . Hmisc: Harrell miscellaneous. Available at: http://CRAN.R‐project.org/package=Hmisc. Published Sept 12, 2014. Last accessed Oct 27, 2014.
- , . Multiple imputation for the fatal accident reporting system. J R Stat Soc Ser C Appl Stat. 1991;40(1):13–29.
- , , , . Purposeful selection of variables in logistic regression. Source Code Biol Med. 2008;3:17.
- , , , , ; CAPO authors. Mortality differences among hospitalized patients with community‐acquired pneumonia in three world regions: results from the Community‐Acquired Pneumonia Organization (CAPO) International Cohort Study. Respir Med. 2013;107(7):1101–1111.
- . Reducing bias in a propensity score matched‐pair sample using greedy matching techniques. In: Proceedings of the 26th Annual SAS Users Group International Conference. Cary, NC: SAS Institute Inc.; 2001:214–226. Available at: http://www2.sas.com/proceedings/sugi26/p214–26.pdf. Last accessed Oct 27, 2014.
- , , , et al. A prediction rule to identify low‐risk patients with community‐acquired pneumonia. N Engl J Med. 1997;336(4):243–250.
- , , , et al. Severity assessment tools for predicting mortality in hospitalised patients with community‐acquired pneumonia. Systematic review and meta‐analysis. Thorax. 2010;65(10):878–883.
- , , , , , . Prevalence and prognostic implications of dysphagia in elderly patients with pneumonia. Age Ageing. 2010;39(1):39–45.
- , . The association between oral microorgansims and aspiration pneumonia in the institutionalized elderly: review and recommendations. Dysphagia. 2010;25(4):307–322.
- , . Histopathology of aspiration pneumonia not associated with food or other particulate matter: a clinicopathologic study of 10 cases diagnosed on biopsy. Am J Surg Pathol. 2011;35(3):426–431.
- , , , , , . Interpreting hospital mortality data. The role of clinical risk adjustment. JAMA. 1988;260(24):3611–3616.
- , , , . Hospitalization for community‐acquired pneumonia: the pneumonia severity index vs clinical judgment. Chest. 2003;124(1):121–124.
Pneumonia is a common clinical syndrome with well‐described epidemiology and microbiology. Aspiration pneumonia comprises 5% to 15% of patients with pneumonia acquired outside of the hospital,[1] but is less well characterized despite being a major syndrome of pneumonia in the elderly.[2, 3] Difficulties in studying aspiration pneumonia include the lack of a sensitive and specific marker for aspiration as well as the potential overlap between aspiration pneumonia and other forms of pneumonia.[4, 5, 6] Additionally, clinicians have difficulty distinguishing between aspiration pneumonia, which develops after the aspiration of oropharyngeal contents, and aspiration pneumonitis, wherein inhalation of gastric contents causes inflammation without the subsequent development of bacterial infection.[7, 8] Central to the study of aspiration pneumonia is whether it should exist as its own entity, or if aspiration is really a designation used for pneumonia in an older patient with greater comorbidities. The ability to clearly understand how a clinician diagnoses aspiration pneumonia, and whether that method has face validity with expert definitions may allow for improved future research, improved generalizability of current or past research, and possibly better clinical care.
Several validated mortality prediction models exist for community‐acquired pneumonia (CAP) using a variety of clinical predictors, but their performance in patients with aspiration pneumonia is less well characterized. Most studies validating pneumonia severity scoring systems excluded aspiration pneumonia from their study population.[9, 10, 11] Severity scoring systems for CAP may not accurately predict disease severity in patients with aspiration pneumonia. The CURB‐65[9] (confusion, uremia, respiratory rate, blood pressure, age 65 years) and the eCURB[12] scoring systems are poor predictors of mortality in patients with aspiration pneumonia, perhaps because they do not account for patient comorbidities.[13] The pneumonia severity index (PSI)[10] might predict mortality better than CURB‐65 in the aspiration population due to the inclusion of comorbidities.
Previous studies have demonstrated that patients with aspiration pneumonia are older and have greater disease severity and more comorbidities.[13, 14, 15] These single‐center studies also demonstrated greater mortality, more frequent admission to an intensive care unit (ICU), and longer hospital lengths of stay in patients with aspiration pneumonia. These studies identified aspiration pneumonia by the presence of a risk factor for aspiration[15] or by physician billing codes.[13] In practice, however, the bedside clinician diagnoses a patient as having aspiration pneumonia, but the logic is likely vague and inconsistent. Despite the potential for variability with individual judgment, an aggregate estimation from independent judgments may perform better than individual judgments.[16] Because there is no gold standard for defining aspiration pneumonia, all previous research has been limited to definitions created by investigators. This multicenter study seeks to determine what clinical characteristics lead physicians to diagnose a patient as having aspiration pneumonia, and whether or not the clinician‐derived diagnosis is distinct and clinically useful.
Our objectives were to: (1) identify covariates associated with bedside clinicians diagnosing a pneumonia patient as having aspiration pneumonia; (2) compare aspiration pneumonia and nonaspiration pneumonia in regard to disease severity, patient demographics, comorbidities, and clinical outcomes; and (3) measure the performance of the PSI in aspiration pneumonia versus nonaspiration pneumonia.
PATIENTS AND METHODS
Study Design and Setting
We performed a secondary analysis of the Community‐Acquired Pneumonia Organization (CAPO) database, which contains retrospectively collected data from 71 hospitals in 16 countries between June 2001 and December 2012. In each participating center, primary investigators selected nonconsecutive, adult hospitalized patients diagnosed with CAP. To decrease systematic selection biases, the selection of patients with CAP for enrollment in the trial was based on the date of hospital admission. Each investigator completed a case report form that was transferred via the internet to the CAPO study center at the University of Louisville (Louisville, KY). A sample of the data collection form is available at the study website (
Inclusion and Exclusion Criteria
Patients 18 years of age and satisfying criteria for CAP were included in this study. A diagnosis of CAP required a new pulmonary infiltrate at time of hospitalization, and at least 1 of the following: new or increased cough; leukocytosis; leukopenia, or left shift pattern on white blood cell count; and temperature >37.8C or <35.6 C. We excluded patients with pneumonia attributed to mycobacterial or fungal infection, and patients infected with human immunodeficiency virus, as we believed these types of pneumonia differ fundamentally from typical CAP.
Patient Variables
Patient variables included presence of aspiration pneumonia, laboratory data, comorbidities, and measures of disease severity, including the PSI. The clinician made a clinical diagnosis of the presence or absence of aspiration for each patient by marking a box on the case report form. Outcomes included in‐hospital mortality, hospital length of stay up to 14 days, and time to clinical stability up to 8 days. All variables were obtained directly from the case report form. In accordance with previously published definitions, we defined clinical stability as the day the following criteria were all met: improved clinical signs (improved cough and shortness of breath), lack of fever for >8 hours, improving leukocytosis (decreased at least 10% from the previous day), and tolerating oral intake.[17, 18]
Statistical Analysis
Baseline characteristics of patients with aspiration and nonaspiration CAP were compared using 2 or Fisher exact tests for categorical variables and the Mann‐Whitney U test for continuous variables.
To determine which patient variables were important in the physician diagnosis of aspiration pneumonia, we performed logistic regression with initial covariates comprising the demographic, comorbidity, and disease severity measurements listed in Table 1. We included interactions between cerebrovascular disease and age, nursing home status, and confusion to improve model fit. We centered all variables (including binary indicators) according to the method outlined by Kraemer and Blasey to improve interpretation of the main effects.[19]
| Aspiration Pneumonia, N=451 | Nonaspiration Pneumonia, N=4,734 | P Value | |
|---|---|---|---|
|
|||
| Demographics | |||
| Age, y | 79 (6587) | 69 (5380) | <0.001 |
| % Male | 59% | 60% | 0.58 |
| Nursing home residence | 25% | 5% | <0.001 |
| Recent (30 days) antibiotic use | 21% | 16% | 0.017 |
| Comorbidities | |||
| Cerebrovascular disease | 35% | 14% | <0.001 |
| Chronic obstructive pulmonary disease | 25% | 27% | 0.62 |
| Congestive heart failure | 23% | 19% | 0.027 |
| Diabetes | 18% | 18% | 0.85 |
| Cancer | 12% | 10% | 0.12 |
| Renal disease | 10% | 11% | 0.53 |
| Liver disease | 6% | 5% | 0.29 |
| Disease severity | |||
| Pneumonia severity index | 123 (99153) | 92 (68117) | <0.001 |
| Confusion | 49% | 12% | <0.001 |
| PaO2 <60 mm Hg | 43% | 33% | <0.001 |
| BUN >30 g/dL | 42% | 23% | <0.001 |
| Multilobar pneumonia | 34% | 28% | 0.003 |
| Pleural effusion | 25% | 21% | 0.07 |
| Respiratory rate >30 breaths/minute | 21% | 20% | 0.95 |
| pH <7.35 | 13% | 5% | <0.001 |
| Hematocrit <30% | 11% | 6% | 0.001 |
| Temperature >37.8C or <35.6C | 9% | 7% | 0.30 |
| Systolic blood pressure <90 mm Hg | 8% | 9% | 0.003 |
| Sodium <130 mEq/L | 8% | 6% | 0.08 |
| Heart rate >125 beats/minute | 8% | 5% | 0.71 |
| Glucose >250 mg/dL | 6% | 7% | 0.06 |
| Cavitary lesion | 0% | 0% | 0.67 |
| Clinical outcomes | |||
| In‐hospital mortality | 23% | 9% | <0.001 |
| Intensive care unit admission | 19% | 13% | 0.002 |
| Hospital length of stay, d | 9 (515) | 7 (412) | <0.001 |
| Time to clinical stability, d | 8 (48) | 4 (38) | <0.001 |
To determine if aspiration pneumonia had worse clinical outcomes compared to nonaspiration pneumonia, multiple methods were used. To compare the differences between the 2 groups with respect to time to clinical stability and length of hospital stay, we constructed Kaplan‐Meier survival curves and Cox proportional hazards regression models. The log‐rank test was used to determine statistical differences between the Kaplan‐Meier survival curves. To compare the impact of aspiration on mortality in patients with CAP, we conducted a propensity scorematched analysis. We chose propensity score matching over traditional logistic regression to balance variables among groups and to avoid the potential for overfit and multicollinearity. We considered a variable balanced after matching if its standardized difference was <10. All variables in the propensity scorematched analysis were balanced.
Although our dataset contained minimal missing data, we imputed any missing values to maintain the full study population in the creation of the propensity score. Missing data were imputed using the aregImpute function of the hmisc package of R (The R Foundation for Statistical Computing, Vienna, Austria).[20, 21] We built the propensity score model using a variable selection algorithm described by Bursac et al.[22] Our model included variables for region (United States/Canada, Europe, Asia/Africa or Latin America) and the variables listed in Table 1, with the exception of the PSI and the 4 clinical outcomes. Given that previous analyses accounting for clustering by physician did not substantially affect our results,[23] our model did not include physician‐level variables and did not account for the clustering effects of physicians. Using the propensity scores generated from this model, we matched a case of aspiration CAP with a case of nonaspiration CAP.[24] We then constructed a general linear model using the matched dataset to obtain the magnitude of effect of aspiration on mortality.
We used receiver operating characteristic curves to define the diagnostic accuracy of the pneumonia severity index for the prediction of mortality among patients with aspiration pneumonia and those with nonaspiration pneumonia. SAS version 9.3 (SAS Institute, Cary, NC) and R version 2.15.3 (The R Foundation for Statistical Computing) were used for all analyses. P values of 0.05 were considered statistically significant in all analyses.
RESULTS
Our initial query, after exclusion criteria, yielded a study population of 5185 patients (Figure 1). We compared 451 patients diagnosed with aspiration pneumonia to 4734 with CAP (Figure 1). Patient characteristics are summarized in Table 1. Patients with aspiration pneumonia were older, more likely to live in a nursing home, had greater disease severity, and were more likely to be admitted to an ICU. Patients with aspiration pneumonia had longer adjusted hospital lengths of stay and took more days to achieve clinical stability than patients with nonaspiration pneumonia (Figure 2). After adjusting for all variables in Table 1, the Cox proportional hazards models demonstrated that aspiration pneumonia was associated with ongoing hospitalization (hazard ratio [HR] for discharge: 0.77, 95% confidence interval [CI]: 0.65‐0.91, P=0.002) and clinical instability (HR for attaining clinical stability: 0.72, 95% CI: 0.61‐0.84, P<0.001). Patients with aspiration pneumonia presented with greater disease severity than those with nonaspiration pneumonia. Although there was no difference between groups in regard to temperature, respiratory rate, hyponatremia, or presence of pleural effusions or cavitary lesions, all other measured indices of disease severity were worse in patients with aspiration pneumonia. Patients with aspiration pneumonia were more likely to have cerebrovascular disease than those with nonaspiration pneumonia. Aspiration pneumonia patients also had increased prevalence of congestive heart failure. There was no appreciable difference between groups among other measured comorbidities.


The patient characteristics most associated with a physician diagnosis of aspiration pneumonia, identified using logistic regression, were confusion, residence in nursing home, and presence of cerebrovascular disease (odds ratio [OR]: of 4.4, 2.9, and 2.3, respectively), whereas renal disease was associated with decreased physician diagnosis of aspiration pneumonia over nonaspiration pneumonia (OR: 0.58) (Table 2).
| Covariate | Odds Ratio | 95% Confidence Intervals | P Value |
|---|---|---|---|
|
|||
| Demographics | |||
| Age, y | 1.00 | 0.991.01 | 0.948 |
| Male | 1.20 | 0.941.54 | 0.148 |
| Nursing home residence | 2.93 | 2.134.00 | <0.001 |
| Comorbidities | |||
| Cerebrovascular disease | 2.26 | 1.533.32 | <0.001 |
| Renal disease | 0.58 | 0.390.85 | 0.006 |
| Disease severity | |||
| Confusion | 4.41 | 3.405.72 | <0.001 |
| Hematocrit <30% | 1.59 | 1.062.33 | 0.020 |
| pH <7.35 | 1.67 | 1.102.47 | 0.013 |
| Temperature >37.8C or <35.6C | 1.60 | 1.072.35 | 0.019 |
| Multilobar pneumonia | 1.29 | 1.001.65 | 0.047 |
| Interaction terms | |||
| Age * cerebrovascular disease | 0.98 | 0.960.99 | 0.011 |
| Nursing home * cerebrovascular disease | 0.51 | 0.270.96 | 0.037 |
| Confusion * cerebrovascular disease | 0.70 | 0.421.17 | 0.175 |
Observed in‐patient mortality of aspiration pneumonia was 23%. This mortality was considerably higher than a mean PSI score of 123 would predict (class IV risk group, with expected 30‐day mortality of 8%9%[25]). The PSI score's ability to predict inpatient mortality in patients with aspiration pneumonia was moderate, with an area under the curve (AUC) of 0.71. This was similar to its performance in patients with nonaspiration pneumonia (AUC of 0.75) (Figure 3). These values are lower than the AUC of 0.81 for the PSI in predicting mortality derived from a meta‐analysis of 31 other studies.[26]

Our regression model after propensity score matching demonstrated that aspiration pneumonia independently confers a 2.3‐fold increased odds for inpatient mortality (95% CI: 1.56‐3.45, P<0.001).
DISCUSSION
Pneumonia patients with confusion, nursing home residence, or cerebrovascular disease are more likely to be diagnosed with aspiration pneumonia by clinicians. Although this is unsurprising, it is notable that these patients are more than twice as likely to die in the inpatient setting, even after accounting for age, comorbidities, and disease severity. These findings are similar to three previously published studies comparing aspiration and nonaspiration pneumonia at single institutions, albeit using different aspiration pneumonia definitions.[13, 14, 15] This study is the first large, multicenter, multinational study to demonstrate these findings.
Central to the interpretation of our results is the method of diagnosing aspiration versus nonaspiration. A bottom‐up method that relies on a clinician to check a box for aspiration may appear poorly reproducible. Because there is no diagnostic gold standard, clinicians may use different criteria to diagnose aspiration, creating potential for idiosyncratic noise. The strength of the wisdom of the crowd method used in this study is that an aggregate estimation from independent judgments may reduce the noise from individual judgments.[16] Although clinicians may vary in why they diagnose a particular patient as having aspiration pneumonia, it appears that the overwhelming reason for diagnosing a patient as having aspiration pneumonia is the presence of confusion, followed by previous nursing home residence or cerebrovascular disease. This finding has some face validity when compared with studies using an investigator definition, as altered mental status, chronic debility, and cerebrovascular disease are either prominent features of the definition of aspiration pneumonia[8] or frequently observed in patients with aspiration pneumonia.[13, 15] The distribution of cerebrovascular disease among our study's aspiration and nonaspiration pneumonia patients was similar to studies that used formal criteria in their definitions.[13, 15] Although nursing home residence was more likely in aspiration pneumonia patients, the majority of aspiration pneumonia patients were residing in the community, suggesting that aspiration is not simply a surrogate for healthcare‐associated pneumonia. Although patients with aspiration pneumonia are typically older than their nonaspiration counterparts, it appears that age is not a key determinant in the diagnosis of aspiration. With aspiration pneumonia, confusion, nursing home residence, and the presence of cerebrovascular disease are the greatest contributors in the clinical diagnosis, more than age.
Our data demonstrate that aspiration pneumonia confers increased odds for mortality, even after adjustment for age, disease severity, and comorbidities. These data suggest that aspiration pneumonia is a distinct entity from nonaspiration pneumonia, and that this disease is worse than nonaspiration CAP. If aspiration pneumonia is distinct from nonaspiration pneumonia, some unrecognized host factor other than age, disease severity, or the captured comorbidities decreases survival in aspiration pneumonia patients. However, it is also possible that aspiration pneumonia is merely a clinical designation for one end of the pneumonia spectrum, and we and others have failed to completely account for all measures of disease severity or all measures of comorbidities. Examples of unmeasured comorbidities would include presence of oropharyngeal dysphagia, which is not assessed in the database but could have a significant effect on clinical diagnosis. Unmeasured covariates can include measures beyond that of disease severity or comorbidity, such as the presence of a do not resuscitate (DNR) order, which could have a significant confounding effect on the observed association. A previous, single‐center study demonstrated that increased 30‐day mortality in aspiration pneumonia was mostly attributable to greater disease severity and comorbidities, although aspiration pneumonia independently conferred greater risk for adverse long‐term outcomes.[15] We propose that aspiration pneumonia represents a clinically distinct entity from nonaspiration pneumonia. Patients with chronic aspiration are often chronically malnourished and may have different oral flora than patients without chronic aspiration.[27, 28] Chronic aspiration has been associated with granulomatous reaction, organizing pneumonia, diffuse alveolar damage, and chronic bronchiolitis.[29] Chronic aspiration may elicit changes in the host physiology, and may render the host more susceptible to the development of secondary bacterial infection with morbid consequences.
The ability of the PSI to predict inpatient mortality was moderate (AUC only 0.7), with no significant additional discrimination between the aspiration and nonaspiration pneumonia groups. Although the PSI had moderate ability to predict inpatient mortality, the observed mortality was considerably higher than predicted. It is possible that the PSI incompletely captures clinically relevant comorbidities (eg, malnutrition). Further study to improve mortality prediction of aspiration pneumonia patients could employ sensitivity analysis to determine optimal thresholds and weighting of the PSI components.
Patients with aspiration pneumonia had longer hospital lengths of stay and took longer to achieve clinical stability than their nonaspiration counterparts. Time to clinical stability has been associated with increased posthospitalization mortality and is associated with time to switch from intravenous to oral antibiotics.[17] Although some component of hospital length‐of‐stay is subject to local practice patterns, time to clinical stability has explicit criteria for clinical improvement and failure, and therefore is less likely to be affected by local practice patterns.
We noted a relatively high (16%21%) incidence of prior antibiotic use among patients in this database. Analysis of antibiotic prescription patterns was limited, given the several different countries from which the database draws its cases. Although we used accepted criteria to define CAP cases, it is possible that this population may have a higher rate of resistant or uncommon pathogens than other studies of CAP that have populations with lower incidence of prior antibiotic use. Although not assessed, we suspect a significant component of the prior antibiotic use represented outpatient pneumonia treatment during the few days prior to visiting the hospital.
This study has several limitations, of which the most important may be that we used clinical determination for defining presence of aspiration pneumonia. This method is susceptible to the subjective perceptions of the treating clinician. We did not account for the effect of individual physicians in our model, although we did adjust for regional differences. The retrospective identification of patients allows for the possibility of selection bias, and therefore we have not attempted to make inferences regarding the relative incidence of pneumonia, nor did we adjust for temporal trends in diagnosis. The ratio of aspiration pneumonia patients to nonaspiration pneumonia patients may not necessarily reflect that observed in reality. Microbiologic and antibiotic data were unavailable for analysis. This study cannot inform on nonhospitalized patients with aspiration pneumonia, as only hospitalized patients were enrolled. The database identified cases of pneumonia, so it is possible for a patient to enter into the database more than once. Detection of mortality was limited to the inpatient setting rather than a set interval of 30 days. Inpatient mortality depends on length‐of‐stay patterns that may bias the mortality endpoint.[30] Also not assessed was the presence of a DNR order. It is possible that an older patient with greater comorbidities and disease severity may have care intentionally limited or withdrawn early by the family or clinicians.
Strengths of the study include its size and its multicenter, multinational population. The CAPO database is a large and well‐described population of patients with CAP.[17, 31] These attributes, as well as the clinician‐determined diagnosis, increase the generalizability of the study compared to a single‐center, single‐country study that employs investigator‐defined criteria.
CONCLUSION
Pneumonia patients with confusion, who are nursing home residence, and have cerebrovascular disease are more likely to be diagnosed with aspiration pneumonia by clinicians. Our clinician‐diagnosed cohort appears similar to those derived using an investigator definition. Patients with aspiration pneumonia are older, and have greater disease severity and more comorbidities than patients with nonaspiration pneumonia. They have greater mortality than their PSI score class would predict. Even after accounting for age, disease severity, and comorbidities, the presence of aspiration pneumonia independently conferred a greater than 2‐fold increase in inpatient mortality. These findings together suggest that aspiration pneumonia should be considered a distinct entity from typical pneumonia, and that additional research should be done in this field.
ACKNOWLEDGMENTS
Disclosures: M.J.L. contributed to the study design, data analysis, statistical analysis, and writing of the manuscript. P.P. contributed to the study design and revision of the manuscript for important intellectual content. T.W. and E.W. contributed to the study design, statistical analysis, and revision of the manuscript for important intellectual content. J.A.R. and N.C.D. contributed to the study design and revision of the manuscript for important intellectual content. All authors read and approved the final manuscript. M.L. takes responsibility for the integrity of the work as a whole, from inception to published article. This investigation was partly supported with funding from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health (grant 8UL1TR000105 [formerly UL1RR025764]). The authors report no conflicts of interest.
Pneumonia is a common clinical syndrome with well‐described epidemiology and microbiology. Aspiration pneumonia comprises 5% to 15% of patients with pneumonia acquired outside of the hospital,[1] but is less well characterized despite being a major syndrome of pneumonia in the elderly.[2, 3] Difficulties in studying aspiration pneumonia include the lack of a sensitive and specific marker for aspiration as well as the potential overlap between aspiration pneumonia and other forms of pneumonia.[4, 5, 6] Additionally, clinicians have difficulty distinguishing between aspiration pneumonia, which develops after the aspiration of oropharyngeal contents, and aspiration pneumonitis, wherein inhalation of gastric contents causes inflammation without the subsequent development of bacterial infection.[7, 8] Central to the study of aspiration pneumonia is whether it should exist as its own entity, or if aspiration is really a designation used for pneumonia in an older patient with greater comorbidities. The ability to clearly understand how a clinician diagnoses aspiration pneumonia, and whether that method has face validity with expert definitions may allow for improved future research, improved generalizability of current or past research, and possibly better clinical care.
Several validated mortality prediction models exist for community‐acquired pneumonia (CAP) using a variety of clinical predictors, but their performance in patients with aspiration pneumonia is less well characterized. Most studies validating pneumonia severity scoring systems excluded aspiration pneumonia from their study population.[9, 10, 11] Severity scoring systems for CAP may not accurately predict disease severity in patients with aspiration pneumonia. The CURB‐65[9] (confusion, uremia, respiratory rate, blood pressure, age 65 years) and the eCURB[12] scoring systems are poor predictors of mortality in patients with aspiration pneumonia, perhaps because they do not account for patient comorbidities.[13] The pneumonia severity index (PSI)[10] might predict mortality better than CURB‐65 in the aspiration population due to the inclusion of comorbidities.
Previous studies have demonstrated that patients with aspiration pneumonia are older and have greater disease severity and more comorbidities.[13, 14, 15] These single‐center studies also demonstrated greater mortality, more frequent admission to an intensive care unit (ICU), and longer hospital lengths of stay in patients with aspiration pneumonia. These studies identified aspiration pneumonia by the presence of a risk factor for aspiration[15] or by physician billing codes.[13] In practice, however, the bedside clinician diagnoses a patient as having aspiration pneumonia, but the logic is likely vague and inconsistent. Despite the potential for variability with individual judgment, an aggregate estimation from independent judgments may perform better than individual judgments.[16] Because there is no gold standard for defining aspiration pneumonia, all previous research has been limited to definitions created by investigators. This multicenter study seeks to determine what clinical characteristics lead physicians to diagnose a patient as having aspiration pneumonia, and whether or not the clinician‐derived diagnosis is distinct and clinically useful.
Our objectives were to: (1) identify covariates associated with bedside clinicians diagnosing a pneumonia patient as having aspiration pneumonia; (2) compare aspiration pneumonia and nonaspiration pneumonia in regard to disease severity, patient demographics, comorbidities, and clinical outcomes; and (3) measure the performance of the PSI in aspiration pneumonia versus nonaspiration pneumonia.
PATIENTS AND METHODS
Study Design and Setting
We performed a secondary analysis of the Community‐Acquired Pneumonia Organization (CAPO) database, which contains retrospectively collected data from 71 hospitals in 16 countries between June 2001 and December 2012. In each participating center, primary investigators selected nonconsecutive, adult hospitalized patients diagnosed with CAP. To decrease systematic selection biases, the selection of patients with CAP for enrollment in the trial was based on the date of hospital admission. Each investigator completed a case report form that was transferred via the internet to the CAPO study center at the University of Louisville (Louisville, KY). A sample of the data collection form is available at the study website (
Inclusion and Exclusion Criteria
Patients 18 years of age and satisfying criteria for CAP were included in this study. A diagnosis of CAP required a new pulmonary infiltrate at time of hospitalization, and at least 1 of the following: new or increased cough; leukocytosis; leukopenia, or left shift pattern on white blood cell count; and temperature >37.8C or <35.6 C. We excluded patients with pneumonia attributed to mycobacterial or fungal infection, and patients infected with human immunodeficiency virus, as we believed these types of pneumonia differ fundamentally from typical CAP.
Patient Variables
Patient variables included presence of aspiration pneumonia, laboratory data, comorbidities, and measures of disease severity, including the PSI. The clinician made a clinical diagnosis of the presence or absence of aspiration for each patient by marking a box on the case report form. Outcomes included in‐hospital mortality, hospital length of stay up to 14 days, and time to clinical stability up to 8 days. All variables were obtained directly from the case report form. In accordance with previously published definitions, we defined clinical stability as the day the following criteria were all met: improved clinical signs (improved cough and shortness of breath), lack of fever for >8 hours, improving leukocytosis (decreased at least 10% from the previous day), and tolerating oral intake.[17, 18]
Statistical Analysis
Baseline characteristics of patients with aspiration and nonaspiration CAP were compared using 2 or Fisher exact tests for categorical variables and the Mann‐Whitney U test for continuous variables.
To determine which patient variables were important in the physician diagnosis of aspiration pneumonia, we performed logistic regression with initial covariates comprising the demographic, comorbidity, and disease severity measurements listed in Table 1. We included interactions between cerebrovascular disease and age, nursing home status, and confusion to improve model fit. We centered all variables (including binary indicators) according to the method outlined by Kraemer and Blasey to improve interpretation of the main effects.[19]
| Aspiration Pneumonia, N=451 | Nonaspiration Pneumonia, N=4,734 | P Value | |
|---|---|---|---|
|
|||
| Demographics | |||
| Age, y | 79 (6587) | 69 (5380) | <0.001 |
| % Male | 59% | 60% | 0.58 |
| Nursing home residence | 25% | 5% | <0.001 |
| Recent (30 days) antibiotic use | 21% | 16% | 0.017 |
| Comorbidities | |||
| Cerebrovascular disease | 35% | 14% | <0.001 |
| Chronic obstructive pulmonary disease | 25% | 27% | 0.62 |
| Congestive heart failure | 23% | 19% | 0.027 |
| Diabetes | 18% | 18% | 0.85 |
| Cancer | 12% | 10% | 0.12 |
| Renal disease | 10% | 11% | 0.53 |
| Liver disease | 6% | 5% | 0.29 |
| Disease severity | |||
| Pneumonia severity index | 123 (99153) | 92 (68117) | <0.001 |
| Confusion | 49% | 12% | <0.001 |
| PaO2 <60 mm Hg | 43% | 33% | <0.001 |
| BUN >30 g/dL | 42% | 23% | <0.001 |
| Multilobar pneumonia | 34% | 28% | 0.003 |
| Pleural effusion | 25% | 21% | 0.07 |
| Respiratory rate >30 breaths/minute | 21% | 20% | 0.95 |
| pH <7.35 | 13% | 5% | <0.001 |
| Hematocrit <30% | 11% | 6% | 0.001 |
| Temperature >37.8C or <35.6C | 9% | 7% | 0.30 |
| Systolic blood pressure <90 mm Hg | 8% | 9% | 0.003 |
| Sodium <130 mEq/L | 8% | 6% | 0.08 |
| Heart rate >125 beats/minute | 8% | 5% | 0.71 |
| Glucose >250 mg/dL | 6% | 7% | 0.06 |
| Cavitary lesion | 0% | 0% | 0.67 |
| Clinical outcomes | |||
| In‐hospital mortality | 23% | 9% | <0.001 |
| Intensive care unit admission | 19% | 13% | 0.002 |
| Hospital length of stay, d | 9 (515) | 7 (412) | <0.001 |
| Time to clinical stability, d | 8 (48) | 4 (38) | <0.001 |
To determine if aspiration pneumonia had worse clinical outcomes compared to nonaspiration pneumonia, multiple methods were used. To compare the differences between the 2 groups with respect to time to clinical stability and length of hospital stay, we constructed Kaplan‐Meier survival curves and Cox proportional hazards regression models. The log‐rank test was used to determine statistical differences between the Kaplan‐Meier survival curves. To compare the impact of aspiration on mortality in patients with CAP, we conducted a propensity scorematched analysis. We chose propensity score matching over traditional logistic regression to balance variables among groups and to avoid the potential for overfit and multicollinearity. We considered a variable balanced after matching if its standardized difference was <10. All variables in the propensity scorematched analysis were balanced.
Although our dataset contained minimal missing data, we imputed any missing values to maintain the full study population in the creation of the propensity score. Missing data were imputed using the aregImpute function of the hmisc package of R (The R Foundation for Statistical Computing, Vienna, Austria).[20, 21] We built the propensity score model using a variable selection algorithm described by Bursac et al.[22] Our model included variables for region (United States/Canada, Europe, Asia/Africa or Latin America) and the variables listed in Table 1, with the exception of the PSI and the 4 clinical outcomes. Given that previous analyses accounting for clustering by physician did not substantially affect our results,[23] our model did not include physician‐level variables and did not account for the clustering effects of physicians. Using the propensity scores generated from this model, we matched a case of aspiration CAP with a case of nonaspiration CAP.[24] We then constructed a general linear model using the matched dataset to obtain the magnitude of effect of aspiration on mortality.
We used receiver operating characteristic curves to define the diagnostic accuracy of the pneumonia severity index for the prediction of mortality among patients with aspiration pneumonia and those with nonaspiration pneumonia. SAS version 9.3 (SAS Institute, Cary, NC) and R version 2.15.3 (The R Foundation for Statistical Computing) were used for all analyses. P values of 0.05 were considered statistically significant in all analyses.
RESULTS
Our initial query, after exclusion criteria, yielded a study population of 5185 patients (Figure 1). We compared 451 patients diagnosed with aspiration pneumonia to 4734 with CAP (Figure 1). Patient characteristics are summarized in Table 1. Patients with aspiration pneumonia were older, more likely to live in a nursing home, had greater disease severity, and were more likely to be admitted to an ICU. Patients with aspiration pneumonia had longer adjusted hospital lengths of stay and took more days to achieve clinical stability than patients with nonaspiration pneumonia (Figure 2). After adjusting for all variables in Table 1, the Cox proportional hazards models demonstrated that aspiration pneumonia was associated with ongoing hospitalization (hazard ratio [HR] for discharge: 0.77, 95% confidence interval [CI]: 0.65‐0.91, P=0.002) and clinical instability (HR for attaining clinical stability: 0.72, 95% CI: 0.61‐0.84, P<0.001). Patients with aspiration pneumonia presented with greater disease severity than those with nonaspiration pneumonia. Although there was no difference between groups in regard to temperature, respiratory rate, hyponatremia, or presence of pleural effusions or cavitary lesions, all other measured indices of disease severity were worse in patients with aspiration pneumonia. Patients with aspiration pneumonia were more likely to have cerebrovascular disease than those with nonaspiration pneumonia. Aspiration pneumonia patients also had increased prevalence of congestive heart failure. There was no appreciable difference between groups among other measured comorbidities.


The patient characteristics most associated with a physician diagnosis of aspiration pneumonia, identified using logistic regression, were confusion, residence in nursing home, and presence of cerebrovascular disease (odds ratio [OR]: of 4.4, 2.9, and 2.3, respectively), whereas renal disease was associated with decreased physician diagnosis of aspiration pneumonia over nonaspiration pneumonia (OR: 0.58) (Table 2).
| Covariate | Odds Ratio | 95% Confidence Intervals | P Value |
|---|---|---|---|
|
|||
| Demographics | |||
| Age, y | 1.00 | 0.991.01 | 0.948 |
| Male | 1.20 | 0.941.54 | 0.148 |
| Nursing home residence | 2.93 | 2.134.00 | <0.001 |
| Comorbidities | |||
| Cerebrovascular disease | 2.26 | 1.533.32 | <0.001 |
| Renal disease | 0.58 | 0.390.85 | 0.006 |
| Disease severity | |||
| Confusion | 4.41 | 3.405.72 | <0.001 |
| Hematocrit <30% | 1.59 | 1.062.33 | 0.020 |
| pH <7.35 | 1.67 | 1.102.47 | 0.013 |
| Temperature >37.8C or <35.6C | 1.60 | 1.072.35 | 0.019 |
| Multilobar pneumonia | 1.29 | 1.001.65 | 0.047 |
| Interaction terms | |||
| Age * cerebrovascular disease | 0.98 | 0.960.99 | 0.011 |
| Nursing home * cerebrovascular disease | 0.51 | 0.270.96 | 0.037 |
| Confusion * cerebrovascular disease | 0.70 | 0.421.17 | 0.175 |
Observed in‐patient mortality of aspiration pneumonia was 23%. This mortality was considerably higher than a mean PSI score of 123 would predict (class IV risk group, with expected 30‐day mortality of 8%9%[25]). The PSI score's ability to predict inpatient mortality in patients with aspiration pneumonia was moderate, with an area under the curve (AUC) of 0.71. This was similar to its performance in patients with nonaspiration pneumonia (AUC of 0.75) (Figure 3). These values are lower than the AUC of 0.81 for the PSI in predicting mortality derived from a meta‐analysis of 31 other studies.[26]

Our regression model after propensity score matching demonstrated that aspiration pneumonia independently confers a 2.3‐fold increased odds for inpatient mortality (95% CI: 1.56‐3.45, P<0.001).
DISCUSSION
Pneumonia patients with confusion, nursing home residence, or cerebrovascular disease are more likely to be diagnosed with aspiration pneumonia by clinicians. Although this is unsurprising, it is notable that these patients are more than twice as likely to die in the inpatient setting, even after accounting for age, comorbidities, and disease severity. These findings are similar to three previously published studies comparing aspiration and nonaspiration pneumonia at single institutions, albeit using different aspiration pneumonia definitions.[13, 14, 15] This study is the first large, multicenter, multinational study to demonstrate these findings.
Central to the interpretation of our results is the method of diagnosing aspiration versus nonaspiration. A bottom‐up method that relies on a clinician to check a box for aspiration may appear poorly reproducible. Because there is no diagnostic gold standard, clinicians may use different criteria to diagnose aspiration, creating potential for idiosyncratic noise. The strength of the wisdom of the crowd method used in this study is that an aggregate estimation from independent judgments may reduce the noise from individual judgments.[16] Although clinicians may vary in why they diagnose a particular patient as having aspiration pneumonia, it appears that the overwhelming reason for diagnosing a patient as having aspiration pneumonia is the presence of confusion, followed by previous nursing home residence or cerebrovascular disease. This finding has some face validity when compared with studies using an investigator definition, as altered mental status, chronic debility, and cerebrovascular disease are either prominent features of the definition of aspiration pneumonia[8] or frequently observed in patients with aspiration pneumonia.[13, 15] The distribution of cerebrovascular disease among our study's aspiration and nonaspiration pneumonia patients was similar to studies that used formal criteria in their definitions.[13, 15] Although nursing home residence was more likely in aspiration pneumonia patients, the majority of aspiration pneumonia patients were residing in the community, suggesting that aspiration is not simply a surrogate for healthcare‐associated pneumonia. Although patients with aspiration pneumonia are typically older than their nonaspiration counterparts, it appears that age is not a key determinant in the diagnosis of aspiration. With aspiration pneumonia, confusion, nursing home residence, and the presence of cerebrovascular disease are the greatest contributors in the clinical diagnosis, more than age.
Our data demonstrate that aspiration pneumonia confers increased odds for mortality, even after adjustment for age, disease severity, and comorbidities. These data suggest that aspiration pneumonia is a distinct entity from nonaspiration pneumonia, and that this disease is worse than nonaspiration CAP. If aspiration pneumonia is distinct from nonaspiration pneumonia, some unrecognized host factor other than age, disease severity, or the captured comorbidities decreases survival in aspiration pneumonia patients. However, it is also possible that aspiration pneumonia is merely a clinical designation for one end of the pneumonia spectrum, and we and others have failed to completely account for all measures of disease severity or all measures of comorbidities. Examples of unmeasured comorbidities would include presence of oropharyngeal dysphagia, which is not assessed in the database but could have a significant effect on clinical diagnosis. Unmeasured covariates can include measures beyond that of disease severity or comorbidity, such as the presence of a do not resuscitate (DNR) order, which could have a significant confounding effect on the observed association. A previous, single‐center study demonstrated that increased 30‐day mortality in aspiration pneumonia was mostly attributable to greater disease severity and comorbidities, although aspiration pneumonia independently conferred greater risk for adverse long‐term outcomes.[15] We propose that aspiration pneumonia represents a clinically distinct entity from nonaspiration pneumonia. Patients with chronic aspiration are often chronically malnourished and may have different oral flora than patients without chronic aspiration.[27, 28] Chronic aspiration has been associated with granulomatous reaction, organizing pneumonia, diffuse alveolar damage, and chronic bronchiolitis.[29] Chronic aspiration may elicit changes in the host physiology, and may render the host more susceptible to the development of secondary bacterial infection with morbid consequences.
The ability of the PSI to predict inpatient mortality was moderate (AUC only 0.7), with no significant additional discrimination between the aspiration and nonaspiration pneumonia groups. Although the PSI had moderate ability to predict inpatient mortality, the observed mortality was considerably higher than predicted. It is possible that the PSI incompletely captures clinically relevant comorbidities (eg, malnutrition). Further study to improve mortality prediction of aspiration pneumonia patients could employ sensitivity analysis to determine optimal thresholds and weighting of the PSI components.
Patients with aspiration pneumonia had longer hospital lengths of stay and took longer to achieve clinical stability than their nonaspiration counterparts. Time to clinical stability has been associated with increased posthospitalization mortality and is associated with time to switch from intravenous to oral antibiotics.[17] Although some component of hospital length‐of‐stay is subject to local practice patterns, time to clinical stability has explicit criteria for clinical improvement and failure, and therefore is less likely to be affected by local practice patterns.
We noted a relatively high (16%21%) incidence of prior antibiotic use among patients in this database. Analysis of antibiotic prescription patterns was limited, given the several different countries from which the database draws its cases. Although we used accepted criteria to define CAP cases, it is possible that this population may have a higher rate of resistant or uncommon pathogens than other studies of CAP that have populations with lower incidence of prior antibiotic use. Although not assessed, we suspect a significant component of the prior antibiotic use represented outpatient pneumonia treatment during the few days prior to visiting the hospital.
This study has several limitations, of which the most important may be that we used clinical determination for defining presence of aspiration pneumonia. This method is susceptible to the subjective perceptions of the treating clinician. We did not account for the effect of individual physicians in our model, although we did adjust for regional differences. The retrospective identification of patients allows for the possibility of selection bias, and therefore we have not attempted to make inferences regarding the relative incidence of pneumonia, nor did we adjust for temporal trends in diagnosis. The ratio of aspiration pneumonia patients to nonaspiration pneumonia patients may not necessarily reflect that observed in reality. Microbiologic and antibiotic data were unavailable for analysis. This study cannot inform on nonhospitalized patients with aspiration pneumonia, as only hospitalized patients were enrolled. The database identified cases of pneumonia, so it is possible for a patient to enter into the database more than once. Detection of mortality was limited to the inpatient setting rather than a set interval of 30 days. Inpatient mortality depends on length‐of‐stay patterns that may bias the mortality endpoint.[30] Also not assessed was the presence of a DNR order. It is possible that an older patient with greater comorbidities and disease severity may have care intentionally limited or withdrawn early by the family or clinicians.
Strengths of the study include its size and its multicenter, multinational population. The CAPO database is a large and well‐described population of patients with CAP.[17, 31] These attributes, as well as the clinician‐determined diagnosis, increase the generalizability of the study compared to a single‐center, single‐country study that employs investigator‐defined criteria.
CONCLUSION
Pneumonia patients with confusion, who are nursing home residence, and have cerebrovascular disease are more likely to be diagnosed with aspiration pneumonia by clinicians. Our clinician‐diagnosed cohort appears similar to those derived using an investigator definition. Patients with aspiration pneumonia are older, and have greater disease severity and more comorbidities than patients with nonaspiration pneumonia. They have greater mortality than their PSI score class would predict. Even after accounting for age, disease severity, and comorbidities, the presence of aspiration pneumonia independently conferred a greater than 2‐fold increase in inpatient mortality. These findings together suggest that aspiration pneumonia should be considered a distinct entity from typical pneumonia, and that additional research should be done in this field.
ACKNOWLEDGMENTS
Disclosures: M.J.L. contributed to the study design, data analysis, statistical analysis, and writing of the manuscript. P.P. contributed to the study design and revision of the manuscript for important intellectual content. T.W. and E.W. contributed to the study design, statistical analysis, and revision of the manuscript for important intellectual content. J.A.R. and N.C.D. contributed to the study design and revision of the manuscript for important intellectual content. All authors read and approved the final manuscript. M.L. takes responsibility for the integrity of the work as a whole, from inception to published article. This investigation was partly supported with funding from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health (grant 8UL1TR000105 [formerly UL1RR025764]). The authors report no conflicts of interest.
- , , , et al. Severe community‐acquired pneumonia. Epidemiology and prognostic factors. Am Rev Respir Dis. 1991;144(2):312–318.
- , , . Risk factors for pneumonia in the elderly. Am J Med. 1994;96(4):313–320.
- , . Aspiration pneumonia and dysphagia in the elderly. Chest. 2003;124(1):328–336.
- , , . Pneumonia versus aspiration pneumonitis in nursing home residents: diagnosis and management. J Am Geriatr Soc. 2003;51(1):17–23.
- . Aspiration pneumonia: mixing apples with oranges and tangerines. Crit Care Med. 2004;32(5):1236; author reply 1236–1237.
- , , , , . Epidemiology and impact of aspiration pneumonia in patients undergoing surgery in Maryland, 1999–2000. Crit Care Med. 2003;31(7):1930–1937.
- . Aspiration syndromes: aspiration pneumonia and pneumonitis. Hosp Pract (Minneap). 2010;38(1):35–42.
- . Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344(9):665–671.
- , , , et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58(5):377–382.
- , , , et al. Comparison of a disease‐specific and a generic severity of illness measure for patients with community‐acquired pneumonia. J Gen Intern Med. 1995;10(7):359–368.
- , , , et al. Development and validation of a clinical prediction rule for severe community‐acquired pneumonia. Am J Respir Crit Care Med. 2006;174(11):1249–1256.
- , , , et al. CURB‐65 pneumonia severity assessment adapted for electronic decision support. Chest. 2011;140(1):156–163.
- , , , . Mortality, morbidity, and disease severity of patients with aspiration pneumonia. J Hosp Med. 2013;8(2):83–90.
- , , , , . Pneumonia Severity Index (PSI), CURB‐65, and mortality in hospitalized elderly patients with aspiration pneumonia [in German]. Z Gerontol Geriatr. 2011;44(4):229–234.
- , , , , . Risk factors for aspiration in community‐acquired pneumonia: analysis of a hospitalized UK cohort. Am J Med. 2013;126(11):995–1001.
- , , , . The wisdom of the crowd in combinatorial problems. Cogn Sci. 2012;36(3):452–470.
- , , , et al. Association between time to clinical stability and outcomes after discharge in hospitalized patients with community‐acquired pneumonia. Chest. 2011;140(2):482–488.
- . Clinical stability and switch therapy in hospitalised patients with community‐acquired pneumonia: are we there yet? Eur Respir J. 2013;41(1):5–6.
- , . Centring in regression analyses: a strategy to prevent errors in statistical inference. Int J Methods Psychiatr Res. 2004;13(3):141–151.
- . Hmisc: Harrell miscellaneous. Available at: http://CRAN.R‐project.org/package=Hmisc. Published Sept 12, 2014. Last accessed Oct 27, 2014.
- , . Multiple imputation for the fatal accident reporting system. J R Stat Soc Ser C Appl Stat. 1991;40(1):13–29.
- , , , . Purposeful selection of variables in logistic regression. Source Code Biol Med. 2008;3:17.
- , , , , ; CAPO authors. Mortality differences among hospitalized patients with community‐acquired pneumonia in three world regions: results from the Community‐Acquired Pneumonia Organization (CAPO) International Cohort Study. Respir Med. 2013;107(7):1101–1111.
- . Reducing bias in a propensity score matched‐pair sample using greedy matching techniques. In: Proceedings of the 26th Annual SAS Users Group International Conference. Cary, NC: SAS Institute Inc.; 2001:214–226. Available at: http://www2.sas.com/proceedings/sugi26/p214–26.pdf. Last accessed Oct 27, 2014.
- , , , et al. A prediction rule to identify low‐risk patients with community‐acquired pneumonia. N Engl J Med. 1997;336(4):243–250.
- , , , et al. Severity assessment tools for predicting mortality in hospitalised patients with community‐acquired pneumonia. Systematic review and meta‐analysis. Thorax. 2010;65(10):878–883.
- , , , , , . Prevalence and prognostic implications of dysphagia in elderly patients with pneumonia. Age Ageing. 2010;39(1):39–45.
- , . The association between oral microorgansims and aspiration pneumonia in the institutionalized elderly: review and recommendations. Dysphagia. 2010;25(4):307–322.
- , . Histopathology of aspiration pneumonia not associated with food or other particulate matter: a clinicopathologic study of 10 cases diagnosed on biopsy. Am J Surg Pathol. 2011;35(3):426–431.
- , , , , , . Interpreting hospital mortality data. The role of clinical risk adjustment. JAMA. 1988;260(24):3611–3616.
- , , , . Hospitalization for community‐acquired pneumonia: the pneumonia severity index vs clinical judgment. Chest. 2003;124(1):121–124.
- , , , et al. Severe community‐acquired pneumonia. Epidemiology and prognostic factors. Am Rev Respir Dis. 1991;144(2):312–318.
- , , . Risk factors for pneumonia in the elderly. Am J Med. 1994;96(4):313–320.
- , . Aspiration pneumonia and dysphagia in the elderly. Chest. 2003;124(1):328–336.
- , , . Pneumonia versus aspiration pneumonitis in nursing home residents: diagnosis and management. J Am Geriatr Soc. 2003;51(1):17–23.
- . Aspiration pneumonia: mixing apples with oranges and tangerines. Crit Care Med. 2004;32(5):1236; author reply 1236–1237.
- , , , , . Epidemiology and impact of aspiration pneumonia in patients undergoing surgery in Maryland, 1999–2000. Crit Care Med. 2003;31(7):1930–1937.
- . Aspiration syndromes: aspiration pneumonia and pneumonitis. Hosp Pract (Minneap). 2010;38(1):35–42.
- . Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344(9):665–671.
- , , , et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58(5):377–382.
- , , , et al. Comparison of a disease‐specific and a generic severity of illness measure for patients with community‐acquired pneumonia. J Gen Intern Med. 1995;10(7):359–368.
- , , , et al. Development and validation of a clinical prediction rule for severe community‐acquired pneumonia. Am J Respir Crit Care Med. 2006;174(11):1249–1256.
- , , , et al. CURB‐65 pneumonia severity assessment adapted for electronic decision support. Chest. 2011;140(1):156–163.
- , , , . Mortality, morbidity, and disease severity of patients with aspiration pneumonia. J Hosp Med. 2013;8(2):83–90.
- , , , , . Pneumonia Severity Index (PSI), CURB‐65, and mortality in hospitalized elderly patients with aspiration pneumonia [in German]. Z Gerontol Geriatr. 2011;44(4):229–234.
- , , , , . Risk factors for aspiration in community‐acquired pneumonia: analysis of a hospitalized UK cohort. Am J Med. 2013;126(11):995–1001.
- , , , . The wisdom of the crowd in combinatorial problems. Cogn Sci. 2012;36(3):452–470.
- , , , et al. Association between time to clinical stability and outcomes after discharge in hospitalized patients with community‐acquired pneumonia. Chest. 2011;140(2):482–488.
- . Clinical stability and switch therapy in hospitalised patients with community‐acquired pneumonia: are we there yet? Eur Respir J. 2013;41(1):5–6.
- , . Centring in regression analyses: a strategy to prevent errors in statistical inference. Int J Methods Psychiatr Res. 2004;13(3):141–151.
- . Hmisc: Harrell miscellaneous. Available at: http://CRAN.R‐project.org/package=Hmisc. Published Sept 12, 2014. Last accessed Oct 27, 2014.
- , . Multiple imputation for the fatal accident reporting system. J R Stat Soc Ser C Appl Stat. 1991;40(1):13–29.
- , , , . Purposeful selection of variables in logistic regression. Source Code Biol Med. 2008;3:17.
- , , , , ; CAPO authors. Mortality differences among hospitalized patients with community‐acquired pneumonia in three world regions: results from the Community‐Acquired Pneumonia Organization (CAPO) International Cohort Study. Respir Med. 2013;107(7):1101–1111.
- . Reducing bias in a propensity score matched‐pair sample using greedy matching techniques. In: Proceedings of the 26th Annual SAS Users Group International Conference. Cary, NC: SAS Institute Inc.; 2001:214–226. Available at: http://www2.sas.com/proceedings/sugi26/p214–26.pdf. Last accessed Oct 27, 2014.
- , , , et al. A prediction rule to identify low‐risk patients with community‐acquired pneumonia. N Engl J Med. 1997;336(4):243–250.
- , , , et al. Severity assessment tools for predicting mortality in hospitalised patients with community‐acquired pneumonia. Systematic review and meta‐analysis. Thorax. 2010;65(10):878–883.
- , , , , , . Prevalence and prognostic implications of dysphagia in elderly patients with pneumonia. Age Ageing. 2010;39(1):39–45.
- , . The association between oral microorgansims and aspiration pneumonia in the institutionalized elderly: review and recommendations. Dysphagia. 2010;25(4):307–322.
- , . Histopathology of aspiration pneumonia not associated with food or other particulate matter: a clinicopathologic study of 10 cases diagnosed on biopsy. Am J Surg Pathol. 2011;35(3):426–431.
- , , , , , . Interpreting hospital mortality data. The role of clinical risk adjustment. JAMA. 1988;260(24):3611–3616.
- , , , . Hospitalization for community‐acquired pneumonia: the pneumonia severity index vs clinical judgment. Chest. 2003;124(1):121–124.
© 2014 Society of Hospital Medicine